User login
Prevalence and Postdischarge Outcomes Associated with Frailty in Medical Inpatients: Impact of Different Frailty Definitions
Frailty is associated with adverse outcomes in hospitalized patients, including longer length of stay, increased risk of institutionalization at discharge, and higher rates of readmissions or death postdischarge.1-4 Multiple tools have been developed to evaluate frailty and in an earlier study,4 we compared the three most common of these and demonstrated that the Clinical Frailty Scale (CFS)5 was the most useful tool clinically as it was most strongly associated with adverse events in the first 30 days after discharge. However, it must be collected prospectively and requires contact with patients or proxies for the evaluator to assign the patient into one of nine categories depending on their disease state, mobility, cognition, and ability to perform instrumental and functional activities of daily living. Recently, a new score has been described which is based on an administrative data algorithm that assigns points to patients having any of 109 ICD-10 codes listed for their index hospitalization and all hospitalizations in the prior two years and can be generated retrospectively without trained observers.6 Although higher Hospital Frailty Risk Scores (HFRS) were associated with greater risk of postdischarge adverse events, the kappa when compared with the CFS was only 0.30 (95% CI 0.22-0.38) in that study.6 However, as the HFRS was developed and validated in patients aged ≥75 years within the UK National Health Service, the authors themselves recommended that it be evaluated in other healthcare systems, other populations, and with comparison to prospectively collected frailty data from cumulative deficit models such as the CFS.
The aim of this study was to compare frailty assessments using the CFS and the HFRS in a population of adult patients hospitalized on general medical wards in North America to determine the impact on prevalence estimates and prediction of outcomes within the first 30 days after hospital discharge (a timeframe highlighted in the Affordable Care Act and used by Centers for Medicare & Medicaid Services as an important hospital quality indicator).
METHODS
As described previously,7 we performed a prospective cohort study of adults without cognitive impairment or life expectancy less than three months being discharged back to the community (not to long-term care facilities) from general medical wards in two teaching hospitals in Edmonton, Alberta, between October 2013 and November 2014. All patients provided signed consent, and the University of Alberta Health Research Ethics board (project ID Pro00036880) approved the study.
Trained observers assessed each patient’s frailty status within 24 hours of discharge based on the patient’s best status in the week prior to becoming ill with the reason for the index hospitalization. The research assistant classified patients into one of the following nine CFS categories: very fit, well, managing well, vulnerable, mildly frail (need help with at least one instrumental activities of daily living such as shopping, finances, meal preparation, or housework), moderately frail (need help with one or two activities of daily living such as bathing and dressing), severely frail (dependent for personal care), very severely frail (bedbound), and terminally ill. According to the CFS validation studies, the last five categories were defined as frail for the purposes of our analyses.
Independent of the trained observer’s assessments, we calculated the HFRS for each participant in our cohort by linking to Alberta administrative data holdings within the Alberta Health Services Data Integration and Measurement Reporting unit and examining all diagnostic codes for the index hospitalization and any other hospitalizations in the prior two years for the 109 ICD-10 codes listed in the original HFRS paper and used the same score cutpoints as they reported (HFRS <5 being low risk, 5-15 defined as intermediate risk, and >15 as high risk for frailty; scores ≥5 were defined as frail).6
All patients were followed after discharge by research personnel blinded to the patient’s frailty assessment. We used patient/caregiver self-report and the provincial electronic health record to collect information on all-cause readmissions or mortality within 30 days.
We have previously reported4,7 the association between frailty defined by the CFS and unplanned readmissions or death within 30 days of discharge but in this study, we examined the correlation between CFS-defined frailty and the HFRS score (classifying those with intermediate or high scores as frail) using chance-corrected kappa coefficients. We also compared the prognostic accuracy of both models for predicting death and/or unplanned readmissions within 30 days using the C statistic and the integrated discrimination improvement index and examined patients aged >65 years as a subgroup.8 We used SAS version 9.4 (SAS Institute, Cary, North Carolina) for analyses, with P values of <.05 considered as statistically significant.
RESULTS
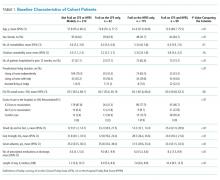
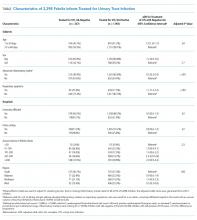
Of the 499 patients in our original cohort,7 we could not link 10 to the administrative data to calculate HFRS, and thus this study sample is only 489 patients (mean age 64 years, 50% women, 52% older than 65 years, a mean of 4.9 comorbidities, and median length of stay five days).
Overall, 276 (56%) patients were deemed frail according to at least one assessment (214 [44%] on the HFRS [35% intermediate risk and 9% high risk] and 161 [33%] on the CFS), and 99 (20%) met both frailty definitions (Appendix Figure). Among the 252 patients aged >65 years, 66 (26%) met both frailty definitions and 166 (66%) were frail according to at least one assessment. Agreement between HFRS and the CFS (kappa 0.24, 95% CI 0.16-0.33) was poor. The CFS definition of frailty was 46% sensitive and 77% specific in classifying frail patients compared with HFRS-defined frailty.
As we reported earlier,4 patients deemed frail were generally similar across scales in that they were older, had more comorbidities, more prescriptions, longer lengths of stay, and poorer quality of life than nonfrail patients (all P < .01, Table 1). However, patients classified as frail on the HFRS only but not meeting the CFS definition were younger, had higher quality of life, and despite a similar Charlson Score and number of comorbidities were much more likely to have been living independently prior to admission than those classified as frail on the CFS.
Death or unplanned readmission within 30 days occurred in 13.3% (65 patients), with most events being readmissions (62, 12.7%). HFRS-defined frail patients exhibited higher 30-day death/readmission rates (16% vs 11% for not frail, P = .08; 14% vs 11% in the elderly, P = .5), which was not statistically significantly different from the nonfrail patients even after adjusting for age and sex (aOR [adjusted odds ratio] 1.62, 95% CI 0.95-2.75 for all adults; aOR 1.24, 95% CI 0.58-2.63 for the elderly). CFS-defined frail patients had significantly higher 30-day readmission/death rates (19% vs 10% for not frail, aOR 2.53, 95% CI 1.40-4.57 for all adults and 21% vs 6% in the elderly, aOR 4.31, 95% CI 1.80-10.31).
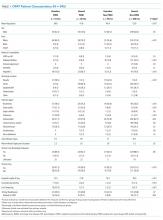
Adding the HFRS results to the CFS-based predictive models added little new information, with an integrated discrimination improvement of only 0.009 that was not statistically significant (P = .09, Table 2). In fact, the HFRS was not an independent predictor of postdischarge outcomes after adjusting for age and sex. Although predictive models incorporating the CFS demonstrated the best C statistics, none of the models had high C statistics (ranging between 0.54 and 0.64 for all adults and between 0.55 and 0.68 for those aged >65 years). Even when the frailty definitions were examined as continuous variables, the C statistics were similar as for the dichotomized analyses (0.64 for CFS and 0.58 for HFRS) and the correlation between the two remained weak (Spearman’s correlation coefficient 0.34).
DISCUSSION
We have demonstrated that the prevalence of frailty in patients being discharged from medical wards was high, with the HFRS (44%) being higher than the CFS (33%), and that only 46% of patients deemed frail on the HFRS were also deemed frail on the CFS. We confirm the report by the developers of the HFRS that there was poor correlation between the CFS cumulative deficit model and the administrative-data-based HFRS model in our cohort, even among those older than 65 years.
Previous studies have reported marked heterogeneity in prevalence estimates between different frailty instruments.2,9 For example, Aguayo et al. found that the prevalence of frailty in the English Longitudinal Study of Aging varied between 0.9% and 68% depending on which of the 35 frailty scales they tested were used, although the prevalence with comprehensive geriatric assessments (the gold standard) was 14.9% (and 15.3% on the CFS).9 Although frail patients are at higher risk for death and/or readmission after discharge, other investigators have also reported similar findings to ours that frailty-based risk models are surprisingly modest at predicting postdischarge readmission or death, with the C statistics ranging between 0.52 and 0.57, although the CFS appears to correlate best with the gold standard of comprehensive geriatric assessment.10-14 This is not surprising since the CFS is multidimensional and as a cumulative deficit model, it incorporates assessment of the patient’s underlying diseases, cognition, function, mobility, and mood in the assignment of their CFS level. Regardless, others15 have pointed out the need for studies such as ours to compare the validity of published frailty scales.
Despite our prospective cohort design and blinded endpoint ascertainment, there are some potential limitations to our study. First, we excluded long-term care residents and patients with foreshortened life expectancy – the frailest of the frail – from our analysis of 30-day outcomes, thereby potentially reducing the magnitude of the association between frailty and adverse outcomes. However, we were interested only in situations where clinicians were faced with equipoise about patient prognosis. Second, we assessed only 30-day readmissions or deaths and cannot comment on the impact of frailty definitions on other postdischarge outcomes (such as discharge locale or need for home care services) or other timeframes. Finally, although the association between the HFRS definition of frailty and the 30-day mortality/readmission was not statistically significant, the 95% confidence intervals were wide and thus we cannot definitively rule out a positive association.
In conclusion, considering that it had the strongest association with postdischarge outcomes and is the fastest and easiest to perform, the most useful of the frailty assessment tools for clinicians at the bedside still appears to be the CFS (both overall and in those patients who are elderly). However, for researchers who are analyzing data retrospectively or policy planners looking at health services data where the CFS was not collected, the HFRS holds promise for risk adjustment in population-level studies comparing processes and outcomes between hospitals.
Acknowledgments
The authors would like to acknowledge Miriam Fradette, Debbie Boyko, Sara Belga, Darren Lau, Jenelle Pederson, and Sharry Kahlon for their important contributions in data acquisition in our original cohort study, as well as all the physicians rotating through the general internal medicine wards at the University of Alberta Hospital for their help in identifying the patients. We also thank Dr. Simon Conroy, MB ChB PhD, University of Leicester, UK, for his helpful comments on an earlier draft of this manuscript.
Disclosures
The authors declare no conflicts of interest. All authors had access to the data and played a role in writing and revising this manuscript.
Funding
Funding for this study was provided by an operating grant from Alberta Innovates - Health Solutions. F.A.M. holds the Chair in Cardiovascular Outcomes Research at the Mazankowski Heart Institute, University of Alberta. The authors have no affiliations or financial interests with any organization or entity with a financial interest in the contents of this manuscript.
1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752-762. doi: 10.1016/S0140-6736(12)62167-9. PubMed
2. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487-1492. doi: 10.1111/j.1532-5415.2012.04054.x. PubMed
3. de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104-114. doi: 10.1016/j.arr.2010.09.001. PubMed
4. Belga S, Majumdar SR, Kahlon S, et al. Comparing three different measures of frailty in medical inpatients: multicenter prospective cohort study examining 30-day risk of readmission or death. J Hosp Med. 2016;11(8):556-562. doi: 10.1002/jhm.2607. PubMed
5. Rockwood K, Andrew M, Mintnitski A. A comparison of two approaches to measuring frailty in elerly people. J Gerontol. 2007;62(7):738-743. doi: 10.1093/gerona/62.7.738. PubMed
6. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8Get. PubMed
7. Kahlon S, Pederson J, Majumdar SR, et al. Association between frailty and 30-day outcomes after discharge from hospital. CMAJ. 2015;187(11):799-804. doi: 10.1503/cmaj.150100. PubMed
8. Pencina MJ, D’ Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the roc curve to reclassification and beyond. Stat Med. 2008;27(2):157-172. doi: 10.1002/sim.2929.
9. Aguayo GA, Donneau A-F, Vaillant MT, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186(4):420-434. doi: 10.1093/aje/kwx061. PubMed
10. Ritt M, Bollheimer LC, Siever CC, Gaßmann KG. Prediction of one-year mortality by five different frailty instruments: a comparative study in hospitalized geriatric patients. Arch Gerontol Geriatr. 2016;66:66-72. doi: 10.1016/j.archger.2016.05.004. PubMed
11. Forti P, Rietti E, Pisacane N, Olivelli V, Maltoni B, Ravaglia G. A comparison of frailty indexes for prediction of adverse health outcomes in a elderly cohort. Arch Gerontol Geriatr. 2012;54(1):16-20. doi: 10.1016/j.archger.2011.01.007. PubMed
12. Wou F, Gladman JR, Bradshaw L, Franklin M, Edmans J, Conroy SP. The predictive properties of frailty-rating scales in the acute medical unit. Age Ageing. 2013;42(6):776-781. doi: 10.1093/ageing/aft055. PubMed
13. Wallis SJ, Wall J, Biram RW, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108(12):943-949. doi: 10.1093/qjmed/hcv066. PubMed
14. Harmand MGC, Meillon C, Bergua V, et al. Comparing the predictive value of three definitions of frailty: results from the Three-City Study. Arch Gerontol Geriatr. 2017;72:153-163. doi: 10.1016/j.archger.2017.06.005. PubMed
15. Bouillon K, Kivimaki M, Hamer M, et al. Measures of frailty in population-based studies: an overview. BMC Geriatrics. 2013;13(1):64. doi: 10.1186/1471-2318-13-64. PubMed
Frailty is associated with adverse outcomes in hospitalized patients, including longer length of stay, increased risk of institutionalization at discharge, and higher rates of readmissions or death postdischarge.1-4 Multiple tools have been developed to evaluate frailty and in an earlier study,4 we compared the three most common of these and demonstrated that the Clinical Frailty Scale (CFS)5 was the most useful tool clinically as it was most strongly associated with adverse events in the first 30 days after discharge. However, it must be collected prospectively and requires contact with patients or proxies for the evaluator to assign the patient into one of nine categories depending on their disease state, mobility, cognition, and ability to perform instrumental and functional activities of daily living. Recently, a new score has been described which is based on an administrative data algorithm that assigns points to patients having any of 109 ICD-10 codes listed for their index hospitalization and all hospitalizations in the prior two years and can be generated retrospectively without trained observers.6 Although higher Hospital Frailty Risk Scores (HFRS) were associated with greater risk of postdischarge adverse events, the kappa when compared with the CFS was only 0.30 (95% CI 0.22-0.38) in that study.6 However, as the HFRS was developed and validated in patients aged ≥75 years within the UK National Health Service, the authors themselves recommended that it be evaluated in other healthcare systems, other populations, and with comparison to prospectively collected frailty data from cumulative deficit models such as the CFS.
The aim of this study was to compare frailty assessments using the CFS and the HFRS in a population of adult patients hospitalized on general medical wards in North America to determine the impact on prevalence estimates and prediction of outcomes within the first 30 days after hospital discharge (a timeframe highlighted in the Affordable Care Act and used by Centers for Medicare & Medicaid Services as an important hospital quality indicator).
METHODS
As described previously,7 we performed a prospective cohort study of adults without cognitive impairment or life expectancy less than three months being discharged back to the community (not to long-term care facilities) from general medical wards in two teaching hospitals in Edmonton, Alberta, between October 2013 and November 2014. All patients provided signed consent, and the University of Alberta Health Research Ethics board (project ID Pro00036880) approved the study.
Trained observers assessed each patient’s frailty status within 24 hours of discharge based on the patient’s best status in the week prior to becoming ill with the reason for the index hospitalization. The research assistant classified patients into one of the following nine CFS categories: very fit, well, managing well, vulnerable, mildly frail (need help with at least one instrumental activities of daily living such as shopping, finances, meal preparation, or housework), moderately frail (need help with one or two activities of daily living such as bathing and dressing), severely frail (dependent for personal care), very severely frail (bedbound), and terminally ill. According to the CFS validation studies, the last five categories were defined as frail for the purposes of our analyses.
Independent of the trained observer’s assessments, we calculated the HFRS for each participant in our cohort by linking to Alberta administrative data holdings within the Alberta Health Services Data Integration and Measurement Reporting unit and examining all diagnostic codes for the index hospitalization and any other hospitalizations in the prior two years for the 109 ICD-10 codes listed in the original HFRS paper and used the same score cutpoints as they reported (HFRS <5 being low risk, 5-15 defined as intermediate risk, and >15 as high risk for frailty; scores ≥5 were defined as frail).6
All patients were followed after discharge by research personnel blinded to the patient’s frailty assessment. We used patient/caregiver self-report and the provincial electronic health record to collect information on all-cause readmissions or mortality within 30 days.
We have previously reported4,7 the association between frailty defined by the CFS and unplanned readmissions or death within 30 days of discharge but in this study, we examined the correlation between CFS-defined frailty and the HFRS score (classifying those with intermediate or high scores as frail) using chance-corrected kappa coefficients. We also compared the prognostic accuracy of both models for predicting death and/or unplanned readmissions within 30 days using the C statistic and the integrated discrimination improvement index and examined patients aged >65 years as a subgroup.8 We used SAS version 9.4 (SAS Institute, Cary, North Carolina) for analyses, with P values of <.05 considered as statistically significant.
RESULTS
Of the 499 patients in our original cohort,7 we could not link 10 to the administrative data to calculate HFRS, and thus this study sample is only 489 patients (mean age 64 years, 50% women, 52% older than 65 years, a mean of 4.9 comorbidities, and median length of stay five days).
Overall, 276 (56%) patients were deemed frail according to at least one assessment (214 [44%] on the HFRS [35% intermediate risk and 9% high risk] and 161 [33%] on the CFS), and 99 (20%) met both frailty definitions (Appendix Figure). Among the 252 patients aged >65 years, 66 (26%) met both frailty definitions and 166 (66%) were frail according to at least one assessment. Agreement between HFRS and the CFS (kappa 0.24, 95% CI 0.16-0.33) was poor. The CFS definition of frailty was 46% sensitive and 77% specific in classifying frail patients compared with HFRS-defined frailty.
As we reported earlier,4 patients deemed frail were generally similar across scales in that they were older, had more comorbidities, more prescriptions, longer lengths of stay, and poorer quality of life than nonfrail patients (all P < .01, Table 1). However, patients classified as frail on the HFRS only but not meeting the CFS definition were younger, had higher quality of life, and despite a similar Charlson Score and number of comorbidities were much more likely to have been living independently prior to admission than those classified as frail on the CFS.
Death or unplanned readmission within 30 days occurred in 13.3% (65 patients), with most events being readmissions (62, 12.7%). HFRS-defined frail patients exhibited higher 30-day death/readmission rates (16% vs 11% for not frail, P = .08; 14% vs 11% in the elderly, P = .5), which was not statistically significantly different from the nonfrail patients even after adjusting for age and sex (aOR [adjusted odds ratio] 1.62, 95% CI 0.95-2.75 for all adults; aOR 1.24, 95% CI 0.58-2.63 for the elderly). CFS-defined frail patients had significantly higher 30-day readmission/death rates (19% vs 10% for not frail, aOR 2.53, 95% CI 1.40-4.57 for all adults and 21% vs 6% in the elderly, aOR 4.31, 95% CI 1.80-10.31).
Adding the HFRS results to the CFS-based predictive models added little new information, with an integrated discrimination improvement of only 0.009 that was not statistically significant (P = .09, Table 2). In fact, the HFRS was not an independent predictor of postdischarge outcomes after adjusting for age and sex. Although predictive models incorporating the CFS demonstrated the best C statistics, none of the models had high C statistics (ranging between 0.54 and 0.64 for all adults and between 0.55 and 0.68 for those aged >65 years). Even when the frailty definitions were examined as continuous variables, the C statistics were similar as for the dichotomized analyses (0.64 for CFS and 0.58 for HFRS) and the correlation between the two remained weak (Spearman’s correlation coefficient 0.34).
DISCUSSION
We have demonstrated that the prevalence of frailty in patients being discharged from medical wards was high, with the HFRS (44%) being higher than the CFS (33%), and that only 46% of patients deemed frail on the HFRS were also deemed frail on the CFS. We confirm the report by the developers of the HFRS that there was poor correlation between the CFS cumulative deficit model and the administrative-data-based HFRS model in our cohort, even among those older than 65 years.
Previous studies have reported marked heterogeneity in prevalence estimates between different frailty instruments.2,9 For example, Aguayo et al. found that the prevalence of frailty in the English Longitudinal Study of Aging varied between 0.9% and 68% depending on which of the 35 frailty scales they tested were used, although the prevalence with comprehensive geriatric assessments (the gold standard) was 14.9% (and 15.3% on the CFS).9 Although frail patients are at higher risk for death and/or readmission after discharge, other investigators have also reported similar findings to ours that frailty-based risk models are surprisingly modest at predicting postdischarge readmission or death, with the C statistics ranging between 0.52 and 0.57, although the CFS appears to correlate best with the gold standard of comprehensive geriatric assessment.10-14 This is not surprising since the CFS is multidimensional and as a cumulative deficit model, it incorporates assessment of the patient’s underlying diseases, cognition, function, mobility, and mood in the assignment of their CFS level. Regardless, others15 have pointed out the need for studies such as ours to compare the validity of published frailty scales.
Despite our prospective cohort design and blinded endpoint ascertainment, there are some potential limitations to our study. First, we excluded long-term care residents and patients with foreshortened life expectancy – the frailest of the frail – from our analysis of 30-day outcomes, thereby potentially reducing the magnitude of the association between frailty and adverse outcomes. However, we were interested only in situations where clinicians were faced with equipoise about patient prognosis. Second, we assessed only 30-day readmissions or deaths and cannot comment on the impact of frailty definitions on other postdischarge outcomes (such as discharge locale or need for home care services) or other timeframes. Finally, although the association between the HFRS definition of frailty and the 30-day mortality/readmission was not statistically significant, the 95% confidence intervals were wide and thus we cannot definitively rule out a positive association.
In conclusion, considering that it had the strongest association with postdischarge outcomes and is the fastest and easiest to perform, the most useful of the frailty assessment tools for clinicians at the bedside still appears to be the CFS (both overall and in those patients who are elderly). However, for researchers who are analyzing data retrospectively or policy planners looking at health services data where the CFS was not collected, the HFRS holds promise for risk adjustment in population-level studies comparing processes and outcomes between hospitals.
Acknowledgments
The authors would like to acknowledge Miriam Fradette, Debbie Boyko, Sara Belga, Darren Lau, Jenelle Pederson, and Sharry Kahlon for their important contributions in data acquisition in our original cohort study, as well as all the physicians rotating through the general internal medicine wards at the University of Alberta Hospital for their help in identifying the patients. We also thank Dr. Simon Conroy, MB ChB PhD, University of Leicester, UK, for his helpful comments on an earlier draft of this manuscript.
Disclosures
The authors declare no conflicts of interest. All authors had access to the data and played a role in writing and revising this manuscript.
Funding
Funding for this study was provided by an operating grant from Alberta Innovates - Health Solutions. F.A.M. holds the Chair in Cardiovascular Outcomes Research at the Mazankowski Heart Institute, University of Alberta. The authors have no affiliations or financial interests with any organization or entity with a financial interest in the contents of this manuscript.
Frailty is associated with adverse outcomes in hospitalized patients, including longer length of stay, increased risk of institutionalization at discharge, and higher rates of readmissions or death postdischarge.1-4 Multiple tools have been developed to evaluate frailty and in an earlier study,4 we compared the three most common of these and demonstrated that the Clinical Frailty Scale (CFS)5 was the most useful tool clinically as it was most strongly associated with adverse events in the first 30 days after discharge. However, it must be collected prospectively and requires contact with patients or proxies for the evaluator to assign the patient into one of nine categories depending on their disease state, mobility, cognition, and ability to perform instrumental and functional activities of daily living. Recently, a new score has been described which is based on an administrative data algorithm that assigns points to patients having any of 109 ICD-10 codes listed for their index hospitalization and all hospitalizations in the prior two years and can be generated retrospectively without trained observers.6 Although higher Hospital Frailty Risk Scores (HFRS) were associated with greater risk of postdischarge adverse events, the kappa when compared with the CFS was only 0.30 (95% CI 0.22-0.38) in that study.6 However, as the HFRS was developed and validated in patients aged ≥75 years within the UK National Health Service, the authors themselves recommended that it be evaluated in other healthcare systems, other populations, and with comparison to prospectively collected frailty data from cumulative deficit models such as the CFS.
The aim of this study was to compare frailty assessments using the CFS and the HFRS in a population of adult patients hospitalized on general medical wards in North America to determine the impact on prevalence estimates and prediction of outcomes within the first 30 days after hospital discharge (a timeframe highlighted in the Affordable Care Act and used by Centers for Medicare & Medicaid Services as an important hospital quality indicator).
METHODS
As described previously,7 we performed a prospective cohort study of adults without cognitive impairment or life expectancy less than three months being discharged back to the community (not to long-term care facilities) from general medical wards in two teaching hospitals in Edmonton, Alberta, between October 2013 and November 2014. All patients provided signed consent, and the University of Alberta Health Research Ethics board (project ID Pro00036880) approved the study.
Trained observers assessed each patient’s frailty status within 24 hours of discharge based on the patient’s best status in the week prior to becoming ill with the reason for the index hospitalization. The research assistant classified patients into one of the following nine CFS categories: very fit, well, managing well, vulnerable, mildly frail (need help with at least one instrumental activities of daily living such as shopping, finances, meal preparation, or housework), moderately frail (need help with one or two activities of daily living such as bathing and dressing), severely frail (dependent for personal care), very severely frail (bedbound), and terminally ill. According to the CFS validation studies, the last five categories were defined as frail for the purposes of our analyses.
Independent of the trained observer’s assessments, we calculated the HFRS for each participant in our cohort by linking to Alberta administrative data holdings within the Alberta Health Services Data Integration and Measurement Reporting unit and examining all diagnostic codes for the index hospitalization and any other hospitalizations in the prior two years for the 109 ICD-10 codes listed in the original HFRS paper and used the same score cutpoints as they reported (HFRS <5 being low risk, 5-15 defined as intermediate risk, and >15 as high risk for frailty; scores ≥5 were defined as frail).6
All patients were followed after discharge by research personnel blinded to the patient’s frailty assessment. We used patient/caregiver self-report and the provincial electronic health record to collect information on all-cause readmissions or mortality within 30 days.
We have previously reported4,7 the association between frailty defined by the CFS and unplanned readmissions or death within 30 days of discharge but in this study, we examined the correlation between CFS-defined frailty and the HFRS score (classifying those with intermediate or high scores as frail) using chance-corrected kappa coefficients. We also compared the prognostic accuracy of both models for predicting death and/or unplanned readmissions within 30 days using the C statistic and the integrated discrimination improvement index and examined patients aged >65 years as a subgroup.8 We used SAS version 9.4 (SAS Institute, Cary, North Carolina) for analyses, with P values of <.05 considered as statistically significant.
RESULTS
Of the 499 patients in our original cohort,7 we could not link 10 to the administrative data to calculate HFRS, and thus this study sample is only 489 patients (mean age 64 years, 50% women, 52% older than 65 years, a mean of 4.9 comorbidities, and median length of stay five days).
Overall, 276 (56%) patients were deemed frail according to at least one assessment (214 [44%] on the HFRS [35% intermediate risk and 9% high risk] and 161 [33%] on the CFS), and 99 (20%) met both frailty definitions (Appendix Figure). Among the 252 patients aged >65 years, 66 (26%) met both frailty definitions and 166 (66%) were frail according to at least one assessment. Agreement between HFRS and the CFS (kappa 0.24, 95% CI 0.16-0.33) was poor. The CFS definition of frailty was 46% sensitive and 77% specific in classifying frail patients compared with HFRS-defined frailty.
As we reported earlier,4 patients deemed frail were generally similar across scales in that they were older, had more comorbidities, more prescriptions, longer lengths of stay, and poorer quality of life than nonfrail patients (all P < .01, Table 1). However, patients classified as frail on the HFRS only but not meeting the CFS definition were younger, had higher quality of life, and despite a similar Charlson Score and number of comorbidities were much more likely to have been living independently prior to admission than those classified as frail on the CFS.
Death or unplanned readmission within 30 days occurred in 13.3% (65 patients), with most events being readmissions (62, 12.7%). HFRS-defined frail patients exhibited higher 30-day death/readmission rates (16% vs 11% for not frail, P = .08; 14% vs 11% in the elderly, P = .5), which was not statistically significantly different from the nonfrail patients even after adjusting for age and sex (aOR [adjusted odds ratio] 1.62, 95% CI 0.95-2.75 for all adults; aOR 1.24, 95% CI 0.58-2.63 for the elderly). CFS-defined frail patients had significantly higher 30-day readmission/death rates (19% vs 10% for not frail, aOR 2.53, 95% CI 1.40-4.57 for all adults and 21% vs 6% in the elderly, aOR 4.31, 95% CI 1.80-10.31).
Adding the HFRS results to the CFS-based predictive models added little new information, with an integrated discrimination improvement of only 0.009 that was not statistically significant (P = .09, Table 2). In fact, the HFRS was not an independent predictor of postdischarge outcomes after adjusting for age and sex. Although predictive models incorporating the CFS demonstrated the best C statistics, none of the models had high C statistics (ranging between 0.54 and 0.64 for all adults and between 0.55 and 0.68 for those aged >65 years). Even when the frailty definitions were examined as continuous variables, the C statistics were similar as for the dichotomized analyses (0.64 for CFS and 0.58 for HFRS) and the correlation between the two remained weak (Spearman’s correlation coefficient 0.34).
DISCUSSION
We have demonstrated that the prevalence of frailty in patients being discharged from medical wards was high, with the HFRS (44%) being higher than the CFS (33%), and that only 46% of patients deemed frail on the HFRS were also deemed frail on the CFS. We confirm the report by the developers of the HFRS that there was poor correlation between the CFS cumulative deficit model and the administrative-data-based HFRS model in our cohort, even among those older than 65 years.
Previous studies have reported marked heterogeneity in prevalence estimates between different frailty instruments.2,9 For example, Aguayo et al. found that the prevalence of frailty in the English Longitudinal Study of Aging varied between 0.9% and 68% depending on which of the 35 frailty scales they tested were used, although the prevalence with comprehensive geriatric assessments (the gold standard) was 14.9% (and 15.3% on the CFS).9 Although frail patients are at higher risk for death and/or readmission after discharge, other investigators have also reported similar findings to ours that frailty-based risk models are surprisingly modest at predicting postdischarge readmission or death, with the C statistics ranging between 0.52 and 0.57, although the CFS appears to correlate best with the gold standard of comprehensive geriatric assessment.10-14 This is not surprising since the CFS is multidimensional and as a cumulative deficit model, it incorporates assessment of the patient’s underlying diseases, cognition, function, mobility, and mood in the assignment of their CFS level. Regardless, others15 have pointed out the need for studies such as ours to compare the validity of published frailty scales.
Despite our prospective cohort design and blinded endpoint ascertainment, there are some potential limitations to our study. First, we excluded long-term care residents and patients with foreshortened life expectancy – the frailest of the frail – from our analysis of 30-day outcomes, thereby potentially reducing the magnitude of the association between frailty and adverse outcomes. However, we were interested only in situations where clinicians were faced with equipoise about patient prognosis. Second, we assessed only 30-day readmissions or deaths and cannot comment on the impact of frailty definitions on other postdischarge outcomes (such as discharge locale or need for home care services) or other timeframes. Finally, although the association between the HFRS definition of frailty and the 30-day mortality/readmission was not statistically significant, the 95% confidence intervals were wide and thus we cannot definitively rule out a positive association.
In conclusion, considering that it had the strongest association with postdischarge outcomes and is the fastest and easiest to perform, the most useful of the frailty assessment tools for clinicians at the bedside still appears to be the CFS (both overall and in those patients who are elderly). However, for researchers who are analyzing data retrospectively or policy planners looking at health services data where the CFS was not collected, the HFRS holds promise for risk adjustment in population-level studies comparing processes and outcomes between hospitals.
Acknowledgments
The authors would like to acknowledge Miriam Fradette, Debbie Boyko, Sara Belga, Darren Lau, Jenelle Pederson, and Sharry Kahlon for their important contributions in data acquisition in our original cohort study, as well as all the physicians rotating through the general internal medicine wards at the University of Alberta Hospital for their help in identifying the patients. We also thank Dr. Simon Conroy, MB ChB PhD, University of Leicester, UK, for his helpful comments on an earlier draft of this manuscript.
Disclosures
The authors declare no conflicts of interest. All authors had access to the data and played a role in writing and revising this manuscript.
Funding
Funding for this study was provided by an operating grant from Alberta Innovates - Health Solutions. F.A.M. holds the Chair in Cardiovascular Outcomes Research at the Mazankowski Heart Institute, University of Alberta. The authors have no affiliations or financial interests with any organization or entity with a financial interest in the contents of this manuscript.
1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752-762. doi: 10.1016/S0140-6736(12)62167-9. PubMed
2. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487-1492. doi: 10.1111/j.1532-5415.2012.04054.x. PubMed
3. de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104-114. doi: 10.1016/j.arr.2010.09.001. PubMed
4. Belga S, Majumdar SR, Kahlon S, et al. Comparing three different measures of frailty in medical inpatients: multicenter prospective cohort study examining 30-day risk of readmission or death. J Hosp Med. 2016;11(8):556-562. doi: 10.1002/jhm.2607. PubMed
5. Rockwood K, Andrew M, Mintnitski A. A comparison of two approaches to measuring frailty in elerly people. J Gerontol. 2007;62(7):738-743. doi: 10.1093/gerona/62.7.738. PubMed
6. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8Get. PubMed
7. Kahlon S, Pederson J, Majumdar SR, et al. Association between frailty and 30-day outcomes after discharge from hospital. CMAJ. 2015;187(11):799-804. doi: 10.1503/cmaj.150100. PubMed
8. Pencina MJ, D’ Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the roc curve to reclassification and beyond. Stat Med. 2008;27(2):157-172. doi: 10.1002/sim.2929.
9. Aguayo GA, Donneau A-F, Vaillant MT, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186(4):420-434. doi: 10.1093/aje/kwx061. PubMed
10. Ritt M, Bollheimer LC, Siever CC, Gaßmann KG. Prediction of one-year mortality by five different frailty instruments: a comparative study in hospitalized geriatric patients. Arch Gerontol Geriatr. 2016;66:66-72. doi: 10.1016/j.archger.2016.05.004. PubMed
11. Forti P, Rietti E, Pisacane N, Olivelli V, Maltoni B, Ravaglia G. A comparison of frailty indexes for prediction of adverse health outcomes in a elderly cohort. Arch Gerontol Geriatr. 2012;54(1):16-20. doi: 10.1016/j.archger.2011.01.007. PubMed
12. Wou F, Gladman JR, Bradshaw L, Franklin M, Edmans J, Conroy SP. The predictive properties of frailty-rating scales in the acute medical unit. Age Ageing. 2013;42(6):776-781. doi: 10.1093/ageing/aft055. PubMed
13. Wallis SJ, Wall J, Biram RW, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108(12):943-949. doi: 10.1093/qjmed/hcv066. PubMed
14. Harmand MGC, Meillon C, Bergua V, et al. Comparing the predictive value of three definitions of frailty: results from the Three-City Study. Arch Gerontol Geriatr. 2017;72:153-163. doi: 10.1016/j.archger.2017.06.005. PubMed
15. Bouillon K, Kivimaki M, Hamer M, et al. Measures of frailty in population-based studies: an overview. BMC Geriatrics. 2013;13(1):64. doi: 10.1186/1471-2318-13-64. PubMed
1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752-762. doi: 10.1016/S0140-6736(12)62167-9. PubMed
2. Collard RM, Boter H, Schoevers RA, Oude Voshaar RC. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc. 2012;60(8):1487-1492. doi: 10.1111/j.1532-5415.2012.04054.x. PubMed
3. de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev. 2011;10(1):104-114. doi: 10.1016/j.arr.2010.09.001. PubMed
4. Belga S, Majumdar SR, Kahlon S, et al. Comparing three different measures of frailty in medical inpatients: multicenter prospective cohort study examining 30-day risk of readmission or death. J Hosp Med. 2016;11(8):556-562. doi: 10.1002/jhm.2607. PubMed
5. Rockwood K, Andrew M, Mintnitski A. A comparison of two approaches to measuring frailty in elerly people. J Gerontol. 2007;62(7):738-743. doi: 10.1093/gerona/62.7.738. PubMed
6. Gilbert T, Neuburger J, Kraindler J, et al. Development and validation of a Hospital Frailty Risk Score focusing on older people in acute care settings using electronic hospital records: an observational study. Lancet. 2018;391(10132):1775-1782. doi: 10.1016/S0140-6736(18)30668-8Get. PubMed
7. Kahlon S, Pederson J, Majumdar SR, et al. Association between frailty and 30-day outcomes after discharge from hospital. CMAJ. 2015;187(11):799-804. doi: 10.1503/cmaj.150100. PubMed
8. Pencina MJ, D’ Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the roc curve to reclassification and beyond. Stat Med. 2008;27(2):157-172. doi: 10.1002/sim.2929.
