User login
Borderline personality disorder diagnosis: To tell or not to tell patients?
News of actor/comedian Pete Davidson expressing relief after finally receiving a diagnosis of borderline personality disorder (BPD) prompted a recent Twitter discussion among physicians regarding the ongoing debate on whether or not to tell a patient he or she has this diagnosis.
“I’ve heard from [many] trainees that they were told never to tell a patient they had BPD, but I can hardly think of anything more paternalistic and stigmatizing,” Amy Barnhorst, MD, vice chair of community psychiatry at University of California, Davis, tweeted.
“Most patients, when I explain it to them, have this kind of reaction – they feel relieved and understood,” she added.
“I was told that as well [not to tell] in one of my practicum placements,” one respondent who identified herself as a clinical/forensic psychologist tweeted back. “I said it anyway and the person was relieved there was a name for what they were living with.”
However, others disagreed with Dr. Barnhorst, noting that BPD is a very serious, stigmatizing, and challenging disorder to treat and, because of this, may cause patients to lose hope.
Still, Dr. Barnhorst stands by her position. Although “there is a negative stigma against a diagnosis of BPD,” that idea more often comes from the clinician instead of the patient, she said.
“I’ve never had a patient say, ‘how dare you call me that!’ like it was an insult,” she said in an interview. Not disclosing a diagnosis “is like you’re not trusting a patient to be a reasonable adult human about this.”
‘Hard diagnosis’
Although BPD is a “hard diagnosis, we would never withhold a diagnosis of cancer or liver disease or something else we knew patients didn’t want but that we were going to try and treat them for,” said Dr. Barnhorst.
BPD is linked to significant morbidity because of its common association with comorbid conditions, such as major depressive disorder, substance use disorders, and dysthymia. A history of self-harm is present in 70%-75% of these patients and some estimates suggest up to 9% of individuals with BPD die by suicide.
In an article published in Innovations in Clinical Neuroscience investigators discussed “ethical and clinical questions psychiatrists should consider” when treating BPD, including whether a diagnosis should be shared with a patient.
After such a diagnosis a patient may “react intensely in negative ways and these responses may be easily triggered,” the researchers wrote.
“A propensity that will likely cause psychiatrists anguish, however, is BPD patients’ increased likelihood of attempting suicide,” they added. Part of the problem has been that, in the past, it was thought that a BPD prognosis was untreatable. However, the researchers note that is no longer the case.
Still, Kaz Nelson, MD, associate professor of psychiatry and behavioral sciences at the University of Minnesota, Minneapolis, has labeled BPD a so-called “asterisk” disorder.
As she wrote in a recent blog, “We tell patients when they meet criteria for a medical diagnosis.* We show compassion and nonjudgmentalism to patients.* We do not discriminate against patients.*” However, the asterisk for each of these statements is: *Except for those with BPD.
Ongoing debate
Starting around the 1980s, the DSM listed personality disorders under the No. 2 Axis, which is for conditions with symptoms that are “not mitigatable,” said Dr. Nelson.
“It really started as well-meaning therapists who care about their patients who wanted to develop some precision in understanding people, and them starting to notice some patterns that can get in the way of optimal function,” she said in an interview.
The thought was not to disclose these diagnoses “because that was for you to understand, and for the patient to discover these patterns over time in the course of your work together,” Dr. Nelson added.
Although treatment for BPD used to be virtually nonexistent, there is now hope – especially with dialectic-behavior therapy (DBT), which uses mindfulness to teach patients how to control emotions and improve relationships.
According to the National Education Alliance for BPD, other useful treatments include mentalization-based therapy, transference-focused therapy, and “good psychiatric management.” Although there are currently no approved medications for BPD, some drugs are used to treat comorbid conditions such as depression or anxiety.
“We now know that people recover, and the whole paradigm has been turned on its head,” Dr. Nelson said. For example, “we no longer categorize these things as treatable or untreatable, which was a very positive move.”
So why is the field still debating the issue of diagnosis disclosure?
“To this day there are different psychiatrists and some medical school curricula that continue to teach that personality disorders are long-term, fixed, and nontreatable – and that it’s kind of disparaging to give this kind of diagnosis to a patient,” Dr. Nelson said.
Dr. Nelson, also the vice chair for education at the University of Minnesota, Minneapolis, medical school, reported that there “we acknowledge BPD’s painful history and that there are these misconceptions. They’re going to be on the front line of combating discrimination and the idea that if you see a patient with possible BPD coming you should run. That’s just unacceptable.”
Dr. Nelson noted that the idea of disclosing a BPD diagnosis is less controversial now than in the past, but “the whole thing is still under debate, and treatment guidelines [on BPD] are old and expired.”
Criteria for BPD were not updated when the DSM-5 was published in 2013, and that needs to be fixed, Dr. Nelson added. “In the meantime, we’re trying to get the word out that it’s okay to interact with people about the diagnosis, discuss treatment plans, and manage it as one would with any other psychiatric or medical illness.”
An evolution, not a debate
Paul Appelbaum, MD, past president of the American Psychiatric Association and current chair of the organization’s DSM steering committee, said in an interview that he hasn’t been involved in any recent debate on this issue.
“I think practice has changed to the point where the general practice is to discuss patient diagnoses with [patients] openly. Patients appreciate that and psychiatrists have come to see the advantages of it,” said Dr. Appelbaum, a professor of psychiatry, medicine, and law at Columbia University, New York.
Dr. Appelbaum noted that patients also increasingly have access to their medical records, “so the reality is that it’s no longer possible in many cases to withhold a diagnosis.”
he said. “Maybe not everyone is entirely on board yet but there has been a sea change in psychiatric practices.”
Asked whether there needs to be some type of guideline update or statement released by the APA regarding BPD, Dr. Appelbaum said he doesn’t think the overall issue is BPD specific but applies to all psychiatric diagnoses.
“To the extent that there are still practitioners today that are telling students or residents [not to disclose], I would guess that they were trained a very long time ago and have not adapted to the new world,” he said.
“I don’t want to speak for the APA, but speaking for myself: I certainly encourage residents that I teach to be open about a diagnosis. It’s not just clinically helpful in some cases, it’s also ethically required from the perspective of allowing patients to make appropriate decisions about their treatment. And arguably it’s legally required as well, as part of the informed consent requirement,” Dr. Appelbaum said.
Regarding DSM updates, he noted that the committee “looks to the field to propose to us additions or changes to the DSM that are warranted by data that have been gathered since the DSM-5 came out.” There is a process set up on the DSM’s website to review such proposals.
In addition, Dr. Appelbaum said that there have been discussions about using a new model “that focuses on dimensions rather than on discreet categories” in order to classify personality disorders.
“There’s a group out there that is formulating a proposal that they will submit to us” on this, he added. “That’s the major discussion that is going on right now and it would clearly have implications for borderline as well as all the other personality disorders.”
In a statement, the APA said practice guidelines for BPD are currently under review and that the organization does not have a “position statement” on BPD for clinicians. The last update to its guideline was in the early 2000s.
A version of this article first appeared on Medscape.com.
News of actor/comedian Pete Davidson expressing relief after finally receiving a diagnosis of borderline personality disorder (BPD) prompted a recent Twitter discussion among physicians regarding the ongoing debate on whether or not to tell a patient he or she has this diagnosis.
“I’ve heard from [many] trainees that they were told never to tell a patient they had BPD, but I can hardly think of anything more paternalistic and stigmatizing,” Amy Barnhorst, MD, vice chair of community psychiatry at University of California, Davis, tweeted.
“Most patients, when I explain it to them, have this kind of reaction – they feel relieved and understood,” she added.
“I was told that as well [not to tell] in one of my practicum placements,” one respondent who identified herself as a clinical/forensic psychologist tweeted back. “I said it anyway and the person was relieved there was a name for what they were living with.”
However, others disagreed with Dr. Barnhorst, noting that BPD is a very serious, stigmatizing, and challenging disorder to treat and, because of this, may cause patients to lose hope.
Still, Dr. Barnhorst stands by her position. Although “there is a negative stigma against a diagnosis of BPD,” that idea more often comes from the clinician instead of the patient, she said.
“I’ve never had a patient say, ‘how dare you call me that!’ like it was an insult,” she said in an interview. Not disclosing a diagnosis “is like you’re not trusting a patient to be a reasonable adult human about this.”
‘Hard diagnosis’
Although BPD is a “hard diagnosis, we would never withhold a diagnosis of cancer or liver disease or something else we knew patients didn’t want but that we were going to try and treat them for,” said Dr. Barnhorst.
BPD is linked to significant morbidity because of its common association with comorbid conditions, such as major depressive disorder, substance use disorders, and dysthymia. A history of self-harm is present in 70%-75% of these patients and some estimates suggest up to 9% of individuals with BPD die by suicide.
In an article published in Innovations in Clinical Neuroscience investigators discussed “ethical and clinical questions psychiatrists should consider” when treating BPD, including whether a diagnosis should be shared with a patient.
After such a diagnosis a patient may “react intensely in negative ways and these responses may be easily triggered,” the researchers wrote.
“A propensity that will likely cause psychiatrists anguish, however, is BPD patients’ increased likelihood of attempting suicide,” they added. Part of the problem has been that, in the past, it was thought that a BPD prognosis was untreatable. However, the researchers note that is no longer the case.
Still, Kaz Nelson, MD, associate professor of psychiatry and behavioral sciences at the University of Minnesota, Minneapolis, has labeled BPD a so-called “asterisk” disorder.
As she wrote in a recent blog, “We tell patients when they meet criteria for a medical diagnosis.* We show compassion and nonjudgmentalism to patients.* We do not discriminate against patients.*” However, the asterisk for each of these statements is: *Except for those with BPD.
Ongoing debate
Starting around the 1980s, the DSM listed personality disorders under the No. 2 Axis, which is for conditions with symptoms that are “not mitigatable,” said Dr. Nelson.
“It really started as well-meaning therapists who care about their patients who wanted to develop some precision in understanding people, and them starting to notice some patterns that can get in the way of optimal function,” she said in an interview.
The thought was not to disclose these diagnoses “because that was for you to understand, and for the patient to discover these patterns over time in the course of your work together,” Dr. Nelson added.
Although treatment for BPD used to be virtually nonexistent, there is now hope – especially with dialectic-behavior therapy (DBT), which uses mindfulness to teach patients how to control emotions and improve relationships.
According to the National Education Alliance for BPD, other useful treatments include mentalization-based therapy, transference-focused therapy, and “good psychiatric management.” Although there are currently no approved medications for BPD, some drugs are used to treat comorbid conditions such as depression or anxiety.
“We now know that people recover, and the whole paradigm has been turned on its head,” Dr. Nelson said. For example, “we no longer categorize these things as treatable or untreatable, which was a very positive move.”
So why is the field still debating the issue of diagnosis disclosure?
“To this day there are different psychiatrists and some medical school curricula that continue to teach that personality disorders are long-term, fixed, and nontreatable – and that it’s kind of disparaging to give this kind of diagnosis to a patient,” Dr. Nelson said.
Dr. Nelson, also the vice chair for education at the University of Minnesota, Minneapolis, medical school, reported that there “we acknowledge BPD’s painful history and that there are these misconceptions. They’re going to be on the front line of combating discrimination and the idea that if you see a patient with possible BPD coming you should run. That’s just unacceptable.”
Dr. Nelson noted that the idea of disclosing a BPD diagnosis is less controversial now than in the past, but “the whole thing is still under debate, and treatment guidelines [on BPD] are old and expired.”
Criteria for BPD were not updated when the DSM-5 was published in 2013, and that needs to be fixed, Dr. Nelson added. “In the meantime, we’re trying to get the word out that it’s okay to interact with people about the diagnosis, discuss treatment plans, and manage it as one would with any other psychiatric or medical illness.”
An evolution, not a debate
Paul Appelbaum, MD, past president of the American Psychiatric Association and current chair of the organization’s DSM steering committee, said in an interview that he hasn’t been involved in any recent debate on this issue.
“I think practice has changed to the point where the general practice is to discuss patient diagnoses with [patients] openly. Patients appreciate that and psychiatrists have come to see the advantages of it,” said Dr. Appelbaum, a professor of psychiatry, medicine, and law at Columbia University, New York.
Dr. Appelbaum noted that patients also increasingly have access to their medical records, “so the reality is that it’s no longer possible in many cases to withhold a diagnosis.”
he said. “Maybe not everyone is entirely on board yet but there has been a sea change in psychiatric practices.”
Asked whether there needs to be some type of guideline update or statement released by the APA regarding BPD, Dr. Appelbaum said he doesn’t think the overall issue is BPD specific but applies to all psychiatric diagnoses.
“To the extent that there are still practitioners today that are telling students or residents [not to disclose], I would guess that they were trained a very long time ago and have not adapted to the new world,” he said.
“I don’t want to speak for the APA, but speaking for myself: I certainly encourage residents that I teach to be open about a diagnosis. It’s not just clinically helpful in some cases, it’s also ethically required from the perspective of allowing patients to make appropriate decisions about their treatment. And arguably it’s legally required as well, as part of the informed consent requirement,” Dr. Appelbaum said.
Regarding DSM updates, he noted that the committee “looks to the field to propose to us additions or changes to the DSM that are warranted by data that have been gathered since the DSM-5 came out.” There is a process set up on the DSM’s website to review such proposals.
