User login
Disease Education
Q) The billing consultant who came to our office said we can increase our reimbursements if we also provide education to our patients with chronic kidney disease (CKD). Is she right?
In 2010, under an omnibus bill, kidney disease education (KDE) classes were added as a Medicare benefit. These are for patients with stage 4 CKD (glomerular filtration rate, 15-30 mL/min) and are to be taught by a qualified instructor (MD, PA, NP, or CNS).
The classes can be taught on the same day as an evaluation/management visit (ie, a regular office visit) and are compensated by the hour. (Side note: Medicare defines an hour as 31 minutes—yes, 31 minutes; Medicare takes for granted that you will also need time to chart!) You can teach two classes in the same day. Thus, if you wanted to, you could have a patient arrive for an office visit, then teach two 31-minute classes, and bill all three for the same day. The entire visit could be 75 minutes (although this may be exhausting for this population).
You can conduct the classes in a number of settings, including nursing homes, hospitals, skilled nursing facilities, the office, or even the patient’s home. Many PAs and NPs have taught these classes to hospitalized patients who have lost kidney function due to an acute insult (ie, medications, dehydration, contrast).
Each Medicare recipient has a lifetime benefit of six KDE classes. The CPT billing code is G0420 for an individual class and G0421 for a group class. You must make sure you also code for the stage 4 CKD diagnosis (code: 585.4).
Congress stipulated KDE classes must include information on causes, symptoms, and treatments and comprise a posttest at a specific health literacy level. To make it simple, the National Kidney Foundation Council of Advanced Practitioners (NKF-CAP) has developed two free Power-Point slide decks for clinicians to use in KDE classes (available at www.kidney.org/professionals/CAP/sub_resources#kde). References and updated peer-reviewed guidelines are included. You can print the slides for your patients and/or share the program with your colleagues.
Many nephrology practitioners teach the two slide sets over and over, because patients only retain one-third of the info we provide them on a given day. So if you teach each slide set three times, you have six lifetime classes—and hopefully the patient will have retained everything.
One caveat: Before you initiate KDE classes for a specific patient, check with the patient’s nephrology group (we hope at stage 4 the patient has a nephrologist) to see if they are providing the education. —KZ and JD
Kim Zuber, PA-C, MSPS, DFAAPA
American Academy of Nephrology PAs
Jane S. Davis, CRNP, DNP
Division of Nephrology at the University of Alabama
National Kidney Foundation's Council of Advanced Practitioners
Q) The billing consultant who came to our office said we can increase our reimbursements if we also provide education to our patients with chronic kidney disease (CKD). Is she right?
In 2010, under an omnibus bill, kidney disease education (KDE) classes were added as a Medicare benefit. These are for patients with stage 4 CKD (glomerular filtration rate, 15-30 mL/min) and are to be taught by a qualified instructor (MD, PA, NP, or CNS).
The classes can be taught on the same day as an evaluation/management visit (ie, a regular office visit) and are compensated by the hour. (Side note: Medicare defines an hour as 31 minutes—yes, 31 minutes; Medicare takes for granted that you will also need time to chart!) You can teach two classes in the same day. Thus, if you wanted to, you could have a patient arrive for an office visit, then teach two 31-minute classes, and bill all three for the same day. The entire visit could be 75 minutes (although this may be exhausting for this population).
You can conduct the classes in a number of settings, including nursing homes, hospitals, skilled nursing facilities, the office, or even the patient’s home. Many PAs and NPs have taught these classes to hospitalized patients who have lost kidney function due to an acute insult (ie, medications, dehydration, contrast).
Each Medicare recipient has a lifetime benefit of six KDE classes. The CPT billing code is G0420 for an individual class and G0421 for a group class. You must make sure you also code for the stage 4 CKD diagnosis (code: 585.4).
Congress stipulated KDE classes must include information on causes, symptoms, and treatments and comprise a posttest at a specific health literacy level. To make it simple, the National Kidney Foundation Council of Advanced Practitioners (NKF-CAP) has developed two free Power-Point slide decks for clinicians to use in KDE classes (available at www.kidney.org/professionals/CAP/sub_resources#kde). References and updated peer-reviewed guidelines are included. You can print the slides for your patients and/or share the program with your colleagues.
Many nephrology practitioners teach the two slide sets over and over, because patients only retain one-third of the info we provide them on a given day. So if you teach each slide set three times, you have six lifetime classes—and hopefully the patient will have retained everything.
One caveat: Before you initiate KDE classes for a specific patient, check with the patient’s nephrology group (we hope at stage 4 the patient has a nephrologist) to see if they are providing the education. —KZ and JD
Kim Zuber, PA-C, MSPS, DFAAPA
American Academy of Nephrology PAs
Jane S. Davis, CRNP, DNP
Division of Nephrology at the University of Alabama
National Kidney Foundation's Council of Advanced Practitioners
Q) The billing consultant who came to our office said we can increase our reimbursements if we also provide education to our patients with chronic kidney disease (CKD). Is she right?
