User login
Navigating new ACR/CHEST guidelines for screening, monitoring, and treatment of SARD-ILD
Interstitial lung disease (ILD) is a frequent complication of systemic autoimmune rheumatic diseases (SARDs) associated with considerable morbidity and mortality.1 The risk of ILD, however, is higher in a subset of SARDs—rheumatoid arthritis (RA), systemic sclerosis (SSc), idiopathic inflammatory myopathies (IIMs), mixed connective tissue disease (MCTD), and Sjögren’s disease (SjD). Accordingly, the American College of Rheumatology (ACR) and American College of Chest Physicians (CHEST) jointly endorsed the recent publication of two separate guidelines detailing recommendations for (1) screening and monitoring and (2) treatment of ILD in adults with SARDs.2,3 These guidelines mark the first of their kind, aiming to promote multidisciplinary collaboration and comprehensive, standardized care. Below, we summarize the major highlights from these guidelines.
Screening and monitoring
For patients with SARD, who should be screened for ILD and how?
The prevalence of ILD is not equally distributed amongst those with SARDs, and the heterogeneity poses a challenge when creating guidelines applicable to all.4 The ACR/CHEST guidelines focus on recommendations for those with SARDs at highest risk of ILD (RA, SSc, IIM, MCTD, and SjD), while excluding pediatric SARDs, sarcoidosis, interstitial pneumonia with autoimmune features, vasculitides, systemic lupus erythematosus, and unclassifiable ILD.2,3 As the guidelines’ recommendations are all conditional and based on low-quality evidence, an individualized ILD screening approach should be implemented for patients with SARDs with regard to risk.
For patients with these high-risk SARDs, screening for ILD with pulmonary function testing (PFT) and high-resolution chest tomography (HRCT) is conditionally recommended at the time of diagnosis. This recommendation was founded on observational studies showing PFTs have low sensitivity and high specificity while HRCT has high sensitivity and low specificity for detection of ILD. The combination was also favored, as it provides complementary information on functional impact (PFTs) and radiologic pattern (HRCT).
The guideline committee conditionally recommended against several routine tests due to poor performance—chest radiography, six-minute walk distance, ambulatory desaturation testing, and bronchoscopy. There was a strong recommendation against pursuing surgical lung biopsy due to high-quality evidence for harm and low-quality evidence for benefit. If initial screening is negative, repeat screening is left to the discretion of the treating physician; nevertheless, for patients with high-risk features, yearly rescreening should be considered through shared decision-making.
How should patients with SARD-ILD be monitored?
Disease monitoring following a SARD-ILD diagnosis is important. PFTs and HRCT were conditionally recommended over PFTs alone; however, the consensus was that HRCT should be less frequent than PFTs. Ambulatory desaturation monitoring was also conditionally recommended. The committee conditionally recommended against chest radiography, six-minute walk distance, and bronchoscopy for screening.
The frequency of monitoring should be guided by patient symptoms, risk profile, and treatment response due to substantial clinical variation. For this reason, the committee made suggestions only to steer clinicians. For patients with IIM-ILD and SSc-ILD, more frequent PFT monitoring was suggested given the high risk of early, aggressive disease. For all SARD-ILDs, more frequent PFT monitoring was suggested early after diagnosis; less frequent testing should be considered for those with stable disease. No suggestion regarding the frequency of monitoring with HRCT was made; however, HRCT may be useful as a complementary test to PFTs in situations of uncertainty.
Treatment
First-line treatment
What are considerations when using glucocorticoids in patients with SARD-ILD?
The decision to treat SARD-ILD should incorporate patient symptoms, disease activity, risk of progression, and goals of care. For almost all SARD-ILDs, short-term glucocorticoids (ie, <3 months) are considered first-line treatment. The exception is SSc-ILD, for which there is a strong recommendation against glucocorticoids as first-line therapy due to concern for precipitating scleroderma renal crisis. Similarly, glucocorticoids should be used cautiously in those patients with MCTD and SSc features or IIM-ILD with SSc antibodies, though they are not strictly contraindicated.
What are the recommended options for a steroid-sparing approach?
An important goal in the treatment of SARD-ILD is tapering off glucocorticoids to avoid toxicity. Steroid-sparing is used for those requiring long-term immunosuppression. Considerations when choosing steroid-sparing agents include contraindications, side-effect profile, and effect on active extrapulmonary symptoms.
The committee conditionally recommended a hierarchy of first-line steroid-sparing agents via a voting consensus. Mycophenolate was conditionally recommended as the preferred agent in all SARD-ILDs for several reasons: (1) positive outcomes in trials of SSc-ILD, (2) additional limited data in other SARDs, (3) favorable side-effect profile, and (4) physicians’ familiarity. Multiple other first-line agents were recommended by disease type. These are summarized in Figure 1.
Progression on first-line treatment
What are considerations for patients with progression despite first-line ILD treatment?
The goal of first-line treatment is to improve or stabilize lung function and symptoms. Unfortunately, some patients with SARD-ILD will progress despite appropriate first-line therapy. Progression of ILD was defined using criteria from the INBUILD trial—a decline in FVC >10% predicted or a FVC decline between 5% and 10% accompanied by worsening respiratory symptoms or radiologic fibrosis within a 24-month period.5 When progression is diagnosed, the goal is to add on or switch to an agent based on patient-specific factors or preferences.
Short-term steroids may have a role, particularly if a patient is experiencing an acute exacerbation; however, long-term steroid therapy (at least three to six months) is not recommended. For those who are on full-dose, first-line therapy but still progressing, addition of an alternative agent should be considered. In some instances, addition of an antifibrotic agent is recommended. If progression continues despite multiple agents, referral for lung transplantation should be discussed.
What are some of the management options for patients with rapidly progressive ILD?
Rapidly progressive (RP)-ILD is considered when a patient exhibits rapid progression in supplemental oxygen needs within days to weeks without an alternative cause. First-line treatment is typically pulse IV methylprednisolone in addition to one to two other immunosuppressive medications; nonsteroidal immunosuppressive options include rituximab, cyclophosphamide, IV immunoglobulin, tacrolimus, mycophenolate, or Janus kinase inhibitors. The guidelines conditionally recommend double or triple therapy for most patients with SARD and RP-ILD (combination of steroids and one or two of the listed agents). For patients with confirmed or suspected anti-melanoma differentiation-associated gene 5 (MDA-5) RP-ILD, triple therapy is conditionally recommended (steroids and two additional agents) due to substantial risk of death. Of note, for patients with SSc and RP-ILD, there is no consensus on whether corticosteroids should be used. Treatment selection ultimately depends on disease severity, concern for infection, and suspected or confirmed MDA-5 RP-ILD. Finally, the committee recommended early referral for lung transplantation for patients whose disease progresses while on optimal medical treatment.
Conclusion
SARDs represent a diverse group of rheumatologic diseases associated with high risk of ILD. The ACR/CHEST guidelines are a first attempt to provide clinicians with evidence-based recommendations for screening, monitoring, and treatment of SARD-ILD. They represent an essential tool for management of SARD-ILD . The studies utilized to create them were mostly observational, and none had examined the relationship between disease screening, monitoring, and patient-centered outcomes. As a result, the recommendations are largely conditional. Additional studies are needed to examine the impact of surveillance in different populations, determine risk factors for RP-ILD in patients with SARD, and further investigate the most effective treatments.
Dr. Castellanos and Dr. Esposito are with the Division of Pulmonary and Critical Care, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL. Dr. Zhao is with the Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL.
References
1. Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380(9842):689-698.
2. Johnson SR, Bernstein EJ, Bolster MB, et al. 2023 American College of Rheumatology (ACR)/American College of Chest Physicians (CHEST) guideline for the screening and monitoring of interstitial lung disease in people with systemic autoimmune rheumatic diseases. Arthritis Care Res. 2024;76(8):1070-1082.
3. Johnson SR, Bernstein EJ, Bolster MB, et al. 2023 American College of Rheumatology (ACR)/American College of Chest Physicians (CHEST) guideline for the treatment of interstitial lung disease in people with systemic autoimmune rheumatic diseases. Arthritis Care Res. 2024;76(8):1051-1069.
4. Jeganathan N, Sathananthan M. Connective tissue disease-related interstitial lung disease: prevalence, patterns, predictors, prognosis, and treatment. Lung. 2020;198(5):735-759.
5. Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718-1727.
Interstitial lung disease (ILD) is a frequent complication of systemic autoimmune rheumatic diseases (SARDs) associated with considerable morbidity and mortality.1 The risk of ILD, however, is higher in a subset of SARDs—rheumatoid arthritis (RA), systemic sclerosis (SSc), idiopathic inflammatory myopathies (IIMs), mixed connective tissue disease (MCTD), and Sjögren’s disease (SjD). Accordingly, the American College of Rheumatology (ACR) and American College of Chest Physicians (CHEST) jointly endorsed the recent publication of two separate guidelines detailing recommendations for (1) screening and monitoring and (2) treatment of ILD in adults with SARDs.2,3 These guidelines mark the first of their kind, aiming to promote multidisciplinary collaboration and comprehensive, standardized care. Below, we summarize the major highlights from these guidelines.
Screening and monitoring
For patients with SARD, who should be screened for ILD and how?
The prevalence of ILD is not equally distributed amongst those with SARDs, and the heterogeneity poses a challenge when creating guidelines applicable to all.4 The ACR/CHEST guidelines focus on recommendations for those with SARDs at highest risk of ILD (RA, SSc, IIM, MCTD, and SjD), while excluding pediatric SARDs, sarcoidosis, interstitial pneumonia with autoimmune features, vasculitides, systemic lupus erythematosus, and unclassifiable ILD.2,3 As the guidelines’ recommendations are all conditional and based on low-quality evidence, an individualized ILD screening approach should be implemented for patients with SARDs with regard to risk.
For patients with these high-risk SARDs, screening for ILD with pulmonary function testing (PFT) and high-resolution chest tomography (HRCT) is conditionally recommended at the time of diagnosis. This recommendation was founded on observational studies showing PFTs have low sensitivity and high specificity while HRCT has high sensitivity and low specificity for detection of ILD. The combination was also favored, as it provides complementary information on functional impact (PFTs) and radiologic pattern (HRCT).
The guideline committee conditionally recommended against several routine tests due to poor performance—chest radiography, six-minute walk distance, ambulatory desaturation testing, and bronchoscopy. There was a strong recommendation against pursuing surgical lung biopsy due to high-quality evidence for harm and low-quality evidence for benefit. If initial screening is negative, repeat screening is left to the discretion of the treating physician; nevertheless, for patients with high-risk features, yearly rescreening should be considered through shared decision-making.
How should patients with SARD-ILD be monitored?
Disease monitoring following a SARD-ILD diagnosis is important. PFTs and HRCT were conditionally recommended over PFTs alone; however, the consensus was that HRCT should be less frequent than PFTs. Ambulatory desaturation monitoring was also conditionally recommended. The committee conditionally recommended against chest radiography, six-minute walk distance, and bronchoscopy for screening.
The frequency of monitoring should be guided by patient symptoms, risk profile, and treatment response due to substantial clinical variation. For this reason, the committee made suggestions only to steer clinicians. For patients with IIM-ILD and SSc-ILD, more frequent PFT monitoring was suggested given the high risk of early, aggressive disease. For all SARD-ILDs, more frequent PFT monitoring was suggested early after diagnosis; less frequent testing should be considered for those with stable disease. No suggestion regarding the frequency of monitoring with HRCT was made; however, HRCT may be useful as a complementary test to PFTs in situations of uncertainty.
Treatment
First-line treatment
What are considerations when using glucocorticoids in patients with SARD-ILD?
The decision to treat SARD-ILD should incorporate patient symptoms, disease activity, risk of progression, and goals of care. For almost all SARD-ILDs, short-term glucocorticoids (ie, <3 months) are considered first-line treatment. The exception is SSc-ILD, for which there is a strong recommendation against glucocorticoids as first-line therapy due to concern for precipitating scleroderma renal crisis. Similarly, glucocorticoids should be used cautiously in those patients with MCTD and SSc features or IIM-ILD with SSc antibodies, though they are not strictly contraindicated.
What are the recommended options for a steroid-sparing approach?
An important goal in the treatment of SARD-ILD is tapering off glucocorticoids to avoid toxicity. Steroid-sparing is used for those requiring long-term immunosuppression. Considerations when choosing steroid-sparing agents include contraindications, side-effect profile, and effect on active extrapulmonary symptoms.
The committee conditionally recommended a hierarchy of first-line steroid-sparing agents via a voting consensus. Mycophenolate was conditionally recommended as the preferred agent in all SARD-ILDs for several reasons: (1) positive outcomes in trials of SSc-ILD, (2) additional limited data in other SARDs, (3) favorable side-effect profile, and (4) physicians’ familiarity. Multiple other first-line agents were recommended by disease type. These are summarized in Figure 1.
Progression on first-line treatment
What are considerations for patients with progression despite first-line ILD treatment?
The goal of first-line treatment is to improve or stabilize lung function and symptoms. Unfortunately, some patients with SARD-ILD will progress despite appropriate first-line therapy. Progression of ILD was defined using criteria from the INBUILD trial—a decline in FVC >10% predicted or a FVC decline between 5% and 10% accompanied by worsening respiratory symptoms or radiologic fibrosis within a 24-month period.5 When progression is diagnosed, the goal is to add on or switch to an agent based on patient-specific factors or preferences.
Short-term steroids may have a role, particularly if a patient is experiencing an acute exacerbation; however, long-term steroid therapy (at least three to six months) is not recommended. For those who are on full-dose, first-line therapy but still progressing, addition of an alternative agent should be considered. In some instances, addition of an antifibrotic agent is recommended. If progression continues despite multiple agents, referral for lung transplantation should be discussed.
What are some of the management options for patients with rapidly progressive ILD?
Rapidly progressive (RP)-ILD is considered when a patient exhibits rapid progression in supplemental oxygen needs within days to weeks without an alternative cause. First-line treatment is typically pulse IV methylprednisolone in addition to one to two other immunosuppressive medications; nonsteroidal immunosuppressive options include rituximab, cyclophosphamide, IV immunoglobulin, tacrolimus, mycophenolate, or Janus kinase inhibitors. The guidelines conditionally recommend double or triple therapy for most patients with SARD and RP-ILD (combination of steroids and one or two of the listed agents). For patients with confirmed or suspected anti-melanoma differentiation-associated gene 5 (MDA-5) RP-ILD, triple therapy is conditionally recommended (steroids and two additional agents) due to substantial risk of death. Of note, for patients with SSc and RP-ILD, there is no consensus on whether corticosteroids should be used. Treatment selection ultimately depends on disease severity, concern for infection, and suspected or confirmed MDA-5 RP-ILD. Finally, the committee recommended early referral for lung transplantation for patients whose disease progresses while on optimal medical treatment.
Conclusion
SARDs represent a diverse group of rheumatologic diseases associated with high risk of ILD. The ACR/CHEST guidelines are a first attempt to provide clinicians with evidence-based recommendations for screening, monitoring, and treatment of SARD-ILD. They represent an essential tool for management of SARD-ILD . The studies utilized to create them were mostly observational, and none had examined the relationship between disease screening, monitoring, and patient-centered outcomes. As a result, the recommendations are largely conditional. Additional studies are needed to examine the impact of surveillance in different populations, determine risk factors for RP-ILD in patients with SARD, and further investigate the most effective treatments.
Dr. Castellanos and Dr. Esposito are with the Division of Pulmonary and Critical Care, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL. Dr. Zhao is with the Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL.
References
1. Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380(9842):689-698.
2. Johnson SR, Bernstein EJ, Bolster MB, et al. 2023 American College of Rheumatology (ACR)/American College of Chest Physicians (CHEST) guideline for the screening and monitoring of interstitial lung disease in people with systemic autoimmune rheumatic diseases. Arthritis Care Res. 2024;76(8):1070-1082.
3. Johnson SR, Bernstein EJ, Bolster MB, et al. 2023 American College of Rheumatology (ACR)/American College of Chest Physicians (CHEST) guideline for the treatment of interstitial lung disease in people with systemic autoimmune rheumatic diseases. Arthritis Care Res. 2024;76(8):1051-1069.
4. Jeganathan N, Sathananthan M. Connective tissue disease-related interstitial lung disease: prevalence, patterns, predictors, prognosis, and treatment. Lung. 2020;198(5):735-759.
5. Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718-1727.
Interstitial lung disease (ILD) is a frequent complication of systemic autoimmune rheumatic diseases (SARDs) associated with considerable morbidity and mortality.1 The risk of ILD, however, is higher in a subset of SARDs—rheumatoid arthritis (RA), systemic sclerosis (SSc), idiopathic inflammatory myopathies (IIMs), mixed connective tissue disease (MCTD), and Sjögren’s disease (SjD). Accordingly, the American College of Rheumatology (ACR) and American College of Chest Physicians (CHEST) jointly endorsed the recent publication of two separate guidelines detailing recommendations for (1) screening and monitoring and (2) treatment of ILD in adults with SARDs.2,3 These guidelines mark the first of their kind, aiming to promote multidisciplinary collaboration and comprehensive, standardized care. Below, we summarize the major highlights from these guidelines.
Screening and monitoring
For patients with SARD, who should be screened for ILD and how?
The prevalence of ILD is not equally distributed amongst those with SARDs, and the heterogeneity poses a challenge when creating guidelines applicable to all.4 The ACR/CHEST guidelines focus on recommendations for those with SARDs at highest risk of ILD (RA, SSc, IIM, MCTD, and SjD), while excluding pediatric SARDs, sarcoidosis, interstitial pneumonia with autoimmune features, vasculitides, systemic lupus erythematosus, and unclassifiable ILD.2,3 As the guidelines’ recommendations are all conditional and based on low-quality evidence, an individualized ILD screening approach should be implemented for patients with SARDs with regard to risk.
For patients with these high-risk SARDs, screening for ILD with pulmonary function testing (PFT) and high-resolution chest tomography (HRCT) is conditionally recommended at the time of diagnosis. This recommendation was founded on observational studies showing PFTs have low sensitivity and high specificity while HRCT has high sensitivity and low specificity for detection of ILD. The combination was also favored, as it provides complementary information on functional impact (PFTs) and radiologic pattern (HRCT).
The guideline committee conditionally recommended against several routine tests due to poor performance—chest radiography, six-minute walk distance, ambulatory desaturation testing, and bronchoscopy. There was a strong recommendation against pursuing surgical lung biopsy due to high-quality evidence for harm and low-quality evidence for benefit. If initial screening is negative, repeat screening is left to the discretion of the treating physician; nevertheless, for patients with high-risk features, yearly rescreening should be considered through shared decision-making.
How should patients with SARD-ILD be monitored?
Disease monitoring following a SARD-ILD diagnosis is important. PFTs and HRCT were conditionally recommended over PFTs alone; however, the consensus was that HRCT should be less frequent than PFTs. Ambulatory desaturation monitoring was also conditionally recommended. The committee conditionally recommended against chest radiography, six-minute walk distance, and bronchoscopy for screening.
The frequency of monitoring should be guided by patient symptoms, risk profile, and treatment response due to substantial clinical variation. For this reason, the committee made suggestions only to steer clinicians. For patients with IIM-ILD and SSc-ILD, more frequent PFT monitoring was suggested given the high risk of early, aggressive disease. For all SARD-ILDs, more frequent PFT monitoring was suggested early after diagnosis; less frequent testing should be considered for those with stable disease. No suggestion regarding the frequency of monitoring with HRCT was made; however, HRCT may be useful as a complementary test to PFTs in situations of uncertainty.
Treatment
First-line treatment
What are considerations when using glucocorticoids in patients with SARD-ILD?
The decision to treat SARD-ILD should incorporate patient symptoms, disease activity, risk of progression, and goals of care. For almost all SARD-ILDs, short-term glucocorticoids (ie, <3 months) are considered first-line treatment. The exception is SSc-ILD, for which there is a strong recommendation against glucocorticoids as first-line therapy due to concern for precipitating scleroderma renal crisis. Similarly, glucocorticoids should be used cautiously in those patients with MCTD and SSc features or IIM-ILD with SSc antibodies, though they are not strictly contraindicated.
What are the recommended options for a steroid-sparing approach?
An important goal in the treatment of SARD-ILD is tapering off glucocorticoids to avoid toxicity. Steroid-sparing is used for those requiring long-term immunosuppression. Considerations when choosing steroid-sparing agents include contraindications, side-effect profile, and effect on active extrapulmonary symptoms.
The committee conditionally recommended a hierarchy of first-line steroid-sparing agents via a voting consensus. Mycophenolate was conditionally recommended as the preferred agent in all SARD-ILDs for several reasons: (1) positive outcomes in trials of SSc-ILD, (2) additional limited data in other SARDs, (3) favorable side-effect profile, and (4) physicians’ familiarity. Multiple other first-line agents were recommended by disease type. These are summarized in Figure 1.
Progression on first-line treatment
What are considerations for patients with progression despite first-line ILD treatment?
The goal of first-line treatment is to improve or stabilize lung function and symptoms. Unfortunately, some patients with SARD-ILD will progress despite appropriate first-line therapy. Progression of ILD was defined using criteria from the INBUILD trial—a decline in FVC >10% predicted or a FVC decline between 5% and 10% accompanied by worsening respiratory symptoms or radiologic fibrosis within a 24-month period.5 When progression is diagnosed, the goal is to add on or switch to an agent based on patient-specific factors or preferences.
Short-term steroids may have a role, particularly if a patient is experiencing an acute exacerbation; however, long-term steroid therapy (at least three to six months) is not recommended. For those who are on full-dose, first-line therapy but still progressing, addition of an alternative agent should be considered. In some instances, addition of an antifibrotic agent is recommended. If progression continues despite multiple agents, referral for lung transplantation should be discussed.
What are some of the management options for patients with rapidly progressive ILD?
Rapidly progressive (RP)-ILD is considered when a patient exhibits rapid progression in supplemental oxygen needs within days to weeks without an alternative cause. First-line treatment is typically pulse IV methylprednisolone in addition to one to two other immunosuppressive medications; nonsteroidal immunosuppressive options include rituximab, cyclophosphamide, IV immunoglobulin, tacrolimus, mycophenolate, or Janus kinase inhibitors. The guidelines conditionally recommend double or triple therapy for most patients with SARD and RP-ILD (combination of steroids and one or two of the listed agents). For patients with confirmed or suspected anti-melanoma differentiation-associated gene 5 (MDA-5) RP-ILD, triple therapy is conditionally recommended (steroids and two additional agents) due to substantial risk of death. Of note, for patients with SSc and RP-ILD, there is no consensus on whether corticosteroids should be used. Treatment selection ultimately depends on disease severity, concern for infection, and suspected or confirmed MDA-5 RP-ILD. Finally, the committee recommended early referral for lung transplantation for patients whose disease progresses while on optimal medical treatment.
