User login
Things We Do for No Reason™: Discontinuing Buprenorphine When Treating Acute Pain

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR™) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR™ series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 40-year-old woman with a history of opioid use disorder (OUD) on buprenorphine-naloxone treatment is admitted to medicine following incision and drainage of a large forearm abscess with surrounding cellulitis. The patient reports severe pain following the procedure, which is not relieved by ibuprofen. The admitting hospitalist orders a pain regimen for the patient, which includes oral and intravenous hydromorphone and discontinues the patient’s buprenorphine-naloxone so that the short-acting opioids can take effect.
BACKGROUND
Medications to treat OUD include methadone, buprenorphine, and extended-release naltrexone. Buprenorphine is a Schedule III medication under the United States Food and Drug Administration that reduces opioid cravings, subsequently decreasing drug use1 and opioid-related overdose deaths.2 It has a favorable safety profile and can be prescribed for OUD in an office-based, outpatient setting since the Drug Addiction Treatment Act of 2000 (DATA 2000). Due to extensive first-pass metabolism, buprenorphine for OUD is typically administered sublingually, either alone or in a fixed combination with naloxone.
WHY YOU MIGHT THINK YOU SHOULD HOLD BUPRENORPHINE WHEN TREATING ACUTE PAIN
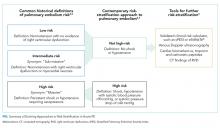
Buprenorphine is a partial opioid agonist with a long half-life and high affinity for the mu opioid receptor. Given these properties, prior recommendations assumed that buprenorphine blocked the effectiveness of additional opioid agonists.3,4 In 2004, guidelines by the Department of Health and Human Service Center for Substance Abuse Treatment recommended discontinuing buprenorphine in patients taking opioid pain medications.5 These suggestions were based on limited case reports describing difficulty controlling pain in patients with OUD with a high opioid tolerance who were receiving buprenorphine.6
Providers may hold buprenorphine when treating acute pain out of concern it could precipitate withdrawal by displacing full opioid agonists from the mu receptor. Providers may also believe that the naloxone component in the most commonly prescribed formulation, buprenorphine-naloxone, blocks the effects of opioid analgesics. Evolving understanding of buprenorphine pharmacology and the absence of high-quality evidence has resulted in providers holding buprenorphine in the setting of acute pain.
Finally, providers without dedicated training may feel they lack the necessary qualifications to prescribe buprenorphine in the inpatient setting. DATA 2000 requires mandatory X waiver training for physicians, nurse practitioners, and physician assistants to prescribe outpatient buprenorphine for OUD treatment outside of specialized opioid treatment programs.
WHY DISCONTINUING BUPRENORPHINE WHEN TREATING ACUTE PAIN IS NOT NECESSARY
Despite buprenorphine’s high affinity at the mu receptor, additional receptors remain available for full opioid agonists to bind and activate,6 providing effective pain relief even in patients using buprenorphine. In contrast to the 2004 Department of Health and Human Service guidelines, subsequent clinical studies have demonstrated that concurrent use of opioid analgesics is effective for patients maintained on buprenorphine, similar to patients on other forms of OUD treatment such as methadone.7,8
Precipitated withdrawal only occurs when buprenorphine is newly introduced to patients with already circulating opioids. Patients receiving buprenorphine-naloxone can also be exposed to opioids without precipitated withdrawal from the naloxone component, as naloxone is not absorbed via sublingual or buccal administration, but only present in the formulation to dissuade intravenous administration of the medication.
Even in the perioperative period, there is insufficient evidence to support the discontinuation of buprenorphine.9 Studies in this patient population have found that patients receiving buprenorphine may require higher doses of short-acting opioids to achieve adequate analgesia, but they experience similar pain control, lengths of stay, and functional outcomes to controls.10 Despite variable perioperative management of buprenorphine,11 protocols at major medical centers now recommend continuing or dose adjusting buprenorphine in the perioperative period rather than discontinuing.12-14
Patients physically dependent on opioid agonists, including buprenorphine, must be maintained on a daily equivalent opioid dose to avoid experiencing withdrawal. This maintenance requirement must be met before any analgesic effect for acute pain is obtained with additional opioids. Temporarily discontinuing buprenorphine introduces unnecessary complexity to a hospitalization, places the patient at risk of exacerbation of pain, opioid withdrawal, and predisposes the patient to return to use and overdose if not resumed before hospital discharge.5
Finally, clinicians do not require additional training or an X waiver to administer buprenorphine to hospitalized patients. These requirements are limited to providers managing buprenorphine in the outpatient setting or those prescribing buprenorphine to patients to take postdischarge. Hospitalists frequently prescribe opioid medications in the inpatient setting with similar or greater safety risk profiles to buprenorphine.
WHEN YOU SHOULD CONSIDER HOLDING BUPRENORPHINE
Providers may consider holding buprenorphine if a patient with OUD has not been taking buprenorphine before hospitalization and has severe acute pain needs. This history can be confirmed with the patient and the state’s online prescription drug monitoring program. If further clarification is needed, this can be accomplished with a pharmacist and urine testing or by verifying with the patient’s opioid treatment program, as some programs provide directly administered buprenorphine.
In cases where a patient may have stopped buprenorphine before admission but wants to restart it in the hospital, it is essential to ascertain when the patient last used an opioid. The buprenorphine reinduction should be timed to a sufficient number of hours since last opioid use and/or to when the patient shows signs of active withdrawal. The re-induction can take place before, during, or after an acute pain episode, depending on the individual circumstances.
Patient preference is extremely important in the management of both pain and OUD. After shared decision-making, some patients may ultimately opt to hold buprenorphine in certain situations or switch to an alternative treatment, such as methadone, during their hospitalization. Such adjustments should be made in conjunction with the patient, primary care provider, and pain or addiction medicine specialty consultation.
WHAT YOU SHOULD DO INSTEAD
For patients on buprenorphine admitted to the hospital with anticipated or unanticipated acute pain needs, hospitalists should continue buprenorphine. Continuation of buprenorphine meets a patient’s baseline opioid requirement while still allowing the use of additional short-acting opioid agonists as needed for pain.15
As with all pain, multimodal pain management should be provided with adjunctive medications such as acetaminophen, nonsteroidal anti-inflammatory drugs, neuropathic agents, topical analgesics, and regional anesthesia.8
Acute pain can be addressed by taking advantage of buprenorphine’s analgesic effects and adding additional short-acting opioids if needed.15 Several options are available, including:
1. Continuing daily buprenorphine and prescribing short-acting opioid agonists, preferably those with high intrinsic activity at the mu receptor (such as morphine, fentanyl, or hydromorphone). Full opioid agonist doses to achieve analgesia for patients on buprenorphine will be higher than in opioid naïve patients due to tolerance.16
2 .Dividing the total daily buprenorphine dose into three or four times per day dosing, since buprenorphine provides an analgesic effect lasting six to eight hours. Short-acting opioid agonists can still be prescribed on an as-needed basis for additional pain needs.
3. Temporarily increasing the total daily buprenorphine dose and dividing into three or four times per day dosing, as above. Short-acting opioid agonists can still be prescribed on an as-needed basis for additional pain needs.
It is essential to make a clear plan with the patient for initiation and discontinuation of short-acting opioid agonists or buprenorphine changes. Patients on buprenorphine should be managed collaboratively with the primary care provider or addiction specialist to coordinate prescribing and follow-up after discharge.
RECOMMENDATIONS
- Continue outpatient buprenorphine treatment for patients admitted with acute pain.
- Use adjunctive nonopioid pain medications and nonpharmacologic modalities to address acute pain.
- Adjust buprenorphine to address acute pain by dividing the total daily amount into three or four times a day dosing, and/or up-titrate the buprenorphine dose (federal prescribing regulations recommend a maximum of 24 mg daily, but state regulations may vary).
- Add short-acting opioid agonists on an as-needed basis in conjunction with a defined plan to discontinue short-acting opioid agonists to avoid a return to use.
- Make plans collaboratively with the patient and outpatient provider, and communicate medication changes and plan at discharge.
CONCLUSION
Concerning our case, the hospitalist can continue the patient’s buprenorphine-naloxone, even with her acute pain needs. The patient has a baseline opioid requirement, fulfilled by continuing buprenorphine. Additional short-acting opioid agonists, such as hydromorphone, will provide analgesia for the patient, though the clinician should be aware that higher doses might be required. The practice of holding buprenorphine during episodes of acute pain is not supported by current evidence and may predispose to inadequate analgesia, opioid withdrawal, and risk of return to use and death.2
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Disclosures
The authors report no conflicts of interest.
1. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(3):CD002207. https://doi.org/10.1002/14651858.CD002207.
2. Sordo L, Barrio G, Bravo M, et al. Morality risk during and after opioid substitution treatment: systemic review and meta-analysis of cohort studies. BMJ. 2017;357:1550. https://doi.org/10.1136/bmj.j1550.
3. Johnson RE, Fudula PJ, Payne R. Buprenorphine: considerations for pain management. J Pain Symptom Manage. 2005;29(3):297-326. https://doi.org/10.1016/j.jpainsymman.2004.07.005.
4. Marcelina JS, Rubinstein A. Continuous perioperative sublingual buprenorphine. J Pain Palliat Care Pharmacother. 2016;30(4):289-293. https://doi.org/10.1080/15360288.2016.1231734.
5. Greenwald MK, Johanson CE, Moody DE, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor binding potential, plasma concentration and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28(11):2000-2009. https://doi.org/10.1038/sj.npp.1300251.
6. Lembke A, Ottestad E, Schmiesing C. Patients maintained on buprenorphine for opioid use disorder should continue buprenorphine through the perioperative period. Pain Med. 2019;20(3):425-428. https://doi.org/10.1093/pm/pny019.
7. Kornfeld H, Manfredi L. Effectiveness of full agonist opioids in patients stabilized on buprenorphine undergoing major surgery: A case series. Am J Ther. 2010;17(5):523-528. https://doi.org/10.1097/MJT.0b013e3181be0804.
8. Harrison TK, Kornfeld H, Aggarwal AK, Lembke A. Perioperative considerations for the patient with opioid use disorder on buprenorphine, methadone, or naltrexone maintenance therapy. Anesthesiol Clin. 2018;36(3):345-359. https://doi.org/10.1016/j.anclin.2018.04.002.
9. Goel A, Azargive S, Lamba W, et al. The perioperative patient on buprenorphine: a systematic review of perioperative management strategies and patient outcomes. Can J Anesth. 2019; 66(2):201-217. https://doi.org/10.1007/s12630-018-1255-3.
10. Hansen LE, Stone GL, Matson CA, Tybor DJ, Pevear ME, Smith EL. Total joint arthroplasty in patients taking methadone or buprenorphine/naloxone preoperatively for prior heroin addiction: a prospective matched cohort study. J Arthroplasty. 2016;31(8):1698-1701. https://doi.org/10.1016/j.arth.2016.01.032.
11. Jonan AB, Kaye AD, Urman RD. Buprenorphine formulations: clinical best practice strategies recommendations for perioperative management of patients undergoing surgical or interventional pain procedures. Pain Physician. 2018;21(1):E1-12. PubMed
12. Quaye AN, Zhang Y. Perioperative management of buprenorphine: solving the conundrum. Pain Med. 2018. https://doi.org/10.1093/pm/pny217.
13. Silva MJ, Rubinstein A. Continuous perioperative sublingual buprenorphine. J Pain Palliative Care Pharmacother. 2016;30(4):289-293. https://doi.org/10.1080/15360288.2016.1231734.
14. Kampman K, Jarvis M. ASAM National practice guidelines for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367. https://doi.org/10.1097/ADM.0000000000000166.
15. Childers JW, Arnold RM. Treatment of pain in patients taking buprenorphine for opioid addiction. J Palliat Med. 2012;15(5):613-614. https://doi.org/10.1089/jpm.2012.9591.
16. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. https://doi.org/10.7326/0003-4819-144-2-200601170-00010

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR™) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR™ series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 40-year-old woman with a history of opioid use disorder (OUD) on buprenorphine-naloxone treatment is admitted to medicine following incision and drainage of a large forearm abscess with surrounding cellulitis. The patient reports severe pain following the procedure, which is not relieved by ibuprofen. The admitting hospitalist orders a pain regimen for the patient, which includes oral and intravenous hydromorphone and discontinues the patient’s buprenorphine-naloxone so that the short-acting opioids can take effect.
BACKGROUND
Medications to treat OUD include methadone, buprenorphine, and extended-release naltrexone. Buprenorphine is a Schedule III medication under the United States Food and Drug Administration that reduces opioid cravings, subsequently decreasing drug use1 and opioid-related overdose deaths.2 It has a favorable safety profile and can be prescribed for OUD in an office-based, outpatient setting since the Drug Addiction Treatment Act of 2000 (DATA 2000). Due to extensive first-pass metabolism, buprenorphine for OUD is typically administered sublingually, either alone or in a fixed combination with naloxone.
WHY YOU MIGHT THINK YOU SHOULD HOLD BUPRENORPHINE WHEN TREATING ACUTE PAIN
Buprenorphine is a partial opioid agonist with a long half-life and high affinity for the mu opioid receptor. Given these properties, prior recommendations assumed that buprenorphine blocked the effectiveness of additional opioid agonists.3,4 In 2004, guidelines by the Department of Health and Human Service Center for Substance Abuse Treatment recommended discontinuing buprenorphine in patients taking opioid pain medications.5 These suggestions were based on limited case reports describing difficulty controlling pain in patients with OUD with a high opioid tolerance who were receiving buprenorphine.6
Providers may hold buprenorphine when treating acute pain out of concern it could precipitate withdrawal by displacing full opioid agonists from the mu receptor. Providers may also believe that the naloxone component in the most commonly prescribed formulation, buprenorphine-naloxone, blocks the effects of opioid analgesics. Evolving understanding of buprenorphine pharmacology and the absence of high-quality evidence has resulted in providers holding buprenorphine in the setting of acute pain.
Finally, providers without dedicated training may feel they lack the necessary qualifications to prescribe buprenorphine in the inpatient setting. DATA 2000 requires mandatory X waiver training for physicians, nurse practitioners, and physician assistants to prescribe outpatient buprenorphine for OUD treatment outside of specialized opioid treatment programs.
WHY DISCONTINUING BUPRENORPHINE WHEN TREATING ACUTE PAIN IS NOT NECESSARY
Despite buprenorphine’s high affinity at the mu receptor, additional receptors remain available for full opioid agonists to bind and activate,6 providing effective pain relief even in patients using buprenorphine. In contrast to the 2004 Department of Health and Human Service guidelines, subsequent clinical studies have demonstrated that concurrent use of opioid analgesics is effective for patients maintained on buprenorphine, similar to patients on other forms of OUD treatment such as methadone.7,8
Precipitated withdrawal only occurs when buprenorphine is newly introduced to patients with already circulating opioids. Patients receiving buprenorphine-naloxone can also be exposed to opioids without precipitated withdrawal from the naloxone component, as naloxone is not absorbed via sublingual or buccal administration, but only present in the formulation to dissuade intravenous administration of the medication.
Even in the perioperative period, there is insufficient evidence to support the discontinuation of buprenorphine.9 Studies in this patient population have found that patients receiving buprenorphine may require higher doses of short-acting opioids to achieve adequate analgesia, but they experience similar pain control, lengths of stay, and functional outcomes to controls.10 Despite variable perioperative management of buprenorphine,11 protocols at major medical centers now recommend continuing or dose adjusting buprenorphine in the perioperative period rather than discontinuing.12-14
Patients physically dependent on opioid agonists, including buprenorphine, must be maintained on a daily equivalent opioid dose to avoid experiencing withdrawal. This maintenance requirement must be met before any analgesic effect for acute pain is obtained with additional opioids. Temporarily discontinuing buprenorphine introduces unnecessary complexity to a hospitalization, places the patient at risk of exacerbation of pain, opioid withdrawal, and predisposes the patient to return to use and overdose if not resumed before hospital discharge.5
Finally, clinicians do not require additional training or an X waiver to administer buprenorphine to hospitalized patients. These requirements are limited to providers managing buprenorphine in the outpatient setting or those prescribing buprenorphine to patients to take postdischarge. Hospitalists frequently prescribe opioid medications in the inpatient setting with similar or greater safety risk profiles to buprenorphine.
WHEN YOU SHOULD CONSIDER HOLDING BUPRENORPHINE
Providers may consider holding buprenorphine if a patient with OUD has not been taking buprenorphine before hospitalization and has severe acute pain needs. This history can be confirmed with the patient and the state’s online prescription drug monitoring program. If further clarification is needed, this can be accomplished with a pharmacist and urine testing or by verifying with the patient’s opioid treatment program, as some programs provide directly administered buprenorphine.
In cases where a patient may have stopped buprenorphine before admission but wants to restart it in the hospital, it is essential to ascertain when the patient last used an opioid. The buprenorphine reinduction should be timed to a sufficient number of hours since last opioid use and/or to when the patient shows signs of active withdrawal. The re-induction can take place before, during, or after an acute pain episode, depending on the individual circumstances.
Patient preference is extremely important in the management of both pain and OUD. After shared decision-making, some patients may ultimately opt to hold buprenorphine in certain situations or switch to an alternative treatment, such as methadone, during their hospitalization. Such adjustments should be made in conjunction with the patient, primary care provider, and pain or addiction medicine specialty consultation.
WHAT YOU SHOULD DO INSTEAD
For patients on buprenorphine admitted to the hospital with anticipated or unanticipated acute pain needs, hospitalists should continue buprenorphine. Continuation of buprenorphine meets a patient’s baseline opioid requirement while still allowing the use of additional short-acting opioid agonists as needed for pain.15
As with all pain, multimodal pain management should be provided with adjunctive medications such as acetaminophen, nonsteroidal anti-inflammatory drugs, neuropathic agents, topical analgesics, and regional anesthesia.8
Acute pain can be addressed by taking advantage of buprenorphine’s analgesic effects and adding additional short-acting opioids if needed.15 Several options are available, including:
1. Continuing daily buprenorphine and prescribing short-acting opioid agonists, preferably those with high intrinsic activity at the mu receptor (such as morphine, fentanyl, or hydromorphone). Full opioid agonist doses to achieve analgesia for patients on buprenorphine will be higher than in opioid naïve patients due to tolerance.16
2 .Dividing the total daily buprenorphine dose into three or four times per day dosing, since buprenorphine provides an analgesic effect lasting six to eight hours. Short-acting opioid agonists can still be prescribed on an as-needed basis for additional pain needs.
3. Temporarily increasing the total daily buprenorphine dose and dividing into three or four times per day dosing, as above. Short-acting opioid agonists can still be prescribed on an as-needed basis for additional pain needs.
It is essential to make a clear plan with the patient for initiation and discontinuation of short-acting opioid agonists or buprenorphine changes. Patients on buprenorphine should be managed collaboratively with the primary care provider or addiction specialist to coordinate prescribing and follow-up after discharge.
RECOMMENDATIONS
- Continue outpatient buprenorphine treatment for patients admitted with acute pain.
- Use adjunctive nonopioid pain medications and nonpharmacologic modalities to address acute pain.
- Adjust buprenorphine to address acute pain by dividing the total daily amount into three or four times a day dosing, and/or up-titrate the buprenorphine dose (federal prescribing regulations recommend a maximum of 24 mg daily, but state regulations may vary).
- Add short-acting opioid agonists on an as-needed basis in conjunction with a defined plan to discontinue short-acting opioid agonists to avoid a return to use.
- Make plans collaboratively with the patient and outpatient provider, and communicate medication changes and plan at discharge.
CONCLUSION
Concerning our case, the hospitalist can continue the patient’s buprenorphine-naloxone, even with her acute pain needs. The patient has a baseline opioid requirement, fulfilled by continuing buprenorphine. Additional short-acting opioid agonists, such as hydromorphone, will provide analgesia for the patient, though the clinician should be aware that higher doses might be required. The practice of holding buprenorphine during episodes of acute pain is not supported by current evidence and may predispose to inadequate analgesia, opioid withdrawal, and risk of return to use and death.2
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Disclosures
The authors report no conflicts of interest.

Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason™” (TWDFNR™) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR™ series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 40-year-old woman with a history of opioid use disorder (OUD) on buprenorphine-naloxone treatment is admitted to medicine following incision and drainage of a large forearm abscess with surrounding cellulitis. The patient reports severe pain following the procedure, which is not relieved by ibuprofen. The admitting hospitalist orders a pain regimen for the patient, which includes oral and intravenous hydromorphone and discontinues the patient’s buprenorphine-naloxone so that the short-acting opioids can take effect.
BACKGROUND
Medications to treat OUD include methadone, buprenorphine, and extended-release naltrexone. Buprenorphine is a Schedule III medication under the United States Food and Drug Administration that reduces opioid cravings, subsequently decreasing drug use1 and opioid-related overdose deaths.2 It has a favorable safety profile and can be prescribed for OUD in an office-based, outpatient setting since the Drug Addiction Treatment Act of 2000 (DATA 2000). Due to extensive first-pass metabolism, buprenorphine for OUD is typically administered sublingually, either alone or in a fixed combination with naloxone.
WHY YOU MIGHT THINK YOU SHOULD HOLD BUPRENORPHINE WHEN TREATING ACUTE PAIN
Buprenorphine is a partial opioid agonist with a long half-life and high affinity for the mu opioid receptor. Given these properties, prior recommendations assumed that buprenorphine blocked the effectiveness of additional opioid agonists.3,4 In 2004, guidelines by the Department of Health and Human Service Center for Substance Abuse Treatment recommended discontinuing buprenorphine in patients taking opioid pain medications.5 These suggestions were based on limited case reports describing difficulty controlling pain in patients with OUD with a high opioid tolerance who were receiving buprenorphine.6
Providers may hold buprenorphine when treating acute pain out of concern it could precipitate withdrawal by displacing full opioid agonists from the mu receptor. Providers may also believe that the naloxone component in the most commonly prescribed formulation, buprenorphine-naloxone, blocks the effects of opioid analgesics. Evolving understanding of buprenorphine pharmacology and the absence of high-quality evidence has resulted in providers holding buprenorphine in the setting of acute pain.
Finally, providers without dedicated training may feel they lack the necessary qualifications to prescribe buprenorphine in the inpatient setting. DATA 2000 requires mandatory X waiver training for physicians, nurse practitioners, and physician assistants to prescribe outpatient buprenorphine for OUD treatment outside of specialized opioid treatment programs.
WHY DISCONTINUING BUPRENORPHINE WHEN TREATING ACUTE PAIN IS NOT NECESSARY
Despite buprenorphine’s high affinity at the mu receptor, additional receptors remain available for full opioid agonists to bind and activate,6 providing effective pain relief even in patients using buprenorphine. In contrast to the 2004 Department of Health and Human Service guidelines, subsequent clinical studies have demonstrated that concurrent use of opioid analgesics is effective for patients maintained on buprenorphine, similar to patients on other forms of OUD treatment such as methadone.7,8
Precipitated withdrawal only occurs when buprenorphine is newly introduced to patients with already circulating opioids. Patients receiving buprenorphine-naloxone can also be exposed to opioids without precipitated withdrawal from the naloxone component, as naloxone is not absorbed via sublingual or buccal administration, but only present in the formulation to dissuade intravenous administration of the medication.
Even in the perioperative period, there is insufficient evidence to support the discontinuation of buprenorphine.9 Studies in this patient population have found that patients receiving buprenorphine may require higher doses of short-acting opioids to achieve adequate analgesia, but they experience similar pain control, lengths of stay, and functional outcomes to controls.10 Despite variable perioperative management of buprenorphine,11 protocols at major medical centers now recommend continuing or dose adjusting buprenorphine in the perioperative period rather than discontinuing.12-14
Patients physically dependent on opioid agonists, including buprenorphine, must be maintained on a daily equivalent opioid dose to avoid experiencing withdrawal. This maintenance requirement must be met before any analgesic effect for acute pain is obtained with additional opioids. Temporarily discontinuing buprenorphine introduces unnecessary complexity to a hospitalization, places the patient at risk of exacerbation of pain, opioid withdrawal, and predisposes the patient to return to use and overdose if not resumed before hospital discharge.5
Finally, clinicians do not require additional training or an X waiver to administer buprenorphine to hospitalized patients. These requirements are limited to providers managing buprenorphine in the outpatient setting or those prescribing buprenorphine to patients to take postdischarge. Hospitalists frequently prescribe opioid medications in the inpatient setting with similar or greater safety risk profiles to buprenorphine.
WHEN YOU SHOULD CONSIDER HOLDING BUPRENORPHINE
Providers may consider holding buprenorphine if a patient with OUD has not been taking buprenorphine before hospitalization and has severe acute pain needs. This history can be confirmed with the patient and the state’s online prescription drug monitoring program. If further clarification is needed, this can be accomplished with a pharmacist and urine testing or by verifying with the patient’s opioid treatment program, as some programs provide directly administered buprenorphine.
In cases where a patient may have stopped buprenorphine before admission but wants to restart it in the hospital, it is essential to ascertain when the patient last used an opioid. The buprenorphine reinduction should be timed to a sufficient number of hours since last opioid use and/or to when the patient shows signs of active withdrawal. The re-induction can take place before, during, or after an acute pain episode, depending on the individual circumstances.
Patient preference is extremely important in the management of both pain and OUD. After shared decision-making, some patients may ultimately opt to hold buprenorphine in certain situations or switch to an alternative treatment, such as methadone, during their hospitalization. Such adjustments should be made in conjunction with the patient, primary care provider, and pain or addiction medicine specialty consultation.
WHAT YOU SHOULD DO INSTEAD
For patients on buprenorphine admitted to the hospital with anticipated or unanticipated acute pain needs, hospitalists should continue buprenorphine. Continuation of buprenorphine meets a patient’s baseline opioid requirement while still allowing the use of additional short-acting opioid agonists as needed for pain.15
As with all pain, multimodal pain management should be provided with adjunctive medications such as acetaminophen, nonsteroidal anti-inflammatory drugs, neuropathic agents, topical analgesics, and regional anesthesia.8
Acute pain can be addressed by taking advantage of buprenorphine’s analgesic effects and adding additional short-acting opioids if needed.15 Several options are available, including:
1. Continuing daily buprenorphine and prescribing short-acting opioid agonists, preferably those with high intrinsic activity at the mu receptor (such as morphine, fentanyl, or hydromorphone). Full opioid agonist doses to achieve analgesia for patients on buprenorphine will be higher than in opioid naïve patients due to tolerance.16
2 .Dividing the total daily buprenorphine dose into three or four times per day dosing, since buprenorphine provides an analgesic effect lasting six to eight hours. Short-acting opioid agonists can still be prescribed on an as-needed basis for additional pain needs.
3. Temporarily increasing the total daily buprenorphine dose and dividing into three or four times per day dosing, as above. Short-acting opioid agonists can still be prescribed on an as-needed basis for additional pain needs.
It is essential to make a clear plan with the patient for initiation and discontinuation of short-acting opioid agonists or buprenorphine changes. Patients on buprenorphine should be managed collaboratively with the primary care provider or addiction specialist to coordinate prescribing and follow-up after discharge.
RECOMMENDATIONS
- Continue outpatient buprenorphine treatment for patients admitted with acute pain.
- Use adjunctive nonopioid pain medications and nonpharmacologic modalities to address acute pain.
- Adjust buprenorphine to address acute pain by dividing the total daily amount into three or four times a day dosing, and/or up-titrate the buprenorphine dose (federal prescribing regulations recommend a maximum of 24 mg daily, but state regulations may vary).
- Add short-acting opioid agonists on an as-needed basis in conjunction with a defined plan to discontinue short-acting opioid agonists to avoid a return to use.
- Make plans collaboratively with the patient and outpatient provider, and communicate medication changes and plan at discharge.
CONCLUSION
Concerning our case, the hospitalist can continue the patient’s buprenorphine-naloxone, even with her acute pain needs. The patient has a baseline opioid requirement, fulfilled by continuing buprenorphine. Additional short-acting opioid agonists, such as hydromorphone, will provide analgesia for the patient, though the clinician should be aware that higher doses might be required. The practice of holding buprenorphine during episodes of acute pain is not supported by current evidence and may predispose to inadequate analgesia, opioid withdrawal, and risk of return to use and death.2
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason™?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason™” topics by emailing [email protected].
Disclosures
The authors report no conflicts of interest.
1. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(3):CD002207. https://doi.org/10.1002/14651858.CD002207.
2. Sordo L, Barrio G, Bravo M, et al. Morality risk during and after opioid substitution treatment: systemic review and meta-analysis of cohort studies. BMJ. 2017;357:1550. https://doi.org/10.1136/bmj.j1550.
3. Johnson RE, Fudula PJ, Payne R. Buprenorphine: considerations for pain management. J Pain Symptom Manage. 2005;29(3):297-326. https://doi.org/10.1016/j.jpainsymman.2004.07.005.
4. Marcelina JS, Rubinstein A. Continuous perioperative sublingual buprenorphine. J Pain Palliat Care Pharmacother. 2016;30(4):289-293. https://doi.org/10.1080/15360288.2016.1231734.
5. Greenwald MK, Johanson CE, Moody DE, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor binding potential, plasma concentration and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28(11):2000-2009. https://doi.org/10.1038/sj.npp.1300251.
6. Lembke A, Ottestad E, Schmiesing C. Patients maintained on buprenorphine for opioid use disorder should continue buprenorphine through the perioperative period. Pain Med. 2019;20(3):425-428. https://doi.org/10.1093/pm/pny019.
7. Kornfeld H, Manfredi L. Effectiveness of full agonist opioids in patients stabilized on buprenorphine undergoing major surgery: A case series. Am J Ther. 2010;17(5):523-528. https://doi.org/10.1097/MJT.0b013e3181be0804.
8. Harrison TK, Kornfeld H, Aggarwal AK, Lembke A. Perioperative considerations for the patient with opioid use disorder on buprenorphine, methadone, or naltrexone maintenance therapy. Anesthesiol Clin. 2018;36(3):345-359. https://doi.org/10.1016/j.anclin.2018.04.002.
9. Goel A, Azargive S, Lamba W, et al. The perioperative patient on buprenorphine: a systematic review of perioperative management strategies and patient outcomes. Can J Anesth. 2019; 66(2):201-217. https://doi.org/10.1007/s12630-018-1255-3.
10. Hansen LE, Stone GL, Matson CA, Tybor DJ, Pevear ME, Smith EL. Total joint arthroplasty in patients taking methadone or buprenorphine/naloxone preoperatively for prior heroin addiction: a prospective matched cohort study. J Arthroplasty. 2016;31(8):1698-1701. https://doi.org/10.1016/j.arth.2016.01.032.
11. Jonan AB, Kaye AD, Urman RD. Buprenorphine formulations: clinical best practice strategies recommendations for perioperative management of patients undergoing surgical or interventional pain procedures. Pain Physician. 2018;21(1):E1-12. PubMed
12. Quaye AN, Zhang Y. Perioperative management of buprenorphine: solving the conundrum. Pain Med. 2018. https://doi.org/10.1093/pm/pny217.
13. Silva MJ, Rubinstein A. Continuous perioperative sublingual buprenorphine. J Pain Palliative Care Pharmacother. 2016;30(4):289-293. https://doi.org/10.1080/15360288.2016.1231734.
14. Kampman K, Jarvis M. ASAM National practice guidelines for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367. https://doi.org/10.1097/ADM.0000000000000166.
15. Childers JW, Arnold RM. Treatment of pain in patients taking buprenorphine for opioid addiction. J Palliat Med. 2012;15(5):613-614. https://doi.org/10.1089/jpm.2012.9591.
16. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. https://doi.org/10.7326/0003-4819-144-2-200601170-00010
1. Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;(3):CD002207. https://doi.org/10.1002/14651858.CD002207.
2. Sordo L, Barrio G, Bravo M, et al. Morality risk during and after opioid substitution treatment: systemic review and meta-analysis of cohort studies. BMJ. 2017;357:1550. https://doi.org/10.1136/bmj.j1550.
3. Johnson RE, Fudula PJ, Payne R. Buprenorphine: considerations for pain management. J Pain Symptom Manage. 2005;29(3):297-326. https://doi.org/10.1016/j.jpainsymman.2004.07.005.
4. Marcelina JS, Rubinstein A. Continuous perioperative sublingual buprenorphine. J Pain Palliat Care Pharmacother. 2016;30(4):289-293. https://doi.org/10.1080/15360288.2016.1231734.
5. Greenwald MK, Johanson CE, Moody DE, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor binding potential, plasma concentration and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28(11):2000-2009. https://doi.org/10.1038/sj.npp.1300251.
6. Lembke A, Ottestad E, Schmiesing C. Patients maintained on buprenorphine for opioid use disorder should continue buprenorphine through the perioperative period. Pain Med. 2019;20(3):425-428. https://doi.org/10.1093/pm/pny019.
7. Kornfeld H, Manfredi L. Effectiveness of full agonist opioids in patients stabilized on buprenorphine undergoing major surgery: A case series. Am J Ther. 2010;17(5):523-528. https://doi.org/10.1097/MJT.0b013e3181be0804.
8. Harrison TK, Kornfeld H, Aggarwal AK, Lembke A. Perioperative considerations for the patient with opioid use disorder on buprenorphine, methadone, or naltrexone maintenance therapy. Anesthesiol Clin. 2018;36(3):345-359. https://doi.org/10.1016/j.anclin.2018.04.002.
9. Goel A, Azargive S, Lamba W, et al. The perioperative patient on buprenorphine: a systematic review of perioperative management strategies and patient outcomes. Can J Anesth. 2019; 66(2):201-217. https://doi.org/10.1007/s12630-018-1255-3.
10. Hansen LE, Stone GL, Matson CA, Tybor DJ, Pevear ME, Smith EL. Total joint arthroplasty in patients taking methadone or buprenorphine/naloxone preoperatively for prior heroin addiction: a prospective matched cohort study. J Arthroplasty. 2016;31(8):1698-1701. https://doi.org/10.1016/j.arth.2016.01.032.
11. Jonan AB, Kaye AD, Urman RD. Buprenorphine formulations: clinical best practice strategies recommendations for perioperative management of patients undergoing surgical or interventional pain procedures. Pain Physician. 2018;21(1):E1-12. PubMed
12. Quaye AN, Zhang Y. Perioperative management of buprenorphine: solving the conundrum. Pain Med. 2018. https://doi.org/10.1093/pm/pny217.
13. Silva MJ, Rubinstein A. Continuous perioperative sublingual buprenorphine. J Pain Palliative Care Pharmacother. 2016;30(4):289-293. https://doi.org/10.1080/15360288.2016.1231734.
14. Kampman K, Jarvis M. ASAM National practice guidelines for the use of medications in the treatment of addiction involving opioid use. J Addict Med. 2015;9(5):358-367. https://doi.org/10.1097/ADM.0000000000000166.
