User login
How Should Hyponatremia Be Evaluated and Managed?
Case
A 67-year-old male patient who has depression and is on sertraline presents with increasing confusion over the past week. Initial plasma sodium is 109 mEq/L. On exam, he weighs 70 kg and is euvolemic. His urine osmolarity (Uosm) is 800 mosm/L with a urine sodium (UNa) of 40 mEq/L. He is somnolent but awakens to sternal rub. How should this patient’s hyponatremia be evaluated and managed?
Overview
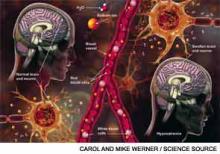
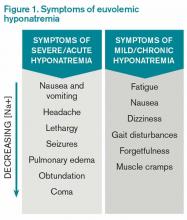
Hyponatremia, a disorder of excess total body water in relation to sodium, occurs in up to 42% of hospitalized patients.1,2 Regardless of the cause, hyponatremia is usually associated with the syndrome of inappropriate antidiuretic hormone secretion (SIADH) or with the appropriate elevation of antidiuretic hormone (ADH), known as hypovolemia. ADH is produced in the hypothalamus and released in the posterior pituitary in response to increasing plasma osmolarity (pOSM) or effective circulating volume depletion. ADH acts in the cortical collecting duct to increase the number of luminal aquaporin channels, increasing water reabsorption and decreasing plasma osmolarity. When hyponatremia is severe, the movement of water into cells causes cellular brain swelling, and clinical symptoms progress from malaise, headache, and nausea to obtundation, seizures, or respiratory arrest (see Figure 1). Even mild, chronic hyponatremia (120-131 mEq/L) is associated with an increased risk of falls due to mild gait and attention impairment.3
Evaluation
Step 1: Plasma osmolarity
The first step in diagnosing the cause of hyponatremia and treating it is to measure pOSM. The majority of patients with hyponatremia have hypoosmolar hyponatremia and therefore have a low pOSM; however, patients may have normal or high osmolarity. Hyponatremia with normal osmolarity can be caused by pseudohyponatremia (i.e., hyperglycemia, paraproteinemia, hyperlipidemia), severe renal failure, ingestion of excess alcohol, or post-transurethral resection of prostate or bladder.
Hyponatremia with high pOSM occurs as a result of elevated levels of an extra solute in the plasma that does not readily enter cells. This draws water into the extracellular fluid and lowers the sodium concentration. This will most commonly result from hyperglycemia or infusion of mannitol.
Step 2: Assess volume status with physical exam, urine sodium (UNa)
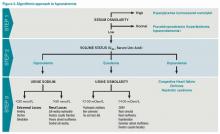
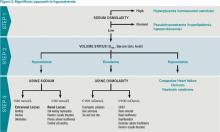
The majority of patients with hyponatremia will have low pOSM. These patients should be categorized by volume status: hypovolemic, euvolemic, or hypervolemic (see Figure 2). On exam, hypervolemia is usually evident, and the cause of hypervolemic hyponatremia is usually elicited from a patient’s history; however, differentiating between hypovolemic and euvolemic hyponatremia by history and physical exam can be difficult, because examination findings are neither sensitive nor specific.4 UNa should always be evaluated, especially when differentiating between hypovolemic and euvolemic. This was illustrated in a study of 58 non-edematous patients with hyponatremia. Investigators determined which patients had hypovolemic hyponatremia based on their response to saline infusion. Of the patients identified as hypovolemic using physical exam, only 47% responded to saline. In contrast, a spot UNa of less than 30 mEq/L was 80% sensitive and 100% specific for saline responsiveness.5 Although the majority of hypovolemic hyponatremia patients will have a low UNa, the following causes of hypovolemic hyponatremia can result in high UNa: diuretics, adrenal insufficiency, salt-wasting nephropathy, and cerebral salt-wasting.
A low serum uric acid can also be useful in differentiating hypovolemic and euvolemic hyponatremia, which is most commonly caused by SIADH. In SIADH, there is urinary wasting of uric acid, which leads to low serum uric acid. In a study of 105 patients with lung cancer, a serum uric acid of less than 4 mg/dL was 75% sensitive and 89% specific for SIADH.6
Step 3: Urine osmolarity
After determining volume status, the physician should determine if there is excess ADH by measuring Uosm. Under normal conditions, hyponatremia should suppress ADH secretion and allow the kidney to excrete water by diluting the urine to less than 100 mosm/L. If Uosm is less than 100 mosm/L, then the kidneys are responding appropriately and can only persist in the following situations: The patient is drinking large volumes of water (e.g. primary polydipsia), there is insufficient solute to excrete free water (e.g. beer potomania, “tea and toast” diet), or the patient has a different set point for ADH suppression (i.e., reset osmostat). After determining volume status, UNa, and Uosm, the physician will have narrowed the cause of hyponatremia significantly (see Figure 2). Of note, when SIADH is diagnosed, it is important to look for and reverse causes (see Figure 3).
Treatment
Severe symptomatic hyponatremia
In patients with severe neurologic symptoms, physicians must balance the need to reduce symptoms quickly with the dangers of overly rapid correction. After its use in marathon runners, several experts have endorsed the following regimen to reduce symptoms rapidly: an intravenous bolus of 100 mL of 3% saline is given and repeated if symptoms persist after 10 minutes.7,8 Once symptoms improve, the basal rate can be calculated using the equation below, but the rate of sodium correction in 24 hours with this regimen should not exceed 6 to 8 mEq/L in 24 hours or 12 to 14 mEq/L in 48 hours.9,10 This is based on several case studies showing that there were no cases of central pontine myelinolysis (CPM) if correction rates were less than 10 mEq/L over 24 hours.11,12
It is important to remember that this is only a rough guide, because the equation assumes the entire infusate is retained and there is no sodium or water output. The best way to avoid overly rapid correction is to check serum sodium every two hours and monitor urine output closely. If the patient is making large volumes of urine, serum sodium may be rising too quickly. If the patient corrects too rapidly, it may be possible to avoid CPM by re-lowering the sodium.13 This can be accomplished by giving desmopressin to slow urinary free water loss while simultaneously giving hypotonic fluids.
Asymptomatic or mildly symptomatic hyponatremia
Hypovolemic hyponatremia: Treatment of hypovolemic hyponatremia is aimed at correcting volume status, the underlying problem that drives ADH secretion. The body will always choose to preserve volume over osmolarity. In most cases, normal saline (NS) should be used to restore intravascular volume, and the rate of infusion can be calculated using the same equation as above. Once volume is replete, ADH release will cease. Patients will be in danger of overly rapid correction of serum sodium, so fluids should be switched to hypotonic solutions, such as ½ NS.
Euvolemic Hyponatremia: Euvolemic hyponatremia, typically caused by SIADH, is characterized by a high Uosm (>100 mosm/L) and a high UNa (>30 mEq/L). All patients require free water restriction, and fluid intake should be at least 500 mL below a patient’s urine output, usually one liter or less. If this is ineffective, salt tabs can be given. Salt tabs will increase the solute load, necessitating an increase in urine output. Patients should be given approximately nine grams of salt tabs in three divided doses (equivalent to 1 L of NS). Patients with highly concentrated urine (Uosm >500 mosm/L) will not respond as well to the salt load, because the kidneys will continue to excrete much of the sodium in a concentrated urine. In such patients, a loop diuretic can be used to help excrete free water, because it decreases the Uosm to about ½ NS (154 mOsm/L). One possible regimen is 20-40 mg of oral furosemide two to three times daily.
Hypervolemic Hyponatremia: Hypervolemic hyponatremia is caused by congestive heart failure (CHF), cirrhosis, or nephrotic syndrome. In all cases, there is excess ADH as a result of the carotid baroreceptors sensing a decrease in effective circulation volume. In the case of CHF and cirrhosis, the degree of hyponatremia is a marker of disease severity, but there is no data to show that correction of hyponatremia improves outcomes. Fluid restriction is the cornerstone of therapy, but if the patient’s volume status is not optimized, then loop diuretics may improve hyponatremia through excretion of diluted urine. In addition, angiotensin-converting enzyme inhibitors can improve hyponatremia in CHF by reducing ADH levels and improving cardiac output via afterload reduction.
There has been recent interest in the use of vasopressin V2 receptor antagonists or “vaptans.” The SALT 1 and 2 trials, which included patients with CHF and cirrhosis, showed that they are effective in increasing serum sodium and improving mental function in the short term. But there are concerns about hepatotoxicity, overly rapid correction of serum sodium, lack of mortality benefit, and cost.14 The latest American Heart Association CHF guidelines recommend (class IIb) vaptans in patients with “hyponatremia that may be causing cognitive symptoms when standard measures have failed.”15 Tolvaptan, in particular, should not be used in cirrhotic patients due to concerns of hepatotoxicity.
Outcome of the Case
Because of the high UNa and Uosm and the use of a selective serotonin reuptake inhibitor (SSRI), the treating physician suspects the patient has SIADH. Given the severe symptoms, he is given 100 mL of 3% hypertonic saline and experiences improvement in his lethargy. Repeat sodium is 112 mEq/L. Using the equation above, a basal rate is calculated:
Change in serum sodium from 1 L of 3% saline= 514 mEq/L -112 mEq/L = 9.4 mEq 43 L
Because the goal correction rate is 6-8 mEq/L in 24 hours and the sodium has already increased by three, the physician elects to increase the sodium by 5 mEq/L for a total of 8 mEq/L for 24 hours:
5.0 mEq x 1000 ml = 532 ml of 3% saline ÷ 24 hours = 22 mL/hr. 9.4 mEq
Serum sodium is checked every two hours. The following day, the sodium is 115 mEq/L and the patient is fully alert. The hypertonic saline is stopped and the patient is maintained on free water restriction. Some 72 hours later, the sodium is 124 mEq/L.
Dr. Chang is co-director of the medicine-geriatrics clerkship, director of education in the division of hospital medicine, and assistant professor in the department of medicine at Mount Sinai Medical Center in New York City. Dr. Madeira is clinical instructor in the department of general internal medicine at the NYU School of Medicine and a hospitalist at the VA NY Harbor Healthcare System.
References
- Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: Treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006;21(1):70-76.
- Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003;337(1-2):169-172.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e1-8.
- McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination: Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
- Passamonte PM. Hypouricemia, inappropriate secretion of antidiuretic hormone, and small cell carcinoma of the lung. Arch Intern Med. 1984;144(8):1569-1570.
- Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1-42.
- Rogers IR, Hook G, Stuempfle KJ, Hoffman MD, Hew-Butler, T. An intervention study of oral versus intravenous hypertonic saline administration in ultramarathon runners with exercise-associated hyponatremia: a preliminary randomized trial. Clin J Sport Med. 2011;21(3):200-203.
- Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
- Tzamaloukas AH, Malhotra D, Rosen BH, Raj DS, Murata GH, Shapiro JI. Principles of management of severe hyponatremia. J Am Heart Assoc. 2013;2(1):e005199.
- Sterns RH. Severe symptomatic hyponatremia: Treatment and outcome. A study of 64 cases. Ann Intern Med. 1987;107(5):656-664.
- Karp BI, Laureno R. Pontine and extrapontine myelinolysis: a neurologic disorder following rapid correction of hyponatremia. Medicine (Baltimore). 1993;72(6):359-373.
- Soupart A, Penninckx R, Crenier L, Stenuit A, Perier O, Decaux G. Prevention of brain demyelination in rats after excessive correction of chronic hyponatremia by serum sodium lowering. Kidney Int. 1994;45(1):193-200.
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-239.
Case
A 67-year-old male patient who has depression and is on sertraline presents with increasing confusion over the past week. Initial plasma sodium is 109 mEq/L. On exam, he weighs 70 kg and is euvolemic. His urine osmolarity (Uosm) is 800 mosm/L with a urine sodium (UNa) of 40 mEq/L. He is somnolent but awakens to sternal rub. How should this patient’s hyponatremia be evaluated and managed?
Overview
Hyponatremia, a disorder of excess total body water in relation to sodium, occurs in up to 42% of hospitalized patients.1,2 Regardless of the cause, hyponatremia is usually associated with the syndrome of inappropriate antidiuretic hormone secretion (SIADH) or with the appropriate elevation of antidiuretic hormone (ADH), known as hypovolemia. ADH is produced in the hypothalamus and released in the posterior pituitary in response to increasing plasma osmolarity (pOSM) or effective circulating volume depletion. ADH acts in the cortical collecting duct to increase the number of luminal aquaporin channels, increasing water reabsorption and decreasing plasma osmolarity. When hyponatremia is severe, the movement of water into cells causes cellular brain swelling, and clinical symptoms progress from malaise, headache, and nausea to obtundation, seizures, or respiratory arrest (see Figure 1). Even mild, chronic hyponatremia (120-131 mEq/L) is associated with an increased risk of falls due to mild gait and attention impairment.3
Evaluation
Step 1: Plasma osmolarity
The first step in diagnosing the cause of hyponatremia and treating it is to measure pOSM. The majority of patients with hyponatremia have hypoosmolar hyponatremia and therefore have a low pOSM; however, patients may have normal or high osmolarity. Hyponatremia with normal osmolarity can be caused by pseudohyponatremia (i.e., hyperglycemia, paraproteinemia, hyperlipidemia), severe renal failure, ingestion of excess alcohol, or post-transurethral resection of prostate or bladder.
Hyponatremia with high pOSM occurs as a result of elevated levels of an extra solute in the plasma that does not readily enter cells. This draws water into the extracellular fluid and lowers the sodium concentration. This will most commonly result from hyperglycemia or infusion of mannitol.
Step 2: Assess volume status with physical exam, urine sodium (UNa)
The majority of patients with hyponatremia will have low pOSM. These patients should be categorized by volume status: hypovolemic, euvolemic, or hypervolemic (see Figure 2). On exam, hypervolemia is usually evident, and the cause of hypervolemic hyponatremia is usually elicited from a patient’s history; however, differentiating between hypovolemic and euvolemic hyponatremia by history and physical exam can be difficult, because examination findings are neither sensitive nor specific.4 UNa should always be evaluated, especially when differentiating between hypovolemic and euvolemic. This was illustrated in a study of 58 non-edematous patients with hyponatremia. Investigators determined which patients had hypovolemic hyponatremia based on their response to saline infusion. Of the patients identified as hypovolemic using physical exam, only 47% responded to saline. In contrast, a spot UNa of less than 30 mEq/L was 80% sensitive and 100% specific for saline responsiveness.5 Although the majority of hypovolemic hyponatremia patients will have a low UNa, the following causes of hypovolemic hyponatremia can result in high UNa: diuretics, adrenal insufficiency, salt-wasting nephropathy, and cerebral salt-wasting.
A low serum uric acid can also be useful in differentiating hypovolemic and euvolemic hyponatremia, which is most commonly caused by SIADH. In SIADH, there is urinary wasting of uric acid, which leads to low serum uric acid. In a study of 105 patients with lung cancer, a serum uric acid of less than 4 mg/dL was 75% sensitive and 89% specific for SIADH.6
Step 3: Urine osmolarity
After determining volume status, the physician should determine if there is excess ADH by measuring Uosm. Under normal conditions, hyponatremia should suppress ADH secretion and allow the kidney to excrete water by diluting the urine to less than 100 mosm/L. If Uosm is less than 100 mosm/L, then the kidneys are responding appropriately and can only persist in the following situations: The patient is drinking large volumes of water (e.g. primary polydipsia), there is insufficient solute to excrete free water (e.g. beer potomania, “tea and toast” diet), or the patient has a different set point for ADH suppression (i.e., reset osmostat). After determining volume status, UNa, and Uosm, the physician will have narrowed the cause of hyponatremia significantly (see Figure 2). Of note, when SIADH is diagnosed, it is important to look for and reverse causes (see Figure 3).
Treatment
Severe symptomatic hyponatremia
In patients with severe neurologic symptoms, physicians must balance the need to reduce symptoms quickly with the dangers of overly rapid correction. After its use in marathon runners, several experts have endorsed the following regimen to reduce symptoms rapidly: an intravenous bolus of 100 mL of 3% saline is given and repeated if symptoms persist after 10 minutes.7,8 Once symptoms improve, the basal rate can be calculated using the equation below, but the rate of sodium correction in 24 hours with this regimen should not exceed 6 to 8 mEq/L in 24 hours or 12 to 14 mEq/L in 48 hours.9,10 This is based on several case studies showing that there were no cases of central pontine myelinolysis (CPM) if correction rates were less than 10 mEq/L over 24 hours.11,12
It is important to remember that this is only a rough guide, because the equation assumes the entire infusate is retained and there is no sodium or water output. The best way to avoid overly rapid correction is to check serum sodium every two hours and monitor urine output closely. If the patient is making large volumes of urine, serum sodium may be rising too quickly. If the patient corrects too rapidly, it may be possible to avoid CPM by re-lowering the sodium.13 This can be accomplished by giving desmopressin to slow urinary free water loss while simultaneously giving hypotonic fluids.
Asymptomatic or mildly symptomatic hyponatremia
Hypovolemic hyponatremia: Treatment of hypovolemic hyponatremia is aimed at correcting volume status, the underlying problem that drives ADH secretion. The body will always choose to preserve volume over osmolarity. In most cases, normal saline (NS) should be used to restore intravascular volume, and the rate of infusion can be calculated using the same equation as above. Once volume is replete, ADH release will cease. Patients will be in danger of overly rapid correction of serum sodium, so fluids should be switched to hypotonic solutions, such as ½ NS.
Euvolemic Hyponatremia: Euvolemic hyponatremia, typically caused by SIADH, is characterized by a high Uosm (>100 mosm/L) and a high UNa (>30 mEq/L). All patients require free water restriction, and fluid intake should be at least 500 mL below a patient’s urine output, usually one liter or less. If this is ineffective, salt tabs can be given. Salt tabs will increase the solute load, necessitating an increase in urine output. Patients should be given approximately nine grams of salt tabs in three divided doses (equivalent to 1 L of NS). Patients with highly concentrated urine (Uosm >500 mosm/L) will not respond as well to the salt load, because the kidneys will continue to excrete much of the sodium in a concentrated urine. In such patients, a loop diuretic can be used to help excrete free water, because it decreases the Uosm to about ½ NS (154 mOsm/L). One possible regimen is 20-40 mg of oral furosemide two to three times daily.
Hypervolemic Hyponatremia: Hypervolemic hyponatremia is caused by congestive heart failure (CHF), cirrhosis, or nephrotic syndrome. In all cases, there is excess ADH as a result of the carotid baroreceptors sensing a decrease in effective circulation volume. In the case of CHF and cirrhosis, the degree of hyponatremia is a marker of disease severity, but there is no data to show that correction of hyponatremia improves outcomes. Fluid restriction is the cornerstone of therapy, but if the patient’s volume status is not optimized, then loop diuretics may improve hyponatremia through excretion of diluted urine. In addition, angiotensin-converting enzyme inhibitors can improve hyponatremia in CHF by reducing ADH levels and improving cardiac output via afterload reduction.
There has been recent interest in the use of vasopressin V2 receptor antagonists or “vaptans.” The SALT 1 and 2 trials, which included patients with CHF and cirrhosis, showed that they are effective in increasing serum sodium and improving mental function in the short term. But there are concerns about hepatotoxicity, overly rapid correction of serum sodium, lack of mortality benefit, and cost.14 The latest American Heart Association CHF guidelines recommend (class IIb) vaptans in patients with “hyponatremia that may be causing cognitive symptoms when standard measures have failed.”15 Tolvaptan, in particular, should not be used in cirrhotic patients due to concerns of hepatotoxicity.
Outcome of the Case
Because of the high UNa and Uosm and the use of a selective serotonin reuptake inhibitor (SSRI), the treating physician suspects the patient has SIADH. Given the severe symptoms, he is given 100 mL of 3% hypertonic saline and experiences improvement in his lethargy. Repeat sodium is 112 mEq/L. Using the equation above, a basal rate is calculated:
Change in serum sodium from 1 L of 3% saline= 514 mEq/L -112 mEq/L = 9.4 mEq 43 L
Because the goal correction rate is 6-8 mEq/L in 24 hours and the sodium has already increased by three, the physician elects to increase the sodium by 5 mEq/L for a total of 8 mEq/L for 24 hours:
5.0 mEq x 1000 ml = 532 ml of 3% saline ÷ 24 hours = 22 mL/hr. 9.4 mEq
Serum sodium is checked every two hours. The following day, the sodium is 115 mEq/L and the patient is fully alert. The hypertonic saline is stopped and the patient is maintained on free water restriction. Some 72 hours later, the sodium is 124 mEq/L.
Dr. Chang is co-director of the medicine-geriatrics clerkship, director of education in the division of hospital medicine, and assistant professor in the department of medicine at Mount Sinai Medical Center in New York City. Dr. Madeira is clinical instructor in the department of general internal medicine at the NYU School of Medicine and a hospitalist at the VA NY Harbor Healthcare System.
References
- Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: Treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006;21(1):70-76.
- Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003;337(1-2):169-172.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e1-8.
- McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination: Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
- Passamonte PM. Hypouricemia, inappropriate secretion of antidiuretic hormone, and small cell carcinoma of the lung. Arch Intern Med. 1984;144(8):1569-1570.
- Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1-42.
- Rogers IR, Hook G, Stuempfle KJ, Hoffman MD, Hew-Butler, T. An intervention study of oral versus intravenous hypertonic saline administration in ultramarathon runners with exercise-associated hyponatremia: a preliminary randomized trial. Clin J Sport Med. 2011;21(3):200-203.
- Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
- Tzamaloukas AH, Malhotra D, Rosen BH, Raj DS, Murata GH, Shapiro JI. Principles of management of severe hyponatremia. J Am Heart Assoc. 2013;2(1):e005199.
- Sterns RH. Severe symptomatic hyponatremia: Treatment and outcome. A study of 64 cases. Ann Intern Med. 1987;107(5):656-664.
- Karp BI, Laureno R. Pontine and extrapontine myelinolysis: a neurologic disorder following rapid correction of hyponatremia. Medicine (Baltimore). 1993;72(6):359-373.
- Soupart A, Penninckx R, Crenier L, Stenuit A, Perier O, Decaux G. Prevention of brain demyelination in rats after excessive correction of chronic hyponatremia by serum sodium lowering. Kidney Int. 1994;45(1):193-200.
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-239.
Case
A 67-year-old male patient who has depression and is on sertraline presents with increasing confusion over the past week. Initial plasma sodium is 109 mEq/L. On exam, he weighs 70 kg and is euvolemic. His urine osmolarity (Uosm) is 800 mosm/L with a urine sodium (UNa) of 40 mEq/L. He is somnolent but awakens to sternal rub. How should this patient’s hyponatremia be evaluated and managed?
Overview
Hyponatremia, a disorder of excess total body water in relation to sodium, occurs in up to 42% of hospitalized patients.1,2 Regardless of the cause, hyponatremia is usually associated with the syndrome of inappropriate antidiuretic hormone secretion (SIADH) or with the appropriate elevation of antidiuretic hormone (ADH), known as hypovolemia. ADH is produced in the hypothalamus and released in the posterior pituitary in response to increasing plasma osmolarity (pOSM) or effective circulating volume depletion. ADH acts in the cortical collecting duct to increase the number of luminal aquaporin channels, increasing water reabsorption and decreasing plasma osmolarity. When hyponatremia is severe, the movement of water into cells causes cellular brain swelling, and clinical symptoms progress from malaise, headache, and nausea to obtundation, seizures, or respiratory arrest (see Figure 1). Even mild, chronic hyponatremia (120-131 mEq/L) is associated with an increased risk of falls due to mild gait and attention impairment.3
Evaluation
Step 1: Plasma osmolarity
The first step in diagnosing the cause of hyponatremia and treating it is to measure pOSM. The majority of patients with hyponatremia have hypoosmolar hyponatremia and therefore have a low pOSM; however, patients may have normal or high osmolarity. Hyponatremia with normal osmolarity can be caused by pseudohyponatremia (i.e., hyperglycemia, paraproteinemia, hyperlipidemia), severe renal failure, ingestion of excess alcohol, or post-transurethral resection of prostate or bladder.
Hyponatremia with high pOSM occurs as a result of elevated levels of an extra solute in the plasma that does not readily enter cells. This draws water into the extracellular fluid and lowers the sodium concentration. This will most commonly result from hyperglycemia or infusion of mannitol.
