User login
Medicare at 50: Is the end near for SGR?
The Sustainable Growth Rate formula. Few aspects of Medicare have been more problematic for physicians.
Designed to control Medicare costs, Congress has spent more since 2003 to delay SGR cuts than it would cost to fund current repeal legislation.
Passed as part of the Balanced Budget Act of 1997, the SGR was designed to make sure Medicare expenditures did not grow faster than the Gross Domestic Product based on four factors: estimated change in physician fees, estimated number of Medicare fee-for-service beneficiaries, estimated 10-year average change in GDP, and estimated changes in expenditures due to law or regulation.
It was not, however, designed to keep up with the Baby Boom. Because it does not adjust for the influx of boomers, it guarantees to produce pay cuts just as demand for physician services grows.
The next pay cut – 21% this time – is slated for April 1.
For doctors, the constant specter of lower payments simply makes it hard to do business.
“Each year, when there is supposedly a cut, it is a consideration for practices that they have to create some contingency thinking, ‘if this were actually to take place, how will I continue to maintain my practice?’ ” Dr. Robert Juhasz, president of the American Osteopathic Association, said in an interview. “If they have a high Medicare population that they take care of, and certainly if there was that kind of a cut, they could not sustain that and would have to make changes.”
Dr. Blase Polite, chair of the American Society of Clinical Oncology Government Relations Committee, agreed. “Ever since the flawed formula was put in place, it has created yearly uncertainty. In recent years, we have had it to the point where the SGR cuts went into effect and then we had to do things like hold bills for a month or 2 months to resubmit them when the SGR would get patched. It became an absolute nightmare from a small business operating standpoint.”
But the tyranny of the SGR may have outgrown itself. In January, the U.S. Department of Health & Human Services announced a major expansion of its efforts to base physicians’ Medicare pay on value instead of volume, calling for half of all Medicare payments to be out of the fee-for-service system by 2018.
“This is the first time in the history of the program that explicit goals for alternative payment models and value-based payment models have been set for Medicare,” HHS Secretary Sylvia Burwell said in an editorial Jan. 26 in the New England Journal of Medicine (doi:10.1056/NEJMp1500445).
The goal is “to move away from the old way of doing things, which amounted to, ‘the more you do, the more you get paid,’ by linking nearly all pay to quality and value in some way to see that we are spending smarter,” Ms. Burwell said in a blog post on the HHS website.
As part of that effort, the department aims to have 30% of Medicare payments tied to quality or value through alternative payment models by the end of 2016.
Further, real efforts to repeal the SGR took hold in the last Congress, with strong support to pass legislation among lawmakers of both parties in the House and in the Senate. H.R. 4015, SGR Repeal and Medicare Provider Payment Modernization Act of 2014, passed the House but was not taken up by the Senate.
“We were cautiously optimistic that this 17th year of trying to repeal the SGR might have been the successful one,” Dr. Patrick T. O’Gara, president of the American College of Cardiology, said in an interview. He said that the sticking point seemed to be that “there was no politically viable way to pay for it.”
Dr. David A. Fleming, president of the American College of Physicians, noted in a statement that finding the money had hung up what otherwise was huge progress: a bill that members of the House and Senate, Republicans and Democrats had put together, and that ultimately passed the House.
Renewed efforts at repeal are underway in the current Congress. The House Energy and Commerce Committee’s Health Subcommittee held 2 days of hearings in January, hearing from doctors and health economists on how to cover the $140 billion cost of SGR repeal. Although experts presented their thoughts on where the health care sector could come up with the money, Rep. Fred Upton (R-Mich.), chairman of the full committee, recently suggested that the funding might come from outside the health care sector, such as from his proposal to legalize Internet poker.
But with time running short, some believe that another SGR patch is in the cards and that repeal will come attached to a broader piece of legislation at the end of the year.
“I don’t think there is any way it gets fixed by the March deadline because the payment offsets are just far too complicated. I think that it has to wait to be incorporated, my guess, in a larger bill,” ASCO’s Dr. Polite said. “That’s how I would do it. You want to include it in a much bigger package of tax reforms, perhaps other entitlement reforms where there’s a lot of pluses and minuses of money flow and the $140 billion for SGR is easy to take care of in that.”
He also cautioned that if it is not taken care of this year, it could be at least another 2 years before the window for repeal is opened.
“There is some talk about it getting delayed 18 months,” Dr. Polite said. “I hope that doesn’t happen because if you kick this can down the road 18 months, you basically put us in the 2016 election cycle and nobody’s coming up with $140 billion during that time. Eighteen months basically means we will see you after the 2016 presidential election. And it would be a shame.”
SGR: A patchwork of fixes
Congress has passed 17 different bills to prevent across-the-board Medicare pay cuts due to the SGR, ranging from 3.3% (2005) to 27.4% (2012). Nearly all have been paid for by some kind of offset. Here’s the sordid history:
• 2003 Consolidated Appropriations Act (4.9%)
• 2004 Medicare Prescription Drug, Improvements, and Modernization Act (4.5%)
• 2005 Medicare Prescription Drug, Improvements, and Modernization Act (3.3%)
• 2006 Deficit Reduction Act of 2005 (4.4%)
• 2007 Tax Relief and Health Care Act (5%)
• 2008 Medicare, Medicaid and SCHIP Extension Act (about 10.%)
• 2009 Medicare Improvements for Patients and Providers Act (16%)
• 2010 DOD Appropriations Act plus two Temporary Extension Acts (21%)
• 2010 Preservation of Access to Care for Medicare Beneficiaries Act (21.2%)
• 2010 Physician Payment and Therapy Relief Act (23% )
• 2011 Medicare and Medicaid Extenders Act (25%)
• 2012 Temporary Payroll Tax Cut Continuation Act (27.4%)
• 2012 Middle Class Tax Relief and Job Creation Act (27.4%)
• 2013 American Tax Payer Relief Act (26.5%)
• 2014 Pathway for SGR Reform Act (20.1%)
• 2014 Protecting Access To Medicare Act (24% )
The Sustainable Growth Rate formula. Few aspects of Medicare have been more problematic for physicians.
Designed to control Medicare costs, Congress has spent more since 2003 to delay SGR cuts than it would cost to fund current repeal legislation.
Passed as part of the Balanced Budget Act of 1997, the SGR was designed to make sure Medicare expenditures did not grow faster than the Gross Domestic Product based on four factors: estimated change in physician fees, estimated number of Medicare fee-for-service beneficiaries, estimated 10-year average change in GDP, and estimated changes in expenditures due to law or regulation.
It was not, however, designed to keep up with the Baby Boom. Because it does not adjust for the influx of boomers, it guarantees to produce pay cuts just as demand for physician services grows.
The next pay cut – 21% this time – is slated for April 1.
For doctors, the constant specter of lower payments simply makes it hard to do business.
“Each year, when there is supposedly a cut, it is a consideration for practices that they have to create some contingency thinking, ‘if this were actually to take place, how will I continue to maintain my practice?’ ” Dr. Robert Juhasz, president of the American Osteopathic Association, said in an interview. “If they have a high Medicare population that they take care of, and certainly if there was that kind of a cut, they could not sustain that and would have to make changes.”
Dr. Blase Polite, chair of the American Society of Clinical Oncology Government Relations Committee, agreed. “Ever since the flawed formula was put in place, it has created yearly uncertainty. In recent years, we have had it to the point where the SGR cuts went into effect and then we had to do things like hold bills for a month or 2 months to resubmit them when the SGR would get patched. It became an absolute nightmare from a small business operating standpoint.”
But the tyranny of the SGR may have outgrown itself. In January, the U.S. Department of Health & Human Services announced a major expansion of its efforts to base physicians’ Medicare pay on value instead of volume, calling for half of all Medicare payments to be out of the fee-for-service system by 2018.
“This is the first time in the history of the program that explicit goals for alternative payment models and value-based payment models have been set for Medicare,” HHS Secretary Sylvia Burwell said in an editorial Jan. 26 in the New England Journal of Medicine (doi:10.1056/NEJMp1500445).
The goal is “to move away from the old way of doing things, which amounted to, ‘the more you do, the more you get paid,’ by linking nearly all pay to quality and value in some way to see that we are spending smarter,” Ms. Burwell said in a blog post on the HHS website.
As part of that effort, the department aims to have 30% of Medicare payments tied to quality or value through alternative payment models by the end of 2016.
Further, real efforts to repeal the SGR took hold in the last Congress, with strong support to pass legislation among lawmakers of both parties in the House and in the Senate. H.R. 4015, SGR Repeal and Medicare Provider Payment Modernization Act of 2014, passed the House but was not taken up by the Senate.
“We were cautiously optimistic that this 17th year of trying to repeal the SGR might have been the successful one,” Dr. Patrick T. O’Gara, president of the American College of Cardiology, said in an interview. He said that the sticking point seemed to be that “there was no politically viable way to pay for it.”
Dr. David A. Fleming, president of the American College of Physicians, noted in a statement that finding the money had hung up what otherwise was huge progress: a bill that members of the House and Senate, Republicans and Democrats had put together, and that ultimately passed the House.
Renewed efforts at repeal are underway in the current Congress. The House Energy and Commerce Committee’s Health Subcommittee held 2 days of hearings in January, hearing from doctors and health economists on how to cover the $140 billion cost of SGR repeal. Although experts presented their thoughts on where the health care sector could come up with the money, Rep. Fred Upton (R-Mich.), chairman of the full committee, recently suggested that the funding might come from outside the health care sector, such as from his proposal to legalize Internet poker.
But with time running short, some believe that another SGR patch is in the cards and that repeal will come attached to a broader piece of legislation at the end of the year.
“I don’t think there is any way it gets fixed by the March deadline because the payment offsets are just far too complicated. I think that it has to wait to be incorporated, my guess, in a larger bill,” ASCO’s Dr. Polite said. “That’s how I would do it. You want to include it in a much bigger package of tax reforms, perhaps other entitlement reforms where there’s a lot of pluses and minuses of money flow and the $140 billion for SGR is easy to take care of in that.”
He also cautioned that if it is not taken care of this year, it could be at least another 2 years before the window for repeal is opened.
“There is some talk about it getting delayed 18 months,” Dr. Polite said. “I hope that doesn’t happen because if you kick this can down the road 18 months, you basically put us in the 2016 election cycle and nobody’s coming up with $140 billion during that time. Eighteen months basically means we will see you after the 2016 presidential election. And it would be a shame.”
SGR: A patchwork of fixes
Congress has passed 17 different bills to prevent across-the-board Medicare pay cuts due to the SGR, ranging from 3.3% (2005) to 27.4% (2012). Nearly all have been paid for by some kind of offset. Here’s the sordid history:
• 2003 Consolidated Appropriations Act (4.9%)
• 2004 Medicare Prescription Drug, Improvements, and Modernization Act (4.5%)
• 2005 Medicare Prescription Drug, Improvements, and Modernization Act (3.3%)
• 2006 Deficit Reduction Act of 2005 (4.4%)
• 2007 Tax Relief and Health Care Act (5%)
• 2008 Medicare, Medicaid and SCHIP Extension Act (about 10.%)
• 2009 Medicare Improvements for Patients and Providers Act (16%)
• 2010 DOD Appropriations Act plus two Temporary Extension Acts (21%)
• 2010 Preservation of Access to Care for Medicare Beneficiaries Act (21.2%)
• 2010 Physician Payment and Therapy Relief Act (23% )
• 2011 Medicare and Medicaid Extenders Act (25%)
• 2012 Temporary Payroll Tax Cut Continuation Act (27.4%)
• 2012 Middle Class Tax Relief and Job Creation Act (27.4%)
• 2013 American Tax Payer Relief Act (26.5%)
• 2014 Pathway for SGR Reform Act (20.1%)
• 2014 Protecting Access To Medicare Act (24% )
The Sustainable Growth Rate formula. Few aspects of Medicare have been more problematic for physicians.
Designed to control Medicare costs, Congress has spent more since 2003 to delay SGR cuts than it would cost to fund current repeal legislation.
Passed as part of the Balanced Budget Act of 1997, the SGR was designed to make sure Medicare expenditures did not grow faster than the Gross Domestic Product based on four factors: estimated change in physician fees, estimated number of Medicare fee-for-service beneficiaries, estimated 10-year average change in GDP, and estimated changes in expenditures due to law or regulation.
It was not, however, designed to keep up with the Baby Boom. Because it does not adjust for the influx of boomers, it guarantees to produce pay cuts just as demand for physician services grows.
The next pay cut – 21% this time – is slated for April 1.
For doctors, the constant specter of lower payments simply makes it hard to do business.
“Each year, when there is supposedly a cut, it is a consideration for practices that they have to create some contingency thinking, ‘if this were actually to take place, how will I continue to maintain my practice?’ ” Dr. Robert Juhasz, president of the American Osteopathic Association, said in an interview. “If they have a high Medicare population that they take care of, and certainly if there was that kind of a cut, they could not sustain that and would have to make changes.”
Dr. Blase Polite, chair of the American Society of Clinical Oncology Government Relations Committee, agreed. “Ever since the flawed formula was put in place, it has created yearly uncertainty. In recent years, we have had it to the point where the SGR cuts went into effect and then we had to do things like hold bills for a month or 2 months to resubmit them when the SGR would get patched. It became an absolute nightmare from a small business operating standpoint.”
But the tyranny of the SGR may have outgrown itself. In January, the U.S. Department of Health & Human Services announced a major expansion of its efforts to base physicians’ Medicare pay on value instead of volume, calling for half of all Medicare payments to be out of the fee-for-service system by 2018.
“This is the first time in the history of the program that explicit goals for alternative payment models and value-based payment models have been set for Medicare,” HHS Secretary Sylvia Burwell said in an editorial Jan. 26 in the New England Journal of Medicine (doi:10.1056/NEJMp1500445).
The goal is “to move away from the old way of doing things, which amounted to, ‘the more you do, the more you get paid,’ by linking nearly all pay to quality and value in some way to see that we are spending smarter,” Ms. Burwell said in a blog post on the HHS website.
As part of that effort, the department aims to have 30% of Medicare payments tied to quality or value through alternative payment models by the end of 2016.
Further, real efforts to repeal the SGR took hold in the last Congress, with strong support to pass legislation among lawmakers of both parties in the House and in the Senate. H.R. 4015, SGR Repeal and Medicare Provider Payment Modernization Act of 2014, passed the House but was not taken up by the Senate.
“We were cautiously optimistic that this 17th year of trying to repeal the SGR might have been the successful one,” Dr. Patrick T. O’Gara, president of the American College of Cardiology, said in an interview. He said that the sticking point seemed to be that “there was no politically viable way to pay for it.”
Dr. David A. Fleming, president of the American College of Physicians, noted in a statement that finding the money had hung up what otherwise was huge progress: a bill that members of the House and Senate, Republicans and Democrats had put together, and that ultimately passed the House.
