User login
Rise in Fatal Drug Overdoses and Drug Misuse- or Abuse-Related ED Visits among Women
Clinical question: How have opioid pain reliever (OPR) prescriptions affected drug misuse or abuse and drug overdose deaths for women in the past decade?
Background: Overdose deaths have increased steadily over the past decade. More men die from drug overdoses, but the percentage of women dying from drug misuse has increased substantially.
Study design: Retrospective analysis.
Setting: Data from the National Vital Statistics System (NVSS) and the Drug Abuse Warning Network (DAWN).
Synopsis: The CDC analyzed death rates based on NVSS multiple causes of death from 1999-2010. Type of drug involved (OPR, cocaine, heroin, benzodiazepines) was based on ICD 10 codes. Analysis showed that deaths from OPRs between 1999 and 2010 increased five-fold in women, compared to 3.6-fold in men.
The CDC also analyzed DAWN data from ED visits by women for drug misuse or abuse between 2004-2010. When compared to data from 2004, the ED visits related to misuse or abuse of OPR among women more than doubled, and the rate of OPR deaths among women increased by 70%.
Limitations of this study include the fact that all drugs used were not identified, and motivation to use was unclear. Also, medical or non-medical reason for use was not always available.
Bottom line: Healthcare providers prescribing OPRs to patients should use their state’s prescription drug monitoring program and regularly screen patients for psychological disorders and use of psychotherapeutic drugs, with or without a prescription.
Citation: Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers and other drugs among women—United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2013;62:537-542.
Clinical question: How have opioid pain reliever (OPR) prescriptions affected drug misuse or abuse and drug overdose deaths for women in the past decade?
Background: Overdose deaths have increased steadily over the past decade. More men die from drug overdoses, but the percentage of women dying from drug misuse has increased substantially.
Study design: Retrospective analysis.
Setting: Data from the National Vital Statistics System (NVSS) and the Drug Abuse Warning Network (DAWN).
Synopsis: The CDC analyzed death rates based on NVSS multiple causes of death from 1999-2010. Type of drug involved (OPR, cocaine, heroin, benzodiazepines) was based on ICD 10 codes. Analysis showed that deaths from OPRs between 1999 and 2010 increased five-fold in women, compared to 3.6-fold in men.
The CDC also analyzed DAWN data from ED visits by women for drug misuse or abuse between 2004-2010. When compared to data from 2004, the ED visits related to misuse or abuse of OPR among women more than doubled, and the rate of OPR deaths among women increased by 70%.
Limitations of this study include the fact that all drugs used were not identified, and motivation to use was unclear. Also, medical or non-medical reason for use was not always available.
Bottom line: Healthcare providers prescribing OPRs to patients should use their state’s prescription drug monitoring program and regularly screen patients for psychological disorders and use of psychotherapeutic drugs, with or without a prescription.
Citation: Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers and other drugs among women—United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2013;62:537-542.
Clinical question: How have opioid pain reliever (OPR) prescriptions affected drug misuse or abuse and drug overdose deaths for women in the past decade?
Background: Overdose deaths have increased steadily over the past decade. More men die from drug overdoses, but the percentage of women dying from drug misuse has increased substantially.
Study design: Retrospective analysis.
Setting: Data from the National Vital Statistics System (NVSS) and the Drug Abuse Warning Network (DAWN).
Synopsis: The CDC analyzed death rates based on NVSS multiple causes of death from 1999-2010. Type of drug involved (OPR, cocaine, heroin, benzodiazepines) was based on ICD 10 codes. Analysis showed that deaths from OPRs between 1999 and 2010 increased five-fold in women, compared to 3.6-fold in men.
The CDC also analyzed DAWN data from ED visits by women for drug misuse or abuse between 2004-2010. When compared to data from 2004, the ED visits related to misuse or abuse of OPR among women more than doubled, and the rate of OPR deaths among women increased by 70%.
Limitations of this study include the fact that all drugs used were not identified, and motivation to use was unclear. Also, medical or non-medical reason for use was not always available.
Bottom line: Healthcare providers prescribing OPRs to patients should use their state’s prescription drug monitoring program and regularly screen patients for psychological disorders and use of psychotherapeutic drugs, with or without a prescription.
Citation: Centers for Disease Control and Prevention (CDC). Vital signs: overdoses of prescription opioid pain relievers and other drugs among women—United States, 1999-2010. MMWR Morb Mortal Wkly Rep. 2013;62:537-542.
“I Cough” Could Reduce Post-Operative Pulmonary Complications among Non-Ventilated Patients
Clinical question: Does the use of a standardized suite of post-operative pulmonary care guidelines decrease the incidence of adverse pulmonary outcomes in non-ventilated patients?
Background: Post-operative pulmonary complications are common and account for high costs and increased length of stay. Best practice guidelines for pulmonary care in general for patients undergoing non-cardiac surgery are scarce, compared to strategies to prevent ventilator-associated pneumonia (VAP).
Study design: Observational study.
Setting: Boston University Medical Center.
Synopsis: The I COUGH program emphasized Incentive spirometry, Coughing and deep breathing, Oral care, Understanding (patient and family education), Getting out of bed at least three times daily, and Head-of-bed elevation.
I COUGH was implemented for one year for all general surgery and vascular surgery patients, and results were compared with the year prior using National Surgical Quality Improvement Program (NSQIP) data. The program reduced the incidence of post-operative pneumonia to 1.6% from 2.6% and the incidence of unplanned intubations to 1.2% from 2.0%. The results did show a trend but did not achieve statistical significance.
Bottom line: Post-operative implementation of I COUGH through consistent education of staff, patients, and family might reduce post-operative pneumonia and unplanned intubations.
Citation: Cassidy MR, Rosenkranz P, McCabe K, Rosen JE, McAneny D. I COUGH: reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg. 2013;148:740-745.
Clinical question: Does the use of a standardized suite of post-operative pulmonary care guidelines decrease the incidence of adverse pulmonary outcomes in non-ventilated patients?
Background: Post-operative pulmonary complications are common and account for high costs and increased length of stay. Best practice guidelines for pulmonary care in general for patients undergoing non-cardiac surgery are scarce, compared to strategies to prevent ventilator-associated pneumonia (VAP).
Study design: Observational study.
Setting: Boston University Medical Center.
Synopsis: The I COUGH program emphasized Incentive spirometry, Coughing and deep breathing, Oral care, Understanding (patient and family education), Getting out of bed at least three times daily, and Head-of-bed elevation.
I COUGH was implemented for one year for all general surgery and vascular surgery patients, and results were compared with the year prior using National Surgical Quality Improvement Program (NSQIP) data. The program reduced the incidence of post-operative pneumonia to 1.6% from 2.6% and the incidence of unplanned intubations to 1.2% from 2.0%. The results did show a trend but did not achieve statistical significance.
Bottom line: Post-operative implementation of I COUGH through consistent education of staff, patients, and family might reduce post-operative pneumonia and unplanned intubations.
Citation: Cassidy MR, Rosenkranz P, McCabe K, Rosen JE, McAneny D. I COUGH: reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg. 2013;148:740-745.
Clinical question: Does the use of a standardized suite of post-operative pulmonary care guidelines decrease the incidence of adverse pulmonary outcomes in non-ventilated patients?
Background: Post-operative pulmonary complications are common and account for high costs and increased length of stay. Best practice guidelines for pulmonary care in general for patients undergoing non-cardiac surgery are scarce, compared to strategies to prevent ventilator-associated pneumonia (VAP).
Study design: Observational study.
Setting: Boston University Medical Center.
Synopsis: The I COUGH program emphasized Incentive spirometry, Coughing and deep breathing, Oral care, Understanding (patient and family education), Getting out of bed at least three times daily, and Head-of-bed elevation.
I COUGH was implemented for one year for all general surgery and vascular surgery patients, and results were compared with the year prior using National Surgical Quality Improvement Program (NSQIP) data. The program reduced the incidence of post-operative pneumonia to 1.6% from 2.6% and the incidence of unplanned intubations to 1.2% from 2.0%. The results did show a trend but did not achieve statistical significance.
Bottom line: Post-operative implementation of I COUGH through consistent education of staff, patients, and family might reduce post-operative pneumonia and unplanned intubations.
Citation: Cassidy MR, Rosenkranz P, McCabe K, Rosen JE, McAneny D. I COUGH: reducing postoperative pulmonary complications with a multidisciplinary patient care program. JAMA Surg. 2013;148:740-745.
Physicians Feel Responsibility to Address Healthcare Costs
Clinical question: What are physicians’ attitudes toward addressing healthcare costs and which strategies do they most enthusiastically support?
Background: Physicians are expected to take a lead role in containing healthcare costs, especially in the face of healthcare reform; however, their attitudes regarding this role are unknown.
Study design: Cross-sectional survey.
Setting: U.S. physicians randomly selected from the AMA master file.
Synopsis: Among 2,556 physicians who responded to the survey (response rate: 65%), most believed stakeholders other than physicians (e.g., lawyers, hospitals, insurers, pharmaceutical manufacturers, and patients) have a “major responsibility” for reducing healthcare costs. Most physicians were likely to support such quality initiatives as enhancing continuity of care and promoting chronic disease care coordination. Physicians were also enthusiastic with regard to expanding the use of electronic health records.
The majority of physicians expressed agreement about their responsibility to address healthcare costs by adhering to clinical guidelines, limiting unnecessary testing, and focusing on the individual patient’s best interest. However, a majority expressed limited enthusiasm for strategies that involved cost cutting to physicians, such as eliminating fee-for-service payment models, reducing compensation for the highest paid specialties, and allowing Medicare payment cuts to doctors.
Of note, in the multivariate model, physicians receiving salary-based compensation were more likely to be enthusiastic about eliminating fee-for-service.
Bottom line: Physicians expressed considerable enthusiasm for addressing healthcare costs and are in general agreement but are not enthusiastic about changes that involve physician payment cuts.
Citation: Tilburt JC, Wynia MK, Sheeler RD, et al. Views of US physicians about controlling health care costs. JAMA. 2013;310:380-388.
Clinical question: What are physicians’ attitudes toward addressing healthcare costs and which strategies do they most enthusiastically support?
Background: Physicians are expected to take a lead role in containing healthcare costs, especially in the face of healthcare reform; however, their attitudes regarding this role are unknown.
Study design: Cross-sectional survey.
Setting: U.S. physicians randomly selected from the AMA master file.
Synopsis: Among 2,556 physicians who responded to the survey (response rate: 65%), most believed stakeholders other than physicians (e.g., lawyers, hospitals, insurers, pharmaceutical manufacturers, and patients) have a “major responsibility” for reducing healthcare costs. Most physicians were likely to support such quality initiatives as enhancing continuity of care and promoting chronic disease care coordination. Physicians were also enthusiastic with regard to expanding the use of electronic health records.
The majority of physicians expressed agreement about their responsibility to address healthcare costs by adhering to clinical guidelines, limiting unnecessary testing, and focusing on the individual patient’s best interest. However, a majority expressed limited enthusiasm for strategies that involved cost cutting to physicians, such as eliminating fee-for-service payment models, reducing compensation for the highest paid specialties, and allowing Medicare payment cuts to doctors.
Of note, in the multivariate model, physicians receiving salary-based compensation were more likely to be enthusiastic about eliminating fee-for-service.
Bottom line: Physicians expressed considerable enthusiasm for addressing healthcare costs and are in general agreement but are not enthusiastic about changes that involve physician payment cuts.
Citation: Tilburt JC, Wynia MK, Sheeler RD, et al. Views of US physicians about controlling health care costs. JAMA. 2013;310:380-388.
Clinical question: What are physicians’ attitudes toward addressing healthcare costs and which strategies do they most enthusiastically support?
Background: Physicians are expected to take a lead role in containing healthcare costs, especially in the face of healthcare reform; however, their attitudes regarding this role are unknown.
Study design: Cross-sectional survey.
Setting: U.S. physicians randomly selected from the AMA master file.
Synopsis: Among 2,556 physicians who responded to the survey (response rate: 65%), most believed stakeholders other than physicians (e.g., lawyers, hospitals, insurers, pharmaceutical manufacturers, and patients) have a “major responsibility” for reducing healthcare costs. Most physicians were likely to support such quality initiatives as enhancing continuity of care and promoting chronic disease care coordination. Physicians were also enthusiastic with regard to expanding the use of electronic health records.
The majority of physicians expressed agreement about their responsibility to address healthcare costs by adhering to clinical guidelines, limiting unnecessary testing, and focusing on the individual patient’s best interest. However, a majority expressed limited enthusiasm for strategies that involved cost cutting to physicians, such as eliminating fee-for-service payment models, reducing compensation for the highest paid specialties, and allowing Medicare payment cuts to doctors.
Of note, in the multivariate model, physicians receiving salary-based compensation were more likely to be enthusiastic about eliminating fee-for-service.
Bottom line: Physicians expressed considerable enthusiasm for addressing healthcare costs and are in general agreement but are not enthusiastic about changes that involve physician payment cuts.
Citation: Tilburt JC, Wynia MK, Sheeler RD, et al. Views of US physicians about controlling health care costs. JAMA. 2013;310:380-388.
Superficial and Deep/Organ-Space Surgical Site Infections Should Not Be Combined for Quality Measurement
Clinical question: What patient-risk factors predict superficial and deep/organ-space surgical site infections (SSIs) following colectomy procedures?
Background: SSIs are often targeted by policymakers for quality improvement and cost saving. Superficial and deep/organ-specific SSIs are traditionally considered a single entity for quality measurement, although they vary by anatomic location and clinical severity.
Study design: Retrospective cohort study.
Setting: American College of Surgeons National Surgical Quality Improvement program (ACS-NSQIP).
Synopsis: Researchers used the ACS-NSQIP registry to identify all patients who underwent colectomy procedures across 305 hospitals. Various patient variables, such as demographics, pre-operative risk factors, comorbidities, and operative information, were collected on all patients. The primary outcome was 30-day post-operative superficial SSI and deep/organ-space SSI.
Overall, 27,011 patients underwent colectomy procedures, of which 6.2% developed a superficial SSI and 4.7% developed deep/organ-space SSI. Open surgical approach (vs. laparoscopic) and current smoking were the only risk factors that predicted the occurrence of both superficial and deep/organ-space SSI. Other risk factors (e.g., post-operative diagnoses, disseminated cancer, and irradiation therapy) had a differential effect and only predicted the occurrence of deep/organ-space SSI. Elevated body mass index was strongly correlated with the occurrence of superficial SSI.
Key limitations of the study included unavailability of infection rates beyond 30 days and grouping of deep and organ-space SSIs, as the latter might vary in magnitude and significance.