9. Aguayo GA, Donneau A-F, Vaillant MT, et al. Agreement between 35 published frailty scores in the general population. Am J Epidemiol. 2017;186(4):420-434. doi: 10.1093/aje/kwx061. PubMed
10. Ritt M, Bollheimer LC, Siever CC, Gaßmann KG. Prediction of one-year mortality by five different frailty instruments: a comparative study in hospitalized geriatric patients. Arch Gerontol Geriatr. 2016;66:66-72. doi: 10.1016/j.archger.2016.05.004. PubMed
11. Forti P, Rietti E, Pisacane N, Olivelli V, Maltoni B, Ravaglia G. A comparison of frailty indexes for prediction of adverse health outcomes in a elderly cohort. Arch Gerontol Geriatr. 2012;54(1):16-20. doi: 10.1016/j.archger.2011.01.007. PubMed
12. Wou F, Gladman JR, Bradshaw L, Franklin M, Edmans J, Conroy SP. The predictive properties of frailty-rating scales in the acute medical unit. Age Ageing. 2013;42(6):776-781. doi: 10.1093/ageing/aft055. PubMed
13. Wallis SJ, Wall J, Biram RW, Romero-Ortuno R. Association of the clinical frailty scale with hospital outcomes. QJM. 2015;108(12):943-949. doi: 10.1093/qjmed/hcv066. PubMed
14. Harmand MGC, Meillon C, Bergua V, et al. Comparing the predictive value of three definitions of frailty: results from the Three-City Study. Arch Gerontol Geriatr. 2017;72:153-163. doi: 10.1016/j.archger.2017.06.005. PubMed
15. Bouillon K, Kivimaki M, Hamer M, et al. Measures of frailty in population-based studies: an overview. BMC Geriatrics. 2013;13(1):64. doi: 10.1186/1471-2318-13-64. PubMed
© 2019 Society of Hospital Medicine
Adherence to Recommended Inpatient Hepatic Encephalopathy Workup
Clinical guidelines are periodically released by medical societies with the overarching goal of improving deliverable medical care by standardizing disease management according to best available published literature and by reducing healthcare expenditure associated with unnecessary and superfluous testing.1 Unfortunately, nonadherence to guidelines is common in clinical practice2 and contributes to the rising cost of healthcare.3 Health resource utilization is particularly relevant in management of cirrhosis, a condition with an annual healthcare expenditure of $13 billion.4 Hepatic encephalopathy (HE), the most common complication of cirrhosis, is characterized by altered sensorium and is the leading indication for hospitalization among cirrhotics. The joint guidelines of the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) for diagnostic workup for HE recommend identification and treatment of potential precipitants.5 The guidelines also recommend against checking serum ammonia levels, which have not been shown to correlate with diagnosis or severity of HE.6-8 Currently, limited data are available on practice patterns regarding guideline adherence and unnecessary serum ammonia testing for initial evaluation of HE in hospitals. To overcome this gap in knowledge, we conducted the present study to provide granular details regarding the diagnostic workup for hospitalized patients with HE.
METHODS
This study adopted a retrospective design and recruited patients admitted to the Virginia Commonwealth University Medical Center between July 1, 2016 and July 1, 2017. The institutional review board approved the study, and the manuscript was reviewed and approved by all authors prior to submission. All chart reviews were performed by hepatologists with access to patients’ electronic medical record (EMR).
Patient Population
Patients were identified from the EMR system by using ICD-9 and ICD-10 codes for cirrhosis, hepatic encephalopathy, and altered mental status. All consecutive admissions with these diagnosis codes were considered for inclusion. Adult patients with cirrhosis resulting from any etiology of chronic liver diseases with primary reason for admission of HE were included. If patients were readmitted for HE during the study period, then only the data from index HE admission was included in the analysis and data from subsequent admissions were excluded. The other exclusion criteria included non-HE causes of confusion, acute liver failure, and those admitted with a preformulated plan (eg, direct hepatology clinic admission or outside hospital transfer). Patients who developed HE during their hospitalization where HE was not the indication for admission were also excluded. Finally, all patients admitted under the direct care of hepatology were excluded.
Diagnostic Workup
The recommendations of the AASLD and the EASL for workup for HE include obtaining detailed history and physical examination supplemented by diagnostic evaluation for potential HE precipitants including infections, electrolyte disturbances, dehydration, renal failure, glycemic disturbances, and toxin ingestion (eg, alcohol, illicit drugs).5 Based on the guideline recommendation, this study defined a “complete workup” as including all of the following elements: infection evaluation (blood culture, urinalysis/urine culture, chest radiograph, diagnostic paracentesis in the presence of ascites), electrolyte/renal evaluation (serum sodium, potassium, creatinine, and glucose), and toxin evaluation (urine drug screening). Any HE admission that was missing elements from the aforementioned battery of tests was defined as “incomplete workup.” In patients admitted with decompensated cirrhosis, serum ammonia testing was considered inappropriate unless there was a nuanced explanation supporting its use documented within the EMR. The frequency and specialty of the physician ordering serum ammonia level tests were determined. The financial burden of unnecessary ammonia testing was estimated by assigning a laboratory charge ($258) for each patient.
Statistical Analysis
Continuous and categorical variables are reported as means (± standard deviation), median (interquartile range or IQR), or proportion (%) as appropriate. Across-group differences were compared using Student t-test for normally distributed continuous variables and Mann-Whitney U test for skewed data. Fisher’s exact test was used to compare proportion. HE evaluations were quantified by the number of patients with complete workup and by the number of patients with missing components of the workup. A nominal P value of less than .05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics version 24.0 (IBM Corporation, Armonk, New York).
RESULTS
Cohort Characteristics
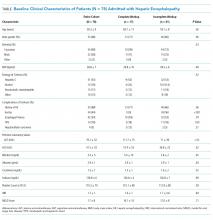
The baseline cohort demographics are listed in the Table. Of the 145 patients identified using diagnostic codes for cirrhosis, 78 subjects met the study criteria. The most common exclusion criteria included non-HE etiology of altered mental status (n = 37) and patients with readmissions for HE during the study period (n = 30). The mean age of the study cohort was 59.3 years, and the most common etiology of cirrhosis was hepatitis C (n = 41), alcohol induced (n = 14), and nonalcoholic steatohepatitis (n = 13).
Initial Diagnostic Evaluation
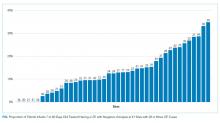
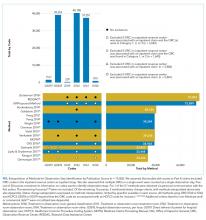
The major precipitants of HE in the study cohort were ineffective lactulose dosing (n = 43), infections (n = 25), and electrolyte disturbances/renal injury (n = 6). At the time of admission, 53 patients were on therapy for HE. Only 17 (22%) patients had complete diagnostic workup within 24 hours of hospital admission. The individual components of the complete workup are shown in the Figure. Notably, 23 (30%) patients were missing blood cultures, 16 (21%) were missing urinalysis, 15 (20%) were missing chest radiograph, and 34 (44%) were missing urine drug screening. Of the 34 patients with ascites on admission, only eight (23%) had diagnostic paracentesis performed on admission to rule out spontaneous bacterial peritonitis.
Serum Ammonia Testing
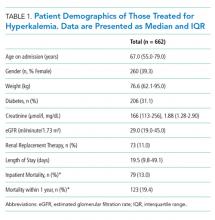
Serum ammonia testing was performed on 74 patients (94.9%), and no patient met the criteria for appropriate testing. Forty patients already had a known diagnosis of HE prior to index admission. Furthermore, 10 (14%) patients had serum ammonia testing repeated after admission without documentation in the EMR to justify repeat testing. Emergency Department (ED) physicians ordered ammonia testing in 57 cases (77%), internists ordered the testing in 11 cases (15%), and intensivists ordered the testing in two cases (3%). The patient’s charges for serum ammonia testing at the time of admission and for repeat testing were $19,092 and $2,580, respectively.
DISCUSSION
This study utilized HE in patients with decompensated cirrhosis as a framework to analyze adherence to societal guidelines. The adherence rate to AALSD/EASL recommended inpatient evaluation of HE is surprisingly low, and most patients are missing key essential elements of the diagnostic work up. While the diagnostic tests that are ordered as part of a panel are completed universally (renal function, electrolytes, and glucose testing), individual testing is less inclined to be ordered (blood cultures, urine culture/urinalysis, CXR, UDS) and procedural testing, such as diagnostic paracentesis, is often missed. This last finding is in line with published literature showing that 40% of patients admitted with ascites or HE did not have diagnostic paracentesis during hospital admission despite 24% reduction of inhospital mortality among patients undergoing the procedure.9
Although serum ammonia testing is not endorsed by the AASLD/EASL guidelines for HE,5 it is ordered nearly universally. The cost of an individual test is relatively low, but the cumulative cost of serum ammonia testing can be substantial because HE is the most common indication for hospitalization among patients with cirrhosis.4 Initiatives, such as the Choosing Wisely® campaign, encourage high-value and evidence-based care by limiting excessive and unnecessary diagnostic testing.10 The Canadian Choosing Wisely campaign specifically includes avoidance of serum ammonia testing for diagnosis of HE to provide high-value care in hepatology.11
Although the exact reasons for nonadherence to recommended HE evaluations are unclear, a potential method to mitigate excessive testing is to utilize the EMR and ordering system.3 EMR-based strategies can curb unnecessary testing in inpatient settings.12 The use of HE order sets, the inclusion of clinical decision support systems, and the restriction of access to specialized testing can be readily incorporated into the EMR to encourage adherence to guideline-based care while limiting unnecessary testing.
This study should be interpreted in the context of study limitations. Given the retrospective design of the study, salient factors in decisions behind diagnostic testing cannot be assessed. Future studies should utilize mixed-model methodology to elucidate reasons behind these decisions. The present study used a strict definition of complete workup including all the mentioned elements of the diagnostic workup for HE; however, in clinical practice, providers could be justified in not ordering certain tests if the specific clinical scenario does not lead to its use (eg, chest X-ray deferred in a patient with clear lung exam, no symptoms, or hypoxia). Similarly, UDS was included as a required element for a complete workup. While it may be ordered in a case-by-case basis to screen for illicit drug abuse, UDS is also a critical element of the workup to screen for opioid use as a precipitant of HE. Finally, considering the strict study entry criteria, we excluded repeated admissions for HE during the study period and therefore likely underestimate the cost burden of serum ammonia testing.
In conclusion, valuable guideline-based diagnostic testing is often missing in patients admitted for HE while serum ammonia testing is nearly universally ordered. These findings underscore the importance of implementing educational strategies, such as the Choosing Wisely® campaign, and EMR-based clinical decision support systems to improve health resource utilization in patients with cirrhosis and HE.
Disclosures
The authors have nothing to disclose.
1. Andrews EJ, Redmond HP. A review of clinical guidelines. Br J Surg. 2004;91:956-964. doi: 10.1002/bjs.4630 PubMed
2. Arts DL, Voncken AG, Medlock S, Abu-Hanna A, van Weert HC. Reasons for intentional guideline non-adherence: a systematic review. Int J Med Inform. 2016;89:55-62. doi: 10.1016/j.ijmedinf.2016.02.009. PubMed
3. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. doi: 10.1001/jamainternmed.2017.5152. PubMed
4. Everhart J. The burden of digestive diseases in the United States. Washington D.C.: US Department of Health and Human Services, Public Health Service, National Institutes of Health. U.S. Government Printing Office; 2008:111-114.
5. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of Liver Diseases. Hepatology . 2014;60:715-735. doi: 10.1002/hep.27210 PubMed
6. Stahl J. Studies of the blood ammonia in liver disease: Its diagnostic, prognostic, and therapeutic significance. Ann Intern Med . 1963;58:1-24. PubMed
7. Ong JP, Aggarwal A, Kreiger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med . 2003;114:188-193. doi: 10.1016/S0002-9343(02)01477-8 PubMed
8. Nicalao F, Efrati C, Masini A, Merli M, Attili AF, Riggio O. Role of determination of partial pressure of ammonia in cirrhotic patients with and without hepatic encephalopathy. J Hepatol. 2003;38:441-446. doi: 10.1016/S0168-8278(02)00436-1 PubMed
9. Orman ES, Hayashi PH, Bataller R, Barritt AS 4th. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12:496-503. doi: 10.1016/j.cgh.2013.08.025. PubMed
10. Cassek CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801-1802. doi: 10.1001/jama.2012.476. PubMed
11. Choosing Wisely Canada. 2018. Five things patients and physicians should question. Available at: https://choosingwiselycanada.org/hepatology/ . Accessed November 18, 2018.
12. Iturrate E, Jubelt L, Volpicelli F, Hochman K. Optimize your electronic medical record to increase value: reducing laboratory overutilization. Am J Med . 2016;129:215-220. doi: 10.1016/j.amjmed.2015.09.009. PubMed
Clinical guidelines are periodically released by medical societies with the overarching goal of improving deliverable medical care by standardizing disease management according to best available published literature and by reducing healthcare expenditure associated with unnecessary and superfluous testing.1 Unfortunately, nonadherence to guidelines is common in clinical practice2 and contributes to the rising cost of healthcare.3 Health resource utilization is particularly relevant in management of cirrhosis, a condition with an annual healthcare expenditure of $13 billion.4 Hepatic encephalopathy (HE), the most common complication of cirrhosis, is characterized by altered sensorium and is the leading indication for hospitalization among cirrhotics. The joint guidelines of the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) for diagnostic workup for HE recommend identification and treatment of potential precipitants.5 The guidelines also recommend against checking serum ammonia levels, which have not been shown to correlate with diagnosis or severity of HE.6-8 Currently, limited data are available on practice patterns regarding guideline adherence and unnecessary serum ammonia testing for initial evaluation of HE in hospitals. To overcome this gap in knowledge, we conducted the present study to provide granular details regarding the diagnostic workup for hospitalized patients with HE.
METHODS
This study adopted a retrospective design and recruited patients admitted to the Virginia Commonwealth University Medical Center between July 1, 2016 and July 1, 2017. The institutional review board approved the study, and the manuscript was reviewed and approved by all authors prior to submission. All chart reviews were performed by hepatologists with access to patients’ electronic medical record (EMR).
Patient Population
Patients were identified from the EMR system by using ICD-9 and ICD-10 codes for cirrhosis, hepatic encephalopathy, and altered mental status. All consecutive admissions with these diagnosis codes were considered for inclusion. Adult patients with cirrhosis resulting from any etiology of chronic liver diseases with primary reason for admission of HE were included. If patients were readmitted for HE during the study period, then only the data from index HE admission was included in the analysis and data from subsequent admissions were excluded. The other exclusion criteria included non-HE causes of confusion, acute liver failure, and those admitted with a preformulated plan (eg, direct hepatology clinic admission or outside hospital transfer). Patients who developed HE during their hospitalization where HE was not the indication for admission were also excluded. Finally, all patients admitted under the direct care of hepatology were excluded.
Diagnostic Workup
The recommendations of the AASLD and the EASL for workup for HE include obtaining detailed history and physical examination supplemented by diagnostic evaluation for potential HE precipitants including infections, electrolyte disturbances, dehydration, renal failure, glycemic disturbances, and toxin ingestion (eg, alcohol, illicit drugs).5 Based on the guideline recommendation, this study defined a “complete workup” as including all of the following elements: infection evaluation (blood culture, urinalysis/urine culture, chest radiograph, diagnostic paracentesis in the presence of ascites), electrolyte/renal evaluation (serum sodium, potassium, creatinine, and glucose), and toxin evaluation (urine drug screening). Any HE admission that was missing elements from the aforementioned battery of tests was defined as “incomplete workup.” In patients admitted with decompensated cirrhosis, serum ammonia testing was considered inappropriate unless there was a nuanced explanation supporting its use documented within the EMR. The frequency and specialty of the physician ordering serum ammonia level tests were determined. The financial burden of unnecessary ammonia testing was estimated by assigning a laboratory charge ($258) for each patient.
Statistical Analysis
Continuous and categorical variables are reported as means (± standard deviation), median (interquartile range or IQR), or proportion (%) as appropriate. Across-group differences were compared using Student t-test for normally distributed continuous variables and Mann-Whitney U test for skewed data. Fisher’s exact test was used to compare proportion. HE evaluations were quantified by the number of patients with complete workup and by the number of patients with missing components of the workup. A nominal P value of less than .05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics version 24.0 (IBM Corporation, Armonk, New York).
RESULTS
Cohort Characteristics
The baseline cohort demographics are listed in the Table. Of the 145 patients identified using diagnostic codes for cirrhosis, 78 subjects met the study criteria. The most common exclusion criteria included non-HE etiology of altered mental status (n = 37) and patients with readmissions for HE during the study period (n = 30). The mean age of the study cohort was 59.3 years, and the most common etiology of cirrhosis was hepatitis C (n = 41), alcohol induced (n = 14), and nonalcoholic steatohepatitis (n = 13).
Initial Diagnostic Evaluation
The major precipitants of HE in the study cohort were ineffective lactulose dosing (n = 43), infections (n = 25), and electrolyte disturbances/renal injury (n = 6). At the time of admission, 53 patients were on therapy for HE. Only 17 (22%) patients had complete diagnostic workup within 24 hours of hospital admission. The individual components of the complete workup are shown in the Figure. Notably, 23 (30%) patients were missing blood cultures, 16 (21%) were missing urinalysis, 15 (20%) were missing chest radiograph, and 34 (44%) were missing urine drug screening. Of the 34 patients with ascites on admission, only eight (23%) had diagnostic paracentesis performed on admission to rule out spontaneous bacterial peritonitis.
Serum Ammonia Testing
Serum ammonia testing was performed on 74 patients (94.9%), and no patient met the criteria for appropriate testing. Forty patients already had a known diagnosis of HE prior to index admission. Furthermore, 10 (14%) patients had serum ammonia testing repeated after admission without documentation in the EMR to justify repeat testing. Emergency Department (ED) physicians ordered ammonia testing in 57 cases (77%), internists ordered the testing in 11 cases (15%), and intensivists ordered the testing in two cases (3%). The patient’s charges for serum ammonia testing at the time of admission and for repeat testing were $19,092 and $2,580, respectively.
DISCUSSION
This study utilized HE in patients with decompensated cirrhosis as a framework to analyze adherence to societal guidelines. The adherence rate to AALSD/EASL recommended inpatient evaluation of HE is surprisingly low, and most patients are missing key essential elements of the diagnostic work up. While the diagnostic tests that are ordered as part of a panel are completed universally (renal function, electrolytes, and glucose testing), individual testing is less inclined to be ordered (blood cultures, urine culture/urinalysis, CXR, UDS) and procedural testing, such as diagnostic paracentesis, is often missed. This last finding is in line with published literature showing that 40% of patients admitted with ascites or HE did not have diagnostic paracentesis during hospital admission despite 24% reduction of inhospital mortality among patients undergoing the procedure.9
Although serum ammonia testing is not endorsed by the AASLD/EASL guidelines for HE,5 it is ordered nearly universally. The cost of an individual test is relatively low, but the cumulative cost of serum ammonia testing can be substantial because HE is the most common indication for hospitalization among patients with cirrhosis.4 Initiatives, such as the Choosing Wisely® campaign, encourage high-value and evidence-based care by limiting excessive and unnecessary diagnostic testing.10 The Canadian Choosing Wisely campaign specifically includes avoidance of serum ammonia testing for diagnosis of HE to provide high-value care in hepatology.11
Although the exact reasons for nonadherence to recommended HE evaluations are unclear, a potential method to mitigate excessive testing is to utilize the EMR and ordering system.3 EMR-based strategies can curb unnecessary testing in inpatient settings.12 The use of HE order sets, the inclusion of clinical decision support systems, and the restriction of access to specialized testing can be readily incorporated into the EMR to encourage adherence to guideline-based care while limiting unnecessary testing.
This study should be interpreted in the context of study limitations. Given the retrospective design of the study, salient factors in decisions behind diagnostic testing cannot be assessed. Future studies should utilize mixed-model methodology to elucidate reasons behind these decisions. The present study used a strict definition of complete workup including all the mentioned elements of the diagnostic workup for HE; however, in clinical practice, providers could be justified in not ordering certain tests if the specific clinical scenario does not lead to its use (eg, chest X-ray deferred in a patient with clear lung exam, no symptoms, or hypoxia). Similarly, UDS was included as a required element for a complete workup. While it may be ordered in a case-by-case basis to screen for illicit drug abuse, UDS is also a critical element of the workup to screen for opioid use as a precipitant of HE. Finally, considering the strict study entry criteria, we excluded repeated admissions for HE during the study period and therefore likely underestimate the cost burden of serum ammonia testing.
In conclusion, valuable guideline-based diagnostic testing is often missing in patients admitted for HE while serum ammonia testing is nearly universally ordered. These findings underscore the importance of implementing educational strategies, such as the Choosing Wisely® campaign, and EMR-based clinical decision support systems to improve health resource utilization in patients with cirrhosis and HE.
Disclosures
The authors have nothing to disclose.
Clinical guidelines are periodically released by medical societies with the overarching goal of improving deliverable medical care by standardizing disease management according to best available published literature and by reducing healthcare expenditure associated with unnecessary and superfluous testing.1 Unfortunately, nonadherence to guidelines is common in clinical practice2 and contributes to the rising cost of healthcare.3 Health resource utilization is particularly relevant in management of cirrhosis, a condition with an annual healthcare expenditure of $13 billion.4 Hepatic encephalopathy (HE), the most common complication of cirrhosis, is characterized by altered sensorium and is the leading indication for hospitalization among cirrhotics. The joint guidelines of the European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) for diagnostic workup for HE recommend identification and treatment of potential precipitants.5 The guidelines also recommend against checking serum ammonia levels, which have not been shown to correlate with diagnosis or severity of HE.6-8 Currently, limited data are available on practice patterns regarding guideline adherence and unnecessary serum ammonia testing for initial evaluation of HE in hospitals. To overcome this gap in knowledge, we conducted the present study to provide granular details regarding the diagnostic workup for hospitalized patients with HE.
METHODS
This study adopted a retrospective design and recruited patients admitted to the Virginia Commonwealth University Medical Center between July 1, 2016 and July 1, 2017. The institutional review board approved the study, and the manuscript was reviewed and approved by all authors prior to submission. All chart reviews were performed by hepatologists with access to patients’ electronic medical record (EMR).
Patient Population
Patients were identified from the EMR system by using ICD-9 and ICD-10 codes for cirrhosis, hepatic encephalopathy, and altered mental status. All consecutive admissions with these diagnosis codes were considered for inclusion. Adult patients with cirrhosis resulting from any etiology of chronic liver diseases with primary reason for admission of HE were included. If patients were readmitted for HE during the study period, then only the data from index HE admission was included in the analysis and data from subsequent admissions were excluded. The other exclusion criteria included non-HE causes of confusion, acute liver failure, and those admitted with a preformulated plan (eg, direct hepatology clinic admission or outside hospital transfer). Patients who developed HE during their hospitalization where HE was not the indication for admission were also excluded. Finally, all patients admitted under the direct care of hepatology were excluded.
Diagnostic Workup
The recommendations of the AASLD and the EASL for workup for HE include obtaining detailed history and physical examination supplemented by diagnostic evaluation for potential HE precipitants including infections, electrolyte disturbances, dehydration, renal failure, glycemic disturbances, and toxin ingestion (eg, alcohol, illicit drugs).5 Based on the guideline recommendation, this study defined a “complete workup” as including all of the following elements: infection evaluation (blood culture, urinalysis/urine culture, chest radiograph, diagnostic paracentesis in the presence of ascites), electrolyte/renal evaluation (serum sodium, potassium, creatinine, and glucose), and toxin evaluation (urine drug screening). Any HE admission that was missing elements from the aforementioned battery of tests was defined as “incomplete workup.” In patients admitted with decompensated cirrhosis, serum ammonia testing was considered inappropriate unless there was a nuanced explanation supporting its use documented within the EMR. The frequency and specialty of the physician ordering serum ammonia level tests were determined. The financial burden of unnecessary ammonia testing was estimated by assigning a laboratory charge ($258) for each patient.
Statistical Analysis
Continuous and categorical variables are reported as means (± standard deviation), median (interquartile range or IQR), or proportion (%) as appropriate. Across-group differences were compared using Student t-test for normally distributed continuous variables and Mann-Whitney U test for skewed data. Fisher’s exact test was used to compare proportion. HE evaluations were quantified by the number of patients with complete workup and by the number of patients with missing components of the workup. A nominal P value of less than .05 was considered statistically significant. All statistical analyses were performed using SPSS Statistics version 24.0 (IBM Corporation, Armonk, New York).
RESULTS
Cohort Characteristics
The baseline cohort demographics are listed in the Table. Of the 145 patients identified using diagnostic codes for cirrhosis, 78 subjects met the study criteria. The most common exclusion criteria included non-HE etiology of altered mental status (n = 37) and patients with readmissions for HE during the study period (n = 30). The mean age of the study cohort was 59.3 years, and the most common etiology of cirrhosis was hepatitis C (n = 41), alcohol induced (n = 14), and nonalcoholic steatohepatitis (n = 13).
Initial Diagnostic Evaluation
The major precipitants of HE in the study cohort were ineffective lactulose dosing (n = 43), infections (n = 25), and electrolyte disturbances/renal injury (n = 6). At the time of admission, 53 patients were on therapy for HE. Only 17 (22%) patients had complete diagnostic workup within 24 hours of hospital admission. The individual components of the complete workup are shown in the Figure. Notably, 23 (30%) patients were missing blood cultures, 16 (21%) were missing urinalysis, 15 (20%) were missing chest radiograph, and 34 (44%) were missing urine drug screening. Of the 34 patients with ascites on admission, only eight (23%) had diagnostic paracentesis performed on admission to rule out spontaneous bacterial peritonitis.
Serum Ammonia Testing
Serum ammonia testing was performed on 74 patients (94.9%), and no patient met the criteria for appropriate testing. Forty patients already had a known diagnosis of HE prior to index admission. Furthermore, 10 (14%) patients had serum ammonia testing repeated after admission without documentation in the EMR to justify repeat testing. Emergency Department (ED) physicians ordered ammonia testing in 57 cases (77%), internists ordered the testing in 11 cases (15%), and intensivists ordered the testing in two cases (3%). The patient’s charges for serum ammonia testing at the time of admission and for repeat testing were $19,092 and $2,580, respectively.
DISCUSSION
This study utilized HE in patients with decompensated cirrhosis as a framework to analyze adherence to societal guidelines. The adherence rate to AALSD/EASL recommended inpatient evaluation of HE is surprisingly low, and most patients are missing key essential elements of the diagnostic work up. While the diagnostic tests that are ordered as part of a panel are completed universally (renal function, electrolytes, and glucose testing), individual testing is less inclined to be ordered (blood cultures, urine culture/urinalysis, CXR, UDS) and procedural testing, such as diagnostic paracentesis, is often missed. This last finding is in line with published literature showing that 40% of patients admitted with ascites or HE did not have diagnostic paracentesis during hospital admission despite 24% reduction of inhospital mortality among patients undergoing the procedure.9
Although serum ammonia testing is not endorsed by the AASLD/EASL guidelines for HE,5 it is ordered nearly universally. The cost of an individual test is relatively low, but the cumulative cost of serum ammonia testing can be substantial because HE is the most common indication for hospitalization among patients with cirrhosis.4 Initiatives, such as the Choosing Wisely® campaign, encourage high-value and evidence-based care by limiting excessive and unnecessary diagnostic testing.10 The Canadian Choosing Wisely campaign specifically includes avoidance of serum ammonia testing for diagnosis of HE to provide high-value care in hepatology.11
Although the exact reasons for nonadherence to recommended HE evaluations are unclear, a potential method to mitigate excessive testing is to utilize the EMR and ordering system.3 EMR-based strategies can curb unnecessary testing in inpatient settings.12 The use of HE order sets, the inclusion of clinical decision support systems, and the restriction of access to specialized testing can be readily incorporated into the EMR to encourage adherence to guideline-based care while limiting unnecessary testing.
This study should be interpreted in the context of study limitations. Given the retrospective design of the study, salient factors in decisions behind diagnostic testing cannot be assessed. Future studies should utilize mixed-model methodology to elucidate reasons behind these decisions. The present study used a strict definition of complete workup including all the mentioned elements of the diagnostic workup for HE; however, in clinical practice, providers could be justified in not ordering certain tests if the specific clinical scenario does not lead to its use (eg, chest X-ray deferred in a patient with clear lung exam, no symptoms, or hypoxia). Similarly, UDS was included as a required element for a complete workup. While it may be ordered in a case-by-case basis to screen for illicit drug abuse, UDS is also a critical element of the workup to screen for opioid use as a precipitant of HE. Finally, considering the strict study entry criteria, we excluded repeated admissions for HE during the study period and therefore likely underestimate the cost burden of serum ammonia testing.
In conclusion, valuable guideline-based diagnostic testing is often missing in patients admitted for HE while serum ammonia testing is nearly universally ordered. These findings underscore the importance of implementing educational strategies, such as the Choosing Wisely® campaign, and EMR-based clinical decision support systems to improve health resource utilization in patients with cirrhosis and HE.
Disclosures
The authors have nothing to disclose.
1. Andrews EJ, Redmond HP. A review of clinical guidelines. Br J Surg. 2004;91:956-964. doi: 10.1002/bjs.4630 PubMed
2. Arts DL, Voncken AG, Medlock S, Abu-Hanna A, van Weert HC. Reasons for intentional guideline non-adherence: a systematic review. Int J Med Inform. 2016;89:55-62. doi: 10.1016/j.ijmedinf.2016.02.009. PubMed
3. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. doi: 10.1001/jamainternmed.2017.5152. PubMed
4. Everhart J. The burden of digestive diseases in the United States. Washington D.C.: US Department of Health and Human Services, Public Health Service, National Institutes of Health. U.S. Government Printing Office; 2008:111-114.
5. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of Liver Diseases. Hepatology . 2014;60:715-735. doi: 10.1002/hep.27210 PubMed
6. Stahl J. Studies of the blood ammonia in liver disease: Its diagnostic, prognostic, and therapeutic significance. Ann Intern Med . 1963;58:1-24. PubMed
7. Ong JP, Aggarwal A, Kreiger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med . 2003;114:188-193. doi: 10.1016/S0002-9343(02)01477-8 PubMed
8. Nicalao F, Efrati C, Masini A, Merli M, Attili AF, Riggio O. Role of determination of partial pressure of ammonia in cirrhotic patients with and without hepatic encephalopathy. J Hepatol. 2003;38:441-446. doi: 10.1016/S0168-8278(02)00436-1 PubMed
9. Orman ES, Hayashi PH, Bataller R, Barritt AS 4th. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12:496-503. doi: 10.1016/j.cgh.2013.08.025. PubMed
10. Cassek CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801-1802. doi: 10.1001/jama.2012.476. PubMed
11. Choosing Wisely Canada. 2018. Five things patients and physicians should question. Available at: https://choosingwiselycanada.org/hepatology/ . Accessed November 18, 2018.
12. Iturrate E, Jubelt L, Volpicelli F, Hochman K. Optimize your electronic medical record to increase value: reducing laboratory overutilization. Am J Med . 2016;129:215-220. doi: 10.1016/j.amjmed.2015.09.009. PubMed
1. Andrews EJ, Redmond HP. A review of clinical guidelines. Br J Surg. 2004;91:956-964. doi: 10.1002/bjs.4630 PubMed
2. Arts DL, Voncken AG, Medlock S, Abu-Hanna A, van Weert HC. Reasons for intentional guideline non-adherence: a systematic review. Int J Med Inform. 2016;89:55-62. doi: 10.1016/j.ijmedinf.2016.02.009. PubMed
3. Eaton KP, Levy K, Soong C, et al. Evidence-based guidelines to eliminate repetitive laboratory testing. JAMA Intern Med. 2017;177(12):1833-1839. doi: 10.1001/jamainternmed.2017.5152. PubMed
4. Everhart J. The burden of digestive diseases in the United States. Washington D.C.: US Department of Health and Human Services, Public Health Service, National Institutes of Health. U.S. Government Printing Office; 2008:111-114.
5. Vilstrup H, Amodio P, Bajaj J, et al. Hepatic encephalopathy in chronic liver disease: 2014 Practice guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of Liver Diseases. Hepatology . 2014;60:715-735. doi: 10.1002/hep.27210 PubMed
6. Stahl J. Studies of the blood ammonia in liver disease: Its diagnostic, prognostic, and therapeutic significance. Ann Intern Med . 1963;58:1-24. PubMed
7. Ong JP, Aggarwal A, Kreiger D, et al. Correlation between ammonia levels and the severity of hepatic encephalopathy. Am J Med . 2003;114:188-193. doi: 10.1016/S0002-9343(02)01477-8 PubMed
8. Nicalao F, Efrati C, Masini A, Merli M, Attili AF, Riggio O. Role of determination of partial pressure of ammonia in cirrhotic patients with and without hepatic encephalopathy. J Hepatol. 2003;38:441-446. doi: 10.1016/S0168-8278(02)00436-1 PubMed
9. Orman ES, Hayashi PH, Bataller R, Barritt AS 4th. Paracentesis is associated with reduced mortality in patients hospitalized with cirrhosis and ascites. Clin Gastroenterol Hepatol. 2014;12:496-503. doi: 10.1016/j.cgh.2013.08.025. PubMed
10. Cassek CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801-1802. doi: 10.1001/jama.2012.476. PubMed
11. Choosing Wisely Canada. 2018. Five things patients and physicians should question. Available at: https://choosingwiselycanada.org/hepatology/ . Accessed November 18, 2018.
12. Iturrate E, Jubelt L, Volpicelli F, Hochman K. Optimize your electronic medical record to increase value: reducing laboratory overutilization. Am J Med . 2016;129:215-220. doi: 10.1016/j.amjmed.2015.09.009. PubMed
© 2019 Society of Hospital Medicine
Preventing Hypoglycemia Following Treatment of Hyperkalemia in Hospitalized Patients
Hyperkalemia is common in hospitalized patients, with an estimated prevalence of 1%-10%.1,2 Hyperkalemia can lead to life-threatening cardiac arrhythmias. The risk of arrhythmias increases with serum potassium values >6.5 mmol/L, and hyperkalemia is associated with increased in-hospital mortality.3 Treatment for hyperkalemia is indicated by a combination of the absolute serum potassium level, the rate of change of potassium level, and the presence of electrocardiogram abnormalities.
Intravenous insulin stimulates the sodium/potassium-ATP pump, leading to intracellular uptake of potassium. Recommendations vary regarding the optimal dosing of insulin and dextrose for the treatment of hyperkalemia.4
Hypoglycemia is a common complication following treatment of hyperkalemia with insulin/dextrose. The reported incidence in hospitalized patients ranges from 6% to 75% depending on the population studied, the doses of insulin/dextrose administered, and the definition of hypoglycemia.5-8 Hypoglycemia itself is associated with increased morbidity and mortality in hospitalized patients.9
The aims of this study were to describe the incidence of hypoglycemia following hyperkalemia treatment with intravenous insulin/dextrose in inpatients in a large (900-bed) UK teaching hospital and to determine the risk factors predisposing to hypoglycemia.
METHODS
We conducted a retrospective, single-center cohort study reviewing the Electronic Patient Records (EPR) of all adult (aged ≥18 years) inpatients (excluding critical care) prescribed treatment for hyperkalemia with intravenous insulin from January 1, 2013, to March 1, 2017. Local hyperkalemia treatment guidelines included administration of 10 units of insulin and 100 ml of 20% glucose intravenously in accordance with national guidelines.10 The study received local approval.
Episodes occurring before May 1, 2015, were excluded because modification to the hyperkalemia prescription care bundle was implemented in April 2015 recommending standardized simultaneous insulin and dextrose administration and hourly capillary blood glucose (CBG) measurement for six hours following treatment. Episodes where no dextrose was prescribed or administered (n = 435) or where no CBG value was recorded within six hours after treatment were excluded (n = 63). All patients included in the analysis received the same insulin/dextrose treatment confirmed by electronic signature of the prescription.
Data extracted included patient demographics, laboratory values, and treatment and administration details. Pretreatment and posttreatment potassium measurements were taken within four hours before and after insulin/dextrose administration, respectively. Serum creatinine and estimated glomerular filtration rate (eGFR) measurements were taken within six hours prior to treatment. Pretreatment CBG levels were measured within two hours of insulin/dextrose administration, and the lowest value within six hours after treatment was used for the analysis. We collected data on length of stay and mortality during one-year follow-up.
Hypoglycemia and severe hypoglycemia were defined as CBG ≤3.9 mmol/l (70 mg/dL) and <3.0 mmol/l (54 mg/dL) in line with definitions used in the National Diabetes Inpatient Audit.11
Descriptive statistics are reported as mean (±SD) or median (interquartile range [IQR]) values for continuous data or numbers and percentages for categorical data. All P values are two-tailed, and P values <.05 were considered to indicate statistical significance. Chi-squared test and Student t test were used to assess differences for categorical and continuous variables between groups. The statistical analysis was performed using the SPSS software, version 25 (IBM).
RESULTS
A total of 662 episodes of hyperkalemia treatment with insulin/dextrose were included in the analysis. These episodes occurred over 445 admissions in 415 individuals. The median number of treatments/patient admission was 1.0 (range 1-11); 108 patients received more than one insulin/dextrose treatment during their admission. Mean pretreatment serum potassium level was 6.4 ± 0.5 mmol/l, and treatment reduced the serum potassium level by 0.6 ± 0.6 mmol/l.