Renewed efforts at repeal are underway in the current Congress. The House Energy and Commerce Committee’s Health Subcommittee held 2 days of hearings in January, hearing from doctors and health economists on how to cover the $140 billion cost of SGR repeal. Although experts presented their thoughts on where the health care sector could come up with the money, Rep. Fred Upton (R-Mich.), chairman of the full committee, recently suggested that the funding might come from outside the health care sector, such as from his proposal to legalize Internet poker.
But with time running short, some believe that another SGR patch is in the cards and that repeal will come attached to a broader piece of legislation at the end of the year.
“I don’t think there is any way it gets fixed by the March deadline because the payment offsets are just far too complicated. I think that it has to wait to be incorporated, my guess, in a larger bill,” ASCO’s Dr. Polite said. “That’s how I would do it. You want to include it in a much bigger package of tax reforms, perhaps other entitlement reforms where there’s a lot of pluses and minuses of money flow and the $140 billion for SGR is easy to take care of in that.”
He also cautioned that if it is not taken care of this year, it could be at least another 2 years before the window for repeal is opened.
“There is some talk about it getting delayed 18 months,” Dr. Polite said. “I hope that doesn’t happen because if you kick this can down the road 18 months, you basically put us in the 2016 election cycle and nobody’s coming up with $140 billion during that time. Eighteen months basically means we will see you after the 2016 presidential election. And it would be a shame.”
SGR: A patchwork of fixes
Congress has passed 17 different bills to prevent across-the-board Medicare pay cuts due to the SGR, ranging from 3.3% (2005) to 27.4% (2012). Nearly all have been paid for by some kind of offset. Here’s the sordid history:
• 2003 Consolidated Appropriations Act (4.9%)
• 2004 Medicare Prescription Drug, Improvements, and Modernization Act (4.5%)
• 2005 Medicare Prescription Drug, Improvements, and Modernization Act (3.3%)
• 2006 Deficit Reduction Act of 2005 (4.4%)
• 2007 Tax Relief and Health Care Act (5%)
• 2008 Medicare, Medicaid and SCHIP Extension Act (about 10.%)
• 2009 Medicare Improvements for Patients and Providers Act (16%)
• 2010 DOD Appropriations Act plus two Temporary Extension Acts (21%)
• 2010 Preservation of Access to Care for Medicare Beneficiaries Act (21.2%)
• 2010 Physician Payment and Therapy Relief Act (23% )
• 2011 Medicare and Medicaid Extenders Act (25%)
• 2012 Temporary Payroll Tax Cut Continuation Act (27.4%)
• 2012 Middle Class Tax Relief and Job Creation Act (27.4%)
• 2013 American Tax Payer Relief Act (26.5%)
• 2014 Pathway for SGR Reform Act (20.1%)
• 2014 Protecting Access To Medicare Act (24% )
Cost of ACA lowers budget deficit
The Congressional Budget Office (CBO) has reduced its budget deficit estimate over the next decade because of lower projected costs for the Affordable Care Act.
Updated projections released March 9 by the CBO find cumulative deficits between 2016 and 2025 will be $431 billion less than the office’s January projection of $7.6 trillion.
Lower costs for ACA provisions driven by smaller spending growth for insurance subsidies, the Children’s Health Insurance Program (CHIP), and Medicaid drove the reduced figure, the CBO said in an online post.
The total projected cost of ACA provisions to the federal government over the next 9 years is $1.2 billion, 11% less than CBO and the Joint Committee on Taxation estimated in January.
However, CBO predicts the annual budget deficit will rise to $486 billion in fiscal 2015, slightly higher than last year’s shortfall. The latest deficit estimate for 2015 is $18 billion higher than CBO had originally projected.
CBO said the estimated rise stems primarily from a projected rise in spending for student loans, Medicaid, and Medicare. The deficit for 2015 represents a slightly lower percentage of gross domestic product of 2.7%, compared with 2.8% last year.
On Twitter @legal_med
The Congressional Budget Office (CBO) has reduced its budget deficit estimate over the next decade because of lower projected costs for the Affordable Care Act.
Updated projections released March 9 by the CBO find cumulative deficits between 2016 and 2025 will be $431 billion less than the office’s January projection of $7.6 trillion.
Lower costs for ACA provisions driven by smaller spending growth for insurance subsidies, the Children’s Health Insurance Program (CHIP), and Medicaid drove the reduced figure, the CBO said in an online post.
The total projected cost of ACA provisions to the federal government over the next 9 years is $1.2 billion, 11% less than CBO and the Joint Committee on Taxation estimated in January.
However, CBO predicts the annual budget deficit will rise to $486 billion in fiscal 2015, slightly higher than last year’s shortfall. The latest deficit estimate for 2015 is $18 billion higher than CBO had originally projected.
CBO said the estimated rise stems primarily from a projected rise in spending for student loans, Medicaid, and Medicare. The deficit for 2015 represents a slightly lower percentage of gross domestic product of 2.7%, compared with 2.8% last year.
On Twitter @legal_med
The Congressional Budget Office (CBO) has reduced its budget deficit estimate over the next decade because of lower projected costs for the Affordable Care Act.
Updated projections released March 9 by the CBO find cumulative deficits between 2016 and 2025 will be $431 billion less than the office’s January projection of $7.6 trillion.
Lower costs for ACA provisions driven by smaller spending growth for insurance subsidies, the Children’s Health Insurance Program (CHIP), and Medicaid drove the reduced figure, the CBO said in an online post.
The total projected cost of ACA provisions to the federal government over the next 9 years is $1.2 billion, 11% less than CBO and the Joint Committee on Taxation estimated in January.
However, CBO predicts the annual budget deficit will rise to $486 billion in fiscal 2015, slightly higher than last year’s shortfall. The latest deficit estimate for 2015 is $18 billion higher than CBO had originally projected.
CBO said the estimated rise stems primarily from a projected rise in spending for student loans, Medicaid, and Medicare. The deficit for 2015 represents a slightly lower percentage of gross domestic product of 2.7%, compared with 2.8% last year.
On Twitter @legal_med
LISTEN NOW: David Pressel, MD, PHD, FHM, discusses violence in hospitals
DAVID PRESSEL, MD, PHD, FHM, medical director of inpatient services at Nemours Children’s Health System, talks about the nature of violence in hospitals and a training program he has helped put into place at his center.
DAVID PRESSEL, MD, PHD, FHM, medical director of inpatient services at Nemours Children’s Health System, talks about the nature of violence in hospitals and a training program he has helped put into place at his center.
DAVID PRESSEL, MD, PHD, FHM, medical director of inpatient services at Nemours Children’s Health System, talks about the nature of violence in hospitals and a training program he has helped put into place at his center.
LISTEN NOW: David Lichtman, PA, explains factors to determine when hospitalists perform procedures
DAVID LICHTMAN, PA, a hospitalist and director of the Johns Hopkins Central Procedure Service, explains the complicated set of factors used by
individual hospitals to determine which procedures fall under the scope of their HM practitioners.
DAVID LICHTMAN, PA, a hospitalist and director of the Johns Hopkins Central Procedure Service, explains the complicated set of factors used by
individual hospitals to determine which procedures fall under the scope of their HM practitioners.
DAVID LICHTMAN, PA, a hospitalist and director of the Johns Hopkins Central Procedure Service, explains the complicated set of factors used by
individual hospitals to determine which procedures fall under the scope of their HM practitioners.
Supreme Court justices appear split on ACA tax subsidies
Supreme Court justices appear to differ over whether the Affordable Care Act allows tax subsidies for patients who purchase insurance through the federal exchange.
During oral arguments March 4 in King v. Burwell, justices expressed mixed perspective not only on the ACA language on tax credits, but also on the ramifications of striking down use of the subsidies in states that rely on the federal exchange.
“One thing that was surprising was that the justices spent a lot of time asking questions about the consequences of agreeing with the petitioners,” said Brian M. Pinheiro, a Philadelphia-based health care and employee benefits attorney who attended the oral arguments. He said that some justices asked questions to find out “ ‘if the court struck down subsidies in the 34 states that had federal exchanges, what would that do to that law? What would that do to the health system, and could Congress have intended that result?’ I think that did trouble several of the justices who were thinking of the ultimate consequences of the decision.”
King v. Burwell centers on whether people who live in states that rely on the federal marketplace are eligible for tax credits to purchase insurance or whether such assistance can go only to residents whose states run their own marketplaces. The ACA states that tax credits apply to insurance purchased through an exchange “established by the state.” Challengers argue the law does not mention the federal exchange. The government interprets the ACA to allow subsidies whenever patients buys insurance on any exchange. Only 16 states and the District of Columbia have established a state-based exchange.
Recent research from the Urban Institute predicts that as many as 6 million Americans could lose their insurance coverage if the court rules against the government and strikes down the federal subsidies. Further, analysts from consultancy Avalere Health predict also that patients could see significant premium increases and health care providers could lose billions due to increased uncompensated care.
During the March 4 debate, the court’s four liberal justices appeared to side with the government’s reading of the law, including Justice Ruth Bader-Ginsberg and Justice Sonia Sotomayor, said Eric J. Segall, professor of law at Georgia State University, Atlanta. Justice Sotomayor said the plaintiffs’ interpretation of the ACA would mean Congress intended to coerce states into creating exchanges.
“The choice the state had was establish your own exchange or let the federal government establish it for you,” Justice Sotomayor said to plaintiff’s attorney Michael Carvin. “If we read it the way you’re saying, then we’re going to read a statute as intruding on the federal/state relationship because then the states are going to be coerced into establishing their own exchanges. In those states that don’t, their citizens don’t receive subsidies. We’re going to have the death spiral that this system was created to avoid.”
But the high court’s more conservative judges, including Justice Samuel Alito and Justice Antonin Scalia, appeared to agree with the plaintiff’s reading. Justice Scalia noted that whether the ACA functions well or not based on King’s interpretation should not be the issue.
“Is it not the case that if the only reasonable interpretation of a particular provision produces disastrous consequences in the rest of the statute, it nonetheless means what it says?” Justice Scalia asked Solicitor General Donald B. Verrilli Jr., who represented the government. Justice Scalia stressed that addressing flaws within a law is the business of legislators. “You really think Congress is just going to sit there while all of these disastrous consequences ensue?”
Justices also grilled attorneys about whether the plaintiffs in the case have standing to sue, an issue that arose late in the litigation. The question surrounds whether all four plaintiffs have legal authority to challenge the ACA since some, or all, may not be penalized if they do not buy health insurance. Mr. Carvin argued the plaintiffs have clear standing to sue, while Mr. Verrilli indicated that the government was not interested in having the case decided on the basis of standing.
Although dismissing the case based on standing would be an easy out for the Supreme Court, the outcome is highly unlikely, Mr. Segall said.
“The court will do what it wants, regardless of the law of standing,” he said in an interview. “It felt like nobody wanted to get rid of this case based on standing. They’re going to basically ignore” the issue.
Mr. Pinheiro said that he believed the government had the stronger case. He predicted the Supreme Court will rule 6-3 in favor of the government.
Ilya Shapiro of the Cato Institute had originally predicted a 6-3 rule in favor of the challengers. However, after the arguments, he now says it’s anyone’s call.
“My only prediction is that it’s a complete toss-up,” he said in an interview. “Whichever side anyone thought had the edge before argument has to temper their expectations, because I would give each side an even 50-50 shot at this point.”
The Justices’ decision is expected in June.
On Twitter @legal_med
Supreme Court justices appear to differ over whether the Affordable Care Act allows tax subsidies for patients who purchase insurance through the federal exchange.
During oral arguments March 4 in King v. Burwell, justices expressed mixed perspective not only on the ACA language on tax credits, but also on the ramifications of striking down use of the subsidies in states that rely on the federal exchange.
“One thing that was surprising was that the justices spent a lot of time asking questions about the consequences of agreeing with the petitioners,” said Brian M. Pinheiro, a Philadelphia-based health care and employee benefits attorney who attended the oral arguments. He said that some justices asked questions to find out “ ‘if the court struck down subsidies in the 34 states that had federal exchanges, what would that do to that law? What would that do to the health system, and could Congress have intended that result?’ I think that did trouble several of the justices who were thinking of the ultimate consequences of the decision.”
King v. Burwell centers on whether people who live in states that rely on the federal marketplace are eligible for tax credits to purchase insurance or whether such assistance can go only to residents whose states run their own marketplaces. The ACA states that tax credits apply to insurance purchased through an exchange “established by the state.” Challengers argue the law does not mention the federal exchange. The government interprets the ACA to allow subsidies whenever patients buys insurance on any exchange. Only 16 states and the District of Columbia have established a state-based exchange.
Recent research from the Urban Institute predicts that as many as 6 million Americans could lose their insurance coverage if the court rules against the government and strikes down the federal subsidies. Further, analysts from consultancy Avalere Health predict also that patients could see significant premium increases and health care providers could lose billions due to increased uncompensated care.
During the March 4 debate, the court’s four liberal justices appeared to side with the government’s reading of the law, including Justice Ruth Bader-Ginsberg and Justice Sonia Sotomayor, said Eric J. Segall, professor of law at Georgia State University, Atlanta. Justice Sotomayor said the plaintiffs’ interpretation of the ACA would mean Congress intended to coerce states into creating exchanges.
“The choice the state had was establish your own exchange or let the federal government establish it for you,” Justice Sotomayor said to plaintiff’s attorney Michael Carvin. “If we read it the way you’re saying, then we’re going to read a statute as intruding on the federal/state relationship because then the states are going to be coerced into establishing their own exchanges. In those states that don’t, their citizens don’t receive subsidies. We’re going to have the death spiral that this system was created to avoid.”
But the high court’s more conservative judges, including Justice Samuel Alito and Justice Antonin Scalia, appeared to agree with the plaintiff’s reading. Justice Scalia noted that whether the ACA functions well or not based on King’s interpretation should not be the issue.
“Is it not the case that if the only reasonable interpretation of a particular provision produces disastrous consequences in the rest of the statute, it nonetheless means what it says?” Justice Scalia asked Solicitor General Donald B. Verrilli Jr., who represented the government. Justice Scalia stressed that addressing flaws within a law is the business of legislators. “You really think Congress is just going to sit there while all of these disastrous consequences ensue?”
Justices also grilled attorneys about whether the plaintiffs in the case have standing to sue, an issue that arose late in the litigation. The question surrounds whether all four plaintiffs have legal authority to challenge the ACA since some, or all, may not be penalized if they do not buy health insurance. Mr. Carvin argued the plaintiffs have clear standing to sue, while Mr. Verrilli indicated that the government was not interested in having the case decided on the basis of standing.
Although dismissing the case based on standing would be an easy out for the Supreme Court, the outcome is highly unlikely, Mr. Segall said.
“The court will do what it wants, regardless of the law of standing,” he said in an interview. “It felt like nobody wanted to get rid of this case based on standing. They’re going to basically ignore” the issue.
Mr. Pinheiro said that he believed the government had the stronger case. He predicted the Supreme Court will rule 6-3 in favor of the government.
Ilya Shapiro of the Cato Institute had originally predicted a 6-3 rule in favor of the challengers. However, after the arguments, he now says it’s anyone’s call.
