User login
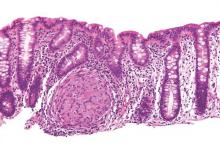
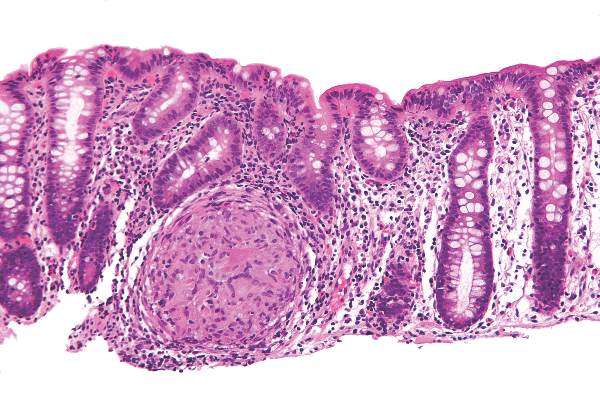
SBRT may be better than RFA for large hepatocellular carcinoma lesions
A retrospective data analysis showed that stereotactic body radiotherapy (SBRT) outperformed radiofrequency ablation (RFA) on tumors larger than 2 cm in patients with hepatocellular carcinoma.
For all tumors treated with RFA, 1- and 2-year freedom from local progression (FFLP) were 83.6% and 80.2%, and for tumors treated with SBRT, rates were 97.4% and 83.8%. On tumors smaller than 2 cm, FFLP was similar for the two methods (HR, 2.50; 95% confidence interval, 0.72-8.67; P = .15) but was significantly worse for RFA treatment of larger tumors (HR, 3.35; 95% CI, 1.17-9.62, P = .025).
“These results suggest that both SBRT and RFA are excellent choices for smaller tumors but that SBRT may be preferred for larger tumors. Prospective, randomized clinical trials are needed to compare these two modalities, especially for larger tumors, although we are unaware of any such trials,” wrote Dr. Daniel R. Wahl, radiation oncologist at the University of Michigan (J Clin Oncol. 2015 Dec. 2. doi:10.1200/JCO.2015.61.4925).
The retrospective study evaluated 224 patients with nonmetastatic hepatocellular carcinoma – 161 patients (249 tumors) who underwent RFA and 63 patients (83 tumors) who underwent SBRT at the University of Michigan from 2004 to 2012. Patients treated with RFA had higher rates of cirrhosis (95% vs. 78%; P less than .001), lower AFP levels (8.8 vs. 18.6; P = .04), and fewer prior liver-directed treatments compared with patients treated with SBRT.
To investigate the impact of fiducial use for image guidance in SBRT, the researchers examined treatment failures. Of the 21 treatments that used fiducials, none had local failure, compared with six failures in 62 treatments without fiducials.
Both methods had similar low rates of late adverse events. Acute adverse events and treatment-related deaths were nonsignificantly greater with RFA, which may suggest SBRT as a better option for medically unfit patients who may not tolerate invasive procedures such as RFA.
The study included only three tumors larger than 5 cm in diameter, so rates of local control for this size tumor cannot be estimated reliably, reported Dr. Wahl and his colleagues.
The National Institutes of Health and the Taubman Institute supported the research. Dr. Wahl reported stock or other ownership in Lycera. Several of his coauthors reported ties to industry.
A retrospective data analysis showed that stereotactic body radiotherapy (SBRT) outperformed radiofrequency ablation (RFA) on tumors larger than 2 cm in patients with hepatocellular carcinoma.
For all tumors treated with RFA, 1- and 2-year freedom from local progression (FFLP) were 83.6% and 80.2%, and for tumors treated with SBRT, rates were 97.4% and 83.8%. On tumors smaller than 2 cm, FFLP was similar for the two methods (HR, 2.50; 95% confidence interval, 0.72-8.67; P = .15) but was significantly worse for RFA treatment of larger tumors (HR, 3.35; 95% CI, 1.17-9.62, P = .025).
“These results suggest that both SBRT and RFA are excellent choices for smaller tumors but that SBRT may be preferred for larger tumors. Prospective, randomized clinical trials are needed to compare these two modalities, especially for larger tumors, although we are unaware of any such trials,” wrote Dr. Daniel R. Wahl, radiation oncologist at the University of Michigan (J Clin Oncol. 2015 Dec. 2. doi:10.1200/JCO.2015.61.4925).
The retrospective study evaluated 224 patients with nonmetastatic hepatocellular carcinoma – 161 patients (249 tumors) who underwent RFA and 63 patients (83 tumors) who underwent SBRT at the University of Michigan from 2004 to 2012. Patients treated with RFA had higher rates of cirrhosis (95% vs. 78%; P less than .001), lower AFP levels (8.8 vs. 18.6; P = .04), and fewer prior liver-directed treatments compared with patients treated with SBRT.
To investigate the impact of fiducial use for image guidance in SBRT, the researchers examined treatment failures. Of the 21 treatments that used fiducials, none had local failure, compared with six failures in 62 treatments without fiducials.
Both methods had similar low rates of late adverse events. Acute adverse events and treatment-related deaths were nonsignificantly greater with RFA, which may suggest SBRT as a better option for medically unfit patients who may not tolerate invasive procedures such as RFA.
The study included only three tumors larger than 5 cm in diameter, so rates of local control for this size tumor cannot be estimated reliably, reported Dr. Wahl and his colleagues.
The National Institutes of Health and the Taubman Institute supported the research. Dr. Wahl reported stock or other ownership in Lycera. Several of his coauthors reported ties to industry.
A retrospective data analysis showed that stereotactic body radiotherapy (SBRT) outperformed radiofrequency ablation (RFA) on tumors larger than 2 cm in patients with hepatocellular carcinoma.
For all tumors treated with RFA, 1- and 2-year freedom from local progression (FFLP) were 83.6% and 80.2%, and for tumors treated with SBRT, rates were 97.4% and 83.8%. On tumors smaller than 2 cm, FFLP was similar for the two methods (HR, 2.50; 95% confidence interval, 0.72-8.67; P = .15) but was significantly worse for RFA treatment of larger tumors (HR, 3.35; 95% CI, 1.17-9.62, P = .025).
“These results suggest that both SBRT and RFA are excellent choices for smaller tumors but that SBRT may be preferred for larger tumors. Prospective, randomized clinical trials are needed to compare these two modalities, especially for larger tumors, although we are unaware of any such trials,” wrote Dr. Daniel R. Wahl, radiation oncologist at the University of Michigan (J Clin Oncol. 2015 Dec. 2. doi:10.1200/JCO.2015.61.4925).
The retrospective study evaluated 224 patients with nonmetastatic hepatocellular carcinoma – 161 patients (249 tumors) who underwent RFA and 63 patients (83 tumors) who underwent SBRT at the University of Michigan from 2004 to 2012. Patients treated with RFA had higher rates of cirrhosis (95% vs. 78%; P less than .001), lower AFP levels (8.8 vs. 18.6; P = .04), and fewer prior liver-directed treatments compared with patients treated with SBRT.
To investigate the impact of fiducial use for image guidance in SBRT, the researchers examined treatment failures. Of the 21 treatments that used fiducials, none had local failure, compared with six failures in 62 treatments without fiducials.
Both methods had similar low rates of late adverse events. Acute adverse events and treatment-related deaths were nonsignificantly greater with RFA, which may suggest SBRT as a better option for medically unfit patients who may not tolerate invasive procedures such as RFA.
The study included only three tumors larger than 5 cm in diameter, so rates of local control for this size tumor cannot be estimated reliably, reported Dr. Wahl and his colleagues.
The National Institutes of Health and the Taubman Institute supported the research. Dr. Wahl reported stock or other ownership in Lycera. Several of his coauthors reported ties to industry.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Stereotactic body radiotherapy (SBRT) and radiofrequency ablation (RFA) both achieved good local control for small hepatocellular carcinoma lesions, but for tumors larger than 2 cm, SBRT showed significantly better freedom from local progression (FFLP).
Major finding: For tumors larger than 2 cm, FFLP was worse for RFA compared with SBRT (hazard ratio, 3.35; 95% CI, 1.17-9.62, P = .025).
Data source: The retrospective study evaluated 161 patients (249 tumors) who underwent RFA and 63 patients (83 tumors) who underwent SBRT at the University of Michigan from 2004 to 2012.
Disclosures: The National Institutes of Health and the Taubman Institute supported the research. Dr. Wahl reported stock or other ownership in Lycera. Several of his coauthors reported ties to industry.
Crohn’s study found no reason to continue immunomodulators after starting anti-TNFs
Baseline exposure to an immunomodulator did not improve the odds of clinical response or remission when starting anti–tumor necrosis factor (anti-TNF) therapy for Crohn’s disease (CD), said authors of a meta-analysis of 11 randomized, controlled trials. Pending better trials, patients with CD and their clinicians will need to carefully weigh the risks and benefits of continuing an immunomodulator when starting anti-TNF therapy, Dr. Jennifer Jones of Dalhousie University in Halifax, Canada and her associates wrote in the December issue of Clinical Gastroenterology and Hepatology.
Intense debate persists about whether patients with CD who have already been exposed to immunomodulators such as azathioprine, 6-mercaptopurine, and methotrexate should stay on them when starting anti-TNF agents. The landmark 2010 SONIC trial could not answer this question because it only enrolled patients who had never received an immunomodulator, and more recent studies (Clin Gastroenterol Hepatol. 2011;9:36-41) have raised concerns about the safety of immunomodulators, the researchers noted. To compare combination immunomodulators and anti-TNF treatment with anti-TNF monotherapy in luminal and fistulizing CD, they analyzed original datasets from 11 randomized, controlled trials published between 1980 and 2008. A total of 625 patients with CD had received an immunomodulator, while 976 patients had not. The investigators excluded trials in which patients were naive to both immunomodulators and anti-TNF agents (Clin Gastroenterol Hepatol. 2015 [doi: 10.1016/j.cgh.2015.06.034]).
In the overall analysis, combination therapy was no better than anti-TNF monotherapy in terms of 6-month remission, maintenance of response, or partial or full fistula closure, Dr. Jones and her associates reported. The same was true for subgroup analyses, but the odds ratio for infliximab reached statistical significance in a sensitivity analysis that included data from the ACCENT 2 (Clin Gastroenterol Hepatol. 2004;2:912-20) trial. “For the infliximab-only analysis, adding ACCENT 2 resulted in minimal change in the point estimate but, as expected, increased the precision of the 95% CIs (the lower CI increased from 0.97 to 1.06), which led to a statistically significant difference in the comparison between infliximab monotherapy and combination therapy,” the researchers commented. While sensitivity analyses have limitations, the finding “does raise the question” of whether the benefits of staying on an immunomodulator depend on the anti-TNF agent, they said.
Combination therapy did not heighten the chances of infusion reactions, malignancies, serious infections, or death, said the investigators. In fact, baseline immunomodulator exposure was associated with fewer injection site reactions among infliximab patients (OR, 0.46; 95% CI, 0.26-0.79). The researchers did not uncover publication bias, and found significant heterogeneity among studies only for the 6-month clinical response endpoint, they added.
The findings “challenge the clinical importance of combination therapy” in the setting of baseline immunomodulator exposure, but “it is hard to ignore the preponderance of data” on anti-TNF pharmacokinetics that support combination therapy over monotherapy, the investigators emphasized. “Whether combination therapy has a greater protective effect against anti-drug antibody development and lower trough levels for all anti-TNF agents or for patients previously exposed to anti-TNF agents is still in question,” they added. They called for a well-designed, randomized, placebo-controlled trial that uses objective measures of disease activity and follow patients long enough to assess efficacy.
The investigators reported no funding sources for the study. Dr. Jones reported having been a speaker for Jansen, Merck, Schering-Plough, Abbott, and AbbVie, and having served on advisory boards for Janssen, Abbott, and Takeda. Nine co-authors reported financial and consulting relationships with Jansen, Merck, Schering-Plough, Abbott, and a number of other pharmaceutical companies.
Source: American Gastroenterological Association
Baseline exposure to an immunomodulator did not improve the odds of clinical response or remission when starting anti–tumor necrosis factor (anti-TNF) therapy for Crohn’s disease (CD), said authors of a meta-analysis of 11 randomized, controlled trials. Pending better trials, patients with CD and their clinicians will need to carefully weigh the risks and benefits of continuing an immunomodulator when starting anti-TNF therapy, Dr. Jennifer Jones of Dalhousie University in Halifax, Canada and her associates wrote in the December issue of Clinical Gastroenterology and Hepatology.
Intense debate persists about whether patients with CD who have already been exposed to immunomodulators such as azathioprine, 6-mercaptopurine, and methotrexate should stay on them when starting anti-TNF agents. The landmark 2010 SONIC trial could not answer this question because it only enrolled patients who had never received an immunomodulator, and more recent studies (Clin Gastroenterol Hepatol. 2011;9:36-41) have raised concerns about the safety of immunomodulators, the researchers noted. To compare combination immunomodulators and anti-TNF treatment with anti-TNF monotherapy in luminal and fistulizing CD, they analyzed original datasets from 11 randomized, controlled trials published between 1980 and 2008. A total of 625 patients with CD had received an immunomodulator, while 976 patients had not. The investigators excluded trials in which patients were naive to both immunomodulators and anti-TNF agents (Clin Gastroenterol Hepatol. 2015 [doi: 10.1016/j.cgh.2015.06.034]).
In the overall analysis, combination therapy was no better than anti-TNF monotherapy in terms of 6-month remission, maintenance of response, or partial or full fistula closure, Dr. Jones and her associates reported. The same was true for subgroup analyses, but the odds ratio for infliximab reached statistical significance in a sensitivity analysis that included data from the ACCENT 2 (Clin Gastroenterol Hepatol. 2004;2:912-20) trial. “For the infliximab-only analysis, adding ACCENT 2 resulted in minimal change in the point estimate but, as expected, increased the precision of the 95% CIs (the lower CI increased from 0.97 to 1.06), which led to a statistically significant difference in the comparison between infliximab monotherapy and combination therapy,” the researchers commented. While sensitivity analyses have limitations, the finding “does raise the question” of whether the benefits of staying on an immunomodulator depend on the anti-TNF agent, they said.