Conclusion
SARDs represent a diverse group of rheumatologic diseases associated with high risk of ILD. The ACR/CHEST guidelines are a first attempt to provide clinicians with evidence-based recommendations for screening, monitoring, and treatment of SARD-ILD. They represent an essential tool for management of SARD-ILD . The studies utilized to create them were mostly observational, and none had examined the relationship between disease screening, monitoring, and patient-centered outcomes. As a result, the recommendations are largely conditional. Additional studies are needed to examine the impact of surveillance in different populations, determine risk factors for RP-ILD in patients with SARD, and further investigate the most effective treatments.
Dr. Castellanos and Dr. Esposito are with the Division of Pulmonary and Critical Care, Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL. Dr. Zhao is with the Department of Medicine, Feinberg School of Medicine, Northwestern University, Chicago, IL.
References
1. Fischer A, du Bois R. Interstitial lung disease in connective tissue disorders. Lancet. 2012;380(9842):689-698.
2. Johnson SR, Bernstein EJ, Bolster MB, et al. 2023 American College of Rheumatology (ACR)/American College of Chest Physicians (CHEST) guideline for the screening and monitoring of interstitial lung disease in people with systemic autoimmune rheumatic diseases. Arthritis Care Res. 2024;76(8):1070-1082.
3. Johnson SR, Bernstein EJ, Bolster MB, et al. 2023 American College of Rheumatology (ACR)/American College of Chest Physicians (CHEST) guideline for the treatment of interstitial lung disease in people with systemic autoimmune rheumatic diseases. Arthritis Care Res. 2024;76(8):1051-1069.
4. Jeganathan N, Sathananthan M. Connective tissue disease-related interstitial lung disease: prevalence, patterns, predictors, prognosis, and treatment. Lung. 2020;198(5):735-759.
5. Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med. 2019;381(18):1718-1727.
Pseudomonas infection in patients with noncystic fibrosis bronchiectasis
Pseudomonas aeruginosa is a clinically important organism that infects patients with noncystic fibrosis bronchiectasis (NCFB). In the United States, the estimated prevalence of NCFB is 213 per 100,000 across all age groups and 813 per 100,000 in the over 65 age group.1 A retrospective cohort study suggests the incidence of NCFB as ascertained from International Classification of Diseases codes may significantly underestimate its true prevalence.2
As the incidence of patients with NCFB continues to increase, the impact of the Pseudomonas infection is expected to grow. A recent retrospective cohort study of commercial claims from IQVIA’s PharMetrics Plus database for the period 2006 to 2020 showed that patients with NCFB and Pseudomonas infection had on average 2.58 hospital admissions per year, with a mean length of stay of 9.94 (± 11.06) days, compared with 1.18 admissions per year, with a mean length of stay of 6.5 (± 8.42) days, in patients with Pseudomonas-negative NCFB. The same trend applied to 30-day readmissions and ICU admissions, 1.32 (± 2.51 days) vs 0.47 (± 1.30 days) and 0.95 (± 1.62 days) vs 0.33 (± 0.76 days), respectively. The differential cost of care per patient per year between patients with NCFB with and without Pseudomonas infection ranged from $55,225 to $315,901.3
Recent data from the United States Bronchiectasis Registry showed the probability of acquiring Pseudomonas aeruginosa was 3% annually.4 The prevalence of Pseudomonas infection in a large, geographically diverse cohort in the United States was quoted at 15%.5 A retrospective analysis of the European Bronchiectasis Registry database showed Pseudomonas infection was the most commonly isolated pathogen (21.8%).6
Given the high incidence and prevalence of NCFB, the high prevalence of Pseudomonas infection in patients with NCFB, and the associated costs and morbidity from infection, identifying effective treatments has become a priority. The British, Spanish (SEPAR), South African, and European bronchiectasis guidelines outline several antibiotic regimens meant to achieve eradication. Generally, there is induction with a (1) quinolone, (2) β-lactam + aminoglycoside, or (3) quinolone with an inhaled antibiotic followed by three months of maintenance inhaled antibiotics.7-10 SEPAR allows for retreatment for recurrence at any time during the first year with any regimen.
For chronic Pseudomonas infection, SEPAR recommends treatment with inhaled antibiotics for patients with more than two exacerbations or one hospitalization, while the threshold in the British and European guidelines is more than three exacerbations. Azithromycin may be used for those who are intolerant or allergic to the nebulized antibiotics. It is worth noting that in the United States, the antibiotics colistin, ciprofloxacin, aztreonam, gentamicin, and tobramycin are administered off label for this indication. A systematic review found a 10% rate of bronchospasm in the treated group compared with 2.3% in the control group, and premedication with albuterol is often needed.11
Unfortunately, the data supporting the listed eradication and suppressive regimens are weak. A systematic review and meta-analysis of six observational studies including 289 patients showed a 12-month eradication rate of only 40% (95% CI, 34-45; P < 0.00001; I2 = 0).12 These results are disappointing and identify a need for further research into the manner in which Pseudomonas infection interacts with the host lung.
We currently know Pseudomonas infection evades antibiotics and host defenses by accumulating mutations and deletions. These include loss-of-function mutations in mucA (mucoidy), lasR (quorum-sensing), mexS (regulates the antibiotic efflux pump), and other genes related to the production of the polysaccharides Psl and Pel (which contribute to biofilm formation).13 There may also be differences in low and high bacteria microbial networks that interact differently with host cytokines to create an unstable environment that predisposes to exacerbation.14
In an attempt to improve our eradication and suppression rates, investigators have begun to target specific aspects of Pseudomonas infection behavior. The GREAT-2 trial compares gremubamab (a bivalent, bispecific, monoclonal antibody targeting Psl exopolysaccharide and the type 3 secretion system component of PcrV) with placebo in patients with chronic Pseudomonas infection. A phase II trial with the phosphodiesterase inhibitor esifentrine, a phase III trial with a reversible DPP1 inhibitor called brensocatib (ASPEN), and a phase II trial with the CatC inhibitor BI 1291583 (Airleaf) are also being conducted. Each of these agents targets mediators of neutrophil inflammation.
In summary, NCFB with Pseudomonas infection is common and leads to an increase in costs, respiratory exacerbations, and hospitalizations. While eradication and suppression are recommended, they are difficult to achieve and require sustained durations of expensive medications that can be difficult to tolerate. Antibiotic therapies will continue to be studied (the ERASE randomized controlled trial to investigate the efficacy and safety of tobramycin to eradicate Pseudomonas infection is currently underway), but targeted therapies represent a promising new approach to combating this stubbornly resistant bacteria. The NCFB community will be watching closely to see whether medicines targeting molecular behavior and host interaction can achieve what antibiotic regimens thus far have not: consistent and sustainable eradication.
Dr. Green is Assistant Professor in Medicine, Medical Director, Bronchiectasis Program, UMass Chan/Baystate Health, Chest Infections Section, Member-at-Large
References
1. Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis. 2017;14(4):377-384. doi: 10.1177/1479972317709649
2. Green O, Liautaud S, Knee A, Modahl L. Measuring accuracy of International Classification of Diseases codes in identification of patients with non-cystic fibrosis bronchiectasis. ERJ Open Res. 2024;10(2):00715-2023. doi: 10.1183/23120541.00715-2023
3. Franklin M, Minshall ME, Pontenani F, Devarajan S. Impact of Pseudomonas aeruginosa on resource utilization and costs in patients with exacerbated non-cystic fibrosis bronchiectasis. J Med Econ. 2024;27(1):671-677. doi: 10.1080/13696998.2024.2340382
4. Aksamit TR, Locantore N, Addrizzo-Harris D, et al. Five-year outcomes among U.S. bronchiectasis and NTM research registry patients. Am J Respir Crit Care Med. Accepted manuscript. Published online April 26, 2024.
5. Dean SG, Blakney RA, Ricotta EE, et al. Bronchiectasis-associated infections and outcomes in a large, geographically diverse electronic health record cohort in the United States. BMC Pulm Med. 2024;24(1):172. doi: 10.1186/s12890-024-02973-3
6. Chalmers JD, Polverino E, Crichton ML, et al. Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis registry (EMBARC). Lancet Respir Med. 2023;11(7):637-649. doi: 10.1016/S2213-2600(23)00093-0
7. Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. doi: 10.1183/13993003.00629-2017
8. Martínez-García MÁ, Máiz L, Olveira C, et al. Spanish guidelines on treatment of bronchiectasis in adults. Arch Bronconeumol. 2018;54(2):88-98. doi: 10.1016/j.arbres.2017.07.016
9. Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society guideline for bronchiectasis in adults. Thorax. 2019;74(Suppl 1):1-69. doi: 10.1136/thoraxjnl-2018-212463
10. Goolam Mahomed A, Maasdorp SD, Barnes R, et al. South African Thoracic Society position statement on the management of non-cystic fibrosis bronchiectasis in adults: 2023. Afr J Thorac Crit Care Med. 2023;29(2):10.7196/AJTCCM. 2023.v29i2.647. doi: 10.7196/AJTCCM.2023.v29i2.647
11. Brodt AM, Stovold E, Zhang L. Inhaled antibiotics for stable non-cystic fibrosis bronchiectasis: a systematic review. Eur Respir J. 2014;44(2):382-393. doi: 10.1183/09031936.00018414
12. Conceição M, Shteinberg M, Goeminne P, Altenburg J, Chalmers JD. Eradication treatment for Pseudomonas aeruginosa infection in adults with bronchiectasis: a systematic review and meta-analysis. Eur Respir Rev. 2024;33(171):230178. doi: 10.1183/16000617.0178-2023
13. Hilliam Y, Moore MP, Lamont IL, et al. Pseudomonas aeruginosa adaptation and diversification in the non-cystic fibrosis bronchiectasis lung. Eur Respir J. 2017;49(4):1602108. doi: 10.1183/13993003.02108-2016
14. Gramegna A, Kumar Narayana J, Amati F, et al. Microbial inflammatory networks in bronchiectasis exacerbators with Pseudomonas aeruginosa. Chest. 2023;164(1):65-68. doi: 10.1016/j.chest.2023.02.014
Pseudomonas aeruginosa is a clinically important organism that infects patients with noncystic fibrosis bronchiectasis (NCFB). In the United States, the estimated prevalence of NCFB is 213 per 100,000 across all age groups and 813 per 100,000 in the over 65 age group.1 A retrospective cohort study suggests the incidence of NCFB as ascertained from International Classification of Diseases codes may significantly underestimate its true prevalence.2
As the incidence of patients with NCFB continues to increase, the impact of the Pseudomonas infection is expected to grow. A recent retrospective cohort study of commercial claims from IQVIA’s PharMetrics Plus database for the period 2006 to 2020 showed that patients with NCFB and Pseudomonas infection had on average 2.58 hospital admissions per year, with a mean length of stay of 9.94 (± 11.06) days, compared with 1.18 admissions per year, with a mean length of stay of 6.5 (± 8.42) days, in patients with Pseudomonas-negative NCFB. The same trend applied to 30-day readmissions and ICU admissions, 1.32 (± 2.51 days) vs 0.47 (± 1.30 days) and 0.95 (± 1.62 days) vs 0.33 (± 0.76 days), respectively. The differential cost of care per patient per year between patients with NCFB with and without Pseudomonas infection ranged from $55,225 to $315,901.3
Recent data from the United States Bronchiectasis Registry showed the probability of acquiring Pseudomonas aeruginosa was 3% annually.4 The prevalence of Pseudomonas infection in a large, geographically diverse cohort in the United States was quoted at 15%.5 A retrospective analysis of the European Bronchiectasis Registry database showed Pseudomonas infection was the most commonly isolated pathogen (21.8%).6
Given the high incidence and prevalence of NCFB, the high prevalence of Pseudomonas infection in patients with NCFB, and the associated costs and morbidity from infection, identifying effective treatments has become a priority. The British, Spanish (SEPAR), South African, and European bronchiectasis guidelines outline several antibiotic regimens meant to achieve eradication. Generally, there is induction with a (1) quinolone, (2) β-lactam + aminoglycoside, or (3) quinolone with an inhaled antibiotic followed by three months of maintenance inhaled antibiotics.7-10 SEPAR allows for retreatment for recurrence at any time during the first year with any regimen.
For chronic Pseudomonas infection, SEPAR recommends treatment with inhaled antibiotics for patients with more than two exacerbations or one hospitalization, while the threshold in the British and European guidelines is more than three exacerbations. Azithromycin may be used for those who are intolerant or allergic to the nebulized antibiotics. It is worth noting that in the United States, the antibiotics colistin, ciprofloxacin, aztreonam, gentamicin, and tobramycin are administered off label for this indication. A systematic review found a 10% rate of bronchospasm in the treated group compared with 2.3% in the control group, and premedication with albuterol is often needed.11
Unfortunately, the data supporting the listed eradication and suppressive regimens are weak. A systematic review and meta-analysis of six observational studies including 289 patients showed a 12-month eradication rate of only 40% (95% CI, 34-45; P < 0.00001; I2 = 0).12 These results are disappointing and identify a need for further research into the manner in which Pseudomonas infection interacts with the host lung.
We currently know Pseudomonas infection evades antibiotics and host defenses by accumulating mutations and deletions. These include loss-of-function mutations in mucA (mucoidy), lasR (quorum-sensing), mexS (regulates the antibiotic efflux pump), and other genes related to the production of the polysaccharides Psl and Pel (which contribute to biofilm formation).13 There may also be differences in low and high bacteria microbial networks that interact differently with host cytokines to create an unstable environment that predisposes to exacerbation.14
In an attempt to improve our eradication and suppression rates, investigators have begun to target specific aspects of Pseudomonas infection behavior. The GREAT-2 trial compares gremubamab (a bivalent, bispecific, monoclonal antibody targeting Psl exopolysaccharide and the type 3 secretion system component of PcrV) with placebo in patients with chronic Pseudomonas infection. A phase II trial with the phosphodiesterase inhibitor esifentrine, a phase III trial with a reversible DPP1 inhibitor called brensocatib (ASPEN), and a phase II trial with the CatC inhibitor BI 1291583 (Airleaf) are also being conducted. Each of these agents targets mediators of neutrophil inflammation.
In summary, NCFB with Pseudomonas infection is common and leads to an increase in costs, respiratory exacerbations, and hospitalizations. While eradication and suppression are recommended, they are difficult to achieve and require sustained durations of expensive medications that can be difficult to tolerate. Antibiotic therapies will continue to be studied (the ERASE randomized controlled trial to investigate the efficacy and safety of tobramycin to eradicate Pseudomonas infection is currently underway), but targeted therapies represent a promising new approach to combating this stubbornly resistant bacteria. The NCFB community will be watching closely to see whether medicines targeting molecular behavior and host interaction can achieve what antibiotic regimens thus far have not: consistent and sustainable eradication.
Dr. Green is Assistant Professor in Medicine, Medical Director, Bronchiectasis Program, UMass Chan/Baystate Health, Chest Infections Section, Member-at-Large
References
1. Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis. 2017;14(4):377-384. doi: 10.1177/1479972317709649
2. Green O, Liautaud S, Knee A, Modahl L. Measuring accuracy of International Classification of Diseases codes in identification of patients with non-cystic fibrosis bronchiectasis. ERJ Open Res. 2024;10(2):00715-2023. doi: 10.1183/23120541.00715-2023
3. Franklin M, Minshall ME, Pontenani F, Devarajan S. Impact of Pseudomonas aeruginosa on resource utilization and costs in patients with exacerbated non-cystic fibrosis bronchiectasis. J Med Econ. 2024;27(1):671-677. doi: 10.1080/13696998.2024.2340382
4. Aksamit TR, Locantore N, Addrizzo-Harris D, et al. Five-year outcomes among U.S. bronchiectasis and NTM research registry patients. Am J Respir Crit Care Med. Accepted manuscript. Published online April 26, 2024.
5. Dean SG, Blakney RA, Ricotta EE, et al. Bronchiectasis-associated infections and outcomes in a large, geographically diverse electronic health record cohort in the United States. BMC Pulm Med. 2024;24(1):172. doi: 10.1186/s12890-024-02973-3
6. Chalmers JD, Polverino E, Crichton ML, et al. Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis registry (EMBARC). Lancet Respir Med. 2023;11(7):637-649. doi: 10.1016/S2213-2600(23)00093-0
7. Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. doi: 10.1183/13993003.00629-2017
8. Martínez-García MÁ, Máiz L, Olveira C, et al. Spanish guidelines on treatment of bronchiectasis in adults. Arch Bronconeumol. 2018;54(2):88-98. doi: 10.1016/j.arbres.2017.07.016
9. Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society guideline for bronchiectasis in adults. Thorax. 2019;74(Suppl 1):1-69. doi: 10.1136/thoraxjnl-2018-212463
10. Goolam Mahomed A, Maasdorp SD, Barnes R, et al. South African Thoracic Society position statement on the management of non-cystic fibrosis bronchiectasis in adults: 2023. Afr J Thorac Crit Care Med. 2023;29(2):10.7196/AJTCCM. 2023.v29i2.647. doi: 10.7196/AJTCCM.2023.v29i2.647
11. Brodt AM, Stovold E, Zhang L. Inhaled antibiotics for stable non-cystic fibrosis bronchiectasis: a systematic review. Eur Respir J. 2014;44(2):382-393. doi: 10.1183/09031936.00018414
12. Conceição M, Shteinberg M, Goeminne P, Altenburg J, Chalmers JD. Eradication treatment for Pseudomonas aeruginosa infection in adults with bronchiectasis: a systematic review and meta-analysis. Eur Respir Rev. 2024;33(171):230178. doi: 10.1183/16000617.0178-2023
13. Hilliam Y, Moore MP, Lamont IL, et al. Pseudomonas aeruginosa adaptation and diversification in the non-cystic fibrosis bronchiectasis lung. Eur Respir J. 2017;49(4):1602108. doi: 10.1183/13993003.02108-2016
14. Gramegna A, Kumar Narayana J, Amati F, et al. Microbial inflammatory networks in bronchiectasis exacerbators with Pseudomonas aeruginosa. Chest. 2023;164(1):65-68. doi: 10.1016/j.chest.2023.02.014
Pseudomonas aeruginosa is a clinically important organism that infects patients with noncystic fibrosis bronchiectasis (NCFB). In the United States, the estimated prevalence of NCFB is 213 per 100,000 across all age groups and 813 per 100,000 in the over 65 age group.1 A retrospective cohort study suggests the incidence of NCFB as ascertained from International Classification of Diseases codes may significantly underestimate its true prevalence.2
As the incidence of patients with NCFB continues to increase, the impact of the Pseudomonas infection is expected to grow. A recent retrospective cohort study of commercial claims from IQVIA’s PharMetrics Plus database for the period 2006 to 2020 showed that patients with NCFB and Pseudomonas infection had on average 2.58 hospital admissions per year, with a mean length of stay of 9.94 (± 11.06) days, compared with 1.18 admissions per year, with a mean length of stay of 6.5 (± 8.42) days, in patients with Pseudomonas-negative NCFB. The same trend applied to 30-day readmissions and ICU admissions, 1.32 (± 2.51 days) vs 0.47 (± 1.30 days) and 0.95 (± 1.62 days) vs 0.33 (± 0.76 days), respectively. The differential cost of care per patient per year between patients with NCFB with and without Pseudomonas infection ranged from $55,225 to $315,901.3
Recent data from the United States Bronchiectasis Registry showed the probability of acquiring Pseudomonas aeruginosa was 3% annually.4 The prevalence of Pseudomonas infection in a large, geographically diverse cohort in the United States was quoted at 15%.5 A retrospective analysis of the European Bronchiectasis Registry database showed Pseudomonas infection was the most commonly isolated pathogen (21.8%).6
Given the high incidence and prevalence of NCFB, the high prevalence of Pseudomonas infection in patients with NCFB, and the associated costs and morbidity from infection, identifying effective treatments has become a priority. The British, Spanish (SEPAR), South African, and European bronchiectasis guidelines outline several antibiotic regimens meant to achieve eradication. Generally, there is induction with a (1) quinolone, (2) β-lactam + aminoglycoside, or (3) quinolone with an inhaled antibiotic followed by three months of maintenance inhaled antibiotics.7-10 SEPAR allows for retreatment for recurrence at any time during the first year with any regimen.
For chronic Pseudomonas infection, SEPAR recommends treatment with inhaled antibiotics for patients with more than two exacerbations or one hospitalization, while the threshold in the British and European guidelines is more than three exacerbations. Azithromycin may be used for those who are intolerant or allergic to the nebulized antibiotics. It is worth noting that in the United States, the antibiotics colistin, ciprofloxacin, aztreonam, gentamicin, and tobramycin are administered off label for this indication. A systematic review found a 10% rate of bronchospasm in the treated group compared with 2.3% in the control group, and premedication with albuterol is often needed.11
Unfortunately, the data supporting the listed eradication and suppressive regimens are weak. A systematic review and meta-analysis of six observational studies including 289 patients showed a 12-month eradication rate of only 40% (95% CI, 34-45; P < 0.00001; I2 = 0).12 These results are disappointing and identify a need for further research into the manner in which Pseudomonas infection interacts with the host lung.
We currently know Pseudomonas infection evades antibiotics and host defenses by accumulating mutations and deletions. These include loss-of-function mutations in mucA (mucoidy), lasR (quorum-sensing), mexS (regulates the antibiotic efflux pump), and other genes related to the production of the polysaccharides Psl and Pel (which contribute to biofilm formation).13 There may also be differences in low and high bacteria microbial networks that interact differently with host cytokines to create an unstable environment that predisposes to exacerbation.14
In an attempt to improve our eradication and suppression rates, investigators have begun to target specific aspects of Pseudomonas infection behavior. The GREAT-2 trial compares gremubamab (a bivalent, bispecific, monoclonal antibody targeting Psl exopolysaccharide and the type 3 secretion system component of PcrV) with placebo in patients with chronic Pseudomonas infection. A phase II trial with the phosphodiesterase inhibitor esifentrine, a phase III trial with a reversible DPP1 inhibitor called brensocatib (ASPEN), and a phase II trial with the CatC inhibitor BI 1291583 (Airleaf) are also being conducted. Each of these agents targets mediators of neutrophil inflammation.
In summary, NCFB with Pseudomonas infection is common and leads to an increase in costs, respiratory exacerbations, and hospitalizations. While eradication and suppression are recommended, they are difficult to achieve and require sustained durations of expensive medications that can be difficult to tolerate. Antibiotic therapies will continue to be studied (the ERASE randomized controlled trial to investigate the efficacy and safety of tobramycin to eradicate Pseudomonas infection is currently underway), but targeted therapies represent a promising new approach to combating this stubbornly resistant bacteria. The NCFB community will be watching closely to see whether medicines targeting molecular behavior and host interaction can achieve what antibiotic regimens thus far have not: consistent and sustainable eradication.