Step 2: Assess volume status with physical exam, urine sodium (UNa)
The majority of patients with hyponatremia will have low pOSM. These patients should be categorized by volume status: hypovolemic, euvolemic, or hypervolemic (see Figure 2). On exam, hypervolemia is usually evident, and the cause of hypervolemic hyponatremia is usually elicited from a patient’s history; however, differentiating between hypovolemic and euvolemic hyponatremia by history and physical exam can be difficult, because examination findings are neither sensitive nor specific.4 UNa should always be evaluated, especially when differentiating between hypovolemic and euvolemic. This was illustrated in a study of 58 non-edematous patients with hyponatremia. Investigators determined which patients had hypovolemic hyponatremia based on their response to saline infusion. Of the patients identified as hypovolemic using physical exam, only 47% responded to saline. In contrast, a spot UNa of less than 30 mEq/L was 80% sensitive and 100% specific for saline responsiveness.5 Although the majority of hypovolemic hyponatremia patients will have a low UNa, the following causes of hypovolemic hyponatremia can result in high UNa: diuretics, adrenal insufficiency, salt-wasting nephropathy, and cerebral salt-wasting.
A low serum uric acid can also be useful in differentiating hypovolemic and euvolemic hyponatremia, which is most commonly caused by SIADH. In SIADH, there is urinary wasting of uric acid, which leads to low serum uric acid. In a study of 105 patients with lung cancer, a serum uric acid of less than 4 mg/dL was 75% sensitive and 89% specific for SIADH.6
Step 3: Urine osmolarity
After determining volume status, the physician should determine if there is excess ADH by measuring Uosm. Under normal conditions, hyponatremia should suppress ADH secretion and allow the kidney to excrete water by diluting the urine to less than 100 mosm/L. If Uosm is less than 100 mosm/L, then the kidneys are responding appropriately and can only persist in the following situations: The patient is drinking large volumes of water (e.g. primary polydipsia), there is insufficient solute to excrete free water (e.g. beer potomania, “tea and toast” diet), or the patient has a different set point for ADH suppression (i.e., reset osmostat). After determining volume status, UNa, and Uosm, the physician will have narrowed the cause of hyponatremia significantly (see Figure 2). Of note, when SIADH is diagnosed, it is important to look for and reverse causes (see Figure 3).
Treatment
Severe symptomatic hyponatremia
In patients with severe neurologic symptoms, physicians must balance the need to reduce symptoms quickly with the dangers of overly rapid correction. After its use in marathon runners, several experts have endorsed the following regimen to reduce symptoms rapidly: an intravenous bolus of 100 mL of 3% saline is given and repeated if symptoms persist after 10 minutes.7,8 Once symptoms improve, the basal rate can be calculated using the equation below, but the rate of sodium correction in 24 hours with this regimen should not exceed 6 to 8 mEq/L in 24 hours or 12 to 14 mEq/L in 48 hours.9,10 This is based on several case studies showing that there were no cases of central pontine myelinolysis (CPM) if correction rates were less than 10 mEq/L over 24 hours.11,12
It is important to remember that this is only a rough guide, because the equation assumes the entire infusate is retained and there is no sodium or water output. The best way to avoid overly rapid correction is to check serum sodium every two hours and monitor urine output closely. If the patient is making large volumes of urine, serum sodium may be rising too quickly. If the patient corrects too rapidly, it may be possible to avoid CPM by re-lowering the sodium.13 This can be accomplished by giving desmopressin to slow urinary free water loss while simultaneously giving hypotonic fluids.
Asymptomatic or mildly symptomatic hyponatremia
Hypovolemic hyponatremia: Treatment of hypovolemic hyponatremia is aimed at correcting volume status, the underlying problem that drives ADH secretion. The body will always choose to preserve volume over osmolarity. In most cases, normal saline (NS) should be used to restore intravascular volume, and the rate of infusion can be calculated using the same equation as above. Once volume is replete, ADH release will cease. Patients will be in danger of overly rapid correction of serum sodium, so fluids should be switched to hypotonic solutions, such as ½ NS.
Euvolemic Hyponatremia: Euvolemic hyponatremia, typically caused by SIADH, is characterized by a high Uosm (>100 mosm/L) and a high UNa (>30 mEq/L). All patients require free water restriction, and fluid intake should be at least 500 mL below a patient’s urine output, usually one liter or less. If this is ineffective, salt tabs can be given. Salt tabs will increase the solute load, necessitating an increase in urine output. Patients should be given approximately nine grams of salt tabs in three divided doses (equivalent to 1 L of NS). Patients with highly concentrated urine (Uosm >500 mosm/L) will not respond as well to the salt load, because the kidneys will continue to excrete much of the sodium in a concentrated urine. In such patients, a loop diuretic can be used to help excrete free water, because it decreases the Uosm to about ½ NS (154 mOsm/L). One possible regimen is 20-40 mg of oral furosemide two to three times daily.
Hypervolemic Hyponatremia: Hypervolemic hyponatremia is caused by congestive heart failure (CHF), cirrhosis, or nephrotic syndrome. In all cases, there is excess ADH as a result of the carotid baroreceptors sensing a decrease in effective circulation volume. In the case of CHF and cirrhosis, the degree of hyponatremia is a marker of disease severity, but there is no data to show that correction of hyponatremia improves outcomes. Fluid restriction is the cornerstone of therapy, but if the patient’s volume status is not optimized, then loop diuretics may improve hyponatremia through excretion of diluted urine. In addition, angiotensin-converting enzyme inhibitors can improve hyponatremia in CHF by reducing ADH levels and improving cardiac output via afterload reduction.
There has been recent interest in the use of vasopressin V2 receptor antagonists or “vaptans.” The SALT 1 and 2 trials, which included patients with CHF and cirrhosis, showed that they are effective in increasing serum sodium and improving mental function in the short term. But there are concerns about hepatotoxicity, overly rapid correction of serum sodium, lack of mortality benefit, and cost.14 The latest American Heart Association CHF guidelines recommend (class IIb) vaptans in patients with “hyponatremia that may be causing cognitive symptoms when standard measures have failed.”15 Tolvaptan, in particular, should not be used in cirrhotic patients due to concerns of hepatotoxicity.
Outcome of the Case
Because of the high UNa and Uosm and the use of a selective serotonin reuptake inhibitor (SSRI), the treating physician suspects the patient has SIADH. Given the severe symptoms, he is given 100 mL of 3% hypertonic saline and experiences improvement in his lethargy. Repeat sodium is 112 mEq/L. Using the equation above, a basal rate is calculated:
Change in serum sodium from 1 L of 3% saline= 514 mEq/L -112 mEq/L = 9.4 mEq 43 L
Because the goal correction rate is 6-8 mEq/L in 24 hours and the sodium has already increased by three, the physician elects to increase the sodium by 5 mEq/L for a total of 8 mEq/L for 24 hours:
5.0 mEq x 1000 ml = 532 ml of 3% saline ÷ 24 hours = 22 mL/hr. 9.4 mEq
Serum sodium is checked every two hours. The following day, the sodium is 115 mEq/L and the patient is fully alert. The hypertonic saline is stopped and the patient is maintained on free water restriction. Some 72 hours later, the sodium is 124 mEq/L.
Dr. Chang is co-director of the medicine-geriatrics clerkship, director of education in the division of hospital medicine, and assistant professor in the department of medicine at Mount Sinai Medical Center in New York City. Dr. Madeira is clinical instructor in the department of general internal medicine at the NYU School of Medicine and a hospitalist at the VA NY Harbor Healthcare System.
References
- Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: Treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006;21(1):70-76.
- Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003;337(1-2):169-172.
- Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71.e1-8.
- McGee S, Abernethy WB 3rd, Simel DL. The rational clinical examination: Is this patient hypovolemic? JAMA. 1999;281(11):1022-1029.
- Chung HM, Kluge R, Schrier RW, Anderson RJ. Clinical assessment of extracellular fluid volume in hyponatremia. Am J Med. 1987;83(5):905-908.
- Passamonte PM. Hypouricemia, inappropriate secretion of antidiuretic hormone, and small cell carcinoma of the lung. Arch Intern Med. 1984;144(8):1569-1570.
- Verbalis JG, Goldsmith SR, Greenberg A, et al. Diagnosis, evaluation, and treatment of hyponatremia: expert panel recommendations. Am J Med. 2013;126(10 Suppl 1):S1-42.
- Rogers IR, Hook G, Stuempfle KJ, Hoffman MD, Hew-Butler, T. An intervention study of oral versus intravenous hypertonic saline administration in ultramarathon runners with exercise-associated hyponatremia: a preliminary randomized trial. Clin J Sport Med. 2011;21(3):200-203.
- Adrogué HJ, Madias NE. Hyponatremia. N Engl J Med. 2000;342(21):1581-1589.
- Tzamaloukas AH, Malhotra D, Rosen BH, Raj DS, Murata GH, Shapiro JI. Principles of management of severe hyponatremia. J Am Heart Assoc. 2013;2(1):e005199.
- Sterns RH. Severe symptomatic hyponatremia: Treatment and outcome. A study of 64 cases. Ann Intern Med. 1987;107(5):656-664.
- Karp BI, Laureno R. Pontine and extrapontine myelinolysis: a neurologic disorder following rapid correction of hyponatremia. Medicine (Baltimore). 1993;72(6):359-373.
- Soupart A, Penninckx R, Crenier L, Stenuit A, Perier O, Decaux G. Prevention of brain demyelination in rats after excessive correction of chronic hyponatremia by serum sodium lowering. Kidney Int. 1994;45(1):193-200.
- Schrier RW, Gross P, Gheorghiade M, et al. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N Engl J Med. 2006;355(20):2099-2112.
- Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62(16):e147-239.
When Should You Suspect Kawasaki Disease as the Cause of Fever in an Infant?
Case
A seven-week-old Hispanic female with a history of prematurity (born at 35 weeks by C-section) presents to the ED with four days of fever as high as 102°F and new-onset cyanotic spells. Cultures of blood, urine, and cerebrospinal fluid obtained 48 hours prior to admission were negative, but she continued to have intermittent fevers and developed a macular, non-pruritic rash on her hands and feet, with associated non-bilious emesis. One day prior to admission, she began to have episodes of apnea, with color change and cyanosis of her lips and eyelids. In the ED, her vital signs include a rectal temperature of 38.4°C, heart rate of 178/min, respiratory rate of 27/min, and blood pressure of 79/66. Examination reveals a non-toxic-appearing infant, with no conjunctival or oropharyngeal abnormalities, unremarkable heart and lung exam, and a blanching, erythematous macular rash on her hands, lower legs, and feet.
When should you suspect Kawasaki disease (KD) as the cause of fever in an infant?
Background
KD is an acute systemic vasculitis of unknown etiology that occurs in children. Affecting the small- and medium-sized arteries, with a striking predilection for coronary arteries, it is the leading cause of acquired pediatric heart disease in Japan and the U.S.1 Occurring predominantly in children younger than five years, KD has been diagnosed in infants and in young adults.2 The incidence of KD is lowest among white children and highest among Asians and Pacific Islanders, with the highest incidence in children of Japanese descent.
A recent epidemiologic study performed in Taiwan showed an incidence of 69 cases per 100,000 per year among children younger than five years, with a male/female ratio of 1.62:1.3 The peak of mortality occurs 15-45 days after onset of fever, although sudden cardiac death may occur many years later. Recurrence rate is approximately 3%. In the U.S., the estimated incidence ranges from nine to 18 per 100,000 children younger than five years per year.4
Review of Data
Because there is no specific diagnostic test or pathognomonic clinical feature, clinical diagnostic criteria have been established to guide physicians. KD diagnosis traditionally requires fever for at least five days and the presence of at least four of the following five principal features:
- bilateral conjunctival injection;
- changes in the mucous membranes of the upper respiratory tract (injected pharynx, infected, fissured lips, strawberry tongue);
- polymorphous rash;
- changes of the extremities (peripheral edema, erythema, periungual desquamation); and
- cervical lymphadenopathy.5
The fever, which is remittent, typically peaks at 39ºC to 40ºC. The mean duration of untreated fever is 11 days; with prompt treatment, fever typically subsides in two days. Bilateral painless non-exudative conjunctival injection begins shortly after onset of fever, involves typically bulbar conjunctiva, and is not associated with edema.
Erythematous rash usually appears within five days of onset of fever and is often a diffuse, nonspecific maculopapular eruption that is commonly pronounced in the perineal region. The appearance might be urticarial, micropustular, or erythema multiforme-like. Changes in extremities include erythema of palms and soles and tender induration of the hands and feet. Subsequently, desquamation begins in the periungual area within two to three weeks after the onset of fever. Typically, peeling begins around the nail folds of fingers, followed by the toes. The least common of the principal clinical features is tender unilateral anterior cervical lymphadenopathy (1.5 cm or greater in diameter).
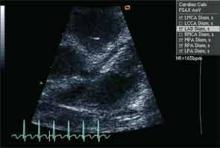
When a patient presents with a history, examination, and laboratory findings consistent with KD without meeting the typical diagnostic standard, incomplete KD should be considered. The term “incomplete” is favored over “atypical” for this pre-sentation, because these patients are otherwise similar to other patients with KD. Patients with fever for five or fewer days and fewer than four principal features can be diagnosed as having KD when coronary artery disease is detected by two-dimensional echocardiography or coronary angiography (see Figure 1, p. 10). In the presence of four or more principal criteria, KD can be diagnosed before day four of the illness by an experienced clinician.6 Features less consistent with KD include the presence of exudative conjunctivitis, exudative pharyngitis, discrete intraoral lesions, bullous or vesicular rash, or generalized adenopathy.
If clinical features are consistent with KD, further risk stratification with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) will determine whether patients are followed daily (if low) or if supplementary laboratory tests should be done (see Figure 1, p. 10). If three or more of supplementary laboratory criteria are present (albumin ≤3.0 g/dL, anemia for age, elevation of alanine aminotransferase (ALT), platelet count after seven days is 450 000/mm3 or greater, white blood cell count is 15,000/mm3 or greater, and urinary sediment containing 10 white blood cells/high-power field or more), echocardiogram should be performed and treatment initiated if abnormal.6
Young infants are more likely to manifest an incomplete presentation of KD, with a polymorphous rash being the most common symptom other than fever in this age group.7 Acute phase symptoms were also more likely to progress rapidly in this age group, with a higher risk of developing cardiac sequelae.8 As a result, any infant under the age of six months with fever for more than seven days and no other clear etiology should be evaluated for KD even in the absence of other diagnostic criteria.9
Other clinical manifestations of KD may include:
- Irritability: more notable in KD than in other febrile illnesses;
- Arthralgia and arthritis: may occur in the first week;
- Gastrointestinal complaints and findings: hepatomegaly, jaundice; and
- Abnormal chest X-ray findings: may be present in as many as 15% of patients.
Cardiovascular manifestations can be prominent in the acute phase of KD and are the leading cause of long-term morbidity and mortality. Coronary artery aneurysms occur in 20% of affected children with KD. Other cardiovascular complications include myocardial ischemia and ensuing depressed contractility and arrhythmias, as well as vascular obstruction in peripheral arteries.
A subset of KD patients develops hemodynamic instability requiring management in a critical care setting. This phenomenon has been named Kawasaki disease shock syndrome, where hemodynamic instability is not related to administration of intravenous immunoglobulin (IVIG). Patients are more likely to be female, to have laboratory findings consistent with greater inflammation, and to have impaired systolic and diastolic function. They also exhibit resistance to IVIG more often and have higher rates of coronary artery dilation and aneurysm formation.10
Differential diagnoses for KD may include viral infections, scarlet fever, staphylococcal scalded skin syndrome, toxic shock syndrome, Rocky Mountain spotted fever, cervical lymphadenitis, drug hypersensitivity, Stevens-Johnson syndrome, systemic idiopathic arthritis, leptospirosis, and mercury hypersensitivity reaction.11
Work-Up
Laboratory evaluation of a patient with suspected KD should include:
- Complete blood count (CBC) with differential: leukocytosis, anemia, thrombocytosis that peaks in the third week is characteristic. A manual differential may reveal an increase in band forms.
- Acute phase reactants: If C-reactive protein (CRP) is 3 mg/dL or greater and erythrocyte sedimentation rate (ESR) is 40 mm/hr or greater, supplementary laboratory work-up should be done. Make sure not to cloud classic with incomplete KD; the stepwise lab evaluation only pertains to the latter.
- Liver panel: Elevated ALT and gamma-glutamyl transferase (GGT), mild hyperbilirubinemia, or hypoalbuminemia may be present.
- Urinalysis: Sterile pyuria may be present; if present, it may be of urethral origin, and catheterized samples could miss this finding.12
Lack of elevated inflammatory markers (CRP is less than 3 mg/dl and ESR is less than 40 mm/hr) and the presence of two or three principal clinical features warrant ongoing daily monitoring of ESR, CRP, and fever until day seven of illness. If the fever resolves but is followed by peeling of extremities, an echocardiogram should be done. Lumbar puncture might help differentiate from CNS infectious etiologies, but about 50% of KD patients have a cerebrospinal fluid pleocytosis.
Echocardiography is the preferred imaging modality for the initial cardiovascular evaluation and follow-up.1 It has a sensitivity of 100% and specificity of 96% for the detection of proximal coronary aneurysms.13 Coronary aneurysms are clinically silent in most cases and can manifest with delayed complications, such as myocardial infarction or sudden death. Imaging plays an important role in the early diagnosis of these aneurysms and in estimating their number, size, and location, important elements in making a therapeutic decision.14
Although the echocardiography should be done as soon as KD is suspected, definitive treatment must not be delayed. Evaluation of all coronary artery segments, as well as cardiac contractility and presence of effusion, should be noted on echocardiography. In the absence of complications, echocardiography is performed at the time of diagnosis and at two weeks and six to eight weeks after disease onset.11
Treatment
Treatment goals for Kawasaki disease in the acute phase are reduction of systemic and coronary arterial inflammation and prevention of coronary thrombosis. The long-term therapy in individuals who develop coronary aneurysms is aimed at preventing myocardial ischemia or infarction.6 The current standard of care for the treatment of children in the U.S. is anti-inflammatory therapy with:
- immunoglobulin (IVIG) in a single 2 g/kg/dose infused over 10–12 hours, accompanied by;
- high-dose aspirin (80–100 mg/kg/day orally in four divided doses).6,15
IVIG administration within 10 days of the onset of fever results in more favorable outcomes. Live virus vaccines should be delayed to 11 months after administration of IVIG. Both aspirin and IVIG have anti-inflammatory effects. This regimen applies to patients without abnormalities on initial echocardiography. High-dose aspirin typically is continued for 48-72 hours after the child becomes afebrile. Thereafter, low-dose aspirin (3-5 mg/kg/day) is prescribed until patient shows no evidence of coronary changes, typically by six to eight weeks after onset of illness. Children with coronary abnormalities should continue aspirin indefinitely.
Approximately 10% of patients are IVIG-resistant and have persistent or recurrent fever for at least 36 hours after completion of the infusion. The current recommendation is to re-treat with IVIG at the same dose. If the patient has fever 36 hours after the second dose of IVIG, this is considered true treatment failure.
Other possible treatments for KD refractory to IVIG include IV methylprednisolone (30 mg/kg over two to three hours daily for three days) or infliximab.16 Even with prompt treatment, 5% of children who have KD develop coronary artery dilation, and 1% develop giant aneurysms.
Back to the Case
Initial laboratory evaluation revealed white blood cell count of 19.0×103 cells/mm3, hemoglobin of 8.9 gm/dL, CRP of 17.9 mg/dL, and ESR of 73 mm/hr. Because of persistent fevers for 48 hours after admission in the absence of another cause to explain the illness, the KD service was consulted. Echocardiography revealed dilatation of the left main (z-score 4.23) and proximal right (z-score 2.59), confirming the diagnosis of KD. Ejection fraction was read as qualitatively normal.
The infant received infliximab and IVIG, as well as high-dose aspirin, clopidogrel, and propranolol. This treatment regimen was directed by a KD expert and was more aggressive than typical therapy due to the severity of presentation. She received blood transfusions for worsening symptomatic anemia (hemoglobin 7.0 gm/dL) with hypoxia.
Following her IVIG infusion, she remained afebrile with progressive reduction in her CRP. She was discharged on hospital day seven on aspirin until her next follow-up, with propranolol for three days to limit potential tachycardia. At her three-week follow-up visit, her ESR had improved to 8 mm/hr. Her echocardiogram revealed a normal ejection fraction. Echocardiography revealed resolution of all abnormalities except for a borderline prominence of the right coronary artery (z-score 2.11). At this time it was recommended that her aspirin be discontinued.
She continues to be followed by the KD service as an outpatient and has done well without cardiovascular symptoms four months after her diagnosis.
Bottom Line
KD can manifest an incomplete presentation, especially in infants under the age of six months. Clinicians should maintain a high level of suspicion for KD in young infants with unexplained fevers lasting more than seven days.
Dr. Gurevich-Panigrahi is a fellow in pediatric hospital medicine at Cleveland Clinic Children’s Hospital. Dr. Kanegaye is a clinical professor of pediatrics at the University of California San Diego (UCSD) School of Medicine and attending physician in the emergency care center at Rady Children’s Hospital San Diego. Dr. Chang is associate clinical professor of pediatrics and medicine at UCSD School of Medicine, a pediatric hospitalist at Rady Children’s, and pediatric editor of The Hospitalist.
References
- Hendaoui L, Stanson AW, Habib Bouhaouala M, Joffre F, eds. Systemic Vasculitis: Imaging Features. New York: Springer; 2012.
- Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009;124(3):e410-e415.
- Huang WC, Huang LM, Chang IS, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123(3):e401-405.
- Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-438.
- Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever Endocarditis, Kawasaki Disease, American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335-336.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
- Shiozawa Y, Inuzuka R, Harita Y, Kagawa J. Age-related differences in the course of the acute phase symptoms of Kawasaki disease. Pediatr Infect Dis J. 2013;32(9):e365-369.
- Genizi J, Miron D, Spiegel R, Fink D, Horowitz Y. Kawasaki disease in very young infants: high prevalence of atypical presentation and coronary arteritis. Clin Pediatr (Phila.). 2003;42(3):263-267.
- Sundel R. Incomplete (atypical) Kawasaki disease. UpToDate. Available at: http://www.uptodate.com/contents/incomplete-atypical-kawasaki-disease. Accessed June 9, 2014.
- Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783-e789.
- Fimbres AM, Shulman ST. Kawasaki disease. Pediatr Rev. 2008;29(9):308-315.
- Shike H, Kanegaye JT, Best BM, Pancheri J, Burns JC. Pyuria associated with acute Kawasaki disease and fever from other causes. Pediatr Infect Dis J. 2009;28(5):440-443.
- Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7(2):355-360.
- Mavrogeni S, Papadopoulos G, Karanasios E, Cokkinos DV. How to image Kawasaki disease: a validation of different imaging techniques. Int J Cardiol. 2008;124(1):27-31.
- Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364(9433):533-544.
- Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. 2012;2(2):71-76.
Case
A seven-week-old Hispanic female with a history of prematurity (born at 35 weeks by C-section) presents to the ED with four days of fever as high as 102°F and new-onset cyanotic spells. Cultures of blood, urine, and cerebrospinal fluid obtained 48 hours prior to admission were negative, but she continued to have intermittent fevers and developed a macular, non-pruritic rash on her hands and feet, with associated non-bilious emesis. One day prior to admission, she began to have episodes of apnea, with color change and cyanosis of her lips and eyelids. In the ED, her vital signs include a rectal temperature of 38.4°C, heart rate of 178/min, respiratory rate of 27/min, and blood pressure of 79/66. Examination reveals a non-toxic-appearing infant, with no conjunctival or oropharyngeal abnormalities, unremarkable heart and lung exam, and a blanching, erythematous macular rash on her hands, lower legs, and feet.
When should you suspect Kawasaki disease (KD) as the cause of fever in an infant?
Background
KD is an acute systemic vasculitis of unknown etiology that occurs in children. Affecting the small- and medium-sized arteries, with a striking predilection for coronary arteries, it is the leading cause of acquired pediatric heart disease in Japan and the U.S.1 Occurring predominantly in children younger than five years, KD has been diagnosed in infants and in young adults.2 The incidence of KD is lowest among white children and highest among Asians and Pacific Islanders, with the highest incidence in children of Japanese descent.
