User login
Depressed and cognitively impaired
CASE Depressed and anxious
Five years ago, Ms. X, age 60, was diagnosed with treatment-resistant major depressive disorder (MDD) with anxiety. This diagnosis was established by a previous psychiatrist. She presents to a clinic for a second opinion.
Since her diagnosis, Ms. X has experienced sad mood, anhedonia, difficulty falling asleep, increased appetite and weight, and decreased concentration and attention. Her anxiety stems from her inability to work, which causes her to worry about her children. In the clinic, the treatment team conducts the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder-7 item scale (GAD-7) with Ms. X. She scores 16 on the PHQ-9, indicating moderately severe depression, and scores 12 on the GAD-7, indicating moderate anxiety.
Ms. X’s current medication regimen consists of venlafaxine extended-release (XR) 225 mg/d, trazodone 100 mg/d at bedtime, and clonazepam 1 mg twice daily. She reports no significant improvement of her symptoms from these medications. Additionally, Ms. X reports that in the past she had been prescribed fluoxetine, citalopram, and duloxetine, but she cannot recall the dosages.
Ms. X appears appropriately groomed, maintains appropriate eye contact, has clear speech, and does not show evidence of internal stimulation; however, she has difficulty following instructions. She makes negative comments about herself such as “I’m worthless” and “Nobody cares about me.” The treatment team decides to taper Ms. X off venlafaxine XR and initiates sertraline 50 mg/d, while continuing trazodone 50 mg/d at bedtime and clonazepam 1 mg twice daily. The team refers her for cognitive-behavioral therapy (CBT) to address her cognitive distortions, sad mood, and anxiety. Ms. X is asked to follow up with Psychiatry in 1 week.
EVALUATION Unusual behavior
At her CBT intake, Ms. X endorses depression and anxiety. Her PHQ-9 score at this visit is 19 (moderately severe depression) and GAD-7 score is 16 (severe anxiety). The psychologist notes that Ms. X is able to complete activities of daily living and instrumental activities of daily living without assistance. Ms. X denies any use of illicit substances or alcohol. No gross memory impairment is noted during this appointment, though Ms. X exhibits unusual behavior, including exiting and re-entering the clinic multiple times to repeatedly ask about follow-up appointments. The psychologist concludes that Ms. X’s presentation and behavior can be explained by MDD and pseudodementia.
[polldaddy:12189562]
The authors’ observations
Pseudodementia gained recognition in clinical research >100 years ago.1 Officially coined by Kiloh in 1961, the term was used broadly to categorize psychiatric cases that present like dementia but are the result of reversible causes. More recently, it has been used to describe older adults who present with cognitive deficits in the context of depressive symptoms.2 The goal of evaluation is to determine if the primary issue is a cognitive disorder or a depressive episode. DSM-5-TR does not classify pseudodementia as a distinct diagnosis, but instead categorizes its symptoms as components under other major diagnostic categories. Patients can present with MDD and associated cognitive symptoms, or with a cognitive disorder with depressive symptoms, which would be diagnosed as a cognitive disorder with a major depressive-like episode.3
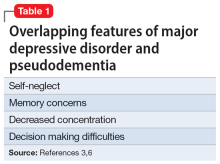
Pseudodementia is rare. Brodaty et al4 found the prevalence of pseudodementia in primary care settings was 0.6%. Older adults (age >65) who live alone are at increased risk of developing pseudodementia, which can be worsened by poor social support and acute psychosocial and environmental changes.5 A key characteristic of this disorder is that as the patient’s depressed mood improves, their memory and cognition also improve.6 Table 13,6 outlines overlapping features of MDD and pseudodementia.
Continue to: EVALUATION Worsening depression
EVALUATION Worsening depression
At her Psychiatry follow-up appointment, Ms. X reports that her mood is worse since she ended the relationship with her partner and she feels anxious because the partner was financially supporting her. Her PHQ-9 score is 24 (severe depression) and her GAD-7 score is 12 (moderate anxiety). Ms. X reports tolerating her transition from venlafaxine XR 225 mg/d to sertraline 50 mg/d well.
Additionally, Ms. X reports her children have called her “useless” since she continues to have difficulties following through on household tasks, even though she has no physical impairments that prevent her from completing them. The Psychiatry team observes that Ms. X has no problems walking or moving her arms or legs.
The Psychiatry team administers the Montreal Cognitive Assessment (MoCA). Ms. X scores 22, indicating mild impairment.
The team recommends a neuropsychological assessment to determine if this MoCA score is due to a cognitive disorder or is rooted in her mood symptoms. The team also recommends an MRI of the brain, complete blood count (CBC), comprehensive metabolic panel (CMP), and urinalysis (UA).
[polldaddy:12189567]
Continue to: The authors' observations
The authors’ observations
Neuropsychological assessments are important tools for exploring the behavioral manifestations of brain dysfunction (Table 2).7 These assessments factor in elements of neurology, psychiatry, and psychology to provide information about the diagnosis, prognosis, and functional status of patients with medical conditions, especially those with neurocognitive and psychiatric disorders. They combine information from the patient and collateral interviews, behavioral observations, a review of patient records, and objective tests of motor, emotional, and cognitive function.
Among other uses, neuropsychological assessments can help identify depression in patients with neurologic impairment, determine the diagnosis and plan of care for patients with concussions, determine the risk of a motor vehicle crash in patients with cognitive impairment, and distinguish Alzheimer disease from vascular dementia.8 Components of such assessments include the Beck Anxiety Inventory (BAI) to assess anxiety, the Dementia Rating Scale-2 and Neuropsychological Assessment Battery-Screening Module to assess dementia, and the Beck Depression Inventory (BDI) to assess depression.9
EVALUATION Continued cognitive decline
A different psychologist performs the neuropsychological assessment, who conducts the Repeatable Battery for the Assessment of Neuropsychological Status Update to determine if Ms. X is experiencing cognitive impairment. Her immediate memory, visuospatial/constructions, language, attention, and delayed memory are significantly impaired for someone her age. The psychologist also administers the Wechsler Adult Intelligence Scale IV and finds Ms. X’s general cognitive ability is within the low average range of intellectual functioning as measured by Full-Scale IQ. Ms. X scores 29 on the BDI-II, indicating significant depressive symptoms, and 13 on the BAI, indicating mild anxiety symptoms.
Ms. X is diagnosed with MDD and an unspecified neurocognitive disorder. The psychologist recommends she start CBT to address her mood and anxiety symptoms.
Upon reviewing the results with Ms. X, the treatment team again recommends a brain MRI, CBC, CMP, and UA to rule out organic causes of her cognitive decline. Ms. X decides against the MRI and laboratory workup and elects to continue her present medication regimen and CBT.
Several weeks later, Ms. X’s family brings her to the emergency department (ED) for evaluation of worsening mood, decreased personal hygiene, increased irritability, and further cognitive decline. They report she is having an increasingly difficult time remembering things such as where she parked her car. The ED team decides to discontinue clonazepam but continues sertraline and trazodone.
Continue to: CBC, CMP, and UA...
CBC, CMP, and UA are unremarkable. Ms. X undergoes a brain CT scan without contrast, which reveals hyperdense lesions in the inferior left tentorium, posterior fossa. A subsequent brain MRI with contrast reveals a dural-based enhancing mass, inferior to the left tentorium, in the left posterior fossa measuring 2.2 cm x 2.1 cm, suggestive of a meningioma. The team orders a Neurosurgery consult.
[polldaddy:12189571]
The authors’ observations
While most brain tumors are secondary to metastasis, meningiomas are the most common primary CNS tumor. Typically, they are asymptomatic; their diagnosis is often delayed until the patient presents with psychiatric symptoms without any focal neurologic findings. The frontal lobe is the most common location of meningioma. Data from 48 case reports of patients with meningiomas and psychiatric symptoms suggest symptoms do not always correlate with specific brain regions.10,11
Indications for neuroimaging in cases such as Ms. X include an abrupt change in behavior or personality, lack of response to psychiatric treatment, presence of focal neurologic signs, and an unusual psychiatric presentation and development of symptoms.11
TREATMENT Neurosurgery
Neurosurgery recommends and performs a suboccipital craniotomy for biopsy and resection. Ms. X tolerates the procedure well. A meningioma is found in the posterior fossa, near the cerebellar convexity. A biopsy finds no evidence of malignancies.
At her postoperative follow-up appointment several days after the procedure, Ms. X reports new-onset hearing loss and tinnitus.
[polldaddy:12189747]
Continue to: The authors' observations
The authors’ observations
Patients who require neurosurgery typically already carry a heavy psychiatric burden, which makes it challenging to determine the exact psychiatric consequences of neurosurgery.12-14 For example, research shows that temporal lobe resection and temporal lobectomy for treatment-resistant epilepsy can lead to an exacerbation of baseline psychiatric symptoms and the development of new symptoms (31% to 34%).15,16 However, Bommakanti et al13 found no new psychiatric symptoms after resection of meningiomas, and surgery seemed to play a role in ameliorating psychiatric symptoms in patients with intracranial tumors. Research attempting to document the psychiatric sequelae of neurosurgery has had mixed results, and it is difficult to determine what effects brain surgery has on mental health.
OUTCOME Minimal improvement
Several weeks after neurosurgery, Ms. X and her family report her mood is improved. Her PHQ-9 score improves to 15, but her GAD-7 score increases to 13, 1 point above her previous score.
The treatment team recommends Ms. X continue taking sertraline 50 mg/d and trazodone 50 mg/d at bedtime. Ms. X’s family reports her cognition and memory have not improved; her MoCA score increases by 1 point to 23. The treatment team discusses with Ms. X and her family the possibility that her cognitive problems maybe better explained as a neurocognitive disorder rather than as a result of the meningioma, since her MoCA score has not significantly improved. Ms. X and her family decide to seek a second opinion from a neurologist.
Bottom Line
Pseudodementia is a term used to describe older adults who present with cognitive issues in the context of depressive symptoms. Even in the absence of focal findings, neuroimaging should be considered as part of the workup in patients who continue to experience a progressive decline in mood and cognitive function.
Related Resources
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: a useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
- Pollak J. Psychological/neuropsychological testing: when to refer for reexamination. Current Psychiatry. 2021;20(9):18- 19,30-31,37. doi:10.12788/cp.0157
Drug Brand Names
Citalopram • Celexa
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Sertraline • Zoloft
Trazodone • Oleptro
Venlafaxine extended- release • Effexor XR
1. Nussbaum PD. (1994). Pseudodementia: a slow death. Neuropsychol Rev. 1994;4(2):71-90. doi:10.1007/BF01874829
2. Kang H, Zhao F, You L, et al. (2014). Pseudo-dementia: a neuropsychological review. Ann Indian Acad Neurol. 17(2):147-154. doi:10.4103/0972-2327.132613
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
4. Brodaty H, Connors MH. Pseudodementia, pseudo-pseudodementia, and pseudodepression. Alzheimers Dement (Amst). 2020;12(1):e12027. doi:10.1002/dad2.12027
5. Sekhon S, Marwaha R. Depressive Cognitive Disorders. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK559256/
6. Brown WA. Pseudodementia: issues in diagnosis. Psychiatric Times. April 9, 2005. Accessed February 3, 2023. www.psychiatrictimes.com/view/pseudodementia-issues-diagnosis
7. Kulas JF, Naugle RI. (2003). Indications for neuropsychological assessment. Cleve Clin J Med. 2003;70(9):785-792.
8. Braun M, Tupper D, Kaufmann P, et al. Neuropsychological assessment: a valuable tool in the diagnosis and management of neurological, neurodevelopmental, medical, and psychiatric disorders. Cogn Behav Neurol. 2011;24(3):107-114.
9. Michels TC, Tiu AY, Graver CJ. Neuropsychological evaluation in primary care. Am Fam Physician. 2010;82(5):495-502.
10. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307-314. doi:10.1007/s11060-010-0386-3
11. Gyawali S, Sharma P, Mahapatra A. Meningioma and psychiatric symptoms: an individual patient data analysis. Asian J Psychiatr. 2019;42:94-103. doi:10.1016/j.ajp.2019.03.029
12. McAllister TW. Neurobehavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatry. 2008;7(1):3-10. doi:10.1002/j.2051-5545.2008.tb00139.x
13. Bommakanti K, Gaddamanugu P, Alladi S, et al. Pre-operative and post-operative psychiatric manifestations in patients with supratentorial meningiomas. Clin Neurol Neurosurg. 2016;147:24-29. doi:10.1016/j.clineuro.2016.05.018
14. Devinsky O, Barr WB, Vickrey BG, et al. Changes in depression and anxiety after resective surgery for epilepsy. Neurology. 2005;65(11):1744-1749. doi:10.1212/01.wnl.0000187114.71524.c3
15. Blumer D, Wakhlu S, Davies K, et al. Psychiatric outcome of temporal lobectomy for epilepsy: incidence and treatment of psychiatric complications. Epilepsia. 1998;39(5):478-486. doi:10.1111/j.1528-1157.1998.tb01409.x
16. Glosser G, Zwil AS, Glosser DS, et al. Psychiatric aspects of temporal lobe epilepsy before and after anterior temporal lobectomy. J Neurol Neurosurg Psychiatry. 2000;68(1):53-58. doi:10.1136/jnnp.68.1.53
CASE Depressed and anxious
Five years ago, Ms. X, age 60, was diagnosed with treatment-resistant major depressive disorder (MDD) with anxiety. This diagnosis was established by a previous psychiatrist. She presents to a clinic for a second opinion.
Since her diagnosis, Ms. X has experienced sad mood, anhedonia, difficulty falling asleep, increased appetite and weight, and decreased concentration and attention. Her anxiety stems from her inability to work, which causes her to worry about her children. In the clinic, the treatment team conducts the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder-7 item scale (GAD-7) with Ms. X. She scores 16 on the PHQ-9, indicating moderately severe depression, and scores 12 on the GAD-7, indicating moderate anxiety.
Ms. X’s current medication regimen consists of venlafaxine extended-release (XR) 225 mg/d, trazodone 100 mg/d at bedtime, and clonazepam 1 mg twice daily. She reports no significant improvement of her symptoms from these medications. Additionally, Ms. X reports that in the past she had been prescribed fluoxetine, citalopram, and duloxetine, but she cannot recall the dosages.
Ms. X appears appropriately groomed, maintains appropriate eye contact, has clear speech, and does not show evidence of internal stimulation; however, she has difficulty following instructions. She makes negative comments about herself such as “I’m worthless” and “Nobody cares about me.” The treatment team decides to taper Ms. X off venlafaxine XR and initiates sertraline 50 mg/d, while continuing trazodone 50 mg/d at bedtime and clonazepam 1 mg twice daily. The team refers her for cognitive-behavioral therapy (CBT) to address her cognitive distortions, sad mood, and anxiety. Ms. X is asked to follow up with Psychiatry in 1 week.
EVALUATION Unusual behavior
At her CBT intake, Ms. X endorses depression and anxiety. Her PHQ-9 score at this visit is 19 (moderately severe depression) and GAD-7 score is 16 (severe anxiety). The psychologist notes that Ms. X is able to complete activities of daily living and instrumental activities of daily living without assistance. Ms. X denies any use of illicit substances or alcohol. No gross memory impairment is noted during this appointment, though Ms. X exhibits unusual behavior, including exiting and re-entering the clinic multiple times to repeatedly ask about follow-up appointments. The psychologist concludes that Ms. X’s presentation and behavior can be explained by MDD and pseudodementia.
[polldaddy:12189562]
The authors’ observations
Pseudodementia gained recognition in clinical research >100 years ago.1 Officially coined by Kiloh in 1961, the term was used broadly to categorize psychiatric cases that present like dementia but are the result of reversible causes. More recently, it has been used to describe older adults who present with cognitive deficits in the context of depressive symptoms.2 The goal of evaluation is to determine if the primary issue is a cognitive disorder or a depressive episode. DSM-5-TR does not classify pseudodementia as a distinct diagnosis, but instead categorizes its symptoms as components under other major diagnostic categories. Patients can present with MDD and associated cognitive symptoms, or with a cognitive disorder with depressive symptoms, which would be diagnosed as a cognitive disorder with a major depressive-like episode.3
Pseudodementia is rare. Brodaty et al4 found the prevalence of pseudodementia in primary care settings was 0.6%. Older adults (age >65) who live alone are at increased risk of developing pseudodementia, which can be worsened by poor social support and acute psychosocial and environmental changes.5 A key characteristic of this disorder is that as the patient’s depressed mood improves, their memory and cognition also improve.6 Table 13,6 outlines overlapping features of MDD and pseudodementia.
Continue to: EVALUATION Worsening depression
EVALUATION Worsening depression
At her Psychiatry follow-up appointment, Ms. X reports that her mood is worse since she ended the relationship with her partner and she feels anxious because the partner was financially supporting her. Her PHQ-9 score is 24 (severe depression) and her GAD-7 score is 12 (moderate anxiety). Ms. X reports tolerating her transition from venlafaxine XR 225 mg/d to sertraline 50 mg/d well.
Additionally, Ms. X reports her children have called her “useless” since she continues to have difficulties following through on household tasks, even though she has no physical impairments that prevent her from completing them. The Psychiatry team observes that Ms. X has no problems walking or moving her arms or legs.
The Psychiatry team administers the Montreal Cognitive Assessment (MoCA). Ms. X scores 22, indicating mild impairment.
The team recommends a neuropsychological assessment to determine if this MoCA score is due to a cognitive disorder or is rooted in her mood symptoms. The team also recommends an MRI of the brain, complete blood count (CBC), comprehensive metabolic panel (CMP), and urinalysis (UA).
[polldaddy:12189567]
Continue to: The authors' observations
The authors’ observations
Neuropsychological assessments are important tools for exploring the behavioral manifestations of brain dysfunction (Table 2).7 These assessments factor in elements of neurology, psychiatry, and psychology to provide information about the diagnosis, prognosis, and functional status of patients with medical conditions, especially those with neurocognitive and psychiatric disorders. They combine information from the patient and collateral interviews, behavioral observations, a review of patient records, and objective tests of motor, emotional, and cognitive function.
Among other uses, neuropsychological assessments can help identify depression in patients with neurologic impairment, determine the diagnosis and plan of care for patients with concussions, determine the risk of a motor vehicle crash in patients with cognitive impairment, and distinguish Alzheimer disease from vascular dementia.8 Components of such assessments include the Beck Anxiety Inventory (BAI) to assess anxiety, the Dementia Rating Scale-2 and Neuropsychological Assessment Battery-Screening Module to assess dementia, and the Beck Depression Inventory (BDI) to assess depression.9
EVALUATION Continued cognitive decline
A different psychologist performs the neuropsychological assessment, who conducts the Repeatable Battery for the Assessment of Neuropsychological Status Update to determine if Ms. X is experiencing cognitive impairment. Her immediate memory, visuospatial/constructions, language, attention, and delayed memory are significantly impaired for someone her age. The psychologist also administers the Wechsler Adult Intelligence Scale IV and finds Ms. X’s general cognitive ability is within the low average range of intellectual functioning as measured by Full-Scale IQ. Ms. X scores 29 on the BDI-II, indicating significant depressive symptoms, and 13 on the BAI, indicating mild anxiety symptoms.
Ms. X is diagnosed with MDD and an unspecified neurocognitive disorder. The psychologist recommends she start CBT to address her mood and anxiety symptoms.
Upon reviewing the results with Ms. X, the treatment team again recommends a brain MRI, CBC, CMP, and UA to rule out organic causes of her cognitive decline. Ms. X decides against the MRI and laboratory workup and elects to continue her present medication regimen and CBT.
Several weeks later, Ms. X’s family brings her to the emergency department (ED) for evaluation of worsening mood, decreased personal hygiene, increased irritability, and further cognitive decline. They report she is having an increasingly difficult time remembering things such as where she parked her car. The ED team decides to discontinue clonazepam but continues sertraline and trazodone.
Continue to: CBC, CMP, and UA...
CBC, CMP, and UA are unremarkable. Ms. X undergoes a brain CT scan without contrast, which reveals hyperdense lesions in the inferior left tentorium, posterior fossa. A subsequent brain MRI with contrast reveals a dural-based enhancing mass, inferior to the left tentorium, in the left posterior fossa measuring 2.2 cm x 2.1 cm, suggestive of a meningioma. The team orders a Neurosurgery consult.
[polldaddy:12189571]
The authors’ observations
While most brain tumors are secondary to metastasis, meningiomas are the most common primary CNS tumor. Typically, they are asymptomatic; their diagnosis is often delayed until the patient presents with psychiatric symptoms without any focal neurologic findings. The frontal lobe is the most common location of meningioma. Data from 48 case reports of patients with meningiomas and psychiatric symptoms suggest symptoms do not always correlate with specific brain regions.10,11
Indications for neuroimaging in cases such as Ms. X include an abrupt change in behavior or personality, lack of response to psychiatric treatment, presence of focal neurologic signs, and an unusual psychiatric presentation and development of symptoms.11
TREATMENT Neurosurgery
Neurosurgery recommends and performs a suboccipital craniotomy for biopsy and resection. Ms. X tolerates the procedure well. A meningioma is found in the posterior fossa, near the cerebellar convexity. A biopsy finds no evidence of malignancies.
At her postoperative follow-up appointment several days after the procedure, Ms. X reports new-onset hearing loss and tinnitus.
[polldaddy:12189747]
Continue to: The authors' observations
The authors’ observations
Patients who require neurosurgery typically already carry a heavy psychiatric burden, which makes it challenging to determine the exact psychiatric consequences of neurosurgery.12-14 For example, research shows that temporal lobe resection and temporal lobectomy for treatment-resistant epilepsy can lead to an exacerbation of baseline psychiatric symptoms and the development of new symptoms (31% to 34%).15,16 However, Bommakanti et al13 found no new psychiatric symptoms after resection of meningiomas, and surgery seemed to play a role in ameliorating psychiatric symptoms in patients with intracranial tumors. Research attempting to document the psychiatric sequelae of neurosurgery has had mixed results, and it is difficult to determine what effects brain surgery has on mental health.
OUTCOME Minimal improvement
Several weeks after neurosurgery, Ms. X and her family report her mood is improved. Her PHQ-9 score improves to 15, but her GAD-7 score increases to 13, 1 point above her previous score.
The treatment team recommends Ms. X continue taking sertraline 50 mg/d and trazodone 50 mg/d at bedtime. Ms. X’s family reports her cognition and memory have not improved; her MoCA score increases by 1 point to 23. The treatment team discusses with Ms. X and her family the possibility that her cognitive problems maybe better explained as a neurocognitive disorder rather than as a result of the meningioma, since her MoCA score has not significantly improved. Ms. X and her family decide to seek a second opinion from a neurologist.
Bottom Line
Pseudodementia is a term used to describe older adults who present with cognitive issues in the context of depressive symptoms. Even in the absence of focal findings, neuroimaging should be considered as part of the workup in patients who continue to experience a progressive decline in mood and cognitive function.
Related Resources
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: a useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
- Pollak J. Psychological/neuropsychological testing: when to refer for reexamination. Current Psychiatry. 2021;20(9):18- 19,30-31,37. doi:10.12788/cp.0157
Drug Brand Names
Citalopram • Celexa
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Sertraline • Zoloft
Trazodone • Oleptro
Venlafaxine extended- release • Effexor XR
CASE Depressed and anxious
Five years ago, Ms. X, age 60, was diagnosed with treatment-resistant major depressive disorder (MDD) with anxiety. This diagnosis was established by a previous psychiatrist. She presents to a clinic for a second opinion.
Since her diagnosis, Ms. X has experienced sad mood, anhedonia, difficulty falling asleep, increased appetite and weight, and decreased concentration and attention. Her anxiety stems from her inability to work, which causes her to worry about her children. In the clinic, the treatment team conducts the Patient Health Questionnaire-9 (PHQ-9) and Generalized Anxiety Disorder-7 item scale (GAD-7) with Ms. X. She scores 16 on the PHQ-9, indicating moderately severe depression, and scores 12 on the GAD-7, indicating moderate anxiety.
Ms. X’s current medication regimen consists of venlafaxine extended-release (XR) 225 mg/d, trazodone 100 mg/d at bedtime, and clonazepam 1 mg twice daily. She reports no significant improvement of her symptoms from these medications. Additionally, Ms. X reports that in the past she had been prescribed fluoxetine, citalopram, and duloxetine, but she cannot recall the dosages.
Ms. X appears appropriately groomed, maintains appropriate eye contact, has clear speech, and does not show evidence of internal stimulation; however, she has difficulty following instructions. She makes negative comments about herself such as “I’m worthless” and “Nobody cares about me.” The treatment team decides to taper Ms. X off venlafaxine XR and initiates sertraline 50 mg/d, while continuing trazodone 50 mg/d at bedtime and clonazepam 1 mg twice daily. The team refers her for cognitive-behavioral therapy (CBT) to address her cognitive distortions, sad mood, and anxiety. Ms. X is asked to follow up with Psychiatry in 1 week.
EVALUATION Unusual behavior
At her CBT intake, Ms. X endorses depression and anxiety. Her PHQ-9 score at this visit is 19 (moderately severe depression) and GAD-7 score is 16 (severe anxiety). The psychologist notes that Ms. X is able to complete activities of daily living and instrumental activities of daily living without assistance. Ms. X denies any use of illicit substances or alcohol. No gross memory impairment is noted during this appointment, though Ms. X exhibits unusual behavior, including exiting and re-entering the clinic multiple times to repeatedly ask about follow-up appointments. The psychologist concludes that Ms. X’s presentation and behavior can be explained by MDD and pseudodementia.
[polldaddy:12189562]
The authors’ observations
Pseudodementia gained recognition in clinical research >100 years ago.1 Officially coined by Kiloh in 1961, the term was used broadly to categorize psychiatric cases that present like dementia but are the result of reversible causes. More recently, it has been used to describe older adults who present with cognitive deficits in the context of depressive symptoms.2 The goal of evaluation is to determine if the primary issue is a cognitive disorder or a depressive episode. DSM-5-TR does not classify pseudodementia as a distinct diagnosis, but instead categorizes its symptoms as components under other major diagnostic categories. Patients can present with MDD and associated cognitive symptoms, or with a cognitive disorder with depressive symptoms, which would be diagnosed as a cognitive disorder with a major depressive-like episode.3
Pseudodementia is rare. Brodaty et al4 found the prevalence of pseudodementia in primary care settings was 0.6%. Older adults (age >65) who live alone are at increased risk of developing pseudodementia, which can be worsened by poor social support and acute psychosocial and environmental changes.5 A key characteristic of this disorder is that as the patient’s depressed mood improves, their memory and cognition also improve.6 Table 13,6 outlines overlapping features of MDD and pseudodementia.
