User login
Kleptomania: Emerging therapies target mood, impulsive behavior
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
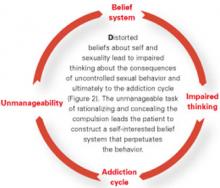
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.
From the Stanley Foundation Bipolar Network: Efficacy of adjunctive therapies for treatment-resistant patients
When choosing psychopharmacologic agents for patients with bipolar disorder, the clinician lacks guidance from controlled clinical trials at virtually every therapeutic step. To help remedy this problem, we in the Stanley Foundation Bipolar Network (SFBN) evaluated compounds with potentially important therapeutic profiles. We began by documenting the demographics and illness characteristics of patients with bipolar disorder. Then we assessed some 16 treatments in naturalistic or formal studies, some of which are complete and others ongoing.
In this second installment, we report our current information about the efficacy of second-generation antidepressants, atypical antipsychotics, and anticonvulsant agents.
Mood stabilizers
As mood stabilizers, the anticonvulsants carbamazepine and valproate are well-accepted alternatives or adjuncts to lithium carbonate, which remains a mainstay treatment for acute episodes and long-term prophylaxis.1
Valproate is approved by the FDA for treatment of acute mania but not for long-term prevention. Open studies in rapid-cycling, treatment-refractory patients demonstrate substantial improvement for both manic and depressive phases with valproate. In the most recent controlled study, valproate did not prevent manic episodes to a statistically significant degree, but it did show greater antidepressant effects than placebo or lithium.2
Carbamazepine has been widely studied in acute mania and compared with lithium for long-term prophylaxis. Most studies show nonsignificant differences between carbamazepine and lithium in preventing manic and depressive episodes, although several studies indicate less-robust antimanic effects of carbamazepine,3 particularly for patients with classic euphoric mania without psychosis, bipolar II, or associated substance abuse.4
In atypical patients, carbamazepine appeared to outperform lithium, suggesting clinical differences in these agents that have different mechanisms of action across neurotransmitter and peptide systems.
Low response rates Most randomized controlled trials and open adjunct studies suggest that 50% or more patients respond to lithium, valproate, or carbamazepine. Outcomes may be far less positive in clinical practice, however. Despite the use of mood stabilizers and a variety of antidepressants, benzodiazepines, and neuroleptics, our patients experienced substantial residual manic and depressive symptoms and functional impairment.
Similarly, clinician ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM) indicated a high rate of breakthrough episodes during carefully monitored and aggressive psychopharmacologic treatment.5 Two-thirds of the first 258 bipolar outpatients that we followed and rated daily for 1 year continued to be substantially impaired. One-quarter of them were ill for more than 75% of the year, despite using an average 4.1 psychopharmacologic agents per patient, including mood stabilizers, antidepressants, antipsychotics, and other agents.
Acute and long-term combination therapy appears more effective than monotherapy, particularly for treatment-refractory rapid cyclers.3 Even so, many patients do not respond to combination therapy. For example, in a 1-year prophylaxis study, 25% or fewer patients were rated “much improved” to “very much improved” on the Clinical Global Impressions (CGI) scale with lithium or carbamazepine as the baseline mood stabilizer and with antidepressants, neuroleptics, and benzodiazepines used as needed. In the third year of combination therapy, the overall response rate, including rapid cyclers, was roughly 50%.3
A low rate of response is apparent for lithium and valproate. While treating rapidly cycling patients, Calabrese and associates found that less than one-quarter of them responded well enough to the two-drug combination that they could be enrolled in a randomized double-blind comparison of each drug in monotherapy. Of those who were eligible, almost all relapsed with either lithium or valproate monotherapy and again required combination therapy.6
Table 1
RATE OF SWITCHING (%) INTO MANIA IN DEPRESSED BIPOLAR PATIENTS DURING ANTIDEPRESSANT TREATMENT*
| Acute treatment (10 wks) | Continuation treatment (52 wks)‡ | |||||
|---|---|---|---|---|---|---|
| Type of switch | Open (n=27) | Blind (n=100) | Total (n=127) | Open (n=12) | Blind (n=55) | Total (n=67) |
| Brief hypomania | 3.7% | 6% | 5.5% | – | 3.6% | 3% |
| Recurrent brief hypomania | 11 | 12 | 11.8 | 8.3% | 18.2 | 16.4 |
| Hypomania | 11 | 13 | 12.6 | 8.3 | 23.6 | 20.9 |
| Mania | 14.8 | 12 | 12.6 | 33.3 | 14.5 | 17.9 |
| Total | 25.8 | 25 | 25.2 | 41.6 | 38.1 | 38.8 |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | ||||||
| ‡ Most patients (80%) dropped out of this phase because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. | ||||||
| Source: Post RM et al. Bipolar Disord 2001;3:259-65, and unpublished data. | ||||||
Taken together, these studies indicate that even with our most effective and most studied agents, many patients respond inadequately. On the basis of this clinical reality, we began to explore additional options that might enhance clinical effectiveness.
Atypical antipsychotics
We assessed the addition of the atypical antipsychotic olanzapine in patients with treatment-refractory bipolar disorder7 and saw improvement in 57% of subjects with manic, mixed, and depressive components. These data foreshadowed more recent controlled findings of olanzapine’s usefulness in depression and mania.8,9 Our preliminary open clinical trial experience allowed for more controlled studies and subsequent approval and wider clinical use of olanzapine in bipolar illness.
Our initial assessment of other atypical antipsychotics found that quetiapine was associated with significantly fewer depressive symptoms on the Inventory of Depressive Symptomatology (IDS) when used as part of the regular treatment regimen. This improvement, which was seen in the first month, was maintained over 4 months of treatment, whereas no significant improvement in depression severity was seen with the use of risperidone or clozapine.10
It would be highly preliminary to conclude from these uncontrolled data that quetiapine had greater effects on bipolar depressive symptoms than did risperidone or clozapine. We cannot rule out the possibility that patients with different degrees of treatment resistance were enrolled and treated with the different concomitant drugs. However, patients did not differ at baseline in severity of their depression.
In this initial look at the atypicals, the severity of depressive symptoms on the IDS was much greater than that of mania, as measured by the Young Mania Rating Scale (YMRS). Thus, the precise patterns of illness being treated and the impact of these antipsychotics on depressive cyclicity and duration await closer examination. We intend to look at the relative efficacy and side-effect profiles of each of the atypical antipsychotics as used in clinical practice.
Second-generation antidepressants
Bupropion, sertraline, and venlafaxine have different mechanisms of action but appear to be of value as adjuncts to bipolar disorder treatment. When we used them as adjuncts to mood stabilizers in a prospective study, we found a 40 to 50% acute response rate and some switching into hypomania and mania (Table 1).11 Many switches appeared to involve only isolated brief bursts of hypomania or recurrent brief hypomanias, but more sustained episodes of hypomania (7 days) could be more clinically problematic. Only 13% of patients treated acutely with these antidepressants had more full-blown manic symptoms (i.e., those associated with moderate or greater dysfunction on the NIMH-LCM).
During the next year, when apparent acute antidepressant responders were offered continuation treatment, 18% switched into mania. However, most patients (about 80%) dropped out because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. The 127 patients who were randomized received 175 acute antidepressant trials. Only 9% were associated with a switch into mania with moderate dysfunction, whereas another 9% showed periods of hypomania lasting longer than 1 week.12
Table 2
RELAPSE INTO DEPRESSION IN BIPOLAR PATIENTS AFTER 1 YEAR WITH OR WITHOUT ADJUNCTIVE ANTIDEPRESSANT TREATMENT*
| Rate of relapse | |
|---|---|
| Continued on antidepressants | |
| Study #1 (n=19) | 35% |
| Study #2 (n=41) | 41% |
| Discontinued antidepressants | |
| Study #1 (n=25) | 68% |
| Study #2 (n=43) | 71% |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | |
| Study #1: Altshuler et al. J Clin Psychiatry 2001;62:612-6. | |
| Study #2: Altshuler et al. (submitted for publication 2002). | |
We have entered 176 patients into this double-blind, randomized comparison of bupropion, sertraline, and venlafaxine, with another 27 randomized openly. The study will attempt to examine any possible between-drug differences. Even without the blind being broken, it appears that these antidepressants are associated with a somewhat lower-than-expected rate of switching into mania with dysfunction, but a direct comparison with a placebo is not available.
In two other series, patients who remained well for 2 months on antidepressants plus mood stabilizers and then continued on their antidepressants had a much lower incidence of depressive relapses over the following year than those who discontinued their antidepressants (Table 2).13,14 Surprisingly, continuing the antidepressant was not associated with an increased rate of switching into mania.
Prospective randomized, controlled studies are required to confirm these findings. Even so, our studies appear to challenge conventional wisdom for the first time. Stopping antidepressant treatment in bipolar patients as soon as possible for fear of inducing a manic episode may not be the optimal recommendation in the admittedly small subgroup (about 15%) of patients who have responded well to an antidepressant for at least 2 months.
Table 3
SIX STUDIES COMPLETED BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number studied | Response rate |
|---|---|---|---|---|
| Antidepressants bupropion | Yes | Double-blind | 173 | ~ 47% |
| vs. sertraline vs. venlafaxine (Post et al, 2001) | Yes | Open | 27 | |
| Total = 200 | ||||
| Olanzapine (McElroy et al, 1998) | No | Open | 14 | ~ 57% |
| Lamotrigine (Suppes et al, 1999) | No | Open | 17 | ~ 65% |
| Gabapentin (Altshuler et al, 1999) | No | Open | 28 | 78% (14/18) for mania or hypomania 100% (n = 5) for depression 20% (1/5) for cycling |
| Topiramate (McElroy et al, 2000) | No | Open | 54 | 63% for mania or cycling 27% for acute depression (weight loss observed) |
| Tiagabine (Suppes et al, 2002) | No | Open | 17 | 18% (3/17) (3 possible seizures) |
Second-generation anticonvulsants
Based on our patients’ substantial residual symptoms, we explored a series of newly approved anticonvulsants for potential use as mood stabilizers.
Lamotrigine Consistent with other open and double-blind, placebo-controlled studies, lamotrigine showed promise as an adjunctive treatment, with improvement in depressive symptoms and cycling.15 One such study showed acute antidepressant effects of lamotrigine monotherapy compared with a placebo at dosages of 50 or 200 mg/d.16
Another double-blind, randomized, placebo-controlled NIMH study (partially funded by the Stanley Foundation) compared lamotrigine with gabapentin and a placebo in patients for whom lithium, carbamazepine, and valproate had failed.17 Most of these treatment-refractory patients received all three agents through three 6-week randomizations. Lamotrigine was much more effective in depression and overall illness than either a placebo or gabapentin. Men with bipolar compared with unipolar illness and patients with fewer unsuccessful clinical trials and hospitalizations for depression showed the greatest response.18
In two recent industry-sponsored controlled trials of long-term prophylaxis (following a recent manic or depressive episode), lamotrigine was significantly more effective than a placebo (Bowden et al and Calabrese et al, unpublished data). Lamotrigine was more effective than lithium in preventing depressive episodes, whereas lithium was more effective than lamotrigine for manic episodes, although lamotrigine was significantly better than the placebo.
These data suggest that lamotrigine may differ from valproate, carbamazepine, and lithium in being a more effective antidepressant than antimanic agent.
Topiramate Adjunctive use of topiramate in rapid-cycling and treatment-resistant patients has been examined in open studies, but controlled studies have not yet been published. In our open-label case series observations, 19 of 30 (63%) patients taking topiramate for mania or cycling were “much” or “very much” improved after 10 weeks, whereas only 3 of 11 (27%) patients taking topiramate for acute depression were so improved.19
Table 4
STUDIES IN PROGRESS BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number enrolled* | Notes |
|---|---|---|---|---|
| Topiramate vs. sibutramine | Yes | Open | 42 | Relative weight loss and mood stabilization |
| Omega-3 fatty acids (6 grams EPA vs. placebo) (for depression cycling) (Keck et al) | Yes Yes | Double-blind Double-blind | 62 60 | Data from both studies being analyzed |
| Acamprosate (for alcohol craving) (Grunze et al) | No | Open | 23 | Goal: 60 patients |
| Levetiracetam (Post et al) | No | Open | 13 | Goal: 30 patients |
| Zonisamide (McElroy et al) | No | Open | 45 | Goal: 60 patients European sites only |
| Tranylcypromine vs. lamotrigine (for refractory depression) (Nolen et al) | Yes | Open | 17 | Single-site study |
| Quetiapine (McElroy et al) | No | Open | 48 | Apparent superior antidepressant effects compared with risperidone or clozapine |
| Risperidone (Grunze et al) | No | Open | 37 | No significant effect on IDS‡ depression ratings |
| Clozapine (Suppes et al) | No | Open | 19 | No significant effect on IDS depression ratings |
| Modafinil vs. placebo (Frye et al) | Yes | Double-blind | Started 4/02 | |
| * Enrollment as of April 2002 n = 696 total patients in tables 3 and 4 | ||||
| ‡ IDS = Inventory of Depressive Symptomatology | ||||
An industry-sponsored double-blind, randomized trial of topiramate in acute mania looked promising in the first 67 patients, but not as encouraging in subsequent subjects. A positive drug effect re-emerged if one eliminated patients whose manic episodes were precipitated by antidepressants.
The acute antidepressant effects of topiramate did not appear promising in our study. On the other hand, a single-blind, randomized study of topiramate versus bupropion as adjunctive therapy for breakthrough bipolar depression suggested excellent acute response (approximately 56%) to both agents.20 These data leave the potential antidepressant effects of topiramate undetermined.
In patients being treated with topiramate, we found significant weight loss (average –0.7 kg at 4 weeks, –1.6 kg at 10 weeks, –4.7 kg at 6 months, and –6.2 kg at 1 year).19 Average weight loss was 4.5±6.7 kg at the final evaluation (body mass index [BMI] change of –6.3% and –5% at 1 year and last evaluation, respectively).
We and others have thus seen substantial weight loss in one-third to one-half of patients taking topiramate for bipolar disorder.21 This weight loss is maintained over relatively long periods, which differentiates topiramate from other weight-loss regimens. Topiramate’s ability to decrease weight—even while it is in the early stages of evaluation as a mood stabilizer—is of considerable interest, given the substantial problem of overweight in the bipolar population and the fact that many drug therapies used in the illness are associated with weight gain.
We are proceeding with a randomized open comparison of the relative weight loss and psychotropic efficacy of topiramate versus the antiobesity agent sibutramine. Sibutramine has been reported to have antidepressant properties and is FDA-approved for weight loss.
Gabapentin and tiagabine Our group and many others have reported substantial overall clinical improvement when gabapentin was used as adjunctive therapy in an open study of patients with inadequate response to previous treatments.22 As monotherapy, however, gabapentin was not more effective than a placebo17 and was less effective than lamotrigine. Similarly, gabapentin was not an effective acute antimanic agent when compared with a placebo,23 although other double-blind, controlled studies have indicated positive effects in anxiety disorders24 and social phobias.25 Thus, the gabapentin literature appears to be divergent—i.e., positive studies support its adjunctive use in bipolar patients, but controlled studies suggest that it is not directly antimanic or mood-stabilizing.
Gabapentin’s effect on syndromes that are highly comorbid with bipolar illness could account for its apparent success as open adjunctive therapy. For example, in addition to its antianxiety effects, gabapentin shows positive effects in alcohol withdrawal, pain syndromes, sleep disorders (including restless leg syndrome), and obsessive-compulsive disorder. Also, combining gabapentin with other psychotropics with other mechanisms of action may have a more robust therapeutic effect than monotherapy.
Our preliminary observations with the selective GABA-reuptake inhibitor tiagabine were not promising. In an open study, only 3 of the first 17 SFBN patients with treatment-resistant affective disorders showed a partial response (moderate improvement on the Clinical Global Impressions Scale for Bipolar Illness [CGI-BP]) to adjunctive tiagabine treatment when the dosage was gradually increased. Serious side effects, including two patients with possible seizures, were noted.26 Similar results were seen when tiagabine doses were rapidly increased in eight patients with acute mania; none responded, and one had a major motor seizure.27
Thus, our initial observations led us to discontinue use of tiagabine and suggest any further clinical consideration of this agent for treatment-resistant affective disorders would require great care. This perspective contrasts with several previous positive case reports. It is unknown whether selected patients or those treated with lower dosages would be more responsive to this agent.
Like valproate, gabapentin and tiagabine increase brain GABA, but their apparent lack of antimanic efficacy strongly suggests that raising brain GABA alone does little to counter mania. These data further support the viewpoint already offered that all anticonvulsants are not alike and that anticonvulsants as a class are not necessarily mood stabilizers.
Zonisamide and levetiracetam Open studies of these two other anticonvulsants are near completion. Each has an interesting mechanistic profile, good tolerability, and the (potentially positive) side effect of inducing some weight loss, based on studies of neurologic patients.
Omega-3 fatty acids Based on positive reports of antidepressant effects of omega-3 fatty acids,28-30 we recently recruited more than 120 patients with bipolar disorder for two double-blind, randomized, controlled trials. Those patients with either an acute depression or a cyclic course will receive 6 grams of omega-3 fatty acid (the eicosapentaenoate form) or a placebo for 4 months of double-blind treatment, followed by an 8-month open, active continuation phase.
If results are positive, omega-3 fatty acids would represent the much-needed addition of a well-tolerated dietary agent to the therapeutic armamentarium directed at bipolar illness.
Conclusion
The Stanley Foundation Bipolar Network has begun to evaluate the usefulness of numerous older and new compounds for patients with bipolar disorder, including second-generation antidepressants, anticonvulsants, atypical antipsychotic agents, and omega-3 fatty acids. Based on initial observations from 16 treatment studies (Table 3), we have drawn these conclusions:
- Second-generation antidepressants such as bupropion, sertraline, and venlafaxine appear to have a lower-than-expected switch rate into mania, compared with the use of the older tricyclic antidepressants.
- If the addition of a second-generation antidepressant results in good antidepressant response for 2 months and no switches into mania, one should consider continuing the antidepressant in this small minority of patients, rather than stopping it as soon as possible as previously recommended.
- Adjunctive use of lamotrigine appears to be of value, particularly for acute depression and prophylaxis, for many patients who have not responded to other treatments.
- While our group and others have reported improvement with adjunctive gabapentin in bipolar patients in uncontrolled case series, its primary antimanic or mood-stabilizing utility (as well as that of tiagabine) does not appear promising.
- Topiramate’s ability to induce substantial weight loss may emerge as clinically therapeutic for patients with problematic weight gain related to other psychotropic drugs, independent of its ultimate utility as a possible mood stabilizer.
We are preparing to analyze other open case series assessing acute and long-term treatment outcomes as well as randomized, double-blind clinical trials. Even though the funding of this unique collaborative network is ending, we look forward to more effective treatment of bipolar disorder in the future with continued development of new psychotropic agents and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and the VA Cooperative studies.31
Related resources
- National Alliance for the Mentally Ill (NAMI). www.nami.org
- National Depressive and Manic-Depressive Association (NDMDA). www.ndmda.org
Drug brand names
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sibutramine • Meridia
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproate • Depakote
- Venlafaxine • Effexor
- Zonisamide • Zonegran
Acknowledgement
The authors are particularly indebted to the generosity of The Stanley Foundation in making funds available for this series of studies and several about to be completed.
Disclosure
Financial disclosure statements have been provided by all authors and are available upon request at [email protected].
1. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press, 1990.
2. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
3. Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997a;58:470-8.
4. Greil W, Kleindienst N. The comparative prophylactic efficacy of lithium and carbamazepine in patients with bipolar I disorder. Int Clin Psychopharmacol 1999;14:277-81.
5. Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for one year with daily prospective ratings on the NIMH-LCM (submitted for publication, 2002).
6. Calabrese JR, Rapport DJ. Mood stabilizers and the evolution of maintenance study designs in bipolar I disorder. J Clin Psychiatry 1999;60(suppl 5):5-13.
7. McElroy SL, Frye M, Denicoff K, et al. Olanzapine in treatment-resistant bipolar disorder. J Affect Disord 1998;49:119-22.
8. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
9. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
10. Keck PE, Jr. New antiepileptics in the treatment of bipolar disorder. Syllabus and Proceedings Summary of the 2001 American Psychiatric Association Meeting, New Orleans, May 5-10, 2001. Abstract 34E, 93.
11. Post RM, Altshuler LL, Frye MA, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001;3:259-65.
12. Post RM, Leverich GS, Nolen WA, et al. A reevaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2002 (in press).
13. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-6.
14. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute remission from bipolar depression on rates of depressive relapse at one-year follow-up (submitted for publication 2002).
15. Suppes T, Brown ES, McElroy SL, et al. Lamotrigine for the treatment of bipolar disorder: a clinical case series. J Affective Disord 1999;53:95-8.
16. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60:79-88.
17. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14.
18. Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biolog Psychiatry 2002;51:253-60.
19. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biolog Psychiatry 2000;47:1025-33.
20. McIntyre RS, Mancini DA, McCann SM, et al. Topiramiate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 2002;4:207-13.
21. Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults. Epilepsia 2000;41(suppl 1):S66-S71.
22. Altshuler LL, Keck PE, Jr, McElroy SL, et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1999;1:61-5.
23. Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord 2000;2:249-55.
24. Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000;20:467-71.
25. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999;19:341-8.
26. Suppes T, Chisholm K, Dhvule D, et al. Tiagabine in bipolar disorder: adverse events in an open case series (submitted for publication, 2002).
27. Grunze H, Erfurth A, Marcuse A, et al. Tiagabine appears not to be efficacious in the treatment of acute mania. J Clin Psychiatry 1999;60:759-62.
28. Stoll AL, Severus WE, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56:407-12.
29. Horrobin DF, Peet M. A dose-ranging study of ethyl-eicosapentaenoate in treatment-unresponsive depression. Biolog Psychiatry 2001;49:37S.-
30. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-9.
31. Bauer MS, Williford WO, Dawson EE, et al. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: Reducing the efficacy-effectiveness gap in bipolar disorder. J Affect Disord 2001;67:61-78.
When choosing psychopharmacologic agents for patients with bipolar disorder, the clinician lacks guidance from controlled clinical trials at virtually every therapeutic step. To help remedy this problem, we in the Stanley Foundation Bipolar Network (SFBN) evaluated compounds with potentially important therapeutic profiles. We began by documenting the demographics and illness characteristics of patients with bipolar disorder. Then we assessed some 16 treatments in naturalistic or formal studies, some of which are complete and others ongoing.
In this second installment, we report our current information about the efficacy of second-generation antidepressants, atypical antipsychotics, and anticonvulsant agents.
Mood stabilizers
As mood stabilizers, the anticonvulsants carbamazepine and valproate are well-accepted alternatives or adjuncts to lithium carbonate, which remains a mainstay treatment for acute episodes and long-term prophylaxis.1
Valproate is approved by the FDA for treatment of acute mania but not for long-term prevention. Open studies in rapid-cycling, treatment-refractory patients demonstrate substantial improvement for both manic and depressive phases with valproate. In the most recent controlled study, valproate did not prevent manic episodes to a statistically significant degree, but it did show greater antidepressant effects than placebo or lithium.2
Carbamazepine has been widely studied in acute mania and compared with lithium for long-term prophylaxis. Most studies show nonsignificant differences between carbamazepine and lithium in preventing manic and depressive episodes, although several studies indicate less-robust antimanic effects of carbamazepine,3 particularly for patients with classic euphoric mania without psychosis, bipolar II, or associated substance abuse.4
In atypical patients, carbamazepine appeared to outperform lithium, suggesting clinical differences in these agents that have different mechanisms of action across neurotransmitter and peptide systems.
Low response rates Most randomized controlled trials and open adjunct studies suggest that 50% or more patients respond to lithium, valproate, or carbamazepine. Outcomes may be far less positive in clinical practice, however. Despite the use of mood stabilizers and a variety of antidepressants, benzodiazepines, and neuroleptics, our patients experienced substantial residual manic and depressive symptoms and functional impairment.
Similarly, clinician ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM) indicated a high rate of breakthrough episodes during carefully monitored and aggressive psychopharmacologic treatment.5 Two-thirds of the first 258 bipolar outpatients that we followed and rated daily for 1 year continued to be substantially impaired. One-quarter of them were ill for more than 75% of the year, despite using an average 4.1 psychopharmacologic agents per patient, including mood stabilizers, antidepressants, antipsychotics, and other agents.
Acute and long-term combination therapy appears more effective than monotherapy, particularly for treatment-refractory rapid cyclers.3 Even so, many patients do not respond to combination therapy. For example, in a 1-year prophylaxis study, 25% or fewer patients were rated “much improved” to “very much improved” on the Clinical Global Impressions (CGI) scale with lithium or carbamazepine as the baseline mood stabilizer and with antidepressants, neuroleptics, and benzodiazepines used as needed. In the third year of combination therapy, the overall response rate, including rapid cyclers, was roughly 50%.3
A low rate of response is apparent for lithium and valproate. While treating rapidly cycling patients, Calabrese and associates found that less than one-quarter of them responded well enough to the two-drug combination that they could be enrolled in a randomized double-blind comparison of each drug in monotherapy. Of those who were eligible, almost all relapsed with either lithium or valproate monotherapy and again required combination therapy.6
Table 1
RATE OF SWITCHING (%) INTO MANIA IN DEPRESSED BIPOLAR PATIENTS DURING ANTIDEPRESSANT TREATMENT*
| Acute treatment (10 wks) | Continuation treatment (52 wks)‡ | |||||
|---|---|---|---|---|---|---|
| Type of switch | Open (n=27) | Blind (n=100) | Total (n=127) | Open (n=12) | Blind (n=55) | Total (n=67) |
| Brief hypomania | 3.7% | 6% | 5.5% | – | 3.6% | 3% |
| Recurrent brief hypomania | 11 | 12 | 11.8 | 8.3% | 18.2 | 16.4 |
| Hypomania | 11 | 13 | 12.6 | 8.3 | 23.6 | 20.9 |
| Mania | 14.8 | 12 | 12.6 | 33.3 | 14.5 | 17.9 |
| Total | 25.8 | 25 | 25.2 | 41.6 | 38.1 | 38.8 |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | ||||||
| ‡ Most patients (80%) dropped out of this phase because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. | ||||||
| Source: Post RM et al. Bipolar Disord 2001;3:259-65, and unpublished data. | ||||||
Taken together, these studies indicate that even with our most effective and most studied agents, many patients respond inadequately. On the basis of this clinical reality, we began to explore additional options that might enhance clinical effectiveness.
Atypical antipsychotics
We assessed the addition of the atypical antipsychotic olanzapine in patients with treatment-refractory bipolar disorder7 and saw improvement in 57% of subjects with manic, mixed, and depressive components. These data foreshadowed more recent controlled findings of olanzapine’s usefulness in depression and mania.8,9 Our preliminary open clinical trial experience allowed for more controlled studies and subsequent approval and wider clinical use of olanzapine in bipolar illness.
Our initial assessment of other atypical antipsychotics found that quetiapine was associated with significantly fewer depressive symptoms on the Inventory of Depressive Symptomatology (IDS) when used as part of the regular treatment regimen. This improvement, which was seen in the first month, was maintained over 4 months of treatment, whereas no significant improvement in depression severity was seen with the use of risperidone or clozapine.10
It would be highly preliminary to conclude from these uncontrolled data that quetiapine had greater effects on bipolar depressive symptoms than did risperidone or clozapine. We cannot rule out the possibility that patients with different degrees of treatment resistance were enrolled and treated with the different concomitant drugs. However, patients did not differ at baseline in severity of their depression.
In this initial look at the atypicals, the severity of depressive symptoms on the IDS was much greater than that of mania, as measured by the Young Mania Rating Scale (YMRS). Thus, the precise patterns of illness being treated and the impact of these antipsychotics on depressive cyclicity and duration await closer examination. We intend to look at the relative efficacy and side-effect profiles of each of the atypical antipsychotics as used in clinical practice.
Second-generation antidepressants
Bupropion, sertraline, and venlafaxine have different mechanisms of action but appear to be of value as adjuncts to bipolar disorder treatment. When we used them as adjuncts to mood stabilizers in a prospective study, we found a 40 to 50% acute response rate and some switching into hypomania and mania (Table 1).11 Many switches appeared to involve only isolated brief bursts of hypomania or recurrent brief hypomanias, but more sustained episodes of hypomania (7 days) could be more clinically problematic. Only 13% of patients treated acutely with these antidepressants had more full-blown manic symptoms (i.e., those associated with moderate or greater dysfunction on the NIMH-LCM).
During the next year, when apparent acute antidepressant responders were offered continuation treatment, 18% switched into mania. However, most patients (about 80%) dropped out because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. The 127 patients who were randomized received 175 acute antidepressant trials. Only 9% were associated with a switch into mania with moderate dysfunction, whereas another 9% showed periods of hypomania lasting longer than 1 week.12
Table 2
RELAPSE INTO DEPRESSION IN BIPOLAR PATIENTS AFTER 1 YEAR WITH OR WITHOUT ADJUNCTIVE ANTIDEPRESSANT TREATMENT*
| Rate of relapse | |
|---|---|
| Continued on antidepressants | |
| Study #1 (n=19) | 35% |
| Study #2 (n=41) | 41% |
| Discontinued antidepressants | |
| Study #1 (n=25) | 68% |
| Study #2 (n=43) | 71% |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | |
| Study #1: Altshuler et al. J Clin Psychiatry 2001;62:612-6. | |
| Study #2: Altshuler et al. (submitted for publication 2002). | |
We have entered 176 patients into this double-blind, randomized comparison of bupropion, sertraline, and venlafaxine, with another 27 randomized openly. The study will attempt to examine any possible between-drug differences. Even without the blind being broken, it appears that these antidepressants are associated with a somewhat lower-than-expected rate of switching into mania with dysfunction, but a direct comparison with a placebo is not available.
In two other series, patients who remained well for 2 months on antidepressants plus mood stabilizers and then continued on their antidepressants had a much lower incidence of depressive relapses over the following year than those who discontinued their antidepressants (Table 2).13,14 Surprisingly, continuing the antidepressant was not associated with an increased rate of switching into mania.
Prospective randomized, controlled studies are required to confirm these findings. Even so, our studies appear to challenge conventional wisdom for the first time. Stopping antidepressant treatment in bipolar patients as soon as possible for fear of inducing a manic episode may not be the optimal recommendation in the admittedly small subgroup (about 15%) of patients who have responded well to an antidepressant for at least 2 months.
Table 3
SIX STUDIES COMPLETED BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number studied | Response rate |
|---|---|---|---|---|
| Antidepressants bupropion | Yes | Double-blind | 173 | ~ 47% |
| vs. sertraline vs. venlafaxine (Post et al, 2001) | Yes | Open | 27 | |
| Total = 200 | ||||
| Olanzapine (McElroy et al, 1998) | No | Open | 14 | ~ 57% |
| Lamotrigine (Suppes et al, 1999) | No | Open | 17 | ~ 65% |
| Gabapentin (Altshuler et al, 1999) | No | Open | 28 | 78% (14/18) for mania or hypomania 100% (n = 5) for depression 20% (1/5) for cycling |
| Topiramate (McElroy et al, 2000) | No | Open | 54 | 63% for mania or cycling 27% for acute depression (weight loss observed) |
| Tiagabine (Suppes et al, 2002) | No | Open | 17 | 18% (3/17) (3 possible seizures) |
Second-generation anticonvulsants
Based on our patients’ substantial residual symptoms, we explored a series of newly approved anticonvulsants for potential use as mood stabilizers.
Lamotrigine Consistent with other open and double-blind, placebo-controlled studies, lamotrigine showed promise as an adjunctive treatment, with improvement in depressive symptoms and cycling.15 One such study showed acute antidepressant effects of lamotrigine monotherapy compared with a placebo at dosages of 50 or 200 mg/d.16
Another double-blind, randomized, placebo-controlled NIMH study (partially funded by the Stanley Foundation) compared lamotrigine with gabapentin and a placebo in patients for whom lithium, carbamazepine, and valproate had failed.17 Most of these treatment-refractory patients received all three agents through three 6-week randomizations. Lamotrigine was much more effective in depression and overall illness than either a placebo or gabapentin. Men with bipolar compared with unipolar illness and patients with fewer unsuccessful clinical trials and hospitalizations for depression showed the greatest response.18
In two recent industry-sponsored controlled trials of long-term prophylaxis (following a recent manic or depressive episode), lamotrigine was significantly more effective than a placebo (Bowden et al and Calabrese et al, unpublished data). Lamotrigine was more effective than lithium in preventing depressive episodes, whereas lithium was more effective than lamotrigine for manic episodes, although lamotrigine was significantly better than the placebo.
These data suggest that lamotrigine may differ from valproate, carbamazepine, and lithium in being a more effective antidepressant than antimanic agent.
Topiramate Adjunctive use of topiramate in rapid-cycling and treatment-resistant patients has been examined in open studies, but controlled studies have not yet been published. In our open-label case series observations, 19 of 30 (63%) patients taking topiramate for mania or cycling were “much” or “very much” improved after 10 weeks, whereas only 3 of 11 (27%) patients taking topiramate for acute depression were so improved.19
Table 4
STUDIES IN PROGRESS BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number enrolled* | Notes |
|---|---|---|---|---|
| Topiramate vs. sibutramine | Yes | Open | 42 | Relative weight loss and mood stabilization |
| Omega-3 fatty acids (6 grams EPA vs. placebo) (for depression cycling) (Keck et al) | Yes Yes | Double-blind Double-blind | 62 60 | Data from both studies being analyzed |
| Acamprosate (for alcohol craving) (Grunze et al) | No | Open | 23 | Goal: 60 patients |
| Levetiracetam (Post et al) | No | Open | 13 | Goal: 30 patients |
| Zonisamide (McElroy et al) | No | Open | 45 | Goal: 60 patients European sites only |
| Tranylcypromine vs. lamotrigine (for refractory depression) (Nolen et al) | Yes | Open | 17 | Single-site study |
| Quetiapine (McElroy et al) | No | Open | 48 | Apparent superior antidepressant effects compared with risperidone or clozapine |
| Risperidone (Grunze et al) | No | Open | 37 | No significant effect on IDS‡ depression ratings |
| Clozapine (Suppes et al) | No | Open | 19 | No significant effect on IDS depression ratings |
| Modafinil vs. placebo (Frye et al) | Yes | Double-blind | Started 4/02 | |
| * Enrollment as of April 2002 n = 696 total patients in tables 3 and 4 | ||||
| ‡ IDS = Inventory of Depressive Symptomatology | ||||
An industry-sponsored double-blind, randomized trial of topiramate in acute mania looked promising in the first 67 patients, but not as encouraging in subsequent subjects. A positive drug effect re-emerged if one eliminated patients whose manic episodes were precipitated by antidepressants.
The acute antidepressant effects of topiramate did not appear promising in our study. On the other hand, a single-blind, randomized study of topiramate versus bupropion as adjunctive therapy for breakthrough bipolar depression suggested excellent acute response (approximately 56%) to both agents.20 These data leave the potential antidepressant effects of topiramate undetermined.
In patients being treated with topiramate, we found significant weight loss (average –0.7 kg at 4 weeks, –1.6 kg at 10 weeks, –4.7 kg at 6 months, and –6.2 kg at 1 year).19 Average weight loss was 4.5±6.7 kg at the final evaluation (body mass index [BMI] change of –6.3% and –5% at 1 year and last evaluation, respectively).
We and others have thus seen substantial weight loss in one-third to one-half of patients taking topiramate for bipolar disorder.21 This weight loss is maintained over relatively long periods, which differentiates topiramate from other weight-loss regimens. Topiramate’s ability to decrease weight—even while it is in the early stages of evaluation as a mood stabilizer—is of considerable interest, given the substantial problem of overweight in the bipolar population and the fact that many drug therapies used in the illness are associated with weight gain.
We are proceeding with a randomized open comparison of the relative weight loss and psychotropic efficacy of topiramate versus the antiobesity agent sibutramine. Sibutramine has been reported to have antidepressant properties and is FDA-approved for weight loss.
Gabapentin and tiagabine Our group and many others have reported substantial overall clinical improvement when gabapentin was used as adjunctive therapy in an open study of patients with inadequate response to previous treatments.22 As monotherapy, however, gabapentin was not more effective than a placebo17 and was less effective than lamotrigine. Similarly, gabapentin was not an effective acute antimanic agent when compared with a placebo,23 although other double-blind, controlled studies have indicated positive effects in anxiety disorders24 and social phobias.25 Thus, the gabapentin literature appears to be divergent—i.e., positive studies support its adjunctive use in bipolar patients, but controlled studies suggest that it is not directly antimanic or mood-stabilizing.
Gabapentin’s effect on syndromes that are highly comorbid with bipolar illness could account for its apparent success as open adjunctive therapy. For example, in addition to its antianxiety effects, gabapentin shows positive effects in alcohol withdrawal, pain syndromes, sleep disorders (including restless leg syndrome), and obsessive-compulsive disorder. Also, combining gabapentin with other psychotropics with other mechanisms of action may have a more robust therapeutic effect than monotherapy.
Our preliminary observations with the selective GABA-reuptake inhibitor tiagabine were not promising. In an open study, only 3 of the first 17 SFBN patients with treatment-resistant affective disorders showed a partial response (moderate improvement on the Clinical Global Impressions Scale for Bipolar Illness [CGI-BP]) to adjunctive tiagabine treatment when the dosage was gradually increased. Serious side effects, including two patients with possible seizures, were noted.26 Similar results were seen when tiagabine doses were rapidly increased in eight patients with acute mania; none responded, and one had a major motor seizure.27
Thus, our initial observations led us to discontinue use of tiagabine and suggest any further clinical consideration of this agent for treatment-resistant affective disorders would require great care. This perspective contrasts with several previous positive case reports. It is unknown whether selected patients or those treated with lower dosages would be more responsive to this agent.
Like valproate, gabapentin and tiagabine increase brain GABA, but their apparent lack of antimanic efficacy strongly suggests that raising brain GABA alone does little to counter mania. These data further support the viewpoint already offered that all anticonvulsants are not alike and that anticonvulsants as a class are not necessarily mood stabilizers.
Zonisamide and levetiracetam Open studies of these two other anticonvulsants are near completion. Each has an interesting mechanistic profile, good tolerability, and the (potentially positive) side effect of inducing some weight loss, based on studies of neurologic patients.
Omega-3 fatty acids Based on positive reports of antidepressant effects of omega-3 fatty acids,28-30 we recently recruited more than 120 patients with bipolar disorder for two double-blind, randomized, controlled trials. Those patients with either an acute depression or a cyclic course will receive 6 grams of omega-3 fatty acid (the eicosapentaenoate form) or a placebo for 4 months of double-blind treatment, followed by an 8-month open, active continuation phase.
If results are positive, omega-3 fatty acids would represent the much-needed addition of a well-tolerated dietary agent to the therapeutic armamentarium directed at bipolar illness.
Conclusion
The Stanley Foundation Bipolar Network has begun to evaluate the usefulness of numerous older and new compounds for patients with bipolar disorder, including second-generation antidepressants, anticonvulsants, atypical antipsychotic agents, and omega-3 fatty acids. Based on initial observations from 16 treatment studies (Table 3), we have drawn these conclusions:
- Second-generation antidepressants such as bupropion, sertraline, and venlafaxine appear to have a lower-than-expected switch rate into mania, compared with the use of the older tricyclic antidepressants.
- If the addition of a second-generation antidepressant results in good antidepressant response for 2 months and no switches into mania, one should consider continuing the antidepressant in this small minority of patients, rather than stopping it as soon as possible as previously recommended.
- Adjunctive use of lamotrigine appears to be of value, particularly for acute depression and prophylaxis, for many patients who have not responded to other treatments.
- While our group and others have reported improvement with adjunctive gabapentin in bipolar patients in uncontrolled case series, its primary antimanic or mood-stabilizing utility (as well as that of tiagabine) does not appear promising.
- Topiramate’s ability to induce substantial weight loss may emerge as clinically therapeutic for patients with problematic weight gain related to other psychotropic drugs, independent of its ultimate utility as a possible mood stabilizer.
We are preparing to analyze other open case series assessing acute and long-term treatment outcomes as well as randomized, double-blind clinical trials. Even though the funding of this unique collaborative network is ending, we look forward to more effective treatment of bipolar disorder in the future with continued development of new psychotropic agents and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and the VA Cooperative studies.31
Related resources
- National Alliance for the Mentally Ill (NAMI). www.nami.org
- National Depressive and Manic-Depressive Association (NDMDA). www.ndmda.org
Drug brand names
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sibutramine • Meridia
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproate • Depakote
- Venlafaxine • Effexor
- Zonisamide • Zonegran
Acknowledgement
The authors are particularly indebted to the generosity of The Stanley Foundation in making funds available for this series of studies and several about to be completed.
Disclosure
Financial disclosure statements have been provided by all authors and are available upon request at [email protected].
When choosing psychopharmacologic agents for patients with bipolar disorder, the clinician lacks guidance from controlled clinical trials at virtually every therapeutic step. To help remedy this problem, we in the Stanley Foundation Bipolar Network (SFBN) evaluated compounds with potentially important therapeutic profiles. We began by documenting the demographics and illness characteristics of patients with bipolar disorder. Then we assessed some 16 treatments in naturalistic or formal studies, some of which are complete and others ongoing.
In this second installment, we report our current information about the efficacy of second-generation antidepressants, atypical antipsychotics, and anticonvulsant agents.
Mood stabilizers
As mood stabilizers, the anticonvulsants carbamazepine and valproate are well-accepted alternatives or adjuncts to lithium carbonate, which remains a mainstay treatment for acute episodes and long-term prophylaxis.1
Valproate is approved by the FDA for treatment of acute mania but not for long-term prevention. Open studies in rapid-cycling, treatment-refractory patients demonstrate substantial improvement for both manic and depressive phases with valproate. In the most recent controlled study, valproate did not prevent manic episodes to a statistically significant degree, but it did show greater antidepressant effects than placebo or lithium.2
Carbamazepine has been widely studied in acute mania and compared with lithium for long-term prophylaxis. Most studies show nonsignificant differences between carbamazepine and lithium in preventing manic and depressive episodes, although several studies indicate less-robust antimanic effects of carbamazepine,3 particularly for patients with classic euphoric mania without psychosis, bipolar II, or associated substance abuse.4
In atypical patients, carbamazepine appeared to outperform lithium, suggesting clinical differences in these agents that have different mechanisms of action across neurotransmitter and peptide systems.
Low response rates Most randomized controlled trials and open adjunct studies suggest that 50% or more patients respond to lithium, valproate, or carbamazepine. Outcomes may be far less positive in clinical practice, however. Despite the use of mood stabilizers and a variety of antidepressants, benzodiazepines, and neuroleptics, our patients experienced substantial residual manic and depressive symptoms and functional impairment.
Similarly, clinician ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM) indicated a high rate of breakthrough episodes during carefully monitored and aggressive psychopharmacologic treatment.5 Two-thirds of the first 258 bipolar outpatients that we followed and rated daily for 1 year continued to be substantially impaired. One-quarter of them were ill for more than 75% of the year, despite using an average 4.1 psychopharmacologic agents per patient, including mood stabilizers, antidepressants, antipsychotics, and other agents.
Acute and long-term combination therapy appears more effective than monotherapy, particularly for treatment-refractory rapid cyclers.3 Even so, many patients do not respond to combination therapy. For example, in a 1-year prophylaxis study, 25% or fewer patients were rated “much improved” to “very much improved” on the Clinical Global Impressions (CGI) scale with lithium or carbamazepine as the baseline mood stabilizer and with antidepressants, neuroleptics, and benzodiazepines used as needed. In the third year of combination therapy, the overall response rate, including rapid cyclers, was roughly 50%.3
A low rate of response is apparent for lithium and valproate. While treating rapidly cycling patients, Calabrese and associates found that less than one-quarter of them responded well enough to the two-drug combination that they could be enrolled in a randomized double-blind comparison of each drug in monotherapy. Of those who were eligible, almost all relapsed with either lithium or valproate monotherapy and again required combination therapy.6
Table 1
RATE OF SWITCHING (%) INTO MANIA IN DEPRESSED BIPOLAR PATIENTS DURING ANTIDEPRESSANT TREATMENT*
| Acute treatment (10 wks) | Continuation treatment (52 wks)‡ | |||||
|---|---|---|---|---|---|---|
| Type of switch | Open (n=27) | Blind (n=100) | Total (n=127) | Open (n=12) | Blind (n=55) | Total (n=67) |
| Brief hypomania | 3.7% | 6% | 5.5% | – | 3.6% | 3% |
| Recurrent brief hypomania | 11 | 12 | 11.8 | 8.3% | 18.2 | 16.4 |
| Hypomania | 11 | 13 | 12.6 | 8.3 | 23.6 | 20.9 |
| Mania | 14.8 | 12 | 12.6 | 33.3 | 14.5 | 17.9 |
| Total | 25.8 | 25 | 25.2 | 41.6 | 38.1 | 38.8 |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | ||||||
| ‡ Most patients (80%) dropped out of this phase because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. | ||||||
| Source: Post RM et al. Bipolar Disord 2001;3:259-65, and unpublished data. | ||||||
Taken together, these studies indicate that even with our most effective and most studied agents, many patients respond inadequately. On the basis of this clinical reality, we began to explore additional options that might enhance clinical effectiveness.
Atypical antipsychotics
We assessed the addition of the atypical antipsychotic olanzapine in patients with treatment-refractory bipolar disorder7 and saw improvement in 57% of subjects with manic, mixed, and depressive components. These data foreshadowed more recent controlled findings of olanzapine’s usefulness in depression and mania.8,9 Our preliminary open clinical trial experience allowed for more controlled studies and subsequent approval and wider clinical use of olanzapine in bipolar illness.
Our initial assessment of other atypical antipsychotics found that quetiapine was associated with significantly fewer depressive symptoms on the Inventory of Depressive Symptomatology (IDS) when used as part of the regular treatment regimen. This improvement, which was seen in the first month, was maintained over 4 months of treatment, whereas no significant improvement in depression severity was seen with the use of risperidone or clozapine.10
It would be highly preliminary to conclude from these uncontrolled data that quetiapine had greater effects on bipolar depressive symptoms than did risperidone or clozapine. We cannot rule out the possibility that patients with different degrees of treatment resistance were enrolled and treated with the different concomitant drugs. However, patients did not differ at baseline in severity of their depression.
In this initial look at the atypicals, the severity of depressive symptoms on the IDS was much greater than that of mania, as measured by the Young Mania Rating Scale (YMRS). Thus, the precise patterns of illness being treated and the impact of these antipsychotics on depressive cyclicity and duration await closer examination. We intend to look at the relative efficacy and side-effect profiles of each of the atypical antipsychotics as used in clinical practice.
Second-generation antidepressants
Bupropion, sertraline, and venlafaxine have different mechanisms of action but appear to be of value as adjuncts to bipolar disorder treatment. When we used them as adjuncts to mood stabilizers in a prospective study, we found a 40 to 50% acute response rate and some switching into hypomania and mania (Table 1).11 Many switches appeared to involve only isolated brief bursts of hypomania or recurrent brief hypomanias, but more sustained episodes of hypomania (7 days) could be more clinically problematic. Only 13% of patients treated acutely with these antidepressants had more full-blown manic symptoms (i.e., those associated with moderate or greater dysfunction on the NIMH-LCM).
During the next year, when apparent acute antidepressant responders were offered continuation treatment, 18% switched into mania. However, most patients (about 80%) dropped out because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. The 127 patients who were randomized received 175 acute antidepressant trials. Only 9% were associated with a switch into mania with moderate dysfunction, whereas another 9% showed periods of hypomania lasting longer than 1 week.12
Table 2
RELAPSE INTO DEPRESSION IN BIPOLAR PATIENTS AFTER 1 YEAR WITH OR WITHOUT ADJUNCTIVE ANTIDEPRESSANT TREATMENT*
| Rate of relapse | |
|---|---|
| Continued on antidepressants | |
| Study #1 (n=19) | 35% |
| Study #2 (n=41) | 41% |
| Discontinued antidepressants | |
| Study #1 (n=25) | 68% |
| Study #2 (n=43) | 71% |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | |
| Study #1: Altshuler et al. J Clin Psychiatry 2001;62:612-6. | |
| Study #2: Altshuler et al. (submitted for publication 2002). | |
We have entered 176 patients into this double-blind, randomized comparison of bupropion, sertraline, and venlafaxine, with another 27 randomized openly. The study will attempt to examine any possible between-drug differences. Even without the blind being broken, it appears that these antidepressants are associated with a somewhat lower-than-expected rate of switching into mania with dysfunction, but a direct comparison with a placebo is not available.
In two other series, patients who remained well for 2 months on antidepressants plus mood stabilizers and then continued on their antidepressants had a much lower incidence of depressive relapses over the following year than those who discontinued their antidepressants (Table 2).13,14 Surprisingly, continuing the antidepressant was not associated with an increased rate of switching into mania.
Prospective randomized, controlled studies are required to confirm these findings. Even so, our studies appear to challenge conventional wisdom for the first time. Stopping antidepressant treatment in bipolar patients as soon as possible for fear of inducing a manic episode may not be the optimal recommendation in the admittedly small subgroup (about 15%) of patients who have responded well to an antidepressant for at least 2 months.
Table 3
SIX STUDIES COMPLETED BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number studied | Response rate |
|---|---|---|---|---|
| Antidepressants bupropion | Yes | Double-blind | 173 | ~ 47% |
| vs. sertraline vs. venlafaxine (Post et al, 2001) | Yes | Open | 27 | |
| Total = 200 | ||||
| Olanzapine (McElroy et al, 1998) | No | Open | 14 | ~ 57% |
| Lamotrigine (Suppes et al, 1999) | No | Open | 17 | ~ 65% |
| Gabapentin (Altshuler et al, 1999) | No | Open | 28 | 78% (14/18) for mania or hypomania 100% (n = 5) for depression 20% (1/5) for cycling |
| Topiramate (McElroy et al, 2000) | No | Open | 54 | 63% for mania or cycling 27% for acute depression (weight loss observed) |
| Tiagabine (Suppes et al, 2002) | No | Open | 17 | 18% (3/17) (3 possible seizures) |
Second-generation anticonvulsants
Based on our patients’ substantial residual symptoms, we explored a series of newly approved anticonvulsants for potential use as mood stabilizers.