“My only prediction is that it’s a complete toss-up,” he said in an interview. “Whichever side anyone thought had the edge before argument has to temper their expectations, because I would give each side an even 50-50 shot at this point.”
The Justices’ decision is expected in June.
On Twitter @legal_med
Supreme Court justices appear to differ over whether the Affordable Care Act allows tax subsidies for patients who purchase insurance through the federal exchange.
During oral arguments March 4 in King v. Burwell, justices expressed mixed perspective not only on the ACA language on tax credits, but also on the ramifications of striking down use of the subsidies in states that rely on the federal exchange.
“One thing that was surprising was that the justices spent a lot of time asking questions about the consequences of agreeing with the petitioners,” said Brian M. Pinheiro, a Philadelphia-based health care and employee benefits attorney who attended the oral arguments. He said that some justices asked questions to find out “ ‘if the court struck down subsidies in the 34 states that had federal exchanges, what would that do to that law? What would that do to the health system, and could Congress have intended that result?’ I think that did trouble several of the justices who were thinking of the ultimate consequences of the decision.”
King v. Burwell centers on whether people who live in states that rely on the federal marketplace are eligible for tax credits to purchase insurance or whether such assistance can go only to residents whose states run their own marketplaces. The ACA states that tax credits apply to insurance purchased through an exchange “established by the state.” Challengers argue the law does not mention the federal exchange. The government interprets the ACA to allow subsidies whenever patients buys insurance on any exchange. Only 16 states and the District of Columbia have established a state-based exchange.
Recent research from the Urban Institute predicts that as many as 6 million Americans could lose their insurance coverage if the court rules against the government and strikes down the federal subsidies. Further, analysts from consultancy Avalere Health predict also that patients could see significant premium increases and health care providers could lose billions due to increased uncompensated care.
During the March 4 debate, the court’s four liberal justices appeared to side with the government’s reading of the law, including Justice Ruth Bader-Ginsberg and Justice Sonia Sotomayor, said Eric J. Segall, professor of law at Georgia State University, Atlanta. Justice Sotomayor said the plaintiffs’ interpretation of the ACA would mean Congress intended to coerce states into creating exchanges.
“The choice the state had was establish your own exchange or let the federal government establish it for you,” Justice Sotomayor said to plaintiff’s attorney Michael Carvin. “If we read it the way you’re saying, then we’re going to read a statute as intruding on the federal/state relationship because then the states are going to be coerced into establishing their own exchanges. In those states that don’t, their citizens don’t receive subsidies. We’re going to have the death spiral that this system was created to avoid.”
But the high court’s more conservative judges, including Justice Samuel Alito and Justice Antonin Scalia, appeared to agree with the plaintiff’s reading. Justice Scalia noted that whether the ACA functions well or not based on King’s interpretation should not be the issue.
“Is it not the case that if the only reasonable interpretation of a particular provision produces disastrous consequences in the rest of the statute, it nonetheless means what it says?” Justice Scalia asked Solicitor General Donald B. Verrilli Jr., who represented the government. Justice Scalia stressed that addressing flaws within a law is the business of legislators. “You really think Congress is just going to sit there while all of these disastrous consequences ensue?”
Justices also grilled attorneys about whether the plaintiffs in the case have standing to sue, an issue that arose late in the litigation. The question surrounds whether all four plaintiffs have legal authority to challenge the ACA since some, or all, may not be penalized if they do not buy health insurance. Mr. Carvin argued the plaintiffs have clear standing to sue, while Mr. Verrilli indicated that the government was not interested in having the case decided on the basis of standing.
Although dismissing the case based on standing would be an easy out for the Supreme Court, the outcome is highly unlikely, Mr. Segall said.
“The court will do what it wants, regardless of the law of standing,” he said in an interview. “It felt like nobody wanted to get rid of this case based on standing. They’re going to basically ignore” the issue.
Mr. Pinheiro said that he believed the government had the stronger case. He predicted the Supreme Court will rule 6-3 in favor of the government.
Ilya Shapiro of the Cato Institute had originally predicted a 6-3 rule in favor of the challengers. However, after the arguments, he now says it’s anyone’s call.
“My only prediction is that it’s a complete toss-up,” he said in an interview. “Whichever side anyone thought had the edge before argument has to temper their expectations, because I would give each side an even 50-50 shot at this point.”
The Justices’ decision is expected in June.
On Twitter @legal_med
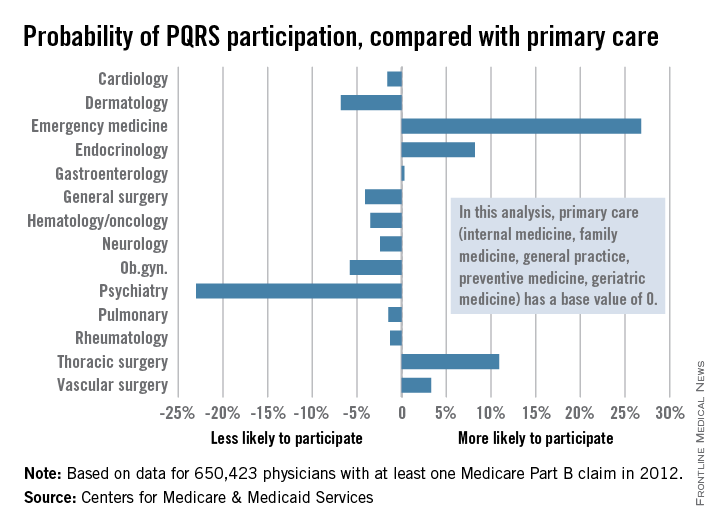
PQRS participation varies by specialty
Participation in the Physician Quality Reporting System (PQRS) varies considerably by specialty, according to an new analysis of 2012 data using primary care physicians as the base, the Centers for Medicare & Medicaid Services reported.
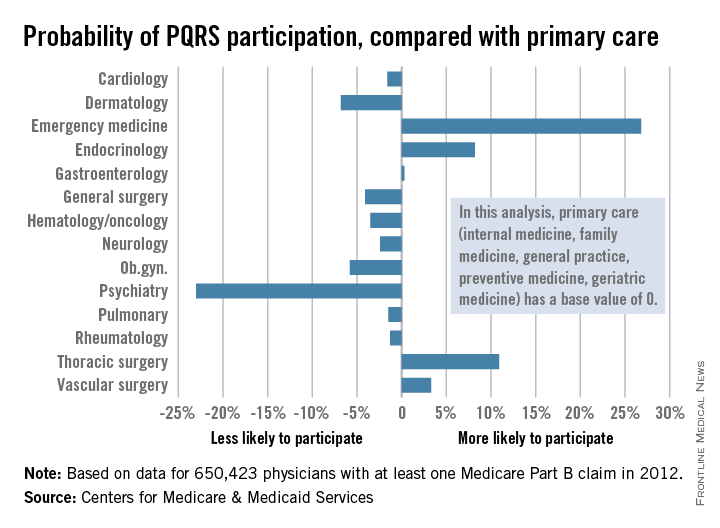
Emergency medicine physicians were almost 27% more likely to participate in PQRS than were physicians in primary care (internal medicine, family medicine, general practice, preventive medicine, and geriatrics). On the other end of the scale were psychiatrists, who were 23% less likely than were primary care physicians to participate in PQRS, according to the CMS.
Among surgical specialties, general surgeons were 4% less likely to participate in PQRS, but thoracic surgeons and vascular surgeons were 11% and 3%, respectively, more likely to participate, compared with primary care physicians.
The PQRS participation rate in 2012 was 41.3% overall for the 650,423 MDs/DOs who submitted at least one Medicare Part B claim that year. Other health care professionals – including podiatrists, chiropractors, nurse practitioners, psychologists, and physical therapists – are eligible for PQRS but were not included in this analysis, the CMS said.
Participation in the Physician Quality Reporting System (PQRS) varies considerably by specialty, according to an new analysis of 2012 data using primary care physicians as the base, the Centers for Medicare & Medicaid Services reported.

Emergency medicine physicians were almost 27% more likely to participate in PQRS than were physicians in primary care (internal medicine, family medicine, general practice, preventive medicine, and geriatrics). On the other end of the scale were psychiatrists, who were 23% less likely than were primary care physicians to participate in PQRS, according to the CMS.
Among surgical specialties, general surgeons were 4% less likely to participate in PQRS, but thoracic surgeons and vascular surgeons were 11% and 3%, respectively, more likely to participate, compared with primary care physicians.
The PQRS participation rate in 2012 was 41.3% overall for the 650,423 MDs/DOs who submitted at least one Medicare Part B claim that year. Other health care professionals – including podiatrists, chiropractors, nurse practitioners, psychologists, and physical therapists – are eligible for PQRS but were not included in this analysis, the CMS said.
Participation in the Physician Quality Reporting System (PQRS) varies considerably by specialty, according to an new analysis of 2012 data using primary care physicians as the base, the Centers for Medicare & Medicaid Services reported.

Emergency medicine physicians were almost 27% more likely to participate in PQRS than were physicians in primary care (internal medicine, family medicine, general practice, preventive medicine, and geriatrics). On the other end of the scale were psychiatrists, who were 23% less likely than were primary care physicians to participate in PQRS, according to the CMS.
Among surgical specialties, general surgeons were 4% less likely to participate in PQRS, but thoracic surgeons and vascular surgeons were 11% and 3%, respectively, more likely to participate, compared with primary care physicians.
The PQRS participation rate in 2012 was 41.3% overall for the 650,423 MDs/DOs who submitted at least one Medicare Part B claim that year. Other health care professionals – including podiatrists, chiropractors, nurse practitioners, psychologists, and physical therapists – are eligible for PQRS but were not included in this analysis, the CMS said.
What Is the Best Approach to a Cavitary Lung Lesion?
Case
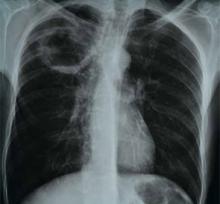
A 66-year-old homeless man with a history of smoking and cirrhosis due to alcoholism presents to the hospital with a productive cough and fever for one month. He has traveled around Arizona and New Mexico but has never left the country. His complete blood count (CBC) is notable for a white blood cell count of 13,000. His chest X-ray reveals a 1.7-cm right upper lobe cavitary lung lesion (see Figure 1). What is the best approach to this patient’s cavitary lung lesion?
Overview
Cavitary lung lesions are relatively common findings on chest imaging and often pose a diagnostic challenge to the hospitalist. Having a standard approach to the evaluation of a cavitary lung lesion can facilitate an expedited workup.
A lung cavity is defined radiographically as a lucent area contained within a consolidation, mass, or nodule.1 Cavities usually are accompanied by thick walls, greater than 4 mm. These should be differentiated from cysts, which are not surrounded by consolidation, mass, or nodule, and are accompanied by a thinner wall.2
The differential diagnosis of a cavitary lung lesion is broad and can be delineated into categories of infectious and noninfectious etiologies (see Figure 2). Infectious causes include bacterial, fungal, and, rarely, parasitic agents. Noninfectious causes encompass malignant, rheumatologic, and other less common etiologies such as infarct related to pulmonary embolism.
The clinical presentation and assessment of risk factors for a particular patient are of the utmost importance in delineating next steps for evaluation and management (see Table 1). For those patients of older age with smoking history, specific occupational or environmental exposures, and weight loss, the most common etiology is neoplasm. Common infectious causes include lung abscess and necrotizing pneumonia, as well as tuberculosis. The approach to diagnosis should be based on a composite of the clinical presentation, patient characteristics, and radiographic appearance of the cavity.
Guidelines for the approach to cavitary lung lesions are lacking, yet a thorough understanding of the initial approach is important for those practicing hospital medicine. Key components in the approach to diagnosis of a solitary cavitary lesion are outlined in this article.
Diagnosis of Infectious Causes
In the initial evaluation of a cavitary lung lesion, it is important to first determine if the cause is an infectious process. The infectious etiologies to consider include lung abscess and necrotizing pneumonia, tuberculosis, and septic emboli. Important components in the clinical presentation include presence of cough, fever, night sweats, chills, and symptoms that have lasted less than one month, as well as comorbid conditions, drug or alcohol abuse, and history of immunocompromise (e.g. HIV, immunosuppressive therapy, or organ transplant).
Given the public health considerations and impact of treatment, tuberculosis (TB) will be discussed in its own category.
Tuberculosis. Given the fact that TB patients require airborne isolation, the disease must be considered early in the evaluation of a cavitary lung lesion. Patients with TB often present with more chronic symptoms, such as fevers, night sweats, weight loss, and hemoptysis. Immunocompromised state, travel to endemic regions, and incarceration increase the likelihood of TB. Nontuberculous mycobacterium (i.e., M. kansasii) should also be considered in endemic areas.
For those patients in whom TB is suspected, airborne isolation must be initiated promptly. The provider should obtain three sputum samples for acid-fast bacillus (AFB) smear and culture when risk factors are present. Most patients with reactivation TB have abnormal chest X-rays, with approximately 20% of those patients having air-fluid levels and the majority of cases affecting the upper lobes.3 Cavities may be seen in patients with primary or reactivation TB.3
Lung abscess and necrotizing pneumonia. Lung abscesses are cavities associated with necrosis caused by a microbial infection. The term necrotizing pneumonia typically is used when there are multiple smaller (smaller than 2 cm) associated lung abscesses, although both lung abscess and necrotizing pneumonia represent a similar pathophysiologic process and are along the same continuum. Lung abscess is suspected with the presence of predisposing risk factors to aspiration (e.g. alcoholism) and poor dentition. History of cough, fever, putrid sputum, night sweats, and weight loss may indicate subacute or chronic development of a lung abscess. Physical examination might be significant for signs of pneumonia and gingivitis.
Organisms that cause lung abscesses include anaerobes (most common), TB, methicillin-resistant Staphylococcus aureus (MRSA), post-influenza illness, endemic fungi, and Nocardia, among others.4 In immunocompromised patients, more common considerations include TB, Mycobacterium avium complex, other mycobacteria, Pseudomonas aeruginosa, Nocardia, Cryptococcus, Aspergillus, endemic fungi (e.g. Coccidiodes in the Southwest and Histoplasma in the Midwest), and, less commonly, Pneumocystis jiroveci.4 The likelihood of each organism is dependent on the patient’s risk factors. Initial laboratory testing includes sputum and blood cultures, as well as serologic testing for endemic fungi, especially in immunocompromised patients.
Imaging may reveal a cavitary lesion in the dependent pulmonary segments (posterior segments of the upper lobes or superior segments of the lower lobes), at times associated with a pleural effusion or infiltrate. The most common appearance of a lung abscess is an asymmetric cavity with an air-fluid level and a wall with a ragged or smooth border. CT scan is often indicated when X-rays are equivocal and when cases are of uncertain cause or are unresponsive to antibiotic therapy. Bronchoscopy is reserved for patients with an immunocompromising condition, atypical presentation, or lack of response to treatment.
