User login
Progress still needed for pregnant and postpartum gastroenterologists
Despite increasing numbers joining the field, women remain a minority group in gastroenterology, where they constitute only 18% of these physicians.1 Additionally, women continue to be underrepresented among senior faculty and in leadership roles in both academic and private practice settings.2 While women now make up a majority of medical school matriculants3,4 women trainees are frequently dissuaded from pursuing specialty fellowships following residency, particularly in procedurally based fields like gastroenterology, because of perceived incompatibility with childbearing and child-rearing.5-8 For many who choose to enter the field despite these challenges, gastroenterology training and early practice often coincide with childbearing years.910 These structural impediments may contribute to the “leaky pipeline” and female physician attrition during the first decade of independent practice after fellowship.11-13 Urgent changes are needed in order to retain and support clinicians and physician-scientists through this period so that they, their offspring, their patients, and the field are able to thrive.
Fertility and pregnancy
The decision to have a child is a major milestone for many physicians and often occurs during gastroenterology training or early practice.10 Medical-training and early-career environments are not yet optimized to support women who become pregnant. At baseline, the formative years of a career are challenging ones, punctuated by long hours and both intellectually and emotionally demanding work. They are also often physically grueling, particularly while one is learning and becoming efficient in endoscopy. The ergonomics in the endoscopy suite (as in other areas of medicine) are not optimized for physicians of shorter stature, smaller hand sizes, and those who may have difficulty pushing a several-hundred-pound endoscopy cart bedside, all of which contribute to increased injury risk for female proceduralists.7,14-16 Methods to reduce endoscopic injuries in pregnant endoscopists have not yet been studied. Additionally, the existence of maternity and gender bias has been well-documented, in our field and beyond.17-20 Not surprisingly, women in gastroenterology commonly report delayed childbearing, with expected consequences, including increased infertility rates, compared with nonphysician peers.21 After 5 and 10 years as attendings, female gastroenterologists continue to report fewer children than male colleagues.22,23 Once pregnant, there are a number of field-specific challenges to navigate. These include decisions about the safety of performing procedures involving fluoroscopy or high infectious risk, particularly early in pregnancy when organogenesis occurs.7,24 Additionally, engaging in appropriate obstetric care can be challenging given the need for regular physician and ultrasound appointments.
Simple, cost-efficient interventions may be effective in decreasing infertility rates, pregnancy loss, and poor physician experiences during pregnancy. For one, all gastroenterology divisions could craft written policies that include a no-tolerance approach to expressions of maternity bias against pregnant or postpartum trainees and faculty.12,25 Additionally, ergonomic improvements, such as standing pads, dial extenders, and adjusted screen heights may decrease injury rates and increase comfort for female endoscopists.26,27 There should also be a no-penalty, no-questions-asked approach for any female endoscopist who defers performance of an obstetrically high-risk procedure to a nonpregnant colleague. Additionally, pregnant gastroenterologists should be supported in obtaining high-quality obstetric care. At an individual level, nonpregnant gastroenterologists, and particularly male allies, can support pregnant colleagues by agreeing to perform higher-risk procedures, stepping in if a fellow is unable to perform endoscopy because of pregnancy, and by offering to push the endoscopy cart on behalf of a pregnant colleague to bedside, if necessary.10,28
Parental leave
Following delivery, parental leave presents an additional challenge for the physician parent. Paid maternal leave has been associated with improved child and maternal outcomes and is widely available to physicians outside the United States.29,30 At present, duration of leave varies significantly by career stage (fellows versus attending), practice setting (academic center versus private practice), and geographic location. The American Academy of Pediatrics recommends a minimum of 12 weeks of leave.31 This length has been associated with lower rates of postpartum depression and higher rates of sustained breastfeeding, with subsequent improved health outcomes for mother and child.32-34 An increasing number of states have passed laws mandating minimum paid and unpaid parental leave time (for example, in Massachusetts, gastroenterology trainees and faculty are afforded 12 weeks of leave, in accordance with state law).35 Recent changes to board eligibility and training requirements via the American Board of Medical Specialties and the American Council for Graduate Medical Education now provide 6 weeks for parental leave. This is an improvement over prior policies which rendered many physician-parents board-ineligible if they took more than 4 weeks of leave, although it must be noted that even the revised policies allow for less time than either that of Obstetricians and Gynecologists or than the American Academy of Pediatrics recommends.
Our data, presented at the 2021 ACG conference, suggest that many trainees report receiving 4 weeks or less of parental leave, despite the ACGME and ABMS policies described above. We also found that physicians were frequently not aware of their institution or division leave policies.10 Ideally, all gastroenterology divisions in the United States would follow the recommended leave duration set forth by the medical societies of specialties that care for pregnant and postpartum mothers and their infants. Additionally, the impact of leave time on graduation and board eligibility, as well as academic and practice promotion, should be made clear at the time of leave and should minimize adverse consequences for the careers of pregnant and postpartum gastroenterologists. Gastroenterology trainees and faculty should be educated in the existence and details of their institution or practice policies, and these policies should be made readily available to all physicians and administrators.
Postpartum period
The transition back to work is a challenging one for mothers in all fields of medicine, particularly for those returning to procedurally based subspecialties such as gastroenterology. This is especially true for trainees and faculty who have returned to work sooner than the recommended 12 weeks and for those who are post cesarean section, for whom physical healing may not be complete. Long days performing endoscopy may be physically challenging or impossible for some women during the postpartum period. Additionally, expressing breast milk, a metabolically intensive activity, also necessitates time, space, and privacy to perform and is frequently made more difficult by insufficient lactation accommodations. The COVID-19 pandemic has increased logistic challenges for lactating mothers, because of the need for well-ventilated lactation spaces to minimize infectious risk.19 Our colleagues have reported pumping in their vehicles, in supply closets, and in spaces that require so much travel time (in addition to time required to express milk, store milk, and clean pump equipment) that the practice was unsustainable, and the physician stopped breastfeeding prematurely.36
The benefits of breastfeeding for mother and infant are well-established, and exclusive breastfeeding for the first 6 months of life is supported by the American College of Obstetricians and Gynecologists, whose position statement reads as follows: “Policies that protect the right of a woman and her child to breastfeed ... and that accommodate milk expression, such as ... paid maternity leave, on-site childcare, break time for expressing milk, and a clean, private location for expressing milk, are essential to sustaining breastfeeding.”37 We would add to these recommendations provision of dedicated milk storage space and establishment of clear, supportive policies that allow lactating physicians to breastfeed and express breast milk if they choose without career penalty. Several institutions offer scheduled protected clinical time and modified work relative value units (RVU) for lactating physicians, such that returning parents can have protected time for expressing breast milk and still meet RVU targets.38 Additionally, many academic institutions offer productivity adjustments for tenure-track faculty who have recently had children.
Creating a more supportive environment for women gastroenterologists who desire children allows the field to be more representative of our patient population and has been shown to positively impact outcomes from improved colorectal cancer screening rates to more guideline-directed informed consent conversations.39-41 Gastroenterology should comprise a physician workforce predicated on clinical and research excellence alone and should not require its practitioners to delay or abstain from pregnancy and child rearing. Robust, clear, and generous parental leave and postpartum accommodations will allow the field to retain and promote talented physicians, who will then contribute to the betterment of patients and the field over decades.
Dr. Rabinowitz is a faculty member in the department of medicine and division of gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston. Dr. Feld is a transplant hepatology fellow, division of gastroenterology, department of medicine, University of Washington, Seattle. Dr. Rabinowitz and Dr. Feld have no conflicts of interest to disclose.
References
1. AAMC. Diversity in Medicine: Facts and Figures 2019. 2018.
2. Colleges AoAM. The State of Women in Academic Medicine: The Pipeline and Pathways to Leadership, 2015-2016. 2016. www.aamc.org/download/481206/data/2015table11.pdf.
3. AAMC. Table B-3: Total U.S. Medical School Enrollment by Race/Ethnicity and Sex, 2014-2015 through 2018-2019, 2019.
4. Rabinowitz LG. Recognizing blind spots – a remedy for gender bias in medicine? (N Engl. J Med. 2018; 378[24]: 2253-5).
5. Douglas PS et al. Career preferences and perceptions of cardiology among US internal medicine trainees: Factors influencing cardiology career choice. JAMA Cardiol 2018; 3(8):682-91.
6. Stack SW et al. Childbearing decisions in residency: A multicenter survey of female residents. Acad Med 2020;95(10):1550-7.
7. David YN et al. Pregnancy and the working gastroenterologist: Perceptions, realities, and systemic challenges. Gastroenterology 2021;161(3):756-60.
8. Rembacken BJ et al. Barriers and bias standing in the way of female trainees wanting to learn advanced endoscopy. United European Gastroenterol J. 2019;7(8):1141-5.
9. Arlow FL et al. Gastroenterology training and career choices: A prospective longitudinal study of the impact of gender and of managed care. Am J Gastroenterol. 2002;97(2):459-69.
10. Feld L et al. Parental leave for gastroenterology fellows: A national survey of current fellows. Am J Gastroenterol. 2021;116:S611-2.
11. Rabinowitz LG et al. Addressing gender in gastroenterology: opportunities for change. Gastrointest Endosc. 2020;91(1):155-61.
12. Feld LD. Baby steps in the right direction: Toward a parental leave policy for gastroenterology fellows. Am J Gastroenterol. 2021;116(3):505-8.
13. Feld LD. Interviewing for two. Am J Gastroenterol. 2020;116(3):445-6
14. Rabinowitz LG et al. Gender dynamics in education and practice of gastroenterology. Gastrointest Endosc. 2021;93(5):1047-56.e5.
15. Harvin G. Review of musculoskeletal injuries and prevention in the endoscopy practitioner. J Clin Gastroenterol. 2014;48(7):590-4.
16. LabX Oecs. www.labx.com/product/endoscopy-cart (accessed 2021 Nov 19.
17. Heilman ME and Okimoto TG. Motherhood: A potential source of bias in employment decisions. J Appl Psychol. 2008;93(1):189-98.
18. Robinson K et al. Racism, bias, and discrimination as modifiable barriers to breastfeeding for African American women: A scoping review of the literature. J Midwifery Womens Health. 2019;64(6):734-42.
19. Rabinowitz LG and Rabinowitz DG. Women on the Frontline: A Changed Workforce and the Fight Against COVID-19. Acad Med. 2021 Jun 1;96(6):808-12.
20. Rabinowitz LG et al. Gender in the endoscopy suite. Lancet Gastroenterol Hepatol. 2020 Dec;5(12):1032-4.
21. Stentz NC et al. Fertility and childbearing among American female physicians. J Womens Health. 2016; 25(10):1059-65.
22. Burke CA et al. Gender disparity in the practice of gastroenterology: The first 5 years of a career. Am J Gastroenterol. 2005;100(2):259-64.
23. Singh A et al. Women in gastroenterology committee of American College of G. Do gender disparities persist in gastroenterology after 10 years of practice? Am J Gastroenterol. 2008;103(7):1589-95.
24. Krueger KJ and Hoffman BJ. Radiation exposure during gastroenterologic fluoroscopy: Risk assessment for pregnant workers. Am J Gastroenterol. 1992;87(4):429-31.
25. Krause ML et al. Impact of pregnancy and gender on internal medicine resident evaluations: A retrospective cohort study. J Gen Intern Med. 2017;32(6):648-53.
26. Pawa S et al. Are all endoscopy-related musculoskeletal injuries created equal? Results of a national gender-based survey. Am J Gastroenterol. 2021;116(3):530-8.
27. David YN et al. Gender-specific factors influencing gastroenterologists to pursue careers in advanced endoscopy: perceptions vs reality. Am J Gastroenterol. 2021;116(3):539-50.
28. Bilal M et al. The need for allyship in achieving gender equity in gastroenterology. Am J Gastroenterol. 2021 Oct 19. doi: 10.14309/ajg.0000000000001508. Online ahead of print.
29. Jou J et al. Paid maternity leave in the United States: Associations with maternal and infant health. Matern Child Health J. 2018;22(2):216-25.
30. Aitken Z et al. The maternal health outcomes of paid maternity leave: A systematic review. Soc Sci Med. 2015;130:32-41.
31. Dodson NA and Talib HJ. Paid parental leave for mothers and fathers can improve physician wellness. AAP News. 2020 Jul 1. https://publications.aap.org/aapnews/news/12432.
32. Kornfeind KR and Sipsma HL. Exploring the link between maternity leave and postpartum depression. Womens Health Issues 2018;28(4):321-6.
33. Navarro-Rosenblatt D and Garmendia ML. Maternity leave and its impact on breastfeeding: A review of the literature. Breastfeed Med 2018;13(9):589-97.
34. Stack SW et al. Maternity leave in residency: A multicenter study of determinants and wellness outcomes. Acad Med. 2019;94(11):1738-45.
35. Mass.gov. Paid Family and Medical Leave Information for Massachusetts Employers. 2020.
36. Ares Segura S et al. en representacion del Comite de Lactancia Materna de la Asociacion Espanola de P. [The importance of maternal nutrition during breastfeeding: Do breastfeeding mothers need nutritional supplements?]. An Pediatr. (Barc) 2016;84(6):347 e1-7.
37. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 658: Optimizing Support for Breastfeeding as Part of Obstetric Practice. Obstet Gynecol. 2016;127(2):e86-92.
38. Porter KK et al. A lactation credit model to support breastfeeding in radiology: The new gold standard to support “liquid gold.” Clin Imaging 2021;80:16-8.
39. Davis J et al. Clinical practice patterns suggest female patients prefer female endoscopists. Dig Dis Sci. 2015;60(10):3149-50.
40. Menees SB et al. Women patients’ preference for women physicians is a barrier to colon cancer screening. Gastrointest Endosc. 2005;62(2):219-23.
41. Feld LD et al. Management of code status in the periendoscopic period: A national survey of current practices and beliefs of U.S. gastroenterologists. Gastrointest Endosc. 2021;94(1):172-7.e2.
Despite increasing numbers joining the field, women remain a minority group in gastroenterology, where they constitute only 18% of these physicians.1 Additionally, women continue to be underrepresented among senior faculty and in leadership roles in both academic and private practice settings.2 While women now make up a majority of medical school matriculants3,4 women trainees are frequently dissuaded from pursuing specialty fellowships following residency, particularly in procedurally based fields like gastroenterology, because of perceived incompatibility with childbearing and child-rearing.5-8 For many who choose to enter the field despite these challenges, gastroenterology training and early practice often coincide with childbearing years.910 These structural impediments may contribute to the “leaky pipeline” and female physician attrition during the first decade of independent practice after fellowship.11-13 Urgent changes are needed in order to retain and support clinicians and physician-scientists through this period so that they, their offspring, their patients, and the field are able to thrive.
Fertility and pregnancy
The decision to have a child is a major milestone for many physicians and often occurs during gastroenterology training or early practice.10 Medical-training and early-career environments are not yet optimized to support women who become pregnant. At baseline, the formative years of a career are challenging ones, punctuated by long hours and both intellectually and emotionally demanding work. They are also often physically grueling, particularly while one is learning and becoming efficient in endoscopy. The ergonomics in the endoscopy suite (as in other areas of medicine) are not optimized for physicians of shorter stature, smaller hand sizes, and those who may have difficulty pushing a several-hundred-pound endoscopy cart bedside, all of which contribute to increased injury risk for female proceduralists.7,14-16 Methods to reduce endoscopic injuries in pregnant endoscopists have not yet been studied. Additionally, the existence of maternity and gender bias has been well-documented, in our field and beyond.17-20 Not surprisingly, women in gastroenterology commonly report delayed childbearing, with expected consequences, including increased infertility rates, compared with nonphysician peers.21 After 5 and 10 years as attendings, female gastroenterologists continue to report fewer children than male colleagues.22,23 Once pregnant, there are a number of field-specific challenges to navigate. These include decisions about the safety of performing procedures involving fluoroscopy or high infectious risk, particularly early in pregnancy when organogenesis occurs.7,24 Additionally, engaging in appropriate obstetric care can be challenging given the need for regular physician and ultrasound appointments.
Simple, cost-efficient interventions may be effective in decreasing infertility rates, pregnancy loss, and poor physician experiences during pregnancy. For one, all gastroenterology divisions could craft written policies that include a no-tolerance approach to expressions of maternity bias against pregnant or postpartum trainees and faculty.12,25 Additionally, ergonomic improvements, such as standing pads, dial extenders, and adjusted screen heights may decrease injury rates and increase comfort for female endoscopists.26,27 There should also be a no-penalty, no-questions-asked approach for any female endoscopist who defers performance of an obstetrically high-risk procedure to a nonpregnant colleague. Additionally, pregnant gastroenterologists should be supported in obtaining high-quality obstetric care. At an individual level, nonpregnant gastroenterologists, and particularly male allies, can support pregnant colleagues by agreeing to perform higher-risk procedures, stepping in if a fellow is unable to perform endoscopy because of pregnancy, and by offering to push the endoscopy cart on behalf of a pregnant colleague to bedside, if necessary.10,28
Parental leave
Following delivery, parental leave presents an additional challenge for the physician parent. Paid maternal leave has been associated with improved child and maternal outcomes and is widely available to physicians outside the United States.29,30 At present, duration of leave varies significantly by career stage (fellows versus attending), practice setting (academic center versus private practice), and geographic location. The American Academy of Pediatrics recommends a minimum of 12 weeks of leave.31 This length has been associated with lower rates of postpartum depression and higher rates of sustained breastfeeding, with subsequent improved health outcomes for mother and child.32-34 An increasing number of states have passed laws mandating minimum paid and unpaid parental leave time (for example, in Massachusetts, gastroenterology trainees and faculty are afforded 12 weeks of leave, in accordance with state law).35 Recent changes to board eligibility and training requirements via the American Board of Medical Specialties and the American Council for Graduate Medical Education now provide 6 weeks for parental leave. This is an improvement over prior policies which rendered many physician-parents board-ineligible if they took more than 4 weeks of leave, although it must be noted that even the revised policies allow for less time than either that of Obstetricians and Gynecologists or than the American Academy of Pediatrics recommends.
Our data, presented at the 2021 ACG conference, suggest that many trainees report receiving 4 weeks or less of parental leave, despite the ACGME and ABMS policies described above. We also found that physicians were frequently not aware of their institution or division leave policies.10 Ideally, all gastroenterology divisions in the United States would follow the recommended leave duration set forth by the medical societies of specialties that care for pregnant and postpartum mothers and their infants. Additionally, the impact of leave time on graduation and board eligibility, as well as academic and practice promotion, should be made clear at the time of leave and should minimize adverse consequences for the careers of pregnant and postpartum gastroenterologists. Gastroenterology trainees and faculty should be educated in the existence and details of their institution or practice policies, and these policies should be made readily available to all physicians and administrators.
Postpartum period
The transition back to work is a challenging one for mothers in all fields of medicine, particularly for those returning to procedurally based subspecialties such as gastroenterology. This is especially true for trainees and faculty who have returned to work sooner than the recommended 12 weeks and for those who are post cesarean section, for whom physical healing may not be complete. Long days performing endoscopy may be physically challenging or impossible for some women during the postpartum period. Additionally, expressing breast milk, a metabolically intensive activity, also necessitates time, space, and privacy to perform and is frequently made more difficult by insufficient lactation accommodations. The COVID-19 pandemic has increased logistic challenges for lactating mothers, because of the need for well-ventilated lactation spaces to minimize infectious risk.19 Our colleagues have reported pumping in their vehicles, in supply closets, and in spaces that require so much travel time (in addition to time required to express milk, store milk, and clean pump equipment) that the practice was unsustainable, and the physician stopped breastfeeding prematurely.36
The benefits of breastfeeding for mother and infant are well-established, and exclusive breastfeeding for the first 6 months of life is supported by the American College of Obstetricians and Gynecologists, whose position statement reads as follows: “Policies that protect the right of a woman and her child to breastfeed ... and that accommodate milk expression, such as ... paid maternity leave, on-site childcare, break time for expressing milk, and a clean, private location for expressing milk, are essential to sustaining breastfeeding.”37 We would add to these recommendations provision of dedicated milk storage space and establishment of clear, supportive policies that allow lactating physicians to breastfeed and express breast milk if they choose without career penalty. Several institutions offer scheduled protected clinical time and modified work relative value units (RVU) for lactating physicians, such that returning parents can have protected time for expressing breast milk and still meet RVU targets.38 Additionally, many academic institutions offer productivity adjustments for tenure-track faculty who have recently had children.
Creating a more supportive environment for women gastroenterologists who desire children allows the field to be more representative of our patient population and has been shown to positively impact outcomes from improved colorectal cancer screening rates to more guideline-directed informed consent conversations.39-41 Gastroenterology should comprise a physician workforce predicated on clinical and research excellence alone and should not require its practitioners to delay or abstain from pregnancy and child rearing. Robust, clear, and generous parental leave and postpartum accommodations will allow the field to retain and promote talented physicians, who will then contribute to the betterment of patients and the field over decades.
Dr. Rabinowitz is a faculty member in the department of medicine and division of gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston. Dr. Feld is a transplant hepatology fellow, division of gastroenterology, department of medicine, University of Washington, Seattle. Dr. Rabinowitz and Dr. Feld have no conflicts of interest to disclose.
References
1. AAMC. Diversity in Medicine: Facts and Figures 2019. 2018.
2. Colleges AoAM. The State of Women in Academic Medicine: The Pipeline and Pathways to Leadership, 2015-2016. 2016. www.aamc.org/download/481206/data/2015table11.pdf.
3. AAMC. Table B-3: Total U.S. Medical School Enrollment by Race/Ethnicity and Sex, 2014-2015 through 2018-2019, 2019.
4. Rabinowitz LG. Recognizing blind spots – a remedy for gender bias in medicine? (N Engl. J Med. 2018; 378[24]: 2253-5).
5. Douglas PS et al. Career preferences and perceptions of cardiology among US internal medicine trainees: Factors influencing cardiology career choice. JAMA Cardiol 2018; 3(8):682-91.
6. Stack SW et al. Childbearing decisions in residency: A multicenter survey of female residents. Acad Med 2020;95(10):1550-7.
7. David YN et al. Pregnancy and the working gastroenterologist: Perceptions, realities, and systemic challenges. Gastroenterology 2021;161(3):756-60.
8. Rembacken BJ et al. Barriers and bias standing in the way of female trainees wanting to learn advanced endoscopy. United European Gastroenterol J. 2019;7(8):1141-5.
9. Arlow FL et al. Gastroenterology training and career choices: A prospective longitudinal study of the impact of gender and of managed care. Am J Gastroenterol. 2002;97(2):459-69.
10. Feld L et al. Parental leave for gastroenterology fellows: A national survey of current fellows. Am J Gastroenterol. 2021;116:S611-2.
11. Rabinowitz LG et al. Addressing gender in gastroenterology: opportunities for change. Gastrointest Endosc. 2020;91(1):155-61.
12. Feld LD. Baby steps in the right direction: Toward a parental leave policy for gastroenterology fellows. Am J Gastroenterol. 2021;116(3):505-8.
13. Feld LD. Interviewing for two. Am J Gastroenterol. 2020;116(3):445-6
14. Rabinowitz LG et al. Gender dynamics in education and practice of gastroenterology. Gastrointest Endosc. 2021;93(5):1047-56.e5.
15. Harvin G. Review of musculoskeletal injuries and prevention in the endoscopy practitioner. J Clin Gastroenterol. 2014;48(7):590-4.
16. LabX Oecs. www.labx.com/product/endoscopy-cart (accessed 2021 Nov 19.
17. Heilman ME and Okimoto TG. Motherhood: A potential source of bias in employment decisions. J Appl Psychol. 2008;93(1):189-98.
18. Robinson K et al. Racism, bias, and discrimination as modifiable barriers to breastfeeding for African American women: A scoping review of the literature. J Midwifery Womens Health. 2019;64(6):734-42.
19. Rabinowitz LG and Rabinowitz DG. Women on the Frontline: A Changed Workforce and the Fight Against COVID-19. Acad Med. 2021 Jun 1;96(6):808-12.
20. Rabinowitz LG et al. Gender in the endoscopy suite. Lancet Gastroenterol Hepatol. 2020 Dec;5(12):1032-4.
21. Stentz NC et al. Fertility and childbearing among American female physicians. J Womens Health. 2016; 25(10):1059-65.
22. Burke CA et al. Gender disparity in the practice of gastroenterology: The first 5 years of a career. Am J Gastroenterol. 2005;100(2):259-64.
23. Singh A et al. Women in gastroenterology committee of American College of G. Do gender disparities persist in gastroenterology after 10 years of practice? Am J Gastroenterol. 2008;103(7):1589-95.
24. Krueger KJ and Hoffman BJ. Radiation exposure during gastroenterologic fluoroscopy: Risk assessment for pregnant workers. Am J Gastroenterol. 1992;87(4):429-31.
25. Krause ML et al. Impact of pregnancy and gender on internal medicine resident evaluations: A retrospective cohort study. J Gen Intern Med. 2017;32(6):648-53.
26. Pawa S et al. Are all endoscopy-related musculoskeletal injuries created equal? Results of a national gender-based survey. Am J Gastroenterol. 2021;116(3):530-8.
27. David YN et al. Gender-specific factors influencing gastroenterologists to pursue careers in advanced endoscopy: perceptions vs reality. Am J Gastroenterol. 2021;116(3):539-50.
28. Bilal M et al. The need for allyship in achieving gender equity in gastroenterology. Am J Gastroenterol. 2021 Oct 19. doi: 10.14309/ajg.0000000000001508. Online ahead of print.
29. Jou J et al. Paid maternity leave in the United States: Associations with maternal and infant health. Matern Child Health J. 2018;22(2):216-25.
30. Aitken Z et al. The maternal health outcomes of paid maternity leave: A systematic review. Soc Sci Med. 2015;130:32-41.
31. Dodson NA and Talib HJ. Paid parental leave for mothers and fathers can improve physician wellness. AAP News. 2020 Jul 1. https://publications.aap.org/aapnews/news/12432.
32. Kornfeind KR and Sipsma HL. Exploring the link between maternity leave and postpartum depression. Womens Health Issues 2018;28(4):321-6.
33. Navarro-Rosenblatt D and Garmendia ML. Maternity leave and its impact on breastfeeding: A review of the literature. Breastfeed Med 2018;13(9):589-97.
34. Stack SW et al. Maternity leave in residency: A multicenter study of determinants and wellness outcomes. Acad Med. 2019;94(11):1738-45.
35. Mass.gov. Paid Family and Medical Leave Information for Massachusetts Employers. 2020.
36. Ares Segura S et al. en representacion del Comite de Lactancia Materna de la Asociacion Espanola de P. [The importance of maternal nutrition during breastfeeding: Do breastfeeding mothers need nutritional supplements?]. An Pediatr. (Barc) 2016;84(6):347 e1-7.
37. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 658: Optimizing Support for Breastfeeding as Part of Obstetric Practice. Obstet Gynecol. 2016;127(2):e86-92.
38. Porter KK et al. A lactation credit model to support breastfeeding in radiology: The new gold standard to support “liquid gold.” Clin Imaging 2021;80:16-8.
39. Davis J et al. Clinical practice patterns suggest female patients prefer female endoscopists. Dig Dis Sci. 2015;60(10):3149-50.
40. Menees SB et al. Women patients’ preference for women physicians is a barrier to colon cancer screening. Gastrointest Endosc. 2005;62(2):219-23.
41. Feld LD et al. Management of code status in the periendoscopic period: A national survey of current practices and beliefs of U.S. gastroenterologists. Gastrointest Endosc. 2021;94(1):172-7.e2.
Despite increasing numbers joining the field, women remain a minority group in gastroenterology, where they constitute only 18% of these physicians.1 Additionally, women continue to be underrepresented among senior faculty and in leadership roles in both academic and private practice settings.2 While women now make up a majority of medical school matriculants3,4 women trainees are frequently dissuaded from pursuing specialty fellowships following residency, particularly in procedurally based fields like gastroenterology, because of perceived incompatibility with childbearing and child-rearing.5-8 For many who choose to enter the field despite these challenges, gastroenterology training and early practice often coincide with childbearing years.910 These structural impediments may contribute to the “leaky pipeline” and female physician attrition during the first decade of independent practice after fellowship.11-13 Urgent changes are needed in order to retain and support clinicians and physician-scientists through this period so that they, their offspring, their patients, and the field are able to thrive.
