User login
Barrett’s esophagus uncommon in patients with uncomplicated GERD
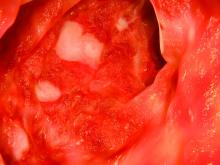
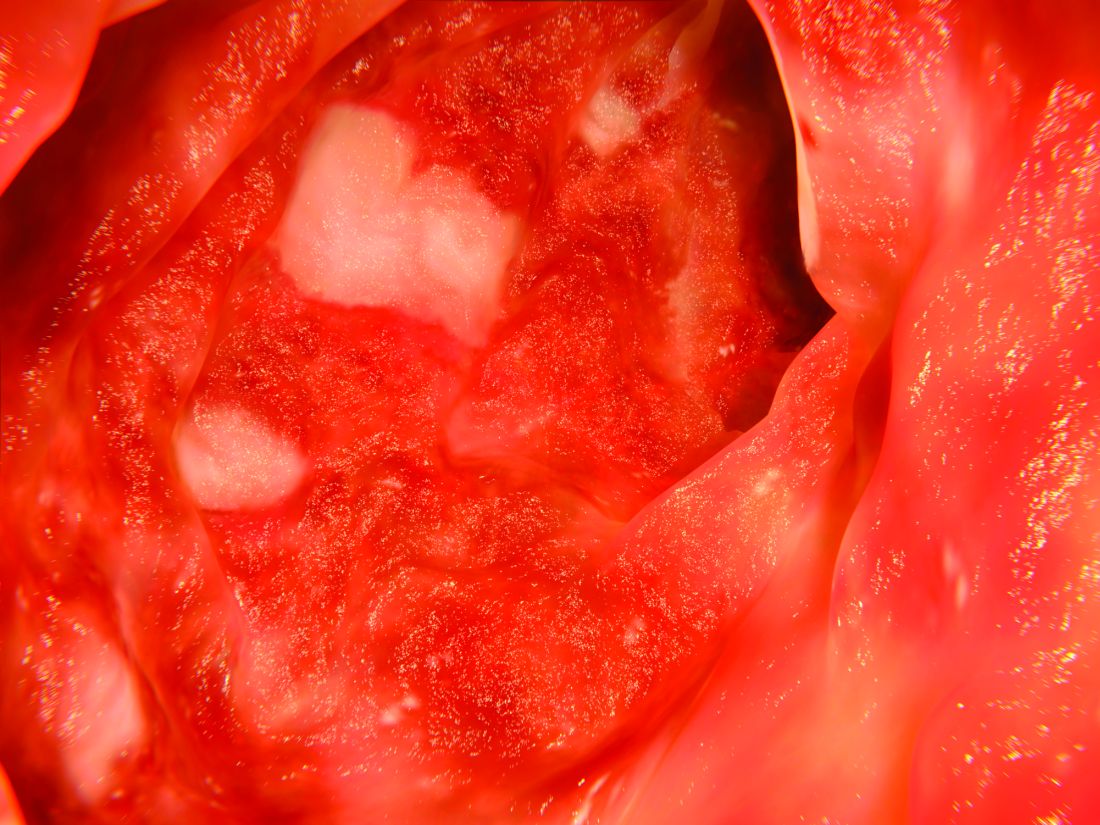
Uncomplicated gastroesophageal reflux disease (GERD) accounted for 13.5% of esophagogastroduodenoscopies, but 5.6% of these patients had suspected Barrett’s esophagus and only 1.4% had suspected long-segment Barrett’s esophagus, researchers reported. The study appears in the April issue of Clinical Gastroenterology and Hepatology.
“The prevalence of suspected Barrett’s esophagus is lower than in prior time periods. This raises questions about the utility of esophagogastroduodenoscopies to detect Barrett’s esophagus in patients with uncomplicated GERD,” wrote Emery C. Lin, MD, of Oregon Health and Science University, Portland, and his associates there and at Massachusetts General Hospital, Boston.
Symptoms of GERD affect more than one in four U.S. adults and are a risk factor for Barrett’s esophagus. However, the prevalence of Barrett’s esophagus is unclear in patients with dysphagia and in the era of proton pump inhibitors, the researchers said. The American Gastroenterological Association strongly discourages reflexively screening patients with GERD for Barrett’s esophagus, but “weakly recommends” screening GERD patients with multiple risk factors for Barrett’s esophagus, including chronic GERD, hiatal hernia, older age (50 years and up), white race, male sex, increased body mass index, and intra-abdominal adiposity.
To understand the prevalence and findings of esophagogastroduodenoscopy in patients with GERD without alarm symptoms (including weight loss, dysphagia, and bleeding), the investigators studied 543,103 of these procedures performed at 82 sites in the United States between 2003 and 2013. The data came from the National Endoscopic Database, which generates endoscopy reports using a structured computer form.
A total of 73,535 esophagogastroduodenoscopies (13.5%) were performed for GERD without alarm symptoms. Among these patients, 4,122 (5.6%) had suspected Barrett’s esophagus, of which 24.2% had suspected long-segment Barrett’s esophagus (3 cm or longer). Among patients with uncomplicated GERD, the prevalence of suspected Barrett’s esophagus was 5.6%, and the prevalence of long-segment disease was 1.4%.
Although male sex, older age, and white race were significant risk factors for suspected Barrett’s esophagus and suspected long-segment disease, 23.6% of esophagogastroduodenoscopies were performed in white men older than 50 years. “We find that low-risk populations with uncomplicated GERD make up a significant number of esophagogastroduodenoscopies done for uncomplicated GERD,” the investigators wrote. “If esophagogastroduodenoscopies were limited to patients that met the AGA criteria of being male, white, and age over 50, we would have detected 34 of 47 (72.3%) of esophageal tumors and found suspected Barrett’s esophagus in nearly 10%, while reducing the burden of endoscopy by more than 75%.”
Hiatal hernia was a significant correlate of suspected Barrett’s esophagus (odds ratio, 1.6), the researchers noted. Esophagitis was not associated with suspected Barrett’s esophagus overall but did correlate with long-segment disease. Esophagitis might mask underlying short-segment Barrett’s esophagus, and short-segment Barrett’s esophagus might be milder in nature and more responsive to antisecretory therapy, the researchers said. They noted that severe (grade C/D) esophagitis was strongly linked with both short-segment and long-segment Barrett’s esophagus.
The National Institute of Diabetes and Digestive and Kidney Diseases provided funding. The researchers reported having no conflicts of interest.
SOURCE: Lin EC et al. Clin Gastroenterol Hepatol. 2019 Apr. doi: 10.1016/j.cgh.2018.08.066.
The utility and cost-effectiveness of screening for Barrett’s esophagus with esophagogastroduodenoscopy (EGD) remain contentious issues. National GI societies currently recommend screening in only a limited high-risk population, mainly white men aged 50 or older with chronic GERD and one or more additional risk factors. It is unclear to what degree those guidelines are adhered to in clinical practice. This study by Lin et al. sheds further light on this issue. The investigators showed that a significant proportion (more than 10%) of EGDs were performed for uncomplicated GERD, with less than one-quarter of those patients meeting the minimal criteria for screening for Barrett’s esophagus. Among this group, the prevalence of Barrett’s esophagus was found to be lower than previously reported. The data offer compelling evidence that screening low-risk patients with uncomplicated GERD by using upper endoscopy is not cost effective, and is at best marginally cost effective if limited to the high-risk group identified by national GI societies. The question arises whether we should abandon screening for Barrett’s esophagus altogether.
The challenge, however, is that the incidence of esophageal adenocarcinoma continues to rise (albeit at a slower pace in recent years), and 5-year survival of patients diagnosed with esophageal adenocarcinoma remains extremely poor. Therefore, prevention remains the optimal strategy. The solution may lie in adopting a lower-cost screening modality that can replace endoscopy for this purpose, and while many such techniques are under investigation, further studies are required to find a widely applicable alternative to EGD.
Nabil M. Mansour, MD, is an assistant professor, department of medicine, section of gastroenterology and hepatology, Baylor College of Medicine, Houston. He has no conflicts of interest.
The utility and cost-effectiveness of screening for Barrett’s esophagus with esophagogastroduodenoscopy (EGD) remain contentious issues. National GI societies currently recommend screening in only a limited high-risk population, mainly white men aged 50 or older with chronic GERD and one or more additional risk factors. It is unclear to what degree those guidelines are adhered to in clinical practice. This study by Lin et al. sheds further light on this issue. The investigators showed that a significant proportion (more than 10%) of EGDs were performed for uncomplicated GERD, with less than one-quarter of those patients meeting the minimal criteria for screening for Barrett’s esophagus. Among this group, the prevalence of Barrett’s esophagus was found to be lower than previously reported. The data offer compelling evidence that screening low-risk patients with uncomplicated GERD by using upper endoscopy is not cost effective, and is at best marginally cost effective if limited to the high-risk group identified by national GI societies. The question arises whether we should abandon screening for Barrett’s esophagus altogether.
The challenge, however, is that the incidence of esophageal adenocarcinoma continues to rise (albeit at a slower pace in recent years), and 5-year survival of patients diagnosed with esophageal adenocarcinoma remains extremely poor. Therefore, prevention remains the optimal strategy. The solution may lie in adopting a lower-cost screening modality that can replace endoscopy for this purpose, and while many such techniques are under investigation, further studies are required to find a widely applicable alternative to EGD.
Nabil M. Mansour, MD, is an assistant professor, department of medicine, section of gastroenterology and hepatology, Baylor College of Medicine, Houston. He has no conflicts of interest.
The utility and cost-effectiveness of screening for Barrett’s esophagus with esophagogastroduodenoscopy (EGD) remain contentious issues. National GI societies currently recommend screening in only a limited high-risk population, mainly white men aged 50 or older with chronic GERD and one or more additional risk factors. It is unclear to what degree those guidelines are adhered to in clinical practice. This study by Lin et al. sheds further light on this issue. The investigators showed that a significant proportion (more than 10%) of EGDs were performed for uncomplicated GERD, with less than one-quarter of those patients meeting the minimal criteria for screening for Barrett’s esophagus. Among this group, the prevalence of Barrett’s esophagus was found to be lower than previously reported. The data offer compelling evidence that screening low-risk patients with uncomplicated GERD by using upper endoscopy is not cost effective, and is at best marginally cost effective if limited to the high-risk group identified by national GI societies. The question arises whether we should abandon screening for Barrett’s esophagus altogether.
The challenge, however, is that the incidence of esophageal adenocarcinoma continues to rise (albeit at a slower pace in recent years), and 5-year survival of patients diagnosed with esophageal adenocarcinoma remains extremely poor. Therefore, prevention remains the optimal strategy. The solution may lie in adopting a lower-cost screening modality that can replace endoscopy for this purpose, and while many such techniques are under investigation, further studies are required to find a widely applicable alternative to EGD.
Nabil M. Mansour, MD, is an assistant professor, department of medicine, section of gastroenterology and hepatology, Baylor College of Medicine, Houston. He has no conflicts of interest.
Uncomplicated gastroesophageal reflux disease (GERD) accounted for 13.5% of esophagogastroduodenoscopies, but 5.6% of these patients had suspected Barrett’s esophagus and only 1.4% had suspected long-segment Barrett’s esophagus, researchers reported. The study appears in the April issue of Clinical Gastroenterology and Hepatology.
“The prevalence of suspected Barrett’s esophagus is lower than in prior time periods. This raises questions about the utility of esophagogastroduodenoscopies to detect Barrett’s esophagus in patients with uncomplicated GERD,” wrote Emery C. Lin, MD, of Oregon Health and Science University, Portland, and his associates there and at Massachusetts General Hospital, Boston.
Symptoms of GERD affect more than one in four U.S. adults and are a risk factor for Barrett’s esophagus. However, the prevalence of Barrett’s esophagus is unclear in patients with dysphagia and in the era of proton pump inhibitors, the researchers said. The American Gastroenterological Association strongly discourages reflexively screening patients with GERD for Barrett’s esophagus, but “weakly recommends” screening GERD patients with multiple risk factors for Barrett’s esophagus, including chronic GERD, hiatal hernia, older age (50 years and up), white race, male sex, increased body mass index, and intra-abdominal adiposity.
To understand the prevalence and findings of esophagogastroduodenoscopy in patients with GERD without alarm symptoms (including weight loss, dysphagia, and bleeding), the investigators studied 543,103 of these procedures performed at 82 sites in the United States between 2003 and 2013. The data came from the National Endoscopic Database, which generates endoscopy reports using a structured computer form.
A total of 73,535 esophagogastroduodenoscopies (13.5%) were performed for GERD without alarm symptoms. Among these patients, 4,122 (5.6%) had suspected Barrett’s esophagus, of which 24.2% had suspected long-segment Barrett’s esophagus (3 cm or longer). Among patients with uncomplicated GERD, the prevalence of suspected Barrett’s esophagus was 5.6%, and the prevalence of long-segment disease was 1.4%.
Although male sex, older age, and white race were significant risk factors for suspected Barrett’s esophagus and suspected long-segment disease, 23.6% of esophagogastroduodenoscopies were performed in white men older than 50 years. “We find that low-risk populations with uncomplicated GERD make up a significant number of esophagogastroduodenoscopies done for uncomplicated GERD,” the investigators wrote. “If esophagogastroduodenoscopies were limited to patients that met the AGA criteria of being male, white, and age over 50, we would have detected 34 of 47 (72.3%) of esophageal tumors and found suspected Barrett’s esophagus in nearly 10%, while reducing the burden of endoscopy by more than 75%.”
Hiatal hernia was a significant correlate of suspected Barrett’s esophagus (odds ratio, 1.6), the researchers noted. Esophagitis was not associated with suspected Barrett’s esophagus overall but did correlate with long-segment disease. Esophagitis might mask underlying short-segment Barrett’s esophagus, and short-segment Barrett’s esophagus might be milder in nature and more responsive to antisecretory therapy, the researchers said. They noted that severe (grade C/D) esophagitis was strongly linked with both short-segment and long-segment Barrett’s esophagus.
The National Institute of Diabetes and Digestive and Kidney Diseases provided funding. The researchers reported having no conflicts of interest.
SOURCE: Lin EC et al. Clin Gastroenterol Hepatol. 2019 Apr. doi: 10.1016/j.cgh.2018.08.066.
Uncomplicated gastroesophageal reflux disease (GERD) accounted for 13.5% of esophagogastroduodenoscopies, but 5.6% of these patients had suspected Barrett’s esophagus and only 1.4% had suspected long-segment Barrett’s esophagus, researchers reported. The study appears in the April issue of Clinical Gastroenterology and Hepatology.
“The prevalence of suspected Barrett’s esophagus is lower than in prior time periods. This raises questions about the utility of esophagogastroduodenoscopies to detect Barrett’s esophagus in patients with uncomplicated GERD,” wrote Emery C. Lin, MD, of Oregon Health and Science University, Portland, and his associates there and at Massachusetts General Hospital, Boston.
Symptoms of GERD affect more than one in four U.S. adults and are a risk factor for Barrett’s esophagus. However, the prevalence of Barrett’s esophagus is unclear in patients with dysphagia and in the era of proton pump inhibitors, the researchers said. The American Gastroenterological Association strongly discourages reflexively screening patients with GERD for Barrett’s esophagus, but “weakly recommends” screening GERD patients with multiple risk factors for Barrett’s esophagus, including chronic GERD, hiatal hernia, older age (50 years and up), white race, male sex, increased body mass index, and intra-abdominal adiposity.
To understand the prevalence and findings of esophagogastroduodenoscopy in patients with GERD without alarm symptoms (including weight loss, dysphagia, and bleeding), the investigators studied 543,103 of these procedures performed at 82 sites in the United States between 2003 and 2013. The data came from the National Endoscopic Database, which generates endoscopy reports using a structured computer form.
A total of 73,535 esophagogastroduodenoscopies (13.5%) were performed for GERD without alarm symptoms. Among these patients, 4,122 (5.6%) had suspected Barrett’s esophagus, of which 24.2% had suspected long-segment Barrett’s esophagus (3 cm or longer). Among patients with uncomplicated GERD, the prevalence of suspected Barrett’s esophagus was 5.6%, and the prevalence of long-segment disease was 1.4%.
Although male sex, older age, and white race were significant risk factors for suspected Barrett’s esophagus and suspected long-segment disease, 23.6% of esophagogastroduodenoscopies were performed in white men older than 50 years. “We find that low-risk populations with uncomplicated GERD make up a significant number of esophagogastroduodenoscopies done for uncomplicated GERD,” the investigators wrote. “If esophagogastroduodenoscopies were limited to patients that met the AGA criteria of being male, white, and age over 50, we would have detected 34 of 47 (72.3%) of esophageal tumors and found suspected Barrett’s esophagus in nearly 10%, while reducing the burden of endoscopy by more than 75%.”
Hiatal hernia was a significant correlate of suspected Barrett’s esophagus (odds ratio, 1.6), the researchers noted. Esophagitis was not associated with suspected Barrett’s esophagus overall but did correlate with long-segment disease. Esophagitis might mask underlying short-segment Barrett’s esophagus, and short-segment Barrett’s esophagus might be milder in nature and more responsive to antisecretory therapy, the researchers said. They noted that severe (grade C/D) esophagitis was strongly linked with both short-segment and long-segment Barrett’s esophagus.
The National Institute of Diabetes and Digestive and Kidney Diseases provided funding. The researchers reported having no conflicts of interest.
SOURCE: Lin EC et al. Clin Gastroenterol Hepatol. 2019 Apr. doi: 10.1016/j.cgh.2018.08.066.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Acceptance and commitment therapy reduced IBD stress, depression
Eight weeks of a mindfulness intervention known as acceptance and commitment therapy (ACT) significantly improved stress and depression among patients with inflammatory bowel disease, and these improvements persisted for at least 12 weeks after therapy ended, according to the results of a randomized, controlled trial.
Source: The American Gastroenterological Association
In the intention-to-treat analysis, stress symptoms, as measured by the Depression Anxiety and Stress Scales (DASS-21), improved by 39% at week 8 and by 45% at week 20, reported Brona Wynne, PhD, of University College Dublin together with her associates. These improvements were highly significant compared with baseline and treatment as usual (P = .001 for both comparisons). “Post hoc analyses indicated that baseline stress levels were similar in control and treatment groups,” the researchers wrote in Gastroenterology. “The results of the per protocol analysis were comparable, with a 43% and 49% reduction in stress in the treatment group from baseline to 8 and 20 weeks.”
