User login
One AA meeting doesn’t fit all: 6 keys to prescribing 12-step programs
“The meeting was like sitting in a chimney – I practically choked to death.”
“I was the only person there without a tattoo.”
Attending the wrong 12-step meeting can turn off some patients, despite the substance abuse treatment support offered by Alcoholics Anonymous (AA) and similar programs. Because of the stigma associated with alcohol or drug addiction, most patients are ambivalent at best about attending their first 12-step meetings. Feeling “out of place”—the most common turn-off—can transform this ambivalence into adamant resistance.
Simply advising an addicted patient to “call AA” is tantamount to giving a depressed patient a copy of the Physicians’ Desk Reference and telling him or her to pick an antidepressant. Not all 12-step meetings are alike; 50,000 AA meetings are held every week in the United States (Box 1).1-7 Recognizing the differences between the groups in your area will help you guide your patients to the best match.
In prescribing a 12-step program, consider these six patient factors: socioeconomic status, gender, age, attitude towards spirituality, smoking status, and drug of choice.
More than 50,000 AA meetings, 20,000 NA meetings, and at least 15,000 Alanon/Alateen meetings are held every week in the United States. Other 12-step fellowships that model the AA approach include Gamblers Anonymous, Sex and Love Addicts Anonymous, Overeaters Anonymous, Cocaine Anonymous, Smokers Anonymous, Debtors Anonymous, Dual Recovery Anonymous, and Co-dependence Anonymous.
The combined membership of AA, NA, and Alanon/Alateen is approximately 2 million. To put this in perspective, if the 12-step approach was a religion—as some have proposed1 —it would have more U.S. congregants than Buddhism and Hinduism combined.
Although 12-step therapy has been a central tenet of community-based substance abuse treatment for more than 50 years,2 only recently has it become a focus of clinical research. Two major national multicenter clinical trials3,4 and several important but smaller clinical studies5-7 have found that 12-step-oriented therapies achieve modestly better abstinence rates than the psychotherapies with which they were compared.
Socioeconomic status
Matching patients with meetings according to socioeconomic status is not elitist—it’s pragmatic. Patients generally feel most comfortable and relate most readily at meetings where they feel they have something in common with the other members. For example, when a newly recovering middle-class alcoholic visits an AA group that is frequented by homeless and unemployed alcoholics, chances are that he will become more ambivalent about attending meetings. After all, he was never “that bad.”
A good practice is to give your patients an up-to-date 12-step meeting directory (Box 2). Suggest that they identify the meetings where they think they will feel most comfortable, based on the neighborhoods in which they are held.
Patients in early recovery often are terrified of encountering someone they know at a 12-step meeting. One strategy for patients concerned about protecting their anonymity—as many are—is to attend meetings outside their own neighborhoods but still in areas that match their socioeconomic status. Similarly, referring patients to meetings that are “closed to members only” might reduce their concerns about exposure.
Once a patient has connected with a 12-step program, matching by socioeconomic status becomes less important. Many begin to see similarities between themselves and other addicted individuals from all walks of life. In the beginning, however, similarities attract.
Your patient’s gender
Though women were once a small minority in AA and Narcotics Anonymous (NA), today they make up about one-third of AA’s membership and more than 40% of NA.8 One factor that may have boosted the number of women attending 12-step programs is the increased availability of women-only meetings.
Most cities have women-only meetings, and they generally will be a good place for your female patients to begin. Evidence indicates that gender-specific treatment enhances treatment outcomes.9,10 Women-only meetings tend to be smaller than mixed groups, and the senior members are often particularly willing to welcome newcomers.
Although it is severely frowned upon, the phenomenon of AA or NA members attempting to become romantically or sexually involved with a newcomer is common enough that 12-step members have coined a term for it: “13-stepping.” Newly recovering patients are often emotionally vulnerable and at risk of becoming enmeshed in a potentially destructive relationship. Beginning recovery in gender-specific meetings helps to reduce this risk.
Your patient’s age
A 12-step meeting dominated by people with gray, blue, or no hair can quickly put off teens and young adults in early recovery. Though these meetings with older members are likely to include persons who have achieved long-term and healthy recovery (making such meetings ideal territory for finding a sponsor), finding peers of a similar age is also important.
Meetings intended for young people are identified in 12-step meeting directories, but many of these “young peoples’ ” meetings have a preponderance of members older than 30—quite ancient by a 16-year-old’s standards. Conversely, some generic 12-step meetings might have a cadre of teenagers that attend regularly—at least for a while.
In AA and NA, teens and young adults tend to travel in nomadic packs, linger for a few months, then move on. For this reason, having contacts familiar with the characteristics of local meetings can be invaluable as you try to match a younger patient with a 12-step meeting.
Attitude toward spirituality
One of patients’ most common complaints about 12-step meetings is their surprise at how “religious” the programs are. Insiders are quick to point out that 12-step programs are “spiritual” and not “religious,” but the distinction is moot to patients who are uneasy with this aspect of meetings. The talk about “God as I understand Him,” the opening and closing of meetings with prayers, and the generous adoption of Judeo-Christian practices can rub agnostic, atheistic, and otherwise spiritually indifferent patients the wrong way.
To protect your patients from being blind-sided, review with them some of the spiritual practices employed in 12-step programs before they attend their first meeting:
- Meetings begin with reading the Twelve Steps (Box 3) and other 12-step literature; all readings are peppered with spiritually-loaded words such as “God,” “Higher Power,” “prayer,” and “meditation.”
- Meetings end with a prayer in which the group stands and holds hands (in AA) or links their arms in a huddle (NA). [I advise patients who might find this activity intolerable to duck out to the rest room 5 minutes before the meeting ends.]
- Group leaders typically collect donations by passing the basket.
Certain meetings have a particularly heavy spiritual focus and might be appropriately prescribed for patients hungering for spiritual growth. But for patients who have had toxic encounters with religion or otherwise are ill-at-ease with spirituality or religious matters, starting out at one of the more spiritually hardcore 12-step meetings could be overwhelming. While your 12-step contact person is your best guide in these matters, the following points also apply:
- Meetings listed as “11th Step” or “God as I understand Him” meetings will have a strong spiritual focus.
- Meetings held on Sunday mornings often have the express purpose of focusing on spirituality.
- “Step” meetings generally have a more spiritual focus, as 11 of the 12 steps are aimed at eliciting a “spiritual awakening.”
- “Speaker” or “topic discussion” meetings tend to have a less spiritual focus, though this will vary with the meeting chairperson’s preferences.
- “Beginners” meetings, when available, are intended for new members and devote more time to helping the newcomer understand the 12-step approach to spirituality.
Unless you regularly attend 12-step meetings, it is impossible to know which groups would be the best match for your patients. Here are suggestions for matching your patient’s needs with local 12-step meetings:
- Use fellowship directories. All 12-step fellowships maintain directories of where and when meetings are held and whether meetings are nonsmoking or have other restrictions (e.g., gay-only, women-only). For directories, call local AA and NA fellowships (in the phone book’s white pages).
- Develop a 12-step contact list. Rehabilitation centers often have counselors on staff who are familiar with local 12-step meetings and can recommend those that match your patients’ characteristics. Counselors who are active AA or NA members can be a valuable resource in identifying subtle differences in meetings.
- Locate 12-step meetings for impaired professionals. Special 12-step meetings for nurses, physicians, and pharmacists are held in many cities. For technical reasons, these are not “official”12-step meetings and are not listed in 12-step directories. Times and locations can generally be obtained from local medical societies, impaired-professional programs, or treatment centers.
- We admitted we were powerless over alcohol—that our lives had become unmanageable.
- Cameto believe that a Power greater than ourselves could restore us to sanity.
- Made a decision to turn our will and our lives over to the care of God as we understood Him.
- Made a searching and fearless moral inventory of ourselves.
- Admitted to God, to ourselves, and to another human being the exact nature of our wrongs.
- Were entirely ready to have God remove all these defects of character.
- Humbly asked Him to remove our shortcomings.
- Made a list of all persons we had harmed, and became willing to make amends to them all.
- Made direct amends to such people wherever possible, except when to do so would injure them or others.
- Continued to take personal inventory and when we were wrong promptly admitted it.
- Sought through prayer and meditation to improve our conscious contact with God as we understood Him, praying only for knowledge of His will for us and the power to carry that out.
- Having had a spiritual awakening as the result of these steps, we tried to carry this message to alcoholics and to practice these principles in all our affairs.
Source: Alcoholics Anonymous
AA’s main text, the so-called “Big Book” (its real title is: Alcoholics Anonymous7) has a chapter titled, “We Agnostics.” AA has many long-time members who have found support in the fellowship but never “found God” or a belief in a higher power other than the fellowship itself. These secular 12-step members demonstrate one of the many ironies of AA and NA—that spiritual fellowships can work even for individuals who reject spirituality.
Patients who resist spirituality are advised to “take what you can use” from the fellowship and “leave the rest.” While 12-step members will propose that the newcomer keep an open mind about spirituality, patients should also be assured that a seat is always waiting for them, regardless.
Whether your patient smokes
Most 12-step meetings today are smoke-free, not because of enlightenment within the fellowships but because meetings are usually held in churches, synagogues, and health care facilities where smoking is banned. The perception that attending 12-step meetings can be harmful to your health is out-of-date. Nonetheless, because most meetings have banned smoking, the few in which smoking is allowed are thick with smoke.
In general, 12-step clubhouses are among the holdouts where smoking is allowed during and after meetings. A clubhouse is typically a storefront rented or acquired by AA/NA members where meetings are held around the clock. Given the evidence that quitting smoking may improve overall health,10,11 patients should be encouraged to begin their involvement in smoke-free fellowships, which are identified in 12-step directories.
Your patient’s drug of choice
As its name implies, AA is intended for persons who desire to stop drinking. In practice, however, much of AA’s membership is addicted to more than one substance, and—in some cases—the drug of choice might not be alcohol.
Narcotics Anonymous—contrary to what its name implies—is for individuals addicted to any drug, not just narcotics. Patients generally should be advised to join the fellowship (AA or NA) that best matches their substance use history. There is, however, at least one exception that might best be illustrated with an example:
After I recommended NA meetings to a middle-class nurse addicted to analgesics, she returned for her next appointment quite angry. She attended three different NA meetings, and “all of the members were either heroin or crack cocaine addicts.” It seemed to her that all of them were on probation or parole. She was very uncomfortable throughout the meetings and upset with my recommendation.
In matching patients with meetings, socioeconomic and cultural factors take precedence over biochemistry. At the neuronal level, a nurse addicted to analgesics has a lot in common with a heroin addict, but her ability to relate to another recovering person—particularly in early recovery—may be limited. Arguing with my patient or countering that other nurses were probably at the meetings she attended would not have eased her reluctance to return to NA or helped our therapeutic alliance.
NA meetings are generally attended by individuals addicted to illicit drugs: amphetamines, crack cocaine, cannabis, and heroin. In larger cities, other 12-step fellowships may focus on specific drugs, such as cocaine, but these are rare. Just as individuals addicted to prescription narcotics are a minority in the treatment population, they are also a minority in NA.
For this reason, our prior recommendation—to match patients to meetings based on socioeconomic status—applies. It’s good policy to recommend that patients addicted to prescription medications try both AA and NA meetings and decide where they feel most comfortable.
The third tradition of AA states, “the only requirement for AA membership is a desire to stop drinking.” Though a purist might suggest that our analgesics-dependent nurse should join NA, her need to connect culturally with similar persons in recovery argues strongly for her to blend in at open AA meetings. A social drinker who never fulfilled the diagnostic criteria for alcohol dependence, she will have a better chance of abstaining from analgesics if she abstains from alcohol as well. For this reason, she should qualify for AA membership because she does, in fact, have “a desire to stop drinking.”
Some professionals addicted to prescription drugs will feel at home in NA meetings, whereas others will react as my patient did. Having access to a 12-step contact person who knows about the demographics of local NA meetings can help you make the best patient/meeting match.
Related resources
- Alcoholics Anonymous. www.alcoholics-anonymous.org
- Narcotics Anonymous. www.na.org
- Alanon-Alateen. www.al-anon.org
1. The Church of God Anonymous (religion of the 12-step movement) http://www.churchofgodanonymous.org/index2.html
2. White W. Slaying the Dragon Bloomington, IL: Chestnut Health Systems, 1998.
3. Crits-Christoph P, Siqueland L, Blaine J, et al. Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Arch Gen Psychiatry 1999;57(6):493-502.
4. Project Match. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. J Studies Alcohol 1997;58(1):7-29.
5. Ouimette PC, Finney JW, Moos RH. Twelve-step and cognitive-behavioral treatment for substance abuse: A comparison of treatment effectiveness. J Consult Clin Psychology 1997;65:230-40.
6. Morgenstern J, Blanchard KA, Morgan TJ, Labouvie E, Hayaki J. Testing the effectiveness of cognitive-behavioral treatment for substance abuse in a community setting: Within treatment and post-treatment findings. J Consult Clin Psychology 2001;69:1007-17.
7. Alcoholics Anonymous (3rd ed). New York: Alcoholics Anonymous World Service, 1976.
8. Emrick CD, Tonigan SJ, Montgomery H, Little L. Alcoholics Anonymous: what is currently known. In: McCrady BS, Miller WR (eds). Research on Alcoholics Anonymous New Brunswick, NJ: Rutgers Center on Alcohol Studies Publications, 1993:45.
9. Blume S. Addiction in women. In: Galanter M, Kleber HD (eds). Textbook of substance abuse treatment (2nd ed). Washington, DC: American Psychiatric Press, 1999;485-91.
10. Jarvis TJ. Implications of gender for alcohol treatment research: a quantitative and qualitative review. Br J Addiction 1992;87:1249-61.
11. Bobo JK, McIlvain HE, Lando HA, Walker RD, Leed-Kelly A. Effect of smoking cessation counseling on recovery from alcoholism: findings from a randomized community intervention trial. Addiction 1998;93:877-87.
12. Burling TA, Marshall GD, Seidner AL. Smoking cessation for substance abuse inpatients. J Subs Abuse 1991;3(3):269-76.
“The meeting was like sitting in a chimney – I practically choked to death.”
“I was the only person there without a tattoo.”
Attending the wrong 12-step meeting can turn off some patients, despite the substance abuse treatment support offered by Alcoholics Anonymous (AA) and similar programs. Because of the stigma associated with alcohol or drug addiction, most patients are ambivalent at best about attending their first 12-step meetings. Feeling “out of place”—the most common turn-off—can transform this ambivalence into adamant resistance.
Simply advising an addicted patient to “call AA” is tantamount to giving a depressed patient a copy of the Physicians’ Desk Reference and telling him or her to pick an antidepressant. Not all 12-step meetings are alike; 50,000 AA meetings are held every week in the United States (Box 1).1-7 Recognizing the differences between the groups in your area will help you guide your patients to the best match.
In prescribing a 12-step program, consider these six patient factors: socioeconomic status, gender, age, attitude towards spirituality, smoking status, and drug of choice.
More than 50,000 AA meetings, 20,000 NA meetings, and at least 15,000 Alanon/Alateen meetings are held every week in the United States. Other 12-step fellowships that model the AA approach include Gamblers Anonymous, Sex and Love Addicts Anonymous, Overeaters Anonymous, Cocaine Anonymous, Smokers Anonymous, Debtors Anonymous, Dual Recovery Anonymous, and Co-dependence Anonymous.
The combined membership of AA, NA, and Alanon/Alateen is approximately 2 million. To put this in perspective, if the 12-step approach was a religion—as some have proposed1 —it would have more U.S. congregants than Buddhism and Hinduism combined.
Although 12-step therapy has been a central tenet of community-based substance abuse treatment for more than 50 years,2 only recently has it become a focus of clinical research. Two major national multicenter clinical trials3,4 and several important but smaller clinical studies5-7 have found that 12-step-oriented therapies achieve modestly better abstinence rates than the psychotherapies with which they were compared.
Socioeconomic status
Matching patients with meetings according to socioeconomic status is not elitist—it’s pragmatic. Patients generally feel most comfortable and relate most readily at meetings where they feel they have something in common with the other members. For example, when a newly recovering middle-class alcoholic visits an AA group that is frequented by homeless and unemployed alcoholics, chances are that he will become more ambivalent about attending meetings. After all, he was never “that bad.”
A good practice is to give your patients an up-to-date 12-step meeting directory (Box 2). Suggest that they identify the meetings where they think they will feel most comfortable, based on the neighborhoods in which they are held.
Patients in early recovery often are terrified of encountering someone they know at a 12-step meeting. One strategy for patients concerned about protecting their anonymity—as many are—is to attend meetings outside their own neighborhoods but still in areas that match their socioeconomic status. Similarly, referring patients to meetings that are “closed to members only” might reduce their concerns about exposure.
Once a patient has connected with a 12-step program, matching by socioeconomic status becomes less important. Many begin to see similarities between themselves and other addicted individuals from all walks of life. In the beginning, however, similarities attract.
Your patient’s gender
Though women were once a small minority in AA and Narcotics Anonymous (NA), today they make up about one-third of AA’s membership and more than 40% of NA.8 One factor that may have boosted the number of women attending 12-step programs is the increased availability of women-only meetings.
Most cities have women-only meetings, and they generally will be a good place for your female patients to begin. Evidence indicates that gender-specific treatment enhances treatment outcomes.9,10 Women-only meetings tend to be smaller than mixed groups, and the senior members are often particularly willing to welcome newcomers.
Although it is severely frowned upon, the phenomenon of AA or NA members attempting to become romantically or sexually involved with a newcomer is common enough that 12-step members have coined a term for it: “13-stepping.” Newly recovering patients are often emotionally vulnerable and at risk of becoming enmeshed in a potentially destructive relationship. Beginning recovery in gender-specific meetings helps to reduce this risk.
Your patient’s age
A 12-step meeting dominated by people with gray, blue, or no hair can quickly put off teens and young adults in early recovery. Though these meetings with older members are likely to include persons who have achieved long-term and healthy recovery (making such meetings ideal territory for finding a sponsor), finding peers of a similar age is also important.
Meetings intended for young people are identified in 12-step meeting directories, but many of these “young peoples’ ” meetings have a preponderance of members older than 30—quite ancient by a 16-year-old’s standards. Conversely, some generic 12-step meetings might have a cadre of teenagers that attend regularly—at least for a while.
In AA and NA, teens and young adults tend to travel in nomadic packs, linger for a few months, then move on. For this reason, having contacts familiar with the characteristics of local meetings can be invaluable as you try to match a younger patient with a 12-step meeting.
Attitude toward spirituality
One of patients’ most common complaints about 12-step meetings is their surprise at how “religious” the programs are. Insiders are quick to point out that 12-step programs are “spiritual” and not “religious,” but the distinction is moot to patients who are uneasy with this aspect of meetings. The talk about “God as I understand Him,” the opening and closing of meetings with prayers, and the generous adoption of Judeo-Christian practices can rub agnostic, atheistic, and otherwise spiritually indifferent patients the wrong way.
To protect your patients from being blind-sided, review with them some of the spiritual practices employed in 12-step programs before they attend their first meeting:
- Meetings begin with reading the Twelve Steps (Box 3) and other 12-step literature; all readings are peppered with spiritually-loaded words such as “God,” “Higher Power,” “prayer,” and “meditation.”
- Meetings end with a prayer in which the group stands and holds hands (in AA) or links their arms in a huddle (NA). [I advise patients who might find this activity intolerable to duck out to the rest room 5 minutes before the meeting ends.]
- Group leaders typically collect donations by passing the basket.
Certain meetings have a particularly heavy spiritual focus and might be appropriately prescribed for patients hungering for spiritual growth. But for patients who have had toxic encounters with religion or otherwise are ill-at-ease with spirituality or religious matters, starting out at one of the more spiritually hardcore 12-step meetings could be overwhelming. While your 12-step contact person is your best guide in these matters, the following points also apply:
- Meetings listed as “11th Step” or “God as I understand Him” meetings will have a strong spiritual focus.
- Meetings held on Sunday mornings often have the express purpose of focusing on spirituality.
- “Step” meetings generally have a more spiritual focus, as 11 of the 12 steps are aimed at eliciting a “spiritual awakening.”
- “Speaker” or “topic discussion” meetings tend to have a less spiritual focus, though this will vary with the meeting chairperson’s preferences.
- “Beginners” meetings, when available, are intended for new members and devote more time to helping the newcomer understand the 12-step approach to spirituality.
Unless you regularly attend 12-step meetings, it is impossible to know which groups would be the best match for your patients. Here are suggestions for matching your patient’s needs with local 12-step meetings:
- Use fellowship directories. All 12-step fellowships maintain directories of where and when meetings are held and whether meetings are nonsmoking or have other restrictions (e.g., gay-only, women-only). For directories, call local AA and NA fellowships (in the phone book’s white pages).
- Develop a 12-step contact list. Rehabilitation centers often have counselors on staff who are familiar with local 12-step meetings and can recommend those that match your patients’ characteristics. Counselors who are active AA or NA members can be a valuable resource in identifying subtle differences in meetings.
- Locate 12-step meetings for impaired professionals. Special 12-step meetings for nurses, physicians, and pharmacists are held in many cities. For technical reasons, these are not “official”12-step meetings and are not listed in 12-step directories. Times and locations can generally be obtained from local medical societies, impaired-professional programs, or treatment centers.
- We admitted we were powerless over alcohol—that our lives had become unmanageable.
- Cameto believe that a Power greater than ourselves could restore us to sanity.
- Made a decision to turn our will and our lives over to the care of God as we understood Him.
- Made a searching and fearless moral inventory of ourselves.
- Admitted to God, to ourselves, and to another human being the exact nature of our wrongs.
- Were entirely ready to have God remove all these defects of character.
- Humbly asked Him to remove our shortcomings.
- Made a list of all persons we had harmed, and became willing to make amends to them all.
- Made direct amends to such people wherever possible, except when to do so would injure them or others.
- Continued to take personal inventory and when we were wrong promptly admitted it.
- Sought through prayer and meditation to improve our conscious contact with God as we understood Him, praying only for knowledge of His will for us and the power to carry that out.
- Having had a spiritual awakening as the result of these steps, we tried to carry this message to alcoholics and to practice these principles in all our affairs.
Source: Alcoholics Anonymous
AA’s main text, the so-called “Big Book” (its real title is: Alcoholics Anonymous7) has a chapter titled, “We Agnostics.” AA has many long-time members who have found support in the fellowship but never “found God” or a belief in a higher power other than the fellowship itself. These secular 12-step members demonstrate one of the many ironies of AA and NA—that spiritual fellowships can work even for individuals who reject spirituality.
Patients who resist spirituality are advised to “take what you can use” from the fellowship and “leave the rest.” While 12-step members will propose that the newcomer keep an open mind about spirituality, patients should also be assured that a seat is always waiting for them, regardless.
Whether your patient smokes
Most 12-step meetings today are smoke-free, not because of enlightenment within the fellowships but because meetings are usually held in churches, synagogues, and health care facilities where smoking is banned. The perception that attending 12-step meetings can be harmful to your health is out-of-date. Nonetheless, because most meetings have banned smoking, the few in which smoking is allowed are thick with smoke.
In general, 12-step clubhouses are among the holdouts where smoking is allowed during and after meetings. A clubhouse is typically a storefront rented or acquired by AA/NA members where meetings are held around the clock. Given the evidence that quitting smoking may improve overall health,10,11 patients should be encouraged to begin their involvement in smoke-free fellowships, which are identified in 12-step directories.
Your patient’s drug of choice
As its name implies, AA is intended for persons who desire to stop drinking. In practice, however, much of AA’s membership is addicted to more than one substance, and—in some cases—the drug of choice might not be alcohol.
Narcotics Anonymous—contrary to what its name implies—is for individuals addicted to any drug, not just narcotics. Patients generally should be advised to join the fellowship (AA or NA) that best matches their substance use history. There is, however, at least one exception that might best be illustrated with an example:
After I recommended NA meetings to a middle-class nurse addicted to analgesics, she returned for her next appointment quite angry. She attended three different NA meetings, and “all of the members were either heroin or crack cocaine addicts.” It seemed to her that all of them were on probation or parole. She was very uncomfortable throughout the meetings and upset with my recommendation.
In matching patients with meetings, socioeconomic and cultural factors take precedence over biochemistry. At the neuronal level, a nurse addicted to analgesics has a lot in common with a heroin addict, but her ability to relate to another recovering person—particularly in early recovery—may be limited. Arguing with my patient or countering that other nurses were probably at the meetings she attended would not have eased her reluctance to return to NA or helped our therapeutic alliance.
NA meetings are generally attended by individuals addicted to illicit drugs: amphetamines, crack cocaine, cannabis, and heroin. In larger cities, other 12-step fellowships may focus on specific drugs, such as cocaine, but these are rare. Just as individuals addicted to prescription narcotics are a minority in the treatment population, they are also a minority in NA.
For this reason, our prior recommendation—to match patients to meetings based on socioeconomic status—applies. It’s good policy to recommend that patients addicted to prescription medications try both AA and NA meetings and decide where they feel most comfortable.
The third tradition of AA states, “the only requirement for AA membership is a desire to stop drinking.” Though a purist might suggest that our analgesics-dependent nurse should join NA, her need to connect culturally with similar persons in recovery argues strongly for her to blend in at open AA meetings. A social drinker who never fulfilled the diagnostic criteria for alcohol dependence, she will have a better chance of abstaining from analgesics if she abstains from alcohol as well. For this reason, she should qualify for AA membership because she does, in fact, have “a desire to stop drinking.”
Some professionals addicted to prescription drugs will feel at home in NA meetings, whereas others will react as my patient did. Having access to a 12-step contact person who knows about the demographics of local NA meetings can help you make the best patient/meeting match.
Related resources
- Alcoholics Anonymous. www.alcoholics-anonymous.org
- Narcotics Anonymous. www.na.org
- Alanon-Alateen. www.al-anon.org
“The meeting was like sitting in a chimney – I practically choked to death.”
“I was the only person there without a tattoo.”
Attending the wrong 12-step meeting can turn off some patients, despite the substance abuse treatment support offered by Alcoholics Anonymous (AA) and similar programs. Because of the stigma associated with alcohol or drug addiction, most patients are ambivalent at best about attending their first 12-step meetings. Feeling “out of place”—the most common turn-off—can transform this ambivalence into adamant resistance.
Simply advising an addicted patient to “call AA” is tantamount to giving a depressed patient a copy of the Physicians’ Desk Reference and telling him or her to pick an antidepressant. Not all 12-step meetings are alike; 50,000 AA meetings are held every week in the United States (Box 1).1-7 Recognizing the differences between the groups in your area will help you guide your patients to the best match.
In prescribing a 12-step program, consider these six patient factors: socioeconomic status, gender, age, attitude towards spirituality, smoking status, and drug of choice.
More than 50,000 AA meetings, 20,000 NA meetings, and at least 15,000 Alanon/Alateen meetings are held every week in the United States. Other 12-step fellowships that model the AA approach include Gamblers Anonymous, Sex and Love Addicts Anonymous, Overeaters Anonymous, Cocaine Anonymous, Smokers Anonymous, Debtors Anonymous, Dual Recovery Anonymous, and Co-dependence Anonymous.
The combined membership of AA, NA, and Alanon/Alateen is approximately 2 million. To put this in perspective, if the 12-step approach was a religion—as some have proposed1 —it would have more U.S. congregants than Buddhism and Hinduism combined.
Although 12-step therapy has been a central tenet of community-based substance abuse treatment for more than 50 years,2 only recently has it become a focus of clinical research. Two major national multicenter clinical trials3,4 and several important but smaller clinical studies5-7 have found that 12-step-oriented therapies achieve modestly better abstinence rates than the psychotherapies with which they were compared.
Socioeconomic status
Matching patients with meetings according to socioeconomic status is not elitist—it’s pragmatic. Patients generally feel most comfortable and relate most readily at meetings where they feel they have something in common with the other members. For example, when a newly recovering middle-class alcoholic visits an AA group that is frequented by homeless and unemployed alcoholics, chances are that he will become more ambivalent about attending meetings. After all, he was never “that bad.”
A good practice is to give your patients an up-to-date 12-step meeting directory (Box 2). Suggest that they identify the meetings where they think they will feel most comfortable, based on the neighborhoods in which they are held.
Patients in early recovery often are terrified of encountering someone they know at a 12-step meeting. One strategy for patients concerned about protecting their anonymity—as many are—is to attend meetings outside their own neighborhoods but still in areas that match their socioeconomic status. Similarly, referring patients to meetings that are “closed to members only” might reduce their concerns about exposure.
Once a patient has connected with a 12-step program, matching by socioeconomic status becomes less important. Many begin to see similarities between themselves and other addicted individuals from all walks of life. In the beginning, however, similarities attract.
Your patient’s gender
Though women were once a small minority in AA and Narcotics Anonymous (NA), today they make up about one-third of AA’s membership and more than 40% of NA.8 One factor that may have boosted the number of women attending 12-step programs is the increased availability of women-only meetings.
Most cities have women-only meetings, and they generally will be a good place for your female patients to begin. Evidence indicates that gender-specific treatment enhances treatment outcomes.9,10 Women-only meetings tend to be smaller than mixed groups, and the senior members are often particularly willing to welcome newcomers.
Although it is severely frowned upon, the phenomenon of AA or NA members attempting to become romantically or sexually involved with a newcomer is common enough that 12-step members have coined a term for it: “13-stepping.” Newly recovering patients are often emotionally vulnerable and at risk of becoming enmeshed in a potentially destructive relationship. Beginning recovery in gender-specific meetings helps to reduce this risk.
Your patient’s age
A 12-step meeting dominated by people with gray, blue, or no hair can quickly put off teens and young adults in early recovery. Though these meetings with older members are likely to include persons who have achieved long-term and healthy recovery (making such meetings ideal territory for finding a sponsor), finding peers of a similar age is also important.
Meetings intended for young people are identified in 12-step meeting directories, but many of these “young peoples’ ” meetings have a preponderance of members older than 30—quite ancient by a 16-year-old’s standards. Conversely, some generic 12-step meetings might have a cadre of teenagers that attend regularly—at least for a while.
In AA and NA, teens and young adults tend to travel in nomadic packs, linger for a few months, then move on. For this reason, having contacts familiar with the characteristics of local meetings can be invaluable as you try to match a younger patient with a 12-step meeting.
Attitude toward spirituality
One of patients’ most common complaints about 12-step meetings is their surprise at how “religious” the programs are. Insiders are quick to point out that 12-step programs are “spiritual” and not “religious,” but the distinction is moot to patients who are uneasy with this aspect of meetings. The talk about “God as I understand Him,” the opening and closing of meetings with prayers, and the generous adoption of Judeo-Christian practices can rub agnostic, atheistic, and otherwise spiritually indifferent patients the wrong way.
To protect your patients from being blind-sided, review with them some of the spiritual practices employed in 12-step programs before they attend their first meeting:
- Meetings begin with reading the Twelve Steps (Box 3) and other 12-step literature; all readings are peppered with spiritually-loaded words such as “God,” “Higher Power,” “prayer,” and “meditation.”
- Meetings end with a prayer in which the group stands and holds hands (in AA) or links their arms in a huddle (NA). [I advise patients who might find this activity intolerable to duck out to the rest room 5 minutes before the meeting ends.]
- Group leaders typically collect donations by passing the basket.
Certain meetings have a particularly heavy spiritual focus and might be appropriately prescribed for patients hungering for spiritual growth. But for patients who have had toxic encounters with religion or otherwise are ill-at-ease with spirituality or religious matters, starting out at one of the more spiritually hardcore 12-step meetings could be overwhelming. While your 12-step contact person is your best guide in these matters, the following points also apply:
- Meetings listed as “11th Step” or “God as I understand Him” meetings will have a strong spiritual focus.
- Meetings held on Sunday mornings often have the express purpose of focusing on spirituality.
- “Step” meetings generally have a more spiritual focus, as 11 of the 12 steps are aimed at eliciting a “spiritual awakening.”
- “Speaker” or “topic discussion” meetings tend to have a less spiritual focus, though this will vary with the meeting chairperson’s preferences.
- “Beginners” meetings, when available, are intended for new members and devote more time to helping the newcomer understand the 12-step approach to spirituality.
Unless you regularly attend 12-step meetings, it is impossible to know which groups would be the best match for your patients. Here are suggestions for matching your patient’s needs with local 12-step meetings:
- Use fellowship directories. All 12-step fellowships maintain directories of where and when meetings are held and whether meetings are nonsmoking or have other restrictions (e.g., gay-only, women-only). For directories, call local AA and NA fellowships (in the phone book’s white pages).
- Develop a 12-step contact list. Rehabilitation centers often have counselors on staff who are familiar with local 12-step meetings and can recommend those that match your patients’ characteristics. Counselors who are active AA or NA members can be a valuable resource in identifying subtle differences in meetings.
- Locate 12-step meetings for impaired professionals. Special 12-step meetings for nurses, physicians, and pharmacists are held in many cities. For technical reasons, these are not “official”12-step meetings and are not listed in 12-step directories. Times and locations can generally be obtained from local medical societies, impaired-professional programs, or treatment centers.
- We admitted we were powerless over alcohol—that our lives had become unmanageable.
- Cameto believe that a Power greater than ourselves could restore us to sanity.
- Made a decision to turn our will and our lives over to the care of God as we understood Him.
- Made a searching and fearless moral inventory of ourselves.
- Admitted to God, to ourselves, and to another human being the exact nature of our wrongs.
- Were entirely ready to have God remove all these defects of character.
- Humbly asked Him to remove our shortcomings.
- Made a list of all persons we had harmed, and became willing to make amends to them all.
- Made direct amends to such people wherever possible, except when to do so would injure them or others.
- Continued to take personal inventory and when we were wrong promptly admitted it.
- Sought through prayer and meditation to improve our conscious contact with God as we understood Him, praying only for knowledge of His will for us and the power to carry that out.
- Having had a spiritual awakening as the result of these steps, we tried to carry this message to alcoholics and to practice these principles in all our affairs.
Source: Alcoholics Anonymous
AA’s main text, the so-called “Big Book” (its real title is: Alcoholics Anonymous7) has a chapter titled, “We Agnostics.” AA has many long-time members who have found support in the fellowship but never “found God” or a belief in a higher power other than the fellowship itself. These secular 12-step members demonstrate one of the many ironies of AA and NA—that spiritual fellowships can work even for individuals who reject spirituality.
Patients who resist spirituality are advised to “take what you can use” from the fellowship and “leave the rest.” While 12-step members will propose that the newcomer keep an open mind about spirituality, patients should also be assured that a seat is always waiting for them, regardless.
Whether your patient smokes
Most 12-step meetings today are smoke-free, not because of enlightenment within the fellowships but because meetings are usually held in churches, synagogues, and health care facilities where smoking is banned. The perception that attending 12-step meetings can be harmful to your health is out-of-date. Nonetheless, because most meetings have banned smoking, the few in which smoking is allowed are thick with smoke.
In general, 12-step clubhouses are among the holdouts where smoking is allowed during and after meetings. A clubhouse is typically a storefront rented or acquired by AA/NA members where meetings are held around the clock. Given the evidence that quitting smoking may improve overall health,10,11 patients should be encouraged to begin their involvement in smoke-free fellowships, which are identified in 12-step directories.
Your patient’s drug of choice
As its name implies, AA is intended for persons who desire to stop drinking. In practice, however, much of AA’s membership is addicted to more than one substance, and—in some cases—the drug of choice might not be alcohol.
Narcotics Anonymous—contrary to what its name implies—is for individuals addicted to any drug, not just narcotics. Patients generally should be advised to join the fellowship (AA or NA) that best matches their substance use history. There is, however, at least one exception that might best be illustrated with an example:
After I recommended NA meetings to a middle-class nurse addicted to analgesics, she returned for her next appointment quite angry. She attended three different NA meetings, and “all of the members were either heroin or crack cocaine addicts.” It seemed to her that all of them were on probation or parole. She was very uncomfortable throughout the meetings and upset with my recommendation.
In matching patients with meetings, socioeconomic and cultural factors take precedence over biochemistry. At the neuronal level, a nurse addicted to analgesics has a lot in common with a heroin addict, but her ability to relate to another recovering person—particularly in early recovery—may be limited. Arguing with my patient or countering that other nurses were probably at the meetings she attended would not have eased her reluctance to return to NA or helped our therapeutic alliance.
NA meetings are generally attended by individuals addicted to illicit drugs: amphetamines, crack cocaine, cannabis, and heroin. In larger cities, other 12-step fellowships may focus on specific drugs, such as cocaine, but these are rare. Just as individuals addicted to prescription narcotics are a minority in the treatment population, they are also a minority in NA.
For this reason, our prior recommendation—to match patients to meetings based on socioeconomic status—applies. It’s good policy to recommend that patients addicted to prescription medications try both AA and NA meetings and decide where they feel most comfortable.
The third tradition of AA states, “the only requirement for AA membership is a desire to stop drinking.” Though a purist might suggest that our analgesics-dependent nurse should join NA, her need to connect culturally with similar persons in recovery argues strongly for her to blend in at open AA meetings. A social drinker who never fulfilled the diagnostic criteria for alcohol dependence, she will have a better chance of abstaining from analgesics if she abstains from alcohol as well. For this reason, she should qualify for AA membership because she does, in fact, have “a desire to stop drinking.”
Some professionals addicted to prescription drugs will feel at home in NA meetings, whereas others will react as my patient did. Having access to a 12-step contact person who knows about the demographics of local NA meetings can help you make the best patient/meeting match.
Related resources
- Alcoholics Anonymous. www.alcoholics-anonymous.org
- Narcotics Anonymous. www.na.org
- Alanon-Alateen. www.al-anon.org
1. The Church of God Anonymous (religion of the 12-step movement) http://www.churchofgodanonymous.org/index2.html
2. White W. Slaying the Dragon Bloomington, IL: Chestnut Health Systems, 1998.
3. Crits-Christoph P, Siqueland L, Blaine J, et al. Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Arch Gen Psychiatry 1999;57(6):493-502.
4. Project Match. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. J Studies Alcohol 1997;58(1):7-29.
5. Ouimette PC, Finney JW, Moos RH. Twelve-step and cognitive-behavioral treatment for substance abuse: A comparison of treatment effectiveness. J Consult Clin Psychology 1997;65:230-40.
6. Morgenstern J, Blanchard KA, Morgan TJ, Labouvie E, Hayaki J. Testing the effectiveness of cognitive-behavioral treatment for substance abuse in a community setting: Within treatment and post-treatment findings. J Consult Clin Psychology 2001;69:1007-17.
7. Alcoholics Anonymous (3rd ed). New York: Alcoholics Anonymous World Service, 1976.
8. Emrick CD, Tonigan SJ, Montgomery H, Little L. Alcoholics Anonymous: what is currently known. In: McCrady BS, Miller WR (eds). Research on Alcoholics Anonymous New Brunswick, NJ: Rutgers Center on Alcohol Studies Publications, 1993:45.
9. Blume S. Addiction in women. In: Galanter M, Kleber HD (eds). Textbook of substance abuse treatment (2nd ed). Washington, DC: American Psychiatric Press, 1999;485-91.
10. Jarvis TJ. Implications of gender for alcohol treatment research: a quantitative and qualitative review. Br J Addiction 1992;87:1249-61.
11. Bobo JK, McIlvain HE, Lando HA, Walker RD, Leed-Kelly A. Effect of smoking cessation counseling on recovery from alcoholism: findings from a randomized community intervention trial. Addiction 1998;93:877-87.
12. Burling TA, Marshall GD, Seidner AL. Smoking cessation for substance abuse inpatients. J Subs Abuse 1991;3(3):269-76.
1. The Church of God Anonymous (religion of the 12-step movement) http://www.churchofgodanonymous.org/index2.html
2. White W. Slaying the Dragon Bloomington, IL: Chestnut Health Systems, 1998.
3. Crits-Christoph P, Siqueland L, Blaine J, et al. Psychosocial treatments for cocaine dependence: National Institute on Drug Abuse Collaborative Cocaine Treatment Study. Arch Gen Psychiatry 1999;57(6):493-502.
4. Project Match. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. J Studies Alcohol 1997;58(1):7-29.
5. Ouimette PC, Finney JW, Moos RH. Twelve-step and cognitive-behavioral treatment for substance abuse: A comparison of treatment effectiveness. J Consult Clin Psychology 1997;65:230-40.
6. Morgenstern J, Blanchard KA, Morgan TJ, Labouvie E, Hayaki J. Testing the effectiveness of cognitive-behavioral treatment for substance abuse in a community setting: Within treatment and post-treatment findings. J Consult Clin Psychology 2001;69:1007-17.
7. Alcoholics Anonymous (3rd ed). New York: Alcoholics Anonymous World Service, 1976.
8. Emrick CD, Tonigan SJ, Montgomery H, Little L. Alcoholics Anonymous: what is currently known. In: McCrady BS, Miller WR (eds). Research on Alcoholics Anonymous New Brunswick, NJ: Rutgers Center on Alcohol Studies Publications, 1993:45.
9. Blume S. Addiction in women. In: Galanter M, Kleber HD (eds). Textbook of substance abuse treatment (2nd ed). Washington, DC: American Psychiatric Press, 1999;485-91.
10. Jarvis TJ. Implications of gender for alcohol treatment research: a quantitative and qualitative review. Br J Addiction 1992;87:1249-61.
11. Bobo JK, McIlvain HE, Lando HA, Walker RD, Leed-Kelly A. Effect of smoking cessation counseling on recovery from alcoholism: findings from a randomized community intervention trial. Addiction 1998;93:877-87.
12. Burling TA, Marshall GD, Seidner AL. Smoking cessation for substance abuse inpatients. J Subs Abuse 1991;3(3):269-76.
Negative symptoms of schizophrenia: How to treat them most effectively
Negative symptoms are the major contributor to low function levels and debilitation in most patients with schizophrenia. Poorly motivated patients cannot function adequately at school or work. Relationships with family and friends decay in the face of unresponsive affect and inattention to social cues. Personal interests yield to the dampening influences of anhedonia, apathy, and inattention.
