User login
Dyssynergic defecation
Introduction
About 40% of the population experiences lower GI symptoms suggestive of gastrointestinal motility disorders.1,2 The global prevalence of chronic constipation is 18%, and the condition includes multiple overlapping subtypes.3 Evacuation disorders affect over half (59%) of patients and include dyssynergic defecation (DD).4 The inability to coordinate the abdominal, rectal, pelvic floor, and anal/puborectalis muscles to evacuate stools causes DD.5 The etiology of DD remains unclear and is often misdiagnosed. Clinically, the symptoms of DD overlap with other lower GI disorders, often leading to unnecessary and invasive procedures.2 We describe the clinical characteristics, diagnostic tools, treatment options, and evidence-based approach for the management of DD.

Clinical presentation
Over two-thirds of patients with DD acquire this disorder during adulthood, and one-third have symptoms from childhood.6 Though there is not usually an inciting event, 29% of patients report that symptoms began after events such as pregnancy or back injury,6 and opioid users have higher prevalence and severity of DD.7
Over 80% of patients report excessive straining, feelings of incomplete evacuation, and hard stools, and 50% report sensation of anal blockage or use of digital maneuvers.2 Other symptoms include infrequent bowel movements, abdominal pain, anal pain, and stool leakage.2 Evaluation of DD includes obtaining a detailed history utilizing the Bristol Stool Form Scale;8 however, patients’ recall of stool habit is often inaccurate, which results in suboptimal care.9,10 Prospective stool diaries can help to provide more objective assessment of patients’ symptoms, eliminate recall bias, and provide more reliable information. Several useful questionnaires are available for clinical and research purposes to characterize lower-GI symptoms, including the Constipation Scoring System,11 Patient Assessment of Constipation Symptoms (PAC-SYM),12 and Patient Assessment of Constipation Quality of Life (PAC-QOL).2,13 The Constipation Stool digital app enhances accuracy of data capture and offers a reliable and user-friendly method for recording bowel symptoms for patients, clinicians, and clinical investigators.14
Diagnosis
The diagnosis of DD requires careful physical and digital rectal examination together with anorectal manometry and a balloon expulsion test. Defecography and colonic transit studies provide additional assessment.
Physical examination
Abdominal examination should include palpation for stool in the colon and identification of abdominal mass or fecal impaction.2A high-quality digital rectal examination can help to identify patients who could benefit from physiological testing to confirm and treat DD.15 Rectal examination is performed by placing examiner’s lubricated gloved right index finger in a patient’s rectum, with the examiner’s left hand on patient’s abdomen, and asking the patient to push and bear down as if defecating.15 The contraction of the abdominal muscles is felt using the left hand, while the anal sphincter relaxation and degree of perineal descent are felt using the right-hand index finger.15 A diagnosis of dyssynergia is suspected if the digital rectal examination reveals two or more of the following abnormalities: inability to contract abdominal muscles (lack of push effort), inability to relax or paradoxical contraction of the anal sphincter and/or puborectalis, or absence of perineal descent.15 Digital rectal examination has good sensitivity (75%), specificity (87%), and positive predictive value (97%) for DD.16
High resolution anorectal manometry
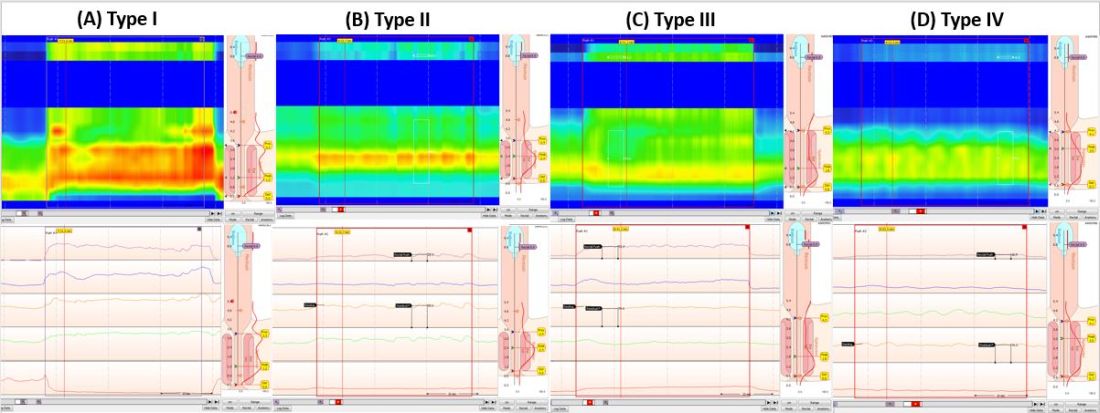
Anorectal manometry (ARM) is the preferred method for the evaluation of defecatory disorders.17,18 ARM is best performed using the high-resolution anorectal manometry (HRAM) systems19 that consist of a flexible probe – 0.5-cm diameter with multiple circumferential sensors along the anal canal – and another two sensors inside a rectal balloon.18 It provides a topographic and waveform display of manometric pressure data (Figure). The 3D high-definition ARM probe is a rigid 1-cm probe that provides 3D topographic profiles.18 ARM is typically performed in both the left lateral position and in a more physiological seated position.20,21 There is considerable variation amongst different institutions on how to perform HRAM, and a recent International Anorectal Physiology Working Group (IAPWG) has provided consensus recommendations for performing this test.22 The procedure for performing HRAM is reviewed elsewhere, but the key elements are summarized below.18
Push maneuver: On HRAM, after the assessment of resting and squeeze anal sphincter pressures, the patient is asked to push or bear down as if to defecate while lying in left lateral decubitus position. The best of two attempts that closely mimics a normal bearing down maneuver is used for categorizing patient’s defecatory pattern.18 In patients with DD, at least four distinct dyssynergia phenotypes have been recognized (Figure),23 though recent studies suggest eight patterns.24 Defecation index (maximum rectal pressure/minimum residual anal pressure when bearing down) greater than 1.2 is considered normal.18
Simulated defecation on commode: The subject is asked to attempt defecation while seated on a commode with intrarectal balloon filled with 60 cc of air, and both the defecation pattern(s) and defecation index are calculated. A lack of coordinated push effort is highly suggestive of DD.21
Rectoanal Inhibitory Reflex (RAIR): RAIR describes the reflex relaxation of the internal anal sphincter after rectal distension. RAIR is dependent on intact autonomic ganglia and myenteric plexus25and is mediated by the release of nitric oxide and vasoactive intestinal peptide.26 The absence of RAIR suggests Hirschsprung disease.22.27.28
Rectal sensory testing: Intermittent balloon distension of the rectum with incremental volumes of air induces a range of rectal sensations that include first sensation, desire to defecate, urgency to defecate, and maximum tolerable volume. Rectal hyposensitivity is diagnosed when two or more sensory thresholds are higher than those seen in normal subjects29.30 and likely results from disruption of afferent gut-brain pathways, cortical perception/rectal wall dysfunction, or both.29 Rectal hyposensitivity affects 40% of patients with constipation30and is associated with DD but not delayed colonic transit.31 Rectal hyposensitivity may also be seen in patients with diabetes or fecal incontinence.18 About two-thirds of patients with rectal hyposensitivity have rectal hypercompliance, and some have megarectum.32 Some patients with DD have coexisting irritable bowel syndrome (IBS) and may have rectal hypersensitivity.18,33 Rectal compliance is measured alongside rectal sensitivity analysis by plotting a graph between the change in intraballoon volume (mL) and change in intrarectal pressures (mm Hg) during incremental balloon distensions.18.34 Rectal hypercompliance may be seen in megarectum and dyssynergic defecation.34,35 Rectal hypocompliance may be seen in patients with inflammatory bowel disease, postpelvic radiation, chronic ischemia, and advanced age.18
Balloon expulsion test: This test is performed by placing a plastic probe with a balloon in the rectum and filling it with 50 cc of warm water. Patients are given 5 minutes to expel the balloon while sitting on a commode. Balloon expulsion time of more than 1 minute suggests a diagnosis of DD,21 although 2 minutes provides a higher level of agreement with manometric findings.36 Balloon type and body position can influence the results.37 Inability to expel the balloon with normal manometric findings is considered an inconclusive finding per the recent London Classification (i.e., it may be associated with generation of anorectal symptoms, but the clinical relevance of this finding is unclear as it may also be seen in healthy subjects).22
Defecography
Defecography is a dynamic fluoroscopic study performed in the sitting position after injecting 150 mL of barium paste into the patient’s rectum. Defecography provides useful information about structural changes (e.g., rectoceles, enteroceles, rectal prolapse, and intussusception), DD, and descending perineum syndrome.38 Methodological differences, radiation exposure, and poor interobserver agreement have limited its wider use; therefore, anorectal manometry and the balloon expulsion test are recommended for the initial evaluation of DD.39 Magnetic resonance defecography may be more useful.17,38
Colonic transit studies
Colonic transit study can be assessed using radiopaque markers, wireless motility capsule, or scintigraphy. Wireless motility capsule and scintigraphy have the advantage of determining gastric, small bowel, and whole gut transit times as well. About two-thirds of patients with DD have slow transit constipation (STC),6 which improves after treatment of DD.40 Hence, in patients with chronic constipation, evaluation and management of DD is recommended first. If symptoms persist, then consider colonic transit assessment.41 Given the overlapping nature of the conditions, documentation of STC at the outset could facilitate treatment of both.
Diagnostic criteria for DD
Patients should fulfill the following criteria for diagnosis of DD:42,43
- Fulfill symptom(s) diagnostic criteria for functional constipation and/or constipation-predominant IBS.
- Demonstrate dyssynergic pattern (Types I-IV; Figure) during attempted defecation on manometry recordings.
- Meet one or more of the following criteria:
- Inability to expel an artificial stool (50 mL water-filled balloon) within 1 minute.
- Inability to evacuate or retention of 50% or more of barium during defecography. (Some institutions use a prolonged colonic transit time: greater than 5 markers or 20% or higher marker retention on a plain abdominal x-Ray at 120 hours after ingestion of one radio-opaque marker capsule containing 24 radio-opaque markers.)
Treatment of DD
The treatment modalities for DD depend on several factors: patient’s age, comorbidities, underlying pathophysiology, and patient expectations. Treatment options include standard management of constipation, but biofeedback therapy is the mainstay.
Standard management
Medications that cause or worsen constipation should be avoided. The patient should consume adequate fluid and exercise regularly. Patients should receive instructions for timed toilet training (twice daily, 30 minutes after meals). Patients should push at about 50%-70% of their ability for no longer than 5 minutes and avoid postponing defecation or use of digital maneuvers to facilitate defecation.42 The patients should take 25 g of soluble fiber (e.g., psyllium) daily. Of note, the benefits of fiber can take days to weeks44 and may be limited in patients with STC and DD.45 Medications including laxatives and intestinal secretagogues (lubiprostone, linaclotide, plecanatide), and enterokinetic agents (prucalopride) can be used as adjunct therapy for management of DD.42 Their use is titrated during and after biofeedback therapy and may decrease after successful treatment.46
Biofeedback therapy
Biofeedback therapy involves operant conditioning techniques using either a solid state anorectal manometry system, electromyography, simulated balloon, or home biofeedback training devices.42,47 The goals of biofeedback therapy are to correct the abdominal pelvic muscle discoordination during defecation and improve rectal sensation to stool if impaired. Biofeedback therapy involves patient education and active training (typically six sessions, 1-2 weeks apart, with each about 30-60 minutes long), followed by a reinforcement stage (three sessions at 3, 6, and 12 months), though there are variations in training protocols.42
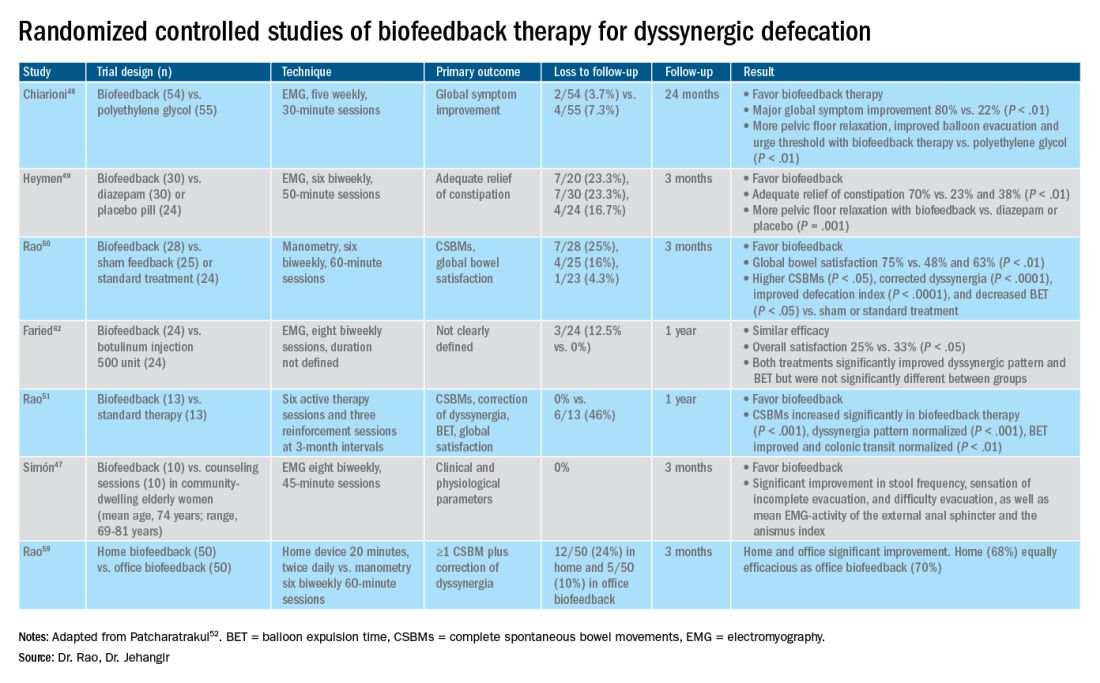
The success of biofeedback therapy depends on the patient’s motivation and the therapist’s skills.42 Compared with standard therapy (diet, exercise, pharmacotherapy), biofeedback therapy provides sustained improvement of bowel symptoms and anorectal function. Up to 70%-80% of DD patients show significant improvement of symptoms in randomized controlled trials (Table).48-52 Biofeedback therapy may also improve dyspeptic symptoms.53 Patients with harder stool consistency, greater willingness to participate, lower baseline bowel satisfaction, lower baseline anal sphincter relaxation, and prolonged balloon expulsion time, as well as patients who used digital maneuvers for defection, more commonly respond to biofeedback therapy.54,55 Longstanding laxative use has been associated with decreased response to biofeedback therapy.56 In patients with rectal hyposensitivity, barostat-assisted sensory training is more effective than a hand-held syringe technique.30 In patients with constipation predominant IBS and rectal hyposensitivity, sensory adaption training is more efficacious and better tolerated than escitalopram.30 Biofeedback therapy was afforded a grade A recommendation for treatment of DD by the American and European Societies of Neurogastroenterology and Motility.57
The access to office-based biofeedback therapy may be limited because of costs and low availability. The time required to attend multiple sessions may be burdensome for some patients, especially if they are taking time off from work. A recent study showed that patients with higher level of education may be less likely to adhere to biofeedback therapy.58 Recently, home biofeedback was shown to be noninferior to office biofeedback and was more cost-effective, which provides an alternative option for treating more patients.59
Endoscopic/surgical options
Other less effective treatment options for DD include botulinum toxin injection and myectomy.60-62 Botulinum toxin injection appears to have mixed effects with less than 50% of patients reporting symptomatic improvement, and it may cause fecal incontinence.60,63
Conclusion
DD is a common yet poorly recognized cause of constipation. Its clinical presentation overlaps with other lower-GI disorders. Its diagnosis requires detailed history, digital rectal examination, prospective stool diaries, anorectal manometry, and balloon expulsion tests. Biofeedback therapy offers excellent and sustained symptomatic improvement; however, access to office-based biofeedback is limited, and there is an urgent need for home-based biofeedback therapy programs.59
Dr. Rao is J. Harold Harrison Distinguished University Chair, professor of medicine, director of neurogastroenterology/motility, and director of digestive health at the Digestive Health Clinical Research Center Augusta (Georgia) University. He is supported by National Institutes of Health grants R01DK121003-02 and U01DK115572. Dr. Jehangir is a gastroenterology and Hepatology Fellow at the Digestive Health Clinical Research Center at Augusta University. They reported having no conflicts of interest.
References
1. Peery AF, et al. Gastroenterology. 2012;143(5):1179-1187.e3 .
2. Curtin B, et al. J Neurogastroenterol Motil. 2020 30;26(4):423-36.
3. Suares NC & Ford AC. Am J Gastroenterol. 2011 Sep;106(9):1582-91.
4. Mertz H, et al. Am J Gastroenterol. 1999;94(3):609-15.
5. Rao SS, et al. Am J Gastroenterol. 1998;93(7):1042-50.
6. Rao SSC, et al. J Clin Gastroenterol. 2004;38(8):680-5.
7. Nojkov B, et al. Am J Gastroenterol. 2019;114(11):1772-7.
8. Heaton KW, et al. Gut. 1992;33(6):818-24.
9. Prichard DO & Bharucha AE. 2018 Oct 15;7:F1000 Faculty Rev-1640.
10. Ashraf W, et al. Am J Gastroenterol. 1996;91(1):26-32.
11. Agachan F, et al.. Dis Colon Rectum. 1996;39(6):681-5.
12. Frank L, et al. Scand J Gastroenterol. 1999;34(9):870-7.
13. Marquis P, et al. Scand J Gastroenterol. 2005;40(5):540-51.
14. Yan Y, et al. Gastroenterology. 2020;158(6):S-400.
15. Rao SSC. Am J Gastroenterol. 2018;113(5):635-8.
16. Tantiphlachiva K, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8(11):955-60.
17. Carrington EV, et al. Nat Rev Gastroenterol Hepatol. 2018;15(5):309-23.
18. Tetangco EP, et al. Performing and analyzing high-resolution anorectal manometry. NeuroGastroLatam Rev. 2018;2:120-32.
19. Lee YY, et al. Curr Gastroenterol Rep. 2013;15(12):360.
20. Sharma M, et al. Neurogastroenterol Motil. 2020;32(10):e13910.
21. Rao SSC, et al.. Am J Gastroenterol. 2006;101(12):2790-6.
22. Carrington EV, et al. Neurogastroenterol Motil. 2020;32(1):e13679.
23. Rao SSC. Gastroenterol Clin North Am. 2008;37(3):569-86, viii.
24. Rao SSC, et al. Gastroenterology. 2016;150(4):S158-9.
25. Guinet A, et al. Int J Colorectal Dis. 2011;26(4):507-13.
26. Rattan S, et al. Gastroenterology. 1992;103(1):43-50.
27. Remes-Troche JM & Rao SSC. 2008;2(3):323-35.
28. Zaafouri H, et al..Int J Surgery. 2015. 2(1):9-17.
29. Remes-Troche JM, et al. Dis Colon Rectum. 2010;53(7):1047-54.
30. Rao SSC, et al. Gastroenterology. 2013;144(5):S-363.
31. Yu T, et al. Medicine (Baltimore). 2016;95(19):e3667.
32. Gladman MA, et al. Neurogastroenterol Motil. 2009;21(5):508-16, e4-5.
33. Lee KJ, et al. Digestion. 2006;73(2-3):133-41 .
34. Rao SSC, et al. Neurogastroenterol Motil. 2002;14(5):553-9.
35. Coss-Adame E, et al.. Clin Gastroenterol Hepatol. 2015;13(6):1143-1150.e1.
36. Chiarioni G, et al. Clin Gastroenterol Hepatol. 2014;12(12):2049-54.
37. Gu G, et al. Gastroenterology. 2018;154(6):S-545–S-546.
38. Savoye-Collet C, et al.. Gastroenterol Clin North Am. 2008;37(3):553-67, viii.
39. Videlock EJ, et al. Neurogastroenterol Motil. 2013;25(6):509-20.
40. Rao SSC, et al. Neurogastroenterol Motil. 2004;16(5):589-96.
41. Wald A, et al. Am J Gastroenterol. 2014;109(8):1141-57 ; (Quiz) 1058.
42. Rao SSC & Patcharatrakul T. J Neurogastroenterol Motil. 2016;22(3):423-35.
43. Rao SS, et al. Functional Anorectal Disorders. Gastroenterology. 2016. S0016-5085(16)00175-X.
44. Bharucha AE, et al.. Gastroenterology. 2013;144(1):218-38.
45. Voderholzer WA, et al. Am J Gastroenterol. 1997;92(1):95-8.
46. Lee HJ, et al. Neurogastroenterol Motil. 2015;27(6):787-95.
47. Simón MA & Bueno AM. J Clin Gastroenterol. 2017;51(10):e90-4.
48. Chiarioni G,et al.. Gastroenterology. 2006;130(3):657-64.
49. Heymen S, et al.. Dis Colon Rectum. 2007;50(4):428-41.
50. Rao SSC, et al. Clin Gastroenterol Hepatol. 2007;5(3):331-8.
51. Rao SSC, et al. Am J Gastroenterol. 2010;105(4):890-6.
52. Patcharatrakul T, et al. Biofeedback therapy. In Clinical and basic neurogastroenterology and motility. India: Stacy Masucci; 2020:517-32.
53. Huaman J-W, et al. Clin Gastroenterol Hepatol. 2020;18(11):2463-2470.e1.
54. Patcharatrakul T, et al. Clin Gastroenterol Hepatol. 2018;16(5):715-21.
55. Chaudhry A, et al. Gastroenterology. 2020;158(6):S-382–S-383.
56. Shim LSE, et al. Aliment Pharmacol Ther. 2011;33(11):1245-51.
57. Rao SSC, et al. Neurogastroenterol Motil. 2015;27(5):594-609.
58. Jangsirikul S, et al. Gastroenterology. 2020;158(6):S-383.
59. Rao SSC, et al. Am J Gastroenterol. 2019;114(6):938-44.
60. Ron Y, et al.. Dis Colon Rectum. 2001;44(12):1821-6.
61. Podzemny V, et al. World J Gastroenterol. 2015;21(4):1053-60.
62. Faried M, et al. J Gastrointest Surg. 2010;14(8):1235-43.
63. Hallan RI, et al. Lancet. 1988;2(8613):714-7.
Introduction
About 40% of the population experiences lower GI symptoms suggestive of gastrointestinal motility disorders.1,2 The global prevalence of chronic constipation is 18%, and the condition includes multiple overlapping subtypes.3 Evacuation disorders affect over half (59%) of patients and include dyssynergic defecation (DD).4 The inability to coordinate the abdominal, rectal, pelvic floor, and anal/puborectalis muscles to evacuate stools causes DD.5 The etiology of DD remains unclear and is often misdiagnosed. Clinically, the symptoms of DD overlap with other lower GI disorders, often leading to unnecessary and invasive procedures.2 We describe the clinical characteristics, diagnostic tools, treatment options, and evidence-based approach for the management of DD.

Clinical presentation
Over two-thirds of patients with DD acquire this disorder during adulthood, and one-third have symptoms from childhood.6 Though there is not usually an inciting event, 29% of patients report that symptoms began after events such as pregnancy or back injury,6 and opioid users have higher prevalence and severity of DD.7
Over 80% of patients report excessive straining, feelings of incomplete evacuation, and hard stools, and 50% report sensation of anal blockage or use of digital maneuvers.2 Other symptoms include infrequent bowel movements, abdominal pain, anal pain, and stool leakage.2 Evaluation of DD includes obtaining a detailed history utilizing the Bristol Stool Form Scale;8 however, patients’ recall of stool habit is often inaccurate, which results in suboptimal care.9,10 Prospective stool diaries can help to provide more objective assessment of patients’ symptoms, eliminate recall bias, and provide more reliable information. Several useful questionnaires are available for clinical and research purposes to characterize lower-GI symptoms, including the Constipation Scoring System,11 Patient Assessment of Constipation Symptoms (PAC-SYM),12 and Patient Assessment of Constipation Quality of Life (PAC-QOL).2,13 The Constipation Stool digital app enhances accuracy of data capture and offers a reliable and user-friendly method for recording bowel symptoms for patients, clinicians, and clinical investigators.14
Diagnosis
The diagnosis of DD requires careful physical and digital rectal examination together with anorectal manometry and a balloon expulsion test. Defecography and colonic transit studies provide additional assessment.
Physical examination
Abdominal examination should include palpation for stool in the colon and identification of abdominal mass or fecal impaction.2A high-quality digital rectal examination can help to identify patients who could benefit from physiological testing to confirm and treat DD.15 Rectal examination is performed by placing examiner’s lubricated gloved right index finger in a patient’s rectum, with the examiner’s left hand on patient’s abdomen, and asking the patient to push and bear down as if defecating.15 The contraction of the abdominal muscles is felt using the left hand, while the anal sphincter relaxation and degree of perineal descent are felt using the right-hand index finger.15 A diagnosis of dyssynergia is suspected if the digital rectal examination reveals two or more of the following abnormalities: inability to contract abdominal muscles (lack of push effort), inability to relax or paradoxical contraction of the anal sphincter and/or puborectalis, or absence of perineal descent.15 Digital rectal examination has good sensitivity (75%), specificity (87%), and positive predictive value (97%) for DD.16
High resolution anorectal manometry

Anorectal manometry (ARM) is the preferred method for the evaluation of defecatory disorders.17,18 ARM is best performed using the high-resolution anorectal manometry (HRAM) systems19 that consist of a flexible probe – 0.5-cm diameter with multiple circumferential sensors along the anal canal – and another two sensors inside a rectal balloon.18 It provides a topographic and waveform display of manometric pressure data (Figure). The 3D high-definition ARM probe is a rigid 1-cm probe that provides 3D topographic profiles.18 ARM is typically performed in both the left lateral position and in a more physiological seated position.20,21 There is considerable variation amongst different institutions on how to perform HRAM, and a recent International Anorectal Physiology Working Group (IAPWG) has provided consensus recommendations for performing this test.22 The procedure for performing HRAM is reviewed elsewhere, but the key elements are summarized below.18
Push maneuver: On HRAM, after the assessment of resting and squeeze anal sphincter pressures, the patient is asked to push or bear down as if to defecate while lying in left lateral decubitus position. The best of two attempts that closely mimics a normal bearing down maneuver is used for categorizing patient’s defecatory pattern.18 In patients with DD, at least four distinct dyssynergia phenotypes have been recognized (Figure),23 though recent studies suggest eight patterns.24 Defecation index (maximum rectal pressure/minimum residual anal pressure when bearing down) greater than 1.2 is considered normal.18
Simulated defecation on commode: The subject is asked to attempt defecation while seated on a commode with intrarectal balloon filled with 60 cc of air, and both the defecation pattern(s) and defecation index are calculated. A lack of coordinated push effort is highly suggestive of DD.21
Rectoanal Inhibitory Reflex (RAIR): RAIR describes the reflex relaxation of the internal anal sphincter after rectal distension. RAIR is dependent on intact autonomic ganglia and myenteric plexus25and is mediated by the release of nitric oxide and vasoactive intestinal peptide.26 The absence of RAIR suggests Hirschsprung disease.22.27.28
Rectal sensory testing: Intermittent balloon distension of the rectum with incremental volumes of air induces a range of rectal sensations that include first sensation, desire to defecate, urgency to defecate, and maximum tolerable volume. Rectal hyposensitivity is diagnosed when two or more sensory thresholds are higher than those seen in normal subjects29.30 and likely results from disruption of afferent gut-brain pathways, cortical perception/rectal wall dysfunction, or both.29 Rectal hyposensitivity affects 40% of patients with constipation30and is associated with DD but not delayed colonic transit.31 Rectal hyposensitivity may also be seen in patients with diabetes or fecal incontinence.18 About two-thirds of patients with rectal hyposensitivity have rectal hypercompliance, and some have megarectum.32 Some patients with DD have coexisting irritable bowel syndrome (IBS) and may have rectal hypersensitivity.18,33 Rectal compliance is measured alongside rectal sensitivity analysis by plotting a graph between the change in intraballoon volume (mL) and change in intrarectal pressures (mm Hg) during incremental balloon distensions.18.34 Rectal hypercompliance may be seen in megarectum and dyssynergic defecation.34,35 Rectal hypocompliance may be seen in patients with inflammatory bowel disease, postpelvic radiation, chronic ischemia, and advanced age.18
Balloon expulsion test: This test is performed by placing a plastic probe with a balloon in the rectum and filling it with 50 cc of warm water. Patients are given 5 minutes to expel the balloon while sitting on a commode. Balloon expulsion time of more than 1 minute suggests a diagnosis of DD,21 although 2 minutes provides a higher level of agreement with manometric findings.36 Balloon type and body position can influence the results.37 Inability to expel the balloon with normal manometric findings is considered an inconclusive finding per the recent London Classification (i.e., it may be associated with generation of anorectal symptoms, but the clinical relevance of this finding is unclear as it may also be seen in healthy subjects).22
Defecography
Defecography is a dynamic fluoroscopic study performed in the sitting position after injecting 150 mL of barium paste into the patient’s rectum. Defecography provides useful information about structural changes (e.g., rectoceles, enteroceles, rectal prolapse, and intussusception), DD, and descending perineum syndrome.38 Methodological differences, radiation exposure, and poor interobserver agreement have limited its wider use; therefore, anorectal manometry and the balloon expulsion test are recommended for the initial evaluation of DD.39 Magnetic resonance defecography may be more useful.17,38
Colonic transit studies
Colonic transit study can be assessed using radiopaque markers, wireless motility capsule, or scintigraphy. Wireless motility capsule and scintigraphy have the advantage of determining gastric, small bowel, and whole gut transit times as well. About two-thirds of patients with DD have slow transit constipation (STC),6 which improves after treatment of DD.40 Hence, in patients with chronic constipation, evaluation and management of DD is recommended first. If symptoms persist, then consider colonic transit assessment.41 Given the overlapping nature of the conditions, documentation of STC at the outset could facilitate treatment of both.