Bottom Line: Risk factors that predict superficial and deep/organ-space SSI differ significantly, suggesting that future quality initiatives and reporting should evaluate different types of SSIs independently.
Citation: Lawson EH, Hall BL, Ko CY. Risk factors for superficial vs. deep/organ-space surgical site infections: implications for quality improvement initiatives [published online ahead of print July 17, 2013]. JAMA Surg.
Clinical question: What patient-risk factors predict superficial and deep/organ-space surgical site infections (SSIs) following colectomy procedures?
Background: SSIs are often targeted by policymakers for quality improvement and cost saving. Superficial and deep/organ-specific SSIs are traditionally considered a single entity for quality measurement, although they vary by anatomic location and clinical severity.
Study design: Retrospective cohort study.
Setting: American College of Surgeons National Surgical Quality Improvement program (ACS-NSQIP).
Synopsis: Researchers used the ACS-NSQIP registry to identify all patients who underwent colectomy procedures across 305 hospitals. Various patient variables, such as demographics, pre-operative risk factors, comorbidities, and operative information, were collected on all patients. The primary outcome was 30-day post-operative superficial SSI and deep/organ-space SSI.
Overall, 27,011 patients underwent colectomy procedures, of which 6.2% developed a superficial SSI and 4.7% developed deep/organ-space SSI. Open surgical approach (vs. laparoscopic) and current smoking were the only risk factors that predicted the occurrence of both superficial and deep/organ-space SSI. Other risk factors (e.g., post-operative diagnoses, disseminated cancer, and irradiation therapy) had a differential effect and only predicted the occurrence of deep/organ-space SSI. Elevated body mass index was strongly correlated with the occurrence of superficial SSI.
Key limitations of the study included unavailability of infection rates beyond 30 days and grouping of deep and organ-space SSIs, as the latter might vary in magnitude and significance.
Bottom Line: Risk factors that predict superficial and deep/organ-space SSI differ significantly, suggesting that future quality initiatives and reporting should evaluate different types of SSIs independently.
Citation: Lawson EH, Hall BL, Ko CY. Risk factors for superficial vs. deep/organ-space surgical site infections: implications for quality improvement initiatives [published online ahead of print July 17, 2013]. JAMA Surg.
Clinical question: What patient-risk factors predict superficial and deep/organ-space surgical site infections (SSIs) following colectomy procedures?
Background: SSIs are often targeted by policymakers for quality improvement and cost saving. Superficial and deep/organ-specific SSIs are traditionally considered a single entity for quality measurement, although they vary by anatomic location and clinical severity.
Study design: Retrospective cohort study.
Setting: American College of Surgeons National Surgical Quality Improvement program (ACS-NSQIP).
Synopsis: Researchers used the ACS-NSQIP registry to identify all patients who underwent colectomy procedures across 305 hospitals. Various patient variables, such as demographics, pre-operative risk factors, comorbidities, and operative information, were collected on all patients. The primary outcome was 30-day post-operative superficial SSI and deep/organ-space SSI.
Overall, 27,011 patients underwent colectomy procedures, of which 6.2% developed a superficial SSI and 4.7% developed deep/organ-space SSI. Open surgical approach (vs. laparoscopic) and current smoking were the only risk factors that predicted the occurrence of both superficial and deep/organ-space SSI. Other risk factors (e.g., post-operative diagnoses, disseminated cancer, and irradiation therapy) had a differential effect and only predicted the occurrence of deep/organ-space SSI. Elevated body mass index was strongly correlated with the occurrence of superficial SSI.
Key limitations of the study included unavailability of infection rates beyond 30 days and grouping of deep and organ-space SSIs, as the latter might vary in magnitude and significance.
Bottom Line: Risk factors that predict superficial and deep/organ-space SSI differ significantly, suggesting that future quality initiatives and reporting should evaluate different types of SSIs independently.
Citation: Lawson EH, Hall BL, Ko CY. Risk factors for superficial vs. deep/organ-space surgical site infections: implications for quality improvement initiatives [published online ahead of print July 17, 2013]. JAMA Surg.
Apixaban Non-Inferior to Standard Therapy to Treat Acute VTE with Favorable Bleeding Risk
Clinical question: Is apixaban non-inferior to standard therapy for treating acute VTE?
Background: Apixaban, a direct Xa inhibitor, has not been tested for efficacy and safety in treating acute VTE. Rivaroxaban, another direct Xa inhibitor, is already FDA-approved for acute VTE treatment.
Study design: Randomized, double-blinded trial.
Setting: International multi-center.
Synopsis: Researchers randomized 5400 patients with acute VTE to receive either apixaban (10 mg po bid x 7 days, then 5 mg po bid x 6 months) + placebo, or lovenox with transition to coumadin, with a goal of international normalized ratio 2-3. Recurrent VTE occurred in 2.3% of the apixaban group, compared with 2.7% in the conventional therapy group (apixaban noninferior). Major bleeding occurred less in the apixaban group than in the conventional therapy group (0.6% compared to 1.8%), as did clinically relevant non-major bleeding (3.8% compared to 8%).
Bottom line: Apixaban is a safe alternative for treating acute VTE (pending FDA approval).
Citation: Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. New Engl J Med. 2013;369:799-808.
Clinical question: Is apixaban non-inferior to standard therapy for treating acute VTE?
Background: Apixaban, a direct Xa inhibitor, has not been tested for efficacy and safety in treating acute VTE. Rivaroxaban, another direct Xa inhibitor, is already FDA-approved for acute VTE treatment.
Study design: Randomized, double-blinded trial.
Setting: International multi-center.
Synopsis: Researchers randomized 5400 patients with acute VTE to receive either apixaban (10 mg po bid x 7 days, then 5 mg po bid x 6 months) + placebo, or lovenox with transition to coumadin, with a goal of international normalized ratio 2-3. Recurrent VTE occurred in 2.3% of the apixaban group, compared with 2.7% in the conventional therapy group (apixaban noninferior). Major bleeding occurred less in the apixaban group than in the conventional therapy group (0.6% compared to 1.8%), as did clinically relevant non-major bleeding (3.8% compared to 8%).
Bottom line: Apixaban is a safe alternative for treating acute VTE (pending FDA approval).
Citation: Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. New Engl J Med. 2013;369:799-808.
Clinical question: Is apixaban non-inferior to standard therapy for treating acute VTE?
Background: Apixaban, a direct Xa inhibitor, has not been tested for efficacy and safety in treating acute VTE. Rivaroxaban, another direct Xa inhibitor, is already FDA-approved for acute VTE treatment.
Study design: Randomized, double-blinded trial.
Setting: International multi-center.
Synopsis: Researchers randomized 5400 patients with acute VTE to receive either apixaban (10 mg po bid x 7 days, then 5 mg po bid x 6 months) + placebo, or lovenox with transition to coumadin, with a goal of international normalized ratio 2-3. Recurrent VTE occurred in 2.3% of the apixaban group, compared with 2.7% in the conventional therapy group (apixaban noninferior). Major bleeding occurred less in the apixaban group than in the conventional therapy group (0.6% compared to 1.8%), as did clinically relevant non-major bleeding (3.8% compared to 8%).
Bottom line: Apixaban is a safe alternative for treating acute VTE (pending FDA approval).
Citation: Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. New Engl J Med. 2013;369:799-808.
Adding Clopidogrel to Aspirin Prevents Recurrent CVA in a Defined Population
Clinical question: Does loading clopidogrel with aspirin reduce recurrent stroke after moderate to high-risk transient ischemic attack (TIA) or minor stroke if started within 24 hours of primary event?
Background: Recurrent stroke risk is highest during the first few weeks after TIA or minor stroke.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Multi-center health system in China.
Synopsis: More than 5100 patients were randomized within 24 hours after minor ischemic stroke (NIHSS<=3) or high-risk TIA (ABCD2>= 4) to loading dose clopidogrel 300 mg, then 75 mg po daily x 90 days in addition to aspirin 75 mg daily for the first 21 days or aspirin 75 mg po daily x 90 days + placebo. Within 90 days, recurrent stroke was higher in aspirin + placebo group compared to aspirin + clopidogrel (11.7% event rate compared with 8.2%). Moderate to severe bleeding risk was the same (0.3%) in both groups.
Strict eligibility criteria in this study might limit generalizability to the general public. This study occurred in China, where the recurrent stroke rate was higher (near 10%) than the rate seen in primary stroke centers in more developed countries (3% to 5%), perhaps because of less emphasis on secondary risk prevention (including hypertension and hyperlipidemia) in China.
Also, the distribution of stroke subtype in China (more intracranial atherosclerosis than in other populations) might have affected the study outcomes. Because of these limitations, more research needs to be done to confirm these findings for other populations.
Bottom line: Adding clopidogrel to aspirin reduced recurrent cerebrovascular event after high-risk TIA or minor ischemic stroke in China, but generalizability to other patient populations is not clear.
Citation: Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New Engl J Med. 2013;369:11-19.
Clinical question: Does loading clopidogrel with aspirin reduce recurrent stroke after moderate to high-risk transient ischemic attack (TIA) or minor stroke if started within 24 hours of primary event?
Background: Recurrent stroke risk is highest during the first few weeks after TIA or minor stroke.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Multi-center health system in China.
Synopsis: More than 5100 patients were randomized within 24 hours after minor ischemic stroke (NIHSS<=3) or high-risk TIA (ABCD2>= 4) to loading dose clopidogrel 300 mg, then 75 mg po daily x 90 days in addition to aspirin 75 mg daily for the first 21 days or aspirin 75 mg po daily x 90 days + placebo. Within 90 days, recurrent stroke was higher in aspirin + placebo group compared to aspirin + clopidogrel (11.7% event rate compared with 8.2%). Moderate to severe bleeding risk was the same (0.3%) in both groups.
Strict eligibility criteria in this study might limit generalizability to the general public. This study occurred in China, where the recurrent stroke rate was higher (near 10%) than the rate seen in primary stroke centers in more developed countries (3% to 5%), perhaps because of less emphasis on secondary risk prevention (including hypertension and hyperlipidemia) in China.
Also, the distribution of stroke subtype in China (more intracranial atherosclerosis than in other populations) might have affected the study outcomes. Because of these limitations, more research needs to be done to confirm these findings for other populations.
Bottom line: Adding clopidogrel to aspirin reduced recurrent cerebrovascular event after high-risk TIA or minor ischemic stroke in China, but generalizability to other patient populations is not clear.
Citation: Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New Engl J Med. 2013;369:11-19.
Clinical question: Does loading clopidogrel with aspirin reduce recurrent stroke after moderate to high-risk transient ischemic attack (TIA) or minor stroke if started within 24 hours of primary event?
Background: Recurrent stroke risk is highest during the first few weeks after TIA or minor stroke.
Study design: Randomized, double-blinded, placebo-controlled trial.
Setting: Multi-center health system in China.
Synopsis: More than 5100 patients were randomized within 24 hours after minor ischemic stroke (NIHSS<=3) or high-risk TIA (ABCD2>= 4) to loading dose clopidogrel 300 mg, then 75 mg po daily x 90 days in addition to aspirin 75 mg daily for the first 21 days or aspirin 75 mg po daily x 90 days + placebo. Within 90 days, recurrent stroke was higher in aspirin + placebo group compared to aspirin + clopidogrel (11.7% event rate compared with 8.2%). Moderate to severe bleeding risk was the same (0.3%) in both groups.
Strict eligibility criteria in this study might limit generalizability to the general public. This study occurred in China, where the recurrent stroke rate was higher (near 10%) than the rate seen in primary stroke centers in more developed countries (3% to 5%), perhaps because of less emphasis on secondary risk prevention (including hypertension and hyperlipidemia) in China.
Also, the distribution of stroke subtype in China (more intracranial atherosclerosis than in other populations) might have affected the study outcomes. Because of these limitations, more research needs to be done to confirm these findings for other populations.
Bottom line: Adding clopidogrel to aspirin reduced recurrent cerebrovascular event after high-risk TIA or minor ischemic stroke in China, but generalizability to other patient populations is not clear.
Citation: Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. New Engl J Med. 2013;369:11-19.
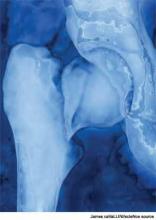
How Should Patients with Acute Hip Fractures Be Managed Perioperatively?
Case
A 91-year-old man with Alzheimer’s dementia presents with severe right hip pain after a fall at his nursing home. His family reports that he is dependent in most of his activities of daily living (ADLs) and can normally ambulate short distances with a walker. He is alert and oriented at baseline but has been more confused since his wife died a week earlier from pneumonia. His only new medication is lorazepam as needed for anxiety. On admission, the patient is diagnosed with a displaced femoral neck fracture, delirium, and healthcare-associated pneumonia, with a new oxygen requirement of 5 L/min. The orthopedic surgery service requests a medicine consult. How should this patient be managed perioperatively?
Overview
Hip fractures are a major health burden on the United States’ geriatric population. The lifetime risk of hip fracture is approximately 17% for Caucasian women and 6% for Caucasian men.1 In 2010, an estimated 258,000 people aged 65 years and older were hospitalized with hip fractures.2 This number is expected to climb to 289,000 by 2030.
In total, hip fractures directly cost the healthcare system about $18 billion per year.1
Hip fractures, like most other geriatric syndromes, are almost invariably multifactorial in etiology. They occur at the intersection of general frailty, bone fragility, and fall risk. Hip fractures too often trigger a further downward spiral in elderly patients, as deconditioning and acute complications compound chronic comorbidities and compromise any remaining physiologic reserve. Mortality after a hip fracture approaches 25% at one year.3 An excess mortality risk persists for at least 10 years.4 Of the patients who survive six months, only 50% can perform their ADLs, and only 25% can perform their instrumental ADLs as well as they could prior to their fracture.5,6
Unsurprisingly, older adults with hip fractures are five times more likely to require nursing home placement at one year.5
Hospitalists frequently encounter patients with hip fractures in the perioperative setting. Given their close collaboration with orthopedic surgeons and emphasis on transitions of care, hospitalists can play an important role in reversing the trajectory of death and disability following hip fractures. Key aspects of inpatient management are outlined below.
Hip Fracture Repair
Hip fractures can be divided into intracapsular (femoral neck) or extracapsular (intratrochanteric or subtrochanteric) fractures. Their relative frequencies are listed in Table 1.7
Surgery types. Femoral neck fractures typically are the most difficult to heal, given a limited regional blood supply.5,7 Displaced femoral neck fractures require either a hemiarthroplasty or total hip arthroplasty. Over time, hemiarthroplasties tend to cause hip pain from acetabular erosion, so they are better suited for less active, elderly patients. Nondisplaced femoral neck, intratrochanteric, and subtrochanteric fractures are usually managed with open reduction and internal fixation.