A recent epidemiologic study performed in Taiwan showed an incidence of 69 cases per 100,000 per year among children younger than five years, with a male/female ratio of 1.62:1.3 The peak of mortality occurs 15-45 days after onset of fever, although sudden cardiac death may occur many years later. Recurrence rate is approximately 3%. In the U.S., the estimated incidence ranges from nine to 18 per 100,000 children younger than five years per year.4
Review of Data
Because there is no specific diagnostic test or pathognomonic clinical feature, clinical diagnostic criteria have been established to guide physicians. KD diagnosis traditionally requires fever for at least five days and the presence of at least four of the following five principal features:
- bilateral conjunctival injection;
- changes in the mucous membranes of the upper respiratory tract (injected pharynx, infected, fissured lips, strawberry tongue);
- polymorphous rash;
- changes of the extremities (peripheral edema, erythema, periungual desquamation); and
- cervical lymphadenopathy.5
The fever, which is remittent, typically peaks at 39ºC to 40ºC. The mean duration of untreated fever is 11 days; with prompt treatment, fever typically subsides in two days. Bilateral painless non-exudative conjunctival injection begins shortly after onset of fever, involves typically bulbar conjunctiva, and is not associated with edema.
Erythematous rash usually appears within five days of onset of fever and is often a diffuse, nonspecific maculopapular eruption that is commonly pronounced in the perineal region. The appearance might be urticarial, micropustular, or erythema multiforme-like. Changes in extremities include erythema of palms and soles and tender induration of the hands and feet. Subsequently, desquamation begins in the periungual area within two to three weeks after the onset of fever. Typically, peeling begins around the nail folds of fingers, followed by the toes. The least common of the principal clinical features is tender unilateral anterior cervical lymphadenopathy (1.5 cm or greater in diameter).
When a patient presents with a history, examination, and laboratory findings consistent with KD without meeting the typical diagnostic standard, incomplete KD should be considered. The term “incomplete” is favored over “atypical” for this pre-sentation, because these patients are otherwise similar to other patients with KD. Patients with fever for five or fewer days and fewer than four principal features can be diagnosed as having KD when coronary artery disease is detected by two-dimensional echocardiography or coronary angiography (see Figure 1, p. 10). In the presence of four or more principal criteria, KD can be diagnosed before day four of the illness by an experienced clinician.6 Features less consistent with KD include the presence of exudative conjunctivitis, exudative pharyngitis, discrete intraoral lesions, bullous or vesicular rash, or generalized adenopathy.
If clinical features are consistent with KD, further risk stratification with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) will determine whether patients are followed daily (if low) or if supplementary laboratory tests should be done (see Figure 1, p. 10). If three or more of supplementary laboratory criteria are present (albumin ≤3.0 g/dL, anemia for age, elevation of alanine aminotransferase (ALT), platelet count after seven days is 450 000/mm3 or greater, white blood cell count is 15,000/mm3 or greater, and urinary sediment containing 10 white blood cells/high-power field or more), echocardiogram should be performed and treatment initiated if abnormal.6
Young infants are more likely to manifest an incomplete presentation of KD, with a polymorphous rash being the most common symptom other than fever in this age group.7 Acute phase symptoms were also more likely to progress rapidly in this age group, with a higher risk of developing cardiac sequelae.8 As a result, any infant under the age of six months with fever for more than seven days and no other clear etiology should be evaluated for KD even in the absence of other diagnostic criteria.9
Other clinical manifestations of KD may include:
- Irritability: more notable in KD than in other febrile illnesses;
- Arthralgia and arthritis: may occur in the first week;
- Gastrointestinal complaints and findings: hepatomegaly, jaundice; and
- Abnormal chest X-ray findings: may be present in as many as 15% of patients.
Cardiovascular manifestations can be prominent in the acute phase of KD and are the leading cause of long-term morbidity and mortality. Coronary artery aneurysms occur in 20% of affected children with KD. Other cardiovascular complications include myocardial ischemia and ensuing depressed contractility and arrhythmias, as well as vascular obstruction in peripheral arteries.
A subset of KD patients develops hemodynamic instability requiring management in a critical care setting. This phenomenon has been named Kawasaki disease shock syndrome, where hemodynamic instability is not related to administration of intravenous immunoglobulin (IVIG). Patients are more likely to be female, to have laboratory findings consistent with greater inflammation, and to have impaired systolic and diastolic function. They also exhibit resistance to IVIG more often and have higher rates of coronary artery dilation and aneurysm formation.10
Differential diagnoses for KD may include viral infections, scarlet fever, staphylococcal scalded skin syndrome, toxic shock syndrome, Rocky Mountain spotted fever, cervical lymphadenitis, drug hypersensitivity, Stevens-Johnson syndrome, systemic idiopathic arthritis, leptospirosis, and mercury hypersensitivity reaction.11
Work-Up
Laboratory evaluation of a patient with suspected KD should include:
- Complete blood count (CBC) with differential: leukocytosis, anemia, thrombocytosis that peaks in the third week is characteristic. A manual differential may reveal an increase in band forms.
- Acute phase reactants: If C-reactive protein (CRP) is 3 mg/dL or greater and erythrocyte sedimentation rate (ESR) is 40 mm/hr or greater, supplementary laboratory work-up should be done. Make sure not to cloud classic with incomplete KD; the stepwise lab evaluation only pertains to the latter.
- Liver panel: Elevated ALT and gamma-glutamyl transferase (GGT), mild hyperbilirubinemia, or hypoalbuminemia may be present.
- Urinalysis: Sterile pyuria may be present; if present, it may be of urethral origin, and catheterized samples could miss this finding.12
Lack of elevated inflammatory markers (CRP is less than 3 mg/dl and ESR is less than 40 mm/hr) and the presence of two or three principal clinical features warrant ongoing daily monitoring of ESR, CRP, and fever until day seven of illness. If the fever resolves but is followed by peeling of extremities, an echocardiogram should be done. Lumbar puncture might help differentiate from CNS infectious etiologies, but about 50% of KD patients have a cerebrospinal fluid pleocytosis.
Echocardiography is the preferred imaging modality for the initial cardiovascular evaluation and follow-up.1 It has a sensitivity of 100% and specificity of 96% for the detection of proximal coronary aneurysms.13 Coronary aneurysms are clinically silent in most cases and can manifest with delayed complications, such as myocardial infarction or sudden death. Imaging plays an important role in the early diagnosis of these aneurysms and in estimating their number, size, and location, important elements in making a therapeutic decision.14
Although the echocardiography should be done as soon as KD is suspected, definitive treatment must not be delayed. Evaluation of all coronary artery segments, as well as cardiac contractility and presence of effusion, should be noted on echocardiography. In the absence of complications, echocardiography is performed at the time of diagnosis and at two weeks and six to eight weeks after disease onset.11
Treatment
Treatment goals for Kawasaki disease in the acute phase are reduction of systemic and coronary arterial inflammation and prevention of coronary thrombosis. The long-term therapy in individuals who develop coronary aneurysms is aimed at preventing myocardial ischemia or infarction.6 The current standard of care for the treatment of children in the U.S. is anti-inflammatory therapy with:
- immunoglobulin (IVIG) in a single 2 g/kg/dose infused over 10–12 hours, accompanied by;
- high-dose aspirin (80–100 mg/kg/day orally in four divided doses).6,15
IVIG administration within 10 days of the onset of fever results in more favorable outcomes. Live virus vaccines should be delayed to 11 months after administration of IVIG. Both aspirin and IVIG have anti-inflammatory effects. This regimen applies to patients without abnormalities on initial echocardiography. High-dose aspirin typically is continued for 48-72 hours after the child becomes afebrile. Thereafter, low-dose aspirin (3-5 mg/kg/day) is prescribed until patient shows no evidence of coronary changes, typically by six to eight weeks after onset of illness. Children with coronary abnormalities should continue aspirin indefinitely.
Approximately 10% of patients are IVIG-resistant and have persistent or recurrent fever for at least 36 hours after completion of the infusion. The current recommendation is to re-treat with IVIG at the same dose. If the patient has fever 36 hours after the second dose of IVIG, this is considered true treatment failure.
Other possible treatments for KD refractory to IVIG include IV methylprednisolone (30 mg/kg over two to three hours daily for three days) or infliximab.16 Even with prompt treatment, 5% of children who have KD develop coronary artery dilation, and 1% develop giant aneurysms.
Back to the Case
Initial laboratory evaluation revealed white blood cell count of 19.0×103 cells/mm3, hemoglobin of 8.9 gm/dL, CRP of 17.9 mg/dL, and ESR of 73 mm/hr. Because of persistent fevers for 48 hours after admission in the absence of another cause to explain the illness, the KD service was consulted. Echocardiography revealed dilatation of the left main (z-score 4.23) and proximal right (z-score 2.59), confirming the diagnosis of KD. Ejection fraction was read as qualitatively normal.
The infant received infliximab and IVIG, as well as high-dose aspirin, clopidogrel, and propranolol. This treatment regimen was directed by a KD expert and was more aggressive than typical therapy due to the severity of presentation. She received blood transfusions for worsening symptomatic anemia (hemoglobin 7.0 gm/dL) with hypoxia.
Following her IVIG infusion, she remained afebrile with progressive reduction in her CRP. She was discharged on hospital day seven on aspirin until her next follow-up, with propranolol for three days to limit potential tachycardia. At her three-week follow-up visit, her ESR had improved to 8 mm/hr. Her echocardiogram revealed a normal ejection fraction. Echocardiography revealed resolution of all abnormalities except for a borderline prominence of the right coronary artery (z-score 2.11). At this time it was recommended that her aspirin be discontinued.
She continues to be followed by the KD service as an outpatient and has done well without cardiovascular symptoms four months after her diagnosis.
Bottom Line
KD can manifest an incomplete presentation, especially in infants under the age of six months. Clinicians should maintain a high level of suspicion for KD in young infants with unexplained fevers lasting more than seven days.
Dr. Gurevich-Panigrahi is a fellow in pediatric hospital medicine at Cleveland Clinic Children’s Hospital. Dr. Kanegaye is a clinical professor of pediatrics at the University of California San Diego (UCSD) School of Medicine and attending physician in the emergency care center at Rady Children’s Hospital San Diego. Dr. Chang is associate clinical professor of pediatrics and medicine at UCSD School of Medicine, a pediatric hospitalist at Rady Children’s, and pediatric editor of The Hospitalist.
References
- Hendaoui L, Stanson AW, Habib Bouhaouala M, Joffre F, eds. Systemic Vasculitis: Imaging Features. New York: Springer; 2012.
- Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009;124(3):e410-e415.
- Huang WC, Huang LM, Chang IS, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123(3):e401-405.
- Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-438.
- Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever Endocarditis, Kawasaki Disease, American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335-336.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
- Shiozawa Y, Inuzuka R, Harita Y, Kagawa J. Age-related differences in the course of the acute phase symptoms of Kawasaki disease. Pediatr Infect Dis J. 2013;32(9):e365-369.
- Genizi J, Miron D, Spiegel R, Fink D, Horowitz Y. Kawasaki disease in very young infants: high prevalence of atypical presentation and coronary arteritis. Clin Pediatr (Phila.). 2003;42(3):263-267.
- Sundel R. Incomplete (atypical) Kawasaki disease. UpToDate. Available at: http://www.uptodate.com/contents/incomplete-atypical-kawasaki-disease. Accessed June 9, 2014.
- Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783-e789.
- Fimbres AM, Shulman ST. Kawasaki disease. Pediatr Rev. 2008;29(9):308-315.
- Shike H, Kanegaye JT, Best BM, Pancheri J, Burns JC. Pyuria associated with acute Kawasaki disease and fever from other causes. Pediatr Infect Dis J. 2009;28(5):440-443.
- Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7(2):355-360.
- Mavrogeni S, Papadopoulos G, Karanasios E, Cokkinos DV. How to image Kawasaki disease: a validation of different imaging techniques. Int J Cardiol. 2008;124(1):27-31.
- Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364(9433):533-544.
- Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. 2012;2(2):71-76.
Case
A seven-week-old Hispanic female with a history of prematurity (born at 35 weeks by C-section) presents to the ED with four days of fever as high as 102°F and new-onset cyanotic spells. Cultures of blood, urine, and cerebrospinal fluid obtained 48 hours prior to admission were negative, but she continued to have intermittent fevers and developed a macular, non-pruritic rash on her hands and feet, with associated non-bilious emesis. One day prior to admission, she began to have episodes of apnea, with color change and cyanosis of her lips and eyelids. In the ED, her vital signs include a rectal temperature of 38.4°C, heart rate of 178/min, respiratory rate of 27/min, and blood pressure of 79/66. Examination reveals a non-toxic-appearing infant, with no conjunctival or oropharyngeal abnormalities, unremarkable heart and lung exam, and a blanching, erythematous macular rash on her hands, lower legs, and feet.
When should you suspect Kawasaki disease (KD) as the cause of fever in an infant?
Background
KD is an acute systemic vasculitis of unknown etiology that occurs in children. Affecting the small- and medium-sized arteries, with a striking predilection for coronary arteries, it is the leading cause of acquired pediatric heart disease in Japan and the U.S.1 Occurring predominantly in children younger than five years, KD has been diagnosed in infants and in young adults.2 The incidence of KD is lowest among white children and highest among Asians and Pacific Islanders, with the highest incidence in children of Japanese descent.
A recent epidemiologic study performed in Taiwan showed an incidence of 69 cases per 100,000 per year among children younger than five years, with a male/female ratio of 1.62:1.3 The peak of mortality occurs 15-45 days after onset of fever, although sudden cardiac death may occur many years later. Recurrence rate is approximately 3%. In the U.S., the estimated incidence ranges from nine to 18 per 100,000 children younger than five years per year.4
Review of Data
Because there is no specific diagnostic test or pathognomonic clinical feature, clinical diagnostic criteria have been established to guide physicians. KD diagnosis traditionally requires fever for at least five days and the presence of at least four of the following five principal features:
- bilateral conjunctival injection;
- changes in the mucous membranes of the upper respiratory tract (injected pharynx, infected, fissured lips, strawberry tongue);
- polymorphous rash;
- changes of the extremities (peripheral edema, erythema, periungual desquamation); and
- cervical lymphadenopathy.5
The fever, which is remittent, typically peaks at 39ºC to 40ºC. The mean duration of untreated fever is 11 days; with prompt treatment, fever typically subsides in two days. Bilateral painless non-exudative conjunctival injection begins shortly after onset of fever, involves typically bulbar conjunctiva, and is not associated with edema.
Erythematous rash usually appears within five days of onset of fever and is often a diffuse, nonspecific maculopapular eruption that is commonly pronounced in the perineal region. The appearance might be urticarial, micropustular, or erythema multiforme-like. Changes in extremities include erythema of palms and soles and tender induration of the hands and feet. Subsequently, desquamation begins in the periungual area within two to three weeks after the onset of fever. Typically, peeling begins around the nail folds of fingers, followed by the toes. The least common of the principal clinical features is tender unilateral anterior cervical lymphadenopathy (1.5 cm or greater in diameter).
When a patient presents with a history, examination, and laboratory findings consistent with KD without meeting the typical diagnostic standard, incomplete KD should be considered. The term “incomplete” is favored over “atypical” for this pre-sentation, because these patients are otherwise similar to other patients with KD. Patients with fever for five or fewer days and fewer than four principal features can be diagnosed as having KD when coronary artery disease is detected by two-dimensional echocardiography or coronary angiography (see Figure 1, p. 10). In the presence of four or more principal criteria, KD can be diagnosed before day four of the illness by an experienced clinician.6 Features less consistent with KD include the presence of exudative conjunctivitis, exudative pharyngitis, discrete intraoral lesions, bullous or vesicular rash, or generalized adenopathy.
If clinical features are consistent with KD, further risk stratification with erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) will determine whether patients are followed daily (if low) or if supplementary laboratory tests should be done (see Figure 1, p. 10). If three or more of supplementary laboratory criteria are present (albumin ≤3.0 g/dL, anemia for age, elevation of alanine aminotransferase (ALT), platelet count after seven days is 450 000/mm3 or greater, white blood cell count is 15,000/mm3 or greater, and urinary sediment containing 10 white blood cells/high-power field or more), echocardiogram should be performed and treatment initiated if abnormal.6
Young infants are more likely to manifest an incomplete presentation of KD, with a polymorphous rash being the most common symptom other than fever in this age group.7 Acute phase symptoms were also more likely to progress rapidly in this age group, with a higher risk of developing cardiac sequelae.8 As a result, any infant under the age of six months with fever for more than seven days and no other clear etiology should be evaluated for KD even in the absence of other diagnostic criteria.9
Other clinical manifestations of KD may include:
- Irritability: more notable in KD than in other febrile illnesses;
- Arthralgia and arthritis: may occur in the first week;
- Gastrointestinal complaints and findings: hepatomegaly, jaundice; and
- Abnormal chest X-ray findings: may be present in as many as 15% of patients.
Cardiovascular manifestations can be prominent in the acute phase of KD and are the leading cause of long-term morbidity and mortality. Coronary artery aneurysms occur in 20% of affected children with KD. Other cardiovascular complications include myocardial ischemia and ensuing depressed contractility and arrhythmias, as well as vascular obstruction in peripheral arteries.
A subset of KD patients develops hemodynamic instability requiring management in a critical care setting. This phenomenon has been named Kawasaki disease shock syndrome, where hemodynamic instability is not related to administration of intravenous immunoglobulin (IVIG). Patients are more likely to be female, to have laboratory findings consistent with greater inflammation, and to have impaired systolic and diastolic function. They also exhibit resistance to IVIG more often and have higher rates of coronary artery dilation and aneurysm formation.10
Differential diagnoses for KD may include viral infections, scarlet fever, staphylococcal scalded skin syndrome, toxic shock syndrome, Rocky Mountain spotted fever, cervical lymphadenitis, drug hypersensitivity, Stevens-Johnson syndrome, systemic idiopathic arthritis, leptospirosis, and mercury hypersensitivity reaction.11
Work-Up
Laboratory evaluation of a patient with suspected KD should include:
- Complete blood count (CBC) with differential: leukocytosis, anemia, thrombocytosis that peaks in the third week is characteristic. A manual differential may reveal an increase in band forms.
- Acute phase reactants: If C-reactive protein (CRP) is 3 mg/dL or greater and erythrocyte sedimentation rate (ESR) is 40 mm/hr or greater, supplementary laboratory work-up should be done. Make sure not to cloud classic with incomplete KD; the stepwise lab evaluation only pertains to the latter.
- Liver panel: Elevated ALT and gamma-glutamyl transferase (GGT), mild hyperbilirubinemia, or hypoalbuminemia may be present.
- Urinalysis: Sterile pyuria may be present; if present, it may be of urethral origin, and catheterized samples could miss this finding.12
Lack of elevated inflammatory markers (CRP is less than 3 mg/dl and ESR is less than 40 mm/hr) and the presence of two or three principal clinical features warrant ongoing daily monitoring of ESR, CRP, and fever until day seven of illness. If the fever resolves but is followed by peeling of extremities, an echocardiogram should be done. Lumbar puncture might help differentiate from CNS infectious etiologies, but about 50% of KD patients have a cerebrospinal fluid pleocytosis.
Echocardiography is the preferred imaging modality for the initial cardiovascular evaluation and follow-up.1 It has a sensitivity of 100% and specificity of 96% for the detection of proximal coronary aneurysms.13 Coronary aneurysms are clinically silent in most cases and can manifest with delayed complications, such as myocardial infarction or sudden death. Imaging plays an important role in the early diagnosis of these aneurysms and in estimating their number, size, and location, important elements in making a therapeutic decision.14
Although the echocardiography should be done as soon as KD is suspected, definitive treatment must not be delayed. Evaluation of all coronary artery segments, as well as cardiac contractility and presence of effusion, should be noted on echocardiography. In the absence of complications, echocardiography is performed at the time of diagnosis and at two weeks and six to eight weeks after disease onset.11
Treatment
Treatment goals for Kawasaki disease in the acute phase are reduction of systemic and coronary arterial inflammation and prevention of coronary thrombosis. The long-term therapy in individuals who develop coronary aneurysms is aimed at preventing myocardial ischemia or infarction.6 The current standard of care for the treatment of children in the U.S. is anti-inflammatory therapy with:
- immunoglobulin (IVIG) in a single 2 g/kg/dose infused over 10–12 hours, accompanied by;
- high-dose aspirin (80–100 mg/kg/day orally in four divided doses).6,15
IVIG administration within 10 days of the onset of fever results in more favorable outcomes. Live virus vaccines should be delayed to 11 months after administration of IVIG. Both aspirin and IVIG have anti-inflammatory effects. This regimen applies to patients without abnormalities on initial echocardiography. High-dose aspirin typically is continued for 48-72 hours after the child becomes afebrile. Thereafter, low-dose aspirin (3-5 mg/kg/day) is prescribed until patient shows no evidence of coronary changes, typically by six to eight weeks after onset of illness. Children with coronary abnormalities should continue aspirin indefinitely.
Approximately 10% of patients are IVIG-resistant and have persistent or recurrent fever for at least 36 hours after completion of the infusion. The current recommendation is to re-treat with IVIG at the same dose. If the patient has fever 36 hours after the second dose of IVIG, this is considered true treatment failure.
Other possible treatments for KD refractory to IVIG include IV methylprednisolone (30 mg/kg over two to three hours daily for three days) or infliximab.16 Even with prompt treatment, 5% of children who have KD develop coronary artery dilation, and 1% develop giant aneurysms.
Back to the Case
Initial laboratory evaluation revealed white blood cell count of 19.0×103 cells/mm3, hemoglobin of 8.9 gm/dL, CRP of 17.9 mg/dL, and ESR of 73 mm/hr. Because of persistent fevers for 48 hours after admission in the absence of another cause to explain the illness, the KD service was consulted. Echocardiography revealed dilatation of the left main (z-score 4.23) and proximal right (z-score 2.59), confirming the diagnosis of KD. Ejection fraction was read as qualitatively normal.
The infant received infliximab and IVIG, as well as high-dose aspirin, clopidogrel, and propranolol. This treatment regimen was directed by a KD expert and was more aggressive than typical therapy due to the severity of presentation. She received blood transfusions for worsening symptomatic anemia (hemoglobin 7.0 gm/dL) with hypoxia.
Following her IVIG infusion, she remained afebrile with progressive reduction in her CRP. She was discharged on hospital day seven on aspirin until her next follow-up, with propranolol for three days to limit potential tachycardia. At her three-week follow-up visit, her ESR had improved to 8 mm/hr. Her echocardiogram revealed a normal ejection fraction. Echocardiography revealed resolution of all abnormalities except for a borderline prominence of the right coronary artery (z-score 2.11). At this time it was recommended that her aspirin be discontinued.
She continues to be followed by the KD service as an outpatient and has done well without cardiovascular symptoms four months after her diagnosis.
Bottom Line
KD can manifest an incomplete presentation, especially in infants under the age of six months. Clinicians should maintain a high level of suspicion for KD in young infants with unexplained fevers lasting more than seven days.
Dr. Gurevich-Panigrahi is a fellow in pediatric hospital medicine at Cleveland Clinic Children’s Hospital. Dr. Kanegaye is a clinical professor of pediatrics at the University of California San Diego (UCSD) School of Medicine and attending physician in the emergency care center at Rady Children’s Hospital San Diego. Dr. Chang is associate clinical professor of pediatrics and medicine at UCSD School of Medicine, a pediatric hospitalist at Rady Children’s, and pediatric editor of The Hospitalist.
References
- Hendaoui L, Stanson AW, Habib Bouhaouala M, Joffre F, eds. Systemic Vasculitis: Imaging Features. New York: Springer; 2012.
- Manlhiot C, Yeung RS, Clarizia NA, Chahal N, McCrindle BW. Kawasaki disease at the extremes of the age spectrum. Pediatrics. 2009;124(3):e410-e415.
- Huang WC, Huang LM, Chang IS, et al. Epidemiologic features of Kawasaki disease in Taiwan, 2003-2006. Pediatrics. 2009;123(3):e401-405.
- Holman RC, Belay ED, Christensen KY, Folkema AM, Steiner CA, Schonberger LB. Hospitalizations for Kawasaki syndrome among children in the United States, 1997-2007. Pediatr Infect Dis J. 2010;29(6):483-438.