Patient Demographics
Median age of the patients was 67 years (IQR 55.0-79.0), and 39.3% of episodes occurred in females (Table 1). Median weight of the patients was 76.6 kg (IQR 62.1-95.0). Diabetes was present in 31.1% of episodes. Renal impairment was common, with median creatinine levels being 166 µmol/l (IQR 113-256) and 1.9 mg/dL (IQR 1.3-2.9) and eGFR being 29.0 ml/min/1.73 m2 (IQR 19.0-45.0), and 11% of episodes occurred in patients requiring acute or chronic dialysis. Median length of stay was 19.5 days (IQR 9.8-49.1). Inpatient mortality was 13%, and one-year mortality was 19.4%.
Incidence
Hypoglycemia occurred following 116 of 662 hyperkalemia treatments administered (17.5%), and severe hypoglycemia occurred after 47 of 662 treatments (7.1%).
Risk Factors
The median age of patients with hypoglycemia was significantly greater than that of patients without hypoglycemia (71.0 years [54.8-83.5] vs 67.0 years [55.0-77.0]; P = .023) (Table 2). There were no significant differences in gender, degree of renal impairment, or requirement for renal replacement therapy between the groups with and without hypoglycemia.
Hypoglycemia occurred in patients who were, on average, 15 kg lighter than those who did not have hypoglycemia (median body weight 66.1 kg [55.4-72.5] vs 81.0 kg [63.1-96.0]; P < .001).
Pretreatment CBG was lower in those who had hypoglycemia following treatment, the levels being 5.8 mmol/l (5.0-7.3), 104 mg/dL (90-131) vs 8.7 mmol/l (6.4-11.4), 157 mg/dL (115-205; P < .001).
There was a nonsignificant trend toward an increased prevalence of diabetes in patients without hypoglycemia (32.6% vs 24.1%; P = .074).
DISCUSSION
This study reports the incidence of iatrogenic hypoglycemia following intravenous insulin treatment for hyperkalemia in a large cohort of general medical and surgical inpatients and describes the risk factors predisposing to this important complication.
The incidence rates of hypoglycemia and severe hypoglycemia in our study were 17.5% and 7.1%, respectively. These rates are greater than previously observed in a smaller US study undertaken in a similar population, which reported an incidence of 8.7% of hypoglycemia and 2.3% of severe hypoglycemia, although it included five different insulin/dextrose prescriptions.8 A similar incidence of hypoglycemia (17%) was reported in patients treated for hyperkalemia in an Emergency Department in the United States.12
Variables that increased the risk of hypoglycemia in the present study included older age, lower body weight, and lower pretreatment CBG level. These risk factors have been reported in previous studies, although inconsistently. Pretreatment CBG is an important predictor of hypoglycemia following treatment for hyperkalemia and has been observed in patients in the emergency department12 and in patients with renal impairment.6,7 In the present study, lower body weight was observed in patients with hypoglycemia compared with those without hypoglycemia. Weight-based insulin dosing (0.1 units/kg) for hyperkalemia has been associated with less hypoglycemia compared with fixed insulin doses (10 units) without affecting the potassium-lowering effect.13
The degree of renal impairment did not affect the risk of hypoglycemia. Chronic kidney disease is associated with increased insulin resistance, which may attenuate the hypoglycemic response to insulin.14 Patients with renal impairment may experience delayed hypoglycemia, which may not have been captured although the posttreatment blood glucose values extended to six hours.
The increased risk of hypoglycemia in older patients treated for hyperkalemia is of concern given the lack of counterregulatory response and reduced symptoms of hypoglycemia in older adults. This association has not been reported in other studies;8,12 however, the average age of subjects in our cohort was higher than that in other studies (67 vs 57 years). Although age did not correlate with weight, older adults may have reduced carbohydrate intake and mild renal impairment affecting insulin clearance.
Hospitalized patients treated for hyperkalemia in the present study had a greater inpatient mortality rate (13%) than the general inpatient population at the same hospital (3%).15 Hyperkalemia often occurs in individuals with comorbidities and is associated with an increased risk of all-cause mortality.3
Mean pretreatment potassium level was 6.4 mmol/l in this study. Evidence-based criteria for treatment thresholds are lacking. Acute potassium increases are associated with cardiac mortality, whereas elevated potassium levels in patients with chronic kidney disease taking renin-angiotensin system drugs are often not treated as emergencies unless significant electrocardiogram (ECG) changes are apparent. It is likely that some hyperkalemia treatments are unnecessary, which is important given the high risk of treatment-related complications.
To our knowledge, this is the largest analysis of hypoglycemic episodes following treatment of hyperkalemia in medical and surgical inpatients. This is a single-center study, thereby limiting its generalizability; however, the patient characteristics are likely similar to those reported from other large, urban institutions.
Treatment prescriptions in our study were consistent due to the electronic prescribing care bundle, allowing us to compare the risk factors for hypoglycemia with standardized prescriptions. Point-of-care CBG measurements can be less accurate at low blood glucose levels; however, laboratory glucose levels are obtained much less frequently, underestimating incidence, due to the need for prompt hypoglycemia treatment without delaying for laboratory glucose measurement.
The dataset was not complete and depended on healthcare professionals entering some data. We did not assess the clinical consequences of hypoglycemia.
FUTURE WORK
An increasing proportion of hospitals are utilizing Electronic Prescribing Systems with the potential to improve patient safety by standardizing prescriptions. However, despite standardized prescriptions, 37% of prescriptions for concurrent dextrose were not administered with insulin and 8.6% of patients had no CBG monitoring within six hours after insulin administration.
We propose to integrate the risk factors identified in this study into a decision support tool embedded within electronic prescriptions and medication administration. This will auto-populate with data from the EPR and identify individuals at high risk of hypoglycemia following hyperkalemia treatment. The decision support tool will then advise a prescription with a higher volume of dextrose and/or lower insulin dose to mitigate this risk.
CONCLUSION
Hyperkalemia treatment with insulin was associated with high incidence of hypoglycemia. Decision support tools highlighting individuals at high risk of hypoglycemia may reduce this incidence.
Disclosures
The authors have nothing to disclose.
1. Stevens MS, Dunlay RW. Hyperkalemia in hospitalized patients. Int Urol Nephrol. 2000;32(2):177-180. doi: 10.1023/A:1007135517950. PubMed
2. Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251-257. doi: 10.5114/aoms.2014.42577. PubMed
3. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162. doi: 10.1001/archinternmed.2009.132. PubMed
4. Harel Z, Kamel KS. Optimal dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS One. 2016;11(5):e0154963. doi: 10.1371/journal.pone.0154963. PubMed
5. Allon M, Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney Int. 1990;38(5):869-872. doi: 10.1038/ki.1990.284. PubMed
6. Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7(3):248-250. doi: 10.1093/ckj/sfu026. PubMed
7. Coca A, Valencia AL, Bustamante J, Mendiluce A, Floege J. Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients with impaired renal function. PLoS One. 2017;12(2):e0172961. doi: 10.1371/journal.pone.0172961. PubMed
8. Schafers S, Naunheim R, Vijayan A, Tobin G. Incidence of hypoglycemia following insulin-based acute stabilization of hyperkalemia treatment. J Hosp Med. 2012;7(3):239-242. doi: 10.1002/jhm.977. PubMed
9. Nirantharakumar K, Marshall T, Kennedy A, Narendran P, Hemming K, Coleman JJ. Hypoglycaemia is associated with increased length of stay and mortality in people with diabetes who are hospitalized. Diabet Med. 2012;29(12):e445-e448. doi: 10.1111/dme.12002. PubMed
10. UK Renal Association Clinical Practice Guidelines: Treatment of acute hyperkalaemia in adults. https://renal.org/guidelines/. Published March 2014. Accessed 1October 2018.
11. National Diabetes Inpatient Audit, England and Wales, 2017 - Full Report. NHS Digital. https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-inpatient-audit/national-diabetes-inpatient-audit-nadia-2017. Published 14 March 2018. Accessed 5 November 2018.
12. Scott NL, Klein L, Cales E, Driver B. Hypoglycemia as a complication of intravenous insulin to treat hyperkalemia in the emergency department. Am J Emerg Med. 2018; 30379-30386. doi: 10.1016/j.ajem.2018.05.016. PubMed
13. Wheeler DT, Schafers SJ, Horwedel TA, Deal EN, Tobin GS. Weight-based insulin dosing for acute hyperkalemia results in less hypoglycemia. J Hosp Med. 2016;11(5):355-357. doi: 10.1002/jhm.2545. PubMed
14. Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol. 2016;311(6):F1087-F1108. doi: 10.1152/ajprenal.00340.2016. PubMed
15. Summary Hospital-level Mortality Indicator (SHMI) - Deaths associated with hospitalisation, England, April 2017 - March 2018. NHS Digital. https://digital.nhs.uk/data-and-information/publications/clinical-indicators/shmi/archive/shmi-april-2017---march-2018. Published 20 September 2018. Accessed 5 November 2018.
Hyperkalemia is common in hospitalized patients, with an estimated prevalence of 1%-10%.1,2 Hyperkalemia can lead to life-threatening cardiac arrhythmias. The risk of arrhythmias increases with serum potassium values >6.5 mmol/L, and hyperkalemia is associated with increased in-hospital mortality.3 Treatment for hyperkalemia is indicated by a combination of the absolute serum potassium level, the rate of change of potassium level, and the presence of electrocardiogram abnormalities.
Intravenous insulin stimulates the sodium/potassium-ATP pump, leading to intracellular uptake of potassium. Recommendations vary regarding the optimal dosing of insulin and dextrose for the treatment of hyperkalemia.4
Hypoglycemia is a common complication following treatment of hyperkalemia with insulin/dextrose. The reported incidence in hospitalized patients ranges from 6% to 75% depending on the population studied, the doses of insulin/dextrose administered, and the definition of hypoglycemia.5-8 Hypoglycemia itself is associated with increased morbidity and mortality in hospitalized patients.9
The aims of this study were to describe the incidence of hypoglycemia following hyperkalemia treatment with intravenous insulin/dextrose in inpatients in a large (900-bed) UK teaching hospital and to determine the risk factors predisposing to hypoglycemia.
METHODS
We conducted a retrospective, single-center cohort study reviewing the Electronic Patient Records (EPR) of all adult (aged ≥18 years) inpatients (excluding critical care) prescribed treatment for hyperkalemia with intravenous insulin from January 1, 2013, to March 1, 2017. Local hyperkalemia treatment guidelines included administration of 10 units of insulin and 100 ml of 20% glucose intravenously in accordance with national guidelines.10 The study received local approval.
Episodes occurring before May 1, 2015, were excluded because modification to the hyperkalemia prescription care bundle was implemented in April 2015 recommending standardized simultaneous insulin and dextrose administration and hourly capillary blood glucose (CBG) measurement for six hours following treatment. Episodes where no dextrose was prescribed or administered (n = 435) or where no CBG value was recorded within six hours after treatment were excluded (n = 63). All patients included in the analysis received the same insulin/dextrose treatment confirmed by electronic signature of the prescription.
Data extracted included patient demographics, laboratory values, and treatment and administration details. Pretreatment and posttreatment potassium measurements were taken within four hours before and after insulin/dextrose administration, respectively. Serum creatinine and estimated glomerular filtration rate (eGFR) measurements were taken within six hours prior to treatment. Pretreatment CBG levels were measured within two hours of insulin/dextrose administration, and the lowest value within six hours after treatment was used for the analysis. We collected data on length of stay and mortality during one-year follow-up.
Hypoglycemia and severe hypoglycemia were defined as CBG ≤3.9 mmol/l (70 mg/dL) and <3.0 mmol/l (54 mg/dL) in line with definitions used in the National Diabetes Inpatient Audit.11
Descriptive statistics are reported as mean (±SD) or median (interquartile range [IQR]) values for continuous data or numbers and percentages for categorical data. All P values are two-tailed, and P values <.05 were considered to indicate statistical significance. Chi-squared test and Student t test were used to assess differences for categorical and continuous variables between groups. The statistical analysis was performed using the SPSS software, version 25 (IBM).
RESULTS
A total of 662 episodes of hyperkalemia treatment with insulin/dextrose were included in the analysis. These episodes occurred over 445 admissions in 415 individuals. The median number of treatments/patient admission was 1.0 (range 1-11); 108 patients received more than one insulin/dextrose treatment during their admission. Mean pretreatment serum potassium level was 6.4 ± 0.5 mmol/l, and treatment reduced the serum potassium level by 0.6 ± 0.6 mmol/l.
Patient Demographics
Median age of the patients was 67 years (IQR 55.0-79.0), and 39.3% of episodes occurred in females (Table 1). Median weight of the patients was 76.6 kg (IQR 62.1-95.0). Diabetes was present in 31.1% of episodes. Renal impairment was common, with median creatinine levels being 166 µmol/l (IQR 113-256) and 1.9 mg/dL (IQR 1.3-2.9) and eGFR being 29.0 ml/min/1.73 m2 (IQR 19.0-45.0), and 11% of episodes occurred in patients requiring acute or chronic dialysis. Median length of stay was 19.5 days (IQR 9.8-49.1). Inpatient mortality was 13%, and one-year mortality was 19.4%.
Incidence
Hypoglycemia occurred following 116 of 662 hyperkalemia treatments administered (17.5%), and severe hypoglycemia occurred after 47 of 662 treatments (7.1%).
Risk Factors
The median age of patients with hypoglycemia was significantly greater than that of patients without hypoglycemia (71.0 years [54.8-83.5] vs 67.0 years [55.0-77.0]; P = .023) (Table 2). There were no significant differences in gender, degree of renal impairment, or requirement for renal replacement therapy between the groups with and without hypoglycemia.
Hypoglycemia occurred in patients who were, on average, 15 kg lighter than those who did not have hypoglycemia (median body weight 66.1 kg [55.4-72.5] vs 81.0 kg [63.1-96.0]; P < .001).
Pretreatment CBG was lower in those who had hypoglycemia following treatment, the levels being 5.8 mmol/l (5.0-7.3), 104 mg/dL (90-131) vs 8.7 mmol/l (6.4-11.4), 157 mg/dL (115-205; P < .001).
There was a nonsignificant trend toward an increased prevalence of diabetes in patients without hypoglycemia (32.6% vs 24.1%; P = .074).
DISCUSSION
This study reports the incidence of iatrogenic hypoglycemia following intravenous insulin treatment for hyperkalemia in a large cohort of general medical and surgical inpatients and describes the risk factors predisposing to this important complication.
The incidence rates of hypoglycemia and severe hypoglycemia in our study were 17.5% and 7.1%, respectively. These rates are greater than previously observed in a smaller US study undertaken in a similar population, which reported an incidence of 8.7% of hypoglycemia and 2.3% of severe hypoglycemia, although it included five different insulin/dextrose prescriptions.8 A similar incidence of hypoglycemia (17%) was reported in patients treated for hyperkalemia in an Emergency Department in the United States.12
Variables that increased the risk of hypoglycemia in the present study included older age, lower body weight, and lower pretreatment CBG level. These risk factors have been reported in previous studies, although inconsistently. Pretreatment CBG is an important predictor of hypoglycemia following treatment for hyperkalemia and has been observed in patients in the emergency department12 and in patients with renal impairment.6,7 In the present study, lower body weight was observed in patients with hypoglycemia compared with those without hypoglycemia. Weight-based insulin dosing (0.1 units/kg) for hyperkalemia has been associated with less hypoglycemia compared with fixed insulin doses (10 units) without affecting the potassium-lowering effect.13
The degree of renal impairment did not affect the risk of hypoglycemia. Chronic kidney disease is associated with increased insulin resistance, which may attenuate the hypoglycemic response to insulin.14 Patients with renal impairment may experience delayed hypoglycemia, which may not have been captured although the posttreatment blood glucose values extended to six hours.
The increased risk of hypoglycemia in older patients treated for hyperkalemia is of concern given the lack of counterregulatory response and reduced symptoms of hypoglycemia in older adults. This association has not been reported in other studies;8,12 however, the average age of subjects in our cohort was higher than that in other studies (67 vs 57 years). Although age did not correlate with weight, older adults may have reduced carbohydrate intake and mild renal impairment affecting insulin clearance.
Hospitalized patients treated for hyperkalemia in the present study had a greater inpatient mortality rate (13%) than the general inpatient population at the same hospital (3%).15 Hyperkalemia often occurs in individuals with comorbidities and is associated with an increased risk of all-cause mortality.3
Mean pretreatment potassium level was 6.4 mmol/l in this study. Evidence-based criteria for treatment thresholds are lacking. Acute potassium increases are associated with cardiac mortality, whereas elevated potassium levels in patients with chronic kidney disease taking renin-angiotensin system drugs are often not treated as emergencies unless significant electrocardiogram (ECG) changes are apparent. It is likely that some hyperkalemia treatments are unnecessary, which is important given the high risk of treatment-related complications.
To our knowledge, this is the largest analysis of hypoglycemic episodes following treatment of hyperkalemia in medical and surgical inpatients. This is a single-center study, thereby limiting its generalizability; however, the patient characteristics are likely similar to those reported from other large, urban institutions.
Treatment prescriptions in our study were consistent due to the electronic prescribing care bundle, allowing us to compare the risk factors for hypoglycemia with standardized prescriptions. Point-of-care CBG measurements can be less accurate at low blood glucose levels; however, laboratory glucose levels are obtained much less frequently, underestimating incidence, due to the need for prompt hypoglycemia treatment without delaying for laboratory glucose measurement.
The dataset was not complete and depended on healthcare professionals entering some data. We did not assess the clinical consequences of hypoglycemia.
FUTURE WORK
An increasing proportion of hospitals are utilizing Electronic Prescribing Systems with the potential to improve patient safety by standardizing prescriptions. However, despite standardized prescriptions, 37% of prescriptions for concurrent dextrose were not administered with insulin and 8.6% of patients had no CBG monitoring within six hours after insulin administration.
We propose to integrate the risk factors identified in this study into a decision support tool embedded within electronic prescriptions and medication administration. This will auto-populate with data from the EPR and identify individuals at high risk of hypoglycemia following hyperkalemia treatment. The decision support tool will then advise a prescription with a higher volume of dextrose and/or lower insulin dose to mitigate this risk.
CONCLUSION
Hyperkalemia treatment with insulin was associated with high incidence of hypoglycemia. Decision support tools highlighting individuals at high risk of hypoglycemia may reduce this incidence.
Disclosures
The authors have nothing to disclose.
Hyperkalemia is common in hospitalized patients, with an estimated prevalence of 1%-10%.1,2 Hyperkalemia can lead to life-threatening cardiac arrhythmias. The risk of arrhythmias increases with serum potassium values >6.5 mmol/L, and hyperkalemia is associated with increased in-hospital mortality.3 Treatment for hyperkalemia is indicated by a combination of the absolute serum potassium level, the rate of change of potassium level, and the presence of electrocardiogram abnormalities.
Intravenous insulin stimulates the sodium/potassium-ATP pump, leading to intracellular uptake of potassium. Recommendations vary regarding the optimal dosing of insulin and dextrose for the treatment of hyperkalemia.4
Hypoglycemia is a common complication following treatment of hyperkalemia with insulin/dextrose. The reported incidence in hospitalized patients ranges from 6% to 75% depending on the population studied, the doses of insulin/dextrose administered, and the definition of hypoglycemia.5-8 Hypoglycemia itself is associated with increased morbidity and mortality in hospitalized patients.9
The aims of this study were to describe the incidence of hypoglycemia following hyperkalemia treatment with intravenous insulin/dextrose in inpatients in a large (900-bed) UK teaching hospital and to determine the risk factors predisposing to hypoglycemia.
METHODS
We conducted a retrospective, single-center cohort study reviewing the Electronic Patient Records (EPR) of all adult (aged ≥18 years) inpatients (excluding critical care) prescribed treatment for hyperkalemia with intravenous insulin from January 1, 2013, to March 1, 2017. Local hyperkalemia treatment guidelines included administration of 10 units of insulin and 100 ml of 20% glucose intravenously in accordance with national guidelines.10 The study received local approval.
Episodes occurring before May 1, 2015, were excluded because modification to the hyperkalemia prescription care bundle was implemented in April 2015 recommending standardized simultaneous insulin and dextrose administration and hourly capillary blood glucose (CBG) measurement for six hours following treatment. Episodes where no dextrose was prescribed or administered (n = 435) or where no CBG value was recorded within six hours after treatment were excluded (n = 63). All patients included in the analysis received the same insulin/dextrose treatment confirmed by electronic signature of the prescription.
Data extracted included patient demographics, laboratory values, and treatment and administration details. Pretreatment and posttreatment potassium measurements were taken within four hours before and after insulin/dextrose administration, respectively. Serum creatinine and estimated glomerular filtration rate (eGFR) measurements were taken within six hours prior to treatment. Pretreatment CBG levels were measured within two hours of insulin/dextrose administration, and the lowest value within six hours after treatment was used for the analysis. We collected data on length of stay and mortality during one-year follow-up.
Hypoglycemia and severe hypoglycemia were defined as CBG ≤3.9 mmol/l (70 mg/dL) and <3.0 mmol/l (54 mg/dL) in line with definitions used in the National Diabetes Inpatient Audit.11
Descriptive statistics are reported as mean (±SD) or median (interquartile range [IQR]) values for continuous data or numbers and percentages for categorical data. All P values are two-tailed, and P values <.05 were considered to indicate statistical significance. Chi-squared test and Student t test were used to assess differences for categorical and continuous variables between groups. The statistical analysis was performed using the SPSS software, version 25 (IBM).
RESULTS
A total of 662 episodes of hyperkalemia treatment with insulin/dextrose were included in the analysis. These episodes occurred over 445 admissions in 415 individuals. The median number of treatments/patient admission was 1.0 (range 1-11); 108 patients received more than one insulin/dextrose treatment during their admission. Mean pretreatment serum potassium level was 6.4 ± 0.5 mmol/l, and treatment reduced the serum potassium level by 0.6 ± 0.6 mmol/l.
Patient Demographics
Median age of the patients was 67 years (IQR 55.0-79.0), and 39.3% of episodes occurred in females (Table 1). Median weight of the patients was 76.6 kg (IQR 62.1-95.0). Diabetes was present in 31.1% of episodes. Renal impairment was common, with median creatinine levels being 166 µmol/l (IQR 113-256) and 1.9 mg/dL (IQR 1.3-2.9) and eGFR being 29.0 ml/min/1.73 m2 (IQR 19.0-45.0), and 11% of episodes occurred in patients requiring acute or chronic dialysis. Median length of stay was 19.5 days (IQR 9.8-49.1). Inpatient mortality was 13%, and one-year mortality was 19.4%.
Incidence
Hypoglycemia occurred following 116 of 662 hyperkalemia treatments administered (17.5%), and severe hypoglycemia occurred after 47 of 662 treatments (7.1%).
Risk Factors
The median age of patients with hypoglycemia was significantly greater than that of patients without hypoglycemia (71.0 years [54.8-83.5] vs 67.0 years [55.0-77.0]; P = .023) (Table 2). There were no significant differences in gender, degree of renal impairment, or requirement for renal replacement therapy between the groups with and without hypoglycemia.
Hypoglycemia occurred in patients who were, on average, 15 kg lighter than those who did not have hypoglycemia (median body weight 66.1 kg [55.4-72.5] vs 81.0 kg [63.1-96.0]; P < .001).
Pretreatment CBG was lower in those who had hypoglycemia following treatment, the levels being 5.8 mmol/l (5.0-7.3), 104 mg/dL (90-131) vs 8.7 mmol/l (6.4-11.4), 157 mg/dL (115-205; P < .001).
There was a nonsignificant trend toward an increased prevalence of diabetes in patients without hypoglycemia (32.6% vs 24.1%; P = .074).
DISCUSSION
This study reports the incidence of iatrogenic hypoglycemia following intravenous insulin treatment for hyperkalemia in a large cohort of general medical and surgical inpatients and describes the risk factors predisposing to this important complication.
The incidence rates of hypoglycemia and severe hypoglycemia in our study were 17.5% and 7.1%, respectively. These rates are greater than previously observed in a smaller US study undertaken in a similar population, which reported an incidence of 8.7% of hypoglycemia and 2.3% of severe hypoglycemia, although it included five different insulin/dextrose prescriptions.8 A similar incidence of hypoglycemia (17%) was reported in patients treated for hyperkalemia in an Emergency Department in the United States.12
Variables that increased the risk of hypoglycemia in the present study included older age, lower body weight, and lower pretreatment CBG level. These risk factors have been reported in previous studies, although inconsistently. Pretreatment CBG is an important predictor of hypoglycemia following treatment for hyperkalemia and has been observed in patients in the emergency department12 and in patients with renal impairment.6,7 In the present study, lower body weight was observed in patients with hypoglycemia compared with those without hypoglycemia. Weight-based insulin dosing (0.1 units/kg) for hyperkalemia has been associated with less hypoglycemia compared with fixed insulin doses (10 units) without affecting the potassium-lowering effect.13
The degree of renal impairment did not affect the risk of hypoglycemia. Chronic kidney disease is associated with increased insulin resistance, which may attenuate the hypoglycemic response to insulin.14 Patients with renal impairment may experience delayed hypoglycemia, which may not have been captured although the posttreatment blood glucose values extended to six hours.
The increased risk of hypoglycemia in older patients treated for hyperkalemia is of concern given the lack of counterregulatory response and reduced symptoms of hypoglycemia in older adults. This association has not been reported in other studies;8,12 however, the average age of subjects in our cohort was higher than that in other studies (67 vs 57 years). Although age did not correlate with weight, older adults may have reduced carbohydrate intake and mild renal impairment affecting insulin clearance.
Hospitalized patients treated for hyperkalemia in the present study had a greater inpatient mortality rate (13%) than the general inpatient population at the same hospital (3%).15 Hyperkalemia often occurs in individuals with comorbidities and is associated with an increased risk of all-cause mortality.3
Mean pretreatment potassium level was 6.4 mmol/l in this study. Evidence-based criteria for treatment thresholds are lacking. Acute potassium increases are associated with cardiac mortality, whereas elevated potassium levels in patients with chronic kidney disease taking renin-angiotensin system drugs are often not treated as emergencies unless significant electrocardiogram (ECG) changes are apparent. It is likely that some hyperkalemia treatments are unnecessary, which is important given the high risk of treatment-related complications.
To our knowledge, this is the largest analysis of hypoglycemic episodes following treatment of hyperkalemia in medical and surgical inpatients. This is a single-center study, thereby limiting its generalizability; however, the patient characteristics are likely similar to those reported from other large, urban institutions.
Treatment prescriptions in our study were consistent due to the electronic prescribing care bundle, allowing us to compare the risk factors for hypoglycemia with standardized prescriptions. Point-of-care CBG measurements can be less accurate at low blood glucose levels; however, laboratory glucose levels are obtained much less frequently, underestimating incidence, due to the need for prompt hypoglycemia treatment without delaying for laboratory glucose measurement.
The dataset was not complete and depended on healthcare professionals entering some data. We did not assess the clinical consequences of hypoglycemia.
FUTURE WORK
An increasing proportion of hospitals are utilizing Electronic Prescribing Systems with the potential to improve patient safety by standardizing prescriptions. However, despite standardized prescriptions, 37% of prescriptions for concurrent dextrose were not administered with insulin and 8.6% of patients had no CBG monitoring within six hours after insulin administration.
We propose to integrate the risk factors identified in this study into a decision support tool embedded within electronic prescriptions and medication administration. This will auto-populate with data from the EPR and identify individuals at high risk of hypoglycemia following hyperkalemia treatment. The decision support tool will then advise a prescription with a higher volume of dextrose and/or lower insulin dose to mitigate this risk.
CONCLUSION
Hyperkalemia treatment with insulin was associated with high incidence of hypoglycemia. Decision support tools highlighting individuals at high risk of hypoglycemia may reduce this incidence.
Disclosures
The authors have nothing to disclose.
1. Stevens MS, Dunlay RW. Hyperkalemia in hospitalized patients. Int Urol Nephrol. 2000;32(2):177-180. doi: 10.1023/A:1007135517950. PubMed
2. Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251-257. doi: 10.5114/aoms.2014.42577. PubMed
3. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162. doi: 10.1001/archinternmed.2009.132. PubMed
4. Harel Z, Kamel KS. Optimal dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS One. 2016;11(5):e0154963. doi: 10.1371/journal.pone.0154963. PubMed
5. Allon M, Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney Int. 1990;38(5):869-872. doi: 10.1038/ki.1990.284. PubMed
6. Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7(3):248-250. doi: 10.1093/ckj/sfu026. PubMed
7. Coca A, Valencia AL, Bustamante J, Mendiluce A, Floege J. Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients with impaired renal function. PLoS One. 2017;12(2):e0172961. doi: 10.1371/journal.pone.0172961. PubMed
8. Schafers S, Naunheim R, Vijayan A, Tobin G. Incidence of hypoglycemia following insulin-based acute stabilization of hyperkalemia treatment. J Hosp Med. 2012;7(3):239-242. doi: 10.1002/jhm.977. PubMed
9. Nirantharakumar K, Marshall T, Kennedy A, Narendran P, Hemming K, Coleman JJ. Hypoglycaemia is associated with increased length of stay and mortality in people with diabetes who are hospitalized. Diabet Med. 2012;29(12):e445-e448. doi: 10.1111/dme.12002. PubMed
10. UK Renal Association Clinical Practice Guidelines: Treatment of acute hyperkalaemia in adults. https://renal.org/guidelines/. Published March 2014. Accessed 1October 2018.
11. National Diabetes Inpatient Audit, England and Wales, 2017 - Full Report. NHS Digital. https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-inpatient-audit/national-diabetes-inpatient-audit-nadia-2017. Published 14 March 2018. Accessed 5 November 2018.
12. Scott NL, Klein L, Cales E, Driver B. Hypoglycemia as a complication of intravenous insulin to treat hyperkalemia in the emergency department. Am J Emerg Med. 2018; 30379-30386. doi: 10.1016/j.ajem.2018.05.016. PubMed
13. Wheeler DT, Schafers SJ, Horwedel TA, Deal EN, Tobin GS. Weight-based insulin dosing for acute hyperkalemia results in less hypoglycemia. J Hosp Med. 2016;11(5):355-357. doi: 10.1002/jhm.2545. PubMed
14. Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol. 2016;311(6):F1087-F1108. doi: 10.1152/ajprenal.00340.2016. PubMed
15. Summary Hospital-level Mortality Indicator (SHMI) - Deaths associated with hospitalisation, England, April 2017 - March 2018. NHS Digital. https://digital.nhs.uk/data-and-information/publications/clinical-indicators/shmi/archive/shmi-april-2017---march-2018. Published 20 September 2018. Accessed 5 November 2018.
1. Stevens MS, Dunlay RW. Hyperkalemia in hospitalized patients. Int Urol Nephrol. 2000;32(2):177-180. doi: 10.1023/A:1007135517950. PubMed
2. Khanagavi J, Gupta T, Aronow WS, et al. Hyperkalemia among hospitalized patients and association between duration of hyperkalemia and outcomes. Arch Med Sci. 2014;10(2):251-257. doi: 10.5114/aoms.2014.42577. PubMed
3. Einhorn LM, Zhan M, Hsu VD, et al. The frequency of hyperkalemia and its significance in chronic kidney disease. Arch Intern Med. 2009;169(12):1156-1162. doi: 10.1001/archinternmed.2009.132. PubMed
4. Harel Z, Kamel KS. Optimal dose and method of administration of intravenous insulin in the management of emergency hyperkalemia: a systematic review. PLoS One. 2016;11(5):e0154963. doi: 10.1371/journal.pone.0154963. PubMed
5. Allon M, Copkney C. Albuterol and insulin for treatment of hyperkalemia in hemodialysis patients. Kidney Int. 1990;38(5):869-872. doi: 10.1038/ki.1990.284. PubMed
6. Apel J, Reutrakul S, Baldwin D. Hypoglycemia in the treatment of hyperkalemia with insulin in patients with end-stage renal disease. Clin Kidney J. 2014;7(3):248-250. doi: 10.1093/ckj/sfu026. PubMed
7. Coca A, Valencia AL, Bustamante J, Mendiluce A, Floege J. Hypoglycemia following intravenous insulin plus glucose for hyperkalemia in patients with impaired renal function. PLoS One. 2017;12(2):e0172961. doi: 10.1371/journal.pone.0172961. PubMed
8. Schafers S, Naunheim R, Vijayan A, Tobin G. Incidence of hypoglycemia following insulin-based acute stabilization of hyperkalemia treatment. J Hosp Med. 2012;7(3):239-242. doi: 10.1002/jhm.977. PubMed
9. Nirantharakumar K, Marshall T, Kennedy A, Narendran P, Hemming K, Coleman JJ. Hypoglycaemia is associated with increased length of stay and mortality in people with diabetes who are hospitalized. Diabet Med. 2012;29(12):e445-e448. doi: 10.1111/dme.12002. PubMed
10. UK Renal Association Clinical Practice Guidelines: Treatment of acute hyperkalaemia in adults. https://renal.org/guidelines/. Published March 2014. Accessed 1October 2018.
11. National Diabetes Inpatient Audit, England and Wales, 2017 - Full Report. NHS Digital. https://digital.nhs.uk/data-and-information/publications/statistical/national-diabetes-inpatient-audit/national-diabetes-inpatient-audit-nadia-2017. Published 14 March 2018. Accessed 5 November 2018.
12. Scott NL, Klein L, Cales E, Driver B. Hypoglycemia as a complication of intravenous insulin to treat hyperkalemia in the emergency department. Am J Emerg Med. 2018; 30379-30386. doi: 10.1016/j.ajem.2018.05.016. PubMed
13. Wheeler DT, Schafers SJ, Horwedel TA, Deal EN, Tobin GS. Weight-based insulin dosing for acute hyperkalemia results in less hypoglycemia. J Hosp Med. 2016;11(5):355-357. doi: 10.1002/jhm.2545. PubMed
14. Spoto B, Pisano A, Zoccali C. Insulin resistance in chronic kidney disease: a systematic review. Am J Physiol Renal Physiol. 2016;311(6):F1087-F1108. doi: 10.1152/ajprenal.00340.2016. PubMed
15. Summary Hospital-level Mortality Indicator (SHMI) - Deaths associated with hospitalisation, England, April 2017 - March 2018. NHS Digital. https://digital.nhs.uk/data-and-information/publications/clinical-indicators/shmi/archive/shmi-april-2017---march-2018. Published 20 September 2018. Accessed 5 November 2018.
© 2019 Society of Hospital Medicine
National Survey of Hospitalists’ Experiences with Incidental Pulmonary Nodules
Pulmonary nodules are common, and their identification is increasing as a result of the use of more sensitive chest imaging modalities.1 Pulmonary nodules are defined on imaging as small (≤30 mm), well-defined lesions, completely surrounded by pulmonary parenchyma.2 Most of the pulmonary nodules detected incidentally (ie, in asymptomatic patients outside the context of chest CT screening for lung cancer) are benign.1 Lesions >30 mm are defined as masses and have higher risks of malignancy.2
Because the majority of patients will not benefit from the identification of incidental pulmonary nodules (IPNs), improving the benefits and minimizing the harms of IPN follow-up are critical. The Fleischner Society3 published their first guideline on the management of solid IPNs in 2005,4 which was supplemented in 2013 with specific guidance for the management of subsolid IPNs.5 In 2017, both guidelines were combined in a single update.6 The Fleischner Society recommendations for imaging surveillance and tissue sampling are based on nodule type (solid vs subsolid), number (single vs multiple), size, appearance, and patient risk for malignancy.
For IPNs identified in the hospital, management may be particularly challenging. For one, the provider initially ordering the chest imaging may not be the provider coordinating the patient’s discharge, leading to a lack of knowledge that the IPN even exists. The hospitalist to primary care provider (PCP) handoff may also have vulnerabilities, including the lack of inclusion of the IPN follow-up in the discharge summary and the nonreceipt of the discharge summary by the PCP. Moreover, because a patient’s acute medical problems often take precedence during a hospitalization, inpatients may not even be made aware of identified IPNs and the need for follow-up. Thus, the absence of standardized approaches to managing IPNs is a threat to patient safety, as well as a legal liability for providers and their institutions.
To better understand the current state of IPN management in our own institution, we examined the management of IPNs identified by chest computed tomographies (CTs) performed for inpatients on our general medicine services over a two-year period.7 Among the 50 inpatients identified with IPNs requiring follow-up, 78% had no follow-up imaging documented. Moreover, 40% had no mention of the IPN in their hospital summary or discharge instructions.
To inform our approach to addressing this challenge, we sought to examine the practices of hospitalist physicians nationally regarding the management of IPNs, including hospitalists’ familiarity with the Fleischner Society guidelines.
METHODS
We developed a 14-item survey to assess hospitalists’ exposure to and management of IPNs. The survey targeted attendees of the 2016 Society of Hospital Medicine (SHM) annual conference and was available for completion on a tablet at the conference registration desk, the SHM kiosk in the exhibit hall, and at the entrance and exit of the morning plenary sessions. Following the annual conference, the survey was e-mailed to conference attendees, with one follow-up e-mailed to nonresponders.