In 2010, under an omnibus bill, kidney disease education (KDE) classes were added as a Medicare benefit. These are for patients with stage 4 CKD (glomerular filtration rate, 15-30 mL/min) and are to be taught by a qualified instructor (MD, PA, NP, or CNS).
The classes can be taught on the same day as an evaluation/management visit (ie, a regular office visit) and are compensated by the hour. (Side note: Medicare defines an hour as 31 minutes—yes, 31 minutes; Medicare takes for granted that you will also need time to chart!) You can teach two classes in the same day. Thus, if you wanted to, you could have a patient arrive for an office visit, then teach two 31-minute classes, and bill all three for the same day. The entire visit could be 75 minutes (although this may be exhausting for this population).
You can conduct the classes in a number of settings, including nursing homes, hospitals, skilled nursing facilities, the office, or even the patient’s home. Many PAs and NPs have taught these classes to hospitalized patients who have lost kidney function due to an acute insult (ie, medications, dehydration, contrast).
Each Medicare recipient has a lifetime benefit of six KDE classes. The CPT billing code is G0420 for an individual class and G0421 for a group class. You must make sure you also code for the stage 4 CKD diagnosis (code: 585.4).
Congress stipulated KDE classes must include information on causes, symptoms, and treatments and comprise a posttest at a specific health literacy level. To make it simple, the National Kidney Foundation Council of Advanced Practitioners (NKF-CAP) has developed two free Power-Point slide decks for clinicians to use in KDE classes (available at www.kidney.org/professionals/CAP/sub_resources#kde). References and updated peer-reviewed guidelines are included. You can print the slides for your patients and/or share the program with your colleagues.
Many nephrology practitioners teach the two slide sets over and over, because patients only retain one-third of the info we provide them on a given day. So if you teach each slide set three times, you have six lifetime classes—and hopefully the patient will have retained everything.
One caveat: Before you initiate KDE classes for a specific patient, check with the patient’s nephrology group (we hope at stage 4 the patient has a nephrologist) to see if they are providing the education. —KZ and JD
Kim Zuber, PA-C, MSPS, DFAAPA
American Academy of Nephrology PAs
Jane S. Davis, CRNP, DNP
Division of Nephrology at the University of Alabama
National Kidney Foundation's Council of Advanced Practitioners
Kidney Transplants
Q) All I hear about is the shortage of kidneys for transplantation. A friend of mine is on the local transplant list, and it is eight years long! Are there any ideas out there to grow your own kidneys?
Eight years is a long time for people dealing with the physical and emotional effects of kidney disease coupled with hemodialysis or peritoneal dialysis. Your friend is one of 110,000 patients (as of January 2015) in the United States on the United Network for Organ Sharing (UNOS) kidney transplant waiting list.1 The UNOS/Organ Procurement and Transplant Network (OPTN) implemented new polices in 2014 to shorten the wait.
Among them: For pediatric patients (those younger than 18), the wait list time starts when the glomerular filtration rate (GFR) is ≤ 20 mL/min or the child starts dialysis. UNOS also has attempted to match posttransplant survival time of the graft with posttransplant survival time of the recipient through use of calculations that classify kidneys on the basis of how long they are likely to function once transplanted. Priority is now given to candidates with high immune system sensitivity or uncommon blood types, as they are less likely to obtain a kidney otherwise.2
The million-dollar question is how to obtain a kidney transplant in a timely fashion. Grave robbing, in case you are wondering, is not a viable option! Nor is transplant tourism (traveling outside the US to obtain an organ transplant), which confers a higher risk for severe infectious complications and acute rejection, possibly related to less extensive donor screening.3
There are other possibilities: Living donors can donate one kidney. Or, as is becoming increasingly common, paired organ transplants can be arranged. These occur when a patient in need of a kidney has a willing but incompatible donor; if a different match can be found, a “swap” is orchestrated, in which Donor A’s kidney is transplanted into Recipient B and Donor B’s kidney is given to Recipient A. This can be and has been done with multiple donors and recipients—in some cases, dozens—allowing the gift of donation to go on and on. (See “Trading Kidneys: Innovative Program Could Save Thousands of Lives” for an overview of how this concept started.)
Some exciting research is going on with regard to 3D printing of kidneys; they are miniature for now but showing survival of the printed cells. Another area of exploration is regenerative medicine; researchers in the field are investigating the bioengineering of organs by taking the “scaffolding” of an organ and implanting a patient’s own cells to “grow” a new organ (which, if successful, would also eliminate the need for immunosuppressants). Other signs of progress include recent news that scientists are getting lab-grown kidneys to work in animals.
