User login
Preventing recurrent staphylococcal skin and soft tissue infection
A frequent referral to our pediatric infectious disease outpatient program at Boston Medical Center is the child with recurrent skin and soft tissue infection. Most often, the child is an infant, toddler, or adolescent; the child is otherwise well but has had two or three prior episodes of skin infection; the infections are typically peri-inguinal including the buttocks, but may involve the face, back, thighs, or scalp. The families are often frustrated and hoping for a solution. Are there effective strategies for reducing recurrences?
Several recent studies provide insights and can be helpful in forming an evidence-based approach that offers modest benefit for reducing the risk of recurrence. Most recently, Kaplan et al. (Clin. Inf. Dis. 2014;58:679-82) reported on a clinical trial of sodium hypochlorite bleach baths combined with hygienic measures (frequent hand washing with soap, cutting fingernails short, using towels or washcloths and clothing without sharing, and daily bathing or showering), compared with hygienic measures alone. The treatment group received twice-weekly hypochlorite baths with 5 mL household bleach (Clorox-Regular 6.0% hypochlorite) per gallon of bath water, followed by moisturizer. Most children were colonized with methicillin-resistant Staphylococcus aureus (MRSA)(approximately 70%) or methicillin-susceptible S. aureus (MSSA)(approximately 30%). In the 12-month follow-up, 20% of children had recurrent skin or soft tissue infection (SSTI). Risk factors for recurrence were young age (<6 years) and burden of colonization (number of colonized sites). A small, nonstatistically significant benefit was observed in the treatment group with a 17% incidence of SSTI, compared with 20.9% in controls (P = 0.15). The authors concluded a bleach bath plus hygiene measures was associated with about a 20% nonstatistically significant decrease in recurrent community-acquired SSTI. No adverse effects of bleach baths were identified.
A second open-label, randomized study by Fritz et al. (Clin. Inf. Dis. 2012;54:743-51) evaluated the value of individual decolonization, compared with household decolonization, in children 6 months through 20 years of age with prior community-acquired SSTI. Cases were randomized to individual decolonization regimens (hygiene, 2% mupirocin for 5 days and 4% chlorhexidine daily body washes) or to household decolonization. Staphylococcal colonization was evaluated at 1, 3, 6, and 12 months. No differences in the rate of eradication of S. aureus were observed between the two strategies, except at 3 months where a greater proportion of children randomized to household decolonization were culture negative. Despite the lack of impact on colonization, SSTI documented by a physician was less common in children where decolonization was householdwide. After 12 months, 36% of children in the household decolonization sites had recurrent SSTI, compared with 55% in the individual decolonization stratum (P = .03). The authors concluded that household decolonization reduces SSTI in both the individual and household contacts.
Another approach to decolonization has been the use of oral antibiotics in combination with mupirocin and hexachloradine. Although data are limited, Miller et al. (Antimicrob. Agents Chemother. 2012;56:1084-6) reported on a small cohort of 31 prospectively evaluated patients with recurrent community-acquired MRSA skin infections. Individuals received nasal mupirocin, topical hexachlorophene body wash, and an oral antibiotic based on susceptibility testing (doxycycline, minocycline, or trimethoprim-sulfamethoxazole). In the 6 months prior to enrollment, the mean rate of SSTI was three infections per person (range, 2-30). The mean number of MRSA infections after the intervention decreased significantly from 0.84 infections per month to 0.03 infections per month during the 5.2-month follow-up. In general, the regimens were well tolerated with minor gastrointestinal complaints. The authors concluded that the combination of systemic and topical antimicrobials was associated with subsequent decreases in community-acquired MRSA SSTI; however, they acknowledged that without a control group, they were unable to be certain that the decrease was due to the prescribed regimen.
Our current approach for children referred with recurrent SSTI is household decolonization with nasal mupirocin and daily hexachloradine baths or showers or hypochlorite baths. The mupirocin is prescribed for 5-10 days; the hexachloradine/hypochlorite baths, for several months. We also stress the need for hygiene, including washing towels and linens in hot water, and cleaning surfaces and items such as remote controls with hypochlorite solutions. Although the value of environmental decontamination is unknown, studies by Uhlemann et al. (PLOS ONE 2011;6: e22407) demonstrated excess contamination of household surfaces in homes of SSTI cases. If recurrences continue, the addition of an antimicrobial agent is considered. We reserve doxycycline for children over 8 years of age and prescribe trimethoprim-sulfamethoxazole for those younger than 8 years. We also will ask about pets although we are aware of only anecdotal reports where treating the family dog or cat has aborted recurrent disease in the patients.
In summary, recurrent SSTI is common, especially among young children. The burden of colonization appears related to both the risk for recurrent disease and the risk for transmission within the household. Reducing colonization is valuable for decreasing the incidence of recurrent SSTI both for the individual as well as the household members. The current strategies demonstrate modest success, but as many as 30%-40% of patients will continue to have recurrent SSTI. Education about the early signs of infection, early evaluation of SSTI, and appropriate management (topical treatment, incision and drainage, or systemic antibiotics) are successful strategies for limiting progression to invasive staphylococcal disease.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Yildirim is a fellow in pediatric infectious disease and an epidemiologist, at Boston Medical Center. To comment, e-mail Dr. Pelton and Dr. Yildirim at [email protected].
A frequent referral to our pediatric infectious disease outpatient program at Boston Medical Center is the child with recurrent skin and soft tissue infection. Most often, the child is an infant, toddler, or adolescent; the child is otherwise well but has had two or three prior episodes of skin infection; the infections are typically peri-inguinal including the buttocks, but may involve the face, back, thighs, or scalp. The families are often frustrated and hoping for a solution. Are there effective strategies for reducing recurrences?
Several recent studies provide insights and can be helpful in forming an evidence-based approach that offers modest benefit for reducing the risk of recurrence. Most recently, Kaplan et al. (Clin. Inf. Dis. 2014;58:679-82) reported on a clinical trial of sodium hypochlorite bleach baths combined with hygienic measures (frequent hand washing with soap, cutting fingernails short, using towels or washcloths and clothing without sharing, and daily bathing or showering), compared with hygienic measures alone. The treatment group received twice-weekly hypochlorite baths with 5 mL household bleach (Clorox-Regular 6.0% hypochlorite) per gallon of bath water, followed by moisturizer. Most children were colonized with methicillin-resistant Staphylococcus aureus (MRSA)(approximately 70%) or methicillin-susceptible S. aureus (MSSA)(approximately 30%). In the 12-month follow-up, 20% of children had recurrent skin or soft tissue infection (SSTI). Risk factors for recurrence were young age (<6 years) and burden of colonization (number of colonized sites). A small, nonstatistically significant benefit was observed in the treatment group with a 17% incidence of SSTI, compared with 20.9% in controls (P = 0.15). The authors concluded a bleach bath plus hygiene measures was associated with about a 20% nonstatistically significant decrease in recurrent community-acquired SSTI. No adverse effects of bleach baths were identified.
A second open-label, randomized study by Fritz et al. (Clin. Inf. Dis. 2012;54:743-51) evaluated the value of individual decolonization, compared with household decolonization, in children 6 months through 20 years of age with prior community-acquired SSTI. Cases were randomized to individual decolonization regimens (hygiene, 2% mupirocin for 5 days and 4% chlorhexidine daily body washes) or to household decolonization. Staphylococcal colonization was evaluated at 1, 3, 6, and 12 months. No differences in the rate of eradication of S. aureus were observed between the two strategies, except at 3 months where a greater proportion of children randomized to household decolonization were culture negative. Despite the lack of impact on colonization, SSTI documented by a physician was less common in children where decolonization was householdwide. After 12 months, 36% of children in the household decolonization sites had recurrent SSTI, compared with 55% in the individual decolonization stratum (P = .03). The authors concluded that household decolonization reduces SSTI in both the individual and household contacts.
Another approach to decolonization has been the use of oral antibiotics in combination with mupirocin and hexachloradine. Although data are limited, Miller et al. (Antimicrob. Agents Chemother. 2012;56:1084-6) reported on a small cohort of 31 prospectively evaluated patients with recurrent community-acquired MRSA skin infections. Individuals received nasal mupirocin, topical hexachlorophene body wash, and an oral antibiotic based on susceptibility testing (doxycycline, minocycline, or trimethoprim-sulfamethoxazole). In the 6 months prior to enrollment, the mean rate of SSTI was three infections per person (range, 2-30). The mean number of MRSA infections after the intervention decreased significantly from 0.84 infections per month to 0.03 infections per month during the 5.2-month follow-up. In general, the regimens were well tolerated with minor gastrointestinal complaints. The authors concluded that the combination of systemic and topical antimicrobials was associated with subsequent decreases in community-acquired MRSA SSTI; however, they acknowledged that without a control group, they were unable to be certain that the decrease was due to the prescribed regimen.
Our current approach for children referred with recurrent SSTI is household decolonization with nasal mupirocin and daily hexachloradine baths or showers or hypochlorite baths. The mupirocin is prescribed for 5-10 days; the hexachloradine/hypochlorite baths, for several months. We also stress the need for hygiene, including washing towels and linens in hot water, and cleaning surfaces and items such as remote controls with hypochlorite solutions. Although the value of environmental decontamination is unknown, studies by Uhlemann et al. (PLOS ONE 2011;6: e22407) demonstrated excess contamination of household surfaces in homes of SSTI cases. If recurrences continue, the addition of an antimicrobial agent is considered. We reserve doxycycline for children over 8 years of age and prescribe trimethoprim-sulfamethoxazole for those younger than 8 years. We also will ask about pets although we are aware of only anecdotal reports where treating the family dog or cat has aborted recurrent disease in the patients.
In summary, recurrent SSTI is common, especially among young children. The burden of colonization appears related to both the risk for recurrent disease and the risk for transmission within the household. Reducing colonization is valuable for decreasing the incidence of recurrent SSTI both for the individual as well as the household members. The current strategies demonstrate modest success, but as many as 30%-40% of patients will continue to have recurrent SSTI. Education about the early signs of infection, early evaluation of SSTI, and appropriate management (topical treatment, incision and drainage, or systemic antibiotics) are successful strategies for limiting progression to invasive staphylococcal disease.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Yildirim is a fellow in pediatric infectious disease and an epidemiologist, at Boston Medical Center. To comment, e-mail Dr. Pelton and Dr. Yildirim at [email protected].
A frequent referral to our pediatric infectious disease outpatient program at Boston Medical Center is the child with recurrent skin and soft tissue infection. Most often, the child is an infant, toddler, or adolescent; the child is otherwise well but has had two or three prior episodes of skin infection; the infections are typically peri-inguinal including the buttocks, but may involve the face, back, thighs, or scalp. The families are often frustrated and hoping for a solution. Are there effective strategies for reducing recurrences?
Several recent studies provide insights and can be helpful in forming an evidence-based approach that offers modest benefit for reducing the risk of recurrence. Most recently, Kaplan et al. (Clin. Inf. Dis. 2014;58:679-82) reported on a clinical trial of sodium hypochlorite bleach baths combined with hygienic measures (frequent hand washing with soap, cutting fingernails short, using towels or washcloths and clothing without sharing, and daily bathing or showering), compared with hygienic measures alone. The treatment group received twice-weekly hypochlorite baths with 5 mL household bleach (Clorox-Regular 6.0% hypochlorite) per gallon of bath water, followed by moisturizer. Most children were colonized with methicillin-resistant Staphylococcus aureus (MRSA)(approximately 70%) or methicillin-susceptible S. aureus (MSSA)(approximately 30%). In the 12-month follow-up, 20% of children had recurrent skin or soft tissue infection (SSTI). Risk factors for recurrence were young age (<6 years) and burden of colonization (number of colonized sites). A small, nonstatistically significant benefit was observed in the treatment group with a 17% incidence of SSTI, compared with 20.9% in controls (P = 0.15). The authors concluded a bleach bath plus hygiene measures was associated with about a 20% nonstatistically significant decrease in recurrent community-acquired SSTI. No adverse effects of bleach baths were identified.
A second open-label, randomized study by Fritz et al. (Clin. Inf. Dis. 2012;54:743-51) evaluated the value of individual decolonization, compared with household decolonization, in children 6 months through 20 years of age with prior community-acquired SSTI. Cases were randomized to individual decolonization regimens (hygiene, 2% mupirocin for 5 days and 4% chlorhexidine daily body washes) or to household decolonization. Staphylococcal colonization was evaluated at 1, 3, 6, and 12 months. No differences in the rate of eradication of S. aureus were observed between the two strategies, except at 3 months where a greater proportion of children randomized to household decolonization were culture negative. Despite the lack of impact on colonization, SSTI documented by a physician was less common in children where decolonization was householdwide. After 12 months, 36% of children in the household decolonization sites had recurrent SSTI, compared with 55% in the individual decolonization stratum (P = .03). The authors concluded that household decolonization reduces SSTI in both the individual and household contacts.
Another approach to decolonization has been the use of oral antibiotics in combination with mupirocin and hexachloradine. Although data are limited, Miller et al. (Antimicrob. Agents Chemother. 2012;56:1084-6) reported on a small cohort of 31 prospectively evaluated patients with recurrent community-acquired MRSA skin infections. Individuals received nasal mupirocin, topical hexachlorophene body wash, and an oral antibiotic based on susceptibility testing (doxycycline, minocycline, or trimethoprim-sulfamethoxazole). In the 6 months prior to enrollment, the mean rate of SSTI was three infections per person (range, 2-30). The mean number of MRSA infections after the intervention decreased significantly from 0.84 infections per month to 0.03 infections per month during the 5.2-month follow-up. In general, the regimens were well tolerated with minor gastrointestinal complaints. The authors concluded that the combination of systemic and topical antimicrobials was associated with subsequent decreases in community-acquired MRSA SSTI; however, they acknowledged that without a control group, they were unable to be certain that the decrease was due to the prescribed regimen.
Our current approach for children referred with recurrent SSTI is household decolonization with nasal mupirocin and daily hexachloradine baths or showers or hypochlorite baths. The mupirocin is prescribed for 5-10 days; the hexachloradine/hypochlorite baths, for several months. We also stress the need for hygiene, including washing towels and linens in hot water, and cleaning surfaces and items such as remote controls with hypochlorite solutions. Although the value of environmental decontamination is unknown, studies by Uhlemann et al. (PLOS ONE 2011;6: e22407) demonstrated excess contamination of household surfaces in homes of SSTI cases. If recurrences continue, the addition of an antimicrobial agent is considered. We reserve doxycycline for children over 8 years of age and prescribe trimethoprim-sulfamethoxazole for those younger than 8 years. We also will ask about pets although we are aware of only anecdotal reports where treating the family dog or cat has aborted recurrent disease in the patients.
In summary, recurrent SSTI is common, especially among young children. The burden of colonization appears related to both the risk for recurrent disease and the risk for transmission within the household. Reducing colonization is valuable for decreasing the incidence of recurrent SSTI both for the individual as well as the household members. The current strategies demonstrate modest success, but as many as 30%-40% of patients will continue to have recurrent SSTI. Education about the early signs of infection, early evaluation of SSTI, and appropriate management (topical treatment, incision and drainage, or systemic antibiotics) are successful strategies for limiting progression to invasive staphylococcal disease.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Yildirim is a fellow in pediatric infectious disease and an epidemiologist, at Boston Medical Center. To comment, e-mail Dr. Pelton and Dr. Yildirim at [email protected].
ID CONSULT: Influenza virus and pneumococci dance together
Most practitioners know that the flu vaccine has been proven to reduce the frequency of middle ear infections, sinusitis, and pneumonia. However, how that happens is not as clear. My group has been studying the details of the interaction between flu virus and pneumococci to unravel the steps in the dance between the flu virus and the pneumococcus in the nasopharynx that results in significant respiratory diseases. Pneumococci live in the posterior part of the nose and upper pharynx as commensal bacteria in all of us, harmlessly present in relatively low numbers. The bacteria are so common that studies to detect pneumococci in the nasopharynx discover their presence in up to 80% of infants and young children, and about 20% of adults at any one time. The bacteria are harmless in patients that have a competent immune system unless an intercurrent viral upper respiratory infection (URI) occurs.
The trigger in pathogenesis of pneumococcal infections is a viral URI, and particularly influenza infection. The combination of pneumococci and flu in the nose can cause compromise in all four aspects of host defense: 1) structural change, 2) physiologic change, 3) innate immunity change, and 4) adaptive immunity change. Structural change is swelling of the nasal passageways, Eustachian tube, osteomeatal sinus pathway, and tracheobronchial tree. Physiologic change is increased mucus production and reduced cilia beat, resulting in stasis of thickened mucus in the respiratory tree. Thus the stage is set for compromise in the immune response.
Innate immunity basically translates to the response of neutrophils, macrophages, and lymphocytes that are resident in the respiratory pathways or migrate there in response to signals from the site of infection that a problem is brewing. To start the process of innate immunity, chemicals are released from resident epithelial cells, lymphocytes, and neutrophils/macrophages. The chemicals are called cytokines and chemokines. The viruses enter the epithelial cells of the nasopharynx and tracheobronchial tree, and leave a change on the surface of the epithelial cells that alerts lymphocytes to kill and destroy those cells harboring virus. Neutrophils and macrophages ingest the bacteria by recognizing surface proteins on the bacteria that are foreign. Sometimes that is all that is needed, and the host clears the infection. But sometimes the innate response is not enough.
The innate response is good and bad. The bad part is that the release of the cytokines and chemokines and the migration of immune cells to the site of infection results in the release of even more cytokines and chemokines that cause increased inflammation. Microbes love inflammation. The inflammation caused by the virus, such as flu virus, creates a very favorable environment for the pneumococci. So the pneumococci start to reproduce in abundance. Then when the secretions of the nose are swept into the Eustachian tube and middle ear or the sinus drainage pathways and then to the sinuses or into the trachea and bronchi and then the lungs, we see the clinical manifestations of acute otitis media, sinusitis, or pneumonia. The innate response failed.
The adaptive response – as the word implies – is when the immune cells recognize and adapt to the presence of foreign microbes by recognizing their presence, migrating to lymph nodes and spleen, communicating with each other, and consequently multiplying into great numbers. The interaction between the immune cells – T cells and B cells – in the lymph node and migration back to the site of infection takes a few days to occur (3-5 days) if the host has prior immunity from prior infections or vaccination. If there is no prior immunity and no vaccination, then it takes 10-14 days for the adaptive immunity response to kick in and clear the infection. During that extra time, the pneumococci are gaining in numbers, causing more inflammation, and we see those clinical signs of fever, redness, and swelling at the site of infection, and pain.
So influenza can cause all of the events above by itself, but when the virus dances with the pneumococci, and the pneumococci benefit from the partnership, that is the most frequent cause of acute otitis media, sinusitis, and pneumonia. And all of that could have been prevented in most of our patients if they only got their annual flu vaccine.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. The study was supported by a National Institutes of Health grant. Dr. Pichichero said he had no relevant financial disclosures. Email him at [email protected].
Most practitioners know that the flu vaccine has been proven to reduce the frequency of middle ear infections, sinusitis, and pneumonia. However, how that happens is not as clear. My group has been studying the details of the interaction between flu virus and pneumococci to unravel the steps in the dance between the flu virus and the pneumococcus in the nasopharynx that results in significant respiratory diseases. Pneumococci live in the posterior part of the nose and upper pharynx as commensal bacteria in all of us, harmlessly present in relatively low numbers. The bacteria are so common that studies to detect pneumococci in the nasopharynx discover their presence in up to 80% of infants and young children, and about 20% of adults at any one time. The bacteria are harmless in patients that have a competent immune system unless an intercurrent viral upper respiratory infection (URI) occurs.