Yet because active psychosis is the most common cause of hospital admission, a primary goal of treatment—and sometimes the only objective of pharmacologic treatment—is to eliminate or reduce positive symptoms. And although controlling positive symptoms is remarkably effective in reducing hospitalizations, patients’ functional capacity improves only minimally as psychosis abates. Even with optimal antipsychotic treatment, negative symptoms tend to persist.
For psychiatrists, the three major challenges of schizophrenia’s negative symptoms are their modest therapeutic response, pervasiveness, and diminution of patients’ quality of life. To help you manage negative symptoms, we suggest the following approach to their assessment and treatment.
Importance of negative symptoms
Schizophrenia is a heterogeneous disorder characterized by positive, negative, cognitive, and mood symptoms. The relative severity of these four pathologic domains varies from case to case and within the same individual over time. Though related, these domains have distinct underlying mechanisms and are differentially related to functional capacity and quality of life. They also show different patterns of response to treatment. Whereas positive symptoms refer to new psychological experiences outside the range of normal (e.g., delusions, hallucinations, suspiciousness, disorganized thinking), negative symptoms represent loss of normal function.
Negative symptoms include blunting of affect, poverty of speech and thought, apathy, anhedonia, reduced social drive, loss of motivation, lack of social interest, and inattention to social or cognitive input. These symptoms have devastating consequences on patients’ lives, and only modest progress has been made in treating them effectively.
From negative to positive. Early investigators1,2 considered negative symptoms to represent the fundamental defect of schizophrenia. Over the years, however, the importance of negative symptoms was progressively downplayed. Positive symptoms were increasingly emphasized because:
- positive symptoms have a more dramatic and easily recognized presentation
- negative symptoms are more difficult to reliably define and document
- antipsychotics, which revolutionized schizophrenia treatment, produce their most dramatic improvement in positive symptoms.
Renewed interest. The almost universal presence and relative persistence of negative symptoms, and the fact that they represent the most debilitating and refractory aspect of schizophrenic psychopathology, make them difficult to ignore. Consequently, interest in negative symptoms resurged in the 1980s-90s, with intense efforts to better understand them and treat them more effectively.3-5
Table
SCHIZOPHRENIA’S NEGATIVE SYMPTOMS: PRIMARY AND SECONDARY COMPONENTS
| Primary Associated with positive symptoms Deficit or primary enduring symptoms (premorbid and deteriorative) |
| Secondary Associated with extrapyramidal symptoms, depression, or environmental deprivation |
| Source: Adapted from DeQuardo JR, Tandon R. J Psychiatr Res 1998;32 (3-4):229-42. |
Negative symptoms are now better (but still incompletely) understood, and their treatment has improved but is still inadequate. Because intense effort yielded only modest success, researchers and clinicians have again begun to pay less attention to negative symptoms and shifted their focus to cognition in schizophrenia. Negative symptoms remain relevant, however, because they constitute the main barrier to a better quality of life for patients with schizophrenia.
Assessment for negative symptoms
The four major clinical subgroups of negative symptoms are affective, communicative, conational, and relational.
Affective. Blunted affect—including deficits in facial expression, eye contact, gestures, and voice pattern—is perhaps the most conspicuous negative symptom. In mild form, gestures may seem artificial or mechanical, and the voice is stilted or lacks normal inflection. Patients with severe blunted affect may appear devoid of facial expression or communicative gestures. They may sit impassively with little spontaneous movement, speak in a monotone, and gaze blankly in no particular direction.
Even when conversation becomes emotional, the patient’s affect does not adjust appropriately to reflect his or her feelings. Nor does the patient display even a basic level of understanding or responsiveness that typically characterize casual human interactions. The ability to experience pleasure (anhedonia) and sense of caring (apathy) are also reduced.
Communicative. The patient’s speech may be reduced in quantity (poverty of speech) and information (poverty of content of speech). In mild forms of impoverished speech (alogia), the patient makes brief, unelaborated statements; in the more severe form, the patient can be virtually mute. Whatever speech is present tends to be vague and overly generalized. Periods of silence may occur, either before the patient answers a question (increased latency) or in the midst of a response (blocking).
Conational. The patient may show a lack of drive or goal-directed behavior (avolition). Personal grooming may be poor. Physical activity may be limited. Patients typically have great difficulty following a work schedule or hospital ward routine. They fail to initiate activities, participate grudgingly, and require frequent direction and encouragement.
Continue to: Relational
Relational. Interest in social activities and relationships is reduced (asociality). Even enjoyable and recreational activities are neglected. Interpersonal relations may be of little interest. Friendships become rare and shallow, with little sharing of intimacy. Contacts with family are neglected. Sexual interest declines. As symptoms progress, patients become increasingly isolated.
Primary and secondary symptoms
Negative symptoms are an intrinsic component of schizophrenic psychopathology, and they can also be caused by secondary factors (Table).6,7 Distinguishing between primary and secondary causes of negative symptoms can help you select appropriate treatment in specific clinical situations.
Primary symptoms. From a longitudinal perspective, the three major components of primary negative symptoms are:
- premorbid negative symptoms (present prior to psychosis onset and associated with poor premorbid functioning)
- psychotic-phase, nonenduring negative symptoms that fluctuate with positive symptoms around periods of psychotic exacerbation
- deteriorative negative symptoms that intensify following each psychotic exacerbation and reflect a decline from premorbid levels of functioning.
Though little can be done to treat the premorbid component, psychotic-phase negative symptoms improve along with positive symptoms (although more slowly).8,9 Therefore, the best strategy for managing negative symptoms is to treat positive symptoms more effectively. Although there is no specific treatment for deteriorative negative symptoms, the severity of this component appears to be related to the “toxicity of psychosis” and can be reduced by early, effective antipsychotic treatment.10,11
Secondary negative symptoms occur in association with (and presumably are caused by) factors such as depression, extrapyramidal symptoms (EPS), and environmental deprivation. Secondary negative symptoms usually respond to treatment of the underlying cause.
Assessment
Symptom severity. Assessing the severity of a patient’s negative symptoms on an ongoing basis is a most important first step towards optimal treatment:
- Our objective is to improve patients’ function and quality of life, and negative symptoms compromise both of these more than any other factor.
- Ongoing assessment can track whether prescribed treatments are improving or worsening a patient’s symptoms.
Tools to assess the severity of negative symptoms include the Brief Psychiatric Rating Scale (BPRS) and Positive and Negative Symptom Scale (PANSS).12 The Scale for the Assessment of Negative Symptoms (SANS)13 measures them exclusively, and others such as the Schedule for the Deficit Syndrome (SDS)14 attempt to classify them into subgroups.
Discussing these instruments is beyond the scope of this article, but they differ greatly in their approach to assessing negative symptoms. Instead of using cumbersome assessment instruments, however, we recommend that you focus on two to four of a patient’s “target” symptoms or behaviors and note their severity on an ongoing basis.
Contributing factors. Determining the overall contribution of different factors to a patient’s negative symptoms allows us to target treatments. Sorting out these relative factors can be difficult, however. For example:
- In a patient on antipsychotic treatment who is experiencing psychotic symptoms (eg, persecutory delusions), depressive symptoms, and prominent negative symptoms, the clinician can only guess whether the negative symptoms are primary or secondary.
- In a patient who is socially withdrawn and delusional, withdrawal may be secondary to delusions or may represent a primary negative symptom.
- In a patient on typical antipsychotics, a flat affect may be caused by antipsychotic-induced EPS or it may be a primary negative symptom.
- A disorganized patient with schizophrenia and depression is often unable to convey his or her feelings coherently, so that negative symptoms secondary to affective disturbance may often be mistaken as primary.
Even in research settings, the distinction between primary and secondary symptoms is quite unreliable; nevertheless, it is of great clinical importance. Two strategies may be helpful:
- Consider whether symptoms are specific to the presumed etiology, such as guilt and sadness in depression or cogwheeling and tremor in EPS.
- Treat empirically, and monitor whether negative symptoms improve. If they improve with antidepressant treatment, for example, then depression was the presumable cause. If they improve with anticholinergics, they were presumably secondary to EPS.
Treatment
Negative symptoms are generally viewed as treatment-resistant, but evidence suggests that they do respond to pharmacologic and social interventions (Box). Most responsive to treatment are negative symptoms that occur in association with positive symptoms (psychotic-phase) and secondary negative symptoms caused by neuroleptic medication, depression, or lack of stimulation.
The most effective treatment for secondary symptoms is to target the underlying cause. Neuroleptic-induced akinesia may respond to anticholinergic agents, reduction in antipsychotic dose, or a change in antipsychotic. Using one of the newer-generation antipsychotics (clozapine, risperidone, olanzapine, quetiapine, or ziprasidone) may prevent EPS.
Apsychosocial approach to schizophrenia builds on relationships between the patient and others and may involve social skills training, vocational rehabilitation, and psychotherapy. Activity-oriented therapies appear to be significantly more effective than verbal therapies.
Goals of psychosocial therapy:
- set realistic expectations for the patient
- stay active in treatment in the face of a protracted illness
- create a benign and supportive environment for the patient and caregivers.
Social skills training, designed to help the patient correctly perceive and respond to social situations, is the most widely studied and applied psychosocial intervention. The training is similar to that used in educational settings but focuses on remedying social rather than academic deficits. In schizophrenia, skills training programs address living skills, communication, conflict resolution, vocational skills, etc.
In early studies of social skills training, patients and their families described enhanced social adjustment, and hospitalization rates improved. More recent studies have confirmed improved social adjustment and relapse rates but suggest that overall symptom improvement is modest.
Continue to: Comorbid depression
Comorbid depression may require adding an antidepressant, or it may respond directly to an antipsychotic. Lack of stimulation is best handled by placing the patient in a more appropriately stimulating (but not overstimulating) and supportive environment. Nonenduring primary or psychotic-phase negative symptoms respond to effective antipsychotic treatment of the positive symptoms.
Atypical antipsychotics. Conventional antipsychotics (e.g., haloperidol, chlorpromazine) clearly offer some benefit in treating negative symptoms, but they have a much greater effect on positive symptoms.15 Using higher-than-appropriate doses diminishes their effect on negative symptoms and may result in severe EPS.
Two-thirds of the approximately 35 studies comparing conventional and atypical antipsychotics in treating negative symptoms have found atypicals to be significantly more effective (regardless of which atypical was used). In general, atypical antipsychotics improve negative symptoms by about 25%, compared with 10 to 15% improvement with conventional agents.16,17
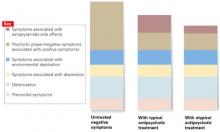
Much of the greater benefit with atypicals appears to be related to their at least equivalent ability to improve positive symptoms without causing EPS. Consequently, the key to improved patient outcomes is appropriate dosing of atypical antipsychotics that reduces positive symptoms optimally without EPS and without the need for an anticholinergic (Figure).
Whether the greater improvement with atypical agents implies an improvement in primary versus secondary negative symptoms is academic.18 From the patient’s perspective, the greater reduction in negative symptoms is meaningful, regardless of why it occurs.
Other medications. Secondary negative symptoms are most effectively treated with medications directed at the primary etiology. For EPS, change the antipsychotic, reduce the dosage, or add an anticholinergic. For depression, try an antidepressant (preferably a selective serotonin reuptake inhibitor). If a likely contributing factor can be identified, then initiate specific treatment.
Figure
ANTIPSYCHOTICS IMPROVE NEGATIVE SYMPTOMS THROUGH THEIR EFFECT ON PSYCHOSIS
Source: Adapted from Tandon et al. J Psychiatric Res. 1993;27:341-347.
Antipsychotics improve negative symptoms through their effect on positive (psychotic) symptoms, but they do not affect secondary components—such as environmental deprivation and depression—or the primary components of deterioration and premorbid symptoms. Typical and atypical antipsychotics have similar effects on positive symptoms, but atypical antipsychotics carry a lower risk of extrapyramidal side effects.
Empiric therapy—trying one agent and then another in an effort to reduce negative symptoms—is appropriate if done systematically and sequentially. Medications that are found not to be helpful should be discontinued. Electroconvulsive therapy is not effective in treating negative symptoms.
Related resources
- Greden JF, Tandon R (eds). Negative schizophrenic symptoms: pathophysiology and clinical implications. Washington, DC: American Psychiatric Press, 1991.
- Keefe RSE, McEvoy JP (eds). Negative symptom and cognitive deficit treatment response in schizophrenia. Washington, DC: American Psychiatric Press, 2001.
Drug brand names
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
1. Kraepelin E. Dementia praecox and paraphrenia. Translated by Barclay RM, Robertson GM. Edinburgh: E&S Livingstone; 1919.
2. Bleuler E. Dementia praecox or the group of schizophrenias. Translated by Zinkin H. New York: International Universities Press; 1911.
3. Crow TJ. Molecular pathology of schizophrenia: More than one disease process. Br Med J. 1980;280:66-68.
4. Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry. 1982;39:784-788.
5. Carpenter WT Jr, Heinrichs DW, Alphs LD. Treatment of negative symptoms. Schizophrenia Bull. 1985;11:440-452.
6. Carpenter WT, Jr, Heinrichs DW, Wagman AMI. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578-583.
7. DeQuardo JR, Tandon R. Do atypical antipsychotic medications favorably alter the long-term course of schizophrenia? J Psychiatric Res. 1998;32:229-242.
8. Tandon R, Greden JF. Cholinergic hyperactivity and negative schizophrenic symptoms. Arch Gen Psychiatry. 1989;46:745-753.
9. Tandon R, et al. Covariance of positive and negative symptoms during neuroleptic treatment in schizophrenia: a replication. Biol Psychiatry. 1993;34(7):495-497.
10. Tandon R, Milner K, Jibson MD. Antipsychotics from theory to practice: integrating clinical and basic data. J Clin Psychiatry. 1999;60(suppl 8):21-28.
11. Jibson MD, Tandon R. Treatment of schizophrenia. Psych Clin North Am Annual of Drug Therapy. 2000;7:83-113.
12. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS). Schizophrenia Bull. 1987;13:261-276.
13. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa; 1983.
14. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT, Jr. The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30(2):119-124.
15. Meltzer HY, Sommers AA, Luchins DJ. The effect of neuroleptics and other psychotropic drugs on negative symptoms in schizophrenia. J Clin Psychopharmacol. 1986;6:329-338.
16. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
17. Tandon R, Goldman R, DeQuardo JR, et al. Positive and negative symptoms covary during clozapine treatment in schizophrenia. J Psychiatric Res. 1993;27:341-347.
18. Breier A, Buchanan RW, Kirkpatrick B, et al. Effect of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151(1):20-26.
Negative symptoms are the major contributor to low function levels and debilitation in most patients with schizophrenia. Poorly motivated patients cannot function adequately at school or work. Relationships with family and friends decay in the face of unresponsive affect and inattention to social cues. Personal interests yield to the dampening influences of anhedonia, apathy, and inattention.
Yet because active psychosis is the most common cause of hospital admission, a primary goal of treatment—and sometimes the only objective of pharmacologic treatment—is to eliminate or reduce positive symptoms. And although controlling positive symptoms is remarkably effective in reducing hospitalizations, patients’ functional capacity improves only minimally as psychosis abates. Even with optimal antipsychotic treatment, negative symptoms tend to persist.
For psychiatrists, the three major challenges of schizophrenia’s negative symptoms are their modest therapeutic response, pervasiveness, and diminution of patients’ quality of life. To help you manage negative symptoms, we suggest the following approach to their assessment and treatment.
Importance of negative symptoms
Schizophrenia is a heterogeneous disorder characterized by positive, negative, cognitive, and mood symptoms. The relative severity of these four pathologic domains varies from case to case and within the same individual over time. Though related, these domains have distinct underlying mechanisms and are differentially related to functional capacity and quality of life. They also show different patterns of response to treatment. Whereas positive symptoms refer to new psychological experiences outside the range of normal (e.g., delusions, hallucinations, suspiciousness, disorganized thinking), negative symptoms represent loss of normal function.
Negative symptoms include blunting of affect, poverty of speech and thought, apathy, anhedonia, reduced social drive, loss of motivation, lack of social interest, and inattention to social or cognitive input. These symptoms have devastating consequences on patients’ lives, and only modest progress has been made in treating them effectively.
From negative to positive. Early investigators1,2 considered negative symptoms to represent the fundamental defect of schizophrenia. Over the years, however, the importance of negative symptoms was progressively downplayed. Positive symptoms were increasingly emphasized because:
- positive symptoms have a more dramatic and easily recognized presentation
- negative symptoms are more difficult to reliably define and document
- antipsychotics, which revolutionized schizophrenia treatment, produce their most dramatic improvement in positive symptoms.
Renewed interest. The almost universal presence and relative persistence of negative symptoms, and the fact that they represent the most debilitating and refractory aspect of schizophrenic psychopathology, make them difficult to ignore. Consequently, interest in negative symptoms resurged in the 1980s-90s, with intense efforts to better understand them and treat them more effectively.3-5
Table
SCHIZOPHRENIA’S NEGATIVE SYMPTOMS: PRIMARY AND SECONDARY COMPONENTS
| Primary Associated with positive symptoms Deficit or primary enduring symptoms (premorbid and deteriorative) |
| Secondary Associated with extrapyramidal symptoms, depression, or environmental deprivation |
| Source: Adapted from DeQuardo JR, Tandon R. J Psychiatr Res 1998;32 (3-4):229-42. |
Negative symptoms are now better (but still incompletely) understood, and their treatment has improved but is still inadequate. Because intense effort yielded only modest success, researchers and clinicians have again begun to pay less attention to negative symptoms and shifted their focus to cognition in schizophrenia. Negative symptoms remain relevant, however, because they constitute the main barrier to a better quality of life for patients with schizophrenia.
Assessment for negative symptoms
The four major clinical subgroups of negative symptoms are affective, communicative, conational, and relational.
Affective. Blunted affect—including deficits in facial expression, eye contact, gestures, and voice pattern—is perhaps the most conspicuous negative symptom. In mild form, gestures may seem artificial or mechanical, and the voice is stilted or lacks normal inflection. Patients with severe blunted affect may appear devoid of facial expression or communicative gestures. They may sit impassively with little spontaneous movement, speak in a monotone, and gaze blankly in no particular direction.
Even when conversation becomes emotional, the patient’s affect does not adjust appropriately to reflect his or her feelings. Nor does the patient display even a basic level of understanding or responsiveness that typically characterize casual human interactions. The ability to experience pleasure (anhedonia) and sense of caring (apathy) are also reduced.
Communicative. The patient’s speech may be reduced in quantity (poverty of speech) and information (poverty of content of speech). In mild forms of impoverished speech (alogia), the patient makes brief, unelaborated statements; in the more severe form, the patient can be virtually mute. Whatever speech is present tends to be vague and overly generalized. Periods of silence may occur, either before the patient answers a question (increased latency) or in the midst of a response (blocking).
Conational. The patient may show a lack of drive or goal-directed behavior (avolition). Personal grooming may be poor. Physical activity may be limited. Patients typically have great difficulty following a work schedule or hospital ward routine. They fail to initiate activities, participate grudgingly, and require frequent direction and encouragement.
Continue to: Relational
Relational. Interest in social activities and relationships is reduced (asociality). Even enjoyable and recreational activities are neglected. Interpersonal relations may be of little interest. Friendships become rare and shallow, with little sharing of intimacy. Contacts with family are neglected. Sexual interest declines. As symptoms progress, patients become increasingly isolated.
Primary and secondary symptoms
Negative symptoms are an intrinsic component of schizophrenic psychopathology, and they can also be caused by secondary factors (Table).6,7 Distinguishing between primary and secondary causes of negative symptoms can help you select appropriate treatment in specific clinical situations.
Primary symptoms. From a longitudinal perspective, the three major components of primary negative symptoms are:
- premorbid negative symptoms (present prior to psychosis onset and associated with poor premorbid functioning)
- psychotic-phase, nonenduring negative symptoms that fluctuate with positive symptoms around periods of psychotic exacerbation
- deteriorative negative symptoms that intensify following each psychotic exacerbation and reflect a decline from premorbid levels of functioning.
Though little can be done to treat the premorbid component, psychotic-phase negative symptoms improve along with positive symptoms (although more slowly).8,9 Therefore, the best strategy for managing negative symptoms is to treat positive symptoms more effectively. Although there is no specific treatment for deteriorative negative symptoms, the severity of this component appears to be related to the “toxicity of psychosis” and can be reduced by early, effective antipsychotic treatment.10,11
Secondary negative symptoms occur in association with (and presumably are caused by) factors such as depression, extrapyramidal symptoms (EPS), and environmental deprivation. Secondary negative symptoms usually respond to treatment of the underlying cause.
Assessment
Symptom severity. Assessing the severity of a patient’s negative symptoms on an ongoing basis is a most important first step towards optimal treatment:
- Our objective is to improve patients’ function and quality of life, and negative symptoms compromise both of these more than any other factor.
- Ongoing assessment can track whether prescribed treatments are improving or worsening a patient’s symptoms.
Tools to assess the severity of negative symptoms include the Brief Psychiatric Rating Scale (BPRS) and Positive and Negative Symptom Scale (PANSS).12 The Scale for the Assessment of Negative Symptoms (SANS)13 measures them exclusively, and others such as the Schedule for the Deficit Syndrome (SDS)14 attempt to classify them into subgroups.
Discussing these instruments is beyond the scope of this article, but they differ greatly in their approach to assessing negative symptoms. Instead of using cumbersome assessment instruments, however, we recommend that you focus on two to four of a patient’s “target” symptoms or behaviors and note their severity on an ongoing basis.
Contributing factors. Determining the overall contribution of different factors to a patient’s negative symptoms allows us to target treatments. Sorting out these relative factors can be difficult, however. For example:
- In a patient on antipsychotic treatment who is experiencing psychotic symptoms (eg, persecutory delusions), depressive symptoms, and prominent negative symptoms, the clinician can only guess whether the negative symptoms are primary or secondary.
- In a patient who is socially withdrawn and delusional, withdrawal may be secondary to delusions or may represent a primary negative symptom.
- In a patient on typical antipsychotics, a flat affect may be caused by antipsychotic-induced EPS or it may be a primary negative symptom.
- A disorganized patient with schizophrenia and depression is often unable to convey his or her feelings coherently, so that negative symptoms secondary to affective disturbance may often be mistaken as primary.
Even in research settings, the distinction between primary and secondary symptoms is quite unreliable; nevertheless, it is of great clinical importance. Two strategies may be helpful:
- Consider whether symptoms are specific to the presumed etiology, such as guilt and sadness in depression or cogwheeling and tremor in EPS.
- Treat empirically, and monitor whether negative symptoms improve. If they improve with antidepressant treatment, for example, then depression was the presumable cause. If they improve with anticholinergics, they were presumably secondary to EPS.
Treatment
Negative symptoms are generally viewed as treatment-resistant, but evidence suggests that they do respond to pharmacologic and social interventions (Box). Most responsive to treatment are negative symptoms that occur in association with positive symptoms (psychotic-phase) and secondary negative symptoms caused by neuroleptic medication, depression, or lack of stimulation.
The most effective treatment for secondary symptoms is to target the underlying cause. Neuroleptic-induced akinesia may respond to anticholinergic agents, reduction in antipsychotic dose, or a change in antipsychotic. Using one of the newer-generation antipsychotics (clozapine, risperidone, olanzapine, quetiapine, or ziprasidone) may prevent EPS.
Apsychosocial approach to schizophrenia builds on relationships between the patient and others and may involve social skills training, vocational rehabilitation, and psychotherapy. Activity-oriented therapies appear to be significantly more effective than verbal therapies.
Goals of psychosocial therapy:
- set realistic expectations for the patient
- stay active in treatment in the face of a protracted illness
- create a benign and supportive environment for the patient and caregivers.
Social skills training, designed to help the patient correctly perceive and respond to social situations, is the most widely studied and applied psychosocial intervention. The training is similar to that used in educational settings but focuses on remedying social rather than academic deficits. In schizophrenia, skills training programs address living skills, communication, conflict resolution, vocational skills, etc.
In early studies of social skills training, patients and their families described enhanced social adjustment, and hospitalization rates improved. More recent studies have confirmed improved social adjustment and relapse rates but suggest that overall symptom improvement is modest.
Continue to: Comorbid depression
Comorbid depression may require adding an antidepressant, or it may respond directly to an antipsychotic. Lack of stimulation is best handled by placing the patient in a more appropriately stimulating (but not overstimulating) and supportive environment. Nonenduring primary or psychotic-phase negative symptoms respond to effective antipsychotic treatment of the positive symptoms.
Atypical antipsychotics. Conventional antipsychotics (e.g., haloperidol, chlorpromazine) clearly offer some benefit in treating negative symptoms, but they have a much greater effect on positive symptoms.15 Using higher-than-appropriate doses diminishes their effect on negative symptoms and may result in severe EPS.
Two-thirds of the approximately 35 studies comparing conventional and atypical antipsychotics in treating negative symptoms have found atypicals to be significantly more effective (regardless of which atypical was used). In general, atypical antipsychotics improve negative symptoms by about 25%, compared with 10 to 15% improvement with conventional agents.16,17
Much of the greater benefit with atypicals appears to be related to their at least equivalent ability to improve positive symptoms without causing EPS. Consequently, the key to improved patient outcomes is appropriate dosing of atypical antipsychotics that reduces positive symptoms optimally without EPS and without the need for an anticholinergic (Figure).
Whether the greater improvement with atypical agents implies an improvement in primary versus secondary negative symptoms is academic.18 From the patient’s perspective, the greater reduction in negative symptoms is meaningful, regardless of why it occurs.
Other medications. Secondary negative symptoms are most effectively treated with medications directed at the primary etiology. For EPS, change the antipsychotic, reduce the dosage, or add an anticholinergic. For depression, try an antidepressant (preferably a selective serotonin reuptake inhibitor). If a likely contributing factor can be identified, then initiate specific treatment.
Figure
ANTIPSYCHOTICS IMPROVE NEGATIVE SYMPTOMS THROUGH THEIR EFFECT ON PSYCHOSIS
Source: Adapted from Tandon et al. J Psychiatric Res. 1993;27:341-347.
Antipsychotics improve negative symptoms through their effect on positive (psychotic) symptoms, but they do not affect secondary components—such as environmental deprivation and depression—or the primary components of deterioration and premorbid symptoms. Typical and atypical antipsychotics have similar effects on positive symptoms, but atypical antipsychotics carry a lower risk of extrapyramidal side effects.
Empiric therapy—trying one agent and then another in an effort to reduce negative symptoms—is appropriate if done systematically and sequentially. Medications that are found not to be helpful should be discontinued. Electroconvulsive therapy is not effective in treating negative symptoms.
Related resources
- Greden JF, Tandon R (eds). Negative schizophrenic symptoms: pathophysiology and clinical implications. Washington, DC: American Psychiatric Press, 1991.
- Keefe RSE, McEvoy JP (eds). Negative symptom and cognitive deficit treatment response in schizophrenia. Washington, DC: American Psychiatric Press, 2001.
Drug brand names
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
Negative symptoms are the major contributor to low function levels and debilitation in most patients with schizophrenia. Poorly motivated patients cannot function adequately at school or work. Relationships with family and friends decay in the face of unresponsive affect and inattention to social cues. Personal interests yield to the dampening influences of anhedonia, apathy, and inattention.
Yet because active psychosis is the most common cause of hospital admission, a primary goal of treatment—and sometimes the only objective of pharmacologic treatment—is to eliminate or reduce positive symptoms. And although controlling positive symptoms is remarkably effective in reducing hospitalizations, patients’ functional capacity improves only minimally as psychosis abates. Even with optimal antipsychotic treatment, negative symptoms tend to persist.
For psychiatrists, the three major challenges of schizophrenia’s negative symptoms are their modest therapeutic response, pervasiveness, and diminution of patients’ quality of life. To help you manage negative symptoms, we suggest the following approach to their assessment and treatment.
Importance of negative symptoms
Schizophrenia is a heterogeneous disorder characterized by positive, negative, cognitive, and mood symptoms. The relative severity of these four pathologic domains varies from case to case and within the same individual over time. Though related, these domains have distinct underlying mechanisms and are differentially related to functional capacity and quality of life. They also show different patterns of response to treatment. Whereas positive symptoms refer to new psychological experiences outside the range of normal (e.g., delusions, hallucinations, suspiciousness, disorganized thinking), negative symptoms represent loss of normal function.
Negative symptoms include blunting of affect, poverty of speech and thought, apathy, anhedonia, reduced social drive, loss of motivation, lack of social interest, and inattention to social or cognitive input. These symptoms have devastating consequences on patients’ lives, and only modest progress has been made in treating them effectively.
From negative to positive. Early investigators1,2 considered negative symptoms to represent the fundamental defect of schizophrenia. Over the years, however, the importance of negative symptoms was progressively downplayed. Positive symptoms were increasingly emphasized because:
- positive symptoms have a more dramatic and easily recognized presentation
- negative symptoms are more difficult to reliably define and document
- antipsychotics, which revolutionized schizophrenia treatment, produce their most dramatic improvement in positive symptoms.
Renewed interest. The almost universal presence and relative persistence of negative symptoms, and the fact that they represent the most debilitating and refractory aspect of schizophrenic psychopathology, make them difficult to ignore. Consequently, interest in negative symptoms resurged in the 1980s-90s, with intense efforts to better understand them and treat them more effectively.3-5
Table
SCHIZOPHRENIA’S NEGATIVE SYMPTOMS: PRIMARY AND SECONDARY COMPONENTS
| Primary Associated with positive symptoms Deficit or primary enduring symptoms (premorbid and deteriorative) |
| Secondary Associated with extrapyramidal symptoms, depression, or environmental deprivation |
| Source: Adapted from DeQuardo JR, Tandon R. J Psychiatr Res 1998;32 (3-4):229-42. |
Negative symptoms are now better (but still incompletely) understood, and their treatment has improved but is still inadequate. Because intense effort yielded only modest success, researchers and clinicians have again begun to pay less attention to negative symptoms and shifted their focus to cognition in schizophrenia. Negative symptoms remain relevant, however, because they constitute the main barrier to a better quality of life for patients with schizophrenia.
Assessment for negative symptoms
The four major clinical subgroups of negative symptoms are affective, communicative, conational, and relational.
Affective. Blunted affect—including deficits in facial expression, eye contact, gestures, and voice pattern—is perhaps the most conspicuous negative symptom. In mild form, gestures may seem artificial or mechanical, and the voice is stilted or lacks normal inflection. Patients with severe blunted affect may appear devoid of facial expression or communicative gestures. They may sit impassively with little spontaneous movement, speak in a monotone, and gaze blankly in no particular direction.
Even when conversation becomes emotional, the patient’s affect does not adjust appropriately to reflect his or her feelings. Nor does the patient display even a basic level of understanding or responsiveness that typically characterize casual human interactions. The ability to experience pleasure (anhedonia) and sense of caring (apathy) are also reduced.
Communicative. The patient’s speech may be reduced in quantity (poverty of speech) and information (poverty of content of speech). In mild forms of impoverished speech (alogia), the patient makes brief, unelaborated statements; in the more severe form, the patient can be virtually mute. Whatever speech is present tends to be vague and overly generalized. Periods of silence may occur, either before the patient answers a question (increased latency) or in the midst of a response (blocking).
Conational. The patient may show a lack of drive or goal-directed behavior (avolition). Personal grooming may be poor. Physical activity may be limited. Patients typically have great difficulty following a work schedule or hospital ward routine. They fail to initiate activities, participate grudgingly, and require frequent direction and encouragement.
Continue to: Relational
Relational. Interest in social activities and relationships is reduced (asociality). Even enjoyable and recreational activities are neglected. Interpersonal relations may be of little interest. Friendships become rare and shallow, with little sharing of intimacy. Contacts with family are neglected. Sexual interest declines. As symptoms progress, patients become increasingly isolated.
Primary and secondary symptoms
Negative symptoms are an intrinsic component of schizophrenic psychopathology, and they can also be caused by secondary factors (Table).6,7 Distinguishing between primary and secondary causes of negative symptoms can help you select appropriate treatment in specific clinical situations.
Primary symptoms. From a longitudinal perspective, the three major components of primary negative symptoms are:
- premorbid negative symptoms (present prior to psychosis onset and associated with poor premorbid functioning)
- psychotic-phase, nonenduring negative symptoms that fluctuate with positive symptoms around periods of psychotic exacerbation
- deteriorative negative symptoms that intensify following each psychotic exacerbation and reflect a decline from premorbid levels of functioning.
Though little can be done to treat the premorbid component, psychotic-phase negative symptoms improve along with positive symptoms (although more slowly).8,9 Therefore, the best strategy for managing negative symptoms is to treat positive symptoms more effectively. Although there is no specific treatment for deteriorative negative symptoms, the severity of this component appears to be related to the “toxicity of psychosis” and can be reduced by early, effective antipsychotic treatment.10,11
Secondary negative symptoms occur in association with (and presumably are caused by) factors such as depression, extrapyramidal symptoms (EPS), and environmental deprivation. Secondary negative symptoms usually respond to treatment of the underlying cause.
Assessment
Symptom severity. Assessing the severity of a patient’s negative symptoms on an ongoing basis is a most important first step towards optimal treatment:
- Our objective is to improve patients’ function and quality of life, and negative symptoms compromise both of these more than any other factor.
- Ongoing assessment can track whether prescribed treatments are improving or worsening a patient’s symptoms.
Tools to assess the severity of negative symptoms include the Brief Psychiatric Rating Scale (BPRS) and Positive and Negative Symptom Scale (PANSS).12 The Scale for the Assessment of Negative Symptoms (SANS)13 measures them exclusively, and others such as the Schedule for the Deficit Syndrome (SDS)14 attempt to classify them into subgroups.
Discussing these instruments is beyond the scope of this article, but they differ greatly in their approach to assessing negative symptoms. Instead of using cumbersome assessment instruments, however, we recommend that you focus on two to four of a patient’s “target” symptoms or behaviors and note their severity on an ongoing basis.
Contributing factors. Determining the overall contribution of different factors to a patient’s negative symptoms allows us to target treatments. Sorting out these relative factors can be difficult, however. For example:
- In a patient on antipsychotic treatment who is experiencing psychotic symptoms (eg, persecutory delusions), depressive symptoms, and prominent negative symptoms, the clinician can only guess whether the negative symptoms are primary or secondary.
- In a patient who is socially withdrawn and delusional, withdrawal may be secondary to delusions or may represent a primary negative symptom.
- In a patient on typical antipsychotics, a flat affect may be caused by antipsychotic-induced EPS or it may be a primary negative symptom.
- A disorganized patient with schizophrenia and depression is often unable to convey his or her feelings coherently, so that negative symptoms secondary to affective disturbance may often be mistaken as primary.
Even in research settings, the distinction between primary and secondary symptoms is quite unreliable; nevertheless, it is of great clinical importance. Two strategies may be helpful:
- Consider whether symptoms are specific to the presumed etiology, such as guilt and sadness in depression or cogwheeling and tremor in EPS.
- Treat empirically, and monitor whether negative symptoms improve. If they improve with antidepressant treatment, for example, then depression was the presumable cause. If they improve with anticholinergics, they were presumably secondary to EPS.
Treatment
Negative symptoms are generally viewed as treatment-resistant, but evidence suggests that they do respond to pharmacologic and social interventions (Box). Most responsive to treatment are negative symptoms that occur in association with positive symptoms (psychotic-phase) and secondary negative symptoms caused by neuroleptic medication, depression, or lack of stimulation.
The most effective treatment for secondary symptoms is to target the underlying cause. Neuroleptic-induced akinesia may respond to anticholinergic agents, reduction in antipsychotic dose, or a change in antipsychotic. Using one of the newer-generation antipsychotics (clozapine, risperidone, olanzapine, quetiapine, or ziprasidone) may prevent EPS.
Apsychosocial approach to schizophrenia builds on relationships between the patient and others and may involve social skills training, vocational rehabilitation, and psychotherapy. Activity-oriented therapies appear to be significantly more effective than verbal therapies.
Goals of psychosocial therapy:
- set realistic expectations for the patient
- stay active in treatment in the face of a protracted illness
- create a benign and supportive environment for the patient and caregivers.
Social skills training, designed to help the patient correctly perceive and respond to social situations, is the most widely studied and applied psychosocial intervention. The training is similar to that used in educational settings but focuses on remedying social rather than academic deficits. In schizophrenia, skills training programs address living skills, communication, conflict resolution, vocational skills, etc.
In early studies of social skills training, patients and their families described enhanced social adjustment, and hospitalization rates improved. More recent studies have confirmed improved social adjustment and relapse rates but suggest that overall symptom improvement is modest.
Continue to: Comorbid depression
Comorbid depression may require adding an antidepressant, or it may respond directly to an antipsychotic. Lack of stimulation is best handled by placing the patient in a more appropriately stimulating (but not overstimulating) and supportive environment. Nonenduring primary or psychotic-phase negative symptoms respond to effective antipsychotic treatment of the positive symptoms.
Atypical antipsychotics. Conventional antipsychotics (e.g., haloperidol, chlorpromazine) clearly offer some benefit in treating negative symptoms, but they have a much greater effect on positive symptoms.15 Using higher-than-appropriate doses diminishes their effect on negative symptoms and may result in severe EPS.
Two-thirds of the approximately 35 studies comparing conventional and atypical antipsychotics in treating negative symptoms have found atypicals to be significantly more effective (regardless of which atypical was used). In general, atypical antipsychotics improve negative symptoms by about 25%, compared with 10 to 15% improvement with conventional agents.16,17
Much of the greater benefit with atypicals appears to be related to their at least equivalent ability to improve positive symptoms without causing EPS. Consequently, the key to improved patient outcomes is appropriate dosing of atypical antipsychotics that reduces positive symptoms optimally without EPS and without the need for an anticholinergic (Figure).
Whether the greater improvement with atypical agents implies an improvement in primary versus secondary negative symptoms is academic.18 From the patient’s perspective, the greater reduction in negative symptoms is meaningful, regardless of why it occurs.
Other medications. Secondary negative symptoms are most effectively treated with medications directed at the primary etiology. For EPS, change the antipsychotic, reduce the dosage, or add an anticholinergic. For depression, try an antidepressant (preferably a selective serotonin reuptake inhibitor). If a likely contributing factor can be identified, then initiate specific treatment.
Figure
ANTIPSYCHOTICS IMPROVE NEGATIVE SYMPTOMS THROUGH THEIR EFFECT ON PSYCHOSIS
Source: Adapted from Tandon et al. J Psychiatric Res. 1993;27:341-347.
Antipsychotics improve negative symptoms through their effect on positive (psychotic) symptoms, but they do not affect secondary components—such as environmental deprivation and depression—or the primary components of deterioration and premorbid symptoms. Typical and atypical antipsychotics have similar effects on positive symptoms, but atypical antipsychotics carry a lower risk of extrapyramidal side effects.
Empiric therapy—trying one agent and then another in an effort to reduce negative symptoms—is appropriate if done systematically and sequentially. Medications that are found not to be helpful should be discontinued. Electroconvulsive therapy is not effective in treating negative symptoms.
Related resources
- Greden JF, Tandon R (eds). Negative schizophrenic symptoms: pathophysiology and clinical implications. Washington, DC: American Psychiatric Press, 1991.
- Keefe RSE, McEvoy JP (eds). Negative symptom and cognitive deficit treatment response in schizophrenia. Washington, DC: American Psychiatric Press, 2001.
Drug brand names
- Chlorpromazine • Thorazine
- Clozapine • Clozaril
- Haloperidol • Haldol
- Olanzapine • Zyprexa
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Ziprasidone • Geodon
1. Kraepelin E. Dementia praecox and paraphrenia. Translated by Barclay RM, Robertson GM. Edinburgh: E&S Livingstone; 1919.
2. Bleuler E. Dementia praecox or the group of schizophrenias. Translated by Zinkin H. New York: International Universities Press; 1911.
3. Crow TJ. Molecular pathology of schizophrenia: More than one disease process. Br Med J. 1980;280:66-68.
4. Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry. 1982;39:784-788.
5. Carpenter WT Jr, Heinrichs DW, Alphs LD. Treatment of negative symptoms. Schizophrenia Bull. 1985;11:440-452.
6. Carpenter WT, Jr, Heinrichs DW, Wagman AMI. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578-583.
7. DeQuardo JR, Tandon R. Do atypical antipsychotic medications favorably alter the long-term course of schizophrenia? J Psychiatric Res. 1998;32:229-242.
8. Tandon R, Greden JF. Cholinergic hyperactivity and negative schizophrenic symptoms. Arch Gen Psychiatry. 1989;46:745-753.
9. Tandon R, et al. Covariance of positive and negative symptoms during neuroleptic treatment in schizophrenia: a replication. Biol Psychiatry. 1993;34(7):495-497.
10. Tandon R, Milner K, Jibson MD. Antipsychotics from theory to practice: integrating clinical and basic data. J Clin Psychiatry. 1999;60(suppl 8):21-28.
11. Jibson MD, Tandon R. Treatment of schizophrenia. Psych Clin North Am Annual of Drug Therapy. 2000;7:83-113.
12. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS). Schizophrenia Bull. 1987;13:261-276.
13. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa; 1983.
14. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT, Jr. The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30(2):119-124.
15. Meltzer HY, Sommers AA, Luchins DJ. The effect of neuroleptics and other psychotropic drugs on negative symptoms in schizophrenia. J Clin Psychopharmacol. 1986;6:329-338.
16. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
17. Tandon R, Goldman R, DeQuardo JR, et al. Positive and negative symptoms covary during clozapine treatment in schizophrenia. J Psychiatric Res. 1993;27:341-347.
18. Breier A, Buchanan RW, Kirkpatrick B, et al. Effect of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151(1):20-26.
