User login
Things We Do For No Reason: Failing to Question a Penicillin Allergy History
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CLINICAL SCENARIO
An 80-year-old male—with a past medical history significant for hypertension, atrial fibrillation, and type II diabetes mellitus—presented to the hospital with fevers, confusion, and urinary outflow tract difficulties. On exam, he was noted to have mild suprapubic tenderness with flank tenderness. Blood and urine cultures grew Enterococcus faecalis sensitive to ampicillin. Because of the patient’s listed penicillin (PCN) allergy, he was started on aztreonam and vancomycin instead of ampicillin.
WHY YOU MIGHT SIMPLY ACCEPT A PCN ALLERGY HISTORY
Ten percent of the population in the United States reports an allergy to penicillin and derivatives—one of the most commonly reported drug allergies.1 Allergic reactions to drugs are distinct immune reactions mediated by drug-specific immunoglobulin E (IgE) that are potentially life-threatening. Specifically these allergic reactions are called IgE-mediated, type 1 hypersensitivity reactions which are characterized by hives; itching; flushing; tissue swelling, especially in areas of the face and neck; bronchospasm; and gastrointestinal (GI) symptoms, including cramping and diarrhea. Head and neck swelling can quickly result in airway compromise. Profound fluid extravasation and release of mediators from mast cells and basophils can rapidly drop blood pressure. Anaphylaxis requires rapid intervention to prevent severe complications and death. Given the life-threatening consequences of anaphylaxis, a cautious approach before administering PCN to PCN-allergic patients is mandatory.
WHY YOU SHOULD QUESTION A REPORTED PCN ALLERGY
While 10% of the adult population and 15% of hospitalized adults report PCN allergy, clinical studies suggest that 90% of all patients reporting a PCN allergy can tolerate PCN antibiotics.1-3 There are several reasons patients initially labeled as PCN allergic may later be able to tolerate this drug. First, patients can lose sensitivity to specific PCN IgE antibodies over time if PCN is avoided.4 Second, non-IgE-mediated immune reactions of skin or GI tract are often wrongly attributed to an IgE-mediated process from a concurrent medication (Table). For example, viral infections can cause exanthems or hives which may be mistaken for an antibiotic-associated IgE-meditated allergic reaction.6 These non-IgE skin reactions include severe manifestations including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis or benign adverse reactions such as GI upset, dizziness, or diarrhea which are often misclassified as an allergy, and this error is perpetuated in the medical record. Third, patients may report a PCN allergy for themselves when a family member is possibly allergic.
PCN allergy has risen to the level of a public health issue as PCN-allergic patients are often relegated to second-line broad-spectrum antibiotics.7 This public health issue is exacerbated when patients with faux or resolved PCN allergy receive the same treatment. Patients labeled as PCN allergic—whether correctly or incorrectly—have poorer outcomes as noted by increased rates of serious infections and tend to have longer hospital stays.8-10 Treatment-related secondary infections from the use of broad-spectrum antibiotics, such as Clostridiiodes difficile and vancomycin-resistant Enterococcus, are identified more frequently in PCN-allergic patients.7 Additionally, pregnant women with PCN allergy, with or without group B streptococcus infections, have higher rates of cesarean sections and longer hospitalizations.11 The misuse and overuse of antibiotics, especially broad-spectrum medications, has led to resistant bacteria that are increasingly difficult to treat.7 Treating with the most narrow-spectrum antibiotic whenever possible is critical. Overall, failure to address and assess PCN allergy can result in treatment failures and unnecessary broad-spectrum antibiotic use.
WHEN YOU SHOULD BELIEVE A REPORTED PCN AND BETA-LACTAMS ALLERGY HISTORY
Avoid beta-lactams for patients with a reported allergy who are medically frail (eg, critically ill intensive care unit patients and those unable to communicate) or have a documented allergic reaction to a beta-lactam within five years. An estimated 50% of patients who had a documented true IgE-mediated allergic reaction within five years of a documented true allergic reaction remain allergic to PCN and are at risk for an allergic reaction with reexposure.1 PCN allergy evaluation with PCN skin testing (PST) and oral challenge in patients who had a reaction within five years have a higher risk of a fatal outcome with an oral challenge despite negative skin testing. PCN allergy evaluation is best handled on a case by case basis in this population.
WHAT YOU SHOULD DO INSTEAD
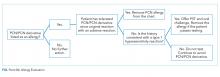
Obtain a thorough drug allergy history. If the history is not consistent with a personal history of an IgE-mediated reaction to PCN ever or if there is documentation that PCN was administered and tolerated since the reaction (eg, a dental prescription), a PCN or beta-lactam can be given. An exception to this rule are patients with a history of an allergic reaction to both a cephalosporin and a PCN—approach this as two separate allergies. Remove the PCN allergy if it is not consistent with the history of IgE-mediated reaction or the patient subsequently had tolerated a PCN/PCN derivative. Regarding the cephalosporin issue, patients are often allergic to a side chain of the cephalosporin and not to the beta-lactam ring. Patients should avoid the specific cephalosporin unless the history is also not consistent with an IgE-mediated reaction or the patient had subsequently tolerated this medication. An allergy evaluation can be useful to discern next steps for cephalosporin allergy. Once the antibiotic is administered and tolerated, the medical record should be updated as well to prevent future mislabeling.
If the symptoms associated with a reported history of a PCN allergy are unknown or consistent with an IgE-mediated reaction, or the patient has not been exposed to PCN since the allergic reaction, the patient should undergo PST followed by a supervised oral test dose to determine whether the allergy exists or persists. PCN allergy evaluation is a simple two-step process of PST followed by an oral challenge of amoxicillin. The use of PCN allergy testing as described is validated and safe.12 A negative skin prick and intradermal test have a negative predictive value that approaches 100%.12,13 Completing the final step—the oral challenge—eliminates concerns for false-negative testing results and patient fears. Additionally, once a patient has had negative skin testing and passed an oral challenge, he/she is not at increased risk of resensitization after PCN/PCN derivative use.14
Although the test takes one and a half hours on average, the benefits that follow are lifelong. Improving future management by disproving a reported allergy affects an individual patient’s clinical course globally, results in cost savings, and increases the use of narrow-spectrum antimicrobials. It is particularly important to test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include, but are not limited to, surgery, transplant, hematology/oncology, and immunosuppressed patients. Inpatients with PCN allergy have higher antibiotic costs—both for medications used during their hospitalization and also for discharge medications.15 A study by Macy and Contreras compared the cost of skin testing to money saved by shortening hospitalization days for 51,582 patients with PCN allergy.7 The cost for testing was $131.37 each (total of $6.7 million). The testing contributed to a $64 million savings for the three-year study period—savings that is 9.5 times larger than the cost of the evaluation.8 A smaller study that looked at cost-effectiveness of PST for 50 patients found an overall cost savings of $11,005 due to the antimicrobial choice alone ($297 per patient switched to a beta-lactam antibiotic).16
RECOMMENDATIONS
- Obtain a thorough drug allergy history as many “allergic reactions” can be removed by history alone. Update the medical record if you can confirm a patient has since tolerated PCN or a PCN derivative to which they were previously allergic. Offer a supervised oral challenge if the patient has any concerns.
- Perform PST if a patient has a PCN allergy listed in their chart and the allergy history is unclear. A negative skin test should be followed by a supervised oral challenge to PCN/PCN derivative if skin testing is negative.
- Test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include surgery, transplant, hematology/oncology, and immunosuppressed patients.
- Implement published protocols from allergists for healthcare systems that lack access to allergy physicians.
- Do not perform PST on patients with a history that is suggestive of a non-IgE-mediated allergic reaction. For these cases, patients are advised to avoid the medication. A supervised graded oral challenge can be considered on a case by case basis if the reaction was not a severe cutaneous adverse reaction syndrome, like SJS, and the benefit of using the medication outweighs the potential harm.
CONCLUSION
The patient, in this case, reported an allergic reaction to PCN over 50 years before this presentation. The reported reaction immediately after receiving IV PCN was a rash—a symptom concerning for an IgE-mediated reaction. Since the patient is well over 10 years from his allergic reaction and would benefit from a PCN derivative, PST testing should be pursued.
The patient passed his skin testing and an oral challenge dose of amoxicillin. With the PCN allergy removed from his chart, his medical team transitioned him from aztreonam and vancomycin to ampicillin. He was then discharged home on amoxicillin and informed that he might be safely treated with PCN/PCN derivatives in the future.
Given the rise in antimicrobial resistance and both the clinical implications and increased costs associated with PCN allergy, it is crucial to offer an allergy evaluation to patients identified as PCN allergic. Hospitalists play a crucial role in obtaining the initial history, determining if the patient has tolerated the antibiotic since the initial reaction, and identifying patients who may benefit from further evaluation for PCN allergy. In hospitals with PST available for inpatients, testing can be performed during the admission. Additionally, it is essential that allergists work with hospitalists and primary care physicians to provide seamless access to outpatient drug allergy evaluations (PST followed by oral challenge) to address the issue of PCN allergy before an acute need for a PCN/PCN derivative antibiotic in the hospital.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing [email protected].
Disclosures
The authors have no conflicts of interest.
Funding
This work is supported by the following NIH Grant: T-32 AI007062-39.
1. American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259-273. https://doi.org/10.1016/j.anai.2010.08.002.
2. American Academy of Allergy AI. Ten things physicians and patients should question Choosing Wisely, ABIM Foundation 2014. http://www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunlogy-non-beta-lactam-antibiotics-penicillin-allergy/. Accessed October 23, 2017.
3. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
4. Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5):918-924. https://doi.org/10.1016/S0091-6749(99)70439-2.
5. Duong TA Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390(10106:1996-2011. doi:10.1016/S0140-6736(16)30378-6.
6. Gonzalez-Estrada A, Radojicic C. Penicillin allergy: a practical guide for clinicians. Cleve Clin J Med. 2015;82(5):295-300. https://doi.org/10.3949/ccjm.82a.14111.
7. Solensky R. Penicillin allergy as a public health measure. J Allergy Clin Immunol. 2014;133(3):797-798. https://doi.org/10.1016/j.jaci.2013.10.032.
8. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. https://doi.org/10.1016/j.jaci.2013.09.021.
9. Chen JR, Khan DA. Evaluation of penicillin allergy in the hospitalized patient: opportunities for antimicrobial stewardship. Curr Allergy Asthma Rep. 2017;17(6):40. https://doi.org/10.1007/s11882-017-0706-1.
10. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
11. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21. https://doi.org/10.7812/TPP/16-080.
12. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1(3):258-263. https://doi.org/10.1016/j.jaip.2013.02.002.
13. Solensky R. The time for penicillin skin testing is here. J Allergy Clin Immunol Pract. 2013;1(3):264-265. https://doi.org/10.1016/j.jaip.2013.03.010.
14. Solensky R, Earl HS, Gruchalla RS. Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002;162(7):822-826.
15. Sade K, Holtzer I, Levo Y, Kivity S. The economic burden of antibiotic treatment of penicillin-allergic patients in internal medicine wards of a general tertiary care hospital. Clin Exp Allergy. 2003;33(4):501-506. https://doi.org/10.1046/j.1365-2222.2003.01638.x.
16. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol. 2016;117(1):67-71. https://doi.org/10.1016/j.anai.2016.04.021.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CLINICAL SCENARIO
An 80-year-old male—with a past medical history significant for hypertension, atrial fibrillation, and type II diabetes mellitus—presented to the hospital with fevers, confusion, and urinary outflow tract difficulties. On exam, he was noted to have mild suprapubic tenderness with flank tenderness. Blood and urine cultures grew Enterococcus faecalis sensitive to ampicillin. Because of the patient’s listed penicillin (PCN) allergy, he was started on aztreonam and vancomycin instead of ampicillin.
WHY YOU MIGHT SIMPLY ACCEPT A PCN ALLERGY HISTORY
Ten percent of the population in the United States reports an allergy to penicillin and derivatives—one of the most commonly reported drug allergies.1 Allergic reactions to drugs are distinct immune reactions mediated by drug-specific immunoglobulin E (IgE) that are potentially life-threatening. Specifically these allergic reactions are called IgE-mediated, type 1 hypersensitivity reactions which are characterized by hives; itching; flushing; tissue swelling, especially in areas of the face and neck; bronchospasm; and gastrointestinal (GI) symptoms, including cramping and diarrhea. Head and neck swelling can quickly result in airway compromise. Profound fluid extravasation and release of mediators from mast cells and basophils can rapidly drop blood pressure. Anaphylaxis requires rapid intervention to prevent severe complications and death. Given the life-threatening consequences of anaphylaxis, a cautious approach before administering PCN to PCN-allergic patients is mandatory.
WHY YOU SHOULD QUESTION A REPORTED PCN ALLERGY
While 10% of the adult population and 15% of hospitalized adults report PCN allergy, clinical studies suggest that 90% of all patients reporting a PCN allergy can tolerate PCN antibiotics.1-3 There are several reasons patients initially labeled as PCN allergic may later be able to tolerate this drug. First, patients can lose sensitivity to specific PCN IgE antibodies over time if PCN is avoided.4 Second, non-IgE-mediated immune reactions of skin or GI tract are often wrongly attributed to an IgE-mediated process from a concurrent medication (Table). For example, viral infections can cause exanthems or hives which may be mistaken for an antibiotic-associated IgE-meditated allergic reaction.6 These non-IgE skin reactions include severe manifestations including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis or benign adverse reactions such as GI upset, dizziness, or diarrhea which are often misclassified as an allergy, and this error is perpetuated in the medical record. Third, patients may report a PCN allergy for themselves when a family member is possibly allergic.
PCN allergy has risen to the level of a public health issue as PCN-allergic patients are often relegated to second-line broad-spectrum antibiotics.7 This public health issue is exacerbated when patients with faux or resolved PCN allergy receive the same treatment. Patients labeled as PCN allergic—whether correctly or incorrectly—have poorer outcomes as noted by increased rates of serious infections and tend to have longer hospital stays.8-10 Treatment-related secondary infections from the use of broad-spectrum antibiotics, such as Clostridiiodes difficile and vancomycin-resistant Enterococcus, are identified more frequently in PCN-allergic patients.7 Additionally, pregnant women with PCN allergy, with or without group B streptococcus infections, have higher rates of cesarean sections and longer hospitalizations.11 The misuse and overuse of antibiotics, especially broad-spectrum medications, has led to resistant bacteria that are increasingly difficult to treat.7 Treating with the most narrow-spectrum antibiotic whenever possible is critical. Overall, failure to address and assess PCN allergy can result in treatment failures and unnecessary broad-spectrum antibiotic use.
WHEN YOU SHOULD BELIEVE A REPORTED PCN AND BETA-LACTAMS ALLERGY HISTORY
Avoid beta-lactams for patients with a reported allergy who are medically frail (eg, critically ill intensive care unit patients and those unable to communicate) or have a documented allergic reaction to a beta-lactam within five years. An estimated 50% of patients who had a documented true IgE-mediated allergic reaction within five years of a documented true allergic reaction remain allergic to PCN and are at risk for an allergic reaction with reexposure.1 PCN allergy evaluation with PCN skin testing (PST) and oral challenge in patients who had a reaction within five years have a higher risk of a fatal outcome with an oral challenge despite negative skin testing. PCN allergy evaluation is best handled on a case by case basis in this population.
WHAT YOU SHOULD DO INSTEAD
Obtain a thorough drug allergy history. If the history is not consistent with a personal history of an IgE-mediated reaction to PCN ever or if there is documentation that PCN was administered and tolerated since the reaction (eg, a dental prescription), a PCN or beta-lactam can be given. An exception to this rule are patients with a history of an allergic reaction to both a cephalosporin and a PCN—approach this as two separate allergies. Remove the PCN allergy if it is not consistent with the history of IgE-mediated reaction or the patient subsequently had tolerated a PCN/PCN derivative. Regarding the cephalosporin issue, patients are often allergic to a side chain of the cephalosporin and not to the beta-lactam ring. Patients should avoid the specific cephalosporin unless the history is also not consistent with an IgE-mediated reaction or the patient had subsequently tolerated this medication. An allergy evaluation can be useful to discern next steps for cephalosporin allergy. Once the antibiotic is administered and tolerated, the medical record should be updated as well to prevent future mislabeling.
If the symptoms associated with a reported history of a PCN allergy are unknown or consistent with an IgE-mediated reaction, or the patient has not been exposed to PCN since the allergic reaction, the patient should undergo PST followed by a supervised oral test dose to determine whether the allergy exists or persists. PCN allergy evaluation is a simple two-step process of PST followed by an oral challenge of amoxicillin. The use of PCN allergy testing as described is validated and safe.12 A negative skin prick and intradermal test have a negative predictive value that approaches 100%.12,13 Completing the final step—the oral challenge—eliminates concerns for false-negative testing results and patient fears. Additionally, once a patient has had negative skin testing and passed an oral challenge, he/she is not at increased risk of resensitization after PCN/PCN derivative use.14
Although the test takes one and a half hours on average, the benefits that follow are lifelong. Improving future management by disproving a reported allergy affects an individual patient’s clinical course globally, results in cost savings, and increases the use of narrow-spectrum antimicrobials. It is particularly important to test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include, but are not limited to, surgery, transplant, hematology/oncology, and immunosuppressed patients. Inpatients with PCN allergy have higher antibiotic costs—both for medications used during their hospitalization and also for discharge medications.15 A study by Macy and Contreras compared the cost of skin testing to money saved by shortening hospitalization days for 51,582 patients with PCN allergy.7 The cost for testing was $131.37 each (total of $6.7 million). The testing contributed to a $64 million savings for the three-year study period—savings that is 9.5 times larger than the cost of the evaluation.8 A smaller study that looked at cost-effectiveness of PST for 50 patients found an overall cost savings of $11,005 due to the antimicrobial choice alone ($297 per patient switched to a beta-lactam antibiotic).16
RECOMMENDATIONS
- Obtain a thorough drug allergy history as many “allergic reactions” can be removed by history alone. Update the medical record if you can confirm a patient has since tolerated PCN or a PCN derivative to which they were previously allergic. Offer a supervised oral challenge if the patient has any concerns.
- Perform PST if a patient has a PCN allergy listed in their chart and the allergy history is unclear. A negative skin test should be followed by a supervised oral challenge to PCN/PCN derivative if skin testing is negative.
- Test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include surgery, transplant, hematology/oncology, and immunosuppressed patients.
- Implement published protocols from allergists for healthcare systems that lack access to allergy physicians.
- Do not perform PST on patients with a history that is suggestive of a non-IgE-mediated allergic reaction. For these cases, patients are advised to avoid the medication. A supervised graded oral challenge can be considered on a case by case basis if the reaction was not a severe cutaneous adverse reaction syndrome, like SJS, and the benefit of using the medication outweighs the potential harm.
CONCLUSION
The patient, in this case, reported an allergic reaction to PCN over 50 years before this presentation. The reported reaction immediately after receiving IV PCN was a rash—a symptom concerning for an IgE-mediated reaction. Since the patient is well over 10 years from his allergic reaction and would benefit from a PCN derivative, PST testing should be pursued.
The patient passed his skin testing and an oral challenge dose of amoxicillin. With the PCN allergy removed from his chart, his medical team transitioned him from aztreonam and vancomycin to ampicillin. He was then discharged home on amoxicillin and informed that he might be safely treated with PCN/PCN derivatives in the future.
Given the rise in antimicrobial resistance and both the clinical implications and increased costs associated with PCN allergy, it is crucial to offer an allergy evaluation to patients identified as PCN allergic. Hospitalists play a crucial role in obtaining the initial history, determining if the patient has tolerated the antibiotic since the initial reaction, and identifying patients who may benefit from further evaluation for PCN allergy. In hospitals with PST available for inpatients, testing can be performed during the admission. Additionally, it is essential that allergists work with hospitalists and primary care physicians to provide seamless access to outpatient drug allergy evaluations (PST followed by oral challenge) to address the issue of PCN allergy before an acute need for a PCN/PCN derivative antibiotic in the hospital.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing [email protected].
Disclosures
The authors have no conflicts of interest.
Funding
This work is supported by the following NIH Grant: T-32 AI007062-39.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.

CLINICAL SCENARIO
An 80-year-old male—with a past medical history significant for hypertension, atrial fibrillation, and type II diabetes mellitus—presented to the hospital with fevers, confusion, and urinary outflow tract difficulties. On exam, he was noted to have mild suprapubic tenderness with flank tenderness. Blood and urine cultures grew Enterococcus faecalis sensitive to ampicillin. Because of the patient’s listed penicillin (PCN) allergy, he was started on aztreonam and vancomycin instead of ampicillin.
WHY YOU MIGHT SIMPLY ACCEPT A PCN ALLERGY HISTORY
Ten percent of the population in the United States reports an allergy to penicillin and derivatives—one of the most commonly reported drug allergies.1 Allergic reactions to drugs are distinct immune reactions mediated by drug-specific immunoglobulin E (IgE) that are potentially life-threatening. Specifically these allergic reactions are called IgE-mediated, type 1 hypersensitivity reactions which are characterized by hives; itching; flushing; tissue swelling, especially in areas of the face and neck; bronchospasm; and gastrointestinal (GI) symptoms, including cramping and diarrhea. Head and neck swelling can quickly result in airway compromise. Profound fluid extravasation and release of mediators from mast cells and basophils can rapidly drop blood pressure. Anaphylaxis requires rapid intervention to prevent severe complications and death. Given the life-threatening consequences of anaphylaxis, a cautious approach before administering PCN to PCN-allergic patients is mandatory.
WHY YOU SHOULD QUESTION A REPORTED PCN ALLERGY
While 10% of the adult population and 15% of hospitalized adults report PCN allergy, clinical studies suggest that 90% of all patients reporting a PCN allergy can tolerate PCN antibiotics.1-3 There are several reasons patients initially labeled as PCN allergic may later be able to tolerate this drug. First, patients can lose sensitivity to specific PCN IgE antibodies over time if PCN is avoided.4 Second, non-IgE-mediated immune reactions of skin or GI tract are often wrongly attributed to an IgE-mediated process from a concurrent medication (Table). For example, viral infections can cause exanthems or hives which may be mistaken for an antibiotic-associated IgE-meditated allergic reaction.6 These non-IgE skin reactions include severe manifestations including Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis or benign adverse reactions such as GI upset, dizziness, or diarrhea which are often misclassified as an allergy, and this error is perpetuated in the medical record. Third, patients may report a PCN allergy for themselves when a family member is possibly allergic.
PCN allergy has risen to the level of a public health issue as PCN-allergic patients are often relegated to second-line broad-spectrum antibiotics.7 This public health issue is exacerbated when patients with faux or resolved PCN allergy receive the same treatment. Patients labeled as PCN allergic—whether correctly or incorrectly—have poorer outcomes as noted by increased rates of serious infections and tend to have longer hospital stays.8-10 Treatment-related secondary infections from the use of broad-spectrum antibiotics, such as Clostridiiodes difficile and vancomycin-resistant Enterococcus, are identified more frequently in PCN-allergic patients.7 Additionally, pregnant women with PCN allergy, with or without group B streptococcus infections, have higher rates of cesarean sections and longer hospitalizations.11 The misuse and overuse of antibiotics, especially broad-spectrum medications, has led to resistant bacteria that are increasingly difficult to treat.7 Treating with the most narrow-spectrum antibiotic whenever possible is critical. Overall, failure to address and assess PCN allergy can result in treatment failures and unnecessary broad-spectrum antibiotic use.
WHEN YOU SHOULD BELIEVE A REPORTED PCN AND BETA-LACTAMS ALLERGY HISTORY
Avoid beta-lactams for patients with a reported allergy who are medically frail (eg, critically ill intensive care unit patients and those unable to communicate) or have a documented allergic reaction to a beta-lactam within five years. An estimated 50% of patients who had a documented true IgE-mediated allergic reaction within five years of a documented true allergic reaction remain allergic to PCN and are at risk for an allergic reaction with reexposure.1 PCN allergy evaluation with PCN skin testing (PST) and oral challenge in patients who had a reaction within five years have a higher risk of a fatal outcome with an oral challenge despite negative skin testing. PCN allergy evaluation is best handled on a case by case basis in this population.
WHAT YOU SHOULD DO INSTEAD
Obtain a thorough drug allergy history. If the history is not consistent with a personal history of an IgE-mediated reaction to PCN ever or if there is documentation that PCN was administered and tolerated since the reaction (eg, a dental prescription), a PCN or beta-lactam can be given. An exception to this rule are patients with a history of an allergic reaction to both a cephalosporin and a PCN—approach this as two separate allergies. Remove the PCN allergy if it is not consistent with the history of IgE-mediated reaction or the patient subsequently had tolerated a PCN/PCN derivative. Regarding the cephalosporin issue, patients are often allergic to a side chain of the cephalosporin and not to the beta-lactam ring. Patients should avoid the specific cephalosporin unless the history is also not consistent with an IgE-mediated reaction or the patient had subsequently tolerated this medication. An allergy evaluation can be useful to discern next steps for cephalosporin allergy. Once the antibiotic is administered and tolerated, the medical record should be updated as well to prevent future mislabeling.
If the symptoms associated with a reported history of a PCN allergy are unknown or consistent with an IgE-mediated reaction, or the patient has not been exposed to PCN since the allergic reaction, the patient should undergo PST followed by a supervised oral test dose to determine whether the allergy exists or persists. PCN allergy evaluation is a simple two-step process of PST followed by an oral challenge of amoxicillin. The use of PCN allergy testing as described is validated and safe.12 A negative skin prick and intradermal test have a negative predictive value that approaches 100%.12,13 Completing the final step—the oral challenge—eliminates concerns for false-negative testing results and patient fears. Additionally, once a patient has had negative skin testing and passed an oral challenge, he/she is not at increased risk of resensitization after PCN/PCN derivative use.14
Although the test takes one and a half hours on average, the benefits that follow are lifelong. Improving future management by disproving a reported allergy affects an individual patient’s clinical course globally, results in cost savings, and increases the use of narrow-spectrum antimicrobials. It is particularly important to test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include, but are not limited to, surgery, transplant, hematology/oncology, and immunosuppressed patients. Inpatients with PCN allergy have higher antibiotic costs—both for medications used during their hospitalization and also for discharge medications.15 A study by Macy and Contreras compared the cost of skin testing to money saved by shortening hospitalization days for 51,582 patients with PCN allergy.7 The cost for testing was $131.37 each (total of $6.7 million). The testing contributed to a $64 million savings for the three-year study period—savings that is 9.5 times larger than the cost of the evaluation.8 A smaller study that looked at cost-effectiveness of PST for 50 patients found an overall cost savings of $11,005 due to the antimicrobial choice alone ($297 per patient switched to a beta-lactam antibiotic).16
RECOMMENDATIONS
- Obtain a thorough drug allergy history as many “allergic reactions” can be removed by history alone. Update the medical record if you can confirm a patient has since tolerated PCN or a PCN derivative to which they were previously allergic. Offer a supervised oral challenge if the patient has any concerns.
- Perform PST if a patient has a PCN allergy listed in their chart and the allergy history is unclear. A negative skin test should be followed by a supervised oral challenge to PCN/PCN derivative if skin testing is negative.
- Test PCN-allergic patients preemptively who are at high risk of requiring PCN/PCN derivative antibiotics. High-risk patients include surgery, transplant, hematology/oncology, and immunosuppressed patients.
- Implement published protocols from allergists for healthcare systems that lack access to allergy physicians.
- Do not perform PST on patients with a history that is suggestive of a non-IgE-mediated allergic reaction. For these cases, patients are advised to avoid the medication. A supervised graded oral challenge can be considered on a case by case basis if the reaction was not a severe cutaneous adverse reaction syndrome, like SJS, and the benefit of using the medication outweighs the potential harm.
CONCLUSION
The patient, in this case, reported an allergic reaction to PCN over 50 years before this presentation. The reported reaction immediately after receiving IV PCN was a rash—a symptom concerning for an IgE-mediated reaction. Since the patient is well over 10 years from his allergic reaction and would benefit from a PCN derivative, PST testing should be pursued.
The patient passed his skin testing and an oral challenge dose of amoxicillin. With the PCN allergy removed from his chart, his medical team transitioned him from aztreonam and vancomycin to ampicillin. He was then discharged home on amoxicillin and informed that he might be safely treated with PCN/PCN derivatives in the future.
Given the rise in antimicrobial resistance and both the clinical implications and increased costs associated with PCN allergy, it is crucial to offer an allergy evaluation to patients identified as PCN allergic. Hospitalists play a crucial role in obtaining the initial history, determining if the patient has tolerated the antibiotic since the initial reaction, and identifying patients who may benefit from further evaluation for PCN allergy. In hospitals with PST available for inpatients, testing can be performed during the admission. Additionally, it is essential that allergists work with hospitalists and primary care physicians to provide seamless access to outpatient drug allergy evaluations (PST followed by oral challenge) to address the issue of PCN allergy before an acute need for a PCN/PCN derivative antibiotic in the hospital.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by e-mailing [email protected].
Disclosures
The authors have no conflicts of interest.
Funding
This work is supported by the following NIH Grant: T-32 AI007062-39.
1. American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259-273. https://doi.org/10.1016/j.anai.2010.08.002.
2. American Academy of Allergy AI. Ten things physicians and patients should question Choosing Wisely, ABIM Foundation 2014. http://www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunlogy-non-beta-lactam-antibiotics-penicillin-allergy/. Accessed October 23, 2017.
3. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
4. Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5):918-924. https://doi.org/10.1016/S0091-6749(99)70439-2.
5. Duong TA Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390(10106:1996-2011. doi:10.1016/S0140-6736(16)30378-6.
6. Gonzalez-Estrada A, Radojicic C. Penicillin allergy: a practical guide for clinicians. Cleve Clin J Med. 2015;82(5):295-300. https://doi.org/10.3949/ccjm.82a.14111.
7. Solensky R. Penicillin allergy as a public health measure. J Allergy Clin Immunol. 2014;133(3):797-798. https://doi.org/10.1016/j.jaci.2013.10.032.
8. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. https://doi.org/10.1016/j.jaci.2013.09.021.
9. Chen JR, Khan DA. Evaluation of penicillin allergy in the hospitalized patient: opportunities for antimicrobial stewardship. Curr Allergy Asthma Rep. 2017;17(6):40. https://doi.org/10.1007/s11882-017-0706-1.
10. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
11. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21. https://doi.org/10.7812/TPP/16-080.
12. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1(3):258-263. https://doi.org/10.1016/j.jaip.2013.02.002.
13. Solensky R. The time for penicillin skin testing is here. J Allergy Clin Immunol Pract. 2013;1(3):264-265. https://doi.org/10.1016/j.jaip.2013.03.010.
14. Solensky R, Earl HS, Gruchalla RS. Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002;162(7):822-826.
15. Sade K, Holtzer I, Levo Y, Kivity S. The economic burden of antibiotic treatment of penicillin-allergic patients in internal medicine wards of a general tertiary care hospital. Clin Exp Allergy. 2003;33(4):501-506. https://doi.org/10.1046/j.1365-2222.2003.01638.x.
16. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol. 2016;117(1):67-71. https://doi.org/10.1016/j.anai.2016.04.021.
1. American Academy of Allergy, Asthma and Immunology, the American College of Allergy, Asthma and Immunology, and the Joint Council of Allergy, Asthma and Immunology. Drug allergy: an updated practice parameter. Ann Allergy Asthma Immunol. 2010;105(4):259-273. https://doi.org/10.1016/j.anai.2010.08.002.
2. American Academy of Allergy AI. Ten things physicians and patients should question Choosing Wisely, ABIM Foundation 2014. http://www.choosingwisely.org/clinician-lists/american-academy-allergy-asthma-immunlogy-non-beta-lactam-antibiotics-penicillin-allergy/. Accessed October 23, 2017.
3. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
4. Blanca M, Torres MJ, Garcia JJ, et al. Natural evolution of skin test sensitivity in patients allergic to beta-lactam antibiotics. J Allergy Clin Immunol. 1999;103(5):918-924. https://doi.org/10.1016/S0091-6749(99)70439-2.
5. Duong TA Valeyrie-Allanore L, Wolkenstein P, Chosidow O. Severe cutaneous adverse reactions to drugs. Lancet. 2017;390(10106:1996-2011. doi:10.1016/S0140-6736(16)30378-6.
6. Gonzalez-Estrada A, Radojicic C. Penicillin allergy: a practical guide for clinicians. Cleve Clin J Med. 2015;82(5):295-300. https://doi.org/10.3949/ccjm.82a.14111.
7. Solensky R. Penicillin allergy as a public health measure. J Allergy Clin Immunol. 2014;133(3):797-798. https://doi.org/10.1016/j.jaci.2013.10.032.
8. Macy E, Contreras R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: a cohort study. J Allergy Clin Immunol. 2014;133(3):790-796. https://doi.org/10.1016/j.jaci.2013.09.021.
9. Chen JR, Khan DA. Evaluation of penicillin allergy in the hospitalized patient: opportunities for antimicrobial stewardship. Curr Allergy Asthma Rep. 2017;17(6):40. https://doi.org/10.1007/s11882-017-0706-1.
10. Blumenthal KG, Wickner PG, Hurwitz S, et al. Tackling inpatient penicillin allergies: Assessing tools for antimicrobial stewardship. J Allergy Clin Immunol. 2017;140(1):154-161. https://doi.org/10.1016/j.jaci.2017.02.005.
11. Desai SH, Kaplan MS, Chen Q, Macy EM. Morbidity in pregnant women associated with unverified penicillin allergies, antibiotic use, and group B Streptococcus infections. Perm J. 2017;21. https://doi.org/10.7812/TPP/16-080.
12. Macy E, Ngor EW. Safely diagnosing clinically significant penicillin allergy using only penicilloyl-poly-lysine, penicillin, and oral amoxicillin. J Allergy Clin Immunol Pract. 2013;1(3):258-263. https://doi.org/10.1016/j.jaip.2013.02.002.
13. Solensky R. The time for penicillin skin testing is here. J Allergy Clin Immunol Pract. 2013;1(3):264-265. https://doi.org/10.1016/j.jaip.2013.03.010.
14. Solensky R, Earl HS, Gruchalla RS. Lack of penicillin resensitization in patients with a history of penicillin allergy after receiving repeated penicillin courses. Arch Intern Med. 2002;162(7):822-826.
15. Sade K, Holtzer I, Levo Y, Kivity S. The economic burden of antibiotic treatment of penicillin-allergic patients in internal medicine wards of a general tertiary care hospital. Clin Exp Allergy. 2003;33(4):501-506. https://doi.org/10.1046/j.1365-2222.2003.01638.x.
16. King EA, Challa S, Curtin P, Bielory L. Penicillin skin testing in hospitalized patients with beta-lactam allergies: effect on antibiotic selection and cost. Ann Allergy Asthma Immunol. 2016;117(1):67-71. https://doi.org/10.1016/j.anai.2016.04.021.
©2019 Society of Hospital Medicine
Things We Do For No Reason: Contact Precautions for MRSA and VRE
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE
A 67-year-old man is admitted to a telemetry ward for an acute myocardial infarction and treated with percutaneous coronary intervention. He is currently on day three of antibiotics for a methicillin-resistant Staphylococcus aureus (MRSA) lower extremity soft tissue infection that is healing without a draining wound. He is placed on contact precautions based on institutional infection control guidelines. The hospitalist overhears members of the team commenting on having to don gowns to see this patient each day and wonders aloud whether care is impacted by the use of contact precautions.
