User login
AGA Gives Guidance on Subepithelial Lesions
The new guidance document, authored by Lionel S. D’Souza, MD, of Stony Brook University Hospital, Stony Brook, New York, and colleagues, offers a framework for deciding between various EFTR techniques based on lesion histology, size, and location.
“EFTR has emerged as a novel treatment option for select SELs,” the update panelists wrote in Gastroenterology. “In this commentary, we reviewed the different techniques and uses of EFTR for the management of SELs.”
They noted that all patients with SELs should first undergo multidisciplinary evaluation in accordance with a separate AGA guidance document on SELs.
The present update focuses specifically on EFTR, first by distinguishing between exposed and nonexposed techniques. While the former involves resection of the mucosa and all other layers of the wall, the latter relies upon a ‘close first, then cut’ method to prevent perforation, or preservation of an overlying flap of mucosa.
The new guidance calls for a nonexposed technique unless the exposed approach is necessary.
“In our opinion, the exposed EFTR technique should be considered for lesions in which other methods (i.e., endoscopic mucosal resection, endoscopic submucosal dissection, and nonexposed EFTR) cannot reliably and completely excise SELs due to larger size or difficult location of the lesion,” the update panelists wrote. “The exposed EFTR technique may be best suited for gastric lesions and as an alternative to other endoscopic approaches for SELs in the rectum. The exposed technique should be avoided in the esophagus and duodenum, as the clinical consequences of a leak can be devastating and endoscopic closure is notoriously challenging.”
Dr. D’Souza and colleagues went on to discuss various nonexposed techniques, including submucosal tunneling and endoscopic resection and peroral endoscopic tunnel resection (STER/POET), device-assisted endoscopic full-thickness resection, and full-thickness resection with an over-the-scope clip with integrated snare (FTRD).
They highlighted how STER/POET encourages traction on the lesion and scope stability while limiting extravasation of luminal contents, and closure tends to be easier than with exposed EFTR. This approach should be reserved for tumors smaller than approximately 3-4 cm, however, with the update noting that lesions larger than 2 cm may present increased risk of incomplete resection. Similarly, device-assisted endoscopic full-thickness resection, which involves pulling or suctioning the lesion into the device, is also limited by lesion size, although fewer data are available to guide size thresholds.
FTRD, which involves “a 23-mm deep cap with a specially designed over-the-scope clip and integrated cautery snare,” also lacks a broad evidence base.
“Although there has been reasonable clinical success reported in most case series, several factors should be considered with the use of the FTRD for SELs,” the update cautions.
Specifically, a recent Dutch and German registry study of FTRD had an adverse event rate of 11.3%, with an approximate 1% perforation rate. More than half of the perforations were due to technical or procedural issues.
“This adverse event rate may improve as individual experience with the device is gained; however, data on this are lacking,” the panelists wrote, also noting that lesions 1.5 cm or larger may carry a higher risk of incomplete resection.
Ultimately, the clinical practice update calls for a personalized approach to EFTR decision-making that considers factors extending beyond the lesion.
“The ‘ideal’ technique will depend on various patient and lesion characteristics, as well as the endoscopist’s preference and available expertise,” Dr. D’Souza and colleagues concluded. “Further research into the efficacy of these resection techniques and the long-term outcomes in patients after endoscopic resection of SELs will be essential in standardizing appropriate resection algorithms.”
This clinical practice update was commissioned and approved by AGA Institute. The investigators disclosed relationships with Olympus, Fujifilm, Apollo Endosurgery, and others.
The new guidance document, authored by Lionel S. D’Souza, MD, of Stony Brook University Hospital, Stony Brook, New York, and colleagues, offers a framework for deciding between various EFTR techniques based on lesion histology, size, and location.
“EFTR has emerged as a novel treatment option for select SELs,” the update panelists wrote in Gastroenterology. “In this commentary, we reviewed the different techniques and uses of EFTR for the management of SELs.”
They noted that all patients with SELs should first undergo multidisciplinary evaluation in accordance with a separate AGA guidance document on SELs.
The present update focuses specifically on EFTR, first by distinguishing between exposed and nonexposed techniques. While the former involves resection of the mucosa and all other layers of the wall, the latter relies upon a ‘close first, then cut’ method to prevent perforation, or preservation of an overlying flap of mucosa.
The new guidance calls for a nonexposed technique unless the exposed approach is necessary.
“In our opinion, the exposed EFTR technique should be considered for lesions in which other methods (i.e., endoscopic mucosal resection, endoscopic submucosal dissection, and nonexposed EFTR) cannot reliably and completely excise SELs due to larger size or difficult location of the lesion,” the update panelists wrote. “The exposed EFTR technique may be best suited for gastric lesions and as an alternative to other endoscopic approaches for SELs in the rectum. The exposed technique should be avoided in the esophagus and duodenum, as the clinical consequences of a leak can be devastating and endoscopic closure is notoriously challenging.”
Dr. D’Souza and colleagues went on to discuss various nonexposed techniques, including submucosal tunneling and endoscopic resection and peroral endoscopic tunnel resection (STER/POET), device-assisted endoscopic full-thickness resection, and full-thickness resection with an over-the-scope clip with integrated snare (FTRD).
They highlighted how STER/POET encourages traction on the lesion and scope stability while limiting extravasation of luminal contents, and closure tends to be easier than with exposed EFTR. This approach should be reserved for tumors smaller than approximately 3-4 cm, however, with the update noting that lesions larger than 2 cm may present increased risk of incomplete resection. Similarly, device-assisted endoscopic full-thickness resection, which involves pulling or suctioning the lesion into the device, is also limited by lesion size, although fewer data are available to guide size thresholds.
FTRD, which involves “a 23-mm deep cap with a specially designed over-the-scope clip and integrated cautery snare,” also lacks a broad evidence base.
“Although there has been reasonable clinical success reported in most case series, several factors should be considered with the use of the FTRD for SELs,” the update cautions.
Specifically, a recent Dutch and German registry study of FTRD had an adverse event rate of 11.3%, with an approximate 1% perforation rate. More than half of the perforations were due to technical or procedural issues.
“This adverse event rate may improve as individual experience with the device is gained; however, data on this are lacking,” the panelists wrote, also noting that lesions 1.5 cm or larger may carry a higher risk of incomplete resection.
Ultimately, the clinical practice update calls for a personalized approach to EFTR decision-making that considers factors extending beyond the lesion.
“The ‘ideal’ technique will depend on various patient and lesion characteristics, as well as the endoscopist’s preference and available expertise,” Dr. D’Souza and colleagues concluded. “Further research into the efficacy of these resection techniques and the long-term outcomes in patients after endoscopic resection of SELs will be essential in standardizing appropriate resection algorithms.”
This clinical practice update was commissioned and approved by AGA Institute. The investigators disclosed relationships with Olympus, Fujifilm, Apollo Endosurgery, and others.
The new guidance document, authored by Lionel S. D’Souza, MD, of Stony Brook University Hospital, Stony Brook, New York, and colleagues, offers a framework for deciding between various EFTR techniques based on lesion histology, size, and location.
“EFTR has emerged as a novel treatment option for select SELs,” the update panelists wrote in Gastroenterology. “In this commentary, we reviewed the different techniques and uses of EFTR for the management of SELs.”
They noted that all patients with SELs should first undergo multidisciplinary evaluation in accordance with a separate AGA guidance document on SELs.
The present update focuses specifically on EFTR, first by distinguishing between exposed and nonexposed techniques. While the former involves resection of the mucosa and all other layers of the wall, the latter relies upon a ‘close first, then cut’ method to prevent perforation, or preservation of an overlying flap of mucosa.
The new guidance calls for a nonexposed technique unless the exposed approach is necessary.
“In our opinion, the exposed EFTR technique should be considered for lesions in which other methods (i.e., endoscopic mucosal resection, endoscopic submucosal dissection, and nonexposed EFTR) cannot reliably and completely excise SELs due to larger size or difficult location of the lesion,” the update panelists wrote. “The exposed EFTR technique may be best suited for gastric lesions and as an alternative to other endoscopic approaches for SELs in the rectum. The exposed technique should be avoided in the esophagus and duodenum, as the clinical consequences of a leak can be devastating and endoscopic closure is notoriously challenging.”
Dr. D’Souza and colleagues went on to discuss various nonexposed techniques, including submucosal tunneling and endoscopic resection and peroral endoscopic tunnel resection (STER/POET), device-assisted endoscopic full-thickness resection, and full-thickness resection with an over-the-scope clip with integrated snare (FTRD).
They highlighted how STER/POET encourages traction on the lesion and scope stability while limiting extravasation of luminal contents, and closure tends to be easier than with exposed EFTR. This approach should be reserved for tumors smaller than approximately 3-4 cm, however, with the update noting that lesions larger than 2 cm may present increased risk of incomplete resection. Similarly, device-assisted endoscopic full-thickness resection, which involves pulling or suctioning the lesion into the device, is also limited by lesion size, although fewer data are available to guide size thresholds.
FTRD, which involves “a 23-mm deep cap with a specially designed over-the-scope clip and integrated cautery snare,” also lacks a broad evidence base.
“Although there has been reasonable clinical success reported in most case series, several factors should be considered with the use of the FTRD for SELs,” the update cautions.
Specifically, a recent Dutch and German registry study of FTRD had an adverse event rate of 11.3%, with an approximate 1% perforation rate. More than half of the perforations were due to technical or procedural issues.
“This adverse event rate may improve as individual experience with the device is gained; however, data on this are lacking,” the panelists wrote, also noting that lesions 1.5 cm or larger may carry a higher risk of incomplete resection.
Ultimately, the clinical practice update calls for a personalized approach to EFTR decision-making that considers factors extending beyond the lesion.
“The ‘ideal’ technique will depend on various patient and lesion characteristics, as well as the endoscopist’s preference and available expertise,” Dr. D’Souza and colleagues concluded. “Further research into the efficacy of these resection techniques and the long-term outcomes in patients after endoscopic resection of SELs will be essential in standardizing appropriate resection algorithms.”
This clinical practice update was commissioned and approved by AGA Institute. The investigators disclosed relationships with Olympus, Fujifilm, Apollo Endosurgery, and others.
FROM GASTROENTEROLOGY
Telephone Best for Switching Patients to New Colonoscopy Intervals
In an article published in Clinical Gastroenterology and Hepatology, a group led by Jeffrey K. Lee, MD, MPH, a gastroenterologist at Kaiser Permanente Medical Center in San Francisco, reported the following 60-day response rates for the three contact methods in potentially transitioning more than 600 post-polypectomy patients to the new interval:
- Telephone: 64.5%
- Secure messaging: 51.7%
- Mailed letter: 31.3%
Compared with letter outreach, overall rate differences were significant for telephone (18.1%) and secure message outreach (13.1%).
Such interventions are widely used, the authors noted , but have not been compared for efficacy terms of communicating updated colonoscopy intervals.
The trial’s aim was to inform low-risk patients of the recommended interval update from 5 years — used since the 1990s — to 7-10 years. Given a choice, more patients opted to transition to the 10-year surveillance interval in the telephone (37%) and secure messaging arms (32.%) compared with mailed-letter arm (18.9%).
In addition to telephone and secure messaging outreach, factors positively associated with adoption of the 10-year interval were a positive fecal immunochemical test–based index colonoscopy and increasing age. Patients with these characteristics may be biased toward avoiding colonoscopy if not medically necessary, the authors conjectured.
Inversely associated factors included Asian or Pacific Islander race (odds ratio .58), Hispanic ethnicity (OR .40), and a higher Charlson comorbidity score of 2 vs 0 (OR .43).
Possible explanations for the race and ethnicity associations include gaps in culturally component care, lack of engagement with the English-based outreach approaches, and medical mistrust, the authors said.
“In this study, we gave all our patients an option to either extend their surveillance interval to current guideline recommendations or continue with their old interval, and some chose to do that,” Dr. Lee said in an interview. “Patients really appreciated having a choice and to be informed about the latest guideline changes.”
“A critical challenge to health systems is how to effectively de-implement outdated surveillance recommendations for low-risk patients who have a 5-year follow-up interval and potentially transition them to the recommended 7- to 10-year interval,” Dr. Lee and colleagues wrote.
More than 5 million surveillance colonoscopies are performed annually in US patients with a history of adenomas, the main precursor lesion for colorectal cancer, the authors noted.
With the recent guidelines issued in 2020 by the US Multi-Society Task Force on Colorectal Cancer lengthening the follow-up interval to 7-10 years , physicians are being advised to reevaluate low-risk patients previously scheduled with 5-year surveillance and provide an updated recommendation for follow-up.
Study Details
The three-arm pragmatic randomized trial was conducted in low-risk patients 54-70 years of age with one or two small (< 10 mm) tubular adenomas at baseline colonoscopy. Participants due for 5-year surveillance in 2022 were randomly assigned to one of three outreach arms: telephone (n = 200], secure messaging (n = 203), and mailed letter (n = 201). Stratified by age, sex, race, and ethnicity, participants could change their assigned interval to 10 years or continue with their previously scheduled 5-year interval.
As to economic considerations, the authors said that telephone may be the costliest form of outreach in terms of staffing resources. “We don’t know because we did not conduct a formal cost-effectiveness analysis,” Dr. Lee said. “However, we do know phone outreach requires a lot of personnel effort, which is why we also explored the less costly option of secure messaging/email.”
But based on the findings, telephone outreach would be a reasonable approach to update patients on post-polypectomy surveillance guideline changes if secure messaging or text messaging isn’t available, he added.
Downsides to Retroactive Changes?
Commenting on the study but not involved in it, Nabil M. Mansour, MD, an assistant professor and director of the McNair General GI Clinic at Baylor College of Medicine in Houston, noted that unlike Kaiser Permanente, his center decided against an overall effort to switch patients colonoscopied before the release of the new guidelines over to the new interval.