Continue to: EVALUATION Worsening depression
EVALUATION Worsening depression
At her Psychiatry follow-up appointment, Ms. X reports that her mood is worse since she ended the relationship with her partner and she feels anxious because the partner was financially supporting her. Her PHQ-9 score is 24 (severe depression) and her GAD-7 score is 12 (moderate anxiety). Ms. X reports tolerating her transition from venlafaxine XR 225 mg/d to sertraline 50 mg/d well.
Additionally, Ms. X reports her children have called her “useless” since she continues to have difficulties following through on household tasks, even though she has no physical impairments that prevent her from completing them. The Psychiatry team observes that Ms. X has no problems walking or moving her arms or legs.
The Psychiatry team administers the Montreal Cognitive Assessment (MoCA). Ms. X scores 22, indicating mild impairment.
The team recommends a neuropsychological assessment to determine if this MoCA score is due to a cognitive disorder or is rooted in her mood symptoms. The team also recommends an MRI of the brain, complete blood count (CBC), comprehensive metabolic panel (CMP), and urinalysis (UA).
[polldaddy:12189567]
Continue to: The authors' observations
The authors’ observations
Neuropsychological assessments are important tools for exploring the behavioral manifestations of brain dysfunction (Table 2).7 These assessments factor in elements of neurology, psychiatry, and psychology to provide information about the diagnosis, prognosis, and functional status of patients with medical conditions, especially those with neurocognitive and psychiatric disorders. They combine information from the patient and collateral interviews, behavioral observations, a review of patient records, and objective tests of motor, emotional, and cognitive function.
Among other uses, neuropsychological assessments can help identify depression in patients with neurologic impairment, determine the diagnosis and plan of care for patients with concussions, determine the risk of a motor vehicle crash in patients with cognitive impairment, and distinguish Alzheimer disease from vascular dementia.8 Components of such assessments include the Beck Anxiety Inventory (BAI) to assess anxiety, the Dementia Rating Scale-2 and Neuropsychological Assessment Battery-Screening Module to assess dementia, and the Beck Depression Inventory (BDI) to assess depression.9
EVALUATION Continued cognitive decline
A different psychologist performs the neuropsychological assessment, who conducts the Repeatable Battery for the Assessment of Neuropsychological Status Update to determine if Ms. X is experiencing cognitive impairment. Her immediate memory, visuospatial/constructions, language, attention, and delayed memory are significantly impaired for someone her age. The psychologist also administers the Wechsler Adult Intelligence Scale IV and finds Ms. X’s general cognitive ability is within the low average range of intellectual functioning as measured by Full-Scale IQ. Ms. X scores 29 on the BDI-II, indicating significant depressive symptoms, and 13 on the BAI, indicating mild anxiety symptoms.
Ms. X is diagnosed with MDD and an unspecified neurocognitive disorder. The psychologist recommends she start CBT to address her mood and anxiety symptoms.
Upon reviewing the results with Ms. X, the treatment team again recommends a brain MRI, CBC, CMP, and UA to rule out organic causes of her cognitive decline. Ms. X decides against the MRI and laboratory workup and elects to continue her present medication regimen and CBT.
Several weeks later, Ms. X’s family brings her to the emergency department (ED) for evaluation of worsening mood, decreased personal hygiene, increased irritability, and further cognitive decline. They report she is having an increasingly difficult time remembering things such as where she parked her car. The ED team decides to discontinue clonazepam but continues sertraline and trazodone.
Continue to: CBC, CMP, and UA...
CBC, CMP, and UA are unremarkable. Ms. X undergoes a brain CT scan without contrast, which reveals hyperdense lesions in the inferior left tentorium, posterior fossa. A subsequent brain MRI with contrast reveals a dural-based enhancing mass, inferior to the left tentorium, in the left posterior fossa measuring 2.2 cm x 2.1 cm, suggestive of a meningioma. The team orders a Neurosurgery consult.
[polldaddy:12189571]
The authors’ observations
While most brain tumors are secondary to metastasis, meningiomas are the most common primary CNS tumor. Typically, they are asymptomatic; their diagnosis is often delayed until the patient presents with psychiatric symptoms without any focal neurologic findings. The frontal lobe is the most common location of meningioma. Data from 48 case reports of patients with meningiomas and psychiatric symptoms suggest symptoms do not always correlate with specific brain regions.10,11
Indications for neuroimaging in cases such as Ms. X include an abrupt change in behavior or personality, lack of response to psychiatric treatment, presence of focal neurologic signs, and an unusual psychiatric presentation and development of symptoms.11
TREATMENT Neurosurgery
Neurosurgery recommends and performs a suboccipital craniotomy for biopsy and resection. Ms. X tolerates the procedure well. A meningioma is found in the posterior fossa, near the cerebellar convexity. A biopsy finds no evidence of malignancies.
At her postoperative follow-up appointment several days after the procedure, Ms. X reports new-onset hearing loss and tinnitus.
[polldaddy:12189747]
Continue to: The authors' observations
The authors’ observations
Patients who require neurosurgery typically already carry a heavy psychiatric burden, which makes it challenging to determine the exact psychiatric consequences of neurosurgery.12-14 For example, research shows that temporal lobe resection and temporal lobectomy for treatment-resistant epilepsy can lead to an exacerbation of baseline psychiatric symptoms and the development of new symptoms (31% to 34%).15,16 However, Bommakanti et al13 found no new psychiatric symptoms after resection of meningiomas, and surgery seemed to play a role in ameliorating psychiatric symptoms in patients with intracranial tumors. Research attempting to document the psychiatric sequelae of neurosurgery has had mixed results, and it is difficult to determine what effects brain surgery has on mental health.
OUTCOME Minimal improvement
Several weeks after neurosurgery, Ms. X and her family report her mood is improved. Her PHQ-9 score improves to 15, but her GAD-7 score increases to 13, 1 point above her previous score.
The treatment team recommends Ms. X continue taking sertraline 50 mg/d and trazodone 50 mg/d at bedtime. Ms. X’s family reports her cognition and memory have not improved; her MoCA score increases by 1 point to 23. The treatment team discusses with Ms. X and her family the possibility that her cognitive problems maybe better explained as a neurocognitive disorder rather than as a result of the meningioma, since her MoCA score has not significantly improved. Ms. X and her family decide to seek a second opinion from a neurologist.
Bottom Line
Pseudodementia is a term used to describe older adults who present with cognitive issues in the context of depressive symptoms. Even in the absence of focal findings, neuroimaging should be considered as part of the workup in patients who continue to experience a progressive decline in mood and cognitive function.
Related Resources
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: a useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
- Pollak J. Psychological/neuropsychological testing: when to refer for reexamination. Current Psychiatry. 2021;20(9):18- 19,30-31,37. doi:10.12788/cp.0157
Drug Brand Names
Citalopram • Celexa
Clonazepam • Klonopin
Duloxetine • Cymbalta
Fluoxetine • Prozac
Sertraline • Zoloft
Trazodone • Oleptro
Venlafaxine extended- release • Effexor XR
1. Nussbaum PD. (1994). Pseudodementia: a slow death. Neuropsychol Rev. 1994;4(2):71-90. doi:10.1007/BF01874829
2. Kang H, Zhao F, You L, et al. (2014). Pseudo-dementia: a neuropsychological review. Ann Indian Acad Neurol. 17(2):147-154. doi:10.4103/0972-2327.132613
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
4. Brodaty H, Connors MH. Pseudodementia, pseudo-pseudodementia, and pseudodepression. Alzheimers Dement (Amst). 2020;12(1):e12027. doi:10.1002/dad2.12027
5. Sekhon S, Marwaha R. Depressive Cognitive Disorders. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK559256/
6. Brown WA. Pseudodementia: issues in diagnosis. Psychiatric Times. April 9, 2005. Accessed February 3, 2023. www.psychiatrictimes.com/view/pseudodementia-issues-diagnosis
7. Kulas JF, Naugle RI. (2003). Indications for neuropsychological assessment. Cleve Clin J Med. 2003;70(9):785-792.
8. Braun M, Tupper D, Kaufmann P, et al. Neuropsychological assessment: a valuable tool in the diagnosis and management of neurological, neurodevelopmental, medical, and psychiatric disorders. Cogn Behav Neurol. 2011;24(3):107-114.
9. Michels TC, Tiu AY, Graver CJ. Neuropsychological evaluation in primary care. Am Fam Physician. 2010;82(5):495-502.
10. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307-314. doi:10.1007/s11060-010-0386-3
11. Gyawali S, Sharma P, Mahapatra A. Meningioma and psychiatric symptoms: an individual patient data analysis. Asian J Psychiatr. 2019;42:94-103. doi:10.1016/j.ajp.2019.03.029
12. McAllister TW. Neurobehavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatry. 2008;7(1):3-10. doi:10.1002/j.2051-5545.2008.tb00139.x
13. Bommakanti K, Gaddamanugu P, Alladi S, et al. Pre-operative and post-operative psychiatric manifestations in patients with supratentorial meningiomas. Clin Neurol Neurosurg. 2016;147:24-29. doi:10.1016/j.clineuro.2016.05.018
14. Devinsky O, Barr WB, Vickrey BG, et al. Changes in depression and anxiety after resective surgery for epilepsy. Neurology. 2005;65(11):1744-1749. doi:10.1212/01.wnl.0000187114.71524.c3
15. Blumer D, Wakhlu S, Davies K, et al. Psychiatric outcome of temporal lobectomy for epilepsy: incidence and treatment of psychiatric complications. Epilepsia. 1998;39(5):478-486. doi:10.1111/j.1528-1157.1998.tb01409.x
16. Glosser G, Zwil AS, Glosser DS, et al. Psychiatric aspects of temporal lobe epilepsy before and after anterior temporal lobectomy. J Neurol Neurosurg Psychiatry. 2000;68(1):53-58. doi:10.1136/jnnp.68.1.53
1. Nussbaum PD. (1994). Pseudodementia: a slow death. Neuropsychol Rev. 1994;4(2):71-90. doi:10.1007/BF01874829
2. Kang H, Zhao F, You L, et al. (2014). Pseudo-dementia: a neuropsychological review. Ann Indian Acad Neurol. 17(2):147-154. doi:10.4103/0972-2327.132613
3. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed, text revision. American Psychiatric Association; 2022.
4. Brodaty H, Connors MH. Pseudodementia, pseudo-pseudodementia, and pseudodepression. Alzheimers Dement (Amst). 2020;12(1):e12027. doi:10.1002/dad2.12027
5. Sekhon S, Marwaha R. Depressive Cognitive Disorders. StatPearls Publishing; 2022. https://www.ncbi.nlm.nih.gov/books/NBK559256/
6. Brown WA. Pseudodementia: issues in diagnosis. Psychiatric Times. April 9, 2005. Accessed February 3, 2023. www.psychiatrictimes.com/view/pseudodementia-issues-diagnosis
7. Kulas JF, Naugle RI. (2003). Indications for neuropsychological assessment. Cleve Clin J Med. 2003;70(9):785-792.
8. Braun M, Tupper D, Kaufmann P, et al. Neuropsychological assessment: a valuable tool in the diagnosis and management of neurological, neurodevelopmental, medical, and psychiatric disorders. Cogn Behav Neurol. 2011;24(3):107-114.
9. Michels TC, Tiu AY, Graver CJ. Neuropsychological evaluation in primary care. Am Fam Physician. 2010;82(5):495-502.
10. Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neurooncol. 2010;99(3):307-314. doi:10.1007/s11060-010-0386-3
11. Gyawali S, Sharma P, Mahapatra A. Meningioma and psychiatric symptoms: an individual patient data analysis. Asian J Psychiatr. 2019;42:94-103. doi:10.1016/j.ajp.2019.03.029
12. McAllister TW. Neurobehavioral sequelae of traumatic brain injury: evaluation and management. World Psychiatry. 2008;7(1):3-10. doi:10.1002/j.2051-5545.2008.tb00139.x
13. Bommakanti K, Gaddamanugu P, Alladi S, et al. Pre-operative and post-operative psychiatric manifestations in patients with supratentorial meningiomas. Clin Neurol Neurosurg. 2016;147:24-29. doi:10.1016/j.clineuro.2016.05.018
14. Devinsky O, Barr WB, Vickrey BG, et al. Changes in depression and anxiety after resective surgery for epilepsy. Neurology. 2005;65(11):1744-1749. doi:10.1212/01.wnl.0000187114.71524.c3
15. Blumer D, Wakhlu S, Davies K, et al. Psychiatric outcome of temporal lobectomy for epilepsy: incidence and treatment of psychiatric complications. Epilepsia. 1998;39(5):478-486. doi:10.1111/j.1528-1157.1998.tb01409.x
16. Glosser G, Zwil AS, Glosser DS, et al. Psychiatric aspects of temporal lobe epilepsy before and after anterior temporal lobectomy. J Neurol Neurosurg Psychiatry. 2000;68(1):53-58. doi:10.1136/jnnp.68.1.53
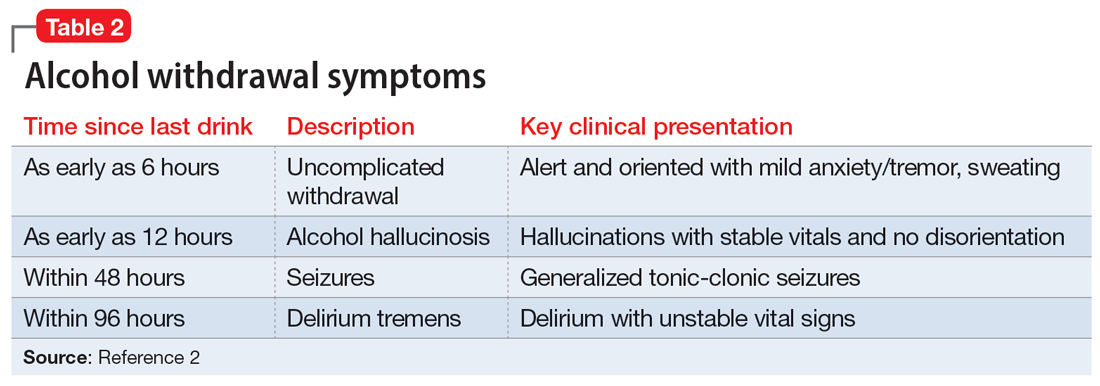
When a patient with chronic alcohol use abruptly stops drinking
CASE A difficult withdrawal
Three days after he stops drinking alcohol, Mr. G, age 49, presents to a detoxification center with his wife, who drove him there because she was concerned about his condition. She says her husband had been drinking alcohol every night for as long as she can remember. Despite numerous admissions to rehabilitation centers, Mr. G usually would resume drinking soon after he was discharged. Three days ago, Mr. G’s wife had told him she “could not take it anymore,” so he got rid of all his alcohol and stopped drinking. Mr. G’s wife felt he was doing fine the first day, but his condition increasingly worsened the second and third days. The triage nurse who attempts to interview Mr. G finds him tremulous, vomiting, and sweating. She notices that he seems preoccupied with pulling at his shirt, appearing to pick at things that are not there.
HISTORY Untreated depression, other comorbidities
Mr. G’s wife says he has never been psychiatrically hospitalized or exhibited suicidal behavior. Mr. G previously received care from a psychiatrist, who diagnosed him with major depressive disorder (MDD) and prescribed an antidepressant, though his wife cannot recall which specific medication. She shares it has been “a long time” since Mr. G has taken the antidepressant and the last time he received treatment for his MDD was 5 years ago. Mr. G’s wife says her husband had once abstained from alcohol use for >6 months following one of his stints at a rehabilitation center. She is not able to share many other details about Mr. G’s previous stays at rehabilitation centers, but says he always had “a rough time.”
She says Mr. G had been drinking an average of 10 drinks each night, usually within 4 hours. He has no history of nicotine or illicit substance use and has held a corporate job for the last 18 years. Several years ago, a physician had diagnosed Mr. G with hypertension and high cholesterol, but he did not follow up for treatment. Mr. G’s wife also recalls a physician told her husband he had a fatty liver. His family history includes heart disease and cancer.
[polldaddy:12041618]
The author’s observations
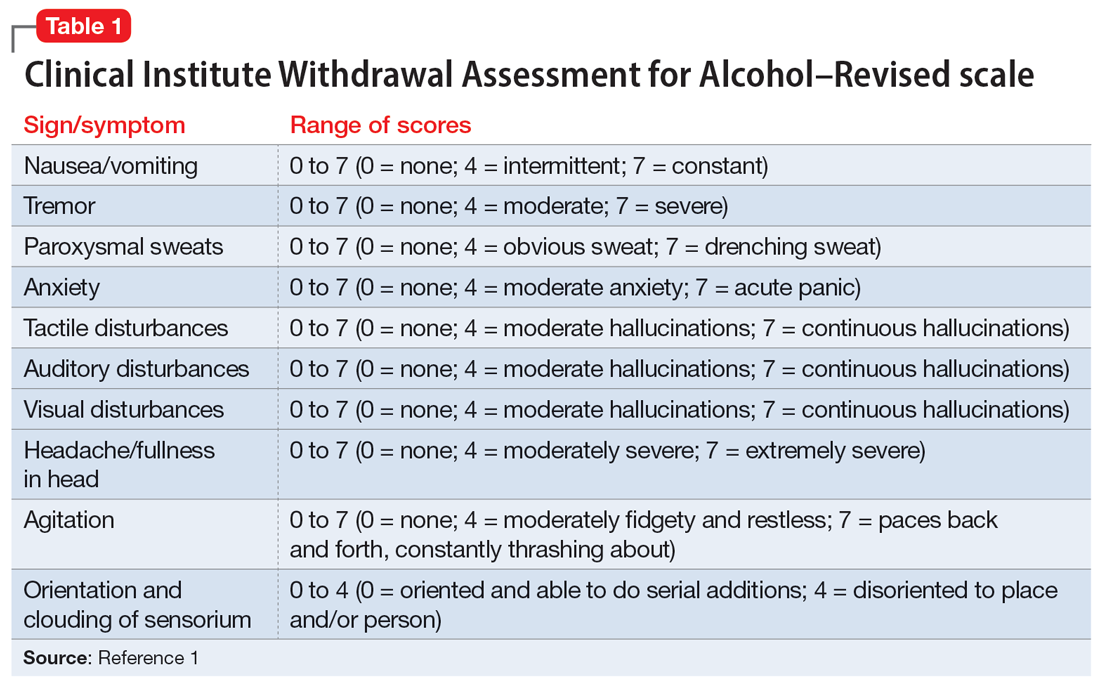
The treatment team observed several elements of alcohol withdrawal and classified Mr. G as a priority patient. If the team had completed the Clinical Institute Withdrawal Assessment for Alcohol–Revised scale (CIWA-Ar) (Table 11), Mr. G would score ≥10. While the protocol for initiating treatment for patients experiencing alcohol withdrawal varies by institution, patients with moderate to severe scores on the CIWA-Ar when experiencing withdrawal typically are managed with pharmacotherapy to address their symptoms.1 Given the timeline of his last drink as reported by his wife, Mr. G is on the brink of experiencing a cascade of symptoms concerning for delirium tremens (DTs).2 Table 22 provides a timeline and symptoms related to alcohol withdrawal. To prevent further exacerbation of symptoms, which could lead to DTs, Mr. G’s treatment team will likely initiate a benzodiazepine, using either scheduled or symptom-driven dosing.3
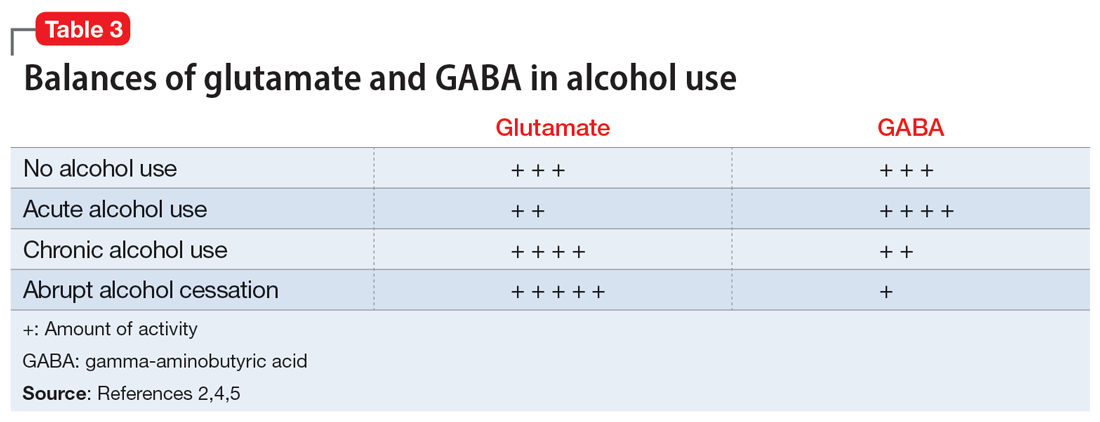
Two neurotransmitters that play a role in DTs are glutamate (excitatory) and GABA (inhibitory). In a normal state, the competing actions of these neurotransmitters balance each other. Acute alcohol intake causes a shift in the excitatory and inhibitory levels, with more inhibition taking place, thus causing disequilibrium. If chronic alcohol use continues, the amount of GABA inhibition reduction is related to downregulation of receptors.2,4 Excitation increases by way of upregulation of the N-methyl-
If alcohol is suddenly removed following chronic use, there is unchecked glutamate excitation related to a blunted GABA state. This added increase in the excitation of glutamate leads to withdrawal symptoms.2,4 Table 32,4,5 depicts the neurotransmitter equilibrium of GABA and glutamate relative to alcohol use.
EVALUATION Bleeding gums and bruising
The treatment team admits Mr. G to the triage bay and contacts the addiction psychiatrist. The physician orders laboratory tests to assess nutritional deficits and electrolyte abnormalities. Mr. G is also placed on routine assessments with symptom-triggered therapy. An assessment reveals bleeding gums and bruises, which are believed to be a result of thrombocytopenia (low blood platelet count).
[polldaddy:12041627]
Continue to: The author's observations
The author’s observations
Though regular clinical assessment of PEth varies, it is considered to have high sensitivity and specificity to detect alcohol use.6 When ethanol is present, the phospholipase D enzyme acts upon phosphatidylcholine, forming a direct biomarker, PEth, on the surface of the red blood cell.6,7 PEth’s half-life ranges from 4.5 to 12 days,6 and it can be detected in blood for 3 to 4 weeks after alcohol ingestion.6,7 A PEth value <20 ng/mL indicates light or no alcohol consumption; 20 to 199 ng/mL indicates significant consumption; and >200 ng/mL indicates heavy consumption.7 Since Mr. G has a history of chronic alcohol use, his PEth level is expected to be >200 ng/mL.
AST/ALT and MCV are indirect biomarkers, meaning the tests are not alcohol-specific and the role of alcohol is instead observed by the damage to the body with excessive use over time.7 The expected AST:ALT ratio is 2:1. This is related to 3 mechanisms. The first is a decrease in ALT usually relative to B6 deficiency in individuals with alcohol use disorder (AUD). Another mechanism is related to alcohol’s propensity to affect mitochondria, which is a source for AST. Additionally, AST is also found in higher proportions in the kidneys, heart, and muscles.8
An MCV <100 fL would be within the normal range (80 to 100 fL) for red blood cells. While the reasons for an enlarged red blood cell (or macrocyte) are extensive, alcohol can be a factor once other causes are excluded. Additional laboratory tests and a peripheral blood smear test can help in this investigation. Alcohol disrupts the complete maturation of red blood cells.9,10 If the cause of the macrocyte is alcohol-related and alcohol use is terminated, those enlarged cells can resolve in an average of 3 months.9
Vitamin B1 levels >200 nmol/L would be within normal range (74 to 222 nmol/L). Mr. G’s chronic alcohol use would likely cause him to be vitamin B1–deficient. The deficiency is usually related to diet, malabsorption, and the cells’ impaired ability to utilize vitamin B1. A consequence of vitamin B1 deficiency is Wernicke-Korsakoff syndrome.11
Due to his chronic alcohol use, Mr. G’s magnesium stores most likely would be below normal range (1.7 to 2.2 mg/dL). Acting as a diuretic, alcohol depletes magnesium and other electrolytes. The intracellular shift that occurs to balance the deficit causes the body to use its normal stores of magnesium, which leads to further magnesium depletion. Other common causes include nutritional deficiency and decreased gastrointestinal absorption.12 The bleeding the physician suspected was a result of drinking likely occurred through direct and indirect mechanisms that affect platelets.9,13 Platelets can show improvement 1 week after drinking cessation. Some evidence suggests the risk of seizure or DTs increases significantly with a platelet count <119,000 µL per unit of blood.13
Continue to: TREATMENT Pharmacotherapy for alcohol use disorder
TREATMENT Pharmacotherapy for alcohol use disorder
As Mr. G’s condition starts to stabilize, he discusses treatment options for AUD with his physician. At the end of the discussion, Mr. G expresses an interest in starting a medication. The doctor reviews his laboratory results and available treatment options.
[polldaddy:12041630]
The author’s observations
Of the 3 FDA-approved medications for treating AUD (disulfiram, acamprosate, and naltrexone), naltrexone has been shown to decrease heavy drinking days5,14 and comes in oral and injectable forms. Reducing drinking is achieved by reducing the rewarding effects of alcohol5,14 and alcohol cravings.5 Disulfiram often has poor adherence, and like acamprosate it may be more helpful for maintenance of abstinence. Neither topiramate nor gabapentin are FDA-approved for AUD but may be used for their affects on GABA.5 Gabapentin may also help patients experiencing alcohol withdrawal syndrome.5,15 Mr. G did not have any concomitant medications or comorbid medical conditions, but these factors as well as any renal or hepatic dysfunction must be considered before initiating any medications.
OUTCOME Improved well-being
Mr. G’s treatment team initiates oral naltrexone 50 mg/d, which he tolerates well without complications. He stops drinking entirely and expresses an interest in transitioning to an injectable form of naltrexone in the future. In addition to taking medication, Mr. G wants to participate in psychotherapy. Mr. G thanks his team for the care he received in the hospital, telling them, “You all saved my life.” As he discusses his past issues with alcohol, Mr. G asks his physician how he could get involved to make changes to reduce excessive alcohol consumption in his community (Box5,15-21).
Box
Alcohol use disorder is undertreated5,15-17 and excessive alcohol use accounts for 1 in 5 deaths in individuals within Mr. G’s age range.18 An April 2011 report from the Community Preventive Services Task Force19 did not recommend privatization of retail alcohol sales as an intervention to reduce excessive alcohol consumption, because it would instead lead to an increase in alcohol consumption per capita, a known gateway to excessive alcohol consumption.20
The Task Force was established in 1996 by the US Department of Health and Human Services. Its objective is to identify scientifically proven interventions to save lives, increase lifespans, and improve quality of life. Recommendations are based on systematic reviews to inform lawmakers, health departments, and other organizations and agencies.21 The Task Force’s recommendations were divided into interventions that have strong evidence, sufficient evidence, or insufficient evidence. If Mr. G wanted to have the greatest impact in his efforts to reduce excessive alcohol consumption in his community, the strongest evidence supporting change focuses on electronic screening and brief intervention, maintaining limits on days of alcohol sale, increasing taxes on alcohol, and establishing dram shop liability (laws that hold retail establishments that sell alcohol liable for the injuries or harms caused by their intoxicated or underage customers).19
Bottom Line
Patients experiencing alcohol withdrawal can present with several layers of complexity. Failure to achieve acute stabilization may be life-threatening. After providing critical care, promptly start alcohol use disorder treatment for patients who expresses a desire to change.
Related Resources
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. https://www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association Publishing; 2018.
Drug Brand Names
Acamprosate • Campral
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone (injection) • Vivitrol
Naltrexone (oral) • ReVia
Topiramate • Topamax
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
3. Holleck JL, Merchant N, Gunderson CG. Symptom-triggered therapy for alcohol withdrawal syndrome: a systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2019;34(6):1018-1024.
4. Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31(4):310-339.
5. Burnette EM, Nieto SJ, Grodin EN, et al. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82(3):251-274.
6. Selim R, Zhou Y, Rupp LB, et al. Availability of PEth testing is associated with reduced eligibility for liver transplant among patients with alcohol-related liver disease. Clin Transplant. 2022;36(5):e14595.
7. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63(6):1634-1640.
8. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117-130.
9. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21(1):42-52.
10. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
11. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134-142.
12. Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med. 2017;377(14):1368-1377.
13. Silczuk A, Habrat B. Alcohol-induced thrombocytopenia: current review. Alcohol. 2020;86:9-16. doi:10.1016/j.alcohol.2020.02.166
14. Pettinati HM, Rabinowitz AR. New pharmacotherapies for treating the neurobiology of alcohol and drug addiction. Psychiatry (Edgmont). 2006;3(5):14-16.
15. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736.
16. Chockalingam L, Burnham EL, Jolley SE. Medication prescribing for alcohol use disorders during alcohol-related encounters in a Colorado regional healthcare system. Alcoholism Clin Exp Res. 2022;46(6):1094-1102.
17. Mintz CM, Hartz SM, Fisher SL, et al. A cascade of care for alcohol use disorder: using 2015-2019 National Survey on Drug Use and Health data to identify gaps in past 12-month care. Alcohol Clin Exp Res. 2021;45(6):1276-1286.
18. Esser MB, Leung G, Sherk A, et al. Estimated deaths attributable to excessive alcohol use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw Open. 2022;5(11):e2239485. doi:10.1001/jamanet workopen.2022.39485
19. The Community Guide. CPSTF Findings for Excessive Alcohol Consumption. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/task-force-findings-excessive-alcohol-consumption.html
20. The Community Guide. Alcohol Excessive Consumption: Privatization of Retail Alcohol Sales. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-privatization-retail-alcohol-sales.html
21. The Community Guide. What is the CPSTF? Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/what-is-the-cpstf.html
CASE A difficult withdrawal
Three days after he stops drinking alcohol, Mr. G, age 49, presents to a detoxification center with his wife, who drove him there because she was concerned about his condition. She says her husband had been drinking alcohol every night for as long as she can remember. Despite numerous admissions to rehabilitation centers, Mr. G usually would resume drinking soon after he was discharged. Three days ago, Mr. G’s wife had told him she “could not take it anymore,” so he got rid of all his alcohol and stopped drinking. Mr. G’s wife felt he was doing fine the first day, but his condition increasingly worsened the second and third days. The triage nurse who attempts to interview Mr. G finds him tremulous, vomiting, and sweating. She notices that he seems preoccupied with pulling at his shirt, appearing to pick at things that are not there.
HISTORY Untreated depression, other comorbidities
Mr. G’s wife says he has never been psychiatrically hospitalized or exhibited suicidal behavior. Mr. G previously received care from a psychiatrist, who diagnosed him with major depressive disorder (MDD) and prescribed an antidepressant, though his wife cannot recall which specific medication. She shares it has been “a long time” since Mr. G has taken the antidepressant and the last time he received treatment for his MDD was 5 years ago. Mr. G’s wife says her husband had once abstained from alcohol use for >6 months following one of his stints at a rehabilitation center. She is not able to share many other details about Mr. G’s previous stays at rehabilitation centers, but says he always had “a rough time.”
She says Mr. G had been drinking an average of 10 drinks each night, usually within 4 hours. He has no history of nicotine or illicit substance use and has held a corporate job for the last 18 years. Several years ago, a physician had diagnosed Mr. G with hypertension and high cholesterol, but he did not follow up for treatment. Mr. G’s wife also recalls a physician told her husband he had a fatty liver. His family history includes heart disease and cancer.
[polldaddy:12041618]
The author’s observations
The treatment team observed several elements of alcohol withdrawal and classified Mr. G as a priority patient. If the team had completed the Clinical Institute Withdrawal Assessment for Alcohol–Revised scale (CIWA-Ar) (Table 11), Mr. G would score ≥10. While the protocol for initiating treatment for patients experiencing alcohol withdrawal varies by institution, patients with moderate to severe scores on the CIWA-Ar when experiencing withdrawal typically are managed with pharmacotherapy to address their symptoms.1 Given the timeline of his last drink as reported by his wife, Mr. G is on the brink of experiencing a cascade of symptoms concerning for delirium tremens (DTs).2 Table 22 provides a timeline and symptoms related to alcohol withdrawal. To prevent further exacerbation of symptoms, which could lead to DTs, Mr. G’s treatment team will likely initiate a benzodiazepine, using either scheduled or symptom-driven dosing.3