BACKGROUND
Contact precautions (CP) for patients with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) infections are common in several hospitals. CP pose a significant burden to health systems, with an estimated 20%-25% of hospitalized patients on CP for MRSA or VRE alone.1 CP are becoming increasingly more prevalent with state laws and the Veterans Affairs (VA) hospital system requiring active surveillance cultures (ASC) and subsequent CP when ASC are positive.2
WHY YOU MIGHT THINK CONTACT PRECAUTIONS ARE HELPFUL FOR MRSA AND VRE
Supporters highlight the utility of CP in preventing the spread of infection, controlling outbreaks, and protecting healthcare workers from certain transmissible diseases. The Centers for Disease Control and Prevention (CDC) recommended CP after prior studies demonstrated their effectiveness during outbreaks of transmissible infections.3 CP were included in bundles alongside interventions such as improving hand hygiene, chlorhexidine gluconate (CHG) bathing, and ASC with targeted or universal decolonization.2 The VA MRSA bundle, for example, demonstrated a reduction of healthcare-associated MRSA in the ICU by 62% after implementation. The Society for Healthcare Epidemiology of America Research Network (SHEA) and the Infectious Diseases Society of America (IDSA) recommend CP for MRSA-infected and colonized patients in acute care settings to control outbreaks.4,5 The CDC also has broad recommendations supporting CP for all patients infected and previously identified as being colonized with target multidrug-resistant organisms (MDROs) without identifying which are considered to be “targets.”6
WHY CONTACT PRECAUTIONS MAY NOT BE HELPFUL FOR MRSA AND VRE
Despite current guidelines, cluster-randomized trials have not shown a benefit of initiating CP over usual care for the prevention of acquiring MRSA or VRE in the hospital. One study demonstrated no change in MRSA and VRE acquisition with broad screening and subsequent CP.7 Another study evaluated a universal gown and glove policy in an ICU setting and found a reduction in MRSA acquisition, but no reduction in VRE acquisition.8 A third study investigated hand hygiene and daily CHG bathing and noted a reduction in MRSA transmission rates, where CP for screened colonized patients had no effect on transmission of MRSA or VRE.9
In addition, a prospective trial at a large academic center over two six-month intervals utilized universal gloving with emollient-impregnated gloves compared with CP and found no difference in MDRO acquisition. Universal gloving was associated with higher hand hygiene rates than CP.10 Another more recent retrospective observational study compared universal contact precautions (UCP) in ICUs to a historical nine-year baseline and concurrently to other nonuniversal CP ICUs. There was no significant decrease in MDROs during the UCP period compared with baseline or with non-UCP units.11Further interest in and scrutiny of CP prompted a recently published meta-analysis of 14 studies in which CP were eliminated. The rates of transmission of MRSA, VRE, or other MDROs studied were not impacted by discontinuation.12 One of the studies included two large academic medical centers and assessed the impact of discontinuing CP for endemic MRSA and VRE. The bundled intervention included the discontinuation of CP for all carriers of MRSA and VRE, except patients with draining wounds, maintaining high hand hygiene rates, and CHG baths for nearly all patients. There was no significant increase in transmission rates, and the intervention saved the health system an estimated $643,776 and 45,277 hours per year in healthcare worker time previously spent on donning and doffing personal protective equipment.13 Another large academic hospital published a time series approach of seven interventions to reduce healthcare-associated infections and noted no increase in MRSA or VRE transmission when CP were discontinued when combined with other horizontal preventions.14 Results were found to be similar in a high-risk population of patients with hematologic malignancies and hematopoietic stem cell transplantation, where both surveillance and CP for VRE were discontinued and did not impact the rates of VRE bacteremia.15
WHY CONTACT PRECAUTIONS MAY BE HARMFUL
Multiple studies have examined the deleterious effects of CP, including a comprehensive systematic literature review of various adverse outcomes linked with CP.16 CP decrease the amount of time that healthcare workers (HCW) spend with patients,17 create delays at admission and discharge,18 increase symptoms of anxiety and depression in patients,19,20 and decrease patient satisfaction with care.21,22 In a study conducted at the Cleveland Clinic Hospital, physician communication, staff responsiveness, patients’ perception of cleanliness, and their willingness to recommend the hospital on the Hospital Consumer Assessment of Healthcare Providers and Systems survey were lower in each category for patients on CP when compared with patients not on CP.22 Patients who are on CP are six times more likely to experience an adverse event in the hospital, including falls and pressure ulcers.23 A recent study from a large academic medical center demonstrated that noninfectious adverse events were reduced by 72% after discontinuing CP for MRSA and VRE. These events included postoperative respiratory failure, hemorrhage or hematoma, thrombosis, wound dehiscence, pressure ulcers, and falls or trauma.24
The financial costs of unnecessary CP have also been studied. A recent retrospective study examining a large cohort of patients on CP for MRSA demonstrated that when compared with nonisolated patients, those on MRSA CP had a 30% increase in length of stay and a 43% increase in costs of care. Patients isolated for MRSA were 4.4% more likely than nonisolated individuals to be readmitted within 30 days after discharge, unrelated to MRSA.25 These data contribute to the growing evidence that a conscientious, patient-centered approach to CP is preferred to overly broad policies that compromise patient safety.
WHEN CONTACT PRECAUTIONS SHOULD BE USED FOR MRSA AND VRE
Contact precautions for MRSA and VRE should be used to interrupt transmission during uncontrolled outbreaks, and in patients with open wounds, uncontained secretions, or incontinent diarrhea.
In addition, there are other commonly encountered organisms for which CP should be continued. CP should be used for active Clostridium difficile infection to prevent transmission. Due to the paucity of data regarding prevention of novel and highly resistant organisms and the complexity in treating these MDROs, it is reasonable to initiate CP in these cases.26 Examples include active infection with multidrug resistance, including carbapenem-resistant Enterobacteriaceae, highly drug-resistant Pseudomonas aeruginosa, and other emerging MDROs such as vancomycin-resistant or -indeterminate S. aureus (VRSA or VISA) and Candida auris.27 Limiting CP to instances where there is clear evidence to support will ensure patient safety and limit the harms associated with CP.
WHAT YOU SHOULD DO INSTEAD
Horizontal prevention aims to reduce the burden of all microorganisms. This includes techniques such as hand hygiene, antimicrobial stewardship, CHG bathing, and environmental cleaning methods to decrease colonization of all MDROs in hospital rooms. Compared with vertical prevention strategies that use active surveillance testing for colonization and CP, horizontal interventions are the most effective means to reduce transmission of MDROs.28 The simplest and the most well-studied method for reducing transmission of all organisms in the hospital remains hand hygiene.29 High institutional hand hygiene rates of at least 90% are critical to the success of any initiative that seeks to eliminate CP.
CHG bathing has also been studied across multiple patient settings for reducing MRSA and VRE acquisition, catheter-associated urinary tract infections, and central line-associated bacterial infections.30 In addition, hospital-wide daily CHG bathing has been associated with decreased C. difficile infection, and the baths were well tolerated by patients.31
SHEA recently released recommendations for timing of discontinuation of CP for patients with MDROs and emphasized that hospital systems must take an individual approach to discontinuing CP that takes into account local prevalence, risk, and resources.32 The decision to not place a patient on CP is one side of this high-value coin. The other side is knowing when it is appropriate to discontinue CP.
RECOMMENDATION
- Discontinue the use of CP for MRSA and VRE in hospitals with low endemic rates and high hand hygiene compliance.
- Improve horizontal preventions by promoting hand hygiene, antimicrobial stewardship, and considering CHG bathing for all patients.
- Create a systematic approach to discontinuing CP and compare transmission of MRSA and VRE rates through microbiology surveillance before and after discontinuation.
CONCLUSION
Contact precautions for MRSA and VRE are another example of a “Thing We Do for No Reason”. For most patients with MRSA and VRE, CP have not been shown to effectively reduce transmission. In addition, CP are expensive and associated with increased rates of patient adverse events. Hospitalists can lead the effort to ensure optimal hand hygiene and work with local infection control teams to reevaluate the utility of CP for patients with MRSA and VRE.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
There are no conflicts of interest for any authors, financial or other.
1. Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163-1172. doi: 10.1017/ice.2015.156. PubMed
2. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419-1430. doi: 10.1056/NEJMoa1007474. PubMed
3. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10):S65-S164. doi: 10.1016/j.ajic.2007.10.007. PubMed
4. Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 Update. Infect Control Hosp Epidemiol. 2014;35(7):772-796. doi: 10.1086/676534. PubMed
5. Mcdonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994. doi: 10.1093/cid/ciy149. PubMed
6. Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in healthcare settings, 2006. Am J Infect Control. 2007;35(10):S165-S193. doi: 10.1016/j.ajic.2007.10.006. PubMed
7. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364(15):1407-1418. doi: 10.1056/NEJMoa1000373. PubMed
8. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310(15):1571-1580. doi: 10.1001/jama.2013.277815. PubMed
9. Derde LPG, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomized trial. Lancet Infect Dis. 2014;14(1):31-39. doi: 10.1016/S1473-3099(13)70295-0. PubMed
10. Bearman G, Rosato AE, Duane TM, et al. Trial of universal gloving with emollient‐impregnated gloves to promote skin health and prevent the transmission of multidrug‐resistant organisms in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2010;31(5):491-497. doi: 10.1086/651671. PubMed
11. Furuya EY, Cohen B, Jia H, Larson EL. Long-term impact of universal contact precautions on rates of multidrug-resistant organisms in ICUs: a comparative effectiveness study. Infect Control Hosp Epidemiol. 2018;39(5):534-540. doi: 10.1017/ice.2018.35. PubMed
12. Marra AR, Edmond MB, Schweizer ML, Ryan GW, Diekema DJ. Discontinuing contact precautions for multidrug-resistant organisms: a systematic literature review and meta-analysis. Am J Infect Control. 2018;46(3):333-340. doi: 10.1016/j.ajic.2017.08.031. PubMed
13. Martin EM, Russell D, Rubin Z, et al. Elimination of routine contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: A retrospective quasi-experimental study. Infect Control Hosp Epidemiol. 2016;37(11):1323-1330. doi: 10.1017/ice.2016.156. PubMed
14. Bearman G, Abbas S, Masroor N, et al. Impact of discontinuing contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an interrupted time series analysis. Infect Control Hosp Epidemiol. 2018;39(6):676-682. doi: 10.1017/ice.2018.57. PubMed
15. Almyroudis NG, Osawa R, Samonis G, et al. Discontinuation of systematic surveillance and contact precautions for vancomycin-resistant Enterococcus (VRE) and its impact on the incidence of VRE faecium bacteremia in patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2016;37(4):398-403. doi: 10.1017/ice.2015.310. PubMed
16. Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: a review of the literature. Am J Infect Control. 2009;37(2):85-93. doi: 10.1016/j.ajic.2008.04.257. PubMed
17. Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31(6):354-356. doi: 10.1016/S0196-6553(02)48250-8. PubMed
18. G oldszer RC, Shadick N, Bardon CG, et al. A program to remove patients from unnecessary contact precautions. J Clin Outcomes Manag. 2002;9(10):553-556.
19. G uilley-Lerondeau B, Bourigault C, Buttes A-CGD, Birgand G, Lepelletier D. Adverse effects of isolation: a prospective matched cohort study including 90 direct interviews of hospitalized patients in a French University Hospital. Eur J Clin Microbiol Infect Dis. 2016;36(1):75-80. doi: 10.1007/s10096-016-2772-z. PubMed
20. Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999;354(9185):1177-1178. doi: 10.1016/S0140-6736(99)04196-3. PubMed
21. Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290(14):1899-1905. doi: 10.1001/jama.290.14.1899. PubMed
22. Vinski J, Bertin M, Sun Z, et al. Impact of isolation on hospital consumer assessment of healthcare providers and systems scores: is isolation isolating? Infect Control Hosp Epidemiol. 2012;33(5):513-516. doi: 10.1086/665314. PubMed
23. Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors a retrospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1118-1120. doi: 10.1086/673153. PubMed
24. Martin EM, Bryant B, Grogan TR, et al. Noninfectious hospital adverse events decline after elimination of contact precautions for MRSA and VRE. Infect Control Hosp Epidemiol. 2018;39(7):788-796. doi: 10.1017/ice.2018.93. PubMed
25. T ran K, Bell C, Stall N, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: A multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-268. doi: 10.1007/s11606-016-3862-4. PubMed
26. Izadpanah M, Khalili H. Antibiotic regimens for treatment of infections due to multidrug-resistant Gram-negative pathogens: an evidence-based literature review. J Res Pharm Pract. 2015;4(3):105-114. doi: 10.4103/2279-042X.162360. PubMed
27. Savard P, Perl TM. Combating the spread of carbapenemases in Enterobacteriaceae: a battle that infection prevention should not lose. Clin Microbiol Infect. 2014;20(9):854-861. doi: 10.1111/1469-0691.12748. PubMed
28. Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. 2010;14(4):S3-S5. doi: 10.1016/j.ijid.2010.05.002. PubMed
29. Pittet D, Allegranzi B, Sax H, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6(10):641-652. doi: 10.1016/S1473-3099(06)70600-4. PubMed
30. Climo MW, Yokoe DS, Warren DK et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533-542. doi: 10.1056/NEJMoa1113849. PubMed
31. Rupp ME, Cavalieri RJ, Lyden E, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012;33(11):1094-1100. doi: 10.1086/668024. PubMed
32. Banach DB, Bearman G, Barnden M, et al. Duration of contact precautions for acute-care settings. Infect Control Hosp Epidemiol. 2018;39(2):127-144. doi: 10.1017/ice.2017.245. PubMed
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE
A 67-year-old man is admitted to a telemetry ward for an acute myocardial infarction and treated with percutaneous coronary intervention. He is currently on day three of antibiotics for a methicillin-resistant Staphylococcus aureus (MRSA) lower extremity soft tissue infection that is healing without a draining wound. He is placed on contact precautions based on institutional infection control guidelines. The hospitalist overhears members of the team commenting on having to don gowns to see this patient each day and wonders aloud whether care is impacted by the use of contact precautions.
BACKGROUND
Contact precautions (CP) for patients with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) infections are common in several hospitals. CP pose a significant burden to health systems, with an estimated 20%-25% of hospitalized patients on CP for MRSA or VRE alone.1 CP are becoming increasingly more prevalent with state laws and the Veterans Affairs (VA) hospital system requiring active surveillance cultures (ASC) and subsequent CP when ASC are positive.2
WHY YOU MIGHT THINK CONTACT PRECAUTIONS ARE HELPFUL FOR MRSA AND VRE
Supporters highlight the utility of CP in preventing the spread of infection, controlling outbreaks, and protecting healthcare workers from certain transmissible diseases. The Centers for Disease Control and Prevention (CDC) recommended CP after prior studies demonstrated their effectiveness during outbreaks of transmissible infections.3 CP were included in bundles alongside interventions such as improving hand hygiene, chlorhexidine gluconate (CHG) bathing, and ASC with targeted or universal decolonization.2 The VA MRSA bundle, for example, demonstrated a reduction of healthcare-associated MRSA in the ICU by 62% after implementation. The Society for Healthcare Epidemiology of America Research Network (SHEA) and the Infectious Diseases Society of America (IDSA) recommend CP for MRSA-infected and colonized patients in acute care settings to control outbreaks.4,5 The CDC also has broad recommendations supporting CP for all patients infected and previously identified as being colonized with target multidrug-resistant organisms (MDROs) without identifying which are considered to be “targets.”6
WHY CONTACT PRECAUTIONS MAY NOT BE HELPFUL FOR MRSA AND VRE
Despite current guidelines, cluster-randomized trials have not shown a benefit of initiating CP over usual care for the prevention of acquiring MRSA or VRE in the hospital. One study demonstrated no change in MRSA and VRE acquisition with broad screening and subsequent CP.7 Another study evaluated a universal gown and glove policy in an ICU setting and found a reduction in MRSA acquisition, but no reduction in VRE acquisition.8 A third study investigated hand hygiene and daily CHG bathing and noted a reduction in MRSA transmission rates, where CP for screened colonized patients had no effect on transmission of MRSA or VRE.9
In addition, a prospective trial at a large academic center over two six-month intervals utilized universal gloving with emollient-impregnated gloves compared with CP and found no difference in MDRO acquisition. Universal gloving was associated with higher hand hygiene rates than CP.10 Another more recent retrospective observational study compared universal contact precautions (UCP) in ICUs to a historical nine-year baseline and concurrently to other nonuniversal CP ICUs. There was no significant decrease in MDROs during the UCP period compared with baseline or with non-UCP units.11Further interest in and scrutiny of CP prompted a recently published meta-analysis of 14 studies in which CP were eliminated. The rates of transmission of MRSA, VRE, or other MDROs studied were not impacted by discontinuation.12 One of the studies included two large academic medical centers and assessed the impact of discontinuing CP for endemic MRSA and VRE. The bundled intervention included the discontinuation of CP for all carriers of MRSA and VRE, except patients with draining wounds, maintaining high hand hygiene rates, and CHG baths for nearly all patients. There was no significant increase in transmission rates, and the intervention saved the health system an estimated $643,776 and 45,277 hours per year in healthcare worker time previously spent on donning and doffing personal protective equipment.13 Another large academic hospital published a time series approach of seven interventions to reduce healthcare-associated infections and noted no increase in MRSA or VRE transmission when CP were discontinued when combined with other horizontal preventions.14 Results were found to be similar in a high-risk population of patients with hematologic malignancies and hematopoietic stem cell transplantation, where both surveillance and CP for VRE were discontinued and did not impact the rates of VRE bacteremia.15
WHY CONTACT PRECAUTIONS MAY BE HARMFUL
Multiple studies have examined the deleterious effects of CP, including a comprehensive systematic literature review of various adverse outcomes linked with CP.16 CP decrease the amount of time that healthcare workers (HCW) spend with patients,17 create delays at admission and discharge,18 increase symptoms of anxiety and depression in patients,19,20 and decrease patient satisfaction with care.21,22 In a study conducted at the Cleveland Clinic Hospital, physician communication, staff responsiveness, patients’ perception of cleanliness, and their willingness to recommend the hospital on the Hospital Consumer Assessment of Healthcare Providers and Systems survey were lower in each category for patients on CP when compared with patients not on CP.22 Patients who are on CP are six times more likely to experience an adverse event in the hospital, including falls and pressure ulcers.23 A recent study from a large academic medical center demonstrated that noninfectious adverse events were reduced by 72% after discontinuing CP for MRSA and VRE. These events included postoperative respiratory failure, hemorrhage or hematoma, thrombosis, wound dehiscence, pressure ulcers, and falls or trauma.24
The financial costs of unnecessary CP have also been studied. A recent retrospective study examining a large cohort of patients on CP for MRSA demonstrated that when compared with nonisolated patients, those on MRSA CP had a 30% increase in length of stay and a 43% increase in costs of care. Patients isolated for MRSA were 4.4% more likely than nonisolated individuals to be readmitted within 30 days after discharge, unrelated to MRSA.25 These data contribute to the growing evidence that a conscientious, patient-centered approach to CP is preferred to overly broad policies that compromise patient safety.
WHEN CONTACT PRECAUTIONS SHOULD BE USED FOR MRSA AND VRE
Contact precautions for MRSA and VRE should be used to interrupt transmission during uncontrolled outbreaks, and in patients with open wounds, uncontained secretions, or incontinent diarrhea.
In addition, there are other commonly encountered organisms for which CP should be continued. CP should be used for active Clostridium difficile infection to prevent transmission. Due to the paucity of data regarding prevention of novel and highly resistant organisms and the complexity in treating these MDROs, it is reasonable to initiate CP in these cases.26 Examples include active infection with multidrug resistance, including carbapenem-resistant Enterobacteriaceae, highly drug-resistant Pseudomonas aeruginosa, and other emerging MDROs such as vancomycin-resistant or -indeterminate S. aureus (VRSA or VISA) and Candida auris.27 Limiting CP to instances where there is clear evidence to support will ensure patient safety and limit the harms associated with CP.
WHAT YOU SHOULD DO INSTEAD
Horizontal prevention aims to reduce the burden of all microorganisms. This includes techniques such as hand hygiene, antimicrobial stewardship, CHG bathing, and environmental cleaning methods to decrease colonization of all MDROs in hospital rooms. Compared with vertical prevention strategies that use active surveillance testing for colonization and CP, horizontal interventions are the most effective means to reduce transmission of MDROs.28 The simplest and the most well-studied method for reducing transmission of all organisms in the hospital remains hand hygiene.29 High institutional hand hygiene rates of at least 90% are critical to the success of any initiative that seeks to eliminate CP.
CHG bathing has also been studied across multiple patient settings for reducing MRSA and VRE acquisition, catheter-associated urinary tract infections, and central line-associated bacterial infections.30 In addition, hospital-wide daily CHG bathing has been associated with decreased C. difficile infection, and the baths were well tolerated by patients.31
SHEA recently released recommendations for timing of discontinuation of CP for patients with MDROs and emphasized that hospital systems must take an individual approach to discontinuing CP that takes into account local prevalence, risk, and resources.32 The decision to not place a patient on CP is one side of this high-value coin. The other side is knowing when it is appropriate to discontinue CP.
RECOMMENDATION
- Discontinue the use of CP for MRSA and VRE in hospitals with low endemic rates and high hand hygiene compliance.
- Improve horizontal preventions by promoting hand hygiene, antimicrobial stewardship, and considering CHG bathing for all patients.
- Create a systematic approach to discontinuing CP and compare transmission of MRSA and VRE rates through microbiology surveillance before and after discontinuation.
CONCLUSION
Contact precautions for MRSA and VRE are another example of a “Thing We Do for No Reason”. For most patients with MRSA and VRE, CP have not been shown to effectively reduce transmission. In addition, CP are expensive and associated with increased rates of patient adverse events. Hospitalists can lead the effort to ensure optimal hand hygiene and work with local infection control teams to reevaluate the utility of CP for patients with MRSA and VRE.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
There are no conflicts of interest for any authors, financial or other.
Inspired by the ABIM Foundation’s Choosing Wisely® campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CASE
A 67-year-old man is admitted to a telemetry ward for an acute myocardial infarction and treated with percutaneous coronary intervention. He is currently on day three of antibiotics for a methicillin-resistant Staphylococcus aureus (MRSA) lower extremity soft tissue infection that is healing without a draining wound. He is placed on contact precautions based on institutional infection control guidelines. The hospitalist overhears members of the team commenting on having to don gowns to see this patient each day and wonders aloud whether care is impacted by the use of contact precautions.
BACKGROUND
Contact precautions (CP) for patients with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE) infections are common in several hospitals. CP pose a significant burden to health systems, with an estimated 20%-25% of hospitalized patients on CP for MRSA or VRE alone.1 CP are becoming increasingly more prevalent with state laws and the Veterans Affairs (VA) hospital system requiring active surveillance cultures (ASC) and subsequent CP when ASC are positive.2
WHY YOU MIGHT THINK CONTACT PRECAUTIONS ARE HELPFUL FOR MRSA AND VRE
Supporters highlight the utility of CP in preventing the spread of infection, controlling outbreaks, and protecting healthcare workers from certain transmissible diseases. The Centers for Disease Control and Prevention (CDC) recommended CP after prior studies demonstrated their effectiveness during outbreaks of transmissible infections.3 CP were included in bundles alongside interventions such as improving hand hygiene, chlorhexidine gluconate (CHG) bathing, and ASC with targeted or universal decolonization.2 The VA MRSA bundle, for example, demonstrated a reduction of healthcare-associated MRSA in the ICU by 62% after implementation. The Society for Healthcare Epidemiology of America Research Network (SHEA) and the Infectious Diseases Society of America (IDSA) recommend CP for MRSA-infected and colonized patients in acute care settings to control outbreaks.4,5 The CDC also has broad recommendations supporting CP for all patients infected and previously identified as being colonized with target multidrug-resistant organisms (MDROs) without identifying which are considered to be “targets.”6
WHY CONTACT PRECAUTIONS MAY NOT BE HELPFUL FOR MRSA AND VRE
Despite current guidelines, cluster-randomized trials have not shown a benefit of initiating CP over usual care for the prevention of acquiring MRSA or VRE in the hospital. One study demonstrated no change in MRSA and VRE acquisition with broad screening and subsequent CP.7 Another study evaluated a universal gown and glove policy in an ICU setting and found a reduction in MRSA acquisition, but no reduction in VRE acquisition.8 A third study investigated hand hygiene and daily CHG bathing and noted a reduction in MRSA transmission rates, where CP for screened colonized patients had no effect on transmission of MRSA or VRE.9
In addition, a prospective trial at a large academic center over two six-month intervals utilized universal gloving with emollient-impregnated gloves compared with CP and found no difference in MDRO acquisition. Universal gloving was associated with higher hand hygiene rates than CP.10 Another more recent retrospective observational study compared universal contact precautions (UCP) in ICUs to a historical nine-year baseline and concurrently to other nonuniversal CP ICUs. There was no significant decrease in MDROs during the UCP period compared with baseline or with non-UCP units.11Further interest in and scrutiny of CP prompted a recently published meta-analysis of 14 studies in which CP were eliminated. The rates of transmission of MRSA, VRE, or other MDROs studied were not impacted by discontinuation.12 One of the studies included two large academic medical centers and assessed the impact of discontinuing CP for endemic MRSA and VRE. The bundled intervention included the discontinuation of CP for all carriers of MRSA and VRE, except patients with draining wounds, maintaining high hand hygiene rates, and CHG baths for nearly all patients. There was no significant increase in transmission rates, and the intervention saved the health system an estimated $643,776 and 45,277 hours per year in healthcare worker time previously spent on donning and doffing personal protective equipment.13 Another large academic hospital published a time series approach of seven interventions to reduce healthcare-associated infections and noted no increase in MRSA or VRE transmission when CP were discontinued when combined with other horizontal preventions.14 Results were found to be similar in a high-risk population of patients with hematologic malignancies and hematopoietic stem cell transplantation, where both surveillance and CP for VRE were discontinued and did not impact the rates of VRE bacteremia.15
WHY CONTACT PRECAUTIONS MAY BE HARMFUL
Multiple studies have examined the deleterious effects of CP, including a comprehensive systematic literature review of various adverse outcomes linked with CP.16 CP decrease the amount of time that healthcare workers (HCW) spend with patients,17 create delays at admission and discharge,18 increase symptoms of anxiety and depression in patients,19,20 and decrease patient satisfaction with care.21,22 In a study conducted at the Cleveland Clinic Hospital, physician communication, staff responsiveness, patients’ perception of cleanliness, and their willingness to recommend the hospital on the Hospital Consumer Assessment of Healthcare Providers and Systems survey were lower in each category for patients on CP when compared with patients not on CP.22 Patients who are on CP are six times more likely to experience an adverse event in the hospital, including falls and pressure ulcers.23 A recent study from a large academic medical center demonstrated that noninfectious adverse events were reduced by 72% after discontinuing CP for MRSA and VRE. These events included postoperative respiratory failure, hemorrhage or hematoma, thrombosis, wound dehiscence, pressure ulcers, and falls or trauma.24
The financial costs of unnecessary CP have also been studied. A recent retrospective study examining a large cohort of patients on CP for MRSA demonstrated that when compared with nonisolated patients, those on MRSA CP had a 30% increase in length of stay and a 43% increase in costs of care. Patients isolated for MRSA were 4.4% more likely than nonisolated individuals to be readmitted within 30 days after discharge, unrelated to MRSA.25 These data contribute to the growing evidence that a conscientious, patient-centered approach to CP is preferred to overly broad policies that compromise patient safety.
WHEN CONTACT PRECAUTIONS SHOULD BE USED FOR MRSA AND VRE
Contact precautions for MRSA and VRE should be used to interrupt transmission during uncontrolled outbreaks, and in patients with open wounds, uncontained secretions, or incontinent diarrhea.
In addition, there are other commonly encountered organisms for which CP should be continued. CP should be used for active Clostridium difficile infection to prevent transmission. Due to the paucity of data regarding prevention of novel and highly resistant organisms and the complexity in treating these MDROs, it is reasonable to initiate CP in these cases.26 Examples include active infection with multidrug resistance, including carbapenem-resistant Enterobacteriaceae, highly drug-resistant Pseudomonas aeruginosa, and other emerging MDROs such as vancomycin-resistant or -indeterminate S. aureus (VRSA or VISA) and Candida auris.27 Limiting CP to instances where there is clear evidence to support will ensure patient safety and limit the harms associated with CP.
WHAT YOU SHOULD DO INSTEAD
Horizontal prevention aims to reduce the burden of all microorganisms. This includes techniques such as hand hygiene, antimicrobial stewardship, CHG bathing, and environmental cleaning methods to decrease colonization of all MDROs in hospital rooms. Compared with vertical prevention strategies that use active surveillance testing for colonization and CP, horizontal interventions are the most effective means to reduce transmission of MDROs.28 The simplest and the most well-studied method for reducing transmission of all organisms in the hospital remains hand hygiene.29 High institutional hand hygiene rates of at least 90% are critical to the success of any initiative that seeks to eliminate CP.
CHG bathing has also been studied across multiple patient settings for reducing MRSA and VRE acquisition, catheter-associated urinary tract infections, and central line-associated bacterial infections.30 In addition, hospital-wide daily CHG bathing has been associated with decreased C. difficile infection, and the baths were well tolerated by patients.31
SHEA recently released recommendations for timing of discontinuation of CP for patients with MDROs and emphasized that hospital systems must take an individual approach to discontinuing CP that takes into account local prevalence, risk, and resources.32 The decision to not place a patient on CP is one side of this high-value coin. The other side is knowing when it is appropriate to discontinue CP.
RECOMMENDATION
- Discontinue the use of CP for MRSA and VRE in hospitals with low endemic rates and high hand hygiene compliance.
- Improve horizontal preventions by promoting hand hygiene, antimicrobial stewardship, and considering CHG bathing for all patients.
- Create a systematic approach to discontinuing CP and compare transmission of MRSA and VRE rates through microbiology surveillance before and after discontinuation.
CONCLUSION
Contact precautions for MRSA and VRE are another example of a “Thing We Do for No Reason”. For most patients with MRSA and VRE, CP have not been shown to effectively reduce transmission. In addition, CP are expensive and associated with increased rates of patient adverse events. Hospitalists can lead the effort to ensure optimal hand hygiene and work with local infection control teams to reevaluate the utility of CP for patients with MRSA and VRE.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
There are no conflicts of interest for any authors, financial or other.
1. Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163-1172. doi: 10.1017/ice.2015.156. PubMed
2. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419-1430. doi: 10.1056/NEJMoa1007474. PubMed
3. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10):S65-S164. doi: 10.1016/j.ajic.2007.10.007. PubMed
4. Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 Update. Infect Control Hosp Epidemiol. 2014;35(7):772-796. doi: 10.1086/676534. PubMed
5. Mcdonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994. doi: 10.1093/cid/ciy149. PubMed
6. Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in healthcare settings, 2006. Am J Infect Control. 2007;35(10):S165-S193. doi: 10.1016/j.ajic.2007.10.006. PubMed
7. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364(15):1407-1418. doi: 10.1056/NEJMoa1000373. PubMed
8. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310(15):1571-1580. doi: 10.1001/jama.2013.277815. PubMed
9. Derde LPG, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomized trial. Lancet Infect Dis. 2014;14(1):31-39. doi: 10.1016/S1473-3099(13)70295-0. PubMed
10. Bearman G, Rosato AE, Duane TM, et al. Trial of universal gloving with emollient‐impregnated gloves to promote skin health and prevent the transmission of multidrug‐resistant organisms in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2010;31(5):491-497. doi: 10.1086/651671. PubMed
11. Furuya EY, Cohen B, Jia H, Larson EL. Long-term impact of universal contact precautions on rates of multidrug-resistant organisms in ICUs: a comparative effectiveness study. Infect Control Hosp Epidemiol. 2018;39(5):534-540. doi: 10.1017/ice.2018.35. PubMed
12. Marra AR, Edmond MB, Schweizer ML, Ryan GW, Diekema DJ. Discontinuing contact precautions for multidrug-resistant organisms: a systematic literature review and meta-analysis. Am J Infect Control. 2018;46(3):333-340. doi: 10.1016/j.ajic.2017.08.031. PubMed
13. Martin EM, Russell D, Rubin Z, et al. Elimination of routine contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: A retrospective quasi-experimental study. Infect Control Hosp Epidemiol. 2016;37(11):1323-1330. doi: 10.1017/ice.2016.156. PubMed
14. Bearman G, Abbas S, Masroor N, et al. Impact of discontinuing contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an interrupted time series analysis. Infect Control Hosp Epidemiol. 2018;39(6):676-682. doi: 10.1017/ice.2018.57. PubMed
15. Almyroudis NG, Osawa R, Samonis G, et al. Discontinuation of systematic surveillance and contact precautions for vancomycin-resistant Enterococcus (VRE) and its impact on the incidence of VRE faecium bacteremia in patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2016;37(4):398-403. doi: 10.1017/ice.2015.310. PubMed
16. Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: a review of the literature. Am J Infect Control. 2009;37(2):85-93. doi: 10.1016/j.ajic.2008.04.257. PubMed
17. Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31(6):354-356. doi: 10.1016/S0196-6553(02)48250-8. PubMed
18. G oldszer RC, Shadick N, Bardon CG, et al. A program to remove patients from unnecessary contact precautions. J Clin Outcomes Manag. 2002;9(10):553-556.