Diagnostic criteria for DD
Patients should fulfill the following criteria for diagnosis of DD:42,43
- Fulfill symptom(s) diagnostic criteria for functional constipation and/or constipation-predominant IBS.
- Demonstrate dyssynergic pattern (Types I-IV; Figure) during attempted defecation on manometry recordings.
- Meet one or more of the following criteria:
- Inability to expel an artificial stool (50 mL water-filled balloon) within 1 minute.
- Inability to evacuate or retention of 50% or more of barium during defecography. (Some institutions use a prolonged colonic transit time: greater than 5 markers or 20% or higher marker retention on a plain abdominal x-Ray at 120 hours after ingestion of one radio-opaque marker capsule containing 24 radio-opaque markers.)
Treatment of DD
The treatment modalities for DD depend on several factors: patient’s age, comorbidities, underlying pathophysiology, and patient expectations. Treatment options include standard management of constipation, but biofeedback therapy is the mainstay.
Standard management
Medications that cause or worsen constipation should be avoided. The patient should consume adequate fluid and exercise regularly. Patients should receive instructions for timed toilet training (twice daily, 30 minutes after meals). Patients should push at about 50%-70% of their ability for no longer than 5 minutes and avoid postponing defecation or use of digital maneuvers to facilitate defecation.42 The patients should take 25 g of soluble fiber (e.g., psyllium) daily. Of note, the benefits of fiber can take days to weeks44 and may be limited in patients with STC and DD.45 Medications including laxatives and intestinal secretagogues (lubiprostone, linaclotide, plecanatide), and enterokinetic agents (prucalopride) can be used as adjunct therapy for management of DD.42 Their use is titrated during and after biofeedback therapy and may decrease after successful treatment.46
Biofeedback therapy
Biofeedback therapy involves operant conditioning techniques using either a solid state anorectal manometry system, electromyography, simulated balloon, or home biofeedback training devices.42,47 The goals of biofeedback therapy are to correct the abdominal pelvic muscle discoordination during defecation and improve rectal sensation to stool if impaired. Biofeedback therapy involves patient education and active training (typically six sessions, 1-2 weeks apart, with each about 30-60 minutes long), followed by a reinforcement stage (three sessions at 3, 6, and 12 months), though there are variations in training protocols.42

The success of biofeedback therapy depends on the patient’s motivation and the therapist’s skills.42 Compared with standard therapy (diet, exercise, pharmacotherapy), biofeedback therapy provides sustained improvement of bowel symptoms and anorectal function. Up to 70%-80% of DD patients show significant improvement of symptoms in randomized controlled trials (Table).48-52 Biofeedback therapy may also improve dyspeptic symptoms.53 Patients with harder stool consistency, greater willingness to participate, lower baseline bowel satisfaction, lower baseline anal sphincter relaxation, and prolonged balloon expulsion time, as well as patients who used digital maneuvers for defection, more commonly respond to biofeedback therapy.54,55 Longstanding laxative use has been associated with decreased response to biofeedback therapy.56 In patients with rectal hyposensitivity, barostat-assisted sensory training is more effective than a hand-held syringe technique.30 In patients with constipation predominant IBS and rectal hyposensitivity, sensory adaption training is more efficacious and better tolerated than escitalopram.30 Biofeedback therapy was afforded a grade A recommendation for treatment of DD by the American and European Societies of Neurogastroenterology and Motility.57
The access to office-based biofeedback therapy may be limited because of costs and low availability. The time required to attend multiple sessions may be burdensome for some patients, especially if they are taking time off from work. A recent study showed that patients with higher level of education may be less likely to adhere to biofeedback therapy.58 Recently, home biofeedback was shown to be noninferior to office biofeedback and was more cost-effective, which provides an alternative option for treating more patients.59
Endoscopic/surgical options
Other less effective treatment options for DD include botulinum toxin injection and myectomy.60-62 Botulinum toxin injection appears to have mixed effects with less than 50% of patients reporting symptomatic improvement, and it may cause fecal incontinence.60,63
Conclusion
DD is a common yet poorly recognized cause of constipation. Its clinical presentation overlaps with other lower-GI disorders. Its diagnosis requires detailed history, digital rectal examination, prospective stool diaries, anorectal manometry, and balloon expulsion tests. Biofeedback therapy offers excellent and sustained symptomatic improvement; however, access to office-based biofeedback is limited, and there is an urgent need for home-based biofeedback therapy programs.59
Dr. Rao is J. Harold Harrison Distinguished University Chair, professor of medicine, director of neurogastroenterology/motility, and director of digestive health at the Digestive Health Clinical Research Center Augusta (Georgia) University. He is supported by National Institutes of Health grants R01DK121003-02 and U01DK115572. Dr. Jehangir is a gastroenterology and Hepatology Fellow at the Digestive Health Clinical Research Center at Augusta University. They reported having no conflicts of interest.
References
1. Peery AF, et al. Gastroenterology. 2012;143(5):1179-1187.e3 .
2. Curtin B, et al. J Neurogastroenterol Motil. 2020 30;26(4):423-36.
3. Suares NC & Ford AC. Am J Gastroenterol. 2011 Sep;106(9):1582-91.
4. Mertz H, et al. Am J Gastroenterol. 1999;94(3):609-15.
5. Rao SS, et al. Am J Gastroenterol. 1998;93(7):1042-50.
6. Rao SSC, et al. J Clin Gastroenterol. 2004;38(8):680-5.
7. Nojkov B, et al. Am J Gastroenterol. 2019;114(11):1772-7.
8. Heaton KW, et al. Gut. 1992;33(6):818-24.
9. Prichard DO & Bharucha AE. 2018 Oct 15;7:F1000 Faculty Rev-1640.
10. Ashraf W, et al. Am J Gastroenterol. 1996;91(1):26-32.
11. Agachan F, et al.. Dis Colon Rectum. 1996;39(6):681-5.
12. Frank L, et al. Scand J Gastroenterol. 1999;34(9):870-7.
13. Marquis P, et al. Scand J Gastroenterol. 2005;40(5):540-51.
14. Yan Y, et al. Gastroenterology. 2020;158(6):S-400.
15. Rao SSC. Am J Gastroenterol. 2018;113(5):635-8.
16. Tantiphlachiva K, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8(11):955-60.
17. Carrington EV, et al. Nat Rev Gastroenterol Hepatol. 2018;15(5):309-23.
18. Tetangco EP, et al. Performing and analyzing high-resolution anorectal manometry. NeuroGastroLatam Rev. 2018;2:120-32.
19. Lee YY, et al. Curr Gastroenterol Rep. 2013;15(12):360.
20. Sharma M, et al. Neurogastroenterol Motil. 2020;32(10):e13910.
21. Rao SSC, et al.. Am J Gastroenterol. 2006;101(12):2790-6.
22. Carrington EV, et al. Neurogastroenterol Motil. 2020;32(1):e13679.
23. Rao SSC. Gastroenterol Clin North Am. 2008;37(3):569-86, viii.
24. Rao SSC, et al. Gastroenterology. 2016;150(4):S158-9.
25. Guinet A, et al. Int J Colorectal Dis. 2011;26(4):507-13.
26. Rattan S, et al. Gastroenterology. 1992;103(1):43-50.
27. Remes-Troche JM & Rao SSC. 2008;2(3):323-35.
28. Zaafouri H, et al..Int J Surgery. 2015. 2(1):9-17.
29. Remes-Troche JM, et al. Dis Colon Rectum. 2010;53(7):1047-54.
30. Rao SSC, et al. Gastroenterology. 2013;144(5):S-363.
31. Yu T, et al. Medicine (Baltimore). 2016;95(19):e3667.
32. Gladman MA, et al. Neurogastroenterol Motil. 2009;21(5):508-16, e4-5.
33. Lee KJ, et al. Digestion. 2006;73(2-3):133-41 .
34. Rao SSC, et al. Neurogastroenterol Motil. 2002;14(5):553-9.
35. Coss-Adame E, et al.. Clin Gastroenterol Hepatol. 2015;13(6):1143-1150.e1.
36. Chiarioni G, et al. Clin Gastroenterol Hepatol. 2014;12(12):2049-54.
37. Gu G, et al. Gastroenterology. 2018;154(6):S-545–S-546.
38. Savoye-Collet C, et al.. Gastroenterol Clin North Am. 2008;37(3):553-67, viii.
39. Videlock EJ, et al. Neurogastroenterol Motil. 2013;25(6):509-20.
40. Rao SSC, et al. Neurogastroenterol Motil. 2004;16(5):589-96.
41. Wald A, et al. Am J Gastroenterol. 2014;109(8):1141-57 ; (Quiz) 1058.
42. Rao SSC & Patcharatrakul T. J Neurogastroenterol Motil. 2016;22(3):423-35.
43. Rao SS, et al. Functional Anorectal Disorders. Gastroenterology. 2016. S0016-5085(16)00175-X.
44. Bharucha AE, et al.. Gastroenterology. 2013;144(1):218-38.
45. Voderholzer WA, et al. Am J Gastroenterol. 1997;92(1):95-8.
46. Lee HJ, et al. Neurogastroenterol Motil. 2015;27(6):787-95.
47. Simón MA & Bueno AM. J Clin Gastroenterol. 2017;51(10):e90-4.
48. Chiarioni G,et al.. Gastroenterology. 2006;130(3):657-64.
49. Heymen S, et al.. Dis Colon Rectum. 2007;50(4):428-41.
50. Rao SSC, et al. Clin Gastroenterol Hepatol. 2007;5(3):331-8.
51. Rao SSC, et al. Am J Gastroenterol. 2010;105(4):890-6.
52. Patcharatrakul T, et al. Biofeedback therapy. In Clinical and basic neurogastroenterology and motility. India: Stacy Masucci; 2020:517-32.
53. Huaman J-W, et al. Clin Gastroenterol Hepatol. 2020;18(11):2463-2470.e1.
54. Patcharatrakul T, et al. Clin Gastroenterol Hepatol. 2018;16(5):715-21.
55. Chaudhry A, et al. Gastroenterology. 2020;158(6):S-382–S-383.
56. Shim LSE, et al. Aliment Pharmacol Ther. 2011;33(11):1245-51.
57. Rao SSC, et al. Neurogastroenterol Motil. 2015;27(5):594-609.
58. Jangsirikul S, et al. Gastroenterology. 2020;158(6):S-383.
59. Rao SSC, et al. Am J Gastroenterol. 2019;114(6):938-44.
60. Ron Y, et al.. Dis Colon Rectum. 2001;44(12):1821-6.
61. Podzemny V, et al. World J Gastroenterol. 2015;21(4):1053-60.
62. Faried M, et al. J Gastrointest Surg. 2010;14(8):1235-43.
63. Hallan RI, et al. Lancet. 1988;2(8613):714-7.
Introduction
About 40% of the population experiences lower GI symptoms suggestive of gastrointestinal motility disorders.1,2 The global prevalence of chronic constipation is 18%, and the condition includes multiple overlapping subtypes.3 Evacuation disorders affect over half (59%) of patients and include dyssynergic defecation (DD).4 The inability to coordinate the abdominal, rectal, pelvic floor, and anal/puborectalis muscles to evacuate stools causes DD.5 The etiology of DD remains unclear and is often misdiagnosed. Clinically, the symptoms of DD overlap with other lower GI disorders, often leading to unnecessary and invasive procedures.2 We describe the clinical characteristics, diagnostic tools, treatment options, and evidence-based approach for the management of DD.

Clinical presentation
Over two-thirds of patients with DD acquire this disorder during adulthood, and one-third have symptoms from childhood.6 Though there is not usually an inciting event, 29% of patients report that symptoms began after events such as pregnancy or back injury,6 and opioid users have higher prevalence and severity of DD.7
Over 80% of patients report excessive straining, feelings of incomplete evacuation, and hard stools, and 50% report sensation of anal blockage or use of digital maneuvers.2 Other symptoms include infrequent bowel movements, abdominal pain, anal pain, and stool leakage.2 Evaluation of DD includes obtaining a detailed history utilizing the Bristol Stool Form Scale;8 however, patients’ recall of stool habit is often inaccurate, which results in suboptimal care.9,10 Prospective stool diaries can help to provide more objective assessment of patients’ symptoms, eliminate recall bias, and provide more reliable information. Several useful questionnaires are available for clinical and research purposes to characterize lower-GI symptoms, including the Constipation Scoring System,11 Patient Assessment of Constipation Symptoms (PAC-SYM),12 and Patient Assessment of Constipation Quality of Life (PAC-QOL).2,13 The Constipation Stool digital app enhances accuracy of data capture and offers a reliable and user-friendly method for recording bowel symptoms for patients, clinicians, and clinical investigators.14
Diagnosis
The diagnosis of DD requires careful physical and digital rectal examination together with anorectal manometry and a balloon expulsion test. Defecography and colonic transit studies provide additional assessment.
Physical examination
Abdominal examination should include palpation for stool in the colon and identification of abdominal mass or fecal impaction.2A high-quality digital rectal examination can help to identify patients who could benefit from physiological testing to confirm and treat DD.15 Rectal examination is performed by placing examiner’s lubricated gloved right index finger in a patient’s rectum, with the examiner’s left hand on patient’s abdomen, and asking the patient to push and bear down as if defecating.15 The contraction of the abdominal muscles is felt using the left hand, while the anal sphincter relaxation and degree of perineal descent are felt using the right-hand index finger.15 A diagnosis of dyssynergia is suspected if the digital rectal examination reveals two or more of the following abnormalities: inability to contract abdominal muscles (lack of push effort), inability to relax or paradoxical contraction of the anal sphincter and/or puborectalis, or absence of perineal descent.15 Digital rectal examination has good sensitivity (75%), specificity (87%), and positive predictive value (97%) for DD.16
High resolution anorectal manometry

Anorectal manometry (ARM) is the preferred method for the evaluation of defecatory disorders.17,18 ARM is best performed using the high-resolution anorectal manometry (HRAM) systems19 that consist of a flexible probe – 0.5-cm diameter with multiple circumferential sensors along the anal canal – and another two sensors inside a rectal balloon.18 It provides a topographic and waveform display of manometric pressure data (Figure). The 3D high-definition ARM probe is a rigid 1-cm probe that provides 3D topographic profiles.18 ARM is typically performed in both the left lateral position and in a more physiological seated position.20,21 There is considerable variation amongst different institutions on how to perform HRAM, and a recent International Anorectal Physiology Working Group (IAPWG) has provided consensus recommendations for performing this test.22 The procedure for performing HRAM is reviewed elsewhere, but the key elements are summarized below.18
Push maneuver: On HRAM, after the assessment of resting and squeeze anal sphincter pressures, the patient is asked to push or bear down as if to defecate while lying in left lateral decubitus position. The best of two attempts that closely mimics a normal bearing down maneuver is used for categorizing patient’s defecatory pattern.18 In patients with DD, at least four distinct dyssynergia phenotypes have been recognized (Figure),23 though recent studies suggest eight patterns.24 Defecation index (maximum rectal pressure/minimum residual anal pressure when bearing down) greater than 1.2 is considered normal.18
Simulated defecation on commode: The subject is asked to attempt defecation while seated on a commode with intrarectal balloon filled with 60 cc of air, and both the defecation pattern(s) and defecation index are calculated. A lack of coordinated push effort is highly suggestive of DD.21
Rectoanal Inhibitory Reflex (RAIR): RAIR describes the reflex relaxation of the internal anal sphincter after rectal distension. RAIR is dependent on intact autonomic ganglia and myenteric plexus25and is mediated by the release of nitric oxide and vasoactive intestinal peptide.26 The absence of RAIR suggests Hirschsprung disease.22.27.28
Rectal sensory testing: Intermittent balloon distension of the rectum with incremental volumes of air induces a range of rectal sensations that include first sensation, desire to defecate, urgency to defecate, and maximum tolerable volume. Rectal hyposensitivity is diagnosed when two or more sensory thresholds are higher than those seen in normal subjects29.30 and likely results from disruption of afferent gut-brain pathways, cortical perception/rectal wall dysfunction, or both.29 Rectal hyposensitivity affects 40% of patients with constipation30and is associated with DD but not delayed colonic transit.31 Rectal hyposensitivity may also be seen in patients with diabetes or fecal incontinence.18 About two-thirds of patients with rectal hyposensitivity have rectal hypercompliance, and some have megarectum.32 Some patients with DD have coexisting irritable bowel syndrome (IBS) and may have rectal hypersensitivity.18,33 Rectal compliance is measured alongside rectal sensitivity analysis by plotting a graph between the change in intraballoon volume (mL) and change in intrarectal pressures (mm Hg) during incremental balloon distensions.18.34 Rectal hypercompliance may be seen in megarectum and dyssynergic defecation.34,35 Rectal hypocompliance may be seen in patients with inflammatory bowel disease, postpelvic radiation, chronic ischemia, and advanced age.18
Balloon expulsion test: This test is performed by placing a plastic probe with a balloon in the rectum and filling it with 50 cc of warm water. Patients are given 5 minutes to expel the balloon while sitting on a commode. Balloon expulsion time of more than 1 minute suggests a diagnosis of DD,21 although 2 minutes provides a higher level of agreement with manometric findings.36 Balloon type and body position can influence the results.37 Inability to expel the balloon with normal manometric findings is considered an inconclusive finding per the recent London Classification (i.e., it may be associated with generation of anorectal symptoms, but the clinical relevance of this finding is unclear as it may also be seen in healthy subjects).22
Defecography
Defecography is a dynamic fluoroscopic study performed in the sitting position after injecting 150 mL of barium paste into the patient’s rectum. Defecography provides useful information about structural changes (e.g., rectoceles, enteroceles, rectal prolapse, and intussusception), DD, and descending perineum syndrome.38 Methodological differences, radiation exposure, and poor interobserver agreement have limited its wider use; therefore, anorectal manometry and the balloon expulsion test are recommended for the initial evaluation of DD.39 Magnetic resonance defecography may be more useful.17,38
Colonic transit studies
Colonic transit study can be assessed using radiopaque markers, wireless motility capsule, or scintigraphy. Wireless motility capsule and scintigraphy have the advantage of determining gastric, small bowel, and whole gut transit times as well. About two-thirds of patients with DD have slow transit constipation (STC),6 which improves after treatment of DD.40 Hence, in patients with chronic constipation, evaluation and management of DD is recommended first. If symptoms persist, then consider colonic transit assessment.41 Given the overlapping nature of the conditions, documentation of STC at the outset could facilitate treatment of both.
Diagnostic criteria for DD
Patients should fulfill the following criteria for diagnosis of DD:42,43
- Fulfill symptom(s) diagnostic criteria for functional constipation and/or constipation-predominant IBS.
- Demonstrate dyssynergic pattern (Types I-IV; Figure) during attempted defecation on manometry recordings.
- Meet one or more of the following criteria:
- Inability to expel an artificial stool (50 mL water-filled balloon) within 1 minute.
- Inability to evacuate or retention of 50% or more of barium during defecography. (Some institutions use a prolonged colonic transit time: greater than 5 markers or 20% or higher marker retention on a plain abdominal x-Ray at 120 hours after ingestion of one radio-opaque marker capsule containing 24 radio-opaque markers.)
Treatment of DD
The treatment modalities for DD depend on several factors: patient’s age, comorbidities, underlying pathophysiology, and patient expectations. Treatment options include standard management of constipation, but biofeedback therapy is the mainstay.
Standard management
Medications that cause or worsen constipation should be avoided. The patient should consume adequate fluid and exercise regularly. Patients should receive instructions for timed toilet training (twice daily, 30 minutes after meals). Patients should push at about 50%-70% of their ability for no longer than 5 minutes and avoid postponing defecation or use of digital maneuvers to facilitate defecation.42 The patients should take 25 g of soluble fiber (e.g., psyllium) daily. Of note, the benefits of fiber can take days to weeks44 and may be limited in patients with STC and DD.45 Medications including laxatives and intestinal secretagogues (lubiprostone, linaclotide, plecanatide), and enterokinetic agents (prucalopride) can be used as adjunct therapy for management of DD.42 Their use is titrated during and after biofeedback therapy and may decrease after successful treatment.46
Biofeedback therapy
Biofeedback therapy involves operant conditioning techniques using either a solid state anorectal manometry system, electromyography, simulated balloon, or home biofeedback training devices.42,47 The goals of biofeedback therapy are to correct the abdominal pelvic muscle discoordination during defecation and improve rectal sensation to stool if impaired. Biofeedback therapy involves patient education and active training (typically six sessions, 1-2 weeks apart, with each about 30-60 minutes long), followed by a reinforcement stage (three sessions at 3, 6, and 12 months), though there are variations in training protocols.42

The success of biofeedback therapy depends on the patient’s motivation and the therapist’s skills.42 Compared with standard therapy (diet, exercise, pharmacotherapy), biofeedback therapy provides sustained improvement of bowel symptoms and anorectal function. Up to 70%-80% of DD patients show significant improvement of symptoms in randomized controlled trials (Table).48-52 Biofeedback therapy may also improve dyspeptic symptoms.53 Patients with harder stool consistency, greater willingness to participate, lower baseline bowel satisfaction, lower baseline anal sphincter relaxation, and prolonged balloon expulsion time, as well as patients who used digital maneuvers for defection, more commonly respond to biofeedback therapy.54,55 Longstanding laxative use has been associated with decreased response to biofeedback therapy.56 In patients with rectal hyposensitivity, barostat-assisted sensory training is more effective than a hand-held syringe technique.30 In patients with constipation predominant IBS and rectal hyposensitivity, sensory adaption training is more efficacious and better tolerated than escitalopram.30 Biofeedback therapy was afforded a grade A recommendation for treatment of DD by the American and European Societies of Neurogastroenterology and Motility.57
The access to office-based biofeedback therapy may be limited because of costs and low availability. The time required to attend multiple sessions may be burdensome for some patients, especially if they are taking time off from work. A recent study showed that patients with higher level of education may be less likely to adhere to biofeedback therapy.58 Recently, home biofeedback was shown to be noninferior to office biofeedback and was more cost-effective, which provides an alternative option for treating more patients.59
Endoscopic/surgical options
Other less effective treatment options for DD include botulinum toxin injection and myectomy.60-62 Botulinum toxin injection appears to have mixed effects with less than 50% of patients reporting symptomatic improvement, and it may cause fecal incontinence.60,63
Conclusion
DD is a common yet poorly recognized cause of constipation. Its clinical presentation overlaps with other lower-GI disorders. Its diagnosis requires detailed history, digital rectal examination, prospective stool diaries, anorectal manometry, and balloon expulsion tests. Biofeedback therapy offers excellent and sustained symptomatic improvement; however, access to office-based biofeedback is limited, and there is an urgent need for home-based biofeedback therapy programs.59
Dr. Rao is J. Harold Harrison Distinguished University Chair, professor of medicine, director of neurogastroenterology/motility, and director of digestive health at the Digestive Health Clinical Research Center Augusta (Georgia) University. He is supported by National Institutes of Health grants R01DK121003-02 and U01DK115572. Dr. Jehangir is a gastroenterology and Hepatology Fellow at the Digestive Health Clinical Research Center at Augusta University. They reported having no conflicts of interest.
References
1. Peery AF, et al. Gastroenterology. 2012;143(5):1179-1187.e3 .
2. Curtin B, et al. J Neurogastroenterol Motil. 2020 30;26(4):423-36.
3. Suares NC & Ford AC. Am J Gastroenterol. 2011 Sep;106(9):1582-91.
4. Mertz H, et al. Am J Gastroenterol. 1999;94(3):609-15.
5. Rao SS, et al. Am J Gastroenterol. 1998;93(7):1042-50.
6. Rao SSC, et al. J Clin Gastroenterol. 2004;38(8):680-5.
7. Nojkov B, et al. Am J Gastroenterol. 2019;114(11):1772-7.
8. Heaton KW, et al. Gut. 1992;33(6):818-24.
9. Prichard DO & Bharucha AE. 2018 Oct 15;7:F1000 Faculty Rev-1640.
10. Ashraf W, et al. Am J Gastroenterol. 1996;91(1):26-32.
11. Agachan F, et al.. Dis Colon Rectum. 1996;39(6):681-5.
12. Frank L, et al. Scand J Gastroenterol. 1999;34(9):870-7.
13. Marquis P, et al. Scand J Gastroenterol. 2005;40(5):540-51.
14. Yan Y, et al. Gastroenterology. 2020;158(6):S-400.
15. Rao SSC. Am J Gastroenterol. 2018;113(5):635-8.
16. Tantiphlachiva K, et al. Digital rectal examination is a useful tool for identifying patients with dyssynergia. Clin Gastroenterol Hepatol. 2010;8(11):955-60.
17. Carrington EV, et al. Nat Rev Gastroenterol Hepatol. 2018;15(5):309-23.
18. Tetangco EP, et al. Performing and analyzing high-resolution anorectal manometry. NeuroGastroLatam Rev. 2018;2:120-32.
19. Lee YY, et al. Curr Gastroenterol Rep. 2013;15(12):360.
20. Sharma M, et al. Neurogastroenterol Motil. 2020;32(10):e13910.
21. Rao SSC, et al.. Am J Gastroenterol. 2006;101(12):2790-6.
22. Carrington EV, et al. Neurogastroenterol Motil. 2020;32(1):e13679.
23. Rao SSC. Gastroenterol Clin North Am. 2008;37(3):569-86, viii.
24. Rao SSC, et al. Gastroenterology. 2016;150(4):S158-9.
25. Guinet A, et al. Int J Colorectal Dis. 2011;26(4):507-13.
26. Rattan S, et al. Gastroenterology. 1992;103(1):43-50.
27. Remes-Troche JM & Rao SSC. 2008;2(3):323-35.
28. Zaafouri H, et al..Int J Surgery. 2015. 2(1):9-17.
29. Remes-Troche JM, et al. Dis Colon Rectum. 2010;53(7):1047-54.
30. Rao SSC, et al. Gastroenterology. 2013;144(5):S-363.
31. Yu T, et al. Medicine (Baltimore). 2016;95(19):e3667.
32. Gladman MA, et al. Neurogastroenterol Motil. 2009;21(5):508-16, e4-5.
33. Lee KJ, et al. Digestion. 2006;73(2-3):133-41 .
34. Rao SSC, et al. Neurogastroenterol Motil. 2002;14(5):553-9.
35. Coss-Adame E, et al.. Clin Gastroenterol Hepatol. 2015;13(6):1143-1150.e1.
36. Chiarioni G, et al. Clin Gastroenterol Hepatol. 2014;12(12):2049-54.
37. Gu G, et al. Gastroenterology. 2018;154(6):S-545–S-546.
38. Savoye-Collet C, et al.. Gastroenterol Clin North Am. 2008;37(3):553-67, viii.
39. Videlock EJ, et al. Neurogastroenterol Motil. 2013;25(6):509-20.
40. Rao SSC, et al. Neurogastroenterol Motil. 2004;16(5):589-96.
41. Wald A, et al. Am J Gastroenterol. 2014;109(8):1141-57 ; (Quiz) 1058.
42. Rao SSC & Patcharatrakul T. J Neurogastroenterol Motil. 2016;22(3):423-35.
43. Rao SS, et al. Functional Anorectal Disorders. Gastroenterology. 2016. S0016-5085(16)00175-X.
44. Bharucha AE, et al.. Gastroenterology. 2013;144(1):218-38.
45. Voderholzer WA, et al. Am J Gastroenterol. 1997;92(1):95-8.
46. Lee HJ, et al. Neurogastroenterol Motil. 2015;27(6):787-95.
47. Simón MA & Bueno AM. J Clin Gastroenterol. 2017;51(10):e90-4.
48. Chiarioni G,et al.. Gastroenterology. 2006;130(3):657-64.
49. Heymen S, et al.. Dis Colon Rectum. 2007;50(4):428-41.
50. Rao SSC, et al. Clin Gastroenterol Hepatol. 2007;5(3):331-8.
51. Rao SSC, et al. Am J Gastroenterol. 2010;105(4):890-6.
52. Patcharatrakul T, et al. Biofeedback therapy. In Clinical and basic neurogastroenterology and motility. India: Stacy Masucci; 2020:517-32.