15. Childers JW, Arnold RM. Treatment of pain in patients taking buprenorphine for opioid addiction. J Palliat Med. 2012;15(5):613-614. https://doi.org/10.1089/jpm.2012.9591.
16. Alford DP, Compton P, Samet JH. Acute pain management for patients receiving maintenance methadone or buprenorphine therapy. Ann Intern Med. 2006;144(2):127-134. https://doi.org/10.7326/0003-4819-144-2-200601170-00010
© 2019 Society of Hospital Medicine
Things We Do for No Reason: Systemic Corticosteroids for Wheezing in Preschool-Aged Children
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CASE PRESENTATION
A four-year-old girl, with a history of one wheezing episode, presents to the emergency department (ED) with wheezing, tachypnea, and respiratory distress. She receives three successive treatments of short-acting bronchodilators and is given one dose of dexamethasone, after which she improves significantly. Because of persistent tachypnea and wheezing, she is admitted for further management. By the next day she is much improved, now requiring bronchodilator treatment every four hours. She receives a second dose of dexamethasone to complete her steroid burst. Was the trajectory of this patient’s illness altered by treatment with systemic corticosteroids (SCS)? Is there any benefit to SCS treatment in a wheezing preschool-aged patient?
BACKGROUND
Wheezing is common in preschool-aged children (ages 2-5 years), with up to half in this age group having experienced a wheezing episode and up to one-third, recurrent wheezing.1,2 Young children with wheezing require ED visits and hospitalizations at much higher rates than older children and adults.3 Several studies have also demonstrated that children in this age group have higher rates of SCS prescriptions compared with older children.4,5 Despite the high prevalence of wheezing in this age group, there is great heterogeneity in the etiology and clinical progression of early childhood wheezing, with up to six described phenotypes each with varying levels of association with the development of asthma.6 Given the high frequency of asthma, preschool-aged children admitted with wheezing are often treated with SCS, as this is the standard of care for an acute asthma exacerbation.7
WHY YOU MIGHT THINK SYSTEMIC CORTICOSTEROIDS WOULD BE HELPFUL IN TREATING PRESCHOOL WHEEZE
The benefit of SCS in school-aged children and adolescents with multitrigger asthma exacerbation is well established and includes shorter time to resolution of acute illness and reduction in relapses.8 Because of these benefits, expert panels and regulatory agencies often include preschool-aged children in treatment recommendations for the older age groups.7,9,10 Consequently, apart from infants diagnosed with bronchiolitis, SCS remain a common and accepted treatment for young children presenting with asthma-like symptoms.4,5
Some data suggest that there may be clinical benefit from treatment with SCS in preschool children who wheeze. A recent trial by Foster et al. included 605 children, aged 24-72 months, presenting to a pediatric ED with wheeze plus viral upper respiratory symptoms.11 Patients were randomized to receive a three-day course of prednisolone (1 mg/kg) or placebo. The primary outcome was length of hospital stay until ready for discharge, which they found was significantly longer for placebo-treated patients (540 minutes) versus prednisolone (370 minutes).
WHY SYSTEMIC CORTICOSTEROIDS ARE NOT ROUTINELY HELPFUL IN PRESCHOOL CHILDREN WHO WHEEZE
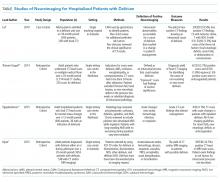
There are few randomized controlled trials evaluating the efficacy of SCS in preschool-aged children with viral-induced wheezing, and these children are often grouped with younger or older children in studies. While limited in number, these studies have evaluated SCS efficacy with acute wheezing in preschool-aged children in outpatient, ED, and inpatient settings (Appendix Table).12-16 The majority of trials of SCS in this age group have shown mixed or negative results.
Admission rates for preschoolers with viral wheezing were not statistically different in those receiving oral prednisolone versus placebo in a study conducted by Oomen et al. evaluating outpatient, parent-initiated prednisolone.14 Tal et al. found overall benefit with reduced admission rate for patients treated in the ED with methylprednisolone versus placebo; however, this finding was not statistically significant in patients 24-54 months old.16
For those requiring hospitalization, length of hospital stay and time until readiness to discharge were the primary outcomes assessed by Panickar et al. and Jartti et al. Neither study found a statistically significant difference between groups who received oral prednisolone versus placebo for 3 or 5 days. Secondary outcomes such as symptom scores, symptom duration, albuterol use, and 60-day relapse rate were also not improved in those taking oral prednisolone compared with placebo.14,15
The mixed results of studies assessing the efficacy of SCS in preschool-aged wheezing children may be attributed to the fact that wheezing in this age group likely represents multiple underlying processes. Most acute wheezing at this age is not associated with atopy and is often triggered by viral respiratory tract infections.17 Furthermore, 90% of wheezing in children under the age of five years does not persist to the asthma phenotype (recurrent episodes with multiple triggers, airway obstruction, and hyper-responsiveness) once they reach school age.18
While SCS are generally not expensive, their use is not without cost. Studies of oral corticosteroid use in children with asthma have shown adverse effects including vomiting, hypertension, and impaired growth.19 Children with recurrent wheeze receiving SCS may demonstrate biochemical hypothalamic-pituitary-axis dysfunction.20 Given the high utilization and SCS prescription rates in this age group, reducing the use of SCS with wheezing episodes could have a large clinical and financial impact.3,4 These medications should be used judiciously in order to balance benefit with potential risks.
WHEN MIGHT SYSTEMIC CORTICOSTEROIDS BE HELPFUL IN WHEEZING PRESCHOOLERS
Given that there is diversity in the phenotype of preschool-aged children who wheeze, it is possible that a subset of these children would benefit from SCS. Some studies have shown that certain groups of patients derive benefit, including those with rhinovirus infection, eczema, and children at higher risk for multitrigger asthma.11,13 Children who have atopic wheeze are more likely to have persistent symptoms that may eventually be diagnosed as asthma.18 These children will have airway inflammation secondary to eosinophilic infiltration and may be responsive to SCS at times of exacerbation. However, attempts to classify preschool children based on risk of asthma have not shown consistent results.
The Asthma Predictive Index (API), a tool developed as a part of the Tucson Children’s Respiratory Study, uses clinical factors including history of wheeze, atopic dermatitis, and allergic rhinitis to determine a young child’s risk of having asthma symptoms after age six years.21 Jartti et al. and Panickar et al. used the API to stratify patients based on future asthma risk.13,15 The high risk group in the Jartti et al. study showed the benefit of SCS, while there was no benefit in the Panickar et al. study. When Oommen et al. also attempted to stratify asthma risk using levels of blood eosinophil proteins, which when elevated, are predictive of persistent wheeze.14 There was no difference in drug efficacy between patients with high and low blood eosinophil proteins. Although Foster et al. demonstrated shorter length of stay (LOS) with SCS overall, this was only seen in the subgroup with a previous diagnosis of asthma.
Patients presenting with severe disease (including those requiring critical care or with the highest symptom scores) have mostly been excluded from these studies. Although patients with severe disease often receive steroids, there is insufficient evidence of the efficacy of SCS in this population.12,13,15,22 Foster et al. did include patients with high symptom scores (although they excluded patients with “critical wheeze”) and found that the efficacy of SCS was clearest for those with severe presentations.11
Finally, some studies have demonstrated a virus-specific effect, with a reduction in time to readiness for discharge and reduction in recurrent wheeze in children treated with prednisolone who were positive for rhinovirus.12,23 Rhinovirus infection has also been associated with allergic sensitization and recurrent wheezing.23,24 However, rhinovirus-specific steroid responsiveness has not been consistently replicated by other investigators.11
WHAT YOU SHOULD DO INSTEAD
The majority of preschool-aged children presenting with wheeze in the care of hospitalists have mild to moderate symptoms, triggered by viral infections.22 It can be helpful to categorize the wheezing child as atopic or nonatopic. Laboratory studies such as allergen-specific IgE, peripheral eosinophil count, and exhaled nitric oxide can aid in predicting response to asthma medications and progression to the classic asthma phenotype.25 In the absence of these diagnostic studies, which are often costly and challenging to obtain in young children, a clinical score such as the API, or the recently validated Pediatric Asthma Risk Score (PARS), can help to assess future risk of developing multitrigger asthma.21,26 A positive API requires a history of more than three episodes of wheeze over the past year as well as one major (physician-diagnosed atopic dermatitis or parental asthma) or two minor (peripheral blood eosinophilia, physician-diagnosed allergic rhinitis, or wheezing apart from colds) criteria.17 It has a sensitivity of 57% and specificity of 81%.26 The PARS uses the presence of parental asthma, eczema, early wheezing, wheezing apart from colds, African-American race, and ≥2 positive skin prick tests to predict asthma. The sensitivity and specificity of PARS are similar to the API at 68% and 79%, respectively.26
Given the mixed results from studies evaluating the benefit of SCS in preschoolers with atopic symptoms and/or a positive API, evidence is lacking to guide decision-making in these children.13-15 However, it is reasonable to treat those at higher risk for future multitrigger asthma with SCS. There is also insufficient evidence to determine whether those with more severe disease or those infected with particular viral pathogens may benefit. Therefore, for the majority of children presenting with viral-induced wheezing, with a negative API or low PARS, there is no evidence that treatment with an SCS provides benefit in the form of reduced LOS, reduction in clinical symptoms, treatment failure, or relapse rate.
RECOMMENDATIONS
- Do not routinely treat with SCS preschool-aged children who have episodic wheezing triggered by viral respiratory tract infections and who do not have risk factors for persistent asthma.
- For preschool-aged children with a history of atopy, a positive API, or elevated PARS, SCS can be considered during admissions for respiratory distress and wheezing.
- Preschool-aged children presenting with severe disease or requiring intensive care may benefit from SCS, but there is insufficient evidence to conclude whether this practice provides benefit.
CONCLUSIONS
Current evidence does not support the routine use of SCS for preschool-aged children admitted for mild to moderate wheezing episodes. The majority of these children have viral episodic wheeze that does not develop into the asthma phenotype. For children with severe disease or at higher risk for asthma, SCS may be considered, although there remains insufficient evidence as to their efficacy. The patient in the introductory case lacks risk factors that would suggest SCS responsiveness (eg, positive API, previous asthma diagnosis, inhaled corticosteroid use, or severe disease) and would likely receive no clinical benefit from dexamethasone treatment.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
Dr. Jennifer O’Toole consulted with and received honoraria payment from the I-PASS Patient Safety Institute. She also holds stock options in the I-PASS Patient Safety Institute, a nonpublicly traded company. Drs. Jones and Hubbell have nothing to disclose.
Funding
Dr. Thomson was supported by the Agency for Healthcare Research and Quality under award number K08HS025138.
1. Mallol J, Garcia-Marcos L, Sole D, Brand P, EISL Study Group. International prevalence of recurrent wheezing during the first year of life: variability, treatment patterns and use of health resources. Thorax. 2010;65(11):1004-1009. https://doi.org/10.1136/thx.2009.115188.
2. Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatric Pulmonol. 2007;48(8):723-728. https://doi.org/10.1002/ppul.20644.
3. Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital signs: asthma in children - United States, 2001-2016. MMWR Morb Mortal Wkly Rep. 2018;67(5):149-155. https://doi.org/10.15585/mmwr.mm6705e1.
4. Arabkhazaeli A, Vijverberg SJ, van der Ent CK, Raaijmakers JA, Maitland-van der Zee AH. High incidence of oral corticosteroids prescriptions in children with asthma in early childhood. J Asthma. 2016;53(10):1012-1017. https://doi.org/10.1080/02770903.2016.1185439.
5. Farber HJ, Silveira EA, Vicere DR, Kothari VD, Giardino AP. Oral corticosteroid prescribing for children with asthma in a medicaid managed care program. Pediatrics. 2017;139(5):139. https://doi.org/10.1542/peds.2016-4146.
6. Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63(11):974-980. https://doi.org/10.1136/thx.2007.093187.
7. National Asthma Education and Prevention Program. Expert Panel Report 3(EPR-3): Guidelines for the Diagnosis and Management of Asthma- Summary Report 2007. J Allergy Clin Immunol. 2007;120(5):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.043.
8. Smith M, Iqbal S, Elliott TM, Everard M, Rowe BH. Corticosteroids for hospitalised children with acute asthma. Cochrane Database Syst Rev. 2003(2):CD002886. https://doi.org/10.1002/14651858.CD002886.
9. Pedersen SE, Hurd SS, Lemanske Rf Jr., et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46(1):1-7. https://doi.org/10.1002/ppul.21321.
10. Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63(1):5-34. https://doi.org/10.1111/j.1398-9995.2007.01586.x.
11. Foster SJ, Cooper MN, Oosterhof S, Borland ML. Oral prednisolone in preschool children with virus-associated wheeze: a prospective, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2018;6(2):97-106. https://doi.org/10.1016/S2213-2600(18)30008-0.
12. Jartti T, Lehtinen P, Vanto T, et al. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25(6):482-488. https://doi.org/10.1097/01.inf.0000215226.69696.0c.
13. Jartti T, Lehtinen P, Vanto T, et al. Atopic characteristics of wheezing children and responses to prednisolone. Pediatr Pulmonol. 2007;42(12):1125-1133. https://doi.org/10.1002/ppul.20706.
14. Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1–5 years: randomised controlled trial. Lancet. 2003;362(9394):1433-1438. https://doi.org/10.1016/S0140-6736(03)14685-5.
15. Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360(4):329-338. https://doi.org/10.1056/NEJMoa0804897.
16. Tal A, Levy N, Bearman JE. Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86(3):350-356 .
17. Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson children’s respiratory study: 1980 to present. J Allergy Clin Immunol. 2003;111(4):661-675. https://doi.org/10.1067/mai.2003.162.
18. Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U, Multicentre Allergy Study (MAS) group. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9537):763-770. https://doi.org/10.1016/S0140-6736(06)69286-6.
19. Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103(7):975-994. https://doi.org/10.1016/j.rmed.2009.01.003.
20. Barra CB, Fontes MJF, Cintra MTG, et al. Oral corticosteroids for asthma exacerbations might be associated with adrenal suppression: are physicians aware of that? Rev Assoc Med Bras. 2017;63(10):899-903. https://doi.org/10.1590/1806-9282.63.10.899..
21. Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4):1403-1406. https://doi.org/10.1164/ajrccm.162.4.9912111.
22. Bush A, Grigg J, Saglani S. Managing wheeze in preschool children. BMJ. 2014;348:g15. https://doi.org/10.1136/bmj.g15.
23. Lukkarinen M, Lukkarinen H, Lehtinen P, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after first rhinovirus wheeze: a 7-year follow-up. Pediatr Allergy Immunol. 2013;24(3):237-243. (1399-3038. https://doi.org/10.1111/pai.12046.
24. Jartti T, Kuusipalo H, Vuorinen T, et al. Allergic sensitization is associated with rhinovirus-, but not other virus-, induced wheezing in children. Pediatr Allergy Immunol. 2010;21(7):1008-1014. https://doi.org/10.1111/j.1399-3038.2010.01059.x.
25. Burbank AJ, Szefler SJ. Current and future management of the young child with early onset wheezing. Curr Opin Allergy Clin Immunol. 2017;17(2):146-152. https://doi.org/10.1097/ACI.0000000000000341
26. Myers JM, Schauberger E, He H, et al. A Pediatric Asthma Risk Score (PARS) to better predict asthma development in young children. J Allergy Clin Immunol. 2018;143(5):1803-1810.e2. https://doi.org/10.1016/j.jaci.2018.09.037.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CASE PRESENTATION
A four-year-old girl, with a history of one wheezing episode, presents to the emergency department (ED) with wheezing, tachypnea, and respiratory distress. She receives three successive treatments of short-acting bronchodilators and is given one dose of dexamethasone, after which she improves significantly. Because of persistent tachypnea and wheezing, she is admitted for further management. By the next day she is much improved, now requiring bronchodilator treatment every four hours. She receives a second dose of dexamethasone to complete her steroid burst. Was the trajectory of this patient’s illness altered by treatment with systemic corticosteroids (SCS)? Is there any benefit to SCS treatment in a wheezing preschool-aged patient?
BACKGROUND
Wheezing is common in preschool-aged children (ages 2-5 years), with up to half in this age group having experienced a wheezing episode and up to one-third, recurrent wheezing.1,2 Young children with wheezing require ED visits and hospitalizations at much higher rates than older children and adults.3 Several studies have also demonstrated that children in this age group have higher rates of SCS prescriptions compared with older children.4,5 Despite the high prevalence of wheezing in this age group, there is great heterogeneity in the etiology and clinical progression of early childhood wheezing, with up to six described phenotypes each with varying levels of association with the development of asthma.6 Given the high frequency of asthma, preschool-aged children admitted with wheezing are often treated with SCS, as this is the standard of care for an acute asthma exacerbation.7
WHY YOU MIGHT THINK SYSTEMIC CORTICOSTEROIDS WOULD BE HELPFUL IN TREATING PRESCHOOL WHEEZE
The benefit of SCS in school-aged children and adolescents with multitrigger asthma exacerbation is well established and includes shorter time to resolution of acute illness and reduction in relapses.8 Because of these benefits, expert panels and regulatory agencies often include preschool-aged children in treatment recommendations for the older age groups.7,9,10 Consequently, apart from infants diagnosed with bronchiolitis, SCS remain a common and accepted treatment for young children presenting with asthma-like symptoms.4,5
Some data suggest that there may be clinical benefit from treatment with SCS in preschool children who wheeze. A recent trial by Foster et al. included 605 children, aged 24-72 months, presenting to a pediatric ED with wheeze plus viral upper respiratory symptoms.11 Patients were randomized to receive a three-day course of prednisolone (1 mg/kg) or placebo. The primary outcome was length of hospital stay until ready for discharge, which they found was significantly longer for placebo-treated patients (540 minutes) versus prednisolone (370 minutes).
WHY SYSTEMIC CORTICOSTEROIDS ARE NOT ROUTINELY HELPFUL IN PRESCHOOL CHILDREN WHO WHEEZE
There are few randomized controlled trials evaluating the efficacy of SCS in preschool-aged children with viral-induced wheezing, and these children are often grouped with younger or older children in studies. While limited in number, these studies have evaluated SCS efficacy with acute wheezing in preschool-aged children in outpatient, ED, and inpatient settings (Appendix Table).12-16 The majority of trials of SCS in this age group have shown mixed or negative results.
Admission rates for preschoolers with viral wheezing were not statistically different in those receiving oral prednisolone versus placebo in a study conducted by Oomen et al. evaluating outpatient, parent-initiated prednisolone.14 Tal et al. found overall benefit with reduced admission rate for patients treated in the ED with methylprednisolone versus placebo; however, this finding was not statistically significant in patients 24-54 months old.16
For those requiring hospitalization, length of hospital stay and time until readiness to discharge were the primary outcomes assessed by Panickar et al. and Jartti et al. Neither study found a statistically significant difference between groups who received oral prednisolone versus placebo for 3 or 5 days. Secondary outcomes such as symptom scores, symptom duration, albuterol use, and 60-day relapse rate were also not improved in those taking oral prednisolone compared with placebo.14,15
The mixed results of studies assessing the efficacy of SCS in preschool-aged wheezing children may be attributed to the fact that wheezing in this age group likely represents multiple underlying processes. Most acute wheezing at this age is not associated with atopy and is often triggered by viral respiratory tract infections.17 Furthermore, 90% of wheezing in children under the age of five years does not persist to the asthma phenotype (recurrent episodes with multiple triggers, airway obstruction, and hyper-responsiveness) once they reach school age.18
While SCS are generally not expensive, their use is not without cost. Studies of oral corticosteroid use in children with asthma have shown adverse effects including vomiting, hypertension, and impaired growth.19 Children with recurrent wheeze receiving SCS may demonstrate biochemical hypothalamic-pituitary-axis dysfunction.20 Given the high utilization and SCS prescription rates in this age group, reducing the use of SCS with wheezing episodes could have a large clinical and financial impact.3,4 These medications should be used judiciously in order to balance benefit with potential risks.
WHEN MIGHT SYSTEMIC CORTICOSTEROIDS BE HELPFUL IN WHEEZING PRESCHOOLERS
Given that there is diversity in the phenotype of preschool-aged children who wheeze, it is possible that a subset of these children would benefit from SCS. Some studies have shown that certain groups of patients derive benefit, including those with rhinovirus infection, eczema, and children at higher risk for multitrigger asthma.11,13 Children who have atopic wheeze are more likely to have persistent symptoms that may eventually be diagnosed as asthma.18 These children will have airway inflammation secondary to eosinophilic infiltration and may be responsive to SCS at times of exacerbation. However, attempts to classify preschool children based on risk of asthma have not shown consistent results.
The Asthma Predictive Index (API), a tool developed as a part of the Tucson Children’s Respiratory Study, uses clinical factors including history of wheeze, atopic dermatitis, and allergic rhinitis to determine a young child’s risk of having asthma symptoms after age six years.21 Jartti et al. and Panickar et al. used the API to stratify patients based on future asthma risk.13,15 The high risk group in the Jartti et al. study showed the benefit of SCS, while there was no benefit in the Panickar et al. study. When Oommen et al. also attempted to stratify asthma risk using levels of blood eosinophil proteins, which when elevated, are predictive of persistent wheeze.14 There was no difference in drug efficacy between patients with high and low blood eosinophil proteins. Although Foster et al. demonstrated shorter length of stay (LOS) with SCS overall, this was only seen in the subgroup with a previous diagnosis of asthma.
Patients presenting with severe disease (including those requiring critical care or with the highest symptom scores) have mostly been excluded from these studies. Although patients with severe disease often receive steroids, there is insufficient evidence of the efficacy of SCS in this population.12,13,15,22 Foster et al. did include patients with high symptom scores (although they excluded patients with “critical wheeze”) and found that the efficacy of SCS was clearest for those with severe presentations.11
Finally, some studies have demonstrated a virus-specific effect, with a reduction in time to readiness for discharge and reduction in recurrent wheeze in children treated with prednisolone who were positive for rhinovirus.12,23 Rhinovirus infection has also been associated with allergic sensitization and recurrent wheezing.23,24 However, rhinovirus-specific steroid responsiveness has not been consistently replicated by other investigators.11
WHAT YOU SHOULD DO INSTEAD
The majority of preschool-aged children presenting with wheeze in the care of hospitalists have mild to moderate symptoms, triggered by viral infections.22 It can be helpful to categorize the wheezing child as atopic or nonatopic. Laboratory studies such as allergen-specific IgE, peripheral eosinophil count, and exhaled nitric oxide can aid in predicting response to asthma medications and progression to the classic asthma phenotype.25 In the absence of these diagnostic studies, which are often costly and challenging to obtain in young children, a clinical score such as the API, or the recently validated Pediatric Asthma Risk Score (PARS), can help to assess future risk of developing multitrigger asthma.21,26 A positive API requires a history of more than three episodes of wheeze over the past year as well as one major (physician-diagnosed atopic dermatitis or parental asthma) or two minor (peripheral blood eosinophilia, physician-diagnosed allergic rhinitis, or wheezing apart from colds) criteria.17 It has a sensitivity of 57% and specificity of 81%.26 The PARS uses the presence of parental asthma, eczema, early wheezing, wheezing apart from colds, African-American race, and ≥2 positive skin prick tests to predict asthma. The sensitivity and specificity of PARS are similar to the API at 68% and 79%, respectively.26
Given the mixed results from studies evaluating the benefit of SCS in preschoolers with atopic symptoms and/or a positive API, evidence is lacking to guide decision-making in these children.13-15 However, it is reasonable to treat those at higher risk for future multitrigger asthma with SCS. There is also insufficient evidence to determine whether those with more severe disease or those infected with particular viral pathogens may benefit. Therefore, for the majority of children presenting with viral-induced wheezing, with a negative API or low PARS, there is no evidence that treatment with an SCS provides benefit in the form of reduced LOS, reduction in clinical symptoms, treatment failure, or relapse rate.
RECOMMENDATIONS
- Do not routinely treat with SCS preschool-aged children who have episodic wheezing triggered by viral respiratory tract infections and who do not have risk factors for persistent asthma.
- For preschool-aged children with a history of atopy, a positive API, or elevated PARS, SCS can be considered during admissions for respiratory distress and wheezing.
- Preschool-aged children presenting with severe disease or requiring intensive care may benefit from SCS, but there is insufficient evidence to conclude whether this practice provides benefit.
CONCLUSIONS
Current evidence does not support the routine use of SCS for preschool-aged children admitted for mild to moderate wheezing episodes. The majority of these children have viral episodic wheeze that does not develop into the asthma phenotype. For children with severe disease or at higher risk for asthma, SCS may be considered, although there remains insufficient evidence as to their efficacy. The patient in the introductory case lacks risk factors that would suggest SCS responsiveness (eg, positive API, previous asthma diagnosis, inhaled corticosteroid use, or severe disease) and would likely receive no clinical benefit from dexamethasone treatment.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
Dr. Jennifer O’Toole consulted with and received honoraria payment from the I-PASS Patient Safety Institute. She also holds stock options in the I-PASS Patient Safety Institute, a nonpublicly traded company. Drs. Jones and Hubbell have nothing to disclose.
Funding
Dr. Thomson was supported by the Agency for Healthcare Research and Quality under award number K08HS025138.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CASE PRESENTATION
A four-year-old girl, with a history of one wheezing episode, presents to the emergency department (ED) with wheezing, tachypnea, and respiratory distress. She receives three successive treatments of short-acting bronchodilators and is given one dose of dexamethasone, after which she improves significantly. Because of persistent tachypnea and wheezing, she is admitted for further management. By the next day she is much improved, now requiring bronchodilator treatment every four hours. She receives a second dose of dexamethasone to complete her steroid burst. Was the trajectory of this patient’s illness altered by treatment with systemic corticosteroids (SCS)? Is there any benefit to SCS treatment in a wheezing preschool-aged patient?
BACKGROUND
Wheezing is common in preschool-aged children (ages 2-5 years), with up to half in this age group having experienced a wheezing episode and up to one-third, recurrent wheezing.1,2 Young children with wheezing require ED visits and hospitalizations at much higher rates than older children and adults.3 Several studies have also demonstrated that children in this age group have higher rates of SCS prescriptions compared with older children.4,5 Despite the high prevalence of wheezing in this age group, there is great heterogeneity in the etiology and clinical progression of early childhood wheezing, with up to six described phenotypes each with varying levels of association with the development of asthma.6 Given the high frequency of asthma, preschool-aged children admitted with wheezing are often treated with SCS, as this is the standard of care for an acute asthma exacerbation.7
WHY YOU MIGHT THINK SYSTEMIC CORTICOSTEROIDS WOULD BE HELPFUL IN TREATING PRESCHOOL WHEEZE
The benefit of SCS in school-aged children and adolescents with multitrigger asthma exacerbation is well established and includes shorter time to resolution of acute illness and reduction in relapses.8 Because of these benefits, expert panels and regulatory agencies often include preschool-aged children in treatment recommendations for the older age groups.7,9,10 Consequently, apart from infants diagnosed with bronchiolitis, SCS remain a common and accepted treatment for young children presenting with asthma-like symptoms.4,5
Some data suggest that there may be clinical benefit from treatment with SCS in preschool children who wheeze. A recent trial by Foster et al. included 605 children, aged 24-72 months, presenting to a pediatric ED with wheeze plus viral upper respiratory symptoms.11 Patients were randomized to receive a three-day course of prednisolone (1 mg/kg) or placebo. The primary outcome was length of hospital stay until ready for discharge, which they found was significantly longer for placebo-treated patients (540 minutes) versus prednisolone (370 minutes).
WHY SYSTEMIC CORTICOSTEROIDS ARE NOT ROUTINELY HELPFUL IN PRESCHOOL CHILDREN WHO WHEEZE
There are few randomized controlled trials evaluating the efficacy of SCS in preschool-aged children with viral-induced wheezing, and these children are often grouped with younger or older children in studies. While limited in number, these studies have evaluated SCS efficacy with acute wheezing in preschool-aged children in outpatient, ED, and inpatient settings (Appendix Table).12-16 The majority of trials of SCS in this age group have shown mixed or negative results.
Admission rates for preschoolers with viral wheezing were not statistically different in those receiving oral prednisolone versus placebo in a study conducted by Oomen et al. evaluating outpatient, parent-initiated prednisolone.14 Tal et al. found overall benefit with reduced admission rate for patients treated in the ED with methylprednisolone versus placebo; however, this finding was not statistically significant in patients 24-54 months old.16
For those requiring hospitalization, length of hospital stay and time until readiness to discharge were the primary outcomes assessed by Panickar et al. and Jartti et al. Neither study found a statistically significant difference between groups who received oral prednisolone versus placebo for 3 or 5 days. Secondary outcomes such as symptom scores, symptom duration, albuterol use, and 60-day relapse rate were also not improved in those taking oral prednisolone compared with placebo.14,15
The mixed results of studies assessing the efficacy of SCS in preschool-aged wheezing children may be attributed to the fact that wheezing in this age group likely represents multiple underlying processes. Most acute wheezing at this age is not associated with atopy and is often triggered by viral respiratory tract infections.17 Furthermore, 90% of wheezing in children under the age of five years does not persist to the asthma phenotype (recurrent episodes with multiple triggers, airway obstruction, and hyper-responsiveness) once they reach school age.18
While SCS are generally not expensive, their use is not without cost. Studies of oral corticosteroid use in children with asthma have shown adverse effects including vomiting, hypertension, and impaired growth.19 Children with recurrent wheeze receiving SCS may demonstrate biochemical hypothalamic-pituitary-axis dysfunction.20 Given the high utilization and SCS prescription rates in this age group, reducing the use of SCS with wheezing episodes could have a large clinical and financial impact.3,4 These medications should be used judiciously in order to balance benefit with potential risks.
WHEN MIGHT SYSTEMIC CORTICOSTEROIDS BE HELPFUL IN WHEEZING PRESCHOOLERS
Given that there is diversity in the phenotype of preschool-aged children who wheeze, it is possible that a subset of these children would benefit from SCS. Some studies have shown that certain groups of patients derive benefit, including those with rhinovirus infection, eczema, and children at higher risk for multitrigger asthma.11,13 Children who have atopic wheeze are more likely to have persistent symptoms that may eventually be diagnosed as asthma.18 These children will have airway inflammation secondary to eosinophilic infiltration and may be responsive to SCS at times of exacerbation. However, attempts to classify preschool children based on risk of asthma have not shown consistent results.
The Asthma Predictive Index (API), a tool developed as a part of the Tucson Children’s Respiratory Study, uses clinical factors including history of wheeze, atopic dermatitis, and allergic rhinitis to determine a young child’s risk of having asthma symptoms after age six years.21 Jartti et al. and Panickar et al. used the API to stratify patients based on future asthma risk.13,15 The high risk group in the Jartti et al. study showed the benefit of SCS, while there was no benefit in the Panickar et al. study. When Oommen et al. also attempted to stratify asthma risk using levels of blood eosinophil proteins, which when elevated, are predictive of persistent wheeze.14 There was no difference in drug efficacy between patients with high and low blood eosinophil proteins. Although Foster et al. demonstrated shorter length of stay (LOS) with SCS overall, this was only seen in the subgroup with a previous diagnosis of asthma.
Patients presenting with severe disease (including those requiring critical care or with the highest symptom scores) have mostly been excluded from these studies. Although patients with severe disease often receive steroids, there is insufficient evidence of the efficacy of SCS in this population.12,13,15,22 Foster et al. did include patients with high symptom scores (although they excluded patients with “critical wheeze”) and found that the efficacy of SCS was clearest for those with severe presentations.11
Finally, some studies have demonstrated a virus-specific effect, with a reduction in time to readiness for discharge and reduction in recurrent wheeze in children treated with prednisolone who were positive for rhinovirus.12,23 Rhinovirus infection has also been associated with allergic sensitization and recurrent wheezing.23,24 However, rhinovirus-specific steroid responsiveness has not been consistently replicated by other investigators.11
WHAT YOU SHOULD DO INSTEAD
The majority of preschool-aged children presenting with wheeze in the care of hospitalists have mild to moderate symptoms, triggered by viral infections.22 It can be helpful to categorize the wheezing child as atopic or nonatopic. Laboratory studies such as allergen-specific IgE, peripheral eosinophil count, and exhaled nitric oxide can aid in predicting response to asthma medications and progression to the classic asthma phenotype.25 In the absence of these diagnostic studies, which are often costly and challenging to obtain in young children, a clinical score such as the API, or the recently validated Pediatric Asthma Risk Score (PARS), can help to assess future risk of developing multitrigger asthma.21,26 A positive API requires a history of more than three episodes of wheeze over the past year as well as one major (physician-diagnosed atopic dermatitis or parental asthma) or two minor (peripheral blood eosinophilia, physician-diagnosed allergic rhinitis, or wheezing apart from colds) criteria.17 It has a sensitivity of 57% and specificity of 81%.26 The PARS uses the presence of parental asthma, eczema, early wheezing, wheezing apart from colds, African-American race, and ≥2 positive skin prick tests to predict asthma. The sensitivity and specificity of PARS are similar to the API at 68% and 79%, respectively.26
Given the mixed results from studies evaluating the benefit of SCS in preschoolers with atopic symptoms and/or a positive API, evidence is lacking to guide decision-making in these children.13-15 However, it is reasonable to treat those at higher risk for future multitrigger asthma with SCS. There is also insufficient evidence to determine whether those with more severe disease or those infected with particular viral pathogens may benefit. Therefore, for the majority of children presenting with viral-induced wheezing, with a negative API or low PARS, there is no evidence that treatment with an SCS provides benefit in the form of reduced LOS, reduction in clinical symptoms, treatment failure, or relapse rate.
RECOMMENDATIONS
- Do not routinely treat with SCS preschool-aged children who have episodic wheezing triggered by viral respiratory tract infections and who do not have risk factors for persistent asthma.
- For preschool-aged children with a history of atopy, a positive API, or elevated PARS, SCS can be considered during admissions for respiratory distress and wheezing.
- Preschool-aged children presenting with severe disease or requiring intensive care may benefit from SCS, but there is insufficient evidence to conclude whether this practice provides benefit.
CONCLUSIONS
Current evidence does not support the routine use of SCS for preschool-aged children admitted for mild to moderate wheezing episodes. The majority of these children have viral episodic wheeze that does not develop into the asthma phenotype. For children with severe disease or at higher risk for asthma, SCS may be considered, although there remains insufficient evidence as to their efficacy. The patient in the introductory case lacks risk factors that would suggest SCS responsiveness (eg, positive API, previous asthma diagnosis, inhaled corticosteroid use, or severe disease) and would likely receive no clinical benefit from dexamethasone treatment.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
Dr. Jennifer O’Toole consulted with and received honoraria payment from the I-PASS Patient Safety Institute. She also holds stock options in the I-PASS Patient Safety Institute, a nonpublicly traded company. Drs. Jones and Hubbell have nothing to disclose.
