User login
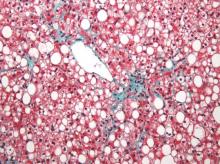
MRI topped transient elastography for staging nonalcoholic fatty liver disease
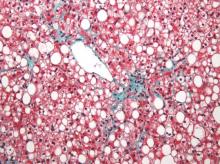
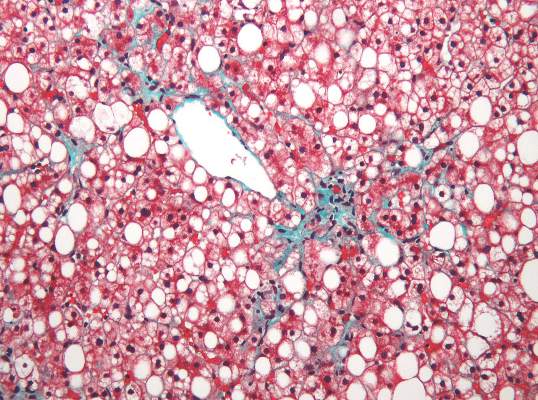
Two magnetic resonance imaging (MRI) techniques topped transient elastography (TE) for diagnosing hepatic fibrosis and steatosis in patients with nonalcoholic fatty liver disease (NAFLD), according to a first-in-kind study.
Magnetic resonance elastography surpassed all other methods for staging fibrosis, while MRI-based measurement of proton density fat fraction (PDFF) was superior for grading steatosis, with liver biopsy used as the comparative gold standard, said Dr. Kento Imajo at Yokohama (Japan) City University Graduate School of Medicine and his associates. “Magnetic resonance imaging–based noninvasive assessment of liver fibrosis and steatosis is a potential alternative to liver biopsy in clinical practice,” the investigators wrote in the March issue of Gastroenterology.
Assessing liver fibrosis and steatosis is important for staging NAFLD. Although “useful” overall, transient elastography can be unreliable in morbidly obese NAFLD patients or those with ascites because of low-frequency vibrations created by the probe, the researchers noted. To compare TE with MRI-based magnetic resonance elastography and PDFF, they evaluated 142 patients with biopsy-confirmed NAFLD and 10 controls, all of whom they also assessed with five clinical scoring systems for fibrosis – the FIB4 index, the NAFLD fibrosis score, the aspartate aminotransferase (AST) to platelet ratio, the AST-to-alanine transaminase (ALT) ratio, and the BARD score (Gastroenterology. 2015 Dec 8. doi: 10.1053/j.gastro.2015.11.048).
Magnetic resonance elastography detected stage 2 or higher hepatic fibrosis with an area under the receiver operating characteristic (AUROC) curve value of 0.91 (95% confidence interval, 0.86-0.96), compared with 0.82 (0.74-0.89) for transient elastography (P = .001), the investigators reported. The AUROC for MRE also significantly exceeded the AUROCs for all five clinical indexes of fibrosis severity. Furthermore, MRI-based measurement of PDFF identified hepatic steatosis of grade 2 or higher with an AUROC curve value of 0.90 (95% CI, 0.82-0.97), which was significantly greater than the AUROC obtained by using TE to measure the controlled attenuation parameter (0.73; 95% CI, 0.64-0.81; P less than .001).
Adding a measure for serum keratin 18 fragments or ALT did not significantly improve the detection of nonalcoholic steatohepatitis or macrovesicular steatosis affecting at least 5% of hepatocytes by either MRI or TE, the researchers noted. While liver biopsy remains the gold standard for assessing NAFLD, it is associated with sampling errors and intra- and interobserver variability, and these errors could have affected their study results, they acknowledged. The study also did not account for hepatic perfusion, which can elevate liver stiffness measurement independently from liver disease.
Both the magnetic resonance elastography and PDFF techniques require specialized hardware and software that are available from several commercial suppliers, the researchers also noted.
The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
Two magnetic resonance imaging (MRI) techniques topped transient elastography (TE) for diagnosing hepatic fibrosis and steatosis in patients with nonalcoholic fatty liver disease (NAFLD), according to a first-in-kind study.
Magnetic resonance elastography surpassed all other methods for staging fibrosis, while MRI-based measurement of proton density fat fraction (PDFF) was superior for grading steatosis, with liver biopsy used as the comparative gold standard, said Dr. Kento Imajo at Yokohama (Japan) City University Graduate School of Medicine and his associates. “Magnetic resonance imaging–based noninvasive assessment of liver fibrosis and steatosis is a potential alternative to liver biopsy in clinical practice,” the investigators wrote in the March issue of Gastroenterology.
Assessing liver fibrosis and steatosis is important for staging NAFLD. Although “useful” overall, transient elastography can be unreliable in morbidly obese NAFLD patients or those with ascites because of low-frequency vibrations created by the probe, the researchers noted. To compare TE with MRI-based magnetic resonance elastography and PDFF, they evaluated 142 patients with biopsy-confirmed NAFLD and 10 controls, all of whom they also assessed with five clinical scoring systems for fibrosis – the FIB4 index, the NAFLD fibrosis score, the aspartate aminotransferase (AST) to platelet ratio, the AST-to-alanine transaminase (ALT) ratio, and the BARD score (Gastroenterology. 2015 Dec 8. doi: 10.1053/j.gastro.2015.11.048).
Magnetic resonance elastography detected stage 2 or higher hepatic fibrosis with an area under the receiver operating characteristic (AUROC) curve value of 0.91 (95% confidence interval, 0.86-0.96), compared with 0.82 (0.74-0.89) for transient elastography (P = .001), the investigators reported. The AUROC for MRE also significantly exceeded the AUROCs for all five clinical indexes of fibrosis severity. Furthermore, MRI-based measurement of PDFF identified hepatic steatosis of grade 2 or higher with an AUROC curve value of 0.90 (95% CI, 0.82-0.97), which was significantly greater than the AUROC obtained by using TE to measure the controlled attenuation parameter (0.73; 95% CI, 0.64-0.81; P less than .001).
Adding a measure for serum keratin 18 fragments or ALT did not significantly improve the detection of nonalcoholic steatohepatitis or macrovesicular steatosis affecting at least 5% of hepatocytes by either MRI or TE, the researchers noted. While liver biopsy remains the gold standard for assessing NAFLD, it is associated with sampling errors and intra- and interobserver variability, and these errors could have affected their study results, they acknowledged. The study also did not account for hepatic perfusion, which can elevate liver stiffness measurement independently from liver disease.
Both the magnetic resonance elastography and PDFF techniques require specialized hardware and software that are available from several commercial suppliers, the researchers also noted.
The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
Two magnetic resonance imaging (MRI) techniques topped transient elastography (TE) for diagnosing hepatic fibrosis and steatosis in patients with nonalcoholic fatty liver disease (NAFLD), according to a first-in-kind study.
Magnetic resonance elastography surpassed all other methods for staging fibrosis, while MRI-based measurement of proton density fat fraction (PDFF) was superior for grading steatosis, with liver biopsy used as the comparative gold standard, said Dr. Kento Imajo at Yokohama (Japan) City University Graduate School of Medicine and his associates. “Magnetic resonance imaging–based noninvasive assessment of liver fibrosis and steatosis is a potential alternative to liver biopsy in clinical practice,” the investigators wrote in the March issue of Gastroenterology.
Assessing liver fibrosis and steatosis is important for staging NAFLD. Although “useful” overall, transient elastography can be unreliable in morbidly obese NAFLD patients or those with ascites because of low-frequency vibrations created by the probe, the researchers noted. To compare TE with MRI-based magnetic resonance elastography and PDFF, they evaluated 142 patients with biopsy-confirmed NAFLD and 10 controls, all of whom they also assessed with five clinical scoring systems for fibrosis – the FIB4 index, the NAFLD fibrosis score, the aspartate aminotransferase (AST) to platelet ratio, the AST-to-alanine transaminase (ALT) ratio, and the BARD score (Gastroenterology. 2015 Dec 8. doi: 10.1053/j.gastro.2015.11.048).
Magnetic resonance elastography detected stage 2 or higher hepatic fibrosis with an area under the receiver operating characteristic (AUROC) curve value of 0.91 (95% confidence interval, 0.86-0.96), compared with 0.82 (0.74-0.89) for transient elastography (P = .001), the investigators reported. The AUROC for MRE also significantly exceeded the AUROCs for all five clinical indexes of fibrosis severity. Furthermore, MRI-based measurement of PDFF identified hepatic steatosis of grade 2 or higher with an AUROC curve value of 0.90 (95% CI, 0.82-0.97), which was significantly greater than the AUROC obtained by using TE to measure the controlled attenuation parameter (0.73; 95% CI, 0.64-0.81; P less than .001).
Adding a measure for serum keratin 18 fragments or ALT did not significantly improve the detection of nonalcoholic steatohepatitis or macrovesicular steatosis affecting at least 5% of hepatocytes by either MRI or TE, the researchers noted. While liver biopsy remains the gold standard for assessing NAFLD, it is associated with sampling errors and intra- and interobserver variability, and these errors could have affected their study results, they acknowledged. The study also did not account for hepatic perfusion, which can elevate liver stiffness measurement independently from liver disease.
Both the magnetic resonance elastography and PDFF techniques require specialized hardware and software that are available from several commercial suppliers, the researchers also noted.
The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Two specialized MRI techniques surpassed transient elastography for staging fibrosis and steatosis in nonalcoholic fatty liver disease.
Major finding: The areas under the curve for magnetic resonance elastography and the proton density fat fraction measure were significantly greater than those for transient elastography and the TE-based controlled attenuation parameter (P is less than .001 for both comparisons).
Data source: A cross-sectional study of 142 patients with nonalcoholic fatty liver disease and 10 controls.
Disclosures: The study was partially supported by the Japanese Ministry of Health, Labor, and Welfare, the Japanese Science and Technology Agency, and Kiban-B, Shingakujuturyouiki. The investigators had no disclosures.
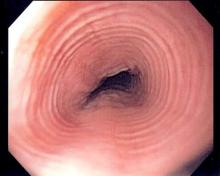
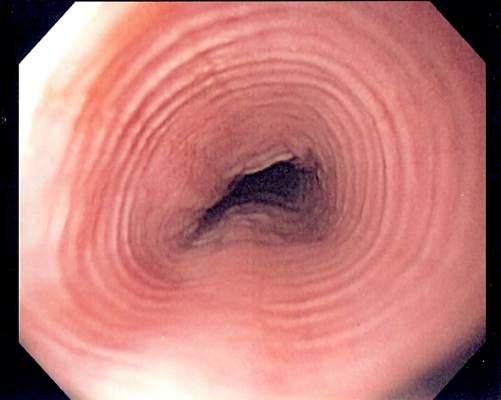
Minor residual staining found adequate for colonoscopy
A Boston Bowel Preparation Scale (BBPS) score of 2 – indicating mild residual staining and small stool fragments – was as good as the optimal preparation score of 3 for visualizing polyps and adenomas larger than 5 mm and advanced adenomas during colonoscopy, researchers said.
A score of 2 might increase the chances of missing smaller polyps, but is adequate for detecting clinically significant masses, Dr. Brian Clark of Yale University, New Haven, Conn., and his associates reported in the February issue of Gastroenterology. But a score of 1 – meaning that there is enough staining or stool to obscure the mucosa – significantly increased the chances of missing adenomas larger than 5 mm, they said. Patients should undergo early repeat colonoscopy if their BBPS score is 1 or 0 in any colon segment, they emphasized.
Source: American Gastroenterological Association
Bowel preparation for colonoscopy is considered adequate if endoscopists can detect polyps larger than 5 mm, but no prior study had quantified the amount of preparation needed. This prospective observational study assessed adequate preparation in terms of the BBPS, which scores each of three colon segments on a scale of 0 (solid stool covering the mucosa) to 3 points (entire mucosa seen well, with no residual staining). Study participants included 438 men aged 50-75 years who underwent screening or surveillance colonoscopy at a single Veterans Affairs center, followed by repeat colonoscopies within 60 days performed by different blinded endoscopists. The investigators excluded patients who scored 0 in all colon segments or had familial polyposis syndrome, inflammatory bowel disease, polyps so large that they could not be completely removed, or a history of colonic or rectal resection. In all, they analyzed 1,161 colon segments (Gastroenterology. 2015 Dec 7. doi: 10.1053/j.gastro.2015.09.041).
Endoscopists missed about 5% of adenomas greater than 5 mm, regardless of whether BBPS scores were 2 or 3 in a model that accounted for age, reason for colonoscopy, colon segment, number of polyps removed in the first examination, and endoscopist performing the procedure, the researchers said. But when BBPS scores were 1, endoscopists missed 16% of adenomas larger than 5 mm, a difference of about 10%. Furthermore, 43% of screening and surveillance intervals would have been incorrect had they been based solely on an initial examination for which scores were 1 in at least one segment. In contrast, only about 15% of intervals would have been incorrect for patients who scored 2 or 3 in all segments.
In all, 80% of patients were sufficiently prepared, having scored at least 2 in all segments on the first examination. “Determining whether a patient’s preparation quality is adequate is one of the most common and important decisions made by gastroenterologists each day,” the researchers said. Between 25% and 30% of screening and surveillance colonoscopies occur at “inappropriately shortened intervals,” often because of uncertainty about what constitutes adequate visualization, they added. Defining adequate visualization based on bowel preparation could save billions of dollars in health care costs every year, minimize complications from unnecessary procedures, and pinpoint those patients who truly need an early repeat colonoscopy to help prevent interval colorectal cancer, they emphasized.
The National Institutes of Health funded the study. The investigators had no disclosures.
We have seen a dramatic increase in attention to improving the adenoma detection rate (ADR) during colonoscopy because patients of endoscopists with a higher ADR have a lower risk of colorectal cancer after colonoscopy. One major contributor to missed adenomas is inadequate bowel preparation, though little was known about how best to define adequacy.

|
| Dr. Jason Domonitz |
Clark and colleagues’ elegant tandem colonoscopy study helps address this knowledge gap using the Boston Bowel Preparation Scale (BBPS), a validated instrument that is easy to implement. They hypothesized that a BBPS colon-segment score of 2 was noninferior to a score of 3 for identifying adenomas greater than 5 mm, but that a BBPS colon-segment score of 1 would be inferior to scores of 2 or 3. Their findings support this hypothesis and give us long overdue data that we can now use to define an adequate bowel preparation. Given that the adenoma miss rate was 16% when the segment score was 1, but only about 5% with higher scores, it is reasonable to recommend repeat colonoscopy within 12 months if any segment score is less than 2. Otherwise, standard surveillance intervals should be recommended. Finally, unless and until other scoring systems are similarly validated, these findings should encourage the widespread adoption of the BBPS.
Dr. Jason A. Dominitz, AGAF, is the national program director for gastroenterology for the Veterans Health Administration and is professor of medicine in the division of gastroenterology at the University of Washington, Seattle. He has no conflicts of interest.
We have seen a dramatic increase in attention to improving the adenoma detection rate (ADR) during colonoscopy because patients of endoscopists with a higher ADR have a lower risk of colorectal cancer after colonoscopy. One major contributor to missed adenomas is inadequate bowel preparation, though little was known about how best to define adequacy.

|
| Dr. Jason Domonitz |
Clark and colleagues’ elegant tandem colonoscopy study helps address this knowledge gap using the Boston Bowel Preparation Scale (BBPS), a validated instrument that is easy to implement. They hypothesized that a BBPS colon-segment score of 2 was noninferior to a score of 3 for identifying adenomas greater than 5 mm, but that a BBPS colon-segment score of 1 would be inferior to scores of 2 or 3. Their findings support this hypothesis and give us long overdue data that we can now use to define an adequate bowel preparation. Given that the adenoma miss rate was 16% when the segment score was 1, but only about 5% with higher scores, it is reasonable to recommend repeat colonoscopy within 12 months if any segment score is less than 2. Otherwise, standard surveillance intervals should be recommended. Finally, unless and until other scoring systems are similarly validated, these findings should encourage the widespread adoption of the BBPS.