Fertility and pregnancy
The decision to have a child is a major milestone for many physicians and often occurs during gastroenterology training or early practice.10 Medical-training and early-career environments are not yet optimized to support women who become pregnant. At baseline, the formative years of a career are challenging ones, punctuated by long hours and both intellectually and emotionally demanding work. They are also often physically grueling, particularly while one is learning and becoming efficient in endoscopy. The ergonomics in the endoscopy suite (as in other areas of medicine) are not optimized for physicians of shorter stature, smaller hand sizes, and those who may have difficulty pushing a several-hundred-pound endoscopy cart bedside, all of which contribute to increased injury risk for female proceduralists.7,14-16 Methods to reduce endoscopic injuries in pregnant endoscopists have not yet been studied. Additionally, the existence of maternity and gender bias has been well-documented, in our field and beyond.17-20 Not surprisingly, women in gastroenterology commonly report delayed childbearing, with expected consequences, including increased infertility rates, compared with nonphysician peers.21 After 5 and 10 years as attendings, female gastroenterologists continue to report fewer children than male colleagues.22,23 Once pregnant, there are a number of field-specific challenges to navigate. These include decisions about the safety of performing procedures involving fluoroscopy or high infectious risk, particularly early in pregnancy when organogenesis occurs.7,24 Additionally, engaging in appropriate obstetric care can be challenging given the need for regular physician and ultrasound appointments.
Simple, cost-efficient interventions may be effective in decreasing infertility rates, pregnancy loss, and poor physician experiences during pregnancy. For one, all gastroenterology divisions could craft written policies that include a no-tolerance approach to expressions of maternity bias against pregnant or postpartum trainees and faculty.12,25 Additionally, ergonomic improvements, such as standing pads, dial extenders, and adjusted screen heights may decrease injury rates and increase comfort for female endoscopists.26,27 There should also be a no-penalty, no-questions-asked approach for any female endoscopist who defers performance of an obstetrically high-risk procedure to a nonpregnant colleague. Additionally, pregnant gastroenterologists should be supported in obtaining high-quality obstetric care. At an individual level, nonpregnant gastroenterologists, and particularly male allies, can support pregnant colleagues by agreeing to perform higher-risk procedures, stepping in if a fellow is unable to perform endoscopy because of pregnancy, and by offering to push the endoscopy cart on behalf of a pregnant colleague to bedside, if necessary.10,28
Parental leave
Following delivery, parental leave presents an additional challenge for the physician parent. Paid maternal leave has been associated with improved child and maternal outcomes and is widely available to physicians outside the United States.29,30 At present, duration of leave varies significantly by career stage (fellows versus attending), practice setting (academic center versus private practice), and geographic location. The American Academy of Pediatrics recommends a minimum of 12 weeks of leave.31 This length has been associated with lower rates of postpartum depression and higher rates of sustained breastfeeding, with subsequent improved health outcomes for mother and child.32-34 An increasing number of states have passed laws mandating minimum paid and unpaid parental leave time (for example, in Massachusetts, gastroenterology trainees and faculty are afforded 12 weeks of leave, in accordance with state law).35 Recent changes to board eligibility and training requirements via the American Board of Medical Specialties and the American Council for Graduate Medical Education now provide 6 weeks for parental leave. This is an improvement over prior policies which rendered many physician-parents board-ineligible if they took more than 4 weeks of leave, although it must be noted that even the revised policies allow for less time than either that of Obstetricians and Gynecologists or than the American Academy of Pediatrics recommends.
Our data, presented at the 2021 ACG conference, suggest that many trainees report receiving 4 weeks or less of parental leave, despite the ACGME and ABMS policies described above. We also found that physicians were frequently not aware of their institution or division leave policies.10 Ideally, all gastroenterology divisions in the United States would follow the recommended leave duration set forth by the medical societies of specialties that care for pregnant and postpartum mothers and their infants. Additionally, the impact of leave time on graduation and board eligibility, as well as academic and practice promotion, should be made clear at the time of leave and should minimize adverse consequences for the careers of pregnant and postpartum gastroenterologists. Gastroenterology trainees and faculty should be educated in the existence and details of their institution or practice policies, and these policies should be made readily available to all physicians and administrators.
Postpartum period
The transition back to work is a challenging one for mothers in all fields of medicine, particularly for those returning to procedurally based subspecialties such as gastroenterology. This is especially true for trainees and faculty who have returned to work sooner than the recommended 12 weeks and for those who are post cesarean section, for whom physical healing may not be complete. Long days performing endoscopy may be physically challenging or impossible for some women during the postpartum period. Additionally, expressing breast milk, a metabolically intensive activity, also necessitates time, space, and privacy to perform and is frequently made more difficult by insufficient lactation accommodations. The COVID-19 pandemic has increased logistic challenges for lactating mothers, because of the need for well-ventilated lactation spaces to minimize infectious risk.19 Our colleagues have reported pumping in their vehicles, in supply closets, and in spaces that require so much travel time (in addition to time required to express milk, store milk, and clean pump equipment) that the practice was unsustainable, and the physician stopped breastfeeding prematurely.36
The benefits of breastfeeding for mother and infant are well-established, and exclusive breastfeeding for the first 6 months of life is supported by the American College of Obstetricians and Gynecologists, whose position statement reads as follows: “Policies that protect the right of a woman and her child to breastfeed ... and that accommodate milk expression, such as ... paid maternity leave, on-site childcare, break time for expressing milk, and a clean, private location for expressing milk, are essential to sustaining breastfeeding.”37 We would add to these recommendations provision of dedicated milk storage space and establishment of clear, supportive policies that allow lactating physicians to breastfeed and express breast milk if they choose without career penalty. Several institutions offer scheduled protected clinical time and modified work relative value units (RVU) for lactating physicians, such that returning parents can have protected time for expressing breast milk and still meet RVU targets.38 Additionally, many academic institutions offer productivity adjustments for tenure-track faculty who have recently had children.
Creating a more supportive environment for women gastroenterologists who desire children allows the field to be more representative of our patient population and has been shown to positively impact outcomes from improved colorectal cancer screening rates to more guideline-directed informed consent conversations.39-41 Gastroenterology should comprise a physician workforce predicated on clinical and research excellence alone and should not require its practitioners to delay or abstain from pregnancy and child rearing. Robust, clear, and generous parental leave and postpartum accommodations will allow the field to retain and promote talented physicians, who will then contribute to the betterment of patients and the field over decades.
Dr. Rabinowitz is a faculty member in the department of medicine and division of gastroenterology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston. Dr. Feld is a transplant hepatology fellow, division of gastroenterology, department of medicine, University of Washington, Seattle. Dr. Rabinowitz and Dr. Feld have no conflicts of interest to disclose.
References
1. AAMC. Diversity in Medicine: Facts and Figures 2019. 2018.
2. Colleges AoAM. The State of Women in Academic Medicine: The Pipeline and Pathways to Leadership, 2015-2016. 2016. www.aamc.org/download/481206/data/2015table11.pdf.
3. AAMC. Table B-3: Total U.S. Medical School Enrollment by Race/Ethnicity and Sex, 2014-2015 through 2018-2019, 2019.
4. Rabinowitz LG. Recognizing blind spots – a remedy for gender bias in medicine? (N Engl. J Med. 2018; 378[24]: 2253-5).
5. Douglas PS et al. Career preferences and perceptions of cardiology among US internal medicine trainees: Factors influencing cardiology career choice. JAMA Cardiol 2018; 3(8):682-91.
6. Stack SW et al. Childbearing decisions in residency: A multicenter survey of female residents. Acad Med 2020;95(10):1550-7.
7. David YN et al. Pregnancy and the working gastroenterologist: Perceptions, realities, and systemic challenges. Gastroenterology 2021;161(3):756-60.
8. Rembacken BJ et al. Barriers and bias standing in the way of female trainees wanting to learn advanced endoscopy. United European Gastroenterol J. 2019;7(8):1141-5.
9. Arlow FL et al. Gastroenterology training and career choices: A prospective longitudinal study of the impact of gender and of managed care. Am J Gastroenterol. 2002;97(2):459-69.
10. Feld L et al. Parental leave for gastroenterology fellows: A national survey of current fellows. Am J Gastroenterol. 2021;116:S611-2.
11. Rabinowitz LG et al. Addressing gender in gastroenterology: opportunities for change. Gastrointest Endosc. 2020;91(1):155-61.
12. Feld LD. Baby steps in the right direction: Toward a parental leave policy for gastroenterology fellows. Am J Gastroenterol. 2021;116(3):505-8.
13. Feld LD. Interviewing for two. Am J Gastroenterol. 2020;116(3):445-6
14. Rabinowitz LG et al. Gender dynamics in education and practice of gastroenterology. Gastrointest Endosc. 2021;93(5):1047-56.e5.
15. Harvin G. Review of musculoskeletal injuries and prevention in the endoscopy practitioner. J Clin Gastroenterol. 2014;48(7):590-4.
16. LabX Oecs. www.labx.com/product/endoscopy-cart (accessed 2021 Nov 19.
17. Heilman ME and Okimoto TG. Motherhood: A potential source of bias in employment decisions. J Appl Psychol. 2008;93(1):189-98.
18. Robinson K et al. Racism, bias, and discrimination as modifiable barriers to breastfeeding for African American women: A scoping review of the literature. J Midwifery Womens Health. 2019;64(6):734-42.
19. Rabinowitz LG and Rabinowitz DG. Women on the Frontline: A Changed Workforce and the Fight Against COVID-19. Acad Med. 2021 Jun 1;96(6):808-12.
20. Rabinowitz LG et al. Gender in the endoscopy suite. Lancet Gastroenterol Hepatol. 2020 Dec;5(12):1032-4.
21. Stentz NC et al. Fertility and childbearing among American female physicians. J Womens Health. 2016; 25(10):1059-65.
22. Burke CA et al. Gender disparity in the practice of gastroenterology: The first 5 years of a career. Am J Gastroenterol. 2005;100(2):259-64.
23. Singh A et al. Women in gastroenterology committee of American College of G. Do gender disparities persist in gastroenterology after 10 years of practice? Am J Gastroenterol. 2008;103(7):1589-95.
24. Krueger KJ and Hoffman BJ. Radiation exposure during gastroenterologic fluoroscopy: Risk assessment for pregnant workers. Am J Gastroenterol. 1992;87(4):429-31.
25. Krause ML et al. Impact of pregnancy and gender on internal medicine resident evaluations: A retrospective cohort study. J Gen Intern Med. 2017;32(6):648-53.
26. Pawa S et al. Are all endoscopy-related musculoskeletal injuries created equal? Results of a national gender-based survey. Am J Gastroenterol. 2021;116(3):530-8.
27. David YN et al. Gender-specific factors influencing gastroenterologists to pursue careers in advanced endoscopy: perceptions vs reality. Am J Gastroenterol. 2021;116(3):539-50.
28. Bilal M et al. The need for allyship in achieving gender equity in gastroenterology. Am J Gastroenterol. 2021 Oct 19. doi: 10.14309/ajg.0000000000001508. Online ahead of print.
29. Jou J et al. Paid maternity leave in the United States: Associations with maternal and infant health. Matern Child Health J. 2018;22(2):216-25.
30. Aitken Z et al. The maternal health outcomes of paid maternity leave: A systematic review. Soc Sci Med. 2015;130:32-41.
31. Dodson NA and Talib HJ. Paid parental leave for mothers and fathers can improve physician wellness. AAP News. 2020 Jul 1. https://publications.aap.org/aapnews/news/12432.
32. Kornfeind KR and Sipsma HL. Exploring the link between maternity leave and postpartum depression. Womens Health Issues 2018;28(4):321-6.
33. Navarro-Rosenblatt D and Garmendia ML. Maternity leave and its impact on breastfeeding: A review of the literature. Breastfeed Med 2018;13(9):589-97.
34. Stack SW et al. Maternity leave in residency: A multicenter study of determinants and wellness outcomes. Acad Med. 2019;94(11):1738-45.
35. Mass.gov. Paid Family and Medical Leave Information for Massachusetts Employers. 2020.
36. Ares Segura S et al. en representacion del Comite de Lactancia Materna de la Asociacion Espanola de P. [The importance of maternal nutrition during breastfeeding: Do breastfeeding mothers need nutritional supplements?]. An Pediatr. (Barc) 2016;84(6):347 e1-7.
37. American College of Obstetricians and Gynecologists, Committee on Obstetric Practice. Committee Opinion No. 658: Optimizing Support for Breastfeeding as Part of Obstetric Practice. Obstet Gynecol. 2016;127(2):e86-92.
38. Porter KK et al. A lactation credit model to support breastfeeding in radiology: The new gold standard to support “liquid gold.” Clin Imaging 2021;80:16-8.
39. Davis J et al. Clinical practice patterns suggest female patients prefer female endoscopists. Dig Dis Sci. 2015;60(10):3149-50.
40. Menees SB et al. Women patients’ preference for women physicians is a barrier to colon cancer screening. Gastrointest Endosc. 2005;62(2):219-23.
41. Feld LD et al. Management of code status in the periendoscopic period: A national survey of current practices and beliefs of U.S. gastroenterologists. Gastrointest Endosc. 2021;94(1):172-7.e2.
AGA News - February 2022
Registration now open: Gut Microbiota for Health World Summit 2022
Registration is now open for the Gut Microbiota for Health (GMFH) World Summit 2022, taking place March 12-13 in Washington, D.C., and virtually.
Organized by AGA and the European Society of Neurogastroenterology and Motility (ESNM), the GMFH World Summit is the preeminent international meeting on the gut microbiome for clinicians, dietitians, and researchers.
Now in its tenth year, this year’s program will focus on “The Gut Microbiome in Precision Nutrition and Medicine.” Join us to gain a deeper understanding of the role of the gut microbiome in precision medicine and discover personalized approaches to modulating the gut microbiome that may promote health and improve patient outcomes for a variety of disorders and diseases.
https://www.gutmicrobiotaforhealth.com/summit
See Gastroenterology’s curated Equity in GI journal collection
Gastroenterology is proud to announce the release of a special collection of articles focused on the intersection of diversity, equity, and inclusion (DEI) and gastroenterology and hepatology. This curated collection, under the guidance of the journal’s new DEI section editor Dr. Chyke Doubeni, includes original research, reviews, commentaries and editorials on matters of health disparities, socioeconomic determinants of health outcomes, and population-based studies on disease incidence among races and ethnicities, among other topics. New articles are added to the collection as they are published.
View the special collection on Gastroenterology’s website, which is designed to help you quickly and easily look over the latest DEI articles and content of interest. Recent articles include:
- How to incorporate health equity training into GI/hepatology fellowships by Jannel Lee-Allen and Brijen J. Shah
- Disparities in preventable mortality from colorectal cancer: are they the result of structural racism? By Chyke A. Doubeni, Kevin Selby and Theodore R. Levin
- COVID-19 pediatric patients: GI symptoms, presentations and disparities by race/ethnicity in a large, multicenter U.S. study by Yusuf Ashktorab, Anas Brim, Antonio Pizuorno, Vijay Gayam, Sahar Nikdel and Hassan Brim
View all of Gastroenterology’s curated article collections.
Registration now open: Gut Microbiota for Health World Summit 2022
Registration is now open for the Gut Microbiota for Health (GMFH) World Summit 2022, taking place March 12-13 in Washington, D.C., and virtually.
Organized by AGA and the European Society of Neurogastroenterology and Motility (ESNM), the GMFH World Summit is the preeminent international meeting on the gut microbiome for clinicians, dietitians, and researchers.
Now in its tenth year, this year’s program will focus on “The Gut Microbiome in Precision Nutrition and Medicine.” Join us to gain a deeper understanding of the role of the gut microbiome in precision medicine and discover personalized approaches to modulating the gut microbiome that may promote health and improve patient outcomes for a variety of disorders and diseases.
https://www.gutmicrobiotaforhealth.com/summit
See Gastroenterology’s curated Equity in GI journal collection
Gastroenterology is proud to announce the release of a special collection of articles focused on the intersection of diversity, equity, and inclusion (DEI) and gastroenterology and hepatology. This curated collection, under the guidance of the journal’s new DEI section editor Dr. Chyke Doubeni, includes original research, reviews, commentaries and editorials on matters of health disparities, socioeconomic determinants of health outcomes, and population-based studies on disease incidence among races and ethnicities, among other topics. New articles are added to the collection as they are published.
View the special collection on Gastroenterology’s website, which is designed to help you quickly and easily look over the latest DEI articles and content of interest. Recent articles include:
- How to incorporate health equity training into GI/hepatology fellowships by Jannel Lee-Allen and Brijen J. Shah
- Disparities in preventable mortality from colorectal cancer: are they the result of structural racism? By Chyke A. Doubeni, Kevin Selby and Theodore R. Levin
- COVID-19 pediatric patients: GI symptoms, presentations and disparities by race/ethnicity in a large, multicenter U.S. study by Yusuf Ashktorab, Anas Brim, Antonio Pizuorno, Vijay Gayam, Sahar Nikdel and Hassan Brim
View all of Gastroenterology’s curated article collections.
Registration now open: Gut Microbiota for Health World Summit 2022
Registration is now open for the Gut Microbiota for Health (GMFH) World Summit 2022, taking place March 12-13 in Washington, D.C., and virtually.
Organized by AGA and the European Society of Neurogastroenterology and Motility (ESNM), the GMFH World Summit is the preeminent international meeting on the gut microbiome for clinicians, dietitians, and researchers.
Now in its tenth year, this year’s program will focus on “The Gut Microbiome in Precision Nutrition and Medicine.” Join us to gain a deeper understanding of the role of the gut microbiome in precision medicine and discover personalized approaches to modulating the gut microbiome that may promote health and improve patient outcomes for a variety of disorders and diseases.
https://www.gutmicrobiotaforhealth.com/summit
See Gastroenterology’s curated Equity in GI journal collection
Gastroenterology is proud to announce the release of a special collection of articles focused on the intersection of diversity, equity, and inclusion (DEI) and gastroenterology and hepatology. This curated collection, under the guidance of the journal’s new DEI section editor Dr. Chyke Doubeni, includes original research, reviews, commentaries and editorials on matters of health disparities, socioeconomic determinants of health outcomes, and population-based studies on disease incidence among races and ethnicities, among other topics. New articles are added to the collection as they are published.
View the special collection on Gastroenterology’s website, which is designed to help you quickly and easily look over the latest DEI articles and content of interest. Recent articles include:
- How to incorporate health equity training into GI/hepatology fellowships by Jannel Lee-Allen and Brijen J. Shah
- Disparities in preventable mortality from colorectal cancer: are they the result of structural racism? By Chyke A. Doubeni, Kevin Selby and Theodore R. Levin
- COVID-19 pediatric patients: GI symptoms, presentations and disparities by race/ethnicity in a large, multicenter U.S. study by Yusuf Ashktorab, Anas Brim, Antonio Pizuorno, Vijay Gayam, Sahar Nikdel and Hassan Brim
View all of Gastroenterology’s curated article collections.
February 2022 – ICYMI
Gastroenterology
November 2021
How to navigate national societal organizations for leadership development and academic promotion: A guide for trainees and young faculty
Aby ES et al. Gastroenterology. 2021 Nov;161(5):1361-1365. doi: 10.1053/j.gastro.2021.08.044.
Value of pH impedance monitoring while on twice-daily proton pump inhibitor therapy to identify need for escalation of reflux management
Gyawali CG et al. Gastroenterology. 2021 Nov;161(5):1412-1422. doi: 10.1053/j.gastro.2021.07.004.
The sulfur microbial diet is associated with increased risk of early-onset colorectal cancer precursors
Nguyen LH et al. Gastroenterology. 2021 Nov;161(5):1423-1432.e4. doi: 10.1053/j.gastro.2021.07.008.
Underwater vs conventional endoscopic mucosal resection of large sessile or flat colorectal polyps: A prospective randomized controlled trial
Nagl S et al. Gastroenterology. 2021 Nov;161(5):1460-1474.e1. doi: 10.1053/j.gastro.2021.07.044.
December 2021
How to approach long-term enteral and parenteral nutrition
Hadefi A, Arvanitakis M. Gastroenterology. 2021 Dec;161(6):1780-1786. doi: 10.1053/j.gastro.2021.09.030.
Regular use of proton pump inhibitor and the risk of inflammatory bowel disease: Pooled analysis of 3 prospective cohorts
Xia B et al. Gastroenterology. 2021 Dec;161(6):1842-1852.e10. doi: 10.1053/j.gastro.2021.08.005.
January 2022
Serologic response to Coronavirus Disease 2019 (COVID-19) vaccination in patients with immune-mediated inflammatory diseases: A systematic review and meta-analysis
Sakuraba A et al. Gastroenterology. 2022 Jan;162(1):88-108.e9. doi: 10.1053/j.gastro.2021.09.055.
Advancing diversity, equity, and inclusion in scientific publishing
Doubeni CA et al. Gastroenterology. 2022 Jan;162(1):59-62.e1. doi: 10.1053/j.gastro.2021.10.043.
How we approach difficult to eradicate Helicobacter pylori
Argueta EA, Moss SF. Gastroenterology. 2022 Jan;162(1):32-37. doi: 10.1053/j.gastro.2021.10.048.
Global incidence of acute pancreatitis is increasing over time: A systematic review and meta-analysis
Iannuzzi JP et al. Gastroenterology. 2022 Jan;162(1):122-134. doi: 10.1053/j.gastro.2021.09.043.
Epidemiology, etiology, and treatment of gastroparesis: Real-world evidence from a large US national claims database
Ye Y et al. Gastroenterology. 2022 Jan;162(1):109-121.e5. doi: 10.1053/j.gastro.2021.09.064.
Clinical Gastroenterology and Hepatology
November 2021
AGA Clinical Practice Update on endoscopic management of perforations in gastrointestinal tract: Expert Review
Lee JH et al. Clin Gastroenterol Hepatol. 2021 Nov;19(11):2252-2261.e2. doi: 10.1016/j.cgh.2021.06.045.
Food allergies and intolerances: A clinical approach to the diagnosis and management of adverse reactions to food
Onyimba F et al. Clin Gastroenterol Hepatol. 2021 Nov;19(11):2230-2240.e1. doi: 10.1016/j.cgh.2021.01.025.
Management of gastrointestinal side effects of immune checkpoint inhibitors
Lui RN et al. Clin Gastroenterol Hepatol. 2021 Nov;19(11):2262-2265. doi: 10.1016/j.cgh.2021.06.038.
December 2021
Optimizing the endoscopic examination in eosinophilic esophagitis
Dellon ES. Clin Gastroenterol Hepatol. 2021 Dec;19(12):2489-2492.e1. doi: 10.1016/j.cgh.2021.07.011.
Diagnostic accuracy of fecal calprotectin concentration in evaluating therapeutic outcomes of patients with ulcerative colitis
Stevens TW et al. Clin Gastroenterol Hepatol. 2021 Nov;19(11):2333-2342. doi: 10.1016/j.cgh.2020.08.019.
Factors associated with inpatient endoscopy delay and its impact on hospital length-of-stay and 30-day readmission
Jacobs CC et al. Clin Gastroenterol Hepatol. 2021 Dec;19(12):2648-2655. doi: 10.1016/j.cgh.2021.06.009.
January 2022
Comparing costs and outcomes of treatments for irritable bowel syndrome with diarrhea: Cost-benefit analysis
Shah ED et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):136-144.e31. doi: 10.1016/j.cgh.2020.09.043.
Next generation academic gastroenterology
Allen JI, Berry S. Clin Gastroenterol Hepatol. 2022 Jan;20(1):5-8. doi: 10.1016/j.cgh.2021.09.038.
Beyond metoclopramide for gastroparesis
Camilleri M. Clin Gastroenterol Hepatol. 2022 Jan;20(1):19-24. doi: 10.1016/j.cgh.2021.08.052.
Comparative safety and effectiveness of vedolizumab to tumor necrosis factor antagonist therapy for ulcerative colitis
Lukin D et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):126-135. doi: 10.1016/j.cgh.2020.10.003.
Techniques and Innovations in Gastrointestinal Endoscopy
Impact of the COVID-19 pandemic on utilization of EGD and colonoscopy in the United States: An analysis of the GIQuIC registry
Calderwood AH et al. Tech Innov Gastrointest Endosc. 2021;23(4):313-321. doi: 10.1016/j.tige.2021.07.003.
How to approach small polyps in colon: Tips and tricks
Mahmood S et al. Tech Inov Gastroinest Endosc. 2021;23(4):238-335. doi: 10.1016/j.tige.2021.06.007
Gastroenterology
November 2021
How to navigate national societal organizations for leadership development and academic promotion: A guide for trainees and young faculty
Aby ES et al. Gastroenterology. 2021 Nov;161(5):1361-1365. doi: 10.1053/j.gastro.2021.08.044.
Value of pH impedance monitoring while on twice-daily proton pump inhibitor therapy to identify need for escalation of reflux management
Gyawali CG et al. Gastroenterology. 2021 Nov;161(5):1412-1422. doi: 10.1053/j.gastro.2021.07.004.
The sulfur microbial diet is associated with increased risk of early-onset colorectal cancer precursors
Nguyen LH et al. Gastroenterology. 2021 Nov;161(5):1423-1432.e4. doi: 10.1053/j.gastro.2021.07.008.
Underwater vs conventional endoscopic mucosal resection of large sessile or flat colorectal polyps: A prospective randomized controlled trial
Nagl S et al. Gastroenterology. 2021 Nov;161(5):1460-1474.e1. doi: 10.1053/j.gastro.2021.07.044.
December 2021
How to approach long-term enteral and parenteral nutrition
Hadefi A, Arvanitakis M. Gastroenterology. 2021 Dec;161(6):1780-1786. doi: 10.1053/j.gastro.2021.09.030.
Regular use of proton pump inhibitor and the risk of inflammatory bowel disease: Pooled analysis of 3 prospective cohorts
Xia B et al. Gastroenterology. 2021 Dec;161(6):1842-1852.e10. doi: 10.1053/j.gastro.2021.08.005.
January 2022
Serologic response to Coronavirus Disease 2019 (COVID-19) vaccination in patients with immune-mediated inflammatory diseases: A systematic review and meta-analysis
Sakuraba A et al. Gastroenterology. 2022 Jan;162(1):88-108.e9. doi: 10.1053/j.gastro.2021.09.055.
Advancing diversity, equity, and inclusion in scientific publishing
Doubeni CA et al. Gastroenterology. 2022 Jan;162(1):59-62.e1. doi: 10.1053/j.gastro.2021.10.043.
How we approach difficult to eradicate Helicobacter pylori
Argueta EA, Moss SF. Gastroenterology. 2022 Jan;162(1):32-37. doi: 10.1053/j.gastro.2021.10.048.
Global incidence of acute pancreatitis is increasing over time: A systematic review and meta-analysis
Iannuzzi JP et al. Gastroenterology. 2022 Jan;162(1):122-134. doi: 10.1053/j.gastro.2021.09.043.
Epidemiology, etiology, and treatment of gastroparesis: Real-world evidence from a large US national claims database
Ye Y et al. Gastroenterology. 2022 Jan;162(1):109-121.e5. doi: 10.1053/j.gastro.2021.09.064.
Clinical Gastroenterology and Hepatology
November 2021
AGA Clinical Practice Update on endoscopic management of perforations in gastrointestinal tract: Expert Review
Lee JH et al. Clin Gastroenterol Hepatol. 2021 Nov;19(11):2252-2261.e2. doi: 10.1016/j.cgh.2021.06.045.
Food allergies and intolerances: A clinical approach to the diagnosis and management of adverse reactions to food
Onyimba F et al. Clin Gastroenterol Hepatol. 2021 Nov;19(11):2230-2240.e1. doi: 10.1016/j.cgh.2021.01.025.
Management of gastrointestinal side effects of immune checkpoint inhibitors
Lui RN et al. Clin Gastroenterol Hepatol. 2021 Nov;19(11):2262-2265. doi: 10.1016/j.cgh.2021.06.038.
December 2021
Optimizing the endoscopic examination in eosinophilic esophagitis
Dellon ES. Clin Gastroenterol Hepatol. 2021 Dec;19(12):2489-2492.e1. doi: 10.1016/j.cgh.2021.07.011.
Diagnostic accuracy of fecal calprotectin concentration in evaluating therapeutic outcomes of patients with ulcerative colitis
Stevens TW et al. Clin Gastroenterol Hepatol. 2021 Nov;19(11):2333-2342. doi: 10.1016/j.cgh.2020.08.019.
Factors associated with inpatient endoscopy delay and its impact on hospital length-of-stay and 30-day readmission
Jacobs CC et al. Clin Gastroenterol Hepatol. 2021 Dec;19(12):2648-2655. doi: 10.1016/j.cgh.2021.06.009.
