User login
Paging goldilocks: How much glycemic control is just right?
There is no doubt that hyperglycemia among hospitalized patients correlates with worse prognosis. Further, there are well‐documented mechanisms by which poor glycemic control may directly impact outcomes. For example, hyperglycemia and insulin deficiency can impair neutrophil function, exacerbate inflammation, and impair endothelium‐mediated dilatation,1, 2 whereas hypoglycemia increases sympathetic tone. And both severe hyperglycemia and hypoglycemia, of course, can precipitate altered mental status. But certainly not all of the morbid outcomes associated with poor glycemic control in the hospitalincluding infection, cardiac events and deathare caused by poor glycemic control in the hospital. Elevated glucose levels in the hospital are often seen in sicker patients with raging stress hormones and in brittle diabetics with a present‐on‐admission condition that has been ravaging their vasculature for years. This means that virtually all observational studies demonstrating worse outcomes in the setting of poor glucose control in the hospital will be severely confounded by comorbid illness, and much confounding will remain even after multivariate adjustment.3
Nonetheless, high‐quality randomized controlled trials that have focused on critically ill patients,4, 5 rather than general medical patients, have generated intense interest and fostered the belief that controlling the glucose level of all hospitalized patients is probably a good idea. (Although, more recently, even the data supporting glycemic control in the critically ill have been challenged.)6 Enthusiasm for implementing aggressive glycemic control protocols outside of the intensive care unit (ICU) appears widespread, as is evident in this issue of JHM.711 In this issue, two articles detail the challenges of implementing glycemia control protocols.7, 8 The research teams employed different protocols and used different metrics, but there are common themes: (1) The process was iterative. Interventions were piloted, then rolled out, and substantial effort was needed to foster continued attention to the interventions. (2) The process was multidisciplinary. Buy‐in and input were needed not only from physicians, but also from nurses, pharmacists, dieticians, clinical data system experts, and probably patients. (3) Impacting process measures was easier than impacting surrogate outcome measures. Specifically, despite dramatic changes in the use of carefully vetted order sets and protocols, the impact on glycemia was modest and sometimes inconsistent.
These studies illustrate that implementing protocols to control glycemia is neither easy, nor consistently associated with improved glycemic controllet alone improved major clinical outcomes. Three complementary observational studies911 further illustrate how hard it is to optimize glycemic control in the hospital setting. Together, the observational and interventional studies demonstrate how difficult it is to measure success. Should we focus on the mean glucose value achieved or the frequency of extreme glucose values (which are, by definition, more dangerous)? Should we look at glycemic control in every patient who is placed on a protocol, even those who barely need any insulin at all, or should we focus our interventions and analyses on those patients with more severe dysglycemia at baseline? This latter issue is fundamentally important, since the rollout of any systemwide glycemia protocol that results in higher catchment rates will appear more effective than it really is by enriching the postintervention data with healthier patients.
Before embarking on time‐intensive efforts to improve care, maybe we should be sure that the evidence supports our efforts.12 Recent recommendations from the American Diabetes Association state that for non‐critically ill patients: there is no clear evidence for specific blood glucose goals.13 (This recommendation, based on expert consensus or clinical experience, further states that because cohort data suggest that outcomes are better in hospitalized patients with fasting glucose <126 mg/dL and all random glucose values <180 to 200 mg/dL, these goals are reasonable if they can be safely achieved.) But given the challenges associated with implementing glycemia protocols, one might argue that hospitalists should invest their quality improvement efforts elsewhere.
So where does this leave us? What target glucose is not too high, not too low, but just right? Given the ever‐increasing number of quality improvement measures and interventions that are expected in the hospital, what amount of time, effort, and money devoted to improving inpatient glycemic control is just right? And what do our patients think? Should we be feeding our patients low glycemic load diets, or letting them indulge in one of the few creature comforts remaining in a semiprivate room?
What is clear from the results of the research published in this issue of JHM (regardless of whether we think that an inpatient pre‐meal glucose of 160 mg/dL is good, bad, or neither), is that we need to continue to develop systems, strategies, and teams to rapidly disseminate quality improvement interventions locally. We need multidisciplinary inputfrom physicians, nurses, dieticians, pharmacists, and patientsto do it right. So, even if the pendulum swings away from tight glycemic control in the hospital, the lessons we learned from these authors' valiant efforts to tame inpatient glycemia may provide us with the tools and knowledge required to successfully tackle other clinical issues such as delirium prevention, pain control, medication reconciliation, and handoffs. The striking obstacles (both in implementation and analysis) faced and overcome by the authors of the articles in this issue of JHM will hopefully embolden them to take on other quality improvement interventions that are perhaps more likely to help hospitalized patients.
- ,,,,.Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose‐binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,,.In search of fewer independent risk factors.Arch Intern Med.2005;165:138–145.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Benefits and risks of tight glucose control in critically ill adults: a meta‐analysis.JAMA.2008;300:933–944.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,,; for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- , ,, et al.Computer‐based insulin infusion protocol improves glycemia control over manual protocol.J Am Med Inform Assoc.2007;14:278–287.
- ,,,,.Estimated risk for undiagnosed diabetes in the emergency department: a multicenter survey.Acad Emerg Med.2007;14:492–495.
- ,,,,,.Evaluation of glycemic control following discontinuation of an intensive insulin protocol.J Hosp Med.2009;28–34.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357:608–613.
- American Diabetes Association. Standards of medical care in diabetes—2008.Diabetes Care.2008;31(Suppl 1):S12–S54.
There is no doubt that hyperglycemia among hospitalized patients correlates with worse prognosis. Further, there are well‐documented mechanisms by which poor glycemic control may directly impact outcomes. For example, hyperglycemia and insulin deficiency can impair neutrophil function, exacerbate inflammation, and impair endothelium‐mediated dilatation,1, 2 whereas hypoglycemia increases sympathetic tone. And both severe hyperglycemia and hypoglycemia, of course, can precipitate altered mental status. But certainly not all of the morbid outcomes associated with poor glycemic control in the hospitalincluding infection, cardiac events and deathare caused by poor glycemic control in the hospital. Elevated glucose levels in the hospital are often seen in sicker patients with raging stress hormones and in brittle diabetics with a present‐on‐admission condition that has been ravaging their vasculature for years. This means that virtually all observational studies demonstrating worse outcomes in the setting of poor glucose control in the hospital will be severely confounded by comorbid illness, and much confounding will remain even after multivariate adjustment.3
Nonetheless, high‐quality randomized controlled trials that have focused on critically ill patients,4, 5 rather than general medical patients, have generated intense interest and fostered the belief that controlling the glucose level of all hospitalized patients is probably a good idea. (Although, more recently, even the data supporting glycemic control in the critically ill have been challenged.)6 Enthusiasm for implementing aggressive glycemic control protocols outside of the intensive care unit (ICU) appears widespread, as is evident in this issue of JHM.711 In this issue, two articles detail the challenges of implementing glycemia control protocols.7, 8 The research teams employed different protocols and used different metrics, but there are common themes: (1) The process was iterative. Interventions were piloted, then rolled out, and substantial effort was needed to foster continued attention to the interventions. (2) The process was multidisciplinary. Buy‐in and input were needed not only from physicians, but also from nurses, pharmacists, dieticians, clinical data system experts, and probably patients. (3) Impacting process measures was easier than impacting surrogate outcome measures. Specifically, despite dramatic changes in the use of carefully vetted order sets and protocols, the impact on glycemia was modest and sometimes inconsistent.
These studies illustrate that implementing protocols to control glycemia is neither easy, nor consistently associated with improved glycemic controllet alone improved major clinical outcomes. Three complementary observational studies911 further illustrate how hard it is to optimize glycemic control in the hospital setting. Together, the observational and interventional studies demonstrate how difficult it is to measure success. Should we focus on the mean glucose value achieved or the frequency of extreme glucose values (which are, by definition, more dangerous)? Should we look at glycemic control in every patient who is placed on a protocol, even those who barely need any insulin at all, or should we focus our interventions and analyses on those patients with more severe dysglycemia at baseline? This latter issue is fundamentally important, since the rollout of any systemwide glycemia protocol that results in higher catchment rates will appear more effective than it really is by enriching the postintervention data with healthier patients.
Before embarking on time‐intensive efforts to improve care, maybe we should be sure that the evidence supports our efforts.12 Recent recommendations from the American Diabetes Association state that for non‐critically ill patients: there is no clear evidence for specific blood glucose goals.13 (This recommendation, based on expert consensus or clinical experience, further states that because cohort data suggest that outcomes are better in hospitalized patients with fasting glucose <126 mg/dL and all random glucose values <180 to 200 mg/dL, these goals are reasonable if they can be safely achieved.) But given the challenges associated with implementing glycemia protocols, one might argue that hospitalists should invest their quality improvement efforts elsewhere.
So where does this leave us? What target glucose is not too high, not too low, but just right? Given the ever‐increasing number of quality improvement measures and interventions that are expected in the hospital, what amount of time, effort, and money devoted to improving inpatient glycemic control is just right? And what do our patients think? Should we be feeding our patients low glycemic load diets, or letting them indulge in one of the few creature comforts remaining in a semiprivate room?
What is clear from the results of the research published in this issue of JHM (regardless of whether we think that an inpatient pre‐meal glucose of 160 mg/dL is good, bad, or neither), is that we need to continue to develop systems, strategies, and teams to rapidly disseminate quality improvement interventions locally. We need multidisciplinary inputfrom physicians, nurses, dieticians, pharmacists, and patientsto do it right. So, even if the pendulum swings away from tight glycemic control in the hospital, the lessons we learned from these authors' valiant efforts to tame inpatient glycemia may provide us with the tools and knowledge required to successfully tackle other clinical issues such as delirium prevention, pain control, medication reconciliation, and handoffs. The striking obstacles (both in implementation and analysis) faced and overcome by the authors of the articles in this issue of JHM will hopefully embolden them to take on other quality improvement interventions that are perhaps more likely to help hospitalized patients.
There is no doubt that hyperglycemia among hospitalized patients correlates with worse prognosis. Further, there are well‐documented mechanisms by which poor glycemic control may directly impact outcomes. For example, hyperglycemia and insulin deficiency can impair neutrophil function, exacerbate inflammation, and impair endothelium‐mediated dilatation,1, 2 whereas hypoglycemia increases sympathetic tone. And both severe hyperglycemia and hypoglycemia, of course, can precipitate altered mental status. But certainly not all of the morbid outcomes associated with poor glycemic control in the hospitalincluding infection, cardiac events and deathare caused by poor glycemic control in the hospital. Elevated glucose levels in the hospital are often seen in sicker patients with raging stress hormones and in brittle diabetics with a present‐on‐admission condition that has been ravaging their vasculature for years. This means that virtually all observational studies demonstrating worse outcomes in the setting of poor glucose control in the hospital will be severely confounded by comorbid illness, and much confounding will remain even after multivariate adjustment.3
Nonetheless, high‐quality randomized controlled trials that have focused on critically ill patients,4, 5 rather than general medical patients, have generated intense interest and fostered the belief that controlling the glucose level of all hospitalized patients is probably a good idea. (Although, more recently, even the data supporting glycemic control in the critically ill have been challenged.)6 Enthusiasm for implementing aggressive glycemic control protocols outside of the intensive care unit (ICU) appears widespread, as is evident in this issue of JHM.711 In this issue, two articles detail the challenges of implementing glycemia control protocols.7, 8 The research teams employed different protocols and used different metrics, but there are common themes: (1) The process was iterative. Interventions were piloted, then rolled out, and substantial effort was needed to foster continued attention to the interventions. (2) The process was multidisciplinary. Buy‐in and input were needed not only from physicians, but also from nurses, pharmacists, dieticians, clinical data system experts, and probably patients. (3) Impacting process measures was easier than impacting surrogate outcome measures. Specifically, despite dramatic changes in the use of carefully vetted order sets and protocols, the impact on glycemia was modest and sometimes inconsistent.
These studies illustrate that implementing protocols to control glycemia is neither easy, nor consistently associated with improved glycemic controllet alone improved major clinical outcomes. Three complementary observational studies911 further illustrate how hard it is to optimize glycemic control in the hospital setting. Together, the observational and interventional studies demonstrate how difficult it is to measure success. Should we focus on the mean glucose value achieved or the frequency of extreme glucose values (which are, by definition, more dangerous)? Should we look at glycemic control in every patient who is placed on a protocol, even those who barely need any insulin at all, or should we focus our interventions and analyses on those patients with more severe dysglycemia at baseline? This latter issue is fundamentally important, since the rollout of any systemwide glycemia protocol that results in higher catchment rates will appear more effective than it really is by enriching the postintervention data with healthier patients.
Before embarking on time‐intensive efforts to improve care, maybe we should be sure that the evidence supports our efforts.12 Recent recommendations from the American Diabetes Association state that for non‐critically ill patients: there is no clear evidence for specific blood glucose goals.13 (This recommendation, based on expert consensus or clinical experience, further states that because cohort data suggest that outcomes are better in hospitalized patients with fasting glucose <126 mg/dL and all random glucose values <180 to 200 mg/dL, these goals are reasonable if they can be safely achieved.) But given the challenges associated with implementing glycemia protocols, one might argue that hospitalists should invest their quality improvement efforts elsewhere.
So where does this leave us? What target glucose is not too high, not too low, but just right? Given the ever‐increasing number of quality improvement measures and interventions that are expected in the hospital, what amount of time, effort, and money devoted to improving inpatient glycemic control is just right? And what do our patients think? Should we be feeding our patients low glycemic load diets, or letting them indulge in one of the few creature comforts remaining in a semiprivate room?
What is clear from the results of the research published in this issue of JHM (regardless of whether we think that an inpatient pre‐meal glucose of 160 mg/dL is good, bad, or neither), is that we need to continue to develop systems, strategies, and teams to rapidly disseminate quality improvement interventions locally. We need multidisciplinary inputfrom physicians, nurses, dieticians, pharmacists, and patientsto do it right. So, even if the pendulum swings away from tight glycemic control in the hospital, the lessons we learned from these authors' valiant efforts to tame inpatient glycemia may provide us with the tools and knowledge required to successfully tackle other clinical issues such as delirium prevention, pain control, medication reconciliation, and handoffs. The striking obstacles (both in implementation and analysis) faced and overcome by the authors of the articles in this issue of JHM will hopefully embolden them to take on other quality improvement interventions that are perhaps more likely to help hospitalized patients.
- ,,,,.Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose‐binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,,.In search of fewer independent risk factors.Arch Intern Med.2005;165:138–145.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Benefits and risks of tight glucose control in critically ill adults: a meta‐analysis.JAMA.2008;300:933–944.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,,; for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- , ,, et al.Computer‐based insulin infusion protocol improves glycemia control over manual protocol.J Am Med Inform Assoc.2007;14:278–287.
- ,,,,.Estimated risk for undiagnosed diabetes in the emergency department: a multicenter survey.Acad Emerg Med.2007;14:492–495.
- ,,,,,.Evaluation of glycemic control following discontinuation of an intensive insulin protocol.J Hosp Med.2009;28–34.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357:608–613.
- American Diabetes Association. Standards of medical care in diabetes—2008.Diabetes Care.2008;31(Suppl 1):S12–S54.
- ,,,,.Intensive insulin therapy exerts antiinflammatory effects in critically ill patients and counteracts the adverse effect of low mannose‐binding lectin levels.J Clin Endocrinol Metab.2003;88:1082–1088.
- ,,,,.Insulin infusion in acute illness.J Clin Invest.2005;115:2069–2072.
- ,,,.In search of fewer independent risk factors.Arch Intern Med.2005;165:138–145.
- ,,, et al.Intensive insulin therapy in the critically ill patients.N Engl J Med.2001;345:1359–1367.
- ,,, et al.Intensive insulin therapy in the medical ICU.N Engl J Med.2006;354:449–461.
- ,,.Benefits and risks of tight glucose control in critically ill adults: a meta‐analysis.JAMA.2008;300:933–944.
- ,,,,.Inpatient management of diabetes and hyperglycemia among general medicine patients at a large teaching hospital.J Hosp Med.2006;1:145–150.
- ,,,; for the SHM Glycemic Control Task Force.Subcutaneous insulin order sets and protocols: effective design and implementation strategies.J Hosp Med.2008;3(S5):29–41.
- , ,, et al.Computer‐based insulin infusion protocol improves glycemia control over manual protocol.J Am Med Inform Assoc.2007;14:278–287.
- ,,,,.Estimated risk for undiagnosed diabetes in the emergency department: a multicenter survey.Acad Emerg Med.2007;14:492–495.
- ,,,,,.Evaluation of glycemic control following discontinuation of an intensive insulin protocol.J Hosp Med.2009;28–34.
- ,,.The tension between needing to improve care and knowing how to do it.N Engl J Med.2007;357:608–613.
- American Diabetes Association. Standards of medical care in diabetes—2008.Diabetes Care.2008;31(Suppl 1):S12–S54.
Engaging Patients at Hospital Discharge
I first met G.M. (a pseudonym) a year ago during a hospitalization for a flare of his Crohn's disease. At the age of 26, he had accrued nearly 400 hospital days in more than 10 institutionsranging from academic to community medical centers from the East Coast to the West Coast. He had been admitted and discharged more than 25 times and endured several surgeries, intermittent struggles with chronic pain and depression, and mishaps due to poor discharge planning. He referred to discharge as the most chaotic time of hospitalization, a comment that prompted a memorable discussion.
He began by describing the emotions he felt when first told about being discharged, using words such as fear and helplessness. He repeatedly talked about the lack of planning and anticipation of discharge as well as the frustration of watching a system that required fixing. Speaking with tremendous emotion and insight, he also pointed out the discharge experiences that maintained his trust and faith in the system. The conversation then shifted to his mother, who pointed out that her experiences as the caretaker were quite different than her son's. She was equally passionate and genuine in trying to characterize the hospital discharge process.
The conversation was so moving that I asked G.M. and his mother to jot down their thoughts on discharge as well as participate in a multidisciplinary patient safety conference. The following are excerpts from our conversation, their letters, and the conference.
THE PATIENT'S VIEW
You never go into the hospital wanting to stay there, but you also worry tremendously about adjusting back to home life. In my case, I was often on heavy pain medications with a PCA (patient‐controlled analgesia), so the transition to orals always created a source of stress, particularly when the transition happened right at discharge. I've had a number of experiences when they told me I was going home, stopped the PCA, and then simply sent me on my way. Nothing is worse than being discharged from the hospital, spending the car ride home doubled over in pain, and then not being able to get pain meds from the pharmacy until the next day. On the other hand, I've had discharges that were better anticipated, so I could participate in the process. This made all the difference in the world. I don't think people realize that when you're on a PCA right up to discharge, you're not really in a state to receive counseling, education, or instructions about follow‐up plansI was just trying to get better.
Many times, I knew I was getting close to discharge, but I often didn't see anyone owning the process. Information would be fragmented or inconsistent, and while I may have been ready for discharge, I wasn't prepared for discharge. This was a combination of paperwork being incomplete or being left to arrange my own follow‐up appointments after getting home. When you're sick and depressed, you fall through the cracks of the system. You just don't have the resolve to make things happen.
Ultimately, a well orchestrated discharge prepared me to be independent on some level. I felt comfortable and ready for life outside the hospital. I didn't feel helpless because I was only responsible for getting wellnot for arranging my follow‐up appointments, ensuring the home care nurse was coming by, and confirming that my primary doctor knew what was going on. In these situations, there was always a discharge planner serving as a patient advocate of sorts. I also can't imagine what I would have done if I didn't have my mom with me all the time. She's my mom, my advocate, and my caretaker and I don't know how patients survive without someone like that.
HIS MOTHER'S VIEW
I wasn't the sick and helpless one but rather the one who was expected to make it all happen: keep tabs on the medications, understand the details of the discharge plan, and ultimately manage the execution of care postdischarge. In the majority of cases when we had a bad discharge experience, it was because the goals were confused. It became about the bed that was needed for the person still sitting in the emergency department. They may not have realized it, but we fully understood the tension, and we very much felt it during the spotty discharge communications. Safety for the patient being discharged seemed to fall off the radar.
The goals of the process must be clear. In good discharges, caregivers clearly outline the transition plan, transfer records to the outpatient physicians, and arrange referrals to specialists as needed. Perhaps equally important is addressing the patient's emotional state for discharge. This isn't about convincing us that he's safe to go home, but a simple acknowledgement of the difficult transitionparticularly after a long hospitalizationgoes a far way in providing reassurance and decreasing fear and anxiety. If the issue is always one about beds and cost, I would think someone would figure out that a good discharge prevents readmissions, which would have to be a cost‐effective investment.
DISCUSSION
The voice of the patient (or family member) is incredibly powerful. Rather than having a trainee present a case history to illustrate teaching points, it is sometimes more meaningful and instructive to let patients tell their own stories. We invited G.M. and his mother to discuss their discharge experiences at a multidisciplinary patient safety conference. There, representative members of the discharge team (eg, house staff, attending, bedside nurse, pharmacist, and discharge planner) responded to their comments and discussed their roles in the discharge process. Ultimately, the patient and his mother taught us the most about what we can do to improve a process fraught with complexity and the potential for errors: communicate and work better as a team.
G.M. and his mother listened to each of the experts discuss the tasks they must complete to ensure a smooth discharge. Each provider expressed how committed they were to safe discharges, yet all of them shared how easy it is for one to go awry. They knew their individual roles, but all relied on each other to make the process completehighlighting that communication failures frequently lead to poor discharge experiences for patients. Engaging patients in the process should not transfer ownership of discharge to them (ie, making them responsible to ensure we do our jobs), though our patient and his mother presented several examples of how they owned the process because it was clear no one else did.
Evaluating our hospital discharge systems must include identifying methods to improve communication with outpatient providers, ensuring medications are available to patients on discharge, and providing written instructions (including follow‐up appointments) to patients before they leave the hospital. G.M. and his mother remind us that the best systems still need to engage patients, make them an active part of the discharge process (rather than an outcome of it), and never underestimate what patients suffer through emotionally prior to discharge.
Providers often feel uneasy when having to explain to patients that they no longer require hospitalization and perhaps avoid emotional engagement in those discussions because of the fear that some patients may become upset about a planned discharge. Communicating with patients about discharge plans should be handled with the same compassion, patience, and skill as delivering bad news. Patients entrust their lives to our clinical decision making, and abandoning this trust just as they leave the hospital is an unintended message that our patient and his mother perceived during their poor discharge experiences.
In my practice, I frequently include trainees in bedside discussions with patients and families, both to illustrate how important these conversations are and to model skills I was taught during my training. I now use discussions about discharge as a specific bedside teaching moment as well, hoping to impress on trainees the overriding message shared by G.M. and his mother: do not forget to engage patients in a process that is designed for them rather than to them.
Many physicians remain dedicated to improving hospital systems, but perhaps we should all be including our patients more in quality improvement activities and hospital committee work, as they provide perspectives not easily captured by administrative data and run charts.
Acknowledgements
The author thanks G.M. and his mother for candidly sharing their thoughts and feelings about the discharge process. He also thanks Erin Hartman, MS, for her invaluable editorial assistance in preparing this manuscript. The patient safety conference described was part of the Triad for Optimal Patient Safety (TOPS), a project funded by the Gorden & Betty Moore Foundation.
I first met G.M. (a pseudonym) a year ago during a hospitalization for a flare of his Crohn's disease. At the age of 26, he had accrued nearly 400 hospital days in more than 10 institutionsranging from academic to community medical centers from the East Coast to the West Coast. He had been admitted and discharged more than 25 times and endured several surgeries, intermittent struggles with chronic pain and depression, and mishaps due to poor discharge planning. He referred to discharge as the most chaotic time of hospitalization, a comment that prompted a memorable discussion.
He began by describing the emotions he felt when first told about being discharged, using words such as fear and helplessness. He repeatedly talked about the lack of planning and anticipation of discharge as well as the frustration of watching a system that required fixing. Speaking with tremendous emotion and insight, he also pointed out the discharge experiences that maintained his trust and faith in the system. The conversation then shifted to his mother, who pointed out that her experiences as the caretaker were quite different than her son's. She was equally passionate and genuine in trying to characterize the hospital discharge process.
The conversation was so moving that I asked G.M. and his mother to jot down their thoughts on discharge as well as participate in a multidisciplinary patient safety conference. The following are excerpts from our conversation, their letters, and the conference.
THE PATIENT'S VIEW
You never go into the hospital wanting to stay there, but you also worry tremendously about adjusting back to home life. In my case, I was often on heavy pain medications with a PCA (patient‐controlled analgesia), so the transition to orals always created a source of stress, particularly when the transition happened right at discharge. I've had a number of experiences when they told me I was going home, stopped the PCA, and then simply sent me on my way. Nothing is worse than being discharged from the hospital, spending the car ride home doubled over in pain, and then not being able to get pain meds from the pharmacy until the next day. On the other hand, I've had discharges that were better anticipated, so I could participate in the process. This made all the difference in the world. I don't think people realize that when you're on a PCA right up to discharge, you're not really in a state to receive counseling, education, or instructions about follow‐up plansI was just trying to get better.
Many times, I knew I was getting close to discharge, but I often didn't see anyone owning the process. Information would be fragmented or inconsistent, and while I may have been ready for discharge, I wasn't prepared for discharge. This was a combination of paperwork being incomplete or being left to arrange my own follow‐up appointments after getting home. When you're sick and depressed, you fall through the cracks of the system. You just don't have the resolve to make things happen.
Ultimately, a well orchestrated discharge prepared me to be independent on some level. I felt comfortable and ready for life outside the hospital. I didn't feel helpless because I was only responsible for getting wellnot for arranging my follow‐up appointments, ensuring the home care nurse was coming by, and confirming that my primary doctor knew what was going on. In these situations, there was always a discharge planner serving as a patient advocate of sorts. I also can't imagine what I would have done if I didn't have my mom with me all the time. She's my mom, my advocate, and my caretaker and I don't know how patients survive without someone like that.
HIS MOTHER'S VIEW
I wasn't the sick and helpless one but rather the one who was expected to make it all happen: keep tabs on the medications, understand the details of the discharge plan, and ultimately manage the execution of care postdischarge. In the majority of cases when we had a bad discharge experience, it was because the goals were confused. It became about the bed that was needed for the person still sitting in the emergency department. They may not have realized it, but we fully understood the tension, and we very much felt it during the spotty discharge communications. Safety for the patient being discharged seemed to fall off the radar.
The goals of the process must be clear. In good discharges, caregivers clearly outline the transition plan, transfer records to the outpatient physicians, and arrange referrals to specialists as needed. Perhaps equally important is addressing the patient's emotional state for discharge. This isn't about convincing us that he's safe to go home, but a simple acknowledgement of the difficult transitionparticularly after a long hospitalizationgoes a far way in providing reassurance and decreasing fear and anxiety. If the issue is always one about beds and cost, I would think someone would figure out that a good discharge prevents readmissions, which would have to be a cost‐effective investment.
DISCUSSION
The voice of the patient (or family member) is incredibly powerful. Rather than having a trainee present a case history to illustrate teaching points, it is sometimes more meaningful and instructive to let patients tell their own stories. We invited G.M. and his mother to discuss their discharge experiences at a multidisciplinary patient safety conference. There, representative members of the discharge team (eg, house staff, attending, bedside nurse, pharmacist, and discharge planner) responded to their comments and discussed their roles in the discharge process. Ultimately, the patient and his mother taught us the most about what we can do to improve a process fraught with complexity and the potential for errors: communicate and work better as a team.
G.M. and his mother listened to each of the experts discuss the tasks they must complete to ensure a smooth discharge. Each provider expressed how committed they were to safe discharges, yet all of them shared how easy it is for one to go awry. They knew their individual roles, but all relied on each other to make the process completehighlighting that communication failures frequently lead to poor discharge experiences for patients. Engaging patients in the process should not transfer ownership of discharge to them (ie, making them responsible to ensure we do our jobs), though our patient and his mother presented several examples of how they owned the process because it was clear no one else did.
Evaluating our hospital discharge systems must include identifying methods to improve communication with outpatient providers, ensuring medications are available to patients on discharge, and providing written instructions (including follow‐up appointments) to patients before they leave the hospital. G.M. and his mother remind us that the best systems still need to engage patients, make them an active part of the discharge process (rather than an outcome of it), and never underestimate what patients suffer through emotionally prior to discharge.
Providers often feel uneasy when having to explain to patients that they no longer require hospitalization and perhaps avoid emotional engagement in those discussions because of the fear that some patients may become upset about a planned discharge. Communicating with patients about discharge plans should be handled with the same compassion, patience, and skill as delivering bad news. Patients entrust their lives to our clinical decision making, and abandoning this trust just as they leave the hospital is an unintended message that our patient and his mother perceived during their poor discharge experiences.
In my practice, I frequently include trainees in bedside discussions with patients and families, both to illustrate how important these conversations are and to model skills I was taught during my training. I now use discussions about discharge as a specific bedside teaching moment as well, hoping to impress on trainees the overriding message shared by G.M. and his mother: do not forget to engage patients in a process that is designed for them rather than to them.
Many physicians remain dedicated to improving hospital systems, but perhaps we should all be including our patients more in quality improvement activities and hospital committee work, as they provide perspectives not easily captured by administrative data and run charts.