For those cavitary lesions in which there is a high degree of suspicion for lung abscess, empiric treatment should include antibiotics active against anaerobes and MRSA if the patient has risk factors. Patients often receive an empiric trial of antibiotics prior to biopsy unless there are clear indications that the cavitary lung lesion is related to cancer. Lung abscesses typically drain spontaneously, and transthoracic or endobronchial drainage is not usually recommended as initial management due to risk of pneumothorax and formation of bronchopleural fistula.
Lung abscesses should be followed to resolution with serial chest imaging. If the lung abscess does not resolve, it would be appropriate to consult thoracic surgery, interventional radiology, or pulmonary, depending on the location of the abscess and the local expertise with transthoracic or endobronchial drainage and surgical resection.
Septic emboli. Septic emboli are a less common cause of cavitary lung lesions. This entity should be considered in patients with a history of IV drug use or infected indwelling devices (central venous catheters, pacemaker wires, and right-sided prosthetic heart valves). Physical examination should include an assessment for signs of endocarditis and inspection for infected indwelling devices. In patients with IV drug use, the likely pathogen is S. aureus.
Oropharyngeal infection or indwelling catheters may predispose patients to septic thrombophlebitis of the internal jugular vein, also known as Lemierre’s syndrome, a rare but important cause of septic emboli.5 Laboratory testing includes culture for sputum and blood and culture of the infected device if applicable. On chest X-ray, septic emboli commonly appear as nodules located in the lung periphery. CT scan is more sensitive for detecting cavitation associated with septic emboli.
Diagnosis of Noninfectious Causes
Upon identification of a cavitary lung lesion, noninfectious etiologies must also be entertained. Noninfectious etiologies include malignancy, rheumatologic diseases, pulmonary embolism, and other causes. Important components in the clinical presentation include the presence of constitutional symptoms (fevers, weight loss, night sweats), smoking history, family history, and an otherwise complete review of systems. Physical exam should include evaluation for lymphadenopathy, cachexia, rash, clubbing, and other symptoms pertinent to the suspected etiology.
Malignancy. Perhaps most important among noninfectious causes of cavitary lung lesions is malignancy, and a high index of suspicion is warranted given that it is commonly the first diagnosis to consider overall.2 Cavities can form in primary lung cancers (e.g. bronchogenic carcinomas), lung tumors such as lymphoma or Kaposi’s sarcoma, or in metastatic disease. Cavitation has been detected in 7%-11% of primary lung cancers by plain radiography and in 22% by computed tomography.5 Cancers of squamous cell origin are the most likely to cavitate; this holds true for both primary lung tumors and metastatic tumors.6 Additionally, cavitation portends a worse prognosis.7
Clinicians should review any available prior chest imaging studies to look for a change in the quality or size of a cavitary lung lesion. Neoplasms are typically of variable size with irregular thick walls (greater than 4 mm) on CT scan, with higher specificity for neoplasm in those with a wall thickness greater than 15 mm.2
When the diagnosis is less clear, the decision to embark on more advanced diagnostic methods, such as biopsy, should rest on the provider’s clinical suspicion for a certain disease process. When a lung cancer is suspected, consultation with pulmonary and interventional radiology should be obtained to determine the best approach for biopsy.
Rheumatologic. Less common causes of cavitary lesions include those related to rheumatologic diseases (e.g. granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis). One study demonstrated that cavitary lung nodules occur in 37% of patients with granulomatosis with polyangiitis.8
Although uncommon, cavitary nodules can also be seen in rheumatoid arthritis and sarcoidosis. Given that patients with rheumatologic diseases are often treated with immunosuppressive agents, infection must remain high on the differential. Suspicion of a rheumatologic cause should prompt the clinician to obtain appropriate serologic testing and consultation as needed.
Pulmonary embolism. Although often not considered in the evaluation of cavitary lung lesions, pulmonary embolism (PE) can lead to infarction and the formation of a cavitary lesion. Pulmonary infarction has been reported to occur in as many as one third of cases of PE.9 Cavitary lesions also have been described in chronic thromboembolic disease.10
Other. Uncommon causes of cavitary lesions include bronchiolitis obliterans with organizing pneumonia, Langerhans cell histiocytosis, and amyloidosis, among others. The hospitalist should keep a broad differential and involve consultants if the diagnosis remains unclear after initial diagnostic evaluation.
Back to the Case
The patient’s fever and productive cough, in combination with recent travel and location of the cavitary lesion, increase his risk for tuberculosis and endemic fungi, such as Coccidioides. This patient was placed on respiratory isolation with AFBs obtained to rule out TB, with Coccidioides antibodies, Cyptococcal antigen titers, and sputum for fungus sent to evaluate for an endemic fungus. He had a chest CT, which revealed a 17-mm cavitary mass within the right upper lobe that contained an air-fluid level indicating lung abscess. Coccidioides, cryptococcal, fungal sputum, and TB studies were negative.
The patient was treated empirically with clindamycin given the high prevalence of anaerobes in lung abscess. He was followed as an outpatient and had a chest X-ray showing resolution of the lesion at six months. The purpose of the X-ray was two-fold: to monitor the effect of antibiotic treatment and to evaluate for persistence of the cavitation given the neoplastic risk factors of older age and smoking.
Bottom Line
The best approach to a patient with a cavitary lung lesion includes assessing the clinical presentation and risk factors, differentiating infectious from noninfectious causes, and then utilizing this information to further direct the diagnostic evaluation. Consultation with a subspecialist or further testing such as biopsy should be considered if the etiology remains undefined after the initial evaluation.
Drs. Rendon, Pizanis, Montanaro, and Kraai are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque.
References
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697-722.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc. 2003;78(6):744-752.
- Barnes PF, Verdegem TD, Vachon LA, Leedom JM, Overturf GD. Chest roentgenogram in pulmonary tuberculosis. New data on an old test. Chest. 1988;94(2):316-320.
- Yazbeck MF, Dahdel M, Kalra A, Browne AS, Pratter MR. Lung abscess: update on microbiology and management. Am J Ther. 2012;21(3):217-221. doi: 10.1097/MJT.0b013e3182383c9b.
- Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21(2):305-333.
- Chiu FT. Cavitation in lung cancers. Aust N Z J Med. 1975;5(6):523-530.
- Kolodziejski LS, Dyczek S, Duda K, Góralczyk J, Wysocki WM, Lobaziewicz W. Cavitated tumor as a clinical subentity in squamous cell lung cancer patients. Neoplasma. 2003;50(1):66-73.
- Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912.
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging. 2006;21(1):1-7.
- Harris H, Barraclough R, Davies C, Armstrong I, Kiely DG, van Beek E Jr. Cavitating lung lesions in chronic thromboembolic pulmonary hypertension. J Radiol Case Rep. 2008;2(3):11-21.
- Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135(6):1269-1271.
Case
A 66-year-old homeless man with a history of smoking and cirrhosis due to alcoholism presents to the hospital with a productive cough and fever for one month. He has traveled around Arizona and New Mexico but has never left the country. His complete blood count (CBC) is notable for a white blood cell count of 13,000. His chest X-ray reveals a 1.7-cm right upper lobe cavitary lung lesion (see Figure 1). What is the best approach to this patient’s cavitary lung lesion?
Overview
Cavitary lung lesions are relatively common findings on chest imaging and often pose a diagnostic challenge to the hospitalist. Having a standard approach to the evaluation of a cavitary lung lesion can facilitate an expedited workup.
A lung cavity is defined radiographically as a lucent area contained within a consolidation, mass, or nodule.1 Cavities usually are accompanied by thick walls, greater than 4 mm. These should be differentiated from cysts, which are not surrounded by consolidation, mass, or nodule, and are accompanied by a thinner wall.2
The differential diagnosis of a cavitary lung lesion is broad and can be delineated into categories of infectious and noninfectious etiologies (see Figure 2). Infectious causes include bacterial, fungal, and, rarely, parasitic agents. Noninfectious causes encompass malignant, rheumatologic, and other less common etiologies such as infarct related to pulmonary embolism.
The clinical presentation and assessment of risk factors for a particular patient are of the utmost importance in delineating next steps for evaluation and management (see Table 1). For those patients of older age with smoking history, specific occupational or environmental exposures, and weight loss, the most common etiology is neoplasm. Common infectious causes include lung abscess and necrotizing pneumonia, as well as tuberculosis. The approach to diagnosis should be based on a composite of the clinical presentation, patient characteristics, and radiographic appearance of the cavity.
Guidelines for the approach to cavitary lung lesions are lacking, yet a thorough understanding of the initial approach is important for those practicing hospital medicine. Key components in the approach to diagnosis of a solitary cavitary lesion are outlined in this article.
Diagnosis of Infectious Causes
In the initial evaluation of a cavitary lung lesion, it is important to first determine if the cause is an infectious process. The infectious etiologies to consider include lung abscess and necrotizing pneumonia, tuberculosis, and septic emboli. Important components in the clinical presentation include presence of cough, fever, night sweats, chills, and symptoms that have lasted less than one month, as well as comorbid conditions, drug or alcohol abuse, and history of immunocompromise (e.g. HIV, immunosuppressive therapy, or organ transplant).
Given the public health considerations and impact of treatment, tuberculosis (TB) will be discussed in its own category.
Tuberculosis. Given the fact that TB patients require airborne isolation, the disease must be considered early in the evaluation of a cavitary lung lesion. Patients with TB often present with more chronic symptoms, such as fevers, night sweats, weight loss, and hemoptysis. Immunocompromised state, travel to endemic regions, and incarceration increase the likelihood of TB. Nontuberculous mycobacterium (i.e., M. kansasii) should also be considered in endemic areas.
For those patients in whom TB is suspected, airborne isolation must be initiated promptly. The provider should obtain three sputum samples for acid-fast bacillus (AFB) smear and culture when risk factors are present. Most patients with reactivation TB have abnormal chest X-rays, with approximately 20% of those patients having air-fluid levels and the majority of cases affecting the upper lobes.3 Cavities may be seen in patients with primary or reactivation TB.3
Lung abscess and necrotizing pneumonia. Lung abscesses are cavities associated with necrosis caused by a microbial infection. The term necrotizing pneumonia typically is used when there are multiple smaller (smaller than 2 cm) associated lung abscesses, although both lung abscess and necrotizing pneumonia represent a similar pathophysiologic process and are along the same continuum. Lung abscess is suspected with the presence of predisposing risk factors to aspiration (e.g. alcoholism) and poor dentition. History of cough, fever, putrid sputum, night sweats, and weight loss may indicate subacute or chronic development of a lung abscess. Physical examination might be significant for signs of pneumonia and gingivitis.
Organisms that cause lung abscesses include anaerobes (most common), TB, methicillin-resistant Staphylococcus aureus (MRSA), post-influenza illness, endemic fungi, and Nocardia, among others.4 In immunocompromised patients, more common considerations include TB, Mycobacterium avium complex, other mycobacteria, Pseudomonas aeruginosa, Nocardia, Cryptococcus, Aspergillus, endemic fungi (e.g. Coccidiodes in the Southwest and Histoplasma in the Midwest), and, less commonly, Pneumocystis jiroveci.4 The likelihood of each organism is dependent on the patient’s risk factors. Initial laboratory testing includes sputum and blood cultures, as well as serologic testing for endemic fungi, especially in immunocompromised patients.
Imaging may reveal a cavitary lesion in the dependent pulmonary segments (posterior segments of the upper lobes or superior segments of the lower lobes), at times associated with a pleural effusion or infiltrate. The most common appearance of a lung abscess is an asymmetric cavity with an air-fluid level and a wall with a ragged or smooth border. CT scan is often indicated when X-rays are equivocal and when cases are of uncertain cause or are unresponsive to antibiotic therapy. Bronchoscopy is reserved for patients with an immunocompromising condition, atypical presentation, or lack of response to treatment.
For those cavitary lesions in which there is a high degree of suspicion for lung abscess, empiric treatment should include antibiotics active against anaerobes and MRSA if the patient has risk factors. Patients often receive an empiric trial of antibiotics prior to biopsy unless there are clear indications that the cavitary lung lesion is related to cancer. Lung abscesses typically drain spontaneously, and transthoracic or endobronchial drainage is not usually recommended as initial management due to risk of pneumothorax and formation of bronchopleural fistula.
Lung abscesses should be followed to resolution with serial chest imaging. If the lung abscess does not resolve, it would be appropriate to consult thoracic surgery, interventional radiology, or pulmonary, depending on the location of the abscess and the local expertise with transthoracic or endobronchial drainage and surgical resection.
Septic emboli. Septic emboli are a less common cause of cavitary lung lesions. This entity should be considered in patients with a history of IV drug use or infected indwelling devices (central venous catheters, pacemaker wires, and right-sided prosthetic heart valves). Physical examination should include an assessment for signs of endocarditis and inspection for infected indwelling devices. In patients with IV drug use, the likely pathogen is S. aureus.
Oropharyngeal infection or indwelling catheters may predispose patients to septic thrombophlebitis of the internal jugular vein, also known as Lemierre’s syndrome, a rare but important cause of septic emboli.5 Laboratory testing includes culture for sputum and blood and culture of the infected device if applicable. On chest X-ray, septic emboli commonly appear as nodules located in the lung periphery. CT scan is more sensitive for detecting cavitation associated with septic emboli.
Diagnosis of Noninfectious Causes
Upon identification of a cavitary lung lesion, noninfectious etiologies must also be entertained. Noninfectious etiologies include malignancy, rheumatologic diseases, pulmonary embolism, and other causes. Important components in the clinical presentation include the presence of constitutional symptoms (fevers, weight loss, night sweats), smoking history, family history, and an otherwise complete review of systems. Physical exam should include evaluation for lymphadenopathy, cachexia, rash, clubbing, and other symptoms pertinent to the suspected etiology.
Malignancy. Perhaps most important among noninfectious causes of cavitary lung lesions is malignancy, and a high index of suspicion is warranted given that it is commonly the first diagnosis to consider overall.2 Cavities can form in primary lung cancers (e.g. bronchogenic carcinomas), lung tumors such as lymphoma or Kaposi’s sarcoma, or in metastatic disease. Cavitation has been detected in 7%-11% of primary lung cancers by plain radiography and in 22% by computed tomography.5 Cancers of squamous cell origin are the most likely to cavitate; this holds true for both primary lung tumors and metastatic tumors.6 Additionally, cavitation portends a worse prognosis.7
Clinicians should review any available prior chest imaging studies to look for a change in the quality or size of a cavitary lung lesion. Neoplasms are typically of variable size with irregular thick walls (greater than 4 mm) on CT scan, with higher specificity for neoplasm in those with a wall thickness greater than 15 mm.2
When the diagnosis is less clear, the decision to embark on more advanced diagnostic methods, such as biopsy, should rest on the provider’s clinical suspicion for a certain disease process. When a lung cancer is suspected, consultation with pulmonary and interventional radiology should be obtained to determine the best approach for biopsy.
