User login
What Are the Indications for a Blood Transfusion?
Case
A 65-year-old man with a history of coronary artery disease (CAD) presents to the ED after a mechanical fall. He was found to have a hip fracture, admitted to orthopedic service, and underwent an uneventful hip repair. His post-operative course was uncomplicated, except for his hemoglobin level of 7.5 g/dL, which had decreased from his pre-operative hemoglobin of 11.2 g/dL. The patient was without cardiac symptoms, was ambulating with assistance, had normal vital signs, and was otherwise having an unremarkable recovery. The orthopedic surgeon, who recently heard that you do not have to transfuse patients unless their hemoglobin is less than 7 g/dL, consulted the hospitalist to help make the decision. What would your recommendation be?
Overview
Blood transfusions are a common medical procedure routinely given in the hospital.1 An estimated 15 million red blood cell (RBC) units are transfused each year in the United States.2 Despite its common use, the clinical indications for transfusion continue to be the subject of considerable debate. Most clinicians would agree that treating a patient with a low hemoglobin level and symptoms of anemia is reasonable.1,3 However, in the absence of overt symptoms, there is debate about when transfusions are appropriate.2,3
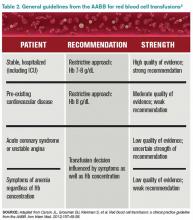
Because tissue oxygen delivery is dependent on hemoglobin and cardiac output, past medical practice has supported the use of the “golden 10/30 rule,” by which patients are transfused to a hemoglobin concentration of 10 g/dL or a hematocrit of 30%, regardless of symptoms. The rationale for this approach is based on physiologic evidence that cardiac output increases when hemoglobin falls below 7 g/dl. In patients with cardiac disease, the ability to increase cardiac output is compromised. Therefore, in order to reduce strain on the heart, hemoglobin levels historically have been kept higher than this threshold.
However, several studies have forced us to re-evaluate this old paradigm, including increasing concern for the infectious and noninfectious complications associated with blood transfusions and the need for cost containment (see Table 1).1,2,4 Due to improved blood screening, infectious complications from transfusions have been greatly reduced; noninfectious complications are 1,000 times more likely than infectious ones.
Review of Data
Although a number of studies have been performed on the indications for blood transfusions, many of the trials conducted in the past were too small to substantiate a certain practice. However, three trials with a large number of participants have allowed for a more evidence-based approach to blood transfusions. The studies address different patient populations to help broaden the restrictive transfusion approach to a larger range of patients.
TRICC trial: critically ill patients5. The TRICC trial was the first major study that compared a liberal transfusion strategy (transfuse when Hb <10 g/dL) to a more conservative approach (transfuse when Hb <7 g/dL). In this multicenter, randomized controlled trial, Hébert et al enrolled 418 critically ill patients and found that there was no significant difference in 30-day all-cause mortality between the restrictive-strategy group (18.7%) and the liberal-strategy group (23.3%).
However, in the pre-determined subgroup analysis, patients who were less severely ill (APACHE II scores of <20) had 30-day all-cause mortality of 8.7%, compared with 16.1% in the liberal-strategy group. Interestingly, there were more cardiac complications (pulmonary edema, angina, MI, and cardiac arrest) in the liberal-strategy group (21%) compared with the restrictive-strategy group (13%). Despite this finding, 30-day mortality was not significantly different in patients with clinically significant cardiac disease (primary or secondary diagnosis of cardiac disease [20.5% restrictive versus 22.9% liberal]).
An average of 2.6 units of RBCs per patient were given in the restrictive group, while 5.6 units were given to patients in the liberal group. This reflects a 54% decrease in the number of transfusions used in the conservative group. All the patients in the liberal group received transfusions, while 33% of the restrictive group’s patients received no blood at all.
The results of this trial suggested that there is no clinical advantage in transfusing ICU patients to Hb values above 9 g/dL, even if they have a history of cardiac disease. In fact, it may be harmful to practice a liberal transfusion strategy in critically ill younger patients (<55 years old) and those who are less severely ill (APACHE II <20).5
FOCUS trial: hip surgery and history of cardiac disease6. The FOCUS trial is a recent study that looked at the optimal hemoglobin level at which an RBC transfusion is beneficial for patients undergoing hip surgery. This study enrolled patients aged 50 or older who had a history or risk factors for cardiovascular disease (clinical evidence of cardiovascular disease: h/o ischemic heart disease, EKG evidence of previous MI, h/o CHF/PVD, h/o stroke/TIA, h/o HTN, DM, hyperlipidemia (TC >200/LDL >130), current tobacco use, or Cr>2.0), who were undergoing primary surgical repair of a hip fracture, and who had Hb <10g/dL within three days after surgery.
More than 2,000 patients were assigned randomly to a liberal-strategy group (transfuse to maintain a Hb >10g/dL) or a restrictive strategy group (transfuse to maintain Hg >8g/dl or for symptoms or signs of anemia). These signs/symptoms included chest pain that was possibly cardiac-related, congestive heart failure, tachycardia, and unresponsive hypotension. The primary outcomes were mortality or inability to walk 10 feet without assistance at 60-day follow-up.
The FOCUS trial found no statistically significant difference in mortality rate (7.6% in the liberal group versus 6.6% in the restrictive group) or in the ability to walk at 60 days (35.2% in the liberal group versus 34.7% in the restrictive group). There were no significant differences in the rates of in-hospital acute MI, unstable angina, or death between the two groups.
Patients in the restrictive-strategy group received 65% fewer units of blood than the liberal group, with 59% receiving no blood after surgery compared with 3% of the liberal group. Overall, the liberal group received 1,866 units of blood, compared with 652 units in the restrictive group.
This trial helps support the findings in previous trials, such as TRICC, by showing that a restrictive transfusion strategy using a trigger point of 8 g/dl does not increase mortality or cardiovascular complications and does not decrease functional ability after orthopedic surgery.
TRAC trial: patients after cardiac surgery7. The TRAC trial was a prospective randomized trial in 502 patients undergoing cardiac surgery that assigned 253 patients to the liberal-transfusion-strategy group (Hb >10g/dl) and 249 to the restrictive-strategy group (Hb >8 g/dl). In this study, the primary endpoint of all-cause 30-day mortality occurred in 10% of the liberal group and 11% of the restrictive group. This difference was not significant.
Subanalysis showed that blood transfusion in both groups was an independent risk factor for the occurrence of respiratory, cardiac, renal, and infectious complications, in addition to the composite end point of 30-day mortality—again highlighting the risk involved in of blood transfusions.
These results support the other trial conclusions that a restrictive transfusion strategy of maintaining a hematocrit of 24% (Hb 8 g/dL) is as safe as a more liberal strategy with a hematocrit of 30% (Hb 10 g/dL). It also offers further evidence of the risks of blood transfusions and supports the view that blood transfusions should never be given simply to correct low hemoglobin levels.
Cochrane Review. A recent Cochrane Review that comprised 19 trials with a combined total of 6,264 patients also supported a restrictive-strategy approach.8 In this review, no difference in mortality was established between the restrictive and liberal transfusion groups, with a trend toward decreased hospital mortality in the restrictive-transfusion group. The authors of the study felt that for most patients, blood transfusion is not necessary until hemoglobin levels drop below 7-8 g/dL but emphasized that this criteria should not be generalized to patients with an acute cardiac issue.
Back to the Case
In this case, the patient is doing well post-operatively and has no cardiac symptoms or hypotension. However, based on the new available data from the FOCUS trial, given the patient’s history of CAD, and the threshold of 8 g/dL used in the study, it was recommended that the patient be transfused.
Bottom Line
Current practice guidelines clearly support clinical judgment as the primary determinant in the decision to transfuse.2 However, current evidence is growing that our threshold for blood transfusions should be a hemoglobin level of 7-8 g/dl.
Dr. Chang is a hospitalist and assistant professor at Mount Sinai Medical Center in New York City, and is co-director of the medicine-geriatrics clerkship at the Icahn School of Medicine at Mount Sinai. Dr. Torgalkar is a hospitalist and assistant professor at Mount Sinai Medical Center.
References
- Sharma S, Sharma P, Tyler L. Transfusion of blood and blood products: indications and complications. Am Fam Physician. 2011;83:719-724.
- Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49-58.
- Valeri CR, Crowley JP, Loscalzo J. The red cell transfusion trigger: has a sin of commission now become a sin of omission? Transfusion. 1998;38:602-610.
- Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370(9585):415-426.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409-17.
- Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-2462.
- Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559-1567.
- Carson JL, Carless PA, Hébert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012; 4:CD002042.
Case
A 65-year-old man with a history of coronary artery disease (CAD) presents to the ED after a mechanical fall. He was found to have a hip fracture, admitted to orthopedic service, and underwent an uneventful hip repair. His post-operative course was uncomplicated, except for his hemoglobin level of 7.5 g/dL, which had decreased from his pre-operative hemoglobin of 11.2 g/dL. The patient was without cardiac symptoms, was ambulating with assistance, had normal vital signs, and was otherwise having an unremarkable recovery. The orthopedic surgeon, who recently heard that you do not have to transfuse patients unless their hemoglobin is less than 7 g/dL, consulted the hospitalist to help make the decision. What would your recommendation be?
Overview
Blood transfusions are a common medical procedure routinely given in the hospital.1 An estimated 15 million red blood cell (RBC) units are transfused each year in the United States.2 Despite its common use, the clinical indications for transfusion continue to be the subject of considerable debate. Most clinicians would agree that treating a patient with a low hemoglobin level and symptoms of anemia is reasonable.1,3 However, in the absence of overt symptoms, there is debate about when transfusions are appropriate.2,3
Because tissue oxygen delivery is dependent on hemoglobin and cardiac output, past medical practice has supported the use of the “golden 10/30 rule,” by which patients are transfused to a hemoglobin concentration of 10 g/dL or a hematocrit of 30%, regardless of symptoms. The rationale for this approach is based on physiologic evidence that cardiac output increases when hemoglobin falls below 7 g/dl. In patients with cardiac disease, the ability to increase cardiac output is compromised. Therefore, in order to reduce strain on the heart, hemoglobin levels historically have been kept higher than this threshold.
However, several studies have forced us to re-evaluate this old paradigm, including increasing concern for the infectious and noninfectious complications associated with blood transfusions and the need for cost containment (see Table 1).1,2,4 Due to improved blood screening, infectious complications from transfusions have been greatly reduced; noninfectious complications are 1,000 times more likely than infectious ones.
Review of Data
Although a number of studies have been performed on the indications for blood transfusions, many of the trials conducted in the past were too small to substantiate a certain practice. However, three trials with a large number of participants have allowed for a more evidence-based approach to blood transfusions. The studies address different patient populations to help broaden the restrictive transfusion approach to a larger range of patients.
TRICC trial: critically ill patients5. The TRICC trial was the first major study that compared a liberal transfusion strategy (transfuse when Hb <10 g/dL) to a more conservative approach (transfuse when Hb <7 g/dL). In this multicenter, randomized controlled trial, Hébert et al enrolled 418 critically ill patients and found that there was no significant difference in 30-day all-cause mortality between the restrictive-strategy group (18.7%) and the liberal-strategy group (23.3%).
However, in the pre-determined subgroup analysis, patients who were less severely ill (APACHE II scores of <20) had 30-day all-cause mortality of 8.7%, compared with 16.1% in the liberal-strategy group. Interestingly, there were more cardiac complications (pulmonary edema, angina, MI, and cardiac arrest) in the liberal-strategy group (21%) compared with the restrictive-strategy group (13%). Despite this finding, 30-day mortality was not significantly different in patients with clinically significant cardiac disease (primary or secondary diagnosis of cardiac disease [20.5% restrictive versus 22.9% liberal]).
An average of 2.6 units of RBCs per patient were given in the restrictive group, while 5.6 units were given to patients in the liberal group. This reflects a 54% decrease in the number of transfusions used in the conservative group. All the patients in the liberal group received transfusions, while 33% of the restrictive group’s patients received no blood at all.
The results of this trial suggested that there is no clinical advantage in transfusing ICU patients to Hb values above 9 g/dL, even if they have a history of cardiac disease. In fact, it may be harmful to practice a liberal transfusion strategy in critically ill younger patients (<55 years old) and those who are less severely ill (APACHE II <20).5
FOCUS trial: hip surgery and history of cardiac disease6. The FOCUS trial is a recent study that looked at the optimal hemoglobin level at which an RBC transfusion is beneficial for patients undergoing hip surgery. This study enrolled patients aged 50 or older who had a history or risk factors for cardiovascular disease (clinical evidence of cardiovascular disease: h/o ischemic heart disease, EKG evidence of previous MI, h/o CHF/PVD, h/o stroke/TIA, h/o HTN, DM, hyperlipidemia (TC >200/LDL >130), current tobacco use, or Cr>2.0), who were undergoing primary surgical repair of a hip fracture, and who had Hb <10g/dL within three days after surgery.
More than 2,000 patients were assigned randomly to a liberal-strategy group (transfuse to maintain a Hb >10g/dL) or a restrictive strategy group (transfuse to maintain Hg >8g/dl or for symptoms or signs of anemia). These signs/symptoms included chest pain that was possibly cardiac-related, congestive heart failure, tachycardia, and unresponsive hypotension. The primary outcomes were mortality or inability to walk 10 feet without assistance at 60-day follow-up.
The FOCUS trial found no statistically significant difference in mortality rate (7.6% in the liberal group versus 6.6% in the restrictive group) or in the ability to walk at 60 days (35.2% in the liberal group versus 34.7% in the restrictive group). There were no significant differences in the rates of in-hospital acute MI, unstable angina, or death between the two groups.
Patients in the restrictive-strategy group received 65% fewer units of blood than the liberal group, with 59% receiving no blood after surgery compared with 3% of the liberal group. Overall, the liberal group received 1,866 units of blood, compared with 652 units in the restrictive group.
This trial helps support the findings in previous trials, such as TRICC, by showing that a restrictive transfusion strategy using a trigger point of 8 g/dl does not increase mortality or cardiovascular complications and does not decrease functional ability after orthopedic surgery.
TRAC trial: patients after cardiac surgery7. The TRAC trial was a prospective randomized trial in 502 patients undergoing cardiac surgery that assigned 253 patients to the liberal-transfusion-strategy group (Hb >10g/dl) and 249 to the restrictive-strategy group (Hb >8 g/dl). In this study, the primary endpoint of all-cause 30-day mortality occurred in 10% of the liberal group and 11% of the restrictive group. This difference was not significant.
Subanalysis showed that blood transfusion in both groups was an independent risk factor for the occurrence of respiratory, cardiac, renal, and infectious complications, in addition to the composite end point of 30-day mortality—again highlighting the risk involved in of blood transfusions.
These results support the other trial conclusions that a restrictive transfusion strategy of maintaining a hematocrit of 24% (Hb 8 g/dL) is as safe as a more liberal strategy with a hematocrit of 30% (Hb 10 g/dL). It also offers further evidence of the risks of blood transfusions and supports the view that blood transfusions should never be given simply to correct low hemoglobin levels.
Cochrane Review. A recent Cochrane Review that comprised 19 trials with a combined total of 6,264 patients also supported a restrictive-strategy approach.8 In this review, no difference in mortality was established between the restrictive and liberal transfusion groups, with a trend toward decreased hospital mortality in the restrictive-transfusion group. The authors of the study felt that for most patients, blood transfusion is not necessary until hemoglobin levels drop below 7-8 g/dL but emphasized that this criteria should not be generalized to patients with an acute cardiac issue.
Back to the Case
In this case, the patient is doing well post-operatively and has no cardiac symptoms or hypotension. However, based on the new available data from the FOCUS trial, given the patient’s history of CAD, and the threshold of 8 g/dL used in the study, it was recommended that the patient be transfused.
Bottom Line
Current practice guidelines clearly support clinical judgment as the primary determinant in the decision to transfuse.2 However, current evidence is growing that our threshold for blood transfusions should be a hemoglobin level of 7-8 g/dl.
Dr. Chang is a hospitalist and assistant professor at Mount Sinai Medical Center in New York City, and is co-director of the medicine-geriatrics clerkship at the Icahn School of Medicine at Mount Sinai. Dr. Torgalkar is a hospitalist and assistant professor at Mount Sinai Medical Center.
References
- Sharma S, Sharma P, Tyler L. Transfusion of blood and blood products: indications and complications. Am Fam Physician. 2011;83:719-724.
- Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49-58.
- Valeri CR, Crowley JP, Loscalzo J. The red cell transfusion trigger: has a sin of commission now become a sin of omission? Transfusion. 1998;38:602-610.
- Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370(9585):415-426.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409-17.
- Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-2462.
- Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559-1567.
- Carson JL, Carless PA, Hébert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012; 4:CD002042.
Case
A 65-year-old man with a history of coronary artery disease (CAD) presents to the ED after a mechanical fall. He was found to have a hip fracture, admitted to orthopedic service, and underwent an uneventful hip repair. His post-operative course was uncomplicated, except for his hemoglobin level of 7.5 g/dL, which had decreased from his pre-operative hemoglobin of 11.2 g/dL. The patient was without cardiac symptoms, was ambulating with assistance, had normal vital signs, and was otherwise having an unremarkable recovery. The orthopedic surgeon, who recently heard that you do not have to transfuse patients unless their hemoglobin is less than 7 g/dL, consulted the hospitalist to help make the decision. What would your recommendation be?
Overview
Blood transfusions are a common medical procedure routinely given in the hospital.1 An estimated 15 million red blood cell (RBC) units are transfused each year in the United States.2 Despite its common use, the clinical indications for transfusion continue to be the subject of considerable debate. Most clinicians would agree that treating a patient with a low hemoglobin level and symptoms of anemia is reasonable.1,3 However, in the absence of overt symptoms, there is debate about when transfusions are appropriate.2,3
Because tissue oxygen delivery is dependent on hemoglobin and cardiac output, past medical practice has supported the use of the “golden 10/30 rule,” by which patients are transfused to a hemoglobin concentration of 10 g/dL or a hematocrit of 30%, regardless of symptoms. The rationale for this approach is based on physiologic evidence that cardiac output increases when hemoglobin falls below 7 g/dl. In patients with cardiac disease, the ability to increase cardiac output is compromised. Therefore, in order to reduce strain on the heart, hemoglobin levels historically have been kept higher than this threshold.
However, several studies have forced us to re-evaluate this old paradigm, including increasing concern for the infectious and noninfectious complications associated with blood transfusions and the need for cost containment (see Table 1).1,2,4 Due to improved blood screening, infectious complications from transfusions have been greatly reduced; noninfectious complications are 1,000 times more likely than infectious ones.
Review of Data
Although a number of studies have been performed on the indications for blood transfusions, many of the trials conducted in the past were too small to substantiate a certain practice. However, three trials with a large number of participants have allowed for a more evidence-based approach to blood transfusions. The studies address different patient populations to help broaden the restrictive transfusion approach to a larger range of patients.
TRICC trial: critically ill patients5. The TRICC trial was the first major study that compared a liberal transfusion strategy (transfuse when Hb <10 g/dL) to a more conservative approach (transfuse when Hb <7 g/dL). In this multicenter, randomized controlled trial, Hébert et al enrolled 418 critically ill patients and found that there was no significant difference in 30-day all-cause mortality between the restrictive-strategy group (18.7%) and the liberal-strategy group (23.3%).
However, in the pre-determined subgroup analysis, patients who were less severely ill (APACHE II scores of <20) had 30-day all-cause mortality of 8.7%, compared with 16.1% in the liberal-strategy group. Interestingly, there were more cardiac complications (pulmonary edema, angina, MI, and cardiac arrest) in the liberal-strategy group (21%) compared with the restrictive-strategy group (13%). Despite this finding, 30-day mortality was not significantly different in patients with clinically significant cardiac disease (primary or secondary diagnosis of cardiac disease [20.5% restrictive versus 22.9% liberal]).
An average of 2.6 units of RBCs per patient were given in the restrictive group, while 5.6 units were given to patients in the liberal group. This reflects a 54% decrease in the number of transfusions used in the conservative group. All the patients in the liberal group received transfusions, while 33% of the restrictive group’s patients received no blood at all.