It will be several years before any of these options will be viable. In the meantime, it never hurts to ask loved ones if they are willing to donate a kidney. Best wishes to your friend. —DC
Della Connor, PhD, RN, FNP-BC
East Texas Nephrology Associates, Lufkin, Texas
REFERENCES
1. Organ Procurement and Transplantation Network. http://optn.transplant.hrsa.gov. Accessed December 10, 2015.
2. Organ Procurement and Transplantation Network. New OPTN requirements and resources for the living donor kidney transplant programs. Prog Transplant. 2013;23(2):117.
3. Gill J, Madhira BR, Gjertson D, et al. Transplant tourism in the United States: a single-center experience. Clin J Am Soc Nephrol. 2008;3(6):1820-1828.
Q) All I hear about is the shortage of kidneys for transplantation. A friend of mine is on the local transplant list, and it is eight years long! Are there any ideas out there to grow your own kidneys?
Eight years is a long time for people dealing with the physical and emotional effects of kidney disease coupled with hemodialysis or peritoneal dialysis. Your friend is one of 110,000 patients (as of January 2015) in the United States on the United Network for Organ Sharing (UNOS) kidney transplant waiting list.1 The UNOS/Organ Procurement and Transplant Network (OPTN) implemented new polices in 2014 to shorten the wait.
Among them: For pediatric patients (those younger than 18), the wait list time starts when the glomerular filtration rate (GFR) is ≤ 20 mL/min or the child starts dialysis. UNOS also has attempted to match posttransplant survival time of the graft with posttransplant survival time of the recipient through use of calculations that classify kidneys on the basis of how long they are likely to function once transplanted. Priority is now given to candidates with high immune system sensitivity or uncommon blood types, as they are less likely to obtain a kidney otherwise.2
The million-dollar question is how to obtain a kidney transplant in a timely fashion. Grave robbing, in case you are wondering, is not a viable option! Nor is transplant tourism (traveling outside the US to obtain an organ transplant), which confers a higher risk for severe infectious complications and acute rejection, possibly related to less extensive donor screening.3
There are other possibilities: Living donors can donate one kidney. Or, as is becoming increasingly common, paired organ transplants can be arranged. These occur when a patient in need of a kidney has a willing but incompatible donor; if a different match can be found, a “swap” is orchestrated, in which Donor A’s kidney is transplanted into Recipient B and Donor B’s kidney is given to Recipient A. This can be and has been done with multiple donors and recipients—in some cases, dozens—allowing the gift of donation to go on and on. (See “Trading Kidneys: Innovative Program Could Save Thousands of Lives” for an overview of how this concept started.)
Some exciting research is going on with regard to 3D printing of kidneys; they are miniature for now but showing survival of the printed cells. Another area of exploration is regenerative medicine; researchers in the field are investigating the bioengineering of organs by taking the “scaffolding” of an organ and implanting a patient’s own cells to “grow” a new organ (which, if successful, would also eliminate the need for immunosuppressants). Other signs of progress include recent news that scientists are getting lab-grown kidneys to work in animals.
It will be several years before any of these options will be viable. In the meantime, it never hurts to ask loved ones if they are willing to donate a kidney. Best wishes to your friend. —DC
Della Connor, PhD, RN, FNP-BC
East Texas Nephrology Associates, Lufkin, Texas
REFERENCES
1. Organ Procurement and Transplantation Network. http://optn.transplant.hrsa.gov. Accessed December 10, 2015.
2. Organ Procurement and Transplantation Network. New OPTN requirements and resources for the living donor kidney transplant programs. Prog Transplant. 2013;23(2):117.
3. Gill J, Madhira BR, Gjertson D, et al. Transplant tourism in the United States: a single-center experience. Clin J Am Soc Nephrol. 2008;3(6):1820-1828.
Q) All I hear about is the shortage of kidneys for transplantation. A friend of mine is on the local transplant list, and it is eight years long! Are there any ideas out there to grow your own kidneys?
Eight years is a long time for people dealing with the physical and emotional effects of kidney disease coupled with hemodialysis or peritoneal dialysis. Your friend is one of 110,000 patients (as of January 2015) in the United States on the United Network for Organ Sharing (UNOS) kidney transplant waiting list.1 The UNOS/Organ Procurement and Transplant Network (OPTN) implemented new polices in 2014 to shorten the wait.
Among them: For pediatric patients (those younger than 18), the wait list time starts when the glomerular filtration rate (GFR) is ≤ 20 mL/min or the child starts dialysis. UNOS also has attempted to match posttransplant survival time of the graft with posttransplant survival time of the recipient through use of calculations that classify kidneys on the basis of how long they are likely to function once transplanted. Priority is now given to candidates with high immune system sensitivity or uncommon blood types, as they are less likely to obtain a kidney otherwise.2
The million-dollar question is how to obtain a kidney transplant in a timely fashion. Grave robbing, in case you are wondering, is not a viable option! Nor is transplant tourism (traveling outside the US to obtain an organ transplant), which confers a higher risk for severe infectious complications and acute rejection, possibly related to less extensive donor screening.3
There are other possibilities: Living donors can donate one kidney. Or, as is becoming increasingly common, paired organ transplants can be arranged. These occur when a patient in need of a kidney has a willing but incompatible donor; if a different match can be found, a “swap” is orchestrated, in which Donor A’s kidney is transplanted into Recipient B and Donor B’s kidney is given to Recipient A. This can be and has been done with multiple donors and recipients—in some cases, dozens—allowing the gift of donation to go on and on. (See “Trading Kidneys: Innovative Program Could Save Thousands of Lives” for an overview of how this concept started.)