January 2022
Comparing costs and outcomes of treatments for irritable bowel syndrome with diarrhea: Cost-benefit analysis
Shah ED et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):136-144.e31. doi: 10.1016/j.cgh.2020.09.043.
Next generation academic gastroenterology
Allen JI, Berry S. Clin Gastroenterol Hepatol. 2022 Jan;20(1):5-8. doi: 10.1016/j.cgh.2021.09.038.
Beyond metoclopramide for gastroparesis
Camilleri M. Clin Gastroenterol Hepatol. 2022 Jan;20(1):19-24. doi: 10.1016/j.cgh.2021.08.052.
Comparative safety and effectiveness of vedolizumab to tumor necrosis factor antagonist therapy for ulcerative colitis
Lukin D et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):126-135. doi: 10.1016/j.cgh.2020.10.003.
Techniques and Innovations in Gastrointestinal Endoscopy
Impact of the COVID-19 pandemic on utilization of EGD and colonoscopy in the United States: An analysis of the GIQuIC registry
Calderwood AH et al. Tech Innov Gastrointest Endosc. 2021;23(4):313-321. doi: 10.1016/j.tige.2021.07.003.
How to approach small polyps in colon: Tips and tricks
Mahmood S et al. Tech Inov Gastroinest Endosc. 2021;23(4):238-335. doi: 10.1016/j.tige.2021.06.007
Gastroenterology
November 2021
How to navigate national societal organizations for leadership development and academic promotion: A guide for trainees and young faculty
Aby ES et al. Gastroenterology. 2021 Nov;161(5):1361-1365. doi: 10.1053/j.gastro.2021.08.044.
Value of pH impedance monitoring while on twice-daily proton pump inhibitor therapy to identify need for escalation of reflux management
Gyawali CG et al. Gastroenterology. 2021 Nov;161(5):1412-1422. doi: 10.1053/j.gastro.2021.07.004.
The sulfur microbial diet is associated with increased risk of early-onset colorectal cancer precursors
Nguyen LH et al. Gastroenterology. 2021 Nov;161(5):1423-1432.e4. doi: 10.1053/j.gastro.2021.07.008.
Underwater vs conventional endoscopic mucosal resection of large sessile or flat colorectal polyps: A prospective randomized controlled trial
Nagl S et al. Gastroenterology. 2021 Nov;161(5):1460-1474.e1. doi: 10.1053/j.gastro.2021.07.044.
December 2021
How to approach long-term enteral and parenteral nutrition
Hadefi A, Arvanitakis M. Gastroenterology. 2021 Dec;161(6):1780-1786. doi: 10.1053/j.gastro.2021.09.030.
Regular use of proton pump inhibitor and the risk of inflammatory bowel disease: Pooled analysis of 3 prospective cohorts
Xia B et al. Gastroenterology. 2021 Dec;161(6):1842-1852.e10. doi: 10.1053/j.gastro.2021.08.005.
January 2022
Serologic response to Coronavirus Disease 2019 (COVID-19) vaccination in patients with immune-mediated inflammatory diseases: A systematic review and meta-analysis
Sakuraba A et al. Gastroenterology. 2022 Jan;162(1):88-108.e9. doi: 10.1053/j.gastro.2021.09.055.
Advancing diversity, equity, and inclusion in scientific publishing
Doubeni CA et al. Gastroenterology. 2022 Jan;162(1):59-62.e1. doi: 10.1053/j.gastro.2021.10.043.
How we approach difficult to eradicate Helicobacter pylori
Argueta EA, Moss SF. Gastroenterology. 2022 Jan;162(1):32-37. doi: 10.1053/j.gastro.2021.10.048.
Global incidence of acute pancreatitis is increasing over time: A systematic review and meta-analysis
Iannuzzi JP et al. Gastroenterology. 2022 Jan;162(1):122-134. doi: 10.1053/j.gastro.2021.09.043.
Epidemiology, etiology, and treatment of gastroparesis: Real-world evidence from a large US national claims database
Ye Y et al. Gastroenterology. 2022 Jan;162(1):109-121.e5. doi: 10.1053/j.gastro.2021.09.064.
Clinical Gastroenterology and Hepatology
November 2021
AGA Clinical Practice Update on endoscopic management of perforations in gastrointestinal tract: Expert Review
Lee JH et al. Clin Gastroenterol Hepatol. 2021 Nov;19(11):2252-2261.e2. doi: 10.1016/j.cgh.2021.06.045.
Food allergies and intolerances: A clinical approach to the diagnosis and management of adverse reactions to food
Onyimba F et al. Clin Gastroenterol Hepatol. 2021 Nov;19(11):2230-2240.e1. doi: 10.1016/j.cgh.2021.01.025.
Management of gastrointestinal side effects of immune checkpoint inhibitors
Lui RN et al. Clin Gastroenterol Hepatol. 2021 Nov;19(11):2262-2265. doi: 10.1016/j.cgh.2021.06.038.
December 2021
Optimizing the endoscopic examination in eosinophilic esophagitis
Dellon ES. Clin Gastroenterol Hepatol. 2021 Dec;19(12):2489-2492.e1. doi: 10.1016/j.cgh.2021.07.011.
Diagnostic accuracy of fecal calprotectin concentration in evaluating therapeutic outcomes of patients with ulcerative colitis
Stevens TW et al. Clin Gastroenterol Hepatol. 2021 Nov;19(11):2333-2342. doi: 10.1016/j.cgh.2020.08.019.
Factors associated with inpatient endoscopy delay and its impact on hospital length-of-stay and 30-day readmission
Jacobs CC et al. Clin Gastroenterol Hepatol. 2021 Dec;19(12):2648-2655. doi: 10.1016/j.cgh.2021.06.009.
January 2022
Comparing costs and outcomes of treatments for irritable bowel syndrome with diarrhea: Cost-benefit analysis
Shah ED et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):136-144.e31. doi: 10.1016/j.cgh.2020.09.043.
Next generation academic gastroenterology
Allen JI, Berry S. Clin Gastroenterol Hepatol. 2022 Jan;20(1):5-8. doi: 10.1016/j.cgh.2021.09.038.
Beyond metoclopramide for gastroparesis
Camilleri M. Clin Gastroenterol Hepatol. 2022 Jan;20(1):19-24. doi: 10.1016/j.cgh.2021.08.052.
Comparative safety and effectiveness of vedolizumab to tumor necrosis factor antagonist therapy for ulcerative colitis
Lukin D et al. Clin Gastroenterol Hepatol. 2022 Jan;20(1):126-135. doi: 10.1016/j.cgh.2020.10.003.
Techniques and Innovations in Gastrointestinal Endoscopy
Impact of the COVID-19 pandemic on utilization of EGD and colonoscopy in the United States: An analysis of the GIQuIC registry
Calderwood AH et al. Tech Innov Gastrointest Endosc. 2021;23(4):313-321. doi: 10.1016/j.tige.2021.07.003.
How to approach small polyps in colon: Tips and tricks
Mahmood S et al. Tech Inov Gastroinest Endosc. 2021;23(4):238-335. doi: 10.1016/j.tige.2021.06.007
Gender-based pay inequity in gastroenterology
In 2017, the number of women students entering medical school surpassed that of men.1 However, the future generation of women doctors is unlikely to be paid the same as their male colleagues for equal work unless something changes in health care. About 34% of gastroenterology fellows are women,2 and there are increasing proportions of women in all academic and community practices, as well as in leadership positions.
Despite this progress, equity in pay between male and female physicians has been unequal in many areas of the country, despite the same level of training.3 Doximity, a social network for physicians, surveyed 65,000 doctors in the United States and found a difference in pay between male and female physicians who worked full time.4 This is an issue that the medical field has been aware of for many years, and articles have been published on this topic in several medical journals.5-11 Doximity found that women physicians are paid less than men, although the extent of the difference varies among regions.
In 2017, per the Doximity report, the field of gastroenterology was one of the top five specialties with the biggest pay gap: Women gastroenterologists earn 19% less (or $86,447) than men gastroenterologists. This study did not differentiate among practice types (academic, private practice, hospital, or multispecialty), but it did break down the data for all physicians into general groups of owner/partner, independent contractor, and employee – it found a gender-based gap in pay among all three of these groups. For owner/partners, the gap was a $114,590 (27.2%) difference.4 According to Doximity survey data from 2018, gastroenterology is no longer in the top five specialties with the largest gender pay gap, indicating the gap is shrinking but still exists.12
A questionnaire sent to gastroenterologists 3, 5, or 10 years after they completed their fellowships (in 1993 or 1995) revealed that after 3 years women earned 23% less per hour than men, and at 5 years, the gap had decreased to 19% less per hour.6-7 The statistical data showed that the mean annual gross income of males was significantly higher at 3 years and 5 years.7 Unfortunately, at 10 years the income gap increased up to 22%.6 The researchers found that female gastroenterologists at academic centers earned 39% less than male gastroenterologists at academic centers, whereas women at nonacademic centers earned 24% less than men, despite similar work hours and call schedules.6-7
Desai and colleauges analyzed health care provider reimbursement data for various medical specialties using the 2014 Medicare Fee-for-Service Provider Utilization and Payment Data Physician and Other Supplier Public Use File, and they found a disparity in reimbursements of female versus male physicians.11 Female physicians received significantly lower Medicare reimbursements in 11 of 13 medical specialties,4 despite adjustments for productivity, work hours, and years of experience. Factors that might affect Medicare reimbursement include variations in payment among different locations, types of service provided, location of procedures performed (hospital vs. clinic), and missing data because of privacy concerns.
Among medical specialties, the gender-based payment gap is highest among vascular surgeons, followed by occupational medicine physicians, gastroenterologists, pediatric endocrinologists, and rheumatologists. In these specialties, men earn approximately 20% more than women (approximately $89,000 more for a male vascular surgeon or about $45,000 more for a male pediatric rheumatologist).4
Gender-based gaps in pay, leadership opportunities, and other opportunities exist in the health care field regardless of whether physicians are employed at academic institutions, community-based private practices, or large health care systems. Women physicians occupy fewer leadership positions, and female physician leaders have greater disparities in pay, compared with men than women who are not in leadership positions.6,10 A 2016 survey of the 50 medical schools with the largest amounts of funding from the National Institutes of Health revealed that only 13% of the department leaders were women.
The Fair Pay Act of 2013 and the Paycheck Fairness Act of 2014 aimed to close the salary gap between men and women.13 So why are women paid less than men for the same work? Some researchers have proposed “gender differences in negotiation skills, lack of opportunities to join networks of influence within organizations, and implicit or explicit bias and discrimination.”8,10
The fee for service model based on relative value units can result in lower pay for female physicians, who spend more time with patients, compared with male physicians, because of fewer billable RVUs per hour and per day.15
What should be done?
The American Medical Women’s Association leadership stated that the key to pay equity is transparency, which has been a struggle. Some states, such as New York, require state contractors, including providers that work with the state health department, to disclose salary information. Because of the persistent gender gap in pay in all medical specialties (even after adjustments for age, experience, faculty rank, and measures of research productivity and clinical revenue), the American Medical Association House of Delegates announced a plan to balance salaries within the AMA, and in medicine overall, by promoting research, action, and advocacy.14 In the American College of Physicians, 37% of the members are women. This organization published a position paper in 2018 on gender disparity in pay, and proposed solutions included reviewing and addressing recruitment and advancement of women and other underrepresented groups.15
The executive director of Indiana University’s National Center of Excellence in Women’s Health in Indianapolis, Theresa Rohr-Kirchgraber, MD, who is a professor of clinical care and pediatrics, said that women physicians should bill and code in ways that better reflect the services they provide. Women should also demand more transparency in salaries and push to remove patient satisfaction scores from being a factor in salary determination.16
It is also important to note that there are medical groups and hospitals at which disparities in gender pay might not be an issue, because of physician compensation models. These include but are not limited to Kaiser Permanente and large private practice groups (such as MNGI Digestive Health). For example, with MNGI Digestive Health, shareholder track, ambulatory surgical center distributions are based on full-time equivalent status and not on production. Shareholder compensation is transparent and communicated to all. For Kaiser Permanente, salary is based on specialty and years of service. We will have the opportunity to evaluate the effects of different compensation models as health care delivery moves toward value-based care.
There is a limitation in data presented, as we were unable to obtain specialty salary data from the Association of American Medical Colleges or Medical Group Management Association to confirm findings from the Doximity survey, etc.
Conclusions
It is important to acknowledge that we have made great strides in ensuring gender diversity in the field of gastroenterology. All professional medical and gastroenterological societies are working to address gender disparities in compensation and leadership opportunities. Medical schools and fellowship programs have incorporated training on negotiation skills into their curriculums. The medical profession and overall society will benefit from providing thriving workplaces to female physicians, allowing them to achieve their full potential by ensuring gender equity in compensation and opportunities.
Dr. Perera is a gastroenterologist at Advocate Aurora Health, Grafton, Wisc. Dr. Toriz is a gastroenterologist, treasurer, and board member, MNGI Digestive Health, Bloomington, Minn. They disclosed having no relevant conflicts of interest.
References
1. The American Association of Medical Colleges. “More Women Than Men Enrolled in U.S. Medical schools in 2017.” 2017 Dec 17. http://news.aamc.org/press-releases/article/applicants-enrollment
2. The American Association of Medical Colleges data. https://aamc.org/downlaod/280338/data/tablel3.pdf
3. CBS Business. “The gender pay gap for women doctors is big – and getting worse.” 2018 Mar 14. https://money.CNN.com/2018/03/14/news/economy/gender-pay-gap-doctors/index.html4. Doximity. “Doxmity 2018 Physician Compensation Report.” 2018 Mar 27. https://blog.doximity.com/articles/doximity-2018-physician-compensation-report
5. Tomer G et al. Gastroenterology. 2015;60: 481-5.
6. Singh A et al. Am J Gastroenterol. 2008 Jul;103(7):1589-95.
7. Burke CA et al. Am J Gastroenterol. 2005 Feb;100(2):259-64.
8. Achkar E. Am J Gastroenterol. 2008 Jul;103(7):1587-8.
9. Hoff TJ. Inquiry. 2004;41(3):301-15.
10. Weaver AC et al. J Hosp Med. 2015 Aug;10(8):486-90.
11. Desai T et al. Postgrad Med J. 2016 Oct;92(1092):571-5.
12. Doximity. “Women in Medicine: The Gender Pay Gap” 2018 Oct 2. https://blog.finder.doximity.info/women-in-medicine-the-gender-pay-gap
13. H.R.438. Fair Pay Act of 2013. 113th Congress (2013-2014)
14. O’Reilly KB. American Medical Association. “Physicians adopt plan to combat pay gap in medicine.” 2018 Jun 13. https://www.ama-assn.org/delivering-care/health-equity/physicians-adopt-plan-combat-pay-gap-medicine
15. Butkus R et al. Ann Intern Med. 2018 May 15;168(10):721-3.
16. Commins J. “5 Reasons Women Doctors Earn Less Than Men.” Health Leaders. 2018 Aug 6. https://www.healthleadersmedia.com/clinical-care /5-reasons-women-doctors-earn-less-men
In 2017, the number of women students entering medical school surpassed that of men.1 However, the future generation of women doctors is unlikely to be paid the same as their male colleagues for equal work unless something changes in health care. About 34% of gastroenterology fellows are women,2 and there are increasing proportions of women in all academic and community practices, as well as in leadership positions.
Despite this progress, equity in pay between male and female physicians has been unequal in many areas of the country, despite the same level of training.3 Doximity, a social network for physicians, surveyed 65,000 doctors in the United States and found a difference in pay between male and female physicians who worked full time.4 This is an issue that the medical field has been aware of for many years, and articles have been published on this topic in several medical journals.5-11 Doximity found that women physicians are paid less than men, although the extent of the difference varies among regions.
In 2017, per the Doximity report, the field of gastroenterology was one of the top five specialties with the biggest pay gap: Women gastroenterologists earn 19% less (or $86,447) than men gastroenterologists. This study did not differentiate among practice types (academic, private practice, hospital, or multispecialty), but it did break down the data for all physicians into general groups of owner/partner, independent contractor, and employee – it found a gender-based gap in pay among all three of these groups. For owner/partners, the gap was a $114,590 (27.2%) difference.4 According to Doximity survey data from 2018, gastroenterology is no longer in the top five specialties with the largest gender pay gap, indicating the gap is shrinking but still exists.12
A questionnaire sent to gastroenterologists 3, 5, or 10 years after they completed their fellowships (in 1993 or 1995) revealed that after 3 years women earned 23% less per hour than men, and at 5 years, the gap had decreased to 19% less per hour.6-7 The statistical data showed that the mean annual gross income of males was significantly higher at 3 years and 5 years.7 Unfortunately, at 10 years the income gap increased up to 22%.6 The researchers found that female gastroenterologists at academic centers earned 39% less than male gastroenterologists at academic centers, whereas women at nonacademic centers earned 24% less than men, despite similar work hours and call schedules.6-7
Desai and colleauges analyzed health care provider reimbursement data for various medical specialties using the 2014 Medicare Fee-for-Service Provider Utilization and Payment Data Physician and Other Supplier Public Use File, and they found a disparity in reimbursements of female versus male physicians.11 Female physicians received significantly lower Medicare reimbursements in 11 of 13 medical specialties,4 despite adjustments for productivity, work hours, and years of experience. Factors that might affect Medicare reimbursement include variations in payment among different locations, types of service provided, location of procedures performed (hospital vs. clinic), and missing data because of privacy concerns.
Among medical specialties, the gender-based payment gap is highest among vascular surgeons, followed by occupational medicine physicians, gastroenterologists, pediatric endocrinologists, and rheumatologists. In these specialties, men earn approximately 20% more than women (approximately $89,000 more for a male vascular surgeon or about $45,000 more for a male pediatric rheumatologist).4
Gender-based gaps in pay, leadership opportunities, and other opportunities exist in the health care field regardless of whether physicians are employed at academic institutions, community-based private practices, or large health care systems. Women physicians occupy fewer leadership positions, and female physician leaders have greater disparities in pay, compared with men than women who are not in leadership positions.6,10 A 2016 survey of the 50 medical schools with the largest amounts of funding from the National Institutes of Health revealed that only 13% of the department leaders were women.
The Fair Pay Act of 2013 and the Paycheck Fairness Act of 2014 aimed to close the salary gap between men and women.13 So why are women paid less than men for the same work? Some researchers have proposed “gender differences in negotiation skills, lack of opportunities to join networks of influence within organizations, and implicit or explicit bias and discrimination.”8,10
The fee for service model based on relative value units can result in lower pay for female physicians, who spend more time with patients, compared with male physicians, because of fewer billable RVUs per hour and per day.15
What should be done?
The American Medical Women’s Association leadership stated that the key to pay equity is transparency, which has been a struggle. Some states, such as New York, require state contractors, including providers that work with the state health department, to disclose salary information. Because of the persistent gender gap in pay in all medical specialties (even after adjustments for age, experience, faculty rank, and measures of research productivity and clinical revenue), the American Medical Association House of Delegates announced a plan to balance salaries within the AMA, and in medicine overall, by promoting research, action, and advocacy.14 In the American College of Physicians, 37% of the members are women. This organization published a position paper in 2018 on gender disparity in pay, and proposed solutions included reviewing and addressing recruitment and advancement of women and other underrepresented groups.15
The executive director of Indiana University’s National Center of Excellence in Women’s Health in Indianapolis, Theresa Rohr-Kirchgraber, MD, who is a professor of clinical care and pediatrics, said that women physicians should bill and code in ways that better reflect the services they provide. Women should also demand more transparency in salaries and push to remove patient satisfaction scores from being a factor in salary determination.16
It is also important to note that there are medical groups and hospitals at which disparities in gender pay might not be an issue, because of physician compensation models. These include but are not limited to Kaiser Permanente and large private practice groups (such as MNGI Digestive Health). For example, with MNGI Digestive Health, shareholder track, ambulatory surgical center distributions are based on full-time equivalent status and not on production. Shareholder compensation is transparent and communicated to all. For Kaiser Permanente, salary is based on specialty and years of service. We will have the opportunity to evaluate the effects of different compensation models as health care delivery moves toward value-based care.
There is a limitation in data presented, as we were unable to obtain specialty salary data from the Association of American Medical Colleges or Medical Group Management Association to confirm findings from the Doximity survey, etc.
Conclusions
It is important to acknowledge that we have made great strides in ensuring gender diversity in the field of gastroenterology. All professional medical and gastroenterological societies are working to address gender disparities in compensation and leadership opportunities. Medical schools and fellowship programs have incorporated training on negotiation skills into their curriculums. The medical profession and overall society will benefit from providing thriving workplaces to female physicians, allowing them to achieve their full potential by ensuring gender equity in compensation and opportunities.
Dr. Perera is a gastroenterologist at Advocate Aurora Health, Grafton, Wisc. Dr. Toriz is a gastroenterologist, treasurer, and board member, MNGI Digestive Health, Bloomington, Minn. They disclosed having no relevant conflicts of interest.
References
1. The American Association of Medical Colleges. “More Women Than Men Enrolled in U.S. Medical schools in 2017.” 2017 Dec 17. http://news.aamc.org/press-releases/article/applicants-enrollment
2. The American Association of Medical Colleges data. https://aamc.org/downlaod/280338/data/tablel3.pdf
3. CBS Business. “The gender pay gap for women doctors is big – and getting worse.” 2018 Mar 14. https://money.CNN.com/2018/03/14/news/economy/gender-pay-gap-doctors/index.html4. Doximity. “Doxmity 2018 Physician Compensation Report.” 2018 Mar 27. https://blog.doximity.com/articles/doximity-2018-physician-compensation-report
5. Tomer G et al. Gastroenterology. 2015;60: 481-5.
6. Singh A et al. Am J Gastroenterol. 2008 Jul;103(7):1589-95.
7. Burke CA et al. Am J Gastroenterol. 2005 Feb;100(2):259-64.
8. Achkar E. Am J Gastroenterol. 2008 Jul;103(7):1587-8.
9. Hoff TJ. Inquiry. 2004;41(3):301-15.
10. Weaver AC et al. J Hosp Med. 2015 Aug;10(8):486-90.
11. Desai T et al. Postgrad Med J. 2016 Oct;92(1092):571-5.
12. Doximity. “Women in Medicine: The Gender Pay Gap” 2018 Oct 2. https://blog.finder.doximity.info/women-in-medicine-the-gender-pay-gap
13. H.R.438. Fair Pay Act of 2013. 113th Congress (2013-2014)
14. O’Reilly KB. American Medical Association. “Physicians adopt plan to combat pay gap in medicine.” 2018 Jun 13. https://www.ama-assn.org/delivering-care/health-equity/physicians-adopt-plan-combat-pay-gap-medicine
15. Butkus R et al. Ann Intern Med. 2018 May 15;168(10):721-3.
16. Commins J. “5 Reasons Women Doctors Earn Less Than Men.” Health Leaders. 2018 Aug 6. https://www.healthleadersmedia.com/clinical-care /5-reasons-women-doctors-earn-less-men
In 2017, the number of women students entering medical school surpassed that of men.1 However, the future generation of women doctors is unlikely to be paid the same as their male colleagues for equal work unless something changes in health care. About 34% of gastroenterology fellows are women,2 and there are increasing proportions of women in all academic and community practices, as well as in leadership positions.
Despite this progress, equity in pay between male and female physicians has been unequal in many areas of the country, despite the same level of training.3 Doximity, a social network for physicians, surveyed 65,000 doctors in the United States and found a difference in pay between male and female physicians who worked full time.4 This is an issue that the medical field has been aware of for many years, and articles have been published on this topic in several medical journals.5-11 Doximity found that women physicians are paid less than men, although the extent of the difference varies among regions.
In 2017, per the Doximity report, the field of gastroenterology was one of the top five specialties with the biggest pay gap: Women gastroenterologists earn 19% less (or $86,447) than men gastroenterologists. This study did not differentiate among practice types (academic, private practice, hospital, or multispecialty), but it did break down the data for all physicians into general groups of owner/partner, independent contractor, and employee – it found a gender-based gap in pay among all three of these groups. For owner/partners, the gap was a $114,590 (27.2%) difference.4 According to Doximity survey data from 2018, gastroenterology is no longer in the top five specialties with the largest gender pay gap, indicating the gap is shrinking but still exists.12
A questionnaire sent to gastroenterologists 3, 5, or 10 years after they completed their fellowships (in 1993 or 1995) revealed that after 3 years women earned 23% less per hour than men, and at 5 years, the gap had decreased to 19% less per hour.6-7 The statistical data showed that the mean annual gross income of males was significantly higher at 3 years and 5 years.7 Unfortunately, at 10 years the income gap increased up to 22%.6 The researchers found that female gastroenterologists at academic centers earned 39% less than male gastroenterologists at academic centers, whereas women at nonacademic centers earned 24% less than men, despite similar work hours and call schedules.6-7
Desai and colleauges analyzed health care provider reimbursement data for various medical specialties using the 2014 Medicare Fee-for-Service Provider Utilization and Payment Data Physician and Other Supplier Public Use File, and they found a disparity in reimbursements of female versus male physicians.11 Female physicians received significantly lower Medicare reimbursements in 11 of 13 medical specialties,4 despite adjustments for productivity, work hours, and years of experience. Factors that might affect Medicare reimbursement include variations in payment among different locations, types of service provided, location of procedures performed (hospital vs. clinic), and missing data because of privacy concerns.
Among medical specialties, the gender-based payment gap is highest among vascular surgeons, followed by occupational medicine physicians, gastroenterologists, pediatric endocrinologists, and rheumatologists. In these specialties, men earn approximately 20% more than women (approximately $89,000 more for a male vascular surgeon or about $45,000 more for a male pediatric rheumatologist).4
Gender-based gaps in pay, leadership opportunities, and other opportunities exist in the health care field regardless of whether physicians are employed at academic institutions, community-based private practices, or large health care systems. Women physicians occupy fewer leadership positions, and female physician leaders have greater disparities in pay, compared with men than women who are not in leadership positions.6,10 A 2016 survey of the 50 medical schools with the largest amounts of funding from the National Institutes of Health revealed that only 13% of the department leaders were women.
The Fair Pay Act of 2013 and the Paycheck Fairness Act of 2014 aimed to close the salary gap between men and women.13 So why are women paid less than men for the same work? Some researchers have proposed “gender differences in negotiation skills, lack of opportunities to join networks of influence within organizations, and implicit or explicit bias and discrimination.”8,10
The fee for service model based on relative value units can result in lower pay for female physicians, who spend more time with patients, compared with male physicians, because of fewer billable RVUs per hour and per day.15
What should be done?
The American Medical Women’s Association leadership stated that the key to pay equity is transparency, which has been a struggle. Some states, such as New York, require state contractors, including providers that work with the state health department, to disclose salary information. Because of the persistent gender gap in pay in all medical specialties (even after adjustments for age, experience, faculty rank, and measures of research productivity and clinical revenue), the American Medical Association House of Delegates announced a plan to balance salaries within the AMA, and in medicine overall, by promoting research, action, and advocacy.14 In the American College of Physicians, 37% of the members are women. This organization published a position paper in 2018 on gender disparity in pay, and proposed solutions included reviewing and addressing recruitment and advancement of women and other underrepresented groups.15
The executive director of Indiana University’s National Center of Excellence in Women’s Health in Indianapolis, Theresa Rohr-Kirchgraber, MD, who is a professor of clinical care and pediatrics, said that women physicians should bill and code in ways that better reflect the services they provide. Women should also demand more transparency in salaries and push to remove patient satisfaction scores from being a factor in salary determination.16
It is also important to note that there are medical groups and hospitals at which disparities in gender pay might not be an issue, because of physician compensation models. These include but are not limited to Kaiser Permanente and large private practice groups (such as MNGI Digestive Health). For example, with MNGI Digestive Health, shareholder track, ambulatory surgical center distributions are based on full-time equivalent status and not on production. Shareholder compensation is transparent and communicated to all. For Kaiser Permanente, salary is based on specialty and years of service. We will have the opportunity to evaluate the effects of different compensation models as health care delivery moves toward value-based care.
There is a limitation in data presented, as we were unable to obtain specialty salary data from the Association of American Medical Colleges or Medical Group Management Association to confirm findings from the Doximity survey, etc.
Conclusions
It is important to acknowledge that we have made great strides in ensuring gender diversity in the field of gastroenterology. All professional medical and gastroenterological societies are working to address gender disparities in compensation and leadership opportunities. Medical schools and fellowship programs have incorporated training on negotiation skills into their curriculums. The medical profession and overall society will benefit from providing thriving workplaces to female physicians, allowing them to achieve their full potential by ensuring gender equity in compensation and opportunities.