In addition, Dr. Appelbaum said that there have been discussions about using a new model “that focuses on dimensions rather than on discreet categories” in order to classify personality disorders.
“There’s a group out there that is formulating a proposal that they will submit to us” on this, he added. “That’s the major discussion that is going on right now and it would clearly have implications for borderline as well as all the other personality disorders.”
In a statement, the APA said practice guidelines for BPD are currently under review and that the organization does not have a “position statement” on BPD for clinicians. The last update to its guideline was in the early 2000s.
A version of this article first appeared on Medscape.com.
News of actor/comedian Pete Davidson expressing relief after finally receiving a diagnosis of borderline personality disorder (BPD) prompted a recent Twitter discussion among physicians regarding the ongoing debate on whether or not to tell a patient he or she has this diagnosis.
“I’ve heard from [many] trainees that they were told never to tell a patient they had BPD, but I can hardly think of anything more paternalistic and stigmatizing,” Amy Barnhorst, MD, vice chair of community psychiatry at University of California, Davis, tweeted.
“Most patients, when I explain it to them, have this kind of reaction – they feel relieved and understood,” she added.
“I was told that as well [not to tell] in one of my practicum placements,” one respondent who identified herself as a clinical/forensic psychologist tweeted back. “I said it anyway and the person was relieved there was a name for what they were living with.”
However, others disagreed with Dr. Barnhorst, noting that BPD is a very serious, stigmatizing, and challenging disorder to treat and, because of this, may cause patients to lose hope.
Still, Dr. Barnhorst stands by her position. Although “there is a negative stigma against a diagnosis of BPD,” that idea more often comes from the clinician instead of the patient, she said.
“I’ve never had a patient say, ‘how dare you call me that!’ like it was an insult,” she said in an interview. Not disclosing a diagnosis “is like you’re not trusting a patient to be a reasonable adult human about this.”
‘Hard diagnosis’
Although BPD is a “hard diagnosis, we would never withhold a diagnosis of cancer or liver disease or something else we knew patients didn’t want but that we were going to try and treat them for,” said Dr. Barnhorst.
BPD is linked to significant morbidity because of its common association with comorbid conditions, such as major depressive disorder, substance use disorders, and dysthymia. A history of self-harm is present in 70%-75% of these patients and some estimates suggest up to 9% of individuals with BPD die by suicide.
In an article published in Innovations in Clinical Neuroscience investigators discussed “ethical and clinical questions psychiatrists should consider” when treating BPD, including whether a diagnosis should be shared with a patient.
After such a diagnosis a patient may “react intensely in negative ways and these responses may be easily triggered,” the researchers wrote.
“A propensity that will likely cause psychiatrists anguish, however, is BPD patients’ increased likelihood of attempting suicide,” they added. Part of the problem has been that, in the past, it was thought that a BPD prognosis was untreatable. However, the researchers note that is no longer the case.
Still, Kaz Nelson, MD, associate professor of psychiatry and behavioral sciences at the University of Minnesota, Minneapolis, has labeled BPD a so-called “asterisk” disorder.
As she wrote in a recent blog, “We tell patients when they meet criteria for a medical diagnosis.* We show compassion and nonjudgmentalism to patients.* We do not discriminate against patients.*” However, the asterisk for each of these statements is: *Except for those with BPD.
Ongoing debate
Starting around the 1980s, the DSM listed personality disorders under the No. 2 Axis, which is for conditions with symptoms that are “not mitigatable,” said Dr. Nelson.
“It really started as well-meaning therapists who care about their patients who wanted to develop some precision in understanding people, and them starting to notice some patterns that can get in the way of optimal function,” she said in an interview.
The thought was not to disclose these diagnoses “because that was for you to understand, and for the patient to discover these patterns over time in the course of your work together,” Dr. Nelson added.
Although treatment for BPD used to be virtually nonexistent, there is now hope – especially with dialectic-behavior therapy (DBT), which uses mindfulness to teach patients how to control emotions and improve relationships.
According to the National Education Alliance for BPD, other useful treatments include mentalization-based therapy, transference-focused therapy, and “good psychiatric management.” Although there are currently no approved medications for BPD, some drugs are used to treat comorbid conditions such as depression or anxiety.
“We now know that people recover, and the whole paradigm has been turned on its head,” Dr. Nelson said. For example, “we no longer categorize these things as treatable or untreatable, which was a very positive move.”
So why is the field still debating the issue of diagnosis disclosure?
“To this day there are different psychiatrists and some medical school curricula that continue to teach that personality disorders are long-term, fixed, and nontreatable – and that it’s kind of disparaging to give this kind of diagnosis to a patient,” Dr. Nelson said.
Dr. Nelson, also the vice chair for education at the University of Minnesota, Minneapolis, medical school, reported that there “we acknowledge BPD’s painful history and that there are these misconceptions. They’re going to be on the front line of combating discrimination and the idea that if you see a patient with possible BPD coming you should run. That’s just unacceptable.”
Dr. Nelson noted that the idea of disclosing a BPD diagnosis is less controversial now than in the past, but “the whole thing is still under debate, and treatment guidelines [on BPD] are old and expired.”
Criteria for BPD were not updated when the DSM-5 was published in 2013, and that needs to be fixed, Dr. Nelson added. “In the meantime, we’re trying to get the word out that it’s okay to interact with people about the diagnosis, discuss treatment plans, and manage it as one would with any other psychiatric or medical illness.”
An evolution, not a debate
Paul Appelbaum, MD, past president of the American Psychiatric Association and current chair of the organization’s DSM steering committee, said in an interview that he hasn’t been involved in any recent debate on this issue.
“I think practice has changed to the point where the general practice is to discuss patient diagnoses with [patients] openly. Patients appreciate that and psychiatrists have come to see the advantages of it,” said Dr. Appelbaum, a professor of psychiatry, medicine, and law at Columbia University, New York.
Dr. Appelbaum noted that patients also increasingly have access to their medical records, “so the reality is that it’s no longer possible in many cases to withhold a diagnosis.”
he said. “Maybe not everyone is entirely on board yet but there has been a sea change in psychiatric practices.”
Asked whether there needs to be some type of guideline update or statement released by the APA regarding BPD, Dr. Appelbaum said he doesn’t think the overall issue is BPD specific but applies to all psychiatric diagnoses.
“To the extent that there are still practitioners today that are telling students or residents [not to disclose], I would guess that they were trained a very long time ago and have not adapted to the new world,” he said.
“I don’t want to speak for the APA, but speaking for myself: I certainly encourage residents that I teach to be open about a diagnosis. It’s not just clinically helpful in some cases, it’s also ethically required from the perspective of allowing patients to make appropriate decisions about their treatment. And arguably it’s legally required as well, as part of the informed consent requirement,” Dr. Appelbaum said.
Regarding DSM updates, he noted that the committee “looks to the field to propose to us additions or changes to the DSM that are warranted by data that have been gathered since the DSM-5 came out.” There is a process set up on the DSM’s website to review such proposals.
In addition, Dr. Appelbaum said that there have been discussions about using a new model “that focuses on dimensions rather than on discreet categories” in order to classify personality disorders.
“There’s a group out there that is formulating a proposal that they will submit to us” on this, he added. “That’s the major discussion that is going on right now and it would clearly have implications for borderline as well as all the other personality disorders.”
In a statement, the APA said practice guidelines for BPD are currently under review and that the organization does not have a “position statement” on BPD for clinicians. The last update to its guideline was in the early 2000s.
A version of this article first appeared on Medscape.com.
Starting April 5, patients can read your notes: 5 things to consider
Change in writing style is not mandated
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, a wide-ranging piece of federal health care legislation. The previous deadline of Nov. 2, 2020, for enacting open notes was extended last year because of the exigencies of the COVID-19 pandemic.
Organizations must provide access via patient portals to the following types of notes: consultations, discharge summaries, histories, physical examination findings, imaging narratives, laboratory and pathology report narratives, and procedure and progress notes. Noncompliant organizations will eventually be subject to fines from the Department of Health & Human Services for “information blocking.”
This news organization reported on the mandate in 2020, and some readers said it was an unwelcome intrusion into practice. Since then, this news organization has run additional open notes stories about physician concerns, a perspective essay addressing those fears, and a reader poll about the phenomenon.
Now, as the legislation turns into a practical clinical matter, there are five key points clinicians should consider.
Clinicians don’t have to change writing style.
The new law mandates timely patient access to notes and test results, but it doesn’t require that clinicians alter their writing, said Scott MacDonald, MD, an internist and electronic health record medical director at University of California Davis Health in Sacramento.
“You don’t have to change your notes,” he said. However, patients are now part of the note audience and some health care systems are directing clinicians to make patient-friendly style changes.
Everyday experience should guide clinicians when writing notes, said one expert.
“When you’re not sure [of how to write a note], just mirror the way you would speak in the office – that’s going to get you right, including for mental health issues,” advised Leonor Fernandez, MD, an internist at Beth Deaconess Israel Medical Center, Boston, in her “take-away” comments in the online video, How to Write an Open Note.
According to a 2020 Medscape poll of 1,050 physicians, a majority (56%) anticipate that they will write notes differently, knowing that patients can read them via open notes. Nearly two-thirds (64%) believe that this new wrinkle in medical records will increase their workload. However, actual practice suggests that this is true for a minority of practitioners, according to the results from a recent study of more than 1,000 physicians in Boston, Seattle, and rural Pennsylvania, who already work in open notes settings. Only about one-third (37%) reported “spending more time on documentation.”
Note writing is going to change because of the addition of the patient reader, and something will be lost, argued Steven Reidbord, MD, a psychiatrist in private practice in San Francisco. By watering down the language for patients, “you are trading away the technical precision and other advantages of having a professional language,” commented Dr. Reidbord, who blogs for Psychology Today and has criticized the open notes movement in the past.
However, years of investigation from OpenNotes, the Boston-based advocacy and research organization, indicates that there are many gains with patient-accessible notes, including improved medical record accuracy, greater medication adherence, and potentially improved health care disparities among a range of patient types. In a 2019 study, researchers said that worry and confusion among note-reading patients are uncommon (5% and 3%, respectively), which addresses two criticisms voiced by multiple people last year.
Some clinical notes can be withheld.
The new rules from the federal government permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties, Tom Delbanco, MD, and Charlotte Blease, PhD, of OpenNotes in Boston wrote in a commentary in February 2021.
There are also state-level laws that can supersede the new U.S. law and block access to notes, points out MacDonald. For example, California law dictates that providers cannot post cancer test results without talking with the patient first.
The OpenNotes organization also points out that, with regard to sensitive psychotherapy notes that are separated from the rest of a medical record, those notes “can be kept from patients without their permission, and such rules vary state by state.”
Some patients are more likely readers.
Some patients are more likely to peer into their files than others, said Liz Salmi, senior strategist at OpenNotes, who is also a brain cancer patient.
“Those patients who have more serious or chronic conditions ... are more likely to read their notes,” she said in an interview.
A new study of nearly 6,000 medical oncology patients at the University of Wisconsin confirmed that opinion. Patients with incurable metastatic disease were much more likely than those with early-stage, curable disease to read notes. Notably, younger patients were more likely than older ones to access notes, likely the result of generational tech savvy.
Despite the unpredictability of serious disease such as cancer, oncology patients find satisfaction in reading their notes, say experts. “We’ve overwhelmingly heard that patients like it,” Thomas LeBlanc, MD, medical oncologist at Duke University, Durham, N.C., where all patients already have access to clinicians’ notes, told this news organization in 2018.
You are part of the avant garde.
The United States and Scandinavian countries are the world leaders in implementing open notes in clinical practice, Dr. Blease said in an interview.
“It’s a phenomenal achievement” to have enacted open notes nationally, she said. For example, there are no open notes in Northern Ireland, Dr. Blease’s home country, or most of Europe.
In the United States, there are more than 200 medical organizations, including at least one in every state, that were voluntarily providing open notes before April 5, including interstate giants such as Banner Health and big-name medical centers such as Cleveland Clinic.
It may be hard for the United States to top Sweden’s embrace of the practice. The national open notes program now has 7.2 million patient accounts in a country of 10 million people, noted Maria Häggland, PhD, of Uppsala (Sweden) MedTech Science Innovation Center during a webinar last year.
The start day will come, and you may not notice.
“When April 5 happens, something brand new is going to happen symbolically,” Ms. Salmi said. Its importance is hard to measure.
“Patients say they trust their doctor more because they understand their thinking with open notes. How do you value that? We don’t have metrics for that,” she said.
Dr. MacDonald suggested that open notes are both new and not new. In the fall of 2020, he predicted that the launch day would come, and few clinicians would notice, in part because many patients already access truncated information via patient portals.
However, there are “sensitive issues,” such as with adolescents and reproductive health, where “we know that some parents have sign-in information for their teen’s portal,” he commented. With clinical notes now on full display, potential problems “may be out of our control.”
Still, the Sacramento-based physician and IT officer acknowledged that concerns about open notes may be a bit inflated. “I’ve been more worried about reassuring physicians that everything will be okay than what’s actually going to happen [as the law takes effect],” Dr. MacDonald said.
The OpenNotes organization is grant funded, and staff disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Change in writing style is not mandated
Change in writing style is not mandated
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, a wide-ranging piece of federal health care legislation. The previous deadline of Nov. 2, 2020, for enacting open notes was extended last year because of the exigencies of the COVID-19 pandemic.
