User login
Product Update: AVYCAZ, Electro Lube, Methergine Oral Tablets, ChartLogic
TREATING GRAM-NEGATIVE BACTERIAL INFECTIONS

Allergan reports an updated label approved by the US Food and Drug Administration (FDA) for AVYCAZ® (ceftazidime and avibactam) in combination with metronidazole for the treatment of complicated intraabdominal infections caused by designated susceptible microorganisms. The label change was made after completion of a Phase 3 trial evaluating the safety and efficacy of AVYCAZ in combination with metronidazole. Results of the trial also included data from subsets of patients with infections due to ceftazidime- nonsusceptible pathogens and with pathogens producing certain extended-spectrum beta-lactamases (ESBLs).
First FDA-approved in February 2015, AVYCAZ is an antibiotic developed to treat certain Gram-negative bacterial infections. It consists of ceftazidime, a third- generation cephalosporin, and avibactam, a non-ß lactam ß-lactamase inhibitor.
Allergan says that AVYCAZ has demonstrated in vitro activity against Enterobacteriaceae in the presence of some beta-lactamases and ESBLs of the following groups: TEM, SHV, CTX-M, Klebsiella pneumoniae carbapenemase, AmpC, and certain oxacillinases. AVYCAZ also demonstrated in vitro activity against Pseudomonas aeruginosa in the presence of some AmpC beta-lactamases, and certain strains lacking outer membrane porin.
FOR MORE INFORMATION, VISIT: www.avycaz.com
ANTI-STICK SOLUTION FOR ELECTROSURGICAL INSTRUMENTS
Electro Lube is a mixture of natural, nonsynthetic, nonflammable, nonallergenic biocompatible phospholipids with no known side effects associated with patient use. It can be used on a variety of monopolar and bipolar instrumentation including: cutting forceps, Kleppingers, bipolar forceps, Bovie pencils, suction coagulators, robots, curved hot scissors, spatula electrodes, and l-hook electrodes.
FOR MORE INFORMATION, VISIT: www.electrolubesurgical.com
ORAL TREATMENT FOR PPH
A semi-synthetic ergot alkaloid, Methergine is indicated for routine management of uterine atony, hemorrhage, and subinvolution of the uterus following delivery of the placenta, and for control of uterine hemorrhage in the second stage of labor following delivery of the anterior shoulder. Methergine provides specific and rapid (onset of action under 10 minutes) uterotonic action on the smooth muscle of the uterus to increase contractions, resulting in restricting blood loss.
The Methergine dosing schedule is one tablet (0.2 mg) 3 or 4 times daily in the puerperium for a maximum of 1 week. The most common adverse event is hypertension, associated in several cases with seizure and/or headache. Methylergonovine maleate is also available as an intramuscular injection.
FOR MORE INFORMATION, VISIT: www.methergine.com
FREE MEDICAL BILLING CALCULATOR

ChartLogic, Inc. has launched its Medical Billing Analysis Calculator, a free, online, interactive tool that provides key billing metrics and compares those results to Medical Group Management Association (MGMA) benchmarks for a practice’s specialty. The calculator consists of an input section where practice specialty, annual charges, annual collections, outstanding accounts receivable (A/R), number of annual encounters, and contractual adjustments are entered. The tool, accessible on desktop or mobile devices, then calculates the practice’s billing analysis, including average reimbursement per encounter, net collection percentages, average monthly collections, and A/R data and displays the results beside specialty benchmarks. After submission, the user can alter the inputs or request more information from a ChartLogic billing expert.
ChartLogic’s goal as a medical billing service is to increase a practice’s performance. Services include a full ambulatory EHR suite with electronic medical records, practice management tools, e-prescribing, a patient portal, and more.
FOR MORE INFORMATION, VISIT: www.chartlogic.com
TREATING GRAM-NEGATIVE BACTERIAL INFECTIONS

Allergan reports an updated label approved by the US Food and Drug Administration (FDA) for AVYCAZ® (ceftazidime and avibactam) in combination with metronidazole for the treatment of complicated intraabdominal infections caused by designated susceptible microorganisms. The label change was made after completion of a Phase 3 trial evaluating the safety and efficacy of AVYCAZ in combination with metronidazole. Results of the trial also included data from subsets of patients with infections due to ceftazidime- nonsusceptible pathogens and with pathogens producing certain extended-spectrum beta-lactamases (ESBLs).
First FDA-approved in February 2015, AVYCAZ is an antibiotic developed to treat certain Gram-negative bacterial infections. It consists of ceftazidime, a third- generation cephalosporin, and avibactam, a non-ß lactam ß-lactamase inhibitor.
Allergan says that AVYCAZ has demonstrated in vitro activity against Enterobacteriaceae in the presence of some beta-lactamases and ESBLs of the following groups: TEM, SHV, CTX-M, Klebsiella pneumoniae carbapenemase, AmpC, and certain oxacillinases. AVYCAZ also demonstrated in vitro activity against Pseudomonas aeruginosa in the presence of some AmpC beta-lactamases, and certain strains lacking outer membrane porin.
FOR MORE INFORMATION, VISIT: www.avycaz.com
ANTI-STICK SOLUTION FOR ELECTROSURGICAL INSTRUMENTS
Electro Lube is a mixture of natural, nonsynthetic, nonflammable, nonallergenic biocompatible phospholipids with no known side effects associated with patient use. It can be used on a variety of monopolar and bipolar instrumentation including: cutting forceps, Kleppingers, bipolar forceps, Bovie pencils, suction coagulators, robots, curved hot scissors, spatula electrodes, and l-hook electrodes.
FOR MORE INFORMATION, VISIT: www.electrolubesurgical.com
ORAL TREATMENT FOR PPH
A semi-synthetic ergot alkaloid, Methergine is indicated for routine management of uterine atony, hemorrhage, and subinvolution of the uterus following delivery of the placenta, and for control of uterine hemorrhage in the second stage of labor following delivery of the anterior shoulder. Methergine provides specific and rapid (onset of action under 10 minutes) uterotonic action on the smooth muscle of the uterus to increase contractions, resulting in restricting blood loss.
The Methergine dosing schedule is one tablet (0.2 mg) 3 or 4 times daily in the puerperium for a maximum of 1 week. The most common adverse event is hypertension, associated in several cases with seizure and/or headache. Methylergonovine maleate is also available as an intramuscular injection.
FOR MORE INFORMATION, VISIT: www.methergine.com
FREE MEDICAL BILLING CALCULATOR

ChartLogic, Inc. has launched its Medical Billing Analysis Calculator, a free, online, interactive tool that provides key billing metrics and compares those results to Medical Group Management Association (MGMA) benchmarks for a practice’s specialty. The calculator consists of an input section where practice specialty, annual charges, annual collections, outstanding accounts receivable (A/R), number of annual encounters, and contractual adjustments are entered. The tool, accessible on desktop or mobile devices, then calculates the practice’s billing analysis, including average reimbursement per encounter, net collection percentages, average monthly collections, and A/R data and displays the results beside specialty benchmarks. After submission, the user can alter the inputs or request more information from a ChartLogic billing expert.
ChartLogic’s goal as a medical billing service is to increase a practice’s performance. Services include a full ambulatory EHR suite with electronic medical records, practice management tools, e-prescribing, a patient portal, and more.
FOR MORE INFORMATION, VISIT: www.chartlogic.com
TREATING GRAM-NEGATIVE BACTERIAL INFECTIONS

Allergan reports an updated label approved by the US Food and Drug Administration (FDA) for AVYCAZ® (ceftazidime and avibactam) in combination with metronidazole for the treatment of complicated intraabdominal infections caused by designated susceptible microorganisms. The label change was made after completion of a Phase 3 trial evaluating the safety and efficacy of AVYCAZ in combination with metronidazole. Results of the trial also included data from subsets of patients with infections due to ceftazidime- nonsusceptible pathogens and with pathogens producing certain extended-spectrum beta-lactamases (ESBLs).
First FDA-approved in February 2015, AVYCAZ is an antibiotic developed to treat certain Gram-negative bacterial infections. It consists of ceftazidime, a third- generation cephalosporin, and avibactam, a non-ß lactam ß-lactamase inhibitor.
Allergan says that AVYCAZ has demonstrated in vitro activity against Enterobacteriaceae in the presence of some beta-lactamases and ESBLs of the following groups: TEM, SHV, CTX-M, Klebsiella pneumoniae carbapenemase, AmpC, and certain oxacillinases. AVYCAZ also demonstrated in vitro activity against Pseudomonas aeruginosa in the presence of some AmpC beta-lactamases, and certain strains lacking outer membrane porin.
FOR MORE INFORMATION, VISIT: www.avycaz.com
ANTI-STICK SOLUTION FOR ELECTROSURGICAL INSTRUMENTS
Electro Lube is a mixture of natural, nonsynthetic, nonflammable, nonallergenic biocompatible phospholipids with no known side effects associated with patient use. It can be used on a variety of monopolar and bipolar instrumentation including: cutting forceps, Kleppingers, bipolar forceps, Bovie pencils, suction coagulators, robots, curved hot scissors, spatula electrodes, and l-hook electrodes.
FOR MORE INFORMATION, VISIT: www.electrolubesurgical.com
ORAL TREATMENT FOR PPH
A semi-synthetic ergot alkaloid, Methergine is indicated for routine management of uterine atony, hemorrhage, and subinvolution of the uterus following delivery of the placenta, and for control of uterine hemorrhage in the second stage of labor following delivery of the anterior shoulder. Methergine provides specific and rapid (onset of action under 10 minutes) uterotonic action on the smooth muscle of the uterus to increase contractions, resulting in restricting blood loss.
The Methergine dosing schedule is one tablet (0.2 mg) 3 or 4 times daily in the puerperium for a maximum of 1 week. The most common adverse event is hypertension, associated in several cases with seizure and/or headache. Methylergonovine maleate is also available as an intramuscular injection.
FOR MORE INFORMATION, VISIT: www.methergine.com
FREE MEDICAL BILLING CALCULATOR