1. Kraepelin E. Dementia praecox and paraphrenia. Translated by Barclay RM, Robertson GM. Edinburgh: E&S Livingstone; 1919.
2. Bleuler E. Dementia praecox or the group of schizophrenias. Translated by Zinkin H. New York: International Universities Press; 1911.
3. Crow TJ. Molecular pathology of schizophrenia: More than one disease process. Br Med J. 1980;280:66-68.
4. Andreasen NC. Negative symptoms in schizophrenia: definition and reliability. Arch Gen Psychiatry. 1982;39:784-788.
5. Carpenter WT Jr, Heinrichs DW, Alphs LD. Treatment of negative symptoms. Schizophrenia Bull. 1985;11:440-452.
6. Carpenter WT, Jr, Heinrichs DW, Wagman AMI. Deficit and nondeficit forms of schizophrenia: the concept. Am J Psychiatry. 1988;145:578-583.
7. DeQuardo JR, Tandon R. Do atypical antipsychotic medications favorably alter the long-term course of schizophrenia? J Psychiatric Res. 1998;32:229-242.
8. Tandon R, Greden JF. Cholinergic hyperactivity and negative schizophrenic symptoms. Arch Gen Psychiatry. 1989;46:745-753.
9. Tandon R, et al. Covariance of positive and negative symptoms during neuroleptic treatment in schizophrenia: a replication. Biol Psychiatry. 1993;34(7):495-497.
10. Tandon R, Milner K, Jibson MD. Antipsychotics from theory to practice: integrating clinical and basic data. J Clin Psychiatry. 1999;60(suppl 8):21-28.
11. Jibson MD, Tandon R. Treatment of schizophrenia. Psych Clin North Am Annual of Drug Therapy. 2000;7:83-113.
12. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS). Schizophrenia Bull. 1987;13:261-276.
13. Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS). Iowa City: University of Iowa; 1983.
14. Kirkpatrick B, Buchanan RW, McKenney PD, Alphs LD, Carpenter WT, Jr. The Schedule for the Deficit Syndrome: an instrument for research in schizophrenia. Psychiatry Res. 1989;30(2):119-124.
15. Meltzer HY, Sommers AA, Luchins DJ. The effect of neuroleptics and other psychotropic drugs on negative symptoms in schizophrenia. J Clin Psychopharmacol. 1986;6:329-338.
16. Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic: a double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45(9):789-796.
17. Tandon R, Goldman R, DeQuardo JR, et al. Positive and negative symptoms covary during clozapine treatment in schizophrenia. J Psychiatric Res. 1993;27:341-347.
18. Breier A, Buchanan RW, Kirkpatrick B, et al. Effect of clozapine on positive and negative symptoms in outpatients with schizophrenia. Am J Psychiatry. 1994;151(1):20-26.
Psychiatric symptoms in Parkinson’s disease: A team approach to successful management
Depression, anxiety, and psychosis are common complications of Parkinson’s disease (PD) and of the medications used in antiparkinsonian treatment. These psychiatric problems impair patients’ functioning throughout the course of the chronic degenerative disease.
Because medication side effects often call for adjustments and trade-offs in PD treatment, a team effort by the psychiatrist, neurologist, patient, and caregiver is the most effective approach to decision-making. From our experience in such collaborations, here’s what you need to know about PD to be a most-valuable player on that treatment team.
Presentation of PD
The classic triad of PD features consists of a pill-rolling tremor, rigidity, and bradykinesia or slowness of movement. Other common features include postural instability, flexed posture, and other motor-freezing phenomena.
Freezing phenomena occur in the later stages of PD, as the response to dopaminergic therapy becomes erratic and unpredictable. Freezing can range from hesitation—such as when the patient tries to turn or is in a doorway—to transient episodes of total inability to move. These episodes are extremely distressing for both patients and caregivers.
Patients rarely present with the full complement of symptoms, but the presence of tremor at rest and/or bradykinesia is essential for the diagnosis. While motor signs dominate the presentation, cognitive symptoms such as shortened attention span, visuospatial impairment, personality changes, and dementia are also frequently present.
Average age of diagnosis is 60, and more men are affected than women (male-to-female ratio is 3:2). Many causative factors—including genetics and environmental toxins—have been implicated, but the disorder’s etiology remains unknown.
Drug treatment side effects
PD results from the loss of neurons in the substantia nigra that produce the neurotransmitter dopamine. Pharmacologic treatment emphasizes dopamine replacement, dopamine receptor stimulation, or prevention of enzymatic breakdown of dopamine in the synaptic cleft. As treatment of PD is symptomatic and not curative, medications are instituted only when the disease begins to cause functional impairment.
Treatment begins with dopamine agonists (Table). As dopamine agonist monotherapy becomes less effective, levodopa therapy is initiated. Blocking the enzymatic breakdown of dopamine with catechol-O-methyltransferase inhibitors is the next therapeutic strategy.
Within 5 years of starting levodopa therapy 75% of patients experience unsatisfactory motor response, from unpredictable fluctuations to wearing-off phenomena (in which a dose of levodopa does not last as long as it once did). Treatment of advanced PD is complicated by the emergence of psychiatric symptoms, such as hallucinations and psychosis, as dopamine levels are increased in an attempt to smooth the motor response.
The significantly distressing level of disability associated with the prominent side effects of pharmacologic treatment has led to interest in surgical interventions. These range from pallidotomy to implantation of basal ganglia stimulators to transplantation of fetal striatal neurons. The possibility of neuroprotection has also been extensively investigated, with mixed results.
Psychiatric complications of PD
Depression. Clearly, the stress of anticipating and coping with a relentless degenerative disease helps to trigger depression and anxiety in patients with PD. Depression is the most common psychiatric syndrome, with prevalence in PD as high as 42%.1 Patients with a history of depression are at particular risk.2 Those with recent deterioration or advancing severity of PD, akinesia, history of falls, or cognitive impairment are also at increased risk for depression.
Table
MEDICATIONS COMMONLY USED IN MANAGING PARKINSON’S DISEASE
| Medication class | Example | Indication for use |
|---|---|---|
| MAO-B inhibitor | Selegiline | ? Neuroprotection |
| Anticholinergic agents | Trihexyphenidyl, benztropine, biperiden, hyoscyamine, diphenhydramine | Tremor |
| Dopamine agonist | Pramipexole, pergolide, ropinirole | ? Neuroprotection Treatment of movement disorder |
| Dopamine replacement | Carbidopa-levodopa | Treatment of movement disorder |
| Catechol-O-methyltransferase inhibitor | Entacapone, tolcapone | Smooth motor fluctuations |
Depression correlates well with the patient’s perception of his or her degree of PD-related disability. Depression symptoms seem to peak early in the illness following diagnosis and in advanced disease.3
Patients may present with symptoms meeting diagnostic criteria ranging from dysthymic disorder to minor depression to major depressive disorder.1,4 Although they will frequently endorse suicidal ideation, patients with PD have a low rate of suicide. Diagnosing depression, however, may be difficult because its symptoms overlap with those of the underlying neurologic disease:
- Diminished affect and psychomotor slowing may be secondary to the motor features of parkinsonism.
- Diminished concentration may be secondary to cognitive decline rather than depression.
Patients also frequently have a chief complaint of diminished energy or fatigue that should trigger further investigation into other depressive symptoms.4,5
In addition to the obvious additional suffering it causes, depression in PD predicts impaired social, physical, and role functioning.6 Depression in the PD patient also results in higher distress for caregivers.7 In one study, depression was identified as a risk factor for development of psychosis in PD patients.8
Anxiety is a frequent problem for PD patients, with a prevalence of 33 to 40%.9,10 Anxiety in PD typically presents with symptoms of panic disorder, generalized anxiety disorder, or social phobia.11 It is comorbid with depression in up to 92% of cases and—like depression—frequently predates the onset of motor symptoms.12
Anxiety symptoms have been correlated, although not consistently, with the on-off motor phenomenon often found in advanced PD.13 They can also be an adverse effect of many of the antiparkinsonian medications, including anticholinergics, dopamine agonists, catechol-O-methyltransferase inhibitors, and selegiline. Both anxiety and depression have been associated with an increased risk for falls.14
Psychotic symptoms. Up to 25% of PD patients experience delusions or hallucinations.15 Risk factors include dementia, sleep disturbance, and—most commonly—the use of dopaminergic agents. Up to one-fifth of patients using dopaminergic drugs experience psychotic symptoms.16
Psychotic symptoms can occur with or without the clouded sensorium characteristic of delirium. Psychotic symptoms with an associated confusional state can be associated with use of anticholinergic agents and drugs such as selegiline and amantadine.17 Catechol-O-methyltransferase inhibitors cause more sustained dopaminergic activity of levodopa, which can result in psychotic symptoms. Therefore, the use of all known classes of antiparkinsonian medications has been associated with drug-induced psychosis.
In advanced PD, paranoid delusions, delusions of spousal infidelity, and visual hallucinations are common, whereas negative symptoms and thought disturbances are not.18 Psychosis may be a more important contributor to caregiver distress than the motor symptoms of PD and may be more likely than any other factor to lead to nursing home placement of the PD patient (Box 1).15
Psychiatric interventions
Goals for psychiatric treatment of depression, anxiety, and psychosis associated with PD seem relatively straightforward:
- improvement or remission of psychiatric symptoms
- restoration of optimal patient functioning.
Ideally, these goals would be achieved without causing sedation, orthostatic hypotension, or exacerbating motor symptoms. The older age of patients and the progressive nature of this neurodegenerative disorder predispose patients to cognitive side effects. Unfortunately, despite the high prevalence of psychiatric disturbances in PD, evidence with which to evaluate treatment efficacy and safety and to guide treatment selection is extremely limited.
For depression associated with PD, extensive clinical experience supports the efficacy of tricyclic antidepressants. Even so, selective serotonin reuptake inhibitors (SSRIs) are the preferred treatment, although only open-label trials and case reports support their efficacy.5,12 Compared with tricyclics, SSRIs exhibit a relative lack of problems with sedation, orthostatic hypotension, and memory-impairing anticholinergic side effects. While case reports have cited worsening of motor symptoms with SSRIs, a recent prospective study found no significant worsening of PD symptoms during treatment with citalopram, fluoxetine, fluvoxamine, or sertraline.19 Co-administration of an SSRI with selegiline is not absolutely contraindicated, but the combination does carry a very small risk of development of serotonin syndrome.1,5
Mr. J had a 6-year history of PD with pronounced bradykinesia and gait disturbance treated with amantadine and carbidopa-levodopa. His rigidity began to worsen, so the dosage of carbidopa-levodopa was increased. His wife then reported that he had increased confusion and balance problems. On evaluation, he was found to have a urinary tract infection. Following antibiotic treatment, mental status and gait returned to usual baseline.
One year later, Mr. J began having trouble getting out of bed, with unpredictable motor freezing episodes. Pramipexole was added to his regimen, and he began having prominent visual hallucinations. Low-dose trifluoperazine was added, and hallucinations improved. The patient became increasingly depressed, and sertraline was started.
Over the next year, his function progressively worsened, with increased motor freezing and unpredictable dyskinesias. Hallucinations complicated attempts to change his medications. Amantadine was stopped without improvement. He was referred for surgical evaluation, but because of his cognitive status and depression was deemed not to be a candidate.
He began to fall repeatedly and developed orthostatic hypotension. His clinical course continued to be complicated by hallucinations and delusions that his wife was being unfaithful. Ongoing psychosis and severe gait instability led to his admission to a nursing home.
Data are even more scant on the safe use of other antidepressants in PD. Electroconvulsive therapy has been proven helpful in refractory cases and sometimes results in transient motor symptom improvement.1,5,12 While clinical experience suggests that psychotherapy frequently helps, no extensive controlled studies exist. One small study suggests the efficacy of structured cognitive psychotherapy.20
Anxiety. No studies have examined the treatment of anxiety in PD patients. Given the extremely high comorbidity of anxiety with depression, antidepressants should probably be considered as a first-line pharmacotherapy. Benzodiazepines should be used cautiously, as they increase the risk of falls, sedation, and confusion in older patients. One small controlled study found that buspirone was well tolerated in PD patients at low dosages (10 to 40 mg/d), but anxiety did not improve. At high dosages (100 mg/d), anxiety worsened.21
Psychosis. Data on use of antipsychotic agents in PD are also limited, but some evidence supports their use in treating PD-related psychotic symptoms. While conventional antipsychotics can help control psychosis, the potential is high for worsening of parkinsonian symptoms due to D2 receptor blockade.
Among the atypical antipsychotics, clozapine has been most extensively studied in PD and has been shown in open and double-blind trials to be effective and well tolerated at low dosages (6.25 to 50 mg/d). A limited number of open studies of some of the newer atypicals have been performed. While extreme caution must be used in comparing data from these studies due to highly variable dosing and other study design issues, clozapine and quetiapine appear to be the agents best tolerated by PD patients.12,18,22 Initial antipsychotic dosing should be low and escalation cautious—regardless of the agent chosen—because of the dose-related potential for worsening of parkinsonian symptoms, sedation, and orthostatic hypotension.
A team approach to treatment
Because psychiatric and PD symptoms and treatments are closely interrelated, the psychiatrist, neurologist, patient, and caregiver must collaborate for the best therapeutic result. A simplistic approach to treatment can result in a catastrophic downward spiral in patient functioning.
Often, compromises must be made between optimal control of parkinsonian and psychiatric symptoms to achieve the best overall patient function. Patients and caregivers must be counseled about possible psychiatric symptoms associated with PD and antiparkinsonian therapy, as well as the potential for adverse effects from psychiatric medications. With this knowledge, patients and caregivers can help assess the severity of symptoms and set treatment priorities, depending on how symptoms may be affecting the patient’s level of functioning. For example, if an effective antiparkinsonian regimen has triggered infrequent, nondistressing hallucinations with preserved insight, intervention may not be required beyond patient and caregiver education (Box 2).
Patient workup. When intervention is required for psychiatric symptoms, it should begin with careful neurologic evaluation. Triggering factors such as infections (commonly urinary tract infections and pneumonia), metabolic disorders (hyperglycemia, hypothyroidism), subdural hematomas (if the patient is falling), and drug interactions should be ruled out or appropriately addressed.
Next, try to sequentially eliminate antiparkinsonian medications until the psychosis resolves or motor function worsens.23 Because of considerable overlap between PD symptoms and depression (psychomotor retardation, fatigue, and anergia), optimizing PD therapy sometimes can result in substantial psychiatric improvement. Some evidence also suggests that the dopamine agonist pramipexole may be effective in treating both PD and depression.5
When psychiatric medications are necessary for depression, anxiety, or psychosis, carefully review target symptoms, treatment expectations, and possible adverse effects with the patient and caregiver. Keep in mind the progressive nature of PD and, in addition to frequent monitoring, educate and encourage caregivers to immediately report any suspected adverse effects.
Any motor function deterioration should trigger a re-evaluation of psychotropic medications before you presume that the patient’s PD is progressing. Because antiparkinsonian drug regimens change over time, review the patient’s medications at each appointment, and alert patients and caregivers to potential psychiatric complications of any new medication.
Caregiver treatment In addition to treating the patient, it is important to monitor the impact of psychiatric symptoms and PD on the patient’s caregiver. Frequently assess whether the caregiver and patient have adequate social supports, and address any emerging needs. Useful interventions include caregiver counseling, referrals to support groups, and respite care.24
Mrs. K had a 4-year history of rapidly progressing PD treated with entacapone, carbidopa-levodopa, and a deep brain stimulator. Increasing periods of motor freezing, which were often accompanied by panic attacks, led her to become increasingly depressed and demanding of her caregiver husband. Eventually, she was admitted to an inpatient psychiatry unit because of suicidal ideation.
After a neurologic evaluation, the dosing times of her carbidopa-levodopa and entacapone were changed, but she continued to have panic attacks and remained depressed. Alprazolam promptly reduced her panic symptoms, and paroxetine was initiated for depression. A discussion with the patient and her husband revealed that they had some longstanding issues in their marriage that were exacerbated by Mrs K’s increasing dependency. The couple was referred for marital therapy, and Mrs. K agreed to begin attending a senior center.
Following discharge, the panic remained controlled and depression improved. Entacapone was replaced with tolcapone to see if motor freezing would decrease. Mrs. K’s movements improved, but her husband reported she had awakened on several nights with visual hallucinations. The hallucinations were infrequent, unaccompanied by agitation, and not distressing to the patient. Following a discussion of therapeutic options with Mrs. K and her husband, antipsychotic therapy was not instituted. The patient continues to live at home and attends the senior center regularly.
Related resources
- Parkinson’s Disease Foundation: http://www.pdf.org
- American Parkinson Disease Association: http://apdaparkinson.com
- National Parkinson Foundation: http://www.parkinson.org
- Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson’s disease 2001: treatment guidelines. Neurology 2001;56:5(suppl):S1-S88.
Drug brand names
- Alprazolam • Xanax
- Amantadine • Symmetrel
- Benztropine • Cogentin
- Biperiden • Akineton
- Buspirone • Buspar
- Carbidopa-levodopa • Sinemet
- Citalopram • Celexa
- Clozapine • Clozaril
- Entacapone • Comtan
- Fluvoxamine • Luvox
- Hyoscyamine • Levsin
- Paroxetine • Paxil
- Pergolide • Permax
- Pramipexole • Mirapex
- Ropinirole • Requip
- Selegeline • Eldepryl
- Sertraline • Zoloft
- Tolcapone • Tasmar
- Trihexyphenidyl • Artane
- Trifluoperazine • Stelazine
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Slaughter JR, Slaughter KA, Nichols D, et al. Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 2001;13:187-96.
2. Starksein SE, Preziosi TJ, Bolduc PL, Robinson RG. Depression in Parkinson’s disease. J Nerv Ment Dis 1990;178:27-31.
3. Schrag A, Jahanshahi M, Quinn P. What contributes to depression in Parkinson’s disease? Psychological Medicine 2001;31:65-73.
4. Poewe W, Luginger E. Depression in Parkinson’s disease: impediments to recognition and treatment options. Neurology 2001;52(7):S002-S006.
5. Okun MS, Watts RL. Depression associated with Parkinson’s disease: clinical features and treatment. Neurology 2002;58:1(suppl):S63-S70.
6. Cole SA, Woodard JL, Juncos JL. Depression and disability in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 1996;8(1):20-5.
7. Aarsland D, Larsen JP, et al. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry 1999;14(10):866-74.
8. Giladi N, Treves TA, Paleacu D, et al. Risk factors for dementia, depression and psychosis in long-standing Parkinson’s disease. J Neural Transm 2000;107(1):59-71.
9. Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of PD. Mov Disord 2001;16(3):507-10.
10. Walsh K, Bennett G. Parkinson’s disease and anxiety. Postgrad Med J 2001;77(904):89-93.
11. Richard IH, Schiffe RB, Kurler R. Anxiety and Parkinson’s disease. J Neuropsychiatry Clin Neurosci 1996;8(4):383-92.
12. Menza MA. Psychiatric aspects of Parkinson’s disease. Psychiatric Ann 2002;32:99-104.
13. Richard IH, Justus AW, Kurlan R. Relationship between mood and motor fluctuations in PD. J Neuropsychiatry Clin Neurosci 2001;13(1):35-41.
14. Ashburn A, Stack E, Pickering CM, Ward CD. A community-dwelling sample of people with Parkinson’s disease: characteristics of fallers and non-fallers. Age Ageing 2001;30(1):47-52.
15. Wolters EC, Berendse HW. Management of psychosis in Parkinson’s disease. Curr Opin Neurol 2001;14(4):499-504.
16. Juncos JL. Management of psychotic aspects of Parkinson’s disease. J Clin Psychiatry 1999;60:8(suppl):42-53.
17. Wolters EC. Dopaminomimetic psychosis in Parkinson’s disease patients. Neurology 1999;52:7(suppl):S010-S013.
18. Friedman JH, Factor SA. Atypical antipsychotics in the treatment of drug-induced Parkinson’s disease. Mov Disord 2000;15(2):201-11.
19. Dell’Agnello G, Ceravolo R, et al. SSRIs do not worsen Parkinson’s disease: evidence from an open-label, prospective study. Clin Neuropharmacol 2001;24(4):221-27.
20. Dreisig H, Beckmann J, Wermuth L, et al. Psychological effects of structured cognitive psychotherapy in young patients with Parkinson’s disease (abstr). Nordic J Psychiatry 1999;53(3):217-21.
21. Ludwig CL, Weinberger DR, Bruno G, et al. Buspirone, Parkinson’s disease and the locus ceruleus. Clin Neuropharmacol 1986;9(4):373-8.
22. Tarsy D, Baldessarini RJ, Tarazi FI. Effects of newer antipsychotics on extrapyramidal function. CNS Drugs 2002;16(1):23-45.
23. Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson’s disease 2001: treatment guidelines. Neurology 2001;56:5(suppl):S1-S88.
24. Ellgring JH. Depression, psychosis, dementia: impact on the family. Neurology 1999;52:7(suppl 3):S17-S20.
Depression, anxiety, and psychosis are common complications of Parkinson’s disease (PD) and of the medications used in antiparkinsonian treatment. These psychiatric problems impair patients’ functioning throughout the course of the chronic degenerative disease.
Because medication side effects often call for adjustments and trade-offs in PD treatment, a team effort by the psychiatrist, neurologist, patient, and caregiver is the most effective approach to decision-making. From our experience in such collaborations, here’s what you need to know about PD to be a most-valuable player on that treatment team.
Presentation of PD
The classic triad of PD features consists of a pill-rolling tremor, rigidity, and bradykinesia or slowness of movement. Other common features include postural instability, flexed posture, and other motor-freezing phenomena.
Freezing phenomena occur in the later stages of PD, as the response to dopaminergic therapy becomes erratic and unpredictable. Freezing can range from hesitation—such as when the patient tries to turn or is in a doorway—to transient episodes of total inability to move. These episodes are extremely distressing for both patients and caregivers.
Patients rarely present with the full complement of symptoms, but the presence of tremor at rest and/or bradykinesia is essential for the diagnosis. While motor signs dominate the presentation, cognitive symptoms such as shortened attention span, visuospatial impairment, personality changes, and dementia are also frequently present.
Average age of diagnosis is 60, and more men are affected than women (male-to-female ratio is 3:2). Many causative factors—including genetics and environmental toxins—have been implicated, but the disorder’s etiology remains unknown.
Drug treatment side effects
PD results from the loss of neurons in the substantia nigra that produce the neurotransmitter dopamine. Pharmacologic treatment emphasizes dopamine replacement, dopamine receptor stimulation, or prevention of enzymatic breakdown of dopamine in the synaptic cleft. As treatment of PD is symptomatic and not curative, medications are instituted only when the disease begins to cause functional impairment.
Treatment begins with dopamine agonists (Table). As dopamine agonist monotherapy becomes less effective, levodopa therapy is initiated. Blocking the enzymatic breakdown of dopamine with catechol-O-methyltransferase inhibitors is the next therapeutic strategy.
Within 5 years of starting levodopa therapy 75% of patients experience unsatisfactory motor response, from unpredictable fluctuations to wearing-off phenomena (in which a dose of levodopa does not last as long as it once did). Treatment of advanced PD is complicated by the emergence of psychiatric symptoms, such as hallucinations and psychosis, as dopamine levels are increased in an attempt to smooth the motor response.
The significantly distressing level of disability associated with the prominent side effects of pharmacologic treatment has led to interest in surgical interventions. These range from pallidotomy to implantation of basal ganglia stimulators to transplantation of fetal striatal neurons. The possibility of neuroprotection has also been extensively investigated, with mixed results.
Psychiatric complications of PD
Depression. Clearly, the stress of anticipating and coping with a relentless degenerative disease helps to trigger depression and anxiety in patients with PD. Depression is the most common psychiatric syndrome, with prevalence in PD as high as 42%.1 Patients with a history of depression are at particular risk.2 Those with recent deterioration or advancing severity of PD, akinesia, history of falls, or cognitive impairment are also at increased risk for depression.
Table
MEDICATIONS COMMONLY USED IN MANAGING PARKINSON’S DISEASE
| Medication class | Example | Indication for use |
|---|---|---|
| MAO-B inhibitor | Selegiline | ? Neuroprotection |
| Anticholinergic agents | Trihexyphenidyl, benztropine, biperiden, hyoscyamine, diphenhydramine | Tremor |
| Dopamine agonist | Pramipexole, pergolide, ropinirole | ? Neuroprotection Treatment of movement disorder |
| Dopamine replacement | Carbidopa-levodopa | Treatment of movement disorder |
| Catechol-O-methyltransferase inhibitor | Entacapone, tolcapone | Smooth motor fluctuations |
Depression correlates well with the patient’s perception of his or her degree of PD-related disability. Depression symptoms seem to peak early in the illness following diagnosis and in advanced disease.3
Patients may present with symptoms meeting diagnostic criteria ranging from dysthymic disorder to minor depression to major depressive disorder.1,4 Although they will frequently endorse suicidal ideation, patients with PD have a low rate of suicide. Diagnosing depression, however, may be difficult because its symptoms overlap with those of the underlying neurologic disease:
- Diminished affect and psychomotor slowing may be secondary to the motor features of parkinsonism.
- Diminished concentration may be secondary to cognitive decline rather than depression.
Patients also frequently have a chief complaint of diminished energy or fatigue that should trigger further investigation into other depressive symptoms.4,5
In addition to the obvious additional suffering it causes, depression in PD predicts impaired social, physical, and role functioning.6 Depression in the PD patient also results in higher distress for caregivers.7 In one study, depression was identified as a risk factor for development of psychosis in PD patients.8
Anxiety is a frequent problem for PD patients, with a prevalence of 33 to 40%.9,10 Anxiety in PD typically presents with symptoms of panic disorder, generalized anxiety disorder, or social phobia.11 It is comorbid with depression in up to 92% of cases and—like depression—frequently predates the onset of motor symptoms.12
Anxiety symptoms have been correlated, although not consistently, with the on-off motor phenomenon often found in advanced PD.13 They can also be an adverse effect of many of the antiparkinsonian medications, including anticholinergics, dopamine agonists, catechol-O-methyltransferase inhibitors, and selegiline. Both anxiety and depression have been associated with an increased risk for falls.14
Psychotic symptoms. Up to 25% of PD patients experience delusions or hallucinations.15 Risk factors include dementia, sleep disturbance, and—most commonly—the use of dopaminergic agents. Up to one-fifth of patients using dopaminergic drugs experience psychotic symptoms.16
Psychotic symptoms can occur with or without the clouded sensorium characteristic of delirium. Psychotic symptoms with an associated confusional state can be associated with use of anticholinergic agents and drugs such as selegiline and amantadine.17 Catechol-O-methyltransferase inhibitors cause more sustained dopaminergic activity of levodopa, which can result in psychotic symptoms. Therefore, the use of all known classes of antiparkinsonian medications has been associated with drug-induced psychosis.
In advanced PD, paranoid delusions, delusions of spousal infidelity, and visual hallucinations are common, whereas negative symptoms and thought disturbances are not.18 Psychosis may be a more important contributor to caregiver distress than the motor symptoms of PD and may be more likely than any other factor to lead to nursing home placement of the PD patient (Box 1).15
Psychiatric interventions
Goals for psychiatric treatment of depression, anxiety, and psychosis associated with PD seem relatively straightforward:
- improvement or remission of psychiatric symptoms
- restoration of optimal patient functioning.
Ideally, these goals would be achieved without causing sedation, orthostatic hypotension, or exacerbating motor symptoms. The older age of patients and the progressive nature of this neurodegenerative disorder predispose patients to cognitive side effects. Unfortunately, despite the high prevalence of psychiatric disturbances in PD, evidence with which to evaluate treatment efficacy and safety and to guide treatment selection is extremely limited.
For depression associated with PD, extensive clinical experience supports the efficacy of tricyclic antidepressants. Even so, selective serotonin reuptake inhibitors (SSRIs) are the preferred treatment, although only open-label trials and case reports support their efficacy.5,12 Compared with tricyclics, SSRIs exhibit a relative lack of problems with sedation, orthostatic hypotension, and memory-impairing anticholinergic side effects. While case reports have cited worsening of motor symptoms with SSRIs, a recent prospective study found no significant worsening of PD symptoms during treatment with citalopram, fluoxetine, fluvoxamine, or sertraline.19 Co-administration of an SSRI with selegiline is not absolutely contraindicated, but the combination does carry a very small risk of development of serotonin syndrome.1,5
Mr. J had a 6-year history of PD with pronounced bradykinesia and gait disturbance treated with amantadine and carbidopa-levodopa. His rigidity began to worsen, so the dosage of carbidopa-levodopa was increased. His wife then reported that he had increased confusion and balance problems. On evaluation, he was found to have a urinary tract infection. Following antibiotic treatment, mental status and gait returned to usual baseline.
One year later, Mr. J began having trouble getting out of bed, with unpredictable motor freezing episodes. Pramipexole was added to his regimen, and he began having prominent visual hallucinations. Low-dose trifluoperazine was added, and hallucinations improved. The patient became increasingly depressed, and sertraline was started.
Over the next year, his function progressively worsened, with increased motor freezing and unpredictable dyskinesias. Hallucinations complicated attempts to change his medications. Amantadine was stopped without improvement. He was referred for surgical evaluation, but because of his cognitive status and depression was deemed not to be a candidate.
He began to fall repeatedly and developed orthostatic hypotension. His clinical course continued to be complicated by hallucinations and delusions that his wife was being unfaithful. Ongoing psychosis and severe gait instability led to his admission to a nursing home.
Data are even more scant on the safe use of other antidepressants in PD. Electroconvulsive therapy has been proven helpful in refractory cases and sometimes results in transient motor symptom improvement.1,5,12 While clinical experience suggests that psychotherapy frequently helps, no extensive controlled studies exist. One small study suggests the efficacy of structured cognitive psychotherapy.20
Anxiety. No studies have examined the treatment of anxiety in PD patients. Given the extremely high comorbidity of anxiety with depression, antidepressants should probably be considered as a first-line pharmacotherapy. Benzodiazepines should be used cautiously, as they increase the risk of falls, sedation, and confusion in older patients. One small controlled study found that buspirone was well tolerated in PD patients at low dosages (10 to 40 mg/d), but anxiety did not improve. At high dosages (100 mg/d), anxiety worsened.21
Psychosis. Data on use of antipsychotic agents in PD are also limited, but some evidence supports their use in treating PD-related psychotic symptoms. While conventional antipsychotics can help control psychosis, the potential is high for worsening of parkinsonian symptoms due to D2 receptor blockade.
Among the atypical antipsychotics, clozapine has been most extensively studied in PD and has been shown in open and double-blind trials to be effective and well tolerated at low dosages (6.25 to 50 mg/d). A limited number of open studies of some of the newer atypicals have been performed. While extreme caution must be used in comparing data from these studies due to highly variable dosing and other study design issues, clozapine and quetiapine appear to be the agents best tolerated by PD patients.12,18,22 Initial antipsychotic dosing should be low and escalation cautious—regardless of the agent chosen—because of the dose-related potential for worsening of parkinsonian symptoms, sedation, and orthostatic hypotension.
A team approach to treatment
Because psychiatric and PD symptoms and treatments are closely interrelated, the psychiatrist, neurologist, patient, and caregiver must collaborate for the best therapeutic result. A simplistic approach to treatment can result in a catastrophic downward spiral in patient functioning.
Often, compromises must be made between optimal control of parkinsonian and psychiatric symptoms to achieve the best overall patient function. Patients and caregivers must be counseled about possible psychiatric symptoms associated with PD and antiparkinsonian therapy, as well as the potential for adverse effects from psychiatric medications. With this knowledge, patients and caregivers can help assess the severity of symptoms and set treatment priorities, depending on how symptoms may be affecting the patient’s level of functioning. For example, if an effective antiparkinsonian regimen has triggered infrequent, nondistressing hallucinations with preserved insight, intervention may not be required beyond patient and caregiver education (Box 2).
Patient workup. When intervention is required for psychiatric symptoms, it should begin with careful neurologic evaluation. Triggering factors such as infections (commonly urinary tract infections and pneumonia), metabolic disorders (hyperglycemia, hypothyroidism), subdural hematomas (if the patient is falling), and drug interactions should be ruled out or appropriately addressed.
Next, try to sequentially eliminate antiparkinsonian medications until the psychosis resolves or motor function worsens.23 Because of considerable overlap between PD symptoms and depression (psychomotor retardation, fatigue, and anergia), optimizing PD therapy sometimes can result in substantial psychiatric improvement. Some evidence also suggests that the dopamine agonist pramipexole may be effective in treating both PD and depression.5
When psychiatric medications are necessary for depression, anxiety, or psychosis, carefully review target symptoms, treatment expectations, and possible adverse effects with the patient and caregiver. Keep in mind the progressive nature of PD and, in addition to frequent monitoring, educate and encourage caregivers to immediately report any suspected adverse effects.
Any motor function deterioration should trigger a re-evaluation of psychotropic medications before you presume that the patient’s PD is progressing. Because antiparkinsonian drug regimens change over time, review the patient’s medications at each appointment, and alert patients and caregivers to potential psychiatric complications of any new medication.
Caregiver treatment In addition to treating the patient, it is important to monitor the impact of psychiatric symptoms and PD on the patient’s caregiver. Frequently assess whether the caregiver and patient have adequate social supports, and address any emerging needs. Useful interventions include caregiver counseling, referrals to support groups, and respite care.24
Mrs. K had a 4-year history of rapidly progressing PD treated with entacapone, carbidopa-levodopa, and a deep brain stimulator. Increasing periods of motor freezing, which were often accompanied by panic attacks, led her to become increasingly depressed and demanding of her caregiver husband. Eventually, she was admitted to an inpatient psychiatry unit because of suicidal ideation.
After a neurologic evaluation, the dosing times of her carbidopa-levodopa and entacapone were changed, but she continued to have panic attacks and remained depressed. Alprazolam promptly reduced her panic symptoms, and paroxetine was initiated for depression. A discussion with the patient and her husband revealed that they had some longstanding issues in their marriage that were exacerbated by Mrs K’s increasing dependency. The couple was referred for marital therapy, and Mrs. K agreed to begin attending a senior center.
Following discharge, the panic remained controlled and depression improved. Entacapone was replaced with tolcapone to see if motor freezing would decrease. Mrs. K’s movements improved, but her husband reported she had awakened on several nights with visual hallucinations. The hallucinations were infrequent, unaccompanied by agitation, and not distressing to the patient. Following a discussion of therapeutic options with Mrs. K and her husband, antipsychotic therapy was not instituted. The patient continues to live at home and attends the senior center regularly.
Related resources
- Parkinson’s Disease Foundation: http://www.pdf.org
- American Parkinson Disease Association: http://apdaparkinson.com
- National Parkinson Foundation: http://www.parkinson.org
- Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson’s disease 2001: treatment guidelines. Neurology 2001;56:5(suppl):S1-S88.
Drug brand names
- Alprazolam • Xanax
- Amantadine • Symmetrel
- Benztropine • Cogentin
- Biperiden • Akineton
- Buspirone • Buspar
- Carbidopa-levodopa • Sinemet
- Citalopram • Celexa
- Clozapine • Clozaril
- Entacapone • Comtan
- Fluvoxamine • Luvox
- Hyoscyamine • Levsin
- Paroxetine • Paxil
- Pergolide • Permax
- Pramipexole • Mirapex
- Ropinirole • Requip
- Selegeline • Eldepryl
- Sertraline • Zoloft
- Tolcapone • Tasmar
- Trihexyphenidyl • Artane
- Trifluoperazine • Stelazine
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Depression, anxiety, and psychosis are common complications of Parkinson’s disease (PD) and of the medications used in antiparkinsonian treatment. These psychiatric problems impair patients’ functioning throughout the course of the chronic degenerative disease.
Because medication side effects often call for adjustments and trade-offs in PD treatment, a team effort by the psychiatrist, neurologist, patient, and caregiver is the most effective approach to decision-making. From our experience in such collaborations, here’s what you need to know about PD to be a most-valuable player on that treatment team.
Presentation of PD
The classic triad of PD features consists of a pill-rolling tremor, rigidity, and bradykinesia or slowness of movement. Other common features include postural instability, flexed posture, and other motor-freezing phenomena.
Freezing phenomena occur in the later stages of PD, as the response to dopaminergic therapy becomes erratic and unpredictable. Freezing can range from hesitation—such as when the patient tries to turn or is in a doorway—to transient episodes of total inability to move. These episodes are extremely distressing for both patients and caregivers.
Patients rarely present with the full complement of symptoms, but the presence of tremor at rest and/or bradykinesia is essential for the diagnosis. While motor signs dominate the presentation, cognitive symptoms such as shortened attention span, visuospatial impairment, personality changes, and dementia are also frequently present.
Average age of diagnosis is 60, and more men are affected than women (male-to-female ratio is 3:2). Many causative factors—including genetics and environmental toxins—have been implicated, but the disorder’s etiology remains unknown.
Drug treatment side effects
PD results from the loss of neurons in the substantia nigra that produce the neurotransmitter dopamine. Pharmacologic treatment emphasizes dopamine replacement, dopamine receptor stimulation, or prevention of enzymatic breakdown of dopamine in the synaptic cleft. As treatment of PD is symptomatic and not curative, medications are instituted only when the disease begins to cause functional impairment.
Treatment begins with dopamine agonists (Table). As dopamine agonist monotherapy becomes less effective, levodopa therapy is initiated. Blocking the enzymatic breakdown of dopamine with catechol-O-methyltransferase inhibitors is the next therapeutic strategy.
Within 5 years of starting levodopa therapy 75% of patients experience unsatisfactory motor response, from unpredictable fluctuations to wearing-off phenomena (in which a dose of levodopa does not last as long as it once did). Treatment of advanced PD is complicated by the emergence of psychiatric symptoms, such as hallucinations and psychosis, as dopamine levels are increased in an attempt to smooth the motor response.
The significantly distressing level of disability associated with the prominent side effects of pharmacologic treatment has led to interest in surgical interventions. These range from pallidotomy to implantation of basal ganglia stimulators to transplantation of fetal striatal neurons. The possibility of neuroprotection has also been extensively investigated, with mixed results.
Psychiatric complications of PD
Depression. Clearly, the stress of anticipating and coping with a relentless degenerative disease helps to trigger depression and anxiety in patients with PD. Depression is the most common psychiatric syndrome, with prevalence in PD as high as 42%.1 Patients with a history of depression are at particular risk.2 Those with recent deterioration or advancing severity of PD, akinesia, history of falls, or cognitive impairment are also at increased risk for depression.
Table
MEDICATIONS COMMONLY USED IN MANAGING PARKINSON’S DISEASE
| Medication class | Example | Indication for use |
|---|---|---|
| MAO-B inhibitor | Selegiline | ? Neuroprotection |
| Anticholinergic agents | Trihexyphenidyl, benztropine, biperiden, hyoscyamine, diphenhydramine | Tremor |
| Dopamine agonist | Pramipexole, pergolide, ropinirole | ? Neuroprotection Treatment of movement disorder |
| Dopamine replacement | Carbidopa-levodopa | Treatment of movement disorder |
| Catechol-O-methyltransferase inhibitor | Entacapone, tolcapone | Smooth motor fluctuations |
Depression correlates well with the patient’s perception of his or her degree of PD-related disability. Depression symptoms seem to peak early in the illness following diagnosis and in advanced disease.3
Patients may present with symptoms meeting diagnostic criteria ranging from dysthymic disorder to minor depression to major depressive disorder.1,4 Although they will frequently endorse suicidal ideation, patients with PD have a low rate of suicide. Diagnosing depression, however, may be difficult because its symptoms overlap with those of the underlying neurologic disease:
- Diminished affect and psychomotor slowing may be secondary to the motor features of parkinsonism.
- Diminished concentration may be secondary to cognitive decline rather than depression.
Patients also frequently have a chief complaint of diminished energy or fatigue that should trigger further investigation into other depressive symptoms.4,5
In addition to the obvious additional suffering it causes, depression in PD predicts impaired social, physical, and role functioning.6 Depression in the PD patient also results in higher distress for caregivers.7 In one study, depression was identified as a risk factor for development of psychosis in PD patients.8
Anxiety is a frequent problem for PD patients, with a prevalence of 33 to 40%.9,10 Anxiety in PD typically presents with symptoms of panic disorder, generalized anxiety disorder, or social phobia.11 It is comorbid with depression in up to 92% of cases and—like depression—frequently predates the onset of motor symptoms.12
Anxiety symptoms have been correlated, although not consistently, with the on-off motor phenomenon often found in advanced PD.13 They can also be an adverse effect of many of the antiparkinsonian medications, including anticholinergics, dopamine agonists, catechol-O-methyltransferase inhibitors, and selegiline. Both anxiety and depression have been associated with an increased risk for falls.14
Psychotic symptoms. Up to 25% of PD patients experience delusions or hallucinations.15 Risk factors include dementia, sleep disturbance, and—most commonly—the use of dopaminergic agents. Up to one-fifth of patients using dopaminergic drugs experience psychotic symptoms.16
Psychotic symptoms can occur with or without the clouded sensorium characteristic of delirium. Psychotic symptoms with an associated confusional state can be associated with use of anticholinergic agents and drugs such as selegiline and amantadine.17 Catechol-O-methyltransferase inhibitors cause more sustained dopaminergic activity of levodopa, which can result in psychotic symptoms. Therefore, the use of all known classes of antiparkinsonian medications has been associated with drug-induced psychosis.
In advanced PD, paranoid delusions, delusions of spousal infidelity, and visual hallucinations are common, whereas negative symptoms and thought disturbances are not.18 Psychosis may be a more important contributor to caregiver distress than the motor symptoms of PD and may be more likely than any other factor to lead to nursing home placement of the PD patient (Box 1).15
Psychiatric interventions
Goals for psychiatric treatment of depression, anxiety, and psychosis associated with PD seem relatively straightforward:
- improvement or remission of psychiatric symptoms
- restoration of optimal patient functioning.