Combination therapy did not heighten the chances of infusion reactions, malignancies, serious infections, or death, said the investigators. In fact, baseline immunomodulator exposure was associated with fewer injection site reactions among infliximab patients (OR, 0.46; 95% CI, 0.26-0.79). The researchers did not uncover publication bias, and found significant heterogeneity among studies only for the 6-month clinical response endpoint, they added.
The findings “challenge the clinical importance of combination therapy” in the setting of baseline immunomodulator exposure, but “it is hard to ignore the preponderance of data” on anti-TNF pharmacokinetics that support combination therapy over monotherapy, the investigators emphasized. “Whether combination therapy has a greater protective effect against anti-drug antibody development and lower trough levels for all anti-TNF agents or for patients previously exposed to anti-TNF agents is still in question,” they added. They called for a well-designed, randomized, placebo-controlled trial that uses objective measures of disease activity and follow patients long enough to assess efficacy.
The investigators reported no funding sources for the study. Dr. Jones reported having been a speaker for Jansen, Merck, Schering-Plough, Abbott, and AbbVie, and having served on advisory boards for Janssen, Abbott, and Takeda. Nine co-authors reported financial and consulting relationships with Jansen, Merck, Schering-Plough, Abbott, and a number of other pharmaceutical companies.
Source: American Gastroenterological Association
Baseline exposure to an immunomodulator did not improve the odds of clinical response or remission when starting anti–tumor necrosis factor (anti-TNF) therapy for Crohn’s disease (CD), said authors of a meta-analysis of 11 randomized, controlled trials. Pending better trials, patients with CD and their clinicians will need to carefully weigh the risks and benefits of continuing an immunomodulator when starting anti-TNF therapy, Dr. Jennifer Jones of Dalhousie University in Halifax, Canada and her associates wrote in the December issue of Clinical Gastroenterology and Hepatology.
Intense debate persists about whether patients with CD who have already been exposed to immunomodulators such as azathioprine, 6-mercaptopurine, and methotrexate should stay on them when starting anti-TNF agents. The landmark 2010 SONIC trial could not answer this question because it only enrolled patients who had never received an immunomodulator, and more recent studies (Clin Gastroenterol Hepatol. 2011;9:36-41) have raised concerns about the safety of immunomodulators, the researchers noted. To compare combination immunomodulators and anti-TNF treatment with anti-TNF monotherapy in luminal and fistulizing CD, they analyzed original datasets from 11 randomized, controlled trials published between 1980 and 2008. A total of 625 patients with CD had received an immunomodulator, while 976 patients had not. The investigators excluded trials in which patients were naive to both immunomodulators and anti-TNF agents (Clin Gastroenterol Hepatol. 2015 [doi: 10.1016/j.cgh.2015.06.034]).
In the overall analysis, combination therapy was no better than anti-TNF monotherapy in terms of 6-month remission, maintenance of response, or partial or full fistula closure, Dr. Jones and her associates reported. The same was true for subgroup analyses, but the odds ratio for infliximab reached statistical significance in a sensitivity analysis that included data from the ACCENT 2 (Clin Gastroenterol Hepatol. 2004;2:912-20) trial. “For the infliximab-only analysis, adding ACCENT 2 resulted in minimal change in the point estimate but, as expected, increased the precision of the 95% CIs (the lower CI increased from 0.97 to 1.06), which led to a statistically significant difference in the comparison between infliximab monotherapy and combination therapy,” the researchers commented. While sensitivity analyses have limitations, the finding “does raise the question” of whether the benefits of staying on an immunomodulator depend on the anti-TNF agent, they said.
Combination therapy did not heighten the chances of infusion reactions, malignancies, serious infections, or death, said the investigators. In fact, baseline immunomodulator exposure was associated with fewer injection site reactions among infliximab patients (OR, 0.46; 95% CI, 0.26-0.79). The researchers did not uncover publication bias, and found significant heterogeneity among studies only for the 6-month clinical response endpoint, they added.
The findings “challenge the clinical importance of combination therapy” in the setting of baseline immunomodulator exposure, but “it is hard to ignore the preponderance of data” on anti-TNF pharmacokinetics that support combination therapy over monotherapy, the investigators emphasized. “Whether combination therapy has a greater protective effect against anti-drug antibody development and lower trough levels for all anti-TNF agents or for patients previously exposed to anti-TNF agents is still in question,” they added. They called for a well-designed, randomized, placebo-controlled trial that uses objective measures of disease activity and follow patients long enough to assess efficacy.
The investigators reported no funding sources for the study. Dr. Jones reported having been a speaker for Jansen, Merck, Schering-Plough, Abbott, and AbbVie, and having served on advisory boards for Janssen, Abbott, and Takeda. Nine co-authors reported financial and consulting relationships with Jansen, Merck, Schering-Plough, Abbott, and a number of other pharmaceutical companies.
Source: American Gastroenterological Association
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Continuing an immunomodulator was no more effective than switching to anti-TNF monotherapy in a meta-analysis of patients with Crohn’s disease.
Major finding: Combination therapy was no more effective than anti-TNF monotherapy in terms of clinical response, remission induction, or fistula closure.
Data source: Meta-analysis of 11 randomized, controlled trials of 1,601 patients with luminal or fistulizing CD.
Disclosures: The investigators reported no funding sources for the study. Dr. Jones reported having been a speaker for Jansen, Merck, Schering-Plough, Abbott, and AbbVie, and serving on advisory boards for Janssen, Abbott, and Takeda. Nine coauthors reported financial and consulting relationships with Jansen, Merck, Schering-Plough, Abbott, and a number of other pharmaceutical companies.
Portal venous blood yielded higher levels of circulating tumor cells
Researchers detected circulating tumor cells (CTCs) in the portal venous blood of all patients with pancreaticobiliary cancer (PBC), but in the peripheral blood of only 22% of patients, according to a small single-center cohort study.
“We have shown that portal venous CTCs are far more common and higher in absolute numbers than peripheral blood CTCs. We also have shown the feasibility of obtaining portal venous CTCs noninvasively via endoscopic ultrasound,” Dr. Daniel Catenacci, Dr. Christopher Chapman, and their associates from the University of Chicago Medicine wrote in the December issue of Gastroenterology. “Portal vein CTCs can be used for molecular characterization of PBCs and share features of metastatic tissue.”
Circulating tumor cells have shown promise in the minimally invasive assessment of solid tumors, but the peripheral bloodstream contains only about one CTC for every 1 billion blood cells, limiting the potential sensitivity of testing. The researchers therefore used EUS guidance to transhepatically collect portal venous blood from 18 patients with PBCs. They quantified CTCs in both their portal venous and peripheral blood by using CellSearch, a commercially available test that uses magnetic beads labeled with antibodies against epithelial cell adhesion molecules. They only counted epithelial-derived cells as CTCs if they were morphologically compatible with tumor cells, CD45-negative, and positive for cytokeratins 8, 18, or 19 and 40,6-diamidino-2-phenylindole (Gastroenterology 2015 [doi: 10.1053/j.gastro.2015.08.050]).
Patients suffered no adverse affects from portal vein sampling, the researchers reported. They detected CTCs in the portal venous blood of all 18 patients, but in the peripheral blood of only four (22%) patients. Average CTC concentrations also were significantly higher in portal venous blood (118.4 ± 36.8 CTCs per 7.5 mL) compared with peripheral blood (0.8 ± 0.4 CTCs per 7.5 mL; P less than .01).
Among nine patients with nonmetastatic, resectable, or borderline-resectable PBC, portal vein CTCs averaged 83.2 per 7.5 mL (median, 62.0), the researchers reported. Whole-genome amplification and KRAS codon sequencing in one patient also showed that CTCs had the same mutations and similar levels of P16, SMAD4, and P53 proteins as tumor cells from a metastatic lymph node.
In addition, magnetic cell sorting identified CTC clusters, which other studies have implicated in the metastatic seeding of distant organs, the researchers said. Indeed, CTCs are now known to include “a heterogeneous population of cells, including apoptotic cells, cells undergoing epithelial-to-mesenchymal transition with loss of epithelial markers, epithelial cells, and cell clusters,” they noted. This heterogeneity might make CTCs useful for studying the pathogenesis and progression of PBCs, as well as for assessing the individual chances of recurrence or metastasis, they added. “Future prospective studies will define the role of portal vein CTCs or predictive biomarkers in the perioperative setting,” the investigators concluded.
The work was funded by the Rolfe Pancreatic Cancer Foundation, officers of the Gerald O. Mann Charitable Foundation, the National Institutes of Health, the University of Chicago Comprehensive Cancer Center, the Cancer Research Foundation, the Alliance for Clinical Trials in Oncology Foundation, and the Live Like Katie Foundation. The researchers reported having no conflicts of interest.
Source: American Gastroenterological Association
Researchers detected circulating tumor cells (CTCs) in the portal venous blood of all patients with pancreaticobiliary cancer (PBC), but in the peripheral blood of only 22% of patients, according to a small single-center cohort study.
“We have shown that portal venous CTCs are far more common and higher in absolute numbers than peripheral blood CTCs. We also have shown the feasibility of obtaining portal venous CTCs noninvasively via endoscopic ultrasound,” Dr. Daniel Catenacci, Dr. Christopher Chapman, and their associates from the University of Chicago Medicine wrote in the December issue of Gastroenterology. “Portal vein CTCs can be used for molecular characterization of PBCs and share features of metastatic tissue.”
Circulating tumor cells have shown promise in the minimally invasive assessment of solid tumors, but the peripheral bloodstream contains only about one CTC for every 1 billion blood cells, limiting the potential sensitivity of testing. The researchers therefore used EUS guidance to transhepatically collect portal venous blood from 18 patients with PBCs. They quantified CTCs in both their portal venous and peripheral blood by using CellSearch, a commercially available test that uses magnetic beads labeled with antibodies against epithelial cell adhesion molecules. They only counted epithelial-derived cells as CTCs if they were morphologically compatible with tumor cells, CD45-negative, and positive for cytokeratins 8, 18, or 19 and 40,6-diamidino-2-phenylindole (Gastroenterology 2015 [doi: 10.1053/j.gastro.2015.08.050]).
Patients suffered no adverse affects from portal vein sampling, the researchers reported. They detected CTCs in the portal venous blood of all 18 patients, but in the peripheral blood of only four (22%) patients. Average CTC concentrations also were significantly higher in portal venous blood (118.4 ± 36.8 CTCs per 7.5 mL) compared with peripheral blood (0.8 ± 0.4 CTCs per 7.5 mL; P less than .01).
Among nine patients with nonmetastatic, resectable, or borderline-resectable PBC, portal vein CTCs averaged 83.2 per 7.5 mL (median, 62.0), the researchers reported. Whole-genome amplification and KRAS codon sequencing in one patient also showed that CTCs had the same mutations and similar levels of P16, SMAD4, and P53 proteins as tumor cells from a metastatic lymph node.
In addition, magnetic cell sorting identified CTC clusters, which other studies have implicated in the metastatic seeding of distant organs, the researchers said. Indeed, CTCs are now known to include “a heterogeneous population of cells, including apoptotic cells, cells undergoing epithelial-to-mesenchymal transition with loss of epithelial markers, epithelial cells, and cell clusters,” they noted. This heterogeneity might make CTCs useful for studying the pathogenesis and progression of PBCs, as well as for assessing the individual chances of recurrence or metastasis, they added. “Future prospective studies will define the role of portal vein CTCs or predictive biomarkers in the perioperative setting,” the investigators concluded.
The work was funded by the Rolfe Pancreatic Cancer Foundation, officers of the Gerald O. Mann Charitable Foundation, the National Institutes of Health, the University of Chicago Comprehensive Cancer Center, the Cancer Research Foundation, the Alliance for Clinical Trials in Oncology Foundation, and the Live Like Katie Foundation. The researchers reported having no conflicts of interest.
Source: American Gastroenterological Association
Researchers detected circulating tumor cells (CTCs) in the portal venous blood of all patients with pancreaticobiliary cancer (PBC), but in the peripheral blood of only 22% of patients, according to a small single-center cohort study.
“We have shown that portal venous CTCs are far more common and higher in absolute numbers than peripheral blood CTCs. We also have shown the feasibility of obtaining portal venous CTCs noninvasively via endoscopic ultrasound,” Dr. Daniel Catenacci, Dr. Christopher Chapman, and their associates from the University of Chicago Medicine wrote in the December issue of Gastroenterology. “Portal vein CTCs can be used for molecular characterization of PBCs and share features of metastatic tissue.”
Circulating tumor cells have shown promise in the minimally invasive assessment of solid tumors, but the peripheral bloodstream contains only about one CTC for every 1 billion blood cells, limiting the potential sensitivity of testing. The researchers therefore used EUS guidance to transhepatically collect portal venous blood from 18 patients with PBCs. They quantified CTCs in both their portal venous and peripheral blood by using CellSearch, a commercially available test that uses magnetic beads labeled with antibodies against epithelial cell adhesion molecules. They only counted epithelial-derived cells as CTCs if they were morphologically compatible with tumor cells, CD45-negative, and positive for cytokeratins 8, 18, or 19 and 40,6-diamidino-2-phenylindole (Gastroenterology 2015 [doi: 10.1053/j.gastro.2015.08.050]).
Patients suffered no adverse affects from portal vein sampling, the researchers reported. They detected CTCs in the portal venous blood of all 18 patients, but in the peripheral blood of only four (22%) patients. Average CTC concentrations also were significantly higher in portal venous blood (118.4 ± 36.8 CTCs per 7.5 mL) compared with peripheral blood (0.8 ± 0.4 CTCs per 7.5 mL; P less than .01).
Among nine patients with nonmetastatic, resectable, or borderline-resectable PBC, portal vein CTCs averaged 83.2 per 7.5 mL (median, 62.0), the researchers reported. Whole-genome amplification and KRAS codon sequencing in one patient also showed that CTCs had the same mutations and similar levels of P16, SMAD4, and P53 proteins as tumor cells from a metastatic lymph node.
In addition, magnetic cell sorting identified CTC clusters, which other studies have implicated in the metastatic seeding of distant organs, the researchers said. Indeed, CTCs are now known to include “a heterogeneous population of cells, including apoptotic cells, cells undergoing epithelial-to-mesenchymal transition with loss of epithelial markers, epithelial cells, and cell clusters,” they noted. This heterogeneity might make CTCs useful for studying the pathogenesis and progression of PBCs, as well as for assessing the individual chances of recurrence or metastasis, they added. “Future prospective studies will define the role of portal vein CTCs or predictive biomarkers in the perioperative setting,” the investigators concluded.