ChartLogic, Inc. has launched its Medical Billing Analysis Calculator, a free, online, interactive tool that provides key billing metrics and compares those results to Medical Group Management Association (MGMA) benchmarks for a practice’s specialty. The calculator consists of an input section where practice specialty, annual charges, annual collections, outstanding accounts receivable (A/R), number of annual encounters, and contractual adjustments are entered. The tool, accessible on desktop or mobile devices, then calculates the practice’s billing analysis, including average reimbursement per encounter, net collection percentages, average monthly collections, and A/R data and displays the results beside specialty benchmarks. After submission, the user can alter the inputs or request more information from a ChartLogic billing expert.
ChartLogic’s goal as a medical billing service is to increase a practice’s performance. Services include a full ambulatory EHR suite with electronic medical records, practice management tools, e-prescribing, a patient portal, and more.
FOR MORE INFORMATION, VISIT: www.chartlogic.com
Product News: 08 2016
CeraVeValeant Pharmaceuticals North America LLC receives The Skin Cancer Foundation (SCF) Seal of Recommendation for 2 products in the CeraVe skin care line. The CeraVe AM Facial Moisturizing Lotion With SPF 30 has earned the daily use SCF seal and the CeraVe SPF 50 Sunscreen Face Lotion has earned the active SCF seal. Products earning the SCF seal have fulfilled strict criteria from photobiologists, and consumers can purchase these products with the added reassurance that they perform as claimed. “We believe that more and more people are becoming aware of the dangers posed by unprotected sun exposure and for these individuals, particularly, the Seal of Recommendation is very meaningful,” said Joe Gordon, general manager of consumer health care at Valeant Pharmaceuticals. Both products are available over-the-counter at major retailers. For more information, visit www.cerave.com.
Differin Gel Galderma Laboratories, LP, announces approval from the US Food and Drug Administration for
over-the-counter use of Differin Gel (adapalene gel 0.1%) for the treatment of acne. “We’re excited to deliver this new level of efficacy without a prescription to consumers, and to bring the first new active ingredient to the category in over 30 years,” said Miles Harrison, president and general manager of Galderma Laboratories. With Differin Gel now available over-the-counter versus by prescription, acne patients will have easier access to this prescription-strength product containing the retinoid adapalene. For more information, visit www.differin.com.
UltraShape Power Syneron Medical Ltd announces US Food and Drug Administration clearance of UltraShape Power, a noninvasive fat destruction device for reduction of abdominal circumference. UltraShape Power uses pulsed mechanical ultrasound energy to target and destroy fat in both large and small areas and the advanced transducer delivers 20% more energy than its predecessor. UltraShape Power offers patients a 32% reduction in subcutaneous fat thickness. Patients have reported favorable results with little pain. A typical regimen is 3 treatments spaced 2 weeks apart. For more information, visit www.syneron-candela.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
CeraVeValeant Pharmaceuticals North America LLC receives The Skin Cancer Foundation (SCF) Seal of Recommendation for 2 products in the CeraVe skin care line. The CeraVe AM Facial Moisturizing Lotion With SPF 30 has earned the daily use SCF seal and the CeraVe SPF 50 Sunscreen Face Lotion has earned the active SCF seal. Products earning the SCF seal have fulfilled strict criteria from photobiologists, and consumers can purchase these products with the added reassurance that they perform as claimed. “We believe that more and more people are becoming aware of the dangers posed by unprotected sun exposure and for these individuals, particularly, the Seal of Recommendation is very meaningful,” said Joe Gordon, general manager of consumer health care at Valeant Pharmaceuticals. Both products are available over-the-counter at major retailers. For more information, visit www.cerave.com.
Differin Gel Galderma Laboratories, LP, announces approval from the US Food and Drug Administration for
over-the-counter use of Differin Gel (adapalene gel 0.1%) for the treatment of acne. “We’re excited to deliver this new level of efficacy without a prescription to consumers, and to bring the first new active ingredient to the category in over 30 years,” said Miles Harrison, president and general manager of Galderma Laboratories. With Differin Gel now available over-the-counter versus by prescription, acne patients will have easier access to this prescription-strength product containing the retinoid adapalene. For more information, visit www.differin.com.
UltraShape Power Syneron Medical Ltd announces US Food and Drug Administration clearance of UltraShape Power, a noninvasive fat destruction device for reduction of abdominal circumference. UltraShape Power uses pulsed mechanical ultrasound energy to target and destroy fat in both large and small areas and the advanced transducer delivers 20% more energy than its predecessor. UltraShape Power offers patients a 32% reduction in subcutaneous fat thickness. Patients have reported favorable results with little pain. A typical regimen is 3 treatments spaced 2 weeks apart. For more information, visit www.syneron-candela.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
CeraVeValeant Pharmaceuticals North America LLC receives The Skin Cancer Foundation (SCF) Seal of Recommendation for 2 products in the CeraVe skin care line. The CeraVe AM Facial Moisturizing Lotion With SPF 30 has earned the daily use SCF seal and the CeraVe SPF 50 Sunscreen Face Lotion has earned the active SCF seal. Products earning the SCF seal have fulfilled strict criteria from photobiologists, and consumers can purchase these products with the added reassurance that they perform as claimed. “We believe that more and more people are becoming aware of the dangers posed by unprotected sun exposure and for these individuals, particularly, the Seal of Recommendation is very meaningful,” said Joe Gordon, general manager of consumer health care at Valeant Pharmaceuticals. Both products are available over-the-counter at major retailers. For more information, visit www.cerave.com.
Differin Gel Galderma Laboratories, LP, announces approval from the US Food and Drug Administration for
over-the-counter use of Differin Gel (adapalene gel 0.1%) for the treatment of acne. “We’re excited to deliver this new level of efficacy without a prescription to consumers, and to bring the first new active ingredient to the category in over 30 years,” said Miles Harrison, president and general manager of Galderma Laboratories. With Differin Gel now available over-the-counter versus by prescription, acne patients will have easier access to this prescription-strength product containing the retinoid adapalene. For more information, visit www.differin.com.
UltraShape Power Syneron Medical Ltd announces US Food and Drug Administration clearance of UltraShape Power, a noninvasive fat destruction device for reduction of abdominal circumference. UltraShape Power uses pulsed mechanical ultrasound energy to target and destroy fat in both large and small areas and the advanced transducer delivers 20% more energy than its predecessor. UltraShape Power offers patients a 32% reduction in subcutaneous fat thickness. Patients have reported favorable results with little pain. A typical regimen is 3 treatments spaced 2 weeks apart. For more information, visit www.syneron-candela.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Product Update: RESECTR disposable handheld resector, PreTRM Test
DISPOSABLE HANDHELD RESECTOR

Distal Access offers nationwide availability of the RESECTR™ 9 French / 3.0 mm High- Performance Disposable Tissue Resector, a disposable, nonpowered, handheld, and hand-driven system designed to combine the benefits of basic manual devices and complex electro-mechanical systems.
The RESECTR platform is “ready-to-use,” says Distal Access, giving clinicians an important tool to see-and-treat lesions in the hospital, clinic, or office. Starting at the cutting tip, aspiration pulls tissue samples into the cutting window where oscillating blades are controlled by the clinician’s index finger and hand. Clinicians can increase or decrease oscillation and cutting based on what they see and feel during the procedure.
According to Distal Access, for small tissue samples, resection time with the RESECTR can be similar to that with electromechanical devices, with a significantly lower cost. The RESECTR is compatible with available fluid management systems and endoscopic devices.
FOR MORE INFORMATION, VISIT: www.resectr.com
PREDICTIVE TOOL FOR PRETERM BIRTH RISK

Sera Prognostics announced that its PreTRM® Test is the first and only clinically validated blood test to predict preterm birth risk in asymptomatic, singleton pregnancies.
Premature birth, defined as birth before 37 weeks, is the leading cause of death and illness in newborns and is associated with an increased risk of major long-term complications. Previously, the 2 best traditional predictors of premature birth were prior preterm birth history and short cervical length, but these identify only a small percentage of women who deliver early, asserts Sera Prognostics. Implemented during gestational weeks 19 and 20, the PreTRM test uses proteomic technology to measure and analyze 2 proteins in the blood that are highly predictive of preterm birth: IBP4, insulin-like growth factor binding protein 4, and SHBG, sex-hormone binding globulin.
According to Sera Prognostics, data from the 5,501-patient Proteomic Assessment of Preterm Risk (PAPR) study, recently published in American Journal of Obstetrics & Gynecology, confirm that the test can help identify a high percentage of women who are at increased risk early in pregnancy before symptoms occur.
FOR MORE INFORMATION, VISIT: www.pretrm.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
DISPOSABLE HANDHELD RESECTOR

Distal Access offers nationwide availability of the RESECTR™ 9 French / 3.0 mm High- Performance Disposable Tissue Resector, a disposable, nonpowered, handheld, and hand-driven system designed to combine the benefits of basic manual devices and complex electro-mechanical systems.
The RESECTR platform is “ready-to-use,” says Distal Access, giving clinicians an important tool to see-and-treat lesions in the hospital, clinic, or office. Starting at the cutting tip, aspiration pulls tissue samples into the cutting window where oscillating blades are controlled by the clinician’s index finger and hand. Clinicians can increase or decrease oscillation and cutting based on what they see and feel during the procedure.
According to Distal Access, for small tissue samples, resection time with the RESECTR can be similar to that with electromechanical devices, with a significantly lower cost. The RESECTR is compatible with available fluid management systems and endoscopic devices.
FOR MORE INFORMATION, VISIT: www.resectr.com
PREDICTIVE TOOL FOR PRETERM BIRTH RISK

