User login
Pediatric Dermatology Consult - August 2016
Dr. Catalina Matiz and David Ginsberg describe the diagnosis and treatment of discoid lupus erythematosus in children.
Discoid lupus erythematosus
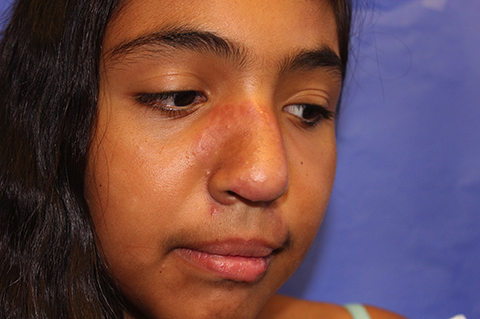
Discoid lupus erythematosus (DLE) is a relatively common form of chronic cutaneous lupus, although its presentation in children is rare. The typical presentation of DLE is well-circumscribed, indurated, sometimes scaly round or oval plaques with pigmentary change, often red to purple in color. DLE also is clinically associated with telangiectasia, scarring, and follicular plugging, which has a characteristic appearance of “carpet tacking” beneath the scale.
When left untreated, these lesions may result in areas of long-term hypo- or hyperpigmentation, as well as atrophy and scarring.1 These cutaneous manifestations often are exacerbated by UV light exposure. This is particularly problematic because DLE most often affects the face, although lesions also can be found on the scalp, ears, trunk, extremities, and in the mouth as well.
Currently, there are few studies looking specifically at DLE in children and, based on the studies that have been done, there appear to be several important differences between the adult and pediatric populations. DLE affects women more than twice as much as men in the adult population, but reports vary as to whether this female predominance carries over to affected children.2,3 Adults with DLE rarely have a family history of systemic lupus erythematosus (SLE), with rates reported between 1% and 4.4%. In children, however, the reported rates of family history increase tenfold to 11%-40%.2
One study showed that children with DLE progress to SLE at a rate of 23.5%-26%, which is higher than reported rates in adults of 5%-20%.2,4 For this reason, repeated laboratory studies are essential in the follow-up of children diagnosed with DLE, given the possibility of a transition to systemic disease, particularly within the first year of diagnosis. Disseminated lesions can be a red flag for future progression to SLE.2
Differential diagnosis
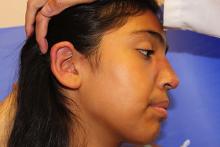
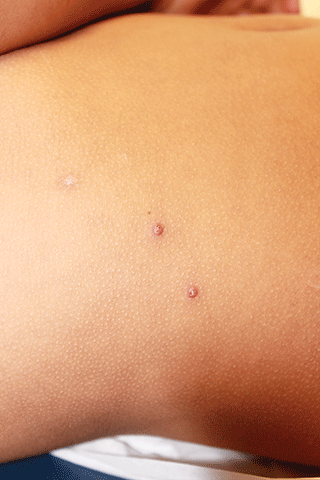
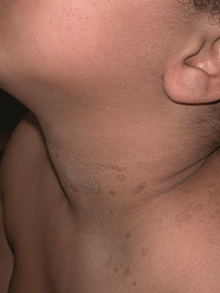
The differential diagnosis of infiltrated annular lesions on the face and ears should include conditions such as tinea faciae, seborrheic dermatitis, granuloma annulare, cutaneous lymphoma, sarcoidosis, and leishmaniasis. When the lesions are present on the ear or nose, as in the case of this patient, relapsing polychonritis also may be considered.
Although histology plays a large part in diagnosing DLE, clinical presentation and recognizing the need for a biopsy are important.5 Tinea faciei, a fungal infection of the face, can present as erythematous scaly plaques.6 A thorough history and physical exam are important in differentiating tinea faciei from DLE because the former often begins as a small scaly papule that annularly expands outward to form larger plaque with scale around the outer rim, as opposed to DLE, which may have an adherent scale across the entire plaque.5 A potassium hydroxide (KOH) preparation of scraped scale from one of the lesions or a fungal culture can confirm the diagnosis of tinea faciei.6
Seborrheic dermatitis can be localized to the face and scalp and presents with greasy yellow scales. Early lesions of DLE can be difficult to differentiate from seborrheic dermatitis.
Infiltrated annular lesions on the face may represent granulomatous conditions such as granuloma annulare or sarcoidosis, but these lesions usually lack the presence of scale that can be seen in DLE.
Relapsing polychondritis presents as intermittent episodes of cartilage inflammation, usually affecting the cartilage of the ear, nose, and respiratory tract. Areas affected do not show changes on the surface of the skin as it occurs in DLE lesions.
As mentioned above, family history of SLE could indicate a potential for DLE in a small percentage of patients, but the clinical feature of the scaly plaques with the carpet tacking underneath the scale, caused by follicular plugging, is helpful in making the diagnosis clinically. Ultimately, the best way to differentiate anything resembling DLE is through histology and direct immunofluorescence (DIF). Histologic findings in DLE include epidermal atrophy, basal membrane cell vacuolization, hyperkeratosis, parakeratosis, corneal plugs, pseudoscysts, and acanthosis.3 The lupus band test is done using DIF and is a widely used tool for making the diagnosis of DLE based on the distribution of immunoglobulin deposition in the basement membrane zone.7
Treatment
Without timely diagnosis and treatment of DLE, the lesions can progress to scarring and atrophy, leading to a decreased quality of life. UV light exposure and smoking can exacerbate DLE, so sun protection and smoking cessation are both recommended in patients with DLE, although, admittedly, the latter is less relevant in the pediatric population.1 Topical, intralesional, or systemic corticosteroids, with or without antimalarials, are the first line therapy for the management of DLE.1 For refractory cases, some reports document the use of topical calcineurin inhibitors, dapsone, methotrexate, and topical or systemic retinoids.1 For severe cases, intravenous immunoglobulin, ustekinumab, and rituximab also may be used.1
References
- Dermatol Ther. 2016 Apr 12 Epub.
- Pediatr Dermatol. 2008 Mar-Apr;25(2):163-7.
- Pediatr Dermatol. 2003 Mar-Apr;20(2):103-7.
- J Am Acad Dermatol. 2015 Apr;72(4):628-33.
- Pediatr Dermatol. 2016 Mar-Apr;33(2):200-8.
- Pediatr Clin North Am. 2014 Apr;61(2):443-55.
- Am J Dermatopathol. 2016 Feb;38(2):121-3.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
Dr. Catalina Matiz and David Ginsberg describe the diagnosis and treatment of discoid lupus erythematosus in children.
Discoid lupus erythematosus
Discoid lupus erythematosus (DLE) is a relatively common form of chronic cutaneous lupus, although its presentation in children is rare. The typical presentation of DLE is well-circumscribed, indurated, sometimes scaly round or oval plaques with pigmentary change, often red to purple in color. DLE also is clinically associated with telangiectasia, scarring, and follicular plugging, which has a characteristic appearance of “carpet tacking” beneath the scale.
When left untreated, these lesions may result in areas of long-term hypo- or hyperpigmentation, as well as atrophy and scarring.1 These cutaneous manifestations often are exacerbated by UV light exposure. This is particularly problematic because DLE most often affects the face, although lesions also can be found on the scalp, ears, trunk, extremities, and in the mouth as well.
Currently, there are few studies looking specifically at DLE in children and, based on the studies that have been done, there appear to be several important differences between the adult and pediatric populations. DLE affects women more than twice as much as men in the adult population, but reports vary as to whether this female predominance carries over to affected children.2,3 Adults with DLE rarely have a family history of systemic lupus erythematosus (SLE), with rates reported between 1% and 4.4%. In children, however, the reported rates of family history increase tenfold to 11%-40%.2
One study showed that children with DLE progress to SLE at a rate of 23.5%-26%, which is higher than reported rates in adults of 5%-20%.2,4 For this reason, repeated laboratory studies are essential in the follow-up of children diagnosed with DLE, given the possibility of a transition to systemic disease, particularly within the first year of diagnosis. Disseminated lesions can be a red flag for future progression to SLE.2
Differential diagnosis
The differential diagnosis of infiltrated annular lesions on the face and ears should include conditions such as tinea faciae, seborrheic dermatitis, granuloma annulare, cutaneous lymphoma, sarcoidosis, and leishmaniasis. When the lesions are present on the ear or nose, as in the case of this patient, relapsing polychonritis also may be considered.
Although histology plays a large part in diagnosing DLE, clinical presentation and recognizing the need for a biopsy are important.5 Tinea faciei, a fungal infection of the face, can present as erythematous scaly plaques.6 A thorough history and physical exam are important in differentiating tinea faciei from DLE because the former often begins as a small scaly papule that annularly expands outward to form larger plaque with scale around the outer rim, as opposed to DLE, which may have an adherent scale across the entire plaque.5 A potassium hydroxide (KOH) preparation of scraped scale from one of the lesions or a fungal culture can confirm the diagnosis of tinea faciei.6
Seborrheic dermatitis can be localized to the face and scalp and presents with greasy yellow scales. Early lesions of DLE can be difficult to differentiate from seborrheic dermatitis.
Infiltrated annular lesions on the face may represent granulomatous conditions such as granuloma annulare or sarcoidosis, but these lesions usually lack the presence of scale that can be seen in DLE.
Relapsing polychondritis presents as intermittent episodes of cartilage inflammation, usually affecting the cartilage of the ear, nose, and respiratory tract. Areas affected do not show changes on the surface of the skin as it occurs in DLE lesions.
As mentioned above, family history of SLE could indicate a potential for DLE in a small percentage of patients, but the clinical feature of the scaly plaques with the carpet tacking underneath the scale, caused by follicular plugging, is helpful in making the diagnosis clinically. Ultimately, the best way to differentiate anything resembling DLE is through histology and direct immunofluorescence (DIF). Histologic findings in DLE include epidermal atrophy, basal membrane cell vacuolization, hyperkeratosis, parakeratosis, corneal plugs, pseudoscysts, and acanthosis.3 The lupus band test is done using DIF and is a widely used tool for making the diagnosis of DLE based on the distribution of immunoglobulin deposition in the basement membrane zone.7
Treatment
Without timely diagnosis and treatment of DLE, the lesions can progress to scarring and atrophy, leading to a decreased quality of life. UV light exposure and smoking can exacerbate DLE, so sun protection and smoking cessation are both recommended in patients with DLE, although, admittedly, the latter is less relevant in the pediatric population.1 Topical, intralesional, or systemic corticosteroids, with or without antimalarials, are the first line therapy for the management of DLE.1 For refractory cases, some reports document the use of topical calcineurin inhibitors, dapsone, methotrexate, and topical or systemic retinoids.1 For severe cases, intravenous immunoglobulin, ustekinumab, and rituximab also may be used.1
References
- Dermatol Ther. 2016 Apr 12 Epub.
- Pediatr Dermatol. 2008 Mar-Apr;25(2):163-7.
- Pediatr Dermatol. 2003 Mar-Apr;20(2):103-7.
- J Am Acad Dermatol. 2015 Apr;72(4):628-33.
- Pediatr Dermatol. 2016 Mar-Apr;33(2):200-8.
- Pediatr Clin North Am. 2014 Apr;61(2):443-55.
- Am J Dermatopathol. 2016 Feb;38(2):121-3.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
Dr. Catalina Matiz and David Ginsberg describe the diagnosis and treatment of discoid lupus erythematosus in children.
Discoid lupus erythematosus
Discoid lupus erythematosus (DLE) is a relatively common form of chronic cutaneous lupus, although its presentation in children is rare. The typical presentation of DLE is well-circumscribed, indurated, sometimes scaly round or oval plaques with pigmentary change, often red to purple in color. DLE also is clinically associated with telangiectasia, scarring, and follicular plugging, which has a characteristic appearance of “carpet tacking” beneath the scale.
When left untreated, these lesions may result in areas of long-term hypo- or hyperpigmentation, as well as atrophy and scarring.1 These cutaneous manifestations often are exacerbated by UV light exposure. This is particularly problematic because DLE most often affects the face, although lesions also can be found on the scalp, ears, trunk, extremities, and in the mouth as well.
Currently, there are few studies looking specifically at DLE in children and, based on the studies that have been done, there appear to be several important differences between the adult and pediatric populations. DLE affects women more than twice as much as men in the adult population, but reports vary as to whether this female predominance carries over to affected children.2,3 Adults with DLE rarely have a family history of systemic lupus erythematosus (SLE), with rates reported between 1% and 4.4%. In children, however, the reported rates of family history increase tenfold to 11%-40%.2
One study showed that children with DLE progress to SLE at a rate of 23.5%-26%, which is higher than reported rates in adults of 5%-20%.2,4 For this reason, repeated laboratory studies are essential in the follow-up of children diagnosed with DLE, given the possibility of a transition to systemic disease, particularly within the first year of diagnosis. Disseminated lesions can be a red flag for future progression to SLE.2
Differential diagnosis
The differential diagnosis of infiltrated annular lesions on the face and ears should include conditions such as tinea faciae, seborrheic dermatitis, granuloma annulare, cutaneous lymphoma, sarcoidosis, and leishmaniasis. When the lesions are present on the ear or nose, as in the case of this patient, relapsing polychonritis also may be considered.
Although histology plays a large part in diagnosing DLE, clinical presentation and recognizing the need for a biopsy are important.5 Tinea faciei, a fungal infection of the face, can present as erythematous scaly plaques.6 A thorough history and physical exam are important in differentiating tinea faciei from DLE because the former often begins as a small scaly papule that annularly expands outward to form larger plaque with scale around the outer rim, as opposed to DLE, which may have an adherent scale across the entire plaque.5 A potassium hydroxide (KOH) preparation of scraped scale from one of the lesions or a fungal culture can confirm the diagnosis of tinea faciei.6
Seborrheic dermatitis can be localized to the face and scalp and presents with greasy yellow scales. Early lesions of DLE can be difficult to differentiate from seborrheic dermatitis.
Infiltrated annular lesions on the face may represent granulomatous conditions such as granuloma annulare or sarcoidosis, but these lesions usually lack the presence of scale that can be seen in DLE.
Relapsing polychondritis presents as intermittent episodes of cartilage inflammation, usually affecting the cartilage of the ear, nose, and respiratory tract. Areas affected do not show changes on the surface of the skin as it occurs in DLE lesions.
As mentioned above, family history of SLE could indicate a potential for DLE in a small percentage of patients, but the clinical feature of the scaly plaques with the carpet tacking underneath the scale, caused by follicular plugging, is helpful in making the diagnosis clinically. Ultimately, the best way to differentiate anything resembling DLE is through histology and direct immunofluorescence (DIF). Histologic findings in DLE include epidermal atrophy, basal membrane cell vacuolization, hyperkeratosis, parakeratosis, corneal plugs, pseudoscysts, and acanthosis.3 The lupus band test is done using DIF and is a widely used tool for making the diagnosis of DLE based on the distribution of immunoglobulin deposition in the basement membrane zone.7
Treatment
Without timely diagnosis and treatment of DLE, the lesions can progress to scarring and atrophy, leading to a decreased quality of life. UV light exposure and smoking can exacerbate DLE, so sun protection and smoking cessation are both recommended in patients with DLE, although, admittedly, the latter is less relevant in the pediatric population.1 Topical, intralesional, or systemic corticosteroids, with or without antimalarials, are the first line therapy for the management of DLE.1 For refractory cases, some reports document the use of topical calcineurin inhibitors, dapsone, methotrexate, and topical or systemic retinoids.1 For severe cases, intravenous immunoglobulin, ustekinumab, and rituximab also may be used.1
References
- Dermatol Ther. 2016 Apr 12 Epub.
- Pediatr Dermatol. 2008 Mar-Apr;25(2):163-7.
- Pediatr Dermatol. 2003 Mar-Apr;20(2):103-7.
- J Am Acad Dermatol. 2015 Apr;72(4):628-33.
- Pediatr Dermatol. 2016 Mar-Apr;33(2):200-8.
- Pediatr Clin North Am. 2014 Apr;61(2):443-55.
- Am J Dermatopathol. 2016 Feb;38(2):121-3.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
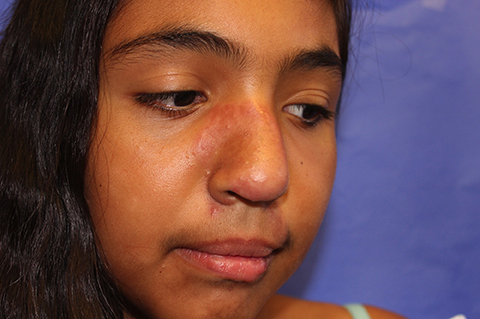
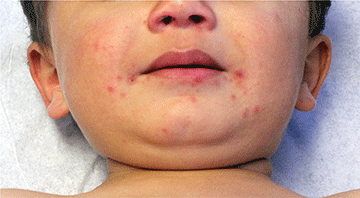
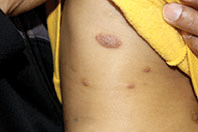
The patient is a well-appearing adolescent in no acute distress, but complaining of continued mild pain of her ear. Upon inspection, she has pink and violaceous indurated annular plaques on her right nasal sidewall and cheek. There is a pink edematous plaque on her right helix and an indurated plaque on the left upper cutaneous lip. The lesions are limited to her face, with no scalp or oral mucosal involvement. Her nails and hair are unaffected.
Pediatric Dermatology Consult - July 2016
By Ellen S. Haddock, MBA, and Lawrence F. Eichenfield, MD
Molluscum
Molluscum typically presents as smooth, flesh-colored, flat-topped papules 2-8 mm in diameter with central whitish area, which is composed of the causative molluscum pox virus. While central depressions called umbilications are common, they may not be present in early molluscum lesions.1,2 Lesions most often occur on the trunk and arms, but can occur anywhere.3-5 Individuals usually have multiple lesions, which may be clustered especially in areas of skin-to-skin contact.5,6 Molluscum lesions can be itchy.
Molluscum is a benign viral skin infection caused by the molluscum contagiosum virus, which is a member of the poxvirus family. Molluscum infections are common, affecting 5%-11% of children.5,7 Molluscum most often affects children younger than 8-years-old,5 with an average age of 5.8 years.8 The infection is spread through skin-to-skin contact with other individuals and by autoinoculation, which means that the infection can be spread from one area of an individual's skin to another when he or she scratches a lesion and then touches another area. It also can be spread by contact with fomites like towels and sponges.1 An association between public swimming pool use and molluscum infection has been reported, but this may have more to do with shared towels and equipment like kick-boards than transmission through the water itself.9 Molluscum sometimes is spread through contact sports like wrestling6 and between children sharing a bath.9 In adults, in whom molluscum is much less common because of acquired immunity,10 molluscum may be sexually transmitted or associated with HIV; however, this is rarely the case in children.1
Children with atopic dermatitis have increased risk of molluscum infection in part because breaks in their skin and pruritus facilitate autoinoculation through scratching.11 Not uncommonly, molluscum lesions become inflamed, with tenderness, erythema, and crust. In a study by Berger et al., 22% of patients had inflamed molluscum lesions.3 The appearance of inflamed molluscum lesions may raise concern about bacterial infection, but more often, the inflammation is a sign that the immune system is reacting to the viral infection and has almost "won the battle."10 After inflamed molluscum lesions develop, the total number of molluscum lesions typically declines,3 and some consider inflamed molluscum lesions to be a "beginning of the end sign," indicating that the infection may soon resolve.10 If the child is afebrile, lesions are itchy and painless, skin culture is negative, and there is no lymphangitis or spreading erythema, the inflammation is more likely a sign of impending resolution than bacterial secondary infection, and the urge to prescribe antibiotics should be resisted.10
As seen in this case, some patients with molluscum (5%) develop a diffuse, monomorphous, papular, or papulovesicular eruption that is an id reaction.3 This may appear to be eczema-like, lichenoid in appearance, or mimicking Gianotti-Crosti syndrome (papular acrodermatitis of childhood).3 It typically affects the arms and legs, is bilateral, and may be pruritic. The id reaction may occur in conjunction with inflamed molluscum, as is true in this case. The diffuse eruption can sometimes be mistaken for a sudden increase in the number of molluscum lesions, but the papular dermatitis lesions do not have the flat-topped dome-shape nor white centers.3 On average, the id reaction lasts about 6 weeks, after which both it and the primary molluscum lesions typically resolve.3 Although not seen in this case, more than a third of molluscum patients develop a pruritic, erythematous, eczematous area around molluscum lesions, termed molluscum dermatitis or eczema molluscatum, which may be more prominent than the molluscum itself.3 The eczematous patch typically surrounds molluscum lesions but also may occur at distant sites.12 This reaction is especially common in patients with atopic dermatitis, 51% of whom develop it.3 It is considered a hypersensitivity reaction and may be asymptomatic or minimally pruritic.12 Molluscum dermatitis suggests that the immune system has taken notice of the infection and is fighting it13; however, it does not necessarily indicate impending resolution.3
Differential diagnosis
The differential diagnosis for molluscum includes herpes simplex, warts, and milia.14 Like molluscum, herpes simplex lesions can have central umbilication, but the lesions are vesicular rather than solid. Warts typically have a rough, jagged surface in contrast to the smooth surface of molluscum lesions. Milia tend to be smaller and not flat topped. They are more common in infants and adults than in children and primarily affect the face.
Inflamed molluscum lesions and molluscum dermatitis can be mistaken for atopic dermatitis, and molluscum-associated id reactions may exacerbate atopic dermatitis. Inflamed molluscum and molluscum with id reaction could be confused with scabies, which may become crusted and also may be accompanied by id reaction. Presence of serpiginous linear burrows would suggest scabies rather than molluscum, and the diagnosis of scabies can be confirmed by scraping a burrow and looking for a mite or its feces under a microscope.
