User login
Transforming vaginal hysterectomy: 7 solutions to the most daunting challenges
Vaginal hysterectomy is the preferred route to benign hysterectomy because it is associated with better outcomes and fewer complications than the laparoscopic and open abdominal approaches.1,2 Yet, despite superior patient outcomes and cost benefits, the rate of vaginal hysterectomy is declining.
According to the Nationwide Inpatient Sample, the use of vaginal hysterectomy declined from 24.8% in 1998 to 16.7% in 2010.3 In fact, more than 80% of surgeons in the United States now perform fewer than five vaginal procedures in a year.4
The increasing use of other minimally invasive routes, such as laparoscopy and robotics, indicates that most practicing surgeons and recent graduates are choosing these approaches over the vaginal route. In only 3 years, the rate of laparoscopy increased by 6% and robotics increased by almost 10%.3
Many surgeons assume that vaginal hysterectomy exists in a state of suspended animation, with nothing much changed in the way it has been performed over the past few decades. Further, vaginal surgery is difficult to teach and learn, given limitations in exposure and visualization, difficulty in securing hemostasis, and challenges in the removal of the large uterus and adnexae. As a result, vaginal hysterectomy often is thought, erroneously, to be indicated only in procedures involving a small and prolapsing uterus.
To increase the rate of vaginal hysterectomy, we can benefit from experience gained in laparoscopy and robotics—whether we are teachers or learners—while maintaining patient safety and containing costs.
In this article, I describe common challenges in vaginal hysterectomy and offer tools and techniques to overcome them:
- achieving and enhancing ergonomics, exposure, and visualization
- the need to work in a long vaginal vault
- the task of securing vascular and thick tissue pedicles when the introitus and vaginal vault are narrow.
The vaginal approach is less costly
Vaginal hysterectomy costs significantly less to perform than other approaches. At a tertiary referral center, vaginal hysterectomy costs approximately $7,000 to $18,000 per case less than laparoscopic, abdominal, and robotic hysterectomy.5 With declining use of vaginal hysterectomy and increasing use of more costly approaches, we face a health-care crisis.
Residents are inadequately trained to perform vaginal hysterectomy
Data reveal that not only are our recent graduates inadequately prepared to perform vaginal hysterectomy, but national health-care dollars and resources are depleted when surgeons choose to perform more costly approaches. As a result, many eligible patients end up deprived of the benefits of a single, concealed, and minimally invasive procedure.
The increase in laparoscopic and robotic approaches to hysterectomy has affected residency training. National case log reports from the Accreditation Council of Graduate Medical Education show that the number of vaginal hysterectomies performed by residents as “primary surgeons” decreased by 40%, from a mean of 35 cases in 2002 to 19 cases in 2012.6 A recent survey found that only 28% of graduating residents were “completely prepared” to perform a vaginal hysterectomy, compared with 58% for abdominal hysterectomy, 22% for laparoscopic hysterectomy, and 3% for the robotic approach.7
The rate of vaginal hysterectomy will continue to decline if we perform it in the same manner it was done 30 years ago. The current generation of practicing gynecologists and graduates is choosing to perform the procedure laparoscopically or robotically because of the advantages these technologies provide. It is time that we incorporate features from these minimally invasive approaches to streamline vaginal hysterectomy while maintaining patient safety and containing costs.
Challenges: Ergonomics, exposure, and visualization
In conventional vaginal surgery, the surgeon often is the person who has the best and, sometimes, the sole view. Two bedside assistants are required to hold retractors during the entire case, which can lead to fatigue and muscle strain. Poor lighting also can greatly limit visualization into the pelvic cavity.
Both laparoscopy and robotics provide a well-illuminated and magnified view, with three-dimensional images now available in both platforms. This view is projected to overhead monitors for the entire surgical team to see. Magnification of the pelvic anatomic structures and projection to an external monitor facilitate teaching and learning, better anticipation of the surgical and procedural needs, and overall patient safety.
From robotics, where ergonomics is exemplified, we also learn the importance of surgeon comfort during the procedure.
Solution #1: A self-retaining retractor
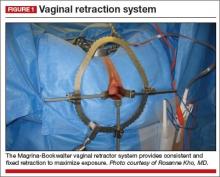
A self-retaining system such as the Magrina-Bookwalter vaginal retractor (Symmetry Surgical, Nashville, Tennessee) (FIGURE 1)
Solution #2: Seat the surgeon for an optimal view
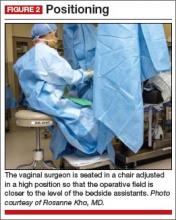
With the patient in the lithotomy position and her legs in candy cane stirrups, the surgeon can be seated on a high chair so that the operative field is at the approximate level of the assistants’ view (FIGURE 2)
Solution #3: Illuminate the cavity
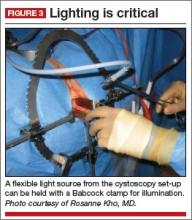
The deep pelvic cavity can be easily illuminated using a lighted suction tip, a flexible light source (as part of the cystoscopy set) held with a Babcock clamp (FIGURE 3), or a malleable illuminating mat taped to the retractor blades (such as Lightmat surgical illuminator, Lumitex, Inc., Strongsville, Ohio).
Solution #4: Project the image
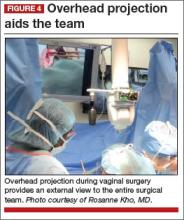
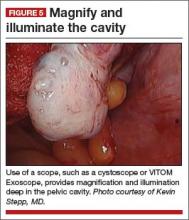
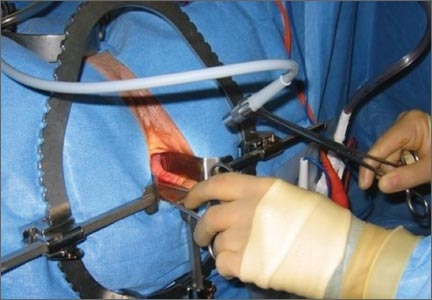
Cameras attached to an overhead boom or operating room light handles (FIGURE 4) and an external telescope with integrated illumination, such as a standard cystoscope or VITOM Exoscope (Karl Storz, El Segundo, California) (FIGURE 5) provide both magnification and projection of the procedure to an overhead monitor.
Glass technology (Google, Mountain View, California) also has been utilized in surgery and can be a good application of simultaneous projection and recording of the procedure to an external monitor (FIGURE 6). Google Glass is a wearable computer with an optical head-mounted display. The device, similar to eyeglasses, is voice-activated, thereby allowing the surgeon to record the procedure hands-free. Simultaneous projection to an external monitor allows the entire team in the operating room to be aware of the flow of the procedure.
Challenge: Working in a narrow vaginal vault
Without correct instrumentation, this challenge can be especially daunting. Laparoscopy and robotics have changed the way we perform pelvic surgery by providing advanced instrumentation.
Solution #5: Adapt your instruments
Modified vaginal instruments can be used to facilitate a case. Watch the accompanying VIDEO on the use of improved vaginal instruments during morcellation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
| Click to enlarge >>> |
Among the instruments adaptable for vaginal surgery:
- curving, articulating instruments
- long, curved, and rounded knife handles, which allow for better ergonomics during prolonged morcellation
- modified long retractors and use of a single long vaginal pack provide retraction of loops of bowel and easy access to secure pedicles deep in the pelvis.
All of these instruments are available through Marina Medical in Sunrise, Florida.
Challenge: Securing vascular and thick tissue pediclesA narrow introitus and vaginal vault can be difficult to manage during vaginal surgery. Another challenge is a uterus that is large or deformed by multiple fibroids.
Solution #6: Vaginal incision
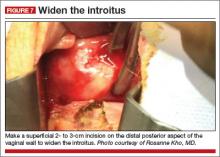
A simple superficial 2- to 3-cm incision on the distal posterior aspect of the vaginal wall can widen the introitus and vault to facilitate the procedure (FIGURE 7)
Solution #7: Vessel-sealing tools
The use of energy is integral to laparoscopy and robotics for dissection and securing vessels. In a meta-analysis that included seven randomized controlled trials, advanced vessel-sealing devices proved useful in vaginal surgery by decreasing blood loss and operative time.8
In the setting of a difficult vaginal hysterectomy with a narrow introitus and large uterus, the use of vessel-sealing technology allows the surgeon to skeletonize the uterine arteries while allowing progressive descensus to secure the upper pedicles.
In my experience, the use of an advanced vessel-sealing device, compared with traditional clamp-cut-tying technique, facilitated successful completion of vaginal hysterectomy in 650 patients with relative contraindications to the vaginal approach, such as nulliparity, a uterus weighing more than 250 g, and a history of cesarean delivery (Mayo Clinic data; yet unpublished).
We must change with the times
The rate of vaginal hysterectomy will continue to decline unless we modify our technique to incorporate new technology. The current generation of practicing gynecologists and recent graduates are choosing the laparoscopic and robotic approaches because of the advantages these technologies offer. It is time we incorporate relevant features from these minimally invasive approaches while maintaining patient safety and containing costs by performing vaginal hysterectomy whenever possible. A willingness to change and ability to think outside the usual box will help us train new generations of vaginal surgeons who can bring back vaginal hysterectomy as the preferred route to the benign hysterectomy.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
3. Wright T, Herzog T, Tsul J, et al. Nationwide trends in inpatient hysterectomy in the United States. Obstet Gynecol. 2013:122(2):233–241.
4. Rogo-Gupta L, Lewyn S, Jum JH, et al. Effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
5. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
6. Washburn EE, Cohen SL, Manoucherie E, Zurawin, RJ, Einarsson JI. Trends in reported residency surgical experience in hysterectomy [published online ahead of print June 4, 2014]. J Minim Invasive Gynecol. doi:10.1016/j.jmig.2014.05.005.
7. Burkett D, Horwitz J, Kennedy V, et al. Assessing current trends in resident hysterectomy training. Female Pelvic Med Reconstr Surg. 2011;17(5):210–214.
8. Kroft J, Selk K. Energy-based vessel sealing in vaginal hysterectomy. A systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
Vaginal hysterectomy is the preferred route to benign hysterectomy because it is associated with better outcomes and fewer complications than the laparoscopic and open abdominal approaches.1,2 Yet, despite superior patient outcomes and cost benefits, the rate of vaginal hysterectomy is declining.
According to the Nationwide Inpatient Sample, the use of vaginal hysterectomy declined from 24.8% in 1998 to 16.7% in 2010.3 In fact, more than 80% of surgeons in the United States now perform fewer than five vaginal procedures in a year.4
The increasing use of other minimally invasive routes, such as laparoscopy and robotics, indicates that most practicing surgeons and recent graduates are choosing these approaches over the vaginal route. In only 3 years, the rate of laparoscopy increased by 6% and robotics increased by almost 10%.3
Many surgeons assume that vaginal hysterectomy exists in a state of suspended animation, with nothing much changed in the way it has been performed over the past few decades. Further, vaginal surgery is difficult to teach and learn, given limitations in exposure and visualization, difficulty in securing hemostasis, and challenges in the removal of the large uterus and adnexae. As a result, vaginal hysterectomy often is thought, erroneously, to be indicated only in procedures involving a small and prolapsing uterus.
To increase the rate of vaginal hysterectomy, we can benefit from experience gained in laparoscopy and robotics—whether we are teachers or learners—while maintaining patient safety and containing costs.
In this article, I describe common challenges in vaginal hysterectomy and offer tools and techniques to overcome them:
- achieving and enhancing ergonomics, exposure, and visualization
- the need to work in a long vaginal vault
- the task of securing vascular and thick tissue pedicles when the introitus and vaginal vault are narrow.
The vaginal approach is less costly
Vaginal hysterectomy costs significantly less to perform than other approaches. At a tertiary referral center, vaginal hysterectomy costs approximately $7,000 to $18,000 per case less than laparoscopic, abdominal, and robotic hysterectomy.5 With declining use of vaginal hysterectomy and increasing use of more costly approaches, we face a health-care crisis.
Residents are inadequately trained to perform vaginal hysterectomy
Data reveal that not only are our recent graduates inadequately prepared to perform vaginal hysterectomy, but national health-care dollars and resources are depleted when surgeons choose to perform more costly approaches. As a result, many eligible patients end up deprived of the benefits of a single, concealed, and minimally invasive procedure.
The increase in laparoscopic and robotic approaches to hysterectomy has affected residency training. National case log reports from the Accreditation Council of Graduate Medical Education show that the number of vaginal hysterectomies performed by residents as “primary surgeons” decreased by 40%, from a mean of 35 cases in 2002 to 19 cases in 2012.6 A recent survey found that only 28% of graduating residents were “completely prepared” to perform a vaginal hysterectomy, compared with 58% for abdominal hysterectomy, 22% for laparoscopic hysterectomy, and 3% for the robotic approach.7
The rate of vaginal hysterectomy will continue to decline if we perform it in the same manner it was done 30 years ago. The current generation of practicing gynecologists and graduates is choosing to perform the procedure laparoscopically or robotically because of the advantages these technologies provide. It is time that we incorporate features from these minimally invasive approaches to streamline vaginal hysterectomy while maintaining patient safety and containing costs.
Challenges: Ergonomics, exposure, and visualization
In conventional vaginal surgery, the surgeon often is the person who has the best and, sometimes, the sole view. Two bedside assistants are required to hold retractors during the entire case, which can lead to fatigue and muscle strain. Poor lighting also can greatly limit visualization into the pelvic cavity.
Both laparoscopy and robotics provide a well-illuminated and magnified view, with three-dimensional images now available in both platforms. This view is projected to overhead monitors for the entire surgical team to see. Magnification of the pelvic anatomic structures and projection to an external monitor facilitate teaching and learning, better anticipation of the surgical and procedural needs, and overall patient safety.
From robotics, where ergonomics is exemplified, we also learn the importance of surgeon comfort during the procedure.
Solution #1: A self-retaining retractor
A self-retaining system such as the Magrina-Bookwalter vaginal retractor (Symmetry Surgical, Nashville, Tennessee) (FIGURE 1)
Solution #2: Seat the surgeon for an optimal view
With the patient in the lithotomy position and her legs in candy cane stirrups, the surgeon can be seated on a high chair so that the operative field is at the approximate level of the assistants’ view (FIGURE 2)
Solution #3: Illuminate the cavity
The deep pelvic cavity can be easily illuminated using a lighted suction tip, a flexible light source (as part of the cystoscopy set) held with a Babcock clamp (FIGURE 3), or a malleable illuminating mat taped to the retractor blades (such as Lightmat surgical illuminator, Lumitex, Inc., Strongsville, Ohio).
Solution #4: Project the image
Cameras attached to an overhead boom or operating room light handles (FIGURE 4) and an external telescope with integrated illumination, such as a standard cystoscope or VITOM Exoscope (Karl Storz, El Segundo, California) (FIGURE 5) provide both magnification and projection of the procedure to an overhead monitor.
Glass technology (Google, Mountain View, California) also has been utilized in surgery and can be a good application of simultaneous projection and recording of the procedure to an external monitor (FIGURE 6). Google Glass is a wearable computer with an optical head-mounted display. The device, similar to eyeglasses, is voice-activated, thereby allowing the surgeon to record the procedure hands-free. Simultaneous projection to an external monitor allows the entire team in the operating room to be aware of the flow of the procedure.
Challenge: Working in a narrow vaginal vault
Without correct instrumentation, this challenge can be especially daunting. Laparoscopy and robotics have changed the way we perform pelvic surgery by providing advanced instrumentation.
Solution #5: Adapt your instruments
Modified vaginal instruments can be used to facilitate a case. Watch the accompanying VIDEO on the use of improved vaginal instruments during morcellation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
| Click to enlarge >>> |
Among the instruments adaptable for vaginal surgery:
- curving, articulating instruments
- long, curved, and rounded knife handles, which allow for better ergonomics during prolonged morcellation
- modified long retractors and use of a single long vaginal pack provide retraction of loops of bowel and easy access to secure pedicles deep in the pelvis.
All of these instruments are available through Marina Medical in Sunrise, Florida.
Challenge: Securing vascular and thick tissue pediclesA narrow introitus and vaginal vault can be difficult to manage during vaginal surgery. Another challenge is a uterus that is large or deformed by multiple fibroids.
Solution #6: Vaginal incision
A simple superficial 2- to 3-cm incision on the distal posterior aspect of the vaginal wall can widen the introitus and vault to facilitate the procedure (FIGURE 7)
Solution #7: Vessel-sealing tools
The use of energy is integral to laparoscopy and robotics for dissection and securing vessels. In a meta-analysis that included seven randomized controlled trials, advanced vessel-sealing devices proved useful in vaginal surgery by decreasing blood loss and operative time.8
In the setting of a difficult vaginal hysterectomy with a narrow introitus and large uterus, the use of vessel-sealing technology allows the surgeon to skeletonize the uterine arteries while allowing progressive descensus to secure the upper pedicles.
In my experience, the use of an advanced vessel-sealing device, compared with traditional clamp-cut-tying technique, facilitated successful completion of vaginal hysterectomy in 650 patients with relative contraindications to the vaginal approach, such as nulliparity, a uterus weighing more than 250 g, and a history of cesarean delivery (Mayo Clinic data; yet unpublished).
We must change with the times
The rate of vaginal hysterectomy will continue to decline unless we modify our technique to incorporate new technology. The current generation of practicing gynecologists and recent graduates are choosing the laparoscopic and robotic approaches because of the advantages these technologies offer. It is time we incorporate relevant features from these minimally invasive approaches while maintaining patient safety and containing costs by performing vaginal hysterectomy whenever possible. A willingness to change and ability to think outside the usual box will help us train new generations of vaginal surgeons who can bring back vaginal hysterectomy as the preferred route to the benign hysterectomy.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
Vaginal hysterectomy is the preferred route to benign hysterectomy because it is associated with better outcomes and fewer complications than the laparoscopic and open abdominal approaches.1,2 Yet, despite superior patient outcomes and cost benefits, the rate of vaginal hysterectomy is declining.
According to the Nationwide Inpatient Sample, the use of vaginal hysterectomy declined from 24.8% in 1998 to 16.7% in 2010.3 In fact, more than 80% of surgeons in the United States now perform fewer than five vaginal procedures in a year.4
The increasing use of other minimally invasive routes, such as laparoscopy and robotics, indicates that most practicing surgeons and recent graduates are choosing these approaches over the vaginal route. In only 3 years, the rate of laparoscopy increased by 6% and robotics increased by almost 10%.3
Many surgeons assume that vaginal hysterectomy exists in a state of suspended animation, with nothing much changed in the way it has been performed over the past few decades. Further, vaginal surgery is difficult to teach and learn, given limitations in exposure and visualization, difficulty in securing hemostasis, and challenges in the removal of the large uterus and adnexae. As a result, vaginal hysterectomy often is thought, erroneously, to be indicated only in procedures involving a small and prolapsing uterus.
To increase the rate of vaginal hysterectomy, we can benefit from experience gained in laparoscopy and robotics—whether we are teachers or learners—while maintaining patient safety and containing costs.
In this article, I describe common challenges in vaginal hysterectomy and offer tools and techniques to overcome them:
- achieving and enhancing ergonomics, exposure, and visualization
- the need to work in a long vaginal vault
- the task of securing vascular and thick tissue pedicles when the introitus and vaginal vault are narrow.
The vaginal approach is less costly
Vaginal hysterectomy costs significantly less to perform than other approaches. At a tertiary referral center, vaginal hysterectomy costs approximately $7,000 to $18,000 per case less than laparoscopic, abdominal, and robotic hysterectomy.5 With declining use of vaginal hysterectomy and increasing use of more costly approaches, we face a health-care crisis.
Residents are inadequately trained to perform vaginal hysterectomy
Data reveal that not only are our recent graduates inadequately prepared to perform vaginal hysterectomy, but national health-care dollars and resources are depleted when surgeons choose to perform more costly approaches. As a result, many eligible patients end up deprived of the benefits of a single, concealed, and minimally invasive procedure.
The increase in laparoscopic and robotic approaches to hysterectomy has affected residency training. National case log reports from the Accreditation Council of Graduate Medical Education show that the number of vaginal hysterectomies performed by residents as “primary surgeons” decreased by 40%, from a mean of 35 cases in 2002 to 19 cases in 2012.6 A recent survey found that only 28% of graduating residents were “completely prepared” to perform a vaginal hysterectomy, compared with 58% for abdominal hysterectomy, 22% for laparoscopic hysterectomy, and 3% for the robotic approach.7
The rate of vaginal hysterectomy will continue to decline if we perform it in the same manner it was done 30 years ago. The current generation of practicing gynecologists and graduates is choosing to perform the procedure laparoscopically or robotically because of the advantages these technologies provide. It is time that we incorporate features from these minimally invasive approaches to streamline vaginal hysterectomy while maintaining patient safety and containing costs.
Challenges: Ergonomics, exposure, and visualization
In conventional vaginal surgery, the surgeon often is the person who has the best and, sometimes, the sole view. Two bedside assistants are required to hold retractors during the entire case, which can lead to fatigue and muscle strain. Poor lighting also can greatly limit visualization into the pelvic cavity.
Both laparoscopy and robotics provide a well-illuminated and magnified view, with three-dimensional images now available in both platforms. This view is projected to overhead monitors for the entire surgical team to see. Magnification of the pelvic anatomic structures and projection to an external monitor facilitate teaching and learning, better anticipation of the surgical and procedural needs, and overall patient safety.
From robotics, where ergonomics is exemplified, we also learn the importance of surgeon comfort during the procedure.
Solution #1: A self-retaining retractor
A self-retaining system such as the Magrina-Bookwalter vaginal retractor (Symmetry Surgical, Nashville, Tennessee) (FIGURE 1)
Solution #2: Seat the surgeon for an optimal view
With the patient in the lithotomy position and her legs in candy cane stirrups, the surgeon can be seated on a high chair so that the operative field is at the approximate level of the assistants’ view (FIGURE 2)
Solution #3: Illuminate the cavity
The deep pelvic cavity can be easily illuminated using a lighted suction tip, a flexible light source (as part of the cystoscopy set) held with a Babcock clamp (FIGURE 3), or a malleable illuminating mat taped to the retractor blades (such as Lightmat surgical illuminator, Lumitex, Inc., Strongsville, Ohio).
Solution #4: Project the image
Cameras attached to an overhead boom or operating room light handles (FIGURE 4) and an external telescope with integrated illumination, such as a standard cystoscope or VITOM Exoscope (Karl Storz, El Segundo, California) (FIGURE 5) provide both magnification and projection of the procedure to an overhead monitor.
Glass technology (Google, Mountain View, California) also has been utilized in surgery and can be a good application of simultaneous projection and recording of the procedure to an external monitor (FIGURE 6). Google Glass is a wearable computer with an optical head-mounted display. The device, similar to eyeglasses, is voice-activated, thereby allowing the surgeon to record the procedure hands-free. Simultaneous projection to an external monitor allows the entire team in the operating room to be aware of the flow of the procedure.
Challenge: Working in a narrow vaginal vault
Without correct instrumentation, this challenge can be especially daunting. Laparoscopy and robotics have changed the way we perform pelvic surgery by providing advanced instrumentation.
Solution #5: Adapt your instruments
Modified vaginal instruments can be used to facilitate a case. Watch the accompanying VIDEO on the use of improved vaginal instruments during morcellation.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel |
| Click to enlarge >>> |
Among the instruments adaptable for vaginal surgery:
- curving, articulating instruments
- long, curved, and rounded knife handles, which allow for better ergonomics during prolonged morcellation
- modified long retractors and use of a single long vaginal pack provide retraction of loops of bowel and easy access to secure pedicles deep in the pelvis.
All of these instruments are available through Marina Medical in Sunrise, Florida.
Challenge: Securing vascular and thick tissue pediclesA narrow introitus and vaginal vault can be difficult to manage during vaginal surgery. Another challenge is a uterus that is large or deformed by multiple fibroids.
Solution #6: Vaginal incision
A simple superficial 2- to 3-cm incision on the distal posterior aspect of the vaginal wall can widen the introitus and vault to facilitate the procedure (FIGURE 7)
Solution #7: Vessel-sealing tools
The use of energy is integral to laparoscopy and robotics for dissection and securing vessels. In a meta-analysis that included seven randomized controlled trials, advanced vessel-sealing devices proved useful in vaginal surgery by decreasing blood loss and operative time.8
In the setting of a difficult vaginal hysterectomy with a narrow introitus and large uterus, the use of vessel-sealing technology allows the surgeon to skeletonize the uterine arteries while allowing progressive descensus to secure the upper pedicles.
In my experience, the use of an advanced vessel-sealing device, compared with traditional clamp-cut-tying technique, facilitated successful completion of vaginal hysterectomy in 650 patients with relative contraindications to the vaginal approach, such as nulliparity, a uterus weighing more than 250 g, and a history of cesarean delivery (Mayo Clinic data; yet unpublished).
We must change with the times
The rate of vaginal hysterectomy will continue to decline unless we modify our technique to incorporate new technology. The current generation of practicing gynecologists and recent graduates are choosing the laparoscopic and robotic approaches because of the advantages these technologies offer. It is time we incorporate relevant features from these minimally invasive approaches while maintaining patient safety and containing costs by performing vaginal hysterectomy whenever possible. A willingness to change and ability to think outside the usual box will help us train new generations of vaginal surgeons who can bring back vaginal hysterectomy as the preferred route to the benign hysterectomy.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
3. Wright T, Herzog T, Tsul J, et al. Nationwide trends in inpatient hysterectomy in the United States. Obstet Gynecol. 2013:122(2):233–241.
4. Rogo-Gupta L, Lewyn S, Jum JH, et al. Effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
5. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
6. Washburn EE, Cohen SL, Manoucherie E, Zurawin, RJ, Einarsson JI. Trends in reported residency surgical experience in hysterectomy [published online ahead of print June 4, 2014]. J Minim Invasive Gynecol. doi:10.1016/j.jmig.2014.05.005.
7. Burkett D, Horwitz J, Kennedy V, et al. Assessing current trends in resident hysterectomy training. Female Pelvic Med Reconstr Surg. 2011;17(5):210–214.
8. Kroft J, Selk K. Energy-based vessel sealing in vaginal hysterectomy. A systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2009;(3):CD003677.
2. American College of Obstetricians and Gynecologists. ACOG Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
3. Wright T, Herzog T, Tsul J, et al. Nationwide trends in inpatient hysterectomy in the United States. Obstet Gynecol. 2013:122(2):233–241.
4. Rogo-Gupta L, Lewyn S, Jum JH, et al. Effect of surgeon volume on outcomes and resource use for vaginal hysterectomy. Obstet Gynecol. 2010;116(6):1341–1347.
5. Wright KN, Jonsdottir GM, Jorgensen S, Shah N, Einarsson JI. Costs and outcomes of abdominal, vaginal, laparoscopic and robotic hysterectomies. JSLS. 2012;16(4):519–524.
6. Washburn EE, Cohen SL, Manoucherie E, Zurawin, RJ, Einarsson JI. Trends in reported residency surgical experience in hysterectomy [published online ahead of print June 4, 2014]. J Minim Invasive Gynecol. doi:10.1016/j.jmig.2014.05.005.
7. Burkett D, Horwitz J, Kennedy V, et al. Assessing current trends in resident hysterectomy training. Female Pelvic Med Reconstr Surg. 2011;17(5):210–214.
8. Kroft J, Selk K. Energy-based vessel sealing in vaginal hysterectomy. A systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1127–1136.
![]()
Dr. Kho presents vaginal morcellation by hand, using advanced instrumentation
Does prenatal acetaminophen exposure increase the risk of behavioral problems in the child?
In the continuing drive to determine the cause of neurobehavioral complications, particularly ADHD and HKD, a number of studies have reported associations with various substances. These include pesticides,1 hormones,2 hormone disrupters,1 and, possibly, genetics. Nevertheless, the etiology of these disorders remains a mystery. ADHD is a complex and heterogeneous disorder. Although we do not yet understand the cause, genetics (or, more accurately, pharmacogenetics) seems likely to play a role.
This study from a large Danish population appears to suggest that the prenatal use of acetaminophen may increase the risk of ADHD and HKD. It is yet another study in which the data indicate and the authors claim that use of a particular drug during pregnancy is responsible for this condition. However, despite the extremely large sample size (which increases the likelihood of positive findings), the hazard ratios were only marginally significant, suggesting that the relevance of the conclusions is questionable.
Details of the study
The 64,322 live-born children and mothers in the Danish National Birth Cohort from 1996 to 2002 were evaluated three ways:
- through parental reports of behavioral problems in children at age 7 using the Strengths and Difficulties Questionnaire
- through retrieval of HKD diagnoses from the Danish National Hospital Registry or the Danish Psychiatric Central Registry prior to 2011
- through prescriptions for ADHD (primarily methylphenidate [Ritalin]) for children from the Danish Prescription Registry.
Liew and colleagues then estimated hazard ratios for receiving a diagnosis of HKD or using a medication for ADHD, as well as risk ratios for behavioral problems in children after prenatal exposure to acetaminophen.
Stronger associations between prenatal acetaminophen use and HKD or ADHD were found when the mother used the medication in more than one trimester. Exposure-response trends increased with the frequency of acetaminophen use during pregnancy for all three outcomes (HKD diagnosis, ADHD-like behaviors, and ADHD medication use; P trend <.001). Results did not appear to be confounded by maternal inflammation, infection during pregnancy, or the mother’s mental health status.
