User login
Product News: 07 2015
Alevicyn SG Antipruritic Spray Gel
IntraDerm Pharmaceuticals, a division of Oculus Innovative Sciences, Inc, receives 510(k) clearance from the US Food and Drug Administration for Alevicyn SG Antipruritic Spray Gel with both prescription and over-the-counter indications. The Alevicyn SG prescription product manages and relieves the burning, itching, and pain experienced with dermatoses such as radiation dermatitis and atopic dermatitis. It also relieves the pain of first- and second-degree burns and helps to relieve dry waxy skin by maintaining a moist wound environment, which is beneficial to the healing process. The over-the-counter product relieves the burning and itching associated with many common types of skin irritation, lacerations, abrasions, and minor burns including sunburn. For more information, visit www.intraderm.com.
Promius Promise
Promius Pharma LLC announces that the Promius Promise program has been expanded to include Cloderm (clocortolone pivalate) Cream 0.1% and Trianex (triamcinolone acetonide) Ointment 0.05%, both for relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. The Promius Promise program was created in 2013 to support patients. This program features a dedicated call center staff to educate patients about their insurance coverage and answer any questions they may have about their out-of-pocket cost, co-pay assistance, and prior authorizations. For more information, visit www.promiuspharma.com.
Radiesse
Merz North America, Inc, receives US Food and Drug Administration approval of Radiesse for correction of volume loss in the dorsum of the hands. Radiesse is an opaque dermal filler composed of calcium hydroxylapatite microspheres suspended in a water-based gel carrier. It provides an immediate volumizing effect and helps reduce the prominence of tendons and veins in the hands, delivering natural-looking results that can last up to 1 year. Radiesse also is indicated for subdermal implantation for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. For more information, visit www.MerzUSA.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Alevicyn SG Antipruritic Spray Gel
IntraDerm Pharmaceuticals, a division of Oculus Innovative Sciences, Inc, receives 510(k) clearance from the US Food and Drug Administration for Alevicyn SG Antipruritic Spray Gel with both prescription and over-the-counter indications. The Alevicyn SG prescription product manages and relieves the burning, itching, and pain experienced with dermatoses such as radiation dermatitis and atopic dermatitis. It also relieves the pain of first- and second-degree burns and helps to relieve dry waxy skin by maintaining a moist wound environment, which is beneficial to the healing process. The over-the-counter product relieves the burning and itching associated with many common types of skin irritation, lacerations, abrasions, and minor burns including sunburn. For more information, visit www.intraderm.com.
Promius Promise
Promius Pharma LLC announces that the Promius Promise program has been expanded to include Cloderm (clocortolone pivalate) Cream 0.1% and Trianex (triamcinolone acetonide) Ointment 0.05%, both for relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. The Promius Promise program was created in 2013 to support patients. This program features a dedicated call center staff to educate patients about their insurance coverage and answer any questions they may have about their out-of-pocket cost, co-pay assistance, and prior authorizations. For more information, visit www.promiuspharma.com.
Radiesse
Merz North America, Inc, receives US Food and Drug Administration approval of Radiesse for correction of volume loss in the dorsum of the hands. Radiesse is an opaque dermal filler composed of calcium hydroxylapatite microspheres suspended in a water-based gel carrier. It provides an immediate volumizing effect and helps reduce the prominence of tendons and veins in the hands, delivering natural-looking results that can last up to 1 year. Radiesse also is indicated for subdermal implantation for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. For more information, visit www.MerzUSA.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Alevicyn SG Antipruritic Spray Gel
IntraDerm Pharmaceuticals, a division of Oculus Innovative Sciences, Inc, receives 510(k) clearance from the US Food and Drug Administration for Alevicyn SG Antipruritic Spray Gel with both prescription and over-the-counter indications. The Alevicyn SG prescription product manages and relieves the burning, itching, and pain experienced with dermatoses such as radiation dermatitis and atopic dermatitis. It also relieves the pain of first- and second-degree burns and helps to relieve dry waxy skin by maintaining a moist wound environment, which is beneficial to the healing process. The over-the-counter product relieves the burning and itching associated with many common types of skin irritation, lacerations, abrasions, and minor burns including sunburn. For more information, visit www.intraderm.com.
Promius Promise
Promius Pharma LLC announces that the Promius Promise program has been expanded to include Cloderm (clocortolone pivalate) Cream 0.1% and Trianex (triamcinolone acetonide) Ointment 0.05%, both for relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses. The Promius Promise program was created in 2013 to support patients. This program features a dedicated call center staff to educate patients about their insurance coverage and answer any questions they may have about their out-of-pocket cost, co-pay assistance, and prior authorizations. For more information, visit www.promiuspharma.com.
Radiesse
Merz North America, Inc, receives US Food and Drug Administration approval of Radiesse for correction of volume loss in the dorsum of the hands. Radiesse is an opaque dermal filler composed of calcium hydroxylapatite microspheres suspended in a water-based gel carrier. It provides an immediate volumizing effect and helps reduce the prominence of tendons and veins in the hands, delivering natural-looking results that can last up to 1 year. Radiesse also is indicated for subdermal implantation for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. For more information, visit www.MerzUSA.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Workshops on Heart Disease and Comorbid Conditions
Older patients with heart disease usually have multiple other health problems—such as multimorbidity, polypharmacy, cognitive impairment, and frailty—but a new website provides the latest information on how to care for them.
Related: Heart Failure and Cognitive Impairment: Breaking It Down
The website contains presentations from workshops cosponsored by the American College of Cardiology, the American Geriatrics Association, and the National Institute on Aging. The first workshop, which was held in February, identified unmet needs, formulated a research agenda, and discussed strategies for translating new research into clinical practice. Topics on the agenda included how to help improve patient-centered care and outcomes for this population. The presenters aimed to answer questions such as “Why do multiple coexisting conditions [MCC] increase with age?” and “Can the risk of MCCs be detected before MCCs appear?”
Related: Factors Affecting Heart Failure Readmission Rates in VA Patients
For more information, visit www.accagsniamultimorbidityworkshop.com.
Older patients with heart disease usually have multiple other health problems—such as multimorbidity, polypharmacy, cognitive impairment, and frailty—but a new website provides the latest information on how to care for them.
Related: Heart Failure and Cognitive Impairment: Breaking It Down
The website contains presentations from workshops cosponsored by the American College of Cardiology, the American Geriatrics Association, and the National Institute on Aging. The first workshop, which was held in February, identified unmet needs, formulated a research agenda, and discussed strategies for translating new research into clinical practice. Topics on the agenda included how to help improve patient-centered care and outcomes for this population. The presenters aimed to answer questions such as “Why do multiple coexisting conditions [MCC] increase with age?” and “Can the risk of MCCs be detected before MCCs appear?”
Related: Factors Affecting Heart Failure Readmission Rates in VA Patients
For more information, visit www.accagsniamultimorbidityworkshop.com.
Older patients with heart disease usually have multiple other health problems—such as multimorbidity, polypharmacy, cognitive impairment, and frailty—but a new website provides the latest information on how to care for them.
Related: Heart Failure and Cognitive Impairment: Breaking It Down
The website contains presentations from workshops cosponsored by the American College of Cardiology, the American Geriatrics Association, and the National Institute on Aging. The first workshop, which was held in February, identified unmet needs, formulated a research agenda, and discussed strategies for translating new research into clinical practice. Topics on the agenda included how to help improve patient-centered care and outcomes for this population. The presenters aimed to answer questions such as “Why do multiple coexisting conditions [MCC] increase with age?” and “Can the risk of MCCs be detected before MCCs appear?”
Related: Factors Affecting Heart Failure Readmission Rates in VA Patients
For more information, visit www.accagsniamultimorbidityworkshop.com.
Product Update: LILETTA, myHDL, KleenSpec, STEPS Forward
NEW, SMALL IUD NOW AVAILABLE
LILETTA™ (levonorgestrel-releasing intrauterine system) 52 mg is now available for use by women to prevent pregnancy for up to 3 years. LILETTA is a small, flexible plastic T-shaped system 32 mm x 32 mm in size. It works to prevent pregnancy by slowly releasing levonorgestrel (LNG), a progestin, at an initial release rate of 18.6 µg/day with an average in vivo release rate of LNG of approximately 15.6 µg/day over a period of 3 years. Generally, LILETTA can be inserted at any time if the provider is reasonably certain that the woman is not pregnant. While LILETTA is intended for use up to 3 years, according to a press release from Actavis, it can be removed by a clinician at any time and can be replaced at the time of removal with a new LILETTA if continued contraceptive protection is desired.
The February 2015 FDA approval of LILETTA was based on the largest hormonal IUD trial conducted in the United States, designed to reflect the US population, says the manufacturer. The IUD was studied in women aged 16 to 45 years who were nulliparous or parous with a BMI of 15.8 kg/m2 to 61.6 kg/m2.
Through a partnership between Actavis and Medicines360 described at www.liletta.com, IUD-appropriate women, regardless of income and insurance coverage, now have access to this IUD at their doctor’s offices and at public health clinics enrolled in the 340B Drug Pricing Program.
FOR MORE INFORMATION, VISIT www.liletta.com
MYHDL APP ON APPLE WATCH, iPAD, iPHONE
The myHDL Physician App from Health Diagnostic Laboratory, Inc. (HDL) is newly available on the Apple Watch, iPhone, and iPad. The app is designed to allow users to view and manage patient cases from their wrist on all models of Apple Watch. HDL says that the myHDL app helps clinicians offer effective, personalized care to their patients, and features include the ability to manage multiple patients’ results, ease of use through color coding and categorized test results, and security and privacy. HDL provides comprehensive biomarker testing and clinical health consulting for earlier disease detection and targeted disease management. The myHDL app is available on iTunes App Store and can be downloaded at www.myhdlapp.com.