53. Huaman J-W, et al. Clin Gastroenterol Hepatol. 2020;18(11):2463-2470.e1.
54. Patcharatrakul T, et al. Clin Gastroenterol Hepatol. 2018;16(5):715-21.
55. Chaudhry A, et al. Gastroenterology. 2020;158(6):S-382–S-383.
56. Shim LSE, et al. Aliment Pharmacol Ther. 2011;33(11):1245-51.
57. Rao SSC, et al. Neurogastroenterol Motil. 2015;27(5):594-609.
58. Jangsirikul S, et al. Gastroenterology. 2020;158(6):S-383.
59. Rao SSC, et al. Am J Gastroenterol. 2019;114(6):938-44.
60. Ron Y, et al.. Dis Colon Rectum. 2001;44(12):1821-6.
61. Podzemny V, et al. World J Gastroenterol. 2015;21(4):1053-60.
62. Faried M, et al. J Gastrointest Surg. 2010;14(8):1235-43.
63. Hallan RI, et al. Lancet. 1988;2(8613):714-7.
AGA News
AGAF applications now open
Applications are now open for the 2022 American Gastroenterological Association Fellowship cohort. AGA is proud to formally recognize its exemplary members whose accomplishments and contributions demonstrate a deep commitment to gastroenterology through the AGA Fellows Program. Those in clinical practice, education, or research (basic or clinical) are encouraged to apply today.
Longstanding members who apply and meet the program criteria are granted the distinguished honor of AGA Fellowship and receive the following:
- The privilege of using the designation “AGAF” in professional activities.
- An official certificate and pin denoting your status.
- International acknowledgment at Digestive Disease Week® (DDW).
- A listing on the AGA website alongside esteemed peers.
- A prewritten, fill-in press release and a digital badge to inform others of your accomplishment.
Apply for consideration and gain recognition worldwide for your commitment to the field. The deadline is Aug. 24.
Call for new AGA guideline topics
The AGA Institute Clinical Guidelines Committee wants your input on the next set of guidelines to be developed. By completing this online form, you can submit recommendations for guideline topics that will be developed within the next two years. The deadline to submit your ideas is Monday, May 3.
It’s easy – just take the following 3 steps to submit a guideline idea:
- Check out the guidelines that AGA has already developed or are in progress.
- Complete the survey. You can submit more than one guideline topic by filling out the form multiple times.
- Stay tuned for follow-up questions in case the committee needs more information on your recommendations.
The AGA Institute Clinical Guidelines Committee will review guideline topics in May, prioritizing and ranking topics based on the following criteria: prevalence of disease, resource utilization, variation in care, other existing guidelines, new data/changes in diagnosis or treatment, and potential for measure/quality development. Once vetted, four or more new guidelines will be recommended for development throughout the year. Complete the online survey at www.surveymonkey.com/r/AGAtopicsubmission
Get to know DDW® 2021 Virtual
The world’s premier meeting for gastroenterology, hepatology, endoscopy, and gastrointestinal surgery professionals will be a fully virtual event, May 21-23, 2021. We invite you to take advantage of this unique opportunity to exchange knowledge with colleagues from all over the world and explore the latest advances in the field – all from the convenience of your home. Plus, your registration grants you access to everything offered at Digestive Disease Week® (DDW) this year (no additional ticketed sessions). Learn more and register at ddw.org.
AGAF applications now open
Applications are now open for the 2022 American Gastroenterological Association Fellowship cohort. AGA is proud to formally recognize its exemplary members whose accomplishments and contributions demonstrate a deep commitment to gastroenterology through the AGA Fellows Program. Those in clinical practice, education, or research (basic or clinical) are encouraged to apply today.
Longstanding members who apply and meet the program criteria are granted the distinguished honor of AGA Fellowship and receive the following:
- The privilege of using the designation “AGAF” in professional activities.
- An official certificate and pin denoting your status.
- International acknowledgment at Digestive Disease Week® (DDW).
- A listing on the AGA website alongside esteemed peers.
- A prewritten, fill-in press release and a digital badge to inform others of your accomplishment.
Apply for consideration and gain recognition worldwide for your commitment to the field. The deadline is Aug. 24.
Call for new AGA guideline topics
The AGA Institute Clinical Guidelines Committee wants your input on the next set of guidelines to be developed. By completing this online form, you can submit recommendations for guideline topics that will be developed within the next two years. The deadline to submit your ideas is Monday, May 3.
It’s easy – just take the following 3 steps to submit a guideline idea:
- Check out the guidelines that AGA has already developed or are in progress.
- Complete the survey. You can submit more than one guideline topic by filling out the form multiple times.
- Stay tuned for follow-up questions in case the committee needs more information on your recommendations.
The AGA Institute Clinical Guidelines Committee will review guideline topics in May, prioritizing and ranking topics based on the following criteria: prevalence of disease, resource utilization, variation in care, other existing guidelines, new data/changes in diagnosis or treatment, and potential for measure/quality development. Once vetted, four or more new guidelines will be recommended for development throughout the year. Complete the online survey at www.surveymonkey.com/r/AGAtopicsubmission
Get to know DDW® 2021 Virtual
The world’s premier meeting for gastroenterology, hepatology, endoscopy, and gastrointestinal surgery professionals will be a fully virtual event, May 21-23, 2021. We invite you to take advantage of this unique opportunity to exchange knowledge with colleagues from all over the world and explore the latest advances in the field – all from the convenience of your home. Plus, your registration grants you access to everything offered at Digestive Disease Week® (DDW) this year (no additional ticketed sessions). Learn more and register at ddw.org.
AGAF applications now open
Applications are now open for the 2022 American Gastroenterological Association Fellowship cohort. AGA is proud to formally recognize its exemplary members whose accomplishments and contributions demonstrate a deep commitment to gastroenterology through the AGA Fellows Program. Those in clinical practice, education, or research (basic or clinical) are encouraged to apply today.
Longstanding members who apply and meet the program criteria are granted the distinguished honor of AGA Fellowship and receive the following:
- The privilege of using the designation “AGAF” in professional activities.
- An official certificate and pin denoting your status.
- International acknowledgment at Digestive Disease Week® (DDW).
- A listing on the AGA website alongside esteemed peers.
- A prewritten, fill-in press release and a digital badge to inform others of your accomplishment.
Apply for consideration and gain recognition worldwide for your commitment to the field. The deadline is Aug. 24.
Call for new AGA guideline topics
The AGA Institute Clinical Guidelines Committee wants your input on the next set of guidelines to be developed. By completing this online form, you can submit recommendations for guideline topics that will be developed within the next two years. The deadline to submit your ideas is Monday, May 3.
It’s easy – just take the following 3 steps to submit a guideline idea:
- Check out the guidelines that AGA has already developed or are in progress.
- Complete the survey. You can submit more than one guideline topic by filling out the form multiple times.
- Stay tuned for follow-up questions in case the committee needs more information on your recommendations.
The AGA Institute Clinical Guidelines Committee will review guideline topics in May, prioritizing and ranking topics based on the following criteria: prevalence of disease, resource utilization, variation in care, other existing guidelines, new data/changes in diagnosis or treatment, and potential for measure/quality development. Once vetted, four or more new guidelines will be recommended for development throughout the year. Complete the online survey at www.surveymonkey.com/r/AGAtopicsubmission
Get to know DDW® 2021 Virtual
The world’s premier meeting for gastroenterology, hepatology, endoscopy, and gastrointestinal surgery professionals will be a fully virtual event, May 21-23, 2021. We invite you to take advantage of this unique opportunity to exchange knowledge with colleagues from all over the world and explore the latest advances in the field – all from the convenience of your home. Plus, your registration grants you access to everything offered at Digestive Disease Week® (DDW) this year (no additional ticketed sessions). Learn more and register at ddw.org.
May 2021 – ICYMI
Gastroenterology
February 2021
Worldwide burden of, risk factors for, and trends in pancreatic cancer
Huang J et al. Gastroenterology. 2021 Mar 1;160(4):744-54. doi: 10.1053/j.gastro.2020.10.007.
Fibrates for Itch (FITCH) in fibrosing cholangiopathies: A double-blind, randomized, placebo-controlled trial
de Vries E et al. Gastroenterology. 2021 Mar 1;160(4):734-43.e6. doi: 10.1053/j.gastro.2020.10.001.
March 2021
How to integrate a medical ethics curriculum into gastroenterology fellowships
Rao VL et al. Gastroenterology. 2021 Mar 1;160(4):1003-6. https://doi.org/10.1053/j.gastro.2021.01.211.
Colonoscopist performance and colorectal cancer risk after adenoma removal to stratify surveillance: two nationwide observational studies
Wieszczy P et al. Gastroenterology. 2021 Mar 1;160(4):1067-74. doi: 10.1053/j.gastro.2020.10.009.
Pregnancy and neonatal outcomes after fetal exposure to biologics and thiopurines among women with inflammatory bowel disease
Mahadevan U et al. Gastroenterology. 2021 Mar 1;160(4):1131-9. doi: 10.1053/j.gastro.2020.11.038.
April 2021
AGA Clinical Practice Guidelines on intragastric balloons in the management of obesity
Muniraj T et al. Gastroenterology. 2021 Apr 1;160(5):1799-808. doi: 10.1053/j.gastro.2021.03.003.
How to strategically build your network for early career gastroenterologists
Gaidos JKJ et al. Gastroenterology. 2021 Apr 1;160(5):1461-6. doi: 10.1053/j.gastro.2021.01.025.
The microbiota-gut-brain axis: From motility to mood
Margolis KG et al. Gastroenterology. 2021 Apr 1;160(5):1486-501. doi: 10.1053/j.gastro.2020.10.066.
The association of histologic and noninvasive tests with adverse clinical and patient-reported outcomes in patients with advanced fibrosis due to nonalcoholic steatohepatitis
Younossi ZM et al. Gastroenterology. 2021 Apr 1;160(5):1608-19. doi: 10.1053/j.gastro.2020.12.003.
Clinical Gastroenterology and Hepatology
February 2021
Management of chronic abdominal distension and bloating
Lacy BE et al. Clin Gastroenterol Hepatol. 2021 Feb 1;19(2):219-31. doi: 10.1016/j.cgh.2020.03.056.
Prevalence of gastric intestinal metaplasia in a multiethnic US veterans population
Nguyen TH et al. Clin Gastroenterol Hepatol. 2021 Feb 1;19(2):269-76. doi: 10.1016/j.cgh.2020.03.015.
Rome IV functional gastrointestinal disorders and health impairment in subjects with hypermobility spectrum disorders or hypermobile Ehlers-Danlos syndrome
Lam CY et al. Clin Gastroenterol Hepatol. 2021 Feb 1;19(2):277-87. doi: 10.1016/j.cgh.2020.02.034.
Factors that affect adequacy of colon cleansing for colonoscopy in hospitalized patients
Fucci L et al. Clin Gastroenterol Hepatol. 2021 Feb 1;19(2):339-48. doi: org/10.1016/j.cgh.2020.02.055.
March 2021
Real-world gluten exposure in patients with celiac disease on gluten-free diets, determined from gliadin immunogenic peptides in urine and fecal samples
Stefanolo JP et al. Clin Gastroenterol Hepatol. 2021 Mar 1;19(3):484-91. doi: 10.1016/j.cgh.2020.03.038.
Factors associated with response to anorectal biofeedback therapy in patients with fecal incontinence
Mazor Y et al. Clin Gastroenterol Hepatol. 2021 Mar 1;19(3):492-502. doi: 10.1016/j.cgh.2020.03.050.
April 2021
Long-term outcome of gastric per-oral endoscopic pyloromyotomy in treatment of gastroparesis
Abdelfatah MM et al. Clin Gastroenterol Hepatol. 2021 Apr 1;19(4):816-24. doi: 10.1016/j.cgh.2020.05.039.
What gastroenterologists should know about COVID-19 vaccines
Rolak S et al. Clin Gastroenterol Hepatol. 2021 Apr 1;19(4):657-61. doi: 10.1016/j.cgh.2021.01.001.
No benefit of concomitant immunomodulator therapy on efficacy of biologics that are not tumor necrosis factor antagonists in patients with inflammatory bowel diseases: A meta-analysis
Yzet C et al. Clin Gastroenterol Hepatol. 2021 Apr 1;19(4):668-78. doi: 10.1016/j.cgh.2020.06.071.
Patient safety reporting in GI: All hands on deck
Wall A and Kothari D. Clin Gastroenterol Hepatol. 2021 Apr 1;19(4):626-32. doi: 10.1016/j.cgh.2020.11.007.
Techniques and Innovations in Gastrointestinal Endoscopy
Barriers and pitfalls for artificial intelligence in gastroenterology: Ethical and regulatory issues
Ahmad OF et al. Tech Innov Gastrointest Endosc. 2020 Apr 1;22(2):80-4. doi: 10.1016/j.tgie.2019.150636.
Development of a scoring system to predict a positive diagnosis on video capsule endoscopy for suspected small bowel bleeding
Marya NB et al. Tech Innov Gastrointest Endosc. 2020 Oct 1;22(4):178-84. doi: 10.1016/j.tige.2020.06.001.
Training for Advanced Endoscopic Imaging in Gastrointestinal Diseases
Hoogenboom SA et al. Tech Innov Gastrointest Endosc. 2021 Jan 1;23(1):99-106. doi: 10.1016/j.tige.2020.09.001.
Chromoendoscopy techniques in imaging of colorectal polyps and cancer: Overview and practical applications for detection and characterization.
Rivero-Sanchez L et al. Tech Innov Gastrointest Endosc. 2021 Jan 1;23(1):30-41. doi: 10.1016/j.tige.2020.10.006.
Gastroenterology
February 2021
Worldwide burden of, risk factors for, and trends in pancreatic cancer
Huang J et al. Gastroenterology. 2021 Mar 1;160(4):744-54. doi: 10.1053/j.gastro.2020.10.007.
Fibrates for Itch (FITCH) in fibrosing cholangiopathies: A double-blind, randomized, placebo-controlled trial
de Vries E et al. Gastroenterology. 2021 Mar 1;160(4):734-43.e6. doi: 10.1053/j.gastro.2020.10.001.
March 2021
How to integrate a medical ethics curriculum into gastroenterology fellowships
Rao VL et al. Gastroenterology. 2021 Mar 1;160(4):1003-6. https://doi.org/10.1053/j.gastro.2021.01.211.
Colonoscopist performance and colorectal cancer risk after adenoma removal to stratify surveillance: two nationwide observational studies
Wieszczy P et al. Gastroenterology. 2021 Mar 1;160(4):1067-74. doi: 10.1053/j.gastro.2020.10.009.
Pregnancy and neonatal outcomes after fetal exposure to biologics and thiopurines among women with inflammatory bowel disease
Mahadevan U et al. Gastroenterology. 2021 Mar 1;160(4):1131-9. doi: 10.1053/j.gastro.2020.11.038.
April 2021
AGA Clinical Practice Guidelines on intragastric balloons in the management of obesity
Muniraj T et al. Gastroenterology. 2021 Apr 1;160(5):1799-808. doi: 10.1053/j.gastro.2021.03.003.
How to strategically build your network for early career gastroenterologists
Gaidos JKJ et al. Gastroenterology. 2021 Apr 1;160(5):1461-6. doi: 10.1053/j.gastro.2021.01.025.
The microbiota-gut-brain axis: From motility to mood
Margolis KG et al. Gastroenterology. 2021 Apr 1;160(5):1486-501. doi: 10.1053/j.gastro.2020.10.066.
The association of histologic and noninvasive tests with adverse clinical and patient-reported outcomes in patients with advanced fibrosis due to nonalcoholic steatohepatitis
Younossi ZM et al. Gastroenterology. 2021 Apr 1;160(5):1608-19. doi: 10.1053/j.gastro.2020.12.003.
Clinical Gastroenterology and Hepatology
February 2021
Management of chronic abdominal distension and bloating
Lacy BE et al. Clin Gastroenterol Hepatol. 2021 Feb 1;19(2):219-31. doi: 10.1016/j.cgh.2020.03.056.
Prevalence of gastric intestinal metaplasia in a multiethnic US veterans population
Nguyen TH et al. Clin Gastroenterol Hepatol. 2021 Feb 1;19(2):269-76. doi: 10.1016/j.cgh.2020.03.015.
Rome IV functional gastrointestinal disorders and health impairment in subjects with hypermobility spectrum disorders or hypermobile Ehlers-Danlos syndrome
Lam CY et al. Clin Gastroenterol Hepatol. 2021 Feb 1;19(2):277-87. doi: 10.1016/j.cgh.2020.02.034.
Factors that affect adequacy of colon cleansing for colonoscopy in hospitalized patients
Fucci L et al. Clin Gastroenterol Hepatol. 2021 Feb 1;19(2):339-48. doi: org/10.1016/j.cgh.2020.02.055.
March 2021
Real-world gluten exposure in patients with celiac disease on gluten-free diets, determined from gliadin immunogenic peptides in urine and fecal samples
Stefanolo JP et al. Clin Gastroenterol Hepatol. 2021 Mar 1;19(3):484-91. doi: 10.1016/j.cgh.2020.03.038.
Factors associated with response to anorectal biofeedback therapy in patients with fecal incontinence
Mazor Y et al. Clin Gastroenterol Hepatol. 2021 Mar 1;19(3):492-502. doi: 10.1016/j.cgh.2020.03.050.
April 2021
Long-term outcome of gastric per-oral endoscopic pyloromyotomy in treatment of gastroparesis
Abdelfatah MM et al. Clin Gastroenterol Hepatol. 2021 Apr 1;19(4):816-24. doi: 10.1016/j.cgh.2020.05.039.
What gastroenterologists should know about COVID-19 vaccines
Rolak S et al. Clin Gastroenterol Hepatol. 2021 Apr 1;19(4):657-61. doi: 10.1016/j.cgh.2021.01.001.
No benefit of concomitant immunomodulator therapy on efficacy of biologics that are not tumor necrosis factor antagonists in patients with inflammatory bowel diseases: A meta-analysis
Yzet C et al. Clin Gastroenterol Hepatol. 2021 Apr 1;19(4):668-78. doi: 10.1016/j.cgh.2020.06.071.
Patient safety reporting in GI: All hands on deck
Wall A and Kothari D. Clin Gastroenterol Hepatol. 2021 Apr 1;19(4):626-32. doi: 10.1016/j.cgh.2020.11.007.
Techniques and Innovations in Gastrointestinal Endoscopy
Barriers and pitfalls for artificial intelligence in gastroenterology: Ethical and regulatory issues
Ahmad OF et al. Tech Innov Gastrointest Endosc. 2020 Apr 1;22(2):80-4. doi: 10.1016/j.tgie.2019.150636.
Development of a scoring system to predict a positive diagnosis on video capsule endoscopy for suspected small bowel bleeding
Marya NB et al. Tech Innov Gastrointest Endosc. 2020 Oct 1;22(4):178-84. doi: 10.1016/j.tige.2020.06.001.
Training for Advanced Endoscopic Imaging in Gastrointestinal Diseases
Hoogenboom SA et al. Tech Innov Gastrointest Endosc. 2021 Jan 1;23(1):99-106. doi: 10.1016/j.tige.2020.09.001.
Chromoendoscopy techniques in imaging of colorectal polyps and cancer: Overview and practical applications for detection and characterization.
Rivero-Sanchez L et al. Tech Innov Gastrointest Endosc. 2021 Jan 1;23(1):30-41. doi: 10.1016/j.tige.2020.10.006.
Gastroenterology
February 2021
Worldwide burden of, risk factors for, and trends in pancreatic cancer
Huang J et al. Gastroenterology. 2021 Mar 1;160(4):744-54. doi: 10.1053/j.gastro.2020.10.007.
Fibrates for Itch (FITCH) in fibrosing cholangiopathies: A double-blind, randomized, placebo-controlled trial
de Vries E et al. Gastroenterology. 2021 Mar 1;160(4):734-43.e6. doi: 10.1053/j.gastro.2020.10.001.
March 2021
How to integrate a medical ethics curriculum into gastroenterology fellowships
Rao VL et al. Gastroenterology. 2021 Mar 1;160(4):1003-6. https://doi.org/10.1053/j.gastro.2021.01.211.
Colonoscopist performance and colorectal cancer risk after adenoma removal to stratify surveillance: two nationwide observational studies
Wieszczy P et al. Gastroenterology. 2021 Mar 1;160(4):1067-74. doi: 10.1053/j.gastro.2020.10.009.
Pregnancy and neonatal outcomes after fetal exposure to biologics and thiopurines among women with inflammatory bowel disease
Mahadevan U et al. Gastroenterology. 2021 Mar 1;160(4):1131-9. doi: 10.1053/j.gastro.2020.11.038.
April 2021
AGA Clinical Practice Guidelines on intragastric balloons in the management of obesity
Muniraj T et al. Gastroenterology. 2021 Apr 1;160(5):1799-808. doi: 10.1053/j.gastro.2021.03.003.
How to strategically build your network for early career gastroenterologists
Gaidos JKJ et al. Gastroenterology. 2021 Apr 1;160(5):1461-6. doi: 10.1053/j.gastro.2021.01.025.
The microbiota-gut-brain axis: From motility to mood
Margolis KG et al. Gastroenterology. 2021 Apr 1;160(5):1486-501. doi: 10.1053/j.gastro.2020.10.066.
The association of histologic and noninvasive tests with adverse clinical and patient-reported outcomes in patients with advanced fibrosis due to nonalcoholic steatohepatitis
Younossi ZM et al. Gastroenterology. 2021 Apr 1;160(5):1608-19. doi: 10.1053/j.gastro.2020.12.003.
Clinical Gastroenterology and Hepatology
February 2021
Management of chronic abdominal distension and bloating
Lacy BE et al. Clin Gastroenterol Hepatol. 2021 Feb 1;19(2):219-31. doi: 10.1016/j.cgh.2020.03.056.
Prevalence of gastric intestinal metaplasia in a multiethnic US veterans population
Nguyen TH et al. Clin Gastroenterol Hepatol. 2021 Feb 1;19(2):269-76. doi: 10.1016/j.cgh.2020.03.015.
Rome IV functional gastrointestinal disorders and health impairment in subjects with hypermobility spectrum disorders or hypermobile Ehlers-Danlos syndrome
Lam CY et al. Clin Gastroenterol Hepatol. 2021 Feb 1;19(2):277-87. doi: 10.1016/j.cgh.2020.02.034.
Factors that affect adequacy of colon cleansing for colonoscopy in hospitalized patients
Fucci L et al. Clin Gastroenterol Hepatol. 2021 Feb 1;19(2):339-48. doi: org/10.1016/j.cgh.2020.02.055.
March 2021
Real-world gluten exposure in patients with celiac disease on gluten-free diets, determined from gliadin immunogenic peptides in urine and fecal samples
Stefanolo JP et al. Clin Gastroenterol Hepatol. 2021 Mar 1;19(3):484-91. doi: 10.1016/j.cgh.2020.03.038.
Factors associated with response to anorectal biofeedback therapy in patients with fecal incontinence
Mazor Y et al. Clin Gastroenterol Hepatol. 2021 Mar 1;19(3):492-502. doi: 10.1016/j.cgh.2020.03.050.
April 2021
Long-term outcome of gastric per-oral endoscopic pyloromyotomy in treatment of gastroparesis
Abdelfatah MM et al. Clin Gastroenterol Hepatol. 2021 Apr 1;19(4):816-24. doi: 10.1016/j.cgh.2020.05.039.
What gastroenterologists should know about COVID-19 vaccines
Rolak S et al. Clin Gastroenterol Hepatol. 2021 Apr 1;19(4):657-61. doi: 10.1016/j.cgh.2021.01.001.
No benefit of concomitant immunomodulator therapy on efficacy of biologics that are not tumor necrosis factor antagonists in patients with inflammatory bowel diseases: A meta-analysis
Yzet C et al. Clin Gastroenterol Hepatol. 2021 Apr 1;19(4):668-78. doi: 10.1016/j.cgh.2020.06.071.
Patient safety reporting in GI: All hands on deck
Wall A and Kothari D. Clin Gastroenterol Hepatol. 2021 Apr 1;19(4):626-32. doi: 10.1016/j.cgh.2020.11.007.
Techniques and Innovations in Gastrointestinal Endoscopy
Barriers and pitfalls for artificial intelligence in gastroenterology: Ethical and regulatory issues
Ahmad OF et al. Tech Innov Gastrointest Endosc. 2020 Apr 1;22(2):80-4. doi: 10.1016/j.tgie.2019.150636.
Development of a scoring system to predict a positive diagnosis on video capsule endoscopy for suspected small bowel bleeding
Marya NB et al. Tech Innov Gastrointest Endosc. 2020 Oct 1;22(4):178-84. doi: 10.1016/j.tige.2020.06.001.
Training for Advanced Endoscopic Imaging in Gastrointestinal Diseases
Hoogenboom SA et al. Tech Innov Gastrointest Endosc. 2021 Jan 1;23(1):99-106. doi: 10.1016/j.tige.2020.09.001.
Chromoendoscopy techniques in imaging of colorectal polyps and cancer: Overview and practical applications for detection and characterization.
Rivero-Sanchez L et al. Tech Innov Gastrointest Endosc. 2021 Jan 1;23(1):30-41. doi: 10.1016/j.tige.2020.10.006.
Our role in colorectal cancer prevention education
Each year in the month of March, advocates, physicians, and health care educators come together to promote the importance of colorectal cancer screening during Colorectal Cancer Awareness Month. As independent GI physicians, we work within our communities to promote colorectal screening year-round.
We also understand that our education efforts do not end with the people in our community who need to be screened. Independent GI practices also engage with primary care physicians who often initiate conversations about available screening tests and when people should be screened.
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States.1 It is expected to kill more than 50,000 Americans this year alone.2 This is why screening for colorectal cancer is so important. The American Cancer Society recommends screening for all average-risk patients aged 45-75 years.3
The good news? If caught early, the survival rate is very high. In fact, when caught early, the five-year survival rate is 90 percent. Unfortunately, one in three Americans who are eligible for screenings do not get screened. For certain groups, there are larger numbers of people who are not getting screened. And there are groups for whom the death rates from colorectal cancer are much higher.
Disparities in colorectal cancer screenings
According to the American Cancer Society, Blacks and Hispanics are less likely to receive prompt follow up after an abnormal CRC screening result and are more likely to be diagnosed with late-stage cancer.4 African Americans have the highest death rate when compared with all other racial groups in the United States. American Indians and Alaska Natives are the only groups for which CRC death rates are not declining.
There are many factors that drive disparities, but the main factors seem to be socioeconomic status and differences in access to early detection and treatment. While some of these issues are complex and difficult to change, increasing awareness and providing education can be easier than you might think.
Working with your community as a private GI practitioner
To address economic factors, Atlanta Gastroenterology Associates has a program that provides resources on a sliding fee scale to people in our community who do not have insurance and are concerned about having to pay for CRC screening out of pocket. This includes the costs for anesthesia, colonoscopy, and pathology services.
We also have a Direct Access Program, which allows people to self-schedule a screening and fill out a survey that assesses their candidacy for screening colonoscopy. This allows our patients to bypass an initial prescreening office visit and associated copays. Patients are provided instructions for colonoscopy prep and show up for the colonoscopy on the day of their procedure. When the colonoscopy is completed, we give them a patient education card on CRC screening to share with friends and family members who need to be screened.
Atlanta is a very diverse city, and representation is important. But, fortunately, the size of Atlanta Gastroenterology Associates allows us to have representation within many communities. We attend a significant number of health fairs and community events, many of which are sourced internally. Our physicians and staff are members of churches and social groups that we work with to provide screening materials and conduct informational events.
Word of mouth is the best advertising, and it works the same way with health education. There are a lot of myths that we must debunk. And in many of our communities, people are worrying about paying the bills to keep the lights on – they are not thinking about getting screened. But, if they hear from a friend or family member that their screening colonoscopy was a good experience and that resources were provided to help pay for the procedure, it really does make a difference.
You do not need to join a large practice to have an impact. All over the country, there are community groups working to increase screening rates, and engaging with those groups is a good start. During the COVID-19 pandemic, we are all using social media and other platforms to connect. You do not need a lot of resources to set up a Zoom meeting with people in your community to discuss CRC screening.
Engaging with referring physicians
As a private practice practitioner, part of growing your practice is engaging with the primary care physicians in your area to ensure that they are up to date on the latest research in CRC screening and that they are discussing available screening options with their patients.
Preventing cancer should always be our first goal. Most CRCs begin as a polyp. Finding, quantifying, localizing, and removing polyps through screening colonoscopy is the most effective strategy for preventing this cancer. That is why colonoscopy remains the preferred method for colon cancer screening.
The Multi-Society Task Force on Colorectal Cancer recommends that, in sequential approaches, physicians should offer colonoscopy first.5 For patients who decline to have a colonoscopy, the FIT test should be offered next, followed by second-tier tests such as Cologuard and CT colonography for patients who decline both first-tier options.