Lamotrigine Consistent with other open and double-blind, placebo-controlled studies, lamotrigine showed promise as an adjunctive treatment, with improvement in depressive symptoms and cycling.15 One such study showed acute antidepressant effects of lamotrigine monotherapy compared with a placebo at dosages of 50 or 200 mg/d.16
Another double-blind, randomized, placebo-controlled NIMH study (partially funded by the Stanley Foundation) compared lamotrigine with gabapentin and a placebo in patients for whom lithium, carbamazepine, and valproate had failed.17 Most of these treatment-refractory patients received all three agents through three 6-week randomizations. Lamotrigine was much more effective in depression and overall illness than either a placebo or gabapentin. Men with bipolar compared with unipolar illness and patients with fewer unsuccessful clinical trials and hospitalizations for depression showed the greatest response.18
In two recent industry-sponsored controlled trials of long-term prophylaxis (following a recent manic or depressive episode), lamotrigine was significantly more effective than a placebo (Bowden et al and Calabrese et al, unpublished data). Lamotrigine was more effective than lithium in preventing depressive episodes, whereas lithium was more effective than lamotrigine for manic episodes, although lamotrigine was significantly better than the placebo.
These data suggest that lamotrigine may differ from valproate, carbamazepine, and lithium in being a more effective antidepressant than antimanic agent.
Topiramate Adjunctive use of topiramate in rapid-cycling and treatment-resistant patients has been examined in open studies, but controlled studies have not yet been published. In our open-label case series observations, 19 of 30 (63%) patients taking topiramate for mania or cycling were “much” or “very much” improved after 10 weeks, whereas only 3 of 11 (27%) patients taking topiramate for acute depression were so improved.19
Table 4
STUDIES IN PROGRESS BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number enrolled* | Notes |
|---|---|---|---|---|
| Topiramate vs. sibutramine | Yes | Open | 42 | Relative weight loss and mood stabilization |
| Omega-3 fatty acids (6 grams EPA vs. placebo) (for depression cycling) (Keck et al) | Yes Yes | Double-blind Double-blind | 62 60 | Data from both studies being analyzed |
| Acamprosate (for alcohol craving) (Grunze et al) | No | Open | 23 | Goal: 60 patients |
| Levetiracetam (Post et al) | No | Open | 13 | Goal: 30 patients |
| Zonisamide (McElroy et al) | No | Open | 45 | Goal: 60 patients European sites only |
| Tranylcypromine vs. lamotrigine (for refractory depression) (Nolen et al) | Yes | Open | 17 | Single-site study |
| Quetiapine (McElroy et al) | No | Open | 48 | Apparent superior antidepressant effects compared with risperidone or clozapine |
| Risperidone (Grunze et al) | No | Open | 37 | No significant effect on IDS‡ depression ratings |
| Clozapine (Suppes et al) | No | Open | 19 | No significant effect on IDS depression ratings |
| Modafinil vs. placebo (Frye et al) | Yes | Double-blind | Started 4/02 | |
| * Enrollment as of April 2002 n = 696 total patients in tables 3 and 4 | ||||
| ‡ IDS = Inventory of Depressive Symptomatology | ||||
An industry-sponsored double-blind, randomized trial of topiramate in acute mania looked promising in the first 67 patients, but not as encouraging in subsequent subjects. A positive drug effect re-emerged if one eliminated patients whose manic episodes were precipitated by antidepressants.
The acute antidepressant effects of topiramate did not appear promising in our study. On the other hand, a single-blind, randomized study of topiramate versus bupropion as adjunctive therapy for breakthrough bipolar depression suggested excellent acute response (approximately 56%) to both agents.20 These data leave the potential antidepressant effects of topiramate undetermined.
In patients being treated with topiramate, we found significant weight loss (average –0.7 kg at 4 weeks, –1.6 kg at 10 weeks, –4.7 kg at 6 months, and –6.2 kg at 1 year).19 Average weight loss was 4.5±6.7 kg at the final evaluation (body mass index [BMI] change of –6.3% and –5% at 1 year and last evaluation, respectively).
We and others have thus seen substantial weight loss in one-third to one-half of patients taking topiramate for bipolar disorder.21 This weight loss is maintained over relatively long periods, which differentiates topiramate from other weight-loss regimens. Topiramate’s ability to decrease weight—even while it is in the early stages of evaluation as a mood stabilizer—is of considerable interest, given the substantial problem of overweight in the bipolar population and the fact that many drug therapies used in the illness are associated with weight gain.
We are proceeding with a randomized open comparison of the relative weight loss and psychotropic efficacy of topiramate versus the antiobesity agent sibutramine. Sibutramine has been reported to have antidepressant properties and is FDA-approved for weight loss.
Gabapentin and tiagabine Our group and many others have reported substantial overall clinical improvement when gabapentin was used as adjunctive therapy in an open study of patients with inadequate response to previous treatments.22 As monotherapy, however, gabapentin was not more effective than a placebo17 and was less effective than lamotrigine. Similarly, gabapentin was not an effective acute antimanic agent when compared with a placebo,23 although other double-blind, controlled studies have indicated positive effects in anxiety disorders24 and social phobias.25 Thus, the gabapentin literature appears to be divergent—i.e., positive studies support its adjunctive use in bipolar patients, but controlled studies suggest that it is not directly antimanic or mood-stabilizing.
Gabapentin’s effect on syndromes that are highly comorbid with bipolar illness could account for its apparent success as open adjunctive therapy. For example, in addition to its antianxiety effects, gabapentin shows positive effects in alcohol withdrawal, pain syndromes, sleep disorders (including restless leg syndrome), and obsessive-compulsive disorder. Also, combining gabapentin with other psychotropics with other mechanisms of action may have a more robust therapeutic effect than monotherapy.
Our preliminary observations with the selective GABA-reuptake inhibitor tiagabine were not promising. In an open study, only 3 of the first 17 SFBN patients with treatment-resistant affective disorders showed a partial response (moderate improvement on the Clinical Global Impressions Scale for Bipolar Illness [CGI-BP]) to adjunctive tiagabine treatment when the dosage was gradually increased. Serious side effects, including two patients with possible seizures, were noted.26 Similar results were seen when tiagabine doses were rapidly increased in eight patients with acute mania; none responded, and one had a major motor seizure.27
Thus, our initial observations led us to discontinue use of tiagabine and suggest any further clinical consideration of this agent for treatment-resistant affective disorders would require great care. This perspective contrasts with several previous positive case reports. It is unknown whether selected patients or those treated with lower dosages would be more responsive to this agent.
Like valproate, gabapentin and tiagabine increase brain GABA, but their apparent lack of antimanic efficacy strongly suggests that raising brain GABA alone does little to counter mania. These data further support the viewpoint already offered that all anticonvulsants are not alike and that anticonvulsants as a class are not necessarily mood stabilizers.
Zonisamide and levetiracetam Open studies of these two other anticonvulsants are near completion. Each has an interesting mechanistic profile, good tolerability, and the (potentially positive) side effect of inducing some weight loss, based on studies of neurologic patients.
Omega-3 fatty acids Based on positive reports of antidepressant effects of omega-3 fatty acids,28-30 we recently recruited more than 120 patients with bipolar disorder for two double-blind, randomized, controlled trials. Those patients with either an acute depression or a cyclic course will receive 6 grams of omega-3 fatty acid (the eicosapentaenoate form) or a placebo for 4 months of double-blind treatment, followed by an 8-month open, active continuation phase.
If results are positive, omega-3 fatty acids would represent the much-needed addition of a well-tolerated dietary agent to the therapeutic armamentarium directed at bipolar illness.
Conclusion
The Stanley Foundation Bipolar Network has begun to evaluate the usefulness of numerous older and new compounds for patients with bipolar disorder, including second-generation antidepressants, anticonvulsants, atypical antipsychotic agents, and omega-3 fatty acids. Based on initial observations from 16 treatment studies (Table 3), we have drawn these conclusions:
- Second-generation antidepressants such as bupropion, sertraline, and venlafaxine appear to have a lower-than-expected switch rate into mania, compared with the use of the older tricyclic antidepressants.
- If the addition of a second-generation antidepressant results in good antidepressant response for 2 months and no switches into mania, one should consider continuing the antidepressant in this small minority of patients, rather than stopping it as soon as possible as previously recommended.
- Adjunctive use of lamotrigine appears to be of value, particularly for acute depression and prophylaxis, for many patients who have not responded to other treatments.
- While our group and others have reported improvement with adjunctive gabapentin in bipolar patients in uncontrolled case series, its primary antimanic or mood-stabilizing utility (as well as that of tiagabine) does not appear promising.
- Topiramate’s ability to induce substantial weight loss may emerge as clinically therapeutic for patients with problematic weight gain related to other psychotropic drugs, independent of its ultimate utility as a possible mood stabilizer.
We are preparing to analyze other open case series assessing acute and long-term treatment outcomes as well as randomized, double-blind clinical trials. Even though the funding of this unique collaborative network is ending, we look forward to more effective treatment of bipolar disorder in the future with continued development of new psychotropic agents and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and the VA Cooperative studies.31
Related resources
- National Alliance for the Mentally Ill (NAMI). www.nami.org
- National Depressive and Manic-Depressive Association (NDMDA). www.ndmda.org
Drug brand names
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sibutramine • Meridia
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproate • Depakote
- Venlafaxine • Effexor
- Zonisamide • Zonegran
Acknowledgement
The authors are particularly indebted to the generosity of The Stanley Foundation in making funds available for this series of studies and several about to be completed.
Disclosure
Financial disclosure statements have been provided by all authors and are available upon request at [email protected].
1. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press, 1990.
2. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
3. Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997a;58:470-8.
4. Greil W, Kleindienst N. The comparative prophylactic efficacy of lithium and carbamazepine in patients with bipolar I disorder. Int Clin Psychopharmacol 1999;14:277-81.
5. Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for one year with daily prospective ratings on the NIMH-LCM (submitted for publication, 2002).
6. Calabrese JR, Rapport DJ. Mood stabilizers and the evolution of maintenance study designs in bipolar I disorder. J Clin Psychiatry 1999;60(suppl 5):5-13.
7. McElroy SL, Frye M, Denicoff K, et al. Olanzapine in treatment-resistant bipolar disorder. J Affect Disord 1998;49:119-22.
8. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
9. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
10. Keck PE, Jr. New antiepileptics in the treatment of bipolar disorder. Syllabus and Proceedings Summary of the 2001 American Psychiatric Association Meeting, New Orleans, May 5-10, 2001. Abstract 34E, 93.
11. Post RM, Altshuler LL, Frye MA, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001;3:259-65.
12. Post RM, Leverich GS, Nolen WA, et al. A reevaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2002 (in press).
13. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-6.
14. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute remission from bipolar depression on rates of depressive relapse at one-year follow-up (submitted for publication 2002).
15. Suppes T, Brown ES, McElroy SL, et al. Lamotrigine for the treatment of bipolar disorder: a clinical case series. J Affective Disord 1999;53:95-8.
16. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60:79-88.
17. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14.
18. Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biolog Psychiatry 2002;51:253-60.
19. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biolog Psychiatry 2000;47:1025-33.
20. McIntyre RS, Mancini DA, McCann SM, et al. Topiramiate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 2002;4:207-13.
21. Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults. Epilepsia 2000;41(suppl 1):S66-S71.
22. Altshuler LL, Keck PE, Jr, McElroy SL, et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1999;1:61-5.
23. Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord 2000;2:249-55.
24. Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000;20:467-71.
25. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999;19:341-8.
26. Suppes T, Chisholm K, Dhvule D, et al. Tiagabine in bipolar disorder: adverse events in an open case series (submitted for publication, 2002).
27. Grunze H, Erfurth A, Marcuse A, et al. Tiagabine appears not to be efficacious in the treatment of acute mania. J Clin Psychiatry 1999;60:759-62.
28. Stoll AL, Severus WE, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56:407-12.
29. Horrobin DF, Peet M. A dose-ranging study of ethyl-eicosapentaenoate in treatment-unresponsive depression. Biolog Psychiatry 2001;49:37S.-
30. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-9.
31. Bauer MS, Williford WO, Dawson EE, et al. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: Reducing the efficacy-effectiveness gap in bipolar disorder. J Affect Disord 2001;67:61-78.
1. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press, 1990.
2. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
3. Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997a;58:470-8.
4. Greil W, Kleindienst N. The comparative prophylactic efficacy of lithium and carbamazepine in patients with bipolar I disorder. Int Clin Psychopharmacol 1999;14:277-81.
5. Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for one year with daily prospective ratings on the NIMH-LCM (submitted for publication, 2002).
6. Calabrese JR, Rapport DJ. Mood stabilizers and the evolution of maintenance study designs in bipolar I disorder. J Clin Psychiatry 1999;60(suppl 5):5-13.
7. McElroy SL, Frye M, Denicoff K, et al. Olanzapine in treatment-resistant bipolar disorder. J Affect Disord 1998;49:119-22.
8. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
9. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
10. Keck PE, Jr. New antiepileptics in the treatment of bipolar disorder. Syllabus and Proceedings Summary of the 2001 American Psychiatric Association Meeting, New Orleans, May 5-10, 2001. Abstract 34E, 93.
11. Post RM, Altshuler LL, Frye MA, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001;3:259-65.
12. Post RM, Leverich GS, Nolen WA, et al. A reevaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2002 (in press).
13. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-6.
14. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute remission from bipolar depression on rates of depressive relapse at one-year follow-up (submitted for publication 2002).
15. Suppes T, Brown ES, McElroy SL, et al. Lamotrigine for the treatment of bipolar disorder: a clinical case series. J Affective Disord 1999;53:95-8.
16. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60:79-88.
17. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14.
18. Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biolog Psychiatry 2002;51:253-60.
19. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biolog Psychiatry 2000;47:1025-33.
20. McIntyre RS, Mancini DA, McCann SM, et al. Topiramiate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 2002;4:207-13.
21. Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults. Epilepsia 2000;41(suppl 1):S66-S71.
22. Altshuler LL, Keck PE, Jr, McElroy SL, et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1999;1:61-5.
23. Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord 2000;2:249-55.
24. Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000;20:467-71.
25. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999;19:341-8.
26. Suppes T, Chisholm K, Dhvule D, et al. Tiagabine in bipolar disorder: adverse events in an open case series (submitted for publication, 2002).
27. Grunze H, Erfurth A, Marcuse A, et al. Tiagabine appears not to be efficacious in the treatment of acute mania. J Clin Psychiatry 1999;60:759-62.
28. Stoll AL, Severus WE, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56:407-12.
29. Horrobin DF, Peet M. A dose-ranging study of ethyl-eicosapentaenoate in treatment-unresponsive depression. Biolog Psychiatry 2001;49:37S.-
30. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-9.
31. Bauer MS, Williford WO, Dawson EE, et al. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: Reducing the efficacy-effectiveness gap in bipolar disorder. J Affect Disord 2001;67:61-78.
From the Stanley Foundation Bipolar Network: Efficacy of adjunctive therapies for treatment-resistant patients
When choosing psychopharmacologic agents for patients with bipolar disorder, the clinician lacks guidance from controlled clinical trials at virtually every therapeutic step. To help remedy this problem, we in the Stanley Foundation Bipolar Network (SFBN) evaluated compounds with potentially important therapeutic profiles. We began by documenting the demographics and illness characteristics of patients with bipolar disorder. Then we assessed some 16 treatments in naturalistic or formal studies, some of which are complete and others ongoing.
In this second installment, we report our current information about the efficacy of second-generation antidepressants, atypical antipsychotics, and anticonvulsant agents.
Mood stabilizers
As mood stabilizers, the anticonvulsants carbamazepine and valproate are well-accepted alternatives or adjuncts to lithium carbonate, which remains a mainstay treatment for acute episodes and long-term prophylaxis.1
Valproate is approved by the FDA for treatment of acute mania but not for long-term prevention. Open studies in rapid-cycling, treatment-refractory patients demonstrate substantial improvement for both manic and depressive phases with valproate. In the most recent controlled study, valproate did not prevent manic episodes to a statistically significant degree, but it did show greater antidepressant effects than placebo or lithium.2
Carbamazepine has been widely studied in acute mania and compared with lithium for long-term prophylaxis. Most studies show nonsignificant differences between carbamazepine and lithium in preventing manic and depressive episodes, although several studies indicate less-robust antimanic effects of carbamazepine,3 particularly for patients with classic euphoric mania without psychosis, bipolar II, or associated substance abuse.4
In atypical patients, carbamazepine appeared to outperform lithium, suggesting clinical differences in these agents that have different mechanisms of action across neurotransmitter and peptide systems.
Low response rates Most randomized controlled trials and open adjunct studies suggest that 50% or more patients respond to lithium, valproate, or carbamazepine. Outcomes may be far less positive in clinical practice, however. Despite the use of mood stabilizers and a variety of antidepressants, benzodiazepines, and neuroleptics, our patients experienced substantial residual manic and depressive symptoms and functional impairment.
Similarly, clinician ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM) indicated a high rate of breakthrough episodes during carefully monitored and aggressive psychopharmacologic treatment.5 Two-thirds of the first 258 bipolar outpatients that we followed and rated daily for 1 year continued to be substantially impaired. One-quarter of them were ill for more than 75% of the year, despite using an average 4.1 psychopharmacologic agents per patient, including mood stabilizers, antidepressants, antipsychotics, and other agents.
Acute and long-term combination therapy appears more effective than monotherapy, particularly for treatment-refractory rapid cyclers.3 Even so, many patients do not respond to combination therapy. For example, in a 1-year prophylaxis study, 25% or fewer patients were rated “much improved” to “very much improved” on the Clinical Global Impressions (CGI) scale with lithium or carbamazepine as the baseline mood stabilizer and with antidepressants, neuroleptics, and benzodiazepines used as needed. In the third year of combination therapy, the overall response rate, including rapid cyclers, was roughly 50%.3
A low rate of response is apparent for lithium and valproate. While treating rapidly cycling patients, Calabrese and associates found that less than one-quarter of them responded well enough to the two-drug combination that they could be enrolled in a randomized double-blind comparison of each drug in monotherapy. Of those who were eligible, almost all relapsed with either lithium or valproate monotherapy and again required combination therapy.6
Table 1
RATE OF SWITCHING (%) INTO MANIA IN DEPRESSED BIPOLAR PATIENTS DURING ANTIDEPRESSANT TREATMENT*
| Acute treatment (10 wks) | Continuation treatment (52 wks)‡ | |||||
|---|---|---|---|---|---|---|
| Type of switch | Open (n=27) | Blind (n=100) | Total (n=127) | Open (n=12) | Blind (n=55) | Total (n=67) |
| Brief hypomania | 3.7% | 6% | 5.5% | – | 3.6% | 3% |
| Recurrent brief hypomania | 11 | 12 | 11.8 | 8.3% | 18.2 | 16.4 |
| Hypomania | 11 | 13 | 12.6 | 8.3 | 23.6 | 20.9 |
| Mania | 14.8 | 12 | 12.6 | 33.3 | 14.5 | 17.9 |
| Total | 25.8 | 25 | 25.2 | 41.6 | 38.1 | 38.8 |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | ||||||
| ‡ Most patients (80%) dropped out of this phase because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. | ||||||
| Source: Post RM et al. Bipolar Disord 2001;3:259-65, and unpublished data. | ||||||
Taken together, these studies indicate that even with our most effective and most studied agents, many patients respond inadequately. On the basis of this clinical reality, we began to explore additional options that might enhance clinical effectiveness.
Atypical antipsychotics
We assessed the addition of the atypical antipsychotic olanzapine in patients with treatment-refractory bipolar disorder7 and saw improvement in 57% of subjects with manic, mixed, and depressive components. These data foreshadowed more recent controlled findings of olanzapine’s usefulness in depression and mania.8,9 Our preliminary open clinical trial experience allowed for more controlled studies and subsequent approval and wider clinical use of olanzapine in bipolar illness.
Our initial assessment of other atypical antipsychotics found that quetiapine was associated with significantly fewer depressive symptoms on the Inventory of Depressive Symptomatology (IDS) when used as part of the regular treatment regimen. This improvement, which was seen in the first month, was maintained over 4 months of treatment, whereas no significant improvement in depression severity was seen with the use of risperidone or clozapine.10
It would be highly preliminary to conclude from these uncontrolled data that quetiapine had greater effects on bipolar depressive symptoms than did risperidone or clozapine. We cannot rule out the possibility that patients with different degrees of treatment resistance were enrolled and treated with the different concomitant drugs. However, patients did not differ at baseline in severity of their depression.
In this initial look at the atypicals, the severity of depressive symptoms on the IDS was much greater than that of mania, as measured by the Young Mania Rating Scale (YMRS). Thus, the precise patterns of illness being treated and the impact of these antipsychotics on depressive cyclicity and duration await closer examination. We intend to look at the relative efficacy and side-effect profiles of each of the atypical antipsychotics as used in clinical practice.
Second-generation antidepressants
Bupropion, sertraline, and venlafaxine have different mechanisms of action but appear to be of value as adjuncts to bipolar disorder treatment. When we used them as adjuncts to mood stabilizers in a prospective study, we found a 40 to 50% acute response rate and some switching into hypomania and mania (Table 1).11 Many switches appeared to involve only isolated brief bursts of hypomania or recurrent brief hypomanias, but more sustained episodes of hypomania (7 days) could be more clinically problematic. Only 13% of patients treated acutely with these antidepressants had more full-blown manic symptoms (i.e., those associated with moderate or greater dysfunction on the NIMH-LCM).
During the next year, when apparent acute antidepressant responders were offered continuation treatment, 18% switched into mania. However, most patients (about 80%) dropped out because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. The 127 patients who were randomized received 175 acute antidepressant trials. Only 9% were associated with a switch into mania with moderate dysfunction, whereas another 9% showed periods of hypomania lasting longer than 1 week.12
Table 2
RELAPSE INTO DEPRESSION IN BIPOLAR PATIENTS AFTER 1 YEAR WITH OR WITHOUT ADJUNCTIVE ANTIDEPRESSANT TREATMENT*
| Rate of relapse | |
|---|---|
| Continued on antidepressants | |
| Study #1 (n=19) | 35% |
| Study #2 (n=41) | 41% |
| Discontinued antidepressants | |
| Study #1 (n=25) | 68% |
| Study #2 (n=43) | 71% |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | |
| Study #1: Altshuler et al. J Clin Psychiatry 2001;62:612-6. | |
| Study #2: Altshuler et al. (submitted for publication 2002). | |
We have entered 176 patients into this double-blind, randomized comparison of bupropion, sertraline, and venlafaxine, with another 27 randomized openly. The study will attempt to examine any possible between-drug differences. Even without the blind being broken, it appears that these antidepressants are associated with a somewhat lower-than-expected rate of switching into mania with dysfunction, but a direct comparison with a placebo is not available.
In two other series, patients who remained well for 2 months on antidepressants plus mood stabilizers and then continued on their antidepressants had a much lower incidence of depressive relapses over the following year than those who discontinued their antidepressants (Table 2).13,14 Surprisingly, continuing the antidepressant was not associated with an increased rate of switching into mania.
Prospective randomized, controlled studies are required to confirm these findings. Even so, our studies appear to challenge conventional wisdom for the first time. Stopping antidepressant treatment in bipolar patients as soon as possible for fear of inducing a manic episode may not be the optimal recommendation in the admittedly small subgroup (about 15%) of patients who have responded well to an antidepressant for at least 2 months.
Table 3
SIX STUDIES COMPLETED BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number studied | Response rate |
|---|---|---|---|---|
| Antidepressants bupropion | Yes | Double-blind | 173 | ~ 47% |
| vs. sertraline vs. venlafaxine (Post et al, 2001) | Yes | Open | 27 | |
| Total = 200 | ||||
| Olanzapine (McElroy et al, 1998) | No | Open | 14 | ~ 57% |
| Lamotrigine (Suppes et al, 1999) | No | Open | 17 | ~ 65% |
| Gabapentin (Altshuler et al, 1999) | No | Open | 28 | 78% (14/18) for mania or hypomania 100% (n = 5) for depression 20% (1/5) for cycling |
| Topiramate (McElroy et al, 2000) | No | Open | 54 | 63% for mania or cycling 27% for acute depression (weight loss observed) |
| Tiagabine (Suppes et al, 2002) | No | Open | 17 | 18% (3/17) (3 possible seizures) |
Second-generation anticonvulsants
Based on our patients’ substantial residual symptoms, we explored a series of newly approved anticonvulsants for potential use as mood stabilizers.
Lamotrigine Consistent with other open and double-blind, placebo-controlled studies, lamotrigine showed promise as an adjunctive treatment, with improvement in depressive symptoms and cycling.15 One such study showed acute antidepressant effects of lamotrigine monotherapy compared with a placebo at dosages of 50 or 200 mg/d.16
Another double-blind, randomized, placebo-controlled NIMH study (partially funded by the Stanley Foundation) compared lamotrigine with gabapentin and a placebo in patients for whom lithium, carbamazepine, and valproate had failed.17 Most of these treatment-refractory patients received all three agents through three 6-week randomizations. Lamotrigine was much more effective in depression and overall illness than either a placebo or gabapentin. Men with bipolar compared with unipolar illness and patients with fewer unsuccessful clinical trials and hospitalizations for depression showed the greatest response.18
In two recent industry-sponsored controlled trials of long-term prophylaxis (following a recent manic or depressive episode), lamotrigine was significantly more effective than a placebo (Bowden et al and Calabrese et al, unpublished data). Lamotrigine was more effective than lithium in preventing depressive episodes, whereas lithium was more effective than lamotrigine for manic episodes, although lamotrigine was significantly better than the placebo.
These data suggest that lamotrigine may differ from valproate, carbamazepine, and lithium in being a more effective antidepressant than antimanic agent.
Topiramate Adjunctive use of topiramate in rapid-cycling and treatment-resistant patients has been examined in open studies, but controlled studies have not yet been published. In our open-label case series observations, 19 of 30 (63%) patients taking topiramate for mania or cycling were “much” or “very much” improved after 10 weeks, whereas only 3 of 11 (27%) patients taking topiramate for acute depression were so improved.19
Table 4
STUDIES IN PROGRESS BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number enrolled* | Notes |
|---|---|---|---|---|
| Topiramate vs. sibutramine | Yes | Open | 42 | Relative weight loss and mood stabilization |
| Omega-3 fatty acids (6 grams EPA vs. placebo) (for depression cycling) (Keck et al) | Yes Yes | Double-blind Double-blind | 62 60 | Data from both studies being analyzed |
| Acamprosate (for alcohol craving) (Grunze et al) | No | Open | 23 | Goal: 60 patients |
| Levetiracetam (Post et al) | No | Open | 13 | Goal: 30 patients |
| Zonisamide (McElroy et al) | No | Open | 45 | Goal: 60 patients European sites only |
| Tranylcypromine vs. lamotrigine (for refractory depression) (Nolen et al) | Yes | Open | 17 | Single-site study |
| Quetiapine (McElroy et al) | No | Open | 48 | Apparent superior antidepressant effects compared with risperidone or clozapine |
| Risperidone (Grunze et al) | No | Open | 37 | No significant effect on IDS‡ depression ratings |
| Clozapine (Suppes et al) | No | Open | 19 | No significant effect on IDS depression ratings |
| Modafinil vs. placebo (Frye et al) | Yes | Double-blind | Started 4/02 | |
| * Enrollment as of April 2002 n = 696 total patients in tables 3 and 4 | ||||
| ‡ IDS = Inventory of Depressive Symptomatology | ||||
An industry-sponsored double-blind, randomized trial of topiramate in acute mania looked promising in the first 67 patients, but not as encouraging in subsequent subjects. A positive drug effect re-emerged if one eliminated patients whose manic episodes were precipitated by antidepressants.
The acute antidepressant effects of topiramate did not appear promising in our study. On the other hand, a single-blind, randomized study of topiramate versus bupropion as adjunctive therapy for breakthrough bipolar depression suggested excellent acute response (approximately 56%) to both agents.20 These data leave the potential antidepressant effects of topiramate undetermined.
In patients being treated with topiramate, we found significant weight loss (average –0.7 kg at 4 weeks, –1.6 kg at 10 weeks, –4.7 kg at 6 months, and –6.2 kg at 1 year).19 Average weight loss was 4.5±6.7 kg at the final evaluation (body mass index [BMI] change of –6.3% and –5% at 1 year and last evaluation, respectively).
We and others have thus seen substantial weight loss in one-third to one-half of patients taking topiramate for bipolar disorder.21 This weight loss is maintained over relatively long periods, which differentiates topiramate from other weight-loss regimens. Topiramate’s ability to decrease weight—even while it is in the early stages of evaluation as a mood stabilizer—is of considerable interest, given the substantial problem of overweight in the bipolar population and the fact that many drug therapies used in the illness are associated with weight gain.
We are proceeding with a randomized open comparison of the relative weight loss and psychotropic efficacy of topiramate versus the antiobesity agent sibutramine. Sibutramine has been reported to have antidepressant properties and is FDA-approved for weight loss.
Gabapentin and tiagabine Our group and many others have reported substantial overall clinical improvement when gabapentin was used as adjunctive therapy in an open study of patients with inadequate response to previous treatments.22 As monotherapy, however, gabapentin was not more effective than a placebo17 and was less effective than lamotrigine. Similarly, gabapentin was not an effective acute antimanic agent when compared with a placebo,23 although other double-blind, controlled studies have indicated positive effects in anxiety disorders24 and social phobias.25 Thus, the gabapentin literature appears to be divergent—i.e., positive studies support its adjunctive use in bipolar patients, but controlled studies suggest that it is not directly antimanic or mood-stabilizing.
Gabapentin’s effect on syndromes that are highly comorbid with bipolar illness could account for its apparent success as open adjunctive therapy. For example, in addition to its antianxiety effects, gabapentin shows positive effects in alcohol withdrawal, pain syndromes, sleep disorders (including restless leg syndrome), and obsessive-compulsive disorder. Also, combining gabapentin with other psychotropics with other mechanisms of action may have a more robust therapeutic effect than monotherapy.
Our preliminary observations with the selective GABA-reuptake inhibitor tiagabine were not promising. In an open study, only 3 of the first 17 SFBN patients with treatment-resistant affective disorders showed a partial response (moderate improvement on the Clinical Global Impressions Scale for Bipolar Illness [CGI-BP]) to adjunctive tiagabine treatment when the dosage was gradually increased. Serious side effects, including two patients with possible seizures, were noted.26 Similar results were seen when tiagabine doses were rapidly increased in eight patients with acute mania; none responded, and one had a major motor seizure.27
Thus, our initial observations led us to discontinue use of tiagabine and suggest any further clinical consideration of this agent for treatment-resistant affective disorders would require great care. This perspective contrasts with several previous positive case reports. It is unknown whether selected patients or those treated with lower dosages would be more responsive to this agent.
Like valproate, gabapentin and tiagabine increase brain GABA, but their apparent lack of antimanic efficacy strongly suggests that raising brain GABA alone does little to counter mania. These data further support the viewpoint already offered that all anticonvulsants are not alike and that anticonvulsants as a class are not necessarily mood stabilizers.
Zonisamide and levetiracetam Open studies of these two other anticonvulsants are near completion. Each has an interesting mechanistic profile, good tolerability, and the (potentially positive) side effect of inducing some weight loss, based on studies of neurologic patients.
Omega-3 fatty acids Based on positive reports of antidepressant effects of omega-3 fatty acids,28-30 we recently recruited more than 120 patients with bipolar disorder for two double-blind, randomized, controlled trials. Those patients with either an acute depression or a cyclic course will receive 6 grams of omega-3 fatty acid (the eicosapentaenoate form) or a placebo for 4 months of double-blind treatment, followed by an 8-month open, active continuation phase.
If results are positive, omega-3 fatty acids would represent the much-needed addition of a well-tolerated dietary agent to the therapeutic armamentarium directed at bipolar illness.
Conclusion
The Stanley Foundation Bipolar Network has begun to evaluate the usefulness of numerous older and new compounds for patients with bipolar disorder, including second-generation antidepressants, anticonvulsants, atypical antipsychotic agents, and omega-3 fatty acids. Based on initial observations from 16 treatment studies (Table 3), we have drawn these conclusions:
- Second-generation antidepressants such as bupropion, sertraline, and venlafaxine appear to have a lower-than-expected switch rate into mania, compared with the use of the older tricyclic antidepressants.
- If the addition of a second-generation antidepressant results in good antidepressant response for 2 months and no switches into mania, one should consider continuing the antidepressant in this small minority of patients, rather than stopping it as soon as possible as previously recommended.
- Adjunctive use of lamotrigine appears to be of value, particularly for acute depression and prophylaxis, for many patients who have not responded to other treatments.
- While our group and others have reported improvement with adjunctive gabapentin in bipolar patients in uncontrolled case series, its primary antimanic or mood-stabilizing utility (as well as that of tiagabine) does not appear promising.
- Topiramate’s ability to induce substantial weight loss may emerge as clinically therapeutic for patients with problematic weight gain related to other psychotropic drugs, independent of its ultimate utility as a possible mood stabilizer.
We are preparing to analyze other open case series assessing acute and long-term treatment outcomes as well as randomized, double-blind clinical trials. Even though the funding of this unique collaborative network is ending, we look forward to more effective treatment of bipolar disorder in the future with continued development of new psychotropic agents and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and the VA Cooperative studies.31
Related resources
- National Alliance for the Mentally Ill (NAMI). www.nami.org
- National Depressive and Manic-Depressive Association (NDMDA). www.ndmda.org
Drug brand names
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sibutramine • Meridia
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproate • Depakote
- Venlafaxine • Effexor
- Zonisamide • Zonegran
Acknowledgement
The authors are particularly indebted to the generosity of The Stanley Foundation in making funds available for this series of studies and several about to be completed.
Disclosure
Financial disclosure statements have been provided by all authors and are available upon request at [email protected].
1. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press, 1990.
2. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
3. Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997a;58:470-8.
4. Greil W, Kleindienst N. The comparative prophylactic efficacy of lithium and carbamazepine in patients with bipolar I disorder. Int Clin Psychopharmacol 1999;14:277-81.
5. Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for one year with daily prospective ratings on the NIMH-LCM (submitted for publication, 2002).
6. Calabrese JR, Rapport DJ. Mood stabilizers and the evolution of maintenance study designs in bipolar I disorder. J Clin Psychiatry 1999;60(suppl 5):5-13.
7. McElroy SL, Frye M, Denicoff K, et al. Olanzapine in treatment-resistant bipolar disorder. J Affect Disord 1998;49:119-22.
8. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
9. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
10. Keck PE, Jr. New antiepileptics in the treatment of bipolar disorder. Syllabus and Proceedings Summary of the 2001 American Psychiatric Association Meeting, New Orleans, May 5-10, 2001. Abstract 34E, 93.
11. Post RM, Altshuler LL, Frye MA, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001;3:259-65.
12. Post RM, Leverich GS, Nolen WA, et al. A reevaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2002 (in press).
13. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-6.
14. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute remission from bipolar depression on rates of depressive relapse at one-year follow-up (submitted for publication 2002).
15. Suppes T, Brown ES, McElroy SL, et al. Lamotrigine for the treatment of bipolar disorder: a clinical case series. J Affective Disord 1999;53:95-8.
16. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60:79-88.
17. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14.
18. Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biolog Psychiatry 2002;51:253-60.
19. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biolog Psychiatry 2000;47:1025-33.
20. McIntyre RS, Mancini DA, McCann SM, et al. Topiramiate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 2002;4:207-13.
21. Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults. Epilepsia 2000;41(suppl 1):S66-S71.
22. Altshuler LL, Keck PE, Jr, McElroy SL, et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1999;1:61-5.
23. Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord 2000;2:249-55.
24. Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000;20:467-71.
25. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999;19:341-8.
26. Suppes T, Chisholm K, Dhvule D, et al. Tiagabine in bipolar disorder: adverse events in an open case series (submitted for publication, 2002).
27. Grunze H, Erfurth A, Marcuse A, et al. Tiagabine appears not to be efficacious in the treatment of acute mania. J Clin Psychiatry 1999;60:759-62.
28. Stoll AL, Severus WE, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56:407-12.
29. Horrobin DF, Peet M. A dose-ranging study of ethyl-eicosapentaenoate in treatment-unresponsive depression. Biolog Psychiatry 2001;49:37S.-
30. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-9.
31. Bauer MS, Williford WO, Dawson EE, et al. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: Reducing the efficacy-effectiveness gap in bipolar disorder. J Affect Disord 2001;67:61-78.
When choosing psychopharmacologic agents for patients with bipolar disorder, the clinician lacks guidance from controlled clinical trials at virtually every therapeutic step. To help remedy this problem, we in the Stanley Foundation Bipolar Network (SFBN) evaluated compounds with potentially important therapeutic profiles. We began by documenting the demographics and illness characteristics of patients with bipolar disorder. Then we assessed some 16 treatments in naturalistic or formal studies, some of which are complete and others ongoing.
In this second installment, we report our current information about the efficacy of second-generation antidepressants, atypical antipsychotics, and anticonvulsant agents.
Mood stabilizers
As mood stabilizers, the anticonvulsants carbamazepine and valproate are well-accepted alternatives or adjuncts to lithium carbonate, which remains a mainstay treatment for acute episodes and long-term prophylaxis.1
Valproate is approved by the FDA for treatment of acute mania but not for long-term prevention. Open studies in rapid-cycling, treatment-refractory patients demonstrate substantial improvement for both manic and depressive phases with valproate. In the most recent controlled study, valproate did not prevent manic episodes to a statistically significant degree, but it did show greater antidepressant effects than placebo or lithium.2
Carbamazepine has been widely studied in acute mania and compared with lithium for long-term prophylaxis. Most studies show nonsignificant differences between carbamazepine and lithium in preventing manic and depressive episodes, although several studies indicate less-robust antimanic effects of carbamazepine,3 particularly for patients with classic euphoric mania without psychosis, bipolar II, or associated substance abuse.4
In atypical patients, carbamazepine appeared to outperform lithium, suggesting clinical differences in these agents that have different mechanisms of action across neurotransmitter and peptide systems.
Low response rates Most randomized controlled trials and open adjunct studies suggest that 50% or more patients respond to lithium, valproate, or carbamazepine. Outcomes may be far less positive in clinical practice, however. Despite the use of mood stabilizers and a variety of antidepressants, benzodiazepines, and neuroleptics, our patients experienced substantial residual manic and depressive symptoms and functional impairment.
Similarly, clinician ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM) indicated a high rate of breakthrough episodes during carefully monitored and aggressive psychopharmacologic treatment.5 Two-thirds of the first 258 bipolar outpatients that we followed and rated daily for 1 year continued to be substantially impaired. One-quarter of them were ill for more than 75% of the year, despite using an average 4.1 psychopharmacologic agents per patient, including mood stabilizers, antidepressants, antipsychotics, and other agents.
Acute and long-term combination therapy appears more effective than monotherapy, particularly for treatment-refractory rapid cyclers.3 Even so, many patients do not respond to combination therapy. For example, in a 1-year prophylaxis study, 25% or fewer patients were rated “much improved” to “very much improved” on the Clinical Global Impressions (CGI) scale with lithium or carbamazepine as the baseline mood stabilizer and with antidepressants, neuroleptics, and benzodiazepines used as needed. In the third year of combination therapy, the overall response rate, including rapid cyclers, was roughly 50%.3
A low rate of response is apparent for lithium and valproate. While treating rapidly cycling patients, Calabrese and associates found that less than one-quarter of them responded well enough to the two-drug combination that they could be enrolled in a randomized double-blind comparison of each drug in monotherapy. Of those who were eligible, almost all relapsed with either lithium or valproate monotherapy and again required combination therapy.6
Table 1
RATE OF SWITCHING (%) INTO MANIA IN DEPRESSED BIPOLAR PATIENTS DURING ANTIDEPRESSANT TREATMENT*
| Acute treatment (10 wks) | Continuation treatment (52 wks)‡ | |||||
|---|---|---|---|---|---|---|
| Type of switch | Open (n=27) | Blind (n=100) | Total (n=127) | Open (n=12) | Blind (n=55) | Total (n=67) |
| Brief hypomania | 3.7% | 6% | 5.5% | – | 3.6% | 3% |
| Recurrent brief hypomania | 11 | 12 | 11.8 | 8.3% | 18.2 | 16.4 |
| Hypomania | 11 | 13 | 12.6 | 8.3 | 23.6 | 20.9 |
| Mania | 14.8 | 12 | 12.6 | 33.3 | 14.5 | 17.9 |
| Total | 25.8 | 25 | 25.2 | 41.6 | 38.1 | 38.8 |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | ||||||
| ‡ Most patients (80%) dropped out of this phase because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. | ||||||
| Source: Post RM et al. Bipolar Disord 2001;3:259-65, and unpublished data. | ||||||
Taken together, these studies indicate that even with our most effective and most studied agents, many patients respond inadequately. On the basis of this clinical reality, we began to explore additional options that might enhance clinical effectiveness.
Atypical antipsychotics
We assessed the addition of the atypical antipsychotic olanzapine in patients with treatment-refractory bipolar disorder7 and saw improvement in 57% of subjects with manic, mixed, and depressive components. These data foreshadowed more recent controlled findings of olanzapine’s usefulness in depression and mania.8,9 Our preliminary open clinical trial experience allowed for more controlled studies and subsequent approval and wider clinical use of olanzapine in bipolar illness.
Our initial assessment of other atypical antipsychotics found that quetiapine was associated with significantly fewer depressive symptoms on the Inventory of Depressive Symptomatology (IDS) when used as part of the regular treatment regimen. This improvement, which was seen in the first month, was maintained over 4 months of treatment, whereas no significant improvement in depression severity was seen with the use of risperidone or clozapine.10
It would be highly preliminary to conclude from these uncontrolled data that quetiapine had greater effects on bipolar depressive symptoms than did risperidone or clozapine. We cannot rule out the possibility that patients with different degrees of treatment resistance were enrolled and treated with the different concomitant drugs. However, patients did not differ at baseline in severity of their depression.
In this initial look at the atypicals, the severity of depressive symptoms on the IDS was much greater than that of mania, as measured by the Young Mania Rating Scale (YMRS). Thus, the precise patterns of illness being treated and the impact of these antipsychotics on depressive cyclicity and duration await closer examination. We intend to look at the relative efficacy and side-effect profiles of each of the atypical antipsychotics as used in clinical practice.
Second-generation antidepressants
Bupropion, sertraline, and venlafaxine have different mechanisms of action but appear to be of value as adjuncts to bipolar disorder treatment. When we used them as adjuncts to mood stabilizers in a prospective study, we found a 40 to 50% acute response rate and some switching into hypomania and mania (Table 1).11 Many switches appeared to involve only isolated brief bursts of hypomania or recurrent brief hypomanias, but more sustained episodes of hypomania (7 days) could be more clinically problematic. Only 13% of patients treated acutely with these antidepressants had more full-blown manic symptoms (i.e., those associated with moderate or greater dysfunction on the NIMH-LCM).
During the next year, when apparent acute antidepressant responders were offered continuation treatment, 18% switched into mania. However, most patients (about 80%) dropped out because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. The 127 patients who were randomized received 175 acute antidepressant trials. Only 9% were associated with a switch into mania with moderate dysfunction, whereas another 9% showed periods of hypomania lasting longer than 1 week.12
Table 2
RELAPSE INTO DEPRESSION IN BIPOLAR PATIENTS AFTER 1 YEAR WITH OR WITHOUT ADJUNCTIVE ANTIDEPRESSANT TREATMENT*
| Rate of relapse | |
|---|---|
| Continued on antidepressants | |
| Study #1 (n=19) | 35% |
| Study #2 (n=41) | 41% |
| Discontinued antidepressants | |
| Study #1 (n=25) | 68% |
| Study #2 (n=43) | 71% |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | |
| Study #1: Altshuler et al. J Clin Psychiatry 2001;62:612-6. | |
| Study #2: Altshuler et al. (submitted for publication 2002). | |
We have entered 176 patients into this double-blind, randomized comparison of bupropion, sertraline, and venlafaxine, with another 27 randomized openly. The study will attempt to examine any possible between-drug differences. Even without the blind being broken, it appears that these antidepressants are associated with a somewhat lower-than-expected rate of switching into mania with dysfunction, but a direct comparison with a placebo is not available.
In two other series, patients who remained well for 2 months on antidepressants plus mood stabilizers and then continued on their antidepressants had a much lower incidence of depressive relapses over the following year than those who discontinued their antidepressants (Table 2).13,14 Surprisingly, continuing the antidepressant was not associated with an increased rate of switching into mania.
Prospective randomized, controlled studies are required to confirm these findings. Even so, our studies appear to challenge conventional wisdom for the first time. Stopping antidepressant treatment in bipolar patients as soon as possible for fear of inducing a manic episode may not be the optimal recommendation in the admittedly small subgroup (about 15%) of patients who have responded well to an antidepressant for at least 2 months.
Table 3
SIX STUDIES COMPLETED BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number studied | Response rate |
|---|---|---|---|---|
| Antidepressants bupropion | Yes | Double-blind | 173 | ~ 47% |
| vs. sertraline vs. venlafaxine (Post et al, 2001) | Yes | Open | 27 | |
| Total = 200 | ||||
| Olanzapine (McElroy et al, 1998) | No | Open | 14 | ~ 57% |
| Lamotrigine (Suppes et al, 1999) | No | Open | 17 | ~ 65% |
| Gabapentin (Altshuler et al, 1999) | No | Open | 28 | 78% (14/18) for mania or hypomania 100% (n = 5) for depression 20% (1/5) for cycling |
| Topiramate (McElroy et al, 2000) | No | Open | 54 | 63% for mania or cycling 27% for acute depression (weight loss observed) |
| Tiagabine (Suppes et al, 2002) | No | Open | 17 | 18% (3/17) (3 possible seizures) |
Second-generation anticonvulsants
Based on our patients’ substantial residual symptoms, we explored a series of newly approved anticonvulsants for potential use as mood stabilizers.
Lamotrigine Consistent with other open and double-blind, placebo-controlled studies, lamotrigine showed promise as an adjunctive treatment, with improvement in depressive symptoms and cycling.15 One such study showed acute antidepressant effects of lamotrigine monotherapy compared with a placebo at dosages of 50 or 200 mg/d.16
Another double-blind, randomized, placebo-controlled NIMH study (partially funded by the Stanley Foundation) compared lamotrigine with gabapentin and a placebo in patients for whom lithium, carbamazepine, and valproate had failed.17 Most of these treatment-refractory patients received all three agents through three 6-week randomizations. Lamotrigine was much more effective in depression and overall illness than either a placebo or gabapentin. Men with bipolar compared with unipolar illness and patients with fewer unsuccessful clinical trials and hospitalizations for depression showed the greatest response.18
In two recent industry-sponsored controlled trials of long-term prophylaxis (following a recent manic or depressive episode), lamotrigine was significantly more effective than a placebo (Bowden et al and Calabrese et al, unpublished data). Lamotrigine was more effective than lithium in preventing depressive episodes, whereas lithium was more effective than lamotrigine for manic episodes, although lamotrigine was significantly better than the placebo.
These data suggest that lamotrigine may differ from valproate, carbamazepine, and lithium in being a more effective antidepressant than antimanic agent.
Topiramate Adjunctive use of topiramate in rapid-cycling and treatment-resistant patients has been examined in open studies, but controlled studies have not yet been published. In our open-label case series observations, 19 of 30 (63%) patients taking topiramate for mania or cycling were “much” or “very much” improved after 10 weeks, whereas only 3 of 11 (27%) patients taking topiramate for acute depression were so improved.19
Table 4
STUDIES IN PROGRESS BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number enrolled* | Notes |
|---|---|---|---|---|
| Topiramate vs. sibutramine | Yes | Open | 42 | Relative weight loss and mood stabilization |
| Omega-3 fatty acids (6 grams EPA vs. placebo) (for depression cycling) (Keck et al) | Yes Yes | Double-blind Double-blind | 62 60 | Data from both studies being analyzed |
| Acamprosate (for alcohol craving) (Grunze et al) | No | Open | 23 | Goal: 60 patients |
| Levetiracetam (Post et al) | No | Open | 13 | Goal: 30 patients |
| Zonisamide (McElroy et al) | No | Open | 45 | Goal: 60 patients European sites only |
| Tranylcypromine vs. lamotrigine (for refractory depression) (Nolen et al) | Yes | Open | 17 | Single-site study |
| Quetiapine (McElroy et al) | No | Open | 48 | Apparent superior antidepressant effects compared with risperidone or clozapine |
| Risperidone (Grunze et al) | No | Open | 37 | No significant effect on IDS‡ depression ratings |
| Clozapine (Suppes et al) | No | Open | 19 | No significant effect on IDS depression ratings |
| Modafinil vs. placebo (Frye et al) | Yes | Double-blind | Started 4/02 | |
| * Enrollment as of April 2002 n = 696 total patients in tables 3 and 4 | ||||
| ‡ IDS = Inventory of Depressive Symptomatology | ||||
An industry-sponsored double-blind, randomized trial of topiramate in acute mania looked promising in the first 67 patients, but not as encouraging in subsequent subjects. A positive drug effect re-emerged if one eliminated patients whose manic episodes were precipitated by antidepressants.
The acute antidepressant effects of topiramate did not appear promising in our study. On the other hand, a single-blind, randomized study of topiramate versus bupropion as adjunctive therapy for breakthrough bipolar depression suggested excellent acute response (approximately 56%) to both agents.20 These data leave the potential antidepressant effects of topiramate undetermined.