“Several of our physicians may have chosen to recommend a 5-year interval specifically for a variety of reasons and we felt going back, and making a blanket change to everyone’s interval retrospectively might create confusion and frustration and might actually delay the colonoscopies of some patients for which their doctors had a very good, legitimate reason to recommend a 5-year interval,” he said in an interview.
Dr. Mansour added that no difficulties were encountered in getting patients to agree to a 10-year interval. In his view telephone communication or in-person clinic visits are likely the most effective ways but both are more labor-intensive than automated patient portal messages. “I do not think traditional snail mail is effective.” His clinic uses automatic EMR reminders.
Offering another perspective on the study, Aditya Sreenivasan, MD, a gastroenterologist at Northwell Health in New York City, said his center has not reached out to correct the old intervals. “When I see a patient who previously had a colonoscopy with another physician, I always follow the previous recommendation for when the next colonoscopy should be, regardless of whether or not it technically meets guideline recommendations,” he told this news organization. “I do this because I was not there during the procedure and am not aware of any circumstances that would require a shorter interval that may not be apparent from the report.”
While he agrees with the new guidelines, Dr. Sreenivasan is “not sure if retroactively changing intervals is beneficial to patients, as the presence of guidelines may subconsciously influence the behavior of the endoscopist at the time of the procedure. For example, if a patient has a technically challenging colonoscopy and the endoscopist is running late, the endoscopist may drop their guard once they find a polyp and miss 1-2 additional small polyps that they would have spent more time looking for if they knew their next one would be in 10 years instead of 5.”
As for notification method, despite the logistical downside of taking dedicated staff time to make telephone calls, Dr. Sreenivasan said, “I think having a conversation with the patient directly is a much better way to communicate this information as it allows the patient to ask and answer questions. Things like tone of voice can provide reassurance that one cannot get via email.” Looking to the future, the study authors acknowledged that combinations of initial and reminder outreach approaches — for example, a mailed letter followed by secure message or telephone call — could potentially yield higher response rates and/or adoption rates than they observed. And a longer follow-up period with additional reminders may have produced higher yields. Additional studies are needed to optimize outreach approaches and to understand patient barriers to adopting the new guideline recommendations in different healthcare settings.
The study was supported by a Delivery Science grant from the Kaiser Permanente Northern California.
The authors disclosed no conflicts of interest. Dr. Mansour and Dr. Sreenivasan disclosed no conflicts of interest relevant to their comments.
In an article published in Clinical Gastroenterology and Hepatology, a group led by Jeffrey K. Lee, MD, MPH, a gastroenterologist at Kaiser Permanente Medical Center in San Francisco, reported the following 60-day response rates for the three contact methods in potentially transitioning more than 600 post-polypectomy patients to the new interval:
- Telephone: 64.5%
- Secure messaging: 51.7%
- Mailed letter: 31.3%
Compared with letter outreach, overall rate differences were significant for telephone (18.1%) and secure message outreach (13.1%).
Such interventions are widely used, the authors noted , but have not been compared for efficacy terms of communicating updated colonoscopy intervals.
The trial’s aim was to inform low-risk patients of the recommended interval update from 5 years — used since the 1990s — to 7-10 years. Given a choice, more patients opted to transition to the 10-year surveillance interval in the telephone (37%) and secure messaging arms (32.%) compared with mailed-letter arm (18.9%).
In addition to telephone and secure messaging outreach, factors positively associated with adoption of the 10-year interval were a positive fecal immunochemical test–based index colonoscopy and increasing age. Patients with these characteristics may be biased toward avoiding colonoscopy if not medically necessary, the authors conjectured.
Inversely associated factors included Asian or Pacific Islander race (odds ratio .58), Hispanic ethnicity (OR .40), and a higher Charlson comorbidity score of 2 vs 0 (OR .43).
Possible explanations for the race and ethnicity associations include gaps in culturally component care, lack of engagement with the English-based outreach approaches, and medical mistrust, the authors said.
“In this study, we gave all our patients an option to either extend their surveillance interval to current guideline recommendations or continue with their old interval, and some chose to do that,” Dr. Lee said in an interview. “Patients really appreciated having a choice and to be informed about the latest guideline changes.”
“A critical challenge to health systems is how to effectively de-implement outdated surveillance recommendations for low-risk patients who have a 5-year follow-up interval and potentially transition them to the recommended 7- to 10-year interval,” Dr. Lee and colleagues wrote.
More than 5 million surveillance colonoscopies are performed annually in US patients with a history of adenomas, the main precursor lesion for colorectal cancer, the authors noted.
With the recent guidelines issued in 2020 by the US Multi-Society Task Force on Colorectal Cancer lengthening the follow-up interval to 7-10 years , physicians are being advised to reevaluate low-risk patients previously scheduled with 5-year surveillance and provide an updated recommendation for follow-up.
Study Details
The three-arm pragmatic randomized trial was conducted in low-risk patients 54-70 years of age with one or two small (< 10 mm) tubular adenomas at baseline colonoscopy. Participants due for 5-year surveillance in 2022 were randomly assigned to one of three outreach arms: telephone (n = 200], secure messaging (n = 203), and mailed letter (n = 201). Stratified by age, sex, race, and ethnicity, participants could change their assigned interval to 10 years or continue with their previously scheduled 5-year interval.
As to economic considerations, the authors said that telephone may be the costliest form of outreach in terms of staffing resources. “We don’t know because we did not conduct a formal cost-effectiveness analysis,” Dr. Lee said. “However, we do know phone outreach requires a lot of personnel effort, which is why we also explored the less costly option of secure messaging/email.”
But based on the findings, telephone outreach would be a reasonable approach to update patients on post-polypectomy surveillance guideline changes if secure messaging or text messaging isn’t available, he added.
Downsides to Retroactive Changes?
Commenting on the study but not involved in it, Nabil M. Mansour, MD, an assistant professor and director of the McNair General GI Clinic at Baylor College of Medicine in Houston, noted that unlike Kaiser Permanente, his center decided against an overall effort to switch patients colonoscopied before the release of the new guidelines over to the new interval.
“Several of our physicians may have chosen to recommend a 5-year interval specifically for a variety of reasons and we felt going back, and making a blanket change to everyone’s interval retrospectively might create confusion and frustration and might actually delay the colonoscopies of some patients for which their doctors had a very good, legitimate reason to recommend a 5-year interval,” he said in an interview.
Dr. Mansour added that no difficulties were encountered in getting patients to agree to a 10-year interval. In his view telephone communication or in-person clinic visits are likely the most effective ways but both are more labor-intensive than automated patient portal messages. “I do not think traditional snail mail is effective.” His clinic uses automatic EMR reminders.
Offering another perspective on the study, Aditya Sreenivasan, MD, a gastroenterologist at Northwell Health in New York City, said his center has not reached out to correct the old intervals. “When I see a patient who previously had a colonoscopy with another physician, I always follow the previous recommendation for when the next colonoscopy should be, regardless of whether or not it technically meets guideline recommendations,” he told this news organization. “I do this because I was not there during the procedure and am not aware of any circumstances that would require a shorter interval that may not be apparent from the report.”
While he agrees with the new guidelines, Dr. Sreenivasan is “not sure if retroactively changing intervals is beneficial to patients, as the presence of guidelines may subconsciously influence the behavior of the endoscopist at the time of the procedure. For example, if a patient has a technically challenging colonoscopy and the endoscopist is running late, the endoscopist may drop their guard once they find a polyp and miss 1-2 additional small polyps that they would have spent more time looking for if they knew their next one would be in 10 years instead of 5.”
As for notification method, despite the logistical downside of taking dedicated staff time to make telephone calls, Dr. Sreenivasan said, “I think having a conversation with the patient directly is a much better way to communicate this information as it allows the patient to ask and answer questions. Things like tone of voice can provide reassurance that one cannot get via email.” Looking to the future, the study authors acknowledged that combinations of initial and reminder outreach approaches — for example, a mailed letter followed by secure message or telephone call — could potentially yield higher response rates and/or adoption rates than they observed. And a longer follow-up period with additional reminders may have produced higher yields. Additional studies are needed to optimize outreach approaches and to understand patient barriers to adopting the new guideline recommendations in different healthcare settings.
The study was supported by a Delivery Science grant from the Kaiser Permanente Northern California.
The authors disclosed no conflicts of interest. Dr. Mansour and Dr. Sreenivasan disclosed no conflicts of interest relevant to their comments.
In an article published in Clinical Gastroenterology and Hepatology, a group led by Jeffrey K. Lee, MD, MPH, a gastroenterologist at Kaiser Permanente Medical Center in San Francisco, reported the following 60-day response rates for the three contact methods in potentially transitioning more than 600 post-polypectomy patients to the new interval:
- Telephone: 64.5%
- Secure messaging: 51.7%
- Mailed letter: 31.3%
Compared with letter outreach, overall rate differences were significant for telephone (18.1%) and secure message outreach (13.1%).
Such interventions are widely used, the authors noted , but have not been compared for efficacy terms of communicating updated colonoscopy intervals.
The trial’s aim was to inform low-risk patients of the recommended interval update from 5 years — used since the 1990s — to 7-10 years. Given a choice, more patients opted to transition to the 10-year surveillance interval in the telephone (37%) and secure messaging arms (32.%) compared with mailed-letter arm (18.9%).
In addition to telephone and secure messaging outreach, factors positively associated with adoption of the 10-year interval were a positive fecal immunochemical test–based index colonoscopy and increasing age. Patients with these characteristics may be biased toward avoiding colonoscopy if not medically necessary, the authors conjectured.
Inversely associated factors included Asian or Pacific Islander race (odds ratio .58), Hispanic ethnicity (OR .40), and a higher Charlson comorbidity score of 2 vs 0 (OR .43).
Possible explanations for the race and ethnicity associations include gaps in culturally component care, lack of engagement with the English-based outreach approaches, and medical mistrust, the authors said.
“In this study, we gave all our patients an option to either extend their surveillance interval to current guideline recommendations or continue with their old interval, and some chose to do that,” Dr. Lee said in an interview. “Patients really appreciated having a choice and to be informed about the latest guideline changes.”
“A critical challenge to health systems is how to effectively de-implement outdated surveillance recommendations for low-risk patients who have a 5-year follow-up interval and potentially transition them to the recommended 7- to 10-year interval,” Dr. Lee and colleagues wrote.
More than 5 million surveillance colonoscopies are performed annually in US patients with a history of adenomas, the main precursor lesion for colorectal cancer, the authors noted.
With the recent guidelines issued in 2020 by the US Multi-Society Task Force on Colorectal Cancer lengthening the follow-up interval to 7-10 years , physicians are being advised to reevaluate low-risk patients previously scheduled with 5-year surveillance and provide an updated recommendation for follow-up.
Study Details
The three-arm pragmatic randomized trial was conducted in low-risk patients 54-70 years of age with one or two small (< 10 mm) tubular adenomas at baseline colonoscopy. Participants due for 5-year surveillance in 2022 were randomly assigned to one of three outreach arms: telephone (n = 200], secure messaging (n = 203), and mailed letter (n = 201). Stratified by age, sex, race, and ethnicity, participants could change their assigned interval to 10 years or continue with their previously scheduled 5-year interval.
As to economic considerations, the authors said that telephone may be the costliest form of outreach in terms of staffing resources. “We don’t know because we did not conduct a formal cost-effectiveness analysis,” Dr. Lee said. “However, we do know phone outreach requires a lot of personnel effort, which is why we also explored the less costly option of secure messaging/email.”
But based on the findings, telephone outreach would be a reasonable approach to update patients on post-polypectomy surveillance guideline changes if secure messaging or text messaging isn’t available, he added.
Downsides to Retroactive Changes?
Commenting on the study but not involved in it, Nabil M. Mansour, MD, an assistant professor and director of the McNair General GI Clinic at Baylor College of Medicine in Houston, noted that unlike Kaiser Permanente, his center decided against an overall effort to switch patients colonoscopied before the release of the new guidelines over to the new interval.
“Several of our physicians may have chosen to recommend a 5-year interval specifically for a variety of reasons and we felt going back, and making a blanket change to everyone’s interval retrospectively might create confusion and frustration and might actually delay the colonoscopies of some patients for which their doctors had a very good, legitimate reason to recommend a 5-year interval,” he said in an interview.
Dr. Mansour added that no difficulties were encountered in getting patients to agree to a 10-year interval. In his view telephone communication or in-person clinic visits are likely the most effective ways but both are more labor-intensive than automated patient portal messages. “I do not think traditional snail mail is effective.” His clinic uses automatic EMR reminders.
Offering another perspective on the study, Aditya Sreenivasan, MD, a gastroenterologist at Northwell Health in New York City, said his center has not reached out to correct the old intervals. “When I see a patient who previously had a colonoscopy with another physician, I always follow the previous recommendation for when the next colonoscopy should be, regardless of whether or not it technically meets guideline recommendations,” he told this news organization. “I do this because I was not there during the procedure and am not aware of any circumstances that would require a shorter interval that may not be apparent from the report.”
While he agrees with the new guidelines, Dr. Sreenivasan is “not sure if retroactively changing intervals is beneficial to patients, as the presence of guidelines may subconsciously influence the behavior of the endoscopist at the time of the procedure. For example, if a patient has a technically challenging colonoscopy and the endoscopist is running late, the endoscopist may drop their guard once they find a polyp and miss 1-2 additional small polyps that they would have spent more time looking for if they knew their next one would be in 10 years instead of 5.”
As for notification method, despite the logistical downside of taking dedicated staff time to make telephone calls, Dr. Sreenivasan said, “I think having a conversation with the patient directly is a much better way to communicate this information as it allows the patient to ask and answer questions. Things like tone of voice can provide reassurance that one cannot get via email.” Looking to the future, the study authors acknowledged that combinations of initial and reminder outreach approaches — for example, a mailed letter followed by secure message or telephone call — could potentially yield higher response rates and/or adoption rates than they observed. And a longer follow-up period with additional reminders may have produced higher yields. Additional studies are needed to optimize outreach approaches and to understand patient barriers to adopting the new guideline recommendations in different healthcare settings.