Dr. Jason A. Dominitz, AGAF, is the national program director for gastroenterology for the Veterans Health Administration and is professor of medicine in the division of gastroenterology at the University of Washington, Seattle. He has no conflicts of interest.
We have seen a dramatic increase in attention to improving the adenoma detection rate (ADR) during colonoscopy because patients of endoscopists with a higher ADR have a lower risk of colorectal cancer after colonoscopy. One major contributor to missed adenomas is inadequate bowel preparation, though little was known about how best to define adequacy.

|
| Dr. Jason Domonitz |
Clark and colleagues’ elegant tandem colonoscopy study helps address this knowledge gap using the Boston Bowel Preparation Scale (BBPS), a validated instrument that is easy to implement. They hypothesized that a BBPS colon-segment score of 2 was noninferior to a score of 3 for identifying adenomas greater than 5 mm, but that a BBPS colon-segment score of 1 would be inferior to scores of 2 or 3. Their findings support this hypothesis and give us long overdue data that we can now use to define an adequate bowel preparation. Given that the adenoma miss rate was 16% when the segment score was 1, but only about 5% with higher scores, it is reasonable to recommend repeat colonoscopy within 12 months if any segment score is less than 2. Otherwise, standard surveillance intervals should be recommended. Finally, unless and until other scoring systems are similarly validated, these findings should encourage the widespread adoption of the BBPS.
Dr. Jason A. Dominitz, AGAF, is the national program director for gastroenterology for the Veterans Health Administration and is professor of medicine in the division of gastroenterology at the University of Washington, Seattle. He has no conflicts of interest.
A Boston Bowel Preparation Scale (BBPS) score of 2 – indicating mild residual staining and small stool fragments – was as good as the optimal preparation score of 3 for visualizing polyps and adenomas larger than 5 mm and advanced adenomas during colonoscopy, researchers said.
A score of 2 might increase the chances of missing smaller polyps, but is adequate for detecting clinically significant masses, Dr. Brian Clark of Yale University, New Haven, Conn., and his associates reported in the February issue of Gastroenterology. But a score of 1 – meaning that there is enough staining or stool to obscure the mucosa – significantly increased the chances of missing adenomas larger than 5 mm, they said. Patients should undergo early repeat colonoscopy if their BBPS score is 1 or 0 in any colon segment, they emphasized.
Source: American Gastroenterological Association
Bowel preparation for colonoscopy is considered adequate if endoscopists can detect polyps larger than 5 mm, but no prior study had quantified the amount of preparation needed. This prospective observational study assessed adequate preparation in terms of the BBPS, which scores each of three colon segments on a scale of 0 (solid stool covering the mucosa) to 3 points (entire mucosa seen well, with no residual staining). Study participants included 438 men aged 50-75 years who underwent screening or surveillance colonoscopy at a single Veterans Affairs center, followed by repeat colonoscopies within 60 days performed by different blinded endoscopists. The investigators excluded patients who scored 0 in all colon segments or had familial polyposis syndrome, inflammatory bowel disease, polyps so large that they could not be completely removed, or a history of colonic or rectal resection. In all, they analyzed 1,161 colon segments (Gastroenterology. 2015 Dec 7. doi: 10.1053/j.gastro.2015.09.041).
Endoscopists missed about 5% of adenomas greater than 5 mm, regardless of whether BBPS scores were 2 or 3 in a model that accounted for age, reason for colonoscopy, colon segment, number of polyps removed in the first examination, and endoscopist performing the procedure, the researchers said. But when BBPS scores were 1, endoscopists missed 16% of adenomas larger than 5 mm, a difference of about 10%. Furthermore, 43% of screening and surveillance intervals would have been incorrect had they been based solely on an initial examination for which scores were 1 in at least one segment. In contrast, only about 15% of intervals would have been incorrect for patients who scored 2 or 3 in all segments.
In all, 80% of patients were sufficiently prepared, having scored at least 2 in all segments on the first examination. “Determining whether a patient’s preparation quality is adequate is one of the most common and important decisions made by gastroenterologists each day,” the researchers said. Between 25% and 30% of screening and surveillance colonoscopies occur at “inappropriately shortened intervals,” often because of uncertainty about what constitutes adequate visualization, they added. Defining adequate visualization based on bowel preparation could save billions of dollars in health care costs every year, minimize complications from unnecessary procedures, and pinpoint those patients who truly need an early repeat colonoscopy to help prevent interval colorectal cancer, they emphasized.
The National Institutes of Health funded the study. The investigators had no disclosures.
A Boston Bowel Preparation Scale (BBPS) score of 2 – indicating mild residual staining and small stool fragments – was as good as the optimal preparation score of 3 for visualizing polyps and adenomas larger than 5 mm and advanced adenomas during colonoscopy, researchers said.
A score of 2 might increase the chances of missing smaller polyps, but is adequate for detecting clinically significant masses, Dr. Brian Clark of Yale University, New Haven, Conn., and his associates reported in the February issue of Gastroenterology. But a score of 1 – meaning that there is enough staining or stool to obscure the mucosa – significantly increased the chances of missing adenomas larger than 5 mm, they said. Patients should undergo early repeat colonoscopy if their BBPS score is 1 or 0 in any colon segment, they emphasized.
Source: American Gastroenterological Association
Bowel preparation for colonoscopy is considered adequate if endoscopists can detect polyps larger than 5 mm, but no prior study had quantified the amount of preparation needed. This prospective observational study assessed adequate preparation in terms of the BBPS, which scores each of three colon segments on a scale of 0 (solid stool covering the mucosa) to 3 points (entire mucosa seen well, with no residual staining). Study participants included 438 men aged 50-75 years who underwent screening or surveillance colonoscopy at a single Veterans Affairs center, followed by repeat colonoscopies within 60 days performed by different blinded endoscopists. The investigators excluded patients who scored 0 in all colon segments or had familial polyposis syndrome, inflammatory bowel disease, polyps so large that they could not be completely removed, or a history of colonic or rectal resection. In all, they analyzed 1,161 colon segments (Gastroenterology. 2015 Dec 7. doi: 10.1053/j.gastro.2015.09.041).
Endoscopists missed about 5% of adenomas greater than 5 mm, regardless of whether BBPS scores were 2 or 3 in a model that accounted for age, reason for colonoscopy, colon segment, number of polyps removed in the first examination, and endoscopist performing the procedure, the researchers said. But when BBPS scores were 1, endoscopists missed 16% of adenomas larger than 5 mm, a difference of about 10%. Furthermore, 43% of screening and surveillance intervals would have been incorrect had they been based solely on an initial examination for which scores were 1 in at least one segment. In contrast, only about 15% of intervals would have been incorrect for patients who scored 2 or 3 in all segments.
In all, 80% of patients were sufficiently prepared, having scored at least 2 in all segments on the first examination. “Determining whether a patient’s preparation quality is adequate is one of the most common and important decisions made by gastroenterologists each day,” the researchers said. Between 25% and 30% of screening and surveillance colonoscopies occur at “inappropriately shortened intervals,” often because of uncertainty about what constitutes adequate visualization, they added. Defining adequate visualization based on bowel preparation could save billions of dollars in health care costs every year, minimize complications from unnecessary procedures, and pinpoint those patients who truly need an early repeat colonoscopy to help prevent interval colorectal cancer, they emphasized.
The National Institutes of Health funded the study. The investigators had no disclosures.
FROM GASTROENTEROLOGY
Key clinical point: Minor residual staining that does not obscure the bowel mucosa is adequate for detection of adenomas greater than 5 mm during surveillance or screening colonoscopy.
Major finding: Endoscopists missed about 5% of clinically significant adenomas, regardless of whether the Boston Bowel Preparation Score was 2 (minor residual staining) or 3 (entire mucosa seen well).
Data source: A blinded prospective observational study of 438 men at a single Veterans Affairs center.
Disclosures: The National Institutes of Health funded the study. The investigators had no disclosures.
Drug combo held up in real-world HCV study
A 12-week, ribavirin-free regimen achieved sustained virologic response for 85% of patients with genotype 1 hepatitis C virus (HCV) infection, researchers reported in the February issue of Gastroenterology.
“This represents one of the first applications of a highly effective HCV regimen outside clinical trials,” said Dr. Mark S. Sulkowski of John Hopkins University in Baltimore and his associates. Adding ribavirin to the simeprevir and sofosbuvir combination regimen did not improve sustained virologic response (SVR), but patients were less likely to achieve it if they had cirrhosis, current or prior hepatic decompensation, or a history of failing other protease inhibitors, the investigators said.
Novel hepatitis C therapies have yielded “substantially lower” rates of SVR and more side effects in everyday practice than in clinical trials, the investigators noted. To better understand how some of newest HCV drugs perform in the real world, they conducted an observational cohort study of the safety, tolerability, and efficacy of simeprevir plus sofosbuvir for treating genotype 1 HCV infections in academic and nonacademic settings (HCV-TARGET) (Gastroenterology 2015 doi: 10.1053/j.gastro.2015.10.013).
A total of 836 patients received once-daily simeprevir (150 mg) and sofosbuvir (400 mg), and 169 of them also received ribavirin. Most (61%) patients had genotype 1a infection and were white (76%), male (61%), and cirrhotic (59%); 13% were black. Patients usually were treatment experienced, having failed peginterferon and ribavirin either with (12%) or without (46%) telaprevir or boceprevir, the researchers said.
In all, 675 (84%) patients achieved SVR after 12 weeks of treatment (SVR12; 95% confidence interval, 81%-87%). Adding ribavirin to the combination PI regimen did not improve SVR, regardless of cirrhosis status, genetic subtype, or treatment history. However, crude SVR12 rates were only 75% for patients with hepatic decompensation and 81% for those with cirrhosis, and these patients had significantly lower adjusted odds of achieving SVR, compared with other patients. In hindsight, decompensated and cirrhotic patients might have needed 24 weeks of treatment, as the Food and Drug Administration now recommends based on the COSMOS trial results (Lancet. 2014;384[9956]:1756-65), the investigators said.
The adjusted model did not uncover a link between genotype 1 subtype and SVR, but only about 10% of patients were tested for the Q80K polymorphism, which is more common in genotype 1a infections and is associated with treatment resistance, the investigators noted. Crude SVR12 rates were 92% for patients with genotype 1b infection and 86% for those with 1a infection, they said.
Only 3% of patients stopped treatment; 2% did so because of side effects, and ribavirin did not significantly affect rates of treatment discontinuation, said the investigators. The most common side effects were fatigue, headache, nausea, rash, and insomnia. Serious adverse events affected 5% of patients and included gastrointestinal bleeding (0.5%), hepatic failure or encephalopathy (1.2%), and infections (1.1%).
Taken together, these results show that simeprevir and sofosbuvir effectively translate from the clinical trial setting into clinical practice, said the researchers. “Additional research is needed to understand which patients may benefit from different treatment regimens or longer treatment durations,” they emphasized.
The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
A 12-week, ribavirin-free regimen achieved sustained virologic response for 85% of patients with genotype 1 hepatitis C virus (HCV) infection, researchers reported in the February issue of Gastroenterology.
“This represents one of the first applications of a highly effective HCV regimen outside clinical trials,” said Dr. Mark S. Sulkowski of John Hopkins University in Baltimore and his associates. Adding ribavirin to the simeprevir and sofosbuvir combination regimen did not improve sustained virologic response (SVR), but patients were less likely to achieve it if they had cirrhosis, current or prior hepatic decompensation, or a history of failing other protease inhibitors, the investigators said.
Novel hepatitis C therapies have yielded “substantially lower” rates of SVR and more side effects in everyday practice than in clinical trials, the investigators noted. To better understand how some of newest HCV drugs perform in the real world, they conducted an observational cohort study of the safety, tolerability, and efficacy of simeprevir plus sofosbuvir for treating genotype 1 HCV infections in academic and nonacademic settings (HCV-TARGET) (Gastroenterology 2015 doi: 10.1053/j.gastro.2015.10.013).
A total of 836 patients received once-daily simeprevir (150 mg) and sofosbuvir (400 mg), and 169 of them also received ribavirin. Most (61%) patients had genotype 1a infection and were white (76%), male (61%), and cirrhotic (59%); 13% were black. Patients usually were treatment experienced, having failed peginterferon and ribavirin either with (12%) or without (46%) telaprevir or boceprevir, the researchers said.
In all, 675 (84%) patients achieved SVR after 12 weeks of treatment (SVR12; 95% confidence interval, 81%-87%). Adding ribavirin to the combination PI regimen did not improve SVR, regardless of cirrhosis status, genetic subtype, or treatment history. However, crude SVR12 rates were only 75% for patients with hepatic decompensation and 81% for those with cirrhosis, and these patients had significantly lower adjusted odds of achieving SVR, compared with other patients. In hindsight, decompensated and cirrhotic patients might have needed 24 weeks of treatment, as the Food and Drug Administration now recommends based on the COSMOS trial results (Lancet. 2014;384[9956]:1756-65), the investigators said.
The adjusted model did not uncover a link between genotype 1 subtype and SVR, but only about 10% of patients were tested for the Q80K polymorphism, which is more common in genotype 1a infections and is associated with treatment resistance, the investigators noted. Crude SVR12 rates were 92% for patients with genotype 1b infection and 86% for those with 1a infection, they said.
Only 3% of patients stopped treatment; 2% did so because of side effects, and ribavirin did not significantly affect rates of treatment discontinuation, said the investigators. The most common side effects were fatigue, headache, nausea, rash, and insomnia. Serious adverse events affected 5% of patients and included gastrointestinal bleeding (0.5%), hepatic failure or encephalopathy (1.2%), and infections (1.1%).
Taken together, these results show that simeprevir and sofosbuvir effectively translate from the clinical trial setting into clinical practice, said the researchers. “Additional research is needed to understand which patients may benefit from different treatment regimens or longer treatment durations,” they emphasized.
The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
A 12-week, ribavirin-free regimen achieved sustained virologic response for 85% of patients with genotype 1 hepatitis C virus (HCV) infection, researchers reported in the February issue of Gastroenterology.
“This represents one of the first applications of a highly effective HCV regimen outside clinical trials,” said Dr. Mark S. Sulkowski of John Hopkins University in Baltimore and his associates. Adding ribavirin to the simeprevir and sofosbuvir combination regimen did not improve sustained virologic response (SVR), but patients were less likely to achieve it if they had cirrhosis, current or prior hepatic decompensation, or a history of failing other protease inhibitors, the investigators said.
Novel hepatitis C therapies have yielded “substantially lower” rates of SVR and more side effects in everyday practice than in clinical trials, the investigators noted. To better understand how some of newest HCV drugs perform in the real world, they conducted an observational cohort study of the safety, tolerability, and efficacy of simeprevir plus sofosbuvir for treating genotype 1 HCV infections in academic and nonacademic settings (HCV-TARGET) (Gastroenterology 2015 doi: 10.1053/j.gastro.2015.10.013).
A total of 836 patients received once-daily simeprevir (150 mg) and sofosbuvir (400 mg), and 169 of them also received ribavirin. Most (61%) patients had genotype 1a infection and were white (76%), male (61%), and cirrhotic (59%); 13% were black. Patients usually were treatment experienced, having failed peginterferon and ribavirin either with (12%) or without (46%) telaprevir or boceprevir, the researchers said.