Acknowledgements
The author thanks G.M. and his mother for candidly sharing their thoughts and feelings about the discharge process. He also thanks Erin Hartman, MS, for her invaluable editorial assistance in preparing this manuscript. The patient safety conference described was part of the Triad for Optimal Patient Safety (TOPS), a project funded by the Gorden & Betty Moore Foundation.
I first met G.M. (a pseudonym) a year ago during a hospitalization for a flare of his Crohn's disease. At the age of 26, he had accrued nearly 400 hospital days in more than 10 institutionsranging from academic to community medical centers from the East Coast to the West Coast. He had been admitted and discharged more than 25 times and endured several surgeries, intermittent struggles with chronic pain and depression, and mishaps due to poor discharge planning. He referred to discharge as the most chaotic time of hospitalization, a comment that prompted a memorable discussion.
He began by describing the emotions he felt when first told about being discharged, using words such as fear and helplessness. He repeatedly talked about the lack of planning and anticipation of discharge as well as the frustration of watching a system that required fixing. Speaking with tremendous emotion and insight, he also pointed out the discharge experiences that maintained his trust and faith in the system. The conversation then shifted to his mother, who pointed out that her experiences as the caretaker were quite different than her son's. She was equally passionate and genuine in trying to characterize the hospital discharge process.
The conversation was so moving that I asked G.M. and his mother to jot down their thoughts on discharge as well as participate in a multidisciplinary patient safety conference. The following are excerpts from our conversation, their letters, and the conference.
THE PATIENT'S VIEW
You never go into the hospital wanting to stay there, but you also worry tremendously about adjusting back to home life. In my case, I was often on heavy pain medications with a PCA (patient‐controlled analgesia), so the transition to orals always created a source of stress, particularly when the transition happened right at discharge. I've had a number of experiences when they told me I was going home, stopped the PCA, and then simply sent me on my way. Nothing is worse than being discharged from the hospital, spending the car ride home doubled over in pain, and then not being able to get pain meds from the pharmacy until the next day. On the other hand, I've had discharges that were better anticipated, so I could participate in the process. This made all the difference in the world. I don't think people realize that when you're on a PCA right up to discharge, you're not really in a state to receive counseling, education, or instructions about follow‐up plansI was just trying to get better.
Many times, I knew I was getting close to discharge, but I often didn't see anyone owning the process. Information would be fragmented or inconsistent, and while I may have been ready for discharge, I wasn't prepared for discharge. This was a combination of paperwork being incomplete or being left to arrange my own follow‐up appointments after getting home. When you're sick and depressed, you fall through the cracks of the system. You just don't have the resolve to make things happen.
Ultimately, a well orchestrated discharge prepared me to be independent on some level. I felt comfortable and ready for life outside the hospital. I didn't feel helpless because I was only responsible for getting wellnot for arranging my follow‐up appointments, ensuring the home care nurse was coming by, and confirming that my primary doctor knew what was going on. In these situations, there was always a discharge planner serving as a patient advocate of sorts. I also can't imagine what I would have done if I didn't have my mom with me all the time. She's my mom, my advocate, and my caretaker and I don't know how patients survive without someone like that.
HIS MOTHER'S VIEW
I wasn't the sick and helpless one but rather the one who was expected to make it all happen: keep tabs on the medications, understand the details of the discharge plan, and ultimately manage the execution of care postdischarge. In the majority of cases when we had a bad discharge experience, it was because the goals were confused. It became about the bed that was needed for the person still sitting in the emergency department. They may not have realized it, but we fully understood the tension, and we very much felt it during the spotty discharge communications. Safety for the patient being discharged seemed to fall off the radar.
The goals of the process must be clear. In good discharges, caregivers clearly outline the transition plan, transfer records to the outpatient physicians, and arrange referrals to specialists as needed. Perhaps equally important is addressing the patient's emotional state for discharge. This isn't about convincing us that he's safe to go home, but a simple acknowledgement of the difficult transitionparticularly after a long hospitalizationgoes a far way in providing reassurance and decreasing fear and anxiety. If the issue is always one about beds and cost, I would think someone would figure out that a good discharge prevents readmissions, which would have to be a cost‐effective investment.
DISCUSSION
The voice of the patient (or family member) is incredibly powerful. Rather than having a trainee present a case history to illustrate teaching points, it is sometimes more meaningful and instructive to let patients tell their own stories. We invited G.M. and his mother to discuss their discharge experiences at a multidisciplinary patient safety conference. There, representative members of the discharge team (eg, house staff, attending, bedside nurse, pharmacist, and discharge planner) responded to their comments and discussed their roles in the discharge process. Ultimately, the patient and his mother taught us the most about what we can do to improve a process fraught with complexity and the potential for errors: communicate and work better as a team.
G.M. and his mother listened to each of the experts discuss the tasks they must complete to ensure a smooth discharge. Each provider expressed how committed they were to safe discharges, yet all of them shared how easy it is for one to go awry. They knew their individual roles, but all relied on each other to make the process completehighlighting that communication failures frequently lead to poor discharge experiences for patients. Engaging patients in the process should not transfer ownership of discharge to them (ie, making them responsible to ensure we do our jobs), though our patient and his mother presented several examples of how they owned the process because it was clear no one else did.
Evaluating our hospital discharge systems must include identifying methods to improve communication with outpatient providers, ensuring medications are available to patients on discharge, and providing written instructions (including follow‐up appointments) to patients before they leave the hospital. G.M. and his mother remind us that the best systems still need to engage patients, make them an active part of the discharge process (rather than an outcome of it), and never underestimate what patients suffer through emotionally prior to discharge.
Providers often feel uneasy when having to explain to patients that they no longer require hospitalization and perhaps avoid emotional engagement in those discussions because of the fear that some patients may become upset about a planned discharge. Communicating with patients about discharge plans should be handled with the same compassion, patience, and skill as delivering bad news. Patients entrust their lives to our clinical decision making, and abandoning this trust just as they leave the hospital is an unintended message that our patient and his mother perceived during their poor discharge experiences.
In my practice, I frequently include trainees in bedside discussions with patients and families, both to illustrate how important these conversations are and to model skills I was taught during my training. I now use discussions about discharge as a specific bedside teaching moment as well, hoping to impress on trainees the overriding message shared by G.M. and his mother: do not forget to engage patients in a process that is designed for them rather than to them.
Many physicians remain dedicated to improving hospital systems, but perhaps we should all be including our patients more in quality improvement activities and hospital committee work, as they provide perspectives not easily captured by administrative data and run charts.
Acknowledgements
The author thanks G.M. and his mother for candidly sharing their thoughts and feelings about the discharge process. He also thanks Erin Hartman, MS, for her invaluable editorial assistance in preparing this manuscript. The patient safety conference described was part of the Triad for Optimal Patient Safety (TOPS), a project funded by the Gorden & Betty Moore Foundation.
Death is a Crafty Old Friend
I will never forget her first words to me: You mispronounced my name.
I remember stopping short, awkwardly looking down at the emergency room chart in my hands and wondering how I could have mispronounced Mrs. Wells. I'm sorry, how should I say your name? I asked her.
Kitty.
Ah. Miss Kitty? I tried. This is, after all, Virginia, and octogenarians here are renowned for their adherence to social niceties.
She shook her head, her frown showing just how hard she was working to be patient with me. Miss Kitty was a character on Gunsmoke, she told me. Well, actually, she was lecturing me. I have never had red hair, I don't own a saloon, and I sure as heck don't have Marshall Dillon to keep me company. Darn my luck.
She was my seventh admission that afternoon, with at least 2 more to follow, but I found a smile for her and started into my usual routine. So, what brings you to the hospital today, Kitty?
I was hoping to meet a cute young doctor, she told me. And I think I succeeded.
This was clearly not going to go quickly. We'll deal with your poor eyesight later. What about the shortness of breath that the nurse wrote on your chart? I did not even mention the audible wheezing or the pursed‐lip breathing I had heard from outside the curtain.
Oh, that's just a ploy, she lied. To get to the meeting the cute young doctor part.
In glancing at her fingers to look for clubbing, I noticed the golden band and the tiny diamond ring, both of which looked huge on her knobby fingers. Kitty, I gently scolded her, I don't have time to deal with jealous husbands.
She laughed as she absently twirled the rings around her finger. Carl won't mind, she told me. He's been dead 14 years now.
But you still wear his rings, I pointed out.
He brought this ring with him when he came back to me from France after the war, she told me, straightening the tiny diamond on her finger. It was all his private's salary could afford, so he gave me the ring and a magnifying glass, and he told me to never look at the ring without using the magnifying glass.
No, this was clearly not going to go quickly at all. But I was pretty sure I was not going to mind.
Over the next 4 days, Kitty and I got to know each other fairly well. Her chronic obstructive pulmonary disease exacerbation improved to the point that I could send her home, and she begrudgingly accepted my recommendation of oxygen at night.
Over the next 2 years, I admitted her 7 more times; to my frustration, this meant that she was seeing me more regularly than she was seeing her primary care physician. After her third admission, I found myself having to convince her that it was time to start using her oxygen continuously. When she came back for her fourth admission, I noticed the cane at her bedside. Are you having trouble walking now, Kitty? I naively asked her.
Of course not, she shot back quickly. This is just in case I see a man I want to get a closer look at. I just hook him around the ankle and pull him in.
That discharge was to a skilled nursing facility because she had no family and no one else to care for her. After her 3 months of skilled benefits, she transitioned to long‐term care, and the next time her emphysema acted up, she thanked me for the introduction to all those handsome gentlemen. On each day of that admission, she asked when I was going to let her out of the hospital. Each day, she made sure that I knew, meant missed opportunities for her to be able to figure out which of the male residents was meant to be Carl's successor.
Her sixth admission was for pneumonia, and we nearly lost her. I am still convinced that it was nothing more than her self‐described abundance of piss and vinegar that pulled her through.
When the emergency room called me less than a month later for her seventh admission, I knew that Kitty and I were getting awfully close to the end of our relationship. She was tired and weak and smaller even than she had been just a few weeks before.
I admitted her to the intensive care unit for the first time on that admission, and I made the mistake of telling her that it was because with her somnolence and with her wish that she not be intubated, I was flirting with the idea of bilevel positive airway pressure (BiPAP) ventilation. She managed to find enough wind to tell me that I should save all my flirting for her.
Three days of antibiotics, frequent bronchodilators, steroids, and as much BiPAP as she could stand did not net us much improvement. On hospital day 4, the intensive care unit nurse caught me before I knocked on Kitty's sliding glass door.
She wants to go home, Lucy told me.
We're working on it, I reminded her.
Lucy shook her head. No, she said. Home with a capital H. Heaven. Lucy relayed the conversation that Kitty had had with her. The one that my old friend had not had with me.
The one that I should have had with her.
I closed Kitty's door behind me and sat down at her side on the bed, noticing how she was drawing each breath as if she had to pay for it.
Lucy told me about your conversation, I said quietly, once Kitty had finally opened her eyes and found me. No more BiPAP, I understand. She nodded. I waited for her to say something more, but she did not. Why didn't you tell me, Kitty? I asked her gently.
She laughed; her laugh was a short, tired little thing that died in her throat. She reached from under the covers to pat my hand. I suppose, she said, that I didn't want to let you down. You always seem so proud of yourself when I come in here, gasping and coughing, and you get me well enough to go back home. She paused to blow off some CO2 and find a sad smile for me. Time to let me go, she told me. Let me be with Carl again.
We talked for quite a while. Well, actually, I did most of the talking. She did not have the wind for it. I admitted to her just how embarrassed I was that she had had to bring this up to me. I have for quite some time been rather comfortable with the notion that death is not the worst thing that can happen to a patient. It was Dr. Tom Smith, at the Medical College of Virginia, who first introduced what was then a wide‐eyed, idealist medical student to the concept of a good death: snatching a victory for compassion from the jaws of a medical defeat.
Therefore, at Kitty's insistence, there was no more BiPAP. When her breathing became labored, we gave her just enough morphine to take the edge off her air hunger. Rather than round on her in the mornings, I simply sat with her.
I would like to say that I was with Kitty when she died. It would bring the story full circle and give our relationship a clean beginning and a clean end. It would be good fiction.
However, it would also be a lie.
Lucy met me at her door again, just 3 days later, and told me about Kitty's quiet passing, in her sleep, just an hour before I had arrived for rounds.
I sat at Kitty's side one last time, holding her now cold hand. She was smiling.
At least, that is how I intend to remember that moment.
The bed sagged just a bit on the other side as I realized that Death had joined me. For those who have not had the pleasure, the part of Death is played by Gwyneth Paltrow.
We sat in silence for several minutes, Death showing me the patience that she had once told me she had.
I rarely see it.
How is she? I finally asked.
Better. She and Carl are catching up a bit. I nodded. He's giving her hell for losing that magnifying glass.
I could not help the smile. Carl was one lucky man.
She says thank you.
Whether it was true or not, it was nice for Death to say it.
She let me have a long stretch of silence before she felt the need to ruin our tender moment. You have any other business for me?
I growled a warning, and she smiled. That was a joke, Doctor. Lighten up a little, would you?
She turned to go, but I stopped her. Hey, I found a great quote for you. The last time that we had chatted, she had been lamenting the plethora of love lines and the paucity of good death ones:
Because I could not stop for Death,
He kindly stopped for me;
The carriage held but just ourselves
and immortality.
Emily Dickinson, I told her.
Huh, she said, frowning and doing that thing with her eyebrow for which Gwyneth gets paid millions. Wonder why she thought I was a he?
Acknowledgements
I thank the editors of the Journal of Hospital Medicine for considering this submission. This is a fictionalized account of a true patient experience. The patient in question has passed away, but while she was alive, she and I discussed many things, including my writing, and she actually asked that I write about her some day. This is the fulfillment of my promise to her.
I will never forget her first words to me: You mispronounced my name.
I remember stopping short, awkwardly looking down at the emergency room chart in my hands and wondering how I could have mispronounced Mrs. Wells. I'm sorry, how should I say your name? I asked her.
Kitty.
Ah. Miss Kitty? I tried. This is, after all, Virginia, and octogenarians here are renowned for their adherence to social niceties.
She shook her head, her frown showing just how hard she was working to be patient with me. Miss Kitty was a character on Gunsmoke, she told me. Well, actually, she was lecturing me. I have never had red hair, I don't own a saloon, and I sure as heck don't have Marshall Dillon to keep me company. Darn my luck.
She was my seventh admission that afternoon, with at least 2 more to follow, but I found a smile for her and started into my usual routine. So, what brings you to the hospital today, Kitty?
I was hoping to meet a cute young doctor, she told me. And I think I succeeded.
This was clearly not going to go quickly. We'll deal with your poor eyesight later. What about the shortness of breath that the nurse wrote on your chart? I did not even mention the audible wheezing or the pursed‐lip breathing I had heard from outside the curtain.
Oh, that's just a ploy, she lied. To get to the meeting the cute young doctor part.
In glancing at her fingers to look for clubbing, I noticed the golden band and the tiny diamond ring, both of which looked huge on her knobby fingers. Kitty, I gently scolded her, I don't have time to deal with jealous husbands.
She laughed as she absently twirled the rings around her finger. Carl won't mind, she told me. He's been dead 14 years now.
But you still wear his rings, I pointed out.
He brought this ring with him when he came back to me from France after the war, she told me, straightening the tiny diamond on her finger. It was all his private's salary could afford, so he gave me the ring and a magnifying glass, and he told me to never look at the ring without using the magnifying glass.
No, this was clearly not going to go quickly at all. But I was pretty sure I was not going to mind.
Over the next 4 days, Kitty and I got to know each other fairly well. Her chronic obstructive pulmonary disease exacerbation improved to the point that I could send her home, and she begrudgingly accepted my recommendation of oxygen at night.
Over the next 2 years, I admitted her 7 more times; to my frustration, this meant that she was seeing me more regularly than she was seeing her primary care physician. After her third admission, I found myself having to convince her that it was time to start using her oxygen continuously. When she came back for her fourth admission, I noticed the cane at her bedside. Are you having trouble walking now, Kitty? I naively asked her.
Of course not, she shot back quickly. This is just in case I see a man I want to get a closer look at. I just hook him around the ankle and pull him in.
That discharge was to a skilled nursing facility because she had no family and no one else to care for her. After her 3 months of skilled benefits, she transitioned to long‐term care, and the next time her emphysema acted up, she thanked me for the introduction to all those handsome gentlemen. On each day of that admission, she asked when I was going to let her out of the hospital. Each day, she made sure that I knew, meant missed opportunities for her to be able to figure out which of the male residents was meant to be Carl's successor.
Her sixth admission was for pneumonia, and we nearly lost her. I am still convinced that it was nothing more than her self‐described abundance of piss and vinegar that pulled her through.
When the emergency room called me less than a month later for her seventh admission, I knew that Kitty and I were getting awfully close to the end of our relationship. She was tired and weak and smaller even than she had been just a few weeks before.
I admitted her to the intensive care unit for the first time on that admission, and I made the mistake of telling her that it was because with her somnolence and with her wish that she not be intubated, I was flirting with the idea of bilevel positive airway pressure (BiPAP) ventilation. She managed to find enough wind to tell me that I should save all my flirting for her.
Three days of antibiotics, frequent bronchodilators, steroids, and as much BiPAP as she could stand did not net us much improvement. On hospital day 4, the intensive care unit nurse caught me before I knocked on Kitty's sliding glass door.
She wants to go home, Lucy told me.
We're working on it, I reminded her.
Lucy shook her head. No, she said. Home with a capital H. Heaven. Lucy relayed the conversation that Kitty had had with her. The one that my old friend had not had with me.
The one that I should have had with her.
I closed Kitty's door behind me and sat down at her side on the bed, noticing how she was drawing each breath as if she had to pay for it.
Lucy told me about your conversation, I said quietly, once Kitty had finally opened her eyes and found me. No more BiPAP, I understand. She nodded. I waited for her to say something more, but she did not. Why didn't you tell me, Kitty? I asked her gently.
She laughed; her laugh was a short, tired little thing that died in her throat. She reached from under the covers to pat my hand. I suppose, she said, that I didn't want to let you down. You always seem so proud of yourself when I come in here, gasping and coughing, and you get me well enough to go back home. She paused to blow off some CO2 and find a sad smile for me. Time to let me go, she told me. Let me be with Carl again.
We talked for quite a while. Well, actually, I did most of the talking. She did not have the wind for it. I admitted to her just how embarrassed I was that she had had to bring this up to me. I have for quite some time been rather comfortable with the notion that death is not the worst thing that can happen to a patient. It was Dr. Tom Smith, at the Medical College of Virginia, who first introduced what was then a wide‐eyed, idealist medical student to the concept of a good death: snatching a victory for compassion from the jaws of a medical defeat.
Therefore, at Kitty's insistence, there was no more BiPAP. When her breathing became labored, we gave her just enough morphine to take the edge off her air hunger. Rather than round on her in the mornings, I simply sat with her.
I would like to say that I was with Kitty when she died. It would bring the story full circle and give our relationship a clean beginning and a clean end. It would be good fiction.
However, it would also be a lie.
Lucy met me at her door again, just 3 days later, and told me about Kitty's quiet passing, in her sleep, just an hour before I had arrived for rounds.
I sat at Kitty's side one last time, holding her now cold hand. She was smiling.
At least, that is how I intend to remember that moment.
The bed sagged just a bit on the other side as I realized that Death had joined me. For those who have not had the pleasure, the part of Death is played by Gwyneth Paltrow.
We sat in silence for several minutes, Death showing me the patience that she had once told me she had.
I rarely see it.
How is she? I finally asked.
Better. She and Carl are catching up a bit. I nodded. He's giving her hell for losing that magnifying glass.
I could not help the smile. Carl was one lucky man.
She says thank you.
Whether it was true or not, it was nice for Death to say it.
She let me have a long stretch of silence before she felt the need to ruin our tender moment. You have any other business for me?
I growled a warning, and she smiled. That was a joke, Doctor. Lighten up a little, would you?
She turned to go, but I stopped her. Hey, I found a great quote for you. The last time that we had chatted, she had been lamenting the plethora of love lines and the paucity of good death ones:
Because I could not stop for Death,
He kindly stopped for me;
The carriage held but just ourselves
and immortality.
Emily Dickinson, I told her.
Huh, she said, frowning and doing that thing with her eyebrow for which Gwyneth gets paid millions. Wonder why she thought I was a he?
Acknowledgements
I thank the editors of the Journal of Hospital Medicine for considering this submission. This is a fictionalized account of a true patient experience. The patient in question has passed away, but while she was alive, she and I discussed many things, including my writing, and she actually asked that I write about her some day. This is the fulfillment of my promise to her.
I will never forget her first words to me: You mispronounced my name.
I remember stopping short, awkwardly looking down at the emergency room chart in my hands and wondering how I could have mispronounced Mrs. Wells. I'm sorry, how should I say your name? I asked her.
Kitty.
Ah. Miss Kitty? I tried. This is, after all, Virginia, and octogenarians here are renowned for their adherence to social niceties.
She shook her head, her frown showing just how hard she was working to be patient with me. Miss Kitty was a character on Gunsmoke, she told me. Well, actually, she was lecturing me. I have never had red hair, I don't own a saloon, and I sure as heck don't have Marshall Dillon to keep me company. Darn my luck.
She was my seventh admission that afternoon, with at least 2 more to follow, but I found a smile for her and started into my usual routine. So, what brings you to the hospital today, Kitty?
I was hoping to meet a cute young doctor, she told me. And I think I succeeded.
This was clearly not going to go quickly. We'll deal with your poor eyesight later. What about the shortness of breath that the nurse wrote on your chart? I did not even mention the audible wheezing or the pursed‐lip breathing I had heard from outside the curtain.
Oh, that's just a ploy, she lied. To get to the meeting the cute young doctor part.
In glancing at her fingers to look for clubbing, I noticed the golden band and the tiny diamond ring, both of which looked huge on her knobby fingers. Kitty, I gently scolded her, I don't have time to deal with jealous husbands.
She laughed as she absently twirled the rings around her finger. Carl won't mind, she told me. He's been dead 14 years now.
But you still wear his rings, I pointed out.
He brought this ring with him when he came back to me from France after the war, she told me, straightening the tiny diamond on her finger. It was all his private's salary could afford, so he gave me the ring and a magnifying glass, and he told me to never look at the ring without using the magnifying glass.
No, this was clearly not going to go quickly at all. But I was pretty sure I was not going to mind.
Over the next 4 days, Kitty and I got to know each other fairly well. Her chronic obstructive pulmonary disease exacerbation improved to the point that I could send her home, and she begrudgingly accepted my recommendation of oxygen at night.
Over the next 2 years, I admitted her 7 more times; to my frustration, this meant that she was seeing me more regularly than she was seeing her primary care physician. After her third admission, I found myself having to convince her that it was time to start using her oxygen continuously. When she came back for her fourth admission, I noticed the cane at her bedside. Are you having trouble walking now, Kitty? I naively asked her.
Of course not, she shot back quickly. This is just in case I see a man I want to get a closer look at. I just hook him around the ankle and pull him in.
That discharge was to a skilled nursing facility because she had no family and no one else to care for her. After her 3 months of skilled benefits, she transitioned to long‐term care, and the next time her emphysema acted up, she thanked me for the introduction to all those handsome gentlemen. On each day of that admission, she asked when I was going to let her out of the hospital. Each day, she made sure that I knew, meant missed opportunities for her to be able to figure out which of the male residents was meant to be Carl's successor.
Her sixth admission was for pneumonia, and we nearly lost her. I am still convinced that it was nothing more than her self‐described abundance of piss and vinegar that pulled her through.
When the emergency room called me less than a month later for her seventh admission, I knew that Kitty and I were getting awfully close to the end of our relationship. She was tired and weak and smaller even than she had been just a few weeks before.
I admitted her to the intensive care unit for the first time on that admission, and I made the mistake of telling her that it was because with her somnolence and with her wish that she not be intubated, I was flirting with the idea of bilevel positive airway pressure (BiPAP) ventilation. She managed to find enough wind to tell me that I should save all my flirting for her.
Three days of antibiotics, frequent bronchodilators, steroids, and as much BiPAP as she could stand did not net us much improvement. On hospital day 4, the intensive care unit nurse caught me before I knocked on Kitty's sliding glass door.
She wants to go home, Lucy told me.
We're working on it, I reminded her.
Lucy shook her head. No, she said. Home with a capital H. Heaven. Lucy relayed the conversation that Kitty had had with her. The one that my old friend had not had with me.
The one that I should have had with her.
I closed Kitty's door behind me and sat down at her side on the bed, noticing how she was drawing each breath as if she had to pay for it.
Lucy told me about your conversation, I said quietly, once Kitty had finally opened her eyes and found me. No more BiPAP, I understand. She nodded. I waited for her to say something more, but she did not. Why didn't you tell me, Kitty? I asked her gently.
She laughed; her laugh was a short, tired little thing that died in her throat. She reached from under the covers to pat my hand. I suppose, she said, that I didn't want to let you down. You always seem so proud of yourself when I come in here, gasping and coughing, and you get me well enough to go back home. She paused to blow off some CO2 and find a sad smile for me. Time to let me go, she told me. Let me be with Carl again.
We talked for quite a while. Well, actually, I did most of the talking. She did not have the wind for it. I admitted to her just how embarrassed I was that she had had to bring this up to me. I have for quite some time been rather comfortable with the notion that death is not the worst thing that can happen to a patient. It was Dr. Tom Smith, at the Medical College of Virginia, who first introduced what was then a wide‐eyed, idealist medical student to the concept of a good death: snatching a victory for compassion from the jaws of a medical defeat.
Therefore, at Kitty's insistence, there was no more BiPAP. When her breathing became labored, we gave her just enough morphine to take the edge off her air hunger. Rather than round on her in the mornings, I simply sat with her.
I would like to say that I was with Kitty when she died. It would bring the story full circle and give our relationship a clean beginning and a clean end. It would be good fiction.
However, it would also be a lie.
Lucy met me at her door again, just 3 days later, and told me about Kitty's quiet passing, in her sleep, just an hour before I had arrived for rounds.
I sat at Kitty's side one last time, holding her now cold hand. She was smiling.
At least, that is how I intend to remember that moment.
The bed sagged just a bit on the other side as I realized that Death had joined me. For those who have not had the pleasure, the part of Death is played by Gwyneth Paltrow.
We sat in silence for several minutes, Death showing me the patience that she had once told me she had.
I rarely see it.
How is she? I finally asked.
Better. She and Carl are catching up a bit. I nodded. He's giving her hell for losing that magnifying glass.
I could not help the smile. Carl was one lucky man.
She says thank you.
Whether it was true or not, it was nice for Death to say it.
She let me have a long stretch of silence before she felt the need to ruin our tender moment. You have any other business for me?
I growled a warning, and she smiled. That was a joke, Doctor. Lighten up a little, would you?
She turned to go, but I stopped her. Hey, I found a great quote for you. The last time that we had chatted, she had been lamenting the plethora of love lines and the paucity of good death ones:
Because I could not stop for Death,
He kindly stopped for me;
The carriage held but just ourselves
and immortality.
Emily Dickinson, I told her.
Huh, she said, frowning and doing that thing with her eyebrow for which Gwyneth gets paid millions. Wonder why she thought I was a he?
Acknowledgements
I thank the editors of the Journal of Hospital Medicine for considering this submission. This is a fictionalized account of a true patient experience. The patient in question has passed away, but while she was alive, she and I discussed many things, including my writing, and she actually asked that I write about her some day. This is the fulfillment of my promise to her.
Evolution and results of aortic valve surgery, and a ‘disruptive’ technology
Disruptive technologies are innovations that are quickly adopted and that change long-established practices. One example is coronary stenting; another that is emerging is percutaneous aortic valve insertion. The latter is already benefiting patients who would not be able to undergo open heart surgery for valve replacement. However, the technology is still so new that we do not yet know how to define who will benefit from it.
VALVE SURGERY CONTINUES TO IMPROVE
Yet despite these excellent results, 30% to 61% of patients with severe symptomatic aortic valve stenosis do not undergo surgical aortic valve replacement because age and comorbid diseases put them at unacceptably high risk, or because they do not want it, or because they were never referred for it.
This concern about high risk is certainly justified, since age and comorbid conditions such as coronary artery disease, oxygen-dependent chronic pulmonary disease, renal disease, and peripheral vascular disease clearly have an adverse affect on outcome. For example, the risk of stroke and death is markedly higher in patients with peripheral vascular disease.1 It was because of the strong influence of comorbid disease in the elderly that we and others4,6 developed the novel approach of replacing the aortic valve with a stented valve via a catheter.
SURGICAL RISK IS HARD TO PREDICT
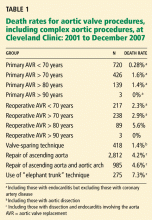
Decisions about which patients are at very high surgical risk or cannot undergo surgery are often somewhat subjective, based on a surgeon’s own experience.4,6 An algorithm for predicting operative outcomes, the Society for Thoracic Surgery equation score, is a reliable way to calculate the risk of death in patients in need of aortic valve replacement. Another method, the EuroScore, has been shown to be less predictive: in an audit of data for the multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial currently under way to analyze results with these procedures, in 4,892 patients undergoing open surgical repair at Cleveland Clinic and considered at high risk (EuroScore > 10), the calculated expected risk of death was 26%, but the observed death rate was 10.9%—only 42% of the expected rate.