The results of this trial suggested that there is no clinical advantage in transfusing ICU patients to Hb values above 9 g/dL, even if they have a history of cardiac disease. In fact, it may be harmful to practice a liberal transfusion strategy in critically ill younger patients (<55 years old) and those who are less severely ill (APACHE II <20).5
FOCUS trial: hip surgery and history of cardiac disease6. The FOCUS trial is a recent study that looked at the optimal hemoglobin level at which an RBC transfusion is beneficial for patients undergoing hip surgery. This study enrolled patients aged 50 or older who had a history or risk factors for cardiovascular disease (clinical evidence of cardiovascular disease: h/o ischemic heart disease, EKG evidence of previous MI, h/o CHF/PVD, h/o stroke/TIA, h/o HTN, DM, hyperlipidemia (TC >200/LDL >130), current tobacco use, or Cr>2.0), who were undergoing primary surgical repair of a hip fracture, and who had Hb <10g/dL within three days after surgery.
More than 2,000 patients were assigned randomly to a liberal-strategy group (transfuse to maintain a Hb >10g/dL) or a restrictive strategy group (transfuse to maintain Hg >8g/dl or for symptoms or signs of anemia). These signs/symptoms included chest pain that was possibly cardiac-related, congestive heart failure, tachycardia, and unresponsive hypotension. The primary outcomes were mortality or inability to walk 10 feet without assistance at 60-day follow-up.
The FOCUS trial found no statistically significant difference in mortality rate (7.6% in the liberal group versus 6.6% in the restrictive group) or in the ability to walk at 60 days (35.2% in the liberal group versus 34.7% in the restrictive group). There were no significant differences in the rates of in-hospital acute MI, unstable angina, or death between the two groups.
Patients in the restrictive-strategy group received 65% fewer units of blood than the liberal group, with 59% receiving no blood after surgery compared with 3% of the liberal group. Overall, the liberal group received 1,866 units of blood, compared with 652 units in the restrictive group.
This trial helps support the findings in previous trials, such as TRICC, by showing that a restrictive transfusion strategy using a trigger point of 8 g/dl does not increase mortality or cardiovascular complications and does not decrease functional ability after orthopedic surgery.
TRAC trial: patients after cardiac surgery7. The TRAC trial was a prospective randomized trial in 502 patients undergoing cardiac surgery that assigned 253 patients to the liberal-transfusion-strategy group (Hb >10g/dl) and 249 to the restrictive-strategy group (Hb >8 g/dl). In this study, the primary endpoint of all-cause 30-day mortality occurred in 10% of the liberal group and 11% of the restrictive group. This difference was not significant.
Subanalysis showed that blood transfusion in both groups was an independent risk factor for the occurrence of respiratory, cardiac, renal, and infectious complications, in addition to the composite end point of 30-day mortality—again highlighting the risk involved in of blood transfusions.
These results support the other trial conclusions that a restrictive transfusion strategy of maintaining a hematocrit of 24% (Hb 8 g/dL) is as safe as a more liberal strategy with a hematocrit of 30% (Hb 10 g/dL). It also offers further evidence of the risks of blood transfusions and supports the view that blood transfusions should never be given simply to correct low hemoglobin levels.
Cochrane Review. A recent Cochrane Review that comprised 19 trials with a combined total of 6,264 patients also supported a restrictive-strategy approach.8 In this review, no difference in mortality was established between the restrictive and liberal transfusion groups, with a trend toward decreased hospital mortality in the restrictive-transfusion group. The authors of the study felt that for most patients, blood transfusion is not necessary until hemoglobin levels drop below 7-8 g/dL but emphasized that this criteria should not be generalized to patients with an acute cardiac issue.
Back to the Case
In this case, the patient is doing well post-operatively and has no cardiac symptoms or hypotension. However, based on the new available data from the FOCUS trial, given the patient’s history of CAD, and the threshold of 8 g/dL used in the study, it was recommended that the patient be transfused.
Bottom Line
Current practice guidelines clearly support clinical judgment as the primary determinant in the decision to transfuse.2 However, current evidence is growing that our threshold for blood transfusions should be a hemoglobin level of 7-8 g/dl.
Dr. Chang is a hospitalist and assistant professor at Mount Sinai Medical Center in New York City, and is co-director of the medicine-geriatrics clerkship at the Icahn School of Medicine at Mount Sinai. Dr. Torgalkar is a hospitalist and assistant professor at Mount Sinai Medical Center.
References
- Sharma S, Sharma P, Tyler L. Transfusion of blood and blood products: indications and complications. Am Fam Physician. 2011;83:719-724.
- Carson JL, Grossman BJ, Kleinman S, et al. Red blood cell transfusion: a clinical practice guideline from the AABB. Ann Intern Med. 2012;157:49-58.
- Valeri CR, Crowley JP, Loscalzo J. The red cell transfusion trigger: has a sin of commission now become a sin of omission? Transfusion. 1998;38:602-610.
- Klein HG, Spahn DR, Carson JL. Red blood cell transfusion in clinical practice. Lancet. 2007;370(9585):415-426.
- Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340:409-17.
- Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365:2453-2462.
- Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304:1559-1567.
- Carson JL, Carless PA, Hébert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012; 4:CD002042.
Intravenous Immunoglobulin Most Common Retreatment Approach for Refractory Kawasaki Disease
Clinical question: How is refractory Kawasaki disease (rKD) treated in the United States?
Background: Kawasaki disease (KD) is an immunologically mediated disease of primarily small to medium-sized arteries. It is the most common cause of acquired heart disease in children in the United States.
The current standard of care for KD treatment is a single 2 g/kg dose of intravenous immunoglobulin (IVIG), infused over 10 to 12 hours, accompanied by aspirin (80 to 100 mg/kg/day by mouth in four divided doses). Fevers persistent more than 36 hours after initial treatment represent refractory Kawasaki disease (rKD). There are no current national guidelines or standards for rKD treatment, although a 2004 joint statement from the American Academy of Pediatrics and the American Heart Association suggested a second dose of IVIG for rKD.
Study design: Multicenter, retrospective, cross-sectional study.
Setting: Forty freestanding children’s hospitals.
Synopsis: Researchers examined data obtained from the Pediatric Health Information System (PHIS), a clinical and financial database of care provided at 43 nonprofit, freestanding children’s hospitals in the United States. Data from 40 of these hospitals were deemed complete enough for analysis and were collected from Jan. 1, 2005, to June 30, 2009. Subjects were included if they received at least one dose of IVIG and had a principal diagnosis of KD. To be considered rKD, the subject must have received additional treatment after the initial diagnosis of rKD.
The most commonly used treatment after initial IVIG treatment was retreatment with IVIG (65%), followed by intravenous methylprednisolone (27%), then infliximab (8%). Significant regional variation was observed, with hospitals in the Northeast using methylprednisolone most frequently for rKD (55%). Infliximab was used at a much higher frequency in the West (29%) compared with other regions.
Bottom line: Retreatment with IVIG is the most common treatment for rKD, but significant regional variation exists, possibly due to the influence of regional experts.
Citation: Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hospital Pediatrics. 2012;2:71-76.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Clinical question: How is refractory Kawasaki disease (rKD) treated in the United States?
Background: Kawasaki disease (KD) is an immunologically mediated disease of primarily small to medium-sized arteries. It is the most common cause of acquired heart disease in children in the United States.
The current standard of care for KD treatment is a single 2 g/kg dose of intravenous immunoglobulin (IVIG), infused over 10 to 12 hours, accompanied by aspirin (80 to 100 mg/kg/day by mouth in four divided doses). Fevers persistent more than 36 hours after initial treatment represent refractory Kawasaki disease (rKD). There are no current national guidelines or standards for rKD treatment, although a 2004 joint statement from the American Academy of Pediatrics and the American Heart Association suggested a second dose of IVIG for rKD.
Study design: Multicenter, retrospective, cross-sectional study.
Setting: Forty freestanding children’s hospitals.
Synopsis: Researchers examined data obtained from the Pediatric Health Information System (PHIS), a clinical and financial database of care provided at 43 nonprofit, freestanding children’s hospitals in the United States. Data from 40 of these hospitals were deemed complete enough for analysis and were collected from Jan. 1, 2005, to June 30, 2009. Subjects were included if they received at least one dose of IVIG and had a principal diagnosis of KD. To be considered rKD, the subject must have received additional treatment after the initial diagnosis of rKD.
The most commonly used treatment after initial IVIG treatment was retreatment with IVIG (65%), followed by intravenous methylprednisolone (27%), then infliximab (8%). Significant regional variation was observed, with hospitals in the Northeast using methylprednisolone most frequently for rKD (55%). Infliximab was used at a much higher frequency in the West (29%) compared with other regions.
Bottom line: Retreatment with IVIG is the most common treatment for rKD, but significant regional variation exists, possibly due to the influence of regional experts.
Citation: Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hospital Pediatrics. 2012;2:71-76.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Clinical question: How is refractory Kawasaki disease (rKD) treated in the United States?
Background: Kawasaki disease (KD) is an immunologically mediated disease of primarily small to medium-sized arteries. It is the most common cause of acquired heart disease in children in the United States.
The current standard of care for KD treatment is a single 2 g/kg dose of intravenous immunoglobulin (IVIG), infused over 10 to 12 hours, accompanied by aspirin (80 to 100 mg/kg/day by mouth in four divided doses). Fevers persistent more than 36 hours after initial treatment represent refractory Kawasaki disease (rKD). There are no current national guidelines or standards for rKD treatment, although a 2004 joint statement from the American Academy of Pediatrics and the American Heart Association suggested a second dose of IVIG for rKD.
Study design: Multicenter, retrospective, cross-sectional study.
Setting: Forty freestanding children’s hospitals.
Synopsis: Researchers examined data obtained from the Pediatric Health Information System (PHIS), a clinical and financial database of care provided at 43 nonprofit, freestanding children’s hospitals in the United States. Data from 40 of these hospitals were deemed complete enough for analysis and were collected from Jan. 1, 2005, to June 30, 2009. Subjects were included if they received at least one dose of IVIG and had a principal diagnosis of KD. To be considered rKD, the subject must have received additional treatment after the initial diagnosis of rKD.
The most commonly used treatment after initial IVIG treatment was retreatment with IVIG (65%), followed by intravenous methylprednisolone (27%), then infliximab (8%). Significant regional variation was observed, with hospitals in the Northeast using methylprednisolone most frequently for rKD (55%). Infliximab was used at a much higher frequency in the West (29%) compared with other regions.
Bottom line: Retreatment with IVIG is the most common treatment for rKD, but significant regional variation exists, possibly due to the influence of regional experts.
Citation: Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hospital Pediatrics. 2012;2:71-76.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Observation Status Designation in Pediatric Hospitals Is Costly
Clinical question: What are the costs associated with observation-status hospital stays compared to inpatient-status stays in pediatric hospitals?
Background: Observation status is a designation for hospitalizations that are typically shorter than 48 hours and do not meet criteria for inpatient status. It is considered to be outpatient for evaluation and management (E/M) coding. A designation of observation status for a hospital stay can have significant effects on out-of-pocket costs for patients and reimbursements to physicians and hospitals. It also can affect readmission and length-of-stay data, as observation-status hospital stays are often excluded from a hospital’s inpatient data.
Study design: Multicenter retrospective cohort study.
Setting: Thirty-three freestanding children’s hospitals.
Synopsis: Researchers reviewed data obtained from the Pediatric Health Information System (PHIS), which contains demographic and resource utilization date from 43 freestanding children’s hospitals in the U.S. Resource utilization data were reviewed from 33 of 43 hospitals in PHIS that reported data regarding observation- versus inpatient-status stays. Data were then limited to observation-status stays £2 days, which made up 97.8% of all observation-status stays. These were then compared to a corresponding cohort of inpatient-status stays of £2 days (47.5% of inpatient-status stays), excluding any patient who had spent time in an ICU.
Hospitalization costs were analyzed and separated into room and nonroom costs, as well as in aggregate. These were further subdivided into costs for four common diagnoses (asthma, gastroenteritis, bronchiolitis, and seizure) and were risk-adjusted.
Observation status was used variably between hospitals (2% to 45%) and within hospitals. There was significant overlap in costs of observation-status and inpatient-status stays, which persisted when accounting for nonroom costs and within the diagnosis subgroups. Although average severity-adjusted costs for observation-status stays were consistently less than those for inpatient-status stays, the dollar amounts were small.
Bottom line: Observation-status designation is used inconsistently in pediatric hospitals, and their costs overlap substantially with inpatient-status stays.
Citation: Fieldston ES, Shah SS, Hall M. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131;1050-1058.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FACP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Clinical question: What are the costs associated with observation-status hospital stays compared to inpatient-status stays in pediatric hospitals?
Background: Observation status is a designation for hospitalizations that are typically shorter than 48 hours and do not meet criteria for inpatient status. It is considered to be outpatient for evaluation and management (E/M) coding. A designation of observation status for a hospital stay can have significant effects on out-of-pocket costs for patients and reimbursements to physicians and hospitals. It also can affect readmission and length-of-stay data, as observation-status hospital stays are often excluded from a hospital’s inpatient data.
Study design: Multicenter retrospective cohort study.
Setting: Thirty-three freestanding children’s hospitals.
Synopsis: Researchers reviewed data obtained from the Pediatric Health Information System (PHIS), which contains demographic and resource utilization date from 43 freestanding children’s hospitals in the U.S. Resource utilization data were reviewed from 33 of 43 hospitals in PHIS that reported data regarding observation- versus inpatient-status stays. Data were then limited to observation-status stays £2 days, which made up 97.8% of all observation-status stays. These were then compared to a corresponding cohort of inpatient-status stays of £2 days (47.5% of inpatient-status stays), excluding any patient who had spent time in an ICU.
Hospitalization costs were analyzed and separated into room and nonroom costs, as well as in aggregate. These were further subdivided into costs for four common diagnoses (asthma, gastroenteritis, bronchiolitis, and seizure) and were risk-adjusted.
Observation status was used variably between hospitals (2% to 45%) and within hospitals. There was significant overlap in costs of observation-status and inpatient-status stays, which persisted when accounting for nonroom costs and within the diagnosis subgroups. Although average severity-adjusted costs for observation-status stays were consistently less than those for inpatient-status stays, the dollar amounts were small.
Bottom line: Observation-status designation is used inconsistently in pediatric hospitals, and their costs overlap substantially with inpatient-status stays.
Citation: Fieldston ES, Shah SS, Hall M. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131;1050-1058.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FACP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Clinical question: What are the costs associated with observation-status hospital stays compared to inpatient-status stays in pediatric hospitals?
Background: Observation status is a designation for hospitalizations that are typically shorter than 48 hours and do not meet criteria for inpatient status. It is considered to be outpatient for evaluation and management (E/M) coding. A designation of observation status for a hospital stay can have significant effects on out-of-pocket costs for patients and reimbursements to physicians and hospitals. It also can affect readmission and length-of-stay data, as observation-status hospital stays are often excluded from a hospital’s inpatient data.
Study design: Multicenter retrospective cohort study.
Setting: Thirty-three freestanding children’s hospitals.
Synopsis: Researchers reviewed data obtained from the Pediatric Health Information System (PHIS), which contains demographic and resource utilization date from 43 freestanding children’s hospitals in the U.S. Resource utilization data were reviewed from 33 of 43 hospitals in PHIS that reported data regarding observation- versus inpatient-status stays. Data were then limited to observation-status stays £2 days, which made up 97.8% of all observation-status stays. These were then compared to a corresponding cohort of inpatient-status stays of £2 days (47.5% of inpatient-status stays), excluding any patient who had spent time in an ICU.
Hospitalization costs were analyzed and separated into room and nonroom costs, as well as in aggregate. These were further subdivided into costs for four common diagnoses (asthma, gastroenteritis, bronchiolitis, and seizure) and were risk-adjusted.
Observation status was used variably between hospitals (2% to 45%) and within hospitals. There was significant overlap in costs of observation-status and inpatient-status stays, which persisted when accounting for nonroom costs and within the diagnosis subgroups. Although average severity-adjusted costs for observation-status stays were consistently less than those for inpatient-status stays, the dollar amounts were small.
Bottom line: Observation-status designation is used inconsistently in pediatric hospitals, and their costs overlap substantially with inpatient-status stays.
Citation: Fieldston ES, Shah SS, Hall M. Resource utilization for observation-status stays at children’s hospitals. Pediatrics. 2013;131;1050-1058.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FACP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
How to Manage Pain in Patients with Renal Insufficiency or End-Stage Renal Disease on Dialysis?
Case
A 70-year-old male with ESRD on hemodialysis presents with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia and ankle pain after a fall. An MRI of his ankle is negative, and he is started on acetaminophen and lidocaine patches, which result in adequate pain relief of the ankle. He later develops significant neuropathic pain in both arms, and a CT scan of the cervical spine reveals a cervical abscess and osteomyelitis. The patient desires pain relief but adamantly refuses narcotics, stating: “I don’t want to get addicted.” How can his pain be managed?
Overview
Pain is a common problem in patients with renal insufficiency and end-stage renal disease (ESRD) and can have a significant effect on the patient’s quality of life.1 When assessing a patient’s pain, assess both the severity of the pain (such as on an analogue scale, 0-10) and the characteristics of the pain. Pain is most commonly characterized as nociceptive, neuropathic, or both. Nociceptive pain can be further classified as arising from either somatic or visceral sources, and is often described as dull, throbbing, cramping, and/or pressurelike.1 Neuropathic pain is often described as tingling, numbing, burning, and/or stabbing.
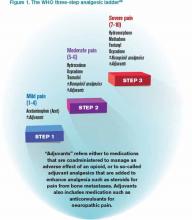
It is a challenge to manage pain in patients with renal insufficiency and dialysis. Renal insufficiency affects the pharmacokinetic properties of most pain medications, including their distribution, clearance, and excretion. The magnitude of the effect of renal insufficiency on drug metabolism varies depending on the agent itself, its metabolite, and the extent of renal failure.3 Multiple factors should be considered when prescribing pain medications for patients on dialysis, including the properties of the parent drug and its metabolites; the physical properties of the dialysis equipment, such as the filter pore size, the flow rate, and the efficiency of the technique used; and the dialysis method (intermittent versus continuous).3 Table 1 provides the recommended dosing of the most commonly prescribed agents, based on the degree of renal impairment. A modified World Health Organization (WHO) ladder has been suggested to treat pain in patients with ESRD, which can lead to effective pain relief in as many as 96% of patients (see Figure 1).2
*Beginning dose: If switching from IR to ER, calculate 24-hour total dose.
**For patients with creatinine clearances (CrCl) of 15 mL/min or less, the daily dosage should be adjusted proportionally (e.g. patients with a CrCl of 7.5 mL/min should receive one-half the dose of a patient with a CrCl of 15 mL/min).
Review of Data
Nonopioid options. Nonopioids, such as acetaminophen and NSAIDs, have no associated tolerance but have a ceiling effect for analgesia, and NSAIDs are associated with dose-dependent acute renal failure, gastrointestinal ulceration and bleeding, and cardiac events. The nonopioids that are considered safe options in patients with renal insufficiency include acetaminophen, ibuprofen, and fenoprofen (Nalfon). However, in the elderly, American Geriatric Society (AGS) guidelines currently recommend avoiding all NSAIDs due to their safety profile in the geriatric population.4 Although all NSAIDs can potentially be used for pain, selected NSAIDs with an FDA indication for acute or chronic pain were included for this review.
Acetaminophen (APAP) is a dialyzable compound that is metabolized in the liver to five inactive metabolites. The terminal elimination half-life of its sulfate and glucuronide metabolites are prolonged in patients with renal failure; therefore, the dosing interval of APAP should be increased to six to eight hours in renally impaired patients.5,6,7 Overall, acetaminophen is considered one of the safest agents to use for the treatment of pain, in renal patients and otherwise, as long as dosing is below the minimal daily dose (see Table 1).