Prognosis and treatment
Molluscum infections typically resolve spontaneously in months to years (average duration, 13 months),14 so treatment may not be required. The goal of treatment is to accelerate the resolution of the infection, but some studies have found that common treatments may not shorten the time to resolution.11 However, if there is substantial pruritus, lesions are cosmetically undesirable, or a child has atopic dermatitis and is at increased risk for autoinoculation, treatment may be warranted.15 Furthermore, molluscum lesions can scar, so prevention of autoinoculation may help minimize scarring.16
Few high-quality studies of the efficacy of molluscum treatments exist, and a 2009 Cochrane review found insufficient evidence to recommend any therapy for molluscum. The most common treatment used by pediatric dermatologists is cantharidin,17 and this treatment also is available to primary care practitioners. This option is preferred over other destructive methods such as curettage or liquid nitrogen cryotherapy because it is not painful or traumatic and is well tolerated by pediatric patients.8 Parent and physician satisfaction with the therapy is high; 78%-95% of parents would use cantharidin treatment again for molluscum recurrence.4,8,18 Originally extracted from the blister beetle but now synthesized commercially,19 cantharidin causes vesiculation at the dermoepidermal junction6 by destroying intercellular connections.4 Vesiculation of the skin causes extrusion of the molluscum body, which facilitates resolution of the lesion.19 The cantharidin formulation is applied directly to molluscum lesions with the wooden end of a cotton-tipped applicator.4 Patients may be directed to wash it off after 4-6 hours. Blistering is an expected, desired outcome. A minority of patients may experience mild temporary pain (7%), more significant blistering (2.5%), burning (less than 1%), pruritus (less than 1%), or irritation (less than 1%).4 There is a risk of scarring and pigmentary changes, but these risks also exist for untreated lesions.19 Cantharidin treatment is repeated approximately every 4 weeks, and 90% of cases resolve after an average of 2.1 treatments.18 Topical retinoids can be used in an attempt to trigger an irritant response by the immune system, and they are the preferred therapy for facial lesions, but they are inconsistently effective.4 Randomized controlled trials found that imiquimod, a previously popular treatment is not effective,20 and the evidence for cimetidine is contradictory.21,22 Molluscum dermatitis and id reaction can be treated with medium strength topical steroids.
References
- Viral diseases of the skin, in "Hurwitz Clinical Pediatric Dermatology," 4 ed. (New York: Elsevier, 2011, pp. 348-69). .
- Molluscum, in "Red Book Report of the Committee on Infectious Diseases," 2015.
Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at Rady Children's Hospital-San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield and Ms. Haddock state they have no relevant financial disclosures.
By Ellen S. Haddock, MBA, and Lawrence F. Eichenfield, MD
Molluscum
Molluscum typically presents as smooth, flesh-colored, flat-topped papules 2-8 mm in diameter with central whitish area, which is composed of the causative molluscum pox virus. While central depressions called umbilications are common, they may not be present in early molluscum lesions.1,2 Lesions most often occur on the trunk and arms, but can occur anywhere.3-5 Individuals usually have multiple lesions, which may be clustered especially in areas of skin-to-skin contact.5,6 Molluscum lesions can be itchy.
Molluscum is a benign viral skin infection caused by the molluscum contagiosum virus, which is a member of the poxvirus family. Molluscum infections are common, affecting 5%-11% of children.5,7 Molluscum most often affects children younger than 8-years-old,5 with an average age of 5.8 years.8 The infection is spread through skin-to-skin contact with other individuals and by autoinoculation, which means that the infection can be spread from one area of an individual's skin to another when he or she scratches a lesion and then touches another area. It also can be spread by contact with fomites like towels and sponges.1 An association between public swimming pool use and molluscum infection has been reported, but this may have more to do with shared towels and equipment like kick-boards than transmission through the water itself.9 Molluscum sometimes is spread through contact sports like wrestling6 and between children sharing a bath.9 In adults, in whom molluscum is much less common because of acquired immunity,10 molluscum may be sexually transmitted or associated with HIV; however, this is rarely the case in children.1
Children with atopic dermatitis have increased risk of molluscum infection in part because breaks in their skin and pruritus facilitate autoinoculation through scratching.11 Not uncommonly, molluscum lesions become inflamed, with tenderness, erythema, and crust. In a study by Berger et al., 22% of patients had inflamed molluscum lesions.3 The appearance of inflamed molluscum lesions may raise concern about bacterial infection, but more often, the inflammation is a sign that the immune system is reacting to the viral infection and has almost "won the battle."10 After inflamed molluscum lesions develop, the total number of molluscum lesions typically declines,3 and some consider inflamed molluscum lesions to be a "beginning of the end sign," indicating that the infection may soon resolve.10 If the child is afebrile, lesions are itchy and painless, skin culture is negative, and there is no lymphangitis or spreading erythema, the inflammation is more likely a sign of impending resolution than bacterial secondary infection, and the urge to prescribe antibiotics should be resisted.10
As seen in this case, some patients with molluscum (5%) develop a diffuse, monomorphous, papular, or papulovesicular eruption that is an id reaction.3 This may appear to be eczema-like, lichenoid in appearance, or mimicking Gianotti-Crosti syndrome (papular acrodermatitis of childhood).3 It typically affects the arms and legs, is bilateral, and may be pruritic. The id reaction may occur in conjunction with inflamed molluscum, as is true in this case. The diffuse eruption can sometimes be mistaken for a sudden increase in the number of molluscum lesions, but the papular dermatitis lesions do not have the flat-topped dome-shape nor white centers.3 On average, the id reaction lasts about 6 weeks, after which both it and the primary molluscum lesions typically resolve.3 Although not seen in this case, more than a third of molluscum patients develop a pruritic, erythematous, eczematous area around molluscum lesions, termed molluscum dermatitis or eczema molluscatum, which may be more prominent than the molluscum itself.3 The eczematous patch typically surrounds molluscum lesions but also may occur at distant sites.12 This reaction is especially common in patients with atopic dermatitis, 51% of whom develop it.3 It is considered a hypersensitivity reaction and may be asymptomatic or minimally pruritic.12 Molluscum dermatitis suggests that the immune system has taken notice of the infection and is fighting it13; however, it does not necessarily indicate impending resolution.3
Differential diagnosis
The differential diagnosis for molluscum includes herpes simplex, warts, and milia.14 Like molluscum, herpes simplex lesions can have central umbilication, but the lesions are vesicular rather than solid. Warts typically have a rough, jagged surface in contrast to the smooth surface of molluscum lesions. Milia tend to be smaller and not flat topped. They are more common in infants and adults than in children and primarily affect the face.
Inflamed molluscum lesions and molluscum dermatitis can be mistaken for atopic dermatitis, and molluscum-associated id reactions may exacerbate atopic dermatitis. Inflamed molluscum and molluscum with id reaction could be confused with scabies, which may become crusted and also may be accompanied by id reaction. Presence of serpiginous linear burrows would suggest scabies rather than molluscum, and the diagnosis of scabies can be confirmed by scraping a burrow and looking for a mite or its feces under a microscope.
Prognosis and treatment
Molluscum infections typically resolve spontaneously in months to years (average duration, 13 months),14 so treatment may not be required. The goal of treatment is to accelerate the resolution of the infection, but some studies have found that common treatments may not shorten the time to resolution.11 However, if there is substantial pruritus, lesions are cosmetically undesirable, or a child has atopic dermatitis and is at increased risk for autoinoculation, treatment may be warranted.15 Furthermore, molluscum lesions can scar, so prevention of autoinoculation may help minimize scarring.16
Few high-quality studies of the efficacy of molluscum treatments exist, and a 2009 Cochrane review found insufficient evidence to recommend any therapy for molluscum. The most common treatment used by pediatric dermatologists is cantharidin,17 and this treatment also is available to primary care practitioners. This option is preferred over other destructive methods such as curettage or liquid nitrogen cryotherapy because it is not painful or traumatic and is well tolerated by pediatric patients.8 Parent and physician satisfaction with the therapy is high; 78%-95% of parents would use cantharidin treatment again for molluscum recurrence.4,8,18 Originally extracted from the blister beetle but now synthesized commercially,19 cantharidin causes vesiculation at the dermoepidermal junction6 by destroying intercellular connections.4 Vesiculation of the skin causes extrusion of the molluscum body, which facilitates resolution of the lesion.19 The cantharidin formulation is applied directly to molluscum lesions with the wooden end of a cotton-tipped applicator.4 Patients may be directed to wash it off after 4-6 hours. Blistering is an expected, desired outcome. A minority of patients may experience mild temporary pain (7%), more significant blistering (2.5%), burning (less than 1%), pruritus (less than 1%), or irritation (less than 1%).4 There is a risk of scarring and pigmentary changes, but these risks also exist for untreated lesions.19 Cantharidin treatment is repeated approximately every 4 weeks, and 90% of cases resolve after an average of 2.1 treatments.18 Topical retinoids can be used in an attempt to trigger an irritant response by the immune system, and they are the preferred therapy for facial lesions, but they are inconsistently effective.4 Randomized controlled trials found that imiquimod, a previously popular treatment is not effective,20 and the evidence for cimetidine is contradictory.21,22 Molluscum dermatitis and id reaction can be treated with medium strength topical steroids.
References
- Viral diseases of the skin, in "Hurwitz Clinical Pediatric Dermatology," 4 ed. (New York: Elsevier, 2011, pp. 348-69). .
- Molluscum, in "Red Book Report of the Committee on Infectious Diseases," 2015.
Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at Rady Children's Hospital-San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield and Ms. Haddock state they have no relevant financial disclosures.
By Ellen S. Haddock, MBA, and Lawrence F. Eichenfield, MD
Molluscum
Molluscum typically presents as smooth, flesh-colored, flat-topped papules 2-8 mm in diameter with central whitish area, which is composed of the causative molluscum pox virus. While central depressions called umbilications are common, they may not be present in early molluscum lesions.1,2 Lesions most often occur on the trunk and arms, but can occur anywhere.3-5 Individuals usually have multiple lesions, which may be clustered especially in areas of skin-to-skin contact.5,6 Molluscum lesions can be itchy.
Molluscum is a benign viral skin infection caused by the molluscum contagiosum virus, which is a member of the poxvirus family. Molluscum infections are common, affecting 5%-11% of children.5,7 Molluscum most often affects children younger than 8-years-old,5 with an average age of 5.8 years.8 The infection is spread through skin-to-skin contact with other individuals and by autoinoculation, which means that the infection can be spread from one area of an individual's skin to another when he or she scratches a lesion and then touches another area. It also can be spread by contact with fomites like towels and sponges.1 An association between public swimming pool use and molluscum infection has been reported, but this may have more to do with shared towels and equipment like kick-boards than transmission through the water itself.9 Molluscum sometimes is spread through contact sports like wrestling6 and between children sharing a bath.9 In adults, in whom molluscum is much less common because of acquired immunity,10 molluscum may be sexually transmitted or associated with HIV; however, this is rarely the case in children.1
Children with atopic dermatitis have increased risk of molluscum infection in part because breaks in their skin and pruritus facilitate autoinoculation through scratching.11 Not uncommonly, molluscum lesions become inflamed, with tenderness, erythema, and crust. In a study by Berger et al., 22% of patients had inflamed molluscum lesions.3 The appearance of inflamed molluscum lesions may raise concern about bacterial infection, but more often, the inflammation is a sign that the immune system is reacting to the viral infection and has almost "won the battle."10 After inflamed molluscum lesions develop, the total number of molluscum lesions typically declines,3 and some consider inflamed molluscum lesions to be a "beginning of the end sign," indicating that the infection may soon resolve.10 If the child is afebrile, lesions are itchy and painless, skin culture is negative, and there is no lymphangitis or spreading erythema, the inflammation is more likely a sign of impending resolution than bacterial secondary infection, and the urge to prescribe antibiotics should be resisted.10
As seen in this case, some patients with molluscum (5%) develop a diffuse, monomorphous, papular, or papulovesicular eruption that is an id reaction.3 This may appear to be eczema-like, lichenoid in appearance, or mimicking Gianotti-Crosti syndrome (papular acrodermatitis of childhood).3 It typically affects the arms and legs, is bilateral, and may be pruritic. The id reaction may occur in conjunction with inflamed molluscum, as is true in this case. The diffuse eruption can sometimes be mistaken for a sudden increase in the number of molluscum lesions, but the papular dermatitis lesions do not have the flat-topped dome-shape nor white centers.3 On average, the id reaction lasts about 6 weeks, after which both it and the primary molluscum lesions typically resolve.3 Although not seen in this case, more than a third of molluscum patients develop a pruritic, erythematous, eczematous area around molluscum lesions, termed molluscum dermatitis or eczema molluscatum, which may be more prominent than the molluscum itself.3 The eczematous patch typically surrounds molluscum lesions but also may occur at distant sites.12 This reaction is especially common in patients with atopic dermatitis, 51% of whom develop it.3 It is considered a hypersensitivity reaction and may be asymptomatic or minimally pruritic.12 Molluscum dermatitis suggests that the immune system has taken notice of the infection and is fighting it13; however, it does not necessarily indicate impending resolution.3
Differential diagnosis
The differential diagnosis for molluscum includes herpes simplex, warts, and milia.14 Like molluscum, herpes simplex lesions can have central umbilication, but the lesions are vesicular rather than solid. Warts typically have a rough, jagged surface in contrast to the smooth surface of molluscum lesions. Milia tend to be smaller and not flat topped. They are more common in infants and adults than in children and primarily affect the face.
Inflamed molluscum lesions and molluscum dermatitis can be mistaken for atopic dermatitis, and molluscum-associated id reactions may exacerbate atopic dermatitis. Inflamed molluscum and molluscum with id reaction could be confused with scabies, which may become crusted and also may be accompanied by id reaction. Presence of serpiginous linear burrows would suggest scabies rather than molluscum, and the diagnosis of scabies can be confirmed by scraping a burrow and looking for a mite or its feces under a microscope.
Prognosis and treatment
Molluscum infections typically resolve spontaneously in months to years (average duration, 13 months),14 so treatment may not be required. The goal of treatment is to accelerate the resolution of the infection, but some studies have found that common treatments may not shorten the time to resolution.11 However, if there is substantial pruritus, lesions are cosmetically undesirable, or a child has atopic dermatitis and is at increased risk for autoinoculation, treatment may be warranted.15 Furthermore, molluscum lesions can scar, so prevention of autoinoculation may help minimize scarring.16
Few high-quality studies of the efficacy of molluscum treatments exist, and a 2009 Cochrane review found insufficient evidence to recommend any therapy for molluscum. The most common treatment used by pediatric dermatologists is cantharidin,17 and this treatment also is available to primary care practitioners. This option is preferred over other destructive methods such as curettage or liquid nitrogen cryotherapy because it is not painful or traumatic and is well tolerated by pediatric patients.8 Parent and physician satisfaction with the therapy is high; 78%-95% of parents would use cantharidin treatment again for molluscum recurrence.4,8,18 Originally extracted from the blister beetle but now synthesized commercially,19 cantharidin causes vesiculation at the dermoepidermal junction6 by destroying intercellular connections.4 Vesiculation of the skin causes extrusion of the molluscum body, which facilitates resolution of the lesion.19 The cantharidin formulation is applied directly to molluscum lesions with the wooden end of a cotton-tipped applicator.4 Patients may be directed to wash it off after 4-6 hours. Blistering is an expected, desired outcome. A minority of patients may experience mild temporary pain (7%), more significant blistering (2.5%), burning (less than 1%), pruritus (less than 1%), or irritation (less than 1%).4 There is a risk of scarring and pigmentary changes, but these risks also exist for untreated lesions.19 Cantharidin treatment is repeated approximately every 4 weeks, and 90% of cases resolve after an average of 2.1 treatments.18 Topical retinoids can be used in an attempt to trigger an irritant response by the immune system, and they are the preferred therapy for facial lesions, but they are inconsistently effective.4 Randomized controlled trials found that imiquimod, a previously popular treatment is not effective,20 and the evidence for cimetidine is contradictory.21,22 Molluscum dermatitis and id reaction can be treated with medium strength topical steroids.
References
- Viral diseases of the skin, in "Hurwitz Clinical Pediatric Dermatology," 4 ed. (New York: Elsevier, 2011, pp. 348-69). .
- Molluscum, in "Red Book Report of the Committee on Infectious Diseases," 2015.
Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at Rady Children's Hospital-San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children's Hospital-San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield and Ms. Haddock state they have no relevant financial disclosures.
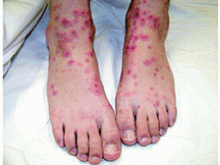
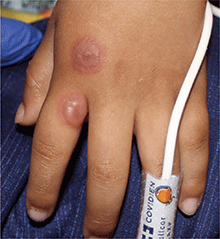
An otherwise healthy 9-year-old girl presented for evaluation of multiple small, skin-colored bumps on her belly, arms, knees, and buttocks. She first noticed a few bumps on her belly 4 months ago. Some of the original bumps have resolved, leaving only two of the originals remaining, but a few weeks ago she developed many additional itchy bumps on her arms, knees, and buttocks. On physical exam, she has two erythematous, flat-topped papules on her abdomen with white centers. (See photo.) A hypopigmented macule also is present on the abdomen. On her legs, arms, and buttocks she has multiple skin-colored to pink papules without white centers.
Pediatric Dermatology Consult - June 2016
Dr. Catalina Matiz and David Ginsberg discuss the diagnosis and treatment of phytophotodermatitis.
Phytophotodermatitis
The term phytophotodermatitis was first used in 1942 by Robert Klaber, but knowledge of this condition dates back as far as 1500 BC.1,2 It is a nonimmune reaction caused by exposure to chemicals called furocoumarins and psoralens, found in a variety of plants and fruits such as lemons, limes, celery, parsnips, figs, carrots, dill, mustard, and rindweed.1,2
When these chemicals get in contact with the skin and are then exposed to UV light from the sun, a phototoxic reaction occurs. This reaction leads to cell membrane damage and cell death, which after the acute insult has resolved results in postinflammatory hyperpigmentation that may last months and is not responsive to bleaching skin treatments.1,2
Studies have shown that long-wave length UV radiation is the best trigger to induce this irritating reaction.2 There have been rare reports of photosensitivity reactions due to ingestion of large quantities of plants containing furocoumarins and psoralens.3 Plants known to cause phytophotodermatitis are found in almost every country across the globe, and exposure does not have to just be to the fruit, but contact with the leaves or sap also can induce the reaction.2-4 Typically after contact with the plant, followed by sun exposure, erythema will begin within 1 to 2 days, followed by bullae and vesicles, which tend to coalesce and burst over the following days. The patient is left with hyperpigmentation.2-4
Differential diagnosis
This condition can be difficult to diagnose and is often confused with type IV hypersensitivity reaction, eczema, herpes simplex virus (HSV), and burns, potentially leading to suspicion of child abuse.4,5 A thorough and detailed history is essential to correctly making the diagnosis and ruling out other potential causes.
Because the cause in children is often due to exposure to certain plants, unsupervised time outdoors leading to a rash may lead parents to think of poison ivy or poison oak, which are type IV hypersensitivity reactions.4,5 Although the rash may appear in a similar time course, the evolution to hyperpigmented patches is a distinguishing feature that helps to make the diagnosis of phytophotodermatitis.4 The postinflammatory hyperpigmentation, along with the clinical course, also can help differentiate it from burns and HSV infections, which it sometimes is mistaken for due to the bullous and vesicular lesions.4,5
Treatment
Counseling for avoidance of the exposure in the future is the most important aspect of treatment in order to prevent recurrences. Depending on the extent of the inflammatory reaction prior to the hyperpigmentation, no treatment may be needed for mild cases, but for more extreme bullous reactions, systemic steroids may be used.4
Topical corticosteroids and sun avoidance are the mainstays for treating mild to moderate cases. UV avoidance can be difficult because the long wave-length UV radiation that causes this reaction is not blocked by windows, and therefore it is important to keep affected areas covered even while indoors during daylight hours.4
There is no effective treatment for the hyperpigmented lesions. Patients need to be informed that this may resolve in months.
References
- Br J Dermatol. 1942;54(7):193-211.
- Clin Dermatol. 1986 Apr-Jun;4(2):102-21.
- Arch Dermatol. 1990 Oct;126(10):1334-6.
- J Am Acad Dermatol. 2007 Nov;57(5 Suppl):S88-91.
- Arch Fam Med. 2000 Jan;9(1):88.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
Dr. Catalina Matiz and David Ginsberg discuss the diagnosis and treatment of phytophotodermatitis.
Phytophotodermatitis
The term phytophotodermatitis was first used in 1942 by Robert Klaber, but knowledge of this condition dates back as far as 1500 BC.1,2 It is a nonimmune reaction caused by exposure to chemicals called furocoumarins and psoralens, found in a variety of plants and fruits such as lemons, limes, celery, parsnips, figs, carrots, dill, mustard, and rindweed.1,2
When these chemicals get in contact with the skin and are then exposed to UV light from the sun, a phototoxic reaction occurs. This reaction leads to cell membrane damage and cell death, which after the acute insult has resolved results in postinflammatory hyperpigmentation that may last months and is not responsive to bleaching skin treatments.1,2
Studies have shown that long-wave length UV radiation is the best trigger to induce this irritating reaction.2 There have been rare reports of photosensitivity reactions due to ingestion of large quantities of plants containing furocoumarins and psoralens.3 Plants known to cause phytophotodermatitis are found in almost every country across the globe, and exposure does not have to just be to the fruit, but contact with the leaves or sap also can induce the reaction.2-4 Typically after contact with the plant, followed by sun exposure, erythema will begin within 1 to 2 days, followed by bullae and vesicles, which tend to coalesce and burst over the following days. The patient is left with hyperpigmentation.2-4
Differential diagnosis
This condition can be difficult to diagnose and is often confused with type IV hypersensitivity reaction, eczema, herpes simplex virus (HSV), and burns, potentially leading to suspicion of child abuse.4,5 A thorough and detailed history is essential to correctly making the diagnosis and ruling out other potential causes.
Because the cause in children is often due to exposure to certain plants, unsupervised time outdoors leading to a rash may lead parents to think of poison ivy or poison oak, which are type IV hypersensitivity reactions.4,5 Although the rash may appear in a similar time course, the evolution to hyperpigmented patches is a distinguishing feature that helps to make the diagnosis of phytophotodermatitis.4 The postinflammatory hyperpigmentation, along with the clinical course, also can help differentiate it from burns and HSV infections, which it sometimes is mistaken for due to the bullous and vesicular lesions.4,5
Treatment
Counseling for avoidance of the exposure in the future is the most important aspect of treatment in order to prevent recurrences. Depending on the extent of the inflammatory reaction prior to the hyperpigmentation, no treatment may be needed for mild cases, but for more extreme bullous reactions, systemic steroids may be used.4
Topical corticosteroids and sun avoidance are the mainstays for treating mild to moderate cases. UV avoidance can be difficult because the long wave-length UV radiation that causes this reaction is not blocked by windows, and therefore it is important to keep affected areas covered even while indoors during daylight hours.4
There is no effective treatment for the hyperpigmented lesions. Patients need to be informed that this may resolve in months.