In my personal audit of the last 594 patients who underwent open surgical aortic valve replacement and were considered to be at high risk, the expected risk of death (as calculated by the EuroScore) was 27%, but the observed risk was considerably lower at 7%—only 26% of the predicted rate.
ENTER THE PERCUTANEOUS DEVICES
In this issue of the Journal, Dr. Singh and colleagues review the options for percutaneous aortic valve insertion in high-risk patients, and their potential outcomes.7 But as the authors note, much study still needs to be done regarding this technique.
In an initial feasibility study of 55 high-risk or inoperable patients undergoing transfemoral aortic valve insertion under a protocol approved by the US Food and Drug Administration (FDA), the mortality rate was 7.2% and the stroke rate was 9.2%. For the FDA-approved study of 40 patients underoing transapical valve placement, the mortality rate was 17%, but no immediate strokes occurred in successful procedures, even though most of these patients were not eligible for transfemoral aortic valve insertion because of peripheral vascular disease.6 Clearly, based on our data,1 the presence of peripheral vascular disease added to the risk of death.1,6
Even if the issues surrounding percutaneous valve insertion remain unresolved for early versions of the devices, one important benefit is that more people who would benefit from treatment are being referred for evaluation. At Cleveland Clinic, we have already noticed that sick patients who would not previously have been referred for surgery are now being referred because of the new technology, although only about 20% of these are eventually enrolled in the PARTNER study. A further 20% undergo conventional open surgery, 20% undergo balloon valvuloplasty, and the remainder are too sick, die during evaluation, or refuse intervention.6 Indeed, none of the patients who underwent high-risk open surgery died.6
Although this new, “disruptive” technology was introduced for patients for whom surgery would pose an unacceptably high risk, it is inevitable that, with further improvements in prosthetic valves and the ways to insert them, percutaneous valve insertion will make inroads in the treatment of aortic valve stenosis.
While most disruptive technologies are cheaper than the technologies they displace, this may not be the case with percutaneous valve insertion: a standard aortic heart valve costs $2,500 to $6,000, whereas percutaneously delivered valves cost $30,000. The hospital stay may turn out to be a little shorter, which may help control the overall cost. But while the hospital stay after percutaneous insertion may be shorter than for surgical valve replacement (3–5 days vs 5–7 days), percutaneous valve insertion is currently labor-intensive and requires a team of 25 to 30 people, compared with five or six for open repair.
Percutaneous valve insertion offers selected high-risk patients one of the most beneficial treatments in cardiovascular medicine that they potentially would never have benefited from—ie, improved quality of life, and more years of life. It has great potential, but the problems of procedural safety and of access to treatment still need to be overcome.
- Svensson LG. Aortic valve stenosis and regurgitation: an overview of management. J Cardiovasc Surg 2008; 49:297–303.
- Svensson LG, Deglurkar I, Ung J, et al. Aortic valve repair and root preservation by remodeling, reimplantation, and tailoring: technical aspects and early outcome. J Card Surg 2007; 22:473–479.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008; 135:180–187.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of transcatheter insertion of a stented aortic valve via left ventricular apex. Ann Thoracic Surg 2008; 86:46–54.
- Singh IM, Shishehbor MH, Christofferson RD, Tuzcu EM, Kapadia SR. Percutaneous treatment of aortic valve stenosis. Cleve Clin J Med 2008; 75:805–812.
Disruptive technologies are innovations that are quickly adopted and that change long-established practices. One example is coronary stenting; another that is emerging is percutaneous aortic valve insertion. The latter is already benefiting patients who would not be able to undergo open heart surgery for valve replacement. However, the technology is still so new that we do not yet know how to define who will benefit from it.
VALVE SURGERY CONTINUES TO IMPROVE
Yet despite these excellent results, 30% to 61% of patients with severe symptomatic aortic valve stenosis do not undergo surgical aortic valve replacement because age and comorbid diseases put them at unacceptably high risk, or because they do not want it, or because they were never referred for it.
This concern about high risk is certainly justified, since age and comorbid conditions such as coronary artery disease, oxygen-dependent chronic pulmonary disease, renal disease, and peripheral vascular disease clearly have an adverse affect on outcome. For example, the risk of stroke and death is markedly higher in patients with peripheral vascular disease.1 It was because of the strong influence of comorbid disease in the elderly that we and others4,6 developed the novel approach of replacing the aortic valve with a stented valve via a catheter.
SURGICAL RISK IS HARD TO PREDICT
Decisions about which patients are at very high surgical risk or cannot undergo surgery are often somewhat subjective, based on a surgeon’s own experience.4,6 An algorithm for predicting operative outcomes, the Society for Thoracic Surgery equation score, is a reliable way to calculate the risk of death in patients in need of aortic valve replacement. Another method, the EuroScore, has been shown to be less predictive: in an audit of data for the multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial currently under way to analyze results with these procedures, in 4,892 patients undergoing open surgical repair at Cleveland Clinic and considered at high risk (EuroScore > 10), the calculated expected risk of death was 26%, but the observed death rate was 10.9%—only 42% of the expected rate.
In my personal audit of the last 594 patients who underwent open surgical aortic valve replacement and were considered to be at high risk, the expected risk of death (as calculated by the EuroScore) was 27%, but the observed risk was considerably lower at 7%—only 26% of the predicted rate.
ENTER THE PERCUTANEOUS DEVICES
In this issue of the Journal, Dr. Singh and colleagues review the options for percutaneous aortic valve insertion in high-risk patients, and their potential outcomes.7 But as the authors note, much study still needs to be done regarding this technique.
In an initial feasibility study of 55 high-risk or inoperable patients undergoing transfemoral aortic valve insertion under a protocol approved by the US Food and Drug Administration (FDA), the mortality rate was 7.2% and the stroke rate was 9.2%. For the FDA-approved study of 40 patients underoing transapical valve placement, the mortality rate was 17%, but no immediate strokes occurred in successful procedures, even though most of these patients were not eligible for transfemoral aortic valve insertion because of peripheral vascular disease.6 Clearly, based on our data,1 the presence of peripheral vascular disease added to the risk of death.1,6
Even if the issues surrounding percutaneous valve insertion remain unresolved for early versions of the devices, one important benefit is that more people who would benefit from treatment are being referred for evaluation. At Cleveland Clinic, we have already noticed that sick patients who would not previously have been referred for surgery are now being referred because of the new technology, although only about 20% of these are eventually enrolled in the PARTNER study. A further 20% undergo conventional open surgery, 20% undergo balloon valvuloplasty, and the remainder are too sick, die during evaluation, or refuse intervention.6 Indeed, none of the patients who underwent high-risk open surgery died.6
Although this new, “disruptive” technology was introduced for patients for whom surgery would pose an unacceptably high risk, it is inevitable that, with further improvements in prosthetic valves and the ways to insert them, percutaneous valve insertion will make inroads in the treatment of aortic valve stenosis.
While most disruptive technologies are cheaper than the technologies they displace, this may not be the case with percutaneous valve insertion: a standard aortic heart valve costs $2,500 to $6,000, whereas percutaneously delivered valves cost $30,000. The hospital stay may turn out to be a little shorter, which may help control the overall cost. But while the hospital stay after percutaneous insertion may be shorter than for surgical valve replacement (3–5 days vs 5–7 days), percutaneous valve insertion is currently labor-intensive and requires a team of 25 to 30 people, compared with five or six for open repair.
Percutaneous valve insertion offers selected high-risk patients one of the most beneficial treatments in cardiovascular medicine that they potentially would never have benefited from—ie, improved quality of life, and more years of life. It has great potential, but the problems of procedural safety and of access to treatment still need to be overcome.
Disruptive technologies are innovations that are quickly adopted and that change long-established practices. One example is coronary stenting; another that is emerging is percutaneous aortic valve insertion. The latter is already benefiting patients who would not be able to undergo open heart surgery for valve replacement. However, the technology is still so new that we do not yet know how to define who will benefit from it.
VALVE SURGERY CONTINUES TO IMPROVE
Yet despite these excellent results, 30% to 61% of patients with severe symptomatic aortic valve stenosis do not undergo surgical aortic valve replacement because age and comorbid diseases put them at unacceptably high risk, or because they do not want it, or because they were never referred for it.
This concern about high risk is certainly justified, since age and comorbid conditions such as coronary artery disease, oxygen-dependent chronic pulmonary disease, renal disease, and peripheral vascular disease clearly have an adverse affect on outcome. For example, the risk of stroke and death is markedly higher in patients with peripheral vascular disease.1 It was because of the strong influence of comorbid disease in the elderly that we and others4,6 developed the novel approach of replacing the aortic valve with a stented valve via a catheter.
SURGICAL RISK IS HARD TO PREDICT
Decisions about which patients are at very high surgical risk or cannot undergo surgery are often somewhat subjective, based on a surgeon’s own experience.4,6 An algorithm for predicting operative outcomes, the Society for Thoracic Surgery equation score, is a reliable way to calculate the risk of death in patients in need of aortic valve replacement. Another method, the EuroScore, has been shown to be less predictive: in an audit of data for the multicenter Placement of Aortic Transcatheter Valves (PARTNER) trial currently under way to analyze results with these procedures, in 4,892 patients undergoing open surgical repair at Cleveland Clinic and considered at high risk (EuroScore > 10), the calculated expected risk of death was 26%, but the observed death rate was 10.9%—only 42% of the expected rate.
In my personal audit of the last 594 patients who underwent open surgical aortic valve replacement and were considered to be at high risk, the expected risk of death (as calculated by the EuroScore) was 27%, but the observed risk was considerably lower at 7%—only 26% of the predicted rate.
ENTER THE PERCUTANEOUS DEVICES
In this issue of the Journal, Dr. Singh and colleagues review the options for percutaneous aortic valve insertion in high-risk patients, and their potential outcomes.7 But as the authors note, much study still needs to be done regarding this technique.
In an initial feasibility study of 55 high-risk or inoperable patients undergoing transfemoral aortic valve insertion under a protocol approved by the US Food and Drug Administration (FDA), the mortality rate was 7.2% and the stroke rate was 9.2%. For the FDA-approved study of 40 patients underoing transapical valve placement, the mortality rate was 17%, but no immediate strokes occurred in successful procedures, even though most of these patients were not eligible for transfemoral aortic valve insertion because of peripheral vascular disease.6 Clearly, based on our data,1 the presence of peripheral vascular disease added to the risk of death.1,6
Even if the issues surrounding percutaneous valve insertion remain unresolved for early versions of the devices, one important benefit is that more people who would benefit from treatment are being referred for evaluation. At Cleveland Clinic, we have already noticed that sick patients who would not previously have been referred for surgery are now being referred because of the new technology, although only about 20% of these are eventually enrolled in the PARTNER study. A further 20% undergo conventional open surgery, 20% undergo balloon valvuloplasty, and the remainder are too sick, die during evaluation, or refuse intervention.6 Indeed, none of the patients who underwent high-risk open surgery died.6
Although this new, “disruptive” technology was introduced for patients for whom surgery would pose an unacceptably high risk, it is inevitable that, with further improvements in prosthetic valves and the ways to insert them, percutaneous valve insertion will make inroads in the treatment of aortic valve stenosis.
While most disruptive technologies are cheaper than the technologies they displace, this may not be the case with percutaneous valve insertion: a standard aortic heart valve costs $2,500 to $6,000, whereas percutaneously delivered valves cost $30,000. The hospital stay may turn out to be a little shorter, which may help control the overall cost. But while the hospital stay after percutaneous insertion may be shorter than for surgical valve replacement (3–5 days vs 5–7 days), percutaneous valve insertion is currently labor-intensive and requires a team of 25 to 30 people, compared with five or six for open repair.
Percutaneous valve insertion offers selected high-risk patients one of the most beneficial treatments in cardiovascular medicine that they potentially would never have benefited from—ie, improved quality of life, and more years of life. It has great potential, but the problems of procedural safety and of access to treatment still need to be overcome.
- Svensson LG. Aortic valve stenosis and regurgitation: an overview of management. J Cardiovasc Surg 2008; 49:297–303.
- Svensson LG, Deglurkar I, Ung J, et al. Aortic valve repair and root preservation by remodeling, reimplantation, and tailoring: technical aspects and early outcome. J Card Surg 2007; 22:473–479.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008; 135:180–187.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of transcatheter insertion of a stented aortic valve via left ventricular apex. Ann Thoracic Surg 2008; 86:46–54.
- Singh IM, Shishehbor MH, Christofferson RD, Tuzcu EM, Kapadia SR. Percutaneous treatment of aortic valve stenosis. Cleve Clin J Med 2008; 75:805–812.
- Svensson LG. Aortic valve stenosis and regurgitation: an overview of management. J Cardiovasc Surg 2008; 49:297–303.
- Svensson LG, Deglurkar I, Ung J, et al. Aortic valve repair and root preservation by remodeling, reimplantation, and tailoring: technical aspects and early outcome. J Card Surg 2007; 22:473–479.
- Svensson LG, Blackstone EH, Cosgrove DM. Surgical options in young adults with aortic valve disease. Curr Probl Cardiol 2003; 28:417–480.
- Dewey TM, Brown D, Ryan WH, Herbert MA, Prince SL, Mack MJ. Reliability of risk algorithms in predicting early and late operative outcomes in high-risk patients undergoing aortic valve replacement. J Thorac Cardiovasc Surg 2008; 135:180–187.
- Varadarajan P, Kapoor N, Bansal RC, Pai RG. Clinical profile and natural history of 453 nonsurgically managed patients with severe aortic stenosis. Ann Thorac Surg 2006; 82:2111–2115.
- Svensson LG, Dewey T, Kapadia S, et al. United States feasibility study of transcatheter insertion of a stented aortic valve via left ventricular apex. Ann Thoracic Surg 2008; 86:46–54.
- Singh IM, Shishehbor MH, Christofferson RD, Tuzcu EM, Kapadia SR. Percutaneous treatment of aortic valve stenosis. Cleve Clin J Med 2008; 75:805–812.
Debates in Hospital Medicine
At a hospital at which I work, every patient who presents to the emergency department with a suspected stroke or transient ischemic attack is evaluated by the stroke team. Per protocol, the team rapidly assesses each patient, orders diagnostic and therapeutic interventions and then refers each and every patient to the hospitalist service for admission and medical comanagement. At no point is any consideration given to whether the patients actually have medical comorbidities, or if a hospitalist will have anything meaningful to add to the care. The firmly set expectation is that hospitalists admit all stroke patients for the purposes of comanagement, while the neurologists consult.
Comanagement has become a mainstay of hospital medicine.1 It is predicated upon the assumption that surgical and specialty patients benefit when their medical comorbidities are managed by hospitalists. It differs conceptually from traditional medical consultation in that hospitalists collaboratively manage patients with surgeons or specialists, sharing responsibility and authority. In practice, however, comanagement varies widely, ranging from a model of care indistinguishable from traditional medical consultation to one where hospitalists admit and assume primary responsibility for surgical and specialty patients. This variability makes it difficult to study and make generalizations about the role and impact of hospitalist comanagement. Nonetheless, recent evidence suggests that hospitalist consultation and comanagement may not be as effective as originally anticipated.
In a 2008 observational cohort study of patients undergoing surgery at an academic medical center, Auerbach et al demonstrated that medical consultation (provided by hospitalists) did not improve glycemic control or increase the likelihood of perioperative beta‐blockade and venous thromboembolism prophylaxis.2 Patients who received consultation had longer adjusted lengths of stay (12.98% longer; 95% confidence interval, 1.61%‐25.61%) and higher adjusted costs (24.36% higher; 95% confidence interval, 13.54%‐36.34%). Notwithstanding the limited generalizability of this study to community hospitals, it has raised concerns that hospitalist consultation does not automatically improve quality of care or cost effectiveness.3
Several other recent trials have also helped to define where hospitalist comanagement may work well and where it may not. In 2004, Huddleston et al published the Hospitalist Orthopedic Team (HOT) trial, the first randomized prospective trial comparing hospitalist‐surgical comanagement to standard care.4 A total of 526 patients undergoing elective hip or knee replacement surgery at the Mayo Clinic were randomized to either standard orthopedic care with consultation as needed, or immediate hospitalist comanagement. The outcomes were disappointing. Hospitalist comanagement reduced minor complications (such as incidence of urinary tract infections, fever, and hyponatremia) but had no effect on moderate or major complications. The HOT intervention modestly reduced adjusted length of stay (LOS), defined as the point at which patients were deemed stable for discharge, by 0.5 days, but had no impact on actual LOS or cost per case. Not surprisingly, orthopedic surgeons and nurses preferred the HOT model of care over the standard model. One year later, Phy et al analyzed outcomes for patients admitted with hip fracture at the same institution.5 This retrospective cohort study compared patients who were admitted to either a standard orthopedic service or to a hospitalist team. In contrast to the HOT trial, hospitalist comanagement of hip fracture patients decreased time to surgery and lowered LOS by 2.2 days without compromising patient outcomes.
How did two trials that occurred roughly simultaneously at the same hospital, involving the same hospitalists and orthopedic surgeons generate such different outcomes? A likely answer is patient selection. Patients who undergo elective joint replacement are usually relatively healthy. They are almost always ambulatory and their comorbidities, when present, are generally reasonably compensated. As a rule, they fare well postoperatively, as evidenced by the 1.3% major complication rate demonstrated in the HOT trial.3 In contrast, hip fracture patients are older, have greater comorbidity and are at remarkably high risk for developing perioperative delirium.3, 4, 6 By definition, their urgent/emergent hip surgery stratifies them to a higher operative risk category than patients who undergo elective joint replacement.7 Half of hip fracture patients do not return to premorbid levels of function, and the 1‐year mortality rate has been estimated to be as high as 25%.6, 8 Given these differences, it is not surprising that hip fracture patients are more likely than elective joint replacement patients to respond favorably to hospitalist comanagement.
In 2007, Simon et al published a retrospective study of 739 pediatric spinal fusion patients at Childrens' Hospital in Denver.9 Beginning in 2004, hospitalists comanaged selected, high‐risk surgical patients (14 of 115 spinal fusion patients, or 12%). Over the course of the study, the mean LOS for low‐risk patients decreased by 21% but the mean LOS for the high‐risk, hospitalist‐comanaged patients decreased by 28%; a 33% relative reduction favoring hospitalist‐managed patients. By targeting selected high‐risk patients, pediatric hospitalists were able to improve upon LOS reductions that occurred systemically across the entire spinal fusion program. Also in 2007, Southern et al compared outcomes for 2,913 patients admitted by full‐time teaching hospitalists vs 6,124 patients admitted by nonhospitalists at Montefiore Medical Center, Bronx, New York.10 Mean LOS for patients admitted to the hospitalist service was 5.01 days vs 5.87 days for the nonhospitalists. Subgroup analysis demonstrated the greatest LOS differentials for patients requiring close clinical monitoring (heart failure, stroke, asthma, or pneumonia) or complex discharge planning.
Although these studies, performed at large academic medical centers, may have limited generalizability, they support the common‐sense notion that hospitalists most benefit patients who are sick, frail, and medically or socially complex. As a corollary, hospitalists probably offer relatively little benefit to surgical and specialty patients who are young or have compensated medical comorbidities and/or straightforward disposition plans. The enormous variability across healthcare institutions makes it difficult if not impossible to define a patient acuity or complexity cutoff below which hospitalist comanagement is unlikely to be beneficial. Nonetheless, some degree of common sense can be applied. As a case in point, a hospitalist probably adds little value to the care of a basically healthy patient with a hemodynamically stable upper gastrointestinal bleed. Despite this, in many institutions, hospitalists admit or comanage all gastroenterology patients, irrespective of their diagnosis, acuity, or complexity.11
One can even hypothesize that hospitalist comanagement may potentially inject risk into patient care. Admitting that patient with a stable upper gastrointestinal bleed to a hospitalist service may delay the gastroenterologist's involvement and initiation of the necessary endoscopy. Having assumed that the hospitalist is running the show, the gastroenterologist may pay insufficient attention to the patient. The hospitalist and gastroenterologist may give conflicting orders and reports that confuse patients, families, and hospital staff, ultimately increasing the likelihood of medical errors.
Ultimately, the risks inherent in adding complexity into patient care must be balanced against the potential benefits. For patients who are sick, frail, or complicated, the risk‐benefit ratio probably tilts in favor of comanagement. However, for generally healthy patients, it is conceivable that adding complexity negates (or worse yet, exceeds) the putative benefits of comanagement.
Given the potential limitations of hospitalist comanagement, why are hospitalists admitting or managing broad and unselected populations of surgical and specialty patients? Hospital leaders have suggested that hospitalist comanagement may protect overstretched surgeons and specialists and extend their capacity. A hospital with only one neurosurgeon on staff might reasonably ask its hospitalists to primarily manage carefully selected low‐acuity neurosurgical patients, allowing the neurosurgeon to serve as a consultant. However, in communities where specialists and surgeons are abundant, this justification is less credible. In such cases, it is difficult not to suspect that the primary reason that hospitalists admit surgical and specialty patients is to enhance the income and quality of life of the surgeons and specialists.
Expanding hospitalist comanagement services for no other reason than to keep specialists and surgeons happy might be justifiable if hospital medicine was not faced with its own critical manpower shortage. Hospital medicine is expected to grow from approximately 20,000 current practitioners to more than 40,000 within a decade.12 The growing shortage of qualified hospitalists has become a preoccupation for hospitalist employers across the country.13 At its 2006 strategic planning retreat, the Board of Directors of the Society of Hospital Medicine identified this issue as one of the greatest threats to the future health of hospital medicine.14 Demand for hospitalists will not abate for at least a decade, which will leave many hospitalist programs significantly understaffed for the foreseeable future. Understaffing forces hospitalist programs to lower hiring standards, jeopardizes patient care, accelerates physician burnout, and may ultimately destabilize hospital medicine.15 Understaffed hospitalist programs should be very circumspect about how and where they expand their clinical coverage.
Another principle underlying hospitalist comanagement is that it improves care by allowing surgeons and specialists to focus on their areas of expertise. Surgeons and specialists who do not have to manage their patients' medical issues can presumably spend more time focusing on their own disciplines. Although this argument is conceptually appealing, there is no evidence that this actually occurs. In fact, it is equally conceivable that hospitalist comanagement could jeopardize care by disengaging surgeons and specialists from their patients' progress (or lack thereof). Furthermore, evidence suggests that hospitalists are underprepared to manage diagnoses that have historically been the purview of surgeons and specialists. Practicing hospitalists who manage acute neurological and neurosurgical conditions, orthopedic trauma, and acute psychiatric illnesses have reported relative undertraining in all of these disease states.10, 16 Generally, hospitalists are expected to deliver this care in the absence of any regime to assess their competence, provide targeted training to fill knowledge gaps, and monitor their progress. At minimum, this should raise concerns about the quality and consistency of care that hospitalists provide to nonmedical patients.
Finally, working collaboratively with other specialties should be a major professional benefit of comanagement. In well‐designed comanagement arrangements, hospitalists and specialists work equitably under clearly defined and mutually agreed upon rules of engagement. They share responsibility for patients, collaborate to improve care, and teach and learn from each other. Unfortunately, in many instances, the power structure becomes lopsided, with surgeons and specialists dictating how, when, and why hospitalists manage their patients.17 Emergency departments have learned to default surgical and specialty patient admissions to hospitalists when surgeons and specialists balk. Hospital administrations may tacitly or overtly expect their financially subsidized hospitalists to cheerfully accept any and all referrals, irrespective of how inappropriate they may be. Practicing hospitalists frequently complain about their subordinate status and inability to control their working conditions, both of which are identified risk factors for career dissatisfaction and burnout.14, 16, 18 Once again, as a specialty facing a critical manpower shortage, hospitalist programs should be very attuned to defining work conditions that foster career satisfaction and physician retention.
REFRAMING COMANAGEMENT
The history of healthcare is laden with examples of new ideas that were widely and indiscriminately adopted only to subsequently fail to withstand rigorous scrutiny.19, 20 The unchecked expansion of hospitalist comanagement has the potential to become another case in point. In the absence of clear definitions of comanagement and good evidence to define best practices, hospitalists are left to use their best judgment to define the parameters of their comanagement services. At minimum, hospitalist leaders should ask some basic questions as they ponder potential comanagement relationships:
-
Why are we being asked to provide this service?
-
Do the patients have comorbidities that require our input?
-
Is there a legitimate quality or efficiency case to be made to support our participation?
-
Do we have the manpower to provide the service? If not, what will suffer as a result?
-
Will the relationship be equitable?
-
What might go wrong?
Comanagement is an appealing construct that has grown to fill many niches of healthcare delivery.10 Given compelling reasons to be skeptical about the purported benefits of comanagement, hospitalists should be circumspect about how and where they offer such services. Comanagement should be applied carefully and methodically, paying close attention to the consequences, intended and unintended. Applying comanagement in a rational, evidence‐based, and sustainable fashion will ultimately better serve patients, the healthcare community, and hospital medicine.
- Society of Hospital Medicine. The Society of Hospital Medicine 2005–2006 Survey: The Authoritative Source on the State of the Hospital Medicine Movement. Published by the, 2006. Executive summary available at http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2167(21):2338–2344.
- .Exceed acceptable: new studies challenge hospitalists to prove our value.Hospitalist.2008;12(2):63.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,,.Treatment and survival among elderly Americans with hip fractures: a population‐based study.Am J Public Health.1994;84:1287–1291.
- ,,, et al.Predicting cardiac complications in patients undergoing non‐cardiac surgery.J Gen Intern Med.1986;1:211–219.
- ,,,,.Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study.J Gerontol.1990;45(3):M101–M107.
- ,,,,,.Pediatric hospitalist comanagement of spinal fusion surgery patients.J Hosp Med.2007;2:23–29.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167:1869–1874.
- ,,,,.The spectrum of community‐based hospitalist practice, a call to tailor internal medicine residency training.Arch Intern Med.2007;167(7):727–728.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNation wide/Growth_of_Hospital_M.htm. Accessed September 2,2008.
- ,,, et al.Rebuilding the future of the private practice of hospital medicine.The Phoenix Group, May2007.
- Society of Hospital Medicine Board of Directors Strategic Planning Retreat: November 28‐29,2006.
- ,,,,,,for the SGIM Career Satisfaction Study Group.Physician stress: results from the physician worklife study.Stress Health.2001;18(1):37–42.
- ,,, et al.Hospitalist's perceptions of their residency training needs: Results of a national survey.Am J Med.2001;111:247–254.
- .Feeling pressure to admit surgical patients? Hospitalists work to set limits on co‐management arrangements.Today's Hospitalist. January2008.
- Society of Hospital Medicine. Career Satisfaction White Paper. http://www.hospitalmedicine.org/AM/Template.cfm?Section=Practice_Resources321:406–412.
- .Pulmonary‐artery catheters—peace at last?N Engl J Med.2006;354(21):2273–2274.
At a hospital at which I work, every patient who presents to the emergency department with a suspected stroke or transient ischemic attack is evaluated by the stroke team. Per protocol, the team rapidly assesses each patient, orders diagnostic and therapeutic interventions and then refers each and every patient to the hospitalist service for admission and medical comanagement. At no point is any consideration given to whether the patients actually have medical comorbidities, or if a hospitalist will have anything meaningful to add to the care. The firmly set expectation is that hospitalists admit all stroke patients for the purposes of comanagement, while the neurologists consult.
Comanagement has become a mainstay of hospital medicine.1 It is predicated upon the assumption that surgical and specialty patients benefit when their medical comorbidities are managed by hospitalists. It differs conceptually from traditional medical consultation in that hospitalists collaboratively manage patients with surgeons or specialists, sharing responsibility and authority. In practice, however, comanagement varies widely, ranging from a model of care indistinguishable from traditional medical consultation to one where hospitalists admit and assume primary responsibility for surgical and specialty patients. This variability makes it difficult to study and make generalizations about the role and impact of hospitalist comanagement. Nonetheless, recent evidence suggests that hospitalist consultation and comanagement may not be as effective as originally anticipated.
In a 2008 observational cohort study of patients undergoing surgery at an academic medical center, Auerbach et al demonstrated that medical consultation (provided by hospitalists) did not improve glycemic control or increase the likelihood of perioperative beta‐blockade and venous thromboembolism prophylaxis.2 Patients who received consultation had longer adjusted lengths of stay (12.98% longer; 95% confidence interval, 1.61%‐25.61%) and higher adjusted costs (24.36% higher; 95% confidence interval, 13.54%‐36.34%). Notwithstanding the limited generalizability of this study to community hospitals, it has raised concerns that hospitalist consultation does not automatically improve quality of care or cost effectiveness.3
Several other recent trials have also helped to define where hospitalist comanagement may work well and where it may not. In 2004, Huddleston et al published the Hospitalist Orthopedic Team (HOT) trial, the first randomized prospective trial comparing hospitalist‐surgical comanagement to standard care.4 A total of 526 patients undergoing elective hip or knee replacement surgery at the Mayo Clinic were randomized to either standard orthopedic care with consultation as needed, or immediate hospitalist comanagement. The outcomes were disappointing. Hospitalist comanagement reduced minor complications (such as incidence of urinary tract infections, fever, and hyponatremia) but had no effect on moderate or major complications. The HOT intervention modestly reduced adjusted length of stay (LOS), defined as the point at which patients were deemed stable for discharge, by 0.5 days, but had no impact on actual LOS or cost per case. Not surprisingly, orthopedic surgeons and nurses preferred the HOT model of care over the standard model. One year later, Phy et al analyzed outcomes for patients admitted with hip fracture at the same institution.5 This retrospective cohort study compared patients who were admitted to either a standard orthopedic service or to a hospitalist team. In contrast to the HOT trial, hospitalist comanagement of hip fracture patients decreased time to surgery and lowered LOS by 2.2 days without compromising patient outcomes.