Multiple studies have documented high levels of stress and psychological dysfunction among patients with Crohn’s disease and ulcerative colitis. Studies of various mindfulness therapy, relaxation, stress management, cognitive-behavioral therapy, and hypnotherapy interventions often failed to collect key clinical data or were underpowered, uncontrolled, and unrandomized. Acceptance and commitment therapy uses mindfulness to identify adverse thoughts and experiences, accept these as part of life, and recommit to “move towards values that have been identified and adopted by the individual,” the investigators wrote. “This can be defined as the ability to contact the present moment more fully as a conscious human being and to change, or persist in, behavior when doing so serves valued ends.”
Their single-center study, which they said was the first to evaluate ACT in IBD patients, included 79 individuals with stable or mildly active Crohn’s disease (38 patients) or ulcerative colitis (41 patients) who were randomly assigned to ACT (37 patients) or control treatment as usual (42 patients). The two comparison groups were demographically and clinically similar. The ACT program involved eight 90-minute, weekly sessions of groups of 14-16 individuals, led by a single psychologist who tailored the course material toward IBD with a focus on lowering stress. An independent psychologist observed each session to assess adherence to protocol.
Not only did ACT meet the primary study endpoint, it also produced a 25% decrease in perceived stress (on a 1-10 scale) by week 8 and a 27% decrease in perceived stress by week 20 (P less than .001 versus treatment as usual). Depression scores in the ACT group also fell by 47% by week 8 and by 45% at week 20 (P = .01 versus treatment as usual). Anxiety levels decreased by 29% at week 8 and by 31% at week 20, but these improvements did not significantly differ from those in the control group (P = .39).
Interestingly, ACT did not significantly improve symptom burden, activities of daily living, disease-related worry, general well-being, C-reactive protein (CRP) levels, fecal calprotectin levels, or scores on the version used of the Clinical Assessment of Depression (CAD) or the short Mayo assessment. Hair cortisol levels showed an association with baseline stress and anxiety, but not with treatment response.
Care programs for IBD increasingly emphasize mental health services despite a lack of robust trials to support these interventions, the investigators noted. Thus, their findings highlight “the need for researchers and clinicians to further develop and optimize the content and delivery of psychological programs for IBD patients.”
Tillotts Pharma and Boston Scientific provided partial funding, but had no other role in the study. The researchers reported having no relevant conflicts of interest.
SOURCE: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
Factors that affect stress level and mood symptoms are vast when it comes to living with inflammatory bowel disease (IBD). Comorbid mood symptoms are common in patients with IBD, and psychological interventions are increasingly recommended as part of holistic, multidisciplinary treatment planning. Additionally, patients are open to GI-focused psychology treatments given the recognition that the complexities of living with IBD strongly influence emotional factors.
What must be acknowledged is the importance of long-term adherence to skills learned during the 8 weeks of ACT. Stress and mood symptoms tend to be more prevalent during times of flare. Given the relapsing and remitting nature of IBD, it must be conveyed that patients will need to continue the practice of this mindfulness-based intervention in the long term. Future studies are encouraged to look at longitudinal data assessing the manner in which these patients used their skill set during periods of flare or disease-related stress.
Factors that affect stress level and mood symptoms are vast when it comes to living with inflammatory bowel disease (IBD). Comorbid mood symptoms are common in patients with IBD, and psychological interventions are increasingly recommended as part of holistic, multidisciplinary treatment planning. Additionally, patients are open to GI-focused psychology treatments given the recognition that the complexities of living with IBD strongly influence emotional factors.
What must be acknowledged is the importance of long-term adherence to skills learned during the 8 weeks of ACT. Stress and mood symptoms tend to be more prevalent during times of flare. Given the relapsing and remitting nature of IBD, it must be conveyed that patients will need to continue the practice of this mindfulness-based intervention in the long term. Future studies are encouraged to look at longitudinal data assessing the manner in which these patients used their skill set during periods of flare or disease-related stress.
Factors that affect stress level and mood symptoms are vast when it comes to living with inflammatory bowel disease (IBD). Comorbid mood symptoms are common in patients with IBD, and psychological interventions are increasingly recommended as part of holistic, multidisciplinary treatment planning. Additionally, patients are open to GI-focused psychology treatments given the recognition that the complexities of living with IBD strongly influence emotional factors.
What must be acknowledged is the importance of long-term adherence to skills learned during the 8 weeks of ACT. Stress and mood symptoms tend to be more prevalent during times of flare. Given the relapsing and remitting nature of IBD, it must be conveyed that patients will need to continue the practice of this mindfulness-based intervention in the long term. Future studies are encouraged to look at longitudinal data assessing the manner in which these patients used their skill set during periods of flare or disease-related stress.
Eight weeks of a mindfulness intervention known as acceptance and commitment therapy (ACT) significantly improved stress and depression among patients with inflammatory bowel disease, and these improvements persisted for at least 12 weeks after therapy ended, according to the results of a randomized, controlled trial.
Source: The American Gastroenterological Association
In the intention-to-treat analysis, stress symptoms, as measured by the Depression Anxiety and Stress Scales (DASS-21), improved by 39% at week 8 and by 45% at week 20, reported Brona Wynne, PhD, of University College Dublin together with her associates. These improvements were highly significant compared with baseline and treatment as usual (P = .001 for both comparisons). “Post hoc analyses indicated that baseline stress levels were similar in control and treatment groups,” the researchers wrote in Gastroenterology. “The results of the per protocol analysis were comparable, with a 43% and 49% reduction in stress in the treatment group from baseline to 8 and 20 weeks.”
Multiple studies have documented high levels of stress and psychological dysfunction among patients with Crohn’s disease and ulcerative colitis. Studies of various mindfulness therapy, relaxation, stress management, cognitive-behavioral therapy, and hypnotherapy interventions often failed to collect key clinical data or were underpowered, uncontrolled, and unrandomized. Acceptance and commitment therapy uses mindfulness to identify adverse thoughts and experiences, accept these as part of life, and recommit to “move towards values that have been identified and adopted by the individual,” the investigators wrote. “This can be defined as the ability to contact the present moment more fully as a conscious human being and to change, or persist in, behavior when doing so serves valued ends.”
Their single-center study, which they said was the first to evaluate ACT in IBD patients, included 79 individuals with stable or mildly active Crohn’s disease (38 patients) or ulcerative colitis (41 patients) who were randomly assigned to ACT (37 patients) or control treatment as usual (42 patients). The two comparison groups were demographically and clinically similar. The ACT program involved eight 90-minute, weekly sessions of groups of 14-16 individuals, led by a single psychologist who tailored the course material toward IBD with a focus on lowering stress. An independent psychologist observed each session to assess adherence to protocol.
Not only did ACT meet the primary study endpoint, it also produced a 25% decrease in perceived stress (on a 1-10 scale) by week 8 and a 27% decrease in perceived stress by week 20 (P less than .001 versus treatment as usual). Depression scores in the ACT group also fell by 47% by week 8 and by 45% at week 20 (P = .01 versus treatment as usual). Anxiety levels decreased by 29% at week 8 and by 31% at week 20, but these improvements did not significantly differ from those in the control group (P = .39).
Interestingly, ACT did not significantly improve symptom burden, activities of daily living, disease-related worry, general well-being, C-reactive protein (CRP) levels, fecal calprotectin levels, or scores on the version used of the Clinical Assessment of Depression (CAD) or the short Mayo assessment. Hair cortisol levels showed an association with baseline stress and anxiety, but not with treatment response.
Care programs for IBD increasingly emphasize mental health services despite a lack of robust trials to support these interventions, the investigators noted. Thus, their findings highlight “the need for researchers and clinicians to further develop and optimize the content and delivery of psychological programs for IBD patients.”
Tillotts Pharma and Boston Scientific provided partial funding, but had no other role in the study. The researchers reported having no relevant conflicts of interest.
SOURCE: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
Eight weeks of a mindfulness intervention known as acceptance and commitment therapy (ACT) significantly improved stress and depression among patients with inflammatory bowel disease, and these improvements persisted for at least 12 weeks after therapy ended, according to the results of a randomized, controlled trial.
Source: The American Gastroenterological Association
In the intention-to-treat analysis, stress symptoms, as measured by the Depression Anxiety and Stress Scales (DASS-21), improved by 39% at week 8 and by 45% at week 20, reported Brona Wynne, PhD, of University College Dublin together with her associates. These improvements were highly significant compared with baseline and treatment as usual (P = .001 for both comparisons). “Post hoc analyses indicated that baseline stress levels were similar in control and treatment groups,” the researchers wrote in Gastroenterology. “The results of the per protocol analysis were comparable, with a 43% and 49% reduction in stress in the treatment group from baseline to 8 and 20 weeks.”
Multiple studies have documented high levels of stress and psychological dysfunction among patients with Crohn’s disease and ulcerative colitis. Studies of various mindfulness therapy, relaxation, stress management, cognitive-behavioral therapy, and hypnotherapy interventions often failed to collect key clinical data or were underpowered, uncontrolled, and unrandomized. Acceptance and commitment therapy uses mindfulness to identify adverse thoughts and experiences, accept these as part of life, and recommit to “move towards values that have been identified and adopted by the individual,” the investigators wrote. “This can be defined as the ability to contact the present moment more fully as a conscious human being and to change, or persist in, behavior when doing so serves valued ends.”
Their single-center study, which they said was the first to evaluate ACT in IBD patients, included 79 individuals with stable or mildly active Crohn’s disease (38 patients) or ulcerative colitis (41 patients) who were randomly assigned to ACT (37 patients) or control treatment as usual (42 patients). The two comparison groups were demographically and clinically similar. The ACT program involved eight 90-minute, weekly sessions of groups of 14-16 individuals, led by a single psychologist who tailored the course material toward IBD with a focus on lowering stress. An independent psychologist observed each session to assess adherence to protocol.
Not only did ACT meet the primary study endpoint, it also produced a 25% decrease in perceived stress (on a 1-10 scale) by week 8 and a 27% decrease in perceived stress by week 20 (P less than .001 versus treatment as usual). Depression scores in the ACT group also fell by 47% by week 8 and by 45% at week 20 (P = .01 versus treatment as usual). Anxiety levels decreased by 29% at week 8 and by 31% at week 20, but these improvements did not significantly differ from those in the control group (P = .39).
Interestingly, ACT did not significantly improve symptom burden, activities of daily living, disease-related worry, general well-being, C-reactive protein (CRP) levels, fecal calprotectin levels, or scores on the version used of the Clinical Assessment of Depression (CAD) or the short Mayo assessment. Hair cortisol levels showed an association with baseline stress and anxiety, but not with treatment response.
Care programs for IBD increasingly emphasize mental health services despite a lack of robust trials to support these interventions, the investigators noted. Thus, their findings highlight “the need for researchers and clinicians to further develop and optimize the content and delivery of psychological programs for IBD patients.”
Tillotts Pharma and Boston Scientific provided partial funding, but had no other role in the study. The researchers reported having no relevant conflicts of interest.
SOURCE: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
FROM GASTROENTEROLOGY
Key clinical point: An 8-week course of acceptance and commitment therapy improved stress and depression in patients with inflammatory bowel disease.
Major finding: Compared with controls, the intervention group experienced significant improvements in stress (P = .001) and depression (P = .01), but not anxiety.
Study details: Randomized controlled trial of 79 patients.
Disclosures: Tillotts Pharma and Boston Scientific provided partial funding but had no other role in the study. The researchers reported having no relevant conflicts of interest.
Source: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
Meta-analysis generally supports LI-RADS classification accuracy
Higher (more severe) Liver Imaging Reporting and Data System (LI-RADS) categories contained increasing proportions of hepatocellular carcinomas and overall malignancies, supporting the general reliability of the system, according to a systematic review and meta-analysis of 17 retrospective studies.
But 13% of LR-2 (“probably benign”) observations were actually hepatocellular carcinomas, as were 38% of LR-3 (“intermediate probability of malignancy”) observations, reported Christian B. van der Pol, MD, of McMaster University, Hamilton, Ont., and Christopher S. Lim, BBS, of Harvard Medical School, Boston, and their associates. Thus, clinicians should consider biopsy of many LR-3s, and LR-2s might need “more active management” than the currently recommended “return to surveillance,” including consideration for biopsy of solid LR-2 nodules measuring 1 cm or more, they wrote in Gastroenterology.
Histopathology confirmed that 93% of CT and MRI observations designated as LR-M (“definite or probable malignancy”) were indeed malignancies and that 36% were hepatocellular carcinomas,
The LI-RADS system, like its counterparts in breast and prostate imaging (BI-RADS and PI-RADS), classifies CT and MRI findings based on level of suspicion for malignancy. These categories include LR-M, LR-3, LR-2, LR-1 (“definitely benign”), LR-TIV (“definitely tumor in vein”), and LR-4 and LR-5 (“probably” and “definitely” hepatocellular carcinoma). However, CT and MRI interpretation is only as useful as it is accurate. To calculate actual percentages of hepatocellular carcinomas and overall malignancies within each LI-RADS category, the investigators analyzed aggregate data from studies found by searching MEDLINE, Embase, Cochrane CENTRAL, and Scopus during 2014-2018.
These 17 studies included 2,760 patients and 3,556 imaging observations. Pathology was the reference standard for LR-M, but for other LI-RADS categories, the researchers accepted strong clinical indicators of hepatocellular carcinoma, such as a 50% increase in lesion size within 6 months, or posttreatment recurrence of a previously confirmed malignancy. They classified observations as negative if they stayed stable in size for at least 12 months, spontaneously diminished in size, or disappeared without treatment.
In all, 94% and 97% of LR-5 observations were (respectively) hepatocellular carcinomas and other malignancies, as were 79% and 92% of LR-TIVs, 36% and 93% of LR-Ms, 74% and 80% of LR-4s, 38% and 40% of LR-3s, and 13% and 14% of LR-2s. No LR-1s were confirmed as malignant.
“Our data suggest biopsy of LI-RADS 3 observations should be considered in many patients, as a risk of 38% of HCC would usually provoke biopsy of a lesion elsewhere in the body,” the researchers wrote. They suggested consideration for biopsy of certain LR-2 lesions, but added that many “are small, perfusional alterations caused by arterioportal shunts, which are often not reported” and would be difficult or impossible to biopsy.
The study did not cover the most recent (2018) LI-RADS system, which featured several changes to simplify and better align it with American Association for the Study of Liver Diseases criteria, the researchers noted. They called for prospective studies to help confirm the accuracy of the LI-RADS system, particularly with regard to intermediate categories, such as LR-2.
The researchers disclosed no funding sources. Dr. van der Pol, Dr. Lim, and three other investigators reported having no conflicts of interest. Five researchers reported that they are members of the LI-RADS Steering Committee and four disclosed ties to pharmaceutical companies.
SOURCE: Van der Pol CB et al. Gastroenterology. 2018 Nov 13. doi: 10.1053/j.gastro.2018.11.020.
Higher (more severe) Liver Imaging Reporting and Data System (LI-RADS) categories contained increasing proportions of hepatocellular carcinomas and overall malignancies, supporting the general reliability of the system, according to a systematic review and meta-analysis of 17 retrospective studies.
But 13% of LR-2 (“probably benign”) observations were actually hepatocellular carcinomas, as were 38% of LR-3 (“intermediate probability of malignancy”) observations, reported Christian B. van der Pol, MD, of McMaster University, Hamilton, Ont., and Christopher S. Lim, BBS, of Harvard Medical School, Boston, and their associates. Thus, clinicians should consider biopsy of many LR-3s, and LR-2s might need “more active management” than the currently recommended “return to surveillance,” including consideration for biopsy of solid LR-2 nodules measuring 1 cm or more, they wrote in Gastroenterology.
Histopathology confirmed that 93% of CT and MRI observations designated as LR-M (“definite or probable malignancy”) were indeed malignancies and that 36% were hepatocellular carcinomas,
The LI-RADS system, like its counterparts in breast and prostate imaging (BI-RADS and PI-RADS), classifies CT and MRI findings based on level of suspicion for malignancy. These categories include LR-M, LR-3, LR-2, LR-1 (“definitely benign”), LR-TIV (“definitely tumor in vein”), and LR-4 and LR-5 (“probably” and “definitely” hepatocellular carcinoma). However, CT and MRI interpretation is only as useful as it is accurate. To calculate actual percentages of hepatocellular carcinomas and overall malignancies within each LI-RADS category, the investigators analyzed aggregate data from studies found by searching MEDLINE, Embase, Cochrane CENTRAL, and Scopus during 2014-2018.
These 17 studies included 2,760 patients and 3,556 imaging observations. Pathology was the reference standard for LR-M, but for other LI-RADS categories, the researchers accepted strong clinical indicators of hepatocellular carcinoma, such as a 50% increase in lesion size within 6 months, or posttreatment recurrence of a previously confirmed malignancy. They classified observations as negative if they stayed stable in size for at least 12 months, spontaneously diminished in size, or disappeared without treatment.
In all, 94% and 97% of LR-5 observations were (respectively) hepatocellular carcinomas and other malignancies, as were 79% and 92% of LR-TIVs, 36% and 93% of LR-Ms, 74% and 80% of LR-4s, 38% and 40% of LR-3s, and 13% and 14% of LR-2s. No LR-1s were confirmed as malignant.
“Our data suggest biopsy of LI-RADS 3 observations should be considered in many patients, as a risk of 38% of HCC would usually provoke biopsy of a lesion elsewhere in the body,” the researchers wrote. They suggested consideration for biopsy of certain LR-2 lesions, but added that many “are small, perfusional alterations caused by arterioportal shunts, which are often not reported” and would be difficult or impossible to biopsy.
The study did not cover the most recent (2018) LI-RADS system, which featured several changes to simplify and better align it with American Association for the Study of Liver Diseases criteria, the researchers noted. They called for prospective studies to help confirm the accuracy of the LI-RADS system, particularly with regard to intermediate categories, such as LR-2.
The researchers disclosed no funding sources. Dr. van der Pol, Dr. Lim, and three other investigators reported having no conflicts of interest. Five researchers reported that they are members of the LI-RADS Steering Committee and four disclosed ties to pharmaceutical companies.
SOURCE: Van der Pol CB et al. Gastroenterology. 2018 Nov 13. doi: 10.1053/j.gastro.2018.11.020.
Higher (more severe) Liver Imaging Reporting and Data System (LI-RADS) categories contained increasing proportions of hepatocellular carcinomas and overall malignancies, supporting the general reliability of the system, according to a systematic review and meta-analysis of 17 retrospective studies.