Dr. Perera is a gastroenterologist at Advocate Aurora Health, Grafton, Wisc. Dr. Toriz is a gastroenterologist, treasurer, and board member, MNGI Digestive Health, Bloomington, Minn. They disclosed having no relevant conflicts of interest.
References
1. The American Association of Medical Colleges. “More Women Than Men Enrolled in U.S. Medical schools in 2017.” 2017 Dec 17. http://news.aamc.org/press-releases/article/applicants-enrollment
2. The American Association of Medical Colleges data. https://aamc.org/downlaod/280338/data/tablel3.pdf
3. CBS Business. “The gender pay gap for women doctors is big – and getting worse.” 2018 Mar 14. https://money.CNN.com/2018/03/14/news/economy/gender-pay-gap-doctors/index.html4. Doximity. “Doxmity 2018 Physician Compensation Report.” 2018 Mar 27. https://blog.doximity.com/articles/doximity-2018-physician-compensation-report
5. Tomer G et al. Gastroenterology. 2015;60: 481-5.
6. Singh A et al. Am J Gastroenterol. 2008 Jul;103(7):1589-95.
7. Burke CA et al. Am J Gastroenterol. 2005 Feb;100(2):259-64.
8. Achkar E. Am J Gastroenterol. 2008 Jul;103(7):1587-8.
9. Hoff TJ. Inquiry. 2004;41(3):301-15.
10. Weaver AC et al. J Hosp Med. 2015 Aug;10(8):486-90.
11. Desai T et al. Postgrad Med J. 2016 Oct;92(1092):571-5.
12. Doximity. “Women in Medicine: The Gender Pay Gap” 2018 Oct 2. https://blog.finder.doximity.info/women-in-medicine-the-gender-pay-gap
13. H.R.438. Fair Pay Act of 2013. 113th Congress (2013-2014)
14. O’Reilly KB. American Medical Association. “Physicians adopt plan to combat pay gap in medicine.” 2018 Jun 13. https://www.ama-assn.org/delivering-care/health-equity/physicians-adopt-plan-combat-pay-gap-medicine
15. Butkus R et al. Ann Intern Med. 2018 May 15;168(10):721-3.
16. Commins J. “5 Reasons Women Doctors Earn Less Than Men.” Health Leaders. 2018 Aug 6. https://www.healthleadersmedia.com/clinical-care /5-reasons-women-doctors-earn-less-men
Your money. Your voice. Your wellness.
I was a third-year gastroenterology fellow when I realized that something had to change. I was on a one-way trip to burnout.
I went through medical school with the sole goal of becoming an excellent physician. Like many physicians, I was six figures deep in student loan debt by the end of training. I remember clearly being told, “You are going to be physicians. Money won’t be a problem.” In fact, in 2021, money remains a taboo topic in medicine, and most of medical education remains void of the fundamentals of money management.
Although I was surrounded by some of the most brilliant minds in medicine, burnout was spreading like a wave. Physicians are becoming increasingly broken, burned out by a system through which we have vowed to care for our patients: For better or for worse. We are required to attend lectures about burnout, yet nothing about money or finances. We can all agree that talking about resilience and burnout during odd hours of the morning are ironic measures that by themselves have done nothing to help us through the crisis that exists.
I noticed that there seemed to be a difference between physicians who had their finances in order and those who didn’t. This eventually made sense as I became more aware of the data that now exists. Healthy financial practices can lead to financial independence, which may in turn decrease burnout-associated stressors.1 This is what we need.
My observation about the difference in satisfaction between physicians led me to decide to explore that path for myself. My hypothesis? Empowering myself financially is an anti-burnout tool that will improve my satisfaction, longevity in medicine, and my well-being. I traded my financial illiteracy for empowerment and I am now on a mission to help physicians become financially empowered. This is an important step toward preventing and recovering from burnout. The surprising part is that it is not difficult. You need to be committed. Our math literacy is already higher than needed. When we physicians are financially independent, we will have the ability to practice medicine in a way that is healthy. In a world where physician suicide, burnout, and dissatisfaction continue to rise, there is an urgent call to financial action. This is a critical key that will help us change the future of medicine.
In this article, I am going to share four myths that are preventing physicians from truly managing their finances.
1. I love medicine. I have no plans of leaving: I love gastroenterology. The ability to use our critical internal medicine skills as well as intervene procedurally is truly a privilege. As a gastroenterologist with a focus on inflammatory bowel diseases, I have the honor of walking patients through seasons of life and making decisions that truly impact their lives. It is an honor. I also believe that good money management allows physicians to become even better physicians. The platforms of medicine continue to change. According to Physician Advocacy Institute, about 70% of physicians report being employed.2 As physicians graduate from training, joining large hospitals, physician autonomy in the practice of medicine is affected. To ensure that we continue to practice medicine at the fullest extent of our oath, it is essential that our finances allow us the ability and capacity to fulfill that oath. Furthermore, the pandemic has shown that physician income is not pandemic-proof. Having a healthy emergency fund and diversifying our income sources is critical as we move forward.
2. I have a financial adviser or planner. They will figure it out for me: Financial advisers and planners are hired professionals with varied levels of training and expertise. A great financial adviser can be an important part of your team. A team that is led by you, the CEO, because no one will care about your finances as much as you do. Investing the time to learn the basics can pay dividends. When I started my financial education journey, I was completely illiterate. I knew I wanted to have money but didn’t know how. One of the first things in my financial competency journey was to hire a financial adviser. Unfortunately, as I learned more about money, I realized that my investments favored him more than they did me. Coincidentally, we had similar starting balances in a different self-management investment account. At the end of our time together, our self-managed funds fared better than his actively picked funds. As humans, we assume that actively picking investments and stocks would be better than passive investments. Based on experience and data, investing in boring, diverse funds such as index funds averagely do better than actively managed funds. Is it wrong then to hire an adviser? No, but you are still the CEO of you-incorporated. Choosing to completely delegate to someone else, avoiding the basic education that would allow you to better screen for effectiveness and competence, may in fact be negligence. After empowering themselves financially, some physicians who have gone through my money curriculum have chosen to keep their advisers; others chose to self-manage. The key is giving yourself the gift of choice: Choosing to have an adviser because you want to rather than because you thought you had no choice.
3. Money management looks complicated. This is one of the most common statements I get for why physicians avoid their own money management. I remember the complex biochemical pathways we learned in medical school. Those were hard and complicated. We chose to stay the course because we believed that, with repetition and simplifying, it would eventually become less difficult. Why then is it any different with money? A physician shared a discussion she once had with a banker. She was told, “Doctors are bad with money.” When did we become the stereotype for being bad with money? If we can learn channelopathies and memorize mechanisms and save lives, we can do money. We have to start somewhere. We may not get it the first time. However, as physicians, we are the more persistent people and are excellent examples of what happens when you commit to learning something new. After coaching hundreds of physicians regarding money management, I have concluded that physicians are not bad with money. We simply may not be committed to learning it. Once we commit, the rest becomes history.
4. I don’t have time. For practicing gastroenterologists dealing with post-lockdown influx of patients, the days can be long. As a gastroenterologist who is also a parent, I know firsthand how time can be tight. When we had two children, we were busy. We thought we were at our capacity on time with two children. Then we had a third. Suddenly, life with two children looked easier than with three. As humans, we have the capacity to create. Things take exactly how much time we commit to them. If I give myself a month to write an article, I will write it in a month. If I give myself 2 weeks, I will be done in 2 weeks. The key is to remember that we all have 24 hours. David Frankel is the author of “The Freedom Formula: How to Succeed in Business Without Sacrificing Your Family, Health, or Life.”3 He analyzed a poll of business owners. He showed that they were wasting an average of 21.8 hours per week. Many times, we talk about our to-do list. We don’t talk enough about our “to don’t list.” This refers to the list of things we need to stop doing so that we can spend time on things that give or add value to our lives. Starting with as little as 30 minutes per day or per week dedicated to learning and/or managing our finances, the result will compound.
As the platform of medicine continues to evolve, it is important for astute gastroenterologists to be part of these conversations. When we are confident in our finances, they become a vehicle that gives strength to the power of our voice. We are less likely to overwork and more likely to find joy and meaning within and outside medicine.
If we want to care for our patients at a high level and keep our oath to do no harm, we have to remember that includes doing no harm to self as well.
Money management tools and empowering ourselves financially should be an essential component of our training; until then, the onus is on you to learn, so that you can be well.
Your voice matters. Your wellness matters. Your time matters. Your money matters.
Dr. Alli-Akintade is a gastroenterologist with Kaiser Permanente South Sacramento (Calif.) Medical Center. She is the CEO of MoneyFitMD, a financial empowerment coaching platform for female physicians. She is also the host of The MoneyFitMD podcast.
References
1. Royce TJ et al. Pract Radiat Oncol. Jul-Aug 2019;9(4):231-8.
2. Physician Advocacy Institute. “COVID-19’s Impact on Acquisitions of Physician Practices and Physician Employment 2019-2020.” 2021 Jun.
3. Finkel D. “New Study Shows You’re Wasting 21.8 hours a Week.” Inc.com. 2018 Mar 1.
I was a third-year gastroenterology fellow when I realized that something had to change. I was on a one-way trip to burnout.
I went through medical school with the sole goal of becoming an excellent physician. Like many physicians, I was six figures deep in student loan debt by the end of training. I remember clearly being told, “You are going to be physicians. Money won’t be a problem.” In fact, in 2021, money remains a taboo topic in medicine, and most of medical education remains void of the fundamentals of money management.
Although I was surrounded by some of the most brilliant minds in medicine, burnout was spreading like a wave. Physicians are becoming increasingly broken, burned out by a system through which we have vowed to care for our patients: For better or for worse. We are required to attend lectures about burnout, yet nothing about money or finances. We can all agree that talking about resilience and burnout during odd hours of the morning are ironic measures that by themselves have done nothing to help us through the crisis that exists.
I noticed that there seemed to be a difference between physicians who had their finances in order and those who didn’t. This eventually made sense as I became more aware of the data that now exists. Healthy financial practices can lead to financial independence, which may in turn decrease burnout-associated stressors.1 This is what we need.
My observation about the difference in satisfaction between physicians led me to decide to explore that path for myself. My hypothesis? Empowering myself financially is an anti-burnout tool that will improve my satisfaction, longevity in medicine, and my well-being. I traded my financial illiteracy for empowerment and I am now on a mission to help physicians become financially empowered. This is an important step toward preventing and recovering from burnout. The surprising part is that it is not difficult. You need to be committed. Our math literacy is already higher than needed. When we physicians are financially independent, we will have the ability to practice medicine in a way that is healthy. In a world where physician suicide, burnout, and dissatisfaction continue to rise, there is an urgent call to financial action. This is a critical key that will help us change the future of medicine.
In this article, I am going to share four myths that are preventing physicians from truly managing their finances.
1. I love medicine. I have no plans of leaving: I love gastroenterology. The ability to use our critical internal medicine skills as well as intervene procedurally is truly a privilege. As a gastroenterologist with a focus on inflammatory bowel diseases, I have the honor of walking patients through seasons of life and making decisions that truly impact their lives. It is an honor. I also believe that good money management allows physicians to become even better physicians. The platforms of medicine continue to change. According to Physician Advocacy Institute, about 70% of physicians report being employed.2 As physicians graduate from training, joining large hospitals, physician autonomy in the practice of medicine is affected. To ensure that we continue to practice medicine at the fullest extent of our oath, it is essential that our finances allow us the ability and capacity to fulfill that oath. Furthermore, the pandemic has shown that physician income is not pandemic-proof. Having a healthy emergency fund and diversifying our income sources is critical as we move forward.
2. I have a financial adviser or planner. They will figure it out for me: Financial advisers and planners are hired professionals with varied levels of training and expertise. A great financial adviser can be an important part of your team. A team that is led by you, the CEO, because no one will care about your finances as much as you do. Investing the time to learn the basics can pay dividends. When I started my financial education journey, I was completely illiterate. I knew I wanted to have money but didn’t know how. One of the first things in my financial competency journey was to hire a financial adviser. Unfortunately, as I learned more about money, I realized that my investments favored him more than they did me. Coincidentally, we had similar starting balances in a different self-management investment account. At the end of our time together, our self-managed funds fared better than his actively picked funds. As humans, we assume that actively picking investments and stocks would be better than passive investments. Based on experience and data, investing in boring, diverse funds such as index funds averagely do better than actively managed funds. Is it wrong then to hire an adviser? No, but you are still the CEO of you-incorporated. Choosing to completely delegate to someone else, avoiding the basic education that would allow you to better screen for effectiveness and competence, may in fact be negligence. After empowering themselves financially, some physicians who have gone through my money curriculum have chosen to keep their advisers; others chose to self-manage. The key is giving yourself the gift of choice: Choosing to have an adviser because you want to rather than because you thought you had no choice.
3. Money management looks complicated. This is one of the most common statements I get for why physicians avoid their own money management. I remember the complex biochemical pathways we learned in medical school. Those were hard and complicated. We chose to stay the course because we believed that, with repetition and simplifying, it would eventually become less difficult. Why then is it any different with money? A physician shared a discussion she once had with a banker. She was told, “Doctors are bad with money.” When did we become the stereotype for being bad with money? If we can learn channelopathies and memorize mechanisms and save lives, we can do money. We have to start somewhere. We may not get it the first time. However, as physicians, we are the more persistent people and are excellent examples of what happens when you commit to learning something new. After coaching hundreds of physicians regarding money management, I have concluded that physicians are not bad with money. We simply may not be committed to learning it. Once we commit, the rest becomes history.
4. I don’t have time. For practicing gastroenterologists dealing with post-lockdown influx of patients, the days can be long. As a gastroenterologist who is also a parent, I know firsthand how time can be tight. When we had two children, we were busy. We thought we were at our capacity on time with two children. Then we had a third. Suddenly, life with two children looked easier than with three. As humans, we have the capacity to create. Things take exactly how much time we commit to them. If I give myself a month to write an article, I will write it in a month. If I give myself 2 weeks, I will be done in 2 weeks. The key is to remember that we all have 24 hours. David Frankel is the author of “The Freedom Formula: How to Succeed in Business Without Sacrificing Your Family, Health, or Life.”3 He analyzed a poll of business owners. He showed that they were wasting an average of 21.8 hours per week. Many times, we talk about our to-do list. We don’t talk enough about our “to don’t list.” This refers to the list of things we need to stop doing so that we can spend time on things that give or add value to our lives. Starting with as little as 30 minutes per day or per week dedicated to learning and/or managing our finances, the result will compound.
As the platform of medicine continues to evolve, it is important for astute gastroenterologists to be part of these conversations. When we are confident in our finances, they become a vehicle that gives strength to the power of our voice. We are less likely to overwork and more likely to find joy and meaning within and outside medicine.
If we want to care for our patients at a high level and keep our oath to do no harm, we have to remember that includes doing no harm to self as well.
Money management tools and empowering ourselves financially should be an essential component of our training; until then, the onus is on you to learn, so that you can be well.
Your voice matters. Your wellness matters. Your time matters. Your money matters.
Dr. Alli-Akintade is a gastroenterologist with Kaiser Permanente South Sacramento (Calif.) Medical Center. She is the CEO of MoneyFitMD, a financial empowerment coaching platform for female physicians. She is also the host of The MoneyFitMD podcast.
References
1. Royce TJ et al. Pract Radiat Oncol. Jul-Aug 2019;9(4):231-8.
2. Physician Advocacy Institute. “COVID-19’s Impact on Acquisitions of Physician Practices and Physician Employment 2019-2020.” 2021 Jun.
3. Finkel D. “New Study Shows You’re Wasting 21.8 hours a Week.” Inc.com. 2018 Mar 1.
I was a third-year gastroenterology fellow when I realized that something had to change. I was on a one-way trip to burnout.
I went through medical school with the sole goal of becoming an excellent physician. Like many physicians, I was six figures deep in student loan debt by the end of training. I remember clearly being told, “You are going to be physicians. Money won’t be a problem.” In fact, in 2021, money remains a taboo topic in medicine, and most of medical education remains void of the fundamentals of money management.
Although I was surrounded by some of the most brilliant minds in medicine, burnout was spreading like a wave. Physicians are becoming increasingly broken, burned out by a system through which we have vowed to care for our patients: For better or for worse. We are required to attend lectures about burnout, yet nothing about money or finances. We can all agree that talking about resilience and burnout during odd hours of the morning are ironic measures that by themselves have done nothing to help us through the crisis that exists.
I noticed that there seemed to be a difference between physicians who had their finances in order and those who didn’t. This eventually made sense as I became more aware of the data that now exists. Healthy financial practices can lead to financial independence, which may in turn decrease burnout-associated stressors.1 This is what we need.
My observation about the difference in satisfaction between physicians led me to decide to explore that path for myself. My hypothesis? Empowering myself financially is an anti-burnout tool that will improve my satisfaction, longevity in medicine, and my well-being. I traded my financial illiteracy for empowerment and I am now on a mission to help physicians become financially empowered. This is an important step toward preventing and recovering from burnout. The surprising part is that it is not difficult. You need to be committed. Our math literacy is already higher than needed. When we physicians are financially independent, we will have the ability to practice medicine in a way that is healthy. In a world where physician suicide, burnout, and dissatisfaction continue to rise, there is an urgent call to financial action. This is a critical key that will help us change the future of medicine.
In this article, I am going to share four myths that are preventing physicians from truly managing their finances.
1. I love medicine. I have no plans of leaving: I love gastroenterology. The ability to use our critical internal medicine skills as well as intervene procedurally is truly a privilege. As a gastroenterologist with a focus on inflammatory bowel diseases, I have the honor of walking patients through seasons of life and making decisions that truly impact their lives. It is an honor. I also believe that good money management allows physicians to become even better physicians. The platforms of medicine continue to change. According to Physician Advocacy Institute, about 70% of physicians report being employed.2 As physicians graduate from training, joining large hospitals, physician autonomy in the practice of medicine is affected. To ensure that we continue to practice medicine at the fullest extent of our oath, it is essential that our finances allow us the ability and capacity to fulfill that oath. Furthermore, the pandemic has shown that physician income is not pandemic-proof. Having a healthy emergency fund and diversifying our income sources is critical as we move forward.
2. I have a financial adviser or planner. They will figure it out for me: Financial advisers and planners are hired professionals with varied levels of training and expertise. A great financial adviser can be an important part of your team. A team that is led by you, the CEO, because no one will care about your finances as much as you do. Investing the time to learn the basics can pay dividends. When I started my financial education journey, I was completely illiterate. I knew I wanted to have money but didn’t know how. One of the first things in my financial competency journey was to hire a financial adviser. Unfortunately, as I learned more about money, I realized that my investments favored him more than they did me. Coincidentally, we had similar starting balances in a different self-management investment account. At the end of our time together, our self-managed funds fared better than his actively picked funds. As humans, we assume that actively picking investments and stocks would be better than passive investments. Based on experience and data, investing in boring, diverse funds such as index funds averagely do better than actively managed funds. Is it wrong then to hire an adviser? No, but you are still the CEO of you-incorporated. Choosing to completely delegate to someone else, avoiding the basic education that would allow you to better screen for effectiveness and competence, may in fact be negligence. After empowering themselves financially, some physicians who have gone through my money curriculum have chosen to keep their advisers; others chose to self-manage. The key is giving yourself the gift of choice: Choosing to have an adviser because you want to rather than because you thought you had no choice.
3. Money management looks complicated. This is one of the most common statements I get for why physicians avoid their own money management. I remember the complex biochemical pathways we learned in medical school. Those were hard and complicated. We chose to stay the course because we believed that, with repetition and simplifying, it would eventually become less difficult. Why then is it any different with money? A physician shared a discussion she once had with a banker. She was told, “Doctors are bad with money.” When did we become the stereotype for being bad with money? If we can learn channelopathies and memorize mechanisms and save lives, we can do money. We have to start somewhere. We may not get it the first time. However, as physicians, we are the more persistent people and are excellent examples of what happens when you commit to learning something new. After coaching hundreds of physicians regarding money management, I have concluded that physicians are not bad with money. We simply may not be committed to learning it. Once we commit, the rest becomes history.
4. I don’t have time. For practicing gastroenterologists dealing with post-lockdown influx of patients, the days can be long. As a gastroenterologist who is also a parent, I know firsthand how time can be tight. When we had two children, we were busy. We thought we were at our capacity on time with two children. Then we had a third. Suddenly, life with two children looked easier than with three. As humans, we have the capacity to create. Things take exactly how much time we commit to them. If I give myself a month to write an article, I will write it in a month. If I give myself 2 weeks, I will be done in 2 weeks. The key is to remember that we all have 24 hours. David Frankel is the author of “The Freedom Formula: How to Succeed in Business Without Sacrificing Your Family, Health, or Life.”3 He analyzed a poll of business owners. He showed that they were wasting an average of 21.8 hours per week. Many times, we talk about our to-do list. We don’t talk enough about our “to don’t list.” This refers to the list of things we need to stop doing so that we can spend time on things that give or add value to our lives. Starting with as little as 30 minutes per day or per week dedicated to learning and/or managing our finances, the result will compound.
As the platform of medicine continues to evolve, it is important for astute gastroenterologists to be part of these conversations. When we are confident in our finances, they become a vehicle that gives strength to the power of our voice. We are less likely to overwork and more likely to find joy and meaning within and outside medicine.
If we want to care for our patients at a high level and keep our oath to do no harm, we have to remember that includes doing no harm to self as well.
Money management tools and empowering ourselves financially should be an essential component of our training; until then, the onus is on you to learn, so that you can be well.
Your voice matters. Your wellness matters. Your time matters. Your money matters.
Dr. Alli-Akintade is a gastroenterologist with Kaiser Permanente South Sacramento (Calif.) Medical Center. She is the CEO of MoneyFitMD, a financial empowerment coaching platform for female physicians. She is also the host of The MoneyFitMD podcast.
References
1. Royce TJ et al. Pract Radiat Oncol. Jul-Aug 2019;9(4):231-8.
2. Physician Advocacy Institute. “COVID-19’s Impact on Acquisitions of Physician Practices and Physician Employment 2019-2020.” 2021 Jun.
3. Finkel D. “New Study Shows You’re Wasting 21.8 hours a Week.” Inc.com. 2018 Mar 1.
Telehealth: The 21st century house call
On March 11, 2020, the World Health Organization declared the novel coronavirus disease (COVID-19) to be a global pandemic. Shortly after, federal regulators temporarily relaxed restrictions, raised Medicare payment for telemedicine visits to the same level as in-person visits, and waived or reduced cost sharing for patients. As pandemic-related regulations expire, policymakers are debating the need to address insurance coverage of telemedicine services going forward. Congress should consider the lessons learned over the past 20 months to ensure that health care providers have the flexibility to meet the needs of patients.
One of my early telehealth visits was with a patient in his 80s who spent nearly a month in the hospital after complex abdominal surgery. While at home with his daughter, it was the first visit to assess his progress after discharge from the hospital. We were able to address his concerns, assess his wounds using the video on his computer, and formulate a plan so he could continue to improve. At the end of the call, his daughter mentioned in passing, “Thank God we did not have to go to the office ... that would have been a nightmare.”
The nightmare would have consisted of driving her frail father 45 minutes to our office, spending 15 minutes to park, waiting for 30 minutes to be seen, and finally speaking with the physician for 30 minutes face-to-face. Following the appointment, my patient and his daughter would spend another 10 minutes checking out before the 45-minute drive home. Instead, they spent a few minutes logging on through a computer prior to the 30-minute visit from the comfort of their couch.
The COVID-19 pandemic has resulted in millions of deaths and trillions of dollars in economic loss, as well as changed the norms of social interaction. One of the many ways it impacted our health care system is through the exponential growth of telehealth – the use of telecommunication modalities, such as telephone and real-time video – to connect patients with clinicians for the purpose of providing health care.
Prior to the pandemic, telehealth was limited to populations with limited access to health care. Our practice had never performed telehealth, yet converted nearly exclusively to telehealth at the height of the pandemic. My colleagues and I were concerned about how patients and physicians would respond to the sudden disruption of norms of patient engagement.
To measure the response, we conducted an online survey of over 500 gastroenterologists and nearly 1,500 patients from March to May 2020 to assess their satisfaction with telehealth. Our published results demonstrated that more than 80% of patients and 90% of physicians surveyed were either satisfied or highly satisfied with telehealth. Surprisingly, these trends were true irrespective of age or the reason for a visit. Greater than 80% of patients also indicated that the provider addressed their concerns and that they were willing to participate in telehealth visits in the future.
In a subsequent survey of nearly 3,000 patients who had experience with telehealth and in-person visits, 73% of respondents indicated that they received a similar quality of care through telehealth as compared to in-person visits and 61% stated that the interaction with their physician was also similar. More than half of the patients (54%) were likely to continue using telehealth services after the pandemic mainly because of shorter wait and travel times (75%), flexibility with personal schedule (56%), and ease of scheduling appointments on a desired date (47%).
During the COVID-19 pandemic, access to health care has been limited for a great number of patients, and telehealth has been a useful and necessary tool in overcoming this challenge. Telehealth also promotes the triple aim of improving health care by improving the care experience, reducing cost, and improving patient and population health outcomes. Our findings showed a high level of overall patient and provider satisfaction following telehealth appointments. Telehealth increases access to care by decreasing travel time and cost, limiting missed workdays, and reducing the need to find alternative caregivers, especially among rural communities and people facing financial hardship. For a small subset of people who lack the resources, access to technology, or ability to do video visits, telephone-only visits are an appropriate option and should be preserved and reimbursed in some capacity.
From a patient perspective, convenience and decreased cost are often cited as major reasons for satisfaction with telehealth. This is of particular importance to people with limited mobility, nontraditional work hours, and lower socioeconomic status. For patients who use public transportation or caregivers to travel to appointments, a short appointment may require hours of logistical planning and may come at significant financial cost. Enabling these patients to interact with their providers from home would make accessing the health care system both less expensive and logistically less challenging.
One unexpected benefit of telehealth that I have experienced is the ability to “visit” the patients in their own surroundings. Many telehealth visits have allowed the doctor to make a “house call” and see the patients in their homes, cars, and break rooms. Observing the chaos in a home or an extremely quiet and dark space has given me insight into the role anxiety and depression might play in health conditions – which may have not been appreciated in a visit to my office.
The most memorable meeting was a man who was sitting in his kitchen while smoking a cigarette and drinking a beer for breakfast whose main complaint was heartburn. His life habits were obviously contributing to his heartburn, and this degree of insight would not have been appreciated during a traditional in-person office visit.
Congress is now contemplating the role telehealth will play in health care once the pandemic is over. The main concerns are abuse of telehealth by providers, leading to a dramatic rise in visits due to the ease of care delivery. This in turn can dramatically increase health care costs. The long-term health outcomes of patients seen through telehealth are also unknown and must be studied.
All these concerns are valid and must be addressed in future studies, but it would be a mistake for Congress to revert telehealth back to prepandemic regulations. We must move forward with this important innovation in care delivery.
The adoption of telehealth is one of few silver linings of the COVID-19 pandemic. It will never replace in-person visits but should be preserved as an additional tool we can use when in-person visits are not the best option. The future of U.S. health care must allow for a hybrid model so that patients and providers can continue to benefit from this valuable innovation. Patients, providers, and families will be forever grateful.
Naresh Gunaratnam MD, AGAF is a practicing gastroenterologist with Huron Gastroenterology in Ann Arbor, Mich. He also serves as the chair of data analytics as a member of the Digestive Health Physicians Association executive committee. Dr. Gunaratnam has no conflicts in telehealth. He is the founder of and CMO of a weight loss device company and service.
On March 11, 2020, the World Health Organization declared the novel coronavirus disease (COVID-19) to be a global pandemic. Shortly after, federal regulators temporarily relaxed restrictions, raised Medicare payment for telemedicine visits to the same level as in-person visits, and waived or reduced cost sharing for patients. As pandemic-related regulations expire, policymakers are debating the need to address insurance coverage of telemedicine services going forward. Congress should consider the lessons learned over the past 20 months to ensure that health care providers have the flexibility to meet the needs of patients.
One of my early telehealth visits was with a patient in his 80s who spent nearly a month in the hospital after complex abdominal surgery. While at home with his daughter, it was the first visit to assess his progress after discharge from the hospital. We were able to address his concerns, assess his wounds using the video on his computer, and formulate a plan so he could continue to improve. At the end of the call, his daughter mentioned in passing, “Thank God we did not have to go to the office ... that would have been a nightmare.”