References
- Br J Dermatol. 1942;54(7):193-211.
- Clin Dermatol. 1986 Apr-Jun;4(2):102-21.
- Arch Dermatol. 1990 Oct;126(10):1334-6.
- J Am Acad Dermatol. 2007 Nov;57(5 Suppl):S88-91.
- Arch Fam Med. 2000 Jan;9(1):88.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
Dr. Catalina Matiz and David Ginsberg discuss the diagnosis and treatment of phytophotodermatitis.
Phytophotodermatitis
The term phytophotodermatitis was first used in 1942 by Robert Klaber, but knowledge of this condition dates back as far as 1500 BC.1,2 It is a nonimmune reaction caused by exposure to chemicals called furocoumarins and psoralens, found in a variety of plants and fruits such as lemons, limes, celery, parsnips, figs, carrots, dill, mustard, and rindweed.1,2
When these chemicals get in contact with the skin and are then exposed to UV light from the sun, a phototoxic reaction occurs. This reaction leads to cell membrane damage and cell death, which after the acute insult has resolved results in postinflammatory hyperpigmentation that may last months and is not responsive to bleaching skin treatments.1,2
Studies have shown that long-wave length UV radiation is the best trigger to induce this irritating reaction.2 There have been rare reports of photosensitivity reactions due to ingestion of large quantities of plants containing furocoumarins and psoralens.3 Plants known to cause phytophotodermatitis are found in almost every country across the globe, and exposure does not have to just be to the fruit, but contact with the leaves or sap also can induce the reaction.2-4 Typically after contact with the plant, followed by sun exposure, erythema will begin within 1 to 2 days, followed by bullae and vesicles, which tend to coalesce and burst over the following days. The patient is left with hyperpigmentation.2-4
Differential diagnosis
This condition can be difficult to diagnose and is often confused with type IV hypersensitivity reaction, eczema, herpes simplex virus (HSV), and burns, potentially leading to suspicion of child abuse.4,5 A thorough and detailed history is essential to correctly making the diagnosis and ruling out other potential causes.
Because the cause in children is often due to exposure to certain plants, unsupervised time outdoors leading to a rash may lead parents to think of poison ivy or poison oak, which are type IV hypersensitivity reactions.4,5 Although the rash may appear in a similar time course, the evolution to hyperpigmented patches is a distinguishing feature that helps to make the diagnosis of phytophotodermatitis.4 The postinflammatory hyperpigmentation, along with the clinical course, also can help differentiate it from burns and HSV infections, which it sometimes is mistaken for due to the bullous and vesicular lesions.4,5
Treatment
Counseling for avoidance of the exposure in the future is the most important aspect of treatment in order to prevent recurrences. Depending on the extent of the inflammatory reaction prior to the hyperpigmentation, no treatment may be needed for mild cases, but for more extreme bullous reactions, systemic steroids may be used.4
Topical corticosteroids and sun avoidance are the mainstays for treating mild to moderate cases. UV avoidance can be difficult because the long wave-length UV radiation that causes this reaction is not blocked by windows, and therefore it is important to keep affected areas covered even while indoors during daylight hours.4
There is no effective treatment for the hyperpigmented lesions. Patients need to be informed that this may resolve in months.
References
- Br J Dermatol. 1942;54(7):193-211.
- Clin Dermatol. 1986 Apr-Jun;4(2):102-21.
- Arch Dermatol. 1990 Oct;126(10):1334-6.
- J Am Acad Dermatol. 2007 Nov;57(5 Suppl):S88-91.
- Arch Fam Med. 2000 Jan;9(1):88.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
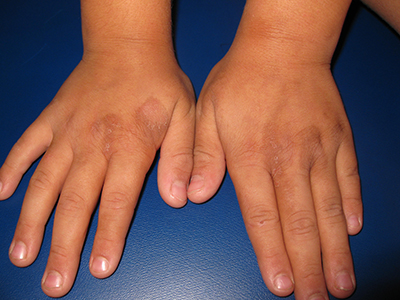
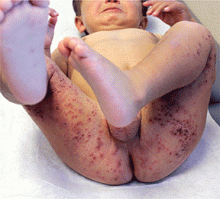
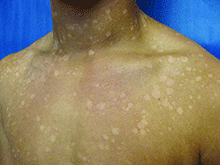
An 8-year-old healthy female presents with a 7-day history of a rash on the dorsum of her hands bilaterally. Her mother reports that it began as redness and swelling followed by blisters. She also reports that after the blisters popped and the redness went away, she noticed some darkened areas around where the blisters had been. The patient reports that there was some slight stinging and burning when the rash first appeared, which has been improving with time. She has had no fevers and has not been ill. The family has a dog, the patient has one older sibling who has atopic dermatitis, and the mother has asthma. The mother reports they live on a farm, and they have a vegetable and flower garden where the daughter plays every afternoon. She has no known allergies. The mother denies any recent travels or family history of bullous disease. Physical exam The patient is a well-appearing child who is attentive and in no apparent distress. On exam, there are several non-tender hyperpigmented patches between 1 cm and 3 cm on the dorsum of the hands, and intertriginous areas with overlying erosions. There are no other lesions on the skin. She is afebrile.
Pediatric Dermatology Consult - May 2016
By Ellen S. Haddock and Lawrence F. Eichenfield, MD
Terra firma-forme dermatosis
The easy removal of this patient’s persistent skin discoloration with an alcohol wipe confirms the diagnosis of terra firma-forme dermatosis. Terra firma-forme dermatosis is a benign skin condition in which dirt-like plaques develop on the skin despite normal hygiene. They cannot be removed with soap and water, but are removed easily with alcohol.
Terra firma means “solid earth” in Latin and refers to the dirt-like appearance of the lesion.1,2 The condition typically presents with brown or black hyperkeratotic plaques or papules. The hyperpigmented lesions may be papillomatous, verrucous (warty), or have a reticulated (net-like) distribution.3 The condition is usually asymptomatic but occasionally itchy.1 In the largest published series of 31 patients by Berk et al., the neck, ankles, and face were most commonly affected, but the condition can occur anywhere on the body.4 Lesions may be single or multiple and tend to be symmetrically distributed.4 The condition seems to be most common in young adults and adolescents, but can occur at any age and affects both genders equally.2,5
It seems to occur more commonly in darker skin, heavier patients, and concave body areas.3,6 The condition can last for months (median duration of 4 months in Berk et al.’s series), and by the time patients present to a physician they usually have tried aggressive scrubbing with multiple different soaps.4 As in this case, it is not uncommon for patients to receive an unnecessary work-up for diabetes due to concern for acanthosis nigricans.4 This work-up can be avoided if practitioners attempt removal with 70% isopropyl alcohol before ordering the diabetic work-up.
Biopsies are not typically performed (and should be abandoned if the lesion disappears when the site is cleansed with alcohol in preparation for biopsy), but hematoxylin and eosin staining shows lamellar hyperkeratosis, orthokeratotic whorls, and increased melanin in the basal layer and hyperkeratotic areas. Keratin globules are seen throughout the stratum corneum.7
Electron microscopy shows that the keratin lamellae is immature in some places, with incomplete keratinization and retention of desmosomal attachments.7 Overall, the histology is nonspecific and may be indistinguishable from other benign papillomatous conditions including confluent and reticulated papillomatosis (CARP), acanthosis nigricans, and epidermal nevi.6
Differential diagnosis
Clinically, the lesion may appear very similar to the brown velvety plaques of acanthosis nigricans, but the latter can be quickly ruled out if the lesion disappears when cleansed with alcohol. The differential diagnosis also includes dermatosis neglecta, CARP, hyperpigmented tinea versicolor, and dirty neck syndrome of atopic dermatitis.4,7
Dermatosis neglecta is caused by poor hygiene with insufficient skin cleaning. In contrast with terra firma-forme dermatitis, it can be removed with soap and water. Patients with dermatosis neglecta lack a history of aggressive attempts at removal by scrubbing. Dermatosis neglecta looks similar to terra firma-forme dermatosis, but may have a waxy “cornflake-like” scale.2 It is most common in the elderly, but also can be seen in children, especially if they have had a cast or brace that prevented washing an area.8
CARP may present as brown hyperkeratotic or verrucous papules, but typically occurs on the trunk and has a reticulated pattern, which is less common in terra firma-forme dermatosis. Typically, it cannot be removed completely with alcohol10-12 and tends to respond best to treatment with minocycline.11
Hyperpigmented tinea versicolor lesions are usually more discrete and have fine scale, which becomes more prominent when the lesions are scraped. When analyzed with KOH under a microscope, hyphae and spores looking like spaghetti and meatballs can be seen.
Dirty neck syndrome is acquired hyperpigmentation that may develop on the neck of patients with atopic dermatitis. It cannot be removed with alcohol.4 It may result from frictional melanosis with melanin incontinence and post-inflammatory hyperpigmentation.12 The hyperpigmentation is patchy or rippled,12 whereas terra firma-forme dermatosis more often presents as plaques.
Etiology
The cause of terra firma-forme dermatosis is uncertain. It is thought to be a disorder of keratinocyte retention, in which delayed keratinocyte maturation causes prolonged cell-to-cell adhesion that impairs shedding.7,8 Discoloration may be due to retained melanin as well as sebum and dirt that build up along with excess keratinocytes.7-9 Alcohol is a better solvent for this retained material than soap and water. Patients using heavy emollients or oily soaps for conditions like atopic dermatitis may be relatively predisposed to the condition if emollients or oily soaps are not removed completely by bathing; the combination of underlying scale and retained skin care products may make skin more adhesive, disrupting normal keratinocyte shedding and allowing dirt and sebum to accumulate.1 Atopic dermatitis was the most common comorbid condition for terra firma-forme dermatosis in Berk et al.’s series (12 of 31 patients).4
Treatment
Wiping with 70% isopropyl alcohol is both diagnostic and therapeutic, removing the lesion completely. Recurrence is uncommon but possible.3 In rare instances of regularly recurrent lesions, the area can be wiped prophylactically with alcohol weekly.1
References
- Indian J Dermatol Venereol Leprol. 2012 May-Jun;78(3):358-60.
- Pediatr Dermatol. 2011 Jan-Feb;28(1):79-81.
- Dermatol Pract Concept. 2015 Jul; 5(3): 29-33.
- Pediatr Dermatol. 2012 May-Jun;29(3):297-300.
- Eur J Intern Med. 2016 Feb. doi: 10.1016/j.ejim.2016.02.009.
- J Cutan Pathol. 2012 Feb;39(2):300-1.
- Arch Dermatol. 1987;123(5):567-9.
- Pediatr Dermatol. 2015;32(2):e50-3.
- Arch Dermatol. 2010;146(6):679-80.
- Arch Dermatol. 2011 Feb;147(2):247-8.
- Am J Clin Dermatol. 2006;7(5):305-313.
- Dermatology. 2014;229:174-182.
Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield and Ms. Haddock state they have no relevant financial disclosures. Email them at [email protected].
By Ellen S. Haddock and Lawrence F. Eichenfield, MD
Terra firma-forme dermatosis
The easy removal of this patient’s persistent skin discoloration with an alcohol wipe confirms the diagnosis of terra firma-forme dermatosis. Terra firma-forme dermatosis is a benign skin condition in which dirt-like plaques develop on the skin despite normal hygiene. They cannot be removed with soap and water, but are removed easily with alcohol.
Terra firma means “solid earth” in Latin and refers to the dirt-like appearance of the lesion.1,2 The condition typically presents with brown or black hyperkeratotic plaques or papules. The hyperpigmented lesions may be papillomatous, verrucous (warty), or have a reticulated (net-like) distribution.3 The condition is usually asymptomatic but occasionally itchy.1 In the largest published series of 31 patients by Berk et al., the neck, ankles, and face were most commonly affected, but the condition can occur anywhere on the body.4 Lesions may be single or multiple and tend to be symmetrically distributed.4 The condition seems to be most common in young adults and adolescents, but can occur at any age and affects both genders equally.2,5
It seems to occur more commonly in darker skin, heavier patients, and concave body areas.3,6 The condition can last for months (median duration of 4 months in Berk et al.’s series), and by the time patients present to a physician they usually have tried aggressive scrubbing with multiple different soaps.4 As in this case, it is not uncommon for patients to receive an unnecessary work-up for diabetes due to concern for acanthosis nigricans.4 This work-up can be avoided if practitioners attempt removal with 70% isopropyl alcohol before ordering the diabetic work-up.
Biopsies are not typically performed (and should be abandoned if the lesion disappears when the site is cleansed with alcohol in preparation for biopsy), but hematoxylin and eosin staining shows lamellar hyperkeratosis, orthokeratotic whorls, and increased melanin in the basal layer and hyperkeratotic areas. Keratin globules are seen throughout the stratum corneum.7
Electron microscopy shows that the keratin lamellae is immature in some places, with incomplete keratinization and retention of desmosomal attachments.7 Overall, the histology is nonspecific and may be indistinguishable from other benign papillomatous conditions including confluent and reticulated papillomatosis (CARP), acanthosis nigricans, and epidermal nevi.6
Differential diagnosis
Clinically, the lesion may appear very similar to the brown velvety plaques of acanthosis nigricans, but the latter can be quickly ruled out if the lesion disappears when cleansed with alcohol. The differential diagnosis also includes dermatosis neglecta, CARP, hyperpigmented tinea versicolor, and dirty neck syndrome of atopic dermatitis.4,7
Dermatosis neglecta is caused by poor hygiene with insufficient skin cleaning. In contrast with terra firma-forme dermatitis, it can be removed with soap and water. Patients with dermatosis neglecta lack a history of aggressive attempts at removal by scrubbing. Dermatosis neglecta looks similar to terra firma-forme dermatosis, but may have a waxy “cornflake-like” scale.2 It is most common in the elderly, but also can be seen in children, especially if they have had a cast or brace that prevented washing an area.8
CARP may present as brown hyperkeratotic or verrucous papules, but typically occurs on the trunk and has a reticulated pattern, which is less common in terra firma-forme dermatosis. Typically, it cannot be removed completely with alcohol10-12 and tends to respond best to treatment with minocycline.11
Hyperpigmented tinea versicolor lesions are usually more discrete and have fine scale, which becomes more prominent when the lesions are scraped. When analyzed with KOH under a microscope, hyphae and spores looking like spaghetti and meatballs can be seen.
Dirty neck syndrome is acquired hyperpigmentation that may develop on the neck of patients with atopic dermatitis. It cannot be removed with alcohol.4 It may result from frictional melanosis with melanin incontinence and post-inflammatory hyperpigmentation.12 The hyperpigmentation is patchy or rippled,12 whereas terra firma-forme dermatosis more often presents as plaques.
Etiology
The cause of terra firma-forme dermatosis is uncertain. It is thought to be a disorder of keratinocyte retention, in which delayed keratinocyte maturation causes prolonged cell-to-cell adhesion that impairs shedding.7,8 Discoloration may be due to retained melanin as well as sebum and dirt that build up along with excess keratinocytes.7-9 Alcohol is a better solvent for this retained material than soap and water. Patients using heavy emollients or oily soaps for conditions like atopic dermatitis may be relatively predisposed to the condition if emollients or oily soaps are not removed completely by bathing; the combination of underlying scale and retained skin care products may make skin more adhesive, disrupting normal keratinocyte shedding and allowing dirt and sebum to accumulate.1 Atopic dermatitis was the most common comorbid condition for terra firma-forme dermatosis in Berk et al.’s series (12 of 31 patients).4
Treatment
Wiping with 70% isopropyl alcohol is both diagnostic and therapeutic, removing the lesion completely. Recurrence is uncommon but possible.3 In rare instances of regularly recurrent lesions, the area can be wiped prophylactically with alcohol weekly.1
References
- Indian J Dermatol Venereol Leprol. 2012 May-Jun;78(3):358-60.
- Pediatr Dermatol. 2011 Jan-Feb;28(1):79-81.
- Dermatol Pract Concept. 2015 Jul; 5(3): 29-33.
- Pediatr Dermatol. 2012 May-Jun;29(3):297-300.
- Eur J Intern Med. 2016 Feb. doi: 10.1016/j.ejim.2016.02.009.
- J Cutan Pathol. 2012 Feb;39(2):300-1.
- Arch Dermatol. 1987;123(5):567-9.
- Pediatr Dermatol. 2015;32(2):e50-3.
- Arch Dermatol. 2010;146(6):679-80.
- Arch Dermatol. 2011 Feb;147(2):247-8.
- Am J Clin Dermatol. 2006;7(5):305-313.
- Dermatology. 2014;229:174-182.
Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield and Ms. Haddock state they have no relevant financial disclosures. Email them at [email protected].
By Ellen S. Haddock and Lawrence F. Eichenfield, MD
Terra firma-forme dermatosis
The easy removal of this patient’s persistent skin discoloration with an alcohol wipe confirms the diagnosis of terra firma-forme dermatosis. Terra firma-forme dermatosis is a benign skin condition in which dirt-like plaques develop on the skin despite normal hygiene. They cannot be removed with soap and water, but are removed easily with alcohol.
Terra firma means “solid earth” in Latin and refers to the dirt-like appearance of the lesion.1,2 The condition typically presents with brown or black hyperkeratotic plaques or papules. The hyperpigmented lesions may be papillomatous, verrucous (warty), or have a reticulated (net-like) distribution.3 The condition is usually asymptomatic but occasionally itchy.1 In the largest published series of 31 patients by Berk et al., the neck, ankles, and face were most commonly affected, but the condition can occur anywhere on the body.4 Lesions may be single or multiple and tend to be symmetrically distributed.4 The condition seems to be most common in young adults and adolescents, but can occur at any age and affects both genders equally.2,5
It seems to occur more commonly in darker skin, heavier patients, and concave body areas.3,6 The condition can last for months (median duration of 4 months in Berk et al.’s series), and by the time patients present to a physician they usually have tried aggressive scrubbing with multiple different soaps.4 As in this case, it is not uncommon for patients to receive an unnecessary work-up for diabetes due to concern for acanthosis nigricans.4 This work-up can be avoided if practitioners attempt removal with 70% isopropyl alcohol before ordering the diabetic work-up.
Biopsies are not typically performed (and should be abandoned if the lesion disappears when the site is cleansed with alcohol in preparation for biopsy), but hematoxylin and eosin staining shows lamellar hyperkeratosis, orthokeratotic whorls, and increased melanin in the basal layer and hyperkeratotic areas. Keratin globules are seen throughout the stratum corneum.7
Electron microscopy shows that the keratin lamellae is immature in some places, with incomplete keratinization and retention of desmosomal attachments.7 Overall, the histology is nonspecific and may be indistinguishable from other benign papillomatous conditions including confluent and reticulated papillomatosis (CARP), acanthosis nigricans, and epidermal nevi.6
Differential diagnosis
Clinically, the lesion may appear very similar to the brown velvety plaques of acanthosis nigricans, but the latter can be quickly ruled out if the lesion disappears when cleansed with alcohol. The differential diagnosis also includes dermatosis neglecta, CARP, hyperpigmented tinea versicolor, and dirty neck syndrome of atopic dermatitis.4,7
Dermatosis neglecta is caused by poor hygiene with insufficient skin cleaning. In contrast with terra firma-forme dermatitis, it can be removed with soap and water. Patients with dermatosis neglecta lack a history of aggressive attempts at removal by scrubbing. Dermatosis neglecta looks similar to terra firma-forme dermatosis, but may have a waxy “cornflake-like” scale.2 It is most common in the elderly, but also can be seen in children, especially if they have had a cast or brace that prevented washing an area.8
CARP may present as brown hyperkeratotic or verrucous papules, but typically occurs on the trunk and has a reticulated pattern, which is less common in terra firma-forme dermatosis. Typically, it cannot be removed completely with alcohol10-12 and tends to respond best to treatment with minocycline.11
Hyperpigmented tinea versicolor lesions are usually more discrete and have fine scale, which becomes more prominent when the lesions are scraped. When analyzed with KOH under a microscope, hyphae and spores looking like spaghetti and meatballs can be seen.
Dirty neck syndrome is acquired hyperpigmentation that may develop on the neck of patients with atopic dermatitis. It cannot be removed with alcohol.4 It may result from frictional melanosis with melanin incontinence and post-inflammatory hyperpigmentation.12 The hyperpigmentation is patchy or rippled,12 whereas terra firma-forme dermatosis more often presents as plaques.
Etiology
The cause of terra firma-forme dermatosis is uncertain. It is thought to be a disorder of keratinocyte retention, in which delayed keratinocyte maturation causes prolonged cell-to-cell adhesion that impairs shedding.7,8 Discoloration may be due to retained melanin as well as sebum and dirt that build up along with excess keratinocytes.7-9 Alcohol is a better solvent for this retained material than soap and water. Patients using heavy emollients or oily soaps for conditions like atopic dermatitis may be relatively predisposed to the condition if emollients or oily soaps are not removed completely by bathing; the combination of underlying scale and retained skin care products may make skin more adhesive, disrupting normal keratinocyte shedding and allowing dirt and sebum to accumulate.1 Atopic dermatitis was the most common comorbid condition for terra firma-forme dermatosis in Berk et al.’s series (12 of 31 patients).4
Treatment
Wiping with 70% isopropyl alcohol is both diagnostic and therapeutic, removing the lesion completely. Recurrence is uncommon but possible.3 In rare instances of regularly recurrent lesions, the area can be wiped prophylactically with alcohol weekly.1
References
- Indian J Dermatol Venereol Leprol. 2012 May-Jun;78(3):358-60.
- Pediatr Dermatol. 2011 Jan-Feb;28(1):79-81.
- Dermatol Pract Concept. 2015 Jul; 5(3): 29-33.
- Pediatr Dermatol. 2012 May-Jun;29(3):297-300.