But 13% of LR-2 (“probably benign”) observations were actually hepatocellular carcinomas, as were 38% of LR-3 (“intermediate probability of malignancy”) observations, reported Christian B. van der Pol, MD, of McMaster University, Hamilton, Ont., and Christopher S. Lim, BBS, of Harvard Medical School, Boston, and their associates. Thus, clinicians should consider biopsy of many LR-3s, and LR-2s might need “more active management” than the currently recommended “return to surveillance,” including consideration for biopsy of solid LR-2 nodules measuring 1 cm or more, they wrote in Gastroenterology.
Histopathology confirmed that 93% of CT and MRI observations designated as LR-M (“definite or probable malignancy”) were indeed malignancies and that 36% were hepatocellular carcinomas,
The LI-RADS system, like its counterparts in breast and prostate imaging (BI-RADS and PI-RADS), classifies CT and MRI findings based on level of suspicion for malignancy. These categories include LR-M, LR-3, LR-2, LR-1 (“definitely benign”), LR-TIV (“definitely tumor in vein”), and LR-4 and LR-5 (“probably” and “definitely” hepatocellular carcinoma). However, CT and MRI interpretation is only as useful as it is accurate. To calculate actual percentages of hepatocellular carcinomas and overall malignancies within each LI-RADS category, the investigators analyzed aggregate data from studies found by searching MEDLINE, Embase, Cochrane CENTRAL, and Scopus during 2014-2018.
These 17 studies included 2,760 patients and 3,556 imaging observations. Pathology was the reference standard for LR-M, but for other LI-RADS categories, the researchers accepted strong clinical indicators of hepatocellular carcinoma, such as a 50% increase in lesion size within 6 months, or posttreatment recurrence of a previously confirmed malignancy. They classified observations as negative if they stayed stable in size for at least 12 months, spontaneously diminished in size, or disappeared without treatment.
In all, 94% and 97% of LR-5 observations were (respectively) hepatocellular carcinomas and other malignancies, as were 79% and 92% of LR-TIVs, 36% and 93% of LR-Ms, 74% and 80% of LR-4s, 38% and 40% of LR-3s, and 13% and 14% of LR-2s. No LR-1s were confirmed as malignant.
“Our data suggest biopsy of LI-RADS 3 observations should be considered in many patients, as a risk of 38% of HCC would usually provoke biopsy of a lesion elsewhere in the body,” the researchers wrote. They suggested consideration for biopsy of certain LR-2 lesions, but added that many “are small, perfusional alterations caused by arterioportal shunts, which are often not reported” and would be difficult or impossible to biopsy.
The study did not cover the most recent (2018) LI-RADS system, which featured several changes to simplify and better align it with American Association for the Study of Liver Diseases criteria, the researchers noted. They called for prospective studies to help confirm the accuracy of the LI-RADS system, particularly with regard to intermediate categories, such as LR-2.
The researchers disclosed no funding sources. Dr. van der Pol, Dr. Lim, and three other investigators reported having no conflicts of interest. Five researchers reported that they are members of the LI-RADS Steering Committee and four disclosed ties to pharmaceutical companies.
SOURCE: Van der Pol CB et al. Gastroenterology. 2018 Nov 13. doi: 10.1053/j.gastro.2018.11.020.
FROM GASTROENTEROLOGY
Key clinical point: Consider biopsy of CT/MRI observations classified as LI-RADS 3 (intermediate probability of malignancy), as well as LI-RADS 2 (probably benign) observations that are solid nodules measuring at least 1 cm.
Major finding: In all, 13% of LR-2 observations were confirmed to be hepatocellular carcinomas, as were 38% of LR-3 observations.
Study details: Systematic review and meta-analysis of 17 retrospective studies.
Disclosures: The researchers disclosed no external funding sources. Dr. van der Pol, Dr. Lim, and three other investigators reported having no conflicts of interest. Five researchers reported that they are members of the LI-RADS Steering Committee and four disclosed ties to pharmaceutical companies.
Source: Van der Pol CB et al. Gastroenterology. 2018 Nov 13. doi: 10.1053/j.gastro.2018.11.020.
Maltodextrin may increase colitis risk
The food additive maltodextrin may increase risk of inflammatory bowel disease, according to a recent study.
Compared with control subjects, mice given drinking water that contained 5% maltodextrin were significantly more likely to develop colitis and lose weight when challenged with dextran sodium sulfate (DSS), reported lead author Federica Laudisi, PhD, of the department of systems medicine at the University of Rome Tor Vergata in Rome, and her colleagues.
Further experiments with murine intestinal crypts and a human cell line echoed these results and offered mechanistic insight. Treatment with maltodextrin stressed the endoplasmic reticulum of goblet cells, predisposing the intestinal epithelium to mucus depletion and inflammation. With these results, maltodextrin joins polysorbate 80 and carboxymethylcellulose on a growing list of food additives in the Western diet with proinflammatory potential.
“Although the U.S. Food and Drug Administration recognizes these dietary elements as safe,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, “their use has been linked to the development of intestinal pathologies in both animals and human beings.
“It also has been shown that the polysaccharide maltodextrin, which is commonly used as a filler and thickener during food processing, can alter microbial phenotype and host antibacterial defenses. Maltodextrin expands the Escherichia coli population in the ileum and induces necrotizing enterocolitis in preterm piglets (Am J Physiol Gastrointest Liver Physiol. 2009 Dec;297:G1115-25).”
The present study began by administering three compounds dissolved in drinking water to wild-type Balb/c mice for 45 days: 5% maltodextrin, 0.5% propylene glycol, or 5 g/L animal gelatin. Control mice drank plain water. None of the treatments triggered clinical or histologic signs of colitis, and stool levels of lipocalin-2 (Lcn-2), a biomarker of intestinal inflammation, remained comparable with that of control mice. However, outcomes changed when mice were challenged with DSS (1.75% in drinking water) on days 35-45 or injected subcutaneously with indomethacin (5 mg/kg) on day 35 and sacrificed 24 hours later. When challenged with DSS, mice in the maltodextrin group developed severe colitis and lost 10%-15% of body weight, compared with minimal colitis and negligible weight loss in the other groups. In addition, compared with other mice, maltodextrin-fed mice had increased colon tissue expression of Lcn-2 and inflammatory cytokine interleukin (IL)-1beta. These initial findings suggested that dietary maltodextrin could increase susceptibility to clinical colitis.
To determine the pathophysiology of this phenomenon, the investigators performed microarray analysis of colonic samples. Multiple genes associated with carbohydrate and lipid metabolism were upregulated in maltodextrin-fed mice, including genes that controlled the unfolded protein response (UPR), a process in which unfolded proteins accumulate in the endoplasmic reticulum (ER) during ER stress. The most prominently expressed among the UPR-related genes was Ern-2, which regulates inositol-requiring enzyme 1beta, found exclusively in the ER of goblet cells in the small intestine and colon. When maltodextrin causes ER stress in goblet cells, it leads to misfolding of mucin glycoprotein Mucin-2 (Muc-2), a major component of gut mucus, causing gut mucus levels to drop. A diminished mucus barrier exposes the intestine to infection and damage, as demonstrated by higher rates of pathogenic bacteria in Muc-2–deficient mice than in control mice, and more severe intestinal damage than in controls when Muc-2 mice are deliberately infected with pathogens.
The investigators found that humans likely have similar responses to dietary maltodextrin. Treating the mucus-secreting HT29-methotrexate treated (HT29-MTX) cell line with 5% maltodextrin resulted in upregulation of Ern-2, which is the same mechanism observed in mice. Additional testing showed that this process was mediated by p38 mitogen-activated protein kinase, and pharmacologic inhibition or knockdown of p38 suppressed RNA expression of Ern-2. The investigators found that p38 was similarly involved in maltodextrin-fed mice.
To show that maltodextrin enhances susceptibility to inflammation via ER stress, the investigators used tauroursodeoxycholic acid (TUDCA) to inhibit ER stress. Indeed, inhibition led to reduced Ern-2 expression in HT29-MTX cells and in mice treated with maltodextrin. Giving TUDCA to maltodextrin-fed mice resulted in less weight loss, improved histology, and lower expression of Lcn-2 and IL-1beta.
The study concluded with three final experiments: The first showed that maltodextrin did not alter mucosa-associated microbiota; the second showed that mice fed 5% maltodextrin long term (for 10 weeks) had low-grade intestinal inflammation on histology, albeit without clinical colitis or weight loss; and the third showed that mice consuming maltodextrin long term had higher 15-hour fasting blood glycemic levels than control mice, supporting recent research suggesting that food additives can disrupt metabolism in a nonsusceptible host.
“In conclusion,” the investigators wrote, “this study shows that a maltodextrin-enriched diet reduces the intestinal content of Muc-2, thus making the host more sensitive to colitogenic stimuli. These data, together with the demonstration that maltodextrin can promote epithelial intestinal adhesion of pathogenic bacteria, supports the hypothesis that Western diets rich in maltodextrin can contribute to gut disease susceptibility.”
The study was funded by the Italian Ministry of Education, Universities, and Research. The authors reported no conflicts of interest.
SOURCE: Laudisi F et al. CMGH. 2019 Jan 18. doi: 10.1016/j.jcmgh.2018.09.002.
Maltodextrin is a polysaccharide derived from starch hydrolysis and broadly used as a thickener and filler in processed food. While it is regarded as inert and considered “generally regarded as safe” by the U.S. Food and Drug Administration, multiple recent studies have demonstrated detrimental roles played by maltodextrin in the intestinal environment, suggesting that this broadly used food additive may play a role in chronic inflammatory diseases.
Importantly, in addition to the use of a murine model of colitis, Laudisi and colleagues also investigated the impact that maltodextrin may have on a “normal” host, i.e. without genetic susceptibility nor induced colitis. While maltodextrin did not induce visible levels of intestinal inflammation, it led to the development of low-grade intestinal inflammation, characterized by subtle but nonetheless consistent elevation in intestinal inflammatory markers, ultimately leading to metabolic abnormalities.
Altogether, these recent results, together with previous reports, suggest that consumption of the food additive maltodextrin may be a risk factor for the IBD-prone population, as well as a factor promoting chronic low-grade intestinal inflammation leading to metabolic abnormalities in the general population. These findings further support the concept that FDA testing of food additives should be performed in disease-prone and resistant host models, designed to detect chronic and low-grade inflammation, as well as consider impacts on the gut microbiota.
Benoit Chassaing, PhD, is an assistant professor in the Neuroscience Institute and Institute for Biomedical Sciences, Georgia State University, Atlanta. He has no conflicts. These remarks are excerpted from an editorial accompanying Dr. Laudisi’s article (CMGH. 2019 Jan 18. doi.org/10.1016/j.jcmgh.2018.09.002).
Maltodextrin is a polysaccharide derived from starch hydrolysis and broadly used as a thickener and filler in processed food. While it is regarded as inert and considered “generally regarded as safe” by the U.S. Food and Drug Administration, multiple recent studies have demonstrated detrimental roles played by maltodextrin in the intestinal environment, suggesting that this broadly used food additive may play a role in chronic inflammatory diseases.
Importantly, in addition to the use of a murine model of colitis, Laudisi and colleagues also investigated the impact that maltodextrin may have on a “normal” host, i.e. without genetic susceptibility nor induced colitis. While maltodextrin did not induce visible levels of intestinal inflammation, it led to the development of low-grade intestinal inflammation, characterized by subtle but nonetheless consistent elevation in intestinal inflammatory markers, ultimately leading to metabolic abnormalities.
Altogether, these recent results, together with previous reports, suggest that consumption of the food additive maltodextrin may be a risk factor for the IBD-prone population, as well as a factor promoting chronic low-grade intestinal inflammation leading to metabolic abnormalities in the general population. These findings further support the concept that FDA testing of food additives should be performed in disease-prone and resistant host models, designed to detect chronic and low-grade inflammation, as well as consider impacts on the gut microbiota.
Benoit Chassaing, PhD, is an assistant professor in the Neuroscience Institute and Institute for Biomedical Sciences, Georgia State University, Atlanta. He has no conflicts. These remarks are excerpted from an editorial accompanying Dr. Laudisi’s article (CMGH. 2019 Jan 18. doi.org/10.1016/j.jcmgh.2018.09.002).
Maltodextrin is a polysaccharide derived from starch hydrolysis and broadly used as a thickener and filler in processed food. While it is regarded as inert and considered “generally regarded as safe” by the U.S. Food and Drug Administration, multiple recent studies have demonstrated detrimental roles played by maltodextrin in the intestinal environment, suggesting that this broadly used food additive may play a role in chronic inflammatory diseases.
Importantly, in addition to the use of a murine model of colitis, Laudisi and colleagues also investigated the impact that maltodextrin may have on a “normal” host, i.e. without genetic susceptibility nor induced colitis. While maltodextrin did not induce visible levels of intestinal inflammation, it led to the development of low-grade intestinal inflammation, characterized by subtle but nonetheless consistent elevation in intestinal inflammatory markers, ultimately leading to metabolic abnormalities.
Altogether, these recent results, together with previous reports, suggest that consumption of the food additive maltodextrin may be a risk factor for the IBD-prone population, as well as a factor promoting chronic low-grade intestinal inflammation leading to metabolic abnormalities in the general population. These findings further support the concept that FDA testing of food additives should be performed in disease-prone and resistant host models, designed to detect chronic and low-grade inflammation, as well as consider impacts on the gut microbiota.
Benoit Chassaing, PhD, is an assistant professor in the Neuroscience Institute and Institute for Biomedical Sciences, Georgia State University, Atlanta. He has no conflicts. These remarks are excerpted from an editorial accompanying Dr. Laudisi’s article (CMGH. 2019 Jan 18. doi.org/10.1016/j.jcmgh.2018.09.002).
The food additive maltodextrin may increase risk of inflammatory bowel disease, according to a recent study.
Compared with control subjects, mice given drinking water that contained 5% maltodextrin were significantly more likely to develop colitis and lose weight when challenged with dextran sodium sulfate (DSS), reported lead author Federica Laudisi, PhD, of the department of systems medicine at the University of Rome Tor Vergata in Rome, and her colleagues.
Further experiments with murine intestinal crypts and a human cell line echoed these results and offered mechanistic insight. Treatment with maltodextrin stressed the endoplasmic reticulum of goblet cells, predisposing the intestinal epithelium to mucus depletion and inflammation. With these results, maltodextrin joins polysorbate 80 and carboxymethylcellulose on a growing list of food additives in the Western diet with proinflammatory potential.
“Although the U.S. Food and Drug Administration recognizes these dietary elements as safe,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, “their use has been linked to the development of intestinal pathologies in both animals and human beings.
“It also has been shown that the polysaccharide maltodextrin, which is commonly used as a filler and thickener during food processing, can alter microbial phenotype and host antibacterial defenses. Maltodextrin expands the Escherichia coli population in the ileum and induces necrotizing enterocolitis in preterm piglets (Am J Physiol Gastrointest Liver Physiol. 2009 Dec;297:G1115-25).”
The present study began by administering three compounds dissolved in drinking water to wild-type Balb/c mice for 45 days: 5% maltodextrin, 0.5% propylene glycol, or 5 g/L animal gelatin. Control mice drank plain water. None of the treatments triggered clinical or histologic signs of colitis, and stool levels of lipocalin-2 (Lcn-2), a biomarker of intestinal inflammation, remained comparable with that of control mice. However, outcomes changed when mice were challenged with DSS (1.75% in drinking water) on days 35-45 or injected subcutaneously with indomethacin (5 mg/kg) on day 35 and sacrificed 24 hours later. When challenged with DSS, mice in the maltodextrin group developed severe colitis and lost 10%-15% of body weight, compared with minimal colitis and negligible weight loss in the other groups. In addition, compared with other mice, maltodextrin-fed mice had increased colon tissue expression of Lcn-2 and inflammatory cytokine interleukin (IL)-1beta. These initial findings suggested that dietary maltodextrin could increase susceptibility to clinical colitis.
To determine the pathophysiology of this phenomenon, the investigators performed microarray analysis of colonic samples. Multiple genes associated with carbohydrate and lipid metabolism were upregulated in maltodextrin-fed mice, including genes that controlled the unfolded protein response (UPR), a process in which unfolded proteins accumulate in the endoplasmic reticulum (ER) during ER stress. The most prominently expressed among the UPR-related genes was Ern-2, which regulates inositol-requiring enzyme 1beta, found exclusively in the ER of goblet cells in the small intestine and colon. When maltodextrin causes ER stress in goblet cells, it leads to misfolding of mucin glycoprotein Mucin-2 (Muc-2), a major component of gut mucus, causing gut mucus levels to drop. A diminished mucus barrier exposes the intestine to infection and damage, as demonstrated by higher rates of pathogenic bacteria in Muc-2–deficient mice than in control mice, and more severe intestinal damage than in controls when Muc-2 mice are deliberately infected with pathogens.
The investigators found that humans likely have similar responses to dietary maltodextrin. Treating the mucus-secreting HT29-methotrexate treated (HT29-MTX) cell line with 5% maltodextrin resulted in upregulation of Ern-2, which is the same mechanism observed in mice. Additional testing showed that this process was mediated by p38 mitogen-activated protein kinase, and pharmacologic inhibition or knockdown of p38 suppressed RNA expression of Ern-2. The investigators found that p38 was similarly involved in maltodextrin-fed mice.
To show that maltodextrin enhances susceptibility to inflammation via ER stress, the investigators used tauroursodeoxycholic acid (TUDCA) to inhibit ER stress. Indeed, inhibition led to reduced Ern-2 expression in HT29-MTX cells and in mice treated with maltodextrin. Giving TUDCA to maltodextrin-fed mice resulted in less weight loss, improved histology, and lower expression of Lcn-2 and IL-1beta.
The study concluded with three final experiments: The first showed that maltodextrin did not alter mucosa-associated microbiota; the second showed that mice fed 5% maltodextrin long term (for 10 weeks) had low-grade intestinal inflammation on histology, albeit without clinical colitis or weight loss; and the third showed that mice consuming maltodextrin long term had higher 15-hour fasting blood glycemic levels than control mice, supporting recent research suggesting that food additives can disrupt metabolism in a nonsusceptible host.
“In conclusion,” the investigators wrote, “this study shows that a maltodextrin-enriched diet reduces the intestinal content of Muc-2, thus making the host more sensitive to colitogenic stimuli. These data, together with the demonstration that maltodextrin can promote epithelial intestinal adhesion of pathogenic bacteria, supports the hypothesis that Western diets rich in maltodextrin can contribute to gut disease susceptibility.”
The study was funded by the Italian Ministry of Education, Universities, and Research. The authors reported no conflicts of interest.
SOURCE: Laudisi F et al. CMGH. 2019 Jan 18. doi: 10.1016/j.jcmgh.2018.09.002.