19. G uilley-Lerondeau B, Bourigault C, Buttes A-CGD, Birgand G, Lepelletier D. Adverse effects of isolation: a prospective matched cohort study including 90 direct interviews of hospitalized patients in a French University Hospital. Eur J Clin Microbiol Infect Dis. 2016;36(1):75-80. doi: 10.1007/s10096-016-2772-z. PubMed
20. Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999;354(9185):1177-1178. doi: 10.1016/S0140-6736(99)04196-3. PubMed
21. Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290(14):1899-1905. doi: 10.1001/jama.290.14.1899. PubMed
22. Vinski J, Bertin M, Sun Z, et al. Impact of isolation on hospital consumer assessment of healthcare providers and systems scores: is isolation isolating? Infect Control Hosp Epidemiol. 2012;33(5):513-516. doi: 10.1086/665314. PubMed
23. Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors a retrospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1118-1120. doi: 10.1086/673153. PubMed
24. Martin EM, Bryant B, Grogan TR, et al. Noninfectious hospital adverse events decline after elimination of contact precautions for MRSA and VRE. Infect Control Hosp Epidemiol. 2018;39(7):788-796. doi: 10.1017/ice.2018.93. PubMed
25. T ran K, Bell C, Stall N, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: A multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-268. doi: 10.1007/s11606-016-3862-4. PubMed
26. Izadpanah M, Khalili H. Antibiotic regimens for treatment of infections due to multidrug-resistant Gram-negative pathogens: an evidence-based literature review. J Res Pharm Pract. 2015;4(3):105-114. doi: 10.4103/2279-042X.162360. PubMed
27. Savard P, Perl TM. Combating the spread of carbapenemases in Enterobacteriaceae: a battle that infection prevention should not lose. Clin Microbiol Infect. 2014;20(9):854-861. doi: 10.1111/1469-0691.12748. PubMed
28. Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. 2010;14(4):S3-S5. doi: 10.1016/j.ijid.2010.05.002. PubMed
29. Pittet D, Allegranzi B, Sax H, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6(10):641-652. doi: 10.1016/S1473-3099(06)70600-4. PubMed
30. Climo MW, Yokoe DS, Warren DK et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533-542. doi: 10.1056/NEJMoa1113849. PubMed
31. Rupp ME, Cavalieri RJ, Lyden E, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012;33(11):1094-1100. doi: 10.1086/668024. PubMed
32. Banach DB, Bearman G, Barnden M, et al. Duration of contact precautions for acute-care settings. Infect Control Hosp Epidemiol. 2018;39(2):127-144. doi: 10.1017/ice.2017.245. PubMed
1. Morgan DJ, Murthy R, Munoz-Price LS, et al. Reconsidering contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus. Infect Control Hosp Epidemiol. 2015;36(10):1163-1172. doi: 10.1017/ice.2015.156. PubMed
2. Jain R, Kralovic SM, Evans ME, et al. Veterans Affairs initiative to prevent methicillin-resistant Staphylococcus aureus infections. N Engl J Med. 2011;364(15):1419-1430. doi: 10.1056/NEJMoa1007474. PubMed
3. Siegel JD, Rhinehart E, Jackson M, Chiarello L. 2007 Guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10):S65-S164. doi: 10.1016/j.ajic.2007.10.007. PubMed
4. Calfee DP, Salgado CD, Milstone AM, et al. Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute care hospitals: 2014 Update. Infect Control Hosp Epidemiol. 2014;35(7):772-796. doi: 10.1086/676534. PubMed
5. Mcdonald LC, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults and children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):987-994. doi: 10.1093/cid/ciy149. PubMed
6. Siegel JD, Rhinehart E, Jackson M, Chiarello L, Healthcare Infection Control Practices Advisory Committee. Management of multidrug-resistant organisms in healthcare settings, 2006. Am J Infect Control. 2007;35(10):S165-S193. doi: 10.1016/j.ajic.2007.10.006. PubMed
7. Huskins WC, Huckabee CM, O’Grady NP, et al. Intervention to reduce transmission of resistant bacteria in intensive care. N Engl J Med. 2011;364(15):1407-1418. doi: 10.1056/NEJMoa1000373. PubMed
8. Harris AD, Pineles L, Belton B, et al. Universal glove and gown use and acquisition of antibiotic-resistant bacteria in the ICU: a randomized trial. JAMA. 2013;310(15):1571-1580. doi: 10.1001/jama.2013.277815. PubMed
9. Derde LPG, Cooper BS, Goossens H, et al. Interventions to reduce colonisation and transmission of antimicrobial-resistant bacteria in intensive care units: an interrupted time series study and cluster randomized trial. Lancet Infect Dis. 2014;14(1):31-39. doi: 10.1016/S1473-3099(13)70295-0. PubMed
10. Bearman G, Rosato AE, Duane TM, et al. Trial of universal gloving with emollient‐impregnated gloves to promote skin health and prevent the transmission of multidrug‐resistant organisms in a surgical intensive care unit. Infect Control Hosp Epidemiol. 2010;31(5):491-497. doi: 10.1086/651671. PubMed
11. Furuya EY, Cohen B, Jia H, Larson EL. Long-term impact of universal contact precautions on rates of multidrug-resistant organisms in ICUs: a comparative effectiveness study. Infect Control Hosp Epidemiol. 2018;39(5):534-540. doi: 10.1017/ice.2018.35. PubMed
12. Marra AR, Edmond MB, Schweizer ML, Ryan GW, Diekema DJ. Discontinuing contact precautions for multidrug-resistant organisms: a systematic literature review and meta-analysis. Am J Infect Control. 2018;46(3):333-340. doi: 10.1016/j.ajic.2017.08.031. PubMed
13. Martin EM, Russell D, Rubin Z, et al. Elimination of routine contact precautions for endemic methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: A retrospective quasi-experimental study. Infect Control Hosp Epidemiol. 2016;37(11):1323-1330. doi: 10.1017/ice.2016.156. PubMed
14. Bearman G, Abbas S, Masroor N, et al. Impact of discontinuing contact precautions for methicillin-resistant Staphylococcus aureus and vancomycin-resistant Enterococcus: an interrupted time series analysis. Infect Control Hosp Epidemiol. 2018;39(6):676-682. doi: 10.1017/ice.2018.57. PubMed
15. Almyroudis NG, Osawa R, Samonis G, et al. Discontinuation of systematic surveillance and contact precautions for vancomycin-resistant Enterococcus (VRE) and its impact on the incidence of VRE faecium bacteremia in patients with hematologic malignancies. Infect Control Hosp Epidemiol. 2016;37(4):398-403. doi: 10.1017/ice.2015.310. PubMed
16. Morgan DJ, Diekema DJ, Sepkowitz K, Perencevich EN. Adverse outcomes associated with contact precautions: a review of the literature. Am J Infect Control. 2009;37(2):85-93. doi: 10.1016/j.ajic.2008.04.257. PubMed
17. Saint S, Higgins LA, Nallamothu BK, Chenoweth C. Do physicians examine patients in contact isolation less frequently? A brief report. Am J Infect Control. 2003;31(6):354-356. doi: 10.1016/S0196-6553(02)48250-8. PubMed
18. G oldszer RC, Shadick N, Bardon CG, et al. A program to remove patients from unnecessary contact precautions. J Clin Outcomes Manag. 2002;9(10):553-556.
19. G uilley-Lerondeau B, Bourigault C, Buttes A-CGD, Birgand G, Lepelletier D. Adverse effects of isolation: a prospective matched cohort study including 90 direct interviews of hospitalized patients in a French University Hospital. Eur J Clin Microbiol Infect Dis. 2016;36(1):75-80. doi: 10.1007/s10096-016-2772-z. PubMed
20. Kirkland KB, Weinstein JM. Adverse effects of contact isolation. Lancet. 1999;354(9185):1177-1178. doi: 10.1016/S0140-6736(99)04196-3. PubMed
21. Stelfox HT, Bates DW, Redelmeier DA. Safety of patients isolated for infection control. JAMA. 2003;290(14):1899-1905. doi: 10.1001/jama.290.14.1899. PubMed
22. Vinski J, Bertin M, Sun Z, et al. Impact of isolation on hospital consumer assessment of healthcare providers and systems scores: is isolation isolating? Infect Control Hosp Epidemiol. 2012;33(5):513-516. doi: 10.1086/665314. PubMed
23. Karki S, Leder K, Cheng AC. Patients under contact precautions have an increased risk of injuries and medication errors a retrospective cohort study. Infect Control Hosp Epidemiol. 2013;34(10):1118-1120. doi: 10.1086/673153. PubMed
24. Martin EM, Bryant B, Grogan TR, et al. Noninfectious hospital adverse events decline after elimination of contact precautions for MRSA and VRE. Infect Control Hosp Epidemiol. 2018;39(7):788-796. doi: 10.1017/ice.2018.93. PubMed
25. T ran K, Bell C, Stall N, et al. The effect of hospital isolation precautions on patient outcomes and cost of care: A multi-site, retrospective, propensity score-matched cohort study. J Gen Intern Med. 2017;32(3):262-268. doi: 10.1007/s11606-016-3862-4. PubMed
26. Izadpanah M, Khalili H. Antibiotic regimens for treatment of infections due to multidrug-resistant Gram-negative pathogens: an evidence-based literature review. J Res Pharm Pract. 2015;4(3):105-114. doi: 10.4103/2279-042X.162360. PubMed
27. Savard P, Perl TM. Combating the spread of carbapenemases in Enterobacteriaceae: a battle that infection prevention should not lose. Clin Microbiol Infect. 2014;20(9):854-861. doi: 10.1111/1469-0691.12748. PubMed
28. Wenzel RP, Edmond MB. Infection control: the case for horizontal rather than vertical interventional programs. Int J Infect Dis. 2010;14(4):S3-S5. doi: 10.1016/j.ijid.2010.05.002. PubMed
29. Pittet D, Allegranzi B, Sax H, et al. Evidence-based model for hand transmission during patient care and the role of improved practices. Lancet Infect Dis. 2006;6(10):641-652. doi: 10.1016/S1473-3099(06)70600-4. PubMed
30. Climo MW, Yokoe DS, Warren DK et al. Effect of daily chlorhexidine bathing on hospital-acquired infection. N Engl J Med. 2013;368(6):533-542. doi: 10.1056/NEJMoa1113849. PubMed
31. Rupp ME, Cavalieri RJ, Lyden E, et al. Effect of hospital-wide chlorhexidine patient bathing on healthcare-associated infections. Infect Control Hosp Epidemiol. 2012;33(11):1094-1100. doi: 10.1086/668024. PubMed
32. Banach DB, Bearman G, Barnden M, et al. Duration of contact precautions for acute-care settings. Infect Control Hosp Epidemiol. 2018;39(2):127-144. doi: 10.1017/ice.2017.245. PubMed
© 2019 Society of Hospital Medicine
Things We Do for No Reason: The Use of Thickened Liquids in Treating Hospitalized Adult Patients with Dysphagia
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with Alzheimer’s dementia and chronic dysphagia with a history of aspiration pneumonia presents with urinary tract infection, hypovolemia, and hypernatremia. He has been on thickened liquids at home for the past several months. As his overall condition improves with intravenous fluids and antibiotics, he requests to drink thin liquids.
BACKGROUND
Dysphagia is defined as difficulty or discomfort with feeding or swallowing1 and is a common clinical problem facing hospitalists. The prevalence of swallowing difficulties is estimated to affect 13 million people in the United States, which is likely to increase as the population ages.2 Dysphagia often results in inadequate fluid consumption, resulting in complications such as dehydration.1 However, the most dreaded complication is pneumonia from aspiration. Aspiration, the entry of material from the oropharynx or the gastrointestinal tract into the larynx and lungs, can be problematic since it is often colonized with pathogens.3-5 It constitutes 5%-15% of the four and a half million cases of community-acquired pneumonia per year with a mortality rate as high as 21%.5,6
Dysphagia is a clinical diagnosis, and assessment tools are available to help establish the mechanism and severity.3 For example, the bedside swallow evaluation uses the administration of water by the clinician to the patient to assess for the presence and severity of dysphagia.1,7 The evaluation is performed by making the patient sit upright at up at 90° and administering either single sips of ≤20 ml of water, consecutive sips with intake up to 100 ml of water, or progressively increasing volumes of water. The clinician then observes for clinical signs of aspiration such as choking or coughing. This evaluation is inexpensive, noninvasive, and time-efficient with a sensitivity as high as 91%, if conducted using the consecutive sips technique.7 A video fluoroscopic swallowing exam (VFSE) includes the administration of various barium consistencies that may be helpful in determining the precise mechanism of dysphagia, particularly in the pharyngeal stage of swallowing.3,8 VFSE is often considered as the standard for dysphagia evaluation, although it is expensive, time-consuming, exposes the patient to radiation, and its translation to functional ability to safely eat and drink is unproven.8
WHY YOU MIGHT THINK THICKENED LIQUIDS ARE HELPFUL FOR ADULT PATIENTS WITH DYSPHAGIA
Modifying oral liquid intake using thickened liquids has been the cornerstone of clinical practice in treating adults with dysphagia.4,9-11 Water, a thin liquid with a low viscosity, flows rapidly from the mouth into the oropharynx. The rapid rate may be too fast for the patient’s pharyngeal muscles to compensate, thus allowing aspiration.10 Thickening the liquids is meant to slow the flow of liquids to allow more time for airway closure, which could potentially reduce the risk of aspiration.10,11
The strongest evidence for thickened liquids originates from a study based on videofluoroscopy findings. Clave et al. studied patients with stroke or traumatic brain injury, patients with neurodegenerative diseases, and healthy volunteers using videofluoroscopy while swallowing liquid, nectar, and pudding boluses.11 Of the 46 patients with stroke or traumatic brain injury, 21.6% had aspiration of liquid into the airway, but this incidence was reduced to 10.5% and 5.3% when the diet was modified to nectar and pudding, respectively. Of the 46 patients with neurodegenerative diseases, 16.2% had aspiration of liquid into the airway, which was reduced to 8.3% and 2.9% when given nectar and pudding boluses, respectively. Thus, thickened liquids significantly improved the videofluoroscopy results, leading to a presumptive decrease in the rate of respiratory complications. Other authors have reached similar conclusions in different settings and selected patient populations.9 These results, although mostly based on imaging findings and in only narrow populations, have been widely extrapolated to routine clinical practice.1,9,12
WHY THICKENED LIQUIDS ARE NOT HELPFUL FOR ADULT PATIENTS WITH DYSPHAGIA
Evidence against thickened liquids dates back to 1994, when a comparative effectiveness trial of stroke patients found that family instruction on appropriate compensatory swallowing techniques without the use of thickened liquids carried no increased risk of pneumonia, dehydration, malnutrition, or death when compared with thickened liquids.13 Recent evidence has established the risk for harm with thickened liquids. Specifically, patients assigned to thickened liquids in one study had a higher rate of dehydration (6%-2%), fever (4%-2%), and urinary tract infections (6%-3%) than those assigned to thin liquids.14 This is presumed to be related to poor fluid and nutritional intake resulting from the thickened liquids.1,9,14
Patients’ perceived quality of life is also lower when on thickened liquids. Studies typically measured this using the validated Swallowing Quality of Life (SWAL-QOL), which is a quality-of-life and quality-of-care outcomes tool designed for patients with oropharyngeal dysphagia.1,15 One study found that those started on thickened liquids had a significant reduction in their SWAL-QOL score by nearly 14 points (P < .05).15 Perhaps because of this reduced quality of life, patient compliance has been reported to be as low as 35% at five days.16
Several systematic reviews support allowing access to free water rather than limiting patients to thickened liquids in the setting of dysphagia. Gillman et al., Kaneoka et al., and Loeb et al. found no statistical difference in the risk of developing aspiration pneumonia in patients granted access to free water when compared to those with thickened liquids.1,9,12,15 In the meta-analysis of Gillman et al. of 206 patients, there was no significant increase in the odds of having lung complications when allowing patients access to free water in comparison to thickened liquids (odds ratio 1.51, 95% confidence interval 0.2-100.03).1 The meta-analysis of Kaneoka et al. showed no significant difference in the odds of developing pneumonia in patients with access to free water compared with thickened liquids in a sample of 135 patients (odds ratio 0.82, 95% confidence interval 0.05-13.42).12 However, the systematic reviews of Gillman et al. and Kaneoka et al. included studies with stringent exclusion criteria, including impaired cognition and mobility limitations, which limits their applicability.1,12
IN WHAT CIRCUMSTANCES MIGHT THICKENED LIQUIDS BE HELPFUL
In patients who have extreme choking with water intake, restricting access to oral water may be reasonable to avoid the physical stress of coughing. Similarly, in end-of-life situations, if coughing is so bothersome to patients or families as to be inconsistent with goals of care, then thickened liquids for comfort measures may be reasonable. Finally, Foley et al. found that combining thickened liquids with texture-modified diets and intensive training sessions with speech-language pathologists focused on swallowing techniques led to a reduced risk for aspiration pneumonia during the first seven days following an acute stroke. Since risk reduction did not persist after seven days, prolonged modification is likely not helpful.4
WHAT WE SHOULD DO INSTEAD
Access to free water is important for hydration, quality of life, and delirium prevention. A collaborative approach with nurses, speech therapists, and caretakers should be employed to focus on strategies to prevent aspiration pneumonia via positioning, oral hygiene, and patient and family education. Postural adjustment with the chin-down posture alters the flow of the bolus during the pharyngeal phase of the swallow.14,17 This technique has shown superior safety when directly compared with thickened liquids without any difference in aspiration pneumonia rates.14 In addition, oral hygiene for patients who cannot perform oral care themselves should be implemented to decrease the amount of pathogenic bacteria in secretions.1,15 Finally, ensuring that patients and families understand the risks and benefits of access to free water is paramount.
Tube feeding (eg, nasogastric and gastric tubes) allows for reliable delivery of enteral nutrition and medications. Tube feeding does not decrease aspiration events compared with oral diets. Moreover, the risk of developing aspiration pneumonia appears to be similar among gastrostomy, nasogastric, and postpyloric feeding tubes.5 This approach may be preferable, though, when the dysphagia is the result of a structural abnormality such as stroke deficit, neoplastic changes, or surgical alteration of the larynx.
Free water protocols use an interdisciplinary approach to safely improve access to water for patients with dysphagia. Free water protocols involve screening high-risk populations such as the elderly, confused, or stroke patients with a bedside swallow evaluation. Those with difficulty following directions, who are unable to limit their drinking to manageable-sized sips, or with excessive cough are restricted to supervised water drinking with access to water only between meals (30 minutes after a meal) and with aggressive oral hygiene. Posturing techniques with the chin-down position may be employed. Patients and their families must be educated on protocol implementation and rationale.1,9,12
Overall, free water protocols have demonstrated an improvement in quality of life, no change in adverse events, and improved water intake. SWAL-QOL scores were significantly improved by nearly three points (P < .05).15 There was no significant difference in the odds of developing aspiration pneumonia when comparing those on thickened liquids to those with access to free water.1,9,12 Furthermore, one study by Loeb et al. even found that those allocated to a thickened liquid group were more likely to develop aspiration pneumonia, although this difference was not statistically significant.9 Finally, those given access to free water had higher amounts of fluid intake by a mean of 180 ml.1
RECOMMENDATIONS
- Allow patients with dysphagia access to free water
- Initiate protocols to ensure adequate oral hygiene, patient and family education, and optimization of positioning strategies
CONCLUSIONS
Our patient is assessed with a bedside swallow evaluation and has issues with minor coughing. Despite this, he repeatedly requests access to free water, and these requests are upsetting to his family. The risks of potential aspiration are explained to him, and he and his family express understanding. He is given supervised access to water between meals and is encouraged to sit upright and brush his teeth prior to drinking. He continues to improve throughout the hospitalization and at the time of discharge, his sodium level is within normal limits and he is delighted to be drinking regular water.
Patients with dysphagia are often restricted to thickened liquids. This approach does alter the liquid flow throughout the oropharynx and minimal clinical evidence supports this practice as a method to reduce aspiration pneumonia. Given the potential harm and the reduced quality of life, we recommend against thickened liquids in this setting. Taken as a whole, available evidence suggests that protocols to facilitate safe access to water,1 family information and education,13 and positioning techniques14 are safe, effective, and preferable to thickened liquids.1,12
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
1. Gillman A, Winkler R, Taylor NF. Implementing the free water protocol does not result in aspiration pneumonia in carefully selected patients with dysphagia: a systematic review. Dysphagia. 2017;32(3):345-361. doi: 10.1007/s00455-016-9761-3. PubMed
2. Bhattacharyya N. The prevalence of dysphagia among adults in the United States. Otolaryngol Head Neck Surg. 2014;151(5):765-769. doi: 10.1177/0194599814549156. PubMed
3. Karagiannis MJP CL, Karagiannis TC. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatrics. 2011;11(2):9. doi: 10.1186/1471-2318-11-9. PubMed
4. Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomised controlled trials. Age Ageing. 2008;37(3):258-264. doi: 10.1093/ageing/afn064. PubMed
5. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. doi: 10.1056/NEJM200103013440908. PubMed
6. Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83-90. doi: 10.1002/jhm.1996. PubMed
7. Brodsky MB, Suiter DM, Gonzalez-Fernandez M, et al. Screening accuracy for aspiration using bedside water swallow tests: a systematic review and meta-analysis. Chest. 2016;150(1):148-163. doi: 10.1016/j.chest.2016.03.059. PubMed
8. Carnaby-Mann G, Lenius K. The bedside examination in dysphagia. Phys Med Rehabil Clin N Am. 2008;19(4):747-768, viii. doi: 10.1016/j.pmr.2008.05.008. PubMed
9. Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022. doi: 10.1046/j.1365-2389.2003.51318.x. PubMed
10. Steele CM, Alsanei WA, Ayanikalath S, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: a systematic review. Dysphagia. 2015;30(1):2-26. doi: 10.1007/s00455-014-9578-x. PubMed
11. Clave P, de Kraa M, Arreola V, et al. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment Pharmacol Ther. 2006;24(9):1385-1394. doi: 10.1111/j.1365-2036.2006.03118.x. PubMed
12. Kaneoka A, Pisegna JM, Saito H, et al. A systematic review and meta-analysis of pneumonia associated with thin liquid vs. thickened liquid intake in patients who aspirate. Clin Rehabil. 2017;31(8):1116-1125. doi: 10.1177/0269215516677739. PubMed
13. DePippo KL, Holas MA, Reding MJ, Mandel FS, Lesser ML. Dysphagia therapy following stroke: a controlled trial. Neurology. 1994;44(9):1655-1660. doi: 10.1212/WNL.44.9.1655. PubMed
14. Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509-518. doi: 10.7326/0003-4819-148-7-200804010-00007. PubMed
15. Carlaw C, Finlayson H, Beggs K, et al. Outcomes of a pilot water protocol project in a rehabilitation setting. Dysphagia. 2012;27(3):297-306. doi: 10.1007/s00455-011-9366-9. PubMed
16. Leiter AE WJ. Compliance of geriatric dysphagic patients with safe-swallowing instructions. J Med Speech Lang Pathol. 1996;4(4):289-300.
17. Ashford J, McCabe D, Wheeler-Hegland K, et al. Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part III--impact of dysphagia treatments on populations with neurological disorders. J Rehabil Res Dev. 2009;46(2):195-204. doi: 10.1682/JRRD.2008.08.0091. PubMed
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with Alzheimer’s dementia and chronic dysphagia with a history of aspiration pneumonia presents with urinary tract infection, hypovolemia, and hypernatremia. He has been on thickened liquids at home for the past several months. As his overall condition improves with intravenous fluids and antibiotics, he requests to drink thin liquids.
BACKGROUND
Dysphagia is defined as difficulty or discomfort with feeding or swallowing1 and is a common clinical problem facing hospitalists. The prevalence of swallowing difficulties is estimated to affect 13 million people in the United States, which is likely to increase as the population ages.2 Dysphagia often results in inadequate fluid consumption, resulting in complications such as dehydration.1 However, the most dreaded complication is pneumonia from aspiration. Aspiration, the entry of material from the oropharynx or the gastrointestinal tract into the larynx and lungs, can be problematic since it is often colonized with pathogens.3-5 It constitutes 5%-15% of the four and a half million cases of community-acquired pneumonia per year with a mortality rate as high as 21%.5,6
Dysphagia is a clinical diagnosis, and assessment tools are available to help establish the mechanism and severity.3 For example, the bedside swallow evaluation uses the administration of water by the clinician to the patient to assess for the presence and severity of dysphagia.1,7 The evaluation is performed by making the patient sit upright at up at 90° and administering either single sips of ≤20 ml of water, consecutive sips with intake up to 100 ml of water, or progressively increasing volumes of water. The clinician then observes for clinical signs of aspiration such as choking or coughing. This evaluation is inexpensive, noninvasive, and time-efficient with a sensitivity as high as 91%, if conducted using the consecutive sips technique.7 A video fluoroscopic swallowing exam (VFSE) includes the administration of various barium consistencies that may be helpful in determining the precise mechanism of dysphagia, particularly in the pharyngeal stage of swallowing.3,8 VFSE is often considered as the standard for dysphagia evaluation, although it is expensive, time-consuming, exposes the patient to radiation, and its translation to functional ability to safely eat and drink is unproven.8
WHY YOU MIGHT THINK THICKENED LIQUIDS ARE HELPFUL FOR ADULT PATIENTS WITH DYSPHAGIA
Modifying oral liquid intake using thickened liquids has been the cornerstone of clinical practice in treating adults with dysphagia.4,9-11 Water, a thin liquid with a low viscosity, flows rapidly from the mouth into the oropharynx. The rapid rate may be too fast for the patient’s pharyngeal muscles to compensate, thus allowing aspiration.10 Thickening the liquids is meant to slow the flow of liquids to allow more time for airway closure, which could potentially reduce the risk of aspiration.10,11
The strongest evidence for thickened liquids originates from a study based on videofluoroscopy findings. Clave et al. studied patients with stroke or traumatic brain injury, patients with neurodegenerative diseases, and healthy volunteers using videofluoroscopy while swallowing liquid, nectar, and pudding boluses.11 Of the 46 patients with stroke or traumatic brain injury, 21.6% had aspiration of liquid into the airway, but this incidence was reduced to 10.5% and 5.3% when the diet was modified to nectar and pudding, respectively. Of the 46 patients with neurodegenerative diseases, 16.2% had aspiration of liquid into the airway, which was reduced to 8.3% and 2.9% when given nectar and pudding boluses, respectively. Thus, thickened liquids significantly improved the videofluoroscopy results, leading to a presumptive decrease in the rate of respiratory complications. Other authors have reached similar conclusions in different settings and selected patient populations.9 These results, although mostly based on imaging findings and in only narrow populations, have been widely extrapolated to routine clinical practice.1,9,12
WHY THICKENED LIQUIDS ARE NOT HELPFUL FOR ADULT PATIENTS WITH DYSPHAGIA
Evidence against thickened liquids dates back to 1994, when a comparative effectiveness trial of stroke patients found that family instruction on appropriate compensatory swallowing techniques without the use of thickened liquids carried no increased risk of pneumonia, dehydration, malnutrition, or death when compared with thickened liquids.13 Recent evidence has established the risk for harm with thickened liquids. Specifically, patients assigned to thickened liquids in one study had a higher rate of dehydration (6%-2%), fever (4%-2%), and urinary tract infections (6%-3%) than those assigned to thin liquids.14 This is presumed to be related to poor fluid and nutritional intake resulting from the thickened liquids.1,9,14
Patients’ perceived quality of life is also lower when on thickened liquids. Studies typically measured this using the validated Swallowing Quality of Life (SWAL-QOL), which is a quality-of-life and quality-of-care outcomes tool designed for patients with oropharyngeal dysphagia.1,15 One study found that those started on thickened liquids had a significant reduction in their SWAL-QOL score by nearly 14 points (P < .05).15 Perhaps because of this reduced quality of life, patient compliance has been reported to be as low as 35% at five days.16
Several systematic reviews support allowing access to free water rather than limiting patients to thickened liquids in the setting of dysphagia. Gillman et al., Kaneoka et al., and Loeb et al. found no statistical difference in the risk of developing aspiration pneumonia in patients granted access to free water when compared to those with thickened liquids.1,9,12,15 In the meta-analysis of Gillman et al. of 206 patients, there was no significant increase in the odds of having lung complications when allowing patients access to free water in comparison to thickened liquids (odds ratio 1.51, 95% confidence interval 0.2-100.03).1 The meta-analysis of Kaneoka et al. showed no significant difference in the odds of developing pneumonia in patients with access to free water compared with thickened liquids in a sample of 135 patients (odds ratio 0.82, 95% confidence interval 0.05-13.42).12 However, the systematic reviews of Gillman et al. and Kaneoka et al. included studies with stringent exclusion criteria, including impaired cognition and mobility limitations, which limits their applicability.1,12
IN WHAT CIRCUMSTANCES MIGHT THICKENED LIQUIDS BE HELPFUL
In patients who have extreme choking with water intake, restricting access to oral water may be reasonable to avoid the physical stress of coughing. Similarly, in end-of-life situations, if coughing is so bothersome to patients or families as to be inconsistent with goals of care, then thickened liquids for comfort measures may be reasonable. Finally, Foley et al. found that combining thickened liquids with texture-modified diets and intensive training sessions with speech-language pathologists focused on swallowing techniques led to a reduced risk for aspiration pneumonia during the first seven days following an acute stroke. Since risk reduction did not persist after seven days, prolonged modification is likely not helpful.4
WHAT WE SHOULD DO INSTEAD
Access to free water is important for hydration, quality of life, and delirium prevention. A collaborative approach with nurses, speech therapists, and caretakers should be employed to focus on strategies to prevent aspiration pneumonia via positioning, oral hygiene, and patient and family education. Postural adjustment with the chin-down posture alters the flow of the bolus during the pharyngeal phase of the swallow.14,17 This technique has shown superior safety when directly compared with thickened liquids without any difference in aspiration pneumonia rates.14 In addition, oral hygiene for patients who cannot perform oral care themselves should be implemented to decrease the amount of pathogenic bacteria in secretions.1,15 Finally, ensuring that patients and families understand the risks and benefits of access to free water is paramount.
Tube feeding (eg, nasogastric and gastric tubes) allows for reliable delivery of enteral nutrition and medications. Tube feeding does not decrease aspiration events compared with oral diets. Moreover, the risk of developing aspiration pneumonia appears to be similar among gastrostomy, nasogastric, and postpyloric feeding tubes.5 This approach may be preferable, though, when the dysphagia is the result of a structural abnormality such as stroke deficit, neoplastic changes, or surgical alteration of the larynx.
Free water protocols use an interdisciplinary approach to safely improve access to water for patients with dysphagia. Free water protocols involve screening high-risk populations such as the elderly, confused, or stroke patients with a bedside swallow evaluation. Those with difficulty following directions, who are unable to limit their drinking to manageable-sized sips, or with excessive cough are restricted to supervised water drinking with access to water only between meals (30 minutes after a meal) and with aggressive oral hygiene. Posturing techniques with the chin-down position may be employed. Patients and their families must be educated on protocol implementation and rationale.1,9,12
Overall, free water protocols have demonstrated an improvement in quality of life, no change in adverse events, and improved water intake. SWAL-QOL scores were significantly improved by nearly three points (P < .05).15 There was no significant difference in the odds of developing aspiration pneumonia when comparing those on thickened liquids to those with access to free water.1,9,12 Furthermore, one study by Loeb et al. even found that those allocated to a thickened liquid group were more likely to develop aspiration pneumonia, although this difference was not statistically significant.9 Finally, those given access to free water had higher amounts of fluid intake by a mean of 180 ml.1
RECOMMENDATIONS
- Allow patients with dysphagia access to free water
- Initiate protocols to ensure adequate oral hygiene, patient and family education, and optimization of positioning strategies
CONCLUSIONS
Our patient is assessed with a bedside swallow evaluation and has issues with minor coughing. Despite this, he repeatedly requests access to free water, and these requests are upsetting to his family. The risks of potential aspiration are explained to him, and he and his family express understanding. He is given supervised access to water between meals and is encouraged to sit upright and brush his teeth prior to drinking. He continues to improve throughout the hospitalization and at the time of discharge, his sodium level is within normal limits and he is delighted to be drinking regular water.
Patients with dysphagia are often restricted to thickened liquids. This approach does alter the liquid flow throughout the oropharynx and minimal clinical evidence supports this practice as a method to reduce aspiration pneumonia. Given the potential harm and the reduced quality of life, we recommend against thickened liquids in this setting. Taken as a whole, available evidence suggests that protocols to facilitate safe access to water,1 family information and education,13 and positioning techniques14 are safe, effective, and preferable to thickened liquids.1,12
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with Alzheimer’s dementia and chronic dysphagia with a history of aspiration pneumonia presents with urinary tract infection, hypovolemia, and hypernatremia. He has been on thickened liquids at home for the past several months. As his overall condition improves with intravenous fluids and antibiotics, he requests to drink thin liquids.
BACKGROUND
Dysphagia is defined as difficulty or discomfort with feeding or swallowing1 and is a common clinical problem facing hospitalists. The prevalence of swallowing difficulties is estimated to affect 13 million people in the United States, which is likely to increase as the population ages.2 Dysphagia often results in inadequate fluid consumption, resulting in complications such as dehydration.1 However, the most dreaded complication is pneumonia from aspiration. Aspiration, the entry of material from the oropharynx or the gastrointestinal tract into the larynx and lungs, can be problematic since it is often colonized with pathogens.3-5 It constitutes 5%-15% of the four and a half million cases of community-acquired pneumonia per year with a mortality rate as high as 21%.5,6
Dysphagia is a clinical diagnosis, and assessment tools are available to help establish the mechanism and severity.3 For example, the bedside swallow evaluation uses the administration of water by the clinician to the patient to assess for the presence and severity of dysphagia.1,7 The evaluation is performed by making the patient sit upright at up at 90° and administering either single sips of ≤20 ml of water, consecutive sips with intake up to 100 ml of water, or progressively increasing volumes of water. The clinician then observes for clinical signs of aspiration such as choking or coughing. This evaluation is inexpensive, noninvasive, and time-efficient with a sensitivity as high as 91%, if conducted using the consecutive sips technique.7 A video fluoroscopic swallowing exam (VFSE) includes the administration of various barium consistencies that may be helpful in determining the precise mechanism of dysphagia, particularly in the pharyngeal stage of swallowing.3,8 VFSE is often considered as the standard for dysphagia evaluation, although it is expensive, time-consuming, exposes the patient to radiation, and its translation to functional ability to safely eat and drink is unproven.8
WHY YOU MIGHT THINK THICKENED LIQUIDS ARE HELPFUL FOR ADULT PATIENTS WITH DYSPHAGIA
Modifying oral liquid intake using thickened liquids has been the cornerstone of clinical practice in treating adults with dysphagia.4,9-11 Water, a thin liquid with a low viscosity, flows rapidly from the mouth into the oropharynx. The rapid rate may be too fast for the patient’s pharyngeal muscles to compensate, thus allowing aspiration.10 Thickening the liquids is meant to slow the flow of liquids to allow more time for airway closure, which could potentially reduce the risk of aspiration.10,11
The strongest evidence for thickened liquids originates from a study based on videofluoroscopy findings. Clave et al. studied patients with stroke or traumatic brain injury, patients with neurodegenerative diseases, and healthy volunteers using videofluoroscopy while swallowing liquid, nectar, and pudding boluses.11 Of the 46 patients with stroke or traumatic brain injury, 21.6% had aspiration of liquid into the airway, but this incidence was reduced to 10.5% and 5.3% when the diet was modified to nectar and pudding, respectively. Of the 46 patients with neurodegenerative diseases, 16.2% had aspiration of liquid into the airway, which was reduced to 8.3% and 2.9% when given nectar and pudding boluses, respectively. Thus, thickened liquids significantly improved the videofluoroscopy results, leading to a presumptive decrease in the rate of respiratory complications. Other authors have reached similar conclusions in different settings and selected patient populations.9 These results, although mostly based on imaging findings and in only narrow populations, have been widely extrapolated to routine clinical practice.1,9,12
WHY THICKENED LIQUIDS ARE NOT HELPFUL FOR ADULT PATIENTS WITH DYSPHAGIA
Evidence against thickened liquids dates back to 1994, when a comparative effectiveness trial of stroke patients found that family instruction on appropriate compensatory swallowing techniques without the use of thickened liquids carried no increased risk of pneumonia, dehydration, malnutrition, or death when compared with thickened liquids.13 Recent evidence has established the risk for harm with thickened liquids. Specifically, patients assigned to thickened liquids in one study had a higher rate of dehydration (6%-2%), fever (4%-2%), and urinary tract infections (6%-3%) than those assigned to thin liquids.14 This is presumed to be related to poor fluid and nutritional intake resulting from the thickened liquids.1,9,14
Patients’ perceived quality of life is also lower when on thickened liquids. Studies typically measured this using the validated Swallowing Quality of Life (SWAL-QOL), which is a quality-of-life and quality-of-care outcomes tool designed for patients with oropharyngeal dysphagia.1,15 One study found that those started on thickened liquids had a significant reduction in their SWAL-QOL score by nearly 14 points (P < .05).15 Perhaps because of this reduced quality of life, patient compliance has been reported to be as low as 35% at five days.16
Several systematic reviews support allowing access to free water rather than limiting patients to thickened liquids in the setting of dysphagia. Gillman et al., Kaneoka et al., and Loeb et al. found no statistical difference in the risk of developing aspiration pneumonia in patients granted access to free water when compared to those with thickened liquids.1,9,12,15 In the meta-analysis of Gillman et al. of 206 patients, there was no significant increase in the odds of having lung complications when allowing patients access to free water in comparison to thickened liquids (odds ratio 1.51, 95% confidence interval 0.2-100.03).1 The meta-analysis of Kaneoka et al. showed no significant difference in the odds of developing pneumonia in patients with access to free water compared with thickened liquids in a sample of 135 patients (odds ratio 0.82, 95% confidence interval 0.05-13.42).12 However, the systematic reviews of Gillman et al. and Kaneoka et al. included studies with stringent exclusion criteria, including impaired cognition and mobility limitations, which limits their applicability.1,12
IN WHAT CIRCUMSTANCES MIGHT THICKENED LIQUIDS BE HELPFUL
In patients who have extreme choking with water intake, restricting access to oral water may be reasonable to avoid the physical stress of coughing. Similarly, in end-of-life situations, if coughing is so bothersome to patients or families as to be inconsistent with goals of care, then thickened liquids for comfort measures may be reasonable. Finally, Foley et al. found that combining thickened liquids with texture-modified diets and intensive training sessions with speech-language pathologists focused on swallowing techniques led to a reduced risk for aspiration pneumonia during the first seven days following an acute stroke. Since risk reduction did not persist after seven days, prolonged modification is likely not helpful.4
WHAT WE SHOULD DO INSTEAD
Access to free water is important for hydration, quality of life, and delirium prevention. A collaborative approach with nurses, speech therapists, and caretakers should be employed to focus on strategies to prevent aspiration pneumonia via positioning, oral hygiene, and patient and family education. Postural adjustment with the chin-down posture alters the flow of the bolus during the pharyngeal phase of the swallow.14,17 This technique has shown superior safety when directly compared with thickened liquids without any difference in aspiration pneumonia rates.14 In addition, oral hygiene for patients who cannot perform oral care themselves should be implemented to decrease the amount of pathogenic bacteria in secretions.1,15 Finally, ensuring that patients and families understand the risks and benefits of access to free water is paramount.