Rheumatologic. Less common causes of cavitary lesions include those related to rheumatologic diseases (e.g. granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis). One study demonstrated that cavitary lung nodules occur in 37% of patients with granulomatosis with polyangiitis.8
Although uncommon, cavitary nodules can also be seen in rheumatoid arthritis and sarcoidosis. Given that patients with rheumatologic diseases are often treated with immunosuppressive agents, infection must remain high on the differential. Suspicion of a rheumatologic cause should prompt the clinician to obtain appropriate serologic testing and consultation as needed.
Pulmonary embolism. Although often not considered in the evaluation of cavitary lung lesions, pulmonary embolism (PE) can lead to infarction and the formation of a cavitary lesion. Pulmonary infarction has been reported to occur in as many as one third of cases of PE.9 Cavitary lesions also have been described in chronic thromboembolic disease.10
Other. Uncommon causes of cavitary lesions include bronchiolitis obliterans with organizing pneumonia, Langerhans cell histiocytosis, and amyloidosis, among others. The hospitalist should keep a broad differential and involve consultants if the diagnosis remains unclear after initial diagnostic evaluation.
Back to the Case
The patient’s fever and productive cough, in combination with recent travel and location of the cavitary lesion, increase his risk for tuberculosis and endemic fungi, such as Coccidioides. This patient was placed on respiratory isolation with AFBs obtained to rule out TB, with Coccidioides antibodies, Cyptococcal antigen titers, and sputum for fungus sent to evaluate for an endemic fungus. He had a chest CT, which revealed a 17-mm cavitary mass within the right upper lobe that contained an air-fluid level indicating lung abscess. Coccidioides, cryptococcal, fungal sputum, and TB studies were negative.
The patient was treated empirically with clindamycin given the high prevalence of anaerobes in lung abscess. He was followed as an outpatient and had a chest X-ray showing resolution of the lesion at six months. The purpose of the X-ray was two-fold: to monitor the effect of antibiotic treatment and to evaluate for persistence of the cavitation given the neoplastic risk factors of older age and smoking.
Bottom Line
The best approach to a patient with a cavitary lung lesion includes assessing the clinical presentation and risk factors, differentiating infectious from noninfectious causes, and then utilizing this information to further direct the diagnostic evaluation. Consultation with a subspecialist or further testing such as biopsy should be considered if the etiology remains undefined after the initial evaluation.
Drs. Rendon, Pizanis, Montanaro, and Kraai are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque.
References
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697-722.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc. 2003;78(6):744-752.
- Barnes PF, Verdegem TD, Vachon LA, Leedom JM, Overturf GD. Chest roentgenogram in pulmonary tuberculosis. New data on an old test. Chest. 1988;94(2):316-320.
- Yazbeck MF, Dahdel M, Kalra A, Browne AS, Pratter MR. Lung abscess: update on microbiology and management. Am J Ther. 2012;21(3):217-221. doi: 10.1097/MJT.0b013e3182383c9b.
- Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21(2):305-333.
- Chiu FT. Cavitation in lung cancers. Aust N Z J Med. 1975;5(6):523-530.
- Kolodziejski LS, Dyczek S, Duda K, Góralczyk J, Wysocki WM, Lobaziewicz W. Cavitated tumor as a clinical subentity in squamous cell lung cancer patients. Neoplasma. 2003;50(1):66-73.
- Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912.
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging. 2006;21(1):1-7.
- Harris H, Barraclough R, Davies C, Armstrong I, Kiely DG, van Beek E Jr. Cavitating lung lesions in chronic thromboembolic pulmonary hypertension. J Radiol Case Rep. 2008;2(3):11-21.
- Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135(6):1269-1271.
Case
A 66-year-old homeless man with a history of smoking and cirrhosis due to alcoholism presents to the hospital with a productive cough and fever for one month. He has traveled around Arizona and New Mexico but has never left the country. His complete blood count (CBC) is notable for a white blood cell count of 13,000. His chest X-ray reveals a 1.7-cm right upper lobe cavitary lung lesion (see Figure 1). What is the best approach to this patient’s cavitary lung lesion?
Overview
Cavitary lung lesions are relatively common findings on chest imaging and often pose a diagnostic challenge to the hospitalist. Having a standard approach to the evaluation of a cavitary lung lesion can facilitate an expedited workup.
A lung cavity is defined radiographically as a lucent area contained within a consolidation, mass, or nodule.1 Cavities usually are accompanied by thick walls, greater than 4 mm. These should be differentiated from cysts, which are not surrounded by consolidation, mass, or nodule, and are accompanied by a thinner wall.2
The differential diagnosis of a cavitary lung lesion is broad and can be delineated into categories of infectious and noninfectious etiologies (see Figure 2). Infectious causes include bacterial, fungal, and, rarely, parasitic agents. Noninfectious causes encompass malignant, rheumatologic, and other less common etiologies such as infarct related to pulmonary embolism.
The clinical presentation and assessment of risk factors for a particular patient are of the utmost importance in delineating next steps for evaluation and management (see Table 1). For those patients of older age with smoking history, specific occupational or environmental exposures, and weight loss, the most common etiology is neoplasm. Common infectious causes include lung abscess and necrotizing pneumonia, as well as tuberculosis. The approach to diagnosis should be based on a composite of the clinical presentation, patient characteristics, and radiographic appearance of the cavity.
Guidelines for the approach to cavitary lung lesions are lacking, yet a thorough understanding of the initial approach is important for those practicing hospital medicine. Key components in the approach to diagnosis of a solitary cavitary lesion are outlined in this article.
Diagnosis of Infectious Causes
In the initial evaluation of a cavitary lung lesion, it is important to first determine if the cause is an infectious process. The infectious etiologies to consider include lung abscess and necrotizing pneumonia, tuberculosis, and septic emboli. Important components in the clinical presentation include presence of cough, fever, night sweats, chills, and symptoms that have lasted less than one month, as well as comorbid conditions, drug or alcohol abuse, and history of immunocompromise (e.g. HIV, immunosuppressive therapy, or organ transplant).
Given the public health considerations and impact of treatment, tuberculosis (TB) will be discussed in its own category.
Tuberculosis. Given the fact that TB patients require airborne isolation, the disease must be considered early in the evaluation of a cavitary lung lesion. Patients with TB often present with more chronic symptoms, such as fevers, night sweats, weight loss, and hemoptysis. Immunocompromised state, travel to endemic regions, and incarceration increase the likelihood of TB. Nontuberculous mycobacterium (i.e., M. kansasii) should also be considered in endemic areas.
For those patients in whom TB is suspected, airborne isolation must be initiated promptly. The provider should obtain three sputum samples for acid-fast bacillus (AFB) smear and culture when risk factors are present. Most patients with reactivation TB have abnormal chest X-rays, with approximately 20% of those patients having air-fluid levels and the majority of cases affecting the upper lobes.3 Cavities may be seen in patients with primary or reactivation TB.3
Lung abscess and necrotizing pneumonia. Lung abscesses are cavities associated with necrosis caused by a microbial infection. The term necrotizing pneumonia typically is used when there are multiple smaller (smaller than 2 cm) associated lung abscesses, although both lung abscess and necrotizing pneumonia represent a similar pathophysiologic process and are along the same continuum. Lung abscess is suspected with the presence of predisposing risk factors to aspiration (e.g. alcoholism) and poor dentition. History of cough, fever, putrid sputum, night sweats, and weight loss may indicate subacute or chronic development of a lung abscess. Physical examination might be significant for signs of pneumonia and gingivitis.
Organisms that cause lung abscesses include anaerobes (most common), TB, methicillin-resistant Staphylococcus aureus (MRSA), post-influenza illness, endemic fungi, and Nocardia, among others.4 In immunocompromised patients, more common considerations include TB, Mycobacterium avium complex, other mycobacteria, Pseudomonas aeruginosa, Nocardia, Cryptococcus, Aspergillus, endemic fungi (e.g. Coccidiodes in the Southwest and Histoplasma in the Midwest), and, less commonly, Pneumocystis jiroveci.4 The likelihood of each organism is dependent on the patient’s risk factors. Initial laboratory testing includes sputum and blood cultures, as well as serologic testing for endemic fungi, especially in immunocompromised patients.
Imaging may reveal a cavitary lesion in the dependent pulmonary segments (posterior segments of the upper lobes or superior segments of the lower lobes), at times associated with a pleural effusion or infiltrate. The most common appearance of a lung abscess is an asymmetric cavity with an air-fluid level and a wall with a ragged or smooth border. CT scan is often indicated when X-rays are equivocal and when cases are of uncertain cause or are unresponsive to antibiotic therapy. Bronchoscopy is reserved for patients with an immunocompromising condition, atypical presentation, or lack of response to treatment.
For those cavitary lesions in which there is a high degree of suspicion for lung abscess, empiric treatment should include antibiotics active against anaerobes and MRSA if the patient has risk factors. Patients often receive an empiric trial of antibiotics prior to biopsy unless there are clear indications that the cavitary lung lesion is related to cancer. Lung abscesses typically drain spontaneously, and transthoracic or endobronchial drainage is not usually recommended as initial management due to risk of pneumothorax and formation of bronchopleural fistula.
Lung abscesses should be followed to resolution with serial chest imaging. If the lung abscess does not resolve, it would be appropriate to consult thoracic surgery, interventional radiology, or pulmonary, depending on the location of the abscess and the local expertise with transthoracic or endobronchial drainage and surgical resection.
Septic emboli. Septic emboli are a less common cause of cavitary lung lesions. This entity should be considered in patients with a history of IV drug use or infected indwelling devices (central venous catheters, pacemaker wires, and right-sided prosthetic heart valves). Physical examination should include an assessment for signs of endocarditis and inspection for infected indwelling devices. In patients with IV drug use, the likely pathogen is S. aureus.
Oropharyngeal infection or indwelling catheters may predispose patients to septic thrombophlebitis of the internal jugular vein, also known as Lemierre’s syndrome, a rare but important cause of septic emboli.5 Laboratory testing includes culture for sputum and blood and culture of the infected device if applicable. On chest X-ray, septic emboli commonly appear as nodules located in the lung periphery. CT scan is more sensitive for detecting cavitation associated with septic emboli.
Diagnosis of Noninfectious Causes
Upon identification of a cavitary lung lesion, noninfectious etiologies must also be entertained. Noninfectious etiologies include malignancy, rheumatologic diseases, pulmonary embolism, and other causes. Important components in the clinical presentation include the presence of constitutional symptoms (fevers, weight loss, night sweats), smoking history, family history, and an otherwise complete review of systems. Physical exam should include evaluation for lymphadenopathy, cachexia, rash, clubbing, and other symptoms pertinent to the suspected etiology.
Malignancy. Perhaps most important among noninfectious causes of cavitary lung lesions is malignancy, and a high index of suspicion is warranted given that it is commonly the first diagnosis to consider overall.2 Cavities can form in primary lung cancers (e.g. bronchogenic carcinomas), lung tumors such as lymphoma or Kaposi’s sarcoma, or in metastatic disease. Cavitation has been detected in 7%-11% of primary lung cancers by plain radiography and in 22% by computed tomography.5 Cancers of squamous cell origin are the most likely to cavitate; this holds true for both primary lung tumors and metastatic tumors.6 Additionally, cavitation portends a worse prognosis.7
Clinicians should review any available prior chest imaging studies to look for a change in the quality or size of a cavitary lung lesion. Neoplasms are typically of variable size with irregular thick walls (greater than 4 mm) on CT scan, with higher specificity for neoplasm in those with a wall thickness greater than 15 mm.2
When the diagnosis is less clear, the decision to embark on more advanced diagnostic methods, such as biopsy, should rest on the provider’s clinical suspicion for a certain disease process. When a lung cancer is suspected, consultation with pulmonary and interventional radiology should be obtained to determine the best approach for biopsy.
Rheumatologic. Less common causes of cavitary lesions include those related to rheumatologic diseases (e.g. granulomatosis with polyangiitis, formerly known as Wegener’s granulomatosis). One study demonstrated that cavitary lung nodules occur in 37% of patients with granulomatosis with polyangiitis.8
Although uncommon, cavitary nodules can also be seen in rheumatoid arthritis and sarcoidosis. Given that patients with rheumatologic diseases are often treated with immunosuppressive agents, infection must remain high on the differential. Suspicion of a rheumatologic cause should prompt the clinician to obtain appropriate serologic testing and consultation as needed.
Pulmonary embolism. Although often not considered in the evaluation of cavitary lung lesions, pulmonary embolism (PE) can lead to infarction and the formation of a cavitary lesion. Pulmonary infarction has been reported to occur in as many as one third of cases of PE.9 Cavitary lesions also have been described in chronic thromboembolic disease.10
Other. Uncommon causes of cavitary lesions include bronchiolitis obliterans with organizing pneumonia, Langerhans cell histiocytosis, and amyloidosis, among others. The hospitalist should keep a broad differential and involve consultants if the diagnosis remains unclear after initial diagnostic evaluation.
Back to the Case
The patient’s fever and productive cough, in combination with recent travel and location of the cavitary lesion, increase his risk for tuberculosis and endemic fungi, such as Coccidioides. This patient was placed on respiratory isolation with AFBs obtained to rule out TB, with Coccidioides antibodies, Cyptococcal antigen titers, and sputum for fungus sent to evaluate for an endemic fungus. He had a chest CT, which revealed a 17-mm cavitary mass within the right upper lobe that contained an air-fluid level indicating lung abscess. Coccidioides, cryptococcal, fungal sputum, and TB studies were negative.
The patient was treated empirically with clindamycin given the high prevalence of anaerobes in lung abscess. He was followed as an outpatient and had a chest X-ray showing resolution of the lesion at six months. The purpose of the X-ray was two-fold: to monitor the effect of antibiotic treatment and to evaluate for persistence of the cavitation given the neoplastic risk factors of older age and smoking.
Bottom Line
The best approach to a patient with a cavitary lung lesion includes assessing the clinical presentation and risk factors, differentiating infectious from noninfectious causes, and then utilizing this information to further direct the diagnostic evaluation. Consultation with a subspecialist or further testing such as biopsy should be considered if the etiology remains undefined after the initial evaluation.
Drs. Rendon, Pizanis, Montanaro, and Kraai are hospitalists in the department of internal medicine at the University of New Mexico School of Medicine in Albuquerque.
References
- Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246(3):697-722.
- Ryu JH, Swensen SJ. Cystic and cavitary lung diseases: focal and diffuse. Mayo Clin Proc. 2003;78(6):744-752.
- Barnes PF, Verdegem TD, Vachon LA, Leedom JM, Overturf GD. Chest roentgenogram in pulmonary tuberculosis. New data on an old test. Chest. 1988;94(2):316-320.
- Yazbeck MF, Dahdel M, Kalra A, Browne AS, Pratter MR. Lung abscess: update on microbiology and management. Am J Ther. 2012;21(3):217-221. doi: 10.1097/MJT.0b013e3182383c9b.
- Gadkowski LB, Stout JE. Cavitary pulmonary disease. Clin Microbiol Rev. 2008;21(2):305-333.