The trigger in pathogenesis of pneumococcal infections is a viral URI, and particularly influenza infection. The combination of pneumococci and flu in the nose can cause compromise in all four aspects of host defense: 1) structural change, 2) physiologic change, 3) innate immunity change, and 4) adaptive immunity change. Structural change is swelling of the nasal passageways, Eustachian tube, osteomeatal sinus pathway, and tracheobronchial tree. Physiologic change is increased mucus production and reduced cilia beat, resulting in stasis of thickened mucus in the respiratory tree. Thus the stage is set for compromise in the immune response.
Innate immunity basically translates to the response of neutrophils, macrophages, and lymphocytes that are resident in the respiratory pathways or migrate there in response to signals from the site of infection that a problem is brewing. To start the process of innate immunity, chemicals are released from resident epithelial cells, lymphocytes, and neutrophils/macrophages. The chemicals are called cytokines and chemokines. The viruses enter the epithelial cells of the nasopharynx and tracheobronchial tree, and leave a change on the surface of the epithelial cells that alerts lymphocytes to kill and destroy those cells harboring virus. Neutrophils and macrophages ingest the bacteria by recognizing surface proteins on the bacteria that are foreign. Sometimes that is all that is needed, and the host clears the infection. But sometimes the innate response is not enough.
The innate response is good and bad. The bad part is that the release of the cytokines and chemokines and the migration of immune cells to the site of infection results in the release of even more cytokines and chemokines that cause increased inflammation. Microbes love inflammation. The inflammation caused by the virus, such as flu virus, creates a very favorable environment for the pneumococci. So the pneumococci start to reproduce in abundance. Then when the secretions of the nose are swept into the Eustachian tube and middle ear or the sinus drainage pathways and then to the sinuses or into the trachea and bronchi and then the lungs, we see the clinical manifestations of acute otitis media, sinusitis, or pneumonia. The innate response failed.
The adaptive response – as the word implies – is when the immune cells recognize and adapt to the presence of foreign microbes by recognizing their presence, migrating to lymph nodes and spleen, communicating with each other, and consequently multiplying into great numbers. The interaction between the immune cells – T cells and B cells – in the lymph node and migration back to the site of infection takes a few days to occur (3-5 days) if the host has prior immunity from prior infections or vaccination. If there is no prior immunity and no vaccination, then it takes 10-14 days for the adaptive immunity response to kick in and clear the infection. During that extra time, the pneumococci are gaining in numbers, causing more inflammation, and we see those clinical signs of fever, redness, and swelling at the site of infection, and pain.
So influenza can cause all of the events above by itself, but when the virus dances with the pneumococci, and the pneumococci benefit from the partnership, that is the most frequent cause of acute otitis media, sinusitis, and pneumonia. And all of that could have been prevented in most of our patients if they only got their annual flu vaccine.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. The study was supported by a National Institutes of Health grant. Dr. Pichichero said he had no relevant financial disclosures. Email him at [email protected].
Most practitioners know that the flu vaccine has been proven to reduce the frequency of middle ear infections, sinusitis, and pneumonia. However, how that happens is not as clear. My group has been studying the details of the interaction between flu virus and pneumococci to unravel the steps in the dance between the flu virus and the pneumococcus in the nasopharynx that results in significant respiratory diseases. Pneumococci live in the posterior part of the nose and upper pharynx as commensal bacteria in all of us, harmlessly present in relatively low numbers. The bacteria are so common that studies to detect pneumococci in the nasopharynx discover their presence in up to 80% of infants and young children, and about 20% of adults at any one time. The bacteria are harmless in patients that have a competent immune system unless an intercurrent viral upper respiratory infection (URI) occurs.
The trigger in pathogenesis of pneumococcal infections is a viral URI, and particularly influenza infection. The combination of pneumococci and flu in the nose can cause compromise in all four aspects of host defense: 1) structural change, 2) physiologic change, 3) innate immunity change, and 4) adaptive immunity change. Structural change is swelling of the nasal passageways, Eustachian tube, osteomeatal sinus pathway, and tracheobronchial tree. Physiologic change is increased mucus production and reduced cilia beat, resulting in stasis of thickened mucus in the respiratory tree. Thus the stage is set for compromise in the immune response.
Innate immunity basically translates to the response of neutrophils, macrophages, and lymphocytes that are resident in the respiratory pathways or migrate there in response to signals from the site of infection that a problem is brewing. To start the process of innate immunity, chemicals are released from resident epithelial cells, lymphocytes, and neutrophils/macrophages. The chemicals are called cytokines and chemokines. The viruses enter the epithelial cells of the nasopharynx and tracheobronchial tree, and leave a change on the surface of the epithelial cells that alerts lymphocytes to kill and destroy those cells harboring virus. Neutrophils and macrophages ingest the bacteria by recognizing surface proteins on the bacteria that are foreign. Sometimes that is all that is needed, and the host clears the infection. But sometimes the innate response is not enough.
The innate response is good and bad. The bad part is that the release of the cytokines and chemokines and the migration of immune cells to the site of infection results in the release of even more cytokines and chemokines that cause increased inflammation. Microbes love inflammation. The inflammation caused by the virus, such as flu virus, creates a very favorable environment for the pneumococci. So the pneumococci start to reproduce in abundance. Then when the secretions of the nose are swept into the Eustachian tube and middle ear or the sinus drainage pathways and then to the sinuses or into the trachea and bronchi and then the lungs, we see the clinical manifestations of acute otitis media, sinusitis, or pneumonia. The innate response failed.
The adaptive response – as the word implies – is when the immune cells recognize and adapt to the presence of foreign microbes by recognizing their presence, migrating to lymph nodes and spleen, communicating with each other, and consequently multiplying into great numbers. The interaction between the immune cells – T cells and B cells – in the lymph node and migration back to the site of infection takes a few days to occur (3-5 days) if the host has prior immunity from prior infections or vaccination. If there is no prior immunity and no vaccination, then it takes 10-14 days for the adaptive immunity response to kick in and clear the infection. During that extra time, the pneumococci are gaining in numbers, causing more inflammation, and we see those clinical signs of fever, redness, and swelling at the site of infection, and pain.
So influenza can cause all of the events above by itself, but when the virus dances with the pneumococci, and the pneumococci benefit from the partnership, that is the most frequent cause of acute otitis media, sinusitis, and pneumonia. And all of that could have been prevented in most of our patients if they only got their annual flu vaccine.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. The study was supported by a National Institutes of Health grant. Dr. Pichichero said he had no relevant financial disclosures. Email him at [email protected].
Hot topics in vaccines
I recently attended the International Interscience Conference of Infectious Diseases and Vaccines, and I would like to share some of the presentations from the session entitled “Hot Topics in Vaccines.”
CNS complications of varicella-zoster virus infection
Dr. Michelle Science of the Hospital for Sick Children, Toronto, and her associates described the spectrum of CNS complications of varicella-zoster virus (VZV) in children admitted to the hospital during 1999-2012 (J. Pediatr. 2014;165:779-85). Clinical syndromes included 26 cases of acute cerebellar ataxia, 17 of encephalitis, 16 isolated seizures, 10 strokes, 10 cases of meningitis, 2 cases of Guillain-Barré syndrome, 2 cases of acute disseminated encephalomyelitis, and 1 case of Ramsay Hunt syndrome. In children with acute nonstroke complications, neurologic symptoms occurred a median 5 days after the onset of rash, but neurologic symptoms predated the onset of rash in five cases and in two cases there were no exanthems. Time between rash onset and stroke ranged from 2 to 26 weeks (median 16 weeks). There were three deaths among the 17 (18%) children with encephalitis. Among the 39 children with follow-up at 1 year, residual neurologic sequelae occurred in 9 (23%). Only four of the children had received a VZV vaccine. Although an effective vaccine exists, neurologic complications of VZV infection continue to occur.
Timely versus delayed early childhood vaccination and seizures
Dr. Simon J. Hambidge of Denver Health, Colorado, and his associates studied a cohort of 323,247 U.S. children from the Vaccine Safety Datalink born during 2004-2008 for an association between the timing of childhood vaccination and the first occurrence of seizures (Pediatrics 2014;133(6):e1492-9). In the first year, there was no association between the timing of infant vaccination and postvaccination seizures. In the second year, the incidence rate ratio for seizures after receiving the first MMR dose at 12-15 months was 2.7, compared with a rate of 6.5 after an MMR dose at 16-23 months; thus there were more seizures when MMR was delayed. The incidence rate ratio for seizures after receiving the first measles-mumps-rubella-varicella vaccine (MMRV) dose at 12-15 months was 4.95, compared with 9.80 after an MMRV dose at 16-23 months. Again, there were more seizures when MMRV was delayed. These findings suggest that on-time vaccination is as safe with regard to seizures as delayed vaccination in year 1, and that delayed vaccination in year 2 is linked to more postvaccination seizures than on-time vaccination with MMR and that risk is doubled with MMRV.
Effective messages in vaccine promotion: a randomized trial
Brendan Nyhan, Ph.D., of Dartmouth College, Hanover, N.H., and his associates tested the efficacy of various informational messages tailored to reduce misperceptions about vaccines and increase MMR vaccination rates (Pediatrics 2014;133:e835-42). Nearly 1,800 parents were randomly assigned to receive one of four interventions: information explaining the lack of evidence that MMR causes autism from the Centers for Disease Control and Prevention; information about the danger of the diseases prevented by MMR from the Vaccine Information Statement; photos of children with diseases prevented by the MMR vaccine; a dramatic narrative about an infant who almost died of measles from a CDC fact sheet. In addition there was a control group. None of the four interventions increased parents’ intention to vaccinate another child if they had one in the future. Although refuting claims of an MMR/autism link did reduce misperceptions that vaccines cause autism, it decreased intent to vaccinate among parents who had the least favorable attitudes toward vaccines. Also, photos of sick children increased belief in an association between vaccines and autism, and the dramatic narrative about an infant in danger increased belief in serious vaccine side effects. Attempts to rectify misperceptions about vaccines may be counterproductive in some populations, so public health communications about vaccines should be tested before being widely disseminated.
Silent reintroduction of wild-type poliovirus to Israel, 2013
Dr. E. Kaliner of the Israeli Ministry of Health, Jerusalem, and associates, reported that Israel has been certified as polio-free by the World Health Organization for decades and its routine immunization schedule, like the United States, consists of inactivated poliovirus vaccine only (Euro. Surveill. 2014;19:20703). At the end of May 2013, the Israeli Ministry of Health confirmed the reintroduction of wild-type poliovirus 1 into the country. Documented ongoing human-to-human transmission required a thorough risk assessment followed by a supplemental immunization campaign using oral polio vaccine.
Trends in otitis media–related health care use in the United States, 2001-2011
Dr. Tal Marom of the University of Texas, Galveston, and associates studied the trend in otitis media–related health care use in the United States during the pneumococcal conjugate vaccine (PCV) era in 2001-2011 (JAMA Pediatr. 2014;168:68-75). An analysis of an insurance claims database of a large, nationwide managed health care plan was conducted; 7.82 million children aged 6 years and under had 6.21 million primary otitis media (OM) visits. There was an overall downward trend in OM-related health care use across the 10-year study. Recurrent OM rates (defined as greater than or equal to three OM visits within 6 months) decreased at 0.003 per child-year in 2001-2009 and at 0.018 per child-year in 2010-2011. Prior to the pneumococcal conjugate vaccine (PCV-13), there was a stable rate ratio of 1.38 between OM visit rates. During the transition year 2010, the RR decreased significantly to 1.32, and in 2011 the RR decreased further to 1.01. Mastoiditis rates significantly decreased from 61 per 100,000 child-years in 2008 to 37 per 100,000 child-years in 2011. The ventilating tube insertion rate decreased by 19% from 2010 to 2011. Tympanic membrane perforation/otorrhea rates increased gradually and significantly from 3,721 per 100,000 OM child-years in 2001 to 4,542 per 100,000 OM child-years in 2011; the reasons for this are unclear.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no financial disclosures relevant to this article. To comment, e-mail him at [email protected].
I recently attended the International Interscience Conference of Infectious Diseases and Vaccines, and I would like to share some of the presentations from the session entitled “Hot Topics in Vaccines.”
CNS complications of varicella-zoster virus infection
Dr. Michelle Science of the Hospital for Sick Children, Toronto, and her associates described the spectrum of CNS complications of varicella-zoster virus (VZV) in children admitted to the hospital during 1999-2012 (J. Pediatr. 2014;165:779-85). Clinical syndromes included 26 cases of acute cerebellar ataxia, 17 of encephalitis, 16 isolated seizures, 10 strokes, 10 cases of meningitis, 2 cases of Guillain-Barré syndrome, 2 cases of acute disseminated encephalomyelitis, and 1 case of Ramsay Hunt syndrome. In children with acute nonstroke complications, neurologic symptoms occurred a median 5 days after the onset of rash, but neurologic symptoms predated the onset of rash in five cases and in two cases there were no exanthems. Time between rash onset and stroke ranged from 2 to 26 weeks (median 16 weeks). There were three deaths among the 17 (18%) children with encephalitis. Among the 39 children with follow-up at 1 year, residual neurologic sequelae occurred in 9 (23%). Only four of the children had received a VZV vaccine. Although an effective vaccine exists, neurologic complications of VZV infection continue to occur.
Timely versus delayed early childhood vaccination and seizures
Dr. Simon J. Hambidge of Denver Health, Colorado, and his associates studied a cohort of 323,247 U.S. children from the Vaccine Safety Datalink born during 2004-2008 for an association between the timing of childhood vaccination and the first occurrence of seizures (Pediatrics 2014;133(6):e1492-9). In the first year, there was no association between the timing of infant vaccination and postvaccination seizures. In the second year, the incidence rate ratio for seizures after receiving the first MMR dose at 12-15 months was 2.7, compared with a rate of 6.5 after an MMR dose at 16-23 months; thus there were more seizures when MMR was delayed. The incidence rate ratio for seizures after receiving the first measles-mumps-rubella-varicella vaccine (MMRV) dose at 12-15 months was 4.95, compared with 9.80 after an MMRV dose at 16-23 months. Again, there were more seizures when MMRV was delayed. These findings suggest that on-time vaccination is as safe with regard to seizures as delayed vaccination in year 1, and that delayed vaccination in year 2 is linked to more postvaccination seizures than on-time vaccination with MMR and that risk is doubled with MMRV.
Effective messages in vaccine promotion: a randomized trial
Brendan Nyhan, Ph.D., of Dartmouth College, Hanover, N.H., and his associates tested the efficacy of various informational messages tailored to reduce misperceptions about vaccines and increase MMR vaccination rates (Pediatrics 2014;133:e835-42). Nearly 1,800 parents were randomly assigned to receive one of four interventions: information explaining the lack of evidence that MMR causes autism from the Centers for Disease Control and Prevention; information about the danger of the diseases prevented by MMR from the Vaccine Information Statement; photos of children with diseases prevented by the MMR vaccine; a dramatic narrative about an infant who almost died of measles from a CDC fact sheet. In addition there was a control group. None of the four interventions increased parents’ intention to vaccinate another child if they had one in the future. Although refuting claims of an MMR/autism link did reduce misperceptions that vaccines cause autism, it decreased intent to vaccinate among parents who had the least favorable attitudes toward vaccines. Also, photos of sick children increased belief in an association between vaccines and autism, and the dramatic narrative about an infant in danger increased belief in serious vaccine side effects. Attempts to rectify misperceptions about vaccines may be counterproductive in some populations, so public health communications about vaccines should be tested before being widely disseminated.
Silent reintroduction of wild-type poliovirus to Israel, 2013
Dr. E. Kaliner of the Israeli Ministry of Health, Jerusalem, and associates, reported that Israel has been certified as polio-free by the World Health Organization for decades and its routine immunization schedule, like the United States, consists of inactivated poliovirus vaccine only (Euro. Surveill. 2014;19:20703). At the end of May 2013, the Israeli Ministry of Health confirmed the reintroduction of wild-type poliovirus 1 into the country. Documented ongoing human-to-human transmission required a thorough risk assessment followed by a supplemental immunization campaign using oral polio vaccine.
Trends in otitis media–related health care use in the United States, 2001-2011
Dr. Tal Marom of the University of Texas, Galveston, and associates studied the trend in otitis media–related health care use in the United States during the pneumococcal conjugate vaccine (PCV) era in 2001-2011 (JAMA Pediatr. 2014;168:68-75). An analysis of an insurance claims database of a large, nationwide managed health care plan was conducted; 7.82 million children aged 6 years and under had 6.21 million primary otitis media (OM) visits. There was an overall downward trend in OM-related health care use across the 10-year study. Recurrent OM rates (defined as greater than or equal to three OM visits within 6 months) decreased at 0.003 per child-year in 2001-2009 and at 0.018 per child-year in 2010-2011. Prior to the pneumococcal conjugate vaccine (PCV-13), there was a stable rate ratio of 1.38 between OM visit rates. During the transition year 2010, the RR decreased significantly to 1.32, and in 2011 the RR decreased further to 1.01. Mastoiditis rates significantly decreased from 61 per 100,000 child-years in 2008 to 37 per 100,000 child-years in 2011. The ventilating tube insertion rate decreased by 19% from 2010 to 2011. Tympanic membrane perforation/otorrhea rates increased gradually and significantly from 3,721 per 100,000 OM child-years in 2001 to 4,542 per 100,000 OM child-years in 2011; the reasons for this are unclear.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no financial disclosures relevant to this article. To comment, e-mail him at [email protected].
I recently attended the International Interscience Conference of Infectious Diseases and Vaccines, and I would like to share some of the presentations from the session entitled “Hot Topics in Vaccines.”
CNS complications of varicella-zoster virus infection
Dr. Michelle Science of the Hospital for Sick Children, Toronto, and her associates described the spectrum of CNS complications of varicella-zoster virus (VZV) in children admitted to the hospital during 1999-2012 (J. Pediatr. 2014;165:779-85). Clinical syndromes included 26 cases of acute cerebellar ataxia, 17 of encephalitis, 16 isolated seizures, 10 strokes, 10 cases of meningitis, 2 cases of Guillain-Barré syndrome, 2 cases of acute disseminated encephalomyelitis, and 1 case of Ramsay Hunt syndrome. In children with acute nonstroke complications, neurologic symptoms occurred a median 5 days after the onset of rash, but neurologic symptoms predated the onset of rash in five cases and in two cases there were no exanthems. Time between rash onset and stroke ranged from 2 to 26 weeks (median 16 weeks). There were three deaths among the 17 (18%) children with encephalitis. Among the 39 children with follow-up at 1 year, residual neurologic sequelae occurred in 9 (23%). Only four of the children had received a VZV vaccine. Although an effective vaccine exists, neurologic complications of VZV infection continue to occur.
Timely versus delayed early childhood vaccination and seizures
Dr. Simon J. Hambidge of Denver Health, Colorado, and his associates studied a cohort of 323,247 U.S. children from the Vaccine Safety Datalink born during 2004-2008 for an association between the timing of childhood vaccination and the first occurrence of seizures (Pediatrics 2014;133(6):e1492-9). In the first year, there was no association between the timing of infant vaccination and postvaccination seizures. In the second year, the incidence rate ratio for seizures after receiving the first MMR dose at 12-15 months was 2.7, compared with a rate of 6.5 after an MMR dose at 16-23 months; thus there were more seizures when MMR was delayed. The incidence rate ratio for seizures after receiving the first measles-mumps-rubella-varicella vaccine (MMRV) dose at 12-15 months was 4.95, compared with 9.80 after an MMRV dose at 16-23 months. Again, there were more seizures when MMRV was delayed. These findings suggest that on-time vaccination is as safe with regard to seizures as delayed vaccination in year 1, and that delayed vaccination in year 2 is linked to more postvaccination seizures than on-time vaccination with MMR and that risk is doubled with MMRV.