Funding
Dr. Thomson was supported by the Agency for Healthcare Research and Quality under award number K08HS025138.
1. Mallol J, Garcia-Marcos L, Sole D, Brand P, EISL Study Group. International prevalence of recurrent wheezing during the first year of life: variability, treatment patterns and use of health resources. Thorax. 2010;65(11):1004-1009. https://doi.org/10.1136/thx.2009.115188.
2. Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatric Pulmonol. 2007;48(8):723-728. https://doi.org/10.1002/ppul.20644.
3. Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital signs: asthma in children - United States, 2001-2016. MMWR Morb Mortal Wkly Rep. 2018;67(5):149-155. https://doi.org/10.15585/mmwr.mm6705e1.
4. Arabkhazaeli A, Vijverberg SJ, van der Ent CK, Raaijmakers JA, Maitland-van der Zee AH. High incidence of oral corticosteroids prescriptions in children with asthma in early childhood. J Asthma. 2016;53(10):1012-1017. https://doi.org/10.1080/02770903.2016.1185439.
5. Farber HJ, Silveira EA, Vicere DR, Kothari VD, Giardino AP. Oral corticosteroid prescribing for children with asthma in a medicaid managed care program. Pediatrics. 2017;139(5):139. https://doi.org/10.1542/peds.2016-4146.
6. Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63(11):974-980. https://doi.org/10.1136/thx.2007.093187.
7. National Asthma Education and Prevention Program. Expert Panel Report 3(EPR-3): Guidelines for the Diagnosis and Management of Asthma- Summary Report 2007. J Allergy Clin Immunol. 2007;120(5):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.043.
8. Smith M, Iqbal S, Elliott TM, Everard M, Rowe BH. Corticosteroids for hospitalised children with acute asthma. Cochrane Database Syst Rev. 2003(2):CD002886. https://doi.org/10.1002/14651858.CD002886.
9. Pedersen SE, Hurd SS, Lemanske Rf Jr., et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46(1):1-7. https://doi.org/10.1002/ppul.21321.
10. Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63(1):5-34. https://doi.org/10.1111/j.1398-9995.2007.01586.x.
11. Foster SJ, Cooper MN, Oosterhof S, Borland ML. Oral prednisolone in preschool children with virus-associated wheeze: a prospective, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2018;6(2):97-106. https://doi.org/10.1016/S2213-2600(18)30008-0.
12. Jartti T, Lehtinen P, Vanto T, et al. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25(6):482-488. https://doi.org/10.1097/01.inf.0000215226.69696.0c.
13. Jartti T, Lehtinen P, Vanto T, et al. Atopic characteristics of wheezing children and responses to prednisolone. Pediatr Pulmonol. 2007;42(12):1125-1133. https://doi.org/10.1002/ppul.20706.
14. Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1–5 years: randomised controlled trial. Lancet. 2003;362(9394):1433-1438. https://doi.org/10.1016/S0140-6736(03)14685-5.
15. Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360(4):329-338. https://doi.org/10.1056/NEJMoa0804897.
16. Tal A, Levy N, Bearman JE. Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86(3):350-356 .
17. Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson children’s respiratory study: 1980 to present. J Allergy Clin Immunol. 2003;111(4):661-675. https://doi.org/10.1067/mai.2003.162.
18. Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U, Multicentre Allergy Study (MAS) group. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9537):763-770. https://doi.org/10.1016/S0140-6736(06)69286-6.
19. Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103(7):975-994. https://doi.org/10.1016/j.rmed.2009.01.003.
20. Barra CB, Fontes MJF, Cintra MTG, et al. Oral corticosteroids for asthma exacerbations might be associated with adrenal suppression: are physicians aware of that? Rev Assoc Med Bras. 2017;63(10):899-903. https://doi.org/10.1590/1806-9282.63.10.899..
21. Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4):1403-1406. https://doi.org/10.1164/ajrccm.162.4.9912111.
22. Bush A, Grigg J, Saglani S. Managing wheeze in preschool children. BMJ. 2014;348:g15. https://doi.org/10.1136/bmj.g15.
23. Lukkarinen M, Lukkarinen H, Lehtinen P, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after first rhinovirus wheeze: a 7-year follow-up. Pediatr Allergy Immunol. 2013;24(3):237-243. (1399-3038. https://doi.org/10.1111/pai.12046.
24. Jartti T, Kuusipalo H, Vuorinen T, et al. Allergic sensitization is associated with rhinovirus-, but not other virus-, induced wheezing in children. Pediatr Allergy Immunol. 2010;21(7):1008-1014. https://doi.org/10.1111/j.1399-3038.2010.01059.x.
25. Burbank AJ, Szefler SJ. Current and future management of the young child with early onset wheezing. Curr Opin Allergy Clin Immunol. 2017;17(2):146-152. https://doi.org/10.1097/ACI.0000000000000341
26. Myers JM, Schauberger E, He H, et al. A Pediatric Asthma Risk Score (PARS) to better predict asthma development in young children. J Allergy Clin Immunol. 2018;143(5):1803-1810.e2. https://doi.org/10.1016/j.jaci.2018.09.037.
1. Mallol J, Garcia-Marcos L, Sole D, Brand P, EISL Study Group. International prevalence of recurrent wheezing during the first year of life: variability, treatment patterns and use of health resources. Thorax. 2010;65(11):1004-1009. https://doi.org/10.1136/thx.2009.115188.
2. Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatric Pulmonol. 2007;48(8):723-728. https://doi.org/10.1002/ppul.20644.
3. Zahran HS, Bailey CM, Damon SA, Garbe PL, Breysse PN. Vital signs: asthma in children - United States, 2001-2016. MMWR Morb Mortal Wkly Rep. 2018;67(5):149-155. https://doi.org/10.15585/mmwr.mm6705e1.
4. Arabkhazaeli A, Vijverberg SJ, van der Ent CK, Raaijmakers JA, Maitland-van der Zee AH. High incidence of oral corticosteroids prescriptions in children with asthma in early childhood. J Asthma. 2016;53(10):1012-1017. https://doi.org/10.1080/02770903.2016.1185439.
5. Farber HJ, Silveira EA, Vicere DR, Kothari VD, Giardino AP. Oral corticosteroid prescribing for children with asthma in a medicaid managed care program. Pediatrics. 2017;139(5):139. https://doi.org/10.1542/peds.2016-4146.
6. Henderson J, Granell R, Heron J, et al. Associations of wheezing phenotypes in the first 6 years of life with atopy, lung function and airway responsiveness in mid-childhood. Thorax. 2008;63(11):974-980. https://doi.org/10.1136/thx.2007.093187.
7. National Asthma Education and Prevention Program. Expert Panel Report 3(EPR-3): Guidelines for the Diagnosis and Management of Asthma- Summary Report 2007. J Allergy Clin Immunol. 2007;120(5):S94-S138. https://doi.org/10.1016/j.jaci.2007.09.043.
8. Smith M, Iqbal S, Elliott TM, Everard M, Rowe BH. Corticosteroids for hospitalised children with acute asthma. Cochrane Database Syst Rev. 2003(2):CD002886. https://doi.org/10.1002/14651858.CD002886.
9. Pedersen SE, Hurd SS, Lemanske Rf Jr., et al. Global strategy for the diagnosis and management of asthma in children 5 years and younger. Pediatr Pulmonol. 2011;46(1):1-7. https://doi.org/10.1002/ppul.21321.
10. Bacharier LB, Boner A, Carlsen KH, et al. Diagnosis and treatment of asthma in childhood: a PRACTALL consensus report. Allergy. 2008;63(1):5-34. https://doi.org/10.1111/j.1398-9995.2007.01586.x.
11. Foster SJ, Cooper MN, Oosterhof S, Borland ML. Oral prednisolone in preschool children with virus-associated wheeze: a prospective, randomised, double-blind, placebo-controlled trial. Lancet Respir Med. 2018;6(2):97-106. https://doi.org/10.1016/S2213-2600(18)30008-0.
12. Jartti T, Lehtinen P, Vanto T, et al. Evaluation of the efficacy of prednisolone in early wheezing induced by rhinovirus or respiratory syncytial virus. Pediatr Infect Dis J. 2006;25(6):482-488. https://doi.org/10.1097/01.inf.0000215226.69696.0c.
13. Jartti T, Lehtinen P, Vanto T, et al. Atopic characteristics of wheezing children and responses to prednisolone. Pediatr Pulmonol. 2007;42(12):1125-1133. https://doi.org/10.1002/ppul.20706.
14. Oommen A, Lambert PC, Grigg J. Efficacy of a short course of parent-initiated oral prednisolone for viral wheeze in children aged 1–5 years: randomised controlled trial. Lancet. 2003;362(9394):1433-1438. https://doi.org/10.1016/S0140-6736(03)14685-5.
15. Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360(4):329-338. https://doi.org/10.1056/NEJMoa0804897.
16. Tal A, Levy N, Bearman JE. Methylprednisolone therapy for acute asthma in infants and toddlers: a controlled clinical trial. Pediatrics. 1990;86(3):350-356 .
17. Taussig LM, Wright AL, Holberg CJ, Halonen M, Morgan WJ, Martinez FD. Tucson children’s respiratory study: 1980 to present. J Allergy Clin Immunol. 2003;111(4):661-675. https://doi.org/10.1067/mai.2003.162.
18. Illi S, von Mutius E, Lau S, Niggemann B, Grüber C, Wahn U, Multicentre Allergy Study (MAS) group. Perennial allergen sensitisation early in life and chronic asthma in children: a birth cohort study. Lancet. 2006;368(9537):763-770. https://doi.org/10.1016/S0140-6736(06)69286-6.
19. Manson SC, Brown RE, Cerulli A, Vidaurre CF. The cumulative burden of oral corticosteroid side effects and the economic implications of steroid use. Respir Med. 2009;103(7):975-994. https://doi.org/10.1016/j.rmed.2009.01.003.
20. Barra CB, Fontes MJF, Cintra MTG, et al. Oral corticosteroids for asthma exacerbations might be associated with adrenal suppression: are physicians aware of that? Rev Assoc Med Bras. 2017;63(10):899-903. https://doi.org/10.1590/1806-9282.63.10.899..
21. Castro-Rodriguez JA, Holberg CJ, Wright AL, Martinez FD. A clinical index to define risk of asthma in young children with recurrent wheezing. Am J Respir Crit Care Med. 2000;162(4):1403-1406. https://doi.org/10.1164/ajrccm.162.4.9912111.
22. Bush A, Grigg J, Saglani S. Managing wheeze in preschool children. BMJ. 2014;348:g15. https://doi.org/10.1136/bmj.g15.
23. Lukkarinen M, Lukkarinen H, Lehtinen P, Vuorinen T, Ruuskanen O, Jartti T. Prednisolone reduces recurrent wheezing after first rhinovirus wheeze: a 7-year follow-up. Pediatr Allergy Immunol. 2013;24(3):237-243. (1399-3038. https://doi.org/10.1111/pai.12046.
24. Jartti T, Kuusipalo H, Vuorinen T, et al. Allergic sensitization is associated with rhinovirus-, but not other virus-, induced wheezing in children. Pediatr Allergy Immunol. 2010;21(7):1008-1014. https://doi.org/10.1111/j.1399-3038.2010.01059.x.
25. Burbank AJ, Szefler SJ. Current and future management of the young child with early onset wheezing. Curr Opin Allergy Clin Immunol. 2017;17(2):146-152. https://doi.org/10.1097/ACI.0000000000000341
26. Myers JM, Schauberger E, He H, et al. A Pediatric Asthma Risk Score (PARS) to better predict asthma development in young children. J Allergy Clin Immunol. 2018;143(5):1803-1810.e2. https://doi.org/10.1016/j.jaci.2018.09.037.
© 2019 Society of Hospital Medicine
Things We Do For No Reason: HIT Testing in Low Probability Patients
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CLINICAL SCENARIO
A 59-year-old man with cirrhosis secondary to nonalcoholic steatohepatitis was admitted to the intensive care unit (ICU) for management of hepatorenal syndrome and work-up for liver transplantation. On admission, his platelet count was 90 × 109/L (normal 150-400 × 109/L), and he was started on thromboprophylaxis with unfractionated heparin (UFH) 5,000 units subcutaneously twice daily. His platelet count began to fall two days after admission. He did have a history of prior heparin exposure associated with his hemodialysis sessions in the past 30 days. During this period, he also had an episode of fever, and antibiotics were initiated for a presumed line infection. He also required periodic vasopressor support for hypotension. His platelet count reached 14 × 109/L by the end of two weeks. He did not have any symptoms of thrombosis, skin necrosis, or reaction to heparin exposure.
BACKGROUND
Thrombocytopenia is common, especially during critical illness, occurring in up to 50% of patients.1 In this population, thrombocytopenia is often due to sepsis, hemorrhage, liver dysfunction, and drug reactions.1,2 Heparin-induced thrombocytopenia (HIT) is an acquired thrombotic drug reaction resulting from platelet activation secondary to antibodies formed against the heparin-modified platelet factor 4 (PF4) complexes.3 This leads to platelet aggregation and dysregulation of the coagulation cascade, which can result in arterial or venous thromboembolic events in up to 50% of patients.3 Mortality associated with HIT can be as high as 30% in this critically ill population.3 Diagnosis of HIT can be made initially through the enzyme-linked immunosorbent assay (ELISA). Management of HIT involves immediate cessation of heparin and initiation of therapeutic anticoagulation with nonheparin agents in order to prevent or treat the thrombotic events.4,5
The true incidence of HIT remains low, occurring in 0.2% to 5% of patients exposed to heparin and less than 1% in the ICU population.2,3,6,7 However, given the high incidence of thrombocytopenia in the ICU, the diagnosis of HIT is often considered, resulting in over-testing in this population. Studies suggest that more than 200 ELISAs are requested per year at many hospitals.8,9 This can lead to significant clinical and economic consequences.
WHY YOU MIGHT THINK HIT TESTING WITH ELISA IS HELPFUL
Thrombocytopenia is common in hospitalized patients while heparin is frequently used for thromboprophylaxis or therapeutic anticoagulation. As a result, a diagnosis of HIT is often considered.1 The high stakes of the inpatient environment, coupled with the increased frequency of thrombocytopenia and heparin exposure, has led to increased use of HIT testing in this population.10
The most widely available diagnostic test for HIT is the ELISA which detects anti-PF4-heparin antibodies but also nonpathogenic antibodies.11 As a result, the ELISA has a sensitivity close to 100%, allowing physicians to rule out HIT if the test is negative, as indicated by an optical density (OD) of less than 0.4.7 Confirmatory testing with the functional serotonin release assay (SRA) is the reference standard as it confers both a high sensitivity and specificity for HIT.11 Due to technical aspects, SRA, unlike the ELISA, is not available in every center and is often outsourced to external labs. Turn-around time for external SRA testing can vary from days to weeks versus hours for the ELISA. The cost for SRA is approximately $120 (USD) per test compared to $30 (USD) per ELISA. Therefore, the ELISA is the recommended initial test due to its quick turn-around time and lower costs.12,13 For these reasons, the SRA test should not be used initially, but rather to confirm the diagnosis of HIT in patients with a positive ELISA.
WHY YOU SHOULD NOT TEST LOW PROBABILITY PATIENTS FOR HIT
The “4T’s” scoring system is a clinical scoring system that estimates the pretest probability of HIT using clinical and basic laboratory parameters (Table).14 The 4T’s score provides a pretest probability for HIT using four parameters: platelet count, timing of platelet fall, presence of thrombotic events, and the likelihood of another cause of thrombocytopenia. Based on these parameters, the pretest probability for HIT can be divided into three categories: low (4T’s score of ≤3), intermediate (score 4-5), or high (score 6-8).14-16
Validation of the 4T’s score has shown that a low probability score carries a negative predictive value of 99% in a patient population with varying HIT prevalence rates.14 Therefore, having a low score is sufficient to rule out HIT without the need for further laboratory testing.14-16 Although the HIT ELISA confers high sensitivity, due to its detection of nonpathogenic antibodies, its specificity can range from 74% to 84%.15 Therefore, in the setting of a low 4T’s score, HIT testing is not only unnecessary, it can be harmful due to the risk of treating a false positive result. For instance, assuming an average HIT prevalence of 1% and a false positive rate of 16% (specificity 84%), 1/17 (5.6%) patients with a positive ELISA will have HIT if testing is pursued in an indiscriminate manner. The American Society of Hematology Choosing Wisely® Campaign has highlighted this concern by advising physicians that they should “not test or treat for suspected HIT in patients with a low pretest probability of HIT.”17
False positive results on HIT tests are not a trivial concern. The most recognizable adverse event associated with HIT treatment is an elevated risk of bleeding while receiving nonheparin agents. Availability of nonheparin anticoagulants vary by center; however, the most commonly used agents include argatroban, danaparoid, bivalirudin, and off-label fondaparinux.4 Due to its short half-life and hepatic clearance, argatroban is commonly used for cases of confirmed or suspected HIT. A retrospective study assessing the bleeding risk of critically ill patients on argatroban therapy suggests a major bleeding risk of 10% within two days of argatroban initiation.18 In addition, factors such as the presence of elevated bilirubin, major surgery, weight >90 kg, and platelet count <70 × 109/L were found to be associated with increased risk for major bleeding.18 These identified risk factors are very common in the inpatient setting. As a result, monitoring and titration of argatroban can be
Over-diagnosis and over-treatment can also lead to significant costs to the healthcare system. A retrospective study assessing the use of HIT testing found that out of 218 HIT ELISA’s sent over a one-year period at a single institution, 161 (74%) were sent inappropriately (ie, in patients with a low pretest probability), with only one resulting in confirmed HIT by SRA. This incurred an additional cost of $33,000 (USD) for testing alone.8 A retrospective study of 85 patients assessed the costs of treating patients with a false positive HIT assay. They found that the average duration of treatment with a nonheparin agent was three days and the total cost per patient was $982 (USD).19 Treatment with a nonheparin agent such as argatroban costs more than $700 (USD) per day while the continuation of unfractionated heparin for prophylaxis costs less than $10 (USD) per day.20Lastly, a diagnosis of HIT can also result in late consequences due to heparin re-exposure. Clinicians may be wary of exposing patients to heparin in situations where heparin may be the most appropriate agent such as cardiovascular surgery, percutaneous interventions, routine thromboprophylaxis, or therapeutic anticoagulation. In these situations when heparin is the agent of choice, determining safety for re-exposure requires further antibody testing which may delay procedures or result in the use of alternative agents with their associated risks and cost implications.4
WHEN HIT TESTING WITH ELISA MAY BE HELPFUL
Laboratory testing for HIT is appropriate when the pretest probability for HIT is intermediate or high based on the 4T’s score.14-16 Studies assessing the application of the 4T’s score have shown that a moderate or high pretest probability carries a probability of having true HIT in 14% and 64% of the cases respectively.14 However, due to the subjective nature of the 4T’s score components, it is important to recognize that in nonexpert hands, the 4T’s scoring system can suffer from a lack of interrater reliability.16
As discussed above, a negative ELISA (OD < 0.4) helps to rule out HIT and allow heparin to be safely reintroduced without any further testing. If ELISA is positive (OD ≥ 0.4) confirmation testing with SRA should be performed.5 However, studies suggest that the magnitude of the OD is associated with increased likelihood for true HIT, with an OD of greater than 2.00 associated with a positive SRA approximately 90% of the time.21 This suggests that if OD values are strongly positive (≥2.00), SRA can be deferred.5
Due to the SRA limited availability, confirmatory testing is not always possible or in some situations, SRA results may be negative despite a positive OD. In both these cases, discussion with the Hematology service is recommended.
WHAT WE SHOULD DO INSTEAD OF SENDING ELISA
When presented with a case of thrombocytopenia, it is important for clinicians to consider a broad approach in their differential diagnosis. Hospitalists should investigate common etiologies, consider the coagulation parameters, liver enzymes, nutritional status, peripheral blood smear, and a detailed history and physical exam to identify other common potential cause such as sepsis.
The 4T’s score should be applied in patients who have had recent heparin exposure. A score of ≤3 indicates a low pretest probability; therefore, HIT is unlikely and further testing is not needed. A score of ≥4 indicates an intermediate or high pretest probability and should prompt clinicians to consider further HIT testing with ELISA. In these situations, heparin should be held, and nonheparin agents should be initiated to prevent thromboembolic complications. In their study of ICU patients, Pierce et al. found that 17% of patients did not have a concurrent cessation of heparin and initiation of alternative agents despite a high clinical suspicion for HIT.1 Lastly, if hospitalists have concerns regarding HIT testing or management, expert consultation with the Hematology service is recommended.
RECOMMENDATIONS
- Consider a broad differential diagnosis when presented with a hospitalized patient with new thrombocytopenia given the low incidence of HIT (<5%).
- Apply the 4T’s score in those who have thrombocytopenia and recent heparin exposure. A low scores 4T’s score (≤3) predicts a low pretest probability and further testing is not required.
- Patients with moderate or high 4T’s score (≥4) should have the ELISA test. During this time, heparin should be discontinued and nonheparin agents initiated while waiting for test results.
- Confirmatory testing with SRA should be performed for all positive ELISAs; however, they can be deferred in patients with strongly positive OD (≥2.00) on ELISA.
CONCLUSION
In the opening clinical scenario, the 4T’s score would have been 2 (1 point for the platelet count, 1 point for the platelet count fall after 10 days, 0 points for thrombosis, and 0 points for an alternative cause of thrombocytopenia), indicating a low pretest probability. Further HIT testing should be deferred as the likelihood for HIT is low. In this case, the more likely etiology for his thrombocytopenia would be sepsis. Therefore, heparin can be safely reinitiated once the platelet count recovers. This case helps to illustrate the importance of keeping a broad differential in cases of thrombocytopenia in the hospitalized patient while concurrently applying the 4T’s score to determine appropriateness for further HIT testing. Ultimately by choosing wisely, we can help reduce the cost and safety implications of a falsely positive HIT diagnosis.
What do you do?
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
Disclosures
The authors report no conflict of interest.
1. Pierce W, Mazur J, Greenberg C, Mueller J, Foster J, Lazarchick J. Evaluation of heparin-induced thrombocytopenia (HIT) laboratory testing and the 4Ts scoring system in the intensive care unit. Ann Clin Lab Sci. 2013;43(4):429-435. PubMed
2. Harada MY, Hoang DM, Zaw AA, et al. Overtreatment of heparin-induced thrombocytopenia in the surgical ICU. Crit Care Med. 2017;45(1):28-34. doi:10.1097/ccm.0000000000002002. PubMed
3. Warkentin TE, Sheppard JAI, Heels-Ansdell D, et al. Heparin-induced thrombocytopenia in medical-surgical critical illness. Chest. 2013;144(3):848-858. doi: 10.1378/chest.13-0057. PubMed
4. Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia. Chest. 2012;141(2):e495S-e530S. doi: 10.1378/chest.11-2303. PubMed
5. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. doi: 10.1182/bloodadvances.2018024489. PubMed
6. Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4(4):759-765. doi: 10.1111/j.1538-7836.2006.01787.x PubMed
7. Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209-2218. doi: 10.1182/blood-2011-11-376293. PubMed
8. Elmer P, Passero FC, Xavier M. Retrospective Analysis of Heparin-Induced Thrombocytopenia Management at a Large Tertiary Hospital. J Hematol. 2014;3(2):27-33. doi: http://dx.doi.org/10.14740/jh157w.
9. Goldman R, Ustun B, Levine RL. Retrospective cost analysis of testing for HIT antibodies in a community hospital. Blood. 2008;112(11):4544.
10. Cuker A. Heparin-induced thrombocytopenia (HIT) in 2011: an epidemic of overdiagnosis. Thromb Haemost. 2011;106(6):993-994. doi: 10.1160/TH11-09-0677.
11. Warkentin TE. Heparin-induced thrombocytopenia in critically ill patients. Semin Thromb Hemost. 2015;41(5):49-60. doi: 10.1055/s-0034-1398381. PubMed
12. Caton S, O’Brien E, Pannelay AJ, Cook RG. Assessing the clinical and cost impact of on-demand immunoassay testing for the diagnosis of heparin-induced thrombocytopenia. Thromb Res. 2016;140:155-162. doi: 10.1016/j.thromres.2016.01.025 PubMed
13. Nanwa N, Mittmann N, Knowles S, et al. The direct medical costs associated with suspected heparin-induced thrombocytopenia. Pharmacoeconomics. 2011;29(6):511-520. doi: 10.2165/11584330-000000000-00000. PubMed
14. Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. doi: 10.1182/blood-2012-07-443051. PubMed
15. Fiorenza MA, Frazee EN, Personett HA, Dierkhising RA, Schramm GE. Assessment of a modified 4T scoring system for heparin-induced thrombocytopenia in critically ill patients. J Crit Care. 2014;29(3):426-431. doi: 10.1016/j.jcrc.2013.12.010. PubMed
16. Crowther M, Cook D, Guyatt G, et al. Heparin-induced thrombocytopenia in the critically ill: interpreting the 4Ts test in a randomized trial. J Crit Care. 2014;29(3):470.e7-470.e15 doi: 10.1016/j.jcrc.2014.02.004. PubMed
17. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely campaign: five hematologic tests and treatments to question. Blood. 2013;122(24):3879-3883. doi: 10.1182/blood-2013-07-518423. PubMed
18. Doepker B, Mount KL, Ryder LJ, Gerlach AT, Murphy CV, Philips GS. Bleeding risk factors associated with argatroban therapy in the critically ill. J Thromb Thrombolysis. 2012;34(4):491-498. doi: 10.1007/s11239-012-0758-y. PubMed
19. Marler J, Unzaga J, Stelts S, Oliphant CS. Consequences of treating false positive heparin-induced thrombocytopenia. J Thromb Thrombolysis. 2015;40(4):512-514. doi: 10.1007/s11239-015-1236-0. PubMed
20. Fowler RA, Mittmann N, Geerts W, et al. Cost-effectiveness of dalteparin vs unfractionated heparin for the prevention of venous thromboembolism in critically ill patients. JAMA. 2014;312(20):2135-2145. doi: 10.1001/jama.2014.15101. PubMed
21. Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost. 2008;6(8):1304-1312. doi: 10.1111/j.1538-7836.2008.03025.x. PubMed
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CLINICAL SCENARIO
A 59-year-old man with cirrhosis secondary to nonalcoholic steatohepatitis was admitted to the intensive care unit (ICU) for management of hepatorenal syndrome and work-up for liver transplantation. On admission, his platelet count was 90 × 109/L (normal 150-400 × 109/L), and he was started on thromboprophylaxis with unfractionated heparin (UFH) 5,000 units subcutaneously twice daily. His platelet count began to fall two days after admission. He did have a history of prior heparin exposure associated with his hemodialysis sessions in the past 30 days. During this period, he also had an episode of fever, and antibiotics were initiated for a presumed line infection. He also required periodic vasopressor support for hypotension. His platelet count reached 14 × 109/L by the end of two weeks. He did not have any symptoms of thrombosis, skin necrosis, or reaction to heparin exposure.
BACKGROUND
Thrombocytopenia is common, especially during critical illness, occurring in up to 50% of patients.1 In this population, thrombocytopenia is often due to sepsis, hemorrhage, liver dysfunction, and drug reactions.1,2 Heparin-induced thrombocytopenia (HIT) is an acquired thrombotic drug reaction resulting from platelet activation secondary to antibodies formed against the heparin-modified platelet factor 4 (PF4) complexes.3 This leads to platelet aggregation and dysregulation of the coagulation cascade, which can result in arterial or venous thromboembolic events in up to 50% of patients.3 Mortality associated with HIT can be as high as 30% in this critically ill population.3 Diagnosis of HIT can be made initially through the enzyme-linked immunosorbent assay (ELISA). Management of HIT involves immediate cessation of heparin and initiation of therapeutic anticoagulation with nonheparin agents in order to prevent or treat the thrombotic events.4,5
The true incidence of HIT remains low, occurring in 0.2% to 5% of patients exposed to heparin and less than 1% in the ICU population.2,3,6,7 However, given the high incidence of thrombocytopenia in the ICU, the diagnosis of HIT is often considered, resulting in over-testing in this population. Studies suggest that more than 200 ELISAs are requested per year at many hospitals.8,9 This can lead to significant clinical and economic consequences.
WHY YOU MIGHT THINK HIT TESTING WITH ELISA IS HELPFUL
Thrombocytopenia is common in hospitalized patients while heparin is frequently used for thromboprophylaxis or therapeutic anticoagulation. As a result, a diagnosis of HIT is often considered.1 The high stakes of the inpatient environment, coupled with the increased frequency of thrombocytopenia and heparin exposure, has led to increased use of HIT testing in this population.10
The most widely available diagnostic test for HIT is the ELISA which detects anti-PF4-heparin antibodies but also nonpathogenic antibodies.11 As a result, the ELISA has a sensitivity close to 100%, allowing physicians to rule out HIT if the test is negative, as indicated by an optical density (OD) of less than 0.4.7 Confirmatory testing with the functional serotonin release assay (SRA) is the reference standard as it confers both a high sensitivity and specificity for HIT.11 Due to technical aspects, SRA, unlike the ELISA, is not available in every center and is often outsourced to external labs. Turn-around time for external SRA testing can vary from days to weeks versus hours for the ELISA. The cost for SRA is approximately $120 (USD) per test compared to $30 (USD) per ELISA. Therefore, the ELISA is the recommended initial test due to its quick turn-around time and lower costs.12,13 For these reasons, the SRA test should not be used initially, but rather to confirm the diagnosis of HIT in patients with a positive ELISA.
WHY YOU SHOULD NOT TEST LOW PROBABILITY PATIENTS FOR HIT
The “4T’s” scoring system is a clinical scoring system that estimates the pretest probability of HIT using clinical and basic laboratory parameters (Table).14 The 4T’s score provides a pretest probability for HIT using four parameters: platelet count, timing of platelet fall, presence of thrombotic events, and the likelihood of another cause of thrombocytopenia. Based on these parameters, the pretest probability for HIT can be divided into three categories: low (4T’s score of ≤3), intermediate (score 4-5), or high (score 6-8).14-16
Validation of the 4T’s score has shown that a low probability score carries a negative predictive value of 99% in a patient population with varying HIT prevalence rates.14 Therefore, having a low score is sufficient to rule out HIT without the need for further laboratory testing.14-16 Although the HIT ELISA confers high sensitivity, due to its detection of nonpathogenic antibodies, its specificity can range from 74% to 84%.15 Therefore, in the setting of a low 4T’s score, HIT testing is not only unnecessary, it can be harmful due to the risk of treating a false positive result. For instance, assuming an average HIT prevalence of 1% and a false positive rate of 16% (specificity 84%), 1/17 (5.6%) patients with a positive ELISA will have HIT if testing is pursued in an indiscriminate manner. The American Society of Hematology Choosing Wisely® Campaign has highlighted this concern by advising physicians that they should “not test or treat for suspected HIT in patients with a low pretest probability of HIT.”17
False positive results on HIT tests are not a trivial concern. The most recognizable adverse event associated with HIT treatment is an elevated risk of bleeding while receiving nonheparin agents. Availability of nonheparin anticoagulants vary by center; however, the most commonly used agents include argatroban, danaparoid, bivalirudin, and off-label fondaparinux.4 Due to its short half-life and hepatic clearance, argatroban is commonly used for cases of confirmed or suspected HIT. A retrospective study assessing the bleeding risk of critically ill patients on argatroban therapy suggests a major bleeding risk of 10% within two days of argatroban initiation.18 In addition, factors such as the presence of elevated bilirubin, major surgery, weight >90 kg, and platelet count <70 × 109/L were found to be associated with increased risk for major bleeding.18 These identified risk factors are very common in the inpatient setting. As a result, monitoring and titration of argatroban can be
Over-diagnosis and over-treatment can also lead to significant costs to the healthcare system. A retrospective study assessing the use of HIT testing found that out of 218 HIT ELISA’s sent over a one-year period at a single institution, 161 (74%) were sent inappropriately (ie, in patients with a low pretest probability), with only one resulting in confirmed HIT by SRA. This incurred an additional cost of $33,000 (USD) for testing alone.8 A retrospective study of 85 patients assessed the costs of treating patients with a false positive HIT assay. They found that the average duration of treatment with a nonheparin agent was three days and the total cost per patient was $982 (USD).19 Treatment with a nonheparin agent such as argatroban costs more than $700 (USD) per day while the continuation of unfractionated heparin for prophylaxis costs less than $10 (USD) per day.20Lastly, a diagnosis of HIT can also result in late consequences due to heparin re-exposure. Clinicians may be wary of exposing patients to heparin in situations where heparin may be the most appropriate agent such as cardiovascular surgery, percutaneous interventions, routine thromboprophylaxis, or therapeutic anticoagulation. In these situations when heparin is the agent of choice, determining safety for re-exposure requires further antibody testing which may delay procedures or result in the use of alternative agents with their associated risks and cost implications.4
WHEN HIT TESTING WITH ELISA MAY BE HELPFUL
Laboratory testing for HIT is appropriate when the pretest probability for HIT is intermediate or high based on the 4T’s score.14-16 Studies assessing the application of the 4T’s score have shown that a moderate or high pretest probability carries a probability of having true HIT in 14% and 64% of the cases respectively.14 However, due to the subjective nature of the 4T’s score components, it is important to recognize that in nonexpert hands, the 4T’s scoring system can suffer from a lack of interrater reliability.16
As discussed above, a negative ELISA (OD < 0.4) helps to rule out HIT and allow heparin to be safely reintroduced without any further testing. If ELISA is positive (OD ≥ 0.4) confirmation testing with SRA should be performed.5 However, studies suggest that the magnitude of the OD is associated with increased likelihood for true HIT, with an OD of greater than 2.00 associated with a positive SRA approximately 90% of the time.21 This suggests that if OD values are strongly positive (≥2.00), SRA can be deferred.5
Due to the SRA limited availability, confirmatory testing is not always possible or in some situations, SRA results may be negative despite a positive OD. In both these cases, discussion with the Hematology service is recommended.
WHAT WE SHOULD DO INSTEAD OF SENDING ELISA
When presented with a case of thrombocytopenia, it is important for clinicians to consider a broad approach in their differential diagnosis. Hospitalists should investigate common etiologies, consider the coagulation parameters, liver enzymes, nutritional status, peripheral blood smear, and a detailed history and physical exam to identify other common potential cause such as sepsis.
The 4T’s score should be applied in patients who have had recent heparin exposure. A score of ≤3 indicates a low pretest probability; therefore, HIT is unlikely and further testing is not needed. A score of ≥4 indicates an intermediate or high pretest probability and should prompt clinicians to consider further HIT testing with ELISA. In these situations, heparin should be held, and nonheparin agents should be initiated to prevent thromboembolic complications. In their study of ICU patients, Pierce et al. found that 17% of patients did not have a concurrent cessation of heparin and initiation of alternative agents despite a high clinical suspicion for HIT.1 Lastly, if hospitalists have concerns regarding HIT testing or management, expert consultation with the Hematology service is recommended.
