User login
ERRATUM: Decreasing Hypoglycemia following Insulin Administration for Inpatient Hyperkalemia
A correction has been made to the Figure. A dosage was incorrect in the Orderset 1.1 (1/1/16-3/19/17) box. The figure listed Insulin 19 Units IV x 1 and should have been Insulin 10 Units IV x 1. Below is the corrected figure..

A correction has been made to the Figure. A dosage was incorrect in the Orderset 1.1 (1/1/16-3/19/17) box. The figure listed Insulin 19 Units IV x 1 and should have been Insulin 10 Units IV x 1. Below is the corrected figure..

A correction has been made to the Figure. A dosage was incorrect in the Orderset 1.1 (1/1/16-3/19/17) box. The figure listed Insulin 19 Units IV x 1 and should have been Insulin 10 Units IV x 1. Below is the corrected figure..

A Traumatic Traveler
A 19-year-old man with Duchenne Muscular Dystrophy (DMD) presented to the Emergency Department (ED) for left knee pain after ejection from his motorized wheelchair at a low velocity. In the ED, he developed increasing respiratory distress.
When addressing a new problem in a patient with a chronic condition, it is crucial to first understand the chronic condition and then consider whether the presenting symptoms relate to that condition or stem from an unrelated inciting event.
Patients with DMD are at risk of pulmonary complications relating to their underlying disease. For instance, dysphagia and ineffective cough can predispose them to recurrent aspiration pneumonitis and/or pneumonia, whereas decreased lung compliance (from scoliosis, atelectasis, and/or pulmonary fibrosis) and respiratory muscle weakness can progress to ventilatory failure. In addition, patients with DMD are at risk for pulmonary thromboembolism in the setting of immobility. Patients with DMD may also develop congestive heart failure resulting from myocardial fibrosis and nonischemic cardiomyopathy.
The ejection from his wheelchair signals potential trauma-associated conditions that could explain his respiratory distress. Respiratory complications of blunt thoracic trauma include pulmonary contusion, pneumothorax, flail chest (resulting from fractured ribs), and acute respiratory distress syndrome (ARDS). Lower extremity injury can result in venous thrombosis and pulmonary thromboembolism. While classically associated with long bone fractures, fat embolism syndrome (FES) may rarely occur with rib fractures and soft-tissue trauma. Respiratory compromise may also result from cervical spinal cord injury or severe anemia from trauma-associated hemorrhage.
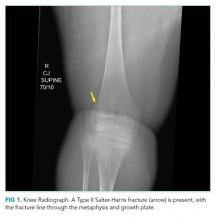
Additional past medical history included growth hormone deficiency, migraine headaches, osteoporosis secondary to chronic steroid use, cardiac fibrosis of the inferolateral wall and septum with a baseline left ventricular ejection fraction of 65%, and atrial fibrillation. His medications included calcium carbonate, vitamin D, omeprazole, lisinopril, metoprolol, prednisone, escitalopram, and testosterone. Physical examination revealed an ill-appearing obese man in respiratory distress. Temperature was 37.3°C, heart rate was 102 beats per minute (bpm), blood pressure was 110/74 mm Hg. His oxygen saturation was 93% with a respiratory rate of 25 breaths per minute while breathing ambient air. His lung sounds were clear, and his heart was without murmur. The left knee was diffusely tender to palpation without specific point tenderness. Strength was 2/5 with flexion and extension at the bilateral knees and hips and 3/5 flexion and extension at the bilateral elbows. He reported this level of weakness was his baseline. Radiographs revealed a minimally displaced Salter Harris II fracture (fracture line through the metaphysis and growth plate) of the left distal femur. His fracture was splinted early in his ED course. During his ED evaluation, the patient had acute worsening of tachycardia to 130 bpm, increased respiratory rate of 34 breaths per minute, and hypoxemia with an oxygen saturation of 83% on ambient air. He was placed on 3 L/min of oxygen via nasal cannula with improvement in his oxygen saturation to 90%. A chest radiograph was unremarkable, without evidence of pneumothorax, effusion, or pneumonia. The patient was admitted to the hospital.
The acute onset of tachypnea, tachycardia, and hypoxia, accompanied by a clear lung exam and normal chest radiograph, increases the likelihood of a pulmonary embolism. Obesity, testosterone therapy, and trauma increase his susceptibility to venous thromboembolism, while a distal femur fracture increases his risk for FES. Acute pulmonary aspiration often presents with initially absent or subtle radiographic findings. An arterial blood gas analysis would determine the presence and extent of an alveolar-arterial (A-a) gradient; a normal A-a gradient is seen in hypoventilation, while an elevated A-a gradient is seen in conditions affecting gas exchange, including pulmonary emboli and alveolar filling processes. His hypoxemia only partially corrects with supplemental oxygen, raising the possibility of capillary or anatomic shunting. Capillary shunting may occur with atelectasis, aspiration/pneumonia and pulmonary edema, whereas anatomic shunting can be intra-cardiac (eg, patent foramen ovale or septic defect) or intrapulmonary (eg, arteriovenous malformations). Patients with pulmonary emboli may also develop right-to-left shunting because of increased pulmonary vascular resistance, although hypoxemia with pulmonary emboli largely relates to ventilation/perfusion mismatch and decreased level of mixed venous blood oxygen (PvO2).
This patient’s complex medical history warrants a broadened differential with consideration of his cardiac history, including myocardial fibrosis and arrhythmia, and the impact of exposure to steroids on his immune and musculoskeletal systems. He has a history of atrial fibrillation, and an electrocardiogram is warranted to determine the underlying rhythm. Prolonged periods of rapid ventricular response may lead to tachycardia-induced cardiomyopathy. Myocardial fibrosis may progress despite use of angiotensin-converting enzyme inhibitors and is associated with systolic and/or diastolic dysfunction, although neither the examination findings provided nor the chest radiograph are suggestive of decompensated heart failure. Chronic exposure to corticosteroids (used in DMD to improve muscle strength and function) may predispose to numerous infectious and metabolic complications. Up to 10%-15% of patients with Pneumocystis jirovecii pneumonia may present with a normal chest radiograph. Acute adrenal insufficiency can present with tachycardia, weakness, and respiratory distress, so recent prednisone dose changes or interruptions should be assessed.
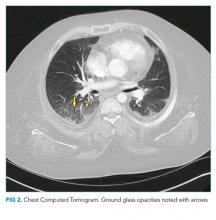
The patient’s respiratory status worsened. In light of his complex medical history, he was transferred to a children’s hospital for a higher level of care with a presumptive diagnosis of aspiration pneumonia. Upon reassessment at the new facility, the patient reported an ongoing and severe headache since his initial injury. NSAIDs had been given prior to transfer. His exam continued to be significant for tachycardia, tachypnea, and hypoxemia. His cardiac and lung examinations were otherwise normal. A comprehensive metabolic panel, procalcitonin, complete blood count with differential, and lactate were normal; his C-reactive protein (CRP) was 46.8 mg/dL (Normal <8 mg/dL). A computed tomography (CT) angiogram of the chest revealed small multifocal nodular ground-glass opacities, especially in the lower lobes, concerning for microatelectasis, multifocal pneumonia, or aspiration pneumonia. After consultation with pediatric pulmonology consultants, antimicrobials were held during the initial phase of work-up.
His headache may reflect a migraine, although further characterization and assessment for the presence and extent of head or neck trauma is warranted. Headache following trauma warrants consideration of cerebral contusion, diffuse axonal injury, intracranial hemorrhage, and carotid or vertebral artery dissection. Screening for concussion should also be performed. Hypoxemia may increase cerebral blood flow and raise intracranial pressure, resulting in headache.
CRP elevation is nonspecific and signals the presence of focal or systemic inflammation and is often elevated to a milder extent in obese patients with DMD. While normal procalcitonin argues against bacterial pneumonia, the precise level can be informative, and serial procalcitonin values may be more helpful than a single value. Although antecedent respiratory symptoms were not mentioned, viral or fungal pneumonia can present insidiously. An occult malignancy may be incidentally discovered when patients present for unrelated issues, although this and other sources of elevated CRP (eg, exacerbation of an autoimmune disease or drug reaction) remain less likely given the acuity of his presentation. Acute pulmonary embolism may be associated with a systemic inflammatory response and elevation in CRP.
In addition to the radiographic differential diagnosis already presented, the appearance of multifocal opacifications with hypoxemia raises the possibility of pulmonary infarcts or noncardiogenic pulmonary edema.
On hospital day 2, the patient continued to complain of “the worst headache of his life” as well as blurry vision and seeing “dark spots.” His headache did not improve with NSAIDs. A noncontrast CT scan of the head was normal. Neurology was consulted. Given his symptoms, history of migraines, stable neurological examination, and normal head CT, he was diagnosed with migraines and administered fluids, prochlorperazine, diphenhydramine, ondansetron, and NSAIDs. His headache continued and he continued to require supplemental oxygen.
The combination of hypoxemia, severe headache, and vision changes remains consistent with systemic emboli caused by thromboembolism or fat embolism. Headache assessment must also involve screening for “red flags,” which include sudden onset, antecedent head trauma, systemic illness (eg, fever or meningismus), focal neurologic findings, papilledema, changes with position or Valsalva, and immunosuppression. Although primary headache syndromes (eg, migraines or tension and cluster headache) may be triggered in the setting of trauma and systemic illness, “the worst headache of my life” is a concerning symptom that warrants urgent attention. While this invokes the possibility of a subarachnoid hemorrhage (SAH), headache severity is nonspecific, and rapid onset (ie, thunderclap headache) would be more suggestive. After 6 hours of symptoms, the sensitivity of head CT for detecting SAH declines, and lumbar puncture would be warranted to evaluate for xanthochromia.
His blurry vision and dark spots require testing of visual acuity and visual fields, as well as fundoscopic examination to assess for embolic phenomena or papilledema. Migraine is classically associated with “positive” or scintillating scotomata, although dark spots may occur. The presence of horizontal diplopia would indicate a cranial nerve VI palsy, which can occur with increased intracranial pressure. Visual-field cuts may also present as blurry vision, and monocular vs binocular deficits signal whether the issue is anterior or involving/posterior to the optic chiasm, respectively. Magnetic resonance imaging (MRI) may reveal the presence or sequelae of cerebral emboli (eg, fat emboli), including vasogenic edema.
Dilated fundus examination revealed Purtscher retinopathy: bilateral cotton-wool spots and larger areas of retinal whitening (Purtscher flecken).
Typical findings of Purtscher retinopathy include Purtscher flecken, cotton-wool spots, retinal hemorrhage, and optic disc edema. Purtscher retinopathy is classically associated with severe head trauma. Without associated head trauma, the term “Purtscher-like retinopathy” is used. Conditions that can cause Purtscher-like retinopathy include pancreatitis, vasculitis, microangiopathy, chronic renal failure, and systemic embolization. The most likely source of systemic embolization remains fat emboli stemming from his femur fracture. Treatment of FES is largely supportive.
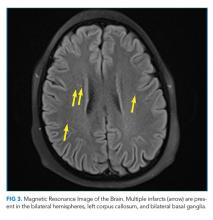
The possibility of fat emboli had been repeatedly raised by the patient’s mother since admission. While providers had considered this a possibility, it was discounted early on because of the minor nature of the patient’s orthopedic trauma, the lack of clear radiographic evidence for pulmonary emboli on chest CT, and the normal head CT. The findings on the ophthalmologist’s fundoscopic examination led the primary team to reconsider FES, along with thromboemboli and pancreatitis. Lipase was normal. MRI of the brain with contrast revealed >20 microinfarcts in the bilateral hemispheres, left corpus callosum, and bilateral basal ganglia. The CT angiogram of the chest was rereviewed; the pediatric radiologists suggested that microinfarcts could explain the patchy small ground glass opacities seen in the lungs. A transthoracic echocardiogram and electrocardiogram were normal. The diagnosis of FES was made, and the patient was started on aspirin and enoxaparin prophylaxis. His headache and respiratory status improved, and he was discharged home with close follow-up.
DISCUSSION
FES is a rare complication associated with long bone fractures and orthopedic manipulation.1,2 The exact mechanism of fat emboli production is unknown, but two theories prevail. The mechanical theory states that an outside mechanical source causes bone marrow contents or adipose tissue contents to be dislodged into the circulation where they travel through the venous circulation to become embedded in the lungs.1,2 These fragments may also migrate to the arterial circulation, through a patent foramen ovale or intrapulmonary shunts, leading to end organ damage.1,2 The biochemical theory suggests that fat emboli in the venous circulation precipitate an inflammatory and prothrombotic cascade that triggers fibrin production, platelet aggregation, and release of free fatty acids into the circulation, predisposing patients to develop multifocal systemic emboli.1
Although the classic triad in FES includes respiratory symptoms, rash, and CNS symptoms, all three findings are only present in 1%-29% of cases.1,2 Respiratory abnormalities, ranging from tachypnea and dyspnea to ARDS and hypoxic respiratory failure, occur in up to 75% of patients with FES.1 Central nervous system (CNS) complications, including headache, confusion, coma, seizures, and death caused by cerebral ischemia, occur in up to 86% of patients.1,2 Petechiae may occur in 20%-60% of patients and are usually located on nondependent regions of the body such as the head, neck, and chest.
Diagnosis of FES is largely clinical and requires a high index of suspicion and elimination of other conditions, including pulmonary thromboembolism, diffuse intravascular coagulation, and sepsis. The CNS complications must be differentiated from CNS infection, stroke, migraine, benign intracranial hypertension, and intracranial hemorrhage. There is no gold standard test for diagnosis. The Gurd and Wilson criteria, modified Gurd criteria, and Schonfeld’s criteria (Table) are commonly used but have not been clinically validated.1,3-5 These use a combination of clinical signs of respiratory distress, neurological symptoms, petechial rash, and various other diagnostic factors. When patients have risk factors, such as trauma, surgery, or predisposing conditions (eg, mobility-limiting neuromuscular disorders) and signs and symptoms potentially consistent with FES, the diagnostic evaluation should include arterial blood gas analysis, complete blood count, chest radiographs, and coagulation studies. Ophthalmological exam to evaluate for cotton-wool spots, brain MRI to detect cerebral emboli, and CT pulmonary angiogram to assess for pulmonary infarcts may help to eliminate other diagnoses and/or confirm FES.
Diagnosis of uncommon conditions that present with nonspecific symptoms, like FES, can be challenging because the symptoms may overlap with many other possible diagnoses. This challenge is further exacerbated in patients with significant medical complexity, as with the patient discussed here. Specifically, this patient had multiple plausible explanations for CNS symptoms and respiratory symptoms. It was ultimately the visual symptoms that began to link his symptoms together into a unifying syndrome and the ophthalmologic examination that prompted confirmatory imaging. It is important to continually revisit and revise the differential diagnosis in patients with medical complexity and avoid the competing temptations to abandon the search for a unifying diagnosis and attribute all symptoms to a patient’s underlying condition.
Treatment of FES is largely supportive with close monitoring of neurological status and providing supplemental oxygen as needed. Corticosteroids have been suggested to help prevent FES in patients with long bone fractures, but there is no evidence to suggest they are helpful once FES is diagnosed.2 There is conflicting evidence for the efficacy of heparin or low-molecular-weight heparin as treatment in FES.2,6 After discussions with consulting physician teams, the patient, and his family, enoxaparin and aspirin were started for this patient in light of his tenuous condition in order to decrease the risk of further embolic complications.
Patients with DMD and other neuromuscular disorders likely have a greater propensity to develop FES even after minor trauma.1,6 This is believed to be caused by patients becoming nonambulatory early in life and receiving substantial corticosteroid therapy, which can lead to osteopenia and fatty replacement of the bone marrow.1,6 This population is also often obese by the second or third decade of life, which contributes to their already increased propensity to fall.1,6
To our knowledge, this patient is 1 of 18 reported cases of FES after trauma in DMD patients. Two-thirds of these cases occurred when an unrestrained patient fell from their wheelchair. The other cases occurred while walking, during physical therapy, and during assisted transfers.6-12 In these cases, FES had a guarded prognosis, with 7 of the 18 patients dying and 1 of the patients remaining in a persistent vegetative state.8,9 While caution is warranted in generalizing these findings, given the small number of reported cases and likely publication bias,education of caregivers and patients on use of restraints and safe transfers is paramount to limit the risk of trauma.