Effective messages in vaccine promotion: a randomized trial
Brendan Nyhan, Ph.D., of Dartmouth College, Hanover, N.H., and his associates tested the efficacy of various informational messages tailored to reduce misperceptions about vaccines and increase MMR vaccination rates (Pediatrics 2014;133:e835-42). Nearly 1,800 parents were randomly assigned to receive one of four interventions: information explaining the lack of evidence that MMR causes autism from the Centers for Disease Control and Prevention; information about the danger of the diseases prevented by MMR from the Vaccine Information Statement; photos of children with diseases prevented by the MMR vaccine; a dramatic narrative about an infant who almost died of measles from a CDC fact sheet. In addition there was a control group. None of the four interventions increased parents’ intention to vaccinate another child if they had one in the future. Although refuting claims of an MMR/autism link did reduce misperceptions that vaccines cause autism, it decreased intent to vaccinate among parents who had the least favorable attitudes toward vaccines. Also, photos of sick children increased belief in an association between vaccines and autism, and the dramatic narrative about an infant in danger increased belief in serious vaccine side effects. Attempts to rectify misperceptions about vaccines may be counterproductive in some populations, so public health communications about vaccines should be tested before being widely disseminated.
Silent reintroduction of wild-type poliovirus to Israel, 2013
Dr. E. Kaliner of the Israeli Ministry of Health, Jerusalem, and associates, reported that Israel has been certified as polio-free by the World Health Organization for decades and its routine immunization schedule, like the United States, consists of inactivated poliovirus vaccine only (Euro. Surveill. 2014;19:20703). At the end of May 2013, the Israeli Ministry of Health confirmed the reintroduction of wild-type poliovirus 1 into the country. Documented ongoing human-to-human transmission required a thorough risk assessment followed by a supplemental immunization campaign using oral polio vaccine.
Trends in otitis media–related health care use in the United States, 2001-2011
Dr. Tal Marom of the University of Texas, Galveston, and associates studied the trend in otitis media–related health care use in the United States during the pneumococcal conjugate vaccine (PCV) era in 2001-2011 (JAMA Pediatr. 2014;168:68-75). An analysis of an insurance claims database of a large, nationwide managed health care plan was conducted; 7.82 million children aged 6 years and under had 6.21 million primary otitis media (OM) visits. There was an overall downward trend in OM-related health care use across the 10-year study. Recurrent OM rates (defined as greater than or equal to three OM visits within 6 months) decreased at 0.003 per child-year in 2001-2009 and at 0.018 per child-year in 2010-2011. Prior to the pneumococcal conjugate vaccine (PCV-13), there was a stable rate ratio of 1.38 between OM visit rates. During the transition year 2010, the RR decreased significantly to 1.32, and in 2011 the RR decreased further to 1.01. Mastoiditis rates significantly decreased from 61 per 100,000 child-years in 2008 to 37 per 100,000 child-years in 2011. The ventilating tube insertion rate decreased by 19% from 2010 to 2011. Tympanic membrane perforation/otorrhea rates increased gradually and significantly from 3,721 per 100,000 OM child-years in 2001 to 4,542 per 100,000 OM child-years in 2011; the reasons for this are unclear.
Dr. Pichichero, a specialist in pediatric infectious diseases, is director of the Research Institute, Rochester (N.Y.) General Hospital. He is also a pediatrician at Legacy Pediatrics in Rochester. Dr. Pichichero said he had no financial disclosures relevant to this article. To comment, e-mail him at [email protected].
ID Consult: National immunization coverage and measles
August was National Immunization Awareness Month. For most pediatricians, it is also a very busy month as patients prepare for the start of the new school year. So how are we doing?
On August 28, 2013, vaccination coverage of U.S. children aged 19-35 months was published in Morbidity and Mortality Weekly Review (2014; 63:741-8) based on results from the National Information Survey (NIS), which provides national, regional, state, and selected local area vaccination coverage estimates. NIS has monitored vaccination coverage since 1994 for all 50 states and assists in tracking the progress of achieving our national goals. It also can identify problem areas that may require special interventions. Survey data was obtained by a random telephone survey using both landline and cellular phones to households that have children born between January 2010 and May 2012. The verbal interview was followed by a survey mailed to the vaccine provider to confirm the verbal vaccine history.
Highlights
Vaccination coverage of at least 90 %, a goal of Healthy People 2020, was achieved for receipt of one or more dose of MMR (91.9%); three or more doses of hepatitis B vaccine (HepB) (90.8 %); three or more doses of poliovirus vaccine (92.7%) and one or more doses of varicella vaccine (91.2%).
Coverage for the following vaccines failed to meet this goal: four or more doses of diphtheria, tetanus, and pertussis vaccine (DTaP) (83.1%); four or more doses of pneumococcal conjugate vaccine (PCV) (82%); and a full series of Haemophilus influenzae type b (Hib) (82%). Coverage for the remaining vaccines also fell short of their respective targeted goals: two or more doses of hepatitis A vaccine (54.7%; target 85%); rotavirus (72.6%; target 80%); and hepatitis B birth dose (74.2%; target 85%).
Compared with 2012, coverage remained stable for the four vaccines that achieved at least 90% coverage. For those that did not, rotavirus was the only vaccine in 2013 that had an increase (4%) in coverage. Of note, there was an increase in the birth dose of 2.6% for Hep B.
Children living at or below the poverty level had lower vaccination coverage, compared with those living at or above this level for several vaccines, including four or more doses of DTaP; full series of Hib vaccine, four or more doses of PCV, and rotavirus vaccine. Coverage was between 8% and 12.6% points lower for these vaccines.
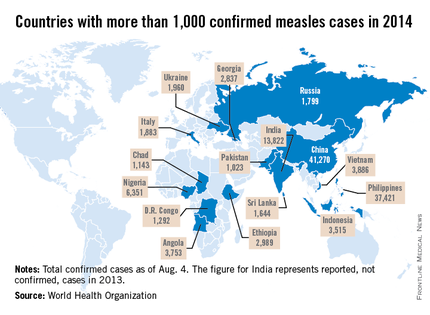
Measles
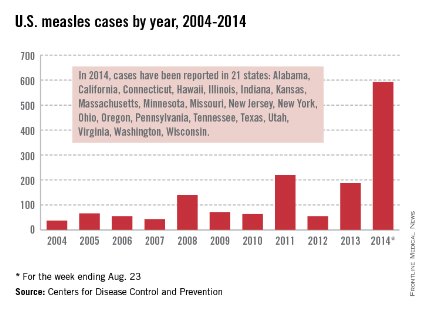
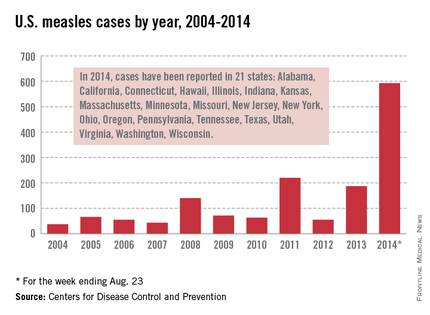
Let’s take a closer look at measles. Nationally, almost 92 % of children received at least one dose of MMR. However, coverage varied by state – an observation unchanged from 2012. New Hampshire had the highest coverage at 96.3% and three states had coverage of only 86% (Colorado, Ohio, and West Virginia). Overall 17 states had immunization rates less than 90%. Additionally, 1 in 12 children did not receive their first dose of MMR on time. Why the concern? In 2013, there were 187 cases of measles including 11 outbreaks. A total of 82% occurred in unvaccinated individuals, and another 9% were unaware of their immunization status.
As of Aug. 25, 2014, there were 595 cases of measles in the United States in 21 states, according to the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases. This is the highest number of cases reported since endemic measles was eliminated in 2000. There were as a result of 18 outbreaks, representing 89% of the reported cases. Cases are occurring even in states where immunization rates are reported to be at least 90% – a reminder that there can be pockets of low or nonimmunizing communities that leave its citizens vulnerable to outbreaks when a highly contagious virus is introduced.
Since endemic measles was eliminated 14 years ago in the United States, many health care providers have never seen a case of measles or may not realize the impact it once had on our public health system. Prior to the initiation of the measles vaccination program in 1963, 3-4 million cases of measles occurred annually in the United States with 400-500 deaths and 48,000 hospitalizations. Approximately another 1,000 individuals were left disabled secondary to measles encephalitis. Once the vaccine was introduced, the incidence of measles declined 98%, according to "Epidemiology and Prevention of Vaccine-Preventable Diseases," 12th ed., second printing. (Washington, D.C: Public Health Foundation, 2012). Between 1989 and 1991, there was a resurgence of measles resulting in approximately 55,000 cases, 11,000 hospitalizations, and 123 deaths. The resurgence was caused primarily by the failure to vaccinate uninsured children at the recommended 12-15 months of age. Children younger than 5 years of age accounted for 45% of all cases. The Vaccines for Children Program was created in 1993 as a direct response to the resurgence of measles. It would ensure that no child would contract a vaccine preventable disease because of inability to pay.
Measles remains endemic in multiple countries worldwide that are travel destinations for many Americans. In 2013, 99% of 159 U.S. cases were import related. An overwhelming majority of infections occurred in unvaccinated individuals. In 2014, this trend continues, with the majority of cases occurring in unvaccinated international travelers who return infected and spread disease to susceptible persons including children in their communities (MMWR 2014:63;496-9). Of the 288 cases reported in by May 23, 2014, 97% were associated with importations from 18 countries.
High immunization coverage must be maintained to prevent and sustain measles elimination in the United States. As a reminder, all children aged 6-11 months should receive one dose of MMR ideally 2 weeks prior to international travel. When the infant is at least 12 months of age, they should receive two additional doses of MMR or MMRV according to the routine immunization schedule. Those children older than 12 months of age should receive two doses of MMR. The second can be administered as soon as 4 weeks after the first dose. It is not uncommon for families to travel internationally and fail to mention it to you. Many have been told their child’s immunizations are up to date, not realizing that international travel may alter that definition. It behooves primary care providers to develop strategies to facilitate discussions regarding sharing international travel plans in a timely manner.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
August was National Immunization Awareness Month. For most pediatricians, it is also a very busy month as patients prepare for the start of the new school year. So how are we doing?
On August 28, 2013, vaccination coverage of U.S. children aged 19-35 months was published in Morbidity and Mortality Weekly Review (2014; 63:741-8) based on results from the National Information Survey (NIS), which provides national, regional, state, and selected local area vaccination coverage estimates. NIS has monitored vaccination coverage since 1994 for all 50 states and assists in tracking the progress of achieving our national goals. It also can identify problem areas that may require special interventions. Survey data was obtained by a random telephone survey using both landline and cellular phones to households that have children born between January 2010 and May 2012. The verbal interview was followed by a survey mailed to the vaccine provider to confirm the verbal vaccine history.

Highlights
Vaccination coverage of at least 90 %, a goal of Healthy People 2020, was achieved for receipt of one or more dose of MMR (91.9%); three or more doses of hepatitis B vaccine (HepB) (90.8 %); three or more doses of poliovirus vaccine (92.7%) and one or more doses of varicella vaccine (91.2%).
Coverage for the following vaccines failed to meet this goal: four or more doses of diphtheria, tetanus, and pertussis vaccine (DTaP) (83.1%); four or more doses of pneumococcal conjugate vaccine (PCV) (82%); and a full series of Haemophilus influenzae type b (Hib) (82%). Coverage for the remaining vaccines also fell short of their respective targeted goals: two or more doses of hepatitis A vaccine (54.7%; target 85%); rotavirus (72.6%; target 80%); and hepatitis B birth dose (74.2%; target 85%).
Compared with 2012, coverage remained stable for the four vaccines that achieved at least 90% coverage. For those that did not, rotavirus was the only vaccine in 2013 that had an increase (4%) in coverage. Of note, there was an increase in the birth dose of 2.6% for Hep B.
Children living at or below the poverty level had lower vaccination coverage, compared with those living at or above this level for several vaccines, including four or more doses of DTaP; full series of Hib vaccine, four or more doses of PCV, and rotavirus vaccine. Coverage was between 8% and 12.6% points lower for these vaccines.

Measles
Let’s take a closer look at measles. Nationally, almost 92 % of children received at least one dose of MMR. However, coverage varied by state – an observation unchanged from 2012. New Hampshire had the highest coverage at 96.3% and three states had coverage of only 86% (Colorado, Ohio, and West Virginia). Overall 17 states had immunization rates less than 90%. Additionally, 1 in 12 children did not receive their first dose of MMR on time. Why the concern? In 2013, there were 187 cases of measles including 11 outbreaks. A total of 82% occurred in unvaccinated individuals, and another 9% were unaware of their immunization status.
As of Aug. 25, 2014, there were 595 cases of measles in the United States in 21 states, according to the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases. This is the highest number of cases reported since endemic measles was eliminated in 2000. There were as a result of 18 outbreaks, representing 89% of the reported cases. Cases are occurring even in states where immunization rates are reported to be at least 90% – a reminder that there can be pockets of low or nonimmunizing communities that leave its citizens vulnerable to outbreaks when a highly contagious virus is introduced.
Since endemic measles was eliminated 14 years ago in the United States, many health care providers have never seen a case of measles or may not realize the impact it once had on our public health system. Prior to the initiation of the measles vaccination program in 1963, 3-4 million cases of measles occurred annually in the United States with 400-500 deaths and 48,000 hospitalizations. Approximately another 1,000 individuals were left disabled secondary to measles encephalitis. Once the vaccine was introduced, the incidence of measles declined 98%, according to "Epidemiology and Prevention of Vaccine-Preventable Diseases," 12th ed., second printing. (Washington, D.C: Public Health Foundation, 2012). Between 1989 and 1991, there was a resurgence of measles resulting in approximately 55,000 cases, 11,000 hospitalizations, and 123 deaths. The resurgence was caused primarily by the failure to vaccinate uninsured children at the recommended 12-15 months of age. Children younger than 5 years of age accounted for 45% of all cases. The Vaccines for Children Program was created in 1993 as a direct response to the resurgence of measles. It would ensure that no child would contract a vaccine preventable disease because of inability to pay.
Measles remains endemic in multiple countries worldwide that are travel destinations for many Americans. In 2013, 99% of 159 U.S. cases were import related. An overwhelming majority of infections occurred in unvaccinated individuals. In 2014, this trend continues, with the majority of cases occurring in unvaccinated international travelers who return infected and spread disease to susceptible persons including children in their communities (MMWR 2014:63;496-9). Of the 288 cases reported in by May 23, 2014, 97% were associated with importations from 18 countries.
High immunization coverage must be maintained to prevent and sustain measles elimination in the United States. As a reminder, all children aged 6-11 months should receive one dose of MMR ideally 2 weeks prior to international travel. When the infant is at least 12 months of age, they should receive two additional doses of MMR or MMRV according to the routine immunization schedule. Those children older than 12 months of age should receive two doses of MMR. The second can be administered as soon as 4 weeks after the first dose. It is not uncommon for families to travel internationally and fail to mention it to you. Many have been told their child’s immunizations are up to date, not realizing that international travel may alter that definition. It behooves primary care providers to develop strategies to facilitate discussions regarding sharing international travel plans in a timely manner.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
August was National Immunization Awareness Month. For most pediatricians, it is also a very busy month as patients prepare for the start of the new school year. So how are we doing?
On August 28, 2013, vaccination coverage of U.S. children aged 19-35 months was published in Morbidity and Mortality Weekly Review (2014; 63:741-8) based on results from the National Information Survey (NIS), which provides national, regional, state, and selected local area vaccination coverage estimates. NIS has monitored vaccination coverage since 1994 for all 50 states and assists in tracking the progress of achieving our national goals. It also can identify problem areas that may require special interventions. Survey data was obtained by a random telephone survey using both landline and cellular phones to households that have children born between January 2010 and May 2012. The verbal interview was followed by a survey mailed to the vaccine provider to confirm the verbal vaccine history.

Highlights
Vaccination coverage of at least 90 %, a goal of Healthy People 2020, was achieved for receipt of one or more dose of MMR (91.9%); three or more doses of hepatitis B vaccine (HepB) (90.8 %); three or more doses of poliovirus vaccine (92.7%) and one or more doses of varicella vaccine (91.2%).
Coverage for the following vaccines failed to meet this goal: four or more doses of diphtheria, tetanus, and pertussis vaccine (DTaP) (83.1%); four or more doses of pneumococcal conjugate vaccine (PCV) (82%); and a full series of Haemophilus influenzae type b (Hib) (82%). Coverage for the remaining vaccines also fell short of their respective targeted goals: two or more doses of hepatitis A vaccine (54.7%; target 85%); rotavirus (72.6%; target 80%); and hepatitis B birth dose (74.2%; target 85%).
Compared with 2012, coverage remained stable for the four vaccines that achieved at least 90% coverage. For those that did not, rotavirus was the only vaccine in 2013 that had an increase (4%) in coverage. Of note, there was an increase in the birth dose of 2.6% for Hep B.
Children living at or below the poverty level had lower vaccination coverage, compared with those living at or above this level for several vaccines, including four or more doses of DTaP; full series of Hib vaccine, four or more doses of PCV, and rotavirus vaccine. Coverage was between 8% and 12.6% points lower for these vaccines.

Measles
Let’s take a closer look at measles. Nationally, almost 92 % of children received at least one dose of MMR. However, coverage varied by state – an observation unchanged from 2012. New Hampshire had the highest coverage at 96.3% and three states had coverage of only 86% (Colorado, Ohio, and West Virginia). Overall 17 states had immunization rates less than 90%. Additionally, 1 in 12 children did not receive their first dose of MMR on time. Why the concern? In 2013, there were 187 cases of measles including 11 outbreaks. A total of 82% occurred in unvaccinated individuals, and another 9% were unaware of their immunization status.
As of Aug. 25, 2014, there were 595 cases of measles in the United States in 21 states, according to the Centers for Disease Control and Prevention’s National Center for Immunization and Respiratory Diseases. This is the highest number of cases reported since endemic measles was eliminated in 2000. There were as a result of 18 outbreaks, representing 89% of the reported cases. Cases are occurring even in states where immunization rates are reported to be at least 90% – a reminder that there can be pockets of low or nonimmunizing communities that leave its citizens vulnerable to outbreaks when a highly contagious virus is introduced.
Since endemic measles was eliminated 14 years ago in the United States, many health care providers have never seen a case of measles or may not realize the impact it once had on our public health system. Prior to the initiation of the measles vaccination program in 1963, 3-4 million cases of measles occurred annually in the United States with 400-500 deaths and 48,000 hospitalizations. Approximately another 1,000 individuals were left disabled secondary to measles encephalitis. Once the vaccine was introduced, the incidence of measles declined 98%, according to "Epidemiology and Prevention of Vaccine-Preventable Diseases," 12th ed., second printing. (Washington, D.C: Public Health Foundation, 2012). Between 1989 and 1991, there was a resurgence of measles resulting in approximately 55,000 cases, 11,000 hospitalizations, and 123 deaths. The resurgence was caused primarily by the failure to vaccinate uninsured children at the recommended 12-15 months of age. Children younger than 5 years of age accounted for 45% of all cases. The Vaccines for Children Program was created in 1993 as a direct response to the resurgence of measles. It would ensure that no child would contract a vaccine preventable disease because of inability to pay.
Measles remains endemic in multiple countries worldwide that are travel destinations for many Americans. In 2013, 99% of 159 U.S. cases were import related. An overwhelming majority of infections occurred in unvaccinated individuals. In 2014, this trend continues, with the majority of cases occurring in unvaccinated international travelers who return infected and spread disease to susceptible persons including children in their communities (MMWR 2014:63;496-9). Of the 288 cases reported in by May 23, 2014, 97% were associated with importations from 18 countries.