Beyond the science of colorectal screening, we want to make sure that our primary care partners are aware of the disparities that exist – and which patients are at higher risk – so that they can engage with their patients to encourage screening.
For example, in our practice, we work with local Asian American community groups to help make sure that the “model minority” myth – that Asian Americans are healthier, wealthier, and better educated than the average American – does not become a barrier to screening. While Asian Americans may have lower overall rates of some types of cancer, there are some cancers that disproportionately affect certain Asian American groups. Rates of CRC in Japanese men, for instance, are 23% higher than in non-Hispanic Whites.
Additionally, we work with our primary care colleagues to help them understand that patients may have insurance considerations when choosing a test. While insurance typically covers 100% of a preventive screening test, a follow-up colonoscopy for a positive stool test is considered a diagnostic or therapeutic service and may not be fully covered. Medicare patients may face a coinsurance bill after their follow-up colonoscopy for a positive stool test. Legislation was passed last year to remove this barrier, but Medicare beneficiaries may have some out-of-pocket costs until it is completely removed in 2030.
Are you joining a practice that supports CRC education? Just ask!
We all want to work for an organization that aligns with our core values, and for GI physicians like us, CRC screening is a core component of our everyday work.
If you are considering joining a private practice, ask how the practice is doing with their CRC awareness programs and if it leads to increases in screenings. Inquire about the groups that are being engaged with and why. Is the practice focused on communities that have disparities in screening and treatment, and is it able to complete the entire screening process for individuals in communities that are more adversely affected by colorectal cancer?
We have found that candidates who have the most success in our practice are people who want to work at Atlanta Gastroenterology Associates but are also active in their communities and have a sense of how they want to be of service in their community. It is a sign of leadership in people – the idea that they are really going to get out and network and build a practice that serves everyone in their community. These actions make a difference in getting more people screened and in decreasing the disparities that exist.
Dr. Aja McCutchen is the chair of the quality committee at Atlanta Gastroenterology Associates and serves as chair of the Digestive Health Physicians Association’s Diversity, Equity and Inclusion Committee. She reports having nothing to disclose.
References
1. Siegel RL et al. CA Cancer J Clin. 2018 Jan;68(1):7-30.
2. Key Statistics for Colorectal Cancer. Cancer.org.
3. Wolf AMD et al. CA Cancer J Clin. 2018 Jul;68(4):250-281.
4. American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022.
5. Rex DK et al. Am J Gastroenterol. 2017;112(7):1016-30.
Each year in the month of March, advocates, physicians, and health care educators come together to promote the importance of colorectal cancer screening during Colorectal Cancer Awareness Month. As independent GI physicians, we work within our communities to promote colorectal screening year-round.
We also understand that our education efforts do not end with the people in our community who need to be screened. Independent GI practices also engage with primary care physicians who often initiate conversations about available screening tests and when people should be screened.
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States.1 It is expected to kill more than 50,000 Americans this year alone.2 This is why screening for colorectal cancer is so important. The American Cancer Society recommends screening for all average-risk patients aged 45-75 years.3
The good news? If caught early, the survival rate is very high. In fact, when caught early, the five-year survival rate is 90 percent. Unfortunately, one in three Americans who are eligible for screenings do not get screened. For certain groups, there are larger numbers of people who are not getting screened. And there are groups for whom the death rates from colorectal cancer are much higher.
Disparities in colorectal cancer screenings
According to the American Cancer Society, Blacks and Hispanics are less likely to receive prompt follow up after an abnormal CRC screening result and are more likely to be diagnosed with late-stage cancer.4 African Americans have the highest death rate when compared with all other racial groups in the United States. American Indians and Alaska Natives are the only groups for which CRC death rates are not declining.
There are many factors that drive disparities, but the main factors seem to be socioeconomic status and differences in access to early detection and treatment. While some of these issues are complex and difficult to change, increasing awareness and providing education can be easier than you might think.
Working with your community as a private GI practitioner
To address economic factors, Atlanta Gastroenterology Associates has a program that provides resources on a sliding fee scale to people in our community who do not have insurance and are concerned about having to pay for CRC screening out of pocket. This includes the costs for anesthesia, colonoscopy, and pathology services.
We also have a Direct Access Program, which allows people to self-schedule a screening and fill out a survey that assesses their candidacy for screening colonoscopy. This allows our patients to bypass an initial prescreening office visit and associated copays. Patients are provided instructions for colonoscopy prep and show up for the colonoscopy on the day of their procedure. When the colonoscopy is completed, we give them a patient education card on CRC screening to share with friends and family members who need to be screened.
Atlanta is a very diverse city, and representation is important. But, fortunately, the size of Atlanta Gastroenterology Associates allows us to have representation within many communities. We attend a significant number of health fairs and community events, many of which are sourced internally. Our physicians and staff are members of churches and social groups that we work with to provide screening materials and conduct informational events.
Word of mouth is the best advertising, and it works the same way with health education. There are a lot of myths that we must debunk. And in many of our communities, people are worrying about paying the bills to keep the lights on – they are not thinking about getting screened. But, if they hear from a friend or family member that their screening colonoscopy was a good experience and that resources were provided to help pay for the procedure, it really does make a difference.
You do not need to join a large practice to have an impact. All over the country, there are community groups working to increase screening rates, and engaging with those groups is a good start. During the COVID-19 pandemic, we are all using social media and other platforms to connect. You do not need a lot of resources to set up a Zoom meeting with people in your community to discuss CRC screening.
Engaging with referring physicians
As a private practice practitioner, part of growing your practice is engaging with the primary care physicians in your area to ensure that they are up to date on the latest research in CRC screening and that they are discussing available screening options with their patients.
Preventing cancer should always be our first goal. Most CRCs begin as a polyp. Finding, quantifying, localizing, and removing polyps through screening colonoscopy is the most effective strategy for preventing this cancer. That is why colonoscopy remains the preferred method for colon cancer screening.
The Multi-Society Task Force on Colorectal Cancer recommends that, in sequential approaches, physicians should offer colonoscopy first.5 For patients who decline to have a colonoscopy, the FIT test should be offered next, followed by second-tier tests such as Cologuard and CT colonography for patients who decline both first-tier options.
Beyond the science of colorectal screening, we want to make sure that our primary care partners are aware of the disparities that exist – and which patients are at higher risk – so that they can engage with their patients to encourage screening.
For example, in our practice, we work with local Asian American community groups to help make sure that the “model minority” myth – that Asian Americans are healthier, wealthier, and better educated than the average American – does not become a barrier to screening. While Asian Americans may have lower overall rates of some types of cancer, there are some cancers that disproportionately affect certain Asian American groups. Rates of CRC in Japanese men, for instance, are 23% higher than in non-Hispanic Whites.
Additionally, we work with our primary care colleagues to help them understand that patients may have insurance considerations when choosing a test. While insurance typically covers 100% of a preventive screening test, a follow-up colonoscopy for a positive stool test is considered a diagnostic or therapeutic service and may not be fully covered. Medicare patients may face a coinsurance bill after their follow-up colonoscopy for a positive stool test. Legislation was passed last year to remove this barrier, but Medicare beneficiaries may have some out-of-pocket costs until it is completely removed in 2030.
Are you joining a practice that supports CRC education? Just ask!
We all want to work for an organization that aligns with our core values, and for GI physicians like us, CRC screening is a core component of our everyday work.
If you are considering joining a private practice, ask how the practice is doing with their CRC awareness programs and if it leads to increases in screenings. Inquire about the groups that are being engaged with and why. Is the practice focused on communities that have disparities in screening and treatment, and is it able to complete the entire screening process for individuals in communities that are more adversely affected by colorectal cancer?
We have found that candidates who have the most success in our practice are people who want to work at Atlanta Gastroenterology Associates but are also active in their communities and have a sense of how they want to be of service in their community. It is a sign of leadership in people – the idea that they are really going to get out and network and build a practice that serves everyone in their community. These actions make a difference in getting more people screened and in decreasing the disparities that exist.
Dr. Aja McCutchen is the chair of the quality committee at Atlanta Gastroenterology Associates and serves as chair of the Digestive Health Physicians Association’s Diversity, Equity and Inclusion Committee. She reports having nothing to disclose.
References
1. Siegel RL et al. CA Cancer J Clin. 2018 Jan;68(1):7-30.
2. Key Statistics for Colorectal Cancer. Cancer.org.
3. Wolf AMD et al. CA Cancer J Clin. 2018 Jul;68(4):250-281.
4. American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022.
5. Rex DK et al. Am J Gastroenterol. 2017;112(7):1016-30.
Each year in the month of March, advocates, physicians, and health care educators come together to promote the importance of colorectal cancer screening during Colorectal Cancer Awareness Month. As independent GI physicians, we work within our communities to promote colorectal screening year-round.
We also understand that our education efforts do not end with the people in our community who need to be screened. Independent GI practices also engage with primary care physicians who often initiate conversations about available screening tests and when people should be screened.
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States.1 It is expected to kill more than 50,000 Americans this year alone.2 This is why screening for colorectal cancer is so important. The American Cancer Society recommends screening for all average-risk patients aged 45-75 years.3
The good news? If caught early, the survival rate is very high. In fact, when caught early, the five-year survival rate is 90 percent. Unfortunately, one in three Americans who are eligible for screenings do not get screened. For certain groups, there are larger numbers of people who are not getting screened. And there are groups for whom the death rates from colorectal cancer are much higher.
Disparities in colorectal cancer screenings
According to the American Cancer Society, Blacks and Hispanics are less likely to receive prompt follow up after an abnormal CRC screening result and are more likely to be diagnosed with late-stage cancer.4 African Americans have the highest death rate when compared with all other racial groups in the United States. American Indians and Alaska Natives are the only groups for which CRC death rates are not declining.
There are many factors that drive disparities, but the main factors seem to be socioeconomic status and differences in access to early detection and treatment. While some of these issues are complex and difficult to change, increasing awareness and providing education can be easier than you might think.
Working with your community as a private GI practitioner
To address economic factors, Atlanta Gastroenterology Associates has a program that provides resources on a sliding fee scale to people in our community who do not have insurance and are concerned about having to pay for CRC screening out of pocket. This includes the costs for anesthesia, colonoscopy, and pathology services.
We also have a Direct Access Program, which allows people to self-schedule a screening and fill out a survey that assesses their candidacy for screening colonoscopy. This allows our patients to bypass an initial prescreening office visit and associated copays. Patients are provided instructions for colonoscopy prep and show up for the colonoscopy on the day of their procedure. When the colonoscopy is completed, we give them a patient education card on CRC screening to share with friends and family members who need to be screened.
Atlanta is a very diverse city, and representation is important. But, fortunately, the size of Atlanta Gastroenterology Associates allows us to have representation within many communities. We attend a significant number of health fairs and community events, many of which are sourced internally. Our physicians and staff are members of churches and social groups that we work with to provide screening materials and conduct informational events.
Word of mouth is the best advertising, and it works the same way with health education. There are a lot of myths that we must debunk. And in many of our communities, people are worrying about paying the bills to keep the lights on – they are not thinking about getting screened. But, if they hear from a friend or family member that their screening colonoscopy was a good experience and that resources were provided to help pay for the procedure, it really does make a difference.
You do not need to join a large practice to have an impact. All over the country, there are community groups working to increase screening rates, and engaging with those groups is a good start. During the COVID-19 pandemic, we are all using social media and other platforms to connect. You do not need a lot of resources to set up a Zoom meeting with people in your community to discuss CRC screening.
Engaging with referring physicians
As a private practice practitioner, part of growing your practice is engaging with the primary care physicians in your area to ensure that they are up to date on the latest research in CRC screening and that they are discussing available screening options with their patients.
Preventing cancer should always be our first goal. Most CRCs begin as a polyp. Finding, quantifying, localizing, and removing polyps through screening colonoscopy is the most effective strategy for preventing this cancer. That is why colonoscopy remains the preferred method for colon cancer screening.
The Multi-Society Task Force on Colorectal Cancer recommends that, in sequential approaches, physicians should offer colonoscopy first.5 For patients who decline to have a colonoscopy, the FIT test should be offered next, followed by second-tier tests such as Cologuard and CT colonography for patients who decline both first-tier options.
Beyond the science of colorectal screening, we want to make sure that our primary care partners are aware of the disparities that exist – and which patients are at higher risk – so that they can engage with their patients to encourage screening.
For example, in our practice, we work with local Asian American community groups to help make sure that the “model minority” myth – that Asian Americans are healthier, wealthier, and better educated than the average American – does not become a barrier to screening. While Asian Americans may have lower overall rates of some types of cancer, there are some cancers that disproportionately affect certain Asian American groups. Rates of CRC in Japanese men, for instance, are 23% higher than in non-Hispanic Whites.
Additionally, we work with our primary care colleagues to help them understand that patients may have insurance considerations when choosing a test. While insurance typically covers 100% of a preventive screening test, a follow-up colonoscopy for a positive stool test is considered a diagnostic or therapeutic service and may not be fully covered. Medicare patients may face a coinsurance bill after their follow-up colonoscopy for a positive stool test. Legislation was passed last year to remove this barrier, but Medicare beneficiaries may have some out-of-pocket costs until it is completely removed in 2030.
Are you joining a practice that supports CRC education? Just ask!
We all want to work for an organization that aligns with our core values, and for GI physicians like us, CRC screening is a core component of our everyday work.
If you are considering joining a private practice, ask how the practice is doing with their CRC awareness programs and if it leads to increases in screenings. Inquire about the groups that are being engaged with and why. Is the practice focused on communities that have disparities in screening and treatment, and is it able to complete the entire screening process for individuals in communities that are more adversely affected by colorectal cancer?
We have found that candidates who have the most success in our practice are people who want to work at Atlanta Gastroenterology Associates but are also active in their communities and have a sense of how they want to be of service in their community. It is a sign of leadership in people – the idea that they are really going to get out and network and build a practice that serves everyone in their community. These actions make a difference in getting more people screened and in decreasing the disparities that exist.
Dr. Aja McCutchen is the chair of the quality committee at Atlanta Gastroenterology Associates and serves as chair of the Digestive Health Physicians Association’s Diversity, Equity and Inclusion Committee. She reports having nothing to disclose.
References
1. Siegel RL et al. CA Cancer J Clin. 2018 Jan;68(1):7-30.
2. Key Statistics for Colorectal Cancer. Cancer.org.
3. Wolf AMD et al. CA Cancer J Clin. 2018 Jul;68(4):250-281.
4. American Cancer Society. Colorectal Cancer Facts & Figures 2020-2022.
5. Rex DK et al. Am J Gastroenterol. 2017;112(7):1016-30.
When to not go with your gut: Modern approaches to abdominal wall pain
Abdominal pain is a commonly seen presenting concern in gastroenterology clinics. Establishing a diagnosis effectively and efficiently can be challenging given the broad differential. Abdominal wall pain is an often-overlooked diagnosis but accounts for up to 30% of cases of chronic abdominal pain1 and up to 10% of patients with chronic idiopathic abdominal pain seen in gastroenterology practices.2 Trigger point injection in the office can be both diagnostic and therapeutic.
The prevalence of chronic abdominal wall pain is highest in the fifth and sixth decades, and it is four times more likely to occur in women than in men. Common comorbid conditions include obesity, gastroesophageal reflux disease, irritable bowel syndrome, and fibromyalgia.3 Abdominal wall pain is often sharp or burning due to somatic innervation of the abdominal wall supplied by the anterior branches of thoracic intercostal nerves (T7 to T11). Abdominal wall pain may originate from entrapment of these nerves.2 Potential causes of entrapment include disruption of insulating fat, localized edema and distension, and scar tissue or fibrosis from prior surgical procedures.3 Symptoms are typically exacerbated with any actions or activities that engage the abdominal wall such as twisting or turning, and pain often improves with rest.
The classic physical exam finding for abdominal wall pain is a positive Carnett sign. This is determined via palpation of the point of maximal tenderness. First, this is done with a single finger while the patient’s abdominal wall is relaxed. The same point is then palpated again while the patient engages their abdominal muscles, most commonly while the patient to performs a “sit up” or lifts their legs off the exam table. Exacerbation of pain with these maneuvers indicates a positive test and suggests the abdominal wall as the underlying etiology.
While performing the maneuver for determining Carnett sign is a simple test in the traditional office visit, the COVID-19 pandemic has led to a burgeoning proportion of telehealth visits, limiting the physician’s ability to perform a direct physical exam. Fortunately, the maneuvers required when testing for Carnett sign are simple enough that a clinician can guide a patient step-by-step on how to perform the test. Ideally, if a family member or friend is available to serve as the clinician’s hands, the test can be performed with ease while directly visualizing proper technique. Sample videos of how the test is performed are readily available on the Internet for patients to view (the authors suggest screening the video yourself before providing a link to patients). The sensitivity and specificity of Carnett sign are very high (>70%) and even better when there is no apparent hernia.1
Management
Trigger point injections with local anesthetic can be both diagnostic and therapeutic in patients with abdominal wall pain. An immediate reduction of pain by at least 50% with injection at the site of maximal tenderness strongly supports the diagnosis of abdominal wall pain.1 Patients should first be thoroughly counseled on potential side effects of local corticosteroid injection to include risk of infection, bleeding, pain, skin hypopigmentation, or thinning and fat atrophy. Repeat injections are rarely needed, and any additional injection should be performed after at least 3 months. Additional adjunct therapies include nonsteroidal anti-inflammatory medications, topical therapies such as lidocaine, and neuroleptic agents such as gabapentin.4 One previously described trigger point injection technique, involves a mix of triamcinolone and lidocaine injected at the point of maximal tenderness.5 This technique is easy to perform in clinic and has minimal risks.
Conclusion
Abdominal wall pain is a common, yet often-overlooked, condition that can be diagnosed with a good clinical history and physical exam. A simple in-office trigger point injection can confirm the diagnosis and offer durable relief for most patients. A shift to virtual medicine does not need to a barrier to diagnosis, particularly in the attentive patient.
Dr. Park is a fellow in the gastroenterology service in the Department of Internal Medicine at Naval Medical Center San Diego and an assistant professor in the department of medicine of the Uniformed Services University in Bethesda, Md. Dr. Singla is a gastroenterologist at Capital Digestive Care in Silver Spring, Md., and an associate professor in the department of medicine at the Uniformed Services University. The authors have no conflicts of interest.
References
1. Glissen Brown JR et al. J Clin Gastroenterol. 2016;50(10):828-35.
2. Srinivasan R, Greenbaum DS. Am J Gastroenterol. 2002;97(4):824-30.
3. Kambox AK et al. Mayo Clin Proc. 2019;94(1):139-44.
4. Scheltinga MR, Roumen RM. Hernia. 2018;22(3):507-16.
5. Singla M, Laczek JT. Am J Gastroenterol. 2020 May;115(5):645-7.
Abdominal pain is a commonly seen presenting concern in gastroenterology clinics. Establishing a diagnosis effectively and efficiently can be challenging given the broad differential. Abdominal wall pain is an often-overlooked diagnosis but accounts for up to 30% of cases of chronic abdominal pain1 and up to 10% of patients with chronic idiopathic abdominal pain seen in gastroenterology practices.2 Trigger point injection in the office can be both diagnostic and therapeutic.
The prevalence of chronic abdominal wall pain is highest in the fifth and sixth decades, and it is four times more likely to occur in women than in men. Common comorbid conditions include obesity, gastroesophageal reflux disease, irritable bowel syndrome, and fibromyalgia.3 Abdominal wall pain is often sharp or burning due to somatic innervation of the abdominal wall supplied by the anterior branches of thoracic intercostal nerves (T7 to T11). Abdominal wall pain may originate from entrapment of these nerves.2 Potential causes of entrapment include disruption of insulating fat, localized edema and distension, and scar tissue or fibrosis from prior surgical procedures.3 Symptoms are typically exacerbated with any actions or activities that engage the abdominal wall such as twisting or turning, and pain often improves with rest.
The classic physical exam finding for abdominal wall pain is a positive Carnett sign. This is determined via palpation of the point of maximal tenderness. First, this is done with a single finger while the patient’s abdominal wall is relaxed. The same point is then palpated again while the patient engages their abdominal muscles, most commonly while the patient to performs a “sit up” or lifts their legs off the exam table. Exacerbation of pain with these maneuvers indicates a positive test and suggests the abdominal wall as the underlying etiology.
While performing the maneuver for determining Carnett sign is a simple test in the traditional office visit, the COVID-19 pandemic has led to a burgeoning proportion of telehealth visits, limiting the physician’s ability to perform a direct physical exam. Fortunately, the maneuvers required when testing for Carnett sign are simple enough that a clinician can guide a patient step-by-step on how to perform the test. Ideally, if a family member or friend is available to serve as the clinician’s hands, the test can be performed with ease while directly visualizing proper technique. Sample videos of how the test is performed are readily available on the Internet for patients to view (the authors suggest screening the video yourself before providing a link to patients). The sensitivity and specificity of Carnett sign are very high (>70%) and even better when there is no apparent hernia.1
Management
Trigger point injections with local anesthetic can be both diagnostic and therapeutic in patients with abdominal wall pain. An immediate reduction of pain by at least 50% with injection at the site of maximal tenderness strongly supports the diagnosis of abdominal wall pain.1 Patients should first be thoroughly counseled on potential side effects of local corticosteroid injection to include risk of infection, bleeding, pain, skin hypopigmentation, or thinning and fat atrophy. Repeat injections are rarely needed, and any additional injection should be performed after at least 3 months. Additional adjunct therapies include nonsteroidal anti-inflammatory medications, topical therapies such as lidocaine, and neuroleptic agents such as gabapentin.4 One previously described trigger point injection technique, involves a mix of triamcinolone and lidocaine injected at the point of maximal tenderness.5 This technique is easy to perform in clinic and has minimal risks.
Conclusion
Abdominal wall pain is a common, yet often-overlooked, condition that can be diagnosed with a good clinical history and physical exam. A simple in-office trigger point injection can confirm the diagnosis and offer durable relief for most patients. A shift to virtual medicine does not need to a barrier to diagnosis, particularly in the attentive patient.
Dr. Park is a fellow in the gastroenterology service in the Department of Internal Medicine at Naval Medical Center San Diego and an assistant professor in the department of medicine of the Uniformed Services University in Bethesda, Md. Dr. Singla is a gastroenterologist at Capital Digestive Care in Silver Spring, Md., and an associate professor in the department of medicine at the Uniformed Services University. The authors have no conflicts of interest.
References
1. Glissen Brown JR et al. J Clin Gastroenterol. 2016;50(10):828-35.
2. Srinivasan R, Greenbaum DS. Am J Gastroenterol. 2002;97(4):824-30.
3. Kambox AK et al. Mayo Clin Proc. 2019;94(1):139-44.
4. Scheltinga MR, Roumen RM. Hernia. 2018;22(3):507-16.
5. Singla M, Laczek JT. Am J Gastroenterol. 2020 May;115(5):645-7.
Abdominal pain is a commonly seen presenting concern in gastroenterology clinics. Establishing a diagnosis effectively and efficiently can be challenging given the broad differential. Abdominal wall pain is an often-overlooked diagnosis but accounts for up to 30% of cases of chronic abdominal pain1 and up to 10% of patients with chronic idiopathic abdominal pain seen in gastroenterology practices.2 Trigger point injection in the office can be both diagnostic and therapeutic.
The prevalence of chronic abdominal wall pain is highest in the fifth and sixth decades, and it is four times more likely to occur in women than in men. Common comorbid conditions include obesity, gastroesophageal reflux disease, irritable bowel syndrome, and fibromyalgia.3 Abdominal wall pain is often sharp or burning due to somatic innervation of the abdominal wall supplied by the anterior branches of thoracic intercostal nerves (T7 to T11). Abdominal wall pain may originate from entrapment of these nerves.2 Potential causes of entrapment include disruption of insulating fat, localized edema and distension, and scar tissue or fibrosis from prior surgical procedures.3 Symptoms are typically exacerbated with any actions or activities that engage the abdominal wall such as twisting or turning, and pain often improves with rest.
The classic physical exam finding for abdominal wall pain is a positive Carnett sign. This is determined via palpation of the point of maximal tenderness. First, this is done with a single finger while the patient’s abdominal wall is relaxed. The same point is then palpated again while the patient engages their abdominal muscles, most commonly while the patient to performs a “sit up” or lifts their legs off the exam table. Exacerbation of pain with these maneuvers indicates a positive test and suggests the abdominal wall as the underlying etiology.
While performing the maneuver for determining Carnett sign is a simple test in the traditional office visit, the COVID-19 pandemic has led to a burgeoning proportion of telehealth visits, limiting the physician’s ability to perform a direct physical exam. Fortunately, the maneuvers required when testing for Carnett sign are simple enough that a clinician can guide a patient step-by-step on how to perform the test. Ideally, if a family member or friend is available to serve as the clinician’s hands, the test can be performed with ease while directly visualizing proper technique. Sample videos of how the test is performed are readily available on the Internet for patients to view (the authors suggest screening the video yourself before providing a link to patients). The sensitivity and specificity of Carnett sign are very high (>70%) and even better when there is no apparent hernia.1
Management
Trigger point injections with local anesthetic can be both diagnostic and therapeutic in patients with abdominal wall pain. An immediate reduction of pain by at least 50% with injection at the site of maximal tenderness strongly supports the diagnosis of abdominal wall pain.1 Patients should first be thoroughly counseled on potential side effects of local corticosteroid injection to include risk of infection, bleeding, pain, skin hypopigmentation, or thinning and fat atrophy. Repeat injections are rarely needed, and any additional injection should be performed after at least 3 months. Additional adjunct therapies include nonsteroidal anti-inflammatory medications, topical therapies such as lidocaine, and neuroleptic agents such as gabapentin.4 One previously described trigger point injection technique, involves a mix of triamcinolone and lidocaine injected at the point of maximal tenderness.5 This technique is easy to perform in clinic and has minimal risks.
Conclusion
Abdominal wall pain is a common, yet often-overlooked, condition that can be diagnosed with a good clinical history and physical exam. A simple in-office trigger point injection can confirm the diagnosis and offer durable relief for most patients. A shift to virtual medicine does not need to a barrier to diagnosis, particularly in the attentive patient.
Dr. Park is a fellow in the gastroenterology service in the Department of Internal Medicine at Naval Medical Center San Diego and an assistant professor in the department of medicine of the Uniformed Services University in Bethesda, Md. Dr. Singla is a gastroenterologist at Capital Digestive Care in Silver Spring, Md., and an associate professor in the department of medicine at the Uniformed Services University. The authors have no conflicts of interest.
References
1. Glissen Brown JR et al. J Clin Gastroenterol. 2016;50(10):828-35.
2. Srinivasan R, Greenbaum DS. Am J Gastroenterol. 2002;97(4):824-30.
3. Kambox AK et al. Mayo Clin Proc. 2019;94(1):139-44.
4. Scheltinga MR, Roumen RM. Hernia. 2018;22(3):507-16.
5. Singla M, Laczek JT. Am J Gastroenterol. 2020 May;115(5):645-7.
Advocating in a pandemic: A fellow’s perspective on AGA Advocacy Day
Gastroenterology fellowship is an exercise in balance. You are learning your way around different parts of the gastrointestinal tract, both cerebrally and anatomically. You are continuously taking care of patients, in the hospital and in the clinic. You attend all kinds of conferences, didactics, and webinars. And though the hours are long, the work is worth seeing a smile from even one patient for whom you have made a tangible difference in health care. Though these moments are priceless, how often they happen is often limited by access to care, health care disparities, and systemic injustice. Fighting for each one of our patients and their health is difficult for even the most seasoned physician. Training during the middle of a pandemic has brought health care disparities to the forefront; delays in colorectal cancer screening and postponements of nonurgent procedures will have downstream impacts. It was with these thoughts in mind that I decided to participate in AGA (American Gastroenterological Association) Advocacy Day in September 2020 as a gastroenterology fellow.
After close to 20 years of in-person Advocacy Days, the AGA decided to take its advocacy efforts to a virtual platform in the fall of 2020. The country remained in the throes of the worst pandemic it had seen in over a century, and social distancing efforts necessitated a different venue than previous years. This year’s online platform was designed to let individuals involved in gastroenterology health care join each other and discuss policies germane to our patients and our profession.
I have to confess that I did not have significant legislative experience, and I signed up for the virtual Advocacy Day with a sense of slight trepidation. Would I be prepared to talk to experts in the field? What did I know about health care policy on the granular level? How could I get across my message cogently and successfully? All I really knew was that I wanted to get engaged with a group of gastroenterologists early on in my career who were not only vociferous advocates for their patients at the bedside, but who were also able to actively support policy changes that would bring about systemic change.