The overall goal of surgery is to return patients to their prior level of functioning. In the short term, surgery also provides pain relief and allows for early mobilization. Nonoperative management is generally reserved for patients with very high operative risk or limited life expectancies or those who are bedridden at baseline.
Timing of surgery. In general, hip fracture repair should be performed within 24-48 hours of admission in patients who are medically stable. Though early surgery may not improve functional outcomes or mortality, it has been associated with improved pain control, decreased length of stay, and fewer major complications.8 Patients with active medical conditions (e.g. pneumonia) should be medically optimized before proceeding with surgery. A 2011 study found that most of the excess in-hospital mortality associated with surgical delays beyond five days was attributable to the active medical issues rather than to the delay itself.9
Prevention of Perioperative Complications
The principles of geriatric medicine should be applied to the care of elderly patients with hip fractures. Emphasis should be placed on early recognition of treatable conditions and avoidance of iatrogenesis. Careful assessment of medical problems, social support, and functional status within an interdisciplinary framework is recommended. Such a multi-faceted approach has been shown to reduce overall complications in hip fracture patients.10 Specific complications are discussed in more detail below.
Delirium. Delirium is the most common complication after hip fracture surgery, with a prevalence of 35%-65%.7 Proper pain control, minimization of polypharmacy, removal of tethers, and frequent reorientation are among the many preventive measures that should be implemented.
Venous thromboembolism (VTE). VTE is a leading cause of morbidity and mortality for hip fracture patients.11 Without prophylaxis, about 1.8% will develop symptomatic deep venous thromboses, and 1% will develop symptomatic pulmonary emboli in the first seven to 14 days after surgery. An estimated 4.3% will develop symptomatic VTE in the first 35 days after surgery.
The American College of Chest Physicians recommends that patients undergoing hip fracture surgery receive VTE prophylaxis for a minimum of 10-14 days postoperatively.11 Extending prophylaxis out to 35 days is reasonable. Low molecular-weight heparin is preferred over low-dose unfractionated heparin, fondaparinux, warfarin, and aspirin. Patients should receive preoperative VTE prophylaxis if surgery is delayed.
Postoperative infections. Urinary tract infections (UTIs) are the most common infectious complication after hip fracture surgery.7 If not caught early, they can result in urosepsis, prosthetic joint infections, and death. After the first 48 hours of urinary catheterization, the risk of a UTI is 5%-10% per day.12
Therefore, catheters should be removed within 24-48 hours of surgery.
Acute blood loss anemia. Anemia is common in hip fracture patients. It may be present on admission or develop as a result of intraoperative blood loss, ongoing drain output, or fluid resuscitation.
The recent FOCUS trial, which helped to clarify the optimal transfusion threshold for patients after hip fracture surgery, compared a liberal versus restrictive transfusion strategy in patients with cardiovascular disease.13 Transfusing for a hemoglobin < 10 g/dL, as opposed to transfusing for symptoms or a hemoglobin < 8 g/dL, did not improve mortality, in-hospital morbidity (including myocardial infarction), or functional status at 60 days.
Pressure ulcers. Patients with hip fractures are at risk of developing decubitus ulcers. One study found the incidence of new pressure ulcers to be 16% at seven days and 36% at 32 days after initial hospitalization.14 Multicomponent interventions have been shown to successfully reduce the rate of hospital-acquired pressure ulcers.15
Medical Management of Osteoporosis
The World Heath Organization defines osteoporosis as a bone mineral density of at least 2.5 standard deviations below that of a “normal” young adult as measured on DEXA scan, or a T-score ≤ -2.5.16 However, it is important to recognize that bone strength depends not only on the quantity of bone but also on the quality. Any patient who sustains a hip fracture with minimal trauma (e.g. a fall from standing height) should be considered to have osteoporosis, regardless of T-score.
Patients with their first hip fracture are 2.5 times more likely to have a future fragility fracture.17 Hospitalists must therefore make secondary prevention a priority. Medical management focuses on maintaining bone strength, slowing further bone loss, and preventing future falls.
Evaluation. A directed history and physical examination should be completed to screen for secondary causes of osteoporosis. A basic laboratory workup is reasonable in the inpatient setting (see Table 2).17 Other tests, such as a serum and urine protein electrophoresis, can be obtained as clinically indicated.
Patients require counseling directed at lifestyle factors, including the importance of weight-bearing exercise, smoking cessation, and avoidance of excessive alcohol intake. A comprehensive falls assessment is also warranted.
Treatment. All patients with hip fractures should be discharged from the hospital on calcium and vitamin D supplementation, unless there is a specific contraindication.18 Guidelines vary by organization, but the National Osteoporosis Foundation’s recommendations are listed in Table 3.17,19,20 Dietary calcium is usually insufficient to meet the daily requirement.
Bisphosphonates are considered first-line therapy for osteoporosis.17 The HORIZON trial was a randomized, placebo-controlled study that evaluated annual zoledronic acid infusions in hip fracture patients who were intolerant of oral bisphosphonates.21 Zoledronic acid reduced the rate of new fractures by 35% after 1.9 years, with a number needed to treat (NNT) of 19. It also improved survival by 28%, for an NNT of 27. All subjects also received calcium and vitamin D supplementation.
Both hospitalists and orthopedists might worry about bisphosphonates adversely affecting bone healing in the acute setting. Subsequent analyses from the HORIZON trial suggest that bisphosphonates can be safely started as soon as two weeks after surgery.22,23
Transitions of care. Despite well-established guidelines for the treatment of osteoporosis, patients with hip fractures often are undertreated. A retrospective study of 420 acute hip fracture patients found that only 37% received calcium, 36% received vitamin D, and 31% received a bisphosphonate on discharge.24 A prospective study of 1,075 women with new osteoporotic fractures found that only 17% had started anti-osteoporosis medications at one year.25
Hospitalists should recognize and address potential barriers to appropriate medical therapy. Patient-related obstacles may include the cost of medications, concerns about side effects, and lack of a PCP.24,25 Hospitalists should document the diagnosis of osteoporosis in the medical record so subsequent providers are attuned to the issue.26 They should also clarify the ownership of osteoporosis across the continuum of care, because medicine consultants, orthopedists, primary care or rehabilitation physicians, and subspecialists may all be involved. Hospitalists can certainly take advantage of this window of opportunity by starting patients on osteoporosis treatment and ensuring smooth transitions of care on discharge.
Back to the Case
The patient was started on intravenous antibiotics for healthcare-associated pneumonia with improvement of his oxygen requirement to 3 L/min. He underwent a right hemiarthroplasty on hospital day five and tolerated the procedure well. His delirium resolved with treatment of his infection, pain control, discontinuation of lorazepam, and other conservative measures. He was given VTE prophylaxis pre- and postoperatively. His urinary catheter was discontinued on day one after surgery. He was started on calcium supplementation and vitamin D repletion after his 25-OH vitamin D level returned low at 14 ng/mL.
The patient progressed well with physical and occupational therapy and was discharged back to his skilled nursing facility, with plans to start a bisphosphonate in two weeks.
Bottom Line
Hospitalists should be familiar with the best practices for the perioperative management of hip fracture patients.
Dr. Anderson and Dr. Wolfe are hospitalists at the University of Colorado Hospital in Aurora. Dr. Anderson directs the medicine consult service, and Dr. Wolfe serves as the associate director.
References
- The Joint Commission. Improving and measuring osteoporosis treatment 2008. Oakbrook Terrace, Il; 2008. Available at: http://www.jointcommission.org/improving_and_measuring_osteoporosis_management/. Accessed September 29, 2013.
- Stevens JA, Rudd RA. The impact of decreasing U.S. hip fracture rates on future hip fracture estimates. Osteoporos Int. 2013;24(10):2725-2728.
- Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality, and costs. J Am Geriatr Soc. 2003;51(3):364-370. Haentjens P, Magaziner J, Colón-Emeric CS, et al.
- Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380-390.
- Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: tailoring care for the older patient. JAMA. 2012;307(20):2185-2194.
- Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. J Gerontol. 1990;45(3):M101-107.
- Bateman L, Vuppala S, Porada P, et al. Medical management in the acute hip fracture patient: a comprehensive review for the internist. Ochsner J. 2012;12(2):101-110.
- Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
- Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233.
- Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482.
- Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
- Wald H, Epstein A, Kramer A. Extended use of indwelling urinary catheters in postoperative hip fracture patients. Med Care. 2005;43(10):1009-1017.
- Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462.
- Baumgarten M, Margolis DJ, Orwig DL, et al. Pressure ulcers in elderly patients with hip fracture across the continuum of care. J Am Geriatr Soc. 2009;57(5):863-870.
- Sullivan N, Schoelles KM. Preventing in-facility pressure ulcers as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):410-416.
- World Health Organization. Prevention and management of osteoporosis: Report of a WHO scientific group 2000. Geneva, Switzerland; 2000. WHO technical report series; 921. Available at: http://whqlibdoc.who.int/trs/who_trs_921.pdf. Accessed July 9, 2013.
- National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis 2013. Washington, D.C.; 2013. Available at: www.nof.org/files/nof/public/content/file/917/upload/481.pdf. Accessed September 29, 2013.
- Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657-666.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary reference intakes for calcium and vitamin D. Washington, D.C.: National Academies Press; 2011. Lyles KW, Colón-Emeric CS, Magaziner JS, et al.
- Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med. 2007;357: nihpa40967.
- Colón-Emeric CS, Nordsletten L, Olson S, et al. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int. 2011;22(8):2329-2336.
- Eriksen EF, Lyles KW, Colón-Emeric CS, et al. Antifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fracture. J Bone Miner Res. 2009;24(7):1308-1313.
- Byszewski A, Lemay G, Molnar F, Azad N, McMartin SE. Closing the osteoporosis care gap in hip fracture patients: an opportunity to decrease recurrent fractures and hospital admissions. J Osteoporos. 2011;2011:404969.
- Greenspan SL, Wyman, A, Hoovan FH, et al. Predictors of treatment with osteoporosis medications after recent fragility fractures in a multinational cohort of postmenopausal women. J Am Geriatr Soc. 2012;60(3):455-461.
- Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med. 2000;109(4):326-328.
Case
A 91-year-old man with Alzheimer’s dementia presents with severe right hip pain after a fall at his nursing home. His family reports that he is dependent in most of his activities of daily living (ADLs) and can normally ambulate short distances with a walker. He is alert and oriented at baseline but has been more confused since his wife died a week earlier from pneumonia. His only new medication is lorazepam as needed for anxiety. On admission, the patient is diagnosed with a displaced femoral neck fracture, delirium, and healthcare-associated pneumonia, with a new oxygen requirement of 5 L/min. The orthopedic surgery service requests a medicine consult. How should this patient be managed perioperatively?
Overview
Hip fractures are a major health burden on the United States’ geriatric population. The lifetime risk of hip fracture is approximately 17% for Caucasian women and 6% for Caucasian men.1 In 2010, an estimated 258,000 people aged 65 years and older were hospitalized with hip fractures.2 This number is expected to climb to 289,000 by 2030.
In total, hip fractures directly cost the healthcare system about $18 billion per year.1
Hip fractures, like most other geriatric syndromes, are almost invariably multifactorial in etiology. They occur at the intersection of general frailty, bone fragility, and fall risk. Hip fractures too often trigger a further downward spiral in elderly patients, as deconditioning and acute complications compound chronic comorbidities and compromise any remaining physiologic reserve. Mortality after a hip fracture approaches 25% at one year.3 An excess mortality risk persists for at least 10 years.4 Of the patients who survive six months, only 50% can perform their ADLs, and only 25% can perform their instrumental ADLs as well as they could prior to their fracture.5,6
Unsurprisingly, older adults with hip fractures are five times more likely to require nursing home placement at one year.5
Hospitalists frequently encounter patients with hip fractures in the perioperative setting. Given their close collaboration with orthopedic surgeons and emphasis on transitions of care, hospitalists can play an important role in reversing the trajectory of death and disability following hip fractures. Key aspects of inpatient management are outlined below.
Hip Fracture Repair
Hip fractures can be divided into intracapsular (femoral neck) or extracapsular (intratrochanteric or subtrochanteric) fractures. Their relative frequencies are listed in Table 1.7
Surgery types. Femoral neck fractures typically are the most difficult to heal, given a limited regional blood supply.5,7 Displaced femoral neck fractures require either a hemiarthroplasty or total hip arthroplasty. Over time, hemiarthroplasties tend to cause hip pain from acetabular erosion, so they are better suited for less active, elderly patients. Nondisplaced femoral neck, intratrochanteric, and subtrochanteric fractures are usually managed with open reduction and internal fixation.
The overall goal of surgery is to return patients to their prior level of functioning. In the short term, surgery also provides pain relief and allows for early mobilization. Nonoperative management is generally reserved for patients with very high operative risk or limited life expectancies or those who are bedridden at baseline.
Timing of surgery. In general, hip fracture repair should be performed within 24-48 hours of admission in patients who are medically stable. Though early surgery may not improve functional outcomes or mortality, it has been associated with improved pain control, decreased length of stay, and fewer major complications.8 Patients with active medical conditions (e.g. pneumonia) should be medically optimized before proceeding with surgery. A 2011 study found that most of the excess in-hospital mortality associated with surgical delays beyond five days was attributable to the active medical issues rather than to the delay itself.9
Prevention of Perioperative Complications
The principles of geriatric medicine should be applied to the care of elderly patients with hip fractures. Emphasis should be placed on early recognition of treatable conditions and avoidance of iatrogenesis. Careful assessment of medical problems, social support, and functional status within an interdisciplinary framework is recommended. Such a multi-faceted approach has been shown to reduce overall complications in hip fracture patients.10 Specific complications are discussed in more detail below.
Delirium. Delirium is the most common complication after hip fracture surgery, with a prevalence of 35%-65%.7 Proper pain control, minimization of polypharmacy, removal of tethers, and frequent reorientation are among the many preventive measures that should be implemented.
Venous thromboembolism (VTE). VTE is a leading cause of morbidity and mortality for hip fracture patients.11 Without prophylaxis, about 1.8% will develop symptomatic deep venous thromboses, and 1% will develop symptomatic pulmonary emboli in the first seven to 14 days after surgery. An estimated 4.3% will develop symptomatic VTE in the first 35 days after surgery.