Tube feeding (eg, nasogastric and gastric tubes) allows for reliable delivery of enteral nutrition and medications. Tube feeding does not decrease aspiration events compared with oral diets. Moreover, the risk of developing aspiration pneumonia appears to be similar among gastrostomy, nasogastric, and postpyloric feeding tubes.5 This approach may be preferable, though, when the dysphagia is the result of a structural abnormality such as stroke deficit, neoplastic changes, or surgical alteration of the larynx.
Free water protocols use an interdisciplinary approach to safely improve access to water for patients with dysphagia. Free water protocols involve screening high-risk populations such as the elderly, confused, or stroke patients with a bedside swallow evaluation. Those with difficulty following directions, who are unable to limit their drinking to manageable-sized sips, or with excessive cough are restricted to supervised water drinking with access to water only between meals (30 minutes after a meal) and with aggressive oral hygiene. Posturing techniques with the chin-down position may be employed. Patients and their families must be educated on protocol implementation and rationale.1,9,12
Overall, free water protocols have demonstrated an improvement in quality of life, no change in adverse events, and improved water intake. SWAL-QOL scores were significantly improved by nearly three points (P < .05).15 There was no significant difference in the odds of developing aspiration pneumonia when comparing those on thickened liquids to those with access to free water.1,9,12 Furthermore, one study by Loeb et al. even found that those allocated to a thickened liquid group were more likely to develop aspiration pneumonia, although this difference was not statistically significant.9 Finally, those given access to free water had higher amounts of fluid intake by a mean of 180 ml.1
RECOMMENDATIONS
- Allow patients with dysphagia access to free water
- Initiate protocols to ensure adequate oral hygiene, patient and family education, and optimization of positioning strategies
CONCLUSIONS
Our patient is assessed with a bedside swallow evaluation and has issues with minor coughing. Despite this, he repeatedly requests access to free water, and these requests are upsetting to his family. The risks of potential aspiration are explained to him, and he and his family express understanding. He is given supervised access to water between meals and is encouraged to sit upright and brush his teeth prior to drinking. He continues to improve throughout the hospitalization and at the time of discharge, his sodium level is within normal limits and he is delighted to be drinking regular water.
Patients with dysphagia are often restricted to thickened liquids. This approach does alter the liquid flow throughout the oropharynx and minimal clinical evidence supports this practice as a method to reduce aspiration pneumonia. Given the potential harm and the reduced quality of life, we recommend against thickened liquids in this setting. Taken as a whole, available evidence suggests that protocols to facilitate safe access to water,1 family information and education,13 and positioning techniques14 are safe, effective, and preferable to thickened liquids.1,12
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
The authors have nothing to disclose.
1. Gillman A, Winkler R, Taylor NF. Implementing the free water protocol does not result in aspiration pneumonia in carefully selected patients with dysphagia: a systematic review. Dysphagia. 2017;32(3):345-361. doi: 10.1007/s00455-016-9761-3. PubMed
2. Bhattacharyya N. The prevalence of dysphagia among adults in the United States. Otolaryngol Head Neck Surg. 2014;151(5):765-769. doi: 10.1177/0194599814549156. PubMed
3. Karagiannis MJP CL, Karagiannis TC. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatrics. 2011;11(2):9. doi: 10.1186/1471-2318-11-9. PubMed
4. Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomised controlled trials. Age Ageing. 2008;37(3):258-264. doi: 10.1093/ageing/afn064. PubMed
5. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. doi: 10.1056/NEJM200103013440908. PubMed
6. Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83-90. doi: 10.1002/jhm.1996. PubMed
7. Brodsky MB, Suiter DM, Gonzalez-Fernandez M, et al. Screening accuracy for aspiration using bedside water swallow tests: a systematic review and meta-analysis. Chest. 2016;150(1):148-163. doi: 10.1016/j.chest.2016.03.059. PubMed
8. Carnaby-Mann G, Lenius K. The bedside examination in dysphagia. Phys Med Rehabil Clin N Am. 2008;19(4):747-768, viii. doi: 10.1016/j.pmr.2008.05.008. PubMed
9. Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022. doi: 10.1046/j.1365-2389.2003.51318.x. PubMed
10. Steele CM, Alsanei WA, Ayanikalath S, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: a systematic review. Dysphagia. 2015;30(1):2-26. doi: 10.1007/s00455-014-9578-x. PubMed
11. Clave P, de Kraa M, Arreola V, et al. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment Pharmacol Ther. 2006;24(9):1385-1394. doi: 10.1111/j.1365-2036.2006.03118.x. PubMed
12. Kaneoka A, Pisegna JM, Saito H, et al. A systematic review and meta-analysis of pneumonia associated with thin liquid vs. thickened liquid intake in patients who aspirate. Clin Rehabil. 2017;31(8):1116-1125. doi: 10.1177/0269215516677739. PubMed
13. DePippo KL, Holas MA, Reding MJ, Mandel FS, Lesser ML. Dysphagia therapy following stroke: a controlled trial. Neurology. 1994;44(9):1655-1660. doi: 10.1212/WNL.44.9.1655. PubMed
14. Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509-518. doi: 10.7326/0003-4819-148-7-200804010-00007. PubMed
15. Carlaw C, Finlayson H, Beggs K, et al. Outcomes of a pilot water protocol project in a rehabilitation setting. Dysphagia. 2012;27(3):297-306. doi: 10.1007/s00455-011-9366-9. PubMed
16. Leiter AE WJ. Compliance of geriatric dysphagic patients with safe-swallowing instructions. J Med Speech Lang Pathol. 1996;4(4):289-300.
17. Ashford J, McCabe D, Wheeler-Hegland K, et al. Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part III--impact of dysphagia treatments on populations with neurological disorders. J Rehabil Res Dev. 2009;46(2):195-204. doi: 10.1682/JRRD.2008.08.0091. PubMed
1. Gillman A, Winkler R, Taylor NF. Implementing the free water protocol does not result in aspiration pneumonia in carefully selected patients with dysphagia: a systematic review. Dysphagia. 2017;32(3):345-361. doi: 10.1007/s00455-016-9761-3. PubMed
2. Bhattacharyya N. The prevalence of dysphagia among adults in the United States. Otolaryngol Head Neck Surg. 2014;151(5):765-769. doi: 10.1177/0194599814549156. PubMed
3. Karagiannis MJP CL, Karagiannis TC. Effects of oral intake of water in patients with oropharyngeal dysphagia. BMC Geriatrics. 2011;11(2):9. doi: 10.1186/1471-2318-11-9. PubMed
4. Foley N, Teasell R, Salter K, Kruger E, Martino R. Dysphagia treatment post stroke: a systematic review of randomised controlled trials. Age Ageing. 2008;37(3):258-264. doi: 10.1093/ageing/afn064. PubMed
5. Marik PE. Aspiration pneumonitis and aspiration pneumonia. N Engl J Med. 2001;344(9):665-671. doi: 10.1056/NEJM200103013440908. PubMed
6. Lanspa MJ, Jones BE, Brown SM, Dean NC. Mortality, morbidity, and disease severity of patients with aspiration pneumonia. J Hosp Med. 2013;8(2):83-90. doi: 10.1002/jhm.1996. PubMed
7. Brodsky MB, Suiter DM, Gonzalez-Fernandez M, et al. Screening accuracy for aspiration using bedside water swallow tests: a systematic review and meta-analysis. Chest. 2016;150(1):148-163. doi: 10.1016/j.chest.2016.03.059. PubMed
8. Carnaby-Mann G, Lenius K. The bedside examination in dysphagia. Phys Med Rehabil Clin N Am. 2008;19(4):747-768, viii. doi: 10.1016/j.pmr.2008.05.008. PubMed
9. Loeb MB, Becker M, Eady A, Walker-Dilks C. Interventions to prevent aspiration pneumonia in older adults: a systematic review. J Am Geriatr Soc. 2003;51(7):1018-1022. doi: 10.1046/j.1365-2389.2003.51318.x. PubMed
10. Steele CM, Alsanei WA, Ayanikalath S, et al. The influence of food texture and liquid consistency modification on swallowing physiology and function: a systematic review. Dysphagia. 2015;30(1):2-26. doi: 10.1007/s00455-014-9578-x. PubMed
11. Clave P, de Kraa M, Arreola V, et al. The effect of bolus viscosity on swallowing function in neurogenic dysphagia. Aliment Pharmacol Ther. 2006;24(9):1385-1394. doi: 10.1111/j.1365-2036.2006.03118.x. PubMed
12. Kaneoka A, Pisegna JM, Saito H, et al. A systematic review and meta-analysis of pneumonia associated with thin liquid vs. thickened liquid intake in patients who aspirate. Clin Rehabil. 2017;31(8):1116-1125. doi: 10.1177/0269215516677739. PubMed
13. DePippo KL, Holas MA, Reding MJ, Mandel FS, Lesser ML. Dysphagia therapy following stroke: a controlled trial. Neurology. 1994;44(9):1655-1660. doi: 10.1212/WNL.44.9.1655. PubMed
14. Robbins J, Gensler G, Hind J, et al. Comparison of 2 interventions for liquid aspiration on pneumonia incidence: a randomized trial. Ann Intern Med. 2008;148(7):509-518. doi: 10.7326/0003-4819-148-7-200804010-00007. PubMed
15. Carlaw C, Finlayson H, Beggs K, et al. Outcomes of a pilot water protocol project in a rehabilitation setting. Dysphagia. 2012;27(3):297-306. doi: 10.1007/s00455-011-9366-9. PubMed
16. Leiter AE WJ. Compliance of geriatric dysphagic patients with safe-swallowing instructions. J Med Speech Lang Pathol. 1996;4(4):289-300.
17. Ashford J, McCabe D, Wheeler-Hegland K, et al. Evidence-based systematic review: Oropharyngeal dysphagia behavioral treatments. Part III--impact of dysphagia treatments on populations with neurological disorders. J Rehabil Res Dev. 2009;46(2):195-204. doi: 10.1682/JRRD.2008.08.0091. PubMed
© 2019 Society of Hospital Medicine
Things We Do for No Reason: Prescribing Docusate for Constipation in Hospitalized Adults
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Click here for the Choosing Wisely website.

CASE PRESENTATION
An 80-year-old woman with no significant past medical history presents with a mechanical fall. X-rays are notable for a right hip fracture. She is treated with morphine for analgesia and evaluated by orthopedic surgery for surgical repair. The hospitalist recognizes that this patient is at high risk for constipation and orders docusate for prevention of constipation.
BACKGROUND
Constipation is a highly prevalent problem in all practice settings, especially in the hospital, affecting two out of five hospitalized patients.1 Multiple factors in the inpatient setting contribute to constipation, including decreased mobility, medical comorbidities, postsurgical ileus, anesthetics, and medications such as opioid analgesics. Furthermore, the inpatient population is aging in parallel with the general population and constipation is more common in the elderly, likely owing to a combination of decreased muscle mass and impaired function of autonomic nerves.2 Consequently, inpatient providers frequently treat constipation or try to prevent it using stool softeners or laxatives.
One of the most commonly prescribed agents, regardless of medical specialty, is docusate, also known as dioctyl sulfosuccinate or by its brand name, Colace. A study from McGill University Health Centre in Montreal, Canada reported that docusate was the most frequently prescribed laxative, accounting for 64% of laxative medication doses, with associated costs approaching $60,000 per year.3 Direct drug costs accounted for a quarter of the expenses, and the remaining three quarters were estimated labor costs for administration. Medical and surgical admissions shared similar proportions of usage, with an average of 10 doses of docusate per admission across 17,064 admissions. Furthermore, half of the patients were prescribed docusate upon discharge. The authors extrapolated their data to suggest that total healthcare spending in North America on docusate products likely exceeds $100,000,000 yearly. A second study from Toronto found that 15% of all hospitalized patients are prescribed at least one dose of docusate, and that one-third of all new inpatient prescriptions are continued at discharge.4
WHY YOU THINK DOCUSATE MIGHT BE HELPFUL FOR CONSTIPATION
Docusate is thought to act as a detergent to retain water in the stool, thereby acting as a stool softener to facilitate stool passage. Physicians have prescribed docusate for decades, and attendings have passed down the practice of prescribing docusate for constipation to medical trainees for generations. The initial docusate studies showed promise, as it softened the stool by increasing its water content and made it easier to pass through the intestines.5 One of the earliest human studies compared docusate to an unspecified placebo in 35 elderly patients with chronic atonic constipation and found a decreased need for enemas.6 Some other observational studies also reported a decreased need for manual disimpactions and enemas in elderly populations.7,8 One randomized, controlled trial from 1968 showed an increased frequency of bowel movements compared to placebo, but it excluded half of the enrolled patients because they had a positive placebo response.9 Since those early studies from the 1950s and 1960s, docusate remains widely accepted as an effective stool softener with positive endorsements from hospital formularies and order sets and patient information sheets such as the JAMA Patient Page.10 Furthermore, the World Health Organization lists docusate as an “essential medicine,” reinforcing the notion that it is effective.11
WHY THERE IS NO REASON TO PRESCRIBE DOCUSATE FOR CONSTIPATION
Despite common practice, the efficacy of docusate as a stool softener has not been borne out by rigorous scientific data. On the contrary, multiple randomized controlled trials have failed to show any significant efficacy of this drug over placebo (Table).
The initial trial in 1976 studied 34 elderly patients on a general medical ward for prophylaxis of constipation.12 They randomized patients to 100 mg twice daily of docusate sodium versus a control group that did not receive any type of laxative. The number of bowel movements and their character served as the measured outcomes. The study demonstrated no statistically significant differences in the frequency and character of bowel movements between the docusate and placebo groups. Even at that time, the authors questioned whether docusate had any efficacy at all: “[w]hether the drug actually offers anything beyond a placebo effect in preventing constipation is in doubt.”
Another trial in 1978 studied 46 elderly, institutionalized patients with chronic functional constipation.13 All patients underwent a two-week placebo period followed by a three-week treatment period with three arms of randomization: docusate sodium 100 mg daily, docusate sodium 100 mg twice daily, or docusate calcium 240 mg daily. Patients received enemas or suppositories if required. All three arms showed an increase in the average number of natural bowel movements when compared to each patient’s own placebo period, but only the arm with docusate calcium reached statistical significance (P < .02). According to the authors, none of the therapies appeared to have a significant effect on stool consistency. The authors hypothesized that the higher dose given to the docusate calcium arm may have been the reason for the apparent efficacy in this cohort. As such, studies with higher doses of docusate calcium would be reasonable.
A third study in 1985 compared docusate sodium 100 mg three times daily versus placebo in six healthy patients with ileostomies and six healthy volunteers.14 Therapy with docusate “had no effect on stool weight, stool frequency, stool water, or mean transit time.”
Another study in 1991 evaluated 15 elderly nursing home residents with a randomized, double-blind crossover design.15 Subjects received 240 mg twice daily of docusate calcium versus placebo for three weeks and then crossed over to other arm after a two-week wash-out period. The investigators found no difference in the number of bowel movements per week or in the need for additional laxatives between the two study periods. There were also no differences in the patients’ subjective experience of constipation or discomfort with defecation.
Larger studies were subsequently initiated in more recent years. In 1998, a randomized controlled trial in 170 subjects with chronic idiopathic constipation compared psyllium 5.1 g twice daily and docusate sodium 100 mg twice daily with a corresponding placebo in each arm for a treatment duration of two weeks after a two-week placebo baseline period.16 Psyllium was found to increase stool water content and stool water weight over the baseline period, while docusate essentially had no effect on stool water content or water weight. Furthermore, by treatment week 2, psyllium demonstrated an increase in the frequency of bowel movements, whereas docusate did not. It should be noted that this study was funded by Procter & Gamble, which manufactures Metamucil, a popular brand of psyllium.
Lastly, the most recent randomized controlled trial was published in 2013. It included 74 hospice patients in Canada, comparing docusate 200 mg and sennosides twice daily versus placebo and sennosides for 10 days. The study found no difference in stool frequency, volume, or consistency between docusate and placebo.17
A number of systematic reviews have studied the literature on bowel regimens and have noted the paucity of high-quality data supporting the efficacy of docusate, despite its widespread use.18-22 With these weak data, multiple authors have advocated for removing docusate from hospital formularies and using hospitalizations as an opportunity to deprescribe this medication to reduce polypharmacy. 3,4,23
Although docusate is considered a benign therapy, there is certainly potential for harm to the patient and detrimental effects on the healthcare system. Patients commonly complain about the unpleasant taste and lingering aftertaste, which may lead to decreased oral intake and worsening nutritional status.23 Furthermore, docusate may impact the absorption and effectiveness of other proven treatments.23 Perhaps the most important harm is that providers needlessly wait for docusate to fail before prescribing effective therapies for constipation. This process negatively impacts patient satisfaction and potentially increases healthcare costs if hospital length of stay is increased. Another important consideration is that patients may refuse truly necessary medications due to the excessive pill burden.
Costs to the healthcare system are increased needlessly when medications that do not improve outcomes are prescribed. Although the individual pill cost is low, the widespread use and the associated pharmacy and nursing resources required for administration create an estimated cost for docusate over $100,000,000 per year for North America alone.3 The staff time required for administration may prevent healthcare personnel from engaging in other more valuable tasks. Additionally, every medication order creates an opportunity for medical error. Lastly, bacteria were recently found contaminating the liquid formulation, which carries its own obvious implications if patients develop iatrogenic infections.24
WHAT YOU SHOULD DO INSTEAD
Instead of using docusate, prescribe agents with established efficacy. In 2006, a systematic review published in the American Journal of Gastroenterology graded the evidence behind different therapies for chronic constipation.21 They found good evidence (Grade A) to support the use of polyethylene glycol (PEG), while psyllium and lactulose had moderate evidence (Grade B) to support their use. All other currently available agents that were reviewed had poor evidence to support their use. A more recent study in people prescribed opioids similarly found evidence to support the use of polyethylene glycol, lactulose, and sennosides.25 Lastly, the 2016 guidelines from the American Society of Colon and Rectal Surgeons do not mention docusate, though they comment on the paucity of data on stool softeners. Their recommendations for laxative therapy are similar to those of the previously discussed reviews.26 Ultimately, the choice of therapy, pharmacological and nonpharmacological, should be individualized for each patient based on the clinical context and cause of constipation. Nonpharmacologic treatments include dietary modification, mobilization, chewing gum, and biofeedback. If pharmacotherapy is required, use laxatives with the strongest evidence.
RECOMMENDATIONS
- In patients with constipation or at risk for constipation, use laxatives with proven efficacy (such as polyethylene glycol, lactulose, psyllium, or sennosides) for treatment or prophylaxis of constipation instead of using docusate.
- Discuss de-prescription for patients using docusate prior to admission.
- Remove docusate from your hospital formulary.
CONCLUSION
Docusate is commonly used for the treatment and prevention of constipation in hospitalized patients, with significant associated costs. This common practice continues despite little evidence supporting its efficacy and many trials failing to show benefits over placebo. Decreased utilization of ineffective therapies such as docusate is recommended. Returning to the case presentation, the hospitalist should start the patient on alternative therapies, instead of docusate, such as polyethylene glycol, lactulose, psyllium, or sennosides, which have better evidence supporting their use.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
All authors deny any relevant conflict of interest with the attached manuscript.
1. Noiesen E, Trosborg I, Bager L, Herning M, Lyngby C, Konradsen H. Constipation--prevalence and incidence among medical patients acutely admitted to hospital with a medical condition. J Clin Nurs. 2014;23(15-16):2295-2302. doi: 10.1111/jocn.12511.
2. De Giorgio R, Ruggeri E, Stanghellini V, Eusebi LH, Bazzoli F, Chiarioni G. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi: 10.1186/s12876-015-0366-3.
3. Lee TC, McDonald EG, Bonnici A, Tamblyn R. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med. 2016;176(8):1216-1217. doi: 10.1001/jamainternmed.2016.2775.
4. MacMillan TE, Kamali R, Cavalcanti RB. Missed opportunity to deprescribe: docusate for constipation in medical inpatients. Am J Med. 2016;129(9):1001 e1001-1007. doi: 10.1016/j.amjmed.2016.04.008.
5. Spiesman MG, Malow L. New fecal softener (doxinate) in the treatment of constipation. J Lancet. 1956;76(6):164-167.
6. Harris R. Constipation in geriatrics; management with dioctyl sodium sulfosuccinate. Am J Dig Dis. Sep 1957;2(9):487-492.
7. Smigel JO, Lowe KJ, Hosp PH, Gibson JH. Constipation in elderly patients; treatment with dioctyl sodium sulfosuccinate and dioctyl sodium sulfosuccinate plus peristim. Med Times. 1958;86(12):1521-1526.
8. Wilson JL, Dickinson DG. Use of dioctyl sodium sulfosuccinate (aerosol O.T.) for severe constipation. J Am Med Assoc. 1955;158(4):261-263. doi: 10.1001/jama.1955.02960040019006a.
9. Hyland CM, Foran JD. Dioctyl sodium sulphosuccinate as a laxative in the elderly. Practitioner. 1968;200(199):698-699.
10. Jin J. JAMA patient page. Over-the-counter laxatives. JAMA. 2014;312(11):1167. doi: 10.1001/jama.2014.2078.
11. 19th WHO Model List of Essential Medicines (April 2015). 2015; http://www.who.int/medicines/publications/essentialmedicines/en/.
12. Goodman J, Pang J, Bessman AN. Dioctyl sodium sulfosuccinate- an ineffective prophylactic laxative. J Chronic Dis. 1976;29(1):59-63. doi: 10.1016/0021-9681(76)90068-0.
13. Fain AM, Susat R, Herring M, Dorton K. Treatment of constipation in geriatric and chronically ill patients: a comparison. South Med J. 1978;71(6):677-680.
14. Chapman RW, Sillery J, Fontana DD, Matthys C, Saunders DR. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89(3):489-493. doi: 10.1016/0016-5085(85)90441-X.
15. Castle SC, Cantrell M, Israel DS, Samuelson MJ. Constipation prevention: empiric use of stool softeners questioned. Geriatrics. 1991;46(11):84-86.
16. McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12(5):491-497. doi: 10.1046/j.1365-2036.1998.00336.x.
17. Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45(1):2-13. doi: 10.1016/j.jpainsymman.2012.02.008.
18. Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015(5):CD003448.
19. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage. 2000;19(2):130-136. doi: 10.1016/S0885-3924(99)00157-8.
20. Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol. 2014;28(10):549-557.
21. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. doi: 10.1111/j.1572-0241.2005.40925.x
22. Health CAfDaTi. Dioctyl sulfosuccinate or docusate (calcium or sodium) for the prevention or management of constipation: a review of the clinical effectiveness. Ottawa (ON)2014.
23. McKee KY, Widera E. Habitual prescribing of laxatives-it’s time to flush outdated protocols down the drain. JAMA Intern Med. 2016;176(8):1217-1219. doi: 10.1001/jamainternmed.2016.2780.
24. Marquez L, Jones KN, Whaley EM, et al. An outbreak of burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol. 2017;38(5):567-573. doi: 10.1017/ice.2017.11.
25. Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010.
26. Paquette IM, Varma M, Ternent C, et al. The American society of colon and rectal surgeons’ clinical practice guideline for the evaluation and management of constipation. Dis Colon Rectum. 2016;59(6):479-492. doi: 10.1097/DCR.0000000000000599
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Click here for the Choosing Wisely website.

CASE PRESENTATION
An 80-year-old woman with no significant past medical history presents with a mechanical fall. X-rays are notable for a right hip fracture. She is treated with morphine for analgesia and evaluated by orthopedic surgery for surgical repair. The hospitalist recognizes that this patient is at high risk for constipation and orders docusate for prevention of constipation.
BACKGROUND
Constipation is a highly prevalent problem in all practice settings, especially in the hospital, affecting two out of five hospitalized patients.1 Multiple factors in the inpatient setting contribute to constipation, including decreased mobility, medical comorbidities, postsurgical ileus, anesthetics, and medications such as opioid analgesics. Furthermore, the inpatient population is aging in parallel with the general population and constipation is more common in the elderly, likely owing to a combination of decreased muscle mass and impaired function of autonomic nerves.2 Consequently, inpatient providers frequently treat constipation or try to prevent it using stool softeners or laxatives.
One of the most commonly prescribed agents, regardless of medical specialty, is docusate, also known as dioctyl sulfosuccinate or by its brand name, Colace. A study from McGill University Health Centre in Montreal, Canada reported that docusate was the most frequently prescribed laxative, accounting for 64% of laxative medication doses, with associated costs approaching $60,000 per year.3 Direct drug costs accounted for a quarter of the expenses, and the remaining three quarters were estimated labor costs for administration. Medical and surgical admissions shared similar proportions of usage, with an average of 10 doses of docusate per admission across 17,064 admissions. Furthermore, half of the patients were prescribed docusate upon discharge. The authors extrapolated their data to suggest that total healthcare spending in North America on docusate products likely exceeds $100,000,000 yearly. A second study from Toronto found that 15% of all hospitalized patients are prescribed at least one dose of docusate, and that one-third of all new inpatient prescriptions are continued at discharge.4
WHY YOU THINK DOCUSATE MIGHT BE HELPFUL FOR CONSTIPATION
Docusate is thought to act as a detergent to retain water in the stool, thereby acting as a stool softener to facilitate stool passage. Physicians have prescribed docusate for decades, and attendings have passed down the practice of prescribing docusate for constipation to medical trainees for generations. The initial docusate studies showed promise, as it softened the stool by increasing its water content and made it easier to pass through the intestines.5 One of the earliest human studies compared docusate to an unspecified placebo in 35 elderly patients with chronic atonic constipation and found a decreased need for enemas.6 Some other observational studies also reported a decreased need for manual disimpactions and enemas in elderly populations.7,8 One randomized, controlled trial from 1968 showed an increased frequency of bowel movements compared to placebo, but it excluded half of the enrolled patients because they had a positive placebo response.9 Since those early studies from the 1950s and 1960s, docusate remains widely accepted as an effective stool softener with positive endorsements from hospital formularies and order sets and patient information sheets such as the JAMA Patient Page.10 Furthermore, the World Health Organization lists docusate as an “essential medicine,” reinforcing the notion that it is effective.11
WHY THERE IS NO REASON TO PRESCRIBE DOCUSATE FOR CONSTIPATION
Despite common practice, the efficacy of docusate as a stool softener has not been borne out by rigorous scientific data. On the contrary, multiple randomized controlled trials have failed to show any significant efficacy of this drug over placebo (Table).
The initial trial in 1976 studied 34 elderly patients on a general medical ward for prophylaxis of constipation.12 They randomized patients to 100 mg twice daily of docusate sodium versus a control group that did not receive any type of laxative. The number of bowel movements and their character served as the measured outcomes. The study demonstrated no statistically significant differences in the frequency and character of bowel movements between the docusate and placebo groups. Even at that time, the authors questioned whether docusate had any efficacy at all: “[w]hether the drug actually offers anything beyond a placebo effect in preventing constipation is in doubt.”
Another trial in 1978 studied 46 elderly, institutionalized patients with chronic functional constipation.13 All patients underwent a two-week placebo period followed by a three-week treatment period with three arms of randomization: docusate sodium 100 mg daily, docusate sodium 100 mg twice daily, or docusate calcium 240 mg daily. Patients received enemas or suppositories if required. All three arms showed an increase in the average number of natural bowel movements when compared to each patient’s own placebo period, but only the arm with docusate calcium reached statistical significance (P < .02). According to the authors, none of the therapies appeared to have a significant effect on stool consistency. The authors hypothesized that the higher dose given to the docusate calcium arm may have been the reason for the apparent efficacy in this cohort. As such, studies with higher doses of docusate calcium would be reasonable.
A third study in 1985 compared docusate sodium 100 mg three times daily versus placebo in six healthy patients with ileostomies and six healthy volunteers.14 Therapy with docusate “had no effect on stool weight, stool frequency, stool water, or mean transit time.”
Another study in 1991 evaluated 15 elderly nursing home residents with a randomized, double-blind crossover design.15 Subjects received 240 mg twice daily of docusate calcium versus placebo for three weeks and then crossed over to other arm after a two-week wash-out period. The investigators found no difference in the number of bowel movements per week or in the need for additional laxatives between the two study periods. There were also no differences in the patients’ subjective experience of constipation or discomfort with defecation.
Larger studies were subsequently initiated in more recent years. In 1998, a randomized controlled trial in 170 subjects with chronic idiopathic constipation compared psyllium 5.1 g twice daily and docusate sodium 100 mg twice daily with a corresponding placebo in each arm for a treatment duration of two weeks after a two-week placebo baseline period.16 Psyllium was found to increase stool water content and stool water weight over the baseline period, while docusate essentially had no effect on stool water content or water weight. Furthermore, by treatment week 2, psyllium demonstrated an increase in the frequency of bowel movements, whereas docusate did not. It should be noted that this study was funded by Procter & Gamble, which manufactures Metamucil, a popular brand of psyllium.
Lastly, the most recent randomized controlled trial was published in 2013. It included 74 hospice patients in Canada, comparing docusate 200 mg and sennosides twice daily versus placebo and sennosides for 10 days. The study found no difference in stool frequency, volume, or consistency between docusate and placebo.17
A number of systematic reviews have studied the literature on bowel regimens and have noted the paucity of high-quality data supporting the efficacy of docusate, despite its widespread use.18-22 With these weak data, multiple authors have advocated for removing docusate from hospital formularies and using hospitalizations as an opportunity to deprescribe this medication to reduce polypharmacy. 3,4,23
Although docusate is considered a benign therapy, there is certainly potential for harm to the patient and detrimental effects on the healthcare system. Patients commonly complain about the unpleasant taste and lingering aftertaste, which may lead to decreased oral intake and worsening nutritional status.23 Furthermore, docusate may impact the absorption and effectiveness of other proven treatments.23 Perhaps the most important harm is that providers needlessly wait for docusate to fail before prescribing effective therapies for constipation. This process negatively impacts patient satisfaction and potentially increases healthcare costs if hospital length of stay is increased. Another important consideration is that patients may refuse truly necessary medications due to the excessive pill burden.
Costs to the healthcare system are increased needlessly when medications that do not improve outcomes are prescribed. Although the individual pill cost is low, the widespread use and the associated pharmacy and nursing resources required for administration create an estimated cost for docusate over $100,000,000 per year for North America alone.3 The staff time required for administration may prevent healthcare personnel from engaging in other more valuable tasks. Additionally, every medication order creates an opportunity for medical error. Lastly, bacteria were recently found contaminating the liquid formulation, which carries its own obvious implications if patients develop iatrogenic infections.24
WHAT YOU SHOULD DO INSTEAD
Instead of using docusate, prescribe agents with established efficacy. In 2006, a systematic review published in the American Journal of Gastroenterology graded the evidence behind different therapies for chronic constipation.21 They found good evidence (Grade A) to support the use of polyethylene glycol (PEG), while psyllium and lactulose had moderate evidence (Grade B) to support their use. All other currently available agents that were reviewed had poor evidence to support their use. A more recent study in people prescribed opioids similarly found evidence to support the use of polyethylene glycol, lactulose, and sennosides.25 Lastly, the 2016 guidelines from the American Society of Colon and Rectal Surgeons do not mention docusate, though they comment on the paucity of data on stool softeners. Their recommendations for laxative therapy are similar to those of the previously discussed reviews.26 Ultimately, the choice of therapy, pharmacological and nonpharmacological, should be individualized for each patient based on the clinical context and cause of constipation. Nonpharmacologic treatments include dietary modification, mobilization, chewing gum, and biofeedback. If pharmacotherapy is required, use laxatives with the strongest evidence.
RECOMMENDATIONS
- In patients with constipation or at risk for constipation, use laxatives with proven efficacy (such as polyethylene glycol, lactulose, psyllium, or sennosides) for treatment or prophylaxis of constipation instead of using docusate.
- Discuss de-prescription for patients using docusate prior to admission.
- Remove docusate from your hospital formulary.
CONCLUSION
Docusate is commonly used for the treatment and prevention of constipation in hospitalized patients, with significant associated costs. This common practice continues despite little evidence supporting its efficacy and many trials failing to show benefits over placebo. Decreased utilization of ineffective therapies such as docusate is recommended. Returning to the case presentation, the hospitalist should start the patient on alternative therapies, instead of docusate, such as polyethylene glycol, lactulose, psyllium, or sennosides, which have better evidence supporting their use.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
All authors deny any relevant conflict of interest with the attached manuscript.
The “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but which may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion.
Click here for the Choosing Wisely website.

CASE PRESENTATION
An 80-year-old woman with no significant past medical history presents with a mechanical fall. X-rays are notable for a right hip fracture. She is treated with morphine for analgesia and evaluated by orthopedic surgery for surgical repair. The hospitalist recognizes that this patient is at high risk for constipation and orders docusate for prevention of constipation.