How did two trials that occurred roughly simultaneously at the same hospital, involving the same hospitalists and orthopedic surgeons generate such different outcomes? A likely answer is patient selection. Patients who undergo elective joint replacement are usually relatively healthy. They are almost always ambulatory and their comorbidities, when present, are generally reasonably compensated. As a rule, they fare well postoperatively, as evidenced by the 1.3% major complication rate demonstrated in the HOT trial.3 In contrast, hip fracture patients are older, have greater comorbidity and are at remarkably high risk for developing perioperative delirium.3, 4, 6 By definition, their urgent/emergent hip surgery stratifies them to a higher operative risk category than patients who undergo elective joint replacement.7 Half of hip fracture patients do not return to premorbid levels of function, and the 1‐year mortality rate has been estimated to be as high as 25%.6, 8 Given these differences, it is not surprising that hip fracture patients are more likely than elective joint replacement patients to respond favorably to hospitalist comanagement.
In 2007, Simon et al published a retrospective study of 739 pediatric spinal fusion patients at Childrens' Hospital in Denver.9 Beginning in 2004, hospitalists comanaged selected, high‐risk surgical patients (14 of 115 spinal fusion patients, or 12%). Over the course of the study, the mean LOS for low‐risk patients decreased by 21% but the mean LOS for the high‐risk, hospitalist‐comanaged patients decreased by 28%; a 33% relative reduction favoring hospitalist‐managed patients. By targeting selected high‐risk patients, pediatric hospitalists were able to improve upon LOS reductions that occurred systemically across the entire spinal fusion program. Also in 2007, Southern et al compared outcomes for 2,913 patients admitted by full‐time teaching hospitalists vs 6,124 patients admitted by nonhospitalists at Montefiore Medical Center, Bronx, New York.10 Mean LOS for patients admitted to the hospitalist service was 5.01 days vs 5.87 days for the nonhospitalists. Subgroup analysis demonstrated the greatest LOS differentials for patients requiring close clinical monitoring (heart failure, stroke, asthma, or pneumonia) or complex discharge planning.
Although these studies, performed at large academic medical centers, may have limited generalizability, they support the common‐sense notion that hospitalists most benefit patients who are sick, frail, and medically or socially complex. As a corollary, hospitalists probably offer relatively little benefit to surgical and specialty patients who are young or have compensated medical comorbidities and/or straightforward disposition plans. The enormous variability across healthcare institutions makes it difficult if not impossible to define a patient acuity or complexity cutoff below which hospitalist comanagement is unlikely to be beneficial. Nonetheless, some degree of common sense can be applied. As a case in point, a hospitalist probably adds little value to the care of a basically healthy patient with a hemodynamically stable upper gastrointestinal bleed. Despite this, in many institutions, hospitalists admit or comanage all gastroenterology patients, irrespective of their diagnosis, acuity, or complexity.11
One can even hypothesize that hospitalist comanagement may potentially inject risk into patient care. Admitting that patient with a stable upper gastrointestinal bleed to a hospitalist service may delay the gastroenterologist's involvement and initiation of the necessary endoscopy. Having assumed that the hospitalist is running the show, the gastroenterologist may pay insufficient attention to the patient. The hospitalist and gastroenterologist may give conflicting orders and reports that confuse patients, families, and hospital staff, ultimately increasing the likelihood of medical errors.
Ultimately, the risks inherent in adding complexity into patient care must be balanced against the potential benefits. For patients who are sick, frail, or complicated, the risk‐benefit ratio probably tilts in favor of comanagement. However, for generally healthy patients, it is conceivable that adding complexity negates (or worse yet, exceeds) the putative benefits of comanagement.
Given the potential limitations of hospitalist comanagement, why are hospitalists admitting or managing broad and unselected populations of surgical and specialty patients? Hospital leaders have suggested that hospitalist comanagement may protect overstretched surgeons and specialists and extend their capacity. A hospital with only one neurosurgeon on staff might reasonably ask its hospitalists to primarily manage carefully selected low‐acuity neurosurgical patients, allowing the neurosurgeon to serve as a consultant. However, in communities where specialists and surgeons are abundant, this justification is less credible. In such cases, it is difficult not to suspect that the primary reason that hospitalists admit surgical and specialty patients is to enhance the income and quality of life of the surgeons and specialists.
Expanding hospitalist comanagement services for no other reason than to keep specialists and surgeons happy might be justifiable if hospital medicine was not faced with its own critical manpower shortage. Hospital medicine is expected to grow from approximately 20,000 current practitioners to more than 40,000 within a decade.12 The growing shortage of qualified hospitalists has become a preoccupation for hospitalist employers across the country.13 At its 2006 strategic planning retreat, the Board of Directors of the Society of Hospital Medicine identified this issue as one of the greatest threats to the future health of hospital medicine.14 Demand for hospitalists will not abate for at least a decade, which will leave many hospitalist programs significantly understaffed for the foreseeable future. Understaffing forces hospitalist programs to lower hiring standards, jeopardizes patient care, accelerates physician burnout, and may ultimately destabilize hospital medicine.15 Understaffed hospitalist programs should be very circumspect about how and where they expand their clinical coverage.
Another principle underlying hospitalist comanagement is that it improves care by allowing surgeons and specialists to focus on their areas of expertise. Surgeons and specialists who do not have to manage their patients' medical issues can presumably spend more time focusing on their own disciplines. Although this argument is conceptually appealing, there is no evidence that this actually occurs. In fact, it is equally conceivable that hospitalist comanagement could jeopardize care by disengaging surgeons and specialists from their patients' progress (or lack thereof). Furthermore, evidence suggests that hospitalists are underprepared to manage diagnoses that have historically been the purview of surgeons and specialists. Practicing hospitalists who manage acute neurological and neurosurgical conditions, orthopedic trauma, and acute psychiatric illnesses have reported relative undertraining in all of these disease states.10, 16 Generally, hospitalists are expected to deliver this care in the absence of any regime to assess their competence, provide targeted training to fill knowledge gaps, and monitor their progress. At minimum, this should raise concerns about the quality and consistency of care that hospitalists provide to nonmedical patients.
Finally, working collaboratively with other specialties should be a major professional benefit of comanagement. In well‐designed comanagement arrangements, hospitalists and specialists work equitably under clearly defined and mutually agreed upon rules of engagement. They share responsibility for patients, collaborate to improve care, and teach and learn from each other. Unfortunately, in many instances, the power structure becomes lopsided, with surgeons and specialists dictating how, when, and why hospitalists manage their patients.17 Emergency departments have learned to default surgical and specialty patient admissions to hospitalists when surgeons and specialists balk. Hospital administrations may tacitly or overtly expect their financially subsidized hospitalists to cheerfully accept any and all referrals, irrespective of how inappropriate they may be. Practicing hospitalists frequently complain about their subordinate status and inability to control their working conditions, both of which are identified risk factors for career dissatisfaction and burnout.14, 16, 18 Once again, as a specialty facing a critical manpower shortage, hospitalist programs should be very attuned to defining work conditions that foster career satisfaction and physician retention.
REFRAMING COMANAGEMENT
The history of healthcare is laden with examples of new ideas that were widely and indiscriminately adopted only to subsequently fail to withstand rigorous scrutiny.19, 20 The unchecked expansion of hospitalist comanagement has the potential to become another case in point. In the absence of clear definitions of comanagement and good evidence to define best practices, hospitalists are left to use their best judgment to define the parameters of their comanagement services. At minimum, hospitalist leaders should ask some basic questions as they ponder potential comanagement relationships:
-
Why are we being asked to provide this service?
-
Do the patients have comorbidities that require our input?
-
Is there a legitimate quality or efficiency case to be made to support our participation?
-
Do we have the manpower to provide the service? If not, what will suffer as a result?
-
Will the relationship be equitable?
-
What might go wrong?
Comanagement is an appealing construct that has grown to fill many niches of healthcare delivery.10 Given compelling reasons to be skeptical about the purported benefits of comanagement, hospitalists should be circumspect about how and where they offer such services. Comanagement should be applied carefully and methodically, paying close attention to the consequences, intended and unintended. Applying comanagement in a rational, evidence‐based, and sustainable fashion will ultimately better serve patients, the healthcare community, and hospital medicine.
At a hospital at which I work, every patient who presents to the emergency department with a suspected stroke or transient ischemic attack is evaluated by the stroke team. Per protocol, the team rapidly assesses each patient, orders diagnostic and therapeutic interventions and then refers each and every patient to the hospitalist service for admission and medical comanagement. At no point is any consideration given to whether the patients actually have medical comorbidities, or if a hospitalist will have anything meaningful to add to the care. The firmly set expectation is that hospitalists admit all stroke patients for the purposes of comanagement, while the neurologists consult.
Comanagement has become a mainstay of hospital medicine.1 It is predicated upon the assumption that surgical and specialty patients benefit when their medical comorbidities are managed by hospitalists. It differs conceptually from traditional medical consultation in that hospitalists collaboratively manage patients with surgeons or specialists, sharing responsibility and authority. In practice, however, comanagement varies widely, ranging from a model of care indistinguishable from traditional medical consultation to one where hospitalists admit and assume primary responsibility for surgical and specialty patients. This variability makes it difficult to study and make generalizations about the role and impact of hospitalist comanagement. Nonetheless, recent evidence suggests that hospitalist consultation and comanagement may not be as effective as originally anticipated.
In a 2008 observational cohort study of patients undergoing surgery at an academic medical center, Auerbach et al demonstrated that medical consultation (provided by hospitalists) did not improve glycemic control or increase the likelihood of perioperative beta‐blockade and venous thromboembolism prophylaxis.2 Patients who received consultation had longer adjusted lengths of stay (12.98% longer; 95% confidence interval, 1.61%‐25.61%) and higher adjusted costs (24.36% higher; 95% confidence interval, 13.54%‐36.34%). Notwithstanding the limited generalizability of this study to community hospitals, it has raised concerns that hospitalist consultation does not automatically improve quality of care or cost effectiveness.3
Several other recent trials have also helped to define where hospitalist comanagement may work well and where it may not. In 2004, Huddleston et al published the Hospitalist Orthopedic Team (HOT) trial, the first randomized prospective trial comparing hospitalist‐surgical comanagement to standard care.4 A total of 526 patients undergoing elective hip or knee replacement surgery at the Mayo Clinic were randomized to either standard orthopedic care with consultation as needed, or immediate hospitalist comanagement. The outcomes were disappointing. Hospitalist comanagement reduced minor complications (such as incidence of urinary tract infections, fever, and hyponatremia) but had no effect on moderate or major complications. The HOT intervention modestly reduced adjusted length of stay (LOS), defined as the point at which patients were deemed stable for discharge, by 0.5 days, but had no impact on actual LOS or cost per case. Not surprisingly, orthopedic surgeons and nurses preferred the HOT model of care over the standard model. One year later, Phy et al analyzed outcomes for patients admitted with hip fracture at the same institution.5 This retrospective cohort study compared patients who were admitted to either a standard orthopedic service or to a hospitalist team. In contrast to the HOT trial, hospitalist comanagement of hip fracture patients decreased time to surgery and lowered LOS by 2.2 days without compromising patient outcomes.
How did two trials that occurred roughly simultaneously at the same hospital, involving the same hospitalists and orthopedic surgeons generate such different outcomes? A likely answer is patient selection. Patients who undergo elective joint replacement are usually relatively healthy. They are almost always ambulatory and their comorbidities, when present, are generally reasonably compensated. As a rule, they fare well postoperatively, as evidenced by the 1.3% major complication rate demonstrated in the HOT trial.3 In contrast, hip fracture patients are older, have greater comorbidity and are at remarkably high risk for developing perioperative delirium.3, 4, 6 By definition, their urgent/emergent hip surgery stratifies them to a higher operative risk category than patients who undergo elective joint replacement.7 Half of hip fracture patients do not return to premorbid levels of function, and the 1‐year mortality rate has been estimated to be as high as 25%.6, 8 Given these differences, it is not surprising that hip fracture patients are more likely than elective joint replacement patients to respond favorably to hospitalist comanagement.
In 2007, Simon et al published a retrospective study of 739 pediatric spinal fusion patients at Childrens' Hospital in Denver.9 Beginning in 2004, hospitalists comanaged selected, high‐risk surgical patients (14 of 115 spinal fusion patients, or 12%). Over the course of the study, the mean LOS for low‐risk patients decreased by 21% but the mean LOS for the high‐risk, hospitalist‐comanaged patients decreased by 28%; a 33% relative reduction favoring hospitalist‐managed patients. By targeting selected high‐risk patients, pediatric hospitalists were able to improve upon LOS reductions that occurred systemically across the entire spinal fusion program. Also in 2007, Southern et al compared outcomes for 2,913 patients admitted by full‐time teaching hospitalists vs 6,124 patients admitted by nonhospitalists at Montefiore Medical Center, Bronx, New York.10 Mean LOS for patients admitted to the hospitalist service was 5.01 days vs 5.87 days for the nonhospitalists. Subgroup analysis demonstrated the greatest LOS differentials for patients requiring close clinical monitoring (heart failure, stroke, asthma, or pneumonia) or complex discharge planning.
Although these studies, performed at large academic medical centers, may have limited generalizability, they support the common‐sense notion that hospitalists most benefit patients who are sick, frail, and medically or socially complex. As a corollary, hospitalists probably offer relatively little benefit to surgical and specialty patients who are young or have compensated medical comorbidities and/or straightforward disposition plans. The enormous variability across healthcare institutions makes it difficult if not impossible to define a patient acuity or complexity cutoff below which hospitalist comanagement is unlikely to be beneficial. Nonetheless, some degree of common sense can be applied. As a case in point, a hospitalist probably adds little value to the care of a basically healthy patient with a hemodynamically stable upper gastrointestinal bleed. Despite this, in many institutions, hospitalists admit or comanage all gastroenterology patients, irrespective of their diagnosis, acuity, or complexity.11
One can even hypothesize that hospitalist comanagement may potentially inject risk into patient care. Admitting that patient with a stable upper gastrointestinal bleed to a hospitalist service may delay the gastroenterologist's involvement and initiation of the necessary endoscopy. Having assumed that the hospitalist is running the show, the gastroenterologist may pay insufficient attention to the patient. The hospitalist and gastroenterologist may give conflicting orders and reports that confuse patients, families, and hospital staff, ultimately increasing the likelihood of medical errors.
Ultimately, the risks inherent in adding complexity into patient care must be balanced against the potential benefits. For patients who are sick, frail, or complicated, the risk‐benefit ratio probably tilts in favor of comanagement. However, for generally healthy patients, it is conceivable that adding complexity negates (or worse yet, exceeds) the putative benefits of comanagement.
Given the potential limitations of hospitalist comanagement, why are hospitalists admitting or managing broad and unselected populations of surgical and specialty patients? Hospital leaders have suggested that hospitalist comanagement may protect overstretched surgeons and specialists and extend their capacity. A hospital with only one neurosurgeon on staff might reasonably ask its hospitalists to primarily manage carefully selected low‐acuity neurosurgical patients, allowing the neurosurgeon to serve as a consultant. However, in communities where specialists and surgeons are abundant, this justification is less credible. In such cases, it is difficult not to suspect that the primary reason that hospitalists admit surgical and specialty patients is to enhance the income and quality of life of the surgeons and specialists.
Expanding hospitalist comanagement services for no other reason than to keep specialists and surgeons happy might be justifiable if hospital medicine was not faced with its own critical manpower shortage. Hospital medicine is expected to grow from approximately 20,000 current practitioners to more than 40,000 within a decade.12 The growing shortage of qualified hospitalists has become a preoccupation for hospitalist employers across the country.13 At its 2006 strategic planning retreat, the Board of Directors of the Society of Hospital Medicine identified this issue as one of the greatest threats to the future health of hospital medicine.14 Demand for hospitalists will not abate for at least a decade, which will leave many hospitalist programs significantly understaffed for the foreseeable future. Understaffing forces hospitalist programs to lower hiring standards, jeopardizes patient care, accelerates physician burnout, and may ultimately destabilize hospital medicine.15 Understaffed hospitalist programs should be very circumspect about how and where they expand their clinical coverage.
Another principle underlying hospitalist comanagement is that it improves care by allowing surgeons and specialists to focus on their areas of expertise. Surgeons and specialists who do not have to manage their patients' medical issues can presumably spend more time focusing on their own disciplines. Although this argument is conceptually appealing, there is no evidence that this actually occurs. In fact, it is equally conceivable that hospitalist comanagement could jeopardize care by disengaging surgeons and specialists from their patients' progress (or lack thereof). Furthermore, evidence suggests that hospitalists are underprepared to manage diagnoses that have historically been the purview of surgeons and specialists. Practicing hospitalists who manage acute neurological and neurosurgical conditions, orthopedic trauma, and acute psychiatric illnesses have reported relative undertraining in all of these disease states.10, 16 Generally, hospitalists are expected to deliver this care in the absence of any regime to assess their competence, provide targeted training to fill knowledge gaps, and monitor their progress. At minimum, this should raise concerns about the quality and consistency of care that hospitalists provide to nonmedical patients.
Finally, working collaboratively with other specialties should be a major professional benefit of comanagement. In well‐designed comanagement arrangements, hospitalists and specialists work equitably under clearly defined and mutually agreed upon rules of engagement. They share responsibility for patients, collaborate to improve care, and teach and learn from each other. Unfortunately, in many instances, the power structure becomes lopsided, with surgeons and specialists dictating how, when, and why hospitalists manage their patients.17 Emergency departments have learned to default surgical and specialty patient admissions to hospitalists when surgeons and specialists balk. Hospital administrations may tacitly or overtly expect their financially subsidized hospitalists to cheerfully accept any and all referrals, irrespective of how inappropriate they may be. Practicing hospitalists frequently complain about their subordinate status and inability to control their working conditions, both of which are identified risk factors for career dissatisfaction and burnout.14, 16, 18 Once again, as a specialty facing a critical manpower shortage, hospitalist programs should be very attuned to defining work conditions that foster career satisfaction and physician retention.
REFRAMING COMANAGEMENT
The history of healthcare is laden with examples of new ideas that were widely and indiscriminately adopted only to subsequently fail to withstand rigorous scrutiny.19, 20 The unchecked expansion of hospitalist comanagement has the potential to become another case in point. In the absence of clear definitions of comanagement and good evidence to define best practices, hospitalists are left to use their best judgment to define the parameters of their comanagement services. At minimum, hospitalist leaders should ask some basic questions as they ponder potential comanagement relationships:
-
Why are we being asked to provide this service?
-
Do the patients have comorbidities that require our input?
-
Is there a legitimate quality or efficiency case to be made to support our participation?
-
Do we have the manpower to provide the service? If not, what will suffer as a result?
-
Will the relationship be equitable?
-
What might go wrong?
Comanagement is an appealing construct that has grown to fill many niches of healthcare delivery.10 Given compelling reasons to be skeptical about the purported benefits of comanagement, hospitalists should be circumspect about how and where they offer such services. Comanagement should be applied carefully and methodically, paying close attention to the consequences, intended and unintended. Applying comanagement in a rational, evidence‐based, and sustainable fashion will ultimately better serve patients, the healthcare community, and hospital medicine.
- Society of Hospital Medicine. The Society of Hospital Medicine 2005–2006 Survey: The Authoritative Source on the State of the Hospital Medicine Movement. Published by the, 2006. Executive summary available at http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2167(21):2338–2344.
- .Exceed acceptable: new studies challenge hospitalists to prove our value.Hospitalist.2008;12(2):63.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,,.Treatment and survival among elderly Americans with hip fractures: a population‐based study.Am J Public Health.1994;84:1287–1291.
- ,,, et al.Predicting cardiac complications in patients undergoing non‐cardiac surgery.J Gen Intern Med.1986;1:211–219.
- ,,,,.Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study.J Gerontol.1990;45(3):M101–M107.
- ,,,,,.Pediatric hospitalist comanagement of spinal fusion surgery patients.J Hosp Med.2007;2:23–29.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167:1869–1874.
- ,,,,.The spectrum of community‐based hospitalist practice, a call to tailor internal medicine residency training.Arch Intern Med.2007;167(7):727–728.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNation wide/Growth_of_Hospital_M.htm. Accessed September 2,2008.
- ,,, et al.Rebuilding the future of the private practice of hospital medicine.The Phoenix Group, May2007.
- Society of Hospital Medicine Board of Directors Strategic Planning Retreat: November 28‐29,2006.
- ,,,,,,for the SGIM Career Satisfaction Study Group.Physician stress: results from the physician worklife study.Stress Health.2001;18(1):37–42.
- ,,, et al.Hospitalist's perceptions of their residency training needs: Results of a national survey.Am J Med.2001;111:247–254.
- .Feeling pressure to admit surgical patients? Hospitalists work to set limits on co‐management arrangements.Today's Hospitalist. January2008.
- Society of Hospital Medicine. Career Satisfaction White Paper. http://www.hospitalmedicine.org/AM/Template.cfm?Section=Practice_Resources321:406–412.
- .Pulmonary‐artery catheters—peace at last?N Engl J Med.2006;354(21):2273–2274.
- Society of Hospital Medicine. The Society of Hospital Medicine 2005–2006 Survey: The Authoritative Source on the State of the Hospital Medicine Movement. Published by the, 2006. Executive summary available at http://www.hospitalmedicine.org/AM/Template.cfm?Section=Surveys2167(21):2338–2344.
- .Exceed acceptable: new studies challenge hospitalists to prove our value.Hospitalist.2008;12(2):63.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty.Ann Intern Med.2004;141:28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165:796–801.
- ,,,.Treatment and survival among elderly Americans with hip fractures: a population‐based study.Am J Public Health.1994;84:1287–1291.
- ,,, et al.Predicting cardiac complications in patients undergoing non‐cardiac surgery.J Gen Intern Med.1986;1:211–219.
- ,,,,.Predictors of functional recovery one year following hospital discharge for hip fracture: a prospective study.J Gerontol.1990;45(3):M101–M107.
- ,,,,,.Pediatric hospitalist comanagement of spinal fusion surgery patients.J Hosp Med.2007;2:23–29.
- ,,,,.Hospitalist care and length of stay in patients requiring complex discharge planning and close clinical monitoring.Arch Intern Med.2007;167:1869–1874.
- ,,,,.The spectrum of community‐based hospitalist practice, a call to tailor internal medicine residency training.Arch Intern Med.2007;167(7):727–728.
- Society of Hospital Medicine. Growth of Hospital Medicine Nationwide. http://www.hospitalmedicine.org/Content/NavigationMenu/Media/GrowthofHospitalMedicineNation wide/Growth_of_Hospital_M.htm. Accessed September 2,2008.
- ,,, et al.Rebuilding the future of the private practice of hospital medicine.The Phoenix Group, May2007.
- Society of Hospital Medicine Board of Directors Strategic Planning Retreat: November 28‐29,2006.
- ,,,,,,for the SGIM Career Satisfaction Study Group.Physician stress: results from the physician worklife study.Stress Health.2001;18(1):37–42.
- ,,, et al.Hospitalist's perceptions of their residency training needs: Results of a national survey.Am J Med.2001;111:247–254.
- .Feeling pressure to admit surgical patients? Hospitalists work to set limits on co‐management arrangements.Today's Hospitalist. January2008.
- Society of Hospital Medicine. Career Satisfaction White Paper. http://www.hospitalmedicine.org/AM/Template.cfm?Section=Practice_Resources321:406–412.
- .Pulmonary‐artery catheters—peace at last?N Engl J Med.2006;354(21):2273–2274.
Editorial
Older Americans comprise approximately half the patients on inpatient medical wards. There are too few geriatricians to care for these patients, and few geriatricians practice hospital medicine. Hospitalists often provide the majority of inpatient geriatric care, and at teaching hospitals, hospitalists also play a pivotal role in educating residents and students to provide high‐quality care for hospitalized geriatric patients. Thus, hospitalists will be the primary clinicians educating many trainees to care for older patients, and the hospitalists must be skilled in addressing the clinical syndromes that are common in these patients, including delirium, dementia, falls, and infection.1 Generalists and geriatricians have anticipated a shortfall in clinicians prepared to educate trainees about geriatrics and called for faculty development for generalists in geriatrics.2, 3
In this issue of the Journal of Hospital Medicine, Podrazik and colleagues present initial results from a major initiative to enhance the quality and quantity of geriatric inpatient education for residents and students.4 The Curriculum for the Hospitalized Aging Medical Patient (CHAMP) at the University of Chicago represents a multifaceted faculty development effort funded in part by the Donald W. Reynolds and John A. Hartford Foundations. In 12 half‐day sessions offered weekly, hospitalist and general internist faculty members learned about four thematic areasthe frail older person, hazards of hospitalization, end‐of‐life issues, and transitions of carewhile also receiving training in engaging and effective teaching strategies. At each session, participants drew on their own experiences attending on the wards to generate clinical examples and test new teaching strategies. CHAMP incorporates the attributes of best practices for integrating geriatrics education into internal medicine residency training: it promotes model care for older hospital patients, uses a train‐the‐trainer model, addresses care transitions, and promotes interdisciplinary teamwork.5
CHAMP achieved its initial goals. Faculty participants were satisfied and CHAMP substantially increased participants' confidence in practicing and teaching geriatric care. Faculty participants also gained confidence in their teaching abilities and presumably learned teaching strategies that could be applied to other topics in inpatient medicine. Faculty participants demonstrated modest improvements in their knowledge of geriatric issues and more positive attitudes about geriatrics at the end of the course than at the beginning. It is worth noting that the hospitalist and general internist ward attending physicians who participated in CHAMP were volunteers and may have started the process with greater interest in learning geriatric care than other attendings. Thus, it is unknown whether CHAMP might have greater or lesser effect on other faculty.
The CHAMP train‐the‐trainer model offers the potential to impact future practitioners. Findings of the CHAMP investigators are consistent with the literature on faculty development programs for educators, which shows that faculty development on teaching yields high participant satisfaction, knowledge gains, and improved self‐assessment of the ability to implement changes in teaching practice.6 The use in CHAMP of a diverse menu of teaching strategies and active learning techniques such as case‐based discussions and the Objective Structured Teaching Exercise in a small group of colleagues should promote learning and retention.
Is the CHAMP curriculum worth the cost? The program requires resources to pay for 48 hours for each faculty participant and for instructors with expertise in geriatrics and teaching skills. We estimate that the cost for 12 faculty participants would be roughly $72,000. We believe this investment will likely pay off in terms of enhancing faculty skills, improving faculty job satisfaction, promoting faculty retention in academic or other teaching positions, and improving care provided by trainees. For example, if CHAMP were to lead to the retention and promotion of even 2 faculty for just 1 year, it would save recruitment costs that would exceed the direct program costs, and other benefits of CHAMP would only further add value. However, analysis of the benefits of CHAMP will require more in‐depth evaluation data of its impact. The program leaders currently contact former participants around the time of ward attending to reinforce teaching concepts and encourage implementation of CHAMP materials, through a Commitment to Change contract. The ultimate downstream educational goal would be that these faculty learners retain and apply this newly acquired knowledge and skills in their clinical practice and teaching activities. Ideally, evidence would confirm that these benefits improve patient care. The long‐term evaluation plan for CHAMP incorporates important additional outcome measures including resident and student geriatric knowledge as well as patient satisfaction and clinical outcomes. We commend the authors for aiming to expand their evaluation plan over time and aspiring for sustained changes in teaching practice. The literature on the impact of hospitalists has similarly evolved from early descriptions of hospitalists and the logistics of developing a hospitalist program to sophisticated analyses of the impact of hospitalists on clinical outcomes such as length of stay and mortality.7, 8
The feasibility of disseminating CHAMP is an open question. The University of Chicago model employs a time‐intensive curriculum that engages participants in part by releasing them from clinical duties for a half day per week. Release time was funded through combined support from external funding sources and the Department of Medicine. This model addresses the major barrier to faculty development in geriatrics for general internists: lack of time.2, 9 The investment in intensive, longitudinal faculty development may generate higher returns than periodic short faculty workshop sessions that do not build in the time for role‐playing, practice, and reinforcement of key concepts. This type of intervention may also be more feasible when done in conjunction with one of the approximately 50 Health Resources and Services Administration (HRSA)supported Geriatrics Education Centers, which can fund teachers and infrastructure for faculty development.