The food additive maltodextrin may increase risk of inflammatory bowel disease, according to a recent study.
Compared with control subjects, mice given drinking water that contained 5% maltodextrin were significantly more likely to develop colitis and lose weight when challenged with dextran sodium sulfate (DSS), reported lead author Federica Laudisi, PhD, of the department of systems medicine at the University of Rome Tor Vergata in Rome, and her colleagues.
Further experiments with murine intestinal crypts and a human cell line echoed these results and offered mechanistic insight. Treatment with maltodextrin stressed the endoplasmic reticulum of goblet cells, predisposing the intestinal epithelium to mucus depletion and inflammation. With these results, maltodextrin joins polysorbate 80 and carboxymethylcellulose on a growing list of food additives in the Western diet with proinflammatory potential.
“Although the U.S. Food and Drug Administration recognizes these dietary elements as safe,” the investigators wrote in Cellular and Molecular Gastroenterology and Hepatology, “their use has been linked to the development of intestinal pathologies in both animals and human beings.
“It also has been shown that the polysaccharide maltodextrin, which is commonly used as a filler and thickener during food processing, can alter microbial phenotype and host antibacterial defenses. Maltodextrin expands the Escherichia coli population in the ileum and induces necrotizing enterocolitis in preterm piglets (Am J Physiol Gastrointest Liver Physiol. 2009 Dec;297:G1115-25).”
The present study began by administering three compounds dissolved in drinking water to wild-type Balb/c mice for 45 days: 5% maltodextrin, 0.5% propylene glycol, or 5 g/L animal gelatin. Control mice drank plain water. None of the treatments triggered clinical or histologic signs of colitis, and stool levels of lipocalin-2 (Lcn-2), a biomarker of intestinal inflammation, remained comparable with that of control mice. However, outcomes changed when mice were challenged with DSS (1.75% in drinking water) on days 35-45 or injected subcutaneously with indomethacin (5 mg/kg) on day 35 and sacrificed 24 hours later. When challenged with DSS, mice in the maltodextrin group developed severe colitis and lost 10%-15% of body weight, compared with minimal colitis and negligible weight loss in the other groups. In addition, compared with other mice, maltodextrin-fed mice had increased colon tissue expression of Lcn-2 and inflammatory cytokine interleukin (IL)-1beta. These initial findings suggested that dietary maltodextrin could increase susceptibility to clinical colitis.
To determine the pathophysiology of this phenomenon, the investigators performed microarray analysis of colonic samples. Multiple genes associated with carbohydrate and lipid metabolism were upregulated in maltodextrin-fed mice, including genes that controlled the unfolded protein response (UPR), a process in which unfolded proteins accumulate in the endoplasmic reticulum (ER) during ER stress. The most prominently expressed among the UPR-related genes was Ern-2, which regulates inositol-requiring enzyme 1beta, found exclusively in the ER of goblet cells in the small intestine and colon. When maltodextrin causes ER stress in goblet cells, it leads to misfolding of mucin glycoprotein Mucin-2 (Muc-2), a major component of gut mucus, causing gut mucus levels to drop. A diminished mucus barrier exposes the intestine to infection and damage, as demonstrated by higher rates of pathogenic bacteria in Muc-2–deficient mice than in control mice, and more severe intestinal damage than in controls when Muc-2 mice are deliberately infected with pathogens.
The investigators found that humans likely have similar responses to dietary maltodextrin. Treating the mucus-secreting HT29-methotrexate treated (HT29-MTX) cell line with 5% maltodextrin resulted in upregulation of Ern-2, which is the same mechanism observed in mice. Additional testing showed that this process was mediated by p38 mitogen-activated protein kinase, and pharmacologic inhibition or knockdown of p38 suppressed RNA expression of Ern-2. The investigators found that p38 was similarly involved in maltodextrin-fed mice.
To show that maltodextrin enhances susceptibility to inflammation via ER stress, the investigators used tauroursodeoxycholic acid (TUDCA) to inhibit ER stress. Indeed, inhibition led to reduced Ern-2 expression in HT29-MTX cells and in mice treated with maltodextrin. Giving TUDCA to maltodextrin-fed mice resulted in less weight loss, improved histology, and lower expression of Lcn-2 and IL-1beta.
The study concluded with three final experiments: The first showed that maltodextrin did not alter mucosa-associated microbiota; the second showed that mice fed 5% maltodextrin long term (for 10 weeks) had low-grade intestinal inflammation on histology, albeit without clinical colitis or weight loss; and the third showed that mice consuming maltodextrin long term had higher 15-hour fasting blood glycemic levels than control mice, supporting recent research suggesting that food additives can disrupt metabolism in a nonsusceptible host.
“In conclusion,” the investigators wrote, “this study shows that a maltodextrin-enriched diet reduces the intestinal content of Muc-2, thus making the host more sensitive to colitogenic stimuli. These data, together with the demonstration that maltodextrin can promote epithelial intestinal adhesion of pathogenic bacteria, supports the hypothesis that Western diets rich in maltodextrin can contribute to gut disease susceptibility.”
The study was funded by the Italian Ministry of Education, Universities, and Research. The authors reported no conflicts of interest.
SOURCE: Laudisi F et al. CMGH. 2019 Jan 18. doi: 10.1016/j.jcmgh.2018.09.002.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The food additive maltodextrin may increase risk of inflammatory bowel disease.
Major finding: When challenged with dextran sulfate sodium, mice consuming maltodextrin developed colitis and lost about 10%-15% of original body weight, compared with negligible inflammation and weight loss in mice not receiving maltodextrin.
Study details: A prospective study involving in vivo experiments with wild-type Balb/c mice and in vitro experiments with murine intestinal crypts and a human intestinal cell line.
Disclosures: The study was funded by the Italian Ministry of Education, Universities, and Research. The investigators reported no conflicts of interest.
Source: Laudisi F et al. CMGH. 2019 Jan 18. doi: 10.1016/j.jcmgh.2018.09.002.
NASH: Fastest-growing cause of liver cancer in transplant candidates
Nonalcoholic steatohepatitis may soon supplant chronic hepatitis C as the leading cause of hepatocellular carcinoma among patients awaiting liver transplantation, according to the findings of a national longitudinal registry study.
The proportion of affected patients with nonalcoholic steatohepatitis (NASH) rose nearly 700% between 2002 and 2017 (P less than .0001), making NASH the only etiology to significantly rise in prevalence, reported Zobair Younossi, MD, MPH, of Inova Health System in Falls Church, Va., and his associates. Chronic hepatitis C remained the most common cause of liver cancer during the study period, but its prevalence fell by more than 10% in the last 3 years (2014-2017). These trends reflect the advent of “new, highly effective antiviral regimens” for hepatitis C, the global epidemic of obesity and metabolic syndrome, and the urgent need for effective, safe treatments for NASH, they wrote in Clinical Gastroenterology and Hepatology.
Historically, hepatocellular carcinoma is usually caused by chronic hepatitis C or B infection, but the global rise of obesity and type 2 diabetes mellitus has led to epidemic levels of NASH, a progressive form of nonalcoholic fatty liver disease that lacks useful predictive noninvasive biomarkers or safe treatments. This phenomenon, coupled with the advent of new, often-curative treatments for viral hepatitis, is making NASH a leading driver of both fibrosis and liver transplantation in the United States. To compare trends in liver cancer etiologies among transplant candidates, Dr. Younossi and his associates analyzed data on 158,347 adults who were wait-listed between 2002 and 2017 and captured by the national Scientific Registry of Transplant Recipients.
A total of 26,121 (16.5%) patients awaiting liver transplant had hepatocellular carcinoma. This proportion nearly quadrupled over the study period, from 6% to 23% (P less than .0001) and rose significantly (P less than .0001) for all liver cancer etiologies (hepatitis C and B, alcoholic liver disease, and NASH). However, the absolute rise in prevalence was far greater for NASH (1050%) than for chronic hepatitis C (more than 500%) or any other etiology.
Furthermore, while most (65%) liver cancer cases involved chronic hepatitis C, the proportion of cases involving NASH rose from 2% in 2002 to 18% in 2017 (P less than .0001). By 2017, NASH topped alcoholic liver disease, comorbid hepatitis C with alcoholic liver disease, and chronic hepatitis B as an etiology of hepatocellular carcinoma among patients listed for transplant. Conversely, by 2017, less than 50% of liver cancers were caused by hepatitis C – a more than 10% drop from 2014. Over the study period, NASH was the only etiology whose prevalence significantly increased among transplant-listed patients with hepatocellular carcinoma.
In this study, etiology of liver cancer did not seem to affect the likelihood of either death or transplantation. However, serious cardiovascular disease or late-stage cancer diagnosis might exclude many NASH patients from transplantation, the researchers wrote. “Thus, the population reported here actually may underestimate the true proportion of hepatocellular carcinoma cases related to nonalcoholic fatty liver disease and NASH in the United States. Because NASH is on a trajectory to become the most common cause of hepatocellular carcinoma in the United States, effective prevention strategies and treatment options are urgently needed for this currently underserved patient population.”
Minneapolis Medical Research Foundation is the contractor for the registry and supplied the data. Dr. Younossi reported ties to Bristol-Myers Squibb, Gilead Sciences, AbbVie, Intercept Pharmaceuticals, and GlaxoSmithKline.
SOURCE: Younossi Z et al. Clin Gastroenterol Hepatol. 2018 Jun 14. doi: 10.1016/j.cgh.2018.05.057.
Nonalcoholic steatohepatitis may soon supplant chronic hepatitis C as the leading cause of hepatocellular carcinoma among patients awaiting liver transplantation, according to the findings of a national longitudinal registry study.
The proportion of affected patients with nonalcoholic steatohepatitis (NASH) rose nearly 700% between 2002 and 2017 (P less than .0001), making NASH the only etiology to significantly rise in prevalence, reported Zobair Younossi, MD, MPH, of Inova Health System in Falls Church, Va., and his associates. Chronic hepatitis C remained the most common cause of liver cancer during the study period, but its prevalence fell by more than 10% in the last 3 years (2014-2017). These trends reflect the advent of “new, highly effective antiviral regimens” for hepatitis C, the global epidemic of obesity and metabolic syndrome, and the urgent need for effective, safe treatments for NASH, they wrote in Clinical Gastroenterology and Hepatology.
Historically, hepatocellular carcinoma is usually caused by chronic hepatitis C or B infection, but the global rise of obesity and type 2 diabetes mellitus has led to epidemic levels of NASH, a progressive form of nonalcoholic fatty liver disease that lacks useful predictive noninvasive biomarkers or safe treatments. This phenomenon, coupled with the advent of new, often-curative treatments for viral hepatitis, is making NASH a leading driver of both fibrosis and liver transplantation in the United States. To compare trends in liver cancer etiologies among transplant candidates, Dr. Younossi and his associates analyzed data on 158,347 adults who were wait-listed between 2002 and 2017 and captured by the national Scientific Registry of Transplant Recipients.
A total of 26,121 (16.5%) patients awaiting liver transplant had hepatocellular carcinoma. This proportion nearly quadrupled over the study period, from 6% to 23% (P less than .0001) and rose significantly (P less than .0001) for all liver cancer etiologies (hepatitis C and B, alcoholic liver disease, and NASH). However, the absolute rise in prevalence was far greater for NASH (1050%) than for chronic hepatitis C (more than 500%) or any other etiology.
Furthermore, while most (65%) liver cancer cases involved chronic hepatitis C, the proportion of cases involving NASH rose from 2% in 2002 to 18% in 2017 (P less than .0001). By 2017, NASH topped alcoholic liver disease, comorbid hepatitis C with alcoholic liver disease, and chronic hepatitis B as an etiology of hepatocellular carcinoma among patients listed for transplant. Conversely, by 2017, less than 50% of liver cancers were caused by hepatitis C – a more than 10% drop from 2014. Over the study period, NASH was the only etiology whose prevalence significantly increased among transplant-listed patients with hepatocellular carcinoma.
In this study, etiology of liver cancer did not seem to affect the likelihood of either death or transplantation. However, serious cardiovascular disease or late-stage cancer diagnosis might exclude many NASH patients from transplantation, the researchers wrote. “Thus, the population reported here actually may underestimate the true proportion of hepatocellular carcinoma cases related to nonalcoholic fatty liver disease and NASH in the United States. Because NASH is on a trajectory to become the most common cause of hepatocellular carcinoma in the United States, effective prevention strategies and treatment options are urgently needed for this currently underserved patient population.”
Minneapolis Medical Research Foundation is the contractor for the registry and supplied the data. Dr. Younossi reported ties to Bristol-Myers Squibb, Gilead Sciences, AbbVie, Intercept Pharmaceuticals, and GlaxoSmithKline.
SOURCE: Younossi Z et al. Clin Gastroenterol Hepatol. 2018 Jun 14. doi: 10.1016/j.cgh.2018.05.057.
Nonalcoholic steatohepatitis may soon supplant chronic hepatitis C as the leading cause of hepatocellular carcinoma among patients awaiting liver transplantation, according to the findings of a national longitudinal registry study.
The proportion of affected patients with nonalcoholic steatohepatitis (NASH) rose nearly 700% between 2002 and 2017 (P less than .0001), making NASH the only etiology to significantly rise in prevalence, reported Zobair Younossi, MD, MPH, of Inova Health System in Falls Church, Va., and his associates. Chronic hepatitis C remained the most common cause of liver cancer during the study period, but its prevalence fell by more than 10% in the last 3 years (2014-2017). These trends reflect the advent of “new, highly effective antiviral regimens” for hepatitis C, the global epidemic of obesity and metabolic syndrome, and the urgent need for effective, safe treatments for NASH, they wrote in Clinical Gastroenterology and Hepatology.
Historically, hepatocellular carcinoma is usually caused by chronic hepatitis C or B infection, but the global rise of obesity and type 2 diabetes mellitus has led to epidemic levels of NASH, a progressive form of nonalcoholic fatty liver disease that lacks useful predictive noninvasive biomarkers or safe treatments. This phenomenon, coupled with the advent of new, often-curative treatments for viral hepatitis, is making NASH a leading driver of both fibrosis and liver transplantation in the United States. To compare trends in liver cancer etiologies among transplant candidates, Dr. Younossi and his associates analyzed data on 158,347 adults who were wait-listed between 2002 and 2017 and captured by the national Scientific Registry of Transplant Recipients.
A total of 26,121 (16.5%) patients awaiting liver transplant had hepatocellular carcinoma. This proportion nearly quadrupled over the study period, from 6% to 23% (P less than .0001) and rose significantly (P less than .0001) for all liver cancer etiologies (hepatitis C and B, alcoholic liver disease, and NASH). However, the absolute rise in prevalence was far greater for NASH (1050%) than for chronic hepatitis C (more than 500%) or any other etiology.
Furthermore, while most (65%) liver cancer cases involved chronic hepatitis C, the proportion of cases involving NASH rose from 2% in 2002 to 18% in 2017 (P less than .0001). By 2017, NASH topped alcoholic liver disease, comorbid hepatitis C with alcoholic liver disease, and chronic hepatitis B as an etiology of hepatocellular carcinoma among patients listed for transplant. Conversely, by 2017, less than 50% of liver cancers were caused by hepatitis C – a more than 10% drop from 2014. Over the study period, NASH was the only etiology whose prevalence significantly increased among transplant-listed patients with hepatocellular carcinoma.
In this study, etiology of liver cancer did not seem to affect the likelihood of either death or transplantation. However, serious cardiovascular disease or late-stage cancer diagnosis might exclude many NASH patients from transplantation, the researchers wrote. “Thus, the population reported here actually may underestimate the true proportion of hepatocellular carcinoma cases related to nonalcoholic fatty liver disease and NASH in the United States. Because NASH is on a trajectory to become the most common cause of hepatocellular carcinoma in the United States, effective prevention strategies and treatment options are urgently needed for this currently underserved patient population.”
Minneapolis Medical Research Foundation is the contractor for the registry and supplied the data. Dr. Younossi reported ties to Bristol-Myers Squibb, Gilead Sciences, AbbVie, Intercept Pharmaceuticals, and GlaxoSmithKline.
SOURCE: Younossi Z et al. Clin Gastroenterol Hepatol. 2018 Jun 14. doi: 10.1016/j.cgh.2018.05.057.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Nonalcoholic steatohepatitis may soon become the leading cause of hepatocellular carcinoma among patients awaiting liver transplantation.
Major finding: The proportion of these patients with NASH rose nearly 700% between 2002 and 2017 (P less than .0001).
Study details: A longitudinal registry study of 26,121 patients listed for liver transplantation in the United States.
Disclosures: Minneapolis Medical Research Foundation is the contractor for the registry and supplied the data. Dr. Younossi reported ties to Bristol-Myers Squibb, Gilead Sciences, AbbVie, Intercept Pharmaceuticals, and GlaxoSmithKline.
Source: Younossi Z et al. Clin Gastroenterol Hepatol. 2018 Jun 14. doi: 10.1016/j.cgh.2018.05.057.
Long-term budesonide oral suspension well tolerated in EoE
Treatment with budesonide oral suspension (BOS) was generally well tolerated and maintained a histologic response in some patients with eosinophilic esophagitis (EoE), according to the results of the 24-week, open-label extension phase of a multicenter, randomized, placebo-controlled, industry-sponsored trial.
Rates of histologic response (up to 6 eosinophils per high-power field) were “modest” – 23% among patients who stayed on BOS throughout the study and 48.5% among patients who initiated BOS after 12 weeks on placebo, reported Evan S. Dellon, MD, MPH, of the University of North Carolina in Chapel Hill and his associates. However, these rates “need to be viewed in the context of a highly symptomatic and histologically severe population with eosinophilic esophagitis,” they contended. A total of 11% of budesonide initiators developed esophageal candidiasis, they reported in Clinical Gastroenterology and Hepatology.
Budesonide oral suspension is a mucoadherent formulation of topical corticosteroid that has recently been developed to treat EoE. Previously, during the randomized, double-blind component of this phase 2 trial, 93 patients aged 11-40 years with active EoE and dysphagia received either BOS (2 mg) or placebo twice daily (Gastroenterology. 2017 Mar;157[4]:776-86). After 12 weeks, rates of histologic response were 39% for BOS versus 3% for placebo, and BOS significantly improved patients’ mean peak eosinophil count and scores on the Dysphagia Symptom Questionnaire, compared with baseline and compared with the response in the placebo group. During the open-label extension phase, 45 BOS continuers and 37 BOS initiators received 2 mg once daily for 12 weeks and then had the option to increase the BOS dose to 1.5-2.0 mg twice daily.