TEACHING POINTS
- FES is a rare condition that most commonly manifests with respiratory, neurological, and cutaneous findings.
- Patients with Duchenne’s Muscular Dystrophy are likely at increased risk for FES even with minor trauma; this makes wheelchair restraints and safe transfers fundamental.
- Patients with medical complexity and their caregivers are key members of the diagnostic team.
1. Fukumoto LE, Fukumoto KD. Fat embolism syndrome. Nurs Clin North Am. 2018;53(3):335-347. https://doi.org/10.1016/j.cnur.2018.04.003.
2. Scarpino M, Lanzo G, Lolli F, Grippo A. From the diagnosis to the therapeutic management: Cerebral fat embolism, a clinical challenge. Int J Gen Med. 2019;2019(12):39-48. https://doi.org/10.2147/IJGM.S177407.
A 19-year-old man with Duchenne Muscular Dystrophy (DMD) presented to the Emergency Department (ED) for left knee pain after ejection from his motorized wheelchair at a low velocity. In the ED, he developed increasing respiratory distress.
When addressing a new problem in a patient with a chronic condition, it is crucial to first understand the chronic condition and then consider whether the presenting symptoms relate to that condition or stem from an unrelated inciting event.
Patients with DMD are at risk of pulmonary complications relating to their underlying disease. For instance, dysphagia and ineffective cough can predispose them to recurrent aspiration pneumonitis and/or pneumonia, whereas decreased lung compliance (from scoliosis, atelectasis, and/or pulmonary fibrosis) and respiratory muscle weakness can progress to ventilatory failure. In addition, patients with DMD are at risk for pulmonary thromboembolism in the setting of immobility. Patients with DMD may also develop congestive heart failure resulting from myocardial fibrosis and nonischemic cardiomyopathy.
The ejection from his wheelchair signals potential trauma-associated conditions that could explain his respiratory distress. Respiratory complications of blunt thoracic trauma include pulmonary contusion, pneumothorax, flail chest (resulting from fractured ribs), and acute respiratory distress syndrome (ARDS). Lower extremity injury can result in venous thrombosis and pulmonary thromboembolism. While classically associated with long bone fractures, fat embolism syndrome (FES) may rarely occur with rib fractures and soft-tissue trauma. Respiratory compromise may also result from cervical spinal cord injury or severe anemia from trauma-associated hemorrhage.
Additional past medical history included growth hormone deficiency, migraine headaches, osteoporosis secondary to chronic steroid use, cardiac fibrosis of the inferolateral wall and septum with a baseline left ventricular ejection fraction of 65%, and atrial fibrillation. His medications included calcium carbonate, vitamin D, omeprazole, lisinopril, metoprolol, prednisone, escitalopram, and testosterone. Physical examination revealed an ill-appearing obese man in respiratory distress. Temperature was 37.3°C, heart rate was 102 beats per minute (bpm), blood pressure was 110/74 mm Hg. His oxygen saturation was 93% with a respiratory rate of 25 breaths per minute while breathing ambient air. His lung sounds were clear, and his heart was without murmur. The left knee was diffusely tender to palpation without specific point tenderness. Strength was 2/5 with flexion and extension at the bilateral knees and hips and 3/5 flexion and extension at the bilateral elbows. He reported this level of weakness was his baseline. Radiographs revealed a minimally displaced Salter Harris II fracture (fracture line through the metaphysis and growth plate) of the left distal femur. His fracture was splinted early in his ED course. During his ED evaluation, the patient had acute worsening of tachycardia to 130 bpm, increased respiratory rate of 34 breaths per minute, and hypoxemia with an oxygen saturation of 83% on ambient air. He was placed on 3 L/min of oxygen via nasal cannula with improvement in his oxygen saturation to 90%. A chest radiograph was unremarkable, without evidence of pneumothorax, effusion, or pneumonia. The patient was admitted to the hospital.
The acute onset of tachypnea, tachycardia, and hypoxia, accompanied by a clear lung exam and normal chest radiograph, increases the likelihood of a pulmonary embolism. Obesity, testosterone therapy, and trauma increase his susceptibility to venous thromboembolism, while a distal femur fracture increases his risk for FES. Acute pulmonary aspiration often presents with initially absent or subtle radiographic findings. An arterial blood gas analysis would determine the presence and extent of an alveolar-arterial (A-a) gradient; a normal A-a gradient is seen in hypoventilation, while an elevated A-a gradient is seen in conditions affecting gas exchange, including pulmonary emboli and alveolar filling processes. His hypoxemia only partially corrects with supplemental oxygen, raising the possibility of capillary or anatomic shunting. Capillary shunting may occur with atelectasis, aspiration/pneumonia and pulmonary edema, whereas anatomic shunting can be intra-cardiac (eg, patent foramen ovale or septic defect) or intrapulmonary (eg, arteriovenous malformations). Patients with pulmonary emboli may also develop right-to-left shunting because of increased pulmonary vascular resistance, although hypoxemia with pulmonary emboli largely relates to ventilation/perfusion mismatch and decreased level of mixed venous blood oxygen (PvO2).
This patient’s complex medical history warrants a broadened differential with consideration of his cardiac history, including myocardial fibrosis and arrhythmia, and the impact of exposure to steroids on his immune and musculoskeletal systems. He has a history of atrial fibrillation, and an electrocardiogram is warranted to determine the underlying rhythm. Prolonged periods of rapid ventricular response may lead to tachycardia-induced cardiomyopathy. Myocardial fibrosis may progress despite use of angiotensin-converting enzyme inhibitors and is associated with systolic and/or diastolic dysfunction, although neither the examination findings provided nor the chest radiograph are suggestive of decompensated heart failure. Chronic exposure to corticosteroids (used in DMD to improve muscle strength and function) may predispose to numerous infectious and metabolic complications. Up to 10%-15% of patients with Pneumocystis jirovecii pneumonia may present with a normal chest radiograph. Acute adrenal insufficiency can present with tachycardia, weakness, and respiratory distress, so recent prednisone dose changes or interruptions should be assessed.
The patient’s respiratory status worsened. In light of his complex medical history, he was transferred to a children’s hospital for a higher level of care with a presumptive diagnosis of aspiration pneumonia. Upon reassessment at the new facility, the patient reported an ongoing and severe headache since his initial injury. NSAIDs had been given prior to transfer. His exam continued to be significant for tachycardia, tachypnea, and hypoxemia. His cardiac and lung examinations were otherwise normal. A comprehensive metabolic panel, procalcitonin, complete blood count with differential, and lactate were normal; his C-reactive protein (CRP) was 46.8 mg/dL (Normal <8 mg/dL). A computed tomography (CT) angiogram of the chest revealed small multifocal nodular ground-glass opacities, especially in the lower lobes, concerning for microatelectasis, multifocal pneumonia, or aspiration pneumonia. After consultation with pediatric pulmonology consultants, antimicrobials were held during the initial phase of work-up.
His headache may reflect a migraine, although further characterization and assessment for the presence and extent of head or neck trauma is warranted. Headache following trauma warrants consideration of cerebral contusion, diffuse axonal injury, intracranial hemorrhage, and carotid or vertebral artery dissection. Screening for concussion should also be performed. Hypoxemia may increase cerebral blood flow and raise intracranial pressure, resulting in headache.
CRP elevation is nonspecific and signals the presence of focal or systemic inflammation and is often elevated to a milder extent in obese patients with DMD. While normal procalcitonin argues against bacterial pneumonia, the precise level can be informative, and serial procalcitonin values may be more helpful than a single value. Although antecedent respiratory symptoms were not mentioned, viral or fungal pneumonia can present insidiously. An occult malignancy may be incidentally discovered when patients present for unrelated issues, although this and other sources of elevated CRP (eg, exacerbation of an autoimmune disease or drug reaction) remain less likely given the acuity of his presentation. Acute pulmonary embolism may be associated with a systemic inflammatory response and elevation in CRP.
In addition to the radiographic differential diagnosis already presented, the appearance of multifocal opacifications with hypoxemia raises the possibility of pulmonary infarcts or noncardiogenic pulmonary edema.
On hospital day 2, the patient continued to complain of “the worst headache of his life” as well as blurry vision and seeing “dark spots.” His headache did not improve with NSAIDs. A noncontrast CT scan of the head was normal. Neurology was consulted. Given his symptoms, history of migraines, stable neurological examination, and normal head CT, he was diagnosed with migraines and administered fluids, prochlorperazine, diphenhydramine, ondansetron, and NSAIDs. His headache continued and he continued to require supplemental oxygen.
The combination of hypoxemia, severe headache, and vision changes remains consistent with systemic emboli caused by thromboembolism or fat embolism. Headache assessment must also involve screening for “red flags,” which include sudden onset, antecedent head trauma, systemic illness (eg, fever or meningismus), focal neurologic findings, papilledema, changes with position or Valsalva, and immunosuppression. Although primary headache syndromes (eg, migraines or tension and cluster headache) may be triggered in the setting of trauma and systemic illness, “the worst headache of my life” is a concerning symptom that warrants urgent attention. While this invokes the possibility of a subarachnoid hemorrhage (SAH), headache severity is nonspecific, and rapid onset (ie, thunderclap headache) would be more suggestive. After 6 hours of symptoms, the sensitivity of head CT for detecting SAH declines, and lumbar puncture would be warranted to evaluate for xanthochromia.
His blurry vision and dark spots require testing of visual acuity and visual fields, as well as fundoscopic examination to assess for embolic phenomena or papilledema. Migraine is classically associated with “positive” or scintillating scotomata, although dark spots may occur. The presence of horizontal diplopia would indicate a cranial nerve VI palsy, which can occur with increased intracranial pressure. Visual-field cuts may also present as blurry vision, and monocular vs binocular deficits signal whether the issue is anterior or involving/posterior to the optic chiasm, respectively. Magnetic resonance imaging (MRI) may reveal the presence or sequelae of cerebral emboli (eg, fat emboli), including vasogenic edema.
Dilated fundus examination revealed Purtscher retinopathy: bilateral cotton-wool spots and larger areas of retinal whitening (Purtscher flecken).
Typical findings of Purtscher retinopathy include Purtscher flecken, cotton-wool spots, retinal hemorrhage, and optic disc edema. Purtscher retinopathy is classically associated with severe head trauma. Without associated head trauma, the term “Purtscher-like retinopathy” is used. Conditions that can cause Purtscher-like retinopathy include pancreatitis, vasculitis, microangiopathy, chronic renal failure, and systemic embolization. The most likely source of systemic embolization remains fat emboli stemming from his femur fracture. Treatment of FES is largely supportive.
The possibility of fat emboli had been repeatedly raised by the patient’s mother since admission. While providers had considered this a possibility, it was discounted early on because of the minor nature of the patient’s orthopedic trauma, the lack of clear radiographic evidence for pulmonary emboli on chest CT, and the normal head CT. The findings on the ophthalmologist’s fundoscopic examination led the primary team to reconsider FES, along with thromboemboli and pancreatitis. Lipase was normal. MRI of the brain with contrast revealed >20 microinfarcts in the bilateral hemispheres, left corpus callosum, and bilateral basal ganglia. The CT angiogram of the chest was rereviewed; the pediatric radiologists suggested that microinfarcts could explain the patchy small ground glass opacities seen in the lungs. A transthoracic echocardiogram and electrocardiogram were normal. The diagnosis of FES was made, and the patient was started on aspirin and enoxaparin prophylaxis. His headache and respiratory status improved, and he was discharged home with close follow-up.
DISCUSSION
FES is a rare complication associated with long bone fractures and orthopedic manipulation.1,2 The exact mechanism of fat emboli production is unknown, but two theories prevail. The mechanical theory states that an outside mechanical source causes bone marrow contents or adipose tissue contents to be dislodged into the circulation where they travel through the venous circulation to become embedded in the lungs.1,2 These fragments may also migrate to the arterial circulation, through a patent foramen ovale or intrapulmonary shunts, leading to end organ damage.1,2 The biochemical theory suggests that fat emboli in the venous circulation precipitate an inflammatory and prothrombotic cascade that triggers fibrin production, platelet aggregation, and release of free fatty acids into the circulation, predisposing patients to develop multifocal systemic emboli.1
Although the classic triad in FES includes respiratory symptoms, rash, and CNS symptoms, all three findings are only present in 1%-29% of cases.1,2 Respiratory abnormalities, ranging from tachypnea and dyspnea to ARDS and hypoxic respiratory failure, occur in up to 75% of patients with FES.1 Central nervous system (CNS) complications, including headache, confusion, coma, seizures, and death caused by cerebral ischemia, occur in up to 86% of patients.1,2 Petechiae may occur in 20%-60% of patients and are usually located on nondependent regions of the body such as the head, neck, and chest.
Diagnosis of FES is largely clinical and requires a high index of suspicion and elimination of other conditions, including pulmonary thromboembolism, diffuse intravascular coagulation, and sepsis. The CNS complications must be differentiated from CNS infection, stroke, migraine, benign intracranial hypertension, and intracranial hemorrhage. There is no gold standard test for diagnosis. The Gurd and Wilson criteria, modified Gurd criteria, and Schonfeld’s criteria (Table) are commonly used but have not been clinically validated.1,3-5 These use a combination of clinical signs of respiratory distress, neurological symptoms, petechial rash, and various other diagnostic factors. When patients have risk factors, such as trauma, surgery, or predisposing conditions (eg, mobility-limiting neuromuscular disorders) and signs and symptoms potentially consistent with FES, the diagnostic evaluation should include arterial blood gas analysis, complete blood count, chest radiographs, and coagulation studies. Ophthalmological exam to evaluate for cotton-wool spots, brain MRI to detect cerebral emboli, and CT pulmonary angiogram to assess for pulmonary infarcts may help to eliminate other diagnoses and/or confirm FES.
Diagnosis of uncommon conditions that present with nonspecific symptoms, like FES, can be challenging because the symptoms may overlap with many other possible diagnoses. This challenge is further exacerbated in patients with significant medical complexity, as with the patient discussed here. Specifically, this patient had multiple plausible explanations for CNS symptoms and respiratory symptoms. It was ultimately the visual symptoms that began to link his symptoms together into a unifying syndrome and the ophthalmologic examination that prompted confirmatory imaging. It is important to continually revisit and revise the differential diagnosis in patients with medical complexity and avoid the competing temptations to abandon the search for a unifying diagnosis and attribute all symptoms to a patient’s underlying condition.
Treatment of FES is largely supportive with close monitoring of neurological status and providing supplemental oxygen as needed. Corticosteroids have been suggested to help prevent FES in patients with long bone fractures, but there is no evidence to suggest they are helpful once FES is diagnosed.2 There is conflicting evidence for the efficacy of heparin or low-molecular-weight heparin as treatment in FES.2,6 After discussions with consulting physician teams, the patient, and his family, enoxaparin and aspirin were started for this patient in light of his tenuous condition in order to decrease the risk of further embolic complications.
Patients with DMD and other neuromuscular disorders likely have a greater propensity to develop FES even after minor trauma.1,6 This is believed to be caused by patients becoming nonambulatory early in life and receiving substantial corticosteroid therapy, which can lead to osteopenia and fatty replacement of the bone marrow.1,6 This population is also often obese by the second or third decade of life, which contributes to their already increased propensity to fall.1,6
To our knowledge, this patient is 1 of 18 reported cases of FES after trauma in DMD patients. Two-thirds of these cases occurred when an unrestrained patient fell from their wheelchair. The other cases occurred while walking, during physical therapy, and during assisted transfers.6-12 In these cases, FES had a guarded prognosis, with 7 of the 18 patients dying and 1 of the patients remaining in a persistent vegetative state.8,9 While caution is warranted in generalizing these findings, given the small number of reported cases and likely publication bias,education of caregivers and patients on use of restraints and safe transfers is paramount to limit the risk of trauma.
TEACHING POINTS
- FES is a rare condition that most commonly manifests with respiratory, neurological, and cutaneous findings.
- Patients with Duchenne’s Muscular Dystrophy are likely at increased risk for FES even with minor trauma; this makes wheelchair restraints and safe transfers fundamental.