Sera Prognostics announced that its PreTRM® Test is the first and only clinically validated blood test to predict preterm birth risk in asymptomatic, singleton pregnancies.
Premature birth, defined as birth before 37 weeks, is the leading cause of death and illness in newborns and is associated with an increased risk of major long-term complications. Previously, the 2 best traditional predictors of premature birth were prior preterm birth history and short cervical length, but these identify only a small percentage of women who deliver early, asserts Sera Prognostics. Implemented during gestational weeks 19 and 20, the PreTRM test uses proteomic technology to measure and analyze 2 proteins in the blood that are highly predictive of preterm birth: IBP4, insulin-like growth factor binding protein 4, and SHBG, sex-hormone binding globulin.
According to Sera Prognostics, data from the 5,501-patient Proteomic Assessment of Preterm Risk (PAPR) study, recently published in American Journal of Obstetrics & Gynecology, confirm that the test can help identify a high percentage of women who are at increased risk early in pregnancy before symptoms occur.
FOR MORE INFORMATION, VISIT: www.pretrm.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
DISPOSABLE HANDHELD RESECTOR

Distal Access offers nationwide availability of the RESECTR™ 9 French / 3.0 mm High- Performance Disposable Tissue Resector, a disposable, nonpowered, handheld, and hand-driven system designed to combine the benefits of basic manual devices and complex electro-mechanical systems.
The RESECTR platform is “ready-to-use,” says Distal Access, giving clinicians an important tool to see-and-treat lesions in the hospital, clinic, or office. Starting at the cutting tip, aspiration pulls tissue samples into the cutting window where oscillating blades are controlled by the clinician’s index finger and hand. Clinicians can increase or decrease oscillation and cutting based on what they see and feel during the procedure.
According to Distal Access, for small tissue samples, resection time with the RESECTR can be similar to that with electromechanical devices, with a significantly lower cost. The RESECTR is compatible with available fluid management systems and endoscopic devices.
FOR MORE INFORMATION, VISIT: www.resectr.com
PREDICTIVE TOOL FOR PRETERM BIRTH RISK