The rate of drug-related adverse events was 19% among BOS initiators and 4% among BOS continuers. One patient in each group developed oral candidiasis, while four BOS initiators (11%) developed esophageal candidiasis. Three BOS continuers had subnormal morning cortisol levels; while these were subclinical cases, they merit attention since long-term corticosteroids for EoE have been linked with possible hypothalamic–pituitary–adrenal axis suppression, the researchers noted.
In addition, while BOS initiators tended to maintain their endoscopic response, only 42% of those with an initial histologic response maintained a histologic response after 36 weeks of treatment or when leaving the study. Post hoc analyses confirmed that prolonged BOS treatment does not increase the chances of histologic or endoscopic response. Prior studies have suggested that EoE can become steroid-refractory over time and that certain molecular and histologic markers might predict resistance, the investigators noted.
Meritage Pharma (now part of Shire) was involved in the study design and conduct, data collection and management, and manuscript review. Dr. Dellon disclosed research funding from Meritage and Shire and a consulting relationship with Shire, along with ties to several other pharmaceutical companies. All six coinvestigators also disclosed ties to Meritage, Shire, or both, and two are Shire employees and stockholders.
*This story was updated on Feb. 7, 2019.
SOURCE: Dellon ES et al. Clin Gastroenterol Hepatol. 2018 Jun 11. doi: 10.1016/j.cgh.2018.05.051.
Guidelines regarding the management of eosinophilic esophagitis (EoE) with topical steroids are still unclear with regard to dosing and duration. Here, Dellon et al. present evidence that long-term budesonide oral suspension (BOS) therapy is safe and efficacious. Both the BOS and placebo cohorts of the initial, 12-week trial demonstrated clinical and histologic improvement on BOS over this 24-week period, with few adverse events. Maintenance of histologic response was only seen in 42% in initial BOS responders, suggesting steroid tolerance or resistance may develop. Another important observation was that peak eosinphil count decreased steroid dosing.
Finally, these data support the notion that initial responders are unlikely to gain response with continued therapy and may be better served with early transition to alternatives. Further research is needed to clarify these issues and which patients may be predisposed to nonresponse or loss of response.
Reena V. Chokshi, MD , is assistant professor of medicine in the department of gastroenterology at Baylor College of Medicine, Houston.
Guidelines regarding the management of eosinophilic esophagitis (EoE) with topical steroids are still unclear with regard to dosing and duration. Here, Dellon et al. present evidence that long-term budesonide oral suspension (BOS) therapy is safe and efficacious. Both the BOS and placebo cohorts of the initial, 12-week trial demonstrated clinical and histologic improvement on BOS over this 24-week period, with few adverse events. Maintenance of histologic response was only seen in 42% in initial BOS responders, suggesting steroid tolerance or resistance may develop. Another important observation was that peak eosinphil count decreased steroid dosing.
Finally, these data support the notion that initial responders are unlikely to gain response with continued therapy and may be better served with early transition to alternatives. Further research is needed to clarify these issues and which patients may be predisposed to nonresponse or loss of response.
Reena V. Chokshi, MD , is assistant professor of medicine in the department of gastroenterology at Baylor College of Medicine, Houston.
Guidelines regarding the management of eosinophilic esophagitis (EoE) with topical steroids are still unclear with regard to dosing and duration. Here, Dellon et al. present evidence that long-term budesonide oral suspension (BOS) therapy is safe and efficacious. Both the BOS and placebo cohorts of the initial, 12-week trial demonstrated clinical and histologic improvement on BOS over this 24-week period, with few adverse events. Maintenance of histologic response was only seen in 42% in initial BOS responders, suggesting steroid tolerance or resistance may develop. Another important observation was that peak eosinphil count decreased steroid dosing.
Finally, these data support the notion that initial responders are unlikely to gain response with continued therapy and may be better served with early transition to alternatives. Further research is needed to clarify these issues and which patients may be predisposed to nonresponse or loss of response.
Reena V. Chokshi, MD , is assistant professor of medicine in the department of gastroenterology at Baylor College of Medicine, Houston.
Treatment with budesonide oral suspension (BOS) was generally well tolerated and maintained a histologic response in some patients with eosinophilic esophagitis (EoE), according to the results of the 24-week, open-label extension phase of a multicenter, randomized, placebo-controlled, industry-sponsored trial.
Rates of histologic response (up to 6 eosinophils per high-power field) were “modest” – 23% among patients who stayed on BOS throughout the study and 48.5% among patients who initiated BOS after 12 weeks on placebo, reported Evan S. Dellon, MD, MPH, of the University of North Carolina in Chapel Hill and his associates. However, these rates “need to be viewed in the context of a highly symptomatic and histologically severe population with eosinophilic esophagitis,” they contended. A total of 11% of budesonide initiators developed esophageal candidiasis, they reported in Clinical Gastroenterology and Hepatology.
Budesonide oral suspension is a mucoadherent formulation of topical corticosteroid that has recently been developed to treat EoE. Previously, during the randomized, double-blind component of this phase 2 trial, 93 patients aged 11-40 years with active EoE and dysphagia received either BOS (2 mg) or placebo twice daily (Gastroenterology. 2017 Mar;157[4]:776-86). After 12 weeks, rates of histologic response were 39% for BOS versus 3% for placebo, and BOS significantly improved patients’ mean peak eosinophil count and scores on the Dysphagia Symptom Questionnaire, compared with baseline and compared with the response in the placebo group. During the open-label extension phase, 45 BOS continuers and 37 BOS initiators received 2 mg once daily for 12 weeks and then had the option to increase the BOS dose to 1.5-2.0 mg twice daily.
The rate of drug-related adverse events was 19% among BOS initiators and 4% among BOS continuers. One patient in each group developed oral candidiasis, while four BOS initiators (11%) developed esophageal candidiasis. Three BOS continuers had subnormal morning cortisol levels; while these were subclinical cases, they merit attention since long-term corticosteroids for EoE have been linked with possible hypothalamic–pituitary–adrenal axis suppression, the researchers noted.
In addition, while BOS initiators tended to maintain their endoscopic response, only 42% of those with an initial histologic response maintained a histologic response after 36 weeks of treatment or when leaving the study. Post hoc analyses confirmed that prolonged BOS treatment does not increase the chances of histologic or endoscopic response. Prior studies have suggested that EoE can become steroid-refractory over time and that certain molecular and histologic markers might predict resistance, the investigators noted.
Meritage Pharma (now part of Shire) was involved in the study design and conduct, data collection and management, and manuscript review. Dr. Dellon disclosed research funding from Meritage and Shire and a consulting relationship with Shire, along with ties to several other pharmaceutical companies. All six coinvestigators also disclosed ties to Meritage, Shire, or both, and two are Shire employees and stockholders.
*This story was updated on Feb. 7, 2019.
SOURCE: Dellon ES et al. Clin Gastroenterol Hepatol. 2018 Jun 11. doi: 10.1016/j.cgh.2018.05.051.
Treatment with budesonide oral suspension (BOS) was generally well tolerated and maintained a histologic response in some patients with eosinophilic esophagitis (EoE), according to the results of the 24-week, open-label extension phase of a multicenter, randomized, placebo-controlled, industry-sponsored trial.
Rates of histologic response (up to 6 eosinophils per high-power field) were “modest” – 23% among patients who stayed on BOS throughout the study and 48.5% among patients who initiated BOS after 12 weeks on placebo, reported Evan S. Dellon, MD, MPH, of the University of North Carolina in Chapel Hill and his associates. However, these rates “need to be viewed in the context of a highly symptomatic and histologically severe population with eosinophilic esophagitis,” they contended. A total of 11% of budesonide initiators developed esophageal candidiasis, they reported in Clinical Gastroenterology and Hepatology.
Budesonide oral suspension is a mucoadherent formulation of topical corticosteroid that has recently been developed to treat EoE. Previously, during the randomized, double-blind component of this phase 2 trial, 93 patients aged 11-40 years with active EoE and dysphagia received either BOS (2 mg) or placebo twice daily (Gastroenterology. 2017 Mar;157[4]:776-86). After 12 weeks, rates of histologic response were 39% for BOS versus 3% for placebo, and BOS significantly improved patients’ mean peak eosinophil count and scores on the Dysphagia Symptom Questionnaire, compared with baseline and compared with the response in the placebo group. During the open-label extension phase, 45 BOS continuers and 37 BOS initiators received 2 mg once daily for 12 weeks and then had the option to increase the BOS dose to 1.5-2.0 mg twice daily.
The rate of drug-related adverse events was 19% among BOS initiators and 4% among BOS continuers. One patient in each group developed oral candidiasis, while four BOS initiators (11%) developed esophageal candidiasis. Three BOS continuers had subnormal morning cortisol levels; while these were subclinical cases, they merit attention since long-term corticosteroids for EoE have been linked with possible hypothalamic–pituitary–adrenal axis suppression, the researchers noted.
In addition, while BOS initiators tended to maintain their endoscopic response, only 42% of those with an initial histologic response maintained a histologic response after 36 weeks of treatment or when leaving the study. Post hoc analyses confirmed that prolonged BOS treatment does not increase the chances of histologic or endoscopic response. Prior studies have suggested that EoE can become steroid-refractory over time and that certain molecular and histologic markers might predict resistance, the investigators noted.
Meritage Pharma (now part of Shire) was involved in the study design and conduct, data collection and management, and manuscript review. Dr. Dellon disclosed research funding from Meritage and Shire and a consulting relationship with Shire, along with ties to several other pharmaceutical companies. All six coinvestigators also disclosed ties to Meritage, Shire, or both, and two are Shire employees and stockholders.
*This story was updated on Feb. 7, 2019.
SOURCE: Dellon ES et al. Clin Gastroenterol Hepatol. 2018 Jun 11. doi: 10.1016/j.cgh.2018.05.051.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Budesonide oral suspension was well tolerated and maintained a histologic response in some patients with eosinophilic esophagitis.
Major finding: A total of 42% of initial histologic responders maintained a histologic response (less than 6 eosinophils per high-power field) after 24 weeks. Treatment was generally well tolerated, but 11% of initiators developed esophageal candidiasis.
Study details: Open-label extension study of a 12-week, multicenter, randomized, double-blind, placebo-controlled trial.
Disclosures: Meritage Pharma (now part of Shire) was involved in the study design and conduct, data collection and management, and manuscript review. Dr. Dellon disclosed research funding from Meritage and Shire and a consulting relationship with Shire, along with ties to several other pharmaceutical companies. All six coinvestigators also disclosed ties to Meritage, Shire, or both, and two are Shire employees and stockholders.
Source: Dellon ES et al. Clin Gastroenterol Hepatol. 2018 Jun 11. https://doi.org/10.1016/j.cgh.2018.05.051
AGA Clinical Practice Update: Functional gastrointestinal symptoms in patients with inflammatory bowel disease
When patients with inflammatory bowel disease report persistent gastrointestinal symptoms, clinicians should perform a thorough clinical assessment and then take a stepwise approach to rule out ongoing inflammation, according to a clinical practice update from the American Gastroenterological Association.
A fecal calprotectin test can be useful because values under 50 mcg/mL may suggest endoscopic remission, which may, in turn, point to another etiology of gastrointestinal symptoms, wrote Jean-Frederic Colombel, MD, of the Icahn School of Medicine at Mount Sinai, New York, together with his associates in Clinical Gastroenterology and Hepatology.
However, a result between 50 and 250 mcg/mL is harder to interpret because the upper limit of normal varies and mild increases can occur secondary to nonspecific low-grade inflammation, according to the experts. For mild gastrointestinal symptoms, they suggested testing fecal calprotectin every 3-6 months to identify flares as early as possible. If a flare is suspected, they advised considering cross-sectional imaging or endoscopy with biopsy.
Imaging also is indicated for patients with obstructive symptoms such as abdominal pain, obstipation, or constipation, the practice update states. Such symptoms can indicate fecal stasis proximal to distal colitis in patients with ulcerative colitis, or intestinal stenosis in patients with Crohn’s disease.
Other pathophysiologies of gastrointestinal symptoms also should be considered based on constellations of symptoms. For example, steatorrhea with chronic abdominal pain may indicate pancreatic exocrine insufficiency, which fecal elastase testing can help confirm. Symptoms of diarrhea-predominant irritable bowel syndrome can result from bile acid diarrhea, for which several screening tests are available. Diarrhea, abdominal pain, and bloating may indicate carbohydrate malabsorption or small-intestinal bacterial overgrowth, which can be evaluated with breath testing.
If patients with inflammatory bowel disease have persistent gastrointestinal symptoms but lack objective evidence of ongoing inflammation or another etiology, then clinicians should increase their suspicion of an overlapping functional gastrointestinal disorder. These conditions actually “share many common pathophysiologic disturbances that, in some inflammatory bowel disease patients, may be a consequence of prior structural and functional bowel damage,” the experts wrote.
For patients with chronic constipation who do not have an underlying obstruction, they suggest osmotic or stimulant laxatives. For chronic diarrhea, they recommend hypomobility agents or bile-acid sequestrants. Patients with fecal symptoms of irritable bowel syndrome also should be evaluated for pelvic floor disorders, which may improve with biofeedback therapy, the experts state.
A low-FODMAP diet (a diet low in lactose, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) also can improve symptoms of irritable bowel syndrome, including patients with inflammatory bowel disease. However, a dietitian always should deliver this restrictive diet because patients with inflammatory bowel disease already are at increased risk for undernutrition.
Patients with functional gastrointestinal pain may benefit from antispasmodics, neuropathic-directed agents, and antidepressants, but they should not receive opiates, the experts emphasized. Anxiety and depression are common in both inflammatory bowel disease and irritable bowel syndrome, and patients may benefit from psychotherapy (cognitive-behavioral therapy, hypnotherapy, and mindfulness therapy), antidepressants, anxiolytics, or combinations of these treatments. The practice update also recommends physical exercise, which has been shown to decrease the risk of recurrent active disease in the setting of inflammatory bowel disease.
Finally, persistent gut symptoms can indicate intestinal barrier dysfunction, even if endoscopy shows mucosal healing. Barrier dysfunction is a potential therapeutic target that needs further study in this setting, the experts noted. They also called for studies of the potential benefits and risks of probiotics and other alternative approaches, such as herbal treatments and supplements, yoga, acupuncture, and moxibustion. Until further evidence, however, they have recommended against complementary or alternative medicine or fecal microbiota transplantation.
Dr. Colombel has served as consultant, advisory board member, or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, and many other pharmaceutical companies. He has received research grants from AbbVie, Takeda, and Janssen and Janssen.
SOURCE: Colombel J-F et al. Clin Gastroenterol Hepatol. 2018 Aug 9. doi: 10.1016/j.cgh.2018.08.001.
When patients with inflammatory bowel disease report persistent gastrointestinal symptoms, clinicians should perform a thorough clinical assessment and then take a stepwise approach to rule out ongoing inflammation, according to a clinical practice update from the American Gastroenterological Association.
A fecal calprotectin test can be useful because values under 50 mcg/mL may suggest endoscopic remission, which may, in turn, point to another etiology of gastrointestinal symptoms, wrote Jean-Frederic Colombel, MD, of the Icahn School of Medicine at Mount Sinai, New York, together with his associates in Clinical Gastroenterology and Hepatology.
However, a result between 50 and 250 mcg/mL is harder to interpret because the upper limit of normal varies and mild increases can occur secondary to nonspecific low-grade inflammation, according to the experts. For mild gastrointestinal symptoms, they suggested testing fecal calprotectin every 3-6 months to identify flares as early as possible. If a flare is suspected, they advised considering cross-sectional imaging or endoscopy with biopsy.
Imaging also is indicated for patients with obstructive symptoms such as abdominal pain, obstipation, or constipation, the practice update states. Such symptoms can indicate fecal stasis proximal to distal colitis in patients with ulcerative colitis, or intestinal stenosis in patients with Crohn’s disease.
Other pathophysiologies of gastrointestinal symptoms also should be considered based on constellations of symptoms. For example, steatorrhea with chronic abdominal pain may indicate pancreatic exocrine insufficiency, which fecal elastase testing can help confirm. Symptoms of diarrhea-predominant irritable bowel syndrome can result from bile acid diarrhea, for which several screening tests are available. Diarrhea, abdominal pain, and bloating may indicate carbohydrate malabsorption or small-intestinal bacterial overgrowth, which can be evaluated with breath testing.
If patients with inflammatory bowel disease have persistent gastrointestinal symptoms but lack objective evidence of ongoing inflammation or another etiology, then clinicians should increase their suspicion of an overlapping functional gastrointestinal disorder. These conditions actually “share many common pathophysiologic disturbances that, in some inflammatory bowel disease patients, may be a consequence of prior structural and functional bowel damage,” the experts wrote.
For patients with chronic constipation who do not have an underlying obstruction, they suggest osmotic or stimulant laxatives. For chronic diarrhea, they recommend hypomobility agents or bile-acid sequestrants. Patients with fecal symptoms of irritable bowel syndrome also should be evaluated for pelvic floor disorders, which may improve with biofeedback therapy, the experts state.
A low-FODMAP diet (a diet low in lactose, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) also can improve symptoms of irritable bowel syndrome, including patients with inflammatory bowel disease. However, a dietitian always should deliver this restrictive diet because patients with inflammatory bowel disease already are at increased risk for undernutrition.
Patients with functional gastrointestinal pain may benefit from antispasmodics, neuropathic-directed agents, and antidepressants, but they should not receive opiates, the experts emphasized. Anxiety and depression are common in both inflammatory bowel disease and irritable bowel syndrome, and patients may benefit from psychotherapy (cognitive-behavioral therapy, hypnotherapy, and mindfulness therapy), antidepressants, anxiolytics, or combinations of these treatments. The practice update also recommends physical exercise, which has been shown to decrease the risk of recurrent active disease in the setting of inflammatory bowel disease.
Finally, persistent gut symptoms can indicate intestinal barrier dysfunction, even if endoscopy shows mucosal healing. Barrier dysfunction is a potential therapeutic target that needs further study in this setting, the experts noted. They also called for studies of the potential benefits and risks of probiotics and other alternative approaches, such as herbal treatments and supplements, yoga, acupuncture, and moxibustion. Until further evidence, however, they have recommended against complementary or alternative medicine or fecal microbiota transplantation.
Dr. Colombel has served as consultant, advisory board member, or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, and many other pharmaceutical companies. He has received research grants from AbbVie, Takeda, and Janssen and Janssen.