In patients being treated with topiramate, we found significant weight loss (average –0.7 kg at 4 weeks, –1.6 kg at 10 weeks, –4.7 kg at 6 months, and –6.2 kg at 1 year).19 Average weight loss was 4.5±6.7 kg at the final evaluation (body mass index [BMI] change of –6.3% and –5% at 1 year and last evaluation, respectively).
We and others have thus seen substantial weight loss in one-third to one-half of patients taking topiramate for bipolar disorder.21 This weight loss is maintained over relatively long periods, which differentiates topiramate from other weight-loss regimens. Topiramate’s ability to decrease weight—even while it is in the early stages of evaluation as a mood stabilizer—is of considerable interest, given the substantial problem of overweight in the bipolar population and the fact that many drug therapies used in the illness are associated with weight gain.
We are proceeding with a randomized open comparison of the relative weight loss and psychotropic efficacy of topiramate versus the antiobesity agent sibutramine. Sibutramine has been reported to have antidepressant properties and is FDA-approved for weight loss.
Gabapentin and tiagabine Our group and many others have reported substantial overall clinical improvement when gabapentin was used as adjunctive therapy in an open study of patients with inadequate response to previous treatments.22 As monotherapy, however, gabapentin was not more effective than a placebo17 and was less effective than lamotrigine. Similarly, gabapentin was not an effective acute antimanic agent when compared with a placebo,23 although other double-blind, controlled studies have indicated positive effects in anxiety disorders24 and social phobias.25 Thus, the gabapentin literature appears to be divergent—i.e., positive studies support its adjunctive use in bipolar patients, but controlled studies suggest that it is not directly antimanic or mood-stabilizing.
Gabapentin’s effect on syndromes that are highly comorbid with bipolar illness could account for its apparent success as open adjunctive therapy. For example, in addition to its antianxiety effects, gabapentin shows positive effects in alcohol withdrawal, pain syndromes, sleep disorders (including restless leg syndrome), and obsessive-compulsive disorder. Also, combining gabapentin with other psychotropics with other mechanisms of action may have a more robust therapeutic effect than monotherapy.
Our preliminary observations with the selective GABA-reuptake inhibitor tiagabine were not promising. In an open study, only 3 of the first 17 SFBN patients with treatment-resistant affective disorders showed a partial response (moderate improvement on the Clinical Global Impressions Scale for Bipolar Illness [CGI-BP]) to adjunctive tiagabine treatment when the dosage was gradually increased. Serious side effects, including two patients with possible seizures, were noted.26 Similar results were seen when tiagabine doses were rapidly increased in eight patients with acute mania; none responded, and one had a major motor seizure.27
Thus, our initial observations led us to discontinue use of tiagabine and suggest any further clinical consideration of this agent for treatment-resistant affective disorders would require great care. This perspective contrasts with several previous positive case reports. It is unknown whether selected patients or those treated with lower dosages would be more responsive to this agent.
Like valproate, gabapentin and tiagabine increase brain GABA, but their apparent lack of antimanic efficacy strongly suggests that raising brain GABA alone does little to counter mania. These data further support the viewpoint already offered that all anticonvulsants are not alike and that anticonvulsants as a class are not necessarily mood stabilizers.
Zonisamide and levetiracetam Open studies of these two other anticonvulsants are near completion. Each has an interesting mechanistic profile, good tolerability, and the (potentially positive) side effect of inducing some weight loss, based on studies of neurologic patients.
Omega-3 fatty acids Based on positive reports of antidepressant effects of omega-3 fatty acids,28-30 we recently recruited more than 120 patients with bipolar disorder for two double-blind, randomized, controlled trials. Those patients with either an acute depression or a cyclic course will receive 6 grams of omega-3 fatty acid (the eicosapentaenoate form) or a placebo for 4 months of double-blind treatment, followed by an 8-month open, active continuation phase.
If results are positive, omega-3 fatty acids would represent the much-needed addition of a well-tolerated dietary agent to the therapeutic armamentarium directed at bipolar illness.
Conclusion
The Stanley Foundation Bipolar Network has begun to evaluate the usefulness of numerous older and new compounds for patients with bipolar disorder, including second-generation antidepressants, anticonvulsants, atypical antipsychotic agents, and omega-3 fatty acids. Based on initial observations from 16 treatment studies (Table 3), we have drawn these conclusions:
- Second-generation antidepressants such as bupropion, sertraline, and venlafaxine appear to have a lower-than-expected switch rate into mania, compared with the use of the older tricyclic antidepressants.
- If the addition of a second-generation antidepressant results in good antidepressant response for 2 months and no switches into mania, one should consider continuing the antidepressant in this small minority of patients, rather than stopping it as soon as possible as previously recommended.
- Adjunctive use of lamotrigine appears to be of value, particularly for acute depression and prophylaxis, for many patients who have not responded to other treatments.
- While our group and others have reported improvement with adjunctive gabapentin in bipolar patients in uncontrolled case series, its primary antimanic or mood-stabilizing utility (as well as that of tiagabine) does not appear promising.
- Topiramate’s ability to induce substantial weight loss may emerge as clinically therapeutic for patients with problematic weight gain related to other psychotropic drugs, independent of its ultimate utility as a possible mood stabilizer.
We are preparing to analyze other open case series assessing acute and long-term treatment outcomes as well as randomized, double-blind clinical trials. Even though the funding of this unique collaborative network is ending, we look forward to more effective treatment of bipolar disorder in the future with continued development of new psychotropic agents and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and the VA Cooperative studies.31
Related resources
- National Alliance for the Mentally Ill (NAMI). www.nami.org
- National Depressive and Manic-Depressive Association (NDMDA). www.ndmda.org
Drug brand names
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sibutramine • Meridia
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproate • Depakote
- Venlafaxine • Effexor
- Zonisamide • Zonegran
Acknowledgement
The authors are particularly indebted to the generosity of The Stanley Foundation in making funds available for this series of studies and several about to be completed.
Disclosure
Financial disclosure statements have been provided by all authors and are available upon request at [email protected].
When choosing psychopharmacologic agents for patients with bipolar disorder, the clinician lacks guidance from controlled clinical trials at virtually every therapeutic step. To help remedy this problem, we in the Stanley Foundation Bipolar Network (SFBN) evaluated compounds with potentially important therapeutic profiles. We began by documenting the demographics and illness characteristics of patients with bipolar disorder. Then we assessed some 16 treatments in naturalistic or formal studies, some of which are complete and others ongoing.
In this second installment, we report our current information about the efficacy of second-generation antidepressants, atypical antipsychotics, and anticonvulsant agents.
Mood stabilizers
As mood stabilizers, the anticonvulsants carbamazepine and valproate are well-accepted alternatives or adjuncts to lithium carbonate, which remains a mainstay treatment for acute episodes and long-term prophylaxis.1
Valproate is approved by the FDA for treatment of acute mania but not for long-term prevention. Open studies in rapid-cycling, treatment-refractory patients demonstrate substantial improvement for both manic and depressive phases with valproate. In the most recent controlled study, valproate did not prevent manic episodes to a statistically significant degree, but it did show greater antidepressant effects than placebo or lithium.2
Carbamazepine has been widely studied in acute mania and compared with lithium for long-term prophylaxis. Most studies show nonsignificant differences between carbamazepine and lithium in preventing manic and depressive episodes, although several studies indicate less-robust antimanic effects of carbamazepine,3 particularly for patients with classic euphoric mania without psychosis, bipolar II, or associated substance abuse.4
In atypical patients, carbamazepine appeared to outperform lithium, suggesting clinical differences in these agents that have different mechanisms of action across neurotransmitter and peptide systems.
Low response rates Most randomized controlled trials and open adjunct studies suggest that 50% or more patients respond to lithium, valproate, or carbamazepine. Outcomes may be far less positive in clinical practice, however. Despite the use of mood stabilizers and a variety of antidepressants, benzodiazepines, and neuroleptics, our patients experienced substantial residual manic and depressive symptoms and functional impairment.
Similarly, clinician ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM) indicated a high rate of breakthrough episodes during carefully monitored and aggressive psychopharmacologic treatment.5 Two-thirds of the first 258 bipolar outpatients that we followed and rated daily for 1 year continued to be substantially impaired. One-quarter of them were ill for more than 75% of the year, despite using an average 4.1 psychopharmacologic agents per patient, including mood stabilizers, antidepressants, antipsychotics, and other agents.
Acute and long-term combination therapy appears more effective than monotherapy, particularly for treatment-refractory rapid cyclers.3 Even so, many patients do not respond to combination therapy. For example, in a 1-year prophylaxis study, 25% or fewer patients were rated “much improved” to “very much improved” on the Clinical Global Impressions (CGI) scale with lithium or carbamazepine as the baseline mood stabilizer and with antidepressants, neuroleptics, and benzodiazepines used as needed. In the third year of combination therapy, the overall response rate, including rapid cyclers, was roughly 50%.3
A low rate of response is apparent for lithium and valproate. While treating rapidly cycling patients, Calabrese and associates found that less than one-quarter of them responded well enough to the two-drug combination that they could be enrolled in a randomized double-blind comparison of each drug in monotherapy. Of those who were eligible, almost all relapsed with either lithium or valproate monotherapy and again required combination therapy.6
Table 1
RATE OF SWITCHING (%) INTO MANIA IN DEPRESSED BIPOLAR PATIENTS DURING ANTIDEPRESSANT TREATMENT*
| Acute treatment (10 wks) | Continuation treatment (52 wks)‡ | |||||
|---|---|---|---|---|---|---|
| Type of switch | Open (n=27) | Blind (n=100) | Total (n=127) | Open (n=12) | Blind (n=55) | Total (n=67) |
| Brief hypomania | 3.7% | 6% | 5.5% | – | 3.6% | 3% |
| Recurrent brief hypomania | 11 | 12 | 11.8 | 8.3% | 18.2 | 16.4 |
| Hypomania | 11 | 13 | 12.6 | 8.3 | 23.6 | 20.9 |
| Mania | 14.8 | 12 | 12.6 | 33.3 | 14.5 | 17.9 |
| Total | 25.8 | 25 | 25.2 | 41.6 | 38.1 | 38.8 |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | ||||||
| ‡ Most patients (80%) dropped out of this phase because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. | ||||||
| Source: Post RM et al. Bipolar Disord 2001;3:259-65, and unpublished data. | ||||||
Taken together, these studies indicate that even with our most effective and most studied agents, many patients respond inadequately. On the basis of this clinical reality, we began to explore additional options that might enhance clinical effectiveness.
Atypical antipsychotics
We assessed the addition of the atypical antipsychotic olanzapine in patients with treatment-refractory bipolar disorder7 and saw improvement in 57% of subjects with manic, mixed, and depressive components. These data foreshadowed more recent controlled findings of olanzapine’s usefulness in depression and mania.8,9 Our preliminary open clinical trial experience allowed for more controlled studies and subsequent approval and wider clinical use of olanzapine in bipolar illness.
Our initial assessment of other atypical antipsychotics found that quetiapine was associated with significantly fewer depressive symptoms on the Inventory of Depressive Symptomatology (IDS) when used as part of the regular treatment regimen. This improvement, which was seen in the first month, was maintained over 4 months of treatment, whereas no significant improvement in depression severity was seen with the use of risperidone or clozapine.10
It would be highly preliminary to conclude from these uncontrolled data that quetiapine had greater effects on bipolar depressive symptoms than did risperidone or clozapine. We cannot rule out the possibility that patients with different degrees of treatment resistance were enrolled and treated with the different concomitant drugs. However, patients did not differ at baseline in severity of their depression.
In this initial look at the atypicals, the severity of depressive symptoms on the IDS was much greater than that of mania, as measured by the Young Mania Rating Scale (YMRS). Thus, the precise patterns of illness being treated and the impact of these antipsychotics on depressive cyclicity and duration await closer examination. We intend to look at the relative efficacy and side-effect profiles of each of the atypical antipsychotics as used in clinical practice.
Second-generation antidepressants
Bupropion, sertraline, and venlafaxine have different mechanisms of action but appear to be of value as adjuncts to bipolar disorder treatment. When we used them as adjuncts to mood stabilizers in a prospective study, we found a 40 to 50% acute response rate and some switching into hypomania and mania (Table 1).11 Many switches appeared to involve only isolated brief bursts of hypomania or recurrent brief hypomanias, but more sustained episodes of hypomania (7 days) could be more clinically problematic. Only 13% of patients treated acutely with these antidepressants had more full-blown manic symptoms (i.e., those associated with moderate or greater dysfunction on the NIMH-LCM).
During the next year, when apparent acute antidepressant responders were offered continuation treatment, 18% switched into mania. However, most patients (about 80%) dropped out because of lack of efficacy (depressive recurrences), intolerance, or for administrative reasons. The 127 patients who were randomized received 175 acute antidepressant trials. Only 9% were associated with a switch into mania with moderate dysfunction, whereas another 9% showed periods of hypomania lasting longer than 1 week.12
Table 2
RELAPSE INTO DEPRESSION IN BIPOLAR PATIENTS AFTER 1 YEAR WITH OR WITHOUT ADJUNCTIVE ANTIDEPRESSANT TREATMENT*
| Rate of relapse | |
|---|---|
| Continued on antidepressants | |
| Study #1 (n=19) | 35% |
| Study #2 (n=41) | 41% |
| Discontinued antidepressants | |
| Study #1 (n=25) | 68% |
| Study #2 (n=43) | 71% |
| * When bupropion, sertraline, or venlafaxine were used as adjuncts to mood stabilizers | |
| Study #1: Altshuler et al. J Clin Psychiatry 2001;62:612-6. | |
| Study #2: Altshuler et al. (submitted for publication 2002). | |
We have entered 176 patients into this double-blind, randomized comparison of bupropion, sertraline, and venlafaxine, with another 27 randomized openly. The study will attempt to examine any possible between-drug differences. Even without the blind being broken, it appears that these antidepressants are associated with a somewhat lower-than-expected rate of switching into mania with dysfunction, but a direct comparison with a placebo is not available.
In two other series, patients who remained well for 2 months on antidepressants plus mood stabilizers and then continued on their antidepressants had a much lower incidence of depressive relapses over the following year than those who discontinued their antidepressants (Table 2).13,14 Surprisingly, continuing the antidepressant was not associated with an increased rate of switching into mania.
Prospective randomized, controlled studies are required to confirm these findings. Even so, our studies appear to challenge conventional wisdom for the first time. Stopping antidepressant treatment in bipolar patients as soon as possible for fear of inducing a manic episode may not be the optimal recommendation in the admittedly small subgroup (about 15%) of patients who have responded well to an antidepressant for at least 2 months.
Table 3
SIX STUDIES COMPLETED BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number studied | Response rate |
|---|---|---|---|---|
| Antidepressants bupropion | Yes | Double-blind | 173 | ~ 47% |
| vs. sertraline vs. venlafaxine (Post et al, 2001) | Yes | Open | 27 | |
| Total = 200 | ||||
| Olanzapine (McElroy et al, 1998) | No | Open | 14 | ~ 57% |
| Lamotrigine (Suppes et al, 1999) | No | Open | 17 | ~ 65% |
| Gabapentin (Altshuler et al, 1999) | No | Open | 28 | 78% (14/18) for mania or hypomania 100% (n = 5) for depression 20% (1/5) for cycling |
| Topiramate (McElroy et al, 2000) | No | Open | 54 | 63% for mania or cycling 27% for acute depression (weight loss observed) |
| Tiagabine (Suppes et al, 2002) | No | Open | 17 | 18% (3/17) (3 possible seizures) |
Second-generation anticonvulsants
Based on our patients’ substantial residual symptoms, we explored a series of newly approved anticonvulsants for potential use as mood stabilizers.
Lamotrigine Consistent with other open and double-blind, placebo-controlled studies, lamotrigine showed promise as an adjunctive treatment, with improvement in depressive symptoms and cycling.15 One such study showed acute antidepressant effects of lamotrigine monotherapy compared with a placebo at dosages of 50 or 200 mg/d.16
Another double-blind, randomized, placebo-controlled NIMH study (partially funded by the Stanley Foundation) compared lamotrigine with gabapentin and a placebo in patients for whom lithium, carbamazepine, and valproate had failed.17 Most of these treatment-refractory patients received all three agents through three 6-week randomizations. Lamotrigine was much more effective in depression and overall illness than either a placebo or gabapentin. Men with bipolar compared with unipolar illness and patients with fewer unsuccessful clinical trials and hospitalizations for depression showed the greatest response.18
In two recent industry-sponsored controlled trials of long-term prophylaxis (following a recent manic or depressive episode), lamotrigine was significantly more effective than a placebo (Bowden et al and Calabrese et al, unpublished data). Lamotrigine was more effective than lithium in preventing depressive episodes, whereas lithium was more effective than lamotrigine for manic episodes, although lamotrigine was significantly better than the placebo.
These data suggest that lamotrigine may differ from valproate, carbamazepine, and lithium in being a more effective antidepressant than antimanic agent.
Topiramate Adjunctive use of topiramate in rapid-cycling and treatment-resistant patients has been examined in open studies, but controlled studies have not yet been published. In our open-label case series observations, 19 of 30 (63%) patients taking topiramate for mania or cycling were “much” or “very much” improved after 10 weeks, whereas only 3 of 11 (27%) patients taking topiramate for acute depression were so improved.19
Table 4
STUDIES IN PROGRESS BY SFBN INVESTIGATORS
| Study (authors) | Randomized | Design | Number enrolled* | Notes |
|---|---|---|---|---|
| Topiramate vs. sibutramine | Yes | Open | 42 | Relative weight loss and mood stabilization |
| Omega-3 fatty acids (6 grams EPA vs. placebo) (for depression cycling) (Keck et al) | Yes Yes | Double-blind Double-blind | 62 60 | Data from both studies being analyzed |
| Acamprosate (for alcohol craving) (Grunze et al) | No | Open | 23 | Goal: 60 patients |
| Levetiracetam (Post et al) | No | Open | 13 | Goal: 30 patients |
| Zonisamide (McElroy et al) | No | Open | 45 | Goal: 60 patients European sites only |
| Tranylcypromine vs. lamotrigine (for refractory depression) (Nolen et al) | Yes | Open | 17 | Single-site study |
| Quetiapine (McElroy et al) | No | Open | 48 | Apparent superior antidepressant effects compared with risperidone or clozapine |
| Risperidone (Grunze et al) | No | Open | 37 | No significant effect on IDS‡ depression ratings |
| Clozapine (Suppes et al) | No | Open | 19 | No significant effect on IDS depression ratings |
| Modafinil vs. placebo (Frye et al) | Yes | Double-blind | Started 4/02 | |
| * Enrollment as of April 2002 n = 696 total patients in tables 3 and 4 | ||||
| ‡ IDS = Inventory of Depressive Symptomatology | ||||
An industry-sponsored double-blind, randomized trial of topiramate in acute mania looked promising in the first 67 patients, but not as encouraging in subsequent subjects. A positive drug effect re-emerged if one eliminated patients whose manic episodes were precipitated by antidepressants.
The acute antidepressant effects of topiramate did not appear promising in our study. On the other hand, a single-blind, randomized study of topiramate versus bupropion as adjunctive therapy for breakthrough bipolar depression suggested excellent acute response (approximately 56%) to both agents.20 These data leave the potential antidepressant effects of topiramate undetermined.
In patients being treated with topiramate, we found significant weight loss (average –0.7 kg at 4 weeks, –1.6 kg at 10 weeks, –4.7 kg at 6 months, and –6.2 kg at 1 year).19 Average weight loss was 4.5±6.7 kg at the final evaluation (body mass index [BMI] change of –6.3% and –5% at 1 year and last evaluation, respectively).
We and others have thus seen substantial weight loss in one-third to one-half of patients taking topiramate for bipolar disorder.21 This weight loss is maintained over relatively long periods, which differentiates topiramate from other weight-loss regimens. Topiramate’s ability to decrease weight—even while it is in the early stages of evaluation as a mood stabilizer—is of considerable interest, given the substantial problem of overweight in the bipolar population and the fact that many drug therapies used in the illness are associated with weight gain.
We are proceeding with a randomized open comparison of the relative weight loss and psychotropic efficacy of topiramate versus the antiobesity agent sibutramine. Sibutramine has been reported to have antidepressant properties and is FDA-approved for weight loss.
Gabapentin and tiagabine Our group and many others have reported substantial overall clinical improvement when gabapentin was used as adjunctive therapy in an open study of patients with inadequate response to previous treatments.22 As monotherapy, however, gabapentin was not more effective than a placebo17 and was less effective than lamotrigine. Similarly, gabapentin was not an effective acute antimanic agent when compared with a placebo,23 although other double-blind, controlled studies have indicated positive effects in anxiety disorders24 and social phobias.25 Thus, the gabapentin literature appears to be divergent—i.e., positive studies support its adjunctive use in bipolar patients, but controlled studies suggest that it is not directly antimanic or mood-stabilizing.
Gabapentin’s effect on syndromes that are highly comorbid with bipolar illness could account for its apparent success as open adjunctive therapy. For example, in addition to its antianxiety effects, gabapentin shows positive effects in alcohol withdrawal, pain syndromes, sleep disorders (including restless leg syndrome), and obsessive-compulsive disorder. Also, combining gabapentin with other psychotropics with other mechanisms of action may have a more robust therapeutic effect than monotherapy.
Our preliminary observations with the selective GABA-reuptake inhibitor tiagabine were not promising. In an open study, only 3 of the first 17 SFBN patients with treatment-resistant affective disorders showed a partial response (moderate improvement on the Clinical Global Impressions Scale for Bipolar Illness [CGI-BP]) to adjunctive tiagabine treatment when the dosage was gradually increased. Serious side effects, including two patients with possible seizures, were noted.26 Similar results were seen when tiagabine doses were rapidly increased in eight patients with acute mania; none responded, and one had a major motor seizure.27
Thus, our initial observations led us to discontinue use of tiagabine and suggest any further clinical consideration of this agent for treatment-resistant affective disorders would require great care. This perspective contrasts with several previous positive case reports. It is unknown whether selected patients or those treated with lower dosages would be more responsive to this agent.
Like valproate, gabapentin and tiagabine increase brain GABA, but their apparent lack of antimanic efficacy strongly suggests that raising brain GABA alone does little to counter mania. These data further support the viewpoint already offered that all anticonvulsants are not alike and that anticonvulsants as a class are not necessarily mood stabilizers.
Zonisamide and levetiracetam Open studies of these two other anticonvulsants are near completion. Each has an interesting mechanistic profile, good tolerability, and the (potentially positive) side effect of inducing some weight loss, based on studies of neurologic patients.
Omega-3 fatty acids Based on positive reports of antidepressant effects of omega-3 fatty acids,28-30 we recently recruited more than 120 patients with bipolar disorder for two double-blind, randomized, controlled trials. Those patients with either an acute depression or a cyclic course will receive 6 grams of omega-3 fatty acid (the eicosapentaenoate form) or a placebo for 4 months of double-blind treatment, followed by an 8-month open, active continuation phase.
If results are positive, omega-3 fatty acids would represent the much-needed addition of a well-tolerated dietary agent to the therapeutic armamentarium directed at bipolar illness.
Conclusion
The Stanley Foundation Bipolar Network has begun to evaluate the usefulness of numerous older and new compounds for patients with bipolar disorder, including second-generation antidepressants, anticonvulsants, atypical antipsychotic agents, and omega-3 fatty acids. Based on initial observations from 16 treatment studies (Table 3), we have drawn these conclusions:
- Second-generation antidepressants such as bupropion, sertraline, and venlafaxine appear to have a lower-than-expected switch rate into mania, compared with the use of the older tricyclic antidepressants.
- If the addition of a second-generation antidepressant results in good antidepressant response for 2 months and no switches into mania, one should consider continuing the antidepressant in this small minority of patients, rather than stopping it as soon as possible as previously recommended.
- Adjunctive use of lamotrigine appears to be of value, particularly for acute depression and prophylaxis, for many patients who have not responded to other treatments.
- While our group and others have reported improvement with adjunctive gabapentin in bipolar patients in uncontrolled case series, its primary antimanic or mood-stabilizing utility (as well as that of tiagabine) does not appear promising.
- Topiramate’s ability to induce substantial weight loss may emerge as clinically therapeutic for patients with problematic weight gain related to other psychotropic drugs, independent of its ultimate utility as a possible mood stabilizer.
We are preparing to analyze other open case series assessing acute and long-term treatment outcomes as well as randomized, double-blind clinical trials. Even though the funding of this unique collaborative network is ending, we look forward to more effective treatment of bipolar disorder in the future with continued development of new psychotropic agents and data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) and the VA Cooperative studies.31
Related resources
- National Alliance for the Mentally Ill (NAMI). www.nami.org
- National Depressive and Manic-Depressive Association (NDMDA). www.ndmda.org
Drug brand names
- Bupropion • Wellbutrin
- Carbamazepine • Tegretol
- Clozapine • Clozaril
- Gabapentin • Neurontin
- Lamotrigine • Lamictal
- Levetiracetam • Keppra
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Sibutramine • Meridia
- Tiagabine • Gabitril
- Topiramate • Topamax
- Valproate • Depakote
- Venlafaxine • Effexor
- Zonisamide • Zonegran
Acknowledgement
The authors are particularly indebted to the generosity of The Stanley Foundation in making funds available for this series of studies and several about to be completed.
Disclosure
Financial disclosure statements have been provided by all authors and are available upon request at [email protected].
1. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press, 1990.
2. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
3. Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997a;58:470-8.
4. Greil W, Kleindienst N. The comparative prophylactic efficacy of lithium and carbamazepine in patients with bipolar I disorder. Int Clin Psychopharmacol 1999;14:277-81.
5. Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for one year with daily prospective ratings on the NIMH-LCM (submitted for publication, 2002).
6. Calabrese JR, Rapport DJ. Mood stabilizers and the evolution of maintenance study designs in bipolar I disorder. J Clin Psychiatry 1999;60(suppl 5):5-13.
7. McElroy SL, Frye M, Denicoff K, et al. Olanzapine in treatment-resistant bipolar disorder. J Affect Disord 1998;49:119-22.
8. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
9. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
10. Keck PE, Jr. New antiepileptics in the treatment of bipolar disorder. Syllabus and Proceedings Summary of the 2001 American Psychiatric Association Meeting, New Orleans, May 5-10, 2001. Abstract 34E, 93.
11. Post RM, Altshuler LL, Frye MA, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001;3:259-65.
12. Post RM, Leverich GS, Nolen WA, et al. A reevaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2002 (in press).
13. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-6.
14. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute remission from bipolar depression on rates of depressive relapse at one-year follow-up (submitted for publication 2002).
15. Suppes T, Brown ES, McElroy SL, et al. Lamotrigine for the treatment of bipolar disorder: a clinical case series. J Affective Disord 1999;53:95-8.
16. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60:79-88.
17. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14.
18. Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biolog Psychiatry 2002;51:253-60.
19. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biolog Psychiatry 2000;47:1025-33.
20. McIntyre RS, Mancini DA, McCann SM, et al. Topiramiate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 2002;4:207-13.
21. Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults. Epilepsia 2000;41(suppl 1):S66-S71.
22. Altshuler LL, Keck PE, Jr, McElroy SL, et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1999;1:61-5.
23. Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord 2000;2:249-55.
24. Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000;20:467-71.
25. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999;19:341-8.
26. Suppes T, Chisholm K, Dhvule D, et al. Tiagabine in bipolar disorder: adverse events in an open case series (submitted for publication, 2002).
27. Grunze H, Erfurth A, Marcuse A, et al. Tiagabine appears not to be efficacious in the treatment of acute mania. J Clin Psychiatry 1999;60:759-62.
28. Stoll AL, Severus WE, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56:407-12.
29. Horrobin DF, Peet M. A dose-ranging study of ethyl-eicosapentaenoate in treatment-unresponsive depression. Biolog Psychiatry 2001;49:37S.-
30. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-9.
31. Bauer MS, Williford WO, Dawson EE, et al. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: Reducing the efficacy-effectiveness gap in bipolar disorder. J Affect Disord 2001;67:61-78.
1. Goodwin FK, Jamison KR. Manic-depressive illness. New York: Oxford University Press, 1990.
2. Bowden CL, Calabrese JR, McElroy SL, et al. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 2000;57:481-9.
3. Denicoff KD, Smith-Jackson EE, Disney ER, et al. Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997a;58:470-8.
4. Greil W, Kleindienst N. The comparative prophylactic efficacy of lithium and carbamazepine in patients with bipolar I disorder. Int Clin Psychopharmacol 1999;14:277-81.
5. Post RM, Denicoff KD, Leverich GS, et al. Morbidity in 258 bipolar outpatients followed for one year with daily prospective ratings on the NIMH-LCM (submitted for publication, 2002).
6. Calabrese JR, Rapport DJ. Mood stabilizers and the evolution of maintenance study designs in bipolar I disorder. J Clin Psychiatry 1999;60(suppl 5):5-13.
7. McElroy SL, Frye M, Denicoff K, et al. Olanzapine in treatment-resistant bipolar disorder. J Affect Disord 1998;49:119-22.
8. Shelton RC, Tollefson GD, Tohen M, et al. A novel augmentation strategy for treating resistant major depression. Am J Psychiatry 2001;158:131-4.
9. Tohen M, Chengappa KN, Suppes T, et al. Efficacy of olanzapine in combination with valproate or lithium in the treatment of mania in patients partially nonresponsive to valproate or lithium monotherapy. Arch Gen Psychiatry 2002;59:62-9.
10. Keck PE, Jr. New antiepileptics in the treatment of bipolar disorder. Syllabus and Proceedings Summary of the 2001 American Psychiatric Association Meeting, New Orleans, May 5-10, 2001. Abstract 34E, 93.
11. Post RM, Altshuler LL, Frye MA, et al. Rate of switch in bipolar patients prospectively treated with second-generation antidepressants as augmentation to mood stabilizers. Bipolar Disord 2001;3:259-65.
12. Post RM, Leverich GS, Nolen WA, et al. A reevaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley Bipolar Treatment Network. Bipolar Disord 2002 (in press).
13. Altshuler L, Kiriakos L, Calcagno J, et al. The impact of antidepressant discontinuation versus antidepressant continuation on 1-year risk for relapse of bipolar depression: a retrospective chart review. J Clin Psychiatry 2001;62:612-6.
14. Altshuler L, Suppes T, Black D, et al. Impact of antidepressant discontinuation after acute remission from bipolar depression on rates of depressive relapse at one-year follow-up (submitted for publication 2002).
15. Suppes T, Brown ES, McElroy SL, et al. Lamotrigine for the treatment of bipolar disorder: a clinical case series. J Affective Disord 1999;53:95-8.
16. Calabrese JR, Bowden CL, Sachs GS, et al. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Lamictal 602 Study Group. J Clin Psychiatry 1999;60:79-88.
17. Frye MA, Ketter TA, Kimbrell TA, et al. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000;20:607-14.
18. Obrocea GV, Dunn RM, Frye MA, et al. Clinical predictors of response to lamotrigine and gabapentin monotherapy in refractory affective disorders. Biolog Psychiatry 2002;51:253-60.
19. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biolog Psychiatry 2000;47:1025-33.
20. McIntyre RS, Mancini DA, McCann SM, et al. Topiramiate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 2002;4:207-13.
21. Reife R, Pledger G, Wu SC. Topiramate as add-on therapy: pooled analysis of randomized controlled trials in adults. Epilepsia 2000;41(suppl 1):S66-S71.
22. Altshuler LL, Keck PE, Jr, McElroy SL, et al. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1999;1:61-5.
23. Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G. Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Gabapentin Bipolar Disorder Study Group. Bipolar Disord 2000;2:249-55.
24. Pande AC, Pollack MH, Crockatt J, et al. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000;20:467-71.
25. Pande AC, Davidson JR, Jefferson JW, et al. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999;19:341-8.
26. Suppes T, Chisholm K, Dhvule D, et al. Tiagabine in bipolar disorder: adverse events in an open case series (submitted for publication, 2002).
27. Grunze H, Erfurth A, Marcuse A, et al. Tiagabine appears not to be efficacious in the treatment of acute mania. J Clin Psychiatry 1999;60:759-62.
28. Stoll AL, Severus WE, et al. Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999;56:407-12.
29. Horrobin DF, Peet M. A dose-ranging study of ethyl-eicosapentaenoate in treatment-unresponsive depression. Biolog Psychiatry 2001;49:37S.-
30. Nemets B, Stahl Z, Belmaker RH. Addition of omega-3 fatty acid to maintenance medication treatment for recurrent unipolar depressive disorder. Am J Psychiatry 2002;159:477-9.
31. Bauer MS, Williford WO, Dawson EE, et al. Principles of effectiveness trials and their implementation in VA Cooperative Study #430: Reducing the efficacy-effectiveness gap in bipolar disorder. J Affect Disord 2001;67:61-78.
From the Stanley Foundation Bipolar Network: New findings on suicide attempts, substance abuse, obesity, and more
Even with psychiatry’s most effective and best studied agents, many patients with bipolar disorder are inadequately treated and respond insufficiently to available psychopharmacologic agents. To address this problem, the Stanley Foundation Bipolar Network (SFBN) was created to explore acute and long-term response to treatment of bipolar disorder.
For the past 6 years, our group (Box) has followed an international cohort of patients with bipolar disorder and offered them opportunities to participate in clinical trials at seven field centers. We have made considerable progress as we focused on the longitudinal course of bipolar illness and evaluated various combinations of psychopharmacologic therapies. The SFBN also has explored factors that increase the risk of suicide, quantified the incidence of anxiety disorder and substance abuse, and looked at other variables that may affect treatment outcome.
This report for the readers of Current Psychiatry provides a brief state-of-the-art overview of what we have learned about the course and management of bipolar disorder. It brings together and summarizes the findings from studies published, in press, or under review. Our goal is to update current findings, which may help you provide optimum care for these at-risk patients.
Since Robert Prien’s pioneering studies in the 1970s, the National Institute of Mental Health (NIMH) had funded little work on prophylaxis of bipolar disorders or acute bipolar depression. Following two NIMH conferences on bipolar illness in 1989 and 1994, the Stanley Foundation Bipolar Network (SFBN) was established to help overcome these research deficiencies, with funding provided by the Theodore and Vada Stanley Foundation.
Patients were recruited into the network from the general community and with the use of newspaper and newsletter advertisements. In contrast to many other clinical populations, few who wished to enter the network were excluded. It was clear, however, that filling out demographic, clinical, and rating forms would require considerable effort from each patient. All patients gave written informed consent to participate in the naturalistic part of the study, and were reconsented for any open or blind randomized clinical trial.
Patients provided daily self-ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM), a unique longitudinal instrument. Clinicians then completed their own separate LCMs, as more severely ill patients often could not complete their self-ratings, and patients often underestimated the severity and functional disability associated with the hypomanic and manic phases of bipolar illness. With the use of the NIMH-LCM, network investigators were able, for the first time, to assess the details of mood fluctuations associated with bipolar illness and to more precisely delineate the qualitative and quantitative aspects of response to psychopharmacologic interventions.
The SFBN includes seven research field centers in the United States, the Netherlands, and Germany. The leaders of those centers collaborated with Robert M. Post, MD, to prepare this report of the network findings. The collaborators are:
| Gabriele S. Leverich, MSW, and Kirk D. Denicoff, MD Biological Psychiatry Branch National Institute of Mental Health Bethesda, MD | Paul E. Keck, Jr., MD, and Susan L. McElroy, MD Biological Psychiatry Program University of Cincinnati College of Medicine Cincinnati, OH |
| Lori L. Altshuler, MD, and Mark A. Frye, MD UCLA Mood Disorders Research Program VA Medical Center Los Angeles, CA | Willem A. Nolen, MD, and Ralph Kupka, MD Altrecht Institute for Mental Health Care University Medical Center Utrecht, The Netherlands |
| John Rush, MD, and Trisha Suppes, MD Department of Psychiatry Southwest Medical Center University of Texas, Dallas | Joerg Walden, MD Department of Psychiatry University of Freiburg Freiburg, Germany |
| Keith Kramlinger, MD Mayo Clinic, Rochester, MN (Administrative center) | Heinz Grunze, MD Psychiatrische Klinik der LMU Munich, Germany |
In part one, we discuss the demographics of our cohort of 676 community-based bipolar patients, including risk factors for suicide attempts, frequency of comorbid psychiatric diagnoses, problems of overweight and obesity, and preliminary findings about thyroiditis.
In part two, we describe our clinical trials assessing mood stabilizers and adjunctive agents such as atypical antipsychotics, second-generation antidepressants, and anticonvulsants. We also will address a soon-to-be analyzed study of omega-3 fatty acids.
Table 1
CHARACTERISTICS OF THE 676 BIPOLAR OUTPATIENTS
| Mean age | 41 | |
| % Female | 43 | |
| Diagnosis (%) | ||
| Bipolar I | 79 | |
| Bipolar II | 16 | |
| Bipolar NOS | 4 | |
| Marital status (%) | ||
| Married/cohabitating | 44 | |
| Divorced/separated | 22 | |
| Income (%) | ||
| <$20,000 | 37 | |
| $20,000-40,000 | 22 | |
| $40,000-100,000 | 32 | |
| >$100,000 | 9 | |
| Education (completed, %) | ||
| High school | 34 | |
| Jr. college/technical | 13 | |
| College, 4-year | 27 | |
| Graduate degree | 16 | |
| Positive family history (1st-degree relatives, %) | ||
| Bipolar disorder | 56 | |
| Unipolar disorder | 64 | |
| Schizophrenia | 24 | |
| Drug dependence | 44 | |
| Other psychiatric disorder | 52 | |
| Alcohol abuse/dependence | 52 | |
| Suicide (complete or serious attempt) | 38 | |
| Environmental adversity (%): | Child/adolescent | Adult |
| Physical abuse | 29 | 8 |
| Sexual abuse | 30 | 8 |
| Illness course and treatment (age in yrs) | ||
| Age of onset (symptoms) | 19 | |
| Early onset (age <14) | 34 | |
| Age at first treatment | 29 | |
| Age at first hospitalization | 30 | |
| Cycling pattern (n = 643, %) | ||
| Rapid (or faster cycling) | 47 | |
| Ultrarapid (or faster cycling) | 30 | |
| Rapid-cycling only | 17 | |
| Ultrarapid cycling only | 10 | |
| Ultradian cycling | 20 | |
Study population
The SFBN recruited outpatients with bipolar disorders I, II, or not otherwise specified (NOS). We did not exclude patients with comorbid illnesses, except those with active substance abuse disorder requiring treatment in another setting.1 Patients were recruited almost exclusively from the community and rarely from inpatient hospitalization. The average age was 41, and 43% were college graduates. Most were employed but indicated that their illness limited their employment level or ability to function at work (Table 1).
One striking finding was a decadelong lag between the onset of symptoms reaching thresholds for diagnosis and the onset of first treatment.2 This long delay could have adverse consequences for illness outcome and represents a major public health target. Age of onset appeared to have both genetic/familial and environmental determinants.3 Subjects with a history of both extreme early life adversities (i.e., physical or sexual abuse) and a positive family history of mood disorder had the earliest onset of bipolar illness. Those who had neither of these factors had late-onset illness.
Suicide attempts
Prior to network entry, 25 to 50% of our study population had made a serious suicide attempt (defined as requiring medical attention and/or hospitalization), pointing to bipolar disorder’s potential lethality.
History of abuse A history of physical or sexual abuse in childhood or adolescence was a prominent risk factor for attempted suicide.4 With either type of abuse there was an approximate 15% increase in incidence of suicide attempts over the baseline rate of about 25%. More than 55% of patients with a history of both physical and sexual abuse had made a serious suicide attempt. The frequency of physical and sexual abuse was also positively related to increased incidence of suicide attempts. Taken together, these data suggest a “stressor dose-response” relationship between adverse childhood or adolescent experiences and an increased incidence of suicide attempts.
Table 2
FACTORS THAT APPEAR TO INCREASE SUICIDE RISK IN BIPOLAR DISORDER*
| Course-of-illness characteristics |
| Early onset |
| More time ill |
| More time depressed |
| More-severe depression |
| Social and medical support |
| Loss of significant other |
| Lack of a confidant |
| Single motherhood |
| Decreased access to health care |
| Lack of medical insurance |
| Demographics |
| Less education |
| Lower income |
| Being single |
| Comorbidities |
| Axis I |
| Eating disorders |
| Anxiety disorders |
| Axis II |
| Cluster A, B, C |
| Medical |
| Traumatic life events |
| Sexual abuse |
| Physical abuse |
| Genetics |
| Family history of suicide and drug abuse |
| * Identified in the SFBN cohort |
Comorbidities Other factors that appear to be associated with bipolar disorder and increased risk of attempted suicide include anxiety and eating disorders, early onset of symptoms, at least four previous hospitalizations for depression, and medical comorbidity (Table 2). Limited access to psychiatric and medical services and inadequate health insurance also were correlated with having made a serious suicide attempt. Thus, lack of medical care might exacerbate demoralization and increase the risk of suicide.
Family history of suicide or serious suicide attempts and drug abuse were associated with an increase in suicide attempts, but family history of unipolar or bipolar illness were not. These data are consistent with other studies suggesting that vulnerability to suicide may have a separate genetic component from that of mood disorders per se.
Severity of illness Increased severity of bipolar illness also was associated with prior psychosocial stressors3 and serious suicide attempts. We confirmed self-reported history, using daily mood charting, the clinician-rated Inventory of Depressive Symptomatology (IDS-C), and the Young Mania Rating Scale (YMRS) to rate increase in percentage of time ill, percentage of time depressed, and severity of depression.
Clinical implications We are uncertain how directly these data regarding suicide attempts relate to completed suicide. However, patients with bipolar disorder are among those at highest risk for completed suicide, and a prior serious attempt is often a precursor to completed suicide.5,6
Factors that appear particularly related to increased risk of suicide in patients with bipolar disorder are family history of suicide, history of early psychosocial stressors, comorbid personality disorders, substance abuse, and a relatively severe course of bipolar illness. Patients with these risk factors need support for two types of psychosocial stressors:
- poor access to health care
- stressors related to concurrent events (e.g., occurring with new affective episodes).
It may also be important for clinicians to consider the prophylactic use of lithium because of its demonstrated ability to reduce mortality in bipolar illness.7 Additionally, we need data on the efficacy of new drugs in preventing affective episodes and reducing suicidal ideation and acts.
Common comorbidities
We found considerable axis I lifetime comorbidities in the SFBN cohort (Table 3), including a 42% incidence of anxiety disorder and a similarly high rate of substance abuse. In addition to bipolar disorder, patients averaged approximately two lifetime comorbid diagnoses:
- 35% had none
- 65% had one or more
- 42% had two or more
- 24% had three or more.
Table 3
PSYCHIATRIC COMORBIDITY IN BIPOLAR OUTPATIENTS (%)*.
| Any substance abuse disorder | 42% |
| Alcohol | 33 |
| Marijuana | 16 |
| Stimulant | 9 |
| Sedative | 8 |
| Opiate | 7 |
| Hallucinogens | 6 |
| Any anxiety disorder | 42% |
| Panic disorder | 20 |
| Social phobia | 16 |
| Simple phobia | 10 |
| OCD | 9 |
| PTSD | 7 |
| GAD | 3 |
| Other | 3 |
| Any eating disorder | 11% |
| Bulimia nervosa | 8 |
| Anorexia nervosa | 4 |
| * Lifetime comorbid axis I disorders based on Structured Clinical Interview for DSM-IV (SCID) interviews with 288 SFBN patients. | |
Men with bipolar disorder had a higher absolute prevalence of alcoholism, but the relative risk compared with the general population was much higher in women (odds ratio 7.35) compared with men (odds ratio 2.77). In women, alcohol use and abuse were related to history of social phobia, greater number of depressive episodes prior to network entry, and history of abusing more than one substance. In men, alcohol comorbidity was related to history of alcohol abuse in first-degree relatives and history of early physical abuse.
These data suggest that female patients, in particular, were self-medicating their affective episodes and comorbid anxiety disorders with alcohol. They suggest the importance of asking patients directly about alcohol use to identify the need for primary and secondary prevention. In an adolescent or young adult, affective illness—and bipolar disorder in particular—should be considered a major risk factor for subsequent problems with alcohol and drug abuse. When taking a careful history, the treating physician should include questions regarding any substance abuse.
Overweight and obesity
One-half of the women and two-thirds of the men in the SFBN were overweight at network entry.8 Although this rate of overweight sounds alarmingly high, it was not significantly different from rates in the general U.S. population. A greater percentage of women than men in the network had morbid obesity, however.
In our cohort, being overweight or obese was associated with an increased incidence of cardiovascular disease, diabetes, arthritis, hypertension, hypothyroidism, and cancer. The degree of obesity also correlated significantly with past use of the numerous psychotropic drugs that have been associated with weight gain. Also, as might be expected, increased weight was associated with physical inactivity.
Many of the psychotropic drugs routinely used to treat bipolar disorder are associated with weight gain. Among these are the mood stabilizers lithium and valproate and the atypical antipsychotics, with the exception of ziprasidone (and, to follow, aripiprazole). Lithium’s liability to cause weight gain may be counterbalanced by its antisuicide effects and its ability to reverse excess medical mortality associated with affective illness. Thus, lithium should continue to play a major therapeutic role in bipolar disorder.
It may be more effective and easier to prevent weight gain than to attempt to reduce weight that has been gained. Recommending exercise and other weight-reduction measures may be helpful, as may co-administering drugs associated with weight loss, such as topiramate.9
Thyroiditis
Antithyroid antibodies were detected in 28% of network patients, compared with rates of 3% in the general population and 18% in psychiatric controls.10 The increased rate was associated with the need to augment thyroid function in 17% of network patients, but it was not related to age, mood state, rapid cycling status, or lithium treatment.
These data indicate that bipolar patients are prone to autoimmune thyroid changes. Further analysis is required to ascertain whether this finding has therapeutic implications, especially related to adjunctive T3 and T4 treatment.11
1. Leverich GS, Nolen WA, Rush AJ, et al. The Stanley Foundation Bipolar Treatment Outcome Network. I. Longitudinal methodology. J Affect Disord 2001;67:33-44.
2. Suppes T, Leverich GS, Keck PE, et al. The Stanley Foundation Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disord 2001b;67:45-59.
3. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological Psychiatry 2002;51:288-97.
4. Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. Manuscript submitted for publication, 2002.
5. Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39:896-9.
6. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch.Gen.Psychiatry 1999;56:617-26.
7. Ahrens B, Muller-Oerlinghausen B, Schou M, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord 1995;33:67-75.
8. McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry 2002;63:207-13.
9. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biological Psychiatry 2000;47:1025-33.
10. Kupka RW, Nolen WA, Post RM, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biological Psychiatry 2002;51:305-11.
11. Bauer M, Priebe S, Berghofer A, et al. Subjective response to and tolerability of long-term supraphysiological doses of levothyroxine in refractory mood disorders. J Affect Disord 2001;64:35-42.
Even with psychiatry’s most effective and best studied agents, many patients with bipolar disorder are inadequately treated and respond insufficiently to available psychopharmacologic agents. To address this problem, the Stanley Foundation Bipolar Network (SFBN) was created to explore acute and long-term response to treatment of bipolar disorder.
For the past 6 years, our group (Box) has followed an international cohort of patients with bipolar disorder and offered them opportunities to participate in clinical trials at seven field centers. We have made considerable progress as we focused on the longitudinal course of bipolar illness and evaluated various combinations of psychopharmacologic therapies. The SFBN also has explored factors that increase the risk of suicide, quantified the incidence of anxiety disorder and substance abuse, and looked at other variables that may affect treatment outcome.
This report for the readers of Current Psychiatry provides a brief state-of-the-art overview of what we have learned about the course and management of bipolar disorder. It brings together and summarizes the findings from studies published, in press, or under review. Our goal is to update current findings, which may help you provide optimum care for these at-risk patients.
Since Robert Prien’s pioneering studies in the 1970s, the National Institute of Mental Health (NIMH) had funded little work on prophylaxis of bipolar disorders or acute bipolar depression. Following two NIMH conferences on bipolar illness in 1989 and 1994, the Stanley Foundation Bipolar Network (SFBN) was established to help overcome these research deficiencies, with funding provided by the Theodore and Vada Stanley Foundation.
Patients were recruited into the network from the general community and with the use of newspaper and newsletter advertisements. In contrast to many other clinical populations, few who wished to enter the network were excluded. It was clear, however, that filling out demographic, clinical, and rating forms would require considerable effort from each patient. All patients gave written informed consent to participate in the naturalistic part of the study, and were reconsented for any open or blind randomized clinical trial.
Patients provided daily self-ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM), a unique longitudinal instrument. Clinicians then completed their own separate LCMs, as more severely ill patients often could not complete their self-ratings, and patients often underestimated the severity and functional disability associated with the hypomanic and manic phases of bipolar illness. With the use of the NIMH-LCM, network investigators were able, for the first time, to assess the details of mood fluctuations associated with bipolar illness and to more precisely delineate the qualitative and quantitative aspects of response to psychopharmacologic interventions.
The SFBN includes seven research field centers in the United States, the Netherlands, and Germany. The leaders of those centers collaborated with Robert M. Post, MD, to prepare this report of the network findings. The collaborators are:
| Gabriele S. Leverich, MSW, and Kirk D. Denicoff, MD Biological Psychiatry Branch National Institute of Mental Health Bethesda, MD | Paul E. Keck, Jr., MD, and Susan L. McElroy, MD Biological Psychiatry Program University of Cincinnati College of Medicine Cincinnati, OH |
| Lori L. Altshuler, MD, and Mark A. Frye, MD UCLA Mood Disorders Research Program VA Medical Center Los Angeles, CA | Willem A. Nolen, MD, and Ralph Kupka, MD Altrecht Institute for Mental Health Care University Medical Center Utrecht, The Netherlands |
| John Rush, MD, and Trisha Suppes, MD Department of Psychiatry Southwest Medical Center University of Texas, Dallas | Joerg Walden, MD Department of Psychiatry University of Freiburg Freiburg, Germany |
| Keith Kramlinger, MD Mayo Clinic, Rochester, MN (Administrative center) | Heinz Grunze, MD Psychiatrische Klinik der LMU Munich, Germany |
In part one, we discuss the demographics of our cohort of 676 community-based bipolar patients, including risk factors for suicide attempts, frequency of comorbid psychiatric diagnoses, problems of overweight and obesity, and preliminary findings about thyroiditis.