- Council on Cardiovascular Disease in the Young, Committee on Rheumatic Fever Endocarditis, Kawasaki Disease, American Heart Association. Diagnostic guidelines for Kawasaki disease. Circulation. 2001;103:335-336.
- Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004;110(17):2747-2771.
- Shiozawa Y, Inuzuka R, Harita Y, Kagawa J. Age-related differences in the course of the acute phase symptoms of Kawasaki disease. Pediatr Infect Dis J. 2013;32(9):e365-369.
- Genizi J, Miron D, Spiegel R, Fink D, Horowitz Y. Kawasaki disease in very young infants: high prevalence of atypical presentation and coronary arteritis. Clin Pediatr (Phila.). 2003;42(3):263-267.
- Sundel R. Incomplete (atypical) Kawasaki disease. UpToDate. Available at: http://www.uptodate.com/contents/incomplete-atypical-kawasaki-disease. Accessed June 9, 2014.
- Kanegaye JT, Wilder MS, Molkara D, et al. Recognition of a Kawasaki disease shock syndrome. Pediatrics. 2009;123(5):e783-e789.
- Fimbres AM, Shulman ST. Kawasaki disease. Pediatr Rev. 2008;29(9):308-315.
- Shike H, Kanegaye JT, Best BM, Pancheri J, Burns JC. Pyuria associated with acute Kawasaki disease and fever from other causes. Pediatr Infect Dis J. 2009;28(5):440-443.
- Capannari TE, Daniels SR, Meyer RA, Schwartz DC, Kaplan S. Sensitivity, specificity and predictive value of two-dimensional echocardiography in detecting coronary artery aneurysms in patients with Kawasaki disease. J Am Coll Cardiol. 1986;7(2):355-360.
- Mavrogeni S, Papadopoulos G, Karanasios E, Cokkinos DV. How to image Kawasaki disease: a validation of different imaging techniques. Int J Cardiol. 2008;124(1):27-31.
- Burns JC, Glodé MP. Kawasaki syndrome. Lancet. 2004;364(9433):533-544.
- Ghelani SJ, Pastor W, Parikh K. Demographic and treatment variability of refractory Kawasaki Disease: a multicenter analysis from 2005 to 2009. Hosp Pediatr. 2012;2(2):71-76.
Which Patients Should be Screened for Hepatitis C Virus Infection?
Case
A 65-year-old male with a history of a motor vehicle accident that required emergency surgery in 1982 is hospitalized for acute renal failure. He reports a distant history of IV heroin use and a brief incarceration. He does not currently use illicit drugs. He has no signs or symptoms of liver disease. Should this patient be screened for chronic hepatitis C virus (HCV) infection?
Brief Overview
HCV is a major public health concern in the United States and worldwide. It is estimated that more than 4.1 million people in the U.S. (1.6% prevalence) and more than 180 million worldwide (2.8% prevalence) are HCV antibody-positive.1,2 The acute infection is most often asymptomatic, and 80% to 100% of patients will remain HCV RNA-positive, 60% to 80% will have persistently elevated liver enzymes, and 16% will develop evidence of cirrhosis at 20 years after initial infection.3
A number of organizations in the United States have released HCV screening guidelines, including the CDC, the American Association for the Study of Liver Disease (AASLD), and the U.S. Preventive Services Task Force (USPSTF); however, despite these established recommendations, an estimated 50% of individuals with chronic HCV infection are unscreened and unaware of their infection status.4 Furthermore, in a recent study of one managed care network, even when one or more risk factors were present, only 29% of individuals underwent screening for HCV antibodies detection.5 The importance of detecting chronic HCV infection will have greater significance as newer and better-tolerated treatment options become available.6
Multiple organizations recommend screening for chronic HCV infection. This screening is recommended for patients with known risk factors and those in populations with a high prevalence of HCV infection.
Risk Factors and High-Prevalence Populations
IV or intranasal drug use. IV drug use is the main identifiable source of HCV infection in the U.S. It is estimated that 60% of new HCV infections occur in people who have injected drugs in the past six months.7 The prevalence of HCV antibodies in current IV drug users is between 72% and 96%.8 Intranasal cocaine use is also associated with a higher prevalence of HCV antibodies than the general population.8
Blood transfusion prior to July 1992. Testing of donor blood was not routinely done until 1990, and more sensitive testing was not implemented until July 1992.8 The prevalence of HCV antibodies in people who received blood transfusions prior to 1990 is 6%.8 Prior to 1990, the risk for transfusion-associated HCV infection was one in 526 units transfused.9 Since implementation of highly sensitive screening techniques, the risk of infection has dropped to less than one in 1.9 million units transfused.10
Clotting factors prior to 1987 or transplanted tissue prior to 1992. Individuals who have received clotting factors, other blood product transfusions, or transplanted tissue prior to 1987 are at an increased risk for developing HCV infection. For instance, individuals with hemophilia treated with clotting factors prior to 1987 had chronic HCV infection rates of up to 90%.8 In 1987, widespread use of protocols to inactivate HCV in clotting factors and other blood products was adopted.8 In addition, widespread screening of potential tissue donors and the use of HCV antibody-negative donors became routine.8
Alanine aminotransferase elevation. This can be considered screening or part of the diagnostic work-up of transaminitis. Regardless of the classification, this is a cohort of people with a high prevalence of HCV antibody. For individuals with one isolated alanine aminotransferase elevation, the prevalence is 3.2%.4 With two or more elevated aminotransferase results, the prevalence rises to 8.2%.4
Hemodialysis. Two major studies have estimated the prevalence of HCV antibody-positive in end-stage renal disease individuals on hemodialysis to be 7.8% and 10.4%.11,12 This prevalence can reach 64% at some dialysis centers.11 The risk of HCV infection has been associated with blood transfusions, longer duration of hemodialysis, and higher rates of HCV infection in the dialysis unit.13 With implementation of infection control practices in dialysis units, the incidence and prevalence of HCV infection are declining.13
Born in the U.S. between 1945 and 1965. The CDC and USPSTF recommend a one-time screening for HCV infection for people born in the U.S. between 1945 and 1965, regardless of the presence or absence of risk factors.6,14 This age group has an increased prevalence of HCV antibodies, at 3.25%.6
Human immunodeficiency virus (HIV). HCV has a prevalence of 30% in people infected with HIV.15 The rate of co-infection is likely secondary to shared routes of transmission. For example, 72.7% of HIV-infected individuals who used IV drugs had HCV antibodies, but only 3.5% of “low-risk” HIV-infected individuals had HCV antibodies.16
Born in a high prevalence country. In the U.S., a significant number of immigrants are from areas with a high endemic rate of HCV infection. High prevalence areas (greater than 3.5%) include Central Asia and East Asia, North Africa, and the Middle East.7 Of note, Egypt is thought to have the highest prevalence of chronic HCV infection in the world, with well over 10% of the population being antibody-positive.17 Although major guidelines do not currently recommend it, the high prevalence of chronic HCV infection in this population may warrant screening.
Other high-risk or high-prevalence populations. The prevalence of HCV infection in people who have had over 10 lifetime sexual partners (3% to 9%), those with a history of sexually transmitted disease (6%), men who have had sex with men (5%), and children born to HCV-infected mothers (5%) is increased compared with the general population.8 Incarcerated people in the U.S. have an HCV antibody prevalence of 16% to 41%.18 In addition, people who have sustained needle-stick injury or mucosal exposure, or those with potential exposures in unregulated tattoo or piercing salons, may also benefit from HCV antibody screening.14
Table 1 reviews HCV screening recommendations for the CDC, AASLD, and USPSTF.1,6,8,14
CDC=Centers for Disease Control and Prevention; USPSTF=U.S. Preventive Services Task Force; AASLD=American Association for the Study of Liver Disease; ALT=alanine aminotransferase; 1=considered diagnostic and not screening test
Screening Method
The most common initial screening test for the diagnosis of chronic HCV infection is the HCV antibody test. A positive antibody test should be followed by an HCV RNA test. In an individual with recent exposure, it takes between four and 10 weeks for the antibody to be detectable. HCV RNA testing can be positive as soon as two to three weeks after infection.8
Hospitalist Role in HCV Screening
None of the U.S.-based guidelines make recommendations on the preferred setting for HCV screening. According to the CDC, 60.4% of HCV screening was done in a physician office and 5.9% was done as a hospital inpatient.19 Traditionally, the PCP is responsible for screening for chronic diseases, including HCV infection; however, the current screening rate is insufficient, as 50% of people with chronic HCV infection remain unscreened.4
Given the insufficient rate of HCV screening at present, hospital medicine (HM) physicians have an opportunity to help improve this rate. Currently, there is no established standard of care for HCV screening in hospitalized patients. HM physicians could use the following strategies:
- Continue the current system and defer screening to outpatient providers;
- Offer screening to selected inpatients at high risk for chronic HCV infection; or
- Offer screening to all inpatients who meet screening criteria based on current guidelines.
Given the shortcomings of the current screening strategies, these authors would recommend widespread screening for chronic HCV infection in hospitalized people who meet screening criteria per current guidelines.
If HM physicians are to take an increased role in HCV screening, there are a number of important considerations. Because hospitalized patients have a limited length of stay, it would be unreasonable to expect HM physicians to test for HCV RNA viral load or genotype for all patients with a positive antibody test, because the duration of the inpatient stay may be shorter than the time it takes for these test results to return. These tests are often indicated after a positive HCV antibody test, however. Thus, communication of HCV antibody results to PCPs or other responsible providers is essential. If no follow-up is available or there are no responsible outpatient providers, HM physicians should continue with a limited screening strategy.
Back to the Case
This individual has multiple indications for chronic HCV infection screening. His risk factors include date of birth between 1945 and 1965, a history of IV drug use, and a history of incarceration. He also notes a history of emergency surgery, for which he may have received blood products prior to 1987. These factors significantly raise the likelihood of chronic HCV infection when compared with the general population. He was screened and found to be HCV antibody-positive. A follow-up HCV RNA viral load was also positive. He did not have any evidence of liver disease but did have a mild transaminitis. He has followed up as an outpatient with plans to start therapy.
Bottom Line
The current screening strategies for individuals with high prevalence of chronic HCV infection are insufficient. HM physicians have an opportunity to improve the rates of screening in this population.
Dr. Theisen-Toupal is an internist, Dr. Rosenthal is a clinical fellow in medicine, and Dr. Carbo is an assistant professor of medicine, all at Beth Israel Deaconess Medical Center in Boston. Dr. Li is an internist and associate professor of medicine at Harvard Medical School and director of the hospital medicine division at Beth Israel Deaconess Medical Center.
References
- Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333-1342.
- Chopra S. Clinical manifestations and natural history of chronic hepatitis C virus infection. UpToDate. Available at: http://www.uptodate.com/contents/clinical-manifestations-and-natural-history-of-chronic-hepatitis-c-virus-infection. Accessed March 5, 2014.
- Spradling PR, Rupp L, Moorman AC, et al. Hepatitis B and C virus infection among 1.2 million people with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55(8):1047-1055.
- Roblin DW, Smith BD, Weinbaum CM, Sabin ME. HCV screening practices and prevalence in an MCO, 2000-2007. Am J Manag Care. 2011;17(8):548-555.
- Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among people born during 1945-1965. MMWR. August 17, 2012. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6104a1.htm. Accessed March 5, 2014.
- Chopra S. Epidemiology and transmission of hepatitis C virus infection. UpToDate. Available at: http://www.uptodate.com/contents/epidemiology-and-transmission-of-hepatitis-c-virus-infection?source=search_result&search=%22Epidemiology+and+transmission+of+hepatitis+C+virus+infection%22&selectedTitle=1~150. Accessed March 5, 2014.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. October 16, 1998. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00055154.htm. Accessed March 5, 2014.
- Donahue JG, Muñoz A, Ness PM, et al. The declining risk of post-transfusion hepatitis C virus infection. N Engl J Med. 1992;327(6):369-373.
- Pomper GJ, Wu Y, Snyder EL. Risks of transfusion-transmitted infections: 2003. Curr Opin Hematol. 2003;10(6):412-418.
- Tokars JI, Miller ER, Alter MJ, Arduino MJ. National surveillance of dialysis associated diseases in the United States, 1995. ASAIO J. 1998;44(1):98-107.
- Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18(1):52-61.
- Natov S, Pereira BJG. Hepatitis C virus infection in patients on maintenance dialysis. UpToDate. Available at: http://www.uptodate.com/contents/hepatitis-c-virus-infection-in-patients-on-maintenance-dialysis?source=search_result&search=Hepatitis+C+virus+infection+in+patients+on+maintenance+dialysis.&selectedTitle=1~150. Accessed March 5, 2014.
- Moyer VA, U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
- Staples CT II, Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29(1):150-154.
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the U.S. adult AIDS clinical trials group. Clin Infect Dis. 2002;34(6):831-837.
- Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55 Suppl 1:S10-15.
- Centers for Disease Control and Prevention. Prevention and control of infections with hepatitis viruses in correctional settings. MMWR. January 24, 2003. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5201a1.htm. Accessed March 5, 2014.
- Centers for Disease Control and Prevention. Locations and reasons for initial testing for hepatitis C infection—chronic hepatitis cohort study, United States, 2006-2010. MMWR. August 16, 2013. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6232a3.htm?s_cid=mm6232a3_w. Accessed March 5, 2014.
Case
A 65-year-old male with a history of a motor vehicle accident that required emergency surgery in 1982 is hospitalized for acute renal failure. He reports a distant history of IV heroin use and a brief incarceration. He does not currently use illicit drugs. He has no signs or symptoms of liver disease. Should this patient be screened for chronic hepatitis C virus (HCV) infection?
Brief Overview
HCV is a major public health concern in the United States and worldwide. It is estimated that more than 4.1 million people in the U.S. (1.6% prevalence) and more than 180 million worldwide (2.8% prevalence) are HCV antibody-positive.1,2 The acute infection is most often asymptomatic, and 80% to 100% of patients will remain HCV RNA-positive, 60% to 80% will have persistently elevated liver enzymes, and 16% will develop evidence of cirrhosis at 20 years after initial infection.3
A number of organizations in the United States have released HCV screening guidelines, including the CDC, the American Association for the Study of Liver Disease (AASLD), and the U.S. Preventive Services Task Force (USPSTF); however, despite these established recommendations, an estimated 50% of individuals with chronic HCV infection are unscreened and unaware of their infection status.4 Furthermore, in a recent study of one managed care network, even when one or more risk factors were present, only 29% of individuals underwent screening for HCV antibodies detection.5 The importance of detecting chronic HCV infection will have greater significance as newer and better-tolerated treatment options become available.6
Multiple organizations recommend screening for chronic HCV infection. This screening is recommended for patients with known risk factors and those in populations with a high prevalence of HCV infection.
Risk Factors and High-Prevalence Populations
IV or intranasal drug use. IV drug use is the main identifiable source of HCV infection in the U.S. It is estimated that 60% of new HCV infections occur in people who have injected drugs in the past six months.7 The prevalence of HCV antibodies in current IV drug users is between 72% and 96%.8 Intranasal cocaine use is also associated with a higher prevalence of HCV antibodies than the general population.8
Blood transfusion prior to July 1992. Testing of donor blood was not routinely done until 1990, and more sensitive testing was not implemented until July 1992.8 The prevalence of HCV antibodies in people who received blood transfusions prior to 1990 is 6%.8 Prior to 1990, the risk for transfusion-associated HCV infection was one in 526 units transfused.9 Since implementation of highly sensitive screening techniques, the risk of infection has dropped to less than one in 1.9 million units transfused.10
Clotting factors prior to 1987 or transplanted tissue prior to 1992. Individuals who have received clotting factors, other blood product transfusions, or transplanted tissue prior to 1987 are at an increased risk for developing HCV infection. For instance, individuals with hemophilia treated with clotting factors prior to 1987 had chronic HCV infection rates of up to 90%.8 In 1987, widespread use of protocols to inactivate HCV in clotting factors and other blood products was adopted.8 In addition, widespread screening of potential tissue donors and the use of HCV antibody-negative donors became routine.8
Alanine aminotransferase elevation. This can be considered screening or part of the diagnostic work-up of transaminitis. Regardless of the classification, this is a cohort of people with a high prevalence of HCV antibody. For individuals with one isolated alanine aminotransferase elevation, the prevalence is 3.2%.4 With two or more elevated aminotransferase results, the prevalence rises to 8.2%.4
Hemodialysis. Two major studies have estimated the prevalence of HCV antibody-positive in end-stage renal disease individuals on hemodialysis to be 7.8% and 10.4%.11,12 This prevalence can reach 64% at some dialysis centers.11 The risk of HCV infection has been associated with blood transfusions, longer duration of hemodialysis, and higher rates of HCV infection in the dialysis unit.13 With implementation of infection control practices in dialysis units, the incidence and prevalence of HCV infection are declining.13
Born in the U.S. between 1945 and 1965. The CDC and USPSTF recommend a one-time screening for HCV infection for people born in the U.S. between 1945 and 1965, regardless of the presence or absence of risk factors.6,14 This age group has an increased prevalence of HCV antibodies, at 3.25%.6
Human immunodeficiency virus (HIV). HCV has a prevalence of 30% in people infected with HIV.15 The rate of co-infection is likely secondary to shared routes of transmission. For example, 72.7% of HIV-infected individuals who used IV drugs had HCV antibodies, but only 3.5% of “low-risk” HIV-infected individuals had HCV antibodies.16
Born in a high prevalence country. In the U.S., a significant number of immigrants are from areas with a high endemic rate of HCV infection. High prevalence areas (greater than 3.5%) include Central Asia and East Asia, North Africa, and the Middle East.7 Of note, Egypt is thought to have the highest prevalence of chronic HCV infection in the world, with well over 10% of the population being antibody-positive.17 Although major guidelines do not currently recommend it, the high prevalence of chronic HCV infection in this population may warrant screening.
Other high-risk or high-prevalence populations. The prevalence of HCV infection in people who have had over 10 lifetime sexual partners (3% to 9%), those with a history of sexually transmitted disease (6%), men who have had sex with men (5%), and children born to HCV-infected mothers (5%) is increased compared with the general population.8 Incarcerated people in the U.S. have an HCV antibody prevalence of 16% to 41%.18 In addition, people who have sustained needle-stick injury or mucosal exposure, or those with potential exposures in unregulated tattoo or piercing salons, may also benefit from HCV antibody screening.14
Table 1 reviews HCV screening recommendations for the CDC, AASLD, and USPSTF.1,6,8,14
CDC=Centers for Disease Control and Prevention; USPSTF=U.S. Preventive Services Task Force; AASLD=American Association for the Study of Liver Disease; ALT=alanine aminotransferase; 1=considered diagnostic and not screening test
Screening Method
The most common initial screening test for the diagnosis of chronic HCV infection is the HCV antibody test. A positive antibody test should be followed by an HCV RNA test. In an individual with recent exposure, it takes between four and 10 weeks for the antibody to be detectable. HCV RNA testing can be positive as soon as two to three weeks after infection.8
Hospitalist Role in HCV Screening
None of the U.S.-based guidelines make recommendations on the preferred setting for HCV screening. According to the CDC, 60.4% of HCV screening was done in a physician office and 5.9% was done as a hospital inpatient.19 Traditionally, the PCP is responsible for screening for chronic diseases, including HCV infection; however, the current screening rate is insufficient, as 50% of people with chronic HCV infection remain unscreened.4
Given the insufficient rate of HCV screening at present, hospital medicine (HM) physicians have an opportunity to help improve this rate. Currently, there is no established standard of care for HCV screening in hospitalized patients. HM physicians could use the following strategies:
- Continue the current system and defer screening to outpatient providers;
- Offer screening to selected inpatients at high risk for chronic HCV infection; or
- Offer screening to all inpatients who meet screening criteria based on current guidelines.
Given the shortcomings of the current screening strategies, these authors would recommend widespread screening for chronic HCV infection in hospitalized people who meet screening criteria per current guidelines.
If HM physicians are to take an increased role in HCV screening, there are a number of important considerations. Because hospitalized patients have a limited length of stay, it would be unreasonable to expect HM physicians to test for HCV RNA viral load or genotype for all patients with a positive antibody test, because the duration of the inpatient stay may be shorter than the time it takes for these test results to return. These tests are often indicated after a positive HCV antibody test, however. Thus, communication of HCV antibody results to PCPs or other responsible providers is essential. If no follow-up is available or there are no responsible outpatient providers, HM physicians should continue with a limited screening strategy.
Back to the Case
This individual has multiple indications for chronic HCV infection screening. His risk factors include date of birth between 1945 and 1965, a history of IV drug use, and a history of incarceration. He also notes a history of emergency surgery, for which he may have received blood products prior to 1987. These factors significantly raise the likelihood of chronic HCV infection when compared with the general population. He was screened and found to be HCV antibody-positive. A follow-up HCV RNA viral load was also positive. He did not have any evidence of liver disease but did have a mild transaminitis. He has followed up as an outpatient with plans to start therapy.
Bottom Line
The current screening strategies for individuals with high prevalence of chronic HCV infection are insufficient. HM physicians have an opportunity to improve the rates of screening in this population.
Dr. Theisen-Toupal is an internist, Dr. Rosenthal is a clinical fellow in medicine, and Dr. Carbo is an assistant professor of medicine, all at Beth Israel Deaconess Medical Center in Boston. Dr. Li is an internist and associate professor of medicine at Harvard Medical School and director of the hospital medicine division at Beth Israel Deaconess Medical Center.
References
- Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333-1342.
- Chopra S. Clinical manifestations and natural history of chronic hepatitis C virus infection. UpToDate. Available at: http://www.uptodate.com/contents/clinical-manifestations-and-natural-history-of-chronic-hepatitis-c-virus-infection. Accessed March 5, 2014.
- Spradling PR, Rupp L, Moorman AC, et al. Hepatitis B and C virus infection among 1.2 million people with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55(8):1047-1055.
- Roblin DW, Smith BD, Weinbaum CM, Sabin ME. HCV screening practices and prevalence in an MCO, 2000-2007. Am J Manag Care. 2011;17(8):548-555.
- Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among people born during 1945-1965. MMWR. August 17, 2012. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6104a1.htm. Accessed March 5, 2014.
- Chopra S. Epidemiology and transmission of hepatitis C virus infection. UpToDate. Available at: http://www.uptodate.com/contents/epidemiology-and-transmission-of-hepatitis-c-virus-infection?source=search_result&search=%22Epidemiology+and+transmission+of+hepatitis+C+virus+infection%22&selectedTitle=1~150. Accessed March 5, 2014.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. October 16, 1998. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00055154.htm. Accessed March 5, 2014.
- Donahue JG, Muñoz A, Ness PM, et al. The declining risk of post-transfusion hepatitis C virus infection. N Engl J Med. 1992;327(6):369-373.
- Pomper GJ, Wu Y, Snyder EL. Risks of transfusion-transmitted infections: 2003. Curr Opin Hematol. 2003;10(6):412-418.
- Tokars JI, Miller ER, Alter MJ, Arduino MJ. National surveillance of dialysis associated diseases in the United States, 1995. ASAIO J. 1998;44(1):98-107.
- Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18(1):52-61.
- Natov S, Pereira BJG. Hepatitis C virus infection in patients on maintenance dialysis. UpToDate. Available at: http://www.uptodate.com/contents/hepatitis-c-virus-infection-in-patients-on-maintenance-dialysis?source=search_result&search=Hepatitis+C+virus+infection+in+patients+on+maintenance+dialysis.&selectedTitle=1~150. Accessed March 5, 2014.
- Moyer VA, U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
- Staples CT II, Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29(1):150-154.
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the U.S. adult AIDS clinical trials group. Clin Infect Dis. 2002;34(6):831-837.
- Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55 Suppl 1:S10-15.
- Centers for Disease Control and Prevention. Prevention and control of infections with hepatitis viruses in correctional settings. MMWR. January 24, 2003. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5201a1.htm. Accessed March 5, 2014.
- Centers for Disease Control and Prevention. Locations and reasons for initial testing for hepatitis C infection—chronic hepatitis cohort study, United States, 2006-2010. MMWR. August 16, 2013. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6232a3.htm?s_cid=mm6232a3_w. Accessed March 5, 2014.