FOR MORE INFORMATION, VISIT www.hdlabinc.com
SINGLE-USE LED SPECULA IN 3 SIZES
Welch Allyn, Inc. recently launched the KleenSpec® Single Use LED Vaginal Specula intended for use in hospital emergency departments, labor and delivery units, urgent care centers, ambulatory care surgery centers, clinics, and other women’s health treatment centers.
Welch Allyn says that the KleenSpec Single Use LED Vaginal Specula are 100% acrylic and designed with smooth, molded edges to deliver maximum patient comfort. Wide handles are meant to provide comfortable ergonomics, ease of use, and good balance. According to Welch Allyn, the cordless device’s LED light source provides enhanced visualization of the examination area by supplying uniform white light for more than 30 minutes. The sealed LED light source and Lithium-primary battery are placed in the handle to reduce patient risk. The LED and battery can be removed for disposal or recycling. The device is ready to use out of the package and has a 5-year shelf life. The KleenSpec Single Use LED Vaginal Specula are available in extra small, small, and medium sizes in a distinctive color scheme to help clinicians identify various sizes.
FOR MORE INFORMATION, VISIT www.welchallyn.com
AMA’S PRACTICE TRANSFORMATION SERIES
AMA STEPS Forward™ is an online, interactive practice transformation series offering innovative strategies to help physicians and their staff refocus their practice. The American Medical Association (AMA) developed this initiative after a recent AMA-RAND report found that the satisfaction physicians derive from their work is eroding as they spend more and more time on administrative tasks. The AMA’s goal by offering STEPS Forward is to help clinicians achieve the “Quadruple Aim” to provide better patient experiences, better population health, lower overall costs, and improved professional satisfaction.
Physicians can access a collection of interactive educational modules to help deal with common practice challenges and also earn CME credit. Currently, 16 modules include steps for implementation, case studies, and downloadable videos, tools, and resources that address: practice efficiency and patient care, patient health, physician health, and technology and innovation. Additional modules are planned.
FOR MORE INFORMATION, VISIT www.stepsforward.org
NEW, SMALL IUD NOW AVAILABLE
LILETTA™ (levonorgestrel-releasing intrauterine system) 52 mg is now available for use by women to prevent pregnancy for up to 3 years. LILETTA is a small, flexible plastic T-shaped system 32 mm x 32 mm in size. It works to prevent pregnancy by slowly releasing levonorgestrel (LNG), a progestin, at an initial release rate of 18.6 µg/day with an average in vivo release rate of LNG of approximately 15.6 µg/day over a period of 3 years. Generally, LILETTA can be inserted at any time if the provider is reasonably certain that the woman is not pregnant. While LILETTA is intended for use up to 3 years, according to a press release from Actavis, it can be removed by a clinician at any time and can be replaced at the time of removal with a new LILETTA if continued contraceptive protection is desired.
The February 2015 FDA approval of LILETTA was based on the largest hormonal IUD trial conducted in the United States, designed to reflect the US population, says the manufacturer. The IUD was studied in women aged 16 to 45 years who were nulliparous or parous with a BMI of 15.8 kg/m2 to 61.6 kg/m2.
Through a partnership between Actavis and Medicines360 described at www.liletta.com, IUD-appropriate women, regardless of income and insurance coverage, now have access to this IUD at their doctor’s offices and at public health clinics enrolled in the 340B Drug Pricing Program.
FOR MORE INFORMATION, VISIT www.liletta.com
MYHDL APP ON APPLE WATCH, iPAD, iPHONE
The myHDL Physician App from Health Diagnostic Laboratory, Inc. (HDL) is newly available on the Apple Watch, iPhone, and iPad. The app is designed to allow users to view and manage patient cases from their wrist on all models of Apple Watch. HDL says that the myHDL app helps clinicians offer effective, personalized care to their patients, and features include the ability to manage multiple patients’ results, ease of use through color coding and categorized test results, and security and privacy. HDL provides comprehensive biomarker testing and clinical health consulting for earlier disease detection and targeted disease management. The myHDL app is available on iTunes App Store and can be downloaded at www.myhdlapp.com.
FOR MORE INFORMATION, VISIT www.hdlabinc.com
SINGLE-USE LED SPECULA IN 3 SIZES
Welch Allyn, Inc. recently launched the KleenSpec® Single Use LED Vaginal Specula intended for use in hospital emergency departments, labor and delivery units, urgent care centers, ambulatory care surgery centers, clinics, and other women’s health treatment centers.
Welch Allyn says that the KleenSpec Single Use LED Vaginal Specula are 100% acrylic and designed with smooth, molded edges to deliver maximum patient comfort. Wide handles are meant to provide comfortable ergonomics, ease of use, and good balance. According to Welch Allyn, the cordless device’s LED light source provides enhanced visualization of the examination area by supplying uniform white light for more than 30 minutes. The sealed LED light source and Lithium-primary battery are placed in the handle to reduce patient risk. The LED and battery can be removed for disposal or recycling. The device is ready to use out of the package and has a 5-year shelf life. The KleenSpec Single Use LED Vaginal Specula are available in extra small, small, and medium sizes in a distinctive color scheme to help clinicians identify various sizes.
FOR MORE INFORMATION, VISIT www.welchallyn.com
AMA’S PRACTICE TRANSFORMATION SERIES
AMA STEPS Forward™ is an online, interactive practice transformation series offering innovative strategies to help physicians and their staff refocus their practice. The American Medical Association (AMA) developed this initiative after a recent AMA-RAND report found that the satisfaction physicians derive from their work is eroding as they spend more and more time on administrative tasks. The AMA’s goal by offering STEPS Forward is to help clinicians achieve the “Quadruple Aim” to provide better patient experiences, better population health, lower overall costs, and improved professional satisfaction.
Physicians can access a collection of interactive educational modules to help deal with common practice challenges and also earn CME credit. Currently, 16 modules include steps for implementation, case studies, and downloadable videos, tools, and resources that address: practice efficiency and patient care, patient health, physician health, and technology and innovation. Additional modules are planned.
FOR MORE INFORMATION, VISIT www.stepsforward.org
NEW, SMALL IUD NOW AVAILABLE
LILETTA™ (levonorgestrel-releasing intrauterine system) 52 mg is now available for use by women to prevent pregnancy for up to 3 years. LILETTA is a small, flexible plastic T-shaped system 32 mm x 32 mm in size. It works to prevent pregnancy by slowly releasing levonorgestrel (LNG), a progestin, at an initial release rate of 18.6 µg/day with an average in vivo release rate of LNG of approximately 15.6 µg/day over a period of 3 years. Generally, LILETTA can be inserted at any time if the provider is reasonably certain that the woman is not pregnant. While LILETTA is intended for use up to 3 years, according to a press release from Actavis, it can be removed by a clinician at any time and can be replaced at the time of removal with a new LILETTA if continued contraceptive protection is desired.
The February 2015 FDA approval of LILETTA was based on the largest hormonal IUD trial conducted in the United States, designed to reflect the US population, says the manufacturer. The IUD was studied in women aged 16 to 45 years who were nulliparous or parous with a BMI of 15.8 kg/m2 to 61.6 kg/m2.
Through a partnership between Actavis and Medicines360 described at www.liletta.com, IUD-appropriate women, regardless of income and insurance coverage, now have access to this IUD at their doctor’s offices and at public health clinics enrolled in the 340B Drug Pricing Program.
FOR MORE INFORMATION, VISIT www.liletta.com
MYHDL APP ON APPLE WATCH, iPAD, iPHONE
The myHDL Physician App from Health Diagnostic Laboratory, Inc. (HDL) is newly available on the Apple Watch, iPhone, and iPad. The app is designed to allow users to view and manage patient cases from their wrist on all models of Apple Watch. HDL says that the myHDL app helps clinicians offer effective, personalized care to their patients, and features include the ability to manage multiple patients’ results, ease of use through color coding and categorized test results, and security and privacy. HDL provides comprehensive biomarker testing and clinical health consulting for earlier disease detection and targeted disease management. The myHDL app is available on iTunes App Store and can be downloaded at www.myhdlapp.com.
FOR MORE INFORMATION, VISIT www.hdlabinc.com
SINGLE-USE LED SPECULA IN 3 SIZES
Welch Allyn, Inc. recently launched the KleenSpec® Single Use LED Vaginal Specula intended for use in hospital emergency departments, labor and delivery units, urgent care centers, ambulatory care surgery centers, clinics, and other women’s health treatment centers.
Welch Allyn says that the KleenSpec Single Use LED Vaginal Specula are 100% acrylic and designed with smooth, molded edges to deliver maximum patient comfort. Wide handles are meant to provide comfortable ergonomics, ease of use, and good balance. According to Welch Allyn, the cordless device’s LED light source provides enhanced visualization of the examination area by supplying uniform white light for more than 30 minutes. The sealed LED light source and Lithium-primary battery are placed in the handle to reduce patient risk. The LED and battery can be removed for disposal or recycling. The device is ready to use out of the package and has a 5-year shelf life. The KleenSpec Single Use LED Vaginal Specula are available in extra small, small, and medium sizes in a distinctive color scheme to help clinicians identify various sizes.
FOR MORE INFORMATION, VISIT www.welchallyn.com
AMA’S PRACTICE TRANSFORMATION SERIES
AMA STEPS Forward™ is an online, interactive practice transformation series offering innovative strategies to help physicians and their staff refocus their practice. The American Medical Association (AMA) developed this initiative after a recent AMA-RAND report found that the satisfaction physicians derive from their work is eroding as they spend more and more time on administrative tasks. The AMA’s goal by offering STEPS Forward is to help clinicians achieve the “Quadruple Aim” to provide better patient experiences, better population health, lower overall costs, and improved professional satisfaction.