In part two, we describe our clinical trials assessing mood stabilizers and adjunctive agents such as atypical antipsychotics, second-generation antidepressants, and anticonvulsants. We also will address a soon-to-be analyzed study of omega-3 fatty acids.
Table 1
CHARACTERISTICS OF THE 676 BIPOLAR OUTPATIENTS
| Mean age | 41 | |
| % Female | 43 | |
| Diagnosis (%) | ||
| Bipolar I | 79 | |
| Bipolar II | 16 | |
| Bipolar NOS | 4 | |
| Marital status (%) | ||
| Married/cohabitating | 44 | |
| Divorced/separated | 22 | |
| Income (%) | ||
| <$20,000 | 37 | |
| $20,000-40,000 | 22 | |
| $40,000-100,000 | 32 | |
| >$100,000 | 9 | |
| Education (completed, %) | ||
| High school | 34 | |
| Jr. college/technical | 13 | |
| College, 4-year | 27 | |
| Graduate degree | 16 | |
| Positive family history (1st-degree relatives, %) | ||
| Bipolar disorder | 56 | |
| Unipolar disorder | 64 | |
| Schizophrenia | 24 | |
| Drug dependence | 44 | |
| Other psychiatric disorder | 52 | |
| Alcohol abuse/dependence | 52 | |
| Suicide (complete or serious attempt) | 38 | |
| Environmental adversity (%): | Child/adolescent | Adult |
| Physical abuse | 29 | 8 |
| Sexual abuse | 30 | 8 |
| Illness course and treatment (age in yrs) | ||
| Age of onset (symptoms) | 19 | |
| Early onset (age <14) | 34 | |
| Age at first treatment | 29 | |
| Age at first hospitalization | 30 | |
| Cycling pattern (n = 643, %) | ||
| Rapid (or faster cycling) | 47 | |
| Ultrarapid (or faster cycling) | 30 | |
| Rapid-cycling only | 17 | |
| Ultrarapid cycling only | 10 | |
| Ultradian cycling | 20 | |
Study population
The SFBN recruited outpatients with bipolar disorders I, II, or not otherwise specified (NOS). We did not exclude patients with comorbid illnesses, except those with active substance abuse disorder requiring treatment in another setting.1 Patients were recruited almost exclusively from the community and rarely from inpatient hospitalization. The average age was 41, and 43% were college graduates. Most were employed but indicated that their illness limited their employment level or ability to function at work (Table 1).
One striking finding was a decadelong lag between the onset of symptoms reaching thresholds for diagnosis and the onset of first treatment.2 This long delay could have adverse consequences for illness outcome and represents a major public health target. Age of onset appeared to have both genetic/familial and environmental determinants.3 Subjects with a history of both extreme early life adversities (i.e., physical or sexual abuse) and a positive family history of mood disorder had the earliest onset of bipolar illness. Those who had neither of these factors had late-onset illness.
Suicide attempts
Prior to network entry, 25 to 50% of our study population had made a serious suicide attempt (defined as requiring medical attention and/or hospitalization), pointing to bipolar disorder’s potential lethality.
History of abuse A history of physical or sexual abuse in childhood or adolescence was a prominent risk factor for attempted suicide.4 With either type of abuse there was an approximate 15% increase in incidence of suicide attempts over the baseline rate of about 25%. More than 55% of patients with a history of both physical and sexual abuse had made a serious suicide attempt. The frequency of physical and sexual abuse was also positively related to increased incidence of suicide attempts. Taken together, these data suggest a “stressor dose-response” relationship between adverse childhood or adolescent experiences and an increased incidence of suicide attempts.
Table 2
FACTORS THAT APPEAR TO INCREASE SUICIDE RISK IN BIPOLAR DISORDER*
| Course-of-illness characteristics |
| Early onset |
| More time ill |
| More time depressed |
| More-severe depression |
| Social and medical support |
| Loss of significant other |
| Lack of a confidant |
| Single motherhood |
| Decreased access to health care |
| Lack of medical insurance |
| Demographics |
| Less education |
| Lower income |
| Being single |
| Comorbidities |
| Axis I |
| Eating disorders |
| Anxiety disorders |
| Axis II |
| Cluster A, B, C |
| Medical |
| Traumatic life events |
| Sexual abuse |
| Physical abuse |
| Genetics |
| Family history of suicide and drug abuse |
| * Identified in the SFBN cohort |
Comorbidities Other factors that appear to be associated with bipolar disorder and increased risk of attempted suicide include anxiety and eating disorders, early onset of symptoms, at least four previous hospitalizations for depression, and medical comorbidity (Table 2). Limited access to psychiatric and medical services and inadequate health insurance also were correlated with having made a serious suicide attempt. Thus, lack of medical care might exacerbate demoralization and increase the risk of suicide.
Family history of suicide or serious suicide attempts and drug abuse were associated with an increase in suicide attempts, but family history of unipolar or bipolar illness were not. These data are consistent with other studies suggesting that vulnerability to suicide may have a separate genetic component from that of mood disorders per se.
Severity of illness Increased severity of bipolar illness also was associated with prior psychosocial stressors3 and serious suicide attempts. We confirmed self-reported history, using daily mood charting, the clinician-rated Inventory of Depressive Symptomatology (IDS-C), and the Young Mania Rating Scale (YMRS) to rate increase in percentage of time ill, percentage of time depressed, and severity of depression.
Clinical implications We are uncertain how directly these data regarding suicide attempts relate to completed suicide. However, patients with bipolar disorder are among those at highest risk for completed suicide, and a prior serious attempt is often a precursor to completed suicide.5,6
Factors that appear particularly related to increased risk of suicide in patients with bipolar disorder are family history of suicide, history of early psychosocial stressors, comorbid personality disorders, substance abuse, and a relatively severe course of bipolar illness. Patients with these risk factors need support for two types of psychosocial stressors:
- poor access to health care
- stressors related to concurrent events (e.g., occurring with new affective episodes).
It may also be important for clinicians to consider the prophylactic use of lithium because of its demonstrated ability to reduce mortality in bipolar illness.7 Additionally, we need data on the efficacy of new drugs in preventing affective episodes and reducing suicidal ideation and acts.
Common comorbidities
We found considerable axis I lifetime comorbidities in the SFBN cohort (Table 3), including a 42% incidence of anxiety disorder and a similarly high rate of substance abuse. In addition to bipolar disorder, patients averaged approximately two lifetime comorbid diagnoses:
- 35% had none
- 65% had one or more
- 42% had two or more
- 24% had three or more.
Table 3
PSYCHIATRIC COMORBIDITY IN BIPOLAR OUTPATIENTS (%)*.
| Any substance abuse disorder | 42% |
| Alcohol | 33 |
| Marijuana | 16 |
| Stimulant | 9 |
| Sedative | 8 |
| Opiate | 7 |
| Hallucinogens | 6 |
| Any anxiety disorder | 42% |
| Panic disorder | 20 |
| Social phobia | 16 |
| Simple phobia | 10 |
| OCD | 9 |
| PTSD | 7 |
| GAD | 3 |
| Other | 3 |
| Any eating disorder | 11% |
| Bulimia nervosa | 8 |
| Anorexia nervosa | 4 |
| * Lifetime comorbid axis I disorders based on Structured Clinical Interview for DSM-IV (SCID) interviews with 288 SFBN patients. | |
Men with bipolar disorder had a higher absolute prevalence of alcoholism, but the relative risk compared with the general population was much higher in women (odds ratio 7.35) compared with men (odds ratio 2.77). In women, alcohol use and abuse were related to history of social phobia, greater number of depressive episodes prior to network entry, and history of abusing more than one substance. In men, alcohol comorbidity was related to history of alcohol abuse in first-degree relatives and history of early physical abuse.
These data suggest that female patients, in particular, were self-medicating their affective episodes and comorbid anxiety disorders with alcohol. They suggest the importance of asking patients directly about alcohol use to identify the need for primary and secondary prevention. In an adolescent or young adult, affective illness—and bipolar disorder in particular—should be considered a major risk factor for subsequent problems with alcohol and drug abuse. When taking a careful history, the treating physician should include questions regarding any substance abuse.
Overweight and obesity
One-half of the women and two-thirds of the men in the SFBN were overweight at network entry.8 Although this rate of overweight sounds alarmingly high, it was not significantly different from rates in the general U.S. population. A greater percentage of women than men in the network had morbid obesity, however.
In our cohort, being overweight or obese was associated with an increased incidence of cardiovascular disease, diabetes, arthritis, hypertension, hypothyroidism, and cancer. The degree of obesity also correlated significantly with past use of the numerous psychotropic drugs that have been associated with weight gain. Also, as might be expected, increased weight was associated with physical inactivity.
Many of the psychotropic drugs routinely used to treat bipolar disorder are associated with weight gain. Among these are the mood stabilizers lithium and valproate and the atypical antipsychotics, with the exception of ziprasidone (and, to follow, aripiprazole). Lithium’s liability to cause weight gain may be counterbalanced by its antisuicide effects and its ability to reverse excess medical mortality associated with affective illness. Thus, lithium should continue to play a major therapeutic role in bipolar disorder.
It may be more effective and easier to prevent weight gain than to attempt to reduce weight that has been gained. Recommending exercise and other weight-reduction measures may be helpful, as may co-administering drugs associated with weight loss, such as topiramate.9
Thyroiditis
Antithyroid antibodies were detected in 28% of network patients, compared with rates of 3% in the general population and 18% in psychiatric controls.10 The increased rate was associated with the need to augment thyroid function in 17% of network patients, but it was not related to age, mood state, rapid cycling status, or lithium treatment.
These data indicate that bipolar patients are prone to autoimmune thyroid changes. Further analysis is required to ascertain whether this finding has therapeutic implications, especially related to adjunctive T3 and T4 treatment.11
Even with psychiatry’s most effective and best studied agents, many patients with bipolar disorder are inadequately treated and respond insufficiently to available psychopharmacologic agents. To address this problem, the Stanley Foundation Bipolar Network (SFBN) was created to explore acute and long-term response to treatment of bipolar disorder.
For the past 6 years, our group (Box) has followed an international cohort of patients with bipolar disorder and offered them opportunities to participate in clinical trials at seven field centers. We have made considerable progress as we focused on the longitudinal course of bipolar illness and evaluated various combinations of psychopharmacologic therapies. The SFBN also has explored factors that increase the risk of suicide, quantified the incidence of anxiety disorder and substance abuse, and looked at other variables that may affect treatment outcome.
This report for the readers of Current Psychiatry provides a brief state-of-the-art overview of what we have learned about the course and management of bipolar disorder. It brings together and summarizes the findings from studies published, in press, or under review. Our goal is to update current findings, which may help you provide optimum care for these at-risk patients.
Since Robert Prien’s pioneering studies in the 1970s, the National Institute of Mental Health (NIMH) had funded little work on prophylaxis of bipolar disorders or acute bipolar depression. Following two NIMH conferences on bipolar illness in 1989 and 1994, the Stanley Foundation Bipolar Network (SFBN) was established to help overcome these research deficiencies, with funding provided by the Theodore and Vada Stanley Foundation.
Patients were recruited into the network from the general community and with the use of newspaper and newsletter advertisements. In contrast to many other clinical populations, few who wished to enter the network were excluded. It was clear, however, that filling out demographic, clinical, and rating forms would require considerable effort from each patient. All patients gave written informed consent to participate in the naturalistic part of the study, and were reconsented for any open or blind randomized clinical trial.
Patients provided daily self-ratings on the National Institute of Mental Health Life Chart Method (NIMH-LCM), a unique longitudinal instrument. Clinicians then completed their own separate LCMs, as more severely ill patients often could not complete their self-ratings, and patients often underestimated the severity and functional disability associated with the hypomanic and manic phases of bipolar illness. With the use of the NIMH-LCM, network investigators were able, for the first time, to assess the details of mood fluctuations associated with bipolar illness and to more precisely delineate the qualitative and quantitative aspects of response to psychopharmacologic interventions.
The SFBN includes seven research field centers in the United States, the Netherlands, and Germany. The leaders of those centers collaborated with Robert M. Post, MD, to prepare this report of the network findings. The collaborators are:
| Gabriele S. Leverich, MSW, and Kirk D. Denicoff, MD Biological Psychiatry Branch National Institute of Mental Health Bethesda, MD | Paul E. Keck, Jr., MD, and Susan L. McElroy, MD Biological Psychiatry Program University of Cincinnati College of Medicine Cincinnati, OH |
| Lori L. Altshuler, MD, and Mark A. Frye, MD UCLA Mood Disorders Research Program VA Medical Center Los Angeles, CA | Willem A. Nolen, MD, and Ralph Kupka, MD Altrecht Institute for Mental Health Care University Medical Center Utrecht, The Netherlands |
| John Rush, MD, and Trisha Suppes, MD Department of Psychiatry Southwest Medical Center University of Texas, Dallas | Joerg Walden, MD Department of Psychiatry University of Freiburg Freiburg, Germany |
| Keith Kramlinger, MD Mayo Clinic, Rochester, MN (Administrative center) | Heinz Grunze, MD Psychiatrische Klinik der LMU Munich, Germany |
In part one, we discuss the demographics of our cohort of 676 community-based bipolar patients, including risk factors for suicide attempts, frequency of comorbid psychiatric diagnoses, problems of overweight and obesity, and preliminary findings about thyroiditis.
In part two, we describe our clinical trials assessing mood stabilizers and adjunctive agents such as atypical antipsychotics, second-generation antidepressants, and anticonvulsants. We also will address a soon-to-be analyzed study of omega-3 fatty acids.
Table 1
CHARACTERISTICS OF THE 676 BIPOLAR OUTPATIENTS
| Mean age | 41 | |
| % Female | 43 | |
| Diagnosis (%) | ||
| Bipolar I | 79 | |
| Bipolar II | 16 | |
| Bipolar NOS | 4 | |
| Marital status (%) | ||
| Married/cohabitating | 44 | |
| Divorced/separated | 22 | |
| Income (%) | ||
| <$20,000 | 37 | |
| $20,000-40,000 | 22 | |
| $40,000-100,000 | 32 | |
| >$100,000 | 9 | |
| Education (completed, %) | ||
| High school | 34 | |
| Jr. college/technical | 13 | |
| College, 4-year | 27 | |
| Graduate degree | 16 | |
| Positive family history (1st-degree relatives, %) | ||
| Bipolar disorder | 56 | |
| Unipolar disorder | 64 | |
| Schizophrenia | 24 | |
| Drug dependence | 44 | |
| Other psychiatric disorder | 52 | |
| Alcohol abuse/dependence | 52 | |
| Suicide (complete or serious attempt) | 38 | |
| Environmental adversity (%): | Child/adolescent | Adult |
| Physical abuse | 29 | 8 |
| Sexual abuse | 30 | 8 |
| Illness course and treatment (age in yrs) | ||
| Age of onset (symptoms) | 19 | |
| Early onset (age <14) | 34 | |
| Age at first treatment | 29 | |
| Age at first hospitalization | 30 | |
| Cycling pattern (n = 643, %) | ||
| Rapid (or faster cycling) | 47 | |
| Ultrarapid (or faster cycling) | 30 | |
| Rapid-cycling only | 17 | |
| Ultrarapid cycling only | 10 | |
| Ultradian cycling | 20 | |
Study population
The SFBN recruited outpatients with bipolar disorders I, II, or not otherwise specified (NOS). We did not exclude patients with comorbid illnesses, except those with active substance abuse disorder requiring treatment in another setting.1 Patients were recruited almost exclusively from the community and rarely from inpatient hospitalization. The average age was 41, and 43% were college graduates. Most were employed but indicated that their illness limited their employment level or ability to function at work (Table 1).
One striking finding was a decadelong lag between the onset of symptoms reaching thresholds for diagnosis and the onset of first treatment.2 This long delay could have adverse consequences for illness outcome and represents a major public health target. Age of onset appeared to have both genetic/familial and environmental determinants.3 Subjects with a history of both extreme early life adversities (i.e., physical or sexual abuse) and a positive family history of mood disorder had the earliest onset of bipolar illness. Those who had neither of these factors had late-onset illness.
Suicide attempts
Prior to network entry, 25 to 50% of our study population had made a serious suicide attempt (defined as requiring medical attention and/or hospitalization), pointing to bipolar disorder’s potential lethality.
History of abuse A history of physical or sexual abuse in childhood or adolescence was a prominent risk factor for attempted suicide.4 With either type of abuse there was an approximate 15% increase in incidence of suicide attempts over the baseline rate of about 25%. More than 55% of patients with a history of both physical and sexual abuse had made a serious suicide attempt. The frequency of physical and sexual abuse was also positively related to increased incidence of suicide attempts. Taken together, these data suggest a “stressor dose-response” relationship between adverse childhood or adolescent experiences and an increased incidence of suicide attempts.
Table 2
FACTORS THAT APPEAR TO INCREASE SUICIDE RISK IN BIPOLAR DISORDER*
| Course-of-illness characteristics |
| Early onset |
| More time ill |
| More time depressed |
| More-severe depression |
| Social and medical support |
| Loss of significant other |
| Lack of a confidant |
| Single motherhood |
| Decreased access to health care |
| Lack of medical insurance |
| Demographics |
| Less education |
| Lower income |
| Being single |
| Comorbidities |
| Axis I |
| Eating disorders |
| Anxiety disorders |
| Axis II |
| Cluster A, B, C |
| Medical |
| Traumatic life events |
| Sexual abuse |
| Physical abuse |
| Genetics |
| Family history of suicide and drug abuse |
| * Identified in the SFBN cohort |
Comorbidities Other factors that appear to be associated with bipolar disorder and increased risk of attempted suicide include anxiety and eating disorders, early onset of symptoms, at least four previous hospitalizations for depression, and medical comorbidity (Table 2). Limited access to psychiatric and medical services and inadequate health insurance also were correlated with having made a serious suicide attempt. Thus, lack of medical care might exacerbate demoralization and increase the risk of suicide.
Family history of suicide or serious suicide attempts and drug abuse were associated with an increase in suicide attempts, but family history of unipolar or bipolar illness were not. These data are consistent with other studies suggesting that vulnerability to suicide may have a separate genetic component from that of mood disorders per se.
Severity of illness Increased severity of bipolar illness also was associated with prior psychosocial stressors3 and serious suicide attempts. We confirmed self-reported history, using daily mood charting, the clinician-rated Inventory of Depressive Symptomatology (IDS-C), and the Young Mania Rating Scale (YMRS) to rate increase in percentage of time ill, percentage of time depressed, and severity of depression.
Clinical implications We are uncertain how directly these data regarding suicide attempts relate to completed suicide. However, patients with bipolar disorder are among those at highest risk for completed suicide, and a prior serious attempt is often a precursor to completed suicide.5,6
Factors that appear particularly related to increased risk of suicide in patients with bipolar disorder are family history of suicide, history of early psychosocial stressors, comorbid personality disorders, substance abuse, and a relatively severe course of bipolar illness. Patients with these risk factors need support for two types of psychosocial stressors:
- poor access to health care
- stressors related to concurrent events (e.g., occurring with new affective episodes).
It may also be important for clinicians to consider the prophylactic use of lithium because of its demonstrated ability to reduce mortality in bipolar illness.7 Additionally, we need data on the efficacy of new drugs in preventing affective episodes and reducing suicidal ideation and acts.
Common comorbidities
We found considerable axis I lifetime comorbidities in the SFBN cohort (Table 3), including a 42% incidence of anxiety disorder and a similarly high rate of substance abuse. In addition to bipolar disorder, patients averaged approximately two lifetime comorbid diagnoses:
- 35% had none
- 65% had one or more
- 42% had two or more
- 24% had three or more.
Table 3
PSYCHIATRIC COMORBIDITY IN BIPOLAR OUTPATIENTS (%)*.
| Any substance abuse disorder | 42% |
| Alcohol | 33 |
| Marijuana | 16 |
| Stimulant | 9 |
| Sedative | 8 |
| Opiate | 7 |
| Hallucinogens | 6 |
| Any anxiety disorder | 42% |
| Panic disorder | 20 |
| Social phobia | 16 |
| Simple phobia | 10 |
| OCD | 9 |
| PTSD | 7 |
| GAD | 3 |
| Other | 3 |
| Any eating disorder | 11% |
| Bulimia nervosa | 8 |
| Anorexia nervosa | 4 |
| * Lifetime comorbid axis I disorders based on Structured Clinical Interview for DSM-IV (SCID) interviews with 288 SFBN patients. | |
Men with bipolar disorder had a higher absolute prevalence of alcoholism, but the relative risk compared with the general population was much higher in women (odds ratio 7.35) compared with men (odds ratio 2.77). In women, alcohol use and abuse were related to history of social phobia, greater number of depressive episodes prior to network entry, and history of abusing more than one substance. In men, alcohol comorbidity was related to history of alcohol abuse in first-degree relatives and history of early physical abuse.
These data suggest that female patients, in particular, were self-medicating their affective episodes and comorbid anxiety disorders with alcohol. They suggest the importance of asking patients directly about alcohol use to identify the need for primary and secondary prevention. In an adolescent or young adult, affective illness—and bipolar disorder in particular—should be considered a major risk factor for subsequent problems with alcohol and drug abuse. When taking a careful history, the treating physician should include questions regarding any substance abuse.
Overweight and obesity
One-half of the women and two-thirds of the men in the SFBN were overweight at network entry.8 Although this rate of overweight sounds alarmingly high, it was not significantly different from rates in the general U.S. population. A greater percentage of women than men in the network had morbid obesity, however.
In our cohort, being overweight or obese was associated with an increased incidence of cardiovascular disease, diabetes, arthritis, hypertension, hypothyroidism, and cancer. The degree of obesity also correlated significantly with past use of the numerous psychotropic drugs that have been associated with weight gain. Also, as might be expected, increased weight was associated with physical inactivity.
Many of the psychotropic drugs routinely used to treat bipolar disorder are associated with weight gain. Among these are the mood stabilizers lithium and valproate and the atypical antipsychotics, with the exception of ziprasidone (and, to follow, aripiprazole). Lithium’s liability to cause weight gain may be counterbalanced by its antisuicide effects and its ability to reverse excess medical mortality associated with affective illness. Thus, lithium should continue to play a major therapeutic role in bipolar disorder.
It may be more effective and easier to prevent weight gain than to attempt to reduce weight that has been gained. Recommending exercise and other weight-reduction measures may be helpful, as may co-administering drugs associated with weight loss, such as topiramate.9
Thyroiditis
Antithyroid antibodies were detected in 28% of network patients, compared with rates of 3% in the general population and 18% in psychiatric controls.10 The increased rate was associated with the need to augment thyroid function in 17% of network patients, but it was not related to age, mood state, rapid cycling status, or lithium treatment.
These data indicate that bipolar patients are prone to autoimmune thyroid changes. Further analysis is required to ascertain whether this finding has therapeutic implications, especially related to adjunctive T3 and T4 treatment.11
1. Leverich GS, Nolen WA, Rush AJ, et al. The Stanley Foundation Bipolar Treatment Outcome Network. I. Longitudinal methodology. J Affect Disord 2001;67:33-44.
2. Suppes T, Leverich GS, Keck PE, et al. The Stanley Foundation Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disord 2001b;67:45-59.
3. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological Psychiatry 2002;51:288-97.
4. Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. Manuscript submitted for publication, 2002.
5. Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39:896-9.
6. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch.Gen.Psychiatry 1999;56:617-26.
7. Ahrens B, Muller-Oerlinghausen B, Schou M, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord 1995;33:67-75.
8. McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry 2002;63:207-13.
9. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biological Psychiatry 2000;47:1025-33.
10. Kupka RW, Nolen WA, Post RM, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biological Psychiatry 2002;51:305-11.
11. Bauer M, Priebe S, Berghofer A, et al. Subjective response to and tolerability of long-term supraphysiological doses of levothyroxine in refractory mood disorders. J Affect Disord 2001;64:35-42.
1. Leverich GS, Nolen WA, Rush AJ, et al. The Stanley Foundation Bipolar Treatment Outcome Network. I. Longitudinal methodology. J Affect Disord 2001;67:33-44.
2. Suppes T, Leverich GS, Keck PE, et al. The Stanley Foundation Bipolar Treatment Outcome Network. II. Demographics and illness characteristics of the first 261 patients. J Affect Disord 2001b;67:45-59.
3. Leverich GS, McElroy SL, Suppes T, et al. Early physical and sexual abuse associated with an adverse course of bipolar illness. Biological Psychiatry 2002;51:288-97.
4. Leverich GS, Altshuler LL, Frye MA, et al. Factors associated with suicide attempts in 648 patients with bipolar disorder in the Stanley Foundation Bipolar Network. Manuscript submitted for publication, 2002.
5. Chen Y-W, Dilsaver SC. Lifetime rates of suicide attempts among subjects with bipolar and unipolar disorders relative to subjects with other axis I disorders. Biological Psychiatry 1996;39:896-9.
6. Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch.Gen.Psychiatry 1999;56:617-26.
7. Ahrens B, Muller-Oerlinghausen B, Schou M, et al. Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord 1995;33:67-75.
8. McElroy SL, Frye MA, Suppes T, et al. Correlates of overweight and obesity in 644 patients with bipolar disorder. J Clin Psychiatry 2002;63:207-13.
9. McElroy SL, Suppes T, Keck PE, et al. Open-label adjunctive topiramate in the treatment of bipolar disorders. Biological Psychiatry 2000;47:1025-33.
10. Kupka RW, Nolen WA, Post RM, et al. High rate of autoimmune thyroiditis in bipolar disorder: lack of association with lithium exposure. Biological Psychiatry 2002;51:305-11.
11. Bauer M, Priebe S, Berghofer A, et al. Subjective response to and tolerability of long-term supraphysiological doses of levothyroxine in refractory mood disorders. J Affect Disord 2001;64:35-42.
Counterpoint: Flaws in the sexual addiction model
It is naïve to think that psychiatry is generally accepted in the treatment of problematic sexual behaviors. Although some might think that psychiatry has a “liberated” view of sexuality born of Freud’s Oedipus, the psychiatrist’s involvement in managing sexual behavior, especially within the criminal justice system, is controversial rather than “celebrated.”
Although some such as Dr. Mahorney may assert an addiction model for problem sexual behavior, that model is seriously flawed. Parallels do exist between problem sexual behavior and other addictive behaviors. Within the broad range of sexual behaviors, however, the line between “addictive” and “alternative” cannot be finely drawn. For example:
- Certain advocacy groups such as the North American Man-Boy Love Association (NAMBLA) believe in legalized pederasty and have exerted a lobby to affect rulings on freedom of expression in “virtual” child pornography over the Internet.
- Representatives of the U.S. Catholic church have publicly “mitigated” the controversy over some priests’ sexually inappropriate behavior by stating that the offenders were “not pedophiles” because their victims were over the arbitrary age of 13.
This discussion might easily encompass the controversial “cures” for homosexuality that were quashed in the late 1960s. How also might we classify the consenting masochist or the philanderer?
Finally, there is the forensic question: Do we allow “mental illness” to “excuse” one’s willful sexual behavior by giving it a label or a treatment?
Sexual offense vs. mental illness
Part of the problem with defining sexual “addiction” is that one first needs to define sexual “normalcy.” No such definition exists. Even so, adding a “sexual addiction” diagnosis to the next Diagnostic and Statistical Manual (DSM) would likely be met with indifference or active denial from the psychiatric community, based solely on its legal ramifications. Attorneys would perceive that an official DSM diagnosis could open the door to acquittal on the basis of insanity for sexually motivated crimes in certain states that retain a “product rule.” It might even exonerate individuals in restrictive states that follow the more cognitive M’Naughten standard (Box).
The “product rule” asserts that a person is not responsible for his or her acts if they were a product of mental illness. The M’Naughten rule holds that a person is not responsible if, by virtue of a mental illness, that person does not understand the wrongfulness of the act.
Assailant John Hinckley Jr. was acquitted on the basis of an insanity plea under the “product rule” in the attempted assassination of President Reagan on March 30, 1981, outside a hotel in Washington, DC. Hinckley, who was obsessed with actress Jodie Foster, shot and wounded Reagan, press secretary James Brady, a Secret Service agent, and a police officer. After Hinckley’s acquittal, most states adopted the more stringent M’Naughten standard.
A number of forensic mental health issues such as sexual predator classifications and sex offender civil commitment have placed professional societies at odds with one another over sexual behavior and mental illness. Mental health organizations such as the National Association of State Mental Health Program Directors and the American Psychiatric Association argue that most sex offenders do not suffer from a mental illness. Translation: “We don’t want to be responsible for handling sex offenders.”
This distinction between sexual offenses and mental illness contrasts with the laws of several states that broadly define mental illness to include mental conditions that result in dangerous and “uncontrollable” behavior. Translation: “We want to keep sex offenders controlled or away from the community.” There has been no rational support for either of these positions, only data that have been loosely contrived to justify one or the other.
Res ipsum loquitur: the thing defines itself
Many “experts” have conjectured about the nature of sex-offending behavior and the phenomenon of “sexual addiction” without subjecting their conclusions to scientific scrutiny. Agendas have abounded, and countertransference rather than objectivity has driven many perceptions within the field. Treatments have often been theory-based rather than tested. The universal problem has been that many providers have invented solutions without defining the problems. Indeed, the problems have been multifaceted.
There has been little reliable outcome data to demonstrate the success in any given treatment, although a few notable exceptions stand out. Medical interventions with hormonal compounds like medroxyprogesterone and cyproterone have reduced recidivism.1 According to the Task Force Report on Sexually Dangerous Offenders, however, psychodynamic treatments in general have been ineffective.1 Most of the supportable psychological treatments have emerged since 1980 and have centered on a cognitive model of relapse prevention.2
The Minnesota Sex Offender Program has been the gold standard as a cognitive-based relapse prevention model for sex offenders.3 The program was developed as an inpatient program with a structured level system and topic-specific modules. It has focused on a relapse prevention cycle that teaches strategies to recognize and avoid cognitive distortions and maladaptive coping responses that eventually lead to re-offense.
Common beliefs about the incidence of mental illness in sex offenders, aside from paraphilias, have also been challenged by careful observation. Our research at the University of Cincinnati revealed high rates of mental illness and substance abuse in a group of sex offenders released from prison to a residential treatment center. Offenders with paraphilias had even higher rates of mood, anxiety, and impulse control disorders (Table).4 Concurrently, Nancy Raymond and her colleagues reported similar findings with a group of residential and outpatient child molesters in Minnesota.5 Data from both these studies suggest that:
- Axis I conditions, especially those related to increased sexual drive or impaired impulse control (such as bipolar disorder), have some importance in treatment.
- Some relationship exists between Axis I disorders and the expression of underlying paraphilic interests.
It is clear that sexual compulsions are not homogeneous, and different characteristics exist across different populations. In our sample, for instance, pedophiles had lower rates of antisocial personality disorder but higher rates of anxiety than other offenders. Pharmacologically, Martin Kafka found that self-identified, noncriminal clients with sexual compulsions responded well to selective serotonin reuptake inhibitors (SSRIs) and stimulants,6 whereas patients at the University of Cincinnati program had a far less robust response to SSRI treatment.7 In essence, one style of intervention may not fit all patients.
Table
COMMON AXIS I DIAGNOSES IN 113 CONVICTED SEX OFFENDERS WITH AND WITHOUT PARAPHILIAS*
| Diagnosis | Total | With paraphilias | Without paraphilias | |||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| Mood disorders (any) | 66 | (58.4) | 61 | (72.6) | 5 | (19.2) |
| Major depressive | 27 | (23.9) | 26 | (31.0) | 1 | (3.8) |
| Bipolar I | 28 | (24.8) | 25 | (29.8) | 2 | (7.7) |
| Substance abuse (any) | 96 | (85.0) | 69 | (82.1) | 26 | (100.0) |
| Anxiety disorders (any) | 26 | (23.0) | 24 | (28.6) | 2 | (7.7) |
| Eating disorders (any) | 10 | (8.8) | 10 | (11.9) | 0 | (0) |
| Impulse control disorders (any) | 43 | (38.1) | 38 | (45.2) | 4 | (15.4) |
| Compulsive buying | 11 | (9.7) | 11 | (13.1) | 0 | (0) |
| * Among the 113 subjects, 110 were evaluated for paraphilias. In that group, 84 were diagnosed as paraphilic and 26 as nonparaphilic. Mood disorders, major depressive disorder, bipolar affective disorder type I, and impulse control disorders were significantly more prevalent among paraphilic sex offenders. Individuals without paraphilias were significantly more likely than those with paraphilias to have a substance abuse disorder. Anxiety disorders and compulsive buying were more prevalent among paraphilic sex offenders. | ||||||
One other problem has plagued the forensic setting: Sex offenders lie about their sexual urges. In our program, they often appeared to “comply” with the program but concealed their sexual urges and fantasies. Sometimes they were embarrassed, ashamed, or simply in denial. We found that serial polygraph testing was essential for gauging treatment because it allowed us to objectively examine the information that the patients offered.
Frequently, I have heard people assert that an individual who seeks treatment actually wants treatment. Few sex offenders self-identify, however, and many more enter treatment as a condition of probation or parole. Thus most have an external motivation to participate. And with sex offenders, voluntary treatment is less successful than mandated treatment.1
Summary
Ultimately, self-help, 12-step, and psychodynamic approaches have an unproven role in managing problem sexual behaviors. The most appropriate intervention is a multidisciplinary team approach with screening for Axis I disorders and paraphilias, a thorough psychological assessment, substance abuse treatment, and a cognitive relapse prevention program. Forensic patients additionally benefit from collateral data such as polygraph testing and ongoing monitoring by community corrections personnel.
1. Zonana H, (chairperson). Dangerous sex offenders. A task force report of the American Psychiatric Association. Washington, DC: American Psychiatric Association Press, 1999.
2. Hanson RK, Gordon A, Harris AJ, et al. First report of the collaborative outcome data project on the effectiveness of psychological treatment for sex offenders. Sex Abuse 2002;14(2):169-94.
3. Schlank A, Cohen F. The sexual predator: law, policy, evaluation, and treatment. Kingston, NJ: Civic Research Institute, 1999.
4. McElroy SL, Soutullo CA, Taylor P, et al. Psychiatric features of 36 persons convicted of sexual offenses. J Clin Psychiatry 1999;60(6):414-22(update by Dunsieth N, et al with data from 113 subjects in progress).
5. Raymond N, Coleman E, Ohlerking F, Christenson GA, Miner M. Psychiatric comorbidity in pedophilic sex offenders. Am J Psychiatry 1999;156:786-8.
6. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry 2000;61(9):664-70.
7. Nelson E, et al. Data unpublished from the University of Cincinnati Biological Psychiatry Program (paper in progress).
It is naïve to think that psychiatry is generally accepted in the treatment of problematic sexual behaviors. Although some might think that psychiatry has a “liberated” view of sexuality born of Freud’s Oedipus, the psychiatrist’s involvement in managing sexual behavior, especially within the criminal justice system, is controversial rather than “celebrated.”
Although some such as Dr. Mahorney may assert an addiction model for problem sexual behavior, that model is seriously flawed. Parallels do exist between problem sexual behavior and other addictive behaviors. Within the broad range of sexual behaviors, however, the line between “addictive” and “alternative” cannot be finely drawn. For example:
- Certain advocacy groups such as the North American Man-Boy Love Association (NAMBLA) believe in legalized pederasty and have exerted a lobby to affect rulings on freedom of expression in “virtual” child pornography over the Internet.
- Representatives of the U.S. Catholic church have publicly “mitigated” the controversy over some priests’ sexually inappropriate behavior by stating that the offenders were “not pedophiles” because their victims were over the arbitrary age of 13.
This discussion might easily encompass the controversial “cures” for homosexuality that were quashed in the late 1960s. How also might we classify the consenting masochist or the philanderer?
Finally, there is the forensic question: Do we allow “mental illness” to “excuse” one’s willful sexual behavior by giving it a label or a treatment?
Sexual offense vs. mental illness
Part of the problem with defining sexual “addiction” is that one first needs to define sexual “normalcy.” No such definition exists. Even so, adding a “sexual addiction” diagnosis to the next Diagnostic and Statistical Manual (DSM) would likely be met with indifference or active denial from the psychiatric community, based solely on its legal ramifications. Attorneys would perceive that an official DSM diagnosis could open the door to acquittal on the basis of insanity for sexually motivated crimes in certain states that retain a “product rule.” It might even exonerate individuals in restrictive states that follow the more cognitive M’Naughten standard (Box).
The “product rule” asserts that a person is not responsible for his or her acts if they were a product of mental illness. The M’Naughten rule holds that a person is not responsible if, by virtue of a mental illness, that person does not understand the wrongfulness of the act.
Assailant John Hinckley Jr. was acquitted on the basis of an insanity plea under the “product rule” in the attempted assassination of President Reagan on March 30, 1981, outside a hotel in Washington, DC. Hinckley, who was obsessed with actress Jodie Foster, shot and wounded Reagan, press secretary James Brady, a Secret Service agent, and a police officer. After Hinckley’s acquittal, most states adopted the more stringent M’Naughten standard.
A number of forensic mental health issues such as sexual predator classifications and sex offender civil commitment have placed professional societies at odds with one another over sexual behavior and mental illness. Mental health organizations such as the National Association of State Mental Health Program Directors and the American Psychiatric Association argue that most sex offenders do not suffer from a mental illness. Translation: “We don’t want to be responsible for handling sex offenders.”
This distinction between sexual offenses and mental illness contrasts with the laws of several states that broadly define mental illness to include mental conditions that result in dangerous and “uncontrollable” behavior. Translation: “We want to keep sex offenders controlled or away from the community.” There has been no rational support for either of these positions, only data that have been loosely contrived to justify one or the other.
Res ipsum loquitur: the thing defines itself
Many “experts” have conjectured about the nature of sex-offending behavior and the phenomenon of “sexual addiction” without subjecting their conclusions to scientific scrutiny. Agendas have abounded, and countertransference rather than objectivity has driven many perceptions within the field. Treatments have often been theory-based rather than tested. The universal problem has been that many providers have invented solutions without defining the problems. Indeed, the problems have been multifaceted.
There has been little reliable outcome data to demonstrate the success in any given treatment, although a few notable exceptions stand out. Medical interventions with hormonal compounds like medroxyprogesterone and cyproterone have reduced recidivism.1 According to the Task Force Report on Sexually Dangerous Offenders, however, psychodynamic treatments in general have been ineffective.1 Most of the supportable psychological treatments have emerged since 1980 and have centered on a cognitive model of relapse prevention.2
The Minnesota Sex Offender Program has been the gold standard as a cognitive-based relapse prevention model for sex offenders.3 The program was developed as an inpatient program with a structured level system and topic-specific modules. It has focused on a relapse prevention cycle that teaches strategies to recognize and avoid cognitive distortions and maladaptive coping responses that eventually lead to re-offense.
Common beliefs about the incidence of mental illness in sex offenders, aside from paraphilias, have also been challenged by careful observation. Our research at the University of Cincinnati revealed high rates of mental illness and substance abuse in a group of sex offenders released from prison to a residential treatment center. Offenders with paraphilias had even higher rates of mood, anxiety, and impulse control disorders (Table).4 Concurrently, Nancy Raymond and her colleagues reported similar findings with a group of residential and outpatient child molesters in Minnesota.5 Data from both these studies suggest that:
- Axis I conditions, especially those related to increased sexual drive or impaired impulse control (such as bipolar disorder), have some importance in treatment.
- Some relationship exists between Axis I disorders and the expression of underlying paraphilic interests.
It is clear that sexual compulsions are not homogeneous, and different characteristics exist across different populations. In our sample, for instance, pedophiles had lower rates of antisocial personality disorder but higher rates of anxiety than other offenders. Pharmacologically, Martin Kafka found that self-identified, noncriminal clients with sexual compulsions responded well to selective serotonin reuptake inhibitors (SSRIs) and stimulants,6 whereas patients at the University of Cincinnati program had a far less robust response to SSRI treatment.7 In essence, one style of intervention may not fit all patients.
Table
COMMON AXIS I DIAGNOSES IN 113 CONVICTED SEX OFFENDERS WITH AND WITHOUT PARAPHILIAS*
| Diagnosis | Total | With paraphilias | Without paraphilias | |||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| Mood disorders (any) | 66 | (58.4) | 61 | (72.6) | 5 | (19.2) |
| Major depressive | 27 | (23.9) | 26 | (31.0) | 1 | (3.8) |
| Bipolar I | 28 | (24.8) | 25 | (29.8) | 2 | (7.7) |
| Substance abuse (any) | 96 | (85.0) | 69 | (82.1) | 26 | (100.0) |
| Anxiety disorders (any) | 26 | (23.0) | 24 | (28.6) | 2 | (7.7) |
| Eating disorders (any) | 10 | (8.8) | 10 | (11.9) | 0 | (0) |
| Impulse control disorders (any) | 43 | (38.1) | 38 | (45.2) | 4 | (15.4) |
| Compulsive buying | 11 | (9.7) | 11 | (13.1) | 0 | (0) |
| * Among the 113 subjects, 110 were evaluated for paraphilias. In that group, 84 were diagnosed as paraphilic and 26 as nonparaphilic. Mood disorders, major depressive disorder, bipolar affective disorder type I, and impulse control disorders were significantly more prevalent among paraphilic sex offenders. Individuals without paraphilias were significantly more likely than those with paraphilias to have a substance abuse disorder. Anxiety disorders and compulsive buying were more prevalent among paraphilic sex offenders. | ||||||
One other problem has plagued the forensic setting: Sex offenders lie about their sexual urges. In our program, they often appeared to “comply” with the program but concealed their sexual urges and fantasies. Sometimes they were embarrassed, ashamed, or simply in denial. We found that serial polygraph testing was essential for gauging treatment because it allowed us to objectively examine the information that the patients offered.
Frequently, I have heard people assert that an individual who seeks treatment actually wants treatment. Few sex offenders self-identify, however, and many more enter treatment as a condition of probation or parole. Thus most have an external motivation to participate. And with sex offenders, voluntary treatment is less successful than mandated treatment.1
Summary
Ultimately, self-help, 12-step, and psychodynamic approaches have an unproven role in managing problem sexual behaviors. The most appropriate intervention is a multidisciplinary team approach with screening for Axis I disorders and paraphilias, a thorough psychological assessment, substance abuse treatment, and a cognitive relapse prevention program. Forensic patients additionally benefit from collateral data such as polygraph testing and ongoing monitoring by community corrections personnel.
It is naïve to think that psychiatry is generally accepted in the treatment of problematic sexual behaviors. Although some might think that psychiatry has a “liberated” view of sexuality born of Freud’s Oedipus, the psychiatrist’s involvement in managing sexual behavior, especially within the criminal justice system, is controversial rather than “celebrated.”
Although some such as Dr. Mahorney may assert an addiction model for problem sexual behavior, that model is seriously flawed. Parallels do exist between problem sexual behavior and other addictive behaviors. Within the broad range of sexual behaviors, however, the line between “addictive” and “alternative” cannot be finely drawn. For example:
- Certain advocacy groups such as the North American Man-Boy Love Association (NAMBLA) believe in legalized pederasty and have exerted a lobby to affect rulings on freedom of expression in “virtual” child pornography over the Internet.
- Representatives of the U.S. Catholic church have publicly “mitigated” the controversy over some priests’ sexually inappropriate behavior by stating that the offenders were “not pedophiles” because their victims were over the arbitrary age of 13.
This discussion might easily encompass the controversial “cures” for homosexuality that were quashed in the late 1960s. How also might we classify the consenting masochist or the philanderer?
Finally, there is the forensic question: Do we allow “mental illness” to “excuse” one’s willful sexual behavior by giving it a label or a treatment?
Sexual offense vs. mental illness
Part of the problem with defining sexual “addiction” is that one first needs to define sexual “normalcy.” No such definition exists. Even so, adding a “sexual addiction” diagnosis to the next Diagnostic and Statistical Manual (DSM) would likely be met with indifference or active denial from the psychiatric community, based solely on its legal ramifications. Attorneys would perceive that an official DSM diagnosis could open the door to acquittal on the basis of insanity for sexually motivated crimes in certain states that retain a “product rule.” It might even exonerate individuals in restrictive states that follow the more cognitive M’Naughten standard (Box).
The “product rule” asserts that a person is not responsible for his or her acts if they were a product of mental illness. The M’Naughten rule holds that a person is not responsible if, by virtue of a mental illness, that person does not understand the wrongfulness of the act.
Assailant John Hinckley Jr. was acquitted on the basis of an insanity plea under the “product rule” in the attempted assassination of President Reagan on March 30, 1981, outside a hotel in Washington, DC. Hinckley, who was obsessed with actress Jodie Foster, shot and wounded Reagan, press secretary James Brady, a Secret Service agent, and a police officer. After Hinckley’s acquittal, most states adopted the more stringent M’Naughten standard.
A number of forensic mental health issues such as sexual predator classifications and sex offender civil commitment have placed professional societies at odds with one another over sexual behavior and mental illness. Mental health organizations such as the National Association of State Mental Health Program Directors and the American Psychiatric Association argue that most sex offenders do not suffer from a mental illness. Translation: “We don’t want to be responsible for handling sex offenders.”
This distinction between sexual offenses and mental illness contrasts with the laws of several states that broadly define mental illness to include mental conditions that result in dangerous and “uncontrollable” behavior. Translation: “We want to keep sex offenders controlled or away from the community.” There has been no rational support for either of these positions, only data that have been loosely contrived to justify one or the other.
Res ipsum loquitur: the thing defines itself
Many “experts” have conjectured about the nature of sex-offending behavior and the phenomenon of “sexual addiction” without subjecting their conclusions to scientific scrutiny. Agendas have abounded, and countertransference rather than objectivity has driven many perceptions within the field. Treatments have often been theory-based rather than tested. The universal problem has been that many providers have invented solutions without defining the problems. Indeed, the problems have been multifaceted.
There has been little reliable outcome data to demonstrate the success in any given treatment, although a few notable exceptions stand out. Medical interventions with hormonal compounds like medroxyprogesterone and cyproterone have reduced recidivism.1 According to the Task Force Report on Sexually Dangerous Offenders, however, psychodynamic treatments in general have been ineffective.1 Most of the supportable psychological treatments have emerged since 1980 and have centered on a cognitive model of relapse prevention.2
The Minnesota Sex Offender Program has been the gold standard as a cognitive-based relapse prevention model for sex offenders.3 The program was developed as an inpatient program with a structured level system and topic-specific modules. It has focused on a relapse prevention cycle that teaches strategies to recognize and avoid cognitive distortions and maladaptive coping responses that eventually lead to re-offense.
Common beliefs about the incidence of mental illness in sex offenders, aside from paraphilias, have also been challenged by careful observation. Our research at the University of Cincinnati revealed high rates of mental illness and substance abuse in a group of sex offenders released from prison to a residential treatment center. Offenders with paraphilias had even higher rates of mood, anxiety, and impulse control disorders (Table).4 Concurrently, Nancy Raymond and her colleagues reported similar findings with a group of residential and outpatient child molesters in Minnesota.5 Data from both these studies suggest that:
- Axis I conditions, especially those related to increased sexual drive or impaired impulse control (such as bipolar disorder), have some importance in treatment.
- Some relationship exists between Axis I disorders and the expression of underlying paraphilic interests.
It is clear that sexual compulsions are not homogeneous, and different characteristics exist across different populations. In our sample, for instance, pedophiles had lower rates of antisocial personality disorder but higher rates of anxiety than other offenders. Pharmacologically, Martin Kafka found that self-identified, noncriminal clients with sexual compulsions responded well to selective serotonin reuptake inhibitors (SSRIs) and stimulants,6 whereas patients at the University of Cincinnati program had a far less robust response to SSRI treatment.7 In essence, one style of intervention may not fit all patients.
Table
COMMON AXIS I DIAGNOSES IN 113 CONVICTED SEX OFFENDERS WITH AND WITHOUT PARAPHILIAS*
| Diagnosis | Total | With paraphilias | Without paraphilias | |||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| Mood disorders (any) | 66 | (58.4) | 61 | (72.6) | 5 | (19.2) |
| Major depressive | 27 | (23.9) | 26 | (31.0) | 1 | (3.8) |
| Bipolar I | 28 | (24.8) | 25 | (29.8) | 2 | (7.7) |
| Substance abuse (any) | 96 | (85.0) | 69 | (82.1) | 26 | (100.0) |
| Anxiety disorders (any) | 26 | (23.0) | 24 | (28.6) | 2 | (7.7) |
| Eating disorders (any) | 10 | (8.8) | 10 | (11.9) | 0 | (0) |
| Impulse control disorders (any) | 43 | (38.1) | 38 | (45.2) | 4 | (15.4) |
| Compulsive buying | 11 | (9.7) | 11 | (13.1) | 0 | (0) |
| * Among the 113 subjects, 110 were evaluated for paraphilias. In that group, 84 were diagnosed as paraphilic and 26 as nonparaphilic. Mood disorders, major depressive disorder, bipolar affective disorder type I, and impulse control disorders were significantly more prevalent among paraphilic sex offenders. Individuals without paraphilias were significantly more likely than those with paraphilias to have a substance abuse disorder. Anxiety disorders and compulsive buying were more prevalent among paraphilic sex offenders. | ||||||
One other problem has plagued the forensic setting: Sex offenders lie about their sexual urges. In our program, they often appeared to “comply” with the program but concealed their sexual urges and fantasies. Sometimes they were embarrassed, ashamed, or simply in denial. We found that serial polygraph testing was essential for gauging treatment because it allowed us to objectively examine the information that the patients offered.