Organizations must provide access via patient portals to the following types of notes: consultations, discharge summaries, histories, physical examination findings, imaging narratives, laboratory and pathology report narratives, and procedure and progress notes. Noncompliant organizations will eventually be subject to fines from the Department of Health & Human Services for “information blocking.”
This news organization reported on the mandate in 2020, and some readers said it was an unwelcome intrusion into practice. Since then, this news organization has run additional open notes stories about physician concerns, a perspective essay addressing those fears, and a reader poll about the phenomenon.
Now, as the legislation turns into a practical clinical matter, there are five key points clinicians should consider.
Clinicians don’t have to change writing style.
The new law mandates timely patient access to notes and test results, but it doesn’t require that clinicians alter their writing, said Scott MacDonald, MD, an internist and electronic health record medical director at University of California Davis Health in Sacramento.
“You don’t have to change your notes,” he said. However, patients are now part of the note audience and some health care systems are directing clinicians to make patient-friendly style changes.
Everyday experience should guide clinicians when writing notes, said one expert.
“When you’re not sure [of how to write a note], just mirror the way you would speak in the office – that’s going to get you right, including for mental health issues,” advised Leonor Fernandez, MD, an internist at Beth Deaconess Israel Medical Center, Boston, in her “take-away” comments in the online video, How to Write an Open Note.
According to a 2020 Medscape poll of 1,050 physicians, a majority (56%) anticipate that they will write notes differently, knowing that patients can read them via open notes. Nearly two-thirds (64%) believe that this new wrinkle in medical records will increase their workload. However, actual practice suggests that this is true for a minority of practitioners, according to the results from a recent study of more than 1,000 physicians in Boston, Seattle, and rural Pennsylvania, who already work in open notes settings. Only about one-third (37%) reported “spending more time on documentation.”
Note writing is going to change because of the addition of the patient reader, and something will be lost, argued Steven Reidbord, MD, a psychiatrist in private practice in San Francisco. By watering down the language for patients, “you are trading away the technical precision and other advantages of having a professional language,” commented Dr. Reidbord, who blogs for Psychology Today and has criticized the open notes movement in the past.
However, years of investigation from OpenNotes, the Boston-based advocacy and research organization, indicates that there are many gains with patient-accessible notes, including improved medical record accuracy, greater medication adherence, and potentially improved health care disparities among a range of patient types. In a 2019 study, researchers said that worry and confusion among note-reading patients are uncommon (5% and 3%, respectively), which addresses two criticisms voiced by multiple people last year.
Some clinical notes can be withheld.
The new rules from the federal government permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties, Tom Delbanco, MD, and Charlotte Blease, PhD, of OpenNotes in Boston wrote in a commentary in February 2021.
There are also state-level laws that can supersede the new U.S. law and block access to notes, points out MacDonald. For example, California law dictates that providers cannot post cancer test results without talking with the patient first.
The OpenNotes organization also points out that, with regard to sensitive psychotherapy notes that are separated from the rest of a medical record, those notes “can be kept from patients without their permission, and such rules vary state by state.”
Some patients are more likely readers.
Some patients are more likely to peer into their files than others, said Liz Salmi, senior strategist at OpenNotes, who is also a brain cancer patient.
“Those patients who have more serious or chronic conditions ... are more likely to read their notes,” she said in an interview.
A new study of nearly 6,000 medical oncology patients at the University of Wisconsin confirmed that opinion. Patients with incurable metastatic disease were much more likely than those with early-stage, curable disease to read notes. Notably, younger patients were more likely than older ones to access notes, likely the result of generational tech savvy.
Despite the unpredictability of serious disease such as cancer, oncology patients find satisfaction in reading their notes, say experts. “We’ve overwhelmingly heard that patients like it,” Thomas LeBlanc, MD, medical oncologist at Duke University, Durham, N.C., where all patients already have access to clinicians’ notes, told this news organization in 2018.
You are part of the avant garde.
The United States and Scandinavian countries are the world leaders in implementing open notes in clinical practice, Dr. Blease said in an interview.
“It’s a phenomenal achievement” to have enacted open notes nationally, she said. For example, there are no open notes in Northern Ireland, Dr. Blease’s home country, or most of Europe.
In the United States, there are more than 200 medical organizations, including at least one in every state, that were voluntarily providing open notes before April 5, including interstate giants such as Banner Health and big-name medical centers such as Cleveland Clinic.
It may be hard for the United States to top Sweden’s embrace of the practice. The national open notes program now has 7.2 million patient accounts in a country of 10 million people, noted Maria Häggland, PhD, of Uppsala (Sweden) MedTech Science Innovation Center during a webinar last year.
The start day will come, and you may not notice.
“When April 5 happens, something brand new is going to happen symbolically,” Ms. Salmi said. Its importance is hard to measure.
“Patients say they trust their doctor more because they understand their thinking with open notes. How do you value that? We don’t have metrics for that,” she said.
Dr. MacDonald suggested that open notes are both new and not new. In the fall of 2020, he predicted that the launch day would come, and few clinicians would notice, in part because many patients already access truncated information via patient portals.
However, there are “sensitive issues,” such as with adolescents and reproductive health, where “we know that some parents have sign-in information for their teen’s portal,” he commented. With clinical notes now on full display, potential problems “may be out of our control.”
Still, the Sacramento-based physician and IT officer acknowledged that concerns about open notes may be a bit inflated. “I’ve been more worried about reassuring physicians that everything will be okay than what’s actually going to happen [as the law takes effect],” Dr. MacDonald said.
The OpenNotes organization is grant funded, and staff disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
The mandate, called “open notes” by many, is part of the 21st Century Cures Act, a wide-ranging piece of federal health care legislation. The previous deadline of Nov. 2, 2020, for enacting open notes was extended last year because of the exigencies of the COVID-19 pandemic.
Organizations must provide access via patient portals to the following types of notes: consultations, discharge summaries, histories, physical examination findings, imaging narratives, laboratory and pathology report narratives, and procedure and progress notes. Noncompliant organizations will eventually be subject to fines from the Department of Health & Human Services for “information blocking.”
This news organization reported on the mandate in 2020, and some readers said it was an unwelcome intrusion into practice. Since then, this news organization has run additional open notes stories about physician concerns, a perspective essay addressing those fears, and a reader poll about the phenomenon.
Now, as the legislation turns into a practical clinical matter, there are five key points clinicians should consider.
Clinicians don’t have to change writing style.
The new law mandates timely patient access to notes and test results, but it doesn’t require that clinicians alter their writing, said Scott MacDonald, MD, an internist and electronic health record medical director at University of California Davis Health in Sacramento.
“You don’t have to change your notes,” he said. However, patients are now part of the note audience and some health care systems are directing clinicians to make patient-friendly style changes.
Everyday experience should guide clinicians when writing notes, said one expert.
“When you’re not sure [of how to write a note], just mirror the way you would speak in the office – that’s going to get you right, including for mental health issues,” advised Leonor Fernandez, MD, an internist at Beth Deaconess Israel Medical Center, Boston, in her “take-away” comments in the online video, How to Write an Open Note.
According to a 2020 Medscape poll of 1,050 physicians, a majority (56%) anticipate that they will write notes differently, knowing that patients can read them via open notes. Nearly two-thirds (64%) believe that this new wrinkle in medical records will increase their workload. However, actual practice suggests that this is true for a minority of practitioners, according to the results from a recent study of more than 1,000 physicians in Boston, Seattle, and rural Pennsylvania, who already work in open notes settings. Only about one-third (37%) reported “spending more time on documentation.”
Note writing is going to change because of the addition of the patient reader, and something will be lost, argued Steven Reidbord, MD, a psychiatrist in private practice in San Francisco. By watering down the language for patients, “you are trading away the technical precision and other advantages of having a professional language,” commented Dr. Reidbord, who blogs for Psychology Today and has criticized the open notes movement in the past.
However, years of investigation from OpenNotes, the Boston-based advocacy and research organization, indicates that there are many gains with patient-accessible notes, including improved medical record accuracy, greater medication adherence, and potentially improved health care disparities among a range of patient types. In a 2019 study, researchers said that worry and confusion among note-reading patients are uncommon (5% and 3%, respectively), which addresses two criticisms voiced by multiple people last year.
Some clinical notes can be withheld.
The new rules from the federal government permit information blocking if there is clear evidence that doing so “will substantially reduce the risk of harm” to patients or to other third parties, Tom Delbanco, MD, and Charlotte Blease, PhD, of OpenNotes in Boston wrote in a commentary in February 2021.
There are also state-level laws that can supersede the new U.S. law and block access to notes, points out MacDonald. For example, California law dictates that providers cannot post cancer test results without talking with the patient first.
The OpenNotes organization also points out that, with regard to sensitive psychotherapy notes that are separated from the rest of a medical record, those notes “can be kept from patients without their permission, and such rules vary state by state.”
Some patients are more likely readers.
Some patients are more likely to peer into their files than others, said Liz Salmi, senior strategist at OpenNotes, who is also a brain cancer patient.
“Those patients who have more serious or chronic conditions ... are more likely to read their notes,” she said in an interview.
A new study of nearly 6,000 medical oncology patients at the University of Wisconsin confirmed that opinion. Patients with incurable metastatic disease were much more likely than those with early-stage, curable disease to read notes. Notably, younger patients were more likely than older ones to access notes, likely the result of generational tech savvy.
Despite the unpredictability of serious disease such as cancer, oncology patients find satisfaction in reading their notes, say experts. “We’ve overwhelmingly heard that patients like it,” Thomas LeBlanc, MD, medical oncologist at Duke University, Durham, N.C., where all patients already have access to clinicians’ notes, told this news organization in 2018.
You are part of the avant garde.
The United States and Scandinavian countries are the world leaders in implementing open notes in clinical practice, Dr. Blease said in an interview.
“It’s a phenomenal achievement” to have enacted open notes nationally, she said. For example, there are no open notes in Northern Ireland, Dr. Blease’s home country, or most of Europe.
In the United States, there are more than 200 medical organizations, including at least one in every state, that were voluntarily providing open notes before April 5, including interstate giants such as Banner Health and big-name medical centers such as Cleveland Clinic.
It may be hard for the United States to top Sweden’s embrace of the practice. The national open notes program now has 7.2 million patient accounts in a country of 10 million people, noted Maria Häggland, PhD, of Uppsala (Sweden) MedTech Science Innovation Center during a webinar last year.
The start day will come, and you may not notice.
“When April 5 happens, something brand new is going to happen symbolically,” Ms. Salmi said. Its importance is hard to measure.
“Patients say they trust their doctor more because they understand their thinking with open notes. How do you value that? We don’t have metrics for that,” she said.
Dr. MacDonald suggested that open notes are both new and not new. In the fall of 2020, he predicted that the launch day would come, and few clinicians would notice, in part because many patients already access truncated information via patient portals.
However, there are “sensitive issues,” such as with adolescents and reproductive health, where “we know that some parents have sign-in information for their teen’s portal,” he commented. With clinical notes now on full display, potential problems “may be out of our control.”
Still, the Sacramento-based physician and IT officer acknowledged that concerns about open notes may be a bit inflated. “I’ve been more worried about reassuring physicians that everything will be okay than what’s actually going to happen [as the law takes effect],” Dr. MacDonald said.
The OpenNotes organization is grant funded, and staff disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Camrelizumab ‘another brick in the wall’ against squamous NSCLC
.
Results of the CAMEL-sq trial showed a progression-free survival (PFS) advantage of 3.6 months with camrelizumab plus chemotherapy, compared with chemotherapy plus placebo (P < .0001). The median overall survival (OS) was not reached in the camrelizumab arm, but it was significantly better than in the placebo arm (P < .0001).
Camrelizumab plus chemotherapy is already a standard of care in China for patients with advanced nonsquamous NSCLC who are negative for EGFR and ALK mutations, study investigator Caicun Zhou, MD, PhD, said when presenting the CAMEL-sq results at the European Lung Cancer Virtual Congress 2021 (Abstract 96O).
The CAMEL-sq findings now support the combination as a “standard first-line treatment for advanced squamous NSCLC,” said Dr. Zhou of Shanghai Pulmonary Hospital and Tongji University.
“The study has kind of changed our daily practice,” he said. “I do think we will have the label, camrelizumab plus chemo as first-line treatment for squamous [NSCLC] in China, maybe in a couple of months.”
“Camrelizumab will most likely be another brick in the wall for our Chinese patients and colleagues to use for patients with squamous histology, non–small cell lung cancer in addition to pembrolizumab,” said Julie Renee Brahmer, MD, of Johns Hopkins Medicine in Baltimore, who was the invited discussant for the trial.
Dr. Brahmer noted that the PFS hazard ratio in this trial – 0.37 – was “impressive.”
Patients and treatment
CAMEL-sq is a phase 3, double-blind, multicenter trial. The 390 patients enrolled had pathologically-confirmed stage IIIB or IV squamous NSCLC, and they had not received any prior treatment.
Patients received four to six cycles of chemotherapy, consisting of carboplatin and paclitaxel given every 3 weeks. Camrelizumab was added to one arm at a dose of 200 mg, and placebo was added to the other.
This was followed by a maintenance phase in which patients remained on active treatment with camrelizumab or placebo for up to 2 years. Patients in the placebo arm could cross over to camrelizumab at progression.