Dr. Green is Assistant Professor in Medicine, Medical Director, Bronchiectasis Program, UMass Chan/Baystate Health, Chest Infections Section, Member-at-Large
References
1. Weycker D, Hansen GL, Seifer FD. Prevalence and incidence of noncystic fibrosis bronchiectasis among US adults in 2013. Chron Respir Dis. 2017;14(4):377-384. doi: 10.1177/1479972317709649
2. Green O, Liautaud S, Knee A, Modahl L. Measuring accuracy of International Classification of Diseases codes in identification of patients with non-cystic fibrosis bronchiectasis. ERJ Open Res. 2024;10(2):00715-2023. doi: 10.1183/23120541.00715-2023
3. Franklin M, Minshall ME, Pontenani F, Devarajan S. Impact of Pseudomonas aeruginosa on resource utilization and costs in patients with exacerbated non-cystic fibrosis bronchiectasis. J Med Econ. 2024;27(1):671-677. doi: 10.1080/13696998.2024.2340382
4. Aksamit TR, Locantore N, Addrizzo-Harris D, et al. Five-year outcomes among U.S. bronchiectasis and NTM research registry patients. Am J Respir Crit Care Med. Accepted manuscript. Published online April 26, 2024.
5. Dean SG, Blakney RA, Ricotta EE, et al. Bronchiectasis-associated infections and outcomes in a large, geographically diverse electronic health record cohort in the United States. BMC Pulm Med. 2024;24(1):172. doi: 10.1186/s12890-024-02973-3
6. Chalmers JD, Polverino E, Crichton ML, et al. Bronchiectasis in Europe: data on disease characteristics from the European Bronchiectasis registry (EMBARC). Lancet Respir Med. 2023;11(7):637-649. doi: 10.1016/S2213-2600(23)00093-0
7. Polverino E, Goeminne PC, McDonnell MJ, et al. European Respiratory Society guidelines for the management of adult bronchiectasis. Eur Respir J. 2017;50(3):1700629. doi: 10.1183/13993003.00629-2017
8. Martínez-García MÁ, Máiz L, Olveira C, et al. Spanish guidelines on treatment of bronchiectasis in adults. Arch Bronconeumol. 2018;54(2):88-98. doi: 10.1016/j.arbres.2017.07.016
9. Hill AT, Sullivan AL, Chalmers JD, et al. British Thoracic Society guideline for bronchiectasis in adults. Thorax. 2019;74(Suppl 1):1-69. doi: 10.1136/thoraxjnl-2018-212463
10. Goolam Mahomed A, Maasdorp SD, Barnes R, et al. South African Thoracic Society position statement on the management of non-cystic fibrosis bronchiectasis in adults: 2023. Afr J Thorac Crit Care Med. 2023;29(2):10.7196/AJTCCM. 2023.v29i2.647. doi: 10.7196/AJTCCM.2023.v29i2.647
11. Brodt AM, Stovold E, Zhang L. Inhaled antibiotics for stable non-cystic fibrosis bronchiectasis: a systematic review. Eur Respir J. 2014;44(2):382-393. doi: 10.1183/09031936.00018414
12. Conceição M, Shteinberg M, Goeminne P, Altenburg J, Chalmers JD. Eradication treatment for Pseudomonas aeruginosa infection in adults with bronchiectasis: a systematic review and meta-analysis. Eur Respir Rev. 2024;33(171):230178. doi: 10.1183/16000617.0178-2023
13. Hilliam Y, Moore MP, Lamont IL, et al. Pseudomonas aeruginosa adaptation and diversification in the non-cystic fibrosis bronchiectasis lung. Eur Respir J. 2017;49(4):1602108. doi: 10.1183/13993003.02108-2016
14. Gramegna A, Kumar Narayana J, Amati F, et al. Microbial inflammatory networks in bronchiectasis exacerbators with Pseudomonas aeruginosa. Chest. 2023;164(1):65-68. doi: 10.1016/j.chest.2023.02.014
Changing the tumor board conversation: Immunotherapy in resectable NSCLC
Without a doubt, immunotherapy has transformed the treatment landscape of non-small cell lung cancer (NSCLC) and enhanced survival rates across the different stages of disease. High recurrence rates following complete surgical resection prompted the study of immune checkpoint inhibitors (ICI) in earlier, operable stages of disease. This shift toward early application of ICI reflects the larger trend toward merging precision oncology with lung cancer staging. The resulting complexity in treatment and decision making creates systemic and logistical challenges that will require health care systems to adapt and improve.
Adjuvant immunotherapy for NSCLC
Prior to recent approvals for adjuvant immunotherapy, it was standard to give chemotherapy following resection of stage IB-IIIA disease, which offered a statistically nonsignificant survival gain. Recurrence in these patients is believed to be related to postsurgical micrometastasis. The utilization of alternative mechanisms to prevent recurrence is increasingly more common.
Atezolizumab, a PD-L1 inhibitor, is currently approved as first-line adjuvant treatment following chemotherapy in post-NSCLC resection patients with PD-L1 scores ≥1%. This category one recommendation by the National Comprehensive Cancer Network (NCCN) is based on results from the IMpower010 trial, which randomized patients to Atezolizumab vs best supportive care. All were early-stage NSCLC, stage IB-IIIA, who underwent resection followed by platinum-based chemotherapy. Statistically significant benefits were found in disease-free survival (DFS) with a trend toward overall survival.1
The PEARLS/KEYNOTE-091 trial evaluated another PD-L1 inhibitor, Pembrolizumab, as adjuvant therapy. Its design largely mirrored the IMPower010 study, but it differed in that the ICI was administered with or without chemotherapy following resection in patients with stage IB-IIIA NSCLC. Improvements in DFS were found in the overall population, leading to FDA approval for adjuvant therapy in 2023.2
These approvals require changes to the management of operable NSCLC. Until recently, it was not routine to send surgical specimens for additional testing because adjuvant treatment meant chemotherapy only. However, it is now essential that all surgically resected malignant tissue be sent for genomic sequencing and PD-L1 testing. Selecting the next form of therapy, whether it is an ICI or targeted drug therapy, depends on it.
From a surgical perspective, quality surgery with accurate nodal staging is crucial. The surgical findings can determine and identify those who are candidates for adjuvant immunotherapy. For these same reasons, it is helpful to advise surgeons preoperatively that targeted adjuvant therapy is being considered after resection.
Neoadjuvant immunotherapy for NSCLC
ICIs have also been used as neoadjuvant treatment for operable NSCLC. In 2021, the Checkmate-816 trial evaluated Nivolumab with platinum doublet chemotherapy prior to resection of stage IB-IIIa NSCLC. When compared with chemotherapy alone, there were significant improvements in EFS, MPR, and time to death or distant metastasis (TTDM) out to 3 years. At a median follow-up time of 41.4 months, only 28% in the nivolumab group had recurrence postsurgery compared with 42% in the chemotherapy-alone group.3 As a result, certain patients who are likely to receive adjuvant chemotherapy may additionally receive neoadjuvant immunotherapy with chemotherapy before surgical resection. In 2023, the KEYNOTE-671 study demonstrated that neoadjuvant Pembrolizumab and chemotherapy in patients with resectable stage II-IIIb (N2 stage) NSCLC improved EFS. At a median follow-up of 25.2 months, the EFS was 62.4% in the Pembrolizumab group vs 40.6% in the placebo group (P < .001).4
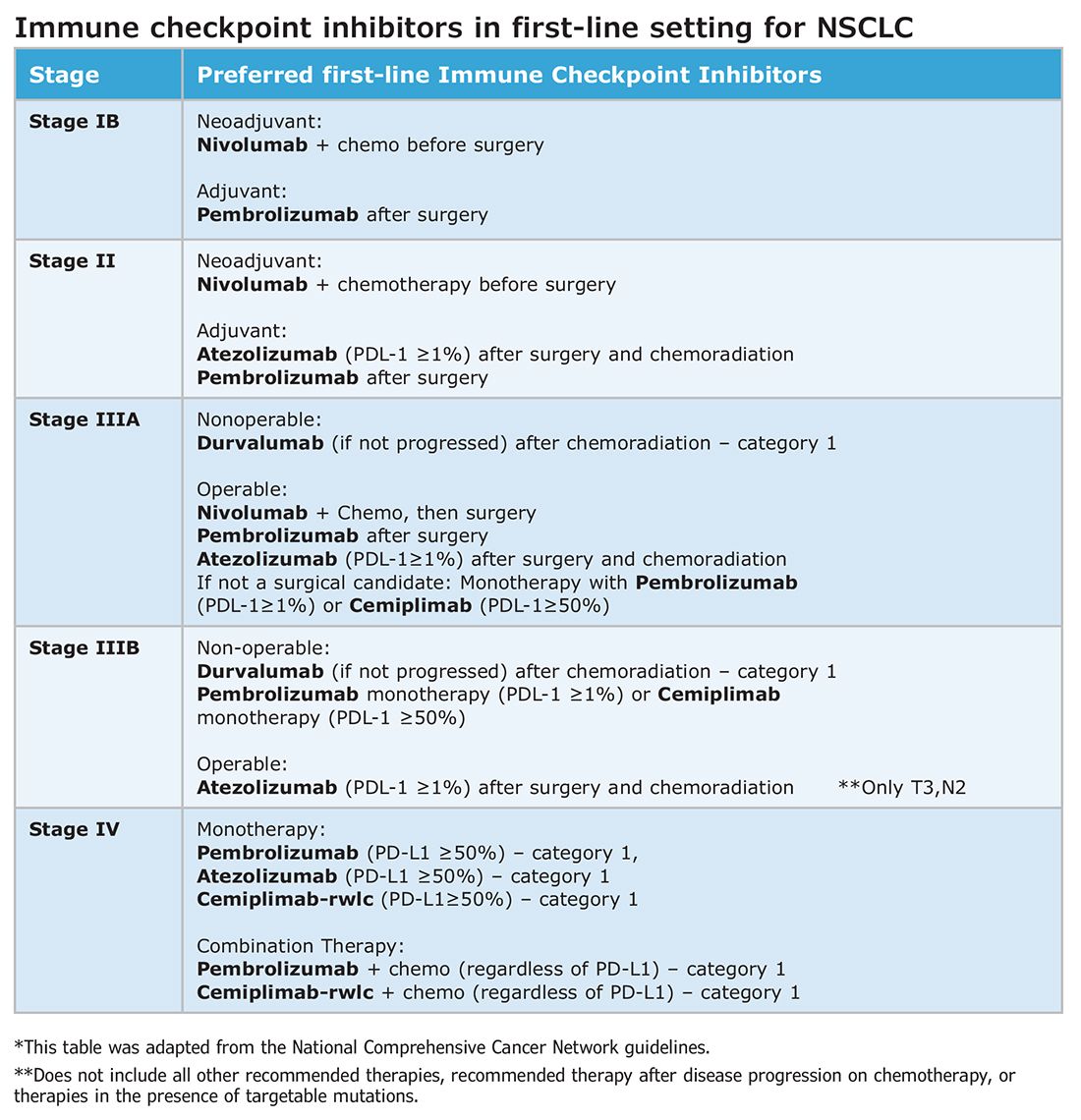
Such changes in treatment options mean patients should be discussed first and simultaneous referrals to oncology and surgery should occur in early-stage NSCLC. Up-front genomic phenotyping and PD-L1 testing may assist in decision making. High PD-L1 levels correlate better with response.
When an ICI-chemotherapy combination is given up front for newly diagnosed NSCLC, there is the potential for large reductions in tumor size and lymph node burden. Although the NCCN does not recommend ICIs to induce resectability, a patient originally deemed inoperable could theoretically become a surgical candidate with neoadjuvant ICI treatment. There is also the potential for toxicity, which could increase the risk of surgery when it does occur. Such scenarios will require frequent tumor board discussions so plans can be adjusted in real time to optimize outcomes as clinical circumstances change.
Perioperative immunotherapy for NSCLC
It is clear that both neoadjuvant and adjuvant immunotherapy can improve outcomes for patients with resectable NSCLC. The combination of neoadjuvant with adjuvant immunotherapy/chemotherapy is currently being studied. Two recent phase III clinical trials, NEOTORCH and AEGAEN, have found statistical improvements in EFS and MPR with this approach.5,6 These studies have not found their way into the NCCN guidelines yet but are sure to be considered in future iterations. Once adopted, the tumor board at each institution will have more options to choose from but many more decisions to make.
References
1. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357. [Published correction appears in Lancet. 2021 Nov 6;398(10312):1686.]
2. O’Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286.
3. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985.
4. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491-503.
5. Lu S, Zhang W, Wu L, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. 2024;331(3):201-211.
6. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684.
Without a doubt, immunotherapy has transformed the treatment landscape of non-small cell lung cancer (NSCLC) and enhanced survival rates across the different stages of disease. High recurrence rates following complete surgical resection prompted the study of immune checkpoint inhibitors (ICI) in earlier, operable stages of disease. This shift toward early application of ICI reflects the larger trend toward merging precision oncology with lung cancer staging. The resulting complexity in treatment and decision making creates systemic and logistical challenges that will require health care systems to adapt and improve.
Adjuvant immunotherapy for NSCLC
Prior to recent approvals for adjuvant immunotherapy, it was standard to give chemotherapy following resection of stage IB-IIIA disease, which offered a statistically nonsignificant survival gain. Recurrence in these patients is believed to be related to postsurgical micrometastasis. The utilization of alternative mechanisms to prevent recurrence is increasingly more common.
Atezolizumab, a PD-L1 inhibitor, is currently approved as first-line adjuvant treatment following chemotherapy in post-NSCLC resection patients with PD-L1 scores ≥1%. This category one recommendation by the National Comprehensive Cancer Network (NCCN) is based on results from the IMpower010 trial, which randomized patients to Atezolizumab vs best supportive care. All were early-stage NSCLC, stage IB-IIIA, who underwent resection followed by platinum-based chemotherapy. Statistically significant benefits were found in disease-free survival (DFS) with a trend toward overall survival.1
The PEARLS/KEYNOTE-091 trial evaluated another PD-L1 inhibitor, Pembrolizumab, as adjuvant therapy. Its design largely mirrored the IMPower010 study, but it differed in that the ICI was administered with or without chemotherapy following resection in patients with stage IB-IIIA NSCLC. Improvements in DFS were found in the overall population, leading to FDA approval for adjuvant therapy in 2023.2
These approvals require changes to the management of operable NSCLC. Until recently, it was not routine to send surgical specimens for additional testing because adjuvant treatment meant chemotherapy only. However, it is now essential that all surgically resected malignant tissue be sent for genomic sequencing and PD-L1 testing. Selecting the next form of therapy, whether it is an ICI or targeted drug therapy, depends on it.
From a surgical perspective, quality surgery with accurate nodal staging is crucial. The surgical findings can determine and identify those who are candidates for adjuvant immunotherapy. For these same reasons, it is helpful to advise surgeons preoperatively that targeted adjuvant therapy is being considered after resection.
Neoadjuvant immunotherapy for NSCLC
ICIs have also been used as neoadjuvant treatment for operable NSCLC. In 2021, the Checkmate-816 trial evaluated Nivolumab with platinum doublet chemotherapy prior to resection of stage IB-IIIa NSCLC. When compared with chemotherapy alone, there were significant improvements in EFS, MPR, and time to death or distant metastasis (TTDM) out to 3 years. At a median follow-up time of 41.4 months, only 28% in the nivolumab group had recurrence postsurgery compared with 42% in the chemotherapy-alone group.3 As a result, certain patients who are likely to receive adjuvant chemotherapy may additionally receive neoadjuvant immunotherapy with chemotherapy before surgical resection. In 2023, the KEYNOTE-671 study demonstrated that neoadjuvant Pembrolizumab and chemotherapy in patients with resectable stage II-IIIb (N2 stage) NSCLC improved EFS. At a median follow-up of 25.2 months, the EFS was 62.4% in the Pembrolizumab group vs 40.6% in the placebo group (P < .001).4

Such changes in treatment options mean patients should be discussed first and simultaneous referrals to oncology and surgery should occur in early-stage NSCLC. Up-front genomic phenotyping and PD-L1 testing may assist in decision making. High PD-L1 levels correlate better with response.
When an ICI-chemotherapy combination is given up front for newly diagnosed NSCLC, there is the potential for large reductions in tumor size and lymph node burden. Although the NCCN does not recommend ICIs to induce resectability, a patient originally deemed inoperable could theoretically become a surgical candidate with neoadjuvant ICI treatment. There is also the potential for toxicity, which could increase the risk of surgery when it does occur. Such scenarios will require frequent tumor board discussions so plans can be adjusted in real time to optimize outcomes as clinical circumstances change.
Perioperative immunotherapy for NSCLC
It is clear that both neoadjuvant and adjuvant immunotherapy can improve outcomes for patients with resectable NSCLC. The combination of neoadjuvant with adjuvant immunotherapy/chemotherapy is currently being studied. Two recent phase III clinical trials, NEOTORCH and AEGAEN, have found statistical improvements in EFS and MPR with this approach.5,6 These studies have not found their way into the NCCN guidelines yet but are sure to be considered in future iterations. Once adopted, the tumor board at each institution will have more options to choose from but many more decisions to make.
References
1. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357. [Published correction appears in Lancet. 2021 Nov 6;398(10312):1686.]
2. O’Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286.
3. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985.
4. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491-503.
5. Lu S, Zhang W, Wu L, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. 2024;331(3):201-211.
6. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684.
Without a doubt, immunotherapy has transformed the treatment landscape of non-small cell lung cancer (NSCLC) and enhanced survival rates across the different stages of disease. High recurrence rates following complete surgical resection prompted the study of immune checkpoint inhibitors (ICI) in earlier, operable stages of disease. This shift toward early application of ICI reflects the larger trend toward merging precision oncology with lung cancer staging. The resulting complexity in treatment and decision making creates systemic and logistical challenges that will require health care systems to adapt and improve.
Adjuvant immunotherapy for NSCLC
Prior to recent approvals for adjuvant immunotherapy, it was standard to give chemotherapy following resection of stage IB-IIIA disease, which offered a statistically nonsignificant survival gain. Recurrence in these patients is believed to be related to postsurgical micrometastasis. The utilization of alternative mechanisms to prevent recurrence is increasingly more common.
Atezolizumab, a PD-L1 inhibitor, is currently approved as first-line adjuvant treatment following chemotherapy in post-NSCLC resection patients with PD-L1 scores ≥1%. This category one recommendation by the National Comprehensive Cancer Network (NCCN) is based on results from the IMpower010 trial, which randomized patients to Atezolizumab vs best supportive care. All were early-stage NSCLC, stage IB-IIIA, who underwent resection followed by platinum-based chemotherapy. Statistically significant benefits were found in disease-free survival (DFS) with a trend toward overall survival.1
The PEARLS/KEYNOTE-091 trial evaluated another PD-L1 inhibitor, Pembrolizumab, as adjuvant therapy. Its design largely mirrored the IMPower010 study, but it differed in that the ICI was administered with or without chemotherapy following resection in patients with stage IB-IIIA NSCLC. Improvements in DFS were found in the overall population, leading to FDA approval for adjuvant therapy in 2023.2
These approvals require changes to the management of operable NSCLC. Until recently, it was not routine to send surgical specimens for additional testing because adjuvant treatment meant chemotherapy only. However, it is now essential that all surgically resected malignant tissue be sent for genomic sequencing and PD-L1 testing. Selecting the next form of therapy, whether it is an ICI or targeted drug therapy, depends on it.
From a surgical perspective, quality surgery with accurate nodal staging is crucial. The surgical findings can determine and identify those who are candidates for adjuvant immunotherapy. For these same reasons, it is helpful to advise surgeons preoperatively that targeted adjuvant therapy is being considered after resection.
Neoadjuvant immunotherapy for NSCLC
ICIs have also been used as neoadjuvant treatment for operable NSCLC. In 2021, the Checkmate-816 trial evaluated Nivolumab with platinum doublet chemotherapy prior to resection of stage IB-IIIa NSCLC. When compared with chemotherapy alone, there were significant improvements in EFS, MPR, and time to death or distant metastasis (TTDM) out to 3 years. At a median follow-up time of 41.4 months, only 28% in the nivolumab group had recurrence postsurgery compared with 42% in the chemotherapy-alone group.3 As a result, certain patients who are likely to receive adjuvant chemotherapy may additionally receive neoadjuvant immunotherapy with chemotherapy before surgical resection. In 2023, the KEYNOTE-671 study demonstrated that neoadjuvant Pembrolizumab and chemotherapy in patients with resectable stage II-IIIb (N2 stage) NSCLC improved EFS. At a median follow-up of 25.2 months, the EFS was 62.4% in the Pembrolizumab group vs 40.6% in the placebo group (P < .001).4

Such changes in treatment options mean patients should be discussed first and simultaneous referrals to oncology and surgery should occur in early-stage NSCLC. Up-front genomic phenotyping and PD-L1 testing may assist in decision making. High PD-L1 levels correlate better with response.
When an ICI-chemotherapy combination is given up front for newly diagnosed NSCLC, there is the potential for large reductions in tumor size and lymph node burden. Although the NCCN does not recommend ICIs to induce resectability, a patient originally deemed inoperable could theoretically become a surgical candidate with neoadjuvant ICI treatment. There is also the potential for toxicity, which could increase the risk of surgery when it does occur. Such scenarios will require frequent tumor board discussions so plans can be adjusted in real time to optimize outcomes as clinical circumstances change.
Perioperative immunotherapy for NSCLC
It is clear that both neoadjuvant and adjuvant immunotherapy can improve outcomes for patients with resectable NSCLC. The combination of neoadjuvant with adjuvant immunotherapy/chemotherapy is currently being studied. Two recent phase III clinical trials, NEOTORCH and AEGAEN, have found statistical improvements in EFS and MPR with this approach.5,6 These studies have not found their way into the NCCN guidelines yet but are sure to be considered in future iterations. Once adopted, the tumor board at each institution will have more options to choose from but many more decisions to make.
References
1. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357. [Published correction appears in Lancet. 2021 Nov 6;398(10312):1686.]
2. O’Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286.
3. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985.
4. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491-503.
5. Lu S, Zhang W, Wu L, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. 2024;331(3):201-211.
6. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684.
Are Beta-Blockers Safe for COPD?