As it turned out, I had nothing to worry about. This advocacy experience was designed for gastroenterology clinical providers to be able to talk intelligently about topics they knew well – research funding, colonoscopy costs, and different levels of therapy for patients with inflammatory bowel disease, among others. To provide an overview of public policy issues, the AGA prepared a legislative briefing book that allowed us to take a deep dive into these topics. I remember reviewing the issue briefs in detail, understanding that I did not need to be an expert but that familiarity with the issues would be a key component of having a successful meeting. I also completed an online advocacy training module that gave me insight into how and why I could advocate for my profession as a future gastroenterologist. Based on our congressional district and state, we were divided into groups of congressional advocates who would speak to specific congressional staff members. During our meetings, we had legislative staff available to help us navigate the finer points of public policy. Each member of my group chose a topic that was personally relevant to them. Throughout our sessions, we shared personal stories, dove in and out of virtual meeting rooms, and made sure we were clear in what we were advocating for.
As a second-year gastroenterology fellow working at the National Institutes of Health, I chose to focus on digestive diseases research funding for the research community. I talked to the congressional staff members about a patient I had seen earlier that year. He was a man in his mid-30s who was diagnosed with hepatitis C more than 10 years ago and was told, at the time of his diagnosis, that there were no good treatments for him. He had resigned himself to that fact until I saw him in my office and spoke to him about the remarkable advances in liver disease treatment that were made over the past few years. I talked to him about how Hepatitis C was a disease that could now be cured – the relief on his face was clear and only reaffirmed in me the understanding that research in digestive diseases has improved the health of our nation’s population through sustained research efforts in gastrointestinal cancers and other life-altering illnesses.
So what did I take away from this adventure in advocacy? Our role as gastroenterologists can go beyond treating one patient in one office in one hospital system at a time. We can effect change by addressing policies that we know are hurting our patients and their health. The learning curve is made much easier under the excellence of the AGA advocacy staff, who takes the time to gather resources and educate us on the specifics of relevant legislative policies, as well as of the congressional members with whom we are speaking. Our advice was sought after because, after years of training, we were the experts in this field. I was proud to have joined this grassroots network of engaged members to speak to our lawmakers. I can only imagine, in the years to come, how wonderful it would be to do this in person in our nation’s capital.
Dr. Asif is a gastroenterology fellow working with the University of Maryland and National Institutes of Health. He has no conflicts of interest to disclose.
Gastroenterology fellowship is an exercise in balance. You are learning your way around different parts of the gastrointestinal tract, both cerebrally and anatomically. You are continuously taking care of patients, in the hospital and in the clinic. You attend all kinds of conferences, didactics, and webinars. And though the hours are long, the work is worth seeing a smile from even one patient for whom you have made a tangible difference in health care. Though these moments are priceless, how often they happen is often limited by access to care, health care disparities, and systemic injustice. Fighting for each one of our patients and their health is difficult for even the most seasoned physician. Training during the middle of a pandemic has brought health care disparities to the forefront; delays in colorectal cancer screening and postponements of nonurgent procedures will have downstream impacts. It was with these thoughts in mind that I decided to participate in AGA (American Gastroenterological Association) Advocacy Day in September 2020 as a gastroenterology fellow.
After close to 20 years of in-person Advocacy Days, the AGA decided to take its advocacy efforts to a virtual platform in the fall of 2020. The country remained in the throes of the worst pandemic it had seen in over a century, and social distancing efforts necessitated a different venue than previous years. This year’s online platform was designed to let individuals involved in gastroenterology health care join each other and discuss policies germane to our patients and our profession.
I have to confess that I did not have significant legislative experience, and I signed up for the virtual Advocacy Day with a sense of slight trepidation. Would I be prepared to talk to experts in the field? What did I know about health care policy on the granular level? How could I get across my message cogently and successfully? All I really knew was that I wanted to get engaged with a group of gastroenterologists early on in my career who were not only vociferous advocates for their patients at the bedside, but who were also able to actively support policy changes that would bring about systemic change.
As it turned out, I had nothing to worry about. This advocacy experience was designed for gastroenterology clinical providers to be able to talk intelligently about topics they knew well – research funding, colonoscopy costs, and different levels of therapy for patients with inflammatory bowel disease, among others. To provide an overview of public policy issues, the AGA prepared a legislative briefing book that allowed us to take a deep dive into these topics. I remember reviewing the issue briefs in detail, understanding that I did not need to be an expert but that familiarity with the issues would be a key component of having a successful meeting. I also completed an online advocacy training module that gave me insight into how and why I could advocate for my profession as a future gastroenterologist. Based on our congressional district and state, we were divided into groups of congressional advocates who would speak to specific congressional staff members. During our meetings, we had legislative staff available to help us navigate the finer points of public policy. Each member of my group chose a topic that was personally relevant to them. Throughout our sessions, we shared personal stories, dove in and out of virtual meeting rooms, and made sure we were clear in what we were advocating for.
As a second-year gastroenterology fellow working at the National Institutes of Health, I chose to focus on digestive diseases research funding for the research community. I talked to the congressional staff members about a patient I had seen earlier that year. He was a man in his mid-30s who was diagnosed with hepatitis C more than 10 years ago and was told, at the time of his diagnosis, that there were no good treatments for him. He had resigned himself to that fact until I saw him in my office and spoke to him about the remarkable advances in liver disease treatment that were made over the past few years. I talked to him about how Hepatitis C was a disease that could now be cured – the relief on his face was clear and only reaffirmed in me the understanding that research in digestive diseases has improved the health of our nation’s population through sustained research efforts in gastrointestinal cancers and other life-altering illnesses.
So what did I take away from this adventure in advocacy? Our role as gastroenterologists can go beyond treating one patient in one office in one hospital system at a time. We can effect change by addressing policies that we know are hurting our patients and their health. The learning curve is made much easier under the excellence of the AGA advocacy staff, who takes the time to gather resources and educate us on the specifics of relevant legislative policies, as well as of the congressional members with whom we are speaking. Our advice was sought after because, after years of training, we were the experts in this field. I was proud to have joined this grassroots network of engaged members to speak to our lawmakers. I can only imagine, in the years to come, how wonderful it would be to do this in person in our nation’s capital.
Dr. Asif is a gastroenterology fellow working with the University of Maryland and National Institutes of Health. He has no conflicts of interest to disclose.
Gastroenterology fellowship is an exercise in balance. You are learning your way around different parts of the gastrointestinal tract, both cerebrally and anatomically. You are continuously taking care of patients, in the hospital and in the clinic. You attend all kinds of conferences, didactics, and webinars. And though the hours are long, the work is worth seeing a smile from even one patient for whom you have made a tangible difference in health care. Though these moments are priceless, how often they happen is often limited by access to care, health care disparities, and systemic injustice. Fighting for each one of our patients and their health is difficult for even the most seasoned physician. Training during the middle of a pandemic has brought health care disparities to the forefront; delays in colorectal cancer screening and postponements of nonurgent procedures will have downstream impacts. It was with these thoughts in mind that I decided to participate in AGA (American Gastroenterological Association) Advocacy Day in September 2020 as a gastroenterology fellow.
After close to 20 years of in-person Advocacy Days, the AGA decided to take its advocacy efforts to a virtual platform in the fall of 2020. The country remained in the throes of the worst pandemic it had seen in over a century, and social distancing efforts necessitated a different venue than previous years. This year’s online platform was designed to let individuals involved in gastroenterology health care join each other and discuss policies germane to our patients and our profession.
I have to confess that I did not have significant legislative experience, and I signed up for the virtual Advocacy Day with a sense of slight trepidation. Would I be prepared to talk to experts in the field? What did I know about health care policy on the granular level? How could I get across my message cogently and successfully? All I really knew was that I wanted to get engaged with a group of gastroenterologists early on in my career who were not only vociferous advocates for their patients at the bedside, but who were also able to actively support policy changes that would bring about systemic change.
As it turned out, I had nothing to worry about. This advocacy experience was designed for gastroenterology clinical providers to be able to talk intelligently about topics they knew well – research funding, colonoscopy costs, and different levels of therapy for patients with inflammatory bowel disease, among others. To provide an overview of public policy issues, the AGA prepared a legislative briefing book that allowed us to take a deep dive into these topics. I remember reviewing the issue briefs in detail, understanding that I did not need to be an expert but that familiarity with the issues would be a key component of having a successful meeting. I also completed an online advocacy training module that gave me insight into how and why I could advocate for my profession as a future gastroenterologist. Based on our congressional district and state, we were divided into groups of congressional advocates who would speak to specific congressional staff members. During our meetings, we had legislative staff available to help us navigate the finer points of public policy. Each member of my group chose a topic that was personally relevant to them. Throughout our sessions, we shared personal stories, dove in and out of virtual meeting rooms, and made sure we were clear in what we were advocating for.
As a second-year gastroenterology fellow working at the National Institutes of Health, I chose to focus on digestive diseases research funding for the research community. I talked to the congressional staff members about a patient I had seen earlier that year. He was a man in his mid-30s who was diagnosed with hepatitis C more than 10 years ago and was told, at the time of his diagnosis, that there were no good treatments for him. He had resigned himself to that fact until I saw him in my office and spoke to him about the remarkable advances in liver disease treatment that were made over the past few years. I talked to him about how Hepatitis C was a disease that could now be cured – the relief on his face was clear and only reaffirmed in me the understanding that research in digestive diseases has improved the health of our nation’s population through sustained research efforts in gastrointestinal cancers and other life-altering illnesses.
So what did I take away from this adventure in advocacy? Our role as gastroenterologists can go beyond treating one patient in one office in one hospital system at a time. We can effect change by addressing policies that we know are hurting our patients and their health. The learning curve is made much easier under the excellence of the AGA advocacy staff, who takes the time to gather resources and educate us on the specifics of relevant legislative policies, as well as of the congressional members with whom we are speaking. Our advice was sought after because, after years of training, we were the experts in this field. I was proud to have joined this grassroots network of engaged members to speak to our lawmakers. I can only imagine, in the years to come, how wonderful it would be to do this in person in our nation’s capital.
Dr. Asif is a gastroenterology fellow working with the University of Maryland and National Institutes of Health. He has no conflicts of interest to disclose.
Microaggressions, racism, and antiracism: The role of gastroenterology
On a busy call day, Oviea (a second-year gastroenterology fellow), paused in the hallway to listen to a conversation between an endoscopy nurse and a patient. The nurse was requesting the patient’s permission for a gastroenterology fellow to participate in their care and the patient, well acquainted with the role from prior procedures, immediately agreed. Oviea entered the patient’s room, introduced himself as “Dr. Akpotaire, the gastroenterology fellow,” as he had with hundreds of other patients during his fellowship, and completed the informed consent. The interaction was brief but pleasant. As Oviea was leaving the room, the patient asked: “When will I meet the doctor”?
This question was familiar to Oviea. Despite always introducing himself by title and wearing matching identification, many patients had dismissed his credentials since graduating from medical school. His answer was equally familiar: “I am a doctor, and Dr. X, the supervising physician, will meet you soon.” With the patient seemingly placated, Oviea delivered the consent form to the procedure room. Minutes later, he was surprised to learn that the patient specifically requested that he not be allowed to participate in their care. This in combination with the patient’s initial dismissal of Oviea’s credentials, left a sting. While none of the other team members outwardly questioned the reason for the patient’s change of heart, Oviea continued to wonder if the patient’s decision was because of his race.
Beyond gastroenterology, similar experiences are common in other spheres. The Twitter thread #BlackintheIvory recounts stories of microaggressions and structural racism in medicine and academia. The cumulative toll of these experiences leads to departures of Black physicians including Uché Blackstock, MD;1 Aysha Khoury, MD, MPH;2 Ben Danielson, MD;3 Princess Dennar, MD;4 and others.
Microaggressions as proxy for bias
The term microaggression was coined by Chester Pierce, MD, the first Black tenured professor at Massachusetts General Hospital in the 1970’s, to describe the frequent, yet subtle dismissals Black Americans experienced in society. Over time, the term has been expanded to include “brief and commonplace daily verbal, behavioral, or environmental indignities, intentional or unintentional, that communicate hostile, derogatory, or negative slights and insults” to any marginalized group.5
While the term microaggressions is useful in contextualizing individual experiences, it narrowly focuses on conscious or unconscious interpersonal prejudices. In medicine, this misdirects attention away from the policies and practices that create and reinforce prejudices; these policies and practices do so by systematically excluding underrepresented minority (URM) physicians,6 defined by the American Association of Medical Colleges as physicians who are Black, Hispanic, Native Americans, and Alaska Natives,7 from the medical workforce. Ultimately, this leads to and exacerbates poor health outcomes for racial and ethnic minority patients.
Microaggressions represent our society’s deepest and oldest biases and are rooted in structural racism, as well as misogyny, homophobia, transphobia, xenophobia, ableism, and other prejudices.8 For URM physicians, experiences like the example above are frequently caused by structural racism.
Structural racism in medicine
Structural racism refers to the policies, practices, cultural representations, and norms that reinforce inequities by providing privileges to White people at the disadvantage of non-White people.9 In 1910, Abraham Flexner, commissioned by the Carnegie Foundation and the American Medical Association, wrote that African American physicians should be trained in hygiene rather than surgery and should primarily serve as “sanitarians” whose purpose was to “protect Whites” from common diseases like tuberculosis.10 The 1910 Flexner Report also emphasized the importance of prerequisite basic sciences education and recommended that only two of the seven existing Black medical schools remain open because Flexner believed that only these schools had the potential to meet the new requirements for medical education.11 A recent analysis found that, had the other five medical schools affiliated with historically Black colleges and universities remained open, this would have resulted in an additional 33,315 Black medical school graduates by 2019.12 Structural racism explains why the majority of practicing physicians, medical educators, National Institutes of Health–funded researchers, and hospital executives are White and, similarly, why White patients are overrepresented in clinical trials, have better health outcomes, and live longer lives than several racial and ethnic minority groups.13
The murders of Ahmaud Arbery, Breonna Taylor, and George Floyd and the inequitable toll of the COVID-19 pandemic on Black, Hispanic, and Native American people renewed the dialogue regarding structural racism in America. Beyond criminal justice and police reform, the current social justice movement demands that structural racism is examined in all spheres. In medicine and health care, acknowledging the history of exclusion and exploitation of Black people and other URM groups is an important first step, but this must be followed by a commitment to an antiracist future for the benefit of all medical professionals and patients.14,15
Antiracism as a path forward
Antiracism refers to actions and policies that seek to dismantle structural racism. While individuals can and should engage in antiracist actions, it is equally important for organizations and government to actively participate in this process as well.
Individual and interpersonal levels
Gastroenterologists should advocate an end to racist practices within their organizations (e.g., unjustified use of race-based corrections in diagnostic algorithms and practice guidelines),16 and interrupt microaggressions and racist actions in real time (e.g., overpolicing of underrepresented groups in health care settings).17 Gastroenterologists from underrepresented groups may also need to unlearn internalized racism, which is defined as acceptance by members of disadvantaged races of the negative messages about their own abilities and intrinsic worth.18
Organizational level
Gastroenterology divisions and practices must ensure that the entire workforce, including leadership, reflects the diversity of our country. Underrepresented groups represent 33% of the U.S. population, but only 9.1% of gastroenterology fellows and 10% of gastroenterology faculty are from underrepresented groups.19 In addition to diversifying the field of gastroenterology through financial and operational support of pipeline educational programs, organizations should also promote the scholarship of URM groups, whose work is often undervalued, and redistribute power by elevating voices that have been historically absent.20 Gastroenterology practices should also collect high-quality patient data disaggregated by demographic factors. Doing so will enable rapid identification of disparate health outcomes by demographic variables and inform interventions to eliminate identified disparities.
Government level
The “Executive Order On Advancing Racial Equity and Support for Underserved Communities Through the Federal Government” issued by President Biden on Jan. 20, 2021, is an example of how government can promote antiracism.21 The executive order states that domestic policies cause group inequities and calls for the removal of systemic barriers in current and future domestic policies. The executive order outlines several additional ways to improve equity in current and future policy, including engagement, consultation, and coordination with members of underserved communities. The details outlined in the executive order should serve as the foundation for establishing new standards at the state, county, and city levels as well. Gastroenterologists can influence government by voting for officials at all levels that support and promote these standards.
Conclusion
Beyond calling out microaggressions in real time, we must also interrogate the biases, policies, and practices that support them in medicine and beyond. As Black gastroenterologists who have experienced microaggressions and overt acts of racism, we ground Oviea’s experience in structural racism and offer strategies that individuals, organizations, and governing institutions can adopt toward an antiracist future. This model can be applied to experiences rooted in misogyny, homophobia, transphobia, xenophobia, ableism, and other prejudices.
As a nation, we must make an active and collective choice to address structural racism. In health care, doing so will strengthen communities, enhance the lived experiences of URM physician colleagues, and save patient lives. Gastroenterologists, as trusted health care providers, are uniquely positioned to lead the way.
Dr. Akpotaire is a second-year GI fellow in the division of gastroenterology at the University of Washington, Seattle. Dr. Issaka is an assistant professor with both the Fred Hutchinson Cancer Research Center, Seattle, and the division of gastroenterology at the University of Washington.
References
1. Blackstock U. “Why Black doctors like me are leaving faculty positions in academic medical centers.” STAT News, 2020.
2. Asare JG. “One Doctor Shares Her Story of Racism in Medicine.” Forbes. 2021 Feb 1.
3. Kroman D. “Revered doctor steps down, accusing Seattle Children’s Hospital of racism.” Crosscut. 2020 Dec 31.
4. United States District Court Eastern District of Louisiana. Princess Dennar, M.D. v. The Administrators of the Tulane Educational Fund, 2020.
5. Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Hoboken, N.J.: Wiley, 2010.
6. Boyd RW. Lancet. 2019 Jun 22;393(10190):2484-5.
7. AAMC. Diversity in Medicine Facts and Figures 2019. Washington, D.C., 2019.
8. Overland MK et al. PM R. 2019 Sep;11(9):1004-12.
9. Jones CP. Ethn Dis. 2018 Aug 9;28(Suppl 1):231-4.
10. Hlavinka E. “Racial Bias in Flexner Report Permeates Medical Education Today.” Medpage Today. 2020 Jun 18.
11. Flexner A. Medical Education in the United States and Canada. New York: 1910. Republished: Bull World Health Organ. 2002;80(7):594-602.
12. Campbell KM et al. JAMA Netw Open. 2020 Aug 3;3(8):e2015220.
13. Malat J et al. Soc Sci Med. 2018 Feb;199:148-56.
14. Kendi IX. How to be an antiracist. New York: Random House Books, 2019.
15. Gray DM 2nd et al. Nat Rev Gastroenterol Hepatol. 2020 Oct;17(10):589-90.
16. Vyas DA et al. N Engl J Med. 2020 Aug 27;383(9):874-82.
17. Green CR et al. J Natl Med Assoc. 2018 Feb;110(1):37-43.
18. Jones CP. Am J Public Health. 2000 Aug;90(8):1212-5.
19. Anyane-Yeboa A et al. Am J Gastroenterol. 2020 Aug;115(8):1147-9.
20. Issaka RB. JAMA. 2020 Aug 11;324(6):556-7.
21. Biden JR. Executive Order On Advancing Racial Equity and Support for Underserved Communities Through the Federal Government. Washington, D.C.: The White House, 2021.
On a busy call day, Oviea (a second-year gastroenterology fellow), paused in the hallway to listen to a conversation between an endoscopy nurse and a patient. The nurse was requesting the patient’s permission for a gastroenterology fellow to participate in their care and the patient, well acquainted with the role from prior procedures, immediately agreed. Oviea entered the patient’s room, introduced himself as “Dr. Akpotaire, the gastroenterology fellow,” as he had with hundreds of other patients during his fellowship, and completed the informed consent. The interaction was brief but pleasant. As Oviea was leaving the room, the patient asked: “When will I meet the doctor”?
This question was familiar to Oviea. Despite always introducing himself by title and wearing matching identification, many patients had dismissed his credentials since graduating from medical school. His answer was equally familiar: “I am a doctor, and Dr. X, the supervising physician, will meet you soon.” With the patient seemingly placated, Oviea delivered the consent form to the procedure room. Minutes later, he was surprised to learn that the patient specifically requested that he not be allowed to participate in their care. This in combination with the patient’s initial dismissal of Oviea’s credentials, left a sting. While none of the other team members outwardly questioned the reason for the patient’s change of heart, Oviea continued to wonder if the patient’s decision was because of his race.
Beyond gastroenterology, similar experiences are common in other spheres. The Twitter thread #BlackintheIvory recounts stories of microaggressions and structural racism in medicine and academia. The cumulative toll of these experiences leads to departures of Black physicians including Uché Blackstock, MD;1 Aysha Khoury, MD, MPH;2 Ben Danielson, MD;3 Princess Dennar, MD;4 and others.
Microaggressions as proxy for bias
The term microaggression was coined by Chester Pierce, MD, the first Black tenured professor at Massachusetts General Hospital in the 1970’s, to describe the frequent, yet subtle dismissals Black Americans experienced in society. Over time, the term has been expanded to include “brief and commonplace daily verbal, behavioral, or environmental indignities, intentional or unintentional, that communicate hostile, derogatory, or negative slights and insults” to any marginalized group.5
While the term microaggressions is useful in contextualizing individual experiences, it narrowly focuses on conscious or unconscious interpersonal prejudices. In medicine, this misdirects attention away from the policies and practices that create and reinforce prejudices; these policies and practices do so by systematically excluding underrepresented minority (URM) physicians,6 defined by the American Association of Medical Colleges as physicians who are Black, Hispanic, Native Americans, and Alaska Natives,7 from the medical workforce. Ultimately, this leads to and exacerbates poor health outcomes for racial and ethnic minority patients.
Microaggressions represent our society’s deepest and oldest biases and are rooted in structural racism, as well as misogyny, homophobia, transphobia, xenophobia, ableism, and other prejudices.8 For URM physicians, experiences like the example above are frequently caused by structural racism.
Structural racism in medicine
Structural racism refers to the policies, practices, cultural representations, and norms that reinforce inequities by providing privileges to White people at the disadvantage of non-White people.9 In 1910, Abraham Flexner, commissioned by the Carnegie Foundation and the American Medical Association, wrote that African American physicians should be trained in hygiene rather than surgery and should primarily serve as “sanitarians” whose purpose was to “protect Whites” from common diseases like tuberculosis.10 The 1910 Flexner Report also emphasized the importance of prerequisite basic sciences education and recommended that only two of the seven existing Black medical schools remain open because Flexner believed that only these schools had the potential to meet the new requirements for medical education.11 A recent analysis found that, had the other five medical schools affiliated with historically Black colleges and universities remained open, this would have resulted in an additional 33,315 Black medical school graduates by 2019.12 Structural racism explains why the majority of practicing physicians, medical educators, National Institutes of Health–funded researchers, and hospital executives are White and, similarly, why White patients are overrepresented in clinical trials, have better health outcomes, and live longer lives than several racial and ethnic minority groups.13
The murders of Ahmaud Arbery, Breonna Taylor, and George Floyd and the inequitable toll of the COVID-19 pandemic on Black, Hispanic, and Native American people renewed the dialogue regarding structural racism in America. Beyond criminal justice and police reform, the current social justice movement demands that structural racism is examined in all spheres. In medicine and health care, acknowledging the history of exclusion and exploitation of Black people and other URM groups is an important first step, but this must be followed by a commitment to an antiracist future for the benefit of all medical professionals and patients.14,15
Antiracism as a path forward
Antiracism refers to actions and policies that seek to dismantle structural racism. While individuals can and should engage in antiracist actions, it is equally important for organizations and government to actively participate in this process as well.
Individual and interpersonal levels
Gastroenterologists should advocate an end to racist practices within their organizations (e.g., unjustified use of race-based corrections in diagnostic algorithms and practice guidelines),16 and interrupt microaggressions and racist actions in real time (e.g., overpolicing of underrepresented groups in health care settings).17 Gastroenterologists from underrepresented groups may also need to unlearn internalized racism, which is defined as acceptance by members of disadvantaged races of the negative messages about their own abilities and intrinsic worth.18
Organizational level
Gastroenterology divisions and practices must ensure that the entire workforce, including leadership, reflects the diversity of our country. Underrepresented groups represent 33% of the U.S. population, but only 9.1% of gastroenterology fellows and 10% of gastroenterology faculty are from underrepresented groups.19 In addition to diversifying the field of gastroenterology through financial and operational support of pipeline educational programs, organizations should also promote the scholarship of URM groups, whose work is often undervalued, and redistribute power by elevating voices that have been historically absent.20 Gastroenterology practices should also collect high-quality patient data disaggregated by demographic factors. Doing so will enable rapid identification of disparate health outcomes by demographic variables and inform interventions to eliminate identified disparities.
Government level
The “Executive Order On Advancing Racial Equity and Support for Underserved Communities Through the Federal Government” issued by President Biden on Jan. 20, 2021, is an example of how government can promote antiracism.21 The executive order states that domestic policies cause group inequities and calls for the removal of systemic barriers in current and future domestic policies. The executive order outlines several additional ways to improve equity in current and future policy, including engagement, consultation, and coordination with members of underserved communities. The details outlined in the executive order should serve as the foundation for establishing new standards at the state, county, and city levels as well. Gastroenterologists can influence government by voting for officials at all levels that support and promote these standards.
Conclusion
Beyond calling out microaggressions in real time, we must also interrogate the biases, policies, and practices that support them in medicine and beyond. As Black gastroenterologists who have experienced microaggressions and overt acts of racism, we ground Oviea’s experience in structural racism and offer strategies that individuals, organizations, and governing institutions can adopt toward an antiracist future. This model can be applied to experiences rooted in misogyny, homophobia, transphobia, xenophobia, ableism, and other prejudices.
As a nation, we must make an active and collective choice to address structural racism. In health care, doing so will strengthen communities, enhance the lived experiences of URM physician colleagues, and save patient lives. Gastroenterologists, as trusted health care providers, are uniquely positioned to lead the way.
Dr. Akpotaire is a second-year GI fellow in the division of gastroenterology at the University of Washington, Seattle. Dr. Issaka is an assistant professor with both the Fred Hutchinson Cancer Research Center, Seattle, and the division of gastroenterology at the University of Washington.
References
1. Blackstock U. “Why Black doctors like me are leaving faculty positions in academic medical centers.” STAT News, 2020.
2. Asare JG. “One Doctor Shares Her Story of Racism in Medicine.” Forbes. 2021 Feb 1.
3. Kroman D. “Revered doctor steps down, accusing Seattle Children’s Hospital of racism.” Crosscut. 2020 Dec 31.
4. United States District Court Eastern District of Louisiana. Princess Dennar, M.D. v. The Administrators of the Tulane Educational Fund, 2020.
5. Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Hoboken, N.J.: Wiley, 2010.
6. Boyd RW. Lancet. 2019 Jun 22;393(10190):2484-5.
7. AAMC. Diversity in Medicine Facts and Figures 2019. Washington, D.C., 2019.
8. Overland MK et al. PM R. 2019 Sep;11(9):1004-12.
9. Jones CP. Ethn Dis. 2018 Aug 9;28(Suppl 1):231-4.
10. Hlavinka E. “Racial Bias in Flexner Report Permeates Medical Education Today.” Medpage Today. 2020 Jun 18.
11. Flexner A. Medical Education in the United States and Canada. New York: 1910. Republished: Bull World Health Organ. 2002;80(7):594-602.
12. Campbell KM et al. JAMA Netw Open. 2020 Aug 3;3(8):e2015220.
13. Malat J et al. Soc Sci Med. 2018 Feb;199:148-56.
14. Kendi IX. How to be an antiracist. New York: Random House Books, 2019.
15. Gray DM 2nd et al. Nat Rev Gastroenterol Hepatol. 2020 Oct;17(10):589-90.
16. Vyas DA et al. N Engl J Med. 2020 Aug 27;383(9):874-82.
17. Green CR et al. J Natl Med Assoc. 2018 Feb;110(1):37-43.
18. Jones CP. Am J Public Health. 2000 Aug;90(8):1212-5.
19. Anyane-Yeboa A et al. Am J Gastroenterol. 2020 Aug;115(8):1147-9.
20. Issaka RB. JAMA. 2020 Aug 11;324(6):556-7.
21. Biden JR. Executive Order On Advancing Racial Equity and Support for Underserved Communities Through the Federal Government. Washington, D.C.: The White House, 2021.
On a busy call day, Oviea (a second-year gastroenterology fellow), paused in the hallway to listen to a conversation between an endoscopy nurse and a patient. The nurse was requesting the patient’s permission for a gastroenterology fellow to participate in their care and the patient, well acquainted with the role from prior procedures, immediately agreed. Oviea entered the patient’s room, introduced himself as “Dr. Akpotaire, the gastroenterology fellow,” as he had with hundreds of other patients during his fellowship, and completed the informed consent. The interaction was brief but pleasant. As Oviea was leaving the room, the patient asked: “When will I meet the doctor”?
This question was familiar to Oviea. Despite always introducing himself by title and wearing matching identification, many patients had dismissed his credentials since graduating from medical school. His answer was equally familiar: “I am a doctor, and Dr. X, the supervising physician, will meet you soon.” With the patient seemingly placated, Oviea delivered the consent form to the procedure room. Minutes later, he was surprised to learn that the patient specifically requested that he not be allowed to participate in their care. This in combination with the patient’s initial dismissal of Oviea’s credentials, left a sting. While none of the other team members outwardly questioned the reason for the patient’s change of heart, Oviea continued to wonder if the patient’s decision was because of his race.