Related articles:
• How can pregnant women safely relieve low-back pain? Roselyn Jan W. Clemente-Fuentes, MD; Heather Pickett, DO; Misty Carney, MLIS; and Paul Crawford, MD (January 2014)
• Perinatal depression: What you can do to reduce its long-term effects. Janelle Yates (February 2014)
• Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”? John T. Repke, MD (Guest Editorial; June 2014)
Why these findings are less than compelling
Acetaminophen is the most commonly used medication during pregnancy, although few investigators have analyzed neurobehavioral complications in children exposed to this drug in utero. Another recent epidemiologic study from Norway also suggests that long-term exposure (>28 days) to acetaminophen increases the risk of poor gross motor functioning, poor communication skills, and externalizing and internalizing behavior problems.3
The rationale behind an association between acetaminophen and ADHD and HKD is that the medication is an endocrine-disrupting agent. The evidence of this status comes primarily from in vitro experiments from one group of researchers, which may not represent in vivo conditions.4,5
Epidemiologic studies frequently are confounded by poor design and methodology. It also should be noted that correlation is not necessarily the same as causation. In this study, the design and methodology were appropriate considering the data available. Researchers often use large databases like this to research “hot topics” such as the association between ADHD and prenatal acetaminophen use. In this study, acetaminophen cannot be associated definitively with an increased risk of ADHD or HKD. Further research is needed, with greater attention to possible confounding factors, such as why these women consumed chronic doses and for what conditions.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
For the time being, you should probably counsel your patients to use acetaminophen sparingly during pregnancy, and certainly not on a daily basis. We also should encourage nonpharmacologic pain management, such as cognitive behavioral therapy, when appropriate, and caution patients against long-term use of analgesics, when possible, during gestation and lactation.
Thomas W. Hale, RPH, PhD; Aarienne Einarson, RN; and Teresa Baker, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Kajta M, Wojtowicz AK. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol Rep. 2013;65(6):1632–1639.
2. de Bruin EI, Verheij F, Wiegman T, Ferdinand RF. Differences in finger length ratio between males with autism, pervasive developmental disorder–not otherwise specified, ADHD, and anxiety disorders. Dev Med Child Neurol. 2006;48(12):962–965.
3. Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: A sibling-controlled cohort study. Int J Epidemiol. 2013;42(6):1702–1713.
4. Kristensen DM, Lesne L, Le Fol V, et al. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid), and indomethacin are anti-androgenic in the rat foetal testis. Int J Androl. 2012;35(3):377–384.
5. Albert O, Desdoits-Lethimonier C, Lesne L, et al. Paracetamol, aspirin, and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod. 2013;28(7):1890–1898.
In the continuing drive to determine the cause of neurobehavioral complications, particularly ADHD and HKD, a number of studies have reported associations with various substances. These include pesticides,1 hormones,2 hormone disrupters,1 and, possibly, genetics. Nevertheless, the etiology of these disorders remains a mystery. ADHD is a complex and heterogeneous disorder. Although we do not yet understand the cause, genetics (or, more accurately, pharmacogenetics) seems likely to play a role.
This study from a large Danish population appears to suggest that the prenatal use of acetaminophen may increase the risk of ADHD and HKD. It is yet another study in which the data indicate and the authors claim that use of a particular drug during pregnancy is responsible for this condition. However, despite the extremely large sample size (which increases the likelihood of positive findings), the hazard ratios were only marginally significant, suggesting that the relevance of the conclusions is questionable.
Details of the study
The 64,322 live-born children and mothers in the Danish National Birth Cohort from 1996 to 2002 were evaluated three ways:
- through parental reports of behavioral problems in children at age 7 using the Strengths and Difficulties Questionnaire
- through retrieval of HKD diagnoses from the Danish National Hospital Registry or the Danish Psychiatric Central Registry prior to 2011
- through prescriptions for ADHD (primarily methylphenidate [Ritalin]) for children from the Danish Prescription Registry.
Liew and colleagues then estimated hazard ratios for receiving a diagnosis of HKD or using a medication for ADHD, as well as risk ratios for behavioral problems in children after prenatal exposure to acetaminophen.
Stronger associations between prenatal acetaminophen use and HKD or ADHD were found when the mother used the medication in more than one trimester. Exposure-response trends increased with the frequency of acetaminophen use during pregnancy for all three outcomes (HKD diagnosis, ADHD-like behaviors, and ADHD medication use; P trend <.001). Results did not appear to be confounded by maternal inflammation, infection during pregnancy, or the mother’s mental health status.
Related articles:
• How can pregnant women safely relieve low-back pain? Roselyn Jan W. Clemente-Fuentes, MD; Heather Pickett, DO; Misty Carney, MLIS; and Paul Crawford, MD (January 2014)
• Perinatal depression: What you can do to reduce its long-term effects. Janelle Yates (February 2014)
• Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”? John T. Repke, MD (Guest Editorial; June 2014)
Why these findings are less than compelling
Acetaminophen is the most commonly used medication during pregnancy, although few investigators have analyzed neurobehavioral complications in children exposed to this drug in utero. Another recent epidemiologic study from Norway also suggests that long-term exposure (>28 days) to acetaminophen increases the risk of poor gross motor functioning, poor communication skills, and externalizing and internalizing behavior problems.3
The rationale behind an association between acetaminophen and ADHD and HKD is that the medication is an endocrine-disrupting agent. The evidence of this status comes primarily from in vitro experiments from one group of researchers, which may not represent in vivo conditions.4,5
Epidemiologic studies frequently are confounded by poor design and methodology. It also should be noted that correlation is not necessarily the same as causation. In this study, the design and methodology were appropriate considering the data available. Researchers often use large databases like this to research “hot topics” such as the association between ADHD and prenatal acetaminophen use. In this study, acetaminophen cannot be associated definitively with an increased risk of ADHD or HKD. Further research is needed, with greater attention to possible confounding factors, such as why these women consumed chronic doses and for what conditions.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
For the time being, you should probably counsel your patients to use acetaminophen sparingly during pregnancy, and certainly not on a daily basis. We also should encourage nonpharmacologic pain management, such as cognitive behavioral therapy, when appropriate, and caution patients against long-term use of analgesics, when possible, during gestation and lactation.
Thomas W. Hale, RPH, PhD; Aarienne Einarson, RN; and Teresa Baker, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
In the continuing drive to determine the cause of neurobehavioral complications, particularly ADHD and HKD, a number of studies have reported associations with various substances. These include pesticides,1 hormones,2 hormone disrupters,1 and, possibly, genetics. Nevertheless, the etiology of these disorders remains a mystery. ADHD is a complex and heterogeneous disorder. Although we do not yet understand the cause, genetics (or, more accurately, pharmacogenetics) seems likely to play a role.
This study from a large Danish population appears to suggest that the prenatal use of acetaminophen may increase the risk of ADHD and HKD. It is yet another study in which the data indicate and the authors claim that use of a particular drug during pregnancy is responsible for this condition. However, despite the extremely large sample size (which increases the likelihood of positive findings), the hazard ratios were only marginally significant, suggesting that the relevance of the conclusions is questionable.
Details of the study
The 64,322 live-born children and mothers in the Danish National Birth Cohort from 1996 to 2002 were evaluated three ways:
- through parental reports of behavioral problems in children at age 7 using the Strengths and Difficulties Questionnaire
- through retrieval of HKD diagnoses from the Danish National Hospital Registry or the Danish Psychiatric Central Registry prior to 2011
- through prescriptions for ADHD (primarily methylphenidate [Ritalin]) for children from the Danish Prescription Registry.
Liew and colleagues then estimated hazard ratios for receiving a diagnosis of HKD or using a medication for ADHD, as well as risk ratios for behavioral problems in children after prenatal exposure to acetaminophen.
Stronger associations between prenatal acetaminophen use and HKD or ADHD were found when the mother used the medication in more than one trimester. Exposure-response trends increased with the frequency of acetaminophen use during pregnancy for all three outcomes (HKD diagnosis, ADHD-like behaviors, and ADHD medication use; P trend <.001). Results did not appear to be confounded by maternal inflammation, infection during pregnancy, or the mother’s mental health status.
Related articles:
• How can pregnant women safely relieve low-back pain? Roselyn Jan W. Clemente-Fuentes, MD; Heather Pickett, DO; Misty Carney, MLIS; and Paul Crawford, MD (January 2014)
• Perinatal depression: What you can do to reduce its long-term effects. Janelle Yates (February 2014)
• Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”? John T. Repke, MD (Guest Editorial; June 2014)
Why these findings are less than compelling
Acetaminophen is the most commonly used medication during pregnancy, although few investigators have analyzed neurobehavioral complications in children exposed to this drug in utero. Another recent epidemiologic study from Norway also suggests that long-term exposure (>28 days) to acetaminophen increases the risk of poor gross motor functioning, poor communication skills, and externalizing and internalizing behavior problems.3
The rationale behind an association between acetaminophen and ADHD and HKD is that the medication is an endocrine-disrupting agent. The evidence of this status comes primarily from in vitro experiments from one group of researchers, which may not represent in vivo conditions.4,5
Epidemiologic studies frequently are confounded by poor design and methodology. It also should be noted that correlation is not necessarily the same as causation. In this study, the design and methodology were appropriate considering the data available. Researchers often use large databases like this to research “hot topics” such as the association between ADHD and prenatal acetaminophen use. In this study, acetaminophen cannot be associated definitively with an increased risk of ADHD or HKD. Further research is needed, with greater attention to possible confounding factors, such as why these women consumed chronic doses and for what conditions.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
For the time being, you should probably counsel your patients to use acetaminophen sparingly during pregnancy, and certainly not on a daily basis. We also should encourage nonpharmacologic pain management, such as cognitive behavioral therapy, when appropriate, and caution patients against long-term use of analgesics, when possible, during gestation and lactation.
Thomas W. Hale, RPH, PhD; Aarienne Einarson, RN; and Teresa Baker, MD
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. Kajta M, Wojtowicz AK. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol Rep. 2013;65(6):1632–1639.
2. de Bruin EI, Verheij F, Wiegman T, Ferdinand RF. Differences in finger length ratio between males with autism, pervasive developmental disorder–not otherwise specified, ADHD, and anxiety disorders. Dev Med Child Neurol. 2006;48(12):962–965.
3. Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: A sibling-controlled cohort study. Int J Epidemiol. 2013;42(6):1702–1713.
4. Kristensen DM, Lesne L, Le Fol V, et al. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid), and indomethacin are anti-androgenic in the rat foetal testis. Int J Androl. 2012;35(3):377–384.
5. Albert O, Desdoits-Lethimonier C, Lesne L, et al. Paracetamol, aspirin, and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod. 2013;28(7):1890–1898.
1. Kajta M, Wojtowicz AK. Impact of endocrine-disrupting chemicals on neural development and the onset of neurological disorders. Pharmacol Rep. 2013;65(6):1632–1639.
2. de Bruin EI, Verheij F, Wiegman T, Ferdinand RF. Differences in finger length ratio between males with autism, pervasive developmental disorder–not otherwise specified, ADHD, and anxiety disorders. Dev Med Child Neurol. 2006;48(12):962–965.
3. Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: A sibling-controlled cohort study. Int J Epidemiol. 2013;42(6):1702–1713.
4. Kristensen DM, Lesne L, Le Fol V, et al. Paracetamol (acetaminophen), aspirin (acetylsalicylic acid), and indomethacin are anti-androgenic in the rat foetal testis. Int J Androl. 2012;35(3):377–384.
5. Albert O, Desdoits-Lethimonier C, Lesne L, et al. Paracetamol, aspirin, and indomethacin display endocrine disrupting properties in the adult human testis in vitro. Hum Reprod. 2013;28(7):1890–1898.
Low-dose aspirin and preeclampsia prevention: Ready for prime time, but as a “re-run” or as a “new series”?
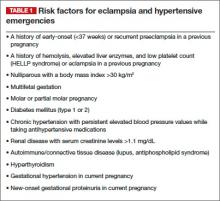
In November 2013, The American College of Obstetricians and Gynecologists (ACOG) published the results of its Task Force on Hypertension in Pregnancy.1 The Task Force aimed to help clinicians become familiar with the state of research in hypertension during pregnancy as well as to assist us in standardizing management approaches to such patients.
The Task Force reported that, worldwide, hypertensive disorders complicate approximately 10% of pregnancies. In addition, in the United States, the past 20 years have brought a 25% increase in the incidence of preeclampsia. According to past ACOG President James N. Martin, Jr, MD, in the last 10 years, the pathophysiology of preeclampsia has become better understood, but the etiology remains unclear and evidence that has emerged to guide therapy has not translated into clinical practice.1
Related articles:
The latest guidance from ACOG on hypertension in pregnancy. Jaimey E. Pauli, MD (Audiocast, January 2014)
Update on Obstetrics. Jaimey E. Pauli, MD, and John T. Repke, MD (January 2014)
The Task Force document contained 60 recommendations for the prevention, prediction, and management of hypertensive disorders of pregnancy, including preeclampsia, gestational hypertension, chronic hypertension, HELLP syndrome, and preeclampsia superimposed on an underlying hypertensive disorder (see box below). One recommendation was that women at high risk for preeclampsia, particularly those with a history of preeclampsia that required delivery before 34 weeks, could possibly benefit from taking aspirin (60–81 mg) daily starting at the end of the first trimester. They further noted that this benefit could include prevention of recurrent severe preeclampsia, or at least a reduction in recurrence risk.
The ACOG Task Force made its recommendation based on results of a meta-analysis of low-dose aspirin trials, involving more than 30,000 patients,2 suggesting a small decrease in the risk of preeclampsia and associated morbidity. More precise risk reduction estimates were difficult to make due to the heterogeneity of the studies reviewed. And the Task Force further stated that this (low-dose aspirin) approach had no demonstrable acute adverse fetal effects, although long-term adverse effects could not be entirely excluded based on the current data.
Unfortunately, according to the ACOG document, the strength of the evidence supporting their recommendation was “moderate” and the strength of the recommendation was “qualified” so, not exactly a resounding endorsement of this approach, but a recommendation nonetheless.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery

John T. Repke, MD, author of this Guest Editorial, recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
Data suggest aspirin for high-risk women could be reasonable
A recent study by Henderson and colleagues presented a systematic review for the US Preventive Services Task Force (USPSTF) on the potential for low-dose aspirin to prevent morbidity and mortality from preeclampsia.3 The design was a meta-analysis of 28 studies: two large, multisite, randomized clinical trials (RCTs); 13 smaller RCTs of high-risk women, of which eight were deemed “good quality”; and six RCTs and two observational studies of average-risk women, of which seven were deemed to be good quality.
The results essentially supported the notion that low-dose aspirin had a beneficial effect with respect to prevention of preeclampsia and perinatal morbidity in women at high risk for preeclampsia. Additionally, no harmful effects were identified, although the authors acknowledged potential rare or long-term harm could not be excluded.
Questions remain
While somewhat gratifying, the results of the USPSTF systematic review still leave many questions. First, the dose of aspirin used in the studies analyzed ranged from 50 mg/d to 150 mg/d. In the United States, “low-dose” aspirin is usually prescribed at 81 mg/d, so the applicability of this review’s findings to US clinical practice is not exact. Second, the authors acknowledged that the putative positive effects observed could be secondary to so-called “small study effects,” and that when only the larger studies were analyzed the effects were less impressive.
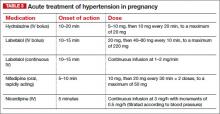
Related article: A stepwise approach to managing eclampsia and other hypertensive emergencies. Baha M. Sibai (October 2013)
In my opinion, both the USPSTF study and the recommendations from the ACOG Task Force provide some reassurance for clinicians that the use of daily, low-dose aspirin by women at high risk for preeclampsia probably does afford some benefit, and seems to be a safe approach—as we have known from the initial Maternal-Fetal Medicine Units (MFMU) trial published in 1993 on low-risk women4 and the follow-up MFMU study on high-risk women.5
The need for additional studies is clear, however. The idea that preeclampsia is the same in every patient would seem to make no more sense than thinking all cancer is the same, with the same risk factors, the same epidemiology and pathophysiology, and the same response to similar treatments. Fundamentally, we need to further explore the different pathways through which preeclampsia develops in women and then apply the strategy best suited to treating (or preventing) their form of the disease—a personalized medicine approach.
In the meantime, most patients who have delivered at 34 weeks or less because of preeclampsia and who are contemplating another pregnancy are really not interested in hearing us tell them that we cannot do anything to prevent recurrent preeclampsia because we are awaiting further studies. At least the ACOG recommendations and the results of the USPSTF’s systematic review provide us with a reasonable, although perhaps not yet optimal, therapeutic option.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia. Baha M. Sibai (November 2011)
The bottom line
In my own practice, I discuss the option of initiating low-dose aspirin (81 mg/d) as early as 12 weeks’ gestation for patients who had either prior early-onset preeclampsia requiring delivery before 34 weeks’ gestation or preeclampsia during more than one pregnancy.
QUICK POLL
Do you offer low-dose aspirin for preeclampsia prevention?
When faced with a patient with prior preeclampsia in more than one pregnancy or with preeclampsia that resulted in delivery prior to 34 weeks, do you offer low-dose aspirin as an option for preventing preeclampsia?
Visit the Quick Poll on the right column of the OBGManagement.com home page to register your answer and see how your colleagues voted.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Duley L, Henderson-Smart DJ, Heher S, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force [published online ahead of print April 8, 2014]. Ann Intern Med. doi:10.7326/M13-2844.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–1218.
- Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705.
In November 2013, The American College of Obstetricians and Gynecologists (ACOG) published the results of its Task Force on Hypertension in Pregnancy.1 The Task Force aimed to help clinicians become familiar with the state of research in hypertension during pregnancy as well as to assist us in standardizing management approaches to such patients.
The Task Force reported that, worldwide, hypertensive disorders complicate approximately 10% of pregnancies. In addition, in the United States, the past 20 years have brought a 25% increase in the incidence of preeclampsia. According to past ACOG President James N. Martin, Jr, MD, in the last 10 years, the pathophysiology of preeclampsia has become better understood, but the etiology remains unclear and evidence that has emerged to guide therapy has not translated into clinical practice.1
Related articles:
The latest guidance from ACOG on hypertension in pregnancy. Jaimey E. Pauli, MD (Audiocast, January 2014)
Update on Obstetrics. Jaimey E. Pauli, MD, and John T. Repke, MD (January 2014)
The Task Force document contained 60 recommendations for the prevention, prediction, and management of hypertensive disorders of pregnancy, including preeclampsia, gestational hypertension, chronic hypertension, HELLP syndrome, and preeclampsia superimposed on an underlying hypertensive disorder (see box below). One recommendation was that women at high risk for preeclampsia, particularly those with a history of preeclampsia that required delivery before 34 weeks, could possibly benefit from taking aspirin (60–81 mg) daily starting at the end of the first trimester. They further noted that this benefit could include prevention of recurrent severe preeclampsia, or at least a reduction in recurrence risk.
The ACOG Task Force made its recommendation based on results of a meta-analysis of low-dose aspirin trials, involving more than 30,000 patients,2 suggesting a small decrease in the risk of preeclampsia and associated morbidity. More precise risk reduction estimates were difficult to make due to the heterogeneity of the studies reviewed. And the Task Force further stated that this (low-dose aspirin) approach had no demonstrable acute adverse fetal effects, although long-term adverse effects could not be entirely excluded based on the current data.
Unfortunately, according to the ACOG document, the strength of the evidence supporting their recommendation was “moderate” and the strength of the recommendation was “qualified” so, not exactly a resounding endorsement of this approach, but a recommendation nonetheless.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery

John T. Repke, MD, author of this Guest Editorial, recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
Data suggest aspirin for high-risk women could be reasonable
A recent study by Henderson and colleagues presented a systematic review for the US Preventive Services Task Force (USPSTF) on the potential for low-dose aspirin to prevent morbidity and mortality from preeclampsia.3 The design was a meta-analysis of 28 studies: two large, multisite, randomized clinical trials (RCTs); 13 smaller RCTs of high-risk women, of which eight were deemed “good quality”; and six RCTs and two observational studies of average-risk women, of which seven were deemed to be good quality.
The results essentially supported the notion that low-dose aspirin had a beneficial effect with respect to prevention of preeclampsia and perinatal morbidity in women at high risk for preeclampsia. Additionally, no harmful effects were identified, although the authors acknowledged potential rare or long-term harm could not be excluded.
Questions remain
While somewhat gratifying, the results of the USPSTF systematic review still leave many questions. First, the dose of aspirin used in the studies analyzed ranged from 50 mg/d to 150 mg/d. In the United States, “low-dose” aspirin is usually prescribed at 81 mg/d, so the applicability of this review’s findings to US clinical practice is not exact. Second, the authors acknowledged that the putative positive effects observed could be secondary to so-called “small study effects,” and that when only the larger studies were analyzed the effects were less impressive.
Related article: A stepwise approach to managing eclampsia and other hypertensive emergencies. Baha M. Sibai (October 2013)
In my opinion, both the USPSTF study and the recommendations from the ACOG Task Force provide some reassurance for clinicians that the use of daily, low-dose aspirin by women at high risk for preeclampsia probably does afford some benefit, and seems to be a safe approach—as we have known from the initial Maternal-Fetal Medicine Units (MFMU) trial published in 1993 on low-risk women4 and the follow-up MFMU study on high-risk women.5
The need for additional studies is clear, however. The idea that preeclampsia is the same in every patient would seem to make no more sense than thinking all cancer is the same, with the same risk factors, the same epidemiology and pathophysiology, and the same response to similar treatments. Fundamentally, we need to further explore the different pathways through which preeclampsia develops in women and then apply the strategy best suited to treating (or preventing) their form of the disease—a personalized medicine approach.
In the meantime, most patients who have delivered at 34 weeks or less because of preeclampsia and who are contemplating another pregnancy are really not interested in hearing us tell them that we cannot do anything to prevent recurrent preeclampsia because we are awaiting further studies. At least the ACOG recommendations and the results of the USPSTF’s systematic review provide us with a reasonable, although perhaps not yet optimal, therapeutic option.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia. Baha M. Sibai (November 2011)
The bottom line
In my own practice, I discuss the option of initiating low-dose aspirin (81 mg/d) as early as 12 weeks’ gestation for patients who had either prior early-onset preeclampsia requiring delivery before 34 weeks’ gestation or preeclampsia during more than one pregnancy.
QUICK POLL
Do you offer low-dose aspirin for preeclampsia prevention?
When faced with a patient with prior preeclampsia in more than one pregnancy or with preeclampsia that resulted in delivery prior to 34 weeks, do you offer low-dose aspirin as an option for preventing preeclampsia?
Visit the Quick Poll on the right column of the OBGManagement.com home page to register your answer and see how your colleagues voted.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
In November 2013, The American College of Obstetricians and Gynecologists (ACOG) published the results of its Task Force on Hypertension in Pregnancy.1 The Task Force aimed to help clinicians become familiar with the state of research in hypertension during pregnancy as well as to assist us in standardizing management approaches to such patients.
The Task Force reported that, worldwide, hypertensive disorders complicate approximately 10% of pregnancies. In addition, in the United States, the past 20 years have brought a 25% increase in the incidence of preeclampsia. According to past ACOG President James N. Martin, Jr, MD, in the last 10 years, the pathophysiology of preeclampsia has become better understood, but the etiology remains unclear and evidence that has emerged to guide therapy has not translated into clinical practice.1
Related articles:
The latest guidance from ACOG on hypertension in pregnancy. Jaimey E. Pauli, MD (Audiocast, January 2014)
Update on Obstetrics. Jaimey E. Pauli, MD, and John T. Repke, MD (January 2014)
The Task Force document contained 60 recommendations for the prevention, prediction, and management of hypertensive disorders of pregnancy, including preeclampsia, gestational hypertension, chronic hypertension, HELLP syndrome, and preeclampsia superimposed on an underlying hypertensive disorder (see box below). One recommendation was that women at high risk for preeclampsia, particularly those with a history of preeclampsia that required delivery before 34 weeks, could possibly benefit from taking aspirin (60–81 mg) daily starting at the end of the first trimester. They further noted that this benefit could include prevention of recurrent severe preeclampsia, or at least a reduction in recurrence risk.
The ACOG Task Force made its recommendation based on results of a meta-analysis of low-dose aspirin trials, involving more than 30,000 patients,2 suggesting a small decrease in the risk of preeclampsia and associated morbidity. More precise risk reduction estimates were difficult to make due to the heterogeneity of the studies reviewed. And the Task Force further stated that this (low-dose aspirin) approach had no demonstrable acute adverse fetal effects, although long-term adverse effects could not be entirely excluded based on the current data.
Unfortunately, according to the ACOG document, the strength of the evidence supporting their recommendation was “moderate” and the strength of the recommendation was “qualified” so, not exactly a resounding endorsement of this approach, but a recommendation nonetheless.
OBSTETRIC PRACTICE CHANGERS 2014
Hypertension and pregnancy and preventing the first cesarean delivery

John T. Repke, MD, author of this Guest Editorial, recently sat down with Errol R. Norwitz, MD, PhD, fellow OBG Management Board of Editors Member and author of this month’s "Update on Operative Vaginal Delivery." Their discussion focused on individual takeaways from ACOG’s Hypertension in Pregnancy guidelines and the recent joint ACOG−Society of Maternal-Fetal Medicine report on emerging clinical and scientific advances in safe prevention of the primary cesarean delivery.
From their conversation:
Dr. Repke: About 60 recommendations came out of ACOG’s Hypertension in Pregnancy document; only six had high-quality supporting evidence, and I think most practitioners already did them. Many really were based on either moderate- or low-quality evidence, with qualified recommendations. I think this has led to confusion.
Dr. Norwitz, how do you answer when a clinician asks you, “Is this gestational hypertension or is this preeclampsia?”
Click here to access the audiocast with full transcript.
Data suggest aspirin for high-risk women could be reasonable
A recent study by Henderson and colleagues presented a systematic review for the US Preventive Services Task Force (USPSTF) on the potential for low-dose aspirin to prevent morbidity and mortality from preeclampsia.3 The design was a meta-analysis of 28 studies: two large, multisite, randomized clinical trials (RCTs); 13 smaller RCTs of high-risk women, of which eight were deemed “good quality”; and six RCTs and two observational studies of average-risk women, of which seven were deemed to be good quality.
The results essentially supported the notion that low-dose aspirin had a beneficial effect with respect to prevention of preeclampsia and perinatal morbidity in women at high risk for preeclampsia. Additionally, no harmful effects were identified, although the authors acknowledged potential rare or long-term harm could not be excluded.
Questions remain
While somewhat gratifying, the results of the USPSTF systematic review still leave many questions. First, the dose of aspirin used in the studies analyzed ranged from 50 mg/d to 150 mg/d. In the United States, “low-dose” aspirin is usually prescribed at 81 mg/d, so the applicability of this review’s findings to US clinical practice is not exact. Second, the authors acknowledged that the putative positive effects observed could be secondary to so-called “small study effects,” and that when only the larger studies were analyzed the effects were less impressive.
Related article: A stepwise approach to managing eclampsia and other hypertensive emergencies. Baha M. Sibai (October 2013)
In my opinion, both the USPSTF study and the recommendations from the ACOG Task Force provide some reassurance for clinicians that the use of daily, low-dose aspirin by women at high risk for preeclampsia probably does afford some benefit, and seems to be a safe approach—as we have known from the initial Maternal-Fetal Medicine Units (MFMU) trial published in 1993 on low-risk women4 and the follow-up MFMU study on high-risk women.5
The need for additional studies is clear, however. The idea that preeclampsia is the same in every patient would seem to make no more sense than thinking all cancer is the same, with the same risk factors, the same epidemiology and pathophysiology, and the same response to similar treatments. Fundamentally, we need to further explore the different pathways through which preeclampsia develops in women and then apply the strategy best suited to treating (or preventing) their form of the disease—a personalized medicine approach.
In the meantime, most patients who have delivered at 34 weeks or less because of preeclampsia and who are contemplating another pregnancy are really not interested in hearing us tell them that we cannot do anything to prevent recurrent preeclampsia because we are awaiting further studies. At least the ACOG recommendations and the results of the USPSTF’s systematic review provide us with a reasonable, although perhaps not yet optimal, therapeutic option.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia. Baha M. Sibai (November 2011)
The bottom line
In my own practice, I discuss the option of initiating low-dose aspirin (81 mg/d) as early as 12 weeks’ gestation for patients who had either prior early-onset preeclampsia requiring delivery before 34 weeks’ gestation or preeclampsia during more than one pregnancy.
QUICK POLL
Do you offer low-dose aspirin for preeclampsia prevention?
When faced with a patient with prior preeclampsia in more than one pregnancy or with preeclampsia that resulted in delivery prior to 34 weeks, do you offer low-dose aspirin as an option for preventing preeclampsia?
Visit the Quick Poll on the right column of the OBGManagement.com home page to register your answer and see how your colleagues voted.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Duley L, Henderson-Smart DJ, Heher S, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force [published online ahead of print April 8, 2014]. Ann Intern Med. doi:10.7326/M13-2844.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–1218.
- Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705.
- ACOG Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–1131.
- Duley L, Henderson-Smart DJ, Heher S, King JF. Antiplatelet agents for preventing preeclampsia and its complications. Cochrane Database Syst Rev. 2007;(2):CD004659.
- Henderson JT, Whitlock EP, O’Connor E, Senger CA, Thompson JH, Rowland MG. Low-dose aspirin for prevention of morbidity and mortality from preeclampsia: A systematic evidence review for the U.S. Preventive Services Task Force [published online ahead of print April 8, 2014]. Ann Intern Med. doi:10.7326/M13-2844.
- Sibai BM, Caritis SN, Thom E, et al. Prevention of preeclampsia with low-dose aspirin in healthy, nulliparous pregnant women. The National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1993;329(17):1213–1218.