Analyses were descriptive and included proportions for categorical variables and median and mean values and standard deviations for continuous variables. In addition, we examined the association between survey items and a response of “yes” to the question “Are you familiar with the Fleischner Society guidelines for the management of incidental pulmonary nodules?”
Associations between familiarity with the Fleischner Society guidelines and survey items were examined using Pearson’s chi-square test for categorical variables, Fisher’s exact test for categorical variables with small sample sizes, the Cochran–Armitage test for trend for ordinal variables, and the t-test for continuous variables. The associations between categorical items were measured by odds ratios with 95% confidence intervals. Statistical tests were two-sided using a P =.05 level for statistical significance. All analyses were performed using R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria), with the R packages MASS, stats, and Publish. Institutional review board exemption was granted.
RESULTS
We received 174 responses from a total of 3,954 conference attendees. The majority were identified as hospitalist physicians, and most of them were internists (Table 1). About half practiced at a university or a teaching hospital, and more than half supervised trainees and practiced for more than five years. Respondents were involved in direct patient care (whether a teaching or a nonteaching service) for a median of 28 weeks annually (mean 31.2 weeks, standard deviation 13.5), and practice regions were geographically diverse. All respondents reported seeing at least one IPN case in the past six months, with most seeing three or more cases (Table 2). Despite this exposure, 42% were unfamiliar with the Fleischner Society guidelines. When determining the need for IPN follow-up, most of them utilized radiology report recommendations or consulted national or international guidelines, and a third spoke with radiologists directly. About a third agreed that determining the need for follow-up was challenging, with 39% citing patient factors (eg, lack of insurance, poor access to healthcare), and 30% citing scheduling of follow-up imaging. Few reported the availability of an automated tracking system at their institution, although most of them desired automatic notifications of results requiring follow-up.
Unadjusted analyses revealed that supervision of trainees and seeing more IPN cases significantly increased the odds of a survey respondent being familiar with the Fleischner Society guidelines (OR 1.96, 95% CI 1.04-3.68, P =.05, and OR 1.55, 95% CI 1.12-2.18, P =.008, respectively; Supplementary Table 1).
DISCUSSION
To our knowledge, the survey reported here is the first to examine hospitalists’ knowledge of the Fleischner Society guidelines and their approach to management of IPNs. Although our data suggest that hospitalists are less familiar with the Fleischner Society recommendations than pulmonologists8 and radiologists,8-10 the majority of hospitalists in our study rely on radiology report recommendations to inform follow-up. This suggests that embedding the Fleischner Society recommendations into radiology reports is an effective method to promote adherence to these recommendations, which has been demonstrated in previous research.11-13 Our study also suggests that hospitalists with more IPN exposure and those who supervise trainees are more likely to be aware of the Fleischner Society recommendations, which is similar to findings from studies examining radiologists and pulmonologists.8-9
Our findings highlight other opportunities for quality improvement in IPN management. Almost a quarter of hospitalists reported formally consulting pulmonologists for IPN management. Hospitalist groups wishing to improve value could partner with their radiology departments and embed the Fleischner Society recommendations into their imaging reports to potentially reduce unnecessary pulmonary consultations. Among the 59 hospitalists who agreed that IPN management was challenging, a majority cited the scheduling process (30%) as a barrier. Redesigning the scheduling process for follow-up imaging could be a focus in local efforts to improve IPN management. Strengthening communication between hospitalists and PCPs may provide additional opportunities for improved IPN follow-up, given the centrality of PCPs to ensuring such follow-up. This might include enhancing direct communication between hospitalists and PCPs for high-risk patients, or creating systems to ensure robust indirect communication, such as the implementation of standardized discharge summaries that uniformly include essential follow-up information.
At our institution, given the large volume of high-risk patients and imaging performed, and the available resources, we have established an IPN consult team to improve follow-up for inpatients with IPNs identified by chest CTs on Medicine services. The team includes a nurse practitioner (NP) and a pulmonologist who consult by default, to notify patients of their findings and recommended follow-up, and communicate results to their PCPs. The IPN consult team also sees patients for follow-up in the ambulatory IPN clinic. This initiative has addressed the most frequently cited challenges identified in our nationwide hospitalist survey by taking the communication and follow-up out of the hospitalists’ hands. To ensure identification of all IPNs by the NP, our radiology department has created a structured template for radiology attendings to document follow-up for all chest CTs reviewed based on the Fleischner Society guidelines. Compliance with use of the template by radiologists is followed monthly. After a run-in period, almost 100% of chest CT reports use the structured template, consistent with published findings from similar initiatives,14 and 100% of patients with new IPNs identified on the inpatient Medicine services have had an IPN consult.
The major limitation of our survey study is the response rate. It is difficult to determine in what direction this could bias our results, as those with and without experience in managing IPNs may have been equally likely to complete the survey. Despite the low response rate, our sample targeted the general cohort of conference attendees (rather than specific forums such as audiences interested in quality or imaging), and the descriptive characteristics of our convenience sample align well with the overall conference attendee demographics (eg, conference attendees were 77% hospitalist attendings and 9% advanced practice providers, as compared with 82% and 7% of survey respondents, respectively), suggesting that our respondents were representative of conference attendees as a whole.
Next steps for this work at our institution include developing systems to ensure appropriate follow-up for those with IPNs identified on chest CTs performed for Medicine outpatients. In addition, our institution is collaborating on a national study to compare outcomes resulting from following the traditional Fleischner Society recommendations compared to the new 2017 recommendations, which recommend more lenient follow-up.15
Acknowledgments
The authors acknowledge Vivek Ahya, Eduardo Barbosa Jr., Tammy Tursi, and Anil Vachani for their leadership of the local quality improvement initiatives described in our Discussion, namely, the development and implementation of the structured templates for radiology reports and the incidental pulmonary nodule consult team.
Disclosures
Dr. Cook reports relevant financial activity outside the submitted work, including royalties from Osler Institute and grants from ACRIN and Beryl Institute. All other authors report no potential conflicts of interest relevant to this study. There was no financial support for this study.
Previous Presentations
Presented as a poster at the 2017 Society of Hospital Medicine Annual Conference, Las Vegas, NV: Wilen J, Garin M, Umscheid CA, Cook TS, Myers JS. Follow-up of incidental pulmonary nodules: a survey of hospitalists nationwide [abstract]. Journal of Hospital Medicine. 2017; 12 (Suppl 2). Available at: https://www.shmabstracts.com/abstract/follow-up-of-incidental-pulmonary-nodules-a-survey-of-hospitalists-nationwide/. Accessed March 18, 2018.
1. Ost D, Fein AM, Feinsilver SH. Clinical Practice: the solitary pulmonary nodule. N Engl J Med. 2003;348(25):2535-2542. doi: 10.1056/NEJMcp012290 PubMed
2. Tuddenham WJ. Glossary of terms for thoracic radiology: recommendations of the Nomenclature Committee of the Fleischner Society. AJR Am J Roentgenol. 1984;143(3):509-517. PubMed
3. Janower ML. A brief history of the Fleischner Society. J Thorac Imaging. 2010;25(1):27-28. doi: 10.1097/RTI.0b013e3181cc4cee. PubMed
4. Macmahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400. doi: 10.1148/radiol.2372041887. PubMed
5. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317. doi: 10.1148/radiol.12120628. PubMed
6. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology.2017;284(1):228-243. doi: 10.1148/radiol.2017161659. PubMed
7. Garin M, Soran C, Cook T, Ferguson M, Day S, Myers JS. Communication and follow-up of incidental lung nodules found on chest CT in a hospitalized and ambulatory patient population. J Hosp Med. 2014:9(2). Available at: https://www.shmabstracts.com/abstract/communication-and-followup-of-incidental-lung-nodules-found-on-chest-ct-in-a-hospitalized-and-ambulatory-patient-population/ Accessed June 14, 2018.
8. Mets OM, de Jong PA, Chung K, Lammers JWJ, van Ginneken B, Schaefer-Prokop CM. Fleischner recommendations for the management of subsolid pulmonary nodules: high awareness but limited conformance – a survey study. Eur Radiol. 2016;26:3840-3849. doi: 10.1007/s00330-016-4249-y. PubMed
9. Eisenberg RL, Bankier AA, Boiselle PM. Compliance with Fleischner Society Guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology. 2010;255(1):218-224. doi: 10.1148/radiol.09091556. PubMed
10. Eisenberg RL. Ways to improve radiologists’ adherence to Fleischner society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441. doi: 10.1016/j.jacr.2012.10.001. PubMed
11. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. doi: 10.1016/j.jacr.2013.08.003. PubMed
12. Woloshin S, Schwartz LM, Dann E, Black WC. Using radiology reports to encourage evidence-based practice in the evaluation of small, incidentally detected pulmonary nodules: a preliminary study. Ann Am Thorac Soc. 2014;11(2):211-214. doi: 10.1513/AnnalsATS.201307-242BC. PubMed
13. McDonald JS, Koo CW, White D, Hartman TE, Bender CE, Sykes AMG. Addition of the Fleischner Society Guidelines to chest CT examination interpretive reports improves adherence to recommended follow-up care for incidental pulmonary nodules. Acad Radiol. 2017;24(3):337-344. doi: 10.1016/j.acra.2016.08.026. PubMed
14. Zygmont ME, Shekhani H, Kerchberger JM, Johnson JO, Hanna TN. Point-of-Care reference materials increase practice compliance with societal guidelines for incidental findings in emergency imaging. J Am Coll Radiol. 2016;13(12):1494-1500. doi: 10.1016/j.jacr.2016.07.032. PubMed
15. Patient-Centered Outcomes Research Institute Portfolio of Funded Projects. Available at: https://www.pcori.org/research-results/2015/pragmatic-trial-more-versus-less-intensive-strategies-active-surveillance#/. Accessed May 22, 2018.
Pulmonary nodules are common, and their identification is increasing as a result of the use of more sensitive chest imaging modalities.1 Pulmonary nodules are defined on imaging as small (≤30 mm), well-defined lesions, completely surrounded by pulmonary parenchyma.2 Most of the pulmonary nodules detected incidentally (ie, in asymptomatic patients outside the context of chest CT screening for lung cancer) are benign.1 Lesions >30 mm are defined as masses and have higher risks of malignancy.2
Because the majority of patients will not benefit from the identification of incidental pulmonary nodules (IPNs), improving the benefits and minimizing the harms of IPN follow-up are critical. The Fleischner Society3 published their first guideline on the management of solid IPNs in 2005,4 which was supplemented in 2013 with specific guidance for the management of subsolid IPNs.5 In 2017, both guidelines were combined in a single update.6 The Fleischner Society recommendations for imaging surveillance and tissue sampling are based on nodule type (solid vs subsolid), number (single vs multiple), size, appearance, and patient risk for malignancy.
For IPNs identified in the hospital, management may be particularly challenging. For one, the provider initially ordering the chest imaging may not be the provider coordinating the patient’s discharge, leading to a lack of knowledge that the IPN even exists. The hospitalist to primary care provider (PCP) handoff may also have vulnerabilities, including the lack of inclusion of the IPN follow-up in the discharge summary and the nonreceipt of the discharge summary by the PCP. Moreover, because a patient’s acute medical problems often take precedence during a hospitalization, inpatients may not even be made aware of identified IPNs and the need for follow-up. Thus, the absence of standardized approaches to managing IPNs is a threat to patient safety, as well as a legal liability for providers and their institutions.
To better understand the current state of IPN management in our own institution, we examined the management of IPNs identified by chest computed tomographies (CTs) performed for inpatients on our general medicine services over a two-year period.7 Among the 50 inpatients identified with IPNs requiring follow-up, 78% had no follow-up imaging documented. Moreover, 40% had no mention of the IPN in their hospital summary or discharge instructions.
To inform our approach to addressing this challenge, we sought to examine the practices of hospitalist physicians nationally regarding the management of IPNs, including hospitalists’ familiarity with the Fleischner Society guidelines.
METHODS
We developed a 14-item survey to assess hospitalists’ exposure to and management of IPNs. The survey targeted attendees of the 2016 Society of Hospital Medicine (SHM) annual conference and was available for completion on a tablet at the conference registration desk, the SHM kiosk in the exhibit hall, and at the entrance and exit of the morning plenary sessions. Following the annual conference, the survey was e-mailed to conference attendees, with one follow-up e-mailed to nonresponders.
Analyses were descriptive and included proportions for categorical variables and median and mean values and standard deviations for continuous variables. In addition, we examined the association between survey items and a response of “yes” to the question “Are you familiar with the Fleischner Society guidelines for the management of incidental pulmonary nodules?”
Associations between familiarity with the Fleischner Society guidelines and survey items were examined using Pearson’s chi-square test for categorical variables, Fisher’s exact test for categorical variables with small sample sizes, the Cochran–Armitage test for trend for ordinal variables, and the t-test for continuous variables. The associations between categorical items were measured by odds ratios with 95% confidence intervals. Statistical tests were two-sided using a P =.05 level for statistical significance. All analyses were performed using R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria), with the R packages MASS, stats, and Publish. Institutional review board exemption was granted.
RESULTS
We received 174 responses from a total of 3,954 conference attendees. The majority were identified as hospitalist physicians, and most of them were internists (Table 1). About half practiced at a university or a teaching hospital, and more than half supervised trainees and practiced for more than five years. Respondents were involved in direct patient care (whether a teaching or a nonteaching service) for a median of 28 weeks annually (mean 31.2 weeks, standard deviation 13.5), and practice regions were geographically diverse. All respondents reported seeing at least one IPN case in the past six months, with most seeing three or more cases (Table 2). Despite this exposure, 42% were unfamiliar with the Fleischner Society guidelines. When determining the need for IPN follow-up, most of them utilized radiology report recommendations or consulted national or international guidelines, and a third spoke with radiologists directly. About a third agreed that determining the need for follow-up was challenging, with 39% citing patient factors (eg, lack of insurance, poor access to healthcare), and 30% citing scheduling of follow-up imaging. Few reported the availability of an automated tracking system at their institution, although most of them desired automatic notifications of results requiring follow-up.
Unadjusted analyses revealed that supervision of trainees and seeing more IPN cases significantly increased the odds of a survey respondent being familiar with the Fleischner Society guidelines (OR 1.96, 95% CI 1.04-3.68, P =.05, and OR 1.55, 95% CI 1.12-2.18, P =.008, respectively; Supplementary Table 1).
DISCUSSION
To our knowledge, the survey reported here is the first to examine hospitalists’ knowledge of the Fleischner Society guidelines and their approach to management of IPNs. Although our data suggest that hospitalists are less familiar with the Fleischner Society recommendations than pulmonologists8 and radiologists,8-10 the majority of hospitalists in our study rely on radiology report recommendations to inform follow-up. This suggests that embedding the Fleischner Society recommendations into radiology reports is an effective method to promote adherence to these recommendations, which has been demonstrated in previous research.11-13 Our study also suggests that hospitalists with more IPN exposure and those who supervise trainees are more likely to be aware of the Fleischner Society recommendations, which is similar to findings from studies examining radiologists and pulmonologists.8-9
Our findings highlight other opportunities for quality improvement in IPN management. Almost a quarter of hospitalists reported formally consulting pulmonologists for IPN management. Hospitalist groups wishing to improve value could partner with their radiology departments and embed the Fleischner Society recommendations into their imaging reports to potentially reduce unnecessary pulmonary consultations. Among the 59 hospitalists who agreed that IPN management was challenging, a majority cited the scheduling process (30%) as a barrier. Redesigning the scheduling process for follow-up imaging could be a focus in local efforts to improve IPN management. Strengthening communication between hospitalists and PCPs may provide additional opportunities for improved IPN follow-up, given the centrality of PCPs to ensuring such follow-up. This might include enhancing direct communication between hospitalists and PCPs for high-risk patients, or creating systems to ensure robust indirect communication, such as the implementation of standardized discharge summaries that uniformly include essential follow-up information.
At our institution, given the large volume of high-risk patients and imaging performed, and the available resources, we have established an IPN consult team to improve follow-up for inpatients with IPNs identified by chest CTs on Medicine services. The team includes a nurse practitioner (NP) and a pulmonologist who consult by default, to notify patients of their findings and recommended follow-up, and communicate results to their PCPs. The IPN consult team also sees patients for follow-up in the ambulatory IPN clinic. This initiative has addressed the most frequently cited challenges identified in our nationwide hospitalist survey by taking the communication and follow-up out of the hospitalists’ hands. To ensure identification of all IPNs by the NP, our radiology department has created a structured template for radiology attendings to document follow-up for all chest CTs reviewed based on the Fleischner Society guidelines. Compliance with use of the template by radiologists is followed monthly. After a run-in period, almost 100% of chest CT reports use the structured template, consistent with published findings from similar initiatives,14 and 100% of patients with new IPNs identified on the inpatient Medicine services have had an IPN consult.
The major limitation of our survey study is the response rate. It is difficult to determine in what direction this could bias our results, as those with and without experience in managing IPNs may have been equally likely to complete the survey. Despite the low response rate, our sample targeted the general cohort of conference attendees (rather than specific forums such as audiences interested in quality or imaging), and the descriptive characteristics of our convenience sample align well with the overall conference attendee demographics (eg, conference attendees were 77% hospitalist attendings and 9% advanced practice providers, as compared with 82% and 7% of survey respondents, respectively), suggesting that our respondents were representative of conference attendees as a whole.
Next steps for this work at our institution include developing systems to ensure appropriate follow-up for those with IPNs identified on chest CTs performed for Medicine outpatients. In addition, our institution is collaborating on a national study to compare outcomes resulting from following the traditional Fleischner Society recommendations compared to the new 2017 recommendations, which recommend more lenient follow-up.15
Acknowledgments
The authors acknowledge Vivek Ahya, Eduardo Barbosa Jr., Tammy Tursi, and Anil Vachani for their leadership of the local quality improvement initiatives described in our Discussion, namely, the development and implementation of the structured templates for radiology reports and the incidental pulmonary nodule consult team.
Disclosures
Dr. Cook reports relevant financial activity outside the submitted work, including royalties from Osler Institute and grants from ACRIN and Beryl Institute. All other authors report no potential conflicts of interest relevant to this study. There was no financial support for this study.
Previous Presentations
Presented as a poster at the 2017 Society of Hospital Medicine Annual Conference, Las Vegas, NV: Wilen J, Garin M, Umscheid CA, Cook TS, Myers JS. Follow-up of incidental pulmonary nodules: a survey of hospitalists nationwide [abstract]. Journal of Hospital Medicine. 2017; 12 (Suppl 2). Available at: https://www.shmabstracts.com/abstract/follow-up-of-incidental-pulmonary-nodules-a-survey-of-hospitalists-nationwide/. Accessed March 18, 2018.
Pulmonary nodules are common, and their identification is increasing as a result of the use of more sensitive chest imaging modalities.1 Pulmonary nodules are defined on imaging as small (≤30 mm), well-defined lesions, completely surrounded by pulmonary parenchyma.2 Most of the pulmonary nodules detected incidentally (ie, in asymptomatic patients outside the context of chest CT screening for lung cancer) are benign.1 Lesions >30 mm are defined as masses and have higher risks of malignancy.2
Because the majority of patients will not benefit from the identification of incidental pulmonary nodules (IPNs), improving the benefits and minimizing the harms of IPN follow-up are critical. The Fleischner Society3 published their first guideline on the management of solid IPNs in 2005,4 which was supplemented in 2013 with specific guidance for the management of subsolid IPNs.5 In 2017, both guidelines were combined in a single update.6 The Fleischner Society recommendations for imaging surveillance and tissue sampling are based on nodule type (solid vs subsolid), number (single vs multiple), size, appearance, and patient risk for malignancy.
For IPNs identified in the hospital, management may be particularly challenging. For one, the provider initially ordering the chest imaging may not be the provider coordinating the patient’s discharge, leading to a lack of knowledge that the IPN even exists. The hospitalist to primary care provider (PCP) handoff may also have vulnerabilities, including the lack of inclusion of the IPN follow-up in the discharge summary and the nonreceipt of the discharge summary by the PCP. Moreover, because a patient’s acute medical problems often take precedence during a hospitalization, inpatients may not even be made aware of identified IPNs and the need for follow-up. Thus, the absence of standardized approaches to managing IPNs is a threat to patient safety, as well as a legal liability for providers and their institutions.
To better understand the current state of IPN management in our own institution, we examined the management of IPNs identified by chest computed tomographies (CTs) performed for inpatients on our general medicine services over a two-year period.7 Among the 50 inpatients identified with IPNs requiring follow-up, 78% had no follow-up imaging documented. Moreover, 40% had no mention of the IPN in their hospital summary or discharge instructions.
To inform our approach to addressing this challenge, we sought to examine the practices of hospitalist physicians nationally regarding the management of IPNs, including hospitalists’ familiarity with the Fleischner Society guidelines.
METHODS
We developed a 14-item survey to assess hospitalists’ exposure to and management of IPNs. The survey targeted attendees of the 2016 Society of Hospital Medicine (SHM) annual conference and was available for completion on a tablet at the conference registration desk, the SHM kiosk in the exhibit hall, and at the entrance and exit of the morning plenary sessions. Following the annual conference, the survey was e-mailed to conference attendees, with one follow-up e-mailed to nonresponders.
Analyses were descriptive and included proportions for categorical variables and median and mean values and standard deviations for continuous variables. In addition, we examined the association between survey items and a response of “yes” to the question “Are you familiar with the Fleischner Society guidelines for the management of incidental pulmonary nodules?”
Associations between familiarity with the Fleischner Society guidelines and survey items were examined using Pearson’s chi-square test for categorical variables, Fisher’s exact test for categorical variables with small sample sizes, the Cochran–Armitage test for trend for ordinal variables, and the t-test for continuous variables. The associations between categorical items were measured by odds ratios with 95% confidence intervals. Statistical tests were two-sided using a P =.05 level for statistical significance. All analyses were performed using R version 3.4.4 (R Foundation for Statistical Computing, Vienna, Austria), with the R packages MASS, stats, and Publish. Institutional review board exemption was granted.
RESULTS
We received 174 responses from a total of 3,954 conference attendees. The majority were identified as hospitalist physicians, and most of them were internists (Table 1). About half practiced at a university or a teaching hospital, and more than half supervised trainees and practiced for more than five years. Respondents were involved in direct patient care (whether a teaching or a nonteaching service) for a median of 28 weeks annually (mean 31.2 weeks, standard deviation 13.5), and practice regions were geographically diverse. All respondents reported seeing at least one IPN case in the past six months, with most seeing three or more cases (Table 2). Despite this exposure, 42% were unfamiliar with the Fleischner Society guidelines. When determining the need for IPN follow-up, most of them utilized radiology report recommendations or consulted national or international guidelines, and a third spoke with radiologists directly. About a third agreed that determining the need for follow-up was challenging, with 39% citing patient factors (eg, lack of insurance, poor access to healthcare), and 30% citing scheduling of follow-up imaging. Few reported the availability of an automated tracking system at their institution, although most of them desired automatic notifications of results requiring follow-up.
Unadjusted analyses revealed that supervision of trainees and seeing more IPN cases significantly increased the odds of a survey respondent being familiar with the Fleischner Society guidelines (OR 1.96, 95% CI 1.04-3.68, P =.05, and OR 1.55, 95% CI 1.12-2.18, P =.008, respectively; Supplementary Table 1).
DISCUSSION
To our knowledge, the survey reported here is the first to examine hospitalists’ knowledge of the Fleischner Society guidelines and their approach to management of IPNs. Although our data suggest that hospitalists are less familiar with the Fleischner Society recommendations than pulmonologists8 and radiologists,8-10 the majority of hospitalists in our study rely on radiology report recommendations to inform follow-up. This suggests that embedding the Fleischner Society recommendations into radiology reports is an effective method to promote adherence to these recommendations, which has been demonstrated in previous research.11-13 Our study also suggests that hospitalists with more IPN exposure and those who supervise trainees are more likely to be aware of the Fleischner Society recommendations, which is similar to findings from studies examining radiologists and pulmonologists.8-9
Our findings highlight other opportunities for quality improvement in IPN management. Almost a quarter of hospitalists reported formally consulting pulmonologists for IPN management. Hospitalist groups wishing to improve value could partner with their radiology departments and embed the Fleischner Society recommendations into their imaging reports to potentially reduce unnecessary pulmonary consultations. Among the 59 hospitalists who agreed that IPN management was challenging, a majority cited the scheduling process (30%) as a barrier. Redesigning the scheduling process for follow-up imaging could be a focus in local efforts to improve IPN management. Strengthening communication between hospitalists and PCPs may provide additional opportunities for improved IPN follow-up, given the centrality of PCPs to ensuring such follow-up. This might include enhancing direct communication between hospitalists and PCPs for high-risk patients, or creating systems to ensure robust indirect communication, such as the implementation of standardized discharge summaries that uniformly include essential follow-up information.
At our institution, given the large volume of high-risk patients and imaging performed, and the available resources, we have established an IPN consult team to improve follow-up for inpatients with IPNs identified by chest CTs on Medicine services. The team includes a nurse practitioner (NP) and a pulmonologist who consult by default, to notify patients of their findings and recommended follow-up, and communicate results to their PCPs. The IPN consult team also sees patients for follow-up in the ambulatory IPN clinic. This initiative has addressed the most frequently cited challenges identified in our nationwide hospitalist survey by taking the communication and follow-up out of the hospitalists’ hands. To ensure identification of all IPNs by the NP, our radiology department has created a structured template for radiology attendings to document follow-up for all chest CTs reviewed based on the Fleischner Society guidelines. Compliance with use of the template by radiologists is followed monthly. After a run-in period, almost 100% of chest CT reports use the structured template, consistent with published findings from similar initiatives,14 and 100% of patients with new IPNs identified on the inpatient Medicine services have had an IPN consult.
The major limitation of our survey study is the response rate. It is difficult to determine in what direction this could bias our results, as those with and without experience in managing IPNs may have been equally likely to complete the survey. Despite the low response rate, our sample targeted the general cohort of conference attendees (rather than specific forums such as audiences interested in quality or imaging), and the descriptive characteristics of our convenience sample align well with the overall conference attendee demographics (eg, conference attendees were 77% hospitalist attendings and 9% advanced practice providers, as compared with 82% and 7% of survey respondents, respectively), suggesting that our respondents were representative of conference attendees as a whole.
Next steps for this work at our institution include developing systems to ensure appropriate follow-up for those with IPNs identified on chest CTs performed for Medicine outpatients. In addition, our institution is collaborating on a national study to compare outcomes resulting from following the traditional Fleischner Society recommendations compared to the new 2017 recommendations, which recommend more lenient follow-up.15
Acknowledgments
The authors acknowledge Vivek Ahya, Eduardo Barbosa Jr., Tammy Tursi, and Anil Vachani for their leadership of the local quality improvement initiatives described in our Discussion, namely, the development and implementation of the structured templates for radiology reports and the incidental pulmonary nodule consult team.
Disclosures
Dr. Cook reports relevant financial activity outside the submitted work, including royalties from Osler Institute and grants from ACRIN and Beryl Institute. All other authors report no potential conflicts of interest relevant to this study. There was no financial support for this study.
Previous Presentations
Presented as a poster at the 2017 Society of Hospital Medicine Annual Conference, Las Vegas, NV: Wilen J, Garin M, Umscheid CA, Cook TS, Myers JS. Follow-up of incidental pulmonary nodules: a survey of hospitalists nationwide [abstract]. Journal of Hospital Medicine. 2017; 12 (Suppl 2). Available at: https://www.shmabstracts.com/abstract/follow-up-of-incidental-pulmonary-nodules-a-survey-of-hospitalists-nationwide/. Accessed March 18, 2018.
1. Ost D, Fein AM, Feinsilver SH. Clinical Practice: the solitary pulmonary nodule. N Engl J Med. 2003;348(25):2535-2542. doi: 10.1056/NEJMcp012290 PubMed
2. Tuddenham WJ. Glossary of terms for thoracic radiology: recommendations of the Nomenclature Committee of the Fleischner Society. AJR Am J Roentgenol. 1984;143(3):509-517. PubMed
3. Janower ML. A brief history of the Fleischner Society. J Thorac Imaging. 2010;25(1):27-28. doi: 10.1097/RTI.0b013e3181cc4cee. PubMed
4. Macmahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400. doi: 10.1148/radiol.2372041887. PubMed
5. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317. doi: 10.1148/radiol.12120628. PubMed
6. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology.2017;284(1):228-243. doi: 10.1148/radiol.2017161659. PubMed
7. Garin M, Soran C, Cook T, Ferguson M, Day S, Myers JS. Communication and follow-up of incidental lung nodules found on chest CT in a hospitalized and ambulatory patient population. J Hosp Med. 2014:9(2). Available at: https://www.shmabstracts.com/abstract/communication-and-followup-of-incidental-lung-nodules-found-on-chest-ct-in-a-hospitalized-and-ambulatory-patient-population/ Accessed June 14, 2018.
8. Mets OM, de Jong PA, Chung K, Lammers JWJ, van Ginneken B, Schaefer-Prokop CM. Fleischner recommendations for the management of subsolid pulmonary nodules: high awareness but limited conformance – a survey study. Eur Radiol. 2016;26:3840-3849. doi: 10.1007/s00330-016-4249-y. PubMed
9. Eisenberg RL, Bankier AA, Boiselle PM. Compliance with Fleischner Society Guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology. 2010;255(1):218-224. doi: 10.1148/radiol.09091556. PubMed
10. Eisenberg RL. Ways to improve radiologists’ adherence to Fleischner society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441. doi: 10.1016/j.jacr.2012.10.001. PubMed
11. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. doi: 10.1016/j.jacr.2013.08.003. PubMed
12. Woloshin S, Schwartz LM, Dann E, Black WC. Using radiology reports to encourage evidence-based practice in the evaluation of small, incidentally detected pulmonary nodules: a preliminary study. Ann Am Thorac Soc. 2014;11(2):211-214. doi: 10.1513/AnnalsATS.201307-242BC. PubMed
13. McDonald JS, Koo CW, White D, Hartman TE, Bender CE, Sykes AMG. Addition of the Fleischner Society Guidelines to chest CT examination interpretive reports improves adherence to recommended follow-up care for incidental pulmonary nodules. Acad Radiol. 2017;24(3):337-344. doi: 10.1016/j.acra.2016.08.026. PubMed
14. Zygmont ME, Shekhani H, Kerchberger JM, Johnson JO, Hanna TN. Point-of-Care reference materials increase practice compliance with societal guidelines for incidental findings in emergency imaging. J Am Coll Radiol. 2016;13(12):1494-1500. doi: 10.1016/j.jacr.2016.07.032. PubMed
15. Patient-Centered Outcomes Research Institute Portfolio of Funded Projects. Available at: https://www.pcori.org/research-results/2015/pragmatic-trial-more-versus-less-intensive-strategies-active-surveillance#/. Accessed May 22, 2018.
1. Ost D, Fein AM, Feinsilver SH. Clinical Practice: the solitary pulmonary nodule. N Engl J Med. 2003;348(25):2535-2542. doi: 10.1056/NEJMcp012290 PubMed
2. Tuddenham WJ. Glossary of terms for thoracic radiology: recommendations of the Nomenclature Committee of the Fleischner Society. AJR Am J Roentgenol. 1984;143(3):509-517. PubMed
3. Janower ML. A brief history of the Fleischner Society. J Thorac Imaging. 2010;25(1):27-28. doi: 10.1097/RTI.0b013e3181cc4cee. PubMed
4. Macmahon H, Austin JH, Gamsu G, et al. Guidelines for management of small pulmonary nodules detected on CT scans: a statement from the Fleischner Society. Radiology. 2005;237(2):395-400. doi: 10.1148/radiol.2372041887. PubMed
5. Naidich DP, Bankier AA, MacMahon H, et al. Recommendations for the management of subsolid pulmonary nodules detected at CT: a statement from the Fleischner Society. Radiology. 2013;266(1):304-317. doi: 10.1148/radiol.12120628. PubMed
6. MacMahon H, Naidich DP, Goo JM, et al. Guidelines for management of incidental pulmonary nodules detected on CT images: from the Fleischner Society 2017. Radiology.2017;284(1):228-243. doi: 10.1148/radiol.2017161659. PubMed
7. Garin M, Soran C, Cook T, Ferguson M, Day S, Myers JS. Communication and follow-up of incidental lung nodules found on chest CT in a hospitalized and ambulatory patient population. J Hosp Med. 2014:9(2). Available at: https://www.shmabstracts.com/abstract/communication-and-followup-of-incidental-lung-nodules-found-on-chest-ct-in-a-hospitalized-and-ambulatory-patient-population/ Accessed June 14, 2018.
8. Mets OM, de Jong PA, Chung K, Lammers JWJ, van Ginneken B, Schaefer-Prokop CM. Fleischner recommendations for the management of subsolid pulmonary nodules: high awareness but limited conformance – a survey study. Eur Radiol. 2016;26:3840-3849. doi: 10.1007/s00330-016-4249-y. PubMed
9. Eisenberg RL, Bankier AA, Boiselle PM. Compliance with Fleischner Society Guidelines for management of small lung nodules: a survey of 834 radiologists. Radiology. 2010;255(1):218-224. doi: 10.1148/radiol.09091556. PubMed
10. Eisenberg RL. Ways to improve radiologists’ adherence to Fleischner society guidelines for management of pulmonary nodules. J Am Coll Radiol. 2013;10(6):439-441. doi: 10.1016/j.jacr.2012.10.001. PubMed
11. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378-383. doi: 10.1016/j.jacr.2013.08.003. PubMed
12. Woloshin S, Schwartz LM, Dann E, Black WC. Using radiology reports to encourage evidence-based practice in the evaluation of small, incidentally detected pulmonary nodules: a preliminary study. Ann Am Thorac Soc. 2014;11(2):211-214. doi: 10.1513/AnnalsATS.201307-242BC. PubMed
13. McDonald JS, Koo CW, White D, Hartman TE, Bender CE, Sykes AMG. Addition of the Fleischner Society Guidelines to chest CT examination interpretive reports improves adherence to recommended follow-up care for incidental pulmonary nodules. Acad Radiol. 2017;24(3):337-344. doi: 10.1016/j.acra.2016.08.026. PubMed
14. Zygmont ME, Shekhani H, Kerchberger JM, Johnson JO, Hanna TN. Point-of-Care reference materials increase practice compliance with societal guidelines for incidental findings in emergency imaging. J Am Coll Radiol. 2016;13(12):1494-1500. doi: 10.1016/j.jacr.2016.07.032. PubMed
15. Patient-Centered Outcomes Research Institute Portfolio of Funded Projects. Available at: https://www.pcori.org/research-results/2015/pragmatic-trial-more-versus-less-intensive-strategies-active-surveillance#/. Accessed May 22, 2018.
© 2019 Society of Hospital Medicine
Outpatient Parenteral Antimicrobial Therapy in Vulnerable Populations—People Who Inject Drugs and the Homeless
Outpatient parenteral antimicrobial therapy (OPAT) programs allow patients to receive antibiotic therapy at home or in other settings.1-3 Bacterial infections among people who inject drugs (PWID) and the homeless are common, leading to complicated treatment strategies. Those with opioid dependence have frequent hospitalizations.4 Bacteremia and endocarditis frequently require intravenous (IV) antibiotics5-7 and may be difficult to treat. Creating outpatient treatment plans for PWID and the homeless is challenging, and there is a paucity of data on OPAT effectiveness in these groups as they are often excluded from OPAT services.1,2,8
We evaluated treatment outcomes in PWID and the homeless in our OPAT program.
METHODS
We conducted a retrospective cohort study of hospitalized adults discharged from Harborview Medical Center (HMC) with OPAT from January 1, 2015 to April 30, 2016. HMC is a county hospital in Seattle, Washington, affiliated with the University of Washington (UW). Infectious disease specialists supervise our OPAT program and provide follow-up care. We partner with a medical respite facility, a discharge option for homeless patients.9 Respite is staffed by HMC nurses, mental health specialists, and case managers.
Patients aged >18 years were enrolled in OPAT if they were discharged with >2 weeks of IV therapy or required laboratory monitoring while on oral antibiotics. Patients with multiple hospitalizations were included for their initial OPAT encounter only. PWID discharged to respite were instructed not to use their vascular access to inject drugs, but drug abstinence was not required. A tamper-evident sticker was placed over lines that nurses evaluated daily. Patients violating line-tampering restrictions were discharged from respite, and OPAT providers developed alternative antibiotic plans.
The two primary exposures evaluated were patient-reported injection drug use and housing status, and our primary exposure measure was the four-category combination: (1) housed non-PWID, (2) housed PWID, (3) homeless non-PWID, and (4) homeless PWID. Current drug use was defined as use within three months of hospitalization. Homelessness was defined as lack of stable housing. Patients receiving chemotherapy, prolonged steroids, biologic agents, or those with organ transplant were considered immunocompromised.