The median age of patients was similar in the camrelizumab and placebo arms (64 years and 62 years, respectively). The majority of study subjects in both arms were men (more than 90%), had a history of smoking (more than 80%), and had stage IV disease (more than 70%).
Efficacy and safety
The median PFS was 8.5 months in the camrelizumab arm and 4.9 months in the placebo arm (HR, 0.37; P < .0001). The median OS was not reached in the camrelizumab arm and was 14.5 months in the placebo arm (HR, 0.55, P < .0001).
The survival benefits were observed in spite of a crossover rate of 46.9%, Dr. Zhou noted.
Furthermore, survival benefits were consistent across all the subgroups tested. Regardless of age, sex, performance status, smoking history, disease stage, presence of liver or brain metastases, or PD-L1 expression, there was an apparent advantage of camrelizumab over placebo.
The objective response rate was higher in the camrelizumab arm than in the placebo arm, at 64.8% and 36.7%, respectively (P < .0001).
The clinical response seen with camrelizumab was “robust and durable,” Dr. Zhou said. Indeed, the duration of response was 13.1 months in the camrelizumab arm and 4.4 months in the placebo arm.
Grade 3/4 treatment-related adverse events (AEs) were reported in a similar percentage of camrelizumab- or placebo-treated patients (73.6% and 71.4%, respectively). However, “the majority of treatment-related adverse effects were chemotherapy related,” Dr. Zhou pointed out. This included decreased total white blood cell, neutrophil, red blood cell, and platelet counts as well as alopecia and increased liver enzymes.
Immune-related AEs occurred in 76.7% of patients in the camrelizumab arm and 20.4% of those in the placebo arm.
“The majority of immune-related adverse events were grade 1 or grade 2; easily manageable in our daily practice,” Dr. Zhou noted.
Putting CAMEL-sq into perspective
Data from other trials of immunotherapy-chemotherapy combinations in squamous NSCLC have been presented recently but with less impressive results, Dr. Brahmer said.
In one trial – ORIENT-12 – sintilimab was combined with gemcitabine and cisplatin (ESMO 2020, Abstract LBA56). The median PFS, per investigators, was 5.5 months with sintilimab and 4.9 months without it, both of which are lower than the 8.5 months seen with camrelizumab plus chemotherapy in the CAMEL-sq trial.
Another trial is KEYNOTE-407, in which patients received pembrolizumab or placebo plus a carboplatin-paclitaxel (or nab-paclitaxel) regimen. Three-year follow-up data from the trial were presented at ELCC 2021 (Abstract 97O). Continued improvements in second PFS (HR, 0.59) and OS (HR, 0.71) were observed with pembrolizumab-chemotherapy versus placebo-chemotherapy.
“We have to remember the high PD-L1-negative disease rate in the CAMEL-sq study, compared to the KEYNOTE-407 rate,” before stacking the two studies against each other, Dr. Brahmer noted. In KEYNOTE-407, almost 35% of patients had PD-L1 expression of less than 1%, compared with nearly 50% in the CAMEL-sq study.
That aside, “very similar impressive 1-year progression-free survival rates are seen on both studies,” Dr. Brahmer said. “I hope that camrelizumab has continued follow-up so we can see how these patients will do long-term.
“My eyebrows were raised a little bit at the camrelizumab immune-related AE rate of almost 76%, compared to the immune-related AE rate of about 36% in the KEYNOTE-407 study,” Dr. Brahmer said.
She noted, however, that almost two-thirds of the immune-related AEs in CAMEL-sq were due to reactive cutaneous capillary endothelial proliferation, which doesn’t appear to have been previously reported with PD-1 or PD-L1 inhibitors. This is a side effect seen in studies of liver cancer and may be linked to PFS, Dr. Brahmer said.
CAMEL-sq is funded by Jiangsu Hengrui Medicine Co. Ltd. Dr. Zhou disclosed honoraria from multiple pharmaceutical companies, including the study sponsor. Two of Dr. Zhou’s coauthors are employees of the company. Dr. Brahmer disclosed relationships with Amgen, Bristol Myers Squibb, Eli Lily, GlaxoSmithKline, Merck, Sanofi, Easi, AstraZeneca, Genentech, Regeneron, and RAPT Therapeutics Inc.
.
Results of the CAMEL-sq trial showed a progression-free survival (PFS) advantage of 3.6 months with camrelizumab plus chemotherapy, compared with chemotherapy plus placebo (P < .0001). The median overall survival (OS) was not reached in the camrelizumab arm, but it was significantly better than in the placebo arm (P < .0001).
Camrelizumab plus chemotherapy is already a standard of care in China for patients with advanced nonsquamous NSCLC who are negative for EGFR and ALK mutations, study investigator Caicun Zhou, MD, PhD, said when presenting the CAMEL-sq results at the European Lung Cancer Virtual Congress 2021 (Abstract 96O).
The CAMEL-sq findings now support the combination as a “standard first-line treatment for advanced squamous NSCLC,” said Dr. Zhou of Shanghai Pulmonary Hospital and Tongji University.
“The study has kind of changed our daily practice,” he said. “I do think we will have the label, camrelizumab plus chemo as first-line treatment for squamous [NSCLC] in China, maybe in a couple of months.”
“Camrelizumab will most likely be another brick in the wall for our Chinese patients and colleagues to use for patients with squamous histology, non–small cell lung cancer in addition to pembrolizumab,” said Julie Renee Brahmer, MD, of Johns Hopkins Medicine in Baltimore, who was the invited discussant for the trial.
Dr. Brahmer noted that the PFS hazard ratio in this trial – 0.37 – was “impressive.”
Patients and treatment
CAMEL-sq is a phase 3, double-blind, multicenter trial. The 390 patients enrolled had pathologically-confirmed stage IIIB or IV squamous NSCLC, and they had not received any prior treatment.
Patients received four to six cycles of chemotherapy, consisting of carboplatin and paclitaxel given every 3 weeks. Camrelizumab was added to one arm at a dose of 200 mg, and placebo was added to the other.
This was followed by a maintenance phase in which patients remained on active treatment with camrelizumab or placebo for up to 2 years. Patients in the placebo arm could cross over to camrelizumab at progression.
The median age of patients was similar in the camrelizumab and placebo arms (64 years and 62 years, respectively). The majority of study subjects in both arms were men (more than 90%), had a history of smoking (more than 80%), and had stage IV disease (more than 70%).
Efficacy and safety
The median PFS was 8.5 months in the camrelizumab arm and 4.9 months in the placebo arm (HR, 0.37; P < .0001). The median OS was not reached in the camrelizumab arm and was 14.5 months in the placebo arm (HR, 0.55, P < .0001).
The survival benefits were observed in spite of a crossover rate of 46.9%, Dr. Zhou noted.
Furthermore, survival benefits were consistent across all the subgroups tested. Regardless of age, sex, performance status, smoking history, disease stage, presence of liver or brain metastases, or PD-L1 expression, there was an apparent advantage of camrelizumab over placebo.
The objective response rate was higher in the camrelizumab arm than in the placebo arm, at 64.8% and 36.7%, respectively (P < .0001).
The clinical response seen with camrelizumab was “robust and durable,” Dr. Zhou said. Indeed, the duration of response was 13.1 months in the camrelizumab arm and 4.4 months in the placebo arm.
Grade 3/4 treatment-related adverse events (AEs) were reported in a similar percentage of camrelizumab- or placebo-treated patients (73.6% and 71.4%, respectively). However, “the majority of treatment-related adverse effects were chemotherapy related,” Dr. Zhou pointed out. This included decreased total white blood cell, neutrophil, red blood cell, and platelet counts as well as alopecia and increased liver enzymes.
Immune-related AEs occurred in 76.7% of patients in the camrelizumab arm and 20.4% of those in the placebo arm.
“The majority of immune-related adverse events were grade 1 or grade 2; easily manageable in our daily practice,” Dr. Zhou noted.
Putting CAMEL-sq into perspective
Data from other trials of immunotherapy-chemotherapy combinations in squamous NSCLC have been presented recently but with less impressive results, Dr. Brahmer said.
In one trial – ORIENT-12 – sintilimab was combined with gemcitabine and cisplatin (ESMO 2020, Abstract LBA56). The median PFS, per investigators, was 5.5 months with sintilimab and 4.9 months without it, both of which are lower than the 8.5 months seen with camrelizumab plus chemotherapy in the CAMEL-sq trial.
Another trial is KEYNOTE-407, in which patients received pembrolizumab or placebo plus a carboplatin-paclitaxel (or nab-paclitaxel) regimen. Three-year follow-up data from the trial were presented at ELCC 2021 (Abstract 97O). Continued improvements in second PFS (HR, 0.59) and OS (HR, 0.71) were observed with pembrolizumab-chemotherapy versus placebo-chemotherapy.
“We have to remember the high PD-L1-negative disease rate in the CAMEL-sq study, compared to the KEYNOTE-407 rate,” before stacking the two studies against each other, Dr. Brahmer noted. In KEYNOTE-407, almost 35% of patients had PD-L1 expression of less than 1%, compared with nearly 50% in the CAMEL-sq study.
That aside, “very similar impressive 1-year progression-free survival rates are seen on both studies,” Dr. Brahmer said. “I hope that camrelizumab has continued follow-up so we can see how these patients will do long-term.
“My eyebrows were raised a little bit at the camrelizumab immune-related AE rate of almost 76%, compared to the immune-related AE rate of about 36% in the KEYNOTE-407 study,” Dr. Brahmer said.
She noted, however, that almost two-thirds of the immune-related AEs in CAMEL-sq were due to reactive cutaneous capillary endothelial proliferation, which doesn’t appear to have been previously reported with PD-1 or PD-L1 inhibitors. This is a side effect seen in studies of liver cancer and may be linked to PFS, Dr. Brahmer said.
CAMEL-sq is funded by Jiangsu Hengrui Medicine Co. Ltd. Dr. Zhou disclosed honoraria from multiple pharmaceutical companies, including the study sponsor. Two of Dr. Zhou’s coauthors are employees of the company. Dr. Brahmer disclosed relationships with Amgen, Bristol Myers Squibb, Eli Lily, GlaxoSmithKline, Merck, Sanofi, Easi, AstraZeneca, Genentech, Regeneron, and RAPT Therapeutics Inc.
.
Results of the CAMEL-sq trial showed a progression-free survival (PFS) advantage of 3.6 months with camrelizumab plus chemotherapy, compared with chemotherapy plus placebo (P < .0001). The median overall survival (OS) was not reached in the camrelizumab arm, but it was significantly better than in the placebo arm (P < .0001).
Camrelizumab plus chemotherapy is already a standard of care in China for patients with advanced nonsquamous NSCLC who are negative for EGFR and ALK mutations, study investigator Caicun Zhou, MD, PhD, said when presenting the CAMEL-sq results at the European Lung Cancer Virtual Congress 2021 (Abstract 96O).
The CAMEL-sq findings now support the combination as a “standard first-line treatment for advanced squamous NSCLC,” said Dr. Zhou of Shanghai Pulmonary Hospital and Tongji University.
“The study has kind of changed our daily practice,” he said. “I do think we will have the label, camrelizumab plus chemo as first-line treatment for squamous [NSCLC] in China, maybe in a couple of months.”
“Camrelizumab will most likely be another brick in the wall for our Chinese patients and colleagues to use for patients with squamous histology, non–small cell lung cancer in addition to pembrolizumab,” said Julie Renee Brahmer, MD, of Johns Hopkins Medicine in Baltimore, who was the invited discussant for the trial.
Dr. Brahmer noted that the PFS hazard ratio in this trial – 0.37 – was “impressive.”
Patients and treatment
CAMEL-sq is a phase 3, double-blind, multicenter trial. The 390 patients enrolled had pathologically-confirmed stage IIIB or IV squamous NSCLC, and they had not received any prior treatment.
Patients received four to six cycles of chemotherapy, consisting of carboplatin and paclitaxel given every 3 weeks. Camrelizumab was added to one arm at a dose of 200 mg, and placebo was added to the other.
This was followed by a maintenance phase in which patients remained on active treatment with camrelizumab or placebo for up to 2 years. Patients in the placebo arm could cross over to camrelizumab at progression.
The median age of patients was similar in the camrelizumab and placebo arms (64 years and 62 years, respectively). The majority of study subjects in both arms were men (more than 90%), had a history of smoking (more than 80%), and had stage IV disease (more than 70%).
Efficacy and safety
The median PFS was 8.5 months in the camrelizumab arm and 4.9 months in the placebo arm (HR, 0.37; P < .0001). The median OS was not reached in the camrelizumab arm and was 14.5 months in the placebo arm (HR, 0.55, P < .0001).
The survival benefits were observed in spite of a crossover rate of 46.9%, Dr. Zhou noted.
Furthermore, survival benefits were consistent across all the subgroups tested. Regardless of age, sex, performance status, smoking history, disease stage, presence of liver or brain metastases, or PD-L1 expression, there was an apparent advantage of camrelizumab over placebo.
The objective response rate was higher in the camrelizumab arm than in the placebo arm, at 64.8% and 36.7%, respectively (P < .0001).
The clinical response seen with camrelizumab was “robust and durable,” Dr. Zhou said. Indeed, the duration of response was 13.1 months in the camrelizumab arm and 4.4 months in the placebo arm.