Physicians can access a collection of interactive educational modules to help deal with common practice challenges and also earn CME credit. Currently, 16 modules include steps for implementation, case studies, and downloadable videos, tools, and resources that address: practice efficiency and patient care, patient health, physician health, and technology and innovation. Additional modules are planned.
FOR MORE INFORMATION, VISIT www.stepsforward.org
Product News: 06 2015
Intensive Eye Treatment
Niadyne, Incorporated, introduces the NIA24 Intensive Eye Treatment, a dual-phase, predosed, multiaction eye mask to reduce the look of fine lines, wrinkles, and dark circles. The treatment begins with the aesthetician combining the predosed treatment base with the activating powder just prior to use. Once combined, the activated components are applied around the eye area. The Intensive Eye Treatment contains Pro-Niacin to help stimulate skin barrier repair, improve cell turnover, and energize skin. Haloxyl tripeptide complex decreases the red and blue color of dark circles under the eyes and acts as an anti-inflammatory agent. Lumispheres skin brightener diminishes the appearance of fine lines. Other ingredients firm and tighten skin, while reducing undereye bags and puffiness. The Intensive Eye Treatment is a professional treatment that is available exclusively through physicians. For more information, visit www.nia24.com/professional-treatments/.
Kybella Injection
Kythera Biopharmaceuticals announces US Food and Drug Administration approval of Kybella (deoxycholic acid), also known as ATX-101, a nonsurgical treatment indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. Kybella is administered by injections into the fat under the chin, which causes destruction of fat cells. Once destroyed, those cells cannot store or accumulate fat. Each in-office treatment session is typically 15 to 20 minutes, and up to 6 treatments may be administered. Many patients experience visible results in 2 to 4 treatments. Once the aesthetic response is achieved, re-treatment is not expected. Kybella physician training programs will be initiated in late summer. For more information, visit www.mykybella.com.
Sun Shield Broad Spectrum Matte Sunscreen Lotion
Obagi Medical Products, Inc, a division of Valeant Pharmaceuticals North America LLC, introduces the Sun Shield Broad Spectrum Matte SPF 50 Sunscreen Lotion. It combines UVB absorption and UVA protection with a completely sheer application. It contains 10.5% zinc oxide and 7.5% octinoxate. The Sun Shield Broad Spectrum Matte Sunscreen Lotion is ideal for use on all skin types and is physician dispensed. For more information, visit www.obagi.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Intensive Eye Treatment
Niadyne, Incorporated, introduces the NIA24 Intensive Eye Treatment, a dual-phase, predosed, multiaction eye mask to reduce the look of fine lines, wrinkles, and dark circles. The treatment begins with the aesthetician combining the predosed treatment base with the activating powder just prior to use. Once combined, the activated components are applied around the eye area. The Intensive Eye Treatment contains Pro-Niacin to help stimulate skin barrier repair, improve cell turnover, and energize skin. Haloxyl tripeptide complex decreases the red and blue color of dark circles under the eyes and acts as an anti-inflammatory agent. Lumispheres skin brightener diminishes the appearance of fine lines. Other ingredients firm and tighten skin, while reducing undereye bags and puffiness. The Intensive Eye Treatment is a professional treatment that is available exclusively through physicians. For more information, visit www.nia24.com/professional-treatments/.
Kybella Injection
Kythera Biopharmaceuticals announces US Food and Drug Administration approval of Kybella (deoxycholic acid), also known as ATX-101, a nonsurgical treatment indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. Kybella is administered by injections into the fat under the chin, which causes destruction of fat cells. Once destroyed, those cells cannot store or accumulate fat. Each in-office treatment session is typically 15 to 20 minutes, and up to 6 treatments may be administered. Many patients experience visible results in 2 to 4 treatments. Once the aesthetic response is achieved, re-treatment is not expected. Kybella physician training programs will be initiated in late summer. For more information, visit www.mykybella.com.
Sun Shield Broad Spectrum Matte Sunscreen Lotion
Obagi Medical Products, Inc, a division of Valeant Pharmaceuticals North America LLC, introduces the Sun Shield Broad Spectrum Matte SPF 50 Sunscreen Lotion. It combines UVB absorption and UVA protection with a completely sheer application. It contains 10.5% zinc oxide and 7.5% octinoxate. The Sun Shield Broad Spectrum Matte Sunscreen Lotion is ideal for use on all skin types and is physician dispensed. For more information, visit www.obagi.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Intensive Eye Treatment
Niadyne, Incorporated, introduces the NIA24 Intensive Eye Treatment, a dual-phase, predosed, multiaction eye mask to reduce the look of fine lines, wrinkles, and dark circles. The treatment begins with the aesthetician combining the predosed treatment base with the activating powder just prior to use. Once combined, the activated components are applied around the eye area. The Intensive Eye Treatment contains Pro-Niacin to help stimulate skin barrier repair, improve cell turnover, and energize skin. Haloxyl tripeptide complex decreases the red and blue color of dark circles under the eyes and acts as an anti-inflammatory agent. Lumispheres skin brightener diminishes the appearance of fine lines. Other ingredients firm and tighten skin, while reducing undereye bags and puffiness. The Intensive Eye Treatment is a professional treatment that is available exclusively through physicians. For more information, visit www.nia24.com/professional-treatments/.
Kybella Injection
Kythera Biopharmaceuticals announces US Food and Drug Administration approval of Kybella (deoxycholic acid), also known as ATX-101, a nonsurgical treatment indicated for improvement in the appearance of moderate to severe convexity or fullness associated with submental fat in adults. Kybella is administered by injections into the fat under the chin, which causes destruction of fat cells. Once destroyed, those cells cannot store or accumulate fat. Each in-office treatment session is typically 15 to 20 minutes, and up to 6 treatments may be administered. Many patients experience visible results in 2 to 4 treatments. Once the aesthetic response is achieved, re-treatment is not expected. Kybella physician training programs will be initiated in late summer. For more information, visit www.mykybella.com.
Sun Shield Broad Spectrum Matte Sunscreen Lotion
Obagi Medical Products, Inc, a division of Valeant Pharmaceuticals North America LLC, introduces the Sun Shield Broad Spectrum Matte SPF 50 Sunscreen Lotion. It combines UVB absorption and UVA protection with a completely sheer application. It contains 10.5% zinc oxide and 7.5% octinoxate. The Sun Shield Broad Spectrum Matte Sunscreen Lotion is ideal for use on all skin types and is physician dispensed. For more information, visit www.obagi.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Living Tobacco Free
Operation Live Well has a plethora of resources for anyone who wants to quit smoking—or help someone quit smoking—including articles, videos, websites, and competitions. For instance, 6 Healthy Base Initiative sites hosted a text message-based trivia contest called Kicking Butts for Points. Players answered questions about the financial burdens and health effects of smoking and ways to get help, researching their answers on the Quit Tobacco website.
Related: Evaluating Patient Barriers to Tobacco Cessation Treatment
A recent addition to the website is the Tobacco-Free Living Community of Interest (COI), which provides information about DoD’s tobacco policies and resources. The online community has data on active-duty and retiree tobacco use and information on the health benefits of quitting.
Related: Secondhand Smoke Problem Persists
The COI site can be accessed by anyone with a Common Access Card, which allows users to participate in discussions, comment on materials, and interact with colleagues.
Operation Live Well has a plethora of resources for anyone who wants to quit smoking—or help someone quit smoking—including articles, videos, websites, and competitions. For instance, 6 Healthy Base Initiative sites hosted a text message-based trivia contest called Kicking Butts for Points. Players answered questions about the financial burdens and health effects of smoking and ways to get help, researching their answers on the Quit Tobacco website.
Related: Evaluating Patient Barriers to Tobacco Cessation Treatment
A recent addition to the website is the Tobacco-Free Living Community of Interest (COI), which provides information about DoD’s tobacco policies and resources. The online community has data on active-duty and retiree tobacco use and information on the health benefits of quitting.
Related: Secondhand Smoke Problem Persists
The COI site can be accessed by anyone with a Common Access Card, which allows users to participate in discussions, comment on materials, and interact with colleagues.
Operation Live Well has a plethora of resources for anyone who wants to quit smoking—or help someone quit smoking—including articles, videos, websites, and competitions. For instance, 6 Healthy Base Initiative sites hosted a text message-based trivia contest called Kicking Butts for Points. Players answered questions about the financial burdens and health effects of smoking and ways to get help, researching their answers on the Quit Tobacco website.
Related: Evaluating Patient Barriers to Tobacco Cessation Treatment
A recent addition to the website is the Tobacco-Free Living Community of Interest (COI), which provides information about DoD’s tobacco policies and resources. The online community has data on active-duty and retiree tobacco use and information on the health benefits of quitting.
Related: Secondhand Smoke Problem Persists
The COI site can be accessed by anyone with a Common Access Card, which allows users to participate in discussions, comment on materials, and interact with colleagues.
Product News: 04 2015
Antirougeurs FORT Relief Concentrate
Pierre Fabre Dermo-Cosmétique USA introduces Avène Antirougeurs FORT Relief Concentrate for chronic redness. This advanced spot treatment is indicated for localized redness and small visible blood vessels, chronic persistent to permanent redness, and rosacea. It is formulated with Ruscus extract (0.3%) to induce contraction of the smooth muscle in blood vessel walls to encourage microcirculation and inhibit the formation of new blood vessels. Results have been seen after 3 months of once nightly application. Antirougeurs FORT Relief Concentrate is available exclusively in physicians’ offices. For more information, visit www.aveneusa.com.