Some exciting research is going on with regard to 3D printing of kidneys; they are miniature for now but showing survival of the printed cells. Another area of exploration is regenerative medicine; researchers in the field are investigating the bioengineering of organs by taking the “scaffolding” of an organ and implanting a patient’s own cells to “grow” a new organ (which, if successful, would also eliminate the need for immunosuppressants). Other signs of progress include recent news that scientists are getting lab-grown kidneys to work in animals.
It will be several years before any of these options will be viable. In the meantime, it never hurts to ask loved ones if they are willing to donate a kidney. Best wishes to your friend. —DC
Della Connor, PhD, RN, FNP-BC
East Texas Nephrology Associates, Lufkin, Texas
REFERENCES
1. Organ Procurement and Transplantation Network. http://optn.transplant.hrsa.gov. Accessed December 10, 2015.
2. Organ Procurement and Transplantation Network. New OPTN requirements and resources for the living donor kidney transplant programs. Prog Transplant. 2013;23(2):117.
3. Gill J, Madhira BR, Gjertson D, et al. Transplant tourism in the United States: a single-center experience. Clin J Am Soc Nephrol. 2008;3(6):1820-1828.
Renal Denervation
Q) I’ve heard a lot of references to “renal denervation” and its use for resistant hypertension. What is it? Does it work? Is it common in the US?
Renal denervation is a minimally invasive endovascular procedure that ablates (or disrupts) the renal nerves in and around the renal arteries with radiofrequency energy.5 Renal denervation has been approved in the US and other countries and is being used clinically in Europe, Canada, and Australia.6
It is thought that renal denervation interrupts the efferent and afferent signals that stimulate the renin-angiotensin-aldosterone system (RAAS) and regulate whole-body sympathetic nervous system activity.5 Similar to surgical sympathectomy, renal denervation should theoretically lower blood pressure. However, Ezzahti et al found that renin levels did not decrease in patients following renal denervation.7
Drug-resistant hypertension is defined as blood pressure that remains greater than 140/90 mm Hg despite treatment with three or more antihypertensive medications, including a diuretic.8 Patients with resistant hypertension have increased cardiovascular risk.9 Clinical trials of renal denervation have focused on treatment of resistant hypertension, in the hope of reducing the associated morbidity and mortality.
Results of the Symplicity HTN-3 trial, which assessed the safety and efficacy of renal denervation, were anxiously awaited, since prior trials yielded mixed results. Although the Symplicity HTN-1 and Symplicity HTN-2 studies demonstrated a possible benefit of renal denervation to lower office measured blood pressure, other studies did not show a decrease in BP in patients who had undergone renal denervation.6,7 These early trials, however, were small and did not randomize patients to a sham procedure.10
The Symplicity HTN-3 trial included 535 patients at 88 centers in the US. Patients were randomly assigned to receive either renal denervation plus baseline antihypertensive medications or a sham procedure plus baseline antihypertensive medications.
The researchers found that the sham procedure was just as effective as the “true” renal denervation in decreasing systolic blood pressure in patients with resistant hypertension.10 In other words, renal denervation did not demonstrate efficacy for this purpose.
In response to the results of this well-designed trial, the FDA has halted approval to perform renal denervation in patients with resistant hypertension in the US. However, clinical investigation will continue among subgroups of hypertensive patients or separate populations.
Despite a lack of efficacy, renal denervation does appear to be well tolerated, as evidenced by safety data from Symplicity HTN-3. —JK
Jessica Knight, ACNP
University of New Mexico Hospital, Albuquerque
REFERENCES
5. Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for the treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126(25):2976-2982.
6. Thukkani AK, Bhatt LD. Renal denervation therapy for hypertension. Circulation. 2013;128:2251-2254.
7. Ezzahti M, Moelker A, Friesema E, et al. Blood pressure and neurohormonal responses to renal nerve ablation in treatment-resistant hypertension. J Hypertens. 2014;32(1):135-141.
8. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
9. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635-1642.
10. Bhatt DL, Kandzari DE, O’Neill WW, et al; Symplicity HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393-1401.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Q) I’ve heard a lot of references to “renal denervation” and its use for resistant hypertension. What is it? Does it work? Is it common in the US?