Beyond gastroenterology, similar experiences are common in other spheres. The Twitter thread #BlackintheIvory recounts stories of microaggressions and structural racism in medicine and academia. The cumulative toll of these experiences leads to departures of Black physicians including Uché Blackstock, MD;1 Aysha Khoury, MD, MPH;2 Ben Danielson, MD;3 Princess Dennar, MD;4 and others.
Microaggressions as proxy for bias
The term microaggression was coined by Chester Pierce, MD, the first Black tenured professor at Massachusetts General Hospital in the 1970’s, to describe the frequent, yet subtle dismissals Black Americans experienced in society. Over time, the term has been expanded to include “brief and commonplace daily verbal, behavioral, or environmental indignities, intentional or unintentional, that communicate hostile, derogatory, or negative slights and insults” to any marginalized group.5
While the term microaggressions is useful in contextualizing individual experiences, it narrowly focuses on conscious or unconscious interpersonal prejudices. In medicine, this misdirects attention away from the policies and practices that create and reinforce prejudices; these policies and practices do so by systematically excluding underrepresented minority (URM) physicians,6 defined by the American Association of Medical Colleges as physicians who are Black, Hispanic, Native Americans, and Alaska Natives,7 from the medical workforce. Ultimately, this leads to and exacerbates poor health outcomes for racial and ethnic minority patients.
Microaggressions represent our society’s deepest and oldest biases and are rooted in structural racism, as well as misogyny, homophobia, transphobia, xenophobia, ableism, and other prejudices.8 For URM physicians, experiences like the example above are frequently caused by structural racism.
Structural racism in medicine
Structural racism refers to the policies, practices, cultural representations, and norms that reinforce inequities by providing privileges to White people at the disadvantage of non-White people.9 In 1910, Abraham Flexner, commissioned by the Carnegie Foundation and the American Medical Association, wrote that African American physicians should be trained in hygiene rather than surgery and should primarily serve as “sanitarians” whose purpose was to “protect Whites” from common diseases like tuberculosis.10 The 1910 Flexner Report also emphasized the importance of prerequisite basic sciences education and recommended that only two of the seven existing Black medical schools remain open because Flexner believed that only these schools had the potential to meet the new requirements for medical education.11 A recent analysis found that, had the other five medical schools affiliated with historically Black colleges and universities remained open, this would have resulted in an additional 33,315 Black medical school graduates by 2019.12 Structural racism explains why the majority of practicing physicians, medical educators, National Institutes of Health–funded researchers, and hospital executives are White and, similarly, why White patients are overrepresented in clinical trials, have better health outcomes, and live longer lives than several racial and ethnic minority groups.13
The murders of Ahmaud Arbery, Breonna Taylor, and George Floyd and the inequitable toll of the COVID-19 pandemic on Black, Hispanic, and Native American people renewed the dialogue regarding structural racism in America. Beyond criminal justice and police reform, the current social justice movement demands that structural racism is examined in all spheres. In medicine and health care, acknowledging the history of exclusion and exploitation of Black people and other URM groups is an important first step, but this must be followed by a commitment to an antiracist future for the benefit of all medical professionals and patients.14,15
Antiracism as a path forward
Antiracism refers to actions and policies that seek to dismantle structural racism. While individuals can and should engage in antiracist actions, it is equally important for organizations and government to actively participate in this process as well.
Individual and interpersonal levels
Gastroenterologists should advocate an end to racist practices within their organizations (e.g., unjustified use of race-based corrections in diagnostic algorithms and practice guidelines),16 and interrupt microaggressions and racist actions in real time (e.g., overpolicing of underrepresented groups in health care settings).17 Gastroenterologists from underrepresented groups may also need to unlearn internalized racism, which is defined as acceptance by members of disadvantaged races of the negative messages about their own abilities and intrinsic worth.18
Organizational level
Gastroenterology divisions and practices must ensure that the entire workforce, including leadership, reflects the diversity of our country. Underrepresented groups represent 33% of the U.S. population, but only 9.1% of gastroenterology fellows and 10% of gastroenterology faculty are from underrepresented groups.19 In addition to diversifying the field of gastroenterology through financial and operational support of pipeline educational programs, organizations should also promote the scholarship of URM groups, whose work is often undervalued, and redistribute power by elevating voices that have been historically absent.20 Gastroenterology practices should also collect high-quality patient data disaggregated by demographic factors. Doing so will enable rapid identification of disparate health outcomes by demographic variables and inform interventions to eliminate identified disparities.
Government level
The “Executive Order On Advancing Racial Equity and Support for Underserved Communities Through the Federal Government” issued by President Biden on Jan. 20, 2021, is an example of how government can promote antiracism.21 The executive order states that domestic policies cause group inequities and calls for the removal of systemic barriers in current and future domestic policies. The executive order outlines several additional ways to improve equity in current and future policy, including engagement, consultation, and coordination with members of underserved communities. The details outlined in the executive order should serve as the foundation for establishing new standards at the state, county, and city levels as well. Gastroenterologists can influence government by voting for officials at all levels that support and promote these standards.
Conclusion
Beyond calling out microaggressions in real time, we must also interrogate the biases, policies, and practices that support them in medicine and beyond. As Black gastroenterologists who have experienced microaggressions and overt acts of racism, we ground Oviea’s experience in structural racism and offer strategies that individuals, organizations, and governing institutions can adopt toward an antiracist future. This model can be applied to experiences rooted in misogyny, homophobia, transphobia, xenophobia, ableism, and other prejudices.
As a nation, we must make an active and collective choice to address structural racism. In health care, doing so will strengthen communities, enhance the lived experiences of URM physician colleagues, and save patient lives. Gastroenterologists, as trusted health care providers, are uniquely positioned to lead the way.
Dr. Akpotaire is a second-year GI fellow in the division of gastroenterology at the University of Washington, Seattle. Dr. Issaka is an assistant professor with both the Fred Hutchinson Cancer Research Center, Seattle, and the division of gastroenterology at the University of Washington.
References
1. Blackstock U. “Why Black doctors like me are leaving faculty positions in academic medical centers.” STAT News, 2020.
2. Asare JG. “One Doctor Shares Her Story of Racism in Medicine.” Forbes. 2021 Feb 1.
3. Kroman D. “Revered doctor steps down, accusing Seattle Children’s Hospital of racism.” Crosscut. 2020 Dec 31.
4. United States District Court Eastern District of Louisiana. Princess Dennar, M.D. v. The Administrators of the Tulane Educational Fund, 2020.
5. Sue DW. Microaggressions in Everyday Life: Race, Gender, and Sexual Orientation. Hoboken, N.J.: Wiley, 2010.
6. Boyd RW. Lancet. 2019 Jun 22;393(10190):2484-5.
7. AAMC. Diversity in Medicine Facts and Figures 2019. Washington, D.C., 2019.
8. Overland MK et al. PM R. 2019 Sep;11(9):1004-12.
9. Jones CP. Ethn Dis. 2018 Aug 9;28(Suppl 1):231-4.
10. Hlavinka E. “Racial Bias in Flexner Report Permeates Medical Education Today.” Medpage Today. 2020 Jun 18.
11. Flexner A. Medical Education in the United States and Canada. New York: 1910. Republished: Bull World Health Organ. 2002;80(7):594-602.
12. Campbell KM et al. JAMA Netw Open. 2020 Aug 3;3(8):e2015220.
13. Malat J et al. Soc Sci Med. 2018 Feb;199:148-56.
14. Kendi IX. How to be an antiracist. New York: Random House Books, 2019.
15. Gray DM 2nd et al. Nat Rev Gastroenterol Hepatol. 2020 Oct;17(10):589-90.
16. Vyas DA et al. N Engl J Med. 2020 Aug 27;383(9):874-82.
17. Green CR et al. J Natl Med Assoc. 2018 Feb;110(1):37-43.
18. Jones CP. Am J Public Health. 2000 Aug;90(8):1212-5.
19. Anyane-Yeboa A et al. Am J Gastroenterol. 2020 Aug;115(8):1147-9.
20. Issaka RB. JAMA. 2020 Aug 11;324(6):556-7.
21. Biden JR. Executive Order On Advancing Racial Equity and Support for Underserved Communities Through the Federal Government. Washington, D.C.: The White House, 2021.
Mindful mentoring
Scenario
A GI faculty member is approached by two medical students who are planning careers in gastroenterology. They are interested in research projects and are very willing to dedicate the necessary time and energy. The faculty member is impressed by their desire and finds themselves recalling their own unsuccessful medical school search for a research mentor. Inspired by their enthusiasm and a desire to “give back,” the faculty member agrees to mentor them and helps them find suitable projects. Primarily because of the students’ hard work and fueled by their desire to produce results that will help their residency applications, the work progresses rapidly. Both students have separate abstracts accepted at a national meeting.
When COVID-19 hits, the faculty member is asked by their department to take on additional administrative and clinical work. They feel they cannot say no. Soon the faculty member finds it difficult to manage these new responsibilities on top of their many research projects, numerous clinical obligations, and additional pressures outside of work. They find they have no time for mentoring or even adequate sleep. Facing burnout, the faculty member is uncertain what to do for these hard-working and very gifted students. How would you recommend they manage their mentoring obligations?
Discussion
Mentorship is a cornerstone of academic medicine. In fact, it has been shown that academic clinicians who serve as mentors publish more papers, get more grants, are promoted faster, and are more likely to stay at their academic institutions with greater career satisfaction.1 However, not every mentor-mentee relationship is mutually beneficial. Usually, it’s the mentees that disproportionately suffer the consequences of a suboptimal relationship.2
Mentorship malpractice occurs when mentors’ behavior crosses a threshold that places the mentees’ success at risk.1,2 While the case above highlights a specific scenario where multiple issues are unfolding, the ability to recognize, address, and most importantly prevent mentorship malpractice ultimately benefits both mentees and mentors.
Understanding the various types of mentorship malpractice is helpful for prevention and course correction. As described by Chopra and colleagues, there are multiple types of passive and active mentorship malpractice.2 The passive forms are characterized by a lack of face-to-face meeting time with mentees and/or a lack of advocacy on the mentees’ behalf. Meanwhile, the active forms occur when the mentor exhibits self-serving behaviors. These can include listing themselves as first author on a mentee’s project or discouraging a mentee from working with other mentors. Mentors must be able to self-check, seek feedback from mentees, and encourage mentees to further their professional networks beyond the boundaries of what the mentor alone can offer. Doing so helps create new opportunities and helps ensure a mutually beneficial relationship.
A great initial step to prevent passive and active mentorship malpractice is to leverage the benefits of team mentorship.2,3 At its core, team mentorship capitalizes on the collective contributions of multiple mentors. Doing so not only provides security during uncertain times, but also allows for a diversity of perspectives, distribution of workload among mentors, and additional support for mentees.3,4 Team mentorship it is particularly important during this current global health crisis, and such an approach from the outset could have significantly improved the scenario above.
For the above scenario, likely a transition in mentorship would be needed. Such transitions, whether short term or long term, require transparency, honesty, and willingness to engage in difficult conversations with mentees. Whether the mentor in the above case engages another faculty to take on the mentees or chooses to find a colleague who will agree to take on other competing demands, it will require time, effort, and energy – all of which are in short supply. When team mentorship is established from the outset, such transitions of mentorship can occur seamlessly and with more ease for all.
Additional considerations for successful mentoring of medical students or early-career physicians include understanding generational differences between the mentor and their mentees. As outlined by Waljee and colleagues, the next generation of trainees and physicians may act in ways that deviate from the norms of academic medicine’s tradition. As a mentor, it is imperative to understand these actions are not intended to disrupt the traditions and norms of health systems.5 For example, the use of technology during rounds can often be misconstrued as disrespectful. However, the underlying intent in many cases is to answer a question or access a helpful reference.
Seeing behavior and actions from the perspective of the mentee is one of the many ways to support and sustain successful mentoring relationships. A mindful approach benefits both mentees and mentors; this includes reflecting on the underlying motives for mentorship and cultivating gratitude for the relationships formed.6 While these steps may seem trivial, gratitude promotes happiness, trust, motivation, and respect. It can be felt by others, including mentees.
As mentors continue to shape the future, they have an ethical obligation to care for themselves, in addition to their mentees. In addition to avoiding mentorship malpractice, engaging in team mentorship, and incorporating mindful mentoring, an emphasis on self-care is critical.7 Taking time to recharge is essential. It allows one to be fully present, while also setting an example for the mentee. Explicitly addressing self-care for both mentor and mentee is a part of mindful mentorship, with benefits for all.6
Three key points:
1. Awareness of mentorship malpractice
2. Importance of team mentorship
3. Benefits of mindful mentorship
Mr. Rodoni is with the University of Michigan Medical School and Stephen M. Ross School of Business, Ann Arbor, Mich. Dr. Fessel is a professor of radiology in the department of radiology at Michigan Medicine, Ann Arbor. They reported having no disclosures relevant to this article.
References:
1. Chopra V et al. JAMA Intern Med. 2018 Feb;178:175-6.
2. Chopra V et al. JAMA. 2016 Apr 12;315:1453-4.
3. Chopra V et al. The Mentoring Guide: Helping Mentors & Mentees Succeed. Ann Arbor: Michigan Publishing, 2019.
4. Rodoni BM et al. Annals of Surgery. 2020 Aug;272(2):e151-2.
5. Waljee JF et al. JAMA. 2018 Apr 17;319(15):1547-8.
6. Chopra V and Saint S. Healthc (Amst). 2020 Mar;8(1):100390.
7. Fessell D et al. “Mentoring During a Crisis.” Harvard Business Review. 2020 Oct 29.
Scenario
A GI faculty member is approached by two medical students who are planning careers in gastroenterology. They are interested in research projects and are very willing to dedicate the necessary time and energy. The faculty member is impressed by their desire and finds themselves recalling their own unsuccessful medical school search for a research mentor. Inspired by their enthusiasm and a desire to “give back,” the faculty member agrees to mentor them and helps them find suitable projects. Primarily because of the students’ hard work and fueled by their desire to produce results that will help their residency applications, the work progresses rapidly. Both students have separate abstracts accepted at a national meeting.
When COVID-19 hits, the faculty member is asked by their department to take on additional administrative and clinical work. They feel they cannot say no. Soon the faculty member finds it difficult to manage these new responsibilities on top of their many research projects, numerous clinical obligations, and additional pressures outside of work. They find they have no time for mentoring or even adequate sleep. Facing burnout, the faculty member is uncertain what to do for these hard-working and very gifted students. How would you recommend they manage their mentoring obligations?
Discussion
Mentorship is a cornerstone of academic medicine. In fact, it has been shown that academic clinicians who serve as mentors publish more papers, get more grants, are promoted faster, and are more likely to stay at their academic institutions with greater career satisfaction.1 However, not every mentor-mentee relationship is mutually beneficial. Usually, it’s the mentees that disproportionately suffer the consequences of a suboptimal relationship.2
Mentorship malpractice occurs when mentors’ behavior crosses a threshold that places the mentees’ success at risk.1,2 While the case above highlights a specific scenario where multiple issues are unfolding, the ability to recognize, address, and most importantly prevent mentorship malpractice ultimately benefits both mentees and mentors.
Understanding the various types of mentorship malpractice is helpful for prevention and course correction. As described by Chopra and colleagues, there are multiple types of passive and active mentorship malpractice.2 The passive forms are characterized by a lack of face-to-face meeting time with mentees and/or a lack of advocacy on the mentees’ behalf. Meanwhile, the active forms occur when the mentor exhibits self-serving behaviors. These can include listing themselves as first author on a mentee’s project or discouraging a mentee from working with other mentors. Mentors must be able to self-check, seek feedback from mentees, and encourage mentees to further their professional networks beyond the boundaries of what the mentor alone can offer. Doing so helps create new opportunities and helps ensure a mutually beneficial relationship.
A great initial step to prevent passive and active mentorship malpractice is to leverage the benefits of team mentorship.2,3 At its core, team mentorship capitalizes on the collective contributions of multiple mentors. Doing so not only provides security during uncertain times, but also allows for a diversity of perspectives, distribution of workload among mentors, and additional support for mentees.3,4 Team mentorship it is particularly important during this current global health crisis, and such an approach from the outset could have significantly improved the scenario above.
For the above scenario, likely a transition in mentorship would be needed. Such transitions, whether short term or long term, require transparency, honesty, and willingness to engage in difficult conversations with mentees. Whether the mentor in the above case engages another faculty to take on the mentees or chooses to find a colleague who will agree to take on other competing demands, it will require time, effort, and energy – all of which are in short supply. When team mentorship is established from the outset, such transitions of mentorship can occur seamlessly and with more ease for all.
Additional considerations for successful mentoring of medical students or early-career physicians include understanding generational differences between the mentor and their mentees. As outlined by Waljee and colleagues, the next generation of trainees and physicians may act in ways that deviate from the norms of academic medicine’s tradition. As a mentor, it is imperative to understand these actions are not intended to disrupt the traditions and norms of health systems.5 For example, the use of technology during rounds can often be misconstrued as disrespectful. However, the underlying intent in many cases is to answer a question or access a helpful reference.
Seeing behavior and actions from the perspective of the mentee is one of the many ways to support and sustain successful mentoring relationships. A mindful approach benefits both mentees and mentors; this includes reflecting on the underlying motives for mentorship and cultivating gratitude for the relationships formed.6 While these steps may seem trivial, gratitude promotes happiness, trust, motivation, and respect. It can be felt by others, including mentees.
As mentors continue to shape the future, they have an ethical obligation to care for themselves, in addition to their mentees. In addition to avoiding mentorship malpractice, engaging in team mentorship, and incorporating mindful mentoring, an emphasis on self-care is critical.7 Taking time to recharge is essential. It allows one to be fully present, while also setting an example for the mentee. Explicitly addressing self-care for both mentor and mentee is a part of mindful mentorship, with benefits for all.6
Three key points:
1. Awareness of mentorship malpractice
2. Importance of team mentorship
3. Benefits of mindful mentorship
Mr. Rodoni is with the University of Michigan Medical School and Stephen M. Ross School of Business, Ann Arbor, Mich. Dr. Fessel is a professor of radiology in the department of radiology at Michigan Medicine, Ann Arbor. They reported having no disclosures relevant to this article.
References:
1. Chopra V et al. JAMA Intern Med. 2018 Feb;178:175-6.
2. Chopra V et al. JAMA. 2016 Apr 12;315:1453-4.
3. Chopra V et al. The Mentoring Guide: Helping Mentors & Mentees Succeed. Ann Arbor: Michigan Publishing, 2019.
4. Rodoni BM et al. Annals of Surgery. 2020 Aug;272(2):e151-2.
5. Waljee JF et al. JAMA. 2018 Apr 17;319(15):1547-8.
6. Chopra V and Saint S. Healthc (Amst). 2020 Mar;8(1):100390.
7. Fessell D et al. “Mentoring During a Crisis.” Harvard Business Review. 2020 Oct 29.
Scenario
A GI faculty member is approached by two medical students who are planning careers in gastroenterology. They are interested in research projects and are very willing to dedicate the necessary time and energy. The faculty member is impressed by their desire and finds themselves recalling their own unsuccessful medical school search for a research mentor. Inspired by their enthusiasm and a desire to “give back,” the faculty member agrees to mentor them and helps them find suitable projects. Primarily because of the students’ hard work and fueled by their desire to produce results that will help their residency applications, the work progresses rapidly. Both students have separate abstracts accepted at a national meeting.
When COVID-19 hits, the faculty member is asked by their department to take on additional administrative and clinical work. They feel they cannot say no. Soon the faculty member finds it difficult to manage these new responsibilities on top of their many research projects, numerous clinical obligations, and additional pressures outside of work. They find they have no time for mentoring or even adequate sleep. Facing burnout, the faculty member is uncertain what to do for these hard-working and very gifted students. How would you recommend they manage their mentoring obligations?
Discussion
Mentorship is a cornerstone of academic medicine. In fact, it has been shown that academic clinicians who serve as mentors publish more papers, get more grants, are promoted faster, and are more likely to stay at their academic institutions with greater career satisfaction.1 However, not every mentor-mentee relationship is mutually beneficial. Usually, it’s the mentees that disproportionately suffer the consequences of a suboptimal relationship.2
Mentorship malpractice occurs when mentors’ behavior crosses a threshold that places the mentees’ success at risk.1,2 While the case above highlights a specific scenario where multiple issues are unfolding, the ability to recognize, address, and most importantly prevent mentorship malpractice ultimately benefits both mentees and mentors.
Understanding the various types of mentorship malpractice is helpful for prevention and course correction. As described by Chopra and colleagues, there are multiple types of passive and active mentorship malpractice.2 The passive forms are characterized by a lack of face-to-face meeting time with mentees and/or a lack of advocacy on the mentees’ behalf. Meanwhile, the active forms occur when the mentor exhibits self-serving behaviors. These can include listing themselves as first author on a mentee’s project or discouraging a mentee from working with other mentors. Mentors must be able to self-check, seek feedback from mentees, and encourage mentees to further their professional networks beyond the boundaries of what the mentor alone can offer. Doing so helps create new opportunities and helps ensure a mutually beneficial relationship.
A great initial step to prevent passive and active mentorship malpractice is to leverage the benefits of team mentorship.2,3 At its core, team mentorship capitalizes on the collective contributions of multiple mentors. Doing so not only provides security during uncertain times, but also allows for a diversity of perspectives, distribution of workload among mentors, and additional support for mentees.3,4 Team mentorship it is particularly important during this current global health crisis, and such an approach from the outset could have significantly improved the scenario above.
For the above scenario, likely a transition in mentorship would be needed. Such transitions, whether short term or long term, require transparency, honesty, and willingness to engage in difficult conversations with mentees. Whether the mentor in the above case engages another faculty to take on the mentees or chooses to find a colleague who will agree to take on other competing demands, it will require time, effort, and energy – all of which are in short supply. When team mentorship is established from the outset, such transitions of mentorship can occur seamlessly and with more ease for all.
Additional considerations for successful mentoring of medical students or early-career physicians include understanding generational differences between the mentor and their mentees. As outlined by Waljee and colleagues, the next generation of trainees and physicians may act in ways that deviate from the norms of academic medicine’s tradition. As a mentor, it is imperative to understand these actions are not intended to disrupt the traditions and norms of health systems.5 For example, the use of technology during rounds can often be misconstrued as disrespectful. However, the underlying intent in many cases is to answer a question or access a helpful reference.
Seeing behavior and actions from the perspective of the mentee is one of the many ways to support and sustain successful mentoring relationships. A mindful approach benefits both mentees and mentors; this includes reflecting on the underlying motives for mentorship and cultivating gratitude for the relationships formed.6 While these steps may seem trivial, gratitude promotes happiness, trust, motivation, and respect. It can be felt by others, including mentees.
As mentors continue to shape the future, they have an ethical obligation to care for themselves, in addition to their mentees. In addition to avoiding mentorship malpractice, engaging in team mentorship, and incorporating mindful mentoring, an emphasis on self-care is critical.7 Taking time to recharge is essential. It allows one to be fully present, while also setting an example for the mentee. Explicitly addressing self-care for both mentor and mentee is a part of mindful mentorship, with benefits for all.6
Three key points:
1. Awareness of mentorship malpractice
2. Importance of team mentorship
3. Benefits of mindful mentorship
Mr. Rodoni is with the University of Michigan Medical School and Stephen M. Ross School of Business, Ann Arbor, Mich. Dr. Fessel is a professor of radiology in the department of radiology at Michigan Medicine, Ann Arbor. They reported having no disclosures relevant to this article.
References:
1. Chopra V et al. JAMA Intern Med. 2018 Feb;178:175-6.
2. Chopra V et al. JAMA. 2016 Apr 12;315:1453-4.
3. Chopra V et al. The Mentoring Guide: Helping Mentors & Mentees Succeed. Ann Arbor: Michigan Publishing, 2019.
4. Rodoni BM et al. Annals of Surgery. 2020 Aug;272(2):e151-2.
5. Waljee JF et al. JAMA. 2018 Apr 17;319(15):1547-8.
6. Chopra V and Saint S. Healthc (Amst). 2020 Mar;8(1):100390.
7. Fessell D et al. “Mentoring During a Crisis.” Harvard Business Review. 2020 Oct 29.
Medical professional liability risk and mitigation: An overview for early-career gastroenterologists
Disclaimer: This article is for educational purposes only. All examples are hypothetical and aim to illustrate common clinical scenarios and challenges gastroenterologists may encounter within their scope of practice. The content herein should not be interpreted as legal advice for individual cases nor a substitute for seeking the advice of an attorney.
There are unique potential stressors faced by the gastroenterologist at each career stage, some more so early on. One such stressor, and one particularly important in a procedure-intensive specialty like GI, is medical professional liability (MPL), historically termed “medical malpractice.” Between 2009 and 2018, GI was the second-highest internal medicine subspecialty in both MPL claims made and claims paid,1 yet instruction on MPL risk and mitigation is scarce in fellowship, as is the available GI-related literature on the topic. This scarcity may generate untoward stress and unnecessarily expose gastroenterologists to avoidable MPL pitfalls. Therefore, it is vital for GI trainees, early-career gastroenterologists, and even seasoned gastroenterologists to have a working and updated knowledge of the general principles of MPL and GI-specific considerations. Such understanding can help preserve physician well-being, increase professional satisfaction, strengthen the doctor-patient relationship, and improve health care outcomes.2
To this end, we herein provide a focused review of the following: key MPL concepts, trends in MPL claims, GI-related MPL risk scenarios and considerations, adverse provider defensive mechanisms, documentation tenets, challenges posed by telemedicine, and the concept of “vicarious liability.”
Key MPL concepts
MPL falls under the umbrella of tort law, which itself falls under the umbrella of civil law; that is, civil (as opposed to criminal) justice governs torts – including but not limited to MPL claims – as well as other areas of law concerning noncriminal injury.3 A “tort” is a “civil wrong that unfairly causes another to experience loss or harm resulting in legal liability.”3 MPL claims assert the tort of negligence (similar to the concept of “incompetence”) and endeavor to compensate the harmed patient/individual while simultaneously dissuading suboptimal medical care by the provider in the future.4,5 A successful MPL claim must prove four overlapping elements: that the tortfeasor (here, the gastroenterologist) owed a duty of care to the injured party and breached that duty, which caused damages.6 Given that MPL cases exist within tort law rather than criminal law, the burden of proof for these cases is not “beyond a reasonable doubt”; instead, it’s “to a reasonable medical probability.”7
Trends in MPL claims
According to data compiled by the MPL Association, 278,220 MPL claims were made in the United States from 1985 to 2012.3,8-10 Among these, 1.8% involved gastroenterologists, which puts it at 17th place out of the 20 specialties surveyed.9 While the number of paid claims over this time frame decreased in GI by 34.6% (from 18.5 to 12.1 cases per 1,000 physician-years), there was a concurrent 23.3% increase in average claim compensation; essentially, there were fewer paid GI-related claims but there were higher payouts per paid claim.11,12 From 2009 to 2018, average legal defense costs for paid GI-related claims were $97,392, and average paid amount was $330,876.1
GI-related MPL risk scenarios and considerations
Many MPL claims relate to situations involving medical errors or adverse events (AEs), be they procedural or nonprocedural. However other aspects of GI also carry MPL risk.
Informed consent
MPL claims may be made not only on the grounds of inadequately informed consent but also inadequately informed refusal.5,13,14 While standards for adequate informed consent vary by state, most states apply the “reasonable patient standard,” i.e., assuming an average patient with enough information to be an active participant in the medical decision-making process. Generally, informed consent should ensure that the patient understands the nature of the procedure/treatment being proposed, there is a discussion of the risks and benefits of undergoing and not undergoing the procedure/treatment, reasonable alternatives are presented, the risks and benefits associated with these alternatives are discussed, and the patient’s comprehension of these things is assessed (Figure).15 Additionally, informed consent should be tailored to each patient and GI procedure/treatment on a case-by-case basis rather than using a one-size-fits-all approach. Moreover, documentation of the patient’s understanding of the (tailored) information provided can concurrently improve quality of the consent and potentially decrease MPL risk (Figure).16
Endoscopic procedures
Procedure-related MPL claims represent approximately 25% of all GI-related claims (8,17). Among these, 52% involve colonoscopy, 16% involve endoscopic retrograde cholangiopancreatography (ERCP), and 11% involve esophagogastroduodenoscopy.8 Albeit generally safe, colonoscopy, as with esophagogastroduodenoscopy, is subject to rare but serious AEs.18,19 Risk of these AEs may be accentuated in certain scenarios (such as severe colonic inflammation or coagulopathy) and, as discussed earlier, may merit tailored informed consent. Regardless of the procedure, in the event of postprocedural development of signs/symptoms (such as tachycardia, fever, chest or abdominal discomfort, or hypotension) indicating a potential AE, stabilizing measures and evaluation (such as blood work and imaging) should be undertaken, and hospital admission (if not already hospitalized) should be considered until discharge is deemed safe.19
ERCP-related MPL claims, for many years, have had the highest average compensation of any GI procedure.11 Though discussion of advanced procedures is beyond the scope of this article, it is worth mentioning the observation that most of such claims involve an allegation that the procedure was not indicated (for example, that it was performed based on inadequate evidence of pancreatobiliary pathology), or was for diagnostic purposes (for example, being done instead of noninvasive imaging) rather than therapeutic.20-23 This emphasizes the importance of appropriate procedure indications.