- Patients with medical complexity and their caregivers are key members of the diagnostic team.
A 19-year-old man with Duchenne Muscular Dystrophy (DMD) presented to the Emergency Department (ED) for left knee pain after ejection from his motorized wheelchair at a low velocity. In the ED, he developed increasing respiratory distress.
When addressing a new problem in a patient with a chronic condition, it is crucial to first understand the chronic condition and then consider whether the presenting symptoms relate to that condition or stem from an unrelated inciting event.
Patients with DMD are at risk of pulmonary complications relating to their underlying disease. For instance, dysphagia and ineffective cough can predispose them to recurrent aspiration pneumonitis and/or pneumonia, whereas decreased lung compliance (from scoliosis, atelectasis, and/or pulmonary fibrosis) and respiratory muscle weakness can progress to ventilatory failure. In addition, patients with DMD are at risk for pulmonary thromboembolism in the setting of immobility. Patients with DMD may also develop congestive heart failure resulting from myocardial fibrosis and nonischemic cardiomyopathy.
The ejection from his wheelchair signals potential trauma-associated conditions that could explain his respiratory distress. Respiratory complications of blunt thoracic trauma include pulmonary contusion, pneumothorax, flail chest (resulting from fractured ribs), and acute respiratory distress syndrome (ARDS). Lower extremity injury can result in venous thrombosis and pulmonary thromboembolism. While classically associated with long bone fractures, fat embolism syndrome (FES) may rarely occur with rib fractures and soft-tissue trauma. Respiratory compromise may also result from cervical spinal cord injury or severe anemia from trauma-associated hemorrhage.
Additional past medical history included growth hormone deficiency, migraine headaches, osteoporosis secondary to chronic steroid use, cardiac fibrosis of the inferolateral wall and septum with a baseline left ventricular ejection fraction of 65%, and atrial fibrillation. His medications included calcium carbonate, vitamin D, omeprazole, lisinopril, metoprolol, prednisone, escitalopram, and testosterone. Physical examination revealed an ill-appearing obese man in respiratory distress. Temperature was 37.3°C, heart rate was 102 beats per minute (bpm), blood pressure was 110/74 mm Hg. His oxygen saturation was 93% with a respiratory rate of 25 breaths per minute while breathing ambient air. His lung sounds were clear, and his heart was without murmur. The left knee was diffusely tender to palpation without specific point tenderness. Strength was 2/5 with flexion and extension at the bilateral knees and hips and 3/5 flexion and extension at the bilateral elbows. He reported this level of weakness was his baseline. Radiographs revealed a minimally displaced Salter Harris II fracture (fracture line through the metaphysis and growth plate) of the left distal femur. His fracture was splinted early in his ED course. During his ED evaluation, the patient had acute worsening of tachycardia to 130 bpm, increased respiratory rate of 34 breaths per minute, and hypoxemia with an oxygen saturation of 83% on ambient air. He was placed on 3 L/min of oxygen via nasal cannula with improvement in his oxygen saturation to 90%. A chest radiograph was unremarkable, without evidence of pneumothorax, effusion, or pneumonia. The patient was admitted to the hospital.
The acute onset of tachypnea, tachycardia, and hypoxia, accompanied by a clear lung exam and normal chest radiograph, increases the likelihood of a pulmonary embolism. Obesity, testosterone therapy, and trauma increase his susceptibility to venous thromboembolism, while a distal femur fracture increases his risk for FES. Acute pulmonary aspiration often presents with initially absent or subtle radiographic findings. An arterial blood gas analysis would determine the presence and extent of an alveolar-arterial (A-a) gradient; a normal A-a gradient is seen in hypoventilation, while an elevated A-a gradient is seen in conditions affecting gas exchange, including pulmonary emboli and alveolar filling processes. His hypoxemia only partially corrects with supplemental oxygen, raising the possibility of capillary or anatomic shunting. Capillary shunting may occur with atelectasis, aspiration/pneumonia and pulmonary edema, whereas anatomic shunting can be intra-cardiac (eg, patent foramen ovale or septic defect) or intrapulmonary (eg, arteriovenous malformations). Patients with pulmonary emboli may also develop right-to-left shunting because of increased pulmonary vascular resistance, although hypoxemia with pulmonary emboli largely relates to ventilation/perfusion mismatch and decreased level of mixed venous blood oxygen (PvO2).
This patient’s complex medical history warrants a broadened differential with consideration of his cardiac history, including myocardial fibrosis and arrhythmia, and the impact of exposure to steroids on his immune and musculoskeletal systems. He has a history of atrial fibrillation, and an electrocardiogram is warranted to determine the underlying rhythm. Prolonged periods of rapid ventricular response may lead to tachycardia-induced cardiomyopathy. Myocardial fibrosis may progress despite use of angiotensin-converting enzyme inhibitors and is associated with systolic and/or diastolic dysfunction, although neither the examination findings provided nor the chest radiograph are suggestive of decompensated heart failure. Chronic exposure to corticosteroids (used in DMD to improve muscle strength and function) may predispose to numerous infectious and metabolic complications. Up to 10%-15% of patients with Pneumocystis jirovecii pneumonia may present with a normal chest radiograph. Acute adrenal insufficiency can present with tachycardia, weakness, and respiratory distress, so recent prednisone dose changes or interruptions should be assessed.
The patient’s respiratory status worsened. In light of his complex medical history, he was transferred to a children’s hospital for a higher level of care with a presumptive diagnosis of aspiration pneumonia. Upon reassessment at the new facility, the patient reported an ongoing and severe headache since his initial injury. NSAIDs had been given prior to transfer. His exam continued to be significant for tachycardia, tachypnea, and hypoxemia. His cardiac and lung examinations were otherwise normal. A comprehensive metabolic panel, procalcitonin, complete blood count with differential, and lactate were normal; his C-reactive protein (CRP) was 46.8 mg/dL (Normal <8 mg/dL). A computed tomography (CT) angiogram of the chest revealed small multifocal nodular ground-glass opacities, especially in the lower lobes, concerning for microatelectasis, multifocal pneumonia, or aspiration pneumonia. After consultation with pediatric pulmonology consultants, antimicrobials were held during the initial phase of work-up.
His headache may reflect a migraine, although further characterization and assessment for the presence and extent of head or neck trauma is warranted. Headache following trauma warrants consideration of cerebral contusion, diffuse axonal injury, intracranial hemorrhage, and carotid or vertebral artery dissection. Screening for concussion should also be performed. Hypoxemia may increase cerebral blood flow and raise intracranial pressure, resulting in headache.
CRP elevation is nonspecific and signals the presence of focal or systemic inflammation and is often elevated to a milder extent in obese patients with DMD. While normal procalcitonin argues against bacterial pneumonia, the precise level can be informative, and serial procalcitonin values may be more helpful than a single value. Although antecedent respiratory symptoms were not mentioned, viral or fungal pneumonia can present insidiously. An occult malignancy may be incidentally discovered when patients present for unrelated issues, although this and other sources of elevated CRP (eg, exacerbation of an autoimmune disease or drug reaction) remain less likely given the acuity of his presentation. Acute pulmonary embolism may be associated with a systemic inflammatory response and elevation in CRP.
In addition to the radiographic differential diagnosis already presented, the appearance of multifocal opacifications with hypoxemia raises the possibility of pulmonary infarcts or noncardiogenic pulmonary edema.
On hospital day 2, the patient continued to complain of “the worst headache of his life” as well as blurry vision and seeing “dark spots.” His headache did not improve with NSAIDs. A noncontrast CT scan of the head was normal. Neurology was consulted. Given his symptoms, history of migraines, stable neurological examination, and normal head CT, he was diagnosed with migraines and administered fluids, prochlorperazine, diphenhydramine, ondansetron, and NSAIDs. His headache continued and he continued to require supplemental oxygen.
The combination of hypoxemia, severe headache, and vision changes remains consistent with systemic emboli caused by thromboembolism or fat embolism. Headache assessment must also involve screening for “red flags,” which include sudden onset, antecedent head trauma, systemic illness (eg, fever or meningismus), focal neurologic findings, papilledema, changes with position or Valsalva, and immunosuppression. Although primary headache syndromes (eg, migraines or tension and cluster headache) may be triggered in the setting of trauma and systemic illness, “the worst headache of my life” is a concerning symptom that warrants urgent attention. While this invokes the possibility of a subarachnoid hemorrhage (SAH), headache severity is nonspecific, and rapid onset (ie, thunderclap headache) would be more suggestive. After 6 hours of symptoms, the sensitivity of head CT for detecting SAH declines, and lumbar puncture would be warranted to evaluate for xanthochromia.
His blurry vision and dark spots require testing of visual acuity and visual fields, as well as fundoscopic examination to assess for embolic phenomena or papilledema. Migraine is classically associated with “positive” or scintillating scotomata, although dark spots may occur. The presence of horizontal diplopia would indicate a cranial nerve VI palsy, which can occur with increased intracranial pressure. Visual-field cuts may also present as blurry vision, and monocular vs binocular deficits signal whether the issue is anterior or involving/posterior to the optic chiasm, respectively. Magnetic resonance imaging (MRI) may reveal the presence or sequelae of cerebral emboli (eg, fat emboli), including vasogenic edema.
Dilated fundus examination revealed Purtscher retinopathy: bilateral cotton-wool spots and larger areas of retinal whitening (Purtscher flecken).
Typical findings of Purtscher retinopathy include Purtscher flecken, cotton-wool spots, retinal hemorrhage, and optic disc edema. Purtscher retinopathy is classically associated with severe head trauma. Without associated head trauma, the term “Purtscher-like retinopathy” is used. Conditions that can cause Purtscher-like retinopathy include pancreatitis, vasculitis, microangiopathy, chronic renal failure, and systemic embolization. The most likely source of systemic embolization remains fat emboli stemming from his femur fracture. Treatment of FES is largely supportive.
The possibility of fat emboli had been repeatedly raised by the patient’s mother since admission. While providers had considered this a possibility, it was discounted early on because of the minor nature of the patient’s orthopedic trauma, the lack of clear radiographic evidence for pulmonary emboli on chest CT, and the normal head CT. The findings on the ophthalmologist’s fundoscopic examination led the primary team to reconsider FES, along with thromboemboli and pancreatitis. Lipase was normal. MRI of the brain with contrast revealed >20 microinfarcts in the bilateral hemispheres, left corpus callosum, and bilateral basal ganglia. The CT angiogram of the chest was rereviewed; the pediatric radiologists suggested that microinfarcts could explain the patchy small ground glass opacities seen in the lungs. A transthoracic echocardiogram and electrocardiogram were normal. The diagnosis of FES was made, and the patient was started on aspirin and enoxaparin prophylaxis. His headache and respiratory status improved, and he was discharged home with close follow-up.
DISCUSSION
FES is a rare complication associated with long bone fractures and orthopedic manipulation.1,2 The exact mechanism of fat emboli production is unknown, but two theories prevail. The mechanical theory states that an outside mechanical source causes bone marrow contents or adipose tissue contents to be dislodged into the circulation where they travel through the venous circulation to become embedded in the lungs.1,2 These fragments may also migrate to the arterial circulation, through a patent foramen ovale or intrapulmonary shunts, leading to end organ damage.1,2 The biochemical theory suggests that fat emboli in the venous circulation precipitate an inflammatory and prothrombotic cascade that triggers fibrin production, platelet aggregation, and release of free fatty acids into the circulation, predisposing patients to develop multifocal systemic emboli.1
Although the classic triad in FES includes respiratory symptoms, rash, and CNS symptoms, all three findings are only present in 1%-29% of cases.1,2 Respiratory abnormalities, ranging from tachypnea and dyspnea to ARDS and hypoxic respiratory failure, occur in up to 75% of patients with FES.1 Central nervous system (CNS) complications, including headache, confusion, coma, seizures, and death caused by cerebral ischemia, occur in up to 86% of patients.1,2 Petechiae may occur in 20%-60% of patients and are usually located on nondependent regions of the body such as the head, neck, and chest.
Diagnosis of FES is largely clinical and requires a high index of suspicion and elimination of other conditions, including pulmonary thromboembolism, diffuse intravascular coagulation, and sepsis. The CNS complications must be differentiated from CNS infection, stroke, migraine, benign intracranial hypertension, and intracranial hemorrhage. There is no gold standard test for diagnosis. The Gurd and Wilson criteria, modified Gurd criteria, and Schonfeld’s criteria (Table) are commonly used but have not been clinically validated.1,3-5 These use a combination of clinical signs of respiratory distress, neurological symptoms, petechial rash, and various other diagnostic factors. When patients have risk factors, such as trauma, surgery, or predisposing conditions (eg, mobility-limiting neuromuscular disorders) and signs and symptoms potentially consistent with FES, the diagnostic evaluation should include arterial blood gas analysis, complete blood count, chest radiographs, and coagulation studies. Ophthalmological exam to evaluate for cotton-wool spots, brain MRI to detect cerebral emboli, and CT pulmonary angiogram to assess for pulmonary infarcts may help to eliminate other diagnoses and/or confirm FES.
Diagnosis of uncommon conditions that present with nonspecific symptoms, like FES, can be challenging because the symptoms may overlap with many other possible diagnoses. This challenge is further exacerbated in patients with significant medical complexity, as with the patient discussed here. Specifically, this patient had multiple plausible explanations for CNS symptoms and respiratory symptoms. It was ultimately the visual symptoms that began to link his symptoms together into a unifying syndrome and the ophthalmologic examination that prompted confirmatory imaging. It is important to continually revisit and revise the differential diagnosis in patients with medical complexity and avoid the competing temptations to abandon the search for a unifying diagnosis and attribute all symptoms to a patient’s underlying condition.
Treatment of FES is largely supportive with close monitoring of neurological status and providing supplemental oxygen as needed. Corticosteroids have been suggested to help prevent FES in patients with long bone fractures, but there is no evidence to suggest they are helpful once FES is diagnosed.2 There is conflicting evidence for the efficacy of heparin or low-molecular-weight heparin as treatment in FES.2,6 After discussions with consulting physician teams, the patient, and his family, enoxaparin and aspirin were started for this patient in light of his tenuous condition in order to decrease the risk of further embolic complications.
Patients with DMD and other neuromuscular disorders likely have a greater propensity to develop FES even after minor trauma.1,6 This is believed to be caused by patients becoming nonambulatory early in life and receiving substantial corticosteroid therapy, which can lead to osteopenia and fatty replacement of the bone marrow.1,6 This population is also often obese by the second or third decade of life, which contributes to their already increased propensity to fall.1,6
To our knowledge, this patient is 1 of 18 reported cases of FES after trauma in DMD patients. Two-thirds of these cases occurred when an unrestrained patient fell from their wheelchair. The other cases occurred while walking, during physical therapy, and during assisted transfers.6-12 In these cases, FES had a guarded prognosis, with 7 of the 18 patients dying and 1 of the patients remaining in a persistent vegetative state.8,9 While caution is warranted in generalizing these findings, given the small number of reported cases and likely publication bias,education of caregivers and patients on use of restraints and safe transfers is paramount to limit the risk of trauma.
TEACHING POINTS
- FES is a rare condition that most commonly manifests with respiratory, neurological, and cutaneous findings.
- Patients with Duchenne’s Muscular Dystrophy are likely at increased risk for FES even with minor trauma; this makes wheelchair restraints and safe transfers fundamental.
- Patients with medical complexity and their caregivers are key members of the diagnostic team.
1. Fukumoto LE, Fukumoto KD. Fat embolism syndrome. Nurs Clin North Am. 2018;53(3):335-347. https://doi.org/10.1016/j.cnur.2018.04.003.
2. Scarpino M, Lanzo G, Lolli F, Grippo A. From the diagnosis to the therapeutic management: Cerebral fat embolism, a clinical challenge. Int J Gen Med. 2019;2019(12):39-48. https://doi.org/10.2147/IJGM.S177407.
1. Fukumoto LE, Fukumoto KD. Fat embolism syndrome. Nurs Clin North Am. 2018;53(3):335-347. https://doi.org/10.1016/j.cnur.2018.04.003.
2. Scarpino M, Lanzo G, Lolli F, Grippo A. From the diagnosis to the therapeutic management: Cerebral fat embolism, a clinical challenge. Int J Gen Med. 2019;2019(12):39-48. https://doi.org/10.2147/IJGM.S177407.