The work was funded by the Rolfe Pancreatic Cancer Foundation, officers of the Gerald O. Mann Charitable Foundation, the National Institutes of Health, the University of Chicago Comprehensive Cancer Center, the Cancer Research Foundation, the Alliance for Clinical Trials in Oncology Foundation, and the Live Like Katie Foundation. The researchers reported having no conflicts of interest.
Source: American Gastroenterological Association
FROM GASTROENTEROLOGY
Key clinical point: Circulating pancreaticobiliary tumor cells were much more common in portal venous blood than in peripheral blood, and were molecularly similar to tumor tissue.
Major finding: Magnetic cell sorting revealed CTCs in the portal venous blood of all patients, but in the peripheral blood of only 22% of patients.
Data source: Prospective cohort study of 18 patients with pancreaticobiliary cancers, with portal venous blood collected under endoscopic guidance.
Disclosures: The work was funded by the Rolfe Pancreatic Cancer Foundation, officers of the Gerald O. Mann Charitable Foundation, the National Institutes of Health, the University of Chicago Comprehensive Cancer Center, the Cancer Research Foundation, the Alliance for Clinical Trials in Oncology Foundation, and the Live Like Katie Foundation. The investigators reported having no conflicts of interest.
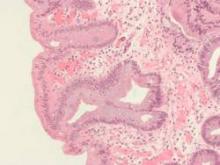
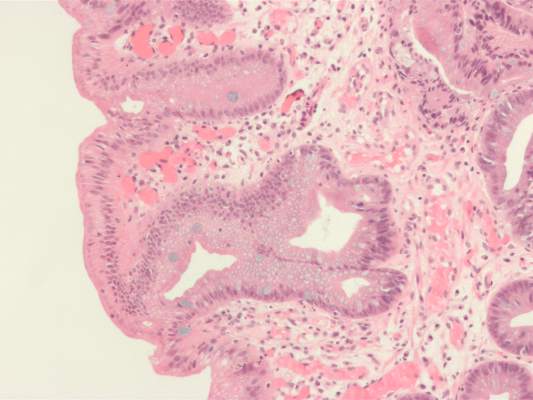
High serum leptin, insulin levels linked to Barrett’s esophagus risk
High serum insulin and leptin levels were significantly associated with Barrett’s esophagus, according to authors of a meta-analysis of nine observational studies published in the December issue of Clinical Gastroenterology and Hepatology.
Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels, and were 1.74 times as likely to have hyperinsulinemia, said Dr. Apoorva Chandar of Case Western Reserve University (Cleveland) and his associates.
Central obesity was known to increase the risk of esophageal inflammation, metaplasia, and adenocarcinoma (Clin Gastroenterol Hepatol 2013 [doi: 10.1016/j.cgh.2013.05.009]), but this meta-analysis helped pinpoint the hormones that might mediate the relationship, the investigators said. However, the link between obesity and Barrett’s esophagus “is likely complex,” meriting additional longitudinal analyses, they added.
Metabolically active fat produces leptin and other adipokines. Elevated serum leptin has anti-apoptotic and angiogenic effects and also is a marker for insulin resistance, the researchers noted. “Several observational studies have examined the association of serum adipokines and insulin with Barrett’s esophagus, but evidence regarding this association remains inconclusive,” they said. Therefore, they reviewed observational studies published through April 2015 that examined relationships between Barrett’s esophagus, adipokines, and insulin. The studies included 10 separate cohorts of 1,432 patients with Barrett’s esophagus and 3,550 controls, enabling the researchers to estimate summary adjusted odds ratios (Clin Gastroenterol Hepatol. 2015 [doi: 10.1016/j.cgh.2015.06.041]).
Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels (adjusted OR, 2.23; 95% confidence interval [CI], 1.31-3.78) and 1.74 times as likely to have elevated serum insulin levels (95% CI, 1.14 to 2.65). Total serum adiponectin was not linked to risk of Barrett’s esophagus, but increased serum levels of high molecular weight (HMW) adiponectin were (aOR, 1.75; 95% CI, 1.16-2.63), and one study reported an inverse correlation between levels of low molecular weight leptin and Barrett’s esophagus risk. Low molecular weight adiponectin has anti-inflammatory effects, while HMW adiponectin is proinflammatory, the researchers noted.
“It is simplistic to assume that the effects of obesity on the development of Barrett’s esophagus are mediated by one single adipokine,” the researchers said. “Leptin and adiponectin seem to crosstalk, and both of these adipokines also affect insulin-signaling pathways.” Obesity is a chronic inflammatory state characterized by increases in other circulating cytokines, such as interleukin-6 and tumor necrosis factor–alpha, they noted. Their findings do not solely implicate leptin among the adipokines, but show that it “might be an important contributor, and support further studies on the effects of leptin on the leptin receptor in the proliferation of Barrett’s epithelium.” They also noted that although women have higher leptin levels than men, men are at much greater risk of Barrett’s esophagus, which their review could not explain. Studies to date are “not adequate” to assess gender-specific relationships between insulin, adipokines, and Barrett’s esophagus, they said.
Other evidence has linked insulin to Barrett’s esophagus, according to the researchers. Insulin and related signaling pathways are upregulated in tissue specimens of Barrett’s esophagus and esophageal adenocarcinoma, and Barrett’s esophagus is more likely to progress to esophageal adenocarcinoma in the setting of insulin resistance, they noted. “Given that recent studies have shown an association between Barrett’s esophagus and measures of central obesity and diabetes mellitus type 2, it is conceivable that hyperinsulinemia and insulin resistance, which are known consequences of central obesity, are associated with Barrett’s esophagus pathogenesis,” they said.
However, their study did not link hyperinsulinemia to Barrett’s esophagus among subjects with GERD, possibly because of confounding or overmatching, they noted. More rigorous studies would be needed to fairly evaluate any relationship between insulin resistance and risk of Barrett’s esophagus, they concluded.
The National Cancer Institute funded the study. The investigators had no conflicts of interest.
Epidemiologic studies have shown that abdominal, especially visceral as opposed to cutaneous, obesity isassociated with increased risk of Barrett’s esophagus. The precise mechanisms are unclear; however, there is increasing evidence that this association is likely mediated through both the mechanical effect of increased abdominal pressure promoting gastroesophageal reflux and the nonmechanical metabolic and inflammatory effects of abdominal obesity. Adipose tissue produces and releases a variety of proinflammatory and anti-inflammatory factors, including the adipokines leptin and adiponectin, as well as cytokines and chemokines. Leptin (higher levels in visceral fat) has proinflammatory effects that promote a low-grade inflammatory state, while adiponectin (less visceral fat) protects against the complications of obesity by exerting anti-inflammatory effects.

|
| Dr. Aaron Thrift |
Results from single-center studies examining associations of circulating adipokines, insulin, and inflammatory cytokines with Barrett’s esophagus have been conflicting, potentially due to methodologic shortcomings. In this article, Dr. Chandar and his colleagues conducted a meta-analysis and report that higher serum levels of leptin and insulin are associated with increased risk of Barrett’s esophagus, while there was no association between serum adiponectin and Barrett’s esophagus. This study highlights the complexity of these associations. For example, only leptin among the adipokines was associated with Barrett’s esophagus. Thus, additional longitudinal studies are required to further tease out these associations, and formal mediation analysis would help quantify how much of the obesity effect is through these hormones. From a clinical perspective, the importance of the findings of this paper is that these may be attractive targets for preventing Barrett’s esophagus.
Dr. Thrift is in the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. He has no conflicts of interest.
Epidemiologic studies have shown that abdominal, especially visceral as opposed to cutaneous, obesity isassociated with increased risk of Barrett’s esophagus. The precise mechanisms are unclear; however, there is increasing evidence that this association is likely mediated through both the mechanical effect of increased abdominal pressure promoting gastroesophageal reflux and the nonmechanical metabolic and inflammatory effects of abdominal obesity. Adipose tissue produces and releases a variety of proinflammatory and anti-inflammatory factors, including the adipokines leptin and adiponectin, as well as cytokines and chemokines. Leptin (higher levels in visceral fat) has proinflammatory effects that promote a low-grade inflammatory state, while adiponectin (less visceral fat) protects against the complications of obesity by exerting anti-inflammatory effects.

|
| Dr. Aaron Thrift |
Results from single-center studies examining associations of circulating adipokines, insulin, and inflammatory cytokines with Barrett’s esophagus have been conflicting, potentially due to methodologic shortcomings. In this article, Dr. Chandar and his colleagues conducted a meta-analysis and report that higher serum levels of leptin and insulin are associated with increased risk of Barrett’s esophagus, while there was no association between serum adiponectin and Barrett’s esophagus. This study highlights the complexity of these associations. For example, only leptin among the adipokines was associated with Barrett’s esophagus. Thus, additional longitudinal studies are required to further tease out these associations, and formal mediation analysis would help quantify how much of the obesity effect is through these hormones. From a clinical perspective, the importance of the findings of this paper is that these may be attractive targets for preventing Barrett’s esophagus.
Dr. Thrift is in the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. He has no conflicts of interest.
Epidemiologic studies have shown that abdominal, especially visceral as opposed to cutaneous, obesity isassociated with increased risk of Barrett’s esophagus. The precise mechanisms are unclear; however, there is increasing evidence that this association is likely mediated through both the mechanical effect of increased abdominal pressure promoting gastroesophageal reflux and the nonmechanical metabolic and inflammatory effects of abdominal obesity. Adipose tissue produces and releases a variety of proinflammatory and anti-inflammatory factors, including the adipokines leptin and adiponectin, as well as cytokines and chemokines. Leptin (higher levels in visceral fat) has proinflammatory effects that promote a low-grade inflammatory state, while adiponectin (less visceral fat) protects against the complications of obesity by exerting anti-inflammatory effects.

|
| Dr. Aaron Thrift |
Results from single-center studies examining associations of circulating adipokines, insulin, and inflammatory cytokines with Barrett’s esophagus have been conflicting, potentially due to methodologic shortcomings. In this article, Dr. Chandar and his colleagues conducted a meta-analysis and report that higher serum levels of leptin and insulin are associated with increased risk of Barrett’s esophagus, while there was no association between serum adiponectin and Barrett’s esophagus. This study highlights the complexity of these associations. For example, only leptin among the adipokines was associated with Barrett’s esophagus. Thus, additional longitudinal studies are required to further tease out these associations, and formal mediation analysis would help quantify how much of the obesity effect is through these hormones. From a clinical perspective, the importance of the findings of this paper is that these may be attractive targets for preventing Barrett’s esophagus.
Dr. Thrift is in the section of gastroenterology and hepatology, department of medicine, Baylor College of Medicine, Houston. He has no conflicts of interest.
High serum insulin and leptin levels were significantly associated with Barrett’s esophagus, according to authors of a meta-analysis of nine observational studies published in the December issue of Clinical Gastroenterology and Hepatology.
Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels, and were 1.74 times as likely to have hyperinsulinemia, said Dr. Apoorva Chandar of Case Western Reserve University (Cleveland) and his associates.
Central obesity was known to increase the risk of esophageal inflammation, metaplasia, and adenocarcinoma (Clin Gastroenterol Hepatol 2013 [doi: 10.1016/j.cgh.2013.05.009]), but this meta-analysis helped pinpoint the hormones that might mediate the relationship, the investigators said. However, the link between obesity and Barrett’s esophagus “is likely complex,” meriting additional longitudinal analyses, they added.
Metabolically active fat produces leptin and other adipokines. Elevated serum leptin has anti-apoptotic and angiogenic effects and also is a marker for insulin resistance, the researchers noted. “Several observational studies have examined the association of serum adipokines and insulin with Barrett’s esophagus, but evidence regarding this association remains inconclusive,” they said. Therefore, they reviewed observational studies published through April 2015 that examined relationships between Barrett’s esophagus, adipokines, and insulin. The studies included 10 separate cohorts of 1,432 patients with Barrett’s esophagus and 3,550 controls, enabling the researchers to estimate summary adjusted odds ratios (Clin Gastroenterol Hepatol. 2015 [doi: 10.1016/j.cgh.2015.06.041]).
Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels (adjusted OR, 2.23; 95% confidence interval [CI], 1.31-3.78) and 1.74 times as likely to have elevated serum insulin levels (95% CI, 1.14 to 2.65). Total serum adiponectin was not linked to risk of Barrett’s esophagus, but increased serum levels of high molecular weight (HMW) adiponectin were (aOR, 1.75; 95% CI, 1.16-2.63), and one study reported an inverse correlation between levels of low molecular weight leptin and Barrett’s esophagus risk. Low molecular weight adiponectin has anti-inflammatory effects, while HMW adiponectin is proinflammatory, the researchers noted.
“It is simplistic to assume that the effects of obesity on the development of Barrett’s esophagus are mediated by one single adipokine,” the researchers said. “Leptin and adiponectin seem to crosstalk, and both of these adipokines also affect insulin-signaling pathways.” Obesity is a chronic inflammatory state characterized by increases in other circulating cytokines, such as interleukin-6 and tumor necrosis factor–alpha, they noted. Their findings do not solely implicate leptin among the adipokines, but show that it “might be an important contributor, and support further studies on the effects of leptin on the leptin receptor in the proliferation of Barrett’s epithelium.” They also noted that although women have higher leptin levels than men, men are at much greater risk of Barrett’s esophagus, which their review could not explain. Studies to date are “not adequate” to assess gender-specific relationships between insulin, adipokines, and Barrett’s esophagus, they said.
Other evidence has linked insulin to Barrett’s esophagus, according to the researchers. Insulin and related signaling pathways are upregulated in tissue specimens of Barrett’s esophagus and esophageal adenocarcinoma, and Barrett’s esophagus is more likely to progress to esophageal adenocarcinoma in the setting of insulin resistance, they noted. “Given that recent studies have shown an association between Barrett’s esophagus and measures of central obesity and diabetes mellitus type 2, it is conceivable that hyperinsulinemia and insulin resistance, which are known consequences of central obesity, are associated with Barrett’s esophagus pathogenesis,” they said.
However, their study did not link hyperinsulinemia to Barrett’s esophagus among subjects with GERD, possibly because of confounding or overmatching, they noted. More rigorous studies would be needed to fairly evaluate any relationship between insulin resistance and risk of Barrett’s esophagus, they concluded.