High immunization coverage must be maintained to prevent and sustain measles elimination in the United States. As a reminder, all children aged 6-11 months should receive one dose of MMR ideally 2 weeks prior to international travel. When the infant is at least 12 months of age, they should receive two additional doses of MMR or MMRV according to the routine immunization schedule. Those children older than 12 months of age should receive two doses of MMR. The second can be administered as soon as 4 weeks after the first dose. It is not uncommon for families to travel internationally and fail to mention it to you. Many have been told their child’s immunizations are up to date, not realizing that international travel may alter that definition. It behooves primary care providers to develop strategies to facilitate discussions regarding sharing international travel plans in a timely manner.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
ACIP and 2014 flu vaccine
The effectiveness of influenza vaccine is recognized to vary widely from season to season. At least two factors are critical for determining the likelihood that flu vaccine will be successful in preventing illness.
First, the demographics of who is being immunized (primarily age and presence of comorbidity) and second, the "match" between the circulating flu viruses and that year’s flu vaccine. When the flu vaccine is a poor match with circulating viruses, less benefit from flu vaccination will be observed; in years when the "match" between vaccine and circulating virus is good, substantial reduction in influenza respiratory illness in children and adults is observed. Recently, a second influenza B antigen has been added (creating quadrivalent vaccines) to improve the match with influenza B strains that may circulate in the community.
In February 2014, the Centers for Disease Control and Prevention reported midseason vaccine effectiveness estimates (MMWR 2014 Feb 21;63:137-42).
The major circulating virus was influenza A "2009 H1N1" virus and the "match" between vaccine strains and circulating strains was considered good. The CDC’s midseason vaccine effectiveness estimate was 61% for all age groups (95% confidence interval, 52%-68%), reinforcing the value of influenza vaccine for disease prevention in both children and adults. Flu vaccine reduced the risk of seeking medical attention for flulike illness by 60% for both children and adults.
Another factor that may determine the effectiveness of influenza vaccine in children is whether the individual receives live attenuated influenza vaccine (LAIV) or trivalent or quadrivalent inactivated influenza vaccine (IIV). The CDC has been considering the question "should LAIV be recommended preferentially over IIV in healthy children 2-8 years of age?" based on data from a limited number of studies. Canada, United Kingdom, Israel, and Germany have each expressed a preference for LAIV in their recent recommendations. The CDC working group evaluated published studies primarily restricted to those focused on healthy children, those with both LAIV and IIV cohorts, those studying the U.S. licensed and similar vaccines, and those in English. Their literature review identified five randomized trials and five additional observational studies. Lab-confirmed influenza in symptomatic children was the primary outcome; influenza related mortality and hospitalization also were considered.
The efficacy of LAIV was originally established in four randomized, placebo-controlled clinical trials. Each study was completed over two influenza seasons.
In the Belshe study (N. Engl. J. Med. 1998;338:1405-12), the efficacy compared with placebo was 93% in the first season and 100% in the second (after revaccination).
In a second study (Pediatrics 2006;118:2298-312), efficacy compared to placebo was 85% in the first season and 89% in the second (after revaccination).
Subsequently, randomized studies comparing LAIV with IIV in children younger than 8 years of age demonstrating the relative benefits of LAIV were reported (N. Engl. J. Med. 2007;356:685-96; Pediatr. Infect. Dis. J. 2006 ;25:870-9). A reduction greater than or equal to 50% in laboratory-confirmed influenza cases in the LAIV cohorts compared with the trivalent inactivated vaccine groups was observed. Greater efficacy was reported both in groups that were influenza vaccine naive as well as those with prior immunization. No reductions in hospitalization and medically-attended acute respiratory illness were reported for the LAIV cohorts; however, the quality of the data was judged to be less robust than for laboratory-confirmed disease. For children aged 9-18 years, no differences in laboratory-confirmed influenza were reported.
The mechanism for improved efficacy of LAIV in young children (2-8 years) is largely unknown. LAIV may elicit long-lasting and broader humoral and cellular responses that more closely resembles natural immunity. It also has been hypothesized that LAIV is more immunogenic than IIV as a priming vaccine, and IIV is more effective in boosting preexisting immunity. It is possible that is one explanation for why LAIV is more effective in young children, and that no differences are observed in older children and adults. It also has been suggested that LAIV may elicit an antibody that is more broadly protective against mismatched influenza strains.
In June, the Advisory Committee on Immunization Practices (ACIP) proposed new recommendations regarding the use of LAIV and IIV for young healthy children. ACIP affirmed that both LAIV and IIV are effective in prevention of influenza in children, but recommended that LAIV be used for healthy children aged 2-8 years when both vaccines are available and there are no contraindications or precautions to its use. When LAIV is not immediately available, IIV should be used. Vaccination should not be delayed to procure LAIV.
ACIP restated previous contraindications and precautions to administration of LAIV. Those with contraindications to LAIV should receive inactivated vaccine. These include:
• Children less than 2 years of age and adults older than 49 years of age.
• Children aged 2-17 years receiving aspirin, persons with allergic reactions to vaccine or vaccine components, pregnant women, immunosuppressed persons, and persons with egg allergy.
• Children aged 2-4 years who have had a wheezing episode noted in the medical record or whose parents report that a health care provider informed them of wheezing or asthma within the last 12 months.
• Individuals who have taken antiviral medications within the previous 48 hours.
Administration to children less than 8 years of age with chronic medical conditions (specifically those associated with increased risk of influenza complications) is considered a precaution as safety has not been established.
Immunization for all children beginning at 6 months of age is still the essential message. However, when both LAIV and IIV (trivalent [TIV] or quadrivalent inactivated influenza vaccines [QIV]) are available, the advisory committee recommended LAIV as a preference in healthy children aged 2-8 years. If only TIV or QIV is available, administration of either one is recommended as delays in receipt are of greater concern than are the differences in vaccine formulations. This recommendation, if approved by the CDC director, will not be official until it is published in the 2014-2015 influenza prevention and control recommendations in the MMWR. In anticipation of this new recommendation, the manufacturer has stated that it will be producing 18 million doses of quadrivalent LAIV for the U.S. market for the 2014-2015 season, up from the 13 million it produced last season. More information when available also will be posted on the CDC influenza website and the American Academy of Pediatrics website.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said that he had no relevant financial disclosures.
The effectiveness of influenza vaccine is recognized to vary widely from season to season. At least two factors are critical for determining the likelihood that flu vaccine will be successful in preventing illness.
First, the demographics of who is being immunized (primarily age and presence of comorbidity) and second, the "match" between the circulating flu viruses and that year’s flu vaccine. When the flu vaccine is a poor match with circulating viruses, less benefit from flu vaccination will be observed; in years when the "match" between vaccine and circulating virus is good, substantial reduction in influenza respiratory illness in children and adults is observed. Recently, a second influenza B antigen has been added (creating quadrivalent vaccines) to improve the match with influenza B strains that may circulate in the community.
In February 2014, the Centers for Disease Control and Prevention reported midseason vaccine effectiveness estimates (MMWR 2014 Feb 21;63:137-42).
The major circulating virus was influenza A "2009 H1N1" virus and the "match" between vaccine strains and circulating strains was considered good. The CDC’s midseason vaccine effectiveness estimate was 61% for all age groups (95% confidence interval, 52%-68%), reinforcing the value of influenza vaccine for disease prevention in both children and adults. Flu vaccine reduced the risk of seeking medical attention for flulike illness by 60% for both children and adults.
Another factor that may determine the effectiveness of influenza vaccine in children is whether the individual receives live attenuated influenza vaccine (LAIV) or trivalent or quadrivalent inactivated influenza vaccine (IIV). The CDC has been considering the question "should LAIV be recommended preferentially over IIV in healthy children 2-8 years of age?" based on data from a limited number of studies. Canada, United Kingdom, Israel, and Germany have each expressed a preference for LAIV in their recent recommendations. The CDC working group evaluated published studies primarily restricted to those focused on healthy children, those with both LAIV and IIV cohorts, those studying the U.S. licensed and similar vaccines, and those in English. Their literature review identified five randomized trials and five additional observational studies. Lab-confirmed influenza in symptomatic children was the primary outcome; influenza related mortality and hospitalization also were considered.
The efficacy of LAIV was originally established in four randomized, placebo-controlled clinical trials. Each study was completed over two influenza seasons.
In the Belshe study (N. Engl. J. Med. 1998;338:1405-12), the efficacy compared with placebo was 93% in the first season and 100% in the second (after revaccination).
In a second study (Pediatrics 2006;118:2298-312), efficacy compared to placebo was 85% in the first season and 89% in the second (after revaccination).
Subsequently, randomized studies comparing LAIV with IIV in children younger than 8 years of age demonstrating the relative benefits of LAIV were reported (N. Engl. J. Med. 2007;356:685-96; Pediatr. Infect. Dis. J. 2006 ;25:870-9). A reduction greater than or equal to 50% in laboratory-confirmed influenza cases in the LAIV cohorts compared with the trivalent inactivated vaccine groups was observed. Greater efficacy was reported both in groups that were influenza vaccine naive as well as those with prior immunization. No reductions in hospitalization and medically-attended acute respiratory illness were reported for the LAIV cohorts; however, the quality of the data was judged to be less robust than for laboratory-confirmed disease. For children aged 9-18 years, no differences in laboratory-confirmed influenza were reported.
The mechanism for improved efficacy of LAIV in young children (2-8 years) is largely unknown. LAIV may elicit long-lasting and broader humoral and cellular responses that more closely resembles natural immunity. It also has been hypothesized that LAIV is more immunogenic than IIV as a priming vaccine, and IIV is more effective in boosting preexisting immunity. It is possible that is one explanation for why LAIV is more effective in young children, and that no differences are observed in older children and adults. It also has been suggested that LAIV may elicit an antibody that is more broadly protective against mismatched influenza strains.
In June, the Advisory Committee on Immunization Practices (ACIP) proposed new recommendations regarding the use of LAIV and IIV for young healthy children. ACIP affirmed that both LAIV and IIV are effective in prevention of influenza in children, but recommended that LAIV be used for healthy children aged 2-8 years when both vaccines are available and there are no contraindications or precautions to its use. When LAIV is not immediately available, IIV should be used. Vaccination should not be delayed to procure LAIV.
ACIP restated previous contraindications and precautions to administration of LAIV. Those with contraindications to LAIV should receive inactivated vaccine. These include:
• Children less than 2 years of age and adults older than 49 years of age.
• Children aged 2-17 years receiving aspirin, persons with allergic reactions to vaccine or vaccine components, pregnant women, immunosuppressed persons, and persons with egg allergy.
• Children aged 2-4 years who have had a wheezing episode noted in the medical record or whose parents report that a health care provider informed them of wheezing or asthma within the last 12 months.
• Individuals who have taken antiviral medications within the previous 48 hours.
Administration to children less than 8 years of age with chronic medical conditions (specifically those associated with increased risk of influenza complications) is considered a precaution as safety has not been established.
Immunization for all children beginning at 6 months of age is still the essential message. However, when both LAIV and IIV (trivalent [TIV] or quadrivalent inactivated influenza vaccines [QIV]) are available, the advisory committee recommended LAIV as a preference in healthy children aged 2-8 years. If only TIV or QIV is available, administration of either one is recommended as delays in receipt are of greater concern than are the differences in vaccine formulations. This recommendation, if approved by the CDC director, will not be official until it is published in the 2014-2015 influenza prevention and control recommendations in the MMWR. In anticipation of this new recommendation, the manufacturer has stated that it will be producing 18 million doses of quadrivalent LAIV for the U.S. market for the 2014-2015 season, up from the 13 million it produced last season. More information when available also will be posted on the CDC influenza website and the American Academy of Pediatrics website.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said that he had no relevant financial disclosures.
The effectiveness of influenza vaccine is recognized to vary widely from season to season. At least two factors are critical for determining the likelihood that flu vaccine will be successful in preventing illness.
First, the demographics of who is being immunized (primarily age and presence of comorbidity) and second, the "match" between the circulating flu viruses and that year’s flu vaccine. When the flu vaccine is a poor match with circulating viruses, less benefit from flu vaccination will be observed; in years when the "match" between vaccine and circulating virus is good, substantial reduction in influenza respiratory illness in children and adults is observed. Recently, a second influenza B antigen has been added (creating quadrivalent vaccines) to improve the match with influenza B strains that may circulate in the community.
In February 2014, the Centers for Disease Control and Prevention reported midseason vaccine effectiveness estimates (MMWR 2014 Feb 21;63:137-42).
The major circulating virus was influenza A "2009 H1N1" virus and the "match" between vaccine strains and circulating strains was considered good. The CDC’s midseason vaccine effectiveness estimate was 61% for all age groups (95% confidence interval, 52%-68%), reinforcing the value of influenza vaccine for disease prevention in both children and adults. Flu vaccine reduced the risk of seeking medical attention for flulike illness by 60% for both children and adults.
Another factor that may determine the effectiveness of influenza vaccine in children is whether the individual receives live attenuated influenza vaccine (LAIV) or trivalent or quadrivalent inactivated influenza vaccine (IIV). The CDC has been considering the question "should LAIV be recommended preferentially over IIV in healthy children 2-8 years of age?" based on data from a limited number of studies. Canada, United Kingdom, Israel, and Germany have each expressed a preference for LAIV in their recent recommendations. The CDC working group evaluated published studies primarily restricted to those focused on healthy children, those with both LAIV and IIV cohorts, those studying the U.S. licensed and similar vaccines, and those in English. Their literature review identified five randomized trials and five additional observational studies. Lab-confirmed influenza in symptomatic children was the primary outcome; influenza related mortality and hospitalization also were considered.
The efficacy of LAIV was originally established in four randomized, placebo-controlled clinical trials. Each study was completed over two influenza seasons.
In the Belshe study (N. Engl. J. Med. 1998;338:1405-12), the efficacy compared with placebo was 93% in the first season and 100% in the second (after revaccination).
In a second study (Pediatrics 2006;118:2298-312), efficacy compared to placebo was 85% in the first season and 89% in the second (after revaccination).
Subsequently, randomized studies comparing LAIV with IIV in children younger than 8 years of age demonstrating the relative benefits of LAIV were reported (N. Engl. J. Med. 2007;356:685-96; Pediatr. Infect. Dis. J. 2006 ;25:870-9). A reduction greater than or equal to 50% in laboratory-confirmed influenza cases in the LAIV cohorts compared with the trivalent inactivated vaccine groups was observed. Greater efficacy was reported both in groups that were influenza vaccine naive as well as those with prior immunization. No reductions in hospitalization and medically-attended acute respiratory illness were reported for the LAIV cohorts; however, the quality of the data was judged to be less robust than for laboratory-confirmed disease. For children aged 9-18 years, no differences in laboratory-confirmed influenza were reported.
The mechanism for improved efficacy of LAIV in young children (2-8 years) is largely unknown. LAIV may elicit long-lasting and broader humoral and cellular responses that more closely resembles natural immunity. It also has been hypothesized that LAIV is more immunogenic than IIV as a priming vaccine, and IIV is more effective in boosting preexisting immunity. It is possible that is one explanation for why LAIV is more effective in young children, and that no differences are observed in older children and adults. It also has been suggested that LAIV may elicit an antibody that is more broadly protective against mismatched influenza strains.
In June, the Advisory Committee on Immunization Practices (ACIP) proposed new recommendations regarding the use of LAIV and IIV for young healthy children. ACIP affirmed that both LAIV and IIV are effective in prevention of influenza in children, but recommended that LAIV be used for healthy children aged 2-8 years when both vaccines are available and there are no contraindications or precautions to its use. When LAIV is not immediately available, IIV should be used. Vaccination should not be delayed to procure LAIV.
ACIP restated previous contraindications and precautions to administration of LAIV. Those with contraindications to LAIV should receive inactivated vaccine. These include:
• Children less than 2 years of age and adults older than 49 years of age.
• Children aged 2-17 years receiving aspirin, persons with allergic reactions to vaccine or vaccine components, pregnant women, immunosuppressed persons, and persons with egg allergy.
• Children aged 2-4 years who have had a wheezing episode noted in the medical record or whose parents report that a health care provider informed them of wheezing or asthma within the last 12 months.
• Individuals who have taken antiviral medications within the previous 48 hours.
Administration to children less than 8 years of age with chronic medical conditions (specifically those associated with increased risk of influenza complications) is considered a precaution as safety has not been established.
Immunization for all children beginning at 6 months of age is still the essential message. However, when both LAIV and IIV (trivalent [TIV] or quadrivalent inactivated influenza vaccines [QIV]) are available, the advisory committee recommended LAIV as a preference in healthy children aged 2-8 years. If only TIV or QIV is available, administration of either one is recommended as delays in receipt are of greater concern than are the differences in vaccine formulations. This recommendation, if approved by the CDC director, will not be official until it is published in the 2014-2015 influenza prevention and control recommendations in the MMWR. In anticipation of this new recommendation, the manufacturer has stated that it will be producing 18 million doses of quadrivalent LAIV for the U.S. market for the 2014-2015 season, up from the 13 million it produced last season. More information when available also will be posted on the CDC influenza website and the American Academy of Pediatrics website.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said that he had no relevant financial disclosures.
Rethinking antimicrobial prophylaxis for UTI
The RIVUR [Randomized Intervention for Children With Vesicoureteral Reflux] trial investigators set out to reevaluate the role of antimicrobial prophylaxis for the prevention of recurrences in children with vesicoureteral reflux. As recent randomized trials have produced conflicting results, the goal of the RIVUR investigators was to determine whether antimicrobial prophylaxis could prevent febrile or symptomatic urinary tract infection and whether prevention would reduce the likelihood of subsequent renal scarring. The results, recently published in the New England Journal of Medicine (2014;370:2367-76), demonstrated that nearly 18% of children, 2 months to 6 years of age, have a febrile or symptomatic recurrence within the first year after the initial or presenting episode. The recurrence rate for febrile or symptomatic episodes was reduced by approximately 50% in the treatment group (trimethoprim-sulfamethoxazole) to nearly 8%.
In addition, the proportion of children considered treatment failures (defined as a combination of febrile or symptomatic UTIs or development of new renal scarring) occurred twice as often in the placebo group as in the treatment group. However, despite the reduction in febrile or symptomatic episodes in the treatment group, approximately 8% of children in both treatment and placebo groups developed new renal scarring, as defined by a decreased uptake of tracer or cortical thinning.
The study confirmed that children with higher grades of reflux (III or IV at baseline) were more likely to have febrile or symptomatic recurrences, that children with bladder and bowel dysfunction (based on a modified Dysfunctional Voiding Symptom Score) also were more likely to have febrile or symptomatic recurrences, and that recurrences in children on prophylaxis were more likely to be resistant to trimethoprim-sulfamethoxazole than were those in children on placebo.
Implications for prevention of UTI
The American Academy of Pediatrics guidelines for the management of UTI in children were updated in 2011 (Pediatrics 2011;128:595-610). The authors contacted the six researchers who had conducted the most recent randomized controlled trials and completed a formal meta-analysis that did not detect a statistically significant benefit of prophylaxis for stopping the recurrence of febrile UTI/pyelonephritis in infants without reflux or those with grades I, II, III, or IV. The 2011 recommendations reflected the findings of an AAP subcommittee that antimicrobial prophylaxis was not effective, as had been presumed in a 1999 report (Pediatrics 1999;103:843-52).
The AAP subcommittee on urinary tract infection of the Steering Committee on Quality Improvement and Management – authors of the 2011 revised guidelines – have recently reviewed the RIVUR study data (AAP News, July 1 2014) and concluded that antimicrobial prophylaxis did not alter the development of new renal scarring/damage, that the benefits of daily antimicrobial prophylaxis were modest, and that the increased likelihood of resistance to trimethoprim-sulfamethoxazole at recurrences was significant. The subcommittee reaffirmed the 2011 guidance concerning a "less aggressive" approach: Renal and bladder ultrasound are adequate for assessment of risk for renal scarring at first episodes, and watchful waiting without performing voiding cystourethrography (VCUG) or initiating prophylaxis is appropriate. VCUG is indicated after a first episode if renal and bladder ultrasonography reveals hydronephrosis, scarring, or other findings that would suggest either high-grade vesicoureteral reflux (VUR) or obstructive uropathy and in other atypical or complex clinical circumstances. As well, VCUG also should be performed if there is a recurrence of a febrile UTI (Pediatrics 2011;128:595-610).