Cosentyx
Novartis Pharmaceuticals Corporation announces US Food and Drug Administration approval of Cosentyx (secukinumab) for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy. Cosentyx is a human monoclonal antibody that selectively binds to IL-17A and inhibits its reaction with the IL-17 receptor. For more information, visit www.novartis.com.
DEJ Face Cream
Revision Skincare adds DEJ face cream to the antiaging collection. It is formulated with rosemary extract, goji fruit extract, coenzyme Q10, and vitamins C and E for antioxidant benefits. The cream also contains peptides palmitoyl tripeptide-38 and acetyl tetrapeptide-2 to tighten the dermoepidermal junction and reduce the appearance of fine lines and wrinkles as well as a copper complex to provide energy to aging cells. A blend of 3 ceramides provides moisturization. Results have shown a reduction in redness, hyperpigmentation, and fine lines and wrinkles, as well as better overall appearance. DEJ face cream is available exclusively through dermatologists, plastic surgeons, and medical spas, and can be layered with other products. For more information, visit www.revisionskincare.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Antirougeurs FORT Relief Concentrate
Pierre Fabre Dermo-Cosmétique USA introduces Avène Antirougeurs FORT Relief Concentrate for chronic redness. This advanced spot treatment is indicated for localized redness and small visible blood vessels, chronic persistent to permanent redness, and rosacea. It is formulated with Ruscus extract (0.3%) to induce contraction of the smooth muscle in blood vessel walls to encourage microcirculation and inhibit the formation of new blood vessels. Results have been seen after 3 months of once nightly application. Antirougeurs FORT Relief Concentrate is available exclusively in physicians’ offices. For more information, visit www.aveneusa.com.
Cosentyx
Novartis Pharmaceuticals Corporation announces US Food and Drug Administration approval of Cosentyx (secukinumab) for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy. Cosentyx is a human monoclonal antibody that selectively binds to IL-17A and inhibits its reaction with the IL-17 receptor. For more information, visit www.novartis.com.
DEJ Face Cream
Revision Skincare adds DEJ face cream to the antiaging collection. It is formulated with rosemary extract, goji fruit extract, coenzyme Q10, and vitamins C and E for antioxidant benefits. The cream also contains peptides palmitoyl tripeptide-38 and acetyl tetrapeptide-2 to tighten the dermoepidermal junction and reduce the appearance of fine lines and wrinkles as well as a copper complex to provide energy to aging cells. A blend of 3 ceramides provides moisturization. Results have shown a reduction in redness, hyperpigmentation, and fine lines and wrinkles, as well as better overall appearance. DEJ face cream is available exclusively through dermatologists, plastic surgeons, and medical spas, and can be layered with other products. For more information, visit www.revisionskincare.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Antirougeurs FORT Relief Concentrate
Pierre Fabre Dermo-Cosmétique USA introduces Avène Antirougeurs FORT Relief Concentrate for chronic redness. This advanced spot treatment is indicated for localized redness and small visible blood vessels, chronic persistent to permanent redness, and rosacea. It is formulated with Ruscus extract (0.3%) to induce contraction of the smooth muscle in blood vessel walls to encourage microcirculation and inhibit the formation of new blood vessels. Results have been seen after 3 months of once nightly application. Antirougeurs FORT Relief Concentrate is available exclusively in physicians’ offices. For more information, visit www.aveneusa.com.
Cosentyx
Novartis Pharmaceuticals Corporation announces US Food and Drug Administration approval of Cosentyx (secukinumab) for the treatment of moderate to severe plaque psoriasis in adult patients who are candidates for systemic therapy or phototherapy. Cosentyx is a human monoclonal antibody that selectively binds to IL-17A and inhibits its reaction with the IL-17 receptor. For more information, visit www.novartis.com.
DEJ Face Cream
Revision Skincare adds DEJ face cream to the antiaging collection. It is formulated with rosemary extract, goji fruit extract, coenzyme Q10, and vitamins C and E for antioxidant benefits. The cream also contains peptides palmitoyl tripeptide-38 and acetyl tetrapeptide-2 to tighten the dermoepidermal junction and reduce the appearance of fine lines and wrinkles as well as a copper complex to provide energy to aging cells. A blend of 3 ceramides provides moisturization. Results have shown a reduction in redness, hyperpigmentation, and fine lines and wrinkles, as well as better overall appearance. DEJ face cream is available exclusively through dermatologists, plastic surgeons, and medical spas, and can be layered with other products. For more information, visit www.revisionskincare.com.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Product News: 03 2015
Avène Hydrating Sunscreen Lotions
Pierre Fabre Dermo-Cosmétique USA introduces 5 Avène Hydrating Sunscreen Lotions with sun protection factor 50+. The Hydrating Sunscreen Lotion contains avobenzone, homosalate, octisalate, and octocrylene for broad-spectrum UVA and UVB protection on the face and body with a smooth finish. The Ultra-Light Hydrating Sunscreen Lotion is suitable for the face with a matte finish. The Ultra-Light Hydrating Sunscreen Lotion Spray is fast absorbing with a sheer finish for application on the body. The Mineral Hydrating Sunscreen Lotions are available in light and ultra-light formulations. They offer sheer, nonwhitening, broad-spectrum UVA and UVB protection with both titanium dioxide and zinc oxide. The light formulation is suitable for the face and body, and the ultra-light formulation is suitable for the face. All of the sunscreens are water resistant for 80 minutes of water activity and are formulated with vitamin E (tocopheryl acetate) to provide antioxidant protection and glycerin for long-lasting hydration. These sunscreens will be available mid-March 2015. For more information, visit www.aveneusa.com.
cosmelan
Mesoestetic introduces cosmelan for the correction of pigmentary imperfections on the skin. This topical treatment eliminates dark spots by affecting melanocytes, which inhibits melanin production in hyperpigmented areas. As a result, spots disappear or are noticeably lightened. Its formula has antioxidant properties to enhance the skin’s radiance and even out skin tone. The cosmelan pack includes an intense depigmenting mask (cosmelan 1) and an oil-removing solution for skin cleansing and exfoliation, both for use in the physician’s office. It also contains a depigmenting maintenance cream (cosmelan 2) and hydra-vital factor k moisturizing cream, both for at-home use. The treatment consists of 2 phases: 1 or 2 sessions at the physician’s office with cosmelan 1, followed by at-home treatment (cosmelan 2) for 6 to 9 months. cosmelan is suitable for all skin types. For more information, visit www.mesoestetic.com.
Cresemba
Astellas Pharma US, Inc, announces approval of the investigational once-daily intravenous and oral broad-spectrum Cresemba (isavuconazonium) by the Anti-infective Drugs Advisory Committee of the US Food and Drug Administration. Cresemba is indicated for the treatment of invasive aspergillosis and invasive mucormycosis, both life-threatening fungal infections that predominately occur in immunocompromised patients. The review of the New Drug Application is expected to be completed in March 2015. For more information, visit www.astellas.us.
Glytone Lipid Recovery Cream
Pierre Fabre Dermo-Cosmétique USA introduces an enhanced Glytone Lipid Recovery Cream for use on inflamed and dry skin following cosmetic procedures such as chemical peels, lasers, microdermabrasion, and fillers. It contains camelina oil to preserve lipid barrier function; retinyl palmitate to repair damaged skin tissue; sodium hyaluronate to help skin cell proliferation and tissue repair; and shea butter, glycerin, and squalane to hydrate and moisturize. This postprocedure cream is now lighter in texture, allowing for faster absorption. Glytone Lipid Recovery Cream is physician dispensed. For more information, visit www.glytone-usa.com.
Opdivo
Bristol-Myers Squibb Company announces US Food and Drug Administration approval of Opdivo (nivolumab) for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor. It works by inhibiting the PD-1 (programmed death receptor-1) protein on cells, which blocks the body’s immune system from attacking melanoma tumors. The indication represents accelerated approval based on tumor response rate and durability of response. For more information, visit www.opdivo.com.
Pazeo
Alcon, a Novartis company, receives US Food and Drug Administration approval of Pazeo (olopatadine hydrochloride ophthalmic solution) 0.7% for daily ocular itch relief associated with allergic conjunctivitis. It is administered one drop in each affected eye once daily and was approved with efficacy data at 24 hours following a dose. Pazeo is anticipated to be available by prescription in the United States in March 2015. For more information, visit www.myalcon.com/products/pharmaceutical/pazeo.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Avène Hydrating Sunscreen Lotions
Pierre Fabre Dermo-Cosmétique USA introduces 5 Avène Hydrating Sunscreen Lotions with sun protection factor 50+. The Hydrating Sunscreen Lotion contains avobenzone, homosalate, octisalate, and octocrylene for broad-spectrum UVA and UVB protection on the face and body with a smooth finish. The Ultra-Light Hydrating Sunscreen Lotion is suitable for the face with a matte finish. The Ultra-Light Hydrating Sunscreen Lotion Spray is fast absorbing with a sheer finish for application on the body. The Mineral Hydrating Sunscreen Lotions are available in light and ultra-light formulations. They offer sheer, nonwhitening, broad-spectrum UVA and UVB protection with both titanium dioxide and zinc oxide. The light formulation is suitable for the face and body, and the ultra-light formulation is suitable for the face. All of the sunscreens are water resistant for 80 minutes of water activity and are formulated with vitamin E (tocopheryl acetate) to provide antioxidant protection and glycerin for long-lasting hydration. These sunscreens will be available mid-March 2015. For more information, visit www.aveneusa.com.
cosmelan
Mesoestetic introduces cosmelan for the correction of pigmentary imperfections on the skin. This topical treatment eliminates dark spots by affecting melanocytes, which inhibits melanin production in hyperpigmented areas. As a result, spots disappear or are noticeably lightened. Its formula has antioxidant properties to enhance the skin’s radiance and even out skin tone. The cosmelan pack includes an intense depigmenting mask (cosmelan 1) and an oil-removing solution for skin cleansing and exfoliation, both for use in the physician’s office. It also contains a depigmenting maintenance cream (cosmelan 2) and hydra-vital factor k moisturizing cream, both for at-home use. The treatment consists of 2 phases: 1 or 2 sessions at the physician’s office with cosmelan 1, followed by at-home treatment (cosmelan 2) for 6 to 9 months. cosmelan is suitable for all skin types. For more information, visit www.mesoestetic.com.