The National Cancer Institute funded the study. The investigators had no conflicts of interest.
High serum insulin and leptin levels were significantly associated with Barrett’s esophagus, according to authors of a meta-analysis of nine observational studies published in the December issue of Clinical Gastroenterology and Hepatology.
Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels, and were 1.74 times as likely to have hyperinsulinemia, said Dr. Apoorva Chandar of Case Western Reserve University (Cleveland) and his associates.
Central obesity was known to increase the risk of esophageal inflammation, metaplasia, and adenocarcinoma (Clin Gastroenterol Hepatol 2013 [doi: 10.1016/j.cgh.2013.05.009]), but this meta-analysis helped pinpoint the hormones that might mediate the relationship, the investigators said. However, the link between obesity and Barrett’s esophagus “is likely complex,” meriting additional longitudinal analyses, they added.
Metabolically active fat produces leptin and other adipokines. Elevated serum leptin has anti-apoptotic and angiogenic effects and also is a marker for insulin resistance, the researchers noted. “Several observational studies have examined the association of serum adipokines and insulin with Barrett’s esophagus, but evidence regarding this association remains inconclusive,” they said. Therefore, they reviewed observational studies published through April 2015 that examined relationships between Barrett’s esophagus, adipokines, and insulin. The studies included 10 separate cohorts of 1,432 patients with Barrett’s esophagus and 3,550 controls, enabling the researchers to estimate summary adjusted odds ratios (Clin Gastroenterol Hepatol. 2015 [doi: 10.1016/j.cgh.2015.06.041]).
Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels (adjusted OR, 2.23; 95% confidence interval [CI], 1.31-3.78) and 1.74 times as likely to have elevated serum insulin levels (95% CI, 1.14 to 2.65). Total serum adiponectin was not linked to risk of Barrett’s esophagus, but increased serum levels of high molecular weight (HMW) adiponectin were (aOR, 1.75; 95% CI, 1.16-2.63), and one study reported an inverse correlation between levels of low molecular weight leptin and Barrett’s esophagus risk. Low molecular weight adiponectin has anti-inflammatory effects, while HMW adiponectin is proinflammatory, the researchers noted.
“It is simplistic to assume that the effects of obesity on the development of Barrett’s esophagus are mediated by one single adipokine,” the researchers said. “Leptin and adiponectin seem to crosstalk, and both of these adipokines also affect insulin-signaling pathways.” Obesity is a chronic inflammatory state characterized by increases in other circulating cytokines, such as interleukin-6 and tumor necrosis factor–alpha, they noted. Their findings do not solely implicate leptin among the adipokines, but show that it “might be an important contributor, and support further studies on the effects of leptin on the leptin receptor in the proliferation of Barrett’s epithelium.” They also noted that although women have higher leptin levels than men, men are at much greater risk of Barrett’s esophagus, which their review could not explain. Studies to date are “not adequate” to assess gender-specific relationships between insulin, adipokines, and Barrett’s esophagus, they said.
Other evidence has linked insulin to Barrett’s esophagus, according to the researchers. Insulin and related signaling pathways are upregulated in tissue specimens of Barrett’s esophagus and esophageal adenocarcinoma, and Barrett’s esophagus is more likely to progress to esophageal adenocarcinoma in the setting of insulin resistance, they noted. “Given that recent studies have shown an association between Barrett’s esophagus and measures of central obesity and diabetes mellitus type 2, it is conceivable that hyperinsulinemia and insulin resistance, which are known consequences of central obesity, are associated with Barrett’s esophagus pathogenesis,” they said.
However, their study did not link hyperinsulinemia to Barrett’s esophagus among subjects with GERD, possibly because of confounding or overmatching, they noted. More rigorous studies would be needed to fairly evaluate any relationship between insulin resistance and risk of Barrett’s esophagus, they concluded.
The National Cancer Institute funded the study. The investigators had no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: High serum levels of leptin and insulin were associated with Barrett’s esophagus in a meta-analysis.
Major finding: Compared with population controls, patients with Barrett’s esophagus were twice as likely to have high serum leptin levels, and were 1.74 times as likely to have hyperinsulinemia.
Data source: Meta-analysis of nine observational studies that included 1,432 Barrett’s esophagus patients and 3,550 controls.
Disclosures: The National Cancer Institute funded the study. The investigators had no conflicts of interest.
Gastrointestinal and liver diseases remain substantial public health burden
Diseases such as Clostridium difficile infection, inflammatory bowel disease, and liver cancer continue to cost billions and cause many thousands of deaths in the United States every year, investigators reported in the December issue of Gastroenterology.
“Gastrointestinal and liver diseases are a source of substantial burden and cost,” said Dr. Anne Peery and her associates at the University of North Carolina School of Medicine and the Gillings School of Public Health, both in Chapel Hill. The Affordable Care Act has extended health insurance to more than 16 million Americans, which is “expected to change the landscape of care for GI illnesses” and intensifies the need for their comprehensive study, the researchers added.
They analyzed health care visits, costs, and deaths from GI, pancreatic, and hepatic diseases for 2007 through 2012 by using surveillance data from the Centers for Disease Control and Prevention, the Agency for Healthcare Research and Quality, and the National Cancer Institute. Chronic hepatitis C virus infection was a leading disease burden, they found. Associated emergency department visits rose by 176% between 2006 and 2012, hospital admissions increased by 225% between 2003 and 2012, and in-hospital mortality approached 6%. These trends reflect the aging of baby boomers, who make up three-quarters of infected patients, the investigators noted. As a result, rates of new liver cancers also are rising, and end-stage liver disease is expected to keep increasing until 2030, they added (Gastroenterology. 2015 Aug 20. doi: 10.1053/j.gastro.2015.08.045). Aging boomers are increasingly seeking care for other age-related GI disorders, the investigators reported. Outpatient visits for hemorrhoids are rising, as are emergency department visits for constipation and lower-GI bleeding, and hospitalizations for acute diverticulitis and C. difficile infection. Gastrointestinal hemorrhage was the most common diagnosis at hospitalization, accounting for more than 500,000 discharges and costing almost $5 billion dollars in 2012 alone, the researchers said.
Despite better treatments, hospital admissions for Crohn’s disease and ulcerative colitis also rose from less than 60,000 in 1993 to about 100,000 in 2012, said Dr. Peery and her associates. “This is congruent with earlier trends using the National Hospital Discharge Survey. Emergency department visits [for inflammatory bowel disease] are also rising,” they added.
In contrast, cases and deaths from colorectal cancer continue to drop, partly because of intensified screening efforts, the investigators said. They called the trend “encouraging,” but noted that CRC still tops cancers of the pancreas, liver, and intrahepatic bile ducts as the leading GI cause of mortality in the United States. In 2012, more than 51,000 Americans died from CRC, and screening efforts captured only 58% of those between 50 and 75 years old. Boosting that percentage to 80% by 2018 http://nccrt.org/tools/80-percent-by-2018/ could prevent 280,000 CRC cases and 200,000 deaths within 20 years, Dr. Peery and her associates noted.
The National Institutes of Health helped fund the work. The investigators reported having no conflicts of interest.
Source: American Gastroenterological Association
In the excellent study by Peery and colleagues, statistics on health care utilization in the ambulatory and hospital settings, incidence and mortality from GI cancers, and mortality associated with other GI illnesses from 2007 to 2012 was collected using data from multiple complementary databases. This is the ideal methodology for this type of study because it quantifies utilization data from several complementary national databases. Of course, these data may be limited by systematic errors in ICD coding and costs are estimated using Medicare’s cost-to-charge ratio. Nevertheless, these data provide the best “snap shot” of trends in the burden of gastrointestinal and liver illness as of 2012.
What are the key points? First, the increase in the burden of GI and liver illness probably reflects the aging of the “baby boomer” population. Furthermore, since the Affordable Care Act is expanding access to health care, the burden on gastroenterologists is also likely to expand. Second, although we’re doing a good job with CRC screening, there is also room for improvement. While the incidence of CRC continues to decrease, only 58% of adults aged 50-75 years old had CRC screening in 2010. Third, HCV-associated hospitalizations have doubled from 2003 to 2012. Since HCV-associated cirrhosis is likely to increase until 2030, insurers and public health officials will have to carefully weigh the initial high cost of using new and highly effective regimens of direct-acting antiviral agents versus the downstream costs of managing these individuals after developing decompensated cirrhosis.
Dr. Philip S. Schoenfeld is professor of medicine and director, training program in GI epidemiology, division of gastroenterology, University of Michigan, Ann Arbor. He has no conflicts of interest.
In the excellent study by Peery and colleagues, statistics on health care utilization in the ambulatory and hospital settings, incidence and mortality from GI cancers, and mortality associated with other GI illnesses from 2007 to 2012 was collected using data from multiple complementary databases. This is the ideal methodology for this type of study because it quantifies utilization data from several complementary national databases. Of course, these data may be limited by systematic errors in ICD coding and costs are estimated using Medicare’s cost-to-charge ratio. Nevertheless, these data provide the best “snap shot” of trends in the burden of gastrointestinal and liver illness as of 2012.
What are the key points? First, the increase in the burden of GI and liver illness probably reflects the aging of the “baby boomer” population. Furthermore, since the Affordable Care Act is expanding access to health care, the burden on gastroenterologists is also likely to expand. Second, although we’re doing a good job with CRC screening, there is also room for improvement. While the incidence of CRC continues to decrease, only 58% of adults aged 50-75 years old had CRC screening in 2010. Third, HCV-associated hospitalizations have doubled from 2003 to 2012. Since HCV-associated cirrhosis is likely to increase until 2030, insurers and public health officials will have to carefully weigh the initial high cost of using new and highly effective regimens of direct-acting antiviral agents versus the downstream costs of managing these individuals after developing decompensated cirrhosis.
Dr. Philip S. Schoenfeld is professor of medicine and director, training program in GI epidemiology, division of gastroenterology, University of Michigan, Ann Arbor. He has no conflicts of interest.
In the excellent study by Peery and colleagues, statistics on health care utilization in the ambulatory and hospital settings, incidence and mortality from GI cancers, and mortality associated with other GI illnesses from 2007 to 2012 was collected using data from multiple complementary databases. This is the ideal methodology for this type of study because it quantifies utilization data from several complementary national databases. Of course, these data may be limited by systematic errors in ICD coding and costs are estimated using Medicare’s cost-to-charge ratio. Nevertheless, these data provide the best “snap shot” of trends in the burden of gastrointestinal and liver illness as of 2012.
What are the key points? First, the increase in the burden of GI and liver illness probably reflects the aging of the “baby boomer” population. Furthermore, since the Affordable Care Act is expanding access to health care, the burden on gastroenterologists is also likely to expand. Second, although we’re doing a good job with CRC screening, there is also room for improvement. While the incidence of CRC continues to decrease, only 58% of adults aged 50-75 years old had CRC screening in 2010. Third, HCV-associated hospitalizations have doubled from 2003 to 2012. Since HCV-associated cirrhosis is likely to increase until 2030, insurers and public health officials will have to carefully weigh the initial high cost of using new and highly effective regimens of direct-acting antiviral agents versus the downstream costs of managing these individuals after developing decompensated cirrhosis.
Dr. Philip S. Schoenfeld is professor of medicine and director, training program in GI epidemiology, division of gastroenterology, University of Michigan, Ann Arbor. He has no conflicts of interest.
Diseases such as Clostridium difficile infection, inflammatory bowel disease, and liver cancer continue to cost billions and cause many thousands of deaths in the United States every year, investigators reported in the December issue of Gastroenterology.
“Gastrointestinal and liver diseases are a source of substantial burden and cost,” said Dr. Anne Peery and her associates at the University of North Carolina School of Medicine and the Gillings School of Public Health, both in Chapel Hill. The Affordable Care Act has extended health insurance to more than 16 million Americans, which is “expected to change the landscape of care for GI illnesses” and intensifies the need for their comprehensive study, the researchers added.
They analyzed health care visits, costs, and deaths from GI, pancreatic, and hepatic diseases for 2007 through 2012 by using surveillance data from the Centers for Disease Control and Prevention, the Agency for Healthcare Research and Quality, and the National Cancer Institute. Chronic hepatitis C virus infection was a leading disease burden, they found. Associated emergency department visits rose by 176% between 2006 and 2012, hospital admissions increased by 225% between 2003 and 2012, and in-hospital mortality approached 6%. These trends reflect the aging of baby boomers, who make up three-quarters of infected patients, the investigators noted. As a result, rates of new liver cancers also are rising, and end-stage liver disease is expected to keep increasing until 2030, they added (Gastroenterology. 2015 Aug 20. doi: 10.1053/j.gastro.2015.08.045). Aging boomers are increasingly seeking care for other age-related GI disorders, the investigators reported. Outpatient visits for hemorrhoids are rising, as are emergency department visits for constipation and lower-GI bleeding, and hospitalizations for acute diverticulitis and C. difficile infection. Gastrointestinal hemorrhage was the most common diagnosis at hospitalization, accounting for more than 500,000 discharges and costing almost $5 billion dollars in 2012 alone, the researchers said.
Despite better treatments, hospital admissions for Crohn’s disease and ulcerative colitis also rose from less than 60,000 in 1993 to about 100,000 in 2012, said Dr. Peery and her associates. “This is congruent with earlier trends using the National Hospital Discharge Survey. Emergency department visits [for inflammatory bowel disease] are also rising,” they added.
In contrast, cases and deaths from colorectal cancer continue to drop, partly because of intensified screening efforts, the investigators said. They called the trend “encouraging,” but noted that CRC still tops cancers of the pancreas, liver, and intrahepatic bile ducts as the leading GI cause of mortality in the United States. In 2012, more than 51,000 Americans died from CRC, and screening efforts captured only 58% of those between 50 and 75 years old. Boosting that percentage to 80% by 2018 http://nccrt.org/tools/80-percent-by-2018/ could prevent 280,000 CRC cases and 200,000 deaths within 20 years, Dr. Peery and her associates noted.
The National Institutes of Health helped fund the work. The investigators reported having no conflicts of interest.
Source: American Gastroenterological Association
Diseases such as Clostridium difficile infection, inflammatory bowel disease, and liver cancer continue to cost billions and cause many thousands of deaths in the United States every year, investigators reported in the December issue of Gastroenterology.