How is this article useful for hospitalist educators? Many hospitalists at academic centers serve important teaching functions, and some will aspire to advance their educational efforts through more scholarly activities such as curriculum design. The CHAMP curriculum represents a successful model for hospitalists aiming to follow a rigorous approach to curriculum design relevant to inpatient medicine, and the extensive CHAMP materials are available online.10 It serves as a practical model that could be applied to other clinical topics related to hospital medicine. Hospitalists are effective and respected teachers for residents and students, and they develop unique expertise in the content and process of inpatient medicine.11 The authors followed the 6 steps of effective curriculum design: problem identification, targeted needs assessment, goals and objectives, education methods, implementation, and evaluation.12
The CHAMP curriculum typifies a set of materials that aligns well with the Society of Hospital Medicine (SHM) Core Competencies.13 As part of their needs assessment, the authors also surveyed hospitalists at a regional SHM meeting to determine the geriatrics topics for which they perceived greatest educational need. The Core Competencies chapters on the care of the elderly patient, delirium and dementia, hospital‐acquired infections, and palliative care highlight the common learning goals shared by hospital medicine and geriatrics. Both disciplines also emphasize the team‐based, multidisciplinary approach to care, particularly during care transitions, that is highlighted in the CHAMP curriculum.
More generally, the CHAMP curriculum can be used to teach and assess the Accreditation Council for Graduate Medical Education (ACGME) competencies, which must be assessed in all ACGME‐accredited residency programs.14 In an initial session on Teaching on Today's Wards, CHAMP participants brainstorm about how to incorporate both geriatrics content and the ACGME competencies into their post‐call rounds. The emphasis in CHAMP on the health care system and interdisciplinary care is evident in topics such as end‐of‐life care and transitions in care, and provides opportunity for assessment of residents' performance in the ACGME competency of systems‐based practice. The organization of the curriculum by ACGME competency makes it more applicable today than some prior geriatric curricula that emphasized similar themes but without the emphasis on demonstrating competency as an outcome.15
Hospitalists partnering with the Donald W. Reynolds and John A. Hartford Foundations and other external organizations may find funding opportunities for educational projects. For example, the Hartford Foundation has partnered with SHM since 2002 to support hospitalists' efforts to improve care for older adults. Products of this collaboration include a Geriatric Toolbox that contains assessment tools designed for use with geriatric patients.16 The tools assess a range of parameters including nutritional, functional, and mental status, and the website supplies guidelines on the advantages and disadvantages and appropriate use of each assessment tool. With support from the Hartford Foundation, hospitalists have also conducted several workshops at SHM meetings on improving assessment and care of geriatric patients and developed a discharge‐planning checklist for older adults.
As hospitalist programs gain traction in academic centers, hospitalists will increasingly serve as key geriatric content educators for trainees. The CHAMP curriculum offers a model of intensive faculty development for hospitalists and general internists that clinician educators find engaging and empowering. The partnerships of geriatricians and hospitalists, and of the SHM with national geriatrics organizations, have the potential for widespread benefits for both learners and elderly patients.
Older Americans comprise approximately half the patients on inpatient medical wards. There are too few geriatricians to care for these patients, and few geriatricians practice hospital medicine. Hospitalists often provide the majority of inpatient geriatric care, and at teaching hospitals, hospitalists also play a pivotal role in educating residents and students to provide high‐quality care for hospitalized geriatric patients. Thus, hospitalists will be the primary clinicians educating many trainees to care for older patients, and the hospitalists must be skilled in addressing the clinical syndromes that are common in these patients, including delirium, dementia, falls, and infection.1 Generalists and geriatricians have anticipated a shortfall in clinicians prepared to educate trainees about geriatrics and called for faculty development for generalists in geriatrics.2, 3
In this issue of the Journal of Hospital Medicine, Podrazik and colleagues present initial results from a major initiative to enhance the quality and quantity of geriatric inpatient education for residents and students.4 The Curriculum for the Hospitalized Aging Medical Patient (CHAMP) at the University of Chicago represents a multifaceted faculty development effort funded in part by the Donald W. Reynolds and John A. Hartford Foundations. In 12 half‐day sessions offered weekly, hospitalist and general internist faculty members learned about four thematic areasthe frail older person, hazards of hospitalization, end‐of‐life issues, and transitions of carewhile also receiving training in engaging and effective teaching strategies. At each session, participants drew on their own experiences attending on the wards to generate clinical examples and test new teaching strategies. CHAMP incorporates the attributes of best practices for integrating geriatrics education into internal medicine residency training: it promotes model care for older hospital patients, uses a train‐the‐trainer model, addresses care transitions, and promotes interdisciplinary teamwork.5
CHAMP achieved its initial goals. Faculty participants were satisfied and CHAMP substantially increased participants' confidence in practicing and teaching geriatric care. Faculty participants also gained confidence in their teaching abilities and presumably learned teaching strategies that could be applied to other topics in inpatient medicine. Faculty participants demonstrated modest improvements in their knowledge of geriatric issues and more positive attitudes about geriatrics at the end of the course than at the beginning. It is worth noting that the hospitalist and general internist ward attending physicians who participated in CHAMP were volunteers and may have started the process with greater interest in learning geriatric care than other attendings. Thus, it is unknown whether CHAMP might have greater or lesser effect on other faculty.
The CHAMP train‐the‐trainer model offers the potential to impact future practitioners. Findings of the CHAMP investigators are consistent with the literature on faculty development programs for educators, which shows that faculty development on teaching yields high participant satisfaction, knowledge gains, and improved self‐assessment of the ability to implement changes in teaching practice.6 The use in CHAMP of a diverse menu of teaching strategies and active learning techniques such as case‐based discussions and the Objective Structured Teaching Exercise in a small group of colleagues should promote learning and retention.
Is the CHAMP curriculum worth the cost? The program requires resources to pay for 48 hours for each faculty participant and for instructors with expertise in geriatrics and teaching skills. We estimate that the cost for 12 faculty participants would be roughly $72,000. We believe this investment will likely pay off in terms of enhancing faculty skills, improving faculty job satisfaction, promoting faculty retention in academic or other teaching positions, and improving care provided by trainees. For example, if CHAMP were to lead to the retention and promotion of even 2 faculty for just 1 year, it would save recruitment costs that would exceed the direct program costs, and other benefits of CHAMP would only further add value. However, analysis of the benefits of CHAMP will require more in‐depth evaluation data of its impact. The program leaders currently contact former participants around the time of ward attending to reinforce teaching concepts and encourage implementation of CHAMP materials, through a Commitment to Change contract. The ultimate downstream educational goal would be that these faculty learners retain and apply this newly acquired knowledge and skills in their clinical practice and teaching activities. Ideally, evidence would confirm that these benefits improve patient care. The long‐term evaluation plan for CHAMP incorporates important additional outcome measures including resident and student geriatric knowledge as well as patient satisfaction and clinical outcomes. We commend the authors for aiming to expand their evaluation plan over time and aspiring for sustained changes in teaching practice. The literature on the impact of hospitalists has similarly evolved from early descriptions of hospitalists and the logistics of developing a hospitalist program to sophisticated analyses of the impact of hospitalists on clinical outcomes such as length of stay and mortality.7, 8
The feasibility of disseminating CHAMP is an open question. The University of Chicago model employs a time‐intensive curriculum that engages participants in part by releasing them from clinical duties for a half day per week. Release time was funded through combined support from external funding sources and the Department of Medicine. This model addresses the major barrier to faculty development in geriatrics for general internists: lack of time.2, 9 The investment in intensive, longitudinal faculty development may generate higher returns than periodic short faculty workshop sessions that do not build in the time for role‐playing, practice, and reinforcement of key concepts. This type of intervention may also be more feasible when done in conjunction with one of the approximately 50 Health Resources and Services Administration (HRSA)supported Geriatrics Education Centers, which can fund teachers and infrastructure for faculty development.
How is this article useful for hospitalist educators? Many hospitalists at academic centers serve important teaching functions, and some will aspire to advance their educational efforts through more scholarly activities such as curriculum design. The CHAMP curriculum represents a successful model for hospitalists aiming to follow a rigorous approach to curriculum design relevant to inpatient medicine, and the extensive CHAMP materials are available online.10 It serves as a practical model that could be applied to other clinical topics related to hospital medicine. Hospitalists are effective and respected teachers for residents and students, and they develop unique expertise in the content and process of inpatient medicine.11 The authors followed the 6 steps of effective curriculum design: problem identification, targeted needs assessment, goals and objectives, education methods, implementation, and evaluation.12
The CHAMP curriculum typifies a set of materials that aligns well with the Society of Hospital Medicine (SHM) Core Competencies.13 As part of their needs assessment, the authors also surveyed hospitalists at a regional SHM meeting to determine the geriatrics topics for which they perceived greatest educational need. The Core Competencies chapters on the care of the elderly patient, delirium and dementia, hospital‐acquired infections, and palliative care highlight the common learning goals shared by hospital medicine and geriatrics. Both disciplines also emphasize the team‐based, multidisciplinary approach to care, particularly during care transitions, that is highlighted in the CHAMP curriculum.
More generally, the CHAMP curriculum can be used to teach and assess the Accreditation Council for Graduate Medical Education (ACGME) competencies, which must be assessed in all ACGME‐accredited residency programs.14 In an initial session on Teaching on Today's Wards, CHAMP participants brainstorm about how to incorporate both geriatrics content and the ACGME competencies into their post‐call rounds. The emphasis in CHAMP on the health care system and interdisciplinary care is evident in topics such as end‐of‐life care and transitions in care, and provides opportunity for assessment of residents' performance in the ACGME competency of systems‐based practice. The organization of the curriculum by ACGME competency makes it more applicable today than some prior geriatric curricula that emphasized similar themes but without the emphasis on demonstrating competency as an outcome.15
Hospitalists partnering with the Donald W. Reynolds and John A. Hartford Foundations and other external organizations may find funding opportunities for educational projects. For example, the Hartford Foundation has partnered with SHM since 2002 to support hospitalists' efforts to improve care for older adults. Products of this collaboration include a Geriatric Toolbox that contains assessment tools designed for use with geriatric patients.16 The tools assess a range of parameters including nutritional, functional, and mental status, and the website supplies guidelines on the advantages and disadvantages and appropriate use of each assessment tool. With support from the Hartford Foundation, hospitalists have also conducted several workshops at SHM meetings on improving assessment and care of geriatric patients and developed a discharge‐planning checklist for older adults.
As hospitalist programs gain traction in academic centers, hospitalists will increasingly serve as key geriatric content educators for trainees. The CHAMP curriculum offers a model of intensive faculty development for hospitalists and general internists that clinician educators find engaging and empowering. The partnerships of geriatricians and hospitalists, and of the SHM with national geriatrics organizations, have the potential for widespread benefits for both learners and elderly patients.
Older Americans comprise approximately half the patients on inpatient medical wards. There are too few geriatricians to care for these patients, and few geriatricians practice hospital medicine. Hospitalists often provide the majority of inpatient geriatric care, and at teaching hospitals, hospitalists also play a pivotal role in educating residents and students to provide high‐quality care for hospitalized geriatric patients. Thus, hospitalists will be the primary clinicians educating many trainees to care for older patients, and the hospitalists must be skilled in addressing the clinical syndromes that are common in these patients, including delirium, dementia, falls, and infection.1 Generalists and geriatricians have anticipated a shortfall in clinicians prepared to educate trainees about geriatrics and called for faculty development for generalists in geriatrics.2, 3
In this issue of the Journal of Hospital Medicine, Podrazik and colleagues present initial results from a major initiative to enhance the quality and quantity of geriatric inpatient education for residents and students.4 The Curriculum for the Hospitalized Aging Medical Patient (CHAMP) at the University of Chicago represents a multifaceted faculty development effort funded in part by the Donald W. Reynolds and John A. Hartford Foundations. In 12 half‐day sessions offered weekly, hospitalist and general internist faculty members learned about four thematic areasthe frail older person, hazards of hospitalization, end‐of‐life issues, and transitions of carewhile also receiving training in engaging and effective teaching strategies. At each session, participants drew on their own experiences attending on the wards to generate clinical examples and test new teaching strategies. CHAMP incorporates the attributes of best practices for integrating geriatrics education into internal medicine residency training: it promotes model care for older hospital patients, uses a train‐the‐trainer model, addresses care transitions, and promotes interdisciplinary teamwork.5
CHAMP achieved its initial goals. Faculty participants were satisfied and CHAMP substantially increased participants' confidence in practicing and teaching geriatric care. Faculty participants also gained confidence in their teaching abilities and presumably learned teaching strategies that could be applied to other topics in inpatient medicine. Faculty participants demonstrated modest improvements in their knowledge of geriatric issues and more positive attitudes about geriatrics at the end of the course than at the beginning. It is worth noting that the hospitalist and general internist ward attending physicians who participated in CHAMP were volunteers and may have started the process with greater interest in learning geriatric care than other attendings. Thus, it is unknown whether CHAMP might have greater or lesser effect on other faculty.
The CHAMP train‐the‐trainer model offers the potential to impact future practitioners. Findings of the CHAMP investigators are consistent with the literature on faculty development programs for educators, which shows that faculty development on teaching yields high participant satisfaction, knowledge gains, and improved self‐assessment of the ability to implement changes in teaching practice.6 The use in CHAMP of a diverse menu of teaching strategies and active learning techniques such as case‐based discussions and the Objective Structured Teaching Exercise in a small group of colleagues should promote learning and retention.
Is the CHAMP curriculum worth the cost? The program requires resources to pay for 48 hours for each faculty participant and for instructors with expertise in geriatrics and teaching skills. We estimate that the cost for 12 faculty participants would be roughly $72,000. We believe this investment will likely pay off in terms of enhancing faculty skills, improving faculty job satisfaction, promoting faculty retention in academic or other teaching positions, and improving care provided by trainees. For example, if CHAMP were to lead to the retention and promotion of even 2 faculty for just 1 year, it would save recruitment costs that would exceed the direct program costs, and other benefits of CHAMP would only further add value. However, analysis of the benefits of CHAMP will require more in‐depth evaluation data of its impact. The program leaders currently contact former participants around the time of ward attending to reinforce teaching concepts and encourage implementation of CHAMP materials, through a Commitment to Change contract. The ultimate downstream educational goal would be that these faculty learners retain and apply this newly acquired knowledge and skills in their clinical practice and teaching activities. Ideally, evidence would confirm that these benefits improve patient care. The long‐term evaluation plan for CHAMP incorporates important additional outcome measures including resident and student geriatric knowledge as well as patient satisfaction and clinical outcomes. We commend the authors for aiming to expand their evaluation plan over time and aspiring for sustained changes in teaching practice. The literature on the impact of hospitalists has similarly evolved from early descriptions of hospitalists and the logistics of developing a hospitalist program to sophisticated analyses of the impact of hospitalists on clinical outcomes such as length of stay and mortality.7, 8
The feasibility of disseminating CHAMP is an open question. The University of Chicago model employs a time‐intensive curriculum that engages participants in part by releasing them from clinical duties for a half day per week. Release time was funded through combined support from external funding sources and the Department of Medicine. This model addresses the major barrier to faculty development in geriatrics for general internists: lack of time.2, 9 The investment in intensive, longitudinal faculty development may generate higher returns than periodic short faculty workshop sessions that do not build in the time for role‐playing, practice, and reinforcement of key concepts. This type of intervention may also be more feasible when done in conjunction with one of the approximately 50 Health Resources and Services Administration (HRSA)supported Geriatrics Education Centers, which can fund teachers and infrastructure for faculty development.
How is this article useful for hospitalist educators? Many hospitalists at academic centers serve important teaching functions, and some will aspire to advance their educational efforts through more scholarly activities such as curriculum design. The CHAMP curriculum represents a successful model for hospitalists aiming to follow a rigorous approach to curriculum design relevant to inpatient medicine, and the extensive CHAMP materials are available online.10 It serves as a practical model that could be applied to other clinical topics related to hospital medicine. Hospitalists are effective and respected teachers for residents and students, and they develop unique expertise in the content and process of inpatient medicine.11 The authors followed the 6 steps of effective curriculum design: problem identification, targeted needs assessment, goals and objectives, education methods, implementation, and evaluation.12
The CHAMP curriculum typifies a set of materials that aligns well with the Society of Hospital Medicine (SHM) Core Competencies.13 As part of their needs assessment, the authors also surveyed hospitalists at a regional SHM meeting to determine the geriatrics topics for which they perceived greatest educational need. The Core Competencies chapters on the care of the elderly patient, delirium and dementia, hospital‐acquired infections, and palliative care highlight the common learning goals shared by hospital medicine and geriatrics. Both disciplines also emphasize the team‐based, multidisciplinary approach to care, particularly during care transitions, that is highlighted in the CHAMP curriculum.
More generally, the CHAMP curriculum can be used to teach and assess the Accreditation Council for Graduate Medical Education (ACGME) competencies, which must be assessed in all ACGME‐accredited residency programs.14 In an initial session on Teaching on Today's Wards, CHAMP participants brainstorm about how to incorporate both geriatrics content and the ACGME competencies into their post‐call rounds. The emphasis in CHAMP on the health care system and interdisciplinary care is evident in topics such as end‐of‐life care and transitions in care, and provides opportunity for assessment of residents' performance in the ACGME competency of systems‐based practice. The organization of the curriculum by ACGME competency makes it more applicable today than some prior geriatric curricula that emphasized similar themes but without the emphasis on demonstrating competency as an outcome.15
Hospitalists partnering with the Donald W. Reynolds and John A. Hartford Foundations and other external organizations may find funding opportunities for educational projects. For example, the Hartford Foundation has partnered with SHM since 2002 to support hospitalists' efforts to improve care for older adults. Products of this collaboration include a Geriatric Toolbox that contains assessment tools designed for use with geriatric patients.16 The tools assess a range of parameters including nutritional, functional, and mental status, and the website supplies guidelines on the advantages and disadvantages and appropriate use of each assessment tool. With support from the Hartford Foundation, hospitalists have also conducted several workshops at SHM meetings on improving assessment and care of geriatric patients and developed a discharge‐planning checklist for older adults.
As hospitalist programs gain traction in academic centers, hospitalists will increasingly serve as key geriatric content educators for trainees. The CHAMP curriculum offers a model of intensive faculty development for hospitalists and general internists that clinician educators find engaging and empowering. The partnerships of geriatricians and hospitalists, and of the SHM with national geriatrics organizations, have the potential for widespread benefits for both learners and elderly patients.
An Unconventional Living Will
Her name was Mrs. Carberry, but her readers knew her as Mary Margaret. Two months earlier, she suffered a debilitating stroke that took away her vibrant life. Previously a prolific writer of advice columns and opinion pieces, the blockage of blood to her brain dammed the steady flow of wisdom to her innumerable fans. They never again would benefit from the words of this intelligent, feisty, and self‐proclaimed Cranky Catholic. Unfortunately, I would not know Mary Margaret for her words, but only her numbers: her vital signs, her urine output, her tube feed residuals.
Not able to communicate with us, we wondered did Mrs. Carberry want this tracheostomy that was placed? Did she want this peg tube that fed her continuously? And even if she would have initially agreed to them, would she still want them now? Especially given she was not improving and had little hope for a meaningful recovery.
We posed these difficult questions to her two sons. One son believed that his mother would want an end to these aggressive measures. The other son disagreed; he said his mother would want to make every effort to stay alive.
In the end, Mary Margaret's voice found its way to us and provided us with the answer. She did not tell us in the conventional manner via a lawyer or a living will. Her friends did not come forth and let us know about serious conversations during their afternoon lunches. Instead, Mary Margaret penned it to her audience of devoted readers in a newspaper column written 14 years earlier. Mary Margaret's poignant words were revealed to her doctors by her son who understood its undeniable significance.
Mary Margaret was an essayist in Chicago whose pieces filled many major newspapers and magazines. She had strong opinions on matters large and small, writing articles addressing topics ranging from her Catholic beliefs to gender‐based inequities in the workplace. One article in particular addressed sickness and death and provided her sons the answer they sought. Having witnessed illness strike two loved ones, it was only natural for Mary Margaret to write about it. Her very personal essay was entitled Tough Questions on Life, Death and a Dog Named Bamboo. The words, resurfacing years later, and now having direct meaning to her own life and death, may have been some of the most profound and prophetic words of her career:
Tough Questions on Life, Death and a Dog Named Bamboo
I sat with her that evening while she was dyingrubbing her back, smoothing her head, whispering that I loved her, trying to be of some small comfort as she snuggled closer, looking up with her mysteriously accepting, somehow understanding brown eyes.
Adjusting herself once more, she half rose, then toppled sideways and simply stopped breathing. She died as a lady ought to be able to diequietly, as easily as possible, in her own bed. In my bed actually. She was Bamboo, my Shar‐pei, and it is difficult to write this even several months later without tears starting to roll.
It was not just that last day, of course. For a bit more than a week, Bamboo had been giving signs of a serious problema heavy doggie cough indicating severe congestion and a firm, stubborn decision not to eat. The kitchen offered a parade of small bowls of her favorite people foods with which I hoped to restore her appetite when she determinedly ignored her regular dog food. She had her choice of cottage cheese, scrambled eggs, ground sirloin, cheddar chunks, ice cream, buttered rice and morea virtual buffet for ants.
After I set each dish out, she would go over to look and sniff admiringly, even wag her tail, but then rather reluctantly return to her favorite resting place, a small rug at the top of the stairs to the front door.
She would only drink a lot of water, bowl after bowl, in which she did also get her medicine, mashed and melted. Late on that last afternoon, however she stopped the drinking, too. I couldn't get her to sip even when I brought the water dish to her or offered the ice cubes she once loved to lick. In her own way, she was saying No.
Lately I have been reflecting again on the experience as a result of having heard some discussion about the death of a woman with whom I once shared friendly commuter chitchat as we trained together into the Loop.
Following a stroke, she had been unconscious, vegetative, tragically for almost as long as I had enjoyed the eight years of Bamboo's delightful companionship. Her husband, I learned, had ultimately gone to court and had been granted permission to remove the feeding tube and let nature take its course. A counteraction to prevent this was filed by some well‐intentioned people; but what I believe as good human and legal sense prevailed. So without the tube feeding, this nice long‐suffering woman finally slipped away to God.
People who oppose the dying being released this way argue that they are being starved to death without the feeding tubes. But I don't buy that, especially after having watched Bamboo decide by some deep natural instinct that it was time for her, first, to stop eating even the treats she loved and, finally, to stop drinking while she waited patiently for what was to come, what was inevitable.
There used to be an advertising slogan: It's not nice to fool Mother Nature. If you believe in God and the promise of eternity, perhaps it is equally not nice to fool dying human bodies into a semblance of living when nature is poised to move them beyond the rim of this life. Nature or God, I mean, and absolutely never, of course, a manipulative Dr. Death.
I don't think my puppy was starving those last few days so much as simply stopping. Simply letting herself be folded into an immutable process. At least this is something to ponder in terms of the will of God overwhelming the hopes of man. I am awed and rather apprehensive and yet somehow comfortable with this conclusion.
A couple of months tops with the tubes and no other reasonable hope, I think I'll tell my kids.
Mary Margaret's words struck a cord with both her sons. Her wishes, neatly laid out in a dusty newspaper, were respected. Mary Margaret entered hospice and died peacefully 2 weeks later.
(Tough Questions on Life, Death and a Dog Named Bamboo originally appeared in the The Catholic New World on July 16, 1993. The article was reprinted with their permission.)
Postscript: The author of the essay (my mother) passed away after a week in hospice care and 7 weeks in hospitals following a stroke. She was a published writer for more than 60 years. For her 61st birthday, my brother and I decided to buy her a wrinkly little puppy to keep her company and bark at anyone who came to her house. It ended up being a terrific watchdog as well as my mom's best friend. The last days with her puppy were translated into the essay, which also helped guide me during my mother's final days. My hope is that she and Bamboo are enjoying the promise of eternity in each other's company.
Patrick Carberry
Her name was Mrs. Carberry, but her readers knew her as Mary Margaret. Two months earlier, she suffered a debilitating stroke that took away her vibrant life. Previously a prolific writer of advice columns and opinion pieces, the blockage of blood to her brain dammed the steady flow of wisdom to her innumerable fans. They never again would benefit from the words of this intelligent, feisty, and self‐proclaimed Cranky Catholic. Unfortunately, I would not know Mary Margaret for her words, but only her numbers: her vital signs, her urine output, her tube feed residuals.
Not able to communicate with us, we wondered did Mrs. Carberry want this tracheostomy that was placed? Did she want this peg tube that fed her continuously? And even if she would have initially agreed to them, would she still want them now? Especially given she was not improving and had little hope for a meaningful recovery.
We posed these difficult questions to her two sons. One son believed that his mother would want an end to these aggressive measures. The other son disagreed; he said his mother would want to make every effort to stay alive.
In the end, Mary Margaret's voice found its way to us and provided us with the answer. She did not tell us in the conventional manner via a lawyer or a living will. Her friends did not come forth and let us know about serious conversations during their afternoon lunches. Instead, Mary Margaret penned it to her audience of devoted readers in a newspaper column written 14 years earlier. Mary Margaret's poignant words were revealed to her doctors by her son who understood its undeniable significance.
Mary Margaret was an essayist in Chicago whose pieces filled many major newspapers and magazines. She had strong opinions on matters large and small, writing articles addressing topics ranging from her Catholic beliefs to gender‐based inequities in the workplace. One article in particular addressed sickness and death and provided her sons the answer they sought. Having witnessed illness strike two loved ones, it was only natural for Mary Margaret to write about it. Her very personal essay was entitled Tough Questions on Life, Death and a Dog Named Bamboo. The words, resurfacing years later, and now having direct meaning to her own life and death, may have been some of the most profound and prophetic words of her career:
Tough Questions on Life, Death and a Dog Named Bamboo
I sat with her that evening while she was dyingrubbing her back, smoothing her head, whispering that I loved her, trying to be of some small comfort as she snuggled closer, looking up with her mysteriously accepting, somehow understanding brown eyes.
Adjusting herself once more, she half rose, then toppled sideways and simply stopped breathing. She died as a lady ought to be able to diequietly, as easily as possible, in her own bed. In my bed actually. She was Bamboo, my Shar‐pei, and it is difficult to write this even several months later without tears starting to roll.
It was not just that last day, of course. For a bit more than a week, Bamboo had been giving signs of a serious problema heavy doggie cough indicating severe congestion and a firm, stubborn decision not to eat. The kitchen offered a parade of small bowls of her favorite people foods with which I hoped to restore her appetite when she determinedly ignored her regular dog food. She had her choice of cottage cheese, scrambled eggs, ground sirloin, cheddar chunks, ice cream, buttered rice and morea virtual buffet for ants.
After I set each dish out, she would go over to look and sniff admiringly, even wag her tail, but then rather reluctantly return to her favorite resting place, a small rug at the top of the stairs to the front door.
She would only drink a lot of water, bowl after bowl, in which she did also get her medicine, mashed and melted. Late on that last afternoon, however she stopped the drinking, too. I couldn't get her to sip even when I brought the water dish to her or offered the ice cubes she once loved to lick. In her own way, she was saying No.
Lately I have been reflecting again on the experience as a result of having heard some discussion about the death of a woman with whom I once shared friendly commuter chitchat as we trained together into the Loop.
Following a stroke, she had been unconscious, vegetative, tragically for almost as long as I had enjoyed the eight years of Bamboo's delightful companionship. Her husband, I learned, had ultimately gone to court and had been granted permission to remove the feeding tube and let nature take its course. A counteraction to prevent this was filed by some well‐intentioned people; but what I believe as good human and legal sense prevailed. So without the tube feeding, this nice long‐suffering woman finally slipped away to God.
People who oppose the dying being released this way argue that they are being starved to death without the feeding tubes. But I don't buy that, especially after having watched Bamboo decide by some deep natural instinct that it was time for her, first, to stop eating even the treats she loved and, finally, to stop drinking while she waited patiently for what was to come, what was inevitable.
There used to be an advertising slogan: It's not nice to fool Mother Nature. If you believe in God and the promise of eternity, perhaps it is equally not nice to fool dying human bodies into a semblance of living when nature is poised to move them beyond the rim of this life. Nature or God, I mean, and absolutely never, of course, a manipulative Dr. Death.
I don't think my puppy was starving those last few days so much as simply stopping. Simply letting herself be folded into an immutable process. At least this is something to ponder in terms of the will of God overwhelming the hopes of man. I am awed and rather apprehensive and yet somehow comfortable with this conclusion.
A couple of months tops with the tubes and no other reasonable hope, I think I'll tell my kids.
Mary Margaret's words struck a cord with both her sons. Her wishes, neatly laid out in a dusty newspaper, were respected. Mary Margaret entered hospice and died peacefully 2 weeks later.
(Tough Questions on Life, Death and a Dog Named Bamboo originally appeared in the The Catholic New World on July 16, 1993. The article was reprinted with their permission.)
Postscript: The author of the essay (my mother) passed away after a week in hospice care and 7 weeks in hospitals following a stroke. She was a published writer for more than 60 years. For her 61st birthday, my brother and I decided to buy her a wrinkly little puppy to keep her company and bark at anyone who came to her house. It ended up being a terrific watchdog as well as my mom's best friend. The last days with her puppy were translated into the essay, which also helped guide me during my mother's final days. My hope is that she and Bamboo are enjoying the promise of eternity in each other's company.