- Caritis S, Sibai B, Hauth J, et al. Low-dose aspirin to prevent preeclampsia in women at high risk. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med. 1998;338(11):701–705.
Have you read Dr. Errol R. Norwitz's Update on Operative Vaginal Delivery? Click here to access.
How I am adapting my morcellation practice: Voices from across the country
Hear from:
Michael Baggish, MD (St. Helena, California)
Rupen Baxi, MD (Royal Oak, Michigan)
Jennifer Hollings, MD (Richmond, Virginia)
Gwinnett Ladson, MD (Nashville, Tennessee)
Rich Persino, MD (McHenry, Illinois)
Teresa Tam, MD (Chicago, Illinois)
Yvonne Wolny, MD (Chicago, Illinois)
Hear from:
Michael Baggish, MD (St. Helena, California)
Rupen Baxi, MD (Royal Oak, Michigan)
Jennifer Hollings, MD (Richmond, Virginia)
Gwinnett Ladson, MD (Nashville, Tennessee)
Rich Persino, MD (McHenry, Illinois)
Teresa Tam, MD (Chicago, Illinois)
Yvonne Wolny, MD (Chicago, Illinois)
Hear from:
Michael Baggish, MD (St. Helena, California)
Rupen Baxi, MD (Royal Oak, Michigan)
Jennifer Hollings, MD (Richmond, Virginia)
Gwinnett Ladson, MD (Nashville, Tennessee)
Rich Persino, MD (McHenry, Illinois)
Teresa Tam, MD (Chicago, Illinois)
Yvonne Wolny, MD (Chicago, Illinois)
2014 Update on cervical disease
Advances in cervical cancer screening continue apace. We are fortunate that these advances are based on a substantial amount of high-quality prospective evidence. Many of these advances are designed to target the women who have clinically relevant disease while minimizing harm and anxiety caused by unnecessary procedures related to cervical screening test abnormalities that have little clinical relevance.
With clinicians being regularly judged on performance and outcomes, adoption of advances and new guidelines should be considered relatively quickly by women’s health providers.
In this article, I focus on two significant advances of the past (and coming) year:
- recent application and unanimous approval by a Food and Drug Administration (FDA) expert panel for the use of the cobas human papillomavirus (HPV) DNA test as a primary cervical cancer screen
- the latest update of guidelines on the management of abnormal cervical screening tests from the American Society for Colposcopy and Cervical Pathology (ASCCP).
cobas HPV TEST IS POISED FOR FDA APPROVAL AS A PRIMARY SCREEN FOR CERVICAL CANCER
Wright TC Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206(1):46.e1–e11.
An FDA expert panel unanimously approved the cobas (Roche Molecular Diagnostics; Pleasanton, California) HPV DNA test on March 12, 2014. The FDA will decide on potential approval within the coming months. Although the FDA sometimes reaches a different decision from one of its advisory committees when it comes to a final vote on a product or device, most often the FDA concurs with the committee’s judgment. Therefore, approval of the cobas HPV test as a primary screen is likely.
Related article: FDA Advisory Committee recommends HPV test as primary screening tool for cervical cancer Deborah Reale (News for your Practice, March 2014)
The cobas HPV test yields a pooled result for 12 high-risk HPV types (hrHPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), as well as individual results for types 16 and 18; it also has an internal control for specimen adequacy. HPV 16 and 18 account for roughly 70% of all cases of cervical cancer, and infection with both types are known to place women at high risk for having clinically relevant disease—more so than the other hrHPV types.
COMMITTEE REVIEWED DATA FROM ATHENA IN VOTING FOR APPROVAL
In considering the cobas HPV test, the advisory committee reviewed data from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial, a prospective, multicenter, US-based study of 47,208 women aged 21 and older. These women were recruited at the time of undergoing routine screening for cervical cancer; only 2.6% had been vaccinated against HPV. All were screened by liquid-based cytology and an HPV test. Those who had abnormal cytology or a positive test for a high-risk HPV type underwent colposcopy, as did a randomly selected group of women aged 25 or older who tested negative on both tests.
The prevalence of abnormal findings was:
- 7.1% for liquid-based cytology
- 12.6% for pooled high-risk HPV
- 2.8% for HPV 16
- 1.0% for HPV 18.
As expected, cytologic abnormalities and infection with high-risk HPV types declined with increasing age. The adjusted prevalence of cervical intraepithelial neoplasia (CIN) grade 2 or higher in women aged 25 to 34 years was 2.3%; it declined to 1.5% among women older than age 34. Of note, approximately 500,000 US women are given a diagnosis of CIN 2 or CIN 3 each year in the United States.
WHY ATHENA IS IMPORTANT
This US-based trial was designed to assess the medical utility of pooled high-risk HPV DNA in addition to genotyping for HPV 16 and 18 in three populations:
- women aged 21 and older with a cytologic finding of atypical squamous cells of undetermined significance (ASC-US)
- women aged 30 and older with normal cytology
- women aged 25 and older in the overall screening population with any cytologic finding.
Investigators were particularly interested in the use of the HPV test as:
- a triage for women with abnormal cytologic findings
- an adjunct to guide clinical management of women with negative cytology results
- a potential front-line test in the screening of women aged 25 and older.
Related article: Endometrial cancer update: The move toward personalized cancer care Lindsay M. Kuroki, MD, and David G. Mutch, MD (October 2013)
The participants of the ATHENA trial were representative of women undergoing screening for cervical cancer in the United States—both in terms of demographics and in the distribution of cytologic findings. For example, recent US census data indicate that the female population is 79% white, 13% black, and 16% Hispanic or Latino—figures comparable to the breakdown of race/ethnicity in the ATHENA trial.
The trial was conducted in a baseline phase (published in 2012) and a 3-year follow-up phase (not yet published). The 3-year data were reviewed by the FDA advisory committee during its consideration of the cobas HPV test as a primary screen.
DESPITE PROBABLE APPROVAL, INCREMENTAL CHANGE IS LIKELY
Although a move to the HPV test as the primary screen is a definite paradigm shift for what has been cytology-based screening since the initiation of cervical cancer screening, the changeover from primary cytology to primary HPV testing likely will be slow. It will require education of clinicians as well as patients, and a shift in many internal procedures for pathology laboratories.
The ATHENA trial also leaves some intriguing questions unanswered:
- How do we transition women into the new screening strategy? Many women today still undergo cytology screening with reflex HPV testing, as appropriate, and an increasing number of women aged 30 and older undergo cotesting with both cytology and HPV testing. When should they begin screening in a primary HPV testing setting? And what screening intervals will be recommended? If a woman already has been screened with cytology, how should she transition into and at what interval should she begin primary HPV screening?
- How should we manage women’s care after the first round of primary HPV testing? The ATHENA trial so far only has outcomes data after one round of HPV testing. While some data are available from Europe, we do not know what happens after two or three rounds of screening with primary HPV testing in a large US-based cohort. We clearly will be identifying and treating many women with preinvasive disease from screening after one round of testing, at a rate likely higher than with cytology alone—a good thing. We also likely will be reducing the number of unnecessary colposcopies for cytology that are not related to hrHPV.
What this EVIDENCE means for practice
Screening women using the cobas HPV test as a primary screen will require considerable education of providers and patients to explain how this change will affect how a woman will be managed after being screened for cervical cancer. Though much remains to be determined about this new cervical cancer screening paradigm (eg, logistics, timing, use of secondary tests), it should reduce the number of screening tests and colposcopies necessary to detect clinically relevant disease.
UPDATED ASCCP GUIDELINES EMPHASIZE EQUAL MANAGEMENT FOR EQUAL RISK
Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27.
In formulating this latest set of guidelines for the management of abnormal cervical cancer screening tests and cancer precursors, the ASCCP led a conference consisting of scientific stakeholders to perform a comprehensive review of the literature. Also, with study investigators at Kaiser Permanente Northern California (KPNC) and the National Cancer Institute, the guidelines panel also modeled and assessed data on risk after abnormal tests from almost 1.4 million women followed over 8 years in the KPNC Medical Care Plan—this cohort has provided us with “big data.”
The sheer size of the Kaiser Permanente population made it possible for the ASCCP-led panel to validate its previous guidelines or to modify them, where needed. It also made risk-based stratification possible for even rare abnormalities and clinical outcomes.
Although findings from the KPNC population may not be fully generalizable to the US population as a whole, they enhance our understanding of the optimal management of abnormal cervical cancer screening tests and cancer precursors. More widely dispersed study cohorts on a similar scale in the United States are unlikely in the near future.
Related article: Update on cervical disease Mark H. Einstein, MD, MS, and J. Thomas Cox, MD (May 2013)
SEVERAL SIGNIFICANT MODIFICATIONS
Although the ASCCP reaffirmed most elements of its 2006 consensus management guidelines, it did make a number of changes:
- Women who have ASC-US cytology but test HPV-negative now should be followed with cotesting at 3 years rather than 5 years before they return to routine screening.
- Women near age 65 who have a negative finding on ASC-US cytology and HPV testing should not exit screening.
- Women who have ASC-US cytology and test HPV-positive should go to immediate colposcopy, regardless of hrHPV results, including genotyping.
- Women who test positive for HPV 16 or 18 but have negative cytology should undergo immediate colposcopy.
- Women aged 21 to 24 years should be managed as conservatively and minimally invasively as possible, especially when an abnormality is minor.
- Endocervical curettage reported as CIN 1 should be managed as CIN 1, not as a positive endocervical curettage.
- When a cytologic sample is unsatisfactory, sampling usually should be repeated, even when HPV cotesting results are known. However, negative cytology that lacks sufficient endocervical cells or a transformation zone component usually can be managed without frequent follow-up.
Related article: New cervical Ca screening guidelines recommend less frequent assessment Janelle Yates (News for your Practice; April 2012)
EQUAL MANAGEMENT SHOULD BE PERFORMED FOR ABNORMAL TESTS THAT INDICATE EQUAL RISK
The ASCCP-led management panel unanimously agreed to several basic assumptions in formulating the updated guidelines. For example, they concurred that achieving zero risk for cancer is impossible and that attempts to achieve zero risk (which typically means more frequent testing) may cause harm. They also cited the 2011 American Cancer Society/ASCCP/American Society for Clinical Pathology consensus screening document, which stated: “Optimal prevention strategies should identify those HPV-related abnormalities likely to progress to invasive cancers while avoiding destructive treatment of abnormalities not destined to become cancerous.”1
The panel also agreed that CIN 3+ is a “reasonable proxy for cancer risk.” When calculating risk, the KPNC data were modeled for all combinations of cytology and HPV testing, using CIN 3+ for many of the outcomes, and when outcomes were rare, using CIN 2+. The theme of equal management for equal risk was the rationale behind the management approaches detailed in the TABLE. Risks were deemed to be low and return to normal screening was recommended when the risks were similar to the rate of CIN 3+ 3 years after negative cytology or 5 years after negative cotesting. However, immediate colposcopy was recommended when the 5-year risk of CIN 3+ for the combination of cytology and hrHPV testing, when indicated, exceeded 5%. A 6-month to 12-month return (intermediate risk) is indicated with a risk of CIN3+ of 2% to 5%.
An emphasis on avoiding harms
Abnormal findings at the time of cervical cancer screening can lead to a number of harms for the patient, including anxiety and emotional distress, particularly when colposcopy is necessary, as well as time lost from home and work life. For this reason, the guidelines panel emphasized that colposcopy and other interventions should be avoided when the risk of CIN 3+ is low and when the cervical screening abnormalities are likely to resolve without treatment.
However, women who experience postcoital bleeding, unexplained abnormal vaginal bleeding, pelvic pain, abnormal discharge, or a visible lesion should be managed promptly on an individualized basis.
Long-term effects of HPV vaccination are unknown
Among the areas that remain to be addressed are the unknown effects of widespread prophylactic HPV vaccination over the long term. We also lack full understanding of whether and how HPV vaccination will alter the incidence and management of cytologic and histologic abnormalities. Given the low rates of vaccination against HPV in the United States at present, this will need to be re-evaluated in the future.
What this EVIDENCE means for practice
The updated ASCCP guidelines are inherently complex, but their complexity arises from a large body of high-quality prospective data from a large population of women. Equal risk should result in equal management of cervical screening test abnormalities. Practitioners need not feel obligated to memorize the guidelines, owing to the availability of algorithms for specific findings in specific populations at the ASCCP Web site (www.asccp.org/consensus2012). Apps also are available for the iPhone, iPad, and Android.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Reference
- Saslow D, Solomon D, Lawson HW, et al;ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
Advances in cervical cancer screening continue apace. We are fortunate that these advances are based on a substantial amount of high-quality prospective evidence. Many of these advances are designed to target the women who have clinically relevant disease while minimizing harm and anxiety caused by unnecessary procedures related to cervical screening test abnormalities that have little clinical relevance.
With clinicians being regularly judged on performance and outcomes, adoption of advances and new guidelines should be considered relatively quickly by women’s health providers.
In this article, I focus on two significant advances of the past (and coming) year:
- recent application and unanimous approval by a Food and Drug Administration (FDA) expert panel for the use of the cobas human papillomavirus (HPV) DNA test as a primary cervical cancer screen
- the latest update of guidelines on the management of abnormal cervical screening tests from the American Society for Colposcopy and Cervical Pathology (ASCCP).
cobas HPV TEST IS POISED FOR FDA APPROVAL AS A PRIMARY SCREEN FOR CERVICAL CANCER
Wright TC Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206(1):46.e1–e11.
An FDA expert panel unanimously approved the cobas (Roche Molecular Diagnostics; Pleasanton, California) HPV DNA test on March 12, 2014. The FDA will decide on potential approval within the coming months. Although the FDA sometimes reaches a different decision from one of its advisory committees when it comes to a final vote on a product or device, most often the FDA concurs with the committee’s judgment. Therefore, approval of the cobas HPV test as a primary screen is likely.
Related article: FDA Advisory Committee recommends HPV test as primary screening tool for cervical cancer Deborah Reale (News for your Practice, March 2014)
The cobas HPV test yields a pooled result for 12 high-risk HPV types (hrHPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), as well as individual results for types 16 and 18; it also has an internal control for specimen adequacy. HPV 16 and 18 account for roughly 70% of all cases of cervical cancer, and infection with both types are known to place women at high risk for having clinically relevant disease—more so than the other hrHPV types.
COMMITTEE REVIEWED DATA FROM ATHENA IN VOTING FOR APPROVAL
In considering the cobas HPV test, the advisory committee reviewed data from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial, a prospective, multicenter, US-based study of 47,208 women aged 21 and older. These women were recruited at the time of undergoing routine screening for cervical cancer; only 2.6% had been vaccinated against HPV. All were screened by liquid-based cytology and an HPV test. Those who had abnormal cytology or a positive test for a high-risk HPV type underwent colposcopy, as did a randomly selected group of women aged 25 or older who tested negative on both tests.
The prevalence of abnormal findings was:
- 7.1% for liquid-based cytology
- 12.6% for pooled high-risk HPV
- 2.8% for HPV 16
- 1.0% for HPV 18.
As expected, cytologic abnormalities and infection with high-risk HPV types declined with increasing age. The adjusted prevalence of cervical intraepithelial neoplasia (CIN) grade 2 or higher in women aged 25 to 34 years was 2.3%; it declined to 1.5% among women older than age 34. Of note, approximately 500,000 US women are given a diagnosis of CIN 2 or CIN 3 each year in the United States.
WHY ATHENA IS IMPORTANT
This US-based trial was designed to assess the medical utility of pooled high-risk HPV DNA in addition to genotyping for HPV 16 and 18 in three populations:
- women aged 21 and older with a cytologic finding of atypical squamous cells of undetermined significance (ASC-US)
- women aged 30 and older with normal cytology
- women aged 25 and older in the overall screening population with any cytologic finding.
Investigators were particularly interested in the use of the HPV test as:
- a triage for women with abnormal cytologic findings
- an adjunct to guide clinical management of women with negative cytology results
- a potential front-line test in the screening of women aged 25 and older.
Related article: Endometrial cancer update: The move toward personalized cancer care Lindsay M. Kuroki, MD, and David G. Mutch, MD (October 2013)
The participants of the ATHENA trial were representative of women undergoing screening for cervical cancer in the United States—both in terms of demographics and in the distribution of cytologic findings. For example, recent US census data indicate that the female population is 79% white, 13% black, and 16% Hispanic or Latino—figures comparable to the breakdown of race/ethnicity in the ATHENA trial.
The trial was conducted in a baseline phase (published in 2012) and a 3-year follow-up phase (not yet published). The 3-year data were reviewed by the FDA advisory committee during its consideration of the cobas HPV test as a primary screen.
DESPITE PROBABLE APPROVAL, INCREMENTAL CHANGE IS LIKELY
Although a move to the HPV test as the primary screen is a definite paradigm shift for what has been cytology-based screening since the initiation of cervical cancer screening, the changeover from primary cytology to primary HPV testing likely will be slow. It will require education of clinicians as well as patients, and a shift in many internal procedures for pathology laboratories.
The ATHENA trial also leaves some intriguing questions unanswered:
- How do we transition women into the new screening strategy? Many women today still undergo cytology screening with reflex HPV testing, as appropriate, and an increasing number of women aged 30 and older undergo cotesting with both cytology and HPV testing. When should they begin screening in a primary HPV testing setting? And what screening intervals will be recommended? If a woman already has been screened with cytology, how should she transition into and at what interval should she begin primary HPV screening?
- How should we manage women’s care after the first round of primary HPV testing? The ATHENA trial so far only has outcomes data after one round of HPV testing. While some data are available from Europe, we do not know what happens after two or three rounds of screening with primary HPV testing in a large US-based cohort. We clearly will be identifying and treating many women with preinvasive disease from screening after one round of testing, at a rate likely higher than with cytology alone—a good thing. We also likely will be reducing the number of unnecessary colposcopies for cytology that are not related to hrHPV.
What this EVIDENCE means for practice
Screening women using the cobas HPV test as a primary screen will require considerable education of providers and patients to explain how this change will affect how a woman will be managed after being screened for cervical cancer. Though much remains to be determined about this new cervical cancer screening paradigm (eg, logistics, timing, use of secondary tests), it should reduce the number of screening tests and colposcopies necessary to detect clinically relevant disease.
UPDATED ASCCP GUIDELINES EMPHASIZE EQUAL MANAGEMENT FOR EQUAL RISK
Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27.
In formulating this latest set of guidelines for the management of abnormal cervical cancer screening tests and cancer precursors, the ASCCP led a conference consisting of scientific stakeholders to perform a comprehensive review of the literature. Also, with study investigators at Kaiser Permanente Northern California (KPNC) and the National Cancer Institute, the guidelines panel also modeled and assessed data on risk after abnormal tests from almost 1.4 million women followed over 8 years in the KPNC Medical Care Plan—this cohort has provided us with “big data.”
The sheer size of the Kaiser Permanente population made it possible for the ASCCP-led panel to validate its previous guidelines or to modify them, where needed. It also made risk-based stratification possible for even rare abnormalities and clinical outcomes.
Although findings from the KPNC population may not be fully generalizable to the US population as a whole, they enhance our understanding of the optimal management of abnormal cervical cancer screening tests and cancer precursors. More widely dispersed study cohorts on a similar scale in the United States are unlikely in the near future.
Related article: Update on cervical disease Mark H. Einstein, MD, MS, and J. Thomas Cox, MD (May 2013)
SEVERAL SIGNIFICANT MODIFICATIONS
Although the ASCCP reaffirmed most elements of its 2006 consensus management guidelines, it did make a number of changes:
- Women who have ASC-US cytology but test HPV-negative now should be followed with cotesting at 3 years rather than 5 years before they return to routine screening.
- Women near age 65 who have a negative finding on ASC-US cytology and HPV testing should not exit screening.
- Women who have ASC-US cytology and test HPV-positive should go to immediate colposcopy, regardless of hrHPV results, including genotyping.
- Women who test positive for HPV 16 or 18 but have negative cytology should undergo immediate colposcopy.
- Women aged 21 to 24 years should be managed as conservatively and minimally invasively as possible, especially when an abnormality is minor.
- Endocervical curettage reported as CIN 1 should be managed as CIN 1, not as a positive endocervical curettage.
- When a cytologic sample is unsatisfactory, sampling usually should be repeated, even when HPV cotesting results are known. However, negative cytology that lacks sufficient endocervical cells or a transformation zone component usually can be managed without frequent follow-up.
Related article: New cervical Ca screening guidelines recommend less frequent assessment Janelle Yates (News for your Practice; April 2012)
EQUAL MANAGEMENT SHOULD BE PERFORMED FOR ABNORMAL TESTS THAT INDICATE EQUAL RISK
The ASCCP-led management panel unanimously agreed to several basic assumptions in formulating the updated guidelines. For example, they concurred that achieving zero risk for cancer is impossible and that attempts to achieve zero risk (which typically means more frequent testing) may cause harm. They also cited the 2011 American Cancer Society/ASCCP/American Society for Clinical Pathology consensus screening document, which stated: “Optimal prevention strategies should identify those HPV-related abnormalities likely to progress to invasive cancers while avoiding destructive treatment of abnormalities not destined to become cancerous.”1
The panel also agreed that CIN 3+ is a “reasonable proxy for cancer risk.” When calculating risk, the KPNC data were modeled for all combinations of cytology and HPV testing, using CIN 3+ for many of the outcomes, and when outcomes were rare, using CIN 2+. The theme of equal management for equal risk was the rationale behind the management approaches detailed in the TABLE. Risks were deemed to be low and return to normal screening was recommended when the risks were similar to the rate of CIN 3+ 3 years after negative cytology or 5 years after negative cotesting. However, immediate colposcopy was recommended when the 5-year risk of CIN 3+ for the combination of cytology and hrHPV testing, when indicated, exceeded 5%. A 6-month to 12-month return (intermediate risk) is indicated with a risk of CIN3+ of 2% to 5%.
An emphasis on avoiding harms
Abnormal findings at the time of cervical cancer screening can lead to a number of harms for the patient, including anxiety and emotional distress, particularly when colposcopy is necessary, as well as time lost from home and work life. For this reason, the guidelines panel emphasized that colposcopy and other interventions should be avoided when the risk of CIN 3+ is low and when the cervical screening abnormalities are likely to resolve without treatment.
However, women who experience postcoital bleeding, unexplained abnormal vaginal bleeding, pelvic pain, abnormal discharge, or a visible lesion should be managed promptly on an individualized basis.
Long-term effects of HPV vaccination are unknown
Among the areas that remain to be addressed are the unknown effects of widespread prophylactic HPV vaccination over the long term. We also lack full understanding of whether and how HPV vaccination will alter the incidence and management of cytologic and histologic abnormalities. Given the low rates of vaccination against HPV in the United States at present, this will need to be re-evaluated in the future.
What this EVIDENCE means for practice
The updated ASCCP guidelines are inherently complex, but their complexity arises from a large body of high-quality prospective data from a large population of women. Equal risk should result in equal management of cervical screening test abnormalities. Practitioners need not feel obligated to memorize the guidelines, owing to the availability of algorithms for specific findings in specific populations at the ASCCP Web site (www.asccp.org/consensus2012). Apps also are available for the iPhone, iPad, and Android.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Advances in cervical cancer screening continue apace. We are fortunate that these advances are based on a substantial amount of high-quality prospective evidence. Many of these advances are designed to target the women who have clinically relevant disease while minimizing harm and anxiety caused by unnecessary procedures related to cervical screening test abnormalities that have little clinical relevance.
With clinicians being regularly judged on performance and outcomes, adoption of advances and new guidelines should be considered relatively quickly by women’s health providers.
In this article, I focus on two significant advances of the past (and coming) year:
- recent application and unanimous approval by a Food and Drug Administration (FDA) expert panel for the use of the cobas human papillomavirus (HPV) DNA test as a primary cervical cancer screen
- the latest update of guidelines on the management of abnormal cervical screening tests from the American Society for Colposcopy and Cervical Pathology (ASCCP).
cobas HPV TEST IS POISED FOR FDA APPROVAL AS A PRIMARY SCREEN FOR CERVICAL CANCER
Wright TC Jr, Stoler MH, Behrens CM, Apple R, Derion T, Wright TL. The ATHENA human papillomavirus study: design, methods, and baseline results. Am J Obstet Gynecol. 2012;206(1):46.e1–e11.
An FDA expert panel unanimously approved the cobas (Roche Molecular Diagnostics; Pleasanton, California) HPV DNA test on March 12, 2014. The FDA will decide on potential approval within the coming months. Although the FDA sometimes reaches a different decision from one of its advisory committees when it comes to a final vote on a product or device, most often the FDA concurs with the committee’s judgment. Therefore, approval of the cobas HPV test as a primary screen is likely.
Related article: FDA Advisory Committee recommends HPV test as primary screening tool for cervical cancer Deborah Reale (News for your Practice, March 2014)
The cobas HPV test yields a pooled result for 12 high-risk HPV types (hrHPV 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68), as well as individual results for types 16 and 18; it also has an internal control for specimen adequacy. HPV 16 and 18 account for roughly 70% of all cases of cervical cancer, and infection with both types are known to place women at high risk for having clinically relevant disease—more so than the other hrHPV types.
COMMITTEE REVIEWED DATA FROM ATHENA IN VOTING FOR APPROVAL
In considering the cobas HPV test, the advisory committee reviewed data from the Addressing the Need for Advanced HPV Diagnostics (ATHENA) trial, a prospective, multicenter, US-based study of 47,208 women aged 21 and older. These women were recruited at the time of undergoing routine screening for cervical cancer; only 2.6% had been vaccinated against HPV. All were screened by liquid-based cytology and an HPV test. Those who had abnormal cytology or a positive test for a high-risk HPV type underwent colposcopy, as did a randomly selected group of women aged 25 or older who tested negative on both tests.
The prevalence of abnormal findings was:
- 7.1% for liquid-based cytology
- 12.6% for pooled high-risk HPV
- 2.8% for HPV 16
- 1.0% for HPV 18.
As expected, cytologic abnormalities and infection with high-risk HPV types declined with increasing age. The adjusted prevalence of cervical intraepithelial neoplasia (CIN) grade 2 or higher in women aged 25 to 34 years was 2.3%; it declined to 1.5% among women older than age 34. Of note, approximately 500,000 US women are given a diagnosis of CIN 2 or CIN 3 each year in the United States.
WHY ATHENA IS IMPORTANT
This US-based trial was designed to assess the medical utility of pooled high-risk HPV DNA in addition to genotyping for HPV 16 and 18 in three populations:
- women aged 21 and older with a cytologic finding of atypical squamous cells of undetermined significance (ASC-US)
- women aged 30 and older with normal cytology
- women aged 25 and older in the overall screening population with any cytologic finding.
Investigators were particularly interested in the use of the HPV test as:
- a triage for women with abnormal cytologic findings
- an adjunct to guide clinical management of women with negative cytology results
- a potential front-line test in the screening of women aged 25 and older.
Related article: Endometrial cancer update: The move toward personalized cancer care Lindsay M. Kuroki, MD, and David G. Mutch, MD (October 2013)
The participants of the ATHENA trial were representative of women undergoing screening for cervical cancer in the United States—both in terms of demographics and in the distribution of cytologic findings. For example, recent US census data indicate that the female population is 79% white, 13% black, and 16% Hispanic or Latino—figures comparable to the breakdown of race/ethnicity in the ATHENA trial.
The trial was conducted in a baseline phase (published in 2012) and a 3-year follow-up phase (not yet published). The 3-year data were reviewed by the FDA advisory committee during its consideration of the cobas HPV test as a primary screen.
DESPITE PROBABLE APPROVAL, INCREMENTAL CHANGE IS LIKELY
Although a move to the HPV test as the primary screen is a definite paradigm shift for what has been cytology-based screening since the initiation of cervical cancer screening, the changeover from primary cytology to primary HPV testing likely will be slow. It will require education of clinicians as well as patients, and a shift in many internal procedures for pathology laboratories.
The ATHENA trial also leaves some intriguing questions unanswered:
- How do we transition women into the new screening strategy? Many women today still undergo cytology screening with reflex HPV testing, as appropriate, and an increasing number of women aged 30 and older undergo cotesting with both cytology and HPV testing. When should they begin screening in a primary HPV testing setting? And what screening intervals will be recommended? If a woman already has been screened with cytology, how should she transition into and at what interval should she begin primary HPV screening?
- How should we manage women’s care after the first round of primary HPV testing? The ATHENA trial so far only has outcomes data after one round of HPV testing. While some data are available from Europe, we do not know what happens after two or three rounds of screening with primary HPV testing in a large US-based cohort. We clearly will be identifying and treating many women with preinvasive disease from screening after one round of testing, at a rate likely higher than with cytology alone—a good thing. We also likely will be reducing the number of unnecessary colposcopies for cytology that are not related to hrHPV.
What this EVIDENCE means for practice
Screening women using the cobas HPV test as a primary screen will require considerable education of providers and patients to explain how this change will affect how a woman will be managed after being screened for cervical cancer. Though much remains to be determined about this new cervical cancer screening paradigm (eg, logistics, timing, use of secondary tests), it should reduce the number of screening tests and colposcopies necessary to detect clinically relevant disease.
UPDATED ASCCP GUIDELINES EMPHASIZE EQUAL MANAGEMENT FOR EQUAL RISK
Massad LS, Einstein MH, Huh WK, et al; 2012 ASCCP Consensus Guidelines Conference. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17(5 Suppl 1):S1–S27.