The American College of Chest Physicians recommends that patients undergoing hip fracture surgery receive VTE prophylaxis for a minimum of 10-14 days postoperatively.11 Extending prophylaxis out to 35 days is reasonable. Low molecular-weight heparin is preferred over low-dose unfractionated heparin, fondaparinux, warfarin, and aspirin. Patients should receive preoperative VTE prophylaxis if surgery is delayed.
Postoperative infections. Urinary tract infections (UTIs) are the most common infectious complication after hip fracture surgery.7 If not caught early, they can result in urosepsis, prosthetic joint infections, and death. After the first 48 hours of urinary catheterization, the risk of a UTI is 5%-10% per day.12
Therefore, catheters should be removed within 24-48 hours of surgery.
Acute blood loss anemia. Anemia is common in hip fracture patients. It may be present on admission or develop as a result of intraoperative blood loss, ongoing drain output, or fluid resuscitation.
The recent FOCUS trial, which helped to clarify the optimal transfusion threshold for patients after hip fracture surgery, compared a liberal versus restrictive transfusion strategy in patients with cardiovascular disease.13 Transfusing for a hemoglobin < 10 g/dL, as opposed to transfusing for symptoms or a hemoglobin < 8 g/dL, did not improve mortality, in-hospital morbidity (including myocardial infarction), or functional status at 60 days.
Pressure ulcers. Patients with hip fractures are at risk of developing decubitus ulcers. One study found the incidence of new pressure ulcers to be 16% at seven days and 36% at 32 days after initial hospitalization.14 Multicomponent interventions have been shown to successfully reduce the rate of hospital-acquired pressure ulcers.15
Medical Management of Osteoporosis
The World Heath Organization defines osteoporosis as a bone mineral density of at least 2.5 standard deviations below that of a “normal” young adult as measured on DEXA scan, or a T-score ≤ -2.5.16 However, it is important to recognize that bone strength depends not only on the quantity of bone but also on the quality. Any patient who sustains a hip fracture with minimal trauma (e.g. a fall from standing height) should be considered to have osteoporosis, regardless of T-score.
Patients with their first hip fracture are 2.5 times more likely to have a future fragility fracture.17 Hospitalists must therefore make secondary prevention a priority. Medical management focuses on maintaining bone strength, slowing further bone loss, and preventing future falls.
Evaluation. A directed history and physical examination should be completed to screen for secondary causes of osteoporosis. A basic laboratory workup is reasonable in the inpatient setting (see Table 2).17 Other tests, such as a serum and urine protein electrophoresis, can be obtained as clinically indicated.
Patients require counseling directed at lifestyle factors, including the importance of weight-bearing exercise, smoking cessation, and avoidance of excessive alcohol intake. A comprehensive falls assessment is also warranted.
Treatment. All patients with hip fractures should be discharged from the hospital on calcium and vitamin D supplementation, unless there is a specific contraindication.18 Guidelines vary by organization, but the National Osteoporosis Foundation’s recommendations are listed in Table 3.17,19,20 Dietary calcium is usually insufficient to meet the daily requirement.
Bisphosphonates are considered first-line therapy for osteoporosis.17 The HORIZON trial was a randomized, placebo-controlled study that evaluated annual zoledronic acid infusions in hip fracture patients who were intolerant of oral bisphosphonates.21 Zoledronic acid reduced the rate of new fractures by 35% after 1.9 years, with a number needed to treat (NNT) of 19. It also improved survival by 28%, for an NNT of 27. All subjects also received calcium and vitamin D supplementation.
Both hospitalists and orthopedists might worry about bisphosphonates adversely affecting bone healing in the acute setting. Subsequent analyses from the HORIZON trial suggest that bisphosphonates can be safely started as soon as two weeks after surgery.22,23
Transitions of care. Despite well-established guidelines for the treatment of osteoporosis, patients with hip fractures often are undertreated. A retrospective study of 420 acute hip fracture patients found that only 37% received calcium, 36% received vitamin D, and 31% received a bisphosphonate on discharge.24 A prospective study of 1,075 women with new osteoporotic fractures found that only 17% had started anti-osteoporosis medications at one year.25
Hospitalists should recognize and address potential barriers to appropriate medical therapy. Patient-related obstacles may include the cost of medications, concerns about side effects, and lack of a PCP.24,25 Hospitalists should document the diagnosis of osteoporosis in the medical record so subsequent providers are attuned to the issue.26 They should also clarify the ownership of osteoporosis across the continuum of care, because medicine consultants, orthopedists, primary care or rehabilitation physicians, and subspecialists may all be involved. Hospitalists can certainly take advantage of this window of opportunity by starting patients on osteoporosis treatment and ensuring smooth transitions of care on discharge.
Back to the Case
The patient was started on intravenous antibiotics for healthcare-associated pneumonia with improvement of his oxygen requirement to 3 L/min. He underwent a right hemiarthroplasty on hospital day five and tolerated the procedure well. His delirium resolved with treatment of his infection, pain control, discontinuation of lorazepam, and other conservative measures. He was given VTE prophylaxis pre- and postoperatively. His urinary catheter was discontinued on day one after surgery. He was started on calcium supplementation and vitamin D repletion after his 25-OH vitamin D level returned low at 14 ng/mL.
The patient progressed well with physical and occupational therapy and was discharged back to his skilled nursing facility, with plans to start a bisphosphonate in two weeks.
Bottom Line
Hospitalists should be familiar with the best practices for the perioperative management of hip fracture patients.
Dr. Anderson and Dr. Wolfe are hospitalists at the University of Colorado Hospital in Aurora. Dr. Anderson directs the medicine consult service, and Dr. Wolfe serves as the associate director.
References
- The Joint Commission. Improving and measuring osteoporosis treatment 2008. Oakbrook Terrace, Il; 2008. Available at: http://www.jointcommission.org/improving_and_measuring_osteoporosis_management/. Accessed September 29, 2013.
- Stevens JA, Rudd RA. The impact of decreasing U.S. hip fracture rates on future hip fracture estimates. Osteoporos Int. 2013;24(10):2725-2728.
- Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality, and costs. J Am Geriatr Soc. 2003;51(3):364-370. Haentjens P, Magaziner J, Colón-Emeric CS, et al.
- Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380-390.
- Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: tailoring care for the older patient. JAMA. 2012;307(20):2185-2194.
- Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. J Gerontol. 1990;45(3):M101-107.
- Bateman L, Vuppala S, Porada P, et al. Medical management in the acute hip fracture patient: a comprehensive review for the internist. Ochsner J. 2012;12(2):101-110.
- Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
- Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233.
- Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482.
- Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
- Wald H, Epstein A, Kramer A. Extended use of indwelling urinary catheters in postoperative hip fracture patients. Med Care. 2005;43(10):1009-1017.
- Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462.
- Baumgarten M, Margolis DJ, Orwig DL, et al. Pressure ulcers in elderly patients with hip fracture across the continuum of care. J Am Geriatr Soc. 2009;57(5):863-870.
- Sullivan N, Schoelles KM. Preventing in-facility pressure ulcers as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):410-416.
- World Health Organization. Prevention and management of osteoporosis: Report of a WHO scientific group 2000. Geneva, Switzerland; 2000. WHO technical report series; 921. Available at: http://whqlibdoc.who.int/trs/who_trs_921.pdf. Accessed July 9, 2013.
- National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis 2013. Washington, D.C.; 2013. Available at: www.nof.org/files/nof/public/content/file/917/upload/481.pdf. Accessed September 29, 2013.
- Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657-666.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary reference intakes for calcium and vitamin D. Washington, D.C.: National Academies Press; 2011. Lyles KW, Colón-Emeric CS, Magaziner JS, et al.
- Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med. 2007;357: nihpa40967.
- Colón-Emeric CS, Nordsletten L, Olson S, et al. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int. 2011;22(8):2329-2336.
- Eriksen EF, Lyles KW, Colón-Emeric CS, et al. Antifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fracture. J Bone Miner Res. 2009;24(7):1308-1313.
- Byszewski A, Lemay G, Molnar F, Azad N, McMartin SE. Closing the osteoporosis care gap in hip fracture patients: an opportunity to decrease recurrent fractures and hospital admissions. J Osteoporos. 2011;2011:404969.
- Greenspan SL, Wyman, A, Hoovan FH, et al. Predictors of treatment with osteoporosis medications after recent fragility fractures in a multinational cohort of postmenopausal women. J Am Geriatr Soc. 2012;60(3):455-461.
- Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med. 2000;109(4):326-328.
Case
A 91-year-old man with Alzheimer’s dementia presents with severe right hip pain after a fall at his nursing home. His family reports that he is dependent in most of his activities of daily living (ADLs) and can normally ambulate short distances with a walker. He is alert and oriented at baseline but has been more confused since his wife died a week earlier from pneumonia. His only new medication is lorazepam as needed for anxiety. On admission, the patient is diagnosed with a displaced femoral neck fracture, delirium, and healthcare-associated pneumonia, with a new oxygen requirement of 5 L/min. The orthopedic surgery service requests a medicine consult. How should this patient be managed perioperatively?
Overview
Hip fractures are a major health burden on the United States’ geriatric population. The lifetime risk of hip fracture is approximately 17% for Caucasian women and 6% for Caucasian men.1 In 2010, an estimated 258,000 people aged 65 years and older were hospitalized with hip fractures.2 This number is expected to climb to 289,000 by 2030.
In total, hip fractures directly cost the healthcare system about $18 billion per year.1
Hip fractures, like most other geriatric syndromes, are almost invariably multifactorial in etiology. They occur at the intersection of general frailty, bone fragility, and fall risk. Hip fractures too often trigger a further downward spiral in elderly patients, as deconditioning and acute complications compound chronic comorbidities and compromise any remaining physiologic reserve. Mortality after a hip fracture approaches 25% at one year.3 An excess mortality risk persists for at least 10 years.4 Of the patients who survive six months, only 50% can perform their ADLs, and only 25% can perform their instrumental ADLs as well as they could prior to their fracture.5,6
Unsurprisingly, older adults with hip fractures are five times more likely to require nursing home placement at one year.5
Hospitalists frequently encounter patients with hip fractures in the perioperative setting. Given their close collaboration with orthopedic surgeons and emphasis on transitions of care, hospitalists can play an important role in reversing the trajectory of death and disability following hip fractures. Key aspects of inpatient management are outlined below.
Hip Fracture Repair
Hip fractures can be divided into intracapsular (femoral neck) or extracapsular (intratrochanteric or subtrochanteric) fractures. Their relative frequencies are listed in Table 1.7
Surgery types. Femoral neck fractures typically are the most difficult to heal, given a limited regional blood supply.5,7 Displaced femoral neck fractures require either a hemiarthroplasty or total hip arthroplasty. Over time, hemiarthroplasties tend to cause hip pain from acetabular erosion, so they are better suited for less active, elderly patients. Nondisplaced femoral neck, intratrochanteric, and subtrochanteric fractures are usually managed with open reduction and internal fixation.
The overall goal of surgery is to return patients to their prior level of functioning. In the short term, surgery also provides pain relief and allows for early mobilization. Nonoperative management is generally reserved for patients with very high operative risk or limited life expectancies or those who are bedridden at baseline.
Timing of surgery. In general, hip fracture repair should be performed within 24-48 hours of admission in patients who are medically stable. Though early surgery may not improve functional outcomes or mortality, it has been associated with improved pain control, decreased length of stay, and fewer major complications.8 Patients with active medical conditions (e.g. pneumonia) should be medically optimized before proceeding with surgery. A 2011 study found that most of the excess in-hospital mortality associated with surgical delays beyond five days was attributable to the active medical issues rather than to the delay itself.9
Prevention of Perioperative Complications
The principles of geriatric medicine should be applied to the care of elderly patients with hip fractures. Emphasis should be placed on early recognition of treatable conditions and avoidance of iatrogenesis. Careful assessment of medical problems, social support, and functional status within an interdisciplinary framework is recommended. Such a multi-faceted approach has been shown to reduce overall complications in hip fracture patients.10 Specific complications are discussed in more detail below.
Delirium. Delirium is the most common complication after hip fracture surgery, with a prevalence of 35%-65%.7 Proper pain control, minimization of polypharmacy, removal of tethers, and frequent reorientation are among the many preventive measures that should be implemented.
Venous thromboembolism (VTE). VTE is a leading cause of morbidity and mortality for hip fracture patients.11 Without prophylaxis, about 1.8% will develop symptomatic deep venous thromboses, and 1% will develop symptomatic pulmonary emboli in the first seven to 14 days after surgery. An estimated 4.3% will develop symptomatic VTE in the first 35 days after surgery.
The American College of Chest Physicians recommends that patients undergoing hip fracture surgery receive VTE prophylaxis for a minimum of 10-14 days postoperatively.11 Extending prophylaxis out to 35 days is reasonable. Low molecular-weight heparin is preferred over low-dose unfractionated heparin, fondaparinux, warfarin, and aspirin. Patients should receive preoperative VTE prophylaxis if surgery is delayed.
Postoperative infections. Urinary tract infections (UTIs) are the most common infectious complication after hip fracture surgery.7 If not caught early, they can result in urosepsis, prosthetic joint infections, and death. After the first 48 hours of urinary catheterization, the risk of a UTI is 5%-10% per day.12
Therefore, catheters should be removed within 24-48 hours of surgery.
Acute blood loss anemia. Anemia is common in hip fracture patients. It may be present on admission or develop as a result of intraoperative blood loss, ongoing drain output, or fluid resuscitation.
The recent FOCUS trial, which helped to clarify the optimal transfusion threshold for patients after hip fracture surgery, compared a liberal versus restrictive transfusion strategy in patients with cardiovascular disease.13 Transfusing for a hemoglobin < 10 g/dL, as opposed to transfusing for symptoms or a hemoglobin < 8 g/dL, did not improve mortality, in-hospital morbidity (including myocardial infarction), or functional status at 60 days.
Pressure ulcers. Patients with hip fractures are at risk of developing decubitus ulcers. One study found the incidence of new pressure ulcers to be 16% at seven days and 36% at 32 days after initial hospitalization.14 Multicomponent interventions have been shown to successfully reduce the rate of hospital-acquired pressure ulcers.15
Medical Management of Osteoporosis
The World Heath Organization defines osteoporosis as a bone mineral density of at least 2.5 standard deviations below that of a “normal” young adult as measured on DEXA scan, or a T-score ≤ -2.5.16 However, it is important to recognize that bone strength depends not only on the quantity of bone but also on the quality. Any patient who sustains a hip fracture with minimal trauma (e.g. a fall from standing height) should be considered to have osteoporosis, regardless of T-score.
Patients with their first hip fracture are 2.5 times more likely to have a future fragility fracture.17 Hospitalists must therefore make secondary prevention a priority. Medical management focuses on maintaining bone strength, slowing further bone loss, and preventing future falls.