Ibuprofen is metabolized in the liver to inactive compounds. It does not accumulate in renal insufficiency, and two of the inactive compounds are dialyzable.8 It is considered a safe option for the treatment of pain in patients with renal insufficiency or dialysis.9
Fenoprofen is metabolized in the liver to inactive compounds. Renal impairment is likely to cause the accumulation of the inactive metabolites but not the parent compound, so dose reduction is not necessary with the use of this agent in renal insufficiency or dialysis.6
Mefenamic acid (Ponstel) is metabolized in the liver. Mefenamic acid can further deteriorate renal function in patients with underlying renal disease.12 However, the nephrotoxic potential of this agent is of little consideration in ESRD patients on dialysis, and therefore no dosage adjustments are necessary in these patients.6
Ketoprofen is metabolized in the liver, where approximately 80% of the dose is excreted in the urine as a glucuronide metabolite. Dose reduction is recommended in renal insufficiency and dialysis, as it not dialyzable.8
Ketorolac accumulates in renal insufficiency; therefore, it is contraindicated in these patients and in patients at risk for renal failure, including those with volume depletion.10 Ketorolac is unlikely to be removed by dialysis and so should be avoided.10,11
Naproxen is metabolized in the liver to inactive compounds. Use of naproxen is not recommended in patients with moderate to severe renal impairment. If therapy must be initiated, close monitoring of the patient’s renal function is recommended.13
Celecoxib is the only cyclooxygenase-2 (COX-2) inhibitor available in the U.S. It is metabolized extensively by the liver and is unlikely to be removed by dialysis. Therefore, use of COX-2 inhibitors should be avoided in severe renal impairment and in those on dialysis.14,15
Opioid options. The use of opioids in the renally impaired population is challenging, as one must balance opioid-related adverse events with adequate pain control. As such, it is recommended to start with lower-than-recommended doses and slowly titrate up the dose while extending the dosing interval. This will help limit adverse effects, such as respiratory depression and hypotension.3
Hydrocodone is metabolized to hydromorphone (Dilaudid), which is then metabolized to its major metabolite hydromorphine-3-glucuronide (H3G) and minor metabolite hydromorphine-6-hydroxy, all of which are excreted renally along with the parent compound. H3G has no analgesic properties, but it can potentially cause neuroexcitation, agitation, confusion, and hallucination. Hydromorphone has been used safely in patients with renal insufficiency and dialysis, as it is expected to be dialyzable. 16,17
Tramadol is metabolized in the liver, producing one active compound. Approximately 30% of the tramadol dose is excreted unchanged in the urine, whereas 60% of the dose is excreted as metabolites. It is recommended to reduce the dose and increase the dosing interval in patients with renal insufficiency, but tramadol is generally well-tolerated in patients with renal insufficiency and dialysis. It is significantly removed by hemodialysis; therefore, redosing after a session may be necessary.18,19
Oxycodone can be used in patients with mild to moderate renal insufficiency but should be used at reduced dosing; it has been associated with significant sedation with usual doses in renal failure patients.16 Its use is generally not recommended in dialysis patients due to lack of data.3
Methadone and its metabolites are excreted in the urine and feces. Methadone has been used safely in patients with renal insufficiency, but it is poorly removed by dialysis and no specific recommendations are available regarding its dosing in dialysis.3,16
Fentanyl is primarily metabolized in the liver to inactive metabolites. Fentanyl clearance is reduced in patients with moderate to severe uremia (BUN >60 mg/dL). It is not expected that fentanyl be dialyzable because of its pharmacokinetic properties (high protein-binding, low water solubility, high molecular weight, and high volume of distribution). Data suggests that fentanyl can be used at usual doses in mild to moderate renal insufficiency and in dialysis patients, although reduced doses may be prudent. Such patients should be monitored for signs of gradual accumulation of the parent drug.3,16
Morphine is metabolized in the liver to morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G), all of which are excreted renally, along with the parent compound. Only M6G has analgesic properties, and when it accumulates, it can lead to CNS depression. M3G is associated with behavioral excitation, a side effect that is further magnified in patients with renal insufficiency. Although morphine is dialyzable, it should generally be avoided in patients with any level of renal insufficiency.16,17,20,21
Codeine is metabolized to several active metabolites, all of which are renally excreted. Lower-than-usual doses are recommended in patients with renal insufficiency, and it should be avoided altogether in dialysis patients.3,16
Meperidine is metabolized in the liver to various metabolites, primarily normeperidine, which is toxic and has a long half-life, five to 10 times longer then meperidine. Meperidine should not be used in patients with renal insufficiency or dialysis.3
Adjunctive therapeutic options. Lidocaine patches currently are only FDA-indicated for postherpetic neuralgia but are used for a wide variety of local pain syndromes. Absorption of lidocaine is determined by the duration of application and the surface area over which it is applied. There is no appreciable accumulation of lidocaine or its metabolites in renal insufficiency; therefore, dose adjustments are not required.22,23
Gabapentin is FDA-indicated for partial seizures and postherpetic neuralgia but is also used for a wide variety of neuropathic pain syndromes, including postoperative pain.24 Gabapentin is not metabolized and is excreted in the urine unchanged. Renal clearance of gabapentin is reduced by 40% and the elimination half-life is increased up to 52 hours in renal insufficiency, but it is dialyzable. Therefore, dose adjustments are required with gabapentin in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.25-27
Pregabalin is structurally related to gabapentin and is indicated for a variety of neuropathic pain conditions. Pregabalin is 90% excreted unchanged in the urine, and approximately 50% of drug is removed after four hours of hemodialysis. Dose adjustments are required in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.28
Antidepressant options. Amitriptyline, nortryptiline, and desipramine are the tricyclic antidepressants (TCAs) commonly used for neuropathic pain. TCAs are metabolized in the liver to inactive metabolites, with the exception of amitriptyline, which is metabolized to nortryptiline. Common side effects reported with TCAs include postural hypotension and anticholinergic side effects, such as constipation, urinary retention, blurred vision, dry mouth, delirium, and sedation. It is unlikely that the TCAs can be removed by dialysis. It is suggested that the dosage be reduced in renal insufficiency and that anticholinergic side effects be monitored.29
Back to the Case
The patient’s ankle pain was controlled with acetaminophen and lidocaine patches. For the neuropathic pain in his upper extremities, tramadol was started at 25 mg oral every 12 hours and increased to 50 mg oral every eight hours (below the maximum of 200 mg a day). The tramadol did not result in adequate pain relief, so gabapentin 100 mg at bedtime was initiated, then increased to twice daily over three days with some relief.
A geriatric consult was obtained to help educate him regarding addiction to opioids, as well as to explore goals of care, but he continued to insist on the use of a non-narcotic regimen for his pain.
Bottom Line
Pain management in patients with renal insufficiency and dialysis can be challenging, but there are a number of safe non-narcotic and narcotic pain regimens that can be safely used in this patient population.
Dr. Harisingani is a board-certified hospitalist at Long Island Jewish Medical Center in New Hyde Park, N.Y., and Drs. Saad and Cassagnol are assistant clinical professors at St. Johns University College of Pharmacy and Health Sciences in Jamaica, N.Y., and clinical pharmacy coordinators at Long Island Jewish Medical Center.
References
- Mid-Atlantic Renal Coalition and the Kidney End-of-Life Coalition. Clinical algorithm & preferred medications to treat pain in dialysis patients. Coalition for Supportive Care of Kidney Patients website. Available at: http://www.kidneysupportivecare.org/Physicians-Clinicians/Pain—Symptom-Management.aspx. Accessed Nov. 18, 2012.
- Barakzoy AS, Moss AH. Efficacy of the World Health Organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17(11):3198-3203.
- Johnson SJ. Opioid safety in patients with renal or hepatic dysfunction. Pain Treatment Topics website. Available at: http://pain-topics.org/pdf/Opioids-Renal-Hepatic-Dysfunction.pdf. Accessed Nov. 28, 2012.
- Ferrell B, Argoff CE, Epplin J, et al. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
- Prescott LF, Speirs GC, Critchley JA, Temple RM, Winney RJ. Paracetamol disposition and metabolite kinetics in patients with chronic renal failure. Eur J Clin Pharmacol. 1989;36(3):291-297.
- Launay-Vacher V, Karie S, Fau JB, Izzedine H, Deray G. Treatment of pain in patients with renal insufficiency: the World Health Organization three-step ladder adapted. J Pain. 2006;6(3):137-148.
- Berg KJ, Djøseland O, Gjellan A, et al. Acute effects of paracetamol on prostaglandin synthesis and renal function in normal man and in patients with renal failure. Clin Nephrol. 1990;34:255-262.
- Delbarre F, Roucayrol JC, Amor B, et al. Pharmacokinetic study of ketoprofen (19.583 R.P.) in man using the tritiated compound. Scand J Rheumatol Suppl. 1976;1976(0):45-52.
- Shen CH, Hung CJ, Wu CC, Huang HW, Ho WM. Rhabdomyolysis-induced acute renal failure after morphine overdose—a case report. Acta Anaesthesiol Sin. 1999;37(3):159-162.
- Ketorolac tromethamine oral tablets [package insert]. St. Louis: Ethex Corp.: 2008.
- Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet. 1992;23:415-427. Erratum in: Clin Pharmacokinet. 1999;24(3):270.
- Ponstel [package insert]. Alpharetta, GA: First Horizon Pharmaceutical Corp.; 2006.
- Naprosyn [package insert]. Nutley, NJ: Roche Laboratories Inc.; 2008.
- Celebrex [package insert]. New York: G.D. Searle LLC; 2011.
- Catella-Lawson F, McAdam B, Morrison BW, et al. Effects of specific inhibition of cyclooygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther. 1999;289:735-741.
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28(5):497-504.
- Lee MA, Leng ME, Tiernan EJ. Retrospective study of the use of hydromorphone in palliative care patients with normal and abnormal urea and creatinine. Palliat Med. 2001;15(1):26-34.
- Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCI. Am J. Med. 1996;101(1A):47S-53S.
- Izzedine H, Launay-Vacher V, Abbara C, Aymard G, Bassilios N, Deray G. Pharmacokinetics of tramadol in a hemodialysis patient. Nephron. 2002;92(3):755-756.
- Hasselström J, Säwe J. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokinet. 1993;24(4):344-354.
- Andersen G, Christrup L, Sjøgren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manage. 2003;25(1):74-91.
- Lidoderm [package insert]. Chadds Ford, PA: Endo Pharmaceuticals Inc.; 2010.
- Carter GT, Galer BS. Advances in the management of neuropathic pain. Phys Med Rehabil Clin N Am. 2001;12(2):447-459.
- Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain—a systematic review of randomized controlled trials. Pain. 2006;15:126(1-3):91-101.
- Neurontin [package insert]. New York: Parke-Davis; 2010.
- Pandey CK, Priye S, Singh S, et al. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can J Anaesth. 2004;51(4):358-363.
- Srivastava U, Kumar A, Saxena S, et al: Effect of preoperative gabapentin on postoperative pain and tramadol consumption after minilap open cholecystectomy: a randomized double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2010;27(N4):331-335.
- Lyrica [package insert]. New York: Pfizer Inc.; 2012.
- Broadbent A, Khor K, Heaney A. Palliation and chronic renal failure: opioid and other palliative medications—dosage guidelines. Progress in Palliative Care. 2003;11(4):183-190(8).
- Nayak-Rao S. Achieving effective pain relief in patients with chronic kidney disease: a review of analgesics in renal failure. J Nephrol. 2011;24(1):35-40.
- Wolters Kluwer Health. Facts & comparisons. Wolters Kluwer Health website. Available at: http://www.factsandcomparisons.com. Accessed Jan. 14, 2013.
- Lexicomp. Lexicomp Online. Lexicomp website. Available at: http://www.lexi.com/institutions/products/online/.
Case
A 70-year-old male with ESRD on hemodialysis presents with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia and ankle pain after a fall. An MRI of his ankle is negative, and he is started on acetaminophen and lidocaine patches, which result in adequate pain relief of the ankle. He later develops significant neuropathic pain in both arms, and a CT scan of the cervical spine reveals a cervical abscess and osteomyelitis. The patient desires pain relief but adamantly refuses narcotics, stating: “I don’t want to get addicted.” How can his pain be managed?
Overview
Pain is a common problem in patients with renal insufficiency and end-stage renal disease (ESRD) and can have a significant effect on the patient’s quality of life.1 When assessing a patient’s pain, assess both the severity of the pain (such as on an analogue scale, 0-10) and the characteristics of the pain. Pain is most commonly characterized as nociceptive, neuropathic, or both. Nociceptive pain can be further classified as arising from either somatic or visceral sources, and is often described as dull, throbbing, cramping, and/or pressurelike.1 Neuropathic pain is often described as tingling, numbing, burning, and/or stabbing.
It is a challenge to manage pain in patients with renal insufficiency and dialysis. Renal insufficiency affects the pharmacokinetic properties of most pain medications, including their distribution, clearance, and excretion. The magnitude of the effect of renal insufficiency on drug metabolism varies depending on the agent itself, its metabolite, and the extent of renal failure.3 Multiple factors should be considered when prescribing pain medications for patients on dialysis, including the properties of the parent drug and its metabolites; the physical properties of the dialysis equipment, such as the filter pore size, the flow rate, and the efficiency of the technique used; and the dialysis method (intermittent versus continuous).3 Table 1 provides the recommended dosing of the most commonly prescribed agents, based on the degree of renal impairment. A modified World Health Organization (WHO) ladder has been suggested to treat pain in patients with ESRD, which can lead to effective pain relief in as many as 96% of patients (see Figure 1).2
*Beginning dose: If switching from IR to ER, calculate 24-hour total dose.
**For patients with creatinine clearances (CrCl) of 15 mL/min or less, the daily dosage should be adjusted proportionally (e.g. patients with a CrCl of 7.5 mL/min should receive one-half the dose of a patient with a CrCl of 15 mL/min).
Review of Data
Nonopioid options. Nonopioids, such as acetaminophen and NSAIDs, have no associated tolerance but have a ceiling effect for analgesia, and NSAIDs are associated with dose-dependent acute renal failure, gastrointestinal ulceration and bleeding, and cardiac events. The nonopioids that are considered safe options in patients with renal insufficiency include acetaminophen, ibuprofen, and fenoprofen (Nalfon). However, in the elderly, American Geriatric Society (AGS) guidelines currently recommend avoiding all NSAIDs due to their safety profile in the geriatric population.4 Although all NSAIDs can potentially be used for pain, selected NSAIDs with an FDA indication for acute or chronic pain were included for this review.
Acetaminophen (APAP) is a dialyzable compound that is metabolized in the liver to five inactive metabolites. The terminal elimination half-life of its sulfate and glucuronide metabolites are prolonged in patients with renal failure; therefore, the dosing interval of APAP should be increased to six to eight hours in renally impaired patients.5,6,7 Overall, acetaminophen is considered one of the safest agents to use for the treatment of pain, in renal patients and otherwise, as long as dosing is below the minimal daily dose (see Table 1).
Ibuprofen is metabolized in the liver to inactive compounds. It does not accumulate in renal insufficiency, and two of the inactive compounds are dialyzable.8 It is considered a safe option for the treatment of pain in patients with renal insufficiency or dialysis.9
Fenoprofen is metabolized in the liver to inactive compounds. Renal impairment is likely to cause the accumulation of the inactive metabolites but not the parent compound, so dose reduction is not necessary with the use of this agent in renal insufficiency or dialysis.6
Mefenamic acid (Ponstel) is metabolized in the liver. Mefenamic acid can further deteriorate renal function in patients with underlying renal disease.12 However, the nephrotoxic potential of this agent is of little consideration in ESRD patients on dialysis, and therefore no dosage adjustments are necessary in these patients.6
Ketoprofen is metabolized in the liver, where approximately 80% of the dose is excreted in the urine as a glucuronide metabolite. Dose reduction is recommended in renal insufficiency and dialysis, as it not dialyzable.8
Ketorolac accumulates in renal insufficiency; therefore, it is contraindicated in these patients and in patients at risk for renal failure, including those with volume depletion.10 Ketorolac is unlikely to be removed by dialysis and so should be avoided.10,11
Naproxen is metabolized in the liver to inactive compounds. Use of naproxen is not recommended in patients with moderate to severe renal impairment. If therapy must be initiated, close monitoring of the patient’s renal function is recommended.13
Celecoxib is the only cyclooxygenase-2 (COX-2) inhibitor available in the U.S. It is metabolized extensively by the liver and is unlikely to be removed by dialysis. Therefore, use of COX-2 inhibitors should be avoided in severe renal impairment and in those on dialysis.14,15
Opioid options. The use of opioids in the renally impaired population is challenging, as one must balance opioid-related adverse events with adequate pain control. As such, it is recommended to start with lower-than-recommended doses and slowly titrate up the dose while extending the dosing interval. This will help limit adverse effects, such as respiratory depression and hypotension.3
Hydrocodone is metabolized to hydromorphone (Dilaudid), which is then metabolized to its major metabolite hydromorphine-3-glucuronide (H3G) and minor metabolite hydromorphine-6-hydroxy, all of which are excreted renally along with the parent compound. H3G has no analgesic properties, but it can potentially cause neuroexcitation, agitation, confusion, and hallucination. Hydromorphone has been used safely in patients with renal insufficiency and dialysis, as it is expected to be dialyzable. 16,17
Tramadol is metabolized in the liver, producing one active compound. Approximately 30% of the tramadol dose is excreted unchanged in the urine, whereas 60% of the dose is excreted as metabolites. It is recommended to reduce the dose and increase the dosing interval in patients with renal insufficiency, but tramadol is generally well-tolerated in patients with renal insufficiency and dialysis. It is significantly removed by hemodialysis; therefore, redosing after a session may be necessary.18,19
Oxycodone can be used in patients with mild to moderate renal insufficiency but should be used at reduced dosing; it has been associated with significant sedation with usual doses in renal failure patients.16 Its use is generally not recommended in dialysis patients due to lack of data.3
Methadone and its metabolites are excreted in the urine and feces. Methadone has been used safely in patients with renal insufficiency, but it is poorly removed by dialysis and no specific recommendations are available regarding its dosing in dialysis.3,16
Fentanyl is primarily metabolized in the liver to inactive metabolites. Fentanyl clearance is reduced in patients with moderate to severe uremia (BUN >60 mg/dL). It is not expected that fentanyl be dialyzable because of its pharmacokinetic properties (high protein-binding, low water solubility, high molecular weight, and high volume of distribution). Data suggests that fentanyl can be used at usual doses in mild to moderate renal insufficiency and in dialysis patients, although reduced doses may be prudent. Such patients should be monitored for signs of gradual accumulation of the parent drug.3,16
Morphine is metabolized in the liver to morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G), all of which are excreted renally, along with the parent compound. Only M6G has analgesic properties, and when it accumulates, it can lead to CNS depression. M3G is associated with behavioral excitation, a side effect that is further magnified in patients with renal insufficiency. Although morphine is dialyzable, it should generally be avoided in patients with any level of renal insufficiency.16,17,20,21
Codeine is metabolized to several active metabolites, all of which are renally excreted. Lower-than-usual doses are recommended in patients with renal insufficiency, and it should be avoided altogether in dialysis patients.3,16
Meperidine is metabolized in the liver to various metabolites, primarily normeperidine, which is toxic and has a long half-life, five to 10 times longer then meperidine. Meperidine should not be used in patients with renal insufficiency or dialysis.3
Adjunctive therapeutic options. Lidocaine patches currently are only FDA-indicated for postherpetic neuralgia but are used for a wide variety of local pain syndromes. Absorption of lidocaine is determined by the duration of application and the surface area over which it is applied. There is no appreciable accumulation of lidocaine or its metabolites in renal insufficiency; therefore, dose adjustments are not required.22,23
Gabapentin is FDA-indicated for partial seizures and postherpetic neuralgia but is also used for a wide variety of neuropathic pain syndromes, including postoperative pain.24 Gabapentin is not metabolized and is excreted in the urine unchanged. Renal clearance of gabapentin is reduced by 40% and the elimination half-life is increased up to 52 hours in renal insufficiency, but it is dialyzable. Therefore, dose adjustments are required with gabapentin in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.25-27
Pregabalin is structurally related to gabapentin and is indicated for a variety of neuropathic pain conditions. Pregabalin is 90% excreted unchanged in the urine, and approximately 50% of drug is removed after four hours of hemodialysis. Dose adjustments are required in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.28
Antidepressant options. Amitriptyline, nortryptiline, and desipramine are the tricyclic antidepressants (TCAs) commonly used for neuropathic pain. TCAs are metabolized in the liver to inactive metabolites, with the exception of amitriptyline, which is metabolized to nortryptiline. Common side effects reported with TCAs include postural hypotension and anticholinergic side effects, such as constipation, urinary retention, blurred vision, dry mouth, delirium, and sedation. It is unlikely that the TCAs can be removed by dialysis. It is suggested that the dosage be reduced in renal insufficiency and that anticholinergic side effects be monitored.29
Back to the Case
The patient’s ankle pain was controlled with acetaminophen and lidocaine patches. For the neuropathic pain in his upper extremities, tramadol was started at 25 mg oral every 12 hours and increased to 50 mg oral every eight hours (below the maximum of 200 mg a day). The tramadol did not result in adequate pain relief, so gabapentin 100 mg at bedtime was initiated, then increased to twice daily over three days with some relief.