In formulating this latest set of guidelines for the management of abnormal cervical cancer screening tests and cancer precursors, the ASCCP led a conference consisting of scientific stakeholders to perform a comprehensive review of the literature. Also, with study investigators at Kaiser Permanente Northern California (KPNC) and the National Cancer Institute, the guidelines panel also modeled and assessed data on risk after abnormal tests from almost 1.4 million women followed over 8 years in the KPNC Medical Care Plan—this cohort has provided us with “big data.”
The sheer size of the Kaiser Permanente population made it possible for the ASCCP-led panel to validate its previous guidelines or to modify them, where needed. It also made risk-based stratification possible for even rare abnormalities and clinical outcomes.
Although findings from the KPNC population may not be fully generalizable to the US population as a whole, they enhance our understanding of the optimal management of abnormal cervical cancer screening tests and cancer precursors. More widely dispersed study cohorts on a similar scale in the United States are unlikely in the near future.
Related article: Update on cervical disease Mark H. Einstein, MD, MS, and J. Thomas Cox, MD (May 2013)
SEVERAL SIGNIFICANT MODIFICATIONS
Although the ASCCP reaffirmed most elements of its 2006 consensus management guidelines, it did make a number of changes:
- Women who have ASC-US cytology but test HPV-negative now should be followed with cotesting at 3 years rather than 5 years before they return to routine screening.
- Women near age 65 who have a negative finding on ASC-US cytology and HPV testing should not exit screening.
- Women who have ASC-US cytology and test HPV-positive should go to immediate colposcopy, regardless of hrHPV results, including genotyping.
- Women who test positive for HPV 16 or 18 but have negative cytology should undergo immediate colposcopy.
- Women aged 21 to 24 years should be managed as conservatively and minimally invasively as possible, especially when an abnormality is minor.
- Endocervical curettage reported as CIN 1 should be managed as CIN 1, not as a positive endocervical curettage.
- When a cytologic sample is unsatisfactory, sampling usually should be repeated, even when HPV cotesting results are known. However, negative cytology that lacks sufficient endocervical cells or a transformation zone component usually can be managed without frequent follow-up.
Related article: New cervical Ca screening guidelines recommend less frequent assessment Janelle Yates (News for your Practice; April 2012)
EQUAL MANAGEMENT SHOULD BE PERFORMED FOR ABNORMAL TESTS THAT INDICATE EQUAL RISK
The ASCCP-led management panel unanimously agreed to several basic assumptions in formulating the updated guidelines. For example, they concurred that achieving zero risk for cancer is impossible and that attempts to achieve zero risk (which typically means more frequent testing) may cause harm. They also cited the 2011 American Cancer Society/ASCCP/American Society for Clinical Pathology consensus screening document, which stated: “Optimal prevention strategies should identify those HPV-related abnormalities likely to progress to invasive cancers while avoiding destructive treatment of abnormalities not destined to become cancerous.”1
The panel also agreed that CIN 3+ is a “reasonable proxy for cancer risk.” When calculating risk, the KPNC data were modeled for all combinations of cytology and HPV testing, using CIN 3+ for many of the outcomes, and when outcomes were rare, using CIN 2+. The theme of equal management for equal risk was the rationale behind the management approaches detailed in the TABLE. Risks were deemed to be low and return to normal screening was recommended when the risks were similar to the rate of CIN 3+ 3 years after negative cytology or 5 years after negative cotesting. However, immediate colposcopy was recommended when the 5-year risk of CIN 3+ for the combination of cytology and hrHPV testing, when indicated, exceeded 5%. A 6-month to 12-month return (intermediate risk) is indicated with a risk of CIN3+ of 2% to 5%.
An emphasis on avoiding harms
Abnormal findings at the time of cervical cancer screening can lead to a number of harms for the patient, including anxiety and emotional distress, particularly when colposcopy is necessary, as well as time lost from home and work life. For this reason, the guidelines panel emphasized that colposcopy and other interventions should be avoided when the risk of CIN 3+ is low and when the cervical screening abnormalities are likely to resolve without treatment.
However, women who experience postcoital bleeding, unexplained abnormal vaginal bleeding, pelvic pain, abnormal discharge, or a visible lesion should be managed promptly on an individualized basis.
Long-term effects of HPV vaccination are unknown
Among the areas that remain to be addressed are the unknown effects of widespread prophylactic HPV vaccination over the long term. We also lack full understanding of whether and how HPV vaccination will alter the incidence and management of cytologic and histologic abnormalities. Given the low rates of vaccination against HPV in the United States at present, this will need to be re-evaluated in the future.
What this EVIDENCE means for practice
The updated ASCCP guidelines are inherently complex, but their complexity arises from a large body of high-quality prospective data from a large population of women. Equal risk should result in equal management of cervical screening test abnormalities. Practitioners need not feel obligated to memorize the guidelines, owing to the availability of algorithms for specific findings in specific populations at the ASCCP Web site (www.asccp.org/consensus2012). Apps also are available for the iPhone, iPad, and Android.
WE WANT TO HEAR FROM YOU!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue. Send your letter to: [email protected] Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
Reference
- Saslow D, Solomon D, Lawson HW, et al;ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
Reference
- Saslow D, Solomon D, Lawson HW, et al;ACS-ASCCP-ASCP Cervical Cancer Guideline Committee. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. CA Cancer J Clin. 2012;62(3):147–172.
Dr. Mark Einstein anticipated final FDA approval of the first HPV test for primary cervical cancer screening and, in this UPDATE ON CERVICAL DISEASE, expands on the data behind the approval and how your practice could change
2014 Update on abnormal uterine bleeding
As recently defined by the International Federation of Gynecology and Obstetrics (FIGO)—and endorsed by the American College of Obstetricians and Gynecologists—the term “abnormal uterine bleeding” (AUB) now describes any departure from normal menstrual bleeding.1 To determine the most appropriate intervention for this widespread problem, FIGO proposed that clinicians consider potential contributors to the clinical problem by investigating and categorizing patients according to the following system:
- Polyp
- Adenomyosis
- Leiomyoma
- Malignancy and hyperplasia
- Coagulopathy
- Ovulatory disorders
- Endometrial dysfunction
- Iatrogenic
- Not otherwise classified.
A given individual may be found to have one or more of these features, but not all of the features may contribute to the AUB. To facilitate their use, these nine causes are more commonly identified using the acronym PALM-COEIN.
In this article, I focus on three of these categories, presenting recent data on AUB associated with leiomyomata (AUB-L) or adenomyosis (AUB-A), and AUB of an iatrogenic nature (AUB-I).
AUB-L: SATISFACTION RATES ARE SIMILAR 5 YEARS AFTER FIBROID TREATMENT BY SURGERY OR UTERINE ARTERY EMBOLIZATION
Gupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2012;5:CD005073. doi:10.1002/14651858.CD005073.pub3.
Women who undergo uterine artery embolization (UAE) for the treatment of symptomatic uterine fibroids are just as satisfied with the outcome as women treated with hysterectomy or myomectomy, according to this 2012 review from the Cochrane Database.
Gupta and colleagues found similar patient-satisfaction rates at 5 years (odds ratio [OR] 0.9; 95% confidence interval [CI], 0.45–1.8), although women undergoing UAE were more likely to require additional interventions within 2 years (56 additional interventions per 1,000 women for surgery vs 250 per 1,000 women for UAE; OR, 5.64).
Details and general findings
Gupta and colleagues selected randomized, controlled trials comparing UAE with surgery:
- three trials of UAE versus abdominal hysterectomy (n = 291)
- one trial of UAE versus hysterectomy or myomectomy (the specific surgery was determined by patient preference) (n = 157)
- one trial of UAE versus myomectomy in women desiring future childbearing (n = 121).
In these trials, UAE was bilateral and involved the use of permanent embolic material.
Among the findings:
- Costs were lower with UAE, as assessed by measuring the duration of the procedure, length of hospitalization, and time to resumption of normal activities.
- Ovarian-failure rates were comparable between women in the UAE and surgery groups. Ovarian function was assessed by measuring follicle-stimulating hormone (FSH), although FSH thresholds varied in some of the studies.
- Pregnancy was less likely after UAE than after myomectomy. In the trial comparing UAE with myomectomy, 26 women later tried to conceive after UAE versus 40 after myomectomy. Significantly fewer women became pregnant after UAE (OR, 0.29; 95% CI, 0.10–0.85).
Related Article: Update on Fertility G. David Adamson, MD; Mary E. Abusief, MD (February 2014)
Bleeding outcomes were not measured
Strengths of this systematic review are its inclusion of high-quality, randomized, controlled trials and its assessment of ovarian-failure rates. However, a major weakness is the fact that its design does not allow for discrete evaluation of bleeding outcomes. Nor can its findings be broken down by the type of leiomyoma being treated.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This review demonstrates that women are satisfied with outcomes five years after UAE and that ovarian failure is not more common after UAE than after surgery. Although the available evidence demonstrates that pregnancy following UAE is possible, women requiring a surgical procedure for AUB-L who are uncertain about their childbearing plans or who are hoping to conceive should be encouraged to select myomectomy as their intervention of choice.
AUB-A: FOR ADENOMYOSIS-ASSOCIATED AUB, CONSIDER THE LNG-IUS AS AN ALTERNATIVE TO HYSTERECTOMY
Ozdegirmenci O, Kayikcioglu F, Akgul MA, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011;95(2):497–502.
In a small randomized, controlled trial of the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena) versus hysterectomy for adenomyosis-associated AUB, women allocated to the LNG-IUS experienced a reduction in bleeding and comparable gains in hemoglobin values during the first year of use. Both the LNG-IUS and hysterectomy improved health-related quality of life, but the LNG-IUS was associated with superior improvements in measures of psychological and social functioning.
Related Article: Update: Minimally invasive gynecology Amy Garcia, MD (April 2013)
Details and general findings of the trial
Eighty-six women were enrolled in the trial after exclusion of endometrial pathology as a cause of their heavy menstrual bleeding and after transvaginal ultrasound and magnetic resonance imaging findings were consistent with the diagnosis of adenomyosis. Participants then were randomly assigned to undergo hysterectomy or insertion of an LNG-IUS (43 women in each group). At baseline, the mean (SD) age was 44.28 (4.36) years among women in the LNG-IUS group versus 46.38 (3.76) years among women undergoing hysterectomy (P = .032), a statistical difference that I suspect is not clinically significant.
Menstrual bleeding, hemoglobin levels, and quality of life were assessed prior to insertion or surgery, and again at 6- and 12-month follow-up. Eleven women in the hysterectomy group were lost to follow-up.
General findings of the trial include:
- Women in the LNG-IUS group had a mean reduction in the volume of menstrual bleeding—as measured by the number of pads used—from two pads to one pad at 6 months, remaining at that level until 12 months. Serum hemoglobin levels increased from a median of just over
11 g/dL at the time of insertion to 13 g/dL at 6 months and slightly higher at 12 months. In the five self-reported quality-of-life domains assessed (physical, psychological, social, environmental, and a national environmental domain), women using the LNG-IUS demonstrated improvement in all five. - Women in the hysterectomy group were treated using an abdominal surgical approach, with one patient experiencing postoperative wound infection that required secondary suture. Postoperative pathologic analysis found that 21 of these women (65.6%) had adenomyosis, six women (18.8%) had myomas, three women (9.4%) had both adenomyosis and a myoma, and two women (6.2%) had a normal uterus. Serum hemoglobin levels increased from a median of roughly 10.5 g/dL at the time of treatment to 13 g/dL at 6 months and slightly higher at 12 months. (There were no statistically significant differences in hemoglobin values between the LNG-IUS and hysterectomy groups at any point in the study.) Quality of life improved in three of the five domains assessed (physical and both environmental domains).
Although 11 women were lost to follow-up, this trial appeared to have an adequate sample size to examine the selected outcomes, and the population was well defined.
Two weaknesses were the limited follow-up (only 12 months) and the use of quality-of-life measures designed for a Turkish population (the trial was conducted in Turkey), which may or may not be fully applicable to a US population.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
The relationship of adenomyosis to gynecologic symptoms, including heavy menstrual bleeding and dysmenorrhea, needs further study. However, this trial confirmed that transvaginal ultrasound is helpful in the nonsurgical diagnosis of adenomyosis and suggests that the LNG-IUS may be as effective at 1 year as hysterectomy for the treatment of adenomyosis-associated heavy menstrual bleeding (AUB-A).
Clinicians who perform office-based ultrasound to assess AUB should familiarize themselves with the criteria for ultrasonic diagnosis of adenomyosis. These criteria include the presence of heterogeneous myometrial echogenicity, a loss of clarity of the endo-myometrial interface, typically radially oriented linear striations, the appearance of myometrial cysts, and an overall globular enlarged uterus characterized by asymmetric thickening of the myometrium.2
In patients with heavy menstrual bleeding who have these findings, particularly if there is coexistent dysmenorrhea and uterine tenderness, it behooves the clinician to consider the LNG-IUS as first-line therapy, especially for women who wish to preserve fertility, but also for women for whom fertility is not an issue.
There is some evidence that the therapeutic effect of the LNG-IUS containing 20 µg of levonorgestrel may start to fade at 2 or 3 years, a possibility that should be shared with patients.3 Other features, such as cavity size, thickness of the myometrium, and the coexistence of clinically relevant leiomyomas, have not been evaluated but may have an impact on the clinical response.
AUB-I: LOW-DOSE DOXYCYCLINE REDUCES THE TIME TO AMENORRHEA IN USERS OF CONTINUOUS ORAL CONTRACEPTIVES
Kaneshiro B, Edelman A, Carlson NE, Nichols M, Forbes MM, Jensen J. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception. 2012;85(4):351–358.
Unscheduled bleeding is the most common complaint among women who use continuous combination oral contraceptives (OCs). Because unscheduled bleeding has been correlated with the upregulation of matrix metalloprotineases (MMPs), Kaneshiro and colleagues conducted a randomized, controlled trial of doxycycline (an MMP inhibitor) versus placebo among users of continuous OCs. The addition of doxycycline to the OC regimen did not significantly reduce unscheduled bleeding during the first 84 days of use, but it did shorten the time required to achieve amenorrhea (mean of 61.7 days for doxycycline vs 85.2 days for placebo; standard error [SE], 7.7 vs 6.7, respectively; P = .03).
Related Article: Big step forward and downward: An OC with 10 μg of estrogen Robert L. Barbieri, MD (Editorial, May 2011)
Details and general findings of the trial
Participants (n = 65) were healthy women aged 18 to 45 years who had no contraindications to continuous use of combination OCs. Prior to enrollment, they all had used cyclic combination contraception (pill, patch, or ring) without unscheduled bleeding, thereby avoiding the “transition bleeding” that often occurs when continuous OCs are initiated.
All women in the trial were started on continuous OCs (20 µg ethinyl estradiol with 100 µg levonorgestrel; Aviane) and then randomly assigned to receive one of the following for 84 days in addition to the OC:
- doxycycline 40 mg daily (controlled-release Oracea), a subantimicrobial dose
- placebo.
After 84 days, doxycycline was discontinued, and participants were observed for an additional 28 days on the OC regimen alone for the documentation of bleeding patterns.
General findings:
- The number of bleeding and spotting days decreased in both groups over the course of the study.
- During the first 84 days of the trial, bleeding and spotting occurred among a median of 11 and 17 women in the doxycycline and placebo groups, respectively, and bleeding alone (without spotting) occurred in a median 3 and 4 women in the doxycycline and placebo groups, respectively.
- During the 28-day observation period, bleeding and spotting occurred among a median of 0 and 6 women in the doxycycline and placebo groups, respectively. Bleeding alone (without spotting) was absent in both groups.
- Women in the doxycycline group were significantly less likely to report side effects such as headache, depressed mood, and abdominal cramping. However, they were more likely to prefer continuous OCs without doxycycline, compared with women receiving placebo (16.1% vs 10.7%).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This trial increases our insight into AUB associated with the use of progestins and suggests that concomitant doxycycline may reduce unscheduled bleeding and spotting in women using continuous combination OCs. The trial was of adequate sample size for the primary outcomes, lending credence to its findings, although longer-term data would be helpful.
I have included this trial for two reasons:
It offers useful information regarding the mechanisms and potential prevention or reduction of AUB-I in users of continuous combined estrogen-progestin contraception.
Doxycycline is one of the agents covered in a Cochrane review of high-quality research into AUB-I in women using progestin-only products, including injectables, implantables, intrauterine systems, and oral agents.4 Estrogens have been shown to have some value in reducing breakthrough bleeding associated with depot medroxyprogesterone acetate, and individual use of tranexamic acid or doxycycline has shown value in terminating an episode of breakthrough bleeding in women using progestin-only contraceptives.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. The FIGO classification for causes of abnormal bleeding in the reproductive years. Fertil Steril. 2011;95(7):2204–2208.
- Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: Systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89(11):1374–1384.
- Cho S, Nam A, Kim H, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198(4):373.e1–e7.
- Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gaffield ML, Gulmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin-only contraceptives. Cochrane Database Syst Rev. 2013;10:CD003449.
As recently defined by the International Federation of Gynecology and Obstetrics (FIGO)—and endorsed by the American College of Obstetricians and Gynecologists—the term “abnormal uterine bleeding” (AUB) now describes any departure from normal menstrual bleeding.1 To determine the most appropriate intervention for this widespread problem, FIGO proposed that clinicians consider potential contributors to the clinical problem by investigating and categorizing patients according to the following system:
- Polyp
- Adenomyosis
- Leiomyoma
- Malignancy and hyperplasia
- Coagulopathy
- Ovulatory disorders
- Endometrial dysfunction
- Iatrogenic
- Not otherwise classified.
A given individual may be found to have one or more of these features, but not all of the features may contribute to the AUB. To facilitate their use, these nine causes are more commonly identified using the acronym PALM-COEIN.
In this article, I focus on three of these categories, presenting recent data on AUB associated with leiomyomata (AUB-L) or adenomyosis (AUB-A), and AUB of an iatrogenic nature (AUB-I).
AUB-L: SATISFACTION RATES ARE SIMILAR 5 YEARS AFTER FIBROID TREATMENT BY SURGERY OR UTERINE ARTERY EMBOLIZATION
Gupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2012;5:CD005073. doi:10.1002/14651858.CD005073.pub3.
Women who undergo uterine artery embolization (UAE) for the treatment of symptomatic uterine fibroids are just as satisfied with the outcome as women treated with hysterectomy or myomectomy, according to this 2012 review from the Cochrane Database.
Gupta and colleagues found similar patient-satisfaction rates at 5 years (odds ratio [OR] 0.9; 95% confidence interval [CI], 0.45–1.8), although women undergoing UAE were more likely to require additional interventions within 2 years (56 additional interventions per 1,000 women for surgery vs 250 per 1,000 women for UAE; OR, 5.64).
Details and general findings
Gupta and colleagues selected randomized, controlled trials comparing UAE with surgery:
- three trials of UAE versus abdominal hysterectomy (n = 291)
- one trial of UAE versus hysterectomy or myomectomy (the specific surgery was determined by patient preference) (n = 157)
- one trial of UAE versus myomectomy in women desiring future childbearing (n = 121).
In these trials, UAE was bilateral and involved the use of permanent embolic material.
Among the findings:
- Costs were lower with UAE, as assessed by measuring the duration of the procedure, length of hospitalization, and time to resumption of normal activities.
- Ovarian-failure rates were comparable between women in the UAE and surgery groups. Ovarian function was assessed by measuring follicle-stimulating hormone (FSH), although FSH thresholds varied in some of the studies.
- Pregnancy was less likely after UAE than after myomectomy. In the trial comparing UAE with myomectomy, 26 women later tried to conceive after UAE versus 40 after myomectomy. Significantly fewer women became pregnant after UAE (OR, 0.29; 95% CI, 0.10–0.85).
Related Article: Update on Fertility G. David Adamson, MD; Mary E. Abusief, MD (February 2014)
Bleeding outcomes were not measured
Strengths of this systematic review are its inclusion of high-quality, randomized, controlled trials and its assessment of ovarian-failure rates. However, a major weakness is the fact that its design does not allow for discrete evaluation of bleeding outcomes. Nor can its findings be broken down by the type of leiomyoma being treated.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This review demonstrates that women are satisfied with outcomes five years after UAE and that ovarian failure is not more common after UAE than after surgery. Although the available evidence demonstrates that pregnancy following UAE is possible, women requiring a surgical procedure for AUB-L who are uncertain about their childbearing plans or who are hoping to conceive should be encouraged to select myomectomy as their intervention of choice.
AUB-A: FOR ADENOMYOSIS-ASSOCIATED AUB, CONSIDER THE LNG-IUS AS AN ALTERNATIVE TO HYSTERECTOMY
Ozdegirmenci O, Kayikcioglu F, Akgul MA, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011;95(2):497–502.
In a small randomized, controlled trial of the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena) versus hysterectomy for adenomyosis-associated AUB, women allocated to the LNG-IUS experienced a reduction in bleeding and comparable gains in hemoglobin values during the first year of use. Both the LNG-IUS and hysterectomy improved health-related quality of life, but the LNG-IUS was associated with superior improvements in measures of psychological and social functioning.
Related Article: Update: Minimally invasive gynecology Amy Garcia, MD (April 2013)
Details and general findings of the trial
Eighty-six women were enrolled in the trial after exclusion of endometrial pathology as a cause of their heavy menstrual bleeding and after transvaginal ultrasound and magnetic resonance imaging findings were consistent with the diagnosis of adenomyosis. Participants then were randomly assigned to undergo hysterectomy or insertion of an LNG-IUS (43 women in each group). At baseline, the mean (SD) age was 44.28 (4.36) years among women in the LNG-IUS group versus 46.38 (3.76) years among women undergoing hysterectomy (P = .032), a statistical difference that I suspect is not clinically significant.
Menstrual bleeding, hemoglobin levels, and quality of life were assessed prior to insertion or surgery, and again at 6- and 12-month follow-up. Eleven women in the hysterectomy group were lost to follow-up.
General findings of the trial include:
- Women in the LNG-IUS group had a mean reduction in the volume of menstrual bleeding—as measured by the number of pads used—from two pads to one pad at 6 months, remaining at that level until 12 months. Serum hemoglobin levels increased from a median of just over
11 g/dL at the time of insertion to 13 g/dL at 6 months and slightly higher at 12 months. In the five self-reported quality-of-life domains assessed (physical, psychological, social, environmental, and a national environmental domain), women using the LNG-IUS demonstrated improvement in all five. - Women in the hysterectomy group were treated using an abdominal surgical approach, with one patient experiencing postoperative wound infection that required secondary suture. Postoperative pathologic analysis found that 21 of these women (65.6%) had adenomyosis, six women (18.8%) had myomas, three women (9.4%) had both adenomyosis and a myoma, and two women (6.2%) had a normal uterus. Serum hemoglobin levels increased from a median of roughly 10.5 g/dL at the time of treatment to 13 g/dL at 6 months and slightly higher at 12 months. (There were no statistically significant differences in hemoglobin values between the LNG-IUS and hysterectomy groups at any point in the study.) Quality of life improved in three of the five domains assessed (physical and both environmental domains).
Although 11 women were lost to follow-up, this trial appeared to have an adequate sample size to examine the selected outcomes, and the population was well defined.
Two weaknesses were the limited follow-up (only 12 months) and the use of quality-of-life measures designed for a Turkish population (the trial was conducted in Turkey), which may or may not be fully applicable to a US population.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
The relationship of adenomyosis to gynecologic symptoms, including heavy menstrual bleeding and dysmenorrhea, needs further study. However, this trial confirmed that transvaginal ultrasound is helpful in the nonsurgical diagnosis of adenomyosis and suggests that the LNG-IUS may be as effective at 1 year as hysterectomy for the treatment of adenomyosis-associated heavy menstrual bleeding (AUB-A).
Clinicians who perform office-based ultrasound to assess AUB should familiarize themselves with the criteria for ultrasonic diagnosis of adenomyosis. These criteria include the presence of heterogeneous myometrial echogenicity, a loss of clarity of the endo-myometrial interface, typically radially oriented linear striations, the appearance of myometrial cysts, and an overall globular enlarged uterus characterized by asymmetric thickening of the myometrium.2
In patients with heavy menstrual bleeding who have these findings, particularly if there is coexistent dysmenorrhea and uterine tenderness, it behooves the clinician to consider the LNG-IUS as first-line therapy, especially for women who wish to preserve fertility, but also for women for whom fertility is not an issue.
There is some evidence that the therapeutic effect of the LNG-IUS containing 20 µg of levonorgestrel may start to fade at 2 or 3 years, a possibility that should be shared with patients.3 Other features, such as cavity size, thickness of the myometrium, and the coexistence of clinically relevant leiomyomas, have not been evaluated but may have an impact on the clinical response.
AUB-I: LOW-DOSE DOXYCYCLINE REDUCES THE TIME TO AMENORRHEA IN USERS OF CONTINUOUS ORAL CONTRACEPTIVES
Kaneshiro B, Edelman A, Carlson NE, Nichols M, Forbes MM, Jensen J. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception. 2012;85(4):351–358.
Unscheduled bleeding is the most common complaint among women who use continuous combination oral contraceptives (OCs). Because unscheduled bleeding has been correlated with the upregulation of matrix metalloprotineases (MMPs), Kaneshiro and colleagues conducted a randomized, controlled trial of doxycycline (an MMP inhibitor) versus placebo among users of continuous OCs. The addition of doxycycline to the OC regimen did not significantly reduce unscheduled bleeding during the first 84 days of use, but it did shorten the time required to achieve amenorrhea (mean of 61.7 days for doxycycline vs 85.2 days for placebo; standard error [SE], 7.7 vs 6.7, respectively; P = .03).
Related Article: Big step forward and downward: An OC with 10 μg of estrogen Robert L. Barbieri, MD (Editorial, May 2011)
Details and general findings of the trial
Participants (n = 65) were healthy women aged 18 to 45 years who had no contraindications to continuous use of combination OCs. Prior to enrollment, they all had used cyclic combination contraception (pill, patch, or ring) without unscheduled bleeding, thereby avoiding the “transition bleeding” that often occurs when continuous OCs are initiated.
All women in the trial were started on continuous OCs (20 µg ethinyl estradiol with 100 µg levonorgestrel; Aviane) and then randomly assigned to receive one of the following for 84 days in addition to the OC:
- doxycycline 40 mg daily (controlled-release Oracea), a subantimicrobial dose
- placebo.
After 84 days, doxycycline was discontinued, and participants were observed for an additional 28 days on the OC regimen alone for the documentation of bleeding patterns.
General findings:
- The number of bleeding and spotting days decreased in both groups over the course of the study.
- During the first 84 days of the trial, bleeding and spotting occurred among a median of 11 and 17 women in the doxycycline and placebo groups, respectively, and bleeding alone (without spotting) occurred in a median 3 and 4 women in the doxycycline and placebo groups, respectively.
- During the 28-day observation period, bleeding and spotting occurred among a median of 0 and 6 women in the doxycycline and placebo groups, respectively. Bleeding alone (without spotting) was absent in both groups.
- Women in the doxycycline group were significantly less likely to report side effects such as headache, depressed mood, and abdominal cramping. However, they were more likely to prefer continuous OCs without doxycycline, compared with women receiving placebo (16.1% vs 10.7%).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This trial increases our insight into AUB associated with the use of progestins and suggests that concomitant doxycycline may reduce unscheduled bleeding and spotting in women using continuous combination OCs. The trial was of adequate sample size for the primary outcomes, lending credence to its findings, although longer-term data would be helpful.
I have included this trial for two reasons:
It offers useful information regarding the mechanisms and potential prevention or reduction of AUB-I in users of continuous combined estrogen-progestin contraception.
Doxycycline is one of the agents covered in a Cochrane review of high-quality research into AUB-I in women using progestin-only products, including injectables, implantables, intrauterine systems, and oral agents.4 Estrogens have been shown to have some value in reducing breakthrough bleeding associated with depot medroxyprogesterone acetate, and individual use of tranexamic acid or doxycycline has shown value in terminating an episode of breakthrough bleeding in women using progestin-only contraceptives.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
As recently defined by the International Federation of Gynecology and Obstetrics (FIGO)—and endorsed by the American College of Obstetricians and Gynecologists—the term “abnormal uterine bleeding” (AUB) now describes any departure from normal menstrual bleeding.1 To determine the most appropriate intervention for this widespread problem, FIGO proposed that clinicians consider potential contributors to the clinical problem by investigating and categorizing patients according to the following system:
- Polyp
- Adenomyosis
- Leiomyoma
- Malignancy and hyperplasia
- Coagulopathy
- Ovulatory disorders
- Endometrial dysfunction
- Iatrogenic
- Not otherwise classified.
A given individual may be found to have one or more of these features, but not all of the features may contribute to the AUB. To facilitate their use, these nine causes are more commonly identified using the acronym PALM-COEIN.
In this article, I focus on three of these categories, presenting recent data on AUB associated with leiomyomata (AUB-L) or adenomyosis (AUB-A), and AUB of an iatrogenic nature (AUB-I).
AUB-L: SATISFACTION RATES ARE SIMILAR 5 YEARS AFTER FIBROID TREATMENT BY SURGERY OR UTERINE ARTERY EMBOLIZATION
Gupta JK, Sinha A, Lumsden MA, Hickey M. Uterine artery embolization for symptomatic uterine fibroids. Cochrane Database Syst Rev. 2012;5:CD005073. doi:10.1002/14651858.CD005073.pub3.
Women who undergo uterine artery embolization (UAE) for the treatment of symptomatic uterine fibroids are just as satisfied with the outcome as women treated with hysterectomy or myomectomy, according to this 2012 review from the Cochrane Database.