Cresemba
Astellas Pharma US, Inc, announces approval of the investigational once-daily intravenous and oral broad-spectrum Cresemba (isavuconazonium) by the Anti-infective Drugs Advisory Committee of the US Food and Drug Administration. Cresemba is indicated for the treatment of invasive aspergillosis and invasive mucormycosis, both life-threatening fungal infections that predominately occur in immunocompromised patients. The review of the New Drug Application is expected to be completed in March 2015. For more information, visit www.astellas.us.
Glytone Lipid Recovery Cream
Pierre Fabre Dermo-Cosmétique USA introduces an enhanced Glytone Lipid Recovery Cream for use on inflamed and dry skin following cosmetic procedures such as chemical peels, lasers, microdermabrasion, and fillers. It contains camelina oil to preserve lipid barrier function; retinyl palmitate to repair damaged skin tissue; sodium hyaluronate to help skin cell proliferation and tissue repair; and shea butter, glycerin, and squalane to hydrate and moisturize. This postprocedure cream is now lighter in texture, allowing for faster absorption. Glytone Lipid Recovery Cream is physician dispensed. For more information, visit www.glytone-usa.com.
Opdivo
Bristol-Myers Squibb Company announces US Food and Drug Administration approval of Opdivo (nivolumab) for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor. It works by inhibiting the PD-1 (programmed death receptor-1) protein on cells, which blocks the body’s immune system from attacking melanoma tumors. The indication represents accelerated approval based on tumor response rate and durability of response. For more information, visit www.opdivo.com.
Pazeo
Alcon, a Novartis company, receives US Food and Drug Administration approval of Pazeo (olopatadine hydrochloride ophthalmic solution) 0.7% for daily ocular itch relief associated with allergic conjunctivitis. It is administered one drop in each affected eye once daily and was approved with efficacy data at 24 hours following a dose. Pazeo is anticipated to be available by prescription in the United States in March 2015. For more information, visit www.myalcon.com/products/pharmaceutical/pazeo.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Avène Hydrating Sunscreen Lotions
Pierre Fabre Dermo-Cosmétique USA introduces 5 Avène Hydrating Sunscreen Lotions with sun protection factor 50+. The Hydrating Sunscreen Lotion contains avobenzone, homosalate, octisalate, and octocrylene for broad-spectrum UVA and UVB protection on the face and body with a smooth finish. The Ultra-Light Hydrating Sunscreen Lotion is suitable for the face with a matte finish. The Ultra-Light Hydrating Sunscreen Lotion Spray is fast absorbing with a sheer finish for application on the body. The Mineral Hydrating Sunscreen Lotions are available in light and ultra-light formulations. They offer sheer, nonwhitening, broad-spectrum UVA and UVB protection with both titanium dioxide and zinc oxide. The light formulation is suitable for the face and body, and the ultra-light formulation is suitable for the face. All of the sunscreens are water resistant for 80 minutes of water activity and are formulated with vitamin E (tocopheryl acetate) to provide antioxidant protection and glycerin for long-lasting hydration. These sunscreens will be available mid-March 2015. For more information, visit www.aveneusa.com.
cosmelan
Mesoestetic introduces cosmelan for the correction of pigmentary imperfections on the skin. This topical treatment eliminates dark spots by affecting melanocytes, which inhibits melanin production in hyperpigmented areas. As a result, spots disappear or are noticeably lightened. Its formula has antioxidant properties to enhance the skin’s radiance and even out skin tone. The cosmelan pack includes an intense depigmenting mask (cosmelan 1) and an oil-removing solution for skin cleansing and exfoliation, both for use in the physician’s office. It also contains a depigmenting maintenance cream (cosmelan 2) and hydra-vital factor k moisturizing cream, both for at-home use. The treatment consists of 2 phases: 1 or 2 sessions at the physician’s office with cosmelan 1, followed by at-home treatment (cosmelan 2) for 6 to 9 months. cosmelan is suitable for all skin types. For more information, visit www.mesoestetic.com.
Cresemba
Astellas Pharma US, Inc, announces approval of the investigational once-daily intravenous and oral broad-spectrum Cresemba (isavuconazonium) by the Anti-infective Drugs Advisory Committee of the US Food and Drug Administration. Cresemba is indicated for the treatment of invasive aspergillosis and invasive mucormycosis, both life-threatening fungal infections that predominately occur in immunocompromised patients. The review of the New Drug Application is expected to be completed in March 2015. For more information, visit www.astellas.us.
Glytone Lipid Recovery Cream
Pierre Fabre Dermo-Cosmétique USA introduces an enhanced Glytone Lipid Recovery Cream for use on inflamed and dry skin following cosmetic procedures such as chemical peels, lasers, microdermabrasion, and fillers. It contains camelina oil to preserve lipid barrier function; retinyl palmitate to repair damaged skin tissue; sodium hyaluronate to help skin cell proliferation and tissue repair; and shea butter, glycerin, and squalane to hydrate and moisturize. This postprocedure cream is now lighter in texture, allowing for faster absorption. Glytone Lipid Recovery Cream is physician dispensed. For more information, visit www.glytone-usa.com.
Opdivo
Bristol-Myers Squibb Company announces US Food and Drug Administration approval of Opdivo (nivolumab) for the treatment of patients with unresectable or metastatic melanoma and disease progression following ipilimumab and, if BRAF V600 mutation positive, a BRAF inhibitor. It works by inhibiting the PD-1 (programmed death receptor-1) protein on cells, which blocks the body’s immune system from attacking melanoma tumors. The indication represents accelerated approval based on tumor response rate and durability of response. For more information, visit www.opdivo.com.
Pazeo
Alcon, a Novartis company, receives US Food and Drug Administration approval of Pazeo (olopatadine hydrochloride ophthalmic solution) 0.7% for daily ocular itch relief associated with allergic conjunctivitis. It is administered one drop in each affected eye once daily and was approved with efficacy data at 24 hours following a dose. Pazeo is anticipated to be available by prescription in the United States in March 2015. For more information, visit www.myalcon.com/products/pharmaceutical/pazeo.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Product Update: InTone, E-Sacs, traxi, Saliva Fertility Monitor
FEMALE URINARY INCONTINENCE DEVICE
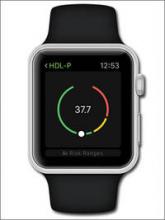
The InTone® system from InControl Medical™ is a noninvasive home-based pelvic-floor rehabilitation program to treat female urinary incontinence. InControl offers two customizable probes. Apex, for women with mild to moderate stress urinary incontinence, delivers electro-stimulation to strengthen pelvic floor muscles and eliminate leakage associated with coughing, laughing, or exercise. ApexM, for women with urgency and mixed urinary incontinence, provides alternating-frequency stimulation to strengthen the pelvic floor muscles and calm detrusor muscle spasms.
The InTone system for moderate to severe incontinence combines muscle stimulation with pelvic-floor exercises. A hand-held control unit allows the patient to listen to prerecorded exercises at home. The control unit also offers visual biofeedback to ensure that the exercises are being completed properly. The patient can present the data from her workouts, stored by the handheld control unit, to her physician, who can then individualize the exercise plan. InTone is indicated for use in 12-minute sessions, six times a week, for 90 days to treat stress urinary incontinence, and for 180 days for urgency or mixed incontinence.
FOR MORE INFORMATION, VISIT www.incontrolmedical.com
TISSUE RETRIEVAL SACS
Espiner Medical offers E-Sacs, a variety of tissue retrieval sacs for minimally invasive surgery. E-Sacs are manufactured from Superamine66™ fabric, a lightweight, ripstop nylon coated with polyurethane designed to make them strong, rupture proof, leak resistant, easy to deploy, and x-ray opaque. Standard E-Sacs, made of one-piece construction with an integral tail that can be deployed using 5-mm instruments, do not require claw forceps to extract. Super E-Sacs are ideal for larger specimens and have colored tabs to facilitate placement. Master E-Sacs open automatically, have stronger arms to reinforce the mouth opening, a drawstring closure, and can accept tissue sizes up to a large spleen, ovary, or kidney. EcoSacs, for bottom-first abdominal entry, have a large open mouth to facilitate tissue capture with a drawstring closure.
FOR MORE INFORMATION, VISIT www.espinermedical.com
PANNICULUS RETRACTOR
traxi can be applied and used as a sterile or nonsterile product in conjunction with both external and internal retractors. The manufacturer explains that traxi works by anchoring at the xiphoid and distributing the patient’s weight rather than applying it fully on the sternum, which can make it difficult for the patient to breathe. traxi should not be left on the patient for longer than 24 hours. traxi is labeled with HOLD and PULL HERE tabs, as well as A, B, and C tabs to guide the clinician through the retractor application process.
FOR MORE INFORMATION, VISIT www.clinicalinnovations.com
SALIVA FERTILITY MONITOR
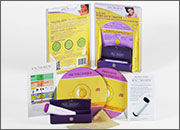
The KNOWHEN® Saliva Fertility Monitor is a handheld mini-microscope that monitors a woman’s ovulation using just a single drop of saliva. A woman’s daily test results can be tracked on an ovulation mobile app provided with the product, allowing her to better plan her sexual activity to achieve fertility goals.