Grade 3/4 treatment-related adverse events (AEs) were reported in a similar percentage of camrelizumab- or placebo-treated patients (73.6% and 71.4%, respectively). However, “the majority of treatment-related adverse effects were chemotherapy related,” Dr. Zhou pointed out. This included decreased total white blood cell, neutrophil, red blood cell, and platelet counts as well as alopecia and increased liver enzymes.
Immune-related AEs occurred in 76.7% of patients in the camrelizumab arm and 20.4% of those in the placebo arm.
“The majority of immune-related adverse events were grade 1 or grade 2; easily manageable in our daily practice,” Dr. Zhou noted.
Putting CAMEL-sq into perspective
Data from other trials of immunotherapy-chemotherapy combinations in squamous NSCLC have been presented recently but with less impressive results, Dr. Brahmer said.
In one trial – ORIENT-12 – sintilimab was combined with gemcitabine and cisplatin (ESMO 2020, Abstract LBA56). The median PFS, per investigators, was 5.5 months with sintilimab and 4.9 months without it, both of which are lower than the 8.5 months seen with camrelizumab plus chemotherapy in the CAMEL-sq trial.
Another trial is KEYNOTE-407, in which patients received pembrolizumab or placebo plus a carboplatin-paclitaxel (or nab-paclitaxel) regimen. Three-year follow-up data from the trial were presented at ELCC 2021 (Abstract 97O). Continued improvements in second PFS (HR, 0.59) and OS (HR, 0.71) were observed with pembrolizumab-chemotherapy versus placebo-chemotherapy.
“We have to remember the high PD-L1-negative disease rate in the CAMEL-sq study, compared to the KEYNOTE-407 rate,” before stacking the two studies against each other, Dr. Brahmer noted. In KEYNOTE-407, almost 35% of patients had PD-L1 expression of less than 1%, compared with nearly 50% in the CAMEL-sq study.
That aside, “very similar impressive 1-year progression-free survival rates are seen on both studies,” Dr. Brahmer said. “I hope that camrelizumab has continued follow-up so we can see how these patients will do long-term.
“My eyebrows were raised a little bit at the camrelizumab immune-related AE rate of almost 76%, compared to the immune-related AE rate of about 36% in the KEYNOTE-407 study,” Dr. Brahmer said.
She noted, however, that almost two-thirds of the immune-related AEs in CAMEL-sq were due to reactive cutaneous capillary endothelial proliferation, which doesn’t appear to have been previously reported with PD-1 or PD-L1 inhibitors. This is a side effect seen in studies of liver cancer and may be linked to PFS, Dr. Brahmer said.
CAMEL-sq is funded by Jiangsu Hengrui Medicine Co. Ltd. Dr. Zhou disclosed honoraria from multiple pharmaceutical companies, including the study sponsor. Two of Dr. Zhou’s coauthors are employees of the company. Dr. Brahmer disclosed relationships with Amgen, Bristol Myers Squibb, Eli Lily, GlaxoSmithKline, Merck, Sanofi, Easi, AstraZeneca, Genentech, Regeneron, and RAPT Therapeutics Inc.
FROM ELCC 2021
The role of aspirin in primary prevention of cardiovascular disease
Background: Previous studies have shown that aspirin reduces the relative risk of cardiovascular disease (CVD) but also increases the relative risk of bleeding. It is unclear if there are patients without known CVD in whom the absolute risk reduction of CVD outweighs the absolute risk of bleeding. Prognostic CVD and bleeding risk models allow for an assessment of absolute risks and primary preventive interventions.
Study design: Individualized risk-benefit analysis based on sex-specific risk scores and estimates from PREDICT cohort data.
Setting: Primary care practices in New Zealand.
Synopsis: Using the New Zealand–based PREDICT online tool, 245,048 patients had their CVD risk assessed and did not meet exclusion criteria. The online tool predicts CVD events avoided and bleeding events caused by aspirin. When one CVD event was equivalent to one major bleeding event, 2.5% of women and 12.1% of men were classified as benefiting from aspirin (more CVD events avoided than bleeding events caused). When one CVD event was equivalent to two major bleeding events, 21.4% of women and 40.7% of men were classified as benefiting from aspirin. The net-benefit subgroups were older, and had higher baseline 5-year CVD risk, fewer risk factors for bleeding, higher systolic blood pressure, and a higher total cholesterol to HDL cholesterol ratio. Ethnicity and socioeconomic index also influenced benefit or harm.
With use of the upper and lower limits of 95% confidence intervals for models, there were considerable ranges of benefit versus harm. Sex-specific risk scores and meta-analysis have intrinsic uncertainties and results potentially not generalizable outside New Zealand population. Ultimate decision to use aspirin requires shared decision making.
Bottom line: Some patients are likely to derive a net benefit from aspirin for primary prevention of CVD. Risk-benefit models with online tools can help providers and patients estimate these factors to inform shared decision making.
Citation: Selak V et al. Personalized prediction of cardiovascular benefits and bleeding harms for aspirin for primary prevention, a benefit-harm analysis. Ann Intern Med. 2019;71(8):529-39.
Dr. Rupp is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
Background: Previous studies have shown that aspirin reduces the relative risk of cardiovascular disease (CVD) but also increases the relative risk of bleeding. It is unclear if there are patients without known CVD in whom the absolute risk reduction of CVD outweighs the absolute risk of bleeding. Prognostic CVD and bleeding risk models allow for an assessment of absolute risks and primary preventive interventions.
Study design: Individualized risk-benefit analysis based on sex-specific risk scores and estimates from PREDICT cohort data.
Setting: Primary care practices in New Zealand.
Synopsis: Using the New Zealand–based PREDICT online tool, 245,048 patients had their CVD risk assessed and did not meet exclusion criteria. The online tool predicts CVD events avoided and bleeding events caused by aspirin. When one CVD event was equivalent to one major bleeding event, 2.5% of women and 12.1% of men were classified as benefiting from aspirin (more CVD events avoided than bleeding events caused). When one CVD event was equivalent to two major bleeding events, 21.4% of women and 40.7% of men were classified as benefiting from aspirin. The net-benefit subgroups were older, and had higher baseline 5-year CVD risk, fewer risk factors for bleeding, higher systolic blood pressure, and a higher total cholesterol to HDL cholesterol ratio. Ethnicity and socioeconomic index also influenced benefit or harm.
With use of the upper and lower limits of 95% confidence intervals for models, there were considerable ranges of benefit versus harm. Sex-specific risk scores and meta-analysis have intrinsic uncertainties and results potentially not generalizable outside New Zealand population. Ultimate decision to use aspirin requires shared decision making.
Bottom line: Some patients are likely to derive a net benefit from aspirin for primary prevention of CVD. Risk-benefit models with online tools can help providers and patients estimate these factors to inform shared decision making.
Citation: Selak V et al. Personalized prediction of cardiovascular benefits and bleeding harms for aspirin for primary prevention, a benefit-harm analysis. Ann Intern Med. 2019;71(8):529-39.
Dr. Rupp is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
Background: Previous studies have shown that aspirin reduces the relative risk of cardiovascular disease (CVD) but also increases the relative risk of bleeding. It is unclear if there are patients without known CVD in whom the absolute risk reduction of CVD outweighs the absolute risk of bleeding. Prognostic CVD and bleeding risk models allow for an assessment of absolute risks and primary preventive interventions.
Study design: Individualized risk-benefit analysis based on sex-specific risk scores and estimates from PREDICT cohort data.
Setting: Primary care practices in New Zealand.
Synopsis: Using the New Zealand–based PREDICT online tool, 245,048 patients had their CVD risk assessed and did not meet exclusion criteria. The online tool predicts CVD events avoided and bleeding events caused by aspirin. When one CVD event was equivalent to one major bleeding event, 2.5% of women and 12.1% of men were classified as benefiting from aspirin (more CVD events avoided than bleeding events caused). When one CVD event was equivalent to two major bleeding events, 21.4% of women and 40.7% of men were classified as benefiting from aspirin. The net-benefit subgroups were older, and had higher baseline 5-year CVD risk, fewer risk factors for bleeding, higher systolic blood pressure, and a higher total cholesterol to HDL cholesterol ratio. Ethnicity and socioeconomic index also influenced benefit or harm.
With use of the upper and lower limits of 95% confidence intervals for models, there were considerable ranges of benefit versus harm. Sex-specific risk scores and meta-analysis have intrinsic uncertainties and results potentially not generalizable outside New Zealand population. Ultimate decision to use aspirin requires shared decision making.
Bottom line: Some patients are likely to derive a net benefit from aspirin for primary prevention of CVD. Risk-benefit models with online tools can help providers and patients estimate these factors to inform shared decision making.
Citation: Selak V et al. Personalized prediction of cardiovascular benefits and bleeding harms for aspirin for primary prevention, a benefit-harm analysis. Ann Intern Med. 2019;71(8):529-39.
Dr. Rupp is a hospitalist and clinical instructor of medicine at the University of Utah, Salt Lake City.
COVID-19 in 2020: Deaths and disparities
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.
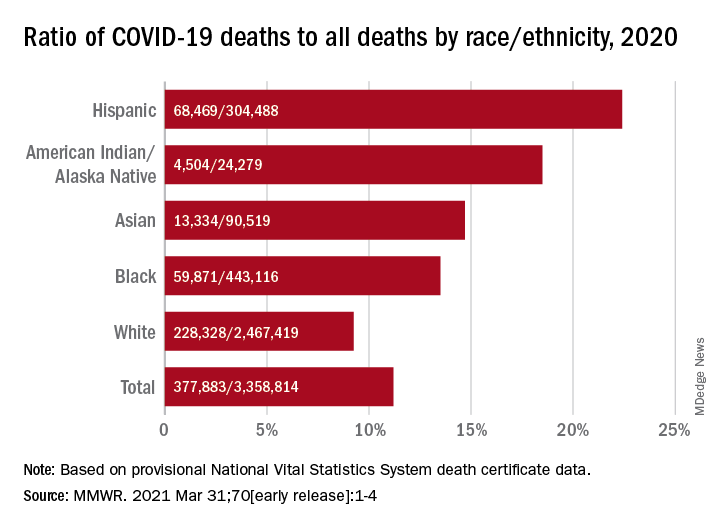
Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
COVID-19 was the third-leading cause of death in the United States in 2020, but that mortality burden did not fall evenly along racial/ethnic lines, according to a provisional report from the Centers for Disease Control and Prevention.

Only heart disease and cancer caused more deaths than SARS-CoV-2, which took the lives of almost 378,000 Americans last year, Farida B. Ahmad, MPH, and associates at the National Center for Health Statistics noted March 31 in the Morbidity and Mortality Weekly Report.
That represents 11.2% of the almost 3.36 million total deaths recorded in 2020. The racial/ethnics demographics, however, show that 22.4% of all deaths among Hispanic Americans were COVID-19–related, as were 18.6% of deaths in American Indians/Alaska Natives. Deaths among Asian persons, at 14.7%, and African Americans, at 13.5%, were closer but still above the national figure, while Whites (9.3%) were the only major subgroup below it, based on data from the National Vital Statistics System.
Age-adjusted death rates tell a somewhat different story: American Indian/Alaska native persons were highest with a rate of 187.8 COVID-19–associated deaths per 100,000 standard population, with Hispanic persons second at 164.3 per 100,000. Blacks were next at 151.1 deaths per 100,000, but Whites had a higher rate (72.5) than did Asian Americans (66.7), the CDC investigators reported.
“During January-December 2020, the estimated 2020 age-adjusted death rate increased for the first time since 2017, with an increase of 15.9% compared with 2019, from 715.2 to 828.7 deaths per 100,000 population,” they wrote, noting that “certain categories of race (i.e., AI/AN and Asian) and Hispanic ethnicity reported on death certificates might have been misclassified, possibly resulting in underestimates of death rates for some groups.”
FROM MMWR
Study: Good overall survival in older patients after liver transplant for HCC
Judicious organ matching for older liver transplant candidates with hepatocellular carcinoma (HCC) leads to survival outcomes similar to those in younger recipients, a case review suggests.
Overall survival (OS) rates among transplant recipients included in a prospective institutional database were 85.5% and 84% at 3 years after liver transplant in patients aged 65 years and under and those over 65 years, respectively. The 5-year survival rates were 73.9% and 77%, respectively (P = .26), Ola Ahmed, MD, of the department of abdominal organ transplantation surgery at Washington University, St. Louis, and colleagues found.
The investigators looked at 1,629 patients diagnosed with HCC between Jan. 1, 2002, and Dec. 31, 2019 of whom 700 were considered for curative surgery, including transplant in 538, and resection in 162.
The patients had a mean age of 62.8 years. Those older than 65 years were less likely to be considered or listed for transplant (27% vs. 73%, P < .01), although oncologic staging and delisting rates were similar in both groups. “This observation still holds true after controlling for other variables, including viral hepatitis and gender in the multivariable analysis (adjusted odds ratio, 0.365),” the investigators reported in the Journal of the American College of Surgeons.
The findings were also reported at the 2020 virtual Western Surgical Association 128th Scientific Session in November.
The issue of resection
Surgical intervention occurred in 597 patients, including 392 and 205 aged 65 years and younger and over 65 years, respectively.
OS was lower among patients who underwent resection, compared with the liver transplant recipients, but was similar in the older and younger age groups (3-year OS, 59% vs. 64.8% and 5-year OS, 44.8% vs. 49%; P = .13). No differences were noted in the development of local or distant metastatic disease after transplant or resection.