Case
A 65-year-old male with a history of a motor vehicle accident that required emergency surgery in 1982 is hospitalized for acute renal failure. He reports a distant history of IV heroin use and a brief incarceration. He does not currently use illicit drugs. He has no signs or symptoms of liver disease. Should this patient be screened for chronic hepatitis C virus (HCV) infection?
Brief Overview
HCV is a major public health concern in the United States and worldwide. It is estimated that more than 4.1 million people in the U.S. (1.6% prevalence) and more than 180 million worldwide (2.8% prevalence) are HCV antibody-positive.1,2 The acute infection is most often asymptomatic, and 80% to 100% of patients will remain HCV RNA-positive, 60% to 80% will have persistently elevated liver enzymes, and 16% will develop evidence of cirrhosis at 20 years after initial infection.3
A number of organizations in the United States have released HCV screening guidelines, including the CDC, the American Association for the Study of Liver Disease (AASLD), and the U.S. Preventive Services Task Force (USPSTF); however, despite these established recommendations, an estimated 50% of individuals with chronic HCV infection are unscreened and unaware of their infection status.4 Furthermore, in a recent study of one managed care network, even when one or more risk factors were present, only 29% of individuals underwent screening for HCV antibodies detection.5 The importance of detecting chronic HCV infection will have greater significance as newer and better-tolerated treatment options become available.6
Multiple organizations recommend screening for chronic HCV infection. This screening is recommended for patients with known risk factors and those in populations with a high prevalence of HCV infection.
Risk Factors and High-Prevalence Populations
IV or intranasal drug use. IV drug use is the main identifiable source of HCV infection in the U.S. It is estimated that 60% of new HCV infections occur in people who have injected drugs in the past six months.7 The prevalence of HCV antibodies in current IV drug users is between 72% and 96%.8 Intranasal cocaine use is also associated with a higher prevalence of HCV antibodies than the general population.8
Blood transfusion prior to July 1992. Testing of donor blood was not routinely done until 1990, and more sensitive testing was not implemented until July 1992.8 The prevalence of HCV antibodies in people who received blood transfusions prior to 1990 is 6%.8 Prior to 1990, the risk for transfusion-associated HCV infection was one in 526 units transfused.9 Since implementation of highly sensitive screening techniques, the risk of infection has dropped to less than one in 1.9 million units transfused.10
Clotting factors prior to 1987 or transplanted tissue prior to 1992. Individuals who have received clotting factors, other blood product transfusions, or transplanted tissue prior to 1987 are at an increased risk for developing HCV infection. For instance, individuals with hemophilia treated with clotting factors prior to 1987 had chronic HCV infection rates of up to 90%.8 In 1987, widespread use of protocols to inactivate HCV in clotting factors and other blood products was adopted.8 In addition, widespread screening of potential tissue donors and the use of HCV antibody-negative donors became routine.8
Alanine aminotransferase elevation. This can be considered screening or part of the diagnostic work-up of transaminitis. Regardless of the classification, this is a cohort of people with a high prevalence of HCV antibody. For individuals with one isolated alanine aminotransferase elevation, the prevalence is 3.2%.4 With two or more elevated aminotransferase results, the prevalence rises to 8.2%.4
Hemodialysis. Two major studies have estimated the prevalence of HCV antibody-positive in end-stage renal disease individuals on hemodialysis to be 7.8% and 10.4%.11,12 This prevalence can reach 64% at some dialysis centers.11 The risk of HCV infection has been associated with blood transfusions, longer duration of hemodialysis, and higher rates of HCV infection in the dialysis unit.13 With implementation of infection control practices in dialysis units, the incidence and prevalence of HCV infection are declining.13
Born in the U.S. between 1945 and 1965. The CDC and USPSTF recommend a one-time screening for HCV infection for people born in the U.S. between 1945 and 1965, regardless of the presence or absence of risk factors.6,14 This age group has an increased prevalence of HCV antibodies, at 3.25%.6
Human immunodeficiency virus (HIV). HCV has a prevalence of 30% in people infected with HIV.15 The rate of co-infection is likely secondary to shared routes of transmission. For example, 72.7% of HIV-infected individuals who used IV drugs had HCV antibodies, but only 3.5% of “low-risk” HIV-infected individuals had HCV antibodies.16
Born in a high prevalence country. In the U.S., a significant number of immigrants are from areas with a high endemic rate of HCV infection. High prevalence areas (greater than 3.5%) include Central Asia and East Asia, North Africa, and the Middle East.7 Of note, Egypt is thought to have the highest prevalence of chronic HCV infection in the world, with well over 10% of the population being antibody-positive.17 Although major guidelines do not currently recommend it, the high prevalence of chronic HCV infection in this population may warrant screening.
Other high-risk or high-prevalence populations. The prevalence of HCV infection in people who have had over 10 lifetime sexual partners (3% to 9%), those with a history of sexually transmitted disease (6%), men who have had sex with men (5%), and children born to HCV-infected mothers (5%) is increased compared with the general population.8 Incarcerated people in the U.S. have an HCV antibody prevalence of 16% to 41%.18 In addition, people who have sustained needle-stick injury or mucosal exposure, or those with potential exposures in unregulated tattoo or piercing salons, may also benefit from HCV antibody screening.14
Table 1 reviews HCV screening recommendations for the CDC, AASLD, and USPSTF.1,6,8,14
CDC=Centers for Disease Control and Prevention; USPSTF=U.S. Preventive Services Task Force; AASLD=American Association for the Study of Liver Disease; ALT=alanine aminotransferase; 1=considered diagnostic and not screening test
Screening Method
The most common initial screening test for the diagnosis of chronic HCV infection is the HCV antibody test. A positive antibody test should be followed by an HCV RNA test. In an individual with recent exposure, it takes between four and 10 weeks for the antibody to be detectable. HCV RNA testing can be positive as soon as two to three weeks after infection.8
Hospitalist Role in HCV Screening
None of the U.S.-based guidelines make recommendations on the preferred setting for HCV screening. According to the CDC, 60.4% of HCV screening was done in a physician office and 5.9% was done as a hospital inpatient.19 Traditionally, the PCP is responsible for screening for chronic diseases, including HCV infection; however, the current screening rate is insufficient, as 50% of people with chronic HCV infection remain unscreened.4
Given the insufficient rate of HCV screening at present, hospital medicine (HM) physicians have an opportunity to help improve this rate. Currently, there is no established standard of care for HCV screening in hospitalized patients. HM physicians could use the following strategies:
- Continue the current system and defer screening to outpatient providers;
- Offer screening to selected inpatients at high risk for chronic HCV infection; or
- Offer screening to all inpatients who meet screening criteria based on current guidelines.
Given the shortcomings of the current screening strategies, these authors would recommend widespread screening for chronic HCV infection in hospitalized people who meet screening criteria per current guidelines.
If HM physicians are to take an increased role in HCV screening, there are a number of important considerations. Because hospitalized patients have a limited length of stay, it would be unreasonable to expect HM physicians to test for HCV RNA viral load or genotype for all patients with a positive antibody test, because the duration of the inpatient stay may be shorter than the time it takes for these test results to return. These tests are often indicated after a positive HCV antibody test, however. Thus, communication of HCV antibody results to PCPs or other responsible providers is essential. If no follow-up is available or there are no responsible outpatient providers, HM physicians should continue with a limited screening strategy.
Back to the Case
This individual has multiple indications for chronic HCV infection screening. His risk factors include date of birth between 1945 and 1965, a history of IV drug use, and a history of incarceration. He also notes a history of emergency surgery, for which he may have received blood products prior to 1987. These factors significantly raise the likelihood of chronic HCV infection when compared with the general population. He was screened and found to be HCV antibody-positive. A follow-up HCV RNA viral load was also positive. He did not have any evidence of liver disease but did have a mild transaminitis. He has followed up as an outpatient with plans to start therapy.
Bottom Line
The current screening strategies for individuals with high prevalence of chronic HCV infection are insufficient. HM physicians have an opportunity to improve the rates of screening in this population.
Dr. Theisen-Toupal is an internist, Dr. Rosenthal is a clinical fellow in medicine, and Dr. Carbo is an assistant professor of medicine, all at Beth Israel Deaconess Medical Center in Boston. Dr. Li is an internist and associate professor of medicine at Harvard Medical School and director of the hospital medicine division at Beth Israel Deaconess Medical Center.
References
- Ghany MG, Strader DB, Thomas DL, Seeff LB; American Association for the Study of Liver Diseases. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49(4):1335-1374.
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatology. 2013;57(4):1333-1342.
- Chopra S. Clinical manifestations and natural history of chronic hepatitis C virus infection. UpToDate. Available at: http://www.uptodate.com/contents/clinical-manifestations-and-natural-history-of-chronic-hepatitis-c-virus-infection. Accessed March 5, 2014.
- Spradling PR, Rupp L, Moorman AC, et al. Hepatitis B and C virus infection among 1.2 million people with access to care: factors associated with testing and infection prevalence. Clin Infect Dis. 2012;55(8):1047-1055.
- Roblin DW, Smith BD, Weinbaum CM, Sabin ME. HCV screening practices and prevalence in an MCO, 2000-2007. Am J Manag Care. 2011;17(8):548-555.
- Centers for Disease Control and Prevention. Recommendations for the identification of chronic hepatitis C virus infection among people born during 1945-1965. MMWR. August 17, 2012. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr6104a1.htm. Accessed March 5, 2014.
- Chopra S. Epidemiology and transmission of hepatitis C virus infection. UpToDate. Available at: http://www.uptodate.com/contents/epidemiology-and-transmission-of-hepatitis-c-virus-infection?source=search_result&search=%22Epidemiology+and+transmission+of+hepatitis+C+virus+infection%22&selectedTitle=1~150. Accessed March 5, 2014.
- Centers for Disease Control and Prevention. Recommendations for prevention and control of hepatitis C virus (HCV) infection and HCV-related chronic disease. MMWR. October 16, 1998. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/00055154.htm. Accessed March 5, 2014.
- Donahue JG, Muñoz A, Ness PM, et al. The declining risk of post-transfusion hepatitis C virus infection. N Engl J Med. 1992;327(6):369-373.
- Pomper GJ, Wu Y, Snyder EL. Risks of transfusion-transmitted infections: 2003. Curr Opin Hematol. 2003;10(6):412-418.
- Tokars JI, Miller ER, Alter MJ, Arduino MJ. National surveillance of dialysis associated diseases in the United States, 1995. ASAIO J. 1998;44(1):98-107.
- Finelli L, Miller JT, Tokars JI, Alter MJ, Arduino MJ. National surveillance of dialysis-associated diseases in the United States, 2002. Semin Dial. 2005;18(1):52-61.
- Natov S, Pereira BJG. Hepatitis C virus infection in patients on maintenance dialysis. UpToDate. Available at: http://www.uptodate.com/contents/hepatitis-c-virus-infection-in-patients-on-maintenance-dialysis?source=search_result&search=Hepatitis+C+virus+infection+in+patients+on+maintenance+dialysis.&selectedTitle=1~150. Accessed March 5, 2014.
- Moyer VA, U.S. Preventive Services Task Force. Screening for hepatitis C virus infection in adults: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159(5):349-357.
- Staples CT II, Rimland D, Dudas D. Hepatitis C in the HIV (human immunodeficiency virus) Atlanta V.A. (Veterans Affairs Medical Center) Cohort Study (HAVACS): the effect of coinfection on survival. Clin Infect Dis. 1999;29(1):150-154.
- Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C virus prevalence among patients infected with human immunodeficiency virus: a cross-sectional analysis of the U.S. adult AIDS clinical trials group. Clin Infect Dis. 2002;34(6):831-837.
- Averhoff FM, Glass N, Holtzman D. Global burden of hepatitis C: considerations for healthcare providers in the United States. Clin Infect Dis. 2012;55 Suppl 1:S10-15.
- Centers for Disease Control and Prevention. Prevention and control of infections with hepatitis viruses in correctional settings. MMWR. January 24, 2003. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/rr5201a1.htm. Accessed March 5, 2014.
- Centers for Disease Control and Prevention. Locations and reasons for initial testing for hepatitis C infection—chronic hepatitis cohort study, United States, 2006-2010. MMWR. August 16, 2013. Available at: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm6232a3.htm?s_cid=mm6232a3_w. Accessed March 5, 2014.
What Patients Undergoing Gastrointestinal Endoscopic Procedures Should Receive Antibiotic Prophylaxis?
Case
You are asked to admit two patients. The first is a 75-year-old male with a prosthetic aortic valve on warfarin who presents with bright red blood per rectum and is scheduled for colonoscopy. The second patient is a 35-year-old female with biliary obstruction due to choledocholithiasis; she is afebrile with normal vital signs and no leukocytosis. She underwent endoscopic retrograde cholangiopancreatography (ERCP), which did not resolve her biliary obstruction. Should you prescribe prophylactic antibiotics for either patient?
Overview
Providers are often confused regarding which patients undergoing gastrointestinal (GI) endoscopic procedures should receive antibiotic prophylaxis. To answer this question, it is important to understand the goal of prophylactic antibiotics. Are we trying to prevent infective endocarditis or a localized infection?
There are few large, prospective, randomized controlled trials that have examined the need for antibiotic prophylaxis with GI endoscopic procedures. Guidelines from professional societies are mainly based on expert opinion, evidence from retrospective case studies, and meta-analysis reviews.
Review of the Data
Infective endocarditis resulting from GI endoscopy has been a concern of physicians for decades. The American Heart Association (AHA) first published its recommendations for antibiotic prophylaxis of GI tract procedures in 1965. The most recent antibacterial prophylaxis guidelines, published in 2007, have simplified recommendations and greatly scaled back the indications for antibiotics. The new guidelines conclude that frequent bacteremia from daily activities is more likely to precipitate endocarditis than a single dental, GI, or genitourinary tract procedure.1
The American Society for Gastrointestinal Endoscopy (ASGE) reports that 14.2 million colonoscopies, 2.8 million flexible sigmoidoscopies, and nearly as many upper endoscopies are performed in the U.S. each year, but only 15 cases of endocarditis have been reported with a temporal association to a procedure.2
The British Society of Gastroenterology (BSG) found, after reviewing the histories of patients with infective endocarditis from 1983 through 2006, that there is not enough evidence to warrant antibiotic prophylaxis prior to endoscopy. They noted less than one case of endocarditis after GI endoscopy per year as well as significant variation in the time interval between the procedure and symptoms. The BSG also recognized that antibiotic prophylaxis does not always protect against infection and that clinical factors unrelated to the endoscopy may play a significant role in the development of endocarditis.3
Upper GI Endoscopy, Colonoscopy with Biopsy, and Esophageal Dilatation. Administering antibiotics to prevent infective endocarditis is not recommended for patients undergoing routine procedures such as endoscopy with biopsy and colonoscopy with polypectomy. Likewise, patients with a history of prosthetic heart valves, valve repair with prosthetic material, endocarditis, congenital heart disease, or cardiac transplant with valvulopathy do not need prophylactic antibiotics before GI endoscopic procedures. However, for patients who are being treated for an active GI infection, antibiotic coverage for enterococcus may be warranted given the increased risk of developing endocarditis. The AHA acknowledges there are no published studies to support the efficacy of antibiotics to prevent enterococcal endocarditis in patients in this clinical setting.1
Unlike routine endoscopy, esophageal dilation is associated with an increased rate of bacteremia (12%-100%).4 Streptococcus viridans has been found in blood cultures up to 79% of the time after esophageal dilation.5 Patients with malignant strictures have higher rates of bacteremia than those with benign strictures (52.9% versus 15.7%). Patients treated with multiple passes with the esophageal dilator compared to those treated with a single dilation have a higher risk of bacteremia.6 All patients undergoing esophageal stricture dilation should receive pre-procedural prophylactic antibiotics.7
Patients with bleeding esophageal varices also have high rates of bacteremia. Up to 20% of patients with cirrhosis and GI bleeding on admission develop an infection within 48 hours of presentation.8 There is evidence that the bacteremia may actually be related to the variceal bleeding rather than the procedure.9 Patients with bleeding esophageal varices treated with antibiotics have improved outcomes, including a decrease in mortality.10 Therefore, all patients with bleeding esophageal varices should be placed on antibiotic therapy regardless of whether an endoscopic intervention is planned.
Percutaneous Endoscopic Gastrostomy (PEG) Placement. Prophylactic antibiotics are recommended before placement of a PEG. The indication for prophylactic antibiotics is to prevent a gastrostomy site infection, not infective endocarditis. Gastrostomy site infection is unfortunately a fairly common infection, affecting 4% to 30% of patients who undergo PEG tube placement. There is significant evidence that antibiotics are beneficial in preventing peristomal infections. A meta-analysis showed that only eight patients need to be treated with prophylactic antibiotics to prevent a single peristomal infection.11 Since these infections are believed to be caused by contamination from the oropharynx, physicians should consider prophylaxis against pathogens from the oral flora.12
More recently, it has been noted that methicillin-resistant Staphylococcus aureus (MRSA) is increasingly cultured from infection sites.13 In centers with endemic MRSA, patients should be screened and then undergo decontamination prior to the PEG placement in positive cases.
Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA). Antibiotic prophylaxis before EUS-FNA of a solid lesion in an organ is generally thought to be unnecessary because the risk of bacteremia with this procedure is low, comparable to routine GI endoscopy with biopsy. The recommendation for prophylactic antibiotics before biopsy of a cystic lesion is different. There is concern that puncturing cystic lesions may create a new infected fluid collection.2 A systematic review of more than 10,000 patients undergoing EUS-FNA with a full range of target organs revealed that, overall, 11.2% of patients experienced a fever and 4.7% of patients had a peri-procedural infection. While it was not possible in this study to determine which patients received prophylactic antibiotics, 93.7% of patients with pancreatic cystic lesions were reported to have been treated with antibiotics.14
A separate, single-center, retrospective trial produced different results. This study examined a population of 253 patients who underwent 266 EUS-FNA of pancreatic cysts and found that prophylactic antibiotics were associated with more adverse events and were not protective for the 3% of the patients with infectious symptoms.15 Despite the conflicting data, guidelines at this time recommend prophylactic antibiotics before drainage of a sterile pancreatic fluid collection that communicates with the pancreatic duct and also for aspiration of cystic lesions along the GI tract and the mediastinum.2
Endoscopic Retrograde Cholangiopancreatography (ERCP). In patients undergoing ERCP, the routine use of prophylactic antibiotics has not been found to be effective in decreasing the risk of post-procedure cholangitis.16 Guidelines recommend the use of prophylactic antibiotics only in those patients in which the ERCP may not completely resolve the biliary obstruction.2 In these patients, the thought is that ERCP can precipitate infection by disturbing bacteria already present in the biliary tree, especially with increased intrabiliary pressure at the time of contrast dye injection.17
Patients with incomplete biliary drainage, including those with primary sclerosing cholangitis (PSC), hilar cholangiocarcinoma, persistent biliary that were not extracted, and strictures that continue to obstruct despite attempted intervention, are thought to be at elevated risk of developing cholangitis post-ERCP. These patients should be placed on prophylactic antibiotics at the time of the procedure to cover biliary flora such as enteric gram negatives and enterococci. Antibiotics should be continued until the biliary obstruction is resolved.2
Additional Populations to Consider. Previously, the International Society for Peritoneal Dialysis recommended that patients on peritoneal dialysis receive prophylactic antibiotics and empty their abdomen of dialysate prior to colonoscopy. This recommendation has been removed from the 2010 guidelines.18 There is also no indication that patients with synthetic vascular grafts or cardiac devices should receive prophylactic antibiotics prior to routine GI endoscopy.19 The American Academy of Orthopaedic Surgeons no longer recommends that patients with joint replacements receive antibiotic prophylaxis prior to GI endoscopy.20
Back to the Case
The older gentleman with a prosthetic valve undergoing colonoscopy should not receive prophylactic antibiotics, because even in the setting of valvulopathy, colonoscopy does not pose a significant risk for infective endocarditis. The young patient with severe choledocholithiasis should be placed on prophylactic antibiotics because she has continued biliary obstruction, which could result in a cholangitis after ERCP.
Bottom Line
Prophylactic antibiotics are not recommended for any patient undergoing routine endoscopy or colonoscopy. They are indicated for patients with bleeding esophageal varices and for patients who undergo esophageal stricture dilation, PEG placement, or pseudocyst or cyst drainage, and those with continued biliary obstruction undergoing ERCP as summarized in Table 1.
Drs. Ritter, Jupiter, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center and Harvard Medical School faculty in Boston.
References
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754.
- Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791-798.
- Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58(6):869-880.
- Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57(4):546-556.
- Zuccaro G Jr., Richter JE, Rice TW, et al. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest Endosc. 1998;48(6):568-573.
- Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest Endosc.1998;48(6):563-567.
- Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58(4):475-482.
- Ho H, Zuckerman MJ, Wassem C. A prospective controlled study of the risk of bacteremia in emergency sclerotherapy of esophageal varices. Gastroenterology. 1991;101(6):1642-1648.
- Rolando N, Gimson A, Philpott-Howard J, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: Role of antibiotic prophylaxis. J Hepatol. 1993;18(3):290-294.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-938.
- Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: Antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25(6):647-656.
- Chuang CH, Hung KH, Chen JR, et al. Airway infection predisposes to peristomal infection after percutaneous endoscopic gastrostomy with high concordance between sputum and wound isolates. J Gastrointest Surg. 2010;14(1):45-51.
- Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. PEG site infections: The emergence of methicillin resistant Staphylococcus aureus as a major pathogen. Am J Gastroenterol. 2002;97(7):1713-1716.
- Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73(2):283-290.
- Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: A retrospective, comparative analysis. Gastrointest Endosc. 2011;74(1):81-86.
- Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: A meta-analysis. Pancreas. 2009;38(2):126-130.
- Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: A sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67(3):471-475.
- Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393-423.
- Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015-2031.
- Rethman MP, Watters W III, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745-747.
Case
You are asked to admit two patients. The first is a 75-year-old male with a prosthetic aortic valve on warfarin who presents with bright red blood per rectum and is scheduled for colonoscopy. The second patient is a 35-year-old female with biliary obstruction due to choledocholithiasis; she is afebrile with normal vital signs and no leukocytosis. She underwent endoscopic retrograde cholangiopancreatography (ERCP), which did not resolve her biliary obstruction. Should you prescribe prophylactic antibiotics for either patient?
Overview
Providers are often confused regarding which patients undergoing gastrointestinal (GI) endoscopic procedures should receive antibiotic prophylaxis. To answer this question, it is important to understand the goal of prophylactic antibiotics. Are we trying to prevent infective endocarditis or a localized infection?
There are few large, prospective, randomized controlled trials that have examined the need for antibiotic prophylaxis with GI endoscopic procedures. Guidelines from professional societies are mainly based on expert opinion, evidence from retrospective case studies, and meta-analysis reviews.
Review of the Data
Infective endocarditis resulting from GI endoscopy has been a concern of physicians for decades. The American Heart Association (AHA) first published its recommendations for antibiotic prophylaxis of GI tract procedures in 1965. The most recent antibacterial prophylaxis guidelines, published in 2007, have simplified recommendations and greatly scaled back the indications for antibiotics. The new guidelines conclude that frequent bacteremia from daily activities is more likely to precipitate endocarditis than a single dental, GI, or genitourinary tract procedure.1
The American Society for Gastrointestinal Endoscopy (ASGE) reports that 14.2 million colonoscopies, 2.8 million flexible sigmoidoscopies, and nearly as many upper endoscopies are performed in the U.S. each year, but only 15 cases of endocarditis have been reported with a temporal association to a procedure.2
The British Society of Gastroenterology (BSG) found, after reviewing the histories of patients with infective endocarditis from 1983 through 2006, that there is not enough evidence to warrant antibiotic prophylaxis prior to endoscopy. They noted less than one case of endocarditis after GI endoscopy per year as well as significant variation in the time interval between the procedure and symptoms. The BSG also recognized that antibiotic prophylaxis does not always protect against infection and that clinical factors unrelated to the endoscopy may play a significant role in the development of endocarditis.3
Upper GI Endoscopy, Colonoscopy with Biopsy, and Esophageal Dilatation. Administering antibiotics to prevent infective endocarditis is not recommended for patients undergoing routine procedures such as endoscopy with biopsy and colonoscopy with polypectomy. Likewise, patients with a history of prosthetic heart valves, valve repair with prosthetic material, endocarditis, congenital heart disease, or cardiac transplant with valvulopathy do not need prophylactic antibiotics before GI endoscopic procedures. However, for patients who are being treated for an active GI infection, antibiotic coverage for enterococcus may be warranted given the increased risk of developing endocarditis. The AHA acknowledges there are no published studies to support the efficacy of antibiotics to prevent enterococcal endocarditis in patients in this clinical setting.1
Unlike routine endoscopy, esophageal dilation is associated with an increased rate of bacteremia (12%-100%).4 Streptococcus viridans has been found in blood cultures up to 79% of the time after esophageal dilation.5 Patients with malignant strictures have higher rates of bacteremia than those with benign strictures (52.9% versus 15.7%). Patients treated with multiple passes with the esophageal dilator compared to those treated with a single dilation have a higher risk of bacteremia.6 All patients undergoing esophageal stricture dilation should receive pre-procedural prophylactic antibiotics.7
Patients with bleeding esophageal varices also have high rates of bacteremia. Up to 20% of patients with cirrhosis and GI bleeding on admission develop an infection within 48 hours of presentation.8 There is evidence that the bacteremia may actually be related to the variceal bleeding rather than the procedure.9 Patients with bleeding esophageal varices treated with antibiotics have improved outcomes, including a decrease in mortality.10 Therefore, all patients with bleeding esophageal varices should be placed on antibiotic therapy regardless of whether an endoscopic intervention is planned.