Sera Prognostics announced that its PreTRM® Test is the first and only clinically validated blood test to predict preterm birth risk in asymptomatic, singleton pregnancies.
Premature birth, defined as birth before 37 weeks, is the leading cause of death and illness in newborns and is associated with an increased risk of major long-term complications. Previously, the 2 best traditional predictors of premature birth were prior preterm birth history and short cervical length, but these identify only a small percentage of women who deliver early, asserts Sera Prognostics. Implemented during gestational weeks 19 and 20, the PreTRM test uses proteomic technology to measure and analyze 2 proteins in the blood that are highly predictive of preterm birth: IBP4, insulin-like growth factor binding protein 4, and SHBG, sex-hormone binding globulin.
According to Sera Prognostics, data from the 5,501-patient Proteomic Assessment of Preterm Risk (PAPR) study, recently published in American Journal of Obstetrics & Gynecology, confirm that the test can help identify a high percentage of women who are at increased risk early in pregnancy before symptoms occur.
FOR MORE INFORMATION, VISIT: www.pretrm.com
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Product Update: MyoSure REACH, Counsyl, Good Clean Love, OB Complete Gold
ACCESS TO HARD-TO-REACH UTERINE CAVITY
Hologic has added the MyoSure REACH device to its tissue removal system to maximize access for resection of fibroids up to 3 cm in size. The MyoSure REACH is designed to access hard-to-reach areas, including the upper third of the uterine cavity. Hologic says that the MyoSure REACH features a shortened distance from the distal tip to the cutting window of less than 1 millimeter to allow for easier access to polyps and fibroids. When compared with the predecessor device, the new design has been shown to get 3 times closer to the uterine wall and removes 25% more tissue in a simulated bench top model. The MyoSure REACH has an outer diameter of 3 mm and working length of 32 cm, with scope compatibility with MyoSure and MyoSure XL. The blade is made of coated stainless steel with ultra-hardness and high-wear resistance.
FOR MORE INFORMATION, VISIT: www.myosure.com/hcp/myosure-reach
DNA TESTING AND GENETIC COUNSELING SERVICE
Counsyl, a DNA testing and genetic counseling service, announced expansion into the oncology market with a focus on advancing cancer risk screening and cancer prevention.
Counsyl’s Inherited Cancer Screen is a prescription-based genetic screening test available for patients to take at the physician’s office or receive via mail. Tests include on-demand genetic counseling that is in-network with most insurance providers. Inherited Cancer Screen helps physicians identify patients who may benefit from more aggressive screening procedures, changes in lifestyle or medication regimens, or preventive actions such as surgery. Inherited Cancer Screen is designed to test for up to 36 genes associated with increased risk of cancers of the breast, ovaries, pancreas, colon, prostate, and skin, with results typically delivered within 2 weeks. To help inform these decisions, Counsyl provides physicians and patients access to a team of more than 40 board-certified genetic counselors.
FirstCare is a web and mobile-friendly software tool to help physicians determine patient eligibility for genetic screening, based on factors such as family history.
Counsyl also provides prepregnancy and prenatal genetic testing. The fee for each test includes a consultation with a genetic counselor.
FOR MORE INFORMATION, VISIT: www.counsyl.com
PERSONAL LUBRICANTS AND WASH
Good Clean Love offers vaginal lubricants and a moisturizing personal wash formulated, according to the manufacturer, to match the ideal pH, salt balance, and type of lactic acid produced by healthy bacteria levels in the vagina.
Good Clean Love’s Personal Lubricants have been developed to mimic the body’s natural mucous production using certified 95% organic and natural ingredients, including aloe and agar, without chemical additives. The 2 choices are Almost Naked Organic Personal Lubricant, lightly scented with lemon and vanilla, and Cinnamon Vanilla Organic Personal Lubricant.
Bio-Match™ Restore™ Moisturizing Personal Lubricant mimics the body’s natural lubricating response, is fertility-friendly, 100% vegan, petroleum-, glycerin-, and paraben-free, and is safe for use with latex condoms and toys.
Bio-Match™ Balance™ Moisturizing Personal Wash is a moisturizing vaginal cleanser made from botanical extracts that Good Clean Love says returns genital pH and salinity to a naturally healthy balance. It is 100% vegan, and petroleum- and paraben-free.
FOR MORE INFORMATION, VISIT: http://goodcleanlove.com
PRENATAL VITAMINS WITH EGG-BASED DHA
OB Complete® Gold are prescription prenatal vitamin supple-ments from Vertical Pharmaceuticals indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and nonlactating women. The supplement delivers gluten-free, sugar-free, and lactose-free multivitamins and minerals in a small softgel taken once a day.
OB Complete Gold prenatal vitamin supplements contain OmEGGa DHA™ (docosahexaenoic acid), an egg-based Omega 3 fatty acid that is believed to play an important role in the development of the brain, retina, and central nervous system of the fetus. According to Vertical Pharmaceuticals, this form of DHA, found naturally in the body and breast milk, is more easily absorbed, digested, and distributed to the body’s tissues than the triglyceride form of DHA developed from marine sources such as fish or algae, which can produce unwanted side effects such as bad breath, a fishy taste, and burping.
FOR MORE INFORMATION, VISIT: www.obcompletegold.com
ACCESS TO HARD-TO-REACH UTERINE CAVITY
Hologic has added the MyoSure REACH device to its tissue removal system to maximize access for resection of fibroids up to 3 cm in size. The MyoSure REACH is designed to access hard-to-reach areas, including the upper third of the uterine cavity. Hologic says that the MyoSure REACH features a shortened distance from the distal tip to the cutting window of less than 1 millimeter to allow for easier access to polyps and fibroids. When compared with the predecessor device, the new design has been shown to get 3 times closer to the uterine wall and removes 25% more tissue in a simulated bench top model. The MyoSure REACH has an outer diameter of 3 mm and working length of 32 cm, with scope compatibility with MyoSure and MyoSure XL. The blade is made of coated stainless steel with ultra-hardness and high-wear resistance.
FOR MORE INFORMATION, VISIT: www.myosure.com/hcp/myosure-reach
DNA TESTING AND GENETIC COUNSELING SERVICE
Counsyl, a DNA testing and genetic counseling service, announced expansion into the oncology market with a focus on advancing cancer risk screening and cancer prevention.
Counsyl’s Inherited Cancer Screen is a prescription-based genetic screening test available for patients to take at the physician’s office or receive via mail. Tests include on-demand genetic counseling that is in-network with most insurance providers. Inherited Cancer Screen helps physicians identify patients who may benefit from more aggressive screening procedures, changes in lifestyle or medication regimens, or preventive actions such as surgery. Inherited Cancer Screen is designed to test for up to 36 genes associated with increased risk of cancers of the breast, ovaries, pancreas, colon, prostate, and skin, with results typically delivered within 2 weeks. To help inform these decisions, Counsyl provides physicians and patients access to a team of more than 40 board-certified genetic counselors.
FirstCare is a web and mobile-friendly software tool to help physicians determine patient eligibility for genetic screening, based on factors such as family history.
Counsyl also provides prepregnancy and prenatal genetic testing. The fee for each test includes a consultation with a genetic counselor.
FOR MORE INFORMATION, VISIT: www.counsyl.com
PERSONAL LUBRICANTS AND WASH
Good Clean Love offers vaginal lubricants and a moisturizing personal wash formulated, according to the manufacturer, to match the ideal pH, salt balance, and type of lactic acid produced by healthy bacteria levels in the vagina.
Good Clean Love’s Personal Lubricants have been developed to mimic the body’s natural mucous production using certified 95% organic and natural ingredients, including aloe and agar, without chemical additives. The 2 choices are Almost Naked Organic Personal Lubricant, lightly scented with lemon and vanilla, and Cinnamon Vanilla Organic Personal Lubricant.
Bio-Match™ Restore™ Moisturizing Personal Lubricant mimics the body’s natural lubricating response, is fertility-friendly, 100% vegan, petroleum-, glycerin-, and paraben-free, and is safe for use with latex condoms and toys.
Bio-Match™ Balance™ Moisturizing Personal Wash is a moisturizing vaginal cleanser made from botanical extracts that Good Clean Love says returns genital pH and salinity to a naturally healthy balance. It is 100% vegan, and petroleum- and paraben-free.
FOR MORE INFORMATION, VISIT: http://goodcleanlove.com
PRENATAL VITAMINS WITH EGG-BASED DHA
OB Complete® Gold are prescription prenatal vitamin supple-ments from Vertical Pharmaceuticals indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and nonlactating women. The supplement delivers gluten-free, sugar-free, and lactose-free multivitamins and minerals in a small softgel taken once a day.
OB Complete Gold prenatal vitamin supplements contain OmEGGa DHA™ (docosahexaenoic acid), an egg-based Omega 3 fatty acid that is believed to play an important role in the development of the brain, retina, and central nervous system of the fetus. According to Vertical Pharmaceuticals, this form of DHA, found naturally in the body and breast milk, is more easily absorbed, digested, and distributed to the body’s tissues than the triglyceride form of DHA developed from marine sources such as fish or algae, which can produce unwanted side effects such as bad breath, a fishy taste, and burping.
FOR MORE INFORMATION, VISIT: www.obcompletegold.com
ACCESS TO HARD-TO-REACH UTERINE CAVITY
Hologic has added the MyoSure REACH device to its tissue removal system to maximize access for resection of fibroids up to 3 cm in size. The MyoSure REACH is designed to access hard-to-reach areas, including the upper third of the uterine cavity. Hologic says that the MyoSure REACH features a shortened distance from the distal tip to the cutting window of less than 1 millimeter to allow for easier access to polyps and fibroids. When compared with the predecessor device, the new design has been shown to get 3 times closer to the uterine wall and removes 25% more tissue in a simulated bench top model. The MyoSure REACH has an outer diameter of 3 mm and working length of 32 cm, with scope compatibility with MyoSure and MyoSure XL. The blade is made of coated stainless steel with ultra-hardness and high-wear resistance.
FOR MORE INFORMATION, VISIT: www.myosure.com/hcp/myosure-reach
DNA TESTING AND GENETIC COUNSELING SERVICE
Counsyl, a DNA testing and genetic counseling service, announced expansion into the oncology market with a focus on advancing cancer risk screening and cancer prevention.
Counsyl’s Inherited Cancer Screen is a prescription-based genetic screening test available for patients to take at the physician’s office or receive via mail. Tests include on-demand genetic counseling that is in-network with most insurance providers. Inherited Cancer Screen helps physicians identify patients who may benefit from more aggressive screening procedures, changes in lifestyle or medication regimens, or preventive actions such as surgery. Inherited Cancer Screen is designed to test for up to 36 genes associated with increased risk of cancers of the breast, ovaries, pancreas, colon, prostate, and skin, with results typically delivered within 2 weeks. To help inform these decisions, Counsyl provides physicians and patients access to a team of more than 40 board-certified genetic counselors.
FirstCare is a web and mobile-friendly software tool to help physicians determine patient eligibility for genetic screening, based on factors such as family history.
Counsyl also provides prepregnancy and prenatal genetic testing. The fee for each test includes a consultation with a genetic counselor.
FOR MORE INFORMATION, VISIT: www.counsyl.com
PERSONAL LUBRICANTS AND WASH
Good Clean Love offers vaginal lubricants and a moisturizing personal wash formulated, according to the manufacturer, to match the ideal pH, salt balance, and type of lactic acid produced by healthy bacteria levels in the vagina.
Good Clean Love’s Personal Lubricants have been developed to mimic the body’s natural mucous production using certified 95% organic and natural ingredients, including aloe and agar, without chemical additives. The 2 choices are Almost Naked Organic Personal Lubricant, lightly scented with lemon and vanilla, and Cinnamon Vanilla Organic Personal Lubricant.
Bio-Match™ Restore™ Moisturizing Personal Lubricant mimics the body’s natural lubricating response, is fertility-friendly, 100% vegan, petroleum-, glycerin-, and paraben-free, and is safe for use with latex condoms and toys.
Bio-Match™ Balance™ Moisturizing Personal Wash is a moisturizing vaginal cleanser made from botanical extracts that Good Clean Love says returns genital pH and salinity to a naturally healthy balance. It is 100% vegan, and petroleum- and paraben-free.
FOR MORE INFORMATION, VISIT: http://goodcleanlove.com
PRENATAL VITAMINS WITH EGG-BASED DHA
OB Complete® Gold are prescription prenatal vitamin supple-ments from Vertical Pharmaceuticals indicated for use in improving the nutritional status of women prior to conception, throughout pregnancy, and in the postnatal period for both lactating and nonlactating women. The supplement delivers gluten-free, sugar-free, and lactose-free multivitamins and minerals in a small softgel taken once a day.
OB Complete Gold prenatal vitamin supplements contain OmEGGa DHA™ (docosahexaenoic acid), an egg-based Omega 3 fatty acid that is believed to play an important role in the development of the brain, retina, and central nervous system of the fetus. According to Vertical Pharmaceuticals, this form of DHA, found naturally in the body and breast milk, is more easily absorbed, digested, and distributed to the body’s tissues than the triglyceride form of DHA developed from marine sources such as fish or algae, which can produce unwanted side effects such as bad breath, a fishy taste, and burping.
FOR MORE INFORMATION, VISIT: www.obcompletegold.com
Engineered Bone Graft
Exactech
Optecure+ccc
(http://www.exac.com/products/biologics/optecure-optecure-ccc)
Autogenous bone graft remains the standard for augmenting the surgical care of severe fractures, promoting spinal fusion, filling bone voids, and treating nonunions. However, lingering problems with donor site morbidity, volume limitation, increased operative time, and increased case complexity have led to the growing use of bone graft substitutes.1 These alternatives include allograft bone, demineralized bone matrix, calcium sulfate and calcium phosphate, bioglass, growth factors (rhBMP-2, rhBMP-7, rhPDGF, and PRP [platelet-rich plasma]), collagen matrix, and new cellular-based compounds using mesenchymal stem cells. Since each individual class of bone substitute falls short of the optimal blend of osteoconduction, osteoinduction, and osteogenesis, novel composite grafts have been developed to combine the convenience, durability, and flexibility of synthetic grafts with the biologic activity of native bone.
Optecure+ccc (Exactech) is an engineered composite bone graft that contains demineralized bone mixed with gamma irradiated cortical cancellous chips in an absorbable synthetic hydrogel matrix (Figure). When mixed with saline, blood, autogenous bone, bone marrow aspirate, or PRP, it becomes a surprisingly robust and malleable 3-dimensional matrix that allows easy bone void filling with excellent osteoconductive and osteoinductive characteristics. Each individual lot is tested for sterility and endotoxin levels to confirm safety as well as in vivo testing in athymic mice to confirm osteoinductive potential. Optecure+ccc has been successfully used to augment healing when combined with bone marrow aspirate in minimally invasive spine fusion surgery.2
Surgical pearl: I treat a large number of bicycle injuries on Nantucket; many are quite serious. I have found Optecure+ccc to be particularly useful during locked volar plating of severe distal radius wrist fractures as a way to restore and support radial length when autogenous bone access is limited. In this application, Optecure’s ability to expand and mold into a functional bone scaffold is critical to create a stable, stress-resistant fracture construct.
After exposure of the comminuted fracture line of the distal radius, gentle axial traction is applied and a small osteotome or freer is used to carefully wedge open the cortex to allow metaphyseal window access. The Optecure+ccc is mixed with either blood or bone marrow aspirate to reach a “grape nuts cereal”-like consistency and then carefully packed into the metaphyseal window to backfill the void. Multiplanar fluoroscopy is used to monitor graft placement and gradual joint line restoration. Traction is then released after the void is filled sufficiently to support the provisional reduction. Additional grafting with standard Optecure without bone chips can be used to fill more difficult-to-access areas. Both forms of Optecure are resistant to diluent migration, giving them good intraoperative behavior. Excess graft can be easily wiped away from the fracture site prior to plate application.
After elevation and restoration of the joint line, the locking volar plate is then affixed, wrist alignment confirmed fluoroscopically, and the procedure completed. The result is a well-filled void and an improved fracture construct. While Optecure+ccc has proven its battle readiness in wrist fracture surgery, I have also found it very helpful in reconstructing complex proximal humerus and clavicle fractures. Its unique combination of intraoperative versatility and durability provides a welcome edge in challenging cases.
1. Rodgers WB, Gerber EJ, Patterson JR. Fusion after minimally disruptive anterior lumbar interbody fusion: analysis of extreme lateral interbody fusion by computed tomography. SAS J. 2010;4(2):63-66.
2. Sasso RC, LeHuec JC, Shaffrey C; Spine Interbody Research Group. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18 Suppl:S77-S81.
Exactech
Optecure+ccc
(http://www.exac.com/products/biologics/optecure-optecure-ccc)
Autogenous bone graft remains the standard for augmenting the surgical care of severe fractures, promoting spinal fusion, filling bone voids, and treating nonunions. However, lingering problems with donor site morbidity, volume limitation, increased operative time, and increased case complexity have led to the growing use of bone graft substitutes.1 These alternatives include allograft bone, demineralized bone matrix, calcium sulfate and calcium phosphate, bioglass, growth factors (rhBMP-2, rhBMP-7, rhPDGF, and PRP [platelet-rich plasma]), collagen matrix, and new cellular-based compounds using mesenchymal stem cells. Since each individual class of bone substitute falls short of the optimal blend of osteoconduction, osteoinduction, and osteogenesis, novel composite grafts have been developed to combine the convenience, durability, and flexibility of synthetic grafts with the biologic activity of native bone.
Optecure+ccc (Exactech) is an engineered composite bone graft that contains demineralized bone mixed with gamma irradiated cortical cancellous chips in an absorbable synthetic hydrogel matrix (Figure). When mixed with saline, blood, autogenous bone, bone marrow aspirate, or PRP, it becomes a surprisingly robust and malleable 3-dimensional matrix that allows easy bone void filling with excellent osteoconductive and osteoinductive characteristics. Each individual lot is tested for sterility and endotoxin levels to confirm safety as well as in vivo testing in athymic mice to confirm osteoinductive potential. Optecure+ccc has been successfully used to augment healing when combined with bone marrow aspirate in minimally invasive spine fusion surgery.2
Surgical pearl: I treat a large number of bicycle injuries on Nantucket; many are quite serious. I have found Optecure+ccc to be particularly useful during locked volar plating of severe distal radius wrist fractures as a way to restore and support radial length when autogenous bone access is limited. In this application, Optecure’s ability to expand and mold into a functional bone scaffold is critical to create a stable, stress-resistant fracture construct.
After exposure of the comminuted fracture line of the distal radius, gentle axial traction is applied and a small osteotome or freer is used to carefully wedge open the cortex to allow metaphyseal window access. The Optecure+ccc is mixed with either blood or bone marrow aspirate to reach a “grape nuts cereal”-like consistency and then carefully packed into the metaphyseal window to backfill the void. Multiplanar fluoroscopy is used to monitor graft placement and gradual joint line restoration. Traction is then released after the void is filled sufficiently to support the provisional reduction. Additional grafting with standard Optecure without bone chips can be used to fill more difficult-to-access areas. Both forms of Optecure are resistant to diluent migration, giving them good intraoperative behavior. Excess graft can be easily wiped away from the fracture site prior to plate application.
After elevation and restoration of the joint line, the locking volar plate is then affixed, wrist alignment confirmed fluoroscopically, and the procedure completed. The result is a well-filled void and an improved fracture construct. While Optecure+ccc has proven its battle readiness in wrist fracture surgery, I have also found it very helpful in reconstructing complex proximal humerus and clavicle fractures. Its unique combination of intraoperative versatility and durability provides a welcome edge in challenging cases.
Exactech
Optecure+ccc
(http://www.exac.com/products/biologics/optecure-optecure-ccc)
Autogenous bone graft remains the standard for augmenting the surgical care of severe fractures, promoting spinal fusion, filling bone voids, and treating nonunions. However, lingering problems with donor site morbidity, volume limitation, increased operative time, and increased case complexity have led to the growing use of bone graft substitutes.1 These alternatives include allograft bone, demineralized bone matrix, calcium sulfate and calcium phosphate, bioglass, growth factors (rhBMP-2, rhBMP-7, rhPDGF, and PRP [platelet-rich plasma]), collagen matrix, and new cellular-based compounds using mesenchymal stem cells. Since each individual class of bone substitute falls short of the optimal blend of osteoconduction, osteoinduction, and osteogenesis, novel composite grafts have been developed to combine the convenience, durability, and flexibility of synthetic grafts with the biologic activity of native bone.
Optecure+ccc (Exactech) is an engineered composite bone graft that contains demineralized bone mixed with gamma irradiated cortical cancellous chips in an absorbable synthetic hydrogel matrix (Figure). When mixed with saline, blood, autogenous bone, bone marrow aspirate, or PRP, it becomes a surprisingly robust and malleable 3-dimensional matrix that allows easy bone void filling with excellent osteoconductive and osteoinductive characteristics. Each individual lot is tested for sterility and endotoxin levels to confirm safety as well as in vivo testing in athymic mice to confirm osteoinductive potential. Optecure+ccc has been successfully used to augment healing when combined with bone marrow aspirate in minimally invasive spine fusion surgery.2