Two neurotransmitters that play a role in DTs are glutamate (excitatory) and GABA (inhibitory). In a normal state, the competing actions of these neurotransmitters balance each other. Acute alcohol intake causes a shift in the excitatory and inhibitory levels, with more inhibition taking place, thus causing disequilibrium. If chronic alcohol use continues, the amount of GABA inhibition reduction is related to downregulation of receptors.2,4 Excitation increases by way of upregulation of the N-methyl-

If alcohol is suddenly removed following chronic use, there is unchecked glutamate excitation related to a blunted GABA state. This added increase in the excitation of glutamate leads to withdrawal symptoms.2,4 Table 32,4,5 depicts the neurotransmitter equilibrium of GABA and glutamate relative to alcohol use.

EVALUATION Bleeding gums and bruising
The treatment team admits Mr. G to the triage bay and contacts the addiction psychiatrist. The physician orders laboratory tests to assess nutritional deficits and electrolyte abnormalities. Mr. G is also placed on routine assessments with symptom-triggered therapy. An assessment reveals bleeding gums and bruises, which are believed to be a result of thrombocytopenia (low blood platelet count).
[polldaddy:12041627]
Continue to: The author's observations
The author’s observations
Though regular clinical assessment of PEth varies, it is considered to have high sensitivity and specificity to detect alcohol use.6 When ethanol is present, the phospholipase D enzyme acts upon phosphatidylcholine, forming a direct biomarker, PEth, on the surface of the red blood cell.6,7 PEth’s half-life ranges from 4.5 to 12 days,6 and it can be detected in blood for 3 to 4 weeks after alcohol ingestion.6,7 A PEth value <20 ng/mL indicates light or no alcohol consumption; 20 to 199 ng/mL indicates significant consumption; and >200 ng/mL indicates heavy consumption.7 Since Mr. G has a history of chronic alcohol use, his PEth level is expected to be >200 ng/mL.
AST/ALT and MCV are indirect biomarkers, meaning the tests are not alcohol-specific and the role of alcohol is instead observed by the damage to the body with excessive use over time.7 The expected AST:ALT ratio is 2:1. This is related to 3 mechanisms. The first is a decrease in ALT usually relative to B6 deficiency in individuals with alcohol use disorder (AUD). Another mechanism is related to alcohol’s propensity to affect mitochondria, which is a source for AST. Additionally, AST is also found in higher proportions in the kidneys, heart, and muscles.8
An MCV <100 fL would be within the normal range (80 to 100 fL) for red blood cells. While the reasons for an enlarged red blood cell (or macrocyte) are extensive, alcohol can be a factor once other causes are excluded. Additional laboratory tests and a peripheral blood smear test can help in this investigation. Alcohol disrupts the complete maturation of red blood cells.9,10 If the cause of the macrocyte is alcohol-related and alcohol use is terminated, those enlarged cells can resolve in an average of 3 months.9
Vitamin B1 levels >200 nmol/L would be within normal range (74 to 222 nmol/L). Mr. G’s chronic alcohol use would likely cause him to be vitamin B1–deficient. The deficiency is usually related to diet, malabsorption, and the cells’ impaired ability to utilize vitamin B1. A consequence of vitamin B1 deficiency is Wernicke-Korsakoff syndrome.11
Due to his chronic alcohol use, Mr. G’s magnesium stores most likely would be below normal range (1.7 to 2.2 mg/dL). Acting as a diuretic, alcohol depletes magnesium and other electrolytes. The intracellular shift that occurs to balance the deficit causes the body to use its normal stores of magnesium, which leads to further magnesium depletion. Other common causes include nutritional deficiency and decreased gastrointestinal absorption.12 The bleeding the physician suspected was a result of drinking likely occurred through direct and indirect mechanisms that affect platelets.9,13 Platelets can show improvement 1 week after drinking cessation. Some evidence suggests the risk of seizure or DTs increases significantly with a platelet count <119,000 µL per unit of blood.13
Continue to: TREATMENT Pharmacotherapy for alcohol use disorder
TREATMENT Pharmacotherapy for alcohol use disorder
As Mr. G’s condition starts to stabilize, he discusses treatment options for AUD with his physician. At the end of the discussion, Mr. G expresses an interest in starting a medication. The doctor reviews his laboratory results and available treatment options.
[polldaddy:12041630]
The author’s observations
Of the 3 FDA-approved medications for treating AUD (disulfiram, acamprosate, and naltrexone), naltrexone has been shown to decrease heavy drinking days5,14 and comes in oral and injectable forms. Reducing drinking is achieved by reducing the rewarding effects of alcohol5,14 and alcohol cravings.5 Disulfiram often has poor adherence, and like acamprosate it may be more helpful for maintenance of abstinence. Neither topiramate nor gabapentin are FDA-approved for AUD but may be used for their affects on GABA.5 Gabapentin may also help patients experiencing alcohol withdrawal syndrome.5,15 Mr. G did not have any concomitant medications or comorbid medical conditions, but these factors as well as any renal or hepatic dysfunction must be considered before initiating any medications.
OUTCOME Improved well-being
Mr. G’s treatment team initiates oral naltrexone 50 mg/d, which he tolerates well without complications. He stops drinking entirely and expresses an interest in transitioning to an injectable form of naltrexone in the future. In addition to taking medication, Mr. G wants to participate in psychotherapy. Mr. G thanks his team for the care he received in the hospital, telling them, “You all saved my life.” As he discusses his past issues with alcohol, Mr. G asks his physician how he could get involved to make changes to reduce excessive alcohol consumption in his community (Box5,15-21).
Box
Alcohol use disorder is undertreated5,15-17 and excessive alcohol use accounts for 1 in 5 deaths in individuals within Mr. G’s age range.18 An April 2011 report from the Community Preventive Services Task Force19 did not recommend privatization of retail alcohol sales as an intervention to reduce excessive alcohol consumption, because it would instead lead to an increase in alcohol consumption per capita, a known gateway to excessive alcohol consumption.20
The Task Force was established in 1996 by the US Department of Health and Human Services. Its objective is to identify scientifically proven interventions to save lives, increase lifespans, and improve quality of life. Recommendations are based on systematic reviews to inform lawmakers, health departments, and other organizations and agencies.21 The Task Force’s recommendations were divided into interventions that have strong evidence, sufficient evidence, or insufficient evidence. If Mr. G wanted to have the greatest impact in his efforts to reduce excessive alcohol consumption in his community, the strongest evidence supporting change focuses on electronic screening and brief intervention, maintaining limits on days of alcohol sale, increasing taxes on alcohol, and establishing dram shop liability (laws that hold retail establishments that sell alcohol liable for the injuries or harms caused by their intoxicated or underage customers).19
Bottom Line
Patients experiencing alcohol withdrawal can present with several layers of complexity. Failure to achieve acute stabilization may be life-threatening. After providing critical care, promptly start alcohol use disorder treatment for patients who expresses a desire to change.
Related Resources
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. https://www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association Publishing; 2018.
Drug Brand Names
Acamprosate • Campral
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone (injection) • Vivitrol
Naltrexone (oral) • ReVia
Topiramate • Topamax
CASE A difficult withdrawal
Three days after he stops drinking alcohol, Mr. G, age 49, presents to a detoxification center with his wife, who drove him there because she was concerned about his condition. She says her husband had been drinking alcohol every night for as long as she can remember. Despite numerous admissions to rehabilitation centers, Mr. G usually would resume drinking soon after he was discharged. Three days ago, Mr. G’s wife had told him she “could not take it anymore,” so he got rid of all his alcohol and stopped drinking. Mr. G’s wife felt he was doing fine the first day, but his condition increasingly worsened the second and third days. The triage nurse who attempts to interview Mr. G finds him tremulous, vomiting, and sweating. She notices that he seems preoccupied with pulling at his shirt, appearing to pick at things that are not there.
HISTORY Untreated depression, other comorbidities
Mr. G’s wife says he has never been psychiatrically hospitalized or exhibited suicidal behavior. Mr. G previously received care from a psychiatrist, who diagnosed him with major depressive disorder (MDD) and prescribed an antidepressant, though his wife cannot recall which specific medication. She shares it has been “a long time” since Mr. G has taken the antidepressant and the last time he received treatment for his MDD was 5 years ago. Mr. G’s wife says her husband had once abstained from alcohol use for >6 months following one of his stints at a rehabilitation center. She is not able to share many other details about Mr. G’s previous stays at rehabilitation centers, but says he always had “a rough time.”
She says Mr. G had been drinking an average of 10 drinks each night, usually within 4 hours. He has no history of nicotine or illicit substance use and has held a corporate job for the last 18 years. Several years ago, a physician had diagnosed Mr. G with hypertension and high cholesterol, but he did not follow up for treatment. Mr. G’s wife also recalls a physician told her husband he had a fatty liver. His family history includes heart disease and cancer.
[polldaddy:12041618]
The author’s observations
The treatment team observed several elements of alcohol withdrawal and classified Mr. G as a priority patient. If the team had completed the Clinical Institute Withdrawal Assessment for Alcohol–Revised scale (CIWA-Ar) (Table 11), Mr. G would score ≥10. While the protocol for initiating treatment for patients experiencing alcohol withdrawal varies by institution, patients with moderate to severe scores on the CIWA-Ar when experiencing withdrawal typically are managed with pharmacotherapy to address their symptoms.1 Given the timeline of his last drink as reported by his wife, Mr. G is on the brink of experiencing a cascade of symptoms concerning for delirium tremens (DTs).2 Table 22 provides a timeline and symptoms related to alcohol withdrawal. To prevent further exacerbation of symptoms, which could lead to DTs, Mr. G’s treatment team will likely initiate a benzodiazepine, using either scheduled or symptom-driven dosing.3

Two neurotransmitters that play a role in DTs are glutamate (excitatory) and GABA (inhibitory). In a normal state, the competing actions of these neurotransmitters balance each other. Acute alcohol intake causes a shift in the excitatory and inhibitory levels, with more inhibition taking place, thus causing disequilibrium. If chronic alcohol use continues, the amount of GABA inhibition reduction is related to downregulation of receptors.2,4 Excitation increases by way of upregulation of the N-methyl-

If alcohol is suddenly removed following chronic use, there is unchecked glutamate excitation related to a blunted GABA state. This added increase in the excitation of glutamate leads to withdrawal symptoms.2,4 Table 32,4,5 depicts the neurotransmitter equilibrium of GABA and glutamate relative to alcohol use.