In all, 675 (84%) patients achieved SVR after 12 weeks of treatment (SVR12; 95% confidence interval, 81%-87%). Adding ribavirin to the combination PI regimen did not improve SVR, regardless of cirrhosis status, genetic subtype, or treatment history. However, crude SVR12 rates were only 75% for patients with hepatic decompensation and 81% for those with cirrhosis, and these patients had significantly lower adjusted odds of achieving SVR, compared with other patients. In hindsight, decompensated and cirrhotic patients might have needed 24 weeks of treatment, as the Food and Drug Administration now recommends based on the COSMOS trial results (Lancet. 2014;384[9956]:1756-65), the investigators said.
The adjusted model did not uncover a link between genotype 1 subtype and SVR, but only about 10% of patients were tested for the Q80K polymorphism, which is more common in genotype 1a infections and is associated with treatment resistance, the investigators noted. Crude SVR12 rates were 92% for patients with genotype 1b infection and 86% for those with 1a infection, they said.
Only 3% of patients stopped treatment; 2% did so because of side effects, and ribavirin did not significantly affect rates of treatment discontinuation, said the investigators. The most common side effects were fatigue, headache, nausea, rash, and insomnia. Serious adverse events affected 5% of patients and included gastrointestinal bleeding (0.5%), hepatic failure or encephalopathy (1.2%), and infections (1.1%).
Taken together, these results show that simeprevir and sofosbuvir effectively translate from the clinical trial setting into clinical practice, said the researchers. “Additional research is needed to understand which patients may benefit from different treatment regimens or longer treatment durations,” they emphasized.
The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
FROM GASTROENTEROLOGY
Key clinical point: Twelve weeks of simeprevir and sofosbuvir cured about 85% of real-world patients with genotype 1 hepatitis C virus infection.
Major finding: The unadjusted rate of SVR12 was 85% (95% CI, 82%-88%).
Data source: An analysis of an observational cohort study of protease inhibitor combination regimen with or without ribavirin for 836 patients (HCV-TARGET).
Disclosures: The study was supported by the University of Florida at Gainesville, the University of North Carolina at Chapel Hill, AbbVie, Bristol-Myers Squibb, Gilead, Janssen, Kadmon, Merck, Vertex, and the National Institutes of Health. Dr. Sulkowski reported grants and personal fees from Gilead, Janssen, Achillion, Abbvie, Merck, and Bristol-Myers Squibb. Of 14 coinvestigators, 13 reported financial relationships with a number of pharmaceutical companies.
Factors within VA control could help prevent missed, canceled appointments
Opt-out scheduling protocols and long appointment lead times contributed significantly to missed and canceled colonoscopy appointments at Veterans Health Administration facilities, researchers reported in the February issue of Clinical Gastroenterology and Hepatology.
These factors are within the control of the Veterans Affairs and could be altered to improve productivity and efficiency, said Melissa Partin, Ph.D., of the Center for Chronic Disease Outcomes Research at the Minneapolis Veterans Affairs Health Care System in Minneapolis, and her associates.
Source: American Gastroenterological Association
Missed and canceled medical appointments are always a concern, but particularly so for colonoscopy clinics, where they incur an average daily net loss of $725, the investigators noted. Most clinics have limited colonoscopy capacity, and even a 30-day wait for diagnostic colonoscopy has been linked to “modest but significantly elevated” chances of detecting cancer on exam, they added. To better understand these problems, they separately examined predictors of missed and canceled appointments among 27,994 patients who had positive fecal occult blood tests with diagnostic colonoscopies scheduled at 69 VA facilities between 2009 and 2011 (Clin Gastroenterol Hepatol. 2015 Aug 21. doi: 10.1016/j.cgh.2015.07.051).
Having a life expectancy of 6 months or less and no personal history of polyps best predicted missing an appointment, with odds ratios of 2.74 for each factor, the researchers said. However, only 0.47% of patients had such a short life expectancy. Other significant predictors of missed appointments included being seen at the largest and most complex facilities (odds ratio, 2.69; P = .007), having both psychiatric and substance abuse disorders (OR, 1.82; P less than .0001), and the use of opt-out scheduling, in which patients were automatically scheduled rather than having to schedule appointments themselves (OR, 1.57; P = .02). Canceled appointments also were linked to opt-out scheduling, as well as to older age and having no history of polyps.
Most appointment lead times were 28 days, and each 12-day increase in lead time increased the odds of missing or canceling appointments by about 15% (P less than .0001). The problem could be curtailed by the Veterans Access, Choice and Accountability Act of 2014, which allows those who cannot schedule VA appointments within 30 days to receive care from eligible non–VA providers, the investigators said. “Future research should focus on assessing the effect of the Choice Act on colonoscopy appointment lead time and on developing and evaluating efficient and effective approaches to implementing the other clinic-level changes supported by our findings,” they added.
The study might have oversimplified or missed changes in protocols because it used single-item survey measures at one point in time, the investigators said. For some patients, the first appointment after the fecal occult blood test may have been for another procedure besides colonoscopy, they added. Furthermore, they did not distinguish between appointments canceled by patients versus clinics. “The VHA is a unique context, characterized by a predominantly male, low-income population with high rates of mental health and substance abuse diagnoses. Therefore, our findings may not generalize to other settings,” they added. “However, our findings do have important implications for a substantial population of health providers and consumers in this country, because the VHA is the largest integrated health care system in the United States.”
The study was funded by the Department of Veterans Affairs Clinical Science Service and Health Services Research & Development Service. The investigators had no disclosures.
Opt-out scheduling protocols and long appointment lead times contributed significantly to missed and canceled colonoscopy appointments at Veterans Health Administration facilities, researchers reported in the February issue of Clinical Gastroenterology and Hepatology.
These factors are within the control of the Veterans Affairs and could be altered to improve productivity and efficiency, said Melissa Partin, Ph.D., of the Center for Chronic Disease Outcomes Research at the Minneapolis Veterans Affairs Health Care System in Minneapolis, and her associates.
Source: American Gastroenterological Association
Missed and canceled medical appointments are always a concern, but particularly so for colonoscopy clinics, where they incur an average daily net loss of $725, the investigators noted. Most clinics have limited colonoscopy capacity, and even a 30-day wait for diagnostic colonoscopy has been linked to “modest but significantly elevated” chances of detecting cancer on exam, they added. To better understand these problems, they separately examined predictors of missed and canceled appointments among 27,994 patients who had positive fecal occult blood tests with diagnostic colonoscopies scheduled at 69 VA facilities between 2009 and 2011 (Clin Gastroenterol Hepatol. 2015 Aug 21. doi: 10.1016/j.cgh.2015.07.051).
Having a life expectancy of 6 months or less and no personal history of polyps best predicted missing an appointment, with odds ratios of 2.74 for each factor, the researchers said. However, only 0.47% of patients had such a short life expectancy. Other significant predictors of missed appointments included being seen at the largest and most complex facilities (odds ratio, 2.69; P = .007), having both psychiatric and substance abuse disorders (OR, 1.82; P less than .0001), and the use of opt-out scheduling, in which patients were automatically scheduled rather than having to schedule appointments themselves (OR, 1.57; P = .02). Canceled appointments also were linked to opt-out scheduling, as well as to older age and having no history of polyps.
Most appointment lead times were 28 days, and each 12-day increase in lead time increased the odds of missing or canceling appointments by about 15% (P less than .0001). The problem could be curtailed by the Veterans Access, Choice and Accountability Act of 2014, which allows those who cannot schedule VA appointments within 30 days to receive care from eligible non–VA providers, the investigators said. “Future research should focus on assessing the effect of the Choice Act on colonoscopy appointment lead time and on developing and evaluating efficient and effective approaches to implementing the other clinic-level changes supported by our findings,” they added.
The study might have oversimplified or missed changes in protocols because it used single-item survey measures at one point in time, the investigators said. For some patients, the first appointment after the fecal occult blood test may have been for another procedure besides colonoscopy, they added. Furthermore, they did not distinguish between appointments canceled by patients versus clinics. “The VHA is a unique context, characterized by a predominantly male, low-income population with high rates of mental health and substance abuse diagnoses. Therefore, our findings may not generalize to other settings,” they added. “However, our findings do have important implications for a substantial population of health providers and consumers in this country, because the VHA is the largest integrated health care system in the United States.”
The study was funded by the Department of Veterans Affairs Clinical Science Service and Health Services Research & Development Service. The investigators had no disclosures.
Opt-out scheduling protocols and long appointment lead times contributed significantly to missed and canceled colonoscopy appointments at Veterans Health Administration facilities, researchers reported in the February issue of Clinical Gastroenterology and Hepatology.
These factors are within the control of the Veterans Affairs and could be altered to improve productivity and efficiency, said Melissa Partin, Ph.D., of the Center for Chronic Disease Outcomes Research at the Minneapolis Veterans Affairs Health Care System in Minneapolis, and her associates.
Source: American Gastroenterological Association
Missed and canceled medical appointments are always a concern, but particularly so for colonoscopy clinics, where they incur an average daily net loss of $725, the investigators noted. Most clinics have limited colonoscopy capacity, and even a 30-day wait for diagnostic colonoscopy has been linked to “modest but significantly elevated” chances of detecting cancer on exam, they added. To better understand these problems, they separately examined predictors of missed and canceled appointments among 27,994 patients who had positive fecal occult blood tests with diagnostic colonoscopies scheduled at 69 VA facilities between 2009 and 2011 (Clin Gastroenterol Hepatol. 2015 Aug 21. doi: 10.1016/j.cgh.2015.07.051).
Having a life expectancy of 6 months or less and no personal history of polyps best predicted missing an appointment, with odds ratios of 2.74 for each factor, the researchers said. However, only 0.47% of patients had such a short life expectancy. Other significant predictors of missed appointments included being seen at the largest and most complex facilities (odds ratio, 2.69; P = .007), having both psychiatric and substance abuse disorders (OR, 1.82; P less than .0001), and the use of opt-out scheduling, in which patients were automatically scheduled rather than having to schedule appointments themselves (OR, 1.57; P = .02). Canceled appointments also were linked to opt-out scheduling, as well as to older age and having no history of polyps.
Most appointment lead times were 28 days, and each 12-day increase in lead time increased the odds of missing or canceling appointments by about 15% (P less than .0001). The problem could be curtailed by the Veterans Access, Choice and Accountability Act of 2014, which allows those who cannot schedule VA appointments within 30 days to receive care from eligible non–VA providers, the investigators said. “Future research should focus on assessing the effect of the Choice Act on colonoscopy appointment lead time and on developing and evaluating efficient and effective approaches to implementing the other clinic-level changes supported by our findings,” they added.
The study might have oversimplified or missed changes in protocols because it used single-item survey measures at one point in time, the investigators said. For some patients, the first appointment after the fecal occult blood test may have been for another procedure besides colonoscopy, they added. Furthermore, they did not distinguish between appointments canceled by patients versus clinics. “The VHA is a unique context, characterized by a predominantly male, low-income population with high rates of mental health and substance abuse diagnoses. Therefore, our findings may not generalize to other settings,” they added. “However, our findings do have important implications for a substantial population of health providers and consumers in this country, because the VHA is the largest integrated health care system in the United States.”
The study was funded by the Department of Veterans Affairs Clinical Science Service and Health Services Research & Development Service. The investigators had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Opt-out scheduling practices and long appointment lead times predicted missed and canceled colonoscopies at the VA.
Major finding: Estimated ratios for these predictors ranged between 1.12 and 1.57, and all were statistically significant.
Data source: An analysis of data from 27,994 patients who had positive fecal occult blood tests with diagnostic colonoscopies scheduled at 69 VA facilities between 2009 and 2011.
Disclosures: The study was funded by the Department of Veterans Affairs Clinical Science Service and Health Services Research and Development Service. The investigators had no disclosures.
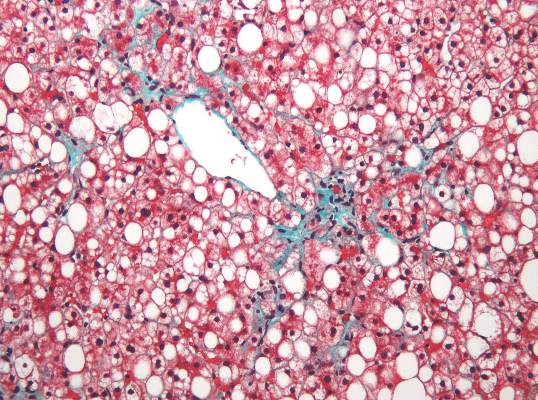
Nonalcoholic fatty liver disease will keep rising ‘in near term’
Nonalcoholic fatty liver disease (NAFLD) almost tripled among United States veterans in a recent 9-year period, investigators reported in the February issue of Clinical Gastroenterology and Hepatology.
The trend “was evident in all racial groups, across all age groups, and in both genders,” said Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston, and her associates. The increasing prevalence of NAFLD “is likely generalizable to nonveterans,” and will probably persist because of a “fairly steady” 2%-3% overall annual incidence and a steeper rise among younger individuals, they added. “Nonalcoholic fatty liver disease will continue to remain a major public health problem in the United States, at least in the near and intermediate future.”
Although NAFLD is the leading cause of chronic liver failure in the United States, few studies have examined its incidence or prevalence over time, which are key to predicting future disease burden. Therefore, the investigators analyzed data for more than 9.78 million patients who visited the VA at least once between 2003 and 2011. They defined NAFLD as at least two elevated alanine aminotransferase (ALT) values (greater than 40 IU/mL) separated by at least 6 months, with no history of positive serology for hepatitis B surface antigen or hepatitis C virus RNA, and no alcohol-related ICD-9 codes or positive AUDIT-C scores within a year of elevated ALT levels (Clin Gastroenterol Hepatol. 2015 Aug 7. doi: 10.1016/j.cgh.2015.08.010).
During the study period, more than 1.3 million patients, or 13.6%, met the definition of NAFLD, said the researchers. Age-adjusted incidence rates dropped slightly from 3.16% in 2003 to 2.5% in 2011, ranging between 2.3% and 2.7% in most years. Prevalence, however, rose from 6.3% in 2003 (95% confidence interval, 6.26%-6.3%) to 17.6% in 2011 (95% CI, 17.58%-17.65%), a 2.8-fold increase. Moreover, about one in five patients with NAFLD who visited the VA in 2011 was at risk for advanced fibrosis.
Among individuals who were younger than 45 years, the incidence of NAFLD rose from 2.3 to 4.3 cases per 100 persons (annual percentage change, 7.4%; 95% CI, 5.7% to 9.2%), the researchers also found. “Although recent studies show that the rate of increase in both obesity and diabetes, which are both major risk factors for NAFLD, may be slowing down in the U.S., this may not be the case in the VA, where the prevalence of obesity and diabetes is in fact higher than in the U.S. population,” they said.
In general, the findings mirror a recent analysis of the National Health and Nutrition Examination Survey (Aliment Pharmacol Ther. 2015 Jan;41[1]:65-76), according to the investigators. “The VA is the largest integrated health care system in the United States,” they added. “We believe that the sheer size of the veteran cohort, combined with a complete dearth of information regarding the burden of NAFLD in the VA, renders our findings highly significant. Furthermore, the VA is in a unique position to test and implement systemic changes in medical care delivery to improve the health care of NAFLD patients.”