SOURCE: Colombel J-F et al. Clin Gastroenterol Hepatol. 2018 Aug 9. doi: 10.1016/j.cgh.2018.08.001.
When patients with inflammatory bowel disease report persistent gastrointestinal symptoms, clinicians should perform a thorough clinical assessment and then take a stepwise approach to rule out ongoing inflammation, according to a clinical practice update from the American Gastroenterological Association.
A fecal calprotectin test can be useful because values under 50 mcg/mL may suggest endoscopic remission, which may, in turn, point to another etiology of gastrointestinal symptoms, wrote Jean-Frederic Colombel, MD, of the Icahn School of Medicine at Mount Sinai, New York, together with his associates in Clinical Gastroenterology and Hepatology.
However, a result between 50 and 250 mcg/mL is harder to interpret because the upper limit of normal varies and mild increases can occur secondary to nonspecific low-grade inflammation, according to the experts. For mild gastrointestinal symptoms, they suggested testing fecal calprotectin every 3-6 months to identify flares as early as possible. If a flare is suspected, they advised considering cross-sectional imaging or endoscopy with biopsy.
Imaging also is indicated for patients with obstructive symptoms such as abdominal pain, obstipation, or constipation, the practice update states. Such symptoms can indicate fecal stasis proximal to distal colitis in patients with ulcerative colitis, or intestinal stenosis in patients with Crohn’s disease.
Other pathophysiologies of gastrointestinal symptoms also should be considered based on constellations of symptoms. For example, steatorrhea with chronic abdominal pain may indicate pancreatic exocrine insufficiency, which fecal elastase testing can help confirm. Symptoms of diarrhea-predominant irritable bowel syndrome can result from bile acid diarrhea, for which several screening tests are available. Diarrhea, abdominal pain, and bloating may indicate carbohydrate malabsorption or small-intestinal bacterial overgrowth, which can be evaluated with breath testing.
If patients with inflammatory bowel disease have persistent gastrointestinal symptoms but lack objective evidence of ongoing inflammation or another etiology, then clinicians should increase their suspicion of an overlapping functional gastrointestinal disorder. These conditions actually “share many common pathophysiologic disturbances that, in some inflammatory bowel disease patients, may be a consequence of prior structural and functional bowel damage,” the experts wrote.
For patients with chronic constipation who do not have an underlying obstruction, they suggest osmotic or stimulant laxatives. For chronic diarrhea, they recommend hypomobility agents or bile-acid sequestrants. Patients with fecal symptoms of irritable bowel syndrome also should be evaluated for pelvic floor disorders, which may improve with biofeedback therapy, the experts state.
A low-FODMAP diet (a diet low in lactose, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols) also can improve symptoms of irritable bowel syndrome, including patients with inflammatory bowel disease. However, a dietitian always should deliver this restrictive diet because patients with inflammatory bowel disease already are at increased risk for undernutrition.
Patients with functional gastrointestinal pain may benefit from antispasmodics, neuropathic-directed agents, and antidepressants, but they should not receive opiates, the experts emphasized. Anxiety and depression are common in both inflammatory bowel disease and irritable bowel syndrome, and patients may benefit from psychotherapy (cognitive-behavioral therapy, hypnotherapy, and mindfulness therapy), antidepressants, anxiolytics, or combinations of these treatments. The practice update also recommends physical exercise, which has been shown to decrease the risk of recurrent active disease in the setting of inflammatory bowel disease.
Finally, persistent gut symptoms can indicate intestinal barrier dysfunction, even if endoscopy shows mucosal healing. Barrier dysfunction is a potential therapeutic target that needs further study in this setting, the experts noted. They also called for studies of the potential benefits and risks of probiotics and other alternative approaches, such as herbal treatments and supplements, yoga, acupuncture, and moxibustion. Until further evidence, however, they have recommended against complementary or alternative medicine or fecal microbiota transplantation.
Dr. Colombel has served as consultant, advisory board member, or speaker for AbbVie, Amgen, Boehringer-Ingelheim, Celgene Corporation, and many other pharmaceutical companies. He has received research grants from AbbVie, Takeda, and Janssen and Janssen.
SOURCE: Colombel J-F et al. Clin Gastroenterol Hepatol. 2018 Aug 9. doi: 10.1016/j.cgh.2018.08.001.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
AGA Guideline: Treatment of mild to moderate ulcerative colitis
For patients with extensive mild to moderate ulcerative colitis, numerous randomized controlled trials support the use of either standard-dose mesalamine (2-3 grams per day) or diazo-bonded 5-aminosalicylic acid (ASA) instead of low-dose mesalamine, sulfasalazine, or no therapy, state new guidelines from the American Gastroenterological Association, published in Gastroenterology.
Sulfasalazine (2-4 grams per day) is less likely to be tolerated but remains a “reasonable option” for remitted patients who are already on it and for patients with prominent arthritis symptoms, especially if alternative treatments are cost prohibitive, wrote Cynthia W. Ko, MD, MS, of the University of Washington, Seattle, and her associates.
According to the guideline, patients with mild to moderate ulcerative colitis have less than four to six bowel movements per day, only mild or moderate rectal bleeding, no constitutional symptoms, and no high overall inflammatory burden or signs of high inflammatory activity on the Mayo Clinic score and Truelove and Witt’s criteria. These patients usually do not require colectomy, but this outcome is more likely when patients are diagnosed before age 40 years or have extensive disease or deep ulcers, extraintestinal manifestations, or elevated inflammatory markers. These higher-risk patients need more aggressive initial treatment and faster treatment intensification in cases of inadequate response, the guideline emphasizes. Even for cases of mild to moderate ulcerative colitis, treatment intensification is preferable to repeated courses of corticosteroids.
The guideline recommends adding rectal mesalamine to oral 5-ASA if patients have extensive or left-sided mild to moderate ulcerative colitis. In randomized controlled trials, this combination was significantly more likely to induce and maintain remission than was standard-dose oral mesalamine monotherapy, the authors noted. “In the maintenance trials, enemas were used twice per week or for 1 week per month. Both oral and topical mesalamine were well tolerated.”
For patients with moderate disease activity or a suboptimal response to standard-dose mesalamine or diazo-bonded 5-ASA, the guideline recommends adding rectal mesalamine to high-dose oral mesalamine (more than 3 grams daily). Combination therapy maximizes the delivery of mesalamine to the affected area of the colon, which optimizes the trial of 5-ASA before opting for treatment escalation, the authors noted. They recommend once-daily oral mesalamine dosing, since this is easier to adhere to and studies have found no benefit of more frequent dosing.
For inducing remission of mild to moderate ulcerative colitis, the guideline recommends standard-dose oral mesalamine or diazo-bonded 5-ASA over budesonide. “Overall, the budesonide preparations are not superior to mesalamine for induction of remission,” the authors wrote. Oral 5-ASAs are preferred, especially given the absence of data on the efficacy or safety of maintenance budesonide therapy.
For patients with mild to moderate ulcerative proctosigmoiditis or proctitis, the guideline conditionally recommends rectal mesalamine over oral mesalamine. Compared with placebo, rectal mesalamine suppositories were significantly more likely to induce remission in randomized trials of patients with mild to moderate ulcerative proctitis. If these patients cannot tolerate or are refractory to mesalamine suppositories, low-quality evidence supports rectal steroid therapy over no treatment, the guideline states. For patients with mild to moderate ulcerative proctosigmoiditis, moderate-quality evidence supports mesalamine enemas over rectal corticosteroids. If these patients want to avoid the difficulties of enemas, the guideline considers rectal corticosteroid foam a reasonable alternative.
Likewise, they cite low-quality evidence for adding oral prednisone or budesonide MMX to 5-ASA if patients are refractory to optimized 5-ASA therapy. No trials have directly compared rates of remission with budesonide MMX versus systemic corticosteroids. In just one placebo-controlled trial, adding budesonide MMX to 5-ASA slightly improved the chances of remission (risk ratio, 0.95; 95% confidence interval, 0.89-1.00). Furthermore, studies of other second-generation corticosteroids found they were better tolerated but no more likely to induce remission than oral prednisone or prednisolone.
Some patients with mild to moderate colitis respond inadequately to these recommended therapies and need systemic corticosteroids, immunomodulators, or biologic therapies to induce and maintain remission, the guideline authors noted. They make no recommendation on immunomodulators or biologics. Studies of probiotics, curcumin, and fecal microbiota transplantation are “urgently needed,” but for now, their use “risks delaying proven effective therapy, with the potential for worsening symptoms or complications,” they wrote. For patients without Clostridium difficile infections, they recommend against fecal microbiota transplantation except in the setting of a clinical trial.
The experts also noted the need for a tool to stratify patients with mild to moderate ulcerative colitis based on their risk of future progression and colectomy.
Finally, they call for studies on who will benefit most from high-dose mesalamine or topical mesalamine and on the relative safety and efficacy of budesonide and systemic corticosteroids in the event of an inadequate response to 5-ASAs.
All members were required to complete the disclosure statement. These statements are maintained at the American Gastroenterological Association headquarters in Bethesda, Maryland, and pertinent disclosures of conflict of interest are published with this report.
SOURCE: Crocket SD et al. Gastro 2019;156(2). doi: org/10.1053/j.gastro.2018.12.009.
For patients with extensive mild to moderate ulcerative colitis, numerous randomized controlled trials support the use of either standard-dose mesalamine (2-3 grams per day) or diazo-bonded 5-aminosalicylic acid (ASA) instead of low-dose mesalamine, sulfasalazine, or no therapy, state new guidelines from the American Gastroenterological Association, published in Gastroenterology.
Sulfasalazine (2-4 grams per day) is less likely to be tolerated but remains a “reasonable option” for remitted patients who are already on it and for patients with prominent arthritis symptoms, especially if alternative treatments are cost prohibitive, wrote Cynthia W. Ko, MD, MS, of the University of Washington, Seattle, and her associates.
According to the guideline, patients with mild to moderate ulcerative colitis have less than four to six bowel movements per day, only mild or moderate rectal bleeding, no constitutional symptoms, and no high overall inflammatory burden or signs of high inflammatory activity on the Mayo Clinic score and Truelove and Witt’s criteria. These patients usually do not require colectomy, but this outcome is more likely when patients are diagnosed before age 40 years or have extensive disease or deep ulcers, extraintestinal manifestations, or elevated inflammatory markers. These higher-risk patients need more aggressive initial treatment and faster treatment intensification in cases of inadequate response, the guideline emphasizes. Even for cases of mild to moderate ulcerative colitis, treatment intensification is preferable to repeated courses of corticosteroids.
The guideline recommends adding rectal mesalamine to oral 5-ASA if patients have extensive or left-sided mild to moderate ulcerative colitis. In randomized controlled trials, this combination was significantly more likely to induce and maintain remission than was standard-dose oral mesalamine monotherapy, the authors noted. “In the maintenance trials, enemas were used twice per week or for 1 week per month. Both oral and topical mesalamine were well tolerated.”
For patients with moderate disease activity or a suboptimal response to standard-dose mesalamine or diazo-bonded 5-ASA, the guideline recommends adding rectal mesalamine to high-dose oral mesalamine (more than 3 grams daily). Combination therapy maximizes the delivery of mesalamine to the affected area of the colon, which optimizes the trial of 5-ASA before opting for treatment escalation, the authors noted. They recommend once-daily oral mesalamine dosing, since this is easier to adhere to and studies have found no benefit of more frequent dosing.
For inducing remission of mild to moderate ulcerative colitis, the guideline recommends standard-dose oral mesalamine or diazo-bonded 5-ASA over budesonide. “Overall, the budesonide preparations are not superior to mesalamine for induction of remission,” the authors wrote. Oral 5-ASAs are preferred, especially given the absence of data on the efficacy or safety of maintenance budesonide therapy.
For patients with mild to moderate ulcerative proctosigmoiditis or proctitis, the guideline conditionally recommends rectal mesalamine over oral mesalamine. Compared with placebo, rectal mesalamine suppositories were significantly more likely to induce remission in randomized trials of patients with mild to moderate ulcerative proctitis. If these patients cannot tolerate or are refractory to mesalamine suppositories, low-quality evidence supports rectal steroid therapy over no treatment, the guideline states. For patients with mild to moderate ulcerative proctosigmoiditis, moderate-quality evidence supports mesalamine enemas over rectal corticosteroids. If these patients want to avoid the difficulties of enemas, the guideline considers rectal corticosteroid foam a reasonable alternative.
Likewise, they cite low-quality evidence for adding oral prednisone or budesonide MMX to 5-ASA if patients are refractory to optimized 5-ASA therapy. No trials have directly compared rates of remission with budesonide MMX versus systemic corticosteroids. In just one placebo-controlled trial, adding budesonide MMX to 5-ASA slightly improved the chances of remission (risk ratio, 0.95; 95% confidence interval, 0.89-1.00). Furthermore, studies of other second-generation corticosteroids found they were better tolerated but no more likely to induce remission than oral prednisone or prednisolone.
Some patients with mild to moderate colitis respond inadequately to these recommended therapies and need systemic corticosteroids, immunomodulators, or biologic therapies to induce and maintain remission, the guideline authors noted. They make no recommendation on immunomodulators or biologics. Studies of probiotics, curcumin, and fecal microbiota transplantation are “urgently needed,” but for now, their use “risks delaying proven effective therapy, with the potential for worsening symptoms or complications,” they wrote. For patients without Clostridium difficile infections, they recommend against fecal microbiota transplantation except in the setting of a clinical trial.
The experts also noted the need for a tool to stratify patients with mild to moderate ulcerative colitis based on their risk of future progression and colectomy.
Finally, they call for studies on who will benefit most from high-dose mesalamine or topical mesalamine and on the relative safety and efficacy of budesonide and systemic corticosteroids in the event of an inadequate response to 5-ASAs.
All members were required to complete the disclosure statement. These statements are maintained at the American Gastroenterological Association headquarters in Bethesda, Maryland, and pertinent disclosures of conflict of interest are published with this report.
SOURCE: Crocket SD et al. Gastro 2019;156(2). doi: org/10.1053/j.gastro.2018.12.009.
For patients with extensive mild to moderate ulcerative colitis, numerous randomized controlled trials support the use of either standard-dose mesalamine (2-3 grams per day) or diazo-bonded 5-aminosalicylic acid (ASA) instead of low-dose mesalamine, sulfasalazine, or no therapy, state new guidelines from the American Gastroenterological Association, published in Gastroenterology.
Sulfasalazine (2-4 grams per day) is less likely to be tolerated but remains a “reasonable option” for remitted patients who are already on it and for patients with prominent arthritis symptoms, especially if alternative treatments are cost prohibitive, wrote Cynthia W. Ko, MD, MS, of the University of Washington, Seattle, and her associates.
According to the guideline, patients with mild to moderate ulcerative colitis have less than four to six bowel movements per day, only mild or moderate rectal bleeding, no constitutional symptoms, and no high overall inflammatory burden or signs of high inflammatory activity on the Mayo Clinic score and Truelove and Witt’s criteria. These patients usually do not require colectomy, but this outcome is more likely when patients are diagnosed before age 40 years or have extensive disease or deep ulcers, extraintestinal manifestations, or elevated inflammatory markers. These higher-risk patients need more aggressive initial treatment and faster treatment intensification in cases of inadequate response, the guideline emphasizes. Even for cases of mild to moderate ulcerative colitis, treatment intensification is preferable to repeated courses of corticosteroids.
The guideline recommends adding rectal mesalamine to oral 5-ASA if patients have extensive or left-sided mild to moderate ulcerative colitis. In randomized controlled trials, this combination was significantly more likely to induce and maintain remission than was standard-dose oral mesalamine monotherapy, the authors noted. “In the maintenance trials, enemas were used twice per week or for 1 week per month. Both oral and topical mesalamine were well tolerated.”
For patients with moderate disease activity or a suboptimal response to standard-dose mesalamine or diazo-bonded 5-ASA, the guideline recommends adding rectal mesalamine to high-dose oral mesalamine (more than 3 grams daily). Combination therapy maximizes the delivery of mesalamine to the affected area of the colon, which optimizes the trial of 5-ASA before opting for treatment escalation, the authors noted. They recommend once-daily oral mesalamine dosing, since this is easier to adhere to and studies have found no benefit of more frequent dosing.
For inducing remission of mild to moderate ulcerative colitis, the guideline recommends standard-dose oral mesalamine or diazo-bonded 5-ASA over budesonide. “Overall, the budesonide preparations are not superior to mesalamine for induction of remission,” the authors wrote. Oral 5-ASAs are preferred, especially given the absence of data on the efficacy or safety of maintenance budesonide therapy.
For patients with mild to moderate ulcerative proctosigmoiditis or proctitis, the guideline conditionally recommends rectal mesalamine over oral mesalamine. Compared with placebo, rectal mesalamine suppositories were significantly more likely to induce remission in randomized trials of patients with mild to moderate ulcerative proctitis. If these patients cannot tolerate or are refractory to mesalamine suppositories, low-quality evidence supports rectal steroid therapy over no treatment, the guideline states. For patients with mild to moderate ulcerative proctosigmoiditis, moderate-quality evidence supports mesalamine enemas over rectal corticosteroids. If these patients want to avoid the difficulties of enemas, the guideline considers rectal corticosteroid foam a reasonable alternative.
Likewise, they cite low-quality evidence for adding oral prednisone or budesonide MMX to 5-ASA if patients are refractory to optimized 5-ASA therapy. No trials have directly compared rates of remission with budesonide MMX versus systemic corticosteroids. In just one placebo-controlled trial, adding budesonide MMX to 5-ASA slightly improved the chances of remission (risk ratio, 0.95; 95% confidence interval, 0.89-1.00). Furthermore, studies of other second-generation corticosteroids found they were better tolerated but no more likely to induce remission than oral prednisone or prednisolone.
Some patients with mild to moderate colitis respond inadequately to these recommended therapies and need systemic corticosteroids, immunomodulators, or biologic therapies to induce and maintain remission, the guideline authors noted. They make no recommendation on immunomodulators or biologics. Studies of probiotics, curcumin, and fecal microbiota transplantation are “urgently needed,” but for now, their use “risks delaying proven effective therapy, with the potential for worsening symptoms or complications,” they wrote. For patients without Clostridium difficile infections, they recommend against fecal microbiota transplantation except in the setting of a clinical trial.
The experts also noted the need for a tool to stratify patients with mild to moderate ulcerative colitis based on their risk of future progression and colectomy.
Finally, they call for studies on who will benefit most from high-dose mesalamine or topical mesalamine and on the relative safety and efficacy of budesonide and systemic corticosteroids in the event of an inadequate response to 5-ASAs.