Renal denervation is a minimally invasive endovascular procedure that ablates (or disrupts) the renal nerves in and around the renal arteries with radiofrequency energy.5 Renal denervation has been approved in the US and other countries and is being used clinically in Europe, Canada, and Australia.6
It is thought that renal denervation interrupts the efferent and afferent signals that stimulate the renin-angiotensin-aldosterone system (RAAS) and regulate whole-body sympathetic nervous system activity.5 Similar to surgical sympathectomy, renal denervation should theoretically lower blood pressure. However, Ezzahti et al found that renin levels did not decrease in patients following renal denervation.7
Drug-resistant hypertension is defined as blood pressure that remains greater than 140/90 mm Hg despite treatment with three or more antihypertensive medications, including a diuretic.8 Patients with resistant hypertension have increased cardiovascular risk.9 Clinical trials of renal denervation have focused on treatment of resistant hypertension, in the hope of reducing the associated morbidity and mortality.
Results of the Symplicity HTN-3 trial, which assessed the safety and efficacy of renal denervation, were anxiously awaited, since prior trials yielded mixed results. Although the Symplicity HTN-1 and Symplicity HTN-2 studies demonstrated a possible benefit of renal denervation to lower office measured blood pressure, other studies did not show a decrease in BP in patients who had undergone renal denervation.6,7 These early trials, however, were small and did not randomize patients to a sham procedure.10
The Symplicity HTN-3 trial included 535 patients at 88 centers in the US. Patients were randomly assigned to receive either renal denervation plus baseline antihypertensive medications or a sham procedure plus baseline antihypertensive medications.
The researchers found that the sham procedure was just as effective as the “true” renal denervation in decreasing systolic blood pressure in patients with resistant hypertension.10 In other words, renal denervation did not demonstrate efficacy for this purpose.
In response to the results of this well-designed trial, the FDA has halted approval to perform renal denervation in patients with resistant hypertension in the US. However, clinical investigation will continue among subgroups of hypertensive patients or separate populations.
Despite a lack of efficacy, renal denervation does appear to be well tolerated, as evidenced by safety data from Symplicity HTN-3. —JK
Jessica Knight, ACNP
University of New Mexico Hospital, Albuquerque
REFERENCES
5. Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for the treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126(25):2976-2982.
6. Thukkani AK, Bhatt LD. Renal denervation therapy for hypertension. Circulation. 2013;128:2251-2254.
7. Ezzahti M, Moelker A, Friesema E, et al. Blood pressure and neurohormonal responses to renal nerve ablation in treatment-resistant hypertension. J Hypertens. 2014;32(1):135-141.
8. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
9. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635-1642.
10. Bhatt DL, Kandzari DE, O’Neill WW, et al; Symplicity HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393-1401.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Q) I’ve heard a lot of references to “renal denervation” and its use for resistant hypertension. What is it? Does it work? Is it common in the US?
Renal denervation is a minimally invasive endovascular procedure that ablates (or disrupts) the renal nerves in and around the renal arteries with radiofrequency energy.5 Renal denervation has been approved in the US and other countries and is being used clinically in Europe, Canada, and Australia.6
It is thought that renal denervation interrupts the efferent and afferent signals that stimulate the renin-angiotensin-aldosterone system (RAAS) and regulate whole-body sympathetic nervous system activity.5 Similar to surgical sympathectomy, renal denervation should theoretically lower blood pressure. However, Ezzahti et al found that renin levels did not decrease in patients following renal denervation.7
Drug-resistant hypertension is defined as blood pressure that remains greater than 140/90 mm Hg despite treatment with three or more antihypertensive medications, including a diuretic.8 Patients with resistant hypertension have increased cardiovascular risk.9 Clinical trials of renal denervation have focused on treatment of resistant hypertension, in the hope of reducing the associated morbidity and mortality.
Results of the Symplicity HTN-3 trial, which assessed the safety and efficacy of renal denervation, were anxiously awaited, since prior trials yielded mixed results. Although the Symplicity HTN-1 and Symplicity HTN-2 studies demonstrated a possible benefit of renal denervation to lower office measured blood pressure, other studies did not show a decrease in BP in patients who had undergone renal denervation.6,7 These early trials, however, were small and did not randomize patients to a sham procedure.10
The Symplicity HTN-3 trial included 535 patients at 88 centers in the US. Patients were randomly assigned to receive either renal denervation plus baseline antihypertensive medications or a sham procedure plus baseline antihypertensive medications.
The researchers found that the sham procedure was just as effective as the “true” renal denervation in decreasing systolic blood pressure in patients with resistant hypertension.10 In other words, renal denervation did not demonstrate efficacy for this purpose.
In response to the results of this well-designed trial, the FDA has halted approval to perform renal denervation in patients with resistant hypertension in the US. However, clinical investigation will continue among subgroups of hypertensive patients or separate populations.
Despite a lack of efficacy, renal denervation does appear to be well tolerated, as evidenced by safety data from Symplicity HTN-3. —JK
Jessica Knight, ACNP
University of New Mexico Hospital, Albuquerque
REFERENCES
5. Esler MD, Krum H, Schlaich M, et al. Renal sympathetic denervation for the treatment of drug-resistant hypertension: one-year results from the Symplicity HTN-2 randomized, controlled trial. Circulation. 2012;126(25):2976-2982.