The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
Kanwal and colleagues present an interesting study assessing the trends in the incidence and prevalence of NAFLD in the United States. Findings suggest that the annual incidence of NAFLD has generally been stable (2.2%-3.2%), while the prevalence of NAFLD has increased by 2.8-fold (6.3%-17.6%). These findings are consistent with the literature and provide additional evidence supporting the increasing burden of NAFLD. Although an important study, there are some limitations to the study design. First, the diagnosis of NAFLD was solely based on elevated liver enzymes, which can underestimate the true incidence and prevalence of NAFLD. In fact, in a recent meta-analysis, NAFLD prevalence based on liver enzymes was 13%, while NAFLD prevalence based on radiologic diagnosis was 25% (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Second, the study subjects came from the VA system, which may not be representative of the U.S. population (Patrick AFB, FL: Defense Equal Opportunity Management Institute, 2010). This is important because sex-specific differences in the prevalence of NAFLD have been reported (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Nevertheless, these limitations do not minimize the important contribution of this study. There appears to be an alarming increase in the burden of NAFLD within all the racial and age groups in the U.S. Further, this increase in the incidence and prevalence of NAFLD is especially significant among the younger age groups (less than 45 years). This finding is in contrast to others who have reported a higher prevalence in older subjects (Presented at AASLD 2015. San Francisco. Abstract #534). If confirmed, this younger cohort of patients with NAFLD can fuel the future burden of liver disease for the next few decades (JAMA. 2012;307:491-7). Given the current lack of an effective treatment for NAFLD, a national strategy to deal with this important and rising cause of chronic liver disease is urgently needed.
Dr. Zobair M. Younossi, MPH, FACG, AGAF, FAASLD, is chairman, department of medicine, Inova Fairfax Hospital; vice president for research, Inova Health System; professor of medicine, VCU-Inova Campus and Beatty Center for Integrated Research, Falls Church, Va. He has consulted for Gilead, AbbVie, Intercept, BMS, and GSK.
Kanwal and colleagues present an interesting study assessing the trends in the incidence and prevalence of NAFLD in the United States. Findings suggest that the annual incidence of NAFLD has generally been stable (2.2%-3.2%), while the prevalence of NAFLD has increased by 2.8-fold (6.3%-17.6%). These findings are consistent with the literature and provide additional evidence supporting the increasing burden of NAFLD. Although an important study, there are some limitations to the study design. First, the diagnosis of NAFLD was solely based on elevated liver enzymes, which can underestimate the true incidence and prevalence of NAFLD. In fact, in a recent meta-analysis, NAFLD prevalence based on liver enzymes was 13%, while NAFLD prevalence based on radiologic diagnosis was 25% (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Second, the study subjects came from the VA system, which may not be representative of the U.S. population (Patrick AFB, FL: Defense Equal Opportunity Management Institute, 2010). This is important because sex-specific differences in the prevalence of NAFLD have been reported (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Nevertheless, these limitations do not minimize the important contribution of this study. There appears to be an alarming increase in the burden of NAFLD within all the racial and age groups in the U.S. Further, this increase in the incidence and prevalence of NAFLD is especially significant among the younger age groups (less than 45 years). This finding is in contrast to others who have reported a higher prevalence in older subjects (Presented at AASLD 2015. San Francisco. Abstract #534). If confirmed, this younger cohort of patients with NAFLD can fuel the future burden of liver disease for the next few decades (JAMA. 2012;307:491-7). Given the current lack of an effective treatment for NAFLD, a national strategy to deal with this important and rising cause of chronic liver disease is urgently needed.
Dr. Zobair M. Younossi, MPH, FACG, AGAF, FAASLD, is chairman, department of medicine, Inova Fairfax Hospital; vice president for research, Inova Health System; professor of medicine, VCU-Inova Campus and Beatty Center for Integrated Research, Falls Church, Va. He has consulted for Gilead, AbbVie, Intercept, BMS, and GSK.
Kanwal and colleagues present an interesting study assessing the trends in the incidence and prevalence of NAFLD in the United States. Findings suggest that the annual incidence of NAFLD has generally been stable (2.2%-3.2%), while the prevalence of NAFLD has increased by 2.8-fold (6.3%-17.6%). These findings are consistent with the literature and provide additional evidence supporting the increasing burden of NAFLD. Although an important study, there are some limitations to the study design. First, the diagnosis of NAFLD was solely based on elevated liver enzymes, which can underestimate the true incidence and prevalence of NAFLD. In fact, in a recent meta-analysis, NAFLD prevalence based on liver enzymes was 13%, while NAFLD prevalence based on radiologic diagnosis was 25% (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Second, the study subjects came from the VA system, which may not be representative of the U.S. population (Patrick AFB, FL: Defense Equal Opportunity Management Institute, 2010). This is important because sex-specific differences in the prevalence of NAFLD have been reported (Hepatology. 2015 Dec 28. doi: 10.1002/hep.28431. [Epub ahead of print]). Nevertheless, these limitations do not minimize the important contribution of this study. There appears to be an alarming increase in the burden of NAFLD within all the racial and age groups in the U.S. Further, this increase in the incidence and prevalence of NAFLD is especially significant among the younger age groups (less than 45 years). This finding is in contrast to others who have reported a higher prevalence in older subjects (Presented at AASLD 2015. San Francisco. Abstract #534). If confirmed, this younger cohort of patients with NAFLD can fuel the future burden of liver disease for the next few decades (JAMA. 2012;307:491-7). Given the current lack of an effective treatment for NAFLD, a national strategy to deal with this important and rising cause of chronic liver disease is urgently needed.
Dr. Zobair M. Younossi, MPH, FACG, AGAF, FAASLD, is chairman, department of medicine, Inova Fairfax Hospital; vice president for research, Inova Health System; professor of medicine, VCU-Inova Campus and Beatty Center for Integrated Research, Falls Church, Va. He has consulted for Gilead, AbbVie, Intercept, BMS, and GSK.
Nonalcoholic fatty liver disease (NAFLD) almost tripled among United States veterans in a recent 9-year period, investigators reported in the February issue of Clinical Gastroenterology and Hepatology.
The trend “was evident in all racial groups, across all age groups, and in both genders,” said Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston, and her associates. The increasing prevalence of NAFLD “is likely generalizable to nonveterans,” and will probably persist because of a “fairly steady” 2%-3% overall annual incidence and a steeper rise among younger individuals, they added. “Nonalcoholic fatty liver disease will continue to remain a major public health problem in the United States, at least in the near and intermediate future.”
Although NAFLD is the leading cause of chronic liver failure in the United States, few studies have examined its incidence or prevalence over time, which are key to predicting future disease burden. Therefore, the investigators analyzed data for more than 9.78 million patients who visited the VA at least once between 2003 and 2011. They defined NAFLD as at least two elevated alanine aminotransferase (ALT) values (greater than 40 IU/mL) separated by at least 6 months, with no history of positive serology for hepatitis B surface antigen or hepatitis C virus RNA, and no alcohol-related ICD-9 codes or positive AUDIT-C scores within a year of elevated ALT levels (Clin Gastroenterol Hepatol. 2015 Aug 7. doi: 10.1016/j.cgh.2015.08.010).
During the study period, more than 1.3 million patients, or 13.6%, met the definition of NAFLD, said the researchers. Age-adjusted incidence rates dropped slightly from 3.16% in 2003 to 2.5% in 2011, ranging between 2.3% and 2.7% in most years. Prevalence, however, rose from 6.3% in 2003 (95% confidence interval, 6.26%-6.3%) to 17.6% in 2011 (95% CI, 17.58%-17.65%), a 2.8-fold increase. Moreover, about one in five patients with NAFLD who visited the VA in 2011 was at risk for advanced fibrosis.
Among individuals who were younger than 45 years, the incidence of NAFLD rose from 2.3 to 4.3 cases per 100 persons (annual percentage change, 7.4%; 95% CI, 5.7% to 9.2%), the researchers also found. “Although recent studies show that the rate of increase in both obesity and diabetes, which are both major risk factors for NAFLD, may be slowing down in the U.S., this may not be the case in the VA, where the prevalence of obesity and diabetes is in fact higher than in the U.S. population,” they said.
In general, the findings mirror a recent analysis of the National Health and Nutrition Examination Survey (Aliment Pharmacol Ther. 2015 Jan;41[1]:65-76), according to the investigators. “The VA is the largest integrated health care system in the United States,” they added. “We believe that the sheer size of the veteran cohort, combined with a complete dearth of information regarding the burden of NAFLD in the VA, renders our findings highly significant. Furthermore, the VA is in a unique position to test and implement systemic changes in medical care delivery to improve the health care of NAFLD patients.”
The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
Nonalcoholic fatty liver disease (NAFLD) almost tripled among United States veterans in a recent 9-year period, investigators reported in the February issue of Clinical Gastroenterology and Hepatology.
The trend “was evident in all racial groups, across all age groups, and in both genders,” said Dr. Fasiha Kanwal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, both in Houston, and her associates. The increasing prevalence of NAFLD “is likely generalizable to nonveterans,” and will probably persist because of a “fairly steady” 2%-3% overall annual incidence and a steeper rise among younger individuals, they added. “Nonalcoholic fatty liver disease will continue to remain a major public health problem in the United States, at least in the near and intermediate future.”
Although NAFLD is the leading cause of chronic liver failure in the United States, few studies have examined its incidence or prevalence over time, which are key to predicting future disease burden. Therefore, the investigators analyzed data for more than 9.78 million patients who visited the VA at least once between 2003 and 2011. They defined NAFLD as at least two elevated alanine aminotransferase (ALT) values (greater than 40 IU/mL) separated by at least 6 months, with no history of positive serology for hepatitis B surface antigen or hepatitis C virus RNA, and no alcohol-related ICD-9 codes or positive AUDIT-C scores within a year of elevated ALT levels (Clin Gastroenterol Hepatol. 2015 Aug 7. doi: 10.1016/j.cgh.2015.08.010).
During the study period, more than 1.3 million patients, or 13.6%, met the definition of NAFLD, said the researchers. Age-adjusted incidence rates dropped slightly from 3.16% in 2003 to 2.5% in 2011, ranging between 2.3% and 2.7% in most years. Prevalence, however, rose from 6.3% in 2003 (95% confidence interval, 6.26%-6.3%) to 17.6% in 2011 (95% CI, 17.58%-17.65%), a 2.8-fold increase. Moreover, about one in five patients with NAFLD who visited the VA in 2011 was at risk for advanced fibrosis.
Among individuals who were younger than 45 years, the incidence of NAFLD rose from 2.3 to 4.3 cases per 100 persons (annual percentage change, 7.4%; 95% CI, 5.7% to 9.2%), the researchers also found. “Although recent studies show that the rate of increase in both obesity and diabetes, which are both major risk factors for NAFLD, may be slowing down in the U.S., this may not be the case in the VA, where the prevalence of obesity and diabetes is in fact higher than in the U.S. population,” they said.
In general, the findings mirror a recent analysis of the National Health and Nutrition Examination Survey (Aliment Pharmacol Ther. 2015 Jan;41[1]:65-76), according to the investigators. “The VA is the largest integrated health care system in the United States,” they added. “We believe that the sheer size of the veteran cohort, combined with a complete dearth of information regarding the burden of NAFLD in the VA, renders our findings highly significant. Furthermore, the VA is in a unique position to test and implement systemic changes in medical care delivery to improve the health care of NAFLD patients.”
The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: The prevalence of nonalcoholic fatty liver disease has risen substantially since 2003, and will probably keep increasing in the near term.
Major finding: Prevalence among veterans rose about 2.8 times between 2003 and 2011, mirroring trends reported in the general population.
Data source: An analysis of data from 9.78 million Veterans Affairs patients.
Disclosures: The study was partially supported by the Michael E. DeBakey Veterans Affairs Medical Center. The researchers had no disclosures.
Hepatitis C virus infection linked to cardiovascular death, disease, and stroke
Patients with hepatitis C virus (HCV) infection face a significantly increased risk of cardiovascular death, subclinical carotid thickening and atherosclerosis, and cerebrocardiovascular events, especially when they also have diabetes and hypertension, according to a systematic review and meta-analysis of 22 studies published in the January issue of Gastroenterology.
“To our knowledge, our meta-analysis clearly highlights, for the first time, that HCV infection increases the risk of cardiovascular disease-related mortality,” wrote Dr. Salvatore Petta and his associates at the University of Palermo, Italy. “We [also] found a twofold higher risk of subclinical carotid plaques among HCV-infected individuals compared to uninfected controls, without significant heterogeneity among studies, as well as an increased risk of carotid thickening. We observed a slightly significant increase in cerebrocardiovascular events among HCV-infected patients, despite the high heterogeneity among studies that was mostly related to the prevalence of diabetes mellitus and hypertension.”
A number of observational studies have reported cardiovascular outcomes in HCV-infected patients, but results have been “ambiguous,” Dr. Petta and his colleagues said. For their meta-analysis, they searched PubMed, Medline, EMBASE, the Cochrane Library, and reference lists of articles to identify studies published through July 2015 that either compared cardiovascular disease between HCV-infected and uninfected patients, or evaluated the prevalence of HCV infection among patients with cardiovascular disease. This literature search identified 12 case-control studies and 10 cohort studies. Outcome measures included carotid atherosclerosis (nine studies), intima media thickness (eight studies), coronary artery disease (seven studies), stroke (six studies), and cardiovascular mortality (three studies) (Gastroenterology. 2015 Sep 18. doi: 10.1053/j.gastro.2015.09.00).In the pooled analysis, the odds of cardiovascular death were 65% higher in HCV-infected patients, compared with uninfected individuals (95% confidence interval for this increase, 1.07%-2.56%). Compared with controls, HCV-infected patients also were at higher risk of carotid plaques (odds ratio, 2.27; 95% CI, 1.76-2.94), especially when they were smokers (P = .02). HCV infection also significantly increased the odds of carotid artery intima-media thickening (OR, 1.20; 95% CI, 1.03-1.40), and cerebrocardiovascular events (OR, 1.30; 95% CI, 1.10-1.55). However, subgroup analyses showed that HCV infection only increased the likelihood of cerebrocardiovascular events in populations with a more than 10% prevalence of diabetes or a more than 20% prevalence of hypertension (OR, 1.71; P less than .001 for both subgroup analyses).
Because the studies of cerebrocardiovascular events were heterogeneous, the researchers also stratified them by study design and by the average age of patients. Pooled odds ratios for the link between HCV infection and cerebrocardiovascular events remained significant at 1.21 for the cohort studies, 2.01 for the case-control studies, 2.46 among patients who averaged more than 50 years of age, and 1.35 among younger patients.
The Egger test for publication bias showed that the literature search was unlikely to have overlooked studies in terms of any of the outcome measures, the investigators noted. “From a clinical standpoint, the results of our meta-analysis suggest that HCV infection increases cardiovascular risk, particularly for individuals who already have cardiovascular risk factors, such as diabetes and hypertension,” they concluded. “Although effective and safe oral antiviral regimens are available, more information is needed to confirm whether anti-HCV medications will decrease cardiovascular risk, as suggested in some studies.”
The researchers reported having no funding sources or conflicts of interest.
Source: American Gastroenterological Association
Patients with hepatitis C virus (HCV) infection face a significantly increased risk of cardiovascular death, subclinical carotid thickening and atherosclerosis, and cerebrocardiovascular events, especially when they also have diabetes and hypertension, according to a systematic review and meta-analysis of 22 studies published in the January issue of Gastroenterology.
“To our knowledge, our meta-analysis clearly highlights, for the first time, that HCV infection increases the risk of cardiovascular disease-related mortality,” wrote Dr. Salvatore Petta and his associates at the University of Palermo, Italy. “We [also] found a twofold higher risk of subclinical carotid plaques among HCV-infected individuals compared to uninfected controls, without significant heterogeneity among studies, as well as an increased risk of carotid thickening. We observed a slightly significant increase in cerebrocardiovascular events among HCV-infected patients, despite the high heterogeneity among studies that was mostly related to the prevalence of diabetes mellitus and hypertension.”