The nightmare would have consisted of driving her frail father 45 minutes to our office, spending 15 minutes to park, waiting for 30 minutes to be seen, and finally speaking with the physician for 30 minutes face-to-face. Following the appointment, my patient and his daughter would spend another 10 minutes checking out before the 45-minute drive home. Instead, they spent a few minutes logging on through a computer prior to the 30-minute visit from the comfort of their couch.
The COVID-19 pandemic has resulted in millions of deaths and trillions of dollars in economic loss, as well as changed the norms of social interaction. One of the many ways it impacted our health care system is through the exponential growth of telehealth – the use of telecommunication modalities, such as telephone and real-time video – to connect patients with clinicians for the purpose of providing health care.
Prior to the pandemic, telehealth was limited to populations with limited access to health care. Our practice had never performed telehealth, yet converted nearly exclusively to telehealth at the height of the pandemic. My colleagues and I were concerned about how patients and physicians would respond to the sudden disruption of norms of patient engagement.
To measure the response, we conducted an online survey of over 500 gastroenterologists and nearly 1,500 patients from March to May 2020 to assess their satisfaction with telehealth. Our published results demonstrated that more than 80% of patients and 90% of physicians surveyed were either satisfied or highly satisfied with telehealth. Surprisingly, these trends were true irrespective of age or the reason for a visit. Greater than 80% of patients also indicated that the provider addressed their concerns and that they were willing to participate in telehealth visits in the future.
In a subsequent survey of nearly 3,000 patients who had experience with telehealth and in-person visits, 73% of respondents indicated that they received a similar quality of care through telehealth as compared to in-person visits and 61% stated that the interaction with their physician was also similar. More than half of the patients (54%) were likely to continue using telehealth services after the pandemic mainly because of shorter wait and travel times (75%), flexibility with personal schedule (56%), and ease of scheduling appointments on a desired date (47%).
During the COVID-19 pandemic, access to health care has been limited for a great number of patients, and telehealth has been a useful and necessary tool in overcoming this challenge. Telehealth also promotes the triple aim of improving health care by improving the care experience, reducing cost, and improving patient and population health outcomes. Our findings showed a high level of overall patient and provider satisfaction following telehealth appointments. Telehealth increases access to care by decreasing travel time and cost, limiting missed workdays, and reducing the need to find alternative caregivers, especially among rural communities and people facing financial hardship. For a small subset of people who lack the resources, access to technology, or ability to do video visits, telephone-only visits are an appropriate option and should be preserved and reimbursed in some capacity.
From a patient perspective, convenience and decreased cost are often cited as major reasons for satisfaction with telehealth. This is of particular importance to people with limited mobility, nontraditional work hours, and lower socioeconomic status. For patients who use public transportation or caregivers to travel to appointments, a short appointment may require hours of logistical planning and may come at significant financial cost. Enabling these patients to interact with their providers from home would make accessing the health care system both less expensive and logistically less challenging.
One unexpected benefit of telehealth that I have experienced is the ability to “visit” the patients in their own surroundings. Many telehealth visits have allowed the doctor to make a “house call” and see the patients in their homes, cars, and break rooms. Observing the chaos in a home or an extremely quiet and dark space has given me insight into the role anxiety and depression might play in health conditions – which may have not been appreciated in a visit to my office.
The most memorable meeting was a man who was sitting in his kitchen while smoking a cigarette and drinking a beer for breakfast whose main complaint was heartburn. His life habits were obviously contributing to his heartburn, and this degree of insight would not have been appreciated during a traditional in-person office visit.
Congress is now contemplating the role telehealth will play in health care once the pandemic is over. The main concerns are abuse of telehealth by providers, leading to a dramatic rise in visits due to the ease of care delivery. This in turn can dramatically increase health care costs. The long-term health outcomes of patients seen through telehealth are also unknown and must be studied.
All these concerns are valid and must be addressed in future studies, but it would be a mistake for Congress to revert telehealth back to prepandemic regulations. We must move forward with this important innovation in care delivery.
The adoption of telehealth is one of few silver linings of the COVID-19 pandemic. It will never replace in-person visits but should be preserved as an additional tool we can use when in-person visits are not the best option. The future of U.S. health care must allow for a hybrid model so that patients and providers can continue to benefit from this valuable innovation. Patients, providers, and families will be forever grateful.
Naresh Gunaratnam MD, AGAF is a practicing gastroenterologist with Huron Gastroenterology in Ann Arbor, Mich. He also serves as the chair of data analytics as a member of the Digestive Health Physicians Association executive committee. Dr. Gunaratnam has no conflicts in telehealth. He is the founder of and CMO of a weight loss device company and service.
On March 11, 2020, the World Health Organization declared the novel coronavirus disease (COVID-19) to be a global pandemic. Shortly after, federal regulators temporarily relaxed restrictions, raised Medicare payment for telemedicine visits to the same level as in-person visits, and waived or reduced cost sharing for patients. As pandemic-related regulations expire, policymakers are debating the need to address insurance coverage of telemedicine services going forward. Congress should consider the lessons learned over the past 20 months to ensure that health care providers have the flexibility to meet the needs of patients.
One of my early telehealth visits was with a patient in his 80s who spent nearly a month in the hospital after complex abdominal surgery. While at home with his daughter, it was the first visit to assess his progress after discharge from the hospital. We were able to address his concerns, assess his wounds using the video on his computer, and formulate a plan so he could continue to improve. At the end of the call, his daughter mentioned in passing, “Thank God we did not have to go to the office ... that would have been a nightmare.”
The nightmare would have consisted of driving her frail father 45 minutes to our office, spending 15 minutes to park, waiting for 30 minutes to be seen, and finally speaking with the physician for 30 minutes face-to-face. Following the appointment, my patient and his daughter would spend another 10 minutes checking out before the 45-minute drive home. Instead, they spent a few minutes logging on through a computer prior to the 30-minute visit from the comfort of their couch.
The COVID-19 pandemic has resulted in millions of deaths and trillions of dollars in economic loss, as well as changed the norms of social interaction. One of the many ways it impacted our health care system is through the exponential growth of telehealth – the use of telecommunication modalities, such as telephone and real-time video – to connect patients with clinicians for the purpose of providing health care.
Prior to the pandemic, telehealth was limited to populations with limited access to health care. Our practice had never performed telehealth, yet converted nearly exclusively to telehealth at the height of the pandemic. My colleagues and I were concerned about how patients and physicians would respond to the sudden disruption of norms of patient engagement.
To measure the response, we conducted an online survey of over 500 gastroenterologists and nearly 1,500 patients from March to May 2020 to assess their satisfaction with telehealth. Our published results demonstrated that more than 80% of patients and 90% of physicians surveyed were either satisfied or highly satisfied with telehealth. Surprisingly, these trends were true irrespective of age or the reason for a visit. Greater than 80% of patients also indicated that the provider addressed their concerns and that they were willing to participate in telehealth visits in the future.
In a subsequent survey of nearly 3,000 patients who had experience with telehealth and in-person visits, 73% of respondents indicated that they received a similar quality of care through telehealth as compared to in-person visits and 61% stated that the interaction with their physician was also similar. More than half of the patients (54%) were likely to continue using telehealth services after the pandemic mainly because of shorter wait and travel times (75%), flexibility with personal schedule (56%), and ease of scheduling appointments on a desired date (47%).
During the COVID-19 pandemic, access to health care has been limited for a great number of patients, and telehealth has been a useful and necessary tool in overcoming this challenge. Telehealth also promotes the triple aim of improving health care by improving the care experience, reducing cost, and improving patient and population health outcomes. Our findings showed a high level of overall patient and provider satisfaction following telehealth appointments. Telehealth increases access to care by decreasing travel time and cost, limiting missed workdays, and reducing the need to find alternative caregivers, especially among rural communities and people facing financial hardship. For a small subset of people who lack the resources, access to technology, or ability to do video visits, telephone-only visits are an appropriate option and should be preserved and reimbursed in some capacity.
From a patient perspective, convenience and decreased cost are often cited as major reasons for satisfaction with telehealth. This is of particular importance to people with limited mobility, nontraditional work hours, and lower socioeconomic status. For patients who use public transportation or caregivers to travel to appointments, a short appointment may require hours of logistical planning and may come at significant financial cost. Enabling these patients to interact with their providers from home would make accessing the health care system both less expensive and logistically less challenging.
One unexpected benefit of telehealth that I have experienced is the ability to “visit” the patients in their own surroundings. Many telehealth visits have allowed the doctor to make a “house call” and see the patients in their homes, cars, and break rooms. Observing the chaos in a home or an extremely quiet and dark space has given me insight into the role anxiety and depression might play in health conditions – which may have not been appreciated in a visit to my office.
The most memorable meeting was a man who was sitting in his kitchen while smoking a cigarette and drinking a beer for breakfast whose main complaint was heartburn. His life habits were obviously contributing to his heartburn, and this degree of insight would not have been appreciated during a traditional in-person office visit.
Congress is now contemplating the role telehealth will play in health care once the pandemic is over. The main concerns are abuse of telehealth by providers, leading to a dramatic rise in visits due to the ease of care delivery. This in turn can dramatically increase health care costs. The long-term health outcomes of patients seen through telehealth are also unknown and must be studied.
All these concerns are valid and must be addressed in future studies, but it would be a mistake for Congress to revert telehealth back to prepandemic regulations. We must move forward with this important innovation in care delivery.
The adoption of telehealth is one of few silver linings of the COVID-19 pandemic. It will never replace in-person visits but should be preserved as an additional tool we can use when in-person visits are not the best option. The future of U.S. health care must allow for a hybrid model so that patients and providers can continue to benefit from this valuable innovation. Patients, providers, and families will be forever grateful.
Naresh Gunaratnam MD, AGAF is a practicing gastroenterologist with Huron Gastroenterology in Ann Arbor, Mich. He also serves as the chair of data analytics as a member of the Digestive Health Physicians Association executive committee. Dr. Gunaratnam has no conflicts in telehealth. He is the founder of and CMO of a weight loss device company and service.
The road less traveled in gastroenterology and hepatology: Becoming a medical educator
How did you realize medical education was the pathway for you?
Near the end of medical school, I recall my friends and I casting predictions about what each person would be doing in twenty years. The projections offered up about my ultimate landing place were unanimous: a clinical researcher leading a gastroenterology division. I was excited when they said this to me. It made sense, as I had already done over 3 years of clinical research on inflammatory bowel disease at the time. But as I began leading various clinical research projects during my internal medicine residency, I realized that they were not generating a strong sense of fulfillment or passion for me. I greatly enjoyed the process of research and writing, but there still was something missing; I could no longer see the role of a funded clinical researcher sustaining me for the length of my medical and academic career.
Thus, at the end of my 2nd year of residency, I began to self-reflect more on the various aspects of my medical journey to elucidate my path forward. This process was jump-started by a humbling recognition from that year’s graduating class of medical students for my contributions to their education over the past 3 years. I had served as a teaching assistant for their pathophysiology course and then subsequently worked alongside many of them on their medicine rotations. I realized that helping foster their growth as physicians in a longitudinal way was unquestionably the most rewarding experience that I had had to date. With further reflection, I recognized that, amid the chaos of a busy call day, I most looked forward to the moments when I could teach the interns and students about the nuances of the patients being admitted. It never felt like an obligation but rather always left me feeling revitalized. So, by the beginning of my 3rd year of residency, I knew that I wanted to pursue a career within medical education.
Once you decided to become a medical educator, what were your next steps?
As I began to vocalize this change in career trajectory, I did not always encounter enthusiastic support. Because the medical educator pathway is more typical amongst the general medicine community, some faculty members advised me to avoid solely focusing on medical education as a specialist because academic success would be difficult to attain. But I had just recognized this could be my vocation within medicine, so I could not turn back now. Thus, I began to seek the mentorship of educators at my institution, and many of them wisely advised me to consider pursuing additional training in medical education to accrue the skill sets needed to lay the groundwork for a lifelong career. So, I participated in a 1-year medical education fellowship in conjunction with my chief residency year. This training was profoundly formative; I learned about the various theories on adult learning, as well as how to create curricula, how to teach effectively in a clinical environment, and how to deliver meaningful feedback to learners. But perhaps most importantly, I learned how to generate tangible evidence of productivity within medical education to allow for advancement in academia. This included rigorously studying the impact of educational interventions. It became clear to me by the end of this year that the pathways of medical education and researcher were not incongruent but could actually be quite complementary. In light of this, I designed and implemented a mandatory inpatient hepatology curriculum for internal medicine residents, for which I studied its immediate and long-term effects throughout my gastroenterology and hepatology fellowships as well as during my time as an attending. Currently, I am also investigating medical students’ exposure to liver disease through a multicenter assessment. Projects such as these would not have been feasible without dedicated mentorship, but as alluded to above, in contrast to the traditional clinical research paradigm, my mentors have often been from outside the fields of gastroenterology and hepatology.
What advice would you offer a junior faculty member interested in a career in medical education within gastroenterology and hepatology?
1. Just before I completed fellowship, I asked Holly Humphrey, MD, the former dean of the Pritzker School of Medicine at the University of Chicago, this same question. Her answer was simple and is worth sharing: “In the beginning, just focus on becoming the best clinician possible. The rest will fall into place with time.” So, I did exactly this. I continually tried to push the limits of my knowledge, always questioning standard clinical practices to understand the evidence behind (or not behind) them. This knowledge then naturally became the content of my teaching for trainees in the clinical environment so that eventually patient care and teaching were seamlessly integrated into the same day-to-day workflow. The more I taught trainees, the more my commitment to education was recognized by my institution.
2. Meet with leadership of your medical school, internal medicine residency program, and gastroenterology and hepatology fellowships early in the course of your career to assert your desire to contribute to their respective educational missions.
3. Create a teaching philosophy that clearly communicates “your fundamental beliefs about teaching and learning, why you hold those values and beliefs, and how you translate these claims into practice.”1 This document will act as a guiding force in your career by highlighting the themes and principles that you have already incorporated and will continue to incorporate into your teaching practices and educational activities. For example, it can provide clarity when you are in doubt of how to address a difficult learning environment or whether to accept a certain position.
4. Because of No. 1 and No. 2, you will start to be offered opportunities to formally become involved in curricula within undergraduate (UME) and graduate medical education (GME). It will likely begin with requests to lecture or precept small group sessions. Use these smaller opportunities not only to refine your teaching skills but to explore whether your career aspirations better align with UME or GME. With hard work and perseverance, the opportunities can progress to invitations to become a course director, join a curriculum committee, or become an associate program director for a residency or fellowship program (which at this point is why you want to know if you prefer working in UME, GME, or both).
5. Seek feedback often from your learners. It is the only way you will continue to improve your teaching skills and the learning environment you create. Furthermore, formal evaluations can be used in the promotion process.
6. Collaborate with and seek mentorship from fellow medical educators both at your own institution and at others. As previously mentioned, these relationships do not need to be (and are often not) with other gastroenterologists or hepatologists.
7. Seek out national opportunities related to medical education. Most of the gastroenterology and hepatology societies have one or more committees focused on medical training. The AGA Academy of Educators is a fantastic community of education-focused individuals within our specialty that provides opportunities for networking, funding, and career development. Furthermore, other general societies (for example, the Association of American Medical Colleges, American College of Physicians) may be interested in including subspecialty members in their educational committees and activities.
Dr. Mikolajczyk is an assistant professor of medicine and an associate program director for the Internal Medicine Residency Program at the University of Illinois Chicago. He is the lead faculty adviser for the Liver Fellow Network. He has no conflicts of interest to disclose.
How did you realize medical education was the pathway for you?
Near the end of medical school, I recall my friends and I casting predictions about what each person would be doing in twenty years. The projections offered up about my ultimate landing place were unanimous: a clinical researcher leading a gastroenterology division. I was excited when they said this to me. It made sense, as I had already done over 3 years of clinical research on inflammatory bowel disease at the time. But as I began leading various clinical research projects during my internal medicine residency, I realized that they were not generating a strong sense of fulfillment or passion for me. I greatly enjoyed the process of research and writing, but there still was something missing; I could no longer see the role of a funded clinical researcher sustaining me for the length of my medical and academic career.
Thus, at the end of my 2nd year of residency, I began to self-reflect more on the various aspects of my medical journey to elucidate my path forward. This process was jump-started by a humbling recognition from that year’s graduating class of medical students for my contributions to their education over the past 3 years. I had served as a teaching assistant for their pathophysiology course and then subsequently worked alongside many of them on their medicine rotations. I realized that helping foster their growth as physicians in a longitudinal way was unquestionably the most rewarding experience that I had had to date. With further reflection, I recognized that, amid the chaos of a busy call day, I most looked forward to the moments when I could teach the interns and students about the nuances of the patients being admitted. It never felt like an obligation but rather always left me feeling revitalized. So, by the beginning of my 3rd year of residency, I knew that I wanted to pursue a career within medical education.
Once you decided to become a medical educator, what were your next steps?
As I began to vocalize this change in career trajectory, I did not always encounter enthusiastic support. Because the medical educator pathway is more typical amongst the general medicine community, some faculty members advised me to avoid solely focusing on medical education as a specialist because academic success would be difficult to attain. But I had just recognized this could be my vocation within medicine, so I could not turn back now. Thus, I began to seek the mentorship of educators at my institution, and many of them wisely advised me to consider pursuing additional training in medical education to accrue the skill sets needed to lay the groundwork for a lifelong career. So, I participated in a 1-year medical education fellowship in conjunction with my chief residency year. This training was profoundly formative; I learned about the various theories on adult learning, as well as how to create curricula, how to teach effectively in a clinical environment, and how to deliver meaningful feedback to learners. But perhaps most importantly, I learned how to generate tangible evidence of productivity within medical education to allow for advancement in academia. This included rigorously studying the impact of educational interventions. It became clear to me by the end of this year that the pathways of medical education and researcher were not incongruent but could actually be quite complementary. In light of this, I designed and implemented a mandatory inpatient hepatology curriculum for internal medicine residents, for which I studied its immediate and long-term effects throughout my gastroenterology and hepatology fellowships as well as during my time as an attending. Currently, I am also investigating medical students’ exposure to liver disease through a multicenter assessment. Projects such as these would not have been feasible without dedicated mentorship, but as alluded to above, in contrast to the traditional clinical research paradigm, my mentors have often been from outside the fields of gastroenterology and hepatology.
What advice would you offer a junior faculty member interested in a career in medical education within gastroenterology and hepatology?
1. Just before I completed fellowship, I asked Holly Humphrey, MD, the former dean of the Pritzker School of Medicine at the University of Chicago, this same question. Her answer was simple and is worth sharing: “In the beginning, just focus on becoming the best clinician possible. The rest will fall into place with time.” So, I did exactly this. I continually tried to push the limits of my knowledge, always questioning standard clinical practices to understand the evidence behind (or not behind) them. This knowledge then naturally became the content of my teaching for trainees in the clinical environment so that eventually patient care and teaching were seamlessly integrated into the same day-to-day workflow. The more I taught trainees, the more my commitment to education was recognized by my institution.
2. Meet with leadership of your medical school, internal medicine residency program, and gastroenterology and hepatology fellowships early in the course of your career to assert your desire to contribute to their respective educational missions.
3. Create a teaching philosophy that clearly communicates “your fundamental beliefs about teaching and learning, why you hold those values and beliefs, and how you translate these claims into practice.”1 This document will act as a guiding force in your career by highlighting the themes and principles that you have already incorporated and will continue to incorporate into your teaching practices and educational activities. For example, it can provide clarity when you are in doubt of how to address a difficult learning environment or whether to accept a certain position.
4. Because of No. 1 and No. 2, you will start to be offered opportunities to formally become involved in curricula within undergraduate (UME) and graduate medical education (GME). It will likely begin with requests to lecture or precept small group sessions. Use these smaller opportunities not only to refine your teaching skills but to explore whether your career aspirations better align with UME or GME. With hard work and perseverance, the opportunities can progress to invitations to become a course director, join a curriculum committee, or become an associate program director for a residency or fellowship program (which at this point is why you want to know if you prefer working in UME, GME, or both).
5. Seek feedback often from your learners. It is the only way you will continue to improve your teaching skills and the learning environment you create. Furthermore, formal evaluations can be used in the promotion process.
6. Collaborate with and seek mentorship from fellow medical educators both at your own institution and at others. As previously mentioned, these relationships do not need to be (and are often not) with other gastroenterologists or hepatologists.
7. Seek out national opportunities related to medical education. Most of the gastroenterology and hepatology societies have one or more committees focused on medical training. The AGA Academy of Educators is a fantastic community of education-focused individuals within our specialty that provides opportunities for networking, funding, and career development. Furthermore, other general societies (for example, the Association of American Medical Colleges, American College of Physicians) may be interested in including subspecialty members in their educational committees and activities.
Dr. Mikolajczyk is an assistant professor of medicine and an associate program director for the Internal Medicine Residency Program at the University of Illinois Chicago. He is the lead faculty adviser for the Liver Fellow Network. He has no conflicts of interest to disclose.
How did you realize medical education was the pathway for you?
Near the end of medical school, I recall my friends and I casting predictions about what each person would be doing in twenty years. The projections offered up about my ultimate landing place were unanimous: a clinical researcher leading a gastroenterology division. I was excited when they said this to me. It made sense, as I had already done over 3 years of clinical research on inflammatory bowel disease at the time. But as I began leading various clinical research projects during my internal medicine residency, I realized that they were not generating a strong sense of fulfillment or passion for me. I greatly enjoyed the process of research and writing, but there still was something missing; I could no longer see the role of a funded clinical researcher sustaining me for the length of my medical and academic career.
Thus, at the end of my 2nd year of residency, I began to self-reflect more on the various aspects of my medical journey to elucidate my path forward. This process was jump-started by a humbling recognition from that year’s graduating class of medical students for my contributions to their education over the past 3 years. I had served as a teaching assistant for their pathophysiology course and then subsequently worked alongside many of them on their medicine rotations. I realized that helping foster their growth as physicians in a longitudinal way was unquestionably the most rewarding experience that I had had to date. With further reflection, I recognized that, amid the chaos of a busy call day, I most looked forward to the moments when I could teach the interns and students about the nuances of the patients being admitted. It never felt like an obligation but rather always left me feeling revitalized. So, by the beginning of my 3rd year of residency, I knew that I wanted to pursue a career within medical education.
Once you decided to become a medical educator, what were your next steps?
As I began to vocalize this change in career trajectory, I did not always encounter enthusiastic support. Because the medical educator pathway is more typical amongst the general medicine community, some faculty members advised me to avoid solely focusing on medical education as a specialist because academic success would be difficult to attain. But I had just recognized this could be my vocation within medicine, so I could not turn back now. Thus, I began to seek the mentorship of educators at my institution, and many of them wisely advised me to consider pursuing additional training in medical education to accrue the skill sets needed to lay the groundwork for a lifelong career. So, I participated in a 1-year medical education fellowship in conjunction with my chief residency year. This training was profoundly formative; I learned about the various theories on adult learning, as well as how to create curricula, how to teach effectively in a clinical environment, and how to deliver meaningful feedback to learners. But perhaps most importantly, I learned how to generate tangible evidence of productivity within medical education to allow for advancement in academia. This included rigorously studying the impact of educational interventions. It became clear to me by the end of this year that the pathways of medical education and researcher were not incongruent but could actually be quite complementary. In light of this, I designed and implemented a mandatory inpatient hepatology curriculum for internal medicine residents, for which I studied its immediate and long-term effects throughout my gastroenterology and hepatology fellowships as well as during my time as an attending. Currently, I am also investigating medical students’ exposure to liver disease through a multicenter assessment. Projects such as these would not have been feasible without dedicated mentorship, but as alluded to above, in contrast to the traditional clinical research paradigm, my mentors have often been from outside the fields of gastroenterology and hepatology.
What advice would you offer a junior faculty member interested in a career in medical education within gastroenterology and hepatology?
1. Just before I completed fellowship, I asked Holly Humphrey, MD, the former dean of the Pritzker School of Medicine at the University of Chicago, this same question. Her answer was simple and is worth sharing: “In the beginning, just focus on becoming the best clinician possible. The rest will fall into place with time.” So, I did exactly this. I continually tried to push the limits of my knowledge, always questioning standard clinical practices to understand the evidence behind (or not behind) them. This knowledge then naturally became the content of my teaching for trainees in the clinical environment so that eventually patient care and teaching were seamlessly integrated into the same day-to-day workflow. The more I taught trainees, the more my commitment to education was recognized by my institution.
2. Meet with leadership of your medical school, internal medicine residency program, and gastroenterology and hepatology fellowships early in the course of your career to assert your desire to contribute to their respective educational missions.
3. Create a teaching philosophy that clearly communicates “your fundamental beliefs about teaching and learning, why you hold those values and beliefs, and how you translate these claims into practice.”1 This document will act as a guiding force in your career by highlighting the themes and principles that you have already incorporated and will continue to incorporate into your teaching practices and educational activities. For example, it can provide clarity when you are in doubt of how to address a difficult learning environment or whether to accept a certain position.
4. Because of No. 1 and No. 2, you will start to be offered opportunities to formally become involved in curricula within undergraduate (UME) and graduate medical education (GME). It will likely begin with requests to lecture or precept small group sessions. Use these smaller opportunities not only to refine your teaching skills but to explore whether your career aspirations better align with UME or GME. With hard work and perseverance, the opportunities can progress to invitations to become a course director, join a curriculum committee, or become an associate program director for a residency or fellowship program (which at this point is why you want to know if you prefer working in UME, GME, or both).
5. Seek feedback often from your learners. It is the only way you will continue to improve your teaching skills and the learning environment you create. Furthermore, formal evaluations can be used in the promotion process.
6. Collaborate with and seek mentorship from fellow medical educators both at your own institution and at others. As previously mentioned, these relationships do not need to be (and are often not) with other gastroenterologists or hepatologists.
7. Seek out national opportunities related to medical education. Most of the gastroenterology and hepatology societies have one or more committees focused on medical training. The AGA Academy of Educators is a fantastic community of education-focused individuals within our specialty that provides opportunities for networking, funding, and career development. Furthermore, other general societies (for example, the Association of American Medical Colleges, American College of Physicians) may be interested in including subspecialty members in their educational committees and activities.
Dr. Mikolajczyk is an assistant professor of medicine and an associate program director for the Internal Medicine Residency Program at the University of Illinois Chicago. He is the lead faculty adviser for the Liver Fellow Network. He has no conflicts of interest to disclose.
The future of training: AGA EndoscopyNow Fellows Forum recap
Introduction
The virtual space has created new opportunities for gastroenterology fellows, but direct conversations about education and career development on the national level have been limited. On Oct. 16, 2021, the American Gastroenterological Association and EndoscopyNow hosted an online Fellows Forum titled “Navigating New Frontiers of Training in Gastroenterology.” Close to 100 fellows attended and had the chance to listen to discussions from a national panel of faculty with expertise in medical education, ask candid questions, and share experiences in breakout rooms specific to their year of training. Reading materials were also provided, which are cited throughout this article. What follows is a rundown of the discussion and points of particular interest for fellows.
What do fellows value?
Dr. Laura Raffals kicked off the event by asking fellows to create word clouds related to their challenges (“Balance” was the most common answer) and joys (“Family”). These answers underscore that, when faced with pressures to be 100% at work and home, it is human connection, particularly family, that sustains us. Fellows, however, worried that spending time with family conflicted with spending time on GI training and that they would be perceived as “that person who always leaves early.”1
Attendees discussed that “there are only 168 hours in a week,” (time is a zero-sum game), and it is important to be self-aware and honest about one’s personal values and commit the commensurate time and energy to those values. Consider personal development exercises.2 Faculty have a crucial role in coaching fellows on time management based on personal values.3
Has COVID-19 reduced fellows’ endoscopic skills?
One brave attendee asked: Is this generation of fellows “weaker” because of limited scoping during the pandemic? Faculty discussed that, even prepandemic, it was “not all about quantity; the quality of exposure matters just as much.” From their perspective, prepared, goal-directed, and helpful fellows would maximize learning during endoscopy blocks (see below). Lawrence Schiller, MD, providing the long view, reassured fellows that with a proactive attitude it all evens out in the end.
Fellows reflected that, although social isolation and burnout were rampant, some individuals stepped up to do extra work, supported colleagues with personal or family health issues, and scoped COVID-positive patients if others could not. In future years, the pandemic will be seen as a case study for those in leadership positions. The decisions that health systems, administrators, and providers made will be remembered, as well as how algorithms for “practice as usual” changed.4
What fellows can do to maximize endoscopic learning (attendings’ perspective):
- Know the patient before the case. Prior endoscopy reports, patient comorbidities, and medical history including details like anticoagulation use or issues with anesthesia.