“Gastrointestinal and liver diseases are a source of substantial burden and cost,” said Dr. Anne Peery and her associates at the University of North Carolina School of Medicine and the Gillings School of Public Health, both in Chapel Hill. The Affordable Care Act has extended health insurance to more than 16 million Americans, which is “expected to change the landscape of care for GI illnesses” and intensifies the need for their comprehensive study, the researchers added.
They analyzed health care visits, costs, and deaths from GI, pancreatic, and hepatic diseases for 2007 through 2012 by using surveillance data from the Centers for Disease Control and Prevention, the Agency for Healthcare Research and Quality, and the National Cancer Institute. Chronic hepatitis C virus infection was a leading disease burden, they found. Associated emergency department visits rose by 176% between 2006 and 2012, hospital admissions increased by 225% between 2003 and 2012, and in-hospital mortality approached 6%. These trends reflect the aging of baby boomers, who make up three-quarters of infected patients, the investigators noted. As a result, rates of new liver cancers also are rising, and end-stage liver disease is expected to keep increasing until 2030, they added (Gastroenterology. 2015 Aug 20. doi: 10.1053/j.gastro.2015.08.045). Aging boomers are increasingly seeking care for other age-related GI disorders, the investigators reported. Outpatient visits for hemorrhoids are rising, as are emergency department visits for constipation and lower-GI bleeding, and hospitalizations for acute diverticulitis and C. difficile infection. Gastrointestinal hemorrhage was the most common diagnosis at hospitalization, accounting for more than 500,000 discharges and costing almost $5 billion dollars in 2012 alone, the researchers said.
Despite better treatments, hospital admissions for Crohn’s disease and ulcerative colitis also rose from less than 60,000 in 1993 to about 100,000 in 2012, said Dr. Peery and her associates. “This is congruent with earlier trends using the National Hospital Discharge Survey. Emergency department visits [for inflammatory bowel disease] are also rising,” they added.
In contrast, cases and deaths from colorectal cancer continue to drop, partly because of intensified screening efforts, the investigators said. They called the trend “encouraging,” but noted that CRC still tops cancers of the pancreas, liver, and intrahepatic bile ducts as the leading GI cause of mortality in the United States. In 2012, more than 51,000 Americans died from CRC, and screening efforts captured only 58% of those between 50 and 75 years old. Boosting that percentage to 80% by 2018 http://nccrt.org/tools/80-percent-by-2018/ could prevent 280,000 CRC cases and 200,000 deaths within 20 years, Dr. Peery and her associates noted.
The National Institutes of Health helped fund the work. The investigators reported having no conflicts of interest.
Source: American Gastroenterological Association
FROM GASTROENTEROLOGY
Key clinical point: Gastrointestinal and liver diseases remain a major cause of health care utilization and associated costs in the United States.
Major finding: Hospital admissions and associated costs for Clostridium difficile infection, inflammatory bowel disease, and liver disease all rose substantially between 1993 and 2012.
Data source: Analysis of surveillance data from the Centers for Disease Control and Prevention, Agency for Healthcare Research and Quality, and National Cancer Institute.
Disclosures: The National Institutes of Health helped fund the work. The investigators reported having no conflicts of interest.
P2X7 receptor implicated in visceral pain caused by chronic pancreatitis
A subtype of purinergic receptor on spinal microglial cells mediated visceral pain hypersensitivity in rats with chronic pancreatitis, and pharmacologic or genetic inhibition of this receptor improved hyperalgesia, according to a report in the November issue of Cellular and Molecular Gastroenterology and Hepatology.
“Our study may be the first to identify that P2X7 receptors in spinal microglia are upregulated in chronic pancreatitis, and that this upregulation is associated with the development of visceral hyperalgesia,” said Dr. Pei-Yi Liu at National Yang-Ming University in Taipei, Taiwan, and her associates. A common laboratory dye known as brilliant blue G, which is an antagonist of P2X7R, “not only attenuated but also prevented CP-related chronic visceral hyperalgesia,” the researchers reported.
Chronic pancreatitis causes intense, recurrent epigastric pain that is “difficult and frustrating” to control and can lead to malnutrition, narcotic analgesic addiction, and social and financial problems, said the researchers. Previously, they had linked visceral pain in murine CP to activation of spinal microglia, the main effector immune cells in the central nervous system. The molecular pathways remained unclear, but some research had implicated extracellular adenosine triphosphate (ATP) as well as purinergic receptors in the CNS. Because a purine receptor subtype known as P2X7 had been linked to neuropathic and inflammatory pain, the researchers wondered if it also facilitated visceral pain (Cell Mol Gastroenterol Hepatol. 2015 Jul 22. doi: 10.1016/j.jcmgh.2015.07.008). To explore that question, they created a CP model by injecting 2% trinitrobenzene sulfonic acid into the pancreatic ducts of male rats. They measured behavioral responses to mechanical and electrical stimulation and quantified spinal cord P2X7R levels with the help of standard laboratory assays. They also watched for changes in pain-related behaviors after blocking spinal cord P2X7R with brilliant blue G or knocking it down with short interfering RNA (siRNA).
Spinal P2X7R expression rose significantly after CP induction, as did levels of the OX-42 microglial marker in the dorsal horn of the spinal cord, said the investigators. Brilliant blue G and genetic knock down suppressed P2X7R expression, inhibited activation of spinal microglia, and “significantly attenuated” nociceptive behaviors, they added.
The researchers also pretreated some rats with brilliant blue G before inducing CP and saw that these rats exhibited significantly lower pain responses to mechanical and electrical stimuli compared with other CP rats. In fact, the nociceptive responses of the pretreated CP rats resembled those of non-CP control rats, the investigators said. Spinal tissue from pretreated rats also lacked signs of P2X7R upregulation, they noted.
Taken together, the data “indicate a critical role of P2X7R expressed in the spinal cord in the development of chronic visceral pain in CP,” concluded the researchers. Brilliant blue G inhibits voltage-gated sodium channels, which are known to contribute to chronic visceral pain, and “may represent an effective drug for the treatment of chronic pain in chronic pancreatitis patients,” they added.
The study was funded by Taipei Veterans General Hospital, National Science Council of Taiwan, and the Taiwan Ministry of Education Aim for Top University Grant. The investigators declared no competing interests.
The traditional approach to treating pain in chronic pancreatitis is as if it were a “plumbing” problem – problems with ductal drainage. More recently, the emphasis has been on sensitization of the sensorineural system (“wiring”), in which the pain responses are greatly exaggerated. An additional consideration is whether this sensitization occurs in peripheral nerves that directly innervate the pancreas, or in the central nervous system, or both. This is clinically important because treatments directed at the periphery, e.g., pancreatectomy, may not be effective in patients in whom central sensitization is dominant.

|
Dr. Pankaj Jay Pasricha |
The findings of Dr. Lui and colleagues show that spinal (hence central) sensitization is important in chronic pancreatitis pain, and that this may be mediated by nonneuronal cells (microglia) in the spinal cord via P2X7R, a nucleotide receptor. This is not surprising, given that this signaling system has shown to be important in other forms of chronic pain. However, some questions remain – is peripheral sensitization driving these changes?
Clearly the “drug” they have used (BBG) is relatively harmless, but it is not practical because it may not be safe in humans (apart from coloring them blue). BBG also affects other channels, notably neuronal voltage-dependent sodium channels. Nevertheless, this study does offer new insight into the pathogenesis of pain in chronic pancreatitis and by itself is an important cautionary message for the growing enthusiasm for total pancreatectomy. It also identifies potential new therapeutic targets for treatment of pain and will, it is hoped, stimulate engagement from the pharmaceutical industry that is developing drugs directed toward glial activation and in particular the P2X7 receptor.
Dr. Pankaj Jay Pasricha, AGAF, is professor of medicine, Johns Hopkins University School of Medicine, director of Johns Hopkins Center for Motility Disorders and Digestive Diseases, and professor of innovation management, The Carey Business School, Johns Hopkins University, Baltimore. He has no conflicts of interest.
The traditional approach to treating pain in chronic pancreatitis is as if it were a “plumbing” problem – problems with ductal drainage. More recently, the emphasis has been on sensitization of the sensorineural system (“wiring”), in which the pain responses are greatly exaggerated. An additional consideration is whether this sensitization occurs in peripheral nerves that directly innervate the pancreas, or in the central nervous system, or both. This is clinically important because treatments directed at the periphery, e.g., pancreatectomy, may not be effective in patients in whom central sensitization is dominant.

|
Dr. Pankaj Jay Pasricha |
The findings of Dr. Lui and colleagues show that spinal (hence central) sensitization is important in chronic pancreatitis pain, and that this may be mediated by nonneuronal cells (microglia) in the spinal cord via P2X7R, a nucleotide receptor. This is not surprising, given that this signaling system has shown to be important in other forms of chronic pain. However, some questions remain – is peripheral sensitization driving these changes?
Clearly the “drug” they have used (BBG) is relatively harmless, but it is not practical because it may not be safe in humans (apart from coloring them blue). BBG also affects other channels, notably neuronal voltage-dependent sodium channels. Nevertheless, this study does offer new insight into the pathogenesis of pain in chronic pancreatitis and by itself is an important cautionary message for the growing enthusiasm for total pancreatectomy. It also identifies potential new therapeutic targets for treatment of pain and will, it is hoped, stimulate engagement from the pharmaceutical industry that is developing drugs directed toward glial activation and in particular the P2X7 receptor.
Dr. Pankaj Jay Pasricha, AGAF, is professor of medicine, Johns Hopkins University School of Medicine, director of Johns Hopkins Center for Motility Disorders and Digestive Diseases, and professor of innovation management, The Carey Business School, Johns Hopkins University, Baltimore. He has no conflicts of interest.
The traditional approach to treating pain in chronic pancreatitis is as if it were a “plumbing” problem – problems with ductal drainage. More recently, the emphasis has been on sensitization of the sensorineural system (“wiring”), in which the pain responses are greatly exaggerated. An additional consideration is whether this sensitization occurs in peripheral nerves that directly innervate the pancreas, or in the central nervous system, or both. This is clinically important because treatments directed at the periphery, e.g., pancreatectomy, may not be effective in patients in whom central sensitization is dominant.

|
Dr. Pankaj Jay Pasricha |
The findings of Dr. Lui and colleagues show that spinal (hence central) sensitization is important in chronic pancreatitis pain, and that this may be mediated by nonneuronal cells (microglia) in the spinal cord via P2X7R, a nucleotide receptor. This is not surprising, given that this signaling system has shown to be important in other forms of chronic pain. However, some questions remain – is peripheral sensitization driving these changes?
Clearly the “drug” they have used (BBG) is relatively harmless, but it is not practical because it may not be safe in humans (apart from coloring them blue). BBG also affects other channels, notably neuronal voltage-dependent sodium channels. Nevertheless, this study does offer new insight into the pathogenesis of pain in chronic pancreatitis and by itself is an important cautionary message for the growing enthusiasm for total pancreatectomy. It also identifies potential new therapeutic targets for treatment of pain and will, it is hoped, stimulate engagement from the pharmaceutical industry that is developing drugs directed toward glial activation and in particular the P2X7 receptor.
Dr. Pankaj Jay Pasricha, AGAF, is professor of medicine, Johns Hopkins University School of Medicine, director of Johns Hopkins Center for Motility Disorders and Digestive Diseases, and professor of innovation management, The Carey Business School, Johns Hopkins University, Baltimore. He has no conflicts of interest.
A subtype of purinergic receptor on spinal microglial cells mediated visceral pain hypersensitivity in rats with chronic pancreatitis, and pharmacologic or genetic inhibition of this receptor improved hyperalgesia, according to a report in the November issue of Cellular and Molecular Gastroenterology and Hepatology.
“Our study may be the first to identify that P2X7 receptors in spinal microglia are upregulated in chronic pancreatitis, and that this upregulation is associated with the development of visceral hyperalgesia,” said Dr. Pei-Yi Liu at National Yang-Ming University in Taipei, Taiwan, and her associates. A common laboratory dye known as brilliant blue G, which is an antagonist of P2X7R, “not only attenuated but also prevented CP-related chronic visceral hyperalgesia,” the researchers reported.
Chronic pancreatitis causes intense, recurrent epigastric pain that is “difficult and frustrating” to control and can lead to malnutrition, narcotic analgesic addiction, and social and financial problems, said the researchers. Previously, they had linked visceral pain in murine CP to activation of spinal microglia, the main effector immune cells in the central nervous system. The molecular pathways remained unclear, but some research had implicated extracellular adenosine triphosphate (ATP) as well as purinergic receptors in the CNS. Because a purine receptor subtype known as P2X7 had been linked to neuropathic and inflammatory pain, the researchers wondered if it also facilitated visceral pain (Cell Mol Gastroenterol Hepatol. 2015 Jul 22. doi: 10.1016/j.jcmgh.2015.07.008). To explore that question, they created a CP model by injecting 2% trinitrobenzene sulfonic acid into the pancreatic ducts of male rats. They measured behavioral responses to mechanical and electrical stimulation and quantified spinal cord P2X7R levels with the help of standard laboratory assays. They also watched for changes in pain-related behaviors after blocking spinal cord P2X7R with brilliant blue G or knocking it down with short interfering RNA (siRNA).
Spinal P2X7R expression rose significantly after CP induction, as did levels of the OX-42 microglial marker in the dorsal horn of the spinal cord, said the investigators. Brilliant blue G and genetic knock down suppressed P2X7R expression, inhibited activation of spinal microglia, and “significantly attenuated” nociceptive behaviors, they added.
The researchers also pretreated some rats with brilliant blue G before inducing CP and saw that these rats exhibited significantly lower pain responses to mechanical and electrical stimuli compared with other CP rats. In fact, the nociceptive responses of the pretreated CP rats resembled those of non-CP control rats, the investigators said. Spinal tissue from pretreated rats also lacked signs of P2X7R upregulation, they noted.
Taken together, the data “indicate a critical role of P2X7R expressed in the spinal cord in the development of chronic visceral pain in CP,” concluded the researchers. Brilliant blue G inhibits voltage-gated sodium channels, which are known to contribute to chronic visceral pain, and “may represent an effective drug for the treatment of chronic pain in chronic pancreatitis patients,” they added.
The study was funded by Taipei Veterans General Hospital, National Science Council of Taiwan, and the Taiwan Ministry of Education Aim for Top University Grant. The investigators declared no competing interests.