Percutaneous Endoscopic Gastrostomy (PEG) Placement. Prophylactic antibiotics are recommended before placement of a PEG. The indication for prophylactic antibiotics is to prevent a gastrostomy site infection, not infective endocarditis. Gastrostomy site infection is unfortunately a fairly common infection, affecting 4% to 30% of patients who undergo PEG tube placement. There is significant evidence that antibiotics are beneficial in preventing peristomal infections. A meta-analysis showed that only eight patients need to be treated with prophylactic antibiotics to prevent a single peristomal infection.11 Since these infections are believed to be caused by contamination from the oropharynx, physicians should consider prophylaxis against pathogens from the oral flora.12
More recently, it has been noted that methicillin-resistant Staphylococcus aureus (MRSA) is increasingly cultured from infection sites.13 In centers with endemic MRSA, patients should be screened and then undergo decontamination prior to the PEG placement in positive cases.
Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA). Antibiotic prophylaxis before EUS-FNA of a solid lesion in an organ is generally thought to be unnecessary because the risk of bacteremia with this procedure is low, comparable to routine GI endoscopy with biopsy. The recommendation for prophylactic antibiotics before biopsy of a cystic lesion is different. There is concern that puncturing cystic lesions may create a new infected fluid collection.2 A systematic review of more than 10,000 patients undergoing EUS-FNA with a full range of target organs revealed that, overall, 11.2% of patients experienced a fever and 4.7% of patients had a peri-procedural infection. While it was not possible in this study to determine which patients received prophylactic antibiotics, 93.7% of patients with pancreatic cystic lesions were reported to have been treated with antibiotics.14
A separate, single-center, retrospective trial produced different results. This study examined a population of 253 patients who underwent 266 EUS-FNA of pancreatic cysts and found that prophylactic antibiotics were associated with more adverse events and were not protective for the 3% of the patients with infectious symptoms.15 Despite the conflicting data, guidelines at this time recommend prophylactic antibiotics before drainage of a sterile pancreatic fluid collection that communicates with the pancreatic duct and also for aspiration of cystic lesions along the GI tract and the mediastinum.2
Endoscopic Retrograde Cholangiopancreatography (ERCP). In patients undergoing ERCP, the routine use of prophylactic antibiotics has not been found to be effective in decreasing the risk of post-procedure cholangitis.16 Guidelines recommend the use of prophylactic antibiotics only in those patients in which the ERCP may not completely resolve the biliary obstruction.2 In these patients, the thought is that ERCP can precipitate infection by disturbing bacteria already present in the biliary tree, especially with increased intrabiliary pressure at the time of contrast dye injection.17
Patients with incomplete biliary drainage, including those with primary sclerosing cholangitis (PSC), hilar cholangiocarcinoma, persistent biliary that were not extracted, and strictures that continue to obstruct despite attempted intervention, are thought to be at elevated risk of developing cholangitis post-ERCP. These patients should be placed on prophylactic antibiotics at the time of the procedure to cover biliary flora such as enteric gram negatives and enterococci. Antibiotics should be continued until the biliary obstruction is resolved.2
Additional Populations to Consider. Previously, the International Society for Peritoneal Dialysis recommended that patients on peritoneal dialysis receive prophylactic antibiotics and empty their abdomen of dialysate prior to colonoscopy. This recommendation has been removed from the 2010 guidelines.18 There is also no indication that patients with synthetic vascular grafts or cardiac devices should receive prophylactic antibiotics prior to routine GI endoscopy.19 The American Academy of Orthopaedic Surgeons no longer recommends that patients with joint replacements receive antibiotic prophylaxis prior to GI endoscopy.20
Back to the Case
The older gentleman with a prosthetic valve undergoing colonoscopy should not receive prophylactic antibiotics, because even in the setting of valvulopathy, colonoscopy does not pose a significant risk for infective endocarditis. The young patient with severe choledocholithiasis should be placed on prophylactic antibiotics because she has continued biliary obstruction, which could result in a cholangitis after ERCP.
Bottom Line
Prophylactic antibiotics are not recommended for any patient undergoing routine endoscopy or colonoscopy. They are indicated for patients with bleeding esophageal varices and for patients who undergo esophageal stricture dilation, PEG placement, or pseudocyst or cyst drainage, and those with continued biliary obstruction undergoing ERCP as summarized in Table 1.
Drs. Ritter, Jupiter, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center and Harvard Medical School faculty in Boston.
References
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754.
- Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791-798.
- Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58(6):869-880.
- Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57(4):546-556.
- Zuccaro G Jr., Richter JE, Rice TW, et al. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest Endosc. 1998;48(6):568-573.
- Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest Endosc.1998;48(6):563-567.
- Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58(4):475-482.
- Ho H, Zuckerman MJ, Wassem C. A prospective controlled study of the risk of bacteremia in emergency sclerotherapy of esophageal varices. Gastroenterology. 1991;101(6):1642-1648.
- Rolando N, Gimson A, Philpott-Howard J, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: Role of antibiotic prophylaxis. J Hepatol. 1993;18(3):290-294.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-938.
- Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: Antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25(6):647-656.
- Chuang CH, Hung KH, Chen JR, et al. Airway infection predisposes to peristomal infection after percutaneous endoscopic gastrostomy with high concordance between sputum and wound isolates. J Gastrointest Surg. 2010;14(1):45-51.
- Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. PEG site infections: The emergence of methicillin resistant Staphylococcus aureus as a major pathogen. Am J Gastroenterol. 2002;97(7):1713-1716.
- Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73(2):283-290.
- Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: A retrospective, comparative analysis. Gastrointest Endosc. 2011;74(1):81-86.
- Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: A meta-analysis. Pancreas. 2009;38(2):126-130.
- Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: A sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67(3):471-475.
- Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393-423.
- Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015-2031.
- Rethman MP, Watters W III, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745-747.
Case
You are asked to admit two patients. The first is a 75-year-old male with a prosthetic aortic valve on warfarin who presents with bright red blood per rectum and is scheduled for colonoscopy. The second patient is a 35-year-old female with biliary obstruction due to choledocholithiasis; she is afebrile with normal vital signs and no leukocytosis. She underwent endoscopic retrograde cholangiopancreatography (ERCP), which did not resolve her biliary obstruction. Should you prescribe prophylactic antibiotics for either patient?
Overview
Providers are often confused regarding which patients undergoing gastrointestinal (GI) endoscopic procedures should receive antibiotic prophylaxis. To answer this question, it is important to understand the goal of prophylactic antibiotics. Are we trying to prevent infective endocarditis or a localized infection?
There are few large, prospective, randomized controlled trials that have examined the need for antibiotic prophylaxis with GI endoscopic procedures. Guidelines from professional societies are mainly based on expert opinion, evidence from retrospective case studies, and meta-analysis reviews.
Review of the Data
Infective endocarditis resulting from GI endoscopy has been a concern of physicians for decades. The American Heart Association (AHA) first published its recommendations for antibiotic prophylaxis of GI tract procedures in 1965. The most recent antibacterial prophylaxis guidelines, published in 2007, have simplified recommendations and greatly scaled back the indications for antibiotics. The new guidelines conclude that frequent bacteremia from daily activities is more likely to precipitate endocarditis than a single dental, GI, or genitourinary tract procedure.1
The American Society for Gastrointestinal Endoscopy (ASGE) reports that 14.2 million colonoscopies, 2.8 million flexible sigmoidoscopies, and nearly as many upper endoscopies are performed in the U.S. each year, but only 15 cases of endocarditis have been reported with a temporal association to a procedure.2
The British Society of Gastroenterology (BSG) found, after reviewing the histories of patients with infective endocarditis from 1983 through 2006, that there is not enough evidence to warrant antibiotic prophylaxis prior to endoscopy. They noted less than one case of endocarditis after GI endoscopy per year as well as significant variation in the time interval between the procedure and symptoms. The BSG also recognized that antibiotic prophylaxis does not always protect against infection and that clinical factors unrelated to the endoscopy may play a significant role in the development of endocarditis.3
Upper GI Endoscopy, Colonoscopy with Biopsy, and Esophageal Dilatation. Administering antibiotics to prevent infective endocarditis is not recommended for patients undergoing routine procedures such as endoscopy with biopsy and colonoscopy with polypectomy. Likewise, patients with a history of prosthetic heart valves, valve repair with prosthetic material, endocarditis, congenital heart disease, or cardiac transplant with valvulopathy do not need prophylactic antibiotics before GI endoscopic procedures. However, for patients who are being treated for an active GI infection, antibiotic coverage for enterococcus may be warranted given the increased risk of developing endocarditis. The AHA acknowledges there are no published studies to support the efficacy of antibiotics to prevent enterococcal endocarditis in patients in this clinical setting.1
Unlike routine endoscopy, esophageal dilation is associated with an increased rate of bacteremia (12%-100%).4 Streptococcus viridans has been found in blood cultures up to 79% of the time after esophageal dilation.5 Patients with malignant strictures have higher rates of bacteremia than those with benign strictures (52.9% versus 15.7%). Patients treated with multiple passes with the esophageal dilator compared to those treated with a single dilation have a higher risk of bacteremia.6 All patients undergoing esophageal stricture dilation should receive pre-procedural prophylactic antibiotics.7
Patients with bleeding esophageal varices also have high rates of bacteremia. Up to 20% of patients with cirrhosis and GI bleeding on admission develop an infection within 48 hours of presentation.8 There is evidence that the bacteremia may actually be related to the variceal bleeding rather than the procedure.9 Patients with bleeding esophageal varices treated with antibiotics have improved outcomes, including a decrease in mortality.10 Therefore, all patients with bleeding esophageal varices should be placed on antibiotic therapy regardless of whether an endoscopic intervention is planned.
Percutaneous Endoscopic Gastrostomy (PEG) Placement. Prophylactic antibiotics are recommended before placement of a PEG. The indication for prophylactic antibiotics is to prevent a gastrostomy site infection, not infective endocarditis. Gastrostomy site infection is unfortunately a fairly common infection, affecting 4% to 30% of patients who undergo PEG tube placement. There is significant evidence that antibiotics are beneficial in preventing peristomal infections. A meta-analysis showed that only eight patients need to be treated with prophylactic antibiotics to prevent a single peristomal infection.11 Since these infections are believed to be caused by contamination from the oropharynx, physicians should consider prophylaxis against pathogens from the oral flora.12
More recently, it has been noted that methicillin-resistant Staphylococcus aureus (MRSA) is increasingly cultured from infection sites.13 In centers with endemic MRSA, patients should be screened and then undergo decontamination prior to the PEG placement in positive cases.
Endoscopic Ultrasound with Fine Needle Aspiration (EUS-FNA). Antibiotic prophylaxis before EUS-FNA of a solid lesion in an organ is generally thought to be unnecessary because the risk of bacteremia with this procedure is low, comparable to routine GI endoscopy with biopsy. The recommendation for prophylactic antibiotics before biopsy of a cystic lesion is different. There is concern that puncturing cystic lesions may create a new infected fluid collection.2 A systematic review of more than 10,000 patients undergoing EUS-FNA with a full range of target organs revealed that, overall, 11.2% of patients experienced a fever and 4.7% of patients had a peri-procedural infection. While it was not possible in this study to determine which patients received prophylactic antibiotics, 93.7% of patients with pancreatic cystic lesions were reported to have been treated with antibiotics.14
A separate, single-center, retrospective trial produced different results. This study examined a population of 253 patients who underwent 266 EUS-FNA of pancreatic cysts and found that prophylactic antibiotics were associated with more adverse events and were not protective for the 3% of the patients with infectious symptoms.15 Despite the conflicting data, guidelines at this time recommend prophylactic antibiotics before drainage of a sterile pancreatic fluid collection that communicates with the pancreatic duct and also for aspiration of cystic lesions along the GI tract and the mediastinum.2
Endoscopic Retrograde Cholangiopancreatography (ERCP). In patients undergoing ERCP, the routine use of prophylactic antibiotics has not been found to be effective in decreasing the risk of post-procedure cholangitis.16 Guidelines recommend the use of prophylactic antibiotics only in those patients in which the ERCP may not completely resolve the biliary obstruction.2 In these patients, the thought is that ERCP can precipitate infection by disturbing bacteria already present in the biliary tree, especially with increased intrabiliary pressure at the time of contrast dye injection.17
Patients with incomplete biliary drainage, including those with primary sclerosing cholangitis (PSC), hilar cholangiocarcinoma, persistent biliary that were not extracted, and strictures that continue to obstruct despite attempted intervention, are thought to be at elevated risk of developing cholangitis post-ERCP. These patients should be placed on prophylactic antibiotics at the time of the procedure to cover biliary flora such as enteric gram negatives and enterococci. Antibiotics should be continued until the biliary obstruction is resolved.2
Additional Populations to Consider. Previously, the International Society for Peritoneal Dialysis recommended that patients on peritoneal dialysis receive prophylactic antibiotics and empty their abdomen of dialysate prior to colonoscopy. This recommendation has been removed from the 2010 guidelines.18 There is also no indication that patients with synthetic vascular grafts or cardiac devices should receive prophylactic antibiotics prior to routine GI endoscopy.19 The American Academy of Orthopaedic Surgeons no longer recommends that patients with joint replacements receive antibiotic prophylaxis prior to GI endoscopy.20
Back to the Case
The older gentleman with a prosthetic valve undergoing colonoscopy should not receive prophylactic antibiotics, because even in the setting of valvulopathy, colonoscopy does not pose a significant risk for infective endocarditis. The young patient with severe choledocholithiasis should be placed on prophylactic antibiotics because she has continued biliary obstruction, which could result in a cholangitis after ERCP.
Bottom Line
Prophylactic antibiotics are not recommended for any patient undergoing routine endoscopy or colonoscopy. They are indicated for patients with bleeding esophageal varices and for patients who undergo esophageal stricture dilation, PEG placement, or pseudocyst or cyst drainage, and those with continued biliary obstruction undergoing ERCP as summarized in Table 1.
Drs. Ritter, Jupiter, Carbo, and Li are hospitalists at Beth Israel Deaconess Medical Center and Harvard Medical School faculty in Boston.
References
- Wilson W, Taubert KA, Gewitz M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754.
- Banerjee S, Shen B, Baron TH, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2008;67(6):791-798.
- Allison MC, Sandoe JA, Tighe R, Simpson IA, Hall RJ, Elliott TS. Antibiotic prophylaxis in gastrointestinal endoscopy. Gut. 2009;58(6):869-880.
- Nelson DB. Infectious disease complications of GI endoscopy: Part I, endogenous infections. Gastrointest Endosc. 2003;57(4):546-556.
- Zuccaro G Jr., Richter JE, Rice TW, et al. Viridans streptococcal bacteremia after esophageal stricture dilation. Gastrointest Endosc. 1998;48(6):568-573.
- Nelson DB, Sanderson SJ, Azar MM. Bacteremia with esophageal dilation. Gastrointest Endosc.1998;48(6):563-567.
- Hirota WK, Petersen K, Baron TH, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58(4):475-482.
- Ho H, Zuckerman MJ, Wassem C. A prospective controlled study of the risk of bacteremia in emergency sclerotherapy of esophageal varices. Gastroenterology. 1991;101(6):1642-1648.
- Rolando N, Gimson A, Philpott-Howard J, et al. Infectious sequelae after endoscopic sclerotherapy of oesophageal varices: Role of antibiotic prophylaxis. J Hepatol. 1993;18(3):290-294.
- Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46(3):922-938.
- Jafri NS, Mahid SS, Minor KS, Idstein SR, Hornung CA, Galandiuk S. Meta-analysis: Antibiotic prophylaxis to prevent peristomal infection following percutaneous endoscopic gastrostomy. Aliment Pharmacol Ther. 2007;25(6):647-656.
- Chuang CH, Hung KH, Chen JR, et al. Airway infection predisposes to peristomal infection after percutaneous endoscopic gastrostomy with high concordance between sputum and wound isolates. J Gastrointest Surg. 2010;14(1):45-51.
- Chaudhary KA, Smith OJ, Cuddy PG, Clarkston WK. PEG site infections: The emergence of methicillin resistant Staphylococcus aureus as a major pathogen. Am J Gastroenterol. 2002;97(7):1713-1716.
- Wang KX, Ben QW, Jin ZD, et al. Assessment of morbidity and mortality associated with EUS-guided FNA: A systematic review. Gastrointest Endosc. 2011;73(2):283-290.
- Guarner-Argente C, Shah P, Buchner A, Ahmad NA, Kochman ML, Ginsberg GG. Use of antimicrobials for EUS-guided FNA of pancreatic cysts: A retrospective, comparative analysis. Gastrointest Endosc. 2011;74(1):81-86.
- Bai Y, Gao F, Gao J, Zou DW, Li ZS. Prophylactic antibiotics cannot prevent endoscopic retrograde cholangiopancreatography-induced cholangitis: A meta-analysis. Pancreas. 2009;38(2):126-130.
- Cotton PB, Connor P, Rawls E, Romagnuolo J. Infection after ERCP, and antibiotic prophylaxis: A sequential quality-improvement approach over 11 years. Gastrointest Endosc. 2008;67(3):471-475.
- Li PK, Szeto CC, Piraino B, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int. 2010;30(4):393-423.
- Baddour LM, Bettmann MA, Bolger AF, et al. Nonvalvular cardiovascular device-related infections. Circulation. 2003;108(16):2015-2031.
- Rethman MP, Watters W III, Abt E, et al. The American Academy of Orthopaedic Surgeons and the American Dental Association clinical practice guideline on the prevention of orthopaedic implant infection in patients undergoing dental procedures. J Bone Joint Surg Am. 2013;95(8):745-747.
When Should Antiplatelet Agents and Anticoagulants Be Restarted after Gastrointestinal Bleed?
Source: Adapted from Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-350S.
Two Cases
A 76-year-old female with a history of hypertension, diabetes, atrial fibrillation, and diverticulosis is admitted with acute onset of dizziness and several episodes of bright red blood per rectum. Her labs show a new anemia at hemoglobin level 6.9 g/dL and an international normalized ratio (INR) of 2.7. She is transfused several units of packed red blood cells and fresh frozen plasma without further bleeding. She undergoes an esophagogastroduodenoscopy (EGD) and colonoscopy, which are notable only for extensive diverticulosis. In preparing the discharge medication reconciliation, you are uncertain what to do with the patient’s anticoagulation.
An 85-year-old male with coronary artery disease status post-percutaneous coronary intervention, with placement of a drug-eluting stent several years prior, is admitted with multiple weeks of epigastric discomfort and acute onset of hematemesis. His laboratory tests are notable for a new anemia at hemoglobin level 6.5 g/dL. Urgent EGD demonstrates a bleeding ulcer, which is cauterized. He is started on a proton-pump inhibitor (PPI). He inquires as to when he can restart his home medications, including aspirin.
Overview
Gastrointestinal (GI) bleeding is a serious complication of anticoagulant and antiplatelet therapy. Risks for GI bleeding include older age, history of peptic ulcer disease, NSAID or steroid use, and the use of antiplatelet or anticoagulation therapy. The estimated incidence of GI bleeding in the general population is 48 to 160 cases (upper GI) and 21 cases (lower GI) per 1,000 adults per year, with a case-mortality rate between 5% and 14%.1
Although there is consensus on ceasing anticoagulant and antiplatelet agents during an acute GI bleed, debate remains over the appropriate approach to restarting these agents.
Anticoagulant Resumption
A recent study published in Archives of Internal Medicine supports a quick resumption of anticoagulation following a GI bleed.2 Although previous studies on restarting anticoagulants were small and demonstrated mixed results, this retrospective cohort study examined more than 442 warfarin-associated GI bleeds. After adjusting for various clinical indicators (e.g. clinical seriousness of bleeding, requirement of transfusions), the investigators found that the decision not to resume warfarin within 90 days of an initial GI hemorrhage was associated with an increased risk of thrombosis and death. Of note, in those patients restarted on warfarin, the mean time to medication initiation was four days following the initial GI bleed. In those not restarted on warfarin, the earliest incidence of thrombosis was documented at eight days following cessation of anticoagulation.2
Though its clinical implications are limited by the retrospective design, this study is helpful in guiding management decisions. Randomized control trials and society recommendations on this topic are lacking, so the decision to resume anticoagulants rests on patient-specific estimates of the risk of recurrent bleeding and the benefits of resuming anticoagulants.
In identifying those patients most likely to benefit from restarting anticoagulation, the risk of thromboembolism should be determined using an established risk stratification framework, such as Antithrombotic Therapy and Prevention of Thrombosis, 9th edition (see Table 1).3 According to the guidelines, patients at highest risk of thromboembolism (in the absence of anticoagulation) are those with:
- mitral valve prostheses;
- atrial fibrillation with a CHADS2 score of five to six or cerebrovascular accidents (CVA) within the last three months; and/or
- venous thromboembolism (VTE) within the last three months or history of severe thrombophilia.
Patients at the lowest risk of thromboembolism are those with:
- mechanical aortic prostheses with no other stroke risk factors;
- atrial fibrillation with a CHADS2 score of zero to two; and/or
- a single VTE that occurred >12 months prior.
There are several approaches to identifying patients at greatest risk for bleeding. Location-specific modeling for upper GI bleeds (e.g. Rockall score) and lower GI bleeds (e.g. BLEED score) focus on the clinical presentation and/or endoscopic findings. General hemorrhage risk scores (e.g. HAS-BLED, ATRIA) focus on medical comorbidities. While easy to use, the predictive value of such scores as part of anticoagulation resumption after a GI hemorrhage remains uncertain.
Based on the above methods of risk stratification, patients at higher risk of thromboembolism and lower risk of bleeding will likely benefit from waiting only a short time interval before restarting anticoagulation. Based on the trial conducted by Witt and colleagues, anticoagulation typically can be reinitiated within four days of obtaining hemostatic and hemodynamic stability.2 Conversely, those at highest risk of bleeding and lower risk of thromboembolism will benefit from a delayed resumption of anticoagulation. Involvement of a specialist, such as a gastroenterologist, could help further clarify the risk of rebleeding.
The ideal approach for patients with a high risk of both bleeding and thromboembolism remains uncertain. Such cases highlight the need for an informed discussion with the patient and any involved caregivers, as well as involvement of inpatient subspecialists and outpatient longitudinal providers.
There remains a lack of evidence on the best method to restart anticoagulation. Based on small and retrospective trials, we recommend restarting warfarin at the patient’s previous home dose. The duration of inpatient monitoring following warfarin initiation should be individualized, but warfarin is not expected to impair coagulation for four to six days after initiation.
Little data is available with respect to the role of novel oral anticoagulants after a GI bleed. Given the lack of reversing agents for these drugs, we recommend exercising caution in populations with a high risk of rebleeding. Theoretically, given that these agents reach peak effect faster than warfarin, waiting an additional four days after the time frame recommended for starting warfarin is a prudent resumption strategy for novel oral anticoagulants.
Source: Adapted from Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-350S.