A geriatric consult was obtained to help educate him regarding addiction to opioids, as well as to explore goals of care, but he continued to insist on the use of a non-narcotic regimen for his pain.
Bottom Line
Pain management in patients with renal insufficiency and dialysis can be challenging, but there are a number of safe non-narcotic and narcotic pain regimens that can be safely used in this patient population.
Dr. Harisingani is a board-certified hospitalist at Long Island Jewish Medical Center in New Hyde Park, N.Y., and Drs. Saad and Cassagnol are assistant clinical professors at St. Johns University College of Pharmacy and Health Sciences in Jamaica, N.Y., and clinical pharmacy coordinators at Long Island Jewish Medical Center.
References
- Mid-Atlantic Renal Coalition and the Kidney End-of-Life Coalition. Clinical algorithm & preferred medications to treat pain in dialysis patients. Coalition for Supportive Care of Kidney Patients website. Available at: http://www.kidneysupportivecare.org/Physicians-Clinicians/Pain—Symptom-Management.aspx. Accessed Nov. 18, 2012.
- Barakzoy AS, Moss AH. Efficacy of the World Health Organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17(11):3198-3203.
- Johnson SJ. Opioid safety in patients with renal or hepatic dysfunction. Pain Treatment Topics website. Available at: http://pain-topics.org/pdf/Opioids-Renal-Hepatic-Dysfunction.pdf. Accessed Nov. 28, 2012.
- Ferrell B, Argoff CE, Epplin J, et al. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
- Prescott LF, Speirs GC, Critchley JA, Temple RM, Winney RJ. Paracetamol disposition and metabolite kinetics in patients with chronic renal failure. Eur J Clin Pharmacol. 1989;36(3):291-297.
- Launay-Vacher V, Karie S, Fau JB, Izzedine H, Deray G. Treatment of pain in patients with renal insufficiency: the World Health Organization three-step ladder adapted. J Pain. 2006;6(3):137-148.
- Berg KJ, Djøseland O, Gjellan A, et al. Acute effects of paracetamol on prostaglandin synthesis and renal function in normal man and in patients with renal failure. Clin Nephrol. 1990;34:255-262.
- Delbarre F, Roucayrol JC, Amor B, et al. Pharmacokinetic study of ketoprofen (19.583 R.P.) in man using the tritiated compound. Scand J Rheumatol Suppl. 1976;1976(0):45-52.
- Shen CH, Hung CJ, Wu CC, Huang HW, Ho WM. Rhabdomyolysis-induced acute renal failure after morphine overdose—a case report. Acta Anaesthesiol Sin. 1999;37(3):159-162.
- Ketorolac tromethamine oral tablets [package insert]. St. Louis: Ethex Corp.: 2008.
- Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet. 1992;23:415-427. Erratum in: Clin Pharmacokinet. 1999;24(3):270.
- Ponstel [package insert]. Alpharetta, GA: First Horizon Pharmaceutical Corp.; 2006.
- Naprosyn [package insert]. Nutley, NJ: Roche Laboratories Inc.; 2008.
- Celebrex [package insert]. New York: G.D. Searle LLC; 2011.
- Catella-Lawson F, McAdam B, Morrison BW, et al. Effects of specific inhibition of cyclooygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther. 1999;289:735-741.
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28(5):497-504.
- Lee MA, Leng ME, Tiernan EJ. Retrospective study of the use of hydromorphone in palliative care patients with normal and abnormal urea and creatinine. Palliat Med. 2001;15(1):26-34.
- Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCI. Am J. Med. 1996;101(1A):47S-53S.
- Izzedine H, Launay-Vacher V, Abbara C, Aymard G, Bassilios N, Deray G. Pharmacokinetics of tramadol in a hemodialysis patient. Nephron. 2002;92(3):755-756.
- Hasselström J, Säwe J. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokinet. 1993;24(4):344-354.
- Andersen G, Christrup L, Sjøgren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manage. 2003;25(1):74-91.
- Lidoderm [package insert]. Chadds Ford, PA: Endo Pharmaceuticals Inc.; 2010.
- Carter GT, Galer BS. Advances in the management of neuropathic pain. Phys Med Rehabil Clin N Am. 2001;12(2):447-459.
- Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain—a systematic review of randomized controlled trials. Pain. 2006;15:126(1-3):91-101.
- Neurontin [package insert]. New York: Parke-Davis; 2010.
- Pandey CK, Priye S, Singh S, et al. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can J Anaesth. 2004;51(4):358-363.
- Srivastava U, Kumar A, Saxena S, et al: Effect of preoperative gabapentin on postoperative pain and tramadol consumption after minilap open cholecystectomy: a randomized double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2010;27(N4):331-335.
- Lyrica [package insert]. New York: Pfizer Inc.; 2012.
- Broadbent A, Khor K, Heaney A. Palliation and chronic renal failure: opioid and other palliative medications—dosage guidelines. Progress in Palliative Care. 2003;11(4):183-190(8).
- Nayak-Rao S. Achieving effective pain relief in patients with chronic kidney disease: a review of analgesics in renal failure. J Nephrol. 2011;24(1):35-40.
- Wolters Kluwer Health. Facts & comparisons. Wolters Kluwer Health website. Available at: http://www.factsandcomparisons.com. Accessed Jan. 14, 2013.
- Lexicomp. Lexicomp Online. Lexicomp website. Available at: http://www.lexi.com/institutions/products/online/.
Case
A 70-year-old male with ESRD on hemodialysis presents with methicillin-resistant Staphylococcus aureus (MRSA) bacteremia and ankle pain after a fall. An MRI of his ankle is negative, and he is started on acetaminophen and lidocaine patches, which result in adequate pain relief of the ankle. He later develops significant neuropathic pain in both arms, and a CT scan of the cervical spine reveals a cervical abscess and osteomyelitis. The patient desires pain relief but adamantly refuses narcotics, stating: “I don’t want to get addicted.” How can his pain be managed?
Overview
Pain is a common problem in patients with renal insufficiency and end-stage renal disease (ESRD) and can have a significant effect on the patient’s quality of life.1 When assessing a patient’s pain, assess both the severity of the pain (such as on an analogue scale, 0-10) and the characteristics of the pain. Pain is most commonly characterized as nociceptive, neuropathic, or both. Nociceptive pain can be further classified as arising from either somatic or visceral sources, and is often described as dull, throbbing, cramping, and/or pressurelike.1 Neuropathic pain is often described as tingling, numbing, burning, and/or stabbing.
It is a challenge to manage pain in patients with renal insufficiency and dialysis. Renal insufficiency affects the pharmacokinetic properties of most pain medications, including their distribution, clearance, and excretion. The magnitude of the effect of renal insufficiency on drug metabolism varies depending on the agent itself, its metabolite, and the extent of renal failure.3 Multiple factors should be considered when prescribing pain medications for patients on dialysis, including the properties of the parent drug and its metabolites; the physical properties of the dialysis equipment, such as the filter pore size, the flow rate, and the efficiency of the technique used; and the dialysis method (intermittent versus continuous).3 Table 1 provides the recommended dosing of the most commonly prescribed agents, based on the degree of renal impairment. A modified World Health Organization (WHO) ladder has been suggested to treat pain in patients with ESRD, which can lead to effective pain relief in as many as 96% of patients (see Figure 1).2
*Beginning dose: If switching from IR to ER, calculate 24-hour total dose.
**For patients with creatinine clearances (CrCl) of 15 mL/min or less, the daily dosage should be adjusted proportionally (e.g. patients with a CrCl of 7.5 mL/min should receive one-half the dose of a patient with a CrCl of 15 mL/min).
Review of Data
Nonopioid options. Nonopioids, such as acetaminophen and NSAIDs, have no associated tolerance but have a ceiling effect for analgesia, and NSAIDs are associated with dose-dependent acute renal failure, gastrointestinal ulceration and bleeding, and cardiac events. The nonopioids that are considered safe options in patients with renal insufficiency include acetaminophen, ibuprofen, and fenoprofen (Nalfon). However, in the elderly, American Geriatric Society (AGS) guidelines currently recommend avoiding all NSAIDs due to their safety profile in the geriatric population.4 Although all NSAIDs can potentially be used for pain, selected NSAIDs with an FDA indication for acute or chronic pain were included for this review.
Acetaminophen (APAP) is a dialyzable compound that is metabolized in the liver to five inactive metabolites. The terminal elimination half-life of its sulfate and glucuronide metabolites are prolonged in patients with renal failure; therefore, the dosing interval of APAP should be increased to six to eight hours in renally impaired patients.5,6,7 Overall, acetaminophen is considered one of the safest agents to use for the treatment of pain, in renal patients and otherwise, as long as dosing is below the minimal daily dose (see Table 1).
Ibuprofen is metabolized in the liver to inactive compounds. It does not accumulate in renal insufficiency, and two of the inactive compounds are dialyzable.8 It is considered a safe option for the treatment of pain in patients with renal insufficiency or dialysis.9
Fenoprofen is metabolized in the liver to inactive compounds. Renal impairment is likely to cause the accumulation of the inactive metabolites but not the parent compound, so dose reduction is not necessary with the use of this agent in renal insufficiency or dialysis.6
Mefenamic acid (Ponstel) is metabolized in the liver. Mefenamic acid can further deteriorate renal function in patients with underlying renal disease.12 However, the nephrotoxic potential of this agent is of little consideration in ESRD patients on dialysis, and therefore no dosage adjustments are necessary in these patients.6
Ketoprofen is metabolized in the liver, where approximately 80% of the dose is excreted in the urine as a glucuronide metabolite. Dose reduction is recommended in renal insufficiency and dialysis, as it not dialyzable.8
Ketorolac accumulates in renal insufficiency; therefore, it is contraindicated in these patients and in patients at risk for renal failure, including those with volume depletion.10 Ketorolac is unlikely to be removed by dialysis and so should be avoided.10,11
Naproxen is metabolized in the liver to inactive compounds. Use of naproxen is not recommended in patients with moderate to severe renal impairment. If therapy must be initiated, close monitoring of the patient’s renal function is recommended.13
Celecoxib is the only cyclooxygenase-2 (COX-2) inhibitor available in the U.S. It is metabolized extensively by the liver and is unlikely to be removed by dialysis. Therefore, use of COX-2 inhibitors should be avoided in severe renal impairment and in those on dialysis.14,15
Opioid options. The use of opioids in the renally impaired population is challenging, as one must balance opioid-related adverse events with adequate pain control. As such, it is recommended to start with lower-than-recommended doses and slowly titrate up the dose while extending the dosing interval. This will help limit adverse effects, such as respiratory depression and hypotension.3
Hydrocodone is metabolized to hydromorphone (Dilaudid), which is then metabolized to its major metabolite hydromorphine-3-glucuronide (H3G) and minor metabolite hydromorphine-6-hydroxy, all of which are excreted renally along with the parent compound. H3G has no analgesic properties, but it can potentially cause neuroexcitation, agitation, confusion, and hallucination. Hydromorphone has been used safely in patients with renal insufficiency and dialysis, as it is expected to be dialyzable. 16,17
Tramadol is metabolized in the liver, producing one active compound. Approximately 30% of the tramadol dose is excreted unchanged in the urine, whereas 60% of the dose is excreted as metabolites. It is recommended to reduce the dose and increase the dosing interval in patients with renal insufficiency, but tramadol is generally well-tolerated in patients with renal insufficiency and dialysis. It is significantly removed by hemodialysis; therefore, redosing after a session may be necessary.18,19
Oxycodone can be used in patients with mild to moderate renal insufficiency but should be used at reduced dosing; it has been associated with significant sedation with usual doses in renal failure patients.16 Its use is generally not recommended in dialysis patients due to lack of data.3
Methadone and its metabolites are excreted in the urine and feces. Methadone has been used safely in patients with renal insufficiency, but it is poorly removed by dialysis and no specific recommendations are available regarding its dosing in dialysis.3,16
Fentanyl is primarily metabolized in the liver to inactive metabolites. Fentanyl clearance is reduced in patients with moderate to severe uremia (BUN >60 mg/dL). It is not expected that fentanyl be dialyzable because of its pharmacokinetic properties (high protein-binding, low water solubility, high molecular weight, and high volume of distribution). Data suggests that fentanyl can be used at usual doses in mild to moderate renal insufficiency and in dialysis patients, although reduced doses may be prudent. Such patients should be monitored for signs of gradual accumulation of the parent drug.3,16
Morphine is metabolized in the liver to morphine-6-glucuronide (M6G) and morphine-3-glucuronide (M3G), all of which are excreted renally, along with the parent compound. Only M6G has analgesic properties, and when it accumulates, it can lead to CNS depression. M3G is associated with behavioral excitation, a side effect that is further magnified in patients with renal insufficiency. Although morphine is dialyzable, it should generally be avoided in patients with any level of renal insufficiency.16,17,20,21
Codeine is metabolized to several active metabolites, all of which are renally excreted. Lower-than-usual doses are recommended in patients with renal insufficiency, and it should be avoided altogether in dialysis patients.3,16
Meperidine is metabolized in the liver to various metabolites, primarily normeperidine, which is toxic and has a long half-life, five to 10 times longer then meperidine. Meperidine should not be used in patients with renal insufficiency or dialysis.3
Adjunctive therapeutic options. Lidocaine patches currently are only FDA-indicated for postherpetic neuralgia but are used for a wide variety of local pain syndromes. Absorption of lidocaine is determined by the duration of application and the surface area over which it is applied. There is no appreciable accumulation of lidocaine or its metabolites in renal insufficiency; therefore, dose adjustments are not required.22,23
Gabapentin is FDA-indicated for partial seizures and postherpetic neuralgia but is also used for a wide variety of neuropathic pain syndromes, including postoperative pain.24 Gabapentin is not metabolized and is excreted in the urine unchanged. Renal clearance of gabapentin is reduced by 40% and the elimination half-life is increased up to 52 hours in renal insufficiency, but it is dialyzable. Therefore, dose adjustments are required with gabapentin in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.25-27
Pregabalin is structurally related to gabapentin and is indicated for a variety of neuropathic pain conditions. Pregabalin is 90% excreted unchanged in the urine, and approximately 50% of drug is removed after four hours of hemodialysis. Dose adjustments are required in patients with moderate to severe renal insufficiency, and supplemental doses should be administered in patients after receiving dialysis.28
Antidepressant options. Amitriptyline, nortryptiline, and desipramine are the tricyclic antidepressants (TCAs) commonly used for neuropathic pain. TCAs are metabolized in the liver to inactive metabolites, with the exception of amitriptyline, which is metabolized to nortryptiline. Common side effects reported with TCAs include postural hypotension and anticholinergic side effects, such as constipation, urinary retention, blurred vision, dry mouth, delirium, and sedation. It is unlikely that the TCAs can be removed by dialysis. It is suggested that the dosage be reduced in renal insufficiency and that anticholinergic side effects be monitored.29
Back to the Case
The patient’s ankle pain was controlled with acetaminophen and lidocaine patches. For the neuropathic pain in his upper extremities, tramadol was started at 25 mg oral every 12 hours and increased to 50 mg oral every eight hours (below the maximum of 200 mg a day). The tramadol did not result in adequate pain relief, so gabapentin 100 mg at bedtime was initiated, then increased to twice daily over three days with some relief.
A geriatric consult was obtained to help educate him regarding addiction to opioids, as well as to explore goals of care, but he continued to insist on the use of a non-narcotic regimen for his pain.
Bottom Line
Pain management in patients with renal insufficiency and dialysis can be challenging, but there are a number of safe non-narcotic and narcotic pain regimens that can be safely used in this patient population.
Dr. Harisingani is a board-certified hospitalist at Long Island Jewish Medical Center in New Hyde Park, N.Y., and Drs. Saad and Cassagnol are assistant clinical professors at St. Johns University College of Pharmacy and Health Sciences in Jamaica, N.Y., and clinical pharmacy coordinators at Long Island Jewish Medical Center.
References
- Mid-Atlantic Renal Coalition and the Kidney End-of-Life Coalition. Clinical algorithm & preferred medications to treat pain in dialysis patients. Coalition for Supportive Care of Kidney Patients website. Available at: http://www.kidneysupportivecare.org/Physicians-Clinicians/Pain—Symptom-Management.aspx. Accessed Nov. 18, 2012.
- Barakzoy AS, Moss AH. Efficacy of the World Health Organization analgesic ladder to treat pain in end-stage renal disease. J Am Soc Nephrol. 2006;17(11):3198-3203.
- Johnson SJ. Opioid safety in patients with renal or hepatic dysfunction. Pain Treatment Topics website. Available at: http://pain-topics.org/pdf/Opioids-Renal-Hepatic-Dysfunction.pdf. Accessed Nov. 28, 2012.
- Ferrell B, Argoff CE, Epplin J, et al. American Geriatrics Society Panel on Pharmacological Management of Persistent Pain in Older Persons. Pharmacological management of persistent pain in older persons. J Am Geriatr Soc. 2009;57(8):1331-1346.
- Prescott LF, Speirs GC, Critchley JA, Temple RM, Winney RJ. Paracetamol disposition and metabolite kinetics in patients with chronic renal failure. Eur J Clin Pharmacol. 1989;36(3):291-297.
- Launay-Vacher V, Karie S, Fau JB, Izzedine H, Deray G. Treatment of pain in patients with renal insufficiency: the World Health Organization three-step ladder adapted. J Pain. 2006;6(3):137-148.
- Berg KJ, Djøseland O, Gjellan A, et al. Acute effects of paracetamol on prostaglandin synthesis and renal function in normal man and in patients with renal failure. Clin Nephrol. 1990;34:255-262.
- Delbarre F, Roucayrol JC, Amor B, et al. Pharmacokinetic study of ketoprofen (19.583 R.P.) in man using the tritiated compound. Scand J Rheumatol Suppl. 1976;1976(0):45-52.
- Shen CH, Hung CJ, Wu CC, Huang HW, Ho WM. Rhabdomyolysis-induced acute renal failure after morphine overdose—a case report. Acta Anaesthesiol Sin. 1999;37(3):159-162.
- Ketorolac tromethamine oral tablets [package insert]. St. Louis: Ethex Corp.: 2008.
- Brocks DR, Jamali F. Clinical pharmacokinetics of ketorolac tromethamine. Clin Pharmacokinet. 1992;23:415-427. Erratum in: Clin Pharmacokinet. 1999;24(3):270.
- Ponstel [package insert]. Alpharetta, GA: First Horizon Pharmaceutical Corp.; 2006.
- Naprosyn [package insert]. Nutley, NJ: Roche Laboratories Inc.; 2008.
- Celebrex [package insert]. New York: G.D. Searle LLC; 2011.
- Catella-Lawson F, McAdam B, Morrison BW, et al. Effects of specific inhibition of cyclooygenase-2 on sodium balance, hemodynamics, and vasoactive eicosanoids. J Pharmacol Exp Ther. 1999;289:735-741.
- Dean M. Opioids in renal failure and dialysis patients. J Pain Symptom Manage. 2004;28(5):497-504.
- Lee MA, Leng ME, Tiernan EJ. Retrospective study of the use of hydromorphone in palliative care patients with normal and abnormal urea and creatinine. Palliat Med. 2001;15(1):26-34.
- Gibson TP. Pharmacokinetics, efficacy, and safety of analgesia with a focus on tramadol HCI. Am J. Med. 1996;101(1A):47S-53S.
- Izzedine H, Launay-Vacher V, Abbara C, Aymard G, Bassilios N, Deray G. Pharmacokinetics of tramadol in a hemodialysis patient. Nephron. 2002;92(3):755-756.
- Hasselström J, Säwe J. Morphine pharmacokinetics and metabolism in humans. Enterohepatic cycling and relative contribution of metabolites to active opioid concentrations. Clin Pharmacokinet. 1993;24(4):344-354.
- Andersen G, Christrup L, Sjøgren P. Relationships among morphine metabolism, pain and side effects during long-term treatment: an update. J Pain Symptom Manage. 2003;25(1):74-91.