© 2020 Society of Hospital Medicine
Expanding the View: Implications of the SHM Position Statement on Ultrasound Use in Vascular Access
Is there a single intervention more important to hospitalized patients than vascular access? Since their advent in the 1950s, small plastic tubes have revolutionized medication administration and become a mainstay of modern medicine. Yet, for much of the last 60 years, nurses and doctors have used the same landmark-guided approaches to acquire peripheral and, more specifically, central access.1 Minor improvements to the Seldinger technique and sterile preparation have been reported.2 However, for such a vital and common procedure, the complication rates of landmark-based approaches to central venous access remain unacceptably high.3
In the position statement released by the Society of Hospital Medicine (SHM), Franco–Sadud et al. outline the transformative effects ultrasound can have in obtaining adult vascular access.4 The authors cite comprehensive evidence, leaving little doubt of the technique’s benefits compared with landmark-based approaches. However, several questions remain: Is vascular access the domain of the hospitalist? If so, how can hospitalists pursue and afford ultrasound training? Finally, how will this shift toward ultrasound-guided vascular access affect patients in resource-limited settings?
Through an expert-driven literature review, the authors present 29 succinct recommendations for ultrasound use in vascular access. Supporting data consistently illustrate the association of ultrasound with increased successful vessel cannulation rates and decreased complication rates for all types of vascular access; including central venous access (internal jugular, subclavian, femoral), arterial line placement, peripherally inserted central catheters, and difficult peripheral venous access. Despite this compelling evidence, however, 20%-55% of all central venous catheters are still placed without ultrasound.5 How, then, can hospitalists expand ultrasound use for vascular access or perform these procedures in general?
Hospitalists likely fall into one of three categories in terms of vascular access: (1) they are proficient in ultrasound use for vascular access, (2) they still routinely use traditional landmark-based approaches, or (3) they have little to no involvement in vascular access and defer to intensivists, interventional radiologists, or nurse specialists. Franco-Sadud et al.’s position statement acknowledges the wide range of hospitalist practices and only asserts that, if providers perform vascular access, they should be trained and use ultrasound to do them. We would advocate further that, regardless of their practice, hospitalists have a role in expanding ultrasound use for vascular access given its direct impact on the patients they care for. Hospitalists who do not directly practice vascular access can still leverage the skills that have established hospital medicine’s reputation as leaders in patient safety and quality improvement. Hospitalists can partner with proceduralists in their institutions to ensure that they are supported and trained in the most evidence-based approaches to vascular access and that their patients have access to the highest quality of care.
For the individual hospitalist, the investment of time and resources to incorporate ultrasound into routine practice can seem daunting. In previous position statements, the SHM has advocated for the robust use of simulation and directly observed assessment in credentialing for all bedside procedures.6 However, the Society also acknowledges that this degree of training and monitoring can constitute significant barriers and has argued that the onus for change lies not only with providers but with healthcare institutions at large. How, then, can hospitalists approach their institutions to successfully solicit support? While the evidence is not yet conclusive, Cohen et al. have shown promising data for potential long-term cost savings through ultrasound-guided vascular access.7 Due to decreased complication rates, downstream benefits of lower resource use, higher patient satisfaction, and, theoretically, even lower clinician burnout rates have been attained. These effects, combined with hospitalists acquiring ultrasound skills translatable to other bedside procedures and fundamentals of diagnostic point of care ultrasound, form a compelling argument for institutional support. Many academic medical centers, typically with increased resources and training programs, have been early adopters; but, how will the shift from landmark-based to ultrasound-guided vascular access affect those in resource-limited settings?
While incredible strides have been made in care quality and patient safety over the last 15 years, improvements clearly do not always benefit patients, clinicians, or institutions equally.8 In fact, those in resource-limited settings often experience disproportionately reduced benefits. While focus on the “quality gap” has transformed the culture of the quality improvement and patient safety fields, an “equity gap” has long undermined and limited the impact of those very improvements. Unfortunately, changes in care driven by costly technological advances such as ultrasound are particularly likely to widen this “equity gap.” While ultrasound technology is rapidly becoming more affordable, a lack of access to machines and appropriate training remain significant barriers in the resource-limited settings that hospitalists are most likely to be performing these procedures. Without a focus on equity, the benefits offered by ultrasound will continue to be limited in their reach.
The SHM position statement by Franco-Sadud et al. is an important step in expanding evidence-based ultrasound use for vascular access and improving patient care. While the recommendations are, at times, aspirational and the barriers are real, hospitalists have shown time and again their ability to overcome these challenges and advance the standard of care for all.
1. Beheshti MV. A concise history of central venous access. Tech Vasc Interv Radiol. 2011;14(4):184-5. https://doi.org/10.1053/j.tvir.2011.05.002.
2. Higgs ZC, Macafee DA, Braithwaite BD, Maxwell-Armstrong CA. The Seldinger technique: 50 years on. Lancet. 2005;366(9494):1407-1409. https://doi.org/10.1016/S0140-6736(05)66878-X.
3. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
4. Franco-Sadud R, D Schnobrich, Mathews BK et al. SHM Point-of-care Ultrasound Task Force. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287.
5. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
6. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2);117-125. https://doi.org/10.12788/jhm.2917.
7. Cohen ER, Feinglass J, Barsuk JH, et al. Cost savings from reduced catheter-related bloodstream infection after simulation-based education for residents in a medical intensive care unit. Simul Healthc. 2010;5(2):98-102. https://doi.org/10.1097/SIH.0b013e3181bc8304.
8. 2017 National Healthcare Quality and Disparities Report.
Is there a single intervention more important to hospitalized patients than vascular access? Since their advent in the 1950s, small plastic tubes have revolutionized medication administration and become a mainstay of modern medicine. Yet, for much of the last 60 years, nurses and doctors have used the same landmark-guided approaches to acquire peripheral and, more specifically, central access.1 Minor improvements to the Seldinger technique and sterile preparation have been reported.2 However, for such a vital and common procedure, the complication rates of landmark-based approaches to central venous access remain unacceptably high.3
In the position statement released by the Society of Hospital Medicine (SHM), Franco–Sadud et al. outline the transformative effects ultrasound can have in obtaining adult vascular access.4 The authors cite comprehensive evidence, leaving little doubt of the technique’s benefits compared with landmark-based approaches. However, several questions remain: Is vascular access the domain of the hospitalist? If so, how can hospitalists pursue and afford ultrasound training? Finally, how will this shift toward ultrasound-guided vascular access affect patients in resource-limited settings?
Through an expert-driven literature review, the authors present 29 succinct recommendations for ultrasound use in vascular access. Supporting data consistently illustrate the association of ultrasound with increased successful vessel cannulation rates and decreased complication rates for all types of vascular access; including central venous access (internal jugular, subclavian, femoral), arterial line placement, peripherally inserted central catheters, and difficult peripheral venous access. Despite this compelling evidence, however, 20%-55% of all central venous catheters are still placed without ultrasound.5 How, then, can hospitalists expand ultrasound use for vascular access or perform these procedures in general?
Hospitalists likely fall into one of three categories in terms of vascular access: (1) they are proficient in ultrasound use for vascular access, (2) they still routinely use traditional landmark-based approaches, or (3) they have little to no involvement in vascular access and defer to intensivists, interventional radiologists, or nurse specialists. Franco-Sadud et al.’s position statement acknowledges the wide range of hospitalist practices and only asserts that, if providers perform vascular access, they should be trained and use ultrasound to do them. We would advocate further that, regardless of their practice, hospitalists have a role in expanding ultrasound use for vascular access given its direct impact on the patients they care for. Hospitalists who do not directly practice vascular access can still leverage the skills that have established hospital medicine’s reputation as leaders in patient safety and quality improvement. Hospitalists can partner with proceduralists in their institutions to ensure that they are supported and trained in the most evidence-based approaches to vascular access and that their patients have access to the highest quality of care.
For the individual hospitalist, the investment of time and resources to incorporate ultrasound into routine practice can seem daunting. In previous position statements, the SHM has advocated for the robust use of simulation and directly observed assessment in credentialing for all bedside procedures.6 However, the Society also acknowledges that this degree of training and monitoring can constitute significant barriers and has argued that the onus for change lies not only with providers but with healthcare institutions at large. How, then, can hospitalists approach their institutions to successfully solicit support? While the evidence is not yet conclusive, Cohen et al. have shown promising data for potential long-term cost savings through ultrasound-guided vascular access.7 Due to decreased complication rates, downstream benefits of lower resource use, higher patient satisfaction, and, theoretically, even lower clinician burnout rates have been attained. These effects, combined with hospitalists acquiring ultrasound skills translatable to other bedside procedures and fundamentals of diagnostic point of care ultrasound, form a compelling argument for institutional support. Many academic medical centers, typically with increased resources and training programs, have been early adopters; but, how will the shift from landmark-based to ultrasound-guided vascular access affect those in resource-limited settings?
While incredible strides have been made in care quality and patient safety over the last 15 years, improvements clearly do not always benefit patients, clinicians, or institutions equally.8 In fact, those in resource-limited settings often experience disproportionately reduced benefits. While focus on the “quality gap” has transformed the culture of the quality improvement and patient safety fields, an “equity gap” has long undermined and limited the impact of those very improvements. Unfortunately, changes in care driven by costly technological advances such as ultrasound are particularly likely to widen this “equity gap.” While ultrasound technology is rapidly becoming more affordable, a lack of access to machines and appropriate training remain significant barriers in the resource-limited settings that hospitalists are most likely to be performing these procedures. Without a focus on equity, the benefits offered by ultrasound will continue to be limited in their reach.
The SHM position statement by Franco-Sadud et al. is an important step in expanding evidence-based ultrasound use for vascular access and improving patient care. While the recommendations are, at times, aspirational and the barriers are real, hospitalists have shown time and again their ability to overcome these challenges and advance the standard of care for all.
Is there a single intervention more important to hospitalized patients than vascular access? Since their advent in the 1950s, small plastic tubes have revolutionized medication administration and become a mainstay of modern medicine. Yet, for much of the last 60 years, nurses and doctors have used the same landmark-guided approaches to acquire peripheral and, more specifically, central access.1 Minor improvements to the Seldinger technique and sterile preparation have been reported.2 However, for such a vital and common procedure, the complication rates of landmark-based approaches to central venous access remain unacceptably high.3
In the position statement released by the Society of Hospital Medicine (SHM), Franco–Sadud et al. outline the transformative effects ultrasound can have in obtaining adult vascular access.4 The authors cite comprehensive evidence, leaving little doubt of the technique’s benefits compared with landmark-based approaches. However, several questions remain: Is vascular access the domain of the hospitalist? If so, how can hospitalists pursue and afford ultrasound training? Finally, how will this shift toward ultrasound-guided vascular access affect patients in resource-limited settings?
Through an expert-driven literature review, the authors present 29 succinct recommendations for ultrasound use in vascular access. Supporting data consistently illustrate the association of ultrasound with increased successful vessel cannulation rates and decreased complication rates for all types of vascular access; including central venous access (internal jugular, subclavian, femoral), arterial line placement, peripherally inserted central catheters, and difficult peripheral venous access. Despite this compelling evidence, however, 20%-55% of all central venous catheters are still placed without ultrasound.5 How, then, can hospitalists expand ultrasound use for vascular access or perform these procedures in general?
Hospitalists likely fall into one of three categories in terms of vascular access: (1) they are proficient in ultrasound use for vascular access, (2) they still routinely use traditional landmark-based approaches, or (3) they have little to no involvement in vascular access and defer to intensivists, interventional radiologists, or nurse specialists. Franco-Sadud et al.’s position statement acknowledges the wide range of hospitalist practices and only asserts that, if providers perform vascular access, they should be trained and use ultrasound to do them. We would advocate further that, regardless of their practice, hospitalists have a role in expanding ultrasound use for vascular access given its direct impact on the patients they care for. Hospitalists who do not directly practice vascular access can still leverage the skills that have established hospital medicine’s reputation as leaders in patient safety and quality improvement. Hospitalists can partner with proceduralists in their institutions to ensure that they are supported and trained in the most evidence-based approaches to vascular access and that their patients have access to the highest quality of care.
For the individual hospitalist, the investment of time and resources to incorporate ultrasound into routine practice can seem daunting. In previous position statements, the SHM has advocated for the robust use of simulation and directly observed assessment in credentialing for all bedside procedures.6 However, the Society also acknowledges that this degree of training and monitoring can constitute significant barriers and has argued that the onus for change lies not only with providers but with healthcare institutions at large. How, then, can hospitalists approach their institutions to successfully solicit support? While the evidence is not yet conclusive, Cohen et al. have shown promising data for potential long-term cost savings through ultrasound-guided vascular access.7 Due to decreased complication rates, downstream benefits of lower resource use, higher patient satisfaction, and, theoretically, even lower clinician burnout rates have been attained. These effects, combined with hospitalists acquiring ultrasound skills translatable to other bedside procedures and fundamentals of diagnostic point of care ultrasound, form a compelling argument for institutional support. Many academic medical centers, typically with increased resources and training programs, have been early adopters; but, how will the shift from landmark-based to ultrasound-guided vascular access affect those in resource-limited settings?
While incredible strides have been made in care quality and patient safety over the last 15 years, improvements clearly do not always benefit patients, clinicians, or institutions equally.8 In fact, those in resource-limited settings often experience disproportionately reduced benefits. While focus on the “quality gap” has transformed the culture of the quality improvement and patient safety fields, an “equity gap” has long undermined and limited the impact of those very improvements. Unfortunately, changes in care driven by costly technological advances such as ultrasound are particularly likely to widen this “equity gap.” While ultrasound technology is rapidly becoming more affordable, a lack of access to machines and appropriate training remain significant barriers in the resource-limited settings that hospitalists are most likely to be performing these procedures. Without a focus on equity, the benefits offered by ultrasound will continue to be limited in their reach.
The SHM position statement by Franco-Sadud et al. is an important step in expanding evidence-based ultrasound use for vascular access and improving patient care. While the recommendations are, at times, aspirational and the barriers are real, hospitalists have shown time and again their ability to overcome these challenges and advance the standard of care for all.
1. Beheshti MV. A concise history of central venous access. Tech Vasc Interv Radiol. 2011;14(4):184-5. https://doi.org/10.1053/j.tvir.2011.05.002.
2. Higgs ZC, Macafee DA, Braithwaite BD, Maxwell-Armstrong CA. The Seldinger technique: 50 years on. Lancet. 2005;366(9494):1407-1409. https://doi.org/10.1016/S0140-6736(05)66878-X.
3. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
4. Franco-Sadud R, D Schnobrich, Mathews BK et al. SHM Point-of-care Ultrasound Task Force. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287.
5. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
6. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2);117-125. https://doi.org/10.12788/jhm.2917.
7. Cohen ER, Feinglass J, Barsuk JH, et al. Cost savings from reduced catheter-related bloodstream infection after simulation-based education for residents in a medical intensive care unit. Simul Healthc. 2010;5(2):98-102. https://doi.org/10.1097/SIH.0b013e3181bc8304.
8. 2017 National Healthcare Quality and Disparities Report.
1. Beheshti MV. A concise history of central venous access. Tech Vasc Interv Radiol. 2011;14(4):184-5. https://doi.org/10.1053/j.tvir.2011.05.002.
2. Higgs ZC, Macafee DA, Braithwaite BD, Maxwell-Armstrong CA. The Seldinger technique: 50 years on. Lancet. 2005;366(9494):1407-1409. https://doi.org/10.1016/S0140-6736(05)66878-X.
3. Parienti JJ, Mongardon N, Mégarbane B, et al. Intravascular complications of central venous catheterization by insertion site. N Engl J Med. 2015;373(13):1220-1229. https://doi.org/10.1056/NEJMoa1500964.
4. Franco-Sadud R, D Schnobrich, Mathews BK et al. SHM Point-of-care Ultrasound Task Force. Recommendations on the use of ultrasound guidance for central and peripheral vascular access in adults: a position statement of the Society of Hospital Medicine. J Hosp Med. 2019;14:E1-E22. https://doi.org/10.12788/jhm.3287.