The study was supported by a Delivery Science grant from the Kaiser Permanente Northern California.
The authors disclosed no conflicts of interest. Dr. Mansour and Dr. Sreenivasan disclosed no conflicts of interest relevant to their comments.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Obesity and lung disease in the era of GLP-1 agonists
Now is the time for pulmonary clinicians to become comfortable counseling patients about and treating obesity. By 2030, half of the US population will have obesity, a quarter of which will be severe (Ward et al. NEJM. 2019;2440-2450).
Many pulmonary diseases, including asthma, COPD, and interstitial pulmonary fibrosis (IPF) are linked to and made worse by obesity with increased exacerbations, patient-reported decreased quality of life, and resistance to therapy (Ray et al. Am Rev Respir Dis. 1983;501-6). Asthma is even recognized as an obesity-related comorbid condition by both the American Society Metabolic and Bariatric Surgery (ASMBS) and the American Association of Clinical Endocrinologists (AACE) when considering indications for early or more aggressive treatment of obesity (Eisenberg et al. Obesity Surg. 2023;3-14) (Garvey et al. Endocr Pract. 2016;1-203).
Obesity has multiple negative effects on pulmonary function due to the physical forces of extra weight on the lungs and inflammation related to adipose tissue (see Figure 1) (Zerah et al. Chest. 1993;1470-6).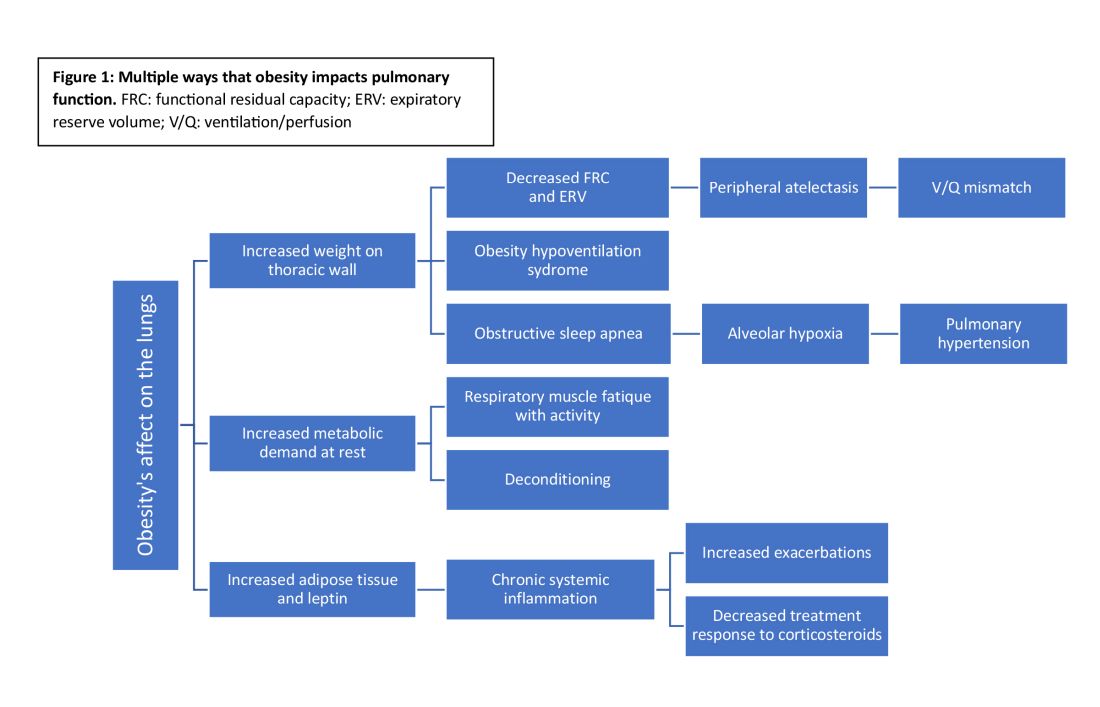
Obesity-related respiratory changes include reduced lung compliance, functional residual capacity (FRC), and expiratory reserve volume (ERV). These changes lead to peripheral atelectasis and V/Q mismatch and increased metabolic demands placed on the respiratory system (Parameswaran et al. Can Respir J. 2006;203-10). The increased weight supported by the thoracic cage alters the equilibrium between the chest wall and lung tissue decreasing FRC and ERV. This reduces lung compliance and increases stiffness by promoting areas of atelectasis and increased alveolar surface tension (Dixon et al. Expert Rev Respir Med. 2018;755-67).
Another biomechanical cost of obesity on respiratory function is the increased consumption of oxygen to sustain ventilation at rest (Koenig SM, Am J Med Sci. 2001;249-79). This can lead to early respiratory muscle fatigue when respiratory rate and tidal volume increase with activity. Patients with obesity are more likely to develop obstructive sleep apnea and obesity hypoventilation syndrome. The resulting alveolar hypoxemia is thought to contribute to the increase in pulmonary hypertension observed in patients with obesity (Shah et al. Breathe. 2023;19[1]). In addition to the biomechanical consequences of obesity, increased adipose tissue can lead to chronic, systemic inflammation that can exacerbate or unmask underlying respiratory disease. Increased leptin and downregulation of adiponectin have been shown to increase systemic cytokine production (Ray et al. Am Rev Respir Dis. 1983;501-6). This inflammatory process contributes to increased airway resistance and an altered response to corticosteroids (inhaled or systemic) in obese patients treated for bronchial hyperresponsiveness. This perhaps reflects the Th2-low phenotype seen in patients with obesity and metabolic syndrome-related asthma (Shah et al. Breathe. 2023;19[1]) (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812).
Multiple studies have demonstrated weight loss through lifestyle changes, medical therapy, and obesity surgery result benefits pulmonary disease (Forno et al. PloS One. 2019;14[4]) (Ardila-Gatas et al. Surg Endosc. 2019;1952-8). Benefits include decreased exacerbation frequency, improved functional testing, and improved patient-reported quality of life. Pulmonary clinicians should be empowered to address obesity as a comorbid condition and treat with appropriate referrals for obesity surgery and initiation of medications when indicated.
GLP-1 receptor agonists
In the past year, glucagon-like peptide receptor agonists (GLP-1RAs) have garnered attention in the medical literature and popular news outlets. GLP-1RAs, including semaglutide, liraglutide, and tirzepatide, are currently FDA approved for the treatment of obesity in patients with a body mass index (BMI) greater than or equal to 30 or a BMI greater than or equal to 27 in the setting of an obesity-related comorbidity, including asthma.
This class of medications acts by increasing the physiologic insulin response to a glucose load, delaying gastric emptying, and reducing production of glucagon. In a phase III study, semaglutide resulted in greater than 15% weight reduction from baseline (Wadden et al. JAMA. 2021;1403-13). In clinical trials, these medications have not only resulted in significant, sustained weight loss but also improved lipid profiles, decreased A1c, and reduced major cardiovascular events (Lincoff et al. N Engl J Med. 2023;389[23]:2221-32) (Verma et al. Circulation. 2018;138[25]:2884-94).
GLP-1RAs and lung disease
GLP-1RAs are associated with ranges of weight loss that lead to symptom improvement. Beyond the anticipated benefits for pulmonary health, there is interest in whether GLP-1RAs may improve specific lung diseases. GLP-1 receptors are found throughout the body (eg, gastrointestinal tract, kidneys, and heart) with the largest proportion located in the lungs (Wu AY and Peebles RS. Expert Rev Clin Immunol. 2021;1053-7). In addition to their known effect on insulin response, GLP-1RAs are hypothesized to reduce proinflammatory cytokine signaling and alter surfactant production potentially improving both airway resistance and lung compliance (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Animal models suggest an antifibrotic effect with delay in the endothelial-mesenchymal transition. If further substantiated, this could impact both acute and chronic lung injury.
Early clinical studies of GLP-1RAs in patients with respiratory diseases have demonstrated improved symptoms and pulmonary function (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Even modest weight loss (2.5 kg in a year) with GLP-1RAs leads to improved symptoms and a reduction in asthma exacerbations. Other asthma literature shows GLP-1RAs improve symptoms and reduce exacerbations independent of changes in weight, supporting the hypothesis that the benefit of GLP-1RAs may be more than biomechanical improvement from weight loss alone (Foer et al. Am J Respir Crit Care Med. 2021;831-40).
GLP-1RAs reduce the proinflammatory cytokine signaling in both TH2-high and TH2-low asthma phenotypes and alter surfactant production, airway resistance, and perhaps even pulmonary vascular resistance (Altintas Dogan et al. Int J Chron Obstruct Pulmon Dis. 2022,405-14). GATA-3 is an ongoing clinical trial examining whether GLP-1RAs reduce airway inflammation via direct effects on of the respiratory tract (NCT05254314).
Drugs developed to treat one condition are often found to impact others during validation studies or postmarketing observation. Some examples are aspirin, sildenafil, minoxidil, hydroxychloroquine, and SGLT-2 inhibitors. Will GLP-1RAs be the latest medication to affect a broad array of physiologic process and end up improving not just metabolic but also lung health?
Now is the time for pulmonary clinicians to become comfortable counseling patients about and treating obesity. By 2030, half of the US population will have obesity, a quarter of which will be severe (Ward et al. NEJM. 2019;2440-2450).
Many pulmonary diseases, including asthma, COPD, and interstitial pulmonary fibrosis (IPF) are linked to and made worse by obesity with increased exacerbations, patient-reported decreased quality of life, and resistance to therapy (Ray et al. Am Rev Respir Dis. 1983;501-6). Asthma is even recognized as an obesity-related comorbid condition by both the American Society Metabolic and Bariatric Surgery (ASMBS) and the American Association of Clinical Endocrinologists (AACE) when considering indications for early or more aggressive treatment of obesity (Eisenberg et al. Obesity Surg. 2023;3-14) (Garvey et al. Endocr Pract. 2016;1-203).
Obesity has multiple negative effects on pulmonary function due to the physical forces of extra weight on the lungs and inflammation related to adipose tissue (see Figure 1) (Zerah et al. Chest. 1993;1470-6).
Obesity-related respiratory changes include reduced lung compliance, functional residual capacity (FRC), and expiratory reserve volume (ERV). These changes lead to peripheral atelectasis and V/Q mismatch and increased metabolic demands placed on the respiratory system (Parameswaran et al. Can Respir J. 2006;203-10). The increased weight supported by the thoracic cage alters the equilibrium between the chest wall and lung tissue decreasing FRC and ERV. This reduces lung compliance and increases stiffness by promoting areas of atelectasis and increased alveolar surface tension (Dixon et al. Expert Rev Respir Med. 2018;755-67).
Another biomechanical cost of obesity on respiratory function is the increased consumption of oxygen to sustain ventilation at rest (Koenig SM, Am J Med Sci. 2001;249-79). This can lead to early respiratory muscle fatigue when respiratory rate and tidal volume increase with activity. Patients with obesity are more likely to develop obstructive sleep apnea and obesity hypoventilation syndrome. The resulting alveolar hypoxemia is thought to contribute to the increase in pulmonary hypertension observed in patients with obesity (Shah et al. Breathe. 2023;19[1]). In addition to the biomechanical consequences of obesity, increased adipose tissue can lead to chronic, systemic inflammation that can exacerbate or unmask underlying respiratory disease. Increased leptin and downregulation of adiponectin have been shown to increase systemic cytokine production (Ray et al. Am Rev Respir Dis. 1983;501-6). This inflammatory process contributes to increased airway resistance and an altered response to corticosteroids (inhaled or systemic) in obese patients treated for bronchial hyperresponsiveness. This perhaps reflects the Th2-low phenotype seen in patients with obesity and metabolic syndrome-related asthma (Shah et al. Breathe. 2023;19[1]) (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812).
Multiple studies have demonstrated weight loss through lifestyle changes, medical therapy, and obesity surgery result benefits pulmonary disease (Forno et al. PloS One. 2019;14[4]) (Ardila-Gatas et al. Surg Endosc. 2019;1952-8). Benefits include decreased exacerbation frequency, improved functional testing, and improved patient-reported quality of life. Pulmonary clinicians should be empowered to address obesity as a comorbid condition and treat with appropriate referrals for obesity surgery and initiation of medications when indicated.
GLP-1 receptor agonists
In the past year, glucagon-like peptide receptor agonists (GLP-1RAs) have garnered attention in the medical literature and popular news outlets. GLP-1RAs, including semaglutide, liraglutide, and tirzepatide, are currently FDA approved for the treatment of obesity in patients with a body mass index (BMI) greater than or equal to 30 or a BMI greater than or equal to 27 in the setting of an obesity-related comorbidity, including asthma.
This class of medications acts by increasing the physiologic insulin response to a glucose load, delaying gastric emptying, and reducing production of glucagon. In a phase III study, semaglutide resulted in greater than 15% weight reduction from baseline (Wadden et al. JAMA. 2021;1403-13). In clinical trials, these medications have not only resulted in significant, sustained weight loss but also improved lipid profiles, decreased A1c, and reduced major cardiovascular events (Lincoff et al. N Engl J Med. 2023;389[23]:2221-32) (Verma et al. Circulation. 2018;138[25]:2884-94).
GLP-1RAs and lung disease
GLP-1RAs are associated with ranges of weight loss that lead to symptom improvement. Beyond the anticipated benefits for pulmonary health, there is interest in whether GLP-1RAs may improve specific lung diseases. GLP-1 receptors are found throughout the body (eg, gastrointestinal tract, kidneys, and heart) with the largest proportion located in the lungs (Wu AY and Peebles RS. Expert Rev Clin Immunol. 2021;1053-7). In addition to their known effect on insulin response, GLP-1RAs are hypothesized to reduce proinflammatory cytokine signaling and alter surfactant production potentially improving both airway resistance and lung compliance (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Animal models suggest an antifibrotic effect with delay in the endothelial-mesenchymal transition. If further substantiated, this could impact both acute and chronic lung injury.