BACKGROUND
Constipation is a highly prevalent problem in all practice settings, especially in the hospital, affecting two out of five hospitalized patients.1 Multiple factors in the inpatient setting contribute to constipation, including decreased mobility, medical comorbidities, postsurgical ileus, anesthetics, and medications such as opioid analgesics. Furthermore, the inpatient population is aging in parallel with the general population and constipation is more common in the elderly, likely owing to a combination of decreased muscle mass and impaired function of autonomic nerves.2 Consequently, inpatient providers frequently treat constipation or try to prevent it using stool softeners or laxatives.
One of the most commonly prescribed agents, regardless of medical specialty, is docusate, also known as dioctyl sulfosuccinate or by its brand name, Colace. A study from McGill University Health Centre in Montreal, Canada reported that docusate was the most frequently prescribed laxative, accounting for 64% of laxative medication doses, with associated costs approaching $60,000 per year.3 Direct drug costs accounted for a quarter of the expenses, and the remaining three quarters were estimated labor costs for administration. Medical and surgical admissions shared similar proportions of usage, with an average of 10 doses of docusate per admission across 17,064 admissions. Furthermore, half of the patients were prescribed docusate upon discharge. The authors extrapolated their data to suggest that total healthcare spending in North America on docusate products likely exceeds $100,000,000 yearly. A second study from Toronto found that 15% of all hospitalized patients are prescribed at least one dose of docusate, and that one-third of all new inpatient prescriptions are continued at discharge.4
WHY YOU THINK DOCUSATE MIGHT BE HELPFUL FOR CONSTIPATION
Docusate is thought to act as a detergent to retain water in the stool, thereby acting as a stool softener to facilitate stool passage. Physicians have prescribed docusate for decades, and attendings have passed down the practice of prescribing docusate for constipation to medical trainees for generations. The initial docusate studies showed promise, as it softened the stool by increasing its water content and made it easier to pass through the intestines.5 One of the earliest human studies compared docusate to an unspecified placebo in 35 elderly patients with chronic atonic constipation and found a decreased need for enemas.6 Some other observational studies also reported a decreased need for manual disimpactions and enemas in elderly populations.7,8 One randomized, controlled trial from 1968 showed an increased frequency of bowel movements compared to placebo, but it excluded half of the enrolled patients because they had a positive placebo response.9 Since those early studies from the 1950s and 1960s, docusate remains widely accepted as an effective stool softener with positive endorsements from hospital formularies and order sets and patient information sheets such as the JAMA Patient Page.10 Furthermore, the World Health Organization lists docusate as an “essential medicine,” reinforcing the notion that it is effective.11
WHY THERE IS NO REASON TO PRESCRIBE DOCUSATE FOR CONSTIPATION
Despite common practice, the efficacy of docusate as a stool softener has not been borne out by rigorous scientific data. On the contrary, multiple randomized controlled trials have failed to show any significant efficacy of this drug over placebo (Table).
The initial trial in 1976 studied 34 elderly patients on a general medical ward for prophylaxis of constipation.12 They randomized patients to 100 mg twice daily of docusate sodium versus a control group that did not receive any type of laxative. The number of bowel movements and their character served as the measured outcomes. The study demonstrated no statistically significant differences in the frequency and character of bowel movements between the docusate and placebo groups. Even at that time, the authors questioned whether docusate had any efficacy at all: “[w]hether the drug actually offers anything beyond a placebo effect in preventing constipation is in doubt.”
Another trial in 1978 studied 46 elderly, institutionalized patients with chronic functional constipation.13 All patients underwent a two-week placebo period followed by a three-week treatment period with three arms of randomization: docusate sodium 100 mg daily, docusate sodium 100 mg twice daily, or docusate calcium 240 mg daily. Patients received enemas or suppositories if required. All three arms showed an increase in the average number of natural bowel movements when compared to each patient’s own placebo period, but only the arm with docusate calcium reached statistical significance (P < .02). According to the authors, none of the therapies appeared to have a significant effect on stool consistency. The authors hypothesized that the higher dose given to the docusate calcium arm may have been the reason for the apparent efficacy in this cohort. As such, studies with higher doses of docusate calcium would be reasonable.
A third study in 1985 compared docusate sodium 100 mg three times daily versus placebo in six healthy patients with ileostomies and six healthy volunteers.14 Therapy with docusate “had no effect on stool weight, stool frequency, stool water, or mean transit time.”
Another study in 1991 evaluated 15 elderly nursing home residents with a randomized, double-blind crossover design.15 Subjects received 240 mg twice daily of docusate calcium versus placebo for three weeks and then crossed over to other arm after a two-week wash-out period. The investigators found no difference in the number of bowel movements per week or in the need for additional laxatives between the two study periods. There were also no differences in the patients’ subjective experience of constipation or discomfort with defecation.
Larger studies were subsequently initiated in more recent years. In 1998, a randomized controlled trial in 170 subjects with chronic idiopathic constipation compared psyllium 5.1 g twice daily and docusate sodium 100 mg twice daily with a corresponding placebo in each arm for a treatment duration of two weeks after a two-week placebo baseline period.16 Psyllium was found to increase stool water content and stool water weight over the baseline period, while docusate essentially had no effect on stool water content or water weight. Furthermore, by treatment week 2, psyllium demonstrated an increase in the frequency of bowel movements, whereas docusate did not. It should be noted that this study was funded by Procter & Gamble, which manufactures Metamucil, a popular brand of psyllium.
Lastly, the most recent randomized controlled trial was published in 2013. It included 74 hospice patients in Canada, comparing docusate 200 mg and sennosides twice daily versus placebo and sennosides for 10 days. The study found no difference in stool frequency, volume, or consistency between docusate and placebo.17
A number of systematic reviews have studied the literature on bowel regimens and have noted the paucity of high-quality data supporting the efficacy of docusate, despite its widespread use.18-22 With these weak data, multiple authors have advocated for removing docusate from hospital formularies and using hospitalizations as an opportunity to deprescribe this medication to reduce polypharmacy. 3,4,23
Although docusate is considered a benign therapy, there is certainly potential for harm to the patient and detrimental effects on the healthcare system. Patients commonly complain about the unpleasant taste and lingering aftertaste, which may lead to decreased oral intake and worsening nutritional status.23 Furthermore, docusate may impact the absorption and effectiveness of other proven treatments.23 Perhaps the most important harm is that providers needlessly wait for docusate to fail before prescribing effective therapies for constipation. This process negatively impacts patient satisfaction and potentially increases healthcare costs if hospital length of stay is increased. Another important consideration is that patients may refuse truly necessary medications due to the excessive pill burden.
Costs to the healthcare system are increased needlessly when medications that do not improve outcomes are prescribed. Although the individual pill cost is low, the widespread use and the associated pharmacy and nursing resources required for administration create an estimated cost for docusate over $100,000,000 per year for North America alone.3 The staff time required for administration may prevent healthcare personnel from engaging in other more valuable tasks. Additionally, every medication order creates an opportunity for medical error. Lastly, bacteria were recently found contaminating the liquid formulation, which carries its own obvious implications if patients develop iatrogenic infections.24
WHAT YOU SHOULD DO INSTEAD
Instead of using docusate, prescribe agents with established efficacy. In 2006, a systematic review published in the American Journal of Gastroenterology graded the evidence behind different therapies for chronic constipation.21 They found good evidence (Grade A) to support the use of polyethylene glycol (PEG), while psyllium and lactulose had moderate evidence (Grade B) to support their use. All other currently available agents that were reviewed had poor evidence to support their use. A more recent study in people prescribed opioids similarly found evidence to support the use of polyethylene glycol, lactulose, and sennosides.25 Lastly, the 2016 guidelines from the American Society of Colon and Rectal Surgeons do not mention docusate, though they comment on the paucity of data on stool softeners. Their recommendations for laxative therapy are similar to those of the previously discussed reviews.26 Ultimately, the choice of therapy, pharmacological and nonpharmacological, should be individualized for each patient based on the clinical context and cause of constipation. Nonpharmacologic treatments include dietary modification, mobilization, chewing gum, and biofeedback. If pharmacotherapy is required, use laxatives with the strongest evidence.
RECOMMENDATIONS
- In patients with constipation or at risk for constipation, use laxatives with proven efficacy (such as polyethylene glycol, lactulose, psyllium, or sennosides) for treatment or prophylaxis of constipation instead of using docusate.
- Discuss de-prescription for patients using docusate prior to admission.
- Remove docusate from your hospital formulary.
CONCLUSION
Docusate is commonly used for the treatment and prevention of constipation in hospitalized patients, with significant associated costs. This common practice continues despite little evidence supporting its efficacy and many trials failing to show benefits over placebo. Decreased utilization of ineffective therapies such as docusate is recommended. Returning to the case presentation, the hospitalist should start the patient on alternative therapies, instead of docusate, such as polyethylene glycol, lactulose, psyllium, or sennosides, which have better evidence supporting their use.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing [email protected].
Disclosures
All authors deny any relevant conflict of interest with the attached manuscript.
1. Noiesen E, Trosborg I, Bager L, Herning M, Lyngby C, Konradsen H. Constipation--prevalence and incidence among medical patients acutely admitted to hospital with a medical condition. J Clin Nurs. 2014;23(15-16):2295-2302. doi: 10.1111/jocn.12511.
2. De Giorgio R, Ruggeri E, Stanghellini V, Eusebi LH, Bazzoli F, Chiarioni G. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi: 10.1186/s12876-015-0366-3.
3. Lee TC, McDonald EG, Bonnici A, Tamblyn R. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med. 2016;176(8):1216-1217. doi: 10.1001/jamainternmed.2016.2775.
4. MacMillan TE, Kamali R, Cavalcanti RB. Missed opportunity to deprescribe: docusate for constipation in medical inpatients. Am J Med. 2016;129(9):1001 e1001-1007. doi: 10.1016/j.amjmed.2016.04.008.
5. Spiesman MG, Malow L. New fecal softener (doxinate) in the treatment of constipation. J Lancet. 1956;76(6):164-167.
6. Harris R. Constipation in geriatrics; management with dioctyl sodium sulfosuccinate. Am J Dig Dis. Sep 1957;2(9):487-492.
7. Smigel JO, Lowe KJ, Hosp PH, Gibson JH. Constipation in elderly patients; treatment with dioctyl sodium sulfosuccinate and dioctyl sodium sulfosuccinate plus peristim. Med Times. 1958;86(12):1521-1526.
8. Wilson JL, Dickinson DG. Use of dioctyl sodium sulfosuccinate (aerosol O.T.) for severe constipation. J Am Med Assoc. 1955;158(4):261-263. doi: 10.1001/jama.1955.02960040019006a.
9. Hyland CM, Foran JD. Dioctyl sodium sulphosuccinate as a laxative in the elderly. Practitioner. 1968;200(199):698-699.
10. Jin J. JAMA patient page. Over-the-counter laxatives. JAMA. 2014;312(11):1167. doi: 10.1001/jama.2014.2078.
11. 19th WHO Model List of Essential Medicines (April 2015). 2015; http://www.who.int/medicines/publications/essentialmedicines/en/.
12. Goodman J, Pang J, Bessman AN. Dioctyl sodium sulfosuccinate- an ineffective prophylactic laxative. J Chronic Dis. 1976;29(1):59-63. doi: 10.1016/0021-9681(76)90068-0.
13. Fain AM, Susat R, Herring M, Dorton K. Treatment of constipation in geriatric and chronically ill patients: a comparison. South Med J. 1978;71(6):677-680.
14. Chapman RW, Sillery J, Fontana DD, Matthys C, Saunders DR. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89(3):489-493. doi: 10.1016/0016-5085(85)90441-X.
15. Castle SC, Cantrell M, Israel DS, Samuelson MJ. Constipation prevention: empiric use of stool softeners questioned. Geriatrics. 1991;46(11):84-86.
16. McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12(5):491-497. doi: 10.1046/j.1365-2036.1998.00336.x.
17. Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45(1):2-13. doi: 10.1016/j.jpainsymman.2012.02.008.
18. Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015(5):CD003448.
19. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage. 2000;19(2):130-136. doi: 10.1016/S0885-3924(99)00157-8.
20. Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol. 2014;28(10):549-557.
21. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. doi: 10.1111/j.1572-0241.2005.40925.x
22. Health CAfDaTi. Dioctyl sulfosuccinate or docusate (calcium or sodium) for the prevention or management of constipation: a review of the clinical effectiveness. Ottawa (ON)2014.
23. McKee KY, Widera E. Habitual prescribing of laxatives-it’s time to flush outdated protocols down the drain. JAMA Intern Med. 2016;176(8):1217-1219. doi: 10.1001/jamainternmed.2016.2780.
24. Marquez L, Jones KN, Whaley EM, et al. An outbreak of burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol. 2017;38(5):567-573. doi: 10.1017/ice.2017.11.
25. Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010.
26. Paquette IM, Varma M, Ternent C, et al. The American society of colon and rectal surgeons’ clinical practice guideline for the evaluation and management of constipation. Dis Colon Rectum. 2016;59(6):479-492. doi: 10.1097/DCR.0000000000000599
1. Noiesen E, Trosborg I, Bager L, Herning M, Lyngby C, Konradsen H. Constipation--prevalence and incidence among medical patients acutely admitted to hospital with a medical condition. J Clin Nurs. 2014;23(15-16):2295-2302. doi: 10.1111/jocn.12511.
2. De Giorgio R, Ruggeri E, Stanghellini V, Eusebi LH, Bazzoli F, Chiarioni G. Chronic constipation in the elderly: a primer for the gastroenterologist. BMC Gastroenterol. 2015;15:130. doi: 10.1186/s12876-015-0366-3.
3. Lee TC, McDonald EG, Bonnici A, Tamblyn R. Pattern of inpatient laxative use: waste not, want not. JAMA Intern Med. 2016;176(8):1216-1217. doi: 10.1001/jamainternmed.2016.2775.
4. MacMillan TE, Kamali R, Cavalcanti RB. Missed opportunity to deprescribe: docusate for constipation in medical inpatients. Am J Med. 2016;129(9):1001 e1001-1007. doi: 10.1016/j.amjmed.2016.04.008.
5. Spiesman MG, Malow L. New fecal softener (doxinate) in the treatment of constipation. J Lancet. 1956;76(6):164-167.
6. Harris R. Constipation in geriatrics; management with dioctyl sodium sulfosuccinate. Am J Dig Dis. Sep 1957;2(9):487-492.
7. Smigel JO, Lowe KJ, Hosp PH, Gibson JH. Constipation in elderly patients; treatment with dioctyl sodium sulfosuccinate and dioctyl sodium sulfosuccinate plus peristim. Med Times. 1958;86(12):1521-1526.
8. Wilson JL, Dickinson DG. Use of dioctyl sodium sulfosuccinate (aerosol O.T.) for severe constipation. J Am Med Assoc. 1955;158(4):261-263. doi: 10.1001/jama.1955.02960040019006a.
9. Hyland CM, Foran JD. Dioctyl sodium sulphosuccinate as a laxative in the elderly. Practitioner. 1968;200(199):698-699.
10. Jin J. JAMA patient page. Over-the-counter laxatives. JAMA. 2014;312(11):1167. doi: 10.1001/jama.2014.2078.
11. 19th WHO Model List of Essential Medicines (April 2015). 2015; http://www.who.int/medicines/publications/essentialmedicines/en/.
12. Goodman J, Pang J, Bessman AN. Dioctyl sodium sulfosuccinate- an ineffective prophylactic laxative. J Chronic Dis. 1976;29(1):59-63. doi: 10.1016/0021-9681(76)90068-0.
13. Fain AM, Susat R, Herring M, Dorton K. Treatment of constipation in geriatric and chronically ill patients: a comparison. South Med J. 1978;71(6):677-680.
14. Chapman RW, Sillery J, Fontana DD, Matthys C, Saunders DR. Effect of oral dioctyl sodium sulfosuccinate on intake-output studies of human small and large intestine. Gastroenterology. 1985;89(3):489-493. doi: 10.1016/0016-5085(85)90441-X.
15. Castle SC, Cantrell M, Israel DS, Samuelson MJ. Constipation prevention: empiric use of stool softeners questioned. Geriatrics. 1991;46(11):84-86.
16. McRorie JW, Daggy BP, Morel JG, Diersing PS, Miner PB, Robinson M. Psyllium is superior to docusate sodium for treatment of chronic constipation. Aliment Pharmacol Ther. 1998;12(5):491-497. doi: 10.1046/j.1365-2036.1998.00336.x.
17. Tarumi Y, Wilson MP, Szafran O, Spooner GR. Randomized, double-blind, placebo-controlled trial of oral docusate in the management of constipation in hospice patients. J Pain Symptom Manage. 2013;45(1):2-13. doi: 10.1016/j.jpainsymman.2012.02.008.
18. Candy B, Jones L, Larkin PJ, Vickerstaff V, Tookman A, Stone P. Laxatives for the management of constipation in people receiving palliative care. Cochrane Database Syst Rev. 2015(5):CD003448.
19. Hurdon V, Viola R, Schroder C. How useful is docusate in patients at risk for constipation? A systematic review of the evidence in the chronically ill. J Pain Symptom Manage. 2000;19(2):130-136. doi: 10.1016/S0885-3924(99)00157-8.
20. Pare P, Fedorak RN. Systematic review of stimulant and nonstimulant laxatives for the treatment of functional constipation. Can J Gastroenterol Hepatol. 2014;28(10):549-557.
21. Ramkumar D, Rao SS. Efficacy and safety of traditional medical therapies for chronic constipation: systematic review. Am J Gastroenterol. 2005;100(4):936-971. doi: 10.1111/j.1572-0241.2005.40925.x
22. Health CAfDaTi. Dioctyl sulfosuccinate or docusate (calcium or sodium) for the prevention or management of constipation: a review of the clinical effectiveness. Ottawa (ON)2014.
23. McKee KY, Widera E. Habitual prescribing of laxatives-it’s time to flush outdated protocols down the drain. JAMA Intern Med. 2016;176(8):1217-1219. doi: 10.1001/jamainternmed.2016.2780.
24. Marquez L, Jones KN, Whaley EM, et al. An outbreak of burkholderia cepacia complex infections associated with contaminated liquid docusate. Infect Control Hosp Epidemiol. 2017;38(5):567-573. doi: 10.1017/ice.2017.11.
25. Ahmedzai SH, Boland J. Constipation in people prescribed opioids. BMJ Clin Evid. 2010;2010.
26. Paquette IM, Varma M, Ternent C, et al. The American society of colon and rectal surgeons’ clinical practice guideline for the evaluation and management of constipation. Dis Colon Rectum. 2016;59(6):479-492. doi: 10.1097/DCR.0000000000000599
© 2019 Society of Hospital Medicine
Things We Do For No Reason: Sliding-Scale Insulin as Monotherapy for Glycemic Control in Hospitalized Patients
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A CLINICAL SCENARIO
A 60-year-old man with a past medical history of obesity and type 2 diabetes presented to the emergency department with one week of myalgias and fever up to 103.5°F (39.7°C). Other vital signs were normal. He had no localizing symptoms, and physical examination was unrevealing, except for a small scab from a tick bite sustained two weeks prior to symptom onset. Before admission, he had been managing his diabetes with metformin 1,000 mg twice a day, and on arrival, his blood sugar level was 275 mg/dL. The admitting provider decided to hold the patient’s metformin and replace it with insulin per a sliding scale. Is monotherapy with sliding-scale insulin the best inpatient management option for this patient’s type 2 diabetes?
WHY YOU MIGHT THINK SLIDING-SCALE INSULIN AS MONOTHERAPY IS HELPFUL
The basic premise of sliding-scale insulin (SSI) is to correct hyperglycemia through the frequent administration of short-acting insulin dosed according to a patient’s blood glucose level with the help of a prespecified rubric. When blood glucose levels are low, patients receive little or no insulin, and when blood glucose levels are high, higher doses are given. This approach to inpatient blood glucose management was first popularized by Joslin in 1934,1 and it remains a common strategy today. For example, a 2007 survey of 44 hospitals in the United States showed that approximately 43% of all noncritically ill patients with hyperglycemia were treated with SSI alone.2 More recently, a single-center study showed that 30% of clinicians continued to use SSI as monotherapy even after the implementation of order sets designed to limit this practice.3
The rationale for SSI as monotherapy appears to have two components. First, guidelines suggest that certain patients should be screened periodically in the hospital for hyperglycemia (blood glucose persistently greater than 180 mg/dL) and that, if identified, hyperglycemia should be treated.4 By pairing finger-stick glucose monitoring with SSI, the diagnosis and treatment—although not the prevention—of hyperglycemia can be accomplished simultaneously. Second, inpatient providers do not want to cause harm in the form of hypoglycemia. SSI as monotherapy is sometimes viewed as a cautious approach in this regard as insulin is administered only if the blood sugar level is high.
Convenience is probably another key contributor to the enduring use of SSI as monotherapy. Several hospitals have ready-made order sets for SSI that are easier to prescribe than a patient-specific regimen including both short- and long-acting insulin. In at least one single-center survey, physicians and staff were found to favor convenience over perceived efficacy when asked about their attitudes toward inpatient glycemic control.5 Although efforts at individual hospitals to change practice patterns among residents have shown promise,6 reform on a broader scale remains elusive.
WHY SSI AS MONOTHERAPY IS NOT HELPFUL
SSI administration does not attempt to replicate normal pancreatic physiology, which involves basal insulin secretion to impair hepatic gluconeogenesis and meal-associated insulin spikes to promote uptake into glucose-avid tissues. SSI is a reactive strategy, not a proactive one, and perhaps unsurprisingly, to our knowledge, it has never been shown to prevent hyperglycemia in hospitalized patients, an impression corroborated by a systematic review of the topic between 1964 and 2003.7 More recently, one multicenter trial analyzed the effect of adding SSI to oral antihyperglycemic medications in hospitalized diabetics and found no differences in rates of hyperglycemia.8 Another study found that 84% of administered SSI doses failed to correct hyperglycemia.9
However, does adding basal insulin to SSI raise a patient’s risk of hypoglycemia? When basal insulin is dosed carefully, the answer appears to be no. In a trial in which diabetic long-term care residents who were receiving SSI at baseline were randomized to either continued SSI or basal-bolus insulin, the investigators found that the basal-bolus group experienced significantly lower average blood glucose levels without an increase in adverse glycemic events.10 Perhaps the most significant milestone to date, however, was the RABBIT 2 multicenter trial, published in 2007, that randomized hospitalized, insulin-naïve diabetics to either a weight-based regimen of basal and prandial insulin or SSI only.11 Rates of hypoglycemia and length of stay did not differ between the groups, and 66% of patients receiving basal-prandial insulin achieved their glycemic control target as opposed to just 38% of patients in the SSI-only group. The SSI group also required more total insulin. A weight-based, basal-bolus strategy was later proven to be similarly effective, without causing severe hypoglycemia, for patients undergoing surgery who could not maintain consistent oral alimentation.12 Basal-bolus insulin was associated with fewer surgical complications, and it produced a cost savings of $751 per day as determined by a post hoc comparative effectiveness study.13
Prolonged use of SSI as monotherapy may be not only ineffective but also harmful. Clearly, the absence of basal insulin will harm type 1 diabetics, who need basal insulin to prevent diabetic ketoacidosis. However, even for type 2 diabetics and nondiabetics, hyperglycemia has been established as a marker for adverse outcomes among hospitalized patients,14 and SSI monotherapy has been associated with a three-fold higher risk of hyperglycemia compared with the use of a sliding scale plus other forms of insulin.15 At least one other study has also linked this practice with a significantly increased length of stay compared with patients who were receiving insulin proactively.16 We believe that the potential for harm is difficult to disregard, especially because safer alternatives are available. Ultimately, it can be stated that in hospitalized patients with persistent hyperglycemia who require insulin, SSI alone should not be the preferred treatment choice regardless of whether the patient carries a known diagnosis of diabetes mellitus or has used insulin previously.
WHEN YOU MIGHT CONSIDER USING SSI AS MONOTHERAPY
As discussed above, there is no known clinical scenario in which SSI as monotherapy has been proven to be effective; however, the use of SSI as monotherapy as a short-term approach has not been well studied. Hospitalized patients who are at risk for adverse glycemic events should be monitored with periodic finger-stick blood glucose draws per guidelines, and in the first 24 hours, it may be reasonable to withhold basal insulin for insulin-naive patients, particularly if the medication reconciliation or other key components of the history are in doubt, or if there are risk factors for hypoglycemia such as a history of bariatric surgery. The amount of insulin received in the first 24 hours of such monitoring may inform subsequent insulin dosing, but this method of “dose finding” has not been validated in the literature.
Uncertain or interrupted alimentation status or stress hyperglycemia may complicate the assessment of a patient’s insulin needs. One of the insights from the RABBIT 2 surgery trial is that even with interrupted alimentation, patients on a weight-based, long-acting insulin regimen did not experience severe hypoglycemia. Nevertheless, if a patient without type 1 diabetes is felt to be at high risk for a severe hypoglycemic event, it may be prudent to withhold long-acting insulin. However, in that situation, adding SSI to finger-stick monitoring is unlikely to be beneficial. Cases of stress hyperglycemia in nondiabetics can also be challenging, as the persistence of hyperglycemia can be difficult to predict. Guidelines state that if hyperglycemia is persistent, then insulin therapy should be initiated and that this therapy is best accomplished in the form of a basal-prandial regimen.17
WHAT YOU SHOULD DO INSTEAD
Current guidelines from the American Diabetes Association17 and the American Association of Clinical Endocrinologists18 for hospitalized patients with hyperglycemia who require insulin recommend against the prolonged use of SSI as monotherapy (category A recommendation) and support the use of basal plus correctional insulin with the addition of nutritional insulin for patients with consistent oral intake (category A recommendation). Although a complete discourse on the determination of the appropriate starting dose of insulin is outside of the scope of this cas presentation, the basic approach begins with calculating a weight-based total daily dose of insulin, approximately half of which can be given as basal insulin with the remainder given with meals along with correctional insulin as needed to account for premeal hyperglycemia.4 For example, the protocol used in the RABBIT 2 trial, which involved known type 2 diabetics, started insulin based on a total daily dose of 0.4 units/kg for patients presenting with blood sugar levels ≤200 mg/dL and 0.5 units/kg for those with higher initial glucose levels.7 Half of the total daily dose was given as basal insulin, and the other half was divided among meals. Caution with insulin dosing may be required in patients aged >70 years, in those with impaired renal function, and in situations in which steroid doses are fluctuating. The Society of Hospital Medicine has formulated an online subcutaneous insulin order implementation guideline, eQUIPS, that can be a helpful resource to centers that are interested in changing their practice patterns.19
RECOMMENDATIONS
- Instead of using SSI monotherapy for hospitalized patients who require insulin, add basal and prandial insulin, using a weight-based approach if necessary for insulin-naive patients.
- Engage with leadership at your center to learn how inpatient hyperglycemia protocols and blood sugar management teams can help provide evidence-based and individualized treatment plans for your patients.
- If no infrastructure exists at your center, the Society of Hospital Medicine offers training and guidance through its eQUIPS inpatient hyperglycemia management program.
CONCLUSION
In the case presentation, the hyperglycemic patient whose metformin was on hold should have been started on a combination of basal and prandial insulin as determined by his weight and current renal function as opposed to monotherapy with SSI. Using SSI as monotherapy for hyperglycemia is a common practice, and although well-intentioned, it is an ineffective and possibly dangerous approach. Continued efforts must be made to address the gap between guidelines and suboptimal practice patterns locally and nationally.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing[email protected].
Acknowledgments
The authors would like to thank Dr. Asem Ali of the Division of Endocrinology at UMass Memorial Medical Center for his review of the manuscript.
Disclosures
The authors have nothing to disclose.
1. Joslin EP. A Diabetic Manual for the Mutual Use of Doctor and Patient. Philadelphia, PA: Lea & Febiger; 1934:108.
2. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. doi: 10.2337/dc06-1715. PubMed
3. Valgardson JD, Merino M, Redgrave J, Hudson JI, Hudson MS. Effectiveness of inpatient insulin order sets using human insulins in noncritically ill patients in a rural hospital. Endocr Pract. 2015;21(7):794-806. doi: 10.4158/EP14153. PubMed
4. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. doi: 10.2337/diacare.27.2.553. PubMed
5. Beliard R, Muzykovsky K, Vincent W, 3rd, Shah B, Davanos E. Perceptions, barriers, and knowledge of inpatient glycemic control: a survey of health care workers. J Pharm Pract. 2016;29(4):348-354. doi: 10.1177/0897190014566309. PubMed
6. Baldwin D, Villanueva G, McNutt R, Bhatnagar S. Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008-1011. doi: 10.2337/diacare.28.12.2987-a. PubMed
7. Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61(15):1611-1614. PubMed
8. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. PubMed
9. Golightly LK, Jones MA, Hamamura DH, Stolpman NM, McDermott MT. Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding-scale insulin therapy. Pharmacotherapy. 2006;26(10):1421-1432. doi: 10.1592/phco.26.10.1421. PubMed
10. Dharmarajan TS, Mahajan D, Zambrano A, et al. Sliding scale insulin vs basal-bolus insulin therapy in long-term care: a 21-day randomized controlled trial comparing efficacy, safety and feasibility. J Am Med Dir Assoc. 2016;17(3):206-213. doi: 10.1016/j.jamda.2015.08.015. PubMed
11. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186. doi: 10.2337/dc07-0295. PubMed
12. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. doi: 10.2337/dc10-1407. PubMed
13. Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. Pharmacoecon Open. 2017;1(2):109-115. doi: 10.1007/s41669-017-0020-9.. PubMed
14. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. doi: 10.1210/jcem.87.3.8341. PubMed
15. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552. PubMed
16. Gearhart JG, Duncan JL, 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322. PubMed
17. American Diabetes A. 14. Diabetes care in the hospital: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S144-S151. doi: 10.2337/dc18-S014. PubMed
18. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369. doi: 10.2337/dc09-9029. PubMed
19. Maynard G, Wesorick DH, O’Malley C, Inzucchi SE, Society of Hospital Medicine Glycemic Control Task F. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29-41. doi: 10.1002/jhm.354. PubMed
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A CLINICAL SCENARIO
A 60-year-old man with a past medical history of obesity and type 2 diabetes presented to the emergency department with one week of myalgias and fever up to 103.5°F (39.7°C). Other vital signs were normal. He had no localizing symptoms, and physical examination was unrevealing, except for a small scab from a tick bite sustained two weeks prior to symptom onset. Before admission, he had been managing his diabetes with metformin 1,000 mg twice a day, and on arrival, his blood sugar level was 275 mg/dL. The admitting provider decided to hold the patient’s metformin and replace it with insulin per a sliding scale. Is monotherapy with sliding-scale insulin the best inpatient management option for this patient’s type 2 diabetes?
WHY YOU MIGHT THINK SLIDING-SCALE INSULIN AS MONOTHERAPY IS HELPFUL
The basic premise of sliding-scale insulin (SSI) is to correct hyperglycemia through the frequent administration of short-acting insulin dosed according to a patient’s blood glucose level with the help of a prespecified rubric. When blood glucose levels are low, patients receive little or no insulin, and when blood glucose levels are high, higher doses are given. This approach to inpatient blood glucose management was first popularized by Joslin in 1934,1 and it remains a common strategy today. For example, a 2007 survey of 44 hospitals in the United States showed that approximately 43% of all noncritically ill patients with hyperglycemia were treated with SSI alone.2 More recently, a single-center study showed that 30% of clinicians continued to use SSI as monotherapy even after the implementation of order sets designed to limit this practice.3
The rationale for SSI as monotherapy appears to have two components. First, guidelines suggest that certain patients should be screened periodically in the hospital for hyperglycemia (blood glucose persistently greater than 180 mg/dL) and that, if identified, hyperglycemia should be treated.4 By pairing finger-stick glucose monitoring with SSI, the diagnosis and treatment—although not the prevention—of hyperglycemia can be accomplished simultaneously. Second, inpatient providers do not want to cause harm in the form of hypoglycemia. SSI as monotherapy is sometimes viewed as a cautious approach in this regard as insulin is administered only if the blood sugar level is high.
Convenience is probably another key contributor to the enduring use of SSI as monotherapy. Several hospitals have ready-made order sets for SSI that are easier to prescribe than a patient-specific regimen including both short- and long-acting insulin. In at least one single-center survey, physicians and staff were found to favor convenience over perceived efficacy when asked about their attitudes toward inpatient glycemic control.5 Although efforts at individual hospitals to change practice patterns among residents have shown promise,6 reform on a broader scale remains elusive.
WHY SSI AS MONOTHERAPY IS NOT HELPFUL
SSI administration does not attempt to replicate normal pancreatic physiology, which involves basal insulin secretion to impair hepatic gluconeogenesis and meal-associated insulin spikes to promote uptake into glucose-avid tissues. SSI is a reactive strategy, not a proactive one, and perhaps unsurprisingly, to our knowledge, it has never been shown to prevent hyperglycemia in hospitalized patients, an impression corroborated by a systematic review of the topic between 1964 and 2003.7 More recently, one multicenter trial analyzed the effect of adding SSI to oral antihyperglycemic medications in hospitalized diabetics and found no differences in rates of hyperglycemia.8 Another study found that 84% of administered SSI doses failed to correct hyperglycemia.9
However, does adding basal insulin to SSI raise a patient’s risk of hypoglycemia? When basal insulin is dosed carefully, the answer appears to be no. In a trial in which diabetic long-term care residents who were receiving SSI at baseline were randomized to either continued SSI or basal-bolus insulin, the investigators found that the basal-bolus group experienced significantly lower average blood glucose levels without an increase in adverse glycemic events.10 Perhaps the most significant milestone to date, however, was the RABBIT 2 multicenter trial, published in 2007, that randomized hospitalized, insulin-naïve diabetics to either a weight-based regimen of basal and prandial insulin or SSI only.11 Rates of hypoglycemia and length of stay did not differ between the groups, and 66% of patients receiving basal-prandial insulin achieved their glycemic control target as opposed to just 38% of patients in the SSI-only group. The SSI group also required more total insulin. A weight-based, basal-bolus strategy was later proven to be similarly effective, without causing severe hypoglycemia, for patients undergoing surgery who could not maintain consistent oral alimentation.12 Basal-bolus insulin was associated with fewer surgical complications, and it produced a cost savings of $751 per day as determined by a post hoc comparative effectiveness study.13
Prolonged use of SSI as monotherapy may be not only ineffective but also harmful. Clearly, the absence of basal insulin will harm type 1 diabetics, who need basal insulin to prevent diabetic ketoacidosis. However, even for type 2 diabetics and nondiabetics, hyperglycemia has been established as a marker for adverse outcomes among hospitalized patients,14 and SSI monotherapy has been associated with a three-fold higher risk of hyperglycemia compared with the use of a sliding scale plus other forms of insulin.15 At least one other study has also linked this practice with a significantly increased length of stay compared with patients who were receiving insulin proactively.16 We believe that the potential for harm is difficult to disregard, especially because safer alternatives are available. Ultimately, it can be stated that in hospitalized patients with persistent hyperglycemia who require insulin, SSI alone should not be the preferred treatment choice regardless of whether the patient carries a known diagnosis of diabetes mellitus or has used insulin previously.