Everyone takes a pharmacology class in medical school that includes a lecture on beta receptors. They’re in the heart (beta-1) and lungs (beta-2), and drug compounds agonize or antagonize one or both. The professor will caution against using antagonists (beta blockade) for patients with chronic obstructive pulmonary disease (COPD) lest they further impair the patient’s irreversibly narrowed airways. Obsequious students mature into obsequious doctors, intent on “doing no harm.” For better or worse, you withhold beta-blockers from your patient with COPD and comorbid cardiac disease.
Perhaps because the pulmonologist isn’t usually the one who decides whether a beta-blocker is prescribed, I’ve been napping on this topic since training. Early in fellowship, I read an ACP Journal Club article about a Cochrane systematic review (yes, I read a review of a review) that concluded that beta-blockers are fine in patients with COPD. The summary appealed to my bias towards evidence-based medicine (EBM) supplanting physiology, medical school, and everything else. I was more apt to believe my stodgy residency attendings than the stodgy pharmacology professor. Even though COPD and cardiovascular disease share multiple risk factors, I had never reinvestigated the relationship between beta-blockers and COPD.
Turns out that while I was sleeping, the debate continued. Go figure. Just last month a prospective, observational study published in JAMA Network Open found that beta-blockers did not increase the risk for cardiovascular or respiratory events among patients with COPD being discharged after hospitalization for acute myocardial infarction. Although this could be viewed as a triumph for EBM over physiology and a validation of my decade-plus of intellectual laziness, the results are actually pretty thin. These studies, in which patients with an indication for a therapy (a beta-blocker in this case) are analyzed by whether or not they received it, are problematic. The fanciest statistics — in this case, they used propensity scores — can’t control for residual confounding. What drove the physicians to prescribe in some cases but not others? We can only guess.
This might be okay if there hadn’t been a randomized controlled trial (RCT) published in 2019 in The New England Journal of Medicine that found that beta-blockers increase the risk for severe COPD exacerbations. In EBM, the RCT trumps all. Ironically, this trial was designed to test whether beta-blockers reduce severe COPD exacerbations. Yes, we’d come full circle. There was enough biologic plausibility to support a positive effect, or so thought the study authors and the Department of Defense (DOD) — for reasons I can’t possibly guess, the DOD funded this RCT. My pharmacology professor must be rolling over in his tenure.
The RCT did leave beta-blockers some wiggle room. The authors purposely excluded anyone with a cardiovascular indication for a beta-blocker. The intent was to ensure beneficial effects were isolated to respiratory and not cardiovascular outcomes. Of course, the reason I’m writing and you’re reading this is that COPD and cardiovascular disease co-occur at a high rate. The RCT notwithstanding, we prescribe beta-blockers to patients with COPD because they have a cardiac indication, not to reduce acute COPD exacerbations. So, it’s possible there’d be a net beta-blocker benefit in patients with COPD and comorbid heart disease.
That’s where the JAMA Network Open study comes in, but as discussed, methodologic weaknesses preclude its being the final word. That said, I think it’s unlikely we’ll see a COPD with comorbid cardiac disease RCT performed to assess whether beta-blockers provide a net benefit, unless maybe the DOD wants to fund another one of these. In the meantime, I’m calling clinical equipoise and punting. Fortunately for me, I don’t have to prescribe beta-blockers.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington, DC. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
Everyone takes a pharmacology class in medical school that includes a lecture on beta receptors. They’re in the heart (beta-1) and lungs (beta-2), and drug compounds agonize or antagonize one or both. The professor will caution against using antagonists (beta blockade) for patients with chronic obstructive pulmonary disease (COPD) lest they further impair the patient’s irreversibly narrowed airways. Obsequious students mature into obsequious doctors, intent on “doing no harm.” For better or worse, you withhold beta-blockers from your patient with COPD and comorbid cardiac disease.
Perhaps because the pulmonologist isn’t usually the one who decides whether a beta-blocker is prescribed, I’ve been napping on this topic since training. Early in fellowship, I read an ACP Journal Club article about a Cochrane systematic review (yes, I read a review of a review) that concluded that beta-blockers are fine in patients with COPD. The summary appealed to my bias towards evidence-based medicine (EBM) supplanting physiology, medical school, and everything else. I was more apt to believe my stodgy residency attendings than the stodgy pharmacology professor. Even though COPD and cardiovascular disease share multiple risk factors, I had never reinvestigated the relationship between beta-blockers and COPD.
Turns out that while I was sleeping, the debate continued. Go figure. Just last month a prospective, observational study published in JAMA Network Open found that beta-blockers did not increase the risk for cardiovascular or respiratory events among patients with COPD being discharged after hospitalization for acute myocardial infarction. Although this could be viewed as a triumph for EBM over physiology and a validation of my decade-plus of intellectual laziness, the results are actually pretty thin. These studies, in which patients with an indication for a therapy (a beta-blocker in this case) are analyzed by whether or not they received it, are problematic. The fanciest statistics — in this case, they used propensity scores — can’t control for residual confounding. What drove the physicians to prescribe in some cases but not others? We can only guess.
This might be okay if there hadn’t been a randomized controlled trial (RCT) published in 2019 in The New England Journal of Medicine that found that beta-blockers increase the risk for severe COPD exacerbations. In EBM, the RCT trumps all. Ironically, this trial was designed to test whether beta-blockers reduce severe COPD exacerbations. Yes, we’d come full circle. There was enough biologic plausibility to support a positive effect, or so thought the study authors and the Department of Defense (DOD) — for reasons I can’t possibly guess, the DOD funded this RCT. My pharmacology professor must be rolling over in his tenure.
The RCT did leave beta-blockers some wiggle room. The authors purposely excluded anyone with a cardiovascular indication for a beta-blocker. The intent was to ensure beneficial effects were isolated to respiratory and not cardiovascular outcomes. Of course, the reason I’m writing and you’re reading this is that COPD and cardiovascular disease co-occur at a high rate. The RCT notwithstanding, we prescribe beta-blockers to patients with COPD because they have a cardiac indication, not to reduce acute COPD exacerbations. So, it’s possible there’d be a net beta-blocker benefit in patients with COPD and comorbid heart disease.
That’s where the JAMA Network Open study comes in, but as discussed, methodologic weaknesses preclude its being the final word. That said, I think it’s unlikely we’ll see a COPD with comorbid cardiac disease RCT performed to assess whether beta-blockers provide a net benefit, unless maybe the DOD wants to fund another one of these. In the meantime, I’m calling clinical equipoise and punting. Fortunately for me, I don’t have to prescribe beta-blockers.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington, DC. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
Everyone takes a pharmacology class in medical school that includes a lecture on beta receptors. They’re in the heart (beta-1) and lungs (beta-2), and drug compounds agonize or antagonize one or both. The professor will caution against using antagonists (beta blockade) for patients with chronic obstructive pulmonary disease (COPD) lest they further impair the patient’s irreversibly narrowed airways. Obsequious students mature into obsequious doctors, intent on “doing no harm.” For better or worse, you withhold beta-blockers from your patient with COPD and comorbid cardiac disease.
Perhaps because the pulmonologist isn’t usually the one who decides whether a beta-blocker is prescribed, I’ve been napping on this topic since training. Early in fellowship, I read an ACP Journal Club article about a Cochrane systematic review (yes, I read a review of a review) that concluded that beta-blockers are fine in patients with COPD. The summary appealed to my bias towards evidence-based medicine (EBM) supplanting physiology, medical school, and everything else. I was more apt to believe my stodgy residency attendings than the stodgy pharmacology professor. Even though COPD and cardiovascular disease share multiple risk factors, I had never reinvestigated the relationship between beta-blockers and COPD.
Turns out that while I was sleeping, the debate continued. Go figure. Just last month a prospective, observational study published in JAMA Network Open found that beta-blockers did not increase the risk for cardiovascular or respiratory events among patients with COPD being discharged after hospitalization for acute myocardial infarction. Although this could be viewed as a triumph for EBM over physiology and a validation of my decade-plus of intellectual laziness, the results are actually pretty thin. These studies, in which patients with an indication for a therapy (a beta-blocker in this case) are analyzed by whether or not they received it, are problematic. The fanciest statistics — in this case, they used propensity scores — can’t control for residual confounding. What drove the physicians to prescribe in some cases but not others? We can only guess.
This might be okay if there hadn’t been a randomized controlled trial (RCT) published in 2019 in The New England Journal of Medicine that found that beta-blockers increase the risk for severe COPD exacerbations. In EBM, the RCT trumps all. Ironically, this trial was designed to test whether beta-blockers reduce severe COPD exacerbations. Yes, we’d come full circle. There was enough biologic plausibility to support a positive effect, or so thought the study authors and the Department of Defense (DOD) — for reasons I can’t possibly guess, the DOD funded this RCT. My pharmacology professor must be rolling over in his tenure.
The RCT did leave beta-blockers some wiggle room. The authors purposely excluded anyone with a cardiovascular indication for a beta-blocker. The intent was to ensure beneficial effects were isolated to respiratory and not cardiovascular outcomes. Of course, the reason I’m writing and you’re reading this is that COPD and cardiovascular disease co-occur at a high rate. The RCT notwithstanding, we prescribe beta-blockers to patients with COPD because they have a cardiac indication, not to reduce acute COPD exacerbations. So, it’s possible there’d be a net beta-blocker benefit in patients with COPD and comorbid heart disease.
That’s where the JAMA Network Open study comes in, but as discussed, methodologic weaknesses preclude its being the final word. That said, I think it’s unlikely we’ll see a COPD with comorbid cardiac disease RCT performed to assess whether beta-blockers provide a net benefit, unless maybe the DOD wants to fund another one of these. In the meantime, I’m calling clinical equipoise and punting. Fortunately for me, I don’t have to prescribe beta-blockers.
Dr. Holley is professor of medicine at Uniformed Services University in Bethesda, Maryland, and a pulmonary/sleep and critical care medicine physician at MedStar Washington Hospital Center in Washington, DC. He reported conflicts of interest with Metapharm, CHEST College, and WebMD.
A version of this article first appeared on Medscape.com.
Military burn pits: Their evidence and implications for respiratory health
Military service is a hazard-ridden profession. It’s easy to recognize the direct dangers from warfighting, such as gunfire and explosions, but the risks from environmental, chemical, and other occupational exposures can be harder to see.
Combustion-based waste management systems, otherwise known as “burn pits,” were used in deployed environments by the US military from the 1990s to the early 2010s. These burn pits were commonly used to eliminate plastics, electronics, munitions, metals, wood, chemicals, and even human waste. At the height of the recent conflicts in Afghanistan, Iraq, and other southwest Asia locations, more than 70% of military installations employed at least one, and nearly 4 million service members were exposed to some degree to their emissions.
Reports of burn pits being related to organic disease have garnered widespread media attention. Initially, this came through anecdotal reports of post-deployment respiratory symptoms. Over time, the conditions attributed to burn pits expanded to include newly diagnosed respiratory diseases and malignancies.
Ultimately, Congress passed the 2022 Promise to Address Comprehensive Toxins (PACT) Act, presumptively linking more than 20 diagnoses to burn pits. The PACT Act provides countless veterans access to low-cost or free medical care for their respective conditions.
What do we know about burn pits and deployment-related respiratory disease?
Data from the Millennium Cohort Study noted an approximately 40% increase in respiratory symptoms among individuals returning from deployment but no increase in the frequency of diagnosed respiratory diseases.1 This study and others definitively established a temporal relationship between deployment and respiratory symptoms. Soon after, a retrospective, observational study of service members with post-deployment respiratory symptoms found a high prevalence of constrictive bronchiolitis (CB) identified by lung biopsy.2 Patients in this group reported exposure to burn pits and a sulfur mine fire in the Mosul area while deployed. Most had normal imaging and pulmonary function testing before biopsy, confounding the clinical significance of the CB finding. The publication of this report led to increased investigation of respiratory function during and after deployment.
In a series of prospective studies that included full pulmonary function testing, impulse oscillometry, cardiopulmonary exercise testing, bronchoscopy, and, occasionally, lung biopsy to evaluate post-deployment dyspnea, only a small minority received a diagnosis of clinically significant lung disease.3,4 Additionally, when comparing spirometry and impulse oscillometry results from before and after deployment, no decline in lung function was observed in a population of service members reporting regular burn pit exposure.5 These studies suggest that at the population level, deployment does not lead to abnormalities in the structure and function of the respiratory system.
The National Academies of Sciences published two separate reviews of burn pit exposure and outcomes in 2011 and 2020.6,7 They found insufficient evidence to support a causal relationship between burn pit exposure and pulmonary disease. They highlighted studies on the composition of emissions from the area surrounding the largest military burn pit in Iraq. Levels of particulate matter, volatile organic compounds, and polycyclic aromatic hydrocarbons were elevated when compared with those of a typical American city but were similar to the pollution levels seen in the region at the time. Given these findings, they suggested ambient air pollution may have contributed more to clinically significant disease than burn pit emissions.
How do we interpret this mixed data?
At the population level, we have yet to find conclusive data directly linking burn pit exposure to the development of any respiratory disease. Does this mean that burn pits are not harmful?
Not necessarily. Research on outcomes related to burn pit exposure is challenging given the heterogeneity in exposure volume. Much of the research is retrospective and subject to recall bias. Relationships may be distorted, and the precision of reported symptoms and exposure levels is altered. Given these challenges, it’s unsurprising that evidence of causality has yet to be proven. In addition, some portion of service members has been diagnosed with respiratory disease that could be related to burn pit exposure.
What is now indisputable is that deployment to southwest Asia leads to an increase in respiratory complaints. Whether veteran respiratory symptoms are due to burn pits, ambient pollution, environmental particulate matter, or dust storms is less clinically relevant. These symptoms require attention, investigation, and management.
What does this mean for the future medical care of service members and veterans?
Many veterans with post-deployment respiratory symptoms undergo extensive evaluations without obtaining a definitive diagnosis. A recent consensus statement on deployment-related respiratory symptoms provides a framework for evaluation in such cases.8 In keeping with that statement, we recommend veterans be referred to centers with expertise in this field, such as the Department of Veterans Affairs (VA) or military health centers, when deployment-related respiratory symptoms are reported. When the evaluation does not lead to a treatable diagnosis, these centers can provide multidisciplinary care to address the symptoms of dyspnea, cough, fatigue, and exercise intolerance to improve functional status.
Despite uncertainty in the evidence or challenges in diagnosis, both the Department of Defense (DoD) and VA remain fully committed to addressing the health concerns of service members and veterans. Notably, the VA has already screened more than 5 million veterans for toxic military exposures in accordance with the PACT Act and is providing ongoing screening and care for veterans with post-deployment respiratory symptoms. Furthermore, the DoD and VA have dedicated large portions of their research budgets to investigating the impacts of exposures during military service and optimizing the care of those with respiratory symptoms. With these commitments to patient care and research, our veterans’ respiratory health can now be optimized, and future risks can be mitigated.
Dr. Haynes is Fellow, Pulmonary and Critical Care Medicine, Walter Reed National Military Medical Center, Assistant Professor of Medicine, Uniformed Services University. Dr. Nations is Pulmonary and Critical Care Medicine, Deputy Chief of Staff for Operations, Washington DC VA Medical Center, Associate Professor of Medicine, Uniformed Services University.
References
1. Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD; Margaret A. K. Ryan for the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170(11):1433-1442. Preprint. Posted online October 22, 2009. PMID: 19850627. doi: 10.1093/aje/kwp287
2. King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365(3):222-230. Erratum in: N Engl J Med. 2011;365(18):1749. PMID: 21774710; PMCID: PMC3296566. doi: 10.1056/NEJMoa1101388
3. Morris MJ, Dodson DW, Lucero PF, et al. Study of active duty military for pulmonary disease related to environmental deployment exposures (STAMPEDE). Am J Respir Crit Care Med. 2014;190(1):77-84. PMID: 24922562. doi: 10.1164/rccm.201402-0372OC
4. Morris MJ, Walter RJ, McCann ET, et al. Clinical evaluation of deployed military personnel with chronic respiratory symptoms: study of active duty military for pulmonary disease related to environmental deployment exposures (STAMPEDE) III. Chest. 2020;157(6):1559-1567. Preprint. Posted online February 1, 2020. PMID: 32017933. doi: 10.1016/j.chest.2020.01.024
5. Morris MJ, Skabelund AJ, Rawlins FA 3rd, Gallup RA, Aden JK, Holley AB. Study of active duty military personnel for environmental deployment exposures: pre- and post-deployment spirometry (STAMPEDE II). Respir Care. 2019;64(5):536-544. Preprint. Posted online January 8, 2019.PMID: 30622173. doi: 10.4187/respcare.06396
6. Institute of Medicine. Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan. The National Academies Press; 2011. https://doi.org/10.17226/13209
7. National Academies of Sciences, Engineering, and Medicine. Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. The National Academies Press; 2020. https://doi.org/10.17226/25837
8. Falvo MJ, Sotolongo AM, Osterholzer JJ, et al. Consensus statements on deployment-related respiratory disease, inclusive of constrictive bronchiolitis: a modified Delphi study. Chest. 2023;163(3):599-609. Preprint. Posted November 4, 2022. PMID: 36343686; PMCID: PMC10154857. doi: 10.1016/j.chest.2022.10.031
Military service is a hazard-ridden profession. It’s easy to recognize the direct dangers from warfighting, such as gunfire and explosions, but the risks from environmental, chemical, and other occupational exposures can be harder to see.
Combustion-based waste management systems, otherwise known as “burn pits,” were used in deployed environments by the US military from the 1990s to the early 2010s. These burn pits were commonly used to eliminate plastics, electronics, munitions, metals, wood, chemicals, and even human waste. At the height of the recent conflicts in Afghanistan, Iraq, and other southwest Asia locations, more than 70% of military installations employed at least one, and nearly 4 million service members were exposed to some degree to their emissions.
Reports of burn pits being related to organic disease have garnered widespread media attention. Initially, this came through anecdotal reports of post-deployment respiratory symptoms. Over time, the conditions attributed to burn pits expanded to include newly diagnosed respiratory diseases and malignancies.
Ultimately, Congress passed the 2022 Promise to Address Comprehensive Toxins (PACT) Act, presumptively linking more than 20 diagnoses to burn pits. The PACT Act provides countless veterans access to low-cost or free medical care for their respective conditions.
What do we know about burn pits and deployment-related respiratory disease?
Data from the Millennium Cohort Study noted an approximately 40% increase in respiratory symptoms among individuals returning from deployment but no increase in the frequency of diagnosed respiratory diseases.1 This study and others definitively established a temporal relationship between deployment and respiratory symptoms. Soon after, a retrospective, observational study of service members with post-deployment respiratory symptoms found a high prevalence of constrictive bronchiolitis (CB) identified by lung biopsy.2 Patients in this group reported exposure to burn pits and a sulfur mine fire in the Mosul area while deployed. Most had normal imaging and pulmonary function testing before biopsy, confounding the clinical significance of the CB finding. The publication of this report led to increased investigation of respiratory function during and after deployment.
In a series of prospective studies that included full pulmonary function testing, impulse oscillometry, cardiopulmonary exercise testing, bronchoscopy, and, occasionally, lung biopsy to evaluate post-deployment dyspnea, only a small minority received a diagnosis of clinically significant lung disease.3,4 Additionally, when comparing spirometry and impulse oscillometry results from before and after deployment, no decline in lung function was observed in a population of service members reporting regular burn pit exposure.5 These studies suggest that at the population level, deployment does not lead to abnormalities in the structure and function of the respiratory system.
The National Academies of Sciences published two separate reviews of burn pit exposure and outcomes in 2011 and 2020.6,7 They found insufficient evidence to support a causal relationship between burn pit exposure and pulmonary disease. They highlighted studies on the composition of emissions from the area surrounding the largest military burn pit in Iraq. Levels of particulate matter, volatile organic compounds, and polycyclic aromatic hydrocarbons were elevated when compared with those of a typical American city but were similar to the pollution levels seen in the region at the time. Given these findings, they suggested ambient air pollution may have contributed more to clinically significant disease than burn pit emissions.
How do we interpret this mixed data?
At the population level, we have yet to find conclusive data directly linking burn pit exposure to the development of any respiratory disease. Does this mean that burn pits are not harmful?
Not necessarily. Research on outcomes related to burn pit exposure is challenging given the heterogeneity in exposure volume. Much of the research is retrospective and subject to recall bias. Relationships may be distorted, and the precision of reported symptoms and exposure levels is altered. Given these challenges, it’s unsurprising that evidence of causality has yet to be proven. In addition, some portion of service members has been diagnosed with respiratory disease that could be related to burn pit exposure.
What is now indisputable is that deployment to southwest Asia leads to an increase in respiratory complaints. Whether veteran respiratory symptoms are due to burn pits, ambient pollution, environmental particulate matter, or dust storms is less clinically relevant. These symptoms require attention, investigation, and management.
What does this mean for the future medical care of service members and veterans?
Many veterans with post-deployment respiratory symptoms undergo extensive evaluations without obtaining a definitive diagnosis. A recent consensus statement on deployment-related respiratory symptoms provides a framework for evaluation in such cases.8 In keeping with that statement, we recommend veterans be referred to centers with expertise in this field, such as the Department of Veterans Affairs (VA) or military health centers, when deployment-related respiratory symptoms are reported. When the evaluation does not lead to a treatable diagnosis, these centers can provide multidisciplinary care to address the symptoms of dyspnea, cough, fatigue, and exercise intolerance to improve functional status.
Despite uncertainty in the evidence or challenges in diagnosis, both the Department of Defense (DoD) and VA remain fully committed to addressing the health concerns of service members and veterans. Notably, the VA has already screened more than 5 million veterans for toxic military exposures in accordance with the PACT Act and is providing ongoing screening and care for veterans with post-deployment respiratory symptoms. Furthermore, the DoD and VA have dedicated large portions of their research budgets to investigating the impacts of exposures during military service and optimizing the care of those with respiratory symptoms. With these commitments to patient care and research, our veterans’ respiratory health can now be optimized, and future risks can be mitigated.
Dr. Haynes is Fellow, Pulmonary and Critical Care Medicine, Walter Reed National Military Medical Center, Assistant Professor of Medicine, Uniformed Services University. Dr. Nations is Pulmonary and Critical Care Medicine, Deputy Chief of Staff for Operations, Washington DC VA Medical Center, Associate Professor of Medicine, Uniformed Services University.