EVALUATION Bleeding gums and bruising
The treatment team admits Mr. G to the triage bay and contacts the addiction psychiatrist. The physician orders laboratory tests to assess nutritional deficits and electrolyte abnormalities. Mr. G is also placed on routine assessments with symptom-triggered therapy. An assessment reveals bleeding gums and bruises, which are believed to be a result of thrombocytopenia (low blood platelet count).
[polldaddy:12041627]
Continue to: The author's observations
The author’s observations
Though regular clinical assessment of PEth varies, it is considered to have high sensitivity and specificity to detect alcohol use.6 When ethanol is present, the phospholipase D enzyme acts upon phosphatidylcholine, forming a direct biomarker, PEth, on the surface of the red blood cell.6,7 PEth’s half-life ranges from 4.5 to 12 days,6 and it can be detected in blood for 3 to 4 weeks after alcohol ingestion.6,7 A PEth value <20 ng/mL indicates light or no alcohol consumption; 20 to 199 ng/mL indicates significant consumption; and >200 ng/mL indicates heavy consumption.7 Since Mr. G has a history of chronic alcohol use, his PEth level is expected to be >200 ng/mL.
AST/ALT and MCV are indirect biomarkers, meaning the tests are not alcohol-specific and the role of alcohol is instead observed by the damage to the body with excessive use over time.7 The expected AST:ALT ratio is 2:1. This is related to 3 mechanisms. The first is a decrease in ALT usually relative to B6 deficiency in individuals with alcohol use disorder (AUD). Another mechanism is related to alcohol’s propensity to affect mitochondria, which is a source for AST. Additionally, AST is also found in higher proportions in the kidneys, heart, and muscles.8
An MCV <100 fL would be within the normal range (80 to 100 fL) for red blood cells. While the reasons for an enlarged red blood cell (or macrocyte) are extensive, alcohol can be a factor once other causes are excluded. Additional laboratory tests and a peripheral blood smear test can help in this investigation. Alcohol disrupts the complete maturation of red blood cells.9,10 If the cause of the macrocyte is alcohol-related and alcohol use is terminated, those enlarged cells can resolve in an average of 3 months.9
Vitamin B1 levels >200 nmol/L would be within normal range (74 to 222 nmol/L). Mr. G’s chronic alcohol use would likely cause him to be vitamin B1–deficient. The deficiency is usually related to diet, malabsorption, and the cells’ impaired ability to utilize vitamin B1. A consequence of vitamin B1 deficiency is Wernicke-Korsakoff syndrome.11
Due to his chronic alcohol use, Mr. G’s magnesium stores most likely would be below normal range (1.7 to 2.2 mg/dL). Acting as a diuretic, alcohol depletes magnesium and other electrolytes. The intracellular shift that occurs to balance the deficit causes the body to use its normal stores of magnesium, which leads to further magnesium depletion. Other common causes include nutritional deficiency and decreased gastrointestinal absorption.12 The bleeding the physician suspected was a result of drinking likely occurred through direct and indirect mechanisms that affect platelets.9,13 Platelets can show improvement 1 week after drinking cessation. Some evidence suggests the risk of seizure or DTs increases significantly with a platelet count <119,000 µL per unit of blood.13
Continue to: TREATMENT Pharmacotherapy for alcohol use disorder
TREATMENT Pharmacotherapy for alcohol use disorder
As Mr. G’s condition starts to stabilize, he discusses treatment options for AUD with his physician. At the end of the discussion, Mr. G expresses an interest in starting a medication. The doctor reviews his laboratory results and available treatment options.
[polldaddy:12041630]
The author’s observations
Of the 3 FDA-approved medications for treating AUD (disulfiram, acamprosate, and naltrexone), naltrexone has been shown to decrease heavy drinking days5,14 and comes in oral and injectable forms. Reducing drinking is achieved by reducing the rewarding effects of alcohol5,14 and alcohol cravings.5 Disulfiram often has poor adherence, and like acamprosate it may be more helpful for maintenance of abstinence. Neither topiramate nor gabapentin are FDA-approved for AUD but may be used for their affects on GABA.5 Gabapentin may also help patients experiencing alcohol withdrawal syndrome.5,15 Mr. G did not have any concomitant medications or comorbid medical conditions, but these factors as well as any renal or hepatic dysfunction must be considered before initiating any medications.
OUTCOME Improved well-being
Mr. G’s treatment team initiates oral naltrexone 50 mg/d, which he tolerates well without complications. He stops drinking entirely and expresses an interest in transitioning to an injectable form of naltrexone in the future. In addition to taking medication, Mr. G wants to participate in psychotherapy. Mr. G thanks his team for the care he received in the hospital, telling them, “You all saved my life.” As he discusses his past issues with alcohol, Mr. G asks his physician how he could get involved to make changes to reduce excessive alcohol consumption in his community (Box5,15-21).
Box
Alcohol use disorder is undertreated5,15-17 and excessive alcohol use accounts for 1 in 5 deaths in individuals within Mr. G’s age range.18 An April 2011 report from the Community Preventive Services Task Force19 did not recommend privatization of retail alcohol sales as an intervention to reduce excessive alcohol consumption, because it would instead lead to an increase in alcohol consumption per capita, a known gateway to excessive alcohol consumption.20
The Task Force was established in 1996 by the US Department of Health and Human Services. Its objective is to identify scientifically proven interventions to save lives, increase lifespans, and improve quality of life. Recommendations are based on systematic reviews to inform lawmakers, health departments, and other organizations and agencies.21 The Task Force’s recommendations were divided into interventions that have strong evidence, sufficient evidence, or insufficient evidence. If Mr. G wanted to have the greatest impact in his efforts to reduce excessive alcohol consumption in his community, the strongest evidence supporting change focuses on electronic screening and brief intervention, maintaining limits on days of alcohol sale, increasing taxes on alcohol, and establishing dram shop liability (laws that hold retail establishments that sell alcohol liable for the injuries or harms caused by their intoxicated or underage customers).19
Bottom Line
Patients experiencing alcohol withdrawal can present with several layers of complexity. Failure to achieve acute stabilization may be life-threatening. After providing critical care, promptly start alcohol use disorder treatment for patients who expresses a desire to change.
Related Resources
- American Society of Addiction Medicine. The ASAM Clinical Practice Guideline on Alcohol Withdrawal Management. https://www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
- American Psychiatric Association. The American Psychiatric Association Practice Guideline for the Pharmacological Treatment of Patients With Alcohol Use Disorder. American Psychiatric Association Publishing; 2018.
Drug Brand Names
Acamprosate • Campral
Disulfiram • Antabuse
Gabapentin • Neurontin
Naltrexone (injection) • Vivitrol
Naltrexone (oral) • ReVia
Topiramate • Topamax
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
3. Holleck JL, Merchant N, Gunderson CG. Symptom-triggered therapy for alcohol withdrawal syndrome: a systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2019;34(6):1018-1024.
4. Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31(4):310-339.
5. Burnette EM, Nieto SJ, Grodin EN, et al. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82(3):251-274.
6. Selim R, Zhou Y, Rupp LB, et al. Availability of PEth testing is associated with reduced eligibility for liver transplant among patients with alcohol-related liver disease. Clin Transplant. 2022;36(5):e14595.
7. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63(6):1634-1640.
8. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117-130.
9. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21(1):42-52.
10. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
11. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134-142.
12. Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med. 2017;377(14):1368-1377.
13. Silczuk A, Habrat B. Alcohol-induced thrombocytopenia: current review. Alcohol. 2020;86:9-16. doi:10.1016/j.alcohol.2020.02.166
14. Pettinati HM, Rabinowitz AR. New pharmacotherapies for treating the neurobiology of alcohol and drug addiction. Psychiatry (Edgmont). 2006;3(5):14-16.
15. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736.
16. Chockalingam L, Burnham EL, Jolley SE. Medication prescribing for alcohol use disorders during alcohol-related encounters in a Colorado regional healthcare system. Alcoholism Clin Exp Res. 2022;46(6):1094-1102.
17. Mintz CM, Hartz SM, Fisher SL, et al. A cascade of care for alcohol use disorder: using 2015-2019 National Survey on Drug Use and Health data to identify gaps in past 12-month care. Alcohol Clin Exp Res. 2021;45(6):1276-1286.
18. Esser MB, Leung G, Sherk A, et al. Estimated deaths attributable to excessive alcohol use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw Open. 2022;5(11):e2239485. doi:10.1001/jamanet workopen.2022.39485
19. The Community Guide. CPSTF Findings for Excessive Alcohol Consumption. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/task-force-findings-excessive-alcohol-consumption.html
20. The Community Guide. Alcohol Excessive Consumption: Privatization of Retail Alcohol Sales. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-privatization-retail-alcohol-sales.html
21. The Community Guide. What is the CPSTF? Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/what-is-the-cpstf.html
1. Sullivan JT, Sykora K, Schneiderman J, et al. Assessment of alcohol withdrawal: the revised clinical institute withdrawal assessment for alcohol scale (CIWA-Ar). Br J Addict. 1989;84(11):1353-1357.
2. Trevisan LA, Boutros N, Petrakis IL, et al. Complications of alcohol withdrawal: pathophysiological insights. Alcohol Health Res World. 1998;22(1):61-66.
3. Holleck JL, Merchant N, Gunderson CG. Symptom-triggered therapy for alcohol withdrawal syndrome: a systematic review and meta-analysis of randomized controlled trials. J Gen Intern Med. 2019;34(6):1018-1024.
4. Clapp P, Bhave SV, Hoffman PL. How adaptation of the brain to alcohol leads to dependence: a pharmacological perspective. Alcohol Res Health. 2008;31(4):310-339.
5. Burnette EM, Nieto SJ, Grodin EN, et al. Novel agents for the pharmacological treatment of alcohol use disorder. Drugs. 2022;82(3):251-274.
6. Selim R, Zhou Y, Rupp LB, et al. Availability of PEth testing is associated with reduced eligibility for liver transplant among patients with alcohol-related liver disease. Clin Transplant. 2022;36(5):e14595.
7. Ulwelling W, Smith K. The PEth blood test in the security environment: what it is; why it is important; and interpretative guidelines. J Forensic Sci. 2018;63(6):1634-1640.
8. Botros M, Sikaris KA. The de ritis ratio: the test of time. Clin Biochem Rev. 2013;34(3):117-130.
9. Ballard HS. The hematological complications of alcoholism. Alcohol Health Res World. 1997;21(1):42-52.
10. Kaferle J, Strzoda CE. Evaluation of macrocytosis. Am Fam Physician. 2009;79(3):203-208.
11. Martin PR, Singleton CK, Hiller-Sturmhöfel S. The role of thiamine deficiency in alcoholic brain disease. Alcohol Res Health. 2003;27(2):134-142.
12. Palmer BF, Clegg DJ. Electrolyte disturbances in patients with chronic alcohol-use disorder. N Engl J Med. 2017;377(14):1368-1377.
13. Silczuk A, Habrat B. Alcohol-induced thrombocytopenia: current review. Alcohol. 2020;86:9-16. doi:10.1016/j.alcohol.2020.02.166
14. Pettinati HM, Rabinowitz AR. New pharmacotherapies for treating the neurobiology of alcohol and drug addiction. Psychiatry (Edgmont). 2006;3(5):14-16.
15. Anton RF, Latham P, Voronin K, et al. Efficacy of gabapentin for the treatment of alcohol use disorder in patients with alcohol withdrawal symptoms: a randomized clinical trial. JAMA Intern Med. 2020;180(5):728-736.
16. Chockalingam L, Burnham EL, Jolley SE. Medication prescribing for alcohol use disorders during alcohol-related encounters in a Colorado regional healthcare system. Alcoholism Clin Exp Res. 2022;46(6):1094-1102.
17. Mintz CM, Hartz SM, Fisher SL, et al. A cascade of care for alcohol use disorder: using 2015-2019 National Survey on Drug Use and Health data to identify gaps in past 12-month care. Alcohol Clin Exp Res. 2021;45(6):1276-1286.
18. Esser MB, Leung G, Sherk A, et al. Estimated deaths attributable to excessive alcohol use among US adults aged 20 to 64 years, 2015 to 2019. JAMA Netw Open. 2022;5(11):e2239485. doi:10.1001/jamanet workopen.2022.39485
19. The Community Guide. CPSTF Findings for Excessive Alcohol Consumption. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/task-force-findings-excessive-alcohol-consumption.html
20. The Community Guide. Alcohol Excessive Consumption: Privatization of Retail Alcohol Sales. Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/findings/alcohol-excessive-consumption-privatization-retail-alcohol-sales.html
21. The Community Guide. What is the CPSTF? Updated June 27, 2022. Accessed December 1, 2022. https://www.thecommunityguide.org/pages/what-is-the-cpstf.html
Increased anxiety and depression after menstruation
CASE Increased anxiety and depression
Ms. C, age 29, has bipolar II disorder (BD II) and generalized anxiety disorder. She presents to her outpatient psychiatrist seeking relief from chronic and significant dips in her mood from Day 5 to Day 15 of her menstrual cycle. During this time, she says she experiences increased anxiety, insomnia, frequent tearfulness, and intermittent suicidal ideation.
Ms. C meticulously charts her menstrual cycle using a smartphone app and reports having a regular 28-day cycle. She says she has experienced this worsening of symptoms since the onset of menarche, but her mood generally stabilizes after Day 14 of her cycle–around the time of ovulation–and remains euthymic throughout the premenstrual period.
HISTORY Depression and a change in medication
Ms. C has a history of major depressive episodes and has experienced hypomanic episodes that lasted 1 to 2 weeks and were associated with an elevated mood, high energy, rapid speech, and increased self-confidence. Ms. C says she has chronically high anxiety associated with trouble sleeping, difficulty focusing, restlessness, and muscle tension. When she was receiving care from previous psychiatrists, treatment with lithium, quetiapine, lamotrigine, sertraline, and fluoxetine was not successful, and Ms. C said she had severe anxiety when she tried sertraline and fluoxetine. After several months of substantial mood instability and high anxiety, Ms. C responded well to pregabalin 100 mg 3 times a day, lurasidone 60 mg/d at bedtime, and gabapentin 500 mg/d at bedtime. Over the last 4 months, she reports that her overall mood has been even, and she has been coping well with her anxiety.
Ms. C is married with no children. She uses condoms for birth control. She previously tried taking a combined estrogen/progestin oral contraceptive, but stopped because she said it made her feel very depressed. Ms. C reports no history of substance use. She is employed, says she has many positive relationships, and does not have a social history suggestive of a personality disorder.
[polldaddy:11818926]
The author’s observations
Many women report worsening of mood during the premenstrual period (luteal phase). Premenstrual dysphoric disorder (PMDD) involves symptoms that develop during the luteal phase and end shortly after menstruation; this condition impacts ≤5% of women.1 The etiology of PMDD appears to involve contributions from genetics, hormones such as estrogen and progesterone, allopregnanolone (a progesterone metabolite), brain-derived neurotrophic factor, brain structural and functional differences, and hypothalamic pathways.2
Researchers have postulated that the precipitous decline in the levels of progesterone and allopregnanolone in the luteal phase may contribute to the mood symptoms of PMDD.2 Allopregnanolone is a modulator of gamma-aminobutyric acid type A (GABA-A) receptors and may exert anxiolytic and sedative effects. Women who experience PMDD may be less sensitive to the effects of allopregnanolone.3 Additionally, early luteal phase levels of estrogen may predict late luteal phase symptoms of PMDD.4 The mechanism involved may be estrogen’s effect on the serotonin system. The HPA axis may also be involved in the etiology of PMDD because patients with this condition appear to have a blunted cortisol response in reaction to stress.5 Research also has implicated immune activation and inflammation in the etiology of PMDD.6
A PMDD diagnosis should be distinguished from a premenstrual exacerbation of an underlying psychiatric condition, which occurs when a patient has an untreated primary mood or anxiety disorder that worsens during the premenstrual period. PMDD is differentiated from premenstrual syndrome by the severity of symptoms.2 The recommended first-line treatment of PMDD is an SSRI, but if an SSRI does not work, is not tolerated, or is not preferred for any other reason, recommended alternatives include combined hormone oral contraceptive pills, dutasteride, gabapentin, or various supplements.7,8 PMDD has been widely studied and is treated by both psychiatrists and gynecologists. In addition, some women report experiencing mood instability around ovulation. Kiesner9 found that 13% of women studied showed an increased negative mood state midcycle, rather than during the premenstrual period.
Continue to: Postmenstrual syndrome
Postmenstrual syndrome
Postmenstrual mood symptoms are atypical. Postmenstrual syndrome is not listed in DSM-5 or formally recognized as a medical diagnosis. Peer-reviewed research or literature on the condition is scarce to nonexistent. However, it has been discussed by physicians in articles in the lay press. One gynecologist and reproductive endocrinologist estimated that approximately 10% of women experience significant physical and emotional symptoms postmenstruation.10 An internist and women’s health specialist suggested that the cause of postmenstrual syndrome might be a surge in levels of estrogen and testosterone and may be associated with insulin resistance and polycystic ovarian syndrome, while another possible contribution could be iron deficiency caused by loss of blood from menstruation.11
TREATMENT Recommending an oral contraceptive
Ms. C’s psychiatrist does not prescribe an SSRI because he is concerned it would destabilize her BD II. The patient also had negative experiences in her past 2 trials of SSRIs.
Because the psychiatrist believes it is prudent to optimize the dosages of a patient’s current medication before starting a new medication or intervention, he considers increasing Ms. C’s dosage of lurasidone or pregabalin. The rationale for optimizing Ms. C’s current medication regimen is that greater overall mood stability would likely result in less severe postmenstrual mood symptoms. However, Ms. C does not want to increase her dosage of either medication because she is concerned about adverse effects.
Ms. C’s psychiatrist discusses the case with 2 gynecologist/obstetrician colleagues. One suggests the patient try a progesterone-only oral contraceptive and the other suggests a trial of Prometrium (a progesterone capsule used to treat endometrial hyperplasia and secondary amenorrhea). Both suggestions are based on the theory that Ms. C may be sensitive to levels of progesterone, which are low during the follicular phase and rise after ovulation; neither recommendation is evidence-based. A low level of allopregnanolone may lead to less GABAergic activity and consequently greater mood dysregulation. Some women are particularly sensitive to low levels of allopregnanolone in the follicular phase, which might lead to postmenstrual mood symptoms. Additionally, Ms. C’s previous treatment with a combined estrogen/progestin oral contraceptive may have decreased her level of allopregnanolone.12 Ultimately, Ms. C’s psychiatrist suggests that she take a progesterone-only oral contraceptive.
The author’s observations
Guidance on how to treat Ms. C’s postmenstrual symptoms came from research on how to treat PMDD in patients who have BD. In a review of managing PMDD in women with BD, Sepede et al13 presented a treatment algorithm that recommends a combined estrogen/progestin oral contraceptive as first-line treatment in euthymic patients who are already receiving an optimal dose of mood stabilizers. Sepede et al13 expressed caution about using SSRIs due to the risk of inducing mood changes, but recommended SSRIs for patients with comorbid PMDD and BD who experience a depressive episode.
Another question is which type of oral contraceptive is most effective for treating PMDD. The combined oral contraceptive drospirenone/ethinyl estradiol has the most evidence for efficacy.14 Combined oral contraceptives carry risks of venous thromboembolism, hypertension, stroke, migraines, and liver complications, and are possibly associated with certain types of cancer, such as breast and cervical cancer.15 Their use is contraindicated in patients with a history of these conditions and for women age >35 who smoke ≥15 cigarettes/d.
The limited research that has examined the efficacy of progestin-only oral contraceptives for treating PMDD has been inconclusive.16 However, progesterone-only oral contraceptives are associated with less overall risk than combined oral contraceptives, and many women opt to use progesterone-only oral contraceptives due to concerns about possible adverse effects of the combined formulations. A substantial drawback of progesterone-only oral contraceptives is they must be taken at the same time every day, and if a dose is taken late, these agents may lose their efficacy in preventing pregnancy (and a backup birth control method must be used17). Additionally, drospirenone, a progestin that is a component of many oral contraceptives, has antimineralocorticoid properties and is contraindicated in patients with kidney or adrenal gland insufficiency or liver disease. As was the case when Ms. C initially took a combined contraceptive, hormonal contraceptives can sometimes cause mood dysregulation.
Continue to: OUTCOME Improved symptoms
OUTCOME Improved symptoms
Ms. C meets with her gynecologist, who prescribes norethindrone, a progestin-only oral contraceptive. Since taking norethindrone, Ms. C reports a dramatic improvement in the mood symptoms she experiences during the postmenstrual period.
Bottom Line
Some women may experience mood symptoms during the postmenstrual period that are similar to the symptoms experienced by patients who have premenstrual dysphoric disorder (PMDD). This phenomenon has been described as postmenstrual syndrome, and though evidence is lacking, treating it similarly to PMDD may be effective.
Related Resources
- Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health. 2020;23(1):113-122. doi:10.1007/s00737-019-0952-4
- Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
Drug Brand Names
Drospirenone/ethinyl estradiol • Yasmin
Dutasteride • Avodart
Fluoxetine • Prozac
Gabapentin • Neurontin
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Norethindrone • Aygestin
Pregabalin • Lyrica
Progesterone • Prometrium
Quetiapine • Seroquel
Sertraline • Zoloft
1. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
2. Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
3. Timby E, Bäckström T, Nyberg S, et al. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls--a pilot study. Psychopharmacology (Berl). 2016;233(11):2109-2117.
4. Yen JY, Lin HC, Lin PC, et al. Early- and late-luteal-phase estrogen and progesterone levels of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. 2019;16(22):4352.
5. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
6. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
7. Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Faculty Rev. 2022:11:(11). doi:10.12703/r/11-11
8. Raffi ER. Premenstrual dysphoric disorder. Current Psychiatry. 2017;16(9). Accessed January 30, 2023. https://www.mdedge.com/psychiatry/article/145089/somatic-disorders/premenstrual-dysphoric-disorder
9. Kiesner J. One woman’s low is another woman’s high: paradoxical effects of the menstrual cycle. Psychoneuroendocrinology. 2011;36(1):68-76.
10. Alnuweiri T. Feel low after your period? Postmenstrual syndrome could be the reason. Accessed January 30, 2023. https://www.wellandgood.com/pms-after-period/
11. Sharkey L. Everything you need to know about post-menstrual syndrome. Healthline. Published April 28, 2020. Accessed January 30, 2023. https://www.healthline.com/health/post-menstrual-syndrome
12. Santoru F, Berretti R, Locci A, et al. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology (Berl). 2014;231(17):3351-3364.
13. Sepede G, Brunetti M, Di Giannantonio M. Comorbid premenstrual dysphoric disorder in women with bipolar disorder: management challenges. Neuropsychiatr Dis Treatment. 2020;16:415-426.
14. Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contraception. 2019;10:27-39. doi:10.2147/OAJC.S183193
15. Roe AH, Bartz DA, Douglas PS. Combined estrogen-progestin contraception: side effects and health concerns. UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/combined-estrogen-progestin-contraception-side-effects-and-health-concerns
16. Ford O, Lethaby A, Roberts H, et al. Progesterone for premenstrual syndrome. Cochrane Database Sys Rev. 2012;3:CD003415. doi:10.1002/14651858.CD003415.pub4
17. Kaunitz AM. Contraception: progestin-only pills (POPs). UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/contraception-progestin-only-pills-pops
CASE Increased anxiety and depression