Frequently, I have heard people assert that an individual who seeks treatment actually wants treatment. Few sex offenders self-identify, however, and many more enter treatment as a condition of probation or parole. Thus most have an external motivation to participate. And with sex offenders, voluntary treatment is less successful than mandated treatment.1
Summary
Ultimately, self-help, 12-step, and psychodynamic approaches have an unproven role in managing problem sexual behaviors. The most appropriate intervention is a multidisciplinary team approach with screening for Axis I disorders and paraphilias, a thorough psychological assessment, substance abuse treatment, and a cognitive relapse prevention program. Forensic patients additionally benefit from collateral data such as polygraph testing and ongoing monitoring by community corrections personnel.
1. Zonana H, (chairperson). Dangerous sex offenders. A task force report of the American Psychiatric Association. Washington, DC: American Psychiatric Association Press, 1999.
2. Hanson RK, Gordon A, Harris AJ, et al. First report of the collaborative outcome data project on the effectiveness of psychological treatment for sex offenders. Sex Abuse 2002;14(2):169-94.
3. Schlank A, Cohen F. The sexual predator: law, policy, evaluation, and treatment. Kingston, NJ: Civic Research Institute, 1999.
4. McElroy SL, Soutullo CA, Taylor P, et al. Psychiatric features of 36 persons convicted of sexual offenses. J Clin Psychiatry 1999;60(6):414-22(update by Dunsieth N, et al with data from 113 subjects in progress).
5. Raymond N, Coleman E, Ohlerking F, Christenson GA, Miner M. Psychiatric comorbidity in pedophilic sex offenders. Am J Psychiatry 1999;156:786-8.
6. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry 2000;61(9):664-70.
7. Nelson E, et al. Data unpublished from the University of Cincinnati Biological Psychiatry Program (paper in progress).
1. Zonana H, (chairperson). Dangerous sex offenders. A task force report of the American Psychiatric Association. Washington, DC: American Psychiatric Association Press, 1999.
2. Hanson RK, Gordon A, Harris AJ, et al. First report of the collaborative outcome data project on the effectiveness of psychological treatment for sex offenders. Sex Abuse 2002;14(2):169-94.
3. Schlank A, Cohen F. The sexual predator: law, policy, evaluation, and treatment. Kingston, NJ: Civic Research Institute, 1999.
4. McElroy SL, Soutullo CA, Taylor P, et al. Psychiatric features of 36 persons convicted of sexual offenses. J Clin Psychiatry 1999;60(6):414-22(update by Dunsieth N, et al with data from 113 subjects in progress).
5. Raymond N, Coleman E, Ohlerking F, Christenson GA, Miner M. Psychiatric comorbidity in pedophilic sex offenders. Am J Psychiatry 1999;156:786-8.
6. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry 2000;61(9):664-70.
7. Nelson E, et al. Data unpublished from the University of Cincinnati Biological Psychiatry Program (paper in progress).
Counterpoint: Flaws in the sexual addiction model
It is naïve to think that psychiatry is generally accepted in the treatment of problematic sexual behaviors. Although some might think that psychiatry has a “liberated” view of sexuality born of Freud’s Oedipus, the psychiatrist’s involvement in managing sexual behavior, especially within the criminal justice system, is controversial rather than “celebrated.”
Although some such as Dr. Mahorney may assert an addiction model for problem sexual behavior, that model is seriously flawed. Parallels do exist between problem sexual behavior and other addictive behaviors. Within the broad range of sexual behaviors, however, the line between “addictive” and “alternative” cannot be finely drawn. For example:
- Certain advocacy groups such as the North American Man-Boy Love Association (NAMBLA) believe in legalized pederasty and have exerted a lobby to affect rulings on freedom of expression in “virtual” child pornography over the Internet.
- Representatives of the U.S. Catholic church have publicly “mitigated” the controversy over some priests’ sexually inappropriate behavior by stating that the offenders were “not pedophiles” because their victims were over the arbitrary age of 13.
This discussion might easily encompass the controversial “cures” for homosexuality that were quashed in the late 1960s. How also might we classify the consenting masochist or the philanderer?
Finally, there is the forensic question: Do we allow “mental illness” to “excuse” one’s willful sexual behavior by giving it a label or a treatment?
Sexual offense vs. mental illness
Part of the problem with defining sexual “addiction” is that one first needs to define sexual “normalcy.” No such definition exists. Even so, adding a “sexual addiction” diagnosis to the next Diagnostic and Statistical Manual (DSM) would likely be met with indifference or active denial from the psychiatric community, based solely on its legal ramifications. Attorneys would perceive that an official DSM diagnosis could open the door to acquittal on the basis of insanity for sexually motivated crimes in certain states that retain a “product rule.” It might even exonerate individuals in restrictive states that follow the more cognitive M’Naughten standard (Box).
The “product rule” asserts that a person is not responsible for his or her acts if they were a product of mental illness. The M’Naughten rule holds that a person is not responsible if, by virtue of a mental illness, that person does not understand the wrongfulness of the act.
Assailant John Hinckley Jr. was acquitted on the basis of an insanity plea under the “product rule” in the attempted assassination of President Reagan on March 30, 1981, outside a hotel in Washington, DC. Hinckley, who was obsessed with actress Jodie Foster, shot and wounded Reagan, press secretary James Brady, a Secret Service agent, and a police officer. After Hinckley’s acquittal, most states adopted the more stringent M’Naughten standard.
A number of forensic mental health issues such as sexual predator classifications and sex offender civil commitment have placed professional societies at odds with one another over sexual behavior and mental illness. Mental health organizations such as the National Association of State Mental Health Program Directors and the American Psychiatric Association argue that most sex offenders do not suffer from a mental illness. Translation: “We don’t want to be responsible for handling sex offenders.”
This distinction between sexual offenses and mental illness contrasts with the laws of several states that broadly define mental illness to include mental conditions that result in dangerous and “uncontrollable” behavior. Translation: “We want to keep sex offenders controlled or away from the community.” There has been no rational support for either of these positions, only data that have been loosely contrived to justify one or the other.
Res ipsum loquitur: the thing defines itself
Many “experts” have conjectured about the nature of sex-offending behavior and the phenomenon of “sexual addiction” without subjecting their conclusions to scientific scrutiny. Agendas have abounded, and countertransference rather than objectivity has driven many perceptions within the field. Treatments have often been theory-based rather than tested. The universal problem has been that many providers have invented solutions without defining the problems. Indeed, the problems have been multifaceted.
There has been little reliable outcome data to demonstrate the success in any given treatment, although a few notable exceptions stand out. Medical interventions with hormonal compounds like medroxyprogesterone and cyproterone have reduced recidivism.1 According to the Task Force Report on Sexually Dangerous Offenders, however, psychodynamic treatments in general have been ineffective.1 Most of the supportable psychological treatments have emerged since 1980 and have centered on a cognitive model of relapse prevention.2
The Minnesota Sex Offender Program has been the gold standard as a cognitive-based relapse prevention model for sex offenders.3 The program was developed as an inpatient program with a structured level system and topic-specific modules. It has focused on a relapse prevention cycle that teaches strategies to recognize and avoid cognitive distortions and maladaptive coping responses that eventually lead to re-offense.
Common beliefs about the incidence of mental illness in sex offenders, aside from paraphilias, have also been challenged by careful observation. Our research at the University of Cincinnati revealed high rates of mental illness and substance abuse in a group of sex offenders released from prison to a residential treatment center. Offenders with paraphilias had even higher rates of mood, anxiety, and impulse control disorders (Table).4 Concurrently, Nancy Raymond and her colleagues reported similar findings with a group of residential and outpatient child molesters in Minnesota.5 Data from both these studies suggest that:
- Axis I conditions, especially those related to increased sexual drive or impaired impulse control (such as bipolar disorder), have some importance in treatment.
- Some relationship exists between Axis I disorders and the expression of underlying paraphilic interests.
It is clear that sexual compulsions are not homogeneous, and different characteristics exist across different populations. In our sample, for instance, pedophiles had lower rates of antisocial personality disorder but higher rates of anxiety than other offenders. Pharmacologically, Martin Kafka found that self-identified, noncriminal clients with sexual compulsions responded well to selective serotonin reuptake inhibitors (SSRIs) and stimulants,6 whereas patients at the University of Cincinnati program had a far less robust response to SSRI treatment.7 In essence, one style of intervention may not fit all patients.
Table
COMMON AXIS I DIAGNOSES IN 113 CONVICTED SEX OFFENDERS WITH AND WITHOUT PARAPHILIAS*
| Diagnosis | Total | With paraphilias | Without paraphilias | |||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| Mood disorders (any) | 66 | (58.4) | 61 | (72.6) | 5 | (19.2) |
| Major depressive | 27 | (23.9) | 26 | (31.0) | 1 | (3.8) |
| Bipolar I | 28 | (24.8) | 25 | (29.8) | 2 | (7.7) |
| Substance abuse (any) | 96 | (85.0) | 69 | (82.1) | 26 | (100.0) |
| Anxiety disorders (any) | 26 | (23.0) | 24 | (28.6) | 2 | (7.7) |
| Eating disorders (any) | 10 | (8.8) | 10 | (11.9) | 0 | (0) |
| Impulse control disorders (any) | 43 | (38.1) | 38 | (45.2) | 4 | (15.4) |
| Compulsive buying | 11 | (9.7) | 11 | (13.1) | 0 | (0) |
| * Among the 113 subjects, 110 were evaluated for paraphilias. In that group, 84 were diagnosed as paraphilic and 26 as nonparaphilic. Mood disorders, major depressive disorder, bipolar affective disorder type I, and impulse control disorders were significantly more prevalent among paraphilic sex offenders. Individuals without paraphilias were significantly more likely than those with paraphilias to have a substance abuse disorder. Anxiety disorders and compulsive buying were more prevalent among paraphilic sex offenders. | ||||||
One other problem has plagued the forensic setting: Sex offenders lie about their sexual urges. In our program, they often appeared to “comply” with the program but concealed their sexual urges and fantasies. Sometimes they were embarrassed, ashamed, or simply in denial. We found that serial polygraph testing was essential for gauging treatment because it allowed us to objectively examine the information that the patients offered.
Frequently, I have heard people assert that an individual who seeks treatment actually wants treatment. Few sex offenders self-identify, however, and many more enter treatment as a condition of probation or parole. Thus most have an external motivation to participate. And with sex offenders, voluntary treatment is less successful than mandated treatment.1
Summary
Ultimately, self-help, 12-step, and psychodynamic approaches have an unproven role in managing problem sexual behaviors. The most appropriate intervention is a multidisciplinary team approach with screening for Axis I disorders and paraphilias, a thorough psychological assessment, substance abuse treatment, and a cognitive relapse prevention program. Forensic patients additionally benefit from collateral data such as polygraph testing and ongoing monitoring by community corrections personnel.
1. Zonana H, (chairperson). Dangerous sex offenders. A task force report of the American Psychiatric Association. Washington, DC: American Psychiatric Association Press, 1999.
2. Hanson RK, Gordon A, Harris AJ, et al. First report of the collaborative outcome data project on the effectiveness of psychological treatment for sex offenders. Sex Abuse 2002;14(2):169-94.
3. Schlank A, Cohen F. The sexual predator: law, policy, evaluation, and treatment. Kingston, NJ: Civic Research Institute, 1999.
4. McElroy SL, Soutullo CA, Taylor P, et al. Psychiatric features of 36 persons convicted of sexual offenses. J Clin Psychiatry 1999;60(6):414-22(update by Dunsieth N, et al with data from 113 subjects in progress).
5. Raymond N, Coleman E, Ohlerking F, Christenson GA, Miner M. Psychiatric comorbidity in pedophilic sex offenders. Am J Psychiatry 1999;156:786-8.
6. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry 2000;61(9):664-70.
7. Nelson E, et al. Data unpublished from the University of Cincinnati Biological Psychiatry Program (paper in progress).
It is naïve to think that psychiatry is generally accepted in the treatment of problematic sexual behaviors. Although some might think that psychiatry has a “liberated” view of sexuality born of Freud’s Oedipus, the psychiatrist’s involvement in managing sexual behavior, especially within the criminal justice system, is controversial rather than “celebrated.”
Although some such as Dr. Mahorney may assert an addiction model for problem sexual behavior, that model is seriously flawed. Parallels do exist between problem sexual behavior and other addictive behaviors. Within the broad range of sexual behaviors, however, the line between “addictive” and “alternative” cannot be finely drawn. For example:
- Certain advocacy groups such as the North American Man-Boy Love Association (NAMBLA) believe in legalized pederasty and have exerted a lobby to affect rulings on freedom of expression in “virtual” child pornography over the Internet.
- Representatives of the U.S. Catholic church have publicly “mitigated” the controversy over some priests’ sexually inappropriate behavior by stating that the offenders were “not pedophiles” because their victims were over the arbitrary age of 13.
This discussion might easily encompass the controversial “cures” for homosexuality that were quashed in the late 1960s. How also might we classify the consenting masochist or the philanderer?
Finally, there is the forensic question: Do we allow “mental illness” to “excuse” one’s willful sexual behavior by giving it a label or a treatment?
Sexual offense vs. mental illness
Part of the problem with defining sexual “addiction” is that one first needs to define sexual “normalcy.” No such definition exists. Even so, adding a “sexual addiction” diagnosis to the next Diagnostic and Statistical Manual (DSM) would likely be met with indifference or active denial from the psychiatric community, based solely on its legal ramifications. Attorneys would perceive that an official DSM diagnosis could open the door to acquittal on the basis of insanity for sexually motivated crimes in certain states that retain a “product rule.” It might even exonerate individuals in restrictive states that follow the more cognitive M’Naughten standard (Box).
The “product rule” asserts that a person is not responsible for his or her acts if they were a product of mental illness. The M’Naughten rule holds that a person is not responsible if, by virtue of a mental illness, that person does not understand the wrongfulness of the act.
Assailant John Hinckley Jr. was acquitted on the basis of an insanity plea under the “product rule” in the attempted assassination of President Reagan on March 30, 1981, outside a hotel in Washington, DC. Hinckley, who was obsessed with actress Jodie Foster, shot and wounded Reagan, press secretary James Brady, a Secret Service agent, and a police officer. After Hinckley’s acquittal, most states adopted the more stringent M’Naughten standard.
A number of forensic mental health issues such as sexual predator classifications and sex offender civil commitment have placed professional societies at odds with one another over sexual behavior and mental illness. Mental health organizations such as the National Association of State Mental Health Program Directors and the American Psychiatric Association argue that most sex offenders do not suffer from a mental illness. Translation: “We don’t want to be responsible for handling sex offenders.”
This distinction between sexual offenses and mental illness contrasts with the laws of several states that broadly define mental illness to include mental conditions that result in dangerous and “uncontrollable” behavior. Translation: “We want to keep sex offenders controlled or away from the community.” There has been no rational support for either of these positions, only data that have been loosely contrived to justify one or the other.
Res ipsum loquitur: the thing defines itself
Many “experts” have conjectured about the nature of sex-offending behavior and the phenomenon of “sexual addiction” without subjecting their conclusions to scientific scrutiny. Agendas have abounded, and countertransference rather than objectivity has driven many perceptions within the field. Treatments have often been theory-based rather than tested. The universal problem has been that many providers have invented solutions without defining the problems. Indeed, the problems have been multifaceted.
There has been little reliable outcome data to demonstrate the success in any given treatment, although a few notable exceptions stand out. Medical interventions with hormonal compounds like medroxyprogesterone and cyproterone have reduced recidivism.1 According to the Task Force Report on Sexually Dangerous Offenders, however, psychodynamic treatments in general have been ineffective.1 Most of the supportable psychological treatments have emerged since 1980 and have centered on a cognitive model of relapse prevention.2
The Minnesota Sex Offender Program has been the gold standard as a cognitive-based relapse prevention model for sex offenders.3 The program was developed as an inpatient program with a structured level system and topic-specific modules. It has focused on a relapse prevention cycle that teaches strategies to recognize and avoid cognitive distortions and maladaptive coping responses that eventually lead to re-offense.
Common beliefs about the incidence of mental illness in sex offenders, aside from paraphilias, have also been challenged by careful observation. Our research at the University of Cincinnati revealed high rates of mental illness and substance abuse in a group of sex offenders released from prison to a residential treatment center. Offenders with paraphilias had even higher rates of mood, anxiety, and impulse control disorders (Table).4 Concurrently, Nancy Raymond and her colleagues reported similar findings with a group of residential and outpatient child molesters in Minnesota.5 Data from both these studies suggest that:
- Axis I conditions, especially those related to increased sexual drive or impaired impulse control (such as bipolar disorder), have some importance in treatment.
- Some relationship exists between Axis I disorders and the expression of underlying paraphilic interests.
It is clear that sexual compulsions are not homogeneous, and different characteristics exist across different populations. In our sample, for instance, pedophiles had lower rates of antisocial personality disorder but higher rates of anxiety than other offenders. Pharmacologically, Martin Kafka found that self-identified, noncriminal clients with sexual compulsions responded well to selective serotonin reuptake inhibitors (SSRIs) and stimulants,6 whereas patients at the University of Cincinnati program had a far less robust response to SSRI treatment.7 In essence, one style of intervention may not fit all patients.
Table
COMMON AXIS I DIAGNOSES IN 113 CONVICTED SEX OFFENDERS WITH AND WITHOUT PARAPHILIAS*
| Diagnosis | Total | With paraphilias | Without paraphilias | |||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| Mood disorders (any) | 66 | (58.4) | 61 | (72.6) | 5 | (19.2) |
| Major depressive | 27 | (23.9) | 26 | (31.0) | 1 | (3.8) |
| Bipolar I | 28 | (24.8) | 25 | (29.8) | 2 | (7.7) |
| Substance abuse (any) | 96 | (85.0) | 69 | (82.1) | 26 | (100.0) |
| Anxiety disorders (any) | 26 | (23.0) | 24 | (28.6) | 2 | (7.7) |
| Eating disorders (any) | 10 | (8.8) | 10 | (11.9) | 0 | (0) |
| Impulse control disorders (any) | 43 | (38.1) | 38 | (45.2) | 4 | (15.4) |
| Compulsive buying | 11 | (9.7) | 11 | (13.1) | 0 | (0) |
| * Among the 113 subjects, 110 were evaluated for paraphilias. In that group, 84 were diagnosed as paraphilic and 26 as nonparaphilic. Mood disorders, major depressive disorder, bipolar affective disorder type I, and impulse control disorders were significantly more prevalent among paraphilic sex offenders. Individuals without paraphilias were significantly more likely than those with paraphilias to have a substance abuse disorder. Anxiety disorders and compulsive buying were more prevalent among paraphilic sex offenders. | ||||||
One other problem has plagued the forensic setting: Sex offenders lie about their sexual urges. In our program, they often appeared to “comply” with the program but concealed their sexual urges and fantasies. Sometimes they were embarrassed, ashamed, or simply in denial. We found that serial polygraph testing was essential for gauging treatment because it allowed us to objectively examine the information that the patients offered.
Frequently, I have heard people assert that an individual who seeks treatment actually wants treatment. Few sex offenders self-identify, however, and many more enter treatment as a condition of probation or parole. Thus most have an external motivation to participate. And with sex offenders, voluntary treatment is less successful than mandated treatment.1
Summary
Ultimately, self-help, 12-step, and psychodynamic approaches have an unproven role in managing problem sexual behaviors. The most appropriate intervention is a multidisciplinary team approach with screening for Axis I disorders and paraphilias, a thorough psychological assessment, substance abuse treatment, and a cognitive relapse prevention program. Forensic patients additionally benefit from collateral data such as polygraph testing and ongoing monitoring by community corrections personnel.
It is naïve to think that psychiatry is generally accepted in the treatment of problematic sexual behaviors. Although some might think that psychiatry has a “liberated” view of sexuality born of Freud’s Oedipus, the psychiatrist’s involvement in managing sexual behavior, especially within the criminal justice system, is controversial rather than “celebrated.”
Although some such as Dr. Mahorney may assert an addiction model for problem sexual behavior, that model is seriously flawed. Parallels do exist between problem sexual behavior and other addictive behaviors. Within the broad range of sexual behaviors, however, the line between “addictive” and “alternative” cannot be finely drawn. For example:
- Certain advocacy groups such as the North American Man-Boy Love Association (NAMBLA) believe in legalized pederasty and have exerted a lobby to affect rulings on freedom of expression in “virtual” child pornography over the Internet.
- Representatives of the U.S. Catholic church have publicly “mitigated” the controversy over some priests’ sexually inappropriate behavior by stating that the offenders were “not pedophiles” because their victims were over the arbitrary age of 13.
This discussion might easily encompass the controversial “cures” for homosexuality that were quashed in the late 1960s. How also might we classify the consenting masochist or the philanderer?
Finally, there is the forensic question: Do we allow “mental illness” to “excuse” one’s willful sexual behavior by giving it a label or a treatment?
Sexual offense vs. mental illness
Part of the problem with defining sexual “addiction” is that one first needs to define sexual “normalcy.” No such definition exists. Even so, adding a “sexual addiction” diagnosis to the next Diagnostic and Statistical Manual (DSM) would likely be met with indifference or active denial from the psychiatric community, based solely on its legal ramifications. Attorneys would perceive that an official DSM diagnosis could open the door to acquittal on the basis of insanity for sexually motivated crimes in certain states that retain a “product rule.” It might even exonerate individuals in restrictive states that follow the more cognitive M’Naughten standard (Box).
The “product rule” asserts that a person is not responsible for his or her acts if they were a product of mental illness. The M’Naughten rule holds that a person is not responsible if, by virtue of a mental illness, that person does not understand the wrongfulness of the act.
Assailant John Hinckley Jr. was acquitted on the basis of an insanity plea under the “product rule” in the attempted assassination of President Reagan on March 30, 1981, outside a hotel in Washington, DC. Hinckley, who was obsessed with actress Jodie Foster, shot and wounded Reagan, press secretary James Brady, a Secret Service agent, and a police officer. After Hinckley’s acquittal, most states adopted the more stringent M’Naughten standard.
A number of forensic mental health issues such as sexual predator classifications and sex offender civil commitment have placed professional societies at odds with one another over sexual behavior and mental illness. Mental health organizations such as the National Association of State Mental Health Program Directors and the American Psychiatric Association argue that most sex offenders do not suffer from a mental illness. Translation: “We don’t want to be responsible for handling sex offenders.”
This distinction between sexual offenses and mental illness contrasts with the laws of several states that broadly define mental illness to include mental conditions that result in dangerous and “uncontrollable” behavior. Translation: “We want to keep sex offenders controlled or away from the community.” There has been no rational support for either of these positions, only data that have been loosely contrived to justify one or the other.
Res ipsum loquitur: the thing defines itself
Many “experts” have conjectured about the nature of sex-offending behavior and the phenomenon of “sexual addiction” without subjecting their conclusions to scientific scrutiny. Agendas have abounded, and countertransference rather than objectivity has driven many perceptions within the field. Treatments have often been theory-based rather than tested. The universal problem has been that many providers have invented solutions without defining the problems. Indeed, the problems have been multifaceted.
There has been little reliable outcome data to demonstrate the success in any given treatment, although a few notable exceptions stand out. Medical interventions with hormonal compounds like medroxyprogesterone and cyproterone have reduced recidivism.1 According to the Task Force Report on Sexually Dangerous Offenders, however, psychodynamic treatments in general have been ineffective.1 Most of the supportable psychological treatments have emerged since 1980 and have centered on a cognitive model of relapse prevention.2
The Minnesota Sex Offender Program has been the gold standard as a cognitive-based relapse prevention model for sex offenders.3 The program was developed as an inpatient program with a structured level system and topic-specific modules. It has focused on a relapse prevention cycle that teaches strategies to recognize and avoid cognitive distortions and maladaptive coping responses that eventually lead to re-offense.
Common beliefs about the incidence of mental illness in sex offenders, aside from paraphilias, have also been challenged by careful observation. Our research at the University of Cincinnati revealed high rates of mental illness and substance abuse in a group of sex offenders released from prison to a residential treatment center. Offenders with paraphilias had even higher rates of mood, anxiety, and impulse control disorders (Table).4 Concurrently, Nancy Raymond and her colleagues reported similar findings with a group of residential and outpatient child molesters in Minnesota.5 Data from both these studies suggest that:
- Axis I conditions, especially those related to increased sexual drive or impaired impulse control (such as bipolar disorder), have some importance in treatment.
- Some relationship exists between Axis I disorders and the expression of underlying paraphilic interests.
It is clear that sexual compulsions are not homogeneous, and different characteristics exist across different populations. In our sample, for instance, pedophiles had lower rates of antisocial personality disorder but higher rates of anxiety than other offenders. Pharmacologically, Martin Kafka found that self-identified, noncriminal clients with sexual compulsions responded well to selective serotonin reuptake inhibitors (SSRIs) and stimulants,6 whereas patients at the University of Cincinnati program had a far less robust response to SSRI treatment.7 In essence, one style of intervention may not fit all patients.
Table
COMMON AXIS I DIAGNOSES IN 113 CONVICTED SEX OFFENDERS WITH AND WITHOUT PARAPHILIAS*
| Diagnosis | Total | With paraphilias | Without paraphilias | |||
|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | |
| Mood disorders (any) | 66 | (58.4) | 61 | (72.6) | 5 | (19.2) |
| Major depressive | 27 | (23.9) | 26 | (31.0) | 1 | (3.8) |
| Bipolar I | 28 | (24.8) | 25 | (29.8) | 2 | (7.7) |
| Substance abuse (any) | 96 | (85.0) | 69 | (82.1) | 26 | (100.0) |
| Anxiety disorders (any) | 26 | (23.0) | 24 | (28.6) | 2 | (7.7) |
| Eating disorders (any) | 10 | (8.8) | 10 | (11.9) | 0 | (0) |
| Impulse control disorders (any) | 43 | (38.1) | 38 | (45.2) | 4 | (15.4) |
| Compulsive buying | 11 | (9.7) | 11 | (13.1) | 0 | (0) |
| * Among the 113 subjects, 110 were evaluated for paraphilias. In that group, 84 were diagnosed as paraphilic and 26 as nonparaphilic. Mood disorders, major depressive disorder, bipolar affective disorder type I, and impulse control disorders were significantly more prevalent among paraphilic sex offenders. Individuals without paraphilias were significantly more likely than those with paraphilias to have a substance abuse disorder. Anxiety disorders and compulsive buying were more prevalent among paraphilic sex offenders. | ||||||
One other problem has plagued the forensic setting: Sex offenders lie about their sexual urges. In our program, they often appeared to “comply” with the program but concealed their sexual urges and fantasies. Sometimes they were embarrassed, ashamed, or simply in denial. We found that serial polygraph testing was essential for gauging treatment because it allowed us to objectively examine the information that the patients offered.
Frequently, I have heard people assert that an individual who seeks treatment actually wants treatment. Few sex offenders self-identify, however, and many more enter treatment as a condition of probation or parole. Thus most have an external motivation to participate. And with sex offenders, voluntary treatment is less successful than mandated treatment.1
Summary
Ultimately, self-help, 12-step, and psychodynamic approaches have an unproven role in managing problem sexual behaviors. The most appropriate intervention is a multidisciplinary team approach with screening for Axis I disorders and paraphilias, a thorough psychological assessment, substance abuse treatment, and a cognitive relapse prevention program. Forensic patients additionally benefit from collateral data such as polygraph testing and ongoing monitoring by community corrections personnel.
1. Zonana H, (chairperson). Dangerous sex offenders. A task force report of the American Psychiatric Association. Washington, DC: American Psychiatric Association Press, 1999.
2. Hanson RK, Gordon A, Harris AJ, et al. First report of the collaborative outcome data project on the effectiveness of psychological treatment for sex offenders. Sex Abuse 2002;14(2):169-94.
3. Schlank A, Cohen F. The sexual predator: law, policy, evaluation, and treatment. Kingston, NJ: Civic Research Institute, 1999.
4. McElroy SL, Soutullo CA, Taylor P, et al. Psychiatric features of 36 persons convicted of sexual offenses. J Clin Psychiatry 1999;60(6):414-22(update by Dunsieth N, et al with data from 113 subjects in progress).
5. Raymond N, Coleman E, Ohlerking F, Christenson GA, Miner M. Psychiatric comorbidity in pedophilic sex offenders. Am J Psychiatry 1999;156:786-8.
6. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry 2000;61(9):664-70.
7. Nelson E, et al. Data unpublished from the University of Cincinnati Biological Psychiatry Program (paper in progress).
1. Zonana H, (chairperson). Dangerous sex offenders. A task force report of the American Psychiatric Association. Washington, DC: American Psychiatric Association Press, 1999.
2. Hanson RK, Gordon A, Harris AJ, et al. First report of the collaborative outcome data project on the effectiveness of psychological treatment for sex offenders. Sex Abuse 2002;14(2):169-94.
3. Schlank A, Cohen F. The sexual predator: law, policy, evaluation, and treatment. Kingston, NJ: Civic Research Institute, 1999.
4. McElroy SL, Soutullo CA, Taylor P, et al. Psychiatric features of 36 persons convicted of sexual offenses. J Clin Psychiatry 1999;60(6):414-22(update by Dunsieth N, et al with data from 113 subjects in progress).
5. Raymond N, Coleman E, Ohlerking F, Christenson GA, Miner M. Psychiatric comorbidity in pedophilic sex offenders. Am J Psychiatry 1999;156:786-8.
6. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibitors in men with paraphilias and paraphilia-related disorders: a case series. J Clin Psychiatry 2000;61(9):664-70.
7. Nelson E, et al. Data unpublished from the University of Cincinnati Biological Psychiatry Program (paper in progress).
Sexual addiction: A diagnosis whose time has come
Sex is in the news. From pedophilic priests to philandering politicians, people at every level of society get into trouble over their sexual behavior. Among clinical disciplines, psychiatry has a celebrated tradition of addressing sexual problems (Box 1).1-4 We can therefore expect to be asked for help when a person’s inappropriate sexual behavior brings him into conflict, whether with his internal values or with society.
Are we prepared to treat these predatory patients and protect their potential victims? As the U.S. Catholic church has clearly demonstrated, many approaches to prevention and treatment—moral, medical, and traditional—are often ineffective. Rather than viewing compulsive sexual behaviors as moral failures, it may be time to conceptualize and treat them as addictions.
Among modern clinical disciplines, psychiatry has one of the more celebrated traditions of addressing sexual problems.
In Three Essays on the Theory of Sexuality,1 Freud identified problem areas as “deviations with respect of the sexual object” and “deviations in respect of the sexual aim.” This early focus on physiologic function as a model of “normal” was succeeded by psychoanalytic attempts to address otherwise normal sexual behavior that was compulsive and, arguably, at odds with societal values.2
Masters and Johnson3 weighed in with Human Sexual Response, physiologically interesting studies of sexual behavior that raised many questions about ethics and underlying psychological processes. Finally, psychiatry’s interest in the subject may have peaked with the publication of Helen Singer Kaplan’s The New Sex Therapy,4 which integrated findings and practices from a number of disciplines.
Sexual addiction as a diagnosis
Patrick Carnes popularized the concept of addictive sexual behavior in the consumer self-help book, Out of the Shadows,5 and in Contrary to Love,6 a volume aimed at clinicians. In characterizing addictive sexual behavior, Carnes cast a large net to include masturbation, heterosexual sex, pornography, prostitution, homosexuality, exhibitionism, voyeurism, and other practices that may be associated with sexual excitement.
The unifying quality was the habitual or compulsive nature of the behavior with the goal of altering mood, regardless of its social, legal, medical, emotional, or other maladaptive consequences (Box 2). As common as these cases are, they tend not to reach the mainstream psychiatry literature until a pharmaceutical treatment is tried.7,8
DSM-IV-TR provides diagnostic criteria for paraphilias such as exhibitionism, fetishism, voyeurism, pedophilia, and sexual masochism or sadism. These are descriptions of behavior, whereas sexual addiction is a cycle of mental and emotional experiences that may have a behavior phase.
A middle-aged clergyman presents for counseling due to repeated heterosexual affairs that have been reported to the head of his district and are a potential cause for dismissal. While the affairs have been largely ego-dystonic, he cannot overcome the compulsion to repeatedly seek and become involved in these superficial relationships.
His job offers him much unstructured time, which he frequently fills with fantasies of pursuing sexual experiences. He uses his pulpit, family visits, counseling sessions, and public service activities to make new sexual contacts. He notes that he is more likely to become preoccupied with such relationships when he is distressed or depressed, and he is aware of craving or feeling states that predispose him to looking for such relationships.
The clergyman has tried prayer, counseling, psychoanalytic psychotherapy, brief courses of cognitive-behavioral therapy (weeks to months), and medication trials with various antidepressant and antianxiety agents. These treatments have been unsuccessful.
The addictive behavior may or may not be legal, and it may or may not involve a victim. The common denominator is that the addictive behavior attempts, in a chronically ineffective way, to upregulate mood and sense of self.
DSM-IV may not recognize the term “sexual addiction,” but many patients will recognize the addiction concept, identify with it, and find it useful to understand their compulsive sexual behaviors. An addiction model is already being used by a variety of 12-step and self-help approaches to sexual addiction that have spun off of the success of Alcoholics Anonymous. This model accepts the unlikelihood of cure while offering hope for rehabilitation.
Advantages As a diagnosis, sexual addiction offers patients and psychiatrists two advantages:
- It recognizes a series of temporal mental, emotional, and behavioral events with which sufferers can identify without prohibitive pain and unbearable damage to self-concept.
- It leads to a potential treatment solution that is widely available, relatively inexpensive, and addresses the volition paradox (controlling the uncontrollable) that confounds other approaches.
The cycle of addiction
The validity of a medical diagnosis is based on patterns of family history, prognosis, treatment response, and course of disease. Sexual addiction, with its predictable course and family history, may meet these diagnostic criteria.
Sexual addicts, like mythical vampires, lead secret lives designed to feed the addiction process rather than life itself. The planning, arrangements, cruising, and cover-ups compete for time with family, friends, career, and hobbies. This creates a chronic situation of unmanageability of circumstances. To cope, the addict creates a strange belief system, such as the pedophile priest’s belief that sex between men and boys is natural and that the trauma lies in reporting and investigating it.9 Belief systems such as these are often socially supported by organizations and in publications by like-minded persons.
Self-interested distortions lead the addict to wrongly perceive that victims are cooperating and the self and others are not being harmed. The result is often gross misinterpretations of reality, such as the man who thinks he is flirting at the office but is charged with sexual exploitation by a co-worker. A sequence of mental events (Figure 1) leads to, and helps the addict cope with, the addiction cycle.
The addiction cycle Carnes describes a four-stage addiction cycle: preoccupation, ritualization, sexual acting out, and despair (Figure 2). Stressful or painful experiences—a narcissistic injury, a disappointment, stress-related anxiety—or the boredom and emptiness of unstructured time may initiate the sequence. The sufferer learns, through repetitive trials and reinforcement mechanisms, to take the maladaptive path into the addiction cycle rather than making a healthy emotional adaptation.
Reasons why one person takes the path to addiction when others do not may include genetics (emotional temperament), developmental and familial circumstances, and state-dependent learning. However the cycle is entered, the preoccupation stage begins the action that characterizes all addictions—diversion of attention from the painful state and anticipation of relief or pleasure.
With all addictive substances, the preoccupation phase exhibits tolerance. Longer time frames require higher doses, and increased stimulation of ritualization is required to produce the same mood-altering effect. The addict begins to devote increasing time and energy to the addiction, consuming resources that persons around him or her realize would be better invested in friends, family, career, or recreation (real life).
It is overstatement to characterize the patient’s denial and distortions as thought disorder. Nevertheless, many patients report waking as if from a dream to realize how their perceptions, rationalizations, and behavior have become inconsistent with their own long-held values and sense of reality.
The acting-out phase is often followed by a brief sense of relief and return to normal thinking which—paradoxically—obscure the emergence of despair and rekindling of the addictive cycle. The psychological phase of despair has several important clinical implications. In alcohol addiction, for example, pseudodepression in a freshly detoxified alcoholic often resolves with abstinence. When superimposed on clinical depression, the phase may take on vegetative signs and symptoms, with dangerously increased suicide potential.
Multiple addictions Another fascinating possibility is the generic nature of the despair phase as opposed to the preoccupation phase. It opens the door to mixed or multiple addictions, in which several addiction cycles are linked and serve to defend and obscure each other (Figure 3).
An example is the alcoholic sex addict who is promiscuous after an alcohol binge. He may explain his drinking at a party as a reaction to the presence of several previous sex partners and attribute his promiscuity to intoxication. Thus, the addiction process underlying both behaviors is concealed, as is the functional relationship between the two. Often there is partial awareness (e.g., the alcohol/sex addict has noticed that his promiscuous acting out frequently occurs after, but only rarely during, an intoxication episode).
Figure 1 BELIEF AND THINKING CYCLE OF SEXUAL ADDICTION
Figure 2 FOUR-STAGE SEXUAL ADDICTION CYCLE
Figure 3 MULTIPLE-ADDICTION CYCLE
Recognizing symptoms
The diagnosis of sexual addiction is often made by the patient who recognizes himself in a description of the cycle of addictive thoughts and behavior. Many patients report being aware that their thinking is distorted or their behavior is out of control. They often can admit that their behavior is inconsistent with their values.
Screening For clinicians, what are the diagnostic signs of sexual addiction? Numbers of sex partners or frequency of sex may not provide adequate information or reliable criteria. The 25 items on Carnes’ Sexual Addiction Screening Test (SAST)6 bring previously off-the-radar subjects into consideration. For example, questions on the survey include:
- Have you subscribed to or regularly purchased sexually explicit magazines like Playboy or Penthouse?
- Do you ever feel bad about your sexual behavior?
- Have you made promises to yourself to quit some aspect of your sexual behavior?6
The SAST questionnaire offers a self-report symptom checklist that can be correlated with normative data on sexual addiction. It yields a likelihood that the disorder—rather than the guilt mobilized by the behaviors—should be considered as a focus of treatment.
Treatment
Although treatment of sexual addiction is beyond the scope of this article, the psychiatrist plays an important role:
- Pharmacologic interventions can be appropriate and helpful for symptoms of anxiety and depression that many addicts develop, particularly in withdrawal states.
- Cognitive-behavioral psychotherapeutic approaches can help restructure distorted thinking and alter behavioral patterns.
- Transference-oriented psychodynamic therapies can help modify the basic faulted sense of self and impaired relationships that foster addiction.10
Treatment is also available through 12-step programs such as Sex Addicts Anonymous, Sex and Love Addicts Anonymous, and Sexaholics Anonymous (“Related resources”).
- Dodes L. The heart of addiction. New York: Harper Collins, 2002.
- Sex Addicts Anonymous. www.sexaa.org
- Sexaholics Anonymous. www.sa.org
- Sex and Love Addicts Anonymous. www.slaafws.org
1. Freud S. Three essays on the theory of sexuality (1905). Complete psychological works (standard ed., vol. 7). London: Hogarth Press, 1953.
2. Hershey D. On a type of heterosexuality, and the fluidity of object relations. J Amer Psychoanal Assn 1989;37(1):147-71.
3. Masters WH, Johnson VE. Human sexual response. Boston: Little, Brown & Co, 1966.
4. Kaplan HS. The new sex therapy. New York: Times Books, 1974.
5. Carnes P. Out of the shadows. Understanding sexual addiction. 2nd ed. Center City, MN: Hazelden Foundation, 1992.
6. Carnes P. Contrary to love. Helping the sexual addict. Center City, MN: Hazelden Foundation, 1989.
7. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibiters in men with paraphilias and paraphilia-related disorders: A case series. J Clin Psychiatry 2000;61(9):664-70.
8. Coleman E, Gratzer T, Nesvacil L, Raymond NC. Nefazodone and the treatment of nonparaphilic compulsive sexual behavior: a retrospective study. J Clin Psychiatry 2000;61(4):282-4.
9. Cannon A, Kelly K, Bentrup N. Is there any end in sight? U.S. News & World Report, April 22, 2002.
10. Dodes L. The heart of addiction. New York: Harper Collins, 2002.
Sex is in the news. From pedophilic priests to philandering politicians, people at every level of society get into trouble over their sexual behavior. Among clinical disciplines, psychiatry has a celebrated tradition of addressing sexual problems (Box 1).1-4 We can therefore expect to be asked for help when a person’s inappropriate sexual behavior brings him into conflict, whether with his internal values or with society.
Are we prepared to treat these predatory patients and protect their potential victims? As the U.S. Catholic church has clearly demonstrated, many approaches to prevention and treatment—moral, medical, and traditional—are often ineffective. Rather than viewing compulsive sexual behaviors as moral failures, it may be time to conceptualize and treat them as addictions.
Among modern clinical disciplines, psychiatry has one of the more celebrated traditions of addressing sexual problems.
In Three Essays on the Theory of Sexuality,1 Freud identified problem areas as “deviations with respect of the sexual object” and “deviations in respect of the sexual aim.” This early focus on physiologic function as a model of “normal” was succeeded by psychoanalytic attempts to address otherwise normal sexual behavior that was compulsive and, arguably, at odds with societal values.2
Masters and Johnson3 weighed in with Human Sexual Response, physiologically interesting studies of sexual behavior that raised many questions about ethics and underlying psychological processes. Finally, psychiatry’s interest in the subject may have peaked with the publication of Helen Singer Kaplan’s The New Sex Therapy,4 which integrated findings and practices from a number of disciplines.
Sexual addiction as a diagnosis
Patrick Carnes popularized the concept of addictive sexual behavior in the consumer self-help book, Out of the Shadows,5 and in Contrary to Love,6 a volume aimed at clinicians. In characterizing addictive sexual behavior, Carnes cast a large net to include masturbation, heterosexual sex, pornography, prostitution, homosexuality, exhibitionism, voyeurism, and other practices that may be associated with sexual excitement.
The unifying quality was the habitual or compulsive nature of the behavior with the goal of altering mood, regardless of its social, legal, medical, emotional, or other maladaptive consequences (Box 2). As common as these cases are, they tend not to reach the mainstream psychiatry literature until a pharmaceutical treatment is tried.7,8
DSM-IV-TR provides diagnostic criteria for paraphilias such as exhibitionism, fetishism, voyeurism, pedophilia, and sexual masochism or sadism. These are descriptions of behavior, whereas sexual addiction is a cycle of mental and emotional experiences that may have a behavior phase.
A middle-aged clergyman presents for counseling due to repeated heterosexual affairs that have been reported to the head of his district and are a potential cause for dismissal. While the affairs have been largely ego-dystonic, he cannot overcome the compulsion to repeatedly seek and become involved in these superficial relationships.
His job offers him much unstructured time, which he frequently fills with fantasies of pursuing sexual experiences. He uses his pulpit, family visits, counseling sessions, and public service activities to make new sexual contacts. He notes that he is more likely to become preoccupied with such relationships when he is distressed or depressed, and he is aware of craving or feeling states that predispose him to looking for such relationships.
The clergyman has tried prayer, counseling, psychoanalytic psychotherapy, brief courses of cognitive-behavioral therapy (weeks to months), and medication trials with various antidepressant and antianxiety agents. These treatments have been unsuccessful.
The addictive behavior may or may not be legal, and it may or may not involve a victim. The common denominator is that the addictive behavior attempts, in a chronically ineffective way, to upregulate mood and sense of self.
DSM-IV may not recognize the term “sexual addiction,” but many patients will recognize the addiction concept, identify with it, and find it useful to understand their compulsive sexual behaviors. An addiction model is already being used by a variety of 12-step and self-help approaches to sexual addiction that have spun off of the success of Alcoholics Anonymous. This model accepts the unlikelihood of cure while offering hope for rehabilitation.
Advantages As a diagnosis, sexual addiction offers patients and psychiatrists two advantages:
- It recognizes a series of temporal mental, emotional, and behavioral events with which sufferers can identify without prohibitive pain and unbearable damage to self-concept.
- It leads to a potential treatment solution that is widely available, relatively inexpensive, and addresses the volition paradox (controlling the uncontrollable) that confounds other approaches.
The cycle of addiction
The validity of a medical diagnosis is based on patterns of family history, prognosis, treatment response, and course of disease. Sexual addiction, with its predictable course and family history, may meet these diagnostic criteria.
Sexual addicts, like mythical vampires, lead secret lives designed to feed the addiction process rather than life itself. The planning, arrangements, cruising, and cover-ups compete for time with family, friends, career, and hobbies. This creates a chronic situation of unmanageability of circumstances. To cope, the addict creates a strange belief system, such as the pedophile priest’s belief that sex between men and boys is natural and that the trauma lies in reporting and investigating it.9 Belief systems such as these are often socially supported by organizations and in publications by like-minded persons.
Self-interested distortions lead the addict to wrongly perceive that victims are cooperating and the self and others are not being harmed. The result is often gross misinterpretations of reality, such as the man who thinks he is flirting at the office but is charged with sexual exploitation by a co-worker. A sequence of mental events (Figure 1) leads to, and helps the addict cope with, the addiction cycle.
The addiction cycle Carnes describes a four-stage addiction cycle: preoccupation, ritualization, sexual acting out, and despair (Figure 2). Stressful or painful experiences—a narcissistic injury, a disappointment, stress-related anxiety—or the boredom and emptiness of unstructured time may initiate the sequence. The sufferer learns, through repetitive trials and reinforcement mechanisms, to take the maladaptive path into the addiction cycle rather than making a healthy emotional adaptation.
Reasons why one person takes the path to addiction when others do not may include genetics (emotional temperament), developmental and familial circumstances, and state-dependent learning. However the cycle is entered, the preoccupation stage begins the action that characterizes all addictions—diversion of attention from the painful state and anticipation of relief or pleasure.
With all addictive substances, the preoccupation phase exhibits tolerance. Longer time frames require higher doses, and increased stimulation of ritualization is required to produce the same mood-altering effect. The addict begins to devote increasing time and energy to the addiction, consuming resources that persons around him or her realize would be better invested in friends, family, career, or recreation (real life).
It is overstatement to characterize the patient’s denial and distortions as thought disorder. Nevertheless, many patients report waking as if from a dream to realize how their perceptions, rationalizations, and behavior have become inconsistent with their own long-held values and sense of reality.
The acting-out phase is often followed by a brief sense of relief and return to normal thinking which—paradoxically—obscure the emergence of despair and rekindling of the addictive cycle. The psychological phase of despair has several important clinical implications. In alcohol addiction, for example, pseudodepression in a freshly detoxified alcoholic often resolves with abstinence. When superimposed on clinical depression, the phase may take on vegetative signs and symptoms, with dangerously increased suicide potential.
Multiple addictions Another fascinating possibility is the generic nature of the despair phase as opposed to the preoccupation phase. It opens the door to mixed or multiple addictions, in which several addiction cycles are linked and serve to defend and obscure each other (Figure 3).
An example is the alcoholic sex addict who is promiscuous after an alcohol binge. He may explain his drinking at a party as a reaction to the presence of several previous sex partners and attribute his promiscuity to intoxication. Thus, the addiction process underlying both behaviors is concealed, as is the functional relationship between the two. Often there is partial awareness (e.g., the alcohol/sex addict has noticed that his promiscuous acting out frequently occurs after, but only rarely during, an intoxication episode).
Figure 1 BELIEF AND THINKING CYCLE OF SEXUAL ADDICTION
Figure 2 FOUR-STAGE SEXUAL ADDICTION CYCLE
Figure 3 MULTIPLE-ADDICTION CYCLE
Recognizing symptoms
The diagnosis of sexual addiction is often made by the patient who recognizes himself in a description of the cycle of addictive thoughts and behavior. Many patients report being aware that their thinking is distorted or their behavior is out of control. They often can admit that their behavior is inconsistent with their values.
Screening For clinicians, what are the diagnostic signs of sexual addiction? Numbers of sex partners or frequency of sex may not provide adequate information or reliable criteria. The 25 items on Carnes’ Sexual Addiction Screening Test (SAST)6 bring previously off-the-radar subjects into consideration. For example, questions on the survey include:
- Have you subscribed to or regularly purchased sexually explicit magazines like Playboy or Penthouse?
- Do you ever feel bad about your sexual behavior?
- Have you made promises to yourself to quit some aspect of your sexual behavior?6
The SAST questionnaire offers a self-report symptom checklist that can be correlated with normative data on sexual addiction. It yields a likelihood that the disorder—rather than the guilt mobilized by the behaviors—should be considered as a focus of treatment.
Treatment
Although treatment of sexual addiction is beyond the scope of this article, the psychiatrist plays an important role:
- Pharmacologic interventions can be appropriate and helpful for symptoms of anxiety and depression that many addicts develop, particularly in withdrawal states.
- Cognitive-behavioral psychotherapeutic approaches can help restructure distorted thinking and alter behavioral patterns.
- Transference-oriented psychodynamic therapies can help modify the basic faulted sense of self and impaired relationships that foster addiction.10
Treatment is also available through 12-step programs such as Sex Addicts Anonymous, Sex and Love Addicts Anonymous, and Sexaholics Anonymous (“Related resources”).
- Dodes L. The heart of addiction. New York: Harper Collins, 2002.
- Sex Addicts Anonymous. www.sexaa.org
- Sexaholics Anonymous. www.sa.org
- Sex and Love Addicts Anonymous. www.slaafws.org
Sex is in the news. From pedophilic priests to philandering politicians, people at every level of society get into trouble over their sexual behavior. Among clinical disciplines, psychiatry has a celebrated tradition of addressing sexual problems (Box 1).1-4 We can therefore expect to be asked for help when a person’s inappropriate sexual behavior brings him into conflict, whether with his internal values or with society.