5. Soni NJ, Reyes LF, Keyt H, et al. Use of ultrasound guidance for central venous catheterization: a national survey of intensivists and hospitalists. J Crit Care. 2016;36:277-283. https://doi.org/10.1016/j.jcrc.2016.07.014.
6. Lucas BP, Tierney DM, Jensen TP, et al. Credentialing of hospitalists in ultrasound-guided bedside procedures: a position statement of the Society of Hospital Medicine. J Hosp Med. 2018;13(2);117-125. https://doi.org/10.12788/jhm.2917.
7. Cohen ER, Feinglass J, Barsuk JH, et al. Cost savings from reduced catheter-related bloodstream infection after simulation-based education for residents in a medical intensive care unit. Simul Healthc. 2010;5(2):98-102. https://doi.org/10.1097/SIH.0b013e3181bc8304.
8. 2017 National Healthcare Quality and Disparities Report.
© 2019 Society of Hospital Medicine
Association Between Anemia and Fatigue in Hospitalized Patients: Does the Measure of Anemia Matter?
Fatigue is the most common clinical symptom of anemia and is a significant concern to patients.1,2 In ambulatory patients, lower hemoglobin (Hb) concentration is associated with increased fatigue.2,3 Accordingly, therapies that treat anemia by increasing Hb concentration, such as erythropoiesis stimulating agents,4-7 often use fatigue as an outcome measure.
In hospitalized patients, transfusion of red blood cell increases Hb concentration and is the primary treatment for anemia. However, the extent to which transfusion and changes in Hb concentration affect hospitalized patients’ fatigue levels is not well established. Guidelines support transfusing patients with symptoms of anemia, such as fatigue, on the assumption that the increased oxygen delivery will improve the symptoms of anemia. While transfusion studies in hospitalized patients have consistently reported that transfusion at lower or “restrictive” Hb concentrations is safe compared with transfusion at higher Hb concentrations,8-10 these studies have mainly used cardiac events and mortality as outcomes rather than patient symptoms, such as fatigue. Nevertheless, they have resulted in hospitals increasingly adopting restrictive transfusion policies that discourage transfusion at higher Hb levels.11,12 Consequently, the rate of transfusion in hospitalized patients has decreased,13 raising questions of whether some patients with lower Hb concentrations may experience increased fatigue as a result of restrictive transfusion policies. Fatigue among hospitalized patients is important not only because it is an adverse symptom but because it may result in decreased activity levels, deconditioning, and losses in functional status.14,15While the effect of alternative transfusion policies on fatigue in hospitalized patients could be answered by a randomized clinical trial using fatigue and functional status as outcomes, an important first step is to assess whether the Hb concentration of hospitalized patients is associated with their fatigue level during hospitalization. Because hospitalized patients often have acute illnesses that can cause fatigue in and of themselves, it is possible that anemia is not associated with fatigue in hospitalized patients despite anemia’s association with fatigue in ambulatory patients. Additionally, Hb concentration varies during hospitalization,16 raising the question of what measures of Hb during hospitalization might be most associated with anemia-related fatigue.
The objective of this study is to explore multiple Hb measures in hospitalized medical patients with anemia and test whether any of these Hb measures are associated with patients’ fatigue level.
METHODS
Study Design
We performed a prospective, observational study of hospitalized patients with anemia on the general medicine services at The University of Chicago Medical Center (UCMC). The institutional review board approved the study procedures, and all study subjects provided informed consent.
Study Eligibility
Between April 2014 and June 2015, all general medicine inpatients were approached for written consent for The University of Chicago Hospitalist Project,17 a research infrastructure at UCMC. Among patients consenting to participate in the Hospitalist Project, patients were eligible if they had Hb <9 g/dL at any point during their hospitalization and were age ≥50 years. Hb concentration of <9 g/dL was chosen to include the range of Hb values covered by most restrictive transfusion policies.8-10,18 Age ≥50 years was an inclusion criteria because anemia is more strongly associated with poor outcomes, including functional impairment, among older patients compared with younger patients.14,19-21 If patients were not eligible for inclusion at the time of consent for the Hospitalist Project, their Hb values were reviewed twice daily until hospital discharge to assess if their Hb was <9 g/dL. Proxies were sought to answer questions for patients who failed the Short Portable Mental Status Questionnaire.22
Patient Demographic Data Collection
Research assistants abstracted patient age and sex from the electronic health record (EHR), and asked patients to self-identify their race. The individual comorbidities included as part of the Charlson Comorbidity Index were identified using International Classification of Diseases, 9th Revision codes from hospital administrative data for each encounter and specifically included the following: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes, hemiplegia and/or paraplegia, renal disease, cancer, and human immunodeficiency virus/acquired immunodeficiency syndrome.23 We also used Healthcare Cost and Utilization Project (www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp) diagnosis categories to identify whether patients had sickle cell (SC) anemia, gastrointestinal bleeding (GIB), or a depressive disorder (DD) because these conditions are associated with anemia (SC and GIB) and fatigue (DD).24
Measuring Anemia
Hb measures were available only when hospital providers ordered them as part of routine practice. The first Hb concentration <9 g/dL during a patient’s hospitalization, which made them eligible for study participation, was obtained through manual review of the EHR. All additional Hb values during the patient’s hospitalization were obtained from the hospital’s administrative data mart. All Hb values collected for each patient during the hospitalization were used to calculate summary measures of Hb during the hospitalization, including the mean Hb, median Hb, minimum Hb, maximum Hb, admission (first recorded) Hb, and discharge (last recorded) Hb. Hb measures were analyzed both as a continuous variable and as a categorical variable created by dividing the continuous Hb measures into integer ranges of 3 groups of approximately the same size.
Measuring Fatigue
Our primary outcome was patients’ level of fatigue reported during hospitalization, measured using the Functional Assessment of Chronic Illness Therapy (FACIT)-Anemia questionnaire. Fatigue was measured using a 13-question fatigue subscale,1,2,25 which measures fatigue within the past 7 days. Scores on the fatigue subscale range from 0 to 52, with lower scores reflecting greater levels of fatigue. As soon as patients met the eligibility criteria for study participation during their hospitalization (age ≥50 years and Hb <9 g/dL), they were approached to answer the FACIT questions. Values for missing data in the fatigue subscale for individual subjects were filled in using a prorated score from their answered questions as long as >50% of the items in the fatigue subscale were answered, in accordance with recommendations for addressing missing data in the FACIT.26 Fatigue was analyzed as a continuous variable and as a dichotomous variable created by dividing the sample into high (FACIT <27) and low (FACIT ≥27) levels of fatigue based on the median FACIT score of the population. Previous literature has shown a FACIT fatigue subscale score between 23 and 26 to be associated with an Eastern Cooperative Oncology Group (ECOG)27 C Performance Status rating of 2 to 33 compared to scores ≥27.
Statistical Analysis
Statistical analysis was performed using Stata statistical software (StataCorp, College Station, TX). Descriptive statistics were used to characterize patient demographics. Analysis of variance was used to test for differences in the mean fatigue levels across Hb measures. χ2 tests were performed to test for associations between high fatigue levels and the Hb measures. Multivariable analysis, including both linear and logistic regression models, were used to test the association of Hb concentration and fatigue. P values <0.05 using a 2-tailed test were deemed statistically significant.
RESULTS
Patient Characteristics
During the study period, 8559 patients were admitted to the general medicine service. Of those, 5073 (59%) consented for participation in the Hospitalist Project, and 3670 (72%) completed the Hospitalist Project inpatient interview. Of these patients, 1292 (35%) had Hb <9 g/dL, and 784 (61%) were 50 years or older and completed the FACIT questionnaire.
Table 1 reports the demographic characteristics and comorbidities for the sample, the mean (standard deviation [SD]) for the 6 Hb measures, and mean (SD) and median FACIT scores.
Bivariate Association of Fatigue and Hb
Categorizing patients into low, middle, or high Hb for each of the 6 Hb measures, minimum Hb was strongly associated with fatigue, with a weaker association for mean Hb and no statistically significant association for the other measures.
Minimum Hb. Patients with a minimum Hb <7 g/dL and patients with Hb 7-8 g/dL had higher fatigue levels (FACIT = 25 for each) than patients with a minimum Hb ≥8 g/dL (FACIT = 29; P < 0.001; Table 2). When excluding patients with SC and/or GIB because their average minimum Hb differed from the average minimum Hb of the full population (P < 0.001), patients with a minimum Hb <7 g/dL or 7-8 g/dL had even higher fatigue levels (FACIT = 23 and FACIT = 24, respectively), with no change in the fatigue level of patients with a minimum Hb ≥8 g/dL (FACIT = 29; P < 0.001; Table 2). Lower minimum Hb continued to be associated with higher fatigue levels when analyzed in 0.5 g/dL increments (Figure).
Mean Hb and Other Measures. Fatigue levels were high for 47% of patients with a mean Hb <8g /dL and 53% of patients with a mean Hb 8-9 g/dL compared with 43% of patients with a mean Hb ≥9 g/dL (P = 0.05). However, the association between high fatigue and mean Hb was not statistically significant when patients with SC and/or GIB were excluded (Table 2). None of the other 4 Hb measures was significantly associated with fatigue.
Linear Regression of Fatigue on Hb
In linear regression models, minimum Hb consistently predicted patient fatigue, mean Hb had a less robust association with fatigue, and the other Hb measures were not associated with patient fatigue. Increases in minimum Hb (analyzed as a continuous variable) were associated with reduced fatigue (higher FACIT score; β = 1.4; P = 0.005). In models in which minimum Hb was a categorical variable, patients with a minimum Hb of <7 g/dL or 7-8 g/dL had greater fatigue (lower FACIT score) than patients whose minimum Hb was ≥8 g/dL (Hb <7 g/dL: β = −4.2; P ≤ 0.001; Hb 7-8 g/dL: β = −4.1; P < 0.001). These results control for patients’ age, sex, individual comorbidities, and whether their minimum Hb occurred before or after the measurement of fatigue during hospitalization (Model 1), and the results are unchanged when also controlling for the number of Hb laboratory draws patients had during their hospitalization (Model 2; Table 3). In a stratified analysis excluding patients with either SC and/or GIB, changes in minimum Hb were associated with larger changes in patient fatigue levels (Supplemental Table 1). We also stratified our analysis to include only patients whose minimum Hb occurred before the measurement of their fatigue level during hospitalization to avoid a spurious association of fatigue with minimum Hb occurring after fatigue was measured. In both Models 1 and 2, minimum Hb remained a predictor of patients’ fatigue levels with similar effect sizes, although in Model 2, the results did not quite reach a statistically significant level, in part due to larger confidence intervals from the smaller sample size of this stratified analysis (Supplemental Table 2a). We further stratified this analysis to include only patients whose transfusion, if they received one, occurred after their minimum Hb and the measurement of their fatigue level to account for the possibility that a transfusion could affect the fatigue level patients report. In this analysis, most of the estimates of the effect of minimum Hb on fatigue were larger than those seen when only analyzing patients whose minimum Hb occurred before the measurement of their fatigue level, although again, the smaller sample size of this additional stratified analysis does produce larger confidence intervals for these estimates (Supplemental Table 2b).
No Hb measure other than minimum or mean had significant association with patient fatigue levels in linear regression models.
Logistic Regression of High Fatigue Level on Hb
Using logistic regression, minimum Hb analyzed as a categorical variable predicted increased odds of a high fatigue level. Patients with a minimum Hb <7 g/dL were 50% (odds ratio [OR] = 1.5; P = 0.03) more likely to have high fatigue and patients with a minimum Hb 7-8 g/dL were 90% (OR = 1.9; P < 0.001) more likely to have high fatigue compared with patients with a minimum Hb ≥8 g/dL in Model 1. These results were similar in Model 2, although the effect was only statistically significant in the 7-8 g/dL Hb group (Table 3). When excluding SC and/or GIB patients, the odds of having high fatigue as minimum Hb decreased were the same or higher for both models compared to the full population of patients. However, again, in Model 2, the effect was only statistically significant in the 7-8 g/dL Hb group (Supplemental Table 1).
Patients with a mean Hb <8 g/dL were 20% to 30% more likely to have high fatigue and patients with mean Hb 8-9 g/dL were 50% more likely to have high fatigue compared with patients with a mean Hb ≥9 g/dL, but the effects were only statistically significant for patients with a mean Hb 8-9 g/dL in both Models 1 and 2 (Table 3). These results were similar when excluding patients with SC and/or GIB, but they were only significant for patients with a mean Hb 8-9 g/dL in Model 1 and patients with a mean Hb <8 g/dL in the Model 2 (Supplemental Table 3).
DISCUSSION
These results demonstrate that minimum Hb during hospitalization is associated with fatigue in hospitalized patients age ≥50 years, and the association is stronger among patients without SC and/or GIB as comorbidities. The analysis of Hb as a continuous and categorical variable and the use of both linear and logistic regression models support the robustness of these associations and illuminate their clinical significance. For example, in linear regression with minimum Hb a continuous variable, the coefficient of 1.4 suggests that an increase of 2 g/dL in Hb, as might be expected from transfusion of 2 units of red blood cells, would be associated with about a 3-point improvement in fatigue. Additionally, as a categorical variable, a minimum Hb ≥8 g/dL compared with a minimum Hb <7 g/dL or 7-8 g/dL is associated with a 3- to 4-point improvement in fatigue. Previous literature suggests that a difference of 3 in the FACIT score is the minimum clinically important difference in fatigue,3 and changes in minimum Hb in either model predict changes in fatigue that are in the range of potential clinical significance.
The clinical significance of the findings is also reflected in the results of the logistic regressions, which may be mapped to potential effects on functional status. Specifically, the odds of having a high fatigue level (FACIT <27) increase 90% for persons with a minimum Hb 7–8 g/dL compared with persons with a minimum Hb ≥8 g/dL. For persons with a minimum Hb <7 g/dL, point estimates suggest a smaller (50%) increase in the odds of high fatigue, but the 95% confidence interval overlaps heavily with the estimate of patients whose minimum Hb is 7-8 g/dL. While it might be expected that patients with a minimum Hb <7 g/dL have greater levels of fatigue compared with patients with a minimum Hb 7-8 g/dL, we did not observe such a pattern. One reason may be that the confidence intervals of our estimated effects are wide enough that we cannot exclude such a pattern. Another possible explanation is that in both groups, the fatigue levels are sufficiently severe, such that the difference in their fatigue levels may not be clinically meaningful. For example, a FACIT score of 23 to 26 has been shown to be associated with an ECOG performance status of 2 to 3, requiring bed rest for at least part of the day.3 Therefore, patients with a minimum Hb 7-8 g/dL (mean FACIT score = 24; Table 2) or a minimum Hb of <7 g/dL (mean FACIT score = 23; Table 2) are already functionally limited to the point of being partially bed bound, such that further decreases in their Hb may not produce additional fatigue in part because they reduce their activity sufficiently to prevent an increase in fatigue. In such cases, the potential benefits of increased Hb may be better assessed by measuring fatigue in response to a specific and provoked activity level, a concept known as fatigability.20
That minimum Hb is more strongly associated with fatigue than any other measure of Hb during hospitalization may not be surprising. Mean, median, maximum, and discharge Hb may all be affected by transfusion during hospitalization that could affect fatigue. Admission Hb may not reflect true oxygen-carrying capacity because of hemoconcentration.
The association between Hb and fatigue in hospitalized patients is important because increased fatigue could contribute to slower clinical recovery in hospitalized patients. Additionally, increased fatigue during hospitalization and at hospital discharge could exacerbate the known deleterious consequences of fatigue on patients and their health outcomes14,15 after hospital discharge. Although one previous study, the Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS)8 trial, did not report differences in patients’ fatigue levels at 30 and 60 days postdischarge when transfused at restrictive (8 g/dL) compared with liberal (10 g/dL) Hb thresholds, confidence in the validity of this finding is reduced by the fact that more than half of the patients were lost to follow-up at the 30- and 60-day time points. Further, patients in the restrictive transfusion arm of FOCUS were transfused to maintain Hb levels at or above 8 g/dL. This transfusion threshold of 8 g/dL may have mitigated the high levels of fatigue that are seen in our study when patients’ Hb drops below 8 g/dL, and maintaining a Hb level of 7 g/dL is now the standard of care in stable hospitalized patients. Lastly, FOCUS was limited to postoperative hip fracture patients, and the generalizability of FOCUS to hospitalized medicine patients with anemia is limited.