Patrick Carberry
Her name was Mrs. Carberry, but her readers knew her as Mary Margaret. Two months earlier, she suffered a debilitating stroke that took away her vibrant life. Previously a prolific writer of advice columns and opinion pieces, the blockage of blood to her brain dammed the steady flow of wisdom to her innumerable fans. They never again would benefit from the words of this intelligent, feisty, and self‐proclaimed Cranky Catholic. Unfortunately, I would not know Mary Margaret for her words, but only her numbers: her vital signs, her urine output, her tube feed residuals.
Not able to communicate with us, we wondered did Mrs. Carberry want this tracheostomy that was placed? Did she want this peg tube that fed her continuously? And even if she would have initially agreed to them, would she still want them now? Especially given she was not improving and had little hope for a meaningful recovery.
We posed these difficult questions to her two sons. One son believed that his mother would want an end to these aggressive measures. The other son disagreed; he said his mother would want to make every effort to stay alive.
In the end, Mary Margaret's voice found its way to us and provided us with the answer. She did not tell us in the conventional manner via a lawyer or a living will. Her friends did not come forth and let us know about serious conversations during their afternoon lunches. Instead, Mary Margaret penned it to her audience of devoted readers in a newspaper column written 14 years earlier. Mary Margaret's poignant words were revealed to her doctors by her son who understood its undeniable significance.
Mary Margaret was an essayist in Chicago whose pieces filled many major newspapers and magazines. She had strong opinions on matters large and small, writing articles addressing topics ranging from her Catholic beliefs to gender‐based inequities in the workplace. One article in particular addressed sickness and death and provided her sons the answer they sought. Having witnessed illness strike two loved ones, it was only natural for Mary Margaret to write about it. Her very personal essay was entitled Tough Questions on Life, Death and a Dog Named Bamboo. The words, resurfacing years later, and now having direct meaning to her own life and death, may have been some of the most profound and prophetic words of her career:
Tough Questions on Life, Death and a Dog Named Bamboo
I sat with her that evening while she was dyingrubbing her back, smoothing her head, whispering that I loved her, trying to be of some small comfort as she snuggled closer, looking up with her mysteriously accepting, somehow understanding brown eyes.
Adjusting herself once more, she half rose, then toppled sideways and simply stopped breathing. She died as a lady ought to be able to diequietly, as easily as possible, in her own bed. In my bed actually. She was Bamboo, my Shar‐pei, and it is difficult to write this even several months later without tears starting to roll.
It was not just that last day, of course. For a bit more than a week, Bamboo had been giving signs of a serious problema heavy doggie cough indicating severe congestion and a firm, stubborn decision not to eat. The kitchen offered a parade of small bowls of her favorite people foods with which I hoped to restore her appetite when she determinedly ignored her regular dog food. She had her choice of cottage cheese, scrambled eggs, ground sirloin, cheddar chunks, ice cream, buttered rice and morea virtual buffet for ants.
After I set each dish out, she would go over to look and sniff admiringly, even wag her tail, but then rather reluctantly return to her favorite resting place, a small rug at the top of the stairs to the front door.
She would only drink a lot of water, bowl after bowl, in which she did also get her medicine, mashed and melted. Late on that last afternoon, however she stopped the drinking, too. I couldn't get her to sip even when I brought the water dish to her or offered the ice cubes she once loved to lick. In her own way, she was saying No.
Lately I have been reflecting again on the experience as a result of having heard some discussion about the death of a woman with whom I once shared friendly commuter chitchat as we trained together into the Loop.
Following a stroke, she had been unconscious, vegetative, tragically for almost as long as I had enjoyed the eight years of Bamboo's delightful companionship. Her husband, I learned, had ultimately gone to court and had been granted permission to remove the feeding tube and let nature take its course. A counteraction to prevent this was filed by some well‐intentioned people; but what I believe as good human and legal sense prevailed. So without the tube feeding, this nice long‐suffering woman finally slipped away to God.
People who oppose the dying being released this way argue that they are being starved to death without the feeding tubes. But I don't buy that, especially after having watched Bamboo decide by some deep natural instinct that it was time for her, first, to stop eating even the treats she loved and, finally, to stop drinking while she waited patiently for what was to come, what was inevitable.
There used to be an advertising slogan: It's not nice to fool Mother Nature. If you believe in God and the promise of eternity, perhaps it is equally not nice to fool dying human bodies into a semblance of living when nature is poised to move them beyond the rim of this life. Nature or God, I mean, and absolutely never, of course, a manipulative Dr. Death.
I don't think my puppy was starving those last few days so much as simply stopping. Simply letting herself be folded into an immutable process. At least this is something to ponder in terms of the will of God overwhelming the hopes of man. I am awed and rather apprehensive and yet somehow comfortable with this conclusion.
A couple of months tops with the tubes and no other reasonable hope, I think I'll tell my kids.
Mary Margaret's words struck a cord with both her sons. Her wishes, neatly laid out in a dusty newspaper, were respected. Mary Margaret entered hospice and died peacefully 2 weeks later.
(Tough Questions on Life, Death and a Dog Named Bamboo originally appeared in the The Catholic New World on July 16, 1993. The article was reprinted with their permission.)
Postscript: The author of the essay (my mother) passed away after a week in hospice care and 7 weeks in hospitals following a stroke. She was a published writer for more than 60 years. For her 61st birthday, my brother and I decided to buy her a wrinkly little puppy to keep her company and bark at anyone who came to her house. It ended up being a terrific watchdog as well as my mom's best friend. The last days with her puppy were translated into the essay, which also helped guide me during my mother's final days. My hope is that she and Bamboo are enjoying the promise of eternity in each other's company.
Patrick Carberry
Surgical Comanagement
With the rapid advance of medicine to its present‐day status in which it evokes the aid of all the natural sciences, an individual is no more able to undertake the more intricate problems alone, without the aid and cooperation of colleagues having special training in each of the various clinical and laboratory branches, than he would be today to make an automobile alone.1
It is ironic that our specialty of hospital‐based medicine grew out of the soil of managed care and a renewed emphasis on generalism.2 Historical precedence clearly confirms the virtue of specialization and multidisciplinary care. Taken in this context, hospitalists have been comanagers from the very start, working with primary care physicians. The unprecedented growth of hospitalists in the United States has been accelerated by forces that pulled generalists out of the hospital and off the hospital wardsnamely the expensive inefficiency of trying to be in 2 places at 1 time. Faced with an expanding scope of practice and increasing outpatient volumes coupled with declining reimbursements, primary care physicians (PCPs) recognized the need to share their patients with inpatient comanagers.
Today, the surgeon is faced with many of the same pressures experienced by PCPs. Surgical productivity, efficiency, and quality are highly valued, yet require the surgeon to be in 2 places at 1 time. In the past, many surgeons in teaching hospitals relied on surgical residents to manage uncomplicated presurgical and postsurgical care and collaborated with internists for more difficult problems. Now, surgical residents are limited by work‐hour restrictions imposed by the Accreditation Council for Graduate Medical Education,3 reducing their ability to respond to patients outside the operating room. Perhaps more importantly, surgical patients today continue to increase in age and complexity, with a projected 50% rise in surgery‐related costs and a 100% rise in surgical complications in the next 2 decades.4 An experienced comanager of surgical patients that does not rely on PCPs or the surgical education system makes great practical and economic sense, and is a natural evolution of the hospitalist concept and skill set. Hospital medicine core competencies highlight perioperative medicine as a body of knowledge and practice germane to hospitalists. In fact, it specifically states that hospitalists should strive to engage in efforts to improve the efficiency and quality of care through innovative models, which may include comanagement of surgical patients in the perioperative period.5
CONSULTATION VERSUS COMANAGEMENT
Historically, in academic settings surgeons and medical practitioners have collaborated via the framework of consultation. If a surgeon needed assistance with uncontrolled diabetes or blood pressure, he or she called the internist to make recommendations on appropriate treatment. If the internist was faced with a potential surgical issue, he or she consulted the surgeon for their evaluation and opinion. In today's chaotic hospital environment, this collaborative framework has obvious inefficiencies. By definition, the consultation involves a formal request, which demands seamless communication that often does not exist. Next, the consultant reviews the chart, evaluates the patient, reviews pertinent clinical data, and provides an assessment with recommendations for management and care. How and whether these recommendations are enacted may be explicitly defined by the requesting service, but often it is not, and a delay in execution of recommendations potentially ensues. An observational cohort study showed that patients receiving medical consultation were no more likely to have tight glycemic control, perioperative beta‐blockers administration, or venous thromboembolism (VTE) prophylaxis; however, patients receiving consultation had a longer length of stay and higher costs of care.6 Comanagement represents a patient care referral, not consultation. A comanager is requested at the outset, but subsequently plays a much more active role, which may involve daily or twice daily visits, writing progress notes and orders, assessing and managing acute issues, and facilitating discharge planning and care transitions. Despite the ability to facilitate care, the basis for comanagement should be the same as for specialty consultation.
In contrast to academic settings, comanagement by PCPs and medical subspecialists occurs routinely in community hospitals. This model works best for patients with few problems who are followed closely by a single comanager, typically the PCP. However, complex patients with multiple comorbidities may decompensate without an attentive and experienced PCP, or wind up with numerous subspecialists making recommendations and writing orders in a disorganized fashion. The extreme of this situation is an unsystematic and inefficient management by committee, where medical specialists pick and choose an area of comanagement, without clear boundaries between the various team members. This approach is fraught with pitfalls in communication and may lead to conflicting recommendations or false assumptions among team members, further increasing patient morbidity.
In both academic and community settings, comanagement by a hospitalist offers advantages of consistent availability and proactive perioperative expertise, both in diagnosing and treating relevant problems and in recognizing the need for subspecialty involvement, thus improving efficiency of care. Although some health care systems may consider automatic patient care referrals to hospitalists for all surgical patients, this approach should be discouraged unless the patient population demands specialty involvement. Best practice would identify comorbid surgical patients during the outpatient preoperative process and then hardwire the patient care referral to the hospitalist upon surgical admission.
COMANAGEMENT MAKES SENSE
The multidisciplinary nature of comanagement can streamline individual patient care from the moment the decision for surgery is made. Preoperative assessment and management by the hospitalist can uncover risks from known conditions requiring optimization; identify new, undiagnosed conditions affecting the perioperative period; and initiate prophylactic and therapeutic regimens that reduce the chances for postoperative complications. Specific examples may include beta‐blockers in higher risk patients, anticoagulation management, and VTE prophylaxis.
The comanaging hospitalist ensures that these strategies are implemented, tailors them to the individual patient, and diagnoses and treats complications promptly when they occur. In addition, hospitalist comanagers can be more involved to facilitate patient transitions to posthospital care venues; this might involve communication with patients, families, case managers, and PCPs, among others. Ultimately, the investment of the comanaging hospitalist in the surgical patient is much greater in both scope and time. This may be expected to improve patient care efficiency, reduce length of stay, and may decrease overall complications. In addition, this investment is often recognized by the other important members of the care team, including nursing, case management, and patients and families, thus improving both patient and nursing satisfaction ratings.
AVAILABLE DATA ON THE BENEFITS OF COMANAGEMENT
Early studies on comanagement focused on orthopedic surgery and geriatric collaboration. Zuckerman et al.7 studied the effects of an interdisciplinary team approach to the hip fracture patient, entitled the Geriatric Hip Fracture Program (GHFP), in the mid‐1980s. They compared 431 patients admitted under the care of the GHFP for surgical repair of hip fracture between 1985 and 1988 with 60 historical controls at the same institution prior to the inception of the program. GHFP patients were evaluated by an orthopedic surgeon and a consulting internist or geriatrician. In addition to therapy service evaluations, each patient was screened by an ophthalmologist for visual impairment, a psychiatrist for preexisting cognitive dysfunction and depression, a social worker, and a case manager. GHFP patients had fewer postoperative complications, fewer intensive care unit transfers for acute medical issues, better ambulatory status and distance ambulated at discharge, and nonsignificant trends toward decreased length of stay and increased likelihood of return to home. A more recent prospective observational study of patients with hip fracture in Australia8 compared a 4‐year period of geriatric comanagement of 447 patients with hip fracture with 3 years of historical control patients (n = 504) prior to the institution of the comanagement service. Postoperative medical complications, mortality, and 6‐month readmission rates were significantly lower in the geriatric comanagement cohort. No differences in median length of hospital stay or in discharge destination were noted. The proportion of patients receiving anti‐osteoporotic therapy (calcium, vitamin D, and bisphosphonates) increased from 12% to 93% after the institution of comanagement. Also, the proportion of patients prescribed pharmacologic VTE prophylaxis increased from 63% to 94%, and symptomatic VTE events (deep vein thrombosis or PE) decreased from 4.6% to 1.3% after implementation. In another geriatrician comanagement study, Marcantonio et al.9 performed a randomized trial in patients with hip fracture comparing geriatric comanagement with a structured treatment care protocol to usual care. Although length of stay was unchanged and costs of care were not reported, geriatric comanagement significantly reduced the number and severity of episodes of delirium.
Macpherson et al.10 studied the effect of internist comanagement of 165 cardiothoracic surgery patients in the Minneaoplis Veteran's Affairs Medical Center in 1990. They found that, compared with the prior year, the implementation of internist comanagement was associated with hospital stays of 6 days shorter length, lower use of resources such as lab and radiology, and a trend toward decreased mortality. Huddleston et al.11 conducted a randomized controlled trial of 526 patients undergoing elective total hip or knee arthroplasty, comparing a comanagement hospitalist‐orthopedic team with standard orthopedic surgery care and internal medicine consultation as needed. Despite comparison to the standard of tightly managed care protocols in elective hip and knee arthroplasty, patients comanaged by hospitalists were more likely to be discharged without postoperative complications, and were ready for discharge half a day sooner when adjusting for skilled facility bed availability. No difference in mortality rates or total cost of care was noted between the 2 models. However, nurses and surgeons both strongly preferred the comanagement model, with providers reporting that care was prompt and coordinated, and there was an enhanced ease of providing care. In a second study, the authors from the same institution12 studied 466 patients over 65 years of age admitted for surgical repair of hip fracture. Patients in the comanagement group went to surgery faster, were discharged sooner after surgery, and had an overall lower length of stay. No differences were noted in inpatient mortality, 30‐day readmission rates, or complication rates. Delirium was diagnosed more often in the comanagement group, but a diagnosis of delirium was associated with an earlier discharge after surgery. This may reflect greater attention to the presence of delirium, better documentation, and more prompt treatment.
Preoperative testing centers staffed by anesthesiologists have been shown to positively impact surgical care.1315 However, there has been little study to specifically evaluate the role of medical comanagement in the preoperative setting. Jaffer et al.16 demonstrated a reduction in postoperative pulmonary complications in a mixed surgical population by utilizing a structured preoperative assessment and management program of hospitalists.
COMANAGEMENT SATISFACTION
Surgical comanagement has been reported to improve surgeon and nurse satisfaction ratings.11 Salerno et al.,17 in their study of consultation preferences of surgeons, internists and family physicians, confirmed that surgeons, especially orthopedic surgeons, favor the comanagement model more than the traditional consultation model. This is not surprising as surgeons in the comanagement model may be expected to spend more time in the operating room as opposed to the hospital floors, thus improving patient access to timely surgery and reducing cancellations and delays. Ultimately, the comanagement model may result in a competitive advantage over traditional care. Improved patient access and throughput may improve patient satisfaction with their surgical experience, which could lead to increased surgical referrals, both patient and PCP initiated. Satisfaction and positive learning experiences of surgical residents with this system of care may improve the likelihood of them joining such a practice, which will then foster the cultural evolution of comanagement. In addition, because of the increased scrutiny and potential financial ties (ie, pay for performance) to quality and safety issues, a comanagement model involving hospitalists is ideally poised to systematically account for these issues. Finally, because of nurse staffing shortages, care processes that promote workplace satisfaction and respect may promote nurse recruitment and retention, thus improving the competitive advantage even further.
CONCLUSION
Surgical comanagement has many distinct advantages for all parties involved, including the surgeon, hospitalist, house staff, nurses, case manager, patient and family, and the health care system overall. As hospitalists have been comanaging medical inpatients with primary care physicians for years, the concept of surgical comanagement is truly a natural evolution of the scope of hospitalist practice.
- .“..To Act as a Unit”: The Story of the Cleveland Clinic.Cleveland, OH:Cleveland Clinic Press;1996:17.
- ,,.Inpatient medicine and the evolution of the hospitalist.Clev Clin J Med.1998;68(11):192–200.
- ,,.New requirements for resident duty hours.JAMA.2002;288(9):1112–1114.
- ,.Why perioperative medicine matters more than ever.Clev Clin J Med.2006:73( ); suppl 1 2006:S1.
- ,,,,.Perioperative Medicine. In: The core competencies in hospital hedicine: a framework for curriculum development.J Hosp Med (Online).2006;1(Suppl 1):30–1.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Intern Med.2007;167(21):2338–2344.
- ,,,.Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program.Clin Orthopaed Relat Res.1992;274:213–225.
- ,,,,,.Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare.J Orthopaed Trauma.2006;20(3):172–178; discussion 9–80.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,,.An internist joins the surgery service: does comanagement make a difference?J Gen Intern Med.1994;9(8):440–444.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165(7):796–801.
- ,,,,,.Value of Preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105(6):1254–1259; discussion 6A.
- ,,,,.Preoperative clinic visits reduce operating room cancellations and delays.Anesthesiology.2005;103(4):855–859.
- .Development and effectiveness of an anesthesia preoperative evaluation clinic in a teaching hospital.Anesthesiology.1996;85(1):196–206.
- ,,, et al.Postoperative pulmonary complications: experience with an outpatient pre‐operative assessment program.J Clin Outcomes Manage.2005;12(10):505–510.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167(3):271–275.
With the rapid advance of medicine to its present‐day status in which it evokes the aid of all the natural sciences, an individual is no more able to undertake the more intricate problems alone, without the aid and cooperation of colleagues having special training in each of the various clinical and laboratory branches, than he would be today to make an automobile alone.1
It is ironic that our specialty of hospital‐based medicine grew out of the soil of managed care and a renewed emphasis on generalism.2 Historical precedence clearly confirms the virtue of specialization and multidisciplinary care. Taken in this context, hospitalists have been comanagers from the very start, working with primary care physicians. The unprecedented growth of hospitalists in the United States has been accelerated by forces that pulled generalists out of the hospital and off the hospital wardsnamely the expensive inefficiency of trying to be in 2 places at 1 time. Faced with an expanding scope of practice and increasing outpatient volumes coupled with declining reimbursements, primary care physicians (PCPs) recognized the need to share their patients with inpatient comanagers.
Today, the surgeon is faced with many of the same pressures experienced by PCPs. Surgical productivity, efficiency, and quality are highly valued, yet require the surgeon to be in 2 places at 1 time. In the past, many surgeons in teaching hospitals relied on surgical residents to manage uncomplicated presurgical and postsurgical care and collaborated with internists for more difficult problems. Now, surgical residents are limited by work‐hour restrictions imposed by the Accreditation Council for Graduate Medical Education,3 reducing their ability to respond to patients outside the operating room. Perhaps more importantly, surgical patients today continue to increase in age and complexity, with a projected 50% rise in surgery‐related costs and a 100% rise in surgical complications in the next 2 decades.4 An experienced comanager of surgical patients that does not rely on PCPs or the surgical education system makes great practical and economic sense, and is a natural evolution of the hospitalist concept and skill set. Hospital medicine core competencies highlight perioperative medicine as a body of knowledge and practice germane to hospitalists. In fact, it specifically states that hospitalists should strive to engage in efforts to improve the efficiency and quality of care through innovative models, which may include comanagement of surgical patients in the perioperative period.5
CONSULTATION VERSUS COMANAGEMENT
Historically, in academic settings surgeons and medical practitioners have collaborated via the framework of consultation. If a surgeon needed assistance with uncontrolled diabetes or blood pressure, he or she called the internist to make recommendations on appropriate treatment. If the internist was faced with a potential surgical issue, he or she consulted the surgeon for their evaluation and opinion. In today's chaotic hospital environment, this collaborative framework has obvious inefficiencies. By definition, the consultation involves a formal request, which demands seamless communication that often does not exist. Next, the consultant reviews the chart, evaluates the patient, reviews pertinent clinical data, and provides an assessment with recommendations for management and care. How and whether these recommendations are enacted may be explicitly defined by the requesting service, but often it is not, and a delay in execution of recommendations potentially ensues. An observational cohort study showed that patients receiving medical consultation were no more likely to have tight glycemic control, perioperative beta‐blockers administration, or venous thromboembolism (VTE) prophylaxis; however, patients receiving consultation had a longer length of stay and higher costs of care.6 Comanagement represents a patient care referral, not consultation. A comanager is requested at the outset, but subsequently plays a much more active role, which may involve daily or twice daily visits, writing progress notes and orders, assessing and managing acute issues, and facilitating discharge planning and care transitions. Despite the ability to facilitate care, the basis for comanagement should be the same as for specialty consultation.
In contrast to academic settings, comanagement by PCPs and medical subspecialists occurs routinely in community hospitals. This model works best for patients with few problems who are followed closely by a single comanager, typically the PCP. However, complex patients with multiple comorbidities may decompensate without an attentive and experienced PCP, or wind up with numerous subspecialists making recommendations and writing orders in a disorganized fashion. The extreme of this situation is an unsystematic and inefficient management by committee, where medical specialists pick and choose an area of comanagement, without clear boundaries between the various team members. This approach is fraught with pitfalls in communication and may lead to conflicting recommendations or false assumptions among team members, further increasing patient morbidity.
In both academic and community settings, comanagement by a hospitalist offers advantages of consistent availability and proactive perioperative expertise, both in diagnosing and treating relevant problems and in recognizing the need for subspecialty involvement, thus improving efficiency of care. Although some health care systems may consider automatic patient care referrals to hospitalists for all surgical patients, this approach should be discouraged unless the patient population demands specialty involvement. Best practice would identify comorbid surgical patients during the outpatient preoperative process and then hardwire the patient care referral to the hospitalist upon surgical admission.
COMANAGEMENT MAKES SENSE
The multidisciplinary nature of comanagement can streamline individual patient care from the moment the decision for surgery is made. Preoperative assessment and management by the hospitalist can uncover risks from known conditions requiring optimization; identify new, undiagnosed conditions affecting the perioperative period; and initiate prophylactic and therapeutic regimens that reduce the chances for postoperative complications. Specific examples may include beta‐blockers in higher risk patients, anticoagulation management, and VTE prophylaxis.
The comanaging hospitalist ensures that these strategies are implemented, tailors them to the individual patient, and diagnoses and treats complications promptly when they occur. In addition, hospitalist comanagers can be more involved to facilitate patient transitions to posthospital care venues; this might involve communication with patients, families, case managers, and PCPs, among others. Ultimately, the investment of the comanaging hospitalist in the surgical patient is much greater in both scope and time. This may be expected to improve patient care efficiency, reduce length of stay, and may decrease overall complications. In addition, this investment is often recognized by the other important members of the care team, including nursing, case management, and patients and families, thus improving both patient and nursing satisfaction ratings.
AVAILABLE DATA ON THE BENEFITS OF COMANAGEMENT
Early studies on comanagement focused on orthopedic surgery and geriatric collaboration. Zuckerman et al.7 studied the effects of an interdisciplinary team approach to the hip fracture patient, entitled the Geriatric Hip Fracture Program (GHFP), in the mid‐1980s. They compared 431 patients admitted under the care of the GHFP for surgical repair of hip fracture between 1985 and 1988 with 60 historical controls at the same institution prior to the inception of the program. GHFP patients were evaluated by an orthopedic surgeon and a consulting internist or geriatrician. In addition to therapy service evaluations, each patient was screened by an ophthalmologist for visual impairment, a psychiatrist for preexisting cognitive dysfunction and depression, a social worker, and a case manager. GHFP patients had fewer postoperative complications, fewer intensive care unit transfers for acute medical issues, better ambulatory status and distance ambulated at discharge, and nonsignificant trends toward decreased length of stay and increased likelihood of return to home. A more recent prospective observational study of patients with hip fracture in Australia8 compared a 4‐year period of geriatric comanagement of 447 patients with hip fracture with 3 years of historical control patients (n = 504) prior to the institution of the comanagement service. Postoperative medical complications, mortality, and 6‐month readmission rates were significantly lower in the geriatric comanagement cohort. No differences in median length of hospital stay or in discharge destination were noted. The proportion of patients receiving anti‐osteoporotic therapy (calcium, vitamin D, and bisphosphonates) increased from 12% to 93% after the institution of comanagement. Also, the proportion of patients prescribed pharmacologic VTE prophylaxis increased from 63% to 94%, and symptomatic VTE events (deep vein thrombosis or PE) decreased from 4.6% to 1.3% after implementation. In another geriatrician comanagement study, Marcantonio et al.9 performed a randomized trial in patients with hip fracture comparing geriatric comanagement with a structured treatment care protocol to usual care. Although length of stay was unchanged and costs of care were not reported, geriatric comanagement significantly reduced the number and severity of episodes of delirium.
Macpherson et al.10 studied the effect of internist comanagement of 165 cardiothoracic surgery patients in the Minneaoplis Veteran's Affairs Medical Center in 1990. They found that, compared with the prior year, the implementation of internist comanagement was associated with hospital stays of 6 days shorter length, lower use of resources such as lab and radiology, and a trend toward decreased mortality. Huddleston et al.11 conducted a randomized controlled trial of 526 patients undergoing elective total hip or knee arthroplasty, comparing a comanagement hospitalist‐orthopedic team with standard orthopedic surgery care and internal medicine consultation as needed. Despite comparison to the standard of tightly managed care protocols in elective hip and knee arthroplasty, patients comanaged by hospitalists were more likely to be discharged without postoperative complications, and were ready for discharge half a day sooner when adjusting for skilled facility bed availability. No difference in mortality rates or total cost of care was noted between the 2 models. However, nurses and surgeons both strongly preferred the comanagement model, with providers reporting that care was prompt and coordinated, and there was an enhanced ease of providing care. In a second study, the authors from the same institution12 studied 466 patients over 65 years of age admitted for surgical repair of hip fracture. Patients in the comanagement group went to surgery faster, were discharged sooner after surgery, and had an overall lower length of stay. No differences were noted in inpatient mortality, 30‐day readmission rates, or complication rates. Delirium was diagnosed more often in the comanagement group, but a diagnosis of delirium was associated with an earlier discharge after surgery. This may reflect greater attention to the presence of delirium, better documentation, and more prompt treatment.
Preoperative testing centers staffed by anesthesiologists have been shown to positively impact surgical care.1315 However, there has been little study to specifically evaluate the role of medical comanagement in the preoperative setting. Jaffer et al.16 demonstrated a reduction in postoperative pulmonary complications in a mixed surgical population by utilizing a structured preoperative assessment and management program of hospitalists.
COMANAGEMENT SATISFACTION
Surgical comanagement has been reported to improve surgeon and nurse satisfaction ratings.11 Salerno et al.,17 in their study of consultation preferences of surgeons, internists and family physicians, confirmed that surgeons, especially orthopedic surgeons, favor the comanagement model more than the traditional consultation model. This is not surprising as surgeons in the comanagement model may be expected to spend more time in the operating room as opposed to the hospital floors, thus improving patient access to timely surgery and reducing cancellations and delays. Ultimately, the comanagement model may result in a competitive advantage over traditional care. Improved patient access and throughput may improve patient satisfaction with their surgical experience, which could lead to increased surgical referrals, both patient and PCP initiated. Satisfaction and positive learning experiences of surgical residents with this system of care may improve the likelihood of them joining such a practice, which will then foster the cultural evolution of comanagement. In addition, because of the increased scrutiny and potential financial ties (ie, pay for performance) to quality and safety issues, a comanagement model involving hospitalists is ideally poised to systematically account for these issues. Finally, because of nurse staffing shortages, care processes that promote workplace satisfaction and respect may promote nurse recruitment and retention, thus improving the competitive advantage even further.
CONCLUSION
Surgical comanagement has many distinct advantages for all parties involved, including the surgeon, hospitalist, house staff, nurses, case manager, patient and family, and the health care system overall. As hospitalists have been comanaging medical inpatients with primary care physicians for years, the concept of surgical comanagement is truly a natural evolution of the scope of hospitalist practice.
With the rapid advance of medicine to its present‐day status in which it evokes the aid of all the natural sciences, an individual is no more able to undertake the more intricate problems alone, without the aid and cooperation of colleagues having special training in each of the various clinical and laboratory branches, than he would be today to make an automobile alone.1
It is ironic that our specialty of hospital‐based medicine grew out of the soil of managed care and a renewed emphasis on generalism.2 Historical precedence clearly confirms the virtue of specialization and multidisciplinary care. Taken in this context, hospitalists have been comanagers from the very start, working with primary care physicians. The unprecedented growth of hospitalists in the United States has been accelerated by forces that pulled generalists out of the hospital and off the hospital wardsnamely the expensive inefficiency of trying to be in 2 places at 1 time. Faced with an expanding scope of practice and increasing outpatient volumes coupled with declining reimbursements, primary care physicians (PCPs) recognized the need to share their patients with inpatient comanagers.