Are we prepared to treat these predatory patients and protect their potential victims? As the U.S. Catholic church has clearly demonstrated, many approaches to prevention and treatment—moral, medical, and traditional—are often ineffective. Rather than viewing compulsive sexual behaviors as moral failures, it may be time to conceptualize and treat them as addictions.
Among modern clinical disciplines, psychiatry has one of the more celebrated traditions of addressing sexual problems.
In Three Essays on the Theory of Sexuality,1 Freud identified problem areas as “deviations with respect of the sexual object” and “deviations in respect of the sexual aim.” This early focus on physiologic function as a model of “normal” was succeeded by psychoanalytic attempts to address otherwise normal sexual behavior that was compulsive and, arguably, at odds with societal values.2
Masters and Johnson3 weighed in with Human Sexual Response, physiologically interesting studies of sexual behavior that raised many questions about ethics and underlying psychological processes. Finally, psychiatry’s interest in the subject may have peaked with the publication of Helen Singer Kaplan’s The New Sex Therapy,4 which integrated findings and practices from a number of disciplines.
Sexual addiction as a diagnosis
Patrick Carnes popularized the concept of addictive sexual behavior in the consumer self-help book, Out of the Shadows,5 and in Contrary to Love,6 a volume aimed at clinicians. In characterizing addictive sexual behavior, Carnes cast a large net to include masturbation, heterosexual sex, pornography, prostitution, homosexuality, exhibitionism, voyeurism, and other practices that may be associated with sexual excitement.
The unifying quality was the habitual or compulsive nature of the behavior with the goal of altering mood, regardless of its social, legal, medical, emotional, or other maladaptive consequences (Box 2). As common as these cases are, they tend not to reach the mainstream psychiatry literature until a pharmaceutical treatment is tried.7,8
DSM-IV-TR provides diagnostic criteria for paraphilias such as exhibitionism, fetishism, voyeurism, pedophilia, and sexual masochism or sadism. These are descriptions of behavior, whereas sexual addiction is a cycle of mental and emotional experiences that may have a behavior phase.
A middle-aged clergyman presents for counseling due to repeated heterosexual affairs that have been reported to the head of his district and are a potential cause for dismissal. While the affairs have been largely ego-dystonic, he cannot overcome the compulsion to repeatedly seek and become involved in these superficial relationships.
His job offers him much unstructured time, which he frequently fills with fantasies of pursuing sexual experiences. He uses his pulpit, family visits, counseling sessions, and public service activities to make new sexual contacts. He notes that he is more likely to become preoccupied with such relationships when he is distressed or depressed, and he is aware of craving or feeling states that predispose him to looking for such relationships.
The clergyman has tried prayer, counseling, psychoanalytic psychotherapy, brief courses of cognitive-behavioral therapy (weeks to months), and medication trials with various antidepressant and antianxiety agents. These treatments have been unsuccessful.
The addictive behavior may or may not be legal, and it may or may not involve a victim. The common denominator is that the addictive behavior attempts, in a chronically ineffective way, to upregulate mood and sense of self.
DSM-IV may not recognize the term “sexual addiction,” but many patients will recognize the addiction concept, identify with it, and find it useful to understand their compulsive sexual behaviors. An addiction model is already being used by a variety of 12-step and self-help approaches to sexual addiction that have spun off of the success of Alcoholics Anonymous. This model accepts the unlikelihood of cure while offering hope for rehabilitation.
Advantages As a diagnosis, sexual addiction offers patients and psychiatrists two advantages:
- It recognizes a series of temporal mental, emotional, and behavioral events with which sufferers can identify without prohibitive pain and unbearable damage to self-concept.
- It leads to a potential treatment solution that is widely available, relatively inexpensive, and addresses the volition paradox (controlling the uncontrollable) that confounds other approaches.
The cycle of addiction
The validity of a medical diagnosis is based on patterns of family history, prognosis, treatment response, and course of disease. Sexual addiction, with its predictable course and family history, may meet these diagnostic criteria.
Sexual addicts, like mythical vampires, lead secret lives designed to feed the addiction process rather than life itself. The planning, arrangements, cruising, and cover-ups compete for time with family, friends, career, and hobbies. This creates a chronic situation of unmanageability of circumstances. To cope, the addict creates a strange belief system, such as the pedophile priest’s belief that sex between men and boys is natural and that the trauma lies in reporting and investigating it.9 Belief systems such as these are often socially supported by organizations and in publications by like-minded persons.
Self-interested distortions lead the addict to wrongly perceive that victims are cooperating and the self and others are not being harmed. The result is often gross misinterpretations of reality, such as the man who thinks he is flirting at the office but is charged with sexual exploitation by a co-worker. A sequence of mental events (Figure 1) leads to, and helps the addict cope with, the addiction cycle.
The addiction cycle Carnes describes a four-stage addiction cycle: preoccupation, ritualization, sexual acting out, and despair (Figure 2). Stressful or painful experiences—a narcissistic injury, a disappointment, stress-related anxiety—or the boredom and emptiness of unstructured time may initiate the sequence. The sufferer learns, through repetitive trials and reinforcement mechanisms, to take the maladaptive path into the addiction cycle rather than making a healthy emotional adaptation.
Reasons why one person takes the path to addiction when others do not may include genetics (emotional temperament), developmental and familial circumstances, and state-dependent learning. However the cycle is entered, the preoccupation stage begins the action that characterizes all addictions—diversion of attention from the painful state and anticipation of relief or pleasure.
With all addictive substances, the preoccupation phase exhibits tolerance. Longer time frames require higher doses, and increased stimulation of ritualization is required to produce the same mood-altering effect. The addict begins to devote increasing time and energy to the addiction, consuming resources that persons around him or her realize would be better invested in friends, family, career, or recreation (real life).
It is overstatement to characterize the patient’s denial and distortions as thought disorder. Nevertheless, many patients report waking as if from a dream to realize how their perceptions, rationalizations, and behavior have become inconsistent with their own long-held values and sense of reality.
The acting-out phase is often followed by a brief sense of relief and return to normal thinking which—paradoxically—obscure the emergence of despair and rekindling of the addictive cycle. The psychological phase of despair has several important clinical implications. In alcohol addiction, for example, pseudodepression in a freshly detoxified alcoholic often resolves with abstinence. When superimposed on clinical depression, the phase may take on vegetative signs and symptoms, with dangerously increased suicide potential.
Multiple addictions Another fascinating possibility is the generic nature of the despair phase as opposed to the preoccupation phase. It opens the door to mixed or multiple addictions, in which several addiction cycles are linked and serve to defend and obscure each other (Figure 3).
An example is the alcoholic sex addict who is promiscuous after an alcohol binge. He may explain his drinking at a party as a reaction to the presence of several previous sex partners and attribute his promiscuity to intoxication. Thus, the addiction process underlying both behaviors is concealed, as is the functional relationship between the two. Often there is partial awareness (e.g., the alcohol/sex addict has noticed that his promiscuous acting out frequently occurs after, but only rarely during, an intoxication episode).
Figure 1 BELIEF AND THINKING CYCLE OF SEXUAL ADDICTION
Figure 2 FOUR-STAGE SEXUAL ADDICTION CYCLE
Figure 3 MULTIPLE-ADDICTION CYCLE
Recognizing symptoms
The diagnosis of sexual addiction is often made by the patient who recognizes himself in a description of the cycle of addictive thoughts and behavior. Many patients report being aware that their thinking is distorted or their behavior is out of control. They often can admit that their behavior is inconsistent with their values.
Screening For clinicians, what are the diagnostic signs of sexual addiction? Numbers of sex partners or frequency of sex may not provide adequate information or reliable criteria. The 25 items on Carnes’ Sexual Addiction Screening Test (SAST)6 bring previously off-the-radar subjects into consideration. For example, questions on the survey include:
- Have you subscribed to or regularly purchased sexually explicit magazines like Playboy or Penthouse?
- Do you ever feel bad about your sexual behavior?
- Have you made promises to yourself to quit some aspect of your sexual behavior?6
The SAST questionnaire offers a self-report symptom checklist that can be correlated with normative data on sexual addiction. It yields a likelihood that the disorder—rather than the guilt mobilized by the behaviors—should be considered as a focus of treatment.
Treatment
Although treatment of sexual addiction is beyond the scope of this article, the psychiatrist plays an important role:
- Pharmacologic interventions can be appropriate and helpful for symptoms of anxiety and depression that many addicts develop, particularly in withdrawal states.
- Cognitive-behavioral psychotherapeutic approaches can help restructure distorted thinking and alter behavioral patterns.
- Transference-oriented psychodynamic therapies can help modify the basic faulted sense of self and impaired relationships that foster addiction.10
Treatment is also available through 12-step programs such as Sex Addicts Anonymous, Sex and Love Addicts Anonymous, and Sexaholics Anonymous (“Related resources”).
- Dodes L. The heart of addiction. New York: Harper Collins, 2002.
- Sex Addicts Anonymous. www.sexaa.org
- Sexaholics Anonymous. www.sa.org
- Sex and Love Addicts Anonymous. www.slaafws.org
1. Freud S. Three essays on the theory of sexuality (1905). Complete psychological works (standard ed., vol. 7). London: Hogarth Press, 1953.
2. Hershey D. On a type of heterosexuality, and the fluidity of object relations. J Amer Psychoanal Assn 1989;37(1):147-71.
3. Masters WH, Johnson VE. Human sexual response. Boston: Little, Brown & Co, 1966.
4. Kaplan HS. The new sex therapy. New York: Times Books, 1974.
5. Carnes P. Out of the shadows. Understanding sexual addiction. 2nd ed. Center City, MN: Hazelden Foundation, 1992.
6. Carnes P. Contrary to love. Helping the sexual addict. Center City, MN: Hazelden Foundation, 1989.
7. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibiters in men with paraphilias and paraphilia-related disorders: A case series. J Clin Psychiatry 2000;61(9):664-70.
8. Coleman E, Gratzer T, Nesvacil L, Raymond NC. Nefazodone and the treatment of nonparaphilic compulsive sexual behavior: a retrospective study. J Clin Psychiatry 2000;61(4):282-4.
9. Cannon A, Kelly K, Bentrup N. Is there any end in sight? U.S. News & World Report, April 22, 2002.
10. Dodes L. The heart of addiction. New York: Harper Collins, 2002.
1. Freud S. Three essays on the theory of sexuality (1905). Complete psychological works (standard ed., vol. 7). London: Hogarth Press, 1953.
2. Hershey D. On a type of heterosexuality, and the fluidity of object relations. J Amer Psychoanal Assn 1989;37(1):147-71.
3. Masters WH, Johnson VE. Human sexual response. Boston: Little, Brown & Co, 1966.
4. Kaplan HS. The new sex therapy. New York: Times Books, 1974.
5. Carnes P. Out of the shadows. Understanding sexual addiction. 2nd ed. Center City, MN: Hazelden Foundation, 1992.
6. Carnes P. Contrary to love. Helping the sexual addict. Center City, MN: Hazelden Foundation, 1989.
7. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibiters in men with paraphilias and paraphilia-related disorders: A case series. J Clin Psychiatry 2000;61(9):664-70.
8. Coleman E, Gratzer T, Nesvacil L, Raymond NC. Nefazodone and the treatment of nonparaphilic compulsive sexual behavior: a retrospective study. J Clin Psychiatry 2000;61(4):282-4.
9. Cannon A, Kelly K, Bentrup N. Is there any end in sight? U.S. News & World Report, April 22, 2002.
10. Dodes L. The heart of addiction. New York: Harper Collins, 2002.
Sexual addiction: A diagnosis whose time has come
Sex is in the news. From pedophilic priests to philandering politicians, people at every level of society get into trouble over their sexual behavior. Among clinical disciplines, psychiatry has a celebrated tradition of addressing sexual problems (Box 1).1-4 We can therefore expect to be asked for help when a person’s inappropriate sexual behavior brings him into conflict, whether with his internal values or with society.
Are we prepared to treat these predatory patients and protect their potential victims? As the U.S. Catholic church has clearly demonstrated, many approaches to prevention and treatment—moral, medical, and traditional—are often ineffective. Rather than viewing compulsive sexual behaviors as moral failures, it may be time to conceptualize and treat them as addictions.
Among modern clinical disciplines, psychiatry has one of the more celebrated traditions of addressing sexual problems.
In Three Essays on the Theory of Sexuality,1 Freud identified problem areas as “deviations with respect of the sexual object” and “deviations in respect of the sexual aim.” This early focus on physiologic function as a model of “normal” was succeeded by psychoanalytic attempts to address otherwise normal sexual behavior that was compulsive and, arguably, at odds with societal values.2
Masters and Johnson3 weighed in with Human Sexual Response, physiologically interesting studies of sexual behavior that raised many questions about ethics and underlying psychological processes. Finally, psychiatry’s interest in the subject may have peaked with the publication of Helen Singer Kaplan’s The New Sex Therapy,4 which integrated findings and practices from a number of disciplines.
Sexual addiction as a diagnosis
Patrick Carnes popularized the concept of addictive sexual behavior in the consumer self-help book, Out of the Shadows,5 and in Contrary to Love,6 a volume aimed at clinicians. In characterizing addictive sexual behavior, Carnes cast a large net to include masturbation, heterosexual sex, pornography, prostitution, homosexuality, exhibitionism, voyeurism, and other practices that may be associated with sexual excitement.
The unifying quality was the habitual or compulsive nature of the behavior with the goal of altering mood, regardless of its social, legal, medical, emotional, or other maladaptive consequences (Box 2). As common as these cases are, they tend not to reach the mainstream psychiatry literature until a pharmaceutical treatment is tried.7,8
DSM-IV-TR provides diagnostic criteria for paraphilias such as exhibitionism, fetishism, voyeurism, pedophilia, and sexual masochism or sadism. These are descriptions of behavior, whereas sexual addiction is a cycle of mental and emotional experiences that may have a behavior phase.
A middle-aged clergyman presents for counseling due to repeated heterosexual affairs that have been reported to the head of his district and are a potential cause for dismissal. While the affairs have been largely ego-dystonic, he cannot overcome the compulsion to repeatedly seek and become involved in these superficial relationships.
His job offers him much unstructured time, which he frequently fills with fantasies of pursuing sexual experiences. He uses his pulpit, family visits, counseling sessions, and public service activities to make new sexual contacts. He notes that he is more likely to become preoccupied with such relationships when he is distressed or depressed, and he is aware of craving or feeling states that predispose him to looking for such relationships.
The clergyman has tried prayer, counseling, psychoanalytic psychotherapy, brief courses of cognitive-behavioral therapy (weeks to months), and medication trials with various antidepressant and antianxiety agents. These treatments have been unsuccessful.
The addictive behavior may or may not be legal, and it may or may not involve a victim. The common denominator is that the addictive behavior attempts, in a chronically ineffective way, to upregulate mood and sense of self.
DSM-IV may not recognize the term “sexual addiction,” but many patients will recognize the addiction concept, identify with it, and find it useful to understand their compulsive sexual behaviors. An addiction model is already being used by a variety of 12-step and self-help approaches to sexual addiction that have spun off of the success of Alcoholics Anonymous. This model accepts the unlikelihood of cure while offering hope for rehabilitation.
Advantages As a diagnosis, sexual addiction offers patients and psychiatrists two advantages:
- It recognizes a series of temporal mental, emotional, and behavioral events with which sufferers can identify without prohibitive pain and unbearable damage to self-concept.
- It leads to a potential treatment solution that is widely available, relatively inexpensive, and addresses the volition paradox (controlling the uncontrollable) that confounds other approaches.
The cycle of addiction
The validity of a medical diagnosis is based on patterns of family history, prognosis, treatment response, and course of disease. Sexual addiction, with its predictable course and family history, may meet these diagnostic criteria.
Sexual addicts, like mythical vampires, lead secret lives designed to feed the addiction process rather than life itself. The planning, arrangements, cruising, and cover-ups compete for time with family, friends, career, and hobbies. This creates a chronic situation of unmanageability of circumstances. To cope, the addict creates a strange belief system, such as the pedophile priest’s belief that sex between men and boys is natural and that the trauma lies in reporting and investigating it.9 Belief systems such as these are often socially supported by organizations and in publications by like-minded persons.
Self-interested distortions lead the addict to wrongly perceive that victims are cooperating and the self and others are not being harmed. The result is often gross misinterpretations of reality, such as the man who thinks he is flirting at the office but is charged with sexual exploitation by a co-worker. A sequence of mental events (Figure 1) leads to, and helps the addict cope with, the addiction cycle.
The addiction cycle Carnes describes a four-stage addiction cycle: preoccupation, ritualization, sexual acting out, and despair (Figure 2). Stressful or painful experiences—a narcissistic injury, a disappointment, stress-related anxiety—or the boredom and emptiness of unstructured time may initiate the sequence. The sufferer learns, through repetitive trials and reinforcement mechanisms, to take the maladaptive path into the addiction cycle rather than making a healthy emotional adaptation.
Reasons why one person takes the path to addiction when others do not may include genetics (emotional temperament), developmental and familial circumstances, and state-dependent learning. However the cycle is entered, the preoccupation stage begins the action that characterizes all addictions—diversion of attention from the painful state and anticipation of relief or pleasure.
With all addictive substances, the preoccupation phase exhibits tolerance. Longer time frames require higher doses, and increased stimulation of ritualization is required to produce the same mood-altering effect. The addict begins to devote increasing time and energy to the addiction, consuming resources that persons around him or her realize would be better invested in friends, family, career, or recreation (real life).
It is overstatement to characterize the patient’s denial and distortions as thought disorder. Nevertheless, many patients report waking as if from a dream to realize how their perceptions, rationalizations, and behavior have become inconsistent with their own long-held values and sense of reality.
The acting-out phase is often followed by a brief sense of relief and return to normal thinking which—paradoxically—obscure the emergence of despair and rekindling of the addictive cycle. The psychological phase of despair has several important clinical implications. In alcohol addiction, for example, pseudodepression in a freshly detoxified alcoholic often resolves with abstinence. When superimposed on clinical depression, the phase may take on vegetative signs and symptoms, with dangerously increased suicide potential.
Multiple addictions Another fascinating possibility is the generic nature of the despair phase as opposed to the preoccupation phase. It opens the door to mixed or multiple addictions, in which several addiction cycles are linked and serve to defend and obscure each other (Figure 3).
An example is the alcoholic sex addict who is promiscuous after an alcohol binge. He may explain his drinking at a party as a reaction to the presence of several previous sex partners and attribute his promiscuity to intoxication. Thus, the addiction process underlying both behaviors is concealed, as is the functional relationship between the two. Often there is partial awareness (e.g., the alcohol/sex addict has noticed that his promiscuous acting out frequently occurs after, but only rarely during, an intoxication episode).
Figure 1 BELIEF AND THINKING CYCLE OF SEXUAL ADDICTION
Figure 2 FOUR-STAGE SEXUAL ADDICTION CYCLE
Figure 3 MULTIPLE-ADDICTION CYCLE
Recognizing symptoms
The diagnosis of sexual addiction is often made by the patient who recognizes himself in a description of the cycle of addictive thoughts and behavior. Many patients report being aware that their thinking is distorted or their behavior is out of control. They often can admit that their behavior is inconsistent with their values.
Screening For clinicians, what are the diagnostic signs of sexual addiction? Numbers of sex partners or frequency of sex may not provide adequate information or reliable criteria. The 25 items on Carnes’ Sexual Addiction Screening Test (SAST)6 bring previously off-the-radar subjects into consideration. For example, questions on the survey include:
- Have you subscribed to or regularly purchased sexually explicit magazines like Playboy or Penthouse?
- Do you ever feel bad about your sexual behavior?
- Have you made promises to yourself to quit some aspect of your sexual behavior?6
The SAST questionnaire offers a self-report symptom checklist that can be correlated with normative data on sexual addiction. It yields a likelihood that the disorder—rather than the guilt mobilized by the behaviors—should be considered as a focus of treatment.
Treatment
Although treatment of sexual addiction is beyond the scope of this article, the psychiatrist plays an important role:
- Pharmacologic interventions can be appropriate and helpful for symptoms of anxiety and depression that many addicts develop, particularly in withdrawal states.
- Cognitive-behavioral psychotherapeutic approaches can help restructure distorted thinking and alter behavioral patterns.
- Transference-oriented psychodynamic therapies can help modify the basic faulted sense of self and impaired relationships that foster addiction.10
Treatment is also available through 12-step programs such as Sex Addicts Anonymous, Sex and Love Addicts Anonymous, and Sexaholics Anonymous (“Related resources”).
- Dodes L. The heart of addiction. New York: Harper Collins, 2002.
- Sex Addicts Anonymous. www.sexaa.org
- Sexaholics Anonymous. www.sa.org
- Sex and Love Addicts Anonymous. www.slaafws.org
1. Freud S. Three essays on the theory of sexuality (1905). Complete psychological works (standard ed., vol. 7). London: Hogarth Press, 1953.
2. Hershey D. On a type of heterosexuality, and the fluidity of object relations. J Amer Psychoanal Assn 1989;37(1):147-71.
3. Masters WH, Johnson VE. Human sexual response. Boston: Little, Brown & Co, 1966.
4. Kaplan HS. The new sex therapy. New York: Times Books, 1974.
5. Carnes P. Out of the shadows. Understanding sexual addiction. 2nd ed. Center City, MN: Hazelden Foundation, 1992.
6. Carnes P. Contrary to love. Helping the sexual addict. Center City, MN: Hazelden Foundation, 1989.
7. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibiters in men with paraphilias and paraphilia-related disorders: A case series. J Clin Psychiatry 2000;61(9):664-70.
8. Coleman E, Gratzer T, Nesvacil L, Raymond NC. Nefazodone and the treatment of nonparaphilic compulsive sexual behavior: a retrospective study. J Clin Psychiatry 2000;61(4):282-4.
9. Cannon A, Kelly K, Bentrup N. Is there any end in sight? U.S. News & World Report, April 22, 2002.
10. Dodes L. The heart of addiction. New York: Harper Collins, 2002.
Sex is in the news. From pedophilic priests to philandering politicians, people at every level of society get into trouble over their sexual behavior. Among clinical disciplines, psychiatry has a celebrated tradition of addressing sexual problems (Box 1).1-4 We can therefore expect to be asked for help when a person’s inappropriate sexual behavior brings him into conflict, whether with his internal values or with society.
Are we prepared to treat these predatory patients and protect their potential victims? As the U.S. Catholic church has clearly demonstrated, many approaches to prevention and treatment—moral, medical, and traditional—are often ineffective. Rather than viewing compulsive sexual behaviors as moral failures, it may be time to conceptualize and treat them as addictions.
Among modern clinical disciplines, psychiatry has one of the more celebrated traditions of addressing sexual problems.
In Three Essays on the Theory of Sexuality,1 Freud identified problem areas as “deviations with respect of the sexual object” and “deviations in respect of the sexual aim.” This early focus on physiologic function as a model of “normal” was succeeded by psychoanalytic attempts to address otherwise normal sexual behavior that was compulsive and, arguably, at odds with societal values.2
Masters and Johnson3 weighed in with Human Sexual Response, physiologically interesting studies of sexual behavior that raised many questions about ethics and underlying psychological processes. Finally, psychiatry’s interest in the subject may have peaked with the publication of Helen Singer Kaplan’s The New Sex Therapy,4 which integrated findings and practices from a number of disciplines.
Sexual addiction as a diagnosis
Patrick Carnes popularized the concept of addictive sexual behavior in the consumer self-help book, Out of the Shadows,5 and in Contrary to Love,6 a volume aimed at clinicians. In characterizing addictive sexual behavior, Carnes cast a large net to include masturbation, heterosexual sex, pornography, prostitution, homosexuality, exhibitionism, voyeurism, and other practices that may be associated with sexual excitement.
The unifying quality was the habitual or compulsive nature of the behavior with the goal of altering mood, regardless of its social, legal, medical, emotional, or other maladaptive consequences (Box 2). As common as these cases are, they tend not to reach the mainstream psychiatry literature until a pharmaceutical treatment is tried.7,8
DSM-IV-TR provides diagnostic criteria for paraphilias such as exhibitionism, fetishism, voyeurism, pedophilia, and sexual masochism or sadism. These are descriptions of behavior, whereas sexual addiction is a cycle of mental and emotional experiences that may have a behavior phase.
A middle-aged clergyman presents for counseling due to repeated heterosexual affairs that have been reported to the head of his district and are a potential cause for dismissal. While the affairs have been largely ego-dystonic, he cannot overcome the compulsion to repeatedly seek and become involved in these superficial relationships.
His job offers him much unstructured time, which he frequently fills with fantasies of pursuing sexual experiences. He uses his pulpit, family visits, counseling sessions, and public service activities to make new sexual contacts. He notes that he is more likely to become preoccupied with such relationships when he is distressed or depressed, and he is aware of craving or feeling states that predispose him to looking for such relationships.
The clergyman has tried prayer, counseling, psychoanalytic psychotherapy, brief courses of cognitive-behavioral therapy (weeks to months), and medication trials with various antidepressant and antianxiety agents. These treatments have been unsuccessful.
The addictive behavior may or may not be legal, and it may or may not involve a victim. The common denominator is that the addictive behavior attempts, in a chronically ineffective way, to upregulate mood and sense of self.
DSM-IV may not recognize the term “sexual addiction,” but many patients will recognize the addiction concept, identify with it, and find it useful to understand their compulsive sexual behaviors. An addiction model is already being used by a variety of 12-step and self-help approaches to sexual addiction that have spun off of the success of Alcoholics Anonymous. This model accepts the unlikelihood of cure while offering hope for rehabilitation.
Advantages As a diagnosis, sexual addiction offers patients and psychiatrists two advantages:
- It recognizes a series of temporal mental, emotional, and behavioral events with which sufferers can identify without prohibitive pain and unbearable damage to self-concept.
- It leads to a potential treatment solution that is widely available, relatively inexpensive, and addresses the volition paradox (controlling the uncontrollable) that confounds other approaches.
The cycle of addiction
The validity of a medical diagnosis is based on patterns of family history, prognosis, treatment response, and course of disease. Sexual addiction, with its predictable course and family history, may meet these diagnostic criteria.
Sexual addicts, like mythical vampires, lead secret lives designed to feed the addiction process rather than life itself. The planning, arrangements, cruising, and cover-ups compete for time with family, friends, career, and hobbies. This creates a chronic situation of unmanageability of circumstances. To cope, the addict creates a strange belief system, such as the pedophile priest’s belief that sex between men and boys is natural and that the trauma lies in reporting and investigating it.9 Belief systems such as these are often socially supported by organizations and in publications by like-minded persons.
Self-interested distortions lead the addict to wrongly perceive that victims are cooperating and the self and others are not being harmed. The result is often gross misinterpretations of reality, such as the man who thinks he is flirting at the office but is charged with sexual exploitation by a co-worker. A sequence of mental events (Figure 1) leads to, and helps the addict cope with, the addiction cycle.
The addiction cycle Carnes describes a four-stage addiction cycle: preoccupation, ritualization, sexual acting out, and despair (Figure 2). Stressful or painful experiences—a narcissistic injury, a disappointment, stress-related anxiety—or the boredom and emptiness of unstructured time may initiate the sequence. The sufferer learns, through repetitive trials and reinforcement mechanisms, to take the maladaptive path into the addiction cycle rather than making a healthy emotional adaptation.
Reasons why one person takes the path to addiction when others do not may include genetics (emotional temperament), developmental and familial circumstances, and state-dependent learning. However the cycle is entered, the preoccupation stage begins the action that characterizes all addictions—diversion of attention from the painful state and anticipation of relief or pleasure.
With all addictive substances, the preoccupation phase exhibits tolerance. Longer time frames require higher doses, and increased stimulation of ritualization is required to produce the same mood-altering effect. The addict begins to devote increasing time and energy to the addiction, consuming resources that persons around him or her realize would be better invested in friends, family, career, or recreation (real life).
It is overstatement to characterize the patient’s denial and distortions as thought disorder. Nevertheless, many patients report waking as if from a dream to realize how their perceptions, rationalizations, and behavior have become inconsistent with their own long-held values and sense of reality.
The acting-out phase is often followed by a brief sense of relief and return to normal thinking which—paradoxically—obscure the emergence of despair and rekindling of the addictive cycle. The psychological phase of despair has several important clinical implications. In alcohol addiction, for example, pseudodepression in a freshly detoxified alcoholic often resolves with abstinence. When superimposed on clinical depression, the phase may take on vegetative signs and symptoms, with dangerously increased suicide potential.
Multiple addictions Another fascinating possibility is the generic nature of the despair phase as opposed to the preoccupation phase. It opens the door to mixed or multiple addictions, in which several addiction cycles are linked and serve to defend and obscure each other (Figure 3).
An example is the alcoholic sex addict who is promiscuous after an alcohol binge. He may explain his drinking at a party as a reaction to the presence of several previous sex partners and attribute his promiscuity to intoxication. Thus, the addiction process underlying both behaviors is concealed, as is the functional relationship between the two. Often there is partial awareness (e.g., the alcohol/sex addict has noticed that his promiscuous acting out frequently occurs after, but only rarely during, an intoxication episode).
Figure 1 BELIEF AND THINKING CYCLE OF SEXUAL ADDICTION
Figure 2 FOUR-STAGE SEXUAL ADDICTION CYCLE
Figure 3 MULTIPLE-ADDICTION CYCLE
Recognizing symptoms
The diagnosis of sexual addiction is often made by the patient who recognizes himself in a description of the cycle of addictive thoughts and behavior. Many patients report being aware that their thinking is distorted or their behavior is out of control. They often can admit that their behavior is inconsistent with their values.
Screening For clinicians, what are the diagnostic signs of sexual addiction? Numbers of sex partners or frequency of sex may not provide adequate information or reliable criteria. The 25 items on Carnes’ Sexual Addiction Screening Test (SAST)6 bring previously off-the-radar subjects into consideration. For example, questions on the survey include:
- Have you subscribed to or regularly purchased sexually explicit magazines like Playboy or Penthouse?
- Do you ever feel bad about your sexual behavior?
- Have you made promises to yourself to quit some aspect of your sexual behavior?6
The SAST questionnaire offers a self-report symptom checklist that can be correlated with normative data on sexual addiction. It yields a likelihood that the disorder—rather than the guilt mobilized by the behaviors—should be considered as a focus of treatment.
Treatment
Although treatment of sexual addiction is beyond the scope of this article, the psychiatrist plays an important role:
- Pharmacologic interventions can be appropriate and helpful for symptoms of anxiety and depression that many addicts develop, particularly in withdrawal states.
- Cognitive-behavioral psychotherapeutic approaches can help restructure distorted thinking and alter behavioral patterns.
- Transference-oriented psychodynamic therapies can help modify the basic faulted sense of self and impaired relationships that foster addiction.10
Treatment is also available through 12-step programs such as Sex Addicts Anonymous, Sex and Love Addicts Anonymous, and Sexaholics Anonymous (“Related resources”).
- Dodes L. The heart of addiction. New York: Harper Collins, 2002.
- Sex Addicts Anonymous. www.sexaa.org
- Sexaholics Anonymous. www.sa.org
- Sex and Love Addicts Anonymous. www.slaafws.org
Sex is in the news. From pedophilic priests to philandering politicians, people at every level of society get into trouble over their sexual behavior. Among clinical disciplines, psychiatry has a celebrated tradition of addressing sexual problems (Box 1).1-4 We can therefore expect to be asked for help when a person’s inappropriate sexual behavior brings him into conflict, whether with his internal values or with society.
Are we prepared to treat these predatory patients and protect their potential victims? As the U.S. Catholic church has clearly demonstrated, many approaches to prevention and treatment—moral, medical, and traditional—are often ineffective. Rather than viewing compulsive sexual behaviors as moral failures, it may be time to conceptualize and treat them as addictions.
Among modern clinical disciplines, psychiatry has one of the more celebrated traditions of addressing sexual problems.
In Three Essays on the Theory of Sexuality,1 Freud identified problem areas as “deviations with respect of the sexual object” and “deviations in respect of the sexual aim.” This early focus on physiologic function as a model of “normal” was succeeded by psychoanalytic attempts to address otherwise normal sexual behavior that was compulsive and, arguably, at odds with societal values.2
Masters and Johnson3 weighed in with Human Sexual Response, physiologically interesting studies of sexual behavior that raised many questions about ethics and underlying psychological processes. Finally, psychiatry’s interest in the subject may have peaked with the publication of Helen Singer Kaplan’s The New Sex Therapy,4 which integrated findings and practices from a number of disciplines.
Sexual addiction as a diagnosis
Patrick Carnes popularized the concept of addictive sexual behavior in the consumer self-help book, Out of the Shadows,5 and in Contrary to Love,6 a volume aimed at clinicians. In characterizing addictive sexual behavior, Carnes cast a large net to include masturbation, heterosexual sex, pornography, prostitution, homosexuality, exhibitionism, voyeurism, and other practices that may be associated with sexual excitement.
The unifying quality was the habitual or compulsive nature of the behavior with the goal of altering mood, regardless of its social, legal, medical, emotional, or other maladaptive consequences (Box 2). As common as these cases are, they tend not to reach the mainstream psychiatry literature until a pharmaceutical treatment is tried.7,8
DSM-IV-TR provides diagnostic criteria for paraphilias such as exhibitionism, fetishism, voyeurism, pedophilia, and sexual masochism or sadism. These are descriptions of behavior, whereas sexual addiction is a cycle of mental and emotional experiences that may have a behavior phase.
A middle-aged clergyman presents for counseling due to repeated heterosexual affairs that have been reported to the head of his district and are a potential cause for dismissal. While the affairs have been largely ego-dystonic, he cannot overcome the compulsion to repeatedly seek and become involved in these superficial relationships.
His job offers him much unstructured time, which he frequently fills with fantasies of pursuing sexual experiences. He uses his pulpit, family visits, counseling sessions, and public service activities to make new sexual contacts. He notes that he is more likely to become preoccupied with such relationships when he is distressed or depressed, and he is aware of craving or feeling states that predispose him to looking for such relationships.
The clergyman has tried prayer, counseling, psychoanalytic psychotherapy, brief courses of cognitive-behavioral therapy (weeks to months), and medication trials with various antidepressant and antianxiety agents. These treatments have been unsuccessful.
The addictive behavior may or may not be legal, and it may or may not involve a victim. The common denominator is that the addictive behavior attempts, in a chronically ineffective way, to upregulate mood and sense of self.
DSM-IV may not recognize the term “sexual addiction,” but many patients will recognize the addiction concept, identify with it, and find it useful to understand their compulsive sexual behaviors. An addiction model is already being used by a variety of 12-step and self-help approaches to sexual addiction that have spun off of the success of Alcoholics Anonymous. This model accepts the unlikelihood of cure while offering hope for rehabilitation.
Advantages As a diagnosis, sexual addiction offers patients and psychiatrists two advantages:
- It recognizes a series of temporal mental, emotional, and behavioral events with which sufferers can identify without prohibitive pain and unbearable damage to self-concept.
- It leads to a potential treatment solution that is widely available, relatively inexpensive, and addresses the volition paradox (controlling the uncontrollable) that confounds other approaches.
The cycle of addiction
The validity of a medical diagnosis is based on patterns of family history, prognosis, treatment response, and course of disease. Sexual addiction, with its predictable course and family history, may meet these diagnostic criteria.
Sexual addicts, like mythical vampires, lead secret lives designed to feed the addiction process rather than life itself. The planning, arrangements, cruising, and cover-ups compete for time with family, friends, career, and hobbies. This creates a chronic situation of unmanageability of circumstances. To cope, the addict creates a strange belief system, such as the pedophile priest’s belief that sex between men and boys is natural and that the trauma lies in reporting and investigating it.9 Belief systems such as these are often socially supported by organizations and in publications by like-minded persons.
Self-interested distortions lead the addict to wrongly perceive that victims are cooperating and the self and others are not being harmed. The result is often gross misinterpretations of reality, such as the man who thinks he is flirting at the office but is charged with sexual exploitation by a co-worker. A sequence of mental events (Figure 1) leads to, and helps the addict cope with, the addiction cycle.
The addiction cycle Carnes describes a four-stage addiction cycle: preoccupation, ritualization, sexual acting out, and despair (Figure 2). Stressful or painful experiences—a narcissistic injury, a disappointment, stress-related anxiety—or the boredom and emptiness of unstructured time may initiate the sequence. The sufferer learns, through repetitive trials and reinforcement mechanisms, to take the maladaptive path into the addiction cycle rather than making a healthy emotional adaptation.
Reasons why one person takes the path to addiction when others do not may include genetics (emotional temperament), developmental and familial circumstances, and state-dependent learning. However the cycle is entered, the preoccupation stage begins the action that characterizes all addictions—diversion of attention from the painful state and anticipation of relief or pleasure.
With all addictive substances, the preoccupation phase exhibits tolerance. Longer time frames require higher doses, and increased stimulation of ritualization is required to produce the same mood-altering effect. The addict begins to devote increasing time and energy to the addiction, consuming resources that persons around him or her realize would be better invested in friends, family, career, or recreation (real life).
It is overstatement to characterize the patient’s denial and distortions as thought disorder. Nevertheless, many patients report waking as if from a dream to realize how their perceptions, rationalizations, and behavior have become inconsistent with their own long-held values and sense of reality.
The acting-out phase is often followed by a brief sense of relief and return to normal thinking which—paradoxically—obscure the emergence of despair and rekindling of the addictive cycle. The psychological phase of despair has several important clinical implications. In alcohol addiction, for example, pseudodepression in a freshly detoxified alcoholic often resolves with abstinence. When superimposed on clinical depression, the phase may take on vegetative signs and symptoms, with dangerously increased suicide potential.
Multiple addictions Another fascinating possibility is the generic nature of the despair phase as opposed to the preoccupation phase. It opens the door to mixed or multiple addictions, in which several addiction cycles are linked and serve to defend and obscure each other (Figure 3).
An example is the alcoholic sex addict who is promiscuous after an alcohol binge. He may explain his drinking at a party as a reaction to the presence of several previous sex partners and attribute his promiscuity to intoxication. Thus, the addiction process underlying both behaviors is concealed, as is the functional relationship between the two. Often there is partial awareness (e.g., the alcohol/sex addict has noticed that his promiscuous acting out frequently occurs after, but only rarely during, an intoxication episode).
Figure 1 BELIEF AND THINKING CYCLE OF SEXUAL ADDICTION
Figure 2 FOUR-STAGE SEXUAL ADDICTION CYCLE
Figure 3 MULTIPLE-ADDICTION CYCLE
Recognizing symptoms
The diagnosis of sexual addiction is often made by the patient who recognizes himself in a description of the cycle of addictive thoughts and behavior. Many patients report being aware that their thinking is distorted or their behavior is out of control. They often can admit that their behavior is inconsistent with their values.
Screening For clinicians, what are the diagnostic signs of sexual addiction? Numbers of sex partners or frequency of sex may not provide adequate information or reliable criteria. The 25 items on Carnes’ Sexual Addiction Screening Test (SAST)6 bring previously off-the-radar subjects into consideration. For example, questions on the survey include:
- Have you subscribed to or regularly purchased sexually explicit magazines like Playboy or Penthouse?
- Do you ever feel bad about your sexual behavior?
- Have you made promises to yourself to quit some aspect of your sexual behavior?6
The SAST questionnaire offers a self-report symptom checklist that can be correlated with normative data on sexual addiction. It yields a likelihood that the disorder—rather than the guilt mobilized by the behaviors—should be considered as a focus of treatment.
Treatment
Although treatment of sexual addiction is beyond the scope of this article, the psychiatrist plays an important role:
- Pharmacologic interventions can be appropriate and helpful for symptoms of anxiety and depression that many addicts develop, particularly in withdrawal states.
- Cognitive-behavioral psychotherapeutic approaches can help restructure distorted thinking and alter behavioral patterns.
- Transference-oriented psychodynamic therapies can help modify the basic faulted sense of self and impaired relationships that foster addiction.10
Treatment is also available through 12-step programs such as Sex Addicts Anonymous, Sex and Love Addicts Anonymous, and Sexaholics Anonymous (“Related resources”).
- Dodes L. The heart of addiction. New York: Harper Collins, 2002.
- Sex Addicts Anonymous. www.sexaa.org
- Sexaholics Anonymous. www.sa.org
- Sex and Love Addicts Anonymous. www.slaafws.org
1. Freud S. Three essays on the theory of sexuality (1905). Complete psychological works (standard ed., vol. 7). London: Hogarth Press, 1953.
2. Hershey D. On a type of heterosexuality, and the fluidity of object relations. J Amer Psychoanal Assn 1989;37(1):147-71.
3. Masters WH, Johnson VE. Human sexual response. Boston: Little, Brown & Co, 1966.
4. Kaplan HS. The new sex therapy. New York: Times Books, 1974.
5. Carnes P. Out of the shadows. Understanding sexual addiction. 2nd ed. Center City, MN: Hazelden Foundation, 1992.
6. Carnes P. Contrary to love. Helping the sexual addict. Center City, MN: Hazelden Foundation, 1989.
7. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibiters in men with paraphilias and paraphilia-related disorders: A case series. J Clin Psychiatry 2000;61(9):664-70.
8. Coleman E, Gratzer T, Nesvacil L, Raymond NC. Nefazodone and the treatment of nonparaphilic compulsive sexual behavior: a retrospective study. J Clin Psychiatry 2000;61(4):282-4.
9. Cannon A, Kelly K, Bentrup N. Is there any end in sight? U.S. News & World Report, April 22, 2002.
10. Dodes L. The heart of addiction. New York: Harper Collins, 2002.
1. Freud S. Three essays on the theory of sexuality (1905). Complete psychological works (standard ed., vol. 7). London: Hogarth Press, 1953.
2. Hershey D. On a type of heterosexuality, and the fluidity of object relations. J Amer Psychoanal Assn 1989;37(1):147-71.
3. Masters WH, Johnson VE. Human sexual response. Boston: Little, Brown & Co, 1966.
4. Kaplan HS. The new sex therapy. New York: Times Books, 1974.
5. Carnes P. Out of the shadows. Understanding sexual addiction. 2nd ed. Center City, MN: Hazelden Foundation, 1992.
6. Carnes P. Contrary to love. Helping the sexual addict. Center City, MN: Hazelden Foundation, 1989.
7. Kafka MP, Hennen J. Psychostimulant augmentation during treatment with selective serotonin reuptake inhibiters in men with paraphilias and paraphilia-related disorders: A case series. J Clin Psychiatry 2000;61(9):664-70.
8. Coleman E, Gratzer T, Nesvacil L, Raymond NC. Nefazodone and the treatment of nonparaphilic compulsive sexual behavior: a retrospective study. J Clin Psychiatry 2000;61(4):282-4.
9. Cannon A, Kelly K, Bentrup N. Is there any end in sight? U.S. News & World Report, April 22, 2002.
10. Dodes L. The heart of addiction. New York: Harper Collins, 2002.
Late-life psychosis: It’s efficacy vs. cost in the tug-of-war over treatment
As psychiatrists, we are being pulled in opposite directions between conflicting goals: to reduce health care costs and to provide our patients with the sophistication and specificity of newly available—and more expensive—biological therapies. In the treatment of late-life psychosis, the evidence is impressive and persuasive that atypical antipsychotic drugs are worth the investment.
Atypical antipsychotics should usually be considered first-line therapy for late-life psychosis, though they cost more than the older neuroleptics. Overall, drugs comprise a minor portion of the cost of treating psychosis but may have a major impact on outcomes.1 With their safer side effect profile, the atypicals have enabled many patients with schizophrenia to “reintegrate” and become contributing members of society. Likewise, the use of atypicals can allow older patients with psychotic symptoms to function longer and more productively in their homes and communities.
Table 1
DIFFERENCES BETWEEN LATE-AND EARLY-ONSET SCHIZOPHRENIA
| Characteristic | Late-onset (age 45 and older) | Early-onset (before age 45) |
|---|---|---|
| Gender | More women | More men |
| Type | Paranoid | Varies |
| Positive symptoms | Severe | Severe |
| Negative symptoms | Less severe | More severe |
| Duration of illness | Chronic | Chronic |
| Cognition | Less impaired | More impaired |
| Neuroimaging | Nonspecific changes | Nonspecific changes |
| Antipsychotic dose | Lower | Higher |
| Mortality rates | High | High |
| Premorbid function | Good | Schizoid traits |
Without appropriate treatment of psychosis in younger patients, we know that recurrences and relapses can cause demonstrable brain changes and lead to residual symptoms.2 Older patients have been shown to lose 0.2 “well years” for every year they have psychotic symptoms.3
One illness or many?
Psychotic symptoms can occur in late life for a variety of reasons, and each diagnosis has different implications for patient work-up and treatment. Causes of psychosis in older patients include late-onset schizophrenia, dementia, affective disorder, delusional disorder, and delirium.
Late-life schizophrenia As life expectancy increases, schizophrenia is increasingly being diagnosed in older persons. In a sample of hospitalized patients with schizophrenia, onset of illness occurred after age 50 in 13%, after age 60 in 7%, and after age 70 in 3%.4 The clinical features of schizophrenia may be modified by the concurrent development of dementia. Thus, this reported increase in psychosis after age 50 may be secondary to an increased incidence of dementia, Parkinson’s disease, cerebrovascular events, and neoplasms.
Early definitions of “paraphrenia” and later schizophrenia described delusions and hallucinations that developed without disturbance of affect, with onset in early adult life.5 The occurrence of disorganized behavior and thinking in late life was ascribed to the effects of “senility” or other organic factors.
In 1943, Bleuler6 reported on a series of 126 patients in whom psychosis developed after age 40. In this group, the illness began after age 60 in 4%.
Current diagnostic criteria for schizophrenia do not exclude or categorize individuals on the basis of age. DSMIV does acknowledge a subgroup of patients with “late-onset” schizophrenia after age 45. However, there are important clinical differences in the presentations of the early- and late-onset types (Table 1). For example, even after adjusting for the greater longevity of women, more women than men develop late-onset schizophrenia. Also, compared with the early-onset type, in late-life schizophrenia:
- Premorbid paranoid or schizoid personality traits appear to be much less prominent.
- Patients may have had better occupational functioning and are more likely to have been married.
- Negative symptoms tend to be less severe, although they do contribute to functional decline.
Visual and hearing loss are among the risk factors correlated with late-onset schizophrenia.7 Sensory loss isolates an older person and leads to misinterpretation and misidentification of environmental cues. Other risk factors, in addition to female gender, include cognitive loss, poor social supports, living alone, and alcohol or drug abuse.
Dementia Between 20 and 50% of patients with vascular and mixed dementias exhibit psychotic symptoms.8 Among those with Alzheimer’s dementia, 30% exhibit persecutory delusions.9 Adding to this population are persons with early-onset schizophrenia, who are living longer and can also develop dementia.
While neuroimaging can sometimes aid in diagnosis, abnormalities seen on neuroimaging of patients with psychotic symptoms do not necessarily correlate with cognitive deficits. The clinical significance of these findings is unclear.10,11 Because of differences in therapy, it is important to establish whether the primary diagnosis is dementia or schizophrenia (Table 2).
Affective psychoses Late-life depression and mania are often unrecognized because of atypical presentations. In the elderly, depression may present with withdrawal, mood-incongruent delusions,12 and symptoms that mimic medical illness. Older manic patients may be misidentified as intrusive, hypersexual, or agitated. A high suspicion index, carefully elicited family history, and past psychiatric illness in the patient usually can clarify the diagnosis.
Delusional disorder Suspiciousness and paranoia are common findings in late life, with an estimated prevalence of 4 to 6% in the older population. Patients with delusional disorder show little evidence of cognitive deficits and—unlike those with schizophrenia—continue to maintain a high level of function.13 Delusions are nonbizarre and well systematized, and hallucinations are not a prominent feature.
Delirium Older persons are particularly vulnerable to developing delirium. Common causes include urinary tract infection, bowel impaction, heart failure, endocrinopathies, and drug toxicity caused by prescribed or over-the-counter drugs. CNS conditions such as subdural hematoma, cerebrovascular accident, slow-growing intracranial tumors, and encephalitis can be associated with the development of hallucinations and delusions without clouding consciousness.
While an agitated, hallucinating patient with clearly evident fluctuations in the sensorium may be readily diagnosed with a delirium, patients who are quiet, withdrawn, and apathetic may be less easy to recognize. Clinicians should therefore be vigilant for both the hyperkinetic and hypokinetic presentations of delirium when assessing older patients.
Work-up
When psychotic symptoms present for the first time in late life, medical causes must be ruled out first. A detailed history, including collateral information from other sources, includes:
- premorbid function
- psychiatric history, including history of affective illness
- family history of schizophrenia, affective illness, and Alzheimer’s disease
- medical history, including risks for cerebrovascular disease.
The examination should include a complete medical and neurologic evaluation, giving special attention to sensory loss, prescribed and over-the-counter drugs, recent changes in drug regimen, and cognitive screening. Laboratory work-up includes:
- in all cases: CBC/differential, BUN, TFT, B12/folate, and RPR
- in selected cases: MRI, HIV screening, toxicology screen, neuropsychological testing
- in rare cases: lumbar puncture, PET or SPECT imaging.
If the suggested work-up leads to diagnosis of medical illness, begin appropriate treatment. Behavioral symptoms during medical illness, such as with delirium, may require concurrent psychotropic medication until the medical illness is controlled.
If the medical work-up is negative, the clinician then needs to make the appropriate psychiatric diagnosis and institute antipsychotic treatment as indicated.