Gupta and colleagues found similar patient-satisfaction rates at 5 years (odds ratio [OR] 0.9; 95% confidence interval [CI], 0.45–1.8), although women undergoing UAE were more likely to require additional interventions within 2 years (56 additional interventions per 1,000 women for surgery vs 250 per 1,000 women for UAE; OR, 5.64).
Details and general findings
Gupta and colleagues selected randomized, controlled trials comparing UAE with surgery:
- three trials of UAE versus abdominal hysterectomy (n = 291)
- one trial of UAE versus hysterectomy or myomectomy (the specific surgery was determined by patient preference) (n = 157)
- one trial of UAE versus myomectomy in women desiring future childbearing (n = 121).
In these trials, UAE was bilateral and involved the use of permanent embolic material.
Among the findings:
- Costs were lower with UAE, as assessed by measuring the duration of the procedure, length of hospitalization, and time to resumption of normal activities.
- Ovarian-failure rates were comparable between women in the UAE and surgery groups. Ovarian function was assessed by measuring follicle-stimulating hormone (FSH), although FSH thresholds varied in some of the studies.
- Pregnancy was less likely after UAE than after myomectomy. In the trial comparing UAE with myomectomy, 26 women later tried to conceive after UAE versus 40 after myomectomy. Significantly fewer women became pregnant after UAE (OR, 0.29; 95% CI, 0.10–0.85).
Related Article: Update on Fertility G. David Adamson, MD; Mary E. Abusief, MD (February 2014)
Bleeding outcomes were not measured
Strengths of this systematic review are its inclusion of high-quality, randomized, controlled trials and its assessment of ovarian-failure rates. However, a major weakness is the fact that its design does not allow for discrete evaluation of bleeding outcomes. Nor can its findings be broken down by the type of leiomyoma being treated.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This review demonstrates that women are satisfied with outcomes five years after UAE and that ovarian failure is not more common after UAE than after surgery. Although the available evidence demonstrates that pregnancy following UAE is possible, women requiring a surgical procedure for AUB-L who are uncertain about their childbearing plans or who are hoping to conceive should be encouraged to select myomectomy as their intervention of choice.
AUB-A: FOR ADENOMYOSIS-ASSOCIATED AUB, CONSIDER THE LNG-IUS AS AN ALTERNATIVE TO HYSTERECTOMY
Ozdegirmenci O, Kayikcioglu F, Akgul MA, et al. Comparison of levonorgestrel intrauterine system versus hysterectomy on efficacy and quality of life in patients with adenomyosis. Fertil Steril. 2011;95(2):497–502.
In a small randomized, controlled trial of the levonorgestrel-releasing intrauterine system (LNG-IUS; Mirena) versus hysterectomy for adenomyosis-associated AUB, women allocated to the LNG-IUS experienced a reduction in bleeding and comparable gains in hemoglobin values during the first year of use. Both the LNG-IUS and hysterectomy improved health-related quality of life, but the LNG-IUS was associated with superior improvements in measures of psychological and social functioning.
Related Article: Update: Minimally invasive gynecology Amy Garcia, MD (April 2013)
Details and general findings of the trial
Eighty-six women were enrolled in the trial after exclusion of endometrial pathology as a cause of their heavy menstrual bleeding and after transvaginal ultrasound and magnetic resonance imaging findings were consistent with the diagnosis of adenomyosis. Participants then were randomly assigned to undergo hysterectomy or insertion of an LNG-IUS (43 women in each group). At baseline, the mean (SD) age was 44.28 (4.36) years among women in the LNG-IUS group versus 46.38 (3.76) years among women undergoing hysterectomy (P = .032), a statistical difference that I suspect is not clinically significant.
Menstrual bleeding, hemoglobin levels, and quality of life were assessed prior to insertion or surgery, and again at 6- and 12-month follow-up. Eleven women in the hysterectomy group were lost to follow-up.
General findings of the trial include:
- Women in the LNG-IUS group had a mean reduction in the volume of menstrual bleeding—as measured by the number of pads used—from two pads to one pad at 6 months, remaining at that level until 12 months. Serum hemoglobin levels increased from a median of just over
11 g/dL at the time of insertion to 13 g/dL at 6 months and slightly higher at 12 months. In the five self-reported quality-of-life domains assessed (physical, psychological, social, environmental, and a national environmental domain), women using the LNG-IUS demonstrated improvement in all five. - Women in the hysterectomy group were treated using an abdominal surgical approach, with one patient experiencing postoperative wound infection that required secondary suture. Postoperative pathologic analysis found that 21 of these women (65.6%) had adenomyosis, six women (18.8%) had myomas, three women (9.4%) had both adenomyosis and a myoma, and two women (6.2%) had a normal uterus. Serum hemoglobin levels increased from a median of roughly 10.5 g/dL at the time of treatment to 13 g/dL at 6 months and slightly higher at 12 months. (There were no statistically significant differences in hemoglobin values between the LNG-IUS and hysterectomy groups at any point in the study.) Quality of life improved in three of the five domains assessed (physical and both environmental domains).
Although 11 women were lost to follow-up, this trial appeared to have an adequate sample size to examine the selected outcomes, and the population was well defined.
Two weaknesses were the limited follow-up (only 12 months) and the use of quality-of-life measures designed for a Turkish population (the trial was conducted in Turkey), which may or may not be fully applicable to a US population.
WHAT THIS EVIDENCE MEANS FOR PRACTICE
The relationship of adenomyosis to gynecologic symptoms, including heavy menstrual bleeding and dysmenorrhea, needs further study. However, this trial confirmed that transvaginal ultrasound is helpful in the nonsurgical diagnosis of adenomyosis and suggests that the LNG-IUS may be as effective at 1 year as hysterectomy for the treatment of adenomyosis-associated heavy menstrual bleeding (AUB-A).
Clinicians who perform office-based ultrasound to assess AUB should familiarize themselves with the criteria for ultrasonic diagnosis of adenomyosis. These criteria include the presence of heterogeneous myometrial echogenicity, a loss of clarity of the endo-myometrial interface, typically radially oriented linear striations, the appearance of myometrial cysts, and an overall globular enlarged uterus characterized by asymmetric thickening of the myometrium.2
In patients with heavy menstrual bleeding who have these findings, particularly if there is coexistent dysmenorrhea and uterine tenderness, it behooves the clinician to consider the LNG-IUS as first-line therapy, especially for women who wish to preserve fertility, but also for women for whom fertility is not an issue.
There is some evidence that the therapeutic effect of the LNG-IUS containing 20 µg of levonorgestrel may start to fade at 2 or 3 years, a possibility that should be shared with patients.3 Other features, such as cavity size, thickness of the myometrium, and the coexistence of clinically relevant leiomyomas, have not been evaluated but may have an impact on the clinical response.
AUB-I: LOW-DOSE DOXYCYCLINE REDUCES THE TIME TO AMENORRHEA IN USERS OF CONTINUOUS ORAL CONTRACEPTIVES
Kaneshiro B, Edelman A, Carlson NE, Nichols M, Forbes MM, Jensen J. A randomized controlled trial of subantimicrobial-dose doxycycline to prevent unscheduled bleeding with continuous oral contraceptive pill use. Contraception. 2012;85(4):351–358.
Unscheduled bleeding is the most common complaint among women who use continuous combination oral contraceptives (OCs). Because unscheduled bleeding has been correlated with the upregulation of matrix metalloprotineases (MMPs), Kaneshiro and colleagues conducted a randomized, controlled trial of doxycycline (an MMP inhibitor) versus placebo among users of continuous OCs. The addition of doxycycline to the OC regimen did not significantly reduce unscheduled bleeding during the first 84 days of use, but it did shorten the time required to achieve amenorrhea (mean of 61.7 days for doxycycline vs 85.2 days for placebo; standard error [SE], 7.7 vs 6.7, respectively; P = .03).
Related Article: Big step forward and downward: An OC with 10 μg of estrogen Robert L. Barbieri, MD (Editorial, May 2011)
Details and general findings of the trial
Participants (n = 65) were healthy women aged 18 to 45 years who had no contraindications to continuous use of combination OCs. Prior to enrollment, they all had used cyclic combination contraception (pill, patch, or ring) without unscheduled bleeding, thereby avoiding the “transition bleeding” that often occurs when continuous OCs are initiated.
All women in the trial were started on continuous OCs (20 µg ethinyl estradiol with 100 µg levonorgestrel; Aviane) and then randomly assigned to receive one of the following for 84 days in addition to the OC:
- doxycycline 40 mg daily (controlled-release Oracea), a subantimicrobial dose
- placebo.
After 84 days, doxycycline was discontinued, and participants were observed for an additional 28 days on the OC regimen alone for the documentation of bleeding patterns.
General findings:
- The number of bleeding and spotting days decreased in both groups over the course of the study.
- During the first 84 days of the trial, bleeding and spotting occurred among a median of 11 and 17 women in the doxycycline and placebo groups, respectively, and bleeding alone (without spotting) occurred in a median 3 and 4 women in the doxycycline and placebo groups, respectively.
- During the 28-day observation period, bleeding and spotting occurred among a median of 0 and 6 women in the doxycycline and placebo groups, respectively. Bleeding alone (without spotting) was absent in both groups.
- Women in the doxycycline group were significantly less likely to report side effects such as headache, depressed mood, and abdominal cramping. However, they were more likely to prefer continuous OCs without doxycycline, compared with women receiving placebo (16.1% vs 10.7%).
WHAT THIS EVIDENCE MEANS FOR PRACTICE
This trial increases our insight into AUB associated with the use of progestins and suggests that concomitant doxycycline may reduce unscheduled bleeding and spotting in women using continuous combination OCs. The trial was of adequate sample size for the primary outcomes, lending credence to its findings, although longer-term data would be helpful.
I have included this trial for two reasons:
It offers useful information regarding the mechanisms and potential prevention or reduction of AUB-I in users of continuous combined estrogen-progestin contraception.
Doxycycline is one of the agents covered in a Cochrane review of high-quality research into AUB-I in women using progestin-only products, including injectables, implantables, intrauterine systems, and oral agents.4 Estrogens have been shown to have some value in reducing breakthrough bleeding associated with depot medroxyprogesterone acetate, and individual use of tranexamic acid or doxycycline has shown value in terminating an episode of breakthrough bleeding in women using progestin-only contraceptives.
TELL US WHAT YOU THINK!
Share your thoughts on this article or on any topic relevant to ObGyns and women’s health practitioners. Tell us which topics you’d like to see covered in future issues, and what challenges you face in daily practice. We will consider publishing your letter and in a future issue.
Send your letter to: [email protected] Please include the city and state in which you practice.
Stay in touch! Your feedback is important to us!
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. The FIGO classification for causes of abnormal bleeding in the reproductive years. Fertil Steril. 2011;95(7):2204–2208.
- Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: Systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89(11):1374–1384.
- Cho S, Nam A, Kim H, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198(4):373.e1–e7.
- Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gaffield ML, Gulmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin-only contraceptives. Cochrane Database Syst Rev. 2013;10:CD003449.
- Munro MG, Critchley HO, Broder MS, Fraser IS; FIGO Working Group on Menstrual Disorders. The FIGO classification for causes of abnormal bleeding in the reproductive years. Fertil Steril. 2011;95(7):2204–2208.
- Champaneria R, Abedin P, Daniels J, Balogun M, Khan KS. Ultrasound scan and magnetic resonance imaging for the diagnosis of adenomyosis: Systematic review comparing test accuracy. Acta Obstet Gynecol Scand. 2010;89(11):1374–1384.
- Cho S, Nam A, Kim H, et al. Clinical effects of the levonorgestrel-releasing intrauterine device in patients with adenomyosis. Am J Obstet Gynecol. 2008;198(4):373.e1–e7.
- Abdel-Aleem H, d’Arcangues C, Vogelsong KM, Gaffield ML, Gulmezoglu AM. Treatment of vaginal bleeding irregularities induced by progestin-only contraceptives. Cochrane Database Syst Rev. 2013;10:CD003449.
Best age to begin screening mammograms: How I manage my patients
Controversy has surrounded the utility of screening mammograms, particularly in women in their 40s. In 2009, the US Preventive Services Task Force recommended that screening mammography begin at age 50 and that women aged 50 to 74 receive a mammogram every 2 years.1 However, the American Cancer Society2 and other professional groups continue to recommend that annual screening begin at age 40, leading to controversy and confusion among women’s health clinicians and our patients.
In a recent study, Webb and colleagues3 used registry data based on a health plan in a single US city to assess the cause of death and mammogram history of 1,705 women who died following a diagnosis of invasive breast cancer from 1990 to 1999. They confirmed that 609 of these deaths were from breast cancer. How many of these patients were screened?
What did they find?
The investigators found that 29% of the 609 women who died from breast cancer had been screened for it—19% of the cancers that caused death were screen-detected and 10% were interval cancers. (Interval cancers were defined as symptomatic or palpable tumors that presented less than 2 years after the prior screening mammogram.) That means that 71% of 609 deaths from breast cancer were among unscreened women, with 6% of the fatal cancers diagnosed more than 2 years after the last mammogram and 65% never found upon screening because screening did not occur.
Among deaths caused (n = 609) and not caused (n = 905) by breast cancer, the median age at diagnosis was 49 and 72 years, respectively. Investigators concluded that regular screening of women younger than age 50 years would lower the death rate from breast cancer.
Related Article: Biennial vs annual mammography: How I manage my patients Andrew M. Kaunitz, MD (June 2013)
Let’s not jump to any conclusions
Although some may find the report by Webb and colleagues persuasive, I am concerned about this study’s limitations, of which there are a few. First, analyses that focus on women diagnosed with breast cancers do not allow comparison of outcomes among screened and unscreened populations.
Moreover, this report provides no information on treatment received by screened and unscreened women. It is likely that women who have never been screened, or who have been screened only infrequently, are considerably less affluent and less educated than women who are regularly screened. Accordingly, upon noting a palpable breast mass, unscreened women may be less likely to seek timely medical attention than regularly screened women, leading to differences in breast cancer outcomes, which are independent of screening history.
How I counsel my patients
For now, I will continue to be laissez-fare in my recommendations about screening mammograms for average-risk women in their 40s by supporting their individual preferences about when to initiate such screening.
- Screening for breast cancer, Topic Page. US Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm. Updated July 2010. Accessed October 28, 2013.
- American Cancer Society Guidelines for the Early Detection of Cancer: Breast cancer. American Cancer Society Web site. http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Updated May 3, 2013. Accessed October 28, 2013.
- Webb ML, Cady B, Michaelson JS, et al. A failure analysis of invasive breast cancer: Most deaths from disease occur in women not regularly screened [published online ahead of print September 9, 2013]. Cancer. doi:10.1002/cncr.28199.
Controversy has surrounded the utility of screening mammograms, particularly in women in their 40s. In 2009, the US Preventive Services Task Force recommended that screening mammography begin at age 50 and that women aged 50 to 74 receive a mammogram every 2 years.1 However, the American Cancer Society2 and other professional groups continue to recommend that annual screening begin at age 40, leading to controversy and confusion among women’s health clinicians and our patients.
In a recent study, Webb and colleagues3 used registry data based on a health plan in a single US city to assess the cause of death and mammogram history of 1,705 women who died following a diagnosis of invasive breast cancer from 1990 to 1999. They confirmed that 609 of these deaths were from breast cancer. How many of these patients were screened?
What did they find?
The investigators found that 29% of the 609 women who died from breast cancer had been screened for it—19% of the cancers that caused death were screen-detected and 10% were interval cancers. (Interval cancers were defined as symptomatic or palpable tumors that presented less than 2 years after the prior screening mammogram.) That means that 71% of 609 deaths from breast cancer were among unscreened women, with 6% of the fatal cancers diagnosed more than 2 years after the last mammogram and 65% never found upon screening because screening did not occur.
Among deaths caused (n = 609) and not caused (n = 905) by breast cancer, the median age at diagnosis was 49 and 72 years, respectively. Investigators concluded that regular screening of women younger than age 50 years would lower the death rate from breast cancer.
Related Article: Biennial vs annual mammography: How I manage my patients Andrew M. Kaunitz, MD (June 2013)
Let’s not jump to any conclusions
Although some may find the report by Webb and colleagues persuasive, I am concerned about this study’s limitations, of which there are a few. First, analyses that focus on women diagnosed with breast cancers do not allow comparison of outcomes among screened and unscreened populations.
Moreover, this report provides no information on treatment received by screened and unscreened women. It is likely that women who have never been screened, or who have been screened only infrequently, are considerably less affluent and less educated than women who are regularly screened. Accordingly, upon noting a palpable breast mass, unscreened women may be less likely to seek timely medical attention than regularly screened women, leading to differences in breast cancer outcomes, which are independent of screening history.
How I counsel my patients
For now, I will continue to be laissez-fare in my recommendations about screening mammograms for average-risk women in their 40s by supporting their individual preferences about when to initiate such screening.
Controversy has surrounded the utility of screening mammograms, particularly in women in their 40s. In 2009, the US Preventive Services Task Force recommended that screening mammography begin at age 50 and that women aged 50 to 74 receive a mammogram every 2 years.1 However, the American Cancer Society2 and other professional groups continue to recommend that annual screening begin at age 40, leading to controversy and confusion among women’s health clinicians and our patients.
In a recent study, Webb and colleagues3 used registry data based on a health plan in a single US city to assess the cause of death and mammogram history of 1,705 women who died following a diagnosis of invasive breast cancer from 1990 to 1999. They confirmed that 609 of these deaths were from breast cancer. How many of these patients were screened?
What did they find?
The investigators found that 29% of the 609 women who died from breast cancer had been screened for it—19% of the cancers that caused death were screen-detected and 10% were interval cancers. (Interval cancers were defined as symptomatic or palpable tumors that presented less than 2 years after the prior screening mammogram.) That means that 71% of 609 deaths from breast cancer were among unscreened women, with 6% of the fatal cancers diagnosed more than 2 years after the last mammogram and 65% never found upon screening because screening did not occur.
Among deaths caused (n = 609) and not caused (n = 905) by breast cancer, the median age at diagnosis was 49 and 72 years, respectively. Investigators concluded that regular screening of women younger than age 50 years would lower the death rate from breast cancer.
Related Article: Biennial vs annual mammography: How I manage my patients Andrew M. Kaunitz, MD (June 2013)
Let’s not jump to any conclusions
Although some may find the report by Webb and colleagues persuasive, I am concerned about this study’s limitations, of which there are a few. First, analyses that focus on women diagnosed with breast cancers do not allow comparison of outcomes among screened and unscreened populations.
Moreover, this report provides no information on treatment received by screened and unscreened women. It is likely that women who have never been screened, or who have been screened only infrequently, are considerably less affluent and less educated than women who are regularly screened. Accordingly, upon noting a palpable breast mass, unscreened women may be less likely to seek timely medical attention than regularly screened women, leading to differences in breast cancer outcomes, which are independent of screening history.
How I counsel my patients
For now, I will continue to be laissez-fare in my recommendations about screening mammograms for average-risk women in their 40s by supporting their individual preferences about when to initiate such screening.
- Screening for breast cancer, Topic Page. US Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm. Updated July 2010. Accessed October 28, 2013.
- American Cancer Society Guidelines for the Early Detection of Cancer: Breast cancer. American Cancer Society Web site. http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Updated May 3, 2013. Accessed October 28, 2013.
- Webb ML, Cady B, Michaelson JS, et al. A failure analysis of invasive breast cancer: Most deaths from disease occur in women not regularly screened [published online ahead of print September 9, 2013]. Cancer. doi:10.1002/cncr.28199.
- Screening for breast cancer, Topic Page. US Preventive Services Task Force. http://www.uspreventiveservicestaskforce.org/uspstf/uspsbrca.htm. Updated July 2010. Accessed October 28, 2013.
- American Cancer Society Guidelines for the Early Detection of Cancer: Breast cancer. American Cancer Society Web site. http://www.cancer.org/healthy/findcancerearly/cancerscreeningguidelines/american-cancer-society-guidelines-for-the-early-detection-of-cancer. Updated May 3, 2013. Accessed October 28, 2013.
- Webb ML, Cady B, Michaelson JS, et al. A failure analysis of invasive breast cancer: Most deaths from disease occur in women not regularly screened [published online ahead of print September 9, 2013]. Cancer. doi:10.1002/cncr.28199.
What do the latest data reveal about the safety of home birth in the United States?
Every morning before I leave for work, I kiss my three children goodbye and tell them, “I love you. Make good choices today.”
This has become my mantra—so much so that, on her way out the door to join her friends at the movies recently, my daughter turned to me and said, “I know, Dad. I know. I’ll make good decisions tonight.”
And what decision is more important than where to deliver your child and who to have in attendance at the birth?
It is said that the passage from the uterus to the outside world that each one of us was forced to negotiate at birth is the most treacherous journey we will ever undertake. Any unnecessary delay or complication can have profound, lifelong consequences.
There is no question that the past few centuries have seen a significant “medicalization” of childbirth, including the relocation of deliveries from the community to a hospital setting, the introduction of male obstetricians, the unfortunate marginalization of midwives and support personnel (doulas), the development of uterotonic drugs, and the evolution of operative vaginal (forceps, vacuum) and cesarean deliveries.
Many of the practices initially introduced by obstetric care providers (including multiple vaginal examinations in labor, induction of labor for a large baby, and active management of labor protocols) have since been shown to be unhelpful in improving pregnancy outcomes, and some practices (such as episiotomy) have even been shown to be harmful.
Related article: Difficult fetal extraction at cesarean delivery: What should you do? Robert L. Barbieri, MD (Editorial, January 2012)
In the midst of this confusion, the one voice that has been lost is that of the patient herself.
Whose birth is it anyway?
The American College of Obstetricians and Gynecologists (ACOG), the American Academy of Pediatrics (AAP), and the American College of Nurse-Midwives (ACNM) all agree that patient autonomy is paramount, and that the final decision of where to deliver and who to have in attendance should be made by the patient herself, ideally in conjunction with her family and her obstetric care provider.1–3 But an informed decision is only as good as the available data. Regrettably, the literature on how planned home birth compares with hospital delivery in terms of pregnancy outcomes in the United States are sparse.
Related article: Lay midwives the the ObGyn: Is collaboration risky? Lucia DiVenere, MA (May 2012)
How safe is home birth in the United States?
Cheng and colleagues attempt to answer this question by reviewing newborn and maternal outcomes among planned home births versus hospital deliveries in a contemporary low-risk birth cohort. Their retrospective study included low-risk women at term with a singleton vertex live birth in 2008 in 27 of the 50 states using information from the Vital Statistics Natality Data provided by the Centers for Disease Control and Prevention.
Of these 2,081,753 women, 0.58% (n = 12,039) had planned home births, and the remainder delivered in a hospital setting. Women who had an “accidental” (unintended) home birth or who delivered in a freestanding birthing center were excluded. The primary outcome was the risk of a 5-minute Apgar score less than 4. Secondary outcomes included the risk of a 5-minute Apgar score less than 7, assisted ventilation for more than 6 hours, neonatal seizures, admission to the NICU, and a series of maternal outcome measures.
Besides the outcomes listed previously (top of page 24), women with a planned home birth had fewer obstetric interventions, including operative vaginal delivery and labor induction or augmentation. They also were less likely to be given antibiotics during labor (although the authors did not distinguish between antibiotics administered for prophylaxis against group B strep or surgical-site infection versus antibiotics to treat infections such as urinary tract infections or chorioamnionitis).
Of special interest is the fact that neither a prior vaginal delivery (multiparity) nor the absence of a prior cesarean delivery was protective against these adverse events.
The women at highest risk of an adverse event were those who delivered at home under the supervision of “other midwives.” Although these providers were not well defined, this term typically refers to community-based lay midwives whose only “training” consists of an unofficial apprenticeship of variable length. Despite the absence of formal training, the lack of certification and standardization of care, and the existence of legislation in many states banning their activity, such lay midwives continue to encourage and support home birth for both low- and high-risk women in the United States.
Related article: Update on Obstetrics John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
Limitations of the study design
Although this dataset contains more than 2 million births, it includes only low-risk women at term and, therefore, is underpowered to measure outcomes such as fetal or neonatal death or birth injuries.
No data were presented on a number of important variables and outcome measures, such as the rate of or indications for cesarean delivery, the mode and frequency of intrapartum fetal monitoring, birth weight, intrapartum complications (uterine rupture, postpartum hemorrhage), blood transfusions, and infectious morbidity. The study also lacks long-term follow-up data on the infants.
That said, the study was well designed and very well written, and many of the limitations listed above are inherent in all retrospective cohort studies.
Putting these findings in context
These data are not novel, but they are remarkably consistent with other publications that have explored pregnancy outcomes in planned home birth versus hospital delivery from the Netherlands, the United Kingdom, Australia, and the United States, all of which show a higher rate of neonatal complications with planned home birth [see Reference 4 for review].4
Moreover, it is likely that the data in the current report significantly underestimate the risks of planned home birth for two reasons:
- Attempted home births that ended in transfer and, ultimately, delivery in a hospital setting (presumably for some unforeseen event such as excessive hemorrhage or uterine rupture or cord prolapse or nonreassuring fetal testing) were classified as hospital births.
- Apgar scores at 5 minutes are assigned by the attending care provider, and there is no way to independently verify their accuracy. Because of their limited training and/or concern about efforts to limit the scope of their practice, “other midwives” may be inclined to assign more favorable Apgar scores.
Who is choosing to deliver at home?
The proportion of US women who delivered outside the hospital setting increased by 29% between 2004 and 2009,5 although home births still constitute a minority of low-risk births (0.58% in the current study).
Related Article: Why are well-educated women more likely to choose home birth? Errol R. Norwitz, MD, PhD (Audiocast, November 2013)
One of the more interesting questions raised by this publication is the issue of who is choosing to deliver at home. In this cohort, women who planned home birth were more likely to be older, married, multiparous, white, and well educated. These aren’t exactly the women you would expect to gamble with the lives of their unborn offspring. So why are they choosing to deliver at home?
It could be that they are not well informed about the risks. Alternatively, they may have concluded that, although the relative risk of an adverse event is significantly higher with home birth, the absolute risk is low and acceptable to them. Or it could be that they are frustrated by the lack of autonomy afforded to them in the decisions surrounding antenatal care and the birthing process.
In recent years, more women are asking for minimally invasive births that are physically, emotionally, and socially supported. As hospital-based obstetric care providers, we do not always respect or meet these expectations. We can and should do better.
Women should not have to choose between a good birth experience and medical safety, between social support and hospital resources, between a sense of autonomy and access to life-saving interventions. Although every effort should be taken to make the birthing experience a positive one for the mother and her family as a whole, it should not be done at the expense of safety. I have yet to hear an asphyxiated and brain-damaged child thank his mother’s obstetric care provider for allowing a wonderful birth experience.
What this evidence means for practice
Even in countries where home births are integrated fully into the medical care system and attended by trained and certified nurse-midwives, they are associated with increased risks, including a twofold to threefold increase in the odds of neonatal death.4 In the US, where no such integration exists, home births are dangerous.
Maternity care has come a long way since the 17th Century, when a woman had a 1 in 6 chance of dying in childbirth and only one of every five children lived to enjoy a first birthday. It is appropriate in this era of Obamacare and cost containment that we explore alternative methods. The option of a safe home delivery may well be part of the solution, as it is for many European countries--but until we can be assured that such an approach is safe for both mothers and infants, let's keep home delivery where it belongs...for pizza!
--Errol R. Norwitz, MD, PHD
We want to hear from you! Tell us what you think.
- Committee on Obstetric Practice; American College of Obstetricians and Gynecologists. Committee Opinion #476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425–428.
- American Academy of Pediatrics. Committee on Fetus and Newborn. Planned home birth. Pediatrics. 2013;131(5):1016–1020.
- American College of Nurse-Midwives. Division of Standards and Practice. Position statement: Home birth. Approved by the ACNM Board of Directors, May 2011. http://midwife.org/ACNM/files/ACNMLibraryData/UPLOADFILENAME/000000000251/Home%20Birth%20Aug%202011.pdf. Accessed October 21, 2013
- Wax JR, Lucas FL, Lamont M, et al. Maternal and newborn outcomes in planned home birth vs planned hospital births: A meta-analysis. Am J Obstet Gynecol. 2012;203(3):243.e1–e8.
- Martin JA, Hamilton BE, Ventura SJ, et al; Division of Vital Statistics. Births: Final data for 2009. Natl Vital Stat Rep. 2011;60(1):1–70.
Every morning before I leave for work, I kiss my three children goodbye and tell them, “I love you. Make good choices today.”
This has become my mantra—so much so that, on her way out the door to join her friends at the movies recently, my daughter turned to me and said, “I know, Dad. I know. I’ll make good decisions tonight.”
And what decision is more important than where to deliver your child and who to have in attendance at the birth?
It is said that the passage from the uterus to the outside world that each one of us was forced to negotiate at birth is the most treacherous journey we will ever undertake. Any unnecessary delay or complication can have profound, lifelong consequences.
There is no question that the past few centuries have seen a significant “medicalization” of childbirth, including the relocation of deliveries from the community to a hospital setting, the introduction of male obstetricians, the unfortunate marginalization of midwives and support personnel (doulas), the development of uterotonic drugs, and the evolution of operative vaginal (forceps, vacuum) and cesarean deliveries.
Many of the practices initially introduced by obstetric care providers (including multiple vaginal examinations in labor, induction of labor for a large baby, and active management of labor protocols) have since been shown to be unhelpful in improving pregnancy outcomes, and some practices (such as episiotomy) have even been shown to be harmful.
Related article: Difficult fetal extraction at cesarean delivery: What should you do? Robert L. Barbieri, MD (Editorial, January 2012)
In the midst of this confusion, the one voice that has been lost is that of the patient herself.