- Lidoderm [package insert]. Chadds Ford, PA: Endo Pharmaceuticals Inc.; 2010.
- Carter GT, Galer BS. Advances in the management of neuropathic pain. Phys Med Rehabil Clin N Am. 2001;12(2):447-459.
- Ho KY, Gan TJ, Habib AS. Gabapentin and postoperative pain—a systematic review of randomized controlled trials. Pain. 2006;15:126(1-3):91-101.
- Neurontin [package insert]. New York: Parke-Davis; 2010.
- Pandey CK, Priye S, Singh S, et al. Preemptive use of gabapentin significantly decreases postoperative pain and rescue analgesic requirements in laparoscopic cholecystectomy. Can J Anaesth. 2004;51(4):358-363.
- Srivastava U, Kumar A, Saxena S, et al: Effect of preoperative gabapentin on postoperative pain and tramadol consumption after minilap open cholecystectomy: a randomized double-blind, placebo-controlled trial. Eur J Anaesthesiol. 2010;27(N4):331-335.
- Lyrica [package insert]. New York: Pfizer Inc.; 2012.
- Broadbent A, Khor K, Heaney A. Palliation and chronic renal failure: opioid and other palliative medications—dosage guidelines. Progress in Palliative Care. 2003;11(4):183-190(8).
- Nayak-Rao S. Achieving effective pain relief in patients with chronic kidney disease: a review of analgesics in renal failure. J Nephrol. 2011;24(1):35-40.
- Wolters Kluwer Health. Facts & comparisons. Wolters Kluwer Health website. Available at: http://www.factsandcomparisons.com. Accessed Jan. 14, 2013.
- Lexicomp. Lexicomp Online. Lexicomp website. Available at: http://www.lexi.com/institutions/products/online/.
A Raw Deal
A 39‐year‐old woman presented to the emergency department (ED) with fever and headache. One to two weeks prior to presentation, she developed nightly fevers that gradually increased to as high as 39.4C. She subsequently developed generalized throbbing headaches, malaise, and diffuse body pain. The headache gradually worsened. The day prior to presentation, she developed photophobia, nausea, and vomiting. She also reported right scalp pain while combing her hair, difficulty emptying her bladder, and left buttock pain radiating down the leg. She denied rash, joint pain, visual changes, dysarthria, cough, chest pain, abdominal pain, or diarrhea.
Fever and headache can be explained by meningitis, encephalitis, or brain abscess. The combination is seen far more frequently, however, in patients with common systemic infections such as influenza. For either bacterial meningitis or influenza, a 2‐week course is prolonged and atypical. The progressive nature of the symptoms and photophobia suggest a chronic meningitis, and the development of nausea and vomiting, although nonspecific, is also consistent with elevated intracranial pressure. In a young woman, subacute fever and aches should prompt consideration of an autoimmune disorder such as systemic lupus erythematosus (SLE), although early central nervous system (CNS) involvement is atypical. Migraine headaches are characterized by light sensitivity, nausea, and vomiting and can be precipitated by a viral syndrome, but in this case, the headaches were present at the outset, and 2 weeks is too long for a migraine attack.
Pain while combing hair is not characteristic of the aforementioned syndromes. The scalp should be examined to confirm that there are no skin lesions associated with herpes zoster and no arterial prominence associated with temporal arteritis. She is young for the latter, which would otherwise be a suitable explanation for fever, headache, scalp tenderness, and visual complaints (usually impairment not photophobia).
Incomplete bladder emptying and left buttock pain suggest that there might be a concomitant lumbosacral myelopathy or radiculopathy. Some nonbacterial causes of meningitis such as cytomegalovirus (CMV), syphilis, and cancer simultaneously involve the CNS and peripheral nerve roots. It is also possible that the scalp tenderness associated with combing reflects a cervical sensory radiculopathy.
She had presented to the ED 2 and 4 days before the current (third) ED visit. Both times her main complaint was left buttock pain and left leg paresthesias. Although she had no skin lesions, she was diagnosed with prodromal herpes zoster in the S2 dermatomal distribution and was prescribed valacyclovir (to be started should eruptions develop, which never occurred).
She reported intermittent self‐limited fevers at 3‐ to 4‐week intervals during the prior 6 months; two fever episodes were accompanied by an influenza‐like illness, and one was associated with gastrointestinal symptoms. Her last fever prior to this evaluation was 6 weeks earlier when she was treated with azithromycin for suspected pneumonia at an outside facility.
Her past medical history included hypothyroidism, gastroesophageal reflux disease, diverticulitis, and gluten intolerance. Her medications included porcine (natural) thyroid, fish oil, ibuprofen, and acetaminophen. She lived in Michigan and traveled to the northeast United States (Maine, Cape Cod, New Hampshire, Connecticut, and Vermont) 7 months prior to this evaluation. She was married and had no pets at home. She denied any tobacco, alcohol, or illicit drug use.
Her illness now appears to be chronic, associated with fever, and multisystem (potentially involving the pulmonary and gastrointestinal tract). None of her medical problems would predispose her to subacute meningitis, myelopathy, or radiculopathy. Hypothyroidism raises the possibility of a concomitant autoimmune disorder which causes meningitis, such as SLE or Behet's disease. Sarcoidosis can cause chronic meningitis and neuropathy with concomitant lung and gastrointestinal involvement and rarely fever.
Residency in the upper Midwest increases exposure to chronic infections that rarely cause subacute meningitis such as histoplasmosis, blastomycosis, or human granulocytic anaplasmosis. Travel to the northeast United States 1 month before the onset of her symptoms raises the possibility of other endemic infections like Lyme disease, babesiosis, and tularemia, which may account for her recurrent fevers. Of these, Lyme is most likely to present as chronic meningitis with cranial neuropathy and radiculoneuropathy.
Although the diagnosis of pneumonia was made late in her 6‐month illness, its etiology and treatment may be relevant. If the recent pneumonia was viral, a subsequent viral meningitis may be manifesting now or may have triggered an autoimmune process, such as acute disseminated encephalomyelitis. Bacterial pneumonia is a common precursor to bacterial meningitis, and treatment with azithromycin for the pneumonia may have delayed the meningitis onset or muted its course; this should be taken into account when interpreting cerebrospinal fluid (CSF) culture results.
On physical examination, her temperature was 39.1C, blood pressure was 135/91 mm Hg, with pulse of 87 beats per minute, respiratory rate of 16 breaths per minute, and oxygenation saturation of 97% on room air. She appeared in distress and was covering her eyes. She was alert and oriented. She had photophobia and mild nuchal rigidity. Pupils were equal and reactive to light, but she could not tolerate the eye exam for papilledema. Lung, heart, and abdominal exam were normal. No cranial nerve abnormalities were noted, and muscle strength was 5/5 in all 4 extremities. She had decreased sensation to light touch with allodynia throughout her lower extremities in addition to the lateral portion of the right scalp, which was also tender to palpation. Deep tendon reflexes were 2+ and symmetric in her bilateral upper and lower extremities. She did not have joint swelling, edema, lymphadenopathy, or a rash.
Her fever, headache, nuchal rigidity and photophobia collectively suggest meningitis, which requires evaluation by a lumbar puncture. There is no rash that supports herpes zoster or SLE. She does not have signs of myelopathy that would explain the urinary complaints, but lower motor neuron involvement has not been excluded. The sensory abnormalities in the scalp and leg are consistent with a polyneuroradiculopathy. Anterior lateral scalp tenderness may signal trigeminal nerve involvement, whereas posterior scalp tenderness would localize to the upper cervical cord nerve roots. The contralateral distribution of the scalp and leg sensory deficits suggests a multifocal peripheral nervous system process rather than a single CNS lesion.
Initial laboratory data showed serum white blood cell count (WBC) of 12,000/mm3 (79% polymorphonuclear leukocytes). Hemoglobin was 14.2 g/dL, and platelets were 251,000/mm3. Electrolytes, renal function, and liver function were normal. Thyroid‐stimulating hormone, erythrocyte sedimentation rate, and C‐reactive protein were normal. Urinalysis was negative. Chest x‐ray was normal. Noncontrast head computed tomography (CT) was normal. The patient was unable to void; 500 mL of urine returned when catheterization was performed.
CSF WBC count was 1,280/mm3 (39% neutrophils and 49% lymphocytes). CSF total protein was 175 mg/dL, and glucose was 48 mg/dL; serum glucose was 104 mg/dL. Opening pressure was not recorded. Gram stain was negative. Ceftriaxone, vancomycin, ampicillin, and acyclovir were administered for presumed bacterial or viral meningitis. Magnetic resonance imaging (MRI) of the brain and spine showed diffuse leptomeningeal enhancement (Figure 1).
The urinary retention in the absence of myelopathic findings on exam or MRI suggests a sacral polyradiculoneuropathy. Diffuse leptomeningeal enhancement is consistent with many, if not all, causes of meningitis. The high WBC count, elevated protein, and low glucosecollectively signaling active inflammation in the CNSare highly compatible with bacterial meningitis, although the lymphocytic predominance and other clinical data point to nonbacterial etiologies. The negative Gram stain further lowers the probability of bacterial meningitis, but it has limited sensitivity, may be affected by recent antibiotics, and is typically negative with Listeria. Enterovirus, acute human immunodeficiency virus (HIV), and herpes viruses (eg, CMV or herpes simplex virus [HSV]) are important considerations, with the latter 2 causing associated polyneuroradiculopathy. Patients with genital HSV (not detected here) can have a concomitant sacral radiculitis leading to urinary retention.
Fungal and mycobacterial meningitis is a possibility (especially with the high protein), but the patient does not have the typical multisystem disease or immunosuppression that frequently accompanies those conditions when CNS disease is present. Autoimmune conditions like SLE, Behet's disease, and sarcoidosis remain important conditions, especially with the polyneuroradiculopathy or mononeuritis multiplex, which may reflect multifocal nerve infarction or invasion. Similarly, lymphomatous or carcinomatous meningitis should be considered, although an isolated manifestation in the CNS is unusual. Based on the multifocal neurologic deficits, I favor a viral, spirochete, or malignant etiology of her meningoencephalitis.
Despite ongoing broad spectrum antibiotics and supportive care, she became confused on hospital day 3 and developed anomia, agitation, and worsening headache. A repeat CT of the brain did not show any new abnormalities, but repeat lumbar puncture demonstrated elevated intracranial pressure (opening pressure of 47 cm water) with 427 WBC/mm3. Blood and CSF cultures remained negative.
Detailed questioning of the family revealed that she had been horseback riding 3 weeks prior to admission; there were no other livestock where she rode horses. In addition, the family reported that she and other family members routinely drank raw milk from a cow share program.
HIV antibody test was negative. Herpes simplex, varicella zoster, enteroviruses, and adenovirus CSF polymerase chain reaction (PCR) were negative. Cytomegalovirus and Epstein‐Barr virus PCR were negative in serum and CSF. Arbovirus, lymphocytic choriomeningitis, Coccidioides, Blastomyces, Histoplasma, Brucella, and Lyme serologies were negative. Cryptococcus neoformans antigen was negative in CSF. Serum QuantiFERON‐TB test was negative. Blood and CSF acid‐fast bacilli smears (and eventually mycobacterial cultures) were also negative. Her CSF flow cytometry and cytology were negative for lymphoma.
Unpasteurized milk conveys multiple infectious risks. Listeriosis is a food‐borne illness that can cause meningoencephalitis, but peripheral neuropathies are not characteristic. Brucellosis is usually characterized by severe bone pain, pancytopenia, and hepatosplenomegaly, which are absent. Infection with Mycobacterium bovis mimics Mycobacterium tuberculosis and can cause multisystem disease, typically involving the lung. Campylobacter infection is characterized by gastroenteritis, which has not been prominent.
Rhodococcus equi is a horse‐related pathogen which leads to pulmonary infections in immunocompromised hosts but not meningitis. Rather than focusing on horse exposure alone, however, it may be useful to consider her at risk for vector‐borne pathogens based on her time outdoors, such as Lyme disease (which can cause radiculopathy and encephalopathy), West Nile virus (although motor weakness rather than sensory symptoms is typical), or eastern equine encephalitis.
The absence of weight loss, cytopenias, lymphadenopathy, and organomegaly with the negative CSF cytology and flow cytometry makes lymphomatous meningitis unlikely. The case for an autoimmune disorder is not strong in the absence of joint pains, rash, or autoimmune serologies. In a young woman with unexplained encephalitis, antibodies to the N‐methyl‐D‐aspartate receptor should be assayed.
Although the CSF leukocytosis is declining, the elevated pressure and clinical deterioration signal that the disease process is not controlled. At this point I am uncertain as to the cause of her progressive meningoencephalitis with polyneuroradiculopathy. The latter feature makes me favor a viral or spirochete etiology.
On hospital day 4, Coxiella burnetii serologies were reported as positive (phase II immunoglobulin [Ig] G 1:256; phase II IgM 1:16; phase I IgG 1:16; phase I IgM 1:16) suggesting acute Q fever. Antibiotics were changed to intravenous doxycycline and ciprofloxacin. Her increased intracranial pressure was managed with serial lumbar punctures. The patient was discharged after 12 days of hospitalization taking oral doxycycline and ciprofloxacin. Her symptoms resolved over 10 weeks. No vegetations were seen on transesophageal echocardiogram. She had no evidence of chronic Q fever on repeat serologies.
I was not aware that Q fever causes meningitis or meningoencephalitis. However, I should have considered it in light of her indirect exposure to cows. It is possible that her pneumonia 6 weeks earlier represented acute Q fever, as pneumonia and hepatitis are among the most typical acute manifestations of this infection.
COMMENTARY
Hospitalists are commonly confronted by the combination of fever, headache, and confusion and are familiar with the diagnostic and therapeutic dilemmas related to prompt discrimination between CNS and non‐CNS processes, particularly infections. At the time of this patient's final ED presentation, her illness unambiguously localized to the CNS. As common and emergent conditions such as acute bacterial meningitis were excluded, the greatest challenge was finding the clue that could direct investigations into less common causes of meningoencephalitis.
The Infectious Disease Society of America has developed clinical practice guidelines for the diagnosis and management of encephalitis which highlight the importance of epidemiology and risk factor assessment.[1] This approach requires the clinician to examine potential clues and to go beyond initial associationsfor instance, not simply linking horseback riding to horse‐associated pathogens, but interpreting horseback riding as a proxy for outdoor exposure, which places her at risk for contact with mosquitos, which transmit West Nile virus or eastern equine encephalitis. Similarly, ingestion of raw milk, which is typically linked to Listeria monocytogenes, Brucella, and other pathogens prompted the infectious disease consultant to think more broadly and include livestock (cow)‐associated pathogens including C. burnetii.
Although involvement of the CNS is common in chronic Q fever endocarditis due to septic embolism, neurologic involvement in acute Q fever varies in prevalence (range of 1.7%22%).[2, 3, 4] The 3 major neurological syndromes of acute Q fever are (1) meningoencephalitis or encephalitis, (2) lymphocytic meningitis, and (3) peripheral neuropathy (myelitis, polyradiculoneuritis, or peripheral neuritis). CSF analysis usually shows mild pleocytosis with a predominance of lymphocytic cells; CSF protein elevation is variable, and glucose is usually normal. Neuroradiologic examination is usually normal, and there are no pathognomonic imaging abnormalities for Q fever meningoencephalitis.[2, 3] The mechanism by which C. burnetii causes neurologic injury and dysfunction is unknown.
The diagnosis of Q fever is usually established by serologic testing. In acute Q fever, antibodies to phase II antigen are higher than the phase I antibody titer. Phase II IgM antibodies are the first to appear, but then decline on average after week 8, often reaching undetectable levels 10 to 12 weeks after disease onset.[5] If this patient's pneumonia 6 weeks prior to this presentation was acute Q fever pneumonia, her IgM titers may have been declining by the time her neurologic illness developed. A false negative test result is also possible; immunofluorescence assays are more specific than sensitive in acute Q fever.[5]
Evaluating this case in isolation may raise some doubt as to the accuracy of the diagnosis as she did not have a 4‐fold rise in the phase II IgG titer and did not have a detectable phase II IgM. However, she was part of a cluster of individuals who regularly consumed raw milk from the same dairy and had evidence of C. burnetii infection. This group included her spouse, who had a robust serologic evidence of C. burnetii, characterized by a >4‐fold rise in phase II IgM and IgG titers.[6]
C. burnetii is found primarily in cattle, sheep, and goats and is shed in large quantities by infected periparturient animals in their urine, feces, and milk.[7] Inhalation of contaminated aerosols is the principal route of transmission.[7, 8] Acute Q fever is underdiagnosed because the majority of acute infections are asymptomatic (60%) or present as a nonspecific flu‐like illness.[7] This case represents a rare manifestation of a rare infection acquired through a rare route of transmission, but highlights the importance of epidemiology and risk factor assessment when clinicians are faced with a diagnostic challenge.
TEACHING POINTS
- Exploration of epidemiology and exposure history is central to diagnosing meningoencephalitis with negative bacterial cultures and undetectable HSV PCR, although the etiology of meningoencephalitis can elude identification even after exhaustive investigation.
- Inhalation of contaminated aerosols is the principal route of transmission for C. burnetii, but it can also be transmitted via infected unpasteurized milk.[7, 9]
- Acute presentations of Q fever, which may warrant admission, include pneumonia, hepatitis, or meningoencephalitis.
- Q fever is diagnosed by serologic testing, and doxycycline is the antibiotic of choice.
Disclosures
This case was presented at the 2012 Annual Meeting of the Society of Hospital Medicine. It was subsequently reported in the epidemiologic report of the outbreak.[6] The authors report no conflicts of interest.
- , , , et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–327.
- , , , et al. Q fever 1985–1998 clinical and epidemiologic features of 1,383 Infections. Medicine. 2000;79:109–123.
- , , , et al. Neurological involvement in acute Q fever: a report of 29 cases and review of the literature. Arch Intern Med. 2002;162:693–700.
- , , . Q fever in Plymouth 1972–88, a review with particular reference to neurological manifestations. Epidemiol Infect. 1990;105:391–408.
- , , . Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834.
- , , . Q fever cluster among raw milk drinkers in Michigan, (2011). Clin Infect Dis. 2012;55:1387–1389.
- , . Q fever. Clin Micorbial Rev. 1999;12:18–53.
- , , , et al. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health. 1998;1:180–187.
- , . A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. 1992;47:35–40.
A 39‐year‐old woman presented to the emergency department (ED) with fever and headache. One to two weeks prior to presentation, she developed nightly fevers that gradually increased to as high as 39.4C. She subsequently developed generalized throbbing headaches, malaise, and diffuse body pain. The headache gradually worsened. The day prior to presentation, she developed photophobia, nausea, and vomiting. She also reported right scalp pain while combing her hair, difficulty emptying her bladder, and left buttock pain radiating down the leg. She denied rash, joint pain, visual changes, dysarthria, cough, chest pain, abdominal pain, or diarrhea.
Fever and headache can be explained by meningitis, encephalitis, or brain abscess. The combination is seen far more frequently, however, in patients with common systemic infections such as influenza. For either bacterial meningitis or influenza, a 2‐week course is prolonged and atypical. The progressive nature of the symptoms and photophobia suggest a chronic meningitis, and the development of nausea and vomiting, although nonspecific, is also consistent with elevated intracranial pressure. In a young woman, subacute fever and aches should prompt consideration of an autoimmune disorder such as systemic lupus erythematosus (SLE), although early central nervous system (CNS) involvement is atypical. Migraine headaches are characterized by light sensitivity, nausea, and vomiting and can be precipitated by a viral syndrome, but in this case, the headaches were present at the outset, and 2 weeks is too long for a migraine attack.
Pain while combing hair is not characteristic of the aforementioned syndromes. The scalp should be examined to confirm that there are no skin lesions associated with herpes zoster and no arterial prominence associated with temporal arteritis. She is young for the latter, which would otherwise be a suitable explanation for fever, headache, scalp tenderness, and visual complaints (usually impairment not photophobia).
Incomplete bladder emptying and left buttock pain suggest that there might be a concomitant lumbosacral myelopathy or radiculopathy. Some nonbacterial causes of meningitis such as cytomegalovirus (CMV), syphilis, and cancer simultaneously involve the CNS and peripheral nerve roots. It is also possible that the scalp tenderness associated with combing reflects a cervical sensory radiculopathy.