- Eur J Intern Med. 2016 Feb. doi: 10.1016/j.ejim.2016.02.009.
- J Cutan Pathol. 2012 Feb;39(2):300-1.
- Arch Dermatol. 1987;123(5):567-9.
- Pediatr Dermatol. 2015;32(2):e50-3.
- Arch Dermatol. 2010;146(6):679-80.
- Arch Dermatol. 2011 Feb;147(2):247-8.
- Am J Clin Dermatol. 2006;7(5):305-313.
- Dermatology. 2014;229:174-182.
Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at Rady Children’s Hospital–San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital–San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Dr. Eichenfield and Ms. Haddock state they have no relevant financial disclosures. Email them at [email protected].
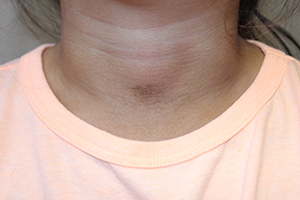
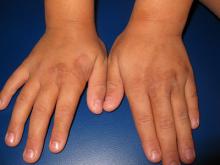
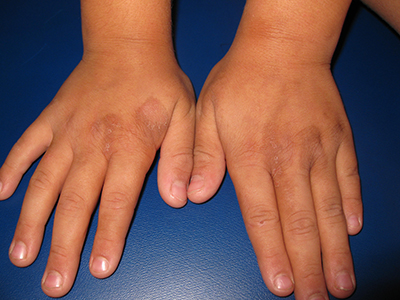
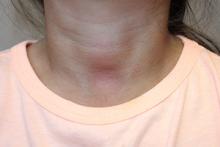
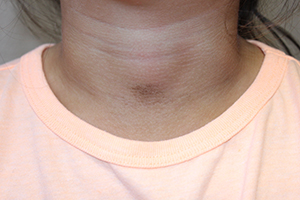
A 4-year-old girl presented for evaluation of a localized hyperpigmented rash of 4 months duration. The family noticed a localized, brown discoloration on her anterior neck. It was asymptomatic. Her mother tried scrubbing the area with several different soaps and baby wipes, but noted no change. Her medical history is notable for atopic dermatitis, well controlled with intermittent topical corticosteroids, and reactive airway disease. There is a family history of atopic dermatitis and type 2 diabetes. On physical exam, the patient is a non-obese (BMI 16 kg/m2) female with a 4 x 3–cm hyperpigmented, rough, slightly elevated plaque on her anterior neck. (See Before photo.) On close inspection, the plaque appears to be composed of hyperpigmented rugations. Viewed through a dermatoscope, the lesion’s polygonal brownish pigmentation looks like cobblestones (Can Med Assoc J. 2016;188[4]:285).The patient had no discoloration on the posterior neck, axillae, groin, or other locations. Blood work, ordered by a practitioner concerned about possible acanthosis nigricans, revealed: • CBC: within normal limits • Glucose: 82 mg/dL (normal 60-110) • Insulin: 3 mU/mL (normal less than 17) • Cholesterol: 136 mg/dL (normal less than 200) • Triglycerides: 79 mg/dL (normal 32-116) • Thyroid-stimulating hormone: 1.72 uIU/mL (normal 0.35-5) Rubbing the hyperpigmented area with a 70% isopropyl alcohol swab in clinic removed it completely.
Pediatric Dermatology Consult - April 2016
By Catalina Matiz, M.D., and David Ginsberg
Eczema cocksackium
Eczema coxsackium (EC) is an atypical variant of hand, foot, and mouth disease (HFMD) that occurs in patients with atopic dermatitis lesions. HFMD is a common viral exanthema seen in pediatric populations, predominantly in children under 5 years of age, and is most commonly caused by coxsackievirus A16 in the United States.1-3 Enterovirus 71 is also a common cause of HFMD in Asian countries, and in rare cases has been linked to more severe neurologic manifestations.2,3
Over the past several years, infection by coxsackievirus A6 (CVA6) has been responsible for HFMD outbreaks in multiple countries around the globe.1-5 This particular strain of coxsackievirus is known for causing atypical HFMD, which has several overlapping variations. The variants of atypical HFMD caused by CVA6 include eczema coxsackium (EC), widespread vesiculobullous and erosive lesions, purpuric lesions, and Gianotti-Crosti-like eruptions.2 EC is the result of a CVA6 infection in a child with a history of atopic dermatitis (AD). The infection in these patients is a more severe form of HFMD, presenting with eczema herpeticum (EH)-like lesions, with some combination of vesicles, papules, and erosions, in areas affected by atopic dermatitis.2,3 This is in contrast to classic HFMD, which commonly presents with oral erosions, and small vesicles limited to the hands, feet, and buttocks.1,2
In addition to the dermatologic manifestations, patients with EC also can present with other symptoms including fever and oropharyngeal pain.2,3 Any of the variants of atypical HFMD can present with lesions, including erosions, vesicles, and bullae, in areas of skin irritation such as sunburns, irritant dermatitis, lacerations, and tinea pedis.2
Differential diagnosis
The differential diagnosis for EC includes EH, impetiginized atopic dermatitis, varicella, and erythema multiforme major.2-4 EH is typically the highest on the differential because the presentation of EC is described as an EH-like rash and on occasion it is difficult to differentiate the two.2 When diagnosing EC, the history plays an important role, and it is imperative to question whether the patient has come into contact with anyone with cold sores to help rule out EH. It is recommended to perform testing for herpes simplex virus (HSV), either by culture, polymerase chain reaction (PCR), or direct immunofluorescence if the diagnosis is not straightforward.2,5 To confirm the diagnosis of EC, CVA6 PCR can be performed.4 The ideal sample would be from vesicular fluid because the same sample also could be used to test for HSV to help rule out EH, but throat swabs and stool samples also can be used.2 As lesions in EH can sometimes be superinfected with bacteria such as Staphylococcus aureus or streptococcus, a bacterial swab for culture and sensitivities is also recommended prior to starting empiric antimicrobial therapy.
Treatment
Because EC was only characterized several years ago, and the majority of the cases reported have been self-limiting, there has been little research done looking into the best treatment. The majority of the literature recommends treating symptomatically in a similar fashion to an eczema flare including daily moisturizing, bleach baths, and topical corticosteroids as needed.6 There has been a report that using wet wraps with lower-strength corticosteroids is an effective treatment method for severe cases.6 Treatment with acyclovir and oral antimicrobials is recommended if the diagnosis of EH is in question and HSV and bacterial testing are pending.
References
- JAMA. 2012;308(4):335.
- Pediatrics. 2013 Jul;132(1):e149-57.
- PIDJ. 2014 Apr;33(4):e92-98.
- Virol J. 2013 Jun 24;10:209.
- Lancet Infect Dis. 2014 Jan;14(1):83-6.
- J Allergy Clin Immunol Pract. 2014 Nov-Dec;2(6):803-4.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
By Catalina Matiz, M.D., and David Ginsberg
Eczema cocksackium
Eczema coxsackium (EC) is an atypical variant of hand, foot, and mouth disease (HFMD) that occurs in patients with atopic dermatitis lesions. HFMD is a common viral exanthema seen in pediatric populations, predominantly in children under 5 years of age, and is most commonly caused by coxsackievirus A16 in the United States.1-3 Enterovirus 71 is also a common cause of HFMD in Asian countries, and in rare cases has been linked to more severe neurologic manifestations.2,3
Over the past several years, infection by coxsackievirus A6 (CVA6) has been responsible for HFMD outbreaks in multiple countries around the globe.1-5 This particular strain of coxsackievirus is known for causing atypical HFMD, which has several overlapping variations. The variants of atypical HFMD caused by CVA6 include eczema coxsackium (EC), widespread vesiculobullous and erosive lesions, purpuric lesions, and Gianotti-Crosti-like eruptions.2 EC is the result of a CVA6 infection in a child with a history of atopic dermatitis (AD). The infection in these patients is a more severe form of HFMD, presenting with eczema herpeticum (EH)-like lesions, with some combination of vesicles, papules, and erosions, in areas affected by atopic dermatitis.2,3 This is in contrast to classic HFMD, which commonly presents with oral erosions, and small vesicles limited to the hands, feet, and buttocks.1,2
In addition to the dermatologic manifestations, patients with EC also can present with other symptoms including fever and oropharyngeal pain.2,3 Any of the variants of atypical HFMD can present with lesions, including erosions, vesicles, and bullae, in areas of skin irritation such as sunburns, irritant dermatitis, lacerations, and tinea pedis.2
Differential diagnosis
The differential diagnosis for EC includes EH, impetiginized atopic dermatitis, varicella, and erythema multiforme major.2-4 EH is typically the highest on the differential because the presentation of EC is described as an EH-like rash and on occasion it is difficult to differentiate the two.2 When diagnosing EC, the history plays an important role, and it is imperative to question whether the patient has come into contact with anyone with cold sores to help rule out EH. It is recommended to perform testing for herpes simplex virus (HSV), either by culture, polymerase chain reaction (PCR), or direct immunofluorescence if the diagnosis is not straightforward.2,5 To confirm the diagnosis of EC, CVA6 PCR can be performed.4 The ideal sample would be from vesicular fluid because the same sample also could be used to test for HSV to help rule out EH, but throat swabs and stool samples also can be used.2 As lesions in EH can sometimes be superinfected with bacteria such as Staphylococcus aureus or streptococcus, a bacterial swab for culture and sensitivities is also recommended prior to starting empiric antimicrobial therapy.
Treatment
Because EC was only characterized several years ago, and the majority of the cases reported have been self-limiting, there has been little research done looking into the best treatment. The majority of the literature recommends treating symptomatically in a similar fashion to an eczema flare including daily moisturizing, bleach baths, and topical corticosteroids as needed.6 There has been a report that using wet wraps with lower-strength corticosteroids is an effective treatment method for severe cases.6 Treatment with acyclovir and oral antimicrobials is recommended if the diagnosis of EH is in question and HSV and bacterial testing are pending.
References
- JAMA. 2012;308(4):335.
- Pediatrics. 2013 Jul;132(1):e149-57.
- PIDJ. 2014 Apr;33(4):e92-98.
- Virol J. 2013 Jun 24;10:209.
- Lancet Infect Dis. 2014 Jan;14(1):83-6.
- J Allergy Clin Immunol Pract. 2014 Nov-Dec;2(6):803-4.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
By Catalina Matiz, M.D., and David Ginsberg
Eczema cocksackium
Eczema coxsackium (EC) is an atypical variant of hand, foot, and mouth disease (HFMD) that occurs in patients with atopic dermatitis lesions. HFMD is a common viral exanthema seen in pediatric populations, predominantly in children under 5 years of age, and is most commonly caused by coxsackievirus A16 in the United States.1-3 Enterovirus 71 is also a common cause of HFMD in Asian countries, and in rare cases has been linked to more severe neurologic manifestations.2,3
Over the past several years, infection by coxsackievirus A6 (CVA6) has been responsible for HFMD outbreaks in multiple countries around the globe.1-5 This particular strain of coxsackievirus is known for causing atypical HFMD, which has several overlapping variations. The variants of atypical HFMD caused by CVA6 include eczema coxsackium (EC), widespread vesiculobullous and erosive lesions, purpuric lesions, and Gianotti-Crosti-like eruptions.2 EC is the result of a CVA6 infection in a child with a history of atopic dermatitis (AD). The infection in these patients is a more severe form of HFMD, presenting with eczema herpeticum (EH)-like lesions, with some combination of vesicles, papules, and erosions, in areas affected by atopic dermatitis.2,3 This is in contrast to classic HFMD, which commonly presents with oral erosions, and small vesicles limited to the hands, feet, and buttocks.1,2
In addition to the dermatologic manifestations, patients with EC also can present with other symptoms including fever and oropharyngeal pain.2,3 Any of the variants of atypical HFMD can present with lesions, including erosions, vesicles, and bullae, in areas of skin irritation such as sunburns, irritant dermatitis, lacerations, and tinea pedis.2
Differential diagnosis
The differential diagnosis for EC includes EH, impetiginized atopic dermatitis, varicella, and erythema multiforme major.2-4 EH is typically the highest on the differential because the presentation of EC is described as an EH-like rash and on occasion it is difficult to differentiate the two.2 When diagnosing EC, the history plays an important role, and it is imperative to question whether the patient has come into contact with anyone with cold sores to help rule out EH. It is recommended to perform testing for herpes simplex virus (HSV), either by culture, polymerase chain reaction (PCR), or direct immunofluorescence if the diagnosis is not straightforward.2,5 To confirm the diagnosis of EC, CVA6 PCR can be performed.4 The ideal sample would be from vesicular fluid because the same sample also could be used to test for HSV to help rule out EH, but throat swabs and stool samples also can be used.2 As lesions in EH can sometimes be superinfected with bacteria such as Staphylococcus aureus or streptococcus, a bacterial swab for culture and sensitivities is also recommended prior to starting empiric antimicrobial therapy.
Treatment
Because EC was only characterized several years ago, and the majority of the cases reported have been self-limiting, there has been little research done looking into the best treatment. The majority of the literature recommends treating symptomatically in a similar fashion to an eczema flare including daily moisturizing, bleach baths, and topical corticosteroids as needed.6 There has been a report that using wet wraps with lower-strength corticosteroids is an effective treatment method for severe cases.6 Treatment with acyclovir and oral antimicrobials is recommended if the diagnosis of EH is in question and HSV and bacterial testing are pending.
References
- JAMA. 2012;308(4):335.
- Pediatrics. 2013 Jul;132(1):e149-57.
- PIDJ. 2014 Apr;33(4):e92-98.
- Virol J. 2013 Jun 24;10:209.
- Lancet Infect Dis. 2014 Jan;14(1):83-6.
- J Allergy Clin Immunol Pract. 2014 Nov-Dec;2(6):803-4.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
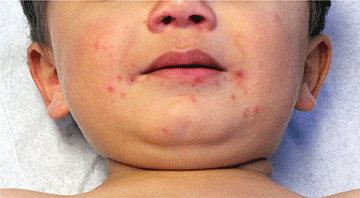
A 16-month-old male with a history of mild atopic dermatitis presents with a 2-day history of a rash on his lower extremities, arms, abdomen, and face. His father reports that it began as a single spot on his leg and has spread over the past 2 days. His other symptoms included a fever of 101.5 F, hypoactivity, and an unwillingness to eat solid foods. The patient has not had contact with anyone with cold sores. The sister is developing similar lesions on her face and knees. Physical exam On exam there are hemorrhagic crusted papules with an erythematous base, as well as grouped vesicles and eroded punched-out papules scattered on the lower extremities bilaterally. The area of involvement extends from his ankles to his hips, involving the posterior thighs, inguinal folds, and calves. There also are some hemorrhagic crusted erythematous grouped papules scattered on the upper extremities. On the face there are few crusted erythematous papules around the mouth. On the soles there are few erythematous oval papules.
Pediatric Dermatology Consult - March 2016
BY ELLEN S. HADDOCK AND LAWRENCE F. EICHENFIELD, M.D.
Pediatric Dermatology Consult: Pernio
Itchy localized areas of swelling on the bilateral hands with erythematous papules and crusting is consistent with pernio, as seen in the presentation of the teen boy described on p. 2. Pernio is a localized abnormal inflammatory response to cold and damp conditions, also known as chilblains, derived from the old English words for chill and sore.1 Damp air is thought to enhance the air conductivity of cold.2 Pernio typically presents with erythematous to blue-violet macules, papules, and nodules on the bilateral fingers and toes. When occurring on the feet, it is sometimes called trench foot or kibes.1 The nose and ears also can be affected.3 Lesions may develop 12-24 hours after exposure to damp or chilly weather, typically at temperatures above freezing.4
This patient’s pernio may have been triggered by recent stormy winter weather; being from within San Diego County, he had no exposure to snow. Lesions often are tender and may be accompanied by pruritus, pain, or a burning sensation, but they can be asymptomatic.3 Lesions may blister and ulcerate, and can become secondarily infected.5 Brownish or yellowish discoloration may be seen.1 Proposed diagnostic criteria requires localized erythema and swelling of the acral sites for more than 24 hours, as well as either onset during the cool months of the year or improvement with warming the affected area.3
Pernio is most common in adults, with a mean age of 38 years in one series.3 It also occurs in children but is uncommon, with only eight cases diagnosed at the University of Colorado over a 10-year period.6 Adult patients are primarily female,3 while the gender distribution in children is equal.6 Because pernio is triggered by cold and damp weather, it is not surprising that pernio occurs more often in cold climates.3 Raynaud’s phenomenon, smoking, and anorexia nervosa (due to lack of insulating fat) seem to be risk factors.1,3
Pernio typically is a benign primary disorder thought to result from cold-induced vasospasm, which leads to hypoxia and triggers a localized inflammatory reaction.7 Lesions of primary pernio usually resolve in a few weeks to months.4,6 However, pernio can be secondarily associated with systemic diseases including lupus (5% of patients in one of the largest series), non-lupus connective tissue disorders (4%), hematologic malignancy (3%), solid organ malignancy (2%), hepatitis, and Epstein-Barr virus.3 In these cases, pernio may be more persistent and hyperviscosity may contribute to its pathogenesis.8,9
Approximately a third of patients have laboratory abnormalities such as anemia, abnormal blood smear, autoantibodies, or serum monoclonal proteins,3 which may facilitate diagnosis of an underlying systemic disease. Not all pernio patients with connective tissue disease autoantibodies have clinical features of connective tissue disease, but these may manifest later.3,9 Laboratory abnormalities including cryoglobulinemia, cold agglutinins, rheumatoid factor, and antineutrophilic antibody also are seen in children;6,10 however, there are no reports of childhood pernio being associated with connective tissue disease or other systemic illness, although long-term studies are lacking.
When lesions are biopsied, histopathology shows nonspecific dermal edema with superficial and deep perivascular lymphocytic infiltrate.3,4
Differential diagnosis
The differential diagnosis for pernio includes Raynaud’s phenomenon, frostbite, herpetic whitlow, and purpura caused by cryoproteinemia. In Raynaud’s, pallor and cyanosis are followed by erythema, but the discoloration is more sharply demarcated and episodes are typically shorter, lasting hours rather than days.1,8 In this case, progression of the lesions over weeks and the lack of sudden skin color change when holding a cold drink make Raynaud’s unlikely. Frostbite, in which the tissue freezes and necroses, can be distinguished by history.11
When lesions have blistered, herpetic whitlow also may be on the differential, but herpetic whitlow vesicles typically cluster or coalesce into a single bulla while pernio lesions are more discrete. Cryoproteinemia causes lesions on acral sites exposed to the cold, but its onset is sudden and lesions are purpuric with a reticular (net-like) pattern.12 In adults, cutaneous thromboemboli also can present similarly to pernio,13 but thromboemboli are unlikely in children.
Clinical findings of pernio in the setting of lupus erythematosus is called chilblains lupus erythematosus. Confusingly, the condition called lupus pernio is actually a cutaneous manifestation of sarcoidosis, not lupus, and its erythematous or violaceous lesions occur on the nose and central face, not the hands and feet.13
Work-up
For pernio patients without systemic symptoms or signs of underlying systemic disease, laboratory workup or skin biopsy are not necessary.3,4 When history or physical exam is concerning for a systemic condition, preliminary workup should include complete blood count, peripheral blood smear, serum protein electrophoresis, cold agglutinins, and antinuclear antibody.3 Rheumatoid factor, antiphospholipid antibodies, and cryoglobulins also can be considered. Laboratory workup should be performed if pernio persists beyond the cold season, as persistent pernio may be associated with systemic illness.4,9
This patient’s recent weight loss was concerning for underlying systemic disease, so a laboratory workup including complete blood count, serum protein electrophoresis, cold agglutinins, antinuclear antibody, rheumatoid factor, and cryoglobulins was performed. Cryoglobulinemia was detected. All other lab values were within normal limits. Although cryoglobulinemia is rare in adults,3,14 it was detected in approximately 40% of the children in two pediatric series.6,10 Cryoglobulins, which can be produced in response to viral infection, may suggest a precipitating viral illness, with transient cryoproteinemia amplifying cold injury.6 Although the significance of laboratory abnormalities in pediatric pernio is unclear, because associated systemic disease has not been reported in children, some practitioners recommend long-term monitoring in light of the association between lab abnormalities and systemic disease in adults.10
Treatment
Most pernio (82% in one series) resolves when affected skin is warmed and dried, without additional treatment required.3 Corticosteroids, such as 0.1% triamcinolone cream, sometimes are given to hasten the healing of the lesions, but their benefit is unproven.6 The second-line treatment for persistent pernio is calcium channel blockers such as nifedipine.3,15 This patient’s pernio quickly improved after he began wearing gloves to keep his hands warm. On re-examination 2 weeks later, his hands were warm and the erythematous nodules had resolved, leaving only some scale at the sites of prior lesions (Figure 3). Some patients relapse annually during the cool months.11
References
- Pediatrics. 2005 Sep;116(3):e472-5. doi: 10.1542/peds.2004-2681.
- Journal of Medical Case Reports. 2014 Nov;8:381. doi: 10.1186/1752-1947-8-381.
- Mayo Clin Proc. 2014 Feb;89(2):207-15.
- Clin Exp Dermatol. 2012 Dec;37(8):844-9.
- J Paediatr Child Health. 2013 Feb;49(2):144-7.
- Pediatr Dermatol. 2000 Mar-Apr;17(2):97-9.
- Am J Med. 2009 Dec;122(12):1152-5.
- J Amer Acad Dermatol. 1990 Aug;23(Part 1):257-62.
- Medicine (Baltimore). 2001 May;80(3):180-8.
- Arch Dis Child. 2010 Jul;95(7):567-8.
- Br J Dermatol. 2010 Sep;163(3):645-6.
- Cutaneous manifestations of microvascular occlusion syndromes, in “Dermatology,” 3rd ed. (Philadelphia, 2012, pp 373-4).
- Environmental and sports-related skin diseases, in “Dermatology,” 3rd ed. (Philadelphia, 2012).
- J Am Acad Dermatol. 2010 Jun;62(6):e21-2.
- Br J Dermatol. 1989 Feb;120(2):267-75.
Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at Rady Children’s Hospital, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, and Professor of Medicine and Pediatrics at the University of California, San Diego. Dr. Eichenfield and Ms. Haddock said they have no relevant financial disclosures.
BY ELLEN S. HADDOCK AND LAWRENCE F. EICHENFIELD, M.D.
Pediatric Dermatology Consult: Pernio
Itchy localized areas of swelling on the bilateral hands with erythematous papules and crusting is consistent with pernio, as seen in the presentation of the teen boy described on p. 2. Pernio is a localized abnormal inflammatory response to cold and damp conditions, also known as chilblains, derived from the old English words for chill and sore.1 Damp air is thought to enhance the air conductivity of cold.2 Pernio typically presents with erythematous to blue-violet macules, papules, and nodules on the bilateral fingers and toes. When occurring on the feet, it is sometimes called trench foot or kibes.1 The nose and ears also can be affected.3 Lesions may develop 12-24 hours after exposure to damp or chilly weather, typically at temperatures above freezing.4
This patient’s pernio may have been triggered by recent stormy winter weather; being from within San Diego County, he had no exposure to snow. Lesions often are tender and may be accompanied by pruritus, pain, or a burning sensation, but they can be asymptomatic.3 Lesions may blister and ulcerate, and can become secondarily infected.5 Brownish or yellowish discoloration may be seen.1 Proposed diagnostic criteria requires localized erythema and swelling of the acral sites for more than 24 hours, as well as either onset during the cool months of the year or improvement with warming the affected area.3
Pernio is most common in adults, with a mean age of 38 years in one series.3 It also occurs in children but is uncommon, with only eight cases diagnosed at the University of Colorado over a 10-year period.6 Adult patients are primarily female,3 while the gender distribution in children is equal.6 Because pernio is triggered by cold and damp weather, it is not surprising that pernio occurs more often in cold climates.3 Raynaud’s phenomenon, smoking, and anorexia nervosa (due to lack of insulating fat) seem to be risk factors.1,3
Pernio typically is a benign primary disorder thought to result from cold-induced vasospasm, which leads to hypoxia and triggers a localized inflammatory reaction.7 Lesions of primary pernio usually resolve in a few weeks to months.4,6 However, pernio can be secondarily associated with systemic diseases including lupus (5% of patients in one of the largest series), non-lupus connective tissue disorders (4%), hematologic malignancy (3%), solid organ malignancy (2%), hepatitis, and Epstein-Barr virus.3 In these cases, pernio may be more persistent and hyperviscosity may contribute to its pathogenesis.8,9
Approximately a third of patients have laboratory abnormalities such as anemia, abnormal blood smear, autoantibodies, or serum monoclonal proteins,3 which may facilitate diagnosis of an underlying systemic disease. Not all pernio patients with connective tissue disease autoantibodies have clinical features of connective tissue disease, but these may manifest later.3,9 Laboratory abnormalities including cryoglobulinemia, cold agglutinins, rheumatoid factor, and antineutrophilic antibody also are seen in children;6,10 however, there are no reports of childhood pernio being associated with connective tissue disease or other systemic illness, although long-term studies are lacking.
When lesions are biopsied, histopathology shows nonspecific dermal edema with superficial and deep perivascular lymphocytic infiltrate.3,4
Differential diagnosis
The differential diagnosis for pernio includes Raynaud’s phenomenon, frostbite, herpetic whitlow, and purpura caused by cryoproteinemia. In Raynaud’s, pallor and cyanosis are followed by erythema, but the discoloration is more sharply demarcated and episodes are typically shorter, lasting hours rather than days.1,8 In this case, progression of the lesions over weeks and the lack of sudden skin color change when holding a cold drink make Raynaud’s unlikely. Frostbite, in which the tissue freezes and necroses, can be distinguished by history.11
When lesions have blistered, herpetic whitlow also may be on the differential, but herpetic whitlow vesicles typically cluster or coalesce into a single bulla while pernio lesions are more discrete. Cryoproteinemia causes lesions on acral sites exposed to the cold, but its onset is sudden and lesions are purpuric with a reticular (net-like) pattern.12 In adults, cutaneous thromboemboli also can present similarly to pernio,13 but thromboemboli are unlikely in children.
Clinical findings of pernio in the setting of lupus erythematosus is called chilblains lupus erythematosus. Confusingly, the condition called lupus pernio is actually a cutaneous manifestation of sarcoidosis, not lupus, and its erythematous or violaceous lesions occur on the nose and central face, not the hands and feet.13
Work-up
For pernio patients without systemic symptoms or signs of underlying systemic disease, laboratory workup or skin biopsy are not necessary.3,4 When history or physical exam is concerning for a systemic condition, preliminary workup should include complete blood count, peripheral blood smear, serum protein electrophoresis, cold agglutinins, and antinuclear antibody.3 Rheumatoid factor, antiphospholipid antibodies, and cryoglobulins also can be considered. Laboratory workup should be performed if pernio persists beyond the cold season, as persistent pernio may be associated with systemic illness.4,9
This patient’s recent weight loss was concerning for underlying systemic disease, so a laboratory workup including complete blood count, serum protein electrophoresis, cold agglutinins, antinuclear antibody, rheumatoid factor, and cryoglobulins was performed. Cryoglobulinemia was detected. All other lab values were within normal limits. Although cryoglobulinemia is rare in adults,3,14 it was detected in approximately 40% of the children in two pediatric series.6,10 Cryoglobulins, which can be produced in response to viral infection, may suggest a precipitating viral illness, with transient cryoproteinemia amplifying cold injury.6 Although the significance of laboratory abnormalities in pediatric pernio is unclear, because associated systemic disease has not been reported in children, some practitioners recommend long-term monitoring in light of the association between lab abnormalities and systemic disease in adults.10
Treatment
Most pernio (82% in one series) resolves when affected skin is warmed and dried, without additional treatment required.3 Corticosteroids, such as 0.1% triamcinolone cream, sometimes are given to hasten the healing of the lesions, but their benefit is unproven.6 The second-line treatment for persistent pernio is calcium channel blockers such as nifedipine.3,15 This patient’s pernio quickly improved after he began wearing gloves to keep his hands warm. On re-examination 2 weeks later, his hands were warm and the erythematous nodules had resolved, leaving only some scale at the sites of prior lesions (Figure 3). Some patients relapse annually during the cool months.11
References
- Pediatrics. 2005 Sep;116(3):e472-5. doi: 10.1542/peds.2004-2681.
- Journal of Medical Case Reports. 2014 Nov;8:381. doi: 10.1186/1752-1947-8-381.
- Mayo Clin Proc. 2014 Feb;89(2):207-15.
- Clin Exp Dermatol. 2012 Dec;37(8):844-9.
- J Paediatr Child Health. 2013 Feb;49(2):144-7.
- Pediatr Dermatol. 2000 Mar-Apr;17(2):97-9.
- Am J Med. 2009 Dec;122(12):1152-5.
- J Amer Acad Dermatol. 1990 Aug;23(Part 1):257-62.
- Medicine (Baltimore). 2001 May;80(3):180-8.
- Arch Dis Child. 2010 Jul;95(7):567-8.
- Br J Dermatol. 2010 Sep;163(3):645-6.
- Cutaneous manifestations of microvascular occlusion syndromes, in “Dermatology,” 3rd ed. (Philadelphia, 2012, pp 373-4).
- Environmental and sports-related skin diseases, in “Dermatology,” 3rd ed. (Philadelphia, 2012).
- J Am Acad Dermatol. 2010 Jun;62(6):e21-2.
- Br J Dermatol. 1989 Feb;120(2):267-75.
Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at Rady Children’s Hospital, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, and Professor of Medicine and Pediatrics at the University of California, San Diego. Dr. Eichenfield and Ms. Haddock said they have no relevant financial disclosures.
BY ELLEN S. HADDOCK AND LAWRENCE F. EICHENFIELD, M.D.
Pediatric Dermatology Consult: Pernio
Itchy localized areas of swelling on the bilateral hands with erythematous papules and crusting is consistent with pernio, as seen in the presentation of the teen boy described on p. 2. Pernio is a localized abnormal inflammatory response to cold and damp conditions, also known as chilblains, derived from the old English words for chill and sore.1 Damp air is thought to enhance the air conductivity of cold.2 Pernio typically presents with erythematous to blue-violet macules, papules, and nodules on the bilateral fingers and toes. When occurring on the feet, it is sometimes called trench foot or kibes.1 The nose and ears also can be affected.3 Lesions may develop 12-24 hours after exposure to damp or chilly weather, typically at temperatures above freezing.4
This patient’s pernio may have been triggered by recent stormy winter weather; being from within San Diego County, he had no exposure to snow. Lesions often are tender and may be accompanied by pruritus, pain, or a burning sensation, but they can be asymptomatic.3 Lesions may blister and ulcerate, and can become secondarily infected.5 Brownish or yellowish discoloration may be seen.1 Proposed diagnostic criteria requires localized erythema and swelling of the acral sites for more than 24 hours, as well as either onset during the cool months of the year or improvement with warming the affected area.3
Pernio is most common in adults, with a mean age of 38 years in one series.3 It also occurs in children but is uncommon, with only eight cases diagnosed at the University of Colorado over a 10-year period.6 Adult patients are primarily female,3 while the gender distribution in children is equal.6 Because pernio is triggered by cold and damp weather, it is not surprising that pernio occurs more often in cold climates.3 Raynaud’s phenomenon, smoking, and anorexia nervosa (due to lack of insulating fat) seem to be risk factors.1,3
Pernio typically is a benign primary disorder thought to result from cold-induced vasospasm, which leads to hypoxia and triggers a localized inflammatory reaction.7 Lesions of primary pernio usually resolve in a few weeks to months.4,6 However, pernio can be secondarily associated with systemic diseases including lupus (5% of patients in one of the largest series), non-lupus connective tissue disorders (4%), hematologic malignancy (3%), solid organ malignancy (2%), hepatitis, and Epstein-Barr virus.3 In these cases, pernio may be more persistent and hyperviscosity may contribute to its pathogenesis.8,9
Approximately a third of patients have laboratory abnormalities such as anemia, abnormal blood smear, autoantibodies, or serum monoclonal proteins,3 which may facilitate diagnosis of an underlying systemic disease. Not all pernio patients with connective tissue disease autoantibodies have clinical features of connective tissue disease, but these may manifest later.3,9 Laboratory abnormalities including cryoglobulinemia, cold agglutinins, rheumatoid factor, and antineutrophilic antibody also are seen in children;6,10 however, there are no reports of childhood pernio being associated with connective tissue disease or other systemic illness, although long-term studies are lacking.
When lesions are biopsied, histopathology shows nonspecific dermal edema with superficial and deep perivascular lymphocytic infiltrate.3,4
Differential diagnosis
The differential diagnosis for pernio includes Raynaud’s phenomenon, frostbite, herpetic whitlow, and purpura caused by cryoproteinemia. In Raynaud’s, pallor and cyanosis are followed by erythema, but the discoloration is more sharply demarcated and episodes are typically shorter, lasting hours rather than days.1,8 In this case, progression of the lesions over weeks and the lack of sudden skin color change when holding a cold drink make Raynaud’s unlikely. Frostbite, in which the tissue freezes and necroses, can be distinguished by history.11
When lesions have blistered, herpetic whitlow also may be on the differential, but herpetic whitlow vesicles typically cluster or coalesce into a single bulla while pernio lesions are more discrete. Cryoproteinemia causes lesions on acral sites exposed to the cold, but its onset is sudden and lesions are purpuric with a reticular (net-like) pattern.12 In adults, cutaneous thromboemboli also can present similarly to pernio,13 but thromboemboli are unlikely in children.
Clinical findings of pernio in the setting of lupus erythematosus is called chilblains lupus erythematosus. Confusingly, the condition called lupus pernio is actually a cutaneous manifestation of sarcoidosis, not lupus, and its erythematous or violaceous lesions occur on the nose and central face, not the hands and feet.13
Work-up
For pernio patients without systemic symptoms or signs of underlying systemic disease, laboratory workup or skin biopsy are not necessary.3,4 When history or physical exam is concerning for a systemic condition, preliminary workup should include complete blood count, peripheral blood smear, serum protein electrophoresis, cold agglutinins, and antinuclear antibody.3 Rheumatoid factor, antiphospholipid antibodies, and cryoglobulins also can be considered. Laboratory workup should be performed if pernio persists beyond the cold season, as persistent pernio may be associated with systemic illness.4,9
This patient’s recent weight loss was concerning for underlying systemic disease, so a laboratory workup including complete blood count, serum protein electrophoresis, cold agglutinins, antinuclear antibody, rheumatoid factor, and cryoglobulins was performed. Cryoglobulinemia was detected. All other lab values were within normal limits. Although cryoglobulinemia is rare in adults,3,14 it was detected in approximately 40% of the children in two pediatric series.6,10 Cryoglobulins, which can be produced in response to viral infection, may suggest a precipitating viral illness, with transient cryoproteinemia amplifying cold injury.6 Although the significance of laboratory abnormalities in pediatric pernio is unclear, because associated systemic disease has not been reported in children, some practitioners recommend long-term monitoring in light of the association between lab abnormalities and systemic disease in adults.10
Treatment
Most pernio (82% in one series) resolves when affected skin is warmed and dried, without additional treatment required.3 Corticosteroids, such as 0.1% triamcinolone cream, sometimes are given to hasten the healing of the lesions, but their benefit is unproven.6 The second-line treatment for persistent pernio is calcium channel blockers such as nifedipine.3,15 This patient’s pernio quickly improved after he began wearing gloves to keep his hands warm. On re-examination 2 weeks later, his hands were warm and the erythematous nodules had resolved, leaving only some scale at the sites of prior lesions (Figure 3). Some patients relapse annually during the cool months.11
References
- Pediatrics. 2005 Sep;116(3):e472-5. doi: 10.1542/peds.2004-2681.
- Journal of Medical Case Reports. 2014 Nov;8:381. doi: 10.1186/1752-1947-8-381.
- Mayo Clin Proc. 2014 Feb;89(2):207-15.
- Clin Exp Dermatol. 2012 Dec;37(8):844-9.
- J Paediatr Child Health. 2013 Feb;49(2):144-7.
- Pediatr Dermatol. 2000 Mar-Apr;17(2):97-9.
- Am J Med. 2009 Dec;122(12):1152-5.
- J Amer Acad Dermatol. 1990 Aug;23(Part 1):257-62.
- Medicine (Baltimore). 2001 May;80(3):180-8.
- Arch Dis Child. 2010 Jul;95(7):567-8.
- Br J Dermatol. 2010 Sep;163(3):645-6.
- Cutaneous manifestations of microvascular occlusion syndromes, in “Dermatology,” 3rd ed. (Philadelphia, 2012, pp 373-4).
- Environmental and sports-related skin diseases, in “Dermatology,” 3rd ed. (Philadelphia, 2012).
- J Am Acad Dermatol. 2010 Jun;62(6):e21-2.
- Br J Dermatol. 1989 Feb;120(2):267-75.
Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at Rady Children’s Hospital, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital, San Diego, and Professor of Medicine and Pediatrics at the University of California, San Diego. Dr. Eichenfield and Ms. Haddock said they have no relevant financial disclosures.

A 13-year-old male presents with a rash that began as purplish spots on several fingers of the right hand and progressed over 2 months to involve all ten fingers. No other parts of his body are affected, and he had never experienced anything like this before. The fingers are intermittently painful and swollen. He is otherwise well, playing video games regularly and playing soccer with normal energy, although he states that a few of his soccer games have been canceled due to winter rains. He denies any sudden changes in the color of his hands with exposure to cold or holding cold drink bottles or cans (no “white, blue, and red changes”). He does not have any muscle or joint aches, but his mom reports that he has lost several pounds over the past 3 months. On physical exam, he has fifteen red, violaceous papules with surrounding swelling and erythema scattered on his dorsal fingers (Figure 1) and several similar lesions on his volar fingers. A few of the lesions are crusted. The skin of his volar fingers is dry, with some fine scale and several thickened, yellow-brown areas (Figure 2). His hands are very cold. His fingernails and feet are normal, and he has no lymphadenopathy.
Pediatric Dermatology Consult - February 2016
By Catalina Matiz, M.D., and David Ginsberg
Nummular eczema
Nummular eczema is not an uncommon dermatosis that presents in pediatric and adult patients; its name, which derives from the Latin word nummulus (coin-like), refers to the coined-shape plaques that characterize this condition. It also has been referred to as discoid eczema and nummular dermatitis.1
The lesions begin as erythematous papules and vesicles that extend into larger oval or circular plaques that often become crusted, and can later progress to dry and scaly plaques.1,2 Patients often complain of intense pruritus.1 The lesions can be single or multiple, and more commonly occur on the extensor extremities as well as the trunk, and rarely affect the neck and the head.1-3 The pathophysiology of nummular eczema is not fully understood. It can occur in patients that exhibit atopic manifestations such as atopic dermatitis and other allergies, but there has been no clear link found between nummular eczema and atopy.3,4
Many theories exist implicating causative factors including Staphylococcus aureus colonization and xerosis.1 Similarly, some physicians believe that patch testing can be useful in these patients because of the potential for exacerbation caused by environmental allergens, but there is still no agreement on the ultimate cause.5 There is a higher incidence in males than females, and in the pediatric population, it is more common among “school aged” children between the ages of 2-12.6 Overall, nummular eczema is more commonly seen in adults, but it can occur at any age.2,3,6
Differential diagnosis
Nummular eczema is commonly mistaken as tinea corporis.1 The coined shape lesions, from which nummular eczema gets its name, can resemble the characteristic annular shape plaques of “ring worm,” but a potassium hydroxide (KOH) test or a fungal culture are simple ways to differentiate between the two conditions.
Nummular eczema occasionally can be confused for psoriasis as both entities can present with oval plaques. Psoriasis lesions tend to be pinker and less erythematous than nummular eczema lesions and most psoriasis plaques present with a characteristic silver scale.7 Clinically, nummular eczema is frequently associated with extreme pruritus, while in psoriasis the pruritus is less prominent.7
A biopsy would yield a more definitive diagnosis in difficult cases. Histologically, nummular eczema resembles other forms of spongiotic dermatitis, while psoriasis has very distinct histological features.7 Differentiating between contact dermatitis and nummular eczema relies on a thorough history of known allergies and potential exposure to environmental allergens. If history alone does not yield a definitive diagnosis and a suspicion for contact allergy is high, patch testing could help support one diagnosis over the other.5
Treatment
The generally accepted first line therapy includes mid to high potency topical corticosteroids in an ointment preparation or else under occlusion.1,4 Other topical agents used include tar preparations and calcineurin inhibitors.4 Intralesional corticosteroid injection can be used to treat isolated lesions that fail to respond to topical treatments.4
As with almost all manifestations of dermatitis, general gentle skin care measures and daily moisturizing are recommended.1 For more severe cases in older children, narrow-band UVB light therapy can be helpful.1 Due to their efficacy in treatment of other forms of refractory dermatitis, systemic therapy with cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate can be used in cases in which phototherapy fails or is not accessible.4
In cases recalcitrant to topical therapies, secondary staphylococcal infection always should be ruled out and treated with systemic antimicrobials such as first generation cephalosporins.1
References
- Eczematous eruptions in childhood in “Hurwitz Clinical Pediatric Dermatology,” 4th ed. (New York, N.Y.: Elsevier, pp. 59-60
- Acta Derm Venereol. 1961;41:453-60.
- Acta Derm Venereol. 1969;49(2):189-96.
- Australas J Dermatol. 2010 May;51(2):128-30.
- Contact Dermatitis. 1997 May;36(5):261-4.
- Ped Dermatol. 2012 Oct;29(5):580-3.
- Dermatol Ther. 2006 Mar-Apr;19(2):73-82.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
By Catalina Matiz, M.D., and David Ginsberg
Nummular eczema
Nummular eczema is not an uncommon dermatosis that presents in pediatric and adult patients; its name, which derives from the Latin word nummulus (coin-like), refers to the coined-shape plaques that characterize this condition. It also has been referred to as discoid eczema and nummular dermatitis.1
The lesions begin as erythematous papules and vesicles that extend into larger oval or circular plaques that often become crusted, and can later progress to dry and scaly plaques.1,2 Patients often complain of intense pruritus.1 The lesions can be single or multiple, and more commonly occur on the extensor extremities as well as the trunk, and rarely affect the neck and the head.1-3 The pathophysiology of nummular eczema is not fully understood. It can occur in patients that exhibit atopic manifestations such as atopic dermatitis and other allergies, but there has been no clear link found between nummular eczema and atopy.3,4
Many theories exist implicating causative factors including Staphylococcus aureus colonization and xerosis.1 Similarly, some physicians believe that patch testing can be useful in these patients because of the potential for exacerbation caused by environmental allergens, but there is still no agreement on the ultimate cause.5 There is a higher incidence in males than females, and in the pediatric population, it is more common among “school aged” children between the ages of 2-12.6 Overall, nummular eczema is more commonly seen in adults, but it can occur at any age.2,3,6
Differential diagnosis
Nummular eczema is commonly mistaken as tinea corporis.1 The coined shape lesions, from which nummular eczema gets its name, can resemble the characteristic annular shape plaques of “ring worm,” but a potassium hydroxide (KOH) test or a fungal culture are simple ways to differentiate between the two conditions.
Nummular eczema occasionally can be confused for psoriasis as both entities can present with oval plaques. Psoriasis lesions tend to be pinker and less erythematous than nummular eczema lesions and most psoriasis plaques present with a characteristic silver scale.7 Clinically, nummular eczema is frequently associated with extreme pruritus, while in psoriasis the pruritus is less prominent.7
A biopsy would yield a more definitive diagnosis in difficult cases. Histologically, nummular eczema resembles other forms of spongiotic dermatitis, while psoriasis has very distinct histological features.7 Differentiating between contact dermatitis and nummular eczema relies on a thorough history of known allergies and potential exposure to environmental allergens. If history alone does not yield a definitive diagnosis and a suspicion for contact allergy is high, patch testing could help support one diagnosis over the other.5
Treatment
The generally accepted first line therapy includes mid to high potency topical corticosteroids in an ointment preparation or else under occlusion.1,4 Other topical agents used include tar preparations and calcineurin inhibitors.4 Intralesional corticosteroid injection can be used to treat isolated lesions that fail to respond to topical treatments.4
As with almost all manifestations of dermatitis, general gentle skin care measures and daily moisturizing are recommended.1 For more severe cases in older children, narrow-band UVB light therapy can be helpful.1 Due to their efficacy in treatment of other forms of refractory dermatitis, systemic therapy with cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate can be used in cases in which phototherapy fails or is not accessible.4
In cases recalcitrant to topical therapies, secondary staphylococcal infection always should be ruled out and treated with systemic antimicrobials such as first generation cephalosporins.1
References
- Eczematous eruptions in childhood in “Hurwitz Clinical Pediatric Dermatology,” 4th ed. (New York, N.Y.: Elsevier, pp. 59-60
- Acta Derm Venereol. 1961;41:453-60.