Ideally, these goals would be achieved without causing sedation, orthostatic hypotension, or exacerbating motor symptoms. The older age of patients and the progressive nature of this neurodegenerative disorder predispose patients to cognitive side effects. Unfortunately, despite the high prevalence of psychiatric disturbances in PD, evidence with which to evaluate treatment efficacy and safety and to guide treatment selection is extremely limited.
For depression associated with PD, extensive clinical experience supports the efficacy of tricyclic antidepressants. Even so, selective serotonin reuptake inhibitors (SSRIs) are the preferred treatment, although only open-label trials and case reports support their efficacy.5,12 Compared with tricyclics, SSRIs exhibit a relative lack of problems with sedation, orthostatic hypotension, and memory-impairing anticholinergic side effects. While case reports have cited worsening of motor symptoms with SSRIs, a recent prospective study found no significant worsening of PD symptoms during treatment with citalopram, fluoxetine, fluvoxamine, or sertraline.19 Co-administration of an SSRI with selegiline is not absolutely contraindicated, but the combination does carry a very small risk of development of serotonin syndrome.1,5
Mr. J had a 6-year history of PD with pronounced bradykinesia and gait disturbance treated with amantadine and carbidopa-levodopa. His rigidity began to worsen, so the dosage of carbidopa-levodopa was increased. His wife then reported that he had increased confusion and balance problems. On evaluation, he was found to have a urinary tract infection. Following antibiotic treatment, mental status and gait returned to usual baseline.
One year later, Mr. J began having trouble getting out of bed, with unpredictable motor freezing episodes. Pramipexole was added to his regimen, and he began having prominent visual hallucinations. Low-dose trifluoperazine was added, and hallucinations improved. The patient became increasingly depressed, and sertraline was started.
Over the next year, his function progressively worsened, with increased motor freezing and unpredictable dyskinesias. Hallucinations complicated attempts to change his medications. Amantadine was stopped without improvement. He was referred for surgical evaluation, but because of his cognitive status and depression was deemed not to be a candidate.
He began to fall repeatedly and developed orthostatic hypotension. His clinical course continued to be complicated by hallucinations and delusions that his wife was being unfaithful. Ongoing psychosis and severe gait instability led to his admission to a nursing home.
Data are even more scant on the safe use of other antidepressants in PD. Electroconvulsive therapy has been proven helpful in refractory cases and sometimes results in transient motor symptom improvement.1,5,12 While clinical experience suggests that psychotherapy frequently helps, no extensive controlled studies exist. One small study suggests the efficacy of structured cognitive psychotherapy.20
Anxiety. No studies have examined the treatment of anxiety in PD patients. Given the extremely high comorbidity of anxiety with depression, antidepressants should probably be considered as a first-line pharmacotherapy. Benzodiazepines should be used cautiously, as they increase the risk of falls, sedation, and confusion in older patients. One small controlled study found that buspirone was well tolerated in PD patients at low dosages (10 to 40 mg/d), but anxiety did not improve. At high dosages (100 mg/d), anxiety worsened.21
Psychosis. Data on use of antipsychotic agents in PD are also limited, but some evidence supports their use in treating PD-related psychotic symptoms. While conventional antipsychotics can help control psychosis, the potential is high for worsening of parkinsonian symptoms due to D2 receptor blockade.
Among the atypical antipsychotics, clozapine has been most extensively studied in PD and has been shown in open and double-blind trials to be effective and well tolerated at low dosages (6.25 to 50 mg/d). A limited number of open studies of some of the newer atypicals have been performed. While extreme caution must be used in comparing data from these studies due to highly variable dosing and other study design issues, clozapine and quetiapine appear to be the agents best tolerated by PD patients.12,18,22 Initial antipsychotic dosing should be low and escalation cautious—regardless of the agent chosen—because of the dose-related potential for worsening of parkinsonian symptoms, sedation, and orthostatic hypotension.
A team approach to treatment
Because psychiatric and PD symptoms and treatments are closely interrelated, the psychiatrist, neurologist, patient, and caregiver must collaborate for the best therapeutic result. A simplistic approach to treatment can result in a catastrophic downward spiral in patient functioning.
Often, compromises must be made between optimal control of parkinsonian and psychiatric symptoms to achieve the best overall patient function. Patients and caregivers must be counseled about possible psychiatric symptoms associated with PD and antiparkinsonian therapy, as well as the potential for adverse effects from psychiatric medications. With this knowledge, patients and caregivers can help assess the severity of symptoms and set treatment priorities, depending on how symptoms may be affecting the patient’s level of functioning. For example, if an effective antiparkinsonian regimen has triggered infrequent, nondistressing hallucinations with preserved insight, intervention may not be required beyond patient and caregiver education (Box 2).
Patient workup. When intervention is required for psychiatric symptoms, it should begin with careful neurologic evaluation. Triggering factors such as infections (commonly urinary tract infections and pneumonia), metabolic disorders (hyperglycemia, hypothyroidism), subdural hematomas (if the patient is falling), and drug interactions should be ruled out or appropriately addressed.
Next, try to sequentially eliminate antiparkinsonian medications until the psychosis resolves or motor function worsens.23 Because of considerable overlap between PD symptoms and depression (psychomotor retardation, fatigue, and anergia), optimizing PD therapy sometimes can result in substantial psychiatric improvement. Some evidence also suggests that the dopamine agonist pramipexole may be effective in treating both PD and depression.5
When psychiatric medications are necessary for depression, anxiety, or psychosis, carefully review target symptoms, treatment expectations, and possible adverse effects with the patient and caregiver. Keep in mind the progressive nature of PD and, in addition to frequent monitoring, educate and encourage caregivers to immediately report any suspected adverse effects.
Any motor function deterioration should trigger a re-evaluation of psychotropic medications before you presume that the patient’s PD is progressing. Because antiparkinsonian drug regimens change over time, review the patient’s medications at each appointment, and alert patients and caregivers to potential psychiatric complications of any new medication.
Caregiver treatment In addition to treating the patient, it is important to monitor the impact of psychiatric symptoms and PD on the patient’s caregiver. Frequently assess whether the caregiver and patient have adequate social supports, and address any emerging needs. Useful interventions include caregiver counseling, referrals to support groups, and respite care.24
Mrs. K had a 4-year history of rapidly progressing PD treated with entacapone, carbidopa-levodopa, and a deep brain stimulator. Increasing periods of motor freezing, which were often accompanied by panic attacks, led her to become increasingly depressed and demanding of her caregiver husband. Eventually, she was admitted to an inpatient psychiatry unit because of suicidal ideation.
After a neurologic evaluation, the dosing times of her carbidopa-levodopa and entacapone were changed, but she continued to have panic attacks and remained depressed. Alprazolam promptly reduced her panic symptoms, and paroxetine was initiated for depression. A discussion with the patient and her husband revealed that they had some longstanding issues in their marriage that were exacerbated by Mrs K’s increasing dependency. The couple was referred for marital therapy, and Mrs. K agreed to begin attending a senior center.
Following discharge, the panic remained controlled and depression improved. Entacapone was replaced with tolcapone to see if motor freezing would decrease. Mrs. K’s movements improved, but her husband reported she had awakened on several nights with visual hallucinations. The hallucinations were infrequent, unaccompanied by agitation, and not distressing to the patient. Following a discussion of therapeutic options with Mrs. K and her husband, antipsychotic therapy was not instituted. The patient continues to live at home and attends the senior center regularly.
Related resources
- Parkinson’s Disease Foundation: http://www.pdf.org
- American Parkinson Disease Association: http://apdaparkinson.com
- National Parkinson Foundation: http://www.parkinson.org
- Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson’s disease 2001: treatment guidelines. Neurology 2001;56:5(suppl):S1-S88.
Drug brand names
- Alprazolam • Xanax
- Amantadine • Symmetrel
- Benztropine • Cogentin
- Biperiden • Akineton
- Buspirone • Buspar
- Carbidopa-levodopa • Sinemet
- Citalopram • Celexa
- Clozapine • Clozaril
- Entacapone • Comtan
- Fluvoxamine • Luvox
- Hyoscyamine • Levsin
- Paroxetine • Paxil
- Pergolide • Permax
- Pramipexole • Mirapex
- Ropinirole • Requip
- Selegeline • Eldepryl
- Sertraline • Zoloft
- Tolcapone • Tasmar
- Trihexyphenidyl • Artane
- Trifluoperazine • Stelazine
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Slaughter JR, Slaughter KA, Nichols D, et al. Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 2001;13:187-96.
2. Starksein SE, Preziosi TJ, Bolduc PL, Robinson RG. Depression in Parkinson’s disease. J Nerv Ment Dis 1990;178:27-31.
3. Schrag A, Jahanshahi M, Quinn P. What contributes to depression in Parkinson’s disease? Psychological Medicine 2001;31:65-73.
4. Poewe W, Luginger E. Depression in Parkinson’s disease: impediments to recognition and treatment options. Neurology 2001;52(7):S002-S006.
5. Okun MS, Watts RL. Depression associated with Parkinson’s disease: clinical features and treatment. Neurology 2002;58:1(suppl):S63-S70.
6. Cole SA, Woodard JL, Juncos JL. Depression and disability in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 1996;8(1):20-5.
7. Aarsland D, Larsen JP, et al. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry 1999;14(10):866-74.
8. Giladi N, Treves TA, Paleacu D, et al. Risk factors for dementia, depression and psychosis in long-standing Parkinson’s disease. J Neural Transm 2000;107(1):59-71.
9. Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of PD. Mov Disord 2001;16(3):507-10.
10. Walsh K, Bennett G. Parkinson’s disease and anxiety. Postgrad Med J 2001;77(904):89-93.
11. Richard IH, Schiffe RB, Kurler R. Anxiety and Parkinson’s disease. J Neuropsychiatry Clin Neurosci 1996;8(4):383-92.
12. Menza MA. Psychiatric aspects of Parkinson’s disease. Psychiatric Ann 2002;32:99-104.
13. Richard IH, Justus AW, Kurlan R. Relationship between mood and motor fluctuations in PD. J Neuropsychiatry Clin Neurosci 2001;13(1):35-41.
14. Ashburn A, Stack E, Pickering CM, Ward CD. A community-dwelling sample of people with Parkinson’s disease: characteristics of fallers and non-fallers. Age Ageing 2001;30(1):47-52.
15. Wolters EC, Berendse HW. Management of psychosis in Parkinson’s disease. Curr Opin Neurol 2001;14(4):499-504.
16. Juncos JL. Management of psychotic aspects of Parkinson’s disease. J Clin Psychiatry 1999;60:8(suppl):42-53.
17. Wolters EC. Dopaminomimetic psychosis in Parkinson’s disease patients. Neurology 1999;52:7(suppl):S010-S013.
18. Friedman JH, Factor SA. Atypical antipsychotics in the treatment of drug-induced Parkinson’s disease. Mov Disord 2000;15(2):201-11.
19. Dell’Agnello G, Ceravolo R, et al. SSRIs do not worsen Parkinson’s disease: evidence from an open-label, prospective study. Clin Neuropharmacol 2001;24(4):221-27.
20. Dreisig H, Beckmann J, Wermuth L, et al. Psychological effects of structured cognitive psychotherapy in young patients with Parkinson’s disease (abstr). Nordic J Psychiatry 1999;53(3):217-21.
21. Ludwig CL, Weinberger DR, Bruno G, et al. Buspirone, Parkinson’s disease and the locus ceruleus. Clin Neuropharmacol 1986;9(4):373-8.
22. Tarsy D, Baldessarini RJ, Tarazi FI. Effects of newer antipsychotics on extrapyramidal function. CNS Drugs 2002;16(1):23-45.
23. Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson’s disease 2001: treatment guidelines. Neurology 2001;56:5(suppl):S1-S88.
24. Ellgring JH. Depression, psychosis, dementia: impact on the family. Neurology 1999;52:7(suppl 3):S17-S20.
1. Slaughter JR, Slaughter KA, Nichols D, et al. Prevalence, clinical manifestations, etiology, and treatment of depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 2001;13:187-96.
2. Starksein SE, Preziosi TJ, Bolduc PL, Robinson RG. Depression in Parkinson’s disease. J Nerv Ment Dis 1990;178:27-31.
3. Schrag A, Jahanshahi M, Quinn P. What contributes to depression in Parkinson’s disease? Psychological Medicine 2001;31:65-73.
4. Poewe W, Luginger E. Depression in Parkinson’s disease: impediments to recognition and treatment options. Neurology 2001;52(7):S002-S006.
5. Okun MS, Watts RL. Depression associated with Parkinson’s disease: clinical features and treatment. Neurology 2002;58:1(suppl):S63-S70.
6. Cole SA, Woodard JL, Juncos JL. Depression and disability in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 1996;8(1):20-5.
7. Aarsland D, Larsen JP, et al. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry 1999;14(10):866-74.
8. Giladi N, Treves TA, Paleacu D, et al. Risk factors for dementia, depression and psychosis in long-standing Parkinson’s disease. J Neural Transm 2000;107(1):59-71.
9. Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of PD. Mov Disord 2001;16(3):507-10.
10. Walsh K, Bennett G. Parkinson’s disease and anxiety. Postgrad Med J 2001;77(904):89-93.
11. Richard IH, Schiffe RB, Kurler R. Anxiety and Parkinson’s disease. J Neuropsychiatry Clin Neurosci 1996;8(4):383-92.
12. Menza MA. Psychiatric aspects of Parkinson’s disease. Psychiatric Ann 2002;32:99-104.
13. Richard IH, Justus AW, Kurlan R. Relationship between mood and motor fluctuations in PD. J Neuropsychiatry Clin Neurosci 2001;13(1):35-41.
14. Ashburn A, Stack E, Pickering CM, Ward CD. A community-dwelling sample of people with Parkinson’s disease: characteristics of fallers and non-fallers. Age Ageing 2001;30(1):47-52.
15. Wolters EC, Berendse HW. Management of psychosis in Parkinson’s disease. Curr Opin Neurol 2001;14(4):499-504.
16. Juncos JL. Management of psychotic aspects of Parkinson’s disease. J Clin Psychiatry 1999;60:8(suppl):42-53.
17. Wolters EC. Dopaminomimetic psychosis in Parkinson’s disease patients. Neurology 1999;52:7(suppl):S010-S013.
18. Friedman JH, Factor SA. Atypical antipsychotics in the treatment of drug-induced Parkinson’s disease. Mov Disord 2000;15(2):201-11.
19. Dell’Agnello G, Ceravolo R, et al. SSRIs do not worsen Parkinson’s disease: evidence from an open-label, prospective study. Clin Neuropharmacol 2001;24(4):221-27.
20. Dreisig H, Beckmann J, Wermuth L, et al. Psychological effects of structured cognitive psychotherapy in young patients with Parkinson’s disease (abstr). Nordic J Psychiatry 1999;53(3):217-21.
21. Ludwig CL, Weinberger DR, Bruno G, et al. Buspirone, Parkinson’s disease and the locus ceruleus. Clin Neuropharmacol 1986;9(4):373-8.
22. Tarsy D, Baldessarini RJ, Tarazi FI. Effects of newer antipsychotics on extrapyramidal function. CNS Drugs 2002;16(1):23-45.
23. Olanow CW, Watts RL, Koller WC. An algorithm (decision tree) for the management of Parkinson’s disease 2001: treatment guidelines. Neurology 2001;56:5(suppl):S1-S88.
24. Ellgring JH. Depression, psychosis, dementia: impact on the family. Neurology 1999;52:7(suppl 3):S17-S20.
Irritable bowel syndrome: Psychotherapy can improve GI symptoms and emotional health
A growing body of evidence suggests that psychiatrists have much to offer patients with severe irritable bowel syndrome (IBS). Behavioral and psychotherapeutic approaches are showing promise in relieving both GI and mood disturbances.
Treating patients with IBS with medications designed to influence only gut function can be frustrating. Those with refractory symptoms may be extremely sensitive to drug side effects, and they often report that medical management worsens or does not improve their symptoms. They experiment with alternative medicines and wander from physician to physician in a disappointing search for a “cure.”
Let’s look at evidence on the efficacy of individual and group behavioral therapies, hypnotherapy, biofeedback, and combination medical and behavioral treatment.
Psychotherapeutic approaches
IBS is a common gastrointestinal disorder that is characterized by abdominal discomfort and changes in bowel habits (Boxes 1 and 2). Patients with severe IBS symptoms often bring significant psychological impairment and psychosocial trauma to clinical encounters.1,2 They respond poorly to standard medical management, and evidence supporting the efficacy of medical treatments for IBS remains weak.3,4
Irritable bowel syndrome (IBS) is the most common disorder seen in gastrointestinal practice, representing more than 40% of all visits to gastroenterologists. Complaints of IBS also account for approximately 23% of office visits to primary care physicians.1
Key symptoms of this functional disorder are a pattern of lower abdominal discomfort and bloating accompanied by variable degrees of altered stool pattern—constipation, diarrhea, or intermittent constipation and diarrhea. IBS is most common in young patients, with onset rarely diagnosed after age 45. Its incidence is equal in men and women, but women are more likely to seek medical care for IBS symptoms.
The cause of IBS is unclear. Recent research suggests that changes in serotonin metabolism cause a pattern of visceral hypersensitivity and an altered sensation of pain. IBS is not a psychiatric disorder, but it can be worsened by comorbid psychopathology, particularly mood and anxiety disorders. Although patients tend to have either diarrhea-predominant or constipation-predominant IBS, the pathophysiology of both patterns seems similar.
In 1983, the first controlled trial of psychodynamic psychotherapy for IBS showed dramatic reductions in symptoms.5 A subsequent series of high-quality articles in the early 1990s also showed that interpersonal psychotherapy (with greater interaction between therapist and patient) could significantly decrease IBS symptoms.
Persistent improvement. In one randomized controlled trial,6 102 patients with IBS received either standard medical treatment or 10 hours of dynamically oriented individual psychotherapy in combination with standard medical treatment. After 3 months, patients who received psychotherapy showed significantly greater improvement in somatic symptoms and emotional well-being, compared with those who received medical treatment only.
Interestingly, this difference persisted 1 year after the study ended. GI symptoms and the emotional well-being of patients who received combination therapy continued to improve, whereas the physical and emotional status of those who received only standard medical treatment deteriorated.
In a second study,7 101 patients with severe IBS symptoms continued to receive medical treatment but were randomly divided into two groups:
- Study subjects received 8 hours of dynamically oriented psychotherapy.
- Control patients met with a psychiatrist who engaged in “supportive listening” but delivered no psychotherapy. This strategy was adopted to control for the effect of the psychiatrist’s presence.
Assessments included patients’ self-reports of symptoms, ratings of GI symptoms by the treating gastroenterologists, and measures of depression, anxiety, and health care utilization. Patients who received psychotherapy reported significant improvements in bowel symptoms (e.g., diarrhea, constipation, bloating, and abdominal pain). Likewise, the gastroenterologist who rated patients’ GI symptoms felt that those who received psychotherapy improved significantly across the entire spectrum of GI symptoms. The improvements were maintained at 1-year follow-up.
By comparison, the control patients reported worsening symptoms, as did subjects who dropped out. Patients who received psychotherapy also made significantly fewer outpatient visits to gastroenterologists, compared with controls (p<0.001).
Cognitive-behavioral therapy
Cognitive-behavioral therapy (CBT) is emerging as a major psychotherapeutic tool for treating mood disorders, anxiety disorders, and somatic syndromes associated with psychosocial distress. CBT also is showing promise for patients with moderate to severe IBS and those with IBS and concomitant anxiety or mood disorders. Studies consistently show that CBT is superior to standard medical management or the use of support groups or other behavioral treatments alone.
Reduced symptoms. In an early trial of CBT, 17 patients with IBS experienced significantly less abdominal pain and diarrhea after participating in a program of progressive relaxation, education about bowel functioning, use of thermal biofeedback, and stress coping techniques based on CBT. Overall, 64% of the patients improved.8
The same investigators then assigned 90 patients to 12 sessions of CBT, given over 8 weeks with or without meditation and biofeedback. Patients with axis I psychiatric diagnoses tended to respond poorly to CBT. The authors concluded that a careful pretreatment psychological workup is important to identify patients with IBS who would benefit most from CBT.9
As a follow-up, these investigators randomly assigned 20 patients to intensive individualized CBT (10 sessions over 8 weeks) or 8 weeks of daily GI symptom monitoring. Patients who received CBT had significantly fewer GI symptoms than did the symptom-monitoring group (p = 0.005). With CBT, 80% improved clinically, compared with only 10% in the control group. Improvements in the CBT group persisted at 3 months’ follow-up. Improved GI symptoms also were correlated with increased positive thoughts and reduced negative automatic thoughts (i.e., negative self-image).10
In the preceding 12 months, the patient has experienced at least 12 weeks or more (need not be consecutive) of abdominal discomfort or pain that has:
Two out of three features
- relieved with defecation, and/or
- onset associated with a change in frequency or stool, and/or
- onset associated with a change in form (appearance) of stool
Symptoms that cumulatively support the diagnosis
- Abnormal stool frequency (for research purposes, “abnormal” may be defined as >3 bowel movements per day and <3 bowel movements per week);
- abnormal stool form (lumpy/hard or loose/watery stool)
- abnormal stool passage (straining, urgency, or feeling of incomplete evacuation)
- passage of mucus
- bloating or abdominal distension.
* In the absence of structural or metabolic abnormalities to explain the symptoms
Source: Drossman DA, Corazziari E, Talley NJ, et al. Rome II: The functional gastrointestinal disorders (2nd ed). McLean, VA: Degnon Associates, 2000.
Group therapy. In a study of group rather than individualized CBT, 45 patients with severe IBS symptoms were randomly assigned to either a group CBT module (n = 25) or a waiting list control group (n = 20). Patients received eight 2-hour group CBT sessions over 3 months, then were followed an average of 2.25 years. Their abdominal pain, number of successful coping behavioral strategies, and avoidance of negative situations improved significantly—compared with the waiting list controls—and persisted during follow-up. The authors concluded that group CBT is effective for alleviating IBS symptoms and improving patients’ coping strategies.11
CBT vs. support groups. In a study comparing the effectiveness of CBT and an IBS self-help support group, 34 patients were randomly assigned to:
- individualized CBT (study group)
- a self-help support group
- or a symptom-monitoring waiting list (control group).
Each group was followed for 8 weeks. GI symptoms were reduced significantly in patients who received individualized CBT, compared with the support group or controls. Anxiety and depression in the CBT group also remained significantly improved 3 months later, compared with both other groups.12
Group psychotherapy
Group psychotherapy for IBS treatment can be more cost-effective than individual therapy, but its success depends on whether patients accept a group format. Data on the use of group psychotherapeutic approaches are largely favorable.
As described previously, one study demonstrated that group CBT reduced abdominal pain and diarrhea and improved symptoms overall.11 In another study, 20 patients with refractory IBS underwent 6 weeks of group behavioral and didactic psychotherapy. GI symptoms, dysphoria, and psychological distress improved significantly in all patients, and their interpersonal sensitivity and hostility were reduced. These improvements persisted 6 months later.12
A recent review suggests that cognitive-behavioral group psychotherapy may be highly effective in patients with IBS. These authors concluded that CBT can improve patients’ mood and other emotional symptoms, reduce their pain, and improve well-being and coping ability.14
Hypnotherapy
Researchers in Manchester, UK, have shown excellent results with hypnotherapy for patients with severe refractory IBS. Although their data seem to have established the ability of hypnotherapy to improve the GI and non-GI symptoms of IBS, corroborating evidence is needed from other centers. In this regard, some preliminary evidence is emerging.
Manchester group. In the first trial by the Manchester group, 30 patients received seven half-hour sessions of hypnotherapy (study group) or supportive psychotherapy and placebo (control group). Patients who received hypnotherapy also were given a tape for home autohypnosis after the third session.
Patients who received hypnotherapy improved dramatically in all GI symptoms— including abdominal pain, bloating, and bowel habits—and in their sense of well-being. This effect persisted 3 months later. The control patients’ abdominal pain, bloating, and well-being improved somewhat, but their bowel habits did not improve.15
The same investigators then studied 30 patients with IBS to assess the effect of hypnotherapy on rectal physiology. Fifteen patients received hypnotherapy, and 15 received relaxation exercises. Rectal sensitivity was measured using anorectal manometry at the start and finish of the intervention.
For patients with diarrhea-predominant IBS, rectal sensitivity was significantly reduced during hypnosis and after the full course of hypnotherapy, compared with controls (p<0.05). In patients with constipation-predominant IBS, a trend towards normalized rectal sensitivity did not reach statistical significance. The investigators concluded that symptomatic improvement of IBS after hypnotherapy may be related to changes in visceral sensitivity of the colon.16
These investigators later studied changes in distal colonic motility in 18 patients undergoing hypnotherapy for IBS. As the patients’ hypnotically induced anger or excitement increased, colonic motility decreased significantly (p<0.01) The investigators concluded that hypnosis may be a useful tool to investigate the effects of emotion on physiologic function.17
Using more contemporary outcome measures and diagnostic criteria, these investigators later showed that hypnotherapy significantly improved:
- IBS symptoms (abdominal pain and bloating, bowel habit, nausea, flatulence, urinary symptoms, lethargy, backache, and dyspareunia)
- quality of life (psychological and physical well-being, mood, locus of control, and work attitude).
Patients treated with hypnosis also took less time away from work (p = 0.02) and visited their physicians less often (p = 0.056), compared with controls.18
Other hypnosis studies. In the United States, another group randomly assigned 12 patients to gut-directed hypnotherapy or symptom monitoring. Subjects were matched by concurrent psychiatric diagnosis, susceptibility to hypnosis, and demographic features.
In findings similar to those of the Manchester group, primary IBS symptoms (abdominal pain, constipation, and flatulence) of patients who received hypnotherapy improved more than those of controls (p = 0.016). Anxiety, as measured by the Spielberger State-Trait Anxiety Inventory (STAXI), also decreased significantly. Treatment-induced gains were well-maintained 2 months later. No significant correlation was found between initial sensitivity to hypnosis and subsequent response to hypnotherapy. A positive relationship was seen between the presence of psychiatric diagnoses and overall levels of improvement.
This study suggests that hypnotherapy can be useful for treating IBS and can be easily adapted to different clinical settings and treatment populations.19 A similar study using gut-directed hypnotherapy found significant improvement in 27 IBS patients treated with short-term hypnosis, and symptom improvement persisted after treatment.20
Biofeedback
The role of biofeedback in IBS treatment remains ill-defined. To be considered as a treatment option, biofeedback must meet or exceed the benefits being achieved with psychotherapy, hypnotherapy, and other behavioral approaches.
In gastroenterology, biofeedback has been used mainly to treat constipation and specifically for outlet constipation due to pelvic floor dysfunction. Anorectal probes to measure rectal pressure in the resting state and during defecation can reveal a pattern of pelvic floor dysfunction. Studies have demonstrated up to 67% improvement in constipation symptoms using biofeedback. Use of anorectal biofeedback in adults with IBS also appears promising, but more controlled trials are needed.21
Anorectal biofeedback has also been used effectively to treat fecal incontinence in children and adults, achieving success rates of 70% or better. In IBS, however, the benefit of biofeedback is less clear. Studies of multicomponent treatment—combining biofeedback with CBT techniques—suggest improvement rates in the 50 to 60% range. However, these findings need to be compared with other treatments for IBS.
Combination treatment
Medical treatment of IBS is progressing. Antidepressant therapy, particularly using tricyclics, has shown moderate benefit.22 Newer medications such as alosetron and tegaserod, which modulate serotonin metabolism in the gut, have been developed. Alosetron and tegaserod have shown significantly greater efficacy compared with placebo for treatment of women with the diarrhea-predominant and constipation-predominant types of IBS, respectively.
Alosetron was voluntarily withdrawn from clinical use in 2000 because of reports of serious GI events associated with its use, including ischemic colitis in a small number of patients (about 3 women in 1,000). Because of the drug’s efficacy in IBS, however, the FDA recently approved its rerelease with a restricted indication—it is to be used only in women with severe diarrhea-predominant IBS who have not responded to conventional IBS treatment. The drug’s manufacturer also is implementing a risk management plan designed to reduce the potential for serious GI side effects.
Table
PSYCHOTHERAPY AS TREATMENT FOR IRRITABLE BOWEL SYNDROME
| Psychotherapeutic approach | Summary of research results |
|---|---|
| Psychodynamic therapy | Shown to be effective in reducing pain and dysphoric mood5 |
| Interpersonal psychotherapy | Effective in reducing pain, bloating, and health care utilization and improving emotional well-being7 |
| Cognitive-behavioral therapy | Improves coping skills and decreases helplessness and somatization14 |
| Group psychotherapy | Seems to be as efficacious as cognitive-behavioral therapy, with the added efficiency of a group model13 |
| Hypnotherapy | Highly effective for a spectrum of IBS symptoms15 |
| Biofeedback | Not useful for IBS per se, but helpful for pelvic floor dysfunction21 |
| Combination therapy | Emerging as a particularly useful strategy, combining medical and behavioral approaches23 |
Tegaserod, a serotonin-4 receptor (5HT4) agonist, was approved by the FDA in July. It is indicated for short-term treatment of women with IBS whose primary bowel symptom is constipation.
Combination therapy—medical management plus psychotherapy—may represent the future of IBS treatment (Table). This approach was examined recently in a randomized study of 24 IBS patients who received standard medical treatment alone or in combination with behavioral therapy. The behavioral component included patient education, helping patients shape a plausible model for their illness, progressive muscle relaxation, cognitive coping strategies, problem-solving education, and assertiveness and social skills training. All patients were treated in 10 individual therapy sessions of approximately 1 hour each by one of two psychotherapists across 10 weeks.
Compared with medical treatment alone, patients treated with combination therapy showed significantly improved GI symptoms (p < 0.001) and psychological symptoms (p = 0.01) in terms of both patient report and objective psychological measures. Outcomes are enhanced for patients with severe IBS, the investigators concluded, when behavioral therapy is added to thoughtful medical management.23
Related resource
- International Foundation for Functional Gastrointestinal Disorders. www.iffgd.org
Drug brand names
- Alosetron • Lotronex
- Tegaserod • Zelnorm
Disclosure
The author reports that he serves as a consultant to Novartis Pharmaceuticals Corp. He reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sandler R. Epidemiology of irritable bowel syndrome in the United States. Gastroenterology 1990;99:409-15.
2. Leserman J, Drossman DA, Li Z, et al. Sexual and physical abuse history in gastroenterology practice: how types of abuse impact health status. Psychosom Med 1996;58:4-15.
3. Klein KB. Controlled treatment trials in the irritable bowel syndrome: a critique. Gastroenterology 1988;95:232-41.
4. Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med 2000;133:136-47.
5. Svedlund J, Sjodin I, Ottosson JO, et al. Controlled study of psychotherapy in irritable bowel syndrome. Lancet 1983;ii:589-92.
6. Guthrie EA, Creed FH, Dawson D, et al. A controlled trial of psychological treatment for the irritable bowel syndrome. Gastroenterology 1991;100:450-7.
7. Guthrie EA, Creed FH, Dawson D, et al. A randomized controlled trial of psychotherapy in patients with refractory irritable bowel syndrome. Br J Psychiatry 1993;163:315-21.
8. Blanchard EB, Schwartz SP. Adaptation of a multi-component treatment program for irritable bowel syndrome to a small group format. Biofeedback Self-Regulation 1987;12:63-9.
9. Blanchard EB, Scharff L, Payne A, et al. Prediction of outcome from cognitive-behavioral treatment of irritable bowel syndrome. Behav Res Ther 1992;30:647-50.
10. Greene B, Blanchard EB. Cognitive therapy for irritable bowel syndrome. J Consult Clin Psychol 1994;62(3):576-82.
11. Van Dulmen AM, Fennis JFM, Bleijenberg G. Cognitive-behavioral group therapy for irritable bowel syndrome: effects and long-term follow-up. Psychosom Med 1996;58:508-14.
12. Payne A, Blanchard EB. A controlled comparison of cognitive therapy and self-help support groups in the treatment of irritable bowel syndrome. J Consult Clin Psychol 1995;63(5):779-86.
13. Wise TN, Cooper JN, Ahmed S. The efficacy of group therapy for patients with irritable bowel syndrome. Psychosomatics 1982;23:465-9.
14. Toner BB, Segal ZV, Emmott S, et al. Cognitive-behavioral group therapy for patients with irritable bowel syndrome. Int J Group Psychotherapy 1998;48(2):215-43.
15. Whorwell PJ, Prior A, Faragher EB. Controlled trial of hypnotherapy in the treatment of severe refractory irritable-bowel syndrome. Lancet 1984;2(8414):1232-43.
16. Prior A, Colgan SM, Whorwell PJ. Changes in rectal sensitivity after hypnotherapy in patients with irritable bowel syndrome. Gut 1990;31:896-8.
17. Whorwell PJ, Houghton LA, Taylor EE, et al. Physiological effects of emotion: assessment via hypnosis. Lancet 1992;340:69-72.
18. Houghton LA, Heyman DJ, Whorwell PJ. Symptomatology, quality of life and economic features of irritable bowel syndrome: the effect of hypnotherapy. Aliment Pharmacol Ther 1996;10(1):91-5.
19. Galovski TE, Blanchard EB. The treatment of irritable bowel syndrome with hypnotherapy. Appl Psychophysiol Biofeedback 1998;23(4):219-32.
20. Vidakovic-Vukic M. Hypnotherapy in the treatment of irritable bowel syndrome: methods and results in Amsterdam. Scandinavian University Press 1999;34(suppl 230):49-51.
21. Bassotti G, Whitehead WE. Biofeedback as a treatment approach to gastrointestinal tract disorders. Am J Gastroenterol 1994;89(2):158-64.
22. Jackson JL, O’Malley PG, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000;108:65-72.
23. Heymann-Mönnikes I, Arnold R, Florin I, et al. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-94.
A growing body of evidence suggests that psychiatrists have much to offer patients with severe irritable bowel syndrome (IBS). Behavioral and psychotherapeutic approaches are showing promise in relieving both GI and mood disturbances.
Treating patients with IBS with medications designed to influence only gut function can be frustrating. Those with refractory symptoms may be extremely sensitive to drug side effects, and they often report that medical management worsens or does not improve their symptoms. They experiment with alternative medicines and wander from physician to physician in a disappointing search for a “cure.”
Let’s look at evidence on the efficacy of individual and group behavioral therapies, hypnotherapy, biofeedback, and combination medical and behavioral treatment.
Psychotherapeutic approaches
IBS is a common gastrointestinal disorder that is characterized by abdominal discomfort and changes in bowel habits (Boxes 1 and 2). Patients with severe IBS symptoms often bring significant psychological impairment and psychosocial trauma to clinical encounters.1,2 They respond poorly to standard medical management, and evidence supporting the efficacy of medical treatments for IBS remains weak.3,4
Irritable bowel syndrome (IBS) is the most common disorder seen in gastrointestinal practice, representing more than 40% of all visits to gastroenterologists. Complaints of IBS also account for approximately 23% of office visits to primary care physicians.1
Key symptoms of this functional disorder are a pattern of lower abdominal discomfort and bloating accompanied by variable degrees of altered stool pattern—constipation, diarrhea, or intermittent constipation and diarrhea. IBS is most common in young patients, with onset rarely diagnosed after age 45. Its incidence is equal in men and women, but women are more likely to seek medical care for IBS symptoms.
The cause of IBS is unclear. Recent research suggests that changes in serotonin metabolism cause a pattern of visceral hypersensitivity and an altered sensation of pain. IBS is not a psychiatric disorder, but it can be worsened by comorbid psychopathology, particularly mood and anxiety disorders. Although patients tend to have either diarrhea-predominant or constipation-predominant IBS, the pathophysiology of both patterns seems similar.
In 1983, the first controlled trial of psychodynamic psychotherapy for IBS showed dramatic reductions in symptoms.5 A subsequent series of high-quality articles in the early 1990s also showed that interpersonal psychotherapy (with greater interaction between therapist and patient) could significantly decrease IBS symptoms.
Persistent improvement. In one randomized controlled trial,6 102 patients with IBS received either standard medical treatment or 10 hours of dynamically oriented individual psychotherapy in combination with standard medical treatment. After 3 months, patients who received psychotherapy showed significantly greater improvement in somatic symptoms and emotional well-being, compared with those who received medical treatment only.
Interestingly, this difference persisted 1 year after the study ended. GI symptoms and the emotional well-being of patients who received combination therapy continued to improve, whereas the physical and emotional status of those who received only standard medical treatment deteriorated.
In a second study,7 101 patients with severe IBS symptoms continued to receive medical treatment but were randomly divided into two groups:
- Study subjects received 8 hours of dynamically oriented psychotherapy.
- Control patients met with a psychiatrist who engaged in “supportive listening” but delivered no psychotherapy. This strategy was adopted to control for the effect of the psychiatrist’s presence.
Assessments included patients’ self-reports of symptoms, ratings of GI symptoms by the treating gastroenterologists, and measures of depression, anxiety, and health care utilization. Patients who received psychotherapy reported significant improvements in bowel symptoms (e.g., diarrhea, constipation, bloating, and abdominal pain). Likewise, the gastroenterologist who rated patients’ GI symptoms felt that those who received psychotherapy improved significantly across the entire spectrum of GI symptoms. The improvements were maintained at 1-year follow-up.
By comparison, the control patients reported worsening symptoms, as did subjects who dropped out. Patients who received psychotherapy also made significantly fewer outpatient visits to gastroenterologists, compared with controls (p<0.001).
Cognitive-behavioral therapy
Cognitive-behavioral therapy (CBT) is emerging as a major psychotherapeutic tool for treating mood disorders, anxiety disorders, and somatic syndromes associated with psychosocial distress. CBT also is showing promise for patients with moderate to severe IBS and those with IBS and concomitant anxiety or mood disorders. Studies consistently show that CBT is superior to standard medical management or the use of support groups or other behavioral treatments alone.
Reduced symptoms. In an early trial of CBT, 17 patients with IBS experienced significantly less abdominal pain and diarrhea after participating in a program of progressive relaxation, education about bowel functioning, use of thermal biofeedback, and stress coping techniques based on CBT. Overall, 64% of the patients improved.8
The same investigators then assigned 90 patients to 12 sessions of CBT, given over 8 weeks with or without meditation and biofeedback. Patients with axis I psychiatric diagnoses tended to respond poorly to CBT. The authors concluded that a careful pretreatment psychological workup is important to identify patients with IBS who would benefit most from CBT.9
As a follow-up, these investigators randomly assigned 20 patients to intensive individualized CBT (10 sessions over 8 weeks) or 8 weeks of daily GI symptom monitoring. Patients who received CBT had significantly fewer GI symptoms than did the symptom-monitoring group (p = 0.005). With CBT, 80% improved clinically, compared with only 10% in the control group. Improvements in the CBT group persisted at 3 months’ follow-up. Improved GI symptoms also were correlated with increased positive thoughts and reduced negative automatic thoughts (i.e., negative self-image).10
In the preceding 12 months, the patient has experienced at least 12 weeks or more (need not be consecutive) of abdominal discomfort or pain that has:
Two out of three features
- relieved with defecation, and/or
- onset associated with a change in frequency or stool, and/or
- onset associated with a change in form (appearance) of stool
Symptoms that cumulatively support the diagnosis
- Abnormal stool frequency (for research purposes, “abnormal” may be defined as >3 bowel movements per day and <3 bowel movements per week);
- abnormal stool form (lumpy/hard or loose/watery stool)
- abnormal stool passage (straining, urgency, or feeling of incomplete evacuation)
- passage of mucus
- bloating or abdominal distension.
* In the absence of structural or metabolic abnormalities to explain the symptoms
Source: Drossman DA, Corazziari E, Talley NJ, et al. Rome II: The functional gastrointestinal disorders (2nd ed). McLean, VA: Degnon Associates, 2000.
Group therapy. In a study of group rather than individualized CBT, 45 patients with severe IBS symptoms were randomly assigned to either a group CBT module (n = 25) or a waiting list control group (n = 20). Patients received eight 2-hour group CBT sessions over 3 months, then were followed an average of 2.25 years. Their abdominal pain, number of successful coping behavioral strategies, and avoidance of negative situations improved significantly—compared with the waiting list controls—and persisted during follow-up. The authors concluded that group CBT is effective for alleviating IBS symptoms and improving patients’ coping strategies.11
CBT vs. support groups. In a study comparing the effectiveness of CBT and an IBS self-help support group, 34 patients were randomly assigned to:
- individualized CBT (study group)
- a self-help support group
- or a symptom-monitoring waiting list (control group).
Each group was followed for 8 weeks. GI symptoms were reduced significantly in patients who received individualized CBT, compared with the support group or controls. Anxiety and depression in the CBT group also remained significantly improved 3 months later, compared with both other groups.12
Group psychotherapy
Group psychotherapy for IBS treatment can be more cost-effective than individual therapy, but its success depends on whether patients accept a group format. Data on the use of group psychotherapeutic approaches are largely favorable.
As described previously, one study demonstrated that group CBT reduced abdominal pain and diarrhea and improved symptoms overall.11 In another study, 20 patients with refractory IBS underwent 6 weeks of group behavioral and didactic psychotherapy. GI symptoms, dysphoria, and psychological distress improved significantly in all patients, and their interpersonal sensitivity and hostility were reduced. These improvements persisted 6 months later.12
A recent review suggests that cognitive-behavioral group psychotherapy may be highly effective in patients with IBS. These authors concluded that CBT can improve patients’ mood and other emotional symptoms, reduce their pain, and improve well-being and coping ability.14
Hypnotherapy
Researchers in Manchester, UK, have shown excellent results with hypnotherapy for patients with severe refractory IBS. Although their data seem to have established the ability of hypnotherapy to improve the GI and non-GI symptoms of IBS, corroborating evidence is needed from other centers. In this regard, some preliminary evidence is emerging.