The primary outcome was clinical cure, defined as completion of antibiotic therapy and resolution of infection, determined by OPAT providers. Patients who were placed on oral suppressive antibiotics or died before treatment completion were considered not cured. Unknown status, including care transfer and lost to follow-up, were noted separately. Lost to follow-up was assumed if patients did not return for care, their care was not formally transferred, and no other medical information was available.
Secondary outcomes included hospital length of stay (LOS), secondary bacteremia, line-tampering, and 30-day readmissions. Secondary bacteremia was defined as bacteremia with a different pathogen from the index illness, which occurred during the initial treatment course. Readmission included readmissions related to OPAT (ie, recurrent or worsening infection, treatment-related toxicities, line-tampering, secondary bacteremia, and line-associated complications).
Data collection was performed using REDCap, a data-capturing software program linked to the electronic medical record (EMR).10 Hospitalization dates and demographics were electronically populated from the EMR. Details regarding drug use, homelessness, comorbidities, diagnosis, discharge complications, clinical cure, and lost to follow-up were manually entered.
Statistical Analysis
Statistical calculations were performed using SAS (v. 9.4). Chi-square testing and analysis of variance were conducted to assess group differences in demographics, infection types, and clinical outcomes.
Primary and secondary outcomes were further evaluated by univariable logistic regression and presented as odds ratios, with the non-PWID housed group serving as the reference. Given the large number of PWID and homeless patients lost to follow-up, sensitivity analyses were conducted using the assumption that patients with unknown clinical outcomes did not achieve cure (ie, chronic infection or death). Multivariable regression was performed on the outcomes of cure and 30-day readmission to OPAT using backward elimination to select a final model, initially including potential confounders of age, sex, and relevant comorbidities (DM and HIV). We assumed that those lost to follow-up were not cured (or readmitted). Other secondary outcomes were either rare events or those of uncertain relevance (eg, hospital LOS) to be evaluated in the multivariable analysis.
Our study did not meet the definition of research by the UW’s institutional review board. It was a quality improvement project funded by a UW Medicine Patient Safety Innovations Program Grant.
RESULTS
Overall, 596 patients received OPAT over 16 months. OPAT patients were categorized into groups as follows: homeless PWID (9%, n = 53), housed PWID (8%, n = 48), homeless non-PWID (8%, n = 45), and housed non-PWID (75%, n = 450).
PWID were younger than non-PWID, and the majority of patients in all groups were men (Table 1). PWID were more likely to have hepatitis C. Non-PWID appeared more likely to have diabetes and be immunosuppressed.
Patients had a total of 960 types of infection (Table 1). Bacteremia was the most common infection among PWID. Osteomyelitis was the most frequent infection in non-PWID.
Discharge location varied widely (P < .001; Table 1). The majority of patients with housing (housed PWID 60.4%, housed non-PWID 59.1%) were discharged to home, although 36.7% of housed non-PWID went to nursing facilities. Among homeless patients, 58.5% of PWID and 42.2% of non-PWID were discharged to respite; 10 patients were discharged to a shelter or street. Data specific to transition from IV to oral therapy were not recorded.
Cure rates among participants with known outcomes did not differ by group (Table 1; P = .85). In a sensitivity analysis of clinical cure, assuming those with unknown outcomes were not cured, housing status and drug use were significantly associated with cure (Table 1; P < .001, in the overall test), with rates lower among housed and homeless PWID groups (50.0% and 47.2%, respectively) compared with housed and homeless non-PWID groups (73.1% and 82.2%, respectively). In the multivariable analysis after backward elimination of noninfluential measures, only PWID and housing status were associated with cure; PWID, whether housed (OR = 0.37) or not (OR = 0.33), had lower odds of cure relative to housed non-PWID (Table 2).
Secondary outcomes, evaluated on all patients regardless of cure, differed by group (Table 1). Mean LOS appeared to be shortest for homeless PWID (15.5 days versus ≥18.0 for other groups; P < .001 for overall test). Homeless PWID patients appeared more likely to have secondary bacteremia (13.2% versus <4.2% in other groups; P < .001 for overall test), line tampering (35.9% versus <2.2% in other groups; P < .001), and 30-day readmission related to OPAT (26.4% versus <16.7% in other groups; P = .004). Compared with housed non-PWID using logistic regression, homeless PWID had a higher risk of secondary bacteremia (OR = 12.9; 95% CI 3.8-37.8; P < .001), line tampering (OR 88.4; 95% CI 24.5-318.3; P < .001), and readmission for OPAT (OR 2.4; 95% CI 1.2-4.6; P = .007). After adjusting for age, sex, and comorbidities, readmission for OPAT remained elevated in homeless PWID (OR = 2.4; 95% CI 1.2-4.6). No significant differences in secondary outcomes were found between housed non-PWID and also between housed PWID and homeless non-PWID.
Among homeless persons, discharge to respite care was not associated with improved outcomes, assuming those lost to follow-up did not achieve cure. Among non-PWID discharged to respite, the cure rate was 74% (14/19) compared with 88% (23/26) discharged elsewhere (P = .20). Among PWID, 48% (15/31) discharged to respite were cured compared with 45% (10/22) discharged elsewhere (P = .83).
DISCUSSION
Our study compares the outcomes of 596 OPAT patients, including PWID and the homeless. Among those retained in care, PWID achieved similar rates of cure compared with non-PWID groups. When assuming that all lost to follow-up had poor outcomes, the cure rates were markedly lower for PWID, with no difference noted by housing status.
Data on PWID and homeless enrolled in OPAT programs are limited.5,11,12 Few studies have reported the outcomes of infections in PWID and the homeless, as these populations often experience significant loss to follow-up due to transiency, lack of care continuity, and effective means of communication.
Cure was achieved in less than half of PWID, when lack of cure was assumed for unknown outcomes. This rate was substantially less than that for non-PWID groups. The assumption that those lost to follow-up did not achieve cure dramatically alters the inference; the truth may lie somewhere between the primary and sensitivity analyses. Homeless PWID remained at the highest risk for lost to follow-up, secondary bacteremia, line-tampering, and 30-day readmission related to OPAT.
PWID have traditionally been considered as a high-risk group for OPAT,1,2,8 but to completely restrict PWID from OPAT may not be appropriate. Ho et al. studied 29 PWID who were selectively enrolled to receive OPAT, and 28 completed IV therapy without any instances of line-tampering, death, or unknown clinical status.6 Recent literature suggests that some candidates can succeed with OPAT, despite drug use.13,14
Homelessness is also considered a barrier to OPAT.1,8 Medical respite is a harm-reduction model implemented for patients who require subacute care.9 In our study, among homeless patients, PWID status was the primary determinant of whether therapy was successful, rather than respite care.
Our study may have limited generalizability to other populations. We are a single-center facility in a large, urban city. PWID and housing status were self-reported but were verified before discharge. Most of our patients were men and white; thus, outcomes may differ for others. Due to the nature of the data, cost effectiveness could not be directly calculated. LOS and readmissions serve as proxy measures.
When patients remain engaged in care, PWID and the homeless achieved comparable clinical cure rates to those of housed non-PWID. Moving forward, OPAT can be more effective in PWID and the homeless with careful patient selection and close clinical support. Access to medication-assisted therapy, such as methadone or buprenorphine,15 may improve follow-up rates and linkage to outpatient care. Additional treatment strategies to improve retention in and adherence to care may promote successful outcomes in these vulnerable populations.
Disclosures
Presented at the Poster Abstract Session: Clinical Practice Issues at ID Week, October 26–30, 2016, New Orleans, LA. No conflicts of interested related to this work for all authors.
Funding
AW and AM received NIH NIAID grant K24 AI 071113-06 and UW Medicine Patient Safety Innovations Program Grant.
1. Tice, AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004; 38(12):1651-1672. doi: 10.1086/420939. PubMed
2. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. doi: 10.1016/j.ijantimicag.2015.07.001. PubMed
3. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70(4);965-970. doi: 10.1093/jac/dku517. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections from 2002-2012. Health Aff (Milwood). 2016;35(5):832-837. doi: 10.1377/hlthaff.2015.1424. PubMed
5. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antibiotic therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. doi: 10.1002/jhm.2597. PubMed
6. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641-2644. doi: 10.1093/jac/dkq355. PubMed
7. Suleyman G, Kenney R, Zervos MJ, Weinmann A. Safety and efficacy of outpatient parenteral antibiotic therapy in an academic infectious disease clinic. J Clin Pharm Ther. 2017;42(1):39-43. doi: 10.1111/jcpt.12465. PubMed
8. Bhavan KP, Brown LS, Haley RW. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med. 2015;12(12):e1001922. doi: 10.1371/journal.pmed. PubMed
9. Seattle-King County Medical Respite. https://www.kingcounty.gov/depts/health/locations/homeless-health/healthcare-for-the-homeless/services/medical-respite.aspx. Accessed October 2, 2018.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-3781. doi: 10.1016/j.jbi.2008.08.010. PubMed
11. Buerhle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis.2017;4(3):ofx102. doi: 10.1093/ofid/ofx102. PubMed
12. Hernandez W, Price C, Knepper B, McLees M, Young H. Outpatient parenteral antimicrobial therapy administration in a homeless population. J Infus Nurs. 2016;39(2):81-85. doi: 10.1097/NAN.0000000000000165. PubMed
13. Sukuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. doi: 10.1093/ofid/ofy194. PubMed
14. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. doi: 10.1093/ofid/ofy056. PubMed
15. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. doi: 10.1016/j.amjmed.2015.09.024. PubMed
Outpatient parenteral antimicrobial therapy (OPAT) programs allow patients to receive antibiotic therapy at home or in other settings.1-3 Bacterial infections among people who inject drugs (PWID) and the homeless are common, leading to complicated treatment strategies. Those with opioid dependence have frequent hospitalizations.4 Bacteremia and endocarditis frequently require intravenous (IV) antibiotics5-7 and may be difficult to treat. Creating outpatient treatment plans for PWID and the homeless is challenging, and there is a paucity of data on OPAT effectiveness in these groups as they are often excluded from OPAT services.1,2,8
We evaluated treatment outcomes in PWID and the homeless in our OPAT program.
METHODS
We conducted a retrospective cohort study of hospitalized adults discharged from Harborview Medical Center (HMC) with OPAT from January 1, 2015 to April 30, 2016. HMC is a county hospital in Seattle, Washington, affiliated with the University of Washington (UW). Infectious disease specialists supervise our OPAT program and provide follow-up care. We partner with a medical respite facility, a discharge option for homeless patients.9 Respite is staffed by HMC nurses, mental health specialists, and case managers.
Patients aged >18 years were enrolled in OPAT if they were discharged with >2 weeks of IV therapy or required laboratory monitoring while on oral antibiotics. Patients with multiple hospitalizations were included for their initial OPAT encounter only. PWID discharged to respite were instructed not to use their vascular access to inject drugs, but drug abstinence was not required. A tamper-evident sticker was placed over lines that nurses evaluated daily. Patients violating line-tampering restrictions were discharged from respite, and OPAT providers developed alternative antibiotic plans.
The two primary exposures evaluated were patient-reported injection drug use and housing status, and our primary exposure measure was the four-category combination: (1) housed non-PWID, (2) housed PWID, (3) homeless non-PWID, and (4) homeless PWID. Current drug use was defined as use within three months of hospitalization. Homelessness was defined as lack of stable housing. Patients receiving chemotherapy, prolonged steroids, biologic agents, or those with organ transplant were considered immunocompromised.
The primary outcome was clinical cure, defined as completion of antibiotic therapy and resolution of infection, determined by OPAT providers. Patients who were placed on oral suppressive antibiotics or died before treatment completion were considered not cured. Unknown status, including care transfer and lost to follow-up, were noted separately. Lost to follow-up was assumed if patients did not return for care, their care was not formally transferred, and no other medical information was available.
Secondary outcomes included hospital length of stay (LOS), secondary bacteremia, line-tampering, and 30-day readmissions. Secondary bacteremia was defined as bacteremia with a different pathogen from the index illness, which occurred during the initial treatment course. Readmission included readmissions related to OPAT (ie, recurrent or worsening infection, treatment-related toxicities, line-tampering, secondary bacteremia, and line-associated complications).
Data collection was performed using REDCap, a data-capturing software program linked to the electronic medical record (EMR).10 Hospitalization dates and demographics were electronically populated from the EMR. Details regarding drug use, homelessness, comorbidities, diagnosis, discharge complications, clinical cure, and lost to follow-up were manually entered.
Statistical Analysis
Statistical calculations were performed using SAS (v. 9.4). Chi-square testing and analysis of variance were conducted to assess group differences in demographics, infection types, and clinical outcomes.
Primary and secondary outcomes were further evaluated by univariable logistic regression and presented as odds ratios, with the non-PWID housed group serving as the reference. Given the large number of PWID and homeless patients lost to follow-up, sensitivity analyses were conducted using the assumption that patients with unknown clinical outcomes did not achieve cure (ie, chronic infection or death). Multivariable regression was performed on the outcomes of cure and 30-day readmission to OPAT using backward elimination to select a final model, initially including potential confounders of age, sex, and relevant comorbidities (DM and HIV). We assumed that those lost to follow-up were not cured (or readmitted). Other secondary outcomes were either rare events or those of uncertain relevance (eg, hospital LOS) to be evaluated in the multivariable analysis.
Our study did not meet the definition of research by the UW’s institutional review board. It was a quality improvement project funded by a UW Medicine Patient Safety Innovations Program Grant.
RESULTS
Overall, 596 patients received OPAT over 16 months. OPAT patients were categorized into groups as follows: homeless PWID (9%, n = 53), housed PWID (8%, n = 48), homeless non-PWID (8%, n = 45), and housed non-PWID (75%, n = 450).
PWID were younger than non-PWID, and the majority of patients in all groups were men (Table 1). PWID were more likely to have hepatitis C. Non-PWID appeared more likely to have diabetes and be immunosuppressed.
Patients had a total of 960 types of infection (Table 1). Bacteremia was the most common infection among PWID. Osteomyelitis was the most frequent infection in non-PWID.
Discharge location varied widely (P < .001; Table 1). The majority of patients with housing (housed PWID 60.4%, housed non-PWID 59.1%) were discharged to home, although 36.7% of housed non-PWID went to nursing facilities. Among homeless patients, 58.5% of PWID and 42.2% of non-PWID were discharged to respite; 10 patients were discharged to a shelter or street. Data specific to transition from IV to oral therapy were not recorded.
Cure rates among participants with known outcomes did not differ by group (Table 1; P = .85). In a sensitivity analysis of clinical cure, assuming those with unknown outcomes were not cured, housing status and drug use were significantly associated with cure (Table 1; P < .001, in the overall test), with rates lower among housed and homeless PWID groups (50.0% and 47.2%, respectively) compared with housed and homeless non-PWID groups (73.1% and 82.2%, respectively). In the multivariable analysis after backward elimination of noninfluential measures, only PWID and housing status were associated with cure; PWID, whether housed (OR = 0.37) or not (OR = 0.33), had lower odds of cure relative to housed non-PWID (Table 2).
Secondary outcomes, evaluated on all patients regardless of cure, differed by group (Table 1). Mean LOS appeared to be shortest for homeless PWID (15.5 days versus ≥18.0 for other groups; P < .001 for overall test). Homeless PWID patients appeared more likely to have secondary bacteremia (13.2% versus <4.2% in other groups; P < .001 for overall test), line tampering (35.9% versus <2.2% in other groups; P < .001), and 30-day readmission related to OPAT (26.4% versus <16.7% in other groups; P = .004). Compared with housed non-PWID using logistic regression, homeless PWID had a higher risk of secondary bacteremia (OR = 12.9; 95% CI 3.8-37.8; P < .001), line tampering (OR 88.4; 95% CI 24.5-318.3; P < .001), and readmission for OPAT (OR 2.4; 95% CI 1.2-4.6; P = .007). After adjusting for age, sex, and comorbidities, readmission for OPAT remained elevated in homeless PWID (OR = 2.4; 95% CI 1.2-4.6). No significant differences in secondary outcomes were found between housed non-PWID and also between housed PWID and homeless non-PWID.
Among homeless persons, discharge to respite care was not associated with improved outcomes, assuming those lost to follow-up did not achieve cure. Among non-PWID discharged to respite, the cure rate was 74% (14/19) compared with 88% (23/26) discharged elsewhere (P = .20). Among PWID, 48% (15/31) discharged to respite were cured compared with 45% (10/22) discharged elsewhere (P = .83).
DISCUSSION
Our study compares the outcomes of 596 OPAT patients, including PWID and the homeless. Among those retained in care, PWID achieved similar rates of cure compared with non-PWID groups. When assuming that all lost to follow-up had poor outcomes, the cure rates were markedly lower for PWID, with no difference noted by housing status.
Data on PWID and homeless enrolled in OPAT programs are limited.5,11,12 Few studies have reported the outcomes of infections in PWID and the homeless, as these populations often experience significant loss to follow-up due to transiency, lack of care continuity, and effective means of communication.
Cure was achieved in less than half of PWID, when lack of cure was assumed for unknown outcomes. This rate was substantially less than that for non-PWID groups. The assumption that those lost to follow-up did not achieve cure dramatically alters the inference; the truth may lie somewhere between the primary and sensitivity analyses. Homeless PWID remained at the highest risk for lost to follow-up, secondary bacteremia, line-tampering, and 30-day readmission related to OPAT.
PWID have traditionally been considered as a high-risk group for OPAT,1,2,8 but to completely restrict PWID from OPAT may not be appropriate. Ho et al. studied 29 PWID who were selectively enrolled to receive OPAT, and 28 completed IV therapy without any instances of line-tampering, death, or unknown clinical status.6 Recent literature suggests that some candidates can succeed with OPAT, despite drug use.13,14
Homelessness is also considered a barrier to OPAT.1,8 Medical respite is a harm-reduction model implemented for patients who require subacute care.9 In our study, among homeless patients, PWID status was the primary determinant of whether therapy was successful, rather than respite care.
Our study may have limited generalizability to other populations. We are a single-center facility in a large, urban city. PWID and housing status were self-reported but were verified before discharge. Most of our patients were men and white; thus, outcomes may differ for others. Due to the nature of the data, cost effectiveness could not be directly calculated. LOS and readmissions serve as proxy measures.
When patients remain engaged in care, PWID and the homeless achieved comparable clinical cure rates to those of housed non-PWID. Moving forward, OPAT can be more effective in PWID and the homeless with careful patient selection and close clinical support. Access to medication-assisted therapy, such as methadone or buprenorphine,15 may improve follow-up rates and linkage to outpatient care. Additional treatment strategies to improve retention in and adherence to care may promote successful outcomes in these vulnerable populations.
Disclosures
Presented at the Poster Abstract Session: Clinical Practice Issues at ID Week, October 26–30, 2016, New Orleans, LA. No conflicts of interested related to this work for all authors.
Funding
AW and AM received NIH NIAID grant K24 AI 071113-06 and UW Medicine Patient Safety Innovations Program Grant.
Outpatient parenteral antimicrobial therapy (OPAT) programs allow patients to receive antibiotic therapy at home or in other settings.1-3 Bacterial infections among people who inject drugs (PWID) and the homeless are common, leading to complicated treatment strategies. Those with opioid dependence have frequent hospitalizations.4 Bacteremia and endocarditis frequently require intravenous (IV) antibiotics5-7 and may be difficult to treat. Creating outpatient treatment plans for PWID and the homeless is challenging, and there is a paucity of data on OPAT effectiveness in these groups as they are often excluded from OPAT services.1,2,8
We evaluated treatment outcomes in PWID and the homeless in our OPAT program.
METHODS
We conducted a retrospective cohort study of hospitalized adults discharged from Harborview Medical Center (HMC) with OPAT from January 1, 2015 to April 30, 2016. HMC is a county hospital in Seattle, Washington, affiliated with the University of Washington (UW). Infectious disease specialists supervise our OPAT program and provide follow-up care. We partner with a medical respite facility, a discharge option for homeless patients.9 Respite is staffed by HMC nurses, mental health specialists, and case managers.
Patients aged >18 years were enrolled in OPAT if they were discharged with >2 weeks of IV therapy or required laboratory monitoring while on oral antibiotics. Patients with multiple hospitalizations were included for their initial OPAT encounter only. PWID discharged to respite were instructed not to use their vascular access to inject drugs, but drug abstinence was not required. A tamper-evident sticker was placed over lines that nurses evaluated daily. Patients violating line-tampering restrictions were discharged from respite, and OPAT providers developed alternative antibiotic plans.
The two primary exposures evaluated were patient-reported injection drug use and housing status, and our primary exposure measure was the four-category combination: (1) housed non-PWID, (2) housed PWID, (3) homeless non-PWID, and (4) homeless PWID. Current drug use was defined as use within three months of hospitalization. Homelessness was defined as lack of stable housing. Patients receiving chemotherapy, prolonged steroids, biologic agents, or those with organ transplant were considered immunocompromised.
The primary outcome was clinical cure, defined as completion of antibiotic therapy and resolution of infection, determined by OPAT providers. Patients who were placed on oral suppressive antibiotics or died before treatment completion were considered not cured. Unknown status, including care transfer and lost to follow-up, were noted separately. Lost to follow-up was assumed if patients did not return for care, their care was not formally transferred, and no other medical information was available.
Secondary outcomes included hospital length of stay (LOS), secondary bacteremia, line-tampering, and 30-day readmissions. Secondary bacteremia was defined as bacteremia with a different pathogen from the index illness, which occurred during the initial treatment course. Readmission included readmissions related to OPAT (ie, recurrent or worsening infection, treatment-related toxicities, line-tampering, secondary bacteremia, and line-associated complications).
Data collection was performed using REDCap, a data-capturing software program linked to the electronic medical record (EMR).10 Hospitalization dates and demographics were electronically populated from the EMR. Details regarding drug use, homelessness, comorbidities, diagnosis, discharge complications, clinical cure, and lost to follow-up were manually entered.
Statistical Analysis
Statistical calculations were performed using SAS (v. 9.4). Chi-square testing and analysis of variance were conducted to assess group differences in demographics, infection types, and clinical outcomes.
Primary and secondary outcomes were further evaluated by univariable logistic regression and presented as odds ratios, with the non-PWID housed group serving as the reference. Given the large number of PWID and homeless patients lost to follow-up, sensitivity analyses were conducted using the assumption that patients with unknown clinical outcomes did not achieve cure (ie, chronic infection or death). Multivariable regression was performed on the outcomes of cure and 30-day readmission to OPAT using backward elimination to select a final model, initially including potential confounders of age, sex, and relevant comorbidities (DM and HIV). We assumed that those lost to follow-up were not cured (or readmitted). Other secondary outcomes were either rare events or those of uncertain relevance (eg, hospital LOS) to be evaluated in the multivariable analysis.
Our study did not meet the definition of research by the UW’s institutional review board. It was a quality improvement project funded by a UW Medicine Patient Safety Innovations Program Grant.
RESULTS
Overall, 596 patients received OPAT over 16 months. OPAT patients were categorized into groups as follows: homeless PWID (9%, n = 53), housed PWID (8%, n = 48), homeless non-PWID (8%, n = 45), and housed non-PWID (75%, n = 450).
PWID were younger than non-PWID, and the majority of patients in all groups were men (Table 1). PWID were more likely to have hepatitis C. Non-PWID appeared more likely to have diabetes and be immunosuppressed.
Patients had a total of 960 types of infection (Table 1). Bacteremia was the most common infection among PWID. Osteomyelitis was the most frequent infection in non-PWID.
Discharge location varied widely (P < .001; Table 1). The majority of patients with housing (housed PWID 60.4%, housed non-PWID 59.1%) were discharged to home, although 36.7% of housed non-PWID went to nursing facilities. Among homeless patients, 58.5% of PWID and 42.2% of non-PWID were discharged to respite; 10 patients were discharged to a shelter or street. Data specific to transition from IV to oral therapy were not recorded.
Cure rates among participants with known outcomes did not differ by group (Table 1; P = .85). In a sensitivity analysis of clinical cure, assuming those with unknown outcomes were not cured, housing status and drug use were significantly associated with cure (Table 1; P < .001, in the overall test), with rates lower among housed and homeless PWID groups (50.0% and 47.2%, respectively) compared with housed and homeless non-PWID groups (73.1% and 82.2%, respectively). In the multivariable analysis after backward elimination of noninfluential measures, only PWID and housing status were associated with cure; PWID, whether housed (OR = 0.37) or not (OR = 0.33), had lower odds of cure relative to housed non-PWID (Table 2).
Secondary outcomes, evaluated on all patients regardless of cure, differed by group (Table 1). Mean LOS appeared to be shortest for homeless PWID (15.5 days versus ≥18.0 for other groups; P < .001 for overall test). Homeless PWID patients appeared more likely to have secondary bacteremia (13.2% versus <4.2% in other groups; P < .001 for overall test), line tampering (35.9% versus <2.2% in other groups; P < .001), and 30-day readmission related to OPAT (26.4% versus <16.7% in other groups; P = .004). Compared with housed non-PWID using logistic regression, homeless PWID had a higher risk of secondary bacteremia (OR = 12.9; 95% CI 3.8-37.8; P < .001), line tampering (OR 88.4; 95% CI 24.5-318.3; P < .001), and readmission for OPAT (OR 2.4; 95% CI 1.2-4.6; P = .007). After adjusting for age, sex, and comorbidities, readmission for OPAT remained elevated in homeless PWID (OR = 2.4; 95% CI 1.2-4.6). No significant differences in secondary outcomes were found between housed non-PWID and also between housed PWID and homeless non-PWID.
Among homeless persons, discharge to respite care was not associated with improved outcomes, assuming those lost to follow-up did not achieve cure. Among non-PWID discharged to respite, the cure rate was 74% (14/19) compared with 88% (23/26) discharged elsewhere (P = .20). Among PWID, 48% (15/31) discharged to respite were cured compared with 45% (10/22) discharged elsewhere (P = .83).
DISCUSSION
Our study compares the outcomes of 596 OPAT patients, including PWID and the homeless. Among those retained in care, PWID achieved similar rates of cure compared with non-PWID groups. When assuming that all lost to follow-up had poor outcomes, the cure rates were markedly lower for PWID, with no difference noted by housing status.
Data on PWID and homeless enrolled in OPAT programs are limited.5,11,12 Few studies have reported the outcomes of infections in PWID and the homeless, as these populations often experience significant loss to follow-up due to transiency, lack of care continuity, and effective means of communication.
Cure was achieved in less than half of PWID, when lack of cure was assumed for unknown outcomes. This rate was substantially less than that for non-PWID groups. The assumption that those lost to follow-up did not achieve cure dramatically alters the inference; the truth may lie somewhere between the primary and sensitivity analyses. Homeless PWID remained at the highest risk for lost to follow-up, secondary bacteremia, line-tampering, and 30-day readmission related to OPAT.
PWID have traditionally been considered as a high-risk group for OPAT,1,2,8 but to completely restrict PWID from OPAT may not be appropriate. Ho et al. studied 29 PWID who were selectively enrolled to receive OPAT, and 28 completed IV therapy without any instances of line-tampering, death, or unknown clinical status.6 Recent literature suggests that some candidates can succeed with OPAT, despite drug use.13,14
Homelessness is also considered a barrier to OPAT.1,8 Medical respite is a harm-reduction model implemented for patients who require subacute care.9 In our study, among homeless patients, PWID status was the primary determinant of whether therapy was successful, rather than respite care.
Our study may have limited generalizability to other populations. We are a single-center facility in a large, urban city. PWID and housing status were self-reported but were verified before discharge. Most of our patients were men and white; thus, outcomes may differ for others. Due to the nature of the data, cost effectiveness could not be directly calculated. LOS and readmissions serve as proxy measures.
When patients remain engaged in care, PWID and the homeless achieved comparable clinical cure rates to those of housed non-PWID. Moving forward, OPAT can be more effective in PWID and the homeless with careful patient selection and close clinical support. Access to medication-assisted therapy, such as methadone or buprenorphine,15 may improve follow-up rates and linkage to outpatient care. Additional treatment strategies to improve retention in and adherence to care may promote successful outcomes in these vulnerable populations.
Disclosures
Presented at the Poster Abstract Session: Clinical Practice Issues at ID Week, October 26–30, 2016, New Orleans, LA. No conflicts of interested related to this work for all authors.
Funding
AW and AM received NIH NIAID grant K24 AI 071113-06 and UW Medicine Patient Safety Innovations Program Grant.
1. Tice, AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004; 38(12):1651-1672. doi: 10.1086/420939. PubMed
2. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. doi: 10.1016/j.ijantimicag.2015.07.001. PubMed
3. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70(4);965-970. doi: 10.1093/jac/dku517. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections from 2002-2012. Health Aff (Milwood). 2016;35(5):832-837. doi: 10.1377/hlthaff.2015.1424. PubMed
5. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antibiotic therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. doi: 10.1002/jhm.2597. PubMed
6. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641-2644. doi: 10.1093/jac/dkq355. PubMed
7. Suleyman G, Kenney R, Zervos MJ, Weinmann A. Safety and efficacy of outpatient parenteral antibiotic therapy in an academic infectious disease clinic. J Clin Pharm Ther. 2017;42(1):39-43. doi: 10.1111/jcpt.12465. PubMed
8. Bhavan KP, Brown LS, Haley RW. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med. 2015;12(12):e1001922. doi: 10.1371/journal.pmed. PubMed
9. Seattle-King County Medical Respite. https://www.kingcounty.gov/depts/health/locations/homeless-health/healthcare-for-the-homeless/services/medical-respite.aspx. Accessed October 2, 2018.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-3781. doi: 10.1016/j.jbi.2008.08.010. PubMed
11. Buerhle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis.2017;4(3):ofx102. doi: 10.1093/ofid/ofx102. PubMed
12. Hernandez W, Price C, Knepper B, McLees M, Young H. Outpatient parenteral antimicrobial therapy administration in a homeless population. J Infus Nurs. 2016;39(2):81-85. doi: 10.1097/NAN.0000000000000165. PubMed
13. Sukuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. doi: 10.1093/ofid/ofy194. PubMed
14. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. doi: 10.1093/ofid/ofy056. PubMed
15. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. doi: 10.1016/j.amjmed.2015.09.024. PubMed
1. Tice, AD, Rehm SJ, Dalovisio JR, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004; 38(12):1651-1672. doi: 10.1086/420939. PubMed
2. Williams DN, Baker CA, Kind AC, Sannes MR. The history and evolution of outpatient parenteral antibiotic therapy (OPAT). Int J Antimicrob Agents. 2015;46(3):307-312. doi: 10.1016/j.ijantimicag.2015.07.001. PubMed
3. Gilchrist M, Seaton RA. Outpatient parenteral antimicrobial therapy and antimicrobial stewardship: challenges and checklists. J Antimicrob Chemother. 2015;70(4);965-970. doi: 10.1093/jac/dku517. PubMed
4. Ronan MV, Herzig SJ. Hospitalizations related to opioid abuse/dependence and associated serious infections from 2002-2012. Health Aff (Milwood). 2016;35(5):832-837. doi: 10.1377/hlthaff.2015.1424. PubMed
5. Beieler AM, Dellit TH, Chan JD, et al. Successful implementation of outpatient parenteral antibiotic therapy at a medical respite facility for homeless patients. J Hosp Med. 2016;11(8):531-535. doi: 10.1002/jhm.2597. PubMed
6. Ho J, Archuleta S, Sulaiman Z, Fisher D. Safe and successful treatment of intravenous drug users with a peripherally inserted central catheter in an outpatient parenteral antibiotic treatment service. J Antimicrob Chemother. 2010;65:2641-2644. doi: 10.1093/jac/dkq355. PubMed
7. Suleyman G, Kenney R, Zervos MJ, Weinmann A. Safety and efficacy of outpatient parenteral antibiotic therapy in an academic infectious disease clinic. J Clin Pharm Ther. 2017;42(1):39-43. doi: 10.1111/jcpt.12465. PubMed
8. Bhavan KP, Brown LS, Haley RW. Self-administered outpatient antimicrobial infusion by uninsured patients discharged from a safety-net hospital: a propensity-score-balanced retrospective cohort study. PLoS Med. 2015;12(12):e1001922. doi: 10.1371/journal.pmed. PubMed
9. Seattle-King County Medical Respite. https://www.kingcounty.gov/depts/health/locations/homeless-health/healthcare-for-the-homeless/services/medical-respite.aspx. Accessed October 2, 2018.
10. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377-3781. doi: 10.1016/j.jbi.2008.08.010. PubMed
11. Buerhle DJ, Shields RK, Shah N, Shoff C, Sheridan K. Risk factors associated with outpatient parenteral antibiotic therapy program failure among intravenous drug users. Open Forum Infect Dis.2017;4(3):ofx102. doi: 10.1093/ofid/ofx102. PubMed
12. Hernandez W, Price C, Knepper B, McLees M, Young H. Outpatient parenteral antimicrobial therapy administration in a homeless population. J Infus Nurs. 2016;39(2):81-85. doi: 10.1097/NAN.0000000000000165. PubMed
13. Sukuki J, Johnson J, Montgomery M, Hayden M, Price C. Outpatient parenteral antimicrobial therapy among people who inject drugs: a review of the literature. Open Forum Infect Dis. 2018;5(9):ofy194. doi: 10.1093/ofid/ofy194. PubMed
14. D’Couto HT, Robbins GK, Ard KL, Wakeman SE, Alves J, Nelson SB. Outcomes according to discharge location for persons who inject drugs receiving outpatient parenteral antimicrobial therapy. Open Forum Infect Dis. 2018;5(5):ofy056. doi: 10.1093/ofid/ofy056. PubMed
15. Rosenthal ES, Karchmer AW, Theisen-Toupal J, Castillo RA, Rowley CF. Suboptimal addiction interventions for patients hospitalized with injection drug use-associated infective endocarditis. Am J Med. 2016;129(5):481-485. doi: 10.1016/j.amjmed.2015.09.024. PubMed
© 2019 Society of Hospital Medicine
Negative Urinalyses in Febrile Infants Age 7 to 60 Days Treated for Urinary Tract Infection
The sensitivity of the urinalysis (UA) in young infants has been reported to be in the 75% to 85% range.1-4 This suboptimal sensitivity has prevented a widespread adoption of the UA as a true screening test for urinary tract infection (UTI). Although infants with a positive urine culture and a negative UA may have asymptomatic bacteriuria (AB) or contamination,5-7 they are often treated for UTI.
Due to these concerns, the American Academy of Pediatrics (AAP) recommended in their 2011 UTI Practice Guidelines that UA criteria should be incorporated into the definition of UTI.1 However, these guidelines were intended for the 2-24 month age range, leaving a gap in our understanding of the appropriate management of infants <2 months. It is unknown how UA results influence the current management of UTI in young, febrile infants. Using data from a large, nationally representative quality improvement project surrounding the management of febrile infants, this investigation aimed to examine how frequently infants are treated for UTI despite having normal UAs and to determine whether infant and hospital characteristics are different in infants treated for UTI with a positive UA as compared to those treated for UTI with a negative UA.
METHODS
Subjects and Setting
This is a secondary analysis of the AAP’s Reducing Excessive Variability in the Infant Sepsis Evaluation (REVISE) project that involved 20,570 well-appearing infants 7-60 days of age evaluated in the emergency department and/or inpatient setting for fever ≥38◦C without a source between September 2015 and November 2017 at 124 community- and university-based hospitals in the United States. Data were collected via chart review and entered into a standardized tool for the project. This project was deemed exempt by the AAP Institutional Review Board. Because all data were de-identified, some sites did not require Institutional Review Board approval while others required data sharing agreements.
Variables and Definitions
A positive UA was defined as having any leukocyte esterase, positive nitrites, or >5 white blood cells (WBCs) per high power field. Treatment for UTI was defined using the question “Did the urine culture grow an organism that was treated as a pathogen with a full course of antibiotics?” Subjects treated for meningitis or bacteremia were excluded in order to focus on uncomplicated UTI. “Abnormal inflammatory markers” were defined as having a WBC count <5,000 or >15,000 cells/mm3, an absolute band count ≥ 1,500 cells/mm3, a band to neutrophil ratio of >0.2, cerebrospinal fluid (CSF) WBC count of >8/mm3, a positive CSF gram stain, or an elevated C-reactive protein or procalcitonin level, as defined by the institutional range. Although technically not an “inflammatory marker,” CSF gram stain was included in this composite variable because in the rare cases that it is positive, the result would likely influence risk stratification and immediate management. Infants’ ages were categorized as either 7-30 days or 31-60 days. Hospital length-of-stay (LOS) was recorded to the nearest hour and infants who were not hospitalized were assigned a LOS of 0 hours. Hospital characteristics were determined through a survey completed by site leads.
Statistics
Proportions were compared using chi-square test. We used multilevel mixed-effects logistic regression to determine associations between patients and hospital characteristics and UA-positivity in subjects treated for UTI. We accounted for the hospital clustering effect with a random effect that did not vary with patient characteristics. We “marginalized” the regression coefficients to reflect the average effect across hospitals.8,9 We tested the overall importance of the hospital clustering effect on the treatment by comparing our multilevel model to a single-level model without hospital random effects using the likelihood ratio test.