Early clinical studies of GLP-1RAs in patients with respiratory diseases have demonstrated improved symptoms and pulmonary function (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Even modest weight loss (2.5 kg in a year) with GLP-1RAs leads to improved symptoms and a reduction in asthma exacerbations. Other asthma literature shows GLP-1RAs improve symptoms and reduce exacerbations independent of changes in weight, supporting the hypothesis that the benefit of GLP-1RAs may be more than biomechanical improvement from weight loss alone (Foer et al. Am J Respir Crit Care Med. 2021;831-40).
GLP-1RAs reduce the proinflammatory cytokine signaling in both TH2-high and TH2-low asthma phenotypes and alter surfactant production, airway resistance, and perhaps even pulmonary vascular resistance (Altintas Dogan et al. Int J Chron Obstruct Pulmon Dis. 2022,405-14). GATA-3 is an ongoing clinical trial examining whether GLP-1RAs reduce airway inflammation via direct effects on of the respiratory tract (NCT05254314).
Drugs developed to treat one condition are often found to impact others during validation studies or postmarketing observation. Some examples are aspirin, sildenafil, minoxidil, hydroxychloroquine, and SGLT-2 inhibitors. Will GLP-1RAs be the latest medication to affect a broad array of physiologic process and end up improving not just metabolic but also lung health?
Now is the time for pulmonary clinicians to become comfortable counseling patients about and treating obesity. By 2030, half of the US population will have obesity, a quarter of which will be severe (Ward et al. NEJM. 2019;2440-2450).
Many pulmonary diseases, including asthma, COPD, and interstitial pulmonary fibrosis (IPF) are linked to and made worse by obesity with increased exacerbations, patient-reported decreased quality of life, and resistance to therapy (Ray et al. Am Rev Respir Dis. 1983;501-6). Asthma is even recognized as an obesity-related comorbid condition by both the American Society Metabolic and Bariatric Surgery (ASMBS) and the American Association of Clinical Endocrinologists (AACE) when considering indications for early or more aggressive treatment of obesity (Eisenberg et al. Obesity Surg. 2023;3-14) (Garvey et al. Endocr Pract. 2016;1-203).
Obesity has multiple negative effects on pulmonary function due to the physical forces of extra weight on the lungs and inflammation related to adipose tissue (see Figure 1) (Zerah et al. Chest. 1993;1470-6).
Obesity-related respiratory changes include reduced lung compliance, functional residual capacity (FRC), and expiratory reserve volume (ERV). These changes lead to peripheral atelectasis and V/Q mismatch and increased metabolic demands placed on the respiratory system (Parameswaran et al. Can Respir J. 2006;203-10). The increased weight supported by the thoracic cage alters the equilibrium between the chest wall and lung tissue decreasing FRC and ERV. This reduces lung compliance and increases stiffness by promoting areas of atelectasis and increased alveolar surface tension (Dixon et al. Expert Rev Respir Med. 2018;755-67).
Another biomechanical cost of obesity on respiratory function is the increased consumption of oxygen to sustain ventilation at rest (Koenig SM, Am J Med Sci. 2001;249-79). This can lead to early respiratory muscle fatigue when respiratory rate and tidal volume increase with activity. Patients with obesity are more likely to develop obstructive sleep apnea and obesity hypoventilation syndrome. The resulting alveolar hypoxemia is thought to contribute to the increase in pulmonary hypertension observed in patients with obesity (Shah et al. Breathe. 2023;19[1]). In addition to the biomechanical consequences of obesity, increased adipose tissue can lead to chronic, systemic inflammation that can exacerbate or unmask underlying respiratory disease. Increased leptin and downregulation of adiponectin have been shown to increase systemic cytokine production (Ray et al. Am Rev Respir Dis. 1983;501-6). This inflammatory process contributes to increased airway resistance and an altered response to corticosteroids (inhaled or systemic) in obese patients treated for bronchial hyperresponsiveness. This perhaps reflects the Th2-low phenotype seen in patients with obesity and metabolic syndrome-related asthma (Shah et al. Breathe. 2023;19[1]) (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812).
Multiple studies have demonstrated weight loss through lifestyle changes, medical therapy, and obesity surgery result benefits pulmonary disease (Forno et al. PloS One. 2019;14[4]) (Ardila-Gatas et al. Surg Endosc. 2019;1952-8). Benefits include decreased exacerbation frequency, improved functional testing, and improved patient-reported quality of life. Pulmonary clinicians should be empowered to address obesity as a comorbid condition and treat with appropriate referrals for obesity surgery and initiation of medications when indicated.
GLP-1 receptor agonists
In the past year, glucagon-like peptide receptor agonists (GLP-1RAs) have garnered attention in the medical literature and popular news outlets. GLP-1RAs, including semaglutide, liraglutide, and tirzepatide, are currently FDA approved for the treatment of obesity in patients with a body mass index (BMI) greater than or equal to 30 or a BMI greater than or equal to 27 in the setting of an obesity-related comorbidity, including asthma.
This class of medications acts by increasing the physiologic insulin response to a glucose load, delaying gastric emptying, and reducing production of glucagon. In a phase III study, semaglutide resulted in greater than 15% weight reduction from baseline (Wadden et al. JAMA. 2021;1403-13). In clinical trials, these medications have not only resulted in significant, sustained weight loss but also improved lipid profiles, decreased A1c, and reduced major cardiovascular events (Lincoff et al. N Engl J Med. 2023;389[23]:2221-32) (Verma et al. Circulation. 2018;138[25]:2884-94).
GLP-1RAs and lung disease
GLP-1RAs are associated with ranges of weight loss that lead to symptom improvement. Beyond the anticipated benefits for pulmonary health, there is interest in whether GLP-1RAs may improve specific lung diseases. GLP-1 receptors are found throughout the body (eg, gastrointestinal tract, kidneys, and heart) with the largest proportion located in the lungs (Wu AY and Peebles RS. Expert Rev Clin Immunol. 2021;1053-7). In addition to their known effect on insulin response, GLP-1RAs are hypothesized to reduce proinflammatory cytokine signaling and alter surfactant production potentially improving both airway resistance and lung compliance (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Animal models suggest an antifibrotic effect with delay in the endothelial-mesenchymal transition. If further substantiated, this could impact both acute and chronic lung injury.
Early clinical studies of GLP-1RAs in patients with respiratory diseases have demonstrated improved symptoms and pulmonary function (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Even modest weight loss (2.5 kg in a year) with GLP-1RAs leads to improved symptoms and a reduction in asthma exacerbations. Other asthma literature shows GLP-1RAs improve symptoms and reduce exacerbations independent of changes in weight, supporting the hypothesis that the benefit of GLP-1RAs may be more than biomechanical improvement from weight loss alone (Foer et al. Am J Respir Crit Care Med. 2021;831-40).
GLP-1RAs reduce the proinflammatory cytokine signaling in both TH2-high and TH2-low asthma phenotypes and alter surfactant production, airway resistance, and perhaps even pulmonary vascular resistance (Altintas Dogan et al. Int J Chron Obstruct Pulmon Dis. 2022,405-14). GATA-3 is an ongoing clinical trial examining whether GLP-1RAs reduce airway inflammation via direct effects on of the respiratory tract (NCT05254314).
Drugs developed to treat one condition are often found to impact others during validation studies or postmarketing observation. Some examples are aspirin, sildenafil, minoxidil, hydroxychloroquine, and SGLT-2 inhibitors. Will GLP-1RAs be the latest medication to affect a broad array of physiologic process and end up improving not just metabolic but also lung health?
New age of CHEST philanthropy to focus on education, impact, community
In a time echoing with the constant call for transformation, CHEST delved deep into its essence, questioning its potential for impact. This pivotal introspection led to a crucial inquiry…
Are we harnessing every opportunity to make a difference?
It’s a familiar question, yet its resonance urged a deeper evaluation.
Philanthropy has long been entwined in CHEST’s identity. Commemorating 25 years of the CHEST Foundation at CHEST 2022 spotlighted our history of generosity. Stories of transformative community initiatives and pivotal clinical research grants narrated a tale of empowered change and fostering healthier communities worldwide.
However, amid these achievements, more pressing inquiries surfaced:
- What unique role can CHEST play?
- Where do unmet needs persist?
- Which causes deeply resonate within our community?
CHEST’s leadership and dedicated staff embarked on a comprehensive review, scrutinizing past triumphs, donor commitments, and the evolving aspirations of our members. Themes of social responsibility, professional diversity, community impact, and expanded partnerships emerged as pivotal points. This extensive process, spanning nearly a year, resembled a reflective pause amid the rapid cadence of change.
Achieving these aspirations meant reimagining our approach, thereby streamlining efforts for maximal impact by…
- Integrating philanthropy as an integral facet of our mission, and amplifying the culture of giving within CHEST
- Consolidating philanthropic initiatives under CHEST to maximize resources for direct, substantial impact
- Defining clear avenues for giving that deeply resonate with our members
With endorsement from the Board of Regents, the CHEST Foundation seamlessly merged into CHEST, inaugurating a new chapter in our philanthropic endeavors.
Central to this transformative shift is the crystallization of our giving strategy, fortified by four pillars: Clinical Research, Community Impact, Support to the Profession, and Dedication to Education. These pillars encapsulate our commitment to nurturing clinicians, supporting trainees, and enhancing patient care.
Clinical Research emerges as the cornerstone, transcending boundaries to empower researchers in their pursuit of groundbreaking insights. Through strategic grants, we embolden early career investigators to delve into uncharted territories, unraveling mysteries that underpin advancements in chest medicine. The ripple effect extends beyond labs; it traverses communities, amplifying equitable health care solutions and bridging disparities in patient care. Our commitment to nurturing this pillar springs from the belief that every breakthrough, regardless of scale, is a catalyst for transformative change.
Community Impact extends CHEST’s reach far beyond clinical settings, fostering alliances with local organizations. Together, we forge a tapestry of collaboration, weaving essential services and imparting knowledge on crucial lung health issues into the fabric of diverse communities. This engagement not only elevates awareness but also empowers individuals and communities to take charge of their respiratory well-being. It’s the grassroots unity that amplifies our impact, creating enduring shifts in local landscapes.
Support of the Profession epitomizes our dedication to fortifying the backbone of pulmonary, critical care, and sleep medicine. By offering unparalleled clinical education and mentorship, we empower emerging clinicians from diverse backgrounds with the latest knowledge and resources. Fueling their professional growth is pivotal to nurturing a robust and inclusive cadre of health care professionals, ensuring comprehensive and culturally sensitive care for patients worldwide.
Dedication to Education isn’t just a commitment—it’s a bridge spanning the gap between knowledge and application, patient and clinician. Strengthening this connection involves equipping clinicians with tools for effective communication and partnering with patient-centered organizations. Our focus transcends textbooks; it embodies a relentless pursuit to refine patient-clinician interactions, enhancing patient understanding and, ultimately, elevating their quality of life.
CHEST’s philanthropic evolution signifies not just growth but a resolute commitment to effecting tangible change in chest medicine and patient care. These pillars stand as guiding beacons, steering us toward a future that mirrors our mission, vision, and values. Each pillar represents a pathway to meaningful, enduring change within chest medicine, ensuring a lasting impact on patient well-being.
In a time echoing with the constant call for transformation, CHEST delved deep into its essence, questioning its potential for impact. This pivotal introspection led to a crucial inquiry…
Are we harnessing every opportunity to make a difference?
It’s a familiar question, yet its resonance urged a deeper evaluation.
Philanthropy has long been entwined in CHEST’s identity. Commemorating 25 years of the CHEST Foundation at CHEST 2022 spotlighted our history of generosity. Stories of transformative community initiatives and pivotal clinical research grants narrated a tale of empowered change and fostering healthier communities worldwide.
However, amid these achievements, more pressing inquiries surfaced:
- What unique role can CHEST play?
- Where do unmet needs persist?
- Which causes deeply resonate within our community?
CHEST’s leadership and dedicated staff embarked on a comprehensive review, scrutinizing past triumphs, donor commitments, and the evolving aspirations of our members. Themes of social responsibility, professional diversity, community impact, and expanded partnerships emerged as pivotal points. This extensive process, spanning nearly a year, resembled a reflective pause amid the rapid cadence of change.
Achieving these aspirations meant reimagining our approach, thereby streamlining efforts for maximal impact by…
- Integrating philanthropy as an integral facet of our mission, and amplifying the culture of giving within CHEST
- Consolidating philanthropic initiatives under CHEST to maximize resources for direct, substantial impact
- Defining clear avenues for giving that deeply resonate with our members
With endorsement from the Board of Regents, the CHEST Foundation seamlessly merged into CHEST, inaugurating a new chapter in our philanthropic endeavors.
Central to this transformative shift is the crystallization of our giving strategy, fortified by four pillars: Clinical Research, Community Impact, Support to the Profession, and Dedication to Education. These pillars encapsulate our commitment to nurturing clinicians, supporting trainees, and enhancing patient care.
Clinical Research emerges as the cornerstone, transcending boundaries to empower researchers in their pursuit of groundbreaking insights. Through strategic grants, we embolden early career investigators to delve into uncharted territories, unraveling mysteries that underpin advancements in chest medicine. The ripple effect extends beyond labs; it traverses communities, amplifying equitable health care solutions and bridging disparities in patient care. Our commitment to nurturing this pillar springs from the belief that every breakthrough, regardless of scale, is a catalyst for transformative change.