References
1. Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD; Margaret A. K. Ryan for the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170(11):1433-1442. Preprint. Posted online October 22, 2009. PMID: 19850627. doi: 10.1093/aje/kwp287
2. King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365(3):222-230. Erratum in: N Engl J Med. 2011;365(18):1749. PMID: 21774710; PMCID: PMC3296566. doi: 10.1056/NEJMoa1101388
3. Morris MJ, Dodson DW, Lucero PF, et al. Study of active duty military for pulmonary disease related to environmental deployment exposures (STAMPEDE). Am J Respir Crit Care Med. 2014;190(1):77-84. PMID: 24922562. doi: 10.1164/rccm.201402-0372OC
4. Morris MJ, Walter RJ, McCann ET, et al. Clinical evaluation of deployed military personnel with chronic respiratory symptoms: study of active duty military for pulmonary disease related to environmental deployment exposures (STAMPEDE) III. Chest. 2020;157(6):1559-1567. Preprint. Posted online February 1, 2020. PMID: 32017933. doi: 10.1016/j.chest.2020.01.024
5. Morris MJ, Skabelund AJ, Rawlins FA 3rd, Gallup RA, Aden JK, Holley AB. Study of active duty military personnel for environmental deployment exposures: pre- and post-deployment spirometry (STAMPEDE II). Respir Care. 2019;64(5):536-544. Preprint. Posted online January 8, 2019.PMID: 30622173. doi: 10.4187/respcare.06396
6. Institute of Medicine. Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan. The National Academies Press; 2011. https://doi.org/10.17226/13209
7. National Academies of Sciences, Engineering, and Medicine. Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. The National Academies Press; 2020. https://doi.org/10.17226/25837
8. Falvo MJ, Sotolongo AM, Osterholzer JJ, et al. Consensus statements on deployment-related respiratory disease, inclusive of constrictive bronchiolitis: a modified Delphi study. Chest. 2023;163(3):599-609. Preprint. Posted November 4, 2022. PMID: 36343686; PMCID: PMC10154857. doi: 10.1016/j.chest.2022.10.031
Military service is a hazard-ridden profession. It’s easy to recognize the direct dangers from warfighting, such as gunfire and explosions, but the risks from environmental, chemical, and other occupational exposures can be harder to see.
Combustion-based waste management systems, otherwise known as “burn pits,” were used in deployed environments by the US military from the 1990s to the early 2010s. These burn pits were commonly used to eliminate plastics, electronics, munitions, metals, wood, chemicals, and even human waste. At the height of the recent conflicts in Afghanistan, Iraq, and other southwest Asia locations, more than 70% of military installations employed at least one, and nearly 4 million service members were exposed to some degree to their emissions.
Reports of burn pits being related to organic disease have garnered widespread media attention. Initially, this came through anecdotal reports of post-deployment respiratory symptoms. Over time, the conditions attributed to burn pits expanded to include newly diagnosed respiratory diseases and malignancies.
Ultimately, Congress passed the 2022 Promise to Address Comprehensive Toxins (PACT) Act, presumptively linking more than 20 diagnoses to burn pits. The PACT Act provides countless veterans access to low-cost or free medical care for their respective conditions.
What do we know about burn pits and deployment-related respiratory disease?
Data from the Millennium Cohort Study noted an approximately 40% increase in respiratory symptoms among individuals returning from deployment but no increase in the frequency of diagnosed respiratory diseases.1 This study and others definitively established a temporal relationship between deployment and respiratory symptoms. Soon after, a retrospective, observational study of service members with post-deployment respiratory symptoms found a high prevalence of constrictive bronchiolitis (CB) identified by lung biopsy.2 Patients in this group reported exposure to burn pits and a sulfur mine fire in the Mosul area while deployed. Most had normal imaging and pulmonary function testing before biopsy, confounding the clinical significance of the CB finding. The publication of this report led to increased investigation of respiratory function during and after deployment.
In a series of prospective studies that included full pulmonary function testing, impulse oscillometry, cardiopulmonary exercise testing, bronchoscopy, and, occasionally, lung biopsy to evaluate post-deployment dyspnea, only a small minority received a diagnosis of clinically significant lung disease.3,4 Additionally, when comparing spirometry and impulse oscillometry results from before and after deployment, no decline in lung function was observed in a population of service members reporting regular burn pit exposure.5 These studies suggest that at the population level, deployment does not lead to abnormalities in the structure and function of the respiratory system.
The National Academies of Sciences published two separate reviews of burn pit exposure and outcomes in 2011 and 2020.6,7 They found insufficient evidence to support a causal relationship between burn pit exposure and pulmonary disease. They highlighted studies on the composition of emissions from the area surrounding the largest military burn pit in Iraq. Levels of particulate matter, volatile organic compounds, and polycyclic aromatic hydrocarbons were elevated when compared with those of a typical American city but were similar to the pollution levels seen in the region at the time. Given these findings, they suggested ambient air pollution may have contributed more to clinically significant disease than burn pit emissions.
How do we interpret this mixed data?
At the population level, we have yet to find conclusive data directly linking burn pit exposure to the development of any respiratory disease. Does this mean that burn pits are not harmful?
Not necessarily. Research on outcomes related to burn pit exposure is challenging given the heterogeneity in exposure volume. Much of the research is retrospective and subject to recall bias. Relationships may be distorted, and the precision of reported symptoms and exposure levels is altered. Given these challenges, it’s unsurprising that evidence of causality has yet to be proven. In addition, some portion of service members has been diagnosed with respiratory disease that could be related to burn pit exposure.
What is now indisputable is that deployment to southwest Asia leads to an increase in respiratory complaints. Whether veteran respiratory symptoms are due to burn pits, ambient pollution, environmental particulate matter, or dust storms is less clinically relevant. These symptoms require attention, investigation, and management.
What does this mean for the future medical care of service members and veterans?
Many veterans with post-deployment respiratory symptoms undergo extensive evaluations without obtaining a definitive diagnosis. A recent consensus statement on deployment-related respiratory symptoms provides a framework for evaluation in such cases.8 In keeping with that statement, we recommend veterans be referred to centers with expertise in this field, such as the Department of Veterans Affairs (VA) or military health centers, when deployment-related respiratory symptoms are reported. When the evaluation does not lead to a treatable diagnosis, these centers can provide multidisciplinary care to address the symptoms of dyspnea, cough, fatigue, and exercise intolerance to improve functional status.
Despite uncertainty in the evidence or challenges in diagnosis, both the Department of Defense (DoD) and VA remain fully committed to addressing the health concerns of service members and veterans. Notably, the VA has already screened more than 5 million veterans for toxic military exposures in accordance with the PACT Act and is providing ongoing screening and care for veterans with post-deployment respiratory symptoms. Furthermore, the DoD and VA have dedicated large portions of their research budgets to investigating the impacts of exposures during military service and optimizing the care of those with respiratory symptoms. With these commitments to patient care and research, our veterans’ respiratory health can now be optimized, and future risks can be mitigated.
Dr. Haynes is Fellow, Pulmonary and Critical Care Medicine, Walter Reed National Military Medical Center, Assistant Professor of Medicine, Uniformed Services University. Dr. Nations is Pulmonary and Critical Care Medicine, Deputy Chief of Staff for Operations, Washington DC VA Medical Center, Associate Professor of Medicine, Uniformed Services University.
References
1. Smith B, Wong CA, Smith TC, Boyko EJ, Gackstetter GD; Margaret A. K. Ryan for the Millennium Cohort Study Team. Newly reported respiratory symptoms and conditions among military personnel deployed to Iraq and Afghanistan: a prospective population-based study. Am J Epidemiol. 2009;170(11):1433-1442. Preprint. Posted online October 22, 2009. PMID: 19850627. doi: 10.1093/aje/kwp287
2. King MS, Eisenberg R, Newman JH, et al. Constrictive bronchiolitis in soldiers returning from Iraq and Afghanistan. N Engl J Med. 2011;365(3):222-230. Erratum in: N Engl J Med. 2011;365(18):1749. PMID: 21774710; PMCID: PMC3296566. doi: 10.1056/NEJMoa1101388
3. Morris MJ, Dodson DW, Lucero PF, et al. Study of active duty military for pulmonary disease related to environmental deployment exposures (STAMPEDE). Am J Respir Crit Care Med. 2014;190(1):77-84. PMID: 24922562. doi: 10.1164/rccm.201402-0372OC
4. Morris MJ, Walter RJ, McCann ET, et al. Clinical evaluation of deployed military personnel with chronic respiratory symptoms: study of active duty military for pulmonary disease related to environmental deployment exposures (STAMPEDE) III. Chest. 2020;157(6):1559-1567. Preprint. Posted online February 1, 2020. PMID: 32017933. doi: 10.1016/j.chest.2020.01.024
5. Morris MJ, Skabelund AJ, Rawlins FA 3rd, Gallup RA, Aden JK, Holley AB. Study of active duty military personnel for environmental deployment exposures: pre- and post-deployment spirometry (STAMPEDE II). Respir Care. 2019;64(5):536-544. Preprint. Posted online January 8, 2019.PMID: 30622173. doi: 10.4187/respcare.06396
6. Institute of Medicine. Long-Term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan. The National Academies Press; 2011. https://doi.org/10.17226/13209
7. National Academies of Sciences, Engineering, and Medicine. Respiratory Health Effects of Airborne Hazards Exposures in the Southwest Asia Theater of Military Operations. The National Academies Press; 2020. https://doi.org/10.17226/25837
8. Falvo MJ, Sotolongo AM, Osterholzer JJ, et al. Consensus statements on deployment-related respiratory disease, inclusive of constrictive bronchiolitis: a modified Delphi study. Chest. 2023;163(3):599-609. Preprint. Posted November 4, 2022. PMID: 36343686; PMCID: PMC10154857. doi: 10.1016/j.chest.2022.10.031
Managing severe asthma exacerbations in the ED: We need answers beyond albuterol
Evidence-based medicine (EBM) stems from making the best patient-centered decision from the highest-quality data available that comports with our understanding of pathophysiology. In some situations, clinicians are forced to draw conclusions from data that are imperfect and apply it to patients who are complex and dynamic.
The Centers for Disease Control and Prevention (CDC) estimates that about 7.7% of the United States population has asthma. There were about 1 million ED visits in 2020, with asthma listed as the primary diagnosis, and only 94,000 required hospitalization.1 There are many tools we employ that have greatly decreased inpatient admissions for asthma. The uptake of inhaled corticosteroids (ICS) has significantly reduced asthma-related morbidity and mortality and reduced exacerbations that require admission to a hospital. This treatment strategy is supported by the Global Initiative for Asthma (GINA) and National Asthma Education and Prevention Program (NAEPP) guidelines.2,3 While we should celebrate the impact that EBM and ICS have had on asthma outcomes, we continue to struggle to control severe asthma.
Bronchodilator therapy in the hospital is ubiquitous. House staff and hospitalists click the bronchodilator order set early and often. However, the optimal frequency, dose, and duration of inhaled bronchodilator therapy for acute asthma exacerbation are unknown. Do frequency, dose, and duration change with exacerbation severity? Nothing gets ED, inpatient, or ICU physicians more jittery than the phrase “exacerbation of asthma on BiPap” or “intubated for asthma.” With its enormous clinical impact and notoriously difficult hospital and ICU course, the lack of evidence we have for managing these patients outside of the initial 24- to 48-hour visit is concerning. Neither NAEPP nor GINA provide management recommendations for the patient with severe asthma exacerbation that necessitates admission.
Albuterol is a commonly used medication for asthma and chronic obstructive airway disease. It is rapid acting and effective—few medications give patients (or clinicians) such instant satisfaction. As an internal medicine resident and pulmonary fellow, I ordered it countless times without ever looking at the dose. Sometimes, patients would come up from the emergency department after receiving a “continuous dose.” I would often wonder exactly what that meant. After some investigation, I found that in my hospital at the time, one dose of albuterol was 2.5 mg in 2 mL, and a continuous nebulization was four doses for a total of 10 mg.
Shrestha et al. found that high-dose albuterol (7.5 mg) administered continuously was superior to 2.5 mg albuterol delivered three times over 1.5 hours. There were demonstrable improvements in FEV1 and no ICU admissions.4 This study is one of many that compared intermittent to continuous and high-dose vs low-dose albuterol in the emergency department. Most are small and occur over the first 24 hours of presentation to the hospital. They often use short-term changes in spirometry as their primary outcome measure. Being a pulmonary and critical care doctor, I see patients who require advanced rescue maneuvers such as noninvasive positive pressure ventilation (NIPPV) or other pharmacologic adjuncts, for which the current evidence is limited.
Because studies of inhaled bronchodilators in acute asthma exacerbation use spirometry as their primary outcome, those with more severe disease and higher acuity are excluded. Patients on NIPPV can’t perform spirometry. There is essentially no literature to guide treatment for a patient with asthma in the adult ICU. In pediatric intensive care units, there are some data to support either continuous or intermittent inhaled bronchodilator that extends beyond the initial ED visit up to about 60 hours.5 Much of the pediatric data revolve about the amount of albuterol given, which can be as high as 75 mg/hr though is typically closer to 10-20 mg/hr.6 This rate is continued until respiratory improvement occurs.
With poor evidence to guide us and no specific direction from major guidelines, how should providers manage severe asthma exacerbation? The amount of drug deposited in the lung varies by the device used to deliver it. For nebulization, only about 10% of the nebulized amount reaches the lungs for effect; this is a smaller amount compared with all other devices one could use, such as MDI or DPI.7 Once a patient with asthma reaches the emergency department, that person is usually placed on some form of nebulizer treatment. But based on local hospital protocols, the amount and duration can vary widely. Sometimes, in patients with severe exacerbation, there is trepidation to continuing albuterol therapy due to ongoing tachycardia. This seems reasonable given increased albuterol administration could beget an ongoing cycle of dyspnea and anxiety. It could also lead to choosing therapies that are less evidence based.
In closing, this seemingly mundane topic takes on new meaning when a patient is in severe exacerbation. Fortunately, providers are not often faced with the decision to wade into the evidence-free territory of severe asthma exacerbation that is unresponsive to first-line treatments. This narrative should serve as a general alert that this pathophysiologic state is understudied. When encountered, thoughtful consideration of pathology, physiology, and pharmacology is required to reverse it.
References
1. Centers for Disease Control and Prevention. (2023, May 10). Most recent national asthma data. Centers for Disease Control and Prevention. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Global Initiative for Asthma - GINA. (2023, August 15). 2023 GINA Main Report - Global Initiative for Asthma - GINA. https://ginasthma.org/2023-gina-main-report/
3. Kiley J, Mensah GA, Boyce CA, et al (A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group). 2020 Focused updates to the: Asthma Management Guidelines. US Department of Health and Human Services, NIH, NHLBI 2020.
4. Shrestha M, Bidadi K, Gourlay S, Hayes J. Continuous vs intermittent albuterol, at high and low doses, in the treatment of severe acute asthma in adults. Chest. 1996 Jul;110(1):42-7. doi: 10.1378/chest.110.1.42. PMID: 8681661.
5. Kulalert P, Phinyo P, Patumanond J, Smathakanee C, Chuenjit W, Nanthapisal S. Continuous versus intermittent short-acting β2-agonists nebulization as first-line therapy in hospitalized children with severe asthma exacerbation: a propensity score matching analysis. Asthma Res Pract. 2020 Jul 2;6:6. doi: 10.1186/s40733-020-00059-5. PMID: 32632352; PMCID: PMC7329360.
6. Phumeetham S, Bahk TJ, Abd-Allah S, Mathur M. Effect of high-dose continuous albuterol nebulization on clinical variables in children with status asthmaticus. Pediatr Crit Care Med. 2015 Feb;16(2):e41-6. doi: 10.1097/PCC.0000000000000314. PMID: 25560428.
7. Gardenhire DS, Burnett D, Strickland S, Myers, TR. A guide to aerosol delivery devices for respiratory therapists. American Association for Respiratory Care, Dallas, Texas 2017.
Evidence-based medicine (EBM) stems from making the best patient-centered decision from the highest-quality data available that comports with our understanding of pathophysiology. In some situations, clinicians are forced to draw conclusions from data that are imperfect and apply it to patients who are complex and dynamic.
The Centers for Disease Control and Prevention (CDC) estimates that about 7.7% of the United States population has asthma. There were about 1 million ED visits in 2020, with asthma listed as the primary diagnosis, and only 94,000 required hospitalization.1 There are many tools we employ that have greatly decreased inpatient admissions for asthma. The uptake of inhaled corticosteroids (ICS) has significantly reduced asthma-related morbidity and mortality and reduced exacerbations that require admission to a hospital. This treatment strategy is supported by the Global Initiative for Asthma (GINA) and National Asthma Education and Prevention Program (NAEPP) guidelines.2,3 While we should celebrate the impact that EBM and ICS have had on asthma outcomes, we continue to struggle to control severe asthma.
Bronchodilator therapy in the hospital is ubiquitous. House staff and hospitalists click the bronchodilator order set early and often. However, the optimal frequency, dose, and duration of inhaled bronchodilator therapy for acute asthma exacerbation are unknown. Do frequency, dose, and duration change with exacerbation severity? Nothing gets ED, inpatient, or ICU physicians more jittery than the phrase “exacerbation of asthma on BiPap” or “intubated for asthma.” With its enormous clinical impact and notoriously difficult hospital and ICU course, the lack of evidence we have for managing these patients outside of the initial 24- to 48-hour visit is concerning. Neither NAEPP nor GINA provide management recommendations for the patient with severe asthma exacerbation that necessitates admission.
Albuterol is a commonly used medication for asthma and chronic obstructive airway disease. It is rapid acting and effective—few medications give patients (or clinicians) such instant satisfaction. As an internal medicine resident and pulmonary fellow, I ordered it countless times without ever looking at the dose. Sometimes, patients would come up from the emergency department after receiving a “continuous dose.” I would often wonder exactly what that meant. After some investigation, I found that in my hospital at the time, one dose of albuterol was 2.5 mg in 2 mL, and a continuous nebulization was four doses for a total of 10 mg.
Shrestha et al. found that high-dose albuterol (7.5 mg) administered continuously was superior to 2.5 mg albuterol delivered three times over 1.5 hours. There were demonstrable improvements in FEV1 and no ICU admissions.4 This study is one of many that compared intermittent to continuous and high-dose vs low-dose albuterol in the emergency department. Most are small and occur over the first 24 hours of presentation to the hospital. They often use short-term changes in spirometry as their primary outcome measure. Being a pulmonary and critical care doctor, I see patients who require advanced rescue maneuvers such as noninvasive positive pressure ventilation (NIPPV) or other pharmacologic adjuncts, for which the current evidence is limited.
Because studies of inhaled bronchodilators in acute asthma exacerbation use spirometry as their primary outcome, those with more severe disease and higher acuity are excluded. Patients on NIPPV can’t perform spirometry. There is essentially no literature to guide treatment for a patient with asthma in the adult ICU. In pediatric intensive care units, there are some data to support either continuous or intermittent inhaled bronchodilator that extends beyond the initial ED visit up to about 60 hours.5 Much of the pediatric data revolve about the amount of albuterol given, which can be as high as 75 mg/hr though is typically closer to 10-20 mg/hr.6 This rate is continued until respiratory improvement occurs.
With poor evidence to guide us and no specific direction from major guidelines, how should providers manage severe asthma exacerbation? The amount of drug deposited in the lung varies by the device used to deliver it. For nebulization, only about 10% of the nebulized amount reaches the lungs for effect; this is a smaller amount compared with all other devices one could use, such as MDI or DPI.7 Once a patient with asthma reaches the emergency department, that person is usually placed on some form of nebulizer treatment. But based on local hospital protocols, the amount and duration can vary widely. Sometimes, in patients with severe exacerbation, there is trepidation to continuing albuterol therapy due to ongoing tachycardia. This seems reasonable given increased albuterol administration could beget an ongoing cycle of dyspnea and anxiety. It could also lead to choosing therapies that are less evidence based.
In closing, this seemingly mundane topic takes on new meaning when a patient is in severe exacerbation. Fortunately, providers are not often faced with the decision to wade into the evidence-free territory of severe asthma exacerbation that is unresponsive to first-line treatments. This narrative should serve as a general alert that this pathophysiologic state is understudied. When encountered, thoughtful consideration of pathology, physiology, and pharmacology is required to reverse it.
References
1. Centers for Disease Control and Prevention. (2023, May 10). Most recent national asthma data. Centers for Disease Control and Prevention. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Global Initiative for Asthma - GINA. (2023, August 15). 2023 GINA Main Report - Global Initiative for Asthma - GINA. https://ginasthma.org/2023-gina-main-report/
3. Kiley J, Mensah GA, Boyce CA, et al (A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group). 2020 Focused updates to the: Asthma Management Guidelines. US Department of Health and Human Services, NIH, NHLBI 2020.
4. Shrestha M, Bidadi K, Gourlay S, Hayes J. Continuous vs intermittent albuterol, at high and low doses, in the treatment of severe acute asthma in adults. Chest. 1996 Jul;110(1):42-7. doi: 10.1378/chest.110.1.42. PMID: 8681661.
5. Kulalert P, Phinyo P, Patumanond J, Smathakanee C, Chuenjit W, Nanthapisal S. Continuous versus intermittent short-acting β2-agonists nebulization as first-line therapy in hospitalized children with severe asthma exacerbation: a propensity score matching analysis. Asthma Res Pract. 2020 Jul 2;6:6. doi: 10.1186/s40733-020-00059-5. PMID: 32632352; PMCID: PMC7329360.
6. Phumeetham S, Bahk TJ, Abd-Allah S, Mathur M. Effect of high-dose continuous albuterol nebulization on clinical variables in children with status asthmaticus. Pediatr Crit Care Med. 2015 Feb;16(2):e41-6. doi: 10.1097/PCC.0000000000000314. PMID: 25560428.
7. Gardenhire DS, Burnett D, Strickland S, Myers, TR. A guide to aerosol delivery devices for respiratory therapists. American Association for Respiratory Care, Dallas, Texas 2017.
Evidence-based medicine (EBM) stems from making the best patient-centered decision from the highest-quality data available that comports with our understanding of pathophysiology. In some situations, clinicians are forced to draw conclusions from data that are imperfect and apply it to patients who are complex and dynamic.
The Centers for Disease Control and Prevention (CDC) estimates that about 7.7% of the United States population has asthma. There were about 1 million ED visits in 2020, with asthma listed as the primary diagnosis, and only 94,000 required hospitalization.1 There are many tools we employ that have greatly decreased inpatient admissions for asthma. The uptake of inhaled corticosteroids (ICS) has significantly reduced asthma-related morbidity and mortality and reduced exacerbations that require admission to a hospital. This treatment strategy is supported by the Global Initiative for Asthma (GINA) and National Asthma Education and Prevention Program (NAEPP) guidelines.2,3 While we should celebrate the impact that EBM and ICS have had on asthma outcomes, we continue to struggle to control severe asthma.