Evaluation. A directed history and physical examination should be completed to screen for secondary causes of osteoporosis. A basic laboratory workup is reasonable in the inpatient setting (see Table 2).17 Other tests, such as a serum and urine protein electrophoresis, can be obtained as clinically indicated.
Patients require counseling directed at lifestyle factors, including the importance of weight-bearing exercise, smoking cessation, and avoidance of excessive alcohol intake. A comprehensive falls assessment is also warranted.
Treatment. All patients with hip fractures should be discharged from the hospital on calcium and vitamin D supplementation, unless there is a specific contraindication.18 Guidelines vary by organization, but the National Osteoporosis Foundation’s recommendations are listed in Table 3.17,19,20 Dietary calcium is usually insufficient to meet the daily requirement.
Bisphosphonates are considered first-line therapy for osteoporosis.17 The HORIZON trial was a randomized, placebo-controlled study that evaluated annual zoledronic acid infusions in hip fracture patients who were intolerant of oral bisphosphonates.21 Zoledronic acid reduced the rate of new fractures by 35% after 1.9 years, with a number needed to treat (NNT) of 19. It also improved survival by 28%, for an NNT of 27. All subjects also received calcium and vitamin D supplementation.
Both hospitalists and orthopedists might worry about bisphosphonates adversely affecting bone healing in the acute setting. Subsequent analyses from the HORIZON trial suggest that bisphosphonates can be safely started as soon as two weeks after surgery.22,23
Transitions of care. Despite well-established guidelines for the treatment of osteoporosis, patients with hip fractures often are undertreated. A retrospective study of 420 acute hip fracture patients found that only 37% received calcium, 36% received vitamin D, and 31% received a bisphosphonate on discharge.24 A prospective study of 1,075 women with new osteoporotic fractures found that only 17% had started anti-osteoporosis medications at one year.25
Hospitalists should recognize and address potential barriers to appropriate medical therapy. Patient-related obstacles may include the cost of medications, concerns about side effects, and lack of a PCP.24,25 Hospitalists should document the diagnosis of osteoporosis in the medical record so subsequent providers are attuned to the issue.26 They should also clarify the ownership of osteoporosis across the continuum of care, because medicine consultants, orthopedists, primary care or rehabilitation physicians, and subspecialists may all be involved. Hospitalists can certainly take advantage of this window of opportunity by starting patients on osteoporosis treatment and ensuring smooth transitions of care on discharge.
Back to the Case
The patient was started on intravenous antibiotics for healthcare-associated pneumonia with improvement of his oxygen requirement to 3 L/min. He underwent a right hemiarthroplasty on hospital day five and tolerated the procedure well. His delirium resolved with treatment of his infection, pain control, discontinuation of lorazepam, and other conservative measures. He was given VTE prophylaxis pre- and postoperatively. His urinary catheter was discontinued on day one after surgery. He was started on calcium supplementation and vitamin D repletion after his 25-OH vitamin D level returned low at 14 ng/mL.
The patient progressed well with physical and occupational therapy and was discharged back to his skilled nursing facility, with plans to start a bisphosphonate in two weeks.
Bottom Line
Hospitalists should be familiar with the best practices for the perioperative management of hip fracture patients.
Dr. Anderson and Dr. Wolfe are hospitalists at the University of Colorado Hospital in Aurora. Dr. Anderson directs the medicine consult service, and Dr. Wolfe serves as the associate director.
References
- The Joint Commission. Improving and measuring osteoporosis treatment 2008. Oakbrook Terrace, Il; 2008. Available at: http://www.jointcommission.org/improving_and_measuring_osteoporosis_management/. Accessed September 29, 2013.
- Stevens JA, Rudd RA. The impact of decreasing U.S. hip fracture rates on future hip fracture estimates. Osteoporos Int. 2013;24(10):2725-2728.
- Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality, and costs. J Am Geriatr Soc. 2003;51(3):364-370. Haentjens P, Magaziner J, Colón-Emeric CS, et al.
- Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med. 2010;152(6):380-390.
- Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: tailoring care for the older patient. JAMA. 2012;307(20):2185-2194.
- Magaziner J, Simonsick EM, Kashner TM, Hebel JR, Kenzora JE. Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study. J Gerontol. 1990;45(3):M101-107.
- Bateman L, Vuppala S, Porada P, et al. Medical management in the acute hip fracture patient: a comprehensive review for the internist. Ochsner J. 2012;12(2):101-110.
- Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738-1743.
- Vidán MT, Sánchez E, Gracia Y, Marañón E, Vaquero J, Serra JA. Causes and effects of surgical delay in patients with hip fracture: a cohort study. Ann Intern Med. 2011;155(4):226-233.
- Vidán M, Serra JA, Moreno C, Riquelme G, Ortiz J. Efficacy of a comprehensive geriatric intervention in older patients hospitalized for hip fracture: a randomized, controlled trial. J Am Geriatr Soc. 2005;53(9):1476-1482.
- Falck-Ytter Y, Francis CW, Johanson NA, et al. Prevention of VTE in orthopedic surgery patients: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e278S-e325S.
- Wald H, Epstein A, Kramer A. Extended use of indwelling urinary catheters in postoperative hip fracture patients. Med Care. 2005;43(10):1009-1017.
- Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462.
- Baumgarten M, Margolis DJ, Orwig DL, et al. Pressure ulcers in elderly patients with hip fracture across the continuum of care. J Am Geriatr Soc. 2009;57(5):863-870.
- Sullivan N, Schoelles KM. Preventing in-facility pressure ulcers as a patient safety strategy: a systematic review. Ann Intern Med. 2013;158(5 Pt 2):410-416.
- World Health Organization. Prevention and management of osteoporosis: Report of a WHO scientific group 2000. Geneva, Switzerland; 2000. WHO technical report series; 921. Available at: http://whqlibdoc.who.int/trs/who_trs_921.pdf. Accessed July 9, 2013.
- National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis 2013. Washington, D.C.; 2013. Available at: www.nof.org/files/nof/public/content/file/917/upload/481.pdf. Accessed September 29, 2013.
- Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370(9588):657-666.
- Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(7):1911-1930.
- Ross AC, Taylor CL, Yaktine AL, Del Valle HB, eds. Dietary reference intakes for calcium and vitamin D. Washington, D.C.: National Academies Press; 2011. Lyles KW, Colón-Emeric CS, Magaziner JS, et al.
- Zoledronic acid in reducing clinical fracture and mortality after hip fracture. N Engl J Med. 2007;357: nihpa40967.
- Colón-Emeric CS, Nordsletten L, Olson S, et al. Association between timing of zoledronic acid infusion and hip fracture healing. Osteoporos Int. 2011;22(8):2329-2336.
- Eriksen EF, Lyles KW, Colón-Emeric CS, et al. Antifracture efficacy and reduction of mortality in relation to timing of the first dose of zoledronic acid after hip fracture. J Bone Miner Res. 2009;24(7):1308-1313.
- Byszewski A, Lemay G, Molnar F, Azad N, McMartin SE. Closing the osteoporosis care gap in hip fracture patients: an opportunity to decrease recurrent fractures and hospital admissions. J Osteoporos. 2011;2011:404969.
- Greenspan SL, Wyman, A, Hoovan FH, et al. Predictors of treatment with osteoporosis medications after recent fragility fractures in a multinational cohort of postmenopausal women. J Am Geriatr Soc. 2012;60(3):455-461.
- Kamel HK, Hussain MS, Tariq S, Perry HM, Morley JE. Failure to diagnose and treat osteoporosis in elderly patients hospitalized with hip fracture. Am J Med. 2000;109(4):326-328.
Blood Culture for Uncomplicated SSTI Not Helpful with Bacteriologic Diagnosis
Clinical question: What is the yield of blood cultures performed on pediatric patients admitted for uncomplicated and complicated skin and soft tissue infections (SSTIs and cSSTIs)?
Background: SSTIs are a common cause of pediatric ED visits and hospitalizations. Current Infectious Diseases Society of America (IDSA) guidelines include obtaining a blood culture for patients who show signs of systemic toxicity. Blood cultures are performed frequently in all pediatric patients hospitalized for SSTI and cSSTI. Little recent data exists about the rate of bacteremia in pediatric SSTI since the widespread emergence of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) and adoption of routine vaccination for Haemophilus influenzae type B (HiB) and varicella.
Study design: Single-center, retrospective case series.
Setting: University-affiliated pediatric hospital at a tertiary medical center.
Synopsis: Researchers used the hospital’s electronic medical record system to search for patients between the ages of 0 and 18 years hospitalized for SSTI/cSSTI. Initial screening of the data utilized ICD-9-CM codes for cellulitis and abscess (682.X), with subsequent review by investigators to exclude miscoded cases, immunocompromised patients, hospital-acquired infection, and incidentally noted SSTI during admissions for other problems. SSTIs were classified as being complicated in the cases of surgical or traumatic wound infection, need for surgical intervention, and infected ulcers or burns. Routine incision and drainage did not constitute surgical intervention.
Of the 580 patients remaining, 482 were classified as having SSTI, of which 455 underwent testing with blood cultures. None of the blood cultures led to pathogenic bacterial growth after 120 hours of incubation; three grew S. epidermidis. Of the 98 patients classified as having cSSTI, 80 underwent blood culture testing, of which 10 (12.5%) were positive.
Pathogens identified in positive blood cultures included MRSA (6), methicillin-sensitive S. aureus (3), and S. pneumococcus (1). Length of stay was significantly longer for patients with SSTI who underwent blood culture testing (3.24 days) compared to those who did not (2.33 days).
Bottom line: Obtaining blood cultures in a child hospitalized with uncomplicated SSTI is highly unlikely to be helpful in obtaining a bacteriologic diagnosis. Even worse, it will likely increase the length of stay for these patients.
Citation: Malone JR, Durica SR, Thompson DM, Bogie A, Naifeh M. Blood cultures in the evaluation of uncomplicated skin and soft tissue infections. Pediatrics. 2013;132:454-459.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Clinical question: What is the yield of blood cultures performed on pediatric patients admitted for uncomplicated and complicated skin and soft tissue infections (SSTIs and cSSTIs)?
Background: SSTIs are a common cause of pediatric ED visits and hospitalizations. Current Infectious Diseases Society of America (IDSA) guidelines include obtaining a blood culture for patients who show signs of systemic toxicity. Blood cultures are performed frequently in all pediatric patients hospitalized for SSTI and cSSTI. Little recent data exists about the rate of bacteremia in pediatric SSTI since the widespread emergence of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) and adoption of routine vaccination for Haemophilus influenzae type B (HiB) and varicella.
Study design: Single-center, retrospective case series.
Setting: University-affiliated pediatric hospital at a tertiary medical center.
Synopsis: Researchers used the hospital’s electronic medical record system to search for patients between the ages of 0 and 18 years hospitalized for SSTI/cSSTI. Initial screening of the data utilized ICD-9-CM codes for cellulitis and abscess (682.X), with subsequent review by investigators to exclude miscoded cases, immunocompromised patients, hospital-acquired infection, and incidentally noted SSTI during admissions for other problems. SSTIs were classified as being complicated in the cases of surgical or traumatic wound infection, need for surgical intervention, and infected ulcers or burns. Routine incision and drainage did not constitute surgical intervention.
Of the 580 patients remaining, 482 were classified as having SSTI, of which 455 underwent testing with blood cultures. None of the blood cultures led to pathogenic bacterial growth after 120 hours of incubation; three grew S. epidermidis. Of the 98 patients classified as having cSSTI, 80 underwent blood culture testing, of which 10 (12.5%) were positive.
Pathogens identified in positive blood cultures included MRSA (6), methicillin-sensitive S. aureus (3), and S. pneumococcus (1). Length of stay was significantly longer for patients with SSTI who underwent blood culture testing (3.24 days) compared to those who did not (2.33 days).
Bottom line: Obtaining blood cultures in a child hospitalized with uncomplicated SSTI is highly unlikely to be helpful in obtaining a bacteriologic diagnosis. Even worse, it will likely increase the length of stay for these patients.
Citation: Malone JR, Durica SR, Thompson DM, Bogie A, Naifeh M. Blood cultures in the evaluation of uncomplicated skin and soft tissue infections. Pediatrics. 2013;132:454-459.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Clinical question: What is the yield of blood cultures performed on pediatric patients admitted for uncomplicated and complicated skin and soft tissue infections (SSTIs and cSSTIs)?
Background: SSTIs are a common cause of pediatric ED visits and hospitalizations. Current Infectious Diseases Society of America (IDSA) guidelines include obtaining a blood culture for patients who show signs of systemic toxicity. Blood cultures are performed frequently in all pediatric patients hospitalized for SSTI and cSSTI. Little recent data exists about the rate of bacteremia in pediatric SSTI since the widespread emergence of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) and adoption of routine vaccination for Haemophilus influenzae type B (HiB) and varicella.
Study design: Single-center, retrospective case series.
Setting: University-affiliated pediatric hospital at a tertiary medical center.
Synopsis: Researchers used the hospital’s electronic medical record system to search for patients between the ages of 0 and 18 years hospitalized for SSTI/cSSTI. Initial screening of the data utilized ICD-9-CM codes for cellulitis and abscess (682.X), with subsequent review by investigators to exclude miscoded cases, immunocompromised patients, hospital-acquired infection, and incidentally noted SSTI during admissions for other problems. SSTIs were classified as being complicated in the cases of surgical or traumatic wound infection, need for surgical intervention, and infected ulcers or burns. Routine incision and drainage did not constitute surgical intervention.
Of the 580 patients remaining, 482 were classified as having SSTI, of which 455 underwent testing with blood cultures. None of the blood cultures led to pathogenic bacterial growth after 120 hours of incubation; three grew S. epidermidis. Of the 98 patients classified as having cSSTI, 80 underwent blood culture testing, of which 10 (12.5%) were positive.
Pathogens identified in positive blood cultures included MRSA (6), methicillin-sensitive S. aureus (3), and S. pneumococcus (1). Length of stay was significantly longer for patients with SSTI who underwent blood culture testing (3.24 days) compared to those who did not (2.33 days).
Bottom line: Obtaining blood cultures in a child hospitalized with uncomplicated SSTI is highly unlikely to be helpful in obtaining a bacteriologic diagnosis. Even worse, it will likely increase the length of stay for these patients.
Citation: Malone JR, Durica SR, Thompson DM, Bogie A, Naifeh M. Blood cultures in the evaluation of uncomplicated skin and soft tissue infections. Pediatrics. 2013;132:454-459.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
What Is the Best Empiric Therapy for Community-Acquired Cellulitis?