Whose birth is it anyway?
The American College of Obstetricians and Gynecologists (ACOG), the American Academy of Pediatrics (AAP), and the American College of Nurse-Midwives (ACNM) all agree that patient autonomy is paramount, and that the final decision of where to deliver and who to have in attendance should be made by the patient herself, ideally in conjunction with her family and her obstetric care provider.1–3 But an informed decision is only as good as the available data. Regrettably, the literature on how planned home birth compares with hospital delivery in terms of pregnancy outcomes in the United States are sparse.
Related article: Lay midwives the the ObGyn: Is collaboration risky? Lucia DiVenere, MA (May 2012)
How safe is home birth in the United States?
Cheng and colleagues attempt to answer this question by reviewing newborn and maternal outcomes among planned home births versus hospital deliveries in a contemporary low-risk birth cohort. Their retrospective study included low-risk women at term with a singleton vertex live birth in 2008 in 27 of the 50 states using information from the Vital Statistics Natality Data provided by the Centers for Disease Control and Prevention.
Of these 2,081,753 women, 0.58% (n = 12,039) had planned home births, and the remainder delivered in a hospital setting. Women who had an “accidental” (unintended) home birth or who delivered in a freestanding birthing center were excluded. The primary outcome was the risk of a 5-minute Apgar score less than 4. Secondary outcomes included the risk of a 5-minute Apgar score less than 7, assisted ventilation for more than 6 hours, neonatal seizures, admission to the NICU, and a series of maternal outcome measures.
Besides the outcomes listed previously (top of page 24), women with a planned home birth had fewer obstetric interventions, including operative vaginal delivery and labor induction or augmentation. They also were less likely to be given antibiotics during labor (although the authors did not distinguish between antibiotics administered for prophylaxis against group B strep or surgical-site infection versus antibiotics to treat infections such as urinary tract infections or chorioamnionitis).
Of special interest is the fact that neither a prior vaginal delivery (multiparity) nor the absence of a prior cesarean delivery was protective against these adverse events.
The women at highest risk of an adverse event were those who delivered at home under the supervision of “other midwives.” Although these providers were not well defined, this term typically refers to community-based lay midwives whose only “training” consists of an unofficial apprenticeship of variable length. Despite the absence of formal training, the lack of certification and standardization of care, and the existence of legislation in many states banning their activity, such lay midwives continue to encourage and support home birth for both low- and high-risk women in the United States.
Related article: Update on Obstetrics John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
Limitations of the study design
Although this dataset contains more than 2 million births, it includes only low-risk women at term and, therefore, is underpowered to measure outcomes such as fetal or neonatal death or birth injuries.
No data were presented on a number of important variables and outcome measures, such as the rate of or indications for cesarean delivery, the mode and frequency of intrapartum fetal monitoring, birth weight, intrapartum complications (uterine rupture, postpartum hemorrhage), blood transfusions, and infectious morbidity. The study also lacks long-term follow-up data on the infants.
That said, the study was well designed and very well written, and many of the limitations listed above are inherent in all retrospective cohort studies.
Putting these findings in context
These data are not novel, but they are remarkably consistent with other publications that have explored pregnancy outcomes in planned home birth versus hospital delivery from the Netherlands, the United Kingdom, Australia, and the United States, all of which show a higher rate of neonatal complications with planned home birth [see Reference 4 for review].4
Moreover, it is likely that the data in the current report significantly underestimate the risks of planned home birth for two reasons:
- Attempted home births that ended in transfer and, ultimately, delivery in a hospital setting (presumably for some unforeseen event such as excessive hemorrhage or uterine rupture or cord prolapse or nonreassuring fetal testing) were classified as hospital births.
- Apgar scores at 5 minutes are assigned by the attending care provider, and there is no way to independently verify their accuracy. Because of their limited training and/or concern about efforts to limit the scope of their practice, “other midwives” may be inclined to assign more favorable Apgar scores.
Who is choosing to deliver at home?
The proportion of US women who delivered outside the hospital setting increased by 29% between 2004 and 2009,5 although home births still constitute a minority of low-risk births (0.58% in the current study).
Related Article: Why are well-educated women more likely to choose home birth? Errol R. Norwitz, MD, PhD (Audiocast, November 2013)
One of the more interesting questions raised by this publication is the issue of who is choosing to deliver at home. In this cohort, women who planned home birth were more likely to be older, married, multiparous, white, and well educated. These aren’t exactly the women you would expect to gamble with the lives of their unborn offspring. So why are they choosing to deliver at home?
It could be that they are not well informed about the risks. Alternatively, they may have concluded that, although the relative risk of an adverse event is significantly higher with home birth, the absolute risk is low and acceptable to them. Or it could be that they are frustrated by the lack of autonomy afforded to them in the decisions surrounding antenatal care and the birthing process.
In recent years, more women are asking for minimally invasive births that are physically, emotionally, and socially supported. As hospital-based obstetric care providers, we do not always respect or meet these expectations. We can and should do better.
Women should not have to choose between a good birth experience and medical safety, between social support and hospital resources, between a sense of autonomy and access to life-saving interventions. Although every effort should be taken to make the birthing experience a positive one for the mother and her family as a whole, it should not be done at the expense of safety. I have yet to hear an asphyxiated and brain-damaged child thank his mother’s obstetric care provider for allowing a wonderful birth experience.
What this evidence means for practice
Even in countries where home births are integrated fully into the medical care system and attended by trained and certified nurse-midwives, they are associated with increased risks, including a twofold to threefold increase in the odds of neonatal death.4 In the US, where no such integration exists, home births are dangerous.
Maternity care has come a long way since the 17th Century, when a woman had a 1 in 6 chance of dying in childbirth and only one of every five children lived to enjoy a first birthday. It is appropriate in this era of Obamacare and cost containment that we explore alternative methods. The option of a safe home delivery may well be part of the solution, as it is for many European countries--but until we can be assured that such an approach is safe for both mothers and infants, let's keep home delivery where it belongs...for pizza!
--Errol R. Norwitz, MD, PHD
We want to hear from you! Tell us what you think.
Every morning before I leave for work, I kiss my three children goodbye and tell them, “I love you. Make good choices today.”
This has become my mantra—so much so that, on her way out the door to join her friends at the movies recently, my daughter turned to me and said, “I know, Dad. I know. I’ll make good decisions tonight.”
And what decision is more important than where to deliver your child and who to have in attendance at the birth?
It is said that the passage from the uterus to the outside world that each one of us was forced to negotiate at birth is the most treacherous journey we will ever undertake. Any unnecessary delay or complication can have profound, lifelong consequences.
There is no question that the past few centuries have seen a significant “medicalization” of childbirth, including the relocation of deliveries from the community to a hospital setting, the introduction of male obstetricians, the unfortunate marginalization of midwives and support personnel (doulas), the development of uterotonic drugs, and the evolution of operative vaginal (forceps, vacuum) and cesarean deliveries.
Many of the practices initially introduced by obstetric care providers (including multiple vaginal examinations in labor, induction of labor for a large baby, and active management of labor protocols) have since been shown to be unhelpful in improving pregnancy outcomes, and some practices (such as episiotomy) have even been shown to be harmful.
Related article: Difficult fetal extraction at cesarean delivery: What should you do? Robert L. Barbieri, MD (Editorial, January 2012)
In the midst of this confusion, the one voice that has been lost is that of the patient herself.
Whose birth is it anyway?
The American College of Obstetricians and Gynecologists (ACOG), the American Academy of Pediatrics (AAP), and the American College of Nurse-Midwives (ACNM) all agree that patient autonomy is paramount, and that the final decision of where to deliver and who to have in attendance should be made by the patient herself, ideally in conjunction with her family and her obstetric care provider.1–3 But an informed decision is only as good as the available data. Regrettably, the literature on how planned home birth compares with hospital delivery in terms of pregnancy outcomes in the United States are sparse.
Related article: Lay midwives the the ObGyn: Is collaboration risky? Lucia DiVenere, MA (May 2012)
How safe is home birth in the United States?
Cheng and colleagues attempt to answer this question by reviewing newborn and maternal outcomes among planned home births versus hospital deliveries in a contemporary low-risk birth cohort. Their retrospective study included low-risk women at term with a singleton vertex live birth in 2008 in 27 of the 50 states using information from the Vital Statistics Natality Data provided by the Centers for Disease Control and Prevention.
Of these 2,081,753 women, 0.58% (n = 12,039) had planned home births, and the remainder delivered in a hospital setting. Women who had an “accidental” (unintended) home birth or who delivered in a freestanding birthing center were excluded. The primary outcome was the risk of a 5-minute Apgar score less than 4. Secondary outcomes included the risk of a 5-minute Apgar score less than 7, assisted ventilation for more than 6 hours, neonatal seizures, admission to the NICU, and a series of maternal outcome measures.
Besides the outcomes listed previously (top of page 24), women with a planned home birth had fewer obstetric interventions, including operative vaginal delivery and labor induction or augmentation. They also were less likely to be given antibiotics during labor (although the authors did not distinguish between antibiotics administered for prophylaxis against group B strep or surgical-site infection versus antibiotics to treat infections such as urinary tract infections or chorioamnionitis).
Of special interest is the fact that neither a prior vaginal delivery (multiparity) nor the absence of a prior cesarean delivery was protective against these adverse events.
The women at highest risk of an adverse event were those who delivered at home under the supervision of “other midwives.” Although these providers were not well defined, this term typically refers to community-based lay midwives whose only “training” consists of an unofficial apprenticeship of variable length. Despite the absence of formal training, the lack of certification and standardization of care, and the existence of legislation in many states banning their activity, such lay midwives continue to encourage and support home birth for both low- and high-risk women in the United States.
Related article: Update on Obstetrics John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
Limitations of the study design
Although this dataset contains more than 2 million births, it includes only low-risk women at term and, therefore, is underpowered to measure outcomes such as fetal or neonatal death or birth injuries.
No data were presented on a number of important variables and outcome measures, such as the rate of or indications for cesarean delivery, the mode and frequency of intrapartum fetal monitoring, birth weight, intrapartum complications (uterine rupture, postpartum hemorrhage), blood transfusions, and infectious morbidity. The study also lacks long-term follow-up data on the infants.
That said, the study was well designed and very well written, and many of the limitations listed above are inherent in all retrospective cohort studies.
Putting these findings in context
These data are not novel, but they are remarkably consistent with other publications that have explored pregnancy outcomes in planned home birth versus hospital delivery from the Netherlands, the United Kingdom, Australia, and the United States, all of which show a higher rate of neonatal complications with planned home birth [see Reference 4 for review].4
Moreover, it is likely that the data in the current report significantly underestimate the risks of planned home birth for two reasons:
- Attempted home births that ended in transfer and, ultimately, delivery in a hospital setting (presumably for some unforeseen event such as excessive hemorrhage or uterine rupture or cord prolapse or nonreassuring fetal testing) were classified as hospital births.
- Apgar scores at 5 minutes are assigned by the attending care provider, and there is no way to independently verify their accuracy. Because of their limited training and/or concern about efforts to limit the scope of their practice, “other midwives” may be inclined to assign more favorable Apgar scores.
Who is choosing to deliver at home?
The proportion of US women who delivered outside the hospital setting increased by 29% between 2004 and 2009,5 although home births still constitute a minority of low-risk births (0.58% in the current study).
Related Article: Why are well-educated women more likely to choose home birth? Errol R. Norwitz, MD, PhD (Audiocast, November 2013)
One of the more interesting questions raised by this publication is the issue of who is choosing to deliver at home. In this cohort, women who planned home birth were more likely to be older, married, multiparous, white, and well educated. These aren’t exactly the women you would expect to gamble with the lives of their unborn offspring. So why are they choosing to deliver at home?
It could be that they are not well informed about the risks. Alternatively, they may have concluded that, although the relative risk of an adverse event is significantly higher with home birth, the absolute risk is low and acceptable to them. Or it could be that they are frustrated by the lack of autonomy afforded to them in the decisions surrounding antenatal care and the birthing process.
In recent years, more women are asking for minimally invasive births that are physically, emotionally, and socially supported. As hospital-based obstetric care providers, we do not always respect or meet these expectations. We can and should do better.
Women should not have to choose between a good birth experience and medical safety, between social support and hospital resources, between a sense of autonomy and access to life-saving interventions. Although every effort should be taken to make the birthing experience a positive one for the mother and her family as a whole, it should not be done at the expense of safety. I have yet to hear an asphyxiated and brain-damaged child thank his mother’s obstetric care provider for allowing a wonderful birth experience.
What this evidence means for practice
Even in countries where home births are integrated fully into the medical care system and attended by trained and certified nurse-midwives, they are associated with increased risks, including a twofold to threefold increase in the odds of neonatal death.4 In the US, where no such integration exists, home births are dangerous.
Maternity care has come a long way since the 17th Century, when a woman had a 1 in 6 chance of dying in childbirth and only one of every five children lived to enjoy a first birthday. It is appropriate in this era of Obamacare and cost containment that we explore alternative methods. The option of a safe home delivery may well be part of the solution, as it is for many European countries--but until we can be assured that such an approach is safe for both mothers and infants, let's keep home delivery where it belongs...for pizza!
--Errol R. Norwitz, MD, PHD
We want to hear from you! Tell us what you think.
- Committee on Obstetric Practice; American College of Obstetricians and Gynecologists. Committee Opinion #476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425–428.
- American Academy of Pediatrics. Committee on Fetus and Newborn. Planned home birth. Pediatrics. 2013;131(5):1016–1020.
- American College of Nurse-Midwives. Division of Standards and Practice. Position statement: Home birth. Approved by the ACNM Board of Directors, May 2011. http://midwife.org/ACNM/files/ACNMLibraryData/UPLOADFILENAME/000000000251/Home%20Birth%20Aug%202011.pdf. Accessed October 21, 2013
- Wax JR, Lucas FL, Lamont M, et al. Maternal and newborn outcomes in planned home birth vs planned hospital births: A meta-analysis. Am J Obstet Gynecol. 2012;203(3):243.e1–e8.
- Martin JA, Hamilton BE, Ventura SJ, et al; Division of Vital Statistics. Births: Final data for 2009. Natl Vital Stat Rep. 2011;60(1):1–70.
- Committee on Obstetric Practice; American College of Obstetricians and Gynecologists. Committee Opinion #476: Planned home birth. Obstet Gynecol. 2011;117(2 Pt 1):425–428.
- American Academy of Pediatrics. Committee on Fetus and Newborn. Planned home birth. Pediatrics. 2013;131(5):1016–1020.
- American College of Nurse-Midwives. Division of Standards and Practice. Position statement: Home birth. Approved by the ACNM Board of Directors, May 2011. http://midwife.org/ACNM/files/ACNMLibraryData/UPLOADFILENAME/000000000251/Home%20Birth%20Aug%202011.pdf. Accessed October 21, 2013
- Wax JR, Lucas FL, Lamont M, et al. Maternal and newborn outcomes in planned home birth vs planned hospital births: A meta-analysis. Am J Obstet Gynecol. 2012;203(3):243.e1–e8.
- Martin JA, Hamilton BE, Ventura SJ, et al; Division of Vital Statistics. Births: Final data for 2009. Natl Vital Stat Rep. 2011;60(1):1–70.

Errol R. Norwitz, MD, PhD (November 2013)
A stepwise approach to managing eclampsia and other hypertensive emergencies
CASE: MISSED PREECLAMPSIA
At her first prenatal visit at 14 weeks’ gestation, a 41-year-old woman (G2P1) presents with a dichorionic twin gestation, blood pressure (BP) of 105/68 mm Hg, and a body mass index (BMI) of 40 kg/m2. The pregnancy was achieved through in vitro fertilization. Ten years earlier, the patient’s first pregnancy was complicated by preeclampsia, requiring preterm delivery at 33 weeks’ gestation.
By 28 weeks’ gestation, the patient has gained 26 lb. Her BP is 120/70 mm Hg, with no proteinuria detected by urine dipstick. By 30 weeks, she has gained an additional 8 lb, her BP is 142/84 mm Hg, and no proteinuria is detected. At 32 weeks, her BP is 140/92 mm Hg, she has gained another 8 lb, and no proteinuria is present. She also reports new-onset headaches that do not respond to over-the-counter analgesics. She is sent to the obstetric triage area for BP monitoring, blood testing for preeclampsia and nonstress test fetal monitoring.
During the 2-hour observation period, the patient continues to report headaches, and swelling of her face and hands is present. Her systolic BP values range from 132 to 152 mm Hg, and diastolic values range from 80 to 96 mm Hg. No proteinuria is detected, blood testing results for preeclampsia (complete blood count, liver enzymes, serum creatinine, and uric acid) are normal, and the nonstress tests are reactive in both fetuses.
The patient is given a diagnosis of gestational hypertension, along with a prescription for oral labetalol 200 mg daily and two tablets of acetaminophen with codeine for the headaches (to be taken every 6 hours as needed). She is sent home with instructions to return to her physician’s office in 1 week.
Two days later, she wakes in the middle of the night with a severe headache, blurred vision, and vomiting. Her husband calls the obstetrician’s answering service and is instructed to call 911 immediately. While waiting for an ambulance, the patient experiences a grand mal eclamptic convulsion. A second convulsion occurs during her transfer to the ED.
This scenario could have been avoided.
The obstetrician in this case was negligent for failing to recognize preeclampsia in a patient who had two clear risk factors for it: multifetal gestation and a history of early-onset (<37 weeks) preeclampsia in an earlier pregnancy (other risk factors are listed in TABLE 1).
As a result, the patient developed eclampsia, a serious condition that can lead to grave maternal complications (TABLE 2), including death. It also can cause fetal complications, including growth restriction, hypoxia, acidosis, preterm birth, long-term developmental deficits, and death.1,2
The obstetrician in this case also overlooked published evidence indicating that, in the setting of hypertension and headaches, as many as 20% to 30% of pregnant women whose tests for proteinuria show a negative or trace result via dipstick will develop eclampsia.3 Instead of initiating outpatient administration of oral antihypertensive agents, the obstetrician should have hospitalized this patient for at least 48 hours, with steroid administration, to determine whether outpatient management was feasible.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia Baha Sibai, MD (November 2011)
Defining eclampsia
Eclampsia is marked by the onset of convulsions (during pregnancy or postpartum) in association with gestational hypertension alone, proteinuria, preeclampsia, or superimposed preeclampsia. Although it is rare, eclampsia is potentially life-threatening. For that reason, obstetricians, anesthesiologists, ED physicians, neurologists, and critical-care physicians should be well versed in its diagnosis and management. In this article, I focus on management.
A few preliminary points
Eclampsia can develop any time during the antenatal period (>16 weeks’ gestation), during labor and delivery, and as long as 6 weeks after delivery. Therefore, we should be vigilant for preeclampsia whenever a pregnant patient visits our office, as well as when she makes unscheduled visits to the ED or obstetric triage area or is hospitalized.
Early recognition of women at high risk for preeclampsia and eclampsia may allow for prompt intervention, including early hospitalization for close observation prior to delivery and postpartum.1,2,4–10
Hospitalization of high-risk women allows for use of antihypertensive agents to treat severe BP, administration of magnesium sulfate to prevent convulsions, and timely delivery of the infant. It also allows for intensive maternal support during and after an eclamptic seizure.
Hospitalization is essential for women who exhibit features that suggest severe disease. More specifically, the presence of gestational hypertension with any of the following features is an indication for immediate hospitalization for evaluation and management:
- persistent severe hypertension (systolic
BP ≥160 mm Hg or diastolic BP ≥110 mm Hg) for at least 1 hour - gestational hypertension requiring oral antihypertensive therapy
- progressive and excessive weight gain (≥20 lb prior to 28 weeks’ gestation)
- generalized swelling (edema of hands or face)
- new-onset or persistent headaches despite analgesics
- persistent visual changes (blurred vision, scotomata, photophobia, double vision)
- shortness of breath, dyspnea, orthopnea, or tightness in the chest
- persistent retrosternal chest pain, severe epigastric or right upper quadrant pain
- persistent nausea, vomiting, malaise
- altered mental state, confusion, numbness, tingling, or motor weakness
- platelet count below 100 3 103 µL
- aspartate aminotransferase (AST), alanine aminotransferase (ALT), or lactic acid dehydrogenase (LDH) levels more than twice the upper limit of normal
- serum creatinine level >1.1 mg/dL
- suspected abruptio placentae.
A stepwise approach to eclampsia
Eclampsia is an obstetric emergency. Inadequate preparation for it or an inappropriate response to maternal and fetal conditions during and after an eclamptic convulsion can be detrimental to the mother and fetus. All obstetric units should have up-to-date protocols in place and should conduct mandatory drills to prepare nursing staff, obstetric providers, and anesthesia staff working in these units to manage eclampsia.
Step 1: Let the seizure run its course
During a seizure, resist the impulse to administer anticonvulsive drugs, including intravenous (IV) magnesium sulfate, because most eclamptic convulsions are self-limiting. Also abstain from administering medications such as IV phenytoin, diazepam, or midazolam, as these drugs are less effective than magnesium sulfate, and some can suppress the laryngeal reflex, increasing the risk of aspiration.
If the patient develops status epilepticus, initiate muscle paralysis and intubate her.
Step 2: Support the maternal condition
It is vital to support maternal respiratory and cardiovascular functions to prevent hypoxia, acidosis, and cardiorespiratory arrest.
Begin by establishing airway patency and maternal oxygenation during and after the convulsion. Administer oxygen via a face mask, with or without a reservoir, at a rate of 8 to 10 L/min.
During the apneic period (see “Profile of an eclamptic seizure” on page 46), the patient will develop hypoxia. Use pulse oximetry to monitor oxygen saturation, with the goal of keeping it above 94%. Arterial blood gas analysis is required if oxygen saturation remains below 92% or if pulmonary edema or aspiration is suspected.
If the patient develops recurrent seizures, status epilepticus, florid alveolar pulmonary edema, or respiratory arrest, intubate her immediately.
Step 3: Prevent maternal injury and aspiration
Secure the side rails of the patient’s bed by elevating them to prevent a fall, and make sure they are padded to prevent trauma during convulsions and afterward, when some women become combative and agitated. Position the patient in a lateral decubitus position to minimize aspiration of oral secretions. If any secretions or vomitus are present, remove them via suction.
Step 4: After the convulsion, give magnesium sulfate
Magnesium is the drug of choice for seizure prophylaxis in women with preeclampsia and severe symptoms, and to prevent recurrent seizures in women with eclampsia.
In the latter group, once the eclamptic convulsion has ended, give a loading dose of IV magnesium (6 g/100 mL over 20 minutes), followed by a continuous infusion of 2 g/h for at least 24 hours. If the patient develops a second seizure during the maintenance infusion, administer another bolus of magnesium (2 g/100 mL over 3–5 minutes).
Step 5: Treat severe hypertension
If severe hypertension persists for 60 minutes or longer, it can lead to injury of the brain, heart, and kidneys. To avoid these complications, it is essential to reduce BP to a safe range and maintain that level without compromising cerebral perfusion pressure and uteroplacental blood flow (which already may be reduced in some patients).
The goal of antihypertensive therapy is to keep systolic BP between 140 and 155 mm Hg and diastolic values between 90 and 105 mm Hg.9 Several agents are available for the treatment of severe hypertension during pregnancy and postpartum. The most commonly used IV medications for this purpose are labetalol and hydralazine. Another option is oral, rapidly acting
nifedipine.
Several randomized trials have compared efficacy and side effects between IV bolus injections of hydralazine; IV labetalol; and oral, rapidly acting nifedipine. In general, the findings of these studies suggest that either IV hydralazine or labetalol or oral nifedipine can be used to treat severe hypertension in pregnancy, as long as the provider is familiar with the dose to be used, the expected onset of action, and potential side effects (TABLE 3).
Women who develop generalized swelling or hemoconcentration (hematocrit ≥40%), or both, usually experience markedly reduced plasma volume. For this reason, these women will benefit from treatment with labetalol. If this is ineffective, then add IV hydralazine. However, delay administration of a rapidly acting vasodilator such as hydralazine to prevent an excessive hypotensive response and a secondary reduction in tissue perfusion and uteroplacental blood flow. Rather, administer a bolus infusion of 250 to 500 mL of isotonic saline before giving a vasodilator.
Additional details about the use of antihypertensive drugs are given in the section on other hypertensive emergencies below.
Step 6: Evaluate the patient for complications
Pulmonary edema can develop in patients with eclampsia or another hypertensive emergency. Suspect it if the patient has respiratory symptoms in association with tachypnea, tachycardia, or sustained oxygen saturation values below 93%, as well as when the patient exhibits basal rales during auscultation of the lungs. Treatment involves the administration of oxygen and IV furosemide (20–40 mg push), repeated as needed.
Some women with eclampsia may develop severe cerebral edema, hemorrhage, or both. The edema can be vasogenic or cytotoxic, leading to increased intracerebral pressure. Suspect edema or hemorrhage if the patient remains unresponsive, continues to experience convulsions despite therapy, or exhibits sensory or motor neurologic deficits. In such cases, neuroimaging is indicated, and the patient should be managed in consultation with neurology or neurosurgery.
Step 7: Begin the process of induction and delivery
Once the patient has been stabilized—and not before—initiate the induction process. Be aware that during and after the convulsion, changes in fetal heart rate (FHR) and uterine monitoring will usually be evident:
- prolonged deceleration or bradycardia (3–10 minutes)
- compensatory tachycardia, decreased beat-to-beat variability
- transient recurrent decelerations
- increased uterine tone and greater frequency of uterine activity.
These changes in FHR and uterine activity usually last 3 to 15 minutes. For this reason, it is important to avoid rushing the patient for cesarean delivery, as FHR and uterine activity are likely to return to normal after maternal resuscitation and stabilization. If not, consider other causes, such as abruptio placentae.
Eclampsia itself is not an indication for cesarean delivery. The selection of mode of delivery should be based on the presence or absence of labor, the cervical Bishop score, fetal gestational age, fetal presentation, and overall fetal condition.
Choosing an anesthetic
Regional analgesia/anesthesia is the method of choice for most women with eclampsia. However, regional anesthesia is to be avoided in the presence of disseminated intravascular coagulation or thrombocytopenia (the threshold platelet count is usually less than 75 x 103 µL. In such a case, IV analgesia can be used during labor, and general anesthesia may be appropriate for cesarean delivery. Both spinal and epidural analgesia and anesthesia are appropriate for women with eclampsia.
How to manage other hypertensive emergencies
A hypertensive emergency during pregnancy or postpartum involves acute-onset, persistent (>15 minutes), severe systolic BP (≥160 mm Hg) or severe diastolic BP (≥110 mm Hg), or both. The first step in such an emergency is to ensure the accurate measurement of BP using standard techniques.
Patients with acute-onset, persistent, severe BP should be hospitalized promptly for evaluation and treatment to prevent organ damage. Once such a patient is hospitalized, BP should be recorded every 15 minutes, with continuous FHR monitoring to ensure fetal viability.
Related article: Failure to diagnose preeclampsia and more (Medical Verdicts, February 2013)
The timing of initiation of antihypertensive medications, as well as determination of the type of medication best suited for the patient, should be based on:
- systolic and diastolic BP levels
- maternal clinical and laboratory findings
- presence of associated symptoms
- preexisting medical comorbidities
- whether the patient is antepartum or postpartum.
For example, a sustained BP level of 200/120 mm Hg requires therapy after 15 minutes, whereas observation may be suitable for as long as 60 minutes for a sustained BP of 160/72 mm Hg during labor.
Rapid reduction of systolic BP can lead to marked reductions in uteroplacental blood flow and a nonreassuring FHR tracing. Moreover, a rapid reduction of severe systolic BP in patients who have constricted plasma volume can reduce perfusion to the kidney, brain, and placenta. However, sustained BP of 165/100 mm Hg in association with central nervous system signs or symptoms, congestive heart failure, thrombocytopenia, or postpartum status requires therapy within 1 hour.
In general, it is difficult to obtain accurate BP recordings using noninvasive electronic instruments during labor because of the effects of labor on systolic BP and the lack of standardized methods for positioning of the arm cuff and the patient.
For these reasons, the decision about when to start acute antihypertensive therapy, based on systolic or diastolic BP, or both, should be individualized. And the choice of antihypertensive agent should be based on maternal clinical findings.
Choosing an antihypertensive agent
Because both hydralazine and nifedipine are associated with tachycardia, avoid them in patients with a heart rate above 110 bpm, using labetalol instead.10
In patients with bradycardia (heart rate <60 bpm), asthma, or congestive heart failure, however, labetalol should be avoided. In these populations, hydralazine or nifedipine is the drug of choice. Nifedipine is associated with improved renal blood flow and a resultant increase in urine output, making it preferable for patients with decreased urine output or severe postpartum hypertension.10
One theoretical concern is that the combined use of nifedipine and magnesium sulfate can cause excessive hypotension and neuromuscular blockage. As a result, some experts recommend that nifedipine be avoided in patients receiving magnesium sulfate. However, a recent review of this subject concluded that combined use of these drugs does not increase the risks of excessive hypotension and neuromuscular blockage in patients with severe hypertension or preeclampsia.
The initial dose of labetalol, when it is your chosen agent, is 20 mg IV, with BP measured 10 minutes later. If the target BP threshold is not achieved, administer 40 mg, 80 mg, and 80 mg at 10-minute intervals, as needed, again measuring BP 10 minutes after every dose. If, after a maximum dose of 240 mg, the desired BP threshold still has not been reached, give 5 to 10 mg IV hydralazine and measure BP 20 minutes later. If the target BP threshold still has not been achieved, it is essential to obtain consultation on the need for continuous infusion of labetalol, nicardipine, or sodium nitroprusside.