The current subcommittee opined that prompt diagnosis and effective treatment of a febrile UTI recurrence may be of greater importance, regardless of whether VUR is present or the child is receiving antimicrobial prophylaxis.
My take
For me, the RIVUR data provide further insights into both the risk of any recurrence (approximately 18% by 12 months, approximately 25% by 24 months) and the risk for multiple recurrences (approximately 10%). The data identify those at highest risk for recurrences (patients with bladder and bowel dysfunction or higher grades of reflux) and provide evidence that trimethoprim-sulfamethoxazole prophylaxis is highly effective in such groups. No serious side effects were observed during the RIVUR trial; however, Stevens-Johnson syndrome is documented to occur rarely after administration of trimethoprim-sulfamethoxazole, and the potential for this life-threatening event should be part of the decision process. I believe the value of the new data is that they provide confidence that antimicrobial prophylaxis can be effective for the prevention of febrile/symptomatic UTI, and that in select children at great risk for recurrences and subsequent renal damage, antimicrobial prophylaxis can be part of our toolbox.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said that he had no relevant financial disclosures.
The RIVUR [Randomized Intervention for Children With Vesicoureteral Reflux] trial investigators set out to reevaluate the role of antimicrobial prophylaxis for the prevention of recurrences in children with vesicoureteral reflux. As recent randomized trials have produced conflicting results, the goal of the RIVUR investigators was to determine whether antimicrobial prophylaxis could prevent febrile or symptomatic urinary tract infection and whether prevention would reduce the likelihood of subsequent renal scarring. The results, recently published in the New England Journal of Medicine (2014;370:2367-76), demonstrated that nearly 18% of children, 2 months to 6 years of age, have a febrile or symptomatic recurrence within the first year after the initial or presenting episode. The recurrence rate for febrile or symptomatic episodes was reduced by approximately 50% in the treatment group (trimethoprim-sulfamethoxazole) to nearly 8%.
In addition, the proportion of children considered treatment failures (defined as a combination of febrile or symptomatic UTIs or development of new renal scarring) occurred twice as often in the placebo group as in the treatment group. However, despite the reduction in febrile or symptomatic episodes in the treatment group, approximately 8% of children in both treatment and placebo groups developed new renal scarring, as defined by a decreased uptake of tracer or cortical thinning.
The study confirmed that children with higher grades of reflux (III or IV at baseline) were more likely to have febrile or symptomatic recurrences, that children with bladder and bowel dysfunction (based on a modified Dysfunctional Voiding Symptom Score) also were more likely to have febrile or symptomatic recurrences, and that recurrences in children on prophylaxis were more likely to be resistant to trimethoprim-sulfamethoxazole than were those in children on placebo.
Implications for prevention of UTI
The American Academy of Pediatrics guidelines for the management of UTI in children were updated in 2011 (Pediatrics 2011;128:595-610). The authors contacted the six researchers who had conducted the most recent randomized controlled trials and completed a formal meta-analysis that did not detect a statistically significant benefit of prophylaxis for stopping the recurrence of febrile UTI/pyelonephritis in infants without reflux or those with grades I, II, III, or IV. The 2011 recommendations reflected the findings of an AAP subcommittee that antimicrobial prophylaxis was not effective, as had been presumed in a 1999 report (Pediatrics 1999;103:843-52).
The AAP subcommittee on urinary tract infection of the Steering Committee on Quality Improvement and Management – authors of the 2011 revised guidelines – have recently reviewed the RIVUR study data (AAP News, July 1 2014) and concluded that antimicrobial prophylaxis did not alter the development of new renal scarring/damage, that the benefits of daily antimicrobial prophylaxis were modest, and that the increased likelihood of resistance to trimethoprim-sulfamethoxazole at recurrences was significant. The subcommittee reaffirmed the 2011 guidance concerning a "less aggressive" approach: Renal and bladder ultrasound are adequate for assessment of risk for renal scarring at first episodes, and watchful waiting without performing voiding cystourethrography (VCUG) or initiating prophylaxis is appropriate. VCUG is indicated after a first episode if renal and bladder ultrasonography reveals hydronephrosis, scarring, or other findings that would suggest either high-grade vesicoureteral reflux (VUR) or obstructive uropathy and in other atypical or complex clinical circumstances. As well, VCUG also should be performed if there is a recurrence of a febrile UTI (Pediatrics 2011;128:595-610).
The current subcommittee opined that prompt diagnosis and effective treatment of a febrile UTI recurrence may be of greater importance, regardless of whether VUR is present or the child is receiving antimicrobial prophylaxis.
My take
For me, the RIVUR data provide further insights into both the risk of any recurrence (approximately 18% by 12 months, approximately 25% by 24 months) and the risk for multiple recurrences (approximately 10%). The data identify those at highest risk for recurrences (patients with bladder and bowel dysfunction or higher grades of reflux) and provide evidence that trimethoprim-sulfamethoxazole prophylaxis is highly effective in such groups. No serious side effects were observed during the RIVUR trial; however, Stevens-Johnson syndrome is documented to occur rarely after administration of trimethoprim-sulfamethoxazole, and the potential for this life-threatening event should be part of the decision process. I believe the value of the new data is that they provide confidence that antimicrobial prophylaxis can be effective for the prevention of febrile/symptomatic UTI, and that in select children at great risk for recurrences and subsequent renal damage, antimicrobial prophylaxis can be part of our toolbox.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said that he had no relevant financial disclosures.
The RIVUR [Randomized Intervention for Children With Vesicoureteral Reflux] trial investigators set out to reevaluate the role of antimicrobial prophylaxis for the prevention of recurrences in children with vesicoureteral reflux. As recent randomized trials have produced conflicting results, the goal of the RIVUR investigators was to determine whether antimicrobial prophylaxis could prevent febrile or symptomatic urinary tract infection and whether prevention would reduce the likelihood of subsequent renal scarring. The results, recently published in the New England Journal of Medicine (2014;370:2367-76), demonstrated that nearly 18% of children, 2 months to 6 years of age, have a febrile or symptomatic recurrence within the first year after the initial or presenting episode. The recurrence rate for febrile or symptomatic episodes was reduced by approximately 50% in the treatment group (trimethoprim-sulfamethoxazole) to nearly 8%.
In addition, the proportion of children considered treatment failures (defined as a combination of febrile or symptomatic UTIs or development of new renal scarring) occurred twice as often in the placebo group as in the treatment group. However, despite the reduction in febrile or symptomatic episodes in the treatment group, approximately 8% of children in both treatment and placebo groups developed new renal scarring, as defined by a decreased uptake of tracer or cortical thinning.
The study confirmed that children with higher grades of reflux (III or IV at baseline) were more likely to have febrile or symptomatic recurrences, that children with bladder and bowel dysfunction (based on a modified Dysfunctional Voiding Symptom Score) also were more likely to have febrile or symptomatic recurrences, and that recurrences in children on prophylaxis were more likely to be resistant to trimethoprim-sulfamethoxazole than were those in children on placebo.
Implications for prevention of UTI
The American Academy of Pediatrics guidelines for the management of UTI in children were updated in 2011 (Pediatrics 2011;128:595-610). The authors contacted the six researchers who had conducted the most recent randomized controlled trials and completed a formal meta-analysis that did not detect a statistically significant benefit of prophylaxis for stopping the recurrence of febrile UTI/pyelonephritis in infants without reflux or those with grades I, II, III, or IV. The 2011 recommendations reflected the findings of an AAP subcommittee that antimicrobial prophylaxis was not effective, as had been presumed in a 1999 report (Pediatrics 1999;103:843-52).
The AAP subcommittee on urinary tract infection of the Steering Committee on Quality Improvement and Management – authors of the 2011 revised guidelines – have recently reviewed the RIVUR study data (AAP News, July 1 2014) and concluded that antimicrobial prophylaxis did not alter the development of new renal scarring/damage, that the benefits of daily antimicrobial prophylaxis were modest, and that the increased likelihood of resistance to trimethoprim-sulfamethoxazole at recurrences was significant. The subcommittee reaffirmed the 2011 guidance concerning a "less aggressive" approach: Renal and bladder ultrasound are adequate for assessment of risk for renal scarring at first episodes, and watchful waiting without performing voiding cystourethrography (VCUG) or initiating prophylaxis is appropriate. VCUG is indicated after a first episode if renal and bladder ultrasonography reveals hydronephrosis, scarring, or other findings that would suggest either high-grade vesicoureteral reflux (VUR) or obstructive uropathy and in other atypical or complex clinical circumstances. As well, VCUG also should be performed if there is a recurrence of a febrile UTI (Pediatrics 2011;128:595-610).
The current subcommittee opined that prompt diagnosis and effective treatment of a febrile UTI recurrence may be of greater importance, regardless of whether VUR is present or the child is receiving antimicrobial prophylaxis.
My take
For me, the RIVUR data provide further insights into both the risk of any recurrence (approximately 18% by 12 months, approximately 25% by 24 months) and the risk for multiple recurrences (approximately 10%). The data identify those at highest risk for recurrences (patients with bladder and bowel dysfunction or higher grades of reflux) and provide evidence that trimethoprim-sulfamethoxazole prophylaxis is highly effective in such groups. No serious side effects were observed during the RIVUR trial; however, Stevens-Johnson syndrome is documented to occur rarely after administration of trimethoprim-sulfamethoxazole, and the potential for this life-threatening event should be part of the decision process. I believe the value of the new data is that they provide confidence that antimicrobial prophylaxis can be effective for the prevention of febrile/symptomatic UTI, and that in select children at great risk for recurrences and subsequent renal damage, antimicrobial prophylaxis can be part of our toolbox.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. He said that he had no relevant financial disclosures.
Approach to newborns exposed to HSV at the time of delivery
Recently the American Academy of Pediatrics issued recommendations that address management of asymptomatic newborns whose mothers have active herpes simplex virus (HSV) lesions noted at the time of delivery. Implementing these recommendations requires proactive coordination between the director of the laboratory and the obstetrical and pediatric providers to ensure success (Pediatrics 2013;131:e635-46).
Approximately 1,500 infants are diagnosed and treated for neonatal HSV infection each year in the United States. Most pediatricians are knowledgeable about the three forms of neonatal HSV infection and the role of prompt diagnosis and utilization of acyclovir. Even so, the outcomes of this disease may be devastating. Skin–eye–mucous membrane disease has the best prognosis (98% neurologically normal), as finding the culprit lesion generally ensures timely diagnosis and treatment. With central nervous system infection or disseminated disease, a skin lesion is not noted in 25%-40% of cases. So the diagnosis is sometimes delayed or missed initially because the initial presentations (seizures in CNS infection; sepsis picture or liver failure in disseminated disease) may sidetrack the provider into considering other working diagnoses, such as bacterial sepsis or metabolic disease. Neurologic sequelae occur in 25% of those with disseminated infection, but in upward of 70% in those with CNS disease.
Ideally, both the obstetrician and the pediatric provider play a role in ensuring appropriate care for the baby whose mother has active HSV lesions at time of delivery. Appropriate care includes preemptive treatment for neonatal HSV infection, which has the potential to improve outcomes and so should be a high priority for all providers.
The new guidance is evidence based and predicated on the availability of HSV typing of the HSV from the maternal lesion and type-specific serology. It allows the provider to define the newborn risk of acquiring HSV infection more explicitly and utilize preemptive evaluation/therapy. Providers should ensure that their hospital laboratory can perform such testing with reasonable turnaround time for results.
Obstetrical role and implications of testing
The obstetric provider should swab the maternal lesion for HSV polymerase chain reaction (PCR) assay/culture and typing (HSV-1 or HSV-2). These data can be utilized with maternal history and serologic results to calculate the neonatal risk for infection.
Calculation of relative neonatal risk
• First episode, primary infection. Defined as the first maternal HSV episode with type-specific serology being negative, this makes the risk of neonatal infection approximately 50%. If maternal history of prior disease is negative AND either the maternal lesion test results or serology results are unavailable, follow the plan of care for first episode primary infection.
• First episode, nonprimary infection. Defined as the first maternal episode but antibody to detected HSV type is not present (e.g., HSV-2 confirmed from lesion, with type 1 but NOT type 2 maternal antibody present; OR HSV-1 confirmed, with type 2 but NOT type 1 maternal antibody present), the risk of neonatal infection is approximately 25%.
• Recurrent. If the mother has a history of genital herpes and the mother’s type-specific antibody is the same as the type detected in the lesions, the risk for neonatal infection is lower and approximately 2%.
Pediatrician’s role and plan of care
The first order of business is to identify neonates who demonstrate signs or symptoms suggestive of HSV infection at birth or in the perinatal period (whether or not any lesions were noted at time of delivery). In this case, all infants should undergo full evaluation for both viral and bacterial causes and should have prompt initiation of preemptive antiviral and antibacterial therapy. The evaluation of an ill-appearing infant at birth should include CBC; liver function studies; blood, urine, and cerebrospinal fluid examination with bacterial cultures of blood, urine, and cerebrospinal fluid, plus blood and cerebrospinal fluid HSV PCR. Also, HSV surface (conjunctivae, nasopharynx, and rectum) and lesion cultures are needed. Infectious disease consultation is recommended if HSV infection is confirmed. Acyclovir should continue for 14 days for skin–eye–mucous membrane disease or 21 days for CNS or disseminated infection. Further evaluation toward the end of therapy can determine if a longer course of therapy should be considered.
The recent guideline addresses care for those infants who are born to mothers with active HSV lesions noted at time of delivery, and should be initiated only if the infant is asymptomatic at birth.
In this situation, for babies whose mothers have primary infection (risk 50% for neonatal infection) or first episode, nonprimary infection (risk 25% for neonatal infection):
• Approximately 24 hours after the infant’s birth, obtain blood HSV DNA PCR and HSV surface cultures of conjunctivae, nasopharynx, and rectum as well as from the scalp electrode site if there was one.
• Cerebrospinal fluid examination with HSV DNA PCR testing should be obtained.
• Acyclovir (20 mg/kg per dose every 8 hours IV) should be initiated. Preemptive therapy (acyclovir 20 mg/kg per dose every 8 hours IV) should be continued for 10 days and until all studies are negative.
For babies whose mothers have recurrent infection:
• Cerebrospinal fluid examination may be deferred.
• But the rest of the workup should be completed and IV acyclovir initiated.
• IV acyclovir can be stopped at the time that studies are negative (usually at 48 hours, assuming negative results of blood PCR and preliminary negative surface cultures), with close follow-up of the infant.
Use of this guideline can improve care of infants only when the laboratory and the obstetrical and pediatric providers have established a good working relationship. This ensures the availability of necessary HSV studies, complete implementation, and proper interpretation of testing to guide the newborn’s care.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. Dr. Jackson was a member of the AAP Committee on Infectious Diseases who wrote the AAP clinical report entitled "Guidance on Management of Asymptomatic Neonates Born to Women With Active Genital Herpes Lesions," but said she had no other conflicts of interest to disclose. E-mail her at [email protected].
Recently the American Academy of Pediatrics issued recommendations that address management of asymptomatic newborns whose mothers have active herpes simplex virus (HSV) lesions noted at the time of delivery. Implementing these recommendations requires proactive coordination between the director of the laboratory and the obstetrical and pediatric providers to ensure success (Pediatrics 2013;131:e635-46).
Approximately 1,500 infants are diagnosed and treated for neonatal HSV infection each year in the United States. Most pediatricians are knowledgeable about the three forms of neonatal HSV infection and the role of prompt diagnosis and utilization of acyclovir. Even so, the outcomes of this disease may be devastating. Skin–eye–mucous membrane disease has the best prognosis (98% neurologically normal), as finding the culprit lesion generally ensures timely diagnosis and treatment. With central nervous system infection or disseminated disease, a skin lesion is not noted in 25%-40% of cases. So the diagnosis is sometimes delayed or missed initially because the initial presentations (seizures in CNS infection; sepsis picture or liver failure in disseminated disease) may sidetrack the provider into considering other working diagnoses, such as bacterial sepsis or metabolic disease. Neurologic sequelae occur in 25% of those with disseminated infection, but in upward of 70% in those with CNS disease.
Ideally, both the obstetrician and the pediatric provider play a role in ensuring appropriate care for the baby whose mother has active HSV lesions at time of delivery. Appropriate care includes preemptive treatment for neonatal HSV infection, which has the potential to improve outcomes and so should be a high priority for all providers.
The new guidance is evidence based and predicated on the availability of HSV typing of the HSV from the maternal lesion and type-specific serology. It allows the provider to define the newborn risk of acquiring HSV infection more explicitly and utilize preemptive evaluation/therapy. Providers should ensure that their hospital laboratory can perform such testing with reasonable turnaround time for results.
Obstetrical role and implications of testing
The obstetric provider should swab the maternal lesion for HSV polymerase chain reaction (PCR) assay/culture and typing (HSV-1 or HSV-2). These data can be utilized with maternal history and serologic results to calculate the neonatal risk for infection.
Calculation of relative neonatal risk
• First episode, primary infection. Defined as the first maternal HSV episode with type-specific serology being negative, this makes the risk of neonatal infection approximately 50%. If maternal history of prior disease is negative AND either the maternal lesion test results or serology results are unavailable, follow the plan of care for first episode primary infection.
• First episode, nonprimary infection. Defined as the first maternal episode but antibody to detected HSV type is not present (e.g., HSV-2 confirmed from lesion, with type 1 but NOT type 2 maternal antibody present; OR HSV-1 confirmed, with type 2 but NOT type 1 maternal antibody present), the risk of neonatal infection is approximately 25%.
• Recurrent. If the mother has a history of genital herpes and the mother’s type-specific antibody is the same as the type detected in the lesions, the risk for neonatal infection is lower and approximately 2%.
Pediatrician’s role and plan of care
The first order of business is to identify neonates who demonstrate signs or symptoms suggestive of HSV infection at birth or in the perinatal period (whether or not any lesions were noted at time of delivery). In this case, all infants should undergo full evaluation for both viral and bacterial causes and should have prompt initiation of preemptive antiviral and antibacterial therapy. The evaluation of an ill-appearing infant at birth should include CBC; liver function studies; blood, urine, and cerebrospinal fluid examination with bacterial cultures of blood, urine, and cerebrospinal fluid, plus blood and cerebrospinal fluid HSV PCR. Also, HSV surface (conjunctivae, nasopharynx, and rectum) and lesion cultures are needed. Infectious disease consultation is recommended if HSV infection is confirmed. Acyclovir should continue for 14 days for skin–eye–mucous membrane disease or 21 days for CNS or disseminated infection. Further evaluation toward the end of therapy can determine if a longer course of therapy should be considered.
The recent guideline addresses care for those infants who are born to mothers with active HSV lesions noted at time of delivery, and should be initiated only if the infant is asymptomatic at birth.
In this situation, for babies whose mothers have primary infection (risk 50% for neonatal infection) or first episode, nonprimary infection (risk 25% for neonatal infection):
• Approximately 24 hours after the infant’s birth, obtain blood HSV DNA PCR and HSV surface cultures of conjunctivae, nasopharynx, and rectum as well as from the scalp electrode site if there was one.
• Cerebrospinal fluid examination with HSV DNA PCR testing should be obtained.
• Acyclovir (20 mg/kg per dose every 8 hours IV) should be initiated. Preemptive therapy (acyclovir 20 mg/kg per dose every 8 hours IV) should be continued for 10 days and until all studies are negative.
For babies whose mothers have recurrent infection:
• Cerebrospinal fluid examination may be deferred.