First thing each morning, before eating, drinking, smoking, or brushing teeth, a woman applies a thick drop of saliva to the glass surface and waits for it to dry (5–15 minutes). Then she compares the image she sees through the lens to the chart supplied with the kit and inputs her results on the KNOWHEN app. If she is not ovulating, dots and circles may appear. When she sees a few fernlike crystals, it means she is starting to ovulate, or has just finished ovulating. Consistent fern-like crystals in the viewer indicate that she is ovulating. During ovulation, high estrogen levels raise the percentage of salt in a woman’s body. In saliva, these salts take the shape of ferns under the 60X magnification of the KNOWHEN microscope. The kit includes the app and educational material, including an instructional video.
FOR MORE INFORMATION, VISIT www.knowhen.com
FEMALE URINARY INCONTINENCE DEVICE

The InTone® system from InControl Medical™ is a noninvasive home-based pelvic-floor rehabilitation program to treat female urinary incontinence. InControl offers two customizable probes. Apex, for women with mild to moderate stress urinary incontinence, delivers electro-stimulation to strengthen pelvic floor muscles and eliminate leakage associated with coughing, laughing, or exercise. ApexM, for women with urgency and mixed urinary incontinence, provides alternating-frequency stimulation to strengthen the pelvic floor muscles and calm detrusor muscle spasms.
The InTone system for moderate to severe incontinence combines muscle stimulation with pelvic-floor exercises. A hand-held control unit allows the patient to listen to prerecorded exercises at home. The control unit also offers visual biofeedback to ensure that the exercises are being completed properly. The patient can present the data from her workouts, stored by the handheld control unit, to her physician, who can then individualize the exercise plan. InTone is indicated for use in 12-minute sessions, six times a week, for 90 days to treat stress urinary incontinence, and for 180 days for urgency or mixed incontinence.
FOR MORE INFORMATION, VISIT www.incontrolmedical.com
TISSUE RETRIEVAL SACS

Espiner Medical offers E-Sacs, a variety of tissue retrieval sacs for minimally invasive surgery. E-Sacs are manufactured from Superamine66™ fabric, a lightweight, ripstop nylon coated with polyurethane designed to make them strong, rupture proof, leak resistant, easy to deploy, and x-ray opaque. Standard E-Sacs, made of one-piece construction with an integral tail that can be deployed using 5-mm instruments, do not require claw forceps to extract. Super E-Sacs are ideal for larger specimens and have colored tabs to facilitate placement. Master E-Sacs open automatically, have stronger arms to reinforce the mouth opening, a drawstring closure, and can accept tissue sizes up to a large spleen, ovary, or kidney. EcoSacs, for bottom-first abdominal entry, have a large open mouth to facilitate tissue capture with a drawstring closure.
FOR MORE INFORMATION, VISIT www.espinermedical.com
PANNICULUS RETRACTOR

traxi can be applied and used as a sterile or nonsterile product in conjunction with both external and internal retractors. The manufacturer explains that traxi works by anchoring at the xiphoid and distributing the patient’s weight rather than applying it fully on the sternum, which can make it difficult for the patient to breathe. traxi should not be left on the patient for longer than 24 hours. traxi is labeled with HOLD and PULL HERE tabs, as well as A, B, and C tabs to guide the clinician through the retractor application process.
FOR MORE INFORMATION, VISIT www.clinicalinnovations.com
SALIVA FERTILITY MONITOR

The KNOWHEN® Saliva Fertility Monitor is a handheld mini-microscope that monitors a woman’s ovulation using just a single drop of saliva. A woman’s daily test results can be tracked on an ovulation mobile app provided with the product, allowing her to better plan her sexual activity to achieve fertility goals.
First thing each morning, before eating, drinking, smoking, or brushing teeth, a woman applies a thick drop of saliva to the glass surface and waits for it to dry (5–15 minutes). Then she compares the image she sees through the lens to the chart supplied with the kit and inputs her results on the KNOWHEN app. If she is not ovulating, dots and circles may appear. When she sees a few fernlike crystals, it means she is starting to ovulate, or has just finished ovulating. Consistent fern-like crystals in the viewer indicate that she is ovulating. During ovulation, high estrogen levels raise the percentage of salt in a woman’s body. In saliva, these salts take the shape of ferns under the 60X magnification of the KNOWHEN microscope. The kit includes the app and educational material, including an instructional video.
FOR MORE INFORMATION, VISIT www.knowhen.com
FEMALE URINARY INCONTINENCE DEVICE

The InTone® system from InControl Medical™ is a noninvasive home-based pelvic-floor rehabilitation program to treat female urinary incontinence. InControl offers two customizable probes. Apex, for women with mild to moderate stress urinary incontinence, delivers electro-stimulation to strengthen pelvic floor muscles and eliminate leakage associated with coughing, laughing, or exercise. ApexM, for women with urgency and mixed urinary incontinence, provides alternating-frequency stimulation to strengthen the pelvic floor muscles and calm detrusor muscle spasms.
The InTone system for moderate to severe incontinence combines muscle stimulation with pelvic-floor exercises. A hand-held control unit allows the patient to listen to prerecorded exercises at home. The control unit also offers visual biofeedback to ensure that the exercises are being completed properly. The patient can present the data from her workouts, stored by the handheld control unit, to her physician, who can then individualize the exercise plan. InTone is indicated for use in 12-minute sessions, six times a week, for 90 days to treat stress urinary incontinence, and for 180 days for urgency or mixed incontinence.
FOR MORE INFORMATION, VISIT www.incontrolmedical.com
TISSUE RETRIEVAL SACS

Espiner Medical offers E-Sacs, a variety of tissue retrieval sacs for minimally invasive surgery. E-Sacs are manufactured from Superamine66™ fabric, a lightweight, ripstop nylon coated with polyurethane designed to make them strong, rupture proof, leak resistant, easy to deploy, and x-ray opaque. Standard E-Sacs, made of one-piece construction with an integral tail that can be deployed using 5-mm instruments, do not require claw forceps to extract. Super E-Sacs are ideal for larger specimens and have colored tabs to facilitate placement. Master E-Sacs open automatically, have stronger arms to reinforce the mouth opening, a drawstring closure, and can accept tissue sizes up to a large spleen, ovary, or kidney. EcoSacs, for bottom-first abdominal entry, have a large open mouth to facilitate tissue capture with a drawstring closure.
FOR MORE INFORMATION, VISIT www.espinermedical.com
PANNICULUS RETRACTOR

traxi can be applied and used as a sterile or nonsterile product in conjunction with both external and internal retractors. The manufacturer explains that traxi works by anchoring at the xiphoid and distributing the patient’s weight rather than applying it fully on the sternum, which can make it difficult for the patient to breathe. traxi should not be left on the patient for longer than 24 hours. traxi is labeled with HOLD and PULL HERE tabs, as well as A, B, and C tabs to guide the clinician through the retractor application process.
FOR MORE INFORMATION, VISIT www.clinicalinnovations.com
SALIVA FERTILITY MONITOR

The KNOWHEN® Saliva Fertility Monitor is a handheld mini-microscope that monitors a woman’s ovulation using just a single drop of saliva. A woman’s daily test results can be tracked on an ovulation mobile app provided with the product, allowing her to better plan her sexual activity to achieve fertility goals.
First thing each morning, before eating, drinking, smoking, or brushing teeth, a woman applies a thick drop of saliva to the glass surface and waits for it to dry (5–15 minutes). Then she compares the image she sees through the lens to the chart supplied with the kit and inputs her results on the KNOWHEN app. If she is not ovulating, dots and circles may appear. When she sees a few fernlike crystals, it means she is starting to ovulate, or has just finished ovulating. Consistent fern-like crystals in the viewer indicate that she is ovulating. During ovulation, high estrogen levels raise the percentage of salt in a woman’s body. In saliva, these salts take the shape of ferns under the 60X magnification of the KNOWHEN microscope. The kit includes the app and educational material, including an instructional video.
FOR MORE INFORMATION, VISIT www.knowhen.com
Product News: 02 2015
Bellafill
Suneva Medical, Inc, announces US Food and Drug Administration approval of the polymethylmethacrylate collagen filler Bellafill for the correction of moderate to severe, atrophic, distensible facial acne scars on the cheek in patients older than 21 years. Bellafill adds volume to the skin to lift and smooth out pitted acne scars to the level of the surrounding skin. Bellafill also is indicated for correction of nasolabial folds. For more information, visit www.bellafill.com.
Benzac
Galderma Laboratories, LP, launches Benzac Acne Solutions, an over-the-counter 3-step acne regimen consisting of a skin balancing foaming cleanser, intensive spot treatment, and blemish clearing hydrator. In addition to salicylic acid, Benzac also contains Kakadu plum (an antioxidant) to brighten the skin, lemon myrtle (an astringent) to reduce excess oil, and zinc to act as a barrier against skin moisture loss. Benzac contains pharmaceutical-grade East Indian sandalwood oil to calm and soothe the skin. For more information, visit www.benzac.com.
The Promius Promise App
Promius Pharma, LLC, marketers of Zenatane (isotretinoin capsules), launches The Promius Promise App designed to help educate and guide patients through the iPLEDGE program from the first visit to the last visit. The app is free for patients who have received a Zenatane prescription through The Promius Promise program. Patients can easily find important information, which may help with treatment compliance. The app will be useful for young adults and teenagers as well as their parents. For more information, visit www.zenatane.com/toolkit/Promius-Promise-Enrollment-Form.php.