RECOMMENDATIONS
- Consider a broad differential diagnosis when presented with a hospitalized patient with new thrombocytopenia given the low incidence of HIT (<5%).
- Apply the 4T’s score in those who have thrombocytopenia and recent heparin exposure. A low scores 4T’s score (≤3) predicts a low pretest probability and further testing is not required.
- Patients with moderate or high 4T’s score (≥4) should have the ELISA test. During this time, heparin should be discontinued and nonheparin agents initiated while waiting for test results.
- Confirmatory testing with SRA should be performed for all positive ELISAs; however, they can be deferred in patients with strongly positive OD (≥2.00) on ELISA.
CONCLUSION
In the opening clinical scenario, the 4T’s score would have been 2 (1 point for the platelet count, 1 point for the platelet count fall after 10 days, 0 points for thrombosis, and 0 points for an alternative cause of thrombocytopenia), indicating a low pretest probability. Further HIT testing should be deferred as the likelihood for HIT is low. In this case, the more likely etiology for his thrombocytopenia would be sepsis. Therefore, heparin can be safely reinitiated once the platelet count recovers. This case helps to illustrate the importance of keeping a broad differential in cases of thrombocytopenia in the hospitalized patient while concurrently applying the 4T’s score to determine appropriateness for further HIT testing. Ultimately by choosing wisely, we can help reduce the cost and safety implications of a falsely positive HIT diagnosis.
What do you do?
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
Disclosures
The authors report no conflict of interest.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CLINICAL SCENARIO
A 59-year-old man with cirrhosis secondary to nonalcoholic steatohepatitis was admitted to the intensive care unit (ICU) for management of hepatorenal syndrome and work-up for liver transplantation. On admission, his platelet count was 90 × 109/L (normal 150-400 × 109/L), and he was started on thromboprophylaxis with unfractionated heparin (UFH) 5,000 units subcutaneously twice daily. His platelet count began to fall two days after admission. He did have a history of prior heparin exposure associated with his hemodialysis sessions in the past 30 days. During this period, he also had an episode of fever, and antibiotics were initiated for a presumed line infection. He also required periodic vasopressor support for hypotension. His platelet count reached 14 × 109/L by the end of two weeks. He did not have any symptoms of thrombosis, skin necrosis, or reaction to heparin exposure.
BACKGROUND
Thrombocytopenia is common, especially during critical illness, occurring in up to 50% of patients.1 In this population, thrombocytopenia is often due to sepsis, hemorrhage, liver dysfunction, and drug reactions.1,2 Heparin-induced thrombocytopenia (HIT) is an acquired thrombotic drug reaction resulting from platelet activation secondary to antibodies formed against the heparin-modified platelet factor 4 (PF4) complexes.3 This leads to platelet aggregation and dysregulation of the coagulation cascade, which can result in arterial or venous thromboembolic events in up to 50% of patients.3 Mortality associated with HIT can be as high as 30% in this critically ill population.3 Diagnosis of HIT can be made initially through the enzyme-linked immunosorbent assay (ELISA). Management of HIT involves immediate cessation of heparin and initiation of therapeutic anticoagulation with nonheparin agents in order to prevent or treat the thrombotic events.4,5
The true incidence of HIT remains low, occurring in 0.2% to 5% of patients exposed to heparin and less than 1% in the ICU population.2,3,6,7 However, given the high incidence of thrombocytopenia in the ICU, the diagnosis of HIT is often considered, resulting in over-testing in this population. Studies suggest that more than 200 ELISAs are requested per year at many hospitals.8,9 This can lead to significant clinical and economic consequences.
WHY YOU MIGHT THINK HIT TESTING WITH ELISA IS HELPFUL
Thrombocytopenia is common in hospitalized patients while heparin is frequently used for thromboprophylaxis or therapeutic anticoagulation. As a result, a diagnosis of HIT is often considered.1 The high stakes of the inpatient environment, coupled with the increased frequency of thrombocytopenia and heparin exposure, has led to increased use of HIT testing in this population.10
The most widely available diagnostic test for HIT is the ELISA which detects anti-PF4-heparin antibodies but also nonpathogenic antibodies.11 As a result, the ELISA has a sensitivity close to 100%, allowing physicians to rule out HIT if the test is negative, as indicated by an optical density (OD) of less than 0.4.7 Confirmatory testing with the functional serotonin release assay (SRA) is the reference standard as it confers both a high sensitivity and specificity for HIT.11 Due to technical aspects, SRA, unlike the ELISA, is not available in every center and is often outsourced to external labs. Turn-around time for external SRA testing can vary from days to weeks versus hours for the ELISA. The cost for SRA is approximately $120 (USD) per test compared to $30 (USD) per ELISA. Therefore, the ELISA is the recommended initial test due to its quick turn-around time and lower costs.12,13 For these reasons, the SRA test should not be used initially, but rather to confirm the diagnosis of HIT in patients with a positive ELISA.
WHY YOU SHOULD NOT TEST LOW PROBABILITY PATIENTS FOR HIT
The “4T’s” scoring system is a clinical scoring system that estimates the pretest probability of HIT using clinical and basic laboratory parameters (Table).14 The 4T’s score provides a pretest probability for HIT using four parameters: platelet count, timing of platelet fall, presence of thrombotic events, and the likelihood of another cause of thrombocytopenia. Based on these parameters, the pretest probability for HIT can be divided into three categories: low (4T’s score of ≤3), intermediate (score 4-5), or high (score 6-8).14-16
Validation of the 4T’s score has shown that a low probability score carries a negative predictive value of 99% in a patient population with varying HIT prevalence rates.14 Therefore, having a low score is sufficient to rule out HIT without the need for further laboratory testing.14-16 Although the HIT ELISA confers high sensitivity, due to its detection of nonpathogenic antibodies, its specificity can range from 74% to 84%.15 Therefore, in the setting of a low 4T’s score, HIT testing is not only unnecessary, it can be harmful due to the risk of treating a false positive result. For instance, assuming an average HIT prevalence of 1% and a false positive rate of 16% (specificity 84%), 1/17 (5.6%) patients with a positive ELISA will have HIT if testing is pursued in an indiscriminate manner. The American Society of Hematology Choosing Wisely® Campaign has highlighted this concern by advising physicians that they should “not test or treat for suspected HIT in patients with a low pretest probability of HIT.”17
False positive results on HIT tests are not a trivial concern. The most recognizable adverse event associated with HIT treatment is an elevated risk of bleeding while receiving nonheparin agents. Availability of nonheparin anticoagulants vary by center; however, the most commonly used agents include argatroban, danaparoid, bivalirudin, and off-label fondaparinux.4 Due to its short half-life and hepatic clearance, argatroban is commonly used for cases of confirmed or suspected HIT. A retrospective study assessing the bleeding risk of critically ill patients on argatroban therapy suggests a major bleeding risk of 10% within two days of argatroban initiation.18 In addition, factors such as the presence of elevated bilirubin, major surgery, weight >90 kg, and platelet count <70 × 109/L were found to be associated with increased risk for major bleeding.18 These identified risk factors are very common in the inpatient setting. As a result, monitoring and titration of argatroban can be
Over-diagnosis and over-treatment can also lead to significant costs to the healthcare system. A retrospective study assessing the use of HIT testing found that out of 218 HIT ELISA’s sent over a one-year period at a single institution, 161 (74%) were sent inappropriately (ie, in patients with a low pretest probability), with only one resulting in confirmed HIT by SRA. This incurred an additional cost of $33,000 (USD) for testing alone.8 A retrospective study of 85 patients assessed the costs of treating patients with a false positive HIT assay. They found that the average duration of treatment with a nonheparin agent was three days and the total cost per patient was $982 (USD).19 Treatment with a nonheparin agent such as argatroban costs more than $700 (USD) per day while the continuation of unfractionated heparin for prophylaxis costs less than $10 (USD) per day.20Lastly, a diagnosis of HIT can also result in late consequences due to heparin re-exposure. Clinicians may be wary of exposing patients to heparin in situations where heparin may be the most appropriate agent such as cardiovascular surgery, percutaneous interventions, routine thromboprophylaxis, or therapeutic anticoagulation. In these situations when heparin is the agent of choice, determining safety for re-exposure requires further antibody testing which may delay procedures or result in the use of alternative agents with their associated risks and cost implications.4
WHEN HIT TESTING WITH ELISA MAY BE HELPFUL
Laboratory testing for HIT is appropriate when the pretest probability for HIT is intermediate or high based on the 4T’s score.14-16 Studies assessing the application of the 4T’s score have shown that a moderate or high pretest probability carries a probability of having true HIT in 14% and 64% of the cases respectively.14 However, due to the subjective nature of the 4T’s score components, it is important to recognize that in nonexpert hands, the 4T’s scoring system can suffer from a lack of interrater reliability.16
As discussed above, a negative ELISA (OD < 0.4) helps to rule out HIT and allow heparin to be safely reintroduced without any further testing. If ELISA is positive (OD ≥ 0.4) confirmation testing with SRA should be performed.5 However, studies suggest that the magnitude of the OD is associated with increased likelihood for true HIT, with an OD of greater than 2.00 associated with a positive SRA approximately 90% of the time.21 This suggests that if OD values are strongly positive (≥2.00), SRA can be deferred.5
Due to the SRA limited availability, confirmatory testing is not always possible or in some situations, SRA results may be negative despite a positive OD. In both these cases, discussion with the Hematology service is recommended.
WHAT WE SHOULD DO INSTEAD OF SENDING ELISA
When presented with a case of thrombocytopenia, it is important for clinicians to consider a broad approach in their differential diagnosis. Hospitalists should investigate common etiologies, consider the coagulation parameters, liver enzymes, nutritional status, peripheral blood smear, and a detailed history and physical exam to identify other common potential cause such as sepsis.
The 4T’s score should be applied in patients who have had recent heparin exposure. A score of ≤3 indicates a low pretest probability; therefore, HIT is unlikely and further testing is not needed. A score of ≥4 indicates an intermediate or high pretest probability and should prompt clinicians to consider further HIT testing with ELISA. In these situations, heparin should be held, and nonheparin agents should be initiated to prevent thromboembolic complications. In their study of ICU patients, Pierce et al. found that 17% of patients did not have a concurrent cessation of heparin and initiation of alternative agents despite a high clinical suspicion for HIT.1 Lastly, if hospitalists have concerns regarding HIT testing or management, expert consultation with the Hematology service is recommended.
RECOMMENDATIONS
- Consider a broad differential diagnosis when presented with a hospitalized patient with new thrombocytopenia given the low incidence of HIT (<5%).
- Apply the 4T’s score in those who have thrombocytopenia and recent heparin exposure. A low scores 4T’s score (≤3) predicts a low pretest probability and further testing is not required.
- Patients with moderate or high 4T’s score (≥4) should have the ELISA test. During this time, heparin should be discontinued and nonheparin agents initiated while waiting for test results.
- Confirmatory testing with SRA should be performed for all positive ELISAs; however, they can be deferred in patients with strongly positive OD (≥2.00) on ELISA.
CONCLUSION
In the opening clinical scenario, the 4T’s score would have been 2 (1 point for the platelet count, 1 point for the platelet count fall after 10 days, 0 points for thrombosis, and 0 points for an alternative cause of thrombocytopenia), indicating a low pretest probability. Further HIT testing should be deferred as the likelihood for HIT is low. In this case, the more likely etiology for his thrombocytopenia would be sepsis. Therefore, heparin can be safely reinitiated once the platelet count recovers. This case helps to illustrate the importance of keeping a broad differential in cases of thrombocytopenia in the hospitalized patient while concurrently applying the 4T’s score to determine appropriateness for further HIT testing. Ultimately by choosing wisely, we can help reduce the cost and safety implications of a falsely positive HIT diagnosis.
What do you do?
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason”? Let us know what you do in your practice and propose ideas for other “Things We Do for No Reason” topics. Please join in the conversation online at Twitter (#TWDFNR)/Facebook and don’t forget to “Like It” on Facebook or retweet it on Twitter.
Disclosures
The authors report no conflict of interest.
1. Pierce W, Mazur J, Greenberg C, Mueller J, Foster J, Lazarchick J. Evaluation of heparin-induced thrombocytopenia (HIT) laboratory testing and the 4Ts scoring system in the intensive care unit. Ann Clin Lab Sci. 2013;43(4):429-435. PubMed
2. Harada MY, Hoang DM, Zaw AA, et al. Overtreatment of heparin-induced thrombocytopenia in the surgical ICU. Crit Care Med. 2017;45(1):28-34. doi:10.1097/ccm.0000000000002002. PubMed
3. Warkentin TE, Sheppard JAI, Heels-Ansdell D, et al. Heparin-induced thrombocytopenia in medical-surgical critical illness. Chest. 2013;144(3):848-858. doi: 10.1378/chest.13-0057. PubMed
4. Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia. Chest. 2012;141(2):e495S-e530S. doi: 10.1378/chest.11-2303. PubMed
5. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. doi: 10.1182/bloodadvances.2018024489. PubMed
6. Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4(4):759-765. doi: 10.1111/j.1538-7836.2006.01787.x PubMed
7. Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209-2218. doi: 10.1182/blood-2011-11-376293. PubMed
8. Elmer P, Passero FC, Xavier M. Retrospective Analysis of Heparin-Induced Thrombocytopenia Management at a Large Tertiary Hospital. J Hematol. 2014;3(2):27-33. doi: http://dx.doi.org/10.14740/jh157w.
9. Goldman R, Ustun B, Levine RL. Retrospective cost analysis of testing for HIT antibodies in a community hospital. Blood. 2008;112(11):4544.
10. Cuker A. Heparin-induced thrombocytopenia (HIT) in 2011: an epidemic of overdiagnosis. Thromb Haemost. 2011;106(6):993-994. doi: 10.1160/TH11-09-0677.
11. Warkentin TE. Heparin-induced thrombocytopenia in critically ill patients. Semin Thromb Hemost. 2015;41(5):49-60. doi: 10.1055/s-0034-1398381. PubMed
12. Caton S, O’Brien E, Pannelay AJ, Cook RG. Assessing the clinical and cost impact of on-demand immunoassay testing for the diagnosis of heparin-induced thrombocytopenia. Thromb Res. 2016;140:155-162. doi: 10.1016/j.thromres.2016.01.025 PubMed
13. Nanwa N, Mittmann N, Knowles S, et al. The direct medical costs associated with suspected heparin-induced thrombocytopenia. Pharmacoeconomics. 2011;29(6):511-520. doi: 10.2165/11584330-000000000-00000. PubMed
14. Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. doi: 10.1182/blood-2012-07-443051. PubMed
15. Fiorenza MA, Frazee EN, Personett HA, Dierkhising RA, Schramm GE. Assessment of a modified 4T scoring system for heparin-induced thrombocytopenia in critically ill patients. J Crit Care. 2014;29(3):426-431. doi: 10.1016/j.jcrc.2013.12.010. PubMed
16. Crowther M, Cook D, Guyatt G, et al. Heparin-induced thrombocytopenia in the critically ill: interpreting the 4Ts test in a randomized trial. J Crit Care. 2014;29(3):470.e7-470.e15 doi: 10.1016/j.jcrc.2014.02.004. PubMed
17. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely campaign: five hematologic tests and treatments to question. Blood. 2013;122(24):3879-3883. doi: 10.1182/blood-2013-07-518423. PubMed
18. Doepker B, Mount KL, Ryder LJ, Gerlach AT, Murphy CV, Philips GS. Bleeding risk factors associated with argatroban therapy in the critically ill. J Thromb Thrombolysis. 2012;34(4):491-498. doi: 10.1007/s11239-012-0758-y. PubMed
19. Marler J, Unzaga J, Stelts S, Oliphant CS. Consequences of treating false positive heparin-induced thrombocytopenia. J Thromb Thrombolysis. 2015;40(4):512-514. doi: 10.1007/s11239-015-1236-0. PubMed
20. Fowler RA, Mittmann N, Geerts W, et al. Cost-effectiveness of dalteparin vs unfractionated heparin for the prevention of venous thromboembolism in critically ill patients. JAMA. 2014;312(20):2135-2145. doi: 10.1001/jama.2014.15101. PubMed
21. Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost. 2008;6(8):1304-1312. doi: 10.1111/j.1538-7836.2008.03025.x. PubMed
1. Pierce W, Mazur J, Greenberg C, Mueller J, Foster J, Lazarchick J. Evaluation of heparin-induced thrombocytopenia (HIT) laboratory testing and the 4Ts scoring system in the intensive care unit. Ann Clin Lab Sci. 2013;43(4):429-435. PubMed
2. Harada MY, Hoang DM, Zaw AA, et al. Overtreatment of heparin-induced thrombocytopenia in the surgical ICU. Crit Care Med. 2017;45(1):28-34. doi:10.1097/ccm.0000000000002002. PubMed
3. Warkentin TE, Sheppard JAI, Heels-Ansdell D, et al. Heparin-induced thrombocytopenia in medical-surgical critical illness. Chest. 2013;144(3):848-858. doi: 10.1378/chest.13-0057. PubMed
4. Linkins LA, Dans AL, Moores LK, et al. Treatment and prevention of heparin-induced thrombocytopenia. Chest. 2012;141(2):e495S-e530S. doi: 10.1378/chest.11-2303. PubMed
5. Cuker A, Arepally GM, Chong BH, et al. American Society of Hematology 2018 guidelines for management of venous thromboembolism: heparin-induced thrombocytopenia. Blood Adv. 2018;2(22):3360-3392. doi: 10.1182/bloodadvances.2018024489. PubMed
6. Lo GK, Juhl D, Warkentin TE, Sigouin CS, Eichler P, Greinacher A. Evaluation of pretest clinical score (4 T’s) for the diagnosis of heparin-induced thrombocytopenia in two clinical settings. J Thromb Haemost. 2006;4(4):759-765. doi: 10.1111/j.1538-7836.2006.01787.x PubMed
7. Cuker A, Cines DB. How I treat heparin-induced thrombocytopenia. Blood. 2012;119(10):2209-2218. doi: 10.1182/blood-2011-11-376293. PubMed
8. Elmer P, Passero FC, Xavier M. Retrospective Analysis of Heparin-Induced Thrombocytopenia Management at a Large Tertiary Hospital. J Hematol. 2014;3(2):27-33. doi: http://dx.doi.org/10.14740/jh157w.
9. Goldman R, Ustun B, Levine RL. Retrospective cost analysis of testing for HIT antibodies in a community hospital. Blood. 2008;112(11):4544.
10. Cuker A. Heparin-induced thrombocytopenia (HIT) in 2011: an epidemic of overdiagnosis. Thromb Haemost. 2011;106(6):993-994. doi: 10.1160/TH11-09-0677.
11. Warkentin TE. Heparin-induced thrombocytopenia in critically ill patients. Semin Thromb Hemost. 2015;41(5):49-60. doi: 10.1055/s-0034-1398381. PubMed
12. Caton S, O’Brien E, Pannelay AJ, Cook RG. Assessing the clinical and cost impact of on-demand immunoassay testing for the diagnosis of heparin-induced thrombocytopenia. Thromb Res. 2016;140:155-162. doi: 10.1016/j.thromres.2016.01.025 PubMed
13. Nanwa N, Mittmann N, Knowles S, et al. The direct medical costs associated with suspected heparin-induced thrombocytopenia. Pharmacoeconomics. 2011;29(6):511-520. doi: 10.2165/11584330-000000000-00000. PubMed
14. Cuker A, Gimotty PA, Crowther MA, Warkentin TE. Predictive value of the 4Ts scoring system for heparin-induced thrombocytopenia: a systematic review and meta-analysis. Blood. 2012;120(20):4160-4167. doi: 10.1182/blood-2012-07-443051. PubMed
15. Fiorenza MA, Frazee EN, Personett HA, Dierkhising RA, Schramm GE. Assessment of a modified 4T scoring system for heparin-induced thrombocytopenia in critically ill patients. J Crit Care. 2014;29(3):426-431. doi: 10.1016/j.jcrc.2013.12.010. PubMed
16. Crowther M, Cook D, Guyatt G, et al. Heparin-induced thrombocytopenia in the critically ill: interpreting the 4Ts test in a randomized trial. J Crit Care. 2014;29(3):470.e7-470.e15 doi: 10.1016/j.jcrc.2014.02.004. PubMed
17. Hicks LK, Bering H, Carson KR, et al. The ASH Choosing Wisely campaign: five hematologic tests and treatments to question. Blood. 2013;122(24):3879-3883. doi: 10.1182/blood-2013-07-518423. PubMed
18. Doepker B, Mount KL, Ryder LJ, Gerlach AT, Murphy CV, Philips GS. Bleeding risk factors associated with argatroban therapy in the critically ill. J Thromb Thrombolysis. 2012;34(4):491-498. doi: 10.1007/s11239-012-0758-y. PubMed
19. Marler J, Unzaga J, Stelts S, Oliphant CS. Consequences of treating false positive heparin-induced thrombocytopenia. J Thromb Thrombolysis. 2015;40(4):512-514. doi: 10.1007/s11239-015-1236-0. PubMed
20. Fowler RA, Mittmann N, Geerts W, et al. Cost-effectiveness of dalteparin vs unfractionated heparin for the prevention of venous thromboembolism in critically ill patients. JAMA. 2014;312(20):2135-2145. doi: 10.1001/jama.2014.15101. PubMed
21. Warkentin TE, Sheppard JI, Moore JC, Sigouin CS, Kelton JG. Quantitative interpretation of optical density measurements using PF4-dependent enzyme-immunoassays. J Thromb Haemost. 2008;6(8):1304-1312. doi: 10.1111/j.1538-7836.2008.03025.x. PubMed
© 2019 Society of Hospital Medicine
Things We Do For No Reason: Prealbumin Testing to Diagnose Malnutrition in the Hospitalized Patient
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 34-year-old man is admitted for a complicated urinary tract infection related to a chronic in-dwelling Foley catheter. The patient suffered a spinal cord injury at the C4/C5 level as a result of a motor vehicle accident 10 years ago and is confined to a motorized wheelchair. He is an engineer and lives independently but has caregivers. His body mass index (BMI) is 18.5 kg/m2, and he reports his weight has been stable. He has slight muscle atrophy of the biceps, triceps, interosseous muscles, and quadriceps. The patient reports that he eats well, has no chronic conditions, and has not had any gastrointestinal symptoms (eg, anorexia, nausea, diarrhea) over the last six months. You consider whether to order a serum prealbumin test to assess for possible malnutrition.
BACKGROUND
The presence of malnutrition in hospitalized patients is widely recognized as an independent predictor of hospital mortality.1 According to the American Society for Parenteral and Enteral Nutrition (ASPEN), malnutrition is defined as “an acute, subacute or chronic state of nutrition, in which varying degrees of overnutrition or undernutrition with or without inflammatory activity have led to a change in body composition and diminished function.”2 In one large European study, patients screening positive for being at risk of malnutrition had a 12-fold increase in hospital mortality.1
Inpatient malnutrition is remarkably underdocumented. Studies using chart reviews have found a prevalence of malnutrition in hospitalized patients of between 20% and 50%, and only 3% of hospital discharges are associated with a diagnostic code for malnutrition.3–5 Appropriate diagnosis and documentation of malnutrition is important given the profound prognostic and management implications of a malnutrition diagnosis. Appropriate documentation benefits health systems as malnutrition documentation increases expected mortality, thereby improving the observed-to-expected mortality ratio.
Serum prealbumin testing is widely available and frequently ordered in the inpatient setting. In a query we performed of the large aggregate Cerner Electronic Health Record database, HealthFacts, which includes data from inpatient encounters for approximately 700 United States hospitals, prealbumin tests were ordered 129,152 times in 2015. This activity corresponds to estimated total charges of $2,562,375 based on the 2015 clinical laboratory fee schedule.6
WHY YOU MIGHT THINK PREALBUMIN DIAGNOSES MALNUTRITION
Prealbumin is synthesized in the liver and released into circulation prior to excretion by the kidneys and gastrointestinal tract. Prealbumin transports thyroxine, triiodothyronine, and holo-retinol binding protein and, as a result, is also known as transthyretin.7 It was first proposed as a nutritional marker in 1972 with the publication of a study that showed low levels of prealbumin in 40 children with kwashiorkor that improved with intensive dietary supplementation.8 The shorter half-life of prealbumin (2.5 days) as compared with other identified nutritional markers, such as albumin, indicate that it would be suitable for detecting rapid changes in nutritional status.
WHY PREALBUMIN IS NOT HELPFUL FOR DIAGNOSING MALNUTRITION
Prealbumin Is Not Specific
An ideal nutritional marker should be specific enough that changes in this marker reflect changes in nutritional status.9 While there are many systemic factors that affect nutritional markers, such as prealbumin (Table 1), the acute phase response triggered by inflammation is the most significant confounder in the acutely ill hospitalized patient.9 This response to infection, stress, and malignancy leads to an increase in proinflammatory cytokines, increased liver synthesis of inflammatory proteins, such as C-reactive protein (CRP), and increased vascular permeability. Prealbumin is a negative acute phase reactant that decreases in concentration during the stress response due to slowed synthesis and extravasation.9 In a study of 24 patients with severe sepsis and trauma, levels of prealbumin inversely correlated with CRP, a reflection of the stress response, and returned to normal when CRP levels normalized. Neither prealbumin nor CRP, however, correlated with total body protein changes.10 Unfortunately, many studies supporting the use of prealbumin as a nutritional marker do not address the role of the acute phase response in their results. These studies include the original report on prealbumin in kwashiorkor, a condition known to be associated with a high rate of infectious diseases that can trigger the acute phase response.9 A consensus statement from the Academy of Nutrition and Dietetics (AND) and ASPEN noted that prealbumin is an indicator of inflammation and lacks the specificity to diagnose malnutrition.11
Prealbumin Is Not Sensitive
A sensitive laboratory test for malnutrition should allow for detection of malnutrition at an early stage.9 However, patients who demonstrate severe malnutrition without a coexisting inflammatory state do not consistently show low levels of prealbumin. In a systematic review of 20 studies in nondiseased malnourished patients, only two studies, both of which assessed patients with anorexia nervosa, had a mean prealbumin below normal (<20 mg/dL), and this finding corresponded to patient populations with mean BMIs less than 12 kg/m2. More importantly, normal prealbumin levels were seen in groups of patients with a mean BMI as low as 12.9 kg/m2.12 Analysis by AND found insufficient evidence to support a correlation between prealbumin and weight loss in anorexia nervosa, calorie restricted diets, or starvation.13 The data suggest that prealbumin lacks sufficient sensitivity to consistently detect cases of malnutrition easily diagnosed by history and/or physical exam.
Prealbumin Is Not Consistently Responsive to Nutritional Interventions
An accurate marker for malnutrition should improve when nutritional intervention results in adequate nutritional intake.9 While some studies have shown improvements in prealbumin in the setting of a nutritional intervention, many of these works are subject to the same limitations related to specificity and lack of control for concurrent inflammatory processes. In a retrospective study, prealbumin increased significantly in 102 patients receiving TPN for one week. Unfortunately, patients with renal or hepatic disease were excluded, and the role of inflammation was not assessed.14 Institutionalized patients with Alzheimer’s disease and normal CRP levels showed a statistically significant increase in weight gain, arm muscle circumference, and triceps skin-fold thickness following a nutritional program without a notable change in prealbumin.15 In a study assessing the relationship of prealbumin, CRP, and nutritional intake, critically ill populations receiving less than or greater than 60% of their estimated caloric needs showed no significant difference in prealbumin. In fact, prealbumin levels were only correlated with CRP levels.16 This finding argues against the routine use of prealbumin for nutrition monitoring in the acutely ill hospitalized patient.
Prealbumin Is Not Consistently Correlated with Health Outcomes
Even if prealbumin increased consistently in response to nutritional intervention, whether this change corresponds to an improvement in clinical outcomes has yet to be demonstrated.9 In 2005, Koretz reviewed 99 clinical trials and concluded that even when changes in nutritional markers are seen with nutritional support, the “changes in nutritional markers do not predict clinical outcomes.”17
WHAT YOU SHOULD DO INSTEAD: USE NONBIOLOGIC METHODS FOR SCREENING AND DIAGNOSING MALNUTRITION
Given the lack of a suitable biologic assay to identify malnutrition, dieticians and clinicians must rely on other means to assess malnutrition. Professional societies, including ASPEN and the European Society for Clinical Nutrition and Metabolism, have proposed different guidelines for the screening and assessment of malnutrition (Table 2).11,18 In 2016, these organizations, along with the Latin American Federation of Nutritional Therapy, Clinical Nutrition, and Metabolism and the Parenteral and Enteral Nutrition Society of Asia, formed The Global Leadership Initiative on Malnutrition (GLIM). In 2017, the GLIM taskforce agreed on clinically relevant diagnostic variables for the screening and assessment of malnutrition, including reduced food intake (anorexia), nonvolitional weight loss, (reduced) lean mass, status of disease burden and inflammation, and low body mass index or underweight status.19
RECOMMENDATIONS
- Do not use prealbumin to screen for or diagnose malnutrition.
- Consult with local dietitians to ensure that your institutional approach is in agreement with consensus recommendations.
CONCLUSION
In revisiting the case above, the patient does not have clear evidence of malnutrition based on his history (stable weight and good reported nutritional intake), although he does have a low BMI of 18.5 kg/m2. Rather than prealbumin testing, which would likely be low secondary to the acute phase response, he would better benefit from a nutrition-focused history and physical exam.
The uncertainties faced by clinicians in diagnosing malnutrition cannot readily be resolved by relying on a solitary laboratory marker (eg, prealbumin) or a stand-alone assessment protocol. The data obtained reflect the need for multidisciplinary teams of dieticians and clinicians to contextualize each patient’s medical history and ensure that the selected metrics are used appropriately to aid in diagnosis and documentation. We advocate that clinicians not routinely use prealbumin to screen for, confirm the diagnosis of, or assess the severity of malnutrition in the hospitalized patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
1. Sorensen J, Kondrup J, Prokopowicz J, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr Edinb Scotl. 2008;27(3):340-349. PubMed
2. Mueller C, Compher C, Ellen DM, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35(1):16-24. PubMed
3. Kaiser MJ, Bauer JM, Rämsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734-1738. PubMed
4. Robinson MK, Trujillo EB, Mogensen KM, Rounds J, McManus K, Jacobs DO. Improving nutritional screening of hospitalized patients: the role of prealbumin. JPEN J Parenter Enteral Nutr. 2003;27(6):389-395; quiz 439. PubMed
5. Corkins MR, Guenter P, DiMaria-Ghalili RA, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. 2014;38(2):186-195. PubMed
6. Clinical Laboratory Fee Schedule Files. cms.org. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Published September 29, 2016. Accessed January 5, 2018.
7. Myron Johnson A, Merlini G, Sheldon J, Ichihara K, Scientific Division Committee on Plasma Proteins (C-PP), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419-426. PubMed
8. Ingenbleek Y, De Visscher M, De Nayer P. Measurement of prealbumin as index of protein-calorie malnutrition. Lancet. 1972;2(7768):106-109. PubMed
9. Barbosa-Silva MCG. Subjective and objective nutritional assessment methods: what do they really assess? Curr Opin Clin Nutr Metab Care. 2008;11(3):248-254. PubMed
10. Clark MA, Hentzen BTH, Plank LD, Hill GL. Sequential changes in insulin-like growth factor 1, plasma proteins, and total body protein in severe sepsis and multiple injury. J Parenter Enter Nutr. 1996;20(5):363-370. PubMed
11. White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. PubMed
12. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-22. PubMed
13. Academy of Nutrition and Dietetics Evidence Analysis Library. Nutrition Screening (NSCR) Systematic Review (2009-2010). https://www.andeal.org/tmp/pdf-print-919C51237950859AE3E15F978CEF49D8.pdf. Accessed August 23, 2017.
14. Sawicky CP, Nippo J, Winkler MF, Albina JE. Adequate energy intake and improved prealbumin concentration as indicators of the response to total parenteral nutrition. J Am Diet Assoc. 1992;92(10):1266-1268. PubMed
15. Van Wymelbeke V, Guédon A, Maniere D, Manckoundia P, Pfitzenmeyer P. A 6-month follow-up of nutritional status in institutionalized patients with Alzheimer’s disease. J Nutr Health Aging. 2004;8(6):505-508. PubMed
16. Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr. 2012;36(2):197-204. PubMed
17. Koretz RL. Death, morbidity and economics are the only end points for trials. Proc Nutr Soc. 2005;64(3):277-284. PubMed
18. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr Edinb Scotl. 2015;34(3):335-340. PubMed
19. Jensen GL, Cederholm T. Global leadership initiative on malnutrition: progress report from ASPEN clinical nutrition week 2017. JPEN J Parenter Enteral Nutr. April 2017:148607117707761. PubMed
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 34-year-old man is admitted for a complicated urinary tract infection related to a chronic in-dwelling Foley catheter. The patient suffered a spinal cord injury at the C4/C5 level as a result of a motor vehicle accident 10 years ago and is confined to a motorized wheelchair. He is an engineer and lives independently but has caregivers. His body mass index (BMI) is 18.5 kg/m2, and he reports his weight has been stable. He has slight muscle atrophy of the biceps, triceps, interosseous muscles, and quadriceps. The patient reports that he eats well, has no chronic conditions, and has not had any gastrointestinal symptoms (eg, anorexia, nausea, diarrhea) over the last six months. You consider whether to order a serum prealbumin test to assess for possible malnutrition.
BACKGROUND
The presence of malnutrition in hospitalized patients is widely recognized as an independent predictor of hospital mortality.1 According to the American Society for Parenteral and Enteral Nutrition (ASPEN), malnutrition is defined as “an acute, subacute or chronic state of nutrition, in which varying degrees of overnutrition or undernutrition with or without inflammatory activity have led to a change in body composition and diminished function.”2 In one large European study, patients screening positive for being at risk of malnutrition had a 12-fold increase in hospital mortality.1
Inpatient malnutrition is remarkably underdocumented. Studies using chart reviews have found a prevalence of malnutrition in hospitalized patients of between 20% and 50%, and only 3% of hospital discharges are associated with a diagnostic code for malnutrition.3–5 Appropriate diagnosis and documentation of malnutrition is important given the profound prognostic and management implications of a malnutrition diagnosis. Appropriate documentation benefits health systems as malnutrition documentation increases expected mortality, thereby improving the observed-to-expected mortality ratio.