• But the rest of the workup should be completed and IV acyclovir initiated.
• IV acyclovir can be stopped at the time that studies are negative (usually at 48 hours, assuming negative results of blood PCR and preliminary negative surface cultures), with close follow-up of the infant.
Use of this guideline can improve care of infants only when the laboratory and the obstetrical and pediatric providers have established a good working relationship. This ensures the availability of necessary HSV studies, complete implementation, and proper interpretation of testing to guide the newborn’s care.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. Dr. Jackson was a member of the AAP Committee on Infectious Diseases who wrote the AAP clinical report entitled "Guidance on Management of Asymptomatic Neonates Born to Women With Active Genital Herpes Lesions," but said she had no other conflicts of interest to disclose. E-mail her at [email protected].
Recently the American Academy of Pediatrics issued recommendations that address management of asymptomatic newborns whose mothers have active herpes simplex virus (HSV) lesions noted at the time of delivery. Implementing these recommendations requires proactive coordination between the director of the laboratory and the obstetrical and pediatric providers to ensure success (Pediatrics 2013;131:e635-46).
Approximately 1,500 infants are diagnosed and treated for neonatal HSV infection each year in the United States. Most pediatricians are knowledgeable about the three forms of neonatal HSV infection and the role of prompt diagnosis and utilization of acyclovir. Even so, the outcomes of this disease may be devastating. Skin–eye–mucous membrane disease has the best prognosis (98% neurologically normal), as finding the culprit lesion generally ensures timely diagnosis and treatment. With central nervous system infection or disseminated disease, a skin lesion is not noted in 25%-40% of cases. So the diagnosis is sometimes delayed or missed initially because the initial presentations (seizures in CNS infection; sepsis picture or liver failure in disseminated disease) may sidetrack the provider into considering other working diagnoses, such as bacterial sepsis or metabolic disease. Neurologic sequelae occur in 25% of those with disseminated infection, but in upward of 70% in those with CNS disease.
Ideally, both the obstetrician and the pediatric provider play a role in ensuring appropriate care for the baby whose mother has active HSV lesions at time of delivery. Appropriate care includes preemptive treatment for neonatal HSV infection, which has the potential to improve outcomes and so should be a high priority for all providers.
The new guidance is evidence based and predicated on the availability of HSV typing of the HSV from the maternal lesion and type-specific serology. It allows the provider to define the newborn risk of acquiring HSV infection more explicitly and utilize preemptive evaluation/therapy. Providers should ensure that their hospital laboratory can perform such testing with reasonable turnaround time for results.
Obstetrical role and implications of testing
The obstetric provider should swab the maternal lesion for HSV polymerase chain reaction (PCR) assay/culture and typing (HSV-1 or HSV-2). These data can be utilized with maternal history and serologic results to calculate the neonatal risk for infection.
Calculation of relative neonatal risk
• First episode, primary infection. Defined as the first maternal HSV episode with type-specific serology being negative, this makes the risk of neonatal infection approximately 50%. If maternal history of prior disease is negative AND either the maternal lesion test results or serology results are unavailable, follow the plan of care for first episode primary infection.
• First episode, nonprimary infection. Defined as the first maternal episode but antibody to detected HSV type is not present (e.g., HSV-2 confirmed from lesion, with type 1 but NOT type 2 maternal antibody present; OR HSV-1 confirmed, with type 2 but NOT type 1 maternal antibody present), the risk of neonatal infection is approximately 25%.
• Recurrent. If the mother has a history of genital herpes and the mother’s type-specific antibody is the same as the type detected in the lesions, the risk for neonatal infection is lower and approximately 2%.
Pediatrician’s role and plan of care
The first order of business is to identify neonates who demonstrate signs or symptoms suggestive of HSV infection at birth or in the perinatal period (whether or not any lesions were noted at time of delivery). In this case, all infants should undergo full evaluation for both viral and bacterial causes and should have prompt initiation of preemptive antiviral and antibacterial therapy. The evaluation of an ill-appearing infant at birth should include CBC; liver function studies; blood, urine, and cerebrospinal fluid examination with bacterial cultures of blood, urine, and cerebrospinal fluid, plus blood and cerebrospinal fluid HSV PCR. Also, HSV surface (conjunctivae, nasopharynx, and rectum) and lesion cultures are needed. Infectious disease consultation is recommended if HSV infection is confirmed. Acyclovir should continue for 14 days for skin–eye–mucous membrane disease or 21 days for CNS or disseminated infection. Further evaluation toward the end of therapy can determine if a longer course of therapy should be considered.
The recent guideline addresses care for those infants who are born to mothers with active HSV lesions noted at time of delivery, and should be initiated only if the infant is asymptomatic at birth.
In this situation, for babies whose mothers have primary infection (risk 50% for neonatal infection) or first episode, nonprimary infection (risk 25% for neonatal infection):
• Approximately 24 hours after the infant’s birth, obtain blood HSV DNA PCR and HSV surface cultures of conjunctivae, nasopharynx, and rectum as well as from the scalp electrode site if there was one.
• Cerebrospinal fluid examination with HSV DNA PCR testing should be obtained.
• Acyclovir (20 mg/kg per dose every 8 hours IV) should be initiated. Preemptive therapy (acyclovir 20 mg/kg per dose every 8 hours IV) should be continued for 10 days and until all studies are negative.
For babies whose mothers have recurrent infection:
• Cerebrospinal fluid examination may be deferred.
• But the rest of the workup should be completed and IV acyclovir initiated.
• IV acyclovir can be stopped at the time that studies are negative (usually at 48 hours, assuming negative results of blood PCR and preliminary negative surface cultures), with close follow-up of the infant.
Use of this guideline can improve care of infants only when the laboratory and the obstetrical and pediatric providers have established a good working relationship. This ensures the availability of necessary HSV studies, complete implementation, and proper interpretation of testing to guide the newborn’s care.
Dr. Jackson is chief of pediatric infectious diseases at Children’s Mercy Hospital, Kansas City, Mo., and professor of pediatrics at the University of Missouri–Kansas City. Dr. Jackson was a member of the AAP Committee on Infectious Diseases who wrote the AAP clinical report entitled "Guidance on Management of Asymptomatic Neonates Born to Women With Active Genital Herpes Lesions," but said she had no other conflicts of interest to disclose. E-mail her at [email protected].
Potpourri of travel medicine tips and updates
School’s out for the summer soon! Many of your patients may have plans to travel to areas where they may be exposed to infectious diseases and other health risks not routinely encountered in the United States. They will join the 29 million Americans, including almost 3 million children, who traveled to overseas destinations in 2013. The potential for exposures to these risks is dependent on several factors, including the traveler’s age, health and immunization status, destination, accommodations, and duration of travel. Leisure travel, including visiting friends and relatives, accounts for approximately 90% of overseas travel. Some adolescents are traveling to resource-limited areas for adventure travel, educational experiences, and volunteerism. Many times they will reside with host families as part of this experience. There are also children who will have prolonged stays as a result of parental job relocation.
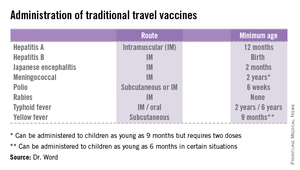
Unfortunately, health precautions often are not considered as many make their travel arrangements. International trips on average are planned at least 105 days in advance; however, many patients wait until the last minute to seek medical advice, if at all. Of 10,032 ill persons who sought post-travel evaluations at participating surveillance facilities (U.S. GeoSentinel sites) between 1997 and 2011, less than half (44%) reported seeking pretravel advice (MMWR 2013;62(SS03):1-15).
Here are some tips that should be useful and easy to implement in your practice for your internationally traveling patients.
• Make sure routine immunizations are up-to-date for age. The exception to this rule is for measles. All children at least 12 months age should receive two doses of MMR prior to departure regardless of their international destination. The second dose of MMR can be administered as early as 4 weeks after the first. Children between 6 and 11 months of age should receive a single dose of MMR prior to departure. If the initial dose is administered at less than 12 months of age, two additional doses will need to be administered to complete the series beginning at 12 months of age.
While measles is no longer endemic in the United States, as of April 25, 2014, there have been 154 cases reported from 14 states. (See measles graphic.) The majority of cases were imported by unvaccinated travelers who became ill after returning home and exposed susceptible individuals. In the last few years, most of the U.S. cases were imported from Western Europe. Currently, there are several countries experiencing record numbers of cases, including Vietnam (3,700) and the Philippines (26,000). This is not to imply that ongoing international outbreaks are limited to these two countries. For additional information, go to cdc.gov/measles.
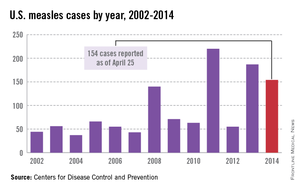
• Identify someone in your area as a local resource for travel-related information and referrals. Make sure they are willing to see children. Develop a system to send out reminders to families to seek pretravel advice, ideally at least 1 month prior to departure. For children with chronic diseases or compromised immune systems, destination selection may need to be adjusted depending on their medical needs, availability of comparable health care at the overseas destination, and ability to receive pretravel vaccine interventions. Involvement prior to booking the trip would be advisable. Many offices successfully send out reminders for well visits and influenza vaccine. Consider incorporating one for overseas travel.
• The timing of initiation of antimalarial prophylaxis is dependent on the medication. Weekly medications such as chloroquine and mefloquine should begin at least 2 weeks prior to exposure. Atovaquone/proguanil and doxycycline are two drugs that are administered daily, and travelers can begin as late as 2 days prior to entry into a malaria-endemic area. This is a great option for the last-minute traveler.
However, there are contraindications for the use of each drug. Some are age dependent, while others are directly related to the presence of a specific medical condition. Areas where chloroquine-sensitive malaria is present are limited. It is always important to prescribe a prophylactic antimalarial agent, but even more prudent to prescribe the appropriate drug and dosage.
Not sure which drug is most appropriate for your patient? Refer to your local travel medicine expert, or visit cdc.gov/malaria.
• The accompanying table lists vaccines that are traditionally considered to be travel vaccines, but pediatricians and family physicians might not consider all to belong in that group. Most are not required for entry into a specific country, but are recommended based on the risk for potential exposure and disease acquisition. In contrast, yellow fever and meningococcal vaccines are required for entry into certain countries. Yellow fever vaccine can be administered only at authorized sites and should be received at least 10 days prior to arrival at the destination. As with routinely administered vaccines, occasionally there are shortages of travel-related vaccines. Most recently, a shortage of yellow fever vaccine has been resolved.
The majority of vaccines should be administered at least 2 weeks prior to departure, while others, such as rabies and Japanese encephalitis, take at least 28 days to complete the series. These are a few additional reasons it behooves your patients to seek advice early.
Travel updates
Chikungunya virus (CHIK V). Local transmission in the Americas was first reported from St. Martin in December 2013. As of May 5, 2014, a total of 12 Caribbean countries have reported locally acquired cases. The disease is transmitted by Aedes species, which are the same species that transmit dengue fever. Disease is characterized by sudden onset of high fever with severe polyarthralgia. Additional symptoms can include headache, myalgias, rash, nausea, and vomiting. Epidemics have historically occurred in Africa, Asia, and islands in the Indian Ocean. Outbreaks also have occurred in Italy and France.
There is no preventive vaccine or drug available. Treatment is symptomatic care. The disease is best prevented by taking adequate mosquito precautions, especially during the daytime. Application of DEET (N,N-diethyl-m-toluamide) and picaridin-containing agents to the skin or treating clothes with a permethrin-containing agent are just two ways to avoid sustaining a mosquito bite.
While no cases Chikungunya virus have been acquired in the United States, there is a potential risk that the virus will be introduced by an infected traveler or mosquito. The Aedes species that transmits the virus is present in several areas of the United States. For additional information, go to cdc.gov/chikungunya.
Polio. While polio has been eliminated in the United States since 1979, it has never been eradicated in Afghanistan, Nigeria, and Pakistan. For a country to be certified as polio free, there cannot be evidence of circulation of wild polio virus for 3 consecutive years. In spite of a massive global initiative to eliminate this disease, in the last 3 months there have been cases confirmed in the following countries: Cameroon, Ethiopia, Equatorial Guinea, Iraq, Kenya, Somalia, and Syria. While no cases of flaccid paralysis have been confirmed in Israel, wild polio virus has been detected in sewage and isolated from stool of asymptomatic individuals.
Completion of the polio series is recommended for those persons inadequately immunized, and a one-time booster dose is recommended for all adults with travel plans to these countries. This should not be an issue for most pediatric patients, except those who may have deferred immunizations. Booster doses are no longer recommended for travel to countries that border countries with active circulation
African tick bite fever. Frequently overshadowed by the appropriate concern for prevention and acquisition of malaria is a rickettsial disease caused by Rickettsia africae, one of the spotted fever group of rickettsial infections. Its geographic distribution is limited to sub-Saharan Africa, and as its name implies, it is transmitted by a tick. It is the most commonly diagnosed rickettsial disease acquired by travelers (Emerg. Infect. Dis. 2009;15:1791-8). Of 280 individuals diagnosed with rickettsiosis, 231 (82.5%) had spotted fever; almost 87% of the spotted fever rickettsiosis cases were acquired in sub-Saharan Africa, and 69% of these patients reported leisure travel to South Africa. In another review, it was the second-leading cause of systemic febrile illnesses acquired in travelers to sub-Saharan Africa. It was surpassed only by malaria (N. Engl. J. Med. 2006;354:119-30). All age groups are at risk.
Transmission occurs most frequently during the spring and summer months, coinciding with increased tick activity and greater outdoor activities. It is commonly acquired by tourists between November and April in South Africa during a safari or game hunting vacation. Because the incubation period is 5 to 14 days, most travelers may not become symptomatic until after their return. This disease should be suspected in any traveler who presents with fever, headache, and myalgias; has an eschar; and indicates they have recently returned from South Africa. Diagnosis is based on clinical history and serology. Therapy with doxycycline is initiated pending laboratory results.
Disease is controlled by prevention of transmission of the organism by the vector to humans. Use of repellents that contain 20%-30% DEET on exposed skin and wearing clothes treated with permethrin are recommended. Pretreated clothing is also available. Travelers should be encouraged to always check their body after exposure and remove ticks if discovered. Many advocate a bath or shower after coming indoors to facilitate finding any ticks.
Parents should check their children thoroughly for ticks under the arms, in and around the ears, inside the belly button, behind the knees, between the legs, around the waist, and especially in their hair.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
School’s out for the summer soon! Many of your patients may have plans to travel to areas where they may be exposed to infectious diseases and other health risks not routinely encountered in the United States. They will join the 29 million Americans, including almost 3 million children, who traveled to overseas destinations in 2013. The potential for exposures to these risks is dependent on several factors, including the traveler’s age, health and immunization status, destination, accommodations, and duration of travel. Leisure travel, including visiting friends and relatives, accounts for approximately 90% of overseas travel. Some adolescents are traveling to resource-limited areas for adventure travel, educational experiences, and volunteerism. Many times they will reside with host families as part of this experience. There are also children who will have prolonged stays as a result of parental job relocation.

Unfortunately, health precautions often are not considered as many make their travel arrangements. International trips on average are planned at least 105 days in advance; however, many patients wait until the last minute to seek medical advice, if at all. Of 10,032 ill persons who sought post-travel evaluations at participating surveillance facilities (U.S. GeoSentinel sites) between 1997 and 2011, less than half (44%) reported seeking pretravel advice (MMWR 2013;62(SS03):1-15).
Here are some tips that should be useful and easy to implement in your practice for your internationally traveling patients.
• Make sure routine immunizations are up-to-date for age. The exception to this rule is for measles. All children at least 12 months age should receive two doses of MMR prior to departure regardless of their international destination. The second dose of MMR can be administered as early as 4 weeks after the first. Children between 6 and 11 months of age should receive a single dose of MMR prior to departure. If the initial dose is administered at less than 12 months of age, two additional doses will need to be administered to complete the series beginning at 12 months of age.
While measles is no longer endemic in the United States, as of April 25, 2014, there have been 154 cases reported from 14 states. (See measles graphic.) The majority of cases were imported by unvaccinated travelers who became ill after returning home and exposed susceptible individuals. In the last few years, most of the U.S. cases were imported from Western Europe. Currently, there are several countries experiencing record numbers of cases, including Vietnam (3,700) and the Philippines (26,000). This is not to imply that ongoing international outbreaks are limited to these two countries. For additional information, go to cdc.gov/measles.

• Identify someone in your area as a local resource for travel-related information and referrals. Make sure they are willing to see children. Develop a system to send out reminders to families to seek pretravel advice, ideally at least 1 month prior to departure. For children with chronic diseases or compromised immune systems, destination selection may need to be adjusted depending on their medical needs, availability of comparable health care at the overseas destination, and ability to receive pretravel vaccine interventions. Involvement prior to booking the trip would be advisable. Many offices successfully send out reminders for well visits and influenza vaccine. Consider incorporating one for overseas travel.
• The timing of initiation of antimalarial prophylaxis is dependent on the medication. Weekly medications such as chloroquine and mefloquine should begin at least 2 weeks prior to exposure. Atovaquone/proguanil and doxycycline are two drugs that are administered daily, and travelers can begin as late as 2 days prior to entry into a malaria-endemic area. This is a great option for the last-minute traveler.
However, there are contraindications for the use of each drug. Some are age dependent, while others are directly related to the presence of a specific medical condition. Areas where chloroquine-sensitive malaria is present are limited. It is always important to prescribe a prophylactic antimalarial agent, but even more prudent to prescribe the appropriate drug and dosage.
Not sure which drug is most appropriate for your patient? Refer to your local travel medicine expert, or visit cdc.gov/malaria.
• The accompanying table lists vaccines that are traditionally considered to be travel vaccines, but pediatricians and family physicians might not consider all to belong in that group. Most are not required for entry into a specific country, but are recommended based on the risk for potential exposure and disease acquisition. In contrast, yellow fever and meningococcal vaccines are required for entry into certain countries. Yellow fever vaccine can be administered only at authorized sites and should be received at least 10 days prior to arrival at the destination. As with routinely administered vaccines, occasionally there are shortages of travel-related vaccines. Most recently, a shortage of yellow fever vaccine has been resolved.
The majority of vaccines should be administered at least 2 weeks prior to departure, while others, such as rabies and Japanese encephalitis, take at least 28 days to complete the series. These are a few additional reasons it behooves your patients to seek advice early.
Travel updates
Chikungunya virus (CHIK V). Local transmission in the Americas was first reported from St. Martin in December 2013. As of May 5, 2014, a total of 12 Caribbean countries have reported locally acquired cases. The disease is transmitted by Aedes species, which are the same species that transmit dengue fever. Disease is characterized by sudden onset of high fever with severe polyarthralgia. Additional symptoms can include headache, myalgias, rash, nausea, and vomiting. Epidemics have historically occurred in Africa, Asia, and islands in the Indian Ocean. Outbreaks also have occurred in Italy and France.
There is no preventive vaccine or drug available. Treatment is symptomatic care. The disease is best prevented by taking adequate mosquito precautions, especially during the daytime. Application of DEET (N,N-diethyl-m-toluamide) and picaridin-containing agents to the skin or treating clothes with a permethrin-containing agent are just two ways to avoid sustaining a mosquito bite.
While no cases Chikungunya virus have been acquired in the United States, there is a potential risk that the virus will be introduced by an infected traveler or mosquito. The Aedes species that transmits the virus is present in several areas of the United States. For additional information, go to cdc.gov/chikungunya.
Polio. While polio has been eliminated in the United States since 1979, it has never been eradicated in Afghanistan, Nigeria, and Pakistan. For a country to be certified as polio free, there cannot be evidence of circulation of wild polio virus for 3 consecutive years. In spite of a massive global initiative to eliminate this disease, in the last 3 months there have been cases confirmed in the following countries: Cameroon, Ethiopia, Equatorial Guinea, Iraq, Kenya, Somalia, and Syria. While no cases of flaccid paralysis have been confirmed in Israel, wild polio virus has been detected in sewage and isolated from stool of asymptomatic individuals.