A subtype of purinergic receptor on spinal microglial cells mediated visceral pain hypersensitivity in rats with chronic pancreatitis, and pharmacologic or genetic inhibition of this receptor improved hyperalgesia, according to a report in the November issue of Cellular and Molecular Gastroenterology and Hepatology.
“Our study may be the first to identify that P2X7 receptors in spinal microglia are upregulated in chronic pancreatitis, and that this upregulation is associated with the development of visceral hyperalgesia,” said Dr. Pei-Yi Liu at National Yang-Ming University in Taipei, Taiwan, and her associates. A common laboratory dye known as brilliant blue G, which is an antagonist of P2X7R, “not only attenuated but also prevented CP-related chronic visceral hyperalgesia,” the researchers reported.
Chronic pancreatitis causes intense, recurrent epigastric pain that is “difficult and frustrating” to control and can lead to malnutrition, narcotic analgesic addiction, and social and financial problems, said the researchers. Previously, they had linked visceral pain in murine CP to activation of spinal microglia, the main effector immune cells in the central nervous system. The molecular pathways remained unclear, but some research had implicated extracellular adenosine triphosphate (ATP) as well as purinergic receptors in the CNS. Because a purine receptor subtype known as P2X7 had been linked to neuropathic and inflammatory pain, the researchers wondered if it also facilitated visceral pain (Cell Mol Gastroenterol Hepatol. 2015 Jul 22. doi: 10.1016/j.jcmgh.2015.07.008). To explore that question, they created a CP model by injecting 2% trinitrobenzene sulfonic acid into the pancreatic ducts of male rats. They measured behavioral responses to mechanical and electrical stimulation and quantified spinal cord P2X7R levels with the help of standard laboratory assays. They also watched for changes in pain-related behaviors after blocking spinal cord P2X7R with brilliant blue G or knocking it down with short interfering RNA (siRNA).
Spinal P2X7R expression rose significantly after CP induction, as did levels of the OX-42 microglial marker in the dorsal horn of the spinal cord, said the investigators. Brilliant blue G and genetic knock down suppressed P2X7R expression, inhibited activation of spinal microglia, and “significantly attenuated” nociceptive behaviors, they added.
The researchers also pretreated some rats with brilliant blue G before inducing CP and saw that these rats exhibited significantly lower pain responses to mechanical and electrical stimuli compared with other CP rats. In fact, the nociceptive responses of the pretreated CP rats resembled those of non-CP control rats, the investigators said. Spinal tissue from pretreated rats also lacked signs of P2X7R upregulation, they noted.
Taken together, the data “indicate a critical role of P2X7R expressed in the spinal cord in the development of chronic visceral pain in CP,” concluded the researchers. Brilliant blue G inhibits voltage-gated sodium channels, which are known to contribute to chronic visceral pain, and “may represent an effective drug for the treatment of chronic pain in chronic pancreatitis patients,” they added.
The study was funded by Taipei Veterans General Hospital, National Science Council of Taiwan, and the Taiwan Ministry of Education Aim for Top University Grant. The investigators declared no competing interests.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: A subtype of purinergic receptor on spinal microglial cells mediated visceral pain hypersensitivity in rats with chronic pancreatitis.
Major finding: Spinal P2X7R expression rose significantly after CP induction, and pharmacologic inhibition and genetic knock down inhibited activation of spinal microglia and “significantly attenuated” nociceptive behaviors.
Data source: Controlled, prospective, molecular and behavioral study of rats with chemically induced chronic pancreatitis.
Disclosures: The study was funded by Taipei Veterans General Hospital, National Science Council of Taiwan, and the Taiwan Ministry of Education Aim for Top University Grant. The investigators declared no competing interests.
No link found between IBS and serologic markers for celiac disease
Irritable bowel syndrome did not increase the likelihood of having serologic markers of celiac disease, according to a study of more than 3,000 residents of Southeastern Minnesota reported in the November issue of Clinical Gastroenterology and Hepatology.
Although several current guidelines list IBS as a risk factor for celiac disease, “our results suggest that testing for celiac disease [CD] in IBS will not have a significantly increased yield over population-based serologic screening,” said Dr. Rok Seon Choung of the Mayo Clinic, Rochester, Minn., and his associates. “In terms of IBS and other major GI syndromes, undetected CD does not appear to be positively associated with GI symptoms in the United States community.”
Despite widely available screening tests for CD, at least 80% of cases go undiagnosed. Testing based only on the presence of malabsorptive signs and symptoms misses many cases because of the trend toward “nonclassic” CD, said the researchers. “Physicians are especially likely to encounter patients with CD who have no classic symptoms while investigating other GI disorders,” they noted. “We aimed to determine whether positive results of serologic testing for CD by using a highly sensitive and specific assaywere associated with IBS and other functional gastrointestinal disorders in a large representative U.S. white population” (Clin Gastroenterol Hepatol. 2015 May doi: 10.1016/j.cgh.2015.05.014).
The investigators sent validated self-report bowel disease questionnaires to randomly chosen adults living in Olmsted County in Southeastern Minnesota. They also performed CD testing on serum from a convenience sample of 47,000 county residents with no prior diagnosis of CD. In all, 3,202 subjects completed questionnaires and had serum available for testing. About 55% of this group reported at least one GI symptom (95% confidence interval, 53%-57%), while 13.6% met criteria for IBS (95% CI, 12%-15%), the researchers said. A total of 1% of respondents had serologic markers for CD (95% CI, 0.7%-1.4%), in keeping with other epidemiologic studies in the United States, they added.
Notably, IBS affected only 3% of CD patients, compared with 14% of patients without CD, although the difference was not statistically significant (OR, 0.2; 95% CI, 0.03-1.5), the investigators said. Seropositive CD patients most often reported abdominal pain, constipation, weight loss, and dyspepsia, but none of these GI symptoms and no functional GI disorders were significantly more prevalent in CD patients than in non-CD patients. “These results may have important management and screening implications,” said the researchers. “Cost-effectiveness data suggest that testing for CD in patients with diarrhea-predominant IBS has an acceptable cost when the prevalence is above 1%, and becomes the dominant strategy when the prevalence exceeds 8%. However, we cannot confirm whether CD testing is a cost-effective approach in our population.”
The findings should be generalizable to white Americans, but not to the U.S. population as a whole because most participants were white, the researchers noted. “The prevalence of CD may vary by ethnic group, but the disease has been shown to be more common in whites than in other races,” they added. Responder bias was also possible, but past studies of the same bowel disease questionnaire uncovered no significant differences in rates of GI symptoms between responders and nonresponders, they noted.
The National Institutes of Health funded part of the work. Coauthor Dr. Nicholas Talley reported having colicensed the questionnaire used in the study. The remaining authors disclosed no financial conflicts.
In the well-designed and rigorous study by Choung et al., the authors conducted a community-based, cross-sectional survey among residents of Olmsted County, Minn., collecting data on symptoms compatible with functional GI disorders, including irritable bowel syndrome; the authors linked these data to prevalence surveys testing for undiagnosed celiac disease using serologic tests conducted among more than 47,000 individuals within the same regio

|
| Dr. Alexander Ford |
Patients with celiac disease may present with GI symptoms such as abdominal pain, bloating, and diarrhea, leading to confusion with IBS and diagnostic delay. Current guidelines, therefore, recommend screening patients consulting with IBS-type symptoms routinely for celiac disease. Despite this, in the study only 3% of individuals with positive celiac serology met the criteria for IBS, compared with 14% of those testing negative. Also of note is that subjects with positive serology were no more likely to report other GI symptoms felt to be typical presenting features of celiac disease, including abdominal pain, diarrhea, bloating, or abdominal distension. This suggests the yield of opportunistic screening of people reporting GI symptoms in the U.S. community is low.
However, current guidelines do not recommend screening people with IBS for celiac disease in the general population, and based their recommendations on studies conducted among patients consulting with GI symptoms. As a result, although the authors concluded, justifiably, that testing in the community is unlikely to have a significantly increased yield over population-based screening, it should not lead to a change in recommendations for practice in either primary or secondary care in other countries.
Dr. Alexander C. Ford is associate professor and honorary consultant gastroenterologist at Leeds Gastroenterology Institute, St. James’s University Hospital, and Leeds (England) Institute of Biomedical and Clinical Sciences, University of Leeds. He had no relevant financial conflicts of interest.
In the well-designed and rigorous study by Choung et al., the authors conducted a community-based, cross-sectional survey among residents of Olmsted County, Minn., collecting data on symptoms compatible with functional GI disorders, including irritable bowel syndrome; the authors linked these data to prevalence surveys testing for undiagnosed celiac disease using serologic tests conducted among more than 47,000 individuals within the same regio

|
| Dr. Alexander Ford |
Patients with celiac disease may present with GI symptoms such as abdominal pain, bloating, and diarrhea, leading to confusion with IBS and diagnostic delay. Current guidelines, therefore, recommend screening patients consulting with IBS-type symptoms routinely for celiac disease. Despite this, in the study only 3% of individuals with positive celiac serology met the criteria for IBS, compared with 14% of those testing negative. Also of note is that subjects with positive serology were no more likely to report other GI symptoms felt to be typical presenting features of celiac disease, including abdominal pain, diarrhea, bloating, or abdominal distension. This suggests the yield of opportunistic screening of people reporting GI symptoms in the U.S. community is low.
However, current guidelines do not recommend screening people with IBS for celiac disease in the general population, and based their recommendations on studies conducted among patients consulting with GI symptoms. As a result, although the authors concluded, justifiably, that testing in the community is unlikely to have a significantly increased yield over population-based screening, it should not lead to a change in recommendations for practice in either primary or secondary care in other countries.
Dr. Alexander C. Ford is associate professor and honorary consultant gastroenterologist at Leeds Gastroenterology Institute, St. James’s University Hospital, and Leeds (England) Institute of Biomedical and Clinical Sciences, University of Leeds. He had no relevant financial conflicts of interest.
In the well-designed and rigorous study by Choung et al., the authors conducted a community-based, cross-sectional survey among residents of Olmsted County, Minn., collecting data on symptoms compatible with functional GI disorders, including irritable bowel syndrome; the authors linked these data to prevalence surveys testing for undiagnosed celiac disease using serologic tests conducted among more than 47,000 individuals within the same regio

|
| Dr. Alexander Ford |
Patients with celiac disease may present with GI symptoms such as abdominal pain, bloating, and diarrhea, leading to confusion with IBS and diagnostic delay. Current guidelines, therefore, recommend screening patients consulting with IBS-type symptoms routinely for celiac disease. Despite this, in the study only 3% of individuals with positive celiac serology met the criteria for IBS, compared with 14% of those testing negative. Also of note is that subjects with positive serology were no more likely to report other GI symptoms felt to be typical presenting features of celiac disease, including abdominal pain, diarrhea, bloating, or abdominal distension. This suggests the yield of opportunistic screening of people reporting GI symptoms in the U.S. community is low.
However, current guidelines do not recommend screening people with IBS for celiac disease in the general population, and based their recommendations on studies conducted among patients consulting with GI symptoms. As a result, although the authors concluded, justifiably, that testing in the community is unlikely to have a significantly increased yield over population-based screening, it should not lead to a change in recommendations for practice in either primary or secondary care in other countries.
Dr. Alexander C. Ford is associate professor and honorary consultant gastroenterologist at Leeds Gastroenterology Institute, St. James’s University Hospital, and Leeds (England) Institute of Biomedical and Clinical Sciences, University of Leeds. He had no relevant financial conflicts of interest.
Irritable bowel syndrome did not increase the likelihood of having serologic markers of celiac disease, according to a study of more than 3,000 residents of Southeastern Minnesota reported in the November issue of Clinical Gastroenterology and Hepatology.
Although several current guidelines list IBS as a risk factor for celiac disease, “our results suggest that testing for celiac disease [CD] in IBS will not have a significantly increased yield over population-based serologic screening,” said Dr. Rok Seon Choung of the Mayo Clinic, Rochester, Minn., and his associates. “In terms of IBS and other major GI syndromes, undetected CD does not appear to be positively associated with GI symptoms in the United States community.”
Despite widely available screening tests for CD, at least 80% of cases go undiagnosed. Testing based only on the presence of malabsorptive signs and symptoms misses many cases because of the trend toward “nonclassic” CD, said the researchers. “Physicians are especially likely to encounter patients with CD who have no classic symptoms while investigating other GI disorders,” they noted. “We aimed to determine whether positive results of serologic testing for CD by using a highly sensitive and specific assaywere associated with IBS and other functional gastrointestinal disorders in a large representative U.S. white population” (Clin Gastroenterol Hepatol. 2015 May doi: 10.1016/j.cgh.2015.05.014).
The investigators sent validated self-report bowel disease questionnaires to randomly chosen adults living in Olmsted County in Southeastern Minnesota. They also performed CD testing on serum from a convenience sample of 47,000 county residents with no prior diagnosis of CD. In all, 3,202 subjects completed questionnaires and had serum available for testing. About 55% of this group reported at least one GI symptom (95% confidence interval, 53%-57%), while 13.6% met criteria for IBS (95% CI, 12%-15%), the researchers said. A total of 1% of respondents had serologic markers for CD (95% CI, 0.7%-1.4%), in keeping with other epidemiologic studies in the United States, they added.
Notably, IBS affected only 3% of CD patients, compared with 14% of patients without CD, although the difference was not statistically significant (OR, 0.2; 95% CI, 0.03-1.5), the investigators said. Seropositive CD patients most often reported abdominal pain, constipation, weight loss, and dyspepsia, but none of these GI symptoms and no functional GI disorders were significantly more prevalent in CD patients than in non-CD patients. “These results may have important management and screening implications,” said the researchers. “Cost-effectiveness data suggest that testing for CD in patients with diarrhea-predominant IBS has an acceptable cost when the prevalence is above 1%, and becomes the dominant strategy when the prevalence exceeds 8%. However, we cannot confirm whether CD testing is a cost-effective approach in our population.”
The findings should be generalizable to white Americans, but not to the U.S. population as a whole because most participants were white, the researchers noted. “The prevalence of CD may vary by ethnic group, but the disease has been shown to be more common in whites than in other races,” they added. Responder bias was also possible, but past studies of the same bowel disease questionnaire uncovered no significant differences in rates of GI symptoms between responders and nonresponders, they noted.