Serum prealbumin testing is widely available and frequently ordered in the inpatient setting. In a query we performed of the large aggregate Cerner Electronic Health Record database, HealthFacts, which includes data from inpatient encounters for approximately 700 United States hospitals, prealbumin tests were ordered 129,152 times in 2015. This activity corresponds to estimated total charges of $2,562,375 based on the 2015 clinical laboratory fee schedule.6
WHY YOU MIGHT THINK PREALBUMIN DIAGNOSES MALNUTRITION
Prealbumin is synthesized in the liver and released into circulation prior to excretion by the kidneys and gastrointestinal tract. Prealbumin transports thyroxine, triiodothyronine, and holo-retinol binding protein and, as a result, is also known as transthyretin.7 It was first proposed as a nutritional marker in 1972 with the publication of a study that showed low levels of prealbumin in 40 children with kwashiorkor that improved with intensive dietary supplementation.8 The shorter half-life of prealbumin (2.5 days) as compared with other identified nutritional markers, such as albumin, indicate that it would be suitable for detecting rapid changes in nutritional status.
WHY PREALBUMIN IS NOT HELPFUL FOR DIAGNOSING MALNUTRITION
Prealbumin Is Not Specific
An ideal nutritional marker should be specific enough that changes in this marker reflect changes in nutritional status.9 While there are many systemic factors that affect nutritional markers, such as prealbumin (Table 1), the acute phase response triggered by inflammation is the most significant confounder in the acutely ill hospitalized patient.9 This response to infection, stress, and malignancy leads to an increase in proinflammatory cytokines, increased liver synthesis of inflammatory proteins, such as C-reactive protein (CRP), and increased vascular permeability. Prealbumin is a negative acute phase reactant that decreases in concentration during the stress response due to slowed synthesis and extravasation.9 In a study of 24 patients with severe sepsis and trauma, levels of prealbumin inversely correlated with CRP, a reflection of the stress response, and returned to normal when CRP levels normalized. Neither prealbumin nor CRP, however, correlated with total body protein changes.10 Unfortunately, many studies supporting the use of prealbumin as a nutritional marker do not address the role of the acute phase response in their results. These studies include the original report on prealbumin in kwashiorkor, a condition known to be associated with a high rate of infectious diseases that can trigger the acute phase response.9 A consensus statement from the Academy of Nutrition and Dietetics (AND) and ASPEN noted that prealbumin is an indicator of inflammation and lacks the specificity to diagnose malnutrition.11
Prealbumin Is Not Sensitive
A sensitive laboratory test for malnutrition should allow for detection of malnutrition at an early stage.9 However, patients who demonstrate severe malnutrition without a coexisting inflammatory state do not consistently show low levels of prealbumin. In a systematic review of 20 studies in nondiseased malnourished patients, only two studies, both of which assessed patients with anorexia nervosa, had a mean prealbumin below normal (<20 mg/dL), and this finding corresponded to patient populations with mean BMIs less than 12 kg/m2. More importantly, normal prealbumin levels were seen in groups of patients with a mean BMI as low as 12.9 kg/m2.12 Analysis by AND found insufficient evidence to support a correlation between prealbumin and weight loss in anorexia nervosa, calorie restricted diets, or starvation.13 The data suggest that prealbumin lacks sufficient sensitivity to consistently detect cases of malnutrition easily diagnosed by history and/or physical exam.
Prealbumin Is Not Consistently Responsive to Nutritional Interventions
An accurate marker for malnutrition should improve when nutritional intervention results in adequate nutritional intake.9 While some studies have shown improvements in prealbumin in the setting of a nutritional intervention, many of these works are subject to the same limitations related to specificity and lack of control for concurrent inflammatory processes. In a retrospective study, prealbumin increased significantly in 102 patients receiving TPN for one week. Unfortunately, patients with renal or hepatic disease were excluded, and the role of inflammation was not assessed.14 Institutionalized patients with Alzheimer’s disease and normal CRP levels showed a statistically significant increase in weight gain, arm muscle circumference, and triceps skin-fold thickness following a nutritional program without a notable change in prealbumin.15 In a study assessing the relationship of prealbumin, CRP, and nutritional intake, critically ill populations receiving less than or greater than 60% of their estimated caloric needs showed no significant difference in prealbumin. In fact, prealbumin levels were only correlated with CRP levels.16 This finding argues against the routine use of prealbumin for nutrition monitoring in the acutely ill hospitalized patient.
Prealbumin Is Not Consistently Correlated with Health Outcomes
Even if prealbumin increased consistently in response to nutritional intervention, whether this change corresponds to an improvement in clinical outcomes has yet to be demonstrated.9 In 2005, Koretz reviewed 99 clinical trials and concluded that even when changes in nutritional markers are seen with nutritional support, the “changes in nutritional markers do not predict clinical outcomes.”17
WHAT YOU SHOULD DO INSTEAD: USE NONBIOLOGIC METHODS FOR SCREENING AND DIAGNOSING MALNUTRITION
Given the lack of a suitable biologic assay to identify malnutrition, dieticians and clinicians must rely on other means to assess malnutrition. Professional societies, including ASPEN and the European Society for Clinical Nutrition and Metabolism, have proposed different guidelines for the screening and assessment of malnutrition (Table 2).11,18 In 2016, these organizations, along with the Latin American Federation of Nutritional Therapy, Clinical Nutrition, and Metabolism and the Parenteral and Enteral Nutrition Society of Asia, formed The Global Leadership Initiative on Malnutrition (GLIM). In 2017, the GLIM taskforce agreed on clinically relevant diagnostic variables for the screening and assessment of malnutrition, including reduced food intake (anorexia), nonvolitional weight loss, (reduced) lean mass, status of disease burden and inflammation, and low body mass index or underweight status.19
RECOMMENDATIONS
- Do not use prealbumin to screen for or diagnose malnutrition.
- Consult with local dietitians to ensure that your institutional approach is in agreement with consensus recommendations.
CONCLUSION
In revisiting the case above, the patient does not have clear evidence of malnutrition based on his history (stable weight and good reported nutritional intake), although he does have a low BMI of 18.5 kg/m2. Rather than prealbumin testing, which would likely be low secondary to the acute phase response, he would better benefit from a nutrition-focused history and physical exam.
The uncertainties faced by clinicians in diagnosing malnutrition cannot readily be resolved by relying on a solitary laboratory marker (eg, prealbumin) or a stand-alone assessment protocol. The data obtained reflect the need for multidisciplinary teams of dieticians and clinicians to contextualize each patient’s medical history and ensure that the selected metrics are used appropriately to aid in diagnosis and documentation. We advocate that clinicians not routinely use prealbumin to screen for, confirm the diagnosis of, or assess the severity of malnutrition in the hospitalized patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
The “Things We Do for No Reason” series reviews practices which have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE PRESENTATION
A 34-year-old man is admitted for a complicated urinary tract infection related to a chronic in-dwelling Foley catheter. The patient suffered a spinal cord injury at the C4/C5 level as a result of a motor vehicle accident 10 years ago and is confined to a motorized wheelchair. He is an engineer and lives independently but has caregivers. His body mass index (BMI) is 18.5 kg/m2, and he reports his weight has been stable. He has slight muscle atrophy of the biceps, triceps, interosseous muscles, and quadriceps. The patient reports that he eats well, has no chronic conditions, and has not had any gastrointestinal symptoms (eg, anorexia, nausea, diarrhea) over the last six months. You consider whether to order a serum prealbumin test to assess for possible malnutrition.
BACKGROUND
The presence of malnutrition in hospitalized patients is widely recognized as an independent predictor of hospital mortality.1 According to the American Society for Parenteral and Enteral Nutrition (ASPEN), malnutrition is defined as “an acute, subacute or chronic state of nutrition, in which varying degrees of overnutrition or undernutrition with or without inflammatory activity have led to a change in body composition and diminished function.”2 In one large European study, patients screening positive for being at risk of malnutrition had a 12-fold increase in hospital mortality.1
Inpatient malnutrition is remarkably underdocumented. Studies using chart reviews have found a prevalence of malnutrition in hospitalized patients of between 20% and 50%, and only 3% of hospital discharges are associated with a diagnostic code for malnutrition.3–5 Appropriate diagnosis and documentation of malnutrition is important given the profound prognostic and management implications of a malnutrition diagnosis. Appropriate documentation benefits health systems as malnutrition documentation increases expected mortality, thereby improving the observed-to-expected mortality ratio.
Serum prealbumin testing is widely available and frequently ordered in the inpatient setting. In a query we performed of the large aggregate Cerner Electronic Health Record database, HealthFacts, which includes data from inpatient encounters for approximately 700 United States hospitals, prealbumin tests were ordered 129,152 times in 2015. This activity corresponds to estimated total charges of $2,562,375 based on the 2015 clinical laboratory fee schedule.6
WHY YOU MIGHT THINK PREALBUMIN DIAGNOSES MALNUTRITION
Prealbumin is synthesized in the liver and released into circulation prior to excretion by the kidneys and gastrointestinal tract. Prealbumin transports thyroxine, triiodothyronine, and holo-retinol binding protein and, as a result, is also known as transthyretin.7 It was first proposed as a nutritional marker in 1972 with the publication of a study that showed low levels of prealbumin in 40 children with kwashiorkor that improved with intensive dietary supplementation.8 The shorter half-life of prealbumin (2.5 days) as compared with other identified nutritional markers, such as albumin, indicate that it would be suitable for detecting rapid changes in nutritional status.
WHY PREALBUMIN IS NOT HELPFUL FOR DIAGNOSING MALNUTRITION
Prealbumin Is Not Specific
An ideal nutritional marker should be specific enough that changes in this marker reflect changes in nutritional status.9 While there are many systemic factors that affect nutritional markers, such as prealbumin (Table 1), the acute phase response triggered by inflammation is the most significant confounder in the acutely ill hospitalized patient.9 This response to infection, stress, and malignancy leads to an increase in proinflammatory cytokines, increased liver synthesis of inflammatory proteins, such as C-reactive protein (CRP), and increased vascular permeability. Prealbumin is a negative acute phase reactant that decreases in concentration during the stress response due to slowed synthesis and extravasation.9 In a study of 24 patients with severe sepsis and trauma, levels of prealbumin inversely correlated with CRP, a reflection of the stress response, and returned to normal when CRP levels normalized. Neither prealbumin nor CRP, however, correlated with total body protein changes.10 Unfortunately, many studies supporting the use of prealbumin as a nutritional marker do not address the role of the acute phase response in their results. These studies include the original report on prealbumin in kwashiorkor, a condition known to be associated with a high rate of infectious diseases that can trigger the acute phase response.9 A consensus statement from the Academy of Nutrition and Dietetics (AND) and ASPEN noted that prealbumin is an indicator of inflammation and lacks the specificity to diagnose malnutrition.11
Prealbumin Is Not Sensitive
A sensitive laboratory test for malnutrition should allow for detection of malnutrition at an early stage.9 However, patients who demonstrate severe malnutrition without a coexisting inflammatory state do not consistently show low levels of prealbumin. In a systematic review of 20 studies in nondiseased malnourished patients, only two studies, both of which assessed patients with anorexia nervosa, had a mean prealbumin below normal (<20 mg/dL), and this finding corresponded to patient populations with mean BMIs less than 12 kg/m2. More importantly, normal prealbumin levels were seen in groups of patients with a mean BMI as low as 12.9 kg/m2.12 Analysis by AND found insufficient evidence to support a correlation between prealbumin and weight loss in anorexia nervosa, calorie restricted diets, or starvation.13 The data suggest that prealbumin lacks sufficient sensitivity to consistently detect cases of malnutrition easily diagnosed by history and/or physical exam.
Prealbumin Is Not Consistently Responsive to Nutritional Interventions
An accurate marker for malnutrition should improve when nutritional intervention results in adequate nutritional intake.9 While some studies have shown improvements in prealbumin in the setting of a nutritional intervention, many of these works are subject to the same limitations related to specificity and lack of control for concurrent inflammatory processes. In a retrospective study, prealbumin increased significantly in 102 patients receiving TPN for one week. Unfortunately, patients with renal or hepatic disease were excluded, and the role of inflammation was not assessed.14 Institutionalized patients with Alzheimer’s disease and normal CRP levels showed a statistically significant increase in weight gain, arm muscle circumference, and triceps skin-fold thickness following a nutritional program without a notable change in prealbumin.15 In a study assessing the relationship of prealbumin, CRP, and nutritional intake, critically ill populations receiving less than or greater than 60% of their estimated caloric needs showed no significant difference in prealbumin. In fact, prealbumin levels were only correlated with CRP levels.16 This finding argues against the routine use of prealbumin for nutrition monitoring in the acutely ill hospitalized patient.
Prealbumin Is Not Consistently Correlated with Health Outcomes
Even if prealbumin increased consistently in response to nutritional intervention, whether this change corresponds to an improvement in clinical outcomes has yet to be demonstrated.9 In 2005, Koretz reviewed 99 clinical trials and concluded that even when changes in nutritional markers are seen with nutritional support, the “changes in nutritional markers do not predict clinical outcomes.”17
WHAT YOU SHOULD DO INSTEAD: USE NONBIOLOGIC METHODS FOR SCREENING AND DIAGNOSING MALNUTRITION
Given the lack of a suitable biologic assay to identify malnutrition, dieticians and clinicians must rely on other means to assess malnutrition. Professional societies, including ASPEN and the European Society for Clinical Nutrition and Metabolism, have proposed different guidelines for the screening and assessment of malnutrition (Table 2).11,18 In 2016, these organizations, along with the Latin American Federation of Nutritional Therapy, Clinical Nutrition, and Metabolism and the Parenteral and Enteral Nutrition Society of Asia, formed The Global Leadership Initiative on Malnutrition (GLIM). In 2017, the GLIM taskforce agreed on clinically relevant diagnostic variables for the screening and assessment of malnutrition, including reduced food intake (anorexia), nonvolitional weight loss, (reduced) lean mass, status of disease burden and inflammation, and low body mass index or underweight status.19
RECOMMENDATIONS
- Do not use prealbumin to screen for or diagnose malnutrition.
- Consult with local dietitians to ensure that your institutional approach is in agreement with consensus recommendations.
CONCLUSION
In revisiting the case above, the patient does not have clear evidence of malnutrition based on his history (stable weight and good reported nutritional intake), although he does have a low BMI of 18.5 kg/m2. Rather than prealbumin testing, which would likely be low secondary to the acute phase response, he would better benefit from a nutrition-focused history and physical exam.
The uncertainties faced by clinicians in diagnosing malnutrition cannot readily be resolved by relying on a solitary laboratory marker (eg, prealbumin) or a stand-alone assessment protocol. The data obtained reflect the need for multidisciplinary teams of dieticians and clinicians to contextualize each patient’s medical history and ensure that the selected metrics are used appropriately to aid in diagnosis and documentation. We advocate that clinicians not routinely use prealbumin to screen for, confirm the diagnosis of, or assess the severity of malnutrition in the hospitalized patient.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
1. Sorensen J, Kondrup J, Prokopowicz J, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr Edinb Scotl. 2008;27(3):340-349. PubMed
2. Mueller C, Compher C, Ellen DM, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35(1):16-24. PubMed
3. Kaiser MJ, Bauer JM, Rämsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734-1738. PubMed
4. Robinson MK, Trujillo EB, Mogensen KM, Rounds J, McManus K, Jacobs DO. Improving nutritional screening of hospitalized patients: the role of prealbumin. JPEN J Parenter Enteral Nutr. 2003;27(6):389-395; quiz 439. PubMed
5. Corkins MR, Guenter P, DiMaria-Ghalili RA, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. 2014;38(2):186-195. PubMed
6. Clinical Laboratory Fee Schedule Files. cms.org. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Published September 29, 2016. Accessed January 5, 2018.
7. Myron Johnson A, Merlini G, Sheldon J, Ichihara K, Scientific Division Committee on Plasma Proteins (C-PP), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419-426. PubMed
8. Ingenbleek Y, De Visscher M, De Nayer P. Measurement of prealbumin as index of protein-calorie malnutrition. Lancet. 1972;2(7768):106-109. PubMed
9. Barbosa-Silva MCG. Subjective and objective nutritional assessment methods: what do they really assess? Curr Opin Clin Nutr Metab Care. 2008;11(3):248-254. PubMed
10. Clark MA, Hentzen BTH, Plank LD, Hill GL. Sequential changes in insulin-like growth factor 1, plasma proteins, and total body protein in severe sepsis and multiple injury. J Parenter Enter Nutr. 1996;20(5):363-370. PubMed
11. White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. PubMed
12. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-22. PubMed
13. Academy of Nutrition and Dietetics Evidence Analysis Library. Nutrition Screening (NSCR) Systematic Review (2009-2010). https://www.andeal.org/tmp/pdf-print-919C51237950859AE3E15F978CEF49D8.pdf. Accessed August 23, 2017.
14. Sawicky CP, Nippo J, Winkler MF, Albina JE. Adequate energy intake and improved prealbumin concentration as indicators of the response to total parenteral nutrition. J Am Diet Assoc. 1992;92(10):1266-1268. PubMed
15. Van Wymelbeke V, Guédon A, Maniere D, Manckoundia P, Pfitzenmeyer P. A 6-month follow-up of nutritional status in institutionalized patients with Alzheimer’s disease. J Nutr Health Aging. 2004;8(6):505-508. PubMed
16. Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr. 2012;36(2):197-204. PubMed
17. Koretz RL. Death, morbidity and economics are the only end points for trials. Proc Nutr Soc. 2005;64(3):277-284. PubMed
18. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr Edinb Scotl. 2015;34(3):335-340. PubMed
19. Jensen GL, Cederholm T. Global leadership initiative on malnutrition: progress report from ASPEN clinical nutrition week 2017. JPEN J Parenter Enteral Nutr. April 2017:148607117707761. PubMed
1. Sorensen J, Kondrup J, Prokopowicz J, et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr Edinb Scotl. 2008;27(3):340-349. PubMed
2. Mueller C, Compher C, Ellen DM, American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) Board of Directors. A.S.P.E.N. clinical guidelines: nutrition screening, assessment, and intervention in adults. JPEN J Parenter Enteral Nutr. 2011;35(1):16-24. PubMed
3. Kaiser MJ, Bauer JM, Rämsch C, et al. Frequency of malnutrition in older adults: a multinational perspective using the mini nutritional assessment. J Am Geriatr Soc. 2010;58(9):1734-1738. PubMed
4. Robinson MK, Trujillo EB, Mogensen KM, Rounds J, McManus K, Jacobs DO. Improving nutritional screening of hospitalized patients: the role of prealbumin. JPEN J Parenter Enteral Nutr. 2003;27(6):389-395; quiz 439. PubMed
5. Corkins MR, Guenter P, DiMaria-Ghalili RA, et al. Malnutrition diagnoses in hospitalized patients: United States, 2010. JPEN J Parenter Enteral Nutr. 2014;38(2):186-195. PubMed
6. Clinical Laboratory Fee Schedule Files. cms.org. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/ClinicalLabFeeSched/Clinical-Laboratory-Fee-Schedule-Files.html. Published September 29, 2016. Accessed January 5, 2018.
7. Myron Johnson A, Merlini G, Sheldon J, Ichihara K, Scientific Division Committee on Plasma Proteins (C-PP), International Federation of Clinical Chemistry and Laboratory Medicine (IFCC). Clinical indications for plasma protein assays: transthyretin (prealbumin) in inflammation and malnutrition. Clin Chem Lab Med. 2007;45(3):419-426. PubMed
8. Ingenbleek Y, De Visscher M, De Nayer P. Measurement of prealbumin as index of protein-calorie malnutrition. Lancet. 1972;2(7768):106-109. PubMed
9. Barbosa-Silva MCG. Subjective and objective nutritional assessment methods: what do they really assess? Curr Opin Clin Nutr Metab Care. 2008;11(3):248-254. PubMed
10. Clark MA, Hentzen BTH, Plank LD, Hill GL. Sequential changes in insulin-like growth factor 1, plasma proteins, and total body protein in severe sepsis and multiple injury. J Parenter Enter Nutr. 1996;20(5):363-370. PubMed
11. White JV, Guenter P, Jensen G, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738. PubMed
12. Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128(9):1023.e1-22. PubMed
13. Academy of Nutrition and Dietetics Evidence Analysis Library. Nutrition Screening (NSCR) Systematic Review (2009-2010). https://www.andeal.org/tmp/pdf-print-919C51237950859AE3E15F978CEF49D8.pdf. Accessed August 23, 2017.
14. Sawicky CP, Nippo J, Winkler MF, Albina JE. Adequate energy intake and improved prealbumin concentration as indicators of the response to total parenteral nutrition. J Am Diet Assoc. 1992;92(10):1266-1268. PubMed
15. Van Wymelbeke V, Guédon A, Maniere D, Manckoundia P, Pfitzenmeyer P. A 6-month follow-up of nutritional status in institutionalized patients with Alzheimer’s disease. J Nutr Health Aging. 2004;8(6):505-508. PubMed
16. Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr. 2012;36(2):197-204. PubMed
17. Koretz RL. Death, morbidity and economics are the only end points for trials. Proc Nutr Soc. 2005;64(3):277-284. PubMed
18. Cederholm T, Bosaeus I, Barazzoni R, et al. Diagnostic criteria for malnutrition - an ESPEN consensus statement. Clin Nutr Edinb Scotl. 2015;34(3):335-340. PubMed
19. Jensen GL, Cederholm T. Global leadership initiative on malnutrition: progress report from ASPEN clinical nutrition week 2017. JPEN J Parenter Enteral Nutr. April 2017:148607117707761. PubMed
© 2018 Society of Hospital Medicine
Things We Do for No Reason: Routine Echocardiography in Hemodynamically Stable Patients with Acute Pulmonary Embolism
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 28 year-old woman presents to the emergency department with acute onset bilateral chest pain and dyspnea. She has a respiratory rate of 28, a heart rate of 106, blood pressure of 110/65 mm Hg, and pulse oximetry of 92% saturation on room air. She has no history of cardiac or pulmonary disease and no personal history of venous thromboembolism. She takes an estrogen-containing oral contraceptive. On examination, she has no jugular venous distention, normal cardiac tones without murmur, and no lower extremity swelling. D-dimer is elevated at 3.4 mg/L (normal < 0.5 mg/L), and she undergoes computed tomography (CT) of the chest, which demonstrates acute segmental pulmonary emboli (PE) in the right upper and middle lobes as well as multiple bilateral subsegmental PEs. The CT suggests right ventricular dysfunction (RVD), and her troponin T is 0.06 ng/mL (normal < 0.01 ng/mL). Bilateral lower extremity venous Doppler ultrasonography demonstrates no acute thrombus.
BACKGROUND
Acute pulmonary embolism (PE) accounts for more than 300,000 inpatient admissions annually in the United States.1 The vast majority of patients with acute PE who receive adequate anticoagulation will have favorable outcomes.2,3 In the past two decades, for example, mortality has decreased significantly among patients admitted with acute PE,2 with 30-day all-cause mortality falling to approximately 5%.3 The risk-adjusted rate of recurrent venous thromboembolism (VTE) within 30 days has concomitantly dropped below 1%.3
Acute PE severity was previously classified as massive or high risk, submassive or intermediate risk, and low risk.4 Massive PE was defined by RVD and persistent hypotension or shock requiring vasopressors. 4 Intermediate-risk or submassive PE typically referred to normotensive patients with RVD and/or myocardial necrosis (eg, elevated troponin).4,5 Low-risk PEs had neither hemodynamic instability nor RVD. This classification scheme, however, has fallen out of favor as PE severity exists on a risk spectrum.6 Instead, recent guidelines from the European Society of Cardiology and the American College of Chest Physicians recommend first parsing PE severity by the presence or absence of hypotension (Figure 1).6,7 Risk assessment can be subsequently enhanced by validated clinical risk prediction scores, imaging-based assessment of RVD, and cardiac biomarker testing.6
In acute PE, hypotension and/or shock are associated with a 12%-35% risk of short-term mortality.2,3,8 Accordingly, patients with high-risk PE, who comprise 3%-12% of hospitalizations for PE,2,3,8 typically receive more intensive monitoring and treatment.2,8,9 In addition to systemic anticoagulation, thrombolysis is generally recommended for hypotensive patients with PE and no contraindications.6,7
Between 7% and 59% of patients with acute PE are hemodynamically stable but have objective evidence of myocardial necrosis and/or RVD.8,10,11 Among these patients, fewer than 10% will have a complicated course as defined by all-cause death, hemodynamic collapse, or recurrent PE in the first month after diagnosis,11 and short-term PE-related mortality rates range from approximately 2%-5%.5,8,11
WHY YOU MIGHT THINK ECHOCARDIOGRAPHY IS HELPFUL IN HEMODYNAMICALLY STABLE ACUTE PE
Echocardiography is a common method for evaluating RVD, and echocardiographic RVD confers an increased risk of adverse outcomes in PE.10-12 In the earliest meta-analysis to evaluate this association, Sanchez et al. combined data from five studies that included 623 patients from emergency room and inpatient settings. They found that echocardiographic RVD conferred an unadjusted relative risk for short-term mortality of 2.53 (95%CI 1.17-5.50).12 A subsequent meta-analysis by Cho et al. pooled data from both prospective and retrospective cohorts to examine short-term mortality in a total of 3,283 hemodynamically stable patients with PE, of whom 1,223 (37.3%) had RVD diagnosed by echocardiogram.10 In this population, RVD was associated with an odds ratio of 2.29 (95%CI 1.61-3.26) for short-term death. Thus, echocardiography could be viewed as a risk stratification tool, even in hemodynamically stable PE.
WHY ECHOCARDIOGRAPHY IN HEMODYNAMICALLY
STABLE ACUTE PE IS NOT AS HELPFUL AS YOU THINK
For most hemodynamically stable patients, echocardiographic findings will not enhance prognostication and/or have a therapeutic impact. The following four reasons explain why echocardiography adds little value to the care of these patients.
First, phenotypic expression of RVD varies from asymptomatic, despite abnormalities on diagnostic testing, to obstructive shock. Unfortunately, available prognostic models classify echocardiographic RVD in a binary fashion (present/absent)4,7,10 whereas RVD exists on a continuum. Consequently, RVD is commonly found in acute PE8,10,11 and has been identified in more than half of patients hospitalized with PE referred for echocardiography.8 Existing data do not allow clinicians to judge the clinical impact of the severity of echocardiographic RVD,8 and only the phenotypic expression of refractory hypotension has clear therapeutic implications.6,7
Second, while echocardiographic RVD is associated with short-term mortality,10-12 absolute rates of adverse outcomes are quite low when RVD is identified. For example, in a study merging multiple prospective cohorts, Becattini et al. demonstrated that RVD diagnosed by echocardiography or CT occurred in 41% of hospitalized patients stratified to low-risk PE by the simplified Pulmonary Embolism Severity Index (sPESI).8 For these patients, the 30-day mortality was 1.2%,8 which approximates the expected mortality from a low-risk sPESI score alone (1.1%).13 Even among intermediate-risk acute PE patients with RVD and/or elevated troponin enrolled in thrombolysis trials, the overall risk of death at 30 days was approximately 2%-3%, irrespective of the treatment arm.5,14,15
Third, RVD identified by echocardiography does not inform or enhance prognostication as compared with cardiac biomarker testing. In a meta-analysis by Sanchez et al., echocardiographic RVD predicted death with a risk ratio of 2.53 (95% CI 1.17-5.50).12 However, both elevated cardiac troponin and brain natriuretic peptide indicated a significantly worse outcome than imaging findings, with risk ratios of 8.3 (95% CI 3.6-19.3) and 9.5 (95% CI 3.2-28.6), respectively.13 More recently, Jiménez derived and validated a multivariable risk prediction model for stable PE.11 In their data, echocardiographic RVD had an unadjusted odds ratio of 2.62 (95% CI 1.54-4.45) for predicting a 30-day complicated course. After multivariable adjustment that included sPESI scores, lower extremity ultrasound results, and cardiac biomarker testing, these odds became insignificant.11 In other words, identifying echocardiographic RVD did not improve prognostication in hemodynamically stable PE patients when other commonly available variables were used.
Finally, in hemodynamically stable patients, echocardiographic RVD might create patient anxiety and cause harm. In a recent retrospective cohort study of 64,037 stable patients with PE, exposure to echocardiography was associated with a five-fold increase in likelihood of having received thrombolysis without any significant differences in risk-adjusted mortality.16 These data suggest that when faced with an abnormal echocardiogram, clinicians and patients may opt for more aggressive, time-sensitive therapies. Basing thrombolysis decisions on echocardiographic RVD potentially subjects patients to harm without decreasing mortality.5,14,15 For example, the PEITHO study, which was the largest randomized trial evaluating thrombolysis in intermediate-risk acute PE, enrolled 1,006 patients and demonstrated that treating 29 intermediate-risk patients with thrombolysis prevented one case of hemodynamic decompensation.5 These benefits were counterbalanced by a number needed to harm of 14 to cause stroke or major bleeding. Ominous echocardiographic findings may also bias clinicians toward more intensive monitoring. Rates of echocardiogram utilization in hemodynamically stable PE are linked to higher rates of ICU admission and longer hospital stays without significant impact on patient outcomes.16
WHEN ECHOCARDIOGRAPHY MIGHT BE HELPFUL IN HEMODYNAMICALLY STABLE PATIENTS WITH PE
Echocardiography should be used to exclude other causes of hypotension in patients with presumed PE-related shock7,9 and to improve clinicians’ confidence prescribing systemic thrombolytics in the face of hemodynamic instability.6,7 Otherwise, echocardiography should be reserved for highly selected intermediate-risk patients with acute PE. Among patients with intermediate-risk PE, those most likely to decompensate or die typically satisfy all of the following conditions: (1) highest-risk PESI or sPESI scores, (2) elevated natriuretic peptides, (3) elevated troponin, and (4) proximal deep vein thrombosis (DVT) on lower extremity ultrasound.11,13 In such patients, the echocardiogram may reveal a critical “tipping point,” such as a right atrial or ventricular thrombus-in-transit, that may warrant more intensive monitoring and multidisciplinary input into the most appropriate treatment plan.
Echocardiography could aid therapeutic decisions when the benefits from thrombolysis may outweigh the risks, such as for patients with minimal physiologic reserve and/or a low risk of major bleeding complications. Prognostic models like sPESI utilize binary variables, such as the presence/absence of chronic cardiopulmonary disease or oxygen saturation above/below 90%. Clearly, these variables exist on a spectrum; intuitively, patients with severe comorbidities and more alarming vital signs have a higher risk of death or decompensation than predicted by sPESI. Analogously, echocardiographic findings of RVD also encompass a spectrum. Because prognostic models and clinical trials cannot guide decisions for each individual patient, clinicians could justify using echocardiography to “fine tune” prognostication and to provide a personalized approach for carefully selected patients.
WHAT SHOULD YOU DO INSTEAD?
Clinicians should use a risk prediction model for all hemodynamically stable patients with confirmed PE.6,7 Validated risk calculators include the sPESI,6,7,14 which relies exclusively on the patient’s history and vital signs, and the eStiMaTe© tool (www.peprognosis.org), which enhances prognostication from sPESI by incorporating troponin, natriuretic peptide, and lower- extremity Doppler results. 11 For patients with symptoms or physical signs of RVD, chest CT and cardiac biomarkers (ie, troponin and/or natriuretic peptides) are sufficient for prognostication.11,14 In intermediate-risk patients with the highest risk for decompensation based on risk prediction scores, the echocardiogram should represent a part of a comprehensive clinical evaluation, not the sole criterion for intensive monitoring and aggressive treatment.
RECOMMENDATIONS
- Clinicians should use a validated tool, such as the sPESI, for initial risk stratification of hemodynamically stable patients with acute pulmonary embolism.
- Hemodynamically unstable patients with confirmed or suspected acute PE may benefit from early echocardiography to confirm RVD as the cause of shock.6,7,9
- The majority of normotensive adults with acute PE should not undergo echocardiography. To identify the patients at the greatest risk for decompensation, clinicians may consider using the eStiMaTe© tool (www.peprognosis.org), which augments risk stratification afforded by sPESI.
- For hemodynamically stable patients with PE who have already undergone echocardiography, clinicians should avoid being biased by the finding of RVD, particularly if other prognostic markers are reassuring.
CONCLUSION
In evaluating the patient described earlier, echocardiography has no clear prognostic implications. Her admission sPESI score equals zero, predicting a 30-day mortality of 1.1%. Including her lower extremity ultrasound and troponin T results into the eStiMaTe© calculator (www.peprognosis.org) surprisingly predicts an even lower rate of 30-day mortality (0.4%) and low risk of a complicated course (2.4%). Assessing for RVD on echocardiography may increase her risk of unnecessary and potentially injurious interventions.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing[email protected].
Disclosures
The authors have no conflicts of interest relevant to this article.
1. Centers for Disease Control and Prevention (CDC). Venous thromboembolism in adult hospitalizations, United States, 2007-2009. Morbidity and mortality weekly report (MMWR). 2012;61(22):401-40. Available: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed May 7, 2018.
2. Stein PD, Matta F, Alrifai A, Rahman A. Trends in case fatality rate in pulmonary embolism according to stability and treatment. Thromb Res. 2012;130(6):841-846. PubMed
3. Jiménez D, de Miguel-Díez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE Registry. J Am Coll Cardiol. 2016;67(2):162-170. PubMed
4. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788-1830. PubMed
5. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-1411. PubMed
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;49(2):315-352. PubMed
7. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033-69, 3069a-3069k. PubMed
8. Becattini C, Agnelli G, Lankeit M, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. 2016;48(3):780-786. PubMed
9. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part II: Cardiac Ultrasonography. Crit Care Med. 2016;44(6):1206-1227. PubMed
10. Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord. 2014;14:64. PubMed
11. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718-726. PubMed
12. Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29(12):1569-1577. PubMed
13. Elias A, Mallett S, Daoud-Elias M, Poggi JN, Clarke M. Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e010324. PubMed
14. Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143-1150. PubMed
15. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. 2014;12(4):459-468. PubMed
16. Cohen DM, Winter M, Lindenauer PK, Walkey AJ. Echocardiogram in the evaluation of hemodynamically stable acute pulmonary embolism: national practices and clinical outcomes. Ann Am Thorac Soc. 2018;15(5):581-588. PubMed
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 28 year-old woman presents to the emergency department with acute onset bilateral chest pain and dyspnea. She has a respiratory rate of 28, a heart rate of 106, blood pressure of 110/65 mm Hg, and pulse oximetry of 92% saturation on room air. She has no history of cardiac or pulmonary disease and no personal history of venous thromboembolism. She takes an estrogen-containing oral contraceptive. On examination, she has no jugular venous distention, normal cardiac tones without murmur, and no lower extremity swelling. D-dimer is elevated at 3.4 mg/L (normal < 0.5 mg/L), and she undergoes computed tomography (CT) of the chest, which demonstrates acute segmental pulmonary emboli (PE) in the right upper and middle lobes as well as multiple bilateral subsegmental PEs. The CT suggests right ventricular dysfunction (RVD), and her troponin T is 0.06 ng/mL (normal < 0.01 ng/mL). Bilateral lower extremity venous Doppler ultrasonography demonstrates no acute thrombus.