Completion of the polio series is recommended for those persons inadequately immunized, and a one-time booster dose is recommended for all adults with travel plans to these countries. This should not be an issue for most pediatric patients, except those who may have deferred immunizations. Booster doses are no longer recommended for travel to countries that border countries with active circulation
African tick bite fever. Frequently overshadowed by the appropriate concern for prevention and acquisition of malaria is a rickettsial disease caused by Rickettsia africae, one of the spotted fever group of rickettsial infections. Its geographic distribution is limited to sub-Saharan Africa, and as its name implies, it is transmitted by a tick. It is the most commonly diagnosed rickettsial disease acquired by travelers (Emerg. Infect. Dis. 2009;15:1791-8). Of 280 individuals diagnosed with rickettsiosis, 231 (82.5%) had spotted fever; almost 87% of the spotted fever rickettsiosis cases were acquired in sub-Saharan Africa, and 69% of these patients reported leisure travel to South Africa. In another review, it was the second-leading cause of systemic febrile illnesses acquired in travelers to sub-Saharan Africa. It was surpassed only by malaria (N. Engl. J. Med. 2006;354:119-30). All age groups are at risk.
Transmission occurs most frequently during the spring and summer months, coinciding with increased tick activity and greater outdoor activities. It is commonly acquired by tourists between November and April in South Africa during a safari or game hunting vacation. Because the incubation period is 5 to 14 days, most travelers may not become symptomatic until after their return. This disease should be suspected in any traveler who presents with fever, headache, and myalgias; has an eschar; and indicates they have recently returned from South Africa. Diagnosis is based on clinical history and serology. Therapy with doxycycline is initiated pending laboratory results.
Disease is controlled by prevention of transmission of the organism by the vector to humans. Use of repellents that contain 20%-30% DEET on exposed skin and wearing clothes treated with permethrin are recommended. Pretreated clothing is also available. Travelers should be encouraged to always check their body after exposure and remove ticks if discovered. Many advocate a bath or shower after coming indoors to facilitate finding any ticks.
Parents should check their children thoroughly for ticks under the arms, in and around the ears, inside the belly button, behind the knees, between the legs, around the waist, and especially in their hair.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
School’s out for the summer soon! Many of your patients may have plans to travel to areas where they may be exposed to infectious diseases and other health risks not routinely encountered in the United States. They will join the 29 million Americans, including almost 3 million children, who traveled to overseas destinations in 2013. The potential for exposures to these risks is dependent on several factors, including the traveler’s age, health and immunization status, destination, accommodations, and duration of travel. Leisure travel, including visiting friends and relatives, accounts for approximately 90% of overseas travel. Some adolescents are traveling to resource-limited areas for adventure travel, educational experiences, and volunteerism. Many times they will reside with host families as part of this experience. There are also children who will have prolonged stays as a result of parental job relocation.

Unfortunately, health precautions often are not considered as many make their travel arrangements. International trips on average are planned at least 105 days in advance; however, many patients wait until the last minute to seek medical advice, if at all. Of 10,032 ill persons who sought post-travel evaluations at participating surveillance facilities (U.S. GeoSentinel sites) between 1997 and 2011, less than half (44%) reported seeking pretravel advice (MMWR 2013;62(SS03):1-15).
Here are some tips that should be useful and easy to implement in your practice for your internationally traveling patients.
• Make sure routine immunizations are up-to-date for age. The exception to this rule is for measles. All children at least 12 months age should receive two doses of MMR prior to departure regardless of their international destination. The second dose of MMR can be administered as early as 4 weeks after the first. Children between 6 and 11 months of age should receive a single dose of MMR prior to departure. If the initial dose is administered at less than 12 months of age, two additional doses will need to be administered to complete the series beginning at 12 months of age.
While measles is no longer endemic in the United States, as of April 25, 2014, there have been 154 cases reported from 14 states. (See measles graphic.) The majority of cases were imported by unvaccinated travelers who became ill after returning home and exposed susceptible individuals. In the last few years, most of the U.S. cases were imported from Western Europe. Currently, there are several countries experiencing record numbers of cases, including Vietnam (3,700) and the Philippines (26,000). This is not to imply that ongoing international outbreaks are limited to these two countries. For additional information, go to cdc.gov/measles.

• Identify someone in your area as a local resource for travel-related information and referrals. Make sure they are willing to see children. Develop a system to send out reminders to families to seek pretravel advice, ideally at least 1 month prior to departure. For children with chronic diseases or compromised immune systems, destination selection may need to be adjusted depending on their medical needs, availability of comparable health care at the overseas destination, and ability to receive pretravel vaccine interventions. Involvement prior to booking the trip would be advisable. Many offices successfully send out reminders for well visits and influenza vaccine. Consider incorporating one for overseas travel.
• The timing of initiation of antimalarial prophylaxis is dependent on the medication. Weekly medications such as chloroquine and mefloquine should begin at least 2 weeks prior to exposure. Atovaquone/proguanil and doxycycline are two drugs that are administered daily, and travelers can begin as late as 2 days prior to entry into a malaria-endemic area. This is a great option for the last-minute traveler.
However, there are contraindications for the use of each drug. Some are age dependent, while others are directly related to the presence of a specific medical condition. Areas where chloroquine-sensitive malaria is present are limited. It is always important to prescribe a prophylactic antimalarial agent, but even more prudent to prescribe the appropriate drug and dosage.
Not sure which drug is most appropriate for your patient? Refer to your local travel medicine expert, or visit cdc.gov/malaria.
• The accompanying table lists vaccines that are traditionally considered to be travel vaccines, but pediatricians and family physicians might not consider all to belong in that group. Most are not required for entry into a specific country, but are recommended based on the risk for potential exposure and disease acquisition. In contrast, yellow fever and meningococcal vaccines are required for entry into certain countries. Yellow fever vaccine can be administered only at authorized sites and should be received at least 10 days prior to arrival at the destination. As with routinely administered vaccines, occasionally there are shortages of travel-related vaccines. Most recently, a shortage of yellow fever vaccine has been resolved.
The majority of vaccines should be administered at least 2 weeks prior to departure, while others, such as rabies and Japanese encephalitis, take at least 28 days to complete the series. These are a few additional reasons it behooves your patients to seek advice early.
Travel updates
Chikungunya virus (CHIK V). Local transmission in the Americas was first reported from St. Martin in December 2013. As of May 5, 2014, a total of 12 Caribbean countries have reported locally acquired cases. The disease is transmitted by Aedes species, which are the same species that transmit dengue fever. Disease is characterized by sudden onset of high fever with severe polyarthralgia. Additional symptoms can include headache, myalgias, rash, nausea, and vomiting. Epidemics have historically occurred in Africa, Asia, and islands in the Indian Ocean. Outbreaks also have occurred in Italy and France.
There is no preventive vaccine or drug available. Treatment is symptomatic care. The disease is best prevented by taking adequate mosquito precautions, especially during the daytime. Application of DEET (N,N-diethyl-m-toluamide) and picaridin-containing agents to the skin or treating clothes with a permethrin-containing agent are just two ways to avoid sustaining a mosquito bite.
While no cases Chikungunya virus have been acquired in the United States, there is a potential risk that the virus will be introduced by an infected traveler or mosquito. The Aedes species that transmits the virus is present in several areas of the United States. For additional information, go to cdc.gov/chikungunya.
Polio. While polio has been eliminated in the United States since 1979, it has never been eradicated in Afghanistan, Nigeria, and Pakistan. For a country to be certified as polio free, there cannot be evidence of circulation of wild polio virus for 3 consecutive years. In spite of a massive global initiative to eliminate this disease, in the last 3 months there have been cases confirmed in the following countries: Cameroon, Ethiopia, Equatorial Guinea, Iraq, Kenya, Somalia, and Syria. While no cases of flaccid paralysis have been confirmed in Israel, wild polio virus has been detected in sewage and isolated from stool of asymptomatic individuals.
Completion of the polio series is recommended for those persons inadequately immunized, and a one-time booster dose is recommended for all adults with travel plans to these countries. This should not be an issue for most pediatric patients, except those who may have deferred immunizations. Booster doses are no longer recommended for travel to countries that border countries with active circulation
African tick bite fever. Frequently overshadowed by the appropriate concern for prevention and acquisition of malaria is a rickettsial disease caused by Rickettsia africae, one of the spotted fever group of rickettsial infections. Its geographic distribution is limited to sub-Saharan Africa, and as its name implies, it is transmitted by a tick. It is the most commonly diagnosed rickettsial disease acquired by travelers (Emerg. Infect. Dis. 2009;15:1791-8). Of 280 individuals diagnosed with rickettsiosis, 231 (82.5%) had spotted fever; almost 87% of the spotted fever rickettsiosis cases were acquired in sub-Saharan Africa, and 69% of these patients reported leisure travel to South Africa. In another review, it was the second-leading cause of systemic febrile illnesses acquired in travelers to sub-Saharan Africa. It was surpassed only by malaria (N. Engl. J. Med. 2006;354:119-30). All age groups are at risk.
Transmission occurs most frequently during the spring and summer months, coinciding with increased tick activity and greater outdoor activities. It is commonly acquired by tourists between November and April in South Africa during a safari or game hunting vacation. Because the incubation period is 5 to 14 days, most travelers may not become symptomatic until after their return. This disease should be suspected in any traveler who presents with fever, headache, and myalgias; has an eschar; and indicates they have recently returned from South Africa. Diagnosis is based on clinical history and serology. Therapy with doxycycline is initiated pending laboratory results.
Disease is controlled by prevention of transmission of the organism by the vector to humans. Use of repellents that contain 20%-30% DEET on exposed skin and wearing clothes treated with permethrin are recommended. Pretreated clothing is also available. Travelers should be encouraged to always check their body after exposure and remove ticks if discovered. Many advocate a bath or shower after coming indoors to facilitate finding any ticks.
Parents should check their children thoroughly for ticks under the arms, in and around the ears, inside the belly button, behind the knees, between the legs, around the waist, and especially in their hair.
Dr. Word is a pediatric infectious disease specialist and director of the Houston Travel Medicine Clinic. She had no relevant financial disclosures. Write to Dr. Word at [email protected].
Clostridium difficile: Not just for adults
The true prevalence and meaning of Clostridium difficile detection in children remains an issue despite a known high prevalence of asymptomatic colonization in children during the first 3 years of life. Distinguishing C. difficile disease from colonization is difficult. Endoscopy can identify some severe C. difficile disease, but what about mild to moderate C. difficile infection?
A passive Centers for Disease Control and Prevention surveillance study (Pediatrics 2014;133:651-8) helps in understanding C. difficile prevalence by documenting the relatively high prevalence of community-acquired C. difficile often associated with use of common oral antibiotics and possibly because of the emergence of the NAP1 strain, which is also emerging in adults. But distinguishing infection from colonization remains an issue. The data have implications for everyday pediatric care.
Methods
Children aged 1-17 years from 10 U.S. states were studied during 2011-2012. C. difficile "cases" were defined via a positive toxin or a molecular test ordered as part of standard care. Standard of care testing for other selected gastrointestinal pathogens and data from medical records were collected. Within 3-6 months of the C. difficile–positive test, a convenience sample of families (about 9%) underwent a telephone interview.
Factors in C. difficile detection
C. difficile was detected in 944 stools from 885 children with no gender difference. The highest rates per 100,000 by race were in whites (23.9) vs. nonwhites (17.4), and in 12- to 23-month-olds (66.3). Overall, 71% of detections were categorized from charted data as community acquired. Only 17% were associated with outpatient health care and 12% with inpatient care.
Antibiotic use in the 14 days before a C. difficile–positive stool was 33% among all cases with no age group differences. Cephalosporins (41%) and amoxicillin/clavulanate (28%) were most common. Among 84 cases also later interviewed by phone, antibiotic use was more frequent (73%); penicillins (39%) and cephalosporins (44%) were the antibiotics most commonly used in this subset of patients. Indications were most often otitis, sinusitis, or upper respiratory infection. In the phone interviews, outpatient office visits were a more frequent (97%) health care exposure than in the overall case population.
Signs and symptoms were mild and similar in all age groups. Diarrhea was not present in 28%. Coinfection with another enteric pathogen was identified in 3% of 535 tested samples: bacterial (n = 12), protozoal (n = 4), and viral (n = 1) – and more common in 2- to 9-year-olds (P = .03). Peripheral WBC counts were abnormal (greater than 15, 000/mm3) in only 7%. There was radiographic evidence of ileus in three and pseudomembranous colitis developed in five cases. Cases were defined as severe in 8% with no age preponderance. There were no deaths.
Infection vs. colonization?
The authors reason that similar clinical presentations and symptom severity at all ages means that detection of C. difficile "likely represents infection" but not colonization. They explain that they expect milder symptoms in the youngest cases if they were only colonized. Is this reasonable?
One could counterargue that in the absence of testing for the most common diarrheagenic pathogen in the United States (norovirus), that diarrhea in at least some of these C. difficile–positive children was likely caused by undetected norovirus. That could partially explain why symptoms were not significantly different by age. One viral coinfection in nearly 500 diarrhea stools (even preselected by C. difficile positivity) seems low. Even if norovirus is not the wildcard here, the similar "disease" at all ages could suggest that something other than C. difficile is the cause. Norovirus and other viral agents testing of samples that were cultured for C. difficile could increase understanding of coinfection rates. Another issue is that 28% of C. difficile children did not have diarrhea, raising concern that these were colonized children.
The authors state that high antibiotic use (73% in phone interviewees) might have contributed to the high C. difficile detection rates. This seems logical, but the phone-derived data came from only about 8% of the total population. The original charted data from the entire population showed 33% antibiotic use. The charted data may have been more reliable because it was collected at the time of the C. difficile–positive stool, not 3-6 months later. Nevertheless, it seems apparent that common outpatient antibiotics could be a factor. If the data were compared with antibiotic use rates for C. difficile–negative children of the same ages, the conclusion would be more powerful.
Children less than 1year of age were not included because up to 73% (Eur. J. Clin. Microbiol. Infect. Dis. 1989;8:390-3) of infants have been reported as asymptomatically colonized. In similar studies, colonized infants were frequent (25% between 6 days and 6 months) up to about 3 years of age when rates dropped off to less than 3%, similar to adults. Inclusion of children in the second and third year of life likely means that not all detections were infections. But there is no way to definitively distinguish infection from colonization in this study.
A further step in filling the knowledge gap on C. difficile would be prospective surveillance with improved definitions of infection vs. colonization and a more complete search for potential concurrent causes of diarrhea. Undoubtedly, many of these C. difficile–positive children had true infection, but it also seems likely that some were colonized, particularly in the second and third year of life. It would be interesting to compare results from healthy controls vs. those with diarrhea using new multiplex molecular assays to gain a better understanding of what proportion of all children have detectable C. difficile with and without other pathogens.
Bottom line
NAP1 C. difficile is emerging in children. C. difficile detection, whether infected or colonized, in this many children is new. These data suggest that our best contributions to reducing the spread of C. difficile are the use of amoxicillin without clavulanate as first line – if antibiotics are needed for acute otitis media and for acute sinusitis – while we refrain from antibiotics for viral upper respiratory infections. As the old knight told Indiana Jones, "Choose wisely."
Factors associated with C. difficile detection in children
1. White race. Question more frequent health care and antibiotic exposure.
2. Age 12 to 23 months. Question whether the population is mix of colonized and infected children. This needs more study.
3. Amoxicillin/clavulanate or oral cephalosporin use for common outpatient infection. Is narrower spectrum, amoxicillin alone better?
4. A recent outpatient health care visit may be a cofactor with #1 and #3.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures. E-mail him at [email protected].
The true prevalence and meaning of Clostridium difficile detection in children remains an issue despite a known high prevalence of asymptomatic colonization in children during the first 3 years of life. Distinguishing C. difficile disease from colonization is difficult. Endoscopy can identify some severe C. difficile disease, but what about mild to moderate C. difficile infection?
A passive Centers for Disease Control and Prevention surveillance study (Pediatrics 2014;133:651-8) helps in understanding C. difficile prevalence by documenting the relatively high prevalence of community-acquired C. difficile often associated with use of common oral antibiotics and possibly because of the emergence of the NAP1 strain, which is also emerging in adults. But distinguishing infection from colonization remains an issue. The data have implications for everyday pediatric care.
Methods
Children aged 1-17 years from 10 U.S. states were studied during 2011-2012. C. difficile "cases" were defined via a positive toxin or a molecular test ordered as part of standard care. Standard of care testing for other selected gastrointestinal pathogens and data from medical records were collected. Within 3-6 months of the C. difficile–positive test, a convenience sample of families (about 9%) underwent a telephone interview.
Factors in C. difficile detection
C. difficile was detected in 944 stools from 885 children with no gender difference. The highest rates per 100,000 by race were in whites (23.9) vs. nonwhites (17.4), and in 12- to 23-month-olds (66.3). Overall, 71% of detections were categorized from charted data as community acquired. Only 17% were associated with outpatient health care and 12% with inpatient care.
Antibiotic use in the 14 days before a C. difficile–positive stool was 33% among all cases with no age group differences. Cephalosporins (41%) and amoxicillin/clavulanate (28%) were most common. Among 84 cases also later interviewed by phone, antibiotic use was more frequent (73%); penicillins (39%) and cephalosporins (44%) were the antibiotics most commonly used in this subset of patients. Indications were most often otitis, sinusitis, or upper respiratory infection. In the phone interviews, outpatient office visits were a more frequent (97%) health care exposure than in the overall case population.
Signs and symptoms were mild and similar in all age groups. Diarrhea was not present in 28%. Coinfection with another enteric pathogen was identified in 3% of 535 tested samples: bacterial (n = 12), protozoal (n = 4), and viral (n = 1) – and more common in 2- to 9-year-olds (P = .03). Peripheral WBC counts were abnormal (greater than 15, 000/mm3) in only 7%. There was radiographic evidence of ileus in three and pseudomembranous colitis developed in five cases. Cases were defined as severe in 8% with no age preponderance. There were no deaths.
Infection vs. colonization?
The authors reason that similar clinical presentations and symptom severity at all ages means that detection of C. difficile "likely represents infection" but not colonization. They explain that they expect milder symptoms in the youngest cases if they were only colonized. Is this reasonable?
One could counterargue that in the absence of testing for the most common diarrheagenic pathogen in the United States (norovirus), that diarrhea in at least some of these C. difficile–positive children was likely caused by undetected norovirus. That could partially explain why symptoms were not significantly different by age. One viral coinfection in nearly 500 diarrhea stools (even preselected by C. difficile positivity) seems low. Even if norovirus is not the wildcard here, the similar "disease" at all ages could suggest that something other than C. difficile is the cause. Norovirus and other viral agents testing of samples that were cultured for C. difficile could increase understanding of coinfection rates. Another issue is that 28% of C. difficile children did not have diarrhea, raising concern that these were colonized children.
The authors state that high antibiotic use (73% in phone interviewees) might have contributed to the high C. difficile detection rates. This seems logical, but the phone-derived data came from only about 8% of the total population. The original charted data from the entire population showed 33% antibiotic use. The charted data may have been more reliable because it was collected at the time of the C. difficile–positive stool, not 3-6 months later. Nevertheless, it seems apparent that common outpatient antibiotics could be a factor. If the data were compared with antibiotic use rates for C. difficile–negative children of the same ages, the conclusion would be more powerful.