WHEN YOU MIGHT CONSIDER USING SSI AS MONOTHERAPY
As discussed above, there is no known clinical scenario in which SSI as monotherapy has been proven to be effective; however, the use of SSI as monotherapy as a short-term approach has not been well studied. Hospitalized patients who are at risk for adverse glycemic events should be monitored with periodic finger-stick blood glucose draws per guidelines, and in the first 24 hours, it may be reasonable to withhold basal insulin for insulin-naive patients, particularly if the medication reconciliation or other key components of the history are in doubt, or if there are risk factors for hypoglycemia such as a history of bariatric surgery. The amount of insulin received in the first 24 hours of such monitoring may inform subsequent insulin dosing, but this method of “dose finding” has not been validated in the literature.
Uncertain or interrupted alimentation status or stress hyperglycemia may complicate the assessment of a patient’s insulin needs. One of the insights from the RABBIT 2 surgery trial is that even with interrupted alimentation, patients on a weight-based, long-acting insulin regimen did not experience severe hypoglycemia. Nevertheless, if a patient without type 1 diabetes is felt to be at high risk for a severe hypoglycemic event, it may be prudent to withhold long-acting insulin. However, in that situation, adding SSI to finger-stick monitoring is unlikely to be beneficial. Cases of stress hyperglycemia in nondiabetics can also be challenging, as the persistence of hyperglycemia can be difficult to predict. Guidelines state that if hyperglycemia is persistent, then insulin therapy should be initiated and that this therapy is best accomplished in the form of a basal-prandial regimen.17
WHAT YOU SHOULD DO INSTEAD
Current guidelines from the American Diabetes Association17 and the American Association of Clinical Endocrinologists18 for hospitalized patients with hyperglycemia who require insulin recommend against the prolonged use of SSI as monotherapy (category A recommendation) and support the use of basal plus correctional insulin with the addition of nutritional insulin for patients with consistent oral intake (category A recommendation). Although a complete discourse on the determination of the appropriate starting dose of insulin is outside of the scope of this cas presentation, the basic approach begins with calculating a weight-based total daily dose of insulin, approximately half of which can be given as basal insulin with the remainder given with meals along with correctional insulin as needed to account for premeal hyperglycemia.4 For example, the protocol used in the RABBIT 2 trial, which involved known type 2 diabetics, started insulin based on a total daily dose of 0.4 units/kg for patients presenting with blood sugar levels ≤200 mg/dL and 0.5 units/kg for those with higher initial glucose levels.7 Half of the total daily dose was given as basal insulin, and the other half was divided among meals. Caution with insulin dosing may be required in patients aged >70 years, in those with impaired renal function, and in situations in which steroid doses are fluctuating. The Society of Hospital Medicine has formulated an online subcutaneous insulin order implementation guideline, eQUIPS, that can be a helpful resource to centers that are interested in changing their practice patterns.19
RECOMMENDATIONS
- Instead of using SSI monotherapy for hospitalized patients who require insulin, add basal and prandial insulin, using a weight-based approach if necessary for insulin-naive patients.
- Engage with leadership at your center to learn how inpatient hyperglycemia protocols and blood sugar management teams can help provide evidence-based and individualized treatment plans for your patients.
- If no infrastructure exists at your center, the Society of Hospital Medicine offers training and guidance through its eQUIPS inpatient hyperglycemia management program.
CONCLUSION
In the case presentation, the hyperglycemic patient whose metformin was on hold should have been started on a combination of basal and prandial insulin as determined by his weight and current renal function as opposed to monotherapy with SSI. Using SSI as monotherapy for hyperglycemia is a common practice, and although well-intentioned, it is an ineffective and possibly dangerous approach. Continued efforts must be made to address the gap between guidelines and suboptimal practice patterns locally and nationally.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing[email protected].
Acknowledgments
The authors would like to thank Dr. Asem Ali of the Division of Endocrinology at UMass Memorial Medical Center for his review of the manuscript.
Disclosures
The authors have nothing to disclose.
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” (TWDFNR) series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

A CLINICAL SCENARIO
A 60-year-old man with a past medical history of obesity and type 2 diabetes presented to the emergency department with one week of myalgias and fever up to 103.5°F (39.7°C). Other vital signs were normal. He had no localizing symptoms, and physical examination was unrevealing, except for a small scab from a tick bite sustained two weeks prior to symptom onset. Before admission, he had been managing his diabetes with metformin 1,000 mg twice a day, and on arrival, his blood sugar level was 275 mg/dL. The admitting provider decided to hold the patient’s metformin and replace it with insulin per a sliding scale. Is monotherapy with sliding-scale insulin the best inpatient management option for this patient’s type 2 diabetes?
WHY YOU MIGHT THINK SLIDING-SCALE INSULIN AS MONOTHERAPY IS HELPFUL
The basic premise of sliding-scale insulin (SSI) is to correct hyperglycemia through the frequent administration of short-acting insulin dosed according to a patient’s blood glucose level with the help of a prespecified rubric. When blood glucose levels are low, patients receive little or no insulin, and when blood glucose levels are high, higher doses are given. This approach to inpatient blood glucose management was first popularized by Joslin in 1934,1 and it remains a common strategy today. For example, a 2007 survey of 44 hospitals in the United States showed that approximately 43% of all noncritically ill patients with hyperglycemia were treated with SSI alone.2 More recently, a single-center study showed that 30% of clinicians continued to use SSI as monotherapy even after the implementation of order sets designed to limit this practice.3
The rationale for SSI as monotherapy appears to have two components. First, guidelines suggest that certain patients should be screened periodically in the hospital for hyperglycemia (blood glucose persistently greater than 180 mg/dL) and that, if identified, hyperglycemia should be treated.4 By pairing finger-stick glucose monitoring with SSI, the diagnosis and treatment—although not the prevention—of hyperglycemia can be accomplished simultaneously. Second, inpatient providers do not want to cause harm in the form of hypoglycemia. SSI as monotherapy is sometimes viewed as a cautious approach in this regard as insulin is administered only if the blood sugar level is high.
Convenience is probably another key contributor to the enduring use of SSI as monotherapy. Several hospitals have ready-made order sets for SSI that are easier to prescribe than a patient-specific regimen including both short- and long-acting insulin. In at least one single-center survey, physicians and staff were found to favor convenience over perceived efficacy when asked about their attitudes toward inpatient glycemic control.5 Although efforts at individual hospitals to change practice patterns among residents have shown promise,6 reform on a broader scale remains elusive.
WHY SSI AS MONOTHERAPY IS NOT HELPFUL
SSI administration does not attempt to replicate normal pancreatic physiology, which involves basal insulin secretion to impair hepatic gluconeogenesis and meal-associated insulin spikes to promote uptake into glucose-avid tissues. SSI is a reactive strategy, not a proactive one, and perhaps unsurprisingly, to our knowledge, it has never been shown to prevent hyperglycemia in hospitalized patients, an impression corroborated by a systematic review of the topic between 1964 and 2003.7 More recently, one multicenter trial analyzed the effect of adding SSI to oral antihyperglycemic medications in hospitalized diabetics and found no differences in rates of hyperglycemia.8 Another study found that 84% of administered SSI doses failed to correct hyperglycemia.9
However, does adding basal insulin to SSI raise a patient’s risk of hypoglycemia? When basal insulin is dosed carefully, the answer appears to be no. In a trial in which diabetic long-term care residents who were receiving SSI at baseline were randomized to either continued SSI or basal-bolus insulin, the investigators found that the basal-bolus group experienced significantly lower average blood glucose levels without an increase in adverse glycemic events.10 Perhaps the most significant milestone to date, however, was the RABBIT 2 multicenter trial, published in 2007, that randomized hospitalized, insulin-naïve diabetics to either a weight-based regimen of basal and prandial insulin or SSI only.11 Rates of hypoglycemia and length of stay did not differ between the groups, and 66% of patients receiving basal-prandial insulin achieved their glycemic control target as opposed to just 38% of patients in the SSI-only group. The SSI group also required more total insulin. A weight-based, basal-bolus strategy was later proven to be similarly effective, without causing severe hypoglycemia, for patients undergoing surgery who could not maintain consistent oral alimentation.12 Basal-bolus insulin was associated with fewer surgical complications, and it produced a cost savings of $751 per day as determined by a post hoc comparative effectiveness study.13
Prolonged use of SSI as monotherapy may be not only ineffective but also harmful. Clearly, the absence of basal insulin will harm type 1 diabetics, who need basal insulin to prevent diabetic ketoacidosis. However, even for type 2 diabetics and nondiabetics, hyperglycemia has been established as a marker for adverse outcomes among hospitalized patients,14 and SSI monotherapy has been associated with a three-fold higher risk of hyperglycemia compared with the use of a sliding scale plus other forms of insulin.15 At least one other study has also linked this practice with a significantly increased length of stay compared with patients who were receiving insulin proactively.16 We believe that the potential for harm is difficult to disregard, especially because safer alternatives are available. Ultimately, it can be stated that in hospitalized patients with persistent hyperglycemia who require insulin, SSI alone should not be the preferred treatment choice regardless of whether the patient carries a known diagnosis of diabetes mellitus or has used insulin previously.
WHEN YOU MIGHT CONSIDER USING SSI AS MONOTHERAPY
As discussed above, there is no known clinical scenario in which SSI as monotherapy has been proven to be effective; however, the use of SSI as monotherapy as a short-term approach has not been well studied. Hospitalized patients who are at risk for adverse glycemic events should be monitored with periodic finger-stick blood glucose draws per guidelines, and in the first 24 hours, it may be reasonable to withhold basal insulin for insulin-naive patients, particularly if the medication reconciliation or other key components of the history are in doubt, or if there are risk factors for hypoglycemia such as a history of bariatric surgery. The amount of insulin received in the first 24 hours of such monitoring may inform subsequent insulin dosing, but this method of “dose finding” has not been validated in the literature.
Uncertain or interrupted alimentation status or stress hyperglycemia may complicate the assessment of a patient’s insulin needs. One of the insights from the RABBIT 2 surgery trial is that even with interrupted alimentation, patients on a weight-based, long-acting insulin regimen did not experience severe hypoglycemia. Nevertheless, if a patient without type 1 diabetes is felt to be at high risk for a severe hypoglycemic event, it may be prudent to withhold long-acting insulin. However, in that situation, adding SSI to finger-stick monitoring is unlikely to be beneficial. Cases of stress hyperglycemia in nondiabetics can also be challenging, as the persistence of hyperglycemia can be difficult to predict. Guidelines state that if hyperglycemia is persistent, then insulin therapy should be initiated and that this therapy is best accomplished in the form of a basal-prandial regimen.17
WHAT YOU SHOULD DO INSTEAD
Current guidelines from the American Diabetes Association17 and the American Association of Clinical Endocrinologists18 for hospitalized patients with hyperglycemia who require insulin recommend against the prolonged use of SSI as monotherapy (category A recommendation) and support the use of basal plus correctional insulin with the addition of nutritional insulin for patients with consistent oral intake (category A recommendation). Although a complete discourse on the determination of the appropriate starting dose of insulin is outside of the scope of this cas presentation, the basic approach begins with calculating a weight-based total daily dose of insulin, approximately half of which can be given as basal insulin with the remainder given with meals along with correctional insulin as needed to account for premeal hyperglycemia.4 For example, the protocol used in the RABBIT 2 trial, which involved known type 2 diabetics, started insulin based on a total daily dose of 0.4 units/kg for patients presenting with blood sugar levels ≤200 mg/dL and 0.5 units/kg for those with higher initial glucose levels.7 Half of the total daily dose was given as basal insulin, and the other half was divided among meals. Caution with insulin dosing may be required in patients aged >70 years, in those with impaired renal function, and in situations in which steroid doses are fluctuating. The Society of Hospital Medicine has formulated an online subcutaneous insulin order implementation guideline, eQUIPS, that can be a helpful resource to centers that are interested in changing their practice patterns.19
RECOMMENDATIONS
- Instead of using SSI monotherapy for hospitalized patients who require insulin, add basal and prandial insulin, using a weight-based approach if necessary for insulin-naive patients.
- Engage with leadership at your center to learn how inpatient hyperglycemia protocols and blood sugar management teams can help provide evidence-based and individualized treatment plans for your patients.
- If no infrastructure exists at your center, the Society of Hospital Medicine offers training and guidance through its eQUIPS inpatient hyperglycemia management program.
CONCLUSION
In the case presentation, the hyperglycemic patient whose metformin was on hold should have been started on a combination of basal and prandial insulin as determined by his weight and current renal function as opposed to monotherapy with SSI. Using SSI as monotherapy for hyperglycemia is a common practice, and although well-intentioned, it is an ineffective and possibly dangerous approach. Continued efforts must be made to address the gap between guidelines and suboptimal practice patterns locally and nationally.
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing[email protected].
Acknowledgments
The authors would like to thank Dr. Asem Ali of the Division of Endocrinology at UMass Memorial Medical Center for his review of the manuscript.
Disclosures
The authors have nothing to disclose.
1. Joslin EP. A Diabetic Manual for the Mutual Use of Doctor and Patient. Philadelphia, PA: Lea & Febiger; 1934:108.
2. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. doi: 10.2337/dc06-1715. PubMed
3. Valgardson JD, Merino M, Redgrave J, Hudson JI, Hudson MS. Effectiveness of inpatient insulin order sets using human insulins in noncritically ill patients in a rural hospital. Endocr Pract. 2015;21(7):794-806. doi: 10.4158/EP14153. PubMed
4. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. doi: 10.2337/diacare.27.2.553. PubMed
5. Beliard R, Muzykovsky K, Vincent W, 3rd, Shah B, Davanos E. Perceptions, barriers, and knowledge of inpatient glycemic control: a survey of health care workers. J Pharm Pract. 2016;29(4):348-354. doi: 10.1177/0897190014566309. PubMed
6. Baldwin D, Villanueva G, McNutt R, Bhatnagar S. Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008-1011. doi: 10.2337/diacare.28.12.2987-a. PubMed
7. Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61(15):1611-1614. PubMed
8. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. PubMed
9. Golightly LK, Jones MA, Hamamura DH, Stolpman NM, McDermott MT. Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding-scale insulin therapy. Pharmacotherapy. 2006;26(10):1421-1432. doi: 10.1592/phco.26.10.1421. PubMed
10. Dharmarajan TS, Mahajan D, Zambrano A, et al. Sliding scale insulin vs basal-bolus insulin therapy in long-term care: a 21-day randomized controlled trial comparing efficacy, safety and feasibility. J Am Med Dir Assoc. 2016;17(3):206-213. doi: 10.1016/j.jamda.2015.08.015. PubMed
11. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186. doi: 10.2337/dc07-0295. PubMed
12. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. doi: 10.2337/dc10-1407. PubMed
13. Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. Pharmacoecon Open. 2017;1(2):109-115. doi: 10.1007/s41669-017-0020-9.. PubMed
14. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. doi: 10.1210/jcem.87.3.8341. PubMed
15. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552. PubMed
16. Gearhart JG, Duncan JL, 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322. PubMed
17. American Diabetes A. 14. Diabetes care in the hospital: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S144-S151. doi: 10.2337/dc18-S014. PubMed
18. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369. doi: 10.2337/dc09-9029. PubMed
19. Maynard G, Wesorick DH, O’Malley C, Inzucchi SE, Society of Hospital Medicine Glycemic Control Task F. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29-41. doi: 10.1002/jhm.354. PubMed
1. Joslin EP. A Diabetic Manual for the Mutual Use of Doctor and Patient. Philadelphia, PA: Lea & Febiger; 1934:108.
2. Wexler DJ, Meigs JB, Cagliero E, Nathan DM, Grant RW. Prevalence of hyper- and hypoglycemia among inpatients with diabetes: a national survey of 44 U.S. hospitals. Diabetes Care. 2007;30(2):367-369. doi: 10.2337/dc06-1715. PubMed
3. Valgardson JD, Merino M, Redgrave J, Hudson JI, Hudson MS. Effectiveness of inpatient insulin order sets using human insulins in noncritically ill patients in a rural hospital. Endocr Pract. 2015;21(7):794-806. doi: 10.4158/EP14153. PubMed
4. Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care. 2004;27(2):553-591. doi: 10.2337/diacare.27.2.553. PubMed
5. Beliard R, Muzykovsky K, Vincent W, 3rd, Shah B, Davanos E. Perceptions, barriers, and knowledge of inpatient glycemic control: a survey of health care workers. J Pharm Pract. 2016;29(4):348-354. doi: 10.1177/0897190014566309. PubMed
6. Baldwin D, Villanueva G, McNutt R, Bhatnagar S. Eliminating inpatient sliding-scale insulin: a reeducation project with medical house staff. Diabetes Care. 2005;28(5):1008-1011. doi: 10.2337/diacare.28.12.2987-a. PubMed
7. Browning LA, Dumo P. Sliding-scale insulin: an antiquated approach to glycemic control in hospitalized patients. Am J Health Syst Pharm. 2004;61(15):1611-1614. PubMed
8. Dickerson LM, Ye X, Sack JL, Hueston WJ. Glycemic control in medical inpatients with type 2 diabetes mellitus receiving sliding scale insulin regimens versus routine diabetes medications: a multicenter randomized controlled trial. Ann Fam Med. 2003;1(1):29-35. PubMed
9. Golightly LK, Jones MA, Hamamura DH, Stolpman NM, McDermott MT. Management of diabetes mellitus in hospitalized patients: efficiency and effectiveness of sliding-scale insulin therapy. Pharmacotherapy. 2006;26(10):1421-1432. doi: 10.1592/phco.26.10.1421. PubMed
10. Dharmarajan TS, Mahajan D, Zambrano A, et al. Sliding scale insulin vs basal-bolus insulin therapy in long-term care: a 21-day randomized controlled trial comparing efficacy, safety and feasibility. J Am Med Dir Assoc. 2016;17(3):206-213. doi: 10.1016/j.jamda.2015.08.015. PubMed
11. Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30(9):2181-2186. doi: 10.2337/dc07-0295. PubMed
12. Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34(2):256-261. doi: 10.2337/dc10-1407. PubMed
13. Phillips VL, Byrd AL, Adeel S, Peng L, Smiley DD, Umpierrez GE. A comparison of inpatient cost per day in general surgery patients with type 2 diabetes treated with basal-bolus versus sliding scale insulin regimens. Pharmacoecon Open. 2017;1(2):109-115. doi: 10.1007/s41669-017-0020-9.. PubMed
14. Umpierrez GE, Isaacs SD, Bazargan N, You X, Thaler LM, Kitabchi AE. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab. 2002;87(3):978-982. doi: 10.1210/jcem.87.3.8341. PubMed
15. Queale WS, Seidler AJ, Brancati FL. Glycemic control and sliding scale insulin use in medical inpatients with diabetes mellitus. Arch Intern Med. 1997;157(5):545-552. PubMed
16. Gearhart JG, Duncan JL, 3rd, Replogle WH, Forbes RC, Walley EJ. Efficacy of sliding-scale insulin therapy: a comparison with prospective regimens. Fam Pract Res J. 1994;14(4):313-322. PubMed
17. American Diabetes A. 14. Diabetes care in the hospital: Standards of medical care in diabetes-2018. Diabetes Care. 2018;41(Suppl 1):S144-S151. doi: 10.2337/dc18-S014. PubMed
18. Moghissi ES, Korytkowski MT, DiNardo M, et al. American Association of Clinical Endocrinologists and American Diabetes Association consensus statement on inpatient glycemic control. Endocr Pract. 2009;15(4):353-369. doi: 10.2337/dc09-9029. PubMed
19. Maynard G, Wesorick DH, O’Malley C, Inzucchi SE, Society of Hospital Medicine Glycemic Control Task F. Subcutaneous insulin order sets and protocols: effective design and implementation strategies. J Hosp Med. 2008;3(5 Suppl):29-41. doi: 10.1002/jhm.354. PubMed
© 2018 Society of Hospital Medicine
Things We Do for No Reason: Intermittent Pneumatic Compression for Medical Ward Patients?
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with a history of diabetes and gastrointestinal bleeding two months prior, presents with nausea/vomiting and diarrhea after eating unrefrigerated leftovers. Body mass index is 25. Labs are unremarkable except for a blood urea nitrogen of 37 mg/dL, serum creatinine of 1.6 mg/dL up from 1.3, and white blood cell count of 12 K/µL. He is afebrile with blood pressure of 100/60 mm Hg. He lives alone and is fully ambulatory at baseline. The Emergency Department physician requests observation admission for “dehydration/gastroenteritis.” The admitting hospitalist orders intermittent pneumatic compression (IPC) for venous thromboembolism (VTE) prophylaxis.
BACKGROUND
The American Public Health Association has called VTE prophylaxis a “public health crisis” due to the gap between existing evidence and implementation.1 The incidence of symptomatic deep venous thrombosis (DVT) and pulmonary embolism (PE) in hospitalized medical patients managed without prophylaxis is 0.96% and 1.2%, respectively,2 whereas that of asymptomatic DVT in hospitalized patients is approximately 1.8%.2,3 IPC is widely used, and an international registry of 15,156 hospitalized acutely ill medical patients found that 22% of United States patients received IPC for VTE prophylaxis compared with 0.2% of patients in other countries.4
WHY YOU MIGHT THINK IPC IS THE BEST OPTION FOR VTE PROPHYLAXIS IN MEDICAL WARD PATIENTS
The main reason clinicians opt to use IPC for VTE prophylaxis is the wish to avoid the bleeding risk associated with heparin. The American College of Chest Physicians antithrombotic guideline 9th edition (ACCP-AT9) recommends mechanical prophylaxis for patients at increased risk for thrombosis who are either bleeding or at “high risk for major bleeding.”5 The guideline considered patients to have an excessive bleeding risk if they had an active gastroduodenal ulcer, bleeding within the past three months, a platelet count below 50,000/ml, or more than one of the following risk factors: age ≥ 85, hepatic failure with INR >1.5, severe renal failure with GFR <30 mL/min/m2, ICU/CCU admission, central venous catheter, rheumatic disease, current cancer, or male gender.5 IPC also avoids the risk of heparin-induced thrombocytopenia, which is a rare but potentially devastating condition.
WHY IPC MIGHT NOT BE AS HELPFUL IN MEDICAL WARD PATIENTS
IPC devices are frequently not worn or turned on. A study at two university-affiliated level one trauma centers found IPC to be functioning properly in only 19% of trauma patients.10 In another study of gynecologic oncology patients, 52% of IPCs were functioning improperly and 25% of patients experienced some discomfort, inconvenience, or problems with external pneumatic compression.11 Redness, itching, or discomfort was cited by 26% of patients, and patients removed IPCs 11% of the time when nurses left the room.11,12 In another study, skin breakdown occurred in 3% of IPC patients as compared with 1% in the control group.7
Concerns about a possible link between IPC and increased fall risk was raised by a 2005 report of 40 falls by the Pennsylvania Patient Safety Reporting System,13 and IPC accounted for 16 of 3,562 hospital falls according to Boelig and colleagues.14 Ritsema et al. found that the most important perceived barriers to IPC compliance according to patient surveys were that the devices “prevented walking or getting up” (47%), “were tethering or tangling” (25%), and “woke the patient from sleep” (15%).15
IPC devices are not created equally, differing in “anatomical location of the sleeve garment, number and location of air bladders, patterns for compression cycles and duration of inflation time and deflation time.”16 Comparative effectiveness may differ. A study comparing a rapid inflation asymmetrical compression device by Venaflow with a sequential circumferential compression device by Kendall in a high-risk post knee replacement population produced DVT rates of 6.9% versus 15%, respectively (P = .007).16,17 Furthermore, the type of sleeve and device may affect comfort and compliance as some sleeves are considered “breathable.”
Perhaps most importantly, data supporting IPC efficacy in general medical ward patients are virtually nonexistent. Ho’s meta-analysis of IPC after excluding surgical patients found a relative risk (RR) of 0.53 (95% CI: 0.35-0.81, P < .01) for DVT in nine trials and a nonstatistically significant RR of 0.64 (95% CI: 0.29-1.42. P = .27) for PE in six trials.6 However, if high-risk populations such as trauma, critical care, and stroke are excluded, then
IPC is expensive. The cost for pneumatic compression boots is quoted in the literature at $120 with a range of $80-$250.21 Furthermore, patients averaged 2.5 pairs per hospitalization.22 An online search of retail prices revealed a pair of knee-length Covidien 5329 compression sleeves at $299.19 per pair23 and knee-length Kendall 7325-2 compression sleeves at $433.76 per pair24 with pumps costing $7,518.07 for Venodyne 610 Advantage,25 $6,965.98 for VenaFlow Elite,26 and $5,750.50 for Covidien 29525 700 series Kendall SCD.27 However, using these prices would be overestimating costs given that hospitals do not pay retail prices. A prior surgical cost/benefit analysis used a prevalence of 6.9% and a 69% reduction of DVT.28 However, recent data showed that VTE incidence in 31,219 medical patients was only 0.57% and RR for a large VTE prevention initiative was a nonsignificant 10% reduction.29 Even if we use a VTE prevalence of 1% for the general medical floor and 0.5% RR reduction, 200 patients would need to be treated to prevent one symptomatic VTE and would cost about $24,000 for IPC sleeves alone (estimating $120 per patient) without factoring in additional costs of pump purchase or rental and six additional episodes of anticipated skin breakdown. In comparison, the cost for VTE treatment ranges from $7,712 to $16,644.30
WHAT SHOULD WE DO INSTEAD?
First, one should consider if VTE prophylaxis is needed based on risk assessment. According to the Agency for Healthcare Research and Quality (AHRQ), the most widely used risk stratification model is the University of California San Diego “3 bucket model” (Table 1) derived from tables in ACCP-AT8 guidelines.31
RECOMMENDATIONS
- The VTE risk of general medicine ward patients should be assessed, preferably with the “3 bucket” or Padua risk assessment models.
- For low-risk patients, no VTE prophylaxis is indicated. Ambulation ought to be encouraged for low-risk patients.
- If prophylaxis is indicated, then bleeding risk should be assessed to determine a contraindication to pharmacologic prophylaxis. If there is excessive bleeding risk, then treatment with IPC may be considered even though there are only data to support this in high-risk populations such as surgical, stroke, trauma, and critical care patients.
- If using IPC, then strategies that ensure compliance and consider patient comfort based on type and location of sleeves should be implemented.
- Combined IPC and pharmacologic prophylaxis should be used for high-risk trauma or surgical patients.
CONCLUSIONS
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing[email protected].
Disclosures
The authors have nothing to disclose.
1. Association APH. Deep-vein thrombosis: advancing awareness to protect patient lives. WHITE Paper. Public Health Leadership Conference on Deep-Vein Thrombosis.
2. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. doi: 10.7326/0003-4819-155-9-201111010-00008. PubMed
3. Zubrow MT, Urie J, Jurkovitz C, et al. Asymptomatic deep vein thrombosis in patients undergoing screening duplex ultrasonography. J Hosp Med. 2014;9(1):19-22. doi: 10.1002/jhm.2112. PubMed
4. Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936-945. doi: 10.1378/chest.06-2993. PubMed
5. Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e185S-e194S. doi: 10.1378/chest.11-2289. PubMed
6. Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013;128(9):1003-1020. doi: 10.1161/CIRCULATIONAHA.113.002690. PubMed
7. CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration, Dennis M, Sandercock P, et al. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013;382(9891):516-524. doi: 10.1016/S0140-6736(13)61050-8. PubMed
8. Park J, Lee JM, Lee JS, Cho YJ. Pharmacological and mechanical thromboprophylaxis in critically ill patients: a network meta-analysis of 12 trials. J Korean Med Sci. 2016;31(11):1828-1837. doi: 10.3346/jkms.2016.31.11.1828. PubMed
9. Kakkos SK, Caprini JA, Geroulakos G, et al. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst Rev. 2016;9:CD005258:CD005258. doi: 10.1002/14651858.CD005258.pub3. PubMed
10. Cornwell EE, 3rd, Chang D, Velmahos G, et al. Compliance with sequential compression device prophylaxis in at-risk trauma patients: a prospective analysis. Am Surg. 2002;68(5):470-473. PubMed
11. Maxwell GL, Synan I, Hayes RP, Clarke-Pearson DL. Preference and compliance in postoperative thromboembolism prophylaxis among gynecologic oncology patients. Obstet Gynecol. 2002;100(3):451-455. doi: 10.1016/S0029-7844(02)02162-2. PubMed
12. Wood KB, Kos PB, Abnet JK, Ista C. Prevention of deep-vein thrombosis after major spinal surgery: a comparison study of external devices. J Spinal Disord. 1997;10(3):209-214. PubMed
13. Unexpected risk from a beneficial device: sequential compression devices and patient falls. PA-PSRS Patient Saf Advis. 2005 Sep;2(3):13-5.
14. Boelig MM, Streiff MB, Hobson DB, Kraus PS, Pronovost PJ, Haut ER. Are sequential compression devices commonly associated with in-hospital falls? A myth-busters review using the patient safety net database. J Patient Saf. 2011;7(2):77-79. doi: 10.1097/PTS.0b013e3182110706. PubMed
15. Ritsema DF, Watson JM, Stiteler AP, Nguyen MM. Sequential compression devices in postoperative urologic patients: an observational trial and survey study on the influence of patient and hospital factors on compliance. BMC Urol. 2013;13:20. doi: 10.1186/1471-2490-13-20. PubMed
16. Pavon JM, Williams JW, Jr, Adam SS, et al. Effectiveness of intermittent pneumatic compression devices for venous thromboembolism prophylaxis in high-risk surgical and medical patients. J Arthroplasty. 2016;31(2):524-532. doi: 10.1016/j.arth.2015.09.043. PubMed
17. Lachiewicz PF, Kelley SS, Haden LR. Two mechanical devices for prophylaxis of thromboembolism after total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 2004;86(8):1137-1141. doi: 10.1302/0301-620X.86B8.15438. PubMed
18. Salzman EW, Sobel M, Lewis J, Sweeney J, Hussey S, Kurland G. Prevention of venous thromboembolism in unstable angina pectoris. N Engl J Med. 1982;306(16):991. doi: 10.1056/NEJM198204223061614. PubMed
19. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S-47S. doi: 10.1378/chest.1412S3. PubMed
20. Qaseem A, Chou R, Humphrey LL, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. doi: 10.7326/0003-4819-155-9-201111010-00011. PubMed
21. Casele H, Grobman WA. Cost-effectiveness of thromboprophylaxis with intermittent pneumatic compression at cesarean delivery. Obstet Gynecol. 2006;108(3 Pt 1):535-540. doi: 10.1097/01.AOG.0000227780.76353.05. PubMed
22. Dennis M, Sandercock P, Graham C, Forbes J, CLOTS Trials Collaboration, Smith J, Smith J. The Clots in Legs or sTockings after Stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess. 2015;19(76):1-90. doi: 10.3310/hta19760. PubMed
23. Amazon.com. Covidien 5329 Sleeve, SCD Knee Length. https://www.amazon.com/Covidien-5329-Sleeve-Knee-Length/dp/B01BSFZM76. Accessed September 14, 2018.
24. Amazon.com. 2270870 SCD Sleeve Knee Length. https://www.amazon.com/s/ref=nb_sb_noss?url=search-alias%3Daps&field-keywords=kendall+7325-2&rh=i%3Aaps%2Ck%3Akendall+7325-2. Accessed September 14, 2018.
25. Amazon.com. 2281540 Venodyne Advantage 610DVT. https://www.amazon.com/Individually-MODEL-610-Microtek-Medical/dp/B00IK4MUUG/ref=sr_1_fkmr0_2?ie=UTF8&qid=1540914574&sr=8-2-fkmr0&keywords=venodyne+scd. Accessed Osctober 30, 2018.
26. Amazon.com. 2339896 Venaflow System w/Battery Elite. https://www.amazon.com/indivdually-Individually-30B-B-DJO-Inc/dp/B00IK4MS3A/ref=sr_1_2?ie=UTF8&qid=1536972486&sr=8-2&keywords=venaflow+elite+system. Accessed September 14, 2018.
27. Amazon.com. Covidien 29525 700 Series Kendall SCD Controller. https://www.amazon.com/Covidien-29525-700-Kendall-Controller/dp/B01BQI5BI0/ref=sr_1_1?ie=UTF8&qid=1536972026&sr=8-1&keywords=covidien+29525. Accessed September 14, 2018.
28. Nicolaides A, Goldhaber SZ, Maxwell GL, et al. Cost benefit of intermittent pneumatic compression for venous thromboembolism prophylaxis in general surgery. Int Angiol. 2008;27(6):500-506. PubMed
29. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. doi: 10.1002/jhm.2658. PubMed
30. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. doi: 10.1592/phco.29.8.943. PubMed
31. Maynard, G. Preventing Hospital-Associated Venous Thromboembolism. A Guide for Effective Quality Improvement. AHRQ Publication No. 16-0001-EF; 2015.
32. Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79. doi: 10.1378/chest.09-3081. PubMed
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with a history of diabetes and gastrointestinal bleeding two months prior, presents with nausea/vomiting and diarrhea after eating unrefrigerated leftovers. Body mass index is 25. Labs are unremarkable except for a blood urea nitrogen of 37 mg/dL, serum creatinine of 1.6 mg/dL up from 1.3, and white blood cell count of 12 K/µL. He is afebrile with blood pressure of 100/60 mm Hg. He lives alone and is fully ambulatory at baseline. The Emergency Department physician requests observation admission for “dehydration/gastroenteritis.” The admitting hospitalist orders intermittent pneumatic compression (IPC) for venous thromboembolism (VTE) prophylaxis.
BACKGROUND
The American Public Health Association has called VTE prophylaxis a “public health crisis” due to the gap between existing evidence and implementation.1 The incidence of symptomatic deep venous thrombosis (DVT) and pulmonary embolism (PE) in hospitalized medical patients managed without prophylaxis is 0.96% and 1.2%, respectively,2 whereas that of asymptomatic DVT in hospitalized patients is approximately 1.8%.2,3 IPC is widely used, and an international registry of 15,156 hospitalized acutely ill medical patients found that 22% of United States patients received IPC for VTE prophylaxis compared with 0.2% of patients in other countries.4
WHY YOU MIGHT THINK IPC IS THE BEST OPTION FOR VTE PROPHYLAXIS IN MEDICAL WARD PATIENTS
The main reason clinicians opt to use IPC for VTE prophylaxis is the wish to avoid the bleeding risk associated with heparin. The American College of Chest Physicians antithrombotic guideline 9th edition (ACCP-AT9) recommends mechanical prophylaxis for patients at increased risk for thrombosis who are either bleeding or at “high risk for major bleeding.”5 The guideline considered patients to have an excessive bleeding risk if they had an active gastroduodenal ulcer, bleeding within the past three months, a platelet count below 50,000/ml, or more than one of the following risk factors: age ≥ 85, hepatic failure with INR >1.5, severe renal failure with GFR <30 mL/min/m2, ICU/CCU admission, central venous catheter, rheumatic disease, current cancer, or male gender.5 IPC also avoids the risk of heparin-induced thrombocytopenia, which is a rare but potentially devastating condition.