Manchester group. In the first trial by the Manchester group, 30 patients received seven half-hour sessions of hypnotherapy (study group) or supportive psychotherapy and placebo (control group). Patients who received hypnotherapy also were given a tape for home autohypnosis after the third session.
Patients who received hypnotherapy improved dramatically in all GI symptoms— including abdominal pain, bloating, and bowel habits—and in their sense of well-being. This effect persisted 3 months later. The control patients’ abdominal pain, bloating, and well-being improved somewhat, but their bowel habits did not improve.15
The same investigators then studied 30 patients with IBS to assess the effect of hypnotherapy on rectal physiology. Fifteen patients received hypnotherapy, and 15 received relaxation exercises. Rectal sensitivity was measured using anorectal manometry at the start and finish of the intervention.
For patients with diarrhea-predominant IBS, rectal sensitivity was significantly reduced during hypnosis and after the full course of hypnotherapy, compared with controls (p<0.05). In patients with constipation-predominant IBS, a trend towards normalized rectal sensitivity did not reach statistical significance. The investigators concluded that symptomatic improvement of IBS after hypnotherapy may be related to changes in visceral sensitivity of the colon.16
These investigators later studied changes in distal colonic motility in 18 patients undergoing hypnotherapy for IBS. As the patients’ hypnotically induced anger or excitement increased, colonic motility decreased significantly (p<0.01) The investigators concluded that hypnosis may be a useful tool to investigate the effects of emotion on physiologic function.17
Using more contemporary outcome measures and diagnostic criteria, these investigators later showed that hypnotherapy significantly improved:
- IBS symptoms (abdominal pain and bloating, bowel habit, nausea, flatulence, urinary symptoms, lethargy, backache, and dyspareunia)
- quality of life (psychological and physical well-being, mood, locus of control, and work attitude).
Patients treated with hypnosis also took less time away from work (p = 0.02) and visited their physicians less often (p = 0.056), compared with controls.18
Other hypnosis studies. In the United States, another group randomly assigned 12 patients to gut-directed hypnotherapy or symptom monitoring. Subjects were matched by concurrent psychiatric diagnosis, susceptibility to hypnosis, and demographic features.
In findings similar to those of the Manchester group, primary IBS symptoms (abdominal pain, constipation, and flatulence) of patients who received hypnotherapy improved more than those of controls (p = 0.016). Anxiety, as measured by the Spielberger State-Trait Anxiety Inventory (STAXI), also decreased significantly. Treatment-induced gains were well-maintained 2 months later. No significant correlation was found between initial sensitivity to hypnosis and subsequent response to hypnotherapy. A positive relationship was seen between the presence of psychiatric diagnoses and overall levels of improvement.
This study suggests that hypnotherapy can be useful for treating IBS and can be easily adapted to different clinical settings and treatment populations.19 A similar study using gut-directed hypnotherapy found significant improvement in 27 IBS patients treated with short-term hypnosis, and symptom improvement persisted after treatment.20
Biofeedback
The role of biofeedback in IBS treatment remains ill-defined. To be considered as a treatment option, biofeedback must meet or exceed the benefits being achieved with psychotherapy, hypnotherapy, and other behavioral approaches.
In gastroenterology, biofeedback has been used mainly to treat constipation and specifically for outlet constipation due to pelvic floor dysfunction. Anorectal probes to measure rectal pressure in the resting state and during defecation can reveal a pattern of pelvic floor dysfunction. Studies have demonstrated up to 67% improvement in constipation symptoms using biofeedback. Use of anorectal biofeedback in adults with IBS also appears promising, but more controlled trials are needed.21
Anorectal biofeedback has also been used effectively to treat fecal incontinence in children and adults, achieving success rates of 70% or better. In IBS, however, the benefit of biofeedback is less clear. Studies of multicomponent treatment—combining biofeedback with CBT techniques—suggest improvement rates in the 50 to 60% range. However, these findings need to be compared with other treatments for IBS.
Combination treatment
Medical treatment of IBS is progressing. Antidepressant therapy, particularly using tricyclics, has shown moderate benefit.22 Newer medications such as alosetron and tegaserod, which modulate serotonin metabolism in the gut, have been developed. Alosetron and tegaserod have shown significantly greater efficacy compared with placebo for treatment of women with the diarrhea-predominant and constipation-predominant types of IBS, respectively.
Alosetron was voluntarily withdrawn from clinical use in 2000 because of reports of serious GI events associated with its use, including ischemic colitis in a small number of patients (about 3 women in 1,000). Because of the drug’s efficacy in IBS, however, the FDA recently approved its rerelease with a restricted indication—it is to be used only in women with severe diarrhea-predominant IBS who have not responded to conventional IBS treatment. The drug’s manufacturer also is implementing a risk management plan designed to reduce the potential for serious GI side effects.
Table
PSYCHOTHERAPY AS TREATMENT FOR IRRITABLE BOWEL SYNDROME
| Psychotherapeutic approach | Summary of research results |
|---|---|
| Psychodynamic therapy | Shown to be effective in reducing pain and dysphoric mood5 |
| Interpersonal psychotherapy | Effective in reducing pain, bloating, and health care utilization and improving emotional well-being7 |
| Cognitive-behavioral therapy | Improves coping skills and decreases helplessness and somatization14 |
| Group psychotherapy | Seems to be as efficacious as cognitive-behavioral therapy, with the added efficiency of a group model13 |
| Hypnotherapy | Highly effective for a spectrum of IBS symptoms15 |
| Biofeedback | Not useful for IBS per se, but helpful for pelvic floor dysfunction21 |
| Combination therapy | Emerging as a particularly useful strategy, combining medical and behavioral approaches23 |
Tegaserod, a serotonin-4 receptor (5HT4) agonist, was approved by the FDA in July. It is indicated for short-term treatment of women with IBS whose primary bowel symptom is constipation.
Combination therapy—medical management plus psychotherapy—may represent the future of IBS treatment (Table). This approach was examined recently in a randomized study of 24 IBS patients who received standard medical treatment alone or in combination with behavioral therapy. The behavioral component included patient education, helping patients shape a plausible model for their illness, progressive muscle relaxation, cognitive coping strategies, problem-solving education, and assertiveness and social skills training. All patients were treated in 10 individual therapy sessions of approximately 1 hour each by one of two psychotherapists across 10 weeks.
Compared with medical treatment alone, patients treated with combination therapy showed significantly improved GI symptoms (p < 0.001) and psychological symptoms (p = 0.01) in terms of both patient report and objective psychological measures. Outcomes are enhanced for patients with severe IBS, the investigators concluded, when behavioral therapy is added to thoughtful medical management.23
Related resource
- International Foundation for Functional Gastrointestinal Disorders. www.iffgd.org
Drug brand names
- Alosetron • Lotronex
- Tegaserod • Zelnorm
Disclosure
The author reports that he serves as a consultant to Novartis Pharmaceuticals Corp. He reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
A growing body of evidence suggests that psychiatrists have much to offer patients with severe irritable bowel syndrome (IBS). Behavioral and psychotherapeutic approaches are showing promise in relieving both GI and mood disturbances.
Treating patients with IBS with medications designed to influence only gut function can be frustrating. Those with refractory symptoms may be extremely sensitive to drug side effects, and they often report that medical management worsens or does not improve their symptoms. They experiment with alternative medicines and wander from physician to physician in a disappointing search for a “cure.”
Let’s look at evidence on the efficacy of individual and group behavioral therapies, hypnotherapy, biofeedback, and combination medical and behavioral treatment.
Psychotherapeutic approaches
IBS is a common gastrointestinal disorder that is characterized by abdominal discomfort and changes in bowel habits (Boxes 1 and 2). Patients with severe IBS symptoms often bring significant psychological impairment and psychosocial trauma to clinical encounters.1,2 They respond poorly to standard medical management, and evidence supporting the efficacy of medical treatments for IBS remains weak.3,4
Irritable bowel syndrome (IBS) is the most common disorder seen in gastrointestinal practice, representing more than 40% of all visits to gastroenterologists. Complaints of IBS also account for approximately 23% of office visits to primary care physicians.1
Key symptoms of this functional disorder are a pattern of lower abdominal discomfort and bloating accompanied by variable degrees of altered stool pattern—constipation, diarrhea, or intermittent constipation and diarrhea. IBS is most common in young patients, with onset rarely diagnosed after age 45. Its incidence is equal in men and women, but women are more likely to seek medical care for IBS symptoms.
The cause of IBS is unclear. Recent research suggests that changes in serotonin metabolism cause a pattern of visceral hypersensitivity and an altered sensation of pain. IBS is not a psychiatric disorder, but it can be worsened by comorbid psychopathology, particularly mood and anxiety disorders. Although patients tend to have either diarrhea-predominant or constipation-predominant IBS, the pathophysiology of both patterns seems similar.
In 1983, the first controlled trial of psychodynamic psychotherapy for IBS showed dramatic reductions in symptoms.5 A subsequent series of high-quality articles in the early 1990s also showed that interpersonal psychotherapy (with greater interaction between therapist and patient) could significantly decrease IBS symptoms.
Persistent improvement. In one randomized controlled trial,6 102 patients with IBS received either standard medical treatment or 10 hours of dynamically oriented individual psychotherapy in combination with standard medical treatment. After 3 months, patients who received psychotherapy showed significantly greater improvement in somatic symptoms and emotional well-being, compared with those who received medical treatment only.
Interestingly, this difference persisted 1 year after the study ended. GI symptoms and the emotional well-being of patients who received combination therapy continued to improve, whereas the physical and emotional status of those who received only standard medical treatment deteriorated.
In a second study,7 101 patients with severe IBS symptoms continued to receive medical treatment but were randomly divided into two groups:
- Study subjects received 8 hours of dynamically oriented psychotherapy.
- Control patients met with a psychiatrist who engaged in “supportive listening” but delivered no psychotherapy. This strategy was adopted to control for the effect of the psychiatrist’s presence.
Assessments included patients’ self-reports of symptoms, ratings of GI symptoms by the treating gastroenterologists, and measures of depression, anxiety, and health care utilization. Patients who received psychotherapy reported significant improvements in bowel symptoms (e.g., diarrhea, constipation, bloating, and abdominal pain). Likewise, the gastroenterologist who rated patients’ GI symptoms felt that those who received psychotherapy improved significantly across the entire spectrum of GI symptoms. The improvements were maintained at 1-year follow-up.
By comparison, the control patients reported worsening symptoms, as did subjects who dropped out. Patients who received psychotherapy also made significantly fewer outpatient visits to gastroenterologists, compared with controls (p<0.001).
Cognitive-behavioral therapy
Cognitive-behavioral therapy (CBT) is emerging as a major psychotherapeutic tool for treating mood disorders, anxiety disorders, and somatic syndromes associated with psychosocial distress. CBT also is showing promise for patients with moderate to severe IBS and those with IBS and concomitant anxiety or mood disorders. Studies consistently show that CBT is superior to standard medical management or the use of support groups or other behavioral treatments alone.
Reduced symptoms. In an early trial of CBT, 17 patients with IBS experienced significantly less abdominal pain and diarrhea after participating in a program of progressive relaxation, education about bowel functioning, use of thermal biofeedback, and stress coping techniques based on CBT. Overall, 64% of the patients improved.8
The same investigators then assigned 90 patients to 12 sessions of CBT, given over 8 weeks with or without meditation and biofeedback. Patients with axis I psychiatric diagnoses tended to respond poorly to CBT. The authors concluded that a careful pretreatment psychological workup is important to identify patients with IBS who would benefit most from CBT.9
As a follow-up, these investigators randomly assigned 20 patients to intensive individualized CBT (10 sessions over 8 weeks) or 8 weeks of daily GI symptom monitoring. Patients who received CBT had significantly fewer GI symptoms than did the symptom-monitoring group (p = 0.005). With CBT, 80% improved clinically, compared with only 10% in the control group. Improvements in the CBT group persisted at 3 months’ follow-up. Improved GI symptoms also were correlated with increased positive thoughts and reduced negative automatic thoughts (i.e., negative self-image).10
In the preceding 12 months, the patient has experienced at least 12 weeks or more (need not be consecutive) of abdominal discomfort or pain that has:
Two out of three features
- relieved with defecation, and/or
- onset associated with a change in frequency or stool, and/or
- onset associated with a change in form (appearance) of stool
Symptoms that cumulatively support the diagnosis
- Abnormal stool frequency (for research purposes, “abnormal” may be defined as >3 bowel movements per day and <3 bowel movements per week);
- abnormal stool form (lumpy/hard or loose/watery stool)
- abnormal stool passage (straining, urgency, or feeling of incomplete evacuation)
- passage of mucus
- bloating or abdominal distension.
* In the absence of structural or metabolic abnormalities to explain the symptoms
Source: Drossman DA, Corazziari E, Talley NJ, et al. Rome II: The functional gastrointestinal disorders (2nd ed). McLean, VA: Degnon Associates, 2000.
Group therapy. In a study of group rather than individualized CBT, 45 patients with severe IBS symptoms were randomly assigned to either a group CBT module (n = 25) or a waiting list control group (n = 20). Patients received eight 2-hour group CBT sessions over 3 months, then were followed an average of 2.25 years. Their abdominal pain, number of successful coping behavioral strategies, and avoidance of negative situations improved significantly—compared with the waiting list controls—and persisted during follow-up. The authors concluded that group CBT is effective for alleviating IBS symptoms and improving patients’ coping strategies.11
CBT vs. support groups. In a study comparing the effectiveness of CBT and an IBS self-help support group, 34 patients were randomly assigned to:
- individualized CBT (study group)
- a self-help support group
- or a symptom-monitoring waiting list (control group).
Each group was followed for 8 weeks. GI symptoms were reduced significantly in patients who received individualized CBT, compared with the support group or controls. Anxiety and depression in the CBT group also remained significantly improved 3 months later, compared with both other groups.12
Group psychotherapy
Group psychotherapy for IBS treatment can be more cost-effective than individual therapy, but its success depends on whether patients accept a group format. Data on the use of group psychotherapeutic approaches are largely favorable.
As described previously, one study demonstrated that group CBT reduced abdominal pain and diarrhea and improved symptoms overall.11 In another study, 20 patients with refractory IBS underwent 6 weeks of group behavioral and didactic psychotherapy. GI symptoms, dysphoria, and psychological distress improved significantly in all patients, and their interpersonal sensitivity and hostility were reduced. These improvements persisted 6 months later.12
A recent review suggests that cognitive-behavioral group psychotherapy may be highly effective in patients with IBS. These authors concluded that CBT can improve patients’ mood and other emotional symptoms, reduce their pain, and improve well-being and coping ability.14
Hypnotherapy
Researchers in Manchester, UK, have shown excellent results with hypnotherapy for patients with severe refractory IBS. Although their data seem to have established the ability of hypnotherapy to improve the GI and non-GI symptoms of IBS, corroborating evidence is needed from other centers. In this regard, some preliminary evidence is emerging.
Manchester group. In the first trial by the Manchester group, 30 patients received seven half-hour sessions of hypnotherapy (study group) or supportive psychotherapy and placebo (control group). Patients who received hypnotherapy also were given a tape for home autohypnosis after the third session.
Patients who received hypnotherapy improved dramatically in all GI symptoms— including abdominal pain, bloating, and bowel habits—and in their sense of well-being. This effect persisted 3 months later. The control patients’ abdominal pain, bloating, and well-being improved somewhat, but their bowel habits did not improve.15
The same investigators then studied 30 patients with IBS to assess the effect of hypnotherapy on rectal physiology. Fifteen patients received hypnotherapy, and 15 received relaxation exercises. Rectal sensitivity was measured using anorectal manometry at the start and finish of the intervention.
For patients with diarrhea-predominant IBS, rectal sensitivity was significantly reduced during hypnosis and after the full course of hypnotherapy, compared with controls (p<0.05). In patients with constipation-predominant IBS, a trend towards normalized rectal sensitivity did not reach statistical significance. The investigators concluded that symptomatic improvement of IBS after hypnotherapy may be related to changes in visceral sensitivity of the colon.16
These investigators later studied changes in distal colonic motility in 18 patients undergoing hypnotherapy for IBS. As the patients’ hypnotically induced anger or excitement increased, colonic motility decreased significantly (p<0.01) The investigators concluded that hypnosis may be a useful tool to investigate the effects of emotion on physiologic function.17
Using more contemporary outcome measures and diagnostic criteria, these investigators later showed that hypnotherapy significantly improved:
- IBS symptoms (abdominal pain and bloating, bowel habit, nausea, flatulence, urinary symptoms, lethargy, backache, and dyspareunia)
- quality of life (psychological and physical well-being, mood, locus of control, and work attitude).
Patients treated with hypnosis also took less time away from work (p = 0.02) and visited their physicians less often (p = 0.056), compared with controls.18
Other hypnosis studies. In the United States, another group randomly assigned 12 patients to gut-directed hypnotherapy or symptom monitoring. Subjects were matched by concurrent psychiatric diagnosis, susceptibility to hypnosis, and demographic features.
In findings similar to those of the Manchester group, primary IBS symptoms (abdominal pain, constipation, and flatulence) of patients who received hypnotherapy improved more than those of controls (p = 0.016). Anxiety, as measured by the Spielberger State-Trait Anxiety Inventory (STAXI), also decreased significantly. Treatment-induced gains were well-maintained 2 months later. No significant correlation was found between initial sensitivity to hypnosis and subsequent response to hypnotherapy. A positive relationship was seen between the presence of psychiatric diagnoses and overall levels of improvement.
This study suggests that hypnotherapy can be useful for treating IBS and can be easily adapted to different clinical settings and treatment populations.19 A similar study using gut-directed hypnotherapy found significant improvement in 27 IBS patients treated with short-term hypnosis, and symptom improvement persisted after treatment.20
Biofeedback
The role of biofeedback in IBS treatment remains ill-defined. To be considered as a treatment option, biofeedback must meet or exceed the benefits being achieved with psychotherapy, hypnotherapy, and other behavioral approaches.
In gastroenterology, biofeedback has been used mainly to treat constipation and specifically for outlet constipation due to pelvic floor dysfunction. Anorectal probes to measure rectal pressure in the resting state and during defecation can reveal a pattern of pelvic floor dysfunction. Studies have demonstrated up to 67% improvement in constipation symptoms using biofeedback. Use of anorectal biofeedback in adults with IBS also appears promising, but more controlled trials are needed.21
Anorectal biofeedback has also been used effectively to treat fecal incontinence in children and adults, achieving success rates of 70% or better. In IBS, however, the benefit of biofeedback is less clear. Studies of multicomponent treatment—combining biofeedback with CBT techniques—suggest improvement rates in the 50 to 60% range. However, these findings need to be compared with other treatments for IBS.
Combination treatment
Medical treatment of IBS is progressing. Antidepressant therapy, particularly using tricyclics, has shown moderate benefit.22 Newer medications such as alosetron and tegaserod, which modulate serotonin metabolism in the gut, have been developed. Alosetron and tegaserod have shown significantly greater efficacy compared with placebo for treatment of women with the diarrhea-predominant and constipation-predominant types of IBS, respectively.
Alosetron was voluntarily withdrawn from clinical use in 2000 because of reports of serious GI events associated with its use, including ischemic colitis in a small number of patients (about 3 women in 1,000). Because of the drug’s efficacy in IBS, however, the FDA recently approved its rerelease with a restricted indication—it is to be used only in women with severe diarrhea-predominant IBS who have not responded to conventional IBS treatment. The drug’s manufacturer also is implementing a risk management plan designed to reduce the potential for serious GI side effects.
Table
PSYCHOTHERAPY AS TREATMENT FOR IRRITABLE BOWEL SYNDROME
| Psychotherapeutic approach | Summary of research results |
|---|---|
| Psychodynamic therapy | Shown to be effective in reducing pain and dysphoric mood5 |
| Interpersonal psychotherapy | Effective in reducing pain, bloating, and health care utilization and improving emotional well-being7 |
| Cognitive-behavioral therapy | Improves coping skills and decreases helplessness and somatization14 |
| Group psychotherapy | Seems to be as efficacious as cognitive-behavioral therapy, with the added efficiency of a group model13 |
| Hypnotherapy | Highly effective for a spectrum of IBS symptoms15 |
| Biofeedback | Not useful for IBS per se, but helpful for pelvic floor dysfunction21 |
| Combination therapy | Emerging as a particularly useful strategy, combining medical and behavioral approaches23 |
Tegaserod, a serotonin-4 receptor (5HT4) agonist, was approved by the FDA in July. It is indicated for short-term treatment of women with IBS whose primary bowel symptom is constipation.
Combination therapy—medical management plus psychotherapy—may represent the future of IBS treatment (Table). This approach was examined recently in a randomized study of 24 IBS patients who received standard medical treatment alone or in combination with behavioral therapy. The behavioral component included patient education, helping patients shape a plausible model for their illness, progressive muscle relaxation, cognitive coping strategies, problem-solving education, and assertiveness and social skills training. All patients were treated in 10 individual therapy sessions of approximately 1 hour each by one of two psychotherapists across 10 weeks.
Compared with medical treatment alone, patients treated with combination therapy showed significantly improved GI symptoms (p < 0.001) and psychological symptoms (p = 0.01) in terms of both patient report and objective psychological measures. Outcomes are enhanced for patients with severe IBS, the investigators concluded, when behavioral therapy is added to thoughtful medical management.23
Related resource
- International Foundation for Functional Gastrointestinal Disorders. www.iffgd.org
Drug brand names
- Alosetron • Lotronex
- Tegaserod • Zelnorm
Disclosure
The author reports that he serves as a consultant to Novartis Pharmaceuticals Corp. He reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Sandler R. Epidemiology of irritable bowel syndrome in the United States. Gastroenterology 1990;99:409-15.
2. Leserman J, Drossman DA, Li Z, et al. Sexual and physical abuse history in gastroenterology practice: how types of abuse impact health status. Psychosom Med 1996;58:4-15.
3. Klein KB. Controlled treatment trials in the irritable bowel syndrome: a critique. Gastroenterology 1988;95:232-41.
4. Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med 2000;133:136-47.
5. Svedlund J, Sjodin I, Ottosson JO, et al. Controlled study of psychotherapy in irritable bowel syndrome. Lancet 1983;ii:589-92.
6. Guthrie EA, Creed FH, Dawson D, et al. A controlled trial of psychological treatment for the irritable bowel syndrome. Gastroenterology 1991;100:450-7.
7. Guthrie EA, Creed FH, Dawson D, et al. A randomized controlled trial of psychotherapy in patients with refractory irritable bowel syndrome. Br J Psychiatry 1993;163:315-21.
8. Blanchard EB, Schwartz SP. Adaptation of a multi-component treatment program for irritable bowel syndrome to a small group format. Biofeedback Self-Regulation 1987;12:63-9.
9. Blanchard EB, Scharff L, Payne A, et al. Prediction of outcome from cognitive-behavioral treatment of irritable bowel syndrome. Behav Res Ther 1992;30:647-50.
10. Greene B, Blanchard EB. Cognitive therapy for irritable bowel syndrome. J Consult Clin Psychol 1994;62(3):576-82.
11. Van Dulmen AM, Fennis JFM, Bleijenberg G. Cognitive-behavioral group therapy for irritable bowel syndrome: effects and long-term follow-up. Psychosom Med 1996;58:508-14.
12. Payne A, Blanchard EB. A controlled comparison of cognitive therapy and self-help support groups in the treatment of irritable bowel syndrome. J Consult Clin Psychol 1995;63(5):779-86.
13. Wise TN, Cooper JN, Ahmed S. The efficacy of group therapy for patients with irritable bowel syndrome. Psychosomatics 1982;23:465-9.
14. Toner BB, Segal ZV, Emmott S, et al. Cognitive-behavioral group therapy for patients with irritable bowel syndrome. Int J Group Psychotherapy 1998;48(2):215-43.
15. Whorwell PJ, Prior A, Faragher EB. Controlled trial of hypnotherapy in the treatment of severe refractory irritable-bowel syndrome. Lancet 1984;2(8414):1232-43.
16. Prior A, Colgan SM, Whorwell PJ. Changes in rectal sensitivity after hypnotherapy in patients with irritable bowel syndrome. Gut 1990;31:896-8.
17. Whorwell PJ, Houghton LA, Taylor EE, et al. Physiological effects of emotion: assessment via hypnosis. Lancet 1992;340:69-72.
18. Houghton LA, Heyman DJ, Whorwell PJ. Symptomatology, quality of life and economic features of irritable bowel syndrome: the effect of hypnotherapy. Aliment Pharmacol Ther 1996;10(1):91-5.
19. Galovski TE, Blanchard EB. The treatment of irritable bowel syndrome with hypnotherapy. Appl Psychophysiol Biofeedback 1998;23(4):219-32.
20. Vidakovic-Vukic M. Hypnotherapy in the treatment of irritable bowel syndrome: methods and results in Amsterdam. Scandinavian University Press 1999;34(suppl 230):49-51.
21. Bassotti G, Whitehead WE. Biofeedback as a treatment approach to gastrointestinal tract disorders. Am J Gastroenterol 1994;89(2):158-64.
22. Jackson JL, O’Malley PG, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000;108:65-72.
23. Heymann-Mönnikes I, Arnold R, Florin I, et al. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-94.
1. Sandler R. Epidemiology of irritable bowel syndrome in the United States. Gastroenterology 1990;99:409-15.
2. Leserman J, Drossman DA, Li Z, et al. Sexual and physical abuse history in gastroenterology practice: how types of abuse impact health status. Psychosom Med 1996;58:4-15.
3. Klein KB. Controlled treatment trials in the irritable bowel syndrome: a critique. Gastroenterology 1988;95:232-41.
4. Jailwala J, Imperiale TF, Kroenke K. Pharmacologic treatment of the irritable bowel syndrome: a systematic review of randomized, controlled trials. Ann Intern Med 2000;133:136-47.
5. Svedlund J, Sjodin I, Ottosson JO, et al. Controlled study of psychotherapy in irritable bowel syndrome. Lancet 1983;ii:589-92.
6. Guthrie EA, Creed FH, Dawson D, et al. A controlled trial of psychological treatment for the irritable bowel syndrome. Gastroenterology 1991;100:450-7.
7. Guthrie EA, Creed FH, Dawson D, et al. A randomized controlled trial of psychotherapy in patients with refractory irritable bowel syndrome. Br J Psychiatry 1993;163:315-21.
8. Blanchard EB, Schwartz SP. Adaptation of a multi-component treatment program for irritable bowel syndrome to a small group format. Biofeedback Self-Regulation 1987;12:63-9.
9. Blanchard EB, Scharff L, Payne A, et al. Prediction of outcome from cognitive-behavioral treatment of irritable bowel syndrome. Behav Res Ther 1992;30:647-50.
10. Greene B, Blanchard EB. Cognitive therapy for irritable bowel syndrome. J Consult Clin Psychol 1994;62(3):576-82.
11. Van Dulmen AM, Fennis JFM, Bleijenberg G. Cognitive-behavioral group therapy for irritable bowel syndrome: effects and long-term follow-up. Psychosom Med 1996;58:508-14.
12. Payne A, Blanchard EB. A controlled comparison of cognitive therapy and self-help support groups in the treatment of irritable bowel syndrome. J Consult Clin Psychol 1995;63(5):779-86.
13. Wise TN, Cooper JN, Ahmed S. The efficacy of group therapy for patients with irritable bowel syndrome. Psychosomatics 1982;23:465-9.
14. Toner BB, Segal ZV, Emmott S, et al. Cognitive-behavioral group therapy for patients with irritable bowel syndrome. Int J Group Psychotherapy 1998;48(2):215-43.
15. Whorwell PJ, Prior A, Faragher EB. Controlled trial of hypnotherapy in the treatment of severe refractory irritable-bowel syndrome. Lancet 1984;2(8414):1232-43.
16. Prior A, Colgan SM, Whorwell PJ. Changes in rectal sensitivity after hypnotherapy in patients with irritable bowel syndrome. Gut 1990;31:896-8.
17. Whorwell PJ, Houghton LA, Taylor EE, et al. Physiological effects of emotion: assessment via hypnosis. Lancet 1992;340:69-72.
18. Houghton LA, Heyman DJ, Whorwell PJ. Symptomatology, quality of life and economic features of irritable bowel syndrome: the effect of hypnotherapy. Aliment Pharmacol Ther 1996;10(1):91-5.
19. Galovski TE, Blanchard EB. The treatment of irritable bowel syndrome with hypnotherapy. Appl Psychophysiol Biofeedback 1998;23(4):219-32.
20. Vidakovic-Vukic M. Hypnotherapy in the treatment of irritable bowel syndrome: methods and results in Amsterdam. Scandinavian University Press 1999;34(suppl 230):49-51.
21. Bassotti G, Whitehead WE. Biofeedback as a treatment approach to gastrointestinal tract disorders. Am J Gastroenterol 1994;89(2):158-64.
22. Jackson JL, O’Malley PG, et al. Treatment of functional gastrointestinal disorders with antidepressant medications: a meta-analysis. Am J Med 2000;108:65-72.
23. Heymann-Mönnikes I, Arnold R, Florin I, et al. The combination of medical treatment plus multicomponent behavioral therapy is superior to medical treatment alone in the therapy of irritable bowel syndrome. Am J Gastroenterol 2000;95:981-94.
Hypertension: Pitfalls to prescribing for patients with high blood pressure
Roughly 50 million adult Americans have hypertension.1 Chances are some of them are—or soon will be—under your care.
Hypertension is common among patients with psychiatric disorders, particularly in those with chronic mental conditions.2 Medication-associated weight gain and other reactions to psychotropics, drug-drug interactions, lack of exercise, adverse dietary habits, and pre-existing medical conditions all predispose psychiatric patients to hypertension.
Yet hypertension often goes undetected in psychiatric patients. Hypertension many times is asymptomatic—about 50% of all people with the disorder don’t even know they have it.3 Some symptoms of uncontrolled hypertension—fatigue, headache, palpitations, and dizziness—are also associated with many psychiatric disorders. As a result, psychiatrists may attempt to manage the symptoms but miss the hypertension.
Psychiatrists need to be alert for hypertension, either as a possible contributing factor to a mental disorder or as a potential side effect of a psychiatric disorder or treatment. The following diagnostic and treatment strategies will help you detect and manage this common condition.
Causes of hypertension in mental illness
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure defines elevated blood pressure as 140 mm Hg systolic and/or 90 mm Hg diastolic. The diagnosis of hypertension should be based on the average of two or more blood pressure readings at each of two or more visits after initial screening.
All patients with elevated blood pressure have an underlying physiologic abnormality that is causing their hypertension. The disorder falls within the following two categories:
- essential hypertension, emanating from an unknown cause
- secondary hypertension, resulting from an underlying, discoverable, often treatable cause.
Researchers have speculated that certain psychiatric disorders might cause, or be risk factors for, hypertension. Anxiety or panic disorders have been associated with acute (and perhaps chronic) blood pressure elevations.2 Some research suggests that patients with alexithymia are at risk for developing hypertension.4
Other studies suggest that hypertensive patients with certain psychological disorders (e.g., depression) or social factors (e.g., substance abuse) are less likely than nonaffected patients to self-report the presence of hypertension and less likely to receive medical attention for it.5
Psychiatric drugs also may affect blood pressure by one of two mechanisms:
- Pharmacodynamic—direct effects at the site of action (e.g., receptors) via physiologic mechanisms (Table 1). For example, amphetamines act directly on the sympathetic nervous system to elevate blood pressure.
- Pharmacokinetic—indirect effects on blood pressure via drug/drug interactions that alter the absorption, distribution, metabolism, or clearance of antihypertensive medications. Thiazide diuretics, angiotensin-converting enzyme (ACE) inhibitors, and salt intake restrictions can raise lithium levels. The calcium-channel blockers verapamil and diltiazem can unpredictably increase or decrease lithium levels, but the combination generally is safe. Verapamil also raises tricyclic antidepressant levels. Monoamine oxidase inhibitors (MAOIs) used in tandem with the antihypertensive reserpine can cause hypomania. Beta-blocker levels are increased when used in concert with selective serotonin reuptake inhibitors. Use of carbamazepine with calcium-channel blockers can elevate carbamazapine levels and diminish the effectiveness of the calcium-channel blocker.
Table 1
POSSIBLE PHARMACODYNAMIC EFFECTS OFPSYCHIATRIC MEDICATIONS ON BLOOD PRESSURE
| Psychiatric medication | Effect on blood pressure |
|---|---|
| Amphetamines | ▲ |
| Benzodiazepines | Withdrawal may cause ▲ |
| Tricyclic antidepressants | ▲ or ▼ (postural hypotension or supine hypertension) |
| Methylphenidate | ▲ |
| Monoamine oxidase inhibitors | ▲ may precipitate an acute hypertensive crisis, especially with foods with high tyramine content (e.g., red wines, aged cheeses) |
| Lithium | ▼ via direct effect on renal concentrating ability |
| Venlafaxine | ▲ dose-related, <1% incidence |
| Antipsychotics (both typical and atypical) | ▼ |
Symptoms, complications of high blood pressure
Symptoms that may be associated with high blood pressure include headaches, dizziness, lightheadedness, fatigue, palpitations, and chest discomfort. Patients may also experience symptoms secondary to end-organ damage (e.g., shortness of breath from congestive heart failure).
Most people, however, experience no symptoms when their blood pressure is elevated. This is one reason most people with hypertension do not adequately control their blood pressure.
Aside from the long-term end-organ damage caused by persistently elevated blood pressure, hypertension also has been found to cause psychiatric disorders, though not directly. For example, post-MI depression is well-recognized. Hypertension may also cause multi-infarct dementia with resultant depression, paranoia, or other psychotic features.
The psychological burden of having chronic and usually incurable (though controllable) hypertension may worsen depression or anxiety disorders. Patients with a chronic psychiatric illness generally have a higher incidence of chronic medical problems.
Likewise, patients with chronic medical disorders have a higher incidence of psychiatric complaints.6
Patient evaluation
When evaluating the patient with elevated blood pressure, it is important to:
- detect and confirm hypertension
- detect target-organ disease (e.g., renal damage or congestive heart failure)
- identify other cardiovascular risk factors (e.g., diabetes mellitus, hyperlipidemia, obesity)
- identify secondary causes of hypertension, such as endocrine abnormalities (e.g., hyperaldosteronism, thyroid disorders), kidney disease, obstructive sleep apnea, and response to medications.
Table 2
ANTIHYPERTENSIVE MEDICATIONS AND SIDE EFFECTS
| Antihypertensive class | Agent(s) | Possible associated psychiatric symptoms |
|---|---|---|
| Beta-adrenergic blocking agents | Propranolol, atenolol, metoprolol, others | Fatigue, depression, psychosis, delirium, anxiety, sexual dysfunction, nightmares, hallucinations* |
| Angiotensin-converting enzyme (ACE) inhibitors | Captopril, enalapril, lisinopril, ramipril, others | Mania, anxiety, hallucinations |
| Angiotensin II receptor blockers (ARBs or AIIAs) | Losartan, valsartan, others | Probably same as ACE inhibitors |
| Diuretics | Hydrochlorothiazide, furosemide | Sexual dysfunction, depression |
| Calcium-channel blockers | Nifedipine, verapamil, diltiazem | Dizziness, headache, flushing, tachycardia, depression |
| Alpha-adrenergic blockers | Prazosin, terazosin, doxazosin | Syncope, dizziness and vertigo, palpitations, drowsiness, weakness, confusion |
| Central alpha-adrenergic agonists | Clonidine, methyldopa | Drowsiness, sedation, fatigue, depression, impotence, delirium, psychosis, nightmares, amnesia |
| Direct vasodilators | Hydralazine, minoxidil | Tachycardia, headache, dizziness |
| Peripheral adrenergic neuron antagonists | Reserpine, guanadrel | Drowsiness, depression, nightmares, tardive dyskinesia |
| *May occur with ophthalmic preparations | ||
A thorough history and physical examination should be performed to assess these four areas. Routine laboratory testing for the hypertensive patient should include a urinalysis, a complete blood count, an assessment of blood chemistries (potassium, sodium, creatinine, fasting glucose, fasting lipid profile), and a 12-lead electrocardiogram.
Treating hypertension
Many medications are used to treat hypertension. Most classes of antihypertensive agents have been shown to be about equally effective in lowering blood pressure.
All other factors being equal, the sixth report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC-VI) recommends initial treatment with a diuretic or beta-blocker. These classes of drugs have been shown to significantly reduce overall hypertension-related mortality.
Most patients with hypertension—particularly the elderly, patients with diabetes mellitus, and those with renal disease—will need two or more agents to control their blood pressure. Avoid prescribing agents that may worsen an existing condition (e.g., beta-blockers may worsen bronchospasm in patients with asthma). Use agents that may help improve comorbid conditions (e.g., beta-blockers have been shown to reduce mortality in patients with previous MI).
The Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC), which has issued six previous reports on hypertension control, is expected to issue updated recommendations within the next year. Angiotensin-converting enzyme inhibitors, calcium-channel blockers, and angiotensin II receptor antagonists may then be recommended as initial treatment options, along with the previously preferred classes of diuretics and beta-blockers.
A wealth of data has been obtained from multiple randomized, controlled studies since JNC released its most recent report in 1997. Turner et al used genetic analysis to identify individuals with essential hypertension who had a common genetic mutation that resulted in a renal absorption defect. Study participants with this mutation responded much better to diuretic therapy (which specifically targeted the underlying defect) than did those without the defect.8
In the future, determination of genetic polymorphism before prescribing medications may reduce side effects and increase efficacy in treating a variety of disorders, including hypertension.
Potential side effects, some of which mimic or are commonly found in psychiatric disorders, must be considered when choosing an antihypertensive agent. Table 2 lists nine classes of antihypertensives and some associated side effects. Also consider the agent’s cost, convenience of administration, direct-to-consumer advertising, and the patient’s age or race. For example, beta-blockers tend to be less effective in black or elderly patients than in other demographic groups.
Nonpharmacologic hypertension management emphasizes weight reduction, moderation of alcohol intake, regular aerobic exercise, dietary restriction of sodium, and smoking cessation. Most studies of these behavioral interventions have demonstrated a short-term benefit in decreasing blood pressure, but long-term adherence to them is disappointing. Relaxation therapies and biofeedback have been advocated for hypertension, but proof of their efficacy is lacking.7
As more is learned about genetic and other causes of hypertension, more-effective treatments for hypertension could become available (Box).
Treating high-risk groups
Special considerations apply to two patient groups with a high prevalence of hypertension—those age 65 and older and those with diabetes.
Older patients. Treatment benefits are more pronounced in patients age 65 or older because of their greater absolute risk for cardiovascular events. Also, systolic blood pressures increase with aging as the arterial tree becomes progressively less distensible.
Older patients often will require more than one drug to control their blood pressure. The initial dosages should be low and gradually titrated upward as needed. To minimize side effects, use smaller doses of multiple agents that are well tolerated instead of high-dose monotherapy.
A diuretic is often recommended as initial treatment for older patients, though a long-acting dihydropyridine calcium-channel blocker is a reasonable alternative. An ACE inhibitor is recommended for older patients with diabetes, systolic congestive heart failure, or previous MI. An alpha blocker should not be used as initial therapy for hypertension in the elderly because of relative lack of efficacy in preventing cardiovascular events.
Patients with diabetes. Aggressive blood pressure control is especially important in the patient with diabetes, which is the leading cause of end-stage renal disease. Most patients with diabetes also have hypertension—which accelerates their renal disease as well as cardiovascular disease. Blood pressure control goals significantly below 140/90 mm Hg are recommended (120 to 135 mm Hg systolic, 80 to 85 mm Hg diastolic) if diabetes is present.
ACE inhibitors or angiotensin receptor blockers are preferred for initial treatment of hypertension in diabetes, especially if proteinuria is present. Some authorities feel the level of blood pressure control in diabetes is more important than the agent(s) chosen to achieve that control. Most patients with hypertension and diabetes are not controlled on a single antihypertensive drug, and a diuretic is often added.
Psychological aspects of hypertension management
The diagnosis of hypertension and a resulting perception of loss of health or longevity may trigger a grief reaction in some patients.
Several psychological aspects to hypertension treatment make it difficult to achieve long-term control. Patients may become discouraged as dosages are increased and more medications are added. Asymptomatic patients may have no incentive to control their blood pressure. Many report, “I don’t feel any better” when their blood pressure comes down.
Because the goal of hypertension therapy is control rather than cure, the patient must commit to long-term treatment. Lifestyle changes such as dietary sodium restriction, smoking cessation, and weight loss may be difficult to achieve, especially for patients already dealing with a psychiatric disorder.
Also, the cost of treatment—the price of medications and initial and follow-up laboratory studies, plus the expense of follow-up office visits (possibly requiring time off work)—may be high.
Psychiatrists can help by offering moral support and encouraging patients to manage their medical problems, risk factors, and overall health. Psychiatrists can also educate patients on the importance of blood pressure control in preventing cardiovascular morbidity and mortality.
Brief cognitive-behaviorial therapy can identify the individual’s state of change (precontemplation, contemplation, preparation, action, or maintenance). Process techniques (such as consciousness-raising, commitment, or self-reevaluation) appropriate to the stage of change may then be employed.
For example, a patient in the precontemplation stage may resist returning to his or her primary care doctor to begin treatment for high blood pressure, employing such reasoning as, “I can’t afford those expensive office visits, and the medications would cost too much anyway.”
The psychiatrist might then apply consciousness-raising to motivate the patient: “How serious do you think it is to have high blood pressure that isn’t controlled? Are you aware that many people with high blood pressure are treated by means other than medications, or that many blood pressure medications are inexpensive?”
Providing relaxation techniques or a 12-week course of buproprion also can enhance the efficacy of smoking cessation efforts.
Related resources
- Drugs for hypertension. The Medical Letter 2001;43(1099):17-22.
- Some drugs that cause psychiatric symptoms. The Medical Letter 1998;40(1020):21-4.