Therefore, our results support guideline suggestions that practitioners incorporate the presence of patient symptoms such as fatigue into transfusion decisions, particularly if patients’ Hb is <8 g/dL.18 Though reasonable, the suggestion to incorporate symptoms such as fatigue into transfusion decisions has not been strongly supported by evidence so far, and it may often be neglected in practice. Definitive evidence to support such recommendations would benefit from study through an optimal trial18 that incorporates symptoms into decision making. Our findings add support for a study of transfusion strategies that incorporates patients’ fatigue level in addition to Hb concentration.
This study has several limitations. Although our sample size is large and includes patients with a range of comorbidities that we believe are representative of hospitalized general medicine patients, as a single-center, observational study, our results may not be generalizable to other centers. Additionally, although these data support a reliable association between hospitalized patients’ minimum Hb and fatigue level, the observational design of this study cannot prove that this relationship is causal. Also, patients’ Hb values were measured at the discretion of their clinician, and therefore, the measures of Hb were not uniformly measured for participating patients. In addition, fatigue was only measured at one time point during a patient’s hospitalization, and it is possible that patients’ fatigue levels change during hospitalization in relation to variables we did not consider. Finally, our study was not designed to assess the association of Hb with longer-term functional outcomes, which may be of greater concern than fatigue.
CONCLUSION
In hospitalized patients ≥50 years old, minimum Hb is reliably associated with patients’ fatigue level. Patients whose minimum Hb is <8 g/dL experience higher fatigue levels compared to patients whose minimum Hb is ≥8 g/dL. Additional studies are warranted to understand if patients may benefit from improved fatigue levels by correcting their anemia through transfusion.
1. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
2. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528-538. doi:10.1002/cncr.10245.
3. Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547-561.
4. Tonelli M, Hemmelgarn B, Reiman T, et al. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ Can Med Assoc J J Assoc Medicale Can. 2009;180(11):E62-E71. doi:10.1503/cmaj.090470.
5. Foley RN, Curtis BM, Parfrey PS. Erythropoietin Therapy, Hemoglobin Targets, and Quality of Life in Healthy Hemodialysis Patients: A Randomized Trial. Clin J Am Soc Nephrol. 2009;4(4):726-733. doi:10.2215/CJN.04950908.
6. Keown PA, Churchill DN, Poulin-Costello M, et al. Dialysis patients treated with Epoetin alfa show improved anemia symptoms: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int Int Symp Home Hemodial. 2010;14(2):168-173. doi:10.1111/j.1542-4758.2009.00422.x.
7. Palmer SC, Saglimbene V, Mavridis D, et al. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis. Cochrane Database Syst Rev. 2014:CD010590.
8. Carson JL, Terrin ML, Noveck H, et al. Liberal or Restrictive Transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462. doi:10.1056/NEJMoa1012452.
9. Holst LB, Haase N, Wetterslev J, et al. Transfusion requirements in septic shock (TRISS) trial – comparing the effects and safety of liberal versus restrictive red blood cell transfusion in septic shock patients in the ICU: protocol for a randomised controlled trial. Trials. 2013;14:150. doi:10.1186/1745-6215-14-150.
10. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340(6):409-417. doi:10.1056/NEJM199902113400601.
11. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: Impact of an education program and a clinical guideline on transfusion practice. J Hosp Med. 2014;9(12):745-749. doi:10.1002/jhm.2237.
12. Saxena, S, editor. The Transfusion Committee: Putting Patient Safety First, 2nd Edition. Bethesda (MD): American Association of Blood Banks; 2013.
13. The 2011 National Blood Collection and Utilization Report. http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf. Accessed August 16, 2017.
14. Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a Representative Population of Older Persons and Its Association With Functional Impairment, Functional Limitation, and Disability. J Gerontol A Biol Sci Med Sci. 2009;64A(1):76-82. doi:10.1093/gerona/gln017.
15. Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135(5):313-321.
16. Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: Prevalence, outcomes, and healthcare implications. J Hosp Med. 2013;8(9):506-512. doi:10.1002/jhm.2061.
17. Meltzer D, Manning WG, Morrison J, et al. Effects of Physician Experience on Costs and Outcomes on an Academic General Medicine Service: Results of a Trial of Hospitalists. Ann Intern Med. 2002;137(11):866-874. doi:10.7326/0003-4819-137-11-200212030-00007.
18. Carson JL, Grossman BJ, Kleinman S, et al. Red Blood Cell Transfusion: A Clinical Practice Guideline From the AABB*. Ann Intern Med. 2012;157(1):49-58. doi:10.7326/0003-4819-157-1-201206190-00429.
19. Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65(8):887-895. doi:10.1093/gerona/glq064.
20. Eldadah BA. Fatigue and Fatigability in Older Adults. PM&R. 2010;2(5):406-413. doi:10.1016/j.pmrj.2010.03.022.
21. Hardy SE, Studenski SA. Fatigue Predicts Mortality among Older Adults. J Am Geriatr Soc. 2008;56(10):1910-1914. doi:10.1111/j.1532-5415.2008.01957.x.
22. Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433-441.
23. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
24. HCUP Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP). 2006-2009. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed November 22, 2016.
25. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol Off J Am Soc Clin Oncol. 1993;11(3):570-579.
26. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi:10.1186/1477-7525-1-79.
27. Oken MMMD a, Creech RHMD b, Tormey DCMD, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. J Clin Oncol. 1982;5(6):649-656.
Fatigue is the most common clinical symptom of anemia and is a significant concern to patients.1,2 In ambulatory patients, lower hemoglobin (Hb) concentration is associated with increased fatigue.2,3 Accordingly, therapies that treat anemia by increasing Hb concentration, such as erythropoiesis stimulating agents,4-7 often use fatigue as an outcome measure.
In hospitalized patients, transfusion of red blood cell increases Hb concentration and is the primary treatment for anemia. However, the extent to which transfusion and changes in Hb concentration affect hospitalized patients’ fatigue levels is not well established. Guidelines support transfusing patients with symptoms of anemia, such as fatigue, on the assumption that the increased oxygen delivery will improve the symptoms of anemia. While transfusion studies in hospitalized patients have consistently reported that transfusion at lower or “restrictive” Hb concentrations is safe compared with transfusion at higher Hb concentrations,8-10 these studies have mainly used cardiac events and mortality as outcomes rather than patient symptoms, such as fatigue. Nevertheless, they have resulted in hospitals increasingly adopting restrictive transfusion policies that discourage transfusion at higher Hb levels.11,12 Consequently, the rate of transfusion in hospitalized patients has decreased,13 raising questions of whether some patients with lower Hb concentrations may experience increased fatigue as a result of restrictive transfusion policies. Fatigue among hospitalized patients is important not only because it is an adverse symptom but because it may result in decreased activity levels, deconditioning, and losses in functional status.14,15While the effect of alternative transfusion policies on fatigue in hospitalized patients could be answered by a randomized clinical trial using fatigue and functional status as outcomes, an important first step is to assess whether the Hb concentration of hospitalized patients is associated with their fatigue level during hospitalization. Because hospitalized patients often have acute illnesses that can cause fatigue in and of themselves, it is possible that anemia is not associated with fatigue in hospitalized patients despite anemia’s association with fatigue in ambulatory patients. Additionally, Hb concentration varies during hospitalization,16 raising the question of what measures of Hb during hospitalization might be most associated with anemia-related fatigue.
The objective of this study is to explore multiple Hb measures in hospitalized medical patients with anemia and test whether any of these Hb measures are associated with patients’ fatigue level.
METHODS
Study Design
We performed a prospective, observational study of hospitalized patients with anemia on the general medicine services at The University of Chicago Medical Center (UCMC). The institutional review board approved the study procedures, and all study subjects provided informed consent.
Study Eligibility
Between April 2014 and June 2015, all general medicine inpatients were approached for written consent for The University of Chicago Hospitalist Project,17 a research infrastructure at UCMC. Among patients consenting to participate in the Hospitalist Project, patients were eligible if they had Hb <9 g/dL at any point during their hospitalization and were age ≥50 years. Hb concentration of <9 g/dL was chosen to include the range of Hb values covered by most restrictive transfusion policies.8-10,18 Age ≥50 years was an inclusion criteria because anemia is more strongly associated with poor outcomes, including functional impairment, among older patients compared with younger patients.14,19-21 If patients were not eligible for inclusion at the time of consent for the Hospitalist Project, their Hb values were reviewed twice daily until hospital discharge to assess if their Hb was <9 g/dL. Proxies were sought to answer questions for patients who failed the Short Portable Mental Status Questionnaire.22
Patient Demographic Data Collection
Research assistants abstracted patient age and sex from the electronic health record (EHR), and asked patients to self-identify their race. The individual comorbidities included as part of the Charlson Comorbidity Index were identified using International Classification of Diseases, 9th Revision codes from hospital administrative data for each encounter and specifically included the following: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes, hemiplegia and/or paraplegia, renal disease, cancer, and human immunodeficiency virus/acquired immunodeficiency syndrome.23 We also used Healthcare Cost and Utilization Project (www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp) diagnosis categories to identify whether patients had sickle cell (SC) anemia, gastrointestinal bleeding (GIB), or a depressive disorder (DD) because these conditions are associated with anemia (SC and GIB) and fatigue (DD).24
Measuring Anemia
Hb measures were available only when hospital providers ordered them as part of routine practice. The first Hb concentration <9 g/dL during a patient’s hospitalization, which made them eligible for study participation, was obtained through manual review of the EHR. All additional Hb values during the patient’s hospitalization were obtained from the hospital’s administrative data mart. All Hb values collected for each patient during the hospitalization were used to calculate summary measures of Hb during the hospitalization, including the mean Hb, median Hb, minimum Hb, maximum Hb, admission (first recorded) Hb, and discharge (last recorded) Hb. Hb measures were analyzed both as a continuous variable and as a categorical variable created by dividing the continuous Hb measures into integer ranges of 3 groups of approximately the same size.
Measuring Fatigue
Our primary outcome was patients’ level of fatigue reported during hospitalization, measured using the Functional Assessment of Chronic Illness Therapy (FACIT)-Anemia questionnaire. Fatigue was measured using a 13-question fatigue subscale,1,2,25 which measures fatigue within the past 7 days. Scores on the fatigue subscale range from 0 to 52, with lower scores reflecting greater levels of fatigue. As soon as patients met the eligibility criteria for study participation during their hospitalization (age ≥50 years and Hb <9 g/dL), they were approached to answer the FACIT questions. Values for missing data in the fatigue subscale for individual subjects were filled in using a prorated score from their answered questions as long as >50% of the items in the fatigue subscale were answered, in accordance with recommendations for addressing missing data in the FACIT.26 Fatigue was analyzed as a continuous variable and as a dichotomous variable created by dividing the sample into high (FACIT <27) and low (FACIT ≥27) levels of fatigue based on the median FACIT score of the population. Previous literature has shown a FACIT fatigue subscale score between 23 and 26 to be associated with an Eastern Cooperative Oncology Group (ECOG)27 C Performance Status rating of 2 to 33 compared to scores ≥27.
Statistical Analysis
Statistical analysis was performed using Stata statistical software (StataCorp, College Station, TX). Descriptive statistics were used to characterize patient demographics. Analysis of variance was used to test for differences in the mean fatigue levels across Hb measures. χ2 tests were performed to test for associations between high fatigue levels and the Hb measures. Multivariable analysis, including both linear and logistic regression models, were used to test the association of Hb concentration and fatigue. P values <0.05 using a 2-tailed test were deemed statistically significant.
RESULTS
Patient Characteristics
During the study period, 8559 patients were admitted to the general medicine service. Of those, 5073 (59%) consented for participation in the Hospitalist Project, and 3670 (72%) completed the Hospitalist Project inpatient interview. Of these patients, 1292 (35%) had Hb <9 g/dL, and 784 (61%) were 50 years or older and completed the FACIT questionnaire.
Table 1 reports the demographic characteristics and comorbidities for the sample, the mean (standard deviation [SD]) for the 6 Hb measures, and mean (SD) and median FACIT scores.
Bivariate Association of Fatigue and Hb
Categorizing patients into low, middle, or high Hb for each of the 6 Hb measures, minimum Hb was strongly associated with fatigue, with a weaker association for mean Hb and no statistically significant association for the other measures.
Minimum Hb. Patients with a minimum Hb <7 g/dL and patients with Hb 7-8 g/dL had higher fatigue levels (FACIT = 25 for each) than patients with a minimum Hb ≥8 g/dL (FACIT = 29; P < 0.001; Table 2). When excluding patients with SC and/or GIB because their average minimum Hb differed from the average minimum Hb of the full population (P < 0.001), patients with a minimum Hb <7 g/dL or 7-8 g/dL had even higher fatigue levels (FACIT = 23 and FACIT = 24, respectively), with no change in the fatigue level of patients with a minimum Hb ≥8 g/dL (FACIT = 29; P < 0.001; Table 2). Lower minimum Hb continued to be associated with higher fatigue levels when analyzed in 0.5 g/dL increments (Figure).
Mean Hb and Other Measures. Fatigue levels were high for 47% of patients with a mean Hb <8g /dL and 53% of patients with a mean Hb 8-9 g/dL compared with 43% of patients with a mean Hb ≥9 g/dL (P = 0.05). However, the association between high fatigue and mean Hb was not statistically significant when patients with SC and/or GIB were excluded (Table 2). None of the other 4 Hb measures was significantly associated with fatigue.
Linear Regression of Fatigue on Hb
In linear regression models, minimum Hb consistently predicted patient fatigue, mean Hb had a less robust association with fatigue, and the other Hb measures were not associated with patient fatigue. Increases in minimum Hb (analyzed as a continuous variable) were associated with reduced fatigue (higher FACIT score; β = 1.4; P = 0.005). In models in which minimum Hb was a categorical variable, patients with a minimum Hb of <7 g/dL or 7-8 g/dL had greater fatigue (lower FACIT score) than patients whose minimum Hb was ≥8 g/dL (Hb <7 g/dL: β = −4.2; P ≤ 0.001; Hb 7-8 g/dL: β = −4.1; P < 0.001). These results control for patients’ age, sex, individual comorbidities, and whether their minimum Hb occurred before or after the measurement of fatigue during hospitalization (Model 1), and the results are unchanged when also controlling for the number of Hb laboratory draws patients had during their hospitalization (Model 2; Table 3). In a stratified analysis excluding patients with either SC and/or GIB, changes in minimum Hb were associated with larger changes in patient fatigue levels (Supplemental Table 1). We also stratified our analysis to include only patients whose minimum Hb occurred before the measurement of their fatigue level during hospitalization to avoid a spurious association of fatigue with minimum Hb occurring after fatigue was measured. In both Models 1 and 2, minimum Hb remained a predictor of patients’ fatigue levels with similar effect sizes, although in Model 2, the results did not quite reach a statistically significant level, in part due to larger confidence intervals from the smaller sample size of this stratified analysis (Supplemental Table 2a). We further stratified this analysis to include only patients whose transfusion, if they received one, occurred after their minimum Hb and the measurement of their fatigue level to account for the possibility that a transfusion could affect the fatigue level patients report. In this analysis, most of the estimates of the effect of minimum Hb on fatigue were larger than those seen when only analyzing patients whose minimum Hb occurred before the measurement of their fatigue level, although again, the smaller sample size of this additional stratified analysis does produce larger confidence intervals for these estimates (Supplemental Table 2b).
No Hb measure other than minimum or mean had significant association with patient fatigue levels in linear regression models.
Logistic Regression of High Fatigue Level on Hb
Using logistic regression, minimum Hb analyzed as a categorical variable predicted increased odds of a high fatigue level. Patients with a minimum Hb <7 g/dL were 50% (odds ratio [OR] = 1.5; P = 0.03) more likely to have high fatigue and patients with a minimum Hb 7-8 g/dL were 90% (OR = 1.9; P < 0.001) more likely to have high fatigue compared with patients with a minimum Hb ≥8 g/dL in Model 1. These results were similar in Model 2, although the effect was only statistically significant in the 7-8 g/dL Hb group (Table 3). When excluding SC and/or GIB patients, the odds of having high fatigue as minimum Hb decreased were the same or higher for both models compared to the full population of patients. However, again, in Model 2, the effect was only statistically significant in the 7-8 g/dL Hb group (Supplemental Table 1).