BACKGROUND
Acute pulmonary embolism (PE) accounts for more than 300,000 inpatient admissions annually in the United States.1 The vast majority of patients with acute PE who receive adequate anticoagulation will have favorable outcomes.2,3 In the past two decades, for example, mortality has decreased significantly among patients admitted with acute PE,2 with 30-day all-cause mortality falling to approximately 5%.3 The risk-adjusted rate of recurrent venous thromboembolism (VTE) within 30 days has concomitantly dropped below 1%.3
Acute PE severity was previously classified as massive or high risk, submassive or intermediate risk, and low risk.4 Massive PE was defined by RVD and persistent hypotension or shock requiring vasopressors. 4 Intermediate-risk or submassive PE typically referred to normotensive patients with RVD and/or myocardial necrosis (eg, elevated troponin).4,5 Low-risk PEs had neither hemodynamic instability nor RVD. This classification scheme, however, has fallen out of favor as PE severity exists on a risk spectrum.6 Instead, recent guidelines from the European Society of Cardiology and the American College of Chest Physicians recommend first parsing PE severity by the presence or absence of hypotension (Figure 1).6,7 Risk assessment can be subsequently enhanced by validated clinical risk prediction scores, imaging-based assessment of RVD, and cardiac biomarker testing.6
In acute PE, hypotension and/or shock are associated with a 12%-35% risk of short-term mortality.2,3,8 Accordingly, patients with high-risk PE, who comprise 3%-12% of hospitalizations for PE,2,3,8 typically receive more intensive monitoring and treatment.2,8,9 In addition to systemic anticoagulation, thrombolysis is generally recommended for hypotensive patients with PE and no contraindications.6,7
Between 7% and 59% of patients with acute PE are hemodynamically stable but have objective evidence of myocardial necrosis and/or RVD.8,10,11 Among these patients, fewer than 10% will have a complicated course as defined by all-cause death, hemodynamic collapse, or recurrent PE in the first month after diagnosis,11 and short-term PE-related mortality rates range from approximately 2%-5%.5,8,11
WHY YOU MIGHT THINK ECHOCARDIOGRAPHY IS HELPFUL IN HEMODYNAMICALLY STABLE ACUTE PE
Echocardiography is a common method for evaluating RVD, and echocardiographic RVD confers an increased risk of adverse outcomes in PE.10-12 In the earliest meta-analysis to evaluate this association, Sanchez et al. combined data from five studies that included 623 patients from emergency room and inpatient settings. They found that echocardiographic RVD conferred an unadjusted relative risk for short-term mortality of 2.53 (95%CI 1.17-5.50).12 A subsequent meta-analysis by Cho et al. pooled data from both prospective and retrospective cohorts to examine short-term mortality in a total of 3,283 hemodynamically stable patients with PE, of whom 1,223 (37.3%) had RVD diagnosed by echocardiogram.10 In this population, RVD was associated with an odds ratio of 2.29 (95%CI 1.61-3.26) for short-term death. Thus, echocardiography could be viewed as a risk stratification tool, even in hemodynamically stable PE.
WHY ECHOCARDIOGRAPHY IN HEMODYNAMICALLY
STABLE ACUTE PE IS NOT AS HELPFUL AS YOU THINK
For most hemodynamically stable patients, echocardiographic findings will not enhance prognostication and/or have a therapeutic impact. The following four reasons explain why echocardiography adds little value to the care of these patients.
First, phenotypic expression of RVD varies from asymptomatic, despite abnormalities on diagnostic testing, to obstructive shock. Unfortunately, available prognostic models classify echocardiographic RVD in a binary fashion (present/absent)4,7,10 whereas RVD exists on a continuum. Consequently, RVD is commonly found in acute PE8,10,11 and has been identified in more than half of patients hospitalized with PE referred for echocardiography.8 Existing data do not allow clinicians to judge the clinical impact of the severity of echocardiographic RVD,8 and only the phenotypic expression of refractory hypotension has clear therapeutic implications.6,7
Second, while echocardiographic RVD is associated with short-term mortality,10-12 absolute rates of adverse outcomes are quite low when RVD is identified. For example, in a study merging multiple prospective cohorts, Becattini et al. demonstrated that RVD diagnosed by echocardiography or CT occurred in 41% of hospitalized patients stratified to low-risk PE by the simplified Pulmonary Embolism Severity Index (sPESI).8 For these patients, the 30-day mortality was 1.2%,8 which approximates the expected mortality from a low-risk sPESI score alone (1.1%).13 Even among intermediate-risk acute PE patients with RVD and/or elevated troponin enrolled in thrombolysis trials, the overall risk of death at 30 days was approximately 2%-3%, irrespective of the treatment arm.5,14,15
Third, RVD identified by echocardiography does not inform or enhance prognostication as compared with cardiac biomarker testing. In a meta-analysis by Sanchez et al., echocardiographic RVD predicted death with a risk ratio of 2.53 (95% CI 1.17-5.50).12 However, both elevated cardiac troponin and brain natriuretic peptide indicated a significantly worse outcome than imaging findings, with risk ratios of 8.3 (95% CI 3.6-19.3) and 9.5 (95% CI 3.2-28.6), respectively.13 More recently, Jiménez derived and validated a multivariable risk prediction model for stable PE.11 In their data, echocardiographic RVD had an unadjusted odds ratio of 2.62 (95% CI 1.54-4.45) for predicting a 30-day complicated course. After multivariable adjustment that included sPESI scores, lower extremity ultrasound results, and cardiac biomarker testing, these odds became insignificant.11 In other words, identifying echocardiographic RVD did not improve prognostication in hemodynamically stable PE patients when other commonly available variables were used.
Finally, in hemodynamically stable patients, echocardiographic RVD might create patient anxiety and cause harm. In a recent retrospective cohort study of 64,037 stable patients with PE, exposure to echocardiography was associated with a five-fold increase in likelihood of having received thrombolysis without any significant differences in risk-adjusted mortality.16 These data suggest that when faced with an abnormal echocardiogram, clinicians and patients may opt for more aggressive, time-sensitive therapies. Basing thrombolysis decisions on echocardiographic RVD potentially subjects patients to harm without decreasing mortality.5,14,15 For example, the PEITHO study, which was the largest randomized trial evaluating thrombolysis in intermediate-risk acute PE, enrolled 1,006 patients and demonstrated that treating 29 intermediate-risk patients with thrombolysis prevented one case of hemodynamic decompensation.5 These benefits were counterbalanced by a number needed to harm of 14 to cause stroke or major bleeding. Ominous echocardiographic findings may also bias clinicians toward more intensive monitoring. Rates of echocardiogram utilization in hemodynamically stable PE are linked to higher rates of ICU admission and longer hospital stays without significant impact on patient outcomes.16
WHEN ECHOCARDIOGRAPHY MIGHT BE HELPFUL IN HEMODYNAMICALLY STABLE PATIENTS WITH PE
Echocardiography should be used to exclude other causes of hypotension in patients with presumed PE-related shock7,9 and to improve clinicians’ confidence prescribing systemic thrombolytics in the face of hemodynamic instability.6,7 Otherwise, echocardiography should be reserved for highly selected intermediate-risk patients with acute PE. Among patients with intermediate-risk PE, those most likely to decompensate or die typically satisfy all of the following conditions: (1) highest-risk PESI or sPESI scores, (2) elevated natriuretic peptides, (3) elevated troponin, and (4) proximal deep vein thrombosis (DVT) on lower extremity ultrasound.11,13 In such patients, the echocardiogram may reveal a critical “tipping point,” such as a right atrial or ventricular thrombus-in-transit, that may warrant more intensive monitoring and multidisciplinary input into the most appropriate treatment plan.
Echocardiography could aid therapeutic decisions when the benefits from thrombolysis may outweigh the risks, such as for patients with minimal physiologic reserve and/or a low risk of major bleeding complications. Prognostic models like sPESI utilize binary variables, such as the presence/absence of chronic cardiopulmonary disease or oxygen saturation above/below 90%. Clearly, these variables exist on a spectrum; intuitively, patients with severe comorbidities and more alarming vital signs have a higher risk of death or decompensation than predicted by sPESI. Analogously, echocardiographic findings of RVD also encompass a spectrum. Because prognostic models and clinical trials cannot guide decisions for each individual patient, clinicians could justify using echocardiography to “fine tune” prognostication and to provide a personalized approach for carefully selected patients.
WHAT SHOULD YOU DO INSTEAD?
Clinicians should use a risk prediction model for all hemodynamically stable patients with confirmed PE.6,7 Validated risk calculators include the sPESI,6,7,14 which relies exclusively on the patient’s history and vital signs, and the eStiMaTe© tool (www.peprognosis.org), which enhances prognostication from sPESI by incorporating troponin, natriuretic peptide, and lower- extremity Doppler results. 11 For patients with symptoms or physical signs of RVD, chest CT and cardiac biomarkers (ie, troponin and/or natriuretic peptides) are sufficient for prognostication.11,14 In intermediate-risk patients with the highest risk for decompensation based on risk prediction scores, the echocardiogram should represent a part of a comprehensive clinical evaluation, not the sole criterion for intensive monitoring and aggressive treatment.
RECOMMENDATIONS
- Clinicians should use a validated tool, such as the sPESI, for initial risk stratification of hemodynamically stable patients with acute pulmonary embolism.
- Hemodynamically unstable patients with confirmed or suspected acute PE may benefit from early echocardiography to confirm RVD as the cause of shock.6,7,9
- The majority of normotensive adults with acute PE should not undergo echocardiography. To identify the patients at the greatest risk for decompensation, clinicians may consider using the eStiMaTe© tool (www.peprognosis.org), which augments risk stratification afforded by sPESI.
- For hemodynamically stable patients with PE who have already undergone echocardiography, clinicians should avoid being biased by the finding of RVD, particularly if other prognostic markers are reassuring.
CONCLUSION
In evaluating the patient described earlier, echocardiography has no clear prognostic implications. Her admission sPESI score equals zero, predicting a 30-day mortality of 1.1%. Including her lower extremity ultrasound and troponin T results into the eStiMaTe© calculator (www.peprognosis.org) surprisingly predicts an even lower rate of 30-day mortality (0.4%) and low risk of a complicated course (2.4%). Assessing for RVD on echocardiography may increase her risk of unnecessary and potentially injurious interventions.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing[email protected].
Disclosures
The authors have no conflicts of interest relevant to this article.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 28 year-old woman presents to the emergency department with acute onset bilateral chest pain and dyspnea. She has a respiratory rate of 28, a heart rate of 106, blood pressure of 110/65 mm Hg, and pulse oximetry of 92% saturation on room air. She has no history of cardiac or pulmonary disease and no personal history of venous thromboembolism. She takes an estrogen-containing oral contraceptive. On examination, she has no jugular venous distention, normal cardiac tones without murmur, and no lower extremity swelling. D-dimer is elevated at 3.4 mg/L (normal < 0.5 mg/L), and she undergoes computed tomography (CT) of the chest, which demonstrates acute segmental pulmonary emboli (PE) in the right upper and middle lobes as well as multiple bilateral subsegmental PEs. The CT suggests right ventricular dysfunction (RVD), and her troponin T is 0.06 ng/mL (normal < 0.01 ng/mL). Bilateral lower extremity venous Doppler ultrasonography demonstrates no acute thrombus.
BACKGROUND
Acute pulmonary embolism (PE) accounts for more than 300,000 inpatient admissions annually in the United States.1 The vast majority of patients with acute PE who receive adequate anticoagulation will have favorable outcomes.2,3 In the past two decades, for example, mortality has decreased significantly among patients admitted with acute PE,2 with 30-day all-cause mortality falling to approximately 5%.3 The risk-adjusted rate of recurrent venous thromboembolism (VTE) within 30 days has concomitantly dropped below 1%.3
Acute PE severity was previously classified as massive or high risk, submassive or intermediate risk, and low risk.4 Massive PE was defined by RVD and persistent hypotension or shock requiring vasopressors. 4 Intermediate-risk or submassive PE typically referred to normotensive patients with RVD and/or myocardial necrosis (eg, elevated troponin).4,5 Low-risk PEs had neither hemodynamic instability nor RVD. This classification scheme, however, has fallen out of favor as PE severity exists on a risk spectrum.6 Instead, recent guidelines from the European Society of Cardiology and the American College of Chest Physicians recommend first parsing PE severity by the presence or absence of hypotension (Figure 1).6,7 Risk assessment can be subsequently enhanced by validated clinical risk prediction scores, imaging-based assessment of RVD, and cardiac biomarker testing.6
In acute PE, hypotension and/or shock are associated with a 12%-35% risk of short-term mortality.2,3,8 Accordingly, patients with high-risk PE, who comprise 3%-12% of hospitalizations for PE,2,3,8 typically receive more intensive monitoring and treatment.2,8,9 In addition to systemic anticoagulation, thrombolysis is generally recommended for hypotensive patients with PE and no contraindications.6,7
Between 7% and 59% of patients with acute PE are hemodynamically stable but have objective evidence of myocardial necrosis and/or RVD.8,10,11 Among these patients, fewer than 10% will have a complicated course as defined by all-cause death, hemodynamic collapse, or recurrent PE in the first month after diagnosis,11 and short-term PE-related mortality rates range from approximately 2%-5%.5,8,11
WHY YOU MIGHT THINK ECHOCARDIOGRAPHY IS HELPFUL IN HEMODYNAMICALLY STABLE ACUTE PE
Echocardiography is a common method for evaluating RVD, and echocardiographic RVD confers an increased risk of adverse outcomes in PE.10-12 In the earliest meta-analysis to evaluate this association, Sanchez et al. combined data from five studies that included 623 patients from emergency room and inpatient settings. They found that echocardiographic RVD conferred an unadjusted relative risk for short-term mortality of 2.53 (95%CI 1.17-5.50).12 A subsequent meta-analysis by Cho et al. pooled data from both prospective and retrospective cohorts to examine short-term mortality in a total of 3,283 hemodynamically stable patients with PE, of whom 1,223 (37.3%) had RVD diagnosed by echocardiogram.10 In this population, RVD was associated with an odds ratio of 2.29 (95%CI 1.61-3.26) for short-term death. Thus, echocardiography could be viewed as a risk stratification tool, even in hemodynamically stable PE.
WHY ECHOCARDIOGRAPHY IN HEMODYNAMICALLY
STABLE ACUTE PE IS NOT AS HELPFUL AS YOU THINK
For most hemodynamically stable patients, echocardiographic findings will not enhance prognostication and/or have a therapeutic impact. The following four reasons explain why echocardiography adds little value to the care of these patients.
First, phenotypic expression of RVD varies from asymptomatic, despite abnormalities on diagnostic testing, to obstructive shock. Unfortunately, available prognostic models classify echocardiographic RVD in a binary fashion (present/absent)4,7,10 whereas RVD exists on a continuum. Consequently, RVD is commonly found in acute PE8,10,11 and has been identified in more than half of patients hospitalized with PE referred for echocardiography.8 Existing data do not allow clinicians to judge the clinical impact of the severity of echocardiographic RVD,8 and only the phenotypic expression of refractory hypotension has clear therapeutic implications.6,7
Second, while echocardiographic RVD is associated with short-term mortality,10-12 absolute rates of adverse outcomes are quite low when RVD is identified. For example, in a study merging multiple prospective cohorts, Becattini et al. demonstrated that RVD diagnosed by echocardiography or CT occurred in 41% of hospitalized patients stratified to low-risk PE by the simplified Pulmonary Embolism Severity Index (sPESI).8 For these patients, the 30-day mortality was 1.2%,8 which approximates the expected mortality from a low-risk sPESI score alone (1.1%).13 Even among intermediate-risk acute PE patients with RVD and/or elevated troponin enrolled in thrombolysis trials, the overall risk of death at 30 days was approximately 2%-3%, irrespective of the treatment arm.5,14,15
Third, RVD identified by echocardiography does not inform or enhance prognostication as compared with cardiac biomarker testing. In a meta-analysis by Sanchez et al., echocardiographic RVD predicted death with a risk ratio of 2.53 (95% CI 1.17-5.50).12 However, both elevated cardiac troponin and brain natriuretic peptide indicated a significantly worse outcome than imaging findings, with risk ratios of 8.3 (95% CI 3.6-19.3) and 9.5 (95% CI 3.2-28.6), respectively.13 More recently, Jiménez derived and validated a multivariable risk prediction model for stable PE.11 In their data, echocardiographic RVD had an unadjusted odds ratio of 2.62 (95% CI 1.54-4.45) for predicting a 30-day complicated course. After multivariable adjustment that included sPESI scores, lower extremity ultrasound results, and cardiac biomarker testing, these odds became insignificant.11 In other words, identifying echocardiographic RVD did not improve prognostication in hemodynamically stable PE patients when other commonly available variables were used.
Finally, in hemodynamically stable patients, echocardiographic RVD might create patient anxiety and cause harm. In a recent retrospective cohort study of 64,037 stable patients with PE, exposure to echocardiography was associated with a five-fold increase in likelihood of having received thrombolysis without any significant differences in risk-adjusted mortality.16 These data suggest that when faced with an abnormal echocardiogram, clinicians and patients may opt for more aggressive, time-sensitive therapies. Basing thrombolysis decisions on echocardiographic RVD potentially subjects patients to harm without decreasing mortality.5,14,15 For example, the PEITHO study, which was the largest randomized trial evaluating thrombolysis in intermediate-risk acute PE, enrolled 1,006 patients and demonstrated that treating 29 intermediate-risk patients with thrombolysis prevented one case of hemodynamic decompensation.5 These benefits were counterbalanced by a number needed to harm of 14 to cause stroke or major bleeding. Ominous echocardiographic findings may also bias clinicians toward more intensive monitoring. Rates of echocardiogram utilization in hemodynamically stable PE are linked to higher rates of ICU admission and longer hospital stays without significant impact on patient outcomes.16
WHEN ECHOCARDIOGRAPHY MIGHT BE HELPFUL IN HEMODYNAMICALLY STABLE PATIENTS WITH PE
Echocardiography should be used to exclude other causes of hypotension in patients with presumed PE-related shock7,9 and to improve clinicians’ confidence prescribing systemic thrombolytics in the face of hemodynamic instability.6,7 Otherwise, echocardiography should be reserved for highly selected intermediate-risk patients with acute PE. Among patients with intermediate-risk PE, those most likely to decompensate or die typically satisfy all of the following conditions: (1) highest-risk PESI or sPESI scores, (2) elevated natriuretic peptides, (3) elevated troponin, and (4) proximal deep vein thrombosis (DVT) on lower extremity ultrasound.11,13 In such patients, the echocardiogram may reveal a critical “tipping point,” such as a right atrial or ventricular thrombus-in-transit, that may warrant more intensive monitoring and multidisciplinary input into the most appropriate treatment plan.
Echocardiography could aid therapeutic decisions when the benefits from thrombolysis may outweigh the risks, such as for patients with minimal physiologic reserve and/or a low risk of major bleeding complications. Prognostic models like sPESI utilize binary variables, such as the presence/absence of chronic cardiopulmonary disease or oxygen saturation above/below 90%. Clearly, these variables exist on a spectrum; intuitively, patients with severe comorbidities and more alarming vital signs have a higher risk of death or decompensation than predicted by sPESI. Analogously, echocardiographic findings of RVD also encompass a spectrum. Because prognostic models and clinical trials cannot guide decisions for each individual patient, clinicians could justify using echocardiography to “fine tune” prognostication and to provide a personalized approach for carefully selected patients.
WHAT SHOULD YOU DO INSTEAD?
Clinicians should use a risk prediction model for all hemodynamically stable patients with confirmed PE.6,7 Validated risk calculators include the sPESI,6,7,14 which relies exclusively on the patient’s history and vital signs, and the eStiMaTe© tool (www.peprognosis.org), which enhances prognostication from sPESI by incorporating troponin, natriuretic peptide, and lower- extremity Doppler results. 11 For patients with symptoms or physical signs of RVD, chest CT and cardiac biomarkers (ie, troponin and/or natriuretic peptides) are sufficient for prognostication.11,14 In intermediate-risk patients with the highest risk for decompensation based on risk prediction scores, the echocardiogram should represent a part of a comprehensive clinical evaluation, not the sole criterion for intensive monitoring and aggressive treatment.
RECOMMENDATIONS
- Clinicians should use a validated tool, such as the sPESI, for initial risk stratification of hemodynamically stable patients with acute pulmonary embolism.
- Hemodynamically unstable patients with confirmed or suspected acute PE may benefit from early echocardiography to confirm RVD as the cause of shock.6,7,9
- The majority of normotensive adults with acute PE should not undergo echocardiography. To identify the patients at the greatest risk for decompensation, clinicians may consider using the eStiMaTe© tool (www.peprognosis.org), which augments risk stratification afforded by sPESI.
- For hemodynamically stable patients with PE who have already undergone echocardiography, clinicians should avoid being biased by the finding of RVD, particularly if other prognostic markers are reassuring.
CONCLUSION
In evaluating the patient described earlier, echocardiography has no clear prognostic implications. Her admission sPESI score equals zero, predicting a 30-day mortality of 1.1%. Including her lower extremity ultrasound and troponin T results into the eStiMaTe© calculator (www.peprognosis.org) surprisingly predicts an even lower rate of 30-day mortality (0.4%) and low risk of a complicated course (2.4%). Assessing for RVD on echocardiography may increase her risk of unnecessary and potentially injurious interventions.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing[email protected].
Disclosures
The authors have no conflicts of interest relevant to this article.
1. Centers for Disease Control and Prevention (CDC). Venous thromboembolism in adult hospitalizations, United States, 2007-2009. Morbidity and mortality weekly report (MMWR). 2012;61(22):401-40. Available: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed May 7, 2018.
2. Stein PD, Matta F, Alrifai A, Rahman A. Trends in case fatality rate in pulmonary embolism according to stability and treatment. Thromb Res. 2012;130(6):841-846. PubMed
3. Jiménez D, de Miguel-Díez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE Registry. J Am Coll Cardiol. 2016;67(2):162-170. PubMed
4. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788-1830. PubMed
5. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-1411. PubMed
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;49(2):315-352. PubMed
7. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033-69, 3069a-3069k. PubMed
8. Becattini C, Agnelli G, Lankeit M, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. 2016;48(3):780-786. PubMed
9. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part II: Cardiac Ultrasonography. Crit Care Med. 2016;44(6):1206-1227. PubMed
10. Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord. 2014;14:64. PubMed
11. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718-726. PubMed
12. Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29(12):1569-1577. PubMed
13. Elias A, Mallett S, Daoud-Elias M, Poggi JN, Clarke M. Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e010324. PubMed
14. Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143-1150. PubMed
15. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. 2014;12(4):459-468. PubMed
16. Cohen DM, Winter M, Lindenauer PK, Walkey AJ. Echocardiogram in the evaluation of hemodynamically stable acute pulmonary embolism: national practices and clinical outcomes. Ann Am Thorac Soc. 2018;15(5):581-588. PubMed
1. Centers for Disease Control and Prevention (CDC). Venous thromboembolism in adult hospitalizations, United States, 2007-2009. Morbidity and mortality weekly report (MMWR). 2012;61(22):401-40. Available: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6122a1.htm. Accessed May 7, 2018.
2. Stein PD, Matta F, Alrifai A, Rahman A. Trends in case fatality rate in pulmonary embolism according to stability and treatment. Thromb Res. 2012;130(6):841-846. PubMed
3. Jiménez D, de Miguel-Díez J, Guijarro R, et al. Trends in the management and outcomes of acute pulmonary embolism: analysis from the RIETE Registry. J Am Coll Cardiol. 2016;67(2):162-170. PubMed
4. Jaff MR, McMurtry MS, Archer SL, et al. Management of massive and submassive pulmonary embolism, iliofemoral deep vein thrombosis, and chronic thromboembolic pulmonary hypertension: a scientific statement from the American Heart Association. Circulation. 2011;123(16):1788-1830. PubMed
5. Meyer G, Vicaut E, Danays T, et al. Fibrinolysis for patients with intermediate-risk pulmonary embolism. N Engl J Med. 2014;370:1402-1411. PubMed
6. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST Guideline and Expert Panel Report. Chest. 2016;49(2):315-352. PubMed
7. Konstantinides SV, Torbicki A, Agnelli G, et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35(43):3033-69, 3069a-3069k. PubMed
8. Becattini C, Agnelli G, Lankeit M, et al. Acute pulmonary embolism: mortality prediction by the 2014 European Society of Cardiology risk stratification model. Eur Respir J. 2016;48(3):780-786. PubMed
9. Levitov A, Frankel HL, Blaivas M, et al. Guidelines for the appropriate use of bedside general and cardiac ultrasonography in the evaluation of critically ill patients-part II: Cardiac Ultrasonography. Crit Care Med. 2016;44(6):1206-1227. PubMed
10. Cho JH, Kutti Sridharan G, Kim SH, et al. Right ventricular dysfunction as an echocardiographic prognostic factor in hemodynamically stable patients with acute pulmonary embolism: a meta-analysis. BMC Cardiovasc Disord. 2014;14:64. PubMed
11. Jiménez D, Kopecna D, Tapson V, et al. Derivation and validation of multimarker prognostication for normotensive patients with acute symptomatic pulmonary embolism. Am J Respir Crit Care Med. 2014;189(6):718-726. PubMed
12. Sanchez O, Trinquart L, Colombet I, et al. Prognostic value of right ventricular dysfunction in patients with haemodynamically stable pulmonary embolism: a systematic review. Eur Heart J. 2008;29(12):1569-1577. PubMed
13. Elias A, Mallett S, Daoud-Elias M, Poggi JN, Clarke M. Prognostic models in acute pulmonary embolism: a systematic review and meta-analysis. BMJ Open. 2016;6(4):e010324. PubMed
14. Konstantinides S, Geibel A, Heusel G, et al. Heparin plus alteplase compared with heparin alone in patients with submassive pulmonary embolism. N Engl J Med. 2002;347(15):1143-1150. PubMed
15. Kline JA, Nordenholz KE, Courtney DM, et al. Treatment of submassive pulmonary embolism with tenecteplase or placebo: cardiopulmonary outcomes at 3 months: multicenter double-blind, placebo-controlled randomized trial. J Thromb Haemost. 2014;12(4):459-468. PubMed
16. Cohen DM, Winter M, Lindenauer PK, Walkey AJ. Echocardiogram in the evaluation of hemodynamically stable acute pulmonary embolism: national practices and clinical outcomes. Ann Am Thorac Soc. 2018;15(5):581-588. PubMed
© 2019 Society of Hospital Medicine
Things We Do For No Reason: Use of Antipsychotic Medications in Patients with Delirium
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No ReasonTM” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNRTM series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CASE
An 86-year-old woman with mild dementia falls at home while preparing a meal. Her son brings her to the emergency department for excruciating pain in her right hip. X-rays reveal a fractured right femur that requires open reduction and internal fixation. On the first postoperative day, she does not participate in therapy and sleeps most of the day. Overnight, a nurse observes her calmly speaking to a hallucination of a family member in the room and picking at the tape around her peripheral intravenous catheter (PIV) causing the PIV to fall out twice. Her vital signs are temperature 36.7°C, pulse 82 beats per minute, respirations 12 breaths per minute, blood pressure 143/72 mm Hg, and pulse oximetry of 99% on room air. She is hypoactive, distractedly picks at her clothing and PIV, inattentive, and unable to say the day of the week or count months backward. Nursing asks for haloperidol for her delirium.
WHY YOU MIGHT THINK ANTIPSYCHOTICS FOR DELIRIUM ARE HELPFUL
Delirium is an acute change in cognition characterized by inattention typically associated with disorganized thinking and/or alteration in consciousness.1 Delirium occurs in almost 25% of hospitalized patients, and clinicians have a limited pharmacologic armamentarium to treat it, given the absence of benefit for acetylcholinesterase inhibitors and concern that benzodiazepine medications cause/exacerbate delirium.2-4 Another treatment option is antipsychotic medications which block dopamine since dopamine excess is a key element in the neurotransmitter pathophysiology of delirium.5 A small 2005 trial of haloperidol prophylaxis in hip fracture patients found that haloperidol reduced the overall severity and duration of delirium.6 Based in part on this trial, a 2007 Cochrane Systematic Review concluded that antipsychotics “may reduce severity and duration of delirium episodes and shorten length of hospital stay in hip surgery.”7 Another study in 2010 demonstrated a 55% faster decline in total Delirium Rating Scale-Revised 98 (DRS-R-98) scores in patients on a general/medical-surgical floor receiving quetiapine treatment compared to those who received placebo.8
Studies show that 10%-30% of patients receive antipsychotics at some point during their hospitalization, usually for delirium.9,10 Variability in antipsychotic prescribing patterns not explained by patient characteristics suggests the local culture may influence antipsychotic prescribing practices when evidence from randomized controlled trials is sparse or conflicting.10
WHY ANTIPSYCHOTIC MEDICATIONS ARE NOT HELPFUL IN PATIENTS WITH DELIRIUM
While few studies have demonstrated positive effects of antipsychotics in delirium treatment, the overall evidence is not persuasive. The results of some studies have not been reproduced while only the positive effects rather than the adverse side effects of antipsychotic medications were highlighted in other articles. For instance, the 2005 hip fracture delirium prophylaxis trial found there was no difference in the incidence of delirium in patients on postoperative day one.7 Furthermore, the 2010 quetiapine study was underpowered for the primary outcome of lower DRS-R-98 scores. Importantly, there was no significant difference in severity of delirium between treatment (quetiapine) and placebo groups on days one, three, or 10.10 These studies show that antipsychotics were neither effective at preventing delirium or in reducing its severity compared to placebo. In 2016, a systemic review in the Journal of the American Geriatric Society included both of the above studies in addition to 17 other studies to assess the efficacy of antipsychotics in preventing and treating delirium. This analysis concluded that antipsychotics did not change the length of delirium or length of stay.11 In addition, the absence of convincing evidence of antipsychotics benefits in postoperative delirium has led the American Geriatrics Society to recommend: “The prescribing practitioner should not prescribe antipsychotic… medications for the treatment of older adults with postoperative delirium who are not agitated and threatening substantial harm to self or others.”12
There is a paucity of data speaking directly to whether antipsychotics reduce patient distress. A recent randomized controlled study compared haloperidol, risperidone, and placebo for delirium treatment in palliative care and hospice patients. With treatment, the patients in the antipsychotic arms demonstrated slightly more severe delirium and a significantly higher incidence of extrapyramidal symptoms (EPS) than the patients receiving placebo.13
Side effects such as EPS, aspiration pneumonia, and arrhythmia are concerns when using antipsychotics for delirium treatment.14 A systematic review and meta-analysis found the difference in EPS incidence between patients treated for delirium with antipsychotics versus no intervention ranged from no difference to over 10%.11 In addition to EPS, patients receiving antipsychotics in a cohort study were at increased risk for aspiration pneumonia compared to patients who did not receive antipsychotics (adjusted odds ratio = 1.5, 95% CI, 1.2-1.9).15 These serious side effects led the Food and Drug Administration (FDA) to issue a black box warning for antipsychotic treatment in dementia-related psychosis. Most importantly, the FDA warns that there is an increased risk of death.16
WHAT YOU SHOULD DO INSTEAD OF USING ANTIPSYCHOTICS
In the first line management of delirium, hospitalists should address underlying modifiable contributions to the condition with attention to medications, pain, electrolytes, ischemia, infection, alcohol withdrawal, and reducing invasive lines. For example, two studies demonstrated a decrease in delirium severity and duration of palliative care in patients by treating delirium triggers, such as dehydration, electrolyte abnormalities, or infection, rather than using antipsychotics.13,17 Furthermore, hospitalists should review the medication list carefully and look for opportunities to deprescribe sedative/hypnotics and anticholinergics.
In addition, hospitalists should implement the core elements of the nursing delirium protocol from the Hospital Elder Life Program (http://www.hospitalelderlifeprogram.org/). The program focuses on orientation, hydration, mobility, sensory aids, and an environment conducive to sleep.18 When not representing an acute threat to the patient or staff, hospitalists should manage transient agitation from blood draws or vital sign checks by having staff members deescalate and re-approach the intervention later. While multicomponent nonpharmacologic interventions have more robust evidence for prevention of delirium than for treatment, they are low risk and still recommended for the patient with established delirium.19,20
A delirious patient picking at PIVs should prompt clinicians to re-evaluate the need for continued PIV access. If still necessary, experience suggests that PIVs can be protected with a combination of well-taped gauze extending from wrist to shoulder with any attached tubing exiting out of reach behind the shoulder. Also “beneficial distraction” with a task or “activity vest” that consists of an apron with zips, ties, and buttons designed to provide harmless objects can occupy the patient’s hands.
WHEN IT IS HELPFUL TO USE ANTIPSYCHOTICS FOR DELIRIUM
The literature does not provide clear evidence for when the use of antipsychotics is warranted. Antipsychotics may have a role for patients who are having severe psychotic symptoms posing an acute safety risk. In those situations, the American Geriatrics Society recommends using the “lowest effective dose for the shortest possible duration to treat patients who are severely agitated or distressed, and are threatening substantial harm to self and/or others…only if behavioral interventions have failed or are not possible.”12 In those patients who are having an acute myocardial infarction, consider atypical antipsychotics since haloperidol carries a small increased risk of mortality in that patient population.21
RECOMMENDATIONS
- Address underlying modifiable contributions to the delirium paying attention to medications, pain, electrolytes, ischemia, infection, alcohol withdrawal, and reducing invasive lines. Deprescribe sedative/hypnotic and anticholinergic medications.
- After addressing modifiable risk factors, attempt behavioral interventions for continuous problematic behaviors or symptoms of delirium.
- Reserve antipsychotics for cases where the patient poses an immediate danger of self-harm or harm to others. Treat for the shortest possible duration with the lowest effective dose of antipsychotic.
CONCLUSION
Returning to our case presentation, the hospitalist should not prescribe antipsychotic medications since there is no immediate risk of harm and antipsychotics do not treat hypoactive delirium. Delirium is a complex condition requiring a review of multifactorial causes. The hospitalist should investigate and address modifiable contributions. Furthermore, the hospitalist can make the PIV less accessible to deter the patient’s efforts to remove it and offer a distracting activity. Resolution of delirium, in all its forms, is still best achieved by treating the underlying etiology. The use of antipsychotics for treatment of patients with delirium in the absence of severe agitation
Do you think this is a low-value practice? Is this truly a “Thing We Do for No ReasonTM?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No ReasonTM” topics by emailing [email protected].
Disclosures
Dr. Pahwa has received compensation for Expert Testimony, royalties from Aquifer, and owns stock/stock options in Pfizer and Aetna outside the submitted work. Dr. Qureshi and Dr. Cumbler have nothing to disclose.