- Chiu FT. Cavitation in lung cancers. Aust N Z J Med. 1975;5(6):523-530.
- Kolodziejski LS, Dyczek S, Duda K, Góralczyk J, Wysocki WM, Lobaziewicz W. Cavitated tumor as a clinical subentity in squamous cell lung cancer patients. Neoplasma. 2003;50(1):66-73.
- Cordier JF, Valeyre D, Guillevin L, Loire R, Brechot JM. Pulmonary Wegener’s granulomatosis. A clinical and imaging study of 77 cases. Chest. 1990;97(4):906-912.
- He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging. 2006;21(1):1-7.
- Harris H, Barraclough R, Davies C, Armstrong I, Kiely DG, van Beek E Jr. Cavitating lung lesions in chronic thromboembolic pulmonary hypertension. J Radiol Case Rep. 2008;2(3):11-21.
- Woodring JH, Fried AM, Chuang VP. Solitary cavities of the lung: diagnostic implications of cavity wall thickness. AJR Am J Roentgenol. 1980;135(6):1269-1271.
Hospitalists Are Frontline Providers in Treating Venous Thromboembolism
“While VTE may not be the No. 1 reason for hospitalization, hospitalists very frequently care for patients with VTE,” says Sowmya Kanikkannan, MD, FACP, SFHM, hospitalist medical director and assistant professor of medicine at Rowan University School of Osteopathic Medicine in Stratford, N.J. “Hospitalists usually are the frontline providers that diagnose and manage hospital-acquired VTEs in hospitalized patients.”
Dr. Kanikkannan, a member of Team Hospitalist, sees a wide range of VTE cases caused by two related conditions—deep vein thrombosis (DVT) and pulmonary embolism (PE).
“Some patients present with a straightforward diagnosis of DVT, while others have extensive DVT,” she says. “In other instances, patients present with acute PE with or without hemodynamic compromise. I’ve also diagnosed and managed hospital-acquired VTEs in medical patients, as well as post-operatively in surgical co-management.”
It is estimated that between 350,000 to 900,000 Americans are affected by DVT or PE each year, with up to 100,000 dying as a result. Twenty to 50% of people who experience DVT develop long-term complications.1VTE costs the U.S. healthcare system more than $1.5 billion annually.2
As lieutenants in the war against VTE, hospitalists are finding that new treatments, continued efforts to standardize VTE prophylaxis, and increased transparency in performance reporting are the tools needed to combat these common conditions—and hospitalists are being held accountable for optimal patient care.
New Treatments Show Promise
Diagnosing and treating VTE early helps to prevent progression and hemodynamic instability. Although the accepted treatment for VTE used to be heparin, or a low molecular weight heparin (LMWH) (Fragmin, Innohep and Lovenox) with a transition to warfarin, three target-specific oral anticoagulants (TSOACs)—dabigatran, rivaroxaban, and apixaban—are now being prescribed. The FDA has approved rivaroxaban and apixaban for the prevention of VTE after knee and hip surgery and for treatment of VTE, while dabigatran is FDA approved only for the treatment of VTE. All three are approved for use in nonvalvular atrial fibrillation (Afib). A fourth TSOAC, edoxaban, received FDA approval in January for VTE treatment and nonvalvular atrial fibrillation.
“The drawback of warfarin is that patients need frequent international normalized ratio (INR) monitoring,” Dr. Kanikkannan says. “Discharge planning is time consuming because patients need to be educated on warfarin, and follow-up appointments need to be arranged before discharge to ensure patient safety.”
“Their [TSOACs] ease of administration and easy dosing helps hospitalists to manage patients with VTE more efficiently,” Dr. Kanikkannan says. “Patients like having lab testing less frequently but equal efficacy in treatment.”
Rivaroxaban used to have the most approved indications by the FDA; however, based on three clinical trials—ADVANCE-1, 2, and 3—apixaban has the same six FDA indications as rivaroxaban.
The majority of clinical trials suggest noninferiority or superiority of the oral agents compared to LMWH, and safety appears to be similar across treatment groups, other than an increased risk in bleeding with oral agents (see Table 1).
“The increased risk of bleeding seen in trials is something hospitalists need to consider,” says Yong Lee, PharmD, BCPS, clinical pharmacy specialist at Parkland Health and Hospital System in Dallas, Texas. Warfarin is easily reversed; the new anticoagulants don’t have any specific reversal agent (see new table about reversal options). Consequently, the American College of Chest Physicians still recommends unfractionated heparin or a LMWH for VTE prophylaxis. “These agents will still likely remain the best available options to hospitalists for VTE prevention,” he adds.
Julianna Lindsey, MD, MBA, FACP, FHM, chief of staff and hospitalist at Victory Medical Center in McKinney, Texas, says there are instances when it would be helpful to know what the therapeutic level of a TSOAC’s anticoagulation effect is, such as in a patient with active bleeding or one who requires major emergent surgery. But there is no coagulation assay to date that is readily available to test the effect of apixaban; the anticoagulation effect for dabigatran can be roughly estimated by the activated partial thromboplastin time (aPTT) and thrombin time (TT), and the anticoagulation effect for rivaroxaban can be roughly estimated by the prothrombin time (PT).

Dr. Lee expects the new FDA approvals to expand the utilization of oral anti-Xa inhibitors in practice. “This will, hopefully, make the new oral anticoagulant market more competitive, driving down their costs,” he says, referring to one of the biggest barriers to current use of these agents. Warfarin still remains the most cost-effective option, despite the need for regular INR monitoring.
“Studies are looking not only at effectiveness but also the safety profile of these anticoagulants,” Dr. Kanikkannan says, as long-term safety data is not yet available on these oral agents.8,9
Researchers also are looking at the comparative effects of other medications. For example, a Journal of Hospital Medicine study concluded that, compared with other anticoagulants, aspirin is associated with a higher risk of DVT following hip fracture repair but similar rates of DVT risk following hip-knee arthroplasty. Bleeding rates with aspirin, however, were substantially lower.10
Improvement Efforts
In an effort to improve VTE prophylaxis in hospitalized patients, The Joint Commission developed a VTE standardized performance measure set in 2009, which has been reported on www.qualitycheck.org since then. The VTE measure set comprises six different measures evaluating the prophylaxis of VTE, treatment of VTE, warfarin discharge education, and hospital-acquired VTE. Since reporting started, most hospitals have implemented VTE risk assessment models and VTE process improvement programs; data trends have shown improvement, says Denise Krusenoski, MSN, RN, CMSRN, CHTS-CP, associate project director at The Joint Commission, which is based in Oakbrook Terrace, Ill.
“While a lot of good, evidence-based data is available, no single VTE risk assessment tool has been prospectively validated as superior,” she says. “Involving key members of medical staff to create and approve protocols based on proven data will increase the buy-in and adoption for using these tools.”
E-Measures Promote Excellence
Many hospitals are now moving from traditional chart abstracting for VTE measures to electronic measures (e-measures), which allow for more rapid and automated reporting of these quality metrics. In order for e-measures to be accurate, documentation necessary for measure computation must be present in defined standardized fields in the medical record. “With no human interpretation, data must be documented in a precise fashion,” Krusenoski says. “Providers will need to be flexible in learning new documentation skills.”
Dr. Lindsey, a member of Team Hospitalist, cautions that e-measures have the potential to increase unwanted events by overutilization of pharmacologic VTE prophylaxis and associated hemorrhagic events.
“We have to continue to make sure that our practice of medicine remains based in evidence and not succumb to the pull of getting a check-box ticked,” she warns.
VTE remains a significant problem in hospitalized patients today. Hospitalists should consider the pros and cons of using newer treatment methods over traditional agents. Efforts are under way to improve VTE prophylaxis by standardizing best practice and moving from traditional chart abstracting to using e-measures for performance reporting.
Karen Appold is a freelance medical writer in Pennsylvania.
References
- Centers for Disease Control and Prevention. Public Health Grand Rounds. Preventing venous thromboembolism. January 15, 2013. Available at: http://www.cdc.gov/cdcgrandrounds/archives/2013/january2013.htm. Accessed February 12, 2015.
- Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953.
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361(6):594-604.
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P; ADVANCE-2 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375(9717):807-815.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487-2498.
- Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368(6):513-523.
- Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365(23):2167-2177.
- Gonsalves WI, Pruthi RK, Patnaik MM. The new oral anticoagulants in clinical practice. Mayo Clin Proc. 2013;88(5):495-511.
- Holster IL, Valkoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology. 2013;145(1):105-112.
- Drescher FS, Sirovich BE, Lee A, Morrison DH, Chiang WH, Larson RJ. Aspirin versus anticoagulation for prevention of venous thromboembolism major lower extremity orthopedic surgery: a systematic review and meta-analysis. J Hosp Med. 2014;9(9):579-585.
- Bullock-Palmer RP, Weiss S, Hyman C. Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital-acquired deep vein thrombosis at a tertiary-care teaching hospital. J Hosp Med. 2008;3(2):148-155.
“While VTE may not be the No. 1 reason for hospitalization, hospitalists very frequently care for patients with VTE,” says Sowmya Kanikkannan, MD, FACP, SFHM, hospitalist medical director and assistant professor of medicine at Rowan University School of Osteopathic Medicine in Stratford, N.J. “Hospitalists usually are the frontline providers that diagnose and manage hospital-acquired VTEs in hospitalized patients.”
Dr. Kanikkannan, a member of Team Hospitalist, sees a wide range of VTE cases caused by two related conditions—deep vein thrombosis (DVT) and pulmonary embolism (PE).
“Some patients present with a straightforward diagnosis of DVT, while others have extensive DVT,” she says. “In other instances, patients present with acute PE with or without hemodynamic compromise. I’ve also diagnosed and managed hospital-acquired VTEs in medical patients, as well as post-operatively in surgical co-management.”
It is estimated that between 350,000 to 900,000 Americans are affected by DVT or PE each year, with up to 100,000 dying as a result. Twenty to 50% of people who experience DVT develop long-term complications.1VTE costs the U.S. healthcare system more than $1.5 billion annually.2
As lieutenants in the war against VTE, hospitalists are finding that new treatments, continued efforts to standardize VTE prophylaxis, and increased transparency in performance reporting are the tools needed to combat these common conditions—and hospitalists are being held accountable for optimal patient care.
New Treatments Show Promise
Diagnosing and treating VTE early helps to prevent progression and hemodynamic instability. Although the accepted treatment for VTE used to be heparin, or a low molecular weight heparin (LMWH) (Fragmin, Innohep and Lovenox) with a transition to warfarin, three target-specific oral anticoagulants (TSOACs)—dabigatran, rivaroxaban, and apixaban—are now being prescribed. The FDA has approved rivaroxaban and apixaban for the prevention of VTE after knee and hip surgery and for treatment of VTE, while dabigatran is FDA approved only for the treatment of VTE. All three are approved for use in nonvalvular atrial fibrillation (Afib). A fourth TSOAC, edoxaban, received FDA approval in January for VTE treatment and nonvalvular atrial fibrillation.
“The drawback of warfarin is that patients need frequent international normalized ratio (INR) monitoring,” Dr. Kanikkannan says. “Discharge planning is time consuming because patients need to be educated on warfarin, and follow-up appointments need to be arranged before discharge to ensure patient safety.”
“Their [TSOACs] ease of administration and easy dosing helps hospitalists to manage patients with VTE more efficiently,” Dr. Kanikkannan says. “Patients like having lab testing less frequently but equal efficacy in treatment.”
Rivaroxaban used to have the most approved indications by the FDA; however, based on three clinical trials—ADVANCE-1, 2, and 3—apixaban has the same six FDA indications as rivaroxaban.
The majority of clinical trials suggest noninferiority or superiority of the oral agents compared to LMWH, and safety appears to be similar across treatment groups, other than an increased risk in bleeding with oral agents (see Table 1).
“The increased risk of bleeding seen in trials is something hospitalists need to consider,” says Yong Lee, PharmD, BCPS, clinical pharmacy specialist at Parkland Health and Hospital System in Dallas, Texas. Warfarin is easily reversed; the new anticoagulants don’t have any specific reversal agent (see new table about reversal options). Consequently, the American College of Chest Physicians still recommends unfractionated heparin or a LMWH for VTE prophylaxis. “These agents will still likely remain the best available options to hospitalists for VTE prevention,” he adds.
Julianna Lindsey, MD, MBA, FACP, FHM, chief of staff and hospitalist at Victory Medical Center in McKinney, Texas, says there are instances when it would be helpful to know what the therapeutic level of a TSOAC’s anticoagulation effect is, such as in a patient with active bleeding or one who requires major emergent surgery. But there is no coagulation assay to date that is readily available to test the effect of apixaban; the anticoagulation effect for dabigatran can be roughly estimated by the activated partial thromboplastin time (aPTT) and thrombin time (TT), and the anticoagulation effect for rivaroxaban can be roughly estimated by the prothrombin time (PT).

Dr. Lee expects the new FDA approvals to expand the utilization of oral anti-Xa inhibitors in practice. “This will, hopefully, make the new oral anticoagulant market more competitive, driving down their costs,” he says, referring to one of the biggest barriers to current use of these agents. Warfarin still remains the most cost-effective option, despite the need for regular INR monitoring.
“Studies are looking not only at effectiveness but also the safety profile of these anticoagulants,” Dr. Kanikkannan says, as long-term safety data is not yet available on these oral agents.8,9
Researchers also are looking at the comparative effects of other medications. For example, a Journal of Hospital Medicine study concluded that, compared with other anticoagulants, aspirin is associated with a higher risk of DVT following hip fracture repair but similar rates of DVT risk following hip-knee arthroplasty. Bleeding rates with aspirin, however, were substantially lower.10
Improvement Efforts
In an effort to improve VTE prophylaxis in hospitalized patients, The Joint Commission developed a VTE standardized performance measure set in 2009, which has been reported on www.qualitycheck.org since then. The VTE measure set comprises six different measures evaluating the prophylaxis of VTE, treatment of VTE, warfarin discharge education, and hospital-acquired VTE. Since reporting started, most hospitals have implemented VTE risk assessment models and VTE process improvement programs; data trends have shown improvement, says Denise Krusenoski, MSN, RN, CMSRN, CHTS-CP, associate project director at The Joint Commission, which is based in Oakbrook Terrace, Ill.
“While a lot of good, evidence-based data is available, no single VTE risk assessment tool has been prospectively validated as superior,” she says. “Involving key members of medical staff to create and approve protocols based on proven data will increase the buy-in and adoption for using these tools.”
E-Measures Promote Excellence
Many hospitals are now moving from traditional chart abstracting for VTE measures to electronic measures (e-measures), which allow for more rapid and automated reporting of these quality metrics. In order for e-measures to be accurate, documentation necessary for measure computation must be present in defined standardized fields in the medical record. “With no human interpretation, data must be documented in a precise fashion,” Krusenoski says. “Providers will need to be flexible in learning new documentation skills.”
Dr. Lindsey, a member of Team Hospitalist, cautions that e-measures have the potential to increase unwanted events by overutilization of pharmacologic VTE prophylaxis and associated hemorrhagic events.