The two age groups had comparable ICU stays (2 days) and total hospital length of stay (6 days). There were no differences in 30- and 90-day hospital readmissions, they noted.
“On additional age analysis, 65% of transplanted patients over 65 years are currently alive and were disease free at the end of the study period, compared to only 18% of their resected counterparts (P < .01),” they wrote.
Justifying transplant
The findings are notable because despite the effectiveness of transplant as an alternative treatment for unresectable HCC, older patients are often excluded from consideration for transplant. Most studies over the past 15 years have focused on patients aged under 60 years and the ability to extrapolate results to older patients has been limited. Further, results have been conflicting in older patients, the authors explained.
“This is particularly apposite at this time with prolonged life expectancy and the growing interest in improving cancer survivorship,” they noted, adding that “there is logic in challenging existing gold standards and traditional norms with real-life medical practice.
Indeed, the current findings suggest – perhaps contrary to common perceptions – that transplant in carefully selected patients “can be justified in older age groups and provide clinically meaningful and longer survival benefits,” they said, adding that “discussions should be guided by the potential for unfair age discriminations and precise terminology of physiologic rather than actual age.
“Such insights highlight the continued need for quality improvement in the surgical management of older patients, raising questions regarding current resource utilization among different age groups and how age can influence patterns of cancer care,” they concluded.
The authors reported having no disclosures.
Judicious organ matching for older liver transplant candidates with hepatocellular carcinoma (HCC) leads to survival outcomes similar to those in younger recipients, a case review suggests.
Overall survival (OS) rates among transplant recipients included in a prospective institutional database were 85.5% and 84% at 3 years after liver transplant in patients aged 65 years and under and those over 65 years, respectively. The 5-year survival rates were 73.9% and 77%, respectively (P = .26), Ola Ahmed, MD, of the department of abdominal organ transplantation surgery at Washington University, St. Louis, and colleagues found.
The investigators looked at 1,629 patients diagnosed with HCC between Jan. 1, 2002, and Dec. 31, 2019 of whom 700 were considered for curative surgery, including transplant in 538, and resection in 162.
The patients had a mean age of 62.8 years. Those older than 65 years were less likely to be considered or listed for transplant (27% vs. 73%, P < .01), although oncologic staging and delisting rates were similar in both groups. “This observation still holds true after controlling for other variables, including viral hepatitis and gender in the multivariable analysis (adjusted odds ratio, 0.365),” the investigators reported in the Journal of the American College of Surgeons.
The findings were also reported at the 2020 virtual Western Surgical Association 128th Scientific Session in November.
The issue of resection
Surgical intervention occurred in 597 patients, including 392 and 205 aged 65 years and younger and over 65 years, respectively.
OS was lower among patients who underwent resection, compared with the liver transplant recipients, but was similar in the older and younger age groups (3-year OS, 59% vs. 64.8% and 5-year OS, 44.8% vs. 49%; P = .13). No differences were noted in the development of local or distant metastatic disease after transplant or resection.
The two age groups had comparable ICU stays (2 days) and total hospital length of stay (6 days). There were no differences in 30- and 90-day hospital readmissions, they noted.
“On additional age analysis, 65% of transplanted patients over 65 years are currently alive and were disease free at the end of the study period, compared to only 18% of their resected counterparts (P < .01),” they wrote.
Justifying transplant
The findings are notable because despite the effectiveness of transplant as an alternative treatment for unresectable HCC, older patients are often excluded from consideration for transplant. Most studies over the past 15 years have focused on patients aged under 60 years and the ability to extrapolate results to older patients has been limited. Further, results have been conflicting in older patients, the authors explained.
“This is particularly apposite at this time with prolonged life expectancy and the growing interest in improving cancer survivorship,” they noted, adding that “there is logic in challenging existing gold standards and traditional norms with real-life medical practice.
Indeed, the current findings suggest – perhaps contrary to common perceptions – that transplant in carefully selected patients “can be justified in older age groups and provide clinically meaningful and longer survival benefits,” they said, adding that “discussions should be guided by the potential for unfair age discriminations and precise terminology of physiologic rather than actual age.
“Such insights highlight the continued need for quality improvement in the surgical management of older patients, raising questions regarding current resource utilization among different age groups and how age can influence patterns of cancer care,” they concluded.
The authors reported having no disclosures.
Judicious organ matching for older liver transplant candidates with hepatocellular carcinoma (HCC) leads to survival outcomes similar to those in younger recipients, a case review suggests.
Overall survival (OS) rates among transplant recipients included in a prospective institutional database were 85.5% and 84% at 3 years after liver transplant in patients aged 65 years and under and those over 65 years, respectively. The 5-year survival rates were 73.9% and 77%, respectively (P = .26), Ola Ahmed, MD, of the department of abdominal organ transplantation surgery at Washington University, St. Louis, and colleagues found.
The investigators looked at 1,629 patients diagnosed with HCC between Jan. 1, 2002, and Dec. 31, 2019 of whom 700 were considered for curative surgery, including transplant in 538, and resection in 162.
The patients had a mean age of 62.8 years. Those older than 65 years were less likely to be considered or listed for transplant (27% vs. 73%, P < .01), although oncologic staging and delisting rates were similar in both groups. “This observation still holds true after controlling for other variables, including viral hepatitis and gender in the multivariable analysis (adjusted odds ratio, 0.365),” the investigators reported in the Journal of the American College of Surgeons.
The findings were also reported at the 2020 virtual Western Surgical Association 128th Scientific Session in November.
The issue of resection
Surgical intervention occurred in 597 patients, including 392 and 205 aged 65 years and younger and over 65 years, respectively.
OS was lower among patients who underwent resection, compared with the liver transplant recipients, but was similar in the older and younger age groups (3-year OS, 59% vs. 64.8% and 5-year OS, 44.8% vs. 49%; P = .13). No differences were noted in the development of local or distant metastatic disease after transplant or resection.
The two age groups had comparable ICU stays (2 days) and total hospital length of stay (6 days). There were no differences in 30- and 90-day hospital readmissions, they noted.
“On additional age analysis, 65% of transplanted patients over 65 years are currently alive and were disease free at the end of the study period, compared to only 18% of their resected counterparts (P < .01),” they wrote.
Justifying transplant
The findings are notable because despite the effectiveness of transplant as an alternative treatment for unresectable HCC, older patients are often excluded from consideration for transplant. Most studies over the past 15 years have focused on patients aged under 60 years and the ability to extrapolate results to older patients has been limited. Further, results have been conflicting in older patients, the authors explained.
“This is particularly apposite at this time with prolonged life expectancy and the growing interest in improving cancer survivorship,” they noted, adding that “there is logic in challenging existing gold standards and traditional norms with real-life medical practice.
Indeed, the current findings suggest – perhaps contrary to common perceptions – that transplant in carefully selected patients “can be justified in older age groups and provide clinically meaningful and longer survival benefits,” they said, adding that “discussions should be guided by the potential for unfair age discriminations and precise terminology of physiologic rather than actual age.
“Such insights highlight the continued need for quality improvement in the surgical management of older patients, raising questions regarding current resource utilization among different age groups and how age can influence patterns of cancer care,” they concluded.
The authors reported having no disclosures.
FROM THE JOURNAL OF THE AMERICAN COLLEGE OF SURGEONS
Steroids can be stopped in some older multiple myeloma patients
For select older patients, it is safe to switch to a lower dose of lenalidomide maintenance therapy and discontinue dexamethasone after 9 months. The regimen is safe and yields outcomes similar to those of standard, continuous lenalidomide/dexamethasone (Rd), according to new findings.
At a median follow-up of 37 months, event-free survival was 10.4 months in the experimental arm in which dexamethasone therapy was stopped (Rd-R) versus 6.9 months for standard therapy. The tailored approach also resulted in fewer adverse effects.
The authors noted that there was no difference in progression-free survival (PFS) and overall survival between the two groups.
“These results may be useful for the treatment of myeloma patients, since approximately one-third of patients not eligible for stem cell transplantation are intermediate fit, the population in our study,” said lead author Alessandra Larocca, MD, PhD, from the department of hematology-oncology of the University Hospital Città della Salute e della Scienza, Torino, Italy.
She said in an interview that they expect that these findings “may help to optimize the treatment of less-fit elderly patients by reducing the occurrence of adverse events and thus improving outcomes and preserving quality of life of these patients.”
This approach is a viable option for clinicians to consider for some patient subgroups. “This steroid-sparing approach can also be used in other combinations,” she said. “Ongoing trials are now evaluating steroid sparing in combination with monoclonal antibodies or the role of frailty-guided treatment.”
The study was published March 19, 2021, in Blood.
Curtailing steroids
Myeloma patients aged 75 years or older or who have comorbidities and functional impairments are an understudied population. They are more susceptible to adverse events that may negatively affect the duration of treatment and outcomes. Steroids are “scarcely tolerated” in the long term, even among younger patients, and “whether sparing dexamethasone is as effective as prolonged steroid exposure remains an open issue,” the authors wrote. There are still no clear data on the advantage of continuous steroid treatment as opposed to fixed-duration treatment for newly diagnosed patients.
In 2010, a study compared high-dose with low-dose dexamethasone. As expected, the rate of adverse events was lower among patients who received the low-dose steroid, but quite unexpectedly, deaths with high-dose dexamethasone were significantly higher than with low-dose dexamethasone.
The 1-year overall survival was 96% among patients who received the low dose of dexamethasone versus 87% with the standard high dose.
S. Vincent Rajkumar, MD, of the Mayo Clinic, Rochester, Minn., who was the lead author of the 2010 study, spoke with this new organization about the current study. “This is an important and practice-changing study,” he said. “We have already changed our practice and recommendations based on this study.”
He explained that, for transplant-ineligible patients, instead of initial therapy with bortezomib-lenalidomide-dexamethasone followed by Rd, they use lenalidomide alone without steroids.
“After 9 months of initial therapy, I now recommend we stop dexamethasone unless we are having problems controlling the myeloma, such as progressive disease,” Dr. Rajkumar said. “I congratulate the authors on a study that will improve the quality of life for our patients.”
Improved event-free survival
In this study, Dr. Larocca and colleagues investigated the efficacy and feasibility of a dose- and schedule-adjusted Rd regimen that was followed by maintenance Rd-R 10 mg/d and compared the regimen with continuous Rd in elderly, intermediate-fit patients who were newly diagnosed with multiple myeloma.
The primary endpoint was event-free survival, defined as progression/death from any cause, lenalidomide discontinuation, and any hematologic grade 4 or nonhematologic grade 3-4 adverse events.
The cohort consisted of 199 patients who were randomly assigned to receive either Rd-R (n = 101) or continuous Rd (n = 98). The median age was 75 years in the Rd-R arm and 76 years in the Rd arm; 52% of patients in the Rd-R group and 43% in the Rd group were classified as being intermediate fit not for age but for geriatric impairments.
With a median follow-up of 37 months, event-free survival was 10.4 months in the Rd-R arm versus 6.9 months in the Rd arm (hazard ratio, 0.70; P = .02). This benefit was maintained beyond nine cycles (median: 19.8 vs. 10.6 months for Rd-R vs. Rd; HR, 0.55; P = .03)
The median PFS was 20.2 months with Rd-R and 18.3 months with Rd (HR, 0.78; P = .16). The median overall survival was not reached. The 3-year overall survival was 74% with Rd-R and 63% with continuous Rd (HR, 0.62; P = .06). Among patients remaining on therapy after nine cycles, no difference in median PFS was observed between the two groups (24.3 vs. 18.7 months; HR, 0.73; P = .19).
Best response was similar for both groups, with an overall response rate of 78% versus 68% (P = .15). The very good partial response rate was 51% in the Rd-R arm versus 39% in the continuous Rd arm (P = .09).
Toxicities were similar between the two groups. Hematologic adverse events of at least grade 3 were reported in 26% of Rd-R patients versus 20% of Rd patients (P = .40). In both groups, the most frequent grade ≥3 hematologic toxicity was neutropenia (21% vs 18%). The most frequent grade ≥3 toxicities were nonhematologic. They occurred in 33% of Rd-R patients and 43% of Rd patients (P = .15). The most frequent nonhematologic toxicities were infections (10% vs. 12%), constitutional (3% vs. 12%), dermatologic (7% vs. 3%), and central nervous toxicities (2% vs. 6%).
The study was sponsored by Fondazione EMN Italy Onlus. Dr. Larocca has received honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and GlaxoSmithKline, and has served on the advisory boards for Bristol-Myers Squibb, Celgene, Janssen, and Takeda. Several coauthors also have disclosed relationships with industry. Dr. Rajkumar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For select older patients, it is safe to switch to a lower dose of lenalidomide maintenance therapy and discontinue dexamethasone after 9 months. The regimen is safe and yields outcomes similar to those of standard, continuous lenalidomide/dexamethasone (Rd), according to new findings.
At a median follow-up of 37 months, event-free survival was 10.4 months in the experimental arm in which dexamethasone therapy was stopped (Rd-R) versus 6.9 months for standard therapy. The tailored approach also resulted in fewer adverse effects.
The authors noted that there was no difference in progression-free survival (PFS) and overall survival between the two groups.
“These results may be useful for the treatment of myeloma patients, since approximately one-third of patients not eligible for stem cell transplantation are intermediate fit, the population in our study,” said lead author Alessandra Larocca, MD, PhD, from the department of hematology-oncology of the University Hospital Città della Salute e della Scienza, Torino, Italy.