Table 2
CLINICAL DIFFERENCES BETWEEN DEMENTIA AND SCHIZOPHRENIA
| Characteristic | Dementia | Late-onset schizophrenia |
|---|---|---|
| Incidence | 50 to 70% | 1 to 2% |
| Bizarre delusions | Uncommon | Common |
| Hallucinations | Usually visual | Usually auditory |
| History of psychosis | Rare | Common |
| Misidentification | Common | Less common |
| Maintenance treatment | Usually unnecessary | Common |
| Antipsychotic dosage | 25% of dosage for adult schizophrenia | 50% of dosage for adult schizophrenia |
Table 3
USE OF ATYPICAL ANTIPSYCHOTICS IN OLDER PATIENTS
| Drug | Administration |
|---|---|
| Clozapine | Use limited by potential for agranulocytosis, need for weekly blood counts Higher risk for diabetes, hyperglycemia, pancreatitis Very sedating “Black box” warning for myocarditis (March 2002) NOT a first-choice drug for older persons |
| Risperidone | Usual geriatric dosage 1 to 2 mg/d Risk of EPS increases significantly with dosages > 2 mg/d Can cause persistent hyperprolactinemia |
| Olanzapine | Usual geriatric dosage 2.5 to 12.5 mg/d (qd dosing) Excellent choice as first-line drug in older patients Anticholinergic side effects ordinarily not a problem in vivo despite receptor profile of M1-M5 antagonism May be weight-neutral in older patients |
| Quetiapine | Geriatric dosage 25 to 400 mg/d Very low potential for EPS Can be a first choice for patients with Lewy body dementia and Parkinson’s disease Sedation increases with dosages > 200 mg/d |
| Ziprasidone | Geriatric dosage 40 to 160 mg/d Significant concern about QT prolongation in older patients, especially women and those with pre-existing cardiac disease, chronic mental illness, hypokalemia, and hypomagnesemia Not recommended as first-line therapy in elderly (“bold” warning) Not approved for IM use in United States; restrictions on use in many European countries |
| EPS: Extrapyramidal symptoms | |
Prescribing antipsychotics
Prescribing psychoactive medications for older patients is similar to pediatric prescribing. You need to distinguish between behaviors that are “disturbed” as a result of a psychotic process or “disturbing,” for which behavioral interventions may be more appropriate. In any case, pharmacologic and behavioral interventions are used concurrently.
When prescribing for older patients, consider the possible interaction of comorbid medical conditions and altered pharmacokinetics and pharmacodynamics, which increase the potential for drug-drug interactions. Start low and go slow to minimize the potential for adverse side effects and to ensure optimal preservation of function.
In general, atypical antipsychotics are considered first-line therapy, unless there is a compelling reason not to use them in an individual patient. Conventional neuroleptic antipsychotics carry a much higher risk of adverse drug reactions and functional loss without providing substantially greater efficacy.14 Atypical antipsychotics differ not only from the older neuroleptics but also from each other—including their safety profiles for use in older patients (Table 3).
Clozapine is a dibenzodiazepine that is indicated for treatment-resistant schizophrenia. The first atypical antipsychotic, clozapine significantly reduced the incidence of extrapyramidal symptoms (EPS) seen with the neuroleptic antipsychotics. Clozapine also was effective against the “negative” symptoms of schizophrenia such as apathy, anhedonia, and emotional blunting, which severely limited the ability of patients with schizophrenia to function in society.
The limitations of clozapine include its potential to cause agranulocytosis and the requirement for weekly blood tests. It is also fairly sedating. A number of reports have suggested a link between the use of clozapine and the development of type 2 diabetes, hyperglycemia, and pancreatitis.15,16 Earlier this year, the FDA issued a “black box” warning linking the use of clozapine with myocarditis. For all of these reasons, clozapine is not recommended for use in older patients.
Risperidone is a benzisoxazole derivative indicated for treatment of psychotic disorders. Risperidone’s antipsychotic efficacy is equivalent to that of haloperidol, but it has a much safer side-effect profile. In one multicenter study comparing risperidone and a placebo at single daily dosages of 0.5, 1.0, and 2.0 mg, risperidone was found to be effective in controlling the psychosis and behavioral disturbance associated with dementia. The authors recommended using a dosage of 1.0 mg/d because higher dosages resulted in excessive sedation and EPS.17
Risk of side effects is dose-dependent, especially in the older patient. Risperidone is the only atypical antipsychotic associated with persistent hyperprolactinemia, which may indirectly contribute to increased osteoporosis and atherogenesis.18,19 Orthostatic hypotension, especially with initial dosages greater than 1.5 mg/d and rapid dose escalation (≥ 25% every 24 to 48 hours), may also limit its use in older patients.
Olanzapine is a thiobenzodiazepine derivative indicated for treatment of psychosis and acute bipolar mania. Olanzapine has a receptor profile that is somewhat analogous with that of clozapine. Its antipsychotic efficacy is equivalent to that of risperidone, but it has a more favorable safety profile. Like risperidone, olanzapine is relatively nonsedating, but it is significantly less likely to cause EPS and orthostatic hypotension, and its use is not associated with persistent hyperprolactinemia.
Based on its vitro muscarinic receptor antagonism profile, some clinicians incorrectly assume that olanzapine is highly anticholinergic. In vivo data and clinical experience have not borne out this contention.20,21 Weight gain with olanzapine is relatively infrequent in older persons. A rapid-dissolve preparation can be useful in resistant and noncompliant patients. An IM preparation has been approved by the FDA but has not yet been made available by the manufacturer.
Quetiapine is a dibenzothiazepine derivative indicated for treatment of psychotic disorders. Despite limited data on its efficacy in late-life psychosis, clinical experience would suggest that its antipsychotic efficacy would at least equal that of other drugs in this category.
The need for twice-daily dosing and titration to a therapeutic response can sometimes limit the use of quetiapine. Sedation can be a problem at dosages greater than 200 mg/d. Because quetiapine has a relatively lower potential among the antipsychotics to cause EPS, it may be a first-line choice in patients with Parkinson’s disease and Lewy body dementia.
Ziprasidone is a benzothiazolylpiperazine indicated for treatment of psychosis. Experience with its use in older patients is limited. Ziprasidone should be avoided in patients with significant cardiovascular disease because of its potential to cause QT prolongation and cardiac arrhythmias. Although rare, this cardiac side effect may be life-threatening, and clinicians must be exceptionally vigilant when using ziprasidone in older patients. Risk factors for QT prolongation include older age, female sex, pre-existing cardiac disease, hypokalemia, and hypomagnesemia.
Ziprasidone can be somewhat sedating. An IM preparation is under development but has not been approved for use by the FDA
Neuroleptics and other options
Occasionally a typical neuroleptic may be the most appropriate first-line drug for older patients with psychotic symptoms. For example, a mid-potency neuroleptic such as perphenazine at an IM dose of 2 to 4 mg may be considered when severe agitation and aggression pose a substantial safety risk for the patient or caregiver and require rapid control. Haloperidol, despite its widespread use in both acute and long-term settings, should be used with caution because it has great potential for causing EPS and can immobilize an older patient, resulting in further functional decline. Regardless of which typical neuroleptic is used, switch the patient to an atypical antipsychotic as soon as agitation is under control.
Antidepressants Affective psychoses in older patients may require the addition of an antidepressant to the antipsychotic drug. The selective serotonin reuptake inhibitors fluoxetine and sertraline have proven track records for efficacy and safety and should be considered first-line agents.
Other options Electroconvulsive therapy is safe and effective for older patients with psychotic depression or late-life mania. Late-life mania also may respond well to the anticonvulsant divalproex sodium. Avoid anxiolytics in older patients because of the potential of these agents to cause sedation or disinhibition and their associated risk of falls and confusion. Buspirone in higher dosages (40 to 60 mg/d) can sometimes help manage chronic anxiety states.
Related resource
- Sadavoy J, Lazarus LW, Jarvik LF, Grossman GT (eds). Comprehensive review of geriatric psychiatry (2nd ed). Washington, DC: American Psychiatric Press, 1996.
Drug brand names
- Buspirone • Buspar
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluoxetine • Prozac
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
Dr. Snow reports no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
Dr. Verma reports that he is on the speakers’ bureau of Eli Lilly and Co. and Abbott Laboratories, serves as a consultant to Eli Lilly and Co., and receives grant support from Eli Lilly and Co. and GlaxoSmithKline.
1. Hargreaves WA, Shumway M. Pharmacoeconomics of drug therapy. J Clin Psych 1996;57(suppl 9):66-76.
2. Lieberman J, Chakos M, Wu H, Alvir J, et al. Longitudinal study of brain morphology in first-episode schizophrenia. Biol Psych 2001;49(6):487-99.
3. Patterson TL, Shaw W, Semple SJ, et al. Health-related quality of life in older patients with schizophrenia and other psychoses: relationships among psychosocial and psychiatric factors. Int J Geriatr Psychiatry 1997;12(4):452-61.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull 1988;14:39-55.
5. Kraeplin E. Dementia, praecox, and paraphrenia (1919). Translated by Barclay RM. Huntingdon NY: Krieger, 1971
6. Bleuler E. Late schizophrenic clinical pictures. Fortschr Neurol Psych 1943;15:259-90.
7. Pearlson G, Rabins P. The late-onset psychoses: possible risk factors. Psychiatr Clin North Am 1988;11(1):15-32.
8. Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer’s type. Biol Psychiatry 1989;25:39-48.
9. Wragg R, Jeste DV. Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry 1989;146:577-87.
10. Lesser IM. Late-onset psychotic disorder not otherwise specified: clinical and neuroimaging findings. Biol Psychiatry 1992;31:419-23.
11. Lesser IM, Miller BL, Swartz JR, et al. Brain imaging in late-life schizophrenia and related psychoses. Schizophr Bull 1993;19:419-23.
12. Jeste DV, Heaton SC, Paulsen JS, Ercoli L, Harris J, Heaton RK. Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry 1996;153(4):490-6.
13. Burns BJ, Larson DB, Goldstrom ID, et al. Mental disorders among nursing home patients: preliminary findings from the National Nursing Home Survey pretest. Int J Geriatr Psychiatry 1988;3:27-35.
14. Schneider LS, Pollock VE, Lyness SA. A meta-analysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc 1990;38:553-63.
15. Popli AP, Konicki PE, Jurjus GJ, et al. Clozapine and associated diabetes mellitus. J Clin Psychiatry 1997;58:108-11.
16. Bergemann N, Ehrig C, Diebold K, Mundt C, von Einsiedel R. Asymptomatic pancreatitis associated with clozapine. Pharmacopsychiatry 1999;32(2):78-80.
17. Katz IR, Jeste DV, Mintzer JE, et al. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psych 1999;60:107-15.
18. Petty RG. Prolactin and antipsychotic medications: mechanisms of action. Schizophr Res 1999;35(suppl):S67-S73.
19. Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res 1999;35(suppl):575-86.
20. Zarate CA, Baldessarini RJ, Siegel AJ, et al:. Risperidone in the elderly: a pharmacoepidemiological study. J Clin Psychiatry 1997;58:311-17.
21. Street JS, Clark WS, Gannon K, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer’s disease in nursing care facilities: a double-blind, placebo-controlled trial. Arch Gen Psychiatry 2000;57(10):968-76.
As psychiatrists, we are being pulled in opposite directions between conflicting goals: to reduce health care costs and to provide our patients with the sophistication and specificity of newly available—and more expensive—biological therapies. In the treatment of late-life psychosis, the evidence is impressive and persuasive that atypical antipsychotic drugs are worth the investment.
Atypical antipsychotics should usually be considered first-line therapy for late-life psychosis, though they cost more than the older neuroleptics. Overall, drugs comprise a minor portion of the cost of treating psychosis but may have a major impact on outcomes.1 With their safer side effect profile, the atypicals have enabled many patients with schizophrenia to “reintegrate” and become contributing members of society. Likewise, the use of atypicals can allow older patients with psychotic symptoms to function longer and more productively in their homes and communities.
Table 1
DIFFERENCES BETWEEN LATE-AND EARLY-ONSET SCHIZOPHRENIA
| Characteristic | Late-onset (age 45 and older) | Early-onset (before age 45) |
|---|---|---|
| Gender | More women | More men |
| Type | Paranoid | Varies |
| Positive symptoms | Severe | Severe |
| Negative symptoms | Less severe | More severe |
| Duration of illness | Chronic | Chronic |
| Cognition | Less impaired | More impaired |
| Neuroimaging | Nonspecific changes | Nonspecific changes |
| Antipsychotic dose | Lower | Higher |
| Mortality rates | High | High |
| Premorbid function | Good | Schizoid traits |
Without appropriate treatment of psychosis in younger patients, we know that recurrences and relapses can cause demonstrable brain changes and lead to residual symptoms.2 Older patients have been shown to lose 0.2 “well years” for every year they have psychotic symptoms.3
One illness or many?
Psychotic symptoms can occur in late life for a variety of reasons, and each diagnosis has different implications for patient work-up and treatment. Causes of psychosis in older patients include late-onset schizophrenia, dementia, affective disorder, delusional disorder, and delirium.
Late-life schizophrenia As life expectancy increases, schizophrenia is increasingly being diagnosed in older persons. In a sample of hospitalized patients with schizophrenia, onset of illness occurred after age 50 in 13%, after age 60 in 7%, and after age 70 in 3%.4 The clinical features of schizophrenia may be modified by the concurrent development of dementia. Thus, this reported increase in psychosis after age 50 may be secondary to an increased incidence of dementia, Parkinson’s disease, cerebrovascular events, and neoplasms.
Early definitions of “paraphrenia” and later schizophrenia described delusions and hallucinations that developed without disturbance of affect, with onset in early adult life.5 The occurrence of disorganized behavior and thinking in late life was ascribed to the effects of “senility” or other organic factors.
In 1943, Bleuler6 reported on a series of 126 patients in whom psychosis developed after age 40. In this group, the illness began after age 60 in 4%.
Current diagnostic criteria for schizophrenia do not exclude or categorize individuals on the basis of age. DSMIV does acknowledge a subgroup of patients with “late-onset” schizophrenia after age 45. However, there are important clinical differences in the presentations of the early- and late-onset types (Table 1). For example, even after adjusting for the greater longevity of women, more women than men develop late-onset schizophrenia. Also, compared with the early-onset type, in late-life schizophrenia:
- Premorbid paranoid or schizoid personality traits appear to be much less prominent.
- Patients may have had better occupational functioning and are more likely to have been married.
- Negative symptoms tend to be less severe, although they do contribute to functional decline.
Visual and hearing loss are among the risk factors correlated with late-onset schizophrenia.7 Sensory loss isolates an older person and leads to misinterpretation and misidentification of environmental cues. Other risk factors, in addition to female gender, include cognitive loss, poor social supports, living alone, and alcohol or drug abuse.
Dementia Between 20 and 50% of patients with vascular and mixed dementias exhibit psychotic symptoms.8 Among those with Alzheimer’s dementia, 30% exhibit persecutory delusions.9 Adding to this population are persons with early-onset schizophrenia, who are living longer and can also develop dementia.
While neuroimaging can sometimes aid in diagnosis, abnormalities seen on neuroimaging of patients with psychotic symptoms do not necessarily correlate with cognitive deficits. The clinical significance of these findings is unclear.10,11 Because of differences in therapy, it is important to establish whether the primary diagnosis is dementia or schizophrenia (Table 2).
Affective psychoses Late-life depression and mania are often unrecognized because of atypical presentations. In the elderly, depression may present with withdrawal, mood-incongruent delusions,12 and symptoms that mimic medical illness. Older manic patients may be misidentified as intrusive, hypersexual, or agitated. A high suspicion index, carefully elicited family history, and past psychiatric illness in the patient usually can clarify the diagnosis.
Delusional disorder Suspiciousness and paranoia are common findings in late life, with an estimated prevalence of 4 to 6% in the older population. Patients with delusional disorder show little evidence of cognitive deficits and—unlike those with schizophrenia—continue to maintain a high level of function.13 Delusions are nonbizarre and well systematized, and hallucinations are not a prominent feature.
Delirium Older persons are particularly vulnerable to developing delirium. Common causes include urinary tract infection, bowel impaction, heart failure, endocrinopathies, and drug toxicity caused by prescribed or over-the-counter drugs. CNS conditions such as subdural hematoma, cerebrovascular accident, slow-growing intracranial tumors, and encephalitis can be associated with the development of hallucinations and delusions without clouding consciousness.
While an agitated, hallucinating patient with clearly evident fluctuations in the sensorium may be readily diagnosed with a delirium, patients who are quiet, withdrawn, and apathetic may be less easy to recognize. Clinicians should therefore be vigilant for both the hyperkinetic and hypokinetic presentations of delirium when assessing older patients.
Work-up
When psychotic symptoms present for the first time in late life, medical causes must be ruled out first. A detailed history, including collateral information from other sources, includes:
- premorbid function
- psychiatric history, including history of affective illness
- family history of schizophrenia, affective illness, and Alzheimer’s disease
- medical history, including risks for cerebrovascular disease.
The examination should include a complete medical and neurologic evaluation, giving special attention to sensory loss, prescribed and over-the-counter drugs, recent changes in drug regimen, and cognitive screening. Laboratory work-up includes:
- in all cases: CBC/differential, BUN, TFT, B12/folate, and RPR
- in selected cases: MRI, HIV screening, toxicology screen, neuropsychological testing
- in rare cases: lumbar puncture, PET or SPECT imaging.
If the suggested work-up leads to diagnosis of medical illness, begin appropriate treatment. Behavioral symptoms during medical illness, such as with delirium, may require concurrent psychotropic medication until the medical illness is controlled.
If the medical work-up is negative, the clinician then needs to make the appropriate psychiatric diagnosis and institute antipsychotic treatment as indicated.
Table 2
CLINICAL DIFFERENCES BETWEEN DEMENTIA AND SCHIZOPHRENIA
| Characteristic | Dementia | Late-onset schizophrenia |
|---|---|---|
| Incidence | 50 to 70% | 1 to 2% |
| Bizarre delusions | Uncommon | Common |
| Hallucinations | Usually visual | Usually auditory |
| History of psychosis | Rare | Common |
| Misidentification | Common | Less common |
| Maintenance treatment | Usually unnecessary | Common |
| Antipsychotic dosage | 25% of dosage for adult schizophrenia | 50% of dosage for adult schizophrenia |
Table 3
USE OF ATYPICAL ANTIPSYCHOTICS IN OLDER PATIENTS
| Drug | Administration |
|---|---|
| Clozapine | Use limited by potential for agranulocytosis, need for weekly blood counts Higher risk for diabetes, hyperglycemia, pancreatitis Very sedating “Black box” warning for myocarditis (March 2002) NOT a first-choice drug for older persons |
| Risperidone | Usual geriatric dosage 1 to 2 mg/d Risk of EPS increases significantly with dosages > 2 mg/d Can cause persistent hyperprolactinemia |
| Olanzapine | Usual geriatric dosage 2.5 to 12.5 mg/d (qd dosing) Excellent choice as first-line drug in older patients Anticholinergic side effects ordinarily not a problem in vivo despite receptor profile of M1-M5 antagonism May be weight-neutral in older patients |
| Quetiapine | Geriatric dosage 25 to 400 mg/d Very low potential for EPS Can be a first choice for patients with Lewy body dementia and Parkinson’s disease Sedation increases with dosages > 200 mg/d |
| Ziprasidone | Geriatric dosage 40 to 160 mg/d Significant concern about QT prolongation in older patients, especially women and those with pre-existing cardiac disease, chronic mental illness, hypokalemia, and hypomagnesemia Not recommended as first-line therapy in elderly (“bold” warning) Not approved for IM use in United States; restrictions on use in many European countries |
| EPS: Extrapyramidal symptoms | |
Prescribing antipsychotics
Prescribing psychoactive medications for older patients is similar to pediatric prescribing. You need to distinguish between behaviors that are “disturbed” as a result of a psychotic process or “disturbing,” for which behavioral interventions may be more appropriate. In any case, pharmacologic and behavioral interventions are used concurrently.
When prescribing for older patients, consider the possible interaction of comorbid medical conditions and altered pharmacokinetics and pharmacodynamics, which increase the potential for drug-drug interactions. Start low and go slow to minimize the potential for adverse side effects and to ensure optimal preservation of function.
In general, atypical antipsychotics are considered first-line therapy, unless there is a compelling reason not to use them in an individual patient. Conventional neuroleptic antipsychotics carry a much higher risk of adverse drug reactions and functional loss without providing substantially greater efficacy.14 Atypical antipsychotics differ not only from the older neuroleptics but also from each other—including their safety profiles for use in older patients (Table 3).
Clozapine is a dibenzodiazepine that is indicated for treatment-resistant schizophrenia. The first atypical antipsychotic, clozapine significantly reduced the incidence of extrapyramidal symptoms (EPS) seen with the neuroleptic antipsychotics. Clozapine also was effective against the “negative” symptoms of schizophrenia such as apathy, anhedonia, and emotional blunting, which severely limited the ability of patients with schizophrenia to function in society.
The limitations of clozapine include its potential to cause agranulocytosis and the requirement for weekly blood tests. It is also fairly sedating. A number of reports have suggested a link between the use of clozapine and the development of type 2 diabetes, hyperglycemia, and pancreatitis.15,16 Earlier this year, the FDA issued a “black box” warning linking the use of clozapine with myocarditis. For all of these reasons, clozapine is not recommended for use in older patients.
Risperidone is a benzisoxazole derivative indicated for treatment of psychotic disorders. Risperidone’s antipsychotic efficacy is equivalent to that of haloperidol, but it has a much safer side-effect profile. In one multicenter study comparing risperidone and a placebo at single daily dosages of 0.5, 1.0, and 2.0 mg, risperidone was found to be effective in controlling the psychosis and behavioral disturbance associated with dementia. The authors recommended using a dosage of 1.0 mg/d because higher dosages resulted in excessive sedation and EPS.17
Risk of side effects is dose-dependent, especially in the older patient. Risperidone is the only atypical antipsychotic associated with persistent hyperprolactinemia, which may indirectly contribute to increased osteoporosis and atherogenesis.18,19 Orthostatic hypotension, especially with initial dosages greater than 1.5 mg/d and rapid dose escalation (≥ 25% every 24 to 48 hours), may also limit its use in older patients.
Olanzapine is a thiobenzodiazepine derivative indicated for treatment of psychosis and acute bipolar mania. Olanzapine has a receptor profile that is somewhat analogous with that of clozapine. Its antipsychotic efficacy is equivalent to that of risperidone, but it has a more favorable safety profile. Like risperidone, olanzapine is relatively nonsedating, but it is significantly less likely to cause EPS and orthostatic hypotension, and its use is not associated with persistent hyperprolactinemia.
Based on its vitro muscarinic receptor antagonism profile, some clinicians incorrectly assume that olanzapine is highly anticholinergic. In vivo data and clinical experience have not borne out this contention.20,21 Weight gain with olanzapine is relatively infrequent in older persons. A rapid-dissolve preparation can be useful in resistant and noncompliant patients. An IM preparation has been approved by the FDA but has not yet been made available by the manufacturer.
Quetiapine is a dibenzothiazepine derivative indicated for treatment of psychotic disorders. Despite limited data on its efficacy in late-life psychosis, clinical experience would suggest that its antipsychotic efficacy would at least equal that of other drugs in this category.
The need for twice-daily dosing and titration to a therapeutic response can sometimes limit the use of quetiapine. Sedation can be a problem at dosages greater than 200 mg/d. Because quetiapine has a relatively lower potential among the antipsychotics to cause EPS, it may be a first-line choice in patients with Parkinson’s disease and Lewy body dementia.
Ziprasidone is a benzothiazolylpiperazine indicated for treatment of psychosis. Experience with its use in older patients is limited. Ziprasidone should be avoided in patients with significant cardiovascular disease because of its potential to cause QT prolongation and cardiac arrhythmias. Although rare, this cardiac side effect may be life-threatening, and clinicians must be exceptionally vigilant when using ziprasidone in older patients. Risk factors for QT prolongation include older age, female sex, pre-existing cardiac disease, hypokalemia, and hypomagnesemia.
Ziprasidone can be somewhat sedating. An IM preparation is under development but has not been approved for use by the FDA
Neuroleptics and other options
Occasionally a typical neuroleptic may be the most appropriate first-line drug for older patients with psychotic symptoms. For example, a mid-potency neuroleptic such as perphenazine at an IM dose of 2 to 4 mg may be considered when severe agitation and aggression pose a substantial safety risk for the patient or caregiver and require rapid control. Haloperidol, despite its widespread use in both acute and long-term settings, should be used with caution because it has great potential for causing EPS and can immobilize an older patient, resulting in further functional decline. Regardless of which typical neuroleptic is used, switch the patient to an atypical antipsychotic as soon as agitation is under control.
Antidepressants Affective psychoses in older patients may require the addition of an antidepressant to the antipsychotic drug. The selective serotonin reuptake inhibitors fluoxetine and sertraline have proven track records for efficacy and safety and should be considered first-line agents.
Other options Electroconvulsive therapy is safe and effective for older patients with psychotic depression or late-life mania. Late-life mania also may respond well to the anticonvulsant divalproex sodium. Avoid anxiolytics in older patients because of the potential of these agents to cause sedation or disinhibition and their associated risk of falls and confusion. Buspirone in higher dosages (40 to 60 mg/d) can sometimes help manage chronic anxiety states.
Related resource
- Sadavoy J, Lazarus LW, Jarvik LF, Grossman GT (eds). Comprehensive review of geriatric psychiatry (2nd ed). Washington, DC: American Psychiatric Press, 1996.
Drug brand names
- Buspirone • Buspar
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluoxetine • Prozac
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
Dr. Snow reports no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
Dr. Verma reports that he is on the speakers’ bureau of Eli Lilly and Co. and Abbott Laboratories, serves as a consultant to Eli Lilly and Co., and receives grant support from Eli Lilly and Co. and GlaxoSmithKline.
As psychiatrists, we are being pulled in opposite directions between conflicting goals: to reduce health care costs and to provide our patients with the sophistication and specificity of newly available—and more expensive—biological therapies. In the treatment of late-life psychosis, the evidence is impressive and persuasive that atypical antipsychotic drugs are worth the investment.
Atypical antipsychotics should usually be considered first-line therapy for late-life psychosis, though they cost more than the older neuroleptics. Overall, drugs comprise a minor portion of the cost of treating psychosis but may have a major impact on outcomes.1 With their safer side effect profile, the atypicals have enabled many patients with schizophrenia to “reintegrate” and become contributing members of society. Likewise, the use of atypicals can allow older patients with psychotic symptoms to function longer and more productively in their homes and communities.
Table 1
DIFFERENCES BETWEEN LATE-AND EARLY-ONSET SCHIZOPHRENIA
| Characteristic | Late-onset (age 45 and older) | Early-onset (before age 45) |
|---|---|---|
| Gender | More women | More men |
| Type | Paranoid | Varies |
| Positive symptoms | Severe | Severe |
| Negative symptoms | Less severe | More severe |
| Duration of illness | Chronic | Chronic |
| Cognition | Less impaired | More impaired |
| Neuroimaging | Nonspecific changes | Nonspecific changes |
| Antipsychotic dose | Lower | Higher |
| Mortality rates | High | High |
| Premorbid function | Good | Schizoid traits |
Without appropriate treatment of psychosis in younger patients, we know that recurrences and relapses can cause demonstrable brain changes and lead to residual symptoms.2 Older patients have been shown to lose 0.2 “well years” for every year they have psychotic symptoms.3
One illness or many?
Psychotic symptoms can occur in late life for a variety of reasons, and each diagnosis has different implications for patient work-up and treatment. Causes of psychosis in older patients include late-onset schizophrenia, dementia, affective disorder, delusional disorder, and delirium.
Late-life schizophrenia As life expectancy increases, schizophrenia is increasingly being diagnosed in older persons. In a sample of hospitalized patients with schizophrenia, onset of illness occurred after age 50 in 13%, after age 60 in 7%, and after age 70 in 3%.4 The clinical features of schizophrenia may be modified by the concurrent development of dementia. Thus, this reported increase in psychosis after age 50 may be secondary to an increased incidence of dementia, Parkinson’s disease, cerebrovascular events, and neoplasms.
Early definitions of “paraphrenia” and later schizophrenia described delusions and hallucinations that developed without disturbance of affect, with onset in early adult life.5 The occurrence of disorganized behavior and thinking in late life was ascribed to the effects of “senility” or other organic factors.
In 1943, Bleuler6 reported on a series of 126 patients in whom psychosis developed after age 40. In this group, the illness began after age 60 in 4%.
Current diagnostic criteria for schizophrenia do not exclude or categorize individuals on the basis of age. DSMIV does acknowledge a subgroup of patients with “late-onset” schizophrenia after age 45. However, there are important clinical differences in the presentations of the early- and late-onset types (Table 1). For example, even after adjusting for the greater longevity of women, more women than men develop late-onset schizophrenia. Also, compared with the early-onset type, in late-life schizophrenia:
- Premorbid paranoid or schizoid personality traits appear to be much less prominent.
- Patients may have had better occupational functioning and are more likely to have been married.
- Negative symptoms tend to be less severe, although they do contribute to functional decline.
Visual and hearing loss are among the risk factors correlated with late-onset schizophrenia.7 Sensory loss isolates an older person and leads to misinterpretation and misidentification of environmental cues. Other risk factors, in addition to female gender, include cognitive loss, poor social supports, living alone, and alcohol or drug abuse.
Dementia Between 20 and 50% of patients with vascular and mixed dementias exhibit psychotic symptoms.8 Among those with Alzheimer’s dementia, 30% exhibit persecutory delusions.9 Adding to this population are persons with early-onset schizophrenia, who are living longer and can also develop dementia.
While neuroimaging can sometimes aid in diagnosis, abnormalities seen on neuroimaging of patients with psychotic symptoms do not necessarily correlate with cognitive deficits. The clinical significance of these findings is unclear.10,11 Because of differences in therapy, it is important to establish whether the primary diagnosis is dementia or schizophrenia (Table 2).
Affective psychoses Late-life depression and mania are often unrecognized because of atypical presentations. In the elderly, depression may present with withdrawal, mood-incongruent delusions,12 and symptoms that mimic medical illness. Older manic patients may be misidentified as intrusive, hypersexual, or agitated. A high suspicion index, carefully elicited family history, and past psychiatric illness in the patient usually can clarify the diagnosis.
Delusional disorder Suspiciousness and paranoia are common findings in late life, with an estimated prevalence of 4 to 6% in the older population. Patients with delusional disorder show little evidence of cognitive deficits and—unlike those with schizophrenia—continue to maintain a high level of function.13 Delusions are nonbizarre and well systematized, and hallucinations are not a prominent feature.
Delirium Older persons are particularly vulnerable to developing delirium. Common causes include urinary tract infection, bowel impaction, heart failure, endocrinopathies, and drug toxicity caused by prescribed or over-the-counter drugs. CNS conditions such as subdural hematoma, cerebrovascular accident, slow-growing intracranial tumors, and encephalitis can be associated with the development of hallucinations and delusions without clouding consciousness.
While an agitated, hallucinating patient with clearly evident fluctuations in the sensorium may be readily diagnosed with a delirium, patients who are quiet, withdrawn, and apathetic may be less easy to recognize. Clinicians should therefore be vigilant for both the hyperkinetic and hypokinetic presentations of delirium when assessing older patients.
Work-up
When psychotic symptoms present for the first time in late life, medical causes must be ruled out first. A detailed history, including collateral information from other sources, includes:
- premorbid function
- psychiatric history, including history of affective illness
- family history of schizophrenia, affective illness, and Alzheimer’s disease
- medical history, including risks for cerebrovascular disease.
The examination should include a complete medical and neurologic evaluation, giving special attention to sensory loss, prescribed and over-the-counter drugs, recent changes in drug regimen, and cognitive screening. Laboratory work-up includes:
- in all cases: CBC/differential, BUN, TFT, B12/folate, and RPR
- in selected cases: MRI, HIV screening, toxicology screen, neuropsychological testing
- in rare cases: lumbar puncture, PET or SPECT imaging.
If the suggested work-up leads to diagnosis of medical illness, begin appropriate treatment. Behavioral symptoms during medical illness, such as with delirium, may require concurrent psychotropic medication until the medical illness is controlled.
If the medical work-up is negative, the clinician then needs to make the appropriate psychiatric diagnosis and institute antipsychotic treatment as indicated.
Table 2
CLINICAL DIFFERENCES BETWEEN DEMENTIA AND SCHIZOPHRENIA
| Characteristic | Dementia | Late-onset schizophrenia |
|---|---|---|
| Incidence | 50 to 70% | 1 to 2% |
| Bizarre delusions | Uncommon | Common |
| Hallucinations | Usually visual | Usually auditory |
| History of psychosis | Rare | Common |
| Misidentification | Common | Less common |
| Maintenance treatment | Usually unnecessary | Common |
| Antipsychotic dosage | 25% of dosage for adult schizophrenia | 50% of dosage for adult schizophrenia |
Table 3
USE OF ATYPICAL ANTIPSYCHOTICS IN OLDER PATIENTS
| Drug | Administration |
|---|---|
| Clozapine | Use limited by potential for agranulocytosis, need for weekly blood counts Higher risk for diabetes, hyperglycemia, pancreatitis Very sedating “Black box” warning for myocarditis (March 2002) NOT a first-choice drug for older persons |
| Risperidone | Usual geriatric dosage 1 to 2 mg/d Risk of EPS increases significantly with dosages > 2 mg/d Can cause persistent hyperprolactinemia |
| Olanzapine | Usual geriatric dosage 2.5 to 12.5 mg/d (qd dosing) Excellent choice as first-line drug in older patients Anticholinergic side effects ordinarily not a problem in vivo despite receptor profile of M1-M5 antagonism May be weight-neutral in older patients |
| Quetiapine | Geriatric dosage 25 to 400 mg/d Very low potential for EPS Can be a first choice for patients with Lewy body dementia and Parkinson’s disease Sedation increases with dosages > 200 mg/d |
| Ziprasidone | Geriatric dosage 40 to 160 mg/d Significant concern about QT prolongation in older patients, especially women and those with pre-existing cardiac disease, chronic mental illness, hypokalemia, and hypomagnesemia Not recommended as first-line therapy in elderly (“bold” warning) Not approved for IM use in United States; restrictions on use in many European countries |
| EPS: Extrapyramidal symptoms | |
Prescribing antipsychotics
Prescribing psychoactive medications for older patients is similar to pediatric prescribing. You need to distinguish between behaviors that are “disturbed” as a result of a psychotic process or “disturbing,” for which behavioral interventions may be more appropriate. In any case, pharmacologic and behavioral interventions are used concurrently.
When prescribing for older patients, consider the possible interaction of comorbid medical conditions and altered pharmacokinetics and pharmacodynamics, which increase the potential for drug-drug interactions. Start low and go slow to minimize the potential for adverse side effects and to ensure optimal preservation of function.
In general, atypical antipsychotics are considered first-line therapy, unless there is a compelling reason not to use them in an individual patient. Conventional neuroleptic antipsychotics carry a much higher risk of adverse drug reactions and functional loss without providing substantially greater efficacy.14 Atypical antipsychotics differ not only from the older neuroleptics but also from each other—including their safety profiles for use in older patients (Table 3).
Clozapine is a dibenzodiazepine that is indicated for treatment-resistant schizophrenia. The first atypical antipsychotic, clozapine significantly reduced the incidence of extrapyramidal symptoms (EPS) seen with the neuroleptic antipsychotics. Clozapine also was effective against the “negative” symptoms of schizophrenia such as apathy, anhedonia, and emotional blunting, which severely limited the ability of patients with schizophrenia to function in society.
The limitations of clozapine include its potential to cause agranulocytosis and the requirement for weekly blood tests. It is also fairly sedating. A number of reports have suggested a link between the use of clozapine and the development of type 2 diabetes, hyperglycemia, and pancreatitis.15,16 Earlier this year, the FDA issued a “black box” warning linking the use of clozapine with myocarditis. For all of these reasons, clozapine is not recommended for use in older patients.
Risperidone is a benzisoxazole derivative indicated for treatment of psychotic disorders. Risperidone’s antipsychotic efficacy is equivalent to that of haloperidol, but it has a much safer side-effect profile. In one multicenter study comparing risperidone and a placebo at single daily dosages of 0.5, 1.0, and 2.0 mg, risperidone was found to be effective in controlling the psychosis and behavioral disturbance associated with dementia. The authors recommended using a dosage of 1.0 mg/d because higher dosages resulted in excessive sedation and EPS.17
Risk of side effects is dose-dependent, especially in the older patient. Risperidone is the only atypical antipsychotic associated with persistent hyperprolactinemia, which may indirectly contribute to increased osteoporosis and atherogenesis.18,19 Orthostatic hypotension, especially with initial dosages greater than 1.5 mg/d and rapid dose escalation (≥ 25% every 24 to 48 hours), may also limit its use in older patients.
Olanzapine is a thiobenzodiazepine derivative indicated for treatment of psychosis and acute bipolar mania. Olanzapine has a receptor profile that is somewhat analogous with that of clozapine. Its antipsychotic efficacy is equivalent to that of risperidone, but it has a more favorable safety profile. Like risperidone, olanzapine is relatively nonsedating, but it is significantly less likely to cause EPS and orthostatic hypotension, and its use is not associated with persistent hyperprolactinemia.
Based on its vitro muscarinic receptor antagonism profile, some clinicians incorrectly assume that olanzapine is highly anticholinergic. In vivo data and clinical experience have not borne out this contention.20,21 Weight gain with olanzapine is relatively infrequent in older persons. A rapid-dissolve preparation can be useful in resistant and noncompliant patients. An IM preparation has been approved by the FDA but has not yet been made available by the manufacturer.
Quetiapine is a dibenzothiazepine derivative indicated for treatment of psychotic disorders. Despite limited data on its efficacy in late-life psychosis, clinical experience would suggest that its antipsychotic efficacy would at least equal that of other drugs in this category.
The need for twice-daily dosing and titration to a therapeutic response can sometimes limit the use of quetiapine. Sedation can be a problem at dosages greater than 200 mg/d. Because quetiapine has a relatively lower potential among the antipsychotics to cause EPS, it may be a first-line choice in patients with Parkinson’s disease and Lewy body dementia.
Ziprasidone is a benzothiazolylpiperazine indicated for treatment of psychosis. Experience with its use in older patients is limited. Ziprasidone should be avoided in patients with significant cardiovascular disease because of its potential to cause QT prolongation and cardiac arrhythmias. Although rare, this cardiac side effect may be life-threatening, and clinicians must be exceptionally vigilant when using ziprasidone in older patients. Risk factors for QT prolongation include older age, female sex, pre-existing cardiac disease, hypokalemia, and hypomagnesemia.
Ziprasidone can be somewhat sedating. An IM preparation is under development but has not been approved for use by the FDA
Neuroleptics and other options
Occasionally a typical neuroleptic may be the most appropriate first-line drug for older patients with psychotic symptoms. For example, a mid-potency neuroleptic such as perphenazine at an IM dose of 2 to 4 mg may be considered when severe agitation and aggression pose a substantial safety risk for the patient or caregiver and require rapid control. Haloperidol, despite its widespread use in both acute and long-term settings, should be used with caution because it has great potential for causing EPS and can immobilize an older patient, resulting in further functional decline. Regardless of which typical neuroleptic is used, switch the patient to an atypical antipsychotic as soon as agitation is under control.
Antidepressants Affective psychoses in older patients may require the addition of an antidepressant to the antipsychotic drug. The selective serotonin reuptake inhibitors fluoxetine and sertraline have proven track records for efficacy and safety and should be considered first-line agents.
Other options Electroconvulsive therapy is safe and effective for older patients with psychotic depression or late-life mania. Late-life mania also may respond well to the anticonvulsant divalproex sodium. Avoid anxiolytics in older patients because of the potential of these agents to cause sedation or disinhibition and their associated risk of falls and confusion. Buspirone in higher dosages (40 to 60 mg/d) can sometimes help manage chronic anxiety states.
Related resource
- Sadavoy J, Lazarus LW, Jarvik LF, Grossman GT (eds). Comprehensive review of geriatric psychiatry (2nd ed). Washington, DC: American Psychiatric Press, 1996.
Drug brand names
- Buspirone • Buspar
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluoxetine • Prozac
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
Dr. Snow reports no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
Dr. Verma reports that he is on the speakers’ bureau of Eli Lilly and Co. and Abbott Laboratories, serves as a consultant to Eli Lilly and Co., and receives grant support from Eli Lilly and Co. and GlaxoSmithKline.
1. Hargreaves WA, Shumway M. Pharmacoeconomics of drug therapy. J Clin Psych 1996;57(suppl 9):66-76.
2. Lieberman J, Chakos M, Wu H, Alvir J, et al. Longitudinal study of brain morphology in first-episode schizophrenia. Biol Psych 2001;49(6):487-99.
3. Patterson TL, Shaw W, Semple SJ, et al. Health-related quality of life in older patients with schizophrenia and other psychoses: relationships among psychosocial and psychiatric factors. Int J Geriatr Psychiatry 1997;12(4):452-61.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull 1988;14:39-55.
5. Kraeplin E. Dementia, praecox, and paraphrenia (1919). Translated by Barclay RM. Huntingdon NY: Krieger, 1971
6. Bleuler E. Late schizophrenic clinical pictures. Fortschr Neurol Psych 1943;15:259-90.
7. Pearlson G, Rabins P. The late-onset psychoses: possible risk factors. Psychiatr Clin North Am 1988;11(1):15-32.
8. Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer’s type. Biol Psychiatry 1989;25:39-48.
9. Wragg R, Jeste DV. Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry 1989;146:577-87.
10. Lesser IM. Late-onset psychotic disorder not otherwise specified: clinical and neuroimaging findings. Biol Psychiatry 1992;31:419-23.
11. Lesser IM, Miller BL, Swartz JR, et al. Brain imaging in late-life schizophrenia and related psychoses. Schizophr Bull 1993;19:419-23.
12. Jeste DV, Heaton SC, Paulsen JS, Ercoli L, Harris J, Heaton RK. Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry 1996;153(4):490-6.
13. Burns BJ, Larson DB, Goldstrom ID, et al. Mental disorders among nursing home patients: preliminary findings from the National Nursing Home Survey pretest. Int J Geriatr Psychiatry 1988;3:27-35.
14. Schneider LS, Pollock VE, Lyness SA. A meta-analysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc 1990;38:553-63.
15. Popli AP, Konicki PE, Jurjus GJ, et al. Clozapine and associated diabetes mellitus. J Clin Psychiatry 1997;58:108-11.
16. Bergemann N, Ehrig C, Diebold K, Mundt C, von Einsiedel R. Asymptomatic pancreatitis associated with clozapine. Pharmacopsychiatry 1999;32(2):78-80.
17. Katz IR, Jeste DV, Mintzer JE, et al. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psych 1999;60:107-15.
18. Petty RG. Prolactin and antipsychotic medications: mechanisms of action. Schizophr Res 1999;35(suppl):S67-S73.
19. Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res 1999;35(suppl):575-86.
20. Zarate CA, Baldessarini RJ, Siegel AJ, et al:. Risperidone in the elderly: a pharmacoepidemiological study. J Clin Psychiatry 1997;58:311-17.
21. Street JS, Clark WS, Gannon K, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer’s disease in nursing care facilities: a double-blind, placebo-controlled trial. Arch Gen Psychiatry 2000;57(10):968-76.
1. Hargreaves WA, Shumway M. Pharmacoeconomics of drug therapy. J Clin Psych 1996;57(suppl 9):66-76.
2. Lieberman J, Chakos M, Wu H, Alvir J, et al. Longitudinal study of brain morphology in first-episode schizophrenia. Biol Psych 2001;49(6):487-99.
3. Patterson TL, Shaw W, Semple SJ, et al. Health-related quality of life in older patients with schizophrenia and other psychoses: relationships among psychosocial and psychiatric factors. Int J Geriatr Psychiatry 1997;12(4):452-61.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull 1988;14:39-55.
5. Kraeplin E. Dementia, praecox, and paraphrenia (1919). Translated by Barclay RM. Huntingdon NY: Krieger, 1971
6. Bleuler E. Late schizophrenic clinical pictures. Fortschr Neurol Psych 1943;15:259-90.
7. Pearlson G, Rabins P. The late-onset psychoses: possible risk factors. Psychiatr Clin North Am 1988;11(1):15-32.
8. Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer’s type. Biol Psychiatry 1989;25:39-48.
9. Wragg R, Jeste DV. Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry 1989;146:577-87.
10. Lesser IM. Late-onset psychotic disorder not otherwise specified: clinical and neuroimaging findings. Biol Psychiatry 1992;31:419-23.
11. Lesser IM, Miller BL, Swartz JR, et al. Brain imaging in late-life schizophrenia and related psychoses. Schizophr Bull 1993;19:419-23.
12. Jeste DV, Heaton SC, Paulsen JS, Ercoli L, Harris J, Heaton RK. Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry 1996;153(4):490-6.
13. Burns BJ, Larson DB, Goldstrom ID, et al. Mental disorders among nursing home patients: preliminary findings from the National Nursing Home Survey pretest. Int J Geriatr Psychiatry 1988;3:27-35.
14. Schneider LS, Pollock VE, Lyness SA. A meta-analysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc 1990;38:553-63.
15. Popli AP, Konicki PE, Jurjus GJ, et al. Clozapine and associated diabetes mellitus. J Clin Psychiatry 1997;58:108-11.
16. Bergemann N, Ehrig C, Diebold K, Mundt C, von Einsiedel R. Asymptomatic pancreatitis associated with clozapine. Pharmacopsychiatry 1999;32(2):78-80.
17. Katz IR, Jeste DV, Mintzer JE, et al. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psych 1999;60:107-15.
18. Petty RG. Prolactin and antipsychotic medications: mechanisms of action. Schizophr Res 1999;35(suppl):S67-S73.
19. Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res 1999;35(suppl):575-86.
20. Zarate CA, Baldessarini RJ, Siegel AJ, et al:. Risperidone in the elderly: a pharmacoepidemiological study. J Clin Psychiatry 1997;58:311-17.
21. Street JS, Clark WS, Gannon K, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer’s disease in nursing care facilities: a double-blind, placebo-controlled trial. Arch Gen Psychiatry 2000;57(10):968-76.
Late-life psychosis: It’s efficacy vs. cost in the tug-of-war over treatment
As psychiatrists, we are being pulled in opposite directions between conflicting goals: to reduce health care costs and to provide our patients with the sophistication and specificity of newly available—and more expensive—biological therapies. In the treatment of late-life psychosis, the evidence is impressive and persuasive that atypical antipsychotic drugs are worth the investment.
Atypical antipsychotics should usually be considered first-line therapy for late-life psychosis, though they cost more than the older neuroleptics. Overall, drugs comprise a minor portion of the cost of treating psychosis but may have a major impact on outcomes.1 With their safer side effect profile, the atypicals have enabled many patients with schizophrenia to “reintegrate” and become contributing members of society. Likewise, the use of atypicals can allow older patients with psychotic symptoms to function longer and more productively in their homes and communities.
Table 1
DIFFERENCES BETWEEN LATE-AND EARLY-ONSET SCHIZOPHRENIA
| Characteristic | Late-onset (age 45 and older) | Early-onset (before age 45) |
|---|---|---|
| Gender | More women | More men |
| Type | Paranoid | Varies |
| Positive symptoms | Severe | Severe |
| Negative symptoms | Less severe | More severe |
| Duration of illness | Chronic | Chronic |
| Cognition | Less impaired | More impaired |
| Neuroimaging | Nonspecific changes | Nonspecific changes |
| Antipsychotic dose | Lower | Higher |
| Mortality rates | High | High |
| Premorbid function | Good | Schizoid traits |
Without appropriate treatment of psychosis in younger patients, we know that recurrences and relapses can cause demonstrable brain changes and lead to residual symptoms.2 Older patients have been shown to lose 0.2 “well years” for every year they have psychotic symptoms.3
One illness or many?
Psychotic symptoms can occur in late life for a variety of reasons, and each diagnosis has different implications for patient work-up and treatment. Causes of psychosis in older patients include late-onset schizophrenia, dementia, affective disorder, delusional disorder, and delirium.
Late-life schizophrenia As life expectancy increases, schizophrenia is increasingly being diagnosed in older persons. In a sample of hospitalized patients with schizophrenia, onset of illness occurred after age 50 in 13%, after age 60 in 7%, and after age 70 in 3%.4 The clinical features of schizophrenia may be modified by the concurrent development of dementia. Thus, this reported increase in psychosis after age 50 may be secondary to an increased incidence of dementia, Parkinson’s disease, cerebrovascular events, and neoplasms.
Early definitions of “paraphrenia” and later schizophrenia described delusions and hallucinations that developed without disturbance of affect, with onset in early adult life.5 The occurrence of disorganized behavior and thinking in late life was ascribed to the effects of “senility” or other organic factors.
In 1943, Bleuler6 reported on a series of 126 patients in whom psychosis developed after age 40. In this group, the illness began after age 60 in 4%.
Current diagnostic criteria for schizophrenia do not exclude or categorize individuals on the basis of age. DSMIV does acknowledge a subgroup of patients with “late-onset” schizophrenia after age 45. However, there are important clinical differences in the presentations of the early- and late-onset types (Table 1). For example, even after adjusting for the greater longevity of women, more women than men develop late-onset schizophrenia. Also, compared with the early-onset type, in late-life schizophrenia:
- Premorbid paranoid or schizoid personality traits appear to be much less prominent.
- Patients may have had better occupational functioning and are more likely to have been married.
- Negative symptoms tend to be less severe, although they do contribute to functional decline.
Visual and hearing loss are among the risk factors correlated with late-onset schizophrenia.7 Sensory loss isolates an older person and leads to misinterpretation and misidentification of environmental cues. Other risk factors, in addition to female gender, include cognitive loss, poor social supports, living alone, and alcohol or drug abuse.
Dementia Between 20 and 50% of patients with vascular and mixed dementias exhibit psychotic symptoms.8 Among those with Alzheimer’s dementia, 30% exhibit persecutory delusions.9 Adding to this population are persons with early-onset schizophrenia, who are living longer and can also develop dementia.