Surgical pearl: I treat a large number of bicycle injuries on Nantucket; many are quite serious. I have found Optecure+ccc to be particularly useful during locked volar plating of severe distal radius wrist fractures as a way to restore and support radial length when autogenous bone access is limited. In this application, Optecure’s ability to expand and mold into a functional bone scaffold is critical to create a stable, stress-resistant fracture construct.
After exposure of the comminuted fracture line of the distal radius, gentle axial traction is applied and a small osteotome or freer is used to carefully wedge open the cortex to allow metaphyseal window access. The Optecure+ccc is mixed with either blood or bone marrow aspirate to reach a “grape nuts cereal”-like consistency and then carefully packed into the metaphyseal window to backfill the void. Multiplanar fluoroscopy is used to monitor graft placement and gradual joint line restoration. Traction is then released after the void is filled sufficiently to support the provisional reduction. Additional grafting with standard Optecure without bone chips can be used to fill more difficult-to-access areas. Both forms of Optecure are resistant to diluent migration, giving them good intraoperative behavior. Excess graft can be easily wiped away from the fracture site prior to plate application.
After elevation and restoration of the joint line, the locking volar plate is then affixed, wrist alignment confirmed fluoroscopically, and the procedure completed. The result is a well-filled void and an improved fracture construct. While Optecure+ccc has proven its battle readiness in wrist fracture surgery, I have also found it very helpful in reconstructing complex proximal humerus and clavicle fractures. Its unique combination of intraoperative versatility and durability provides a welcome edge in challenging cases.
1. Rodgers WB, Gerber EJ, Patterson JR. Fusion after minimally disruptive anterior lumbar interbody fusion: analysis of extreme lateral interbody fusion by computed tomography. SAS J. 2010;4(2):63-66.
2. Sasso RC, LeHuec JC, Shaffrey C; Spine Interbody Research Group. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18 Suppl:S77-S81.
1. Rodgers WB, Gerber EJ, Patterson JR. Fusion after minimally disruptive anterior lumbar interbody fusion: analysis of extreme lateral interbody fusion by computed tomography. SAS J. 2010;4(2):63-66.
2. Sasso RC, LeHuec JC, Shaffrey C; Spine Interbody Research Group. Iliac crest bone graft donor site pain after anterior lumbar interbody fusion: a prospective patient satisfaction outcome assessment. J Spinal Disord Tech. 2005;18 Suppl:S77-S81.
Product News: 07 2016
Carmex Grant
Carma Labs Inc, the maker of Carmex lip balms, announced a $10,000 grant program to support an entrepreneur with an Unstoppable Dream. Participants can enter through Facebook or Instagram by posting a 30-second video depicting their aspiration related to a business or product. All entries must include the hashtags #InPursuitOf and #Contest to be considered. Entries can be submitted through July 15, and a winner will be selected and announced in August. For more information, visit www.mycarmex.com.
Juvéderm Volbella XC
Allergan announces US Food and Drug Administration approval to market Juvéderm Volbella XC for lip augmentation and for correction of perioral lines in adults older than 21 years. Juvéderm Volbella XC increases lip fullness and softens the appearance of lines around the mouth. It is formulated with Vycross, a proprietary filler technology that contributes to the product’s smoothness. Juvéderm Volbella XC has been customized with a lower hyaluronic acid concentration (15 mg/mL), while still providing the long-lasting results of other Juvéderm products. It will be available to patients in the United States in October 2016. For more information, visit www.juvederm.com.
Teflaro
Allergan announces US Food and Drug Administration approval of a supplemental new drug application for Teflaro (ceftaroline fosamil) to expand the label to include the treatment of children aged 2 months to less than 18 years with acute bacterial skin and skin structure infections (ABSSSI) including infections caused by methicillin-resistant Staphylococcus aureus and community-acquired bacterial pneumonia (CABP) caused by Staphylococcus pneumoniae and other designated susceptible bacteria. Teflaro is a bactericidal cephalosporin with activity against both gram-positive and gram-negative pathogens. Teflaro is already approved for ABSSSI and CABP in adult patients. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Carmex Grant
Carma Labs Inc, the maker of Carmex lip balms, announced a $10,000 grant program to support an entrepreneur with an Unstoppable Dream. Participants can enter through Facebook or Instagram by posting a 30-second video depicting their aspiration related to a business or product. All entries must include the hashtags #InPursuitOf and #Contest to be considered. Entries can be submitted through July 15, and a winner will be selected and announced in August. For more information, visit www.mycarmex.com.
Juvéderm Volbella XC
Allergan announces US Food and Drug Administration approval to market Juvéderm Volbella XC for lip augmentation and for correction of perioral lines in adults older than 21 years. Juvéderm Volbella XC increases lip fullness and softens the appearance of lines around the mouth. It is formulated with Vycross, a proprietary filler technology that contributes to the product’s smoothness. Juvéderm Volbella XC has been customized with a lower hyaluronic acid concentration (15 mg/mL), while still providing the long-lasting results of other Juvéderm products. It will be available to patients in the United States in October 2016. For more information, visit www.juvederm.com.
Teflaro
Allergan announces US Food and Drug Administration approval of a supplemental new drug application for Teflaro (ceftaroline fosamil) to expand the label to include the treatment of children aged 2 months to less than 18 years with acute bacterial skin and skin structure infections (ABSSSI) including infections caused by methicillin-resistant Staphylococcus aureus and community-acquired bacterial pneumonia (CABP) caused by Staphylococcus pneumoniae and other designated susceptible bacteria. Teflaro is a bactericidal cephalosporin with activity against both gram-positive and gram-negative pathogens. Teflaro is already approved for ABSSSI and CABP in adult patients. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Carmex Grant
Carma Labs Inc, the maker of Carmex lip balms, announced a $10,000 grant program to support an entrepreneur with an Unstoppable Dream. Participants can enter through Facebook or Instagram by posting a 30-second video depicting their aspiration related to a business or product. All entries must include the hashtags #InPursuitOf and #Contest to be considered. Entries can be submitted through July 15, and a winner will be selected and announced in August. For more information, visit www.mycarmex.com.
Juvéderm Volbella XC
Allergan announces US Food and Drug Administration approval to market Juvéderm Volbella XC for lip augmentation and for correction of perioral lines in adults older than 21 years. Juvéderm Volbella XC increases lip fullness and softens the appearance of lines around the mouth. It is formulated with Vycross, a proprietary filler technology that contributes to the product’s smoothness. Juvéderm Volbella XC has been customized with a lower hyaluronic acid concentration (15 mg/mL), while still providing the long-lasting results of other Juvéderm products. It will be available to patients in the United States in October 2016. For more information, visit www.juvederm.com.
Teflaro
Allergan announces US Food and Drug Administration approval of a supplemental new drug application for Teflaro (ceftaroline fosamil) to expand the label to include the treatment of children aged 2 months to less than 18 years with acute bacterial skin and skin structure infections (ABSSSI) including infections caused by methicillin-resistant Staphylococcus aureus and community-acquired bacterial pneumonia (CABP) caused by Staphylococcus pneumoniae and other designated susceptible bacteria. Teflaro is a bactericidal cephalosporin with activity against both gram-positive and gram-negative pathogens. Teflaro is already approved for ABSSSI and CABP in adult patients. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Product News: 05 2016
Aczone Gel 7.5%
Allergan announces US Food and Drug Administration approval of Aczone (dapsone) Gel 7.5% for the treatment of acne vulgaris in patients 12 years and older. The new formula contains a higher concentration of dapsone (versus the 5% concentration) with the same tolerability and enhanced efficacy to treat both inflammatory and noninflammatory acne. Once-daily application and the pump delivery system aid in improving adherence to treatment. For more information, visit www.aczonehcp.com.
Cutanea Life Sciences
Cutanea Life Sciences, Inc (CLS), an emerging US prescription product development company, unveils its new website, www.cutanea.com, to the dermatology community. The new digital presence demonstrates the company’s intent to change the way customers think about a valued dermatology partner. The CLS management team is well versed in the development and commercialization of dermatologic therapies. With product candidates in various stages of development that cover an array of skin conditions such as acne, rosacea, psoriasis, and warts caused by human papillomavirus, CLS is committed to focusing on underserved patient needs, which will help health care professionals optimize their practice time through CLS’s dermatologic products and services.
Sernivo Spray 0.05%
Promius Pharma, LLC, announces US Food and Drug Administration approval of Sernivo (betamethasone dipropionate) Spray 0.05%, a corticosteroid for the treatment of mild to moderate plaque psoriasis in patients 18 years and older. Sernivo Spray should be used twice daily for 4 weeks and not beyond. In clinical trials more participants showed treatment success with Sernivo Spray versus vehicle at day 15 and day 29. For more information, visit www.promiuspharma.com.
Teflaro
Allergan announces that the US Food and Drug Administration has accepted for filing a supplemental new drug application for Teflaro (ceftaroline fosamil) to expand the label to include the treatment of children 2 months and older with acute bacterial skin and skin structure infections (ABSSSI) including infections caused by methicillin-resistant Staphylococcus aureus and community-acquired bacterial pneumonia (CABP) caused by Staphylococcus pneumoniae and other designated susceptible bacteria. Teflaro is a bactericidal cephalosporin with activity against both gram-positive and gram-negative pathogens. Teflaro is already approved for ABSSSI and CABP in adult patients. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Aczone Gel 7.5%
Allergan announces US Food and Drug Administration approval of Aczone (dapsone) Gel 7.5% for the treatment of acne vulgaris in patients 12 years and older. The new formula contains a higher concentration of dapsone (versus the 5% concentration) with the same tolerability and enhanced efficacy to treat both inflammatory and noninflammatory acne. Once-daily application and the pump delivery system aid in improving adherence to treatment. For more information, visit www.aczonehcp.com.
Cutanea Life Sciences
Cutanea Life Sciences, Inc (CLS), an emerging US prescription product development company, unveils its new website, www.cutanea.com, to the dermatology community. The new digital presence demonstrates the company’s intent to change the way customers think about a valued dermatology partner. The CLS management team is well versed in the development and commercialization of dermatologic therapies. With product candidates in various stages of development that cover an array of skin conditions such as acne, rosacea, psoriasis, and warts caused by human papillomavirus, CLS is committed to focusing on underserved patient needs, which will help health care professionals optimize their practice time through CLS’s dermatologic products and services.
Sernivo Spray 0.05%
Promius Pharma, LLC, announces US Food and Drug Administration approval of Sernivo (betamethasone dipropionate) Spray 0.05%, a corticosteroid for the treatment of mild to moderate plaque psoriasis in patients 18 years and older. Sernivo Spray should be used twice daily for 4 weeks and not beyond. In clinical trials more participants showed treatment success with Sernivo Spray versus vehicle at day 15 and day 29. For more information, visit www.promiuspharma.com.
Teflaro
Allergan announces that the US Food and Drug Administration has accepted for filing a supplemental new drug application for Teflaro (ceftaroline fosamil) to expand the label to include the treatment of children 2 months and older with acute bacterial skin and skin structure infections (ABSSSI) including infections caused by methicillin-resistant Staphylococcus aureus and community-acquired bacterial pneumonia (CABP) caused by Staphylococcus pneumoniae and other designated susceptible bacteria. Teflaro is a bactericidal cephalosporin with activity against both gram-positive and gram-negative pathogens. Teflaro is already approved for ABSSSI and CABP in adult patients. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Aczone Gel 7.5%
Allergan announces US Food and Drug Administration approval of Aczone (dapsone) Gel 7.5% for the treatment of acne vulgaris in patients 12 years and older. The new formula contains a higher concentration of dapsone (versus the 5% concentration) with the same tolerability and enhanced efficacy to treat both inflammatory and noninflammatory acne. Once-daily application and the pump delivery system aid in improving adherence to treatment. For more information, visit www.aczonehcp.com.
Cutanea Life Sciences
Cutanea Life Sciences, Inc (CLS), an emerging US prescription product development company, unveils its new website, www.cutanea.com, to the dermatology community. The new digital presence demonstrates the company’s intent to change the way customers think about a valued dermatology partner. The CLS management team is well versed in the development and commercialization of dermatologic therapies. With product candidates in various stages of development that cover an array of skin conditions such as acne, rosacea, psoriasis, and warts caused by human papillomavirus, CLS is committed to focusing on underserved patient needs, which will help health care professionals optimize their practice time through CLS’s dermatologic products and services.
Sernivo Spray 0.05%
Promius Pharma, LLC, announces US Food and Drug Administration approval of Sernivo (betamethasone dipropionate) Spray 0.05%, a corticosteroid for the treatment of mild to moderate plaque psoriasis in patients 18 years and older. Sernivo Spray should be used twice daily for 4 weeks and not beyond. In clinical trials more participants showed treatment success with Sernivo Spray versus vehicle at day 15 and day 29. For more information, visit www.promiuspharma.com.
Teflaro
Allergan announces that the US Food and Drug Administration has accepted for filing a supplemental new drug application for Teflaro (ceftaroline fosamil) to expand the label to include the treatment of children 2 months and older with acute bacterial skin and skin structure infections (ABSSSI) including infections caused by methicillin-resistant Staphylococcus aureus and community-acquired bacterial pneumonia (CABP) caused by Staphylococcus pneumoniae and other designated susceptible bacteria. Teflaro is a bactericidal cephalosporin with activity against both gram-positive and gram-negative pathogens. Teflaro is already approved for ABSSSI and CABP in adult patients. For more information, visit www.teflaro.com.
If you would like your product included in Product News, please email a press release to the Editorial Office at [email protected].
Suture Anchor
DePuy Synthes Mitek Sports Medicine
(https://www.depuysynthes.com/hcp/mitek-sports-medicine)
Gryphon® Suture Anchor with Proknot™ Technology
Paul Favorito, MD, Wellington Orthopaedic and Sports Medicine, Cincinnati, OH
The Gryphon® suture anchor with Proknot™ technology is a doubled No. 1 Permacord® high-strength orthopedic suture with a proprietary pre-tied sliding knot. The suture construct is loaded onto a 3.0-mm Gryphonsuture anchor (Peek or Biocryl Rapide® biocomposite material) and has clinical indications for labral repair of the shoulder and hip. In a laboratory setting, Proknot technology has been tested against other high-tensile sutures and commonly tied arthroscopic knots.1 Proknot technology demonstrated higher ultimate strength, significantly less knot volume, and better reproducibility among surgeons.
Surgical pearl: I use the Gryphon Proknot suture anchor for all shoulder Bankart and superior labral anterior to posterior (SLAP) repairs. I have colleagues who also use this anchor for hip arthroscopy.
Once opened on the back table, the surgical assistant may ink the free limb of suture for easy arthroscopic identification. The anchor is placed and, in the case of hard bone frequently encountered in younger patients, a 2.5-mm drill bit may be substituted for the usual 2.4-mm. One important goal of any labral repair is to position knots away from the articular surface. The free suture limb is passed through the labrum, retrieved, and delivered through the open, pre-tied knot on the suture card.
Once the knot is released and dressed, the knot pusher is placed over the suture and the knot is advanced and preliminarily tensioned medial to the articular surface. The suture limbs are separated and one limb of the suture is removed from the knot pusher. As few as 1, or up to 3, half hitches may be placed to secure the knot, taking care to direct it away from the joint surface. The result is a strong but well-positioned knot with minimal mass securing the soft tissue.
1. Rodes SA, Favorito PJ, Piccirillo JM, Spivey JT. Performance comparison of a prettied suture knot with three conventional arthroscopic knots. Arthroscopy. 2015;31(11):2183-2190.
DePuy Synthes Mitek Sports Medicine
(https://www.depuysynthes.com/hcp/mitek-sports-medicine)
Gryphon® Suture Anchor with Proknot™ Technology
Paul Favorito, MD, Wellington Orthopaedic and Sports Medicine, Cincinnati, OH
The Gryphon® suture anchor with Proknot™ technology is a doubled No. 1 Permacord® high-strength orthopedic suture with a proprietary pre-tied sliding knot. The suture construct is loaded onto a 3.0-mm Gryphonsuture anchor (Peek or Biocryl Rapide® biocomposite material) and has clinical indications for labral repair of the shoulder and hip. In a laboratory setting, Proknot technology has been tested against other high-tensile sutures and commonly tied arthroscopic knots.1 Proknot technology demonstrated higher ultimate strength, significantly less knot volume, and better reproducibility among surgeons.
Surgical pearl: I use the Gryphon Proknot suture anchor for all shoulder Bankart and superior labral anterior to posterior (SLAP) repairs. I have colleagues who also use this anchor for hip arthroscopy.
Once opened on the back table, the surgical assistant may ink the free limb of suture for easy arthroscopic identification. The anchor is placed and, in the case of hard bone frequently encountered in younger patients, a 2.5-mm drill bit may be substituted for the usual 2.4-mm. One important goal of any labral repair is to position knots away from the articular surface. The free suture limb is passed through the labrum, retrieved, and delivered through the open, pre-tied knot on the suture card.
Once the knot is released and dressed, the knot pusher is placed over the suture and the knot is advanced and preliminarily tensioned medial to the articular surface. The suture limbs are separated and one limb of the suture is removed from the knot pusher. As few as 1, or up to 3, half hitches may be placed to secure the knot, taking care to direct it away from the joint surface. The result is a strong but well-positioned knot with minimal mass securing the soft tissue.
DePuy Synthes Mitek Sports Medicine
(https://www.depuysynthes.com/hcp/mitek-sports-medicine)
Gryphon® Suture Anchor with Proknot™ Technology
Paul Favorito, MD, Wellington Orthopaedic and Sports Medicine, Cincinnati, OH
The Gryphon® suture anchor with Proknot™ technology is a doubled No. 1 Permacord® high-strength orthopedic suture with a proprietary pre-tied sliding knot. The suture construct is loaded onto a 3.0-mm Gryphonsuture anchor (Peek or Biocryl Rapide® biocomposite material) and has clinical indications for labral repair of the shoulder and hip. In a laboratory setting, Proknot technology has been tested against other high-tensile sutures and commonly tied arthroscopic knots.1 Proknot technology demonstrated higher ultimate strength, significantly less knot volume, and better reproducibility among surgeons.
Surgical pearl: I use the Gryphon Proknot suture anchor for all shoulder Bankart and superior labral anterior to posterior (SLAP) repairs. I have colleagues who also use this anchor for hip arthroscopy.
Once opened on the back table, the surgical assistant may ink the free limb of suture for easy arthroscopic identification. The anchor is placed and, in the case of hard bone frequently encountered in younger patients, a 2.5-mm drill bit may be substituted for the usual 2.4-mm. One important goal of any labral repair is to position knots away from the articular surface. The free suture limb is passed through the labrum, retrieved, and delivered through the open, pre-tied knot on the suture card.
Once the knot is released and dressed, the knot pusher is placed over the suture and the knot is advanced and preliminarily tensioned medial to the articular surface. The suture limbs are separated and one limb of the suture is removed from the knot pusher. As few as 1, or up to 3, half hitches may be placed to secure the knot, taking care to direct it away from the joint surface. The result is a strong but well-positioned knot with minimal mass securing the soft tissue.
1. Rodes SA, Favorito PJ, Piccirillo JM, Spivey JT. Performance comparison of a prettied suture knot with three conventional arthroscopic knots. Arthroscopy. 2015;31(11):2183-2190.
1. Rodes SA, Favorito PJ, Piccirillo JM, Spivey JT. Performance comparison of a prettied suture knot with three conventional arthroscopic knots. Arthroscopy. 2015;31(11):2183-2190.
Product Update: Banyan Medical's Vaginal/Rectal Probes, Doctablet, Pre-Seed Lubricant, SonoSure
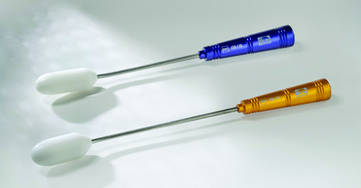
VAGINAL/RECTAL PROBES FOR LAPAROSCOPYBanyan Medical’s Vaginal/Rectal Probes are low-cost reusable instruments used to enhance accurate anatomical visualization during gynecologic surgery. They are designed to be easily positioned to maximize anatomical visualization during excision of lesions from the posterior vagina, rectum, and cul-de-sac. According to the manufacturer, use of one of their probes ensures maximum accuracy; assists in opening the rectovaginal pouch and mapping of the cul-de-sac; can be used to help identify open vessels and other injuries; and acts as a mobilizer when dissecting, cauterizing, and suturing.
Banyan Medical assures that its vaginal/rectal probes are easy to sterilize and ergonomic. Probes have color-coded handles, surgical-grade stainless steel shafts, and atraumatic tips made of high-grade polymer.
FOR MORE INFORMATION, VISIT: http://banyanmedllc.com