Community Impact extends CHEST’s reach far beyond clinical settings, fostering alliances with local organizations. Together, we forge a tapestry of collaboration, weaving essential services and imparting knowledge on crucial lung health issues into the fabric of diverse communities. This engagement not only elevates awareness but also empowers individuals and communities to take charge of their respiratory well-being. It’s the grassroots unity that amplifies our impact, creating enduring shifts in local landscapes.
Support of the Profession epitomizes our dedication to fortifying the backbone of pulmonary, critical care, and sleep medicine. By offering unparalleled clinical education and mentorship, we empower emerging clinicians from diverse backgrounds with the latest knowledge and resources. Fueling their professional growth is pivotal to nurturing a robust and inclusive cadre of health care professionals, ensuring comprehensive and culturally sensitive care for patients worldwide.
Dedication to Education isn’t just a commitment—it’s a bridge spanning the gap between knowledge and application, patient and clinician. Strengthening this connection involves equipping clinicians with tools for effective communication and partnering with patient-centered organizations. Our focus transcends textbooks; it embodies a relentless pursuit to refine patient-clinician interactions, enhancing patient understanding and, ultimately, elevating their quality of life.
CHEST’s philanthropic evolution signifies not just growth but a resolute commitment to effecting tangible change in chest medicine and patient care. These pillars stand as guiding beacons, steering us toward a future that mirrors our mission, vision, and values. Each pillar represents a pathway to meaningful, enduring change within chest medicine, ensuring a lasting impact on patient well-being.
In a time echoing with the constant call for transformation, CHEST delved deep into its essence, questioning its potential for impact. This pivotal introspection led to a crucial inquiry…
Are we harnessing every opportunity to make a difference?
It’s a familiar question, yet its resonance urged a deeper evaluation.
Philanthropy has long been entwined in CHEST’s identity. Commemorating 25 years of the CHEST Foundation at CHEST 2022 spotlighted our history of generosity. Stories of transformative community initiatives and pivotal clinical research grants narrated a tale of empowered change and fostering healthier communities worldwide.
However, amid these achievements, more pressing inquiries surfaced:
- What unique role can CHEST play?
- Where do unmet needs persist?
- Which causes deeply resonate within our community?
CHEST’s leadership and dedicated staff embarked on a comprehensive review, scrutinizing past triumphs, donor commitments, and the evolving aspirations of our members. Themes of social responsibility, professional diversity, community impact, and expanded partnerships emerged as pivotal points. This extensive process, spanning nearly a year, resembled a reflective pause amid the rapid cadence of change.
Achieving these aspirations meant reimagining our approach, thereby streamlining efforts for maximal impact by…
- Integrating philanthropy as an integral facet of our mission, and amplifying the culture of giving within CHEST
- Consolidating philanthropic initiatives under CHEST to maximize resources for direct, substantial impact
- Defining clear avenues for giving that deeply resonate with our members
With endorsement from the Board of Regents, the CHEST Foundation seamlessly merged into CHEST, inaugurating a new chapter in our philanthropic endeavors.
Central to this transformative shift is the crystallization of our giving strategy, fortified by four pillars: Clinical Research, Community Impact, Support to the Profession, and Dedication to Education. These pillars encapsulate our commitment to nurturing clinicians, supporting trainees, and enhancing patient care.
Clinical Research emerges as the cornerstone, transcending boundaries to empower researchers in their pursuit of groundbreaking insights. Through strategic grants, we embolden early career investigators to delve into uncharted territories, unraveling mysteries that underpin advancements in chest medicine. The ripple effect extends beyond labs; it traverses communities, amplifying equitable health care solutions and bridging disparities in patient care. Our commitment to nurturing this pillar springs from the belief that every breakthrough, regardless of scale, is a catalyst for transformative change.
Community Impact extends CHEST’s reach far beyond clinical settings, fostering alliances with local organizations. Together, we forge a tapestry of collaboration, weaving essential services and imparting knowledge on crucial lung health issues into the fabric of diverse communities. This engagement not only elevates awareness but also empowers individuals and communities to take charge of their respiratory well-being. It’s the grassroots unity that amplifies our impact, creating enduring shifts in local landscapes.
Support of the Profession epitomizes our dedication to fortifying the backbone of pulmonary, critical care, and sleep medicine. By offering unparalleled clinical education and mentorship, we empower emerging clinicians from diverse backgrounds with the latest knowledge and resources. Fueling their professional growth is pivotal to nurturing a robust and inclusive cadre of health care professionals, ensuring comprehensive and culturally sensitive care for patients worldwide.
Dedication to Education isn’t just a commitment—it’s a bridge spanning the gap between knowledge and application, patient and clinician. Strengthening this connection involves equipping clinicians with tools for effective communication and partnering with patient-centered organizations. Our focus transcends textbooks; it embodies a relentless pursuit to refine patient-clinician interactions, enhancing patient understanding and, ultimately, elevating their quality of life.
CHEST’s philanthropic evolution signifies not just growth but a resolute commitment to effecting tangible change in chest medicine and patient care. These pillars stand as guiding beacons, steering us toward a future that mirrors our mission, vision, and values. Each pillar represents a pathway to meaningful, enduring change within chest medicine, ensuring a lasting impact on patient well-being.
Biomarker checklist seeks to expedite NSCLC diagnoses
Drs. Tamer Said Ahmed and Adam Fox receive funding for quality improvement projects in biomarker testing
Establishing a systematic biomarker testing program for patients with suspected non-small cell lung cancer (NSCLC) takes both time and collaboration across specialties. To standardize this process, the American College of Chest Physicians (CHEST) created two clinician checklists for use in practice.
The case-by-case checklist helps guide physicians to ensure timely and comprehensive biomarker testing for individual patients, and the programmatic/institutional checklist is for multidisciplinary teams to enable clear expectations and processes across hand-offs to aid in the testing process.
To substantiate best practices for ordering biomarker tests using the checklists, CHEST issued quality improvement demonstration grants for implementation at two institutions. This year, Tamer Said Ahmed, MD, FCCP, pulmonary and sleep physician at Toledo Hospital (ProMedica Health System) and Assistant Professor at the University of Toledo, and Adam Fox, MD, MS, Assistant Professor of Medicine at the Medical University of South Carolina, will begin projects to improve biomarker testing.
“Biomarker testing allows for tailored treatment plans that drastically impact the progression of lung cancer, but every hospital system and practice is following a different procedure for testing,” Dr. Said Ahmed said. “To best serve the patient, our project aims to streamline the approach to biomarker testing to bridge health care inconsistencies. Given the intense progression of some forms of lung cancer where every week matters, the more streamlined we can make the biomarker testing process, the earlier we will get to an accurate diagnosis, begin treatment, and likely extend the life of a patient.”
Discrepancies in the testing process stem from existing silos between specialties, including pathology, oncology, interventional radiology, and more. Care is fragmented, leading to delays like repeat biopsies because a large enough sample was not taken the first time.
This is the exact problem that checklist implementation will seek to solve.
“By intent, these checklists help to provide a systematic approach to timely and comprehensive biomarker testing,” said Dr. Fox, who was also part of the team that developed the checklists. “What we need now is to implement them into clinical practice to gain metrics that can be studied, identified, and will lead to the process being widely accepted. To truly impact practice, we need to be able to provide strong evidence for interventions that work for clinicians to implement.”
To learn more and download the checklists, visit CHEST’s Thoracic Oncology Topic Collection onlineThis project is supported in part by AstraZeneca, Sanofi, and Pfizer.
Drs. Tamer Said Ahmed and Adam Fox receive funding for quality improvement projects in biomarker testing
Drs. Tamer Said Ahmed and Adam Fox receive funding for quality improvement projects in biomarker testing
Establishing a systematic biomarker testing program for patients with suspected non-small cell lung cancer (NSCLC) takes both time and collaboration across specialties. To standardize this process, the American College of Chest Physicians (CHEST) created two clinician checklists for use in practice.
The case-by-case checklist helps guide physicians to ensure timely and comprehensive biomarker testing for individual patients, and the programmatic/institutional checklist is for multidisciplinary teams to enable clear expectations and processes across hand-offs to aid in the testing process.
To substantiate best practices for ordering biomarker tests using the checklists, CHEST issued quality improvement demonstration grants for implementation at two institutions. This year, Tamer Said Ahmed, MD, FCCP, pulmonary and sleep physician at Toledo Hospital (ProMedica Health System) and Assistant Professor at the University of Toledo, and Adam Fox, MD, MS, Assistant Professor of Medicine at the Medical University of South Carolina, will begin projects to improve biomarker testing.
“Biomarker testing allows for tailored treatment plans that drastically impact the progression of lung cancer, but every hospital system and practice is following a different procedure for testing,” Dr. Said Ahmed said. “To best serve the patient, our project aims to streamline the approach to biomarker testing to bridge health care inconsistencies. Given the intense progression of some forms of lung cancer where every week matters, the more streamlined we can make the biomarker testing process, the earlier we will get to an accurate diagnosis, begin treatment, and likely extend the life of a patient.”
Discrepancies in the testing process stem from existing silos between specialties, including pathology, oncology, interventional radiology, and more. Care is fragmented, leading to delays like repeat biopsies because a large enough sample was not taken the first time.
This is the exact problem that checklist implementation will seek to solve.
“By intent, these checklists help to provide a systematic approach to timely and comprehensive biomarker testing,” said Dr. Fox, who was also part of the team that developed the checklists. “What we need now is to implement them into clinical practice to gain metrics that can be studied, identified, and will lead to the process being widely accepted. To truly impact practice, we need to be able to provide strong evidence for interventions that work for clinicians to implement.”
To learn more and download the checklists, visit CHEST’s Thoracic Oncology Topic Collection onlineThis project is supported in part by AstraZeneca, Sanofi, and Pfizer.
Establishing a systematic biomarker testing program for patients with suspected non-small cell lung cancer (NSCLC) takes both time and collaboration across specialties. To standardize this process, the American College of Chest Physicians (CHEST) created two clinician checklists for use in practice.
The case-by-case checklist helps guide physicians to ensure timely and comprehensive biomarker testing for individual patients, and the programmatic/institutional checklist is for multidisciplinary teams to enable clear expectations and processes across hand-offs to aid in the testing process.
To substantiate best practices for ordering biomarker tests using the checklists, CHEST issued quality improvement demonstration grants for implementation at two institutions. This year, Tamer Said Ahmed, MD, FCCP, pulmonary and sleep physician at Toledo Hospital (ProMedica Health System) and Assistant Professor at the University of Toledo, and Adam Fox, MD, MS, Assistant Professor of Medicine at the Medical University of South Carolina, will begin projects to improve biomarker testing.
“Biomarker testing allows for tailored treatment plans that drastically impact the progression of lung cancer, but every hospital system and practice is following a different procedure for testing,” Dr. Said Ahmed said. “To best serve the patient, our project aims to streamline the approach to biomarker testing to bridge health care inconsistencies. Given the intense progression of some forms of lung cancer where every week matters, the more streamlined we can make the biomarker testing process, the earlier we will get to an accurate diagnosis, begin treatment, and likely extend the life of a patient.”
Discrepancies in the testing process stem from existing silos between specialties, including pathology, oncology, interventional radiology, and more. Care is fragmented, leading to delays like repeat biopsies because a large enough sample was not taken the first time.
This is the exact problem that checklist implementation will seek to solve.
“By intent, these checklists help to provide a systematic approach to timely and comprehensive biomarker testing,” said Dr. Fox, who was also part of the team that developed the checklists. “What we need now is to implement them into clinical practice to gain metrics that can be studied, identified, and will lead to the process being widely accepted. To truly impact practice, we need to be able to provide strong evidence for interventions that work for clinicians to implement.”
To learn more and download the checklists, visit CHEST’s Thoracic Oncology Topic Collection onlineThis project is supported in part by AstraZeneca, Sanofi, and Pfizer.
Examining the past and looking toward the future: The need for quality data in interventional pulmonology
THORACIC ONCOLOGY AND CHEST PROCEDURES NETWORK
Interventional Procedures Section
During the last decade, the explosion of technological advancements in the field of interventional pulmonary (IP) has afforded patients the opportunity to undergo novel, minimally invasive diagnostic and therapeutic procedures. However, these unprecedented technological advances have often been introduced without the support of high-quality research on safety and efficacy, and without evaluating their impact on meaningful patient outcomes. Encouraging and participating in high-quality IP research should remain a top priority for those practicing in the field.
Structured research networks, such as the UK Pleural Society and more recently the Interventional Pulmonary Outcome Group, have facilitated the transition of IP research from observational case series and single-center experiences to multicenter, randomized controlled trials to generate level I evidence and inform patient care (Laskawiec-Szkonter M, et al. Br J Hosp Med (Lond). 2019 Apr 2;80[4]:186-7) (Maldonado F, et al. J Bronchology Interv Pulmonol. 2019 Jul;26(3):150-2). In the bronchoscopy space, important investigator-initiated clinical trial results anticipated in 2024 include VERITAS (NCT04250194), FROSTBITE2 (NCT05751278), and RELIANT (NCT05705544), among others. These research efforts complement industry-sponsored clinical trials (such as RheSolve, NCT04677465) and aim to emulate the extraordinary track record achieved in the field of pleural disease that has led to recently updated evidence-based guidelines for the management of challenging diseases like malignant pleural effusions, pleural space infections, and pneumothorax (Davies HE, et a l. JAMA. 2012 Jun 13;307[22]:2383-9, Mishra EK, et al. Am J Respir Crit Care Med. 2018 Feb 15;197[4]:502-8) (Rahman NM, et al. N Engl J Med. 2011 Aug 11;365[6]:518-26) (Hallifax RJ, et al. Lancet. 2020 Jul 4;396[10243]:39-49).
Ultimately, the rapidly evolving technological advancements in interventional pulmonology must be supported by research based on high-quality clinical trials, which will be contingent on appropriate trial funding requiring partnership with industry and federal funding agencies. Only through such collaboration can researchers design robust clinical trials based on complex methodology, which will advance patient care and lead to improved patient outcomes.