“We have to continue to make sure that our practice of medicine remains based in evidence and not succumb to the pull of getting a check-box ticked,” she warns.
VTE remains a significant problem in hospitalized patients today. Hospitalists should consider the pros and cons of using newer treatment methods over traditional agents. Efforts are under way to improve VTE prophylaxis by standardizing best practice and moving from traditional chart abstracting to using e-measures for performance reporting.
Karen Appold is a freelance medical writer in Pennsylvania.
References
- Centers for Disease Control and Prevention. Public Health Grand Rounds. Preventing venous thromboembolism. January 15, 2013. Available at: http://www.cdc.gov/cdcgrandrounds/archives/2013/january2013.htm. Accessed February 12, 2015.
- Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953.
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361(6):594-604.
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P; ADVANCE-2 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375(9717):807-815.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487-2498.
- Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368(6):513-523.
- Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365(23):2167-2177.
- Gonsalves WI, Pruthi RK, Patnaik MM. The new oral anticoagulants in clinical practice. Mayo Clin Proc. 2013;88(5):495-511.
- Holster IL, Valkoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology. 2013;145(1):105-112.
- Drescher FS, Sirovich BE, Lee A, Morrison DH, Chiang WH, Larson RJ. Aspirin versus anticoagulation for prevention of venous thromboembolism major lower extremity orthopedic surgery: a systematic review and meta-analysis. J Hosp Med. 2014;9(9):579-585.
- Bullock-Palmer RP, Weiss S, Hyman C. Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital-acquired deep vein thrombosis at a tertiary-care teaching hospital. J Hosp Med. 2008;3(2):148-155.
“While VTE may not be the No. 1 reason for hospitalization, hospitalists very frequently care for patients with VTE,” says Sowmya Kanikkannan, MD, FACP, SFHM, hospitalist medical director and assistant professor of medicine at Rowan University School of Osteopathic Medicine in Stratford, N.J. “Hospitalists usually are the frontline providers that diagnose and manage hospital-acquired VTEs in hospitalized patients.”
Dr. Kanikkannan, a member of Team Hospitalist, sees a wide range of VTE cases caused by two related conditions—deep vein thrombosis (DVT) and pulmonary embolism (PE).
“Some patients present with a straightforward diagnosis of DVT, while others have extensive DVT,” she says. “In other instances, patients present with acute PE with or without hemodynamic compromise. I’ve also diagnosed and managed hospital-acquired VTEs in medical patients, as well as post-operatively in surgical co-management.”
It is estimated that between 350,000 to 900,000 Americans are affected by DVT or PE each year, with up to 100,000 dying as a result. Twenty to 50% of people who experience DVT develop long-term complications.1VTE costs the U.S. healthcare system more than $1.5 billion annually.2
As lieutenants in the war against VTE, hospitalists are finding that new treatments, continued efforts to standardize VTE prophylaxis, and increased transparency in performance reporting are the tools needed to combat these common conditions—and hospitalists are being held accountable for optimal patient care.
New Treatments Show Promise
Diagnosing and treating VTE early helps to prevent progression and hemodynamic instability. Although the accepted treatment for VTE used to be heparin, or a low molecular weight heparin (LMWH) (Fragmin, Innohep and Lovenox) with a transition to warfarin, three target-specific oral anticoagulants (TSOACs)—dabigatran, rivaroxaban, and apixaban—are now being prescribed. The FDA has approved rivaroxaban and apixaban for the prevention of VTE after knee and hip surgery and for treatment of VTE, while dabigatran is FDA approved only for the treatment of VTE. All three are approved for use in nonvalvular atrial fibrillation (Afib). A fourth TSOAC, edoxaban, received FDA approval in January for VTE treatment and nonvalvular atrial fibrillation.
“The drawback of warfarin is that patients need frequent international normalized ratio (INR) monitoring,” Dr. Kanikkannan says. “Discharge planning is time consuming because patients need to be educated on warfarin, and follow-up appointments need to be arranged before discharge to ensure patient safety.”
“Their [TSOACs] ease of administration and easy dosing helps hospitalists to manage patients with VTE more efficiently,” Dr. Kanikkannan says. “Patients like having lab testing less frequently but equal efficacy in treatment.”
Rivaroxaban used to have the most approved indications by the FDA; however, based on three clinical trials—ADVANCE-1, 2, and 3—apixaban has the same six FDA indications as rivaroxaban.
The majority of clinical trials suggest noninferiority or superiority of the oral agents compared to LMWH, and safety appears to be similar across treatment groups, other than an increased risk in bleeding with oral agents (see Table 1).
“The increased risk of bleeding seen in trials is something hospitalists need to consider,” says Yong Lee, PharmD, BCPS, clinical pharmacy specialist at Parkland Health and Hospital System in Dallas, Texas. Warfarin is easily reversed; the new anticoagulants don’t have any specific reversal agent (see new table about reversal options). Consequently, the American College of Chest Physicians still recommends unfractionated heparin or a LMWH for VTE prophylaxis. “These agents will still likely remain the best available options to hospitalists for VTE prevention,” he adds.
Julianna Lindsey, MD, MBA, FACP, FHM, chief of staff and hospitalist at Victory Medical Center in McKinney, Texas, says there are instances when it would be helpful to know what the therapeutic level of a TSOAC’s anticoagulation effect is, such as in a patient with active bleeding or one who requires major emergent surgery. But there is no coagulation assay to date that is readily available to test the effect of apixaban; the anticoagulation effect for dabigatran can be roughly estimated by the activated partial thromboplastin time (aPTT) and thrombin time (TT), and the anticoagulation effect for rivaroxaban can be roughly estimated by the prothrombin time (PT).

Dr. Lee expects the new FDA approvals to expand the utilization of oral anti-Xa inhibitors in practice. “This will, hopefully, make the new oral anticoagulant market more competitive, driving down their costs,” he says, referring to one of the biggest barriers to current use of these agents. Warfarin still remains the most cost-effective option, despite the need for regular INR monitoring.
“Studies are looking not only at effectiveness but also the safety profile of these anticoagulants,” Dr. Kanikkannan says, as long-term safety data is not yet available on these oral agents.8,9
Researchers also are looking at the comparative effects of other medications. For example, a Journal of Hospital Medicine study concluded that, compared with other anticoagulants, aspirin is associated with a higher risk of DVT following hip fracture repair but similar rates of DVT risk following hip-knee arthroplasty. Bleeding rates with aspirin, however, were substantially lower.10
Improvement Efforts
In an effort to improve VTE prophylaxis in hospitalized patients, The Joint Commission developed a VTE standardized performance measure set in 2009, which has been reported on www.qualitycheck.org since then. The VTE measure set comprises six different measures evaluating the prophylaxis of VTE, treatment of VTE, warfarin discharge education, and hospital-acquired VTE. Since reporting started, most hospitals have implemented VTE risk assessment models and VTE process improvement programs; data trends have shown improvement, says Denise Krusenoski, MSN, RN, CMSRN, CHTS-CP, associate project director at The Joint Commission, which is based in Oakbrook Terrace, Ill.
“While a lot of good, evidence-based data is available, no single VTE risk assessment tool has been prospectively validated as superior,” she says. “Involving key members of medical staff to create and approve protocols based on proven data will increase the buy-in and adoption for using these tools.”
E-Measures Promote Excellence
Many hospitals are now moving from traditional chart abstracting for VTE measures to electronic measures (e-measures), which allow for more rapid and automated reporting of these quality metrics. In order for e-measures to be accurate, documentation necessary for measure computation must be present in defined standardized fields in the medical record. “With no human interpretation, data must be documented in a precise fashion,” Krusenoski says. “Providers will need to be flexible in learning new documentation skills.”
Dr. Lindsey, a member of Team Hospitalist, cautions that e-measures have the potential to increase unwanted events by overutilization of pharmacologic VTE prophylaxis and associated hemorrhagic events.
“We have to continue to make sure that our practice of medicine remains based in evidence and not succumb to the pull of getting a check-box ticked,” she warns.
VTE remains a significant problem in hospitalized patients today. Hospitalists should consider the pros and cons of using newer treatment methods over traditional agents. Efforts are under way to improve VTE prophylaxis by standardizing best practice and moving from traditional chart abstracting to using e-measures for performance reporting.
Karen Appold is a freelance medical writer in Pennsylvania.
References
- Centers for Disease Control and Prevention. Public Health Grand Rounds. Preventing venous thromboembolism. January 15, 2013. Available at: http://www.cdc.gov/cdcgrandrounds/archives/2013/january2013.htm. Accessed February 12, 2015.
- Dobesh PP. Economic burden of venous thromboembolism in hospitalized patients. Pharmacotherapy. 2009;29(8):943-953.
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Portman RJ. Apixaban or enoxaparin for thromboprophylaxis after knee replacement. N Engl J Med. 2009;361(6):594-604.
- Lassen MR, Raskob GE, Gallus A, Pineo G, Chen D, Hornick P; ADVANCE-2 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after knee replacement (ADVANCE-2): a randomised double-blind trial. Lancet. 2010;375(9717):807-815.
- Lassen MR, Gallus A, Raskob GE, Pineo G, Chen D, Ramirez LM; ADVANCE-3 Investigators. Apixaban versus enoxaparin for thromboprophylaxis after hip replacement. N Engl J Med. 2010;363(26):2487-2498.
- Cohen AT, Spiro TE, Büller HR, et al. Rivaroxaban for thromboprophylaxis in acutely ill medical patients. N Engl J Med. 2013;368(6):513-523.
- Goldhaber SZ, Leizorovicz A, Kakkar AK, et al. Apixaban versus enoxaparin for thromboprophylaxis in medically ill patients. N Engl J Med. 2011;365(23):2167-2177.
- Gonsalves WI, Pruthi RK, Patnaik MM. The new oral anticoagulants in clinical practice. Mayo Clin Proc. 2013;88(5):495-511.
- Holster IL, Valkoff VE, Kuipers EJ, Tjwa ET. New oral anticoagulants increase risk for gastrointestinal bleeding: a systematic review and meta-analysis. Gastroenterology. 2013;145(1):105-112.
- Drescher FS, Sirovich BE, Lee A, Morrison DH, Chiang WH, Larson RJ. Aspirin versus anticoagulation for prevention of venous thromboembolism major lower extremity orthopedic surgery: a systematic review and meta-analysis. J Hosp Med. 2014;9(9):579-585.
- Bullock-Palmer RP, Weiss S, Hyman C. Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital-acquired deep vein thrombosis at a tertiary-care teaching hospital. J Hosp Med. 2008;3(2):148-155.
Hospitals Launch Bedside Procedure Services
A dedicated procedure team or service can give hospitals needed expertise without requiring a one-size-fits-all approach. In many cases, hospitalists run procedure services, but interventional radiologists and pulmonary critical care specialists also oversee some of them.
At The Johns Hopkins Hospital, the bedside procedure service began in the department of medicine and has since expanded throughout the hospital.
“I think a proceduralist service is as important as the hospitalist service,” says David Lichtman, PA, director of the service. He calls it “essential” for good patient care because it can allow experienced providers to be consistently involved in the process, whether proceduralists, medical students, or new interns perform the procedure.
“Patients have the benefit of expert care, and the trainees have the ability to learn and do without having to worry about working without a safety net,” he says. As a result, the service keeps patients safe while maximizing medical education.
At many institutions, a service or team can also meet a pressing need. In its seven years of existence, for example, the hospitalist-led procedures team at the University of Miami Jackson Memorial Hospital Medical Campus has been called upon to do more than 7,500 procedures.
“The idea behind procedure services is that you consolidate the expertise and training within a few people, be it a few hospitalists or a few proceduralists,” says Michelle Mourad, MD, director of quality improvement and patient safety for the division of hospital medicine at the University of California
San Francisco (UCSF). But a successful service can require significant investments in infrastructure and other resources. When they run the numbers, many hospitalist groups are forced to conclude that they simply don’t have sufficient demand to justify the expense of maintaining provider competency.
“People are really struggling with this,” she says.

The few studies conducted on procedure services, however, suggest that hospitals can benefit from improved patient satisfaction and a potential reduction in some complications.
“We were worried that that use of trainees and the teaching that went on at the bedside might be a concern for patients,” Dr. Mourad says of the UCSF procedures program. “We found that, instead, patients were reassured by having a designated expert in the room and recognized that it hadn’t always been the case in the past.” Accordingly, she says, a survey of satisfaction recorded “exceptionally high” rates.3
Initial research also suggests a reduction in such complications as thoracentesis-related pneumothorax.
“We have some inkling that perhaps the rigor with which we approach procedures, the high level of experience that we bring to procedures, and the presence of an expert in the room for every procedure may have decreased the complication rate for thoracentesis at our institution,” Dr. Mourad says.
At Boston University, the procedure service is based in the department of pulmonary critical care, and the department’s attending physicians supervise internal medicine residents. It was developed after “identifying some potential patient safety concerns with unsupervised resident procedures,” says Melissa Tukey, MD, MSc, now a pulmonology critical care physician at Lahey Clinic in Burlington, Mass. A major aim of the procedure service, she says, is to provide supervision and teaching to medical house staff performing the procedures.
To test whether the service was delivering on those goals, Dr. Tukey and colleagues studied thoracentesis, paracentesis, central line, and lumbar puncture procedures.4 The study, an 18-month comparison of the procedures performed by the dedicated procedure service versus those done by other providers, found no significant difference in what were already quite low complication rates.
Unexpectedly, the researchers didn’t see higher levels of resident engagement in procedures performed by the procedure team, but they did find improvement in “best practice safety process measures,” such as whether ultrasound use followed established recommendations.
“I think that whenever you’re looking at quality improvement initiatives, you have to have an understanding of what might be the potential benefits,”
Dr. Tukey says. Her study, at least, suggests that launching a procedure service primarily to reduce the number of severe complications may not be the most appropriate goal. On the other hand, she says, the data do support the “very realistic goals” of improving residency education and maintaining procedure quality.
A dedicated service may not be a cure-all, in other words. And it’s certainly not for everyone. But given enough resources and buy-in, experts say, it could at least help put a hospital’s ailing bedside procedure strategy on the road to recovery without overextending its providers.
A dedicated procedure team or service can give hospitals needed expertise without requiring a one-size-fits-all approach. In many cases, hospitalists run procedure services, but interventional radiologists and pulmonary critical care specialists also oversee some of them.
At The Johns Hopkins Hospital, the bedside procedure service began in the department of medicine and has since expanded throughout the hospital.
“I think a proceduralist service is as important as the hospitalist service,” says David Lichtman, PA, director of the service. He calls it “essential” for good patient care because it can allow experienced providers to be consistently involved in the process, whether proceduralists, medical students, or new interns perform the procedure.
“Patients have the benefit of expert care, and the trainees have the ability to learn and do without having to worry about working without a safety net,” he says. As a result, the service keeps patients safe while maximizing medical education.