Editor’s note: This month’s KCQ first appeared in July 2009, and since that time it has been one of our website’s most-read articles, generating 23,000-plus pageviews.
Case
A previously healthy 55-year-old white female presents to the ED with a three-day history of pain and erythema in her right hand. Examination reveals fluctuance as well. She is diagnosed with an abscess with surrounding cellulitis. The abscess is incised, drained, and cultured, and she is sent home on oral trimethoprim/sulfamethoxazole. The following day, her cellulitis has worsened. She is hospitalized and commenced on intravenous vancomycin. What is the best empiric therapy for community-acquired cellulitis?
Background
Cellulitis is defined as a skin and soft-tissue infection (SSTI), which develops as a result of bacterial entry via breaches in the skin barrier. Typically, it involves the dermis and subcutaneous tissue and is associated with local tenderness, erythema, swelling and fever. Cellulitis usually affects the lower extremities, but it can affect other locations, resulting in diagnoses such as periorbital, abdominal wall, buccal, and perianal cellulitis.1,2
Gram-positive organisms, especially Staphylococcus aureus and beta hemolytic streptococci, are the most common causes of cellulitis. Although it is less common, cellulitis can be caused by gram-negative organisms. The recent significant increase in the prevalence of SSTIs due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has led to changes in the selection of antibiotics that were most commonly utilized to empirically treat cellulitis.
The diagnosis of cellulitis is based primarily on clinical manifestations. Due to low diagnostic yields, blood cultures, needle aspiration, or punch biopsy specimens usually are not helpful in the setting of simple cellulitis.3 Therefore, antibiotic therapy is almost universally started empirically. Starting appropriate initial antibiotic therapy improves patient outcomes by reducing mortality rates, length of stay, and inpatient costs.4
Cellulitis incidence is about two cases per 1,000 patient-years.5 This rather high incidence, coupled with escalating rates of SSTIs due to CA-MRSA, demands reliable and cost-effective treatment strategies for the management of community-acquired cellulitis.
Review of the Data
The treatment of community-acquired cellulitis was straightforward until the past decade, as physicians saw a significant increase in CA-MRSA incidence.6 MRSA was reported initially in 1961, only two years after methicillin was introduced into clinical practice.7,8 Subsequently, MRSA prevalence increased dramatically, and by the beginning of this decade, more than 50% of the Staphylococcus aureus hospital strains were resistant to methicillin.8 Furthermore, 60% to 80% of community-acquired Staphylococcus aureus strains in the U.S. are methicillin-resistant.8
The two major types of MRSA infections are healthcare-acquired (HA-MRSA) and community-acquired (CA-MRSA). The HA-MRSA infection group is further subdivided into those strains that develop during a period of hospitalization and those that develop following contact with healthcare facilities (e.g. hospitalization or surgery within the previous year). This subgroup includes HA-MRSA infections in hemodialysis patients, residents of long-term-care facilities, and individuals who have a vascular catheter or other indwelling device.9,10
CA-MRSA infections, on the other hand, occur in individuals who have not had any contact with healthcare facilities. Higher rates of CA-MRSA infection are observed in settings where individuals have close contact with each other, including military trainees, athletes involved in contact sports, patients age 65 and older, men who have sex with other men, and parenteral substance abusers.8,11-13 However, in view of the high prevalence of CA-MRSA in the U.S., most patients, including those without any apparent risk factors, are at risk.8
HA-MRSA has the ability to survive on inanimate objects for extended time periods, increasing the likelihood of transmission to persons who come into contact with those objects. Although evidence has not confirmed that CA-MRSA has a similar capacity, it seems plausible that such spread does contribute to the propagation of CA-MRSA.12
The increasing importance of CA-MRSA also is evident in hospital settings, where it is replacing HA-MRSA as the most common type of Staphylococcus aureus. Because CA-MRSA tends to be susceptible to a larger number of antibiotics than HA-MRSA is, this has led to a reduced incidence of multidrug resistance. Fortunately, unlike HA-MRSA, CA-MRSA is susceptible to non-beta-lactam antibiotics, including tetracyclines, sulfonamides, and clindamycin.9
CA-MRSA most often causes SSTIs, and a tender abscess is a typical presentation.8 Patients commonly misinterpret early skin lesions as an insect or spider bite.12,14 When cutaneous CA-MRSA presents as an abscess, an incision and drainage procedure is essential for adequate treatment of the infection. For some CA-MRSA infections, particularly those characterized by the presence of a relatively small abscess, it might be adequate to do only an incision and drainage procedure, and not administer antibiotics.8,15 However, in most instances, especially when there is an area of cellulitis around the abscess, the initiation of antibiotic therapy improves patients’ clinical outcomes.9,16
When there is no apparent drainable purulent fluid collection, which often occurs with cellulitis, antibiotics should be the mainstay of therapy. The decision about which antibiotic to start can present some challenges, because the organism causing the cellulitis usually is not identified. This is because blood cultures are positive in less than 5% of cases. Also, positive culture results from needle aspiration are only helpful 5% to 40% of the time. Meanwhile, culture of punch biopsy specimens yields a pathogen in only 20% to 30% of cases.3,17-19
Due to increased CA-MRSA incidence, cephalexin should not be prescribed to treat cellulitis in the outpatient setting because it does not provide coverage for the pathogen.13 Instead, oral antibiotics (e.g. clindamycin or trimethoprim/sulfamethoxazole) should be prescribed. Doxycycline, minocycline, rifampin (usually prescribed in combination with fusidic acid to prevent resistance development), and linezolid are additional therapeutic options.
Trimethoprim/sulfamethoxazole and clindamycin have several advantages: good oral bioavailability, familiarity to physicians, and general affordability. A disadvantage to using both trimethoprim/sulfamethoxazole and doxycycline is that they provide inadequate coverage for group A streptococci, which are a common cause of cellulitis. Therefore, the simultaneous use of a beta-lactam antibiotic with either of these medications may improve outcomes for “nonpurulent” cellulitis.13,15 Linezolid has proven effective for SSTIs caused by MRSA, even though it is not bactericidal.
Excellent oral bioavailability of this drug is an attractive characteristic, as it facilitates the transition from the use of intravenous to oral antibiotic therapy later in a patient’s hospital course. Although oral linezolid has been studied in clinical trials and provides good coverage for MRSA, its use in the outpatient setting is relatively limited, largely due to its significant cost.20 In 2008, the cost of 10 days of treatment with oral linezolid was $1,286.80. In comparison, the generic trimethoprim/sulfamethoxazole cost $9.40, and generic clindamycin cost $95.10.8 The lack of routine availability in many outpatient pharmacies also hinders the widespread use of linezolid.13
To date, with the exception of linezolid, no randomized prospective clinical trials clearly demonstrate the efficacy of the oral agents that are commonly used for the outpatient treatment of cellulitis.20
When patients require hospitalization for the optimal treatment of cellulitis, it is important to select a parenteral antibiotic that provides coverage for MRSA.8 Vancomycin, daptomycin, linezolid, and tigecycline are the most commonly used agents.6
In the inpatient setting, failure to initiate appropriate medical therapy can result in longer hospital admissions, which significantly increase inpatient costs. Inadequate antibiotic therapy creates a significant financial burden and has been associated with increased mortality.4 Historically, vancomycin is used whenever a MRSA infection is suspected. However, there is concern about the declining efficacy of vancomycin related to a gradual increase in the rate of relative resistance—a minimal inhibitory concentration (MIC) increase—in MRSA strains. This MIC creep is noted in some medical centers and can lead to a failure to respond to vancomycin.13,20
Daptomycin is rapidly bactericidal against MRSA; in some institutions, its use may be preferred over vancomycin because the former antibiotic is associated with a significantly more rapid clinical response, which may shorten the required length of hospitalization.21 The once-daily dosing requirement for daptomycin allows for ease of use in both hospital and outpatient settings, and therefore may facilitate early hospital discharge or prevent the need for hospitalization altogether. Clinical experience also suggests potential economic advantages with the use of daptomycin.22
Tigecycline is a bacteriostatic antibiotic that achieves low serum concentrations. However, it penetrates the skin well and has a similar effectiveness to combination therapy with vancomycin and aztreonam. Thus far, tigecycline is not widely used for the treatment of MRSA infections, and it has been suggested that it may be preferred for polymicrobial infections or for patients who exhibit allergies to more commonly used agents.8
When selecting an antibiotic therapy, cost considerations play an important role in the decision-making process. For intravenous agents commonly used to treat CA-MRSA infections, the 2008 cost for 10 days of treatment with generic vancomycin was $182.80; daptomycin cost $1,660.80. For tigecycline and linezolid, the same duration of treatment cost $1,362 and $1,560, respectively.8
Back to the Case
Our patient, an otherwise healthy female, presented with no apparent risk factors for developing a CA-MRSA SSTI. However, more detailed history revealed that she regularly used sports equipment at her local fitness center. Based on her clinical presentation and concerns about the high local prevalence of CA-MRSA, an incision and drainage procedure was performed, and she was started empirically on IV vancomycin. She had a positive clinical response to this treatment.
Wound culture results confirmed CA-MRSA abscess and cellulitis, susceptible to trimethoprim/sulfamethoxazole. She was discharged on the oral formulation of this antibiotic to complete a 10-day course of treatment, including the days she received intravenous antibiotics.
Few well-designed trials have compared different lengths of cellulitis therapy. Most authorities recommend five to 10 days of treatment; however, longer courses might be required for more severe or complicated diseases.
Bottom line
Because of the high prevalence of CA-MRSA, initial antibiotic therapy for the treatment of community-acquired cellulitis must provide coverage for this organism.
Dr. Clarke is a hospitalist and assistant professor of medicine at Emory University School of Medicine, Atlanta. Dr. Dressler is a professor of medicine, hospital medicine associate division director for education, and associate program director for the J. Willis Hurst Internal Medicine Residency Program. Dr. Purohit, formerly an instructor in clinical medicine at Emory, is a hospitalist at WakeMed Health and Hospitals in Raleigh, N.C.
References
- Barzilai A, Choen HA. Isolation of group A streptococci from children with perianal cellulitis and from their siblings. Pediatr Infect Dis J. 1998;17(4):358-360.
- Thorsteinsdottir B, Tleyjeh IM, Baddour LM. Abdominal wall cellulitis in the morbidly obese. Scand J Infect Dis. 2005;37(8):605-608.
- Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350(9):904-912.
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160-169.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. Incidence of lower extremity cellulitis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2007;82(7):817-821.
- Moellering RC. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(7):1032-1037.
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7(2):178-182.
- Moellering RC. A 39-year-old man with a skin infection. JAMA. 2008;299(1):79-87.
- Ruhe J, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44(6):777-784.
- David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197(9):1235-1243.
- Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854-858.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(42):919-922.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380-390.
- Dominguez TJ. It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract. 2004;17(3):220-226.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
- Jetton L. Therapy for methicillin-resistant Staphylococcus aureus. N Engl J Med. 2006;355(20):2153-2155.
- Hook EW, Hooton TM, Horton CA, et al. Microbiologic evaluation of cutaneous cellulitis in adults. Arch Intern Med. 1986;146(2):295-297. Duvanel T, Auckenthaler R, Rohner P, Harms M,
- Saurat JH. Quantitative cultures of biopsy specimens from cutaneous cellulitis. Arch Intern Med. 1989;149(2):293-296.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol. 1988; 26(3):401-404.
- Loffler CA, Macdougall C. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti Infect Ther. 2007;5(6):961-981.
- Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611-1618.
- Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15-23.
Editor’s note: This month’s KCQ first appeared in July 2009, and since that time it has been one of our website’s most-read articles, generating 23,000-plus pageviews.
Case
A previously healthy 55-year-old white female presents to the ED with a three-day history of pain and erythema in her right hand. Examination reveals fluctuance as well. She is diagnosed with an abscess with surrounding cellulitis. The abscess is incised, drained, and cultured, and she is sent home on oral trimethoprim/sulfamethoxazole. The following day, her cellulitis has worsened. She is hospitalized and commenced on intravenous vancomycin. What is the best empiric therapy for community-acquired cellulitis?
Background
Cellulitis is defined as a skin and soft-tissue infection (SSTI), which develops as a result of bacterial entry via breaches in the skin barrier. Typically, it involves the dermis and subcutaneous tissue and is associated with local tenderness, erythema, swelling and fever. Cellulitis usually affects the lower extremities, but it can affect other locations, resulting in diagnoses such as periorbital, abdominal wall, buccal, and perianal cellulitis.1,2
Gram-positive organisms, especially Staphylococcus aureus and beta hemolytic streptococci, are the most common causes of cellulitis. Although it is less common, cellulitis can be caused by gram-negative organisms. The recent significant increase in the prevalence of SSTIs due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has led to changes in the selection of antibiotics that were most commonly utilized to empirically treat cellulitis.
The diagnosis of cellulitis is based primarily on clinical manifestations. Due to low diagnostic yields, blood cultures, needle aspiration, or punch biopsy specimens usually are not helpful in the setting of simple cellulitis.3 Therefore, antibiotic therapy is almost universally started empirically. Starting appropriate initial antibiotic therapy improves patient outcomes by reducing mortality rates, length of stay, and inpatient costs.4
Cellulitis incidence is about two cases per 1,000 patient-years.5 This rather high incidence, coupled with escalating rates of SSTIs due to CA-MRSA, demands reliable and cost-effective treatment strategies for the management of community-acquired cellulitis.