Ms. C, age 29, has bipolar II disorder (BD II) and generalized anxiety disorder. She presents to her outpatient psychiatrist seeking relief from chronic and significant dips in her mood from Day 5 to Day 15 of her menstrual cycle. During this time, she says she experiences increased anxiety, insomnia, frequent tearfulness, and intermittent suicidal ideation.
Ms. C meticulously charts her menstrual cycle using a smartphone app and reports having a regular 28-day cycle. She says she has experienced this worsening of symptoms since the onset of menarche, but her mood generally stabilizes after Day 14 of her cycle–around the time of ovulation–and remains euthymic throughout the premenstrual period.
HISTORY Depression and a change in medication
Ms. C has a history of major depressive episodes and has experienced hypomanic episodes that lasted 1 to 2 weeks and were associated with an elevated mood, high energy, rapid speech, and increased self-confidence. Ms. C says she has chronically high anxiety associated with trouble sleeping, difficulty focusing, restlessness, and muscle tension. When she was receiving care from previous psychiatrists, treatment with lithium, quetiapine, lamotrigine, sertraline, and fluoxetine was not successful, and Ms. C said she had severe anxiety when she tried sertraline and fluoxetine. After several months of substantial mood instability and high anxiety, Ms. C responded well to pregabalin 100 mg 3 times a day, lurasidone 60 mg/d at bedtime, and gabapentin 500 mg/d at bedtime. Over the last 4 months, she reports that her overall mood has been even, and she has been coping well with her anxiety.
Ms. C is married with no children. She uses condoms for birth control. She previously tried taking a combined estrogen/progestin oral contraceptive, but stopped because she said it made her feel very depressed. Ms. C reports no history of substance use. She is employed, says she has many positive relationships, and does not have a social history suggestive of a personality disorder.
[polldaddy:11818926]
The author’s observations
Many women report worsening of mood during the premenstrual period (luteal phase). Premenstrual dysphoric disorder (PMDD) involves symptoms that develop during the luteal phase and end shortly after menstruation; this condition impacts ≤5% of women.1 The etiology of PMDD appears to involve contributions from genetics, hormones such as estrogen and progesterone, allopregnanolone (a progesterone metabolite), brain-derived neurotrophic factor, brain structural and functional differences, and hypothalamic pathways.2
Researchers have postulated that the precipitous decline in the levels of progesterone and allopregnanolone in the luteal phase may contribute to the mood symptoms of PMDD.2 Allopregnanolone is a modulator of gamma-aminobutyric acid type A (GABA-A) receptors and may exert anxiolytic and sedative effects. Women who experience PMDD may be less sensitive to the effects of allopregnanolone.3 Additionally, early luteal phase levels of estrogen may predict late luteal phase symptoms of PMDD.4 The mechanism involved may be estrogen’s effect on the serotonin system. The HPA axis may also be involved in the etiology of PMDD because patients with this condition appear to have a blunted cortisol response in reaction to stress.5 Research also has implicated immune activation and inflammation in the etiology of PMDD.6
A PMDD diagnosis should be distinguished from a premenstrual exacerbation of an underlying psychiatric condition, which occurs when a patient has an untreated primary mood or anxiety disorder that worsens during the premenstrual period. PMDD is differentiated from premenstrual syndrome by the severity of symptoms.2 The recommended first-line treatment of PMDD is an SSRI, but if an SSRI does not work, is not tolerated, or is not preferred for any other reason, recommended alternatives include combined hormone oral contraceptive pills, dutasteride, gabapentin, or various supplements.7,8 PMDD has been widely studied and is treated by both psychiatrists and gynecologists. In addition, some women report experiencing mood instability around ovulation. Kiesner9 found that 13% of women studied showed an increased negative mood state midcycle, rather than during the premenstrual period.
Continue to: Postmenstrual syndrome
Postmenstrual syndrome
Postmenstrual mood symptoms are atypical. Postmenstrual syndrome is not listed in DSM-5 or formally recognized as a medical diagnosis. Peer-reviewed research or literature on the condition is scarce to nonexistent. However, it has been discussed by physicians in articles in the lay press. One gynecologist and reproductive endocrinologist estimated that approximately 10% of women experience significant physical and emotional symptoms postmenstruation.10 An internist and women’s health specialist suggested that the cause of postmenstrual syndrome might be a surge in levels of estrogen and testosterone and may be associated with insulin resistance and polycystic ovarian syndrome, while another possible contribution could be iron deficiency caused by loss of blood from menstruation.11
TREATMENT Recommending an oral contraceptive
Ms. C’s psychiatrist does not prescribe an SSRI because he is concerned it would destabilize her BD II. The patient also had negative experiences in her past 2 trials of SSRIs.
Because the psychiatrist believes it is prudent to optimize the dosages of a patient’s current medication before starting a new medication or intervention, he considers increasing Ms. C’s dosage of lurasidone or pregabalin. The rationale for optimizing Ms. C’s current medication regimen is that greater overall mood stability would likely result in less severe postmenstrual mood symptoms. However, Ms. C does not want to increase her dosage of either medication because she is concerned about adverse effects.
Ms. C’s psychiatrist discusses the case with 2 gynecologist/obstetrician colleagues. One suggests the patient try a progesterone-only oral contraceptive and the other suggests a trial of Prometrium (a progesterone capsule used to treat endometrial hyperplasia and secondary amenorrhea). Both suggestions are based on the theory that Ms. C may be sensitive to levels of progesterone, which are low during the follicular phase and rise after ovulation; neither recommendation is evidence-based. A low level of allopregnanolone may lead to less GABAergic activity and consequently greater mood dysregulation. Some women are particularly sensitive to low levels of allopregnanolone in the follicular phase, which might lead to postmenstrual mood symptoms. Additionally, Ms. C’s previous treatment with a combined estrogen/progestin oral contraceptive may have decreased her level of allopregnanolone.12 Ultimately, Ms. C’s psychiatrist suggests that she take a progesterone-only oral contraceptive.
The author’s observations
Guidance on how to treat Ms. C’s postmenstrual symptoms came from research on how to treat PMDD in patients who have BD. In a review of managing PMDD in women with BD, Sepede et al13 presented a treatment algorithm that recommends a combined estrogen/progestin oral contraceptive as first-line treatment in euthymic patients who are already receiving an optimal dose of mood stabilizers. Sepede et al13 expressed caution about using SSRIs due to the risk of inducing mood changes, but recommended SSRIs for patients with comorbid PMDD and BD who experience a depressive episode.
Another question is which type of oral contraceptive is most effective for treating PMDD. The combined oral contraceptive drospirenone/ethinyl estradiol has the most evidence for efficacy.14 Combined oral contraceptives carry risks of venous thromboembolism, hypertension, stroke, migraines, and liver complications, and are possibly associated with certain types of cancer, such as breast and cervical cancer.15 Their use is contraindicated in patients with a history of these conditions and for women age >35 who smoke ≥15 cigarettes/d.
The limited research that has examined the efficacy of progestin-only oral contraceptives for treating PMDD has been inconclusive.16 However, progesterone-only oral contraceptives are associated with less overall risk than combined oral contraceptives, and many women opt to use progesterone-only oral contraceptives due to concerns about possible adverse effects of the combined formulations. A substantial drawback of progesterone-only oral contraceptives is they must be taken at the same time every day, and if a dose is taken late, these agents may lose their efficacy in preventing pregnancy (and a backup birth control method must be used17). Additionally, drospirenone, a progestin that is a component of many oral contraceptives, has antimineralocorticoid properties and is contraindicated in patients with kidney or adrenal gland insufficiency or liver disease. As was the case when Ms. C initially took a combined contraceptive, hormonal contraceptives can sometimes cause mood dysregulation.
Continue to: OUTCOME Improved symptoms
OUTCOME Improved symptoms
Ms. C meets with her gynecologist, who prescribes norethindrone, a progestin-only oral contraceptive. Since taking norethindrone, Ms. C reports a dramatic improvement in the mood symptoms she experiences during the postmenstrual period.
Bottom Line
Some women may experience mood symptoms during the postmenstrual period that are similar to the symptoms experienced by patients who have premenstrual dysphoric disorder (PMDD). This phenomenon has been described as postmenstrual syndrome, and though evidence is lacking, treating it similarly to PMDD may be effective.
Related Resources
- Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health. 2020;23(1):113-122. doi:10.1007/s00737-019-0952-4
- Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
Drug Brand Names
Drospirenone/ethinyl estradiol • Yasmin
Dutasteride • Avodart
Fluoxetine • Prozac
Gabapentin • Neurontin
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Norethindrone • Aygestin
Pregabalin • Lyrica
Progesterone • Prometrium
Quetiapine • Seroquel
Sertraline • Zoloft
CASE Increased anxiety and depression
Ms. C, age 29, has bipolar II disorder (BD II) and generalized anxiety disorder. She presents to her outpatient psychiatrist seeking relief from chronic and significant dips in her mood from Day 5 to Day 15 of her menstrual cycle. During this time, she says she experiences increased anxiety, insomnia, frequent tearfulness, and intermittent suicidal ideation.
Ms. C meticulously charts her menstrual cycle using a smartphone app and reports having a regular 28-day cycle. She says she has experienced this worsening of symptoms since the onset of menarche, but her mood generally stabilizes after Day 14 of her cycle–around the time of ovulation–and remains euthymic throughout the premenstrual period.
HISTORY Depression and a change in medication
Ms. C has a history of major depressive episodes and has experienced hypomanic episodes that lasted 1 to 2 weeks and were associated with an elevated mood, high energy, rapid speech, and increased self-confidence. Ms. C says she has chronically high anxiety associated with trouble sleeping, difficulty focusing, restlessness, and muscle tension. When she was receiving care from previous psychiatrists, treatment with lithium, quetiapine, lamotrigine, sertraline, and fluoxetine was not successful, and Ms. C said she had severe anxiety when she tried sertraline and fluoxetine. After several months of substantial mood instability and high anxiety, Ms. C responded well to pregabalin 100 mg 3 times a day, lurasidone 60 mg/d at bedtime, and gabapentin 500 mg/d at bedtime. Over the last 4 months, she reports that her overall mood has been even, and she has been coping well with her anxiety.
Ms. C is married with no children. She uses condoms for birth control. She previously tried taking a combined estrogen/progestin oral contraceptive, but stopped because she said it made her feel very depressed. Ms. C reports no history of substance use. She is employed, says she has many positive relationships, and does not have a social history suggestive of a personality disorder.
[polldaddy:11818926]
The author’s observations
Many women report worsening of mood during the premenstrual period (luteal phase). Premenstrual dysphoric disorder (PMDD) involves symptoms that develop during the luteal phase and end shortly after menstruation; this condition impacts ≤5% of women.1 The etiology of PMDD appears to involve contributions from genetics, hormones such as estrogen and progesterone, allopregnanolone (a progesterone metabolite), brain-derived neurotrophic factor, brain structural and functional differences, and hypothalamic pathways.2
Researchers have postulated that the precipitous decline in the levels of progesterone and allopregnanolone in the luteal phase may contribute to the mood symptoms of PMDD.2 Allopregnanolone is a modulator of gamma-aminobutyric acid type A (GABA-A) receptors and may exert anxiolytic and sedative effects. Women who experience PMDD may be less sensitive to the effects of allopregnanolone.3 Additionally, early luteal phase levels of estrogen may predict late luteal phase symptoms of PMDD.4 The mechanism involved may be estrogen’s effect on the serotonin system. The HPA axis may also be involved in the etiology of PMDD because patients with this condition appear to have a blunted cortisol response in reaction to stress.5 Research also has implicated immune activation and inflammation in the etiology of PMDD.6
A PMDD diagnosis should be distinguished from a premenstrual exacerbation of an underlying psychiatric condition, which occurs when a patient has an untreated primary mood or anxiety disorder that worsens during the premenstrual period. PMDD is differentiated from premenstrual syndrome by the severity of symptoms.2 The recommended first-line treatment of PMDD is an SSRI, but if an SSRI does not work, is not tolerated, or is not preferred for any other reason, recommended alternatives include combined hormone oral contraceptive pills, dutasteride, gabapentin, or various supplements.7,8 PMDD has been widely studied and is treated by both psychiatrists and gynecologists. In addition, some women report experiencing mood instability around ovulation. Kiesner9 found that 13% of women studied showed an increased negative mood state midcycle, rather than during the premenstrual period.
Continue to: Postmenstrual syndrome
Postmenstrual syndrome
Postmenstrual mood symptoms are atypical. Postmenstrual syndrome is not listed in DSM-5 or formally recognized as a medical diagnosis. Peer-reviewed research or literature on the condition is scarce to nonexistent. However, it has been discussed by physicians in articles in the lay press. One gynecologist and reproductive endocrinologist estimated that approximately 10% of women experience significant physical and emotional symptoms postmenstruation.10 An internist and women’s health specialist suggested that the cause of postmenstrual syndrome might be a surge in levels of estrogen and testosterone and may be associated with insulin resistance and polycystic ovarian syndrome, while another possible contribution could be iron deficiency caused by loss of blood from menstruation.11
TREATMENT Recommending an oral contraceptive
Ms. C’s psychiatrist does not prescribe an SSRI because he is concerned it would destabilize her BD II. The patient also had negative experiences in her past 2 trials of SSRIs.
Because the psychiatrist believes it is prudent to optimize the dosages of a patient’s current medication before starting a new medication or intervention, he considers increasing Ms. C’s dosage of lurasidone or pregabalin. The rationale for optimizing Ms. C’s current medication regimen is that greater overall mood stability would likely result in less severe postmenstrual mood symptoms. However, Ms. C does not want to increase her dosage of either medication because she is concerned about adverse effects.
Ms. C’s psychiatrist discusses the case with 2 gynecologist/obstetrician colleagues. One suggests the patient try a progesterone-only oral contraceptive and the other suggests a trial of Prometrium (a progesterone capsule used to treat endometrial hyperplasia and secondary amenorrhea). Both suggestions are based on the theory that Ms. C may be sensitive to levels of progesterone, which are low during the follicular phase and rise after ovulation; neither recommendation is evidence-based. A low level of allopregnanolone may lead to less GABAergic activity and consequently greater mood dysregulation. Some women are particularly sensitive to low levels of allopregnanolone in the follicular phase, which might lead to postmenstrual mood symptoms. Additionally, Ms. C’s previous treatment with a combined estrogen/progestin oral contraceptive may have decreased her level of allopregnanolone.12 Ultimately, Ms. C’s psychiatrist suggests that she take a progesterone-only oral contraceptive.
The author’s observations
Guidance on how to treat Ms. C’s postmenstrual symptoms came from research on how to treat PMDD in patients who have BD. In a review of managing PMDD in women with BD, Sepede et al13 presented a treatment algorithm that recommends a combined estrogen/progestin oral contraceptive as first-line treatment in euthymic patients who are already receiving an optimal dose of mood stabilizers. Sepede et al13 expressed caution about using SSRIs due to the risk of inducing mood changes, but recommended SSRIs for patients with comorbid PMDD and BD who experience a depressive episode.
Another question is which type of oral contraceptive is most effective for treating PMDD. The combined oral contraceptive drospirenone/ethinyl estradiol has the most evidence for efficacy.14 Combined oral contraceptives carry risks of venous thromboembolism, hypertension, stroke, migraines, and liver complications, and are possibly associated with certain types of cancer, such as breast and cervical cancer.15 Their use is contraindicated in patients with a history of these conditions and for women age >35 who smoke ≥15 cigarettes/d.
The limited research that has examined the efficacy of progestin-only oral contraceptives for treating PMDD has been inconclusive.16 However, progesterone-only oral contraceptives are associated with less overall risk than combined oral contraceptives, and many women opt to use progesterone-only oral contraceptives due to concerns about possible adverse effects of the combined formulations. A substantial drawback of progesterone-only oral contraceptives is they must be taken at the same time every day, and if a dose is taken late, these agents may lose their efficacy in preventing pregnancy (and a backup birth control method must be used17). Additionally, drospirenone, a progestin that is a component of many oral contraceptives, has antimineralocorticoid properties and is contraindicated in patients with kidney or adrenal gland insufficiency or liver disease. As was the case when Ms. C initially took a combined contraceptive, hormonal contraceptives can sometimes cause mood dysregulation.
Continue to: OUTCOME Improved symptoms
OUTCOME Improved symptoms
Ms. C meets with her gynecologist, who prescribes norethindrone, a progestin-only oral contraceptive. Since taking norethindrone, Ms. C reports a dramatic improvement in the mood symptoms she experiences during the postmenstrual period.
Bottom Line
Some women may experience mood symptoms during the postmenstrual period that are similar to the symptoms experienced by patients who have premenstrual dysphoric disorder (PMDD). This phenomenon has been described as postmenstrual syndrome, and though evidence is lacking, treating it similarly to PMDD may be effective.
Related Resources
- Ray P, Mandal N, Sinha VK. Change of symptoms of schizophrenia across phases of menstrual cycle. Arch Womens Ment Health. 2020;23(1):113-122. doi:10.1007/s00737-019-0952-4
- Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
Drug Brand Names
Drospirenone/ethinyl estradiol • Yasmin
Dutasteride • Avodart
Fluoxetine • Prozac
Gabapentin • Neurontin
Lamotrigine • Lamictal
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Norethindrone • Aygestin
Pregabalin • Lyrica
Progesterone • Prometrium
Quetiapine • Seroquel
Sertraline • Zoloft
1. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
2. Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
3. Timby E, Bäckström T, Nyberg S, et al. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls--a pilot study. Psychopharmacology (Berl). 2016;233(11):2109-2117.
4. Yen JY, Lin HC, Lin PC, et al. Early- and late-luteal-phase estrogen and progesterone levels of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. 2019;16(22):4352.
5. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
6. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
7. Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Faculty Rev. 2022:11:(11). doi:10.12703/r/11-11
8. Raffi ER. Premenstrual dysphoric disorder. Current Psychiatry. 2017;16(9). Accessed January 30, 2023. https://www.mdedge.com/psychiatry/article/145089/somatic-disorders/premenstrual-dysphoric-disorder
9. Kiesner J. One woman’s low is another woman’s high: paradoxical effects of the menstrual cycle. Psychoneuroendocrinology. 2011;36(1):68-76.
10. Alnuweiri T. Feel low after your period? Postmenstrual syndrome could be the reason. Accessed January 30, 2023. https://www.wellandgood.com/pms-after-period/
11. Sharkey L. Everything you need to know about post-menstrual syndrome. Healthline. Published April 28, 2020. Accessed January 30, 2023. https://www.healthline.com/health/post-menstrual-syndrome
12. Santoru F, Berretti R, Locci A, et al. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology (Berl). 2014;231(17):3351-3364.
13. Sepede G, Brunetti M, Di Giannantonio M. Comorbid premenstrual dysphoric disorder in women with bipolar disorder: management challenges. Neuropsychiatr Dis Treatment. 2020;16:415-426.
14. Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contraception. 2019;10:27-39. doi:10.2147/OAJC.S183193
15. Roe AH, Bartz DA, Douglas PS. Combined estrogen-progestin contraception: side effects and health concerns. UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/combined-estrogen-progestin-contraception-side-effects-and-health-concerns
16. Ford O, Lethaby A, Roberts H, et al. Progesterone for premenstrual syndrome. Cochrane Database Sys Rev. 2012;3:CD003415. doi:10.1002/14651858.CD003415.pub4
17. Kaunitz AM. Contraception: progestin-only pills (POPs). UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/contraception-progestin-only-pills-pops
1. Epperson CN, Steiner M, Hartlage SA, et al. Premenstrual dysphoric disorder: evidence for a new category for DSM-5. Am J Psychiatry. 2012;169(5):465-475.
2. Raffi ER, Freeman MP. The etiology of premenstrual dysphoric disorder: 5 interwoven pieces. Current Psychiatry. 2017;16(9):20-28.
3. Timby E, Bäckström T, Nyberg S, et al. Women with premenstrual dysphoric disorder have altered sensitivity to allopregnanolone over the menstrual cycle compared to controls--a pilot study. Psychopharmacology (Berl). 2016;233(11):2109-2117.
4. Yen JY, Lin HC, Lin PC, et al. Early- and late-luteal-phase estrogen and progesterone levels of women with premenstrual dysphoric disorder. Int J Environ Res Public Health. 2019;16(22):4352.
5. Huang Y, Zhou R, Wu M, et al. Premenstrual syndrome is associated with blunted cortisol reactivity to the TSST. Stress. 2015;18(2):160-168.
6. Hantsoo L, Epperson CN. Premenstrual dysphoric disorder: epidemiology and treatment. Curr Psychiatry Rep. 2015;17(11):87.
7. Tiranini L, Nappi RE. Recent advances in understanding/management of premenstrual dysphoric disorder/premenstrual syndrome. Faculty Rev. 2022:11:(11). doi:10.12703/r/11-11
8. Raffi ER. Premenstrual dysphoric disorder. Current Psychiatry. 2017;16(9). Accessed January 30, 2023. https://www.mdedge.com/psychiatry/article/145089/somatic-disorders/premenstrual-dysphoric-disorder
9. Kiesner J. One woman’s low is another woman’s high: paradoxical effects of the menstrual cycle. Psychoneuroendocrinology. 2011;36(1):68-76.
10. Alnuweiri T. Feel low after your period? Postmenstrual syndrome could be the reason. Accessed January 30, 2023. https://www.wellandgood.com/pms-after-period/
11. Sharkey L. Everything you need to know about post-menstrual syndrome. Healthline. Published April 28, 2020. Accessed January 30, 2023. https://www.healthline.com/health/post-menstrual-syndrome
12. Santoru F, Berretti R, Locci A, et al. Decreased allopregnanolone induced by hormonal contraceptives is associated with a reduction in social behavior and sexual motivation in female rats. Psychopharmacology (Berl). 2014;231(17):3351-3364.
13. Sepede G, Brunetti M, Di Giannantonio M. Comorbid premenstrual dysphoric disorder in women with bipolar disorder: management challenges. Neuropsychiatr Dis Treatment. 2020;16:415-426.
14. Rapkin AJ, Korotkaya Y, Taylor KC. Contraception counseling for women with premenstrual dysphoric disorder (PMDD): current perspectives. Open Access J Contraception. 2019;10:27-39. doi:10.2147/OAJC.S183193
15. Roe AH, Bartz DA, Douglas PS. Combined estrogen-progestin contraception: side effects and health concerns. UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/combined-estrogen-progestin-contraception-side-effects-and-health-concerns
16. Ford O, Lethaby A, Roberts H, et al. Progesterone for premenstrual syndrome. Cochrane Database Sys Rev. 2012;3:CD003415. doi:10.1002/14651858.CD003415.pub4
17. Kaunitz AM. Contraception: progestin-only pills (POPs). UpToDate. Accessed February 1, 2023. https://www.uptodate.com/contents/contraception-progestin-only-pills-pops
Subtle cognitive decline in a patient with depression and anxiety
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.
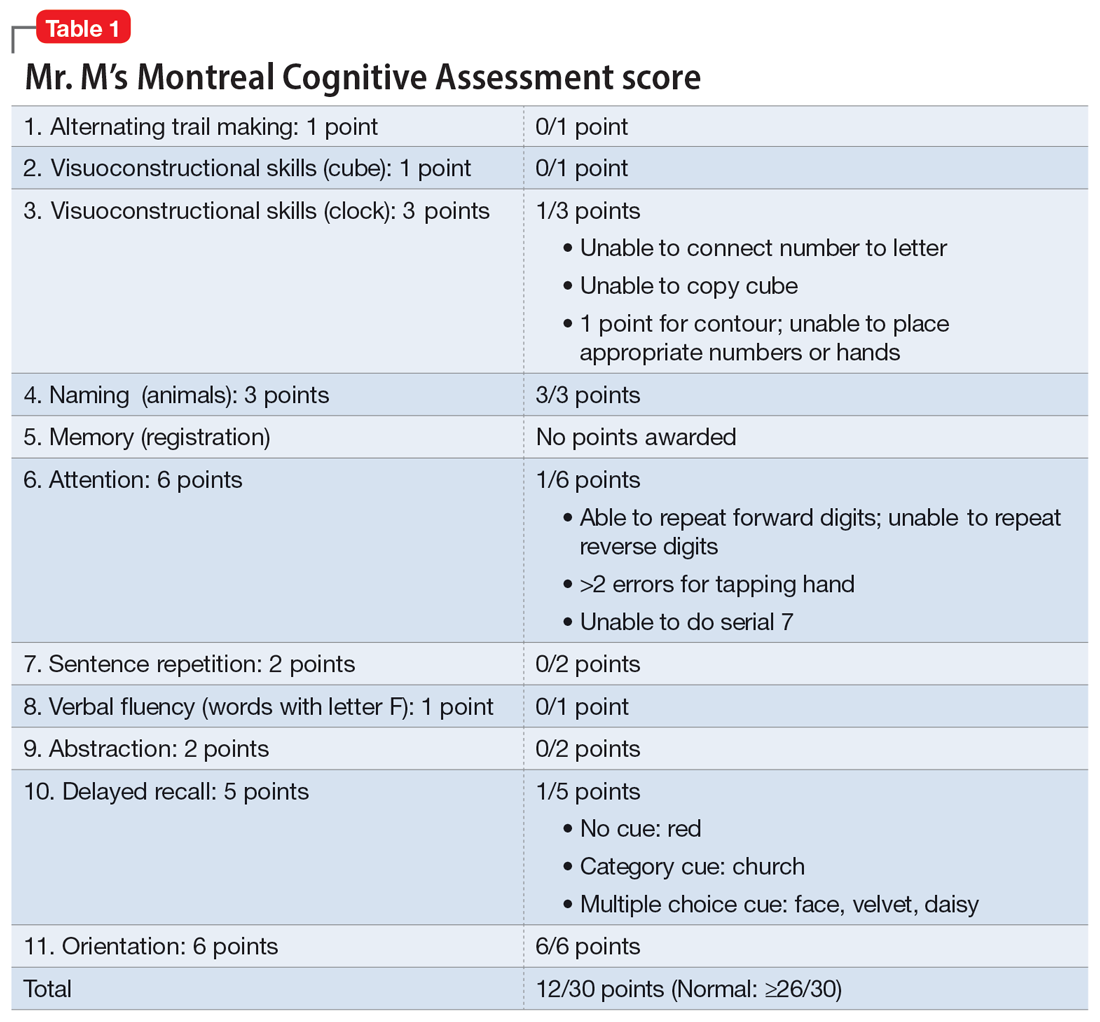
When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.
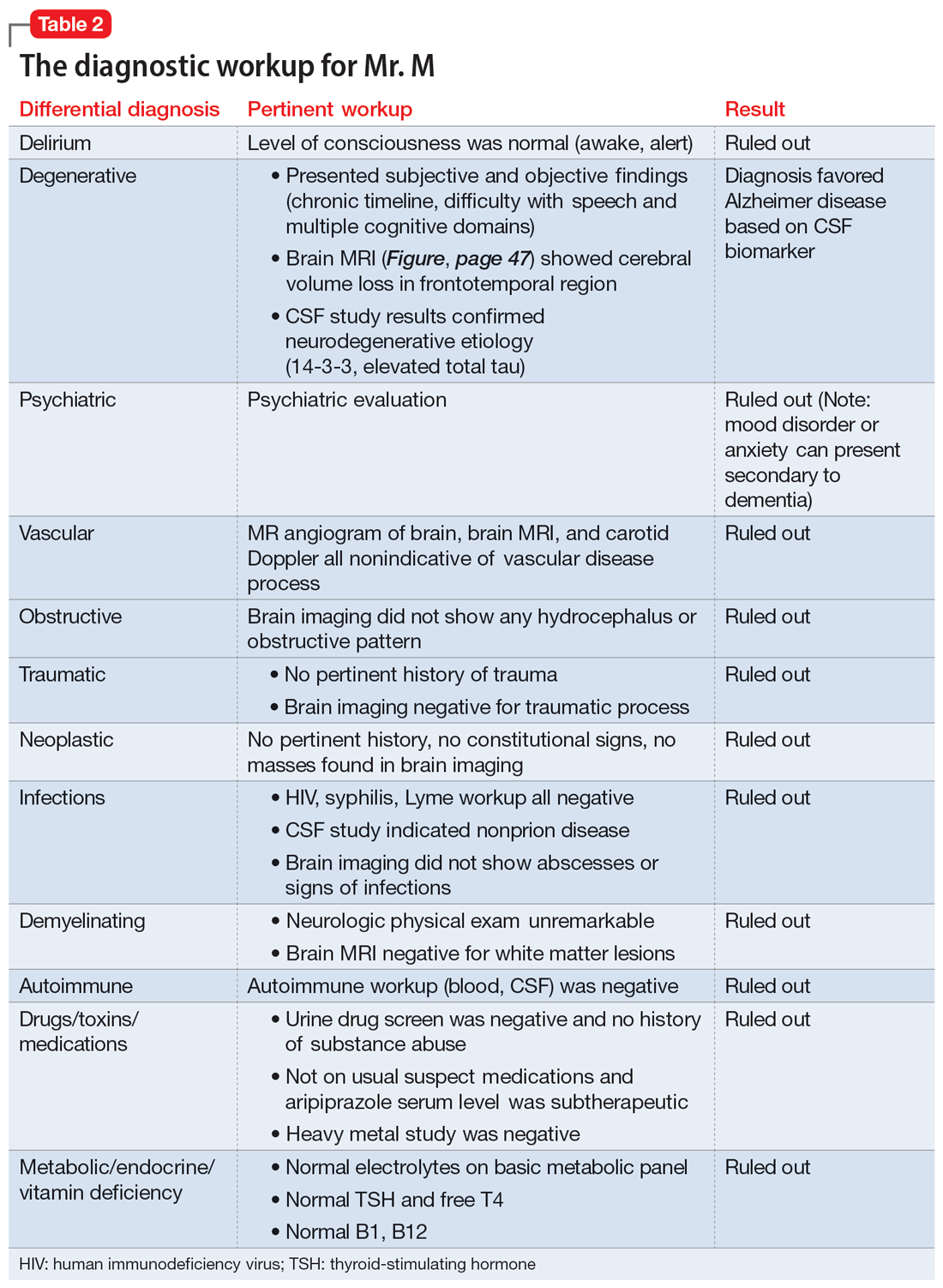
Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.
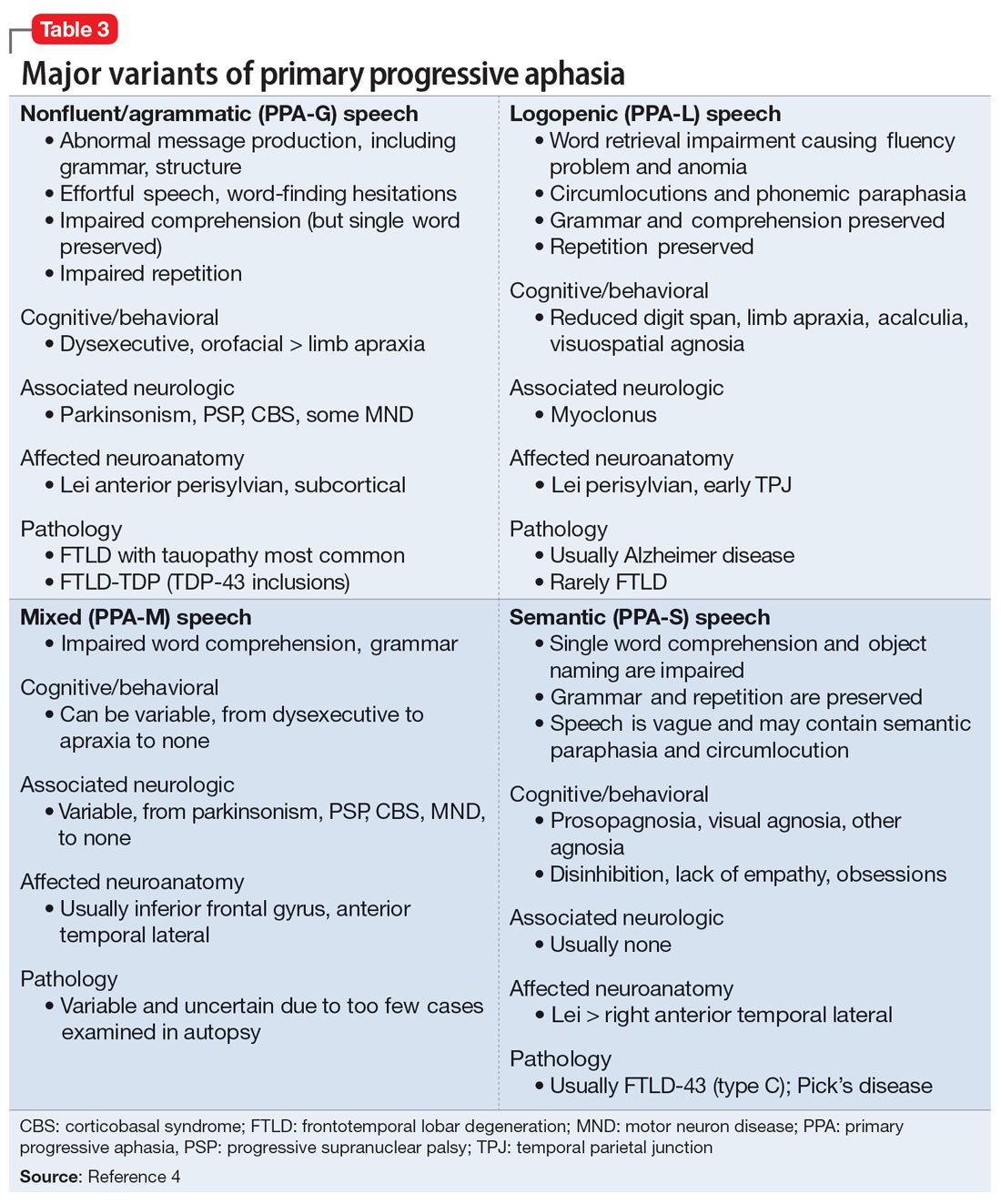
The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.
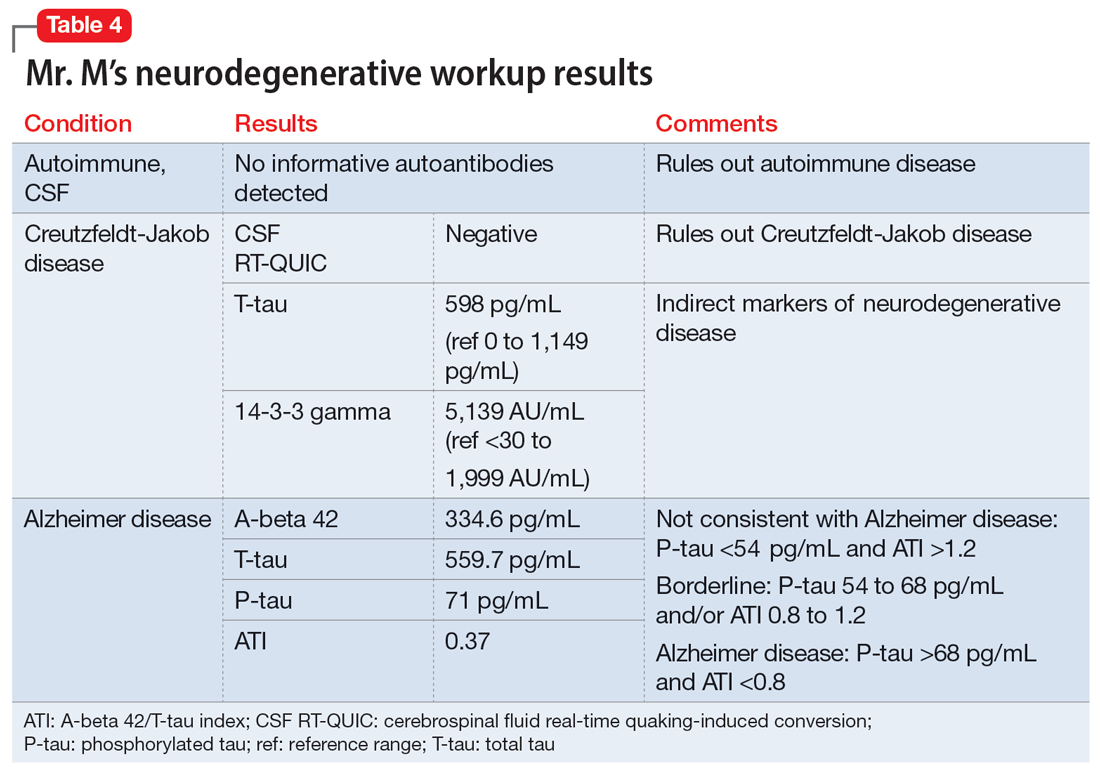
[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7
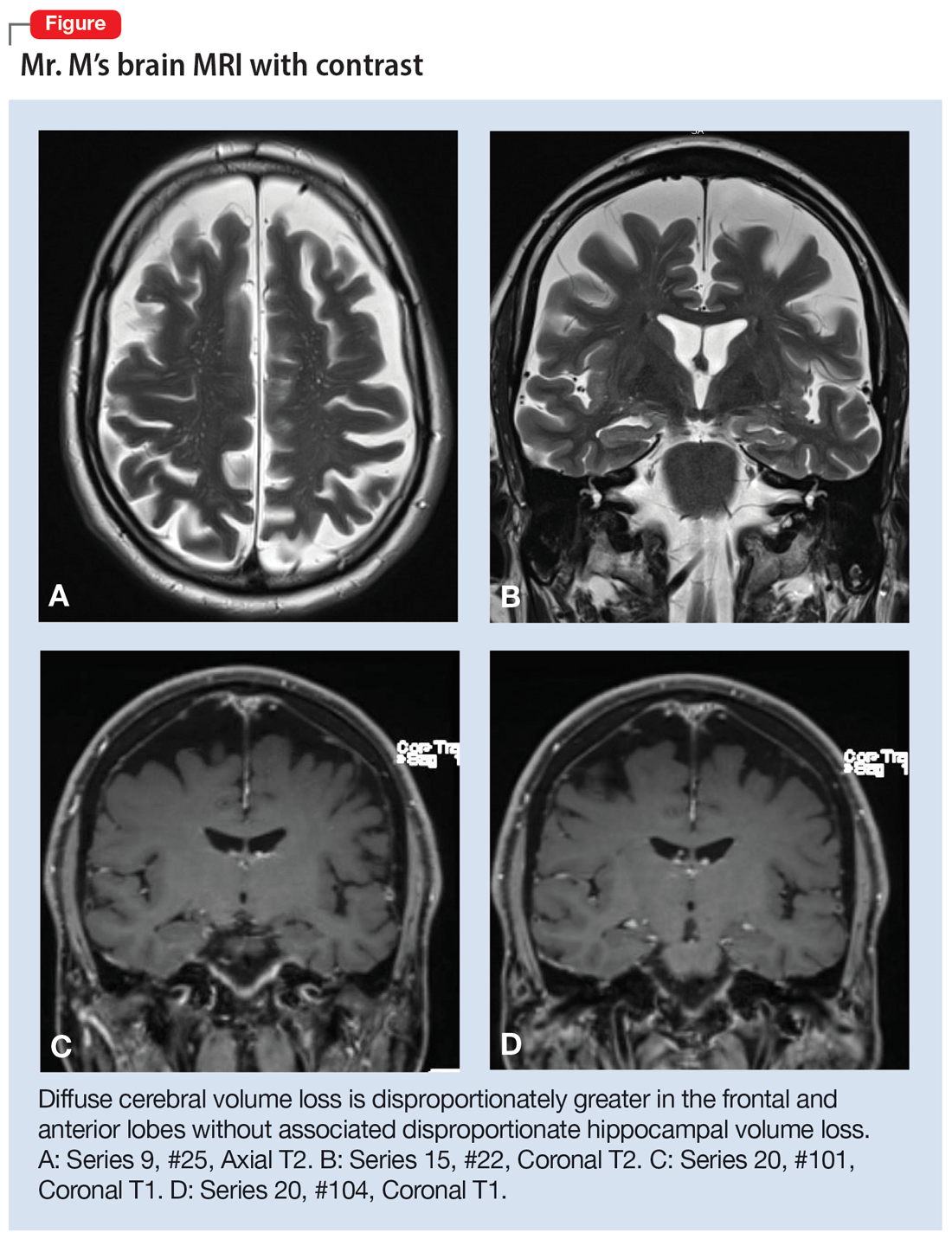
A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.