Bronchodilator therapy in the hospital is ubiquitous. House staff and hospitalists click the bronchodilator order set early and often. However, the optimal frequency, dose, and duration of inhaled bronchodilator therapy for acute asthma exacerbation are unknown. Do frequency, dose, and duration change with exacerbation severity? Nothing gets ED, inpatient, or ICU physicians more jittery than the phrase “exacerbation of asthma on BiPap” or “intubated for asthma.” With its enormous clinical impact and notoriously difficult hospital and ICU course, the lack of evidence we have for managing these patients outside of the initial 24- to 48-hour visit is concerning. Neither NAEPP nor GINA provide management recommendations for the patient with severe asthma exacerbation that necessitates admission.
Albuterol is a commonly used medication for asthma and chronic obstructive airway disease. It is rapid acting and effective—few medications give patients (or clinicians) such instant satisfaction. As an internal medicine resident and pulmonary fellow, I ordered it countless times without ever looking at the dose. Sometimes, patients would come up from the emergency department after receiving a “continuous dose.” I would often wonder exactly what that meant. After some investigation, I found that in my hospital at the time, one dose of albuterol was 2.5 mg in 2 mL, and a continuous nebulization was four doses for a total of 10 mg.
Shrestha et al. found that high-dose albuterol (7.5 mg) administered continuously was superior to 2.5 mg albuterol delivered three times over 1.5 hours. There were demonstrable improvements in FEV1 and no ICU admissions.4 This study is one of many that compared intermittent to continuous and high-dose vs low-dose albuterol in the emergency department. Most are small and occur over the first 24 hours of presentation to the hospital. They often use short-term changes in spirometry as their primary outcome measure. Being a pulmonary and critical care doctor, I see patients who require advanced rescue maneuvers such as noninvasive positive pressure ventilation (NIPPV) or other pharmacologic adjuncts, for which the current evidence is limited.
Because studies of inhaled bronchodilators in acute asthma exacerbation use spirometry as their primary outcome, those with more severe disease and higher acuity are excluded. Patients on NIPPV can’t perform spirometry. There is essentially no literature to guide treatment for a patient with asthma in the adult ICU. In pediatric intensive care units, there are some data to support either continuous or intermittent inhaled bronchodilator that extends beyond the initial ED visit up to about 60 hours.5 Much of the pediatric data revolve about the amount of albuterol given, which can be as high as 75 mg/hr though is typically closer to 10-20 mg/hr.6 This rate is continued until respiratory improvement occurs.
With poor evidence to guide us and no specific direction from major guidelines, how should providers manage severe asthma exacerbation? The amount of drug deposited in the lung varies by the device used to deliver it. For nebulization, only about 10% of the nebulized amount reaches the lungs for effect; this is a smaller amount compared with all other devices one could use, such as MDI or DPI.7 Once a patient with asthma reaches the emergency department, that person is usually placed on some form of nebulizer treatment. But based on local hospital protocols, the amount and duration can vary widely. Sometimes, in patients with severe exacerbation, there is trepidation to continuing albuterol therapy due to ongoing tachycardia. This seems reasonable given increased albuterol administration could beget an ongoing cycle of dyspnea and anxiety. It could also lead to choosing therapies that are less evidence based.
In closing, this seemingly mundane topic takes on new meaning when a patient is in severe exacerbation. Fortunately, providers are not often faced with the decision to wade into the evidence-free territory of severe asthma exacerbation that is unresponsive to first-line treatments. This narrative should serve as a general alert that this pathophysiologic state is understudied. When encountered, thoughtful consideration of pathology, physiology, and pharmacology is required to reverse it.
References
1. Centers for Disease Control and Prevention. (2023, May 10). Most recent national asthma data. Centers for Disease Control and Prevention. https://www.cdc.gov/asthma/most_recent_national_asthma_data.htm
2. Global Initiative for Asthma - GINA. (2023, August 15). 2023 GINA Main Report - Global Initiative for Asthma - GINA. https://ginasthma.org/2023-gina-main-report/
3. Kiley J, Mensah GA, Boyce CA, et al (A Report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group). 2020 Focused updates to the: Asthma Management Guidelines. US Department of Health and Human Services, NIH, NHLBI 2020.
4. Shrestha M, Bidadi K, Gourlay S, Hayes J. Continuous vs intermittent albuterol, at high and low doses, in the treatment of severe acute asthma in adults. Chest. 1996 Jul;110(1):42-7. doi: 10.1378/chest.110.1.42. PMID: 8681661.
5. Kulalert P, Phinyo P, Patumanond J, Smathakanee C, Chuenjit W, Nanthapisal S. Continuous versus intermittent short-acting β2-agonists nebulization as first-line therapy in hospitalized children with severe asthma exacerbation: a propensity score matching analysis. Asthma Res Pract. 2020 Jul 2;6:6. doi: 10.1186/s40733-020-00059-5. PMID: 32632352; PMCID: PMC7329360.
6. Phumeetham S, Bahk TJ, Abd-Allah S, Mathur M. Effect of high-dose continuous albuterol nebulization on clinical variables in children with status asthmaticus. Pediatr Crit Care Med. 2015 Feb;16(2):e41-6. doi: 10.1097/PCC.0000000000000314. PMID: 25560428.
7. Gardenhire DS, Burnett D, Strickland S, Myers, TR. A guide to aerosol delivery devices for respiratory therapists. American Association for Respiratory Care, Dallas, Texas 2017.
Obesity and lung disease in the era of GLP-1 agonists
Now is the time for pulmonary clinicians to become comfortable counseling patients about and treating obesity. By 2030, half of the US population will have obesity, a quarter of which will be severe (Ward et al. NEJM. 2019;2440-2450).
Many pulmonary diseases, including asthma, COPD, and interstitial pulmonary fibrosis (IPF) are linked to and made worse by obesity with increased exacerbations, patient-reported decreased quality of life, and resistance to therapy (Ray et al. Am Rev Respir Dis. 1983;501-6). Asthma is even recognized as an obesity-related comorbid condition by both the American Society Metabolic and Bariatric Surgery (ASMBS) and the American Association of Clinical Endocrinologists (AACE) when considering indications for early or more aggressive treatment of obesity (Eisenberg et al. Obesity Surg. 2023;3-14) (Garvey et al. Endocr Pract. 2016;1-203).
Obesity has multiple negative effects on pulmonary function due to the physical forces of extra weight on the lungs and inflammation related to adipose tissue (see Figure 1) (Zerah et al. Chest. 1993;1470-6).
Obesity-related respiratory changes include reduced lung compliance, functional residual capacity (FRC), and expiratory reserve volume (ERV). These changes lead to peripheral atelectasis and V/Q mismatch and increased metabolic demands placed on the respiratory system (Parameswaran et al. Can Respir J. 2006;203-10). The increased weight supported by the thoracic cage alters the equilibrium between the chest wall and lung tissue decreasing FRC and ERV. This reduces lung compliance and increases stiffness by promoting areas of atelectasis and increased alveolar surface tension (Dixon et al. Expert Rev Respir Med. 2018;755-67).
Another biomechanical cost of obesity on respiratory function is the increased consumption of oxygen to sustain ventilation at rest (Koenig SM, Am J Med Sci. 2001;249-79). This can lead to early respiratory muscle fatigue when respiratory rate and tidal volume increase with activity. Patients with obesity are more likely to develop obstructive sleep apnea and obesity hypoventilation syndrome. The resulting alveolar hypoxemia is thought to contribute to the increase in pulmonary hypertension observed in patients with obesity (Shah et al. Breathe. 2023;19[1]). In addition to the biomechanical consequences of obesity, increased adipose tissue can lead to chronic, systemic inflammation that can exacerbate or unmask underlying respiratory disease. Increased leptin and downregulation of adiponectin have been shown to increase systemic cytokine production (Ray et al. Am Rev Respir Dis. 1983;501-6). This inflammatory process contributes to increased airway resistance and an altered response to corticosteroids (inhaled or systemic) in obese patients treated for bronchial hyperresponsiveness. This perhaps reflects the Th2-low phenotype seen in patients with obesity and metabolic syndrome-related asthma (Shah et al. Breathe. 2023;19[1]) (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812).
Multiple studies have demonstrated weight loss through lifestyle changes, medical therapy, and obesity surgery result benefits pulmonary disease (Forno et al. PloS One. 2019;14[4]) (Ardila-Gatas et al. Surg Endosc. 2019;1952-8). Benefits include decreased exacerbation frequency, improved functional testing, and improved patient-reported quality of life. Pulmonary clinicians should be empowered to address obesity as a comorbid condition and treat with appropriate referrals for obesity surgery and initiation of medications when indicated.
GLP-1 receptor agonists
In the past year, glucagon-like peptide receptor agonists (GLP-1RAs) have garnered attention in the medical literature and popular news outlets. GLP-1RAs, including semaglutide, liraglutide, and tirzepatide, are currently FDA approved for the treatment of obesity in patients with a body mass index (BMI) greater than or equal to 30 or a BMI greater than or equal to 27 in the setting of an obesity-related comorbidity, including asthma.
This class of medications acts by increasing the physiologic insulin response to a glucose load, delaying gastric emptying, and reducing production of glucagon. In a phase III study, semaglutide resulted in greater than 15% weight reduction from baseline (Wadden et al. JAMA. 2021;1403-13). In clinical trials, these medications have not only resulted in significant, sustained weight loss but also improved lipid profiles, decreased A1c, and reduced major cardiovascular events (Lincoff et al. N Engl J Med. 2023;389[23]:2221-32) (Verma et al. Circulation. 2018;138[25]:2884-94).
GLP-1RAs and lung disease
GLP-1RAs are associated with ranges of weight loss that lead to symptom improvement. Beyond the anticipated benefits for pulmonary health, there is interest in whether GLP-1RAs may improve specific lung diseases. GLP-1 receptors are found throughout the body (eg, gastrointestinal tract, kidneys, and heart) with the largest proportion located in the lungs (Wu AY and Peebles RS. Expert Rev Clin Immunol. 2021;1053-7). In addition to their known effect on insulin response, GLP-1RAs are hypothesized to reduce proinflammatory cytokine signaling and alter surfactant production potentially improving both airway resistance and lung compliance (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Animal models suggest an antifibrotic effect with delay in the endothelial-mesenchymal transition. If further substantiated, this could impact both acute and chronic lung injury.
Early clinical studies of GLP-1RAs in patients with respiratory diseases have demonstrated improved symptoms and pulmonary function (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Even modest weight loss (2.5 kg in a year) with GLP-1RAs leads to improved symptoms and a reduction in asthma exacerbations. Other asthma literature shows GLP-1RAs improve symptoms and reduce exacerbations independent of changes in weight, supporting the hypothesis that the benefit of GLP-1RAs may be more than biomechanical improvement from weight loss alone (Foer et al. Am J Respir Crit Care Med. 2021;831-40).
GLP-1RAs reduce the proinflammatory cytokine signaling in both TH2-high and TH2-low asthma phenotypes and alter surfactant production, airway resistance, and perhaps even pulmonary vascular resistance (Altintas Dogan et al. Int J Chron Obstruct Pulmon Dis. 2022,405-14). GATA-3 is an ongoing clinical trial examining whether GLP-1RAs reduce airway inflammation via direct effects on of the respiratory tract (NCT05254314).
Drugs developed to treat one condition are often found to impact others during validation studies or postmarketing observation. Some examples are aspirin, sildenafil, minoxidil, hydroxychloroquine, and SGLT-2 inhibitors. Will GLP-1RAs be the latest medication to affect a broad array of physiologic process and end up improving not just metabolic but also lung health?
Now is the time for pulmonary clinicians to become comfortable counseling patients about and treating obesity. By 2030, half of the US population will have obesity, a quarter of which will be severe (Ward et al. NEJM. 2019;2440-2450).
Many pulmonary diseases, including asthma, COPD, and interstitial pulmonary fibrosis (IPF) are linked to and made worse by obesity with increased exacerbations, patient-reported decreased quality of life, and resistance to therapy (Ray et al. Am Rev Respir Dis. 1983;501-6). Asthma is even recognized as an obesity-related comorbid condition by both the American Society Metabolic and Bariatric Surgery (ASMBS) and the American Association of Clinical Endocrinologists (AACE) when considering indications for early or more aggressive treatment of obesity (Eisenberg et al. Obesity Surg. 2023;3-14) (Garvey et al. Endocr Pract. 2016;1-203).
Obesity has multiple negative effects on pulmonary function due to the physical forces of extra weight on the lungs and inflammation related to adipose tissue (see Figure 1) (Zerah et al. Chest. 1993;1470-6).
Obesity-related respiratory changes include reduced lung compliance, functional residual capacity (FRC), and expiratory reserve volume (ERV). These changes lead to peripheral atelectasis and V/Q mismatch and increased metabolic demands placed on the respiratory system (Parameswaran et al. Can Respir J. 2006;203-10). The increased weight supported by the thoracic cage alters the equilibrium between the chest wall and lung tissue decreasing FRC and ERV. This reduces lung compliance and increases stiffness by promoting areas of atelectasis and increased alveolar surface tension (Dixon et al. Expert Rev Respir Med. 2018;755-67).
Another biomechanical cost of obesity on respiratory function is the increased consumption of oxygen to sustain ventilation at rest (Koenig SM, Am J Med Sci. 2001;249-79). This can lead to early respiratory muscle fatigue when respiratory rate and tidal volume increase with activity. Patients with obesity are more likely to develop obstructive sleep apnea and obesity hypoventilation syndrome. The resulting alveolar hypoxemia is thought to contribute to the increase in pulmonary hypertension observed in patients with obesity (Shah et al. Breathe. 2023;19[1]). In addition to the biomechanical consequences of obesity, increased adipose tissue can lead to chronic, systemic inflammation that can exacerbate or unmask underlying respiratory disease. Increased leptin and downregulation of adiponectin have been shown to increase systemic cytokine production (Ray et al. Am Rev Respir Dis. 1983;501-6). This inflammatory process contributes to increased airway resistance and an altered response to corticosteroids (inhaled or systemic) in obese patients treated for bronchial hyperresponsiveness. This perhaps reflects the Th2-low phenotype seen in patients with obesity and metabolic syndrome-related asthma (Shah et al. Breathe. 2023;19[1]) (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812).
Multiple studies have demonstrated weight loss through lifestyle changes, medical therapy, and obesity surgery result benefits pulmonary disease (Forno et al. PloS One. 2019;14[4]) (Ardila-Gatas et al. Surg Endosc. 2019;1952-8). Benefits include decreased exacerbation frequency, improved functional testing, and improved patient-reported quality of life. Pulmonary clinicians should be empowered to address obesity as a comorbid condition and treat with appropriate referrals for obesity surgery and initiation of medications when indicated.
GLP-1 receptor agonists
In the past year, glucagon-like peptide receptor agonists (GLP-1RAs) have garnered attention in the medical literature and popular news outlets. GLP-1RAs, including semaglutide, liraglutide, and tirzepatide, are currently FDA approved for the treatment of obesity in patients with a body mass index (BMI) greater than or equal to 30 or a BMI greater than or equal to 27 in the setting of an obesity-related comorbidity, including asthma.
This class of medications acts by increasing the physiologic insulin response to a glucose load, delaying gastric emptying, and reducing production of glucagon. In a phase III study, semaglutide resulted in greater than 15% weight reduction from baseline (Wadden et al. JAMA. 2021;1403-13). In clinical trials, these medications have not only resulted in significant, sustained weight loss but also improved lipid profiles, decreased A1c, and reduced major cardiovascular events (Lincoff et al. N Engl J Med. 2023;389[23]:2221-32) (Verma et al. Circulation. 2018;138[25]:2884-94).
GLP-1RAs and lung disease
GLP-1RAs are associated with ranges of weight loss that lead to symptom improvement. Beyond the anticipated benefits for pulmonary health, there is interest in whether GLP-1RAs may improve specific lung diseases. GLP-1 receptors are found throughout the body (eg, gastrointestinal tract, kidneys, and heart) with the largest proportion located in the lungs (Wu AY and Peebles RS. Expert Rev Clin Immunol. 2021;1053-7). In addition to their known effect on insulin response, GLP-1RAs are hypothesized to reduce proinflammatory cytokine signaling and alter surfactant production potentially improving both airway resistance and lung compliance (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Animal models suggest an antifibrotic effect with delay in the endothelial-mesenchymal transition. If further substantiated, this could impact both acute and chronic lung injury.
Early clinical studies of GLP-1RAs in patients with respiratory diseases have demonstrated improved symptoms and pulmonary function (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Even modest weight loss (2.5 kg in a year) with GLP-1RAs leads to improved symptoms and a reduction in asthma exacerbations. Other asthma literature shows GLP-1RAs improve symptoms and reduce exacerbations independent of changes in weight, supporting the hypothesis that the benefit of GLP-1RAs may be more than biomechanical improvement from weight loss alone (Foer et al. Am J Respir Crit Care Med. 2021;831-40).
GLP-1RAs reduce the proinflammatory cytokine signaling in both TH2-high and TH2-low asthma phenotypes and alter surfactant production, airway resistance, and perhaps even pulmonary vascular resistance (Altintas Dogan et al. Int J Chron Obstruct Pulmon Dis. 2022,405-14). GATA-3 is an ongoing clinical trial examining whether GLP-1RAs reduce airway inflammation via direct effects on of the respiratory tract (NCT05254314).
Drugs developed to treat one condition are often found to impact others during validation studies or postmarketing observation. Some examples are aspirin, sildenafil, minoxidil, hydroxychloroquine, and SGLT-2 inhibitors. Will GLP-1RAs be the latest medication to affect a broad array of physiologic process and end up improving not just metabolic but also lung health?
Now is the time for pulmonary clinicians to become comfortable counseling patients about and treating obesity. By 2030, half of the US population will have obesity, a quarter of which will be severe (Ward et al. NEJM. 2019;2440-2450).
Many pulmonary diseases, including asthma, COPD, and interstitial pulmonary fibrosis (IPF) are linked to and made worse by obesity with increased exacerbations, patient-reported decreased quality of life, and resistance to therapy (Ray et al. Am Rev Respir Dis. 1983;501-6). Asthma is even recognized as an obesity-related comorbid condition by both the American Society Metabolic and Bariatric Surgery (ASMBS) and the American Association of Clinical Endocrinologists (AACE) when considering indications for early or more aggressive treatment of obesity (Eisenberg et al. Obesity Surg. 2023;3-14) (Garvey et al. Endocr Pract. 2016;1-203).
Obesity has multiple negative effects on pulmonary function due to the physical forces of extra weight on the lungs and inflammation related to adipose tissue (see Figure 1) (Zerah et al. Chest. 1993;1470-6).
Obesity-related respiratory changes include reduced lung compliance, functional residual capacity (FRC), and expiratory reserve volume (ERV). These changes lead to peripheral atelectasis and V/Q mismatch and increased metabolic demands placed on the respiratory system (Parameswaran et al. Can Respir J. 2006;203-10). The increased weight supported by the thoracic cage alters the equilibrium between the chest wall and lung tissue decreasing FRC and ERV. This reduces lung compliance and increases stiffness by promoting areas of atelectasis and increased alveolar surface tension (Dixon et al. Expert Rev Respir Med. 2018;755-67).
Another biomechanical cost of obesity on respiratory function is the increased consumption of oxygen to sustain ventilation at rest (Koenig SM, Am J Med Sci. 2001;249-79). This can lead to early respiratory muscle fatigue when respiratory rate and tidal volume increase with activity. Patients with obesity are more likely to develop obstructive sleep apnea and obesity hypoventilation syndrome. The resulting alveolar hypoxemia is thought to contribute to the increase in pulmonary hypertension observed in patients with obesity (Shah et al. Breathe. 2023;19[1]). In addition to the biomechanical consequences of obesity, increased adipose tissue can lead to chronic, systemic inflammation that can exacerbate or unmask underlying respiratory disease. Increased leptin and downregulation of adiponectin have been shown to increase systemic cytokine production (Ray et al. Am Rev Respir Dis. 1983;501-6). This inflammatory process contributes to increased airway resistance and an altered response to corticosteroids (inhaled or systemic) in obese patients treated for bronchial hyperresponsiveness. This perhaps reflects the Th2-low phenotype seen in patients with obesity and metabolic syndrome-related asthma (Shah et al. Breathe. 2023;19[1]) (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812).
Multiple studies have demonstrated weight loss through lifestyle changes, medical therapy, and obesity surgery result benefits pulmonary disease (Forno et al. PloS One. 2019;14[4]) (Ardila-Gatas et al. Surg Endosc. 2019;1952-8). Benefits include decreased exacerbation frequency, improved functional testing, and improved patient-reported quality of life. Pulmonary clinicians should be empowered to address obesity as a comorbid condition and treat with appropriate referrals for obesity surgery and initiation of medications when indicated.
GLP-1 receptor agonists
In the past year, glucagon-like peptide receptor agonists (GLP-1RAs) have garnered attention in the medical literature and popular news outlets. GLP-1RAs, including semaglutide, liraglutide, and tirzepatide, are currently FDA approved for the treatment of obesity in patients with a body mass index (BMI) greater than or equal to 30 or a BMI greater than or equal to 27 in the setting of an obesity-related comorbidity, including asthma.
This class of medications acts by increasing the physiologic insulin response to a glucose load, delaying gastric emptying, and reducing production of glucagon. In a phase III study, semaglutide resulted in greater than 15% weight reduction from baseline (Wadden et al. JAMA. 2021;1403-13). In clinical trials, these medications have not only resulted in significant, sustained weight loss but also improved lipid profiles, decreased A1c, and reduced major cardiovascular events (Lincoff et al. N Engl J Med. 2023;389[23]:2221-32) (Verma et al. Circulation. 2018;138[25]:2884-94).
GLP-1RAs and lung disease
GLP-1RAs are associated with ranges of weight loss that lead to symptom improvement. Beyond the anticipated benefits for pulmonary health, there is interest in whether GLP-1RAs may improve specific lung diseases. GLP-1 receptors are found throughout the body (eg, gastrointestinal tract, kidneys, and heart) with the largest proportion located in the lungs (Wu AY and Peebles RS. Expert Rev Clin Immunol. 2021;1053-7). In addition to their known effect on insulin response, GLP-1RAs are hypothesized to reduce proinflammatory cytokine signaling and alter surfactant production potentially improving both airway resistance and lung compliance (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Animal models suggest an antifibrotic effect with delay in the endothelial-mesenchymal transition. If further substantiated, this could impact both acute and chronic lung injury.