A number of observational studies have reported cardiovascular outcomes in HCV-infected patients, but results have been “ambiguous,” Dr. Petta and his colleagues said. For their meta-analysis, they searched PubMed, Medline, EMBASE, the Cochrane Library, and reference lists of articles to identify studies published through July 2015 that either compared cardiovascular disease between HCV-infected and uninfected patients, or evaluated the prevalence of HCV infection among patients with cardiovascular disease. This literature search identified 12 case-control studies and 10 cohort studies. Outcome measures included carotid atherosclerosis (nine studies), intima media thickness (eight studies), coronary artery disease (seven studies), stroke (six studies), and cardiovascular mortality (three studies) (Gastroenterology. 2015 Sep 18. doi: 10.1053/j.gastro.2015.09.00).In the pooled analysis, the odds of cardiovascular death were 65% higher in HCV-infected patients, compared with uninfected individuals (95% confidence interval for this increase, 1.07%-2.56%). Compared with controls, HCV-infected patients also were at higher risk of carotid plaques (odds ratio, 2.27; 95% CI, 1.76-2.94), especially when they were smokers (P = .02). HCV infection also significantly increased the odds of carotid artery intima-media thickening (OR, 1.20; 95% CI, 1.03-1.40), and cerebrocardiovascular events (OR, 1.30; 95% CI, 1.10-1.55). However, subgroup analyses showed that HCV infection only increased the likelihood of cerebrocardiovascular events in populations with a more than 10% prevalence of diabetes or a more than 20% prevalence of hypertension (OR, 1.71; P less than .001 for both subgroup analyses).
Because the studies of cerebrocardiovascular events were heterogeneous, the researchers also stratified them by study design and by the average age of patients. Pooled odds ratios for the link between HCV infection and cerebrocardiovascular events remained significant at 1.21 for the cohort studies, 2.01 for the case-control studies, 2.46 among patients who averaged more than 50 years of age, and 1.35 among younger patients.
The Egger test for publication bias showed that the literature search was unlikely to have overlooked studies in terms of any of the outcome measures, the investigators noted. “From a clinical standpoint, the results of our meta-analysis suggest that HCV infection increases cardiovascular risk, particularly for individuals who already have cardiovascular risk factors, such as diabetes and hypertension,” they concluded. “Although effective and safe oral antiviral regimens are available, more information is needed to confirm whether anti-HCV medications will decrease cardiovascular risk, as suggested in some studies.”
The researchers reported having no funding sources or conflicts of interest.
Source: American Gastroenterological Association
Patients with hepatitis C virus (HCV) infection face a significantly increased risk of cardiovascular death, subclinical carotid thickening and atherosclerosis, and cerebrocardiovascular events, especially when they also have diabetes and hypertension, according to a systematic review and meta-analysis of 22 studies published in the January issue of Gastroenterology.
“To our knowledge, our meta-analysis clearly highlights, for the first time, that HCV infection increases the risk of cardiovascular disease-related mortality,” wrote Dr. Salvatore Petta and his associates at the University of Palermo, Italy. “We [also] found a twofold higher risk of subclinical carotid plaques among HCV-infected individuals compared to uninfected controls, without significant heterogeneity among studies, as well as an increased risk of carotid thickening. We observed a slightly significant increase in cerebrocardiovascular events among HCV-infected patients, despite the high heterogeneity among studies that was mostly related to the prevalence of diabetes mellitus and hypertension.”
A number of observational studies have reported cardiovascular outcomes in HCV-infected patients, but results have been “ambiguous,” Dr. Petta and his colleagues said. For their meta-analysis, they searched PubMed, Medline, EMBASE, the Cochrane Library, and reference lists of articles to identify studies published through July 2015 that either compared cardiovascular disease between HCV-infected and uninfected patients, or evaluated the prevalence of HCV infection among patients with cardiovascular disease. This literature search identified 12 case-control studies and 10 cohort studies. Outcome measures included carotid atherosclerosis (nine studies), intima media thickness (eight studies), coronary artery disease (seven studies), stroke (six studies), and cardiovascular mortality (three studies) (Gastroenterology. 2015 Sep 18. doi: 10.1053/j.gastro.2015.09.00).In the pooled analysis, the odds of cardiovascular death were 65% higher in HCV-infected patients, compared with uninfected individuals (95% confidence interval for this increase, 1.07%-2.56%). Compared with controls, HCV-infected patients also were at higher risk of carotid plaques (odds ratio, 2.27; 95% CI, 1.76-2.94), especially when they were smokers (P = .02). HCV infection also significantly increased the odds of carotid artery intima-media thickening (OR, 1.20; 95% CI, 1.03-1.40), and cerebrocardiovascular events (OR, 1.30; 95% CI, 1.10-1.55). However, subgroup analyses showed that HCV infection only increased the likelihood of cerebrocardiovascular events in populations with a more than 10% prevalence of diabetes or a more than 20% prevalence of hypertension (OR, 1.71; P less than .001 for both subgroup analyses).
Because the studies of cerebrocardiovascular events were heterogeneous, the researchers also stratified them by study design and by the average age of patients. Pooled odds ratios for the link between HCV infection and cerebrocardiovascular events remained significant at 1.21 for the cohort studies, 2.01 for the case-control studies, 2.46 among patients who averaged more than 50 years of age, and 1.35 among younger patients.
The Egger test for publication bias showed that the literature search was unlikely to have overlooked studies in terms of any of the outcome measures, the investigators noted. “From a clinical standpoint, the results of our meta-analysis suggest that HCV infection increases cardiovascular risk, particularly for individuals who already have cardiovascular risk factors, such as diabetes and hypertension,” they concluded. “Although effective and safe oral antiviral regimens are available, more information is needed to confirm whether anti-HCV medications will decrease cardiovascular risk, as suggested in some studies.”
The researchers reported having no funding sources or conflicts of interest.
Source: American Gastroenterological Association
FROM GASTROENTEROLOGY
Key clinical point: Patients with HCV infection are at increased risk of cardiovascular death, stroke, and subclinical carotid atherosclerosis and thickening.
Major finding: The pooled odds of cardiovascular death were 65% higher among infected, compared with uninfected individuals.
Data source: A systematic review and meta-analysis of 22 observational studies.
Disclosures: The researchers reported having no funding sources or conflicts of interest.
Nonalcoholic fatty liver disease linked to liver cancer without cirrhosis
About 13% of U.S. veterans with hepatocellular carcinoma had no evidence of preexisting cirrhosis, according to a report published in the January issue of Clinical Gastroenterology and Hepatology.
“The main risk factors for this entity were nonalcoholic fatty liver disease [NAFLD] or metabolic syndrome” – not hepatitis C virus infection [HCV], HBV [hepatitis B virus] infection, or alcohol abuse, said Dr. Sahil Mittal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine in Houston. Screening all patients with NAFLD for hepatocellular carcinoma [HCC] is impractical, so studies should seek “actionable risk factors” or biomarkers that reliably identify NAFLD patients who are at particular risk of HCC, wrote Dr. Mittal and his coinvestigators.
Researchers have debated whether chronic HCV infection or alcohol abuse can lead to HCC in the absence of cirrhosis, while at least one study has shown that NAFLD can predispose patients to this disease entity (Arch Pathol Lab Med. 2008;132:1761-6).
But few studies have systematically examined risk factors for HCC without cirrhosis in the general population, the investigators said. Therefore, they randomly selected 1,500 patients from the U.S. Veterans Affairs system who were diagnosed with HCC between 2005 and 2010 on the basis of histopathology or established imaging criteria (Hepatology 2005;42:1208-36).
They reviewed complete medical records for these patients, and classified those who did not have cirrhosis according to the quality of supporting histology, laboratory, and imaging data (Clin Gastroenterol Hepatol. 2015. doi: 0.1016/j.cgh.2015.07.019).
In all, 3% of the cohort had level 1 (“highest-quality”) evidence for not having cirrhosis, while another 10% had level 2 evidence for no cirrhosis, the investigators said. “Compared with HCC in the presence of cirrhosis, these patients were more likely to have metabolic syndrome or NAFLD or no identifiable risk factor, and less likely to have alcohol abuse or HCV infection,” they added. Only two-thirds of NAFLD patients with HCC had cirrhosis, compared with 91% of patients with chronic HCV infection, 92% of HCV-infected patients, and 88% of patients with an alcohol use disorder. Notably, the odds of HCC in the absence of cirrhosis were more than five times higher when patients had NAFLD (odds ratio [OR], 5.4; 95% confidence interval [CI], 3.4-8.5) or metabolic syndrome (OR, 5.0; 95% CI, 3.1-7.8) compared with HCV infection.
Patients with cirrhosis often go unscreened for HCC even though they are at greatest risk of this cancer. Therefore, trying to screen all patients with NAFLD for HCC would be “logistically impractical,” particularly when the absolute risk of HCC in noncirrhotic patients is unknown and no one has examined the best ways to screen this population, the investigators said. Instead, clinicians could prioritize screening and treating NAFLD patients for diabetes mellitus and obesity, both of which are associated with HCC. “There is evidence to suggest that metformin reduces the risk of HCC among diabetics,” they added. “Studies of these and other risk factors of HCC among NAFLD patients with and without cirrhosis are needed.”
Most patients in the study were male, potentially limiting the generalizability of the findings, the researchers noted.
The National Cancer Institute, the Houston Veterans Affairs Health Services Research and Development Center of Excellence, the Michael E. DeBakey Veterans Affairs Medical Center, and the Dan Duncan Cancer Center funded the study. The researchers had no disclosures.
Source: American Gastroenterological Association
About 13% of U.S. veterans with hepatocellular carcinoma had no evidence of preexisting cirrhosis, according to a report published in the January issue of Clinical Gastroenterology and Hepatology.
“The main risk factors for this entity were nonalcoholic fatty liver disease [NAFLD] or metabolic syndrome” – not hepatitis C virus infection [HCV], HBV [hepatitis B virus] infection, or alcohol abuse, said Dr. Sahil Mittal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine in Houston. Screening all patients with NAFLD for hepatocellular carcinoma [HCC] is impractical, so studies should seek “actionable risk factors” or biomarkers that reliably identify NAFLD patients who are at particular risk of HCC, wrote Dr. Mittal and his coinvestigators.
Researchers have debated whether chronic HCV infection or alcohol abuse can lead to HCC in the absence of cirrhosis, while at least one study has shown that NAFLD can predispose patients to this disease entity (Arch Pathol Lab Med. 2008;132:1761-6).
But few studies have systematically examined risk factors for HCC without cirrhosis in the general population, the investigators said. Therefore, they randomly selected 1,500 patients from the U.S. Veterans Affairs system who were diagnosed with HCC between 2005 and 2010 on the basis of histopathology or established imaging criteria (Hepatology 2005;42:1208-36).
They reviewed complete medical records for these patients, and classified those who did not have cirrhosis according to the quality of supporting histology, laboratory, and imaging data (Clin Gastroenterol Hepatol. 2015. doi: 0.1016/j.cgh.2015.07.019).
In all, 3% of the cohort had level 1 (“highest-quality”) evidence for not having cirrhosis, while another 10% had level 2 evidence for no cirrhosis, the investigators said. “Compared with HCC in the presence of cirrhosis, these patients were more likely to have metabolic syndrome or NAFLD or no identifiable risk factor, and less likely to have alcohol abuse or HCV infection,” they added. Only two-thirds of NAFLD patients with HCC had cirrhosis, compared with 91% of patients with chronic HCV infection, 92% of HCV-infected patients, and 88% of patients with an alcohol use disorder. Notably, the odds of HCC in the absence of cirrhosis were more than five times higher when patients had NAFLD (odds ratio [OR], 5.4; 95% confidence interval [CI], 3.4-8.5) or metabolic syndrome (OR, 5.0; 95% CI, 3.1-7.8) compared with HCV infection.
Patients with cirrhosis often go unscreened for HCC even though they are at greatest risk of this cancer. Therefore, trying to screen all patients with NAFLD for HCC would be “logistically impractical,” particularly when the absolute risk of HCC in noncirrhotic patients is unknown and no one has examined the best ways to screen this population, the investigators said. Instead, clinicians could prioritize screening and treating NAFLD patients for diabetes mellitus and obesity, both of which are associated with HCC. “There is evidence to suggest that metformin reduces the risk of HCC among diabetics,” they added. “Studies of these and other risk factors of HCC among NAFLD patients with and without cirrhosis are needed.”
Most patients in the study were male, potentially limiting the generalizability of the findings, the researchers noted.
The National Cancer Institute, the Houston Veterans Affairs Health Services Research and Development Center of Excellence, the Michael E. DeBakey Veterans Affairs Medical Center, and the Dan Duncan Cancer Center funded the study. The researchers had no disclosures.
Source: American Gastroenterological Association
About 13% of U.S. veterans with hepatocellular carcinoma had no evidence of preexisting cirrhosis, according to a report published in the January issue of Clinical Gastroenterology and Hepatology.
“The main risk factors for this entity were nonalcoholic fatty liver disease [NAFLD] or metabolic syndrome” – not hepatitis C virus infection [HCV], HBV [hepatitis B virus] infection, or alcohol abuse, said Dr. Sahil Mittal of the Michael E. DeBakey Veterans Affairs Medical Center and Baylor College of Medicine in Houston. Screening all patients with NAFLD for hepatocellular carcinoma [HCC] is impractical, so studies should seek “actionable risk factors” or biomarkers that reliably identify NAFLD patients who are at particular risk of HCC, wrote Dr. Mittal and his coinvestigators.
Researchers have debated whether chronic HCV infection or alcohol abuse can lead to HCC in the absence of cirrhosis, while at least one study has shown that NAFLD can predispose patients to this disease entity (Arch Pathol Lab Med. 2008;132:1761-6).
But few studies have systematically examined risk factors for HCC without cirrhosis in the general population, the investigators said. Therefore, they randomly selected 1,500 patients from the U.S. Veterans Affairs system who were diagnosed with HCC between 2005 and 2010 on the basis of histopathology or established imaging criteria (Hepatology 2005;42:1208-36).
They reviewed complete medical records for these patients, and classified those who did not have cirrhosis according to the quality of supporting histology, laboratory, and imaging data (Clin Gastroenterol Hepatol. 2015. doi: 0.1016/j.cgh.2015.07.019).
In all, 3% of the cohort had level 1 (“highest-quality”) evidence for not having cirrhosis, while another 10% had level 2 evidence for no cirrhosis, the investigators said. “Compared with HCC in the presence of cirrhosis, these patients were more likely to have metabolic syndrome or NAFLD or no identifiable risk factor, and less likely to have alcohol abuse or HCV infection,” they added. Only two-thirds of NAFLD patients with HCC had cirrhosis, compared with 91% of patients with chronic HCV infection, 92% of HCV-infected patients, and 88% of patients with an alcohol use disorder. Notably, the odds of HCC in the absence of cirrhosis were more than five times higher when patients had NAFLD (odds ratio [OR], 5.4; 95% confidence interval [CI], 3.4-8.5) or metabolic syndrome (OR, 5.0; 95% CI, 3.1-7.8) compared with HCV infection.
Patients with cirrhosis often go unscreened for HCC even though they are at greatest risk of this cancer. Therefore, trying to screen all patients with NAFLD for HCC would be “logistically impractical,” particularly when the absolute risk of HCC in noncirrhotic patients is unknown and no one has examined the best ways to screen this population, the investigators said. Instead, clinicians could prioritize screening and treating NAFLD patients for diabetes mellitus and obesity, both of which are associated with HCC. “There is evidence to suggest that metformin reduces the risk of HCC among diabetics,” they added. “Studies of these and other risk factors of HCC among NAFLD patients with and without cirrhosis are needed.”
Most patients in the study were male, potentially limiting the generalizability of the findings, the researchers noted.
The National Cancer Institute, the Houston Veterans Affairs Health Services Research and Development Center of Excellence, the Michael E. DeBakey Veterans Affairs Medical Center, and the Dan Duncan Cancer Center funded the study. The researchers had no disclosures.