Soolantra
Galderma Laboratories, LP, announces US Food and Drug Administration approval of Soolantra Cream 1% for the once-daily treatment of inflammatory lesions of rosacea. Ivermectin, the active ingredient in Soolantra, has both anti-inflammatory and antiparasitic activity. The basis for the cream formulation is Cetaphil Moisturizing Cream. Soolantra offers patients improvement as early as week 2 of treatment. For more information, visit www.soolantra.com/hcp.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Bellafill
Suneva Medical, Inc, announces US Food and Drug Administration approval of the polymethylmethacrylate collagen filler Bellafill for the correction of moderate to severe, atrophic, distensible facial acne scars on the cheek in patients older than 21 years. Bellafill adds volume to the skin to lift and smooth out pitted acne scars to the level of the surrounding skin. Bellafill also is indicated for correction of nasolabial folds. For more information, visit www.bellafill.com.
Benzac
Galderma Laboratories, LP, launches Benzac Acne Solutions, an over-the-counter 3-step acne regimen consisting of a skin balancing foaming cleanser, intensive spot treatment, and blemish clearing hydrator. In addition to salicylic acid, Benzac also contains Kakadu plum (an antioxidant) to brighten the skin, lemon myrtle (an astringent) to reduce excess oil, and zinc to act as a barrier against skin moisture loss. Benzac contains pharmaceutical-grade East Indian sandalwood oil to calm and soothe the skin. For more information, visit www.benzac.com.
The Promius Promise App
Promius Pharma, LLC, marketers of Zenatane (isotretinoin capsules), launches The Promius Promise App designed to help educate and guide patients through the iPLEDGE program from the first visit to the last visit. The app is free for patients who have received a Zenatane prescription through The Promius Promise program. Patients can easily find important information, which may help with treatment compliance. The app will be useful for young adults and teenagers as well as their parents. For more information, visit www.zenatane.com/toolkit/Promius-Promise-Enrollment-Form.php.
Soolantra
Galderma Laboratories, LP, announces US Food and Drug Administration approval of Soolantra Cream 1% for the once-daily treatment of inflammatory lesions of rosacea. Ivermectin, the active ingredient in Soolantra, has both anti-inflammatory and antiparasitic activity. The basis for the cream formulation is Cetaphil Moisturizing Cream. Soolantra offers patients improvement as early as week 2 of treatment. For more information, visit www.soolantra.com/hcp.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Bellafill
Suneva Medical, Inc, announces US Food and Drug Administration approval of the polymethylmethacrylate collagen filler Bellafill for the correction of moderate to severe, atrophic, distensible facial acne scars on the cheek in patients older than 21 years. Bellafill adds volume to the skin to lift and smooth out pitted acne scars to the level of the surrounding skin. Bellafill also is indicated for correction of nasolabial folds. For more information, visit www.bellafill.com.
Benzac
Galderma Laboratories, LP, launches Benzac Acne Solutions, an over-the-counter 3-step acne regimen consisting of a skin balancing foaming cleanser, intensive spot treatment, and blemish clearing hydrator. In addition to salicylic acid, Benzac also contains Kakadu plum (an antioxidant) to brighten the skin, lemon myrtle (an astringent) to reduce excess oil, and zinc to act as a barrier against skin moisture loss. Benzac contains pharmaceutical-grade East Indian sandalwood oil to calm and soothe the skin. For more information, visit www.benzac.com.
The Promius Promise App
Promius Pharma, LLC, marketers of Zenatane (isotretinoin capsules), launches The Promius Promise App designed to help educate and guide patients through the iPLEDGE program from the first visit to the last visit. The app is free for patients who have received a Zenatane prescription through The Promius Promise program. Patients can easily find important information, which may help with treatment compliance. The app will be useful for young adults and teenagers as well as their parents. For more information, visit www.zenatane.com/toolkit/Promius-Promise-Enrollment-Form.php.
Soolantra
Galderma Laboratories, LP, announces US Food and Drug Administration approval of Soolantra Cream 1% for the once-daily treatment of inflammatory lesions of rosacea. Ivermectin, the active ingredient in Soolantra, has both anti-inflammatory and antiparasitic activity. The basis for the cream formulation is Cetaphil Moisturizing Cream. Soolantra offers patients improvement as early as week 2 of treatment. For more information, visit www.soolantra.com/hcp.
If you would like your product included in Product News, please e-mail a press release to the Editorial Office at [email protected].
Product Update
LITHOTOMY STIRRUPS FOR PATIENT COMFORT
Frontier Medical Innovations says its new GStirrup® is designed to provide patients stable and comfortable foot positioning for extended periods of time during office-based surgical procedures. The GStirrup is a pair of cushioned lithotomy boots that attach to examination-table foot rests. Straps secure the patient’s feet and legs; weights in the base make it difficult for the patient to lift the boot off the footrest. GStirrups fit most footrests and are helpful for patients who have hip or knee replacement, low back pain, arthritis, or neurologic conditions such as multiple sclerosis or Parkinson’s disease.
FOR MORE INFORMATION, VISIT www.gstirrup.com
PREGNANCY, BIRTH, AND BREASTFEEDING APP
Totally Pregnant, an app for pregnant women and health-care providers, is now partnering with Lamaze International to provide access to online parenting classes for pregnancy, childbirth, and early parenting. By using the Totally Pregnant app, women can personalize their pregnancy experience and clinicians can connect with their patients. Available for iPhone, iPad, Android, and desktop.
FOR MORE INFORMATION, VISIT www.iamtotally.com
PREDICTING IVF SUCCESS
Univfy®offers fertility predictive analytics to help prospective parents understand the probability for the success of in vitro fertilization (IVF) and estimated costs. The PreIVF™ calculator helps to decide whether or when to start IVF; the PredictIVF™ helps determine if another IVF cycle is the right option. The IVF Cost Calculator offers a cost comparison tailored to the patient’s IVF success rate. Your Fertility™ is an interactive multimedia blog offering educational material.
FOR MORE INFORMATION, VISIT www.univfy.com
SECURE WAY TO CARRY MEDS
FusionWrap is a waist/hip wrap with two 7-inch pockets to carry small personal belongings. Besides providing secure space for identification and money, it also allows those with asthma, diabetes, allergies, or other chronic diseases to carry medications at all times. Made of stretch fabric that is moisture wicking and antimicrobial, FusionWrap comes in various colors and sizes for women, men, and children.
FOR MORE INFORMATION, VISIT www.fusion-wrap.com
BREAST IMAGING TOOLS
Volpara Solutions offers multiple volumetric breast-imaging software tools designed to improve clinical decision making and the early detection of breast cancer. Volpara®Density™ is a breast-density assessment tool available for clinical use with 2D mammography and digital breast tomosynthesis (3D mammography) platforms from multiple manufacturers.
FOR MORE INFORMATION, VISIT www.volparadensity.com
SMOKE EVACUATION PENCIL
The PlumePen® Elite is an electrosurgical smoke pencil with a compact, ergonomic design that is smaller but offers more flow than competitive smoke pencils, claims Buffalo Filter. The adjustable capture port allows for optimum visibility regardless of blade length. The one-piece molded design prevents buttons from sticking and improves grip. The PlumePen Elite connects to most surgical plume evacuators and generators.
FOR MORE INFORMATION, VISIT www.buffalofilter.com
SURGICAL CO2 LASER
Lumenis designed the AcuPulse Smart CO2 Laser with SurgiTouch™ Automation System for tissue ablation during gynecologic surgery to increase speed, precision, and convenience over other electrosurgical devices. Robotic, computer-controlled laser-beam movement provides more precision than hand-held lasers and offers reproducible outcomes. Brief laser-tissue interaction reduces thermal damage.
FOR MORE INFORMATION, VISIT www.lumenis.com
REUSABLE FORNIX FOR LAP HYSTERECTOMY
The Banyan Colpo-Port Vaginal Fornix Delineator is a reusable uterine elevator/vaginal fornix delineator for laparoscopic hysterectomy. Inserted vaginally, the distal tip has a canted, beveled cup that fits securely in the vaginal fornix. The Calibrated Uterine Elevator (CUE) passes through the inner diameter of the delineator body, through the cervix, and into the uterine cavity. The CUE can be locked at preset depths to prevent uterine perforation. The device is easy to clean using standard sterilization procedures.
FOR MORE INFORMATION, VISIT www.banyanmedllc.com
OVARIAN MALIGNANCY ALGORITHM
The Risk of Ovarian Malignancy Algorithm (ROMA™) from Fujirebio is a quantitative serum test intended to assess the risk of finding malignancy at surgery in a premenopausal or postmenopausal woman with an ovarian mass. ROMA, a risk stratification tool, combines the results of human epididymis protein 4 (HE4), cancer antigen 125 (CA125), and menopausal status into a numerical score.
FOR MORE INFORMATION, VISIT www.he4test.com
PORT-SITE CLOSURE DEVICE
neoClose® AnchorGuide facilitates rapid trocar exchange and precise AutoAnchor placement to help prevent port-site hernia. The AnchorGuide design facilitates the delivery of absorbable AutoAnchors through soft tissue during surgery; allows for the VectorX method of port approximation for reduced tension at wound sites; and helps desufflate to remove CO2 at the end of surgery. AnchorGuide comes in 8–15 mm and 5–12 mm sizes compatible with 8 mm robotic ports.
FOR MORE INFORMATION, VISIT www.neosurgical.com
LITHOTOMY STIRRUPS FOR PATIENT COMFORT
Frontier Medical Innovations says its new GStirrup® is designed to provide patients stable and comfortable foot positioning for extended periods of time during office-based surgical procedures. The GStirrup is a pair of cushioned lithotomy boots that attach to examination-table foot rests. Straps secure the patient’s feet and legs; weights in the base make it difficult for the patient to lift the boot off the footrest. GStirrups fit most footrests and are helpful for patients who have hip or knee replacement, low back pain, arthritis, or neurologic conditions such as multiple sclerosis or Parkinson’s disease.