While neuroimaging can sometimes aid in diagnosis, abnormalities seen on neuroimaging of patients with psychotic symptoms do not necessarily correlate with cognitive deficits. The clinical significance of these findings is unclear.10,11 Because of differences in therapy, it is important to establish whether the primary diagnosis is dementia or schizophrenia (Table 2).
Affective psychoses Late-life depression and mania are often unrecognized because of atypical presentations. In the elderly, depression may present with withdrawal, mood-incongruent delusions,12 and symptoms that mimic medical illness. Older manic patients may be misidentified as intrusive, hypersexual, or agitated. A high suspicion index, carefully elicited family history, and past psychiatric illness in the patient usually can clarify the diagnosis.
Delusional disorder Suspiciousness and paranoia are common findings in late life, with an estimated prevalence of 4 to 6% in the older population. Patients with delusional disorder show little evidence of cognitive deficits and—unlike those with schizophrenia—continue to maintain a high level of function.13 Delusions are nonbizarre and well systematized, and hallucinations are not a prominent feature.
Delirium Older persons are particularly vulnerable to developing delirium. Common causes include urinary tract infection, bowel impaction, heart failure, endocrinopathies, and drug toxicity caused by prescribed or over-the-counter drugs. CNS conditions such as subdural hematoma, cerebrovascular accident, slow-growing intracranial tumors, and encephalitis can be associated with the development of hallucinations and delusions without clouding consciousness.
While an agitated, hallucinating patient with clearly evident fluctuations in the sensorium may be readily diagnosed with a delirium, patients who are quiet, withdrawn, and apathetic may be less easy to recognize. Clinicians should therefore be vigilant for both the hyperkinetic and hypokinetic presentations of delirium when assessing older patients.
Work-up
When psychotic symptoms present for the first time in late life, medical causes must be ruled out first. A detailed history, including collateral information from other sources, includes:
- premorbid function
- psychiatric history, including history of affective illness
- family history of schizophrenia, affective illness, and Alzheimer’s disease
- medical history, including risks for cerebrovascular disease.
The examination should include a complete medical and neurologic evaluation, giving special attention to sensory loss, prescribed and over-the-counter drugs, recent changes in drug regimen, and cognitive screening. Laboratory work-up includes:
- in all cases: CBC/differential, BUN, TFT, B12/folate, and RPR
- in selected cases: MRI, HIV screening, toxicology screen, neuropsychological testing
- in rare cases: lumbar puncture, PET or SPECT imaging.
If the suggested work-up leads to diagnosis of medical illness, begin appropriate treatment. Behavioral symptoms during medical illness, such as with delirium, may require concurrent psychotropic medication until the medical illness is controlled.
If the medical work-up is negative, the clinician then needs to make the appropriate psychiatric diagnosis and institute antipsychotic treatment as indicated.
Table 2
CLINICAL DIFFERENCES BETWEEN DEMENTIA AND SCHIZOPHRENIA
| Characteristic | Dementia | Late-onset schizophrenia |
|---|---|---|
| Incidence | 50 to 70% | 1 to 2% |
| Bizarre delusions | Uncommon | Common |
| Hallucinations | Usually visual | Usually auditory |
| History of psychosis | Rare | Common |
| Misidentification | Common | Less common |
| Maintenance treatment | Usually unnecessary | Common |
| Antipsychotic dosage | 25% of dosage for adult schizophrenia | 50% of dosage for adult schizophrenia |
Table 3
USE OF ATYPICAL ANTIPSYCHOTICS IN OLDER PATIENTS
| Drug | Administration |
|---|---|
| Clozapine | Use limited by potential for agranulocytosis, need for weekly blood counts Higher risk for diabetes, hyperglycemia, pancreatitis Very sedating “Black box” warning for myocarditis (March 2002) NOT a first-choice drug for older persons |
| Risperidone | Usual geriatric dosage 1 to 2 mg/d Risk of EPS increases significantly with dosages > 2 mg/d Can cause persistent hyperprolactinemia |
| Olanzapine | Usual geriatric dosage 2.5 to 12.5 mg/d (qd dosing) Excellent choice as first-line drug in older patients Anticholinergic side effects ordinarily not a problem in vivo despite receptor profile of M1-M5 antagonism May be weight-neutral in older patients |
| Quetiapine | Geriatric dosage 25 to 400 mg/d Very low potential for EPS Can be a first choice for patients with Lewy body dementia and Parkinson’s disease Sedation increases with dosages > 200 mg/d |
| Ziprasidone | Geriatric dosage 40 to 160 mg/d Significant concern about QT prolongation in older patients, especially women and those with pre-existing cardiac disease, chronic mental illness, hypokalemia, and hypomagnesemia Not recommended as first-line therapy in elderly (“bold” warning) Not approved for IM use in United States; restrictions on use in many European countries |
| EPS: Extrapyramidal symptoms | |
Prescribing antipsychotics
Prescribing psychoactive medications for older patients is similar to pediatric prescribing. You need to distinguish between behaviors that are “disturbed” as a result of a psychotic process or “disturbing,” for which behavioral interventions may be more appropriate. In any case, pharmacologic and behavioral interventions are used concurrently.
When prescribing for older patients, consider the possible interaction of comorbid medical conditions and altered pharmacokinetics and pharmacodynamics, which increase the potential for drug-drug interactions. Start low and go slow to minimize the potential for adverse side effects and to ensure optimal preservation of function.
In general, atypical antipsychotics are considered first-line therapy, unless there is a compelling reason not to use them in an individual patient. Conventional neuroleptic antipsychotics carry a much higher risk of adverse drug reactions and functional loss without providing substantially greater efficacy.14 Atypical antipsychotics differ not only from the older neuroleptics but also from each other—including their safety profiles for use in older patients (Table 3).
Clozapine is a dibenzodiazepine that is indicated for treatment-resistant schizophrenia. The first atypical antipsychotic, clozapine significantly reduced the incidence of extrapyramidal symptoms (EPS) seen with the neuroleptic antipsychotics. Clozapine also was effective against the “negative” symptoms of schizophrenia such as apathy, anhedonia, and emotional blunting, which severely limited the ability of patients with schizophrenia to function in society.
The limitations of clozapine include its potential to cause agranulocytosis and the requirement for weekly blood tests. It is also fairly sedating. A number of reports have suggested a link between the use of clozapine and the development of type 2 diabetes, hyperglycemia, and pancreatitis.15,16 Earlier this year, the FDA issued a “black box” warning linking the use of clozapine with myocarditis. For all of these reasons, clozapine is not recommended for use in older patients.
Risperidone is a benzisoxazole derivative indicated for treatment of psychotic disorders. Risperidone’s antipsychotic efficacy is equivalent to that of haloperidol, but it has a much safer side-effect profile. In one multicenter study comparing risperidone and a placebo at single daily dosages of 0.5, 1.0, and 2.0 mg, risperidone was found to be effective in controlling the psychosis and behavioral disturbance associated with dementia. The authors recommended using a dosage of 1.0 mg/d because higher dosages resulted in excessive sedation and EPS.17
Risk of side effects is dose-dependent, especially in the older patient. Risperidone is the only atypical antipsychotic associated with persistent hyperprolactinemia, which may indirectly contribute to increased osteoporosis and atherogenesis.18,19 Orthostatic hypotension, especially with initial dosages greater than 1.5 mg/d and rapid dose escalation (≥ 25% every 24 to 48 hours), may also limit its use in older patients.
Olanzapine is a thiobenzodiazepine derivative indicated for treatment of psychosis and acute bipolar mania. Olanzapine has a receptor profile that is somewhat analogous with that of clozapine. Its antipsychotic efficacy is equivalent to that of risperidone, but it has a more favorable safety profile. Like risperidone, olanzapine is relatively nonsedating, but it is significantly less likely to cause EPS and orthostatic hypotension, and its use is not associated with persistent hyperprolactinemia.
Based on its vitro muscarinic receptor antagonism profile, some clinicians incorrectly assume that olanzapine is highly anticholinergic. In vivo data and clinical experience have not borne out this contention.20,21 Weight gain with olanzapine is relatively infrequent in older persons. A rapid-dissolve preparation can be useful in resistant and noncompliant patients. An IM preparation has been approved by the FDA but has not yet been made available by the manufacturer.
Quetiapine is a dibenzothiazepine derivative indicated for treatment of psychotic disorders. Despite limited data on its efficacy in late-life psychosis, clinical experience would suggest that its antipsychotic efficacy would at least equal that of other drugs in this category.
The need for twice-daily dosing and titration to a therapeutic response can sometimes limit the use of quetiapine. Sedation can be a problem at dosages greater than 200 mg/d. Because quetiapine has a relatively lower potential among the antipsychotics to cause EPS, it may be a first-line choice in patients with Parkinson’s disease and Lewy body dementia.
Ziprasidone is a benzothiazolylpiperazine indicated for treatment of psychosis. Experience with its use in older patients is limited. Ziprasidone should be avoided in patients with significant cardiovascular disease because of its potential to cause QT prolongation and cardiac arrhythmias. Although rare, this cardiac side effect may be life-threatening, and clinicians must be exceptionally vigilant when using ziprasidone in older patients. Risk factors for QT prolongation include older age, female sex, pre-existing cardiac disease, hypokalemia, and hypomagnesemia.
Ziprasidone can be somewhat sedating. An IM preparation is under development but has not been approved for use by the FDA
Neuroleptics and other options
Occasionally a typical neuroleptic may be the most appropriate first-line drug for older patients with psychotic symptoms. For example, a mid-potency neuroleptic such as perphenazine at an IM dose of 2 to 4 mg may be considered when severe agitation and aggression pose a substantial safety risk for the patient or caregiver and require rapid control. Haloperidol, despite its widespread use in both acute and long-term settings, should be used with caution because it has great potential for causing EPS and can immobilize an older patient, resulting in further functional decline. Regardless of which typical neuroleptic is used, switch the patient to an atypical antipsychotic as soon as agitation is under control.
Antidepressants Affective psychoses in older patients may require the addition of an antidepressant to the antipsychotic drug. The selective serotonin reuptake inhibitors fluoxetine and sertraline have proven track records for efficacy and safety and should be considered first-line agents.
Other options Electroconvulsive therapy is safe and effective for older patients with psychotic depression or late-life mania. Late-life mania also may respond well to the anticonvulsant divalproex sodium. Avoid anxiolytics in older patients because of the potential of these agents to cause sedation or disinhibition and their associated risk of falls and confusion. Buspirone in higher dosages (40 to 60 mg/d) can sometimes help manage chronic anxiety states.
Related resource
- Sadavoy J, Lazarus LW, Jarvik LF, Grossman GT (eds). Comprehensive review of geriatric psychiatry (2nd ed). Washington, DC: American Psychiatric Press, 1996.
Drug brand names
- Buspirone • Buspar
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluoxetine • Prozac
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
Dr. Snow reports no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
Dr. Verma reports that he is on the speakers’ bureau of Eli Lilly and Co. and Abbott Laboratories, serves as a consultant to Eli Lilly and Co., and receives grant support from Eli Lilly and Co. and GlaxoSmithKline.
1. Hargreaves WA, Shumway M. Pharmacoeconomics of drug therapy. J Clin Psych 1996;57(suppl 9):66-76.
2. Lieberman J, Chakos M, Wu H, Alvir J, et al. Longitudinal study of brain morphology in first-episode schizophrenia. Biol Psych 2001;49(6):487-99.
3. Patterson TL, Shaw W, Semple SJ, et al. Health-related quality of life in older patients with schizophrenia and other psychoses: relationships among psychosocial and psychiatric factors. Int J Geriatr Psychiatry 1997;12(4):452-61.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull 1988;14:39-55.
5. Kraeplin E. Dementia, praecox, and paraphrenia (1919). Translated by Barclay RM. Huntingdon NY: Krieger, 1971
6. Bleuler E. Late schizophrenic clinical pictures. Fortschr Neurol Psych 1943;15:259-90.
7. Pearlson G, Rabins P. The late-onset psychoses: possible risk factors. Psychiatr Clin North Am 1988;11(1):15-32.
8. Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer’s type. Biol Psychiatry 1989;25:39-48.
9. Wragg R, Jeste DV. Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry 1989;146:577-87.
10. Lesser IM. Late-onset psychotic disorder not otherwise specified: clinical and neuroimaging findings. Biol Psychiatry 1992;31:419-23.
11. Lesser IM, Miller BL, Swartz JR, et al. Brain imaging in late-life schizophrenia and related psychoses. Schizophr Bull 1993;19:419-23.
12. Jeste DV, Heaton SC, Paulsen JS, Ercoli L, Harris J, Heaton RK. Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry 1996;153(4):490-6.
13. Burns BJ, Larson DB, Goldstrom ID, et al. Mental disorders among nursing home patients: preliminary findings from the National Nursing Home Survey pretest. Int J Geriatr Psychiatry 1988;3:27-35.
14. Schneider LS, Pollock VE, Lyness SA. A meta-analysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc 1990;38:553-63.
15. Popli AP, Konicki PE, Jurjus GJ, et al. Clozapine and associated diabetes mellitus. J Clin Psychiatry 1997;58:108-11.
16. Bergemann N, Ehrig C, Diebold K, Mundt C, von Einsiedel R. Asymptomatic pancreatitis associated with clozapine. Pharmacopsychiatry 1999;32(2):78-80.
17. Katz IR, Jeste DV, Mintzer JE, et al. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psych 1999;60:107-15.
18. Petty RG. Prolactin and antipsychotic medications: mechanisms of action. Schizophr Res 1999;35(suppl):S67-S73.
19. Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res 1999;35(suppl):575-86.
20. Zarate CA, Baldessarini RJ, Siegel AJ, et al:. Risperidone in the elderly: a pharmacoepidemiological study. J Clin Psychiatry 1997;58:311-17.
21. Street JS, Clark WS, Gannon K, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer’s disease in nursing care facilities: a double-blind, placebo-controlled trial. Arch Gen Psychiatry 2000;57(10):968-76.
As psychiatrists, we are being pulled in opposite directions between conflicting goals: to reduce health care costs and to provide our patients with the sophistication and specificity of newly available—and more expensive—biological therapies. In the treatment of late-life psychosis, the evidence is impressive and persuasive that atypical antipsychotic drugs are worth the investment.
Atypical antipsychotics should usually be considered first-line therapy for late-life psychosis, though they cost more than the older neuroleptics. Overall, drugs comprise a minor portion of the cost of treating psychosis but may have a major impact on outcomes.1 With their safer side effect profile, the atypicals have enabled many patients with schizophrenia to “reintegrate” and become contributing members of society. Likewise, the use of atypicals can allow older patients with psychotic symptoms to function longer and more productively in their homes and communities.
Table 1
DIFFERENCES BETWEEN LATE-AND EARLY-ONSET SCHIZOPHRENIA
| Characteristic | Late-onset (age 45 and older) | Early-onset (before age 45) |
|---|---|---|
| Gender | More women | More men |
| Type | Paranoid | Varies |
| Positive symptoms | Severe | Severe |
| Negative symptoms | Less severe | More severe |
| Duration of illness | Chronic | Chronic |
| Cognition | Less impaired | More impaired |
| Neuroimaging | Nonspecific changes | Nonspecific changes |
| Antipsychotic dose | Lower | Higher |
| Mortality rates | High | High |
| Premorbid function | Good | Schizoid traits |
Without appropriate treatment of psychosis in younger patients, we know that recurrences and relapses can cause demonstrable brain changes and lead to residual symptoms.2 Older patients have been shown to lose 0.2 “well years” for every year they have psychotic symptoms.3
One illness or many?
Psychotic symptoms can occur in late life for a variety of reasons, and each diagnosis has different implications for patient work-up and treatment. Causes of psychosis in older patients include late-onset schizophrenia, dementia, affective disorder, delusional disorder, and delirium.
Late-life schizophrenia As life expectancy increases, schizophrenia is increasingly being diagnosed in older persons. In a sample of hospitalized patients with schizophrenia, onset of illness occurred after age 50 in 13%, after age 60 in 7%, and after age 70 in 3%.4 The clinical features of schizophrenia may be modified by the concurrent development of dementia. Thus, this reported increase in psychosis after age 50 may be secondary to an increased incidence of dementia, Parkinson’s disease, cerebrovascular events, and neoplasms.
Early definitions of “paraphrenia” and later schizophrenia described delusions and hallucinations that developed without disturbance of affect, with onset in early adult life.5 The occurrence of disorganized behavior and thinking in late life was ascribed to the effects of “senility” or other organic factors.
In 1943, Bleuler6 reported on a series of 126 patients in whom psychosis developed after age 40. In this group, the illness began after age 60 in 4%.
Current diagnostic criteria for schizophrenia do not exclude or categorize individuals on the basis of age. DSMIV does acknowledge a subgroup of patients with “late-onset” schizophrenia after age 45. However, there are important clinical differences in the presentations of the early- and late-onset types (Table 1). For example, even after adjusting for the greater longevity of women, more women than men develop late-onset schizophrenia. Also, compared with the early-onset type, in late-life schizophrenia:
- Premorbid paranoid or schizoid personality traits appear to be much less prominent.
- Patients may have had better occupational functioning and are more likely to have been married.
- Negative symptoms tend to be less severe, although they do contribute to functional decline.
Visual and hearing loss are among the risk factors correlated with late-onset schizophrenia.7 Sensory loss isolates an older person and leads to misinterpretation and misidentification of environmental cues. Other risk factors, in addition to female gender, include cognitive loss, poor social supports, living alone, and alcohol or drug abuse.
Dementia Between 20 and 50% of patients with vascular and mixed dementias exhibit psychotic symptoms.8 Among those with Alzheimer’s dementia, 30% exhibit persecutory delusions.9 Adding to this population are persons with early-onset schizophrenia, who are living longer and can also develop dementia.
While neuroimaging can sometimes aid in diagnosis, abnormalities seen on neuroimaging of patients with psychotic symptoms do not necessarily correlate with cognitive deficits. The clinical significance of these findings is unclear.10,11 Because of differences in therapy, it is important to establish whether the primary diagnosis is dementia or schizophrenia (Table 2).
Affective psychoses Late-life depression and mania are often unrecognized because of atypical presentations. In the elderly, depression may present with withdrawal, mood-incongruent delusions,12 and symptoms that mimic medical illness. Older manic patients may be misidentified as intrusive, hypersexual, or agitated. A high suspicion index, carefully elicited family history, and past psychiatric illness in the patient usually can clarify the diagnosis.
Delusional disorder Suspiciousness and paranoia are common findings in late life, with an estimated prevalence of 4 to 6% in the older population. Patients with delusional disorder show little evidence of cognitive deficits and—unlike those with schizophrenia—continue to maintain a high level of function.13 Delusions are nonbizarre and well systematized, and hallucinations are not a prominent feature.
Delirium Older persons are particularly vulnerable to developing delirium. Common causes include urinary tract infection, bowel impaction, heart failure, endocrinopathies, and drug toxicity caused by prescribed or over-the-counter drugs. CNS conditions such as subdural hematoma, cerebrovascular accident, slow-growing intracranial tumors, and encephalitis can be associated with the development of hallucinations and delusions without clouding consciousness.
While an agitated, hallucinating patient with clearly evident fluctuations in the sensorium may be readily diagnosed with a delirium, patients who are quiet, withdrawn, and apathetic may be less easy to recognize. Clinicians should therefore be vigilant for both the hyperkinetic and hypokinetic presentations of delirium when assessing older patients.
Work-up
When psychotic symptoms present for the first time in late life, medical causes must be ruled out first. A detailed history, including collateral information from other sources, includes:
- premorbid function
- psychiatric history, including history of affective illness
- family history of schizophrenia, affective illness, and Alzheimer’s disease
- medical history, including risks for cerebrovascular disease.
The examination should include a complete medical and neurologic evaluation, giving special attention to sensory loss, prescribed and over-the-counter drugs, recent changes in drug regimen, and cognitive screening. Laboratory work-up includes:
- in all cases: CBC/differential, BUN, TFT, B12/folate, and RPR
- in selected cases: MRI, HIV screening, toxicology screen, neuropsychological testing
- in rare cases: lumbar puncture, PET or SPECT imaging.
If the suggested work-up leads to diagnosis of medical illness, begin appropriate treatment. Behavioral symptoms during medical illness, such as with delirium, may require concurrent psychotropic medication until the medical illness is controlled.
If the medical work-up is negative, the clinician then needs to make the appropriate psychiatric diagnosis and institute antipsychotic treatment as indicated.
Table 2
CLINICAL DIFFERENCES BETWEEN DEMENTIA AND SCHIZOPHRENIA
| Characteristic | Dementia | Late-onset schizophrenia |
|---|---|---|
| Incidence | 50 to 70% | 1 to 2% |
| Bizarre delusions | Uncommon | Common |
| Hallucinations | Usually visual | Usually auditory |
| History of psychosis | Rare | Common |
| Misidentification | Common | Less common |
| Maintenance treatment | Usually unnecessary | Common |
| Antipsychotic dosage | 25% of dosage for adult schizophrenia | 50% of dosage for adult schizophrenia |
Table 3
USE OF ATYPICAL ANTIPSYCHOTICS IN OLDER PATIENTS
| Drug | Administration |
|---|---|
| Clozapine | Use limited by potential for agranulocytosis, need for weekly blood counts Higher risk for diabetes, hyperglycemia, pancreatitis Very sedating “Black box” warning for myocarditis (March 2002) NOT a first-choice drug for older persons |
| Risperidone | Usual geriatric dosage 1 to 2 mg/d Risk of EPS increases significantly with dosages > 2 mg/d Can cause persistent hyperprolactinemia |
| Olanzapine | Usual geriatric dosage 2.5 to 12.5 mg/d (qd dosing) Excellent choice as first-line drug in older patients Anticholinergic side effects ordinarily not a problem in vivo despite receptor profile of M1-M5 antagonism May be weight-neutral in older patients |
| Quetiapine | Geriatric dosage 25 to 400 mg/d Very low potential for EPS Can be a first choice for patients with Lewy body dementia and Parkinson’s disease Sedation increases with dosages > 200 mg/d |
| Ziprasidone | Geriatric dosage 40 to 160 mg/d Significant concern about QT prolongation in older patients, especially women and those with pre-existing cardiac disease, chronic mental illness, hypokalemia, and hypomagnesemia Not recommended as first-line therapy in elderly (“bold” warning) Not approved for IM use in United States; restrictions on use in many European countries |
| EPS: Extrapyramidal symptoms | |
Prescribing antipsychotics
Prescribing psychoactive medications for older patients is similar to pediatric prescribing. You need to distinguish between behaviors that are “disturbed” as a result of a psychotic process or “disturbing,” for which behavioral interventions may be more appropriate. In any case, pharmacologic and behavioral interventions are used concurrently.
When prescribing for older patients, consider the possible interaction of comorbid medical conditions and altered pharmacokinetics and pharmacodynamics, which increase the potential for drug-drug interactions. Start low and go slow to minimize the potential for adverse side effects and to ensure optimal preservation of function.
In general, atypical antipsychotics are considered first-line therapy, unless there is a compelling reason not to use them in an individual patient. Conventional neuroleptic antipsychotics carry a much higher risk of adverse drug reactions and functional loss without providing substantially greater efficacy.14 Atypical antipsychotics differ not only from the older neuroleptics but also from each other—including their safety profiles for use in older patients (Table 3).
Clozapine is a dibenzodiazepine that is indicated for treatment-resistant schizophrenia. The first atypical antipsychotic, clozapine significantly reduced the incidence of extrapyramidal symptoms (EPS) seen with the neuroleptic antipsychotics. Clozapine also was effective against the “negative” symptoms of schizophrenia such as apathy, anhedonia, and emotional blunting, which severely limited the ability of patients with schizophrenia to function in society.
The limitations of clozapine include its potential to cause agranulocytosis and the requirement for weekly blood tests. It is also fairly sedating. A number of reports have suggested a link between the use of clozapine and the development of type 2 diabetes, hyperglycemia, and pancreatitis.15,16 Earlier this year, the FDA issued a “black box” warning linking the use of clozapine with myocarditis. For all of these reasons, clozapine is not recommended for use in older patients.
Risperidone is a benzisoxazole derivative indicated for treatment of psychotic disorders. Risperidone’s antipsychotic efficacy is equivalent to that of haloperidol, but it has a much safer side-effect profile. In one multicenter study comparing risperidone and a placebo at single daily dosages of 0.5, 1.0, and 2.0 mg, risperidone was found to be effective in controlling the psychosis and behavioral disturbance associated with dementia. The authors recommended using a dosage of 1.0 mg/d because higher dosages resulted in excessive sedation and EPS.17
Risk of side effects is dose-dependent, especially in the older patient. Risperidone is the only atypical antipsychotic associated with persistent hyperprolactinemia, which may indirectly contribute to increased osteoporosis and atherogenesis.18,19 Orthostatic hypotension, especially with initial dosages greater than 1.5 mg/d and rapid dose escalation (≥ 25% every 24 to 48 hours), may also limit its use in older patients.
Olanzapine is a thiobenzodiazepine derivative indicated for treatment of psychosis and acute bipolar mania. Olanzapine has a receptor profile that is somewhat analogous with that of clozapine. Its antipsychotic efficacy is equivalent to that of risperidone, but it has a more favorable safety profile. Like risperidone, olanzapine is relatively nonsedating, but it is significantly less likely to cause EPS and orthostatic hypotension, and its use is not associated with persistent hyperprolactinemia.
Based on its vitro muscarinic receptor antagonism profile, some clinicians incorrectly assume that olanzapine is highly anticholinergic. In vivo data and clinical experience have not borne out this contention.20,21 Weight gain with olanzapine is relatively infrequent in older persons. A rapid-dissolve preparation can be useful in resistant and noncompliant patients. An IM preparation has been approved by the FDA but has not yet been made available by the manufacturer.
Quetiapine is a dibenzothiazepine derivative indicated for treatment of psychotic disorders. Despite limited data on its efficacy in late-life psychosis, clinical experience would suggest that its antipsychotic efficacy would at least equal that of other drugs in this category.
The need for twice-daily dosing and titration to a therapeutic response can sometimes limit the use of quetiapine. Sedation can be a problem at dosages greater than 200 mg/d. Because quetiapine has a relatively lower potential among the antipsychotics to cause EPS, it may be a first-line choice in patients with Parkinson’s disease and Lewy body dementia.
Ziprasidone is a benzothiazolylpiperazine indicated for treatment of psychosis. Experience with its use in older patients is limited. Ziprasidone should be avoided in patients with significant cardiovascular disease because of its potential to cause QT prolongation and cardiac arrhythmias. Although rare, this cardiac side effect may be life-threatening, and clinicians must be exceptionally vigilant when using ziprasidone in older patients. Risk factors for QT prolongation include older age, female sex, pre-existing cardiac disease, hypokalemia, and hypomagnesemia.
Ziprasidone can be somewhat sedating. An IM preparation is under development but has not been approved for use by the FDA
Neuroleptics and other options
Occasionally a typical neuroleptic may be the most appropriate first-line drug for older patients with psychotic symptoms. For example, a mid-potency neuroleptic such as perphenazine at an IM dose of 2 to 4 mg may be considered when severe agitation and aggression pose a substantial safety risk for the patient or caregiver and require rapid control. Haloperidol, despite its widespread use in both acute and long-term settings, should be used with caution because it has great potential for causing EPS and can immobilize an older patient, resulting in further functional decline. Regardless of which typical neuroleptic is used, switch the patient to an atypical antipsychotic as soon as agitation is under control.
Antidepressants Affective psychoses in older patients may require the addition of an antidepressant to the antipsychotic drug. The selective serotonin reuptake inhibitors fluoxetine and sertraline have proven track records for efficacy and safety and should be considered first-line agents.
Other options Electroconvulsive therapy is safe and effective for older patients with psychotic depression or late-life mania. Late-life mania also may respond well to the anticonvulsant divalproex sodium. Avoid anxiolytics in older patients because of the potential of these agents to cause sedation or disinhibition and their associated risk of falls and confusion. Buspirone in higher dosages (40 to 60 mg/d) can sometimes help manage chronic anxiety states.
Related resource
- Sadavoy J, Lazarus LW, Jarvik LF, Grossman GT (eds). Comprehensive review of geriatric psychiatry (2nd ed). Washington, DC: American Psychiatric Press, 1996.
Drug brand names
- Buspirone • Buspar
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluoxetine • Prozac
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
Dr. Snow reports no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
Dr. Verma reports that he is on the speakers’ bureau of Eli Lilly and Co. and Abbott Laboratories, serves as a consultant to Eli Lilly and Co., and receives grant support from Eli Lilly and Co. and GlaxoSmithKline.
As psychiatrists, we are being pulled in opposite directions between conflicting goals: to reduce health care costs and to provide our patients with the sophistication and specificity of newly available—and more expensive—biological therapies. In the treatment of late-life psychosis, the evidence is impressive and persuasive that atypical antipsychotic drugs are worth the investment.
Atypical antipsychotics should usually be considered first-line therapy for late-life psychosis, though they cost more than the older neuroleptics. Overall, drugs comprise a minor portion of the cost of treating psychosis but may have a major impact on outcomes.1 With their safer side effect profile, the atypicals have enabled many patients with schizophrenia to “reintegrate” and become contributing members of society. Likewise, the use of atypicals can allow older patients with psychotic symptoms to function longer and more productively in their homes and communities.
Table 1
DIFFERENCES BETWEEN LATE-AND EARLY-ONSET SCHIZOPHRENIA
| Characteristic | Late-onset (age 45 and older) | Early-onset (before age 45) |
|---|---|---|
| Gender | More women | More men |
| Type | Paranoid | Varies |
| Positive symptoms | Severe | Severe |
| Negative symptoms | Less severe | More severe |
| Duration of illness | Chronic | Chronic |
| Cognition | Less impaired | More impaired |
| Neuroimaging | Nonspecific changes | Nonspecific changes |
| Antipsychotic dose | Lower | Higher |
| Mortality rates | High | High |
| Premorbid function | Good | Schizoid traits |
Without appropriate treatment of psychosis in younger patients, we know that recurrences and relapses can cause demonstrable brain changes and lead to residual symptoms.2 Older patients have been shown to lose 0.2 “well years” for every year they have psychotic symptoms.3
One illness or many?
Psychotic symptoms can occur in late life for a variety of reasons, and each diagnosis has different implications for patient work-up and treatment. Causes of psychosis in older patients include late-onset schizophrenia, dementia, affective disorder, delusional disorder, and delirium.
Late-life schizophrenia As life expectancy increases, schizophrenia is increasingly being diagnosed in older persons. In a sample of hospitalized patients with schizophrenia, onset of illness occurred after age 50 in 13%, after age 60 in 7%, and after age 70 in 3%.4 The clinical features of schizophrenia may be modified by the concurrent development of dementia. Thus, this reported increase in psychosis after age 50 may be secondary to an increased incidence of dementia, Parkinson’s disease, cerebrovascular events, and neoplasms.
Early definitions of “paraphrenia” and later schizophrenia described delusions and hallucinations that developed without disturbance of affect, with onset in early adult life.5 The occurrence of disorganized behavior and thinking in late life was ascribed to the effects of “senility” or other organic factors.
In 1943, Bleuler6 reported on a series of 126 patients in whom psychosis developed after age 40. In this group, the illness began after age 60 in 4%.
Current diagnostic criteria for schizophrenia do not exclude or categorize individuals on the basis of age. DSMIV does acknowledge a subgroup of patients with “late-onset” schizophrenia after age 45. However, there are important clinical differences in the presentations of the early- and late-onset types (Table 1). For example, even after adjusting for the greater longevity of women, more women than men develop late-onset schizophrenia. Also, compared with the early-onset type, in late-life schizophrenia:
- Premorbid paranoid or schizoid personality traits appear to be much less prominent.
- Patients may have had better occupational functioning and are more likely to have been married.
- Negative symptoms tend to be less severe, although they do contribute to functional decline.
Visual and hearing loss are among the risk factors correlated with late-onset schizophrenia.7 Sensory loss isolates an older person and leads to misinterpretation and misidentification of environmental cues. Other risk factors, in addition to female gender, include cognitive loss, poor social supports, living alone, and alcohol or drug abuse.
Dementia Between 20 and 50% of patients with vascular and mixed dementias exhibit psychotic symptoms.8 Among those with Alzheimer’s dementia, 30% exhibit persecutory delusions.9 Adding to this population are persons with early-onset schizophrenia, who are living longer and can also develop dementia.
While neuroimaging can sometimes aid in diagnosis, abnormalities seen on neuroimaging of patients with psychotic symptoms do not necessarily correlate with cognitive deficits. The clinical significance of these findings is unclear.10,11 Because of differences in therapy, it is important to establish whether the primary diagnosis is dementia or schizophrenia (Table 2).
Affective psychoses Late-life depression and mania are often unrecognized because of atypical presentations. In the elderly, depression may present with withdrawal, mood-incongruent delusions,12 and symptoms that mimic medical illness. Older manic patients may be misidentified as intrusive, hypersexual, or agitated. A high suspicion index, carefully elicited family history, and past psychiatric illness in the patient usually can clarify the diagnosis.
Delusional disorder Suspiciousness and paranoia are common findings in late life, with an estimated prevalence of 4 to 6% in the older population. Patients with delusional disorder show little evidence of cognitive deficits and—unlike those with schizophrenia—continue to maintain a high level of function.13 Delusions are nonbizarre and well systematized, and hallucinations are not a prominent feature.
Delirium Older persons are particularly vulnerable to developing delirium. Common causes include urinary tract infection, bowel impaction, heart failure, endocrinopathies, and drug toxicity caused by prescribed or over-the-counter drugs. CNS conditions such as subdural hematoma, cerebrovascular accident, slow-growing intracranial tumors, and encephalitis can be associated with the development of hallucinations and delusions without clouding consciousness.
While an agitated, hallucinating patient with clearly evident fluctuations in the sensorium may be readily diagnosed with a delirium, patients who are quiet, withdrawn, and apathetic may be less easy to recognize. Clinicians should therefore be vigilant for both the hyperkinetic and hypokinetic presentations of delirium when assessing older patients.
Work-up
When psychotic symptoms present for the first time in late life, medical causes must be ruled out first. A detailed history, including collateral information from other sources, includes:
- premorbid function
- psychiatric history, including history of affective illness
- family history of schizophrenia, affective illness, and Alzheimer’s disease
- medical history, including risks for cerebrovascular disease.
The examination should include a complete medical and neurologic evaluation, giving special attention to sensory loss, prescribed and over-the-counter drugs, recent changes in drug regimen, and cognitive screening. Laboratory work-up includes:
- in all cases: CBC/differential, BUN, TFT, B12/folate, and RPR
- in selected cases: MRI, HIV screening, toxicology screen, neuropsychological testing
- in rare cases: lumbar puncture, PET or SPECT imaging.
If the suggested work-up leads to diagnosis of medical illness, begin appropriate treatment. Behavioral symptoms during medical illness, such as with delirium, may require concurrent psychotropic medication until the medical illness is controlled.
If the medical work-up is negative, the clinician then needs to make the appropriate psychiatric diagnosis and institute antipsychotic treatment as indicated.
Table 2
CLINICAL DIFFERENCES BETWEEN DEMENTIA AND SCHIZOPHRENIA
| Characteristic | Dementia | Late-onset schizophrenia |
|---|---|---|
| Incidence | 50 to 70% | 1 to 2% |
| Bizarre delusions | Uncommon | Common |
| Hallucinations | Usually visual | Usually auditory |
| History of psychosis | Rare | Common |
| Misidentification | Common | Less common |
| Maintenance treatment | Usually unnecessary | Common |
| Antipsychotic dosage | 25% of dosage for adult schizophrenia | 50% of dosage for adult schizophrenia |
Table 3
USE OF ATYPICAL ANTIPSYCHOTICS IN OLDER PATIENTS
| Drug | Administration |
|---|---|
| Clozapine | Use limited by potential for agranulocytosis, need for weekly blood counts Higher risk for diabetes, hyperglycemia, pancreatitis Very sedating “Black box” warning for myocarditis (March 2002) NOT a first-choice drug for older persons |
| Risperidone | Usual geriatric dosage 1 to 2 mg/d Risk of EPS increases significantly with dosages > 2 mg/d Can cause persistent hyperprolactinemia |
| Olanzapine | Usual geriatric dosage 2.5 to 12.5 mg/d (qd dosing) Excellent choice as first-line drug in older patients Anticholinergic side effects ordinarily not a problem in vivo despite receptor profile of M1-M5 antagonism May be weight-neutral in older patients |
| Quetiapine | Geriatric dosage 25 to 400 mg/d Very low potential for EPS Can be a first choice for patients with Lewy body dementia and Parkinson’s disease Sedation increases with dosages > 200 mg/d |
| Ziprasidone | Geriatric dosage 40 to 160 mg/d Significant concern about QT prolongation in older patients, especially women and those with pre-existing cardiac disease, chronic mental illness, hypokalemia, and hypomagnesemia Not recommended as first-line therapy in elderly (“bold” warning) Not approved for IM use in United States; restrictions on use in many European countries |
| EPS: Extrapyramidal symptoms | |
Prescribing antipsychotics
Prescribing psychoactive medications for older patients is similar to pediatric prescribing. You need to distinguish between behaviors that are “disturbed” as a result of a psychotic process or “disturbing,” for which behavioral interventions may be more appropriate. In any case, pharmacologic and behavioral interventions are used concurrently.
When prescribing for older patients, consider the possible interaction of comorbid medical conditions and altered pharmacokinetics and pharmacodynamics, which increase the potential for drug-drug interactions. Start low and go slow to minimize the potential for adverse side effects and to ensure optimal preservation of function.
In general, atypical antipsychotics are considered first-line therapy, unless there is a compelling reason not to use them in an individual patient. Conventional neuroleptic antipsychotics carry a much higher risk of adverse drug reactions and functional loss without providing substantially greater efficacy.14 Atypical antipsychotics differ not only from the older neuroleptics but also from each other—including their safety profiles for use in older patients (Table 3).
Clozapine is a dibenzodiazepine that is indicated for treatment-resistant schizophrenia. The first atypical antipsychotic, clozapine significantly reduced the incidence of extrapyramidal symptoms (EPS) seen with the neuroleptic antipsychotics. Clozapine also was effective against the “negative” symptoms of schizophrenia such as apathy, anhedonia, and emotional blunting, which severely limited the ability of patients with schizophrenia to function in society.
The limitations of clozapine include its potential to cause agranulocytosis and the requirement for weekly blood tests. It is also fairly sedating. A number of reports have suggested a link between the use of clozapine and the development of type 2 diabetes, hyperglycemia, and pancreatitis.15,16 Earlier this year, the FDA issued a “black box” warning linking the use of clozapine with myocarditis. For all of these reasons, clozapine is not recommended for use in older patients.
Risperidone is a benzisoxazole derivative indicated for treatment of psychotic disorders. Risperidone’s antipsychotic efficacy is equivalent to that of haloperidol, but it has a much safer side-effect profile. In one multicenter study comparing risperidone and a placebo at single daily dosages of 0.5, 1.0, and 2.0 mg, risperidone was found to be effective in controlling the psychosis and behavioral disturbance associated with dementia. The authors recommended using a dosage of 1.0 mg/d because higher dosages resulted in excessive sedation and EPS.17
Risk of side effects is dose-dependent, especially in the older patient. Risperidone is the only atypical antipsychotic associated with persistent hyperprolactinemia, which may indirectly contribute to increased osteoporosis and atherogenesis.18,19 Orthostatic hypotension, especially with initial dosages greater than 1.5 mg/d and rapid dose escalation (≥ 25% every 24 to 48 hours), may also limit its use in older patients.
Olanzapine is a thiobenzodiazepine derivative indicated for treatment of psychosis and acute bipolar mania. Olanzapine has a receptor profile that is somewhat analogous with that of clozapine. Its antipsychotic efficacy is equivalent to that of risperidone, but it has a more favorable safety profile. Like risperidone, olanzapine is relatively nonsedating, but it is significantly less likely to cause EPS and orthostatic hypotension, and its use is not associated with persistent hyperprolactinemia.
Based on its vitro muscarinic receptor antagonism profile, some clinicians incorrectly assume that olanzapine is highly anticholinergic. In vivo data and clinical experience have not borne out this contention.20,21 Weight gain with olanzapine is relatively infrequent in older persons. A rapid-dissolve preparation can be useful in resistant and noncompliant patients. An IM preparation has been approved by the FDA but has not yet been made available by the manufacturer.
Quetiapine is a dibenzothiazepine derivative indicated for treatment of psychotic disorders. Despite limited data on its efficacy in late-life psychosis, clinical experience would suggest that its antipsychotic efficacy would at least equal that of other drugs in this category.
The need for twice-daily dosing and titration to a therapeutic response can sometimes limit the use of quetiapine. Sedation can be a problem at dosages greater than 200 mg/d. Because quetiapine has a relatively lower potential among the antipsychotics to cause EPS, it may be a first-line choice in patients with Parkinson’s disease and Lewy body dementia.
Ziprasidone is a benzothiazolylpiperazine indicated for treatment of psychosis. Experience with its use in older patients is limited. Ziprasidone should be avoided in patients with significant cardiovascular disease because of its potential to cause QT prolongation and cardiac arrhythmias. Although rare, this cardiac side effect may be life-threatening, and clinicians must be exceptionally vigilant when using ziprasidone in older patients. Risk factors for QT prolongation include older age, female sex, pre-existing cardiac disease, hypokalemia, and hypomagnesemia.
Ziprasidone can be somewhat sedating. An IM preparation is under development but has not been approved for use by the FDA
Neuroleptics and other options
Occasionally a typical neuroleptic may be the most appropriate first-line drug for older patients with psychotic symptoms. For example, a mid-potency neuroleptic such as perphenazine at an IM dose of 2 to 4 mg may be considered when severe agitation and aggression pose a substantial safety risk for the patient or caregiver and require rapid control. Haloperidol, despite its widespread use in both acute and long-term settings, should be used with caution because it has great potential for causing EPS and can immobilize an older patient, resulting in further functional decline. Regardless of which typical neuroleptic is used, switch the patient to an atypical antipsychotic as soon as agitation is under control.
Antidepressants Affective psychoses in older patients may require the addition of an antidepressant to the antipsychotic drug. The selective serotonin reuptake inhibitors fluoxetine and sertraline have proven track records for efficacy and safety and should be considered first-line agents.
Other options Electroconvulsive therapy is safe and effective for older patients with psychotic depression or late-life mania. Late-life mania also may respond well to the anticonvulsant divalproex sodium. Avoid anxiolytics in older patients because of the potential of these agents to cause sedation or disinhibition and their associated risk of falls and confusion. Buspirone in higher dosages (40 to 60 mg/d) can sometimes help manage chronic anxiety states.
Related resource
- Sadavoy J, Lazarus LW, Jarvik LF, Grossman GT (eds). Comprehensive review of geriatric psychiatry (2nd ed). Washington, DC: American Psychiatric Press, 1996.
Drug brand names
- Buspirone • Buspar
- Clozapine • Clozaril
- Divalproex • Depakote
- Fluoxetine • Prozac
- Olanzapine • Zyprexa
- Perphenazine • Trilafon
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sertraline • Zoloft
- Ziprasidone • Geodon
Disclosure
Dr. Snow reports no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
Dr. Verma reports that he is on the speakers’ bureau of Eli Lilly and Co. and Abbott Laboratories, serves as a consultant to Eli Lilly and Co., and receives grant support from Eli Lilly and Co. and GlaxoSmithKline.
1. Hargreaves WA, Shumway M. Pharmacoeconomics of drug therapy. J Clin Psych 1996;57(suppl 9):66-76.
2. Lieberman J, Chakos M, Wu H, Alvir J, et al. Longitudinal study of brain morphology in first-episode schizophrenia. Biol Psych 2001;49(6):487-99.
3. Patterson TL, Shaw W, Semple SJ, et al. Health-related quality of life in older patients with schizophrenia and other psychoses: relationships among psychosocial and psychiatric factors. Int J Geriatr Psychiatry 1997;12(4):452-61.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull 1988;14:39-55.
5. Kraeplin E. Dementia, praecox, and paraphrenia (1919). Translated by Barclay RM. Huntingdon NY: Krieger, 1971
6. Bleuler E. Late schizophrenic clinical pictures. Fortschr Neurol Psych 1943;15:259-90.
7. Pearlson G, Rabins P. The late-onset psychoses: possible risk factors. Psychiatr Clin North Am 1988;11(1):15-32.
8. Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer’s type. Biol Psychiatry 1989;25:39-48.
9. Wragg R, Jeste DV. Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry 1989;146:577-87.
10. Lesser IM. Late-onset psychotic disorder not otherwise specified: clinical and neuroimaging findings. Biol Psychiatry 1992;31:419-23.
11. Lesser IM, Miller BL, Swartz JR, et al. Brain imaging in late-life schizophrenia and related psychoses. Schizophr Bull 1993;19:419-23.
12. Jeste DV, Heaton SC, Paulsen JS, Ercoli L, Harris J, Heaton RK. Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry 1996;153(4):490-6.
13. Burns BJ, Larson DB, Goldstrom ID, et al. Mental disorders among nursing home patients: preliminary findings from the National Nursing Home Survey pretest. Int J Geriatr Psychiatry 1988;3:27-35.
14. Schneider LS, Pollock VE, Lyness SA. A meta-analysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc 1990;38:553-63.
15. Popli AP, Konicki PE, Jurjus GJ, et al. Clozapine and associated diabetes mellitus. J Clin Psychiatry 1997;58:108-11.
16. Bergemann N, Ehrig C, Diebold K, Mundt C, von Einsiedel R. Asymptomatic pancreatitis associated with clozapine. Pharmacopsychiatry 1999;32(2):78-80.
17. Katz IR, Jeste DV, Mintzer JE, et al. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psych 1999;60:107-15.
18. Petty RG. Prolactin and antipsychotic medications: mechanisms of action. Schizophr Res 1999;35(suppl):S67-S73.
19. Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res 1999;35(suppl):575-86.
20. Zarate CA, Baldessarini RJ, Siegel AJ, et al:. Risperidone in the elderly: a pharmacoepidemiological study. J Clin Psychiatry 1997;58:311-17.
21. Street JS, Clark WS, Gannon K, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer’s disease in nursing care facilities: a double-blind, placebo-controlled trial. Arch Gen Psychiatry 2000;57(10):968-76.
1. Hargreaves WA, Shumway M. Pharmacoeconomics of drug therapy. J Clin Psych 1996;57(suppl 9):66-76.
2. Lieberman J, Chakos M, Wu H, Alvir J, et al. Longitudinal study of brain morphology in first-episode schizophrenia. Biol Psych 2001;49(6):487-99.
3. Patterson TL, Shaw W, Semple SJ, et al. Health-related quality of life in older patients with schizophrenia and other psychoses: relationships among psychosocial and psychiatric factors. Int J Geriatr Psychiatry 1997;12(4):452-61.
4. Harris MJ, Jeste DV. Late-onset schizophrenia: an overview. Schizophr Bull 1988;14:39-55.
5. Kraeplin E. Dementia, praecox, and paraphrenia (1919). Translated by Barclay RM. Huntingdon NY: Krieger, 1971
6. Bleuler E. Late schizophrenic clinical pictures. Fortschr Neurol Psych 1943;15:259-90.
7. Pearlson G, Rabins P. The late-onset psychoses: possible risk factors. Psychiatr Clin North Am 1988;11(1):15-32.
8. Drevets WC, Rubin EH. Psychotic symptoms and the longitudinal course of senile dementia of the Alzheimer’s type. Biol Psychiatry 1989;25:39-48.
9. Wragg R, Jeste DV. Overview of depression and psychosis in Alzheimer’s disease. Am J Psychiatry 1989;146:577-87.
10. Lesser IM. Late-onset psychotic disorder not otherwise specified: clinical and neuroimaging findings. Biol Psychiatry 1992;31:419-23.
11. Lesser IM, Miller BL, Swartz JR, et al. Brain imaging in late-life schizophrenia and related psychoses. Schizophr Bull 1993;19:419-23.
12. Jeste DV, Heaton SC, Paulsen JS, Ercoli L, Harris J, Heaton RK. Clinical and neuropsychological comparison of psychotic depression with nonpsychotic depression and schizophrenia. Am J Psychiatry 1996;153(4):490-6.
13. Burns BJ, Larson DB, Goldstrom ID, et al. Mental disorders among nursing home patients: preliminary findings from the National Nursing Home Survey pretest. Int J Geriatr Psychiatry 1988;3:27-35.
14. Schneider LS, Pollock VE, Lyness SA. A meta-analysis of controlled trials of neuroleptic treatment in dementia. J Am Geriatr Soc 1990;38:553-63.
15. Popli AP, Konicki PE, Jurjus GJ, et al. Clozapine and associated diabetes mellitus. J Clin Psychiatry 1997;58:108-11.
16. Bergemann N, Ehrig C, Diebold K, Mundt C, von Einsiedel R. Asymptomatic pancreatitis associated with clozapine. Pharmacopsychiatry 1999;32(2):78-80.
17. Katz IR, Jeste DV, Mintzer JE, et al. Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. J Clin Psych 1999;60:107-15.
18. Petty RG. Prolactin and antipsychotic medications: mechanisms of action. Schizophr Res 1999;35(suppl):S67-S73.
19. Dickson RA, Glazer WM. Neuroleptic-induced hyperprolactinemia. Schizophr Res 1999;35(suppl):575-86.
20. Zarate CA, Baldessarini RJ, Siegel AJ, et al:. Risperidone in the elderly: a pharmacoepidemiological study. J Clin Psychiatry 1997;58:311-17.
21. Street JS, Clark WS, Gannon K, et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer’s disease in nursing care facilities: a double-blind, placebo-controlled trial. Arch Gen Psychiatry 2000;57(10):968-76.