Source: American Gastroenterological Association
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Nonalcoholic fatty liver disease and metabolic syndrome are risk factors for hepatocellular carcinoma in the absence of cirrhosis.
Major finding: Patients with these diseases were more than five times as likely to develop noncirrhotic HCC compared with patients with chronic hepatitis C virus infection.
Data source: A study of 1,500 randomly selected U.S. veterans diagnosed with HCC between 2005 and 2010.
Disclosures: The National Cancer Institute, the Houston Veterans Affairs Health Services Research and Development Center of Excellence, the Michael E. DeBakey Veterans Affairs Medical Center, and the Dan Duncan Cancer Center funded the study. The researchers had no disclosures.
Study lays groundwork for autologous nerve cell transplantation in Hirschsprung disease
Neural progenitor cells from the proximal colons of patients with Hirschsprung disease divided and formed neurons and glia in their own distal aganglionic colon tissue, according to a study published in the January issue of Cellular and Molecular Gastroenterology and Hepatology.
The approach could lead to “a promising therapeutic strategy” for Hirschsprung disease, because autologous transplantation of nerve progenitor cells would prevent immune rejection, said Dr. Benjamin Rollo of Murdoch Children’s Research Institute in Victoria, Australia, and his associates. But they cautioned against reading too much into their findings, “as there are distributional hurdles to surmount” before cell therapy can be used alone or in combination with surgery.
Hirschsprung disease is a congenital disorder in which embryonic neural crest cells fail to form the distal part of the enteric nervous system. The resulting lack of bowel motility leads to severe constipation and potentially fatal megacolon. Treatment involves surgically resecting the aganglionic distal bowel and anastomosing the normal proximal bowel to the anorectum, but this approach itself can cause constipation as well as fecal soiling, the investigators noted (Cell Molec Gastroenterol Hepatol. 2015 Oct. 22 [doi: 10.1016/j.jcmgh.2015.09.007]).
For the study, they harvested tissue from the proximal (neuronal) and distal (aneuronal) margins of resection of the colons of 31 infants and children with Hirschsprung disease. They cultured cells from the myenteric plexus of the proximal colon, and separated out nerve cells by using flow cytometry for the p75 neural crest marker. To test whether these nerve cells could colonize aganglionic colon, they co-cultured them with aneural avian embryo gut and with patients’ own aneuronal colon muscle. In addition, they co-cultured embryonic mouse enteric nernous system cells with human aneuronal colon muscle. They used quantitative reverse transcriptase PCR and several other standard laboratory techniques to detect cellular markers and mitosis.
Ganglia from the patients’ proximal colons expressed the neural crest markers p75, SOX10, and HNK1, as well as several neuronal, neurite, and glial markers, the researchers reported. The proximal colon specimens also contained ENS progenitor cells, which were SOX10-positive but lacked neuronal or glial markers. The progenitor cells comprised less than 5% of all cells from the proximal colon, but proliferated fourfold more after supplementation with CHIR-99021, a selective inhibitor of glycogen synthase kinase 3. They successfully colonized avian aneural embryonic gut and autologous postnatal aneuronal colon tissue, and the latter also was colonized successfully by mouse enteric neural crest cells.
The study “fulfilled three key requirements” for transplantation – harvesting enteric nervous system cells from the proximal colons of patients with Hirschsprung disease; confirming that postnatal aneuronal colon tissue could support an enteric nervous system; and showing that enteric nervous system–derived cells could colonize both embryonic gut and autologous postnatal colon tissue, said the researchers. More work is needed to sufficiently expand embryonic neural crest stem or progenitor cells and transplant them over a large area of colon, although supplementation with growth factors and use of elastic polymer substrates might help, they added.
The National Health and Medical Research Council, Murdoch Children’s Research Institute, Graham Burke and Yvonne Spencer, the Federation of Chinese Associations, Fonds du Service de chirurgie pédiatrique et de Perfectionnement du CHUV, Fondation SICPA, and Société Académique Vaudoise funded the study. The researchers disclosed no conflicts of interest.
Hirschsprung disease results from a failure of complete neural crest cell migration into the distal colon. Current therapy relies on surgical resection of the aganglionic distal colon. However, for many children, particularly those with large aganglionic segments, surgery often fails to completely normalize function.
One therapeutic approach might be to transplant new enteric neural cells into the aganglionic colon. This has been partially accomplished in rodent and avian models. However, it is not known if human smooth muscle can be colonized or if human postnatal enteric nervous system cells are capable of migration, expansion, and differentiation.
Rollo et al. show that human postnatal enteric nervous system cells isolated from the proximal, i.e., ganglionic, margin of Hirschsprung disease resection specimens can migrate and spread to colonize aganglionic smooth muscle from the distal margin of the same specimen. Remarkably, the transplanted cells differentiated into neurons and glia and formed normal-appearing neural structures.
Many questions remain. If neural cells isolated from the proximal margin can migrate ex vivo, why didn’t they migrate during in utero development? Do the transplanted cells restore normal motility, which requires a complex series of events that must be precisely orchestrated?
Nevertheless, the demonstration that human postnatal neural cells can be isolated from surgical specimens and used to colonize aganglionic smooth muscle suggests that it may be possible to conserve and restore motility and, potentially, to successfully treat Hirschprung disease patients without the need for surgery.
Marion France, Ph.D., is a postdoctoral research fellow at Brigham and Women’s Hospital and Harvard Medical School, Boston. She has no conflicts of interest.
Hirschsprung disease results from a failure of complete neural crest cell migration into the distal colon. Current therapy relies on surgical resection of the aganglionic distal colon. However, for many children, particularly those with large aganglionic segments, surgery often fails to completely normalize function.
One therapeutic approach might be to transplant new enteric neural cells into the aganglionic colon. This has been partially accomplished in rodent and avian models. However, it is not known if human smooth muscle can be colonized or if human postnatal enteric nervous system cells are capable of migration, expansion, and differentiation.
Rollo et al. show that human postnatal enteric nervous system cells isolated from the proximal, i.e., ganglionic, margin of Hirschsprung disease resection specimens can migrate and spread to colonize aganglionic smooth muscle from the distal margin of the same specimen. Remarkably, the transplanted cells differentiated into neurons and glia and formed normal-appearing neural structures.
Many questions remain. If neural cells isolated from the proximal margin can migrate ex vivo, why didn’t they migrate during in utero development? Do the transplanted cells restore normal motility, which requires a complex series of events that must be precisely orchestrated?
Nevertheless, the demonstration that human postnatal neural cells can be isolated from surgical specimens and used to colonize aganglionic smooth muscle suggests that it may be possible to conserve and restore motility and, potentially, to successfully treat Hirschprung disease patients without the need for surgery.
Marion France, Ph.D., is a postdoctoral research fellow at Brigham and Women’s Hospital and Harvard Medical School, Boston. She has no conflicts of interest.
Hirschsprung disease results from a failure of complete neural crest cell migration into the distal colon. Current therapy relies on surgical resection of the aganglionic distal colon. However, for many children, particularly those with large aganglionic segments, surgery often fails to completely normalize function.
One therapeutic approach might be to transplant new enteric neural cells into the aganglionic colon. This has been partially accomplished in rodent and avian models. However, it is not known if human smooth muscle can be colonized or if human postnatal enteric nervous system cells are capable of migration, expansion, and differentiation.
Rollo et al. show that human postnatal enteric nervous system cells isolated from the proximal, i.e., ganglionic, margin of Hirschsprung disease resection specimens can migrate and spread to colonize aganglionic smooth muscle from the distal margin of the same specimen. Remarkably, the transplanted cells differentiated into neurons and glia and formed normal-appearing neural structures.
Many questions remain. If neural cells isolated from the proximal margin can migrate ex vivo, why didn’t they migrate during in utero development? Do the transplanted cells restore normal motility, which requires a complex series of events that must be precisely orchestrated?
Nevertheless, the demonstration that human postnatal neural cells can be isolated from surgical specimens and used to colonize aganglionic smooth muscle suggests that it may be possible to conserve and restore motility and, potentially, to successfully treat Hirschprung disease patients without the need for surgery.
Marion France, Ph.D., is a postdoctoral research fellow at Brigham and Women’s Hospital and Harvard Medical School, Boston. She has no conflicts of interest.
Neural progenitor cells from the proximal colons of patients with Hirschsprung disease divided and formed neurons and glia in their own distal aganglionic colon tissue, according to a study published in the January issue of Cellular and Molecular Gastroenterology and Hepatology.
The approach could lead to “a promising therapeutic strategy” for Hirschsprung disease, because autologous transplantation of nerve progenitor cells would prevent immune rejection, said Dr. Benjamin Rollo of Murdoch Children’s Research Institute in Victoria, Australia, and his associates. But they cautioned against reading too much into their findings, “as there are distributional hurdles to surmount” before cell therapy can be used alone or in combination with surgery.
Hirschsprung disease is a congenital disorder in which embryonic neural crest cells fail to form the distal part of the enteric nervous system. The resulting lack of bowel motility leads to severe constipation and potentially fatal megacolon. Treatment involves surgically resecting the aganglionic distal bowel and anastomosing the normal proximal bowel to the anorectum, but this approach itself can cause constipation as well as fecal soiling, the investigators noted (Cell Molec Gastroenterol Hepatol. 2015 Oct. 22 [doi: 10.1016/j.jcmgh.2015.09.007]).
For the study, they harvested tissue from the proximal (neuronal) and distal (aneuronal) margins of resection of the colons of 31 infants and children with Hirschsprung disease. They cultured cells from the myenteric plexus of the proximal colon, and separated out nerve cells by using flow cytometry for the p75 neural crest marker. To test whether these nerve cells could colonize aganglionic colon, they co-cultured them with aneural avian embryo gut and with patients’ own aneuronal colon muscle. In addition, they co-cultured embryonic mouse enteric nernous system cells with human aneuronal colon muscle. They used quantitative reverse transcriptase PCR and several other standard laboratory techniques to detect cellular markers and mitosis.
Ganglia from the patients’ proximal colons expressed the neural crest markers p75, SOX10, and HNK1, as well as several neuronal, neurite, and glial markers, the researchers reported. The proximal colon specimens also contained ENS progenitor cells, which were SOX10-positive but lacked neuronal or glial markers. The progenitor cells comprised less than 5% of all cells from the proximal colon, but proliferated fourfold more after supplementation with CHIR-99021, a selective inhibitor of glycogen synthase kinase 3. They successfully colonized avian aneural embryonic gut and autologous postnatal aneuronal colon tissue, and the latter also was colonized successfully by mouse enteric neural crest cells.
The study “fulfilled three key requirements” for transplantation – harvesting enteric nervous system cells from the proximal colons of patients with Hirschsprung disease; confirming that postnatal aneuronal colon tissue could support an enteric nervous system; and showing that enteric nervous system–derived cells could colonize both embryonic gut and autologous postnatal colon tissue, said the researchers. More work is needed to sufficiently expand embryonic neural crest stem or progenitor cells and transplant them over a large area of colon, although supplementation with growth factors and use of elastic polymer substrates might help, they added.
The National Health and Medical Research Council, Murdoch Children’s Research Institute, Graham Burke and Yvonne Spencer, the Federation of Chinese Associations, Fonds du Service de chirurgie pédiatrique et de Perfectionnement du CHUV, Fondation SICPA, and Société Académique Vaudoise funded the study. The researchers disclosed no conflicts of interest.
Neural progenitor cells from the proximal colons of patients with Hirschsprung disease divided and formed neurons and glia in their own distal aganglionic colon tissue, according to a study published in the January issue of Cellular and Molecular Gastroenterology and Hepatology.
The approach could lead to “a promising therapeutic strategy” for Hirschsprung disease, because autologous transplantation of nerve progenitor cells would prevent immune rejection, said Dr. Benjamin Rollo of Murdoch Children’s Research Institute in Victoria, Australia, and his associates. But they cautioned against reading too much into their findings, “as there are distributional hurdles to surmount” before cell therapy can be used alone or in combination with surgery.
Hirschsprung disease is a congenital disorder in which embryonic neural crest cells fail to form the distal part of the enteric nervous system. The resulting lack of bowel motility leads to severe constipation and potentially fatal megacolon. Treatment involves surgically resecting the aganglionic distal bowel and anastomosing the normal proximal bowel to the anorectum, but this approach itself can cause constipation as well as fecal soiling, the investigators noted (Cell Molec Gastroenterol Hepatol. 2015 Oct. 22 [doi: 10.1016/j.jcmgh.2015.09.007]).
For the study, they harvested tissue from the proximal (neuronal) and distal (aneuronal) margins of resection of the colons of 31 infants and children with Hirschsprung disease. They cultured cells from the myenteric plexus of the proximal colon, and separated out nerve cells by using flow cytometry for the p75 neural crest marker. To test whether these nerve cells could colonize aganglionic colon, they co-cultured them with aneural avian embryo gut and with patients’ own aneuronal colon muscle. In addition, they co-cultured embryonic mouse enteric nernous system cells with human aneuronal colon muscle. They used quantitative reverse transcriptase PCR and several other standard laboratory techniques to detect cellular markers and mitosis.
Ganglia from the patients’ proximal colons expressed the neural crest markers p75, SOX10, and HNK1, as well as several neuronal, neurite, and glial markers, the researchers reported. The proximal colon specimens also contained ENS progenitor cells, which were SOX10-positive but lacked neuronal or glial markers. The progenitor cells comprised less than 5% of all cells from the proximal colon, but proliferated fourfold more after supplementation with CHIR-99021, a selective inhibitor of glycogen synthase kinase 3. They successfully colonized avian aneural embryonic gut and autologous postnatal aneuronal colon tissue, and the latter also was colonized successfully by mouse enteric neural crest cells.
The study “fulfilled three key requirements” for transplantation – harvesting enteric nervous system cells from the proximal colons of patients with Hirschsprung disease; confirming that postnatal aneuronal colon tissue could support an enteric nervous system; and showing that enteric nervous system–derived cells could colonize both embryonic gut and autologous postnatal colon tissue, said the researchers. More work is needed to sufficiently expand embryonic neural crest stem or progenitor cells and transplant them over a large area of colon, although supplementation with growth factors and use of elastic polymer substrates might help, they added.
The National Health and Medical Research Council, Murdoch Children’s Research Institute, Graham Burke and Yvonne Spencer, the Federation of Chinese Associations, Fonds du Service de chirurgie pédiatrique et de Perfectionnement du CHUV, Fondation SICPA, and Société Académique Vaudoise funded the study. The researchers disclosed no conflicts of interest.
FROM CELLULAR AND MOLECULAR GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: A preclinical study took several key steps toward autologous transplantation of nerve progenitor cells to treat Hirschsprung disease.
Major finding: Neural progenitor cells from the proximal colons of patients with Hirschsprung disease divided and formed neurons and glia in their own distal aganglionic colon tissue.
Data source: Flow cytometry, reverse transcriptase-PCR, immunohistochemistry, and cell culture of proximal (neuronal) and distal (aneuronal) colon tissue from 31 affected patients, and co-culture with avian embryonic gut and mouse enteric nervous system cells.
Disclosures: The National Health and Medical Research Council, Murdoch Children’s Research Institute, Graham Burke and Yvonne Spencer, the Federation of Chinese Associations, Fonds du Service de chirurgie pédiatrique et de Perfectionnement du CHUV, Fondation SICPA, and Société Académique Vaudoise funded the study. The researchers disclosed no conflicts of interest.
PPIs caused remission in about half of esophageal eosinophilia cases
About half of patients with symptomatic esophageal eosinophilia achieved complete clinical and histologic remission on proton pump inhibitors (PPIs), according to a systematic review and meta-analysis of 33 studies.