She had presented to the ED 2 and 4 days before the current (third) ED visit. Both times her main complaint was left buttock pain and left leg paresthesias. Although she had no skin lesions, she was diagnosed with prodromal herpes zoster in the S2 dermatomal distribution and was prescribed valacyclovir (to be started should eruptions develop, which never occurred).
She reported intermittent self‐limited fevers at 3‐ to 4‐week intervals during the prior 6 months; two fever episodes were accompanied by an influenza‐like illness, and one was associated with gastrointestinal symptoms. Her last fever prior to this evaluation was 6 weeks earlier when she was treated with azithromycin for suspected pneumonia at an outside facility.
Her past medical history included hypothyroidism, gastroesophageal reflux disease, diverticulitis, and gluten intolerance. Her medications included porcine (natural) thyroid, fish oil, ibuprofen, and acetaminophen. She lived in Michigan and traveled to the northeast United States (Maine, Cape Cod, New Hampshire, Connecticut, and Vermont) 7 months prior to this evaluation. She was married and had no pets at home. She denied any tobacco, alcohol, or illicit drug use.
Her illness now appears to be chronic, associated with fever, and multisystem (potentially involving the pulmonary and gastrointestinal tract). None of her medical problems would predispose her to subacute meningitis, myelopathy, or radiculopathy. Hypothyroidism raises the possibility of a concomitant autoimmune disorder which causes meningitis, such as SLE or Behet's disease. Sarcoidosis can cause chronic meningitis and neuropathy with concomitant lung and gastrointestinal involvement and rarely fever.
Residency in the upper Midwest increases exposure to chronic infections that rarely cause subacute meningitis such as histoplasmosis, blastomycosis, or human granulocytic anaplasmosis. Travel to the northeast United States 1 month before the onset of her symptoms raises the possibility of other endemic infections like Lyme disease, babesiosis, and tularemia, which may account for her recurrent fevers. Of these, Lyme is most likely to present as chronic meningitis with cranial neuropathy and radiculoneuropathy.
Although the diagnosis of pneumonia was made late in her 6‐month illness, its etiology and treatment may be relevant. If the recent pneumonia was viral, a subsequent viral meningitis may be manifesting now or may have triggered an autoimmune process, such as acute disseminated encephalomyelitis. Bacterial pneumonia is a common precursor to bacterial meningitis, and treatment with azithromycin for the pneumonia may have delayed the meningitis onset or muted its course; this should be taken into account when interpreting cerebrospinal fluid (CSF) culture results.
On physical examination, her temperature was 39.1C, blood pressure was 135/91 mm Hg, with pulse of 87 beats per minute, respiratory rate of 16 breaths per minute, and oxygenation saturation of 97% on room air. She appeared in distress and was covering her eyes. She was alert and oriented. She had photophobia and mild nuchal rigidity. Pupils were equal and reactive to light, but she could not tolerate the eye exam for papilledema. Lung, heart, and abdominal exam were normal. No cranial nerve abnormalities were noted, and muscle strength was 5/5 in all 4 extremities. She had decreased sensation to light touch with allodynia throughout her lower extremities in addition to the lateral portion of the right scalp, which was also tender to palpation. Deep tendon reflexes were 2+ and symmetric in her bilateral upper and lower extremities. She did not have joint swelling, edema, lymphadenopathy, or a rash.
Her fever, headache, nuchal rigidity and photophobia collectively suggest meningitis, which requires evaluation by a lumbar puncture. There is no rash that supports herpes zoster or SLE. She does not have signs of myelopathy that would explain the urinary complaints, but lower motor neuron involvement has not been excluded. The sensory abnormalities in the scalp and leg are consistent with a polyneuroradiculopathy. Anterior lateral scalp tenderness may signal trigeminal nerve involvement, whereas posterior scalp tenderness would localize to the upper cervical cord nerve roots. The contralateral distribution of the scalp and leg sensory deficits suggests a multifocal peripheral nervous system process rather than a single CNS lesion.
Initial laboratory data showed serum white blood cell count (WBC) of 12,000/mm3 (79% polymorphonuclear leukocytes). Hemoglobin was 14.2 g/dL, and platelets were 251,000/mm3. Electrolytes, renal function, and liver function were normal. Thyroid‐stimulating hormone, erythrocyte sedimentation rate, and C‐reactive protein were normal. Urinalysis was negative. Chest x‐ray was normal. Noncontrast head computed tomography (CT) was normal. The patient was unable to void; 500 mL of urine returned when catheterization was performed.
CSF WBC count was 1,280/mm3 (39% neutrophils and 49% lymphocytes). CSF total protein was 175 mg/dL, and glucose was 48 mg/dL; serum glucose was 104 mg/dL. Opening pressure was not recorded. Gram stain was negative. Ceftriaxone, vancomycin, ampicillin, and acyclovir were administered for presumed bacterial or viral meningitis. Magnetic resonance imaging (MRI) of the brain and spine showed diffuse leptomeningeal enhancement (Figure 1).

The urinary retention in the absence of myelopathic findings on exam or MRI suggests a sacral polyradiculoneuropathy. Diffuse leptomeningeal enhancement is consistent with many, if not all, causes of meningitis. The high WBC count, elevated protein, and low glucosecollectively signaling active inflammation in the CNSare highly compatible with bacterial meningitis, although the lymphocytic predominance and other clinical data point to nonbacterial etiologies. The negative Gram stain further lowers the probability of bacterial meningitis, but it has limited sensitivity, may be affected by recent antibiotics, and is typically negative with Listeria. Enterovirus, acute human immunodeficiency virus (HIV), and herpes viruses (eg, CMV or herpes simplex virus [HSV]) are important considerations, with the latter 2 causing associated polyneuroradiculopathy. Patients with genital HSV (not detected here) can have a concomitant sacral radiculitis leading to urinary retention.
Fungal and mycobacterial meningitis is a possibility (especially with the high protein), but the patient does not have the typical multisystem disease or immunosuppression that frequently accompanies those conditions when CNS disease is present. Autoimmune conditions like SLE, Behet's disease, and sarcoidosis remain important conditions, especially with the polyneuroradiculopathy or mononeuritis multiplex, which may reflect multifocal nerve infarction or invasion. Similarly, lymphomatous or carcinomatous meningitis should be considered, although an isolated manifestation in the CNS is unusual. Based on the multifocal neurologic deficits, I favor a viral, spirochete, or malignant etiology of her meningoencephalitis.
Despite ongoing broad spectrum antibiotics and supportive care, she became confused on hospital day 3 and developed anomia, agitation, and worsening headache. A repeat CT of the brain did not show any new abnormalities, but repeat lumbar puncture demonstrated elevated intracranial pressure (opening pressure of 47 cm water) with 427 WBC/mm3. Blood and CSF cultures remained negative.
Detailed questioning of the family revealed that she had been horseback riding 3 weeks prior to admission; there were no other livestock where she rode horses. In addition, the family reported that she and other family members routinely drank raw milk from a cow share program.
HIV antibody test was negative. Herpes simplex, varicella zoster, enteroviruses, and adenovirus CSF polymerase chain reaction (PCR) were negative. Cytomegalovirus and Epstein‐Barr virus PCR were negative in serum and CSF. Arbovirus, lymphocytic choriomeningitis, Coccidioides, Blastomyces, Histoplasma, Brucella, and Lyme serologies were negative. Cryptococcus neoformans antigen was negative in CSF. Serum QuantiFERON‐TB test was negative. Blood and CSF acid‐fast bacilli smears (and eventually mycobacterial cultures) were also negative. Her CSF flow cytometry and cytology were negative for lymphoma.
Unpasteurized milk conveys multiple infectious risks. Listeriosis is a food‐borne illness that can cause meningoencephalitis, but peripheral neuropathies are not characteristic. Brucellosis is usually characterized by severe bone pain, pancytopenia, and hepatosplenomegaly, which are absent. Infection with Mycobacterium bovis mimics Mycobacterium tuberculosis and can cause multisystem disease, typically involving the lung. Campylobacter infection is characterized by gastroenteritis, which has not been prominent.
Rhodococcus equi is a horse‐related pathogen which leads to pulmonary infections in immunocompromised hosts but not meningitis. Rather than focusing on horse exposure alone, however, it may be useful to consider her at risk for vector‐borne pathogens based on her time outdoors, such as Lyme disease (which can cause radiculopathy and encephalopathy), West Nile virus (although motor weakness rather than sensory symptoms is typical), or eastern equine encephalitis.
The absence of weight loss, cytopenias, lymphadenopathy, and organomegaly with the negative CSF cytology and flow cytometry makes lymphomatous meningitis unlikely. The case for an autoimmune disorder is not strong in the absence of joint pains, rash, or autoimmune serologies. In a young woman with unexplained encephalitis, antibodies to the N‐methyl‐D‐aspartate receptor should be assayed.
Although the CSF leukocytosis is declining, the elevated pressure and clinical deterioration signal that the disease process is not controlled. At this point I am uncertain as to the cause of her progressive meningoencephalitis with polyneuroradiculopathy. The latter feature makes me favor a viral or spirochete etiology.
On hospital day 4, Coxiella burnetii serologies were reported as positive (phase II immunoglobulin [Ig] G 1:256; phase II IgM 1:16; phase I IgG 1:16; phase I IgM 1:16) suggesting acute Q fever. Antibiotics were changed to intravenous doxycycline and ciprofloxacin. Her increased intracranial pressure was managed with serial lumbar punctures. The patient was discharged after 12 days of hospitalization taking oral doxycycline and ciprofloxacin. Her symptoms resolved over 10 weeks. No vegetations were seen on transesophageal echocardiogram. She had no evidence of chronic Q fever on repeat serologies.
I was not aware that Q fever causes meningitis or meningoencephalitis. However, I should have considered it in light of her indirect exposure to cows. It is possible that her pneumonia 6 weeks earlier represented acute Q fever, as pneumonia and hepatitis are among the most typical acute manifestations of this infection.
COMMENTARY
Hospitalists are commonly confronted by the combination of fever, headache, and confusion and are familiar with the diagnostic and therapeutic dilemmas related to prompt discrimination between CNS and non‐CNS processes, particularly infections. At the time of this patient's final ED presentation, her illness unambiguously localized to the CNS. As common and emergent conditions such as acute bacterial meningitis were excluded, the greatest challenge was finding the clue that could direct investigations into less common causes of meningoencephalitis.
The Infectious Disease Society of America has developed clinical practice guidelines for the diagnosis and management of encephalitis which highlight the importance of epidemiology and risk factor assessment.[1] This approach requires the clinician to examine potential clues and to go beyond initial associationsfor instance, not simply linking horseback riding to horse‐associated pathogens, but interpreting horseback riding as a proxy for outdoor exposure, which places her at risk for contact with mosquitos, which transmit West Nile virus or eastern equine encephalitis. Similarly, ingestion of raw milk, which is typically linked to Listeria monocytogenes, Brucella, and other pathogens prompted the infectious disease consultant to think more broadly and include livestock (cow)‐associated pathogens including C. burnetii.
Although involvement of the CNS is common in chronic Q fever endocarditis due to septic embolism, neurologic involvement in acute Q fever varies in prevalence (range of 1.7%22%).[2, 3, 4] The 3 major neurological syndromes of acute Q fever are (1) meningoencephalitis or encephalitis, (2) lymphocytic meningitis, and (3) peripheral neuropathy (myelitis, polyradiculoneuritis, or peripheral neuritis). CSF analysis usually shows mild pleocytosis with a predominance of lymphocytic cells; CSF protein elevation is variable, and glucose is usually normal. Neuroradiologic examination is usually normal, and there are no pathognomonic imaging abnormalities for Q fever meningoencephalitis.[2, 3] The mechanism by which C. burnetii causes neurologic injury and dysfunction is unknown.
The diagnosis of Q fever is usually established by serologic testing. In acute Q fever, antibodies to phase II antigen are higher than the phase I antibody titer. Phase II IgM antibodies are the first to appear, but then decline on average after week 8, often reaching undetectable levels 10 to 12 weeks after disease onset.[5] If this patient's pneumonia 6 weeks prior to this presentation was acute Q fever pneumonia, her IgM titers may have been declining by the time her neurologic illness developed. A false negative test result is also possible; immunofluorescence assays are more specific than sensitive in acute Q fever.[5]
Evaluating this case in isolation may raise some doubt as to the accuracy of the diagnosis as she did not have a 4‐fold rise in the phase II IgG titer and did not have a detectable phase II IgM. However, she was part of a cluster of individuals who regularly consumed raw milk from the same dairy and had evidence of C. burnetii infection. This group included her spouse, who had a robust serologic evidence of C. burnetii, characterized by a >4‐fold rise in phase II IgM and IgG titers.[6]
C. burnetii is found primarily in cattle, sheep, and goats and is shed in large quantities by infected periparturient animals in their urine, feces, and milk.[7] Inhalation of contaminated aerosols is the principal route of transmission.[7, 8] Acute Q fever is underdiagnosed because the majority of acute infections are asymptomatic (60%) or present as a nonspecific flu‐like illness.[7] This case represents a rare manifestation of a rare infection acquired through a rare route of transmission, but highlights the importance of epidemiology and risk factor assessment when clinicians are faced with a diagnostic challenge.
TEACHING POINTS
- Exploration of epidemiology and exposure history is central to diagnosing meningoencephalitis with negative bacterial cultures and undetectable HSV PCR, although the etiology of meningoencephalitis can elude identification even after exhaustive investigation.
- Inhalation of contaminated aerosols is the principal route of transmission for C. burnetii, but it can also be transmitted via infected unpasteurized milk.[7, 9]
- Acute presentations of Q fever, which may warrant admission, include pneumonia, hepatitis, or meningoencephalitis.
- Q fever is diagnosed by serologic testing, and doxycycline is the antibiotic of choice.
Disclosures
This case was presented at the 2012 Annual Meeting of the Society of Hospital Medicine. It was subsequently reported in the epidemiologic report of the outbreak.[6] The authors report no conflicts of interest.
A 39‐year‐old woman presented to the emergency department (ED) with fever and headache. One to two weeks prior to presentation, she developed nightly fevers that gradually increased to as high as 39.4C. She subsequently developed generalized throbbing headaches, malaise, and diffuse body pain. The headache gradually worsened. The day prior to presentation, she developed photophobia, nausea, and vomiting. She also reported right scalp pain while combing her hair, difficulty emptying her bladder, and left buttock pain radiating down the leg. She denied rash, joint pain, visual changes, dysarthria, cough, chest pain, abdominal pain, or diarrhea.
Fever and headache can be explained by meningitis, encephalitis, or brain abscess. The combination is seen far more frequently, however, in patients with common systemic infections such as influenza. For either bacterial meningitis or influenza, a 2‐week course is prolonged and atypical. The progressive nature of the symptoms and photophobia suggest a chronic meningitis, and the development of nausea and vomiting, although nonspecific, is also consistent with elevated intracranial pressure. In a young woman, subacute fever and aches should prompt consideration of an autoimmune disorder such as systemic lupus erythematosus (SLE), although early central nervous system (CNS) involvement is atypical. Migraine headaches are characterized by light sensitivity, nausea, and vomiting and can be precipitated by a viral syndrome, but in this case, the headaches were present at the outset, and 2 weeks is too long for a migraine attack.
Pain while combing hair is not characteristic of the aforementioned syndromes. The scalp should be examined to confirm that there are no skin lesions associated with herpes zoster and no arterial prominence associated with temporal arteritis. She is young for the latter, which would otherwise be a suitable explanation for fever, headache, scalp tenderness, and visual complaints (usually impairment not photophobia).
Incomplete bladder emptying and left buttock pain suggest that there might be a concomitant lumbosacral myelopathy or radiculopathy. Some nonbacterial causes of meningitis such as cytomegalovirus (CMV), syphilis, and cancer simultaneously involve the CNS and peripheral nerve roots. It is also possible that the scalp tenderness associated with combing reflects a cervical sensory radiculopathy.
She had presented to the ED 2 and 4 days before the current (third) ED visit. Both times her main complaint was left buttock pain and left leg paresthesias. Although she had no skin lesions, she was diagnosed with prodromal herpes zoster in the S2 dermatomal distribution and was prescribed valacyclovir (to be started should eruptions develop, which never occurred).
She reported intermittent self‐limited fevers at 3‐ to 4‐week intervals during the prior 6 months; two fever episodes were accompanied by an influenza‐like illness, and one was associated with gastrointestinal symptoms. Her last fever prior to this evaluation was 6 weeks earlier when she was treated with azithromycin for suspected pneumonia at an outside facility.
Her past medical history included hypothyroidism, gastroesophageal reflux disease, diverticulitis, and gluten intolerance. Her medications included porcine (natural) thyroid, fish oil, ibuprofen, and acetaminophen. She lived in Michigan and traveled to the northeast United States (Maine, Cape Cod, New Hampshire, Connecticut, and Vermont) 7 months prior to this evaluation. She was married and had no pets at home. She denied any tobacco, alcohol, or illicit drug use.
Her illness now appears to be chronic, associated with fever, and multisystem (potentially involving the pulmonary and gastrointestinal tract). None of her medical problems would predispose her to subacute meningitis, myelopathy, or radiculopathy. Hypothyroidism raises the possibility of a concomitant autoimmune disorder which causes meningitis, such as SLE or Behet's disease. Sarcoidosis can cause chronic meningitis and neuropathy with concomitant lung and gastrointestinal involvement and rarely fever.
Residency in the upper Midwest increases exposure to chronic infections that rarely cause subacute meningitis such as histoplasmosis, blastomycosis, or human granulocytic anaplasmosis. Travel to the northeast United States 1 month before the onset of her symptoms raises the possibility of other endemic infections like Lyme disease, babesiosis, and tularemia, which may account for her recurrent fevers. Of these, Lyme is most likely to present as chronic meningitis with cranial neuropathy and radiculoneuropathy.
Although the diagnosis of pneumonia was made late in her 6‐month illness, its etiology and treatment may be relevant. If the recent pneumonia was viral, a subsequent viral meningitis may be manifesting now or may have triggered an autoimmune process, such as acute disseminated encephalomyelitis. Bacterial pneumonia is a common precursor to bacterial meningitis, and treatment with azithromycin for the pneumonia may have delayed the meningitis onset or muted its course; this should be taken into account when interpreting cerebrospinal fluid (CSF) culture results.
On physical examination, her temperature was 39.1C, blood pressure was 135/91 mm Hg, with pulse of 87 beats per minute, respiratory rate of 16 breaths per minute, and oxygenation saturation of 97% on room air. She appeared in distress and was covering her eyes. She was alert and oriented. She had photophobia and mild nuchal rigidity. Pupils were equal and reactive to light, but she could not tolerate the eye exam for papilledema. Lung, heart, and abdominal exam were normal. No cranial nerve abnormalities were noted, and muscle strength was 5/5 in all 4 extremities. She had decreased sensation to light touch with allodynia throughout her lower extremities in addition to the lateral portion of the right scalp, which was also tender to palpation. Deep tendon reflexes were 2+ and symmetric in her bilateral upper and lower extremities. She did not have joint swelling, edema, lymphadenopathy, or a rash.
Her fever, headache, nuchal rigidity and photophobia collectively suggest meningitis, which requires evaluation by a lumbar puncture. There is no rash that supports herpes zoster or SLE. She does not have signs of myelopathy that would explain the urinary complaints, but lower motor neuron involvement has not been excluded. The sensory abnormalities in the scalp and leg are consistent with a polyneuroradiculopathy. Anterior lateral scalp tenderness may signal trigeminal nerve involvement, whereas posterior scalp tenderness would localize to the upper cervical cord nerve roots. The contralateral distribution of the scalp and leg sensory deficits suggests a multifocal peripheral nervous system process rather than a single CNS lesion.
Initial laboratory data showed serum white blood cell count (WBC) of 12,000/mm3 (79% polymorphonuclear leukocytes). Hemoglobin was 14.2 g/dL, and platelets were 251,000/mm3. Electrolytes, renal function, and liver function were normal. Thyroid‐stimulating hormone, erythrocyte sedimentation rate, and C‐reactive protein were normal. Urinalysis was negative. Chest x‐ray was normal. Noncontrast head computed tomography (CT) was normal. The patient was unable to void; 500 mL of urine returned when catheterization was performed.