ONLINE EDUCATIONAL VIDEO RESOURCEDoctablet is an online educational video resource that offers animated narratives in English and Spanish that describe medical conditions to patients. Jose M. Taveras, MD, a cardiologist at Montefiore Center for Heart and Vascular Care in New York and Christopher R. Palmeiro, MD, chairman of endocrinology at HealthAlliance of the Hudson Valley in Kingston, New York, have developed this free educational platform, which they describe as easily accessible, comprehensive, and entertaining.
Drs. Taveras and Palmeiro note that they created Doctablet to educate people about crucial health issues. They cite the pressures of shrinking budgets and the fact that physicians have less time for individualized care and education and that all too often a patient leaves her physician’s office without clear and direct crucial information.
“I’ve found myself telling little simple stories to my patients to illustrate various heart conditions. They’ve really helped—I’ve seen people quit smoking and seen people become proactive in taking their medication,” says Dr. Taveras. “The way to seriously impact patients, and reduce the need for procedures, is to change the way people live. You do that through taking the time to educate them in a way that makes sense to them.”
The patient-oriented videos are “jargon-free,” approximately 3 minutes in length, and cover health topics such as prediabetes and diabetes, nutrition, and cardiac care and heart attacks.
FOR MORE INFORMATION, VISIT: www.doctablet.com
FERTILITY-FRIENDLY LUBRICANTPre-Seed® Fertility-Friendly Lubricant is specially formulated for couples trying to conceive. Pre-Seed was invented by a sperm physiologist who discovered that many couples were using lubricants that killed sperm. The National Institutes of Health funded research to develop Pre-Seed’s patented sperm-safe lubricant formula. In a January 2014 clinical study published in The Journal of Assisted Reproduction and Genetics, Mowat and colleagues analyzed the effects of 9 lubricants on sperm motility, vitality, and DNA fragmentation. The study authors named Pre-Seed as the leading lubricant of choice for couples who are trying to get pregnant.
Pre-Seed’s design mimics fertile cervical mucus in its pH, ion concentration, and consistency, says its inventor. In addition, Pre-Seed is glycerin-free to allow sperm to swim freely and formulated with antioxidants to help support sperm on their journey to fertilize the egg. Pre-Seed is available nationally at major drug and discount stores, and is offered by select regional and Internet retailers.
FOR MORE INFORMATION, VISIT: www.preseed.com
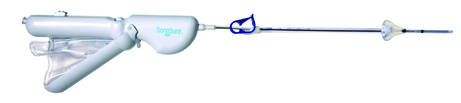
SONOHYSTEROGRAPHY DEVICECrossBay Medical Inc.™ offers SonoSure™, a single-use, disposable device to access the uterine cavity for saline infusion sonohysterography (SIS) and endometrial sampling. The product is designed to assist in the evaluation of a patient with abnormal uterine bleeding (AUB) or heavy menstrual periods.
CrossBay says that SonoSure is meant for single-handed use during concurrent ultrasonography. The device features a built-in deployed endometrial sampling brush, a low profile malleable insertion catheter, a repositionable cervical sealing mechanism for uterine distension designed to not obscure the lower uterine segment, and a 50-cc refillable fluid injection bag.
During SIS, the shapeable catheter with soft acorn tip can be used with anteversion or retroversion placement and provides uterine distention upon infusion and release for draining without the use of forceps. The echogenic tip and brush allow for visualization during insertion and placement. The blunt tip is designed to avoid uterine perforation.
FOR MORE INFORMATION, VISIT: http://crossbaymedicalinc.com/products/crossbay-women/sonosure