Early clinical studies of GLP-1RAs in patients with respiratory diseases have demonstrated improved symptoms and pulmonary function (Kanwar et al. Cureus. 2022 Oct 28. doi: 10.7759/cureus.30812). Even modest weight loss (2.5 kg in a year) with GLP-1RAs leads to improved symptoms and a reduction in asthma exacerbations. Other asthma literature shows GLP-1RAs improve symptoms and reduce exacerbations independent of changes in weight, supporting the hypothesis that the benefit of GLP-1RAs may be more than biomechanical improvement from weight loss alone (Foer et al. Am J Respir Crit Care Med. 2021;831-40).
GLP-1RAs reduce the proinflammatory cytokine signaling in both TH2-high and TH2-low asthma phenotypes and alter surfactant production, airway resistance, and perhaps even pulmonary vascular resistance (Altintas Dogan et al. Int J Chron Obstruct Pulmon Dis. 2022,405-14). GATA-3 is an ongoing clinical trial examining whether GLP-1RAs reduce airway inflammation via direct effects on of the respiratory tract (NCT05254314).
Drugs developed to treat one condition are often found to impact others during validation studies or postmarketing observation. Some examples are aspirin, sildenafil, minoxidil, hydroxychloroquine, and SGLT-2 inhibitors. Will GLP-1RAs be the latest medication to affect a broad array of physiologic process and end up improving not just metabolic but also lung health?
The double-edged sword of virtual pulmonary rehabilitation
It offers a comprehensive approach to improving lung health and overall quality of life using a combination of tailored exercise routines, educational sessions, and emotional support. It empowers our patients to better manage their conditions, improve their fitness level, and regain a sense of control over their lives. However, the response to the COVID-19 pandemic increased the use of telemedicine as a method for providing health care (Shaver J. Prim Care. 2022;49[4]:517).
Many patients have welcomed the convenience offered by virtual care, and studies have demonstrated high levels of patient satisfaction (Polinski JM, et al. Gen Intern Med. 2016;31[3]:269). Geography also drives telehealth use. In urban areas in the United States, the median travel distance is 7.5 miles one way with a resulting travel time of 3 to 25 minutes. In rural areas, the estimated travel distance is three times as long. Distance and travel time have been recognized as major barriers to attending PR (Keating A, et al. Chron Respir Dis. 2011;8[2]:89).
Access to PR is also hindered by lack of program availability. As of 2019, there were only 831 pulmonary rehab centers in the United States serving roughly 24 million patients with COPD. Only 561 of these centers are certified by the American Association of Cardiovascular and Pulmonary Rehabilitation, leaving only one certified center for every 43,000 patients with COPD (Chan L, et al. J Rural Health. 2006;22[2]:140). As such, virtual PR is one option for augmenting availability and accessibility.
While virtual PR programs offer numerous advantages, including accessibility and convenience, there are inherent risks and challenges. There is also concern that they are inferior to in-person PR. They offer less supervision by trained health care professionals and no immediate access to medical assistance. Combined with the absence of real-time monitoring of vitals or symptoms, there may be a higher risk of adverse events despite the incorporation of safety measures. Furthermore, the lack of accountability forces an increased reliance on self-motivation, which may hinder progress (Spruit MA, et al. Am J Respir Crit Care Med. 2013;188[8]:e13).
Although the digital divide is narrowing rapidly, reliable access to technology, combined with poor internet connections or computer literacy, will prevent adoption by some patients. Even in well-resourced areas, technical issues can disrupt continuity. Finally, virtual PR lacks the intangible benefits from in-person group sessions. Social interactions in this already isolated subset of patients are lost in virtual PR, and the cultivation of motivation and support to seek a common goal goes unrealized.
While these concerns are appreciated, PR is currently highly underutilized and essentially unavailable to most pulmonary patients. As such, further study is needed to shape the future design of quality virtual PR programs. In the March 2023 issue of the journal CHEST, Huynh and colleagues published an observational cohort study comparing virtual with traditional PR programs (Huynh VC, et al. Chest. 2023; Mar;163[3]:529). Of the 554 participants in the study, 171 were enrolled in virtual and 383 to in-person PR. Attendance and drop-out rates did not differ, CAT scores significantly improved in both programs, and there were no adverse events during virtual PR. Participants in the virtual group received a TheraBand and were required to have a sturdy chair, three large step-lengths of empty space surrounding their chair, and access to internet/Zoom. They had one-on-one Zoom meetings but relied mostly on staff-made or online videos. These results replicate past investigations that have demonstrated low adverse event rates, positive overall patient satisfaction, and noninferiority in patient-centered outcomes with PR. The total volume of data remains limited though (Cox NS, et al. Cochrane Database Syst Rev. 2021;Issue 1;Art No: CD013040).
PR is an essential resource for the management of chronic lung diseases. Given existing barriers and the growing number of eligible patients, we must embrace alternative delivery strategies, all the while ensuring that a quality and useful product is deployed (Rochester CL, et al. Am J Respir Crit Care Med. 2015;192[11]:1373). Additional study is needed to standardize and validate the implementation of virtual PR. Ultimately, virtual and alternative methods of care delivery may help optimize outcomes for our patients where more traditional methods fall short.
The views and opinions of authors expressed herein do not necessarily reflect those of the Department of Veterans Affairs or the U.S. government. Dr. Cagle and Dr. Gartman are with the Warren Alpert Medical School of Brown University and Providence VA Medical Center, Division of Pulmonary, Critical Care, and Sleep Medicine. Providence, R.I.
It offers a comprehensive approach to improving lung health and overall quality of life using a combination of tailored exercise routines, educational sessions, and emotional support. It empowers our patients to better manage their conditions, improve their fitness level, and regain a sense of control over their lives. However, the response to the COVID-19 pandemic increased the use of telemedicine as a method for providing health care (Shaver J. Prim Care. 2022;49[4]:517).
Many patients have welcomed the convenience offered by virtual care, and studies have demonstrated high levels of patient satisfaction (Polinski JM, et al. Gen Intern Med. 2016;31[3]:269). Geography also drives telehealth use. In urban areas in the United States, the median travel distance is 7.5 miles one way with a resulting travel time of 3 to 25 minutes. In rural areas, the estimated travel distance is three times as long. Distance and travel time have been recognized as major barriers to attending PR (Keating A, et al. Chron Respir Dis. 2011;8[2]:89).
Access to PR is also hindered by lack of program availability. As of 2019, there were only 831 pulmonary rehab centers in the United States serving roughly 24 million patients with COPD. Only 561 of these centers are certified by the American Association of Cardiovascular and Pulmonary Rehabilitation, leaving only one certified center for every 43,000 patients with COPD (Chan L, et al. J Rural Health. 2006;22[2]:140). As such, virtual PR is one option for augmenting availability and accessibility.
While virtual PR programs offer numerous advantages, including accessibility and convenience, there are inherent risks and challenges. There is also concern that they are inferior to in-person PR. They offer less supervision by trained health care professionals and no immediate access to medical assistance. Combined with the absence of real-time monitoring of vitals or symptoms, there may be a higher risk of adverse events despite the incorporation of safety measures. Furthermore, the lack of accountability forces an increased reliance on self-motivation, which may hinder progress (Spruit MA, et al. Am J Respir Crit Care Med. 2013;188[8]:e13).
Although the digital divide is narrowing rapidly, reliable access to technology, combined with poor internet connections or computer literacy, will prevent adoption by some patients. Even in well-resourced areas, technical issues can disrupt continuity. Finally, virtual PR lacks the intangible benefits from in-person group sessions. Social interactions in this already isolated subset of patients are lost in virtual PR, and the cultivation of motivation and support to seek a common goal goes unrealized.
While these concerns are appreciated, PR is currently highly underutilized and essentially unavailable to most pulmonary patients. As such, further study is needed to shape the future design of quality virtual PR programs. In the March 2023 issue of the journal CHEST, Huynh and colleagues published an observational cohort study comparing virtual with traditional PR programs (Huynh VC, et al. Chest. 2023; Mar;163[3]:529). Of the 554 participants in the study, 171 were enrolled in virtual and 383 to in-person PR. Attendance and drop-out rates did not differ, CAT scores significantly improved in both programs, and there were no adverse events during virtual PR. Participants in the virtual group received a TheraBand and were required to have a sturdy chair, three large step-lengths of empty space surrounding their chair, and access to internet/Zoom. They had one-on-one Zoom meetings but relied mostly on staff-made or online videos. These results replicate past investigations that have demonstrated low adverse event rates, positive overall patient satisfaction, and noninferiority in patient-centered outcomes with PR. The total volume of data remains limited though (Cox NS, et al. Cochrane Database Syst Rev. 2021;Issue 1;Art No: CD013040).
PR is an essential resource for the management of chronic lung diseases. Given existing barriers and the growing number of eligible patients, we must embrace alternative delivery strategies, all the while ensuring that a quality and useful product is deployed (Rochester CL, et al. Am J Respir Crit Care Med. 2015;192[11]:1373). Additional study is needed to standardize and validate the implementation of virtual PR. Ultimately, virtual and alternative methods of care delivery may help optimize outcomes for our patients where more traditional methods fall short.
The views and opinions of authors expressed herein do not necessarily reflect those of the Department of Veterans Affairs or the U.S. government. Dr. Cagle and Dr. Gartman are with the Warren Alpert Medical School of Brown University and Providence VA Medical Center, Division of Pulmonary, Critical Care, and Sleep Medicine. Providence, R.I.
It offers a comprehensive approach to improving lung health and overall quality of life using a combination of tailored exercise routines, educational sessions, and emotional support. It empowers our patients to better manage their conditions, improve their fitness level, and regain a sense of control over their lives. However, the response to the COVID-19 pandemic increased the use of telemedicine as a method for providing health care (Shaver J. Prim Care. 2022;49[4]:517).
Many patients have welcomed the convenience offered by virtual care, and studies have demonstrated high levels of patient satisfaction (Polinski JM, et al. Gen Intern Med. 2016;31[3]:269). Geography also drives telehealth use. In urban areas in the United States, the median travel distance is 7.5 miles one way with a resulting travel time of 3 to 25 minutes. In rural areas, the estimated travel distance is three times as long. Distance and travel time have been recognized as major barriers to attending PR (Keating A, et al. Chron Respir Dis. 2011;8[2]:89).
Access to PR is also hindered by lack of program availability. As of 2019, there were only 831 pulmonary rehab centers in the United States serving roughly 24 million patients with COPD. Only 561 of these centers are certified by the American Association of Cardiovascular and Pulmonary Rehabilitation, leaving only one certified center for every 43,000 patients with COPD (Chan L, et al. J Rural Health. 2006;22[2]:140). As such, virtual PR is one option for augmenting availability and accessibility.
While virtual PR programs offer numerous advantages, including accessibility and convenience, there are inherent risks and challenges. There is also concern that they are inferior to in-person PR. They offer less supervision by trained health care professionals and no immediate access to medical assistance. Combined with the absence of real-time monitoring of vitals or symptoms, there may be a higher risk of adverse events despite the incorporation of safety measures. Furthermore, the lack of accountability forces an increased reliance on self-motivation, which may hinder progress (Spruit MA, et al. Am J Respir Crit Care Med. 2013;188[8]:e13).
Although the digital divide is narrowing rapidly, reliable access to technology, combined with poor internet connections or computer literacy, will prevent adoption by some patients. Even in well-resourced areas, technical issues can disrupt continuity. Finally, virtual PR lacks the intangible benefits from in-person group sessions. Social interactions in this already isolated subset of patients are lost in virtual PR, and the cultivation of motivation and support to seek a common goal goes unrealized.
While these concerns are appreciated, PR is currently highly underutilized and essentially unavailable to most pulmonary patients. As such, further study is needed to shape the future design of quality virtual PR programs. In the March 2023 issue of the journal CHEST, Huynh and colleagues published an observational cohort study comparing virtual with traditional PR programs (Huynh VC, et al. Chest. 2023; Mar;163[3]:529). Of the 554 participants in the study, 171 were enrolled in virtual and 383 to in-person PR. Attendance and drop-out rates did not differ, CAT scores significantly improved in both programs, and there were no adverse events during virtual PR. Participants in the virtual group received a TheraBand and were required to have a sturdy chair, three large step-lengths of empty space surrounding their chair, and access to internet/Zoom. They had one-on-one Zoom meetings but relied mostly on staff-made or online videos. These results replicate past investigations that have demonstrated low adverse event rates, positive overall patient satisfaction, and noninferiority in patient-centered outcomes with PR. The total volume of data remains limited though (Cox NS, et al. Cochrane Database Syst Rev. 2021;Issue 1;Art No: CD013040).
PR is an essential resource for the management of chronic lung diseases. Given existing barriers and the growing number of eligible patients, we must embrace alternative delivery strategies, all the while ensuring that a quality and useful product is deployed (Rochester CL, et al. Am J Respir Crit Care Med. 2015;192[11]:1373). Additional study is needed to standardize and validate the implementation of virtual PR. Ultimately, virtual and alternative methods of care delivery may help optimize outcomes for our patients where more traditional methods fall short.
The views and opinions of authors expressed herein do not necessarily reflect those of the Department of Veterans Affairs or the U.S. government. Dr. Cagle and Dr. Gartman are with the Warren Alpert Medical School of Brown University and Providence VA Medical Center, Division of Pulmonary, Critical Care, and Sleep Medicine. Providence, R.I.
Home oxygen therapy: What does the data show?
Inhalers, nebulizers, antibiotics, and steroids – these are some of the most common tools in our pulmonary arsenal that we deploy on a daily basis. But, there is no treatment more fundamental to a pulmonary practitioner than oxygen. So how is it that something that naturally occurs and comprises 21% of ambient air has become so medicalized?
It is difficult (perhaps impossible) to find a pulmonologist or a hospitalist who has not included the phrase “obtain ambulatory saturation to qualify the patient for home oxygen” in at least one of their progress notes on a daily basis. Chronic obstructive pulmonary disease (COPD) is the most common reason for the prescription of long-term oxygen therapy (LTOT), a large industry tightly regulated by the Centers for Medicare & Medicaid Services (CMS).
The evidence for the use of LTOT in patients with COPD dates back to two seminal papers published in 1980 and 1981. The British Medical Research Council Working Party conducted the BMRC trial, in which 87 patients with a Pa
Another study published around the same time, the Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease (NOTT) trial (Ann Intern Med. 1980;93[3]:391-8) directly compared continuous 24-hour to nocturnal home oxygen therapy in patients with COPD and severe hypoxemia with a Pa
Afterward, it became universally accepted dogma that patients with COPD and severe hypoxemia stood to substantially benefit from LTOT. For years, it was the only therapy associated with a mortality reduction. The LOTT study (Albert RK, et al. N Engl J Med. 2016;375[17]:1617-27) included 768 patients with stable COPD and a resting or nocturnal Sp
The INOX (Lacasse Y, et al. N Engl J Med. 2020;383[12]:1129-38) trial, in which 243 patients with oxygen saturation less than 90% for at least 30% of the night were assigned to receive nocturnal vs sham oxygen, found similar results. There was no difference in the composite outcome of all-cause mortality and progression to 24-7 oxygen requirement (according to the criteria originally defined by NOTT). A 2022 systematic review and meta-analysis including six studies designed to assess the role of LTOT in patients with COPD and moderate desaturation, including LOTT and INOX, found no benefit to providing LTOT (Lacasse Y, et al. Lancet Respir Med. 2022;10[11]:1029-37).
Based on these studies, a resting Sp
COPD management has changed significantly in the 40 years since NOTT was published. In the early 1980s, standard of care included an inhaled beta-agonist and oral theophylline. We now prescribe a regimen of modern-day inhaler combinations, which can lead to a mortality benefit in the correct population. Additionally, rates of smoking are markedly lower now than they were in 1980. In the Minnesota Heart Survey, the prevalence of being an ever-smoking man or woman in 1980 compared with 2009 dropped from 71.6% and 54.7% to 44.2% and 39.6%, respectively (Filion KB, et al. Am J Public Health. 2012;102[4]:705-13). Treatment of common comorbid conditions has also dramatically improved.
A report containing all fee-for-service data published in 2021 by CMS reported oxygen therapy accounted for 9.8% of all DME costs covered by CMS and totaled approximately $800,000,000 (Centers for Medicare & Medicaid Services. FFS Data. 2021. This represents a significant financial burden to our health system and government.
Two of the eligible groups per CMS (those with isolated ambulatory or nocturnal hypoxemia) do not benefit from LTOT in RCTs. The other two groups are eligible based on trial data from a small number of patients who were studied more than 40 years ago. These facts raise serious questions about the cost-efficacy of LTOT.
So where does this leave us?
There are significant barriers to repeating large randomized oxygen trials. Due to broad inclusion criteria for LTOT by CMS, there are undoubtedly many people prescribed LTOT for whom there is minimal to no benefit. Patients often feel restricted in their mobility and may feel isolated being tethered to medical equipment. It is good practice to think about LTOT the same way we do any other therapy we provide - as a medicine with associated risks, benefits, and costs.
Despite its ubiquity, oxygen remains an important therapeutic tool. Still, choosing wisely means recognizing that not all patients who qualify for LTOT by CMS criteria will benefit.
Drs. Kreisel and Sonti are with the Division of Pulmonary, Critical Care, and Sleep Medicine, MedStar Georgetown University Hospital, Washington, DC.
Inhalers, nebulizers, antibiotics, and steroids – these are some of the most common tools in our pulmonary arsenal that we deploy on a daily basis. But, there is no treatment more fundamental to a pulmonary practitioner than oxygen. So how is it that something that naturally occurs and comprises 21% of ambient air has become so medicalized?
It is difficult (perhaps impossible) to find a pulmonologist or a hospitalist who has not included the phrase “obtain ambulatory saturation to qualify the patient for home oxygen” in at least one of their progress notes on a daily basis. Chronic obstructive pulmonary disease (COPD) is the most common reason for the prescription of long-term oxygen therapy (LTOT), a large industry tightly regulated by the Centers for Medicare & Medicaid Services (CMS).
The evidence for the use of LTOT in patients with COPD dates back to two seminal papers published in 1980 and 1981. The British Medical Research Council Working Party conducted the BMRC trial, in which 87 patients with a Pa
Another study published around the same time, the Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease (NOTT) trial (Ann Intern Med. 1980;93[3]:391-8) directly compared continuous 24-hour to nocturnal home oxygen therapy in patients with COPD and severe hypoxemia with a Pa
Afterward, it became universally accepted dogma that patients with COPD and severe hypoxemia stood to substantially benefit from LTOT. For years, it was the only therapy associated with a mortality reduction. The LOTT study (Albert RK, et al. N Engl J Med. 2016;375[17]:1617-27) included 768 patients with stable COPD and a resting or nocturnal Sp
The INOX (Lacasse Y, et al. N Engl J Med. 2020;383[12]:1129-38) trial, in which 243 patients with oxygen saturation less than 90% for at least 30% of the night were assigned to receive nocturnal vs sham oxygen, found similar results. There was no difference in the composite outcome of all-cause mortality and progression to 24-7 oxygen requirement (according to the criteria originally defined by NOTT). A 2022 systematic review and meta-analysis including six studies designed to assess the role of LTOT in patients with COPD and moderate desaturation, including LOTT and INOX, found no benefit to providing LTOT (Lacasse Y, et al. Lancet Respir Med. 2022;10[11]:1029-37).
Based on these studies, a resting Sp
COPD management has changed significantly in the 40 years since NOTT was published. In the early 1980s, standard of care included an inhaled beta-agonist and oral theophylline. We now prescribe a regimen of modern-day inhaler combinations, which can lead to a mortality benefit in the correct population. Additionally, rates of smoking are markedly lower now than they were in 1980. In the Minnesota Heart Survey, the prevalence of being an ever-smoking man or woman in 1980 compared with 2009 dropped from 71.6% and 54.7% to 44.2% and 39.6%, respectively (Filion KB, et al. Am J Public Health. 2012;102[4]:705-13). Treatment of common comorbid conditions has also dramatically improved.
A report containing all fee-for-service data published in 2021 by CMS reported oxygen therapy accounted for 9.8% of all DME costs covered by CMS and totaled approximately $800,000,000 (Centers for Medicare & Medicaid Services. FFS Data. 2021. This represents a significant financial burden to our health system and government.
Two of the eligible groups per CMS (those with isolated ambulatory or nocturnal hypoxemia) do not benefit from LTOT in RCTs. The other two groups are eligible based on trial data from a small number of patients who were studied more than 40 years ago. These facts raise serious questions about the cost-efficacy of LTOT.
So where does this leave us?
There are significant barriers to repeating large randomized oxygen trials. Due to broad inclusion criteria for LTOT by CMS, there are undoubtedly many people prescribed LTOT for whom there is minimal to no benefit. Patients often feel restricted in their mobility and may feel isolated being tethered to medical equipment. It is good practice to think about LTOT the same way we do any other therapy we provide - as a medicine with associated risks, benefits, and costs.
Despite its ubiquity, oxygen remains an important therapeutic tool. Still, choosing wisely means recognizing that not all patients who qualify for LTOT by CMS criteria will benefit.
Drs. Kreisel and Sonti are with the Division of Pulmonary, Critical Care, and Sleep Medicine, MedStar Georgetown University Hospital, Washington, DC.
Inhalers, nebulizers, antibiotics, and steroids – these are some of the most common tools in our pulmonary arsenal that we deploy on a daily basis. But, there is no treatment more fundamental to a pulmonary practitioner than oxygen. So how is it that something that naturally occurs and comprises 21% of ambient air has become so medicalized?
It is difficult (perhaps impossible) to find a pulmonologist or a hospitalist who has not included the phrase “obtain ambulatory saturation to qualify the patient for home oxygen” in at least one of their progress notes on a daily basis. Chronic obstructive pulmonary disease (COPD) is the most common reason for the prescription of long-term oxygen therapy (LTOT), a large industry tightly regulated by the Centers for Medicare & Medicaid Services (CMS).
The evidence for the use of LTOT in patients with COPD dates back to two seminal papers published in 1980 and 1981. The British Medical Research Council Working Party conducted the BMRC trial, in which 87 patients with a Pa
Another study published around the same time, the Continuous or nocturnal oxygen therapy in hypoxemic chronic obstructive lung disease (NOTT) trial (Ann Intern Med. 1980;93[3]:391-8) directly compared continuous 24-hour to nocturnal home oxygen therapy in patients with COPD and severe hypoxemia with a Pa
Afterward, it became universally accepted dogma that patients with COPD and severe hypoxemia stood to substantially benefit from LTOT. For years, it was the only therapy associated with a mortality reduction. The LOTT study (Albert RK, et al. N Engl J Med. 2016;375[17]:1617-27) included 768 patients with stable COPD and a resting or nocturnal Sp
The INOX (Lacasse Y, et al. N Engl J Med. 2020;383[12]:1129-38) trial, in which 243 patients with oxygen saturation less than 90% for at least 30% of the night were assigned to receive nocturnal vs sham oxygen, found similar results. There was no difference in the composite outcome of all-cause mortality and progression to 24-7 oxygen requirement (according to the criteria originally defined by NOTT). A 2022 systematic review and meta-analysis including six studies designed to assess the role of LTOT in patients with COPD and moderate desaturation, including LOTT and INOX, found no benefit to providing LTOT (Lacasse Y, et al. Lancet Respir Med. 2022;10[11]:1029-37).