Today, the surgeon is faced with many of the same pressures experienced by PCPs. Surgical productivity, efficiency, and quality are highly valued, yet require the surgeon to be in 2 places at 1 time. In the past, many surgeons in teaching hospitals relied on surgical residents to manage uncomplicated presurgical and postsurgical care and collaborated with internists for more difficult problems. Now, surgical residents are limited by work‐hour restrictions imposed by the Accreditation Council for Graduate Medical Education,3 reducing their ability to respond to patients outside the operating room. Perhaps more importantly, surgical patients today continue to increase in age and complexity, with a projected 50% rise in surgery‐related costs and a 100% rise in surgical complications in the next 2 decades.4 An experienced comanager of surgical patients that does not rely on PCPs or the surgical education system makes great practical and economic sense, and is a natural evolution of the hospitalist concept and skill set. Hospital medicine core competencies highlight perioperative medicine as a body of knowledge and practice germane to hospitalists. In fact, it specifically states that hospitalists should strive to engage in efforts to improve the efficiency and quality of care through innovative models, which may include comanagement of surgical patients in the perioperative period.5
CONSULTATION VERSUS COMANAGEMENT
Historically, in academic settings surgeons and medical practitioners have collaborated via the framework of consultation. If a surgeon needed assistance with uncontrolled diabetes or blood pressure, he or she called the internist to make recommendations on appropriate treatment. If the internist was faced with a potential surgical issue, he or she consulted the surgeon for their evaluation and opinion. In today's chaotic hospital environment, this collaborative framework has obvious inefficiencies. By definition, the consultation involves a formal request, which demands seamless communication that often does not exist. Next, the consultant reviews the chart, evaluates the patient, reviews pertinent clinical data, and provides an assessment with recommendations for management and care. How and whether these recommendations are enacted may be explicitly defined by the requesting service, but often it is not, and a delay in execution of recommendations potentially ensues. An observational cohort study showed that patients receiving medical consultation were no more likely to have tight glycemic control, perioperative beta‐blockers administration, or venous thromboembolism (VTE) prophylaxis; however, patients receiving consultation had a longer length of stay and higher costs of care.6 Comanagement represents a patient care referral, not consultation. A comanager is requested at the outset, but subsequently plays a much more active role, which may involve daily or twice daily visits, writing progress notes and orders, assessing and managing acute issues, and facilitating discharge planning and care transitions. Despite the ability to facilitate care, the basis for comanagement should be the same as for specialty consultation.
In contrast to academic settings, comanagement by PCPs and medical subspecialists occurs routinely in community hospitals. This model works best for patients with few problems who are followed closely by a single comanager, typically the PCP. However, complex patients with multiple comorbidities may decompensate without an attentive and experienced PCP, or wind up with numerous subspecialists making recommendations and writing orders in a disorganized fashion. The extreme of this situation is an unsystematic and inefficient management by committee, where medical specialists pick and choose an area of comanagement, without clear boundaries between the various team members. This approach is fraught with pitfalls in communication and may lead to conflicting recommendations or false assumptions among team members, further increasing patient morbidity.
In both academic and community settings, comanagement by a hospitalist offers advantages of consistent availability and proactive perioperative expertise, both in diagnosing and treating relevant problems and in recognizing the need for subspecialty involvement, thus improving efficiency of care. Although some health care systems may consider automatic patient care referrals to hospitalists for all surgical patients, this approach should be discouraged unless the patient population demands specialty involvement. Best practice would identify comorbid surgical patients during the outpatient preoperative process and then hardwire the patient care referral to the hospitalist upon surgical admission.
COMANAGEMENT MAKES SENSE
The multidisciplinary nature of comanagement can streamline individual patient care from the moment the decision for surgery is made. Preoperative assessment and management by the hospitalist can uncover risks from known conditions requiring optimization; identify new, undiagnosed conditions affecting the perioperative period; and initiate prophylactic and therapeutic regimens that reduce the chances for postoperative complications. Specific examples may include beta‐blockers in higher risk patients, anticoagulation management, and VTE prophylaxis.
The comanaging hospitalist ensures that these strategies are implemented, tailors them to the individual patient, and diagnoses and treats complications promptly when they occur. In addition, hospitalist comanagers can be more involved to facilitate patient transitions to posthospital care venues; this might involve communication with patients, families, case managers, and PCPs, among others. Ultimately, the investment of the comanaging hospitalist in the surgical patient is much greater in both scope and time. This may be expected to improve patient care efficiency, reduce length of stay, and may decrease overall complications. In addition, this investment is often recognized by the other important members of the care team, including nursing, case management, and patients and families, thus improving both patient and nursing satisfaction ratings.
AVAILABLE DATA ON THE BENEFITS OF COMANAGEMENT
Early studies on comanagement focused on orthopedic surgery and geriatric collaboration. Zuckerman et al.7 studied the effects of an interdisciplinary team approach to the hip fracture patient, entitled the Geriatric Hip Fracture Program (GHFP), in the mid‐1980s. They compared 431 patients admitted under the care of the GHFP for surgical repair of hip fracture between 1985 and 1988 with 60 historical controls at the same institution prior to the inception of the program. GHFP patients were evaluated by an orthopedic surgeon and a consulting internist or geriatrician. In addition to therapy service evaluations, each patient was screened by an ophthalmologist for visual impairment, a psychiatrist for preexisting cognitive dysfunction and depression, a social worker, and a case manager. GHFP patients had fewer postoperative complications, fewer intensive care unit transfers for acute medical issues, better ambulatory status and distance ambulated at discharge, and nonsignificant trends toward decreased length of stay and increased likelihood of return to home. A more recent prospective observational study of patients with hip fracture in Australia8 compared a 4‐year period of geriatric comanagement of 447 patients with hip fracture with 3 years of historical control patients (n = 504) prior to the institution of the comanagement service. Postoperative medical complications, mortality, and 6‐month readmission rates were significantly lower in the geriatric comanagement cohort. No differences in median length of hospital stay or in discharge destination were noted. The proportion of patients receiving anti‐osteoporotic therapy (calcium, vitamin D, and bisphosphonates) increased from 12% to 93% after the institution of comanagement. Also, the proportion of patients prescribed pharmacologic VTE prophylaxis increased from 63% to 94%, and symptomatic VTE events (deep vein thrombosis or PE) decreased from 4.6% to 1.3% after implementation. In another geriatrician comanagement study, Marcantonio et al.9 performed a randomized trial in patients with hip fracture comparing geriatric comanagement with a structured treatment care protocol to usual care. Although length of stay was unchanged and costs of care were not reported, geriatric comanagement significantly reduced the number and severity of episodes of delirium.
Macpherson et al.10 studied the effect of internist comanagement of 165 cardiothoracic surgery patients in the Minneaoplis Veteran's Affairs Medical Center in 1990. They found that, compared with the prior year, the implementation of internist comanagement was associated with hospital stays of 6 days shorter length, lower use of resources such as lab and radiology, and a trend toward decreased mortality. Huddleston et al.11 conducted a randomized controlled trial of 526 patients undergoing elective total hip or knee arthroplasty, comparing a comanagement hospitalist‐orthopedic team with standard orthopedic surgery care and internal medicine consultation as needed. Despite comparison to the standard of tightly managed care protocols in elective hip and knee arthroplasty, patients comanaged by hospitalists were more likely to be discharged without postoperative complications, and were ready for discharge half a day sooner when adjusting for skilled facility bed availability. No difference in mortality rates or total cost of care was noted between the 2 models. However, nurses and surgeons both strongly preferred the comanagement model, with providers reporting that care was prompt and coordinated, and there was an enhanced ease of providing care. In a second study, the authors from the same institution12 studied 466 patients over 65 years of age admitted for surgical repair of hip fracture. Patients in the comanagement group went to surgery faster, were discharged sooner after surgery, and had an overall lower length of stay. No differences were noted in inpatient mortality, 30‐day readmission rates, or complication rates. Delirium was diagnosed more often in the comanagement group, but a diagnosis of delirium was associated with an earlier discharge after surgery. This may reflect greater attention to the presence of delirium, better documentation, and more prompt treatment.
Preoperative testing centers staffed by anesthesiologists have been shown to positively impact surgical care.1315 However, there has been little study to specifically evaluate the role of medical comanagement in the preoperative setting. Jaffer et al.16 demonstrated a reduction in postoperative pulmonary complications in a mixed surgical population by utilizing a structured preoperative assessment and management program of hospitalists.
COMANAGEMENT SATISFACTION
Surgical comanagement has been reported to improve surgeon and nurse satisfaction ratings.11 Salerno et al.,17 in their study of consultation preferences of surgeons, internists and family physicians, confirmed that surgeons, especially orthopedic surgeons, favor the comanagement model more than the traditional consultation model. This is not surprising as surgeons in the comanagement model may be expected to spend more time in the operating room as opposed to the hospital floors, thus improving patient access to timely surgery and reducing cancellations and delays. Ultimately, the comanagement model may result in a competitive advantage over traditional care. Improved patient access and throughput may improve patient satisfaction with their surgical experience, which could lead to increased surgical referrals, both patient and PCP initiated. Satisfaction and positive learning experiences of surgical residents with this system of care may improve the likelihood of them joining such a practice, which will then foster the cultural evolution of comanagement. In addition, because of the increased scrutiny and potential financial ties (ie, pay for performance) to quality and safety issues, a comanagement model involving hospitalists is ideally poised to systematically account for these issues. Finally, because of nurse staffing shortages, care processes that promote workplace satisfaction and respect may promote nurse recruitment and retention, thus improving the competitive advantage even further.
CONCLUSION
Surgical comanagement has many distinct advantages for all parties involved, including the surgeon, hospitalist, house staff, nurses, case manager, patient and family, and the health care system overall. As hospitalists have been comanaging medical inpatients with primary care physicians for years, the concept of surgical comanagement is truly a natural evolution of the scope of hospitalist practice.
- .“..To Act as a Unit”: The Story of the Cleveland Clinic.Cleveland, OH:Cleveland Clinic Press;1996:17.
- ,,.Inpatient medicine and the evolution of the hospitalist.Clev Clin J Med.1998;68(11):192–200.
- ,,.New requirements for resident duty hours.JAMA.2002;288(9):1112–1114.
- ,.Why perioperative medicine matters more than ever.Clev Clin J Med.2006:73( ); suppl 1 2006:S1.
- ,,,,.Perioperative Medicine. In: The core competencies in hospital hedicine: a framework for curriculum development.J Hosp Med (Online).2006;1(Suppl 1):30–1.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Intern Med.2007;167(21):2338–2344.
- ,,,.Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program.Clin Orthopaed Relat Res.1992;274:213–225.
- ,,,,,.Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare.J Orthopaed Trauma.2006;20(3):172–178; discussion 9–80.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,,.An internist joins the surgery service: does comanagement make a difference?J Gen Intern Med.1994;9(8):440–444.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165(7):796–801.
- ,,,,,.Value of Preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105(6):1254–1259; discussion 6A.
- ,,,,.Preoperative clinic visits reduce operating room cancellations and delays.Anesthesiology.2005;103(4):855–859.
- .Development and effectiveness of an anesthesia preoperative evaluation clinic in a teaching hospital.Anesthesiology.1996;85(1):196–206.
- ,,, et al.Postoperative pulmonary complications: experience with an outpatient pre‐operative assessment program.J Clin Outcomes Manage.2005;12(10):505–510.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167(3):271–275.
- .“..To Act as a Unit”: The Story of the Cleveland Clinic.Cleveland, OH:Cleveland Clinic Press;1996:17.
- ,,.Inpatient medicine and the evolution of the hospitalist.Clev Clin J Med.1998;68(11):192–200.
- ,,.New requirements for resident duty hours.JAMA.2002;288(9):1112–1114.
- ,.Why perioperative medicine matters more than ever.Clev Clin J Med.2006:73( ); suppl 1 2006:S1.
- ,,,,.Perioperative Medicine. In: The core competencies in hospital hedicine: a framework for curriculum development.J Hosp Med (Online).2006;1(Suppl 1):30–1.
- ,,,,,.Opportunity missed: medical consultation, resource use, and quality of care of patients undergoing major surgery.Arch Intern Med.2007;167(21):2338–2344.
- ,,,.Hip fractures in geriatric patients. Results of an interdisciplinary hospital care program.Clin Orthopaed Relat Res.1992;274:213–225.
- ,,,,,.Outcomes for older patients with hip fractures: the impact of orthopedic and geriatric medicine cocare.J Orthopaed Trauma.2006;20(3):172–178; discussion 9–80.
- ,,,.Reducing delirium after hip fracture: a randomized trial.J Am Geriatr Soc.2001;49(5):516–522.
- ,,,,.An internist joins the surgery service: does comanagement make a difference?J Gen Intern Med.1994;9(8):440–444.
- ,,, et al.Medical and surgical comanagement after elective hip and knee arthroplasty: a randomized, controlled trial.Ann Intern Med.2004;141(1):28–38.
- ,,, et al.Effects of a hospitalist model on elderly patients with hip fracture.Arch Intern Med.2005;165(7):796–801.
- ,,,,,.Value of Preoperative clinic visits in identifying issues with potential impact on operating room efficiency.Anesthesiology.2006;105(6):1254–1259; discussion 6A.
- ,,,,.Preoperative clinic visits reduce operating room cancellations and delays.Anesthesiology.2005;103(4):855–859.
- .Development and effectiveness of an anesthesia preoperative evaluation clinic in a teaching hospital.Anesthesiology.1996;85(1):196–206.
- ,,, et al.Postoperative pulmonary complications: experience with an outpatient pre‐operative assessment program.J Clin Outcomes Manage.2005;12(10):505–510.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167(3):271–275.
Biochemical markers of bone turnover: Useful but underused
Biochemical markers of bone turnover are commonly used as tests in the management of bone disorders, as explained very elegantly by Drs. Singer and Eyre in this issue of the Journal.1
These tests assess the activity of osteoblastic cells or osteoclastic cells in a variety of bone diseases. Such tests do not establish the diagnosis of a disease, but rather they reflect the activity of the skeleton. Because the activity of osteoblasts and the activity of osteoclasts are chemically coupled, markers of blastic and clastic activity move in the same direction. In states of high bone metabolism or turnover, marker levels are high, predicting bone loss and fracture risk. Therapies that slow down bone metabolism make these levels decrease; anabolic drugs that stimulate bone growth do the opposite.
The utility of these markers in general practice is not well appreciated. In part this is because the results can vary if the tests are not appropriately done, causing frustration for some clinicians, who erroneously conclude that these markers lack utility.
WILL INSURANCE PAY FOR TESTING?
In addition, these tests are a source of confrontation with third-party payers who refuse to pay for them, even though they are approved by Medicare and have appropriate Current Procedural Terminology codes assigned to them. The reasons cited for denying payment are that the tests are not diagnostic, that they do not predict risk, and that they are not useful in patient management.
Wrong on all counts! First of all, these markers were never meant to diagnose a specific bone disease. They reflect high bone activity or turnover and potential bone loss, and high levels indicate that further assessment is needed. (In much the same way, an elevated prostate-specific antigen level may or may not mean the patient has prostate cancer, but it does mean further assessment is needed.)
Second, these tests do address fracture risk, either when used alone or when combined with bone densitometry measurements. A high level of a turnover marker indicates a risk of fracture similar to that of a T score lower than −2.5, with an odds ratio in the range of 2.4 to 2.8. 2 Moreover, if a patient has a low T score and a high marker level, his or her risk is even higher, with an odds ratio of 4.1.
Third, the argument about the tests’ lack of ability to help in patient management is completely untrue, as shown by information reviewed by Drs. Singer and Eyre, 1 and by other data recently published. 3 These tests can indicate whether bone physiology is responding to antiresorptive and anabolic drug therapy: marker activity should decline with antiresorptive drugs and increase with anabolic agents.
And this occurs months to years before bone densitometry even reflects a change! The failure of test values to respond appropriately should prompt physicians to find out why. Is the patient not taking the medicine appropriately? Or more worrisome, is he or she not taking it at all?
AN ADDED BENEFIT: BETTER ADHERENCE
The latter point brings up a common problem seen in practice—lack of adherence to drug therapy. Studies have repeatedly shown that only 50% to 60% of osteoporotic patients actually continue taking their oral medicine for a year or so. 4, 5 The reasons are unclear but may include cost, side effects, inconvenience in administration, and lack of any sign that the drug is doing anything. Bone densitometry may not always show changes that encourage patients to continue using expensive medicines.
Bone markers may be a solution to this dilemma. Changes in a bone marker help clinicians know that the patient is properly using therapy. 6 Moreover, these changes tell the patient that treatment is working. In my experience, relaying this type of information to the patient encourages adherence. Studies have indicated that markers do indeed help patients stay adherent to therapy and avoid fractures. 7, 8 Hence, these markers can indicate the risk of fracture and are useful in managing patients and promoting compliance.
It is unclear when third-party carriers will begin reading the appropriate literature to confirm these points, but practitioners need to recognize that there is a valid reason for using these tests.
- Singer FR, Eyre DR. Using biochemical markers of bone turnover in clinical practice. Cleve Clin J Med. 2008; 75:739–750.
- Johnell O, Odén A, De Laet C, Garnero P, Delmas PD, Kanis JA. Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporos Int. 2002; 13:523–526.
- Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan JCommittee of Scientific Advisors of the International Osteoporosis Foundation. The use of biochemical markers of bone turnover in osteoporosis. Osteoporos Int 2000; 11(suppl 6):S2–S17.
- Ivaska KK, Lenora J, Gerdhem P, Akesson K, Väänänen HK, Obrant KJ. Serial assessment of serum bone metabolism markers identifies women with the highest rate of bone loss and osteoporosis risk. J Clin Endocrinol Metab. 2008; 93:2622–2632.
- Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008; 28:437–443.
- Eastell R, Krege JH, Chen P, Glass EV, Reginster JY. Development of an algorithm for using PINP to monitor treatment of patients with teriparatide. Curr Med Res Opin. 2006; 22:61–66.
- Briesacher BA, Andrade SE, Yood RA, Kahler KH. Consequences of poor compliance with bisphosphonates. Bone. 2007; 41:882–887.
- Delmas PD, Vrijens B, Eastell R, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007; 92:1296–1304.
Biochemical markers of bone turnover are commonly used as tests in the management of bone disorders, as explained very elegantly by Drs. Singer and Eyre in this issue of the Journal.1
These tests assess the activity of osteoblastic cells or osteoclastic cells in a variety of bone diseases. Such tests do not establish the diagnosis of a disease, but rather they reflect the activity of the skeleton. Because the activity of osteoblasts and the activity of osteoclasts are chemically coupled, markers of blastic and clastic activity move in the same direction. In states of high bone metabolism or turnover, marker levels are high, predicting bone loss and fracture risk. Therapies that slow down bone metabolism make these levels decrease; anabolic drugs that stimulate bone growth do the opposite.
The utility of these markers in general practice is not well appreciated. In part this is because the results can vary if the tests are not appropriately done, causing frustration for some clinicians, who erroneously conclude that these markers lack utility.
WILL INSURANCE PAY FOR TESTING?
In addition, these tests are a source of confrontation with third-party payers who refuse to pay for them, even though they are approved by Medicare and have appropriate Current Procedural Terminology codes assigned to them. The reasons cited for denying payment are that the tests are not diagnostic, that they do not predict risk, and that they are not useful in patient management.
Wrong on all counts! First of all, these markers were never meant to diagnose a specific bone disease. They reflect high bone activity or turnover and potential bone loss, and high levels indicate that further assessment is needed. (In much the same way, an elevated prostate-specific antigen level may or may not mean the patient has prostate cancer, but it does mean further assessment is needed.)
Second, these tests do address fracture risk, either when used alone or when combined with bone densitometry measurements. A high level of a turnover marker indicates a risk of fracture similar to that of a T score lower than −2.5, with an odds ratio in the range of 2.4 to 2.8. 2 Moreover, if a patient has a low T score and a high marker level, his or her risk is even higher, with an odds ratio of 4.1.
Third, the argument about the tests’ lack of ability to help in patient management is completely untrue, as shown by information reviewed by Drs. Singer and Eyre, 1 and by other data recently published. 3 These tests can indicate whether bone physiology is responding to antiresorptive and anabolic drug therapy: marker activity should decline with antiresorptive drugs and increase with anabolic agents.
And this occurs months to years before bone densitometry even reflects a change! The failure of test values to respond appropriately should prompt physicians to find out why. Is the patient not taking the medicine appropriately? Or more worrisome, is he or she not taking it at all?
AN ADDED BENEFIT: BETTER ADHERENCE
The latter point brings up a common problem seen in practice—lack of adherence to drug therapy. Studies have repeatedly shown that only 50% to 60% of osteoporotic patients actually continue taking their oral medicine for a year or so. 4, 5 The reasons are unclear but may include cost, side effects, inconvenience in administration, and lack of any sign that the drug is doing anything. Bone densitometry may not always show changes that encourage patients to continue using expensive medicines.
Bone markers may be a solution to this dilemma. Changes in a bone marker help clinicians know that the patient is properly using therapy. 6 Moreover, these changes tell the patient that treatment is working. In my experience, relaying this type of information to the patient encourages adherence. Studies have indicated that markers do indeed help patients stay adherent to therapy and avoid fractures. 7, 8 Hence, these markers can indicate the risk of fracture and are useful in managing patients and promoting compliance.
It is unclear when third-party carriers will begin reading the appropriate literature to confirm these points, but practitioners need to recognize that there is a valid reason for using these tests.
Biochemical markers of bone turnover are commonly used as tests in the management of bone disorders, as explained very elegantly by Drs. Singer and Eyre in this issue of the Journal.1
These tests assess the activity of osteoblastic cells or osteoclastic cells in a variety of bone diseases. Such tests do not establish the diagnosis of a disease, but rather they reflect the activity of the skeleton. Because the activity of osteoblasts and the activity of osteoclasts are chemically coupled, markers of blastic and clastic activity move in the same direction. In states of high bone metabolism or turnover, marker levels are high, predicting bone loss and fracture risk. Therapies that slow down bone metabolism make these levels decrease; anabolic drugs that stimulate bone growth do the opposite.
The utility of these markers in general practice is not well appreciated. In part this is because the results can vary if the tests are not appropriately done, causing frustration for some clinicians, who erroneously conclude that these markers lack utility.
WILL INSURANCE PAY FOR TESTING?
In addition, these tests are a source of confrontation with third-party payers who refuse to pay for them, even though they are approved by Medicare and have appropriate Current Procedural Terminology codes assigned to them. The reasons cited for denying payment are that the tests are not diagnostic, that they do not predict risk, and that they are not useful in patient management.
Wrong on all counts! First of all, these markers were never meant to diagnose a specific bone disease. They reflect high bone activity or turnover and potential bone loss, and high levels indicate that further assessment is needed. (In much the same way, an elevated prostate-specific antigen level may or may not mean the patient has prostate cancer, but it does mean further assessment is needed.)
Second, these tests do address fracture risk, either when used alone or when combined with bone densitometry measurements. A high level of a turnover marker indicates a risk of fracture similar to that of a T score lower than −2.5, with an odds ratio in the range of 2.4 to 2.8. 2 Moreover, if a patient has a low T score and a high marker level, his or her risk is even higher, with an odds ratio of 4.1.
Third, the argument about the tests’ lack of ability to help in patient management is completely untrue, as shown by information reviewed by Drs. Singer and Eyre, 1 and by other data recently published. 3 These tests can indicate whether bone physiology is responding to antiresorptive and anabolic drug therapy: marker activity should decline with antiresorptive drugs and increase with anabolic agents.
And this occurs months to years before bone densitometry even reflects a change! The failure of test values to respond appropriately should prompt physicians to find out why. Is the patient not taking the medicine appropriately? Or more worrisome, is he or she not taking it at all?
AN ADDED BENEFIT: BETTER ADHERENCE
The latter point brings up a common problem seen in practice—lack of adherence to drug therapy. Studies have repeatedly shown that only 50% to 60% of osteoporotic patients actually continue taking their oral medicine for a year or so. 4, 5 The reasons are unclear but may include cost, side effects, inconvenience in administration, and lack of any sign that the drug is doing anything. Bone densitometry may not always show changes that encourage patients to continue using expensive medicines.
Bone markers may be a solution to this dilemma. Changes in a bone marker help clinicians know that the patient is properly using therapy. 6 Moreover, these changes tell the patient that treatment is working. In my experience, relaying this type of information to the patient encourages adherence. Studies have indicated that markers do indeed help patients stay adherent to therapy and avoid fractures. 7, 8 Hence, these markers can indicate the risk of fracture and are useful in managing patients and promoting compliance.
It is unclear when third-party carriers will begin reading the appropriate literature to confirm these points, but practitioners need to recognize that there is a valid reason for using these tests.
- Singer FR, Eyre DR. Using biochemical markers of bone turnover in clinical practice. Cleve Clin J Med. 2008; 75:739–750.
- Johnell O, Odén A, De Laet C, Garnero P, Delmas PD, Kanis JA. Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporos Int. 2002; 13:523–526.
- Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan JCommittee of Scientific Advisors of the International Osteoporosis Foundation. The use of biochemical markers of bone turnover in osteoporosis. Osteoporos Int 2000; 11(suppl 6):S2–S17.
- Ivaska KK, Lenora J, Gerdhem P, Akesson K, Väänänen HK, Obrant KJ. Serial assessment of serum bone metabolism markers identifies women with the highest rate of bone loss and osteoporosis risk. J Clin Endocrinol Metab. 2008; 93:2622–2632.
- Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008; 28:437–443.
- Eastell R, Krege JH, Chen P, Glass EV, Reginster JY. Development of an algorithm for using PINP to monitor treatment of patients with teriparatide. Curr Med Res Opin. 2006; 22:61–66.
- Briesacher BA, Andrade SE, Yood RA, Kahler KH. Consequences of poor compliance with bisphosphonates. Bone. 2007; 41:882–887.
- Delmas PD, Vrijens B, Eastell R, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007; 92:1296–1304.
- Singer FR, Eyre DR. Using biochemical markers of bone turnover in clinical practice. Cleve Clin J Med. 2008; 75:739–750.
- Johnell O, Odén A, De Laet C, Garnero P, Delmas PD, Kanis JA. Biochemical indices of bone turnover and the assessment of fracture probability. Osteoporos Int. 2002; 13:523–526.
- Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan JCommittee of Scientific Advisors of the International Osteoporosis Foundation. The use of biochemical markers of bone turnover in osteoporosis. Osteoporos Int 2000; 11(suppl 6):S2–S17.
- Ivaska KK, Lenora J, Gerdhem P, Akesson K, Väänänen HK, Obrant KJ. Serial assessment of serum bone metabolism markers identifies women with the highest rate of bone loss and osteoporosis risk. J Clin Endocrinol Metab. 2008; 93:2622–2632.
- Briesacher BA, Andrade SE, Fouayzi H, Chan KA. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy. 2008; 28:437–443.
- Eastell R, Krege JH, Chen P, Glass EV, Reginster JY. Development of an algorithm for using PINP to monitor treatment of patients with teriparatide. Curr Med Res Opin. 2006; 22:61–66.
- Briesacher BA, Andrade SE, Yood RA, Kahler KH. Consequences of poor compliance with bisphosphonates. Bone. 2007; 41:882–887.
- Delmas PD, Vrijens B, Eastell R, et al. Effect of monitoring bone turnover markers on persistence with risedronate treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab. 2007; 92:1296–1304.
Preventing renal disease progression: Can complete renin-angiotensin-aldosterone blockade work?
Perhaps the most daunting challenge for any primary care physician, nephrologist, or other internal medicine specialist is how to prevent the progression of chronic kidney disease.
A MAJOR HEALTH CARE CRISIS
Ten to 20 million people in the United States have chronic kidney disease, with diabetic nephropathy and arterial hypertension accounting for two-thirds of cases. In 2007, the US Renal Data System1 reported that, at the end of 2005, 341,319 patients were receiving dialysis and another 143,693 had received renal transplants.
The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiatives2 has raised the level of awareness of chronic kidney disease among physicians and the general public. We have become more adept at diagnosing chronic kidney disease, in particular by calculating the estimated glomerular filtration rate, and we are starting to learn how to sort out the patients designated as having chronic kidney disease by this calculation but without “true” kidney disease. Nevertheless, the medical profession is still struggling to determine the best way to prevent progression in chronic kidney disease, and no single innovative approach currently exists. Should the emphasis be on the blood pressure target, the level of proteinuria reduction, the classes of medications to be used, or on other factors such as lipid control, vitamin D repletion,3 or glycemic control?
WHY INHIBIT THE RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM?
Over the last 20 years, investigators have devoted much effort to controlling the adverse effects of the renin-angiotensin-aldosterone system on the renal vasculature and parenchyma. We now understand that this system is a complex cascade and that angiotensin II plays a key role.
Angiotensin II enhances the vascular tone of both the afferent and the efferent glomerular arterioles, helps regulate intraglomerular pressure and glomerular filtration, and stimulates the adrenal cortex to release aldosterone. In addition, it has several nonhemodynamic effects. In particular, it may alter the selective permeability of the glomerular capillary barrier by influencing podocyte morphology and by directing a reorganization of its actin cytostructure.