– Jennifer D. Duke, MD
Section Fellow-in-Training
– Fabien Maldonado, MD, MSc, FCCP
Section Member
THORACIC ONCOLOGY AND CHEST PROCEDURES NETWORK
Interventional Procedures Section
During the last decade, the explosion of technological advancements in the field of interventional pulmonary (IP) has afforded patients the opportunity to undergo novel, minimally invasive diagnostic and therapeutic procedures. However, these unprecedented technological advances have often been introduced without the support of high-quality research on safety and efficacy, and without evaluating their impact on meaningful patient outcomes. Encouraging and participating in high-quality IP research should remain a top priority for those practicing in the field.
Structured research networks, such as the UK Pleural Society and more recently the Interventional Pulmonary Outcome Group, have facilitated the transition of IP research from observational case series and single-center experiences to multicenter, randomized controlled trials to generate level I evidence and inform patient care (Laskawiec-Szkonter M, et al. Br J Hosp Med (Lond). 2019 Apr 2;80[4]:186-7) (Maldonado F, et al. J Bronchology Interv Pulmonol. 2019 Jul;26(3):150-2). In the bronchoscopy space, important investigator-initiated clinical trial results anticipated in 2024 include VERITAS (NCT04250194), FROSTBITE2 (NCT05751278), and RELIANT (NCT05705544), among others. These research efforts complement industry-sponsored clinical trials (such as RheSolve, NCT04677465) and aim to emulate the extraordinary track record achieved in the field of pleural disease that has led to recently updated evidence-based guidelines for the management of challenging diseases like malignant pleural effusions, pleural space infections, and pneumothorax (Davies HE, et a l. JAMA. 2012 Jun 13;307[22]:2383-9, Mishra EK, et al. Am J Respir Crit Care Med. 2018 Feb 15;197[4]:502-8) (Rahman NM, et al. N Engl J Med. 2011 Aug 11;365[6]:518-26) (Hallifax RJ, et al. Lancet. 2020 Jul 4;396[10243]:39-49).
Ultimately, the rapidly evolving technological advancements in interventional pulmonology must be supported by research based on high-quality clinical trials, which will be contingent on appropriate trial funding requiring partnership with industry and federal funding agencies. Only through such collaboration can researchers design robust clinical trials based on complex methodology, which will advance patient care and lead to improved patient outcomes.
– Jennifer D. Duke, MD
Section Fellow-in-Training
– Fabien Maldonado, MD, MSc, FCCP
Section Member
THORACIC ONCOLOGY AND CHEST PROCEDURES NETWORK
Interventional Procedures Section
During the last decade, the explosion of technological advancements in the field of interventional pulmonary (IP) has afforded patients the opportunity to undergo novel, minimally invasive diagnostic and therapeutic procedures. However, these unprecedented technological advances have often been introduced without the support of high-quality research on safety and efficacy, and without evaluating their impact on meaningful patient outcomes. Encouraging and participating in high-quality IP research should remain a top priority for those practicing in the field.
Structured research networks, such as the UK Pleural Society and more recently the Interventional Pulmonary Outcome Group, have facilitated the transition of IP research from observational case series and single-center experiences to multicenter, randomized controlled trials to generate level I evidence and inform patient care (Laskawiec-Szkonter M, et al. Br J Hosp Med (Lond). 2019 Apr 2;80[4]:186-7) (Maldonado F, et al. J Bronchology Interv Pulmonol. 2019 Jul;26(3):150-2). In the bronchoscopy space, important investigator-initiated clinical trial results anticipated in 2024 include VERITAS (NCT04250194), FROSTBITE2 (NCT05751278), and RELIANT (NCT05705544), among others. These research efforts complement industry-sponsored clinical trials (such as RheSolve, NCT04677465) and aim to emulate the extraordinary track record achieved in the field of pleural disease that has led to recently updated evidence-based guidelines for the management of challenging diseases like malignant pleural effusions, pleural space infections, and pneumothorax (Davies HE, et a l. JAMA. 2012 Jun 13;307[22]:2383-9, Mishra EK, et al. Am J Respir Crit Care Med. 2018 Feb 15;197[4]:502-8) (Rahman NM, et al. N Engl J Med. 2011 Aug 11;365[6]:518-26) (Hallifax RJ, et al. Lancet. 2020 Jul 4;396[10243]:39-49).
Ultimately, the rapidly evolving technological advancements in interventional pulmonology must be supported by research based on high-quality clinical trials, which will be contingent on appropriate trial funding requiring partnership with industry and federal funding agencies. Only through such collaboration can researchers design robust clinical trials based on complex methodology, which will advance patient care and lead to improved patient outcomes.
– Jennifer D. Duke, MD
Section Fellow-in-Training
– Fabien Maldonado, MD, MSc, FCCP
Section Member
Updates in evidence for rituximab in interstitial lung disease
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Interstitial Lung Disease Section
Interstitial lung diseases (ILD) are a heterogeneous group of fibro-inflammatory disorders that can be progressive despite available therapies. The cornerstones of pharmacologic therapy include immunosuppression and antifibrotics.
Data on the use of rituximab, a B-lymphocyte-depleting monoclonal antibody, often utilized as rescue therapy in progressive and severe ILD, was limited until recently. The RECITAL trial reported the first randomized controlled trial investigating rituximab in severe or progressive autoimmune ILD. Though rituximab was not superior to cyclophosphamide, both agents improved forced vital capacity (FVC) at 24 weeks and respiratory-related quality of life. Rituximab was associated with less adverse events and lower corticosteroid exposure (Maher et al. Lancet Respir Med. 2023;11:45-54). In the DESIRES trial, patients with systemic sclerosis-associated ILD treated with rituximab had preservation of FVC at 24 and 48 weeks compared to placebo (Ebata et al. Lancet Rheumatol. 2021;3:e489-97; Lancet Rheumatol. 2022;4:e546-55). The EVER-ILD investigators compared mycophenolate mofetil (MMF) alone vs addition of rituximab in patients with autoimmune and idiopathic nonspecific interstitial pneumonia (NSIP). Combination therapy was superior to MMF alone in improving FVC and progression-free survival. Combination regimen was well tolerated though nonserious viral and bacterial infections were more frequent (Mankikian et al. Eur Respir J. 2023;61[6]:2202071).
These findings, primarily in autoimmune ILD, are promising and provide clinicians with evidence for utilizing rituximab in patients with severe and progressive ILD. Nonetheless, they highlight the need for additional research and standardized guidance regarding the target population who stands to most benefit from rituximab.
–Tessy K. Paul, MD
Section Member-at-Large
–Tejaswini Kulkarni, MD, MBBS, FCCP
Section Chair
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Interstitial Lung Disease Section
Interstitial lung diseases (ILD) are a heterogeneous group of fibro-inflammatory disorders that can be progressive despite available therapies. The cornerstones of pharmacologic therapy include immunosuppression and antifibrotics.
Data on the use of rituximab, a B-lymphocyte-depleting monoclonal antibody, often utilized as rescue therapy in progressive and severe ILD, was limited until recently. The RECITAL trial reported the first randomized controlled trial investigating rituximab in severe or progressive autoimmune ILD. Though rituximab was not superior to cyclophosphamide, both agents improved forced vital capacity (FVC) at 24 weeks and respiratory-related quality of life. Rituximab was associated with less adverse events and lower corticosteroid exposure (Maher et al. Lancet Respir Med. 2023;11:45-54). In the DESIRES trial, patients with systemic sclerosis-associated ILD treated with rituximab had preservation of FVC at 24 and 48 weeks compared to placebo (Ebata et al. Lancet Rheumatol. 2021;3:e489-97; Lancet Rheumatol. 2022;4:e546-55). The EVER-ILD investigators compared mycophenolate mofetil (MMF) alone vs addition of rituximab in patients with autoimmune and idiopathic nonspecific interstitial pneumonia (NSIP). Combination therapy was superior to MMF alone in improving FVC and progression-free survival. Combination regimen was well tolerated though nonserious viral and bacterial infections were more frequent (Mankikian et al. Eur Respir J. 2023;61[6]:2202071).
These findings, primarily in autoimmune ILD, are promising and provide clinicians with evidence for utilizing rituximab in patients with severe and progressive ILD. Nonetheless, they highlight the need for additional research and standardized guidance regarding the target population who stands to most benefit from rituximab.
–Tessy K. Paul, MD
Section Member-at-Large
–Tejaswini Kulkarni, MD, MBBS, FCCP
Section Chair
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Interstitial Lung Disease Section
Interstitial lung diseases (ILD) are a heterogeneous group of fibro-inflammatory disorders that can be progressive despite available therapies. The cornerstones of pharmacologic therapy include immunosuppression and antifibrotics.
Data on the use of rituximab, a B-lymphocyte-depleting monoclonal antibody, often utilized as rescue therapy in progressive and severe ILD, was limited until recently. The RECITAL trial reported the first randomized controlled trial investigating rituximab in severe or progressive autoimmune ILD. Though rituximab was not superior to cyclophosphamide, both agents improved forced vital capacity (FVC) at 24 weeks and respiratory-related quality of life. Rituximab was associated with less adverse events and lower corticosteroid exposure (Maher et al. Lancet Respir Med. 2023;11:45-54). In the DESIRES trial, patients with systemic sclerosis-associated ILD treated with rituximab had preservation of FVC at 24 and 48 weeks compared to placebo (Ebata et al. Lancet Rheumatol. 2021;3:e489-97; Lancet Rheumatol. 2022;4:e546-55). The EVER-ILD investigators compared mycophenolate mofetil (MMF) alone vs addition of rituximab in patients with autoimmune and idiopathic nonspecific interstitial pneumonia (NSIP). Combination therapy was superior to MMF alone in improving FVC and progression-free survival. Combination regimen was well tolerated though nonserious viral and bacterial infections were more frequent (Mankikian et al. Eur Respir J. 2023;61[6]:2202071).
These findings, primarily in autoimmune ILD, are promising and provide clinicians with evidence for utilizing rituximab in patients with severe and progressive ILD. Nonetheless, they highlight the need for additional research and standardized guidance regarding the target population who stands to most benefit from rituximab.
–Tessy K. Paul, MD
Section Member-at-Large
–Tejaswini Kulkarni, MD, MBBS, FCCP
Section Chair
The emergence of postgraduate training programs for APPs in pulmonary and critical care
APP Intersection
Postgraduate training for advanced practice providers (APPs) has existed in one form or another since the genesis of the allied professions. They are typically referred to as residencies, fellowships, postgraduate programs, and transition-to-practice.
The desire and necessity for these programs has increased in the past decade with workforce changes; namely the increasing number of nurse practitioners (NPs) graduating with fewer years of experience at the bedside compared with previous eras, a similar decrease in patient contact hours for graduating PAs, the transition of physician colleagues from employers to employees and the subsequent change in priorities in training new graduate APPs, and resident work hour restrictions necessitating more APPs to staff inpatient units and work in various specialties.
The goal of these programs is to provide postgraduate training to physician assistants/associates (PAs) and NPs across myriad medical specialties to both newly graduated APPs and those looking to transition specialties. Current programs exist in family medicine, emergency medicine, urgent care, critical care medicine, pulmonary medicine, oncology, surgery, and various surgical subspecialties, to name a few. Program length is highly variable, though most programs advertise as lasting around 12 months, with varying ratios of clinical and didactic education. Postgraduate APP programs are largely advertise as salaried, benefitted positions, though usually at a rate below that of a so-called “direct hire” due to the protected learning time associated with the postgraduate training year.
Accreditation for these programs is still disjointed, although unifying efforts have been made as of late, and is currently available through the Advanced Practice Provider Fellowship Accreditation, Association of Postgraduate Physician Assistant Programs, ARC-PA, the Accreditation Commission for Education in Nursing, and the Consortium for Advanced Practice Providers. Other organizations, such as the Association of Post Graduate APRN Programs, host regular conferences to discuss the formulation of postgraduate APP education curricula and program development.
While accreditation offers guidance for fledgling programs, many utilize the standards published by the American College of Graduate Medical Education to ensure that appropriate clinical milestones are being met and that a common language among APPs and physicians who are involved in the evaluation of the postgraduate APP trainee is being used. Programs also seek to utilize other well-established curricula and certification programs published by various national and international organizations. A key distinction from physician postgraduate training is that there is currently no fiscal or legislative support for postgraduate APP programs; these issues have been cited as reasons for the limited scope and number of programs.
When starting APP Fellowship programs, it is important to consider why this would be beneficial to a specific division and health care organization. Usually, fellowship programs develop out of a need to train and retain APPs. It is no secret that turnover and retention of skilled APPs is a nationwide problem associated with significant costs to organizations. The ability to retain fellowship-trained APPs will result in cost savings due to the reduction in onboarding time and orientation costs, as these APP fellows finish their programs ready to be fully productive team members.
Additional considerations for the development of an APP fellowship include improving access to care and increasing the quality of the care provided. Fellowship programs encourage a smoother transition to practice by offering more support through education, closer evaluation, and frequent feedback, which improves competence and confidence of these providers. A supported APP is more likely to practice to the fullest extent of their license and have improved personal and professional satisfaction, leading to employee retention and better patient care.
When developing a budget for these types of programs, it is important to include the full-time equivalent (FTE) for the fellow, benefits, onboarding/licensure, simulations, and fellowship faculty costs.
Faculty compensation varies by institution but can include salary support, FTE reduction, and nonclinical appointments. Tracking metrics such as fellow billing, length of stay, and access to care during the fellowship year are helpful to highlight the benefit of these programs to the organization.
Initiating a program like those described may seem like a Herculean feat, but motivated individuals have been able to accomplish similar goals in both adequately and poorly resourced areas. For those aspiring to start a postgraduate APP program at their instruction, these authors suggest the following approach.