Resumption of Antiplatelet Agents
The decision to resume antiplatelet therapy should also be highly individualized. In addition to weighing the risk of bleeding (as described in the previous section), the physician must also estimate the benefits of antiplatelet therapy in decreasing the risk of cardiovascular events.
In low-risk patients on antiplatelet therapy (i.e., for primary cardiovascular prevention) reinitiation after a bleeding episode can be reasonably delayed, because the risk of rebleeding likely outweighs the potential benefit of restarting therapy.
For patients who are at intermediate risk (i.e., those on antiplatelet agents for secondary prevention of cardiovascular disease), emerging evidence argues for early reinstitution after a GI bleed. In a trial published in Annals of Internal Medicine, Sung and colleagues randomized 156 patients to aspirin or placebo therapy immediately following endoscopically obtained hemostasis for peptic ulcer bleeding.4 All patients received PPIs. There was no significant difference in bleeding rates between the two groups, but delayed resumption of aspirin was associated with a significant increase in all-cause mortality.
Two recent meta-analyses provide further insight into the risks of withholding aspirin therapy. The first, which included 50,279 patients on aspirin for secondary prevention, found that aspirin non-adherence or withdrawal after a GI bleed was associated with a three-fold higher risk of major adverse cardiac events.5 Cardiac event rates were highest in the subgroup of patients with a history of prior percutaneous coronary stenting.
A second meta-analysis evaluated patients who had aspirin held perioperatively. In a population of patients on aspirin for secondary prevention, the mean time after withholding aspirin was 8.5 days to coronary events, 14.3 days to cerebrovascular events, and 25.8 days to peripheral arterial events.6 Events occurred as early as five days after withdrawal of aspirin.
Patients with recent intracoronary stenting are at highest risk of thrombosis. In patients with a bare metal stent placed within six weeks, or a drug-eluting stent placed within six months, every effort should be made to minimize interruptions of dual antiplatelet therapy.
Based on the data presented above, for patients at intermediate and/or high risk of adverse cardiac events, we recommend reinstitution of aspirin as soon as possible following a GI hemorrhage, preferably within five days. PPI co-therapy is a mainstay for secondary prevention of upper GI bleeding in patients on antiplatelet therapy. Current research and guidelines have not addressed specifically the role of withholding and reinitiating aspirin in lower GI bleeding, non-peptic ulcer, or upper-GI bleeding, however, a similar strategy is likely appropriate. As with the decision for restarting anticoagulants, discussion with relevant specialists is essential to best define the risk of re-bleeding.
Back to the Cases
Given her CHADS2 score of three, the patient with a diverticular bleed has a 9.6% annual risk of stroke if she does not resume anticoagulation. Using the HAS-BLED and ATRIA scores, this patient has 2.6% to 5.8% annual risk of hemorrhage. We recommend resuming warfarin anticoagulation therapy within four days of achieving hemostasis.
For the patient with coronary artery disease with remote drug-eluting stent placement and upper GI bleed, evidence supports early resumption of appropriate antiplatelet therapy following endoscopic therapy and hemostasis. We recommend resuming aspirin during the current hospitalization and concomitant treatment with a PPI indefinitely.
Bottom Line
Following a GI bleed, the risks and benefits of restarting anticoagulant and antiplatelet agents need to be carefully considered. In patients on oral anticoagulants at high risk for thromboembolism and low risk for rebleeding, consider restarting anticoagulation within four to five days. Patients on antiplatelet agents for secondary prevention should have the medication restarted during hospitalization after endoscopically obtained hemostasis of a peptic ulcer.
In all cases, hospitalists should engage the patient, gastroenterologist, and outpatient provider to best determine when resumption of anticoagulant and/or antiplatelet agents should occur.
Dr. Allen-Dicker is a hospitalist and clinical instructor at Mount Sinai Medical Center in New York City. Dr. Briones is director of perioperative services in the division of hospital medicine and an assistant professor; Dr. Berman is a hospitalist and a clinical instructor, and Dr. Dunn is a professor of medicine and chief of the division of hospital medicine, all at Mount Sinai Medical Center.
References
- Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152(2):101-113.
- Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172(19):1484-1491.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-350S.
- Sung JJ, Lau JY, Ching JY, et al. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: A randomized trial. Ann Intern Med. 2010;152(1):1-9.
- Biondi-Zoccai GG, Lotrionte M, Agostoni P, et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J. 2006;27(22):2667-2674.
- Burger W, Chemnitius JM, Kneissl GD, Rücker G. Low-dose aspirin for secondary cardiovascular prevention – cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation – review and meta-analysis. J Intern Med. 2005;257(5):399-414.
Source: Adapted from Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-350S.
Two Cases
A 76-year-old female with a history of hypertension, diabetes, atrial fibrillation, and diverticulosis is admitted with acute onset of dizziness and several episodes of bright red blood per rectum. Her labs show a new anemia at hemoglobin level 6.9 g/dL and an international normalized ratio (INR) of 2.7. She is transfused several units of packed red blood cells and fresh frozen plasma without further bleeding. She undergoes an esophagogastroduodenoscopy (EGD) and colonoscopy, which are notable only for extensive diverticulosis. In preparing the discharge medication reconciliation, you are uncertain what to do with the patient’s anticoagulation.
An 85-year-old male with coronary artery disease status post-percutaneous coronary intervention, with placement of a drug-eluting stent several years prior, is admitted with multiple weeks of epigastric discomfort and acute onset of hematemesis. His laboratory tests are notable for a new anemia at hemoglobin level 6.5 g/dL. Urgent EGD demonstrates a bleeding ulcer, which is cauterized. He is started on a proton-pump inhibitor (PPI). He inquires as to when he can restart his home medications, including aspirin.
Overview
Gastrointestinal (GI) bleeding is a serious complication of anticoagulant and antiplatelet therapy. Risks for GI bleeding include older age, history of peptic ulcer disease, NSAID or steroid use, and the use of antiplatelet or anticoagulation therapy. The estimated incidence of GI bleeding in the general population is 48 to 160 cases (upper GI) and 21 cases (lower GI) per 1,000 adults per year, with a case-mortality rate between 5% and 14%.1
Although there is consensus on ceasing anticoagulant and antiplatelet agents during an acute GI bleed, debate remains over the appropriate approach to restarting these agents.
Anticoagulant Resumption
A recent study published in Archives of Internal Medicine supports a quick resumption of anticoagulation following a GI bleed.2 Although previous studies on restarting anticoagulants were small and demonstrated mixed results, this retrospective cohort study examined more than 442 warfarin-associated GI bleeds. After adjusting for various clinical indicators (e.g. clinical seriousness of bleeding, requirement of transfusions), the investigators found that the decision not to resume warfarin within 90 days of an initial GI hemorrhage was associated with an increased risk of thrombosis and death. Of note, in those patients restarted on warfarin, the mean time to medication initiation was four days following the initial GI bleed. In those not restarted on warfarin, the earliest incidence of thrombosis was documented at eight days following cessation of anticoagulation.2
Though its clinical implications are limited by the retrospective design, this study is helpful in guiding management decisions. Randomized control trials and society recommendations on this topic are lacking, so the decision to resume anticoagulants rests on patient-specific estimates of the risk of recurrent bleeding and the benefits of resuming anticoagulants.
In identifying those patients most likely to benefit from restarting anticoagulation, the risk of thromboembolism should be determined using an established risk stratification framework, such as Antithrombotic Therapy and Prevention of Thrombosis, 9th edition (see Table 1).3 According to the guidelines, patients at highest risk of thromboembolism (in the absence of anticoagulation) are those with:
- mitral valve prostheses;
- atrial fibrillation with a CHADS2 score of five to six or cerebrovascular accidents (CVA) within the last three months; and/or
- venous thromboembolism (VTE) within the last three months or history of severe thrombophilia.
Patients at the lowest risk of thromboembolism are those with:
- mechanical aortic prostheses with no other stroke risk factors;
- atrial fibrillation with a CHADS2 score of zero to two; and/or
- a single VTE that occurred >12 months prior.
There are several approaches to identifying patients at greatest risk for bleeding. Location-specific modeling for upper GI bleeds (e.g. Rockall score) and lower GI bleeds (e.g. BLEED score) focus on the clinical presentation and/or endoscopic findings. General hemorrhage risk scores (e.g. HAS-BLED, ATRIA) focus on medical comorbidities. While easy to use, the predictive value of such scores as part of anticoagulation resumption after a GI hemorrhage remains uncertain.
Based on the above methods of risk stratification, patients at higher risk of thromboembolism and lower risk of bleeding will likely benefit from waiting only a short time interval before restarting anticoagulation. Based on the trial conducted by Witt and colleagues, anticoagulation typically can be reinitiated within four days of obtaining hemostatic and hemodynamic stability.2 Conversely, those at highest risk of bleeding and lower risk of thromboembolism will benefit from a delayed resumption of anticoagulation. Involvement of a specialist, such as a gastroenterologist, could help further clarify the risk of rebleeding.
The ideal approach for patients with a high risk of both bleeding and thromboembolism remains uncertain. Such cases highlight the need for an informed discussion with the patient and any involved caregivers, as well as involvement of inpatient subspecialists and outpatient longitudinal providers.
There remains a lack of evidence on the best method to restart anticoagulation. Based on small and retrospective trials, we recommend restarting warfarin at the patient’s previous home dose. The duration of inpatient monitoring following warfarin initiation should be individualized, but warfarin is not expected to impair coagulation for four to six days after initiation.
Little data is available with respect to the role of novel oral anticoagulants after a GI bleed. Given the lack of reversing agents for these drugs, we recommend exercising caution in populations with a high risk of rebleeding. Theoretically, given that these agents reach peak effect faster than warfarin, waiting an additional four days after the time frame recommended for starting warfarin is a prudent resumption strategy for novel oral anticoagulants.
Source: Adapted from Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-350S.
Resumption of Antiplatelet Agents
The decision to resume antiplatelet therapy should also be highly individualized. In addition to weighing the risk of bleeding (as described in the previous section), the physician must also estimate the benefits of antiplatelet therapy in decreasing the risk of cardiovascular events.
In low-risk patients on antiplatelet therapy (i.e., for primary cardiovascular prevention) reinitiation after a bleeding episode can be reasonably delayed, because the risk of rebleeding likely outweighs the potential benefit of restarting therapy.
For patients who are at intermediate risk (i.e., those on antiplatelet agents for secondary prevention of cardiovascular disease), emerging evidence argues for early reinstitution after a GI bleed. In a trial published in Annals of Internal Medicine, Sung and colleagues randomized 156 patients to aspirin or placebo therapy immediately following endoscopically obtained hemostasis for peptic ulcer bleeding.4 All patients received PPIs. There was no significant difference in bleeding rates between the two groups, but delayed resumption of aspirin was associated with a significant increase in all-cause mortality.
Two recent meta-analyses provide further insight into the risks of withholding aspirin therapy. The first, which included 50,279 patients on aspirin for secondary prevention, found that aspirin non-adherence or withdrawal after a GI bleed was associated with a three-fold higher risk of major adverse cardiac events.5 Cardiac event rates were highest in the subgroup of patients with a history of prior percutaneous coronary stenting.
A second meta-analysis evaluated patients who had aspirin held perioperatively. In a population of patients on aspirin for secondary prevention, the mean time after withholding aspirin was 8.5 days to coronary events, 14.3 days to cerebrovascular events, and 25.8 days to peripheral arterial events.6 Events occurred as early as five days after withdrawal of aspirin.
Patients with recent intracoronary stenting are at highest risk of thrombosis. In patients with a bare metal stent placed within six weeks, or a drug-eluting stent placed within six months, every effort should be made to minimize interruptions of dual antiplatelet therapy.
Based on the data presented above, for patients at intermediate and/or high risk of adverse cardiac events, we recommend reinstitution of aspirin as soon as possible following a GI hemorrhage, preferably within five days. PPI co-therapy is a mainstay for secondary prevention of upper GI bleeding in patients on antiplatelet therapy. Current research and guidelines have not addressed specifically the role of withholding and reinitiating aspirin in lower GI bleeding, non-peptic ulcer, or upper-GI bleeding, however, a similar strategy is likely appropriate. As with the decision for restarting anticoagulants, discussion with relevant specialists is essential to best define the risk of re-bleeding.
Back to the Cases
Given her CHADS2 score of three, the patient with a diverticular bleed has a 9.6% annual risk of stroke if she does not resume anticoagulation. Using the HAS-BLED and ATRIA scores, this patient has 2.6% to 5.8% annual risk of hemorrhage. We recommend resuming warfarin anticoagulation therapy within four days of achieving hemostasis.
For the patient with coronary artery disease with remote drug-eluting stent placement and upper GI bleed, evidence supports early resumption of appropriate antiplatelet therapy following endoscopic therapy and hemostasis. We recommend resuming aspirin during the current hospitalization and concomitant treatment with a PPI indefinitely.
Bottom Line
Following a GI bleed, the risks and benefits of restarting anticoagulant and antiplatelet agents need to be carefully considered. In patients on oral anticoagulants at high risk for thromboembolism and low risk for rebleeding, consider restarting anticoagulation within four to five days. Patients on antiplatelet agents for secondary prevention should have the medication restarted during hospitalization after endoscopically obtained hemostasis of a peptic ulcer.
In all cases, hospitalists should engage the patient, gastroenterologist, and outpatient provider to best determine when resumption of anticoagulant and/or antiplatelet agents should occur.
Dr. Allen-Dicker is a hospitalist and clinical instructor at Mount Sinai Medical Center in New York City. Dr. Briones is director of perioperative services in the division of hospital medicine and an assistant professor; Dr. Berman is a hospitalist and a clinical instructor, and Dr. Dunn is a professor of medicine and chief of the division of hospital medicine, all at Mount Sinai Medical Center.
References
- Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152(2):101-113.
- Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172(19):1484-1491.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-350S.
- Sung JJ, Lau JY, Ching JY, et al. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: A randomized trial. Ann Intern Med. 2010;152(1):1-9.
- Biondi-Zoccai GG, Lotrionte M, Agostoni P, et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J. 2006;27(22):2667-2674.
- Burger W, Chemnitius JM, Kneissl GD, Rücker G. Low-dose aspirin for secondary cardiovascular prevention – cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation – review and meta-analysis. J Intern Med. 2005;257(5):399-414.
Source: Adapted from Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-350S.
Two Cases
A 76-year-old female with a history of hypertension, diabetes, atrial fibrillation, and diverticulosis is admitted with acute onset of dizziness and several episodes of bright red blood per rectum. Her labs show a new anemia at hemoglobin level 6.9 g/dL and an international normalized ratio (INR) of 2.7. She is transfused several units of packed red blood cells and fresh frozen plasma without further bleeding. She undergoes an esophagogastroduodenoscopy (EGD) and colonoscopy, which are notable only for extensive diverticulosis. In preparing the discharge medication reconciliation, you are uncertain what to do with the patient’s anticoagulation.
An 85-year-old male with coronary artery disease status post-percutaneous coronary intervention, with placement of a drug-eluting stent several years prior, is admitted with multiple weeks of epigastric discomfort and acute onset of hematemesis. His laboratory tests are notable for a new anemia at hemoglobin level 6.5 g/dL. Urgent EGD demonstrates a bleeding ulcer, which is cauterized. He is started on a proton-pump inhibitor (PPI). He inquires as to when he can restart his home medications, including aspirin.
Overview
Gastrointestinal (GI) bleeding is a serious complication of anticoagulant and antiplatelet therapy. Risks for GI bleeding include older age, history of peptic ulcer disease, NSAID or steroid use, and the use of antiplatelet or anticoagulation therapy. The estimated incidence of GI bleeding in the general population is 48 to 160 cases (upper GI) and 21 cases (lower GI) per 1,000 adults per year, with a case-mortality rate between 5% and 14%.1
Although there is consensus on ceasing anticoagulant and antiplatelet agents during an acute GI bleed, debate remains over the appropriate approach to restarting these agents.
Anticoagulant Resumption
A recent study published in Archives of Internal Medicine supports a quick resumption of anticoagulation following a GI bleed.2 Although previous studies on restarting anticoagulants were small and demonstrated mixed results, this retrospective cohort study examined more than 442 warfarin-associated GI bleeds. After adjusting for various clinical indicators (e.g. clinical seriousness of bleeding, requirement of transfusions), the investigators found that the decision not to resume warfarin within 90 days of an initial GI hemorrhage was associated with an increased risk of thrombosis and death. Of note, in those patients restarted on warfarin, the mean time to medication initiation was four days following the initial GI bleed. In those not restarted on warfarin, the earliest incidence of thrombosis was documented at eight days following cessation of anticoagulation.2
Though its clinical implications are limited by the retrospective design, this study is helpful in guiding management decisions. Randomized control trials and society recommendations on this topic are lacking, so the decision to resume anticoagulants rests on patient-specific estimates of the risk of recurrent bleeding and the benefits of resuming anticoagulants.
In identifying those patients most likely to benefit from restarting anticoagulation, the risk of thromboembolism should be determined using an established risk stratification framework, such as Antithrombotic Therapy and Prevention of Thrombosis, 9th edition (see Table 1).3 According to the guidelines, patients at highest risk of thromboembolism (in the absence of anticoagulation) are those with:
- mitral valve prostheses;
- atrial fibrillation with a CHADS2 score of five to six or cerebrovascular accidents (CVA) within the last three months; and/or
- venous thromboembolism (VTE) within the last three months or history of severe thrombophilia.
Patients at the lowest risk of thromboembolism are those with:
- mechanical aortic prostheses with no other stroke risk factors;
- atrial fibrillation with a CHADS2 score of zero to two; and/or
- a single VTE that occurred >12 months prior.
There are several approaches to identifying patients at greatest risk for bleeding. Location-specific modeling for upper GI bleeds (e.g. Rockall score) and lower GI bleeds (e.g. BLEED score) focus on the clinical presentation and/or endoscopic findings. General hemorrhage risk scores (e.g. HAS-BLED, ATRIA) focus on medical comorbidities. While easy to use, the predictive value of such scores as part of anticoagulation resumption after a GI hemorrhage remains uncertain.
Based on the above methods of risk stratification, patients at higher risk of thromboembolism and lower risk of bleeding will likely benefit from waiting only a short time interval before restarting anticoagulation. Based on the trial conducted by Witt and colleagues, anticoagulation typically can be reinitiated within four days of obtaining hemostatic and hemodynamic stability.2 Conversely, those at highest risk of bleeding and lower risk of thromboembolism will benefit from a delayed resumption of anticoagulation. Involvement of a specialist, such as a gastroenterologist, could help further clarify the risk of rebleeding.
The ideal approach for patients with a high risk of both bleeding and thromboembolism remains uncertain. Such cases highlight the need for an informed discussion with the patient and any involved caregivers, as well as involvement of inpatient subspecialists and outpatient longitudinal providers.
There remains a lack of evidence on the best method to restart anticoagulation. Based on small and retrospective trials, we recommend restarting warfarin at the patient’s previous home dose. The duration of inpatient monitoring following warfarin initiation should be individualized, but warfarin is not expected to impair coagulation for four to six days after initiation.
Little data is available with respect to the role of novel oral anticoagulants after a GI bleed. Given the lack of reversing agents for these drugs, we recommend exercising caution in populations with a high risk of rebleeding. Theoretically, given that these agents reach peak effect faster than warfarin, waiting an additional four days after the time frame recommended for starting warfarin is a prudent resumption strategy for novel oral anticoagulants.
Source: Adapted from Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-350S.
Resumption of Antiplatelet Agents
The decision to resume antiplatelet therapy should also be highly individualized. In addition to weighing the risk of bleeding (as described in the previous section), the physician must also estimate the benefits of antiplatelet therapy in decreasing the risk of cardiovascular events.
In low-risk patients on antiplatelet therapy (i.e., for primary cardiovascular prevention) reinitiation after a bleeding episode can be reasonably delayed, because the risk of rebleeding likely outweighs the potential benefit of restarting therapy.
For patients who are at intermediate risk (i.e., those on antiplatelet agents for secondary prevention of cardiovascular disease), emerging evidence argues for early reinstitution after a GI bleed. In a trial published in Annals of Internal Medicine, Sung and colleagues randomized 156 patients to aspirin or placebo therapy immediately following endoscopically obtained hemostasis for peptic ulcer bleeding.4 All patients received PPIs. There was no significant difference in bleeding rates between the two groups, but delayed resumption of aspirin was associated with a significant increase in all-cause mortality.
Two recent meta-analyses provide further insight into the risks of withholding aspirin therapy. The first, which included 50,279 patients on aspirin for secondary prevention, found that aspirin non-adherence or withdrawal after a GI bleed was associated with a three-fold higher risk of major adverse cardiac events.5 Cardiac event rates were highest in the subgroup of patients with a history of prior percutaneous coronary stenting.
A second meta-analysis evaluated patients who had aspirin held perioperatively. In a population of patients on aspirin for secondary prevention, the mean time after withholding aspirin was 8.5 days to coronary events, 14.3 days to cerebrovascular events, and 25.8 days to peripheral arterial events.6 Events occurred as early as five days after withdrawal of aspirin.
Patients with recent intracoronary stenting are at highest risk of thrombosis. In patients with a bare metal stent placed within six weeks, or a drug-eluting stent placed within six months, every effort should be made to minimize interruptions of dual antiplatelet therapy.
Based on the data presented above, for patients at intermediate and/or high risk of adverse cardiac events, we recommend reinstitution of aspirin as soon as possible following a GI hemorrhage, preferably within five days. PPI co-therapy is a mainstay for secondary prevention of upper GI bleeding in patients on antiplatelet therapy. Current research and guidelines have not addressed specifically the role of withholding and reinitiating aspirin in lower GI bleeding, non-peptic ulcer, or upper-GI bleeding, however, a similar strategy is likely appropriate. As with the decision for restarting anticoagulants, discussion with relevant specialists is essential to best define the risk of re-bleeding.
Back to the Cases
Given her CHADS2 score of three, the patient with a diverticular bleed has a 9.6% annual risk of stroke if she does not resume anticoagulation. Using the HAS-BLED and ATRIA scores, this patient has 2.6% to 5.8% annual risk of hemorrhage. We recommend resuming warfarin anticoagulation therapy within four days of achieving hemostasis.
For the patient with coronary artery disease with remote drug-eluting stent placement and upper GI bleed, evidence supports early resumption of appropriate antiplatelet therapy following endoscopic therapy and hemostasis. We recommend resuming aspirin during the current hospitalization and concomitant treatment with a PPI indefinitely.
Bottom Line
Following a GI bleed, the risks and benefits of restarting anticoagulant and antiplatelet agents need to be carefully considered. In patients on oral anticoagulants at high risk for thromboembolism and low risk for rebleeding, consider restarting anticoagulation within four to five days. Patients on antiplatelet agents for secondary prevention should have the medication restarted during hospitalization after endoscopically obtained hemostasis of a peptic ulcer.
In all cases, hospitalists should engage the patient, gastroenterologist, and outpatient provider to best determine when resumption of anticoagulant and/or antiplatelet agents should occur.
Dr. Allen-Dicker is a hospitalist and clinical instructor at Mount Sinai Medical Center in New York City. Dr. Briones is director of perioperative services in the division of hospital medicine and an assistant professor; Dr. Berman is a hospitalist and a clinical instructor, and Dr. Dunn is a professor of medicine and chief of the division of hospital medicine, all at Mount Sinai Medical Center.
References
- Barkun AN, Bardou M, Kuipers EJ, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Intern Med. 2010;152(2):101-113.
- Witt DM, Delate T, Garcia DA, et al. Risk of thromboembolism, recurrent hemorrhage, and death after warfarin therapy interruption for gastrointestinal tract bleeding. Arch Intern Med. 2012;172(19):1484-1491.
- Douketis JD, Spyropoulos AC, Spencer FA, et al. Perioperative management of antithrombotic therapy: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S-350S.
- Sung JJ, Lau JY, Ching JY, et al. Continuation of low-dose aspirin therapy in peptic ulcer bleeding: A randomized trial. Ann Intern Med. 2010;152(1):1-9.
- Biondi-Zoccai GG, Lotrionte M, Agostoni P, et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50,279 patients at risk for coronary artery disease. Eur Heart J. 2006;27(22):2667-2674.
- Burger W, Chemnitius JM, Kneissl GD, Rücker G. Low-dose aspirin for secondary cardiovascular prevention – cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation – review and meta-analysis. J Intern Med. 2005;257(5):399-414.