All members were required to complete the disclosure statement. These statements are maintained at the American Gastroenterological Association headquarters in Bethesda, Maryland, and pertinent disclosures of conflict of interest are published with this report.
SOURCE: Crocket SD et al. Gastro 2019;156(2). doi: org/10.1053/j.gastro.2018.12.009.
FROM GASTROENTEROLOGY
Dietary aluminum may trigger IBS
Aluminum ingested in small amounts causes visceral hypersensitivity in rats, suggesting that dietary levels of aluminum may trigger irritable bowel syndrome (IBS) in humans, according to a study published in Cellular and Molecular Gastroenterology and Hepatology.
Rats given oral aluminum exhibited dose-dependent visceral pain along with activation of proteinase-activated receptor-2 (PAR2) and mast cell degranulation, a combination of events that mirror clinical signs and molecular mechanisms of IBS in humans, reported lead author, Nicolas Esquerre, PhD, of Lille Inflammation Research International Center at Université Lille in France, and his colleagues. The study contributes to ongoing research surrounding causes and mechanisms of IBS, which may vary among patients because of disease subsets. These findings suggest that some patients with IBS may benefit from dietary aluminum restriction or chelation therapy.
“[T]he question of the initial trigger [of IBS] still remains unresolved,” the investigators wrote. “A more precise link between food and IBS has been demonstrated for gluten and other wheat proteins, lactose, and nickel, highlighting particular subsets of IBS patients now diagnosed as nonceliac gluten/wheat sensitivity, lactose intolerance, and nickel-allergic contact mucositis,” they added. “Here, we evaluated the effect of aluminum, a common contaminant of food and water, on abdominal pain.”
Aluminum may enter the diet as a food additive, or it may contaminate foods grown in aluminum-rich soil. Other sources of oral exposure include packaging and kitchenware. A previous study showed that most Americans ingest 0.01-1.4 mg/kg of aluminum daily, and 5% ingest 1.58 mg/kg daily (i.e., 95 mg per day for a 60-kg person).
Based on these statistics, rats in the present study received oral aluminum citrate (AlCi) corresponding with three doses of aluminum: 0.5 mg/kg, 1.5 mg/kg, or 3.0 mg/kg. Treatment continued for 30 days, with colorectal distension (CRD) measured on days 2, 4, 8, 15, and 30.
Results showed a dose-dependent relationship between aluminum ingestion and visceral hypersensitivity. Within 2 days, rats receiving 3.0 mg/kg of aluminum exhibited a significantly lower pain threshold, and within 8 days, rats receiving 0.5 mg/kg and 1.5 mg/kg also showed increased visceral hypersensitivity.
After 1 month of treatment, rats receiving 1.5 mg/kg per day demonstrated a 30% increase in pain compared with control animals. In the same group, visceral hypersensitivity began to wane 7 days after cessation of treatment; 4 more weeks were needed to return to baseline. When treatment was restarted, visceral hypersensitivity occurred within 2 days, compared with 8 days upon initial administration. These findings are particularly relevant to some people, as the 1.5-mg/kg dose corresponds with the daily amount of aluminum ingested by 5% of Americans. Similar patterns of response and sensitization were observed in rats ingesting 0.5 mg/kg and 3.0 mg/kg. Female rats were more sensitive to aluminum than were male rats, a sex pattern that mimics human IBS.
Further testing showed that rats treated with zinc citrate (ZnCi) did not exhibit changes to pain threshold, thereby excluding citrate as an aggravating factor. Rat models of noninflammatory and inflammatory colonic hypersensitivity (butyrate enema or intrathecal injection of 25%-50% ethanol in combination with 2,4,6-trinitrobenzenesulfonic acid, respectively) had visceral hypersensitivity similar to that of rats in the 1.5-mg/kg AlCi group.
Testing of colonic tissue from AlCi-treated rats did not reveal inflammatory changes according to a variety of qualifiers, including histology, myeloperoxidase activity, mRNA expression of several inflammatory cytokines, or infiltration of eosinophils or macrophages. Noninflammatory effects of aluminum, however, were found. For instance, treated rats had lower serotonin levels in enteroendocrine cells.
“Enteroendocrine cells are specialized epithelial cells that respond to luminal stimuli by releasing various biologically active compounds,” the investigators wrote. “They regulate several physiological and homeostatic functions of the gastrointestinal tract, such as postprandial secretion, motility, immune responses, and sensory functions. A reduced number of enteroendocrine cells has been observed in the duodenum, ileum, and colon of some patients with IBS.”
In addition to changes in enteroendocrine cells, AlCi-treated rats had greater colonic mast cell degranulation and histamine with upregulation of histidine decarboxylase transcripts, suggesting that aluminum activated mast cells.
To determine the role of mast cell activation in visceral hypersensitivity, rats were given AlCi with cromoglycate, an inhibitor of mast cell degranulation. This treatment reduced mast cell degranulation and visceral pain threshold, compared with AlCi-treated rats not receiving cromoglycate, suggesting that mast cell degranulation is a primary driver of visceral hypersensitivity. This observation was confirmed by a mast cell–deficient mouse strain (Kit W-sh/W-sh), that had a normal number of mast cells incapable of degranulation. Treating the mast cell–deficient mice with AlCi did not induce visceral hypersensitivity, thereby confirming the role of mast cell degranulation.
Along with mast cell degranulation, AlCi treatment led to PAR2 activation. Investigators explored the significance of this finding with PAR2 knockout mice. When treated with AlCi, PAR2 knockout mice showed no increase in visceral hypersensitivity, suggesting that hypersensitivity is dependent on PAR2 activation. Further testing revealed that mast cell–deficient mice (Kit W-sh/W-sh) did not have PAR2 upregulation either, connecting a sequence in which aluminum triggers mast cell degranulation, mast cell degranulation drives PAR2 upregulation, and PAR2 upregulation causes visceral hypersensitivity. The latter two events in this chain – mast cell degranulation and PAR2 upregulation – mirror molecular mechanisms of IBS in humans.
“We speculate that aluminum activates mast cells to release mediators that can increase excitability of nociceptive afferences contributing to the visceral pain phenotype,” the investigators wrote. “Taken together, our results linked aluminum to several mechanisms implicated in IBS pathophysiology, highlighting a possible role for aluminum as a triggering factor in IBS development.”
The investigators suggested that these findings could influence preventive or therapeutic strategies: “Aluminum might be the first identified dietary risk factor for IBS, implying that measures to limit aluminum dietary consumption or to chelate aluminum may represent novel pathways of prevention and treatment of IBS in some susceptible patients,” they wrote.
The study was funded by the European Fund for Regional Economic Development; the Hauts de France Region, Ministère de l’Enseignement Supérieur et de la Recherche (CPER IRENI); and Digestscience (European Research Foundation on Intestinal Diseases and Nutrition).
SOURCE: Esquerre N et al. Cell Mol Gastroenterol Hepatol. 2019 Sep 20. doi: 10.1016/j.jcmgh.2018.09.012.
Irritable bowel syndrome is a chronic functional gastrointestinal disorder, characterized by relapsing/remitting diarrhea, constipation, and visceral pain. IBS afflicts 10%-25% of the population in developed countries.
Despite histologically normal intestinal biopsy specimens, biological signatures of IBS include alterations in intestinal gene expression, increased gut permeability, and changes in gut microbiota composition. Thus, although the cause or causes of IBS are not defined, these and other data highlight the enormous breadth of factors that might play a role in this disorder. Similar alterations also are associated with inflammatory bowel disease (IBD), although the magnitude of changes is typically greater in IBD. Nevertheless, these data suggest that IBS and IBD may share triggers and pathogenetic mechanisms. That prevalence of both IBS and IBD have shown marked increases in incidence, roughly paralleling the modernization of society that accelerated in the mid-20th century, raises the possibility that environmental factors associated with human activity may be a driver of both diseases. Recent findings suggest that aluminum may be one such trigger. While humans have always been exposed to aluminum, the most abundant metal on earth, industrialization has increased the magnitude of exposure owing to the use of aluminum salts as stabilizers in processed foods and the concentration of ground water aluminum in agricultural products. Mimicking estimated average human ingestion of aluminum via administering it orally to rats increases their perception of visceral pain. These results suggest a possible role for increased exposure to aluminum in driving the post–mid-20th-century increased incidence of IBS. Unfortunately, only broad societal estimates of aluminum exposure are available, and aluminum levels are difficult to measure in individuals, making it difficult to epidemiologically investigate the role of aluminum in promoting GI disease in humans. Hence, I submit that levels of aluminum ingestion by humans should be more closely monitored and the potential of aluminum to promote GI disease carefully scrutinized.
Andrew Ted Gewirtz, PhD, distinguished university center professor, Georgia State University’s Institute for Biomedical Sciences’ Center for Inflammation, Immunity and Infection, Atlanta.
Irritable bowel syndrome is a chronic functional gastrointestinal disorder, characterized by relapsing/remitting diarrhea, constipation, and visceral pain. IBS afflicts 10%-25% of the population in developed countries.
Despite histologically normal intestinal biopsy specimens, biological signatures of IBS include alterations in intestinal gene expression, increased gut permeability, and changes in gut microbiota composition. Thus, although the cause or causes of IBS are not defined, these and other data highlight the enormous breadth of factors that might play a role in this disorder. Similar alterations also are associated with inflammatory bowel disease (IBD), although the magnitude of changes is typically greater in IBD. Nevertheless, these data suggest that IBS and IBD may share triggers and pathogenetic mechanisms. That prevalence of both IBS and IBD have shown marked increases in incidence, roughly paralleling the modernization of society that accelerated in the mid-20th century, raises the possibility that environmental factors associated with human activity may be a driver of both diseases. Recent findings suggest that aluminum may be one such trigger. While humans have always been exposed to aluminum, the most abundant metal on earth, industrialization has increased the magnitude of exposure owing to the use of aluminum salts as stabilizers in processed foods and the concentration of ground water aluminum in agricultural products. Mimicking estimated average human ingestion of aluminum via administering it orally to rats increases their perception of visceral pain. These results suggest a possible role for increased exposure to aluminum in driving the post–mid-20th-century increased incidence of IBS. Unfortunately, only broad societal estimates of aluminum exposure are available, and aluminum levels are difficult to measure in individuals, making it difficult to epidemiologically investigate the role of aluminum in promoting GI disease in humans. Hence, I submit that levels of aluminum ingestion by humans should be more closely monitored and the potential of aluminum to promote GI disease carefully scrutinized.
Andrew Ted Gewirtz, PhD, distinguished university center professor, Georgia State University’s Institute for Biomedical Sciences’ Center for Inflammation, Immunity and Infection, Atlanta.
Irritable bowel syndrome is a chronic functional gastrointestinal disorder, characterized by relapsing/remitting diarrhea, constipation, and visceral pain. IBS afflicts 10%-25% of the population in developed countries.
Despite histologically normal intestinal biopsy specimens, biological signatures of IBS include alterations in intestinal gene expression, increased gut permeability, and changes in gut microbiota composition. Thus, although the cause or causes of IBS are not defined, these and other data highlight the enormous breadth of factors that might play a role in this disorder. Similar alterations also are associated with inflammatory bowel disease (IBD), although the magnitude of changes is typically greater in IBD. Nevertheless, these data suggest that IBS and IBD may share triggers and pathogenetic mechanisms. That prevalence of both IBS and IBD have shown marked increases in incidence, roughly paralleling the modernization of society that accelerated in the mid-20th century, raises the possibility that environmental factors associated with human activity may be a driver of both diseases. Recent findings suggest that aluminum may be one such trigger. While humans have always been exposed to aluminum, the most abundant metal on earth, industrialization has increased the magnitude of exposure owing to the use of aluminum salts as stabilizers in processed foods and the concentration of ground water aluminum in agricultural products. Mimicking estimated average human ingestion of aluminum via administering it orally to rats increases their perception of visceral pain. These results suggest a possible role for increased exposure to aluminum in driving the post–mid-20th-century increased incidence of IBS. Unfortunately, only broad societal estimates of aluminum exposure are available, and aluminum levels are difficult to measure in individuals, making it difficult to epidemiologically investigate the role of aluminum in promoting GI disease in humans. Hence, I submit that levels of aluminum ingestion by humans should be more closely monitored and the potential of aluminum to promote GI disease carefully scrutinized.
Andrew Ted Gewirtz, PhD, distinguished university center professor, Georgia State University’s Institute for Biomedical Sciences’ Center for Inflammation, Immunity and Infection, Atlanta.
Aluminum ingested in small amounts causes visceral hypersensitivity in rats, suggesting that dietary levels of aluminum may trigger irritable bowel syndrome (IBS) in humans, according to a study published in Cellular and Molecular Gastroenterology and Hepatology.
Rats given oral aluminum exhibited dose-dependent visceral pain along with activation of proteinase-activated receptor-2 (PAR2) and mast cell degranulation, a combination of events that mirror clinical signs and molecular mechanisms of IBS in humans, reported lead author, Nicolas Esquerre, PhD, of Lille Inflammation Research International Center at Université Lille in France, and his colleagues. The study contributes to ongoing research surrounding causes and mechanisms of IBS, which may vary among patients because of disease subsets. These findings suggest that some patients with IBS may benefit from dietary aluminum restriction or chelation therapy.
“[T]he question of the initial trigger [of IBS] still remains unresolved,” the investigators wrote. “A more precise link between food and IBS has been demonstrated for gluten and other wheat proteins, lactose, and nickel, highlighting particular subsets of IBS patients now diagnosed as nonceliac gluten/wheat sensitivity, lactose intolerance, and nickel-allergic contact mucositis,” they added. “Here, we evaluated the effect of aluminum, a common contaminant of food and water, on abdominal pain.”
Aluminum may enter the diet as a food additive, or it may contaminate foods grown in aluminum-rich soil. Other sources of oral exposure include packaging and kitchenware. A previous study showed that most Americans ingest 0.01-1.4 mg/kg of aluminum daily, and 5% ingest 1.58 mg/kg daily (i.e., 95 mg per day for a 60-kg person).
Based on these statistics, rats in the present study received oral aluminum citrate (AlCi) corresponding with three doses of aluminum: 0.5 mg/kg, 1.5 mg/kg, or 3.0 mg/kg. Treatment continued for 30 days, with colorectal distension (CRD) measured on days 2, 4, 8, 15, and 30.
Results showed a dose-dependent relationship between aluminum ingestion and visceral hypersensitivity. Within 2 days, rats receiving 3.0 mg/kg of aluminum exhibited a significantly lower pain threshold, and within 8 days, rats receiving 0.5 mg/kg and 1.5 mg/kg also showed increased visceral hypersensitivity.
After 1 month of treatment, rats receiving 1.5 mg/kg per day demonstrated a 30% increase in pain compared with control animals. In the same group, visceral hypersensitivity began to wane 7 days after cessation of treatment; 4 more weeks were needed to return to baseline. When treatment was restarted, visceral hypersensitivity occurred within 2 days, compared with 8 days upon initial administration. These findings are particularly relevant to some people, as the 1.5-mg/kg dose corresponds with the daily amount of aluminum ingested by 5% of Americans. Similar patterns of response and sensitization were observed in rats ingesting 0.5 mg/kg and 3.0 mg/kg. Female rats were more sensitive to aluminum than were male rats, a sex pattern that mimics human IBS.
Further testing showed that rats treated with zinc citrate (ZnCi) did not exhibit changes to pain threshold, thereby excluding citrate as an aggravating factor. Rat models of noninflammatory and inflammatory colonic hypersensitivity (butyrate enema or intrathecal injection of 25%-50% ethanol in combination with 2,4,6-trinitrobenzenesulfonic acid, respectively) had visceral hypersensitivity similar to that of rats in the 1.5-mg/kg AlCi group.
Testing of colonic tissue from AlCi-treated rats did not reveal inflammatory changes according to a variety of qualifiers, including histology, myeloperoxidase activity, mRNA expression of several inflammatory cytokines, or infiltration of eosinophils or macrophages. Noninflammatory effects of aluminum, however, were found. For instance, treated rats had lower serotonin levels in enteroendocrine cells.
“Enteroendocrine cells are specialized epithelial cells that respond to luminal stimuli by releasing various biologically active compounds,” the investigators wrote. “They regulate several physiological and homeostatic functions of the gastrointestinal tract, such as postprandial secretion, motility, immune responses, and sensory functions. A reduced number of enteroendocrine cells has been observed in the duodenum, ileum, and colon of some patients with IBS.”
In addition to changes in enteroendocrine cells, AlCi-treated rats had greater colonic mast cell degranulation and histamine with upregulation of histidine decarboxylase transcripts, suggesting that aluminum activated mast cells.
To determine the role of mast cell activation in visceral hypersensitivity, rats were given AlCi with cromoglycate, an inhibitor of mast cell degranulation. This treatment reduced mast cell degranulation and visceral pain threshold, compared with AlCi-treated rats not receiving cromoglycate, suggesting that mast cell degranulation is a primary driver of visceral hypersensitivity. This observation was confirmed by a mast cell–deficient mouse strain (Kit W-sh/W-sh), that had a normal number of mast cells incapable of degranulation. Treating the mast cell–deficient mice with AlCi did not induce visceral hypersensitivity, thereby confirming the role of mast cell degranulation.
Along with mast cell degranulation, AlCi treatment led to PAR2 activation. Investigators explored the significance of this finding with PAR2 knockout mice. When treated with AlCi, PAR2 knockout mice showed no increase in visceral hypersensitivity, suggesting that hypersensitivity is dependent on PAR2 activation. Further testing revealed that mast cell–deficient mice (Kit W-sh/W-sh) did not have PAR2 upregulation either, connecting a sequence in which aluminum triggers mast cell degranulation, mast cell degranulation drives PAR2 upregulation, and PAR2 upregulation causes visceral hypersensitivity. The latter two events in this chain – mast cell degranulation and PAR2 upregulation – mirror molecular mechanisms of IBS in humans.
“We speculate that aluminum activates mast cells to release mediators that can increase excitability of nociceptive afferences contributing to the visceral pain phenotype,” the investigators wrote. “Taken together, our results linked aluminum to several mechanisms implicated in IBS pathophysiology, highlighting a possible role for aluminum as a triggering factor in IBS development.”
The investigators suggested that these findings could influence preventive or therapeutic strategies: “Aluminum might be the first identified dietary risk factor for IBS, implying that measures to limit aluminum dietary consumption or to chelate aluminum may represent novel pathways of prevention and treatment of IBS in some susceptible patients,” they wrote.