Percutaneous endoscopic gastrostomy (PEG) placement merits special mention given it can be complicated by ethical challenges (for example, needing a surrogate decision-maker’s consent or representing medical futility) and has a relatively high potential for MPL claims. PEG placement carries a low AE rate (0.1%-1%), but these AEs may result in high morbidity/mortality, in part because of the underlying comorbidities of patients needing PEG placement.24,25 Also, timing of a patient’s demise may coincide with PEG placement, thereby prompting (possibly unfounded) perceptions of causality.24-27 Therefore, such scenarios merit unique additional preprocedure safeguards. For instance, for patients lacking capacity to provide informed consent, especially when family members may differ on whether PEG should be placed, it is advisable to ask the family to select one surrogate decision-maker (if there’s no advance directive) to whom the gastroenterologist should discuss both the risks, benefits, and goals of PEG placement in the context of the patient’s overall clinical trajectory/life expectancy and the need for consent (or refusal) based on what the patient would have wished. In addition, having a medical professional witness this discussion may be useful.27
Antithrombotic agents
Periprocedural management of antithrombotics, including anticoagulants and antiplatelets, can pose challenges for the gastroenterologist. While clinical practice guidelines exist to guide decision-making in this regard, the variables involved may extend beyond the expertise of the gastroenterologist.28 For instance, in addition to the procedural risk for bleeding, the indication for antithrombotic therapy, risk of a thrombotic event, duration of action of the antithrombotic, and available bridging options should all be considered according to recommendations.28,29 While requiring more time on the part of the gastroenterologist, the optimal periprocedural management of antithrombotic agents would usually involve discussion with the provider managing antithrombotic therapy to best conduct a risk-benefit assessment regarding if (and how long) the antithrombotic therapy should be held (Figure). This shared decision-making, which should also include the patient, may help decrease MPL risk and improve outcomes.
Provider defense mechanisms
Physicians may engage in various defensive behaviors in an attempt to mitigate MPL risk; however, these behaviors may, paradoxically, increase risk.30,31
Assurance behaviors
Assurance behaviors refer to the practice of recommending or performing additional services (such as medications, imaging, procedures, and referrals) that are not clearly indicated.2,30,31 Assurance behaviors are driven by fear of MPL risk and/or missing a potential diagnosis. Recent studies have estimated that more than 50% of gastroenterologists worldwide have performed additional invasive procedures without clear indications, and that nearly one-third of endoscopic procedures annually have questionable indications.30,32 While assurance behaviors may seem likely to decrease MPL risk, overall, they may inadvertently increase AE and MPL risk, as well as health care expenditures.3,30,32
Avoidance behaviors
Avoidance behaviors refer to providers avoiding participation in potentially high-risk clinical interventions (for example, the actual procedures), including those for which they are credentialed/certified proficient.30,31 Two clinical scenarios that illustrate this behavior include the following: An advanced endoscopist credentialed to perform ERCP might refer a “high-risk” elderly patient with cholangitis to another provider to perform said ERCP or for percutaneous transhepatic drainage (in the absence of a clear benefit to such), or a gastroenterologist might refer a patient to interventional gastroenterology for resection of a large polyp even though gastroenterologists are usually proficient in this skill and may feel comfortable performing the resection themselves. Avoidance behaviors are driven by a fear of MPL risk and can have several negative consequences.33 For example, patients may not receive indicated interventions. Additionally, patients may have to wait longer for an intervention because they are referred to another provider, which also increases potential for loss to follow-up.2,30,31 This may be viewed as noncompliance with the standard of care, among other hazards, thereby increasing MPL risk.
Documentation tenets
Thorough documentation can decrease MPL risk, especially since it is often used as legal evidence.16 Documenting, for instance, preprocedure discussion of potential risk of AEs (such as bleeding or perforation) or procedural failure (for example, missed lesions)can protect gastroenterologists (Figure).16 While, as discussed previously, these should be covered in the informed consent process (which itself reduces MPL risk), proof of compliance in providing adequate informed consent must come in the form of documentation that indicates that the process took place and specifically what topics were discussed therein. MPL risk may be further decreased by documenting steps taken during a procedure and anatomic landmarks encountered to offer proof of technical competency and compliance with standards of care (Figure).16,34 In this context, it is worth recalling the adage: “If it’s not documented, it did not occur.”
Curbside consults versus consultation
Also germane here is the topic of whether documentation is needed for “curbside consults.” The uncertainty is, in part, semantic; that is, at what point does a “curbside” become a consultation? A curbside is a general question or query (such as anything that could also be answered by searching the Internet or reference materials) in response to which information is provided; once it involves provision of medical advice for a specific patient (for example, when patient identifiers have been shared or their EHR has been accessed), it constitutes a consultation. Based on these definitions, a curbside need not be documented, whereas a consultation – even if seemingly trivial – should be.
Consideration of language and cultural factors
Language barriers should be considered when the gastroenterologist is communicating with the patient, and such efforts, whenever made, should be documented to best protect against MPL.16,35 These considerations arise not only during the consent process but when obtaining a history, providing postprocedure instructions, and during follow-ups. To this end, 24/7 telephone interpreter services may assist the gastroenterologist (when one is communicating with non–English speakers and is not medically certified in the patient’s native/preferred language) and strengthen trust in the provider-patient relationship.36 Additionally, written materials (such as consent forms, procedural information) in patients’ native/preferred languages should be provided, when available, to enhance patient understanding and participation in care (Figure).35
Challenges posed by telemedicine
The COVID-19 pandemic has rapidly led to more virtual encounters. While increased utilization of telemedicine platforms may make health care more accessible, it does not lessen the clinicians’ duty to patients and may actually expose them to greater MPL risk.18,37,38 Therefore, the provider must be cognizant of two key principles to mitigate MPL risk in the context of telemedicine encounters. First, the same standard of care applies to virtual and in-person encounters.18,37,38 Second, patient privacy and HIPAA regulations are not waived during telemedicine encounters, and breaches of such may result in an MPL claim.18,37,38
With regard to the first principle, for patients who have not been physically examined (such as when a telemedicine visit was substituted for an in-person clinic encounter), gastroenterologists should not overlook requesting timely preprocedure anesthesia consultation or obtaining additional laboratory studies as needed to ensure safety and the same standard of care. Moreover, particularly in the context of pandemic-related decreased procedural capacity, triaging procedures can be especially challenging. Standardized institutional criteria which prioritize certain diagnoses/conditions over others, leaving room for justifiable exceptions, are advisable.
Vicarious liability
“Vicarious liability” is defined as that extending to persons who have not committed a wrong but on whose behalf wrongdoers acted.39 Therefore, gastroenterologists may be liable not only for their own actions but also for those of personnel they supervise (such as fellow trainees and non–physician practitioners).39 Vicarious liability aims to ensure that systemic checks and balances are in place so that, if failure occurs, harm can still be mitigated and/or avoided, as illustrated by Reason’s “Swiss Cheese Model.”40
Conclusion
Any gastroenterologist can experience an MPL claim. Such an experience can be especially stressful and confusing to early-career clinicians, especially if they’re unfamiliar with legal proceedings. Although MPL principles are not often taught in medical school or residency, it is important for gastroenterologists to be informed regarding tenets of MPL and cognizant of clinical situations which have relatively higher MPL risk. This can assuage untoward angst regarding MPL and highlight proactive risk-mitigation strategies. In general, gastroenterologist practices that can mitigate MPL risk include effective communication; adequate informed consent/refusal; documentation of preprocedure counseling, periprocedure events, and postprocedure recommendations; and maintenance of proper certification and privileging.
Dr. Azizian and Dr. Dalai are with the University of California, Los Angeles and the department of medicine at Olive View–UCLA Medical Center, Sylmar, Calif. They are co–first authors of this paper. Dr. Dalai is also with the division of gastroenterology at the University of New Mexico, Albuquerque. Dr. Adams is with the Center for Clinical Management Research in Veterans Affairs Ann Arbor Healthcare System, the division of gastroenterology at the University of Michigan Health System, and the Institute for Healthcare Policy and Innovation, all in Ann Arbor, Mich. Dr. Tabibian is with UCLA and the division of gastroenterology at Olive View–UCLA Medical Center. The authors have no conflicts of interest.
References
1. 2020 Data Sharing Project Gastroenterology 2009-2018. Inside Medical Liability: Second Quarter. Accessed 2020 Dec 6.
2. Mello MM et al. Health Aff (Millwood). 2004 Jul-Aug;23(4):42-53.
3. Adams MA et al. JAMA. 2014 Oct;312(13):1348-9.
4. Pegalis SE. American Law of Medical Malpractice 3d, Vol. 2. St. Paul, Minn.: Thomson Reuters, 2005.
5. Feld LD et al. Am J Gastroenterol. 2018 Nov;113(11):1577-9.
6. Sawyer v. Wight, 196 F. Supp. 2d 220, 226 (E.D.N.Y. 2002).
7. Michael A. Sita v. Long Island Jewish-Hillside Medical Center, 22 A.D.3d 743 (N.Y. App. Div. 2005).
8. Conklin LS et al. Clin Gastroenterol Hepatol. 2008 Jun;6(6):677-81.
9. Jena AB et al. N Engl J Med. 2011 Aug 18;365(7):629-36.
10. Kane CK. “Policy Research Perspectives Medical Liability Claim Frequency: A 2007-2008 Snapshot of Physicians.” Chicago: American Medical Association, 2010.
11. Hernandez LV et al. World J Gastrointest Endosc. 2013 Apr 16;5(4):169-73.
12. Schaffer AC et al. JAMA Intern Med. 2017 May 1;177(5):710-8.
13. Natanson v. Kline, 186 Kan. 393, 409, 350 P.2d 1093, 1106, decision clarified on denial of reh’g, 187 Kan. 186, 354 P.2d 670 (1960).
14. Truman v. Thomas, 27 Cal. 3d 285, 292, 611 P.2d 902, 906 (1980).
15. Shah P et al. Informed Consent, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Aug 22.
16. Rex DK. Clin Gastroenterol Hepatol. 2013 Jul;11(7):768-73.
17. Gerstenberger PD, Plumeri PA. Gastrointest Endosc. Mar-Apr 1993;39(2):132-8.
18. Adams MA and Allen JI. Clin Gastroenterol Hepatol. 2019 Nov;17(12):2392-6.e1.
19. Ahlawat R et al. Esophagogastroduodenoscopy, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Dec 9.
20. Cotton PB. Gastrointest Endosc. 2006 Mar;63(3):378-82.
21. Cotton PB. Gastrointest Endosc. 2010 Oct;72(4):904.
22. Adamson TE et al. West J Med. 1989 Mar;150(3):356-60.
23. Trap R et al. Endoscopy. 1999 Feb;31(2):125-30.
24. Funaki B. Semin Intervent Radiol. 2015 Mar;32(1):61-4.
25. Feeding Tube Nursing Home and Hospital Malpractice. Miller & Zois, Attorneys at Law. Accessed 2020 Jun 20.
26. Medical Malpractice Lawsuit Brings $750,000 Settlement: Death of 82-year-old woman from sepsis due to improper placement of feeding tube. Lubin & Meyers PC. Accessed 2020 Jun 20.
27. Brendel RW et al. Med Clin North Am. 2010 Nov;94(6):1229-40, xi-ii.
28. ASGE Standards of Practice Committee; Acosta RD et al. Gastrointest Endosc. 2016 Jan;83(1):3-16.
29. Saleem S and Thomas AL. Cureus. 2018 Jun 25;10(6):e2878.
30. Hiyama T et al. World J Gastroenterol. 2006 Dec 21;12(47):7671-5.
31. Studdert DM et al. JAMA. 2005 Jun 1;293(21):2609-17.
32. Shaheen NJ et al. Gastroenterology. 2018 May;154(7):1993-2003.
33. Oza VM et al. Clin Gastroenterol Hepatol. 2016 Feb;14(2):172-4.
34. Feld AD. Gastrointest Endosc Clin N Am. 2002 Jan;12(1):171-9, viii-ix.
35. Lee JS et al. J Gen Intern Med. 2017 Aug;32(8):863-70.
36. Forrow L and Kontrimas JC. J Gen Intern Med. 2017 Aug;32(8):855-7.
37. Moses RE et al. Am J Gastroenterol. 2014 Aug;109(8):1128-32.
38. Tabibian JH. “The Evolution of Telehealth.” Guidepoint: Legal Solutions Blog. Accessed 2020 Aug 12.
39. Feld AD. Am J Gastroenterol. 2004 Sep;99(9):1641-4.
40. Reason J. BMJ. 2000;320(7237):768‐70.
Disclaimer: This article is for educational purposes only. All examples are hypothetical and aim to illustrate common clinical scenarios and challenges gastroenterologists may encounter within their scope of practice. The content herein should not be interpreted as legal advice for individual cases nor a substitute for seeking the advice of an attorney.
There are unique potential stressors faced by the gastroenterologist at each career stage, some more so early on. One such stressor, and one particularly important in a procedure-intensive specialty like GI, is medical professional liability (MPL), historically termed “medical malpractice.” Between 2009 and 2018, GI was the second-highest internal medicine subspecialty in both MPL claims made and claims paid,1 yet instruction on MPL risk and mitigation is scarce in fellowship, as is the available GI-related literature on the topic. This scarcity may generate untoward stress and unnecessarily expose gastroenterologists to avoidable MPL pitfalls. Therefore, it is vital for GI trainees, early-career gastroenterologists, and even seasoned gastroenterologists to have a working and updated knowledge of the general principles of MPL and GI-specific considerations. Such understanding can help preserve physician well-being, increase professional satisfaction, strengthen the doctor-patient relationship, and improve health care outcomes.2
To this end, we herein provide a focused review of the following: key MPL concepts, trends in MPL claims, GI-related MPL risk scenarios and considerations, adverse provider defensive mechanisms, documentation tenets, challenges posed by telemedicine, and the concept of “vicarious liability.”
Key MPL concepts
MPL falls under the umbrella of tort law, which itself falls under the umbrella of civil law; that is, civil (as opposed to criminal) justice governs torts – including but not limited to MPL claims – as well as other areas of law concerning noncriminal injury.3 A “tort” is a “civil wrong that unfairly causes another to experience loss or harm resulting in legal liability.”3 MPL claims assert the tort of negligence (similar to the concept of “incompetence”) and endeavor to compensate the harmed patient/individual while simultaneously dissuading suboptimal medical care by the provider in the future.4,5 A successful MPL claim must prove four overlapping elements: that the tortfeasor (here, the gastroenterologist) owed a duty of care to the injured party and breached that duty, which caused damages.6 Given that MPL cases exist within tort law rather than criminal law, the burden of proof for these cases is not “beyond a reasonable doubt”; instead, it’s “to a reasonable medical probability.”7
Trends in MPL claims
According to data compiled by the MPL Association, 278,220 MPL claims were made in the United States from 1985 to 2012.3,8-10 Among these, 1.8% involved gastroenterologists, which puts it at 17th place out of the 20 specialties surveyed.9 While the number of paid claims over this time frame decreased in GI by 34.6% (from 18.5 to 12.1 cases per 1,000 physician-years), there was a concurrent 23.3% increase in average claim compensation; essentially, there were fewer paid GI-related claims but there were higher payouts per paid claim.11,12 From 2009 to 2018, average legal defense costs for paid GI-related claims were $97,392, and average paid amount was $330,876.1
GI-related MPL risk scenarios and considerations
Many MPL claims relate to situations involving medical errors or adverse events (AEs), be they procedural or nonprocedural. However other aspects of GI also carry MPL risk.

Informed consent
MPL claims may be made not only on the grounds of inadequately informed consent but also inadequately informed refusal.5,13,14 While standards for adequate informed consent vary by state, most states apply the “reasonable patient standard,” i.e., assuming an average patient with enough information to be an active participant in the medical decision-making process. Generally, informed consent should ensure that the patient understands the nature of the procedure/treatment being proposed, there is a discussion of the risks and benefits of undergoing and not undergoing the procedure/treatment, reasonable alternatives are presented, the risks and benefits associated with these alternatives are discussed, and the patient’s comprehension of these things is assessed (Figure).15 Additionally, informed consent should be tailored to each patient and GI procedure/treatment on a case-by-case basis rather than using a one-size-fits-all approach. Moreover, documentation of the patient’s understanding of the (tailored) information provided can concurrently improve quality of the consent and potentially decrease MPL risk (Figure).16
Endoscopic procedures
Procedure-related MPL claims represent approximately 25% of all GI-related claims (8,17). Among these, 52% involve colonoscopy, 16% involve endoscopic retrograde cholangiopancreatography (ERCP), and 11% involve esophagogastroduodenoscopy.8 Albeit generally safe, colonoscopy, as with esophagogastroduodenoscopy, is subject to rare but serious AEs.18,19 Risk of these AEs may be accentuated in certain scenarios (such as severe colonic inflammation or coagulopathy) and, as discussed earlier, may merit tailored informed consent. Regardless of the procedure, in the event of postprocedural development of signs/symptoms (such as tachycardia, fever, chest or abdominal discomfort, or hypotension) indicating a potential AE, stabilizing measures and evaluation (such as blood work and imaging) should be undertaken, and hospital admission (if not already hospitalized) should be considered until discharge is deemed safe.19
ERCP-related MPL claims, for many years, have had the highest average compensation of any GI procedure.11 Though discussion of advanced procedures is beyond the scope of this article, it is worth mentioning the observation that most of such claims involve an allegation that the procedure was not indicated (for example, that it was performed based on inadequate evidence of pancreatobiliary pathology), or was for diagnostic purposes (for example, being done instead of noninvasive imaging) rather than therapeutic.20-23 This emphasizes the importance of appropriate procedure indications.
Percutaneous endoscopic gastrostomy (PEG) placement merits special mention given it can be complicated by ethical challenges (for example, needing a surrogate decision-maker’s consent or representing medical futility) and has a relatively high potential for MPL claims. PEG placement carries a low AE rate (0.1%-1%), but these AEs may result in high morbidity/mortality, in part because of the underlying comorbidities of patients needing PEG placement.24,25 Also, timing of a patient’s demise may coincide with PEG placement, thereby prompting (possibly unfounded) perceptions of causality.24-27 Therefore, such scenarios merit unique additional preprocedure safeguards. For instance, for patients lacking capacity to provide informed consent, especially when family members may differ on whether PEG should be placed, it is advisable to ask the family to select one surrogate decision-maker (if there’s no advance directive) to whom the gastroenterologist should discuss both the risks, benefits, and goals of PEG placement in the context of the patient’s overall clinical trajectory/life expectancy and the need for consent (or refusal) based on what the patient would have wished. In addition, having a medical professional witness this discussion may be useful.27
Antithrombotic agents
Periprocedural management of antithrombotics, including anticoagulants and antiplatelets, can pose challenges for the gastroenterologist. While clinical practice guidelines exist to guide decision-making in this regard, the variables involved may extend beyond the expertise of the gastroenterologist.28 For instance, in addition to the procedural risk for bleeding, the indication for antithrombotic therapy, risk of a thrombotic event, duration of action of the antithrombotic, and available bridging options should all be considered according to recommendations.28,29 While requiring more time on the part of the gastroenterologist, the optimal periprocedural management of antithrombotic agents would usually involve discussion with the provider managing antithrombotic therapy to best conduct a risk-benefit assessment regarding if (and how long) the antithrombotic therapy should be held (Figure). This shared decision-making, which should also include the patient, may help decrease MPL risk and improve outcomes.
Provider defense mechanisms
Physicians may engage in various defensive behaviors in an attempt to mitigate MPL risk; however, these behaviors may, paradoxically, increase risk.30,31
Assurance behaviors
Assurance behaviors refer to the practice of recommending or performing additional services (such as medications, imaging, procedures, and referrals) that are not clearly indicated.2,30,31 Assurance behaviors are driven by fear of MPL risk and/or missing a potential diagnosis. Recent studies have estimated that more than 50% of gastroenterologists worldwide have performed additional invasive procedures without clear indications, and that nearly one-third of endoscopic procedures annually have questionable indications.30,32 While assurance behaviors may seem likely to decrease MPL risk, overall, they may inadvertently increase AE and MPL risk, as well as health care expenditures.3,30,32
Avoidance behaviors
Avoidance behaviors refer to providers avoiding participation in potentially high-risk clinical interventions (for example, the actual procedures), including those for which they are credentialed/certified proficient.30,31 Two clinical scenarios that illustrate this behavior include the following: An advanced endoscopist credentialed to perform ERCP might refer a “high-risk” elderly patient with cholangitis to another provider to perform said ERCP or for percutaneous transhepatic drainage (in the absence of a clear benefit to such), or a gastroenterologist might refer a patient to interventional gastroenterology for resection of a large polyp even though gastroenterologists are usually proficient in this skill and may feel comfortable performing the resection themselves. Avoidance behaviors are driven by a fear of MPL risk and can have several negative consequences.33 For example, patients may not receive indicated interventions. Additionally, patients may have to wait longer for an intervention because they are referred to another provider, which also increases potential for loss to follow-up.2,30,31 This may be viewed as noncompliance with the standard of care, among other hazards, thereby increasing MPL risk.
Documentation tenets
Thorough documentation can decrease MPL risk, especially since it is often used as legal evidence.16 Documenting, for instance, preprocedure discussion of potential risk of AEs (such as bleeding or perforation) or procedural failure (for example, missed lesions)can protect gastroenterologists (Figure).16 While, as discussed previously, these should be covered in the informed consent process (which itself reduces MPL risk), proof of compliance in providing adequate informed consent must come in the form of documentation that indicates that the process took place and specifically what topics were discussed therein. MPL risk may be further decreased by documenting steps taken during a procedure and anatomic landmarks encountered to offer proof of technical competency and compliance with standards of care (Figure).16,34 In this context, it is worth recalling the adage: “If it’s not documented, it did not occur.”
Curbside consults versus consultation
Also germane here is the topic of whether documentation is needed for “curbside consults.” The uncertainty is, in part, semantic; that is, at what point does a “curbside” become a consultation? A curbside is a general question or query (such as anything that could also be answered by searching the Internet or reference materials) in response to which information is provided; once it involves provision of medical advice for a specific patient (for example, when patient identifiers have been shared or their EHR has been accessed), it constitutes a consultation. Based on these definitions, a curbside need not be documented, whereas a consultation – even if seemingly trivial – should be.
Consideration of language and cultural factors
Language barriers should be considered when the gastroenterologist is communicating with the patient, and such efforts, whenever made, should be documented to best protect against MPL.16,35 These considerations arise not only during the consent process but when obtaining a history, providing postprocedure instructions, and during follow-ups. To this end, 24/7 telephone interpreter services may assist the gastroenterologist (when one is communicating with non–English speakers and is not medically certified in the patient’s native/preferred language) and strengthen trust in the provider-patient relationship.36 Additionally, written materials (such as consent forms, procedural information) in patients’ native/preferred languages should be provided, when available, to enhance patient understanding and participation in care (Figure).35
Challenges posed by telemedicine
The COVID-19 pandemic has rapidly led to more virtual encounters. While increased utilization of telemedicine platforms may make health care more accessible, it does not lessen the clinicians’ duty to patients and may actually expose them to greater MPL risk.18,37,38 Therefore, the provider must be cognizant of two key principles to mitigate MPL risk in the context of telemedicine encounters. First, the same standard of care applies to virtual and in-person encounters.18,37,38 Second, patient privacy and HIPAA regulations are not waived during telemedicine encounters, and breaches of such may result in an MPL claim.18,37,38
With regard to the first principle, for patients who have not been physically examined (such as when a telemedicine visit was substituted for an in-person clinic encounter), gastroenterologists should not overlook requesting timely preprocedure anesthesia consultation or obtaining additional laboratory studies as needed to ensure safety and the same standard of care. Moreover, particularly in the context of pandemic-related decreased procedural capacity, triaging procedures can be especially challenging. Standardized institutional criteria which prioritize certain diagnoses/conditions over others, leaving room for justifiable exceptions, are advisable.
Vicarious liability
“Vicarious liability” is defined as that extending to persons who have not committed a wrong but on whose behalf wrongdoers acted.39 Therefore, gastroenterologists may be liable not only for their own actions but also for those of personnel they supervise (such as fellow trainees and non–physician practitioners).39 Vicarious liability aims to ensure that systemic checks and balances are in place so that, if failure occurs, harm can still be mitigated and/or avoided, as illustrated by Reason’s “Swiss Cheese Model.”40
Conclusion
Any gastroenterologist can experience an MPL claim. Such an experience can be especially stressful and confusing to early-career clinicians, especially if they’re unfamiliar with legal proceedings. Although MPL principles are not often taught in medical school or residency, it is important for gastroenterologists to be informed regarding tenets of MPL and cognizant of clinical situations which have relatively higher MPL risk. This can assuage untoward angst regarding MPL and highlight proactive risk-mitigation strategies. In general, gastroenterologist practices that can mitigate MPL risk include effective communication; adequate informed consent/refusal; documentation of preprocedure counseling, periprocedure events, and postprocedure recommendations; and maintenance of proper certification and privileging.
Dr. Azizian and Dr. Dalai are with the University of California, Los Angeles and the department of medicine at Olive View–UCLA Medical Center, Sylmar, Calif. They are co–first authors of this paper. Dr. Dalai is also with the division of gastroenterology at the University of New Mexico, Albuquerque. Dr. Adams is with the Center for Clinical Management Research in Veterans Affairs Ann Arbor Healthcare System, the division of gastroenterology at the University of Michigan Health System, and the Institute for Healthcare Policy and Innovation, all in Ann Arbor, Mich. Dr. Tabibian is with UCLA and the division of gastroenterology at Olive View–UCLA Medical Center. The authors have no conflicts of interest.
References
1. 2020 Data Sharing Project Gastroenterology 2009-2018. Inside Medical Liability: Second Quarter. Accessed 2020 Dec 6.
2. Mello MM et al. Health Aff (Millwood). 2004 Jul-Aug;23(4):42-53.
3. Adams MA et al. JAMA. 2014 Oct;312(13):1348-9.
4. Pegalis SE. American Law of Medical Malpractice 3d, Vol. 2. St. Paul, Minn.: Thomson Reuters, 2005.
5. Feld LD et al. Am J Gastroenterol. 2018 Nov;113(11):1577-9.
6. Sawyer v. Wight, 196 F. Supp. 2d 220, 226 (E.D.N.Y. 2002).
7. Michael A. Sita v. Long Island Jewish-Hillside Medical Center, 22 A.D.3d 743 (N.Y. App. Div. 2005).
8. Conklin LS et al. Clin Gastroenterol Hepatol. 2008 Jun;6(6):677-81.
9. Jena AB et al. N Engl J Med. 2011 Aug 18;365(7):629-36.
10. Kane CK. “Policy Research Perspectives Medical Liability Claim Frequency: A 2007-2008 Snapshot of Physicians.” Chicago: American Medical Association, 2010.
11. Hernandez LV et al. World J Gastrointest Endosc. 2013 Apr 16;5(4):169-73.
12. Schaffer AC et al. JAMA Intern Med. 2017 May 1;177(5):710-8.
13. Natanson v. Kline, 186 Kan. 393, 409, 350 P.2d 1093, 1106, decision clarified on denial of reh’g, 187 Kan. 186, 354 P.2d 670 (1960).
14. Truman v. Thomas, 27 Cal. 3d 285, 292, 611 P.2d 902, 906 (1980).
15. Shah P et al. Informed Consent, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Aug 22.
16. Rex DK. Clin Gastroenterol Hepatol. 2013 Jul;11(7):768-73.
17. Gerstenberger PD, Plumeri PA. Gastrointest Endosc. Mar-Apr 1993;39(2):132-8.
18. Adams MA and Allen JI. Clin Gastroenterol Hepatol. 2019 Nov;17(12):2392-6.e1.
19. Ahlawat R et al. Esophagogastroduodenoscopy, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Dec 9.
20. Cotton PB. Gastrointest Endosc. 2006 Mar;63(3):378-82.
21. Cotton PB. Gastrointest Endosc. 2010 Oct;72(4):904.
22. Adamson TE et al. West J Med. 1989 Mar;150(3):356-60.
23. Trap R et al. Endoscopy. 1999 Feb;31(2):125-30.
24. Funaki B. Semin Intervent Radiol. 2015 Mar;32(1):61-4.
25. Feeding Tube Nursing Home and Hospital Malpractice. Miller & Zois, Attorneys at Law. Accessed 2020 Jun 20.
26. Medical Malpractice Lawsuit Brings $750,000 Settlement: Death of 82-year-old woman from sepsis due to improper placement of feeding tube. Lubin & Meyers PC. Accessed 2020 Jun 20.