1. American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C.: American Psychiatric Association; 2013.
2. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. https://doi.org/10.1002/14651858.CD006379.pub2.
3. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1):CD005317. https://doi.org/10.1002/14651858.CD005317.pub2.
4. Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015;41(12):2130-2137. https://doi.org/10.1007/s00134-015-4063-z.
5. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222. https://doi.org/10.1016/j.jagp.2013.09.005.
6. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666. https://doi.org/10.1111/j.1532-5415.2005.53503.x
7. Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalized patients. Cochrane Database Syst Rev. 2007;(2):CD005563. https://doi.org/10.1002/14651858.CD005563.pub2
8. Tahir TA, Eeles E, Karapareddy V, et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. J Psychosom Res. 2010;69(5):485-490. https://doi.org/10.1016/j.jpsychores.2010.05.006.
9. Loh KP, Ramdass S, Garb JL, Brennan MJ, Lindenauer PK, Lagu T. From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med. 2014;9(12):802-804. https://doi.org/10.1002/jhm.2277.
10. Herzig SJ, Rothberg MB, Guess JR, Gurwitz JH, Marcantonio ER. Antipsychotic medication utilization in nonpsychiatric hospitalizations. J Hosp Med. 2016;11(8):543-549. https://doi.org/10.1002/jhm.2596.
11. Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(4):705-714. https://doi.org/10.1111/jgs.14076.
12. American Geriatrics Society Expert Panel on Postoperative Delirium in Older A. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142-150. https://doi.org/10.1111/jgs.13281
13. Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34-42. https://doi.org/10.1001/jamainternmed.2016.7491.
14. Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry. 2014;29(3):253-262. https://doi.org/10.1002/gps.3999.
15. Herzig SJ, LaSalvia MT, Naidus E, et al. Antipsychotics and the risk of aspiration pneumonia in individuals hospitalized for nonpsychiatric conditions: a cohort study. J Am Geriatr Soc. 2017;65(12):2580-2586. https://doi.org/10.1111/jgs.15066.
16. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970. https://doi.org/10.1038/sj.npp.1301492
17. Hui D, Frisbee-Hume S, Wilson A, et al. Effect of lorazepam with haloperidol vs haloperidol alone on agitated delirium in patients with advanced cancer receiving palliative care: a randomized clinical trial. JAMA. 2017;318(11):1047-1056. https://doi.org/10.1001/jama.2017.11468.
18. Inouye SK, Bogardus ST, Jr., Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901
19. Milisen K, Lemiengre J, Braes T, Foreman MD. Multicomponent intervention strategies for managing delirium in hospitalized older people: systematic review. J Adv Nurs. 2005;52(1):79-90. https://doi.org/10.1111/j.1365-2648.2005.03557.x
20. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. https://doi.org/10.1056/NEJMcp1605501.
21. Park Y, Bateman BT, Kim DH, et al. Use of haloperidol versus atypical antipsychotics and risk of in-hospital death in patients with acute myocardial infarction: cohort study. BMJ. 2018;360. https://doi.org/10.1136/bmj.k1218.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No ReasonTM” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNRTM series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CASE
An 86-year-old woman with mild dementia falls at home while preparing a meal. Her son brings her to the emergency department for excruciating pain in her right hip. X-rays reveal a fractured right femur that requires open reduction and internal fixation. On the first postoperative day, she does not participate in therapy and sleeps most of the day. Overnight, a nurse observes her calmly speaking to a hallucination of a family member in the room and picking at the tape around her peripheral intravenous catheter (PIV) causing the PIV to fall out twice. Her vital signs are temperature 36.7°C, pulse 82 beats per minute, respirations 12 breaths per minute, blood pressure 143/72 mm Hg, and pulse oximetry of 99% on room air. She is hypoactive, distractedly picks at her clothing and PIV, inattentive, and unable to say the day of the week or count months backward. Nursing asks for haloperidol for her delirium.
WHY YOU MIGHT THINK ANTIPSYCHOTICS FOR DELIRIUM ARE HELPFUL
Delirium is an acute change in cognition characterized by inattention typically associated with disorganized thinking and/or alteration in consciousness.1 Delirium occurs in almost 25% of hospitalized patients, and clinicians have a limited pharmacologic armamentarium to treat it, given the absence of benefit for acetylcholinesterase inhibitors and concern that benzodiazepine medications cause/exacerbate delirium.2-4 Another treatment option is antipsychotic medications which block dopamine since dopamine excess is a key element in the neurotransmitter pathophysiology of delirium.5 A small 2005 trial of haloperidol prophylaxis in hip fracture patients found that haloperidol reduced the overall severity and duration of delirium.6 Based in part on this trial, a 2007 Cochrane Systematic Review concluded that antipsychotics “may reduce severity and duration of delirium episodes and shorten length of hospital stay in hip surgery.”7 Another study in 2010 demonstrated a 55% faster decline in total Delirium Rating Scale-Revised 98 (DRS-R-98) scores in patients on a general/medical-surgical floor receiving quetiapine treatment compared to those who received placebo.8
Studies show that 10%-30% of patients receive antipsychotics at some point during their hospitalization, usually for delirium.9,10 Variability in antipsychotic prescribing patterns not explained by patient characteristics suggests the local culture may influence antipsychotic prescribing practices when evidence from randomized controlled trials is sparse or conflicting.10
WHY ANTIPSYCHOTIC MEDICATIONS ARE NOT HELPFUL IN PATIENTS WITH DELIRIUM
While few studies have demonstrated positive effects of antipsychotics in delirium treatment, the overall evidence is not persuasive. The results of some studies have not been reproduced while only the positive effects rather than the adverse side effects of antipsychotic medications were highlighted in other articles. For instance, the 2005 hip fracture delirium prophylaxis trial found there was no difference in the incidence of delirium in patients on postoperative day one.7 Furthermore, the 2010 quetiapine study was underpowered for the primary outcome of lower DRS-R-98 scores. Importantly, there was no significant difference in severity of delirium between treatment (quetiapine) and placebo groups on days one, three, or 10.10 These studies show that antipsychotics were neither effective at preventing delirium or in reducing its severity compared to placebo. In 2016, a systemic review in the Journal of the American Geriatric Society included both of the above studies in addition to 17 other studies to assess the efficacy of antipsychotics in preventing and treating delirium. This analysis concluded that antipsychotics did not change the length of delirium or length of stay.11 In addition, the absence of convincing evidence of antipsychotics benefits in postoperative delirium has led the American Geriatrics Society to recommend: “The prescribing practitioner should not prescribe antipsychotic… medications for the treatment of older adults with postoperative delirium who are not agitated and threatening substantial harm to self or others.”12
There is a paucity of data speaking directly to whether antipsychotics reduce patient distress. A recent randomized controlled study compared haloperidol, risperidone, and placebo for delirium treatment in palliative care and hospice patients. With treatment, the patients in the antipsychotic arms demonstrated slightly more severe delirium and a significantly higher incidence of extrapyramidal symptoms (EPS) than the patients receiving placebo.13
Side effects such as EPS, aspiration pneumonia, and arrhythmia are concerns when using antipsychotics for delirium treatment.14 A systematic review and meta-analysis found the difference in EPS incidence between patients treated for delirium with antipsychotics versus no intervention ranged from no difference to over 10%.11 In addition to EPS, patients receiving antipsychotics in a cohort study were at increased risk for aspiration pneumonia compared to patients who did not receive antipsychotics (adjusted odds ratio = 1.5, 95% CI, 1.2-1.9).15 These serious side effects led the Food and Drug Administration (FDA) to issue a black box warning for antipsychotic treatment in dementia-related psychosis. Most importantly, the FDA warns that there is an increased risk of death.16
WHAT YOU SHOULD DO INSTEAD OF USING ANTIPSYCHOTICS
In the first line management of delirium, hospitalists should address underlying modifiable contributions to the condition with attention to medications, pain, electrolytes, ischemia, infection, alcohol withdrawal, and reducing invasive lines. For example, two studies demonstrated a decrease in delirium severity and duration of palliative care in patients by treating delirium triggers, such as dehydration, electrolyte abnormalities, or infection, rather than using antipsychotics.13,17 Furthermore, hospitalists should review the medication list carefully and look for opportunities to deprescribe sedative/hypnotics and anticholinergics.
In addition, hospitalists should implement the core elements of the nursing delirium protocol from the Hospital Elder Life Program (http://www.hospitalelderlifeprogram.org/). The program focuses on orientation, hydration, mobility, sensory aids, and an environment conducive to sleep.18 When not representing an acute threat to the patient or staff, hospitalists should manage transient agitation from blood draws or vital sign checks by having staff members deescalate and re-approach the intervention later. While multicomponent nonpharmacologic interventions have more robust evidence for prevention of delirium than for treatment, they are low risk and still recommended for the patient with established delirium.19,20
A delirious patient picking at PIVs should prompt clinicians to re-evaluate the need for continued PIV access. If still necessary, experience suggests that PIVs can be protected with a combination of well-taped gauze extending from wrist to shoulder with any attached tubing exiting out of reach behind the shoulder. Also “beneficial distraction” with a task or “activity vest” that consists of an apron with zips, ties, and buttons designed to provide harmless objects can occupy the patient’s hands.
WHEN IT IS HELPFUL TO USE ANTIPSYCHOTICS FOR DELIRIUM
The literature does not provide clear evidence for when the use of antipsychotics is warranted. Antipsychotics may have a role for patients who are having severe psychotic symptoms posing an acute safety risk. In those situations, the American Geriatrics Society recommends using the “lowest effective dose for the shortest possible duration to treat patients who are severely agitated or distressed, and are threatening substantial harm to self and/or others…only if behavioral interventions have failed or are not possible.”12 In those patients who are having an acute myocardial infarction, consider atypical antipsychotics since haloperidol carries a small increased risk of mortality in that patient population.21
RECOMMENDATIONS
- Address underlying modifiable contributions to the delirium paying attention to medications, pain, electrolytes, ischemia, infection, alcohol withdrawal, and reducing invasive lines. Deprescribe sedative/hypnotic and anticholinergic medications.
- After addressing modifiable risk factors, attempt behavioral interventions for continuous problematic behaviors or symptoms of delirium.
- Reserve antipsychotics for cases where the patient poses an immediate danger of self-harm or harm to others. Treat for the shortest possible duration with the lowest effective dose of antipsychotic.
CONCLUSION
Returning to our case presentation, the hospitalist should not prescribe antipsychotic medications since there is no immediate risk of harm and antipsychotics do not treat hypoactive delirium. Delirium is a complex condition requiring a review of multifactorial causes. The hospitalist should investigate and address modifiable contributions. Furthermore, the hospitalist can make the PIV less accessible to deter the patient’s efforts to remove it and offer a distracting activity. Resolution of delirium, in all its forms, is still best achieved by treating the underlying etiology. The use of antipsychotics for treatment of patients with delirium in the absence of severe agitation
Do you think this is a low-value practice? Is this truly a “Thing We Do for No ReasonTM?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No ReasonTM” topics by emailing [email protected].
Disclosures
Dr. Pahwa has received compensation for Expert Testimony, royalties from Aquifer, and owns stock/stock options in Pfizer and Aetna outside the submitted work. Dr. Qureshi and Dr. Cumbler have nothing to disclose.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No ReasonTM” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNRTM series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CASE
An 86-year-old woman with mild dementia falls at home while preparing a meal. Her son brings her to the emergency department for excruciating pain in her right hip. X-rays reveal a fractured right femur that requires open reduction and internal fixation. On the first postoperative day, she does not participate in therapy and sleeps most of the day. Overnight, a nurse observes her calmly speaking to a hallucination of a family member in the room and picking at the tape around her peripheral intravenous catheter (PIV) causing the PIV to fall out twice. Her vital signs are temperature 36.7°C, pulse 82 beats per minute, respirations 12 breaths per minute, blood pressure 143/72 mm Hg, and pulse oximetry of 99% on room air. She is hypoactive, distractedly picks at her clothing and PIV, inattentive, and unable to say the day of the week or count months backward. Nursing asks for haloperidol for her delirium.
WHY YOU MIGHT THINK ANTIPSYCHOTICS FOR DELIRIUM ARE HELPFUL
Delirium is an acute change in cognition characterized by inattention typically associated with disorganized thinking and/or alteration in consciousness.1 Delirium occurs in almost 25% of hospitalized patients, and clinicians have a limited pharmacologic armamentarium to treat it, given the absence of benefit for acetylcholinesterase inhibitors and concern that benzodiazepine medications cause/exacerbate delirium.2-4 Another treatment option is antipsychotic medications which block dopamine since dopamine excess is a key element in the neurotransmitter pathophysiology of delirium.5 A small 2005 trial of haloperidol prophylaxis in hip fracture patients found that haloperidol reduced the overall severity and duration of delirium.6 Based in part on this trial, a 2007 Cochrane Systematic Review concluded that antipsychotics “may reduce severity and duration of delirium episodes and shorten length of hospital stay in hip surgery.”7 Another study in 2010 demonstrated a 55% faster decline in total Delirium Rating Scale-Revised 98 (DRS-R-98) scores in patients on a general/medical-surgical floor receiving quetiapine treatment compared to those who received placebo.8
Studies show that 10%-30% of patients receive antipsychotics at some point during their hospitalization, usually for delirium.9,10 Variability in antipsychotic prescribing patterns not explained by patient characteristics suggests the local culture may influence antipsychotic prescribing practices when evidence from randomized controlled trials is sparse or conflicting.10
WHY ANTIPSYCHOTIC MEDICATIONS ARE NOT HELPFUL IN PATIENTS WITH DELIRIUM
While few studies have demonstrated positive effects of antipsychotics in delirium treatment, the overall evidence is not persuasive. The results of some studies have not been reproduced while only the positive effects rather than the adverse side effects of antipsychotic medications were highlighted in other articles. For instance, the 2005 hip fracture delirium prophylaxis trial found there was no difference in the incidence of delirium in patients on postoperative day one.7 Furthermore, the 2010 quetiapine study was underpowered for the primary outcome of lower DRS-R-98 scores. Importantly, there was no significant difference in severity of delirium between treatment (quetiapine) and placebo groups on days one, three, or 10.10 These studies show that antipsychotics were neither effective at preventing delirium or in reducing its severity compared to placebo. In 2016, a systemic review in the Journal of the American Geriatric Society included both of the above studies in addition to 17 other studies to assess the efficacy of antipsychotics in preventing and treating delirium. This analysis concluded that antipsychotics did not change the length of delirium or length of stay.11 In addition, the absence of convincing evidence of antipsychotics benefits in postoperative delirium has led the American Geriatrics Society to recommend: “The prescribing practitioner should not prescribe antipsychotic… medications for the treatment of older adults with postoperative delirium who are not agitated and threatening substantial harm to self or others.”12
There is a paucity of data speaking directly to whether antipsychotics reduce patient distress. A recent randomized controlled study compared haloperidol, risperidone, and placebo for delirium treatment in palliative care and hospice patients. With treatment, the patients in the antipsychotic arms demonstrated slightly more severe delirium and a significantly higher incidence of extrapyramidal symptoms (EPS) than the patients receiving placebo.13
Side effects such as EPS, aspiration pneumonia, and arrhythmia are concerns when using antipsychotics for delirium treatment.14 A systematic review and meta-analysis found the difference in EPS incidence between patients treated for delirium with antipsychotics versus no intervention ranged from no difference to over 10%.11 In addition to EPS, patients receiving antipsychotics in a cohort study were at increased risk for aspiration pneumonia compared to patients who did not receive antipsychotics (adjusted odds ratio = 1.5, 95% CI, 1.2-1.9).15 These serious side effects led the Food and Drug Administration (FDA) to issue a black box warning for antipsychotic treatment in dementia-related psychosis. Most importantly, the FDA warns that there is an increased risk of death.16
WHAT YOU SHOULD DO INSTEAD OF USING ANTIPSYCHOTICS
In the first line management of delirium, hospitalists should address underlying modifiable contributions to the condition with attention to medications, pain, electrolytes, ischemia, infection, alcohol withdrawal, and reducing invasive lines. For example, two studies demonstrated a decrease in delirium severity and duration of palliative care in patients by treating delirium triggers, such as dehydration, electrolyte abnormalities, or infection, rather than using antipsychotics.13,17 Furthermore, hospitalists should review the medication list carefully and look for opportunities to deprescribe sedative/hypnotics and anticholinergics.
In addition, hospitalists should implement the core elements of the nursing delirium protocol from the Hospital Elder Life Program (http://www.hospitalelderlifeprogram.org/). The program focuses on orientation, hydration, mobility, sensory aids, and an environment conducive to sleep.18 When not representing an acute threat to the patient or staff, hospitalists should manage transient agitation from blood draws or vital sign checks by having staff members deescalate and re-approach the intervention later. While multicomponent nonpharmacologic interventions have more robust evidence for prevention of delirium than for treatment, they are low risk and still recommended for the patient with established delirium.19,20
A delirious patient picking at PIVs should prompt clinicians to re-evaluate the need for continued PIV access. If still necessary, experience suggests that PIVs can be protected with a combination of well-taped gauze extending from wrist to shoulder with any attached tubing exiting out of reach behind the shoulder. Also “beneficial distraction” with a task or “activity vest” that consists of an apron with zips, ties, and buttons designed to provide harmless objects can occupy the patient’s hands.
WHEN IT IS HELPFUL TO USE ANTIPSYCHOTICS FOR DELIRIUM
The literature does not provide clear evidence for when the use of antipsychotics is warranted. Antipsychotics may have a role for patients who are having severe psychotic symptoms posing an acute safety risk. In those situations, the American Geriatrics Society recommends using the “lowest effective dose for the shortest possible duration to treat patients who are severely agitated or distressed, and are threatening substantial harm to self and/or others…only if behavioral interventions have failed or are not possible.”12 In those patients who are having an acute myocardial infarction, consider atypical antipsychotics since haloperidol carries a small increased risk of mortality in that patient population.21
RECOMMENDATIONS
- Address underlying modifiable contributions to the delirium paying attention to medications, pain, electrolytes, ischemia, infection, alcohol withdrawal, and reducing invasive lines. Deprescribe sedative/hypnotic and anticholinergic medications.
- After addressing modifiable risk factors, attempt behavioral interventions for continuous problematic behaviors or symptoms of delirium.
- Reserve antipsychotics for cases where the patient poses an immediate danger of self-harm or harm to others. Treat for the shortest possible duration with the lowest effective dose of antipsychotic.
CONCLUSION
Returning to our case presentation, the hospitalist should not prescribe antipsychotic medications since there is no immediate risk of harm and antipsychotics do not treat hypoactive delirium. Delirium is a complex condition requiring a review of multifactorial causes. The hospitalist should investigate and address modifiable contributions. Furthermore, the hospitalist can make the PIV less accessible to deter the patient’s efforts to remove it and offer a distracting activity. Resolution of delirium, in all its forms, is still best achieved by treating the underlying etiology. The use of antipsychotics for treatment of patients with delirium in the absence of severe agitation
Do you think this is a low-value practice? Is this truly a “Thing We Do for No ReasonTM?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No ReasonTM” topics by emailing [email protected].
Disclosures
Dr. Pahwa has received compensation for Expert Testimony, royalties from Aquifer, and owns stock/stock options in Pfizer and Aetna outside the submitted work. Dr. Qureshi and Dr. Cumbler have nothing to disclose.
1. American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C.: American Psychiatric Association; 2013.
2. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. https://doi.org/10.1002/14651858.CD006379.pub2.
3. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1):CD005317. https://doi.org/10.1002/14651858.CD005317.pub2.
4. Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015;41(12):2130-2137. https://doi.org/10.1007/s00134-015-4063-z.
5. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222. https://doi.org/10.1016/j.jagp.2013.09.005.
6. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666. https://doi.org/10.1111/j.1532-5415.2005.53503.x
7. Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalized patients. Cochrane Database Syst Rev. 2007;(2):CD005563. https://doi.org/10.1002/14651858.CD005563.pub2
8. Tahir TA, Eeles E, Karapareddy V, et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. J Psychosom Res. 2010;69(5):485-490. https://doi.org/10.1016/j.jpsychores.2010.05.006.
9. Loh KP, Ramdass S, Garb JL, Brennan MJ, Lindenauer PK, Lagu T. From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med. 2014;9(12):802-804. https://doi.org/10.1002/jhm.2277.
10. Herzig SJ, Rothberg MB, Guess JR, Gurwitz JH, Marcantonio ER. Antipsychotic medication utilization in nonpsychiatric hospitalizations. J Hosp Med. 2016;11(8):543-549. https://doi.org/10.1002/jhm.2596.
11. Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(4):705-714. https://doi.org/10.1111/jgs.14076.
12. American Geriatrics Society Expert Panel on Postoperative Delirium in Older A. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142-150. https://doi.org/10.1111/jgs.13281
13. Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34-42. https://doi.org/10.1001/jamainternmed.2016.7491.
14. Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry. 2014;29(3):253-262. https://doi.org/10.1002/gps.3999.
15. Herzig SJ, LaSalvia MT, Naidus E, et al. Antipsychotics and the risk of aspiration pneumonia in individuals hospitalized for nonpsychiatric conditions: a cohort study. J Am Geriatr Soc. 2017;65(12):2580-2586. https://doi.org/10.1111/jgs.15066.
16. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970. https://doi.org/10.1038/sj.npp.1301492
17. Hui D, Frisbee-Hume S, Wilson A, et al. Effect of lorazepam with haloperidol vs haloperidol alone on agitated delirium in patients with advanced cancer receiving palliative care: a randomized clinical trial. JAMA. 2017;318(11):1047-1056. https://doi.org/10.1001/jama.2017.11468.
18. Inouye SK, Bogardus ST, Jr., Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901
19. Milisen K, Lemiengre J, Braes T, Foreman MD. Multicomponent intervention strategies for managing delirium in hospitalized older people: systematic review. J Adv Nurs. 2005;52(1):79-90. https://doi.org/10.1111/j.1365-2648.2005.03557.x
20. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. https://doi.org/10.1056/NEJMcp1605501.
21. Park Y, Bateman BT, Kim DH, et al. Use of haloperidol versus atypical antipsychotics and risk of in-hospital death in patients with acute myocardial infarction: cohort study. BMJ. 2018;360. https://doi.org/10.1136/bmj.k1218.
1. American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Washington, D.C.: American Psychiatric Association; 2013.
2. Lonergan E, Luxenberg J, Areosa Sastre A. Benzodiazepines for delirium. Cochrane Database Syst Rev. 2009;(4):CD006379. https://doi.org/10.1002/14651858.CD006379.pub2.
3. Overshott R, Karim S, Burns A. Cholinesterase inhibitors for delirium. Cochrane Database Syst Rev. 2008;(1):CD005317. https://doi.org/10.1002/14651858.CD005317.pub2.
4. Zaal IJ, Devlin JW, Hazelbag M, et al. Benzodiazepine-associated delirium in critically ill adults. Intensive Care Med. 2015;41(12):2130-2137. https://doi.org/10.1007/s00134-015-4063-z.
5. Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190-1222. https://doi.org/10.1016/j.jagp.2013.09.005.
6. Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53(10):1658-1666. https://doi.org/10.1111/j.1532-5415.2005.53503.x
7. Siddiqi N, Stockdale R, Britton AM, Holmes J. Interventions for preventing delirium in hospitalized patients. Cochrane Database Syst Rev. 2007;(2):CD005563. https://doi.org/10.1002/14651858.CD005563.pub2
8. Tahir TA, Eeles E, Karapareddy V, et al. A randomized controlled trial of quetiapine versus placebo in the treatment of delirium. J Psychosom Res. 2010;69(5):485-490. https://doi.org/10.1016/j.jpsychores.2010.05.006.
9. Loh KP, Ramdass S, Garb JL, Brennan MJ, Lindenauer PK, Lagu T. From hospital to community: use of antipsychotics in hospitalized elders. J Hosp Med. 2014;9(12):802-804. https://doi.org/10.1002/jhm.2277.
10. Herzig SJ, Rothberg MB, Guess JR, Gurwitz JH, Marcantonio ER. Antipsychotic medication utilization in nonpsychiatric hospitalizations. J Hosp Med. 2016;11(8):543-549. https://doi.org/10.1002/jhm.2596.
11. Neufeld KJ, Yue J, Robinson TN, Inouye SK, Needham DM. Antipsychotic medication for prevention and treatment of delirium in hospitalized adults: a systematic review and meta-analysis. J Am Geriatr Soc. 2016;64(4):705-714. https://doi.org/10.1111/jgs.14076.
12. American Geriatrics Society Expert Panel on Postoperative Delirium in Older A. American Geriatrics Society abstracted clinical practice guideline for postoperative delirium in older adults. J Am Geriatr Soc. 2015;63(1):142-150. https://doi.org/10.1111/jgs.13281
13. Agar MR, Lawlor PG, Quinn S, et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: a randomized clinical trial. JAMA Intern Med. 2017;177(1):34-42. https://doi.org/10.1001/jamainternmed.2016.7491.
14. Hatta K, Kishi Y, Wada K, et al. Antipsychotics for delirium in the general hospital setting in consecutive 2453 inpatients: a prospective observational study. Int J Geriatr Psychiatry. 2014;29(3):253-262. https://doi.org/10.1002/gps.3999.
15. Herzig SJ, LaSalvia MT, Naidus E, et al. Antipsychotics and the risk of aspiration pneumonia in individuals hospitalized for nonpsychiatric conditions: a cohort study. J Am Geriatr Soc. 2017;65(12):2580-2586. https://doi.org/10.1111/jgs.15066.
16. Jeste DV, Blazer D, Casey D, et al. ACNP White Paper: update on use of antipsychotic drugs in elderly persons with dementia. Neuropsychopharmacology. 2008;33(5):957-970. https://doi.org/10.1038/sj.npp.1301492
17. Hui D, Frisbee-Hume S, Wilson A, et al. Effect of lorazepam with haloperidol vs haloperidol alone on agitated delirium in patients with advanced cancer receiving palliative care: a randomized clinical trial. JAMA. 2017;318(11):1047-1056. https://doi.org/10.1001/jama.2017.11468.
18. Inouye SK, Bogardus ST, Jr., Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340(9):669-676. https://doi.org/10.1056/NEJM199903043400901
19. Milisen K, Lemiengre J, Braes T, Foreman MD. Multicomponent intervention strategies for managing delirium in hospitalized older people: systematic review. J Adv Nurs. 2005;52(1):79-90. https://doi.org/10.1111/j.1365-2648.2005.03557.x
20. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. https://doi.org/10.1056/NEJMcp1605501.
21. Park Y, Bateman BT, Kim DH, et al. Use of haloperidol versus atypical antipsychotics and risk of in-hospital death in patients with acute myocardial infarction: cohort study. BMJ. 2018;360. https://doi.org/10.1136/bmj.k1218.
Amit K Pahwa, MD, FAAP; E-mail: [email protected]; Telephone: 410-502-1934.
© 2019 Society of Hospital Medicine
Things We Do for No Reason: Neuroimaging for Hospitalized Patients with Delirium
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 67-year-old woman with a history of hypertension and osteoarthritis was hospitalized for fever, flank pain, and dysuria with pyuria on urinalysis. She was diagnosed with acute pyelonephritis and started ceftriaxone, ondansetron for nausea, and oxycodone for pain. On hospital day two, she developed acute confusion that waxed and waned in severity throughout the day. On examination, she appeared mildly agitated, inattentive, and was noted to pick at her linens and garment. She was oriented to person only and had a nonfocal neurologic examination. Her nurse reported no recent falls or trauma. As part of the patient’s evaluation, her attending physician ordered a head computed tomography (CT) scan.
BACKGROUND
Delirium is commonly diagnosed in hospitalized patients. It has a prevalence of 29%-64% and is associated with longer lengths of stay, higher mortality, and costs of over $164 billion per year in the United States.1 While a number of practice guidelines have been created to help guide delirium diagnosis and management, there is not a clear consensus on when neuroimaging should be performed during the evaluation.2-4 It should also be noted that numerous guidelines for delirium management exist, with variable quality and a heavy reliance on expert opinion.5 Perhaps due to this lack of consensus, neuroimaging is performed in 33% to 67% of hospitalized patients with delirium.6,7
WHY YOU MAY THINK NEUROIMAGING IS HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
Delirium is known to be associated with intracranial processes. For example, delirium occurs in 13% to 48% of patients with acute stroke8 and conversely 7% of patients with new confusion evaluated in emergency departments or inpatient settings were found to have an acute stroke.9 The inclusion of neuroimaging as part of a delirium evaluation is supported in certain circumstances, such as in patients with recent falls, focal neurologic signs (including papilledema), systemic anticoagulation,2 or increased risk of intracranial processes such as metastatic malignancy.4
WHY NEUROIMAGING IS NOT HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
A number of studies have evaluated the diagnostic yield of neuroimaging in hospitalized patients with delirium (Table).6,7,10,11 Two studies included patients with delirium that developed after hospitalization10,11 and two included patients with delirium at admission.6,7
Theisen-Toupal et al. conducted a retrospective study of 220 hospitalized general medical patients who underwent head CT scans for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence or unresponsiveness.10 Patients were excluded if they had a history of falls, head trauma, or new neurologic deficits in the preceding two weeks or if the admitting diagnosis was stroke or cerebral hemorrhage. Additionally, the authors limited patients to those who developed delirium 24 hours or more after admission. There were 6/220 (2.7%) patients identified with an acute intracranial process. Of these six patients, three were receiving anticoagulation. An additional 4/220 (1.8%) head CT scans were identified as equivocal, prompting further neuroimaging, which ultimately showed chronic findings.
Vijayakrishnan et al. performed a retrospective review of 400 hospitalized patients who underwent inpatient CT scans, then limited to those with new delirium.11 They identified 36 patients, of which four (11%) had acute findings on CT: one case each of acute hemorrhage, subdural hematoma, brain metastases, and septic emboli. The authors state “all the four patients had preimaging clinical symptoms and signs, which warranted imaging as per guidelines suggested by the British Geriatrics Society and the Australian and New Zealand Society for Geriatric Medicine,” though they do not provide further details. The strength of this paper is that it isolated patients who developed delirium while hospitalized; however, conclusions were limited by the small sample size.
Lai et al.’s case-control study evaluated 300 consecutive patients admitted to a delirium unit over 18 months.6 Of these 300 patients, 200 (67%) had CT performed; 29/200 (14.5%) had intracranial findings on CT that explained their delirium, including 13 ischemic strokes, seven subdural hemorrhages, nine intracerebral hemorrhages, and three additional ischemic strokes that evolved on follow-up imaging but were not present on the initial scans. The authors performed univariate and multivariate analyses to identify risk factors for an intracranial cause of delirium. Only 3/29 patients with a positive scan did not have one of three main risk factors the authors identified: a fall in the preceding two weeks, new neurologic findings, or sudden deterioration of consciousness. It should be noted that authors did not define “deterioration of consciousness” and that all patients had confusion on admission to the unit, rather than developing during hospitalization.
Hijazi et al. conducted a retrospective cohort study over a 20-month period of 1,653 patients with delirium at the time of admission or during their hospitalization. Patients with delirium due to drug or medication withdrawal or “psychiatric reasons” were excluded. Overall, 538 (32.5%) patients underwent CT, MRI or both, and 78 (14.5%) patients had a positive finding on neuroimaging. This study’s 14.5 % overall yield matches that of Lai et al. Unfortunately, the study included all patients with delirium and did not report the rates of fall, neurologic deficits, and/or use of anticoagulation among those with positive neuroimaging. This limits the generalizability of the findings to a cohort of patients without intracranial pathology risk factors.
The reported yield of neuroimaging for hospitalized patients with delirium ranged from 2.7% to 14.5% across studies. However, in studies taking into account specific patient risk factors; the reported yields in patients without focal neurologic findings, new decline in mental status, systemic anticoagulation, or recent falls were 0%,11 1.4%,10 and 1.5%.6 While a rate of 1.5% may appear high for a serious outcome such as stroke or intracranial bleeding, it is comparable to rates reported for missed major cardiac events in clinical algorithms for evaluating chest pain.12 It should also be noted that neuroimaging is imperfect for acute stroke, and thus the positive or negative predictive value may be poor in the setting of low prevalence. For example, for detection of any acute stroke, the sensitivity/specificity of MRI and CT are 83%/97% and 26%/98% respectively.13
Neuroimaging is expensive and has risks. The average charge for a head CT is approximately $1,400 at academic institutions.14 Moreover, computed tomography exposes patients to significant radiation and up to 2% of malignancies in the United States may be attributable to prior tomography exposure.15 Additionally, there are non-negligible rates of incidental findings during neuroimaging, 1% for CT16 and 2.7%-13.7% for MRI,17,18 which may result in further evaluation or treatment that causes significant patient anxiety. Obtaining neuroimaging on delirious patients can be time consuming and labor intensive, which could delay care to other patients. Additionally, sedating medications are often administered to agitated patients prior to imaging, which risk worsening delirium. Ordering neuroimaging for all patients with acute delirium, therefore, exposes the large majority to unnecessary costs and potential harms.
WHEN NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS COULD BE REASONABLE
The diagnostic yield of head CT in the evaluation of delirium is significantly higher in patients with specific risk factors. Lai et al. found adjusted odds ratios for abnormal CT of 18.2 in patients with new focal deficits, 5.6 with a fall in the preceding two weeks and 4.6 in patients with deterioration in consciousness. Patients with systemic anticoagulation had higher unadjusted, (OR 2.4) though not adjusted odds of having an abnormal CT.6 Thiesen-Toupal et al. excluded patients with recent falls or neurologic deficits but reported that three out of six delirious patients with abnormal neuroimaging were anticoagulated.10 Vijayakrishnan et al. found that all four delirious patients with intracranial findings met guideline criteria for neuroimaging.11 Thus, current recommendations for neuroimaging in delirious patients with falls, focal neurologic deficits, or systemic anticoagulation are appropriate. In situations when a provider lacks an accurate history and is unable to determine if risk factors are present (for example a confused patient found sitting on the floor next to the bed), it may also be reasonable to consider neuroimaging.
Data are limited, but some authors advocate for neuroimaging in cases of delirium that do not improve with treatment.6 Additionally, it may be reasonable to consider neuroimaging in delirium patients with predispositions to embolic or metastatic intracranial processes such as endovascular infections and certain malignancies.4
WHAT YOU SHOULD DO INSTEAD OF NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS
Hospitalized patients with acute confusion should be assessed for delirium with a validated instrument such as the Confusion Assessment Method (CAM).19,20 The original CAM included several components: acute change in mental status with a fluctuating course and inattention, plus either disorganized thinking and/or altered level of consciousness. Multiple delirium assessment tools have been created and validated, all of which include inattention as a required feature. A recent hospital-based study using a two item bedside test asking the patient to name the day of the week and list the months of the year backwards detected delirium with a sensitivity of 93% and specificity of 64%.21 Once the diagnosis of delirium is established, evaluation should begin with a careful history and physical examination focused on the identification of risk factors such as physical restraints, indwelling urinary catheters, and drugs known to precipitate delirium, particularly those with withdrawal potential, anticholinergic properties, and sedative-hypnotic agents.22-24 Delirium may be the first harbinger of serious medical illness and specific testing should be guided by clinical suspicion. In general, a thorough physical examination should look for focal neurologic deficits, hypoxia, signs of infection, and other inflammatory or painful processes that could precipitate delirium.25 Targeted laboratory evaluation may include a basic metabolic panel to identify electrolyte (including calcium) and metabolic derangements, complete blood count, and urinalysis if infection is suspected.