- Help the work flow (and reduce the attending’s stress level). Consent patients and complete preprocedure paperwork if possible.
- Come into the scope block with a plan. Example: “I want to get to the cecum. The attending can withdraw, and I will take out polyps we find.”
- Ask about decision-making. Example: “Why did you choose to place a clip over that polypectomy site?” or “Why did you choose that instrument and not the other?”
- Give feedback on problematic behavior. Attendings that treat fellows like burdens and undermine fellow scope time should be reported. Fellows may be concerned about being perceived as a “troublemaker,” but discussing these situations with program directors is a civic duty.
How can we improve diversity?
We cannot wait for, and must instead proactively recruit, diverse trainees, as well as create inclusive environments. Mentorship is key. However, recent work showing imbalances in gender of mentor-mentees and extra pressure on women mentors raises concerns about sustainability.5,6 The panel suggested that interested fellows could engage students earlier in the pipeline, participate in community awareness and exposure programs, and dedicate education time to health equity.7 Fellows raised concerns about barriers for international medical graduates, which would require institutional and federal policy changes would to implement change.
How can fellows develop better practice patterns?
Sri Komanduri, MD, focused on complex endoscopic cases.8 Having live video, using polls, and listening to other attendings comment on cases was illuminating and sometimes humbling. The panel discussed that simulation labs could strongly enhance endoscopic skill training, but if unavailable, companies are often willing to sponsor events for teaching purposes.9 If training on specific topics is not offered at an institution, regional weekend courses are also an option.
Raman Muthusamy, MD, MS, discussed his philosophy towards endoscopic complications: be prepared and follow your instinct if something feels off. He and Dr. Ikuo Hirano emphasized the importance of following up with patients after a complication. The panel also suggested that fellows can build quality improvement experience by contributing to GI morbidity and mortality conferences or start them if not already offered.
Where is the future headed?
Amrita Sethi, MD, outlined the trend towards virtual platforms and getting the global GI community involved in education efforts. She pointed out the need for a gold standard on assessing competency in endoscopy. From a practice perspective, implementation of telemedicine in GI merits further study, as so far this technology has been attractive to providers and patients alike. Todd Baron, MD, stressed that newer technologies, including artificial intelligence, will not replace the endoscopist but may reduce the need for screening procedures and instead increase demand for specific diagnostic and therapeutic procedures. He used the examples of therapeutic applications of endoscopic ultrasound and the development of single-use duodenoscopes.
Concerns about transitioning from training to independent practice
During the third-year breakout session, fellows discussed anxieties about starting practice and living up to expectations: “What if it’s my first week and there’s something I can’t do?” Faculty recommended getting to know colleagues at a new institution, being confident in your training, and staying engaged with your own complications.10 Fellows described the surprising amount of time and energy they dedicated to the job search and got counseling from Dr. Schiller, who recommended defining what “success” and “satisfaction” look like (again, defining one’s values). He recommended that, for fellows looking at private practice positions, one should ask: How much autonomy do I want? How much business risk am I willing to accept? Fellows need more formal education on practice management and the “business side” of gastroenterology.11
Conclusions
The 2021 AGA EndoscopyNow forum was unique in its discussion of issues impacting GI fellows. The forum revealed that worries about personal well-being, training quality, and future career prospects have affected fellows everywhere: you are not alone. Presentations and lively conversation between seasoned faculty who reflected on career development, education, and medical management demonstrate the importance of seeking advice from colleagues and mentorship. Based on this event, future sessions with conversations between faculty and fellows to assess needs and set priorities for directions in training would be welcome.
Dr. Liu is a gastroenterology fellow, Northwestern University, Chicago. The author has no conflicts of interest to disclose.
References
1. Katzka DA and Proctor DD. Gastroenterology. 2009;136(4):1147-8.
2. Sull D and Houlder D. Do Your Commitments Match Your Convictions? Harv Bus Rev. 2005 Jan 1. https://hbr.org/2005/01/do-your-commitments-match-your-convictions.
3. Keswani RN et al. Gastroenterology. 2020;159(1):26-9.
4. Sethi A et al. Clin Gastroenterol Hepatol. 2020;18(8):1673-81.
5. Rabinowitz LG et al. Gastrointest Endosc. 2021;93(5):1047-56.e5.
6. Rabinowitz LG et al. Gastrointest Endosc. 2020;91(1):155-61.
7. Lee-Allen J, Shah BJ. Gastroenterology. 2021;160(6):1924-8.
8. Richter JM et al. Am J Gastroenterol. 2016;111(3):348-52.
9. Muthusamy VR and Komanduri S. Clin Gastroenterol Hepatol. 2019 Mar;17(4):580-3.
10. Liu H and Boyatzis RE. Front Psychol. 2021. doi: 10.3389/fpsyg.2021.685829.
11. Amann ST et al. “Words” to practice by: A guide to understand the business vernacular of a healthy practice. https://webfiles.gi.org/links/pm/TheHealthOfMyPracticeToolboxPMCommitteeToolbox.pdf.
Introduction
The virtual space has created new opportunities for gastroenterology fellows, but direct conversations about education and career development on the national level have been limited. On Oct. 16, 2021, the American Gastroenterological Association and EndoscopyNow hosted an online Fellows Forum titled “Navigating New Frontiers of Training in Gastroenterology.” Close to 100 fellows attended and had the chance to listen to discussions from a national panel of faculty with expertise in medical education, ask candid questions, and share experiences in breakout rooms specific to their year of training. Reading materials were also provided, which are cited throughout this article. What follows is a rundown of the discussion and points of particular interest for fellows.
What do fellows value?
Dr. Laura Raffals kicked off the event by asking fellows to create word clouds related to their challenges (“Balance” was the most common answer) and joys (“Family”). These answers underscore that, when faced with pressures to be 100% at work and home, it is human connection, particularly family, that sustains us. Fellows, however, worried that spending time with family conflicted with spending time on GI training and that they would be perceived as “that person who always leaves early.”1
Attendees discussed that “there are only 168 hours in a week,” (time is a zero-sum game), and it is important to be self-aware and honest about one’s personal values and commit the commensurate time and energy to those values. Consider personal development exercises.2 Faculty have a crucial role in coaching fellows on time management based on personal values.3
Has COVID-19 reduced fellows’ endoscopic skills?
One brave attendee asked: Is this generation of fellows “weaker” because of limited scoping during the pandemic? Faculty discussed that, even prepandemic, it was “not all about quantity; the quality of exposure matters just as much.” From their perspective, prepared, goal-directed, and helpful fellows would maximize learning during endoscopy blocks (see below). Lawrence Schiller, MD, providing the long view, reassured fellows that with a proactive attitude it all evens out in the end.
Fellows reflected that, although social isolation and burnout were rampant, some individuals stepped up to do extra work, supported colleagues with personal or family health issues, and scoped COVID-positive patients if others could not. In future years, the pandemic will be seen as a case study for those in leadership positions. The decisions that health systems, administrators, and providers made will be remembered, as well as how algorithms for “practice as usual” changed.4
What fellows can do to maximize endoscopic learning (attendings’ perspective):
- Know the patient before the case. Prior endoscopy reports, patient comorbidities, and medical history including details like anticoagulation use or issues with anesthesia.
- Help the work flow (and reduce the attending’s stress level). Consent patients and complete preprocedure paperwork if possible.
- Come into the scope block with a plan. Example: “I want to get to the cecum. The attending can withdraw, and I will take out polyps we find.”
- Ask about decision-making. Example: “Why did you choose to place a clip over that polypectomy site?” or “Why did you choose that instrument and not the other?”
- Give feedback on problematic behavior. Attendings that treat fellows like burdens and undermine fellow scope time should be reported. Fellows may be concerned about being perceived as a “troublemaker,” but discussing these situations with program directors is a civic duty.
How can we improve diversity?
We cannot wait for, and must instead proactively recruit, diverse trainees, as well as create inclusive environments. Mentorship is key. However, recent work showing imbalances in gender of mentor-mentees and extra pressure on women mentors raises concerns about sustainability.5,6 The panel suggested that interested fellows could engage students earlier in the pipeline, participate in community awareness and exposure programs, and dedicate education time to health equity.7 Fellows raised concerns about barriers for international medical graduates, which would require institutional and federal policy changes would to implement change.
How can fellows develop better practice patterns?
Sri Komanduri, MD, focused on complex endoscopic cases.8 Having live video, using polls, and listening to other attendings comment on cases was illuminating and sometimes humbling. The panel discussed that simulation labs could strongly enhance endoscopic skill training, but if unavailable, companies are often willing to sponsor events for teaching purposes.9 If training on specific topics is not offered at an institution, regional weekend courses are also an option.
Raman Muthusamy, MD, MS, discussed his philosophy towards endoscopic complications: be prepared and follow your instinct if something feels off. He and Dr. Ikuo Hirano emphasized the importance of following up with patients after a complication. The panel also suggested that fellows can build quality improvement experience by contributing to GI morbidity and mortality conferences or start them if not already offered.
Where is the future headed?
Amrita Sethi, MD, outlined the trend towards virtual platforms and getting the global GI community involved in education efforts. She pointed out the need for a gold standard on assessing competency in endoscopy. From a practice perspective, implementation of telemedicine in GI merits further study, as so far this technology has been attractive to providers and patients alike. Todd Baron, MD, stressed that newer technologies, including artificial intelligence, will not replace the endoscopist but may reduce the need for screening procedures and instead increase demand for specific diagnostic and therapeutic procedures. He used the examples of therapeutic applications of endoscopic ultrasound and the development of single-use duodenoscopes.
Concerns about transitioning from training to independent practice
During the third-year breakout session, fellows discussed anxieties about starting practice and living up to expectations: “What if it’s my first week and there’s something I can’t do?” Faculty recommended getting to know colleagues at a new institution, being confident in your training, and staying engaged with your own complications.10 Fellows described the surprising amount of time and energy they dedicated to the job search and got counseling from Dr. Schiller, who recommended defining what “success” and “satisfaction” look like (again, defining one’s values). He recommended that, for fellows looking at private practice positions, one should ask: How much autonomy do I want? How much business risk am I willing to accept? Fellows need more formal education on practice management and the “business side” of gastroenterology.11
Conclusions
The 2021 AGA EndoscopyNow forum was unique in its discussion of issues impacting GI fellows. The forum revealed that worries about personal well-being, training quality, and future career prospects have affected fellows everywhere: you are not alone. Presentations and lively conversation between seasoned faculty who reflected on career development, education, and medical management demonstrate the importance of seeking advice from colleagues and mentorship. Based on this event, future sessions with conversations between faculty and fellows to assess needs and set priorities for directions in training would be welcome.
Dr. Liu is a gastroenterology fellow, Northwestern University, Chicago. The author has no conflicts of interest to disclose.
References
1. Katzka DA and Proctor DD. Gastroenterology. 2009;136(4):1147-8.
2. Sull D and Houlder D. Do Your Commitments Match Your Convictions? Harv Bus Rev. 2005 Jan 1. https://hbr.org/2005/01/do-your-commitments-match-your-convictions.
3. Keswani RN et al. Gastroenterology. 2020;159(1):26-9.
4. Sethi A et al. Clin Gastroenterol Hepatol. 2020;18(8):1673-81.
5. Rabinowitz LG et al. Gastrointest Endosc. 2021;93(5):1047-56.e5.
6. Rabinowitz LG et al. Gastrointest Endosc. 2020;91(1):155-61.
7. Lee-Allen J, Shah BJ. Gastroenterology. 2021;160(6):1924-8.
8. Richter JM et al. Am J Gastroenterol. 2016;111(3):348-52.
9. Muthusamy VR and Komanduri S. Clin Gastroenterol Hepatol. 2019 Mar;17(4):580-3.
10. Liu H and Boyatzis RE. Front Psychol. 2021. doi: 10.3389/fpsyg.2021.685829.
11. Amann ST et al. “Words” to practice by: A guide to understand the business vernacular of a healthy practice. https://webfiles.gi.org/links/pm/TheHealthOfMyPracticeToolboxPMCommitteeToolbox.pdf.
Introduction
The virtual space has created new opportunities for gastroenterology fellows, but direct conversations about education and career development on the national level have been limited. On Oct. 16, 2021, the American Gastroenterological Association and EndoscopyNow hosted an online Fellows Forum titled “Navigating New Frontiers of Training in Gastroenterology.” Close to 100 fellows attended and had the chance to listen to discussions from a national panel of faculty with expertise in medical education, ask candid questions, and share experiences in breakout rooms specific to their year of training. Reading materials were also provided, which are cited throughout this article. What follows is a rundown of the discussion and points of particular interest for fellows.
What do fellows value?
Dr. Laura Raffals kicked off the event by asking fellows to create word clouds related to their challenges (“Balance” was the most common answer) and joys (“Family”). These answers underscore that, when faced with pressures to be 100% at work and home, it is human connection, particularly family, that sustains us. Fellows, however, worried that spending time with family conflicted with spending time on GI training and that they would be perceived as “that person who always leaves early.”1
Attendees discussed that “there are only 168 hours in a week,” (time is a zero-sum game), and it is important to be self-aware and honest about one’s personal values and commit the commensurate time and energy to those values. Consider personal development exercises.2 Faculty have a crucial role in coaching fellows on time management based on personal values.3
Has COVID-19 reduced fellows’ endoscopic skills?
One brave attendee asked: Is this generation of fellows “weaker” because of limited scoping during the pandemic? Faculty discussed that, even prepandemic, it was “not all about quantity; the quality of exposure matters just as much.” From their perspective, prepared, goal-directed, and helpful fellows would maximize learning during endoscopy blocks (see below). Lawrence Schiller, MD, providing the long view, reassured fellows that with a proactive attitude it all evens out in the end.
Fellows reflected that, although social isolation and burnout were rampant, some individuals stepped up to do extra work, supported colleagues with personal or family health issues, and scoped COVID-positive patients if others could not. In future years, the pandemic will be seen as a case study for those in leadership positions. The decisions that health systems, administrators, and providers made will be remembered, as well as how algorithms for “practice as usual” changed.4
What fellows can do to maximize endoscopic learning (attendings’ perspective):
- Know the patient before the case. Prior endoscopy reports, patient comorbidities, and medical history including details like anticoagulation use or issues with anesthesia.
- Help the work flow (and reduce the attending’s stress level). Consent patients and complete preprocedure paperwork if possible.
- Come into the scope block with a plan. Example: “I want to get to the cecum. The attending can withdraw, and I will take out polyps we find.”
- Ask about decision-making. Example: “Why did you choose to place a clip over that polypectomy site?” or “Why did you choose that instrument and not the other?”
- Give feedback on problematic behavior. Attendings that treat fellows like burdens and undermine fellow scope time should be reported. Fellows may be concerned about being perceived as a “troublemaker,” but discussing these situations with program directors is a civic duty.
How can we improve diversity?
We cannot wait for, and must instead proactively recruit, diverse trainees, as well as create inclusive environments. Mentorship is key. However, recent work showing imbalances in gender of mentor-mentees and extra pressure on women mentors raises concerns about sustainability.5,6 The panel suggested that interested fellows could engage students earlier in the pipeline, participate in community awareness and exposure programs, and dedicate education time to health equity.7 Fellows raised concerns about barriers for international medical graduates, which would require institutional and federal policy changes would to implement change.
How can fellows develop better practice patterns?
Sri Komanduri, MD, focused on complex endoscopic cases.8 Having live video, using polls, and listening to other attendings comment on cases was illuminating and sometimes humbling. The panel discussed that simulation labs could strongly enhance endoscopic skill training, but if unavailable, companies are often willing to sponsor events for teaching purposes.9 If training on specific topics is not offered at an institution, regional weekend courses are also an option.
Raman Muthusamy, MD, MS, discussed his philosophy towards endoscopic complications: be prepared and follow your instinct if something feels off. He and Dr. Ikuo Hirano emphasized the importance of following up with patients after a complication. The panel also suggested that fellows can build quality improvement experience by contributing to GI morbidity and mortality conferences or start them if not already offered.
Where is the future headed?
Amrita Sethi, MD, outlined the trend towards virtual platforms and getting the global GI community involved in education efforts. She pointed out the need for a gold standard on assessing competency in endoscopy. From a practice perspective, implementation of telemedicine in GI merits further study, as so far this technology has been attractive to providers and patients alike. Todd Baron, MD, stressed that newer technologies, including artificial intelligence, will not replace the endoscopist but may reduce the need for screening procedures and instead increase demand for specific diagnostic and therapeutic procedures. He used the examples of therapeutic applications of endoscopic ultrasound and the development of single-use duodenoscopes.
Concerns about transitioning from training to independent practice
During the third-year breakout session, fellows discussed anxieties about starting practice and living up to expectations: “What if it’s my first week and there’s something I can’t do?” Faculty recommended getting to know colleagues at a new institution, being confident in your training, and staying engaged with your own complications.10 Fellows described the surprising amount of time and energy they dedicated to the job search and got counseling from Dr. Schiller, who recommended defining what “success” and “satisfaction” look like (again, defining one’s values). He recommended that, for fellows looking at private practice positions, one should ask: How much autonomy do I want? How much business risk am I willing to accept? Fellows need more formal education on practice management and the “business side” of gastroenterology.11
Conclusions
The 2021 AGA EndoscopyNow forum was unique in its discussion of issues impacting GI fellows. The forum revealed that worries about personal well-being, training quality, and future career prospects have affected fellows everywhere: you are not alone. Presentations and lively conversation between seasoned faculty who reflected on career development, education, and medical management demonstrate the importance of seeking advice from colleagues and mentorship. Based on this event, future sessions with conversations between faculty and fellows to assess needs and set priorities for directions in training would be welcome.
Dr. Liu is a gastroenterology fellow, Northwestern University, Chicago. The author has no conflicts of interest to disclose.
References
1. Katzka DA and Proctor DD. Gastroenterology. 2009;136(4):1147-8.
2. Sull D and Houlder D. Do Your Commitments Match Your Convictions? Harv Bus Rev. 2005 Jan 1. https://hbr.org/2005/01/do-your-commitments-match-your-convictions.
3. Keswani RN et al. Gastroenterology. 2020;159(1):26-9.
4. Sethi A et al. Clin Gastroenterol Hepatol. 2020;18(8):1673-81.
5. Rabinowitz LG et al. Gastrointest Endosc. 2021;93(5):1047-56.e5.
6. Rabinowitz LG et al. Gastrointest Endosc. 2020;91(1):155-61.
7. Lee-Allen J, Shah BJ. Gastroenterology. 2021;160(6):1924-8.
8. Richter JM et al. Am J Gastroenterol. 2016;111(3):348-52.
9. Muthusamy VR and Komanduri S. Clin Gastroenterol Hepatol. 2019 Mar;17(4):580-3.
10. Liu H and Boyatzis RE. Front Psychol. 2021. doi: 10.3389/fpsyg.2021.685829.
11. Amann ST et al. “Words” to practice by: A guide to understand the business vernacular of a healthy practice. https://webfiles.gi.org/links/pm/TheHealthOfMyPracticeToolboxPMCommitteeToolbox.pdf.
Definitive diverticular hemorrhage: Diagnosis and management
Diverticular hemorrhage is the most common cause of colonic bleeding, accounting for 20%-65% of cases of severe lower intestinal bleeding in adults.1 Urgent colonoscopy after purging the colon of blood, clots, and stool is the most accurate method of diagnosing and guiding treatment of definitive diverticular hemorrhage.2-5 The diagnosis of definitive diverticular hemorrhage depends upon identification of some stigmata of recent hemorrhage (SRH) in a single diverticulum (TIC), which can include active arterial bleeding, oozing, non-bleeding visible vessel, adherent clot, or flat spot.2-4 Although other approaches, such as nuclear medicine scans and angiography of various types (CT, MRI, or standard angiography), for the early diagnosis of patients with severe hematochezia are utilized in many medical centers, only active bleeding can be detected by these techniques. However, as subsequently discussed, this SRH is documented in only 26% of definitive diverticular bleeds found on urgent colonoscopy, so diagnostic yields of these techniques will be low.2-5
The diagnosis of patients with severe hematochezia and diverticulosis, as well as triage of all of them to specific medical, endoscopic, radiologic, or surgical management, is facilitated by an urgent endoscopic approach.2-5 Patients who are diagnosed with definitive diverticular hemorrhage on colonoscopy represent about 30% of all true TIC bleeds when urgent colonoscopy is the management approach.2-5 That is because approximately 50% of all patients with colon diverticulosis and first presentation of severe hematochezia have incidental diverticulosis; they have colonic diverticulosis, but another site of bleeding is identified as the cause of hemorrhage in the gastrointestinal tract.2-4 Presumptive diverticular hemorrhage is diagnosed when colonic diverticulosis without TIC stigmata are found but no other GI bleeding source is found on colonoscopy, anoscopy, enteroscopy, or capsule endoscopy.2-5 In our experience with urgent colonoscopy, the presumptive diverticular bleed group accounts for about 70% of patients with documented diverticular hemorrhage (e.g., not including incidental diverticulosis bleeds but combining subgroups of patients with either definitive or presumptive TIC diagnoses as documented TIC hemorrhage).
Clinical presentation
Patients with diverticular hemorrhage present with severe, painless large volume hematochezia. Hematochezia may be self-limited and spontaneously resolve in 75%-80% of all patients but with high rebleeding rates up to 40%.5-7 Of all patients with diverticulosis, only about 3%-5% develop diverticular hemorrhage.8 Risk factors for diverticular hemorrhage include medications (e.g., nonsteroidal anti-inflammatory drugs – NSAIDs, antiplatelet drugs, and anticoagulants) and other clinical factors, such as older age, low-fiber diet, and chronic constipation.9,10 On urgent colonoscopy, more than 70% of diverticulosis in U.S. patients are located anatomically in the descending colon or more distally. In contrast, about 60% of definitive diverticular hemorrhage cases in our experience had diverticula with stigmata identified at or proximal to the splenic flexure.2,4,11
Pathophysiology
Colonic diverticula are herniations of mucosa and submucosa with colonic arteries that penetrate the muscular wall. Bleeding can occur when there is asymmetric rupture of the vasa recta at either the base of the diverticulum or the neck.4 Thinning of the mucosa on the luminal surface (such as that resulting from impacted fecaliths and stool) can cause injury to the site of the penetrating vessels, resulting in hemorrhage.12
Initial management
Patients with acute, severe hematochezia should be triaged to an inpatient setting with a monitored bed. Admission to an intensive care unit should be considered for patients with hemodynamic instability, persistent bleeding, and/or significant comorbidities. Patients with TIC hemorrhage often require resuscitation with crystalloids and packed red blood cell transfusions for hemoglobin less than 8 g/dl.4 Unlike upper GI hemorrhage, which has been extensively reported on, data regarding a more restrictive transfusion threshold, compared with a liberal transfusion threshold, in lower intestinal bleeding are very limited. Correction of underlying coagulopathies is recommended but should be individualized, particularly in those patients on antithrombotic agents or with underlying bleeding disorders.
Urgent diagnosis and hemostasis
Urgent colonoscopy within 24 hours is the most accurate way to make a diagnosis of definitive diverticular hemorrhage and to effectively and safely treat them.2-4,10,11 For patients with severe hematochezia, when the colonoscopy is either not available in a medical center or does not reveal the source of bleeding, nuclear scintigraphy or angiography (CT, MRI, or interventional radiology [IR]) are recommended. CT angiography may be particularly helpful to diagnose patients with hemodynamic instability who are suspected to have active TIC bleeding and are not able to complete a bowel preparation. However, these imaging techniques require active bleeding at the time of the study to be diagnostic. This SRH is also uncommon for definitive diverticular hemorrhage, so the diagnostic yield is usually quite low.2-5,10,11 An additional limitation of scintigraphy and CT or MRI angiography is that, if active bleeding is found, some other type of treatment, such as colonoscopy, IR angiography, or surgery, will be required for definitive hemostasis.
For urgent colonoscopy, adequate colon preparation with a large volume preparation (6-8 liters of polyethylene glycol-based solution) is recommended to clear stool, blood, and clots to allow endoscopic visualization and localization of the bleeding source. Use of a nasogastric tube should be considered if the patient is unable to drink enough prep.2-4,13 Additionally, administration of a prokinetic agent, such as Metoclopramide, may improve gastric emptying and tolerance of the prep. During colonoscopy, careful inspection of the colonic mucosa during insertion and withdrawal is important since lesions may bleed intermittently and SRH can be missed. An adult or pediatric colonoscope with a large working channel (at least 3.3 mm) is recommended to facilitate suctioning of blood clots and stool, as well as allow the passage of endoscopic hemostasis accessories. Targeted water-jet irrigation, an expert colonoscopist, a cap attachment, and adequate colon preparation are all predictors for improved diagnosis of definitive diverticular hemorrhage.4,14
SRH in definitive TIC bleeds all have a high risk of TIC rebleeding,2-4,10,11 including active bleeding, nonbleeding visible vessel, adherent clot, and a flat spot (See Figure).