27. Brendel RW et al. Med Clin North Am. 2010 Nov;94(6):1229-40, xi-ii.
28. ASGE Standards of Practice Committee; Acosta RD et al. Gastrointest Endosc. 2016 Jan;83(1):3-16.
29. Saleem S and Thomas AL. Cureus. 2018 Jun 25;10(6):e2878.
30. Hiyama T et al. World J Gastroenterol. 2006 Dec 21;12(47):7671-5.
31. Studdert DM et al. JAMA. 2005 Jun 1;293(21):2609-17.
32. Shaheen NJ et al. Gastroenterology. 2018 May;154(7):1993-2003.
33. Oza VM et al. Clin Gastroenterol Hepatol. 2016 Feb;14(2):172-4.
34. Feld AD. Gastrointest Endosc Clin N Am. 2002 Jan;12(1):171-9, viii-ix.
35. Lee JS et al. J Gen Intern Med. 2017 Aug;32(8):863-70.
36. Forrow L and Kontrimas JC. J Gen Intern Med. 2017 Aug;32(8):855-7.
37. Moses RE et al. Am J Gastroenterol. 2014 Aug;109(8):1128-32.
38. Tabibian JH. “The Evolution of Telehealth.” Guidepoint: Legal Solutions Blog. Accessed 2020 Aug 12.
39. Feld AD. Am J Gastroenterol. 2004 Sep;99(9):1641-4.
40. Reason J. BMJ. 2000;320(7237):768‐70.
Disclaimer: This article is for educational purposes only. All examples are hypothetical and aim to illustrate common clinical scenarios and challenges gastroenterologists may encounter within their scope of practice. The content herein should not be interpreted as legal advice for individual cases nor a substitute for seeking the advice of an attorney.
There are unique potential stressors faced by the gastroenterologist at each career stage, some more so early on. One such stressor, and one particularly important in a procedure-intensive specialty like GI, is medical professional liability (MPL), historically termed “medical malpractice.” Between 2009 and 2018, GI was the second-highest internal medicine subspecialty in both MPL claims made and claims paid,1 yet instruction on MPL risk and mitigation is scarce in fellowship, as is the available GI-related literature on the topic. This scarcity may generate untoward stress and unnecessarily expose gastroenterologists to avoidable MPL pitfalls. Therefore, it is vital for GI trainees, early-career gastroenterologists, and even seasoned gastroenterologists to have a working and updated knowledge of the general principles of MPL and GI-specific considerations. Such understanding can help preserve physician well-being, increase professional satisfaction, strengthen the doctor-patient relationship, and improve health care outcomes.2
To this end, we herein provide a focused review of the following: key MPL concepts, trends in MPL claims, GI-related MPL risk scenarios and considerations, adverse provider defensive mechanisms, documentation tenets, challenges posed by telemedicine, and the concept of “vicarious liability.”
Key MPL concepts
MPL falls under the umbrella of tort law, which itself falls under the umbrella of civil law; that is, civil (as opposed to criminal) justice governs torts – including but not limited to MPL claims – as well as other areas of law concerning noncriminal injury.3 A “tort” is a “civil wrong that unfairly causes another to experience loss or harm resulting in legal liability.”3 MPL claims assert the tort of negligence (similar to the concept of “incompetence”) and endeavor to compensate the harmed patient/individual while simultaneously dissuading suboptimal medical care by the provider in the future.4,5 A successful MPL claim must prove four overlapping elements: that the tortfeasor (here, the gastroenterologist) owed a duty of care to the injured party and breached that duty, which caused damages.6 Given that MPL cases exist within tort law rather than criminal law, the burden of proof for these cases is not “beyond a reasonable doubt”; instead, it’s “to a reasonable medical probability.”7
Trends in MPL claims
According to data compiled by the MPL Association, 278,220 MPL claims were made in the United States from 1985 to 2012.3,8-10 Among these, 1.8% involved gastroenterologists, which puts it at 17th place out of the 20 specialties surveyed.9 While the number of paid claims over this time frame decreased in GI by 34.6% (from 18.5 to 12.1 cases per 1,000 physician-years), there was a concurrent 23.3% increase in average claim compensation; essentially, there were fewer paid GI-related claims but there were higher payouts per paid claim.11,12 From 2009 to 2018, average legal defense costs for paid GI-related claims were $97,392, and average paid amount was $330,876.1
GI-related MPL risk scenarios and considerations
Many MPL claims relate to situations involving medical errors or adverse events (AEs), be they procedural or nonprocedural. However other aspects of GI also carry MPL risk.

Informed consent
MPL claims may be made not only on the grounds of inadequately informed consent but also inadequately informed refusal.5,13,14 While standards for adequate informed consent vary by state, most states apply the “reasonable patient standard,” i.e., assuming an average patient with enough information to be an active participant in the medical decision-making process. Generally, informed consent should ensure that the patient understands the nature of the procedure/treatment being proposed, there is a discussion of the risks and benefits of undergoing and not undergoing the procedure/treatment, reasonable alternatives are presented, the risks and benefits associated with these alternatives are discussed, and the patient’s comprehension of these things is assessed (Figure).15 Additionally, informed consent should be tailored to each patient and GI procedure/treatment on a case-by-case basis rather than using a one-size-fits-all approach. Moreover, documentation of the patient’s understanding of the (tailored) information provided can concurrently improve quality of the consent and potentially decrease MPL risk (Figure).16
Endoscopic procedures
Procedure-related MPL claims represent approximately 25% of all GI-related claims (8,17). Among these, 52% involve colonoscopy, 16% involve endoscopic retrograde cholangiopancreatography (ERCP), and 11% involve esophagogastroduodenoscopy.8 Albeit generally safe, colonoscopy, as with esophagogastroduodenoscopy, is subject to rare but serious AEs.18,19 Risk of these AEs may be accentuated in certain scenarios (such as severe colonic inflammation or coagulopathy) and, as discussed earlier, may merit tailored informed consent. Regardless of the procedure, in the event of postprocedural development of signs/symptoms (such as tachycardia, fever, chest or abdominal discomfort, or hypotension) indicating a potential AE, stabilizing measures and evaluation (such as blood work and imaging) should be undertaken, and hospital admission (if not already hospitalized) should be considered until discharge is deemed safe.19
ERCP-related MPL claims, for many years, have had the highest average compensation of any GI procedure.11 Though discussion of advanced procedures is beyond the scope of this article, it is worth mentioning the observation that most of such claims involve an allegation that the procedure was not indicated (for example, that it was performed based on inadequate evidence of pancreatobiliary pathology), or was for diagnostic purposes (for example, being done instead of noninvasive imaging) rather than therapeutic.20-23 This emphasizes the importance of appropriate procedure indications.
Percutaneous endoscopic gastrostomy (PEG) placement merits special mention given it can be complicated by ethical challenges (for example, needing a surrogate decision-maker’s consent or representing medical futility) and has a relatively high potential for MPL claims. PEG placement carries a low AE rate (0.1%-1%), but these AEs may result in high morbidity/mortality, in part because of the underlying comorbidities of patients needing PEG placement.24,25 Also, timing of a patient’s demise may coincide with PEG placement, thereby prompting (possibly unfounded) perceptions of causality.24-27 Therefore, such scenarios merit unique additional preprocedure safeguards. For instance, for patients lacking capacity to provide informed consent, especially when family members may differ on whether PEG should be placed, it is advisable to ask the family to select one surrogate decision-maker (if there’s no advance directive) to whom the gastroenterologist should discuss both the risks, benefits, and goals of PEG placement in the context of the patient’s overall clinical trajectory/life expectancy and the need for consent (or refusal) based on what the patient would have wished. In addition, having a medical professional witness this discussion may be useful.27
Antithrombotic agents
Periprocedural management of antithrombotics, including anticoagulants and antiplatelets, can pose challenges for the gastroenterologist. While clinical practice guidelines exist to guide decision-making in this regard, the variables involved may extend beyond the expertise of the gastroenterologist.28 For instance, in addition to the procedural risk for bleeding, the indication for antithrombotic therapy, risk of a thrombotic event, duration of action of the antithrombotic, and available bridging options should all be considered according to recommendations.28,29 While requiring more time on the part of the gastroenterologist, the optimal periprocedural management of antithrombotic agents would usually involve discussion with the provider managing antithrombotic therapy to best conduct a risk-benefit assessment regarding if (and how long) the antithrombotic therapy should be held (Figure). This shared decision-making, which should also include the patient, may help decrease MPL risk and improve outcomes.
Provider defense mechanisms
Physicians may engage in various defensive behaviors in an attempt to mitigate MPL risk; however, these behaviors may, paradoxically, increase risk.30,31
Assurance behaviors
Assurance behaviors refer to the practice of recommending or performing additional services (such as medications, imaging, procedures, and referrals) that are not clearly indicated.2,30,31 Assurance behaviors are driven by fear of MPL risk and/or missing a potential diagnosis. Recent studies have estimated that more than 50% of gastroenterologists worldwide have performed additional invasive procedures without clear indications, and that nearly one-third of endoscopic procedures annually have questionable indications.30,32 While assurance behaviors may seem likely to decrease MPL risk, overall, they may inadvertently increase AE and MPL risk, as well as health care expenditures.3,30,32
Avoidance behaviors
Avoidance behaviors refer to providers avoiding participation in potentially high-risk clinical interventions (for example, the actual procedures), including those for which they are credentialed/certified proficient.30,31 Two clinical scenarios that illustrate this behavior include the following: An advanced endoscopist credentialed to perform ERCP might refer a “high-risk” elderly patient with cholangitis to another provider to perform said ERCP or for percutaneous transhepatic drainage (in the absence of a clear benefit to such), or a gastroenterologist might refer a patient to interventional gastroenterology for resection of a large polyp even though gastroenterologists are usually proficient in this skill and may feel comfortable performing the resection themselves. Avoidance behaviors are driven by a fear of MPL risk and can have several negative consequences.33 For example, patients may not receive indicated interventions. Additionally, patients may have to wait longer for an intervention because they are referred to another provider, which also increases potential for loss to follow-up.2,30,31 This may be viewed as noncompliance with the standard of care, among other hazards, thereby increasing MPL risk.
Documentation tenets
Thorough documentation can decrease MPL risk, especially since it is often used as legal evidence.16 Documenting, for instance, preprocedure discussion of potential risk of AEs (such as bleeding or perforation) or procedural failure (for example, missed lesions)can protect gastroenterologists (Figure).16 While, as discussed previously, these should be covered in the informed consent process (which itself reduces MPL risk), proof of compliance in providing adequate informed consent must come in the form of documentation that indicates that the process took place and specifically what topics were discussed therein. MPL risk may be further decreased by documenting steps taken during a procedure and anatomic landmarks encountered to offer proof of technical competency and compliance with standards of care (Figure).16,34 In this context, it is worth recalling the adage: “If it’s not documented, it did not occur.”
Curbside consults versus consultation
Also germane here is the topic of whether documentation is needed for “curbside consults.” The uncertainty is, in part, semantic; that is, at what point does a “curbside” become a consultation? A curbside is a general question or query (such as anything that could also be answered by searching the Internet or reference materials) in response to which information is provided; once it involves provision of medical advice for a specific patient (for example, when patient identifiers have been shared or their EHR has been accessed), it constitutes a consultation. Based on these definitions, a curbside need not be documented, whereas a consultation – even if seemingly trivial – should be.
Consideration of language and cultural factors
Language barriers should be considered when the gastroenterologist is communicating with the patient, and such efforts, whenever made, should be documented to best protect against MPL.16,35 These considerations arise not only during the consent process but when obtaining a history, providing postprocedure instructions, and during follow-ups. To this end, 24/7 telephone interpreter services may assist the gastroenterologist (when one is communicating with non–English speakers and is not medically certified in the patient’s native/preferred language) and strengthen trust in the provider-patient relationship.36 Additionally, written materials (such as consent forms, procedural information) in patients’ native/preferred languages should be provided, when available, to enhance patient understanding and participation in care (Figure).35
Challenges posed by telemedicine
The COVID-19 pandemic has rapidly led to more virtual encounters. While increased utilization of telemedicine platforms may make health care more accessible, it does not lessen the clinicians’ duty to patients and may actually expose them to greater MPL risk.18,37,38 Therefore, the provider must be cognizant of two key principles to mitigate MPL risk in the context of telemedicine encounters. First, the same standard of care applies to virtual and in-person encounters.18,37,38 Second, patient privacy and HIPAA regulations are not waived during telemedicine encounters, and breaches of such may result in an MPL claim.18,37,38
With regard to the first principle, for patients who have not been physically examined (such as when a telemedicine visit was substituted for an in-person clinic encounter), gastroenterologists should not overlook requesting timely preprocedure anesthesia consultation or obtaining additional laboratory studies as needed to ensure safety and the same standard of care. Moreover, particularly in the context of pandemic-related decreased procedural capacity, triaging procedures can be especially challenging. Standardized institutional criteria which prioritize certain diagnoses/conditions over others, leaving room for justifiable exceptions, are advisable.
Vicarious liability
“Vicarious liability” is defined as that extending to persons who have not committed a wrong but on whose behalf wrongdoers acted.39 Therefore, gastroenterologists may be liable not only for their own actions but also for those of personnel they supervise (such as fellow trainees and non–physician practitioners).39 Vicarious liability aims to ensure that systemic checks and balances are in place so that, if failure occurs, harm can still be mitigated and/or avoided, as illustrated by Reason’s “Swiss Cheese Model.”40
Conclusion
Any gastroenterologist can experience an MPL claim. Such an experience can be especially stressful and confusing to early-career clinicians, especially if they’re unfamiliar with legal proceedings. Although MPL principles are not often taught in medical school or residency, it is important for gastroenterologists to be informed regarding tenets of MPL and cognizant of clinical situations which have relatively higher MPL risk. This can assuage untoward angst regarding MPL and highlight proactive risk-mitigation strategies. In general, gastroenterologist practices that can mitigate MPL risk include effective communication; adequate informed consent/refusal; documentation of preprocedure counseling, periprocedure events, and postprocedure recommendations; and maintenance of proper certification and privileging.
Dr. Azizian and Dr. Dalai are with the University of California, Los Angeles and the department of medicine at Olive View–UCLA Medical Center, Sylmar, Calif. They are co–first authors of this paper. Dr. Dalai is also with the division of gastroenterology at the University of New Mexico, Albuquerque. Dr. Adams is with the Center for Clinical Management Research in Veterans Affairs Ann Arbor Healthcare System, the division of gastroenterology at the University of Michigan Health System, and the Institute for Healthcare Policy and Innovation, all in Ann Arbor, Mich. Dr. Tabibian is with UCLA and the division of gastroenterology at Olive View–UCLA Medical Center. The authors have no conflicts of interest.
References
1. 2020 Data Sharing Project Gastroenterology 2009-2018. Inside Medical Liability: Second Quarter. Accessed 2020 Dec 6.
2. Mello MM et al. Health Aff (Millwood). 2004 Jul-Aug;23(4):42-53.
3. Adams MA et al. JAMA. 2014 Oct;312(13):1348-9.
4. Pegalis SE. American Law of Medical Malpractice 3d, Vol. 2. St. Paul, Minn.: Thomson Reuters, 2005.
5. Feld LD et al. Am J Gastroenterol. 2018 Nov;113(11):1577-9.
6. Sawyer v. Wight, 196 F. Supp. 2d 220, 226 (E.D.N.Y. 2002).
7. Michael A. Sita v. Long Island Jewish-Hillside Medical Center, 22 A.D.3d 743 (N.Y. App. Div. 2005).
8. Conklin LS et al. Clin Gastroenterol Hepatol. 2008 Jun;6(6):677-81.
9. Jena AB et al. N Engl J Med. 2011 Aug 18;365(7):629-36.
10. Kane CK. “Policy Research Perspectives Medical Liability Claim Frequency: A 2007-2008 Snapshot of Physicians.” Chicago: American Medical Association, 2010.
11. Hernandez LV et al. World J Gastrointest Endosc. 2013 Apr 16;5(4):169-73.
12. Schaffer AC et al. JAMA Intern Med. 2017 May 1;177(5):710-8.
13. Natanson v. Kline, 186 Kan. 393, 409, 350 P.2d 1093, 1106, decision clarified on denial of reh’g, 187 Kan. 186, 354 P.2d 670 (1960).
14. Truman v. Thomas, 27 Cal. 3d 285, 292, 611 P.2d 902, 906 (1980).
15. Shah P et al. Informed Consent, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Aug 22.
16. Rex DK. Clin Gastroenterol Hepatol. 2013 Jul;11(7):768-73.
17. Gerstenberger PD, Plumeri PA. Gastrointest Endosc. Mar-Apr 1993;39(2):132-8.
18. Adams MA and Allen JI. Clin Gastroenterol Hepatol. 2019 Nov;17(12):2392-6.e1.
19. Ahlawat R et al. Esophagogastroduodenoscopy, in “StatPearls.” Treasure Island, Fla.: StatPearls Publishing, 2020 Jan. Updated 2020 Dec 9.
20. Cotton PB. Gastrointest Endosc. 2006 Mar;63(3):378-82.
21. Cotton PB. Gastrointest Endosc. 2010 Oct;72(4):904.
22. Adamson TE et al. West J Med. 1989 Mar;150(3):356-60.
23. Trap R et al. Endoscopy. 1999 Feb;31(2):125-30.
24. Funaki B. Semin Intervent Radiol. 2015 Mar;32(1):61-4.
25. Feeding Tube Nursing Home and Hospital Malpractice. Miller & Zois, Attorneys at Law. Accessed 2020 Jun 20.
26. Medical Malpractice Lawsuit Brings $750,000 Settlement: Death of 82-year-old woman from sepsis due to improper placement of feeding tube. Lubin & Meyers PC. Accessed 2020 Jun 20.
27. Brendel RW et al. Med Clin North Am. 2010 Nov;94(6):1229-40, xi-ii.
28. ASGE Standards of Practice Committee; Acosta RD et al. Gastrointest Endosc. 2016 Jan;83(1):3-16.
29. Saleem S and Thomas AL. Cureus. 2018 Jun 25;10(6):e2878.
30. Hiyama T et al. World J Gastroenterol. 2006 Dec 21;12(47):7671-5.
31. Studdert DM et al. JAMA. 2005 Jun 1;293(21):2609-17.
32. Shaheen NJ et al. Gastroenterology. 2018 May;154(7):1993-2003.
33. Oza VM et al. Clin Gastroenterol Hepatol. 2016 Feb;14(2):172-4.
34. Feld AD. Gastrointest Endosc Clin N Am. 2002 Jan;12(1):171-9, viii-ix.
35. Lee JS et al. J Gen Intern Med. 2017 Aug;32(8):863-70.
36. Forrow L and Kontrimas JC. J Gen Intern Med. 2017 Aug;32(8):855-7.
37. Moses RE et al. Am J Gastroenterol. 2014 Aug;109(8):1128-32.
38. Tabibian JH. “The Evolution of Telehealth.” Guidepoint: Legal Solutions Blog. Accessed 2020 Aug 12.
39. Feld AD. Am J Gastroenterol. 2004 Sep;99(9):1641-4.
40. Reason J. BMJ. 2000;320(7237):768‐70.
New year, new hopes
Dear colleagues,
I’m pleased to introduce the winter edition of The New Gastroenterologist – the first issue of 2021! The start of the new year has been very much anticipated because many hope that this year will bring some resolution to the challenges we faced in 2020.
With the pandemic came the widespread use of telemedicine, a feature of patient care that is likely here to stay. As physicians, it is imperative that we understand the legal implications of virtual medicine. Experienced medical malpractice lawyers Ashton Hyde and Grace Johnson (Younker Hyde Macfarlane) offer advice on this rapidly evolving realm of medicine.
Early career gastroenterologists often fall victim to self-doubt in a phenomenon referred to as impostor syndrome. Dr. Kimberly Brown (Wayne State University) discusses this important topic: what it is, how to recognize it, and how to mitigate it. One way to temper the effects of impostor syndrome is utilizing the art of coaching. Dr. Ami N. Shah (Rush) takes us through her journey and reviews the personal and professional benefits of implementing coaching in medicine.
Consults about feedings tubes can be daunting because experience with the placement and management of feeding tubes can be limited during training. This quarter’s “In Focus” article, written by Dr. John Fang and Dr. Gregory Toy (University of Utah) reviews the indications for placement, type of tubes available, and common complications and how to troubleshoot them. This is an absolute must-read for any new gastroenterologist.
How do you approach the patient who shows up for an open access endoscopy, but a quick chart review leads you to the realization that the procedure, is in fact, not indicated? There tends to be a lot of inertia which prevents cancellation of cases like this because the patient is already in the endoscopy suite, prepped, and has planned for this procedure in the preceding weeks or months. Dr. Laurel R. Fisher (University of Pennsylvania) unpacks the ethical considerations of this familiar scenario in this fantastic addition to our ethics case series.
In our postfellowship pathways section, Dr. Rena Yadlapati (University of California San Diego) and Dr. Kelli DeLay (University of Colorado) guide us through the path to becoming an esophagologist. In the DHPA Private Practice Perspectives article this quarter, Dr. Nadeem Baig (Allied Digestive Care) and Kevin Harlen (Capital Digestive Care) explain how clinical productivity is measured and how this translates into compensation in practice.
A silver lining of the pandemic is the way in which social media has been used to connect colleagues around the world in fostering medical education. Dr. Sultan Mahmood (State University of New York at Buffalo), Dr. Atoosa Rabiee (Washington DC VA Medical Center), Dr. Sunil Amin (University of Miami), Dr. Allon Kahn (Mayo Clinic Scottsdale), and Dr. Ijlal Akbar Ali (University of Oklahoma) discuss the inception of @GIJournal, a Twitter-based online journal club, and how it has gained popularity in recent months.
The AGA launched a new podcast, “Small Talk, Big Topics,” geared toward trainees and early career gastroenterologists, and through a brief question and answer session, we get to know the hosts: Dr. Matthew Whitson (Zucker School of Medicine at Hofstra-Northwell), Dr. Nina Nandy (Presbyterian Medical Group), and Dr. C.S. Tse (Brown University).
Lastly, I’d like to take a moment to recognize Lora McGlade, who has been instrumental in The New Gastroenterologist as the Medical Communications Editor for our publisher, Frontline. She assumed a new role at the end of last year, and I cannot thank her enough for her contributions in making this publication a success.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m pleased to introduce the winter edition of The New Gastroenterologist – the first issue of 2021! The start of the new year has been very much anticipated because many hope that this year will bring some resolution to the challenges we faced in 2020.
With the pandemic came the widespread use of telemedicine, a feature of patient care that is likely here to stay. As physicians, it is imperative that we understand the legal implications of virtual medicine. Experienced medical malpractice lawyers Ashton Hyde and Grace Johnson (Younker Hyde Macfarlane) offer advice on this rapidly evolving realm of medicine.
Early career gastroenterologists often fall victim to self-doubt in a phenomenon referred to as impostor syndrome. Dr. Kimberly Brown (Wayne State University) discusses this important topic: what it is, how to recognize it, and how to mitigate it. One way to temper the effects of impostor syndrome is utilizing the art of coaching. Dr. Ami N. Shah (Rush) takes us through her journey and reviews the personal and professional benefits of implementing coaching in medicine.
Consults about feedings tubes can be daunting because experience with the placement and management of feeding tubes can be limited during training. This quarter’s “In Focus” article, written by Dr. John Fang and Dr. Gregory Toy (University of Utah) reviews the indications for placement, type of tubes available, and common complications and how to troubleshoot them. This is an absolute must-read for any new gastroenterologist.
How do you approach the patient who shows up for an open access endoscopy, but a quick chart review leads you to the realization that the procedure, is in fact, not indicated? There tends to be a lot of inertia which prevents cancellation of cases like this because the patient is already in the endoscopy suite, prepped, and has planned for this procedure in the preceding weeks or months. Dr. Laurel R. Fisher (University of Pennsylvania) unpacks the ethical considerations of this familiar scenario in this fantastic addition to our ethics case series.
In our postfellowship pathways section, Dr. Rena Yadlapati (University of California San Diego) and Dr. Kelli DeLay (University of Colorado) guide us through the path to becoming an esophagologist. In the DHPA Private Practice Perspectives article this quarter, Dr. Nadeem Baig (Allied Digestive Care) and Kevin Harlen (Capital Digestive Care) explain how clinical productivity is measured and how this translates into compensation in practice.
A silver lining of the pandemic is the way in which social media has been used to connect colleagues around the world in fostering medical education. Dr. Sultan Mahmood (State University of New York at Buffalo), Dr. Atoosa Rabiee (Washington DC VA Medical Center), Dr. Sunil Amin (University of Miami), Dr. Allon Kahn (Mayo Clinic Scottsdale), and Dr. Ijlal Akbar Ali (University of Oklahoma) discuss the inception of @GIJournal, a Twitter-based online journal club, and how it has gained popularity in recent months.
The AGA launched a new podcast, “Small Talk, Big Topics,” geared toward trainees and early career gastroenterologists, and through a brief question and answer session, we get to know the hosts: Dr. Matthew Whitson (Zucker School of Medicine at Hofstra-Northwell), Dr. Nina Nandy (Presbyterian Medical Group), and Dr. C.S. Tse (Brown University).
Lastly, I’d like to take a moment to recognize Lora McGlade, who has been instrumental in The New Gastroenterologist as the Medical Communications Editor for our publisher, Frontline. She assumed a new role at the end of last year, and I cannot thank her enough for her contributions in making this publication a success.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition
Dear colleagues,
I’m pleased to introduce the winter edition of The New Gastroenterologist – the first issue of 2021! The start of the new year has been very much anticipated because many hope that this year will bring some resolution to the challenges we faced in 2020.
With the pandemic came the widespread use of telemedicine, a feature of patient care that is likely here to stay. As physicians, it is imperative that we understand the legal implications of virtual medicine. Experienced medical malpractice lawyers Ashton Hyde and Grace Johnson (Younker Hyde Macfarlane) offer advice on this rapidly evolving realm of medicine.
Early career gastroenterologists often fall victim to self-doubt in a phenomenon referred to as impostor syndrome. Dr. Kimberly Brown (Wayne State University) discusses this important topic: what it is, how to recognize it, and how to mitigate it. One way to temper the effects of impostor syndrome is utilizing the art of coaching. Dr. Ami N. Shah (Rush) takes us through her journey and reviews the personal and professional benefits of implementing coaching in medicine.
Consults about feedings tubes can be daunting because experience with the placement and management of feeding tubes can be limited during training. This quarter’s “In Focus” article, written by Dr. John Fang and Dr. Gregory Toy (University of Utah) reviews the indications for placement, type of tubes available, and common complications and how to troubleshoot them. This is an absolute must-read for any new gastroenterologist.
How do you approach the patient who shows up for an open access endoscopy, but a quick chart review leads you to the realization that the procedure, is in fact, not indicated? There tends to be a lot of inertia which prevents cancellation of cases like this because the patient is already in the endoscopy suite, prepped, and has planned for this procedure in the preceding weeks or months. Dr. Laurel R. Fisher (University of Pennsylvania) unpacks the ethical considerations of this familiar scenario in this fantastic addition to our ethics case series.
In our postfellowship pathways section, Dr. Rena Yadlapati (University of California San Diego) and Dr. Kelli DeLay (University of Colorado) guide us through the path to becoming an esophagologist. In the DHPA Private Practice Perspectives article this quarter, Dr. Nadeem Baig (Allied Digestive Care) and Kevin Harlen (Capital Digestive Care) explain how clinical productivity is measured and how this translates into compensation in practice.
A silver lining of the pandemic is the way in which social media has been used to connect colleagues around the world in fostering medical education. Dr. Sultan Mahmood (State University of New York at Buffalo), Dr. Atoosa Rabiee (Washington DC VA Medical Center), Dr. Sunil Amin (University of Miami), Dr. Allon Kahn (Mayo Clinic Scottsdale), and Dr. Ijlal Akbar Ali (University of Oklahoma) discuss the inception of @GIJournal, a Twitter-based online journal club, and how it has gained popularity in recent months.
The AGA launched a new podcast, “Small Talk, Big Topics,” geared toward trainees and early career gastroenterologists, and through a brief question and answer session, we get to know the hosts: Dr. Matthew Whitson (Zucker School of Medicine at Hofstra-Northwell), Dr. Nina Nandy (Presbyterian Medical Group), and Dr. C.S. Tse (Brown University).
Lastly, I’d like to take a moment to recognize Lora McGlade, who has been instrumental in The New Gastroenterologist as the Medical Communications Editor for our publisher, Frontline. She assumed a new role at the end of last year, and I cannot thank her enough for her contributions in making this publication a success.
If you have interest in contributing or have ideas for future TNG topics, please contact me ([email protected]) or Ryan Farrell ([email protected]), managing editor of TNG.
Stay well,
Vijaya L. Rao, MD
Editor-in-Chief
Assistant Professor of Medicine, University of Chicago, Section of Gastroenterology, Hepatology & Nutrition





