The initial dose of hydralazine, when it is your chosen agent, is 5 to 10 mg IV, with BP measured 20 minutes later. If needed, give another 10 mg and measure BP after another 20-minute interval. After a maximum dose of hydralazine 20 mg, switch to IV labetalol, using the regimen described above for labetalol, if the BP threshold still has not been achieved.
Nitroglycerin may be helpful in carefully selected patients
This drug is an arterial—but mostly venous—dilator. It is administered via IV infusion at an initial rate of 5 µg/min, with the rate gradually increased every 3 to 5 minutes (titrated to BP) to a maximum dose of 100 µg/min. It is the drug of choice in any hypertensive emergency associated with pulmonary edema and for control of hypertension associated with tracheal manipulation during intubation and extubation with general anesthesia.
Nitroglycerin is contraindicated in hypertensive encephalopathy because it increases cerebral blood flow and intracranial pressure. This drug should be administered only under the supervision of an experienced obstetric intensivist.
Sodium nitroprusside: Only in an ICU
This agent causes arterial and venous relaxation by interfering with the influx and intracellular activation of calcium. It is the drug of choice in hypertensive encephalopathy because it controls both afterload (vascular resistance) and preload (fluid status). It should be used only in the setting of intensive care.
The recommended dose is IV infusion at a rate of 0.25 to 5.00 µg/kg/min. Sodium nitroprusside has an immediate onset of action and may continue to exert an effect 3 to 5 minutes after discontinuation. Any hypotension caused by the drug should subside within minutes after discontinuation of the drip, due to the drug’s short half-life.
Nitroprusside is metabolized into thiocyanate and excreted in the urine. Cyanide can accumulate with large doses (>10 µg/kg/min) or prolonged administration (>48 hours), or if the patient has renal insufficiency or decreased hepatic metabolism. Signs of toxicity include anorexia, disorientation, headache, fatigue, restlessness, tinnitus, delirium, hallucinations, nausea, vomiting, and metabolic acidosis. When infused at a rate of less than 2 µg/kg/min, however, cyanide toxicity is unlikely.
As is the case with nitroglycerin, this drug should be administered only under the supervision of an experienced obstetric intensivist.
Case: Resolved
Upon arrival at the ED, the patient exhibits shallow, rapid breathing and foaming from the mouth. She is placed in a lateral decubitus position, an oral airway is established, and all secretions are suctioned. Oxygen is administered via face mask at a rate of 8 L/min. Her initial oxygen saturation level is 92%. IV access is secured, and a loading dose of magnesium sulfate 6 g is given over 20 minutes. Oxygen saturation increases to 94% to 96%. Auscultation of both lungs is normal.
The patient remains in a postictal state for about 15 minutes, but then orients to name, place, and time. FHR monitoring of both fetuses reveals a normal baseline with moderate variability, as well as variable decelerations in the presenting twin.
A maintenance dose of magnesium sulfate is initiated at a rate of 2 g/h, with the BP level recorded every 15 minutes. Systolic values remain between 170 and 180 mm Hg, and diastolic values between 108 and 112 mm Hg for 60 minutes. The obstetrician administers IV labetalol (20 mg) over 2 minutes. About 15 minutes later, the BP level is 154/100 mm Hg, with values remaining in the range of 150 to 156 mm Hg systolic and 92 to 104 mm Hg diastolic.
Ultrasonography reveals that the presenting twin is in a breech position, with estimated fetal weight below the 10th percentile and oligohydramnios. As a result, the obstetrician elects to proceed to cesarean delivery. The twins are delivered by cesarean section using spinal anesthesia. Although the infants are premature, there are no complications.Profile of an eclamptic seizure
Witnessing an eclamptic convulsion can be a frightening experience for nurses and medical providers. The convulsion usually lasts 60 to 90 seconds and occurs in two phases:
- Phase 1 (15–25 seconds) involves facial twitching, rolling of the eyes, and stiffening of the body, with generalized muscular contractions.
- Phase 2 (20–50 seconds) involves alternate contraction and relaxation of the muscles of the body in rapid succession, starting in the face and spreading throughout the body. Foaming at the mouth also occurs, and the patient may bite her tongue if it isn’t protected.
Apnea develops during and immediately after the convulsion, lasting about 120 seconds. A period of hyperventilation follows to compensate for the respiratory acidosis during the apneic period.
A postictal state follows the convulsion, and the patient usually remembers nothing of the episode. Some patients also become restless, combative, and agitated, requiring sedation. Aspiration is possible during or after the convulsion.
We want to hear from you! Tell us what you think.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–410.
- Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):182–192.
- Meyer NL, Mercer BM, Friedman SA, Sibai BM. Urinary dipstick protein: a poor predictor of absent or severe proteinuria. Am J Obstet Gynecol. 1994;170(1 Pt 1):137–141.
- Knight M; UK Obstetric Surveillance System (UKOSS). Eclampsia in the United Kingdom 2005. BJOG. 2007;114(9):1072–1078.
- ACOG Practice Bulletin #33: Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167.
- Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia–eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e1–e7.
- Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206(6):470–475.
- ACOG Committee Opinion #514: Emergent therapy for acute-onset, severe hypertension with preeclampsia or eclampsia. Obstet Gynecol. 2011;118:1465–1468.
- Liu S, Joseph KS, Liston RM, et al. Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118(5):987–994.
- Raheem IA, Saaid R, Omar Sz, Tan PC. Oral nifedipine versus intravenous labetalol for acute blood pressure control in hypertensive emergencies of pregnancy: a randomized trial. BJOG. 2012;119(1):78–85.
CASE: MISSED PREECLAMPSIA
At her first prenatal visit at 14 weeks’ gestation, a 41-year-old woman (G2P1) presents with a dichorionic twin gestation, blood pressure (BP) of 105/68 mm Hg, and a body mass index (BMI) of 40 kg/m2. The pregnancy was achieved through in vitro fertilization. Ten years earlier, the patient’s first pregnancy was complicated by preeclampsia, requiring preterm delivery at 33 weeks’ gestation.
By 28 weeks’ gestation, the patient has gained 26 lb. Her BP is 120/70 mm Hg, with no proteinuria detected by urine dipstick. By 30 weeks, she has gained an additional 8 lb, her BP is 142/84 mm Hg, and no proteinuria is detected. At 32 weeks, her BP is 140/92 mm Hg, she has gained another 8 lb, and no proteinuria is present. She also reports new-onset headaches that do not respond to over-the-counter analgesics. She is sent to the obstetric triage area for BP monitoring, blood testing for preeclampsia and nonstress test fetal monitoring.
During the 2-hour observation period, the patient continues to report headaches, and swelling of her face and hands is present. Her systolic BP values range from 132 to 152 mm Hg, and diastolic values range from 80 to 96 mm Hg. No proteinuria is detected, blood testing results for preeclampsia (complete blood count, liver enzymes, serum creatinine, and uric acid) are normal, and the nonstress tests are reactive in both fetuses.
The patient is given a diagnosis of gestational hypertension, along with a prescription for oral labetalol 200 mg daily and two tablets of acetaminophen with codeine for the headaches (to be taken every 6 hours as needed). She is sent home with instructions to return to her physician’s office in 1 week.
Two days later, she wakes in the middle of the night with a severe headache, blurred vision, and vomiting. Her husband calls the obstetrician’s answering service and is instructed to call 911 immediately. While waiting for an ambulance, the patient experiences a grand mal eclamptic convulsion. A second convulsion occurs during her transfer to the ED.
This scenario could have been avoided.
The obstetrician in this case was negligent for failing to recognize preeclampsia in a patient who had two clear risk factors for it: multifetal gestation and a history of early-onset (<37 weeks) preeclampsia in an earlier pregnancy (other risk factors are listed in TABLE 1).
As a result, the patient developed eclampsia, a serious condition that can lead to grave maternal complications (TABLE 2), including death. It also can cause fetal complications, including growth restriction, hypoxia, acidosis, preterm birth, long-term developmental deficits, and death.1,2
The obstetrician in this case also overlooked published evidence indicating that, in the setting of hypertension and headaches, as many as 20% to 30% of pregnant women whose tests for proteinuria show a negative or trace result via dipstick will develop eclampsia.3 Instead of initiating outpatient administration of oral antihypertensive agents, the obstetrician should have hospitalized this patient for at least 48 hours, with steroid administration, to determine whether outpatient management was feasible.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia Baha Sibai, MD (November 2011)
Defining eclampsia
Eclampsia is marked by the onset of convulsions (during pregnancy or postpartum) in association with gestational hypertension alone, proteinuria, preeclampsia, or superimposed preeclampsia. Although it is rare, eclampsia is potentially life-threatening. For that reason, obstetricians, anesthesiologists, ED physicians, neurologists, and critical-care physicians should be well versed in its diagnosis and management. In this article, I focus on management.
A few preliminary points
Eclampsia can develop any time during the antenatal period (>16 weeks’ gestation), during labor and delivery, and as long as 6 weeks after delivery. Therefore, we should be vigilant for preeclampsia whenever a pregnant patient visits our office, as well as when she makes unscheduled visits to the ED or obstetric triage area or is hospitalized.
Early recognition of women at high risk for preeclampsia and eclampsia may allow for prompt intervention, including early hospitalization for close observation prior to delivery and postpartum.1,2,4–10
Hospitalization of high-risk women allows for use of antihypertensive agents to treat severe BP, administration of magnesium sulfate to prevent convulsions, and timely delivery of the infant. It also allows for intensive maternal support during and after an eclamptic seizure.
Hospitalization is essential for women who exhibit features that suggest severe disease. More specifically, the presence of gestational hypertension with any of the following features is an indication for immediate hospitalization for evaluation and management:
- persistent severe hypertension (systolic
BP ≥160 mm Hg or diastolic BP ≥110 mm Hg) for at least 1 hour - gestational hypertension requiring oral antihypertensive therapy
- progressive and excessive weight gain (≥20 lb prior to 28 weeks’ gestation)
- generalized swelling (edema of hands or face)
- new-onset or persistent headaches despite analgesics
- persistent visual changes (blurred vision, scotomata, photophobia, double vision)
- shortness of breath, dyspnea, orthopnea, or tightness in the chest
- persistent retrosternal chest pain, severe epigastric or right upper quadrant pain
- persistent nausea, vomiting, malaise
- altered mental state, confusion, numbness, tingling, or motor weakness
- platelet count below 100 3 103 µL
- aspartate aminotransferase (AST), alanine aminotransferase (ALT), or lactic acid dehydrogenase (LDH) levels more than twice the upper limit of normal
- serum creatinine level >1.1 mg/dL
- suspected abruptio placentae.
A stepwise approach to eclampsia
Eclampsia is an obstetric emergency. Inadequate preparation for it or an inappropriate response to maternal and fetal conditions during and after an eclamptic convulsion can be detrimental to the mother and fetus. All obstetric units should have up-to-date protocols in place and should conduct mandatory drills to prepare nursing staff, obstetric providers, and anesthesia staff working in these units to manage eclampsia.
Step 1: Let the seizure run its course
During a seizure, resist the impulse to administer anticonvulsive drugs, including intravenous (IV) magnesium sulfate, because most eclamptic convulsions are self-limiting. Also abstain from administering medications such as IV phenytoin, diazepam, or midazolam, as these drugs are less effective than magnesium sulfate, and some can suppress the laryngeal reflex, increasing the risk of aspiration.
If the patient develops status epilepticus, initiate muscle paralysis and intubate her.
Step 2: Support the maternal condition
It is vital to support maternal respiratory and cardiovascular functions to prevent hypoxia, acidosis, and cardiorespiratory arrest.
Begin by establishing airway patency and maternal oxygenation during and after the convulsion. Administer oxygen via a face mask, with or without a reservoir, at a rate of 8 to 10 L/min.
During the apneic period (see “Profile of an eclamptic seizure” on page 46), the patient will develop hypoxia. Use pulse oximetry to monitor oxygen saturation, with the goal of keeping it above 94%. Arterial blood gas analysis is required if oxygen saturation remains below 92% or if pulmonary edema or aspiration is suspected.
If the patient develops recurrent seizures, status epilepticus, florid alveolar pulmonary edema, or respiratory arrest, intubate her immediately.
Step 3: Prevent maternal injury and aspiration
Secure the side rails of the patient’s bed by elevating them to prevent a fall, and make sure they are padded to prevent trauma during convulsions and afterward, when some women become combative and agitated. Position the patient in a lateral decubitus position to minimize aspiration of oral secretions. If any secretions or vomitus are present, remove them via suction.
Step 4: After the convulsion, give magnesium sulfate
Magnesium is the drug of choice for seizure prophylaxis in women with preeclampsia and severe symptoms, and to prevent recurrent seizures in women with eclampsia.
In the latter group, once the eclamptic convulsion has ended, give a loading dose of IV magnesium (6 g/100 mL over 20 minutes), followed by a continuous infusion of 2 g/h for at least 24 hours. If the patient develops a second seizure during the maintenance infusion, administer another bolus of magnesium (2 g/100 mL over 3–5 minutes).
Step 5: Treat severe hypertension
If severe hypertension persists for 60 minutes or longer, it can lead to injury of the brain, heart, and kidneys. To avoid these complications, it is essential to reduce BP to a safe range and maintain that level without compromising cerebral perfusion pressure and uteroplacental blood flow (which already may be reduced in some patients).
The goal of antihypertensive therapy is to keep systolic BP between 140 and 155 mm Hg and diastolic values between 90 and 105 mm Hg.9 Several agents are available for the treatment of severe hypertension during pregnancy and postpartum. The most commonly used IV medications for this purpose are labetalol and hydralazine. Another option is oral, rapidly acting
nifedipine.
Several randomized trials have compared efficacy and side effects between IV bolus injections of hydralazine; IV labetalol; and oral, rapidly acting nifedipine. In general, the findings of these studies suggest that either IV hydralazine or labetalol or oral nifedipine can be used to treat severe hypertension in pregnancy, as long as the provider is familiar with the dose to be used, the expected onset of action, and potential side effects (TABLE 3).
Women who develop generalized swelling or hemoconcentration (hematocrit ≥40%), or both, usually experience markedly reduced plasma volume. For this reason, these women will benefit from treatment with labetalol. If this is ineffective, then add IV hydralazine. However, delay administration of a rapidly acting vasodilator such as hydralazine to prevent an excessive hypotensive response and a secondary reduction in tissue perfusion and uteroplacental blood flow. Rather, administer a bolus infusion of 250 to 500 mL of isotonic saline before giving a vasodilator.
Additional details about the use of antihypertensive drugs are given in the section on other hypertensive emergencies below.
Step 6: Evaluate the patient for complications
Pulmonary edema can develop in patients with eclampsia or another hypertensive emergency. Suspect it if the patient has respiratory symptoms in association with tachypnea, tachycardia, or sustained oxygen saturation values below 93%, as well as when the patient exhibits basal rales during auscultation of the lungs. Treatment involves the administration of oxygen and IV furosemide (20–40 mg push), repeated as needed.
Some women with eclampsia may develop severe cerebral edema, hemorrhage, or both. The edema can be vasogenic or cytotoxic, leading to increased intracerebral pressure. Suspect edema or hemorrhage if the patient remains unresponsive, continues to experience convulsions despite therapy, or exhibits sensory or motor neurologic deficits. In such cases, neuroimaging is indicated, and the patient should be managed in consultation with neurology or neurosurgery.
Step 7: Begin the process of induction and delivery
Once the patient has been stabilized—and not before—initiate the induction process. Be aware that during and after the convulsion, changes in fetal heart rate (FHR) and uterine monitoring will usually be evident:
- prolonged deceleration or bradycardia (3–10 minutes)
- compensatory tachycardia, decreased beat-to-beat variability
- transient recurrent decelerations
- increased uterine tone and greater frequency of uterine activity.
These changes in FHR and uterine activity usually last 3 to 15 minutes. For this reason, it is important to avoid rushing the patient for cesarean delivery, as FHR and uterine activity are likely to return to normal after maternal resuscitation and stabilization. If not, consider other causes, such as abruptio placentae.
Eclampsia itself is not an indication for cesarean delivery. The selection of mode of delivery should be based on the presence or absence of labor, the cervical Bishop score, fetal gestational age, fetal presentation, and overall fetal condition.
Choosing an anesthetic
Regional analgesia/anesthesia is the method of choice for most women with eclampsia. However, regional anesthesia is to be avoided in the presence of disseminated intravascular coagulation or thrombocytopenia (the threshold platelet count is usually less than 75 x 103 µL. In such a case, IV analgesia can be used during labor, and general anesthesia may be appropriate for cesarean delivery. Both spinal and epidural analgesia and anesthesia are appropriate for women with eclampsia.
How to manage other hypertensive emergencies
A hypertensive emergency during pregnancy or postpartum involves acute-onset, persistent (>15 minutes), severe systolic BP (≥160 mm Hg) or severe diastolic BP (≥110 mm Hg), or both. The first step in such an emergency is to ensure the accurate measurement of BP using standard techniques.
Patients with acute-onset, persistent, severe BP should be hospitalized promptly for evaluation and treatment to prevent organ damage. Once such a patient is hospitalized, BP should be recorded every 15 minutes, with continuous FHR monitoring to ensure fetal viability.
Related article: Failure to diagnose preeclampsia and more (Medical Verdicts, February 2013)
The timing of initiation of antihypertensive medications, as well as determination of the type of medication best suited for the patient, should be based on:
- systolic and diastolic BP levels
- maternal clinical and laboratory findings
- presence of associated symptoms
- preexisting medical comorbidities
- whether the patient is antepartum or postpartum.
For example, a sustained BP level of 200/120 mm Hg requires therapy after 15 minutes, whereas observation may be suitable for as long as 60 minutes for a sustained BP of 160/72 mm Hg during labor.
Rapid reduction of systolic BP can lead to marked reductions in uteroplacental blood flow and a nonreassuring FHR tracing. Moreover, a rapid reduction of severe systolic BP in patients who have constricted plasma volume can reduce perfusion to the kidney, brain, and placenta. However, sustained BP of 165/100 mm Hg in association with central nervous system signs or symptoms, congestive heart failure, thrombocytopenia, or postpartum status requires therapy within 1 hour.
In general, it is difficult to obtain accurate BP recordings using noninvasive electronic instruments during labor because of the effects of labor on systolic BP and the lack of standardized methods for positioning of the arm cuff and the patient.
For these reasons, the decision about when to start acute antihypertensive therapy, based on systolic or diastolic BP, or both, should be individualized. And the choice of antihypertensive agent should be based on maternal clinical findings.
Choosing an antihypertensive agent
Because both hydralazine and nifedipine are associated with tachycardia, avoid them in patients with a heart rate above 110 bpm, using labetalol instead.10
In patients with bradycardia (heart rate <60 bpm), asthma, or congestive heart failure, however, labetalol should be avoided. In these populations, hydralazine or nifedipine is the drug of choice. Nifedipine is associated with improved renal blood flow and a resultant increase in urine output, making it preferable for patients with decreased urine output or severe postpartum hypertension.10
One theoretical concern is that the combined use of nifedipine and magnesium sulfate can cause excessive hypotension and neuromuscular blockage. As a result, some experts recommend that nifedipine be avoided in patients receiving magnesium sulfate. However, a recent review of this subject concluded that combined use of these drugs does not increase the risks of excessive hypotension and neuromuscular blockage in patients with severe hypertension or preeclampsia.
The initial dose of labetalol, when it is your chosen agent, is 20 mg IV, with BP measured 10 minutes later. If the target BP threshold is not achieved, administer 40 mg, 80 mg, and 80 mg at 10-minute intervals, as needed, again measuring BP 10 minutes after every dose. If, after a maximum dose of 240 mg, the desired BP threshold still has not been reached, give 5 to 10 mg IV hydralazine and measure BP 20 minutes later. If the target BP threshold still has not been achieved, it is essential to obtain consultation on the need for continuous infusion of labetalol, nicardipine, or sodium nitroprusside.
The initial dose of hydralazine, when it is your chosen agent, is 5 to 10 mg IV, with BP measured 20 minutes later. If needed, give another 10 mg and measure BP after another 20-minute interval. After a maximum dose of hydralazine 20 mg, switch to IV labetalol, using the regimen described above for labetalol, if the BP threshold still has not been achieved.
Nitroglycerin may be helpful in carefully selected patients
This drug is an arterial—but mostly venous—dilator. It is administered via IV infusion at an initial rate of 5 µg/min, with the rate gradually increased every 3 to 5 minutes (titrated to BP) to a maximum dose of 100 µg/min. It is the drug of choice in any hypertensive emergency associated with pulmonary edema and for control of hypertension associated with tracheal manipulation during intubation and extubation with general anesthesia.
Nitroglycerin is contraindicated in hypertensive encephalopathy because it increases cerebral blood flow and intracranial pressure. This drug should be administered only under the supervision of an experienced obstetric intensivist.
Sodium nitroprusside: Only in an ICU
This agent causes arterial and venous relaxation by interfering with the influx and intracellular activation of calcium. It is the drug of choice in hypertensive encephalopathy because it controls both afterload (vascular resistance) and preload (fluid status). It should be used only in the setting of intensive care.
The recommended dose is IV infusion at a rate of 0.25 to 5.00 µg/kg/min. Sodium nitroprusside has an immediate onset of action and may continue to exert an effect 3 to 5 minutes after discontinuation. Any hypotension caused by the drug should subside within minutes after discontinuation of the drip, due to the drug’s short half-life.
Nitroprusside is metabolized into thiocyanate and excreted in the urine. Cyanide can accumulate with large doses (>10 µg/kg/min) or prolonged administration (>48 hours), or if the patient has renal insufficiency or decreased hepatic metabolism. Signs of toxicity include anorexia, disorientation, headache, fatigue, restlessness, tinnitus, delirium, hallucinations, nausea, vomiting, and metabolic acidosis. When infused at a rate of less than 2 µg/kg/min, however, cyanide toxicity is unlikely.
As is the case with nitroglycerin, this drug should be administered only under the supervision of an experienced obstetric intensivist.
Case: Resolved
Upon arrival at the ED, the patient exhibits shallow, rapid breathing and foaming from the mouth. She is placed in a lateral decubitus position, an oral airway is established, and all secretions are suctioned. Oxygen is administered via face mask at a rate of 8 L/min. Her initial oxygen saturation level is 92%. IV access is secured, and a loading dose of magnesium sulfate 6 g is given over 20 minutes. Oxygen saturation increases to 94% to 96%. Auscultation of both lungs is normal.
The patient remains in a postictal state for about 15 minutes, but then orients to name, place, and time. FHR monitoring of both fetuses reveals a normal baseline with moderate variability, as well as variable decelerations in the presenting twin.
A maintenance dose of magnesium sulfate is initiated at a rate of 2 g/h, with the BP level recorded every 15 minutes. Systolic values remain between 170 and 180 mm Hg, and diastolic values between 108 and 112 mm Hg for 60 minutes. The obstetrician administers IV labetalol (20 mg) over 2 minutes. About 15 minutes later, the BP level is 154/100 mm Hg, with values remaining in the range of 150 to 156 mm Hg systolic and 92 to 104 mm Hg diastolic.
Ultrasonography reveals that the presenting twin is in a breech position, with estimated fetal weight below the 10th percentile and oligohydramnios. As a result, the obstetrician elects to proceed to cesarean delivery. The twins are delivered by cesarean section using spinal anesthesia. Although the infants are premature, there are no complications.Profile of an eclamptic seizure
Witnessing an eclamptic convulsion can be a frightening experience for nurses and medical providers. The convulsion usually lasts 60 to 90 seconds and occurs in two phases:
- Phase 1 (15–25 seconds) involves facial twitching, rolling of the eyes, and stiffening of the body, with generalized muscular contractions.
- Phase 2 (20–50 seconds) involves alternate contraction and relaxation of the muscles of the body in rapid succession, starting in the face and spreading throughout the body. Foaming at the mouth also occurs, and the patient may bite her tongue if it isn’t protected.
Apnea develops during and immediately after the convulsion, lasting about 120 seconds. A period of hyperventilation follows to compensate for the respiratory acidosis during the apneic period.
A postictal state follows the convulsion, and the patient usually remembers nothing of the episode. Some patients also become restless, combative, and agitated, requiring sedation. Aspiration is possible during or after the convulsion.
We want to hear from you! Tell us what you think.
CASE: MISSED PREECLAMPSIA
At her first prenatal visit at 14 weeks’ gestation, a 41-year-old woman (G2P1) presents with a dichorionic twin gestation, blood pressure (BP) of 105/68 mm Hg, and a body mass index (BMI) of 40 kg/m2. The pregnancy was achieved through in vitro fertilization. Ten years earlier, the patient’s first pregnancy was complicated by preeclampsia, requiring preterm delivery at 33 weeks’ gestation.
By 28 weeks’ gestation, the patient has gained 26 lb. Her BP is 120/70 mm Hg, with no proteinuria detected by urine dipstick. By 30 weeks, she has gained an additional 8 lb, her BP is 142/84 mm Hg, and no proteinuria is detected. At 32 weeks, her BP is 140/92 mm Hg, she has gained another 8 lb, and no proteinuria is present. She also reports new-onset headaches that do not respond to over-the-counter analgesics. She is sent to the obstetric triage area for BP monitoring, blood testing for preeclampsia and nonstress test fetal monitoring.
During the 2-hour observation period, the patient continues to report headaches, and swelling of her face and hands is present. Her systolic BP values range from 132 to 152 mm Hg, and diastolic values range from 80 to 96 mm Hg. No proteinuria is detected, blood testing results for preeclampsia (complete blood count, liver enzymes, serum creatinine, and uric acid) are normal, and the nonstress tests are reactive in both fetuses.
The patient is given a diagnosis of gestational hypertension, along with a prescription for oral labetalol 200 mg daily and two tablets of acetaminophen with codeine for the headaches (to be taken every 6 hours as needed). She is sent home with instructions to return to her physician’s office in 1 week.
Two days later, she wakes in the middle of the night with a severe headache, blurred vision, and vomiting. Her husband calls the obstetrician’s answering service and is instructed to call 911 immediately. While waiting for an ambulance, the patient experiences a grand mal eclamptic convulsion. A second convulsion occurs during her transfer to the ED.
This scenario could have been avoided.
The obstetrician in this case was negligent for failing to recognize preeclampsia in a patient who had two clear risk factors for it: multifetal gestation and a history of early-onset (<37 weeks) preeclampsia in an earlier pregnancy (other risk factors are listed in TABLE 1).
As a result, the patient developed eclampsia, a serious condition that can lead to grave maternal complications (TABLE 2), including death. It also can cause fetal complications, including growth restriction, hypoxia, acidosis, preterm birth, long-term developmental deficits, and death.1,2
The obstetrician in this case also overlooked published evidence indicating that, in the setting of hypertension and headaches, as many as 20% to 30% of pregnant women whose tests for proteinuria show a negative or trace result via dipstick will develop eclampsia.3 Instead of initiating outpatient administration of oral antihypertensive agents, the obstetrician should have hospitalized this patient for at least 48 hours, with steroid administration, to determine whether outpatient management was feasible.
Related article: 10 practical, evidence-based recommendations to improve outcomes in women who have eclampsia Baha Sibai, MD (November 2011)
Defining eclampsia
Eclampsia is marked by the onset of convulsions (during pregnancy or postpartum) in association with gestational hypertension alone, proteinuria, preeclampsia, or superimposed preeclampsia. Although it is rare, eclampsia is potentially life-threatening. For that reason, obstetricians, anesthesiologists, ED physicians, neurologists, and critical-care physicians should be well versed in its diagnosis and management. In this article, I focus on management.
A few preliminary points
Eclampsia can develop any time during the antenatal period (>16 weeks’ gestation), during labor and delivery, and as long as 6 weeks after delivery. Therefore, we should be vigilant for preeclampsia whenever a pregnant patient visits our office, as well as when she makes unscheduled visits to the ED or obstetric triage area or is hospitalized.
Early recognition of women at high risk for preeclampsia and eclampsia may allow for prompt intervention, including early hospitalization for close observation prior to delivery and postpartum.1,2,4–10
Hospitalization of high-risk women allows for use of antihypertensive agents to treat severe BP, administration of magnesium sulfate to prevent convulsions, and timely delivery of the infant. It also allows for intensive maternal support during and after an eclamptic seizure.
Hospitalization is essential for women who exhibit features that suggest severe disease. More specifically, the presence of gestational hypertension with any of the following features is an indication for immediate hospitalization for evaluation and management:
- persistent severe hypertension (systolic
BP ≥160 mm Hg or diastolic BP ≥110 mm Hg) for at least 1 hour - gestational hypertension requiring oral antihypertensive therapy
- progressive and excessive weight gain (≥20 lb prior to 28 weeks’ gestation)
- generalized swelling (edema of hands or face)
- new-onset or persistent headaches despite analgesics
- persistent visual changes (blurred vision, scotomata, photophobia, double vision)
- shortness of breath, dyspnea, orthopnea, or tightness in the chest
- persistent retrosternal chest pain, severe epigastric or right upper quadrant pain
- persistent nausea, vomiting, malaise
- altered mental state, confusion, numbness, tingling, or motor weakness
- platelet count below 100 3 103 µL
- aspartate aminotransferase (AST), alanine aminotransferase (ALT), or lactic acid dehydrogenase (LDH) levels more than twice the upper limit of normal
- serum creatinine level >1.1 mg/dL
- suspected abruptio placentae.