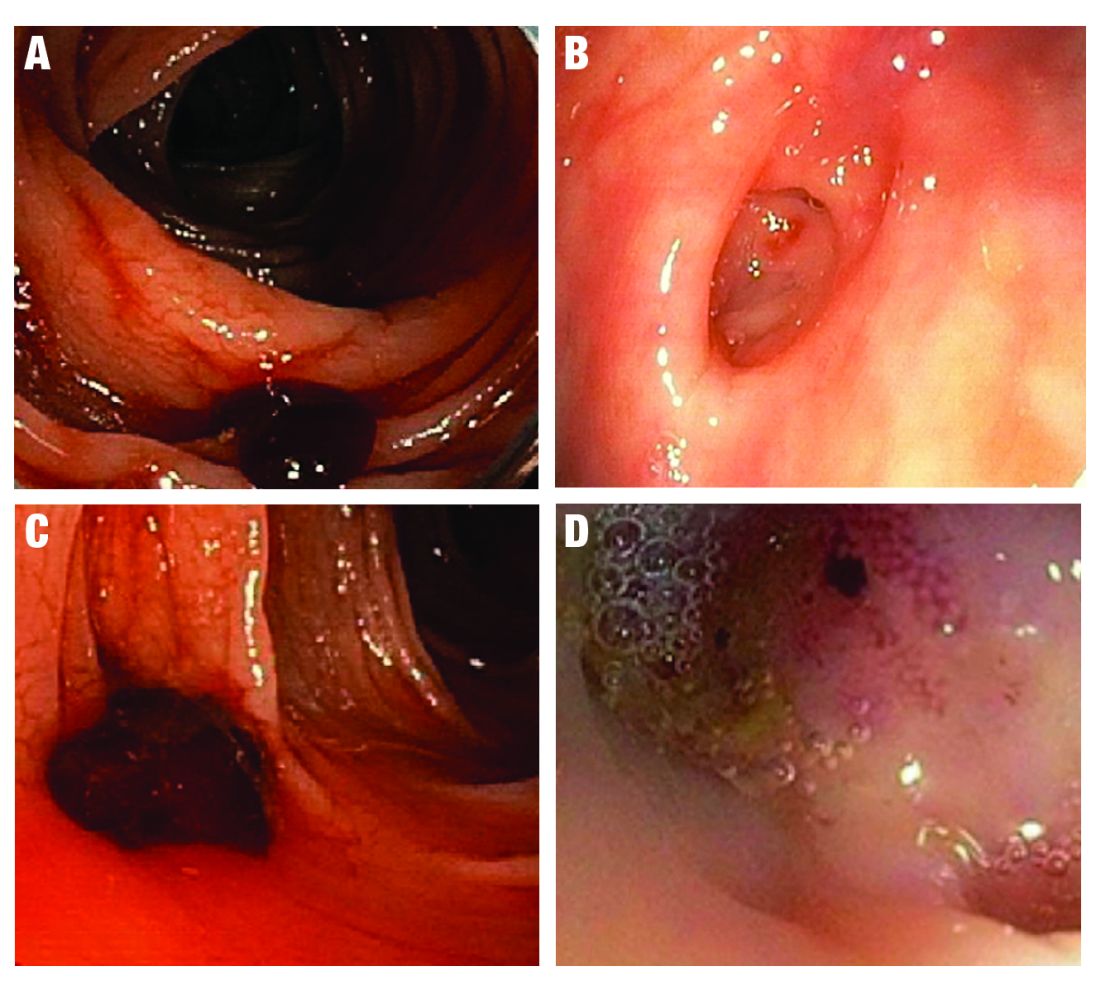
Based on CURE Hemostasis Group data of 118 definitive TIC bleeds, 26% had active bleeding, 24% had a nonbleeding visible vessel, 37% had an adherent clot, and 13% had a flat spot (with underlying arterial blood flow by Doppler probe monitoring).4 Approximately 50% of the SRH were found in the neck of the TIC and 50% at the base, with actively bleeding cases more often from the base. In CURE Doppler endoscopic probe studies, 90% of all stigmata had an underlying arterial blood flow detected with the Doppler probe.4,10 The Doppler probe is reported to be very useful for risk stratification and to confirm obliteration of the arterial blood flow underlying SRH for definitive hemostasis.4,10
Endoscopic treatment
Given high rates of rebleeding with medical management alone, definitive TIC hemorrhage can be effectively and safely treated with endoscopic therapies once SRH are localized.4,10 Endoscopic therapies that have been reported in the literature include electrocoagulation, hemoclip, band ligation, and over-the-scope clip. Four-quadrant injection of 1:20,000 epinephrine around the SRH can improve visualization of SRH and provide temporary control of bleeding, but it should be combined with other modalities because of risk of rebleeding with epinephrine alone.15 Results from studies reporting rates of both early rebleeding (occurring within 30 days) and late rebleeding (occurring after 30 days) are listed in the Table. 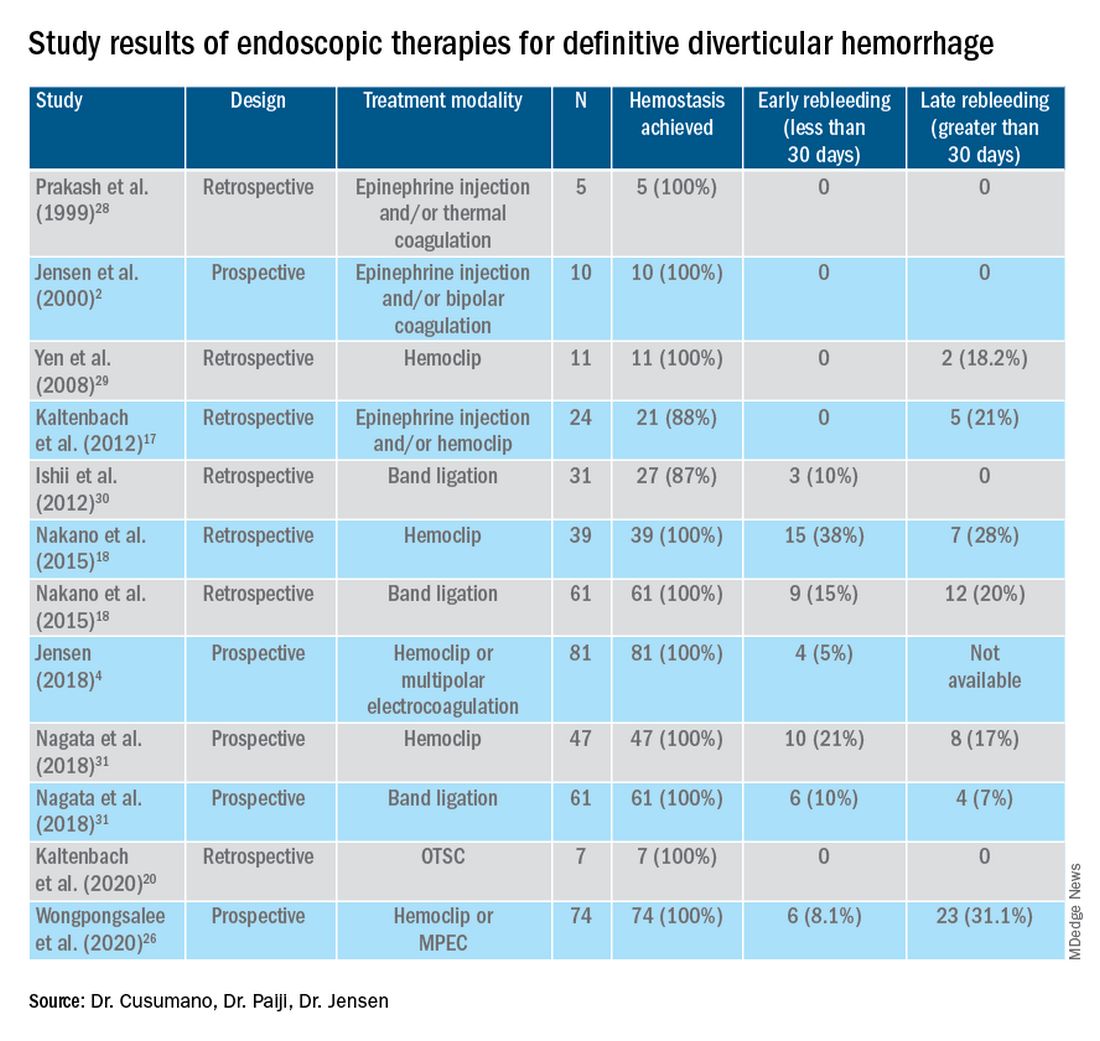
Multipolar electrocoagulation (MPEC), which utilizes a focal electric current to generate heat, can coaptively coagulate small TIC arteries.16 For SRH in the neck of TIC, MPEC is effective for coaptive coagulation at a power of 12-15 watts in 1-2 second pulses with moderate laterally applied tamponade pressure. MPEC should be avoided for treating SRH at the TIC base because of lack of muscularis propria and higher risk of perforation.
Hemoclip therapy has been reported to be safe and efficacious in treatment of definitive TIC hemorrhage, by causing mechanical hemostasis with occlusion of the bleeding artery.16 Hemoclips are recommended to treat stigmata in the base of TICs and should be targeted on either side of visible vessel in order to occlude the artery underneath it.4,10 With a cap on the tip of the colonoscope, suctioning can evert TICs, allowing more precise placement of hemoclip on SRH in the base of the TIC.17 Hemoclip retention rates vary with different models and can range from less than 7 days to more than 4 weeks. Hemoclips can also mark the site if early rebleeding occurs; then, reintervention (e.g., repeat endoscopy or angioembolization) is facilitated.
Another treatment is endoscopic band ligation, which provides mechanical hemostasis. Endoscopic band ligation has been reported to be efficacious for TIC hemorrhage.18 Suctioning the TIC with the SRH into the distal cap and deploying a band leads to obliteration of vessels and potentially necrosis and disappearance of banded TIC.16 This technique carries a risk of perforation because of the thin walls of TICs. This risk may be higher for right-sided colon lesions since an exvivo colon specimen study reported serosal entrapment and inclusion of muscularis propria postband ligation, both of which may result in ischemia of intestinal wall and delayed perforation.19
Over-the-scope clip (OTSC) has been reported in case series for treatment of definitive TIC hemorrhage. With a distal cap and large clip, suctioning can evert TICs and facilitate deployment over the SRH.20,21 OTSC can grasp an entire TIC with the SRH and obliterate the arterial blood flow with a single clip.20,21 No complications have been reported yet for treatment of TIC hemorrhage. However, the OTSC system is relatively expensive when compared with other modalities.
After endoscopic treatment is performed, four-quadrant spot tattooing is recommended adjacent to the TIC with the SRH. This step will facilitate localization and treatment in the case of TIC rebleeding.4,10
Outcomes following endoscopic treatment
Following endoscopic treatment, patients should be monitored for early and late rebleeding. In a pooled analysis of case series composed of 847 patients with TIC bleeding, among the 137 patients in which endoscopic hemostasis was initially achieved, early rebleeding occurred in 8% and late rebleeding occurred in 12% of patients.22 Risk factors for TIC rebleeding within 30 days were residual arterial blood flow following hemostasis and early reinitiation of antiplatelet agents.
Remote treatment of TIC hemorrhage distant from the SRH is a significant risk factor for early TIC rebleeding.4, 10 For example, using hemoclips to close the mouth of a TIC when active bleeding or an SRH is located in the TIC base often fails because arterial flow remains open in the base and the artery is larger there.4,10 This example highlights the importance of focal obliteration of arterial blood flow underlying SRH in order to achieve definitive hemostasis.4,10
Salvage treatments
For TIC hemorrhage that is not controlled by endoscopic therapy, transcatheter arterial embolization (TAE) is recommended. If bleeding rate is high enough (at least 0.5 milliliters per minute) to be detected by angiography, TAE can serve as an effective method of diagnosis and immediate hemostasis.23 However, the most common major complication of embolization is intestinal ischemia. The incidence of intestinal ischemia has been reported as high as 10%, with highest risk with embolization of at least three vasa recta.24
Surgery is also recommended if TIC hemorrhage cannot be controlled with endoscopic therapy or TAE. Segmental colectomy is recommended if the bleeding site can be localized before surgery with colonoscopy or angiography resulting from significantly lower perioperative morbidity than subtotal colectomy.25 However, subtotal colectomy may be necessary if preoperative localization of bleeding is unsuccessful.
There are very few reports of short- or long-term results that compare endoscopy, TAE, and surgery for management of TIC bleeding. However, a recent retrospective study reported better outcomes with endoscopic treatment of definitive TIC bleeding.26 Patients who underwent endoscopic treatment had fewer RBC transfusions, shorter hospitalizations, and lower rates of postprocedure complications.
Management after cessation of hemorrhage
Medical management is important following an episode of TIC hemorrhage. A mainstay is daily fiber supplementation every morning and stool softener in the evening. Furthermore, patients are advised to drink an extra liter of fluids (not containing alcohol or caffeine) daily. By reducing colon transit time and increasing stool weight, these measures can help control constipation and prevent future complications of TIC disease.27
Patients with recurrent TIC hemorrhage should undergo evaluation for elective surgery, provided they are appropriate surgical candidates. If preoperative localization of bleeding site is successful, segmental colectomy is preferred. Segmental resection is associated with significantly decreased rebleeding rate, with lower rates of morbidity compared with subtotal colectomy.32
Chronic NSAIDs, aspirin, and antiplatelet drugs are risk factors for recurrent TIC hemorrhage, and avoiding these medications is recommended if possible.33,34 Although anticoagulants have shown to be associated with increased risk of all-cause gastrointestinal bleeding, these agents have not been shown to increase risk of recurrent TIC hemorrhage in recent large retrospective studies. Since antiplatelet and anticoagulation agents serve to reduce risk of thromboembolic events, the clinician who recommended these medications should be consulted after a TIC bleed to re-evaluate whether these medications can be discontinued or reduced in dose.
Conclusion
The most effective way to diagnose and treat definitive TIC hemorrhage is to perform an urgent colonoscopy within 24 hours to identify and treat TIC SRH. This procedure requires thoroughly cleansing the colon first, as well as an experienced colonoscopist who can identify and treat TIC SRH to obliterate arterial blood flow underneath SRH and achieve definitive TIC hemostasis. Other approaches to early diagnosis include nuclear medicine scintigraphy or angiography (CT, MRI, or IR). However, these techniques can only detect active bleeding which is documented in only 26% of colonoscopically diagnosed definitive TIC hemorrhages. So, the expected diagnostic yield of these tests will be low. When urgent colonoscopy fails to make a diagnosis or TIC bleeding continues, TAE and/or surgery are recommended. After definitive hemostasis of TIC hemorrhage and for long term management, control of constipation and discontinuation of chronic NSAIDs and antiplatelet drugs (if possible) are recommended to prevent recurrent TIC hemorrhage.
Dr. Cusumano and Dr. Paiji are fellow physicians in the Vatche and Tamar Manoukian Division of Digestive Diseases at University of California Los Angeles. Dr. Jensen is a professor of medicine in Vatche and Tamar Manoukian Division of Digestive Diseases and is with the CURE Digestive Diseases Research Center at the VA Greater Los Angeles Healthcare System, Calif. All authors declare that they have no competing interests or disclosures.
References
1. Longstreth GF. Am J Gastroenterol. 1997;92(3):419-24.
2. Jensen DM et al. The New England Journal of Medicine. 2000;342(2):78-82.
3. Jensen DM et al. Techniques in Gastrointestinal Endoscopy. 2001;3(4):192-8.
4. Jensen DM. Am J Gastroenterol. 2018;113(11):1570-3.
5. Zuckerman GR et al. Gastrointestinal Endoscopy. 1999;49(2):228-38.
6. Stollman N et al. Lancet. 2004;363(9409):631-9.
7. McGuire HH et al. Ann Surg. 1994;220(5):653-6.
8. McGuire HH et al. Ann Surg. 1972;175(6):847-55.
9. Strate LL et al. Clinical gastroenterology and hepatol. 2008;6(9):1004-10.
10. Jensen DM et al. Gastrointestinal endoscopy. 2016;83(2):416-23.
11. Jensen DM et al. Gastrointest Endosc Clin N Am. 1997;7(3):477-98.
12. Maykel JA et al. Clin Colon Rectal Surg. 2004;17(3):195-204.
13. Green BT et al. Am J Gastroenterol. 2005;100(11):2395-402.
14. Niikura R et al. Journal of Clinical Gastroenterol. 2015;49(3):e24-30.
15. Bloomfeld RS et al. Am J Gastroenterol. 2001;96(8):2367-72.
16. Parsi MA,et al. VideoGIE. 2019;4(7):285-99.
17. Kaltenbach T et al. Clinical Gastroenterology and Hepatol. 2012;10(2):131-7.
18. Nakano K et al. Endosc Int Open. 2015;3(5):E529-33.
19. Barker KB et al. Gastrointestinal Endoscopy. 2005;62(2):224-7.
20. Kaltenbach T et al. Gastrointest Endosc Clin N Am. 2020;30(1):13-23.
21. Yamazaki K et al. VideoGIE. 2020;5(6):252-4.
22. Strate LL et al. Clinical Gastroenterology and Hepatol. 2010;8(4):333-43.
23. Evangelista et al. J Vasc Interv Radiol. 2000;11(5):601-6.
24. Kodani M et al. J Vasc Interv Radiol. 2016;27(6):824-30.
25. Mohammed et al. Clin Colon Rectal Surg. 2018;31(4):243-50.
26. Wongpongsalee T et al. Gastrointestinal Endoscopy. 2020;91(6):AB471-2.
27. Böhm SK. Viszeralmedizin. 2015;31(2):84-94.
28. Prakash C et al. Endoscopy. 1999;31(6):460-3.
29. Yen EF et al. Digestive Diseases and Sciences. 2008;53(9):2480-5.
30. Ishii N et al. Gastrointestinal Endoscopy. 2012;75(2):382-7.
31. Nagata N et al. Gastrointestinal Endoscopy. 2018;88(5):841-53.e4.
32. Parkes BM et al. Am Surg. 1993;59(10):676-8.
33. Vajravelu RK et al. Gastroenterology. 2018;155(5):1416-27.
34. Oakland K et al. Clin Gastroenterol Hepatol. 2019;17(7):1276-84.e3.
35. Yamada A et al. Dis Colon Rectum. 2008;51(1):116-20.
36. Coleman CI et al. Int J Clin Pract. 2012;66(1):53-63.
37. Holster IL et al. Gastroenterology. 2013;145(1):105-12.e15.
Diverticular hemorrhage is the most common cause of colonic bleeding, accounting for 20%-65% of cases of severe lower intestinal bleeding in adults.1 Urgent colonoscopy after purging the colon of blood, clots, and stool is the most accurate method of diagnosing and guiding treatment of definitive diverticular hemorrhage.2-5 The diagnosis of definitive diverticular hemorrhage depends upon identification of some stigmata of recent hemorrhage (SRH) in a single diverticulum (TIC), which can include active arterial bleeding, oozing, non-bleeding visible vessel, adherent clot, or flat spot.2-4 Although other approaches, such as nuclear medicine scans and angiography of various types (CT, MRI, or standard angiography), for the early diagnosis of patients with severe hematochezia are utilized in many medical centers, only active bleeding can be detected by these techniques. However, as subsequently discussed, this SRH is documented in only 26% of definitive diverticular bleeds found on urgent colonoscopy, so diagnostic yields of these techniques will be low.2-5
The diagnosis of patients with severe hematochezia and diverticulosis, as well as triage of all of them to specific medical, endoscopic, radiologic, or surgical management, is facilitated by an urgent endoscopic approach.2-5 Patients who are diagnosed with definitive diverticular hemorrhage on colonoscopy represent about 30% of all true TIC bleeds when urgent colonoscopy is the management approach.2-5 That is because approximately 50% of all patients with colon diverticulosis and first presentation of severe hematochezia have incidental diverticulosis; they have colonic diverticulosis, but another site of bleeding is identified as the cause of hemorrhage in the gastrointestinal tract.2-4 Presumptive diverticular hemorrhage is diagnosed when colonic diverticulosis without TIC stigmata are found but no other GI bleeding source is found on colonoscopy, anoscopy, enteroscopy, or capsule endoscopy.2-5 In our experience with urgent colonoscopy, the presumptive diverticular bleed group accounts for about 70% of patients with documented diverticular hemorrhage (e.g., not including incidental diverticulosis bleeds but combining subgroups of patients with either definitive or presumptive TIC diagnoses as documented TIC hemorrhage).
Clinical presentation
Patients with diverticular hemorrhage present with severe, painless large volume hematochezia. Hematochezia may be self-limited and spontaneously resolve in 75%-80% of all patients but with high rebleeding rates up to 40%.5-7 Of all patients with diverticulosis, only about 3%-5% develop diverticular hemorrhage.8 Risk factors for diverticular hemorrhage include medications (e.g., nonsteroidal anti-inflammatory drugs – NSAIDs, antiplatelet drugs, and anticoagulants) and other clinical factors, such as older age, low-fiber diet, and chronic constipation.9,10 On urgent colonoscopy, more than 70% of diverticulosis in U.S. patients are located anatomically in the descending colon or more distally. In contrast, about 60% of definitive diverticular hemorrhage cases in our experience had diverticula with stigmata identified at or proximal to the splenic flexure.2,4,11
Pathophysiology
Colonic diverticula are herniations of mucosa and submucosa with colonic arteries that penetrate the muscular wall. Bleeding can occur when there is asymmetric rupture of the vasa recta at either the base of the diverticulum or the neck.4 Thinning of the mucosa on the luminal surface (such as that resulting from impacted fecaliths and stool) can cause injury to the site of the penetrating vessels, resulting in hemorrhage.12
Initial management
Patients with acute, severe hematochezia should be triaged to an inpatient setting with a monitored bed. Admission to an intensive care unit should be considered for patients with hemodynamic instability, persistent bleeding, and/or significant comorbidities. Patients with TIC hemorrhage often require resuscitation with crystalloids and packed red blood cell transfusions for hemoglobin less than 8 g/dl.4 Unlike upper GI hemorrhage, which has been extensively reported on, data regarding a more restrictive transfusion threshold, compared with a liberal transfusion threshold, in lower intestinal bleeding are very limited. Correction of underlying coagulopathies is recommended but should be individualized, particularly in those patients on antithrombotic agents or with underlying bleeding disorders.
Urgent diagnosis and hemostasis
Urgent colonoscopy within 24 hours is the most accurate way to make a diagnosis of definitive diverticular hemorrhage and to effectively and safely treat them.2-4,10,11 For patients with severe hematochezia, when the colonoscopy is either not available in a medical center or does not reveal the source of bleeding, nuclear scintigraphy or angiography (CT, MRI, or interventional radiology [IR]) are recommended. CT angiography may be particularly helpful to diagnose patients with hemodynamic instability who are suspected to have active TIC bleeding and are not able to complete a bowel preparation. However, these imaging techniques require active bleeding at the time of the study to be diagnostic. This SRH is also uncommon for definitive diverticular hemorrhage, so the diagnostic yield is usually quite low.2-5,10,11 An additional limitation of scintigraphy and CT or MRI angiography is that, if active bleeding is found, some other type of treatment, such as colonoscopy, IR angiography, or surgery, will be required for definitive hemostasis.
For urgent colonoscopy, adequate colon preparation with a large volume preparation (6-8 liters of polyethylene glycol-based solution) is recommended to clear stool, blood, and clots to allow endoscopic visualization and localization of the bleeding source. Use of a nasogastric tube should be considered if the patient is unable to drink enough prep.2-4,13 Additionally, administration of a prokinetic agent, such as Metoclopramide, may improve gastric emptying and tolerance of the prep. During colonoscopy, careful inspection of the colonic mucosa during insertion and withdrawal is important since lesions may bleed intermittently and SRH can be missed. An adult or pediatric colonoscope with a large working channel (at least 3.3 mm) is recommended to facilitate suctioning of blood clots and stool, as well as allow the passage of endoscopic hemostasis accessories. Targeted water-jet irrigation, an expert colonoscopist, a cap attachment, and adequate colon preparation are all predictors for improved diagnosis of definitive diverticular hemorrhage.4,14
SRH in definitive TIC bleeds all have a high risk of TIC rebleeding,2-4,10,11 including active bleeding, nonbleeding visible vessel, adherent clot, and a flat spot (See Figure).

Based on CURE Hemostasis Group data of 118 definitive TIC bleeds, 26% had active bleeding, 24% had a nonbleeding visible vessel, 37% had an adherent clot, and 13% had a flat spot (with underlying arterial blood flow by Doppler probe monitoring).4 Approximately 50% of the SRH were found in the neck of the TIC and 50% at the base, with actively bleeding cases more often from the base. In CURE Doppler endoscopic probe studies, 90% of all stigmata had an underlying arterial blood flow detected with the Doppler probe.4,10 The Doppler probe is reported to be very useful for risk stratification and to confirm obliteration of the arterial blood flow underlying SRH for definitive hemostasis.4,10
Endoscopic treatment
Given high rates of rebleeding with medical management alone, definitive TIC hemorrhage can be effectively and safely treated with endoscopic therapies once SRH are localized.4,10 Endoscopic therapies that have been reported in the literature include electrocoagulation, hemoclip, band ligation, and over-the-scope clip. Four-quadrant injection of 1:20,000 epinephrine around the SRH can improve visualization of SRH and provide temporary control of bleeding, but it should be combined with other modalities because of risk of rebleeding with epinephrine alone.15 Results from studies reporting rates of both early rebleeding (occurring within 30 days) and late rebleeding (occurring after 30 days) are listed in the Table. 
Multipolar electrocoagulation (MPEC), which utilizes a focal electric current to generate heat, can coaptively coagulate small TIC arteries.16 For SRH in the neck of TIC, MPEC is effective for coaptive coagulation at a power of 12-15 watts in 1-2 second pulses with moderate laterally applied tamponade pressure. MPEC should be avoided for treating SRH at the TIC base because of lack of muscularis propria and higher risk of perforation.
Hemoclip therapy has been reported to be safe and efficacious in treatment of definitive TIC hemorrhage, by causing mechanical hemostasis with occlusion of the bleeding artery.16 Hemoclips are recommended to treat stigmata in the base of TICs and should be targeted on either side of visible vessel in order to occlude the artery underneath it.4,10 With a cap on the tip of the colonoscope, suctioning can evert TICs, allowing more precise placement of hemoclip on SRH in the base of the TIC.17 Hemoclip retention rates vary with different models and can range from less than 7 days to more than 4 weeks. Hemoclips can also mark the site if early rebleeding occurs; then, reintervention (e.g., repeat endoscopy or angioembolization) is facilitated.
Another treatment is endoscopic band ligation, which provides mechanical hemostasis. Endoscopic band ligation has been reported to be efficacious for TIC hemorrhage.18 Suctioning the TIC with the SRH into the distal cap and deploying a band leads to obliteration of vessels and potentially necrosis and disappearance of banded TIC.16 This technique carries a risk of perforation because of the thin walls of TICs. This risk may be higher for right-sided colon lesions since an exvivo colon specimen study reported serosal entrapment and inclusion of muscularis propria postband ligation, both of which may result in ischemia of intestinal wall and delayed perforation.19
Over-the-scope clip (OTSC) has been reported in case series for treatment of definitive TIC hemorrhage. With a distal cap and large clip, suctioning can evert TICs and facilitate deployment over the SRH.20,21 OTSC can grasp an entire TIC with the SRH and obliterate the arterial blood flow with a single clip.20,21 No complications have been reported yet for treatment of TIC hemorrhage. However, the OTSC system is relatively expensive when compared with other modalities.
After endoscopic treatment is performed, four-quadrant spot tattooing is recommended adjacent to the TIC with the SRH. This step will facilitate localization and treatment in the case of TIC rebleeding.4,10
Outcomes following endoscopic treatment
Following endoscopic treatment, patients should be monitored for early and late rebleeding. In a pooled analysis of case series composed of 847 patients with TIC bleeding, among the 137 patients in which endoscopic hemostasis was initially achieved, early rebleeding occurred in 8% and late rebleeding occurred in 12% of patients.22 Risk factors for TIC rebleeding within 30 days were residual arterial blood flow following hemostasis and early reinitiation of antiplatelet agents.
Remote treatment of TIC hemorrhage distant from the SRH is a significant risk factor for early TIC rebleeding.4, 10 For example, using hemoclips to close the mouth of a TIC when active bleeding or an SRH is located in the TIC base often fails because arterial flow remains open in the base and the artery is larger there.4,10 This example highlights the importance of focal obliteration of arterial blood flow underlying SRH in order to achieve definitive hemostasis.4,10
Salvage treatments
For TIC hemorrhage that is not controlled by endoscopic therapy, transcatheter arterial embolization (TAE) is recommended. If bleeding rate is high enough (at least 0.5 milliliters per minute) to be detected by angiography, TAE can serve as an effective method of diagnosis and immediate hemostasis.23 However, the most common major complication of embolization is intestinal ischemia. The incidence of intestinal ischemia has been reported as high as 10%, with highest risk with embolization of at least three vasa recta.24
Surgery is also recommended if TIC hemorrhage cannot be controlled with endoscopic therapy or TAE. Segmental colectomy is recommended if the bleeding site can be localized before surgery with colonoscopy or angiography resulting from significantly lower perioperative morbidity than subtotal colectomy.25 However, subtotal colectomy may be necessary if preoperative localization of bleeding is unsuccessful.
There are very few reports of short- or long-term results that compare endoscopy, TAE, and surgery for management of TIC bleeding. However, a recent retrospective study reported better outcomes with endoscopic treatment of definitive TIC bleeding.26 Patients who underwent endoscopic treatment had fewer RBC transfusions, shorter hospitalizations, and lower rates of postprocedure complications.
Management after cessation of hemorrhage
Medical management is important following an episode of TIC hemorrhage. A mainstay is daily fiber supplementation every morning and stool softener in the evening. Furthermore, patients are advised to drink an extra liter of fluids (not containing alcohol or caffeine) daily. By reducing colon transit time and increasing stool weight, these measures can help control constipation and prevent future complications of TIC disease.27
Patients with recurrent TIC hemorrhage should undergo evaluation for elective surgery, provided they are appropriate surgical candidates. If preoperative localization of bleeding site is successful, segmental colectomy is preferred. Segmental resection is associated with significantly decreased rebleeding rate, with lower rates of morbidity compared with subtotal colectomy.32
Chronic NSAIDs, aspirin, and antiplatelet drugs are risk factors for recurrent TIC hemorrhage, and avoiding these medications is recommended if possible.33,34 Although anticoagulants have shown to be associated with increased risk of all-cause gastrointestinal bleeding, these agents have not been shown to increase risk of recurrent TIC hemorrhage in recent large retrospective studies. Since antiplatelet and anticoagulation agents serve to reduce risk of thromboembolic events, the clinician who recommended these medications should be consulted after a TIC bleed to re-evaluate whether these medications can be discontinued or reduced in dose.
Conclusion
The most effective way to diagnose and treat definitive TIC hemorrhage is to perform an urgent colonoscopy within 24 hours to identify and treat TIC SRH. This procedure requires thoroughly cleansing the colon first, as well as an experienced colonoscopist who can identify and treat TIC SRH to obliterate arterial blood flow underneath SRH and achieve definitive TIC hemostasis. Other approaches to early diagnosis include nuclear medicine scintigraphy or angiography (CT, MRI, or IR). However, these techniques can only detect active bleeding which is documented in only 26% of colonoscopically diagnosed definitive TIC hemorrhages. So, the expected diagnostic yield of these tests will be low. When urgent colonoscopy fails to make a diagnosis or TIC bleeding continues, TAE and/or surgery are recommended. After definitive hemostasis of TIC hemorrhage and for long term management, control of constipation and discontinuation of chronic NSAIDs and antiplatelet drugs (if possible) are recommended to prevent recurrent TIC hemorrhage.
Dr. Cusumano and Dr. Paiji are fellow physicians in the Vatche and Tamar Manoukian Division of Digestive Diseases at University of California Los Angeles. Dr. Jensen is a professor of medicine in Vatche and Tamar Manoukian Division of Digestive Diseases and is with the CURE Digestive Diseases Research Center at the VA Greater Los Angeles Healthcare System, Calif. All authors declare that they have no competing interests or disclosures.