- Acta Derm Venereol. 1969;49(2):189-96.
- Australas J Dermatol. 2010 May;51(2):128-30.
- Contact Dermatitis. 1997 May;36(5):261-4.
- Ped Dermatol. 2012 Oct;29(5):580-3.
- Dermatol Ther. 2006 Mar-Apr;19(2):73-82.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
By Catalina Matiz, M.D., and David Ginsberg
Nummular eczema
Nummular eczema is not an uncommon dermatosis that presents in pediatric and adult patients; its name, which derives from the Latin word nummulus (coin-like), refers to the coined-shape plaques that characterize this condition. It also has been referred to as discoid eczema and nummular dermatitis.1
The lesions begin as erythematous papules and vesicles that extend into larger oval or circular plaques that often become crusted, and can later progress to dry and scaly plaques.1,2 Patients often complain of intense pruritus.1 The lesions can be single or multiple, and more commonly occur on the extensor extremities as well as the trunk, and rarely affect the neck and the head.1-3 The pathophysiology of nummular eczema is not fully understood. It can occur in patients that exhibit atopic manifestations such as atopic dermatitis and other allergies, but there has been no clear link found between nummular eczema and atopy.3,4
Many theories exist implicating causative factors including Staphylococcus aureus colonization and xerosis.1 Similarly, some physicians believe that patch testing can be useful in these patients because of the potential for exacerbation caused by environmental allergens, but there is still no agreement on the ultimate cause.5 There is a higher incidence in males than females, and in the pediatric population, it is more common among “school aged” children between the ages of 2-12.6 Overall, nummular eczema is more commonly seen in adults, but it can occur at any age.2,3,6
Differential diagnosis
Nummular eczema is commonly mistaken as tinea corporis.1 The coined shape lesions, from which nummular eczema gets its name, can resemble the characteristic annular shape plaques of “ring worm,” but a potassium hydroxide (KOH) test or a fungal culture are simple ways to differentiate between the two conditions.
Nummular eczema occasionally can be confused for psoriasis as both entities can present with oval plaques. Psoriasis lesions tend to be pinker and less erythematous than nummular eczema lesions and most psoriasis plaques present with a characteristic silver scale.7 Clinically, nummular eczema is frequently associated with extreme pruritus, while in psoriasis the pruritus is less prominent.7
A biopsy would yield a more definitive diagnosis in difficult cases. Histologically, nummular eczema resembles other forms of spongiotic dermatitis, while psoriasis has very distinct histological features.7 Differentiating between contact dermatitis and nummular eczema relies on a thorough history of known allergies and potential exposure to environmental allergens. If history alone does not yield a definitive diagnosis and a suspicion for contact allergy is high, patch testing could help support one diagnosis over the other.5
Treatment
The generally accepted first line therapy includes mid to high potency topical corticosteroids in an ointment preparation or else under occlusion.1,4 Other topical agents used include tar preparations and calcineurin inhibitors.4 Intralesional corticosteroid injection can be used to treat isolated lesions that fail to respond to topical treatments.4
As with almost all manifestations of dermatitis, general gentle skin care measures and daily moisturizing are recommended.1 For more severe cases in older children, narrow-band UVB light therapy can be helpful.1 Due to their efficacy in treatment of other forms of refractory dermatitis, systemic therapy with cyclosporine, azathioprine, mycophenolate mofetil, and methotrexate can be used in cases in which phototherapy fails or is not accessible.4
In cases recalcitrant to topical therapies, secondary staphylococcal infection always should be ruled out and treated with systemic antimicrobials such as first generation cephalosporins.1
References
- Eczematous eruptions in childhood in “Hurwitz Clinical Pediatric Dermatology,” 4th ed. (New York, N.Y.: Elsevier, pp. 59-60
- Acta Derm Venereol. 1961;41:453-60.
- Acta Derm Venereol. 1969;49(2):189-96.
- Australas J Dermatol. 2010 May;51(2):128-30.
- Contact Dermatitis. 1997 May;36(5):261-4.
- Ped Dermatol. 2012 Oct;29(5):580-3.
- Dermatol Ther. 2006 Mar-Apr;19(2):73-82.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
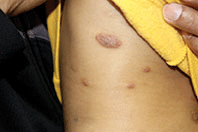
A 9-month-old male with no significant previous medical history presents with a very itchy rash that has been present for 6 weeks. His mother reports that the lesions began as small red bumps on the extremities and his torso, that developed over the course of a few weeks into large round, red, very pruritic plaques. He has been treated with an antifungal cream for several weeks without resolution, and most recently his mother has been applying hydrocortisone 2.5% cream with no improvement either. On exam, the patient is a well appearing infant, who is visibly irritated due to the pruritus accompanying his rash. There are several 1-cm to 4-cm round and oval dry, scaly, erythematous plaques on the trunk (see photo) and a few on the extremities. There is no generalized xerosis.
Pediatric Dermatology Consult - January 2016
By Ellen S. Haddock and Lawrence F. Eichenfield, M.D.
Urticaria multiforme
Although not the most classic presentation, this patient’s migrating rash is most consistent with urticaria (hives). Urticaria is dermal edema which causes transient edematous and usually pruritic wheals.1,2 Each individual lesion lasts less than 24 hours and disappears without leaving a mark. Urticaria is caused by mast cell activation, which leads to release of antihistamines and other substances that increase capillary and venule permeability, allowing fluid to leak into the extravascular space.1 In children, mast cell activation is usually triggered by infections, drugs, or foods.1
Classic urticaria consists of large, pruritic plaques and may be associated with airway edema. However, urticaria also can present with annular and polycyclic lesions, which may be less pruritic and are not associated with airway edema.3 This “multiple redness” is distinct from erythema multiforme (EM), although often urticaria is confused with EM. Lesions of EM are annular and typically have purpuric or dusky centers, with each lesion lasting a minimum of 1 week.4 Annular lesions in urticaria usually do not have central duskiness or blisters. Often the centers of annular urticaria lesions are relatively normal and edges are raised. Some urticaria, especially in younger children as in this case, is sometimes called “urticaria multiforme” because the ecchymotic centers are reminiscent of, but distinct from, classic target lesions of EM. Urticaria multiforme is commonly misdiagnosed as EM, with 29% of patients originally misdiagnosed in one study.3
Urticaria multiforme occurs most commonly in infants and preschool-aged children,5 although it has been diagnosed in patients as old as 18 years.6 Patients often have had an antecedent bacterial or viral illness, recent treatment with antibiotics, or recent vaccination (67%, 44%, and 11% of patients, respectively, in one series).4 In contrast with classic urticaria, urticaria multiforme has not been associated with food allergy.4
In this case, urticaria multiforme was likely caused by a hypersensitivity reaction to amoxicillin. A reaction to nitrofurantoin was less likely because the patient had been taking it continuously for months without any complications.
Differential diagnosis
The differential diagnosis for urticaria multiforme includes EM and a serum sickness–like reaction. The main clue that this patient’s rash was a subtype of urticaria rather than EM was its transience, with individual lesions appearing and disappearing in less than a day.4 In contrast, the lesions of EM are fixed, persisting for a week or longer. While urticaria multiforme may have central ecchymosis (termed “hemorrhagic urticaria”) that looks similar to the dusky centers of EM lesions and persists longer than the transient edematous plaques,1 it resolves quickly with appropriate treatment.4 In contrast, the dusky centers of EM, which are caused by epidermal necrosis, take longer to resolve.4 Dermatographism, if present, would support a diagnosis of urticaria rather than EM. Similarly, facial or acral edema, if present, would support a diagnosis of urticaria multiforme; they are uncommon in EM. In contrast, any necrosis, blistering, or erosions in the centers of the annular lesions or on mucosal membranes would suggest EM, as necrosis, blistering, erosions, and mucosal involvement do not occur in urticaria multiforme.4 We stress that in EM, “the center of the lesion is the center of the action,” while in urticaria, wheals often have relatively normal centers.
Although both urticaria and EM lesions may be pruritic, any burning sensation is more suggestive of EM.4 Urticaria multiforme is often associated with antibiotics, vaccinations, and upper respiratory infections, while EM is most commonly associated with herpes simplex infection.4,7
Urticaria multiforme also may appear similar to a serum sickness–like reaction, which is another kind of hypersensitivity reaction triggered by the administration of antibiotics. It is most commonly associated with cefaclor, but also is associated with other antibiotics including amoxicillin.8 As with urticaria, hypersensitivity drug eruptions and serum sickness-like reactions may present with purpuric, polycyclic wheals with central clearing. However, as with EM, the lesions of serum sickness–like reactions are fixed, lasting for days to weeks.4 Facial or acral angioedema may occur in both urticaria multiforme and serum sickness–like reactions, but serum sickness–like reactions are not associated with dermatographism.4 Furthermore, serum sickness–like reactions are typically associated with high-grade fever, myalgia, arthralgia, and lymphadenopathy, which are not seen in urticaria multiforme.4,5
Diagnosis of urticaria multiforme usually can be made by history and physical exam, so lab testing and skin biopsy typically are not necessary.5 If performed, lab work may show modest elevation in erythrocyte sedimentation rate and C-reactive protein, but often these acute-phase reactants are within normal limits, and complete blood count and complete metabolic panel are unremarkable.3,5 Although urticaria multiforme often is associated with antecedent viral or bacterial infections, work-up for infectious etiology typically is not fruitful or helpful.4 If lesions are biopsied, the histology of urticaria multiforme is indistinguishable from other types of acute urticaria, showing dermal edema with perivascular lymphocytic infiltrate.9 In contrast, EM shows exocytosis, spongiosis, and epidermal necrosis.9
Treatment
The first step in managing urticaria multiforme is discontinuing any unnecessary antibiotic that could be triggering the hypersensitivity reaction. Urticaria multiforme typically resolves within 2 weeks without any treatment and responds to treatment with antihistamines within 24-28 hours.5 Treatment with a histamine1 (H1) blocker such as hydroxyzine, cetirizine, or diphenhydramine may be sufficient to resolve the eruption, but combination therapy with both an H1 blocker and an H2 blocker such as ranitidine can be helpful.4 Treatment with systemic corticosteroids usually is not necessary and should be reserved for severely symptomatic or refractory cases.4,9
One of the reasons that it is important to distinguish urticaria multiforme from EM is to avoid overtreatment with systemic steroids,3 which are rarely required for urticaria multiforme but are sometimes useful, although controversial, for EM.1 Additionally, the correct diagnosis is important for providing anticipatory guidance.6 Patients diagnosed with serum sickness–like reactions should be counseled to avoid unnecessary exposure to the culprit antibiotic in the future. Patients with urticaria multiforme who were taking an antibiotic at the onset of the eruption may consider avoiding the potential culprit antibiotic in the future, but it is important to keep in mind that urticaria multiforme is more strongly associated with antecedent infection than with antibiotic use, and so antibiotic avoidance may not be necessary unless justified by formal allergy testing. EM minor is more commonly associated with a herpes simplex virus infection than a drug reaction, so antibiotic use is less concerning, but patients should be counseled that recurrence is common and prophylactic treatment with acyclovir may be advised for recurrent disease.1
References
- “Neonatal and Infant Dermatology” (Elsevier Health Sciences: New York, 2014, pp. 456-70).
- CRIAI. 2006;30(1):003-012.
- Pediatr Dermatol. 1997;14(3):231-4.
- Pediatrics. 2007;119(5):e1177-83.
- Pediatr Dermatol. 2011;28(4):436-8.
- The Journal of Allergy and Clinical Immunology in Practice. 2013;1(5):520-1.
- Arch Dermatol. 1993;129(1):92-6.
- “The Hypersensitivity Syndromes” in Hurwitz Clinical Pediatric Dermatology. 4 ed. Elsevier: New York, 2011, pp. 455-84.
- J Clin Aesthet Dermatol. 2013;6(3):34-9.
Ms. Haddock is a medical student at University of California, San Diego School of Medicine and a research associate at Rady Children’s Hospital, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego and professor of medicine and pediatrics at UC San Diego School of Medicine. Dr. Eichenfield and Ms. Haddock said they have no relevant financial disclosures. Email [email protected].
By Ellen S. Haddock and Lawrence F. Eichenfield, M.D.
Urticaria multiforme
Although not the most classic presentation, this patient’s migrating rash is most consistent with urticaria (hives). Urticaria is dermal edema which causes transient edematous and usually pruritic wheals.1,2 Each individual lesion lasts less than 24 hours and disappears without leaving a mark. Urticaria is caused by mast cell activation, which leads to release of antihistamines and other substances that increase capillary and venule permeability, allowing fluid to leak into the extravascular space.1 In children, mast cell activation is usually triggered by infections, drugs, or foods.1
Classic urticaria consists of large, pruritic plaques and may be associated with airway edema. However, urticaria also can present with annular and polycyclic lesions, which may be less pruritic and are not associated with airway edema.3 This “multiple redness” is distinct from erythema multiforme (EM), although often urticaria is confused with EM. Lesions of EM are annular and typically have purpuric or dusky centers, with each lesion lasting a minimum of 1 week.4 Annular lesions in urticaria usually do not have central duskiness or blisters. Often the centers of annular urticaria lesions are relatively normal and edges are raised. Some urticaria, especially in younger children as in this case, is sometimes called “urticaria multiforme” because the ecchymotic centers are reminiscent of, but distinct from, classic target lesions of EM. Urticaria multiforme is commonly misdiagnosed as EM, with 29% of patients originally misdiagnosed in one study.3
Urticaria multiforme occurs most commonly in infants and preschool-aged children,5 although it has been diagnosed in patients as old as 18 years.6 Patients often have had an antecedent bacterial or viral illness, recent treatment with antibiotics, or recent vaccination (67%, 44%, and 11% of patients, respectively, in one series).4 In contrast with classic urticaria, urticaria multiforme has not been associated with food allergy.4
In this case, urticaria multiforme was likely caused by a hypersensitivity reaction to amoxicillin. A reaction to nitrofurantoin was less likely because the patient had been taking it continuously for months without any complications.
Differential diagnosis
The differential diagnosis for urticaria multiforme includes EM and a serum sickness–like reaction. The main clue that this patient’s rash was a subtype of urticaria rather than EM was its transience, with individual lesions appearing and disappearing in less than a day.4 In contrast, the lesions of EM are fixed, persisting for a week or longer. While urticaria multiforme may have central ecchymosis (termed “hemorrhagic urticaria”) that looks similar to the dusky centers of EM lesions and persists longer than the transient edematous plaques,1 it resolves quickly with appropriate treatment.4 In contrast, the dusky centers of EM, which are caused by epidermal necrosis, take longer to resolve.4 Dermatographism, if present, would support a diagnosis of urticaria rather than EM. Similarly, facial or acral edema, if present, would support a diagnosis of urticaria multiforme; they are uncommon in EM. In contrast, any necrosis, blistering, or erosions in the centers of the annular lesions or on mucosal membranes would suggest EM, as necrosis, blistering, erosions, and mucosal involvement do not occur in urticaria multiforme.4 We stress that in EM, “the center of the lesion is the center of the action,” while in urticaria, wheals often have relatively normal centers.
Although both urticaria and EM lesions may be pruritic, any burning sensation is more suggestive of EM.4 Urticaria multiforme is often associated with antibiotics, vaccinations, and upper respiratory infections, while EM is most commonly associated with herpes simplex infection.4,7
Urticaria multiforme also may appear similar to a serum sickness–like reaction, which is another kind of hypersensitivity reaction triggered by the administration of antibiotics. It is most commonly associated with cefaclor, but also is associated with other antibiotics including amoxicillin.8 As with urticaria, hypersensitivity drug eruptions and serum sickness-like reactions may present with purpuric, polycyclic wheals with central clearing. However, as with EM, the lesions of serum sickness–like reactions are fixed, lasting for days to weeks.4 Facial or acral angioedema may occur in both urticaria multiforme and serum sickness–like reactions, but serum sickness–like reactions are not associated with dermatographism.4 Furthermore, serum sickness–like reactions are typically associated with high-grade fever, myalgia, arthralgia, and lymphadenopathy, which are not seen in urticaria multiforme.4,5
Diagnosis of urticaria multiforme usually can be made by history and physical exam, so lab testing and skin biopsy typically are not necessary.5 If performed, lab work may show modest elevation in erythrocyte sedimentation rate and C-reactive protein, but often these acute-phase reactants are within normal limits, and complete blood count and complete metabolic panel are unremarkable.3,5 Although urticaria multiforme often is associated with antecedent viral or bacterial infections, work-up for infectious etiology typically is not fruitful or helpful.4 If lesions are biopsied, the histology of urticaria multiforme is indistinguishable from other types of acute urticaria, showing dermal edema with perivascular lymphocytic infiltrate.9 In contrast, EM shows exocytosis, spongiosis, and epidermal necrosis.9
Treatment
The first step in managing urticaria multiforme is discontinuing any unnecessary antibiotic that could be triggering the hypersensitivity reaction. Urticaria multiforme typically resolves within 2 weeks without any treatment and responds to treatment with antihistamines within 24-28 hours.5 Treatment with a histamine1 (H1) blocker such as hydroxyzine, cetirizine, or diphenhydramine may be sufficient to resolve the eruption, but combination therapy with both an H1 blocker and an H2 blocker such as ranitidine can be helpful.4 Treatment with systemic corticosteroids usually is not necessary and should be reserved for severely symptomatic or refractory cases.4,9
One of the reasons that it is important to distinguish urticaria multiforme from EM is to avoid overtreatment with systemic steroids,3 which are rarely required for urticaria multiforme but are sometimes useful, although controversial, for EM.1 Additionally, the correct diagnosis is important for providing anticipatory guidance.6 Patients diagnosed with serum sickness–like reactions should be counseled to avoid unnecessary exposure to the culprit antibiotic in the future. Patients with urticaria multiforme who were taking an antibiotic at the onset of the eruption may consider avoiding the potential culprit antibiotic in the future, but it is important to keep in mind that urticaria multiforme is more strongly associated with antecedent infection than with antibiotic use, and so antibiotic avoidance may not be necessary unless justified by formal allergy testing. EM minor is more commonly associated with a herpes simplex virus infection than a drug reaction, so antibiotic use is less concerning, but patients should be counseled that recurrence is common and prophylactic treatment with acyclovir may be advised for recurrent disease.1
References
- “Neonatal and Infant Dermatology” (Elsevier Health Sciences: New York, 2014, pp. 456-70).
- CRIAI. 2006;30(1):003-012.
- Pediatr Dermatol. 1997;14(3):231-4.
- Pediatrics. 2007;119(5):e1177-83.
- Pediatr Dermatol. 2011;28(4):436-8.
- The Journal of Allergy and Clinical Immunology in Practice. 2013;1(5):520-1.
- Arch Dermatol. 1993;129(1):92-6.
- “The Hypersensitivity Syndromes” in Hurwitz Clinical Pediatric Dermatology. 4 ed. Elsevier: New York, 2011, pp. 455-84.
- J Clin Aesthet Dermatol. 2013;6(3):34-9.
Ms. Haddock is a medical student at University of California, San Diego School of Medicine and a research associate at Rady Children’s Hospital, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego and professor of medicine and pediatrics at UC San Diego School of Medicine. Dr. Eichenfield and Ms. Haddock said they have no relevant financial disclosures. Email [email protected].
By Ellen S. Haddock and Lawrence F. Eichenfield, M.D.
Urticaria multiforme
Although not the most classic presentation, this patient’s migrating rash is most consistent with urticaria (hives). Urticaria is dermal edema which causes transient edematous and usually pruritic wheals.1,2 Each individual lesion lasts less than 24 hours and disappears without leaving a mark. Urticaria is caused by mast cell activation, which leads to release of antihistamines and other substances that increase capillary and venule permeability, allowing fluid to leak into the extravascular space.1 In children, mast cell activation is usually triggered by infections, drugs, or foods.1
Classic urticaria consists of large, pruritic plaques and may be associated with airway edema. However, urticaria also can present with annular and polycyclic lesions, which may be less pruritic and are not associated with airway edema.3 This “multiple redness” is distinct from erythema multiforme (EM), although often urticaria is confused with EM. Lesions of EM are annular and typically have purpuric or dusky centers, with each lesion lasting a minimum of 1 week.4 Annular lesions in urticaria usually do not have central duskiness or blisters. Often the centers of annular urticaria lesions are relatively normal and edges are raised. Some urticaria, especially in younger children as in this case, is sometimes called “urticaria multiforme” because the ecchymotic centers are reminiscent of, but distinct from, classic target lesions of EM. Urticaria multiforme is commonly misdiagnosed as EM, with 29% of patients originally misdiagnosed in one study.3
Urticaria multiforme occurs most commonly in infants and preschool-aged children,5 although it has been diagnosed in patients as old as 18 years.6 Patients often have had an antecedent bacterial or viral illness, recent treatment with antibiotics, or recent vaccination (67%, 44%, and 11% of patients, respectively, in one series).4 In contrast with classic urticaria, urticaria multiforme has not been associated with food allergy.4
In this case, urticaria multiforme was likely caused by a hypersensitivity reaction to amoxicillin. A reaction to nitrofurantoin was less likely because the patient had been taking it continuously for months without any complications.