RESULTS
A total of 20,570 infants from 124 hospitals were enrolled in the REVISE project, and 648 (3.2%) were treated for bacteremia and/or meningitis. Of the remaining 19,922 infants, 2,407 (12.1%) were treated for UTI, of whom 2,298 (95.5%) had an initial UA performed. Urine cultures were obtained by catheterization or suprapubic aspirate in 90.3% and “other/unknown” in 9.7% of these 2,298 subjects.
UAs were negative in 337/2,298 (14.7%) treated subjects. UA-negative subjects were more likely to be 7-30 days old (adjusted odds ratio [aOR] 1.3, 95% CI 1.02-1.7) and have upper respiratory symptoms (aOR 1.7, 95% CI 1.3-2.3) and were less likely to have abnormal inflammatory markers (aOR 0.3, 95% CI 0.3-0.4) than UA+ subjects (Table). Even after accounting for the hospital characteristics depicted in the Table, treatment of UA-negative UTI was affected by the hospital (P < .001), and the intraclass correlation coefficient was 6% (95% CI, 3% to 14%). The Figure illustrates substantial site variability in the proportion of infants treated for UTIs that were UA-negative, ranging from 0% to 35% in hospitals with ≥20 UTI cases.
There was no significant difference in the proportion of catheterized specimens in infants treated for UTIs with negative versus positive UAs (90% vs 92%, P = .26). The median hospital (interquartile range) LOS in infants treated for UTI with positive UAs was 58 (45-78) hours, compared to 54 (38-76) hours in infants treated for UTI with negative UAs and 34 (0-49) hours in infants who were not treated for UTI, meningitis, or bacteremia.
DISCUSSION
In this large, nationally representative sample of febrile infants 7-60 days of age, we demonstrate that nearly 15% of young febrile infants who are treated for UTIs have normal UAs. This proportion varied considerably among hospitals, suggesting that there are institutional differences in the approach to the UA. Infants treated for UA-negative UTIs were more likely to have respiratory symptoms and less likely to have abnormal inflammatory markers than infants treated for UA-positive UTIs, indicating that these infants are either developing a milder inflammatory response to their underlying illness and/or might not have true UTIs (eg due to AB or contamination).
The AAP recently updated their UTI practice parameter to recommend inclusion of UA results as diagnostic criteria for UTI.1 However, the fact that these guidelines do not include infants <2 months creates a gap in our understanding of the appropriate diagnostic criteria in this age group, as reflected by the site variability demonstrated in our investigation. The fact that up to 35% of infants treated for UTI at these different sites have normal UAs suggests that many practitioners continue to treat positive urine cultures regardless of UA values.
Several prior studies provide insight into the clinical significance of a positive urine culture in the absence of pyuria. Wettergren et al.6,7,10 reported growth from suprapubic aspirate in 1.4% of infants who were screened periodically with urine cultures obtained by bag at well-child checks over the course of the first year (with a point prevalence as high as 1.5% in boys aged 0.25 to 1.9 months).10 These infants were not more likely to have subsequent UTIs7 or renal damage6 than infants without asymptomatic growth, leading the authors to conclude that this growth likely represented AB. These findings emphasize that the probability of a positive urine culture in any infant, even asymptomatic infants, is not insignificant.
Hoberman et al.11 demonstrated that dimercaptosuccinic acid scans did not reveal signs of pyelonephritis in 14/15 children < 2 years of age with urine cultures growing >50,000 CFU/mL but no pyuria on UA, and concluded that AB was the most likely explanation for this combination of findings. Schroeder et al.5 and Tzimenatos et al.12 examined infants <2-3 months with UTI and bacteremia caused by the same organism (and hence a true infection that cannot be explained by AB or contamination) and demonstrated that the UA sensitivity in this population was 99.5% and 100%, respectively, suggesting that the prior lower estimates of UA sensitivity in UTI in general, may have been biased by inclusion of positive urine cultures that did not represent UTI.
On the other hand, Shaikh et al.13 recently demonstrated that the sensitivity of the UA appears to vary by organism, with lower reported sensitivity in non-Escherichia coli organisms, leading the authors to conclude that this variability is evidence of suboptimal UA sensitivity. However, an alternative explanation for their findings is that non-E coli organisms may be more likely to cause AB or contamination.14 The fact that follow-up suprapubic aspirates on infants with untreated catheterized cultures yielding these organisms are often negative supports this alternative explanation.15
The median LOS in infants with UA-negative UTI was nearly one day longer than infants not treated for serious bacterial infection. These infants may have also undergone urinary imaging and possibly prophylactic antibiotics, indicating high resource burden created by this subgroup of infants. Expanding AAP UTI guidelines to infants <2 months of age would likely reduce resource utilization, but continued research is needed to assess the safety of this approach. Young infants have immature immune systems and may not develop a timely inflammatory response to UTI, which raises concerns about missing bacterial infections.
Our investigation has several strengths, including the large, nationally representative sample that includes both children’s and non-children’s hospitals. Similar febrile infant investigations of this size have previously been possible only using administrative databases, but our investigation required chart review for all enrolled infants, ensuring that the subjects were febrile, well-appearing, and were treated for UTI. However, our findings are limited in that data were collected primarily as part of a quality improvement initiative, and some of our thresholds for “abnormal” laboratory values might be controversial. For example, urine WBC thresholds differ across studies, and our CSF WBC threshold of >8/mm3 may be somewhat low given prior reports that values slightly above this threshold might be normal in infants under one month of age.16 The original intent of the inflammatory marker composite variable was to aid in risk stratification, but we were unable to collect granular data for all potentially relevant variables. In planning the REVISE project, we attempted to create straightforward, unambiguous variables to facilitate the anticipated high volume of chart reviews. Although patients categorized as having UTI might not have had true UTIs, by linking the “UTI” variable to practitioner management (rather than UA and microbiologic definitions), our data reflect real-world practice.
Acknowledgments
The authors would like to thank all of the site directors who participated in the REVISE project, and Brittany Jennings, Naji Hattar, Faiza Wasif, and Vanessa Shorte at the American Academy of Pediatrics for their leadership and management.
Disclosures
Dr. Schroeder has received honoraria for grand rounds presentations on the subject of urinary tract infections, and Dr. Biondi has received consulting fees from McKesson Inc. The other authors have no financial relationships to disclose.
1. Roberts KB. Urinary tract infection: Clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610. doi: 10.1542/peds.2011-1330. PubMed
2. Bachur R, Harper MB. Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch Pediatr Adolesc Med. 2001;155(1):60. doi: 10.1001/archpedi.155.1.60. PubMed
3. Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age. Pediatr Infect Dis J. 2014;33(4):342-344. doi: 10.1097/inf.0000000000000110. PubMed
4. Hoberman A, Wald ER. Urinary tract infections in young febrile children. Pediatr Infect Dis J. 1997;16(1):11-17. doi: 10.1097/00006454-199701000-00004. PubMed
5. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135(6). doi: 10.1542/peds.2015-0012d. PubMed
6. Wettergren B, Hellstrom M, Stokland E, Jodal U. Six-year follow up of infants with bacteriuria on screening. BMJ. 1990;301(6756):845-848. doi: 10.1136/bmj.301.6756.845. PubMed
7. Wettergren B, Jodal U. Spontaneous clearance of asymptomatic bacteriuria in infants. Acta Paediatrica. 1990;79(3):300-304. doi: 10.1111/j.1651-2227.1990.tb11460.x. PubMed
8. Hedeker D, Toit SHCD, Demirtas H, Gibbons RD. A note on the marginalization of regression parameters from mixed models of binary outcomes. Biometrics. 2017;74(1):354-361. doi: 10.1111/biom.12707. PubMed
9. Neuhaus JM, Kalbfleisch JD, Hauck WW. A comparison of cluster-specific and population-averaged approaches for analyzing correlated binary data. Int Stat Rev. 1991;59(1):25. doi: 10.2307/1403572.
10. Wettergren B, Jodal U, Jonasson G. Epidemiology of bacteriuria during the first year of life. Acta Paediatrica. 1985;74(6):925-933. doi: 10.1111/j.1651-2227.1985.tb10059.x. PubMed
11. Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Is urine culture necessary to rule out urinary tract infection in young febrile children? Pediatr Infect Dis J. 1996;15(4):304-309. doi: 10.1097/00006454-199604000-00005. PubMed
12. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2). doi: 10.1542/peds.2017-3068. PubMed
13. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association between uropathogen and pyuria. Pediatrics. 2016;138(1). doi: 10.1542/peds.2016-0087. PubMed
14. Schroeder AR. UTI and faulty gold standards. Pediatrics. 2017;139(3). doi: 10.1542/peds.2016-3814a. PubMed
15. Eliacik K, Kanik A, Yavascan O, et al. A comparison of bladder catheterization and suprapubic aspiration methods for urine sample collection from infants with a suspected urinary tract infection. Clinical Pediatrics. 2016;55(9):819-824. doi: 10.1177/0009922815608278. PubMed
16. Thomson J, Sucharew H, Cruz AT, et al. Cerebrospinal fluid reference values for young infants undergoing lumbar puncture. Pediatrics. 2018;141(3). doi: 10.1542/peds.2017-3405. PubMed
The sensitivity of the urinalysis (UA) in young infants has been reported to be in the 75% to 85% range.1-4 This suboptimal sensitivity has prevented a widespread adoption of the UA as a true screening test for urinary tract infection (UTI). Although infants with a positive urine culture and a negative UA may have asymptomatic bacteriuria (AB) or contamination,5-7 they are often treated for UTI.
Due to these concerns, the American Academy of Pediatrics (AAP) recommended in their 2011 UTI Practice Guidelines that UA criteria should be incorporated into the definition of UTI.1 However, these guidelines were intended for the 2-24 month age range, leaving a gap in our understanding of the appropriate management of infants <2 months. It is unknown how UA results influence the current management of UTI in young, febrile infants. Using data from a large, nationally representative quality improvement project surrounding the management of febrile infants, this investigation aimed to examine how frequently infants are treated for UTI despite having normal UAs and to determine whether infant and hospital characteristics are different in infants treated for UTI with a positive UA as compared to those treated for UTI with a negative UA.
METHODS
Subjects and Setting
This is a secondary analysis of the AAP’s Reducing Excessive Variability in the Infant Sepsis Evaluation (REVISE) project that involved 20,570 well-appearing infants 7-60 days of age evaluated in the emergency department and/or inpatient setting for fever ≥38◦C without a source between September 2015 and November 2017 at 124 community- and university-based hospitals in the United States. Data were collected via chart review and entered into a standardized tool for the project. This project was deemed exempt by the AAP Institutional Review Board. Because all data were de-identified, some sites did not require Institutional Review Board approval while others required data sharing agreements.
Variables and Definitions
A positive UA was defined as having any leukocyte esterase, positive nitrites, or >5 white blood cells (WBCs) per high power field. Treatment for UTI was defined using the question “Did the urine culture grow an organism that was treated as a pathogen with a full course of antibiotics?” Subjects treated for meningitis or bacteremia were excluded in order to focus on uncomplicated UTI. “Abnormal inflammatory markers” were defined as having a WBC count <5,000 or >15,000 cells/mm3, an absolute band count ≥ 1,500 cells/mm3, a band to neutrophil ratio of >0.2, cerebrospinal fluid (CSF) WBC count of >8/mm3, a positive CSF gram stain, or an elevated C-reactive protein or procalcitonin level, as defined by the institutional range. Although technically not an “inflammatory marker,” CSF gram stain was included in this composite variable because in the rare cases that it is positive, the result would likely influence risk stratification and immediate management. Infants’ ages were categorized as either 7-30 days or 31-60 days. Hospital length-of-stay (LOS) was recorded to the nearest hour and infants who were not hospitalized were assigned a LOS of 0 hours. Hospital characteristics were determined through a survey completed by site leads.
Statistics
Proportions were compared using chi-square test. We used multilevel mixed-effects logistic regression to determine associations between patients and hospital characteristics and UA-positivity in subjects treated for UTI. We accounted for the hospital clustering effect with a random effect that did not vary with patient characteristics. We “marginalized” the regression coefficients to reflect the average effect across hospitals.8,9 We tested the overall importance of the hospital clustering effect on the treatment by comparing our multilevel model to a single-level model without hospital random effects using the likelihood ratio test.
RESULTS
A total of 20,570 infants from 124 hospitals were enrolled in the REVISE project, and 648 (3.2%) were treated for bacteremia and/or meningitis. Of the remaining 19,922 infants, 2,407 (12.1%) were treated for UTI, of whom 2,298 (95.5%) had an initial UA performed. Urine cultures were obtained by catheterization or suprapubic aspirate in 90.3% and “other/unknown” in 9.7% of these 2,298 subjects.
UAs were negative in 337/2,298 (14.7%) treated subjects. UA-negative subjects were more likely to be 7-30 days old (adjusted odds ratio [aOR] 1.3, 95% CI 1.02-1.7) and have upper respiratory symptoms (aOR 1.7, 95% CI 1.3-2.3) and were less likely to have abnormal inflammatory markers (aOR 0.3, 95% CI 0.3-0.4) than UA+ subjects (Table). Even after accounting for the hospital characteristics depicted in the Table, treatment of UA-negative UTI was affected by the hospital (P < .001), and the intraclass correlation coefficient was 6% (95% CI, 3% to 14%). The Figure illustrates substantial site variability in the proportion of infants treated for UTIs that were UA-negative, ranging from 0% to 35% in hospitals with ≥20 UTI cases.
There was no significant difference in the proportion of catheterized specimens in infants treated for UTIs with negative versus positive UAs (90% vs 92%, P = .26). The median hospital (interquartile range) LOS in infants treated for UTI with positive UAs was 58 (45-78) hours, compared to 54 (38-76) hours in infants treated for UTI with negative UAs and 34 (0-49) hours in infants who were not treated for UTI, meningitis, or bacteremia.
DISCUSSION
In this large, nationally representative sample of febrile infants 7-60 days of age, we demonstrate that nearly 15% of young febrile infants who are treated for UTIs have normal UAs. This proportion varied considerably among hospitals, suggesting that there are institutional differences in the approach to the UA. Infants treated for UA-negative UTIs were more likely to have respiratory symptoms and less likely to have abnormal inflammatory markers than infants treated for UA-positive UTIs, indicating that these infants are either developing a milder inflammatory response to their underlying illness and/or might not have true UTIs (eg due to AB or contamination).
The AAP recently updated their UTI practice parameter to recommend inclusion of UA results as diagnostic criteria for UTI.1 However, the fact that these guidelines do not include infants <2 months creates a gap in our understanding of the appropriate diagnostic criteria in this age group, as reflected by the site variability demonstrated in our investigation. The fact that up to 35% of infants treated for UTI at these different sites have normal UAs suggests that many practitioners continue to treat positive urine cultures regardless of UA values.
Several prior studies provide insight into the clinical significance of a positive urine culture in the absence of pyuria. Wettergren et al.6,7,10 reported growth from suprapubic aspirate in 1.4% of infants who were screened periodically with urine cultures obtained by bag at well-child checks over the course of the first year (with a point prevalence as high as 1.5% in boys aged 0.25 to 1.9 months).10 These infants were not more likely to have subsequent UTIs7 or renal damage6 than infants without asymptomatic growth, leading the authors to conclude that this growth likely represented AB. These findings emphasize that the probability of a positive urine culture in any infant, even asymptomatic infants, is not insignificant.
Hoberman et al.11 demonstrated that dimercaptosuccinic acid scans did not reveal signs of pyelonephritis in 14/15 children < 2 years of age with urine cultures growing >50,000 CFU/mL but no pyuria on UA, and concluded that AB was the most likely explanation for this combination of findings. Schroeder et al.5 and Tzimenatos et al.12 examined infants <2-3 months with UTI and bacteremia caused by the same organism (and hence a true infection that cannot be explained by AB or contamination) and demonstrated that the UA sensitivity in this population was 99.5% and 100%, respectively, suggesting that the prior lower estimates of UA sensitivity in UTI in general, may have been biased by inclusion of positive urine cultures that did not represent UTI.
On the other hand, Shaikh et al.13 recently demonstrated that the sensitivity of the UA appears to vary by organism, with lower reported sensitivity in non-Escherichia coli organisms, leading the authors to conclude that this variability is evidence of suboptimal UA sensitivity. However, an alternative explanation for their findings is that non-E coli organisms may be more likely to cause AB or contamination.14 The fact that follow-up suprapubic aspirates on infants with untreated catheterized cultures yielding these organisms are often negative supports this alternative explanation.15
The median LOS in infants with UA-negative UTI was nearly one day longer than infants not treated for serious bacterial infection. These infants may have also undergone urinary imaging and possibly prophylactic antibiotics, indicating high resource burden created by this subgroup of infants. Expanding AAP UTI guidelines to infants <2 months of age would likely reduce resource utilization, but continued research is needed to assess the safety of this approach. Young infants have immature immune systems and may not develop a timely inflammatory response to UTI, which raises concerns about missing bacterial infections.
Our investigation has several strengths, including the large, nationally representative sample that includes both children’s and non-children’s hospitals. Similar febrile infant investigations of this size have previously been possible only using administrative databases, but our investigation required chart review for all enrolled infants, ensuring that the subjects were febrile, well-appearing, and were treated for UTI. However, our findings are limited in that data were collected primarily as part of a quality improvement initiative, and some of our thresholds for “abnormal” laboratory values might be controversial. For example, urine WBC thresholds differ across studies, and our CSF WBC threshold of >8/mm3 may be somewhat low given prior reports that values slightly above this threshold might be normal in infants under one month of age.16 The original intent of the inflammatory marker composite variable was to aid in risk stratification, but we were unable to collect granular data for all potentially relevant variables. In planning the REVISE project, we attempted to create straightforward, unambiguous variables to facilitate the anticipated high volume of chart reviews. Although patients categorized as having UTI might not have had true UTIs, by linking the “UTI” variable to practitioner management (rather than UA and microbiologic definitions), our data reflect real-world practice.
Acknowledgments
The authors would like to thank all of the site directors who participated in the REVISE project, and Brittany Jennings, Naji Hattar, Faiza Wasif, and Vanessa Shorte at the American Academy of Pediatrics for their leadership and management.
Disclosures
Dr. Schroeder has received honoraria for grand rounds presentations on the subject of urinary tract infections, and Dr. Biondi has received consulting fees from McKesson Inc. The other authors have no financial relationships to disclose.
The sensitivity of the urinalysis (UA) in young infants has been reported to be in the 75% to 85% range.1-4 This suboptimal sensitivity has prevented a widespread adoption of the UA as a true screening test for urinary tract infection (UTI). Although infants with a positive urine culture and a negative UA may have asymptomatic bacteriuria (AB) or contamination,5-7 they are often treated for UTI.
Due to these concerns, the American Academy of Pediatrics (AAP) recommended in their 2011 UTI Practice Guidelines that UA criteria should be incorporated into the definition of UTI.1 However, these guidelines were intended for the 2-24 month age range, leaving a gap in our understanding of the appropriate management of infants <2 months. It is unknown how UA results influence the current management of UTI in young, febrile infants. Using data from a large, nationally representative quality improvement project surrounding the management of febrile infants, this investigation aimed to examine how frequently infants are treated for UTI despite having normal UAs and to determine whether infant and hospital characteristics are different in infants treated for UTI with a positive UA as compared to those treated for UTI with a negative UA.
METHODS
Subjects and Setting
This is a secondary analysis of the AAP’s Reducing Excessive Variability in the Infant Sepsis Evaluation (REVISE) project that involved 20,570 well-appearing infants 7-60 days of age evaluated in the emergency department and/or inpatient setting for fever ≥38◦C without a source between September 2015 and November 2017 at 124 community- and university-based hospitals in the United States. Data were collected via chart review and entered into a standardized tool for the project. This project was deemed exempt by the AAP Institutional Review Board. Because all data were de-identified, some sites did not require Institutional Review Board approval while others required data sharing agreements.
Variables and Definitions
A positive UA was defined as having any leukocyte esterase, positive nitrites, or >5 white blood cells (WBCs) per high power field. Treatment for UTI was defined using the question “Did the urine culture grow an organism that was treated as a pathogen with a full course of antibiotics?” Subjects treated for meningitis or bacteremia were excluded in order to focus on uncomplicated UTI. “Abnormal inflammatory markers” were defined as having a WBC count <5,000 or >15,000 cells/mm3, an absolute band count ≥ 1,500 cells/mm3, a band to neutrophil ratio of >0.2, cerebrospinal fluid (CSF) WBC count of >8/mm3, a positive CSF gram stain, or an elevated C-reactive protein or procalcitonin level, as defined by the institutional range. Although technically not an “inflammatory marker,” CSF gram stain was included in this composite variable because in the rare cases that it is positive, the result would likely influence risk stratification and immediate management. Infants’ ages were categorized as either 7-30 days or 31-60 days. Hospital length-of-stay (LOS) was recorded to the nearest hour and infants who were not hospitalized were assigned a LOS of 0 hours. Hospital characteristics were determined through a survey completed by site leads.
Statistics
Proportions were compared using chi-square test. We used multilevel mixed-effects logistic regression to determine associations between patients and hospital characteristics and UA-positivity in subjects treated for UTI. We accounted for the hospital clustering effect with a random effect that did not vary with patient characteristics. We “marginalized” the regression coefficients to reflect the average effect across hospitals.8,9 We tested the overall importance of the hospital clustering effect on the treatment by comparing our multilevel model to a single-level model without hospital random effects using the likelihood ratio test.
RESULTS
A total of 20,570 infants from 124 hospitals were enrolled in the REVISE project, and 648 (3.2%) were treated for bacteremia and/or meningitis. Of the remaining 19,922 infants, 2,407 (12.1%) were treated for UTI, of whom 2,298 (95.5%) had an initial UA performed. Urine cultures were obtained by catheterization or suprapubic aspirate in 90.3% and “other/unknown” in 9.7% of these 2,298 subjects.
UAs were negative in 337/2,298 (14.7%) treated subjects. UA-negative subjects were more likely to be 7-30 days old (adjusted odds ratio [aOR] 1.3, 95% CI 1.02-1.7) and have upper respiratory symptoms (aOR 1.7, 95% CI 1.3-2.3) and were less likely to have abnormal inflammatory markers (aOR 0.3, 95% CI 0.3-0.4) than UA+ subjects (Table). Even after accounting for the hospital characteristics depicted in the Table, treatment of UA-negative UTI was affected by the hospital (P < .001), and the intraclass correlation coefficient was 6% (95% CI, 3% to 14%). The Figure illustrates substantial site variability in the proportion of infants treated for UTIs that were UA-negative, ranging from 0% to 35% in hospitals with ≥20 UTI cases.
There was no significant difference in the proportion of catheterized specimens in infants treated for UTIs with negative versus positive UAs (90% vs 92%, P = .26). The median hospital (interquartile range) LOS in infants treated for UTI with positive UAs was 58 (45-78) hours, compared to 54 (38-76) hours in infants treated for UTI with negative UAs and 34 (0-49) hours in infants who were not treated for UTI, meningitis, or bacteremia.
DISCUSSION
In this large, nationally representative sample of febrile infants 7-60 days of age, we demonstrate that nearly 15% of young febrile infants who are treated for UTIs have normal UAs. This proportion varied considerably among hospitals, suggesting that there are institutional differences in the approach to the UA. Infants treated for UA-negative UTIs were more likely to have respiratory symptoms and less likely to have abnormal inflammatory markers than infants treated for UA-positive UTIs, indicating that these infants are either developing a milder inflammatory response to their underlying illness and/or might not have true UTIs (eg due to AB or contamination).
The AAP recently updated their UTI practice parameter to recommend inclusion of UA results as diagnostic criteria for UTI.1 However, the fact that these guidelines do not include infants <2 months creates a gap in our understanding of the appropriate diagnostic criteria in this age group, as reflected by the site variability demonstrated in our investigation. The fact that up to 35% of infants treated for UTI at these different sites have normal UAs suggests that many practitioners continue to treat positive urine cultures regardless of UA values.
Several prior studies provide insight into the clinical significance of a positive urine culture in the absence of pyuria. Wettergren et al.6,7,10 reported growth from suprapubic aspirate in 1.4% of infants who were screened periodically with urine cultures obtained by bag at well-child checks over the course of the first year (with a point prevalence as high as 1.5% in boys aged 0.25 to 1.9 months).10 These infants were not more likely to have subsequent UTIs7 or renal damage6 than infants without asymptomatic growth, leading the authors to conclude that this growth likely represented AB. These findings emphasize that the probability of a positive urine culture in any infant, even asymptomatic infants, is not insignificant.
Hoberman et al.11 demonstrated that dimercaptosuccinic acid scans did not reveal signs of pyelonephritis in 14/15 children < 2 years of age with urine cultures growing >50,000 CFU/mL but no pyuria on UA, and concluded that AB was the most likely explanation for this combination of findings. Schroeder et al.5 and Tzimenatos et al.12 examined infants <2-3 months with UTI and bacteremia caused by the same organism (and hence a true infection that cannot be explained by AB or contamination) and demonstrated that the UA sensitivity in this population was 99.5% and 100%, respectively, suggesting that the prior lower estimates of UA sensitivity in UTI in general, may have been biased by inclusion of positive urine cultures that did not represent UTI.
On the other hand, Shaikh et al.13 recently demonstrated that the sensitivity of the UA appears to vary by organism, with lower reported sensitivity in non-Escherichia coli organisms, leading the authors to conclude that this variability is evidence of suboptimal UA sensitivity. However, an alternative explanation for their findings is that non-E coli organisms may be more likely to cause AB or contamination.14 The fact that follow-up suprapubic aspirates on infants with untreated catheterized cultures yielding these organisms are often negative supports this alternative explanation.15
The median LOS in infants with UA-negative UTI was nearly one day longer than infants not treated for serious bacterial infection. These infants may have also undergone urinary imaging and possibly prophylactic antibiotics, indicating high resource burden created by this subgroup of infants. Expanding AAP UTI guidelines to infants <2 months of age would likely reduce resource utilization, but continued research is needed to assess the safety of this approach. Young infants have immature immune systems and may not develop a timely inflammatory response to UTI, which raises concerns about missing bacterial infections.
Our investigation has several strengths, including the large, nationally representative sample that includes both children’s and non-children’s hospitals. Similar febrile infant investigations of this size have previously been possible only using administrative databases, but our investigation required chart review for all enrolled infants, ensuring that the subjects were febrile, well-appearing, and were treated for UTI. However, our findings are limited in that data were collected primarily as part of a quality improvement initiative, and some of our thresholds for “abnormal” laboratory values might be controversial. For example, urine WBC thresholds differ across studies, and our CSF WBC threshold of >8/mm3 may be somewhat low given prior reports that values slightly above this threshold might be normal in infants under one month of age.16 The original intent of the inflammatory marker composite variable was to aid in risk stratification, but we were unable to collect granular data for all potentially relevant variables. In planning the REVISE project, we attempted to create straightforward, unambiguous variables to facilitate the anticipated high volume of chart reviews. Although patients categorized as having UTI might not have had true UTIs, by linking the “UTI” variable to practitioner management (rather than UA and microbiologic definitions), our data reflect real-world practice.
Acknowledgments
The authors would like to thank all of the site directors who participated in the REVISE project, and Brittany Jennings, Naji Hattar, Faiza Wasif, and Vanessa Shorte at the American Academy of Pediatrics for their leadership and management.
Disclosures
Dr. Schroeder has received honoraria for grand rounds presentations on the subject of urinary tract infections, and Dr. Biondi has received consulting fees from McKesson Inc. The other authors have no financial relationships to disclose.
1. Roberts KB. Urinary tract infection: Clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610. doi: 10.1542/peds.2011-1330. PubMed
2. Bachur R, Harper MB. Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch Pediatr Adolesc Med. 2001;155(1):60. doi: 10.1001/archpedi.155.1.60. PubMed
3. Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age. Pediatr Infect Dis J. 2014;33(4):342-344. doi: 10.1097/inf.0000000000000110. PubMed
4. Hoberman A, Wald ER. Urinary tract infections in young febrile children. Pediatr Infect Dis J. 1997;16(1):11-17. doi: 10.1097/00006454-199701000-00004. PubMed
5. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135(6). doi: 10.1542/peds.2015-0012d. PubMed
6. Wettergren B, Hellstrom M, Stokland E, Jodal U. Six-year follow up of infants with bacteriuria on screening. BMJ. 1990;301(6756):845-848. doi: 10.1136/bmj.301.6756.845. PubMed
7. Wettergren B, Jodal U. Spontaneous clearance of asymptomatic bacteriuria in infants. Acta Paediatrica. 1990;79(3):300-304. doi: 10.1111/j.1651-2227.1990.tb11460.x. PubMed
8. Hedeker D, Toit SHCD, Demirtas H, Gibbons RD. A note on the marginalization of regression parameters from mixed models of binary outcomes. Biometrics. 2017;74(1):354-361. doi: 10.1111/biom.12707. PubMed
9. Neuhaus JM, Kalbfleisch JD, Hauck WW. A comparison of cluster-specific and population-averaged approaches for analyzing correlated binary data. Int Stat Rev. 1991;59(1):25. doi: 10.2307/1403572.
10. Wettergren B, Jodal U, Jonasson G. Epidemiology of bacteriuria during the first year of life. Acta Paediatrica. 1985;74(6):925-933. doi: 10.1111/j.1651-2227.1985.tb10059.x. PubMed
11. Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Is urine culture necessary to rule out urinary tract infection in young febrile children? Pediatr Infect Dis J. 1996;15(4):304-309. doi: 10.1097/00006454-199604000-00005. PubMed
12. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2). doi: 10.1542/peds.2017-3068. PubMed
13. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association between uropathogen and pyuria. Pediatrics. 2016;138(1). doi: 10.1542/peds.2016-0087. PubMed
14. Schroeder AR. UTI and faulty gold standards. Pediatrics. 2017;139(3). doi: 10.1542/peds.2016-3814a. PubMed
15. Eliacik K, Kanik A, Yavascan O, et al. A comparison of bladder catheterization and suprapubic aspiration methods for urine sample collection from infants with a suspected urinary tract infection. Clinical Pediatrics. 2016;55(9):819-824. doi: 10.1177/0009922815608278. PubMed
16. Thomson J, Sucharew H, Cruz AT, et al. Cerebrospinal fluid reference values for young infants undergoing lumbar puncture. Pediatrics. 2018;141(3). doi: 10.1542/peds.2017-3405. PubMed
1. Roberts KB. Urinary tract infection: Clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128(3):595-610. doi: 10.1542/peds.2011-1330. PubMed
2. Bachur R, Harper MB. Reliability of the urinalysis for predicting urinary tract infections in young febrile children. Arch Pediatr Adolesc Med. 2001;155(1):60. doi: 10.1001/archpedi.155.1.60. PubMed
3. Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age. Pediatr Infect Dis J. 2014;33(4):342-344. doi: 10.1097/inf.0000000000000110. PubMed
4. Hoberman A, Wald ER. Urinary tract infections in young febrile children. Pediatr Infect Dis J. 1997;16(1):11-17. doi: 10.1097/00006454-199701000-00004. PubMed
5. Schroeder AR, Chang PW, Shen MW, Biondi EA, Greenhow TL. Diagnostic accuracy of the urinalysis for urinary tract infection in infants <3 months of age. Pediatrics. 2015;135(6). doi: 10.1542/peds.2015-0012d. PubMed
6. Wettergren B, Hellstrom M, Stokland E, Jodal U. Six-year follow up of infants with bacteriuria on screening. BMJ. 1990;301(6756):845-848. doi: 10.1136/bmj.301.6756.845. PubMed
7. Wettergren B, Jodal U. Spontaneous clearance of asymptomatic bacteriuria in infants. Acta Paediatrica. 1990;79(3):300-304. doi: 10.1111/j.1651-2227.1990.tb11460.x. PubMed
8. Hedeker D, Toit SHCD, Demirtas H, Gibbons RD. A note on the marginalization of regression parameters from mixed models of binary outcomes. Biometrics. 2017;74(1):354-361. doi: 10.1111/biom.12707. PubMed
9. Neuhaus JM, Kalbfleisch JD, Hauck WW. A comparison of cluster-specific and population-averaged approaches for analyzing correlated binary data. Int Stat Rev. 1991;59(1):25. doi: 10.2307/1403572.
10. Wettergren B, Jodal U, Jonasson G. Epidemiology of bacteriuria during the first year of life. Acta Paediatrica. 1985;74(6):925-933. doi: 10.1111/j.1651-2227.1985.tb10059.x. PubMed
11. Hoberman A, Wald ER, Reynolds EA, Penchansky L, Charron M. Is urine culture necessary to rule out urinary tract infection in young febrile children? Pediatr Infect Dis J. 1996;15(4):304-309. doi: 10.1097/00006454-199604000-00005. PubMed
12. Tzimenatos L, Mahajan P, Dayan PS, et al. Accuracy of the urinalysis for urinary tract infections in febrile infants 60 days and younger. Pediatrics. 2018;141(2). doi: 10.1542/peds.2017-3068. PubMed
13. Shaikh N, Shope TR, Hoberman A, Vigliotti A, Kurs-Lasky M, Martin JM. Association between uropathogen and pyuria. Pediatrics. 2016;138(1). doi: 10.1542/peds.2016-0087. PubMed
14. Schroeder AR. UTI and faulty gold standards. Pediatrics. 2017;139(3). doi: 10.1542/peds.2016-3814a. PubMed
15. Eliacik K, Kanik A, Yavascan O, et al. A comparison of bladder catheterization and suprapubic aspiration methods for urine sample collection from infants with a suspected urinary tract infection. Clinical Pediatrics. 2016;55(9):819-824. doi: 10.1177/0009922815608278. PubMed
16. Thomson J, Sucharew H, Cruz AT, et al. Cerebrospinal fluid reference values for young infants undergoing lumbar puncture. Pediatrics. 2018;141(3). doi: 10.1542/peds.2017-3405. PubMed
© 2019 Society of Hospital Medicine
Identifying Observation Stays in Medicare Data: Policy Implications of a Definition
Medicare observation stays are increasingly common. From 2006 to 2012, Medicare observation stays increased by 88%,1 whereas inpatient discharges decreased by 13.9%.2 In 2012, 1.7 million Medicare observation stays were recorded, and an additional 700,000 inpatient stays were preceded by observation services; the latter represented a 96% increase in status change since 2006.1 Yet no standard research methodology for identifying observation stays exists, including methods to identify and properly characterize “status change” events, which are hospital stays where initial and final inpatient or outpatient (observation) statuses differ.
With the increasing number of hospitalized patients classified as observation, a standard methodology for Medicare claims research is needed so that observation stays can be consistently identified and potentially included in future quality measures and outcomes. Existing research studies and government reports use widely varying criteria to identify observation stays, often lack detailed methods on observation stay case finding, and contain limited information on how status changes between inpatient and outpatient (observation) statuses are incorporated. This variability in approach may lead to omission and/or miscategorization of events and raises concern about the comparability of prior work.
This study aimed to determine the claims patterns of Medicare observation stays, define comprehensive claims-based methodology for future Medicare observation research and data reporting, and identify policy implications of such definition. We are poised to do this work because of our access to the nationally generalizable Centers for Medicare & Medicaid Services (CMS) linked Part A inpatient and outpatient data sets for 2013 and 2014, as well as our prior expertise in hospital observation research and Medicare claims studies.
METHODS
General Methods and Data Source
A 2014 national 20% random sample Part A and B Medicare data set was used. In accordance with the Centers for Medicare & Medicaid (CMS) data use agreement, all included beneficiaries had at least 1 acute care inpatient hospitalization. Included beneficiaries were enrolled for 12 months prior to their first 2014 inpatient stay. Those with Medicare Advantage or railroad benefits were excluded because of incomplete data per prior methods.3 The University of Wisconsin Institutional Review Board approved this study.
Comparison of Methods
The PubMED query “Medicare AND (observation OR observation unit),” limited to English and publication between January 1, 2010 and October 1, 2017, was conducted to determine the universe of prior observation stay definitions used in research for comparison (Appendix).4-20 The Office of Inspector General report,21 the Research Data Assistance Center (ResDAC),22 and Medicare Claims Processing Manual (MCPM)23 were also included. Methods stated in each publication were used to extrapolate observation stay case finding to the study data set.
Observation Stay Case Finding
Inpatient and outpatient revenue centers were queried for observation revenue center (ORC) codes identified by ResDAC,22 including 0760 (Treatment or observation room - general classification), 0761 (Treatment or observation room - treatment room), 0762 (Treatment or observation room – observation room), and 0769 (Treatment or observation room – other) occurring within 30 days of an inpatient stay. Healthcare Common Procedure Coding System (HCPCS) codes G0378 (Hospital observation service, per hour) and G0379 (Direct referral of patient for hospital observation care) were included per MCPM.23 A combination of these ORC and HCPCS codes was also used to identify observation stays in every Medicare claims observation study since 2010. When more than one ORC code per event was found, each ORC was recorded as part of that event. Presence of HCPCS G0378 and/or G0379 was determined for each event in association with event ORC(s), as was association of ORC codes with inpatient claims. In this manuscript, “observation stay” refers to an observation hospital stay, and “event” refers to a hospitalization that may include inpatient and/or outpatient (observation) services and ORC codes.