References
1. Longstreth GF. Am J Gastroenterol. 1997;92(3):419-24.
2. Jensen DM et al. The New England Journal of Medicine. 2000;342(2):78-82.
3. Jensen DM et al. Techniques in Gastrointestinal Endoscopy. 2001;3(4):192-8.
4. Jensen DM. Am J Gastroenterol. 2018;113(11):1570-3.
5. Zuckerman GR et al. Gastrointestinal Endoscopy. 1999;49(2):228-38.
6. Stollman N et al. Lancet. 2004;363(9409):631-9.
7. McGuire HH et al. Ann Surg. 1994;220(5):653-6.
8. McGuire HH et al. Ann Surg. 1972;175(6):847-55.
9. Strate LL et al. Clinical gastroenterology and hepatol. 2008;6(9):1004-10.
10. Jensen DM et al. Gastrointestinal endoscopy. 2016;83(2):416-23.
11. Jensen DM et al. Gastrointest Endosc Clin N Am. 1997;7(3):477-98.
12. Maykel JA et al. Clin Colon Rectal Surg. 2004;17(3):195-204.
13. Green BT et al. Am J Gastroenterol. 2005;100(11):2395-402.
14. Niikura R et al. Journal of Clinical Gastroenterol. 2015;49(3):e24-30.
15. Bloomfeld RS et al. Am J Gastroenterol. 2001;96(8):2367-72.
16. Parsi MA,et al. VideoGIE. 2019;4(7):285-99.
17. Kaltenbach T et al. Clinical Gastroenterology and Hepatol. 2012;10(2):131-7.
18. Nakano K et al. Endosc Int Open. 2015;3(5):E529-33.
19. Barker KB et al. Gastrointestinal Endoscopy. 2005;62(2):224-7.
20. Kaltenbach T et al. Gastrointest Endosc Clin N Am. 2020;30(1):13-23.
21. Yamazaki K et al. VideoGIE. 2020;5(6):252-4.
22. Strate LL et al. Clinical Gastroenterology and Hepatol. 2010;8(4):333-43.
23. Evangelista et al. J Vasc Interv Radiol. 2000;11(5):601-6.
24. Kodani M et al. J Vasc Interv Radiol. 2016;27(6):824-30.
25. Mohammed et al. Clin Colon Rectal Surg. 2018;31(4):243-50.
26. Wongpongsalee T et al. Gastrointestinal Endoscopy. 2020;91(6):AB471-2.
27. Böhm SK. Viszeralmedizin. 2015;31(2):84-94.
28. Prakash C et al. Endoscopy. 1999;31(6):460-3.
29. Yen EF et al. Digestive Diseases and Sciences. 2008;53(9):2480-5.
30. Ishii N et al. Gastrointestinal Endoscopy. 2012;75(2):382-7.
31. Nagata N et al. Gastrointestinal Endoscopy. 2018;88(5):841-53.e4.
32. Parkes BM et al. Am Surg. 1993;59(10):676-8.
33. Vajravelu RK et al. Gastroenterology. 2018;155(5):1416-27.
34. Oakland K et al. Clin Gastroenterol Hepatol. 2019;17(7):1276-84.e3.
35. Yamada A et al. Dis Colon Rectum. 2008;51(1):116-20.
36. Coleman CI et al. Int J Clin Pract. 2012;66(1):53-63.
37. Holster IL et al. Gastroenterology. 2013;145(1):105-12.e15.
Diverticular hemorrhage is the most common cause of colonic bleeding, accounting for 20%-65% of cases of severe lower intestinal bleeding in adults.1 Urgent colonoscopy after purging the colon of blood, clots, and stool is the most accurate method of diagnosing and guiding treatment of definitive diverticular hemorrhage.2-5 The diagnosis of definitive diverticular hemorrhage depends upon identification of some stigmata of recent hemorrhage (SRH) in a single diverticulum (TIC), which can include active arterial bleeding, oozing, non-bleeding visible vessel, adherent clot, or flat spot.2-4 Although other approaches, such as nuclear medicine scans and angiography of various types (CT, MRI, or standard angiography), for the early diagnosis of patients with severe hematochezia are utilized in many medical centers, only active bleeding can be detected by these techniques. However, as subsequently discussed, this SRH is documented in only 26% of definitive diverticular bleeds found on urgent colonoscopy, so diagnostic yields of these techniques will be low.2-5
The diagnosis of patients with severe hematochezia and diverticulosis, as well as triage of all of them to specific medical, endoscopic, radiologic, or surgical management, is facilitated by an urgent endoscopic approach.2-5 Patients who are diagnosed with definitive diverticular hemorrhage on colonoscopy represent about 30% of all true TIC bleeds when urgent colonoscopy is the management approach.2-5 That is because approximately 50% of all patients with colon diverticulosis and first presentation of severe hematochezia have incidental diverticulosis; they have colonic diverticulosis, but another site of bleeding is identified as the cause of hemorrhage in the gastrointestinal tract.2-4 Presumptive diverticular hemorrhage is diagnosed when colonic diverticulosis without TIC stigmata are found but no other GI bleeding source is found on colonoscopy, anoscopy, enteroscopy, or capsule endoscopy.2-5 In our experience with urgent colonoscopy, the presumptive diverticular bleed group accounts for about 70% of patients with documented diverticular hemorrhage (e.g., not including incidental diverticulosis bleeds but combining subgroups of patients with either definitive or presumptive TIC diagnoses as documented TIC hemorrhage).
Clinical presentation
Patients with diverticular hemorrhage present with severe, painless large volume hematochezia. Hematochezia may be self-limited and spontaneously resolve in 75%-80% of all patients but with high rebleeding rates up to 40%.5-7 Of all patients with diverticulosis, only about 3%-5% develop diverticular hemorrhage.8 Risk factors for diverticular hemorrhage include medications (e.g., nonsteroidal anti-inflammatory drugs – NSAIDs, antiplatelet drugs, and anticoagulants) and other clinical factors, such as older age, low-fiber diet, and chronic constipation.9,10 On urgent colonoscopy, more than 70% of diverticulosis in U.S. patients are located anatomically in the descending colon or more distally. In contrast, about 60% of definitive diverticular hemorrhage cases in our experience had diverticula with stigmata identified at or proximal to the splenic flexure.2,4,11
Pathophysiology
Colonic diverticula are herniations of mucosa and submucosa with colonic arteries that penetrate the muscular wall. Bleeding can occur when there is asymmetric rupture of the vasa recta at either the base of the diverticulum or the neck.4 Thinning of the mucosa on the luminal surface (such as that resulting from impacted fecaliths and stool) can cause injury to the site of the penetrating vessels, resulting in hemorrhage.12
Initial management
Patients with acute, severe hematochezia should be triaged to an inpatient setting with a monitored bed. Admission to an intensive care unit should be considered for patients with hemodynamic instability, persistent bleeding, and/or significant comorbidities. Patients with TIC hemorrhage often require resuscitation with crystalloids and packed red blood cell transfusions for hemoglobin less than 8 g/dl.4 Unlike upper GI hemorrhage, which has been extensively reported on, data regarding a more restrictive transfusion threshold, compared with a liberal transfusion threshold, in lower intestinal bleeding are very limited. Correction of underlying coagulopathies is recommended but should be individualized, particularly in those patients on antithrombotic agents or with underlying bleeding disorders.
Urgent diagnosis and hemostasis
Urgent colonoscopy within 24 hours is the most accurate way to make a diagnosis of definitive diverticular hemorrhage and to effectively and safely treat them.2-4,10,11 For patients with severe hematochezia, when the colonoscopy is either not available in a medical center or does not reveal the source of bleeding, nuclear scintigraphy or angiography (CT, MRI, or interventional radiology [IR]) are recommended. CT angiography may be particularly helpful to diagnose patients with hemodynamic instability who are suspected to have active TIC bleeding and are not able to complete a bowel preparation. However, these imaging techniques require active bleeding at the time of the study to be diagnostic. This SRH is also uncommon for definitive diverticular hemorrhage, so the diagnostic yield is usually quite low.2-5,10,11 An additional limitation of scintigraphy and CT or MRI angiography is that, if active bleeding is found, some other type of treatment, such as colonoscopy, IR angiography, or surgery, will be required for definitive hemostasis.
For urgent colonoscopy, adequate colon preparation with a large volume preparation (6-8 liters of polyethylene glycol-based solution) is recommended to clear stool, blood, and clots to allow endoscopic visualization and localization of the bleeding source. Use of a nasogastric tube should be considered if the patient is unable to drink enough prep.2-4,13 Additionally, administration of a prokinetic agent, such as Metoclopramide, may improve gastric emptying and tolerance of the prep. During colonoscopy, careful inspection of the colonic mucosa during insertion and withdrawal is important since lesions may bleed intermittently and SRH can be missed. An adult or pediatric colonoscope with a large working channel (at least 3.3 mm) is recommended to facilitate suctioning of blood clots and stool, as well as allow the passage of endoscopic hemostasis accessories. Targeted water-jet irrigation, an expert colonoscopist, a cap attachment, and adequate colon preparation are all predictors for improved diagnosis of definitive diverticular hemorrhage.4,14
SRH in definitive TIC bleeds all have a high risk of TIC rebleeding,2-4,10,11 including active bleeding, nonbleeding visible vessel, adherent clot, and a flat spot (See Figure).

Based on CURE Hemostasis Group data of 118 definitive TIC bleeds, 26% had active bleeding, 24% had a nonbleeding visible vessel, 37% had an adherent clot, and 13% had a flat spot (with underlying arterial blood flow by Doppler probe monitoring).4 Approximately 50% of the SRH were found in the neck of the TIC and 50% at the base, with actively bleeding cases more often from the base. In CURE Doppler endoscopic probe studies, 90% of all stigmata had an underlying arterial blood flow detected with the Doppler probe.4,10 The Doppler probe is reported to be very useful for risk stratification and to confirm obliteration of the arterial blood flow underlying SRH for definitive hemostasis.4,10
Endoscopic treatment
Given high rates of rebleeding with medical management alone, definitive TIC hemorrhage can be effectively and safely treated with endoscopic therapies once SRH are localized.4,10 Endoscopic therapies that have been reported in the literature include electrocoagulation, hemoclip, band ligation, and over-the-scope clip. Four-quadrant injection of 1:20,000 epinephrine around the SRH can improve visualization of SRH and provide temporary control of bleeding, but it should be combined with other modalities because of risk of rebleeding with epinephrine alone.15 Results from studies reporting rates of both early rebleeding (occurring within 30 days) and late rebleeding (occurring after 30 days) are listed in the Table. 
Multipolar electrocoagulation (MPEC), which utilizes a focal electric current to generate heat, can coaptively coagulate small TIC arteries.16 For SRH in the neck of TIC, MPEC is effective for coaptive coagulation at a power of 12-15 watts in 1-2 second pulses with moderate laterally applied tamponade pressure. MPEC should be avoided for treating SRH at the TIC base because of lack of muscularis propria and higher risk of perforation.
Hemoclip therapy has been reported to be safe and efficacious in treatment of definitive TIC hemorrhage, by causing mechanical hemostasis with occlusion of the bleeding artery.16 Hemoclips are recommended to treat stigmata in the base of TICs and should be targeted on either side of visible vessel in order to occlude the artery underneath it.4,10 With a cap on the tip of the colonoscope, suctioning can evert TICs, allowing more precise placement of hemoclip on SRH in the base of the TIC.17 Hemoclip retention rates vary with different models and can range from less than 7 days to more than 4 weeks. Hemoclips can also mark the site if early rebleeding occurs; then, reintervention (e.g., repeat endoscopy or angioembolization) is facilitated.
Another treatment is endoscopic band ligation, which provides mechanical hemostasis. Endoscopic band ligation has been reported to be efficacious for TIC hemorrhage.18 Suctioning the TIC with the SRH into the distal cap and deploying a band leads to obliteration of vessels and potentially necrosis and disappearance of banded TIC.16 This technique carries a risk of perforation because of the thin walls of TICs. This risk may be higher for right-sided colon lesions since an exvivo colon specimen study reported serosal entrapment and inclusion of muscularis propria postband ligation, both of which may result in ischemia of intestinal wall and delayed perforation.19
Over-the-scope clip (OTSC) has been reported in case series for treatment of definitive TIC hemorrhage. With a distal cap and large clip, suctioning can evert TICs and facilitate deployment over the SRH.20,21 OTSC can grasp an entire TIC with the SRH and obliterate the arterial blood flow with a single clip.20,21 No complications have been reported yet for treatment of TIC hemorrhage. However, the OTSC system is relatively expensive when compared with other modalities.
After endoscopic treatment is performed, four-quadrant spot tattooing is recommended adjacent to the TIC with the SRH. This step will facilitate localization and treatment in the case of TIC rebleeding.4,10
Outcomes following endoscopic treatment
Following endoscopic treatment, patients should be monitored for early and late rebleeding. In a pooled analysis of case series composed of 847 patients with TIC bleeding, among the 137 patients in which endoscopic hemostasis was initially achieved, early rebleeding occurred in 8% and late rebleeding occurred in 12% of patients.22 Risk factors for TIC rebleeding within 30 days were residual arterial blood flow following hemostasis and early reinitiation of antiplatelet agents.
Remote treatment of TIC hemorrhage distant from the SRH is a significant risk factor for early TIC rebleeding.4, 10 For example, using hemoclips to close the mouth of a TIC when active bleeding or an SRH is located in the TIC base often fails because arterial flow remains open in the base and the artery is larger there.4,10 This example highlights the importance of focal obliteration of arterial blood flow underlying SRH in order to achieve definitive hemostasis.4,10
Salvage treatments
For TIC hemorrhage that is not controlled by endoscopic therapy, transcatheter arterial embolization (TAE) is recommended. If bleeding rate is high enough (at least 0.5 milliliters per minute) to be detected by angiography, TAE can serve as an effective method of diagnosis and immediate hemostasis.23 However, the most common major complication of embolization is intestinal ischemia. The incidence of intestinal ischemia has been reported as high as 10%, with highest risk with embolization of at least three vasa recta.24
Surgery is also recommended if TIC hemorrhage cannot be controlled with endoscopic therapy or TAE. Segmental colectomy is recommended if the bleeding site can be localized before surgery with colonoscopy or angiography resulting from significantly lower perioperative morbidity than subtotal colectomy.25 However, subtotal colectomy may be necessary if preoperative localization of bleeding is unsuccessful.
There are very few reports of short- or long-term results that compare endoscopy, TAE, and surgery for management of TIC bleeding. However, a recent retrospective study reported better outcomes with endoscopic treatment of definitive TIC bleeding.26 Patients who underwent endoscopic treatment had fewer RBC transfusions, shorter hospitalizations, and lower rates of postprocedure complications.
Management after cessation of hemorrhage
Medical management is important following an episode of TIC hemorrhage. A mainstay is daily fiber supplementation every morning and stool softener in the evening. Furthermore, patients are advised to drink an extra liter of fluids (not containing alcohol or caffeine) daily. By reducing colon transit time and increasing stool weight, these measures can help control constipation and prevent future complications of TIC disease.27
Patients with recurrent TIC hemorrhage should undergo evaluation for elective surgery, provided they are appropriate surgical candidates. If preoperative localization of bleeding site is successful, segmental colectomy is preferred. Segmental resection is associated with significantly decreased rebleeding rate, with lower rates of morbidity compared with subtotal colectomy.32
Chronic NSAIDs, aspirin, and antiplatelet drugs are risk factors for recurrent TIC hemorrhage, and avoiding these medications is recommended if possible.33,34 Although anticoagulants have shown to be associated with increased risk of all-cause gastrointestinal bleeding, these agents have not been shown to increase risk of recurrent TIC hemorrhage in recent large retrospective studies. Since antiplatelet and anticoagulation agents serve to reduce risk of thromboembolic events, the clinician who recommended these medications should be consulted after a TIC bleed to re-evaluate whether these medications can be discontinued or reduced in dose.
Conclusion
The most effective way to diagnose and treat definitive TIC hemorrhage is to perform an urgent colonoscopy within 24 hours to identify and treat TIC SRH. This procedure requires thoroughly cleansing the colon first, as well as an experienced colonoscopist who can identify and treat TIC SRH to obliterate arterial blood flow underneath SRH and achieve definitive TIC hemostasis. Other approaches to early diagnosis include nuclear medicine scintigraphy or angiography (CT, MRI, or IR). However, these techniques can only detect active bleeding which is documented in only 26% of colonoscopically diagnosed definitive TIC hemorrhages. So, the expected diagnostic yield of these tests will be low. When urgent colonoscopy fails to make a diagnosis or TIC bleeding continues, TAE and/or surgery are recommended. After definitive hemostasis of TIC hemorrhage and for long term management, control of constipation and discontinuation of chronic NSAIDs and antiplatelet drugs (if possible) are recommended to prevent recurrent TIC hemorrhage.
Dr. Cusumano and Dr. Paiji are fellow physicians in the Vatche and Tamar Manoukian Division of Digestive Diseases at University of California Los Angeles. Dr. Jensen is a professor of medicine in Vatche and Tamar Manoukian Division of Digestive Diseases and is with the CURE Digestive Diseases Research Center at the VA Greater Los Angeles Healthcare System, Calif. All authors declare that they have no competing interests or disclosures.
References
1. Longstreth GF. Am J Gastroenterol. 1997;92(3):419-24.
2. Jensen DM et al. The New England Journal of Medicine. 2000;342(2):78-82.
3. Jensen DM et al. Techniques in Gastrointestinal Endoscopy. 2001;3(4):192-8.
4. Jensen DM. Am J Gastroenterol. 2018;113(11):1570-3.
5. Zuckerman GR et al. Gastrointestinal Endoscopy. 1999;49(2):228-38.
6. Stollman N et al. Lancet. 2004;363(9409):631-9.
7. McGuire HH et al. Ann Surg. 1994;220(5):653-6.
8. McGuire HH et al. Ann Surg. 1972;175(6):847-55.
9. Strate LL et al. Clinical gastroenterology and hepatol. 2008;6(9):1004-10.
10. Jensen DM et al. Gastrointestinal endoscopy. 2016;83(2):416-23.
11. Jensen DM et al. Gastrointest Endosc Clin N Am. 1997;7(3):477-98.
12. Maykel JA et al. Clin Colon Rectal Surg. 2004;17(3):195-204.
13. Green BT et al. Am J Gastroenterol. 2005;100(11):2395-402.
14. Niikura R et al. Journal of Clinical Gastroenterol. 2015;49(3):e24-30.
15. Bloomfeld RS et al. Am J Gastroenterol. 2001;96(8):2367-72.
16. Parsi MA,et al. VideoGIE. 2019;4(7):285-99.
17. Kaltenbach T et al. Clinical Gastroenterology and Hepatol. 2012;10(2):131-7.
18. Nakano K et al. Endosc Int Open. 2015;3(5):E529-33.
19. Barker KB et al. Gastrointestinal Endoscopy. 2005;62(2):224-7.
20. Kaltenbach T et al. Gastrointest Endosc Clin N Am. 2020;30(1):13-23.
21. Yamazaki K et al. VideoGIE. 2020;5(6):252-4.
22. Strate LL et al. Clinical Gastroenterology and Hepatol. 2010;8(4):333-43.
23. Evangelista et al. J Vasc Interv Radiol. 2000;11(5):601-6.
24. Kodani M et al. J Vasc Interv Radiol. 2016;27(6):824-30.
25. Mohammed et al. Clin Colon Rectal Surg. 2018;31(4):243-50.
26. Wongpongsalee T et al. Gastrointestinal Endoscopy. 2020;91(6):AB471-2.
27. Böhm SK. Viszeralmedizin. 2015;31(2):84-94.
28. Prakash C et al. Endoscopy. 1999;31(6):460-3.
29. Yen EF et al. Digestive Diseases and Sciences. 2008;53(9):2480-5.
30. Ishii N et al. Gastrointestinal Endoscopy. 2012;75(2):382-7.
31. Nagata N et al. Gastrointestinal Endoscopy. 2018;88(5):841-53.e4.
32. Parkes BM et al. Am Surg. 1993;59(10):676-8.
33. Vajravelu RK et al. Gastroenterology. 2018;155(5):1416-27.
34. Oakland K et al. Clin Gastroenterol Hepatol. 2019;17(7):1276-84.e3.
35. Yamada A et al. Dis Colon Rectum. 2008;51(1):116-20.
36. Coleman CI et al. Int J Clin Pract. 2012;66(1):53-63.
37. Holster IL et al. Gastroenterology. 2013;145(1):105-12.e15.
Emerging realities
Dear colleagues,
Welcome to the November edition of The New Gastroenterologist! Our fall newsletter features a particularly interesting compilation of articles. As the pandemic lingers on, we are forced to face the realities of coexisting with COVID-19 as the virus certainly seems to be here to stay.
To protect against ongoing risk of exposure, health care workers and other high-risk subsets of patients are now being offered booster shots. For our patients with inflammatory bowel disease (IBD) on immune-modifying therapies, there has always been a question of vaccine efficacy. Dr. Freddy Caldera and Dr. Trevor Schell (University of Wisconsin-Madison) shed some much needed light on recommendations on the COVID-19 vaccine for IBD patients.
In April of 2021, a federal rule was implemented mandating that patients have immediate and free access to their electronic health information – which includes all documentation from their health care providers. Some physicians have been concerned about this practice, namely how patients will respond and whether this will increase the burden on clinicians. Clearly, this issue is multifaceted: Dr. Sachin Shah (University of Chicago) discusses the ethical implications from a clinical standpoint, while attorney Valerie Guttman Koch (University of Houston Law Center, MacLean Center for Clinical Medical Ethics, University of Chicago) shares a riveting legal perspective.
Colonic diverticular bleeding is the most common etiology of overt lower gastrointestinal bleeding and one of the most frequent consults we receive as gastroenterologists. However, even with the use of colonoscopy, obtaining a definitive diagnosis can often be difficult. Our “In Focus” feature for November, is an excellent piece written by Dr. Vivy Cusumano, Dr. Christopher Paiji, and Dr. Dennis Jensen (all with University of California, Los Angeles), detailing the pathophysiology, diagnosis, and treatment.
Navigating pregnancy and parental leave during training is difficult. Drs. Joy Liu, Keith Summa, Ronak Patel, Erica Donnan, Amanda Guentner, and Leila Kia (all with Northwestern University) share their program’s experience, providing incredibly helpful and practical recommendations for both gastroenterology trainees and fellowship directors.
The Association of Black Gastroenterologists and Hepatologists emerged against the backdrop of recent social and health care injustices. Dr. Kafayat Busari (Florida State University) and Dr. Alexandra Guillaume (Stony Brook University Hospital) discuss the critical importance and mission of this association and how it will help shape the field of gastroenterology in the years to come.
Medical pancreatology is a subspecialty that most gastroenterology fellows have little, if any, exposure to. In our post-fellowship pathways section, Dr. Sajan Nagpal (University of Chicago) details his own experiences in addition to discussing the important role of a medical pancreatologist within a gastroenterology division.
Lastly, our DHPA Private Practice Perspectives article, written by Dr. Sanjay Sandhir (Dayton [Ohio] Gastroenterology), discusses the importance of education and screening for nonalcoholic fatty liver disease.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
Welcome to the November edition of The New Gastroenterologist! Our fall newsletter features a particularly interesting compilation of articles. As the pandemic lingers on, we are forced to face the realities of coexisting with COVID-19 as the virus certainly seems to be here to stay.
To protect against ongoing risk of exposure, health care workers and other high-risk subsets of patients are now being offered booster shots. For our patients with inflammatory bowel disease (IBD) on immune-modifying therapies, there has always been a question of vaccine efficacy. Dr. Freddy Caldera and Dr. Trevor Schell (University of Wisconsin-Madison) shed some much needed light on recommendations on the COVID-19 vaccine for IBD patients.
In April of 2021, a federal rule was implemented mandating that patients have immediate and free access to their electronic health information – which includes all documentation from their health care providers. Some physicians have been concerned about this practice, namely how patients will respond and whether this will increase the burden on clinicians. Clearly, this issue is multifaceted: Dr. Sachin Shah (University of Chicago) discusses the ethical implications from a clinical standpoint, while attorney Valerie Guttman Koch (University of Houston Law Center, MacLean Center for Clinical Medical Ethics, University of Chicago) shares a riveting legal perspective.
Colonic diverticular bleeding is the most common etiology of overt lower gastrointestinal bleeding and one of the most frequent consults we receive as gastroenterologists. However, even with the use of colonoscopy, obtaining a definitive diagnosis can often be difficult. Our “In Focus” feature for November, is an excellent piece written by Dr. Vivy Cusumano, Dr. Christopher Paiji, and Dr. Dennis Jensen (all with University of California, Los Angeles), detailing the pathophysiology, diagnosis, and treatment.
Navigating pregnancy and parental leave during training is difficult. Drs. Joy Liu, Keith Summa, Ronak Patel, Erica Donnan, Amanda Guentner, and Leila Kia (all with Northwestern University) share their program’s experience, providing incredibly helpful and practical recommendations for both gastroenterology trainees and fellowship directors.
The Association of Black Gastroenterologists and Hepatologists emerged against the backdrop of recent social and health care injustices. Dr. Kafayat Busari (Florida State University) and Dr. Alexandra Guillaume (Stony Brook University Hospital) discuss the critical importance and mission of this association and how it will help shape the field of gastroenterology in the years to come.
Medical pancreatology is a subspecialty that most gastroenterology fellows have little, if any, exposure to. In our post-fellowship pathways section, Dr. Sajan Nagpal (University of Chicago) details his own experiences in addition to discussing the important role of a medical pancreatologist within a gastroenterology division.
Lastly, our DHPA Private Practice Perspectives article, written by Dr. Sanjay Sandhir (Dayton [Ohio] Gastroenterology), discusses the importance of education and screening for nonalcoholic fatty liver disease.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
Welcome to the November edition of The New Gastroenterologist! Our fall newsletter features a particularly interesting compilation of articles. As the pandemic lingers on, we are forced to face the realities of coexisting with COVID-19 as the virus certainly seems to be here to stay.
To protect against ongoing risk of exposure, health care workers and other high-risk subsets of patients are now being offered booster shots. For our patients with inflammatory bowel disease (IBD) on immune-modifying therapies, there has always been a question of vaccine efficacy. Dr. Freddy Caldera and Dr. Trevor Schell (University of Wisconsin-Madison) shed some much needed light on recommendations on the COVID-19 vaccine for IBD patients.
In April of 2021, a federal rule was implemented mandating that patients have immediate and free access to their electronic health information – which includes all documentation from their health care providers. Some physicians have been concerned about this practice, namely how patients will respond and whether this will increase the burden on clinicians. Clearly, this issue is multifaceted: Dr. Sachin Shah (University of Chicago) discusses the ethical implications from a clinical standpoint, while attorney Valerie Guttman Koch (University of Houston Law Center, MacLean Center for Clinical Medical Ethics, University of Chicago) shares a riveting legal perspective.
Colonic diverticular bleeding is the most common etiology of overt lower gastrointestinal bleeding and one of the most frequent consults we receive as gastroenterologists. However, even with the use of colonoscopy, obtaining a definitive diagnosis can often be difficult. Our “In Focus” feature for November, is an excellent piece written by Dr. Vivy Cusumano, Dr. Christopher Paiji, and Dr. Dennis Jensen (all with University of California, Los Angeles), detailing the pathophysiology, diagnosis, and treatment.
Navigating pregnancy and parental leave during training is difficult. Drs. Joy Liu, Keith Summa, Ronak Patel, Erica Donnan, Amanda Guentner, and Leila Kia (all with Northwestern University) share their program’s experience, providing incredibly helpful and practical recommendations for both gastroenterology trainees and fellowship directors.
The Association of Black Gastroenterologists and Hepatologists emerged against the backdrop of recent social and health care injustices. Dr. Kafayat Busari (Florida State University) and Dr. Alexandra Guillaume (Stony Brook University Hospital) discuss the critical importance and mission of this association and how it will help shape the field of gastroenterology in the years to come.
Medical pancreatology is a subspecialty that most gastroenterology fellows have little, if any, exposure to. In our post-fellowship pathways section, Dr. Sajan Nagpal (University of Chicago) details his own experiences in addition to discussing the important role of a medical pancreatologist within a gastroenterology division.
Lastly, our DHPA Private Practice Perspectives article, written by Dr. Sanjay Sandhir (Dayton [Ohio] Gastroenterology), discusses the importance of education and screening for nonalcoholic fatty liver disease.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]), or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition



