The National Institutes of Health funded part of the work. Coauthor Dr. Nicholas Talley reported having colicensed the questionnaire used in the study. The remaining authors disclosed no financial conflicts.
Irritable bowel syndrome did not increase the likelihood of having serologic markers of celiac disease, according to a study of more than 3,000 residents of Southeastern Minnesota reported in the November issue of Clinical Gastroenterology and Hepatology.
Although several current guidelines list IBS as a risk factor for celiac disease, “our results suggest that testing for celiac disease [CD] in IBS will not have a significantly increased yield over population-based serologic screening,” said Dr. Rok Seon Choung of the Mayo Clinic, Rochester, Minn., and his associates. “In terms of IBS and other major GI syndromes, undetected CD does not appear to be positively associated with GI symptoms in the United States community.”
Despite widely available screening tests for CD, at least 80% of cases go undiagnosed. Testing based only on the presence of malabsorptive signs and symptoms misses many cases because of the trend toward “nonclassic” CD, said the researchers. “Physicians are especially likely to encounter patients with CD who have no classic symptoms while investigating other GI disorders,” they noted. “We aimed to determine whether positive results of serologic testing for CD by using a highly sensitive and specific assaywere associated with IBS and other functional gastrointestinal disorders in a large representative U.S. white population” (Clin Gastroenterol Hepatol. 2015 May doi: 10.1016/j.cgh.2015.05.014).
The investigators sent validated self-report bowel disease questionnaires to randomly chosen adults living in Olmsted County in Southeastern Minnesota. They also performed CD testing on serum from a convenience sample of 47,000 county residents with no prior diagnosis of CD. In all, 3,202 subjects completed questionnaires and had serum available for testing. About 55% of this group reported at least one GI symptom (95% confidence interval, 53%-57%), while 13.6% met criteria for IBS (95% CI, 12%-15%), the researchers said. A total of 1% of respondents had serologic markers for CD (95% CI, 0.7%-1.4%), in keeping with other epidemiologic studies in the United States, they added.
Notably, IBS affected only 3% of CD patients, compared with 14% of patients without CD, although the difference was not statistically significant (OR, 0.2; 95% CI, 0.03-1.5), the investigators said. Seropositive CD patients most often reported abdominal pain, constipation, weight loss, and dyspepsia, but none of these GI symptoms and no functional GI disorders were significantly more prevalent in CD patients than in non-CD patients. “These results may have important management and screening implications,” said the researchers. “Cost-effectiveness data suggest that testing for CD in patients with diarrhea-predominant IBS has an acceptable cost when the prevalence is above 1%, and becomes the dominant strategy when the prevalence exceeds 8%. However, we cannot confirm whether CD testing is a cost-effective approach in our population.”
The findings should be generalizable to white Americans, but not to the U.S. population as a whole because most participants were white, the researchers noted. “The prevalence of CD may vary by ethnic group, but the disease has been shown to be more common in whites than in other races,” they added. Responder bias was also possible, but past studies of the same bowel disease questionnaire uncovered no significant differences in rates of GI symptoms between responders and nonresponders, they noted.
The National Institutes of Health funded part of the work. Coauthor Dr. Nicholas Talley reported having colicensed the questionnaire used in the study. The remaining authors disclosed no financial conflicts.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Irritable bowel syndrome did not increase the likelihood of seropositivity for celiac disease.
Major finding: Patients with IBS were no more likely than others to have serologic markers for celiac disease (odds ratio, 0.2; 95% confidence interval, 0.03-1.5).
Data source: An analysis of bowel symptom surveys and serum samples from 3,202 residents of one county.
Disclosures: The National Institutes of Health funded part of the work. Coauthor Dr. Nicholas Talley reported having colicensed the questionnaire used in the study. The remaining authors disclosed no conflicts.
Low-FODMAP and traditional IBS diets found equally effective for symptom reduction
Advising patients with irritable bowel syndrome to cut their intake of fermentable short-chain carbohydrates improved GI symptoms as much as “traditional” recommendations to reduce meal size, gas-producing foods, insoluble fiber, fat, and caffeine, investigators reported in a randomized, multicenter, single-blinded study that appears in the November issue of Gastroenterology.
“Combining elements from these two strategies might further reduce symptoms of IBS,” said Lena Böhn, a registered dietician at the University of Gothenburg (Sweden) and her associates. Clinicians, however, should be aware that patients may cut calories in response to dietary advice even if they do not need to do so, which could eventually lead to malnutrition. “Monitoring calorie and nutrient intake in patients who follow dietary advice seems important,” the investigators wrote.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) such as apples, beans, white bread, and milk are poorly absorbed in the small intestine, which can trigger bouts of gas from colonic bacterial fermentation and diarrhea because of osmotic water transfer into the lumen of the colon. Several recent studies had linked FODMAPs to GI symptoms in IBS, but no prior randomized controlled trial had compared real-world recommendations to follow either a low-FODMAP or traditional IBS diet, the researchers noted (Gastroenterology 2015. doi: 10.1053/j.gastro.2015.07.056).
For the study, they randomized 75 patients who met Rome III IBS criteria to either the low-FODMAP or traditional IBS diet for 4 weeks. They used the IBS severity scoring system (Aliment Pharmacol Ther. 1997;11[2]:395-402) to assess symptomatic response and studied food diaries completed before and after the interventions to understand how closely patients followed the dietary advice.
A total of 67 patients completed the study, including 56 women and 14 men, Ms. Böhn and her associates reported. Both diets led to similarly significant (P < .0001) decreases in IBS symptoms, with no clear differences between them. Half the patients in the low-FODMAP group experienced at least a 50-point improvement in their IBS severity score, compared with 46% of patients in the traditional IBS diet cohort (P = .72).
Food diaries showed that patients adhered well to their diets, the investigators said, but “an unwanted and somewhat surprising finding” was that patients cut their caloric intake – by an average of 442 kcal/day on the low-FODMAP diet and almost 200 kcal/day on the traditional diet. “We hypothesize that even though patients were not advised to reduce calorie intake, receiving detailed dietary advice [to] limit intake of certain food constituents may result in this unwanted effect,” said the investigators. “In the short term, this should not be harmful, but a lesson from this trial is that calorie and nutrient intake needs to be supervised in order to avoid malnutrition if long-term dietary changes are initiated.”
The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
Advising patients with irritable bowel syndrome to cut their intake of fermentable short-chain carbohydrates improved GI symptoms as much as “traditional” recommendations to reduce meal size, gas-producing foods, insoluble fiber, fat, and caffeine, investigators reported in a randomized, multicenter, single-blinded study that appears in the November issue of Gastroenterology.
“Combining elements from these two strategies might further reduce symptoms of IBS,” said Lena Böhn, a registered dietician at the University of Gothenburg (Sweden) and her associates. Clinicians, however, should be aware that patients may cut calories in response to dietary advice even if they do not need to do so, which could eventually lead to malnutrition. “Monitoring calorie and nutrient intake in patients who follow dietary advice seems important,” the investigators wrote.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) such as apples, beans, white bread, and milk are poorly absorbed in the small intestine, which can trigger bouts of gas from colonic bacterial fermentation and diarrhea because of osmotic water transfer into the lumen of the colon. Several recent studies had linked FODMAPs to GI symptoms in IBS, but no prior randomized controlled trial had compared real-world recommendations to follow either a low-FODMAP or traditional IBS diet, the researchers noted (Gastroenterology 2015. doi: 10.1053/j.gastro.2015.07.056).
For the study, they randomized 75 patients who met Rome III IBS criteria to either the low-FODMAP or traditional IBS diet for 4 weeks. They used the IBS severity scoring system (Aliment Pharmacol Ther. 1997;11[2]:395-402) to assess symptomatic response and studied food diaries completed before and after the interventions to understand how closely patients followed the dietary advice.
A total of 67 patients completed the study, including 56 women and 14 men, Ms. Böhn and her associates reported. Both diets led to similarly significant (P < .0001) decreases in IBS symptoms, with no clear differences between them. Half the patients in the low-FODMAP group experienced at least a 50-point improvement in their IBS severity score, compared with 46% of patients in the traditional IBS diet cohort (P = .72).
Food diaries showed that patients adhered well to their diets, the investigators said, but “an unwanted and somewhat surprising finding” was that patients cut their caloric intake – by an average of 442 kcal/day on the low-FODMAP diet and almost 200 kcal/day on the traditional diet. “We hypothesize that even though patients were not advised to reduce calorie intake, receiving detailed dietary advice [to] limit intake of certain food constituents may result in this unwanted effect,” said the investigators. “In the short term, this should not be harmful, but a lesson from this trial is that calorie and nutrient intake needs to be supervised in order to avoid malnutrition if long-term dietary changes are initiated.”
The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
Advising patients with irritable bowel syndrome to cut their intake of fermentable short-chain carbohydrates improved GI symptoms as much as “traditional” recommendations to reduce meal size, gas-producing foods, insoluble fiber, fat, and caffeine, investigators reported in a randomized, multicenter, single-blinded study that appears in the November issue of Gastroenterology.
“Combining elements from these two strategies might further reduce symptoms of IBS,” said Lena Böhn, a registered dietician at the University of Gothenburg (Sweden) and her associates. Clinicians, however, should be aware that patients may cut calories in response to dietary advice even if they do not need to do so, which could eventually lead to malnutrition. “Monitoring calorie and nutrient intake in patients who follow dietary advice seems important,” the investigators wrote.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) such as apples, beans, white bread, and milk are poorly absorbed in the small intestine, which can trigger bouts of gas from colonic bacterial fermentation and diarrhea because of osmotic water transfer into the lumen of the colon. Several recent studies had linked FODMAPs to GI symptoms in IBS, but no prior randomized controlled trial had compared real-world recommendations to follow either a low-FODMAP or traditional IBS diet, the researchers noted (Gastroenterology 2015. doi: 10.1053/j.gastro.2015.07.056).
For the study, they randomized 75 patients who met Rome III IBS criteria to either the low-FODMAP or traditional IBS diet for 4 weeks. They used the IBS severity scoring system (Aliment Pharmacol Ther. 1997;11[2]:395-402) to assess symptomatic response and studied food diaries completed before and after the interventions to understand how closely patients followed the dietary advice.
A total of 67 patients completed the study, including 56 women and 14 men, Ms. Böhn and her associates reported. Both diets led to similarly significant (P < .0001) decreases in IBS symptoms, with no clear differences between them. Half the patients in the low-FODMAP group experienced at least a 50-point improvement in their IBS severity score, compared with 46% of patients in the traditional IBS diet cohort (P = .72).
Food diaries showed that patients adhered well to their diets, the investigators said, but “an unwanted and somewhat surprising finding” was that patients cut their caloric intake – by an average of 442 kcal/day on the low-FODMAP diet and almost 200 kcal/day on the traditional diet. “We hypothesize that even though patients were not advised to reduce calorie intake, receiving detailed dietary advice [to] limit intake of certain food constituents may result in this unwanted effect,” said the investigators. “In the short term, this should not be harmful, but a lesson from this trial is that calorie and nutrient intake needs to be supervised in order to avoid malnutrition if long-term dietary changes are initiated.”
The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
FROM GASTROENTEROLOGY
Key clinical point: Diets low in fermentable short-chain carbohydrates cut irritable bowel disease symptoms as effectively as did “traditional” IBS diets.
Major finding: After 4 weeks, patients in both groups experienced similar and significant (P < .0001) decreases in IBS symptoms.
Data source: A randomized, multicenter, parallel-group, single-blinded study of 75 patients.
Disclosures: The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
Low-FODMAP and traditional IBS diets found equally effective for symptom reduction
Advising patients with irritable bowel syndrome to cut their intake of fermentable short-chain carbohydrates improved GI symptoms as much as “traditional” recommendations to reduce meal size, gas-producing foods, insoluble fiber, fat, and caffeine, investigators reported in a randomized, multicenter, single-blinded study that appears in the November issue of Gastroenterology.
“Combining elements from these two strategies might further reduce symptoms of IBS,” said Lena Böhn, a registered dietician at the University of Gothenburg (Sweden) and her associates. Clinicians, however, should be aware that patients may cut calories in response to dietary advice even if they do not need to do so, which could eventually lead to malnutrition. “Monitoring calorie and nutrient intake in patients who follow dietary advice seems important,” the investigators wrote.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) such as apples, beans, white bread, and milk are poorly absorbed in the small intestine, which can trigger bouts of gas from colonic bacterial fermentation and diarrhea because of osmotic water transfer into the lumen of the colon. Several recent studies had linked FODMAPs to GI symptoms in IBS, but no prior randomized controlled trial had compared real-world recommendations to follow either a low-FODMAP or traditional IBS diet, the researchers noted (Gastroenterology 2015. doi: 10.1053/j.gastro.2015.07.056).
For the study, they randomized 75 patients who met Rome III IBS criteria to either the low-FODMAP or traditional IBS diet for 4 weeks. They used the IBS severity scoring system (Aliment Pharmacol Ther. 1997;11[2]:395-402) to assess symptomatic response and studied food diaries completed before and after the interventions to understand how closely patients followed the dietary advice.
A total of 67 patients completed the study, including 56 women and 14 men, Ms. Böhn and her associates reported. Both diets led to similarly significant (P < .0001) decreases in IBS symptoms, with no clear differences between them. Half the patients in the low-FODMAP group experienced at least a 50-point improvement in their IBS severity score, compared with 46% of patients in the traditional IBS diet cohort (P = .72).
Food diaries showed that patients adhered well to their diets, the investigators said, but “an unwanted and somewhat surprising finding” was that patients cut their caloric intake – by an average of 442 kcal/day on the low-FODMAP diet and almost 200 kcal/day on the traditional diet. “We hypothesize that even though patients were not advised to reduce calorie intake, receiving detailed dietary advice [to] limit intake of certain food constituents may result in this unwanted effect,” said the investigators. “In the short term, this should not be harmful, but a lesson from this trial is that calorie and nutrient intake needs to be supervised in order to avoid malnutrition if long-term dietary changes are initiated.”
The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
Advising patients with irritable bowel syndrome to cut their intake of fermentable short-chain carbohydrates improved GI symptoms as much as “traditional” recommendations to reduce meal size, gas-producing foods, insoluble fiber, fat, and caffeine, investigators reported in a randomized, multicenter, single-blinded study that appears in the November issue of Gastroenterology.