A stepwise approach to eclampsia
Eclampsia is an obstetric emergency. Inadequate preparation for it or an inappropriate response to maternal and fetal conditions during and after an eclamptic convulsion can be detrimental to the mother and fetus. All obstetric units should have up-to-date protocols in place and should conduct mandatory drills to prepare nursing staff, obstetric providers, and anesthesia staff working in these units to manage eclampsia.
Step 1: Let the seizure run its course
During a seizure, resist the impulse to administer anticonvulsive drugs, including intravenous (IV) magnesium sulfate, because most eclamptic convulsions are self-limiting. Also abstain from administering medications such as IV phenytoin, diazepam, or midazolam, as these drugs are less effective than magnesium sulfate, and some can suppress the laryngeal reflex, increasing the risk of aspiration.
If the patient develops status epilepticus, initiate muscle paralysis and intubate her.
Step 2: Support the maternal condition
It is vital to support maternal respiratory and cardiovascular functions to prevent hypoxia, acidosis, and cardiorespiratory arrest.
Begin by establishing airway patency and maternal oxygenation during and after the convulsion. Administer oxygen via a face mask, with or without a reservoir, at a rate of 8 to 10 L/min.
During the apneic period (see “Profile of an eclamptic seizure” on page 46), the patient will develop hypoxia. Use pulse oximetry to monitor oxygen saturation, with the goal of keeping it above 94%. Arterial blood gas analysis is required if oxygen saturation remains below 92% or if pulmonary edema or aspiration is suspected.
If the patient develops recurrent seizures, status epilepticus, florid alveolar pulmonary edema, or respiratory arrest, intubate her immediately.
Step 3: Prevent maternal injury and aspiration
Secure the side rails of the patient’s bed by elevating them to prevent a fall, and make sure they are padded to prevent trauma during convulsions and afterward, when some women become combative and agitated. Position the patient in a lateral decubitus position to minimize aspiration of oral secretions. If any secretions or vomitus are present, remove them via suction.
Step 4: After the convulsion, give magnesium sulfate
Magnesium is the drug of choice for seizure prophylaxis in women with preeclampsia and severe symptoms, and to prevent recurrent seizures in women with eclampsia.
In the latter group, once the eclamptic convulsion has ended, give a loading dose of IV magnesium (6 g/100 mL over 20 minutes), followed by a continuous infusion of 2 g/h for at least 24 hours. If the patient develops a second seizure during the maintenance infusion, administer another bolus of magnesium (2 g/100 mL over 3–5 minutes).
Step 5: Treat severe hypertension
If severe hypertension persists for 60 minutes or longer, it can lead to injury of the brain, heart, and kidneys. To avoid these complications, it is essential to reduce BP to a safe range and maintain that level without compromising cerebral perfusion pressure and uteroplacental blood flow (which already may be reduced in some patients).
The goal of antihypertensive therapy is to keep systolic BP between 140 and 155 mm Hg and diastolic values between 90 and 105 mm Hg.9 Several agents are available for the treatment of severe hypertension during pregnancy and postpartum. The most commonly used IV medications for this purpose are labetalol and hydralazine. Another option is oral, rapidly acting
nifedipine.
Several randomized trials have compared efficacy and side effects between IV bolus injections of hydralazine; IV labetalol; and oral, rapidly acting nifedipine. In general, the findings of these studies suggest that either IV hydralazine or labetalol or oral nifedipine can be used to treat severe hypertension in pregnancy, as long as the provider is familiar with the dose to be used, the expected onset of action, and potential side effects (TABLE 3).
Women who develop generalized swelling or hemoconcentration (hematocrit ≥40%), or both, usually experience markedly reduced plasma volume. For this reason, these women will benefit from treatment with labetalol. If this is ineffective, then add IV hydralazine. However, delay administration of a rapidly acting vasodilator such as hydralazine to prevent an excessive hypotensive response and a secondary reduction in tissue perfusion and uteroplacental blood flow. Rather, administer a bolus infusion of 250 to 500 mL of isotonic saline before giving a vasodilator.
Additional details about the use of antihypertensive drugs are given in the section on other hypertensive emergencies below.
Step 6: Evaluate the patient for complications
Pulmonary edema can develop in patients with eclampsia or another hypertensive emergency. Suspect it if the patient has respiratory symptoms in association with tachypnea, tachycardia, or sustained oxygen saturation values below 93%, as well as when the patient exhibits basal rales during auscultation of the lungs. Treatment involves the administration of oxygen and IV furosemide (20–40 mg push), repeated as needed.
Some women with eclampsia may develop severe cerebral edema, hemorrhage, or both. The edema can be vasogenic or cytotoxic, leading to increased intracerebral pressure. Suspect edema or hemorrhage if the patient remains unresponsive, continues to experience convulsions despite therapy, or exhibits sensory or motor neurologic deficits. In such cases, neuroimaging is indicated, and the patient should be managed in consultation with neurology or neurosurgery.
Step 7: Begin the process of induction and delivery
Once the patient has been stabilized—and not before—initiate the induction process. Be aware that during and after the convulsion, changes in fetal heart rate (FHR) and uterine monitoring will usually be evident:
- prolonged deceleration or bradycardia (3–10 minutes)
- compensatory tachycardia, decreased beat-to-beat variability
- transient recurrent decelerations
- increased uterine tone and greater frequency of uterine activity.
These changes in FHR and uterine activity usually last 3 to 15 minutes. For this reason, it is important to avoid rushing the patient for cesarean delivery, as FHR and uterine activity are likely to return to normal after maternal resuscitation and stabilization. If not, consider other causes, such as abruptio placentae.
Eclampsia itself is not an indication for cesarean delivery. The selection of mode of delivery should be based on the presence or absence of labor, the cervical Bishop score, fetal gestational age, fetal presentation, and overall fetal condition.
Choosing an anesthetic
Regional analgesia/anesthesia is the method of choice for most women with eclampsia. However, regional anesthesia is to be avoided in the presence of disseminated intravascular coagulation or thrombocytopenia (the threshold platelet count is usually less than 75 x 103 µL. In such a case, IV analgesia can be used during labor, and general anesthesia may be appropriate for cesarean delivery. Both spinal and epidural analgesia and anesthesia are appropriate for women with eclampsia.
How to manage other hypertensive emergencies
A hypertensive emergency during pregnancy or postpartum involves acute-onset, persistent (>15 minutes), severe systolic BP (≥160 mm Hg) or severe diastolic BP (≥110 mm Hg), or both. The first step in such an emergency is to ensure the accurate measurement of BP using standard techniques.
Patients with acute-onset, persistent, severe BP should be hospitalized promptly for evaluation and treatment to prevent organ damage. Once such a patient is hospitalized, BP should be recorded every 15 minutes, with continuous FHR monitoring to ensure fetal viability.
Related article: Failure to diagnose preeclampsia and more (Medical Verdicts, February 2013)
The timing of initiation of antihypertensive medications, as well as determination of the type of medication best suited for the patient, should be based on:
- systolic and diastolic BP levels
- maternal clinical and laboratory findings
- presence of associated symptoms
- preexisting medical comorbidities
- whether the patient is antepartum or postpartum.
For example, a sustained BP level of 200/120 mm Hg requires therapy after 15 minutes, whereas observation may be suitable for as long as 60 minutes for a sustained BP of 160/72 mm Hg during labor.
Rapid reduction of systolic BP can lead to marked reductions in uteroplacental blood flow and a nonreassuring FHR tracing. Moreover, a rapid reduction of severe systolic BP in patients who have constricted plasma volume can reduce perfusion to the kidney, brain, and placenta. However, sustained BP of 165/100 mm Hg in association with central nervous system signs or symptoms, congestive heart failure, thrombocytopenia, or postpartum status requires therapy within 1 hour.
In general, it is difficult to obtain accurate BP recordings using noninvasive electronic instruments during labor because of the effects of labor on systolic BP and the lack of standardized methods for positioning of the arm cuff and the patient.
For these reasons, the decision about when to start acute antihypertensive therapy, based on systolic or diastolic BP, or both, should be individualized. And the choice of antihypertensive agent should be based on maternal clinical findings.
Choosing an antihypertensive agent
Because both hydralazine and nifedipine are associated with tachycardia, avoid them in patients with a heart rate above 110 bpm, using labetalol instead.10
In patients with bradycardia (heart rate <60 bpm), asthma, or congestive heart failure, however, labetalol should be avoided. In these populations, hydralazine or nifedipine is the drug of choice. Nifedipine is associated with improved renal blood flow and a resultant increase in urine output, making it preferable for patients with decreased urine output or severe postpartum hypertension.10
One theoretical concern is that the combined use of nifedipine and magnesium sulfate can cause excessive hypotension and neuromuscular blockage. As a result, some experts recommend that nifedipine be avoided in patients receiving magnesium sulfate. However, a recent review of this subject concluded that combined use of these drugs does not increase the risks of excessive hypotension and neuromuscular blockage in patients with severe hypertension or preeclampsia.
The initial dose of labetalol, when it is your chosen agent, is 20 mg IV, with BP measured 10 minutes later. If the target BP threshold is not achieved, administer 40 mg, 80 mg, and 80 mg at 10-minute intervals, as needed, again measuring BP 10 minutes after every dose. If, after a maximum dose of 240 mg, the desired BP threshold still has not been reached, give 5 to 10 mg IV hydralazine and measure BP 20 minutes later. If the target BP threshold still has not been achieved, it is essential to obtain consultation on the need for continuous infusion of labetalol, nicardipine, or sodium nitroprusside.
The initial dose of hydralazine, when it is your chosen agent, is 5 to 10 mg IV, with BP measured 20 minutes later. If needed, give another 10 mg and measure BP after another 20-minute interval. After a maximum dose of hydralazine 20 mg, switch to IV labetalol, using the regimen described above for labetalol, if the BP threshold still has not been achieved.
Nitroglycerin may be helpful in carefully selected patients
This drug is an arterial—but mostly venous—dilator. It is administered via IV infusion at an initial rate of 5 µg/min, with the rate gradually increased every 3 to 5 minutes (titrated to BP) to a maximum dose of 100 µg/min. It is the drug of choice in any hypertensive emergency associated with pulmonary edema and for control of hypertension associated with tracheal manipulation during intubation and extubation with general anesthesia.
Nitroglycerin is contraindicated in hypertensive encephalopathy because it increases cerebral blood flow and intracranial pressure. This drug should be administered only under the supervision of an experienced obstetric intensivist.
Sodium nitroprusside: Only in an ICU
This agent causes arterial and venous relaxation by interfering with the influx and intracellular activation of calcium. It is the drug of choice in hypertensive encephalopathy because it controls both afterload (vascular resistance) and preload (fluid status). It should be used only in the setting of intensive care.
The recommended dose is IV infusion at a rate of 0.25 to 5.00 µg/kg/min. Sodium nitroprusside has an immediate onset of action and may continue to exert an effect 3 to 5 minutes after discontinuation. Any hypotension caused by the drug should subside within minutes after discontinuation of the drip, due to the drug’s short half-life.
Nitroprusside is metabolized into thiocyanate and excreted in the urine. Cyanide can accumulate with large doses (>10 µg/kg/min) or prolonged administration (>48 hours), or if the patient has renal insufficiency or decreased hepatic metabolism. Signs of toxicity include anorexia, disorientation, headache, fatigue, restlessness, tinnitus, delirium, hallucinations, nausea, vomiting, and metabolic acidosis. When infused at a rate of less than 2 µg/kg/min, however, cyanide toxicity is unlikely.
As is the case with nitroglycerin, this drug should be administered only under the supervision of an experienced obstetric intensivist.
Case: Resolved
Upon arrival at the ED, the patient exhibits shallow, rapid breathing and foaming from the mouth. She is placed in a lateral decubitus position, an oral airway is established, and all secretions are suctioned. Oxygen is administered via face mask at a rate of 8 L/min. Her initial oxygen saturation level is 92%. IV access is secured, and a loading dose of magnesium sulfate 6 g is given over 20 minutes. Oxygen saturation increases to 94% to 96%. Auscultation of both lungs is normal.
The patient remains in a postictal state for about 15 minutes, but then orients to name, place, and time. FHR monitoring of both fetuses reveals a normal baseline with moderate variability, as well as variable decelerations in the presenting twin.
A maintenance dose of magnesium sulfate is initiated at a rate of 2 g/h, with the BP level recorded every 15 minutes. Systolic values remain between 170 and 180 mm Hg, and diastolic values between 108 and 112 mm Hg for 60 minutes. The obstetrician administers IV labetalol (20 mg) over 2 minutes. About 15 minutes later, the BP level is 154/100 mm Hg, with values remaining in the range of 150 to 156 mm Hg systolic and 92 to 104 mm Hg diastolic.
Ultrasonography reveals that the presenting twin is in a breech position, with estimated fetal weight below the 10th percentile and oligohydramnios. As a result, the obstetrician elects to proceed to cesarean delivery. The twins are delivered by cesarean section using spinal anesthesia. Although the infants are premature, there are no complications.Profile of an eclamptic seizure
Witnessing an eclamptic convulsion can be a frightening experience for nurses and medical providers. The convulsion usually lasts 60 to 90 seconds and occurs in two phases:
- Phase 1 (15–25 seconds) involves facial twitching, rolling of the eyes, and stiffening of the body, with generalized muscular contractions.
- Phase 2 (20–50 seconds) involves alternate contraction and relaxation of the muscles of the body in rapid succession, starting in the face and spreading throughout the body. Foaming at the mouth also occurs, and the patient may bite her tongue if it isn’t protected.
Apnea develops during and immediately after the convulsion, lasting about 120 seconds. A period of hyperventilation follows to compensate for the respiratory acidosis during the apneic period.
A postictal state follows the convulsion, and the patient usually remembers nothing of the episode. Some patients also become restless, combative, and agitated, requiring sedation. Aspiration is possible during or after the convulsion.
We want to hear from you! Tell us what you think.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–410.
- Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):182–192.
- Meyer NL, Mercer BM, Friedman SA, Sibai BM. Urinary dipstick protein: a poor predictor of absent or severe proteinuria. Am J Obstet Gynecol. 1994;170(1 Pt 1):137–141.
- Knight M; UK Obstetric Surveillance System (UKOSS). Eclampsia in the United Kingdom 2005. BJOG. 2007;114(9):1072–1078.
- ACOG Practice Bulletin #33: Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167.
- Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia–eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e1–e7.
- Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206(6):470–475.
- ACOG Committee Opinion #514: Emergent therapy for acute-onset, severe hypertension with preeclampsia or eclampsia. Obstet Gynecol. 2011;118:1465–1468.
- Liu S, Joseph KS, Liston RM, et al. Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118(5):987–994.
- Raheem IA, Saaid R, Omar Sz, Tan PC. Oral nifedipine versus intravenous labetalol for acute blood pressure control in hypertensive emergencies of pregnancy: a randomized trial. BJOG. 2012;119(1):78–85.
- Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–410.
- Sibai BM. Diagnosis and management of gestational hypertension and preeclampsia. Obstet Gynecol. 2003;102(1):182–192.
- Meyer NL, Mercer BM, Friedman SA, Sibai BM. Urinary dipstick protein: a poor predictor of absent or severe proteinuria. Am J Obstet Gynecol. 1994;170(1 Pt 1):137–141.
- Knight M; UK Obstetric Surveillance System (UKOSS). Eclampsia in the United Kingdom 2005. BJOG. 2007;114(9):1072–1078.
- ACOG Practice Bulletin #33: Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167.
- Sibai BM, Stella CL. Diagnosis and management of atypical preeclampsia–eclampsia. Am J Obstet Gynecol. 2009;200(5):481.e1–e7.
- Sibai BM. Etiology and management of postpartum hypertension-preeclampsia. Am J Obstet Gynecol. 2012;206(6):470–475.
- ACOG Committee Opinion #514: Emergent therapy for acute-onset, severe hypertension with preeclampsia or eclampsia. Obstet Gynecol. 2011;118:1465–1468.
- Liu S, Joseph KS, Liston RM, et al. Incidence, risk factors, and associated complications of eclampsia. Obstet Gynecol. 2011;118(5):987–994.
- Raheem IA, Saaid R, Omar Sz, Tan PC. Oral nifedipine versus intravenous labetalol for acute blood pressure control in hypertensive emergencies of pregnancy: a randomized trial. BJOG. 2012;119(1):78–85.
Which abnormal ovarian findings can be followed by serial TVUS?
Ovarian cancer causes more deaths than any other cancer affecting the female reproductive system.1 One reason it’s so deadly: It usually isn’t detected until it has reached an advanced stage. No clear-cut symptoms point definitively to ovarian malignancy, and no feasible screening strategy has been found to increase detection at an early stage.
Among the strategies that have been utilized to detect ovarian cancer are bimanual examination of the adnexae (primarily in postmenopausal women), measurement of cancer antigen (CA) 125, and transvaginal ultrasonography (TVUS) of the ovaries. The last two strategies sometimes are combined in high-risk women.
TVUS can highlight ovarian abnormalities and provide information about their structure. The question then becomes which abnormalities are likely to resolve without treatment, and which should be scrutinized more closely. In this study, Pavlik and colleagues reviewed TVUS findings from 39,337 women enrolled in the University of Kentucky Ovarian Cancer Screening Program, which involved 221,576 baseline and interval TVUS scans.
Details of the study
Women in this study were screened with annual TVUS scans between 1987 and 2002. The population included:
- asymptomatic women aged 50 or older
- asymptomatic women over age 25 who had a first- or second-degree relative with documented ovarian cancer.
The initial TVUS scan was normal in almost 90% of women, and only about 10% subsequently experienced an abnormal scan. About half (46.7%) of the ovarian abnormalities identified via TVUS were found on the very first scan. Of these, 63.2% resolved during follow-up with no treatment.
Approximately 80% of women had no abnormal TVUS findings at any time during the observation period. This is notable because participants had a high risk for ovarian cancer by virtue of advanced age or family history.
TVUS abnormalities had a higher prevalence in premenopausal women (35%) than in postmenopausal women (17%; P<.001). The incidence of ovarian cysts also was significantly higher among premenopausal women (15.3% vs 8.2%; P<.001). These differences are to be expected, owing to the functional nature of premenopausal ovaries in regard to folliculogenesis, ovulation, and endometriosis.
Positive predictive values ranged from 15.3% to 24.7%
Over the 25 years covered by this study, our understanding of the malignant potential of various ovarian masses has evolved considerably. We have long known that unilocular cysts are extremely unlikely to be malignant, but now we are aware that even septated cysts are unlikely to represent cancer.
As for the success of this ovarian cancer-screening program, which identified 85 true malignancies and 472 nonmalignancies in surgical specimens, it had an overall positive predictive value of 15.3%. After January 1, 2008, however, when serial observation expanded to include septated cysts (because published data confirmed these masses to have low malignant potential), positive predictive value improved to 24.7%.
Pavlik and colleagues also discussed findings from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, which relied on a single TVUS abnormality to trigger a recommendation for surgery, with a positive predictive value of only 5.1%.2
Most cancers were diagnosed at an early stage
Of the invasive epithelial cancers identified in this study, the stage distribution at diagnosis was:
- Stage 1: 45%
- Stage 2: 23%
- Stage 3: 32%
- Stage 4: None.
This finding is notable, given statistics from the “real world,” where about 80% of ovarian cancers are diagnosed at Stage 3 or Stage 4.
Among benign findings that were managed surgically, 47% were serous cystadenomas, 13% were hemorrhagic cysts, 9% were fibromas, thecomas, or Brenner tumors, and the rest were fairly equally divided between hydrosalpinx or paratubal cysts; endometriomas; and mucinous cystadenomas, leiomyomas, and cystic teratomas.
What this evidence means for practice
In general, unilocular or septate cysts can be followed every 6 months by TVUS. Although more complex tumors may resolve spontaneously, they should be followed with serial TVUS, with caution, at intervals of 6 weeks to 3 months. The findings of each scan should determine the subsequent course of action, which could involve further monitoring or surgical extirpation.
Regrettably, this study did not utilize color flow Doppler imaging. Because malignant tumors are rich in neovascularity, and the vessels laid down by such tumors often lack a normal media layer, they often exhibit very low resistance to flow. Although neovascularity is not a perfect diagnostic indicator of malignancy, the presence of abundant blood flow and low resistance can raise the index of suspicion. In my opinion, color flow Doppler should be incorporated into ultrasonographic evaluation of potential ovarian malignancies.
—Steven R. Goldstein, MD
Tell us what you think, at [email protected]. Please include your name and city and state.
1. American Cancer Society. Cancer Facts and Figures, 2013. http://www.cancer.org/acs/groups/content/@e p i d e m i o l o g y s u r v e i l a n c e / d o c u m e n t s / d o c u m e n t/acspc-036845.pdf. Accessed August 20, 2013.
2. Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality—the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295–2303.
Ovarian cancer causes more deaths than any other cancer affecting the female reproductive system.1 One reason it’s so deadly: It usually isn’t detected until it has reached an advanced stage. No clear-cut symptoms point definitively to ovarian malignancy, and no feasible screening strategy has been found to increase detection at an early stage.
Among the strategies that have been utilized to detect ovarian cancer are bimanual examination of the adnexae (primarily in postmenopausal women), measurement of cancer antigen (CA) 125, and transvaginal ultrasonography (TVUS) of the ovaries. The last two strategies sometimes are combined in high-risk women.
TVUS can highlight ovarian abnormalities and provide information about their structure. The question then becomes which abnormalities are likely to resolve without treatment, and which should be scrutinized more closely. In this study, Pavlik and colleagues reviewed TVUS findings from 39,337 women enrolled in the University of Kentucky Ovarian Cancer Screening Program, which involved 221,576 baseline and interval TVUS scans.
Details of the study
Women in this study were screened with annual TVUS scans between 1987 and 2002. The population included:
- asymptomatic women aged 50 or older
- asymptomatic women over age 25 who had a first- or second-degree relative with documented ovarian cancer.
The initial TVUS scan was normal in almost 90% of women, and only about 10% subsequently experienced an abnormal scan. About half (46.7%) of the ovarian abnormalities identified via TVUS were found on the very first scan. Of these, 63.2% resolved during follow-up with no treatment.
Approximately 80% of women had no abnormal TVUS findings at any time during the observation period. This is notable because participants had a high risk for ovarian cancer by virtue of advanced age or family history.
TVUS abnormalities had a higher prevalence in premenopausal women (35%) than in postmenopausal women (17%; P<.001). The incidence of ovarian cysts also was significantly higher among premenopausal women (15.3% vs 8.2%; P<.001). These differences are to be expected, owing to the functional nature of premenopausal ovaries in regard to folliculogenesis, ovulation, and endometriosis.
Positive predictive values ranged from 15.3% to 24.7%
Over the 25 years covered by this study, our understanding of the malignant potential of various ovarian masses has evolved considerably. We have long known that unilocular cysts are extremely unlikely to be malignant, but now we are aware that even septated cysts are unlikely to represent cancer.
As for the success of this ovarian cancer-screening program, which identified 85 true malignancies and 472 nonmalignancies in surgical specimens, it had an overall positive predictive value of 15.3%. After January 1, 2008, however, when serial observation expanded to include septated cysts (because published data confirmed these masses to have low malignant potential), positive predictive value improved to 24.7%.
Pavlik and colleagues also discussed findings from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, which relied on a single TVUS abnormality to trigger a recommendation for surgery, with a positive predictive value of only 5.1%.2
Most cancers were diagnosed at an early stage
Of the invasive epithelial cancers identified in this study, the stage distribution at diagnosis was:
- Stage 1: 45%
- Stage 2: 23%
- Stage 3: 32%
- Stage 4: None.
This finding is notable, given statistics from the “real world,” where about 80% of ovarian cancers are diagnosed at Stage 3 or Stage 4.
Among benign findings that were managed surgically, 47% were serous cystadenomas, 13% were hemorrhagic cysts, 9% were fibromas, thecomas, or Brenner tumors, and the rest were fairly equally divided between hydrosalpinx or paratubal cysts; endometriomas; and mucinous cystadenomas, leiomyomas, and cystic teratomas.
What this evidence means for practice
In general, unilocular or septate cysts can be followed every 6 months by TVUS. Although more complex tumors may resolve spontaneously, they should be followed with serial TVUS, with caution, at intervals of 6 weeks to 3 months. The findings of each scan should determine the subsequent course of action, which could involve further monitoring or surgical extirpation.
Regrettably, this study did not utilize color flow Doppler imaging. Because malignant tumors are rich in neovascularity, and the vessels laid down by such tumors often lack a normal media layer, they often exhibit very low resistance to flow. Although neovascularity is not a perfect diagnostic indicator of malignancy, the presence of abundant blood flow and low resistance can raise the index of suspicion. In my opinion, color flow Doppler should be incorporated into ultrasonographic evaluation of potential ovarian malignancies.
—Steven R. Goldstein, MD
Tell us what you think, at [email protected]. Please include your name and city and state.
Ovarian cancer causes more deaths than any other cancer affecting the female reproductive system.1 One reason it’s so deadly: It usually isn’t detected until it has reached an advanced stage. No clear-cut symptoms point definitively to ovarian malignancy, and no feasible screening strategy has been found to increase detection at an early stage.
Among the strategies that have been utilized to detect ovarian cancer are bimanual examination of the adnexae (primarily in postmenopausal women), measurement of cancer antigen (CA) 125, and transvaginal ultrasonography (TVUS) of the ovaries. The last two strategies sometimes are combined in high-risk women.
TVUS can highlight ovarian abnormalities and provide information about their structure. The question then becomes which abnormalities are likely to resolve without treatment, and which should be scrutinized more closely. In this study, Pavlik and colleagues reviewed TVUS findings from 39,337 women enrolled in the University of Kentucky Ovarian Cancer Screening Program, which involved 221,576 baseline and interval TVUS scans.
Details of the study
Women in this study were screened with annual TVUS scans between 1987 and 2002. The population included:
- asymptomatic women aged 50 or older
- asymptomatic women over age 25 who had a first- or second-degree relative with documented ovarian cancer.
The initial TVUS scan was normal in almost 90% of women, and only about 10% subsequently experienced an abnormal scan. About half (46.7%) of the ovarian abnormalities identified via TVUS were found on the very first scan. Of these, 63.2% resolved during follow-up with no treatment.
Approximately 80% of women had no abnormal TVUS findings at any time during the observation period. This is notable because participants had a high risk for ovarian cancer by virtue of advanced age or family history.
TVUS abnormalities had a higher prevalence in premenopausal women (35%) than in postmenopausal women (17%; P<.001). The incidence of ovarian cysts also was significantly higher among premenopausal women (15.3% vs 8.2%; P<.001). These differences are to be expected, owing to the functional nature of premenopausal ovaries in regard to folliculogenesis, ovulation, and endometriosis.
Positive predictive values ranged from 15.3% to 24.7%
Over the 25 years covered by this study, our understanding of the malignant potential of various ovarian masses has evolved considerably. We have long known that unilocular cysts are extremely unlikely to be malignant, but now we are aware that even septated cysts are unlikely to represent cancer.
As for the success of this ovarian cancer-screening program, which identified 85 true malignancies and 472 nonmalignancies in surgical specimens, it had an overall positive predictive value of 15.3%. After January 1, 2008, however, when serial observation expanded to include septated cysts (because published data confirmed these masses to have low malignant potential), positive predictive value improved to 24.7%.
Pavlik and colleagues also discussed findings from the Prostate, Lung, Colorectal, and Ovarian (PLCO) Cancer Screening Trial, which relied on a single TVUS abnormality to trigger a recommendation for surgery, with a positive predictive value of only 5.1%.2
Most cancers were diagnosed at an early stage
Of the invasive epithelial cancers identified in this study, the stage distribution at diagnosis was:
- Stage 1: 45%
- Stage 2: 23%
- Stage 3: 32%
- Stage 4: None.
This finding is notable, given statistics from the “real world,” where about 80% of ovarian cancers are diagnosed at Stage 3 or Stage 4.
Among benign findings that were managed surgically, 47% were serous cystadenomas, 13% were hemorrhagic cysts, 9% were fibromas, thecomas, or Brenner tumors, and the rest were fairly equally divided between hydrosalpinx or paratubal cysts; endometriomas; and mucinous cystadenomas, leiomyomas, and cystic teratomas.
What this evidence means for practice
In general, unilocular or septate cysts can be followed every 6 months by TVUS. Although more complex tumors may resolve spontaneously, they should be followed with serial TVUS, with caution, at intervals of 6 weeks to 3 months. The findings of each scan should determine the subsequent course of action, which could involve further monitoring or surgical extirpation.
Regrettably, this study did not utilize color flow Doppler imaging. Because malignant tumors are rich in neovascularity, and the vessels laid down by such tumors often lack a normal media layer, they often exhibit very low resistance to flow. Although neovascularity is not a perfect diagnostic indicator of malignancy, the presence of abundant blood flow and low resistance can raise the index of suspicion. In my opinion, color flow Doppler should be incorporated into ultrasonographic evaluation of potential ovarian malignancies.
—Steven R. Goldstein, MD
Tell us what you think, at [email protected]. Please include your name and city and state.
1. American Cancer Society. Cancer Facts and Figures, 2013. http://www.cancer.org/acs/groups/content/@e p i d e m i o l o g y s u r v e i l a n c e / d o c u m e n t s / d o c u m e n t/acspc-036845.pdf. Accessed August 20, 2013.
2. Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality—the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295–2303.
1. American Cancer Society. Cancer Facts and Figures, 2013. http://www.cancer.org/acs/groups/content/@e p i d e m i o l o g y s u r v e i l a n c e / d o c u m e n t s / d o c u m e n t/acspc-036845.pdf. Accessed August 20, 2013.
2. Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality—the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Randomized Controlled Trial. JAMA. 2011;305(22):2295–2303.