When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.

Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.

The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.

[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7

A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
CASE Anxious and confused
Mr. M, age 53, a surgeon, presents to the emergency department (ED) following a panic attack and concerns from his staff that he appears confused. Specifically, staff members report that in the past 4 months, Mr. M was observed having problems completing some postoperative tasks related to chart documentation. Mr. M has a history of major depressive disorder (MDD), hypertension, hyperlipidemia, and type 2 diabetes.
HISTORY A long-standing diagnosis of depression
Mr. M reports that 30 years ago, he received care from a psychiatrist to address symptoms of MDD. He says that around the time he arrived at the ED, he had noticed subtle but gradual changes in his cognition, which led him to skip words and often struggle to find the correct words. These episodes left him confused. Mr. M started getting anxious about these cognitive issues because they disrupted his work and forced him to reduce his duties. He does not have any known family history of mental illness, is single, and lives alone.
EVALUATION After stroke is ruled out, a psychiatric workup
In the ED, a comprehensive exam rules out an acute cerebrovascular event. A neurologic evaluation notes some delay in processing information and observes Mr. M having difficulty following simple commands. Laboratory investigations, including a comprehensive metabolic panel, are unremarkable. An MRI of Mr. M’s brain, with and without contrast, notes no acute findings. He is discharged from the ED with a diagnosis of MDD.
Before he presented to the ED, Mr. M’s medication regimen included duloxetine 60 mg/d, buspirone 10 mg 3 times a day, and aripiprazole 5 mg/d for MDD and anxiety. After the ED visit, Mr. M’s physician refers him to an outpatient psychiatrist for management of worsening depression and panic attacks. During the psychiatrist’s evaluation, Mr. M reports a decreased interest in activities, decreased motivation, being easily fatigued, and having poor sleep. He denies having a depressed mood, difficulty concentrating, or having problems with his appetite. He also denies suicidal thoughts, both past and present.
Mr. M describes his mood as anxious, primarily surrounding his recent cognitive changes. He does not have a substance use disorder, psychotic illness, mania or hypomania, posttraumatic stress disorder, or obsessive-compulsive disorder. He reports adherence to his psychiatric medications. A mental status exam reveals Mr. M to be anxious. His attention is not well sustained, and he has difficulty describing details of his cognitive struggles, providing vague descriptions such as “skipping thought” and “skipping words.” Mr. M’s affect is congruent to his mood with some restriction and the psychiatrist notes that he is experiencing thought latency, poverty of content of thoughts, word-finding difficulties, and circumlocution. Mr. M denies any perceptual abnormalities, and there is no evidence of delusions.
[polldaddy:11320112]
The authors’ observations
Mr. M’s symptoms are significant for subacute cognitive decline that is subtle but gradual and can be easily missed, especially in the beginning. Though his ED evaluation—including brain imaging—ruled out acute or focal neurologic findings and his primary psychiatric presentation was anxiety, Mr. M’s medical history and mental status exam were suggestive of cognitive deficits.
Collateral information was obtained from his work colleagues, which confirmed both cognitive problems and comorbid anxiety. Additionally, given Mr. M’s high cognitive baseline as a surgeon, the new-onset cognitive changes over 4 months warranted further cognitive and neurologic evaluation. There are many causes of cognitive impairment (vascular, cancer, infection, autoimmune, medications, substances or toxins, neurodegenerative, psychiatric, vitamin deficiencies), all of which need to be considered in a patient with a nonspecific presentation such as Mr. M’s. The psychiatrist confirmed Mr. M’s current medication regimen, and discussed tapering aripiprazole while continuing duloxetine and buspirone.
Continue to: EVALUATION A closer look at cognitive deficits
EVALUATION A closer look at cognitive deficits
Mr. M scores 12/30 on the Montreal Cognitive Assessment (MoCA), indicating moderate cognitive impairment (Table 1). The psychiatrist refers Mr. M to Neurology. During his neurologic evaluation, Mr. M continues to report feeling anxious that “something is wrong” and skips his words. The neurologist confirms Mr. M’s symptoms may have started 2 to 3 months before he presented to the ED. Mr. M reports unusual eating habits, including yogurt and cookies for breakfast, Mexican food for lunch, and more cookies for dinner. He denies having a fever, gaining or losing weight, rashes, headaches, neck stiffness, tingling or weakness or stiffness of limbs, vertigo, visual changes, photophobia, unsteady gait, bowel or bladder incontinence, or tremors.