VAGINAL/RECTAL PROBES FOR LAPAROSCOPYBanyan Medical’s Vaginal/Rectal Probes are low-cost reusable instruments used to enhance accurate anatomical visualization during gynecologic surgery. They are designed to be easily positioned to maximize anatomical visualization during excision of lesions from the posterior vagina, rectum, and cul-de-sac. According to the manufacturer, use of one of their probes ensures maximum accuracy; assists in opening the rectovaginal pouch and mapping of the cul-de-sac; can be used to help identify open vessels and other injuries; and acts as a mobilizer when dissecting, cauterizing, and suturing.
Banyan Medical assures that its vaginal/rectal probes are easy to sterilize and ergonomic. Probes have color-coded handles, surgical-grade stainless steel shafts, and atraumatic tips made of high-grade polymer.
FOR MORE INFORMATION, VISIT: http://banyanmedllc.com

ONLINE EDUCATIONAL VIDEO RESOURCEDoctablet is an online educational video resource that offers animated narratives in English and Spanish that describe medical conditions to patients. Jose M. Taveras, MD, a cardiologist at Montefiore Center for Heart and Vascular Care in New York and Christopher R. Palmeiro, MD, chairman of endocrinology at HealthAlliance of the Hudson Valley in Kingston, New York, have developed this free educational platform, which they describe as easily accessible, comprehensive, and entertaining.
Drs. Taveras and Palmeiro note that they created Doctablet to educate people about crucial health issues. They cite the pressures of shrinking budgets and the fact that physicians have less time for individualized care and education and that all too often a patient leaves her physician’s office without clear and direct crucial information.
“I’ve found myself telling little simple stories to my patients to illustrate various heart conditions. They’ve really helped—I’ve seen people quit smoking and seen people become proactive in taking their medication,” says Dr. Taveras. “The way to seriously impact patients, and reduce the need for procedures, is to change the way people live. You do that through taking the time to educate them in a way that makes sense to them.”
The patient-oriented videos are “jargon-free,” approximately 3 minutes in length, and cover health topics such as prediabetes and diabetes, nutrition, and cardiac care and heart attacks.
FOR MORE INFORMATION, VISIT: www.doctablet.com
FERTILITY-FRIENDLY LUBRICANTPre-Seed® Fertility-Friendly Lubricant is specially formulated for couples trying to conceive. Pre-Seed was invented by a sperm physiologist who discovered that many couples were using lubricants that killed sperm. The National Institutes of Health funded research to develop Pre-Seed’s patented sperm-safe lubricant formula. In a January 2014 clinical study published in The Journal of Assisted Reproduction and Genetics, Mowat and colleagues analyzed the effects of 9 lubricants on sperm motility, vitality, and DNA fragmentation. The study authors named Pre-Seed as the leading lubricant of choice for couples who are trying to get pregnant.
Pre-Seed’s design mimics fertile cervical mucus in its pH, ion concentration, and consistency, says its inventor. In addition, Pre-Seed is glycerin-free to allow sperm to swim freely and formulated with antioxidants to help support sperm on their journey to fertilize the egg. Pre-Seed is available nationally at major drug and discount stores, and is offered by select regional and Internet retailers.
FOR MORE INFORMATION, VISIT: www.preseed.com