6. Thukkani AK, Bhatt LD. Renal denervation therapy for hypertension. Circulation. 2013;128:2251-2254.
7. Ezzahti M, Moelker A, Friesema E, et al. Blood pressure and neurohormonal responses to renal nerve ablation in treatment-resistant hypertension. J Hypertens. 2014;32(1):135-141.
8. Calhoun DA, Jones D, Textor S, et al. Resistant hypertension: Diagnosis, evaluation, and treatment: A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51(6):1403-1419.
9. Daugherty SL, Powers JD, Magid DJ, et al. Incidence and prognosis of resistant hypertension in hypertensive patients. Circulation. 2012;125(13):1635-1642.
10. Bhatt DL, Kandzari DE, O’Neill WW, et al; Symplicity HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393-1401.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Predictive Factors for CKD
Q) Quite a few of my teenage patients are overweight. I know they are at risk for diabetes, but does their weight also affect their kidneys? Isn’t diabetes the main cause of kidney failure?
The number one cause of chronic kidney disease (CKD) in the United States and worldwide is diabetes, but it is certainly not the only risk factor. Studies have shown a link between obesity and CKD; even in the absence of kidney disease, obesity may cause glomerular dysfunction and an increase in glomerular size.1
Obesity during adolescence has been identified as a strong predictor of CKD in adulthood. Other diseases and conditions that, if present in adolescence, indicate future risk for kidney disease include diabetes, hypertension, inflammation, and proteinuria.
A recent Swedish study followed patients from adolescence to adulthood to identify markers that would predict later kidney disease. In this study, the most predictive factor of kidney failure in adulthood was proteinuria in adolescence (odds ratio, 7.72). These results may be limited by the homogeneity of the predominantly white, male study population, but the extensive follow-up period, which “highlights the long natural history” of kidney disease, is one strength of this study.2
Based on these and other findings, you know that if your teenage patients have proteinuria, they are much more likely to develop kidney failure as an adult. Yet, in the US, the American Academy of Pediatrics and the US Preventive Services Task Force do not recommend urine screening for asymptomatic children.3
Interestingly, however, a survey of pediatric practices revealed that 58% of pediatricians screen adolescents with urinalysis, even if they are asymptomatic.4 In other words, they ignore the guidelines. If they did not, we would likely miss what is possibly the most important predictive factor for kidney failure in adults. —TAH
Tia Austin Hayes, FNP-C
UMMC/JMM Outpatient Dialysis/Renal Clinic, Jackson, Mississippi
REFERENCES
1. Rocchini A. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346(11):854-855.
2. Sundin PO, Udumyan R, Sjöström P, Montgomery S. Predictors in adolescence of ESRD in middle-aged men. Am J Kidney Dis. 2014;64(5):723-729.
3. Kaplan RE, Springate JE, Feld LG. Screening dipstick urinalysis: a time to change. Pediatrics. 1997;100(6):919-921.
4. Sox CM, Christakis DA. Pediatricians’ screening urinalysis practices. J Pediatr. 2005; 147(3):362-365.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Q) Quite a few of my teenage patients are overweight. I know they are at risk for diabetes, but does their weight also affect their kidneys? Isn’t diabetes the main cause of kidney failure?
The number one cause of chronic kidney disease (CKD) in the United States and worldwide is diabetes, but it is certainly not the only risk factor. Studies have shown a link between obesity and CKD; even in the absence of kidney disease, obesity may cause glomerular dysfunction and an increase in glomerular size.1
Obesity during adolescence has been identified as a strong predictor of CKD in adulthood. Other diseases and conditions that, if present in adolescence, indicate future risk for kidney disease include diabetes, hypertension, inflammation, and proteinuria.
A recent Swedish study followed patients from adolescence to adulthood to identify markers that would predict later kidney disease. In this study, the most predictive factor of kidney failure in adulthood was proteinuria in adolescence (odds ratio, 7.72). These results may be limited by the homogeneity of the predominantly white, male study population, but the extensive follow-up period, which “highlights the long natural history” of kidney disease, is one strength of this study.2
Based on these and other findings, you know that if your teenage patients have proteinuria, they are much more likely to develop kidney failure as an adult. Yet, in the US, the American Academy of Pediatrics and the US Preventive Services Task Force do not recommend urine screening for asymptomatic children.3
Interestingly, however, a survey of pediatric practices revealed that 58% of pediatricians screen adolescents with urinalysis, even if they are asymptomatic.4 In other words, they ignore the guidelines. If they did not, we would likely miss what is possibly the most important predictive factor for kidney failure in adults. —TAH
Tia Austin Hayes, FNP-C
UMMC/JMM Outpatient Dialysis/Renal Clinic, Jackson, Mississippi
REFERENCES
1. Rocchini A. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346(11):854-855.
2. Sundin PO, Udumyan R, Sjöström P, Montgomery S. Predictors in adolescence of ESRD in middle-aged men. Am J Kidney Dis. 2014;64(5):723-729.