At many institutions, a service or team can also meet a pressing need. In its seven years of existence, for example, the hospitalist-led procedures team at the University of Miami Jackson Memorial Hospital Medical Campus has been called upon to do more than 7,500 procedures.
“The idea behind procedure services is that you consolidate the expertise and training within a few people, be it a few hospitalists or a few proceduralists,” says Michelle Mourad, MD, director of quality improvement and patient safety for the division of hospital medicine at the University of California
San Francisco (UCSF). But a successful service can require significant investments in infrastructure and other resources. When they run the numbers, many hospitalist groups are forced to conclude that they simply don’t have sufficient demand to justify the expense of maintaining provider competency.
“People are really struggling with this,” she says.

The few studies conducted on procedure services, however, suggest that hospitals can benefit from improved patient satisfaction and a potential reduction in some complications.
“We were worried that that use of trainees and the teaching that went on at the bedside might be a concern for patients,” Dr. Mourad says of the UCSF procedures program. “We found that, instead, patients were reassured by having a designated expert in the room and recognized that it hadn’t always been the case in the past.” Accordingly, she says, a survey of satisfaction recorded “exceptionally high” rates.3
Initial research also suggests a reduction in such complications as thoracentesis-related pneumothorax.
“We have some inkling that perhaps the rigor with which we approach procedures, the high level of experience that we bring to procedures, and the presence of an expert in the room for every procedure may have decreased the complication rate for thoracentesis at our institution,” Dr. Mourad says.
At Boston University, the procedure service is based in the department of pulmonary critical care, and the department’s attending physicians supervise internal medicine residents. It was developed after “identifying some potential patient safety concerns with unsupervised resident procedures,” says Melissa Tukey, MD, MSc, now a pulmonology critical care physician at Lahey Clinic in Burlington, Mass. A major aim of the procedure service, she says, is to provide supervision and teaching to medical house staff performing the procedures.
To test whether the service was delivering on those goals, Dr. Tukey and colleagues studied thoracentesis, paracentesis, central line, and lumbar puncture procedures.4 The study, an 18-month comparison of the procedures performed by the dedicated procedure service versus those done by other providers, found no significant difference in what were already quite low complication rates.
Unexpectedly, the researchers didn’t see higher levels of resident engagement in procedures performed by the procedure team, but they did find improvement in “best practice safety process measures,” such as whether ultrasound use followed established recommendations.
“I think that whenever you’re looking at quality improvement initiatives, you have to have an understanding of what might be the potential benefits,”
Dr. Tukey says. Her study, at least, suggests that launching a procedure service primarily to reduce the number of severe complications may not be the most appropriate goal. On the other hand, she says, the data do support the “very realistic goals” of improving residency education and maintaining procedure quality.
A dedicated service may not be a cure-all, in other words. And it’s certainly not for everyone. But given enough resources and buy-in, experts say, it could at least help put a hospital’s ailing bedside procedure strategy on the road to recovery without overextending its providers.
A dedicated procedure team or service can give hospitals needed expertise without requiring a one-size-fits-all approach. In many cases, hospitalists run procedure services, but interventional radiologists and pulmonary critical care specialists also oversee some of them.
At The Johns Hopkins Hospital, the bedside procedure service began in the department of medicine and has since expanded throughout the hospital.
“I think a proceduralist service is as important as the hospitalist service,” says David Lichtman, PA, director of the service. He calls it “essential” for good patient care because it can allow experienced providers to be consistently involved in the process, whether proceduralists, medical students, or new interns perform the procedure.
“Patients have the benefit of expert care, and the trainees have the ability to learn and do without having to worry about working without a safety net,” he says. As a result, the service keeps patients safe while maximizing medical education.
At many institutions, a service or team can also meet a pressing need. In its seven years of existence, for example, the hospitalist-led procedures team at the University of Miami Jackson Memorial Hospital Medical Campus has been called upon to do more than 7,500 procedures.
“The idea behind procedure services is that you consolidate the expertise and training within a few people, be it a few hospitalists or a few proceduralists,” says Michelle Mourad, MD, director of quality improvement and patient safety for the division of hospital medicine at the University of California
San Francisco (UCSF). But a successful service can require significant investments in infrastructure and other resources. When they run the numbers, many hospitalist groups are forced to conclude that they simply don’t have sufficient demand to justify the expense of maintaining provider competency.
“People are really struggling with this,” she says.

The few studies conducted on procedure services, however, suggest that hospitals can benefit from improved patient satisfaction and a potential reduction in some complications.
“We were worried that that use of trainees and the teaching that went on at the bedside might be a concern for patients,” Dr. Mourad says of the UCSF procedures program. “We found that, instead, patients were reassured by having a designated expert in the room and recognized that it hadn’t always been the case in the past.” Accordingly, she says, a survey of satisfaction recorded “exceptionally high” rates.3
Initial research also suggests a reduction in such complications as thoracentesis-related pneumothorax.
“We have some inkling that perhaps the rigor with which we approach procedures, the high level of experience that we bring to procedures, and the presence of an expert in the room for every procedure may have decreased the complication rate for thoracentesis at our institution,” Dr. Mourad says.
At Boston University, the procedure service is based in the department of pulmonary critical care, and the department’s attending physicians supervise internal medicine residents. It was developed after “identifying some potential patient safety concerns with unsupervised resident procedures,” says Melissa Tukey, MD, MSc, now a pulmonology critical care physician at Lahey Clinic in Burlington, Mass. A major aim of the procedure service, she says, is to provide supervision and teaching to medical house staff performing the procedures.
To test whether the service was delivering on those goals, Dr. Tukey and colleagues studied thoracentesis, paracentesis, central line, and lumbar puncture procedures.4 The study, an 18-month comparison of the procedures performed by the dedicated procedure service versus those done by other providers, found no significant difference in what were already quite low complication rates.
Unexpectedly, the researchers didn’t see higher levels of resident engagement in procedures performed by the procedure team, but they did find improvement in “best practice safety process measures,” such as whether ultrasound use followed established recommendations.
“I think that whenever you’re looking at quality improvement initiatives, you have to have an understanding of what might be the potential benefits,”
Dr. Tukey says. Her study, at least, suggests that launching a procedure service primarily to reduce the number of severe complications may not be the most appropriate goal. On the other hand, she says, the data do support the “very realistic goals” of improving residency education and maintaining procedure quality.
A dedicated service may not be a cure-all, in other words. And it’s certainly not for everyone. But given enough resources and buy-in, experts say, it could at least help put a hospital’s ailing bedside procedure strategy on the road to recovery without overextending its providers.
How Academic Hospitalists Can Balance Teaching, Nonteaching Roles
As a group director at a growing, university-based hospitalist program, I often interview aspiring academic hospitalists. Inevitably, the conversation turns to a coveted aspect of the job. I’m not talking about the salary. Applicants want to know, “How much time will I spend on teaching services?”
Because hospitalists at academic institutions typically are passionate about their work as instructors and mentors, they highly value time with trainees. Unfortunately, the 2011 Accreditation Council for Graduate Medical Education (ACGME) work hour rules triggered an expansion in non-teaching services at many teaching hospitals, forcing groups either to divide teaching service among an increasing number of attending physicians or to allocate this commodity unevenly on grounds such as seniority. For many group leaders, striking the right balance between teaching and non-teaching service can be an important contributor to recruitment and retention. During these interviews, I’ve often wondered how our group compares to others around the country.
The 2014 State of Hospital Medicine report (SOHM) shines light on this topic.
Among the 422 groups that only care for adults, 52 self-reported as academic groups. The groups were then asked to describe how they distribute work duties. In these academic practices, about half (52.5%) of the group’s full-time equivalents (FTEs) were devoted to clinical work in which the attending supervises learners delivering care. The remaining FTEs were devoted to a mix of clinical work on non-teaching services, administration, and protected time for research.
Interestingly, the portion devoted to clinical teaching differs substantially between university-based and affiliated community teaching hospitals (36.2% vs. 79.1%), suggesting that hospitalists face tough competition for teaching time at the main campuses of academic systems but might have more opportunities to teach at the bedside in faculty jobs at affiliated hospitals.
The above FTE figures represent averages, which don’t tell the whole story. Groups might not distribute teaching time evenly; the approach to allocation ranges from a completely egalitarian approach to a system with two tiers that separate teaching and non-teaching hospitalists.
To address the ranges, the State of Hospital Medicine survey asked respondents to divide their faculty into five categories of individual job types, ranging from “No clinical activity with trainees” to “>75% of clinical activity with trainees” (see Figure 1). The results show a broad array of teaching responsibilities, with 20% of academic hospitalists spending no time teaching and another 21% spending almost all of their time teaching.
I suspect this distribution partially reflects the underlying interests of the individual hospitalists, but it is also a product of the available opportunities. A few factors might influence those opportunities, such as decisions by the hospital to hire hospitalists rather than nurse practitioners and physicians assistants to cover new services, or the presence of specialists and general internists who share teaching service slots with hospitalists.
One of the great things about SHM’s State of Hospital Medicine report is how it depicts the wide variety of careers available to hospitalists; the teaching environment is no exception. Although I strive to help my colleagues tailor positions to suit their interests, we never have quite enough resident service time to meet the demands of our enthusiastic teachers. Fortunately, this report allows me to discuss our job openings with candidates knowing how we stack up against academic programs around the country.
Dr. White is assistant professor of medicine at the University of Washington and hospitalist group director at the University of Washington Medical Center in Seattle.
As a group director at a growing, university-based hospitalist program, I often interview aspiring academic hospitalists. Inevitably, the conversation turns to a coveted aspect of the job. I’m not talking about the salary. Applicants want to know, “How much time will I spend on teaching services?”
Because hospitalists at academic institutions typically are passionate about their work as instructors and mentors, they highly value time with trainees. Unfortunately, the 2011 Accreditation Council for Graduate Medical Education (ACGME) work hour rules triggered an expansion in non-teaching services at many teaching hospitals, forcing groups either to divide teaching service among an increasing number of attending physicians or to allocate this commodity unevenly on grounds such as seniority. For many group leaders, striking the right balance between teaching and non-teaching service can be an important contributor to recruitment and retention. During these interviews, I’ve often wondered how our group compares to others around the country.
The 2014 State of Hospital Medicine report (SOHM) shines light on this topic.
Among the 422 groups that only care for adults, 52 self-reported as academic groups. The groups were then asked to describe how they distribute work duties. In these academic practices, about half (52.5%) of the group’s full-time equivalents (FTEs) were devoted to clinical work in which the attending supervises learners delivering care. The remaining FTEs were devoted to a mix of clinical work on non-teaching services, administration, and protected time for research.
Interestingly, the portion devoted to clinical teaching differs substantially between university-based and affiliated community teaching hospitals (36.2% vs. 79.1%), suggesting that hospitalists face tough competition for teaching time at the main campuses of academic systems but might have more opportunities to teach at the bedside in faculty jobs at affiliated hospitals.
The above FTE figures represent averages, which don’t tell the whole story. Groups might not distribute teaching time evenly; the approach to allocation ranges from a completely egalitarian approach to a system with two tiers that separate teaching and non-teaching hospitalists.
To address the ranges, the State of Hospital Medicine survey asked respondents to divide their faculty into five categories of individual job types, ranging from “No clinical activity with trainees” to “>75% of clinical activity with trainees” (see Figure 1). The results show a broad array of teaching responsibilities, with 20% of academic hospitalists spending no time teaching and another 21% spending almost all of their time teaching.
I suspect this distribution partially reflects the underlying interests of the individual hospitalists, but it is also a product of the available opportunities. A few factors might influence those opportunities, such as decisions by the hospital to hire hospitalists rather than nurse practitioners and physicians assistants to cover new services, or the presence of specialists and general internists who share teaching service slots with hospitalists.
One of the great things about SHM’s State of Hospital Medicine report is how it depicts the wide variety of careers available to hospitalists; the teaching environment is no exception. Although I strive to help my colleagues tailor positions to suit their interests, we never have quite enough resident service time to meet the demands of our enthusiastic teachers. Fortunately, this report allows me to discuss our job openings with candidates knowing how we stack up against academic programs around the country.
Dr. White is assistant professor of medicine at the University of Washington and hospitalist group director at the University of Washington Medical Center in Seattle.
As a group director at a growing, university-based hospitalist program, I often interview aspiring academic hospitalists. Inevitably, the conversation turns to a coveted aspect of the job. I’m not talking about the salary. Applicants want to know, “How much time will I spend on teaching services?”
Because hospitalists at academic institutions typically are passionate about their work as instructors and mentors, they highly value time with trainees. Unfortunately, the 2011 Accreditation Council for Graduate Medical Education (ACGME) work hour rules triggered an expansion in non-teaching services at many teaching hospitals, forcing groups either to divide teaching service among an increasing number of attending physicians or to allocate this commodity unevenly on grounds such as seniority. For many group leaders, striking the right balance between teaching and non-teaching service can be an important contributor to recruitment and retention. During these interviews, I’ve often wondered how our group compares to others around the country.
The 2014 State of Hospital Medicine report (SOHM) shines light on this topic.
Among the 422 groups that only care for adults, 52 self-reported as academic groups. The groups were then asked to describe how they distribute work duties. In these academic practices, about half (52.5%) of the group’s full-time equivalents (FTEs) were devoted to clinical work in which the attending supervises learners delivering care. The remaining FTEs were devoted to a mix of clinical work on non-teaching services, administration, and protected time for research.
Interestingly, the portion devoted to clinical teaching differs substantially between university-based and affiliated community teaching hospitals (36.2% vs. 79.1%), suggesting that hospitalists face tough competition for teaching time at the main campuses of academic systems but might have more opportunities to teach at the bedside in faculty jobs at affiliated hospitals.
The above FTE figures represent averages, which don’t tell the whole story. Groups might not distribute teaching time evenly; the approach to allocation ranges from a completely egalitarian approach to a system with two tiers that separate teaching and non-teaching hospitalists.
To address the ranges, the State of Hospital Medicine survey asked respondents to divide their faculty into five categories of individual job types, ranging from “No clinical activity with trainees” to “>75% of clinical activity with trainees” (see Figure 1). The results show a broad array of teaching responsibilities, with 20% of academic hospitalists spending no time teaching and another 21% spending almost all of their time teaching.
I suspect this distribution partially reflects the underlying interests of the individual hospitalists, but it is also a product of the available opportunities. A few factors might influence those opportunities, such as decisions by the hospital to hire hospitalists rather than nurse practitioners and physicians assistants to cover new services, or the presence of specialists and general internists who share teaching service slots with hospitalists.
One of the great things about SHM’s State of Hospital Medicine report is how it depicts the wide variety of careers available to hospitalists; the teaching environment is no exception. Although I strive to help my colleagues tailor positions to suit their interests, we never have quite enough resident service time to meet the demands of our enthusiastic teachers. Fortunately, this report allows me to discuss our job openings with candidates knowing how we stack up against academic programs around the country.
Dr. White is assistant professor of medicine at the University of Washington and hospitalist group director at the University of Washington Medical Center in Seattle.