She said in an interview that they expect that these findings “may help to optimize the treatment of less-fit elderly patients by reducing the occurrence of adverse events and thus improving outcomes and preserving quality of life of these patients.”
This approach is a viable option for clinicians to consider for some patient subgroups. “This steroid-sparing approach can also be used in other combinations,” she said. “Ongoing trials are now evaluating steroid sparing in combination with monoclonal antibodies or the role of frailty-guided treatment.”
The study was published March 19, 2021, in Blood.
Curtailing steroids
Myeloma patients aged 75 years or older or who have comorbidities and functional impairments are an understudied population. They are more susceptible to adverse events that may negatively affect the duration of treatment and outcomes. Steroids are “scarcely tolerated” in the long term, even among younger patients, and “whether sparing dexamethasone is as effective as prolonged steroid exposure remains an open issue,” the authors wrote. There are still no clear data on the advantage of continuous steroid treatment as opposed to fixed-duration treatment for newly diagnosed patients.
In 2010, a study compared high-dose with low-dose dexamethasone. As expected, the rate of adverse events was lower among patients who received the low-dose steroid, but quite unexpectedly, deaths with high-dose dexamethasone were significantly higher than with low-dose dexamethasone.
The 1-year overall survival was 96% among patients who received the low dose of dexamethasone versus 87% with the standard high dose.
S. Vincent Rajkumar, MD, of the Mayo Clinic, Rochester, Minn., who was the lead author of the 2010 study, spoke with this new organization about the current study. “This is an important and practice-changing study,” he said. “We have already changed our practice and recommendations based on this study.”
He explained that, for transplant-ineligible patients, instead of initial therapy with bortezomib-lenalidomide-dexamethasone followed by Rd, they use lenalidomide alone without steroids.
“After 9 months of initial therapy, I now recommend we stop dexamethasone unless we are having problems controlling the myeloma, such as progressive disease,” Dr. Rajkumar said. “I congratulate the authors on a study that will improve the quality of life for our patients.”
Improved event-free survival
In this study, Dr. Larocca and colleagues investigated the efficacy and feasibility of a dose- and schedule-adjusted Rd regimen that was followed by maintenance Rd-R 10 mg/d and compared the regimen with continuous Rd in elderly, intermediate-fit patients who were newly diagnosed with multiple myeloma.
The primary endpoint was event-free survival, defined as progression/death from any cause, lenalidomide discontinuation, and any hematologic grade 4 or nonhematologic grade 3-4 adverse events.
The cohort consisted of 199 patients who were randomly assigned to receive either Rd-R (n = 101) or continuous Rd (n = 98). The median age was 75 years in the Rd-R arm and 76 years in the Rd arm; 52% of patients in the Rd-R group and 43% in the Rd group were classified as being intermediate fit not for age but for geriatric impairments.
With a median follow-up of 37 months, event-free survival was 10.4 months in the Rd-R arm versus 6.9 months in the Rd arm (hazard ratio, 0.70; P = .02). This benefit was maintained beyond nine cycles (median: 19.8 vs. 10.6 months for Rd-R vs. Rd; HR, 0.55; P = .03)
The median PFS was 20.2 months with Rd-R and 18.3 months with Rd (HR, 0.78; P = .16). The median overall survival was not reached. The 3-year overall survival was 74% with Rd-R and 63% with continuous Rd (HR, 0.62; P = .06). Among patients remaining on therapy after nine cycles, no difference in median PFS was observed between the two groups (24.3 vs. 18.7 months; HR, 0.73; P = .19).
Best response was similar for both groups, with an overall response rate of 78% versus 68% (P = .15). The very good partial response rate was 51% in the Rd-R arm versus 39% in the continuous Rd arm (P = .09).
Toxicities were similar between the two groups. Hematologic adverse events of at least grade 3 were reported in 26% of Rd-R patients versus 20% of Rd patients (P = .40). In both groups, the most frequent grade ≥3 hematologic toxicity was neutropenia (21% vs 18%). The most frequent grade ≥3 toxicities were nonhematologic. They occurred in 33% of Rd-R patients and 43% of Rd patients (P = .15). The most frequent nonhematologic toxicities were infections (10% vs. 12%), constitutional (3% vs. 12%), dermatologic (7% vs. 3%), and central nervous toxicities (2% vs. 6%).
The study was sponsored by Fondazione EMN Italy Onlus. Dr. Larocca has received honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and GlaxoSmithKline, and has served on the advisory boards for Bristol-Myers Squibb, Celgene, Janssen, and Takeda. Several coauthors also have disclosed relationships with industry. Dr. Rajkumar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
For select older patients, it is safe to switch to a lower dose of lenalidomide maintenance therapy and discontinue dexamethasone after 9 months. The regimen is safe and yields outcomes similar to those of standard, continuous lenalidomide/dexamethasone (Rd), according to new findings.
At a median follow-up of 37 months, event-free survival was 10.4 months in the experimental arm in which dexamethasone therapy was stopped (Rd-R) versus 6.9 months for standard therapy. The tailored approach also resulted in fewer adverse effects.
The authors noted that there was no difference in progression-free survival (PFS) and overall survival between the two groups.
“These results may be useful for the treatment of myeloma patients, since approximately one-third of patients not eligible for stem cell transplantation are intermediate fit, the population in our study,” said lead author Alessandra Larocca, MD, PhD, from the department of hematology-oncology of the University Hospital Città della Salute e della Scienza, Torino, Italy.
She said in an interview that they expect that these findings “may help to optimize the treatment of less-fit elderly patients by reducing the occurrence of adverse events and thus improving outcomes and preserving quality of life of these patients.”
This approach is a viable option for clinicians to consider for some patient subgroups. “This steroid-sparing approach can also be used in other combinations,” she said. “Ongoing trials are now evaluating steroid sparing in combination with monoclonal antibodies or the role of frailty-guided treatment.”
The study was published March 19, 2021, in Blood.
Curtailing steroids
Myeloma patients aged 75 years or older or who have comorbidities and functional impairments are an understudied population. They are more susceptible to adverse events that may negatively affect the duration of treatment and outcomes. Steroids are “scarcely tolerated” in the long term, even among younger patients, and “whether sparing dexamethasone is as effective as prolonged steroid exposure remains an open issue,” the authors wrote. There are still no clear data on the advantage of continuous steroid treatment as opposed to fixed-duration treatment for newly diagnosed patients.
In 2010, a study compared high-dose with low-dose dexamethasone. As expected, the rate of adverse events was lower among patients who received the low-dose steroid, but quite unexpectedly, deaths with high-dose dexamethasone were significantly higher than with low-dose dexamethasone.
The 1-year overall survival was 96% among patients who received the low dose of dexamethasone versus 87% with the standard high dose.
S. Vincent Rajkumar, MD, of the Mayo Clinic, Rochester, Minn., who was the lead author of the 2010 study, spoke with this new organization about the current study. “This is an important and practice-changing study,” he said. “We have already changed our practice and recommendations based on this study.”
He explained that, for transplant-ineligible patients, instead of initial therapy with bortezomib-lenalidomide-dexamethasone followed by Rd, they use lenalidomide alone without steroids.
“After 9 months of initial therapy, I now recommend we stop dexamethasone unless we are having problems controlling the myeloma, such as progressive disease,” Dr. Rajkumar said. “I congratulate the authors on a study that will improve the quality of life for our patients.”
Improved event-free survival
In this study, Dr. Larocca and colleagues investigated the efficacy and feasibility of a dose- and schedule-adjusted Rd regimen that was followed by maintenance Rd-R 10 mg/d and compared the regimen with continuous Rd in elderly, intermediate-fit patients who were newly diagnosed with multiple myeloma.
The primary endpoint was event-free survival, defined as progression/death from any cause, lenalidomide discontinuation, and any hematologic grade 4 or nonhematologic grade 3-4 adverse events.
The cohort consisted of 199 patients who were randomly assigned to receive either Rd-R (n = 101) or continuous Rd (n = 98). The median age was 75 years in the Rd-R arm and 76 years in the Rd arm; 52% of patients in the Rd-R group and 43% in the Rd group were classified as being intermediate fit not for age but for geriatric impairments.
With a median follow-up of 37 months, event-free survival was 10.4 months in the Rd-R arm versus 6.9 months in the Rd arm (hazard ratio, 0.70; P = .02). This benefit was maintained beyond nine cycles (median: 19.8 vs. 10.6 months for Rd-R vs. Rd; HR, 0.55; P = .03)
The median PFS was 20.2 months with Rd-R and 18.3 months with Rd (HR, 0.78; P = .16). The median overall survival was not reached. The 3-year overall survival was 74% with Rd-R and 63% with continuous Rd (HR, 0.62; P = .06). Among patients remaining on therapy after nine cycles, no difference in median PFS was observed between the two groups (24.3 vs. 18.7 months; HR, 0.73; P = .19).
Best response was similar for both groups, with an overall response rate of 78% versus 68% (P = .15). The very good partial response rate was 51% in the Rd-R arm versus 39% in the continuous Rd arm (P = .09).
Toxicities were similar between the two groups. Hematologic adverse events of at least grade 3 were reported in 26% of Rd-R patients versus 20% of Rd patients (P = .40). In both groups, the most frequent grade ≥3 hematologic toxicity was neutropenia (21% vs 18%). The most frequent grade ≥3 toxicities were nonhematologic. They occurred in 33% of Rd-R patients and 43% of Rd patients (P = .15). The most frequent nonhematologic toxicities were infections (10% vs. 12%), constitutional (3% vs. 12%), dermatologic (7% vs. 3%), and central nervous toxicities (2% vs. 6%).
The study was sponsored by Fondazione EMN Italy Onlus. Dr. Larocca has received honoraria from Amgen, Bristol-Myers Squibb, Celgene, Janssen, and GlaxoSmithKline, and has served on the advisory boards for Bristol-Myers Squibb, Celgene, Janssen, and Takeda. Several coauthors also have disclosed relationships with industry. Dr. Rajkumar disclosed no relevant financial relationships.
A version of this article first appeared on Medscape.com.
Mishap ruins millions of J&J COVID vaccine doses
About 15 million doses of the Johnson & Johnson COVID-19 vaccine were ruined after workers at a manufacturing plant mixed up ingredients, The New York Times reported.
The Baltimore plant is operated by a company called Emergent BioSolutions, the Times said. The company works with both Johnson & Johnson and AstraZeneca.
The mistake has stopped shipments of the vaccine until the FDA investigates, the paper said. The mishap, however, does not affect doses of the J&J one-shot vaccine already delivered and being used.
The problem is that tens of millions of doses were supposed to come from the Baltimore plant.
The Associated Press reported that Emergent has had numerous problems with the FDA, with the agency citing the company for poorly trained employees, cracked vials and mold.
The records cover inspections at Emergent facilities, including Bayview, since 2017. Following a December 2017 inspection at an Emergent plant in Canton, Massachusetts, the FDA said the company hadn’t corrected “continued low level mold and yeast isolates” found in the facility. Nearly a year later, agency investigators questioned why Emergent had “an unwritten policy of not conducting routine compliance audits” at a separate plant in Baltimore, known as Camden, where an anthrax vaccine is filled into vials.
Meanwhile, in a statement, Johnson & Johnson said its own quality control process identified the problem in one batch of ingredients. The company said the Emergent plant in Baltimore is “not yet authorized to manufacture drug substance for our COVID-19 vaccine. This batch was never advanced to the filling and finishing stages of our manufacturing process.”
The company said it plans to still seek emergency use authorization for a different Emergent facility and will provide more experts on site at Emergent.
The Times reports that President Joe Biden’s team still believes the administration can meet its commitment to have enough vaccine doses to immunize every adult by the end of May.
Johnson & Johnson said it still plans to deliver an additional 24 million doses through April.
A version of this article first appeared on WebMD.com.
This article was updated 4/1/21.
About 15 million doses of the Johnson & Johnson COVID-19 vaccine were ruined after workers at a manufacturing plant mixed up ingredients, The New York Times reported.
The Baltimore plant is operated by a company called Emergent BioSolutions, the Times said. The company works with both Johnson & Johnson and AstraZeneca.
The mistake has stopped shipments of the vaccine until the FDA investigates, the paper said. The mishap, however, does not affect doses of the J&J one-shot vaccine already delivered and being used.
The problem is that tens of millions of doses were supposed to come from the Baltimore plant.
The Associated Press reported that Emergent has had numerous problems with the FDA, with the agency citing the company for poorly trained employees, cracked vials and mold.
The records cover inspections at Emergent facilities, including Bayview, since 2017. Following a December 2017 inspection at an Emergent plant in Canton, Massachusetts, the FDA said the company hadn’t corrected “continued low level mold and yeast isolates” found in the facility. Nearly a year later, agency investigators questioned why Emergent had “an unwritten policy of not conducting routine compliance audits” at a separate plant in Baltimore, known as Camden, where an anthrax vaccine is filled into vials.
Meanwhile, in a statement, Johnson & Johnson said its own quality control process identified the problem in one batch of ingredients. The company said the Emergent plant in Baltimore is “not yet authorized to manufacture drug substance for our COVID-19 vaccine. This batch was never advanced to the filling and finishing stages of our manufacturing process.”