First, identify your institution’s need for such a program. Next, define your curriculum, evaluation process, and expectations. Then, create buy-in from stakeholders, including administrative and clinical personnel. Finally, focus on recruitment. Seeking accreditation may be challenging for new programs, but identifying the accreditation standard you plan to pursue early will pay dividends when the time comes for the program to apply. Those starting down this path should realistically expect an 18- to 24-month period between their first efforts and the start of the first class.
“APP Intersection” is a new quarterly column focusing on areas of interest for the entire chest medicine health care team.
APP Intersection
Postgraduate training for advanced practice providers (APPs) has existed in one form or another since the genesis of the allied professions. They are typically referred to as residencies, fellowships, postgraduate programs, and transition-to-practice.
The desire and necessity for these programs has increased in the past decade with workforce changes; namely the increasing number of nurse practitioners (NPs) graduating with fewer years of experience at the bedside compared with previous eras, a similar decrease in patient contact hours for graduating PAs, the transition of physician colleagues from employers to employees and the subsequent change in priorities in training new graduate APPs, and resident work hour restrictions necessitating more APPs to staff inpatient units and work in various specialties.
The goal of these programs is to provide postgraduate training to physician assistants/associates (PAs) and NPs across myriad medical specialties to both newly graduated APPs and those looking to transition specialties. Current programs exist in family medicine, emergency medicine, urgent care, critical care medicine, pulmonary medicine, oncology, surgery, and various surgical subspecialties, to name a few. Program length is highly variable, though most programs advertise as lasting around 12 months, with varying ratios of clinical and didactic education. Postgraduate APP programs are largely advertise as salaried, benefitted positions, though usually at a rate below that of a so-called “direct hire” due to the protected learning time associated with the postgraduate training year.
Accreditation for these programs is still disjointed, although unifying efforts have been made as of late, and is currently available through the Advanced Practice Provider Fellowship Accreditation, Association of Postgraduate Physician Assistant Programs, ARC-PA, the Accreditation Commission for Education in Nursing, and the Consortium for Advanced Practice Providers. Other organizations, such as the Association of Post Graduate APRN Programs, host regular conferences to discuss the formulation of postgraduate APP education curricula and program development.
While accreditation offers guidance for fledgling programs, many utilize the standards published by the American College of Graduate Medical Education to ensure that appropriate clinical milestones are being met and that a common language among APPs and physicians who are involved in the evaluation of the postgraduate APP trainee is being used. Programs also seek to utilize other well-established curricula and certification programs published by various national and international organizations. A key distinction from physician postgraduate training is that there is currently no fiscal or legislative support for postgraduate APP programs; these issues have been cited as reasons for the limited scope and number of programs.
When starting APP Fellowship programs, it is important to consider why this would be beneficial to a specific division and health care organization. Usually, fellowship programs develop out of a need to train and retain APPs. It is no secret that turnover and retention of skilled APPs is a nationwide problem associated with significant costs to organizations. The ability to retain fellowship-trained APPs will result in cost savings due to the reduction in onboarding time and orientation costs, as these APP fellows finish their programs ready to be fully productive team members.
Additional considerations for the development of an APP fellowship include improving access to care and increasing the quality of the care provided. Fellowship programs encourage a smoother transition to practice by offering more support through education, closer evaluation, and frequent feedback, which improves competence and confidence of these providers. A supported APP is more likely to practice to the fullest extent of their license and have improved personal and professional satisfaction, leading to employee retention and better patient care.
When developing a budget for these types of programs, it is important to include the full-time equivalent (FTE) for the fellow, benefits, onboarding/licensure, simulations, and fellowship faculty costs.
Faculty compensation varies by institution but can include salary support, FTE reduction, and nonclinical appointments. Tracking metrics such as fellow billing, length of stay, and access to care during the fellowship year are helpful to highlight the benefit of these programs to the organization.
Initiating a program like those described may seem like a Herculean feat, but motivated individuals have been able to accomplish similar goals in both adequately and poorly resourced areas. For those aspiring to start a postgraduate APP program at their instruction, these authors suggest the following approach.
First, identify your institution’s need for such a program. Next, define your curriculum, evaluation process, and expectations. Then, create buy-in from stakeholders, including administrative and clinical personnel. Finally, focus on recruitment. Seeking accreditation may be challenging for new programs, but identifying the accreditation standard you plan to pursue early will pay dividends when the time comes for the program to apply. Those starting down this path should realistically expect an 18- to 24-month period between their first efforts and the start of the first class.
“APP Intersection” is a new quarterly column focusing on areas of interest for the entire chest medicine health care team.
APP Intersection
Postgraduate training for advanced practice providers (APPs) has existed in one form or another since the genesis of the allied professions. They are typically referred to as residencies, fellowships, postgraduate programs, and transition-to-practice.
The desire and necessity for these programs has increased in the past decade with workforce changes; namely the increasing number of nurse practitioners (NPs) graduating with fewer years of experience at the bedside compared with previous eras, a similar decrease in patient contact hours for graduating PAs, the transition of physician colleagues from employers to employees and the subsequent change in priorities in training new graduate APPs, and resident work hour restrictions necessitating more APPs to staff inpatient units and work in various specialties.
The goal of these programs is to provide postgraduate training to physician assistants/associates (PAs) and NPs across myriad medical specialties to both newly graduated APPs and those looking to transition specialties. Current programs exist in family medicine, emergency medicine, urgent care, critical care medicine, pulmonary medicine, oncology, surgery, and various surgical subspecialties, to name a few. Program length is highly variable, though most programs advertise as lasting around 12 months, with varying ratios of clinical and didactic education. Postgraduate APP programs are largely advertise as salaried, benefitted positions, though usually at a rate below that of a so-called “direct hire” due to the protected learning time associated with the postgraduate training year.
Accreditation for these programs is still disjointed, although unifying efforts have been made as of late, and is currently available through the Advanced Practice Provider Fellowship Accreditation, Association of Postgraduate Physician Assistant Programs, ARC-PA, the Accreditation Commission for Education in Nursing, and the Consortium for Advanced Practice Providers. Other organizations, such as the Association of Post Graduate APRN Programs, host regular conferences to discuss the formulation of postgraduate APP education curricula and program development.
While accreditation offers guidance for fledgling programs, many utilize the standards published by the American College of Graduate Medical Education to ensure that appropriate clinical milestones are being met and that a common language among APPs and physicians who are involved in the evaluation of the postgraduate APP trainee is being used. Programs also seek to utilize other well-established curricula and certification programs published by various national and international organizations. A key distinction from physician postgraduate training is that there is currently no fiscal or legislative support for postgraduate APP programs; these issues have been cited as reasons for the limited scope and number of programs.
When starting APP Fellowship programs, it is important to consider why this would be beneficial to a specific division and health care organization. Usually, fellowship programs develop out of a need to train and retain APPs. It is no secret that turnover and retention of skilled APPs is a nationwide problem associated with significant costs to organizations. The ability to retain fellowship-trained APPs will result in cost savings due to the reduction in onboarding time and orientation costs, as these APP fellows finish their programs ready to be fully productive team members.
Additional considerations for the development of an APP fellowship include improving access to care and increasing the quality of the care provided. Fellowship programs encourage a smoother transition to practice by offering more support through education, closer evaluation, and frequent feedback, which improves competence and confidence of these providers. A supported APP is more likely to practice to the fullest extent of their license and have improved personal and professional satisfaction, leading to employee retention and better patient care.
When developing a budget for these types of programs, it is important to include the full-time equivalent (FTE) for the fellow, benefits, onboarding/licensure, simulations, and fellowship faculty costs.
Faculty compensation varies by institution but can include salary support, FTE reduction, and nonclinical appointments. Tracking metrics such as fellow billing, length of stay, and access to care during the fellowship year are helpful to highlight the benefit of these programs to the organization.
Initiating a program like those described may seem like a Herculean feat, but motivated individuals have been able to accomplish similar goals in both adequately and poorly resourced areas. For those aspiring to start a postgraduate APP program at their instruction, these authors suggest the following approach.
First, identify your institution’s need for such a program. Next, define your curriculum, evaluation process, and expectations. Then, create buy-in from stakeholders, including administrative and clinical personnel. Finally, focus on recruitment. Seeking accreditation may be challenging for new programs, but identifying the accreditation standard you plan to pursue early will pay dividends when the time comes for the program to apply. Those starting down this path should realistically expect an 18- to 24-month period between their first efforts and the start of the first class.
“APP Intersection” is a new quarterly column focusing on areas of interest for the entire chest medicine health care team.
Calcium Pyrophosphate Deposition Disease Nearly Doubles Fracture Risk
Patients with calcium pyrophosphate deposition (CPPD) disease, also known as pseudogout, have an 80% higher risk for fracture than individuals who do not have the disease, according to a new analysis.
This trend was driven by wrist fractures, where there was a more than threefold increased risk.
Previous studies identified an association between CPPD and low bone mineral density, and there is growing evidence suggesting that the dysregulation of osteoprotegerin — a molecule that is important in the regulation of osteoclasts — may be associated with early-onset CPPD, noted Sara K. Tedeschi, MD, MPH, the lead author of the study and head of crystal-induced arthritic diseases at Brigham and Women’s Hospital, Boston, Massachusetts.
However, CPPD’s association with fracture risk has yet to be explored.
In the study, Dr. Tedeschi and colleagues used Mass General Brigham electronic health record (EHR) data from 1991 to 2023 to identify 1148 individuals with acute calcium pyrophosphate (CPP) crystal arthritis. The index date was defined as the first documentation of pseudogout or synovial fluid CPP crystals. These patients were matched to 3730 comparators based on healthcare encounters within 30 days of the index date of a patient with CPPD. Patients were also matched based on the year of their first EHR encounter. Patients with a fracture documented prior to the index date were excluded from the analysis.
The primary outcome was the first fracture of the humerus, knee, wrist, hip, or pelvis, detected via published algorithms using diagnostic and procedural codes.
The research was published on January 14 in Arthritis & Rheumatology.
Although participants were not matched on age or sex, the average age was 73, and most participants were female. In total, 83.1% of participants in the CPPD group and 80.0% of those in the control group were White.
After adjustment for confounding factors including age, sex, comorbidities, and glucocorticoid use, CPPD was associated with an 80% higher risk for any fracture (hazard risk [HR], 1.8). Fracture risk was highest for the wrist (HR, 3.6).
Patients with CPPD had a 40% higher risk to experience a humerus or pelvis fracture and a 30% higher risk for hip fractures, but the results were not statistically significant.
The results were similar for sensitivity analyses that excluded patients who were prescribed glucocorticoids, treatment for osteoporosis, or had a diagnosis of rheumatoid arthritis.
Asked to comment, John D. FitzGerald, MD, PhD, clinical chief of rheumatology at the University of California, Los Angeles, noted that these associations were “convincing and strong. I thought it was a very good study and important work. CPPD is common and osteoporosis is common, so better understanding the connection is important.”
It’s still not clear why the risk for wrist fractures was highest, but Dr. Tedeschi had two hypotheses. The researchers were unable to assess for falls in this dataset, but it’s possible that patients with CPPD experiencing joint pain could fall and try to brace themselves with an outstretched arm, leading to a wrist fracture.
CPPD also commonly affects the wrist, “so it’s possible that if CPPD is affecting the wrist and if there is an association between CPPD and low bone density, maybe there’s particularly low bone density at the wrist,” she said.
Dr. FitzGerald agreed that both hypotheses were plausible, but “with the retrospective study, there could be a lot of things that are unobserved or unexplained,” he added.
Dr. Tedeschi is interested in exploring what could be causing the association with an increased fracture risk in future research.
“I hope this draws attention to the fact that people with CPPD can have related medical problems that are outside of their joints,” added Dr. Tedeschi. “Thinking about routine screening for osteopenia and osteoporosis could be a good first step in patients with CPPD.”
The study was funded by grants from the US National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Tedeschi has worked as a consultant for Novartis. Dr. FitzGerald reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Patients with calcium pyrophosphate deposition (CPPD) disease, also known as pseudogout, have an 80% higher risk for fracture than individuals who do not have the disease, according to a new analysis.
This trend was driven by wrist fractures, where there was a more than threefold increased risk.
Previous studies identified an association between CPPD and low bone mineral density, and there is growing evidence suggesting that the dysregulation of osteoprotegerin — a molecule that is important in the regulation of osteoclasts — may be associated with early-onset CPPD, noted Sara K. Tedeschi, MD, MPH, the lead author of the study and head of crystal-induced arthritic diseases at Brigham and Women’s Hospital, Boston, Massachusetts.
However, CPPD’s association with fracture risk has yet to be explored.
In the study, Dr. Tedeschi and colleagues used Mass General Brigham electronic health record (EHR) data from 1991 to 2023 to identify 1148 individuals with acute calcium pyrophosphate (CPP) crystal arthritis. The index date was defined as the first documentation of pseudogout or synovial fluid CPP crystals. These patients were matched to 3730 comparators based on healthcare encounters within 30 days of the index date of a patient with CPPD. Patients were also matched based on the year of their first EHR encounter. Patients with a fracture documented prior to the index date were excluded from the analysis.
The primary outcome was the first fracture of the humerus, knee, wrist, hip, or pelvis, detected via published algorithms using diagnostic and procedural codes.
The research was published on January 14 in Arthritis & Rheumatology.
Although participants were not matched on age or sex, the average age was 73, and most participants were female. In total, 83.1% of participants in the CPPD group and 80.0% of those in the control group were White.
After adjustment for confounding factors including age, sex, comorbidities, and glucocorticoid use, CPPD was associated with an 80% higher risk for any fracture (hazard risk [HR], 1.8). Fracture risk was highest for the wrist (HR, 3.6).