FOR MORE INFORMATION, VISIT www.gstirrup.com
PREGNANCY, BIRTH, AND BREASTFEEDING APP
Totally Pregnant, an app for pregnant women and health-care providers, is now partnering with Lamaze International to provide access to online parenting classes for pregnancy, childbirth, and early parenting. By using the Totally Pregnant app, women can personalize their pregnancy experience and clinicians can connect with their patients. Available for iPhone, iPad, Android, and desktop.
FOR MORE INFORMATION, VISIT www.iamtotally.com
PREDICTING IVF SUCCESS
Univfy®offers fertility predictive analytics to help prospective parents understand the probability for the success of in vitro fertilization (IVF) and estimated costs. The PreIVF™ calculator helps to decide whether or when to start IVF; the PredictIVF™ helps determine if another IVF cycle is the right option. The IVF Cost Calculator offers a cost comparison tailored to the patient’s IVF success rate. Your Fertility™ is an interactive multimedia blog offering educational material.
FOR MORE INFORMATION, VISIT www.univfy.com
SECURE WAY TO CARRY MEDS
FusionWrap is a waist/hip wrap with two 7-inch pockets to carry small personal belongings. Besides providing secure space for identification and money, it also allows those with asthma, diabetes, allergies, or other chronic diseases to carry medications at all times. Made of stretch fabric that is moisture wicking and antimicrobial, FusionWrap comes in various colors and sizes for women, men, and children.
FOR MORE INFORMATION, VISIT www.fusion-wrap.com
BREAST IMAGING TOOLS
Volpara Solutions offers multiple volumetric breast-imaging software tools designed to improve clinical decision making and the early detection of breast cancer. Volpara®Density™ is a breast-density assessment tool available for clinical use with 2D mammography and digital breast tomosynthesis (3D mammography) platforms from multiple manufacturers.
FOR MORE INFORMATION, VISIT www.volparadensity.com
SMOKE EVACUATION PENCIL
The PlumePen® Elite is an electrosurgical smoke pencil with a compact, ergonomic design that is smaller but offers more flow than competitive smoke pencils, claims Buffalo Filter. The adjustable capture port allows for optimum visibility regardless of blade length. The one-piece molded design prevents buttons from sticking and improves grip. The PlumePen Elite connects to most surgical plume evacuators and generators.
FOR MORE INFORMATION, VISIT www.buffalofilter.com
SURGICAL CO2 LASER
Lumenis designed the AcuPulse Smart CO2 Laser with SurgiTouch™ Automation System for tissue ablation during gynecologic surgery to increase speed, precision, and convenience over other electrosurgical devices. Robotic, computer-controlled laser-beam movement provides more precision than hand-held lasers and offers reproducible outcomes. Brief laser-tissue interaction reduces thermal damage.
FOR MORE INFORMATION, VISIT www.lumenis.com
REUSABLE FORNIX FOR LAP HYSTERECTOMY
The Banyan Colpo-Port Vaginal Fornix Delineator is a reusable uterine elevator/vaginal fornix delineator for laparoscopic hysterectomy. Inserted vaginally, the distal tip has a canted, beveled cup that fits securely in the vaginal fornix. The Calibrated Uterine Elevator (CUE) passes through the inner diameter of the delineator body, through the cervix, and into the uterine cavity. The CUE can be locked at preset depths to prevent uterine perforation. The device is easy to clean using standard sterilization procedures.
FOR MORE INFORMATION, VISIT www.banyanmedllc.com
OVARIAN MALIGNANCY ALGORITHM
The Risk of Ovarian Malignancy Algorithm (ROMA™) from Fujirebio is a quantitative serum test intended to assess the risk of finding malignancy at surgery in a premenopausal or postmenopausal woman with an ovarian mass. ROMA, a risk stratification tool, combines the results of human epididymis protein 4 (HE4), cancer antigen 125 (CA125), and menopausal status into a numerical score.
FOR MORE INFORMATION, VISIT www.he4test.com
PORT-SITE CLOSURE DEVICE
neoClose® AnchorGuide facilitates rapid trocar exchange and precise AutoAnchor placement to help prevent port-site hernia. The AnchorGuide design facilitates the delivery of absorbable AutoAnchors through soft tissue during surgery; allows for the VectorX method of port approximation for reduced tension at wound sites; and helps desufflate to remove CO2 at the end of surgery. AnchorGuide comes in 8–15 mm and 5–12 mm sizes compatible with 8 mm robotic ports.
FOR MORE INFORMATION, VISIT www.neosurgical.com
LITHOTOMY STIRRUPS FOR PATIENT COMFORT
Frontier Medical Innovations says its new GStirrup® is designed to provide patients stable and comfortable foot positioning for extended periods of time during office-based surgical procedures. The GStirrup is a pair of cushioned lithotomy boots that attach to examination-table foot rests. Straps secure the patient’s feet and legs; weights in the base make it difficult for the patient to lift the boot off the footrest. GStirrups fit most footrests and are helpful for patients who have hip or knee replacement, low back pain, arthritis, or neurologic conditions such as multiple sclerosis or Parkinson’s disease.
FOR MORE INFORMATION, VISIT www.gstirrup.com
PREGNANCY, BIRTH, AND BREASTFEEDING APP
Totally Pregnant, an app for pregnant women and health-care providers, is now partnering with Lamaze International to provide access to online parenting classes for pregnancy, childbirth, and early parenting. By using the Totally Pregnant app, women can personalize their pregnancy experience and clinicians can connect with their patients. Available for iPhone, iPad, Android, and desktop.
FOR MORE INFORMATION, VISIT www.iamtotally.com
PREDICTING IVF SUCCESS
Univfy®offers fertility predictive analytics to help prospective parents understand the probability for the success of in vitro fertilization (IVF) and estimated costs. The PreIVF™ calculator helps to decide whether or when to start IVF; the PredictIVF™ helps determine if another IVF cycle is the right option. The IVF Cost Calculator offers a cost comparison tailored to the patient’s IVF success rate. Your Fertility™ is an interactive multimedia blog offering educational material.
FOR MORE INFORMATION, VISIT www.univfy.com
SECURE WAY TO CARRY MEDS
FusionWrap is a waist/hip wrap with two 7-inch pockets to carry small personal belongings. Besides providing secure space for identification and money, it also allows those with asthma, diabetes, allergies, or other chronic diseases to carry medications at all times. Made of stretch fabric that is moisture wicking and antimicrobial, FusionWrap comes in various colors and sizes for women, men, and children.
FOR MORE INFORMATION, VISIT www.fusion-wrap.com
BREAST IMAGING TOOLS
Volpara Solutions offers multiple volumetric breast-imaging software tools designed to improve clinical decision making and the early detection of breast cancer. Volpara®Density™ is a breast-density assessment tool available for clinical use with 2D mammography and digital breast tomosynthesis (3D mammography) platforms from multiple manufacturers.
FOR MORE INFORMATION, VISIT www.volparadensity.com
SMOKE EVACUATION PENCIL
The PlumePen® Elite is an electrosurgical smoke pencil with a compact, ergonomic design that is smaller but offers more flow than competitive smoke pencils, claims Buffalo Filter. The adjustable capture port allows for optimum visibility regardless of blade length. The one-piece molded design prevents buttons from sticking and improves grip. The PlumePen Elite connects to most surgical plume evacuators and generators.
FOR MORE INFORMATION, VISIT www.buffalofilter.com
SURGICAL CO2 LASER
Lumenis designed the AcuPulse Smart CO2 Laser with SurgiTouch™ Automation System for tissue ablation during gynecologic surgery to increase speed, precision, and convenience over other electrosurgical devices. Robotic, computer-controlled laser-beam movement provides more precision than hand-held lasers and offers reproducible outcomes. Brief laser-tissue interaction reduces thermal damage.
FOR MORE INFORMATION, VISIT www.lumenis.com
REUSABLE FORNIX FOR LAP HYSTERECTOMY
The Banyan Colpo-Port Vaginal Fornix Delineator is a reusable uterine elevator/vaginal fornix delineator for laparoscopic hysterectomy. Inserted vaginally, the distal tip has a canted, beveled cup that fits securely in the vaginal fornix. The Calibrated Uterine Elevator (CUE) passes through the inner diameter of the delineator body, through the cervix, and into the uterine cavity. The CUE can be locked at preset depths to prevent uterine perforation. The device is easy to clean using standard sterilization procedures.
FOR MORE INFORMATION, VISIT www.banyanmedllc.com
OVARIAN MALIGNANCY ALGORITHM
The Risk of Ovarian Malignancy Algorithm (ROMA™) from Fujirebio is a quantitative serum test intended to assess the risk of finding malignancy at surgery in a premenopausal or postmenopausal woman with an ovarian mass. ROMA, a risk stratification tool, combines the results of human epididymis protein 4 (HE4), cancer antigen 125 (CA125), and menopausal status into a numerical score.
FOR MORE INFORMATION, VISIT www.he4test.com
PORT-SITE CLOSURE DEVICE
neoClose® AnchorGuide facilitates rapid trocar exchange and precise AutoAnchor placement to help prevent port-site hernia. The AnchorGuide design facilitates the delivery of absorbable AutoAnchors through soft tissue during surgery; allows for the VectorX method of port approximation for reduced tension at wound sites; and helps desufflate to remove CO2 at the end of surgery. AnchorGuide comes in 8–15 mm and 5–12 mm sizes compatible with 8 mm robotic ports.
FOR MORE INFORMATION, VISIT www.neosurgical.com