When the neurologist repeats the MoCA, Mr. M again scores 12. The neurologist notes that Mr. M answers questions a little slowly and pauses for thoughts when unable to find an answer. Mr. M has difficulty following some simple commands, such as “touch a finger to your nose.” Other in-office neurologic physical exams (cranial nerves, involuntary movements or tremors, sensation, muscle strength, reflexes, cerebellar signs) are unremarkable except for mildly decreased vibration sense of his toes. The neurologist concludes that Mr. M’s presentation is suggestive of subacute to chronic bradyphrenia and orders additional evaluation, including neuropsychological testing.
[polldaddy:11320114]
The authors’ observations
Physical and neurologic exams were not suggestive of any obvious causes of cognitive decline. Both the mental status exam and 2 serial MoCAs suggested deficits in executive function, language, and memory. Each of the differential diagnoses considered was ruled out with workup or exams (Table 2), which led to a most likely diagnosis of neurodegenerative disorder with PPA. Neuropsychological testing confirmed the diagnosis of nonfluent PPA.

Primary progressive aphasia
PPA is an uncommon, heterogeneous group of disorders stemming from focal degeneration of language-governing centers of the brain.1,2 The estimated prevalence of PPA is 3 in 100,000 cases.2,3 There are 4 major variants of PPA (Table 34), and each presents with distinct language, cognitive, neuroanatomical, and neuropathological characteristics.4 PPA is usually diagnosed in late middle life; however, diagnosis is often delayed due to the relative obscurity of the disorder.4 In Mr. M’s case, it took approximately 4 months of evaluations by various specialists before a diagnosis was confirmed.

The initial phase of PPA can present as a diagnostic challenge because patients can have difficulty articulating their cognitive and language deficits. PPA can be commonly mistaken for a primary psychiatric disorder such as MDD or anxiety, which can further delay an accurate diagnosis and treatment. Special attention to the mental status exam, close observation of the patient’s language, and assessment of cognitive abilities using standardized screenings such as the MoCA or Mini-Mental State Examination can be helpful in clarifying the diagnosis. It is also important to rule out developmental problems (eg, dyslexia) and hearing difficulties, particularly in older patients.
Continue to: TREATMENT Adjusting the medication regimen
TREATMENT Adjusting the medication regimen
The neurologist completes additional examinations to rule out causes of rare neurodegenerative disorders, including CSF autoimmune disorders, Creutzfeldt-Jakob disease, and Alzheimer disease (AD) (Table 4). Mr. M continues to follow up with his outpatient psychiatrist and his medication regimen is adjusted. Aripiprazole and buspirone are discontinued, and duloxetine is titrated to 60 mg twice a day. During follow-up visits, Mr. M discusses his understanding of his neurologic condition. His concerns shift to his illness and prognosis. During these visits, he continues to deny suicidality.

[polldaddy:11320115]
The authors’ observations
Mr. M’s neurodegenerative workup identified an intriguing diagnostic challenge. A repeat brain MRI (Figure) showed atrophy patterns suggestive of frontotemporal lobar degeneration (FTLD). On the other hand, his CSF ATI (A-beta 42/T-tau index, a value used to aid in the diagnosis of AD) was <1, suggesting early-onset AD.5,6 Although significant advances have been made to distinguish AD and FTLD following an autopsy, there are still no reliable or definitive biomarkers to distinguish AD from FTLD (particularly in the early stages of FTLD). This can often leave the confirmatory diagnosis as a question.7

A PPA diagnosis (and other dementias) can have a significant impact on the patient and their family due to the uncertain nature of the progression of the disease and quality-of-life issues related to language and other cognitive deficits. Early identification and accurate diagnosis of PPA and its etiology (ie, AD vs FTLD) is important to avoid unnecessary exposure to medications or the use of polypharmacy to treat an inaccurate diagnosis of a primary psychiatric illness. For example, Mr. M was being treated with 3 psychiatric medications (aripiprazole, buspirone, and duloxetine) for depression and anxiety prior to the diagnosis of PPA.
Nonpharmacologic interventions can play an important role in the management of patients with PPA. These include educating the patient and their family about the diagnosis and discussions about future planning, including appropriate social support, employment, and finances.4 Pharmacologic interventions may be limited, as there are currently no disease-modifying treatments for PPA or FTLD. For patients with nonfluent PPA or AD, cholinesterase inhibitors such as donepezil or N-methyl
Psychiatrists should continue to treat patients with PPA for comorbid anxiety or depression, with appropriate medications and/or supportive therapy to guide the patient through the process of grief. Assessing for suicide risk is also important in patients diagnosed with dementia. A retrospective cohort study of patients age ≥60 with a diagnosis of dementia suggested that the majority of suicides occurred in those with a new dementia diagnosis.9 End-of-life decisions such as advanced directives should be made when the patient still has legal capacity, ideally as soon as possible after diagnosis.10
OUTCOME Remaining engaged in treatment
Mr. M continues to follow-up with the Neurology team. He has also been regularly seeing his psychiatric team for medication management and supportive therapy, and his psychiatric medications have been optimized to reduce polypharmacy. During his sessions, Mr. M discusses his grief and plans for the future. Despite his anxiety about the uncertainty of his prognosis, Mr. M continues to report that he is doing reasonably well and remains engaged in treatment.
Bottom Line
Patients with primary progressive aphasia and rare neurodegenerative disorders may present to an outpatient or emergency setting with symptoms of anxiety and confusion. They are frequently misdiagnosed with a primary psychiatric disorder due to the nature of cognitive and language deficits, particularly in the early stages of the disease. Paying close attention to language and conducting cognitive screening are critical in identifying the true cause of a patient’s symptoms.
Related Resources
- Primary progressive aphasia. National Center for Advancing Translational Sciences. Genetic and Rare Diseases Information Center. https://rarediseases.info.nih.gov/diseases/8541/primary-progressive-aphasia
- Moller MD, Parmenter BA, Lane DW. Neuropsychological testing: A useful but underutilized resource. Current Psychiatry. 2019;18(11):40-46,51.
Drug Brand Names
Aripiprazole • Abilify
Donepezil • Aricept
Duloxetine • Cymbalta
Memantine • Namenda
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
1. Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat Rev Neurol. 2010;6(2):88-97. doi:10.1038/nrneurol.2009.216
2. Mesulam M-M, Rogalski EJ, Wieneke C, et al. Primary progressive aphasia and the evolving neurology of the language network. Nat Rev Neurol. 2014;10(10):554-569. doi:10.1038/nrneurol.2014.159
3. Coyle-Gilchrist ITS, Dick KM, Patterson K, et al. Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology. 2016;86(18):1736-1743. doi:10.1212/WNL.0000000000002638
4. Marshall CR, Hardy CJD, Volkmer A, et al. Primary progressive aphasia: a clinical approach. J Neurol. 2018;265(6):1474-1490. doi:10.1007/s00415-018-8762-6
5. Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1(2):213-225. doi:10.1602/neurorx.1.2.213
6. Hulstaert F, Blennow K, Ivanoiu A, et al. Improved discrimination of AD patients using beta-amyloid(1-42) and tau levels in CSF. Neurology. 1999;52(8):1555-1562. doi:10.1212/wnl.52.8.1555
7. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387-397. doi:10.1038/s41591-020-0762-2
8. Newhart M, Davis C, Kannan V, et al. Therapy for naming deficits in two variants of primary progressive aphasia. Aphasiology. 2009;23(7-8):823-834. doi:10.1080/02687030802661762
9. Seyfried LS, Kales HC, Ignacio RV, et al. Predictors of suicide in patients with dementia. Alzheimers Dement. 2011;7(6):567-573. doi:10.1016/j.jalz.2011.01.006
10. Porteri C. Advance directives as a tool to respect patients’ values and preferences: discussion on the case of Alzheimer’s disease. BMC Med Ethics. 2018;19(1):9. doi:10.1186/s12910-018-0249-6
Suicidality in an older patient with chronic kidney disease
CASE Depressed, anxious, and suicidal
Mr. J, age 72, is brought to the emergency department by law enforcement at his wife’s request due to worsening suicidal thoughts and anxiety. He has a history of major depressive disorder (MDD) and chronic kidney disease (CKD). Mr. J has been compliant with his medications, but they seem to no longer be effective. He is admitted to the geriatric psychiatry unit.
HISTORY Increased debilitation
Over the past several years, Mr. J has experienced increasing debilitation at home, including difficulty walking and an inability to perform activities of daily life. Recently, he has begun to ask for multiple pills in an attempt to take his own life.
Mr. J has been previously treated in a psychiatric clinic with duloxetine 60 mg/d, mirtazapine 30 mg/d at bedtime, buspirone 15 mg 3 times a day, and trazodone 50 mg/d at bedtime. He is also taking amlodipine 5 mg twice daily for hypertension, lisinopril 2.5 mg/d for hypertension, furosemide 20 mg/d orally for CKD, and potassium chloride 10 mEq/d for hypokalemia secondary to CKD and furosemide use. Over the past year, his psychiatric medications have been steadily increased to target his MDD and anxiety.
EVALUATION Disorientation and Stage 3A CKD
In the psychiatric unit, Mr. J describes panic, feelings of impending doom, and profound anxiety. He states he has increasing anxiety related to “being a burden” on his family and wife. Additionally, he describes decreased appetite, difficulty sleeping, low energy, difficulty concentrating, no interest in outside activities, and feelings of hopelessness.
Mr. J’s temperature is 39.2o C; heart rate is 109 beats per minute; respiratory rate is 18 breaths per minute; blood pressure is 157/83 mm Hg; and pulse oximetry is 97%. Laboratory screening indicates a red blood cell count of 3.57, hemoglobin 11.2, hematocrit 33.8, red blood cell distribution width 17.5, blood urea nitrogen 45, creatinine 1.5 with no known baseline, and an estimated glomerular filtration rate (GFR) of 46 mL/min, indicating Stage 3A CKD (Table 11). Additional testing rules out other potential causes of delirium and psychosis.
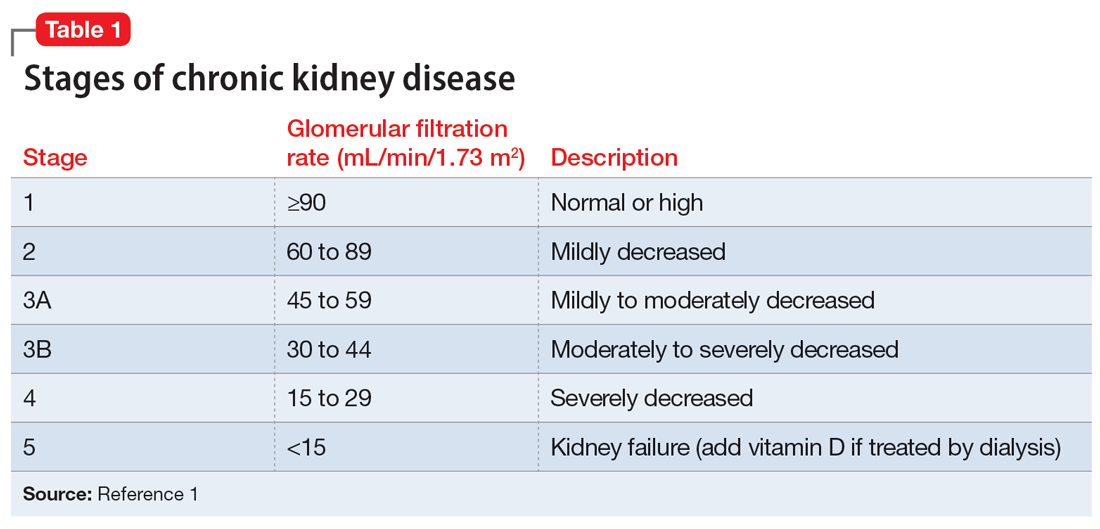
A physical exam reveals Mr. J has a fine tremor, myoclonus, muscle rigidity, and hyperreflexia. He is oriented to name, but not to date, place, or situation, and is easily confused. Mr. J uses a walker but has significant tremors while walking and immediately asks for assistance due to profound anxiety related to a fear of falling. Mr. J’s mood and affect are labile with tearful and anxious episodes. His anxiety focuses on overvalued thoughts of minor or irrelevant concerns. Additionally, he has poor insight and judgment. When asked about the cause of his anxiety, Mr. J says, “I don’t know why I’m anxious; I’m just a worrywart.” His memory is impaired, and he does not know why he is in the hospital. Mr. J scores 24 on the Montreal Cognitive Assessment, which indicates mild impairment.
Mr. J continues to endorse suicidal ideation but denies homicidal thoughts. Based on these symptoms, the differential diagnosis includes serotonin syndrome, MDD with suicidal ideation, generalized anxiety disorder, and panic disorder.
Continue to: The authors' observations
[polldaddy:11273789]
The authors’ observations
GFR is used to determine the level of renal impairment. Mr. J’s GFR of 46 mL/min indicates Stage 3A CKD (Table 11 ). Additionally, he displayed anemia and increased creatinine due to CKD. Twenty percent of patients with CKD also experience MDD.2 In a prospective observational cohort study, Hedayati et al3 found that Stage 2 to Stage 5 CKD with MDD leads to an increased risk of death, hospitalization, or progression to dialysis. It is important to properly manage Mr. J’s MDD and CKD to prevent future comorbidities. Renal impairment is common in people age >65.4 Even when GFR is normal, it is recommended to decrease dosing of medications in older adults due to age-related decreased renal excretion. As kidneys decrease in function, their ability to excrete normal amounts of medications also decreases, leading to increased serum levels and potential toxicity.
A combination of 4 serotonergic psychotropic medications may not be unusual to address treatment-resistant depression in a healthy, nongeriatric adult. However, Mr. J displayed signs of serotonin toxicity, such as hyperthermia, tachycardia, increased blood pressure, increased tremors, myoclonus, hyperreflexia, and muscle rigidity. These are classic signs of serotonin toxicity. For Mr. J, serotonin toxicity can be treated with the removal of serotonergic medications and lorazepam for symptom relief. If symptoms persist, cyproheptadine, a serotonin antagonist, can be used. Mr. J’s psychotropic medications were increased in an outpatient setting and he was unable to renally excrete higher doses of these serotonergic agents, which lead to chronic serotonin toxicity.
It is important to rule out other causes of psychosis or delirium in geriatric patients. A study by Marcantonio et al5 found that >40% of patients referred to a consulting psychiatrist for depression ultimately had delirium, and this was more likely in geriatric patients.
TREATMENT Adjustments to the medication regimen
The treatment team decides to taper and discontinue duloxetine, buspirone, and trazodone and reduce mirtazapine to 15 mg/d at bedtime. Additionally, oral lorazepam 1 mg as needed is prescribed to alleviate agitation and correct vital signs. Mr. J’s vital signs improve, with decreased temperature and normal cardiac and respiratory rhythms.
Mr. J’s Stage 3A CKD is treated with oral fluids, and his hypertension is managed with an increase of lisinopril from 2.5 mg/d to 10 mg/d. After 10 days on the psychiatric unit, he shows improvement, decreased anxiety, and remission of suicidal ideation.
Continue to: The authors' observations
[polldaddy:11273790]
The authors’ observations
In 2019, the American Geriatric Society (AGS) updated the Beers Criteria for potentially inappropriate medication use in older adults.4 The Beers Criteria were created to educate clinicians about the use of potentially inappropriate medications that have an unfavorable balance of benefits and risks compared to alternative treatments. The AGS lists medications that should be avoided or have their dosage reduced with varying levels of kidney function in older adults. Duloxetine is one of the medications listed with the recommendation to avoid for patients with a creatinine clearance <30 mL/min. Creatinine clearance is an estimation of GFR.
Although duloxetine is mentioned in the Beers Criteria, many other antidepressants have metabolites excreted by the kidneys.6 Potential adverse effects include increased bleeding, nausea, vomiting, and serotonin toxicity symptoms.7 Mr. J has Stage 3A CKD and takes 4 psychotropics, which will additively increase the serum concentration of serotonergic medications. In terms of treatment for serotonin toxicity, it is important to remove the causative medications. After discontinuing serotonergic medications, lorazepam can be administered as needed. If a patient continues to have symptoms, cyproheptadine is an option.
For patients with impaired renal function, adding nonpharmacologic options should be considered, such as cognitive-behavioral therapy, electroconvulsive therapy, and transcranial magnetic stimulation. Table 24,8-18 lists the minimum effective doses for well-known medications for treating MDD.
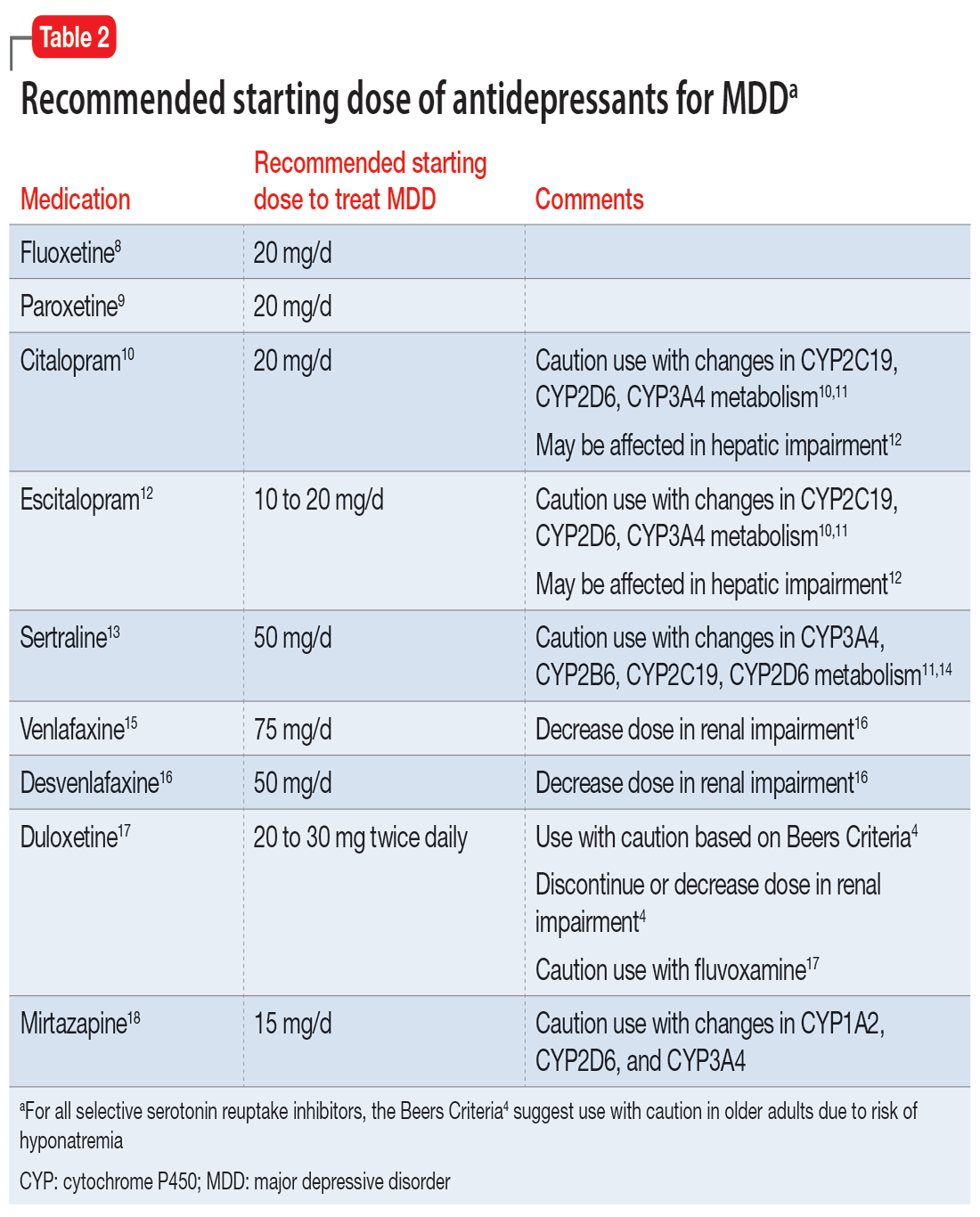
OUTCOME Improvement and discharge
Mr. J’s confusion improves, his heart rate decreases, and his feelings of panic and doom improve. He continues to have depressive symptoms, but his suicidal ideation stops. At discharge, Mr. J is receiving mirtazapine 15 mg/d, potassium chloride 10 mEq/d orally, lisinopril 20 mg/d orally at bedtime, furosemide 20 mg/d orally, and amlodipine 5 mg orally twice a day. Additionally, the treatment team recommends psychotherapy to Mr. J to address his anxiety and depression.
Bottom Line
Older patients are more sensitive to psychotropic medications, regardless of any comorbidities. It is important to review each patient’s glomerular filtration rate to better understand their renal function and adjust medications accordingly.
Related Resources
- Whittaker P, Vordenberg SE, Coe AB. Deprescribing in older adults: an overview. Current Psychiatry. 2022;21(5):40-43. doi:10.12788/cp.0246
- Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
- Barr R, Miskle B, Thomas C. Management of major depressive disorder with psychotic features. Current Psychiatry. 2021;20(2):30-33. doi:10.12788/cp.0092
Drug Brand Names
Amlodipine • Norvasc
Buspirone • BuSpar
Citalopram • Celexa
Cyproheptadine • Periactin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Furosemide • Lasix
Lisinopril • Zestril
Lorazepam • Ativan
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
Trazodone • Desyrel
Venlafaxine • Effexor
1. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-S266.
2. Shirazian S, Grant CD, Aina O, et al. Depression in chronic kidney disease and end-stage renal disease: similarities and differences in diagnosis, epidemiology, and management. Kidney Int Rep. 2017;2(1):94-107.
3. Hedayati SS, Minhajuddin AT, Afshar M, et al. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303(19):1946-1953.
4. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
5. Marcantonio E, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50(5):850-857.
6. Cukor D, Cohen, SD, Peterson RA, et al. Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol. 2007;18(12):3042-3055.
7. Cohen SD, Norris L, Acquaviva K, et al. Screening, diagnosis, and treatment of depression in patients with end-stage renal disease. Clin J Am Soc Nephrol. 2007;2(6):1332-1342.
8. Sommi RW, Crismon ML, Bowden CL. Fluoxetine: a serotonin-specific, second-generation antidepressant. Pharmacotherapy. 1987;7(1):1-15.
9. Jenner PN. Paroxetine: an overview of dosage, tolerability, and safety. Int Clin Psychopharmacol. 1992;6(Suppl 4):69-80.
10. Montgomery SA. Selecting the optimum therapeutic dose of serotonin reuptake inhibitors: studies with citalopram. Int Clin Psychopharmacol. 1995;10(Suppl 1):23-27.
11. Milosavljevic F, Bukvic N, Pavlovic Z, et al. Association of CYP2C19 and CYP2D6 poor and intermediate metabolizer status with antidepressant and antipsychotic exposure: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(3):270-280.
12. Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46(4):281-290.
13. Preskorn SH, Lane RM. Sertraline 50 mg daily: the optimal dose in the treatment of depression. Int Clin Psychopharmacol. 1995;10(3):129-141.
14. Huddart R, Hicks JK, Ramsey LB, et al. PharmGKB summary: sertraline pathway, pharmacokinetics. Pharmacogenet Genomics. 2020;30(2):26-33.
15. Furukawa TA, Cipriani A, Cowen PJ, et al. Optimal dose of selective serotonin reuptake inhibitors, venlafaxine, and mirtazapine in major depression: a systematic review and dose-response meta-analysis. Lancet Psychiatry. 2019;6(7):601-609.
16. Norman TR, Olver JS. Desvenlafaxine in the treatment of major depression: an updated overview. Expert Opin Pharmacother. 2021;22(9):1087-1097.
17. Knadler MP, Lobo E, Chappell J, et al. Duloxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2011;50(5):281-294.
18. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264.
CASE Depressed, anxious, and suicidal
Mr. J, age 72, is brought to the emergency department by law enforcement at his wife’s request due to worsening suicidal thoughts and anxiety. He has a history of major depressive disorder (MDD) and chronic kidney disease (CKD). Mr. J has been compliant with his medications, but they seem to no longer be effective. He is admitted to the geriatric psychiatry unit.
HISTORY Increased debilitation
Over the past several years, Mr. J has experienced increasing debilitation at home, including difficulty walking and an inability to perform activities of daily life. Recently, he has begun to ask for multiple pills in an attempt to take his own life.
Mr. J has been previously treated in a psychiatric clinic with duloxetine 60 mg/d, mirtazapine 30 mg/d at bedtime, buspirone 15 mg 3 times a day, and trazodone 50 mg/d at bedtime. He is also taking amlodipine 5 mg twice daily for hypertension, lisinopril 2.5 mg/d for hypertension, furosemide 20 mg/d orally for CKD, and potassium chloride 10 mEq/d for hypokalemia secondary to CKD and furosemide use. Over the past year, his psychiatric medications have been steadily increased to target his MDD and anxiety.
EVALUATION Disorientation and Stage 3A CKD
In the psychiatric unit, Mr. J describes panic, feelings of impending doom, and profound anxiety. He states he has increasing anxiety related to “being a burden” on his family and wife. Additionally, he describes decreased appetite, difficulty sleeping, low energy, difficulty concentrating, no interest in outside activities, and feelings of hopelessness.
Mr. J’s temperature is 39.2o C; heart rate is 109 beats per minute; respiratory rate is 18 breaths per minute; blood pressure is 157/83 mm Hg; and pulse oximetry is 97%. Laboratory screening indicates a red blood cell count of 3.57, hemoglobin 11.2, hematocrit 33.8, red blood cell distribution width 17.5, blood urea nitrogen 45, creatinine 1.5 with no known baseline, and an estimated glomerular filtration rate (GFR) of 46 mL/min, indicating Stage 3A CKD (Table 11). Additional testing rules out other potential causes of delirium and psychosis.