Differential diagnosis
The differential diagnosis for urticaria multiforme includes EM and a serum sickness–like reaction. The main clue that this patient’s rash was a subtype of urticaria rather than EM was its transience, with individual lesions appearing and disappearing in less than a day.4 In contrast, the lesions of EM are fixed, persisting for a week or longer. While urticaria multiforme may have central ecchymosis (termed “hemorrhagic urticaria”) that looks similar to the dusky centers of EM lesions and persists longer than the transient edematous plaques,1 it resolves quickly with appropriate treatment.4 In contrast, the dusky centers of EM, which are caused by epidermal necrosis, take longer to resolve.4 Dermatographism, if present, would support a diagnosis of urticaria rather than EM. Similarly, facial or acral edema, if present, would support a diagnosis of urticaria multiforme; they are uncommon in EM. In contrast, any necrosis, blistering, or erosions in the centers of the annular lesions or on mucosal membranes would suggest EM, as necrosis, blistering, erosions, and mucosal involvement do not occur in urticaria multiforme.4 We stress that in EM, “the center of the lesion is the center of the action,” while in urticaria, wheals often have relatively normal centers.
Although both urticaria and EM lesions may be pruritic, any burning sensation is more suggestive of EM.4 Urticaria multiforme is often associated with antibiotics, vaccinations, and upper respiratory infections, while EM is most commonly associated with herpes simplex infection.4,7
Urticaria multiforme also may appear similar to a serum sickness–like reaction, which is another kind of hypersensitivity reaction triggered by the administration of antibiotics. It is most commonly associated with cefaclor, but also is associated with other antibiotics including amoxicillin.8 As with urticaria, hypersensitivity drug eruptions and serum sickness-like reactions may present with purpuric, polycyclic wheals with central clearing. However, as with EM, the lesions of serum sickness–like reactions are fixed, lasting for days to weeks.4 Facial or acral angioedema may occur in both urticaria multiforme and serum sickness–like reactions, but serum sickness–like reactions are not associated with dermatographism.4 Furthermore, serum sickness–like reactions are typically associated with high-grade fever, myalgia, arthralgia, and lymphadenopathy, which are not seen in urticaria multiforme.4,5
Diagnosis of urticaria multiforme usually can be made by history and physical exam, so lab testing and skin biopsy typically are not necessary.5 If performed, lab work may show modest elevation in erythrocyte sedimentation rate and C-reactive protein, but often these acute-phase reactants are within normal limits, and complete blood count and complete metabolic panel are unremarkable.3,5 Although urticaria multiforme often is associated with antecedent viral or bacterial infections, work-up for infectious etiology typically is not fruitful or helpful.4 If lesions are biopsied, the histology of urticaria multiforme is indistinguishable from other types of acute urticaria, showing dermal edema with perivascular lymphocytic infiltrate.9 In contrast, EM shows exocytosis, spongiosis, and epidermal necrosis.9
Treatment
The first step in managing urticaria multiforme is discontinuing any unnecessary antibiotic that could be triggering the hypersensitivity reaction. Urticaria multiforme typically resolves within 2 weeks without any treatment and responds to treatment with antihistamines within 24-28 hours.5 Treatment with a histamine1 (H1) blocker such as hydroxyzine, cetirizine, or diphenhydramine may be sufficient to resolve the eruption, but combination therapy with both an H1 blocker and an H2 blocker such as ranitidine can be helpful.4 Treatment with systemic corticosteroids usually is not necessary and should be reserved for severely symptomatic or refractory cases.4,9
One of the reasons that it is important to distinguish urticaria multiforme from EM is to avoid overtreatment with systemic steroids,3 which are rarely required for urticaria multiforme but are sometimes useful, although controversial, for EM.1 Additionally, the correct diagnosis is important for providing anticipatory guidance.6 Patients diagnosed with serum sickness–like reactions should be counseled to avoid unnecessary exposure to the culprit antibiotic in the future. Patients with urticaria multiforme who were taking an antibiotic at the onset of the eruption may consider avoiding the potential culprit antibiotic in the future, but it is important to keep in mind that urticaria multiforme is more strongly associated with antecedent infection than with antibiotic use, and so antibiotic avoidance may not be necessary unless justified by formal allergy testing. EM minor is more commonly associated with a herpes simplex virus infection than a drug reaction, so antibiotic use is less concerning, but patients should be counseled that recurrence is common and prophylactic treatment with acyclovir may be advised for recurrent disease.1
References
- “Neonatal and Infant Dermatology” (Elsevier Health Sciences: New York, 2014, pp. 456-70).
- CRIAI. 2006;30(1):003-012.
- Pediatr Dermatol. 1997;14(3):231-4.
- Pediatrics. 2007;119(5):e1177-83.
- Pediatr Dermatol. 2011;28(4):436-8.
- The Journal of Allergy and Clinical Immunology in Practice. 2013;1(5):520-1.
- Arch Dermatol. 1993;129(1):92-6.
- “The Hypersensitivity Syndromes” in Hurwitz Clinical Pediatric Dermatology. 4 ed. Elsevier: New York, 2011, pp. 455-84.
- J Clin Aesthet Dermatol. 2013;6(3):34-9.
Ms. Haddock is a medical student at University of California, San Diego School of Medicine and a research associate at Rady Children’s Hospital, San Diego. Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego and professor of medicine and pediatrics at UC San Diego School of Medicine. Dr. Eichenfield and Ms. Haddock said they have no relevant financial disclosures. Email [email protected].
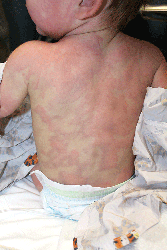
A 15-month-old male presents with a rash that began on his trunk 2 days ago and has spread to his arms, legs, and face. His parents say that the rash seems to migrate, with individual spots seeming to disappear and then reappear in new locations. The patient is playful and seems unbothered by the rash. He has a history of vesicoureteral reflux, for which he takes prophylactic nitrofurantoin daily. He was diagnosed with otitis media 7 days ago, for which he has been taking amoxicillin. On physical exam, the patient is afebrile. He has pink, edematous annular (ring-shaped) and polycyclic (composed of overlapping circles) plaques on his face, chest, abdomen, back, and upper and lower extremities. Some lesions appear vaguely targetoid with central clearing and raised borders. There is no mucosal involvement, joint involvement, or lymphadenopathy.
Pediatric Dermatology Consult - December 2015
Ancillary testing
Complete blood count showed leukocytosis (13.1 th/mcL), with normal differential. The C-reactive protein was elevated (10 mg/dL). The comprehensive metabolic panel was within normal limits. Mycoplasma pneumoniae antibody, IgM was positive, and herpes virus cultures were negative. The chest x-ray showed a dense right upper lobe airspace opacity, concerning for pneumonia, likely with a component of atelectasis.
Discussion
Mycoplasma pneumoniae–induced rash and mucositis (MIRM) is a syndrome of mucocutaneous involvement in conjunction with an M. pneumoniae infection. M. pneumoniae is a bacteria that commonly causes respiratory tract infections. In children, it is a leading cause of atypical pneumonia.1,2 Extrapulmonary manifestations are common and can involve the skin, heart, kidneys, gastrointestinal system, and nervous system.3 There is a 1-3 week incubation period for infections, which always begin with respiratory symptoms. The most common early symptoms of this infection are cough, malaise, fever, and headaches. Studies show that between 25%-38% of M. pneumoniae infections have mucocutaneous manifestations.3,4
An overwhelming majority of cases have mucosal involvement while the degree of skin involvement varies. Cutaneous manifestations, which are usually polymorphic, present with vesiculobullous lesions, targetoid macules and papules, or morbilliform eruptions.3 MIRM is seen most often in children and adolescents, but a few adult cases have been reported. About 66% of cases are seen in males, but the reason for this gender predilection is not well understood.3 Overall, MIRM has an excellent prognosis, compared with the other disorders on the differential diagnosis.
Differential diagnosis
MIRM used to be considered along the spectrum of bullous diseases that have mucosal involvement including erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrosis (TEN).3-6 The distinction can be difficult to identify, given the similarities that all of these diseases share, and many published articles referencing cases of SJS, TEN, and EM actually fit the criteria for MIRM.6 What sets MIRM apart is the predominance of mucosal involvement with minimal skin involvement and the overwhelming overall good prognosis compared with drug induced SJS.3
Although rare cases have moderate cutaneous involvement, MIRM is most notable for oral lesions, which include ulcers, erosions, and vesiculobullae of the lips and buccal mucosa, as well as ocular and genital mucosal involvement in a lower degree.3 For cases with moderate or even mild involvement of the skin, ruling out other diagnoses becomes more difficult, especially since the dermatologic manifestations of targetoid papules and bullae are common to all of them. Testing for M. pneumoniae will strengthen the diagnosis and can be done in a number of ways. Using polymerase chain reaction to measure for M. pneumoniae DNA offers the benefit of being able to detect an infection early in its course because it does not rely on antibody formation.4 Other testing methods include M. pneumoniae antigen detection, and IgM or IgG titers.4
Treatment
The mainstay of treatment of this bacterial infection is, of course, antibiotics. Azithromycin and erythromycin are the drugs of choice for children. While tetracyclines and fluoroquinolones can be used to treat M. pneumoniae, their use is not recommended in young children.4 Systemic corticosteroids can be used in addition to antibiotics, but there is limited data to support its efficacy in these patients.7 In milder cases, reports have documented that the course can be self-limited and may improve with only supportive care. Because of the severity of mucosal involvement in some cases, such as the one in our patient, supportive care may include intravenous hydration and total parenteral nutrition if the patient is unable to tolerate oral intake. In more severe cases, IVIG can be beneficial.8 This has been shown to induce rapid improvement in refractory mucositis when antibiotics and supportive mucosal care alone have not been sufficient.8
References
- (J Clin Microbiol. 2015 Jan;53[1]:124-30.)
- (Clin Microbiol. 2004;17[4]:697-728.)
- (J Am Acad Dermatol. 2015;72[2]: 239-54.)
- (Int J Dermatol. 2009 Jul;48[7]:673-80.)
- (J Eur Acad Dermatol Venereol. 2015 Mar;29[3]:595-8.)
- (J Am Acad Dermatol. 2005 Feb;52[2]:312-5.)
- (Pediatr Dermatol. 2014 Nov-Dec;31[6]:664-9.)
- (Acta Paediatr. 2011 Nov;100[11]:e238-40.)
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California San Diego and Mr. Ginsberg is a research associate at the hospital. They said they had no relevant financial disclosures.
Email them at [email protected].
Ancillary testing
Complete blood count showed leukocytosis (13.1 th/mcL), with normal differential. The C-reactive protein was elevated (10 mg/dL). The comprehensive metabolic panel was within normal limits. Mycoplasma pneumoniae antibody, IgM was positive, and herpes virus cultures were negative. The chest x-ray showed a dense right upper lobe airspace opacity, concerning for pneumonia, likely with a component of atelectasis.
Discussion
Mycoplasma pneumoniae–induced rash and mucositis (MIRM) is a syndrome of mucocutaneous involvement in conjunction with an M. pneumoniae infection. M. pneumoniae is a bacteria that commonly causes respiratory tract infections. In children, it is a leading cause of atypical pneumonia.1,2 Extrapulmonary manifestations are common and can involve the skin, heart, kidneys, gastrointestinal system, and nervous system.3 There is a 1-3 week incubation period for infections, which always begin with respiratory symptoms. The most common early symptoms of this infection are cough, malaise, fever, and headaches. Studies show that between 25%-38% of M. pneumoniae infections have mucocutaneous manifestations.3,4
An overwhelming majority of cases have mucosal involvement while the degree of skin involvement varies. Cutaneous manifestations, which are usually polymorphic, present with vesiculobullous lesions, targetoid macules and papules, or morbilliform eruptions.3 MIRM is seen most often in children and adolescents, but a few adult cases have been reported. About 66% of cases are seen in males, but the reason for this gender predilection is not well understood.3 Overall, MIRM has an excellent prognosis, compared with the other disorders on the differential diagnosis.
Differential diagnosis
MIRM used to be considered along the spectrum of bullous diseases that have mucosal involvement including erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrosis (TEN).3-6 The distinction can be difficult to identify, given the similarities that all of these diseases share, and many published articles referencing cases of SJS, TEN, and EM actually fit the criteria for MIRM.6 What sets MIRM apart is the predominance of mucosal involvement with minimal skin involvement and the overwhelming overall good prognosis compared with drug induced SJS.3
Although rare cases have moderate cutaneous involvement, MIRM is most notable for oral lesions, which include ulcers, erosions, and vesiculobullae of the lips and buccal mucosa, as well as ocular and genital mucosal involvement in a lower degree.3 For cases with moderate or even mild involvement of the skin, ruling out other diagnoses becomes more difficult, especially since the dermatologic manifestations of targetoid papules and bullae are common to all of them. Testing for M. pneumoniae will strengthen the diagnosis and can be done in a number of ways. Using polymerase chain reaction to measure for M. pneumoniae DNA offers the benefit of being able to detect an infection early in its course because it does not rely on antibody formation.4 Other testing methods include M. pneumoniae antigen detection, and IgM or IgG titers.4
Treatment
The mainstay of treatment of this bacterial infection is, of course, antibiotics. Azithromycin and erythromycin are the drugs of choice for children. While tetracyclines and fluoroquinolones can be used to treat M. pneumoniae, their use is not recommended in young children.4 Systemic corticosteroids can be used in addition to antibiotics, but there is limited data to support its efficacy in these patients.7 In milder cases, reports have documented that the course can be self-limited and may improve with only supportive care. Because of the severity of mucosal involvement in some cases, such as the one in our patient, supportive care may include intravenous hydration and total parenteral nutrition if the patient is unable to tolerate oral intake. In more severe cases, IVIG can be beneficial.8 This has been shown to induce rapid improvement in refractory mucositis when antibiotics and supportive mucosal care alone have not been sufficient.8
References
- (J Clin Microbiol. 2015 Jan;53[1]:124-30.)
- (Clin Microbiol. 2004;17[4]:697-728.)
- (J Am Acad Dermatol. 2015;72[2]: 239-54.)
- (Int J Dermatol. 2009 Jul;48[7]:673-80.)
- (J Eur Acad Dermatol Venereol. 2015 Mar;29[3]:595-8.)
- (J Am Acad Dermatol. 2005 Feb;52[2]:312-5.)
- (Pediatr Dermatol. 2014 Nov-Dec;31[6]:664-9.)
- (Acta Paediatr. 2011 Nov;100[11]:e238-40.)
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California San Diego and Mr. Ginsberg is a research associate at the hospital. They said they had no relevant financial disclosures.
Email them at [email protected].
Ancillary testing
Complete blood count showed leukocytosis (13.1 th/mcL), with normal differential. The C-reactive protein was elevated (10 mg/dL). The comprehensive metabolic panel was within normal limits. Mycoplasma pneumoniae antibody, IgM was positive, and herpes virus cultures were negative. The chest x-ray showed a dense right upper lobe airspace opacity, concerning for pneumonia, likely with a component of atelectasis.
Discussion
Mycoplasma pneumoniae–induced rash and mucositis (MIRM) is a syndrome of mucocutaneous involvement in conjunction with an M. pneumoniae infection. M. pneumoniae is a bacteria that commonly causes respiratory tract infections. In children, it is a leading cause of atypical pneumonia.1,2 Extrapulmonary manifestations are common and can involve the skin, heart, kidneys, gastrointestinal system, and nervous system.3 There is a 1-3 week incubation period for infections, which always begin with respiratory symptoms. The most common early symptoms of this infection are cough, malaise, fever, and headaches. Studies show that between 25%-38% of M. pneumoniae infections have mucocutaneous manifestations.3,4
An overwhelming majority of cases have mucosal involvement while the degree of skin involvement varies. Cutaneous manifestations, which are usually polymorphic, present with vesiculobullous lesions, targetoid macules and papules, or morbilliform eruptions.3 MIRM is seen most often in children and adolescents, but a few adult cases have been reported. About 66% of cases are seen in males, but the reason for this gender predilection is not well understood.3 Overall, MIRM has an excellent prognosis, compared with the other disorders on the differential diagnosis.
Differential diagnosis
MIRM used to be considered along the spectrum of bullous diseases that have mucosal involvement including erythema multiforme (EM), Stevens-Johnson syndrome (SJS), and toxic epidermal necrosis (TEN).3-6 The distinction can be difficult to identify, given the similarities that all of these diseases share, and many published articles referencing cases of SJS, TEN, and EM actually fit the criteria for MIRM.6 What sets MIRM apart is the predominance of mucosal involvement with minimal skin involvement and the overwhelming overall good prognosis compared with drug induced SJS.3
Although rare cases have moderate cutaneous involvement, MIRM is most notable for oral lesions, which include ulcers, erosions, and vesiculobullae of the lips and buccal mucosa, as well as ocular and genital mucosal involvement in a lower degree.3 For cases with moderate or even mild involvement of the skin, ruling out other diagnoses becomes more difficult, especially since the dermatologic manifestations of targetoid papules and bullae are common to all of them. Testing for M. pneumoniae will strengthen the diagnosis and can be done in a number of ways. Using polymerase chain reaction to measure for M. pneumoniae DNA offers the benefit of being able to detect an infection early in its course because it does not rely on antibody formation.4 Other testing methods include M. pneumoniae antigen detection, and IgM or IgG titers.4
Treatment
The mainstay of treatment of this bacterial infection is, of course, antibiotics. Azithromycin and erythromycin are the drugs of choice for children. While tetracyclines and fluoroquinolones can be used to treat M. pneumoniae, their use is not recommended in young children.4 Systemic corticosteroids can be used in addition to antibiotics, but there is limited data to support its efficacy in these patients.7 In milder cases, reports have documented that the course can be self-limited and may improve with only supportive care. Because of the severity of mucosal involvement in some cases, such as the one in our patient, supportive care may include intravenous hydration and total parenteral nutrition if the patient is unable to tolerate oral intake. In more severe cases, IVIG can be beneficial.8 This has been shown to induce rapid improvement in refractory mucositis when antibiotics and supportive mucosal care alone have not been sufficient.8
References
- (J Clin Microbiol. 2015 Jan;53[1]:124-30.)
- (Clin Microbiol. 2004;17[4]:697-728.)
- (J Am Acad Dermatol. 2015;72[2]: 239-54.)
- (Int J Dermatol. 2009 Jul;48[7]:673-80.)
- (J Eur Acad Dermatol Venereol. 2015 Mar;29[3]:595-8.)
- (J Am Acad Dermatol. 2005 Feb;52[2]:312-5.)
- (Pediatr Dermatol. 2014 Nov-Dec;31[6]:664-9.)
- (Acta Paediatr. 2011 Nov;100[11]:e238-40.)
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California San Diego and Mr. Ginsberg is a research associate at the hospital. They said they had no relevant financial disclosures.
Email them at [email protected].
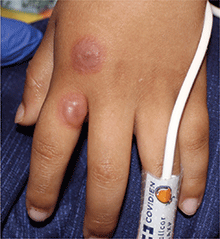
A 4-year-old male with a past history of asthma, presents with a 10-day history of cough, wheezing, rhinorrhea, and fever. He was treated with ibuprofen and albuterol without much improvement. Two days prior to presentation, he developed swollen and cracked lips as well as pink conjunctiva. Since then, he has been hypoactive with poor oral intake. He was evaluated by his pediatrician and was given dexamethasone and diphenhydramine, with no improvement. His oral and ocular symptoms worsen, which is why he was taken to the emergency department. He has no sick contacts. On the day of presentation, he developed several target erythematous lesions on his arms and legs that progressed to bulla (See photo).
Pediatric Dermatology Consult - November 2015
Tinea versicolor
Tinea versicolor – also called pityriasis versicolor – is a benign superficial fungal skin infection caused by Malassezia. It presents as well-demarcated, oval, finely scaling macules, patches, or thin plaques, which can be hypopigmented, hyperpigmented, or erythematous.1,2,3 . The name, tinea versicolor, highlights the variability in the color of lesions.4
Scale may be minimal, but becomes more noticeable when lesions are scraped, which is called the “evoked scale sign.”5 The lesions may be asymptomatic or slightly pruritic.1 Lesions range in size from several millimeters to several centimeters and may coalesce.6 They are most commonly found on the chest, back, upper arms, and neck,2,7 but in children the face may be affected.1,3,8 Hypopigmented lesions may be most noticeable during the summer when the surrounding uninvolved skin darkens with sun exposure.9 Tinea versicolor is not contagious, but pigmentary changes may cause cosmetic concerns, and the condition may persist for years if not treated.2,4
Malassezia is a dimorphic fungus that is part of the normal skin flora in its yeast form, but if Malassezia converts to its hyphal form, it is able penetrate the stratum corneum and cause the tinea versicolor rash.1,10 The reason for the conversion from yeast to hyphal form is not fully understood.11Malassezia is lipophilic, so it thrives when sebum production is high, which is why tinea versicolor most commonly develops in adolescence or young adulthood, although it may be seen in younger children and older adults.12 Genetic predisposition, warm and humid environments, oily skin, use of oily creams, use of corticosteroids, hyperhidrosis, physical activity, malnutrition, immunosuppression, and exposure to sunlight increase susceptibility.1,13,14
Tinea versicolor is most commonly caused by Malassezia globosa and Malassezia furfur.13,15,16 Hypopigmentation may be caused by Malassezia’s production of azelaic acid, which inhibits the dopa-tyrosinase reaction that is part of melanin synthesis.15,17,18 Hyperpigmentation may result from inflammation.15,18 The evoked scale sign results from the production of keratinase, which disrupts the stratum corneum.5
Tinea versicolor often can be diagnosed by its characteristic clinical appearance and may fluoresce golden under a Wood’s ultraviolet lamp.19 Diagnosis can be confirmed by microscopic examination of skin scrapings treated with potassium hydroxide (KOH), which will have a “spaghetti and meatball” appearance, with the hyphae resembling spaghetti and spores resembling meatballs.1 For young children, removing scale with transparent tape can be a good alternative to scraping skin with a blade.2,19
Differential diagnosis
Postinflammatory pigment changes, both hypo and hyper, usually lack scale, may be anywhere on the body, and should have the same distribution as some original inflammation.