CSF WBC count was 1,280/mm3 (39% neutrophils and 49% lymphocytes). CSF total protein was 175 mg/dL, and glucose was 48 mg/dL; serum glucose was 104 mg/dL. Opening pressure was not recorded. Gram stain was negative. Ceftriaxone, vancomycin, ampicillin, and acyclovir were administered for presumed bacterial or viral meningitis. Magnetic resonance imaging (MRI) of the brain and spine showed diffuse leptomeningeal enhancement (Figure 1).

The urinary retention in the absence of myelopathic findings on exam or MRI suggests a sacral polyradiculoneuropathy. Diffuse leptomeningeal enhancement is consistent with many, if not all, causes of meningitis. The high WBC count, elevated protein, and low glucosecollectively signaling active inflammation in the CNSare highly compatible with bacterial meningitis, although the lymphocytic predominance and other clinical data point to nonbacterial etiologies. The negative Gram stain further lowers the probability of bacterial meningitis, but it has limited sensitivity, may be affected by recent antibiotics, and is typically negative with Listeria. Enterovirus, acute human immunodeficiency virus (HIV), and herpes viruses (eg, CMV or herpes simplex virus [HSV]) are important considerations, with the latter 2 causing associated polyneuroradiculopathy. Patients with genital HSV (not detected here) can have a concomitant sacral radiculitis leading to urinary retention.
Fungal and mycobacterial meningitis is a possibility (especially with the high protein), but the patient does not have the typical multisystem disease or immunosuppression that frequently accompanies those conditions when CNS disease is present. Autoimmune conditions like SLE, Behet's disease, and sarcoidosis remain important conditions, especially with the polyneuroradiculopathy or mononeuritis multiplex, which may reflect multifocal nerve infarction or invasion. Similarly, lymphomatous or carcinomatous meningitis should be considered, although an isolated manifestation in the CNS is unusual. Based on the multifocal neurologic deficits, I favor a viral, spirochete, or malignant etiology of her meningoencephalitis.
Despite ongoing broad spectrum antibiotics and supportive care, she became confused on hospital day 3 and developed anomia, agitation, and worsening headache. A repeat CT of the brain did not show any new abnormalities, but repeat lumbar puncture demonstrated elevated intracranial pressure (opening pressure of 47 cm water) with 427 WBC/mm3. Blood and CSF cultures remained negative.
Detailed questioning of the family revealed that she had been horseback riding 3 weeks prior to admission; there were no other livestock where she rode horses. In addition, the family reported that she and other family members routinely drank raw milk from a cow share program.
HIV antibody test was negative. Herpes simplex, varicella zoster, enteroviruses, and adenovirus CSF polymerase chain reaction (PCR) were negative. Cytomegalovirus and Epstein‐Barr virus PCR were negative in serum and CSF. Arbovirus, lymphocytic choriomeningitis, Coccidioides, Blastomyces, Histoplasma, Brucella, and Lyme serologies were negative. Cryptococcus neoformans antigen was negative in CSF. Serum QuantiFERON‐TB test was negative. Blood and CSF acid‐fast bacilli smears (and eventually mycobacterial cultures) were also negative. Her CSF flow cytometry and cytology were negative for lymphoma.
Unpasteurized milk conveys multiple infectious risks. Listeriosis is a food‐borne illness that can cause meningoencephalitis, but peripheral neuropathies are not characteristic. Brucellosis is usually characterized by severe bone pain, pancytopenia, and hepatosplenomegaly, which are absent. Infection with Mycobacterium bovis mimics Mycobacterium tuberculosis and can cause multisystem disease, typically involving the lung. Campylobacter infection is characterized by gastroenteritis, which has not been prominent.
Rhodococcus equi is a horse‐related pathogen which leads to pulmonary infections in immunocompromised hosts but not meningitis. Rather than focusing on horse exposure alone, however, it may be useful to consider her at risk for vector‐borne pathogens based on her time outdoors, such as Lyme disease (which can cause radiculopathy and encephalopathy), West Nile virus (although motor weakness rather than sensory symptoms is typical), or eastern equine encephalitis.
The absence of weight loss, cytopenias, lymphadenopathy, and organomegaly with the negative CSF cytology and flow cytometry makes lymphomatous meningitis unlikely. The case for an autoimmune disorder is not strong in the absence of joint pains, rash, or autoimmune serologies. In a young woman with unexplained encephalitis, antibodies to the N‐methyl‐D‐aspartate receptor should be assayed.
Although the CSF leukocytosis is declining, the elevated pressure and clinical deterioration signal that the disease process is not controlled. At this point I am uncertain as to the cause of her progressive meningoencephalitis with polyneuroradiculopathy. The latter feature makes me favor a viral or spirochete etiology.
On hospital day 4, Coxiella burnetii serologies were reported as positive (phase II immunoglobulin [Ig] G 1:256; phase II IgM 1:16; phase I IgG 1:16; phase I IgM 1:16) suggesting acute Q fever. Antibiotics were changed to intravenous doxycycline and ciprofloxacin. Her increased intracranial pressure was managed with serial lumbar punctures. The patient was discharged after 12 days of hospitalization taking oral doxycycline and ciprofloxacin. Her symptoms resolved over 10 weeks. No vegetations were seen on transesophageal echocardiogram. She had no evidence of chronic Q fever on repeat serologies.
I was not aware that Q fever causes meningitis or meningoencephalitis. However, I should have considered it in light of her indirect exposure to cows. It is possible that her pneumonia 6 weeks earlier represented acute Q fever, as pneumonia and hepatitis are among the most typical acute manifestations of this infection.
COMMENTARY
Hospitalists are commonly confronted by the combination of fever, headache, and confusion and are familiar with the diagnostic and therapeutic dilemmas related to prompt discrimination between CNS and non‐CNS processes, particularly infections. At the time of this patient's final ED presentation, her illness unambiguously localized to the CNS. As common and emergent conditions such as acute bacterial meningitis were excluded, the greatest challenge was finding the clue that could direct investigations into less common causes of meningoencephalitis.
The Infectious Disease Society of America has developed clinical practice guidelines for the diagnosis and management of encephalitis which highlight the importance of epidemiology and risk factor assessment.[1] This approach requires the clinician to examine potential clues and to go beyond initial associationsfor instance, not simply linking horseback riding to horse‐associated pathogens, but interpreting horseback riding as a proxy for outdoor exposure, which places her at risk for contact with mosquitos, which transmit West Nile virus or eastern equine encephalitis. Similarly, ingestion of raw milk, which is typically linked to Listeria monocytogenes, Brucella, and other pathogens prompted the infectious disease consultant to think more broadly and include livestock (cow)‐associated pathogens including C. burnetii.
Although involvement of the CNS is common in chronic Q fever endocarditis due to septic embolism, neurologic involvement in acute Q fever varies in prevalence (range of 1.7%22%).[2, 3, 4] The 3 major neurological syndromes of acute Q fever are (1) meningoencephalitis or encephalitis, (2) lymphocytic meningitis, and (3) peripheral neuropathy (myelitis, polyradiculoneuritis, or peripheral neuritis). CSF analysis usually shows mild pleocytosis with a predominance of lymphocytic cells; CSF protein elevation is variable, and glucose is usually normal. Neuroradiologic examination is usually normal, and there are no pathognomonic imaging abnormalities for Q fever meningoencephalitis.[2, 3] The mechanism by which C. burnetii causes neurologic injury and dysfunction is unknown.
The diagnosis of Q fever is usually established by serologic testing. In acute Q fever, antibodies to phase II antigen are higher than the phase I antibody titer. Phase II IgM antibodies are the first to appear, but then decline on average after week 8, often reaching undetectable levels 10 to 12 weeks after disease onset.[5] If this patient's pneumonia 6 weeks prior to this presentation was acute Q fever pneumonia, her IgM titers may have been declining by the time her neurologic illness developed. A false negative test result is also possible; immunofluorescence assays are more specific than sensitive in acute Q fever.[5]
Evaluating this case in isolation may raise some doubt as to the accuracy of the diagnosis as she did not have a 4‐fold rise in the phase II IgG titer and did not have a detectable phase II IgM. However, she was part of a cluster of individuals who regularly consumed raw milk from the same dairy and had evidence of C. burnetii infection. This group included her spouse, who had a robust serologic evidence of C. burnetii, characterized by a >4‐fold rise in phase II IgM and IgG titers.[6]
C. burnetii is found primarily in cattle, sheep, and goats and is shed in large quantities by infected periparturient animals in their urine, feces, and milk.[7] Inhalation of contaminated aerosols is the principal route of transmission.[7, 8] Acute Q fever is underdiagnosed because the majority of acute infections are asymptomatic (60%) or present as a nonspecific flu‐like illness.[7] This case represents a rare manifestation of a rare infection acquired through a rare route of transmission, but highlights the importance of epidemiology and risk factor assessment when clinicians are faced with a diagnostic challenge.
TEACHING POINTS
- Exploration of epidemiology and exposure history is central to diagnosing meningoencephalitis with negative bacterial cultures and undetectable HSV PCR, although the etiology of meningoencephalitis can elude identification even after exhaustive investigation.
- Inhalation of contaminated aerosols is the principal route of transmission for C. burnetii, but it can also be transmitted via infected unpasteurized milk.[7, 9]
- Acute presentations of Q fever, which may warrant admission, include pneumonia, hepatitis, or meningoencephalitis.
- Q fever is diagnosed by serologic testing, and doxycycline is the antibiotic of choice.
Disclosures
This case was presented at the 2012 Annual Meeting of the Society of Hospital Medicine. It was subsequently reported in the epidemiologic report of the outbreak.[6] The authors report no conflicts of interest.
- , , , et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–327.
- , , , et al. Q fever 1985–1998 clinical and epidemiologic features of 1,383 Infections. Medicine. 2000;79:109–123.
- , , , et al. Neurological involvement in acute Q fever: a report of 29 cases and review of the literature. Arch Intern Med. 2002;162:693–700.
- , , . Q fever in Plymouth 1972–88, a review with particular reference to neurological manifestations. Epidemiol Infect. 1990;105:391–408.
- , , . Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834.
- , , . Q fever cluster among raw milk drinkers in Michigan, (2011). Clin Infect Dis. 2012;55:1387–1389.
- , . Q fever. Clin Micorbial Rev. 1999;12:18–53.
- , , , et al. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health. 1998;1:180–187.
- , . A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. 1992;47:35–40.
- , , , et al. The management of encephalitis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2008;47:303–327.
- , , , et al. Q fever 1985–1998 clinical and epidemiologic features of 1,383 Infections. Medicine. 2000;79:109–123.
- , , , et al. Neurological involvement in acute Q fever: a report of 29 cases and review of the literature. Arch Intern Med. 2002;162:693–700.
- , , . Q fever in Plymouth 1972–88, a review with particular reference to neurological manifestations. Epidemiol Infect. 1990;105:391–408.
- , , . Diagnosis of Q fever. J Clin Microbiol. 1998;36:1823–1834.
- , , . Q fever cluster among raw milk drinkers in Michigan, (2011). Clin Infect Dis. 2012;55:1387–1389.
- , . Q fever. Clin Micorbial Rev. 1999;12:18–53.
- , , , et al. A large outbreak of Q fever in the West Midlands: windborne spread into a metropolitan area? Commun Dis Public Health. 1998;1:180–187.
- , . A cluster of Coxiella burnetii infections associated with exposure to vaccinated goats and their unpasteurized dairy products. Am J Trop Med Hyg. 1992;47:35–40.
Proton-Pump Inhibitors Associated with Increased Mortality Risk
Clinical question: Is the use of proton-pump inhibitors (PPIs) associated with risk of mortality or combined risk of death or rehospitalization in older patients discharged from acute-care hospitals?
Background: Previous studies have shown that the use of PPIs could be associated with increased mortality in institutionalized older people and in patients discharged from acute-care hospitals. Older patients could be more vulnerable to adverse effects of PPIs, such as drug-drug interactions and absorption of nutrients, because of the higher incidence of polypharmacy and malnutrition in the elderly.
Study design: Prospective cohort.
Setting: Eleven acute-care medical wards participating in the Italian study Pharmacosurveillance in the Elderly Care.
Synopsis: All patients aged 65 years or older consecutively admitted to participating wards from April to June 2007 underwent screening. Excluding patients who refused, died during hospitalization, or were admitted to long-term care or rehabilitation units, a total of 491 patients were analyzed. The study team administered questionnaires during admission and conducted follow-up visits every three months for one year after discharge. Outcomes included one-year survival of patients discharged from acute-care medical wards and the combined endpoint of death or rehospitalization.
Overall, 174 patients (35.4%) had PPI exposure. After adjusting for age, cognitive impairment, disability, comorbidities, nutritional status, and number of drugs prescribed, patients exposed to PPIs had a significantly increased risk of death (adjusted HR 1.51, 95% CI 1.03-2.77). This association was strongest among patients receiving high-dose PPIs. No such association was found when considering the combined endpoint (HR 1.49, 95% CI 0.98-2.17). Limitations of the study include observational design, small size, potential for confounding by indication for PPI, and indeterminate PPI use prior to index hospitalization. Finally, the finding of an association between PPIs and increased mortality does not equate to a causative relationship between the two variables.
Bottom line: Proton-pump inhibitor use in older patients discharged from acute-care hospitals is associated with increased all-cause mortality at one year.
Citation: Maggio M, Corsonello A, Ceda GP, et al. Proton-pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173(7):518-523.
Clinical question: Is the use of proton-pump inhibitors (PPIs) associated with risk of mortality or combined risk of death or rehospitalization in older patients discharged from acute-care hospitals?
Background: Previous studies have shown that the use of PPIs could be associated with increased mortality in institutionalized older people and in patients discharged from acute-care hospitals. Older patients could be more vulnerable to adverse effects of PPIs, such as drug-drug interactions and absorption of nutrients, because of the higher incidence of polypharmacy and malnutrition in the elderly.
Study design: Prospective cohort.
Setting: Eleven acute-care medical wards participating in the Italian study Pharmacosurveillance in the Elderly Care.
Synopsis: All patients aged 65 years or older consecutively admitted to participating wards from April to June 2007 underwent screening. Excluding patients who refused, died during hospitalization, or were admitted to long-term care or rehabilitation units, a total of 491 patients were analyzed. The study team administered questionnaires during admission and conducted follow-up visits every three months for one year after discharge. Outcomes included one-year survival of patients discharged from acute-care medical wards and the combined endpoint of death or rehospitalization.
Overall, 174 patients (35.4%) had PPI exposure. After adjusting for age, cognitive impairment, disability, comorbidities, nutritional status, and number of drugs prescribed, patients exposed to PPIs had a significantly increased risk of death (adjusted HR 1.51, 95% CI 1.03-2.77). This association was strongest among patients receiving high-dose PPIs. No such association was found when considering the combined endpoint (HR 1.49, 95% CI 0.98-2.17). Limitations of the study include observational design, small size, potential for confounding by indication for PPI, and indeterminate PPI use prior to index hospitalization. Finally, the finding of an association between PPIs and increased mortality does not equate to a causative relationship between the two variables.
Bottom line: Proton-pump inhibitor use in older patients discharged from acute-care hospitals is associated with increased all-cause mortality at one year.
Citation: Maggio M, Corsonello A, Ceda GP, et al. Proton-pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173(7):518-523.
Clinical question: Is the use of proton-pump inhibitors (PPIs) associated with risk of mortality or combined risk of death or rehospitalization in older patients discharged from acute-care hospitals?
Background: Previous studies have shown that the use of PPIs could be associated with increased mortality in institutionalized older people and in patients discharged from acute-care hospitals. Older patients could be more vulnerable to adverse effects of PPIs, such as drug-drug interactions and absorption of nutrients, because of the higher incidence of polypharmacy and malnutrition in the elderly.
Study design: Prospective cohort.
Setting: Eleven acute-care medical wards participating in the Italian study Pharmacosurveillance in the Elderly Care.
Synopsis: All patients aged 65 years or older consecutively admitted to participating wards from April to June 2007 underwent screening. Excluding patients who refused, died during hospitalization, or were admitted to long-term care or rehabilitation units, a total of 491 patients were analyzed. The study team administered questionnaires during admission and conducted follow-up visits every three months for one year after discharge. Outcomes included one-year survival of patients discharged from acute-care medical wards and the combined endpoint of death or rehospitalization.
Overall, 174 patients (35.4%) had PPI exposure. After adjusting for age, cognitive impairment, disability, comorbidities, nutritional status, and number of drugs prescribed, patients exposed to PPIs had a significantly increased risk of death (adjusted HR 1.51, 95% CI 1.03-2.77). This association was strongest among patients receiving high-dose PPIs. No such association was found when considering the combined endpoint (HR 1.49, 95% CI 0.98-2.17). Limitations of the study include observational design, small size, potential for confounding by indication for PPI, and indeterminate PPI use prior to index hospitalization. Finally, the finding of an association between PPIs and increased mortality does not equate to a causative relationship between the two variables.
Bottom line: Proton-pump inhibitor use in older patients discharged from acute-care hospitals is associated with increased all-cause mortality at one year.
Citation: Maggio M, Corsonello A, Ceda GP, et al. Proton-pump inhibitors and risk of 1-year mortality and rehospitalization in older patients discharged from acute care hospitals. JAMA Intern Med. 2013;173(7):518-523.
Diabetes Mellitus Does Not Increase Risk of Surgical Complications after Elective Total Knee Replacement Surgery
Clinical question: Does uncontrolled diabetes mellitus increase risk for post-operative complications after elective joint replacement surgery?
Background: Several previous studies suggested that patients with uncontrolled diabetes could be at higher risk of postoperative complications and have worse functional outcomes after joint replacement surgery than patients without diabetes. Preoperative glycemic control is a potentially modifiable risk factor in patients undergoing elective joint replacement surgery. Demand for elective joint replacement is expected to increase over time, and reducing the risk of postoperative complications is essential in order to optimize functional outcomes and reduce healthcare costs.
Study design: Retrospective cohort.
Setting: Five regions of the Kaiser Permanente healthcare system.
Synopsis: The study included 40,491 patients aged 18 years and older who underwent primary knee replacement between January 2001 and December 2009 in five regions of the Kaiser Permanente system. Patients were identified using the Kaiser Permanente Total Joint Replacement Registry. Clinical information on each patient was collected from two years before the procedure to one year after the procedure using Kaiser Permanente electronic health records. Subjects were classified as nondiabetic (81.3%), diabetic with good glycemic control (12.5%), or diabetic with poor glycemic control (6.2%). Glycemic control status was assessed using the latest hemoglobin A1c (HbA1c) value measured prior to the date of the index surgery, with HbA1c <7.0% defined as good glycemic control. Outcomes included revision arthroplasty, deep infection, DVT or PE, incident myocardial infarction, and rehospitalization.
There was no significant association identified between uncontrolled diabetes and any of the five outcomes.
Limitations of the study include retrospective design, rarity of all outcomes except all-cause rehospitalization, and the small number of patients with uncontrolled diabetes in the cohort. In addition, functional outcomes were not assessed in this study.
Bottom line: The effect of uncontrolled diabetes on the risk of adverse surgical outcomes following elective joint replacement remains unclear based on currently published data; more studies are needed.
Citation: Adams AL, Paxton EW, Wang JQ, et al. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001-2009. J Bone Joint Surg Am. 2013;95:481-487.
Clinical question: Does uncontrolled diabetes mellitus increase risk for post-operative complications after elective joint replacement surgery?
Background: Several previous studies suggested that patients with uncontrolled diabetes could be at higher risk of postoperative complications and have worse functional outcomes after joint replacement surgery than patients without diabetes. Preoperative glycemic control is a potentially modifiable risk factor in patients undergoing elective joint replacement surgery. Demand for elective joint replacement is expected to increase over time, and reducing the risk of postoperative complications is essential in order to optimize functional outcomes and reduce healthcare costs.
Study design: Retrospective cohort.
Setting: Five regions of the Kaiser Permanente healthcare system.
Synopsis: The study included 40,491 patients aged 18 years and older who underwent primary knee replacement between January 2001 and December 2009 in five regions of the Kaiser Permanente system. Patients were identified using the Kaiser Permanente Total Joint Replacement Registry. Clinical information on each patient was collected from two years before the procedure to one year after the procedure using Kaiser Permanente electronic health records. Subjects were classified as nondiabetic (81.3%), diabetic with good glycemic control (12.5%), or diabetic with poor glycemic control (6.2%). Glycemic control status was assessed using the latest hemoglobin A1c (HbA1c) value measured prior to the date of the index surgery, with HbA1c <7.0% defined as good glycemic control. Outcomes included revision arthroplasty, deep infection, DVT or PE, incident myocardial infarction, and rehospitalization.