SONOHYSTEROGRAPHY DEVICECrossBay Medical Inc.™ offers SonoSure™, a single-use, disposable device to access the uterine cavity for saline infusion sonohysterography (SIS) and endometrial sampling. The product is designed to assist in the evaluation of a patient with abnormal uterine bleeding (AUB) or heavy menstrual periods.
CrossBay says that SonoSure is meant for single-handed use during concurrent ultrasonography. The device features a built-in deployed endometrial sampling brush, a low profile malleable insertion catheter, a repositionable cervical sealing mechanism for uterine distension designed to not obscure the lower uterine segment, and a 50-cc refillable fluid injection bag.
During SIS, the shapeable catheter with soft acorn tip can be used with anteversion or retroversion placement and provides uterine distention upon infusion and release for draining without the use of forceps. The echogenic tip and brush allow for visualization during insertion and placement. The blunt tip is designed to avoid uterine perforation.
FOR MORE INFORMATION, VISIT: http://crossbaymedicalinc.com/products/crossbay-women/sonosure

VAGINAL/RECTAL PROBES FOR LAPAROSCOPYBanyan Medical’s Vaginal/Rectal Probes are low-cost reusable instruments used to enhance accurate anatomical visualization during gynecologic surgery. They are designed to be easily positioned to maximize anatomical visualization during excision of lesions from the posterior vagina, rectum, and cul-de-sac. According to the manufacturer, use of one of their probes ensures maximum accuracy; assists in opening the rectovaginal pouch and mapping of the cul-de-sac; can be used to help identify open vessels and other injuries; and acts as a mobilizer when dissecting, cauterizing, and suturing.
Banyan Medical assures that its vaginal/rectal probes are easy to sterilize and ergonomic. Probes have color-coded handles, surgical-grade stainless steel shafts, and atraumatic tips made of high-grade polymer.
FOR MORE INFORMATION, VISIT: http://banyanmedllc.com

ONLINE EDUCATIONAL VIDEO RESOURCEDoctablet is an online educational video resource that offers animated narratives in English and Spanish that describe medical conditions to patients. Jose M. Taveras, MD, a cardiologist at Montefiore Center for Heart and Vascular Care in New York and Christopher R. Palmeiro, MD, chairman of endocrinology at HealthAlliance of the Hudson Valley in Kingston, New York, have developed this free educational platform, which they describe as easily accessible, comprehensive, and entertaining.
Drs. Taveras and Palmeiro note that they created Doctablet to educate people about crucial health issues. They cite the pressures of shrinking budgets and the fact that physicians have less time for individualized care and education and that all too often a patient leaves her physician’s office without clear and direct crucial information.
“I’ve found myself telling little simple stories to my patients to illustrate various heart conditions. They’ve really helped—I’ve seen people quit smoking and seen people become proactive in taking their medication,” says Dr. Taveras. “The way to seriously impact patients, and reduce the need for procedures, is to change the way people live. You do that through taking the time to educate them in a way that makes sense to them.”
The patient-oriented videos are “jargon-free,” approximately 3 minutes in length, and cover health topics such as prediabetes and diabetes, nutrition, and cardiac care and heart attacks.
FOR MORE INFORMATION, VISIT: www.doctablet.com
FERTILITY-FRIENDLY LUBRICANTPre-Seed® Fertility-Friendly Lubricant is specially formulated for couples trying to conceive. Pre-Seed was invented by a sperm physiologist who discovered that many couples were using lubricants that killed sperm. The National Institutes of Health funded research to develop Pre-Seed’s patented sperm-safe lubricant formula. In a January 2014 clinical study published in The Journal of Assisted Reproduction and Genetics, Mowat and colleagues analyzed the effects of 9 lubricants on sperm motility, vitality, and DNA fragmentation. The study authors named Pre-Seed as the leading lubricant of choice for couples who are trying to get pregnant.
Pre-Seed’s design mimics fertile cervical mucus in its pH, ion concentration, and consistency, says its inventor. In addition, Pre-Seed is glycerin-free to allow sperm to swim freely and formulated with antioxidants to help support sperm on their journey to fertilize the egg. Pre-Seed is available nationally at major drug and discount stores, and is offered by select regional and Internet retailers.
FOR MORE INFORMATION, VISIT: www.preseed.com

SONOHYSTEROGRAPHY DEVICECrossBay Medical Inc.™ offers SonoSure™, a single-use, disposable device to access the uterine cavity for saline infusion sonohysterography (SIS) and endometrial sampling. The product is designed to assist in the evaluation of a patient with abnormal uterine bleeding (AUB) or heavy menstrual periods.
CrossBay says that SonoSure is meant for single-handed use during concurrent ultrasonography. The device features a built-in deployed endometrial sampling brush, a low profile malleable insertion catheter, a repositionable cervical sealing mechanism for uterine distension designed to not obscure the lower uterine segment, and a 50-cc refillable fluid injection bag.
During SIS, the shapeable catheter with soft acorn tip can be used with anteversion or retroversion placement and provides uterine distention upon infusion and release for draining without the use of forceps. The echogenic tip and brush allow for visualization during insertion and placement. The blunt tip is designed to avoid uterine perforation.
FOR MORE INFORMATION, VISIT: http://crossbaymedicalinc.com/products/crossbay-women/sonosure
Cryo-Compression Therapy
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.
CoolSystems, Inc. (www.gameready.com)
The Game Ready Injury Treatment System
Peter Millett, MD, The Steadman Clinic, Vail, CO; Consultant, Major League Baseball Players’ Association
At the Steadman Clinic, we have developed best-practice techniques and protocols to accelerate our patients’ recoveries. Game Ready helps my patients recover faster. The Game Ready device has the most advanced level of rehab technology with the cost-effective cryotherapy delivery system, intermittent compression, and ergonomically designed wraps tailored for specific areas of the body. It just works better than ice alone or other cryotherapy devices. Game Ready reduces swelling and gets patients back faster.
I prescribe Game Ready after surgical procedures because it decreases pain, reduces the need for pain medication, and results in a faster recovery. For my overhead athletes, I routinely use the shoulder and elbow wraps for labral tears, shoulder instability, biceps tendon disorders, and rotator cuff problems.
J.W. Thomas Byrd, MD, Nashville Sports Medicine and Orthopaedics, Orthopaedic Surgical Consultant, various Major League Baseball Clubs
Performing hip arthroscopy procedures for Major League Baseball pitchers over the last 3 decades, I have come to realize the importance of choosing the most effective recovery therapy device. We have trialed numerous products and found the Game Ready cold-intermittent-compression device to be an incredible asset in the recovery and pain management strategy.
During the rehab process, pain control is essential to the athlete’s ability to participate and achieve optimal recovery. Hip procedures can be painful because they usually revolve around restoring the acetabular labrum, which is richly innervated with nociceptive fibers. In order to control discomfort following surgery, regional anesthetic nerve blocks are sometimes necessary. However, these blocks can hinder an athlete’s ability to participate in, and benefit from, the early postoperative rehabilitation process. Applying the Game Ready led to a noticeable drop in postoperative pain, obviating the need for a block.
Kenneth Akizuki, MD, SOAR, San Francisco, CA, Team Physician, San Francisco Giants
Among pro players, Tommy John surgery is a common procedure. The day after surgery, we start the player on the Game Ready system to relieve pain and quickly control swelling. We typically start with cold therapy, then add compression about a week in, and use it throughout recovery.
The players love the comfort of the ergonomic wrap designs and I really like the flexed elbow wrap. The cold is adjustable so we don’t get overcooling, and the wrap design keeps the surgery site dry, which cuts the risk of infection. The pre-set treatment programs are another big advantage. They take the hassle out of application. Whether a professional athlete or not, all our patients want convenience, and we want to see progress. Progress is motivating, it encourages compliance—and that improves outcomes.