RECOMMENDATIONS
- Use a validated instrument such as CAM to evaluate hospitalized patients who develop altered mental status.
- Delirious patients should undergo a thorough history including a review of medications, physical exam, and targeted laboratory testing aimed at identifying common risk factors and precipitants of delirium that should be addressed.
- Perform neuroimaging if there is a history of fall or head trauma in the preceding two weeks, any new focal abnormalities on neurologic exam or if the patient is receiving systemic anticoagulation.
- It may be reasonable to consider neuroimaging for patients with an atypical course of delirium, such as a sudden decline in the level of consciousness, persistence despite addressing identified factors, or if there is a high degree of suspicion for embolic or metastatic processes.
CONCLUSIONS
Performing neuroimaging in undifferentiated patients who develop delirium while hospitalized has a low diagnostic yield, is costly, and is potentially harmful. Neuroimaging should be reserved for those with identified risk factors for intracranial pathology. For the patient described in the initial vignette with no risk factors for intracranial cause, neuroimaging would be unlikely to contribute to her care. To change provider beliefs and behaviors regarding neuroimaging, prospective studies evaluating guideline implementation are needed. However, based on the current evidence, neuroimaging should be reserved for those with identified risk factors.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing [email protected].
Disclosures
The authors have no conflicts of interest relevant to this article to disclose.
1. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. doi: 10.1016/S0140-6736(13)60688-1. PubMed
2. Clinical practice guidelines for the management of delirium in older people. 2006; https://www2.health.vic.gov.au/Api/downloadmedia/%7BDAA8404B-FEE7-4BDA-8A1E-A32494783B7F%7D. Accessed June 4, 2018.
3. Delirium: prevention, diagnosis and management. NICE Guidance 2010; https://www.nice.org.uk/guidance/cg103. Accessed June 4, 2018.
4. Michaud L, Bula C, Berney A, et al. Delirium: guidelines for general hospitals. J Psychosom Res. 2007;62(3):371-383. doi: 10.1016/j.jpsychores.2006.10.004. PubMed
5. Bush SH, Marchington KL, Agar M, Davis DH, Sikora L, Tsang TW. Quality of clinical practice guidelines in delirium: a systematic appraisal. BMJ Open. 2017;7(3):e013809. doi: 10.1136/bmjopen-2016-013809. PubMed
6. Lai MM, Wong Tin Niam DM. Intracranial cause of delirium: computed tomography yield and predictive factors. Intern Med J. 2012;42(4):422-427. doi: 10.1111/j.1445-5994.2010.02400.x. PubMed
7. Hijazi Z, Lange P, Watson R, Maier AB. The use of cerebral imaging for investigating delirium aetiology. Eur J Intern Med. 2018;52:35-39. doi: 10.1016/j.ejim.2018.01.024. PubMed
8. Oldenbeuving AW, de Kort PL, Jansen BP, Roks G, Kappelle LJ. Delirium in acute stroke: a review. Int J Stroke. 2007;2(4):270-275. doi: 10.1111/j.1747-4949.2007.00163.x. PubMed
9. Benbadis SR, Sila CA, Cristea RL. Mental status changes and stroke. J Stroke Cerebrovasc Dis. 1994;4(4):216-219. doi: 10.1016/S1052-3057(10)80093-X. PubMed
10. Theisen-Toupal J, Breu AC, Mattison ML, Arnaout R. Diagnostic yield of head computed tomography for the hospitalized medical patient with delirium. J Hosp Med. 2014;9(8):497-501. doi: 10.1002/jhm.2198. PubMed
11. Vijayakrishnan R, Ramasubramanian A, Dhand S. Utility of head CT scan for acute inpatient delirium. Hosp Top. 2015;93(1):9-12. doi: 10.1080/00185868.2015.1012928. PubMed
12. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. doi: 10.1016/j.ijcard.2013.01.255. PubMed
13. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293-298. doi: 10.1016/S0140-6736(07)60151-2. PubMed
14. Paul AB, Oklu R, Saini S, Prabhakar AM. How much is that head CT? Price transparency and variability in radiology. J Am Coll Radiol. 2015;12(5):453-457. doi: 10.1016/j.jacr.2014.12.016. PubMed
15. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi: 10.1056/NEJMra072149. PubMed
16. Eskandary H, Sabbagh M, Khajehpour F, Eskandari M. Incidental findings in brain computed tomography scans of 3000 head trauma patients. Surg Neurol. 2005;63(6):550-553; discussion 553. doi: 10.1016/j.surneu.2004.07.049. PubMed
17. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821-1828. doi: 10.1056/NEJMoa070972. PubMed
18. Morris Z, Whiteley WN, Longstreth WT, Jr., et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi: 10.1136/bmj.b3016. PubMed
19. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi: 10.7326/0003-4819-113-12-941. PubMed
20. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830. doi: 10.1111/j.1532-5415.2008.01674.x. PubMed
21. Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med. 2015;10(10):645-650. doi: 10.1002/jhm.2418. PubMed
22. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing the risk of delirium. Am J Psychiatry. 1992;149(10):1393-1394. doi: 10.1176/ajp.149.10.1393. PubMed
23. Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care. Am J Med. 1999;106(5):565-573. doi: 0.1016/S0002-9343(99)00070-4. PubMed
24. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. doi: 10.1056/NEJMc1714932. PubMed
25. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857. doi: 10.1001/jama.1996.03530350034031. PubMed
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 67-year-old woman with a history of hypertension and osteoarthritis was hospitalized for fever, flank pain, and dysuria with pyuria on urinalysis. She was diagnosed with acute pyelonephritis and started ceftriaxone, ondansetron for nausea, and oxycodone for pain. On hospital day two, she developed acute confusion that waxed and waned in severity throughout the day. On examination, she appeared mildly agitated, inattentive, and was noted to pick at her linens and garment. She was oriented to person only and had a nonfocal neurologic examination. Her nurse reported no recent falls or trauma. As part of the patient’s evaluation, her attending physician ordered a head computed tomography (CT) scan.
BACKGROUND
Delirium is commonly diagnosed in hospitalized patients. It has a prevalence of 29%-64% and is associated with longer lengths of stay, higher mortality, and costs of over $164 billion per year in the United States.1 While a number of practice guidelines have been created to help guide delirium diagnosis and management, there is not a clear consensus on when neuroimaging should be performed during the evaluation.2-4 It should also be noted that numerous guidelines for delirium management exist, with variable quality and a heavy reliance on expert opinion.5 Perhaps due to this lack of consensus, neuroimaging is performed in 33% to 67% of hospitalized patients with delirium.6,7
WHY YOU MAY THINK NEUROIMAGING IS HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
Delirium is known to be associated with intracranial processes. For example, delirium occurs in 13% to 48% of patients with acute stroke8 and conversely 7% of patients with new confusion evaluated in emergency departments or inpatient settings were found to have an acute stroke.9 The inclusion of neuroimaging as part of a delirium evaluation is supported in certain circumstances, such as in patients with recent falls, focal neurologic signs (including papilledema), systemic anticoagulation,2 or increased risk of intracranial processes such as metastatic malignancy.4
WHY NEUROIMAGING IS NOT HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
A number of studies have evaluated the diagnostic yield of neuroimaging in hospitalized patients with delirium (Table).6,7,10,11 Two studies included patients with delirium that developed after hospitalization10,11 and two included patients with delirium at admission.6,7
Theisen-Toupal et al. conducted a retrospective study of 220 hospitalized general medical patients who underwent head CT scans for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence or unresponsiveness.10 Patients were excluded if they had a history of falls, head trauma, or new neurologic deficits in the preceding two weeks or if the admitting diagnosis was stroke or cerebral hemorrhage. Additionally, the authors limited patients to those who developed delirium 24 hours or more after admission. There were 6/220 (2.7%) patients identified with an acute intracranial process. Of these six patients, three were receiving anticoagulation. An additional 4/220 (1.8%) head CT scans were identified as equivocal, prompting further neuroimaging, which ultimately showed chronic findings.
Vijayakrishnan et al. performed a retrospective review of 400 hospitalized patients who underwent inpatient CT scans, then limited to those with new delirium.11 They identified 36 patients, of which four (11%) had acute findings on CT: one case each of acute hemorrhage, subdural hematoma, brain metastases, and septic emboli. The authors state “all the four patients had preimaging clinical symptoms and signs, which warranted imaging as per guidelines suggested by the British Geriatrics Society and the Australian and New Zealand Society for Geriatric Medicine,” though they do not provide further details. The strength of this paper is that it isolated patients who developed delirium while hospitalized; however, conclusions were limited by the small sample size.
Lai et al.’s case-control study evaluated 300 consecutive patients admitted to a delirium unit over 18 months.6 Of these 300 patients, 200 (67%) had CT performed; 29/200 (14.5%) had intracranial findings on CT that explained their delirium, including 13 ischemic strokes, seven subdural hemorrhages, nine intracerebral hemorrhages, and three additional ischemic strokes that evolved on follow-up imaging but were not present on the initial scans. The authors performed univariate and multivariate analyses to identify risk factors for an intracranial cause of delirium. Only 3/29 patients with a positive scan did not have one of three main risk factors the authors identified: a fall in the preceding two weeks, new neurologic findings, or sudden deterioration of consciousness. It should be noted that authors did not define “deterioration of consciousness” and that all patients had confusion on admission to the unit, rather than developing during hospitalization.
Hijazi et al. conducted a retrospective cohort study over a 20-month period of 1,653 patients with delirium at the time of admission or during their hospitalization. Patients with delirium due to drug or medication withdrawal or “psychiatric reasons” were excluded. Overall, 538 (32.5%) patients underwent CT, MRI or both, and 78 (14.5%) patients had a positive finding on neuroimaging. This study’s 14.5 % overall yield matches that of Lai et al. Unfortunately, the study included all patients with delirium and did not report the rates of fall, neurologic deficits, and/or use of anticoagulation among those with positive neuroimaging. This limits the generalizability of the findings to a cohort of patients without intracranial pathology risk factors.
The reported yield of neuroimaging for hospitalized patients with delirium ranged from 2.7% to 14.5% across studies. However, in studies taking into account specific patient risk factors; the reported yields in patients without focal neurologic findings, new decline in mental status, systemic anticoagulation, or recent falls were 0%,11 1.4%,10 and 1.5%.6 While a rate of 1.5% may appear high for a serious outcome such as stroke or intracranial bleeding, it is comparable to rates reported for missed major cardiac events in clinical algorithms for evaluating chest pain.12 It should also be noted that neuroimaging is imperfect for acute stroke, and thus the positive or negative predictive value may be poor in the setting of low prevalence. For example, for detection of any acute stroke, the sensitivity/specificity of MRI and CT are 83%/97% and 26%/98% respectively.13
Neuroimaging is expensive and has risks. The average charge for a head CT is approximately $1,400 at academic institutions.14 Moreover, computed tomography exposes patients to significant radiation and up to 2% of malignancies in the United States may be attributable to prior tomography exposure.15 Additionally, there are non-negligible rates of incidental findings during neuroimaging, 1% for CT16 and 2.7%-13.7% for MRI,17,18 which may result in further evaluation or treatment that causes significant patient anxiety. Obtaining neuroimaging on delirious patients can be time consuming and labor intensive, which could delay care to other patients. Additionally, sedating medications are often administered to agitated patients prior to imaging, which risk worsening delirium. Ordering neuroimaging for all patients with acute delirium, therefore, exposes the large majority to unnecessary costs and potential harms.
WHEN NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS COULD BE REASONABLE
The diagnostic yield of head CT in the evaluation of delirium is significantly higher in patients with specific risk factors. Lai et al. found adjusted odds ratios for abnormal CT of 18.2 in patients with new focal deficits, 5.6 with a fall in the preceding two weeks and 4.6 in patients with deterioration in consciousness. Patients with systemic anticoagulation had higher unadjusted, (OR 2.4) though not adjusted odds of having an abnormal CT.6 Thiesen-Toupal et al. excluded patients with recent falls or neurologic deficits but reported that three out of six delirious patients with abnormal neuroimaging were anticoagulated.10 Vijayakrishnan et al. found that all four delirious patients with intracranial findings met guideline criteria for neuroimaging.11 Thus, current recommendations for neuroimaging in delirious patients with falls, focal neurologic deficits, or systemic anticoagulation are appropriate. In situations when a provider lacks an accurate history and is unable to determine if risk factors are present (for example a confused patient found sitting on the floor next to the bed), it may also be reasonable to consider neuroimaging.
Data are limited, but some authors advocate for neuroimaging in cases of delirium that do not improve with treatment.6 Additionally, it may be reasonable to consider neuroimaging in delirium patients with predispositions to embolic or metastatic intracranial processes such as endovascular infections and certain malignancies.4
WHAT YOU SHOULD DO INSTEAD OF NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS
Hospitalized patients with acute confusion should be assessed for delirium with a validated instrument such as the Confusion Assessment Method (CAM).19,20 The original CAM included several components: acute change in mental status with a fluctuating course and inattention, plus either disorganized thinking and/or altered level of consciousness. Multiple delirium assessment tools have been created and validated, all of which include inattention as a required feature. A recent hospital-based study using a two item bedside test asking the patient to name the day of the week and list the months of the year backwards detected delirium with a sensitivity of 93% and specificity of 64%.21 Once the diagnosis of delirium is established, evaluation should begin with a careful history and physical examination focused on the identification of risk factors such as physical restraints, indwelling urinary catheters, and drugs known to precipitate delirium, particularly those with withdrawal potential, anticholinergic properties, and sedative-hypnotic agents.22-24 Delirium may be the first harbinger of serious medical illness and specific testing should be guided by clinical suspicion. In general, a thorough physical examination should look for focal neurologic deficits, hypoxia, signs of infection, and other inflammatory or painful processes that could precipitate delirium.25 Targeted laboratory evaluation may include a basic metabolic panel to identify electrolyte (including calcium) and metabolic derangements, complete blood count, and urinalysis if infection is suspected.
RECOMMENDATIONS
- Use a validated instrument such as CAM to evaluate hospitalized patients who develop altered mental status.
- Delirious patients should undergo a thorough history including a review of medications, physical exam, and targeted laboratory testing aimed at identifying common risk factors and precipitants of delirium that should be addressed.
- Perform neuroimaging if there is a history of fall or head trauma in the preceding two weeks, any new focal abnormalities on neurologic exam or if the patient is receiving systemic anticoagulation.
- It may be reasonable to consider neuroimaging for patients with an atypical course of delirium, such as a sudden decline in the level of consciousness, persistence despite addressing identified factors, or if there is a high degree of suspicion for embolic or metastatic processes.
CONCLUSIONS
Performing neuroimaging in undifferentiated patients who develop delirium while hospitalized has a low diagnostic yield, is costly, and is potentially harmful. Neuroimaging should be reserved for those with identified risk factors for intracranial pathology. For the patient described in the initial vignette with no risk factors for intracranial cause, neuroimaging would be unlikely to contribute to her care. To change provider beliefs and behaviors regarding neuroimaging, prospective studies evaluating guideline implementation are needed. However, based on the current evidence, neuroimaging should be reserved for those with identified risk factors.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing [email protected].
Disclosures
The authors have no conflicts of interest relevant to this article to disclose.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
CLINICAL SCENARIO
A 67-year-old woman with a history of hypertension and osteoarthritis was hospitalized for fever, flank pain, and dysuria with pyuria on urinalysis. She was diagnosed with acute pyelonephritis and started ceftriaxone, ondansetron for nausea, and oxycodone for pain. On hospital day two, she developed acute confusion that waxed and waned in severity throughout the day. On examination, she appeared mildly agitated, inattentive, and was noted to pick at her linens and garment. She was oriented to person only and had a nonfocal neurologic examination. Her nurse reported no recent falls or trauma. As part of the patient’s evaluation, her attending physician ordered a head computed tomography (CT) scan.
BACKGROUND
Delirium is commonly diagnosed in hospitalized patients. It has a prevalence of 29%-64% and is associated with longer lengths of stay, higher mortality, and costs of over $164 billion per year in the United States.1 While a number of practice guidelines have been created to help guide delirium diagnosis and management, there is not a clear consensus on when neuroimaging should be performed during the evaluation.2-4 It should also be noted that numerous guidelines for delirium management exist, with variable quality and a heavy reliance on expert opinion.5 Perhaps due to this lack of consensus, neuroimaging is performed in 33% to 67% of hospitalized patients with delirium.6,7
WHY YOU MAY THINK NEUROIMAGING IS HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
Delirium is known to be associated with intracranial processes. For example, delirium occurs in 13% to 48% of patients with acute stroke8 and conversely 7% of patients with new confusion evaluated in emergency departments or inpatient settings were found to have an acute stroke.9 The inclusion of neuroimaging as part of a delirium evaluation is supported in certain circumstances, such as in patients with recent falls, focal neurologic signs (including papilledema), systemic anticoagulation,2 or increased risk of intracranial processes such as metastatic malignancy.4
WHY NEUROIMAGING IS NOT HELPFUL IN EVALUATING UNDIFFERENTIATED HOSPITALIZED PATIENTS WITH DELIRIUM
A number of studies have evaluated the diagnostic yield of neuroimaging in hospitalized patients with delirium (Table).6,7,10,11 Two studies included patients with delirium that developed after hospitalization10,11 and two included patients with delirium at admission.6,7
Theisen-Toupal et al. conducted a retrospective study of 220 hospitalized general medical patients who underwent head CT scans for an indication of delirium, altered mental status, confusion, encephalopathy, somnolence or unresponsiveness.10 Patients were excluded if they had a history of falls, head trauma, or new neurologic deficits in the preceding two weeks or if the admitting diagnosis was stroke or cerebral hemorrhage. Additionally, the authors limited patients to those who developed delirium 24 hours or more after admission. There were 6/220 (2.7%) patients identified with an acute intracranial process. Of these six patients, three were receiving anticoagulation. An additional 4/220 (1.8%) head CT scans were identified as equivocal, prompting further neuroimaging, which ultimately showed chronic findings.
Vijayakrishnan et al. performed a retrospective review of 400 hospitalized patients who underwent inpatient CT scans, then limited to those with new delirium.11 They identified 36 patients, of which four (11%) had acute findings on CT: one case each of acute hemorrhage, subdural hematoma, brain metastases, and septic emboli. The authors state “all the four patients had preimaging clinical symptoms and signs, which warranted imaging as per guidelines suggested by the British Geriatrics Society and the Australian and New Zealand Society for Geriatric Medicine,” though they do not provide further details. The strength of this paper is that it isolated patients who developed delirium while hospitalized; however, conclusions were limited by the small sample size.
Lai et al.’s case-control study evaluated 300 consecutive patients admitted to a delirium unit over 18 months.6 Of these 300 patients, 200 (67%) had CT performed; 29/200 (14.5%) had intracranial findings on CT that explained their delirium, including 13 ischemic strokes, seven subdural hemorrhages, nine intracerebral hemorrhages, and three additional ischemic strokes that evolved on follow-up imaging but were not present on the initial scans. The authors performed univariate and multivariate analyses to identify risk factors for an intracranial cause of delirium. Only 3/29 patients with a positive scan did not have one of three main risk factors the authors identified: a fall in the preceding two weeks, new neurologic findings, or sudden deterioration of consciousness. It should be noted that authors did not define “deterioration of consciousness” and that all patients had confusion on admission to the unit, rather than developing during hospitalization.
Hijazi et al. conducted a retrospective cohort study over a 20-month period of 1,653 patients with delirium at the time of admission or during their hospitalization. Patients with delirium due to drug or medication withdrawal or “psychiatric reasons” were excluded. Overall, 538 (32.5%) patients underwent CT, MRI or both, and 78 (14.5%) patients had a positive finding on neuroimaging. This study’s 14.5 % overall yield matches that of Lai et al. Unfortunately, the study included all patients with delirium and did not report the rates of fall, neurologic deficits, and/or use of anticoagulation among those with positive neuroimaging. This limits the generalizability of the findings to a cohort of patients without intracranial pathology risk factors.
The reported yield of neuroimaging for hospitalized patients with delirium ranged from 2.7% to 14.5% across studies. However, in studies taking into account specific patient risk factors; the reported yields in patients without focal neurologic findings, new decline in mental status, systemic anticoagulation, or recent falls were 0%,11 1.4%,10 and 1.5%.6 While a rate of 1.5% may appear high for a serious outcome such as stroke or intracranial bleeding, it is comparable to rates reported for missed major cardiac events in clinical algorithms for evaluating chest pain.12 It should also be noted that neuroimaging is imperfect for acute stroke, and thus the positive or negative predictive value may be poor in the setting of low prevalence. For example, for detection of any acute stroke, the sensitivity/specificity of MRI and CT are 83%/97% and 26%/98% respectively.13
Neuroimaging is expensive and has risks. The average charge for a head CT is approximately $1,400 at academic institutions.14 Moreover, computed tomography exposes patients to significant radiation and up to 2% of malignancies in the United States may be attributable to prior tomography exposure.15 Additionally, there are non-negligible rates of incidental findings during neuroimaging, 1% for CT16 and 2.7%-13.7% for MRI,17,18 which may result in further evaluation or treatment that causes significant patient anxiety. Obtaining neuroimaging on delirious patients can be time consuming and labor intensive, which could delay care to other patients. Additionally, sedating medications are often administered to agitated patients prior to imaging, which risk worsening delirium. Ordering neuroimaging for all patients with acute delirium, therefore, exposes the large majority to unnecessary costs and potential harms.
WHEN NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS COULD BE REASONABLE
The diagnostic yield of head CT in the evaluation of delirium is significantly higher in patients with specific risk factors. Lai et al. found adjusted odds ratios for abnormal CT of 18.2 in patients with new focal deficits, 5.6 with a fall in the preceding two weeks and 4.6 in patients with deterioration in consciousness. Patients with systemic anticoagulation had higher unadjusted, (OR 2.4) though not adjusted odds of having an abnormal CT.6 Thiesen-Toupal et al. excluded patients with recent falls or neurologic deficits but reported that three out of six delirious patients with abnormal neuroimaging were anticoagulated.10 Vijayakrishnan et al. found that all four delirious patients with intracranial findings met guideline criteria for neuroimaging.11 Thus, current recommendations for neuroimaging in delirious patients with falls, focal neurologic deficits, or systemic anticoagulation are appropriate. In situations when a provider lacks an accurate history and is unable to determine if risk factors are present (for example a confused patient found sitting on the floor next to the bed), it may also be reasonable to consider neuroimaging.
Data are limited, but some authors advocate for neuroimaging in cases of delirium that do not improve with treatment.6 Additionally, it may be reasonable to consider neuroimaging in delirium patients with predispositions to embolic or metastatic intracranial processes such as endovascular infections and certain malignancies.4
WHAT YOU SHOULD DO INSTEAD OF NEUROIMAGING TO EVALUATE DELIRIUM IN HOSPITALIZED PATIENTS
Hospitalized patients with acute confusion should be assessed for delirium with a validated instrument such as the Confusion Assessment Method (CAM).19,20 The original CAM included several components: acute change in mental status with a fluctuating course and inattention, plus either disorganized thinking and/or altered level of consciousness. Multiple delirium assessment tools have been created and validated, all of which include inattention as a required feature. A recent hospital-based study using a two item bedside test asking the patient to name the day of the week and list the months of the year backwards detected delirium with a sensitivity of 93% and specificity of 64%.21 Once the diagnosis of delirium is established, evaluation should begin with a careful history and physical examination focused on the identification of risk factors such as physical restraints, indwelling urinary catheters, and drugs known to precipitate delirium, particularly those with withdrawal potential, anticholinergic properties, and sedative-hypnotic agents.22-24 Delirium may be the first harbinger of serious medical illness and specific testing should be guided by clinical suspicion. In general, a thorough physical examination should look for focal neurologic deficits, hypoxia, signs of infection, and other inflammatory or painful processes that could precipitate delirium.25 Targeted laboratory evaluation may include a basic metabolic panel to identify electrolyte (including calcium) and metabolic derangements, complete blood count, and urinalysis if infection is suspected.
RECOMMENDATIONS
- Use a validated instrument such as CAM to evaluate hospitalized patients who develop altered mental status.
- Delirious patients should undergo a thorough history including a review of medications, physical exam, and targeted laboratory testing aimed at identifying common risk factors and precipitants of delirium that should be addressed.
- Perform neuroimaging if there is a history of fall or head trauma in the preceding two weeks, any new focal abnormalities on neurologic exam or if the patient is receiving systemic anticoagulation.
- It may be reasonable to consider neuroimaging for patients with an atypical course of delirium, such as a sudden decline in the level of consciousness, persistence despite addressing identified factors, or if there is a high degree of suspicion for embolic or metastatic processes.
CONCLUSIONS
Performing neuroimaging in undifferentiated patients who develop delirium while hospitalized has a low diagnostic yield, is costly, and is potentially harmful. Neuroimaging should be reserved for those with identified risk factors for intracranial pathology. For the patient described in the initial vignette with no risk factors for intracranial cause, neuroimaging would be unlikely to contribute to her care. To change provider beliefs and behaviors regarding neuroimaging, prospective studies evaluating guideline implementation are needed. However, based on the current evidence, neuroimaging should be reserved for those with identified risk factors.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing [email protected].
Disclosures
The authors have no conflicts of interest relevant to this article to disclose.
1. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. doi: 10.1016/S0140-6736(13)60688-1. PubMed
2. Clinical practice guidelines for the management of delirium in older people. 2006; https://www2.health.vic.gov.au/Api/downloadmedia/%7BDAA8404B-FEE7-4BDA-8A1E-A32494783B7F%7D. Accessed June 4, 2018.
3. Delirium: prevention, diagnosis and management. NICE Guidance 2010; https://www.nice.org.uk/guidance/cg103. Accessed June 4, 2018.
4. Michaud L, Bula C, Berney A, et al. Delirium: guidelines for general hospitals. J Psychosom Res. 2007;62(3):371-383. doi: 10.1016/j.jpsychores.2006.10.004. PubMed
5. Bush SH, Marchington KL, Agar M, Davis DH, Sikora L, Tsang TW. Quality of clinical practice guidelines in delirium: a systematic appraisal. BMJ Open. 2017;7(3):e013809. doi: 10.1136/bmjopen-2016-013809. PubMed
6. Lai MM, Wong Tin Niam DM. Intracranial cause of delirium: computed tomography yield and predictive factors. Intern Med J. 2012;42(4):422-427. doi: 10.1111/j.1445-5994.2010.02400.x. PubMed
7. Hijazi Z, Lange P, Watson R, Maier AB. The use of cerebral imaging for investigating delirium aetiology. Eur J Intern Med. 2018;52:35-39. doi: 10.1016/j.ejim.2018.01.024. PubMed
8. Oldenbeuving AW, de Kort PL, Jansen BP, Roks G, Kappelle LJ. Delirium in acute stroke: a review. Int J Stroke. 2007;2(4):270-275. doi: 10.1111/j.1747-4949.2007.00163.x. PubMed
9. Benbadis SR, Sila CA, Cristea RL. Mental status changes and stroke. J Stroke Cerebrovasc Dis. 1994;4(4):216-219. doi: 10.1016/S1052-3057(10)80093-X. PubMed
10. Theisen-Toupal J, Breu AC, Mattison ML, Arnaout R. Diagnostic yield of head computed tomography for the hospitalized medical patient with delirium. J Hosp Med. 2014;9(8):497-501. doi: 10.1002/jhm.2198. PubMed
11. Vijayakrishnan R, Ramasubramanian A, Dhand S. Utility of head CT scan for acute inpatient delirium. Hosp Top. 2015;93(1):9-12. doi: 10.1080/00185868.2015.1012928. PubMed
12. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. doi: 10.1016/j.ijcard.2013.01.255. PubMed
13. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293-298. doi: 10.1016/S0140-6736(07)60151-2. PubMed
14. Paul AB, Oklu R, Saini S, Prabhakar AM. How much is that head CT? Price transparency and variability in radiology. J Am Coll Radiol. 2015;12(5):453-457. doi: 10.1016/j.jacr.2014.12.016. PubMed
15. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi: 10.1056/NEJMra072149. PubMed
16. Eskandary H, Sabbagh M, Khajehpour F, Eskandari M. Incidental findings in brain computed tomography scans of 3000 head trauma patients. Surg Neurol. 2005;63(6):550-553; discussion 553. doi: 10.1016/j.surneu.2004.07.049. PubMed
17. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821-1828. doi: 10.1056/NEJMoa070972. PubMed
18. Morris Z, Whiteley WN, Longstreth WT, Jr., et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi: 10.1136/bmj.b3016. PubMed
19. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi: 10.7326/0003-4819-113-12-941. PubMed
20. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830. doi: 10.1111/j.1532-5415.2008.01674.x. PubMed
21. Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med. 2015;10(10):645-650. doi: 10.1002/jhm.2418. PubMed
22. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing the risk of delirium. Am J Psychiatry. 1992;149(10):1393-1394. doi: 10.1176/ajp.149.10.1393. PubMed
23. Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care. Am J Med. 1999;106(5):565-573. doi: 0.1016/S0002-9343(99)00070-4. PubMed
24. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. doi: 10.1056/NEJMc1714932. PubMed
25. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857. doi: 10.1001/jama.1996.03530350034031. PubMed
1. Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911-922. doi: 10.1016/S0140-6736(13)60688-1. PubMed
2. Clinical practice guidelines for the management of delirium in older people. 2006; https://www2.health.vic.gov.au/Api/downloadmedia/%7BDAA8404B-FEE7-4BDA-8A1E-A32494783B7F%7D. Accessed June 4, 2018.
3. Delirium: prevention, diagnosis and management. NICE Guidance 2010; https://www.nice.org.uk/guidance/cg103. Accessed June 4, 2018.
4. Michaud L, Bula C, Berney A, et al. Delirium: guidelines for general hospitals. J Psychosom Res. 2007;62(3):371-383. doi: 10.1016/j.jpsychores.2006.10.004. PubMed
5. Bush SH, Marchington KL, Agar M, Davis DH, Sikora L, Tsang TW. Quality of clinical practice guidelines in delirium: a systematic appraisal. BMJ Open. 2017;7(3):e013809. doi: 10.1136/bmjopen-2016-013809. PubMed
6. Lai MM, Wong Tin Niam DM. Intracranial cause of delirium: computed tomography yield and predictive factors. Intern Med J. 2012;42(4):422-427. doi: 10.1111/j.1445-5994.2010.02400.x. PubMed
7. Hijazi Z, Lange P, Watson R, Maier AB. The use of cerebral imaging for investigating delirium aetiology. Eur J Intern Med. 2018;52:35-39. doi: 10.1016/j.ejim.2018.01.024. PubMed
8. Oldenbeuving AW, de Kort PL, Jansen BP, Roks G, Kappelle LJ. Delirium in acute stroke: a review. Int J Stroke. 2007;2(4):270-275. doi: 10.1111/j.1747-4949.2007.00163.x. PubMed
9. Benbadis SR, Sila CA, Cristea RL. Mental status changes and stroke. J Stroke Cerebrovasc Dis. 1994;4(4):216-219. doi: 10.1016/S1052-3057(10)80093-X. PubMed
10. Theisen-Toupal J, Breu AC, Mattison ML, Arnaout R. Diagnostic yield of head computed tomography for the hospitalized medical patient with delirium. J Hosp Med. 2014;9(8):497-501. doi: 10.1002/jhm.2198. PubMed
11. Vijayakrishnan R, Ramasubramanian A, Dhand S. Utility of head CT scan for acute inpatient delirium. Hosp Top. 2015;93(1):9-12. doi: 10.1080/00185868.2015.1012928. PubMed
12. Backus BE, Six AJ, Kelder JC, et al. A prospective validation of the HEART score for chest pain patients at the emergency department. Int J Cardiol. 2013;168(3):2153-2158. doi: 10.1016/j.ijcard.2013.01.255. PubMed
13. Chalela JA, Kidwell CS, Nentwich LM, et al. Magnetic resonance imaging and computed tomography in emergency assessment of patients with suspected acute stroke: a prospective comparison. Lancet. 2007;369(9558):293-298. doi: 10.1016/S0140-6736(07)60151-2. PubMed
14. Paul AB, Oklu R, Saini S, Prabhakar AM. How much is that head CT? Price transparency and variability in radiology. J Am Coll Radiol. 2015;12(5):453-457. doi: 10.1016/j.jacr.2014.12.016. PubMed
15. Brenner DJ, Hall EJ. Computed tomography--an increasing source of radiation exposure. N Engl J Med. 2007;357(22):2277-2284. doi: 10.1056/NEJMra072149. PubMed
16. Eskandary H, Sabbagh M, Khajehpour F, Eskandari M. Incidental findings in brain computed tomography scans of 3000 head trauma patients. Surg Neurol. 2005;63(6):550-553; discussion 553. doi: 10.1016/j.surneu.2004.07.049. PubMed
17. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821-1828. doi: 10.1056/NEJMoa070972. PubMed
18. Morris Z, Whiteley WN, Longstreth WT, Jr., et al. Incidental findings on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ. 2009;339:b3016. doi: 10.1136/bmj.b3016. PubMed
19. Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948. doi: 10.7326/0003-4819-113-12-941. PubMed
20. Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830. doi: 10.1111/j.1532-5415.2008.01674.x. PubMed
21. Fick DM, Inouye SK, Guess J, et al. Preliminary development of an ultrabrief two-item bedside test for delirium. J Hosp Med. 2015;10(10):645-650. doi: 10.1002/jhm.2418. PubMed
22. Tune L, Carr S, Hoag E, Cooper T. Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing the risk of delirium. Am J Psychiatry. 1992;149(10):1393-1394. doi: 10.1176/ajp.149.10.1393. PubMed
23. Inouye SK, Schlesinger MJ, Lydon TJ. Delirium: a symptom of how hospital care is failing older persons and a window to improve the quality of hospital care. Am J Med. 1999;106(5):565-573. doi: 0.1016/S0002-9343(99)00070-4. PubMed
24. Marcantonio ER. Delirium in hospitalized older adults. N Engl J Med. 2017;377(15):1456-1466. doi: 10.1056/NEJMc1714932. PubMed
25. Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857. doi: 10.1001/jama.1996.03530350034031. PubMed
© 2019 Society of Hospital Medicine