There was no significant association identified between uncontrolled diabetes and any of the five outcomes.
Limitations of the study include retrospective design, rarity of all outcomes except all-cause rehospitalization, and the small number of patients with uncontrolled diabetes in the cohort. In addition, functional outcomes were not assessed in this study.
Bottom line: The effect of uncontrolled diabetes on the risk of adverse surgical outcomes following elective joint replacement remains unclear based on currently published data; more studies are needed.
Citation: Adams AL, Paxton EW, Wang JQ, et al. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001-2009. J Bone Joint Surg Am. 2013;95:481-487.
Clinical question: Does uncontrolled diabetes mellitus increase risk for post-operative complications after elective joint replacement surgery?
Background: Several previous studies suggested that patients with uncontrolled diabetes could be at higher risk of postoperative complications and have worse functional outcomes after joint replacement surgery than patients without diabetes. Preoperative glycemic control is a potentially modifiable risk factor in patients undergoing elective joint replacement surgery. Demand for elective joint replacement is expected to increase over time, and reducing the risk of postoperative complications is essential in order to optimize functional outcomes and reduce healthcare costs.
Study design: Retrospective cohort.
Setting: Five regions of the Kaiser Permanente healthcare system.
Synopsis: The study included 40,491 patients aged 18 years and older who underwent primary knee replacement between January 2001 and December 2009 in five regions of the Kaiser Permanente system. Patients were identified using the Kaiser Permanente Total Joint Replacement Registry. Clinical information on each patient was collected from two years before the procedure to one year after the procedure using Kaiser Permanente electronic health records. Subjects were classified as nondiabetic (81.3%), diabetic with good glycemic control (12.5%), or diabetic with poor glycemic control (6.2%). Glycemic control status was assessed using the latest hemoglobin A1c (HbA1c) value measured prior to the date of the index surgery, with HbA1c <7.0% defined as good glycemic control. Outcomes included revision arthroplasty, deep infection, DVT or PE, incident myocardial infarction, and rehospitalization.
There was no significant association identified between uncontrolled diabetes and any of the five outcomes.
Limitations of the study include retrospective design, rarity of all outcomes except all-cause rehospitalization, and the small number of patients with uncontrolled diabetes in the cohort. In addition, functional outcomes were not assessed in this study.
Bottom line: The effect of uncontrolled diabetes on the risk of adverse surgical outcomes following elective joint replacement remains unclear based on currently published data; more studies are needed.
Citation: Adams AL, Paxton EW, Wang JQ, et al. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001-2009. J Bone Joint Surg Am. 2013;95:481-487.
Elevated Lactate Levels Correlate with Adverse Outcomes in Acute PE
Clinical question: Are high plasma lactate levels associated with mortality and clinical deterioration among patients with acute pulmonary embolism (PE)?
Background: Prognostic clinical markers are limited in patients presenting with acute PE, especially among normotensive individuals. Plasma lactate concentration is a marker of tissue hypoperfusion that has been used to risk-stratify patients with sepsis and trauma. It is unknown whether elevated lactate levels predict poor outcomes in acute PE.
Study design: Prospective cohort.
Setting: ED in a large teaching hospital in Italy.
Synopsis: Consecutive adult patients with acute PE diagnosed by spiral computed tomography or lung scan were included. Plasma lactate levels were tested in all patients at presentation, and levels of ≥2 mmol/L were considered abnormal. The primary endpoint was all-cause death within 30 days, and the secondary endpoint was the composite of all-cause death and PE-related clinical deterioration and death.
Of the 270 patients, 81 (30%) had abnormal lactate levels, though only 12 (4.4%) had shock or hypotension. Patients with elevated lactate had higher mortality compared with patients with lower levels (17.3% vs. 1.6%, OR 12.95, 95% CI 3.43-58.73). Plasma lactate ≥2 mmol/L was associated with higher all-cause mortality (HR 11.67, 95% CI 3.32-41.03) and the composite endpoint (HR 8.14, 95% CI 3.83-17.34). This association was independent of the presence of hypotension, right ventricular dysfunction, or elevated troponin.
Limitations include the single study site (which limits generalizability of the findings) and that lactate levels were only checked once (which might not have fully reflected each patient’s clinical picture). The authors suggest that plasma lactate levels might have utility in determining which patients should be treated more aggressively for PE.
Bottom line: In patients presenting with acute PE, elevated plasma lactate levels are associated with increased risk of short-term mortality and morbidity, independent of the presence of hypotension or markers of right ventricular injury.
Citation: Vanni S, Viviani G, Baioni M, et al. Prognostic value of plasma lactate levels among patients with acute pulmonary embolism: the thrombo-embolism lactate outcome study. Ann Emerg Med. 2013;61:330-338.
Clinical question: Are high plasma lactate levels associated with mortality and clinical deterioration among patients with acute pulmonary embolism (PE)?
Background: Prognostic clinical markers are limited in patients presenting with acute PE, especially among normotensive individuals. Plasma lactate concentration is a marker of tissue hypoperfusion that has been used to risk-stratify patients with sepsis and trauma. It is unknown whether elevated lactate levels predict poor outcomes in acute PE.
Study design: Prospective cohort.
Setting: ED in a large teaching hospital in Italy.
Synopsis: Consecutive adult patients with acute PE diagnosed by spiral computed tomography or lung scan were included. Plasma lactate levels were tested in all patients at presentation, and levels of ≥2 mmol/L were considered abnormal. The primary endpoint was all-cause death within 30 days, and the secondary endpoint was the composite of all-cause death and PE-related clinical deterioration and death.
Of the 270 patients, 81 (30%) had abnormal lactate levels, though only 12 (4.4%) had shock or hypotension. Patients with elevated lactate had higher mortality compared with patients with lower levels (17.3% vs. 1.6%, OR 12.95, 95% CI 3.43-58.73). Plasma lactate ≥2 mmol/L was associated with higher all-cause mortality (HR 11.67, 95% CI 3.32-41.03) and the composite endpoint (HR 8.14, 95% CI 3.83-17.34). This association was independent of the presence of hypotension, right ventricular dysfunction, or elevated troponin.
Limitations include the single study site (which limits generalizability of the findings) and that lactate levels were only checked once (which might not have fully reflected each patient’s clinical picture). The authors suggest that plasma lactate levels might have utility in determining which patients should be treated more aggressively for PE.
Bottom line: In patients presenting with acute PE, elevated plasma lactate levels are associated with increased risk of short-term mortality and morbidity, independent of the presence of hypotension or markers of right ventricular injury.
Citation: Vanni S, Viviani G, Baioni M, et al. Prognostic value of plasma lactate levels among patients with acute pulmonary embolism: the thrombo-embolism lactate outcome study. Ann Emerg Med. 2013;61:330-338.
Clinical question: Are high plasma lactate levels associated with mortality and clinical deterioration among patients with acute pulmonary embolism (PE)?
Background: Prognostic clinical markers are limited in patients presenting with acute PE, especially among normotensive individuals. Plasma lactate concentration is a marker of tissue hypoperfusion that has been used to risk-stratify patients with sepsis and trauma. It is unknown whether elevated lactate levels predict poor outcomes in acute PE.
Study design: Prospective cohort.
Setting: ED in a large teaching hospital in Italy.
Synopsis: Consecutive adult patients with acute PE diagnosed by spiral computed tomography or lung scan were included. Plasma lactate levels were tested in all patients at presentation, and levels of ≥2 mmol/L were considered abnormal. The primary endpoint was all-cause death within 30 days, and the secondary endpoint was the composite of all-cause death and PE-related clinical deterioration and death.
Of the 270 patients, 81 (30%) had abnormal lactate levels, though only 12 (4.4%) had shock or hypotension. Patients with elevated lactate had higher mortality compared with patients with lower levels (17.3% vs. 1.6%, OR 12.95, 95% CI 3.43-58.73). Plasma lactate ≥2 mmol/L was associated with higher all-cause mortality (HR 11.67, 95% CI 3.32-41.03) and the composite endpoint (HR 8.14, 95% CI 3.83-17.34). This association was independent of the presence of hypotension, right ventricular dysfunction, or elevated troponin.
Limitations include the single study site (which limits generalizability of the findings) and that lactate levels were only checked once (which might not have fully reflected each patient’s clinical picture). The authors suggest that plasma lactate levels might have utility in determining which patients should be treated more aggressively for PE.
Bottom line: In patients presenting with acute PE, elevated plasma lactate levels are associated with increased risk of short-term mortality and morbidity, independent of the presence of hypotension or markers of right ventricular injury.
Citation: Vanni S, Viviani G, Baioni M, et al. Prognostic value of plasma lactate levels among patients with acute pulmonary embolism: the thrombo-embolism lactate outcome study. Ann Emerg Med. 2013;61:330-338.
Direct Provider Communication Not Associated with 30-Day Readmissions
Clinical question: How often do inpatient providers report direct communication with outpatient providers, and how is direct communication associated with 30-day readmissions?
Background: Studies have demonstrated that adverse events and errors occurring after hospital discharge can result from poor provider communication between the inpatient and outpatient setting.
Study design: Prospective cohort.
Setting: Johns Hopkins Hospital, Baltimore.
Synopsis: The presence or absence of direct communication between inpatient and outpatient healthcare providers was captured from a required field in an electronic discharge worksheet completed by the discharging physician. Of 6,635 hospitalizations studied, successful direct communication was reported in 36.7% of cases. Predictors of successful direct communication included patients cared for by hospitalists without house staff (OR 1.81, 95% CI 1.59-2.08), high expected 30-day readmission rate (OR 1.18, 95% CI 1.10-1.28), and insurance by Medicare (OR 1.35, 95% CI 1.16-1.56) and private insurance companies (OR 1.35, 95% CI 1.18-1.56). In adjusted analyses, direct communication between the inpatient and outpatient providers was not associated with 30-day readmissions (OR 1.08, 95% CI 0.92-1.26).
There were several limitations in this study. Only the primary team was surveyed; thus, it is not known if consulting providers might have contacted the outpatient providers. Only readmissions to the same medical center were studied, and therefore it is not known if patients were readmitted to other facilities. Additionally, information regarding discharge communication was self-reported, which might have introduced bias.
Bottom line: Self-reported direct communication between inpatient and outpatient providers occurred infrequently and was not associated with 30-day same-hospital readmission.
Citation: Oduyebo I, Lehmann C, Pollack C, et al. Association of self-reported hospital discharge handoffs with 30-day readmissions. JAMA Intern Med. 2013;173:624-629.
Clinical question: How often do inpatient providers report direct communication with outpatient providers, and how is direct communication associated with 30-day readmissions?
Background: Studies have demonstrated that adverse events and errors occurring after hospital discharge can result from poor provider communication between the inpatient and outpatient setting.
Study design: Prospective cohort.
Setting: Johns Hopkins Hospital, Baltimore.
Synopsis: The presence or absence of direct communication between inpatient and outpatient healthcare providers was captured from a required field in an electronic discharge worksheet completed by the discharging physician. Of 6,635 hospitalizations studied, successful direct communication was reported in 36.7% of cases. Predictors of successful direct communication included patients cared for by hospitalists without house staff (OR 1.81, 95% CI 1.59-2.08), high expected 30-day readmission rate (OR 1.18, 95% CI 1.10-1.28), and insurance by Medicare (OR 1.35, 95% CI 1.16-1.56) and private insurance companies (OR 1.35, 95% CI 1.18-1.56). In adjusted analyses, direct communication between the inpatient and outpatient providers was not associated with 30-day readmissions (OR 1.08, 95% CI 0.92-1.26).
There were several limitations in this study. Only the primary team was surveyed; thus, it is not known if consulting providers might have contacted the outpatient providers. Only readmissions to the same medical center were studied, and therefore it is not known if patients were readmitted to other facilities. Additionally, information regarding discharge communication was self-reported, which might have introduced bias.
Bottom line: Self-reported direct communication between inpatient and outpatient providers occurred infrequently and was not associated with 30-day same-hospital readmission.
Citation: Oduyebo I, Lehmann C, Pollack C, et al. Association of self-reported hospital discharge handoffs with 30-day readmissions. JAMA Intern Med. 2013;173:624-629.
Clinical question: How often do inpatient providers report direct communication with outpatient providers, and how is direct communication associated with 30-day readmissions?
Background: Studies have demonstrated that adverse events and errors occurring after hospital discharge can result from poor provider communication between the inpatient and outpatient setting.
Study design: Prospective cohort.
Setting: Johns Hopkins Hospital, Baltimore.
Synopsis: The presence or absence of direct communication between inpatient and outpatient healthcare providers was captured from a required field in an electronic discharge worksheet completed by the discharging physician. Of 6,635 hospitalizations studied, successful direct communication was reported in 36.7% of cases. Predictors of successful direct communication included patients cared for by hospitalists without house staff (OR 1.81, 95% CI 1.59-2.08), high expected 30-day readmission rate (OR 1.18, 95% CI 1.10-1.28), and insurance by Medicare (OR 1.35, 95% CI 1.16-1.56) and private insurance companies (OR 1.35, 95% CI 1.18-1.56). In adjusted analyses, direct communication between the inpatient and outpatient providers was not associated with 30-day readmissions (OR 1.08, 95% CI 0.92-1.26).
There were several limitations in this study. Only the primary team was surveyed; thus, it is not known if consulting providers might have contacted the outpatient providers. Only readmissions to the same medical center were studied, and therefore it is not known if patients were readmitted to other facilities. Additionally, information regarding discharge communication was self-reported, which might have introduced bias.
Bottom line: Self-reported direct communication between inpatient and outpatient providers occurred infrequently and was not associated with 30-day same-hospital readmission.
Citation: Oduyebo I, Lehmann C, Pollack C, et al. Association of self-reported hospital discharge handoffs with 30-day readmissions. JAMA Intern Med. 2013;173:624-629.
Suboptimal Outcomes Using IVC Filters for VTE Prophylaxis, Treatment
Clinical question: In patients who undergo inferior vena cava (IVC) filter placement for venous thromboembolism (VTE) prophylaxis or treatment, what are the associated patient characteristics, indications for placement, complications, retrieval date, and use of concomitant anticoagulant therapy?
Background: Retrievable IVC filters were designed to provide short-term protection from pulmonary embolism but are often left in place indefinitely. Retrievable IVC filters that are not removed can carry significant long-term risks. Further, the use of filters for VTE prophylaxis is controversial, and there are multiple sets of conflicting guidelines for filter insertion provided by various professional groups.
Study design: Retrospective review of IVC filter use over an eight-year period.
Setting: Boston Medical Center.
Synopsis: Medical records from all patients at Boston Medical Center who had a billing code for placement of an IVC filter between August 2003 and February 2011 were manually reviewed. Nine hundred fifty-two medical records were evaluated, of which 679 (71.3%) patients had retrievable IVC filters placed. The most common indications for filter placement were trauma (50.2%), malignancy (15.9%), and bleeding during anticoagulation (11.8%).
In total, 448 patients (47.1%) had filters placed for prophylactic purposes in the absence of documented VTE. Seventy-four patients developed VTE after filter placement; 48.2% of post-filter insertion VTEs occurred in patients who had no VTE prior to the filter; and 89.4% occurred in patients not receiving anticoagulants. An attempt was made to remove 71 of 679 (10.5%) retrievable filters, and 58 (8.5%) attempts were successful. There were 10 serious complications related to mechanical filter failure, including migration or fracture of the filter.
In this study, there was a high volume of filter use by the trauma service; thus, the patient population might be different from the hospital medicine patient population. The study also lacked systematic imaging and follow-up data. Further studies are needed to analyze the risks associated with IVC filter placement.
Bottom line: Use of IVC filters for VTE treatment and prophylaxis, in the context of low filter retrieval rates and lack of appropriate anticoagulant therapy, results in suboptimal outcomes.
Citation: Sarosiek S, Crowther M, Sloan M. Indications, complications, and management of inferior vena cava filters: the experience in 952 patients at an academic hospital with a level I trauma center. JAMA Intern Med. 2013;173:513-517.
Clinical question: In patients who undergo inferior vena cava (IVC) filter placement for venous thromboembolism (VTE) prophylaxis or treatment, what are the associated patient characteristics, indications for placement, complications, retrieval date, and use of concomitant anticoagulant therapy?
Background: Retrievable IVC filters were designed to provide short-term protection from pulmonary embolism but are often left in place indefinitely. Retrievable IVC filters that are not removed can carry significant long-term risks. Further, the use of filters for VTE prophylaxis is controversial, and there are multiple sets of conflicting guidelines for filter insertion provided by various professional groups.
Study design: Retrospective review of IVC filter use over an eight-year period.
Setting: Boston Medical Center.
Synopsis: Medical records from all patients at Boston Medical Center who had a billing code for placement of an IVC filter between August 2003 and February 2011 were manually reviewed. Nine hundred fifty-two medical records were evaluated, of which 679 (71.3%) patients had retrievable IVC filters placed. The most common indications for filter placement were trauma (50.2%), malignancy (15.9%), and bleeding during anticoagulation (11.8%).
In total, 448 patients (47.1%) had filters placed for prophylactic purposes in the absence of documented VTE. Seventy-four patients developed VTE after filter placement; 48.2% of post-filter insertion VTEs occurred in patients who had no VTE prior to the filter; and 89.4% occurred in patients not receiving anticoagulants. An attempt was made to remove 71 of 679 (10.5%) retrievable filters, and 58 (8.5%) attempts were successful. There were 10 serious complications related to mechanical filter failure, including migration or fracture of the filter.
In this study, there was a high volume of filter use by the trauma service; thus, the patient population might be different from the hospital medicine patient population. The study also lacked systematic imaging and follow-up data. Further studies are needed to analyze the risks associated with IVC filter placement.
Bottom line: Use of IVC filters for VTE treatment and prophylaxis, in the context of low filter retrieval rates and lack of appropriate anticoagulant therapy, results in suboptimal outcomes.
Citation: Sarosiek S, Crowther M, Sloan M. Indications, complications, and management of inferior vena cava filters: the experience in 952 patients at an academic hospital with a level I trauma center. JAMA Intern Med. 2013;173:513-517.
Clinical question: In patients who undergo inferior vena cava (IVC) filter placement for venous thromboembolism (VTE) prophylaxis or treatment, what are the associated patient characteristics, indications for placement, complications, retrieval date, and use of concomitant anticoagulant therapy?
Background: Retrievable IVC filters were designed to provide short-term protection from pulmonary embolism but are often left in place indefinitely. Retrievable IVC filters that are not removed can carry significant long-term risks. Further, the use of filters for VTE prophylaxis is controversial, and there are multiple sets of conflicting guidelines for filter insertion provided by various professional groups.
Study design: Retrospective review of IVC filter use over an eight-year period.
Setting: Boston Medical Center.
Synopsis: Medical records from all patients at Boston Medical Center who had a billing code for placement of an IVC filter between August 2003 and February 2011 were manually reviewed. Nine hundred fifty-two medical records were evaluated, of which 679 (71.3%) patients had retrievable IVC filters placed. The most common indications for filter placement were trauma (50.2%), malignancy (15.9%), and bleeding during anticoagulation (11.8%).
In total, 448 patients (47.1%) had filters placed for prophylactic purposes in the absence of documented VTE. Seventy-four patients developed VTE after filter placement; 48.2% of post-filter insertion VTEs occurred in patients who had no VTE prior to the filter; and 89.4% occurred in patients not receiving anticoagulants. An attempt was made to remove 71 of 679 (10.5%) retrievable filters, and 58 (8.5%) attempts were successful. There were 10 serious complications related to mechanical filter failure, including migration or fracture of the filter.
In this study, there was a high volume of filter use by the trauma service; thus, the patient population might be different from the hospital medicine patient population. The study also lacked systematic imaging and follow-up data. Further studies are needed to analyze the risks associated with IVC filter placement.
Bottom line: Use of IVC filters for VTE treatment and prophylaxis, in the context of low filter retrieval rates and lack of appropriate anticoagulant therapy, results in suboptimal outcomes.
Citation: Sarosiek S, Crowther M, Sloan M. Indications, complications, and management of inferior vena cava filters: the experience in 952 patients at an academic hospital with a level I trauma center. JAMA Intern Med. 2013;173:513-517.