Children less than 1year of age were not included because up to 73% (Eur. J. Clin. Microbiol. Infect. Dis. 1989;8:390-3) of infants have been reported as asymptomatically colonized. In similar studies, colonized infants were frequent (25% between 6 days and 6 months) up to about 3 years of age when rates dropped off to less than 3%, similar to adults. Inclusion of children in the second and third year of life likely means that not all detections were infections. But there is no way to definitively distinguish infection from colonization in this study.
A further step in filling the knowledge gap on C. difficile would be prospective surveillance with improved definitions of infection vs. colonization and a more complete search for potential concurrent causes of diarrhea. Undoubtedly, many of these C. difficile–positive children had true infection, but it also seems likely that some were colonized, particularly in the second and third year of life. It would be interesting to compare results from healthy controls vs. those with diarrhea using new multiplex molecular assays to gain a better understanding of what proportion of all children have detectable C. difficile with and without other pathogens.
Bottom line
NAP1 C. difficile is emerging in children. C. difficile detection, whether infected or colonized, in this many children is new. These data suggest that our best contributions to reducing the spread of C. difficile are the use of amoxicillin without clavulanate as first line – if antibiotics are needed for acute otitis media and for acute sinusitis – while we refrain from antibiotics for viral upper respiratory infections. As the old knight told Indiana Jones, "Choose wisely."
Factors associated with C. difficile detection in children
1. White race. Question more frequent health care and antibiotic exposure.
2. Age 12 to 23 months. Question whether the population is mix of colonized and infected children. This needs more study.
3. Amoxicillin/clavulanate or oral cephalosporin use for common outpatient infection. Is narrower spectrum, amoxicillin alone better?
4. A recent outpatient health care visit may be a cofactor with #1 and #3.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures. E-mail him at [email protected].
The true prevalence and meaning of Clostridium difficile detection in children remains an issue despite a known high prevalence of asymptomatic colonization in children during the first 3 years of life. Distinguishing C. difficile disease from colonization is difficult. Endoscopy can identify some severe C. difficile disease, but what about mild to moderate C. difficile infection?
A passive Centers for Disease Control and Prevention surveillance study (Pediatrics 2014;133:651-8) helps in understanding C. difficile prevalence by documenting the relatively high prevalence of community-acquired C. difficile often associated with use of common oral antibiotics and possibly because of the emergence of the NAP1 strain, which is also emerging in adults. But distinguishing infection from colonization remains an issue. The data have implications for everyday pediatric care.
Methods
Children aged 1-17 years from 10 U.S. states were studied during 2011-2012. C. difficile "cases" were defined via a positive toxin or a molecular test ordered as part of standard care. Standard of care testing for other selected gastrointestinal pathogens and data from medical records were collected. Within 3-6 months of the C. difficile–positive test, a convenience sample of families (about 9%) underwent a telephone interview.
Factors in C. difficile detection
C. difficile was detected in 944 stools from 885 children with no gender difference. The highest rates per 100,000 by race were in whites (23.9) vs. nonwhites (17.4), and in 12- to 23-month-olds (66.3). Overall, 71% of detections were categorized from charted data as community acquired. Only 17% were associated with outpatient health care and 12% with inpatient care.
Antibiotic use in the 14 days before a C. difficile–positive stool was 33% among all cases with no age group differences. Cephalosporins (41%) and amoxicillin/clavulanate (28%) were most common. Among 84 cases also later interviewed by phone, antibiotic use was more frequent (73%); penicillins (39%) and cephalosporins (44%) were the antibiotics most commonly used in this subset of patients. Indications were most often otitis, sinusitis, or upper respiratory infection. In the phone interviews, outpatient office visits were a more frequent (97%) health care exposure than in the overall case population.
Signs and symptoms were mild and similar in all age groups. Diarrhea was not present in 28%. Coinfection with another enteric pathogen was identified in 3% of 535 tested samples: bacterial (n = 12), protozoal (n = 4), and viral (n = 1) – and more common in 2- to 9-year-olds (P = .03). Peripheral WBC counts were abnormal (greater than 15, 000/mm3) in only 7%. There was radiographic evidence of ileus in three and pseudomembranous colitis developed in five cases. Cases were defined as severe in 8% with no age preponderance. There were no deaths.
Infection vs. colonization?
The authors reason that similar clinical presentations and symptom severity at all ages means that detection of C. difficile "likely represents infection" but not colonization. They explain that they expect milder symptoms in the youngest cases if they were only colonized. Is this reasonable?
One could counterargue that in the absence of testing for the most common diarrheagenic pathogen in the United States (norovirus), that diarrhea in at least some of these C. difficile–positive children was likely caused by undetected norovirus. That could partially explain why symptoms were not significantly different by age. One viral coinfection in nearly 500 diarrhea stools (even preselected by C. difficile positivity) seems low. Even if norovirus is not the wildcard here, the similar "disease" at all ages could suggest that something other than C. difficile is the cause. Norovirus and other viral agents testing of samples that were cultured for C. difficile could increase understanding of coinfection rates. Another issue is that 28% of C. difficile children did not have diarrhea, raising concern that these were colonized children.
The authors state that high antibiotic use (73% in phone interviewees) might have contributed to the high C. difficile detection rates. This seems logical, but the phone-derived data came from only about 8% of the total population. The original charted data from the entire population showed 33% antibiotic use. The charted data may have been more reliable because it was collected at the time of the C. difficile–positive stool, not 3-6 months later. Nevertheless, it seems apparent that common outpatient antibiotics could be a factor. If the data were compared with antibiotic use rates for C. difficile–negative children of the same ages, the conclusion would be more powerful.
Children less than 1year of age were not included because up to 73% (Eur. J. Clin. Microbiol. Infect. Dis. 1989;8:390-3) of infants have been reported as asymptomatically colonized. In similar studies, colonized infants were frequent (25% between 6 days and 6 months) up to about 3 years of age when rates dropped off to less than 3%, similar to adults. Inclusion of children in the second and third year of life likely means that not all detections were infections. But there is no way to definitively distinguish infection from colonization in this study.
A further step in filling the knowledge gap on C. difficile would be prospective surveillance with improved definitions of infection vs. colonization and a more complete search for potential concurrent causes of diarrhea. Undoubtedly, many of these C. difficile–positive children had true infection, but it also seems likely that some were colonized, particularly in the second and third year of life. It would be interesting to compare results from healthy controls vs. those with diarrhea using new multiplex molecular assays to gain a better understanding of what proportion of all children have detectable C. difficile with and without other pathogens.
Bottom line
NAP1 C. difficile is emerging in children. C. difficile detection, whether infected or colonized, in this many children is new. These data suggest that our best contributions to reducing the spread of C. difficile are the use of amoxicillin without clavulanate as first line – if antibiotics are needed for acute otitis media and for acute sinusitis – while we refrain from antibiotics for viral upper respiratory infections. As the old knight told Indiana Jones, "Choose wisely."
Factors associated with C. difficile detection in children
1. White race. Question more frequent health care and antibiotic exposure.
2. Age 12 to 23 months. Question whether the population is mix of colonized and infected children. This needs more study.
3. Amoxicillin/clavulanate or oral cephalosporin use for common outpatient infection. Is narrower spectrum, amoxicillin alone better?
4. A recent outpatient health care visit may be a cofactor with #1 and #3.
Dr. Harrison is professor of pediatrics and pediatric infectious diseases at Children’s Mercy Hospitals and Clinics, Kansas City, Mo. Dr. Harrison said he has no relevant financial disclosures. E-mail him at [email protected].
Managing fever in the first month
Febrile neonates represent a challenge to clinicians as the risk for serious bacterial infections is highest at this age, the presence of discriminating clinical signs are often absent, and outcomes can be poor in the absence of early treatment. For this reason, most experts recommend that all neonates with a rectal temperature 38°C or higher have blood, urine, and cerebrospinal fluid cultures regardless of clinical appearance (Ann. Emerg. Med. 1993;22:1198-1210). Such neonates should be admitted to the hospital and treated with empiric antibiotics.
In a study of 41,890 neonates (up to 28 days of age) evaluated in 36 pediatric emergency departments, 2,253 (5.4%) were febrile. Three hundred sixty-nine (16%) infants were seen, then discharged from the ED; the remaining 1,884 (84%) were seen and admitted.
As with prior studies, a high rate of serious infection (12%) was documented; urinary tract infection (27%), meningitis (19%), bacteremia and sepsis (14%), cellulitis and soft tissue infections (6%), and pneumonia (3%) were most common. Of the 369 infants discharged, 3 (1%) had serious infection; of the 1,884 admitted, 266 (14%) did.
The study demonstrated significant variability in the approach used to evaluate and treat febrile neonates, with 16% of infants being discharged from the emergency department, the majority of whom (97%) did not get antimicrobial therapy. Sixty-four (3%) of all febrile infants were discharged without any laboratory evaluation or treatment. Eighty-four percent of febrile infants were admitted to the hospital, and 96% of those admitted received antimicrobial treatment (Pediatrics 2014;133:187).
Prior studies reported that serious bacterial infection was uncommon in febrile neonates who met the following six low-risk criteria: 1. an unremarkable medical history, 2. a healthy, nontoxic appearance, 3. no focal signs of infection, 4. an erythrocyte sedimentation rate less than 30 mm at the end of the first hour, 5. a white blood cell count of 5,000-15,000/mcL, and 6. a normal urine analysis (Arch. Dis. Child Fetal Neonatal Ed. 2007;92:F15-8).
Although it is unclear what criteria were used to discharge febrile neonates from the pediatric ED in the current study, only 1 of the 369 neonates discharged from the pediatric ED subsequently returned to the same pediatric ED and was diagnosed with serious infection; however, only 10 in total returned for evaluation. How many subsequently were diagnosed with serious infection at a different facility is unknown. These results were consistent with the initial studies of the "low-risk criteria," which indicates these criteria are not sufficiently reliable to exclude the presence of serious infection.
The study demonstrates that there remains disagreement about how febrile neonates should be evaluated and managed in the ED setting, and how much reliance should be placed on clinical and laboratory parameters. Unlike children older than 3 months of age, in whom immunization with Haemophilus influenzae type b and 13-valent pneumococcal conjugate vaccines has dramatically reduced the incidence of invasive disease, serious infection in febrile neonates up to 28 days of age remains common.
The current spectrum of pathogens and disease – gram-negative uropathogens, staphylococcal and streptococcal skin and soft tissue infections, group B Streptococcus and Staphylococcus aureus bacteremia, and CNS infection – have not been significantly impacted by efforts to prevent "early-onset" neonatal sepsis and by vaccine strategies that target primarily older children. Age remains a risk, with a decreasing incidence of serious bacterial infection as each week of life passes. However, in another study, the rate of serious bacterial infection in febrile neonates 15-21 days of age was found to be sufficiently high to warrant comparable management to that given younger neonates (Pediatr. Inf. Dis. J. 2012;31:455-8).
Thus, currently there seem to be few strategies that would protect febrile neonates from delays in therapy and preventable outcomes, other than the traditional practice of thorough medical evaluation, laboratory testing to include blood, urine, and cerebrospinal fluid cultures, chest x-ray when respiratory tract signs/symptoms are present, and presumptive treatment with parenteral antibiotic therapy.
Office-based studies report greater reliance on clinical judgment with the belief that reliance on clinical guidelines would have only a small benefit, if any, but would result in greater hospitalization and laboratory testing (JAMA 2004;291:1203-12). Still the high rate of disease (14%) in those admitted to the hospital underscore the vulnerability of this age group, the significance of fever, and the potential for a poor outcome without thorough evaluation of each child and presumptive treatment for serious bacterial infection.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. E-mail him at [email protected].
Febrile neonates represent a challenge to clinicians as the risk for serious bacterial infections is highest at this age, the presence of discriminating clinical signs are often absent, and outcomes can be poor in the absence of early treatment. For this reason, most experts recommend that all neonates with a rectal temperature 38°C or higher have blood, urine, and cerebrospinal fluid cultures regardless of clinical appearance (Ann. Emerg. Med. 1993;22:1198-1210). Such neonates should be admitted to the hospital and treated with empiric antibiotics.
In a study of 41,890 neonates (up to 28 days of age) evaluated in 36 pediatric emergency departments, 2,253 (5.4%) were febrile. Three hundred sixty-nine (16%) infants were seen, then discharged from the ED; the remaining 1,884 (84%) were seen and admitted.
As with prior studies, a high rate of serious infection (12%) was documented; urinary tract infection (27%), meningitis (19%), bacteremia and sepsis (14%), cellulitis and soft tissue infections (6%), and pneumonia (3%) were most common. Of the 369 infants discharged, 3 (1%) had serious infection; of the 1,884 admitted, 266 (14%) did.
The study demonstrated significant variability in the approach used to evaluate and treat febrile neonates, with 16% of infants being discharged from the emergency department, the majority of whom (97%) did not get antimicrobial therapy. Sixty-four (3%) of all febrile infants were discharged without any laboratory evaluation or treatment. Eighty-four percent of febrile infants were admitted to the hospital, and 96% of those admitted received antimicrobial treatment (Pediatrics 2014;133:187).
Prior studies reported that serious bacterial infection was uncommon in febrile neonates who met the following six low-risk criteria: 1. an unremarkable medical history, 2. a healthy, nontoxic appearance, 3. no focal signs of infection, 4. an erythrocyte sedimentation rate less than 30 mm at the end of the first hour, 5. a white blood cell count of 5,000-15,000/mcL, and 6. a normal urine analysis (Arch. Dis. Child Fetal Neonatal Ed. 2007;92:F15-8).
Although it is unclear what criteria were used to discharge febrile neonates from the pediatric ED in the current study, only 1 of the 369 neonates discharged from the pediatric ED subsequently returned to the same pediatric ED and was diagnosed with serious infection; however, only 10 in total returned for evaluation. How many subsequently were diagnosed with serious infection at a different facility is unknown. These results were consistent with the initial studies of the "low-risk criteria," which indicates these criteria are not sufficiently reliable to exclude the presence of serious infection.
The study demonstrates that there remains disagreement about how febrile neonates should be evaluated and managed in the ED setting, and how much reliance should be placed on clinical and laboratory parameters. Unlike children older than 3 months of age, in whom immunization with Haemophilus influenzae type b and 13-valent pneumococcal conjugate vaccines has dramatically reduced the incidence of invasive disease, serious infection in febrile neonates up to 28 days of age remains common.
The current spectrum of pathogens and disease – gram-negative uropathogens, staphylococcal and streptococcal skin and soft tissue infections, group B Streptococcus and Staphylococcus aureus bacteremia, and CNS infection – have not been significantly impacted by efforts to prevent "early-onset" neonatal sepsis and by vaccine strategies that target primarily older children. Age remains a risk, with a decreasing incidence of serious bacterial infection as each week of life passes. However, in another study, the rate of serious bacterial infection in febrile neonates 15-21 days of age was found to be sufficiently high to warrant comparable management to that given younger neonates (Pediatr. Inf. Dis. J. 2012;31:455-8).
Thus, currently there seem to be few strategies that would protect febrile neonates from delays in therapy and preventable outcomes, other than the traditional practice of thorough medical evaluation, laboratory testing to include blood, urine, and cerebrospinal fluid cultures, chest x-ray when respiratory tract signs/symptoms are present, and presumptive treatment with parenteral antibiotic therapy.
Office-based studies report greater reliance on clinical judgment with the belief that reliance on clinical guidelines would have only a small benefit, if any, but would result in greater hospitalization and laboratory testing (JAMA 2004;291:1203-12). Still the high rate of disease (14%) in those admitted to the hospital underscore the vulnerability of this age group, the significance of fever, and the potential for a poor outcome without thorough evaluation of each child and presumptive treatment for serious bacterial infection.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. E-mail him at [email protected].
Febrile neonates represent a challenge to clinicians as the risk for serious bacterial infections is highest at this age, the presence of discriminating clinical signs are often absent, and outcomes can be poor in the absence of early treatment. For this reason, most experts recommend that all neonates with a rectal temperature 38°C or higher have blood, urine, and cerebrospinal fluid cultures regardless of clinical appearance (Ann. Emerg. Med. 1993;22:1198-1210). Such neonates should be admitted to the hospital and treated with empiric antibiotics.
In a study of 41,890 neonates (up to 28 days of age) evaluated in 36 pediatric emergency departments, 2,253 (5.4%) were febrile. Three hundred sixty-nine (16%) infants were seen, then discharged from the ED; the remaining 1,884 (84%) were seen and admitted.
As with prior studies, a high rate of serious infection (12%) was documented; urinary tract infection (27%), meningitis (19%), bacteremia and sepsis (14%), cellulitis and soft tissue infections (6%), and pneumonia (3%) were most common. Of the 369 infants discharged, 3 (1%) had serious infection; of the 1,884 admitted, 266 (14%) did.
The study demonstrated significant variability in the approach used to evaluate and treat febrile neonates, with 16% of infants being discharged from the emergency department, the majority of whom (97%) did not get antimicrobial therapy. Sixty-four (3%) of all febrile infants were discharged without any laboratory evaluation or treatment. Eighty-four percent of febrile infants were admitted to the hospital, and 96% of those admitted received antimicrobial treatment (Pediatrics 2014;133:187).
Prior studies reported that serious bacterial infection was uncommon in febrile neonates who met the following six low-risk criteria: 1. an unremarkable medical history, 2. a healthy, nontoxic appearance, 3. no focal signs of infection, 4. an erythrocyte sedimentation rate less than 30 mm at the end of the first hour, 5. a white blood cell count of 5,000-15,000/mcL, and 6. a normal urine analysis (Arch. Dis. Child Fetal Neonatal Ed. 2007;92:F15-8).
Although it is unclear what criteria were used to discharge febrile neonates from the pediatric ED in the current study, only 1 of the 369 neonates discharged from the pediatric ED subsequently returned to the same pediatric ED and was diagnosed with serious infection; however, only 10 in total returned for evaluation. How many subsequently were diagnosed with serious infection at a different facility is unknown. These results were consistent with the initial studies of the "low-risk criteria," which indicates these criteria are not sufficiently reliable to exclude the presence of serious infection.
The study demonstrates that there remains disagreement about how febrile neonates should be evaluated and managed in the ED setting, and how much reliance should be placed on clinical and laboratory parameters. Unlike children older than 3 months of age, in whom immunization with Haemophilus influenzae type b and 13-valent pneumococcal conjugate vaccines has dramatically reduced the incidence of invasive disease, serious infection in febrile neonates up to 28 days of age remains common.
The current spectrum of pathogens and disease – gram-negative uropathogens, staphylococcal and streptococcal skin and soft tissue infections, group B Streptococcus and Staphylococcus aureus bacteremia, and CNS infection – have not been significantly impacted by efforts to prevent "early-onset" neonatal sepsis and by vaccine strategies that target primarily older children. Age remains a risk, with a decreasing incidence of serious bacterial infection as each week of life passes. However, in another study, the rate of serious bacterial infection in febrile neonates 15-21 days of age was found to be sufficiently high to warrant comparable management to that given younger neonates (Pediatr. Inf. Dis. J. 2012;31:455-8).
Thus, currently there seem to be few strategies that would protect febrile neonates from delays in therapy and preventable outcomes, other than the traditional practice of thorough medical evaluation, laboratory testing to include blood, urine, and cerebrospinal fluid cultures, chest x-ray when respiratory tract signs/symptoms are present, and presumptive treatment with parenteral antibiotic therapy.
Office-based studies report greater reliance on clinical judgment with the belief that reliance on clinical guidelines would have only a small benefit, if any, but would result in greater hospitalization and laboratory testing (JAMA 2004;291:1203-12). Still the high rate of disease (14%) in those admitted to the hospital underscore the vulnerability of this age group, the significance of fever, and the potential for a poor outcome without thorough evaluation of each child and presumptive treatment for serious bacterial infection.
Dr. Pelton is chief of pediatric infectious disease and coordinator of the maternal-child HIV program at Boston Medical Center. Dr. Pelton said he had no relevant financial disclosures. E-mail him at [email protected].