Status Change
All ORC codes found in the inpatient revenue center were assumed to represent status changes from outpatient (observation) to inpatient, as ORC codes may remain in claims data when the status changes to inpatient.24 Therefore, all events with ORC codes in the inpatient revenue center were considered inpatient hospitalizations.
For each ORC code found in the outpatient revenue center and also associated with an inpatient claim, timing of the ORC code in the inpatient claim was grouped into four categories to determine events with the final status of outpatient (observation stay). ResDAC defines the “From” date as “…the first day on the billing statement covering services rendered to the beneficiary.”24 For most hospitals, this is a three-day period prior to an inpatient admission where outpatient services are included in the Part A claim.25 We defined Category 1 as ORC codes occurring prior to claim “From” date; Category 2 as ORC codes on the inpatient “From” date, between the inpatient “From” date and admission date, or on the admission date; Category 3 as ORC codes between admission and discharge dates; and Category 4 as ORC codes occurring on or after the discharge date. Given that Category 4 represents the final hospitalization status, we considered Category 4 ORC codes in the outpatient revenue center associated with inpatient claims to be observation stays that had undergone a status change from inpatient to outpatient (observation).
University of Wisconsin Method
After excluding ORC codes in the inpatient revenue center as true inpatient hospitalizations, we defined an observation stay as 0760 and/or 0761 and/or 0762 and/or 0769 in the outpatient revenue center and having no association with an inpatient claim. To address a status change from inpatient to outpatient (observation), for those ORC codes in the outpatient revenue center also associated with an inpatient claim, claims with ORC codes in Category 4 were also considered observation stays.
RESULTS
Of 1,667,660 hospital events, 125,920 (7.6%) had an ORC code within 30 days of an inpatient hospitalization, of which 50,418 (3.0%) were found in the inpatient revenue center and 75,502 (4.5%) were from the outpatient revenue center. A total of 59,529 (47.3%) ORC codes occurred with an inpatient claim (50,418 in the inpatient revenue center and 9,111 in the outpatient revenue center), 5,628 (4.5%) had more than one ORC code on a single hospitalization, and more than 90% of codes were 0761 or 0762. These results illustrated variability in claims submissions as measured by the claims themselves and demonstrated a high rate of status changes (Table).
Observation stay definitions varied in the literature, with different methods capturing variable numbers of observation stays (Figure, Appendix). No methods clearly identified how to categorize events with status changes, directly addressed ORC codes in the inpatient revenue center, or discussed events with more than one ORC code. As such, some assumptions were made to extrapolate observation stay case findings as detailed in the Figure (see also Appendix). Notably, reference 4 methods were obtained via personal communication with the manuscript’s first author. The University of Wisconsin definition offers a comprehensive definition that classifies status change events, yielding 72,858 of 75,502 (96.5%) potential observation events as observation stays (Figure). These observation stays include 66,391 stays with ORC codes in the outpatient revenue center without status change or relation to inpatient claim, and 6467 (71.0%) of 9111 events with ORC codes in the outpatient revenue center were associated with an inpatient claim where ORC code(s) is located in Category 4.
CONCLUSIONS
This study confirmed the importance of a standard observation stay case finding methodology. Variability in prior methodology resulted in studies that may have included less than half of potential observation stays. In addition, most studies did not include, or were unclear, on how to address the increasing number of status changes. Others may have erroneously included hospitalizations that were ultimately billed as inpatient, and some publications lacked sufficient detailed methodology to extrapolate results with absolute certainty, a limitation of our comparative results. Although excluding some ORC codes in the outpatient revenue center associated with inpatient claims may possibly miss some observation stays, or including certain ORC codes, such as 0761 (treatment or observation room - treatment room), may erroneously include a different type of observation stay, the proposed University of Wisconsin method could be used as a comprehensive and reproducible method for observation stay case finding, including encounters with status change.
This study has other important policy implications. More than 90% of ORC codes were either 0761 or 0762, and in almost one in 20 claims, two or more distinct codes were identified. Given the lack of clinical relevance of terms “treatment” or “observation” room, and the frequency of more than 1 ORC code per claim, CMS may consider simplification to a single ORC code. Studies of observation encounter length of stay by hour may require G0378 in addition to an ORC code to define an observation stay, but doing so eliminates nearly half of observation claims. Additionally, G0379 adds minimal value to G0378 in case finding.
Finally, this study illustrates overall confusion with outpatient (observation) and inpatient status designations, with almost half (47.3%) of all hospitalizations with ORC codes also associated with an inpatient claim, demonstrating a high status change rate. More than 40% of all nurse case manager job postings are now for status determination work, shifting duties from patient care and quality improvement.26 We previously demonstrated a need for 5.1 FTE combined physician, attorney, and other personnel to manage the status, audit, and appeals process per institution.27 The frequency of status changes and personnel needed to maintain a two-tiered billing system argues for a single hospital status.
In summary, our study highlights the need for federal observation policy reform. We propose a standardized and reproducible approach for Medicare observation claims research, including status changes that can be used for further studies of observation stays.
Acknowledgments
The authors thank Jinn-ing Liou for analyst support, Jen Birstler for figure creation, and Carol Hermann for technical support. This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (Dr. Kind).
Disclosures
The authors have no relevant conflicts of interest to disclose.Funding: This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (
1. MedPAC Report to Congress. June 2015, Chapter 7. Hospital short-stay policy issues. http://medpac.gov/docs/default-source/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf?sfvrsn=0. Accessed December 21, 2017.
2. MedPAC Report to Congress. March 2017, Chapter 3. Hospital inpatient and outpatient services. http://medpac.gov/docs/default-source/reports/mar17_entirereport224610adfa9c665e80adff00009edf9c.pdf?sfvrsn=0. Accessed December 21, 2017.
3. Kind A, Jencks S, Crock J, et al. Neighborhood socioecomonic disadvantage and 30-day reshospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765-774. doi: 10.7326/M13-2946. PubMed
4. Zuckerman R, Sheingold S, Orav E, Ruhter J, Epstein A. Readmissions, observation, and the Hospital Readmissions Reduction Program. NEJM. 2016;374(16):1543-1551. doi: 10.1056/NEJMsa1513024. PubMed
5. Hockenberry J, Mutter R, Barrett M, Parlato J, Ross M. Factors associated with prolonged observation services stays and the impact of long stays on patient cost. Health Serv Res. 2014;49(3):893-909. 10.1111/1475-6773.12143. PubMed
6. Goldstein J, Zhang Z, Schwartz S, Hicks L. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2017;131(1):e9-101.e15. doi: 10.1016/j.amjmed.2017.07.013. PubMed
7. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff. 2012;31(6):1251-1259. doi: 10.1377/hlthaff.2012.0129. PubMed
8. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care. 2014;52(9):796-800. doi: 10.1097/MLR.0000000000000179 PubMed
9. Wright B, Jung H-Y, Feng Z, Mor V. Hospital, patient, and local health system characteristics associated with the prevalence and duration of observation care. HSR. 2014;49(4):1088-1107. doi: 10.1111/1475-6773.12166. PubMed
10. Overman R, Freburger J, Assimon M, Li X, Brookhart MA. Observation stays in administrative claims databases: underestimation of hospitalized cases. Pharmacoepidemiol Drug Saf. 2014;23(9):902-910. doi: 10.1002/pds.3647. PubMed
11. Vashi A, Cafardi S, Powers C, Ross J, Shrank W. Observation encounters and subsequent nursing facility stays. Am J Manag Care. 2015;21(4):e276-e281. PubMed
12. Venkatesh A, Wang C, Ross J, et al. Hospital use of observation stays: cross-sectional study of the impact on readmission rates. Med Care. 2016;54(12):1070-1077. doi: 10.1097/MLR.0000000000000601 PubMed
13. Gerhardt G, Yemane A, Apostle K, Oelschlaeger A, Rollins E, Brennan N. Evaluating whether changes in utilization of hospital outpatient services contributed to lower Medicare readmission rate. MMRR. 2014;4(1):E1-E13. doi: 10.5600/mmrr2014-004-01-b03 PubMed
14. Lipitz-Snyderman A, Klotz A, Gennarelli R, Groeger J. A population-based assessment of emergency department observation status for older adults with cancer. J Natl Compr Canc Netw. 2017;15(10):1234-1239. doi: 10.6004/jnccn.2017.0160. PubMed
15. Kangovi S, Cafardi S, Smith R, Kulkarni R, Grande D. Patient financial responsibility for observation care. J Hosp Med. 2015;10:718-723. doi: 10.1002/jhm.2436. PubMed
16. Dharmarajan K, Qin L, Bierlein M, et al. Outcomes after observation stays among older adult Medicare beneficiaries in the USA: retrospective cohort study. BMJ. 2017;357:j2616. doi: 10.1136/bmj.j2616 PubMed
17. Baier R, Gardner R, Coleman E, Jencks S, Mor V, Gravenstein S. Shifting the dialogue from hospital readmissions to unplanned care. Am J Manag Care. 2013;19(6):450-453. PubMed
18. Cafardi S, Pines J, Deb P, Powers C, Shrank W. Increased observation services in Medicare beneficiaries with chest pain. Am J Emergency Med. 2016;34(1):16-19. doi: 10.1016/j.ajem.2015.08.049. PubMed
19. Nuckols T, Fingar K, Barrett M, Steiner C, Stocks C, Owens P. The shifting landscape in utilization of inpatient, observation, and emergency department services across payors. J Hosp Med. 2017;12(6):443-446. doi: 10.12788/jhm.2751. PubMed
20. Wright B, Jung H-Y, Feng Z, Mor V. Trends in observation care among Medicare fee-for-service beneficiaries at critical access hospitals, 2007-2009. J Rural Health. 2013;29(1):s1-s6. doi: 10.1111/jrh.12007 PubMed
21. Office of Inspector General. Vulnerabilites remain under Medicare’s 2-Midnight hospital policy. 12-9-2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.asp. Accessed December 27, 2017. PubMed
22. Research Data Assistance Center (ResDAC). Revenue center table. https://www.resdac.org/sites/resdac.umn.edu/files/Revenue%20Center%20Table.txt. Accessed December 26, 2017.
23. Medicare Claims Processing Manual, Chapter 4, Section 290, Outpatient Observation Services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c04.pdf. Accessed December 26, 2017.
24. Research Data Assistance Center (ResDAC). Identifying observation stays for those beneficiaries admitted to the hospital. https://www.resdac.org/resconnect/articles/172. Accessed December 27, 2017.
25. Medicare Claims Processing Manual, Chapter 3, Section 40.B. Outpatient services treated as inpatient services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c03.pdf. Accessed December 26, 2017.
26. Reynolds J. Another look at roles and functions: has hospital case management lost its way? Prof Case Manag. 2013;18(5):246-254. doi: 10.1097/NCM.0b013e31829c8aa8. PubMed
27. Sheehy A, Locke C, Engel J, et al. Recovery audit contractor audit and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. doi: 10.1002/jhm.2332. PubMed
Medicare observation stays are increasingly common. From 2006 to 2012, Medicare observation stays increased by 88%,1 whereas inpatient discharges decreased by 13.9%.2 In 2012, 1.7 million Medicare observation stays were recorded, and an additional 700,000 inpatient stays were preceded by observation services; the latter represented a 96% increase in status change since 2006.1 Yet no standard research methodology for identifying observation stays exists, including methods to identify and properly characterize “status change” events, which are hospital stays where initial and final inpatient or outpatient (observation) statuses differ.
With the increasing number of hospitalized patients classified as observation, a standard methodology for Medicare claims research is needed so that observation stays can be consistently identified and potentially included in future quality measures and outcomes. Existing research studies and government reports use widely varying criteria to identify observation stays, often lack detailed methods on observation stay case finding, and contain limited information on how status changes between inpatient and outpatient (observation) statuses are incorporated. This variability in approach may lead to omission and/or miscategorization of events and raises concern about the comparability of prior work.
This study aimed to determine the claims patterns of Medicare observation stays, define comprehensive claims-based methodology for future Medicare observation research and data reporting, and identify policy implications of such definition. We are poised to do this work because of our access to the nationally generalizable Centers for Medicare & Medicaid Services (CMS) linked Part A inpatient and outpatient data sets for 2013 and 2014, as well as our prior expertise in hospital observation research and Medicare claims studies.
METHODS
General Methods and Data Source
A 2014 national 20% random sample Part A and B Medicare data set was used. In accordance with the Centers for Medicare & Medicaid (CMS) data use agreement, all included beneficiaries had at least 1 acute care inpatient hospitalization. Included beneficiaries were enrolled for 12 months prior to their first 2014 inpatient stay. Those with Medicare Advantage or railroad benefits were excluded because of incomplete data per prior methods.3 The University of Wisconsin Institutional Review Board approved this study.
Comparison of Methods
The PubMED query “Medicare AND (observation OR observation unit),” limited to English and publication between January 1, 2010 and October 1, 2017, was conducted to determine the universe of prior observation stay definitions used in research for comparison (Appendix).4-20 The Office of Inspector General report,21 the Research Data Assistance Center (ResDAC),22 and Medicare Claims Processing Manual (MCPM)23 were also included. Methods stated in each publication were used to extrapolate observation stay case finding to the study data set.
Observation Stay Case Finding
Inpatient and outpatient revenue centers were queried for observation revenue center (ORC) codes identified by ResDAC,22 including 0760 (Treatment or observation room - general classification), 0761 (Treatment or observation room - treatment room), 0762 (Treatment or observation room – observation room), and 0769 (Treatment or observation room – other) occurring within 30 days of an inpatient stay. Healthcare Common Procedure Coding System (HCPCS) codes G0378 (Hospital observation service, per hour) and G0379 (Direct referral of patient for hospital observation care) were included per MCPM.23 A combination of these ORC and HCPCS codes was also used to identify observation stays in every Medicare claims observation study since 2010. When more than one ORC code per event was found, each ORC was recorded as part of that event. Presence of HCPCS G0378 and/or G0379 was determined for each event in association with event ORC(s), as was association of ORC codes with inpatient claims. In this manuscript, “observation stay” refers to an observation hospital stay, and “event” refers to a hospitalization that may include inpatient and/or outpatient (observation) services and ORC codes.
Status Change
All ORC codes found in the inpatient revenue center were assumed to represent status changes from outpatient (observation) to inpatient, as ORC codes may remain in claims data when the status changes to inpatient.24 Therefore, all events with ORC codes in the inpatient revenue center were considered inpatient hospitalizations.
For each ORC code found in the outpatient revenue center and also associated with an inpatient claim, timing of the ORC code in the inpatient claim was grouped into four categories to determine events with the final status of outpatient (observation stay). ResDAC defines the “From” date as “…the first day on the billing statement covering services rendered to the beneficiary.”24 For most hospitals, this is a three-day period prior to an inpatient admission where outpatient services are included in the Part A claim.25 We defined Category 1 as ORC codes occurring prior to claim “From” date; Category 2 as ORC codes on the inpatient “From” date, between the inpatient “From” date and admission date, or on the admission date; Category 3 as ORC codes between admission and discharge dates; and Category 4 as ORC codes occurring on or after the discharge date. Given that Category 4 represents the final hospitalization status, we considered Category 4 ORC codes in the outpatient revenue center associated with inpatient claims to be observation stays that had undergone a status change from inpatient to outpatient (observation).
University of Wisconsin Method
After excluding ORC codes in the inpatient revenue center as true inpatient hospitalizations, we defined an observation stay as 0760 and/or 0761 and/or 0762 and/or 0769 in the outpatient revenue center and having no association with an inpatient claim. To address a status change from inpatient to outpatient (observation), for those ORC codes in the outpatient revenue center also associated with an inpatient claim, claims with ORC codes in Category 4 were also considered observation stays.
RESULTS
Of 1,667,660 hospital events, 125,920 (7.6%) had an ORC code within 30 days of an inpatient hospitalization, of which 50,418 (3.0%) were found in the inpatient revenue center and 75,502 (4.5%) were from the outpatient revenue center. A total of 59,529 (47.3%) ORC codes occurred with an inpatient claim (50,418 in the inpatient revenue center and 9,111 in the outpatient revenue center), 5,628 (4.5%) had more than one ORC code on a single hospitalization, and more than 90% of codes were 0761 or 0762. These results illustrated variability in claims submissions as measured by the claims themselves and demonstrated a high rate of status changes (Table).
Observation stay definitions varied in the literature, with different methods capturing variable numbers of observation stays (Figure, Appendix). No methods clearly identified how to categorize events with status changes, directly addressed ORC codes in the inpatient revenue center, or discussed events with more than one ORC code. As such, some assumptions were made to extrapolate observation stay case findings as detailed in the Figure (see also Appendix). Notably, reference 4 methods were obtained via personal communication with the manuscript’s first author. The University of Wisconsin definition offers a comprehensive definition that classifies status change events, yielding 72,858 of 75,502 (96.5%) potential observation events as observation stays (Figure). These observation stays include 66,391 stays with ORC codes in the outpatient revenue center without status change or relation to inpatient claim, and 6467 (71.0%) of 9111 events with ORC codes in the outpatient revenue center were associated with an inpatient claim where ORC code(s) is located in Category 4.
CONCLUSIONS
This study confirmed the importance of a standard observation stay case finding methodology. Variability in prior methodology resulted in studies that may have included less than half of potential observation stays. In addition, most studies did not include, or were unclear, on how to address the increasing number of status changes. Others may have erroneously included hospitalizations that were ultimately billed as inpatient, and some publications lacked sufficient detailed methodology to extrapolate results with absolute certainty, a limitation of our comparative results. Although excluding some ORC codes in the outpatient revenue center associated with inpatient claims may possibly miss some observation stays, or including certain ORC codes, such as 0761 (treatment or observation room - treatment room), may erroneously include a different type of observation stay, the proposed University of Wisconsin method could be used as a comprehensive and reproducible method for observation stay case finding, including encounters with status change.
This study has other important policy implications. More than 90% of ORC codes were either 0761 or 0762, and in almost one in 20 claims, two or more distinct codes were identified. Given the lack of clinical relevance of terms “treatment” or “observation” room, and the frequency of more than 1 ORC code per claim, CMS may consider simplification to a single ORC code. Studies of observation encounter length of stay by hour may require G0378 in addition to an ORC code to define an observation stay, but doing so eliminates nearly half of observation claims. Additionally, G0379 adds minimal value to G0378 in case finding.
Finally, this study illustrates overall confusion with outpatient (observation) and inpatient status designations, with almost half (47.3%) of all hospitalizations with ORC codes also associated with an inpatient claim, demonstrating a high status change rate. More than 40% of all nurse case manager job postings are now for status determination work, shifting duties from patient care and quality improvement.26 We previously demonstrated a need for 5.1 FTE combined physician, attorney, and other personnel to manage the status, audit, and appeals process per institution.27 The frequency of status changes and personnel needed to maintain a two-tiered billing system argues for a single hospital status.
In summary, our study highlights the need for federal observation policy reform. We propose a standardized and reproducible approach for Medicare observation claims research, including status changes that can be used for further studies of observation stays.
Acknowledgments
The authors thank Jinn-ing Liou for analyst support, Jen Birstler for figure creation, and Carol Hermann for technical support. This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (Dr. Kind).
Disclosures
The authors have no relevant conflicts of interest to disclose.Funding: This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (
Medicare observation stays are increasingly common. From 2006 to 2012, Medicare observation stays increased by 88%,1 whereas inpatient discharges decreased by 13.9%.2 In 2012, 1.7 million Medicare observation stays were recorded, and an additional 700,000 inpatient stays were preceded by observation services; the latter represented a 96% increase in status change since 2006.1 Yet no standard research methodology for identifying observation stays exists, including methods to identify and properly characterize “status change” events, which are hospital stays where initial and final inpatient or outpatient (observation) statuses differ.
With the increasing number of hospitalized patients classified as observation, a standard methodology for Medicare claims research is needed so that observation stays can be consistently identified and potentially included in future quality measures and outcomes. Existing research studies and government reports use widely varying criteria to identify observation stays, often lack detailed methods on observation stay case finding, and contain limited information on how status changes between inpatient and outpatient (observation) statuses are incorporated. This variability in approach may lead to omission and/or miscategorization of events and raises concern about the comparability of prior work.
This study aimed to determine the claims patterns of Medicare observation stays, define comprehensive claims-based methodology for future Medicare observation research and data reporting, and identify policy implications of such definition. We are poised to do this work because of our access to the nationally generalizable Centers for Medicare & Medicaid Services (CMS) linked Part A inpatient and outpatient data sets for 2013 and 2014, as well as our prior expertise in hospital observation research and Medicare claims studies.
METHODS
General Methods and Data Source
A 2014 national 20% random sample Part A and B Medicare data set was used. In accordance with the Centers for Medicare & Medicaid (CMS) data use agreement, all included beneficiaries had at least 1 acute care inpatient hospitalization. Included beneficiaries were enrolled for 12 months prior to their first 2014 inpatient stay. Those with Medicare Advantage or railroad benefits were excluded because of incomplete data per prior methods.3 The University of Wisconsin Institutional Review Board approved this study.
Comparison of Methods
The PubMED query “Medicare AND (observation OR observation unit),” limited to English and publication between January 1, 2010 and October 1, 2017, was conducted to determine the universe of prior observation stay definitions used in research for comparison (Appendix).4-20 The Office of Inspector General report,21 the Research Data Assistance Center (ResDAC),22 and Medicare Claims Processing Manual (MCPM)23 were also included. Methods stated in each publication were used to extrapolate observation stay case finding to the study data set.
Observation Stay Case Finding
Inpatient and outpatient revenue centers were queried for observation revenue center (ORC) codes identified by ResDAC,22 including 0760 (Treatment or observation room - general classification), 0761 (Treatment or observation room - treatment room), 0762 (Treatment or observation room – observation room), and 0769 (Treatment or observation room – other) occurring within 30 days of an inpatient stay. Healthcare Common Procedure Coding System (HCPCS) codes G0378 (Hospital observation service, per hour) and G0379 (Direct referral of patient for hospital observation care) were included per MCPM.23 A combination of these ORC and HCPCS codes was also used to identify observation stays in every Medicare claims observation study since 2010. When more than one ORC code per event was found, each ORC was recorded as part of that event. Presence of HCPCS G0378 and/or G0379 was determined for each event in association with event ORC(s), as was association of ORC codes with inpatient claims. In this manuscript, “observation stay” refers to an observation hospital stay, and “event” refers to a hospitalization that may include inpatient and/or outpatient (observation) services and ORC codes.
Status Change
All ORC codes found in the inpatient revenue center were assumed to represent status changes from outpatient (observation) to inpatient, as ORC codes may remain in claims data when the status changes to inpatient.24 Therefore, all events with ORC codes in the inpatient revenue center were considered inpatient hospitalizations.
For each ORC code found in the outpatient revenue center and also associated with an inpatient claim, timing of the ORC code in the inpatient claim was grouped into four categories to determine events with the final status of outpatient (observation stay). ResDAC defines the “From” date as “…the first day on the billing statement covering services rendered to the beneficiary.”24 For most hospitals, this is a three-day period prior to an inpatient admission where outpatient services are included in the Part A claim.25 We defined Category 1 as ORC codes occurring prior to claim “From” date; Category 2 as ORC codes on the inpatient “From” date, between the inpatient “From” date and admission date, or on the admission date; Category 3 as ORC codes between admission and discharge dates; and Category 4 as ORC codes occurring on or after the discharge date. Given that Category 4 represents the final hospitalization status, we considered Category 4 ORC codes in the outpatient revenue center associated with inpatient claims to be observation stays that had undergone a status change from inpatient to outpatient (observation).
University of Wisconsin Method
After excluding ORC codes in the inpatient revenue center as true inpatient hospitalizations, we defined an observation stay as 0760 and/or 0761 and/or 0762 and/or 0769 in the outpatient revenue center and having no association with an inpatient claim. To address a status change from inpatient to outpatient (observation), for those ORC codes in the outpatient revenue center also associated with an inpatient claim, claims with ORC codes in Category 4 were also considered observation stays.
RESULTS
Of 1,667,660 hospital events, 125,920 (7.6%) had an ORC code within 30 days of an inpatient hospitalization, of which 50,418 (3.0%) were found in the inpatient revenue center and 75,502 (4.5%) were from the outpatient revenue center. A total of 59,529 (47.3%) ORC codes occurred with an inpatient claim (50,418 in the inpatient revenue center and 9,111 in the outpatient revenue center), 5,628 (4.5%) had more than one ORC code on a single hospitalization, and more than 90% of codes were 0761 or 0762. These results illustrated variability in claims submissions as measured by the claims themselves and demonstrated a high rate of status changes (Table).
Observation stay definitions varied in the literature, with different methods capturing variable numbers of observation stays (Figure, Appendix). No methods clearly identified how to categorize events with status changes, directly addressed ORC codes in the inpatient revenue center, or discussed events with more than one ORC code. As such, some assumptions were made to extrapolate observation stay case findings as detailed in the Figure (see also Appendix). Notably, reference 4 methods were obtained via personal communication with the manuscript’s first author. The University of Wisconsin definition offers a comprehensive definition that classifies status change events, yielding 72,858 of 75,502 (96.5%) potential observation events as observation stays (Figure). These observation stays include 66,391 stays with ORC codes in the outpatient revenue center without status change or relation to inpatient claim, and 6467 (71.0%) of 9111 events with ORC codes in the outpatient revenue center were associated with an inpatient claim where ORC code(s) is located in Category 4.
CONCLUSIONS
This study confirmed the importance of a standard observation stay case finding methodology. Variability in prior methodology resulted in studies that may have included less than half of potential observation stays. In addition, most studies did not include, or were unclear, on how to address the increasing number of status changes. Others may have erroneously included hospitalizations that were ultimately billed as inpatient, and some publications lacked sufficient detailed methodology to extrapolate results with absolute certainty, a limitation of our comparative results. Although excluding some ORC codes in the outpatient revenue center associated with inpatient claims may possibly miss some observation stays, or including certain ORC codes, such as 0761 (treatment or observation room - treatment room), may erroneously include a different type of observation stay, the proposed University of Wisconsin method could be used as a comprehensive and reproducible method for observation stay case finding, including encounters with status change.
This study has other important policy implications. More than 90% of ORC codes were either 0761 or 0762, and in almost one in 20 claims, two or more distinct codes were identified. Given the lack of clinical relevance of terms “treatment” or “observation” room, and the frequency of more than 1 ORC code per claim, CMS may consider simplification to a single ORC code. Studies of observation encounter length of stay by hour may require G0378 in addition to an ORC code to define an observation stay, but doing so eliminates nearly half of observation claims. Additionally, G0379 adds minimal value to G0378 in case finding.
Finally, this study illustrates overall confusion with outpatient (observation) and inpatient status designations, with almost half (47.3%) of all hospitalizations with ORC codes also associated with an inpatient claim, demonstrating a high status change rate. More than 40% of all nurse case manager job postings are now for status determination work, shifting duties from patient care and quality improvement.26 We previously demonstrated a need for 5.1 FTE combined physician, attorney, and other personnel to manage the status, audit, and appeals process per institution.27 The frequency of status changes and personnel needed to maintain a two-tiered billing system argues for a single hospital status.
In summary, our study highlights the need for federal observation policy reform. We propose a standardized and reproducible approach for Medicare observation claims research, including status changes that can be used for further studies of observation stays.
Acknowledgments
The authors thank Jinn-ing Liou for analyst support, Jen Birstler for figure creation, and Carol Hermann for technical support. This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (Dr. Kind).
Disclosures
The authors have no relevant conflicts of interest to disclose.Funding: This work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under Award Number R01MD010243 (
1. MedPAC Report to Congress. June 2015, Chapter 7. Hospital short-stay policy issues. http://medpac.gov/docs/default-source/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf?sfvrsn=0. Accessed December 21, 2017.
2. MedPAC Report to Congress. March 2017, Chapter 3. Hospital inpatient and outpatient services. http://medpac.gov/docs/default-source/reports/mar17_entirereport224610adfa9c665e80adff00009edf9c.pdf?sfvrsn=0. Accessed December 21, 2017.
3. Kind A, Jencks S, Crock J, et al. Neighborhood socioecomonic disadvantage and 30-day reshospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765-774. doi: 10.7326/M13-2946. PubMed
4. Zuckerman R, Sheingold S, Orav E, Ruhter J, Epstein A. Readmissions, observation, and the Hospital Readmissions Reduction Program. NEJM. 2016;374(16):1543-1551. doi: 10.1056/NEJMsa1513024. PubMed
5. Hockenberry J, Mutter R, Barrett M, Parlato J, Ross M. Factors associated with prolonged observation services stays and the impact of long stays on patient cost. Health Serv Res. 2014;49(3):893-909. 10.1111/1475-6773.12143. PubMed
6. Goldstein J, Zhang Z, Schwartz S, Hicks L. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2017;131(1):e9-101.e15. doi: 10.1016/j.amjmed.2017.07.013. PubMed
7. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff. 2012;31(6):1251-1259. doi: 10.1377/hlthaff.2012.0129. PubMed
8. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care. 2014;52(9):796-800. doi: 10.1097/MLR.0000000000000179 PubMed
9. Wright B, Jung H-Y, Feng Z, Mor V. Hospital, patient, and local health system characteristics associated with the prevalence and duration of observation care. HSR. 2014;49(4):1088-1107. doi: 10.1111/1475-6773.12166. PubMed
10. Overman R, Freburger J, Assimon M, Li X, Brookhart MA. Observation stays in administrative claims databases: underestimation of hospitalized cases. Pharmacoepidemiol Drug Saf. 2014;23(9):902-910. doi: 10.1002/pds.3647. PubMed
11. Vashi A, Cafardi S, Powers C, Ross J, Shrank W. Observation encounters and subsequent nursing facility stays. Am J Manag Care. 2015;21(4):e276-e281. PubMed
12. Venkatesh A, Wang C, Ross J, et al. Hospital use of observation stays: cross-sectional study of the impact on readmission rates. Med Care. 2016;54(12):1070-1077. doi: 10.1097/MLR.0000000000000601 PubMed
13. Gerhardt G, Yemane A, Apostle K, Oelschlaeger A, Rollins E, Brennan N. Evaluating whether changes in utilization of hospital outpatient services contributed to lower Medicare readmission rate. MMRR. 2014;4(1):E1-E13. doi: 10.5600/mmrr2014-004-01-b03 PubMed
14. Lipitz-Snyderman A, Klotz A, Gennarelli R, Groeger J. A population-based assessment of emergency department observation status for older adults with cancer. J Natl Compr Canc Netw. 2017;15(10):1234-1239. doi: 10.6004/jnccn.2017.0160. PubMed
15. Kangovi S, Cafardi S, Smith R, Kulkarni R, Grande D. Patient financial responsibility for observation care. J Hosp Med. 2015;10:718-723. doi: 10.1002/jhm.2436. PubMed
16. Dharmarajan K, Qin L, Bierlein M, et al. Outcomes after observation stays among older adult Medicare beneficiaries in the USA: retrospective cohort study. BMJ. 2017;357:j2616. doi: 10.1136/bmj.j2616 PubMed
17. Baier R, Gardner R, Coleman E, Jencks S, Mor V, Gravenstein S. Shifting the dialogue from hospital readmissions to unplanned care. Am J Manag Care. 2013;19(6):450-453. PubMed
18. Cafardi S, Pines J, Deb P, Powers C, Shrank W. Increased observation services in Medicare beneficiaries with chest pain. Am J Emergency Med. 2016;34(1):16-19. doi: 10.1016/j.ajem.2015.08.049. PubMed
19. Nuckols T, Fingar K, Barrett M, Steiner C, Stocks C, Owens P. The shifting landscape in utilization of inpatient, observation, and emergency department services across payors. J Hosp Med. 2017;12(6):443-446. doi: 10.12788/jhm.2751. PubMed
20. Wright B, Jung H-Y, Feng Z, Mor V. Trends in observation care among Medicare fee-for-service beneficiaries at critical access hospitals, 2007-2009. J Rural Health. 2013;29(1):s1-s6. doi: 10.1111/jrh.12007 PubMed
21. Office of Inspector General. Vulnerabilites remain under Medicare’s 2-Midnight hospital policy. 12-9-2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.asp. Accessed December 27, 2017. PubMed
22. Research Data Assistance Center (ResDAC). Revenue center table. https://www.resdac.org/sites/resdac.umn.edu/files/Revenue%20Center%20Table.txt. Accessed December 26, 2017.
23. Medicare Claims Processing Manual, Chapter 4, Section 290, Outpatient Observation Services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c04.pdf. Accessed December 26, 2017.
24. Research Data Assistance Center (ResDAC). Identifying observation stays for those beneficiaries admitted to the hospital. https://www.resdac.org/resconnect/articles/172. Accessed December 27, 2017.
25. Medicare Claims Processing Manual, Chapter 3, Section 40.B. Outpatient services treated as inpatient services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c03.pdf. Accessed December 26, 2017.
26. Reynolds J. Another look at roles and functions: has hospital case management lost its way? Prof Case Manag. 2013;18(5):246-254. doi: 10.1097/NCM.0b013e31829c8aa8. PubMed
27. Sheehy A, Locke C, Engel J, et al. Recovery audit contractor audit and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. doi: 10.1002/jhm.2332. PubMed
1. MedPAC Report to Congress. June 2015, Chapter 7. Hospital short-stay policy issues. http://medpac.gov/docs/default-source/reports/june-2015-report-to-the-congress-medicare-and-the-health-care-delivery-system.pdf?sfvrsn=0. Accessed December 21, 2017.
2. MedPAC Report to Congress. March 2017, Chapter 3. Hospital inpatient and outpatient services. http://medpac.gov/docs/default-source/reports/mar17_entirereport224610adfa9c665e80adff00009edf9c.pdf?sfvrsn=0. Accessed December 21, 2017.
3. Kind A, Jencks S, Crock J, et al. Neighborhood socioecomonic disadvantage and 30-day reshospitalization: a retrospective cohort study. Ann Intern Med. 2014;161(11):765-774. doi: 10.7326/M13-2946. PubMed
4. Zuckerman R, Sheingold S, Orav E, Ruhter J, Epstein A. Readmissions, observation, and the Hospital Readmissions Reduction Program. NEJM. 2016;374(16):1543-1551. doi: 10.1056/NEJMsa1513024. PubMed
5. Hockenberry J, Mutter R, Barrett M, Parlato J, Ross M. Factors associated with prolonged observation services stays and the impact of long stays on patient cost. Health Serv Res. 2014;49(3):893-909. 10.1111/1475-6773.12143. PubMed
6. Goldstein J, Zhang Z, Schwartz S, Hicks L. Observation status, poverty, and high financial liability among Medicare beneficiaries. Am J Med. 2017;131(1):e9-101.e15. doi: 10.1016/j.amjmed.2017.07.013. PubMed
7. Feng Z, Wright B, Mor V. Sharp rise in Medicare enrollees being held in hospitals for observation raises concerns about causes and consequences. Health Aff. 2012;31(6):1251-1259. doi: 10.1377/hlthaff.2012.0129. PubMed
8. Feng Z, Jung H-Y, Wright B, Mor V. The origin and disposition of Medicare observation stays. Med Care. 2014;52(9):796-800. doi: 10.1097/MLR.0000000000000179 PubMed
9. Wright B, Jung H-Y, Feng Z, Mor V. Hospital, patient, and local health system characteristics associated with the prevalence and duration of observation care. HSR. 2014;49(4):1088-1107. doi: 10.1111/1475-6773.12166. PubMed
10. Overman R, Freburger J, Assimon M, Li X, Brookhart MA. Observation stays in administrative claims databases: underestimation of hospitalized cases. Pharmacoepidemiol Drug Saf. 2014;23(9):902-910. doi: 10.1002/pds.3647. PubMed
11. Vashi A, Cafardi S, Powers C, Ross J, Shrank W. Observation encounters and subsequent nursing facility stays. Am J Manag Care. 2015;21(4):e276-e281. PubMed
12. Venkatesh A, Wang C, Ross J, et al. Hospital use of observation stays: cross-sectional study of the impact on readmission rates. Med Care. 2016;54(12):1070-1077. doi: 10.1097/MLR.0000000000000601 PubMed
13. Gerhardt G, Yemane A, Apostle K, Oelschlaeger A, Rollins E, Brennan N. Evaluating whether changes in utilization of hospital outpatient services contributed to lower Medicare readmission rate. MMRR. 2014;4(1):E1-E13. doi: 10.5600/mmrr2014-004-01-b03 PubMed
14. Lipitz-Snyderman A, Klotz A, Gennarelli R, Groeger J. A population-based assessment of emergency department observation status for older adults with cancer. J Natl Compr Canc Netw. 2017;15(10):1234-1239. doi: 10.6004/jnccn.2017.0160. PubMed
15. Kangovi S, Cafardi S, Smith R, Kulkarni R, Grande D. Patient financial responsibility for observation care. J Hosp Med. 2015;10:718-723. doi: 10.1002/jhm.2436. PubMed
16. Dharmarajan K, Qin L, Bierlein M, et al. Outcomes after observation stays among older adult Medicare beneficiaries in the USA: retrospective cohort study. BMJ. 2017;357:j2616. doi: 10.1136/bmj.j2616 PubMed
17. Baier R, Gardner R, Coleman E, Jencks S, Mor V, Gravenstein S. Shifting the dialogue from hospital readmissions to unplanned care. Am J Manag Care. 2013;19(6):450-453. PubMed
18. Cafardi S, Pines J, Deb P, Powers C, Shrank W. Increased observation services in Medicare beneficiaries with chest pain. Am J Emergency Med. 2016;34(1):16-19. doi: 10.1016/j.ajem.2015.08.049. PubMed
19. Nuckols T, Fingar K, Barrett M, Steiner C, Stocks C, Owens P. The shifting landscape in utilization of inpatient, observation, and emergency department services across payors. J Hosp Med. 2017;12(6):443-446. doi: 10.12788/jhm.2751. PubMed
20. Wright B, Jung H-Y, Feng Z, Mor V. Trends in observation care among Medicare fee-for-service beneficiaries at critical access hospitals, 2007-2009. J Rural Health. 2013;29(1):s1-s6. doi: 10.1111/jrh.12007 PubMed
21. Office of Inspector General. Vulnerabilites remain under Medicare’s 2-Midnight hospital policy. 12-9-2016. https://oig.hhs.gov/oei/reports/oei-02-15-00020.asp. Accessed December 27, 2017. PubMed
22. Research Data Assistance Center (ResDAC). Revenue center table. https://www.resdac.org/sites/resdac.umn.edu/files/Revenue%20Center%20Table.txt. Accessed December 26, 2017.