“Our results support the concept of PPIs as first-line therapy in both children and adults,” Dr. Alfredo Lucendo of the Servicio de Salud de Castillo–La Mancha, Hospital General de Tomelloso, Albacete, Spain, and his associates wrote in the January issue of Clinical Gastroenterology and Hepatology. “Other effective alternatives, such as dietary or topical steroid therapy, likely might be set aside as second-line treatment, owing to long-term safety concerns (topical steroid therapy) and impairment of quality of life and nutritional inadequacy (dietary interventions).”
The study also confirmed that esophageal pH monitoring does not accurately predict therapeutic response to PPI therapy. “The performance of this test before histologic reevaluation on PPI therapy should be discouraged,” according to the researchers (Clin Gastroenterol Hepatol. 2015. [doi:10.1016/j.cgh.2015.07.041]). Eosinophilic esophagitis was first described as a distinct disorder about 20 years ago, but only recently was understood to be the most common cause of chronic esophageal symptoms among children and young adults. Some cases are now known to respond to PPI therapy, but reported remission rates have varied depending on study design and patient population, the investigators said. In addition, no one had systematically reviewed studies of PPI-responsive esophageal eosinophilia for quality or to determine the optimal type of PPI, dose, or treatment duration.
Therefore, the investigators searched MEDLINE, EMBASE, SCOPUS, and abstracts from the annual meetings of the American Gastroenterological Association, the American College of Gastroenterology, and United European Gastroenterology, identifying 33 studies of 619 patients with symptomatic esophageal eosinophilia. Eleven of the studies were prospective, of which only two were randomized controlled trials. The researchers defined a histologic response as less than 15 eosinophils per high-powered frame after PPI therapy. “Missing data regarding PPI therapy were common and prevented us from drawing conclusions on the most effective PPI drug and doses,” they said. In addition, most studies lacked structured or objective survey tools or other measures of clinical improvement, making it impossible to rule out self-adapted coping strategies as a main cause of improvement over time.
With those caveats in mind, about 61% of patients in the pooled analysis had a clinical response to PPI therapy (95% confidence interval, 48%-72%), and half achieved clinical and histologic remission (95% CI, 42%-59%), the investigators reported. Therapy was somewhat more effective when administered twice a day instead of once daily, when clinicians used esophageal pH monitoring, and when studies were prospective instead of retrospective, but the differences were not significant. Nor did therapeutic response significantly differ based on the age of patients, type of report, or quality of the study.
The overall findings “should be interpreted with caution because of poor-quality evidence, heterogeneity, and publication bias,” the researchers said. Prospective studies are needed to examine the best PPI, dose, and dosing interval to use in an initial trial in the clinic; to clarify long-term effects and dosing strategies; to assess the ability of PPIs to reverse fibrotic esophageal remodeling; and to examine the effects of the CYP2C19 genotype on clinical and histologic response, they added. “More quality evidence on pediatric PPI-responsive eosinophilic esophagitis is needed urgently,” they emphasized.
The authors reported no funding sources and had no disclosures.
Proton pump inhibitor–responsive esophageal eosinophilia (PPI-REE) is a condition in which patients have symptoms of esophageal dysfunction (often dysphagia or heartburn), biopsies with at least 15 eosinophils per high-power field (eos/hpf), and symptomatic and histologic resolution after a PPI trial, typically at twice-daily dosing. Currently, PPI-REE and eosinophilic esophagitis (EoE) overlap substantially, but in the most recent guidelines, they are still considered to be distinct entities. PPI-REE was first reported almost 10 years ago, and since then multiple prospective and retrospective studies in both children and adults have further characterized it. The study by Dr. Lucendo and colleagues, a comprehensive and rigorously conducted systematic review and meta-analysis of 33 studies accounting for 619 patients, found that just over 50% of patients with esophageal eosinophilia had histologic remission (less than 15 eos/hpf) and just over 60% had symptomatic improvement after PPI use. Moreover, similar responses were seen whether or not there was pathologic acid exposure on pH testing.

|
| Dr. Evan S. Dellon |
While there was heterogeneity between studies on meta-analysis, there are several important messages from this study. First, PPI-REE is commonly seen in patients with esophageal eosinophilia, and is not always simply due to reflux. Second, PPIs have a potent antieosinophil effect in these patients. Interestingly, novel acid-independent mechanisms for this anti-inflammatory action recently have been described in other studies. Third, a PPI trial remains important prior to confirming the diagnosis of EoE, and PPIs should be considered the first-line treatment when esophageal eosinophilia is identified. However, it bears emphasizing that all esophageal eosinophilia is not due to EoE. If a patient responds to the PPI trial, there is no clear need to move toward topical steroid or dietary elimination therapy specifically for EoE, and starting multiple antieosinophil treatments concomitantly precludes determining which is most effective. In the future, understanding which patients with esophageal eosinophilia will most benefit from a PPI trial will be important, as we are currently unable to predict this from clinical, endoscopic, and histologic factors. Future studies and guidelines will also need to address whether EoE and PPI-REE are distinct diseases or manifestations of the same underlying process.
Dr. Evan S. Dellon, MPH, is associate professor of medicine and epidemiology at the Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine at Chapel Hill. He has received research funding from Meritage, Miraca, Receptos, and Regeneron and consulted for Aptalis, Banner, Novartis, Receptos, Regeneron, and Roche.
Proton pump inhibitor–responsive esophageal eosinophilia (PPI-REE) is a condition in which patients have symptoms of esophageal dysfunction (often dysphagia or heartburn), biopsies with at least 15 eosinophils per high-power field (eos/hpf), and symptomatic and histologic resolution after a PPI trial, typically at twice-daily dosing. Currently, PPI-REE and eosinophilic esophagitis (EoE) overlap substantially, but in the most recent guidelines, they are still considered to be distinct entities. PPI-REE was first reported almost 10 years ago, and since then multiple prospective and retrospective studies in both children and adults have further characterized it. The study by Dr. Lucendo and colleagues, a comprehensive and rigorously conducted systematic review and meta-analysis of 33 studies accounting for 619 patients, found that just over 50% of patients with esophageal eosinophilia had histologic remission (less than 15 eos/hpf) and just over 60% had symptomatic improvement after PPI use. Moreover, similar responses were seen whether or not there was pathologic acid exposure on pH testing.

|
| Dr. Evan S. Dellon |
While there was heterogeneity between studies on meta-analysis, there are several important messages from this study. First, PPI-REE is commonly seen in patients with esophageal eosinophilia, and is not always simply due to reflux. Second, PPIs have a potent antieosinophil effect in these patients. Interestingly, novel acid-independent mechanisms for this anti-inflammatory action recently have been described in other studies. Third, a PPI trial remains important prior to confirming the diagnosis of EoE, and PPIs should be considered the first-line treatment when esophageal eosinophilia is identified. However, it bears emphasizing that all esophageal eosinophilia is not due to EoE. If a patient responds to the PPI trial, there is no clear need to move toward topical steroid or dietary elimination therapy specifically for EoE, and starting multiple antieosinophil treatments concomitantly precludes determining which is most effective. In the future, understanding which patients with esophageal eosinophilia will most benefit from a PPI trial will be important, as we are currently unable to predict this from clinical, endoscopic, and histologic factors. Future studies and guidelines will also need to address whether EoE and PPI-REE are distinct diseases or manifestations of the same underlying process.
Dr. Evan S. Dellon, MPH, is associate professor of medicine and epidemiology at the Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine at Chapel Hill. He has received research funding from Meritage, Miraca, Receptos, and Regeneron and consulted for Aptalis, Banner, Novartis, Receptos, Regeneron, and Roche.
Proton pump inhibitor–responsive esophageal eosinophilia (PPI-REE) is a condition in which patients have symptoms of esophageal dysfunction (often dysphagia or heartburn), biopsies with at least 15 eosinophils per high-power field (eos/hpf), and symptomatic and histologic resolution after a PPI trial, typically at twice-daily dosing. Currently, PPI-REE and eosinophilic esophagitis (EoE) overlap substantially, but in the most recent guidelines, they are still considered to be distinct entities. PPI-REE was first reported almost 10 years ago, and since then multiple prospective and retrospective studies in both children and adults have further characterized it. The study by Dr. Lucendo and colleagues, a comprehensive and rigorously conducted systematic review and meta-analysis of 33 studies accounting for 619 patients, found that just over 50% of patients with esophageal eosinophilia had histologic remission (less than 15 eos/hpf) and just over 60% had symptomatic improvement after PPI use. Moreover, similar responses were seen whether or not there was pathologic acid exposure on pH testing.

|
| Dr. Evan S. Dellon |
While there was heterogeneity between studies on meta-analysis, there are several important messages from this study. First, PPI-REE is commonly seen in patients with esophageal eosinophilia, and is not always simply due to reflux. Second, PPIs have a potent antieosinophil effect in these patients. Interestingly, novel acid-independent mechanisms for this anti-inflammatory action recently have been described in other studies. Third, a PPI trial remains important prior to confirming the diagnosis of EoE, and PPIs should be considered the first-line treatment when esophageal eosinophilia is identified. However, it bears emphasizing that all esophageal eosinophilia is not due to EoE. If a patient responds to the PPI trial, there is no clear need to move toward topical steroid or dietary elimination therapy specifically for EoE, and starting multiple antieosinophil treatments concomitantly precludes determining which is most effective. In the future, understanding which patients with esophageal eosinophilia will most benefit from a PPI trial will be important, as we are currently unable to predict this from clinical, endoscopic, and histologic factors. Future studies and guidelines will also need to address whether EoE and PPI-REE are distinct diseases or manifestations of the same underlying process.
Dr. Evan S. Dellon, MPH, is associate professor of medicine and epidemiology at the Center for Esophageal Diseases and Swallowing, division of gastroenterology and hepatology, University of North Carolina School of Medicine at Chapel Hill. He has received research funding from Meritage, Miraca, Receptos, and Regeneron and consulted for Aptalis, Banner, Novartis, Receptos, Regeneron, and Roche.
About half of patients with symptomatic esophageal eosinophilia achieved complete clinical and histologic remission on proton pump inhibitors (PPIs), according to a systematic review and meta-analysis of 33 studies.
“Our results support the concept of PPIs as first-line therapy in both children and adults,” Dr. Alfredo Lucendo of the Servicio de Salud de Castillo–La Mancha, Hospital General de Tomelloso, Albacete, Spain, and his associates wrote in the January issue of Clinical Gastroenterology and Hepatology. “Other effective alternatives, such as dietary or topical steroid therapy, likely might be set aside as second-line treatment, owing to long-term safety concerns (topical steroid therapy) and impairment of quality of life and nutritional inadequacy (dietary interventions).”
The study also confirmed that esophageal pH monitoring does not accurately predict therapeutic response to PPI therapy. “The performance of this test before histologic reevaluation on PPI therapy should be discouraged,” according to the researchers (Clin Gastroenterol Hepatol. 2015. [doi:10.1016/j.cgh.2015.07.041]). Eosinophilic esophagitis was first described as a distinct disorder about 20 years ago, but only recently was understood to be the most common cause of chronic esophageal symptoms among children and young adults. Some cases are now known to respond to PPI therapy, but reported remission rates have varied depending on study design and patient population, the investigators said. In addition, no one had systematically reviewed studies of PPI-responsive esophageal eosinophilia for quality or to determine the optimal type of PPI, dose, or treatment duration.
Therefore, the investigators searched MEDLINE, EMBASE, SCOPUS, and abstracts from the annual meetings of the American Gastroenterological Association, the American College of Gastroenterology, and United European Gastroenterology, identifying 33 studies of 619 patients with symptomatic esophageal eosinophilia. Eleven of the studies were prospective, of which only two were randomized controlled trials. The researchers defined a histologic response as less than 15 eosinophils per high-powered frame after PPI therapy. “Missing data regarding PPI therapy were common and prevented us from drawing conclusions on the most effective PPI drug and doses,” they said. In addition, most studies lacked structured or objective survey tools or other measures of clinical improvement, making it impossible to rule out self-adapted coping strategies as a main cause of improvement over time.
With those caveats in mind, about 61% of patients in the pooled analysis had a clinical response to PPI therapy (95% confidence interval, 48%-72%), and half achieved clinical and histologic remission (95% CI, 42%-59%), the investigators reported. Therapy was somewhat more effective when administered twice a day instead of once daily, when clinicians used esophageal pH monitoring, and when studies were prospective instead of retrospective, but the differences were not significant. Nor did therapeutic response significantly differ based on the age of patients, type of report, or quality of the study.
The overall findings “should be interpreted with caution because of poor-quality evidence, heterogeneity, and publication bias,” the researchers said. Prospective studies are needed to examine the best PPI, dose, and dosing interval to use in an initial trial in the clinic; to clarify long-term effects and dosing strategies; to assess the ability of PPIs to reverse fibrotic esophageal remodeling; and to examine the effects of the CYP2C19 genotype on clinical and histologic response, they added. “More quality evidence on pediatric PPI-responsive eosinophilic esophagitis is needed urgently,” they emphasized.
The authors reported no funding sources and had no disclosures.
About half of patients with symptomatic esophageal eosinophilia achieved complete clinical and histologic remission on proton pump inhibitors (PPIs), according to a systematic review and meta-analysis of 33 studies.
“Our results support the concept of PPIs as first-line therapy in both children and adults,” Dr. Alfredo Lucendo of the Servicio de Salud de Castillo–La Mancha, Hospital General de Tomelloso, Albacete, Spain, and his associates wrote in the January issue of Clinical Gastroenterology and Hepatology. “Other effective alternatives, such as dietary or topical steroid therapy, likely might be set aside as second-line treatment, owing to long-term safety concerns (topical steroid therapy) and impairment of quality of life and nutritional inadequacy (dietary interventions).”
The study also confirmed that esophageal pH monitoring does not accurately predict therapeutic response to PPI therapy. “The performance of this test before histologic reevaluation on PPI therapy should be discouraged,” according to the researchers (Clin Gastroenterol Hepatol. 2015. [doi:10.1016/j.cgh.2015.07.041]). Eosinophilic esophagitis was first described as a distinct disorder about 20 years ago, but only recently was understood to be the most common cause of chronic esophageal symptoms among children and young adults. Some cases are now known to respond to PPI therapy, but reported remission rates have varied depending on study design and patient population, the investigators said. In addition, no one had systematically reviewed studies of PPI-responsive esophageal eosinophilia for quality or to determine the optimal type of PPI, dose, or treatment duration.
Therefore, the investigators searched MEDLINE, EMBASE, SCOPUS, and abstracts from the annual meetings of the American Gastroenterological Association, the American College of Gastroenterology, and United European Gastroenterology, identifying 33 studies of 619 patients with symptomatic esophageal eosinophilia. Eleven of the studies were prospective, of which only two were randomized controlled trials. The researchers defined a histologic response as less than 15 eosinophils per high-powered frame after PPI therapy. “Missing data regarding PPI therapy were common and prevented us from drawing conclusions on the most effective PPI drug and doses,” they said. In addition, most studies lacked structured or objective survey tools or other measures of clinical improvement, making it impossible to rule out self-adapted coping strategies as a main cause of improvement over time.
With those caveats in mind, about 61% of patients in the pooled analysis had a clinical response to PPI therapy (95% confidence interval, 48%-72%), and half achieved clinical and histologic remission (95% CI, 42%-59%), the investigators reported. Therapy was somewhat more effective when administered twice a day instead of once daily, when clinicians used esophageal pH monitoring, and when studies were prospective instead of retrospective, but the differences were not significant. Nor did therapeutic response significantly differ based on the age of patients, type of report, or quality of the study.