The company said it plans to still seek emergency use authorization for a different Emergent facility and will provide more experts on site at Emergent.
The Times reports that President Joe Biden’s team still believes the administration can meet its commitment to have enough vaccine doses to immunize every adult by the end of May.
Johnson & Johnson said it still plans to deliver an additional 24 million doses through April.
A version of this article first appeared on WebMD.com.
This article was updated 4/1/21.
About 15 million doses of the Johnson & Johnson COVID-19 vaccine were ruined after workers at a manufacturing plant mixed up ingredients, The New York Times reported.
The Baltimore plant is operated by a company called Emergent BioSolutions, the Times said. The company works with both Johnson & Johnson and AstraZeneca.
The mistake has stopped shipments of the vaccine until the FDA investigates, the paper said. The mishap, however, does not affect doses of the J&J one-shot vaccine already delivered and being used.
The problem is that tens of millions of doses were supposed to come from the Baltimore plant.
The Associated Press reported that Emergent has had numerous problems with the FDA, with the agency citing the company for poorly trained employees, cracked vials and mold.
The records cover inspections at Emergent facilities, including Bayview, since 2017. Following a December 2017 inspection at an Emergent plant in Canton, Massachusetts, the FDA said the company hadn’t corrected “continued low level mold and yeast isolates” found in the facility. Nearly a year later, agency investigators questioned why Emergent had “an unwritten policy of not conducting routine compliance audits” at a separate plant in Baltimore, known as Camden, where an anthrax vaccine is filled into vials.
Meanwhile, in a statement, Johnson & Johnson said its own quality control process identified the problem in one batch of ingredients. The company said the Emergent plant in Baltimore is “not yet authorized to manufacture drug substance for our COVID-19 vaccine. This batch was never advanced to the filling and finishing stages of our manufacturing process.”
The company said it plans to still seek emergency use authorization for a different Emergent facility and will provide more experts on site at Emergent.
The Times reports that President Joe Biden’s team still believes the administration can meet its commitment to have enough vaccine doses to immunize every adult by the end of May.
Johnson & Johnson said it still plans to deliver an additional 24 million doses through April.
A version of this article first appeared on WebMD.com.
This article was updated 4/1/21.
Ob.Gyn. giant Dr. Charles Hammond dies
Ob.Gyn. News acknowledges the passing of Charles B. Hammond, MD, a longtime editorial board member, who died on Feb. 1, 2021.
Dr. Hammond served on the Ob.Gyn. News Advisory Board for 33 years. His service as a board member was one of many leadership roles to which he dedicated his time and expertise. Dr. Hammond served as president of the American College of Obstetrics & Gynecology (ACOG) from 2002 to 2003, and received a Lifetime Achievement Award from ACOG in 2015. He also served as president of the American Society of Reproductive Medicine, president of the American Gynecological and Obstetrical Society, and president of the North Carolina Obstetrical and Gynecological Society.
Dr. Hammond was honored by Duke University, Durham, N.C., as E.C. Hamblen Professor Emeritus in 2010, after more than 40 years in academia. He held the title of Edwin Crowell Hamblen Distinguished Professor of Reproductive Biology and Family Planning and Chair of the Department of Obstetrics & Gynecology from 1980 to 2002. During this time, he distinguished himself for his work in pioneering treatments for gestational trophoblastic disease. As an extension of this research, he was a founder of the Southeast Regional Trophoblastic Disease Center. In addition, Dr. Hammond was often consulted for his expertise on issues related to menopause and hormone replacement therapy.
Dr. Hammond began his medical career at Duke University after graduating from The Citadel with a bachelor of science degree in 1958. After earning his medical degree in 1961, he remained at Duke as a resident in obstetrics and gynecology, followed by completion of a fellowship in reproductive endocrinology in 1964. From 1964 to 1966, he served at the National Institutes of Health as a fellow in the National Cancer Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A few years later, in 1969, Dr. Hammond launched his long and distinguished academic career with an assistant professor position in the department of obstetrics & gynecology at Duke.
Dr. Hammond’s many honors include a National Association for Women’s Health Lifetime Achievement Award and membership in the Institute of Medicine, now the National Academy of Medicine. He also was named a fellow of the Royal College of Obstetricians and Gynaecologists, and an honorary member of the Canadian Society of Obstetrics and Gynecology. Dr. Hammond will be remembered not only as a physician, researcher, educator, and mentor, but also an advocate for women’s health.
Ob.Gyn. News acknowledges the passing of Charles B. Hammond, MD, a longtime editorial board member, who died on Feb. 1, 2021.
Dr. Hammond served on the Ob.Gyn. News Advisory Board for 33 years. His service as a board member was one of many leadership roles to which he dedicated his time and expertise. Dr. Hammond served as president of the American College of Obstetrics & Gynecology (ACOG) from 2002 to 2003, and received a Lifetime Achievement Award from ACOG in 2015. He also served as president of the American Society of Reproductive Medicine, president of the American Gynecological and Obstetrical Society, and president of the North Carolina Obstetrical and Gynecological Society.
Dr. Hammond was honored by Duke University, Durham, N.C., as E.C. Hamblen Professor Emeritus in 2010, after more than 40 years in academia. He held the title of Edwin Crowell Hamblen Distinguished Professor of Reproductive Biology and Family Planning and Chair of the Department of Obstetrics & Gynecology from 1980 to 2002. During this time, he distinguished himself for his work in pioneering treatments for gestational trophoblastic disease. As an extension of this research, he was a founder of the Southeast Regional Trophoblastic Disease Center. In addition, Dr. Hammond was often consulted for his expertise on issues related to menopause and hormone replacement therapy.
Dr. Hammond began his medical career at Duke University after graduating from The Citadel with a bachelor of science degree in 1958. After earning his medical degree in 1961, he remained at Duke as a resident in obstetrics and gynecology, followed by completion of a fellowship in reproductive endocrinology in 1964. From 1964 to 1966, he served at the National Institutes of Health as a fellow in the National Cancer Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A few years later, in 1969, Dr. Hammond launched his long and distinguished academic career with an assistant professor position in the department of obstetrics & gynecology at Duke.
Dr. Hammond’s many honors include a National Association for Women’s Health Lifetime Achievement Award and membership in the Institute of Medicine, now the National Academy of Medicine. He also was named a fellow of the Royal College of Obstetricians and Gynaecologists, and an honorary member of the Canadian Society of Obstetrics and Gynecology. Dr. Hammond will be remembered not only as a physician, researcher, educator, and mentor, but also an advocate for women’s health.
Ob.Gyn. News acknowledges the passing of Charles B. Hammond, MD, a longtime editorial board member, who died on Feb. 1, 2021.
Dr. Hammond served on the Ob.Gyn. News Advisory Board for 33 years. His service as a board member was one of many leadership roles to which he dedicated his time and expertise. Dr. Hammond served as president of the American College of Obstetrics & Gynecology (ACOG) from 2002 to 2003, and received a Lifetime Achievement Award from ACOG in 2015. He also served as president of the American Society of Reproductive Medicine, president of the American Gynecological and Obstetrical Society, and president of the North Carolina Obstetrical and Gynecological Society.
Dr. Hammond was honored by Duke University, Durham, N.C., as E.C. Hamblen Professor Emeritus in 2010, after more than 40 years in academia. He held the title of Edwin Crowell Hamblen Distinguished Professor of Reproductive Biology and Family Planning and Chair of the Department of Obstetrics & Gynecology from 1980 to 2002. During this time, he distinguished himself for his work in pioneering treatments for gestational trophoblastic disease. As an extension of this research, he was a founder of the Southeast Regional Trophoblastic Disease Center. In addition, Dr. Hammond was often consulted for his expertise on issues related to menopause and hormone replacement therapy.
Dr. Hammond began his medical career at Duke University after graduating from The Citadel with a bachelor of science degree in 1958. After earning his medical degree in 1961, he remained at Duke as a resident in obstetrics and gynecology, followed by completion of a fellowship in reproductive endocrinology in 1964. From 1964 to 1966, he served at the National Institutes of Health as a fellow in the National Cancer Institute and the Eunice Kennedy Shriver National Institute of Child Health and Human Development.
A few years later, in 1969, Dr. Hammond launched his long and distinguished academic career with an assistant professor position in the department of obstetrics & gynecology at Duke.
Dr. Hammond’s many honors include a National Association for Women’s Health Lifetime Achievement Award and membership in the Institute of Medicine, now the National Academy of Medicine. He also was named a fellow of the Royal College of Obstetricians and Gynaecologists, and an honorary member of the Canadian Society of Obstetrics and Gynecology. Dr. Hammond will be remembered not only as a physician, researcher, educator, and mentor, but also an advocate for women’s health.
Children could become eligible for a COVID-19 vaccine by fall, expert predicts
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
If everything goes as planned,
According to Yvonne Maldonado, MD, Pfizer has fully enrolled adolescent trials and Moderna is currently enrolling 3,000 adolescents in a safety and reactogenicity trial known as TeenCOVE, in which participants will receive an intramuscular injection of 100 mcg mRNA-1273 on day 1 and on day 29. Meanwhile, Johnson & Johnson and AstraZeneca will be starting to enroll older children and adolescents into studies within the next several weeks.
The companies are also planning to enroll younger children, Dr. Maldonado, the Taube professor of global health and infectious diseases at Stanford (Calif.) University, said during the Society for Pediatric Dermatology pre-AAD meeting. “At least two of the vaccine companies have indicated that they would like to start enrolling children as young as 2-5 years of age and eventually getting down to infants and toddlers if the vaccines prove to be safe and effective in the older children. Eventually, we hope to get to the level where we can have several vaccine candidates for all children 6 months of age and older.”
In the future, she said, infectious disease experts hope to see antiviral, immunomodulatory, anti-inflammatory, and monoclonal therapies for all populations including children, although trials in this population have not begun. “Clinical trials must be flexible and adaptive to deal with children and adolescents,” added Dr. Maldonado, who is also senior associate dean for faculty development and diversity at Stanford.
“We would ideally like to have new correlates of protection, as well as biomarkers to follow for evidence of effectiveness. We also would love to see vaccines in the pediatric population as soon as possible, because herd immunity is the ultimate goal for protection against this disease and prevention of additional transmission over time.” However, she said, the degree and durability of immunity has yet to be determined, and vaccine-associated immune effects are unknown. In the meantime, infectious disease researchers expect nonpharmacologic interventions, such as wearing face masks and social distancing to continue for an undefined period.
(Less than 2 weeks after Dr. Maldonado spoke at the SPD meeting, Pfizer announced in a press release that, in phase 3 clinical trials, the company’s coronavirus vaccine was 100% effective in protecting children aged 12-15 years from infection, with a “robust” antibody responses and side effects similar to those experienced by those aged 16-25 years. The company also announced that it plans to seek Food and Drug Administration EUA for this age group. Asked to comment on this update, Dr. Maldonado said the results released by Pfizer “suggest that their COVID-19 vaccine is very safe and highly effective in preventing COVID-19 among children 12-15 years of age.” She added that additional data from the Pfizer trials as well as from Moderna and Johnson & Johnson vaccine trials “will hopefully lead to FDA EUA review in the coming weeks,” and that COVID-19 vaccinations for children “may be possible by this summer.”)
Children with underlying diseases or on immune suppressants
At the SPD meeting, an attendee asked if there were any pediatric patients for whom she would not recommend receiving a COVID-19 vaccine because of an underlying disease or concurrent therapy with immune suppressants. “We don’t have those data yet,” Dr. Maldonado said. “Based on what we’re seeing with adults, it does appear that those with underlying conditions are at somewhat higher risk of developing severe infection and may therefore most likely to need vaccination. Most of those risks are cardiovascular, obesity, and other factors, but not necessarily immunocompromising conditions. More likely what we’re seeing is that people with underlying immunocompromising conditions may not mount a good response to the vaccines at this time. It doesn’t mean we shouldn’t give the vaccines, but we need to learn more about that.”
Dr. Maldonado went on to note that, as vaccine manufacturers commence pediatric trials, healthy children will be tested first, followed in due time with children who have immunocompromised conditions. “The question will be whether or not we should give monoclonal antibodies to those particular children to help boost their immunity to SARS-CoV-2, because they might not have a good response to the vaccines,” she said. “Those things need to be sorted out, but there’s no safety signal or concerns at this point for vaccine to be given to immunocompromised individuals.”
Another meeting attendee asked Dr. Maldonado if she thinks there is a practical role for assessing markers of T-cell immunity when evaluating suspected COVID-19 patients who may test negative on serology, Dr. Maldonado said that she and her colleagues are seeking pediatric patients who were treated for COVID-19 at Stanford, in an effort to sort this out.
They are checking peripheral blood mononuclear cells in these patients “to try and tease out what the immune response is in kids who have serious disease, versus those who came in with acute disease, versus those who are asymptomatic,” and comparing them with children who don’t have infection, she explained. “The question is, what is the role of T cells and how much do they contribute? One of the biggest questions we have is, do we have an immune correlate? Can we detect a particular level of neutralizing antibody that seems to be protective? If so, how long is it protective, and can we look for T- and B-cell memory cells and effector vector cells and see how long those effector vector cells can be active in protection? Those are studies that are ongoing now.”
Dr. Maldonado disclosed that she is a member of the data safety monitoring board for a non–COVID-19 vaccine being developed by Pfizer.
FROM THE SPD PRE-AAD MEETING