Pityriasis alba presents with hypopigmented patches, typically on the face, and has a more subtle “blotchy” appearance, without discrete oval patches. Pityriasis rosea may appear similar to tinea versicolor with erythema and scale, but it typically begins with a single, large herald patch, and scale is primarily at the outer border of the lesions.1
Tinea corporis (“ringworm”), which is caused by a dermatophyte, is more distinctly ring shaped with a scaly, vesicular, papular, or pustular border and there is often a clear center that may not scale when scraped.5,9 It is much more commonly localized, except in immunosuppressed patients or if mistreated with topical corticosteroids. Vitiligo lesions are completely depigmented, rather than just hypopigmented, and lack scale.1 Psoriasis scale is thicker and is visible without any provocation.
Treatment
First-line treatments for tinea versicolor include ketoconazole shampoo, selenium sulfide lotion or shampoo, and zinc pyrithione shampoo, which are left on for 5-10 minutes before rinsing.1,20 Any of these treatments is a fine first choice, as all are effective, and there are no robust data establishing the superiority of any single treatment.20 The typical treatment duration is 1-4 weeks.1 Longer treatment durations yield better cure rates.20 Ketoconazole and selenium sulfide also are available in foam formulations.11 Shampoo and foam formulations have the benefit of easily covering a large affected area.
Alternatively, terbinafine cream can be applied twice daily for a week or ketoconazole cream can be applied twice daily for 1-4 weeks.1,21 It is advisable to treat the whole trunk, neck, arms, and legs down to the knees, even if only a small area is involved.14,22 Antifungal treatments are well tolerated, with skin irritation and contact allergy being the most common adverse effects.1 Selenium sulfide has a strong odor.1
Hypopigmentation and hyperpigmentation can persist for months after the active infection has resolved and do not necessarily indicate a treatment failure.2,20 However, because Malassezia is a part of the normal skin flora, recurrence is common, occurring in 60%-80% of patients within 2 years.14 Recurrence or persistence of an active infection can be proven by a positive KOH scrape test. If a first treatment fails, a different first-line topical medication should be tried.1 Referral to a dermatologist is recommended if the eruption is unresponsive to two treatments.1
Oral antifungals such as itraconazole, fluconazole, and pramiconazole are effective for tinea versicolor, but have more adverse effects than topicals and interact with other medications because of their inhibition of the cytochrome P450 system, so they are used only for refractory or widespread disease.1,11 In 2013, the Food and Drug Administration issued a black box warning against oral ketoconazole due its ability to cause life-threatening hepatotoxicity and adrenal insufficiency1,23,24; it should not be used to treat tinea versicolor. Topical ketoconazole is safe and remains a first-line treatment for tinea versicolor, as discussed above.24 Oral terbinafine is not effective for tinea versicolor despite its efficacy as a topical treatment.11
Patients with recurrent tinea versicolor can try preventive therapy with ketoconazole shampoo, zinc pyrithione shampoo, or selenium sulfide lotion or shampoo one to four times per month.1 Oral antifungals also are effective for prevention of recurrence, but should be used only if the condition is refractory to topical prophylaxis.20,25 It is important to remember that hypopigmentation and hyperpigmentation may persist for months after resolution of active infection; absence of hyphae on skin scraping prepared with KOH confirms absence of active disease.15,16
References
- BMJ. 2015;350:h1394. doi:10.1136/bmj.h1394.
- Lancet. 2004 Sep 25-Oct 1;364(9440):1173-82.
- Pediatr Dermatol. 1991;8(1):9-12.
- J Eur Acad Dermatol Venereol. 2002;16(1):19-33.
- Arch Dermatol. 2009;145(9):1078.
- Vitiligo and other disorders of pigmentation, in: “Dermatology,” Vol 1. 3rd ed. (Philadelphia: Elsevier Saunders, 2012, pp.1041-2.)
- Am J Clin Dermatol. 2000 Mar-Apr;1(2):75-80.
- Mycoses. 1995;38(5-6):227-8.
- “Skin Disorders Due to Fungi,” in: Hurwitz Clinical Pediatric Dermatology 4th ed. (Philadelphia: Saunders, 2011, pp. 396-403).
- Infect Control Hosp Epidemiol. 2002 Apr;23(4):212-6.
- Expert Opin Pharmacother. 2014 Aug;15(12):1707-13.
- Clin Microbiol Rev. 2002 Jan;15(1):21-57.
- Clin Dermatol. 2010 Mar 4;28(2):185-9.
- J Am Acad Dermatol. 1994 Sep;31(3 Pt 2):S18-20.
- Int J Dermatol. 2014 Feb;53(2):137-41
- Mycopathologia. 2006 Dec;162(6):373-6.
- J Invest Dermatol. 1978 Sep;71(3):205-8.
- Int J Dermatol. 1992 Apr;31(4):253-6.
- Pediatr Dermatol. 2000 Jan-Feb;17(1):68-9.
- Arch Dermatol. 2010 Oct;146(10):1132-40.
- Dermatology (Basel). 1997;194(1):22-4. doi:10.1159/000246179.
- Red Book Plus: 2009 Report of the Committee on Infectious Disease.
- http://www.fda.gov/Drugs/DrugSafety/ucm362415.htm
- J Cutan Med Surg. 2015 Jul-Aug;19(4):352-7.
- Arch Dermatol. 2002 Jan;138(1):69-73.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at the hospital. Dr. Eichenfield and Ms. Haddock said they have no relevant financial disclosures.
Email to [email protected].
Tinea versicolor
Tinea versicolor – also called pityriasis versicolor – is a benign superficial fungal skin infection caused by Malassezia. It presents as well-demarcated, oval, finely scaling macules, patches, or thin plaques, which can be hypopigmented, hyperpigmented, or erythematous.1,2,3 . The name, tinea versicolor, highlights the variability in the color of lesions.4
Scale may be minimal, but becomes more noticeable when lesions are scraped, which is called the “evoked scale sign.”5 The lesions may be asymptomatic or slightly pruritic.1 Lesions range in size from several millimeters to several centimeters and may coalesce.6 They are most commonly found on the chest, back, upper arms, and neck,2,7 but in children the face may be affected.1,3,8 Hypopigmented lesions may be most noticeable during the summer when the surrounding uninvolved skin darkens with sun exposure.9 Tinea versicolor is not contagious, but pigmentary changes may cause cosmetic concerns, and the condition may persist for years if not treated.2,4
Malassezia is a dimorphic fungus that is part of the normal skin flora in its yeast form, but if Malassezia converts to its hyphal form, it is able penetrate the stratum corneum and cause the tinea versicolor rash.1,10 The reason for the conversion from yeast to hyphal form is not fully understood.11Malassezia is lipophilic, so it thrives when sebum production is high, which is why tinea versicolor most commonly develops in adolescence or young adulthood, although it may be seen in younger children and older adults.12 Genetic predisposition, warm and humid environments, oily skin, use of oily creams, use of corticosteroids, hyperhidrosis, physical activity, malnutrition, immunosuppression, and exposure to sunlight increase susceptibility.1,13,14
Tinea versicolor is most commonly caused by Malassezia globosa and Malassezia furfur.13,15,16 Hypopigmentation may be caused by Malassezia’s production of azelaic acid, which inhibits the dopa-tyrosinase reaction that is part of melanin synthesis.15,17,18 Hyperpigmentation may result from inflammation.15,18 The evoked scale sign results from the production of keratinase, which disrupts the stratum corneum.5
Tinea versicolor often can be diagnosed by its characteristic clinical appearance and may fluoresce golden under a Wood’s ultraviolet lamp.19 Diagnosis can be confirmed by microscopic examination of skin scrapings treated with potassium hydroxide (KOH), which will have a “spaghetti and meatball” appearance, with the hyphae resembling spaghetti and spores resembling meatballs.1 For young children, removing scale with transparent tape can be a good alternative to scraping skin with a blade.2,19
Differential diagnosis
Postinflammatory pigment changes, both hypo and hyper, usually lack scale, may be anywhere on the body, and should have the same distribution as some original inflammation.
Pityriasis alba presents with hypopigmented patches, typically on the face, and has a more subtle “blotchy” appearance, without discrete oval patches. Pityriasis rosea may appear similar to tinea versicolor with erythema and scale, but it typically begins with a single, large herald patch, and scale is primarily at the outer border of the lesions.1
Tinea corporis (“ringworm”), which is caused by a dermatophyte, is more distinctly ring shaped with a scaly, vesicular, papular, or pustular border and there is often a clear center that may not scale when scraped.5,9 It is much more commonly localized, except in immunosuppressed patients or if mistreated with topical corticosteroids. Vitiligo lesions are completely depigmented, rather than just hypopigmented, and lack scale.1 Psoriasis scale is thicker and is visible without any provocation.
Treatment
First-line treatments for tinea versicolor include ketoconazole shampoo, selenium sulfide lotion or shampoo, and zinc pyrithione shampoo, which are left on for 5-10 minutes before rinsing.1,20 Any of these treatments is a fine first choice, as all are effective, and there are no robust data establishing the superiority of any single treatment.20 The typical treatment duration is 1-4 weeks.1 Longer treatment durations yield better cure rates.20 Ketoconazole and selenium sulfide also are available in foam formulations.11 Shampoo and foam formulations have the benefit of easily covering a large affected area.
Alternatively, terbinafine cream can be applied twice daily for a week or ketoconazole cream can be applied twice daily for 1-4 weeks.1,21 It is advisable to treat the whole trunk, neck, arms, and legs down to the knees, even if only a small area is involved.14,22 Antifungal treatments are well tolerated, with skin irritation and contact allergy being the most common adverse effects.1 Selenium sulfide has a strong odor.1
Hypopigmentation and hyperpigmentation can persist for months after the active infection has resolved and do not necessarily indicate a treatment failure.2,20 However, because Malassezia is a part of the normal skin flora, recurrence is common, occurring in 60%-80% of patients within 2 years.14 Recurrence or persistence of an active infection can be proven by a positive KOH scrape test. If a first treatment fails, a different first-line topical medication should be tried.1 Referral to a dermatologist is recommended if the eruption is unresponsive to two treatments.1
Oral antifungals such as itraconazole, fluconazole, and pramiconazole are effective for tinea versicolor, but have more adverse effects than topicals and interact with other medications because of their inhibition of the cytochrome P450 system, so they are used only for refractory or widespread disease.1,11 In 2013, the Food and Drug Administration issued a black box warning against oral ketoconazole due its ability to cause life-threatening hepatotoxicity and adrenal insufficiency1,23,24; it should not be used to treat tinea versicolor. Topical ketoconazole is safe and remains a first-line treatment for tinea versicolor, as discussed above.24 Oral terbinafine is not effective for tinea versicolor despite its efficacy as a topical treatment.11
Patients with recurrent tinea versicolor can try preventive therapy with ketoconazole shampoo, zinc pyrithione shampoo, or selenium sulfide lotion or shampoo one to four times per month.1 Oral antifungals also are effective for prevention of recurrence, but should be used only if the condition is refractory to topical prophylaxis.20,25 It is important to remember that hypopigmentation and hyperpigmentation may persist for months after resolution of active infection; absence of hyphae on skin scraping prepared with KOH confirms absence of active disease.15,16
References
- BMJ. 2015;350:h1394. doi:10.1136/bmj.h1394.
- Lancet. 2004 Sep 25-Oct 1;364(9440):1173-82.
- Pediatr Dermatol. 1991;8(1):9-12.
- J Eur Acad Dermatol Venereol. 2002;16(1):19-33.
- Arch Dermatol. 2009;145(9):1078.
- Vitiligo and other disorders of pigmentation, in: “Dermatology,” Vol 1. 3rd ed. (Philadelphia: Elsevier Saunders, 2012, pp.1041-2.)
- Am J Clin Dermatol. 2000 Mar-Apr;1(2):75-80.
- Mycoses. 1995;38(5-6):227-8.
- “Skin Disorders Due to Fungi,” in: Hurwitz Clinical Pediatric Dermatology 4th ed. (Philadelphia: Saunders, 2011, pp. 396-403).
- Infect Control Hosp Epidemiol. 2002 Apr;23(4):212-6.
- Expert Opin Pharmacother. 2014 Aug;15(12):1707-13.
- Clin Microbiol Rev. 2002 Jan;15(1):21-57.
- Clin Dermatol. 2010 Mar 4;28(2):185-9.
- J Am Acad Dermatol. 1994 Sep;31(3 Pt 2):S18-20.
- Int J Dermatol. 2014 Feb;53(2):137-41
- Mycopathologia. 2006 Dec;162(6):373-6.
- J Invest Dermatol. 1978 Sep;71(3):205-8.
- Int J Dermatol. 1992 Apr;31(4):253-6.
- Pediatr Dermatol. 2000 Jan-Feb;17(1):68-9.
- Arch Dermatol. 2010 Oct;146(10):1132-40.
- Dermatology (Basel). 1997;194(1):22-4. doi:10.1159/000246179.
- Red Book Plus: 2009 Report of the Committee on Infectious Disease.
- http://www.fda.gov/Drugs/DrugSafety/ucm362415.htm
- J Cutan Med Surg. 2015 Jul-Aug;19(4):352-7.
- Arch Dermatol. 2002 Jan;138(1):69-73.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at the hospital. Dr. Eichenfield and Ms. Haddock said they have no relevant financial disclosures.
Email to [email protected].
Tinea versicolor
Tinea versicolor – also called pityriasis versicolor – is a benign superficial fungal skin infection caused by Malassezia. It presents as well-demarcated, oval, finely scaling macules, patches, or thin plaques, which can be hypopigmented, hyperpigmented, or erythematous.1,2,3 . The name, tinea versicolor, highlights the variability in the color of lesions.4
Scale may be minimal, but becomes more noticeable when lesions are scraped, which is called the “evoked scale sign.”5 The lesions may be asymptomatic or slightly pruritic.1 Lesions range in size from several millimeters to several centimeters and may coalesce.6 They are most commonly found on the chest, back, upper arms, and neck,2,7 but in children the face may be affected.1,3,8 Hypopigmented lesions may be most noticeable during the summer when the surrounding uninvolved skin darkens with sun exposure.9 Tinea versicolor is not contagious, but pigmentary changes may cause cosmetic concerns, and the condition may persist for years if not treated.2,4
Malassezia is a dimorphic fungus that is part of the normal skin flora in its yeast form, but if Malassezia converts to its hyphal form, it is able penetrate the stratum corneum and cause the tinea versicolor rash.1,10 The reason for the conversion from yeast to hyphal form is not fully understood.11Malassezia is lipophilic, so it thrives when sebum production is high, which is why tinea versicolor most commonly develops in adolescence or young adulthood, although it may be seen in younger children and older adults.12 Genetic predisposition, warm and humid environments, oily skin, use of oily creams, use of corticosteroids, hyperhidrosis, physical activity, malnutrition, immunosuppression, and exposure to sunlight increase susceptibility.1,13,14
Tinea versicolor is most commonly caused by Malassezia globosa and Malassezia furfur.13,15,16 Hypopigmentation may be caused by Malassezia’s production of azelaic acid, which inhibits the dopa-tyrosinase reaction that is part of melanin synthesis.15,17,18 Hyperpigmentation may result from inflammation.15,18 The evoked scale sign results from the production of keratinase, which disrupts the stratum corneum.5
Tinea versicolor often can be diagnosed by its characteristic clinical appearance and may fluoresce golden under a Wood’s ultraviolet lamp.19 Diagnosis can be confirmed by microscopic examination of skin scrapings treated with potassium hydroxide (KOH), which will have a “spaghetti and meatball” appearance, with the hyphae resembling spaghetti and spores resembling meatballs.1 For young children, removing scale with transparent tape can be a good alternative to scraping skin with a blade.2,19
Differential diagnosis
Postinflammatory pigment changes, both hypo and hyper, usually lack scale, may be anywhere on the body, and should have the same distribution as some original inflammation.
Pityriasis alba presents with hypopigmented patches, typically on the face, and has a more subtle “blotchy” appearance, without discrete oval patches. Pityriasis rosea may appear similar to tinea versicolor with erythema and scale, but it typically begins with a single, large herald patch, and scale is primarily at the outer border of the lesions.1
Tinea corporis (“ringworm”), which is caused by a dermatophyte, is more distinctly ring shaped with a scaly, vesicular, papular, or pustular border and there is often a clear center that may not scale when scraped.5,9 It is much more commonly localized, except in immunosuppressed patients or if mistreated with topical corticosteroids. Vitiligo lesions are completely depigmented, rather than just hypopigmented, and lack scale.1 Psoriasis scale is thicker and is visible without any provocation.
Treatment
First-line treatments for tinea versicolor include ketoconazole shampoo, selenium sulfide lotion or shampoo, and zinc pyrithione shampoo, which are left on for 5-10 minutes before rinsing.1,20 Any of these treatments is a fine first choice, as all are effective, and there are no robust data establishing the superiority of any single treatment.20 The typical treatment duration is 1-4 weeks.1 Longer treatment durations yield better cure rates.20 Ketoconazole and selenium sulfide also are available in foam formulations.11 Shampoo and foam formulations have the benefit of easily covering a large affected area.
Alternatively, terbinafine cream can be applied twice daily for a week or ketoconazole cream can be applied twice daily for 1-4 weeks.1,21 It is advisable to treat the whole trunk, neck, arms, and legs down to the knees, even if only a small area is involved.14,22 Antifungal treatments are well tolerated, with skin irritation and contact allergy being the most common adverse effects.1 Selenium sulfide has a strong odor.1
Hypopigmentation and hyperpigmentation can persist for months after the active infection has resolved and do not necessarily indicate a treatment failure.2,20 However, because Malassezia is a part of the normal skin flora, recurrence is common, occurring in 60%-80% of patients within 2 years.14 Recurrence or persistence of an active infection can be proven by a positive KOH scrape test. If a first treatment fails, a different first-line topical medication should be tried.1 Referral to a dermatologist is recommended if the eruption is unresponsive to two treatments.1
Oral antifungals such as itraconazole, fluconazole, and pramiconazole are effective for tinea versicolor, but have more adverse effects than topicals and interact with other medications because of their inhibition of the cytochrome P450 system, so they are used only for refractory or widespread disease.1,11 In 2013, the Food and Drug Administration issued a black box warning against oral ketoconazole due its ability to cause life-threatening hepatotoxicity and adrenal insufficiency1,23,24; it should not be used to treat tinea versicolor. Topical ketoconazole is safe and remains a first-line treatment for tinea versicolor, as discussed above.24 Oral terbinafine is not effective for tinea versicolor despite its efficacy as a topical treatment.11
Patients with recurrent tinea versicolor can try preventive therapy with ketoconazole shampoo, zinc pyrithione shampoo, or selenium sulfide lotion or shampoo one to four times per month.1 Oral antifungals also are effective for prevention of recurrence, but should be used only if the condition is refractory to topical prophylaxis.20,25 It is important to remember that hypopigmentation and hyperpigmentation may persist for months after resolution of active infection; absence of hyphae on skin scraping prepared with KOH confirms absence of active disease.15,16
References
- BMJ. 2015;350:h1394. doi:10.1136/bmj.h1394.
- Lancet. 2004 Sep 25-Oct 1;364(9440):1173-82.
- Pediatr Dermatol. 1991;8(1):9-12.
- J Eur Acad Dermatol Venereol. 2002;16(1):19-33.
- Arch Dermatol. 2009;145(9):1078.
- Vitiligo and other disorders of pigmentation, in: “Dermatology,” Vol 1. 3rd ed. (Philadelphia: Elsevier Saunders, 2012, pp.1041-2.)
- Am J Clin Dermatol. 2000 Mar-Apr;1(2):75-80.
- Mycoses. 1995;38(5-6):227-8.
- “Skin Disorders Due to Fungi,” in: Hurwitz Clinical Pediatric Dermatology 4th ed. (Philadelphia: Saunders, 2011, pp. 396-403).
- Infect Control Hosp Epidemiol. 2002 Apr;23(4):212-6.
- Expert Opin Pharmacother. 2014 Aug;15(12):1707-13.
- Clin Microbiol Rev. 2002 Jan;15(1):21-57.
- Clin Dermatol. 2010 Mar 4;28(2):185-9.
- J Am Acad Dermatol. 1994 Sep;31(3 Pt 2):S18-20.
- Int J Dermatol. 2014 Feb;53(2):137-41
- Mycopathologia. 2006 Dec;162(6):373-6.
- J Invest Dermatol. 1978 Sep;71(3):205-8.
- Int J Dermatol. 1992 Apr;31(4):253-6.
- Pediatr Dermatol. 2000 Jan-Feb;17(1):68-9.
- Arch Dermatol. 2010 Oct;146(10):1132-40.
- Dermatology (Basel). 1997;194(1):22-4. doi:10.1159/000246179.
- Red Book Plus: 2009 Report of the Committee on Infectious Disease.
- http://www.fda.gov/Drugs/DrugSafety/ucm362415.htm
- J Cutan Med Surg. 2015 Jul-Aug;19(4):352-7.
- Arch Dermatol. 2002 Jan;138(1):69-73.
Dr. Eichenfield is chief of pediatric and adolescent dermatology at Rady Children’s Hospital-San Diego and professor of dermatology and pediatrics at the University of California, San Diego. Ms. Haddock is a medical student at the University of California, San Diego, and a research associate at the hospital. Dr. Eichenfield and Ms. Haddock said they have no relevant financial disclosures.
Email to [email protected].
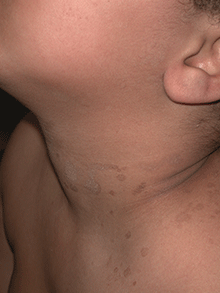
A 16-year-old male youth presents for evaluation of a worsening rash of pale spots on his chest, shoulders, neck, and back. He first noticed spots on his chest a month ago during his summer break from high school. The spots do not itch or cause any discomfort, but they have spread to his neck, back, and shoulders, and his friends on the basketball team have begun making comments about them after practice. He has no other history of skin disease, and he is otherwise healthy, with no recent illness. There is a family history of vitiligo in his father and paternal uncle. On exam, the patient is a healthy male with Fitzpatrick type V brownish skin. He has scattered hypopigmented patches ranging in size from 1 mm to 3 mm on his chest, shoulders, neck, and back. The lesions are slightly erythematous, and some of them overlap. He has a few small hypopigmented patches on his cheeks. His lesions do not appear scaly initially, but a fine scale becomes visible when the lesions are scraped with the edge of a glass slide. He has a few comedones on his forehead consistent with mild acne.