WHY IPC MIGHT NOT BE AS HELPFUL IN MEDICAL WARD PATIENTS
IPC devices are frequently not worn or turned on. A study at two university-affiliated level one trauma centers found IPC to be functioning properly in only 19% of trauma patients.10 In another study of gynecologic oncology patients, 52% of IPCs were functioning improperly and 25% of patients experienced some discomfort, inconvenience, or problems with external pneumatic compression.11 Redness, itching, or discomfort was cited by 26% of patients, and patients removed IPCs 11% of the time when nurses left the room.11,12 In another study, skin breakdown occurred in 3% of IPC patients as compared with 1% in the control group.7
Concerns about a possible link between IPC and increased fall risk was raised by a 2005 report of 40 falls by the Pennsylvania Patient Safety Reporting System,13 and IPC accounted for 16 of 3,562 hospital falls according to Boelig and colleagues.14 Ritsema et al. found that the most important perceived barriers to IPC compliance according to patient surveys were that the devices “prevented walking or getting up” (47%), “were tethering or tangling” (25%), and “woke the patient from sleep” (15%).15
IPC devices are not created equally, differing in “anatomical location of the sleeve garment, number and location of air bladders, patterns for compression cycles and duration of inflation time and deflation time.”16 Comparative effectiveness may differ. A study comparing a rapid inflation asymmetrical compression device by Venaflow with a sequential circumferential compression device by Kendall in a high-risk post knee replacement population produced DVT rates of 6.9% versus 15%, respectively (P = .007).16,17 Furthermore, the type of sleeve and device may affect comfort and compliance as some sleeves are considered “breathable.”
Perhaps most importantly, data supporting IPC efficacy in general medical ward patients are virtually nonexistent. Ho’s meta-analysis of IPC after excluding surgical patients found a relative risk (RR) of 0.53 (95% CI: 0.35-0.81, P < .01) for DVT in nine trials and a nonstatistically significant RR of 0.64 (95% CI: 0.29-1.42. P = .27) for PE in six trials.6 However, if high-risk populations such as trauma, critical care, and stroke are excluded, then
IPC is expensive. The cost for pneumatic compression boots is quoted in the literature at $120 with a range of $80-$250.21 Furthermore, patients averaged 2.5 pairs per hospitalization.22 An online search of retail prices revealed a pair of knee-length Covidien 5329 compression sleeves at $299.19 per pair23 and knee-length Kendall 7325-2 compression sleeves at $433.76 per pair24 with pumps costing $7,518.07 for Venodyne 610 Advantage,25 $6,965.98 for VenaFlow Elite,26 and $5,750.50 for Covidien 29525 700 series Kendall SCD.27 However, using these prices would be overestimating costs given that hospitals do not pay retail prices. A prior surgical cost/benefit analysis used a prevalence of 6.9% and a 69% reduction of DVT.28 However, recent data showed that VTE incidence in 31,219 medical patients was only 0.57% and RR for a large VTE prevention initiative was a nonsignificant 10% reduction.29 Even if we use a VTE prevalence of 1% for the general medical floor and 0.5% RR reduction, 200 patients would need to be treated to prevent one symptomatic VTE and would cost about $24,000 for IPC sleeves alone (estimating $120 per patient) without factoring in additional costs of pump purchase or rental and six additional episodes of anticipated skin breakdown. In comparison, the cost for VTE treatment ranges from $7,712 to $16,644.30
WHAT SHOULD WE DO INSTEAD?
First, one should consider if VTE prophylaxis is needed based on risk assessment. According to the Agency for Healthcare Research and Quality (AHRQ), the most widely used risk stratification model is the University of California San Diego “3 bucket model” (Table 1) derived from tables in ACCP-AT8 guidelines.31
RECOMMENDATIONS
- The VTE risk of general medicine ward patients should be assessed, preferably with the “3 bucket” or Padua risk assessment models.
- For low-risk patients, no VTE prophylaxis is indicated. Ambulation ought to be encouraged for low-risk patients.
- If prophylaxis is indicated, then bleeding risk should be assessed to determine a contraindication to pharmacologic prophylaxis. If there is excessive bleeding risk, then treatment with IPC may be considered even though there are only data to support this in high-risk populations such as surgical, stroke, trauma, and critical care patients.
- If using IPC, then strategies that ensure compliance and consider patient comfort based on type and location of sleeves should be implemented.
- Combined IPC and pharmacologic prophylaxis should be used for high-risk trauma or surgical patients.
CONCLUSIONS
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing[email protected].
Disclosures
The authors have nothing to disclose.
Inspired by the ABIM Foundation's Choosing Wisely campaign, the “Things We Do for No Reason” series reviews practices that have become common parts of hospital care but may provide little value to our patients. Practices reviewed in the TWDFNR series do not represent “black and white” conclusions or clinical practice standards, but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 74-year-old man with a history of diabetes and gastrointestinal bleeding two months prior, presents with nausea/vomiting and diarrhea after eating unrefrigerated leftovers. Body mass index is 25. Labs are unremarkable except for a blood urea nitrogen of 37 mg/dL, serum creatinine of 1.6 mg/dL up from 1.3, and white blood cell count of 12 K/µL. He is afebrile with blood pressure of 100/60 mm Hg. He lives alone and is fully ambulatory at baseline. The Emergency Department physician requests observation admission for “dehydration/gastroenteritis.” The admitting hospitalist orders intermittent pneumatic compression (IPC) for venous thromboembolism (VTE) prophylaxis.
BACKGROUND
The American Public Health Association has called VTE prophylaxis a “public health crisis” due to the gap between existing evidence and implementation.1 The incidence of symptomatic deep venous thrombosis (DVT) and pulmonary embolism (PE) in hospitalized medical patients managed without prophylaxis is 0.96% and 1.2%, respectively,2 whereas that of asymptomatic DVT in hospitalized patients is approximately 1.8%.2,3 IPC is widely used, and an international registry of 15,156 hospitalized acutely ill medical patients found that 22% of United States patients received IPC for VTE prophylaxis compared with 0.2% of patients in other countries.4
WHY YOU MIGHT THINK IPC IS THE BEST OPTION FOR VTE PROPHYLAXIS IN MEDICAL WARD PATIENTS
The main reason clinicians opt to use IPC for VTE prophylaxis is the wish to avoid the bleeding risk associated with heparin. The American College of Chest Physicians antithrombotic guideline 9th edition (ACCP-AT9) recommends mechanical prophylaxis for patients at increased risk for thrombosis who are either bleeding or at “high risk for major bleeding.”5 The guideline considered patients to have an excessive bleeding risk if they had an active gastroduodenal ulcer, bleeding within the past three months, a platelet count below 50,000/ml, or more than one of the following risk factors: age ≥ 85, hepatic failure with INR >1.5, severe renal failure with GFR <30 mL/min/m2, ICU/CCU admission, central venous catheter, rheumatic disease, current cancer, or male gender.5 IPC also avoids the risk of heparin-induced thrombocytopenia, which is a rare but potentially devastating condition.
WHY IPC MIGHT NOT BE AS HELPFUL IN MEDICAL WARD PATIENTS
IPC devices are frequently not worn or turned on. A study at two university-affiliated level one trauma centers found IPC to be functioning properly in only 19% of trauma patients.10 In another study of gynecologic oncology patients, 52% of IPCs were functioning improperly and 25% of patients experienced some discomfort, inconvenience, or problems with external pneumatic compression.11 Redness, itching, or discomfort was cited by 26% of patients, and patients removed IPCs 11% of the time when nurses left the room.11,12 In another study, skin breakdown occurred in 3% of IPC patients as compared with 1% in the control group.7
Concerns about a possible link between IPC and increased fall risk was raised by a 2005 report of 40 falls by the Pennsylvania Patient Safety Reporting System,13 and IPC accounted for 16 of 3,562 hospital falls according to Boelig and colleagues.14 Ritsema et al. found that the most important perceived barriers to IPC compliance according to patient surveys were that the devices “prevented walking or getting up” (47%), “were tethering or tangling” (25%), and “woke the patient from sleep” (15%).15
IPC devices are not created equally, differing in “anatomical location of the sleeve garment, number and location of air bladders, patterns for compression cycles and duration of inflation time and deflation time.”16 Comparative effectiveness may differ. A study comparing a rapid inflation asymmetrical compression device by Venaflow with a sequential circumferential compression device by Kendall in a high-risk post knee replacement population produced DVT rates of 6.9% versus 15%, respectively (P = .007).16,17 Furthermore, the type of sleeve and device may affect comfort and compliance as some sleeves are considered “breathable.”
Perhaps most importantly, data supporting IPC efficacy in general medical ward patients are virtually nonexistent. Ho’s meta-analysis of IPC after excluding surgical patients found a relative risk (RR) of 0.53 (95% CI: 0.35-0.81, P < .01) for DVT in nine trials and a nonstatistically significant RR of 0.64 (95% CI: 0.29-1.42. P = .27) for PE in six trials.6 However, if high-risk populations such as trauma, critical care, and stroke are excluded, then
IPC is expensive. The cost for pneumatic compression boots is quoted in the literature at $120 with a range of $80-$250.21 Furthermore, patients averaged 2.5 pairs per hospitalization.22 An online search of retail prices revealed a pair of knee-length Covidien 5329 compression sleeves at $299.19 per pair23 and knee-length Kendall 7325-2 compression sleeves at $433.76 per pair24 with pumps costing $7,518.07 for Venodyne 610 Advantage,25 $6,965.98 for VenaFlow Elite,26 and $5,750.50 for Covidien 29525 700 series Kendall SCD.27 However, using these prices would be overestimating costs given that hospitals do not pay retail prices. A prior surgical cost/benefit analysis used a prevalence of 6.9% and a 69% reduction of DVT.28 However, recent data showed that VTE incidence in 31,219 medical patients was only 0.57% and RR for a large VTE prevention initiative was a nonsignificant 10% reduction.29 Even if we use a VTE prevalence of 1% for the general medical floor and 0.5% RR reduction, 200 patients would need to be treated to prevent one symptomatic VTE and would cost about $24,000 for IPC sleeves alone (estimating $120 per patient) without factoring in additional costs of pump purchase or rental and six additional episodes of anticipated skin breakdown. In comparison, the cost for VTE treatment ranges from $7,712 to $16,644.30
WHAT SHOULD WE DO INSTEAD?
First, one should consider if VTE prophylaxis is needed based on risk assessment. According to the Agency for Healthcare Research and Quality (AHRQ), the most widely used risk stratification model is the University of California San Diego “3 bucket model” (Table 1) derived from tables in ACCP-AT8 guidelines.31
RECOMMENDATIONS
- The VTE risk of general medicine ward patients should be assessed, preferably with the “3 bucket” or Padua risk assessment models.
- For low-risk patients, no VTE prophylaxis is indicated. Ambulation ought to be encouraged for low-risk patients.
- If prophylaxis is indicated, then bleeding risk should be assessed to determine a contraindication to pharmacologic prophylaxis. If there is excessive bleeding risk, then treatment with IPC may be considered even though there are only data to support this in high-risk populations such as surgical, stroke, trauma, and critical care patients.
- If using IPC, then strategies that ensure compliance and consider patient comfort based on type and location of sleeves should be implemented.
- Combined IPC and pharmacologic prophylaxis should be used for high-risk trauma or surgical patients.
CONCLUSIONS
Do you think this is a low-value practice? Is this truly a “Thing We Do for No Reason?” Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other “Things We Do for No Reason” topics by emailing[email protected].
Disclosures
The authors have nothing to disclose.
1. Association APH. Deep-vein thrombosis: advancing awareness to protect patient lives. WHITE Paper. Public Health Leadership Conference on Deep-Vein Thrombosis.
2. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. doi: 10.7326/0003-4819-155-9-201111010-00008. PubMed
3. Zubrow MT, Urie J, Jurkovitz C, et al. Asymptomatic deep vein thrombosis in patients undergoing screening duplex ultrasonography. J Hosp Med. 2014;9(1):19-22. doi: 10.1002/jhm.2112. PubMed
4. Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936-945. doi: 10.1378/chest.06-2993. PubMed
5. Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e185S-e194S. doi: 10.1378/chest.11-2289. PubMed
6. Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013;128(9):1003-1020. doi: 10.1161/CIRCULATIONAHA.113.002690. PubMed
7. CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration, Dennis M, Sandercock P, et al. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013;382(9891):516-524. doi: 10.1016/S0140-6736(13)61050-8. PubMed
8. Park J, Lee JM, Lee JS, Cho YJ. Pharmacological and mechanical thromboprophylaxis in critically ill patients: a network meta-analysis of 12 trials. J Korean Med Sci. 2016;31(11):1828-1837. doi: 10.3346/jkms.2016.31.11.1828. PubMed
9. Kakkos SK, Caprini JA, Geroulakos G, et al. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst Rev. 2016;9:CD005258:CD005258. doi: 10.1002/14651858.CD005258.pub3. PubMed
10. Cornwell EE, 3rd, Chang D, Velmahos G, et al. Compliance with sequential compression device prophylaxis in at-risk trauma patients: a prospective analysis. Am Surg. 2002;68(5):470-473. PubMed
11. Maxwell GL, Synan I, Hayes RP, Clarke-Pearson DL. Preference and compliance in postoperative thromboembolism prophylaxis among gynecologic oncology patients. Obstet Gynecol. 2002;100(3):451-455. doi: 10.1016/S0029-7844(02)02162-2. PubMed
12. Wood KB, Kos PB, Abnet JK, Ista C. Prevention of deep-vein thrombosis after major spinal surgery: a comparison study of external devices. J Spinal Disord. 1997;10(3):209-214. PubMed
13. Unexpected risk from a beneficial device: sequential compression devices and patient falls. PA-PSRS Patient Saf Advis. 2005 Sep;2(3):13-5.
14. Boelig MM, Streiff MB, Hobson DB, Kraus PS, Pronovost PJ, Haut ER. Are sequential compression devices commonly associated with in-hospital falls? A myth-busters review using the patient safety net database. J Patient Saf. 2011;7(2):77-79. doi: 10.1097/PTS.0b013e3182110706. PubMed
15. Ritsema DF, Watson JM, Stiteler AP, Nguyen MM. Sequential compression devices in postoperative urologic patients: an observational trial and survey study on the influence of patient and hospital factors on compliance. BMC Urol. 2013;13:20. doi: 10.1186/1471-2490-13-20. PubMed
16. Pavon JM, Williams JW, Jr, Adam SS, et al. Effectiveness of intermittent pneumatic compression devices for venous thromboembolism prophylaxis in high-risk surgical and medical patients. J Arthroplasty. 2016;31(2):524-532. doi: 10.1016/j.arth.2015.09.043. PubMed
17. Lachiewicz PF, Kelley SS, Haden LR. Two mechanical devices for prophylaxis of thromboembolism after total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 2004;86(8):1137-1141. doi: 10.1302/0301-620X.86B8.15438. PubMed
18. Salzman EW, Sobel M, Lewis J, Sweeney J, Hussey S, Kurland G. Prevention of venous thromboembolism in unstable angina pectoris. N Engl J Med. 1982;306(16):991. doi: 10.1056/NEJM198204223061614. PubMed
19. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S-47S. doi: 10.1378/chest.1412S3. PubMed
20. Qaseem A, Chou R, Humphrey LL, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. doi: 10.7326/0003-4819-155-9-201111010-00011. PubMed
21. Casele H, Grobman WA. Cost-effectiveness of thromboprophylaxis with intermittent pneumatic compression at cesarean delivery. Obstet Gynecol. 2006;108(3 Pt 1):535-540. doi: 10.1097/01.AOG.0000227780.76353.05. PubMed
22. Dennis M, Sandercock P, Graham C, Forbes J, CLOTS Trials Collaboration, Smith J, Smith J. The Clots in Legs or sTockings after Stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess. 2015;19(76):1-90. doi: 10.3310/hta19760. PubMed
23. Amazon.com. Covidien 5329 Sleeve, SCD Knee Length. https://www.amazon.com/Covidien-5329-Sleeve-Knee-Length/dp/B01BSFZM76. Accessed September 14, 2018.
24. Amazon.com. 2270870 SCD Sleeve Knee Length. https://www.amazon.com/s/ref=nb_sb_noss?url=search-alias%3Daps&field-keywords=kendall+7325-2&rh=i%3Aaps%2Ck%3Akendall+7325-2. Accessed September 14, 2018.
25. Amazon.com. 2281540 Venodyne Advantage 610DVT. https://www.amazon.com/Individually-MODEL-610-Microtek-Medical/dp/B00IK4MUUG/ref=sr_1_fkmr0_2?ie=UTF8&qid=1540914574&sr=8-2-fkmr0&keywords=venodyne+scd. Accessed Osctober 30, 2018.
26. Amazon.com. 2339896 Venaflow System w/Battery Elite. https://www.amazon.com/indivdually-Individually-30B-B-DJO-Inc/dp/B00IK4MS3A/ref=sr_1_2?ie=UTF8&qid=1536972486&sr=8-2&keywords=venaflow+elite+system. Accessed September 14, 2018.
27. Amazon.com. Covidien 29525 700 Series Kendall SCD Controller. https://www.amazon.com/Covidien-29525-700-Kendall-Controller/dp/B01BQI5BI0/ref=sr_1_1?ie=UTF8&qid=1536972026&sr=8-1&keywords=covidien+29525. Accessed September 14, 2018.
28. Nicolaides A, Goldhaber SZ, Maxwell GL, et al. Cost benefit of intermittent pneumatic compression for venous thromboembolism prophylaxis in general surgery. Int Angiol. 2008;27(6):500-506. PubMed
29. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. doi: 10.1002/jhm.2658. PubMed
30. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. doi: 10.1592/phco.29.8.943. PubMed
31. Maynard, G. Preventing Hospital-Associated Venous Thromboembolism. A Guide for Effective Quality Improvement. AHRQ Publication No. 16-0001-EF; 2015.
32. Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79. doi: 10.1378/chest.09-3081. PubMed
1. Association APH. Deep-vein thrombosis: advancing awareness to protect patient lives. WHITE Paper. Public Health Leadership Conference on Deep-Vein Thrombosis.
2. Lederle FA, Zylla D, MacDonald R, Wilt TJ. Venous thromboembolism prophylaxis in hospitalized medical patients and those with stroke: a background review for an American College of Physicians Clinical Practice Guideline. Ann Intern Med. 2011;155(9):602-615. doi: 10.7326/0003-4819-155-9-201111010-00008. PubMed
3. Zubrow MT, Urie J, Jurkovitz C, et al. Asymptomatic deep vein thrombosis in patients undergoing screening duplex ultrasonography. J Hosp Med. 2014;9(1):19-22. doi: 10.1002/jhm.2112. PubMed
4. Tapson VF, Decousus H, Pini M, et al. Venous thromboembolism prophylaxis in acutely ill hospitalized medical patients: findings from the International Medical Prevention Registry on Venous Thromboembolism. Chest. 2007;132(3):936-945. doi: 10.1378/chest.06-2993. PubMed
5. Guyatt GH, Eikelboom JW, Gould MK, et al. Approach to outcome measurement in the prevention of thrombosis in surgical and medical patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e185S-e194S. doi: 10.1378/chest.11-2289. PubMed
6. Ho KM, Tan JA. Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation. 2013;128(9):1003-1020. doi: 10.1161/CIRCULATIONAHA.113.002690. PubMed
7. CLOTS (Clots in Legs Or sTockings after Stroke) Trials Collaboration, Dennis M, Sandercock P, et al. Effectiveness of intermittent pneumatic compression in reduction of risk of deep vein thrombosis in patients who have had a stroke (CLOTS 3): a multicentre randomised controlled trial. Lancet. 2013;382(9891):516-524. doi: 10.1016/S0140-6736(13)61050-8. PubMed
8. Park J, Lee JM, Lee JS, Cho YJ. Pharmacological and mechanical thromboprophylaxis in critically ill patients: a network meta-analysis of 12 trials. J Korean Med Sci. 2016;31(11):1828-1837. doi: 10.3346/jkms.2016.31.11.1828. PubMed
9. Kakkos SK, Caprini JA, Geroulakos G, et al. Combined intermittent pneumatic leg compression and pharmacological prophylaxis for prevention of venous thromboembolism. Cochrane Database Syst Rev. 2016;9:CD005258:CD005258. doi: 10.1002/14651858.CD005258.pub3. PubMed
10. Cornwell EE, 3rd, Chang D, Velmahos G, et al. Compliance with sequential compression device prophylaxis in at-risk trauma patients: a prospective analysis. Am Surg. 2002;68(5):470-473. PubMed
11. Maxwell GL, Synan I, Hayes RP, Clarke-Pearson DL. Preference and compliance in postoperative thromboembolism prophylaxis among gynecologic oncology patients. Obstet Gynecol. 2002;100(3):451-455. doi: 10.1016/S0029-7844(02)02162-2. PubMed
12. Wood KB, Kos PB, Abnet JK, Ista C. Prevention of deep-vein thrombosis after major spinal surgery: a comparison study of external devices. J Spinal Disord. 1997;10(3):209-214. PubMed
13. Unexpected risk from a beneficial device: sequential compression devices and patient falls. PA-PSRS Patient Saf Advis. 2005 Sep;2(3):13-5.
14. Boelig MM, Streiff MB, Hobson DB, Kraus PS, Pronovost PJ, Haut ER. Are sequential compression devices commonly associated with in-hospital falls? A myth-busters review using the patient safety net database. J Patient Saf. 2011;7(2):77-79. doi: 10.1097/PTS.0b013e3182110706. PubMed
15. Ritsema DF, Watson JM, Stiteler AP, Nguyen MM. Sequential compression devices in postoperative urologic patients: an observational trial and survey study on the influence of patient and hospital factors on compliance. BMC Urol. 2013;13:20. doi: 10.1186/1471-2490-13-20. PubMed
16. Pavon JM, Williams JW, Jr, Adam SS, et al. Effectiveness of intermittent pneumatic compression devices for venous thromboembolism prophylaxis in high-risk surgical and medical patients. J Arthroplasty. 2016;31(2):524-532. doi: 10.1016/j.arth.2015.09.043. PubMed
17. Lachiewicz PF, Kelley SS, Haden LR. Two mechanical devices for prophylaxis of thromboembolism after total knee arthroplasty. A prospective, randomised study. J Bone Joint Surg Br. 2004;86(8):1137-1141. doi: 10.1302/0301-620X.86B8.15438. PubMed
18. Salzman EW, Sobel M, Lewis J, Sweeney J, Hussey S, Kurland G. Prevention of venous thromboembolism in unstable angina pectoris. N Engl J Med. 1982;306(16):991. doi: 10.1056/NEJM198204223061614. PubMed
19. Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ, American College of Chest Physicians Antithrombotic Therapy and Prevention of Thrombosis Panel. Executive summary: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S-47S. doi: 10.1378/chest.1412S3. PubMed
20. Qaseem A, Chou R, Humphrey LL, Starkey M, Shekelle P, Clinical Guidelines Committee of the American College of Physicians. Venous thromboembolism prophylaxis in hospitalized patients: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2011;155(9):625-632. doi: 10.7326/0003-4819-155-9-201111010-00011. PubMed
21. Casele H, Grobman WA. Cost-effectiveness of thromboprophylaxis with intermittent pneumatic compression at cesarean delivery. Obstet Gynecol. 2006;108(3 Pt 1):535-540. doi: 10.1097/01.AOG.0000227780.76353.05. PubMed
22. Dennis M, Sandercock P, Graham C, Forbes J, CLOTS Trials Collaboration, Smith J, Smith J. The Clots in Legs or sTockings after Stroke (CLOTS) 3 trial: a randomised controlled trial to determine whether or not intermittent pneumatic compression reduces the risk of post-stroke deep vein thrombosis and to estimate its cost-effectiveness. Health Technol Assess. 2015;19(76):1-90. doi: 10.3310/hta19760. PubMed
23. Amazon.com. Covidien 5329 Sleeve, SCD Knee Length. https://www.amazon.com/Covidien-5329-Sleeve-Knee-Length/dp/B01BSFZM76. Accessed September 14, 2018.
24. Amazon.com. 2270870 SCD Sleeve Knee Length. https://www.amazon.com/s/ref=nb_sb_noss?url=search-alias%3Daps&field-keywords=kendall+7325-2&rh=i%3Aaps%2Ck%3Akendall+7325-2. Accessed September 14, 2018.
25. Amazon.com. 2281540 Venodyne Advantage 610DVT. https://www.amazon.com/Individually-MODEL-610-Microtek-Medical/dp/B00IK4MUUG/ref=sr_1_fkmr0_2?ie=UTF8&qid=1540914574&sr=8-2-fkmr0&keywords=venodyne+scd. Accessed Osctober 30, 2018.
26. Amazon.com. 2339896 Venaflow System w/Battery Elite. https://www.amazon.com/indivdually-Individually-30B-B-DJO-Inc/dp/B00IK4MS3A/ref=sr_1_2?ie=UTF8&qid=1536972486&sr=8-2&keywords=venaflow+elite+system. Accessed September 14, 2018.
27. Amazon.com. Covidien 29525 700 Series Kendall SCD Controller. https://www.amazon.com/Covidien-29525-700-Kendall-Controller/dp/B01BQI5BI0/ref=sr_1_1?ie=UTF8&qid=1536972026&sr=8-1&keywords=covidien+29525. Accessed September 14, 2018.
28. Nicolaides A, Goldhaber SZ, Maxwell GL, et al. Cost benefit of intermittent pneumatic compression for venous thromboembolism prophylaxis in general surgery. Int Angiol. 2008;27(6):500-506. PubMed
29. Jenkins IH, White RH, Amin AN, et al. Reducing the incidence of hospital-associated venous thromboembolism within a network of academic hospitals: findings from five University of California medical centers. J Hosp Med. 2016;11(Suppl 2):S22-S28. doi: 10.1002/jhm.2658. PubMed
30. Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953. doi: 10.1592/phco.29.8.943. PubMed
31. Maynard, G. Preventing Hospital-Associated Venous Thromboembolism. A Guide for Effective Quality Improvement. AHRQ Publication No. 16-0001-EF; 2015.
32. Decousus H, Tapson VF, Bergmann JF, et al. Factors at admission associated with bleeding risk in medical patients: findings from the IMPROVE investigators. Chest. 2011;139(1):69-79. doi: 10.1378/chest.09-3081. PubMed
© 2019 Society of Hospital Medicine
Acute Treatment of Hypertensive Urgency
The "Things We Do for No Reason" (TWDFNR) series reviews practices which have become common parts of hospital care but provide little value to our patients. Practices reviewed in the TWDFNR series do not represent "black and white" conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 67-year-old man is hospitalized with community-acquired pneumonia. He has a history of hypertension and is prescribed two antihypertensive medications (amlodipine and chlorthalidone) as an outpatient. On the evening of hospital day two, he is found to have a blood pressure of 192/95 on a scheduled vital signs check. He reports no symptoms other than cough, which is not new or worsening. The covering hospitalist reviews the documented blood pressures since admission and notes that many have been elevated despite continuation of his home regimen. The patient's nurse inquires about treating the patient with additional "as-needed" antihypertensive medications.
BACKGROUND
Hypertensive crises are common in hospitalized patients, with approximately one in seven patients experiencing an episode of hypertensive emergency and/or hypertensive urgency.1 Hypertensive emergency is typically defined as (1) a systolic blood pressure ≥180 mm Hg and/or a diastolic blood pressure ≥120 mm Hg with (2) evidence of new or worsening end-organ damage. The organs most commonly affected by severe hypertension are the brain (headache, confusion, stroke), heart (chest pain, myocardial infarction, pulmonary edema), large blood vessels (aortic dissection), and kidneys (acute hypertensive nephrosclerosis).2 With hypertensive urgency, patients experience similarly elevated blood pressure but have no symptoms or signs suggesting acute end-organ damage. Acute treatment with intravenous (IV) or immediate-acting oral medications is common; a single-center study showed that 7.4% of hospitalized patients had an order for "as needed" IV hydralazine or labetalol, with 60.3% receiving at least one dose.3 Among internal medicine and family medicine trainees in one survey, nearly half reported that they would use IV medications in a scenario where an inpatient had an asymptomatic blood pressure above 180 mm Hg.4
WHY YOU MIGHT THINK TREATING HYPERTENSIVE URGENCY IS NECESSARY
Treating patients with hypertensive urgency is based on an assumption: If one does not treat immediately, something bad (ie, end-organ damage) will occur over the next few hours. Data from the 1930s showed that patients with untreated hypertensive emergency had a one-year mortality rate >79% and a median survival of 10.4 months.5 More recent studies suggest that the in-hospital and one-year mortality for those with hypertensive emergency are 13% and 39%, respectively.6 These data demonstrate that patients with hypertensive emergency are at risk in both the short- and long-term.
Patients with hypertensive urgency are also at increased risk for long-term morbidity and mortality. The one-year mortality for those experiencing an episode of hypertensive urgency is approximately 9%.6 Given the concerns about poor outcomes, it remains a common practice in many facilities to acutely lower the blood pressure in patients with hypertensive urgency. This is highlighted by recommendations of a commonly used point-of-care medical resource, which suggests that "potential legal ramifications partially motivate lowering the blood pressure over several hours."7
WHY TREATING HYPERTENSIVE URGENCY IS UNNECESSARY AND POTENTIALLY HARMFUL
Concerns regarding overtreatment of hypertensive urgency relate to overestimated rates of hypertensive complications, the pathophysiology of hypertension itself, and the potential for adverse events related to treatment. Given that there are few trials examining hospitalized patients with hypertensive urgency, much of the data supporting a conservative approach are drawn from studies of outpatients or emergency department patients. In addition, there is little data suggesting that outcomes are different for patients presenting with a chief complaint of hypertensive urgency and those presenting with an alternate diagnosis but who are found to have blood pressures that meet the threshold for diagnosis of hypertensive urgency.
The landmark 1967 Veterans Affairs Cooperative Trial demonstrated the long-term benefits of treating patients with chronic hypertensive urgency.8 Importantly though, benefits accrued over a period of months to years, not hours. The time to the first adverse event in the placebo arm was two months, suggesting that even those with blood pressures chronically in the range of hypertensive urgency are unlikely to experience hyperacute (ie, within hours) events, even without treatment.
A more recent study, conducted by Patel et al., examined 58,836 patients seen in outpatient clinics and found to have blood pressures meeting the criteria for hypertensive urgency.9 This study included patients whose primary issue was hypertensive urgency and patients in whom the diagnosis was secondary. A total of 426 patients were referred to the hospital and only 100 (0.17%) were subsequently admitted. At seven days, the rates of the primary outcome (a composite of myocardial infarction, stroke, and/or transient ischemic attack) were 0.1% in those sent home and 0.5% in those sent to the hospital. In those patients with a systolic blood pressure ≥220 mm Hg, two out of 977 (0.2%) of those sent home and zero out of 81 of those sent to the hospital experienced the primary outcome. These data reinforce the message that, in patients with hypertensive urgency, rates of adverse events at seven days are low, even with extreme blood pressure elevation.
The human body has adapted to withstand wide variations in blood pressure.10 For example, through arteriolar constriction and reflex vasodilation, cerebral autoregulation maintains a constant cerebral blood flow within a wide range of perfusion pressures, ensuring that the brain is protected from higher mean arterial pressures.11 While this process is protective, over time the autoregulatory system becomes impaired, especially in patients with cerebrovascular disease. This places patients at risk for cerebral and/or cardiac ischemia with even slight drops in perfusion pressure.12,13 Indeed, in assessing treatment-related adverse events in a series of patients treated with intravenous nicardipine or nitroprusside for hypertensive emergency, Brooks and colleagues reported that 57% (27 of 47) of patients had overly large reductions in blood pressure (>25% reduction in mean arterial pressure) within the first 30 minutes of treatment.14 Two patients had acute ischemic events attributed to treatment with antihypertensive medications. Myocardial infarction and stroke have both been reported,12 and medication classes such as calcium channel blockers (sublingual nifedipine in particular), beta-blockers (eg, labetolol), angiotensin-converting-enzyme inhibitors (eg, captopril), and clonidine have all been implicated in treatment-related adverse events.12,15-17 Another potential issue derives from the observation that blood pressures obtained in the hospital setting are often inaccurate, owing to inappropriate patient preparation, faulty equipment, and inadequate training of staff obtaining the measurement.18
National guidelines support a cautious approach to the treatment of hypertensive urgency. The seventh Report of the Joint National Committee on Detection, Evaluation, and Treatment of Hypertension, published in 2003, noted that "patients with markedly elevated BP but without acute target-organ damage usually do not require hospitalization, but they should receive immediate combination oral antihypertensive therapy" and that "there is no evidence to suggest that failure to aggressively lower BP in the [emergency department] is associated with any increased short-term risk to the patient who presents with severe hypertension." JNC 7 also laments contemporary terminology: "Unfortunately, the term 'urgency' has led to overly aggressive management of many patients with severe, uncomplicated hypertension. Aggressive dosing with intravenous drugs or even oral agents, to rapidly lower BP is not without risk."19 The most recent JNC guideline does not comment on hypertensive urgency,20 and the 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults argues that, "¬there is no indication for referral to the emergency department, immediate reduction in BP in the emergency department, or hospitalization for [patients with hypertensive urgency]."21
WHAT CLINICIANS SHOULD DO INSTEAD
After it is confirmed that a patient has no end-organ damage (ie, the patient has hypertensive urgency, not emergency), treatable causes of hypertension should be assessed. In hospitalized patients, these include missed or held doses of outpatient medications, pain, nausea, alcohol and/or benzodiazepine withdrawal, delirium, and obstructive sleep apnea.22 If no remediable cause is identified, patients should be allowed to rest for at least 30 minutes without the administration of additional antihypertensive medications, after which time the blood pressure should be measured using the correct technique.2 Clinical trials have shown that rest is effective at lowering blood pressure in patients with hypertensive urgency.23,24 One study initially treated 549 emergency department patients with a 30-minute rest period, after which time 32% of patients had responded (defined as a SBP <180 mm Hg and DBP <110 mm Hg, with at least a 20 mm Hg reduction in baseline SBP and/or a 10 mm Hg reduction in DBP).23 Another study randomized 138 patients with hypertensive urgency to either rest or active treatment with telmisartan. Blood pressures were checked every 30 minutes for four hours. The primary endpoint (reduction of MAP of 10%-35%) was similar in both groups (68.5% in the rest group and 69.1% in the telmisartan group).24 Even if rest is ineffective, the risk-benefit ratio of acutely lowering blood pressure will typically favor withholding acute treatment in asymptomatic patients. If blood pressure remains consistently elevated, augmentation of the home regimen (eg, increasing the dose of their next scheduled antihypertensive) of oral medications may be warranted. Though not all agree with management of antihypertensives in hospitalized patients,25 acute hospitalizations afford an opportunity to modify and observe chronic hypertension.26
RECOMMENDATIONS
- Ensure that patients do not have symptoms and/or signs of end-organ damage. This can be done with a brief review of systems and a physical examination. In select cases, an electrocardiogram and a chest x-ray may be warranted.
- Search for common causes of treatable hypertension in hospitalized patients; these include pain, nausea, withdrawal syndromes, and holding of usual antihypertensive medications.
- In those patients without symptoms and/or signs of end-organ damage, allow rest, followed by reassessment.
- Do not administer intravenous or immediate-acting oral antihypertensive medications to acutely lower blood pressure. Instead, address the issues raised in Recommendation #2 and consider modifying the chronic oral antihypertensive regimen in patients who are uncontrolled as outpatients or who are not treated as outpatients. Coordinate early postdischarge follow-up for repeat blood pressure evaluation and continued modification of a patient's chronic antihypertensive regimen.