Patients with CPPD had a 40% higher risk to experience a humerus or pelvis fracture and a 30% higher risk for hip fractures, but the results were not statistically significant.
The results were similar for sensitivity analyses that excluded patients who were prescribed glucocorticoids, treatment for osteoporosis, or had a diagnosis of rheumatoid arthritis.
Asked to comment, John D. FitzGerald, MD, PhD, clinical chief of rheumatology at the University of California, Los Angeles, noted that these associations were “convincing and strong. I thought it was a very good study and important work. CPPD is common and osteoporosis is common, so better understanding the connection is important.”
It’s still not clear why the risk for wrist fractures was highest, but Dr. Tedeschi had two hypotheses. The researchers were unable to assess for falls in this dataset, but it’s possible that patients with CPPD experiencing joint pain could fall and try to brace themselves with an outstretched arm, leading to a wrist fracture.
CPPD also commonly affects the wrist, “so it’s possible that if CPPD is affecting the wrist and if there is an association between CPPD and low bone density, maybe there’s particularly low bone density at the wrist,” she said.
Dr. FitzGerald agreed that both hypotheses were plausible, but “with the retrospective study, there could be a lot of things that are unobserved or unexplained,” he added.
Dr. Tedeschi is interested in exploring what could be causing the association with an increased fracture risk in future research.
“I hope this draws attention to the fact that people with CPPD can have related medical problems that are outside of their joints,” added Dr. Tedeschi. “Thinking about routine screening for osteopenia and osteoporosis could be a good first step in patients with CPPD.”
The study was funded by grants from the US National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Tedeschi has worked as a consultant for Novartis. Dr. FitzGerald reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
Patients with calcium pyrophosphate deposition (CPPD) disease, also known as pseudogout, have an 80% higher risk for fracture than individuals who do not have the disease, according to a new analysis.
This trend was driven by wrist fractures, where there was a more than threefold increased risk.
Previous studies identified an association between CPPD and low bone mineral density, and there is growing evidence suggesting that the dysregulation of osteoprotegerin — a molecule that is important in the regulation of osteoclasts — may be associated with early-onset CPPD, noted Sara K. Tedeschi, MD, MPH, the lead author of the study and head of crystal-induced arthritic diseases at Brigham and Women’s Hospital, Boston, Massachusetts.
However, CPPD’s association with fracture risk has yet to be explored.
In the study, Dr. Tedeschi and colleagues used Mass General Brigham electronic health record (EHR) data from 1991 to 2023 to identify 1148 individuals with acute calcium pyrophosphate (CPP) crystal arthritis. The index date was defined as the first documentation of pseudogout or synovial fluid CPP crystals. These patients were matched to 3730 comparators based on healthcare encounters within 30 days of the index date of a patient with CPPD. Patients were also matched based on the year of their first EHR encounter. Patients with a fracture documented prior to the index date were excluded from the analysis.
The primary outcome was the first fracture of the humerus, knee, wrist, hip, or pelvis, detected via published algorithms using diagnostic and procedural codes.
The research was published on January 14 in Arthritis & Rheumatology.
Although participants were not matched on age or sex, the average age was 73, and most participants were female. In total, 83.1% of participants in the CPPD group and 80.0% of those in the control group were White.
After adjustment for confounding factors including age, sex, comorbidities, and glucocorticoid use, CPPD was associated with an 80% higher risk for any fracture (hazard risk [HR], 1.8). Fracture risk was highest for the wrist (HR, 3.6).
Patients with CPPD had a 40% higher risk to experience a humerus or pelvis fracture and a 30% higher risk for hip fractures, but the results were not statistically significant.
The results were similar for sensitivity analyses that excluded patients who were prescribed glucocorticoids, treatment for osteoporosis, or had a diagnosis of rheumatoid arthritis.
Asked to comment, John D. FitzGerald, MD, PhD, clinical chief of rheumatology at the University of California, Los Angeles, noted that these associations were “convincing and strong. I thought it was a very good study and important work. CPPD is common and osteoporosis is common, so better understanding the connection is important.”
It’s still not clear why the risk for wrist fractures was highest, but Dr. Tedeschi had two hypotheses. The researchers were unable to assess for falls in this dataset, but it’s possible that patients with CPPD experiencing joint pain could fall and try to brace themselves with an outstretched arm, leading to a wrist fracture.
CPPD also commonly affects the wrist, “so it’s possible that if CPPD is affecting the wrist and if there is an association between CPPD and low bone density, maybe there’s particularly low bone density at the wrist,” she said.
Dr. FitzGerald agreed that both hypotheses were plausible, but “with the retrospective study, there could be a lot of things that are unobserved or unexplained,” he added.
Dr. Tedeschi is interested in exploring what could be causing the association with an increased fracture risk in future research.
“I hope this draws attention to the fact that people with CPPD can have related medical problems that are outside of their joints,” added Dr. Tedeschi. “Thinking about routine screening for osteopenia and osteoporosis could be a good first step in patients with CPPD.”
The study was funded by grants from the US National Institute of Arthritis and Musculoskeletal and Skin Diseases. Dr. Tedeschi has worked as a consultant for Novartis. Dr. FitzGerald reported no relevant financial relationships.
A version of this article appeared on Medscape.com.
FROM ARTHRITIS & RHEUMATOLOGY
Two-Step Screening Uncovers Heart Failure Risk in Diabetes
TOPLINE:
A two-step screening, using a risk score and biomarkers, can identify patients with diabetes at a higher risk for heart failure who will most likely benefit from preventive drugs.
METHODOLOGY:
- Researchers compared screening methods and downstream risk for heart failure in 5 years, particularly those without atherosclerotic cardiovascular disease (ASCVD).
- They pooled data from 4889 patients (age ≥ 40 years, about half women) with diabetes, no heart failure at baseline, and no signs of ASCVD. All patients had undergone screening to determine their heart failure risk level.
- Researchers assessed the heart failure risk for patients without ASCVD with one-step screening strategies:
- —Clinical risk score (WATCH-DM risk score)
- —Biomarker tests (N-terminal pro-B-type natriuretic peptide [NT-proBNP]) or high-sensitivity cardiac troponin [hs-cTn)
- —Echocardiography
- They next assessed a sequential two-step strategy, using the second test only for those deemed low risk by the first, with a combination of two tests (WATCH-DM/NT-proBNP, NT-proBNP/hs-cTn, or NT-proBNP/echocardiography), the second used for those deemed low-risk by the first test.
- The primary outcome was incident heart failure during the 5-year follow-up. The researchers also assessed the cost-effectiveness of screening and subsequent treatment of high-risk patients with a sodium-glucose cotransporter 2 inhibitor.
TAKEAWAY:
- Overall, 301 (6.2%) heart failure events occurred among participants without ASCVD.
- Of the heart failure events, 53%-71% occurred among participants deemed high risk by a one-step screening strategy, but 75%-89% occurred among patients assessed as high risk in two steps.
- The risk for incident heart failure was 3.0- to 3.6-fold higher in the high- vs low-risk group identified using a two-step screening approach.
- Among the two-step strategies, the WATCH-DM score first, followed by selective NT-proBNP testing for patients deemed low risk by the first test, was the most efficient, with the fewest tests and lowest screening cost.
IN PRACTICE:
“Matching effective but expensive preventive therapies to the highest-risk individuals who are most likely to benefit would be an efficient and cost-effective strategy for heart failure prevention,” the authors wrote.
SOURCE:
The study, led by Kershaw Patel of the Houston Methodist Academic Institute, was published online in Circulation.
LIMITATIONS:
The study findings may not be generalized, as the study included older adults with a high burden of comorbidities. This study may have missed some individuals with diabetes by defining it with fasting plasma glucose, which was consistently available across cohort studies, instead of with the limited A1c data. Moreover, the screening strategies used did not consider other important prognostic factors, such as diabetes duration and socioeconomic status.
DISCLOSURES:
Two authors declared receiving research support from the National Heart, Lung, and Blood Institute. Several authors disclosed financial relationships with multiple pharmaceutical device and medical publishing companies in the form of receiving personal fees; serving in various capacities such as consultants, members of advisory boards, steering committees, or executive committees; and other ties.
A version of this article appeared on Medscape.com.
TOPLINE:
A two-step screening, using a risk score and biomarkers, can identify patients with diabetes at a higher risk for heart failure who will most likely benefit from preventive drugs.
METHODOLOGY:
- Researchers compared screening methods and downstream risk for heart failure in 5 years, particularly those without atherosclerotic cardiovascular disease (ASCVD).
- They pooled data from 4889 patients (age ≥ 40 years, about half women) with diabetes, no heart failure at baseline, and no signs of ASCVD. All patients had undergone screening to determine their heart failure risk level.
- Researchers assessed the heart failure risk for patients without ASCVD with one-step screening strategies:
- —Clinical risk score (WATCH-DM risk score)
- —Biomarker tests (N-terminal pro-B-type natriuretic peptide [NT-proBNP]) or high-sensitivity cardiac troponin [hs-cTn)
- —Echocardiography
- They next assessed a sequential two-step strategy, using the second test only for those deemed low risk by the first, with a combination of two tests (WATCH-DM/NT-proBNP, NT-proBNP/hs-cTn, or NT-proBNP/echocardiography), the second used for those deemed low-risk by the first test.
- The primary outcome was incident heart failure during the 5-year follow-up. The researchers also assessed the cost-effectiveness of screening and subsequent treatment of high-risk patients with a sodium-glucose cotransporter 2 inhibitor.
TAKEAWAY:
- Overall, 301 (6.2%) heart failure events occurred among participants without ASCVD.
- Of the heart failure events, 53%-71% occurred among participants deemed high risk by a one-step screening strategy, but 75%-89% occurred among patients assessed as high risk in two steps.
- The risk for incident heart failure was 3.0- to 3.6-fold higher in the high- vs low-risk group identified using a two-step screening approach.
- Among the two-step strategies, the WATCH-DM score first, followed by selective NT-proBNP testing for patients deemed low risk by the first test, was the most efficient, with the fewest tests and lowest screening cost.
IN PRACTICE:
“Matching effective but expensive preventive therapies to the highest-risk individuals who are most likely to benefit would be an efficient and cost-effective strategy for heart failure prevention,” the authors wrote.
SOURCE:
The study, led by Kershaw Patel of the Houston Methodist Academic Institute, was published online in Circulation.
LIMITATIONS:
The study findings may not be generalized, as the study included older adults with a high burden of comorbidities. This study may have missed some individuals with diabetes by defining it with fasting plasma glucose, which was consistently available across cohort studies, instead of with the limited A1c data. Moreover, the screening strategies used did not consider other important prognostic factors, such as diabetes duration and socioeconomic status.
DISCLOSURES:
Two authors declared receiving research support from the National Heart, Lung, and Blood Institute. Several authors disclosed financial relationships with multiple pharmaceutical device and medical publishing companies in the form of receiving personal fees; serving in various capacities such as consultants, members of advisory boards, steering committees, or executive committees; and other ties.
A version of this article appeared on Medscape.com.
TOPLINE:
A two-step screening, using a risk score and biomarkers, can identify patients with diabetes at a higher risk for heart failure who will most likely benefit from preventive drugs.
METHODOLOGY:
- Researchers compared screening methods and downstream risk for heart failure in 5 years, particularly those without atherosclerotic cardiovascular disease (ASCVD).
- They pooled data from 4889 patients (age ≥ 40 years, about half women) with diabetes, no heart failure at baseline, and no signs of ASCVD. All patients had undergone screening to determine their heart failure risk level.
- Researchers assessed the heart failure risk for patients without ASCVD with one-step screening strategies:
- —Clinical risk score (WATCH-DM risk score)
- —Biomarker tests (N-terminal pro-B-type natriuretic peptide [NT-proBNP]) or high-sensitivity cardiac troponin [hs-cTn)
- —Echocardiography
- They next assessed a sequential two-step strategy, using the second test only for those deemed low risk by the first, with a combination of two tests (WATCH-DM/NT-proBNP, NT-proBNP/hs-cTn, or NT-proBNP/echocardiography), the second used for those deemed low-risk by the first test.
- The primary outcome was incident heart failure during the 5-year follow-up. The researchers also assessed the cost-effectiveness of screening and subsequent treatment of high-risk patients with a sodium-glucose cotransporter 2 inhibitor.
TAKEAWAY:
- Overall, 301 (6.2%) heart failure events occurred among participants without ASCVD.
- Of the heart failure events, 53%-71% occurred among participants deemed high risk by a one-step screening strategy, but 75%-89% occurred among patients assessed as high risk in two steps.
- The risk for incident heart failure was 3.0- to 3.6-fold higher in the high- vs low-risk group identified using a two-step screening approach.
- Among the two-step strategies, the WATCH-DM score first, followed by selective NT-proBNP testing for patients deemed low risk by the first test, was the most efficient, with the fewest tests and lowest screening cost.
IN PRACTICE:
“Matching effective but expensive preventive therapies to the highest-risk individuals who are most likely to benefit would be an efficient and cost-effective strategy for heart failure prevention,” the authors wrote.
SOURCE:
The study, led by Kershaw Patel of the Houston Methodist Academic Institute, was published online in Circulation.
LIMITATIONS:
The study findings may not be generalized, as the study included older adults with a high burden of comorbidities. This study may have missed some individuals with diabetes by defining it with fasting plasma glucose, which was consistently available across cohort studies, instead of with the limited A1c data. Moreover, the screening strategies used did not consider other important prognostic factors, such as diabetes duration and socioeconomic status.
DISCLOSURES:
Two authors declared receiving research support from the National Heart, Lung, and Blood Institute. Several authors disclosed financial relationships with multiple pharmaceutical device and medical publishing companies in the form of receiving personal fees; serving in various capacities such as consultants, members of advisory boards, steering committees, or executive committees; and other ties.
A version of this article appeared on Medscape.com.




