The overall findings “should be interpreted with caution because of poor-quality evidence, heterogeneity, and publication bias,” the researchers said. Prospective studies are needed to examine the best PPI, dose, and dosing interval to use in an initial trial in the clinic; to clarify long-term effects and dosing strategies; to assess the ability of PPIs to reverse fibrotic esophageal remodeling; and to examine the effects of the CYP2C19 genotype on clinical and histologic response, they added. “More quality evidence on pediatric PPI-responsive eosinophilic esophagitis is needed urgently,” they emphasized.
The authors reported no funding sources and had no disclosures.
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Proton pump inhibitors should be considered in the first-line treatment of esophageal eosinophilia.
Major finding: Half of patients achieved clinical and histologic remission after a trial of PPIs.
Data source: Meta-analysis of 33 studies of 619 patients with symptomatic esophageal eosinophilia indicative of eosinophilic esophagitis.
Disclosures: The authors reported no funding sources and had no disclosures.
Measure found significant variations in outpatient colonoscopy quality
Facilities that performed outpatient colonoscopies varied significantly in terms of subsequent unplanned hospital visits, even after patient-level risk factors were adjusted for, according to a Medicare claims analysis reported in the January issue of Gastroenterology.
This first-in-kind quality measure will help “make transparent the full range of adverse events requiring hospital care that patients experience post-[colonoscopy], inform patient choice, create incentives for improved care, and assist providers’ quality improvement efforts, wrote Dr. Isuru Ranasinghe of Yale–New Haven (Conn.) Hospital and his associates. Preventing unplanned hospitals visits after colonoscopy could also help improve patient outcomes and cut health care costs, they added.
More than 90% of colonoscopies occur in outpatient settings, where it is difficult to follow-up and track serious adverse events such as colonic perforation or gastrointestinal bleeding. Furthermore, there are no publicly available quality reports of providers or facilities that perform outpatient colonoscopies. To help fill that gap, the researchers tracked unplanned hospital visits within 7 days after colonoscopy as part of an outpatient quality initiative by the Centers for Medicare & Medicaid Services. The analysis included 20% of Medicare outpatient colonoscopy claims in 2010, which amounted to 331,880 colonoscopies performed at 8,140 facilities (Gastroenterology 2015 [doi: 10.1053/j.gastro.2015.09.009]).
In 2010, there were 16.3 unplanned hospital visits for every 1,000 Medicare claims for outpatient colonoscopies, which equated to 27,000 such visits nationwide, the investigators said. Patients who were older than 85 years were significantly more likely to have an unplanned hospital visit than were patients aged 65-69 years (odds ratio, 1.87; 95% confidence interval, 1.54-2.28). The other most significant predictors included having an electrolyte or acid/base imbalance (OR, 1.43) or a psychiatric disorder (OR, 1.34). After adjusting for these factors, unplanned admission rates varied significantly among facilities in all four states with available data, including New York, California, Florida, and Nebraska, the researchers said.
These findings might not apply to patients who are too young to be enrolled in Medicare, which “is important, as most colonoscopies are performed among patients aged less than 65 years, although older patients are more likely to suffer adverse events,” Dr. Ranasinghe and his associates noted. The study also did not assess deaths, the one-third of GI bleeding events that occur more than a week after colonoscopy, or the various factors that might affect quality of care, such as type of anesthesia, polypectomy technique, and access to follow-up care. “The goal of the measure is to reveal the opportunity for improvement, and to prompt facilities to examine their practices in depth to identify ways to lower hospital visit rates to the level achieved by the best providers,” the researchers emphasized.
The work was supported by the Centers for Medicare & Medicaid Services, the National Health and Medical Research Council, the National Heart Foundation of Australia, the National Institute on Aging, the American Federation for Aging Research. Dr. Ranasinghe and nine coinvestigators reported working under contract with CMS to develop and maintain performance measures. Three coinvestigators reported financial relationships with AbbVie, qMed, Pentax, Olympus, Myriad Genetics, UnitedHealth, Medtronic, and Johnson & Johnson.
Source: American Gastroenterological Association
As part of the Centers for Medicare & Medicaid Services initiative to develop outpatient quality measures, Dr. Ranasinghe and his colleagues designed a risk-adjusted measure to capture serious adverse events characterized by unplanned hospitalization within 7 days after colonoscopy.
While no measure is perfect, this measure captures many important adverse outcomes after colonoscopy. Their methods carefully excluded hospitalizations that were elective or could be attributable to other conditions. This is an important quality measure because the endoscopist is often unaware of hospitalizations that occur post colonoscopy (Arch Intern Med. 2010;170:1752-7), and therefore cannot target quality improvement (QI) efforts at preventing such unplanned outcomes.

|
| Dr. Jeremy Adler |
This measure can be calculated from available data without chart audit and will fulfill CMS reporting requirements. However, it will be important to keep in mind that it was developed for patients 65 years and older, and may not be applicable to younger patients. Furthermore, to make this measure meaningful, it should be used to improve quality, not just assess quality.
The authors demonstrated that they were able to identify practices that were outliers, even when risk adjusted and adjusted for practice volume. This could allow practices to become self-aware and provide benchmarks against which to compare and improve their performance.
However, one major limitation to using this measure for QI is that the Healthcare Cost and Utilization Project only provides updated data yearly, after a substantial time lag, making it unsuitable for short-term or rapid-cycle QI efforts. In order for individual practices to target their improvement efforts, they would need to review their own local postcolonoscopy hospitalization data as an outcome measure more frequently. They could then review each case in depth to learn details that could further help target improvement efforts.
Dr. Jeremy Adler, M.Sc. is assistant professor, division of pediatric gastroenterology and Child Health Evaluation and Research Unit, University of Michigan, Ann Arbor. He has no conflicts of interest.
As part of the Centers for Medicare & Medicaid Services initiative to develop outpatient quality measures, Dr. Ranasinghe and his colleagues designed a risk-adjusted measure to capture serious adverse events characterized by unplanned hospitalization within 7 days after colonoscopy.
While no measure is perfect, this measure captures many important adverse outcomes after colonoscopy. Their methods carefully excluded hospitalizations that were elective or could be attributable to other conditions. This is an important quality measure because the endoscopist is often unaware of hospitalizations that occur post colonoscopy (Arch Intern Med. 2010;170:1752-7), and therefore cannot target quality improvement (QI) efforts at preventing such unplanned outcomes.

|
| Dr. Jeremy Adler |
This measure can be calculated from available data without chart audit and will fulfill CMS reporting requirements. However, it will be important to keep in mind that it was developed for patients 65 years and older, and may not be applicable to younger patients. Furthermore, to make this measure meaningful, it should be used to improve quality, not just assess quality.
The authors demonstrated that they were able to identify practices that were outliers, even when risk adjusted and adjusted for practice volume. This could allow practices to become self-aware and provide benchmarks against which to compare and improve their performance.
However, one major limitation to using this measure for QI is that the Healthcare Cost and Utilization Project only provides updated data yearly, after a substantial time lag, making it unsuitable for short-term or rapid-cycle QI efforts. In order for individual practices to target their improvement efforts, they would need to review their own local postcolonoscopy hospitalization data as an outcome measure more frequently. They could then review each case in depth to learn details that could further help target improvement efforts.
Dr. Jeremy Adler, M.Sc. is assistant professor, division of pediatric gastroenterology and Child Health Evaluation and Research Unit, University of Michigan, Ann Arbor. He has no conflicts of interest.
As part of the Centers for Medicare & Medicaid Services initiative to develop outpatient quality measures, Dr. Ranasinghe and his colleagues designed a risk-adjusted measure to capture serious adverse events characterized by unplanned hospitalization within 7 days after colonoscopy.
While no measure is perfect, this measure captures many important adverse outcomes after colonoscopy. Their methods carefully excluded hospitalizations that were elective or could be attributable to other conditions. This is an important quality measure because the endoscopist is often unaware of hospitalizations that occur post colonoscopy (Arch Intern Med. 2010;170:1752-7), and therefore cannot target quality improvement (QI) efforts at preventing such unplanned outcomes.

|
| Dr. Jeremy Adler |
This measure can be calculated from available data without chart audit and will fulfill CMS reporting requirements. However, it will be important to keep in mind that it was developed for patients 65 years and older, and may not be applicable to younger patients. Furthermore, to make this measure meaningful, it should be used to improve quality, not just assess quality.
The authors demonstrated that they were able to identify practices that were outliers, even when risk adjusted and adjusted for practice volume. This could allow practices to become self-aware and provide benchmarks against which to compare and improve their performance.
However, one major limitation to using this measure for QI is that the Healthcare Cost and Utilization Project only provides updated data yearly, after a substantial time lag, making it unsuitable for short-term or rapid-cycle QI efforts. In order for individual practices to target their improvement efforts, they would need to review their own local postcolonoscopy hospitalization data as an outcome measure more frequently. They could then review each case in depth to learn details that could further help target improvement efforts.
Dr. Jeremy Adler, M.Sc. is assistant professor, division of pediatric gastroenterology and Child Health Evaluation and Research Unit, University of Michigan, Ann Arbor. He has no conflicts of interest.
Facilities that performed outpatient colonoscopies varied significantly in terms of subsequent unplanned hospital visits, even after patient-level risk factors were adjusted for, according to a Medicare claims analysis reported in the January issue of Gastroenterology.
This first-in-kind quality measure will help “make transparent the full range of adverse events requiring hospital care that patients experience post-[colonoscopy], inform patient choice, create incentives for improved care, and assist providers’ quality improvement efforts, wrote Dr. Isuru Ranasinghe of Yale–New Haven (Conn.) Hospital and his associates. Preventing unplanned hospitals visits after colonoscopy could also help improve patient outcomes and cut health care costs, they added.
More than 90% of colonoscopies occur in outpatient settings, where it is difficult to follow-up and track serious adverse events such as colonic perforation or gastrointestinal bleeding. Furthermore, there are no publicly available quality reports of providers or facilities that perform outpatient colonoscopies. To help fill that gap, the researchers tracked unplanned hospital visits within 7 days after colonoscopy as part of an outpatient quality initiative by the Centers for Medicare & Medicaid Services. The analysis included 20% of Medicare outpatient colonoscopy claims in 2010, which amounted to 331,880 colonoscopies performed at 8,140 facilities (Gastroenterology 2015 [doi: 10.1053/j.gastro.2015.09.009]).
In 2010, there were 16.3 unplanned hospital visits for every 1,000 Medicare claims for outpatient colonoscopies, which equated to 27,000 such visits nationwide, the investigators said. Patients who were older than 85 years were significantly more likely to have an unplanned hospital visit than were patients aged 65-69 years (odds ratio, 1.87; 95% confidence interval, 1.54-2.28). The other most significant predictors included having an electrolyte or acid/base imbalance (OR, 1.43) or a psychiatric disorder (OR, 1.34). After adjusting for these factors, unplanned admission rates varied significantly among facilities in all four states with available data, including New York, California, Florida, and Nebraska, the researchers said.
These findings might not apply to patients who are too young to be enrolled in Medicare, which “is important, as most colonoscopies are performed among patients aged less than 65 years, although older patients are more likely to suffer adverse events,” Dr. Ranasinghe and his associates noted. The study also did not assess deaths, the one-third of GI bleeding events that occur more than a week after colonoscopy, or the various factors that might affect quality of care, such as type of anesthesia, polypectomy technique, and access to follow-up care. “The goal of the measure is to reveal the opportunity for improvement, and to prompt facilities to examine their practices in depth to identify ways to lower hospital visit rates to the level achieved by the best providers,” the researchers emphasized.
The work was supported by the Centers for Medicare & Medicaid Services, the National Health and Medical Research Council, the National Heart Foundation of Australia, the National Institute on Aging, the American Federation for Aging Research. Dr. Ranasinghe and nine coinvestigators reported working under contract with CMS to develop and maintain performance measures. Three coinvestigators reported financial relationships with AbbVie, qMed, Pentax, Olympus, Myriad Genetics, UnitedHealth, Medtronic, and Johnson & Johnson.
Source: American Gastroenterological Association
Facilities that performed outpatient colonoscopies varied significantly in terms of subsequent unplanned hospital visits, even after patient-level risk factors were adjusted for, according to a Medicare claims analysis reported in the January issue of Gastroenterology.
This first-in-kind quality measure will help “make transparent the full range of adverse events requiring hospital care that patients experience post-[colonoscopy], inform patient choice, create incentives for improved care, and assist providers’ quality improvement efforts, wrote Dr. Isuru Ranasinghe of Yale–New Haven (Conn.) Hospital and his associates. Preventing unplanned hospitals visits after colonoscopy could also help improve patient outcomes and cut health care costs, they added.
More than 90% of colonoscopies occur in outpatient settings, where it is difficult to follow-up and track serious adverse events such as colonic perforation or gastrointestinal bleeding. Furthermore, there are no publicly available quality reports of providers or facilities that perform outpatient colonoscopies. To help fill that gap, the researchers tracked unplanned hospital visits within 7 days after colonoscopy as part of an outpatient quality initiative by the Centers for Medicare & Medicaid Services. The analysis included 20% of Medicare outpatient colonoscopy claims in 2010, which amounted to 331,880 colonoscopies performed at 8,140 facilities (Gastroenterology 2015 [doi: 10.1053/j.gastro.2015.09.009]).
In 2010, there were 16.3 unplanned hospital visits for every 1,000 Medicare claims for outpatient colonoscopies, which equated to 27,000 such visits nationwide, the investigators said. Patients who were older than 85 years were significantly more likely to have an unplanned hospital visit than were patients aged 65-69 years (odds ratio, 1.87; 95% confidence interval, 1.54-2.28). The other most significant predictors included having an electrolyte or acid/base imbalance (OR, 1.43) or a psychiatric disorder (OR, 1.34). After adjusting for these factors, unplanned admission rates varied significantly among facilities in all four states with available data, including New York, California, Florida, and Nebraska, the researchers said.
These findings might not apply to patients who are too young to be enrolled in Medicare, which “is important, as most colonoscopies are performed among patients aged less than 65 years, although older patients are more likely to suffer adverse events,” Dr. Ranasinghe and his associates noted. The study also did not assess deaths, the one-third of GI bleeding events that occur more than a week after colonoscopy, or the various factors that might affect quality of care, such as type of anesthesia, polypectomy technique, and access to follow-up care. “The goal of the measure is to reveal the opportunity for improvement, and to prompt facilities to examine their practices in depth to identify ways to lower hospital visit rates to the level achieved by the best providers,” the researchers emphasized.
The work was supported by the Centers for Medicare & Medicaid Services, the National Health and Medical Research Council, the National Heart Foundation of Australia, the National Institute on Aging, the American Federation for Aging Research. Dr. Ranasinghe and nine coinvestigators reported working under contract with CMS to develop and maintain performance measures. Three coinvestigators reported financial relationships with AbbVie, qMed, Pentax, Olympus, Myriad Genetics, UnitedHealth, Medtronic, and Johnson & Johnson.
Source: American Gastroenterological Association
FROM GASTROENTEROLOGY
Key clinical point: Researchers validated a new measure to evaluate the quality of outpatient colonoscopies.
Major finding: Risk-adjusted rates of these visits varied significantly among health care facilities in all four states with available data.
Data source: An analysis of 20% of Medicare outpatient colonoscopy claims in 2010, which included 331,880 procedures performed at 8,140 facilities.
Disclosures: The work was supported by the Centers for Medicare & Medicaid Services, the National Health and Medical Research Council, the National Heart Foundation of Australia, the National Institute on Aging, and the American Federation for Aging Research. Dr. Ranasinghe and nine coinvestigators reported working under contract with CMS to develop and maintain performance measures. Three coinvestigators reported financial relationships with AbbVie, gMed, Pentax, Olympus, Myriad Genetics, UnitedHealth, Medtronic, and Johnson & Johnson.