Based on these studies, a resting Sp
COPD management has changed significantly in the 40 years since NOTT was published. In the early 1980s, standard of care included an inhaled beta-agonist and oral theophylline. We now prescribe a regimen of modern-day inhaler combinations, which can lead to a mortality benefit in the correct population. Additionally, rates of smoking are markedly lower now than they were in 1980. In the Minnesota Heart Survey, the prevalence of being an ever-smoking man or woman in 1980 compared with 2009 dropped from 71.6% and 54.7% to 44.2% and 39.6%, respectively (Filion KB, et al. Am J Public Health. 2012;102[4]:705-13). Treatment of common comorbid conditions has also dramatically improved.
A report containing all fee-for-service data published in 2021 by CMS reported oxygen therapy accounted for 9.8% of all DME costs covered by CMS and totaled approximately $800,000,000 (Centers for Medicare & Medicaid Services. FFS Data. 2021. This represents a significant financial burden to our health system and government.
Two of the eligible groups per CMS (those with isolated ambulatory or nocturnal hypoxemia) do not benefit from LTOT in RCTs. The other two groups are eligible based on trial data from a small number of patients who were studied more than 40 years ago. These facts raise serious questions about the cost-efficacy of LTOT.
So where does this leave us?
There are significant barriers to repeating large randomized oxygen trials. Due to broad inclusion criteria for LTOT by CMS, there are undoubtedly many people prescribed LTOT for whom there is minimal to no benefit. Patients often feel restricted in their mobility and may feel isolated being tethered to medical equipment. It is good practice to think about LTOT the same way we do any other therapy we provide - as a medicine with associated risks, benefits, and costs.
Despite its ubiquity, oxygen remains an important therapeutic tool. Still, choosing wisely means recognizing that not all patients who qualify for LTOT by CMS criteria will benefit.
Drs. Kreisel and Sonti are with the Division of Pulmonary, Critical Care, and Sleep Medicine, MedStar Georgetown University Hospital, Washington, DC.
Which biologic therapy should I use in patients who have moderate to severe asthma with associated comorbidities?
Dr. Hossri and Dr. Ivashchuk are with UTHealth Houston –Texas Medical Center, Department of Internal Medicine; Division of Pulmonary, Critical Care, and Sleep Medicine.
As new treatments for specific moderate to severe asthma phenotypes have been developed, management decisions have grown more complicated. The treatment indications for asthma are clear; however, there is overlap with certain therapeutics that target the same pathway with similar end results. because it is not a one-size-fits-all approach that follows a rigid algorithm. Instead, it is a customized treatment plan that accounts for patient-specific risk factors and comorbidities.
Comorbidities commonly associated with asthma include atopic dermatitis, chronic rhinosinusitis with nasal polyposis, eosinophilic granulomatosis with polyangiitis, eosinophilic esophagitis, bronchiectasis and allergic bronchopulmonary aspergillosis. While we lack consensus or a universally accepted treatment algorithm for treating asthma when these comorbidities are present, recent evidence helps guide us to which therapies work best.
Atopic dermatitis
There is a higher prevalence of asthma in patients with atopic dermatitis. A concept called the “atopic march” refers to the progression of childhood atopic dermatitis to manifestations such as asthma, food allergies, and hay fever. The more severe the atopic dermatitis is in childhood, the higher the risk for asthma later on in life. The data on the biologic pathogenesis of atopic dermatitis point to the involvement of interleukins – interleukin (IL)-4 and IL 13 (Silverberg JI. Ann Allergy Asthma Immunol. 2019;123[2]:144-51).
These same interleukins are active in what is called “Th2-high” asthma. The activation of Th2 cells in the inflammatory pathway occurs in atopic dermatitis and asthma irrespective of immunoglobulin E levels. Preliminary data show therapies that target IL-13 alone are effective for treating asthma with comorbid atopic dermatitis but those blocking both IL-4 and IL-13, like dupilumab, are superior. Both interleukins are considered pivotal in the Th-2 pathway. This suggests that dual inhibition is an integral component in the treatment of moderate to severe atopic dermatitis with asthma. Analysis of other Th2 mediators, such as mepolizumab (IL-5 antagonist) and omalizumab (anti-IgE) have shown minimal efficacy, further supporting the use of dupilumab (Guttman-Yassky E, et al. J Allergy Clin. Immunol. 2019 Jan;143[1]:155-72).
Chronic rhinosinusitis with nasal polyposis
The “unified airway” concept holds that because the upper airways (nasal mucosa, pharynx, and larynx) are in direct communication with the lower airways (bronchi and bronchioles). This would explain the correlation between chronic rhinosinusitis with nasal polyposis (CRSwNP) and asthma. Many studies also show the severity of one disease increases the severity of the other.
Patients with both CRSwNP and asthma typically experience a more treatment-resistant course characterized by higher rates of corticosteroid dependence and nasal polyposis recurrences when compared with asthma alone (Laidlaw TM, et al. J Allergy Clin Immunol. 2021 Mar;9[3]:1133-41). They typically have Th2-high asthma and are usually eosinophilic. The optimal treatment approach is mindful of the unified airway concept. Large-scale studies demonstrate significant benefit when targeting IL-5, especially in those with bilateral nasal polyps, need for systemic steroids in the past 2 years, significant impairment in quality of life, loss of smell, and a concomitant diagnosis of asthma (Fokkens WJ, et al. Allergy. 2019 Dec;74[12]:2312). Although data are inconsistent, there is enough evidence to suggest dupilumab be considered for those with eosinophilic asthma and CRSwNP along with atopy, atopic dermatitis, and/or high FeNO levels. In those without atopic symptoms, an anti-IL5/anti-IL5R (mainly mepolizumab and benralizumab) is preferred. Having said this, direct comparative analyses between biologics are lacking, and the above approach relies on an indirect assessment of existing data coupled with clinical experience. The approach may change as new data become available.
Eosinophilic granulomatosis with polyangiitis
Eosinophilic granulomatosis with polyangiitis (EGPA) is a vasculitis characterized by disseminated necrotizing eosinophilic granulomas. EGPA is driven by a response similar to that seen in Th2-high asthma. Adult-onset asthma with sinusitis and allergic rhinitis is the most common EGPA presentation. Of all the biologics, mepolizumab has been best studied as treatment for those with EGPA and asthma symptoms. One small study demonstrated disease remission in 8 of 10 cases (Moosig F, et al. Ann Intern Med. 2011 Sep 6;155[5]:341-3). However, many of these patients relapsed after discontinuing therapy.
Eosinophilic esophagitis
Recent reports demonstrated a large portion of adults with a
diagnosis of eosinophilic
esophagitis (EoE) also have a history of asthma. Currently, standard treatment is proton pump
inhibitors and diet modifications. The prevalence of EoE has increased with growing awareness of the disease. Unrecognized and untreated EoE can lead to devastating complications such as esophageal fibrosis, strictures, and food impaction. Similar to some of the above-mentioned syndromes,
EoE is also driven by a Th2 response and eosinophilic inflammation. A recent study in 2022 showed that 31% to 38% of
people with EoE had concomitant asthma (Dellon ES, et al. N Engl J Med. 2022 Dec 22;387 [25]:2317-30). In this population, a weekly dose of dupilumab, 300 mg, led
to a significant improvement in dysphagia symptoms and
histology when compared with placebo.
Allergic bronchopulmonary aspergillosis
Despite its low prevalence worldwide, allergic bronchopulmonary aspergillosis (ABPA) is frequently encountered when managing severe asthma. Current treatment is long-term, relatively high dose systemic corticosteroids. In light of their unfavorable side effect profile, steroid-sparing approaches are being sought. Dupilumab, omalizumab, mepolizumab, and benralizumab have all been tested for their effects on ABPA. Thus far, mepolizumab has the most convincing evidence to support its use for asthma with concomitant ABPA, mainly because it has the most rapid onset of action. Up to 90% of patients with ABPA were able to stop systemic steroids between 2 and 14 months after starting mepolizumab (Schleich F, et al. J Allergy Clin Immunol. 2020 Jul-Aug;8[7]:2412-3.e2).
Bronchiectasis
Asthma and bronchiectasis can coexist in up to 77% of patients. Typically, the pathophysiology behind bronchiectasis is focused around neutrophilic inflammation. New evidence suggests some patients with bronchiectasis, usually in the setting of comorbid adult-onset asthma, demonstrate an eosinophilic Th-2 response. The association is seen more commonly in female patients, the elderly, and nonsmokers. A small prospective study with four patients with severe asthma and bronchiectasis showed significant improvement with less exacerbations, increased pre-bronchodilator FEV1, and a reduction of serum and sputum eosinophils after starting mepolizumab treatment (Carpagnano GE, et al. J Asthma Allergy. 2019 Mar 5;12:83-90). Clinical trials designed to clarify the role for biologics for asthma with co-morbid bronchiectasis are currently underway.
Dr. Hossri and Dr. Ivashchuk are with UTHealth Houston –Texas Medical Center, Department of Internal Medicine; Division of Pulmonary, Critical Care, and Sleep Medicine.
As new treatments for specific moderate to severe asthma phenotypes have been developed, management decisions have grown more complicated. The treatment indications for asthma are clear; however, there is overlap with certain therapeutics that target the same pathway with similar end results. because it is not a one-size-fits-all approach that follows a rigid algorithm. Instead, it is a customized treatment plan that accounts for patient-specific risk factors and comorbidities.
Comorbidities commonly associated with asthma include atopic dermatitis, chronic rhinosinusitis with nasal polyposis, eosinophilic granulomatosis with polyangiitis, eosinophilic esophagitis, bronchiectasis and allergic bronchopulmonary aspergillosis. While we lack consensus or a universally accepted treatment algorithm for treating asthma when these comorbidities are present, recent evidence helps guide us to which therapies work best.
Atopic dermatitis
There is a higher prevalence of asthma in patients with atopic dermatitis. A concept called the “atopic march” refers to the progression of childhood atopic dermatitis to manifestations such as asthma, food allergies, and hay fever. The more severe the atopic dermatitis is in childhood, the higher the risk for asthma later on in life. The data on the biologic pathogenesis of atopic dermatitis point to the involvement of interleukins – interleukin (IL)-4 and IL 13 (Silverberg JI. Ann Allergy Asthma Immunol. 2019;123[2]:144-51).
These same interleukins are active in what is called “Th2-high” asthma. The activation of Th2 cells in the inflammatory pathway occurs in atopic dermatitis and asthma irrespective of immunoglobulin E levels. Preliminary data show therapies that target IL-13 alone are effective for treating asthma with comorbid atopic dermatitis but those blocking both IL-4 and IL-13, like dupilumab, are superior. Both interleukins are considered pivotal in the Th-2 pathway. This suggests that dual inhibition is an integral component in the treatment of moderate to severe atopic dermatitis with asthma. Analysis of other Th2 mediators, such as mepolizumab (IL-5 antagonist) and omalizumab (anti-IgE) have shown minimal efficacy, further supporting the use of dupilumab (Guttman-Yassky E, et al. J Allergy Clin. Immunol. 2019 Jan;143[1]:155-72).
Chronic rhinosinusitis with nasal polyposis
The “unified airway” concept holds that because the upper airways (nasal mucosa, pharynx, and larynx) are in direct communication with the lower airways (bronchi and bronchioles). This would explain the correlation between chronic rhinosinusitis with nasal polyposis (CRSwNP) and asthma. Many studies also show the severity of one disease increases the severity of the other.
Patients with both CRSwNP and asthma typically experience a more treatment-resistant course characterized by higher rates of corticosteroid dependence and nasal polyposis recurrences when compared with asthma alone (Laidlaw TM, et al. J Allergy Clin Immunol. 2021 Mar;9[3]:1133-41). They typically have Th2-high asthma and are usually eosinophilic. The optimal treatment approach is mindful of the unified airway concept. Large-scale studies demonstrate significant benefit when targeting IL-5, especially in those with bilateral nasal polyps, need for systemic steroids in the past 2 years, significant impairment in quality of life, loss of smell, and a concomitant diagnosis of asthma (Fokkens WJ, et al. Allergy. 2019 Dec;74[12]:2312). Although data are inconsistent, there is enough evidence to suggest dupilumab be considered for those with eosinophilic asthma and CRSwNP along with atopy, atopic dermatitis, and/or high FeNO levels. In those without atopic symptoms, an anti-IL5/anti-IL5R (mainly mepolizumab and benralizumab) is preferred. Having said this, direct comparative analyses between biologics are lacking, and the above approach relies on an indirect assessment of existing data coupled with clinical experience. The approach may change as new data become available.
Eosinophilic granulomatosis with polyangiitis
Eosinophilic granulomatosis with polyangiitis (EGPA) is a vasculitis characterized by disseminated necrotizing eosinophilic granulomas. EGPA is driven by a response similar to that seen in Th2-high asthma. Adult-onset asthma with sinusitis and allergic rhinitis is the most common EGPA presentation. Of all the biologics, mepolizumab has been best studied as treatment for those with EGPA and asthma symptoms. One small study demonstrated disease remission in 8 of 10 cases (Moosig F, et al. Ann Intern Med. 2011 Sep 6;155[5]:341-3). However, many of these patients relapsed after discontinuing therapy.
Eosinophilic esophagitis
Recent reports demonstrated a large portion of adults with a
diagnosis of eosinophilic
esophagitis (EoE) also have a history of asthma. Currently, standard treatment is proton pump
inhibitors and diet modifications. The prevalence of EoE has increased with growing awareness of the disease. Unrecognized and untreated EoE can lead to devastating complications such as esophageal fibrosis, strictures, and food impaction. Similar to some of the above-mentioned syndromes,
EoE is also driven by a Th2 response and eosinophilic inflammation. A recent study in 2022 showed that 31% to 38% of
people with EoE had concomitant asthma (Dellon ES, et al. N Engl J Med. 2022 Dec 22;387 [25]:2317-30). In this population, a weekly dose of dupilumab, 300 mg, led
to a significant improvement in dysphagia symptoms and
histology when compared with placebo.
Allergic bronchopulmonary aspergillosis
Despite its low prevalence worldwide, allergic bronchopulmonary aspergillosis (ABPA) is frequently encountered when managing severe asthma. Current treatment is long-term, relatively high dose systemic corticosteroids. In light of their unfavorable side effect profile, steroid-sparing approaches are being sought. Dupilumab, omalizumab, mepolizumab, and benralizumab have all been tested for their effects on ABPA. Thus far, mepolizumab has the most convincing evidence to support its use for asthma with concomitant ABPA, mainly because it has the most rapid onset of action. Up to 90% of patients with ABPA were able to stop systemic steroids between 2 and 14 months after starting mepolizumab (Schleich F, et al. J Allergy Clin Immunol. 2020 Jul-Aug;8[7]:2412-3.e2).
Bronchiectasis
Asthma and bronchiectasis can coexist in up to 77% of patients. Typically, the pathophysiology behind bronchiectasis is focused around neutrophilic inflammation. New evidence suggests some patients with bronchiectasis, usually in the setting of comorbid adult-onset asthma, demonstrate an eosinophilic Th-2 response. The association is seen more commonly in female patients, the elderly, and nonsmokers. A small prospective study with four patients with severe asthma and bronchiectasis showed significant improvement with less exacerbations, increased pre-bronchodilator FEV1, and a reduction of serum and sputum eosinophils after starting mepolizumab treatment (Carpagnano GE, et al. J Asthma Allergy. 2019 Mar 5;12:83-90). Clinical trials designed to clarify the role for biologics for asthma with co-morbid bronchiectasis are currently underway.
Dr. Hossri and Dr. Ivashchuk are with UTHealth Houston –Texas Medical Center, Department of Internal Medicine; Division of Pulmonary, Critical Care, and Sleep Medicine.
As new treatments for specific moderate to severe asthma phenotypes have been developed, management decisions have grown more complicated. The treatment indications for asthma are clear; however, there is overlap with certain therapeutics that target the same pathway with similar end results. because it is not a one-size-fits-all approach that follows a rigid algorithm. Instead, it is a customized treatment plan that accounts for patient-specific risk factors and comorbidities.
Comorbidities commonly associated with asthma include atopic dermatitis, chronic rhinosinusitis with nasal polyposis, eosinophilic granulomatosis with polyangiitis, eosinophilic esophagitis, bronchiectasis and allergic bronchopulmonary aspergillosis. While we lack consensus or a universally accepted treatment algorithm for treating asthma when these comorbidities are present, recent evidence helps guide us to which therapies work best.
Atopic dermatitis
There is a higher prevalence of asthma in patients with atopic dermatitis. A concept called the “atopic march” refers to the progression of childhood atopic dermatitis to manifestations such as asthma, food allergies, and hay fever. The more severe the atopic dermatitis is in childhood, the higher the risk for asthma later on in life. The data on the biologic pathogenesis of atopic dermatitis point to the involvement of interleukins – interleukin (IL)-4 and IL 13 (Silverberg JI. Ann Allergy Asthma Immunol. 2019;123[2]:144-51).
These same interleukins are active in what is called “Th2-high” asthma. The activation of Th2 cells in the inflammatory pathway occurs in atopic dermatitis and asthma irrespective of immunoglobulin E levels. Preliminary data show therapies that target IL-13 alone are effective for treating asthma with comorbid atopic dermatitis but those blocking both IL-4 and IL-13, like dupilumab, are superior. Both interleukins are considered pivotal in the Th-2 pathway. This suggests that dual inhibition is an integral component in the treatment of moderate to severe atopic dermatitis with asthma. Analysis of other Th2 mediators, such as mepolizumab (IL-5 antagonist) and omalizumab (anti-IgE) have shown minimal efficacy, further supporting the use of dupilumab (Guttman-Yassky E, et al. J Allergy Clin. Immunol. 2019 Jan;143[1]:155-72).
Chronic rhinosinusitis with nasal polyposis
The “unified airway” concept holds that because the upper airways (nasal mucosa, pharynx, and larynx) are in direct communication with the lower airways (bronchi and bronchioles). This would explain the correlation between chronic rhinosinusitis with nasal polyposis (CRSwNP) and asthma. Many studies also show the severity of one disease increases the severity of the other.
Patients with both CRSwNP and asthma typically experience a more treatment-resistant course characterized by higher rates of corticosteroid dependence and nasal polyposis recurrences when compared with asthma alone (Laidlaw TM, et al. J Allergy Clin Immunol. 2021 Mar;9[3]:1133-41). They typically have Th2-high asthma and are usually eosinophilic. The optimal treatment approach is mindful of the unified airway concept. Large-scale studies demonstrate significant benefit when targeting IL-5, especially in those with bilateral nasal polyps, need for systemic steroids in the past 2 years, significant impairment in quality of life, loss of smell, and a concomitant diagnosis of asthma (Fokkens WJ, et al. Allergy. 2019 Dec;74[12]:2312). Although data are inconsistent, there is enough evidence to suggest dupilumab be considered for those with eosinophilic asthma and CRSwNP along with atopy, atopic dermatitis, and/or high FeNO levels. In those without atopic symptoms, an anti-IL5/anti-IL5R (mainly mepolizumab and benralizumab) is preferred. Having said this, direct comparative analyses between biologics are lacking, and the above approach relies on an indirect assessment of existing data coupled with clinical experience. The approach may change as new data become available.
Eosinophilic granulomatosis with polyangiitis
Eosinophilic granulomatosis with polyangiitis (EGPA) is a vasculitis characterized by disseminated necrotizing eosinophilic granulomas. EGPA is driven by a response similar to that seen in Th2-high asthma. Adult-onset asthma with sinusitis and allergic rhinitis is the most common EGPA presentation. Of all the biologics, mepolizumab has been best studied as treatment for those with EGPA and asthma symptoms. One small study demonstrated disease remission in 8 of 10 cases (Moosig F, et al. Ann Intern Med. 2011 Sep 6;155[5]:341-3). However, many of these patients relapsed after discontinuing therapy.
Eosinophilic esophagitis
Recent reports demonstrated a large portion of adults with a
diagnosis of eosinophilic
esophagitis (EoE) also have a history of asthma. Currently, standard treatment is proton pump
inhibitors and diet modifications. The prevalence of EoE has increased with growing awareness of the disease. Unrecognized and untreated EoE can lead to devastating complications such as esophageal fibrosis, strictures, and food impaction. Similar to some of the above-mentioned syndromes,
EoE is also driven by a Th2 response and eosinophilic inflammation. A recent study in 2022 showed that 31% to 38% of
people with EoE had concomitant asthma (Dellon ES, et al. N Engl J Med. 2022 Dec 22;387 [25]:2317-30). In this population, a weekly dose of dupilumab, 300 mg, led
to a significant improvement in dysphagia symptoms and
histology when compared with placebo.
Allergic bronchopulmonary aspergillosis
Despite its low prevalence worldwide, allergic bronchopulmonary aspergillosis (ABPA) is frequently encountered when managing severe asthma. Current treatment is long-term, relatively high dose systemic corticosteroids. In light of their unfavorable side effect profile, steroid-sparing approaches are being sought. Dupilumab, omalizumab, mepolizumab, and benralizumab have all been tested for their effects on ABPA. Thus far, mepolizumab has the most convincing evidence to support its use for asthma with concomitant ABPA, mainly because it has the most rapid onset of action. Up to 90% of patients with ABPA were able to stop systemic steroids between 2 and 14 months after starting mepolizumab (Schleich F, et al. J Allergy Clin Immunol. 2020 Jul-Aug;8[7]:2412-3.e2).
Bronchiectasis
Asthma and bronchiectasis can coexist in up to 77% of patients. Typically, the pathophysiology behind bronchiectasis is focused around neutrophilic inflammation. New evidence suggests some patients with bronchiectasis, usually in the setting of comorbid adult-onset asthma, demonstrate an eosinophilic Th-2 response. The association is seen more commonly in female patients, the elderly, and nonsmokers. A small prospective study with four patients with severe asthma and bronchiectasis showed significant improvement with less exacerbations, increased pre-bronchodilator FEV1, and a reduction of serum and sputum eosinophils after starting mepolizumab treatment (Carpagnano GE, et al. J Asthma Allergy. 2019 Mar 5;12:83-90). Clinical trials designed to clarify the role for biologics for asthma with co-morbid bronchiectasis are currently underway.

