23. Medicare Claims Processing Manual, Chapter 4, Section 290, Outpatient Observation Services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/downloads/clm104c04.pdf. Accessed December 26, 2017.
24. Research Data Assistance Center (ResDAC). Identifying observation stays for those beneficiaries admitted to the hospital. https://www.resdac.org/resconnect/articles/172. Accessed December 27, 2017.
25. Medicare Claims Processing Manual, Chapter 3, Section 40.B. Outpatient services treated as inpatient services. https://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/clm104c03.pdf. Accessed December 26, 2017.
26. Reynolds J. Another look at roles and functions: has hospital case management lost its way? Prof Case Manag. 2013;18(5):246-254. doi: 10.1097/NCM.0b013e31829c8aa8. PubMed
27. Sheehy A, Locke C, Engel J, et al. Recovery audit contractor audit and appeals at three academic medical centers. J Hosp Med. 2015;10(4):212-219. doi: 10.1002/jhm.2332. PubMed
© 2019 Society of Hospital Medicine
Effectiveness of SIESTA on Objective and Subjective Metrics of Nighttime Hospital Sleep Disruptors
Although sleep is critical to patient recovery in the hospital, hospitalization is not restful,1,2 and inpatient sleep deprivation has been linked to poor health outcomes.1-4 The American Academy of Nursing’s Choosing Wisely® campaign recommends nurses reduce unnecessary nocturnal care.5 However, interventions to improve inpatient sleep are not widely implemented.6 Targeting routine disruptions, such as overnight vital signs, by changing default settings in the electronic health record (EHR)with “nudges” could be a cost-effective strategy to improve inpatient sleep.4,7
We created Sleep for Inpatients: Empowering Staff to Act (SIESTA), which pairs nudges in the EHR with interprofessional education and empowerment,8 and tested its effectiveness on objectively and subjectively measured nocturnal sleep disruptors.
METHODS
Study Design
Two 18-room University of Chicago Medicine general-medicine units were used in this prospective study. The SIESTA-enhanced unit underwent the full sleep intervention: nursing education and empowerment, physician education, and EHR changes. The standard unit did not receive nursing interventions but received all other forms of intervention. Because physicians simultaneously cared for patients on both units, all internal medicine residents and hospitalists received the same education. The study population included physicians, nurses, and awake English-speaking patients who were cognitively intact and admitted to these two units. The University of Chicago Institutional Review Board approved this study (12-1766; 16685B).
Development of SIESTA
To develop SIESTA, patients were surveyed, and focus groups of staff were conducted; overnight vitals, medications, and phlebotomy were identified as major barriers to patient sleep.9 We found that physicians did not know how to change the default vital signs order “every 4 hours” or how to batch-order morning phlebotomy at a time other than 4:00
Behavioral Nudges
The SIESTA team worked with clinical informaticists to change the default orders in EpicTM (Epic Systems Corporation, 2017, Verona, Wisconsin) in September 2015 so that physicians would be asked, “Continue vital signs throughout the night?”10 Previously, this question was marked “Yes” by default and hidden. While the default protocol for heparin q8h was maintained, heparin q12h (9:00
SIESTA Physician Education
We created a 20-minute presentation on the consequences and causes of in-hospital sleep deprivation and evidence-based behavioral modification. We distributed pocket cards describing the mnemonic SIESTA (Screen patients for sleep disorders, Instruct patients on sleep hygiene, Eliminate disruptions, Shut doors, Treat pain, and Alarm and noise control). Physicians were instructed to consider forgoing overnight vitals, using clinical judgment to identify stable patients, use a sleep-promoting VTE prophylaxis option, and order daily labs at 10:00
SIESTA-Enhanced Unit
In the SIESTA-enhanced unit, nurses received education using pocket cards and were coached to collaborate with physicians to implement sleep-friendly orders. Customized signage depicting empowered nurses advocating for patients was posted near the huddle board. Because these nurses suggested adding SIESTA to the nurses’ ongoing daily huddles at 4:00
Data Collection
Objectively Measured Sleep Disruptors
Adoption of SIESTA orders from March 2015 to March 2016 was assessed with a monthly EpicTM Clarity report. From August 1, 2015 to April 1, 2016, nocturnal room entries were recorded using the GOJO SMARTLINKTM Hand Hygiene system (GOJO Industries Inc., 2017, Akron, Ohio). This system includes two components: the hand-sanitizer dispensers, which track dispenses (numerator), and door-mounted Activity Counters, which use heat sensors that react to body heat emitted by a person passing through the doorway (denominator for hand-hygiene compliance). For our analysis, we only used Activity Counter data, which count room entries and exits, regardless of whether sanitizer was dispensed.
Patient-Reported Nighttime Sleep Disruptions
From June 2015 to March 2016, research assistants administered a 10-item Potential Hospital Sleep Disruptions and Noises Questionnaire (PHSDNQ) to patients in both units. Responses to this questionnaire correlate with actigraphy-based sleep measurements.9,12,13 Surveys were administered every other weekday to patients available to participate (eg, willing to participate, on the unit, awake). Survey data were stored on the REDCap Database (Version 6.14.0; Vanderbilt University, 2016, Nashville, Tennessee). Pre- and post-intervention Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) “top-box ratings” for percent quiet at night and percent pain well controlled were also compared.
Data Analysis
Objectively Measured Potential Sleep Disruptors
The proportion of sleep-friendly orders was analyzed using a two-sample test for proportions pre-post for the SIESTA-enhanced and standard units. The difference in use of SIESTA orders between units was analyzed via multivariable logistic regression, testing for independent associations between post-period, SIESTA-enhanced unit, and an interaction term (post-period × SIESTA unit) on use of sleep-friendly orders.
Room entries per night (11:00
Patient-Reported Nighttime Sleep Disruptions
Per prior studies, we defined a score 2 or higher as “sleep disruption.”9 Differences between units were evaluated via multivariable logistic regression to examine the association between the interaction of post-period × SIESTA-enhanced unit and odds of not reporting a sleep disruption. Significance was denoted as P = .05.
RESULTS
Between March 2015 and March 2016, 1,083 general-medicine patients were admitted to the SIESTA-enhanced and standard units (Table).
Nocturnal Orders
From March 2015 to March 2016, 1,669 EpicTM general medicine orders were reviewed (Figure). In the SIESTA-enhanced unit, the mean percentage of sleep-friendly orders rose for both vital signs (+31% [95% CI = 25%, 36%]; P < .001, npre = 306, npost = 306] and VTE prophylaxis (+28% [95% CI = 18%, 37%]; P < .001, npre = 158, npost = 173]. Similar changes were observed in the standard unit for sleep-friendly vital signs (+20% [95% CI = 14%, 25%]; P < .001, npre = 252, npost = 219) and VTE prophylaxis (+16% [95% CI = 6%, 25%]; P = .002, npre = 130, npost = 125). Differences between the two units were not statistically significant, and no significant change in timing of laboratory orders postintervention was found.
Nighttime Room Entries
Immediately after SIESTA launch, an average decrease of 114 total entries/night were noted in the SIESTA-enhanced unit, ([95% CI = −138, −91]; P < .001), corresponding to a 44% reduction (−6.3 entries/room) from the mean of 14.3 entries per patient room at baseline (Figure). No statistically significant change was seen in the standard unit. After SIESTA was incorporated into nursing huddles, total disruptions/night decreased by 1.31 disruptions/night ([95% CI = −1.64, −0.98]; P < .001) in the SIESTA-enhanced unit; by comparison, no significant changes were observed in the standard unit.
Patient-Reported Nighttime Sleep Disruptions
Between June 2015 and March 2016, 201 patient surveys were collected. A significant interaction was observed between the SIESTA-enhanced unit and post-period, and patients in the SIESTA-enhanced unit were more likely to report not being disrupted by medications (OR 4.08 [95% CI = 1.13–14.07]; P = .031) and vital signs (OR 3.35 [95% CI = 1.00–11.2]; P = .05) than those in the standard unit. HCAHPS top-box scores for the SIESTA unit increased by 7% for the “Quiet at night” category and 9% for the “Pain well controlled” category; by comparison, no major changes (>5%) were observed in the standard unit.
DISCUSSION
The present SIESTA intervention demonstrated that physician education coupled with EHR default changes are associated with a significant reduction in orders for overnight vital signs and medication administration in both units. However, addition of nursing education and empowerment in the SIESTA-enhanced unit was associated with fewer nocturnal room entries and improvements in patient-reported outcomes compared with those in the standard unit.
This study presents several implications for hospital initiatives aiming to improve patient sleep.14 Our study is consistent with other research highlighting the hypothesis that altering the default settings of EHR systems can influence physician behavior in a sustainable manner.15 However, our study also finds that, even when sleep-friendly orders are present, creating a sleep-friendly environment likely depends on the unit-based nurses championing the cause. While the initial decrease in nocturnal room entries post-SIESTA eventually faded, sustainable changes were observed only after SIESTA was added to nursing huddles, which illustrates the importance of using multiple methods to nudge staff.
Our study includes a number of limitations. It is not a randomized controlled trial, we cannot assume causality, and contamination was assumed, as residents and hospitalists worked in both units. Our single-site study may not be generalizable. Low HCAHPS response rates (10%-20%) also prevent demonstration of statistically significant differences. Finally, our convenience sampling strategy means not all inpatients were surveyed, and objective sleep duration was not measured.
In summary, at the University of Chicago, SIESTA could be associated with adoption of sleep-friendly vitals and medication orders, a decrease in nighttime room entries, and improved patient experience.
Disclosures
The authors have nothing to disclose.
Funding
This study was funded by the National Institute on Aging (NIA Grant No. T35AG029795) and the National Heart, Lung, and Blood Institute (NHLBI Grant Nos. R25HL116372 and K24HL136859).
1. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients - a clinical review [published online ahead of print February 26, 2016]. Ann Intensive Care. 2015;5(3). doi: 10.1186/s13613-015-0043-2. PubMed
2. Arora VM, Chang KL, Fazal AZ, et al. Objective sleep duration and quality in hospitalized older adults: associations with blood pressure and mood. J Am Geriatr Soc. 2011;59(11):2185-2186. doi: 10.1111/j.1532-5415.2011.03644.x. PubMed
3. Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163-178. doi: 10.1016/j.smrv.2007.01.002. PubMed
4. Manian FA, Manian CJ. Sleep quality in adult hospitalized patients with infection: an observational study. Am J Med Sci. 2015;349(1):56-60. doi: 10.1097/MAJ.0000000000000355. PubMed
5. American Academy of Nursing announced engagement in National Choosing Wisely Campaign. Nurs Outlook. 2015;63(1):96-98. doi: 10.1016/j.outlook.2014.12.017. PubMed
6. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi: 10.1002/jhm.2578. PubMed
7. Fillary J, Chaplin H, Jones G, Thompson A, Holme A, Wilson P. Noise at night in hospital general wards: a mapping of the literature. Br J Nurs. 2015;24(10):536-540. doi: 10.12968/bjon.2015.24.10.536. PubMed
8. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. Yale University Press; 2008.
9. Grossman MN, Anderson SL, Worku A, et al. Awakenings? Patient and hospital staff perceptions of nighttime disruptions and their effect on patient sleep. J Clin Sleep Med. 2017;13(2):301-306. doi: 10.5664/jcsm.6468. PubMed
10. Yoder JC, Yuen TC, Churpek MM, Arora VM, Edelson DP. A prospective study of nighttime vital sign monitoring frequency and risk of clinical deterioration. JAMA Intern Med. 2013;173(16):1554-1555. doi: 10.1001/jamainternmed.2013.7791. PubMed
11. Phung OJ, Kahn SR, Cook DJ, Murad MH. Dosing frequency of unfractionated heparin thromboprophylaxis: a meta-analysis. Chest. 2011;140(2):374-381. doi: 10.1378/chest.10-3084. PubMed
12. Gabor JY, Cooper AB, Hanly PJ. Sleep disruption in the intensive care unit. Curr Opin Crit Care. 2001;7(1):21-27. PubMed
13. Topf M. Personal and environmental predictors of patient disturbance due to hospital noise. J Appl Psychol. 1985;70(1):22-28. doi: 10.1037/0021-9010.70.1.22. PubMed
14. Cho HJ, Wray CM, Maione S, et al. Right care in hospital medicine: co-creation of ten opportunities in overuse and underuse for improving value in hospital medicine. J Gen Intern Med. 2018;33(6):804-806. doi: 10.1007/s11606-018-4371-4. PubMed
15. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. doi: 10.1056/NEJMsb071595. PubMed
Although sleep is critical to patient recovery in the hospital, hospitalization is not restful,1,2 and inpatient sleep deprivation has been linked to poor health outcomes.1-4 The American Academy of Nursing’s Choosing Wisely® campaign recommends nurses reduce unnecessary nocturnal care.5 However, interventions to improve inpatient sleep are not widely implemented.6 Targeting routine disruptions, such as overnight vital signs, by changing default settings in the electronic health record (EHR)with “nudges” could be a cost-effective strategy to improve inpatient sleep.4,7
We created Sleep for Inpatients: Empowering Staff to Act (SIESTA), which pairs nudges in the EHR with interprofessional education and empowerment,8 and tested its effectiveness on objectively and subjectively measured nocturnal sleep disruptors.
METHODS
Study Design
Two 18-room University of Chicago Medicine general-medicine units were used in this prospective study. The SIESTA-enhanced unit underwent the full sleep intervention: nursing education and empowerment, physician education, and EHR changes. The standard unit did not receive nursing interventions but received all other forms of intervention. Because physicians simultaneously cared for patients on both units, all internal medicine residents and hospitalists received the same education. The study population included physicians, nurses, and awake English-speaking patients who were cognitively intact and admitted to these two units. The University of Chicago Institutional Review Board approved this study (12-1766; 16685B).
Development of SIESTA
To develop SIESTA, patients were surveyed, and focus groups of staff were conducted; overnight vitals, medications, and phlebotomy were identified as major barriers to patient sleep.9 We found that physicians did not know how to change the default vital signs order “every 4 hours” or how to batch-order morning phlebotomy at a time other than 4:00
Behavioral Nudges
The SIESTA team worked with clinical informaticists to change the default orders in EpicTM (Epic Systems Corporation, 2017, Verona, Wisconsin) in September 2015 so that physicians would be asked, “Continue vital signs throughout the night?”10 Previously, this question was marked “Yes” by default and hidden. While the default protocol for heparin q8h was maintained, heparin q12h (9:00
SIESTA Physician Education
We created a 20-minute presentation on the consequences and causes of in-hospital sleep deprivation and evidence-based behavioral modification. We distributed pocket cards describing the mnemonic SIESTA (Screen patients for sleep disorders, Instruct patients on sleep hygiene, Eliminate disruptions, Shut doors, Treat pain, and Alarm and noise control). Physicians were instructed to consider forgoing overnight vitals, using clinical judgment to identify stable patients, use a sleep-promoting VTE prophylaxis option, and order daily labs at 10:00
SIESTA-Enhanced Unit
In the SIESTA-enhanced unit, nurses received education using pocket cards and were coached to collaborate with physicians to implement sleep-friendly orders. Customized signage depicting empowered nurses advocating for patients was posted near the huddle board. Because these nurses suggested adding SIESTA to the nurses’ ongoing daily huddles at 4:00
Data Collection
Objectively Measured Sleep Disruptors
Adoption of SIESTA orders from March 2015 to March 2016 was assessed with a monthly EpicTM Clarity report. From August 1, 2015 to April 1, 2016, nocturnal room entries were recorded using the GOJO SMARTLINKTM Hand Hygiene system (GOJO Industries Inc., 2017, Akron, Ohio). This system includes two components: the hand-sanitizer dispensers, which track dispenses (numerator), and door-mounted Activity Counters, which use heat sensors that react to body heat emitted by a person passing through the doorway (denominator for hand-hygiene compliance). For our analysis, we only used Activity Counter data, which count room entries and exits, regardless of whether sanitizer was dispensed.
Patient-Reported Nighttime Sleep Disruptions
From June 2015 to March 2016, research assistants administered a 10-item Potential Hospital Sleep Disruptions and Noises Questionnaire (PHSDNQ) to patients in both units. Responses to this questionnaire correlate with actigraphy-based sleep measurements.9,12,13 Surveys were administered every other weekday to patients available to participate (eg, willing to participate, on the unit, awake). Survey data were stored on the REDCap Database (Version 6.14.0; Vanderbilt University, 2016, Nashville, Tennessee). Pre- and post-intervention Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) “top-box ratings” for percent quiet at night and percent pain well controlled were also compared.
Data Analysis
Objectively Measured Potential Sleep Disruptors
The proportion of sleep-friendly orders was analyzed using a two-sample test for proportions pre-post for the SIESTA-enhanced and standard units. The difference in use of SIESTA orders between units was analyzed via multivariable logistic regression, testing for independent associations between post-period, SIESTA-enhanced unit, and an interaction term (post-period × SIESTA unit) on use of sleep-friendly orders.
Room entries per night (11:00
Patient-Reported Nighttime Sleep Disruptions
Per prior studies, we defined a score 2 or higher as “sleep disruption.”9 Differences between units were evaluated via multivariable logistic regression to examine the association between the interaction of post-period × SIESTA-enhanced unit and odds of not reporting a sleep disruption. Significance was denoted as P = .05.
RESULTS
Between March 2015 and March 2016, 1,083 general-medicine patients were admitted to the SIESTA-enhanced and standard units (Table).
Nocturnal Orders
From March 2015 to March 2016, 1,669 EpicTM general medicine orders were reviewed (Figure). In the SIESTA-enhanced unit, the mean percentage of sleep-friendly orders rose for both vital signs (+31% [95% CI = 25%, 36%]; P < .001, npre = 306, npost = 306] and VTE prophylaxis (+28% [95% CI = 18%, 37%]; P < .001, npre = 158, npost = 173]. Similar changes were observed in the standard unit for sleep-friendly vital signs (+20% [95% CI = 14%, 25%]; P < .001, npre = 252, npost = 219) and VTE prophylaxis (+16% [95% CI = 6%, 25%]; P = .002, npre = 130, npost = 125). Differences between the two units were not statistically significant, and no significant change in timing of laboratory orders postintervention was found.
Nighttime Room Entries
Immediately after SIESTA launch, an average decrease of 114 total entries/night were noted in the SIESTA-enhanced unit, ([95% CI = −138, −91]; P < .001), corresponding to a 44% reduction (−6.3 entries/room) from the mean of 14.3 entries per patient room at baseline (Figure). No statistically significant change was seen in the standard unit. After SIESTA was incorporated into nursing huddles, total disruptions/night decreased by 1.31 disruptions/night ([95% CI = −1.64, −0.98]; P < .001) in the SIESTA-enhanced unit; by comparison, no significant changes were observed in the standard unit.
Patient-Reported Nighttime Sleep Disruptions
Between June 2015 and March 2016, 201 patient surveys were collected. A significant interaction was observed between the SIESTA-enhanced unit and post-period, and patients in the SIESTA-enhanced unit were more likely to report not being disrupted by medications (OR 4.08 [95% CI = 1.13–14.07]; P = .031) and vital signs (OR 3.35 [95% CI = 1.00–11.2]; P = .05) than those in the standard unit. HCAHPS top-box scores for the SIESTA unit increased by 7% for the “Quiet at night” category and 9% for the “Pain well controlled” category; by comparison, no major changes (>5%) were observed in the standard unit.
DISCUSSION
The present SIESTA intervention demonstrated that physician education coupled with EHR default changes are associated with a significant reduction in orders for overnight vital signs and medication administration in both units. However, addition of nursing education and empowerment in the SIESTA-enhanced unit was associated with fewer nocturnal room entries and improvements in patient-reported outcomes compared with those in the standard unit.
This study presents several implications for hospital initiatives aiming to improve patient sleep.14 Our study is consistent with other research highlighting the hypothesis that altering the default settings of EHR systems can influence physician behavior in a sustainable manner.15 However, our study also finds that, even when sleep-friendly orders are present, creating a sleep-friendly environment likely depends on the unit-based nurses championing the cause. While the initial decrease in nocturnal room entries post-SIESTA eventually faded, sustainable changes were observed only after SIESTA was added to nursing huddles, which illustrates the importance of using multiple methods to nudge staff.
Our study includes a number of limitations. It is not a randomized controlled trial, we cannot assume causality, and contamination was assumed, as residents and hospitalists worked in both units. Our single-site study may not be generalizable. Low HCAHPS response rates (10%-20%) also prevent demonstration of statistically significant differences. Finally, our convenience sampling strategy means not all inpatients were surveyed, and objective sleep duration was not measured.
In summary, at the University of Chicago, SIESTA could be associated with adoption of sleep-friendly vitals and medication orders, a decrease in nighttime room entries, and improved patient experience.
Disclosures
The authors have nothing to disclose.
Funding
This study was funded by the National Institute on Aging (NIA Grant No. T35AG029795) and the National Heart, Lung, and Blood Institute (NHLBI Grant Nos. R25HL116372 and K24HL136859).
Although sleep is critical to patient recovery in the hospital, hospitalization is not restful,1,2 and inpatient sleep deprivation has been linked to poor health outcomes.1-4 The American Academy of Nursing’s Choosing Wisely® campaign recommends nurses reduce unnecessary nocturnal care.5 However, interventions to improve inpatient sleep are not widely implemented.6 Targeting routine disruptions, such as overnight vital signs, by changing default settings in the electronic health record (EHR)with “nudges” could be a cost-effective strategy to improve inpatient sleep.4,7
We created Sleep for Inpatients: Empowering Staff to Act (SIESTA), which pairs nudges in the EHR with interprofessional education and empowerment,8 and tested its effectiveness on objectively and subjectively measured nocturnal sleep disruptors.
METHODS
Study Design
Two 18-room University of Chicago Medicine general-medicine units were used in this prospective study. The SIESTA-enhanced unit underwent the full sleep intervention: nursing education and empowerment, physician education, and EHR changes. The standard unit did not receive nursing interventions but received all other forms of intervention. Because physicians simultaneously cared for patients on both units, all internal medicine residents and hospitalists received the same education. The study population included physicians, nurses, and awake English-speaking patients who were cognitively intact and admitted to these two units. The University of Chicago Institutional Review Board approved this study (12-1766; 16685B).
Development of SIESTA
To develop SIESTA, patients were surveyed, and focus groups of staff were conducted; overnight vitals, medications, and phlebotomy were identified as major barriers to patient sleep.9 We found that physicians did not know how to change the default vital signs order “every 4 hours” or how to batch-order morning phlebotomy at a time other than 4:00
Behavioral Nudges
The SIESTA team worked with clinical informaticists to change the default orders in EpicTM (Epic Systems Corporation, 2017, Verona, Wisconsin) in September 2015 so that physicians would be asked, “Continue vital signs throughout the night?”10 Previously, this question was marked “Yes” by default and hidden. While the default protocol for heparin q8h was maintained, heparin q12h (9:00
SIESTA Physician Education
We created a 20-minute presentation on the consequences and causes of in-hospital sleep deprivation and evidence-based behavioral modification. We distributed pocket cards describing the mnemonic SIESTA (Screen patients for sleep disorders, Instruct patients on sleep hygiene, Eliminate disruptions, Shut doors, Treat pain, and Alarm and noise control). Physicians were instructed to consider forgoing overnight vitals, using clinical judgment to identify stable patients, use a sleep-promoting VTE prophylaxis option, and order daily labs at 10:00
SIESTA-Enhanced Unit
In the SIESTA-enhanced unit, nurses received education using pocket cards and were coached to collaborate with physicians to implement sleep-friendly orders. Customized signage depicting empowered nurses advocating for patients was posted near the huddle board. Because these nurses suggested adding SIESTA to the nurses’ ongoing daily huddles at 4:00
Data Collection
Objectively Measured Sleep Disruptors
Adoption of SIESTA orders from March 2015 to March 2016 was assessed with a monthly EpicTM Clarity report. From August 1, 2015 to April 1, 2016, nocturnal room entries were recorded using the GOJO SMARTLINKTM Hand Hygiene system (GOJO Industries Inc., 2017, Akron, Ohio). This system includes two components: the hand-sanitizer dispensers, which track dispenses (numerator), and door-mounted Activity Counters, which use heat sensors that react to body heat emitted by a person passing through the doorway (denominator for hand-hygiene compliance). For our analysis, we only used Activity Counter data, which count room entries and exits, regardless of whether sanitizer was dispensed.
Patient-Reported Nighttime Sleep Disruptions
From June 2015 to March 2016, research assistants administered a 10-item Potential Hospital Sleep Disruptions and Noises Questionnaire (PHSDNQ) to patients in both units. Responses to this questionnaire correlate with actigraphy-based sleep measurements.9,12,13 Surveys were administered every other weekday to patients available to participate (eg, willing to participate, on the unit, awake). Survey data were stored on the REDCap Database (Version 6.14.0; Vanderbilt University, 2016, Nashville, Tennessee). Pre- and post-intervention Hospital Consumer Assessment of Healthcare Providers and Systems (HCAHPS) “top-box ratings” for percent quiet at night and percent pain well controlled were also compared.
Data Analysis
Objectively Measured Potential Sleep Disruptors
The proportion of sleep-friendly orders was analyzed using a two-sample test for proportions pre-post for the SIESTA-enhanced and standard units. The difference in use of SIESTA orders between units was analyzed via multivariable logistic regression, testing for independent associations between post-period, SIESTA-enhanced unit, and an interaction term (post-period × SIESTA unit) on use of sleep-friendly orders.
Room entries per night (11:00
Patient-Reported Nighttime Sleep Disruptions
Per prior studies, we defined a score 2 or higher as “sleep disruption.”9 Differences between units were evaluated via multivariable logistic regression to examine the association between the interaction of post-period × SIESTA-enhanced unit and odds of not reporting a sleep disruption. Significance was denoted as P = .05.
RESULTS
Between March 2015 and March 2016, 1,083 general-medicine patients were admitted to the SIESTA-enhanced and standard units (Table).
Nocturnal Orders
From March 2015 to March 2016, 1,669 EpicTM general medicine orders were reviewed (Figure). In the SIESTA-enhanced unit, the mean percentage of sleep-friendly orders rose for both vital signs (+31% [95% CI = 25%, 36%]; P < .001, npre = 306, npost = 306] and VTE prophylaxis (+28% [95% CI = 18%, 37%]; P < .001, npre = 158, npost = 173]. Similar changes were observed in the standard unit for sleep-friendly vital signs (+20% [95% CI = 14%, 25%]; P < .001, npre = 252, npost = 219) and VTE prophylaxis (+16% [95% CI = 6%, 25%]; P = .002, npre = 130, npost = 125). Differences between the two units were not statistically significant, and no significant change in timing of laboratory orders postintervention was found.
Nighttime Room Entries
Immediately after SIESTA launch, an average decrease of 114 total entries/night were noted in the SIESTA-enhanced unit, ([95% CI = −138, −91]; P < .001), corresponding to a 44% reduction (−6.3 entries/room) from the mean of 14.3 entries per patient room at baseline (Figure). No statistically significant change was seen in the standard unit. After SIESTA was incorporated into nursing huddles, total disruptions/night decreased by 1.31 disruptions/night ([95% CI = −1.64, −0.98]; P < .001) in the SIESTA-enhanced unit; by comparison, no significant changes were observed in the standard unit.
Patient-Reported Nighttime Sleep Disruptions
Between June 2015 and March 2016, 201 patient surveys were collected. A significant interaction was observed between the SIESTA-enhanced unit and post-period, and patients in the SIESTA-enhanced unit were more likely to report not being disrupted by medications (OR 4.08 [95% CI = 1.13–14.07]; P = .031) and vital signs (OR 3.35 [95% CI = 1.00–11.2]; P = .05) than those in the standard unit. HCAHPS top-box scores for the SIESTA unit increased by 7% for the “Quiet at night” category and 9% for the “Pain well controlled” category; by comparison, no major changes (>5%) were observed in the standard unit.
DISCUSSION
The present SIESTA intervention demonstrated that physician education coupled with EHR default changes are associated with a significant reduction in orders for overnight vital signs and medication administration in both units. However, addition of nursing education and empowerment in the SIESTA-enhanced unit was associated with fewer nocturnal room entries and improvements in patient-reported outcomes compared with those in the standard unit.
This study presents several implications for hospital initiatives aiming to improve patient sleep.14 Our study is consistent with other research highlighting the hypothesis that altering the default settings of EHR systems can influence physician behavior in a sustainable manner.15 However, our study also finds that, even when sleep-friendly orders are present, creating a sleep-friendly environment likely depends on the unit-based nurses championing the cause. While the initial decrease in nocturnal room entries post-SIESTA eventually faded, sustainable changes were observed only after SIESTA was added to nursing huddles, which illustrates the importance of using multiple methods to nudge staff.
Our study includes a number of limitations. It is not a randomized controlled trial, we cannot assume causality, and contamination was assumed, as residents and hospitalists worked in both units. Our single-site study may not be generalizable. Low HCAHPS response rates (10%-20%) also prevent demonstration of statistically significant differences. Finally, our convenience sampling strategy means not all inpatients were surveyed, and objective sleep duration was not measured.
In summary, at the University of Chicago, SIESTA could be associated with adoption of sleep-friendly vitals and medication orders, a decrease in nighttime room entries, and improved patient experience.
Disclosures
The authors have nothing to disclose.
Funding
This study was funded by the National Institute on Aging (NIA Grant No. T35AG029795) and the National Heart, Lung, and Blood Institute (NHLBI Grant Nos. R25HL116372 and K24HL136859).
1. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients - a clinical review [published online ahead of print February 26, 2016]. Ann Intensive Care. 2015;5(3). doi: 10.1186/s13613-015-0043-2. PubMed
2. Arora VM, Chang KL, Fazal AZ, et al. Objective sleep duration and quality in hospitalized older adults: associations with blood pressure and mood. J Am Geriatr Soc. 2011;59(11):2185-2186. doi: 10.1111/j.1532-5415.2011.03644.x. PubMed
3. Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163-178. doi: 10.1016/j.smrv.2007.01.002. PubMed
4. Manian FA, Manian CJ. Sleep quality in adult hospitalized patients with infection: an observational study. Am J Med Sci. 2015;349(1):56-60. doi: 10.1097/MAJ.0000000000000355. PubMed
5. American Academy of Nursing announced engagement in National Choosing Wisely Campaign. Nurs Outlook. 2015;63(1):96-98. doi: 10.1016/j.outlook.2014.12.017. PubMed
6. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi: 10.1002/jhm.2578. PubMed
7. Fillary J, Chaplin H, Jones G, Thompson A, Holme A, Wilson P. Noise at night in hospital general wards: a mapping of the literature. Br J Nurs. 2015;24(10):536-540. doi: 10.12968/bjon.2015.24.10.536. PubMed
8. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. Yale University Press; 2008.
9. Grossman MN, Anderson SL, Worku A, et al. Awakenings? Patient and hospital staff perceptions of nighttime disruptions and their effect on patient sleep. J Clin Sleep Med. 2017;13(2):301-306. doi: 10.5664/jcsm.6468. PubMed
10. Yoder JC, Yuen TC, Churpek MM, Arora VM, Edelson DP. A prospective study of nighttime vital sign monitoring frequency and risk of clinical deterioration. JAMA Intern Med. 2013;173(16):1554-1555. doi: 10.1001/jamainternmed.2013.7791. PubMed
11. Phung OJ, Kahn SR, Cook DJ, Murad MH. Dosing frequency of unfractionated heparin thromboprophylaxis: a meta-analysis. Chest. 2011;140(2):374-381. doi: 10.1378/chest.10-3084. PubMed
12. Gabor JY, Cooper AB, Hanly PJ. Sleep disruption in the intensive care unit. Curr Opin Crit Care. 2001;7(1):21-27. PubMed
13. Topf M. Personal and environmental predictors of patient disturbance due to hospital noise. J Appl Psychol. 1985;70(1):22-28. doi: 10.1037/0021-9010.70.1.22. PubMed
14. Cho HJ, Wray CM, Maione S, et al. Right care in hospital medicine: co-creation of ten opportunities in overuse and underuse for improving value in hospital medicine. J Gen Intern Med. 2018;33(6):804-806. doi: 10.1007/s11606-018-4371-4. PubMed
15. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. doi: 10.1056/NEJMsb071595. PubMed
1. Delaney LJ, Van Haren F, Lopez V. Sleeping on a problem: the impact of sleep disturbance on intensive care patients - a clinical review [published online ahead of print February 26, 2016]. Ann Intensive Care. 2015;5(3). doi: 10.1186/s13613-015-0043-2. PubMed
2. Arora VM, Chang KL, Fazal AZ, et al. Objective sleep duration and quality in hospitalized older adults: associations with blood pressure and mood. J Am Geriatr Soc. 2011;59(11):2185-2186. doi: 10.1111/j.1532-5415.2011.03644.x. PubMed
3. Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163-178. doi: 10.1016/j.smrv.2007.01.002. PubMed
4. Manian FA, Manian CJ. Sleep quality in adult hospitalized patients with infection: an observational study. Am J Med Sci. 2015;349(1):56-60. doi: 10.1097/MAJ.0000000000000355. PubMed
5. American Academy of Nursing announced engagement in National Choosing Wisely Campaign. Nurs Outlook. 2015;63(1):96-98. doi: 10.1016/j.outlook.2014.12.017. PubMed
6. Gathecha E, Rios R, Buenaver LF, Landis R, Howell E, Wright S. Pilot study aiming to support sleep quality and duration during hospitalizations. J Hosp Med. 2016;11(7):467-472. doi: 10.1002/jhm.2578. PubMed
7. Fillary J, Chaplin H, Jones G, Thompson A, Holme A, Wilson P. Noise at night in hospital general wards: a mapping of the literature. Br J Nurs. 2015;24(10):536-540. doi: 10.12968/bjon.2015.24.10.536. PubMed
8. Thaler R, Sunstein C. Nudge: Improving Decisions About Health, Wealth and Happiness. Yale University Press; 2008.
9. Grossman MN, Anderson SL, Worku A, et al. Awakenings? Patient and hospital staff perceptions of nighttime disruptions and their effect on patient sleep. J Clin Sleep Med. 2017;13(2):301-306. doi: 10.5664/jcsm.6468. PubMed
10. Yoder JC, Yuen TC, Churpek MM, Arora VM, Edelson DP. A prospective study of nighttime vital sign monitoring frequency and risk of clinical deterioration. JAMA Intern Med. 2013;173(16):1554-1555. doi: 10.1001/jamainternmed.2013.7791. PubMed
11. Phung OJ, Kahn SR, Cook DJ, Murad MH. Dosing frequency of unfractionated heparin thromboprophylaxis: a meta-analysis. Chest. 2011;140(2):374-381. doi: 10.1378/chest.10-3084. PubMed
12. Gabor JY, Cooper AB, Hanly PJ. Sleep disruption in the intensive care unit. Curr Opin Crit Care. 2001;7(1):21-27. PubMed
13. Topf M. Personal and environmental predictors of patient disturbance due to hospital noise. J Appl Psychol. 1985;70(1):22-28. doi: 10.1037/0021-9010.70.1.22. PubMed
14. Cho HJ, Wray CM, Maione S, et al. Right care in hospital medicine: co-creation of ten opportunities in overuse and underuse for improving value in hospital medicine. J Gen Intern Med. 2018;33(6):804-806. doi: 10.1007/s11606-018-4371-4. PubMed
15. Halpern SD, Ubel PA, Asch DA. Harnessing the power of default options to improve health care. N Engl J Med. 2007;357(13):1340-1344. doi: 10.1056/NEJMsb071595. PubMed
© 2019 Society of Hospital Medicine