Podocytes are highly differentiated pericyte-like cells that are essential for normal kidney function, but they have limited regenerative ability. Angiotensin II stimulation can lead to podocyte injury via mechanical stress due to increased intraglomerular pressure or an increase in cytosolic calcium,4 formation of bridging between the parietal basement membrane and the glomerular basement membrane,5 and extension of the extracapillary disease process to the glomerular-proximal tubular junction.6 These alterations can result in progressive atrophy, cell death, subsequent fibrosis, and irreversible loss in functioning renal parenchyma.
EVIDENCE FOR AND AGAINST COMBINATION THERAPY
In theory, by completely inhibiting the renin-angiotensin-aldosterone system in some patients with proteinuric chronic kidney disease (as Dr. Sheldon Hirsch suggests in this issue of the Cleveland Clinic Journal of Medicine7), we might be better able to prevent progressive renal injury than with an incomplete blockade of this system.
The rationale for complete blockade stems from evidence that long-term treatment with an angiotensin-converting enzyme (ACE) inhibitor results in the accumulation of angiotensin I, the escape of angiotensin II generation by ACE-independent enzymes (chymases), and the inhibition of angiotensin-(1–7) formation that partially antagonizes the effects of angiotensin II. In addition, aldosterone may injure the kidney by its rapid nongenomic effect on the renal vasculature, resulting in increased renal vascular resistance, with afferent and efferent vasoconstriction. Therefore, treatment with either an ACE inhibitor or an angiotensin receptor blocker (ARB) by itself may delay but not prevent end-stage renal disease for most patients with proteinuric chronic kidney disease.8
Combining an ACE inhibitor and an ARB
Regimens in which an ACE inhibitor is combined with an ARB may achieve their therapeutic benefit of lowering proteinuria by modulating the compensatory events in kidney injury that stress “normal” nephrons, inhibiting the podocyte injury responsible for contiguous damage in the tubulointerstitial area, and limiting fibrosis and inflammation. However, few trials actually showed that combining an ACE inhibitor with an ARB leads to greater renal protection in the long term than with either agent alone, despite a greater chance of lowering the protein excretion rate.9,10
The COOPERATE study. The Combination Treatment of Angiotensin II Receptor Blocker and Angiotensin-Converting-Enzyme Inhibitor in Non-diabetic Renal Disease (COOPERATE) study11 evaluated the renoprotective effects of the combination of trandolapril (Mavik, an ACE inhibitor) and losartan (Cozaar, an ARB). Significantly fewer patients reached one of the end points (doubling of the serum creatinine concentration or end-stage renal disease) with the combined therapy than with either agent alone.
Kunz et al12 recently performed a meta-analysis, which indicated that the combination of an ACE inhibitor and an ARB reduces proteinuria to a greater extent than either drug alone. However, the total number of patients in each trial was less than 30 on average, the duration of therapy rarely exceeded 1 year, and the effect on changes in the glomerular filtration rate or the need for dialysis was not reported.
ONTARGET. In the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET),13 combination therapy had no clear benefit in the group at the highest renal risk (ie, with overt diabetic nephropathy), and it was associated with a trend toward worse results in the low-risk group. Most participants in ONTARGET did not have microalbuminuria or macroalbuminuria, and of interest, these patients without protein excretion were at increased risk for renal events, such as acute renal failure requiring dialysis.
Phillips et al14 recently reported on the safety profile of patients with symptomatic left ventricular dysfunction treated with the combination of an ACE inhibitor and an ARB. Even in these nonrenal patients there was a significantly higher risk of worsening renal dysfunction (relative risk 4.87, 95% confidence interval 2.39–9.94) and hyperkalemia (relative risk 4.87, 95% confidence interval 2.39–9.94) with combination therapy.
Adding an aldosterone blocker to an ACE inhibitor, ARB, or both
There is little evidence that aldosterone plays a role in the progression of chronic kidney disease. However, several studies found that combining an aldosterone blocker with an ACE inhibitor, ARB, or both had an additional impact on reducing proteinuria and modulating the rate of change in the glomerular filtration rate.15–17
When aldosterone antagonists were added to an ACE inhibitor, an ARB, or both combined, proteinuria was reduced, but there was little effect on preserving the glomerular filtration rate.17 However, most of the studies were small, with short observation periods. Hyperkalemia is a risk when using aldosterone antagonists in combination with ACE inhibitors and ARBs, especially in patients with glomerular filtration rates less than 30 mL/minute.18
Adding a renin inhibitor to an ACE inhibitor or an ARB
Few studies have examined combination therapy with either an ACE inhibitor or ARB plus a renin inhibitor, the newest class of agents that block this system.
Parving et al19 recently reported the results of combining aliskiren (Tekturna, a renin inhibitor) with losartan in 599 patients with type 2 diabetes and nephropathy. At 6 months, the renin inhibitor showed a renoprotective effect that was independent of its blood-pressure-lowering effect in those who were receiving maximal recommended doses of the ARB.
OTHER FACTORS ALSO INFLUENCE PROGRESSION
Even though there is broad agreement that an approach that neutralizes the effects of the renin-angiotensin-aldosterone system on the kidney would lower blood pressure and protein excretion rates, whether it would change the natural history of chronic kidney disease and prevent progression is less clear. In reality, a number of factors other than the renin-angiotensin-aldosterone system are responsible for the progression of chronic kidney disease. These other factors may help explain why control of this system does not totally prevent deterioration of chronic kidney disease, although the rate may be slowed.
MORE QUESTIONS THAN ANSWERS
A number of provocative questions arise from Dr. Hirsch’s discussion of complete renin-angiotensin-aldosterone system blockade to prevent disease progression:
- Will decreasing proteinuria to a specific target (< 500 mg/day) prevent progression?
- How low should the blood pressure target be set to modulate progression, and should it be the same in all age groups?
- Should complete blockade be applied all at once or in a stepwise fashion depending on the glomerular filtration rate, the level of proteinuria, or both?
- Which patients would benefit most from complete blockade?
- Is direct renin inhibition a critical component of complete blockade?
- What model of chronic disease management is required to avoid unexpected complications if this treatment approach is embraced?
Currently, therefore, there are more questions than answers. This strategy is an intriguing, opinion-based option, but for now it should only be applied to patients with proteinuria and evidence of early progression despite standard therapy who can be closely monitored, and it is not for the faint of heart. In view of the risks of hyperkalemia, hypotension, and perhaps even worsening renal function, more data from carefully designed trials are needed before the general medical community widely applies a complete blockade of the renin-angiotensin-aldosterone pathway to prevent progressive chronic kidney disease.
- United States Renal Data System. Annual data report. www.usrds.org/adr.htm. Accessed 9/5/2008.
- National Kidney Foundation. NKF K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. www.kidney.org/Professionals/Kdoqi/guidelines_ckd/toc.htm. Accessed 9/5/2008.
- Remuzzi A. Vitamin D, insulin resistance, and renal disease. Kidney Int. 2007; 71:96–98.
- Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003; 83:253–307.
- Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int 1998; 54:687–697.
- Endlich N, Endlich K. Stretch, tension and adhesion—adaptive mechanisms of the actin cytoskeleton in podocytes. Eur J Cell Biol. 2006; 85:229–234.
- Hirsch S. An update on proteinuric chronic kidney disease: the dual-goal approach. Cleve Clin J Med. 2008; 75:705–713.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993; 329:1456–1462.
- Wolf G, Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney Int. 2005; 67:799–812.
- Campbell R, Sangalli F, Perticucci E, et al. Effects of combined ACE inhibitor and angiotensin II antagonist treatment in human chronic nephropathies. Kidney Int. 2003; 63:1094–1103.
- Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003; 361:117–124.
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008; 148:30–48.
- Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008; 372:547–553.
- Phillips CO, Kashani A, Ko DK, Francis G, Krumholz HM. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: a quantitative review of data from randomized clinical trials. Arch Intern Med. 2007; 167:1930–1936.
- Epstein M. Adding spironolactone to conventional antihypertensives reduces albuminuria in patients with diabetic nephropathy. Nat Clin Pract Nephrol. 2006; 2:310–311.
- Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over study. Diabetes Care. 2005; 28:2106–2112.
- Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006; 70:2116–2123.
- Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis. 2008; 51:199–211.
- Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008; 358:2433–2446.
Perhaps the most daunting challenge for any primary care physician, nephrologist, or other internal medicine specialist is how to prevent the progression of chronic kidney disease.
A MAJOR HEALTH CARE CRISIS
Ten to 20 million people in the United States have chronic kidney disease, with diabetic nephropathy and arterial hypertension accounting for two-thirds of cases. In 2007, the US Renal Data System1 reported that, at the end of 2005, 341,319 patients were receiving dialysis and another 143,693 had received renal transplants.
The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiatives2 has raised the level of awareness of chronic kidney disease among physicians and the general public. We have become more adept at diagnosing chronic kidney disease, in particular by calculating the estimated glomerular filtration rate, and we are starting to learn how to sort out the patients designated as having chronic kidney disease by this calculation but without “true” kidney disease. Nevertheless, the medical profession is still struggling to determine the best way to prevent progression in chronic kidney disease, and no single innovative approach currently exists. Should the emphasis be on the blood pressure target, the level of proteinuria reduction, the classes of medications to be used, or on other factors such as lipid control, vitamin D repletion,3 or glycemic control?
WHY INHIBIT THE RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM?
Over the last 20 years, investigators have devoted much effort to controlling the adverse effects of the renin-angiotensin-aldosterone system on the renal vasculature and parenchyma. We now understand that this system is a complex cascade and that angiotensin II plays a key role.
Angiotensin II enhances the vascular tone of both the afferent and the efferent glomerular arterioles, helps regulate intraglomerular pressure and glomerular filtration, and stimulates the adrenal cortex to release aldosterone. In addition, it has several nonhemodynamic effects. In particular, it may alter the selective permeability of the glomerular capillary barrier by influencing podocyte morphology and by directing a reorganization of its actin cytostructure.
Podocytes are highly differentiated pericyte-like cells that are essential for normal kidney function, but they have limited regenerative ability. Angiotensin II stimulation can lead to podocyte injury via mechanical stress due to increased intraglomerular pressure or an increase in cytosolic calcium,4 formation of bridging between the parietal basement membrane and the glomerular basement membrane,5 and extension of the extracapillary disease process to the glomerular-proximal tubular junction.6 These alterations can result in progressive atrophy, cell death, subsequent fibrosis, and irreversible loss in functioning renal parenchyma.
EVIDENCE FOR AND AGAINST COMBINATION THERAPY
In theory, by completely inhibiting the renin-angiotensin-aldosterone system in some patients with proteinuric chronic kidney disease (as Dr. Sheldon Hirsch suggests in this issue of the Cleveland Clinic Journal of Medicine7), we might be better able to prevent progressive renal injury than with an incomplete blockade of this system.
The rationale for complete blockade stems from evidence that long-term treatment with an angiotensin-converting enzyme (ACE) inhibitor results in the accumulation of angiotensin I, the escape of angiotensin II generation by ACE-independent enzymes (chymases), and the inhibition of angiotensin-(1–7) formation that partially antagonizes the effects of angiotensin II. In addition, aldosterone may injure the kidney by its rapid nongenomic effect on the renal vasculature, resulting in increased renal vascular resistance, with afferent and efferent vasoconstriction. Therefore, treatment with either an ACE inhibitor or an angiotensin receptor blocker (ARB) by itself may delay but not prevent end-stage renal disease for most patients with proteinuric chronic kidney disease.8
Combining an ACE inhibitor and an ARB
Regimens in which an ACE inhibitor is combined with an ARB may achieve their therapeutic benefit of lowering proteinuria by modulating the compensatory events in kidney injury that stress “normal” nephrons, inhibiting the podocyte injury responsible for contiguous damage in the tubulointerstitial area, and limiting fibrosis and inflammation. However, few trials actually showed that combining an ACE inhibitor with an ARB leads to greater renal protection in the long term than with either agent alone, despite a greater chance of lowering the protein excretion rate.9,10
The COOPERATE study. The Combination Treatment of Angiotensin II Receptor Blocker and Angiotensin-Converting-Enzyme Inhibitor in Non-diabetic Renal Disease (COOPERATE) study11 evaluated the renoprotective effects of the combination of trandolapril (Mavik, an ACE inhibitor) and losartan (Cozaar, an ARB). Significantly fewer patients reached one of the end points (doubling of the serum creatinine concentration or end-stage renal disease) with the combined therapy than with either agent alone.
Kunz et al12 recently performed a meta-analysis, which indicated that the combination of an ACE inhibitor and an ARB reduces proteinuria to a greater extent than either drug alone. However, the total number of patients in each trial was less than 30 on average, the duration of therapy rarely exceeded 1 year, and the effect on changes in the glomerular filtration rate or the need for dialysis was not reported.
ONTARGET. In the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET),13 combination therapy had no clear benefit in the group at the highest renal risk (ie, with overt diabetic nephropathy), and it was associated with a trend toward worse results in the low-risk group. Most participants in ONTARGET did not have microalbuminuria or macroalbuminuria, and of interest, these patients without protein excretion were at increased risk for renal events, such as acute renal failure requiring dialysis.
Phillips et al14 recently reported on the safety profile of patients with symptomatic left ventricular dysfunction treated with the combination of an ACE inhibitor and an ARB. Even in these nonrenal patients there was a significantly higher risk of worsening renal dysfunction (relative risk 4.87, 95% confidence interval 2.39–9.94) and hyperkalemia (relative risk 4.87, 95% confidence interval 2.39–9.94) with combination therapy.
Adding an aldosterone blocker to an ACE inhibitor, ARB, or both
There is little evidence that aldosterone plays a role in the progression of chronic kidney disease. However, several studies found that combining an aldosterone blocker with an ACE inhibitor, ARB, or both had an additional impact on reducing proteinuria and modulating the rate of change in the glomerular filtration rate.15–17
When aldosterone antagonists were added to an ACE inhibitor, an ARB, or both combined, proteinuria was reduced, but there was little effect on preserving the glomerular filtration rate.17 However, most of the studies were small, with short observation periods. Hyperkalemia is a risk when using aldosterone antagonists in combination with ACE inhibitors and ARBs, especially in patients with glomerular filtration rates less than 30 mL/minute.18
Adding a renin inhibitor to an ACE inhibitor or an ARB
Few studies have examined combination therapy with either an ACE inhibitor or ARB plus a renin inhibitor, the newest class of agents that block this system.
Parving et al19 recently reported the results of combining aliskiren (Tekturna, a renin inhibitor) with losartan in 599 patients with type 2 diabetes and nephropathy. At 6 months, the renin inhibitor showed a renoprotective effect that was independent of its blood-pressure-lowering effect in those who were receiving maximal recommended doses of the ARB.
OTHER FACTORS ALSO INFLUENCE PROGRESSION
Even though there is broad agreement that an approach that neutralizes the effects of the renin-angiotensin-aldosterone system on the kidney would lower blood pressure and protein excretion rates, whether it would change the natural history of chronic kidney disease and prevent progression is less clear. In reality, a number of factors other than the renin-angiotensin-aldosterone system are responsible for the progression of chronic kidney disease. These other factors may help explain why control of this system does not totally prevent deterioration of chronic kidney disease, although the rate may be slowed.
MORE QUESTIONS THAN ANSWERS
A number of provocative questions arise from Dr. Hirsch’s discussion of complete renin-angiotensin-aldosterone system blockade to prevent disease progression:
- Will decreasing proteinuria to a specific target (< 500 mg/day) prevent progression?
- How low should the blood pressure target be set to modulate progression, and should it be the same in all age groups?
- Should complete blockade be applied all at once or in a stepwise fashion depending on the glomerular filtration rate, the level of proteinuria, or both?
- Which patients would benefit most from complete blockade?
- Is direct renin inhibition a critical component of complete blockade?
- What model of chronic disease management is required to avoid unexpected complications if this treatment approach is embraced?
Currently, therefore, there are more questions than answers. This strategy is an intriguing, opinion-based option, but for now it should only be applied to patients with proteinuria and evidence of early progression despite standard therapy who can be closely monitored, and it is not for the faint of heart. In view of the risks of hyperkalemia, hypotension, and perhaps even worsening renal function, more data from carefully designed trials are needed before the general medical community widely applies a complete blockade of the renin-angiotensin-aldosterone pathway to prevent progressive chronic kidney disease.
Perhaps the most daunting challenge for any primary care physician, nephrologist, or other internal medicine specialist is how to prevent the progression of chronic kidney disease.
A MAJOR HEALTH CARE CRISIS
Ten to 20 million people in the United States have chronic kidney disease, with diabetic nephropathy and arterial hypertension accounting for two-thirds of cases. In 2007, the US Renal Data System1 reported that, at the end of 2005, 341,319 patients were receiving dialysis and another 143,693 had received renal transplants.
The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiatives2 has raised the level of awareness of chronic kidney disease among physicians and the general public. We have become more adept at diagnosing chronic kidney disease, in particular by calculating the estimated glomerular filtration rate, and we are starting to learn how to sort out the patients designated as having chronic kidney disease by this calculation but without “true” kidney disease. Nevertheless, the medical profession is still struggling to determine the best way to prevent progression in chronic kidney disease, and no single innovative approach currently exists. Should the emphasis be on the blood pressure target, the level of proteinuria reduction, the classes of medications to be used, or on other factors such as lipid control, vitamin D repletion,3 or glycemic control?
WHY INHIBIT THE RENIN-ANGIOTENSIN-ALDOSTERONE SYSTEM?
Over the last 20 years, investigators have devoted much effort to controlling the adverse effects of the renin-angiotensin-aldosterone system on the renal vasculature and parenchyma. We now understand that this system is a complex cascade and that angiotensin II plays a key role.
Angiotensin II enhances the vascular tone of both the afferent and the efferent glomerular arterioles, helps regulate intraglomerular pressure and glomerular filtration, and stimulates the adrenal cortex to release aldosterone. In addition, it has several nonhemodynamic effects. In particular, it may alter the selective permeability of the glomerular capillary barrier by influencing podocyte morphology and by directing a reorganization of its actin cytostructure.
Podocytes are highly differentiated pericyte-like cells that are essential for normal kidney function, but they have limited regenerative ability. Angiotensin II stimulation can lead to podocyte injury via mechanical stress due to increased intraglomerular pressure or an increase in cytosolic calcium,4 formation of bridging between the parietal basement membrane and the glomerular basement membrane,5 and extension of the extracapillary disease process to the glomerular-proximal tubular junction.6 These alterations can result in progressive atrophy, cell death, subsequent fibrosis, and irreversible loss in functioning renal parenchyma.
EVIDENCE FOR AND AGAINST COMBINATION THERAPY
In theory, by completely inhibiting the renin-angiotensin-aldosterone system in some patients with proteinuric chronic kidney disease (as Dr. Sheldon Hirsch suggests in this issue of the Cleveland Clinic Journal of Medicine7), we might be better able to prevent progressive renal injury than with an incomplete blockade of this system.
The rationale for complete blockade stems from evidence that long-term treatment with an angiotensin-converting enzyme (ACE) inhibitor results in the accumulation of angiotensin I, the escape of angiotensin II generation by ACE-independent enzymes (chymases), and the inhibition of angiotensin-(1–7) formation that partially antagonizes the effects of angiotensin II. In addition, aldosterone may injure the kidney by its rapid nongenomic effect on the renal vasculature, resulting in increased renal vascular resistance, with afferent and efferent vasoconstriction. Therefore, treatment with either an ACE inhibitor or an angiotensin receptor blocker (ARB) by itself may delay but not prevent end-stage renal disease for most patients with proteinuric chronic kidney disease.8
Combining an ACE inhibitor and an ARB
Regimens in which an ACE inhibitor is combined with an ARB may achieve their therapeutic benefit of lowering proteinuria by modulating the compensatory events in kidney injury that stress “normal” nephrons, inhibiting the podocyte injury responsible for contiguous damage in the tubulointerstitial area, and limiting fibrosis and inflammation. However, few trials actually showed that combining an ACE inhibitor with an ARB leads to greater renal protection in the long term than with either agent alone, despite a greater chance of lowering the protein excretion rate.9,10
The COOPERATE study. The Combination Treatment of Angiotensin II Receptor Blocker and Angiotensin-Converting-Enzyme Inhibitor in Non-diabetic Renal Disease (COOPERATE) study11 evaluated the renoprotective effects of the combination of trandolapril (Mavik, an ACE inhibitor) and losartan (Cozaar, an ARB). Significantly fewer patients reached one of the end points (doubling of the serum creatinine concentration or end-stage renal disease) with the combined therapy than with either agent alone.
Kunz et al12 recently performed a meta-analysis, which indicated that the combination of an ACE inhibitor and an ARB reduces proteinuria to a greater extent than either drug alone. However, the total number of patients in each trial was less than 30 on average, the duration of therapy rarely exceeded 1 year, and the effect on changes in the glomerular filtration rate or the need for dialysis was not reported.
ONTARGET. In the Ongoing Telmisartan Alone and in Combination With Ramipril Global Endpoint Trial (ONTARGET),13 combination therapy had no clear benefit in the group at the highest renal risk (ie, with overt diabetic nephropathy), and it was associated with a trend toward worse results in the low-risk group. Most participants in ONTARGET did not have microalbuminuria or macroalbuminuria, and of interest, these patients without protein excretion were at increased risk for renal events, such as acute renal failure requiring dialysis.
Phillips et al14 recently reported on the safety profile of patients with symptomatic left ventricular dysfunction treated with the combination of an ACE inhibitor and an ARB. Even in these nonrenal patients there was a significantly higher risk of worsening renal dysfunction (relative risk 4.87, 95% confidence interval 2.39–9.94) and hyperkalemia (relative risk 4.87, 95% confidence interval 2.39–9.94) with combination therapy.
Adding an aldosterone blocker to an ACE inhibitor, ARB, or both
There is little evidence that aldosterone plays a role in the progression of chronic kidney disease. However, several studies found that combining an aldosterone blocker with an ACE inhibitor, ARB, or both had an additional impact on reducing proteinuria and modulating the rate of change in the glomerular filtration rate.15–17
When aldosterone antagonists were added to an ACE inhibitor, an ARB, or both combined, proteinuria was reduced, but there was little effect on preserving the glomerular filtration rate.17 However, most of the studies were small, with short observation periods. Hyperkalemia is a risk when using aldosterone antagonists in combination with ACE inhibitors and ARBs, especially in patients with glomerular filtration rates less than 30 mL/minute.18
Adding a renin inhibitor to an ACE inhibitor or an ARB
Few studies have examined combination therapy with either an ACE inhibitor or ARB plus a renin inhibitor, the newest class of agents that block this system.
Parving et al19 recently reported the results of combining aliskiren (Tekturna, a renin inhibitor) with losartan in 599 patients with type 2 diabetes and nephropathy. At 6 months, the renin inhibitor showed a renoprotective effect that was independent of its blood-pressure-lowering effect in those who were receiving maximal recommended doses of the ARB.
OTHER FACTORS ALSO INFLUENCE PROGRESSION
Even though there is broad agreement that an approach that neutralizes the effects of the renin-angiotensin-aldosterone system on the kidney would lower blood pressure and protein excretion rates, whether it would change the natural history of chronic kidney disease and prevent progression is less clear. In reality, a number of factors other than the renin-angiotensin-aldosterone system are responsible for the progression of chronic kidney disease. These other factors may help explain why control of this system does not totally prevent deterioration of chronic kidney disease, although the rate may be slowed.
MORE QUESTIONS THAN ANSWERS
A number of provocative questions arise from Dr. Hirsch’s discussion of complete renin-angiotensin-aldosterone system blockade to prevent disease progression:
- Will decreasing proteinuria to a specific target (< 500 mg/day) prevent progression?
- How low should the blood pressure target be set to modulate progression, and should it be the same in all age groups?
- Should complete blockade be applied all at once or in a stepwise fashion depending on the glomerular filtration rate, the level of proteinuria, or both?
- Which patients would benefit most from complete blockade?
- Is direct renin inhibition a critical component of complete blockade?
- What model of chronic disease management is required to avoid unexpected complications if this treatment approach is embraced?
Currently, therefore, there are more questions than answers. This strategy is an intriguing, opinion-based option, but for now it should only be applied to patients with proteinuria and evidence of early progression despite standard therapy who can be closely monitored, and it is not for the faint of heart. In view of the risks of hyperkalemia, hypotension, and perhaps even worsening renal function, more data from carefully designed trials are needed before the general medical community widely applies a complete blockade of the renin-angiotensin-aldosterone pathway to prevent progressive chronic kidney disease.
- United States Renal Data System. Annual data report. www.usrds.org/adr.htm. Accessed 9/5/2008.
- National Kidney Foundation. NKF K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. www.kidney.org/Professionals/Kdoqi/guidelines_ckd/toc.htm. Accessed 9/5/2008.
- Remuzzi A. Vitamin D, insulin resistance, and renal disease. Kidney Int. 2007; 71:96–98.
- Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003; 83:253–307.
- Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int 1998; 54:687–697.
- Endlich N, Endlich K. Stretch, tension and adhesion—adaptive mechanisms of the actin cytoskeleton in podocytes. Eur J Cell Biol. 2006; 85:229–234.
- Hirsch S. An update on proteinuric chronic kidney disease: the dual-goal approach. Cleve Clin J Med. 2008; 75:705–713.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993; 329:1456–1462.
- Wolf G, Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney Int. 2005; 67:799–812.
- Campbell R, Sangalli F, Perticucci E, et al. Effects of combined ACE inhibitor and angiotensin II antagonist treatment in human chronic nephropathies. Kidney Int. 2003; 63:1094–1103.
- Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003; 361:117–124.
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008; 148:30–48.
- Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008; 372:547–553.
- Phillips CO, Kashani A, Ko DK, Francis G, Krumholz HM. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: a quantitative review of data from randomized clinical trials. Arch Intern Med. 2007; 167:1930–1936.
- Epstein M. Adding spironolactone to conventional antihypertensives reduces albuminuria in patients with diabetic nephropathy. Nat Clin Pract Nephrol. 2006; 2:310–311.
- Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over study. Diabetes Care. 2005; 28:2106–2112.
- Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006; 70:2116–2123.
- Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis. 2008; 51:199–211.
- Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008; 358:2433–2446.
- United States Renal Data System. Annual data report. www.usrds.org/adr.htm. Accessed 9/5/2008.
- National Kidney Foundation. NKF K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. www.kidney.org/Professionals/Kdoqi/guidelines_ckd/toc.htm. Accessed 9/5/2008.
- Remuzzi A. Vitamin D, insulin resistance, and renal disease. Kidney Int. 2007; 71:96–98.
- Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003; 83:253–307.
- Kriz W, Gretz N, Lemley KV. Progression of glomerular diseases: is the podocyte the culprit? Kidney Int 1998; 54:687–697.
- Endlich N, Endlich K. Stretch, tension and adhesion—adaptive mechanisms of the actin cytoskeleton in podocytes. Eur J Cell Biol. 2006; 85:229–234.
- Hirsch S. An update on proteinuric chronic kidney disease: the dual-goal approach. Cleve Clin J Med. 2008; 75:705–713.
- Lewis EJ, Hunsicker LG, Bain RP, Rohde RD. The effect of angiotensin-converting-enzyme inhibition on diabetic nephropathy. The Collaborative Study Group. N Engl J Med. 1993; 329:1456–1462.
- Wolf G, Ritz E. Combination therapy with ACE inhibitors and angiotensin II receptor blockers to halt progression of chronic renal disease: pathophysiology and indications. Kidney Int. 2005; 67:799–812.
- Campbell R, Sangalli F, Perticucci E, et al. Effects of combined ACE inhibitor and angiotensin II antagonist treatment in human chronic nephropathies. Kidney Int. 2003; 63:1094–1103.
- Nakao N, Yoshimura A, Morita H, Takada M, Kayano T, Ideura T. Combination treatment of angiotensin-II receptor blocker and angiotensin-converting-enzyme inhibitor in non-diabetic renal disease (COOPERATE): a randomised controlled trial. Lancet. 2003; 361:117–124.
- Kunz R, Friedrich C, Wolbers M, Mann JF. Meta-analysis: effect of monotherapy and combination therapy with inhibitors of the renin angiotensin system on proteinuria in renal disease. Ann Intern Med. 2008; 148:30–48.
- Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008; 372:547–553.
- Phillips CO, Kashani A, Ko DK, Francis G, Krumholz HM. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: a quantitative review of data from randomized clinical trials. Arch Intern Med. 2007; 167:1930–1936.
- Epstein M. Adding spironolactone to conventional antihypertensives reduces albuminuria in patients with diabetic nephropathy. Nat Clin Pract Nephrol. 2006; 2:310–311.
- Rossing K, Schjoedt KJ, Smidt UM, Boomsma F, Parving HH. Beneficial effects of adding spironolactone to recommended antihypertensive treatment in diabetic nephropathy: a randomized, double-masked, cross-over study. Diabetes Care. 2005; 28:2106–2112.
- Bianchi S, Bigazzi R, Campese VM. Long-term effects of spironolactone on proteinuria and kidney function in patients with chronic kidney disease. Kidney Int. 2006; 70:2116–2123.
- Bomback AS, Kshirsagar AV, Amamoo MA, Klemmer PJ. Change in proteinuria after adding aldosterone blockers to ACE inhibitors or angiotensin receptor blockers in CKD: a systematic review. Am J Kidney Dis. 2008; 51:199–211.
- Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med. 2008; 358:2433–2446.