Patients with a mean Hb <8 g/dL were 20% to 30% more likely to have high fatigue and patients with mean Hb 8-9 g/dL were 50% more likely to have high fatigue compared with patients with a mean Hb ≥9 g/dL, but the effects were only statistically significant for patients with a mean Hb 8-9 g/dL in both Models 1 and 2 (Table 3). These results were similar when excluding patients with SC and/or GIB, but they were only significant for patients with a mean Hb 8-9 g/dL in Model 1 and patients with a mean Hb <8 g/dL in the Model 2 (Supplemental Table 3).
DISCUSSION
These results demonstrate that minimum Hb during hospitalization is associated with fatigue in hospitalized patients age ≥50 years, and the association is stronger among patients without SC and/or GIB as comorbidities. The analysis of Hb as a continuous and categorical variable and the use of both linear and logistic regression models support the robustness of these associations and illuminate their clinical significance. For example, in linear regression with minimum Hb a continuous variable, the coefficient of 1.4 suggests that an increase of 2 g/dL in Hb, as might be expected from transfusion of 2 units of red blood cells, would be associated with about a 3-point improvement in fatigue. Additionally, as a categorical variable, a minimum Hb ≥8 g/dL compared with a minimum Hb <7 g/dL or 7-8 g/dL is associated with a 3- to 4-point improvement in fatigue. Previous literature suggests that a difference of 3 in the FACIT score is the minimum clinically important difference in fatigue,3 and changes in minimum Hb in either model predict changes in fatigue that are in the range of potential clinical significance.
The clinical significance of the findings is also reflected in the results of the logistic regressions, which may be mapped to potential effects on functional status. Specifically, the odds of having a high fatigue level (FACIT <27) increase 90% for persons with a minimum Hb 7–8 g/dL compared with persons with a minimum Hb ≥8 g/dL. For persons with a minimum Hb <7 g/dL, point estimates suggest a smaller (50%) increase in the odds of high fatigue, but the 95% confidence interval overlaps heavily with the estimate of patients whose minimum Hb is 7-8 g/dL. While it might be expected that patients with a minimum Hb <7 g/dL have greater levels of fatigue compared with patients with a minimum Hb 7-8 g/dL, we did not observe such a pattern. One reason may be that the confidence intervals of our estimated effects are wide enough that we cannot exclude such a pattern. Another possible explanation is that in both groups, the fatigue levels are sufficiently severe, such that the difference in their fatigue levels may not be clinically meaningful. For example, a FACIT score of 23 to 26 has been shown to be associated with an ECOG performance status of 2 to 3, requiring bed rest for at least part of the day.3 Therefore, patients with a minimum Hb 7-8 g/dL (mean FACIT score = 24; Table 2) or a minimum Hb of <7 g/dL (mean FACIT score = 23; Table 2) are already functionally limited to the point of being partially bed bound, such that further decreases in their Hb may not produce additional fatigue in part because they reduce their activity sufficiently to prevent an increase in fatigue. In such cases, the potential benefits of increased Hb may be better assessed by measuring fatigue in response to a specific and provoked activity level, a concept known as fatigability.20
That minimum Hb is more strongly associated with fatigue than any other measure of Hb during hospitalization may not be surprising. Mean, median, maximum, and discharge Hb may all be affected by transfusion during hospitalization that could affect fatigue. Admission Hb may not reflect true oxygen-carrying capacity because of hemoconcentration.
The association between Hb and fatigue in hospitalized patients is important because increased fatigue could contribute to slower clinical recovery in hospitalized patients. Additionally, increased fatigue during hospitalization and at hospital discharge could exacerbate the known deleterious consequences of fatigue on patients and their health outcomes14,15 after hospital discharge. Although one previous study, the Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS)8 trial, did not report differences in patients’ fatigue levels at 30 and 60 days postdischarge when transfused at restrictive (8 g/dL) compared with liberal (10 g/dL) Hb thresholds, confidence in the validity of this finding is reduced by the fact that more than half of the patients were lost to follow-up at the 30- and 60-day time points. Further, patients in the restrictive transfusion arm of FOCUS were transfused to maintain Hb levels at or above 8 g/dL. This transfusion threshold of 8 g/dL may have mitigated the high levels of fatigue that are seen in our study when patients’ Hb drops below 8 g/dL, and maintaining a Hb level of 7 g/dL is now the standard of care in stable hospitalized patients. Lastly, FOCUS was limited to postoperative hip fracture patients, and the generalizability of FOCUS to hospitalized medicine patients with anemia is limited.
Therefore, our results support guideline suggestions that practitioners incorporate the presence of patient symptoms such as fatigue into transfusion decisions, particularly if patients’ Hb is <8 g/dL.18 Though reasonable, the suggestion to incorporate symptoms such as fatigue into transfusion decisions has not been strongly supported by evidence so far, and it may often be neglected in practice. Definitive evidence to support such recommendations would benefit from study through an optimal trial18 that incorporates symptoms into decision making. Our findings add support for a study of transfusion strategies that incorporates patients’ fatigue level in addition to Hb concentration.
This study has several limitations. Although our sample size is large and includes patients with a range of comorbidities that we believe are representative of hospitalized general medicine patients, as a single-center, observational study, our results may not be generalizable to other centers. Additionally, although these data support a reliable association between hospitalized patients’ minimum Hb and fatigue level, the observational design of this study cannot prove that this relationship is causal. Also, patients’ Hb values were measured at the discretion of their clinician, and therefore, the measures of Hb were not uniformly measured for participating patients. In addition, fatigue was only measured at one time point during a patient’s hospitalization, and it is possible that patients’ fatigue levels change during hospitalization in relation to variables we did not consider. Finally, our study was not designed to assess the association of Hb with longer-term functional outcomes, which may be of greater concern than fatigue.
CONCLUSION
In hospitalized patients ≥50 years old, minimum Hb is reliably associated with patients’ fatigue level. Patients whose minimum Hb is <8 g/dL experience higher fatigue levels compared to patients whose minimum Hb is ≥8 g/dL. Additional studies are warranted to understand if patients may benefit from improved fatigue levels by correcting their anemia through transfusion.
Fatigue is the most common clinical symptom of anemia and is a significant concern to patients.1,2 In ambulatory patients, lower hemoglobin (Hb) concentration is associated with increased fatigue.2,3 Accordingly, therapies that treat anemia by increasing Hb concentration, such as erythropoiesis stimulating agents,4-7 often use fatigue as an outcome measure.
In hospitalized patients, transfusion of red blood cell increases Hb concentration and is the primary treatment for anemia. However, the extent to which transfusion and changes in Hb concentration affect hospitalized patients’ fatigue levels is not well established. Guidelines support transfusing patients with symptoms of anemia, such as fatigue, on the assumption that the increased oxygen delivery will improve the symptoms of anemia. While transfusion studies in hospitalized patients have consistently reported that transfusion at lower or “restrictive” Hb concentrations is safe compared with transfusion at higher Hb concentrations,8-10 these studies have mainly used cardiac events and mortality as outcomes rather than patient symptoms, such as fatigue. Nevertheless, they have resulted in hospitals increasingly adopting restrictive transfusion policies that discourage transfusion at higher Hb levels.11,12 Consequently, the rate of transfusion in hospitalized patients has decreased,13 raising questions of whether some patients with lower Hb concentrations may experience increased fatigue as a result of restrictive transfusion policies. Fatigue among hospitalized patients is important not only because it is an adverse symptom but because it may result in decreased activity levels, deconditioning, and losses in functional status.14,15While the effect of alternative transfusion policies on fatigue in hospitalized patients could be answered by a randomized clinical trial using fatigue and functional status as outcomes, an important first step is to assess whether the Hb concentration of hospitalized patients is associated with their fatigue level during hospitalization. Because hospitalized patients often have acute illnesses that can cause fatigue in and of themselves, it is possible that anemia is not associated with fatigue in hospitalized patients despite anemia’s association with fatigue in ambulatory patients. Additionally, Hb concentration varies during hospitalization,16 raising the question of what measures of Hb during hospitalization might be most associated with anemia-related fatigue.
The objective of this study is to explore multiple Hb measures in hospitalized medical patients with anemia and test whether any of these Hb measures are associated with patients’ fatigue level.
METHODS
Study Design
We performed a prospective, observational study of hospitalized patients with anemia on the general medicine services at The University of Chicago Medical Center (UCMC). The institutional review board approved the study procedures, and all study subjects provided informed consent.
Study Eligibility
Between April 2014 and June 2015, all general medicine inpatients were approached for written consent for The University of Chicago Hospitalist Project,17 a research infrastructure at UCMC. Among patients consenting to participate in the Hospitalist Project, patients were eligible if they had Hb <9 g/dL at any point during their hospitalization and were age ≥50 years. Hb concentration of <9 g/dL was chosen to include the range of Hb values covered by most restrictive transfusion policies.8-10,18 Age ≥50 years was an inclusion criteria because anemia is more strongly associated with poor outcomes, including functional impairment, among older patients compared with younger patients.14,19-21 If patients were not eligible for inclusion at the time of consent for the Hospitalist Project, their Hb values were reviewed twice daily until hospital discharge to assess if their Hb was <9 g/dL. Proxies were sought to answer questions for patients who failed the Short Portable Mental Status Questionnaire.22
Patient Demographic Data Collection
Research assistants abstracted patient age and sex from the electronic health record (EHR), and asked patients to self-identify their race. The individual comorbidities included as part of the Charlson Comorbidity Index were identified using International Classification of Diseases, 9th Revision codes from hospital administrative data for each encounter and specifically included the following: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, diabetes, hemiplegia and/or paraplegia, renal disease, cancer, and human immunodeficiency virus/acquired immunodeficiency syndrome.23 We also used Healthcare Cost and Utilization Project (www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp) diagnosis categories to identify whether patients had sickle cell (SC) anemia, gastrointestinal bleeding (GIB), or a depressive disorder (DD) because these conditions are associated with anemia (SC and GIB) and fatigue (DD).24
Measuring Anemia
Hb measures were available only when hospital providers ordered them as part of routine practice. The first Hb concentration <9 g/dL during a patient’s hospitalization, which made them eligible for study participation, was obtained through manual review of the EHR. All additional Hb values during the patient’s hospitalization were obtained from the hospital’s administrative data mart. All Hb values collected for each patient during the hospitalization were used to calculate summary measures of Hb during the hospitalization, including the mean Hb, median Hb, minimum Hb, maximum Hb, admission (first recorded) Hb, and discharge (last recorded) Hb. Hb measures were analyzed both as a continuous variable and as a categorical variable created by dividing the continuous Hb measures into integer ranges of 3 groups of approximately the same size.
Measuring Fatigue
Our primary outcome was patients’ level of fatigue reported during hospitalization, measured using the Functional Assessment of Chronic Illness Therapy (FACIT)-Anemia questionnaire. Fatigue was measured using a 13-question fatigue subscale,1,2,25 which measures fatigue within the past 7 days. Scores on the fatigue subscale range from 0 to 52, with lower scores reflecting greater levels of fatigue. As soon as patients met the eligibility criteria for study participation during their hospitalization (age ≥50 years and Hb <9 g/dL), they were approached to answer the FACIT questions. Values for missing data in the fatigue subscale for individual subjects were filled in using a prorated score from their answered questions as long as >50% of the items in the fatigue subscale were answered, in accordance with recommendations for addressing missing data in the FACIT.26 Fatigue was analyzed as a continuous variable and as a dichotomous variable created by dividing the sample into high (FACIT <27) and low (FACIT ≥27) levels of fatigue based on the median FACIT score of the population. Previous literature has shown a FACIT fatigue subscale score between 23 and 26 to be associated with an Eastern Cooperative Oncology Group (ECOG)27 C Performance Status rating of 2 to 33 compared to scores ≥27.
Statistical Analysis
Statistical analysis was performed using Stata statistical software (StataCorp, College Station, TX). Descriptive statistics were used to characterize patient demographics. Analysis of variance was used to test for differences in the mean fatigue levels across Hb measures. χ2 tests were performed to test for associations between high fatigue levels and the Hb measures. Multivariable analysis, including both linear and logistic regression models, were used to test the association of Hb concentration and fatigue. P values <0.05 using a 2-tailed test were deemed statistically significant.
RESULTS
Patient Characteristics
During the study period, 8559 patients were admitted to the general medicine service. Of those, 5073 (59%) consented for participation in the Hospitalist Project, and 3670 (72%) completed the Hospitalist Project inpatient interview. Of these patients, 1292 (35%) had Hb <9 g/dL, and 784 (61%) were 50 years or older and completed the FACIT questionnaire.
Table 1 reports the demographic characteristics and comorbidities for the sample, the mean (standard deviation [SD]) for the 6 Hb measures, and mean (SD) and median FACIT scores.
Bivariate Association of Fatigue and Hb
Categorizing patients into low, middle, or high Hb for each of the 6 Hb measures, minimum Hb was strongly associated with fatigue, with a weaker association for mean Hb and no statistically significant association for the other measures.
Minimum Hb. Patients with a minimum Hb <7 g/dL and patients with Hb 7-8 g/dL had higher fatigue levels (FACIT = 25 for each) than patients with a minimum Hb ≥8 g/dL (FACIT = 29; P < 0.001; Table 2). When excluding patients with SC and/or GIB because their average minimum Hb differed from the average minimum Hb of the full population (P < 0.001), patients with a minimum Hb <7 g/dL or 7-8 g/dL had even higher fatigue levels (FACIT = 23 and FACIT = 24, respectively), with no change in the fatigue level of patients with a minimum Hb ≥8 g/dL (FACIT = 29; P < 0.001; Table 2). Lower minimum Hb continued to be associated with higher fatigue levels when analyzed in 0.5 g/dL increments (Figure).
Mean Hb and Other Measures. Fatigue levels were high for 47% of patients with a mean Hb <8g /dL and 53% of patients with a mean Hb 8-9 g/dL compared with 43% of patients with a mean Hb ≥9 g/dL (P = 0.05). However, the association between high fatigue and mean Hb was not statistically significant when patients with SC and/or GIB were excluded (Table 2). None of the other 4 Hb measures was significantly associated with fatigue.
Linear Regression of Fatigue on Hb
In linear regression models, minimum Hb consistently predicted patient fatigue, mean Hb had a less robust association with fatigue, and the other Hb measures were not associated with patient fatigue. Increases in minimum Hb (analyzed as a continuous variable) were associated with reduced fatigue (higher FACIT score; β = 1.4; P = 0.005). In models in which minimum Hb was a categorical variable, patients with a minimum Hb of <7 g/dL or 7-8 g/dL had greater fatigue (lower FACIT score) than patients whose minimum Hb was ≥8 g/dL (Hb <7 g/dL: β = −4.2; P ≤ 0.001; Hb 7-8 g/dL: β = −4.1; P < 0.001). These results control for patients’ age, sex, individual comorbidities, and whether their minimum Hb occurred before or after the measurement of fatigue during hospitalization (Model 1), and the results are unchanged when also controlling for the number of Hb laboratory draws patients had during their hospitalization (Model 2; Table 3). In a stratified analysis excluding patients with either SC and/or GIB, changes in minimum Hb were associated with larger changes in patient fatigue levels (Supplemental Table 1). We also stratified our analysis to include only patients whose minimum Hb occurred before the measurement of their fatigue level during hospitalization to avoid a spurious association of fatigue with minimum Hb occurring after fatigue was measured. In both Models 1 and 2, minimum Hb remained a predictor of patients’ fatigue levels with similar effect sizes, although in Model 2, the results did not quite reach a statistically significant level, in part due to larger confidence intervals from the smaller sample size of this stratified analysis (Supplemental Table 2a). We further stratified this analysis to include only patients whose transfusion, if they received one, occurred after their minimum Hb and the measurement of their fatigue level to account for the possibility that a transfusion could affect the fatigue level patients report. In this analysis, most of the estimates of the effect of minimum Hb on fatigue were larger than those seen when only analyzing patients whose minimum Hb occurred before the measurement of their fatigue level, although again, the smaller sample size of this additional stratified analysis does produce larger confidence intervals for these estimates (Supplemental Table 2b).