The study was funded by the European Fund for Regional Economic Development; the Hauts de France Region, Ministère de l’Enseignement Supérieur et de la Recherche (CPER IRENI); and Digestscience (European Research Foundation on Intestinal Diseases and Nutrition).
SOURCE: Esquerre N et al. Cell Mol Gastroenterol Hepatol. 2019 Sep 20. doi: 10.1016/j.jcmgh.2018.09.012.
Aluminum ingested in small amounts causes visceral hypersensitivity in rats, suggesting that dietary levels of aluminum may trigger irritable bowel syndrome (IBS) in humans, according to a study published in Cellular and Molecular Gastroenterology and Hepatology.
Rats given oral aluminum exhibited dose-dependent visceral pain along with activation of proteinase-activated receptor-2 (PAR2) and mast cell degranulation, a combination of events that mirror clinical signs and molecular mechanisms of IBS in humans, reported lead author, Nicolas Esquerre, PhD, of Lille Inflammation Research International Center at Université Lille in France, and his colleagues. The study contributes to ongoing research surrounding causes and mechanisms of IBS, which may vary among patients because of disease subsets. These findings suggest that some patients with IBS may benefit from dietary aluminum restriction or chelation therapy.
“[T]he question of the initial trigger [of IBS] still remains unresolved,” the investigators wrote. “A more precise link between food and IBS has been demonstrated for gluten and other wheat proteins, lactose, and nickel, highlighting particular subsets of IBS patients now diagnosed as nonceliac gluten/wheat sensitivity, lactose intolerance, and nickel-allergic contact mucositis,” they added. “Here, we evaluated the effect of aluminum, a common contaminant of food and water, on abdominal pain.”
Aluminum may enter the diet as a food additive, or it may contaminate foods grown in aluminum-rich soil. Other sources of oral exposure include packaging and kitchenware. A previous study showed that most Americans ingest 0.01-1.4 mg/kg of aluminum daily, and 5% ingest 1.58 mg/kg daily (i.e., 95 mg per day for a 60-kg person).
Based on these statistics, rats in the present study received oral aluminum citrate (AlCi) corresponding with three doses of aluminum: 0.5 mg/kg, 1.5 mg/kg, or 3.0 mg/kg. Treatment continued for 30 days, with colorectal distension (CRD) measured on days 2, 4, 8, 15, and 30.
Results showed a dose-dependent relationship between aluminum ingestion and visceral hypersensitivity. Within 2 days, rats receiving 3.0 mg/kg of aluminum exhibited a significantly lower pain threshold, and within 8 days, rats receiving 0.5 mg/kg and 1.5 mg/kg also showed increased visceral hypersensitivity.
After 1 month of treatment, rats receiving 1.5 mg/kg per day demonstrated a 30% increase in pain compared with control animals. In the same group, visceral hypersensitivity began to wane 7 days after cessation of treatment; 4 more weeks were needed to return to baseline. When treatment was restarted, visceral hypersensitivity occurred within 2 days, compared with 8 days upon initial administration. These findings are particularly relevant to some people, as the 1.5-mg/kg dose corresponds with the daily amount of aluminum ingested by 5% of Americans. Similar patterns of response and sensitization were observed in rats ingesting 0.5 mg/kg and 3.0 mg/kg. Female rats were more sensitive to aluminum than were male rats, a sex pattern that mimics human IBS.
Further testing showed that rats treated with zinc citrate (ZnCi) did not exhibit changes to pain threshold, thereby excluding citrate as an aggravating factor. Rat models of noninflammatory and inflammatory colonic hypersensitivity (butyrate enema or intrathecal injection of 25%-50% ethanol in combination with 2,4,6-trinitrobenzenesulfonic acid, respectively) had visceral hypersensitivity similar to that of rats in the 1.5-mg/kg AlCi group.
Testing of colonic tissue from AlCi-treated rats did not reveal inflammatory changes according to a variety of qualifiers, including histology, myeloperoxidase activity, mRNA expression of several inflammatory cytokines, or infiltration of eosinophils or macrophages. Noninflammatory effects of aluminum, however, were found. For instance, treated rats had lower serotonin levels in enteroendocrine cells.
“Enteroendocrine cells are specialized epithelial cells that respond to luminal stimuli by releasing various biologically active compounds,” the investigators wrote. “They regulate several physiological and homeostatic functions of the gastrointestinal tract, such as postprandial secretion, motility, immune responses, and sensory functions. A reduced number of enteroendocrine cells has been observed in the duodenum, ileum, and colon of some patients with IBS.”
In addition to changes in enteroendocrine cells, AlCi-treated rats had greater colonic mast cell degranulation and histamine with upregulation of histidine decarboxylase transcripts, suggesting that aluminum activated mast cells.
To determine the role of mast cell activation in visceral hypersensitivity, rats were given AlCi with cromoglycate, an inhibitor of mast cell degranulation. This treatment reduced mast cell degranulation and visceral pain threshold, compared with AlCi-treated rats not receiving cromoglycate, suggesting that mast cell degranulation is a primary driver of visceral hypersensitivity. This observation was confirmed by a mast cell–deficient mouse strain (Kit W-sh/W-sh), that had a normal number of mast cells incapable of degranulation. Treating the mast cell–deficient mice with AlCi did not induce visceral hypersensitivity, thereby confirming the role of mast cell degranulation.
Along with mast cell degranulation, AlCi treatment led to PAR2 activation. Investigators explored the significance of this finding with PAR2 knockout mice. When treated with AlCi, PAR2 knockout mice showed no increase in visceral hypersensitivity, suggesting that hypersensitivity is dependent on PAR2 activation. Further testing revealed that mast cell–deficient mice (Kit W-sh/W-sh) did not have PAR2 upregulation either, connecting a sequence in which aluminum triggers mast cell degranulation, mast cell degranulation drives PAR2 upregulation, and PAR2 upregulation causes visceral hypersensitivity. The latter two events in this chain – mast cell degranulation and PAR2 upregulation – mirror molecular mechanisms of IBS in humans.
“We speculate that aluminum activates mast cells to release mediators that can increase excitability of nociceptive afferences contributing to the visceral pain phenotype,” the investigators wrote. “Taken together, our results linked aluminum to several mechanisms implicated in IBS pathophysiology, highlighting a possible role for aluminum as a triggering factor in IBS development.”
The investigators suggested that these findings could influence preventive or therapeutic strategies: “Aluminum might be the first identified dietary risk factor for IBS, implying that measures to limit aluminum dietary consumption or to chelate aluminum may represent novel pathways of prevention and treatment of IBS in some susceptible patients,” they wrote.
The study was funded by the European Fund for Regional Economic Development; the Hauts de France Region, Ministère de l’Enseignement Supérieur et de la Recherche (CPER IRENI); and Digestscience (European Research Foundation on Intestinal Diseases and Nutrition).
SOURCE: Esquerre N et al. Cell Mol Gastroenterol Hepatol. 2019 Sep 20. doi: 10.1016/j.jcmgh.2018.09.012.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Aluminum ingestion triggers visceral hypersensitivity in rats, suggesting that dietary levels of aluminum may contribute to development of irritable bowel syndrome in humans.
Major finding: In rodents, 1 month of oral aluminum administration led to a 30% increase in pain during colorectal distension, compared with control subjects.
Study details: A rodent study including noninflammatory and inflammatory IBS rat models, mast cell–deficient mice, and PAR2 knockout mice.
Disclosures: The study was funded by the European Fund for Regional Economic Development; the Hauts de France Region, Ministère de l’Enseignement Supérieur et de la Recherche (CPER IRENI); and Digestscience (European Research Foundation on Intestinal Diseases and Nutrition).
Source: Esquerre N et al. Cell Mol Gastroenterol Hepatol. 2019 Sep 20. doi: 10.1016/j.jcmgh.2018.09.012.
Childhood inflammatory bowel disease linked to increased mortality
Children who developed inflammatory bowel disease before the age of 18 had a three- to fivefold increase in risk of death, compared with others in a large, retrospective registry study.
The study, which spanned a recent 50-year period, found “no evidence that [these] hazard ratios have changed since the introduction of immunomodulators and biologics,” wrote Ola Olén, MD, PhD, of Karolinska University Hospital in Sola, Sweden, together with her associates. Malignancy was the most frequent cause of death among patients with childhood-onset inflammatory bowel disease, followed by digestive diseases and infections. Absolute numbers of premature deaths were low, but the increase in relative risk was highest among patients with childhood-onset ulcerative colitis with primary sclerosing cholangitis and patients who had a first-degree relative with ulcerative colitis. The findings were published in the February issue of Gastroenterology.
Inflammatory bowel disease is thought to be more severe when it begins in childhood, but data on mortality for these patients are lacking. Using national Swedish health registries, Dr. Olén and her associates compared deaths among 9,442 children and adolescents with inflammatory bowel disease with those among 93,180 others matched by sex, age, and place of residence. Both groups were typically followed through age 30 years, and the study covered 1964 through 2014.
In all, there were 294 deaths among patients with childhood-onset inflammatory bowel disease (2.1 deaths per 1,000 person-years) and 940 deaths among matched individuals (0.7 deaths per 1,000 person-years), for a statistically significant adjusted hazard ratio of 3.2 (95% confidence interval, 2.8-3.7). For every 694 patients with childhood-onset inflammatory bowel disease who were followed through adulthood, there was one additional death per year, compared with a demographically similar population, the researchers determined.
Among the 294 deaths, 133 were because of cancer. Consequently, individuals with childhood-onset inflammatory bowel disease had a more than sixfold greater risk of dying from cancer than the general population (HR, 6.6; 95% CI, 5.3-8.2). The risk of death from malignancy was higher among individuals with ulcerative colitis (HR, 9.7) than among those with Crohn’s disease (HR, 3.1). Deaths from conditions of the digestive system were next most common, and these included deaths from liver failure.
In all, 27 individuals with childhood-onset inflammatory bowel disease died before their 18th birthday, for a fivefold increase in the adjusted hazard of death, compared with the general population of children and adolescents (HR, 4.9; 95% CI, 3.0-7.7). There was no significant trend in hazard of death according to calendar period, either among children and adolescents, or young adults (followed through age 25 years), the researchers said.
Additionally, childhood-onset inflammatory bowel disease was associated with a 2.2-year shorter life expectancy in patients followed through age 65 years, they reported. Thus, a diagnosis of childhood-onset inflammatory bowel disease merits careful follow-up, especially if patients have ulcerative colitis and primary sclerosing cholangitis, which was the strongest correlate of fatal intestinal cancer in this study.
Funders included the Swedish Medical Society, the Swedish Cancer Society, the Swedish Research Council, the Swedish Foundation for Strategic Research, Magtarmfonden, the Jane and Dan Olsson Foundation, the Mjölkdroppen Foundation, and the Karolinska Institutet Foundation. Dr. Olén disclosed investigator-initiated grants from Janssen and Pfizer. Other investigators also disclosed ties to Janssen, Pfizer, AstraZeneca, Ferring, Celgene, and Takeda.
SOURCE: Olén O et al. Gastroenterology. 2018 Oct 17. doi: 10.1053/j.gastro.2018.10.028.
Children who developed inflammatory bowel disease before the age of 18 had a three- to fivefold increase in risk of death, compared with others in a large, retrospective registry study.
The study, which spanned a recent 50-year period, found “no evidence that [these] hazard ratios have changed since the introduction of immunomodulators and biologics,” wrote Ola Olén, MD, PhD, of Karolinska University Hospital in Sola, Sweden, together with her associates. Malignancy was the most frequent cause of death among patients with childhood-onset inflammatory bowel disease, followed by digestive diseases and infections. Absolute numbers of premature deaths were low, but the increase in relative risk was highest among patients with childhood-onset ulcerative colitis with primary sclerosing cholangitis and patients who had a first-degree relative with ulcerative colitis. The findings were published in the February issue of Gastroenterology.
Inflammatory bowel disease is thought to be more severe when it begins in childhood, but data on mortality for these patients are lacking. Using national Swedish health registries, Dr. Olén and her associates compared deaths among 9,442 children and adolescents with inflammatory bowel disease with those among 93,180 others matched by sex, age, and place of residence. Both groups were typically followed through age 30 years, and the study covered 1964 through 2014.
In all, there were 294 deaths among patients with childhood-onset inflammatory bowel disease (2.1 deaths per 1,000 person-years) and 940 deaths among matched individuals (0.7 deaths per 1,000 person-years), for a statistically significant adjusted hazard ratio of 3.2 (95% confidence interval, 2.8-3.7). For every 694 patients with childhood-onset inflammatory bowel disease who were followed through adulthood, there was one additional death per year, compared with a demographically similar population, the researchers determined.
Among the 294 deaths, 133 were because of cancer. Consequently, individuals with childhood-onset inflammatory bowel disease had a more than sixfold greater risk of dying from cancer than the general population (HR, 6.6; 95% CI, 5.3-8.2). The risk of death from malignancy was higher among individuals with ulcerative colitis (HR, 9.7) than among those with Crohn’s disease (HR, 3.1). Deaths from conditions of the digestive system were next most common, and these included deaths from liver failure.
In all, 27 individuals with childhood-onset inflammatory bowel disease died before their 18th birthday, for a fivefold increase in the adjusted hazard of death, compared with the general population of children and adolescents (HR, 4.9; 95% CI, 3.0-7.7). There was no significant trend in hazard of death according to calendar period, either among children and adolescents, or young adults (followed through age 25 years), the researchers said.
Additionally, childhood-onset inflammatory bowel disease was associated with a 2.2-year shorter life expectancy in patients followed through age 65 years, they reported. Thus, a diagnosis of childhood-onset inflammatory bowel disease merits careful follow-up, especially if patients have ulcerative colitis and primary sclerosing cholangitis, which was the strongest correlate of fatal intestinal cancer in this study.
Funders included the Swedish Medical Society, the Swedish Cancer Society, the Swedish Research Council, the Swedish Foundation for Strategic Research, Magtarmfonden, the Jane and Dan Olsson Foundation, the Mjölkdroppen Foundation, and the Karolinska Institutet Foundation. Dr. Olén disclosed investigator-initiated grants from Janssen and Pfizer. Other investigators also disclosed ties to Janssen, Pfizer, AstraZeneca, Ferring, Celgene, and Takeda.
SOURCE: Olén O et al. Gastroenterology. 2018 Oct 17. doi: 10.1053/j.gastro.2018.10.028.
Children who developed inflammatory bowel disease before the age of 18 had a three- to fivefold increase in risk of death, compared with others in a large, retrospective registry study.
The study, which spanned a recent 50-year period, found “no evidence that [these] hazard ratios have changed since the introduction of immunomodulators and biologics,” wrote Ola Olén, MD, PhD, of Karolinska University Hospital in Sola, Sweden, together with her associates. Malignancy was the most frequent cause of death among patients with childhood-onset inflammatory bowel disease, followed by digestive diseases and infections. Absolute numbers of premature deaths were low, but the increase in relative risk was highest among patients with childhood-onset ulcerative colitis with primary sclerosing cholangitis and patients who had a first-degree relative with ulcerative colitis. The findings were published in the February issue of Gastroenterology.
Inflammatory bowel disease is thought to be more severe when it begins in childhood, but data on mortality for these patients are lacking. Using national Swedish health registries, Dr. Olén and her associates compared deaths among 9,442 children and adolescents with inflammatory bowel disease with those among 93,180 others matched by sex, age, and place of residence. Both groups were typically followed through age 30 years, and the study covered 1964 through 2014.
In all, there were 294 deaths among patients with childhood-onset inflammatory bowel disease (2.1 deaths per 1,000 person-years) and 940 deaths among matched individuals (0.7 deaths per 1,000 person-years), for a statistically significant adjusted hazard ratio of 3.2 (95% confidence interval, 2.8-3.7). For every 694 patients with childhood-onset inflammatory bowel disease who were followed through adulthood, there was one additional death per year, compared with a demographically similar population, the researchers determined.
Among the 294 deaths, 133 were because of cancer. Consequently, individuals with childhood-onset inflammatory bowel disease had a more than sixfold greater risk of dying from cancer than the general population (HR, 6.6; 95% CI, 5.3-8.2). The risk of death from malignancy was higher among individuals with ulcerative colitis (HR, 9.7) than among those with Crohn’s disease (HR, 3.1). Deaths from conditions of the digestive system were next most common, and these included deaths from liver failure.
In all, 27 individuals with childhood-onset inflammatory bowel disease died before their 18th birthday, for a fivefold increase in the adjusted hazard of death, compared with the general population of children and adolescents (HR, 4.9; 95% CI, 3.0-7.7). There was no significant trend in hazard of death according to calendar period, either among children and adolescents, or young adults (followed through age 25 years), the researchers said.
Additionally, childhood-onset inflammatory bowel disease was associated with a 2.2-year shorter life expectancy in patients followed through age 65 years, they reported. Thus, a diagnosis of childhood-onset inflammatory bowel disease merits careful follow-up, especially if patients have ulcerative colitis and primary sclerosing cholangitis, which was the strongest correlate of fatal intestinal cancer in this study.
Funders included the Swedish Medical Society, the Swedish Cancer Society, the Swedish Research Council, the Swedish Foundation for Strategic Research, Magtarmfonden, the Jane and Dan Olsson Foundation, the Mjölkdroppen Foundation, and the Karolinska Institutet Foundation. Dr. Olén disclosed investigator-initiated grants from Janssen and Pfizer. Other investigators also disclosed ties to Janssen, Pfizer, AstraZeneca, Ferring, Celgene, and Takeda.
SOURCE: Olén O et al. Gastroenterology. 2018 Oct 17. doi: 10.1053/j.gastro.2018.10.028.
FROM GASTROENTEROLOGY
Key clinical point: Childhood-onset inflammatory bowel disease is associated with an increased risk of death.
Major finding: The risk was approximately threefold higher than in the general population in those with childhood-onset inflammatory bowel disease aged under 25 years (aHR, 3.2; 95% CI, 2.8-3.7).
Study details: Retrospective cohort study of 9,442 patients with childhood-onset inflammatory bowel disease and 93,180 individuals from the general population.
Disclosures: Funders included the Swedish Medical Society, the Swedish Cancer Society, the Swedish Research Council, the Swedish Foundation for Strategic Research, Magtarmfonden, the Jane and Dan Olsson Foundation, the Mjölkdroppen Foundation, and the Karolinska Institutet Foundation. Dr. Olén disclosed investigator-initiated grants from Janssen and Pfizer. Other investigators also disclosed ties to Janssen, Pfizer, AstraZeneca, Ferring, Celgene, and Takeda.
Source: Olén O et al. Gastroenterology. 2018 Oct 17.