- Hypertension—Journal of the American Heart Association. http://hyper.ahajournals.org/
- National Heart, Lung, and Blood institute’s Cardiovascular information site. http://www.nhlbi.nih.gov/health/public/heart/index.htm#hbp
Drug brand names
- Bupropion • Wellbutrin
- Guanadrel • Hylorel
- Lisinopril • Prinivil, Zestril
- Losartan • Hyzaar
- Ramipril • Altace
- Reserpine • Diutensen-R
- Valsartan • Diovan
- Venlafaxine • Effexor
- (Numerous other drugs mentioned in this article are available generically)
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kaplan NK. Hypertension in the population at large. In: NK Kaplan, ed. Clinical hypertension (7th ed). Baltimore: Williams & Wilkins, 1998.
2. Yates WR, et al. Cardiovascular risk factors and psychiatric illness. Medical Update for Psychiatrists 1998;3(6):196-201.
3. Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure (JNC VI). Arch Intern Med 1997;157:2413-46.
4. Todarello O, Taylor GJ, Parker JD, Fanelli M. Alexithymia in essential hypertensive and psychiatric outpatients: a compartative study. J Psychosom Res 1995;39(8):987-94.
5. Horwitz S, Prados-Torres A, et al. The influence of psychological and social factors on accuracy of self reported blood pressure. J Clin Epidemiol 1997;50(4):411-18.
6. Adamis D, Ball C. Physical morbidity in elderly psychiatric inpatients: prevalence and possible relations between the major mental disorders and physical illness. Int J Geriatr Psychiatry 2000;15:248-53.
7. Dubbert PM. Behavioral (life-style) modification in the prevention and treatment of hypertension. Clin Psychol Rev 1995;15(3):187-216.
8. Turner ST, et al. C825T polymorphism of the G protein beta(3)-subunit and antihypertensive response to a thiazide diuretic. Hypertension 2001;37(2 Part 2):739-43.
Roughly 50 million adult Americans have hypertension.1 Chances are some of them are—or soon will be—under your care.
Hypertension is common among patients with psychiatric disorders, particularly in those with chronic mental conditions.2 Medication-associated weight gain and other reactions to psychotropics, drug-drug interactions, lack of exercise, adverse dietary habits, and pre-existing medical conditions all predispose psychiatric patients to hypertension.
Yet hypertension often goes undetected in psychiatric patients. Hypertension many times is asymptomatic—about 50% of all people with the disorder don’t even know they have it.3 Some symptoms of uncontrolled hypertension—fatigue, headache, palpitations, and dizziness—are also associated with many psychiatric disorders. As a result, psychiatrists may attempt to manage the symptoms but miss the hypertension.
Psychiatrists need to be alert for hypertension, either as a possible contributing factor to a mental disorder or as a potential side effect of a psychiatric disorder or treatment. The following diagnostic and treatment strategies will help you detect and manage this common condition.
Causes of hypertension in mental illness
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure defines elevated blood pressure as 140 mm Hg systolic and/or 90 mm Hg diastolic. The diagnosis of hypertension should be based on the average of two or more blood pressure readings at each of two or more visits after initial screening.
All patients with elevated blood pressure have an underlying physiologic abnormality that is causing their hypertension. The disorder falls within the following two categories:
- essential hypertension, emanating from an unknown cause
- secondary hypertension, resulting from an underlying, discoverable, often treatable cause.
Researchers have speculated that certain psychiatric disorders might cause, or be risk factors for, hypertension. Anxiety or panic disorders have been associated with acute (and perhaps chronic) blood pressure elevations.2 Some research suggests that patients with alexithymia are at risk for developing hypertension.4
Other studies suggest that hypertensive patients with certain psychological disorders (e.g., depression) or social factors (e.g., substance abuse) are less likely than nonaffected patients to self-report the presence of hypertension and less likely to receive medical attention for it.5
Psychiatric drugs also may affect blood pressure by one of two mechanisms:
- Pharmacodynamic—direct effects at the site of action (e.g., receptors) via physiologic mechanisms (Table 1). For example, amphetamines act directly on the sympathetic nervous system to elevate blood pressure.
- Pharmacokinetic—indirect effects on blood pressure via drug/drug interactions that alter the absorption, distribution, metabolism, or clearance of antihypertensive medications. Thiazide diuretics, angiotensin-converting enzyme (ACE) inhibitors, and salt intake restrictions can raise lithium levels. The calcium-channel blockers verapamil and diltiazem can unpredictably increase or decrease lithium levels, but the combination generally is safe. Verapamil also raises tricyclic antidepressant levels. Monoamine oxidase inhibitors (MAOIs) used in tandem with the antihypertensive reserpine can cause hypomania. Beta-blocker levels are increased when used in concert with selective serotonin reuptake inhibitors. Use of carbamazepine with calcium-channel blockers can elevate carbamazapine levels and diminish the effectiveness of the calcium-channel blocker.
Table 1
POSSIBLE PHARMACODYNAMIC EFFECTS OFPSYCHIATRIC MEDICATIONS ON BLOOD PRESSURE
| Psychiatric medication | Effect on blood pressure |
|---|---|
| Amphetamines | ▲ |
| Benzodiazepines | Withdrawal may cause ▲ |
| Tricyclic antidepressants | ▲ or ▼ (postural hypotension or supine hypertension) |
| Methylphenidate | ▲ |
| Monoamine oxidase inhibitors | ▲ may precipitate an acute hypertensive crisis, especially with foods with high tyramine content (e.g., red wines, aged cheeses) |
| Lithium | ▼ via direct effect on renal concentrating ability |
| Venlafaxine | ▲ dose-related, <1% incidence |
| Antipsychotics (both typical and atypical) | ▼ |
Symptoms, complications of high blood pressure
Symptoms that may be associated with high blood pressure include headaches, dizziness, lightheadedness, fatigue, palpitations, and chest discomfort. Patients may also experience symptoms secondary to end-organ damage (e.g., shortness of breath from congestive heart failure).
Most people, however, experience no symptoms when their blood pressure is elevated. This is one reason most people with hypertension do not adequately control their blood pressure.
Aside from the long-term end-organ damage caused by persistently elevated blood pressure, hypertension also has been found to cause psychiatric disorders, though not directly. For example, post-MI depression is well-recognized. Hypertension may also cause multi-infarct dementia with resultant depression, paranoia, or other psychotic features.
The psychological burden of having chronic and usually incurable (though controllable) hypertension may worsen depression or anxiety disorders. Patients with a chronic psychiatric illness generally have a higher incidence of chronic medical problems.
Likewise, patients with chronic medical disorders have a higher incidence of psychiatric complaints.6
Patient evaluation
When evaluating the patient with elevated blood pressure, it is important to:
- detect and confirm hypertension
- detect target-organ disease (e.g., renal damage or congestive heart failure)
- identify other cardiovascular risk factors (e.g., diabetes mellitus, hyperlipidemia, obesity)
- identify secondary causes of hypertension, such as endocrine abnormalities (e.g., hyperaldosteronism, thyroid disorders), kidney disease, obstructive sleep apnea, and response to medications.
Table 2
ANTIHYPERTENSIVE MEDICATIONS AND SIDE EFFECTS
| Antihypertensive class | Agent(s) | Possible associated psychiatric symptoms |
|---|---|---|
| Beta-adrenergic blocking agents | Propranolol, atenolol, metoprolol, others | Fatigue, depression, psychosis, delirium, anxiety, sexual dysfunction, nightmares, hallucinations* |
| Angiotensin-converting enzyme (ACE) inhibitors | Captopril, enalapril, lisinopril, ramipril, others | Mania, anxiety, hallucinations |
| Angiotensin II receptor blockers (ARBs or AIIAs) | Losartan, valsartan, others | Probably same as ACE inhibitors |
| Diuretics | Hydrochlorothiazide, furosemide | Sexual dysfunction, depression |
| Calcium-channel blockers | Nifedipine, verapamil, diltiazem | Dizziness, headache, flushing, tachycardia, depression |
| Alpha-adrenergic blockers | Prazosin, terazosin, doxazosin | Syncope, dizziness and vertigo, palpitations, drowsiness, weakness, confusion |
| Central alpha-adrenergic agonists | Clonidine, methyldopa | Drowsiness, sedation, fatigue, depression, impotence, delirium, psychosis, nightmares, amnesia |
| Direct vasodilators | Hydralazine, minoxidil | Tachycardia, headache, dizziness |
| Peripheral adrenergic neuron antagonists | Reserpine, guanadrel | Drowsiness, depression, nightmares, tardive dyskinesia |
| *May occur with ophthalmic preparations | ||
A thorough history and physical examination should be performed to assess these four areas. Routine laboratory testing for the hypertensive patient should include a urinalysis, a complete blood count, an assessment of blood chemistries (potassium, sodium, creatinine, fasting glucose, fasting lipid profile), and a 12-lead electrocardiogram.
Treating hypertension
Many medications are used to treat hypertension. Most classes of antihypertensive agents have been shown to be about equally effective in lowering blood pressure.
All other factors being equal, the sixth report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC-VI) recommends initial treatment with a diuretic or beta-blocker. These classes of drugs have been shown to significantly reduce overall hypertension-related mortality.
Most patients with hypertension—particularly the elderly, patients with diabetes mellitus, and those with renal disease—will need two or more agents to control their blood pressure. Avoid prescribing agents that may worsen an existing condition (e.g., beta-blockers may worsen bronchospasm in patients with asthma). Use agents that may help improve comorbid conditions (e.g., beta-blockers have been shown to reduce mortality in patients with previous MI).
The Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC), which has issued six previous reports on hypertension control, is expected to issue updated recommendations within the next year. Angiotensin-converting enzyme inhibitors, calcium-channel blockers, and angiotensin II receptor antagonists may then be recommended as initial treatment options, along with the previously preferred classes of diuretics and beta-blockers.
A wealth of data has been obtained from multiple randomized, controlled studies since JNC released its most recent report in 1997. Turner et al used genetic analysis to identify individuals with essential hypertension who had a common genetic mutation that resulted in a renal absorption defect. Study participants with this mutation responded much better to diuretic therapy (which specifically targeted the underlying defect) than did those without the defect.8
In the future, determination of genetic polymorphism before prescribing medications may reduce side effects and increase efficacy in treating a variety of disorders, including hypertension.
Potential side effects, some of which mimic or are commonly found in psychiatric disorders, must be considered when choosing an antihypertensive agent. Table 2 lists nine classes of antihypertensives and some associated side effects. Also consider the agent’s cost, convenience of administration, direct-to-consumer advertising, and the patient’s age or race. For example, beta-blockers tend to be less effective in black or elderly patients than in other demographic groups.
Nonpharmacologic hypertension management emphasizes weight reduction, moderation of alcohol intake, regular aerobic exercise, dietary restriction of sodium, and smoking cessation. Most studies of these behavioral interventions have demonstrated a short-term benefit in decreasing blood pressure, but long-term adherence to them is disappointing. Relaxation therapies and biofeedback have been advocated for hypertension, but proof of their efficacy is lacking.7
As more is learned about genetic and other causes of hypertension, more-effective treatments for hypertension could become available (Box).
Treating high-risk groups
Special considerations apply to two patient groups with a high prevalence of hypertension—those age 65 and older and those with diabetes.
Older patients. Treatment benefits are more pronounced in patients age 65 or older because of their greater absolute risk for cardiovascular events. Also, systolic blood pressures increase with aging as the arterial tree becomes progressively less distensible.
Older patients often will require more than one drug to control their blood pressure. The initial dosages should be low and gradually titrated upward as needed. To minimize side effects, use smaller doses of multiple agents that are well tolerated instead of high-dose monotherapy.
A diuretic is often recommended as initial treatment for older patients, though a long-acting dihydropyridine calcium-channel blocker is a reasonable alternative. An ACE inhibitor is recommended for older patients with diabetes, systolic congestive heart failure, or previous MI. An alpha blocker should not be used as initial therapy for hypertension in the elderly because of relative lack of efficacy in preventing cardiovascular events.
Patients with diabetes. Aggressive blood pressure control is especially important in the patient with diabetes, which is the leading cause of end-stage renal disease. Most patients with diabetes also have hypertension—which accelerates their renal disease as well as cardiovascular disease. Blood pressure control goals significantly below 140/90 mm Hg are recommended (120 to 135 mm Hg systolic, 80 to 85 mm Hg diastolic) if diabetes is present.
ACE inhibitors or angiotensin receptor blockers are preferred for initial treatment of hypertension in diabetes, especially if proteinuria is present. Some authorities feel the level of blood pressure control in diabetes is more important than the agent(s) chosen to achieve that control. Most patients with hypertension and diabetes are not controlled on a single antihypertensive drug, and a diuretic is often added.
Psychological aspects of hypertension management
The diagnosis of hypertension and a resulting perception of loss of health or longevity may trigger a grief reaction in some patients.
Several psychological aspects to hypertension treatment make it difficult to achieve long-term control. Patients may become discouraged as dosages are increased and more medications are added. Asymptomatic patients may have no incentive to control their blood pressure. Many report, “I don’t feel any better” when their blood pressure comes down.
Because the goal of hypertension therapy is control rather than cure, the patient must commit to long-term treatment. Lifestyle changes such as dietary sodium restriction, smoking cessation, and weight loss may be difficult to achieve, especially for patients already dealing with a psychiatric disorder.
Also, the cost of treatment—the price of medications and initial and follow-up laboratory studies, plus the expense of follow-up office visits (possibly requiring time off work)—may be high.
Psychiatrists can help by offering moral support and encouraging patients to manage their medical problems, risk factors, and overall health. Psychiatrists can also educate patients on the importance of blood pressure control in preventing cardiovascular morbidity and mortality.
Brief cognitive-behaviorial therapy can identify the individual’s state of change (precontemplation, contemplation, preparation, action, or maintenance). Process techniques (such as consciousness-raising, commitment, or self-reevaluation) appropriate to the stage of change may then be employed.
For example, a patient in the precontemplation stage may resist returning to his or her primary care doctor to begin treatment for high blood pressure, employing such reasoning as, “I can’t afford those expensive office visits, and the medications would cost too much anyway.”
The psychiatrist might then apply consciousness-raising to motivate the patient: “How serious do you think it is to have high blood pressure that isn’t controlled? Are you aware that many people with high blood pressure are treated by means other than medications, or that many blood pressure medications are inexpensive?”
Providing relaxation techniques or a 12-week course of buproprion also can enhance the efficacy of smoking cessation efforts.
Related resources
- Drugs for hypertension. The Medical Letter 2001;43(1099):17-22.
- Some drugs that cause psychiatric symptoms. The Medical Letter 1998;40(1020):21-4.
- Hypertension—Journal of the American Heart Association. http://hyper.ahajournals.org/
- National Heart, Lung, and Blood institute’s Cardiovascular information site. http://www.nhlbi.nih.gov/health/public/heart/index.htm#hbp
Drug brand names
- Bupropion • Wellbutrin
- Guanadrel • Hylorel
- Lisinopril • Prinivil, Zestril
- Losartan • Hyzaar
- Ramipril • Altace
- Reserpine • Diutensen-R
- Valsartan • Diovan
- Venlafaxine • Effexor
- (Numerous other drugs mentioned in this article are available generically)
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Roughly 50 million adult Americans have hypertension.1 Chances are some of them are—or soon will be—under your care.
Hypertension is common among patients with psychiatric disorders, particularly in those with chronic mental conditions.2 Medication-associated weight gain and other reactions to psychotropics, drug-drug interactions, lack of exercise, adverse dietary habits, and pre-existing medical conditions all predispose psychiatric patients to hypertension.
Yet hypertension often goes undetected in psychiatric patients. Hypertension many times is asymptomatic—about 50% of all people with the disorder don’t even know they have it.3 Some symptoms of uncontrolled hypertension—fatigue, headache, palpitations, and dizziness—are also associated with many psychiatric disorders. As a result, psychiatrists may attempt to manage the symptoms but miss the hypertension.
Psychiatrists need to be alert for hypertension, either as a possible contributing factor to a mental disorder or as a potential side effect of a psychiatric disorder or treatment. The following diagnostic and treatment strategies will help you detect and manage this common condition.
Causes of hypertension in mental illness
The Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure defines elevated blood pressure as 140 mm Hg systolic and/or 90 mm Hg diastolic. The diagnosis of hypertension should be based on the average of two or more blood pressure readings at each of two or more visits after initial screening.
All patients with elevated blood pressure have an underlying physiologic abnormality that is causing their hypertension. The disorder falls within the following two categories:
- essential hypertension, emanating from an unknown cause
- secondary hypertension, resulting from an underlying, discoverable, often treatable cause.
Researchers have speculated that certain psychiatric disorders might cause, or be risk factors for, hypertension. Anxiety or panic disorders have been associated with acute (and perhaps chronic) blood pressure elevations.2 Some research suggests that patients with alexithymia are at risk for developing hypertension.4
Other studies suggest that hypertensive patients with certain psychological disorders (e.g., depression) or social factors (e.g., substance abuse) are less likely than nonaffected patients to self-report the presence of hypertension and less likely to receive medical attention for it.5
Psychiatric drugs also may affect blood pressure by one of two mechanisms:
- Pharmacodynamic—direct effects at the site of action (e.g., receptors) via physiologic mechanisms (Table 1). For example, amphetamines act directly on the sympathetic nervous system to elevate blood pressure.
- Pharmacokinetic—indirect effects on blood pressure via drug/drug interactions that alter the absorption, distribution, metabolism, or clearance of antihypertensive medications. Thiazide diuretics, angiotensin-converting enzyme (ACE) inhibitors, and salt intake restrictions can raise lithium levels. The calcium-channel blockers verapamil and diltiazem can unpredictably increase or decrease lithium levels, but the combination generally is safe. Verapamil also raises tricyclic antidepressant levels. Monoamine oxidase inhibitors (MAOIs) used in tandem with the antihypertensive reserpine can cause hypomania. Beta-blocker levels are increased when used in concert with selective serotonin reuptake inhibitors. Use of carbamazepine with calcium-channel blockers can elevate carbamazapine levels and diminish the effectiveness of the calcium-channel blocker.
Table 1
POSSIBLE PHARMACODYNAMIC EFFECTS OFPSYCHIATRIC MEDICATIONS ON BLOOD PRESSURE
| Psychiatric medication | Effect on blood pressure |
|---|---|
| Amphetamines | ▲ |
| Benzodiazepines | Withdrawal may cause ▲ |
| Tricyclic antidepressants | ▲ or ▼ (postural hypotension or supine hypertension) |
| Methylphenidate | ▲ |
| Monoamine oxidase inhibitors | ▲ may precipitate an acute hypertensive crisis, especially with foods with high tyramine content (e.g., red wines, aged cheeses) |
| Lithium | ▼ via direct effect on renal concentrating ability |
| Venlafaxine | ▲ dose-related, <1% incidence |
| Antipsychotics (both typical and atypical) | ▼ |
Symptoms, complications of high blood pressure
Symptoms that may be associated with high blood pressure include headaches, dizziness, lightheadedness, fatigue, palpitations, and chest discomfort. Patients may also experience symptoms secondary to end-organ damage (e.g., shortness of breath from congestive heart failure).
Most people, however, experience no symptoms when their blood pressure is elevated. This is one reason most people with hypertension do not adequately control their blood pressure.
Aside from the long-term end-organ damage caused by persistently elevated blood pressure, hypertension also has been found to cause psychiatric disorders, though not directly. For example, post-MI depression is well-recognized. Hypertension may also cause multi-infarct dementia with resultant depression, paranoia, or other psychotic features.
The psychological burden of having chronic and usually incurable (though controllable) hypertension may worsen depression or anxiety disorders. Patients with a chronic psychiatric illness generally have a higher incidence of chronic medical problems.
Likewise, patients with chronic medical disorders have a higher incidence of psychiatric complaints.6
Patient evaluation
When evaluating the patient with elevated blood pressure, it is important to:
- detect and confirm hypertension
- detect target-organ disease (e.g., renal damage or congestive heart failure)
- identify other cardiovascular risk factors (e.g., diabetes mellitus, hyperlipidemia, obesity)
- identify secondary causes of hypertension, such as endocrine abnormalities (e.g., hyperaldosteronism, thyroid disorders), kidney disease, obstructive sleep apnea, and response to medications.
Table 2
ANTIHYPERTENSIVE MEDICATIONS AND SIDE EFFECTS
| Antihypertensive class | Agent(s) | Possible associated psychiatric symptoms |
|---|---|---|
| Beta-adrenergic blocking agents | Propranolol, atenolol, metoprolol, others | Fatigue, depression, psychosis, delirium, anxiety, sexual dysfunction, nightmares, hallucinations* |
| Angiotensin-converting enzyme (ACE) inhibitors | Captopril, enalapril, lisinopril, ramipril, others | Mania, anxiety, hallucinations |
| Angiotensin II receptor blockers (ARBs or AIIAs) | Losartan, valsartan, others | Probably same as ACE inhibitors |
| Diuretics | Hydrochlorothiazide, furosemide | Sexual dysfunction, depression |
| Calcium-channel blockers | Nifedipine, verapamil, diltiazem | Dizziness, headache, flushing, tachycardia, depression |
| Alpha-adrenergic blockers | Prazosin, terazosin, doxazosin | Syncope, dizziness and vertigo, palpitations, drowsiness, weakness, confusion |
| Central alpha-adrenergic agonists | Clonidine, methyldopa | Drowsiness, sedation, fatigue, depression, impotence, delirium, psychosis, nightmares, amnesia |
| Direct vasodilators | Hydralazine, minoxidil | Tachycardia, headache, dizziness |
| Peripheral adrenergic neuron antagonists | Reserpine, guanadrel | Drowsiness, depression, nightmares, tardive dyskinesia |
| *May occur with ophthalmic preparations | ||
A thorough history and physical examination should be performed to assess these four areas. Routine laboratory testing for the hypertensive patient should include a urinalysis, a complete blood count, an assessment of blood chemistries (potassium, sodium, creatinine, fasting glucose, fasting lipid profile), and a 12-lead electrocardiogram.
Treating hypertension
Many medications are used to treat hypertension. Most classes of antihypertensive agents have been shown to be about equally effective in lowering blood pressure.
All other factors being equal, the sixth report of the Joint National Committee on Prevention, Detection, Evaluation and Treatment of High Blood Pressure (JNC-VI) recommends initial treatment with a diuretic or beta-blocker. These classes of drugs have been shown to significantly reduce overall hypertension-related mortality.
Most patients with hypertension—particularly the elderly, patients with diabetes mellitus, and those with renal disease—will need two or more agents to control their blood pressure. Avoid prescribing agents that may worsen an existing condition (e.g., beta-blockers may worsen bronchospasm in patients with asthma). Use agents that may help improve comorbid conditions (e.g., beta-blockers have been shown to reduce mortality in patients with previous MI).
The Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC), which has issued six previous reports on hypertension control, is expected to issue updated recommendations within the next year. Angiotensin-converting enzyme inhibitors, calcium-channel blockers, and angiotensin II receptor antagonists may then be recommended as initial treatment options, along with the previously preferred classes of diuretics and beta-blockers.
A wealth of data has been obtained from multiple randomized, controlled studies since JNC released its most recent report in 1997. Turner et al used genetic analysis to identify individuals with essential hypertension who had a common genetic mutation that resulted in a renal absorption defect. Study participants with this mutation responded much better to diuretic therapy (which specifically targeted the underlying defect) than did those without the defect.8
In the future, determination of genetic polymorphism before prescribing medications may reduce side effects and increase efficacy in treating a variety of disorders, including hypertension.
Potential side effects, some of which mimic or are commonly found in psychiatric disorders, must be considered when choosing an antihypertensive agent. Table 2 lists nine classes of antihypertensives and some associated side effects. Also consider the agent’s cost, convenience of administration, direct-to-consumer advertising, and the patient’s age or race. For example, beta-blockers tend to be less effective in black or elderly patients than in other demographic groups.
Nonpharmacologic hypertension management emphasizes weight reduction, moderation of alcohol intake, regular aerobic exercise, dietary restriction of sodium, and smoking cessation. Most studies of these behavioral interventions have demonstrated a short-term benefit in decreasing blood pressure, but long-term adherence to them is disappointing. Relaxation therapies and biofeedback have been advocated for hypertension, but proof of their efficacy is lacking.7
As more is learned about genetic and other causes of hypertension, more-effective treatments for hypertension could become available (Box).
Treating high-risk groups
Special considerations apply to two patient groups with a high prevalence of hypertension—those age 65 and older and those with diabetes.
Older patients. Treatment benefits are more pronounced in patients age 65 or older because of their greater absolute risk for cardiovascular events. Also, systolic blood pressures increase with aging as the arterial tree becomes progressively less distensible.
Older patients often will require more than one drug to control their blood pressure. The initial dosages should be low and gradually titrated upward as needed. To minimize side effects, use smaller doses of multiple agents that are well tolerated instead of high-dose monotherapy.
A diuretic is often recommended as initial treatment for older patients, though a long-acting dihydropyridine calcium-channel blocker is a reasonable alternative. An ACE inhibitor is recommended for older patients with diabetes, systolic congestive heart failure, or previous MI. An alpha blocker should not be used as initial therapy for hypertension in the elderly because of relative lack of efficacy in preventing cardiovascular events.
Patients with diabetes. Aggressive blood pressure control is especially important in the patient with diabetes, which is the leading cause of end-stage renal disease. Most patients with diabetes also have hypertension—which accelerates their renal disease as well as cardiovascular disease. Blood pressure control goals significantly below 140/90 mm Hg are recommended (120 to 135 mm Hg systolic, 80 to 85 mm Hg diastolic) if diabetes is present.
ACE inhibitors or angiotensin receptor blockers are preferred for initial treatment of hypertension in diabetes, especially if proteinuria is present. Some authorities feel the level of blood pressure control in diabetes is more important than the agent(s) chosen to achieve that control. Most patients with hypertension and diabetes are not controlled on a single antihypertensive drug, and a diuretic is often added.
Psychological aspects of hypertension management
The diagnosis of hypertension and a resulting perception of loss of health or longevity may trigger a grief reaction in some patients.
Several psychological aspects to hypertension treatment make it difficult to achieve long-term control. Patients may become discouraged as dosages are increased and more medications are added. Asymptomatic patients may have no incentive to control their blood pressure. Many report, “I don’t feel any better” when their blood pressure comes down.
Because the goal of hypertension therapy is control rather than cure, the patient must commit to long-term treatment. Lifestyle changes such as dietary sodium restriction, smoking cessation, and weight loss may be difficult to achieve, especially for patients already dealing with a psychiatric disorder.
Also, the cost of treatment—the price of medications and initial and follow-up laboratory studies, plus the expense of follow-up office visits (possibly requiring time off work)—may be high.
Psychiatrists can help by offering moral support and encouraging patients to manage their medical problems, risk factors, and overall health. Psychiatrists can also educate patients on the importance of blood pressure control in preventing cardiovascular morbidity and mortality.
Brief cognitive-behaviorial therapy can identify the individual’s state of change (precontemplation, contemplation, preparation, action, or maintenance). Process techniques (such as consciousness-raising, commitment, or self-reevaluation) appropriate to the stage of change may then be employed.
For example, a patient in the precontemplation stage may resist returning to his or her primary care doctor to begin treatment for high blood pressure, employing such reasoning as, “I can’t afford those expensive office visits, and the medications would cost too much anyway.”
The psychiatrist might then apply consciousness-raising to motivate the patient: “How serious do you think it is to have high blood pressure that isn’t controlled? Are you aware that many people with high blood pressure are treated by means other than medications, or that many blood pressure medications are inexpensive?”
Providing relaxation techniques or a 12-week course of buproprion also can enhance the efficacy of smoking cessation efforts.
Related resources
- Drugs for hypertension. The Medical Letter 2001;43(1099):17-22.
- Some drugs that cause psychiatric symptoms. The Medical Letter 1998;40(1020):21-4.
- Hypertension—Journal of the American Heart Association. http://hyper.ahajournals.org/
- National Heart, Lung, and Blood institute’s Cardiovascular information site. http://www.nhlbi.nih.gov/health/public/heart/index.htm#hbp
Drug brand names
- Bupropion • Wellbutrin
- Guanadrel • Hylorel
- Lisinopril • Prinivil, Zestril
- Losartan • Hyzaar
- Ramipril • Altace
- Reserpine • Diutensen-R
- Valsartan • Diovan
- Venlafaxine • Effexor
- (Numerous other drugs mentioned in this article are available generically)
Disclosure
The author reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. Kaplan NK. Hypertension in the population at large. In: NK Kaplan, ed. Clinical hypertension (7th ed). Baltimore: Williams & Wilkins, 1998.
2. Yates WR, et al. Cardiovascular risk factors and psychiatric illness. Medical Update for Psychiatrists 1998;3(6):196-201.
3. Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure (JNC VI). Arch Intern Med 1997;157:2413-46.
4. Todarello O, Taylor GJ, Parker JD, Fanelli M. Alexithymia in essential hypertensive and psychiatric outpatients: a compartative study. J Psychosom Res 1995;39(8):987-94.
5. Horwitz S, Prados-Torres A, et al. The influence of psychological and social factors on accuracy of self reported blood pressure. J Clin Epidemiol 1997;50(4):411-18.
6. Adamis D, Ball C. Physical morbidity in elderly psychiatric inpatients: prevalence and possible relations between the major mental disorders and physical illness. Int J Geriatr Psychiatry 2000;15:248-53.
7. Dubbert PM. Behavioral (life-style) modification in the prevention and treatment of hypertension. Clin Psychol Rev 1995;15(3):187-216.
8. Turner ST, et al. C825T polymorphism of the G protein beta(3)-subunit and antihypertensive response to a thiazide diuretic. Hypertension 2001;37(2 Part 2):739-43.
1. Kaplan NK. Hypertension in the population at large. In: NK Kaplan, ed. Clinical hypertension (7th ed). Baltimore: Williams & Wilkins, 1998.
2. Yates WR, et al. Cardiovascular risk factors and psychiatric illness. Medical Update for Psychiatrists 1998;3(6):196-201.
3. Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure. The sixth report of the Joint National Committee on Detection, Evaluation and Treatment of High Blood Pressure (JNC VI). Arch Intern Med 1997;157:2413-46.
4. Todarello O, Taylor GJ, Parker JD, Fanelli M. Alexithymia in essential hypertensive and psychiatric outpatients: a compartative study. J Psychosom Res 1995;39(8):987-94.
5. Horwitz S, Prados-Torres A, et al. The influence of psychological and social factors on accuracy of self reported blood pressure. J Clin Epidemiol 1997;50(4):411-18.
6. Adamis D, Ball C. Physical morbidity in elderly psychiatric inpatients: prevalence and possible relations between the major mental disorders and physical illness. Int J Geriatr Psychiatry 2000;15:248-53.
7. Dubbert PM. Behavioral (life-style) modification in the prevention and treatment of hypertension. Clin Psychol Rev 1995;15(3):187-216.
8. Turner ST, et al. C825T polymorphism of the G protein beta(3)-subunit and antihypertensive response to a thiazide diuretic. Hypertension 2001;37(2 Part 2):739-43.
EEGs and epilepsy: When seizures mimic psychiatric illness
Behaviors during seizures can mimic psychiatric disorders, and patients with epilepsy have higher-than-normal rates of many types of psychiatric illness. That’s why it is important for psychiatrists to be familiar with epilepsy and electroencephalography (EEG)—the key diagnostic tool for epileptic disorders.
As a neurologist who specializes in epilepsy treatment, I offer five case studies that highlight basic concepts about epilepsy and EEGs. My goal is to help psychiatrists answer common clinical questions such as:
- If a patient with bipolar illness has an abnormal EEG, should this guide the treatment choice?
- In a patient with episodes of fear, tachycardia, and other autonomic symptoms, how does one differentiate between panic attacks, complex partial seizures, and psychogenic nonepileptic seizures?
- When is EEG indicated in a patient with attention-deficit/hyperactivity disorder (ADHD)?
- Can complex partial status epilepticus present as a psychiatric disorder?
- In patients with epilepsy, why is it important to categorize psychiatric symptoms as ictal (occurring at the time of seizure), interictal (between seizures), or postictal (following seizures)?
How EEG is used today
EEG is used mainly to evaluate epilepsy and diffuse brain dysfunction (e.g., coma and confusional states). Modern brain imaging, including magnetic resonance imaging (MRI) and computerized tomography (CT), has replaced EEG for evaluating structural brain abnormalities.
Two basic EEG findings with which psychiatrists should be familiar are slowing and epileptiform activity:
Slowing is a nonspecific finding that indicates dysfunction of the underlying white matter, with or without gray matter involvement. Focal slowing indicates a focal area of cortical dysfunction usually caused by a focal structural lesion (tumor, stroke, trauma, etc.), although a lesion is not always found. Brain imaging, usually MRI, is indicated.
Epileptiform activity, which is seen as spikes or sharp waves, indicates potential for epileptic seizures (Box). EEG technologists may use activation procedures such as hyperventilation and photic stimulation to enhance the ability of EEG to detect epileptiform activity. Special electrodes (e.g., anterior temporal electrodes) may be used to improve recordings taken from the temporal lobe. In selected inpatients, epilepsy centers may use sphenoidal electrodes—wires inserted under the skin of the cheek to record temporal lobe activity.
Video/EEG monitoring has been used since the 1960s and is the gold standard in evaluating patients with seizures or episodes that resemble seizures. The technique involves simultaneously recording brain activity on an EEG and behavior on tape or digital video. Usually patients are admitted to a specialized hospital unit, medications are reduced or discontinued, and the seizures or other behaviors are recorded. Neurologists with special training in EEG and epilepsy evaluate the EEG for changes before, during, and after the behavioral event. Clinical characteristics of seizures and nonepileptic events detected on the video also help with the evaluation.
Video/EEG is most helpful in:
- determining whether the events are epileptic or nonepileptic
- determining—if epileptic—the precise seizure type for treatment decisions
- localizing the site of seizure onset in patients with medication-resistant epilepsy who may be candidates for epilepsy surgery
Normal variants occur frequently on EEG and may be misinterpreted because they resemble epileptiform activity. They include:
- benign epileptiform transients of sleep
- mu rhythm
- rhythmic midtemporal variant
- subclinical rhythmic epileptiform discharge in adults.
Experienced electroencephalographers can readily identify these normal variants, but some neurologists may misidentify or misinterpret these EEG findings, potentially leading to unnecessary treatment with antiepileptic drugs.
Hundreds of medications can alter an EEG, usually by increasing either slowing or beta activity. The most common change is excessive beta activity, which is seen in most patients taking benzodiazepines or barbiturates. Enhanced beta activity is an appropriate response of a normal brain to certain medications and does not indicate underlying brain pathology.
If a patient with psychiatric illness has seizure-like episodes, an abnormal EEG may help in diagnosis. A neurologist can help direct the patient evaluation. Whether or not interictal EEG abnormalities are present, video/EEG monitoring can often make the diagnosis by capturing the events.
Case 1: Does this patient have epilepsy?
For Mr. A, age 27, lithium has stabilized his bipolar I disorder for 2 years without significant adverse effects. An EEG ordered for unknown reasons by his primary care physician shows large amounts of beta activity and a single sharp wave from the right temporal region. Should you add an antiepileptic drug to his regimen?
In this patient, lithium probably caused the large amounts of beta activity. A rule of thumb says that if an EEG shows increased beta activity, a medication is almost certainly the cause. If an EEG finds increased generalized slowing, medication effect is one of many possible causes.
The single sharp wave from the right temporal lobe raises the possibility of increased susceptibility to seizures but is not diagnostic of epilepsy. There is no indication to change the patient’s treatment regimen if he is well controlled without adverse effects from his current medications and there is no clear history of clinical seizures. In short, treat the patient, not the EEG.
On an EEG, epileptiform activity is seen as spikes or sharp waves and indicates potential for epileptic seizures.
- Focal epileptiform activity is consistent with seizures of focal origin (e.g., simple partial, complex partial, or partial onset seizure with secondary generalization into tonic-clonic seizure).
- Generalized epileptiform activity is consistent with seizures of generalized onset (e.g., absence, myoclonic, or primary generalized tonic-clonic seizure).
Interictal spikes (between seizures) are consistent with, but not diagnostic of, seizures and epilepsy. An ictal discharge (rhythmic, persistent epileptiform activity) on an EEG accompanied by a clinical change in behavior is diagnostic of a seizure.
Focal epileptiform activity
Generalized epileptiform activity
Based on early studies, EEG results can show epileptiform activity in some normal brains.1 On the other hand, only about 50% of patients with epilepsy will show epileptiform activity on a single EEG. Thus, some EEG abnormalities in psychiatric patients may not be related to either epilepsy or the psychiatric disorder, and a normal EEG does not exclude the possibility of epileptic seizures.
Question the patient for a history of other factors that may predispose him or her to seizures, such as:
- family history of seizures
- previous traumatic brain injury
- structural brain abnormality.
In light of this EEG, brain MRI is indicated. In this case the patient could be counseled to avoid seizure triggers (e.g., sleep deprivation). Because a long list of medications can lower the seizure threshold, the clinician must weigh the risks and benefits of using any medication in patients with increased susceptibility for seizures.
Case 2: Seizures or panic attacks?
Mr. B, age 31, is referred by his primary care physician for “spells” that began several years ago and recently increased in frequency to three to four times per week. The episodes start with a feeling of fear, “butterflies” in his stomach, and hyperventilation. These feelings intensify within minutes; each episode lasts 10 to 30 minutes.
The patient is usually aware of his surroundings during the episodes but twice has lost consciousness for less than 1 minute. There is no evidence of incontinence or tongue biting. Four years ago, the patient was involved in a motor vehicle accident and lost consciousness for about 30 minutes.
An EEG shows intermittent slowing from the left temporal region, and brain MRI is normal. Treatment with a benzodiazepine shortens the attacks. Where do you proceed from here?
Mr. B presents a diagnostic dilemma. Characteristics of his episodes may be seen in panic attacks, temporal lobe complex partial seizures, and psychogenic nonepileptic seizures, which is the preferred term for pseudoseizures (Table).2
One option would be to see how he responds to an agent that would treat seizures but not panic attacks (e.g., carbamazepine) or one that would treat panic attacks but not seizures (e.g., a selective serotonin reuptake inhibitor). Because his episodes are frequent, however, a more appropriate option would be to admit him for video/EEG monitoring, which can distinguish among these possible diagnoses with almost 100% accuracy.
Case 3: Attention disorder or epilepsy?
Miss C, age 9, is referred for possible ADHD. Her teachers notice she has difficulty following lessons in class but is intelligent and usually motivated. Her grades have been dropping. Her family reports she has episodes during which she stares and is unable to answer questions for 3 to 5 seconds, but she exhibits no other seizure-like manifestations. A trial of methylphenidate has not improved the symptoms. What should you try next?
This patient presents with symptoms that could be consistent with absence seizures. EEG would be diagnostic if it showed generalized spike and wave. Absence seizures (sometimes called petit mal) usually present in childhood and are characterized by recurrent brief staring spells with no postictal confusion or other clinical manifestations.
Patients with childhood absence epilepsy are usually of normal intelligence and only rarely have other associated seizure types (e.g., myoclonus or tonic-clonic). Seizures of approximately 3 to 5 seconds may occur up to 100 times a day and thus may be mistaken for attention problems.
EEG shows characteristic generalized three-per-second spike and wave, which is often precipitated by hyperventilation or photic stimulation. Ethosuximide or valproate can completely control seizures in most cases.
Case 4: Emergency use of EEG
Ms. D, age 21, is brought to the emergency department by her mother with symptoms of confusion. Ms. D has a long history of temporal lobe complex partial seizures, and her mother thinks she may have missed some doses of her antiepileptic drugs (carbamazepine and valproate). Yesterday Ms. D had two complex partial seizures but returned to baseline. Today she had three complex partial seizures within 2 hours and has not returned to baseline.
When you interview Ms. D she is alert but only intermittently oriented to date and location. She makes a variety of paraphasic speech errors. She is talking about heaven and angels and several times asks if you are the devil. Evaluation by the emergency physician discloses no toxic, metabolic, or infectious cause for her symptoms. Other than mental status, her neurologic examination is normal and shows no evidence of motor seizure activity. What would you suggest next?
This patient presents with complex partial seizures occurring in sequence without return to normal between seizures, followed by a continuous state of altered consciousness. This cluster of symptoms and signs is consistent with complex partial status epilepticus.
Emergency EEG is diagnostic. If the EEG is positive for status epilepticus, recommended treatment is IV benzodiazepines along with other IV antiepileptic drugs.
Complex partial status epilepticus can have a variety of triggers, including drug overdose, hyperthyroidism, brain tumor, or carcinomatous meningitis.3 In this case, the most likely trigger is medication noncompliance.
Complex partial or other forms of nonconvulsive status epilepticus occur in up to 37% of hospitalized patients with altered consciousness of uncertain etiology.3 EEG is required to confirm the diagnosis, but many hospitals do not have EEG technologists on call nights and weekends.
Table
THE FEARFUL PATIENT: PANIC ATTACK, SEIZURE DISORDER, OR PSYCHOGENIC?
| Panic attacks |
|
| Temporal lobe complex partial seizures |
|
| Psychogenic nonepileptic seizures |
|
In my experience, a patient who later was referred to me for seizure control was once admitted to a psychiatry ward for 4 days because of unidentified complex partial status epilepticus. When someone finally restarted her carbamazepine, she returned to normal in a few days. Six months after she became my patient, she presented with identical symptoms. An EEG within hours of symptom onset showed continuous EEG ictal activity from the left temporal lobe. When we administered IV lorazepam, her EEG normalized within minutes and her confusion and delusional symptoms resolved in 20 minutes.