Review of the Data
The treatment of community-acquired cellulitis was straightforward until the past decade, as physicians saw a significant increase in CA-MRSA incidence.6 MRSA was reported initially in 1961, only two years after methicillin was introduced into clinical practice.7,8 Subsequently, MRSA prevalence increased dramatically, and by the beginning of this decade, more than 50% of the Staphylococcus aureus hospital strains were resistant to methicillin.8 Furthermore, 60% to 80% of community-acquired Staphylococcus aureus strains in the U.S. are methicillin-resistant.8
The two major types of MRSA infections are healthcare-acquired (HA-MRSA) and community-acquired (CA-MRSA). The HA-MRSA infection group is further subdivided into those strains that develop during a period of hospitalization and those that develop following contact with healthcare facilities (e.g. hospitalization or surgery within the previous year). This subgroup includes HA-MRSA infections in hemodialysis patients, residents of long-term-care facilities, and individuals who have a vascular catheter or other indwelling device.9,10
CA-MRSA infections, on the other hand, occur in individuals who have not had any contact with healthcare facilities. Higher rates of CA-MRSA infection are observed in settings where individuals have close contact with each other, including military trainees, athletes involved in contact sports, patients age 65 and older, men who have sex with other men, and parenteral substance abusers.8,11-13 However, in view of the high prevalence of CA-MRSA in the U.S., most patients, including those without any apparent risk factors, are at risk.8
HA-MRSA has the ability to survive on inanimate objects for extended time periods, increasing the likelihood of transmission to persons who come into contact with those objects. Although evidence has not confirmed that CA-MRSA has a similar capacity, it seems plausible that such spread does contribute to the propagation of CA-MRSA.12
The increasing importance of CA-MRSA also is evident in hospital settings, where it is replacing HA-MRSA as the most common type of Staphylococcus aureus. Because CA-MRSA tends to be susceptible to a larger number of antibiotics than HA-MRSA is, this has led to a reduced incidence of multidrug resistance. Fortunately, unlike HA-MRSA, CA-MRSA is susceptible to non-beta-lactam antibiotics, including tetracyclines, sulfonamides, and clindamycin.9
CA-MRSA most often causes SSTIs, and a tender abscess is a typical presentation.8 Patients commonly misinterpret early skin lesions as an insect or spider bite.12,14 When cutaneous CA-MRSA presents as an abscess, an incision and drainage procedure is essential for adequate treatment of the infection. For some CA-MRSA infections, particularly those characterized by the presence of a relatively small abscess, it might be adequate to do only an incision and drainage procedure, and not administer antibiotics.8,15 However, in most instances, especially when there is an area of cellulitis around the abscess, the initiation of antibiotic therapy improves patients’ clinical outcomes.9,16
When there is no apparent drainable purulent fluid collection, which often occurs with cellulitis, antibiotics should be the mainstay of therapy. The decision about which antibiotic to start can present some challenges, because the organism causing the cellulitis usually is not identified. This is because blood cultures are positive in less than 5% of cases. Also, positive culture results from needle aspiration are only helpful 5% to 40% of the time. Meanwhile, culture of punch biopsy specimens yields a pathogen in only 20% to 30% of cases.3,17-19
Due to increased CA-MRSA incidence, cephalexin should not be prescribed to treat cellulitis in the outpatient setting because it does not provide coverage for the pathogen.13 Instead, oral antibiotics (e.g. clindamycin or trimethoprim/sulfamethoxazole) should be prescribed. Doxycycline, minocycline, rifampin (usually prescribed in combination with fusidic acid to prevent resistance development), and linezolid are additional therapeutic options.
Trimethoprim/sulfamethoxazole and clindamycin have several advantages: good oral bioavailability, familiarity to physicians, and general affordability. A disadvantage to using both trimethoprim/sulfamethoxazole and doxycycline is that they provide inadequate coverage for group A streptococci, which are a common cause of cellulitis. Therefore, the simultaneous use of a beta-lactam antibiotic with either of these medications may improve outcomes for “nonpurulent” cellulitis.13,15 Linezolid has proven effective for SSTIs caused by MRSA, even though it is not bactericidal.
Excellent oral bioavailability of this drug is an attractive characteristic, as it facilitates the transition from the use of intravenous to oral antibiotic therapy later in a patient’s hospital course. Although oral linezolid has been studied in clinical trials and provides good coverage for MRSA, its use in the outpatient setting is relatively limited, largely due to its significant cost.20 In 2008, the cost of 10 days of treatment with oral linezolid was $1,286.80. In comparison, the generic trimethoprim/sulfamethoxazole cost $9.40, and generic clindamycin cost $95.10.8 The lack of routine availability in many outpatient pharmacies also hinders the widespread use of linezolid.13
To date, with the exception of linezolid, no randomized prospective clinical trials clearly demonstrate the efficacy of the oral agents that are commonly used for the outpatient treatment of cellulitis.20
When patients require hospitalization for the optimal treatment of cellulitis, it is important to select a parenteral antibiotic that provides coverage for MRSA.8 Vancomycin, daptomycin, linezolid, and tigecycline are the most commonly used agents.6
In the inpatient setting, failure to initiate appropriate medical therapy can result in longer hospital admissions, which significantly increase inpatient costs. Inadequate antibiotic therapy creates a significant financial burden and has been associated with increased mortality.4 Historically, vancomycin is used whenever a MRSA infection is suspected. However, there is concern about the declining efficacy of vancomycin related to a gradual increase in the rate of relative resistance—a minimal inhibitory concentration (MIC) increase—in MRSA strains. This MIC creep is noted in some medical centers and can lead to a failure to respond to vancomycin.13,20
Daptomycin is rapidly bactericidal against MRSA; in some institutions, its use may be preferred over vancomycin because the former antibiotic is associated with a significantly more rapid clinical response, which may shorten the required length of hospitalization.21 The once-daily dosing requirement for daptomycin allows for ease of use in both hospital and outpatient settings, and therefore may facilitate early hospital discharge or prevent the need for hospitalization altogether. Clinical experience also suggests potential economic advantages with the use of daptomycin.22
Tigecycline is a bacteriostatic antibiotic that achieves low serum concentrations. However, it penetrates the skin well and has a similar effectiveness to combination therapy with vancomycin and aztreonam. Thus far, tigecycline is not widely used for the treatment of MRSA infections, and it has been suggested that it may be preferred for polymicrobial infections or for patients who exhibit allergies to more commonly used agents.8
When selecting an antibiotic therapy, cost considerations play an important role in the decision-making process. For intravenous agents commonly used to treat CA-MRSA infections, the 2008 cost for 10 days of treatment with generic vancomycin was $182.80; daptomycin cost $1,660.80. For tigecycline and linezolid, the same duration of treatment cost $1,362 and $1,560, respectively.8
Back to the Case
Our patient, an otherwise healthy female, presented with no apparent risk factors for developing a CA-MRSA SSTI. However, more detailed history revealed that she regularly used sports equipment at her local fitness center. Based on her clinical presentation and concerns about the high local prevalence of CA-MRSA, an incision and drainage procedure was performed, and she was started empirically on IV vancomycin. She had a positive clinical response to this treatment.
Wound culture results confirmed CA-MRSA abscess and cellulitis, susceptible to trimethoprim/sulfamethoxazole. She was discharged on the oral formulation of this antibiotic to complete a 10-day course of treatment, including the days she received intravenous antibiotics.
Few well-designed trials have compared different lengths of cellulitis therapy. Most authorities recommend five to 10 days of treatment; however, longer courses might be required for more severe or complicated diseases.
Bottom line
Because of the high prevalence of CA-MRSA, initial antibiotic therapy for the treatment of community-acquired cellulitis must provide coverage for this organism.
Dr. Clarke is a hospitalist and assistant professor of medicine at Emory University School of Medicine, Atlanta. Dr. Dressler is a professor of medicine, hospital medicine associate division director for education, and associate program director for the J. Willis Hurst Internal Medicine Residency Program. Dr. Purohit, formerly an instructor in clinical medicine at Emory, is a hospitalist at WakeMed Health and Hospitals in Raleigh, N.C.
References
- Barzilai A, Choen HA. Isolation of group A streptococci from children with perianal cellulitis and from their siblings. Pediatr Infect Dis J. 1998;17(4):358-360.
- Thorsteinsdottir B, Tleyjeh IM, Baddour LM. Abdominal wall cellulitis in the morbidly obese. Scand J Infect Dis. 2005;37(8):605-608.
- Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350(9):904-912.
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160-169.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. Incidence of lower extremity cellulitis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2007;82(7):817-821.
- Moellering RC. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(7):1032-1037.
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7(2):178-182.
- Moellering RC. A 39-year-old man with a skin infection. JAMA. 2008;299(1):79-87.
- Ruhe J, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44(6):777-784.
- David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197(9):1235-1243.
- Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854-858.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(42):919-922.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380-390.
- Dominguez TJ. It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract. 2004;17(3):220-226.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
- Jetton L. Therapy for methicillin-resistant Staphylococcus aureus. N Engl J Med. 2006;355(20):2153-2155.
- Hook EW, Hooton TM, Horton CA, et al. Microbiologic evaluation of cutaneous cellulitis in adults. Arch Intern Med. 1986;146(2):295-297. Duvanel T, Auckenthaler R, Rohner P, Harms M,
- Saurat JH. Quantitative cultures of biopsy specimens from cutaneous cellulitis. Arch Intern Med. 1989;149(2):293-296.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol. 1988; 26(3):401-404.
- Loffler CA, Macdougall C. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti Infect Ther. 2007;5(6):961-981.
- Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611-1618.
- Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15-23.
Editor’s note: This month’s KCQ first appeared in July 2009, and since that time it has been one of our website’s most-read articles, generating 23,000-plus pageviews.
Case
A previously healthy 55-year-old white female presents to the ED with a three-day history of pain and erythema in her right hand. Examination reveals fluctuance as well. She is diagnosed with an abscess with surrounding cellulitis. The abscess is incised, drained, and cultured, and she is sent home on oral trimethoprim/sulfamethoxazole. The following day, her cellulitis has worsened. She is hospitalized and commenced on intravenous vancomycin. What is the best empiric therapy for community-acquired cellulitis?
Background
Cellulitis is defined as a skin and soft-tissue infection (SSTI), which develops as a result of bacterial entry via breaches in the skin barrier. Typically, it involves the dermis and subcutaneous tissue and is associated with local tenderness, erythema, swelling and fever. Cellulitis usually affects the lower extremities, but it can affect other locations, resulting in diagnoses such as periorbital, abdominal wall, buccal, and perianal cellulitis.1,2
Gram-positive organisms, especially Staphylococcus aureus and beta hemolytic streptococci, are the most common causes of cellulitis. Although it is less common, cellulitis can be caused by gram-negative organisms. The recent significant increase in the prevalence of SSTIs due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) has led to changes in the selection of antibiotics that were most commonly utilized to empirically treat cellulitis.
The diagnosis of cellulitis is based primarily on clinical manifestations. Due to low diagnostic yields, blood cultures, needle aspiration, or punch biopsy specimens usually are not helpful in the setting of simple cellulitis.3 Therefore, antibiotic therapy is almost universally started empirically. Starting appropriate initial antibiotic therapy improves patient outcomes by reducing mortality rates, length of stay, and inpatient costs.4
Cellulitis incidence is about two cases per 1,000 patient-years.5 This rather high incidence, coupled with escalating rates of SSTIs due to CA-MRSA, demands reliable and cost-effective treatment strategies for the management of community-acquired cellulitis.
Review of the Data
The treatment of community-acquired cellulitis was straightforward until the past decade, as physicians saw a significant increase in CA-MRSA incidence.6 MRSA was reported initially in 1961, only two years after methicillin was introduced into clinical practice.7,8 Subsequently, MRSA prevalence increased dramatically, and by the beginning of this decade, more than 50% of the Staphylococcus aureus hospital strains were resistant to methicillin.8 Furthermore, 60% to 80% of community-acquired Staphylococcus aureus strains in the U.S. are methicillin-resistant.8
The two major types of MRSA infections are healthcare-acquired (HA-MRSA) and community-acquired (CA-MRSA). The HA-MRSA infection group is further subdivided into those strains that develop during a period of hospitalization and those that develop following contact with healthcare facilities (e.g. hospitalization or surgery within the previous year). This subgroup includes HA-MRSA infections in hemodialysis patients, residents of long-term-care facilities, and individuals who have a vascular catheter or other indwelling device.9,10
CA-MRSA infections, on the other hand, occur in individuals who have not had any contact with healthcare facilities. Higher rates of CA-MRSA infection are observed in settings where individuals have close contact with each other, including military trainees, athletes involved in contact sports, patients age 65 and older, men who have sex with other men, and parenteral substance abusers.8,11-13 However, in view of the high prevalence of CA-MRSA in the U.S., most patients, including those without any apparent risk factors, are at risk.8
HA-MRSA has the ability to survive on inanimate objects for extended time periods, increasing the likelihood of transmission to persons who come into contact with those objects. Although evidence has not confirmed that CA-MRSA has a similar capacity, it seems plausible that such spread does contribute to the propagation of CA-MRSA.12
The increasing importance of CA-MRSA also is evident in hospital settings, where it is replacing HA-MRSA as the most common type of Staphylococcus aureus. Because CA-MRSA tends to be susceptible to a larger number of antibiotics than HA-MRSA is, this has led to a reduced incidence of multidrug resistance. Fortunately, unlike HA-MRSA, CA-MRSA is susceptible to non-beta-lactam antibiotics, including tetracyclines, sulfonamides, and clindamycin.9
CA-MRSA most often causes SSTIs, and a tender abscess is a typical presentation.8 Patients commonly misinterpret early skin lesions as an insect or spider bite.12,14 When cutaneous CA-MRSA presents as an abscess, an incision and drainage procedure is essential for adequate treatment of the infection. For some CA-MRSA infections, particularly those characterized by the presence of a relatively small abscess, it might be adequate to do only an incision and drainage procedure, and not administer antibiotics.8,15 However, in most instances, especially when there is an area of cellulitis around the abscess, the initiation of antibiotic therapy improves patients’ clinical outcomes.9,16
When there is no apparent drainable purulent fluid collection, which often occurs with cellulitis, antibiotics should be the mainstay of therapy. The decision about which antibiotic to start can present some challenges, because the organism causing the cellulitis usually is not identified. This is because blood cultures are positive in less than 5% of cases. Also, positive culture results from needle aspiration are only helpful 5% to 40% of the time. Meanwhile, culture of punch biopsy specimens yields a pathogen in only 20% to 30% of cases.3,17-19
Due to increased CA-MRSA incidence, cephalexin should not be prescribed to treat cellulitis in the outpatient setting because it does not provide coverage for the pathogen.13 Instead, oral antibiotics (e.g. clindamycin or trimethoprim/sulfamethoxazole) should be prescribed. Doxycycline, minocycline, rifampin (usually prescribed in combination with fusidic acid to prevent resistance development), and linezolid are additional therapeutic options.
Trimethoprim/sulfamethoxazole and clindamycin have several advantages: good oral bioavailability, familiarity to physicians, and general affordability. A disadvantage to using both trimethoprim/sulfamethoxazole and doxycycline is that they provide inadequate coverage for group A streptococci, which are a common cause of cellulitis. Therefore, the simultaneous use of a beta-lactam antibiotic with either of these medications may improve outcomes for “nonpurulent” cellulitis.13,15 Linezolid has proven effective for SSTIs caused by MRSA, even though it is not bactericidal.