“Combining elements from these two strategies might further reduce symptoms of IBS,” said Lena Böhn, a registered dietician at the University of Gothenburg (Sweden) and her associates. Clinicians, however, should be aware that patients may cut calories in response to dietary advice even if they do not need to do so, which could eventually lead to malnutrition. “Monitoring calorie and nutrient intake in patients who follow dietary advice seems important,” the investigators wrote.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) such as apples, beans, white bread, and milk are poorly absorbed in the small intestine, which can trigger bouts of gas from colonic bacterial fermentation and diarrhea because of osmotic water transfer into the lumen of the colon. Several recent studies had linked FODMAPs to GI symptoms in IBS, but no prior randomized controlled trial had compared real-world recommendations to follow either a low-FODMAP or traditional IBS diet, the researchers noted (Gastroenterology 2015. doi: 10.1053/j.gastro.2015.07.056).
For the study, they randomized 75 patients who met Rome III IBS criteria to either the low-FODMAP or traditional IBS diet for 4 weeks. They used the IBS severity scoring system (Aliment Pharmacol Ther. 1997;11[2]:395-402) to assess symptomatic response and studied food diaries completed before and after the interventions to understand how closely patients followed the dietary advice.
A total of 67 patients completed the study, including 56 women and 14 men, Ms. Böhn and her associates reported. Both diets led to similarly significant (P < .0001) decreases in IBS symptoms, with no clear differences between them. Half the patients in the low-FODMAP group experienced at least a 50-point improvement in their IBS severity score, compared with 46% of patients in the traditional IBS diet cohort (P = .72).
Food diaries showed that patients adhered well to their diets, the investigators said, but “an unwanted and somewhat surprising finding” was that patients cut their caloric intake – by an average of 442 kcal/day on the low-FODMAP diet and almost 200 kcal/day on the traditional diet. “We hypothesize that even though patients were not advised to reduce calorie intake, receiving detailed dietary advice [to] limit intake of certain food constituents may result in this unwanted effect,” said the investigators. “In the short term, this should not be harmful, but a lesson from this trial is that calorie and nutrient intake needs to be supervised in order to avoid malnutrition if long-term dietary changes are initiated.”
The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
Advising patients with irritable bowel syndrome to cut their intake of fermentable short-chain carbohydrates improved GI symptoms as much as “traditional” recommendations to reduce meal size, gas-producing foods, insoluble fiber, fat, and caffeine, investigators reported in a randomized, multicenter, single-blinded study that appears in the November issue of Gastroenterology.
“Combining elements from these two strategies might further reduce symptoms of IBS,” said Lena Böhn, a registered dietician at the University of Gothenburg (Sweden) and her associates. Clinicians, however, should be aware that patients may cut calories in response to dietary advice even if they do not need to do so, which could eventually lead to malnutrition. “Monitoring calorie and nutrient intake in patients who follow dietary advice seems important,” the investigators wrote.
Fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs) such as apples, beans, white bread, and milk are poorly absorbed in the small intestine, which can trigger bouts of gas from colonic bacterial fermentation and diarrhea because of osmotic water transfer into the lumen of the colon. Several recent studies had linked FODMAPs to GI symptoms in IBS, but no prior randomized controlled trial had compared real-world recommendations to follow either a low-FODMAP or traditional IBS diet, the researchers noted (Gastroenterology 2015. doi: 10.1053/j.gastro.2015.07.056).
For the study, they randomized 75 patients who met Rome III IBS criteria to either the low-FODMAP or traditional IBS diet for 4 weeks. They used the IBS severity scoring system (Aliment Pharmacol Ther. 1997;11[2]:395-402) to assess symptomatic response and studied food diaries completed before and after the interventions to understand how closely patients followed the dietary advice.
A total of 67 patients completed the study, including 56 women and 14 men, Ms. Böhn and her associates reported. Both diets led to similarly significant (P < .0001) decreases in IBS symptoms, with no clear differences between them. Half the patients in the low-FODMAP group experienced at least a 50-point improvement in their IBS severity score, compared with 46% of patients in the traditional IBS diet cohort (P = .72).
Food diaries showed that patients adhered well to their diets, the investigators said, but “an unwanted and somewhat surprising finding” was that patients cut their caloric intake – by an average of 442 kcal/day on the low-FODMAP diet and almost 200 kcal/day on the traditional diet. “We hypothesize that even though patients were not advised to reduce calorie intake, receiving detailed dietary advice [to] limit intake of certain food constituents may result in this unwanted effect,” said the investigators. “In the short term, this should not be harmful, but a lesson from this trial is that calorie and nutrient intake needs to be supervised in order to avoid malnutrition if long-term dietary changes are initiated.”
The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
FROM GASTROENTEROLOGY
Key clinical point: Diets low in fermentable short-chain carbohydrates cut irritable bowel disease symptoms as effectively as did “traditional” IBS diets.
Major finding: After 4 weeks, patients in both groups experienced similar and significant (P < .0001) decreases in IBS symptoms.
Data source: A randomized, multicenter, parallel-group, single-blinded study of 75 patients.
Disclosures: The study was supported by the Swedish Medical Research Council and by the University of Gothenburg’s Marianne and Marcus Wallenberg Foundation, Centre for Person-Centered Care, and Faculty of Medicine. The investigators declared no competing interests.
No link found between immunosuppression and anal dysplasia in IBD
Immunosuppression did not affect the probability of abnormal anal cytology among patients with inflammatory bowel disease (IBD), according to a prospective, single-center, cross-sectional study of 270 adults published in the November issue of Clinical Gastroenterology and Hepatology.
“Although our study suggested that immunosuppression may not play a role in the risk of dysplasia, a question remains as to whether it contributes to malignant transformation in patients with dysplasia. More research needs to be performed to identify the utility of wider anal dysplasia screening programs in high-risk populations, and the role of HPV vaccine in prevention,” Dr. Shamita Shah of Stanford (Calif.) University and her associates wrote in Clinical Gastroenterology and Hepatology.
Immunosuppression is a cornerstone of IBD management. Because immunosuppressive medications inhibit cell-mediated immunity, patients are at increased risk of opportunistic infections and neoplasias, the researchers noted. One study reported a greater risk of cervical dysplasia among immunosuppressed women with IBD, but the risk of anal dysplasia and cancer in IBD has not been well studied, they said (Clinical Gasteroenterol Hepatol. 2015. doi: 10.1016/j.cgh.2015.05.031).
To examine associations between anal dysplasia and IBD, human papillomavirus infection, and immunosuppression, the researchers analyzed anal Pap tests from 100 IBD patients who were immunosuppressed, 94 IBD patients who were not immunosuppressed, and 76 healthy controls. They identified 19 cases of atypical squamous cells of undetermined significance (ASCUS). The prevalence of ASCUS was somewhat higher among IBD patients (8.8%) than controls (2.6%; P = 0.1), but did not vary based on immunosuppression status or HPV infection. High-risk HPV occurred in 2% of the entire cohort, including 11% of patients with ASCUS and 1.5% of patients with normal Pap cytology (P = .01). High-resolution anoscopy of six patients with ASCUS revealed two cases of condylomatous disease, but no biopsy-positive dysplasia, the investigators reported.
Patients with Crohn’s disease had a significantly higher prevalence of ASCUS than other study participants (P = .02), but patients with ulcerative colitis or unspecified IBD did not, said the researchers. Having had Crohn’s disease for at least 10 years was associated with a fivefold increase in the odds of ASCUS in the multivariate analysis (95% confidence interval, 1.9-13.6), and female sex was also a risk factor (odds ratio, 3.3; P = .047). Notably, women with long-standing Crohn’s disease were almost five times more likely to have abnormal anal Pap cytology than other subjects (P = .0038), the investigators said. “One proposed mechanism for this was a reduction in human defensins in Crohn’s disease patients,” they added. Defensins – antiviral proteins that are found in immune cells – are known to inhibit cutaneous and mucosal HPV, and can be decreased in patients with Crohn’s disease for several reasons, they said. “Although more research is needed in this area, the hindered ability for an IBD patient to defend against viral illness may be responsible for their susceptibility to HPV-related cervical and anal neoplasias.”
Hologic donated the anal Pap tests used in the study. The researchers disclosed no conflicts of interest.
Immunosuppression did not affect the probability of abnormal anal cytology among patients with inflammatory bowel disease (IBD), according to a prospective, single-center, cross-sectional study of 270 adults published in the November issue of Clinical Gastroenterology and Hepatology.
“Although our study suggested that immunosuppression may not play a role in the risk of dysplasia, a question remains as to whether it contributes to malignant transformation in patients with dysplasia. More research needs to be performed to identify the utility of wider anal dysplasia screening programs in high-risk populations, and the role of HPV vaccine in prevention,” Dr. Shamita Shah of Stanford (Calif.) University and her associates wrote in Clinical Gastroenterology and Hepatology.
Immunosuppression is a cornerstone of IBD management. Because immunosuppressive medications inhibit cell-mediated immunity, patients are at increased risk of opportunistic infections and neoplasias, the researchers noted. One study reported a greater risk of cervical dysplasia among immunosuppressed women with IBD, but the risk of anal dysplasia and cancer in IBD has not been well studied, they said (Clinical Gasteroenterol Hepatol. 2015. doi: 10.1016/j.cgh.2015.05.031).
To examine associations between anal dysplasia and IBD, human papillomavirus infection, and immunosuppression, the researchers analyzed anal Pap tests from 100 IBD patients who were immunosuppressed, 94 IBD patients who were not immunosuppressed, and 76 healthy controls. They identified 19 cases of atypical squamous cells of undetermined significance (ASCUS). The prevalence of ASCUS was somewhat higher among IBD patients (8.8%) than controls (2.6%; P = 0.1), but did not vary based on immunosuppression status or HPV infection. High-risk HPV occurred in 2% of the entire cohort, including 11% of patients with ASCUS and 1.5% of patients with normal Pap cytology (P = .01). High-resolution anoscopy of six patients with ASCUS revealed two cases of condylomatous disease, but no biopsy-positive dysplasia, the investigators reported.
Patients with Crohn’s disease had a significantly higher prevalence of ASCUS than other study participants (P = .02), but patients with ulcerative colitis or unspecified IBD did not, said the researchers. Having had Crohn’s disease for at least 10 years was associated with a fivefold increase in the odds of ASCUS in the multivariate analysis (95% confidence interval, 1.9-13.6), and female sex was also a risk factor (odds ratio, 3.3; P = .047). Notably, women with long-standing Crohn’s disease were almost five times more likely to have abnormal anal Pap cytology than other subjects (P = .0038), the investigators said. “One proposed mechanism for this was a reduction in human defensins in Crohn’s disease patients,” they added. Defensins – antiviral proteins that are found in immune cells – are known to inhibit cutaneous and mucosal HPV, and can be decreased in patients with Crohn’s disease for several reasons, they said. “Although more research is needed in this area, the hindered ability for an IBD patient to defend against viral illness may be responsible for their susceptibility to HPV-related cervical and anal neoplasias.”
Hologic donated the anal Pap tests used in the study. The researchers disclosed no conflicts of interest.
Immunosuppression did not affect the probability of abnormal anal cytology among patients with inflammatory bowel disease (IBD), according to a prospective, single-center, cross-sectional study of 270 adults published in the November issue of Clinical Gastroenterology and Hepatology.
“Although our study suggested that immunosuppression may not play a role in the risk of dysplasia, a question remains as to whether it contributes to malignant transformation in patients with dysplasia. More research needs to be performed to identify the utility of wider anal dysplasia screening programs in high-risk populations, and the role of HPV vaccine in prevention,” Dr. Shamita Shah of Stanford (Calif.) University and her associates wrote in Clinical Gastroenterology and Hepatology.
Immunosuppression is a cornerstone of IBD management. Because immunosuppressive medications inhibit cell-mediated immunity, patients are at increased risk of opportunistic infections and neoplasias, the researchers noted. One study reported a greater risk of cervical dysplasia among immunosuppressed women with IBD, but the risk of anal dysplasia and cancer in IBD has not been well studied, they said (Clinical Gasteroenterol Hepatol. 2015. doi: 10.1016/j.cgh.2015.05.031).
To examine associations between anal dysplasia and IBD, human papillomavirus infection, and immunosuppression, the researchers analyzed anal Pap tests from 100 IBD patients who were immunosuppressed, 94 IBD patients who were not immunosuppressed, and 76 healthy controls. They identified 19 cases of atypical squamous cells of undetermined significance (ASCUS). The prevalence of ASCUS was somewhat higher among IBD patients (8.8%) than controls (2.6%; P = 0.1), but did not vary based on immunosuppression status or HPV infection. High-risk HPV occurred in 2% of the entire cohort, including 11% of patients with ASCUS and 1.5% of patients with normal Pap cytology (P = .01). High-resolution anoscopy of six patients with ASCUS revealed two cases of condylomatous disease, but no biopsy-positive dysplasia, the investigators reported.
Patients with Crohn’s disease had a significantly higher prevalence of ASCUS than other study participants (P = .02), but patients with ulcerative colitis or unspecified IBD did not, said the researchers. Having had Crohn’s disease for at least 10 years was associated with a fivefold increase in the odds of ASCUS in the multivariate analysis (95% confidence interval, 1.9-13.6), and female sex was also a risk factor (odds ratio, 3.3; P = .047). Notably, women with long-standing Crohn’s disease were almost five times more likely to have abnormal anal Pap cytology than other subjects (P = .0038), the investigators said. “One proposed mechanism for this was a reduction in human defensins in Crohn’s disease patients,” they added. Defensins – antiviral proteins that are found in immune cells – are known to inhibit cutaneous and mucosal HPV, and can be decreased in patients with Crohn’s disease for several reasons, they said. “Although more research is needed in this area, the hindered ability for an IBD patient to defend against viral illness may be responsible for their susceptibility to HPV-related cervical and anal neoplasias.”
Hologic donated the anal Pap tests used in the study. The researchers disclosed no conflicts of interest.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Immunosuppression did not affect the probability of abnormal anal cytology among patients with inflammatory bowel disease.
Major finding: Almost 9% of patients had atypical squamous cells on anal Pap testing, with no difference in prevalence based on immunosuppression.
Data source: A prospective, single-center, cross-sectional study of 270 subjects.
Disclosures: Hologic donated the anal Pap tests used in the study. The researchers disclosed no conflicts of interest.