Case 5: Seizure clusters followed by agitation
Mr. E, 32, has had complex partial and tonic-clonic seizures since age 14. He presents with chronic depressive symptoms and independent episodes of agitated behavior with psychotic features lasting hours after seizure clusters. His seizures have continued despite trials of different antiepileptic drugs, and he currently is receiving phenytoin and valproate. He is referred to you to diagnose and treat the psychiatric symptoms.
He scores a 28 on the Beck Depression Inventory, which indicates moderate depression,4 but he is not suicidal. He reports that although his depression symptoms are constant, his psychotic features occur only after a series of closely-spaced seizures. How do you approach this problem?
Based on the history, this patient has both mild interictal depression and postictal psychosis. In patients with epilepsy, psychiatric symptoms are categorized as:
- ictal (occur only during a seizure)
- interictal (may wax and wane but are present chronically, usually with no relation to seizure occurrence)
- or postictal (appear within 7 days after a lucid interval following a seizure or—more often—a series of seizures).5
Interictal psychiatric disorders include depression, bipolar disorder, and psychotic disorders. If the psychiatric disorder is truly interictal and has no clear relation to seizure occurrence, it should be treated like any other psychiatric illness with appropriate medications. One should not automatically add another antiepileptic drug (AED), because AEDs as a class have more adverse effects and more drug interactions than commonly used antidepressants.
Begin by examining the AEDs the patient is receiving. For example, phenobarbital and, to a lesser extent, phenytoin are associated with depression and should be used in patients with depression only when other AEDs have failed.
Ictal and postictal psychiatric symptoms should be treated acutely. Postictal symptoms may include psychosis, depression, mania, or anxiety. A short course of benzodiazepines is often helpful; the use of neuroleptics is dictated by the intensity and quality of the postictal symptoms.
Ictal depression-like symptoms can be seen with temporal lobe complex partial seizures. Many patients with these seizures have simple partial seizures (auras) that manifest as brief (usually less than 1 minute) intense feelings of fear or impending doom, or of “the life drained out” of them. These symptoms may arise from seizures involving mesial temporal regions. They do not usually require treatment besides AEDs.
The idea that patients with temporal lobe epilepsy may have a particular personality type is controversial. Retrospective data first suggested that certain traits (e.g., altered sexual behavior, anger, emotionality and obsessionalism, hypergraphia, and hyper-religiosity) could be found consistently in patients with temporal lobe epilepsy.6 This personality inventory was sometimes used to diagnose epilepsy. Subsequent studies have not validated the original data, although the cluster of personality traits can certainly occur in some patients.7-9
Related resources
- Epilepsy Foundation. www.epilepsyfoundation.org
- Engel J, Pedley TA (eds). Epilepsy: A comprehensive textbook. Philadelphia: Lippincott-Raven, 1998.
Drug brand names
- Carbamazepine • Tegretol
- Ethosuximide • Zarontin
- Lorazepam • Ativan
- Phenytoin • Dilantin
- Valproate • Depakote
Disclosure
The author reports that he receives research/grant support from, is a consultant to, and is on the speakers’ bureau of GlaxoSmithKline, UCBPharma, and Ortho-McNeil Pharmaceuticals; receives research/grant support from and is a consultant to Schwarz Pharma; is a consultant to Pfizer Inc., Elan Pharmaceuticals, and Shire Pharmaceuticals; and is on the speakers’ bureau of Abbott Laboratories, Cyberonics, and Shire Pharmaceuticals.
1. Zivin L, Ajmone-Marson C. Incidence and prognostic significance of epileptiform activity in the EEG of nonepileptic subjects. Brain 1969;91:751-78.
2. Arnold LM, Privitera MD. Psychopathology and trauma in epileptic and psychogenic seizures. Psychosomatics 1996;37(5):438-43.
3. Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res 1994;18(2):155-66.
4. Lambert MV, Robertson MM. Depression in epilepsy: etiology, phenomenology, and treatment. Epilepsia 1999;40(suppl 10):S21-47.
5. Logsdail SJ, Toone BK. Post-ictal psychoses: a clinical and phenomenological description. Br J Psychiatry 1988;152:246-52.
6. Bear DM, Fedio P. Quantitative analysis of interictal behavior in temporal lobe epilepsy. Arch Neurol 1977;34:454-67.
7. Devinsky O, Najjar S. Evidence against the existence of a temporal lobe epilepsy personality. Neurology 1999;53(5, suppl 2):S13-25.
8. Blumer D. Evidence supporting the temporal lobe epilepsy personality. Neurology 1999;53 (5, suppl 2):S9-12.
9. Mungas D. Interictal behavior abnormality in temporal lobe epilepsy. A specific syndrome or nonspecific psychopathology? Arch Gen Psychiatry 1982;39(1):108-11.
Behaviors during seizures can mimic psychiatric disorders, and patients with epilepsy have higher-than-normal rates of many types of psychiatric illness. That’s why it is important for psychiatrists to be familiar with epilepsy and electroencephalography (EEG)—the key diagnostic tool for epileptic disorders.
As a neurologist who specializes in epilepsy treatment, I offer five case studies that highlight basic concepts about epilepsy and EEGs. My goal is to help psychiatrists answer common clinical questions such as:
- If a patient with bipolar illness has an abnormal EEG, should this guide the treatment choice?
- In a patient with episodes of fear, tachycardia, and other autonomic symptoms, how does one differentiate between panic attacks, complex partial seizures, and psychogenic nonepileptic seizures?
- When is EEG indicated in a patient with attention-deficit/hyperactivity disorder (ADHD)?
- Can complex partial status epilepticus present as a psychiatric disorder?
- In patients with epilepsy, why is it important to categorize psychiatric symptoms as ictal (occurring at the time of seizure), interictal (between seizures), or postictal (following seizures)?
How EEG is used today
EEG is used mainly to evaluate epilepsy and diffuse brain dysfunction (e.g., coma and confusional states). Modern brain imaging, including magnetic resonance imaging (MRI) and computerized tomography (CT), has replaced EEG for evaluating structural brain abnormalities.
Two basic EEG findings with which psychiatrists should be familiar are slowing and epileptiform activity:
Slowing is a nonspecific finding that indicates dysfunction of the underlying white matter, with or without gray matter involvement. Focal slowing indicates a focal area of cortical dysfunction usually caused by a focal structural lesion (tumor, stroke, trauma, etc.), although a lesion is not always found. Brain imaging, usually MRI, is indicated.
Epileptiform activity, which is seen as spikes or sharp waves, indicates potential for epileptic seizures (Box). EEG technologists may use activation procedures such as hyperventilation and photic stimulation to enhance the ability of EEG to detect epileptiform activity. Special electrodes (e.g., anterior temporal electrodes) may be used to improve recordings taken from the temporal lobe. In selected inpatients, epilepsy centers may use sphenoidal electrodes—wires inserted under the skin of the cheek to record temporal lobe activity.
Video/EEG monitoring has been used since the 1960s and is the gold standard in evaluating patients with seizures or episodes that resemble seizures. The technique involves simultaneously recording brain activity on an EEG and behavior on tape or digital video. Usually patients are admitted to a specialized hospital unit, medications are reduced or discontinued, and the seizures or other behaviors are recorded. Neurologists with special training in EEG and epilepsy evaluate the EEG for changes before, during, and after the behavioral event. Clinical characteristics of seizures and nonepileptic events detected on the video also help with the evaluation.
Video/EEG is most helpful in:
- determining whether the events are epileptic or nonepileptic
- determining—if epileptic—the precise seizure type for treatment decisions
- localizing the site of seizure onset in patients with medication-resistant epilepsy who may be candidates for epilepsy surgery
Normal variants occur frequently on EEG and may be misinterpreted because they resemble epileptiform activity. They include:
- benign epileptiform transients of sleep
- mu rhythm
- rhythmic midtemporal variant
- subclinical rhythmic epileptiform discharge in adults.
Experienced electroencephalographers can readily identify these normal variants, but some neurologists may misidentify or misinterpret these EEG findings, potentially leading to unnecessary treatment with antiepileptic drugs.
Hundreds of medications can alter an EEG, usually by increasing either slowing or beta activity. The most common change is excessive beta activity, which is seen in most patients taking benzodiazepines or barbiturates. Enhanced beta activity is an appropriate response of a normal brain to certain medications and does not indicate underlying brain pathology.
If a patient with psychiatric illness has seizure-like episodes, an abnormal EEG may help in diagnosis. A neurologist can help direct the patient evaluation. Whether or not interictal EEG abnormalities are present, video/EEG monitoring can often make the diagnosis by capturing the events.
Case 1: Does this patient have epilepsy?
For Mr. A, age 27, lithium has stabilized his bipolar I disorder for 2 years without significant adverse effects. An EEG ordered for unknown reasons by his primary care physician shows large amounts of beta activity and a single sharp wave from the right temporal region. Should you add an antiepileptic drug to his regimen?
In this patient, lithium probably caused the large amounts of beta activity. A rule of thumb says that if an EEG shows increased beta activity, a medication is almost certainly the cause. If an EEG finds increased generalized slowing, medication effect is one of many possible causes.
The single sharp wave from the right temporal lobe raises the possibility of increased susceptibility to seizures but is not diagnostic of epilepsy. There is no indication to change the patient’s treatment regimen if he is well controlled without adverse effects from his current medications and there is no clear history of clinical seizures. In short, treat the patient, not the EEG.
On an EEG, epileptiform activity is seen as spikes or sharp waves and indicates potential for epileptic seizures.
- Focal epileptiform activity is consistent with seizures of focal origin (e.g., simple partial, complex partial, or partial onset seizure with secondary generalization into tonic-clonic seizure).
- Generalized epileptiform activity is consistent with seizures of generalized onset (e.g., absence, myoclonic, or primary generalized tonic-clonic seizure).
Interictal spikes (between seizures) are consistent with, but not diagnostic of, seizures and epilepsy. An ictal discharge (rhythmic, persistent epileptiform activity) on an EEG accompanied by a clinical change in behavior is diagnostic of a seizure.
Focal epileptiform activity
Generalized epileptiform activity
Based on early studies, EEG results can show epileptiform activity in some normal brains.1 On the other hand, only about 50% of patients with epilepsy will show epileptiform activity on a single EEG. Thus, some EEG abnormalities in psychiatric patients may not be related to either epilepsy or the psychiatric disorder, and a normal EEG does not exclude the possibility of epileptic seizures.
Question the patient for a history of other factors that may predispose him or her to seizures, such as:
- family history of seizures
- previous traumatic brain injury
- structural brain abnormality.
In light of this EEG, brain MRI is indicated. In this case the patient could be counseled to avoid seizure triggers (e.g., sleep deprivation). Because a long list of medications can lower the seizure threshold, the clinician must weigh the risks and benefits of using any medication in patients with increased susceptibility for seizures.
Case 2: Seizures or panic attacks?
Mr. B, age 31, is referred by his primary care physician for “spells” that began several years ago and recently increased in frequency to three to four times per week. The episodes start with a feeling of fear, “butterflies” in his stomach, and hyperventilation. These feelings intensify within minutes; each episode lasts 10 to 30 minutes.
The patient is usually aware of his surroundings during the episodes but twice has lost consciousness for less than 1 minute. There is no evidence of incontinence or tongue biting. Four years ago, the patient was involved in a motor vehicle accident and lost consciousness for about 30 minutes.
An EEG shows intermittent slowing from the left temporal region, and brain MRI is normal. Treatment with a benzodiazepine shortens the attacks. Where do you proceed from here?
Mr. B presents a diagnostic dilemma. Characteristics of his episodes may be seen in panic attacks, temporal lobe complex partial seizures, and psychogenic nonepileptic seizures, which is the preferred term for pseudoseizures (Table).2
One option would be to see how he responds to an agent that would treat seizures but not panic attacks (e.g., carbamazepine) or one that would treat panic attacks but not seizures (e.g., a selective serotonin reuptake inhibitor). Because his episodes are frequent, however, a more appropriate option would be to admit him for video/EEG monitoring, which can distinguish among these possible diagnoses with almost 100% accuracy.
Case 3: Attention disorder or epilepsy?
Miss C, age 9, is referred for possible ADHD. Her teachers notice she has difficulty following lessons in class but is intelligent and usually motivated. Her grades have been dropping. Her family reports she has episodes during which she stares and is unable to answer questions for 3 to 5 seconds, but she exhibits no other seizure-like manifestations. A trial of methylphenidate has not improved the symptoms. What should you try next?
This patient presents with symptoms that could be consistent with absence seizures. EEG would be diagnostic if it showed generalized spike and wave. Absence seizures (sometimes called petit mal) usually present in childhood and are characterized by recurrent brief staring spells with no postictal confusion or other clinical manifestations.
Patients with childhood absence epilepsy are usually of normal intelligence and only rarely have other associated seizure types (e.g., myoclonus or tonic-clonic). Seizures of approximately 3 to 5 seconds may occur up to 100 times a day and thus may be mistaken for attention problems.
EEG shows characteristic generalized three-per-second spike and wave, which is often precipitated by hyperventilation or photic stimulation. Ethosuximide or valproate can completely control seizures in most cases.
Case 4: Emergency use of EEG
Ms. D, age 21, is brought to the emergency department by her mother with symptoms of confusion. Ms. D has a long history of temporal lobe complex partial seizures, and her mother thinks she may have missed some doses of her antiepileptic drugs (carbamazepine and valproate). Yesterday Ms. D had two complex partial seizures but returned to baseline. Today she had three complex partial seizures within 2 hours and has not returned to baseline.
When you interview Ms. D she is alert but only intermittently oriented to date and location. She makes a variety of paraphasic speech errors. She is talking about heaven and angels and several times asks if you are the devil. Evaluation by the emergency physician discloses no toxic, metabolic, or infectious cause for her symptoms. Other than mental status, her neurologic examination is normal and shows no evidence of motor seizure activity. What would you suggest next?
This patient presents with complex partial seizures occurring in sequence without return to normal between seizures, followed by a continuous state of altered consciousness. This cluster of symptoms and signs is consistent with complex partial status epilepticus.
Emergency EEG is diagnostic. If the EEG is positive for status epilepticus, recommended treatment is IV benzodiazepines along with other IV antiepileptic drugs.
Complex partial status epilepticus can have a variety of triggers, including drug overdose, hyperthyroidism, brain tumor, or carcinomatous meningitis.3 In this case, the most likely trigger is medication noncompliance.
Complex partial or other forms of nonconvulsive status epilepticus occur in up to 37% of hospitalized patients with altered consciousness of uncertain etiology.3 EEG is required to confirm the diagnosis, but many hospitals do not have EEG technologists on call nights and weekends.
Table
THE FEARFUL PATIENT: PANIC ATTACK, SEIZURE DISORDER, OR PSYCHOGENIC?
| Panic attacks |
|
| Temporal lobe complex partial seizures |
|
| Psychogenic nonepileptic seizures |
|
In my experience, a patient who later was referred to me for seizure control was once admitted to a psychiatry ward for 4 days because of unidentified complex partial status epilepticus. When someone finally restarted her carbamazepine, she returned to normal in a few days. Six months after she became my patient, she presented with identical symptoms. An EEG within hours of symptom onset showed continuous EEG ictal activity from the left temporal lobe. When we administered IV lorazepam, her EEG normalized within minutes and her confusion and delusional symptoms resolved in 20 minutes.
Case 5: Seizure clusters followed by agitation
Mr. E, 32, has had complex partial and tonic-clonic seizures since age 14. He presents with chronic depressive symptoms and independent episodes of agitated behavior with psychotic features lasting hours after seizure clusters. His seizures have continued despite trials of different antiepileptic drugs, and he currently is receiving phenytoin and valproate. He is referred to you to diagnose and treat the psychiatric symptoms.
He scores a 28 on the Beck Depression Inventory, which indicates moderate depression,4 but he is not suicidal. He reports that although his depression symptoms are constant, his psychotic features occur only after a series of closely-spaced seizures. How do you approach this problem?
Based on the history, this patient has both mild interictal depression and postictal psychosis. In patients with epilepsy, psychiatric symptoms are categorized as:
- ictal (occur only during a seizure)
- interictal (may wax and wane but are present chronically, usually with no relation to seizure occurrence)
- or postictal (appear within 7 days after a lucid interval following a seizure or—more often—a series of seizures).5
Interictal psychiatric disorders include depression, bipolar disorder, and psychotic disorders. If the psychiatric disorder is truly interictal and has no clear relation to seizure occurrence, it should be treated like any other psychiatric illness with appropriate medications. One should not automatically add another antiepileptic drug (AED), because AEDs as a class have more adverse effects and more drug interactions than commonly used antidepressants.
Begin by examining the AEDs the patient is receiving. For example, phenobarbital and, to a lesser extent, phenytoin are associated with depression and should be used in patients with depression only when other AEDs have failed.
Ictal and postictal psychiatric symptoms should be treated acutely. Postictal symptoms may include psychosis, depression, mania, or anxiety. A short course of benzodiazepines is often helpful; the use of neuroleptics is dictated by the intensity and quality of the postictal symptoms.
Ictal depression-like symptoms can be seen with temporal lobe complex partial seizures. Many patients with these seizures have simple partial seizures (auras) that manifest as brief (usually less than 1 minute) intense feelings of fear or impending doom, or of “the life drained out” of them. These symptoms may arise from seizures involving mesial temporal regions. They do not usually require treatment besides AEDs.
The idea that patients with temporal lobe epilepsy may have a particular personality type is controversial. Retrospective data first suggested that certain traits (e.g., altered sexual behavior, anger, emotionality and obsessionalism, hypergraphia, and hyper-religiosity) could be found consistently in patients with temporal lobe epilepsy.6 This personality inventory was sometimes used to diagnose epilepsy. Subsequent studies have not validated the original data, although the cluster of personality traits can certainly occur in some patients.7-9
Related resources
- Epilepsy Foundation. www.epilepsyfoundation.org
- Engel J, Pedley TA (eds). Epilepsy: A comprehensive textbook. Philadelphia: Lippincott-Raven, 1998.
Drug brand names
- Carbamazepine • Tegretol
- Ethosuximide • Zarontin
- Lorazepam • Ativan
- Phenytoin • Dilantin
- Valproate • Depakote
Disclosure
The author reports that he receives research/grant support from, is a consultant to, and is on the speakers’ bureau of GlaxoSmithKline, UCBPharma, and Ortho-McNeil Pharmaceuticals; receives research/grant support from and is a consultant to Schwarz Pharma; is a consultant to Pfizer Inc., Elan Pharmaceuticals, and Shire Pharmaceuticals; and is on the speakers’ bureau of Abbott Laboratories, Cyberonics, and Shire Pharmaceuticals.
Behaviors during seizures can mimic psychiatric disorders, and patients with epilepsy have higher-than-normal rates of many types of psychiatric illness. That’s why it is important for psychiatrists to be familiar with epilepsy and electroencephalography (EEG)—the key diagnostic tool for epileptic disorders.
As a neurologist who specializes in epilepsy treatment, I offer five case studies that highlight basic concepts about epilepsy and EEGs. My goal is to help psychiatrists answer common clinical questions such as:
- If a patient with bipolar illness has an abnormal EEG, should this guide the treatment choice?
- In a patient with episodes of fear, tachycardia, and other autonomic symptoms, how does one differentiate between panic attacks, complex partial seizures, and psychogenic nonepileptic seizures?
- When is EEG indicated in a patient with attention-deficit/hyperactivity disorder (ADHD)?
- Can complex partial status epilepticus present as a psychiatric disorder?
- In patients with epilepsy, why is it important to categorize psychiatric symptoms as ictal (occurring at the time of seizure), interictal (between seizures), or postictal (following seizures)?
How EEG is used today
EEG is used mainly to evaluate epilepsy and diffuse brain dysfunction (e.g., coma and confusional states). Modern brain imaging, including magnetic resonance imaging (MRI) and computerized tomography (CT), has replaced EEG for evaluating structural brain abnormalities.
Two basic EEG findings with which psychiatrists should be familiar are slowing and epileptiform activity:
Slowing is a nonspecific finding that indicates dysfunction of the underlying white matter, with or without gray matter involvement. Focal slowing indicates a focal area of cortical dysfunction usually caused by a focal structural lesion (tumor, stroke, trauma, etc.), although a lesion is not always found. Brain imaging, usually MRI, is indicated.
Epileptiform activity, which is seen as spikes or sharp waves, indicates potential for epileptic seizures (Box). EEG technologists may use activation procedures such as hyperventilation and photic stimulation to enhance the ability of EEG to detect epileptiform activity. Special electrodes (e.g., anterior temporal electrodes) may be used to improve recordings taken from the temporal lobe. In selected inpatients, epilepsy centers may use sphenoidal electrodes—wires inserted under the skin of the cheek to record temporal lobe activity.
Video/EEG monitoring has been used since the 1960s and is the gold standard in evaluating patients with seizures or episodes that resemble seizures. The technique involves simultaneously recording brain activity on an EEG and behavior on tape or digital video. Usually patients are admitted to a specialized hospital unit, medications are reduced or discontinued, and the seizures or other behaviors are recorded. Neurologists with special training in EEG and epilepsy evaluate the EEG for changes before, during, and after the behavioral event. Clinical characteristics of seizures and nonepileptic events detected on the video also help with the evaluation.
Video/EEG is most helpful in:
- determining whether the events are epileptic or nonepileptic
- determining—if epileptic—the precise seizure type for treatment decisions
- localizing the site of seizure onset in patients with medication-resistant epilepsy who may be candidates for epilepsy surgery
Normal variants occur frequently on EEG and may be misinterpreted because they resemble epileptiform activity. They include:
- benign epileptiform transients of sleep
- mu rhythm
- rhythmic midtemporal variant
- subclinical rhythmic epileptiform discharge in adults.
Experienced electroencephalographers can readily identify these normal variants, but some neurologists may misidentify or misinterpret these EEG findings, potentially leading to unnecessary treatment with antiepileptic drugs.
Hundreds of medications can alter an EEG, usually by increasing either slowing or beta activity. The most common change is excessive beta activity, which is seen in most patients taking benzodiazepines or barbiturates. Enhanced beta activity is an appropriate response of a normal brain to certain medications and does not indicate underlying brain pathology.
If a patient with psychiatric illness has seizure-like episodes, an abnormal EEG may help in diagnosis. A neurologist can help direct the patient evaluation. Whether or not interictal EEG abnormalities are present, video/EEG monitoring can often make the diagnosis by capturing the events.
Case 1: Does this patient have epilepsy?
For Mr. A, age 27, lithium has stabilized his bipolar I disorder for 2 years without significant adverse effects. An EEG ordered for unknown reasons by his primary care physician shows large amounts of beta activity and a single sharp wave from the right temporal region. Should you add an antiepileptic drug to his regimen?
In this patient, lithium probably caused the large amounts of beta activity. A rule of thumb says that if an EEG shows increased beta activity, a medication is almost certainly the cause. If an EEG finds increased generalized slowing, medication effect is one of many possible causes.
The single sharp wave from the right temporal lobe raises the possibility of increased susceptibility to seizures but is not diagnostic of epilepsy. There is no indication to change the patient’s treatment regimen if he is well controlled without adverse effects from his current medications and there is no clear history of clinical seizures. In short, treat the patient, not the EEG.
On an EEG, epileptiform activity is seen as spikes or sharp waves and indicates potential for epileptic seizures.
- Focal epileptiform activity is consistent with seizures of focal origin (e.g., simple partial, complex partial, or partial onset seizure with secondary generalization into tonic-clonic seizure).
- Generalized epileptiform activity is consistent with seizures of generalized onset (e.g., absence, myoclonic, or primary generalized tonic-clonic seizure).
Interictal spikes (between seizures) are consistent with, but not diagnostic of, seizures and epilepsy. An ictal discharge (rhythmic, persistent epileptiform activity) on an EEG accompanied by a clinical change in behavior is diagnostic of a seizure.
Focal epileptiform activity
Generalized epileptiform activity
Based on early studies, EEG results can show epileptiform activity in some normal brains.1 On the other hand, only about 50% of patients with epilepsy will show epileptiform activity on a single EEG. Thus, some EEG abnormalities in psychiatric patients may not be related to either epilepsy or the psychiatric disorder, and a normal EEG does not exclude the possibility of epileptic seizures.
Question the patient for a history of other factors that may predispose him or her to seizures, such as:
- family history of seizures
- previous traumatic brain injury
- structural brain abnormality.
In light of this EEG, brain MRI is indicated. In this case the patient could be counseled to avoid seizure triggers (e.g., sleep deprivation). Because a long list of medications can lower the seizure threshold, the clinician must weigh the risks and benefits of using any medication in patients with increased susceptibility for seizures.
Case 2: Seizures or panic attacks?
Mr. B, age 31, is referred by his primary care physician for “spells” that began several years ago and recently increased in frequency to three to four times per week. The episodes start with a feeling of fear, “butterflies” in his stomach, and hyperventilation. These feelings intensify within minutes; each episode lasts 10 to 30 minutes.
The patient is usually aware of his surroundings during the episodes but twice has lost consciousness for less than 1 minute. There is no evidence of incontinence or tongue biting. Four years ago, the patient was involved in a motor vehicle accident and lost consciousness for about 30 minutes.
An EEG shows intermittent slowing from the left temporal region, and brain MRI is normal. Treatment with a benzodiazepine shortens the attacks. Where do you proceed from here?
Mr. B presents a diagnostic dilemma. Characteristics of his episodes may be seen in panic attacks, temporal lobe complex partial seizures, and psychogenic nonepileptic seizures, which is the preferred term for pseudoseizures (Table).2
One option would be to see how he responds to an agent that would treat seizures but not panic attacks (e.g., carbamazepine) or one that would treat panic attacks but not seizures (e.g., a selective serotonin reuptake inhibitor). Because his episodes are frequent, however, a more appropriate option would be to admit him for video/EEG monitoring, which can distinguish among these possible diagnoses with almost 100% accuracy.
Case 3: Attention disorder or epilepsy?
Miss C, age 9, is referred for possible ADHD. Her teachers notice she has difficulty following lessons in class but is intelligent and usually motivated. Her grades have been dropping. Her family reports she has episodes during which she stares and is unable to answer questions for 3 to 5 seconds, but she exhibits no other seizure-like manifestations. A trial of methylphenidate has not improved the symptoms. What should you try next?
This patient presents with symptoms that could be consistent with absence seizures. EEG would be diagnostic if it showed generalized spike and wave. Absence seizures (sometimes called petit mal) usually present in childhood and are characterized by recurrent brief staring spells with no postictal confusion or other clinical manifestations.
Patients with childhood absence epilepsy are usually of normal intelligence and only rarely have other associated seizure types (e.g., myoclonus or tonic-clonic). Seizures of approximately 3 to 5 seconds may occur up to 100 times a day and thus may be mistaken for attention problems.
EEG shows characteristic generalized three-per-second spike and wave, which is often precipitated by hyperventilation or photic stimulation. Ethosuximide or valproate can completely control seizures in most cases.
Case 4: Emergency use of EEG
Ms. D, age 21, is brought to the emergency department by her mother with symptoms of confusion. Ms. D has a long history of temporal lobe complex partial seizures, and her mother thinks she may have missed some doses of her antiepileptic drugs (carbamazepine and valproate). Yesterday Ms. D had two complex partial seizures but returned to baseline. Today she had three complex partial seizures within 2 hours and has not returned to baseline.
When you interview Ms. D she is alert but only intermittently oriented to date and location. She makes a variety of paraphasic speech errors. She is talking about heaven and angels and several times asks if you are the devil. Evaluation by the emergency physician discloses no toxic, metabolic, or infectious cause for her symptoms. Other than mental status, her neurologic examination is normal and shows no evidence of motor seizure activity. What would you suggest next?
This patient presents with complex partial seizures occurring in sequence without return to normal between seizures, followed by a continuous state of altered consciousness. This cluster of symptoms and signs is consistent with complex partial status epilepticus.
Emergency EEG is diagnostic. If the EEG is positive for status epilepticus, recommended treatment is IV benzodiazepines along with other IV antiepileptic drugs.
Complex partial status epilepticus can have a variety of triggers, including drug overdose, hyperthyroidism, brain tumor, or carcinomatous meningitis.3 In this case, the most likely trigger is medication noncompliance.
Complex partial or other forms of nonconvulsive status epilepticus occur in up to 37% of hospitalized patients with altered consciousness of uncertain etiology.3 EEG is required to confirm the diagnosis, but many hospitals do not have EEG technologists on call nights and weekends.
Table
THE FEARFUL PATIENT: PANIC ATTACK, SEIZURE DISORDER, OR PSYCHOGENIC?
| Panic attacks |
|
| Temporal lobe complex partial seizures |
|
| Psychogenic nonepileptic seizures |
|
In my experience, a patient who later was referred to me for seizure control was once admitted to a psychiatry ward for 4 days because of unidentified complex partial status epilepticus. When someone finally restarted her carbamazepine, she returned to normal in a few days. Six months after she became my patient, she presented with identical symptoms. An EEG within hours of symptom onset showed continuous EEG ictal activity from the left temporal lobe. When we administered IV lorazepam, her EEG normalized within minutes and her confusion and delusional symptoms resolved in 20 minutes.
Case 5: Seizure clusters followed by agitation
Mr. E, 32, has had complex partial and tonic-clonic seizures since age 14. He presents with chronic depressive symptoms and independent episodes of agitated behavior with psychotic features lasting hours after seizure clusters. His seizures have continued despite trials of different antiepileptic drugs, and he currently is receiving phenytoin and valproate. He is referred to you to diagnose and treat the psychiatric symptoms.
He scores a 28 on the Beck Depression Inventory, which indicates moderate depression,4 but he is not suicidal. He reports that although his depression symptoms are constant, his psychotic features occur only after a series of closely-spaced seizures. How do you approach this problem?
Based on the history, this patient has both mild interictal depression and postictal psychosis. In patients with epilepsy, psychiatric symptoms are categorized as:
- ictal (occur only during a seizure)
- interictal (may wax and wane but are present chronically, usually with no relation to seizure occurrence)
- or postictal (appear within 7 days after a lucid interval following a seizure or—more often—a series of seizures).5
Interictal psychiatric disorders include depression, bipolar disorder, and psychotic disorders. If the psychiatric disorder is truly interictal and has no clear relation to seizure occurrence, it should be treated like any other psychiatric illness with appropriate medications. One should not automatically add another antiepileptic drug (AED), because AEDs as a class have more adverse effects and more drug interactions than commonly used antidepressants.
Begin by examining the AEDs the patient is receiving. For example, phenobarbital and, to a lesser extent, phenytoin are associated with depression and should be used in patients with depression only when other AEDs have failed.
Ictal and postictal psychiatric symptoms should be treated acutely. Postictal symptoms may include psychosis, depression, mania, or anxiety. A short course of benzodiazepines is often helpful; the use of neuroleptics is dictated by the intensity and quality of the postictal symptoms.
Ictal depression-like symptoms can be seen with temporal lobe complex partial seizures. Many patients with these seizures have simple partial seizures (auras) that manifest as brief (usually less than 1 minute) intense feelings of fear or impending doom, or of “the life drained out” of them. These symptoms may arise from seizures involving mesial temporal regions. They do not usually require treatment besides AEDs.
The idea that patients with temporal lobe epilepsy may have a particular personality type is controversial. Retrospective data first suggested that certain traits (e.g., altered sexual behavior, anger, emotionality and obsessionalism, hypergraphia, and hyper-religiosity) could be found consistently in patients with temporal lobe epilepsy.6 This personality inventory was sometimes used to diagnose epilepsy. Subsequent studies have not validated the original data, although the cluster of personality traits can certainly occur in some patients.7-9
Related resources
- Epilepsy Foundation. www.epilepsyfoundation.org
- Engel J, Pedley TA (eds). Epilepsy: A comprehensive textbook. Philadelphia: Lippincott-Raven, 1998.
Drug brand names
- Carbamazepine • Tegretol
- Ethosuximide • Zarontin
- Lorazepam • Ativan
- Phenytoin • Dilantin
- Valproate • Depakote
Disclosure
The author reports that he receives research/grant support from, is a consultant to, and is on the speakers’ bureau of GlaxoSmithKline, UCBPharma, and Ortho-McNeil Pharmaceuticals; receives research/grant support from and is a consultant to Schwarz Pharma; is a consultant to Pfizer Inc., Elan Pharmaceuticals, and Shire Pharmaceuticals; and is on the speakers’ bureau of Abbott Laboratories, Cyberonics, and Shire Pharmaceuticals.
1. Zivin L, Ajmone-Marson C. Incidence and prognostic significance of epileptiform activity in the EEG of nonepileptic subjects. Brain 1969;91:751-78.
2. Arnold LM, Privitera MD. Psychopathology and trauma in epileptic and psychogenic seizures. Psychosomatics 1996;37(5):438-43.
3. Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res 1994;18(2):155-66.
4. Lambert MV, Robertson MM. Depression in epilepsy: etiology, phenomenology, and treatment. Epilepsia 1999;40(suppl 10):S21-47.
5. Logsdail SJ, Toone BK. Post-ictal psychoses: a clinical and phenomenological description. Br J Psychiatry 1988;152:246-52.
6. Bear DM, Fedio P. Quantitative analysis of interictal behavior in temporal lobe epilepsy. Arch Neurol 1977;34:454-67.
7. Devinsky O, Najjar S. Evidence against the existence of a temporal lobe epilepsy personality. Neurology 1999;53(5, suppl 2):S13-25.
8. Blumer D. Evidence supporting the temporal lobe epilepsy personality. Neurology 1999;53 (5, suppl 2):S9-12.
9. Mungas D. Interictal behavior abnormality in temporal lobe epilepsy. A specific syndrome or nonspecific psychopathology? Arch Gen Psychiatry 1982;39(1):108-11.
1. Zivin L, Ajmone-Marson C. Incidence and prognostic significance of epileptiform activity in the EEG of nonepileptic subjects. Brain 1969;91:751-78.
2. Arnold LM, Privitera MD. Psychopathology and trauma in epileptic and psychogenic seizures. Psychosomatics 1996;37(5):438-43.
3. Privitera M, Hoffman M, Moore JL, Jester D. EEG detection of nontonic-clonic status epilepticus in patients with altered consciousness. Epilepsy Res 1994;18(2):155-66.
4. Lambert MV, Robertson MM. Depression in epilepsy: etiology, phenomenology, and treatment. Epilepsia 1999;40(suppl 10):S21-47.
5. Logsdail SJ, Toone BK. Post-ictal psychoses: a clinical and phenomenological description. Br J Psychiatry 1988;152:246-52.
6. Bear DM, Fedio P. Quantitative analysis of interictal behavior in temporal lobe epilepsy. Arch Neurol 1977;34:454-67.
7. Devinsky O, Najjar S. Evidence against the existence of a temporal lobe epilepsy personality. Neurology 1999;53(5, suppl 2):S13-25.
8. Blumer D. Evidence supporting the temporal lobe epilepsy personality. Neurology 1999;53 (5, suppl 2):S9-12.
9. Mungas D. Interictal behavior abnormality in temporal lobe epilepsy. A specific syndrome or nonspecific psychopathology? Arch Gen Psychiatry 1982;39(1):108-11.
In a virtual world, games can be therapeutic
Many of us—and our patients—enjoy computer games, and at first glance computer gaming and psychiatry appear to have little in common. Yet computer gaming has spurred the growth of cyber technology by demanding high-level capabilities in computer hardware and software. Games initially were developed and played in two dimensions, but—with improved graphic cards and software rendering engines—they can now be three-dimensional. Some games are realistic enough to stimulate nausea and vertigo.
Overexposure to especially graphic computer games has been blamed for causing violent behavior in some individuals.1 Jeanne B. Funk, PhD, of the University of Toledo department of psychology, testified before the U.S. Senate Commerce Committee regarding the impact of interactive violence on children.2
Avatar psychotherapy. Some computer games also have therapeutic properties, however. John Suler, PhD, of the psychology department at Rider University (Lawrenceville, NJ), writes about “Avatar psychotherapy,” in which an avatar—a personal manifestation in a virtual world—can be used to facilitate psychotherapy.3 Such an environment can permit role-playing, enable fantasies, and allow psychiatrists to explore transference and countertransference issues.
“The Sims,” a popular people simulator game (http://thesims.ea.com/), has also been considered useful for therapy as a “technology of self.”4 One resident physician at the UC Davis psychiatry department uses this game in therapy with adolescents to facilitate expression of family dynamics. Although this technology does not replace traditional psychotherapy, it clearly augments and provides unique benefits.
Virtual fears. In 1995, members of the Georgia Tech computer science department and Emory University department of psychiatry in Atlanta created the Graphics Visualization & Usability Center,5 a project using virtual reality and exposure therapy. Patients wear a head-mounted display and other devices to track their movements in the virtual world. With virtual reality technology, patients can be exposed to a feared stimulus in a safe, computer-generated environment.
This technology has been used to treat acrophobia, fear of flying, and posttraumatic stress disorder. Its benefits include cost effectiveness, high patient acceptance, and effective therapy for patients with imagination deficits.
The developers of virtrual reality therapy have now formed a company called Virtually Better to provide this technology to other therapists.6 Although the technology currently is not applicable to the individual psychiatrist, this tool is expected to be widely available in the coming years with ever-improving and more affordable computing power.
Telemedicine. Virtual reality is also being used to link providers and patients through telemedicine or video conferencing. In clinical practice, telemedicine offers many advantages, such as the ability to reach patients in wide geographic areas, cost effectiveness, and linking of specialists to primary providers.7
Patients appreciate traveling less and are quite satisfied with their virtual visits. In fact, patients with schizophrenia prefer telemedicine to real office visits.8
One of telemedicine’s downsides has been its expense, requiring dedicated ISDN lines and specialized equipment. Other issues include licensing, confidentiality, reimbursement, and adherence to practice guidelines.9 For readers interested in this technology, the American Telemedicine Association Web site (www.americantelemed.org) is a good starting point. As high-speed Internet access becomes more widely available, telemedicine is poised to overtake e-mail as the next communication tool.
Summary. These virtual methods are still considered quite novel and are not yet part of mainstream psychiatry. The technology is not quite mature but is rapidly improving with new hardware and software developments. Its cost, although a barrier today, is diminishing fast. Patient acceptance is likely to grow over time among our increasingly technology-savvy public. With Internet connectivity and improved visual and audio capabilities of computers at affordable prices, virtual reality could soon play a significant new role in psychiatric care.
1. Sources about Role Playing Games: http://www.rpg.net/252/quellen/sources.html. Accessed Aug. 8, 2002.
2. Testimony of Jeanne B. Funk, PhD, before the U.S. Senate Commerce Committee on violent computer games. Available at: http://www.utoledo.edu/psychology/funktestimony.html. Accessed Aug. 8, 2002.
3. Avatar Psychotherapy: http://www.rider.edu/users/suler/psycyber/avatarther.html.
4. Tufts University: The SIMS—the people simulator game—as a technology of the self. Available at: http://www.tufts.edu/~istamm01/The%20SIMS3.htm. Accessed Aug. 8, 2002.
5. Georgia Institute of Technology, Graphics Visualization & Usability Center: http://www.cc.gatech.edu/gvu/virtual/index.html. Accessed Aug. 8, 2002.
6. Virtually Better: http://www.virtuallybetter.com. Accessed Aug. 8, 2002.
7. Hilty DM, Luo JS, Morache C, Marcelo DA, Nesbitt TS. Telepsychiatry: an overview for psychiatrists. CNS Drugs 2002;16(8):527-48.
8. Zarate CA, Jr, et al. Applicability of telemedicine for assessing patients with schizophrenia: acceptance and reliability. J Clin Psychiatry 1997;58(1):22-5.
9. The American Psychiatric Association Resource Document on Telepsychiatry by Videoconferencing. Available at: http://www.psych.org/pract_of_psych/tp_paper.cfm. Accessed Aug. 8, 2002.
Many of us—and our patients—enjoy computer games, and at first glance computer gaming and psychiatry appear to have little in common. Yet computer gaming has spurred the growth of cyber technology by demanding high-level capabilities in computer hardware and software. Games initially were developed and played in two dimensions, but—with improved graphic cards and software rendering engines—they can now be three-dimensional. Some games are realistic enough to stimulate nausea and vertigo.
Overexposure to especially graphic computer games has been blamed for causing violent behavior in some individuals.1 Jeanne B. Funk, PhD, of the University of Toledo department of psychology, testified before the U.S. Senate Commerce Committee regarding the impact of interactive violence on children.2
Avatar psychotherapy. Some computer games also have therapeutic properties, however. John Suler, PhD, of the psychology department at Rider University (Lawrenceville, NJ), writes about “Avatar psychotherapy,” in which an avatar—a personal manifestation in a virtual world—can be used to facilitate psychotherapy.3 Such an environment can permit role-playing, enable fantasies, and allow psychiatrists to explore transference and countertransference issues.
“The Sims,” a popular people simulator game (http://thesims.ea.com/), has also been considered useful for therapy as a “technology of self.”4 One resident physician at the UC Davis psychiatry department uses this game in therapy with adolescents to facilitate expression of family dynamics. Although this technology does not replace traditional psychotherapy, it clearly augments and provides unique benefits.
Virtual fears. In 1995, members of the Georgia Tech computer science department and Emory University department of psychiatry in Atlanta created the Graphics Visualization & Usability Center,5 a project using virtual reality and exposure therapy. Patients wear a head-mounted display and other devices to track their movements in the virtual world. With virtual reality technology, patients can be exposed to a feared stimulus in a safe, computer-generated environment.
This technology has been used to treat acrophobia, fear of flying, and posttraumatic stress disorder. Its benefits include cost effectiveness, high patient acceptance, and effective therapy for patients with imagination deficits.
The developers of virtrual reality therapy have now formed a company called Virtually Better to provide this technology to other therapists.6 Although the technology currently is not applicable to the individual psychiatrist, this tool is expected to be widely available in the coming years with ever-improving and more affordable computing power.