No Hb measure other than minimum or mean had significant association with patient fatigue levels in linear regression models.
Logistic Regression of High Fatigue Level on Hb
Using logistic regression, minimum Hb analyzed as a categorical variable predicted increased odds of a high fatigue level. Patients with a minimum Hb <7 g/dL were 50% (odds ratio [OR] = 1.5; P = 0.03) more likely to have high fatigue and patients with a minimum Hb 7-8 g/dL were 90% (OR = 1.9; P < 0.001) more likely to have high fatigue compared with patients with a minimum Hb ≥8 g/dL in Model 1. These results were similar in Model 2, although the effect was only statistically significant in the 7-8 g/dL Hb group (Table 3). When excluding SC and/or GIB patients, the odds of having high fatigue as minimum Hb decreased were the same or higher for both models compared to the full population of patients. However, again, in Model 2, the effect was only statistically significant in the 7-8 g/dL Hb group (Supplemental Table 1).
Patients with a mean Hb <8 g/dL were 20% to 30% more likely to have high fatigue and patients with mean Hb 8-9 g/dL were 50% more likely to have high fatigue compared with patients with a mean Hb ≥9 g/dL, but the effects were only statistically significant for patients with a mean Hb 8-9 g/dL in both Models 1 and 2 (Table 3). These results were similar when excluding patients with SC and/or GIB, but they were only significant for patients with a mean Hb 8-9 g/dL in Model 1 and patients with a mean Hb <8 g/dL in the Model 2 (Supplemental Table 3).
DISCUSSION
These results demonstrate that minimum Hb during hospitalization is associated with fatigue in hospitalized patients age ≥50 years, and the association is stronger among patients without SC and/or GIB as comorbidities. The analysis of Hb as a continuous and categorical variable and the use of both linear and logistic regression models support the robustness of these associations and illuminate their clinical significance. For example, in linear regression with minimum Hb a continuous variable, the coefficient of 1.4 suggests that an increase of 2 g/dL in Hb, as might be expected from transfusion of 2 units of red blood cells, would be associated with about a 3-point improvement in fatigue. Additionally, as a categorical variable, a minimum Hb ≥8 g/dL compared with a minimum Hb <7 g/dL or 7-8 g/dL is associated with a 3- to 4-point improvement in fatigue. Previous literature suggests that a difference of 3 in the FACIT score is the minimum clinically important difference in fatigue,3 and changes in minimum Hb in either model predict changes in fatigue that are in the range of potential clinical significance.
The clinical significance of the findings is also reflected in the results of the logistic regressions, which may be mapped to potential effects on functional status. Specifically, the odds of having a high fatigue level (FACIT <27) increase 90% for persons with a minimum Hb 7–8 g/dL compared with persons with a minimum Hb ≥8 g/dL. For persons with a minimum Hb <7 g/dL, point estimates suggest a smaller (50%) increase in the odds of high fatigue, but the 95% confidence interval overlaps heavily with the estimate of patients whose minimum Hb is 7-8 g/dL. While it might be expected that patients with a minimum Hb <7 g/dL have greater levels of fatigue compared with patients with a minimum Hb 7-8 g/dL, we did not observe such a pattern. One reason may be that the confidence intervals of our estimated effects are wide enough that we cannot exclude such a pattern. Another possible explanation is that in both groups, the fatigue levels are sufficiently severe, such that the difference in their fatigue levels may not be clinically meaningful. For example, a FACIT score of 23 to 26 has been shown to be associated with an ECOG performance status of 2 to 3, requiring bed rest for at least part of the day.3 Therefore, patients with a minimum Hb 7-8 g/dL (mean FACIT score = 24; Table 2) or a minimum Hb of <7 g/dL (mean FACIT score = 23; Table 2) are already functionally limited to the point of being partially bed bound, such that further decreases in their Hb may not produce additional fatigue in part because they reduce their activity sufficiently to prevent an increase in fatigue. In such cases, the potential benefits of increased Hb may be better assessed by measuring fatigue in response to a specific and provoked activity level, a concept known as fatigability.20
That minimum Hb is more strongly associated with fatigue than any other measure of Hb during hospitalization may not be surprising. Mean, median, maximum, and discharge Hb may all be affected by transfusion during hospitalization that could affect fatigue. Admission Hb may not reflect true oxygen-carrying capacity because of hemoconcentration.
The association between Hb and fatigue in hospitalized patients is important because increased fatigue could contribute to slower clinical recovery in hospitalized patients. Additionally, increased fatigue during hospitalization and at hospital discharge could exacerbate the known deleterious consequences of fatigue on patients and their health outcomes14,15 after hospital discharge. Although one previous study, the Functional Outcomes in Cardiovascular Patients Undergoing Surgical Hip Fracture Repair (FOCUS)8 trial, did not report differences in patients’ fatigue levels at 30 and 60 days postdischarge when transfused at restrictive (8 g/dL) compared with liberal (10 g/dL) Hb thresholds, confidence in the validity of this finding is reduced by the fact that more than half of the patients were lost to follow-up at the 30- and 60-day time points. Further, patients in the restrictive transfusion arm of FOCUS were transfused to maintain Hb levels at or above 8 g/dL. This transfusion threshold of 8 g/dL may have mitigated the high levels of fatigue that are seen in our study when patients’ Hb drops below 8 g/dL, and maintaining a Hb level of 7 g/dL is now the standard of care in stable hospitalized patients. Lastly, FOCUS was limited to postoperative hip fracture patients, and the generalizability of FOCUS to hospitalized medicine patients with anemia is limited.
Therefore, our results support guideline suggestions that practitioners incorporate the presence of patient symptoms such as fatigue into transfusion decisions, particularly if patients’ Hb is <8 g/dL.18 Though reasonable, the suggestion to incorporate symptoms such as fatigue into transfusion decisions has not been strongly supported by evidence so far, and it may often be neglected in practice. Definitive evidence to support such recommendations would benefit from study through an optimal trial18 that incorporates symptoms into decision making. Our findings add support for a study of transfusion strategies that incorporates patients’ fatigue level in addition to Hb concentration.
This study has several limitations. Although our sample size is large and includes patients with a range of comorbidities that we believe are representative of hospitalized general medicine patients, as a single-center, observational study, our results may not be generalizable to other centers. Additionally, although these data support a reliable association between hospitalized patients’ minimum Hb and fatigue level, the observational design of this study cannot prove that this relationship is causal. Also, patients’ Hb values were measured at the discretion of their clinician, and therefore, the measures of Hb were not uniformly measured for participating patients. In addition, fatigue was only measured at one time point during a patient’s hospitalization, and it is possible that patients’ fatigue levels change during hospitalization in relation to variables we did not consider. Finally, our study was not designed to assess the association of Hb with longer-term functional outcomes, which may be of greater concern than fatigue.
CONCLUSION
In hospitalized patients ≥50 years old, minimum Hb is reliably associated with patients’ fatigue level. Patients whose minimum Hb is <8 g/dL experience higher fatigue levels compared to patients whose minimum Hb is ≥8 g/dL. Additional studies are warranted to understand if patients may benefit from improved fatigue levels by correcting their anemia through transfusion.
1. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
2. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528-538. doi:10.1002/cncr.10245.
3. Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547-561.
4. Tonelli M, Hemmelgarn B, Reiman T, et al. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ Can Med Assoc J J Assoc Medicale Can. 2009;180(11):E62-E71. doi:10.1503/cmaj.090470.
5. Foley RN, Curtis BM, Parfrey PS. Erythropoietin Therapy, Hemoglobin Targets, and Quality of Life in Healthy Hemodialysis Patients: A Randomized Trial. Clin J Am Soc Nephrol. 2009;4(4):726-733. doi:10.2215/CJN.04950908.
6. Keown PA, Churchill DN, Poulin-Costello M, et al. Dialysis patients treated with Epoetin alfa show improved anemia symptoms: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int Int Symp Home Hemodial. 2010;14(2):168-173. doi:10.1111/j.1542-4758.2009.00422.x.
7. Palmer SC, Saglimbene V, Mavridis D, et al. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis. Cochrane Database Syst Rev. 2014:CD010590.
8. Carson JL, Terrin ML, Noveck H, et al. Liberal or Restrictive Transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462. doi:10.1056/NEJMoa1012452.
9. Holst LB, Haase N, Wetterslev J, et al. Transfusion requirements in septic shock (TRISS) trial – comparing the effects and safety of liberal versus restrictive red blood cell transfusion in septic shock patients in the ICU: protocol for a randomised controlled trial. Trials. 2013;14:150. doi:10.1186/1745-6215-14-150.
10. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340(6):409-417. doi:10.1056/NEJM199902113400601.
11. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: Impact of an education program and a clinical guideline on transfusion practice. J Hosp Med. 2014;9(12):745-749. doi:10.1002/jhm.2237.
12. Saxena, S, editor. The Transfusion Committee: Putting Patient Safety First, 2nd Edition. Bethesda (MD): American Association of Blood Banks; 2013.
13. The 2011 National Blood Collection and Utilization Report. http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf. Accessed August 16, 2017.
14. Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a Representative Population of Older Persons and Its Association With Functional Impairment, Functional Limitation, and Disability. J Gerontol A Biol Sci Med Sci. 2009;64A(1):76-82. doi:10.1093/gerona/gln017.
15. Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135(5):313-321.
16. Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: Prevalence, outcomes, and healthcare implications. J Hosp Med. 2013;8(9):506-512. doi:10.1002/jhm.2061.
17. Meltzer D, Manning WG, Morrison J, et al. Effects of Physician Experience on Costs and Outcomes on an Academic General Medicine Service: Results of a Trial of Hospitalists. Ann Intern Med. 2002;137(11):866-874. doi:10.7326/0003-4819-137-11-200212030-00007.
18. Carson JL, Grossman BJ, Kleinman S, et al. Red Blood Cell Transfusion: A Clinical Practice Guideline From the AABB*. Ann Intern Med. 2012;157(1):49-58. doi:10.7326/0003-4819-157-1-201206190-00429.
19. Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65(8):887-895. doi:10.1093/gerona/glq064.
20. Eldadah BA. Fatigue and Fatigability in Older Adults. PM&R. 2010;2(5):406-413. doi:10.1016/j.pmrj.2010.03.022.
21. Hardy SE, Studenski SA. Fatigue Predicts Mortality among Older Adults. J Am Geriatr Soc. 2008;56(10):1910-1914. doi:10.1111/j.1532-5415.2008.01957.x.
22. Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433-441.
23. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
24. HCUP Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP). 2006-2009. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed November 22, 2016.
25. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol Off J Am Soc Clin Oncol. 1993;11(3):570-579.
26. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi:10.1186/1477-7525-1-79.
27. Oken MMMD a, Creech RHMD b, Tormey DCMD, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. J Clin Oncol. 1982;5(6):649-656.
1. Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage. 1997;13(2):63-74.
2. Cella D, Lai JS, Chang CH, Peterman A, Slavin M. Fatigue in cancer patients compared with fatigue in the general United States population. Cancer. 2002;94(2):528-538. doi:10.1002/cncr.10245.
3. Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24(6):547-561.
4. Tonelli M, Hemmelgarn B, Reiman T, et al. Benefits and harms of erythropoiesis-stimulating agents for anemia related to cancer: a meta-analysis. CMAJ Can Med Assoc J J Assoc Medicale Can. 2009;180(11):E62-E71. doi:10.1503/cmaj.090470.
5. Foley RN, Curtis BM, Parfrey PS. Erythropoietin Therapy, Hemoglobin Targets, and Quality of Life in Healthy Hemodialysis Patients: A Randomized Trial. Clin J Am Soc Nephrol. 2009;4(4):726-733. doi:10.2215/CJN.04950908.
6. Keown PA, Churchill DN, Poulin-Costello M, et al. Dialysis patients treated with Epoetin alfa show improved anemia symptoms: A new analysis of the Canadian Erythropoietin Study Group trial. Hemodial Int Int Symp Home Hemodial. 2010;14(2):168-173. doi:10.1111/j.1542-4758.2009.00422.x.
7. Palmer SC, Saglimbene V, Mavridis D, et al. Erythropoiesis-stimulating agents for anaemia in adults with chronic kidney disease: a network meta-analysis. Cochrane Database Syst Rev. 2014:CD010590.
8. Carson JL, Terrin ML, Noveck H, et al. Liberal or Restrictive Transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453-2462. doi:10.1056/NEJMoa1012452.
9. Holst LB, Haase N, Wetterslev J, et al. Transfusion requirements in septic shock (TRISS) trial – comparing the effects and safety of liberal versus restrictive red blood cell transfusion in septic shock patients in the ICU: protocol for a randomised controlled trial. Trials. 2013;14:150. doi:10.1186/1745-6215-14-150.
10. Hébert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. N Engl J Med. 1999;340(6):409-417. doi:10.1056/NEJM199902113400601.
11. Corwin HL, Theus JW, Cargile CS, Lang NP. Red blood cell transfusion: Impact of an education program and a clinical guideline on transfusion practice. J Hosp Med. 2014;9(12):745-749. doi:10.1002/jhm.2237.
12. Saxena, S, editor. The Transfusion Committee: Putting Patient Safety First, 2nd Edition. Bethesda (MD): American Association of Blood Banks; 2013.
13. The 2011 National Blood Collection and Utilization Report. http://www.hhs.gov/ash/bloodsafety/2011-nbcus.pdf. Accessed August 16, 2017.
14. Vestergaard S, Nayfield SG, Patel KV, et al. Fatigue in a Representative Population of Older Persons and Its Association With Functional Impairment, Functional Limitation, and Disability. J Gerontol A Biol Sci Med Sci. 2009;64A(1):76-82. doi:10.1093/gerona/gln017.
15. Gill TM, Desai MM, Gahbauer EA, Holford TR, Williams CS. Restricted activity among community-living older persons: incidence, precipitants, and health care utilization. Ann Intern Med. 2001;135(5):313-321.
16. Koch CG, Li L, Sun Z, et al. Hospital-acquired anemia: Prevalence, outcomes, and healthcare implications. J Hosp Med. 2013;8(9):506-512. doi:10.1002/jhm.2061.
17. Meltzer D, Manning WG, Morrison J, et al. Effects of Physician Experience on Costs and Outcomes on an Academic General Medicine Service: Results of a Trial of Hospitalists. Ann Intern Med. 2002;137(11):866-874. doi:10.7326/0003-4819-137-11-200212030-00007.
18. Carson JL, Grossman BJ, Kleinman S, et al. Red Blood Cell Transfusion: A Clinical Practice Guideline From the AABB*. Ann Intern Med. 2012;157(1):49-58. doi:10.7326/0003-4819-157-1-201206190-00429.
19. Moreh E, Jacobs JM, Stessman J. Fatigue, function, and mortality in older adults. J Gerontol A Biol Sci Med Sci. 2010;65(8):887-895. doi:10.1093/gerona/glq064.
20. Eldadah BA. Fatigue and Fatigability in Older Adults. PM&R. 2010;2(5):406-413. doi:10.1016/j.pmrj.2010.03.022.
21. Hardy SE, Studenski SA. Fatigue Predicts Mortality among Older Adults. J Am Geriatr Soc. 2008;56(10):1910-1914. doi:10.1111/j.1532-5415.2008.01957.x.
22. Pfeiffer E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. 1975;23(10):433-441.
23. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139.
24. HCUP Clinical Classifications Software (CCS) for ICD-9-CM. Healthcare Cost and Utilization Project (HCUP). 2006-2009. Agency for Healthcare Research and Quality, Rockville, MD. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed November 22, 2016.
25. Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol Off J Am Soc Clin Oncol. 1993;11(3):570-579.
26. Webster K, Cella D, Yost K. The Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System: properties, applications, and interpretation. Health Qual Life Outcomes. 2003;1:79. doi:10.1186/1477-7525-1-79.
27. Oken MMMD a, Creech RHMD b, Tormey DCMD, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. J Clin Oncol. 1982;5(6):649-656.
© 2017 Society of Hospital Medicine