Excellent oral bioavailability of this drug is an attractive characteristic, as it facilitates the transition from the use of intravenous to oral antibiotic therapy later in a patient’s hospital course. Although oral linezolid has been studied in clinical trials and provides good coverage for MRSA, its use in the outpatient setting is relatively limited, largely due to its significant cost.20 In 2008, the cost of 10 days of treatment with oral linezolid was $1,286.80. In comparison, the generic trimethoprim/sulfamethoxazole cost $9.40, and generic clindamycin cost $95.10.8 The lack of routine availability in many outpatient pharmacies also hinders the widespread use of linezolid.13
To date, with the exception of linezolid, no randomized prospective clinical trials clearly demonstrate the efficacy of the oral agents that are commonly used for the outpatient treatment of cellulitis.20
When patients require hospitalization for the optimal treatment of cellulitis, it is important to select a parenteral antibiotic that provides coverage for MRSA.8 Vancomycin, daptomycin, linezolid, and tigecycline are the most commonly used agents.6
In the inpatient setting, failure to initiate appropriate medical therapy can result in longer hospital admissions, which significantly increase inpatient costs. Inadequate antibiotic therapy creates a significant financial burden and has been associated with increased mortality.4 Historically, vancomycin is used whenever a MRSA infection is suspected. However, there is concern about the declining efficacy of vancomycin related to a gradual increase in the rate of relative resistance—a minimal inhibitory concentration (MIC) increase—in MRSA strains. This MIC creep is noted in some medical centers and can lead to a failure to respond to vancomycin.13,20
Daptomycin is rapidly bactericidal against MRSA; in some institutions, its use may be preferred over vancomycin because the former antibiotic is associated with a significantly more rapid clinical response, which may shorten the required length of hospitalization.21 The once-daily dosing requirement for daptomycin allows for ease of use in both hospital and outpatient settings, and therefore may facilitate early hospital discharge or prevent the need for hospitalization altogether. Clinical experience also suggests potential economic advantages with the use of daptomycin.22
Tigecycline is a bacteriostatic antibiotic that achieves low serum concentrations. However, it penetrates the skin well and has a similar effectiveness to combination therapy with vancomycin and aztreonam. Thus far, tigecycline is not widely used for the treatment of MRSA infections, and it has been suggested that it may be preferred for polymicrobial infections or for patients who exhibit allergies to more commonly used agents.8
When selecting an antibiotic therapy, cost considerations play an important role in the decision-making process. For intravenous agents commonly used to treat CA-MRSA infections, the 2008 cost for 10 days of treatment with generic vancomycin was $182.80; daptomycin cost $1,660.80. For tigecycline and linezolid, the same duration of treatment cost $1,362 and $1,560, respectively.8
Back to the Case
Our patient, an otherwise healthy female, presented with no apparent risk factors for developing a CA-MRSA SSTI. However, more detailed history revealed that she regularly used sports equipment at her local fitness center. Based on her clinical presentation and concerns about the high local prevalence of CA-MRSA, an incision and drainage procedure was performed, and she was started empirically on IV vancomycin. She had a positive clinical response to this treatment.
Wound culture results confirmed CA-MRSA abscess and cellulitis, susceptible to trimethoprim/sulfamethoxazole. She was discharged on the oral formulation of this antibiotic to complete a 10-day course of treatment, including the days she received intravenous antibiotics.
Few well-designed trials have compared different lengths of cellulitis therapy. Most authorities recommend five to 10 days of treatment; however, longer courses might be required for more severe or complicated diseases.
Bottom line
Because of the high prevalence of CA-MRSA, initial antibiotic therapy for the treatment of community-acquired cellulitis must provide coverage for this organism.
Dr. Clarke is a hospitalist and assistant professor of medicine at Emory University School of Medicine, Atlanta. Dr. Dressler is a professor of medicine, hospital medicine associate division director for education, and associate program director for the J. Willis Hurst Internal Medicine Residency Program. Dr. Purohit, formerly an instructor in clinical medicine at Emory, is a hospitalist at WakeMed Health and Hospitals in Raleigh, N.C.
References
- Barzilai A, Choen HA. Isolation of group A streptococci from children with perianal cellulitis and from their siblings. Pediatr Infect Dis J. 1998;17(4):358-360.
- Thorsteinsdottir B, Tleyjeh IM, Baddour LM. Abdominal wall cellulitis in the morbidly obese. Scand J Infect Dis. 2005;37(8):605-608.
- Swartz MN. Clinical practice. Cellulitis. N Engl J Med. 2004;350(9):904-912.
- Edelsberg J, Berger A, Weber DJ, et al. Clinical and economic consequences of failure of initial antibiotic therapy for hospitalized patients with complicated skin and skin-structure infections. Infect Control Hosp Epidemiol. 2008;29(2):160-169.
- McNamara DR, Tleyjeh IM, Berbari EF, et al. Incidence of lower extremity cellulitis: a population-based study in Olmsted County, Minnesota. Mayo Clin Proc. 2007;82(7):817-821.
- Moellering RC. Current treatment options for community-acquired methicillin-resistant Staphylococcus aureus infection. Clin Infect Dis. 2008;46(7):1032-1037.
- Chambers HF. The changing epidemiology of Staphylococcus aureus. Emerg Infect Dis. 2001;7(2):178-182.
- Moellering RC. A 39-year-old man with a skin infection. JAMA. 2008;299(1):79-87.
- Ruhe J, Smith N, Bradsher RW, Menon A. Community-onset methicillin-resistant Staphylococcus aureus skin and soft tissue infections: impact of antimicrobial therapy on outcome. Clin Infect Dis. 2007;44(6):777-784.
- David MZ, Glikman D, Crawford SE, et al. What is community-associated methicillin-resistant Staphylococcus aureus? J Infect Dis. 2008;197(9):1235-1243.
- Iyer S, Jones DH. Community-acquired methicillin-resistant Staphylococcus aureus skin infection: a retrospective analysis of clinical presentation and treatment of a local outbreak. J Am Acad Dermatol. 2004;50(6):854-858.
- Centers for Disease Control and Prevention. Methicillin-resistant Staphylococcus aureus skin or soft tissue infections in a state prison—Mississippi, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(42):919-922.
- Daum RS. Clinical practice. Skin and soft-tissue infections caused by methicillin-resistant Staphylococcus aureus. N Engl J Med. 2007;357(4):380-390.
- Dominguez TJ. It’s not a spider bite, it’s community-acquired methicillin-resistant Staphylococcus aureus. J Am Board Fam Pract. 2004;17(3):220-226.
- Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355(7):666-674.
- Jetton L. Therapy for methicillin-resistant Staphylococcus aureus. N Engl J Med. 2006;355(20):2153-2155.
- Hook EW, Hooton TM, Horton CA, et al. Microbiologic evaluation of cutaneous cellulitis in adults. Arch Intern Med. 1986;146(2):295-297. Duvanel T, Auckenthaler R, Rohner P, Harms M,
- Saurat JH. Quantitative cultures of biopsy specimens from cutaneous cellulitis. Arch Intern Med. 1989;149(2):293-296.
- Newell PM, Norden CW. Value of needle aspiration in bacteriologic diagnosis of cellulitis in adults. J Clin Microbiol. 1988; 26(3):401-404.
- Loffler CA, Macdougall C. Update on prevalence and treatment of methicillin-resistant Staphylococcus aureus infections. Expert Rev Anti Infect Ther. 2007;5(6):961-981.
- Davis SL, McKinnon PS, Hall LM, et al. Daptomycin versus vancomycin for complicated skin and skin structure infections: clinical and economic outcomes. Pharmacotherapy. 2007;27(12):1611-1618.
- Seaton RA. Daptomycin: rationale and role in the management of skin and soft tissue infections. J Antimicrob Chemother. 2008;62(Suppl 3):iii15-23.
Hospital-Based Palliative Care Reduces Length of Stay, Charges, Invasive Procedures, ICU Deaths
Clinical question: What are the characteristics of children who died in children’s hospitals while receiving palliative care (PC) compared to those who did not?
Background: Approximately 44,000 children die annually in hospitals in the U.S. Since the American Academy of Pediatrics (AAP) released a statement in August 2000 that presented an integrated model for providing PC to children with life-threatening conditions, pediatric PC programs have increased steadily in number. Children who receive PC services are commonly afflicted by genetic/congenital disorders, neuromuscular disorders, and cancer diagnoses. Although it is estimated that 6,320 people under the age of 24 received PC services in 2010, little data exist comparing pediatric inpatients receiving PC and those who do not.
Study design: Multicenter retrospective cohort study.
Setting: More than 40 freestanding children’s hospitals.
Synopsis: Using the Pediatric Health Information System (PHIS) database, which collects administrative and clinical data from more than 40 freestanding children’s hospitals belonging to the Children’s Hospital Association, researchers analyzed the characteristics of children under the age of 18 who died in the hospital more than five days after admission from 2001 to 2011. They extracted demographic data and categorized patients using major diagnostic categories (MDC) based on major organ system or etiology of disease. Identification of patients receiving PC services was by ICD-9 codes, and utilization of medications and procedures was identified by clinical transaction codes (CTC) and ICD-9 codes. The unit billing the last hospital day determined location of death.
Of the 24,342 children studied, only 3.8% received PC services based on coding. Patients less likely to receive PC services included black children (2.3%), those with circulatory diseases (2.8%), and those with neonatal diseases (1.9%). Children who did receive PC services had a significantly lower median length of stay (17 vs. 21 days), average daily charges ($9,348 vs. $11,806), received significantly fewer interventions (mechanical ventilation, invasive monitoring, surgical procedures), and died less frequently in an ICU setting (60% vs. 88%). PC services disproportionately altered the care of children with lymphatic/hematopoietic diseases, significantly decreasing use of mechanical ventilation (75% to 22%) and death in an ICU setting (66% to 21%).
Bottom line: Provision of PC services to children dying in children’s hospitals remains low. It is even lower for children with certain racial backgrounds and disease processes. When provided, PC services reduce length of stay, average daily charges, invasive procedures, and death in an ICU setting.
Citation: Keele L, Keenan HT, Sheetz J. Differences in characteristics of dying children who receive and do not receive palliative care. Pediatrics. 2013;132(1):72-78.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Clinical question: What are the characteristics of children who died in children’s hospitals while receiving palliative care (PC) compared to those who did not?
Background: Approximately 44,000 children die annually in hospitals in the U.S. Since the American Academy of Pediatrics (AAP) released a statement in August 2000 that presented an integrated model for providing PC to children with life-threatening conditions, pediatric PC programs have increased steadily in number. Children who receive PC services are commonly afflicted by genetic/congenital disorders, neuromuscular disorders, and cancer diagnoses. Although it is estimated that 6,320 people under the age of 24 received PC services in 2010, little data exist comparing pediatric inpatients receiving PC and those who do not.
Study design: Multicenter retrospective cohort study.
Setting: More than 40 freestanding children’s hospitals.
Synopsis: Using the Pediatric Health Information System (PHIS) database, which collects administrative and clinical data from more than 40 freestanding children’s hospitals belonging to the Children’s Hospital Association, researchers analyzed the characteristics of children under the age of 18 who died in the hospital more than five days after admission from 2001 to 2011. They extracted demographic data and categorized patients using major diagnostic categories (MDC) based on major organ system or etiology of disease. Identification of patients receiving PC services was by ICD-9 codes, and utilization of medications and procedures was identified by clinical transaction codes (CTC) and ICD-9 codes. The unit billing the last hospital day determined location of death.
Of the 24,342 children studied, only 3.8% received PC services based on coding. Patients less likely to receive PC services included black children (2.3%), those with circulatory diseases (2.8%), and those with neonatal diseases (1.9%). Children who did receive PC services had a significantly lower median length of stay (17 vs. 21 days), average daily charges ($9,348 vs. $11,806), received significantly fewer interventions (mechanical ventilation, invasive monitoring, surgical procedures), and died less frequently in an ICU setting (60% vs. 88%). PC services disproportionately altered the care of children with lymphatic/hematopoietic diseases, significantly decreasing use of mechanical ventilation (75% to 22%) and death in an ICU setting (66% to 21%).
Bottom line: Provision of PC services to children dying in children’s hospitals remains low. It is even lower for children with certain racial backgrounds and disease processes. When provided, PC services reduce length of stay, average daily charges, invasive procedures, and death in an ICU setting.
Citation: Keele L, Keenan HT, Sheetz J. Differences in characteristics of dying children who receive and do not receive palliative care. Pediatrics. 2013;132(1):72-78.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Clinical question: What are the characteristics of children who died in children’s hospitals while receiving palliative care (PC) compared to those who did not?
Background: Approximately 44,000 children die annually in hospitals in the U.S. Since the American Academy of Pediatrics (AAP) released a statement in August 2000 that presented an integrated model for providing PC to children with life-threatening conditions, pediatric PC programs have increased steadily in number. Children who receive PC services are commonly afflicted by genetic/congenital disorders, neuromuscular disorders, and cancer diagnoses. Although it is estimated that 6,320 people under the age of 24 received PC services in 2010, little data exist comparing pediatric inpatients receiving PC and those who do not.
Study design: Multicenter retrospective cohort study.
Setting: More than 40 freestanding children’s hospitals.
Synopsis: Using the Pediatric Health Information System (PHIS) database, which collects administrative and clinical data from more than 40 freestanding children’s hospitals belonging to the Children’s Hospital Association, researchers analyzed the characteristics of children under the age of 18 who died in the hospital more than five days after admission from 2001 to 2011. They extracted demographic data and categorized patients using major diagnostic categories (MDC) based on major organ system or etiology of disease. Identification of patients receiving PC services was by ICD-9 codes, and utilization of medications and procedures was identified by clinical transaction codes (CTC) and ICD-9 codes. The unit billing the last hospital day determined location of death.
Of the 24,342 children studied, only 3.8% received PC services based on coding. Patients less likely to receive PC services included black children (2.3%), those with circulatory diseases (2.8%), and those with neonatal diseases (1.9%). Children who did receive PC services had a significantly lower median length of stay (17 vs. 21 days), average daily charges ($9,348 vs. $11,806), received significantly fewer interventions (mechanical ventilation, invasive monitoring, surgical procedures), and died less frequently in an ICU setting (60% vs. 88%). PC services disproportionately altered the care of children with lymphatic/hematopoietic diseases, significantly decreasing use of mechanical ventilation (75% to 22%) and death in an ICU setting (66% to 21%).
Bottom line: Provision of PC services to children dying in children’s hospitals remains low. It is even lower for children with certain racial backgrounds and disease processes. When provided, PC services reduce length of stay, average daily charges, invasive procedures, and death in an ICU setting.
Citation: Keele L, Keenan HT, Sheetz J. Differences in characteristics of dying children who receive and do not receive palliative care. Pediatrics. 2013;132(1):72-78.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.