Telemedicine. Virtual reality is also being used to link providers and patients through telemedicine or video conferencing. In clinical practice, telemedicine offers many advantages, such as the ability to reach patients in wide geographic areas, cost effectiveness, and linking of specialists to primary providers.7
Patients appreciate traveling less and are quite satisfied with their virtual visits. In fact, patients with schizophrenia prefer telemedicine to real office visits.8
One of telemedicine’s downsides has been its expense, requiring dedicated ISDN lines and specialized equipment. Other issues include licensing, confidentiality, reimbursement, and adherence to practice guidelines.9 For readers interested in this technology, the American Telemedicine Association Web site (www.americantelemed.org) is a good starting point. As high-speed Internet access becomes more widely available, telemedicine is poised to overtake e-mail as the next communication tool.
Summary. These virtual methods are still considered quite novel and are not yet part of mainstream psychiatry. The technology is not quite mature but is rapidly improving with new hardware and software developments. Its cost, although a barrier today, is diminishing fast. Patient acceptance is likely to grow over time among our increasingly technology-savvy public. With Internet connectivity and improved visual and audio capabilities of computers at affordable prices, virtual reality could soon play a significant new role in psychiatric care.
Many of us—and our patients—enjoy computer games, and at first glance computer gaming and psychiatry appear to have little in common. Yet computer gaming has spurred the growth of cyber technology by demanding high-level capabilities in computer hardware and software. Games initially were developed and played in two dimensions, but—with improved graphic cards and software rendering engines—they can now be three-dimensional. Some games are realistic enough to stimulate nausea and vertigo.
Overexposure to especially graphic computer games has been blamed for causing violent behavior in some individuals.1 Jeanne B. Funk, PhD, of the University of Toledo department of psychology, testified before the U.S. Senate Commerce Committee regarding the impact of interactive violence on children.2
Avatar psychotherapy. Some computer games also have therapeutic properties, however. John Suler, PhD, of the psychology department at Rider University (Lawrenceville, NJ), writes about “Avatar psychotherapy,” in which an avatar—a personal manifestation in a virtual world—can be used to facilitate psychotherapy.3 Such an environment can permit role-playing, enable fantasies, and allow psychiatrists to explore transference and countertransference issues.
“The Sims,” a popular people simulator game (http://thesims.ea.com/), has also been considered useful for therapy as a “technology of self.”4 One resident physician at the UC Davis psychiatry department uses this game in therapy with adolescents to facilitate expression of family dynamics. Although this technology does not replace traditional psychotherapy, it clearly augments and provides unique benefits.
Virtual fears. In 1995, members of the Georgia Tech computer science department and Emory University department of psychiatry in Atlanta created the Graphics Visualization & Usability Center,5 a project using virtual reality and exposure therapy. Patients wear a head-mounted display and other devices to track their movements in the virtual world. With virtual reality technology, patients can be exposed to a feared stimulus in a safe, computer-generated environment.
This technology has been used to treat acrophobia, fear of flying, and posttraumatic stress disorder. Its benefits include cost effectiveness, high patient acceptance, and effective therapy for patients with imagination deficits.
The developers of virtrual reality therapy have now formed a company called Virtually Better to provide this technology to other therapists.6 Although the technology currently is not applicable to the individual psychiatrist, this tool is expected to be widely available in the coming years with ever-improving and more affordable computing power.
Telemedicine. Virtual reality is also being used to link providers and patients through telemedicine or video conferencing. In clinical practice, telemedicine offers many advantages, such as the ability to reach patients in wide geographic areas, cost effectiveness, and linking of specialists to primary providers.7
Patients appreciate traveling less and are quite satisfied with their virtual visits. In fact, patients with schizophrenia prefer telemedicine to real office visits.8
One of telemedicine’s downsides has been its expense, requiring dedicated ISDN lines and specialized equipment. Other issues include licensing, confidentiality, reimbursement, and adherence to practice guidelines.9 For readers interested in this technology, the American Telemedicine Association Web site (www.americantelemed.org) is a good starting point. As high-speed Internet access becomes more widely available, telemedicine is poised to overtake e-mail as the next communication tool.
Summary. These virtual methods are still considered quite novel and are not yet part of mainstream psychiatry. The technology is not quite mature but is rapidly improving with new hardware and software developments. Its cost, although a barrier today, is diminishing fast. Patient acceptance is likely to grow over time among our increasingly technology-savvy public. With Internet connectivity and improved visual and audio capabilities of computers at affordable prices, virtual reality could soon play a significant new role in psychiatric care.
1. Sources about Role Playing Games: http://www.rpg.net/252/quellen/sources.html. Accessed Aug. 8, 2002.
2. Testimony of Jeanne B. Funk, PhD, before the U.S. Senate Commerce Committee on violent computer games. Available at: http://www.utoledo.edu/psychology/funktestimony.html. Accessed Aug. 8, 2002.
3. Avatar Psychotherapy: http://www.rider.edu/users/suler/psycyber/avatarther.html.
4. Tufts University: The SIMS—the people simulator game—as a technology of the self. Available at: http://www.tufts.edu/~istamm01/The%20SIMS3.htm. Accessed Aug. 8, 2002.
5. Georgia Institute of Technology, Graphics Visualization & Usability Center: http://www.cc.gatech.edu/gvu/virtual/index.html. Accessed Aug. 8, 2002.
6. Virtually Better: http://www.virtuallybetter.com. Accessed Aug. 8, 2002.
7. Hilty DM, Luo JS, Morache C, Marcelo DA, Nesbitt TS. Telepsychiatry: an overview for psychiatrists. CNS Drugs 2002;16(8):527-48.
8. Zarate CA, Jr, et al. Applicability of telemedicine for assessing patients with schizophrenia: acceptance and reliability. J Clin Psychiatry 1997;58(1):22-5.
9. The American Psychiatric Association Resource Document on Telepsychiatry by Videoconferencing. Available at: http://www.psych.org/pract_of_psych/tp_paper.cfm. Accessed Aug. 8, 2002.
1. Sources about Role Playing Games: http://www.rpg.net/252/quellen/sources.html. Accessed Aug. 8, 2002.
2. Testimony of Jeanne B. Funk, PhD, before the U.S. Senate Commerce Committee on violent computer games. Available at: http://www.utoledo.edu/psychology/funktestimony.html. Accessed Aug. 8, 2002.
3. Avatar Psychotherapy: http://www.rider.edu/users/suler/psycyber/avatarther.html.
4. Tufts University: The SIMS—the people simulator game—as a technology of the self. Available at: http://www.tufts.edu/~istamm01/The%20SIMS3.htm. Accessed Aug. 8, 2002.
5. Georgia Institute of Technology, Graphics Visualization & Usability Center: http://www.cc.gatech.edu/gvu/virtual/index.html. Accessed Aug. 8, 2002.
6. Virtually Better: http://www.virtuallybetter.com. Accessed Aug. 8, 2002.
7. Hilty DM, Luo JS, Morache C, Marcelo DA, Nesbitt TS. Telepsychiatry: an overview for psychiatrists. CNS Drugs 2002;16(8):527-48.
8. Zarate CA, Jr, et al. Applicability of telemedicine for assessing patients with schizophrenia: acceptance and reliability. J Clin Psychiatry 1997;58(1):22-5.
9. The American Psychiatric Association Resource Document on Telepsychiatry by Videoconferencing. Available at: http://www.psych.org/pract_of_psych/tp_paper.cfm. Accessed Aug. 8, 2002.
Weight control and antipsychotics: How to tip the scales away from diabetes and heart disease
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Weight control and antipsychotics: How to tip the scales away from diabetes and heart disease
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
Weight gain is a potential problem for all patients who require treatment with antipsychotics. Those with schizophrenia face double jeopardy. Both the disorder and the use of virtually any available antipsychotic drug may be associated with weight gain, new-onset glucose intolerance, and type 2 diabetes mellitus.
Because of the cardiovascular risks and other morbidity associated with weight gain and glucose dysregulation,1 the psychiatrist must remain vigilant and manage these complications aggressively. In this article, we offer insights into the prevention and management of metabolic complications associated with the use of antipsychotic agents in patients with schizophrenia.
Weight gain and antipsychotics
Weight change was recognized as a feature of schizophrenia even before antipsychotic drugs were introduced in the 1950s.2 Schizophrenia—independent of drug treatment—also is a risk factor for the development of type 2 diabetes. In persons with schizophrenia, serum glucose levels increase more slowly, decline more gradually, and represent higher-than-normal reference values.3
Figure 1 WEIGHT GAIN ASSOCIATED WITH ANTIPSYCHOTIC DRUG ADMINISTRATION
Values represent estimates of drug-induced weight gain after 10 weeks of drug administration.
Source: Allison et al. Am J Psychiatry 1999;156:1686-96; Brecher et al. Int J Psychiatry Clin Pract 2000;4:287-92.In 1999, Allison et al assessed the effects of conventional and atypical antipsychotics on body weight. Using 81 published articles, they estimated and compared weight changes associated with 10 antipsychotic agents and a placebo when given at standard dosages for 10 weeks.4 Comparative data on quetiapine, which were insufficient in 1999, have since been added (Figure 1).5
Patients who received a placebo lost 0.74 kg across 10 weeks. Weight changes with the conventional agents ranged from a reduction of 0.39 kg with molindone to an increase of 3.19 kg with thioridazine. Weight gains also were seen with all of the newer atypical agents, including clozapine (+4.45 kg), olanzapine (+4.15 kg), risperidone (+2.10 kg), and ziprasidone (+0.04 kg).
Fontaine et al have estimated that weight gain in patients with schizophrenia has its greatest impact on mortality in two scenarios:
- when patients are overweight before they start antipsychotic medication
- with greater degrees of weight gain across 10 years (Figure 2).
Whatever a patient’s starting weight, substantial weight gain with antipsychotic therapy increases the risk of impaired glucose tolerance and hypertension (Figure 3).6
Schizophrenia and diabetes
The prevalence of type 2 diabetes in patients with schizophrenia increased from 4.2% in 1956 to 17.2% in 1968, related in part to the introduction of phenothiazines.7 A recent study of data collected by the Schizophrenia Patient Outcomes Research Team (PORT)2 found higher rates of diabetes in persons with schizophrenia (lifetime prevalence, 14.9%) than in the general population (approximately 7.3%).1 Most patients in the PORT study were taking older antipsychotics, the use of which has occasionally been associated with carbohydrate dysregulation.
Figure 2 INCREASED MORTALITY ASSOCIATED WITH WEIGHT GAIN
Number of deaths associated with weight gains of 2.5 and 12 kg over 10 years, as related to all body mass index measurements (BMIs) and BMIs >27 (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.The prevalence of new-onset diabetes with use of specific antipsychotics is unknown. Most information is contained in case reports, and proper epidemiologic studies await publication.
The most detailed report—a pooled study of published cases related to clozapine use—comes from the FDA’s Center for Drug Evaluation and Research.8 In this study, the authors identified 384 reports of diabetes that developed (in 242 patients) or was exacerbated (in 54 patients) in association with clozapine. Patient mean age was 40, and diabetes occurred more commonly in women than in men.
Diabetes developed most commonly within 6 months of starting treatment with clozapine, and one patient developed diabetes after a single 500-mg dose. Metabolic acidosis or ketosis occurred in 80 cases, and 25 subjects died during hyperglycemic episodes. Stopping clozapine or reducing the dosage improved glycemic control in 46 patients.8
Figure 3 INCREASED MORBIDITY ASSOCIATED WITH WEIGHT GAIN
New cases of impaired glucose tolerance and hypertension that developed with weight gains of 2.5 and 12 kg over 10 years (per 100,000 persons in U.S. population).
Source: Fontaine et al. Psychiatry Res 2001;101:277-88.During antipsychotic therapy, it is important to measure patients’ fasting plasma glucose at least annually—and more often for high-risk patients (Table 1). The American Diabetes Association defines diabetes as a fasting serum or plasma glucose 126 mg/dl or a 2-hour postprandial serum or plasma glucose 200 mg/dl. In all patients, these tests should be repeated to confirm the diagnosis. Oral glucose tolerance testing is less convenient than fasting plasma glucose testing but more sensitive in identifying changes in carbohydrate metabolism.
As with weight gain, it is easier to prevent diabetes than to treat it. The psychiatrist can best help the patient with emerging carbohydrate dysregulation by collaborating with an internist, family physician, or endocrinologist.
Table 1
FACTORS RELATED TO HIGH RISK OF DEVELOPING TYPE 2 DIABETES
|
| Source: American Diabetes Association |
Weight gain with diabetes drugs Weight gain is associated not only with the use of antipsychotics but also with four classes of oral agents used to treat type 2 diabetes: sulfonylureas, meglitinides, phenylalanine derivatives, and thiazolidinediones. One class—biguanides—contributes to weight reduction, and one—alpha-glucosidase inhibitors—has a variable effect on body weight. These drugs also vary in their effects on serum lipids, including total cholesterol, LDL and HDL cholesterol, and triglycerides (Table 2).9
Many patients with type 2 diabetes require more than one agent to control plasma glucose. With time, insulin deficiency becomes more marked, and insulin therapy is frequently added to the regimen. Hypertension and hyperlipidemia are also very common in patients with type 2 diabetes and require medication to reduce the risk of cardiovascular events.10 As a result, the diabetic patient requiring antipsychotic drugs will likely need polypharmacy, and many of the drugs that might be used may lead to weight gain.
Assessing, managing weight gain
During each visit for the patient with schizophrenia, it is important to routinely weigh those receiving antipsychotics and ask about polydipsia and polyuria, which are early signs of incipient diabetes. A patient who is gaining significant weight (7% of baseline) while taking an antipsychotic and has risk factors for cardiovascular events (e.g., smoking, hypertension, hypertriglyceridemia) is a candidate for a change in antipsychotics.
Try to weigh patients at approximately the same time of day at each visit to compensate for possible diurnal weight changes related to polydipsia-hyponatremia syndrome.11 Patients with this syndrome can gain 5 to 10 lbs (or more) per day and excrete the retained fluid at night. It occurs in 5 to 10% of chronically psychotic patients requiring institutional care and in 1 to 2% of outpatients. Patients with schizophrenia complicated by this syndrome may manifest polydipsia and polyuria secondary to psychosis rather than emerging diabetes. Thus, the clinician must be alert to both diabetes and the polydipsia-hyponatremia syndrome in this setting.
Weight-control approaches
Patients who are taking sedating antipsychotics (e.g., clozapine, olanzapine, or low-potency phenothiazines) may gain up to 30 lbs per year if they become physically inactive and do not reduce their food consumption. Thus, it is important to work with such patients to decrease their caloric intake.
A weight-loss program that produces a loss of 0.5 to 1% of body weight per week is considered safe and acceptable.12 Mild to moderate obesity may be managed by reducing food intake by 500 calories and exercising 30 minutes each day.
CBT Cognitive-behavioral therapy (CBT) may help stem weight gain associated with antipsychotic use. Umbricht et al provided CBT to six patients with chronic psychosis who were receiving clozapine or olanzapine. Therapists in group and individual sessions focused on the causes of weight gain, lowcalorie nutrition, weight-loss guidelines, exercise programs, and relaxation strategies. Across 8 weeks, patients’ mean BMI decreased from 29.6 to 25.1 kg/m2
Table 2
METABOLIC EFFECTS OF ORAL ANTIHYPERGLYCEMIC DRUGS
| Class | Body weight | Total cholesterol | LDL | HDL | Triglycerides |
|---|---|---|---|---|---|
| Sulfonylureas Glipizide Glyburide Glimepiride | ▲ | ◄► | ◄► | ◄► | ◄► |
| Meglitinides Repaglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Phenylalanine derivatives Nateglinide | ▲ | ◄► | ◄► | ◄► | ◄► |
| Biguanides Metformin | ▼ | ▼ | ▼ | ▲ | ▼ |
| Thiazolidinediones Pioglitazone Rosiglitazone | ▲ | ▲ | ▲ | ▲ | ▼ |
| Alpha-glucosidase inhibitors Acarbose Miglitol | ◄► | ◄► | ◄► | ◄► | ◄► |
| ▲ Increase ▼ Decrease ◄► Neutral effect/no change | |||||
Weight management program The Weight Watchers weight management program has shown mild success when offered to men and women with schizophrenia or schizoaffective disorder. Twenty-one patients who had gained an average of 32 lbs while taking olanzapine were enrolled in a Weight Watchers program at a psychiatric center.14 Mean starting BMI was 32 kg/m2 among the 11 patients who completed the 10-week program. Those 11 lost an average of 5 lbs.
All seven men lost weight. Three of the four women gained weight, and one woman lost 13 lbs. Study subjects remained clinically stable during the 10-week study. Two of the three women who did not lose weight had disabling psychiatric symptoms. Participation rates were similar to those of typical Weight Watchers clientele, suggesting that patients requiring antipsychotics might benefit from treatments used for other obese patients.
Patient education Educating patients about nutrition and exercise may help them control their rate of weight gain during antipsychotic therapy.
Littrell et al provided such an educational program for 1 hour per week for 4 months to six men and six women taking olanzapine for schizophrenia or schizoaffective disorder.15 Patients in the behavioral group gained 0.5 kg, compared with a control group that gained 2.9 kg. Mean increase in BMI was less for the behavioral group (0.3 kg/m2) than for the control patients (0.9 kg/m2). Men in both groups gained more weight than did women.
Pharmacologic approaches
Antiobesity medications are generally reserved for patients with a BMI 30 kg/m2 (threshold for obesity) or for those with a BMI 27 kg/m2 (threshold for overweight is 25 kg/m2) who have additional risk factors for cardiovascular disease, stroke, or diabetes.16
For patients with schizophrenia, who typically have a BMI 27 kg/m2, the presence of these risk factors alone may be enough to warrant consideration of an antiobesity agent. Adding any new drug to a patient’s regimen, however, increases the risk of an adverse interaction.
Antiobesity drugs work by a variety of mechanisms, including decreasing appetite, decreasing fat absorption, and increasing energy expenditure. Drugs may reduce caloric intake by decreasing appetite (anorectic drugs) or increasing satiety (appetite suppressants). Centrally-acting sympathomimetics or serotonergic drugs may suppress appetite.
In studies up to 2 years, the appetite suppressant sibutramine, with mixed serotonergic and noradrenergic reuptake inhibition properties, has been shown to cause more weight loss than a placebo in populations without schizophrenia.17 According to one case report, sibutramine use was associated with new-onset psychosis.18
Common side effects of sibutramine include headache, dry mouth, anorexia, constipation, and insomnia. Regular monitoring of blood pressure is required. Do not prescribe this drug for patients with cardiovascular disease, and avoid co-prescribing with MAO inhibitors and serotonergics.
Orlistat reduces fat absorption from the GI tract.19 Common side effects are largely confined to the GI tract and include oily spotting, flatulence, fecal urgency, fatty/oily stool, and oily evacuation.
Combination therapies
Researchers are studying whether adding adjunctive agents to antipsychotics reduces weight gain.
Clozapine plus quetiapine A group of 65 patients who experienced a mean body weight increase of 6.5 kg while taking clozapine for 6 months were then given clozapine plus quetiapine at chlorpromazine-equivalent dosing during the next 10 months. The patients lost a mean of 4.2 kg, and their glycemic control improved. Elevated glycosylated hemoglobin (HbA1c) became normal in those subjects (20% of participants) who had developed type 2 diabetes while taking clozapine alone. The authors theorized that the weight loss diminished insulin resistance, leading to better control of serum glucose levels.20
Olanzapine plus amantadine A group of 12 outpatients with axis I or II diagnoses had responded well clinically to olanzapine but had gained an average 7.3 kg over 1 to 11 months. In an open-label study, they continued their dosages of olanzapine and also were given amantadine, 100 to 300 mg/d. Amantadine was chosen for this trial because of its possible release of dopamine.
No dietary changes were made, but subjects gained no additional weight after amantadine was added. Over the next 3 to 6 months, they lost a mean 3.5 kg, which was 50% of the weight gain associated with olanzapine administration.21
Clozapine plus topiramate In clinical trials, the anticonvulsant topiramate has been associated with significant weight loss for up to 12 months in patients with seizure disorders.22 This agent, which also has mood-stabilizing effects, may be useful both for mood stabilization and weight loss in tandem with antipsychotic therapy.
In a case study,23 a 29-year-old man with schizophrenia who failed several trials of antipsychotic drugs experienced significant improvement with clozapine, 800 mg/d. Over 2 years, however, he developed myoclonic jerks and gained 45.5 kg (a 49% increase over baseline). When topiramate was added, starting with 25 mg/d and increasing to 125 mg/d, his mood improved and the myoclonic jerks stopped. During 5 months of combination therapy, the patient lost 21 kg without changing his eating habits.
Olanzapine and nizatidine Agents that block histamine (H 2) receptors in the digestive tract may be associated with weight loss when given at high doses, although the mechanism by which they contribute to weight loss is unclear. In a double-blind, placebo-controlled study,24 the H 2 blocker nizatidine was given to patients with schizophrenia who were taking olanzapine, 5 to 20 mg/d. In a 16-week trial, 132 patients were randomized to receive adjunctive treatment with low-dose nizatidine (150 mg bid), high-dose nizatidine (300 mg bid), or a placebo.
After 16 weeks, nizatidine demonstrated a dose-response effect when combined with olanzapine. Average weight gain was:
- 5.51 kg with a placebo
- 4.41 kg with low-dose nizatidine
- 2.76 kg with high-dose nizatidine (p =0.02 compared with a placebo).
In the high-dose nizatidine group, only 6% of patients gained more than 10 kg, and weight gain leveled off by week eight. Adverse events and clinical improvements were similar in the three groups.
Related resources
- Weight gain: A growing problem in schizophrenia management. J Clin Psychiatry 2001;62(suppl 7).
- Weight gain associated with the use of psychotropic medications. J Clin Psychiatry 1999;60(suppl 2).
- Effects of atypical antipsychotics on body weight and glucose regulation. J Clin Psychiatry 2001;62(suppl 23).
- National Heart, Lung, and Blood Institute. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. www.nhlbi.nih.gov/guidelines/obesity/ob_home.htm
Drug brand names
- Amantadine • Symmetrel
- Clozapine • Clozaril
- Nizatidine • Axid
- Olanzapine • Zyprexa
- Orlistat • Xenical
- Quetiapine • Seroquel
- Risperidone • Risperdal
- Sibutramine • Meridia
- Topiramate • Topamax
- Ziprasidone • Geodon
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
1. Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA 2001;286:1195-1200.
2. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull 2000;26:903-12.
3. Braceland FJ, Meduna LJ, Vaichulis JA. Delayed action of insulin in schizophrenia. Am J Psychiatry 1945;102:108-10.
4. Allison DB, Mentore JL, Heo M, et al. Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999;156:1686-96.
5. Brecher M, Rak IW, Westhead EK. The long-term effect of quetiapine (“Seroquel’) monotherapy on weight in patients with schizophrenia. Int J Psychiatry Clin Pract 2000;4:287-92.
6. Fontaine KR, Heo M, Harrigan EP, Shear CL, Lakshiminarayanan M. Estimating the consequences of anti-psychotic induced weight gain on health and mortality rate. Psychiatry Res 2001;101:277-88.
7. Theonnard-Neumann E. Phenothiazines and diabetes in hospitalized women. Am J Psychiatry 1968;124:978-82.
8. Koller E, Schneider B, Bennett K, Dubitsky G. Clozapine-associated diabetes. Am J Med 2001;111:716-23.
9. Pendergrass ML. Pathophysiology and management of type 2 diabetes. In: Giles TD, Sowers JR, Weber MA (eds). Diabetes & cardiovascular disease: a practical primer. New Orleans: Institute of Professional Education, 2000;15-40.
10. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults. Findings from the Third National Health and Nutrition Examination Survey. JAMA 2002;287:356-9.
11. Vieweg WVR, Leadbetter RA. The polydipsia-hyponatremia syndrome. Epidemiology, clinical features, and treatment. CNS Drugs 1997;7:121-38.
12. Thomas PR. Weighing the options: criteria for evaluating weight management programs. Washington, DC: National Academy Press, 1995.
13. Umbricht D, Flury H, Bridler R. Cognitive behavioral therapy for weight gain. Am J Psychiatry 2001;158:971.-
14. Ball M, Coons V, Buchanan R. A program for treating olanzapine-related weight gain. Psychiatric Services 2001;52:967-9.
15. Littrell KH, Petty RG, Hilligoss NM, Peabody CD, Johnson CG. Educational interventions for the management of antipsychotic-related weight gain. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31, 2001.
16. Greenberg I, Chan S, Blackburn GL. Nonpharmacologic and pharmacologic management of weight gain. J Clin Psychiatry 1999;60(suppl 21):31-6.
17. Wirth A, Krause J. Long-term weight loss with sibutramine: a randomized controlled trial. JAMA 2001;286:1331-9.
18. Taflinski T, Chojnacka J. Sibutramine-associated psychotic episode. Am J Psychiatry 2001;157:2057-8.
19. Glazer G. Long-term pharmacotherapy of obesity 2000. Arch Intern Med 2001;161:1814-24.
20. Reinstein M, Sirotovskaya L, Jones L. Effect of clozapine-quetiapine combination therapy on weight and glycaemic control. Clin Drug Invest 1999;18:99-104.
21. Floris M, Lejeune J, Deberdt W. Effect of amantadine on weight gain during olanzapine treatment. Eur Neuropsychopharmacol 2001;11:181-2.
22. Norton J, Potter D, Edwards K. Sustained weight loss associated with topiramate [abstract]. Epilepsia 1997;38(suppl 3):60.-
23. Dursun SM, Devarajan S. Clozapine weight gain, plus topiramate weight loss. Can J Psychiatry 2000;45:198.-
24. Breier A, Tanaka Y, Roychowdhury S, Clark WS. Nizatidine for the prevention of olanzapine-associated weight gain in schizophrenia and related disorders. A randomized controlled double blind study. 41st annual meeting of the New Clinical Drug Evaluation Unit, Phoenix, AZ, May 28-31 2001.
Kleptomania: Emerging therapies target mood, impulsive behavior
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
What is kleptomania? An independent illness, a symptom of other psychiatric disorders, or merely criminal behavior? Kleptomania—a disorder defined by an inability to resist the impulse to steal—is one of psychiatry’s most poorly understood diagnoses, even though it has been recognized in the literature for almost 200 years.
Kleptomania causes notable distress and impaired functioning.1 People with kleptomania often suffer from comorbid mood, anxiety, substance use, and other impulse-control disorders.14 They experience the humiliation of repeated arrests, which leads to guilt, depression, and even suicide.1,5 Yet kleptomania usually goes undiagnosed and untreated, despite a lifetime prevalence as high as 0.6%.6
Case report: ‘I’m a thief’
“I’m a thief,” began Susan, age 39. “I steal something four or five times every week. I steal from grocery stores and clothing stores. Sometimes I might steal something like vanilla extract; other times an expensive men’s tie. I probably steal $200 worth of items every week.
“You probably won’t believe this, but I don’t want or need the stuff I take. I have plenty of money. I have no idea why I take the things I do. That’s why I’m so depressed. What kind of person does something like this?
The urge to steal “I was probably 14 when I started stealing. I would go to stores with my mother. When I saw certain objects, I would get urges to steal them. The odd thing was that the items I stole were so ridiculous. I remember stealing key chains for several months, maybe three or four times a week. When I got older, things got worse. I was having urges more often, and so I needed to steal more often.
| Myth | Fact |
|---|---|
| Only little old ladies are kleptomaniacs. | Men and women of all ages suffer from kleptomania. Most patients report that the disorder began in adolescence. |
| It’s just a phase kids go through. | Parents of adolescents might see stealing as a phase. In many cases this might be true, but stealing may also suggest an underlying psychopathology. |
| People who steal are “bad.” | People with kleptomania steal because of urges to steal, not because of moral weakness. Treatment, not judgment, is the appropriate response. |
“My entire life has been torment. Each day I worry about having the urges, and then I worry about being caught stealing. I can’t relax. I’ve been married for 17 years, and I haven’t told my husband. My secrecy is tearing our marriage apart. My husband thinks I’m having an affair because I’ve distanced myself emotionally from him.”
Table 1
SCREENING TEST FOR KLEPTOMANIA
| Yes | No | |
|---|---|---|
| 1. Do you steal or have urges to steal? | ○ | ○ |
| 2. Do thoughts of stealing or urges to steal preoccupy you? That is, do you often think about stealing or have urges to steal and wish the thoughts or urges occurred less often? | ○ | ○ |
| 3. Do you feel tense or anxious before you steal or when you have urges to steal? | ○ | ○ |
| 4. Do you feel pleasure or a sense of calm when you steal something? | ○ | ○ |
| 5. Has the stealing or urges to steal caused you much distress? | ○ | ○ |
| 6. Has the stealing or urges to steal significantly interfered with your life in some way? | ○ | ○ |
| A patient who answers “yes” to questions 1 through 4 and to question 5 or 6 is likely to have kleptomania. | ||
| Adapted from DSM-IV criteria, American Psychiatric Association, 2000 | ||
Susan described urges to steal almost every day. When the urges were mild, she could resist them. Other days they were severe, and Susan felt unable to control her behavior. At work, her urges distracted her from completing projects, and her performance suffered. The urge to steal would often compel Susan to leave work early so she could get to a store.
Calm, then guilt “Every time I steal something I feel both a thrill and a great sense of calm,” she said. “It feels good. The problem is that almost immediately after each theft, I feel guilty and ashamed. After I steal, I usually donate the items to the Salvation Army, throw them away, or give them away as gifts.”
Drug trials We started treating Susan with the selective serotonin reuptake inhibitor (SSRI), citalopram. She reported notable improvement in her mood after 3 weeks on a dosage of 60 mg/d and she had been attending weekly psychotherapy, although her stealing continued unchanged. The addition of naltrexone, 200 mg/d for 2 weeks, decreased the frequency of Susan’s stealing and reduced her urges to steal, but her symptoms continued to interfere significantly with her overall functioning.
We then added the atypical antipsychotic quetiapine, 100 mg bid, and Susan’s urges to steal and stealing behavior went into remission within 3 weeks. She has refrained from stealing for the last 9 months.
Making the diagnosis
In our clinic, we have treated more than 50 patients with kleptomania. Rather than coming to us through the criminal justice system, they are usually self-referred. Often they contact us after discovering on the Internet that we specialize in treating persons with this disorder.
In our experience, kleptomania typically goes undiagnosed in clinical settings, in part because patients are ashamed and embarrassed to discuss their symptoms with physicians unless specifically asked.1 If left untreated, however, kleptomania frequently becomes chronic.4 If persons with kleptomania are to seek treatment, it is important that family, friends, and mental health professionals understand the myths and facts about this disorder (Box).
To make the diagnosis, we use the simple screening instrument shown in Table 1.7 In general, because of high comorbidity with certain disorders, we screen every patient presenting to our clinic with a mood, substance use, anxiety, or eating disorder, or who has a problem with impulse control. Kleptomania is likely if the patient answers “yes” to questions 1 through 4 and to question 5 or 6. Stealing may be a symptom of several other psychiatric disorders, however, and misdiagnosis is fairly common (Table 2).6-8
Data suggest that the female-to-male ratio in kleptomania is approximately 2:1, with onset in adolescence. Typical individuals with kleptomania steal because they have urges to steal, often triggered by specific stimuli such as the sights and sounds of stores or feelings of loneliness or stress.1 Most patients with kleptomania are fairly specific about the types of stores from which they steal and the items they steal, and most hoard stolen items.1
Although many patients with kleptomania function quite well, others are severely debilitated in social and occupational realms. In a series of 22 patients with kleptomania:
- 64% had been apprehended
- 23% had served jail time
- 27% had been hospitalized because of their kleptomania symptoms
- 18% had considered or attempted suicide because of the distress associated with their kleptomania.1
Treatment recommendations
Patient history With patients who steal, we begin by identifying the motivation behind the stealing. Most patients with kleptomania report urges to steal. Some of these patients may have comorbid depression; for them, stealing makes them feel less depressed. Anger or irritability may point to borderline personality disorder. Stealing for the enjoyment of risk may suggest bipolar disorder.
Table 2
DIFFERENTIAL DIAGNOSIS: DISORDERS THAT MAY INVOLVE STEALING
| Misdiagnosis | How to distinguish from kleptomania |
|---|---|
| Bipolar disorder | Patients with bipolar disorder may steal as a result of the impulsivity of mania. In fact, the diagnostic criteria for kleptomania require the exclusion of mania as the cause of stealing.7 Patients with bipolar disorder report an elevated, expansive, or irritable mood while stealing. Patients with kleptomania tend to report a depressed mood when not stealing |
| Borderline personality disorder | Unlike patients with borderline personality disorder, patients with kleptomania do not report long histories of unstable relationships or negative self-image; inappropriate anger and “psychotic-like” symptoms are rare in patients with kleptomania |
| Antisocial personality disorder (ASPD, or conduct disorder) | Patients with kleptomania suffer intense shame and guilt, unlike those with ASPD. Also, most patients with kleptomania do not report other illegal or antisocial behavior. |
| Eating disorders | Data suggests that about one-third of patients with an eating disorder also steal.6,8 Patients with kleptomania, however, do not have disordered eating patterns or distorted body images common to patients with eating disorders. |
Many patients with kleptomania have comorbid mood, substance, or anxiety disorders. Treating these other symptoms while ignoring the symptoms of kleptomania may be unsuccessful. Comorbidity also may influence the choice of medication.
Medical assessment Case reports have associated the onset of kleptomania with a variety of medical conditions, including presenile cortical atrophy in a 25-year-old, a parietal tumor that caused blackouts and obliterated any memory of stealing episodes, narcolepsy, and an insulinoma that caused severe hypoglycemia.9 The relationship of these conditions with the onset of kleptomania is unclear, but the reports suggest that medical causes—although unlikely—should be ruled out before you consider kleptomania as a psychiatric illness.
Patient education Persons with kleptomania often feel that no one else has the same problem. They do not think of their behavior as being an illness. It is helpful to explain that kleptomania is treatable and to connect patients with educational books, self-help groups, and Web sites providing information and support (see “Related resources”).
Cognitive-behavioral therapy (CBT) Although the evidence is quite limited, covert sensitization, exposure and response prevention, and imaginal desensitization have all been shown effective in case reports.10
What medications are effective?
Only case reports, a case series of five subjects, and a single open-label treatment study involving 10 subjects with kleptomania have been done.
So far, uses of tricyclic antidepressants (imipramine, nortriptyline), SSRIs (fluoxetine, fluvoxamine, paroxetine), the opioid antagonist naltrexone, and mood stabilizers (lithium, valproate) have met with varying degrees of success. Strategies targeting urge and behavior reduction and mechanisms for coping with urges and behavior (e.g., cognitive-behavioral therapies) may represent important adjunctive components.2,11-17
No medications are FDA-approved for treating kleptomania. Therefore, it is important to inform patients of any off-label use of medications for this disorder, as well as the empirical basis for considering pharmacologic treatment.
SSRIs Only case reports exist on the use of SSRIs in treating kleptomania. The disorder may share a common pathology with pathologic gambling, and in our clinical experience appears to respond to similar treatments.18 We draw on research of pathologic gambling as well as our clinical experience in choosing SSRIs as first-line treatment, especially for patients with significant mood symptoms.19
We suggest titrating SSRIs to the maximum recommended dosage. As in the treatment of pathologic gambling, dosages of SSRIs required to treat kleptomania symptoms appear to be higher than average dosages required to treat depressive disorders. An SSRI should not be considered ineffective unless it has been tried for at least 10 to 12 weeks and the highest dosage tolerated or recommended by the manufacturer has been reached.
Response to SSRIs usually is characterized by decreased thoughts about stealing, decreased stealing behavior, and improvement in social and occupational functioning. If an SSRI is only partially effective, we consider augmentation with naltrexone, buspirone, or a mood stabilizer.
Naltrexone Patients taking naltrexone often report less-intense urges to steal. The urges may not disappear but are often sufficiently reduced so that the patient can resist them more easily. Patients also report that the thrill associated with stealing is reduced or eliminated.
Naltrexone was used in the first medication study of kleptomania and showed a significant decline in the intensity of urges to steal, stealing thoughts, and stealing behavior. Average dosage was 150 mg/d;11 a reduced dosage (e.g., 50 mg/d) may work in adolescents with kleptomania.20
Nausea as a side effect can be reduced by starting patients on 25 mg/d for the first 3 or 4 days and possibly adding ondansetron, 4 to 8 mg/d. Nausea and diarrhea are usually mild and resolve within the first week. Clinically, most patients respond to naltrexone within 2 weeks. After that, the dosage usually needs to be adjusted.
In patients with comorbid depression, augmentation with an SSRI may prevent worsening of untreated depressive symptoms. It is prudent to obtain liver function tests prior to naltrexone administration and again 3 to 4 weeks after starting the drug.21 Repeat testing should be performed at 2-to 4-week intervals for the next 2 months, then once a month for the following 3 months. After 6 months, testing three to four times a year is usually sufficient.
Nonsteroidal analgesics should not be used with high dosages of naltrexone (>50 mg/d), as concurrent use may increase the risk of hepatic transaminase elevation.21
Mood stabilizers Responses to lithium and valproate have been described in two case reports of patients with kleptomania.14,15 In the case of valproate, the effective dosage was 2,000 mg/d, whereas lithium reduced stealing urges at a serum level of 0.5 mEq/L.
Although it would be premature to recommend the use of mood stabilizers, their possible benefit may be related to their efficacy in bipolar disorder treatment and the existence of features (e.g., impulsivity) shared by kleptomania and bipolar disorder.
Atypical antipsychotics Although there is no evidence that atypical antipsychotics are useful in kleptomania, augmenting an SSRI with an atypical neuroleptic may be beneficial. Atypical antipsychotics have been explored as augmenting agents in the treatment of nonpsychotic disorders and behaviors, including pathologic gambling and obsessive-compulsive disorder.
The role of psychotherapy
Cognitive-behavioral therapy Based on the evidence of its effectiveness in treating pathologic gambling, CBT may hold promise as monotherapy for mild cases of kleptomania.
Combination therapy Combined pharmacologic and behavioral therapy may be the optimal treatment strategy for kleptomania. In our experience, patients who respond only partially or fail to respond to pharmacotherapy alone are more likely to find relief with a combination of drug and cognitive-behavioral therapies.
- Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, N New Horizon Press, 1998.
- Shoplifters Alternative http://www.shoplifters.org
- Impulse Control Disorders Clinic, University of Minnesota http://www.med.umn.edu/psychiatry/research/impulse.htm
Drug brand names
- Citalopram • Celexa
- Fluvoxamine • Luvox
- Imipramine • Tofranil
- Naltrexone • Revia
- Nortriptyline • Aventyl, Pamelor
- Paroxetine • Paxil
- Quetiapine • Seroquel
- Valproic acid • Depakote
Disclosure
The authors report no affiliation or financial arrangement with any of the companies whose products are mentioned in this article.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.
1. Grant JE, Kim SW. Clinical characteristics and associated psychopathology in 22 cases of kleptomania. Comp Psychiatry (in press).
2. McElroy SL, Pope HG, Hudson JI, Keck PE, White KL. Kleptomania: a report of 20 cases. Am J Psychiatry 1991;148:652-7.
3. Presta S, Marazziti D, Dell’Osso L, et al. Kleptomania: clinical features and comorbidity in an Italian sample. Comp Psychiatry 2002;43:7-12.
4. McElroy SL, Keck PE, Phillips KA. Kleptomania, compulsive buying, and binge-eating disorder. J Clin Psychiatry 1995;56:14-26.
5. McElroy SL, Hudson JI, Pope HG, Keck PE. Kleptomania: clinical characteristics and associated psychopathology. Psychol Med 1991;21:93-108.
6. Goldman MJ. Kleptomania: an overview. Psychiatric Ann 1992;22:68-71.
7. American Psychiatric Association Committee on Nomenclature and Statistics Diagnostic and statistical manual of mental disorders (4th ed, text rev). Washington, DC: American Psychiatric Association, 2000.
8. Krahn DD, Nairn K, Gosnell BA, Drewnowski A. Stealing in eating disordered patients. J Clin Psychiatry 1991;52:112-5.
9. Goldman MJ. Kleptomania: making sense of the nonsensical. Am J Psychiatry 1991;148:986-96.
10. Goldman MJ. Kleptomania: the compulsion to steal—what can be done? Far Hills, NJ: New Horizon Press, 1998.
11. Grant JE, Kim SW. An open-label study of naltrexone in the treatment of kleptomania. J Clin Psychiatry 2002;63:349-56.
12. Chong SA, Low BL. Treatment of kleptomania with fluvoxamine. Acta Psychiatr Scand 1996;93:314-5.
13. Kraus JE. Treatment of kleptomania with paroxetine. J Clin Psychiatry 1999;60:793.-
14. Burstein A. Fluoxetine lithium treatment for kleptomania. J Clin Psychiatry 1992;53:28-9.
15. Kmetz GF, McElroy SL, Collins DJ. Response of kleptomania and mixed mania to valproate. Am J Psychiatry 1997;154:580-1.
16. Lepkifker E, Dannon PN, Ziv R, Iancu I, Horesh N, Kotler M. The treatment of kleptomania with serotonin reuptake inhibitors. Clin Neuropharmacol 1999;22:40-3.
17. Durst R, Katz G, Knobler HY. Buspirone augmentation of fluvoxamine in the treatment of kleptomania. J Nerv Ment Dis 1997;185:586-8.
18. Kim SW. Opioid antagonists in the treatment of impulse-control disorders. J Clin Psychiatry 1998;59:159-64.
19. Grant JE, Kim SW. Pharmacotherapy of pathological gambling. Psychiatric Ann 2002;32:186-91.
20. Grant JE, Kim SW. Adolescent kleptomania treated with naltrexone: a case report. Eur Child Adolescent Psychiatry 2002;11:92-5.
21. Kim SW, Grant JE, et al. A preliminary report on a possible naltrexone and nonsteroidal analgesics interaction. J Clin Psychopharmacol 2001;21:632-4.