A physical exam reveals Mr. J has a fine tremor, myoclonus, muscle rigidity, and hyperreflexia. He is oriented to name, but not to date, place, or situation, and is easily confused. Mr. J uses a walker but has significant tremors while walking and immediately asks for assistance due to profound anxiety related to a fear of falling. Mr. J’s mood and affect are labile with tearful and anxious episodes. His anxiety focuses on overvalued thoughts of minor or irrelevant concerns. Additionally, he has poor insight and judgment. When asked about the cause of his anxiety, Mr. J says, “I don’t know why I’m anxious; I’m just a worrywart.” His memory is impaired, and he does not know why he is in the hospital. Mr. J scores 24 on the Montreal Cognitive Assessment, which indicates mild impairment.
Mr. J continues to endorse suicidal ideation but denies homicidal thoughts. Based on these symptoms, the differential diagnosis includes serotonin syndrome, MDD with suicidal ideation, generalized anxiety disorder, and panic disorder.
Continue to: The authors' observations
[polldaddy:11273789]
The authors’ observations
GFR is used to determine the level of renal impairment. Mr. J’s GFR of 46 mL/min indicates Stage 3A CKD (Table 11 ). Additionally, he displayed anemia and increased creatinine due to CKD. Twenty percent of patients with CKD also experience MDD.2 In a prospective observational cohort study, Hedayati et al3 found that Stage 2 to Stage 5 CKD with MDD leads to an increased risk of death, hospitalization, or progression to dialysis. It is important to properly manage Mr. J’s MDD and CKD to prevent future comorbidities. Renal impairment is common in people age >65.4 Even when GFR is normal, it is recommended to decrease dosing of medications in older adults due to age-related decreased renal excretion. As kidneys decrease in function, their ability to excrete normal amounts of medications also decreases, leading to increased serum levels and potential toxicity.
A combination of 4 serotonergic psychotropic medications may not be unusual to address treatment-resistant depression in a healthy, nongeriatric adult. However, Mr. J displayed signs of serotonin toxicity, such as hyperthermia, tachycardia, increased blood pressure, increased tremors, myoclonus, hyperreflexia, and muscle rigidity. These are classic signs of serotonin toxicity. For Mr. J, serotonin toxicity can be treated with the removal of serotonergic medications and lorazepam for symptom relief. If symptoms persist, cyproheptadine, a serotonin antagonist, can be used. Mr. J’s psychotropic medications were increased in an outpatient setting and he was unable to renally excrete higher doses of these serotonergic agents, which lead to chronic serotonin toxicity.
It is important to rule out other causes of psychosis or delirium in geriatric patients. A study by Marcantonio et al5 found that >40% of patients referred to a consulting psychiatrist for depression ultimately had delirium, and this was more likely in geriatric patients.
TREATMENT Adjustments to the medication regimen
The treatment team decides to taper and discontinue duloxetine, buspirone, and trazodone and reduce mirtazapine to 15 mg/d at bedtime. Additionally, oral lorazepam 1 mg as needed is prescribed to alleviate agitation and correct vital signs. Mr. J’s vital signs improve, with decreased temperature and normal cardiac and respiratory rhythms.
Mr. J’s Stage 3A CKD is treated with oral fluids, and his hypertension is managed with an increase of lisinopril from 2.5 mg/d to 10 mg/d. After 10 days on the psychiatric unit, he shows improvement, decreased anxiety, and remission of suicidal ideation.
Continue to: The authors' observations
[polldaddy:11273790]
The authors’ observations
In 2019, the American Geriatric Society (AGS) updated the Beers Criteria for potentially inappropriate medication use in older adults.4 The Beers Criteria were created to educate clinicians about the use of potentially inappropriate medications that have an unfavorable balance of benefits and risks compared to alternative treatments. The AGS lists medications that should be avoided or have their dosage reduced with varying levels of kidney function in older adults. Duloxetine is one of the medications listed with the recommendation to avoid for patients with a creatinine clearance <30 mL/min. Creatinine clearance is an estimation of GFR.
Although duloxetine is mentioned in the Beers Criteria, many other antidepressants have metabolites excreted by the kidneys.6 Potential adverse effects include increased bleeding, nausea, vomiting, and serotonin toxicity symptoms.7 Mr. J has Stage 3A CKD and takes 4 psychotropics, which will additively increase the serum concentration of serotonergic medications. In terms of treatment for serotonin toxicity, it is important to remove the causative medications. After discontinuing serotonergic medications, lorazepam can be administered as needed. If a patient continues to have symptoms, cyproheptadine is an option.
For patients with impaired renal function, adding nonpharmacologic options should be considered, such as cognitive-behavioral therapy, electroconvulsive therapy, and transcranial magnetic stimulation. Table 24,8-18 lists the minimum effective doses for well-known medications for treating MDD.

OUTCOME Improvement and discharge
Mr. J’s confusion improves, his heart rate decreases, and his feelings of panic and doom improve. He continues to have depressive symptoms, but his suicidal ideation stops. At discharge, Mr. J is receiving mirtazapine 15 mg/d, potassium chloride 10 mEq/d orally, lisinopril 20 mg/d orally at bedtime, furosemide 20 mg/d orally, and amlodipine 5 mg orally twice a day. Additionally, the treatment team recommends psychotherapy to Mr. J to address his anxiety and depression.
Bottom Line
Older patients are more sensitive to psychotropic medications, regardless of any comorbidities. It is important to review each patient’s glomerular filtration rate to better understand their renal function and adjust medications accordingly.
Related Resources
- Whittaker P, Vordenberg SE, Coe AB. Deprescribing in older adults: an overview. Current Psychiatry. 2022;21(5):40-43. doi:10.12788/cp.0246
- Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
- Barr R, Miskle B, Thomas C. Management of major depressive disorder with psychotic features. Current Psychiatry. 2021;20(2):30-33. doi:10.12788/cp.0092
Drug Brand Names
Amlodipine • Norvasc
Buspirone • BuSpar
Citalopram • Celexa
Cyproheptadine • Periactin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Furosemide • Lasix
Lisinopril • Zestril
Lorazepam • Ativan
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
Trazodone • Desyrel
Venlafaxine • Effexor
CASE Depressed, anxious, and suicidal
Mr. J, age 72, is brought to the emergency department by law enforcement at his wife’s request due to worsening suicidal thoughts and anxiety. He has a history of major depressive disorder (MDD) and chronic kidney disease (CKD). Mr. J has been compliant with his medications, but they seem to no longer be effective. He is admitted to the geriatric psychiatry unit.
HISTORY Increased debilitation
Over the past several years, Mr. J has experienced increasing debilitation at home, including difficulty walking and an inability to perform activities of daily life. Recently, he has begun to ask for multiple pills in an attempt to take his own life.
Mr. J has been previously treated in a psychiatric clinic with duloxetine 60 mg/d, mirtazapine 30 mg/d at bedtime, buspirone 15 mg 3 times a day, and trazodone 50 mg/d at bedtime. He is also taking amlodipine 5 mg twice daily for hypertension, lisinopril 2.5 mg/d for hypertension, furosemide 20 mg/d orally for CKD, and potassium chloride 10 mEq/d for hypokalemia secondary to CKD and furosemide use. Over the past year, his psychiatric medications have been steadily increased to target his MDD and anxiety.
EVALUATION Disorientation and Stage 3A CKD
In the psychiatric unit, Mr. J describes panic, feelings of impending doom, and profound anxiety. He states he has increasing anxiety related to “being a burden” on his family and wife. Additionally, he describes decreased appetite, difficulty sleeping, low energy, difficulty concentrating, no interest in outside activities, and feelings of hopelessness.
Mr. J’s temperature is 39.2o C; heart rate is 109 beats per minute; respiratory rate is 18 breaths per minute; blood pressure is 157/83 mm Hg; and pulse oximetry is 97%. Laboratory screening indicates a red blood cell count of 3.57, hemoglobin 11.2, hematocrit 33.8, red blood cell distribution width 17.5, blood urea nitrogen 45, creatinine 1.5 with no known baseline, and an estimated glomerular filtration rate (GFR) of 46 mL/min, indicating Stage 3A CKD (Table 11). Additional testing rules out other potential causes of delirium and psychosis.

A physical exam reveals Mr. J has a fine tremor, myoclonus, muscle rigidity, and hyperreflexia. He is oriented to name, but not to date, place, or situation, and is easily confused. Mr. J uses a walker but has significant tremors while walking and immediately asks for assistance due to profound anxiety related to a fear of falling. Mr. J’s mood and affect are labile with tearful and anxious episodes. His anxiety focuses on overvalued thoughts of minor or irrelevant concerns. Additionally, he has poor insight and judgment. When asked about the cause of his anxiety, Mr. J says, “I don’t know why I’m anxious; I’m just a worrywart.” His memory is impaired, and he does not know why he is in the hospital. Mr. J scores 24 on the Montreal Cognitive Assessment, which indicates mild impairment.
Mr. J continues to endorse suicidal ideation but denies homicidal thoughts. Based on these symptoms, the differential diagnosis includes serotonin syndrome, MDD with suicidal ideation, generalized anxiety disorder, and panic disorder.
Continue to: The authors' observations
[polldaddy:11273789]
The authors’ observations
GFR is used to determine the level of renal impairment. Mr. J’s GFR of 46 mL/min indicates Stage 3A CKD (Table 11 ). Additionally, he displayed anemia and increased creatinine due to CKD. Twenty percent of patients with CKD also experience MDD.2 In a prospective observational cohort study, Hedayati et al3 found that Stage 2 to Stage 5 CKD with MDD leads to an increased risk of death, hospitalization, or progression to dialysis. It is important to properly manage Mr. J’s MDD and CKD to prevent future comorbidities. Renal impairment is common in people age >65.4 Even when GFR is normal, it is recommended to decrease dosing of medications in older adults due to age-related decreased renal excretion. As kidneys decrease in function, their ability to excrete normal amounts of medications also decreases, leading to increased serum levels and potential toxicity.
A combination of 4 serotonergic psychotropic medications may not be unusual to address treatment-resistant depression in a healthy, nongeriatric adult. However, Mr. J displayed signs of serotonin toxicity, such as hyperthermia, tachycardia, increased blood pressure, increased tremors, myoclonus, hyperreflexia, and muscle rigidity. These are classic signs of serotonin toxicity. For Mr. J, serotonin toxicity can be treated with the removal of serotonergic medications and lorazepam for symptom relief. If symptoms persist, cyproheptadine, a serotonin antagonist, can be used. Mr. J’s psychotropic medications were increased in an outpatient setting and he was unable to renally excrete higher doses of these serotonergic agents, which lead to chronic serotonin toxicity.
It is important to rule out other causes of psychosis or delirium in geriatric patients. A study by Marcantonio et al5 found that >40% of patients referred to a consulting psychiatrist for depression ultimately had delirium, and this was more likely in geriatric patients.
TREATMENT Adjustments to the medication regimen
The treatment team decides to taper and discontinue duloxetine, buspirone, and trazodone and reduce mirtazapine to 15 mg/d at bedtime. Additionally, oral lorazepam 1 mg as needed is prescribed to alleviate agitation and correct vital signs. Mr. J’s vital signs improve, with decreased temperature and normal cardiac and respiratory rhythms.
Mr. J’s Stage 3A CKD is treated with oral fluids, and his hypertension is managed with an increase of lisinopril from 2.5 mg/d to 10 mg/d. After 10 days on the psychiatric unit, he shows improvement, decreased anxiety, and remission of suicidal ideation.
Continue to: The authors' observations
[polldaddy:11273790]
The authors’ observations
In 2019, the American Geriatric Society (AGS) updated the Beers Criteria for potentially inappropriate medication use in older adults.4 The Beers Criteria were created to educate clinicians about the use of potentially inappropriate medications that have an unfavorable balance of benefits and risks compared to alternative treatments. The AGS lists medications that should be avoided or have their dosage reduced with varying levels of kidney function in older adults. Duloxetine is one of the medications listed with the recommendation to avoid for patients with a creatinine clearance <30 mL/min. Creatinine clearance is an estimation of GFR.
Although duloxetine is mentioned in the Beers Criteria, many other antidepressants have metabolites excreted by the kidneys.6 Potential adverse effects include increased bleeding, nausea, vomiting, and serotonin toxicity symptoms.7 Mr. J has Stage 3A CKD and takes 4 psychotropics, which will additively increase the serum concentration of serotonergic medications. In terms of treatment for serotonin toxicity, it is important to remove the causative medications. After discontinuing serotonergic medications, lorazepam can be administered as needed. If a patient continues to have symptoms, cyproheptadine is an option.
For patients with impaired renal function, adding nonpharmacologic options should be considered, such as cognitive-behavioral therapy, electroconvulsive therapy, and transcranial magnetic stimulation. Table 24,8-18 lists the minimum effective doses for well-known medications for treating MDD.

OUTCOME Improvement and discharge
Mr. J’s confusion improves, his heart rate decreases, and his feelings of panic and doom improve. He continues to have depressive symptoms, but his suicidal ideation stops. At discharge, Mr. J is receiving mirtazapine 15 mg/d, potassium chloride 10 mEq/d orally, lisinopril 20 mg/d orally at bedtime, furosemide 20 mg/d orally, and amlodipine 5 mg orally twice a day. Additionally, the treatment team recommends psychotherapy to Mr. J to address his anxiety and depression.
Bottom Line
Older patients are more sensitive to psychotropic medications, regardless of any comorbidities. It is important to review each patient’s glomerular filtration rate to better understand their renal function and adjust medications accordingly.
Related Resources
- Whittaker P, Vordenberg SE, Coe AB. Deprescribing in older adults: an overview. Current Psychiatry. 2022;21(5):40-43. doi:10.12788/cp.0246
- Gibson G, Kennedy LH, Barlow G. Polypharmacy in older adults. Current Psychiatry. 2020;19(4):40-46.
- Barr R, Miskle B, Thomas C. Management of major depressive disorder with psychotic features. Current Psychiatry. 2021;20(2):30-33. doi:10.12788/cp.0092
Drug Brand Names
Amlodipine • Norvasc
Buspirone • BuSpar
Citalopram • Celexa
Cyproheptadine • Periactin
Desvenlafaxine • Pristiq
Duloxetine • Cymbalta
Escitalopram • Lexapro
Fluoxetine • Prozac
Furosemide • Lasix
Lisinopril • Zestril
Lorazepam • Ativan
Mirtazapine • Remeron
Paroxetine • Paxil
Sertraline • Zoloft
Trazodone • Desyrel
Venlafaxine • Effexor
1. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-S266.
2. Shirazian S, Grant CD, Aina O, et al. Depression in chronic kidney disease and end-stage renal disease: similarities and differences in diagnosis, epidemiology, and management. Kidney Int Rep. 2017;2(1):94-107.
3. Hedayati SS, Minhajuddin AT, Afshar M, et al. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303(19):1946-1953.
4. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
5. Marcantonio E, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50(5):850-857.
6. Cukor D, Cohen, SD, Peterson RA, et al. Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol. 2007;18(12):3042-3055.
7. Cohen SD, Norris L, Acquaviva K, et al. Screening, diagnosis, and treatment of depression in patients with end-stage renal disease. Clin J Am Soc Nephrol. 2007;2(6):1332-1342.
8. Sommi RW, Crismon ML, Bowden CL. Fluoxetine: a serotonin-specific, second-generation antidepressant. Pharmacotherapy. 1987;7(1):1-15.
9. Jenner PN. Paroxetine: an overview of dosage, tolerability, and safety. Int Clin Psychopharmacol. 1992;6(Suppl 4):69-80.
10. Montgomery SA. Selecting the optimum therapeutic dose of serotonin reuptake inhibitors: studies with citalopram. Int Clin Psychopharmacol. 1995;10(Suppl 1):23-27.
11. Milosavljevic F, Bukvic N, Pavlovic Z, et al. Association of CYP2C19 and CYP2D6 poor and intermediate metabolizer status with antidepressant and antipsychotic exposure: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(3):270-280.
12. Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46(4):281-290.
13. Preskorn SH, Lane RM. Sertraline 50 mg daily: the optimal dose in the treatment of depression. Int Clin Psychopharmacol. 1995;10(3):129-141.
14. Huddart R, Hicks JK, Ramsey LB, et al. PharmGKB summary: sertraline pathway, pharmacokinetics. Pharmacogenet Genomics. 2020;30(2):26-33.
15. Furukawa TA, Cipriani A, Cowen PJ, et al. Optimal dose of selective serotonin reuptake inhibitors, venlafaxine, and mirtazapine in major depression: a systematic review and dose-response meta-analysis. Lancet Psychiatry. 2019;6(7):601-609.
16. Norman TR, Olver JS. Desvenlafaxine in the treatment of major depression: an updated overview. Expert Opin Pharmacother. 2021;22(9):1087-1097.
17. Knadler MP, Lobo E, Chappell J, et al. Duloxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2011;50(5):281-294.
18. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264.
1. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39(2 Suppl 1):S1-S266.
2. Shirazian S, Grant CD, Aina O, et al. Depression in chronic kidney disease and end-stage renal disease: similarities and differences in diagnosis, epidemiology, and management. Kidney Int Rep. 2017;2(1):94-107.
3. Hedayati SS, Minhajuddin AT, Afshar M, et al. Association between major depressive episodes in patients with chronic kidney disease and initiation of dialysis, hospitalization, or death. JAMA. 2010;303(19):1946-1953.
4. 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67(4):674-694.
5. Marcantonio E, Ta T, Duthie E, et al. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50(5):850-857.
6. Cukor D, Cohen, SD, Peterson RA, et al. Psychosocial aspects of chronic disease: ESRD as a paradigmatic illness. J Am Soc Nephrol. 2007;18(12):3042-3055.
7. Cohen SD, Norris L, Acquaviva K, et al. Screening, diagnosis, and treatment of depression in patients with end-stage renal disease. Clin J Am Soc Nephrol. 2007;2(6):1332-1342.
8. Sommi RW, Crismon ML, Bowden CL. Fluoxetine: a serotonin-specific, second-generation antidepressant. Pharmacotherapy. 1987;7(1):1-15.
9. Jenner PN. Paroxetine: an overview of dosage, tolerability, and safety. Int Clin Psychopharmacol. 1992;6(Suppl 4):69-80.
10. Montgomery SA. Selecting the optimum therapeutic dose of serotonin reuptake inhibitors: studies with citalopram. Int Clin Psychopharmacol. 1995;10(Suppl 1):23-27.
11. Milosavljevic F, Bukvic N, Pavlovic Z, et al. Association of CYP2C19 and CYP2D6 poor and intermediate metabolizer status with antidepressant and antipsychotic exposure: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(3):270-280.
12. Rao N. The clinical pharmacokinetics of escitalopram. Clin Pharmacokinet. 2007;46(4):281-290.
13. Preskorn SH, Lane RM. Sertraline 50 mg daily: the optimal dose in the treatment of depression. Int Clin Psychopharmacol. 1995;10(3):129-141.
14. Huddart R, Hicks JK, Ramsey LB, et al. PharmGKB summary: sertraline pathway, pharmacokinetics. Pharmacogenet Genomics. 2020;30(2):26-33.
15. Furukawa TA, Cipriani A, Cowen PJ, et al. Optimal dose of selective serotonin reuptake inhibitors, venlafaxine, and mirtazapine in major depression: a systematic review and dose-response meta-analysis. Lancet Psychiatry. 2019;6(7):601-609.
16. Norman TR, Olver JS. Desvenlafaxine in the treatment of major depression: an updated overview. Expert Opin Pharmacother. 2021;22(9):1087-1097.
17. Knadler MP, Lobo E, Chappell J, et al. Duloxetine: clinical pharmacokinetics and drug interactions. Clin Pharmacokinet. 2011;50(5):281-294.
18. Anttila SA, Leinonen EV. A review of the pharmacological and clinical profile of mirtazapine. CNS Drug Rev. 2001;7(3):249-264.