CONCLUSION
Although patients with hypertensive urgency are often treated with medications to acutely lower their blood pressure, there is no evidence to support this practice, and a strong pathophysiologic basis suggests that harm may result. The patient in the case described above should be allowed to rest for at least 30 minutes, with reevaluation of his blood pressure. If it remains elevated and no treatable secondary causes are found, the treating hospitalist should consider altering his chronic antihypertensive regimen to promote long-term blood pressure control.
Do you think this is a low-value practice? Is this truly a "Thing We Do for No Reason?" Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other "Things We Do for No Reason" topics by emailing [email protected].
Disclosures
The authors have no conflicts of interest.
1. Shorr AF, Zilberberg MD, Sun X, et al. Severe acute hypertension among inpatients admitted from the emergency department. J Hosp Med. 2012;7(3):203-210. doi: 10.1002/jhm.969. PubMed
2. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the Prevention, detection, evaluation, and management of High blood pressure in adults: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2017. PubMed
3. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
4. Axon RN, Garrell R, Pfahl K, et al. Attitudes and practices of resident physicians regarding hypertension in the inpatient setting. J Clin Hypertens (Greenwich). 2010;12(9):698-705. doi: 10.1111/j.1751-7176.2010.00309.x. PubMed
5. Keith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Med Sci. 1974;268(6):336-345. doi: 10.1097/00000441-197412000-00004. PubMed
6. Guiga H, Decroux C, Michelet P, et al. Hospital and out-of-hospital mortality in 670 hypertensive emergencies and urgencies. J Clin Hypertens (Greenwich). 2017;19(11):1137-1142. doi: 10.1111/jch.13083. PubMed
7. Varon J, Williams EJ. Management of severe asymptomatic hypertension (hypertensive urgencies) in adults. In: Post T, ed. UpToDate, Waltham, MA. (Accessed February 13, 2018). PubMed
8. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202(11):1028-1034. soi: 10.1001/jama.1967.03130240070013 PubMed
9. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509. PubMed
10. MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58(3):785-790. doi: 10.1152/jappl.1985.58.3.785. PubMed
11. Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. Br Med J. 1973;1(5852):507-510. doi: 10.1136/bmj.1.5852.507. PubMed
12. Fischberg GM, Lozano E, Rajamani K, Ameriso S, Fisher MJ. Stroke precipitated by moderate blood pressure reduction. J Emerg Med. 2000;19(4):339-346. doi: 10.1016/S0736-4679(00)00267-5. PubMed
13. Ross RS. Pathophysiology of coronary circulation. Br Heart J. 1971;33(2):173-184. doi: 10.1136/hrt.33.2.173. PubMed
14. Brooks TW, Finch CK, Lobo BL, Deaton PR, Varner CF. Blood pressure management in acute hypertensive emergency. Am J Health Syst Pharm. 2007;64(24):2579-2582. doi: 10.2146/ajhp070105. PubMed
15. Grossman E, Messerli FH, Grodzicki T, Kowey P. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-1331. doi: 10.1001/jama.1996.03540160050032 PubMed
16. Hodsman GP, Isles CG, Murray GD et al. Factors related to first dose hypotensive effect of captopril: prediction and treatment. Br Med J (Clin Res Ed). 1983;286(6368):832-834. doi: 10.1136/bmj.286.6368.832. PubMed
17. Zeller KR, Von Kuhnert L, Matthews C. Rapid reduction of severe asymptomatic hypertension. A prospective, controlled trial. Arch Intern Med. 1989;149(10):2186-2189. doi: 10.1001/archinte.149.10.2186. PubMed
18. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697-716. doi: 10.1161/01.CIR.0000154900.76284.F6. PubMed
19. Chobanian AV, Bakris GL, Black HR, et al. The seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. doi: 10.1001/jama.289.19.2560. PubMed
20. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427 PubMed
21. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the Prevention, detection, evaluation, and management of High blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017. PubMed
22. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94. doi: 10.1007/s11886-015-0648-y. PubMed
23. Grassi D, O'Flaherty M, Pellizzari M, et al. Hypertensive urgencies in the emergency department: evaluating blood pressure response to rest and to antihypertensive drugs with different profiles. J Clin Hypertens (Greenwich). 2008;10(9):662-667. doi: 10.1111/j.1751-7176.2008.00001.x. PubMed
24. Park SK, Lee DY, Kim WJ, et al. Comparing the clinical efficacy of resting and antihypertensive medication in patients of hypertensive urgency: a randomized, control trial. J Hypertens. 2017;35(7):1474-1480. doi: 10.1097/HJH.0000000000001340. PubMed
25. Steinman MA, Auerbach AD. Managing chronic disease in hospitalized patients. JAMA Intern Med. 2013;173(20):1857-1858. doi: 10.1001/jamainternmed.2013.9511. PubMed
26. Breu AC, Allen-Dicker J, Mueller S et al. Hospitalist and primary care physician perspectives on medication management of chronic conditions for hospitalized patients. J Hosp Med. 2014;9(5):303-309. doi: 10.1002/jhm.2137. PubMed
The "Things We Do for No Reason" (TWDFNR) series reviews practices which have become common parts of hospital care but provide little value to our patients. Practices reviewed in the TWDFNR series do not represent "black and white" conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 67-year-old man is hospitalized with community-acquired pneumonia. He has a history of hypertension and is prescribed two antihypertensive medications (amlodipine and chlorthalidone) as an outpatient. On the evening of hospital day two, he is found to have a blood pressure of 192/95 on a scheduled vital signs check. He reports no symptoms other than cough, which is not new or worsening. The covering hospitalist reviews the documented blood pressures since admission and notes that many have been elevated despite continuation of his home regimen. The patient's nurse inquires about treating the patient with additional "as-needed" antihypertensive medications.
BACKGROUND
Hypertensive crises are common in hospitalized patients, with approximately one in seven patients experiencing an episode of hypertensive emergency and/or hypertensive urgency.1 Hypertensive emergency is typically defined as (1) a systolic blood pressure ≥180 mm Hg and/or a diastolic blood pressure ≥120 mm Hg with (2) evidence of new or worsening end-organ damage. The organs most commonly affected by severe hypertension are the brain (headache, confusion, stroke), heart (chest pain, myocardial infarction, pulmonary edema), large blood vessels (aortic dissection), and kidneys (acute hypertensive nephrosclerosis).2 With hypertensive urgency, patients experience similarly elevated blood pressure but have no symptoms or signs suggesting acute end-organ damage. Acute treatment with intravenous (IV) or immediate-acting oral medications is common; a single-center study showed that 7.4% of hospitalized patients had an order for "as needed" IV hydralazine or labetalol, with 60.3% receiving at least one dose.3 Among internal medicine and family medicine trainees in one survey, nearly half reported that they would use IV medications in a scenario where an inpatient had an asymptomatic blood pressure above 180 mm Hg.4
WHY YOU MIGHT THINK TREATING HYPERTENSIVE URGENCY IS NECESSARY
Treating patients with hypertensive urgency is based on an assumption: If one does not treat immediately, something bad (ie, end-organ damage) will occur over the next few hours. Data from the 1930s showed that patients with untreated hypertensive emergency had a one-year mortality rate >79% and a median survival of 10.4 months.5 More recent studies suggest that the in-hospital and one-year mortality for those with hypertensive emergency are 13% and 39%, respectively.6 These data demonstrate that patients with hypertensive emergency are at risk in both the short- and long-term.
Patients with hypertensive urgency are also at increased risk for long-term morbidity and mortality. The one-year mortality for those experiencing an episode of hypertensive urgency is approximately 9%.6 Given the concerns about poor outcomes, it remains a common practice in many facilities to acutely lower the blood pressure in patients with hypertensive urgency. This is highlighted by recommendations of a commonly used point-of-care medical resource, which suggests that "potential legal ramifications partially motivate lowering the blood pressure over several hours."7
WHY TREATING HYPERTENSIVE URGENCY IS UNNECESSARY AND POTENTIALLY HARMFUL
Concerns regarding overtreatment of hypertensive urgency relate to overestimated rates of hypertensive complications, the pathophysiology of hypertension itself, and the potential for adverse events related to treatment. Given that there are few trials examining hospitalized patients with hypertensive urgency, much of the data supporting a conservative approach are drawn from studies of outpatients or emergency department patients. In addition, there is little data suggesting that outcomes are different for patients presenting with a chief complaint of hypertensive urgency and those presenting with an alternate diagnosis but who are found to have blood pressures that meet the threshold for diagnosis of hypertensive urgency.
The landmark 1967 Veterans Affairs Cooperative Trial demonstrated the long-term benefits of treating patients with chronic hypertensive urgency.8 Importantly though, benefits accrued over a period of months to years, not hours. The time to the first adverse event in the placebo arm was two months, suggesting that even those with blood pressures chronically in the range of hypertensive urgency are unlikely to experience hyperacute (ie, within hours) events, even without treatment.
A more recent study, conducted by Patel et al., examined 58,836 patients seen in outpatient clinics and found to have blood pressures meeting the criteria for hypertensive urgency.9 This study included patients whose primary issue was hypertensive urgency and patients in whom the diagnosis was secondary. A total of 426 patients were referred to the hospital and only 100 (0.17%) were subsequently admitted. At seven days, the rates of the primary outcome (a composite of myocardial infarction, stroke, and/or transient ischemic attack) were 0.1% in those sent home and 0.5% in those sent to the hospital. In those patients with a systolic blood pressure ≥220 mm Hg, two out of 977 (0.2%) of those sent home and zero out of 81 of those sent to the hospital experienced the primary outcome. These data reinforce the message that, in patients with hypertensive urgency, rates of adverse events at seven days are low, even with extreme blood pressure elevation.
The human body has adapted to withstand wide variations in blood pressure.10 For example, through arteriolar constriction and reflex vasodilation, cerebral autoregulation maintains a constant cerebral blood flow within a wide range of perfusion pressures, ensuring that the brain is protected from higher mean arterial pressures.11 While this process is protective, over time the autoregulatory system becomes impaired, especially in patients with cerebrovascular disease. This places patients at risk for cerebral and/or cardiac ischemia with even slight drops in perfusion pressure.12,13 Indeed, in assessing treatment-related adverse events in a series of patients treated with intravenous nicardipine or nitroprusside for hypertensive emergency, Brooks and colleagues reported that 57% (27 of 47) of patients had overly large reductions in blood pressure (>25% reduction in mean arterial pressure) within the first 30 minutes of treatment.14 Two patients had acute ischemic events attributed to treatment with antihypertensive medications. Myocardial infarction and stroke have both been reported,12 and medication classes such as calcium channel blockers (sublingual nifedipine in particular), beta-blockers (eg, labetolol), angiotensin-converting-enzyme inhibitors (eg, captopril), and clonidine have all been implicated in treatment-related adverse events.12,15-17 Another potential issue derives from the observation that blood pressures obtained in the hospital setting are often inaccurate, owing to inappropriate patient preparation, faulty equipment, and inadequate training of staff obtaining the measurement.18
National guidelines support a cautious approach to the treatment of hypertensive urgency. The seventh Report of the Joint National Committee on Detection, Evaluation, and Treatment of Hypertension, published in 2003, noted that "patients with markedly elevated BP but without acute target-organ damage usually do not require hospitalization, but they should receive immediate combination oral antihypertensive therapy" and that "there is no evidence to suggest that failure to aggressively lower BP in the [emergency department] is associated with any increased short-term risk to the patient who presents with severe hypertension." JNC 7 also laments contemporary terminology: "Unfortunately, the term 'urgency' has led to overly aggressive management of many patients with severe, uncomplicated hypertension. Aggressive dosing with intravenous drugs or even oral agents, to rapidly lower BP is not without risk."19 The most recent JNC guideline does not comment on hypertensive urgency,20 and the 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults argues that, "¬there is no indication for referral to the emergency department, immediate reduction in BP in the emergency department, or hospitalization for [patients with hypertensive urgency]."21
WHAT CLINICIANS SHOULD DO INSTEAD
After it is confirmed that a patient has no end-organ damage (ie, the patient has hypertensive urgency, not emergency), treatable causes of hypertension should be assessed. In hospitalized patients, these include missed or held doses of outpatient medications, pain, nausea, alcohol and/or benzodiazepine withdrawal, delirium, and obstructive sleep apnea.22 If no remediable cause is identified, patients should be allowed to rest for at least 30 minutes without the administration of additional antihypertensive medications, after which time the blood pressure should be measured using the correct technique.2 Clinical trials have shown that rest is effective at lowering blood pressure in patients with hypertensive urgency.23,24 One study initially treated 549 emergency department patients with a 30-minute rest period, after which time 32% of patients had responded (defined as a SBP <180 mm Hg and DBP <110 mm Hg, with at least a 20 mm Hg reduction in baseline SBP and/or a 10 mm Hg reduction in DBP).23 Another study randomized 138 patients with hypertensive urgency to either rest or active treatment with telmisartan. Blood pressures were checked every 30 minutes for four hours. The primary endpoint (reduction of MAP of 10%-35%) was similar in both groups (68.5% in the rest group and 69.1% in the telmisartan group).24 Even if rest is ineffective, the risk-benefit ratio of acutely lowering blood pressure will typically favor withholding acute treatment in asymptomatic patients. If blood pressure remains consistently elevated, augmentation of the home regimen (eg, increasing the dose of their next scheduled antihypertensive) of oral medications may be warranted. Though not all agree with management of antihypertensives in hospitalized patients,25 acute hospitalizations afford an opportunity to modify and observe chronic hypertension.26
RECOMMENDATIONS
- Ensure that patients do not have symptoms and/or signs of end-organ damage. This can be done with a brief review of systems and a physical examination. In select cases, an electrocardiogram and a chest x-ray may be warranted.
- Search for common causes of treatable hypertension in hospitalized patients; these include pain, nausea, withdrawal syndromes, and holding of usual antihypertensive medications.
- In those patients without symptoms and/or signs of end-organ damage, allow rest, followed by reassessment.
- Do not administer intravenous or immediate-acting oral antihypertensive medications to acutely lower blood pressure. Instead, address the issues raised in Recommendation #2 and consider modifying the chronic oral antihypertensive regimen in patients who are uncontrolled as outpatients or who are not treated as outpatients. Coordinate early postdischarge follow-up for repeat blood pressure evaluation and continued modification of a patient's chronic antihypertensive regimen.
CONCLUSION
Although patients with hypertensive urgency are often treated with medications to acutely lower their blood pressure, there is no evidence to support this practice, and a strong pathophysiologic basis suggests that harm may result. The patient in the case described above should be allowed to rest for at least 30 minutes, with reevaluation of his blood pressure. If it remains elevated and no treatable secondary causes are found, the treating hospitalist should consider altering his chronic antihypertensive regimen to promote long-term blood pressure control.
Do you think this is a low-value practice? Is this truly a "Thing We Do for No Reason?" Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other "Things We Do for No Reason" topics by emailing [email protected].
Disclosures
The authors have no conflicts of interest.
The "Things We Do for No Reason" (TWDFNR) series reviews practices which have become common parts of hospital care but provide little value to our patients. Practices reviewed in the TWDFNR series do not represent "black and white" conclusions or clinical practice standards but are meant as a starting place for research and active discussions among hospitalists and patients. We invite you to be part of that discussion. https://www.choosingwisely.org/

CLINICAL SCENARIO
A 67-year-old man is hospitalized with community-acquired pneumonia. He has a history of hypertension and is prescribed two antihypertensive medications (amlodipine and chlorthalidone) as an outpatient. On the evening of hospital day two, he is found to have a blood pressure of 192/95 on a scheduled vital signs check. He reports no symptoms other than cough, which is not new or worsening. The covering hospitalist reviews the documented blood pressures since admission and notes that many have been elevated despite continuation of his home regimen. The patient's nurse inquires about treating the patient with additional "as-needed" antihypertensive medications.
BACKGROUND
Hypertensive crises are common in hospitalized patients, with approximately one in seven patients experiencing an episode of hypertensive emergency and/or hypertensive urgency.1 Hypertensive emergency is typically defined as (1) a systolic blood pressure ≥180 mm Hg and/or a diastolic blood pressure ≥120 mm Hg with (2) evidence of new or worsening end-organ damage. The organs most commonly affected by severe hypertension are the brain (headache, confusion, stroke), heart (chest pain, myocardial infarction, pulmonary edema), large blood vessels (aortic dissection), and kidneys (acute hypertensive nephrosclerosis).2 With hypertensive urgency, patients experience similarly elevated blood pressure but have no symptoms or signs suggesting acute end-organ damage. Acute treatment with intravenous (IV) or immediate-acting oral medications is common; a single-center study showed that 7.4% of hospitalized patients had an order for "as needed" IV hydralazine or labetalol, with 60.3% receiving at least one dose.3 Among internal medicine and family medicine trainees in one survey, nearly half reported that they would use IV medications in a scenario where an inpatient had an asymptomatic blood pressure above 180 mm Hg.4
WHY YOU MIGHT THINK TREATING HYPERTENSIVE URGENCY IS NECESSARY
Treating patients with hypertensive urgency is based on an assumption: If one does not treat immediately, something bad (ie, end-organ damage) will occur over the next few hours. Data from the 1930s showed that patients with untreated hypertensive emergency had a one-year mortality rate >79% and a median survival of 10.4 months.5 More recent studies suggest that the in-hospital and one-year mortality for those with hypertensive emergency are 13% and 39%, respectively.6 These data demonstrate that patients with hypertensive emergency are at risk in both the short- and long-term.
Patients with hypertensive urgency are also at increased risk for long-term morbidity and mortality. The one-year mortality for those experiencing an episode of hypertensive urgency is approximately 9%.6 Given the concerns about poor outcomes, it remains a common practice in many facilities to acutely lower the blood pressure in patients with hypertensive urgency. This is highlighted by recommendations of a commonly used point-of-care medical resource, which suggests that "potential legal ramifications partially motivate lowering the blood pressure over several hours."7
WHY TREATING HYPERTENSIVE URGENCY IS UNNECESSARY AND POTENTIALLY HARMFUL
Concerns regarding overtreatment of hypertensive urgency relate to overestimated rates of hypertensive complications, the pathophysiology of hypertension itself, and the potential for adverse events related to treatment. Given that there are few trials examining hospitalized patients with hypertensive urgency, much of the data supporting a conservative approach are drawn from studies of outpatients or emergency department patients. In addition, there is little data suggesting that outcomes are different for patients presenting with a chief complaint of hypertensive urgency and those presenting with an alternate diagnosis but who are found to have blood pressures that meet the threshold for diagnosis of hypertensive urgency.
The landmark 1967 Veterans Affairs Cooperative Trial demonstrated the long-term benefits of treating patients with chronic hypertensive urgency.8 Importantly though, benefits accrued over a period of months to years, not hours. The time to the first adverse event in the placebo arm was two months, suggesting that even those with blood pressures chronically in the range of hypertensive urgency are unlikely to experience hyperacute (ie, within hours) events, even without treatment.
A more recent study, conducted by Patel et al., examined 58,836 patients seen in outpatient clinics and found to have blood pressures meeting the criteria for hypertensive urgency.9 This study included patients whose primary issue was hypertensive urgency and patients in whom the diagnosis was secondary. A total of 426 patients were referred to the hospital and only 100 (0.17%) were subsequently admitted. At seven days, the rates of the primary outcome (a composite of myocardial infarction, stroke, and/or transient ischemic attack) were 0.1% in those sent home and 0.5% in those sent to the hospital. In those patients with a systolic blood pressure ≥220 mm Hg, two out of 977 (0.2%) of those sent home and zero out of 81 of those sent to the hospital experienced the primary outcome. These data reinforce the message that, in patients with hypertensive urgency, rates of adverse events at seven days are low, even with extreme blood pressure elevation.
The human body has adapted to withstand wide variations in blood pressure.10 For example, through arteriolar constriction and reflex vasodilation, cerebral autoregulation maintains a constant cerebral blood flow within a wide range of perfusion pressures, ensuring that the brain is protected from higher mean arterial pressures.11 While this process is protective, over time the autoregulatory system becomes impaired, especially in patients with cerebrovascular disease. This places patients at risk for cerebral and/or cardiac ischemia with even slight drops in perfusion pressure.12,13 Indeed, in assessing treatment-related adverse events in a series of patients treated with intravenous nicardipine or nitroprusside for hypertensive emergency, Brooks and colleagues reported that 57% (27 of 47) of patients had overly large reductions in blood pressure (>25% reduction in mean arterial pressure) within the first 30 minutes of treatment.14 Two patients had acute ischemic events attributed to treatment with antihypertensive medications. Myocardial infarction and stroke have both been reported,12 and medication classes such as calcium channel blockers (sublingual nifedipine in particular), beta-blockers (eg, labetolol), angiotensin-converting-enzyme inhibitors (eg, captopril), and clonidine have all been implicated in treatment-related adverse events.12,15-17 Another potential issue derives from the observation that blood pressures obtained in the hospital setting are often inaccurate, owing to inappropriate patient preparation, faulty equipment, and inadequate training of staff obtaining the measurement.18
National guidelines support a cautious approach to the treatment of hypertensive urgency. The seventh Report of the Joint National Committee on Detection, Evaluation, and Treatment of Hypertension, published in 2003, noted that "patients with markedly elevated BP but without acute target-organ damage usually do not require hospitalization, but they should receive immediate combination oral antihypertensive therapy" and that "there is no evidence to suggest that failure to aggressively lower BP in the [emergency department] is associated with any increased short-term risk to the patient who presents with severe hypertension." JNC 7 also laments contemporary terminology: "Unfortunately, the term 'urgency' has led to overly aggressive management of many patients with severe, uncomplicated hypertension. Aggressive dosing with intravenous drugs or even oral agents, to rapidly lower BP is not without risk."19 The most recent JNC guideline does not comment on hypertensive urgency,20 and the 2017 American College of Cardiology/American Heart Association Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults argues that, "¬there is no indication for referral to the emergency department, immediate reduction in BP in the emergency department, or hospitalization for [patients with hypertensive urgency]."21
WHAT CLINICIANS SHOULD DO INSTEAD
After it is confirmed that a patient has no end-organ damage (ie, the patient has hypertensive urgency, not emergency), treatable causes of hypertension should be assessed. In hospitalized patients, these include missed or held doses of outpatient medications, pain, nausea, alcohol and/or benzodiazepine withdrawal, delirium, and obstructive sleep apnea.22 If no remediable cause is identified, patients should be allowed to rest for at least 30 minutes without the administration of additional antihypertensive medications, after which time the blood pressure should be measured using the correct technique.2 Clinical trials have shown that rest is effective at lowering blood pressure in patients with hypertensive urgency.23,24 One study initially treated 549 emergency department patients with a 30-minute rest period, after which time 32% of patients had responded (defined as a SBP <180 mm Hg and DBP <110 mm Hg, with at least a 20 mm Hg reduction in baseline SBP and/or a 10 mm Hg reduction in DBP).23 Another study randomized 138 patients with hypertensive urgency to either rest or active treatment with telmisartan. Blood pressures were checked every 30 minutes for four hours. The primary endpoint (reduction of MAP of 10%-35%) was similar in both groups (68.5% in the rest group and 69.1% in the telmisartan group).24 Even if rest is ineffective, the risk-benefit ratio of acutely lowering blood pressure will typically favor withholding acute treatment in asymptomatic patients. If blood pressure remains consistently elevated, augmentation of the home regimen (eg, increasing the dose of their next scheduled antihypertensive) of oral medications may be warranted. Though not all agree with management of antihypertensives in hospitalized patients,25 acute hospitalizations afford an opportunity to modify and observe chronic hypertension.26
RECOMMENDATIONS
- Ensure that patients do not have symptoms and/or signs of end-organ damage. This can be done with a brief review of systems and a physical examination. In select cases, an electrocardiogram and a chest x-ray may be warranted.
- Search for common causes of treatable hypertension in hospitalized patients; these include pain, nausea, withdrawal syndromes, and holding of usual antihypertensive medications.
- In those patients without symptoms and/or signs of end-organ damage, allow rest, followed by reassessment.
- Do not administer intravenous or immediate-acting oral antihypertensive medications to acutely lower blood pressure. Instead, address the issues raised in Recommendation #2 and consider modifying the chronic oral antihypertensive regimen in patients who are uncontrolled as outpatients or who are not treated as outpatients. Coordinate early postdischarge follow-up for repeat blood pressure evaluation and continued modification of a patient's chronic antihypertensive regimen.
CONCLUSION
Although patients with hypertensive urgency are often treated with medications to acutely lower their blood pressure, there is no evidence to support this practice, and a strong pathophysiologic basis suggests that harm may result. The patient in the case described above should be allowed to rest for at least 30 minutes, with reevaluation of his blood pressure. If it remains elevated and no treatable secondary causes are found, the treating hospitalist should consider altering his chronic antihypertensive regimen to promote long-term blood pressure control.
Do you think this is a low-value practice? Is this truly a "Thing We Do for No Reason?" Share what you do in your practice and join in the conversation online by retweeting it on Twitter (#TWDFNR) and liking it on Facebook. We invite you to propose ideas for other "Things We Do for No Reason" topics by emailing [email protected].
Disclosures
The authors have no conflicts of interest.
1. Shorr AF, Zilberberg MD, Sun X, et al. Severe acute hypertension among inpatients admitted from the emergency department. J Hosp Med. 2012;7(3):203-210. doi: 10.1002/jhm.969. PubMed
2. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the Prevention, detection, evaluation, and management of High blood pressure in adults: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2017. PubMed
3. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
4. Axon RN, Garrell R, Pfahl K, et al. Attitudes and practices of resident physicians regarding hypertension in the inpatient setting. J Clin Hypertens (Greenwich). 2010;12(9):698-705. doi: 10.1111/j.1751-7176.2010.00309.x. PubMed
5. Keith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Med Sci. 1974;268(6):336-345. doi: 10.1097/00000441-197412000-00004. PubMed
6. Guiga H, Decroux C, Michelet P, et al. Hospital and out-of-hospital mortality in 670 hypertensive emergencies and urgencies. J Clin Hypertens (Greenwich). 2017;19(11):1137-1142. doi: 10.1111/jch.13083. PubMed
7. Varon J, Williams EJ. Management of severe asymptomatic hypertension (hypertensive urgencies) in adults. In: Post T, ed. UpToDate, Waltham, MA. (Accessed February 13, 2018). PubMed
8. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202(11):1028-1034. soi: 10.1001/jama.1967.03130240070013 PubMed
9. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509. PubMed
10. MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58(3):785-790. doi: 10.1152/jappl.1985.58.3.785. PubMed
11. Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. Br Med J. 1973;1(5852):507-510. doi: 10.1136/bmj.1.5852.507. PubMed
12. Fischberg GM, Lozano E, Rajamani K, Ameriso S, Fisher MJ. Stroke precipitated by moderate blood pressure reduction. J Emerg Med. 2000;19(4):339-346. doi: 10.1016/S0736-4679(00)00267-5. PubMed
13. Ross RS. Pathophysiology of coronary circulation. Br Heart J. 1971;33(2):173-184. doi: 10.1136/hrt.33.2.173. PubMed
14. Brooks TW, Finch CK, Lobo BL, Deaton PR, Varner CF. Blood pressure management in acute hypertensive emergency. Am J Health Syst Pharm. 2007;64(24):2579-2582. doi: 10.2146/ajhp070105. PubMed
15. Grossman E, Messerli FH, Grodzicki T, Kowey P. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-1331. doi: 10.1001/jama.1996.03540160050032 PubMed
16. Hodsman GP, Isles CG, Murray GD et al. Factors related to first dose hypotensive effect of captopril: prediction and treatment. Br Med J (Clin Res Ed). 1983;286(6368):832-834. doi: 10.1136/bmj.286.6368.832. PubMed
17. Zeller KR, Von Kuhnert L, Matthews C. Rapid reduction of severe asymptomatic hypertension. A prospective, controlled trial. Arch Intern Med. 1989;149(10):2186-2189. doi: 10.1001/archinte.149.10.2186. PubMed
18. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697-716. doi: 10.1161/01.CIR.0000154900.76284.F6. PubMed
19. Chobanian AV, Bakris GL, Black HR, et al. The seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. doi: 10.1001/jama.289.19.2560. PubMed
20. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427 PubMed
21. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the Prevention, detection, evaluation, and management of High blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017. PubMed
22. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94. doi: 10.1007/s11886-015-0648-y. PubMed
23. Grassi D, O'Flaherty M, Pellizzari M, et al. Hypertensive urgencies in the emergency department: evaluating blood pressure response to rest and to antihypertensive drugs with different profiles. J Clin Hypertens (Greenwich). 2008;10(9):662-667. doi: 10.1111/j.1751-7176.2008.00001.x. PubMed
24. Park SK, Lee DY, Kim WJ, et al. Comparing the clinical efficacy of resting and antihypertensive medication in patients of hypertensive urgency: a randomized, control trial. J Hypertens. 2017;35(7):1474-1480. doi: 10.1097/HJH.0000000000001340. PubMed
25. Steinman MA, Auerbach AD. Managing chronic disease in hospitalized patients. JAMA Intern Med. 2013;173(20):1857-1858. doi: 10.1001/jamainternmed.2013.9511. PubMed
26. Breu AC, Allen-Dicker J, Mueller S et al. Hospitalist and primary care physician perspectives on medication management of chronic conditions for hospitalized patients. J Hosp Med. 2014;9(5):303-309. doi: 10.1002/jhm.2137. PubMed
1. Shorr AF, Zilberberg MD, Sun X, et al. Severe acute hypertension among inpatients admitted from the emergency department. J Hosp Med. 2012;7(3):203-210. doi: 10.1002/jhm.969. PubMed
2. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the Prevention, detection, evaluation, and management of High blood pressure in adults: A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Hypertension. 2017. PubMed
3. Weder AB, Erickson S. Treatment of hypertension in the inpatient setting: use of intravenous labetalol and hydralazine. J Clin Hypertens (Greenwich). 2010;12(1):29-33. doi: 10.1111/j.1751-7176.2009.00196.x. PubMed
4. Axon RN, Garrell R, Pfahl K, et al. Attitudes and practices of resident physicians regarding hypertension in the inpatient setting. J Clin Hypertens (Greenwich). 2010;12(9):698-705. doi: 10.1111/j.1751-7176.2010.00309.x. PubMed
5. Keith NM, Wagener HP, Barker NW. Some different types of essential hypertension: their course and prognosis. Am J Med Sci. 1974;268(6):336-345. doi: 10.1097/00000441-197412000-00004. PubMed
6. Guiga H, Decroux C, Michelet P, et al. Hospital and out-of-hospital mortality in 670 hypertensive emergencies and urgencies. J Clin Hypertens (Greenwich). 2017;19(11):1137-1142. doi: 10.1111/jch.13083. PubMed
7. Varon J, Williams EJ. Management of severe asymptomatic hypertension (hypertensive urgencies) in adults. In: Post T, ed. UpToDate, Waltham, MA. (Accessed February 13, 2018). PubMed
8. Effects of treatment on morbidity in hypertension. Results in patients with diastolic blood pressures averaging 115 through 129 mm Hg. JAMA. 1967;202(11):1028-1034. soi: 10.1001/jama.1967.03130240070013 PubMed
9. Patel KK, Young L, Howell EH, et al. Characteristics and outcomes of patients presenting with hypertensive urgency in the office setting. JAMA Intern Med. 2016;176(7):981-988. doi: 10.1001/jamainternmed.2016.1509. PubMed
10. MacDougall JD, Tuxen D, Sale DG, Moroz JR, Sutton JR. Arterial blood pressure response to heavy resistance exercise. J Appl Physiol. 1985;58(3):785-790. doi: 10.1152/jappl.1985.58.3.785. PubMed
11. Strandgaard S, Olesen J, Skinhoj E, Lassen NA. Autoregulation of brain circulation in severe arterial hypertension. Br Med J. 1973;1(5852):507-510. doi: 10.1136/bmj.1.5852.507. PubMed
12. Fischberg GM, Lozano E, Rajamani K, Ameriso S, Fisher MJ. Stroke precipitated by moderate blood pressure reduction. J Emerg Med. 2000;19(4):339-346. doi: 10.1016/S0736-4679(00)00267-5. PubMed
13. Ross RS. Pathophysiology of coronary circulation. Br Heart J. 1971;33(2):173-184. doi: 10.1136/hrt.33.2.173. PubMed
14. Brooks TW, Finch CK, Lobo BL, Deaton PR, Varner CF. Blood pressure management in acute hypertensive emergency. Am J Health Syst Pharm. 2007;64(24):2579-2582. doi: 10.2146/ajhp070105. PubMed
15. Grossman E, Messerli FH, Grodzicki T, Kowey P. Should a moratorium be placed on sublingual nifedipine capsules given for hypertensive emergencies and pseudoemergencies? JAMA. 1996;276(16):1328-1331. doi: 10.1001/jama.1996.03540160050032 PubMed
16. Hodsman GP, Isles CG, Murray GD et al. Factors related to first dose hypotensive effect of captopril: prediction and treatment. Br Med J (Clin Res Ed). 1983;286(6368):832-834. doi: 10.1136/bmj.286.6368.832. PubMed
17. Zeller KR, Von Kuhnert L, Matthews C. Rapid reduction of severe asymptomatic hypertension. A prospective, controlled trial. Arch Intern Med. 1989;149(10):2186-2189. doi: 10.1001/archinte.149.10.2186. PubMed
18. Pickering TG, Hall JE, Appel LJ, et al. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: Blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Circulation. 2005;111(5):697-716. doi: 10.1161/01.CIR.0000154900.76284.F6. PubMed
19. Chobanian AV, Bakris GL, Black HR, et al. The seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High blood pressure: the JNC 7 report. JAMA. 2003;289(19):2560-2572. doi: 10.1001/jama.289.19.2560. PubMed
20. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311(5):507-520. doi: 10.1001/jama.2013.284427 PubMed
21. Whelton PK, Carey RM, Aronow WS, et al. ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the Prevention, detection, evaluation, and management of High blood pressure in adults: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2017. PubMed
22. Axon RN, Turner M, Buckley R. An update on inpatient hypertension management. Curr Cardiol Rep. 2015;17(11):94. doi: 10.1007/s11886-015-0648-y. PubMed
23. Grassi D, O'Flaherty M, Pellizzari M, et al. Hypertensive urgencies in the emergency department: evaluating blood pressure response to rest and to antihypertensive drugs with different profiles. J Clin Hypertens (Greenwich). 2008;10(9):662-667. doi: 10.1111/j.1751-7176.2008.00001.x. PubMed
24. Park SK, Lee DY, Kim WJ, et al. Comparing the clinical efficacy of resting and antihypertensive medication in patients of hypertensive urgency: a randomized, control trial. J Hypertens. 2017;35(7):1474-1480. doi: 10.1097/HJH.0000000000001340. PubMed
25. Steinman MA, Auerbach AD. Managing chronic disease in hospitalized patients. JAMA Intern Med. 2013;173(20):1857-1858. doi: 10.1001/jamainternmed.2013.9511. PubMed
26. Breu AC, Allen-Dicker J, Mueller S et al. Hospitalist and primary care physician perspectives on medication management of chronic conditions for hospitalized patients. J Hosp Med. 2014;9(5):303-309. doi: 10.1002/jhm.2137. PubMed
© 2018 Society of Hospital Medicine