Oseltamivir for Healthy Pediatric Inpatients with Influenza Unlikely to Yield Measurable Benefit
Clinical question: What is the benefit of oseltamivir treatment to previously healthy children hospitalized with confirmed influenza infection?
Background: Influenza is generally a mild, self-limited infection that affects all ages but can lead to more serious illness. During the 2012-2013 season, laboratory-confirmed influenza in children zero to four years was associated with a hospitalization rate of 66.4 per 100,000 and 164 pediatric deaths. Currently, the American Academy of Pediatrics and the CDC recommend treatment with either oseltamivir or zanamivir for children hospitalized with influenza. However, despite the recommendations, data are limited regarding the benefits resulting from treating otherwise healthy children hospitalized with influenza.
Study design: Multi-center retrospective study.
Setting: Ten public hospitals in Madrid, Spain, between September 2010 and June 2012.
Synopsis: Researchers identified children ≤14 years admitted to participating hospitals by positive testing with either rapid diagnostic testing or polymerase chain reaction assay of nasal washings. Patients at high risk of serious disease were excluded, including those with chronic disease, immunodeficiency, prematurity (less than 32 weeks gestation), age less than six months at admission, nosocomial infection, and those requiring ICU-level care upon admission. Patients with asthma were included. Decision to treat with oseltamivir was determined by hospital guidelines, with some hospitals treating all hospitalized children with confirmed influenza and others only treating patients with risk factors for severe disease. In addition, only children who had manifested symptoms within 48 hours of admission were included.
Of the 287 children included in the final analysis, 93 were treated with oseltamivir (32%). There were no significant differences between treatment and non-treatment groups in duration of fever, duration of hypoxia, length of stay, or rates of ICU transfer. In addition, the lack of differences in outcomes persisted after subgroup analysis of patients less than one year old.
Bottom line: Treatment with oseltamivir of otherwise healthy children hospitalized with influenza not requiring ICU care is unlikely to yield measurable benefit.
Citation: Bueno M, Calvo C, Mendez-Echevarría A, et al. Oseltamivir treatment for influenza in hospitalized children without underlying diseases. Pediatr Infect Dis J. 2013;32(10):1066-1069.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Clinical question: What is the benefit of oseltamivir treatment to previously healthy children hospitalized with confirmed influenza infection?
Background: Influenza is generally a mild, self-limited infection that affects all ages but can lead to more serious illness. During the 2012-2013 season, laboratory-confirmed influenza in children zero to four years was associated with a hospitalization rate of 66.4 per 100,000 and 164 pediatric deaths. Currently, the American Academy of Pediatrics and the CDC recommend treatment with either oseltamivir or zanamivir for children hospitalized with influenza. However, despite the recommendations, data are limited regarding the benefits resulting from treating otherwise healthy children hospitalized with influenza.
Study design: Multi-center retrospective study.
Setting: Ten public hospitals in Madrid, Spain, between September 2010 and June 2012.
Synopsis: Researchers identified children ≤14 years admitted to participating hospitals by positive testing with either rapid diagnostic testing or polymerase chain reaction assay of nasal washings. Patients at high risk of serious disease were excluded, including those with chronic disease, immunodeficiency, prematurity (less than 32 weeks gestation), age less than six months at admission, nosocomial infection, and those requiring ICU-level care upon admission. Patients with asthma were included. Decision to treat with oseltamivir was determined by hospital guidelines, with some hospitals treating all hospitalized children with confirmed influenza and others only treating patients with risk factors for severe disease. In addition, only children who had manifested symptoms within 48 hours of admission were included.
Of the 287 children included in the final analysis, 93 were treated with oseltamivir (32%). There were no significant differences between treatment and non-treatment groups in duration of fever, duration of hypoxia, length of stay, or rates of ICU transfer. In addition, the lack of differences in outcomes persisted after subgroup analysis of patients less than one year old.
Bottom line: Treatment with oseltamivir of otherwise healthy children hospitalized with influenza not requiring ICU care is unlikely to yield measurable benefit.
Citation: Bueno M, Calvo C, Mendez-Echevarría A, et al. Oseltamivir treatment for influenza in hospitalized children without underlying diseases. Pediatr Infect Dis J. 2013;32(10):1066-1069.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Clinical question: What is the benefit of oseltamivir treatment to previously healthy children hospitalized with confirmed influenza infection?
Background: Influenza is generally a mild, self-limited infection that affects all ages but can lead to more serious illness. During the 2012-2013 season, laboratory-confirmed influenza in children zero to four years was associated with a hospitalization rate of 66.4 per 100,000 and 164 pediatric deaths. Currently, the American Academy of Pediatrics and the CDC recommend treatment with either oseltamivir or zanamivir for children hospitalized with influenza. However, despite the recommendations, data are limited regarding the benefits resulting from treating otherwise healthy children hospitalized with influenza.
Study design: Multi-center retrospective study.
Setting: Ten public hospitals in Madrid, Spain, between September 2010 and June 2012.
Synopsis: Researchers identified children ≤14 years admitted to participating hospitals by positive testing with either rapid diagnostic testing or polymerase chain reaction assay of nasal washings. Patients at high risk of serious disease were excluded, including those with chronic disease, immunodeficiency, prematurity (less than 32 weeks gestation), age less than six months at admission, nosocomial infection, and those requiring ICU-level care upon admission. Patients with asthma were included. Decision to treat with oseltamivir was determined by hospital guidelines, with some hospitals treating all hospitalized children with confirmed influenza and others only treating patients with risk factors for severe disease. In addition, only children who had manifested symptoms within 48 hours of admission were included.
Of the 287 children included in the final analysis, 93 were treated with oseltamivir (32%). There were no significant differences between treatment and non-treatment groups in duration of fever, duration of hypoxia, length of stay, or rates of ICU transfer. In addition, the lack of differences in outcomes persisted after subgroup analysis of patients less than one year old.
Bottom line: Treatment with oseltamivir of otherwise healthy children hospitalized with influenza not requiring ICU care is unlikely to yield measurable benefit.
Citation: Bueno M, Calvo C, Mendez-Echevarría A, et al. Oseltamivir treatment for influenza in hospitalized children without underlying diseases. Pediatr Infect Dis J. 2013;32(10):1066-1069.
Reviewed by Pediatric Editor Weijen Chang, MD, SFHM, FAAP, associate clinical professor of medicine and pediatrics at the University of California at San Diego School of Medicine, and a hospitalist at both UCSD Medical Center and Rady Children’s Hospital.
Observation-Status Patients Are Clinically Heterogeneous, Costly to Hospitals
Clinical question: What are the characteristics of a large cohort of patients under observation status?
Background: The use of observation hospital services has increased significantly. The Centers for Medicare and Medicaid Services (CMS) defines observation status as a “well-defined set of specific, clinically appropriate services,” usually lasting <24 hours and exceeding 48 hours in “only rare and exceptional cases.”
Study design: Retrospective descriptive study.
Setting: University of Wisconsin Hospital and Clinics, a 566-bed tertiary academic medical center.
Synopsis: A total of 43,853 hospitalizations were reviewed during the study period. Of those, 4578 (10.4%) were observation. The mean observation LOS was 33.3 hours, which included 16.5% of patients with LOS >48 hours. Although chest pain was the top observation diagnosis, 1141 distinct observation diagnosis codes were found.
These findings illustrate a significant disparity between the CMS definition for observation stay and the description of observation patients in this cohort, despite using CMS-endorsed InterQual criteria to determine status. While the cost per encounter for observation care was less than inpatient care, reimbursement was insufficient to cover those reduced costs. Ultimately, the net loss of revenue per observation encounter was $331, compared to a net gain in revenue per inpatient encounter of $2,163. This operating loss for hospitals is coupled with the fact that some of this cost is being transferred to patients.
Bottom line: Definitions and reimbursement models for observation status warrant continued research and discussion in the context of our evolving healthcare climate.
Citation: Sheehy AM, Graf B, Gangireddy S, et al. Hospitalized but not admitted: characteristics of patients with “observation status” at an academic medical center [published online ahead of print July 8, 2013]. JAMA Intern Med.
Clinical question: What are the characteristics of a large cohort of patients under observation status?
Background: The use of observation hospital services has increased significantly. The Centers for Medicare and Medicaid Services (CMS) defines observation status as a “well-defined set of specific, clinically appropriate services,” usually lasting <24 hours and exceeding 48 hours in “only rare and exceptional cases.”
Study design: Retrospective descriptive study.
Setting: University of Wisconsin Hospital and Clinics, a 566-bed tertiary academic medical center.
Synopsis: A total of 43,853 hospitalizations were reviewed during the study period. Of those, 4578 (10.4%) were observation. The mean observation LOS was 33.3 hours, which included 16.5% of patients with LOS >48 hours. Although chest pain was the top observation diagnosis, 1141 distinct observation diagnosis codes were found.
These findings illustrate a significant disparity between the CMS definition for observation stay and the description of observation patients in this cohort, despite using CMS-endorsed InterQual criteria to determine status. While the cost per encounter for observation care was less than inpatient care, reimbursement was insufficient to cover those reduced costs. Ultimately, the net loss of revenue per observation encounter was $331, compared to a net gain in revenue per inpatient encounter of $2,163. This operating loss for hospitals is coupled with the fact that some of this cost is being transferred to patients.
Bottom line: Definitions and reimbursement models for observation status warrant continued research and discussion in the context of our evolving healthcare climate.
Citation: Sheehy AM, Graf B, Gangireddy S, et al. Hospitalized but not admitted: characteristics of patients with “observation status” at an academic medical center [published online ahead of print July 8, 2013]. JAMA Intern Med.
Clinical question: What are the characteristics of a large cohort of patients under observation status?
Background: The use of observation hospital services has increased significantly. The Centers for Medicare and Medicaid Services (CMS) defines observation status as a “well-defined set of specific, clinically appropriate services,” usually lasting <24 hours and exceeding 48 hours in “only rare and exceptional cases.”
Study design: Retrospective descriptive study.
Setting: University of Wisconsin Hospital and Clinics, a 566-bed tertiary academic medical center.
Synopsis: A total of 43,853 hospitalizations were reviewed during the study period. Of those, 4578 (10.4%) were observation. The mean observation LOS was 33.3 hours, which included 16.5% of patients with LOS >48 hours. Although chest pain was the top observation diagnosis, 1141 distinct observation diagnosis codes were found.
These findings illustrate a significant disparity between the CMS definition for observation stay and the description of observation patients in this cohort, despite using CMS-endorsed InterQual criteria to determine status. While the cost per encounter for observation care was less than inpatient care, reimbursement was insufficient to cover those reduced costs. Ultimately, the net loss of revenue per observation encounter was $331, compared to a net gain in revenue per inpatient encounter of $2,163. This operating loss for hospitals is coupled with the fact that some of this cost is being transferred to patients.
Bottom line: Definitions and reimbursement models for observation status warrant continued research and discussion in the context of our evolving healthcare climate.
Citation: Sheehy AM, Graf B, Gangireddy S, et al. Hospitalized but not admitted: characteristics of patients with “observation status” at an academic medical center [published online ahead of print July 8, 2013]. JAMA Intern Med.
Early Surgery Might Not Provide Survival Benefit in All Patients with Prosthetic Valve Endocarditis
Clinical question: Is early surgery associated with better survival in patients with prosthetic valve endocarditis (PVE)?
Background: PVE occurs in 3% to 6% of patients within five years of valve implantation. Consensus guidelines, based on expert opinion, recommend surgical intervention with debridement and valve replacement in PVE, especially for patients with complications that are unlikely to be successfully treated with medical therapy, such as valve dysfunction, heart failure, and cardiac abscesses. Studies comparing survival with medical therapy versus surgery have reported conflicting results.
Study Design: Multi-center, prospective, cohort study.
Setting: International, multi-center cohort of patients from tertiary care hospitals.
Synopsis: The International Collaboration on Endocarditis—Prospective Cohort Study (ICE-PCS) cohort consisted of 1025 patients with PVE, 490 of whom underwent early surgery and 535 of whom received medical therapy alone.
Unadjusted initial analysis showed early surgery was associated with lower mortality; however, this survival benefit was not evident after the data was adjusted for treatment selection bias and survivor bias for in-hospital mortality and one-year mortality. The hazard ratios were 0.9 (95% CI 0.76 -1.07) and 1.04 (95% CI 0.89 -1.23), respectively.
Subgroup analysis indicated that early surgery in patients with high-risk features was associated with fewer poor outcomes compared to medical therapy: 28% versus 50% (P=.007)
Bottom line: Early surgery may not be associated with mortality benefits for PVE. High-risk patients, however, still might benefit from early surgery.
Citation: Lalani T, Chu VH, Park LP, et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med. 2013:173:1495-1504.
Clinical question: Is early surgery associated with better survival in patients with prosthetic valve endocarditis (PVE)?
Background: PVE occurs in 3% to 6% of patients within five years of valve implantation. Consensus guidelines, based on expert opinion, recommend surgical intervention with debridement and valve replacement in PVE, especially for patients with complications that are unlikely to be successfully treated with medical therapy, such as valve dysfunction, heart failure, and cardiac abscesses. Studies comparing survival with medical therapy versus surgery have reported conflicting results.
Study Design: Multi-center, prospective, cohort study.
Setting: International, multi-center cohort of patients from tertiary care hospitals.
Synopsis: The International Collaboration on Endocarditis—Prospective Cohort Study (ICE-PCS) cohort consisted of 1025 patients with PVE, 490 of whom underwent early surgery and 535 of whom received medical therapy alone.
Unadjusted initial analysis showed early surgery was associated with lower mortality; however, this survival benefit was not evident after the data was adjusted for treatment selection bias and survivor bias for in-hospital mortality and one-year mortality. The hazard ratios were 0.9 (95% CI 0.76 -1.07) and 1.04 (95% CI 0.89 -1.23), respectively.
Subgroup analysis indicated that early surgery in patients with high-risk features was associated with fewer poor outcomes compared to medical therapy: 28% versus 50% (P=.007)
Bottom line: Early surgery may not be associated with mortality benefits for PVE. High-risk patients, however, still might benefit from early surgery.
Citation: Lalani T, Chu VH, Park LP, et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med. 2013:173:1495-1504.
Clinical question: Is early surgery associated with better survival in patients with prosthetic valve endocarditis (PVE)?
Background: PVE occurs in 3% to 6% of patients within five years of valve implantation. Consensus guidelines, based on expert opinion, recommend surgical intervention with debridement and valve replacement in PVE, especially for patients with complications that are unlikely to be successfully treated with medical therapy, such as valve dysfunction, heart failure, and cardiac abscesses. Studies comparing survival with medical therapy versus surgery have reported conflicting results.
Study Design: Multi-center, prospective, cohort study.
Setting: International, multi-center cohort of patients from tertiary care hospitals.
Synopsis: The International Collaboration on Endocarditis—Prospective Cohort Study (ICE-PCS) cohort consisted of 1025 patients with PVE, 490 of whom underwent early surgery and 535 of whom received medical therapy alone.
Unadjusted initial analysis showed early surgery was associated with lower mortality; however, this survival benefit was not evident after the data was adjusted for treatment selection bias and survivor bias for in-hospital mortality and one-year mortality. The hazard ratios were 0.9 (95% CI 0.76 -1.07) and 1.04 (95% CI 0.89 -1.23), respectively.
Subgroup analysis indicated that early surgery in patients with high-risk features was associated with fewer poor outcomes compared to medical therapy: 28% versus 50% (P=.007)
Bottom line: Early surgery may not be associated with mortality benefits for PVE. High-risk patients, however, still might benefit from early surgery.
Citation: Lalani T, Chu VH, Park LP, et al. In-hospital and 1-year mortality in patients undergoing early surgery for prosthetic valve endocarditis. JAMA Intern Med. 2013:173:1495-1504.
Hospital Strategies for Decreasing Readmissions for Heart Failure Patients
Clinical question: What steps can hospitals take to reduce readmission rates in patients with heart failure?
Background: Evidence about various hospital strategies to decrease readmissions in patients with heart failure is limited.
Study Design: Cross-sectional study using a web-based survey.
Setting: Survey of 599 hospitals participating in quality initiatives to reduce readmissions.
Synopsis: Readmission of patients with heart failure is common and costly. Hospitals with high readmissions can lose up to 3% of their Medicare reimbursements by 2015.
This study found six strategies associated with lower risk-standardized 30-day readmission rates.
- Partnering with community physicians and physician groups (0.33%; P=0.017);
- Partnering with local hospitals (0.34%; P=0.020);
- Having nurses responsible for medication reconciliation (0.18%; P=0.002);
- Arranging follow-up visit before discharge (0.19%; P=0.037);
- Having a process in place to send all discharge summaries directly to the patient’s primary care physician (0.21%; P=0.004); and
- Assigning staff to follow up on test results after the patient is discharged (0.26%; P=0.049).
Individually, the magnitude of the effects was modest, but implementing multiple strategies was more beneficial (0.34% additional benefit for each additional strategy). Only 7% of the hospitals surveyed implemented all six strategies, highlighting substantial opportunities for improvement.
Bottom line: Implementing multiple strategies may help reduce readmission in patients with heart failure.
Citation: Bradley EH, Curry L, Horwitz LI, et al. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6:444-450.
Clinical question: What steps can hospitals take to reduce readmission rates in patients with heart failure?
Background: Evidence about various hospital strategies to decrease readmissions in patients with heart failure is limited.
Study Design: Cross-sectional study using a web-based survey.
Setting: Survey of 599 hospitals participating in quality initiatives to reduce readmissions.
Synopsis: Readmission of patients with heart failure is common and costly. Hospitals with high readmissions can lose up to 3% of their Medicare reimbursements by 2015.
This study found six strategies associated with lower risk-standardized 30-day readmission rates.
- Partnering with community physicians and physician groups (0.33%; P=0.017);
- Partnering with local hospitals (0.34%; P=0.020);
- Having nurses responsible for medication reconciliation (0.18%; P=0.002);
- Arranging follow-up visit before discharge (0.19%; P=0.037);
- Having a process in place to send all discharge summaries directly to the patient’s primary care physician (0.21%; P=0.004); and
- Assigning staff to follow up on test results after the patient is discharged (0.26%; P=0.049).
Individually, the magnitude of the effects was modest, but implementing multiple strategies was more beneficial (0.34% additional benefit for each additional strategy). Only 7% of the hospitals surveyed implemented all six strategies, highlighting substantial opportunities for improvement.
Bottom line: Implementing multiple strategies may help reduce readmission in patients with heart failure.
Citation: Bradley EH, Curry L, Horwitz LI, et al. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6:444-450.
Clinical question: What steps can hospitals take to reduce readmission rates in patients with heart failure?
Background: Evidence about various hospital strategies to decrease readmissions in patients with heart failure is limited.
Study Design: Cross-sectional study using a web-based survey.
Setting: Survey of 599 hospitals participating in quality initiatives to reduce readmissions.
Synopsis: Readmission of patients with heart failure is common and costly. Hospitals with high readmissions can lose up to 3% of their Medicare reimbursements by 2015.
This study found six strategies associated with lower risk-standardized 30-day readmission rates.
- Partnering with community physicians and physician groups (0.33%; P=0.017);
- Partnering with local hospitals (0.34%; P=0.020);
- Having nurses responsible for medication reconciliation (0.18%; P=0.002);
- Arranging follow-up visit before discharge (0.19%; P=0.037);
- Having a process in place to send all discharge summaries directly to the patient’s primary care physician (0.21%; P=0.004); and
- Assigning staff to follow up on test results after the patient is discharged (0.26%; P=0.049).
Individually, the magnitude of the effects was modest, but implementing multiple strategies was more beneficial (0.34% additional benefit for each additional strategy). Only 7% of the hospitals surveyed implemented all six strategies, highlighting substantial opportunities for improvement.
Bottom line: Implementing multiple strategies may help reduce readmission in patients with heart failure.
Citation: Bradley EH, Curry L, Horwitz LI, et al. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6:444-450.
If Delivered Systematically, In-Hospital Smoking Cessation Strategies Are Effective
Clinical question: Do programs that systematically provide smoking cessation support to admitted patients improve smoking cessation rates?
Background: Hospitalization is a good setting for initiation of smoking cessation. It is well known that conventional behavioral and pharmacotherapy interventions are effective. Intensive behavioral intervention provided to willing hospitalized patients is known to be useful; however, there is no established systematic delivery of these interventions.
Study design: Open, cluster-randomized, controlled trial.
Setting: Acute medical wards in a large teaching hospital in the United Kingdom.
Synopsis: More than 1,000 patients admitted between October 2010 and August 2011 were eligible for the study, of which 264 were included in the intervention and 229 in the usual care group (determination of smoking status and non-obligatory offer of cessation support). All of those in intervention received advice to quit smoking, compared to only 46% in the usual care group. Four-week smoking cessation was achieved by 38% of patients from the intervention group, compared to 17% from the usual care group. Secondary outcomes (use of behavioral cessation support, pharmacotherapy, and referral to and use of the local stop smoking service) were all significantly higher in the intervention group compared to the usual care group (P<0.001 in all cases).
This study shows that simple measures, when systematically delivered, are effective in initiating smoking cessation.
Bottom line: In-hospital systematic delivery of smoking cessation strategies is effective.
Citation: Murray RL, Leonardi-Bee J, Marsh J, et al. Systematic identification and treatment of smokers by hospital based cessation practitioners in a secondary care setting: cluster randomised controlled trial. BMJ. 2013;347:f4004.
Clinical question: Do programs that systematically provide smoking cessation support to admitted patients improve smoking cessation rates?
Background: Hospitalization is a good setting for initiation of smoking cessation. It is well known that conventional behavioral and pharmacotherapy interventions are effective. Intensive behavioral intervention provided to willing hospitalized patients is known to be useful; however, there is no established systematic delivery of these interventions.
Study design: Open, cluster-randomized, controlled trial.
Setting: Acute medical wards in a large teaching hospital in the United Kingdom.
Synopsis: More than 1,000 patients admitted between October 2010 and August 2011 were eligible for the study, of which 264 were included in the intervention and 229 in the usual care group (determination of smoking status and non-obligatory offer of cessation support). All of those in intervention received advice to quit smoking, compared to only 46% in the usual care group. Four-week smoking cessation was achieved by 38% of patients from the intervention group, compared to 17% from the usual care group. Secondary outcomes (use of behavioral cessation support, pharmacotherapy, and referral to and use of the local stop smoking service) were all significantly higher in the intervention group compared to the usual care group (P<0.001 in all cases).
This study shows that simple measures, when systematically delivered, are effective in initiating smoking cessation.
Bottom line: In-hospital systematic delivery of smoking cessation strategies is effective.
Citation: Murray RL, Leonardi-Bee J, Marsh J, et al. Systematic identification and treatment of smokers by hospital based cessation practitioners in a secondary care setting: cluster randomised controlled trial. BMJ. 2013;347:f4004.
Clinical question: Do programs that systematically provide smoking cessation support to admitted patients improve smoking cessation rates?
Background: Hospitalization is a good setting for initiation of smoking cessation. It is well known that conventional behavioral and pharmacotherapy interventions are effective. Intensive behavioral intervention provided to willing hospitalized patients is known to be useful; however, there is no established systematic delivery of these interventions.
Study design: Open, cluster-randomized, controlled trial.
Setting: Acute medical wards in a large teaching hospital in the United Kingdom.
Synopsis: More than 1,000 patients admitted between October 2010 and August 2011 were eligible for the study, of which 264 were included in the intervention and 229 in the usual care group (determination of smoking status and non-obligatory offer of cessation support). All of those in intervention received advice to quit smoking, compared to only 46% in the usual care group. Four-week smoking cessation was achieved by 38% of patients from the intervention group, compared to 17% from the usual care group. Secondary outcomes (use of behavioral cessation support, pharmacotherapy, and referral to and use of the local stop smoking service) were all significantly higher in the intervention group compared to the usual care group (P<0.001 in all cases).
This study shows that simple measures, when systematically delivered, are effective in initiating smoking cessation.
Bottom line: In-hospital systematic delivery of smoking cessation strategies is effective.
Citation: Murray RL, Leonardi-Bee J, Marsh J, et al. Systematic identification and treatment of smokers by hospital based cessation practitioners in a secondary care setting: cluster randomised controlled trial. BMJ. 2013;347:f4004.