3. Kaplan RE, Springate JE, Feld LG. Screening dipstick urinalysis: a time to change. Pediatrics. 1997;100(6):919-921.
4. Sox CM, Christakis DA. Pediatricians’ screening urinalysis practices. J Pediatr. 2005; 147(3):362-365.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Q) Quite a few of my teenage patients are overweight. I know they are at risk for diabetes, but does their weight also affect their kidneys? Isn’t diabetes the main cause of kidney failure?
The number one cause of chronic kidney disease (CKD) in the United States and worldwide is diabetes, but it is certainly not the only risk factor. Studies have shown a link between obesity and CKD; even in the absence of kidney disease, obesity may cause glomerular dysfunction and an increase in glomerular size.1
Obesity during adolescence has been identified as a strong predictor of CKD in adulthood. Other diseases and conditions that, if present in adolescence, indicate future risk for kidney disease include diabetes, hypertension, inflammation, and proteinuria.
A recent Swedish study followed patients from adolescence to adulthood to identify markers that would predict later kidney disease. In this study, the most predictive factor of kidney failure in adulthood was proteinuria in adolescence (odds ratio, 7.72). These results may be limited by the homogeneity of the predominantly white, male study population, but the extensive follow-up period, which “highlights the long natural history” of kidney disease, is one strength of this study.2
Based on these and other findings, you know that if your teenage patients have proteinuria, they are much more likely to develop kidney failure as an adult. Yet, in the US, the American Academy of Pediatrics and the US Preventive Services Task Force do not recommend urine screening for asymptomatic children.3
Interestingly, however, a survey of pediatric practices revealed that 58% of pediatricians screen adolescents with urinalysis, even if they are asymptomatic.4 In other words, they ignore the guidelines. If they did not, we would likely miss what is possibly the most important predictive factor for kidney failure in adults. —TAH
Tia Austin Hayes, FNP-C
UMMC/JMM Outpatient Dialysis/Renal Clinic, Jackson, Mississippi
REFERENCES
1. Rocchini A. Childhood obesity and a diabetes epidemic. N Engl J Med. 2002;346(11):854-855.
2. Sundin PO, Udumyan R, Sjöström P, Montgomery S. Predictors in adolescence of ESRD in middle-aged men. Am J Kidney Dis. 2014;64(5):723-729.
3. Kaplan RE, Springate JE, Feld LG. Screening dipstick urinalysis: a time to change. Pediatrics. 1997;100(6):919-921.
4. Sox CM, Christakis DA. Pediatricians’ screening urinalysis practices. J Pediatr. 2005; 147(3):362-365.
The author would like to thank Eric Judd, MD, of the University of Alabama at Birmingham, for his advice on the preparation of this response.
Problematic Medications: "Stomach Medicine"
Q) I am getting calls from patients saying they heard a “stomach medicine” would hurt their kidneys. What is the basis, and how should I respond?
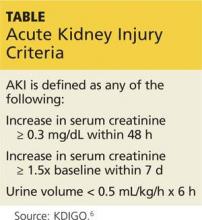
Emerging evidence is suggestive of a causal association between proton pump inhibitor (PPI) use and acute kidney injury/interstitial nephritis. Acute kidney injury is defined as either a decrease in urine output to less than 0.5 mL/kg/h for six hours, a rise in serum creatinine of 0.3 mg/dL or more within 48 hours, or an increase in creatinine of 50% or more above baseline within a week. Acute interstitial nephritis is often definitively diagnosed by renal biopsy, with findings of acute inflammatory cells, interstitial edema, and infiltration. Medications are the most common etiology for acute interstitial nephritis and account for more than 75% of cases.5
According to results published in the American Journal of Kidney Diseases, a retrospective study of 133 biopsy-proven cases of acute interstitial nephritis found 70% were associated with medication use. Of these, 14% were linked to use of a PPI (other drug culprits included antibiotics and NSAIDs, responsible for 49% and 11% of cases, respectively). Overall, omeprazole was the top drug cause, at 12%.6
In a nested case-control study of 572,661 subjects (mean age, 65.4) taking either lansoprazole, omeprazole, or pantoprazole, 46 definite cases and 26 probable cases of first-time acute interstitial nephritis were identified. Omeprazole was the most commonly dispensed PPI in this study. The crude incidence rate per 100,000 person-years for current use of a PPI was 11.98 and for past use, 1.68.7
Another nested case-control study of 184,480 subjects (ages 18 and older) reported 854 cases of acute kidney injury, with a positive association between use of a PPI and development of renal disease, even after controlling for confounding factors (P < .0001). Of note, no significant relationship was found between acute renal injury and use of H2 blocker therapy.8—CAS
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants PLLC, South Charleston, West Virginia
REFERENCES
1. Velazquez H, Perazella MA, Wright FS, Ellison DH. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:296-301.
2. Horn JR, Hansten PD. Trimethoprim and potassium-sparing drugs: a risk for hyperkalemia. www.pharmacytimes.com/publications/issue/2011/February2011/DrugInteractions-0211. Accessed August 24, 2015.
3. Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776-782.
4. Fralick M, Macdonald EM, Gomes T, et al. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ. 2014;349:g6196.
5. Gilbert SJ, Weiner DE, Gipson DS, et al. National Kidney Foundation’s Primer on Kidney Diseases. Philadelphia, PA: Elsevier; 2014.
6. Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
7. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
8. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrology. 2013;14:150.
Q) I am getting calls from patients saying they heard a “stomach medicine” would hurt their kidneys. What is the basis, and how should I respond?
Emerging evidence is suggestive of a causal association between proton pump inhibitor (PPI) use and acute kidney injury/interstitial nephritis. Acute kidney injury is defined as either a decrease in urine output to less than 0.5 mL/kg/h for six hours, a rise in serum creatinine of 0.3 mg/dL or more within 48 hours, or an increase in creatinine of 50% or more above baseline within a week. Acute interstitial nephritis is often definitively diagnosed by renal biopsy, with findings of acute inflammatory cells, interstitial edema, and infiltration. Medications are the most common etiology for acute interstitial nephritis and account for more than 75% of cases.5
According to results published in the American Journal of Kidney Diseases, a retrospective study of 133 biopsy-proven cases of acute interstitial nephritis found 70% were associated with medication use. Of these, 14% were linked to use of a PPI (other drug culprits included antibiotics and NSAIDs, responsible for 49% and 11% of cases, respectively). Overall, omeprazole was the top drug cause, at 12%.6
In a nested case-control study of 572,661 subjects (mean age, 65.4) taking either lansoprazole, omeprazole, or pantoprazole, 46 definite cases and 26 probable cases of first-time acute interstitial nephritis were identified. Omeprazole was the most commonly dispensed PPI in this study. The crude incidence rate per 100,000 person-years for current use of a PPI was 11.98 and for past use, 1.68.7
Another nested case-control study of 184,480 subjects (ages 18 and older) reported 854 cases of acute kidney injury, with a positive association between use of a PPI and development of renal disease, even after controlling for confounding factors (P < .0001). Of note, no significant relationship was found between acute renal injury and use of H2 blocker therapy.8—CAS
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants PLLC, South Charleston, West Virginia
REFERENCES
1. Velazquez H, Perazella MA, Wright FS, Ellison DH. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:296-301.
2. Horn JR, Hansten PD. Trimethoprim and potassium-sparing drugs: a risk for hyperkalemia. www.pharmacytimes.com/publications/issue/2011/February2011/DrugInteractions-0211. Accessed August 24, 2015.
3. Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776-782.
4. Fralick M, Macdonald EM, Gomes T, et al. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ. 2014;349:g6196.
5. Gilbert SJ, Weiner DE, Gipson DS, et al. National Kidney Foundation’s Primer on Kidney Diseases. Philadelphia, PA: Elsevier; 2014.
6. Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
7. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
8. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrology. 2013;14:150.
Q) I am getting calls from patients saying they heard a “stomach medicine” would hurt their kidneys. What is the basis, and how should I respond?
Emerging evidence is suggestive of a causal association between proton pump inhibitor (PPI) use and acute kidney injury/interstitial nephritis. Acute kidney injury is defined as either a decrease in urine output to less than 0.5 mL/kg/h for six hours, a rise in serum creatinine of 0.3 mg/dL or more within 48 hours, or an increase in creatinine of 50% or more above baseline within a week. Acute interstitial nephritis is often definitively diagnosed by renal biopsy, with findings of acute inflammatory cells, interstitial edema, and infiltration. Medications are the most common etiology for acute interstitial nephritis and account for more than 75% of cases.5
According to results published in the American Journal of Kidney Diseases, a retrospective study of 133 biopsy-proven cases of acute interstitial nephritis found 70% were associated with medication use. Of these, 14% were linked to use of a PPI (other drug culprits included antibiotics and NSAIDs, responsible for 49% and 11% of cases, respectively). Overall, omeprazole was the top drug cause, at 12%.6
In a nested case-control study of 572,661 subjects (mean age, 65.4) taking either lansoprazole, omeprazole, or pantoprazole, 46 definite cases and 26 probable cases of first-time acute interstitial nephritis were identified. Omeprazole was the most commonly dispensed PPI in this study. The crude incidence rate per 100,000 person-years for current use of a PPI was 11.98 and for past use, 1.68.7
Another nested case-control study of 184,480 subjects (ages 18 and older) reported 854 cases of acute kidney injury, with a positive association between use of a PPI and development of renal disease, even after controlling for confounding factors (P < .0001). Of note, no significant relationship was found between acute renal injury and use of H2 blocker therapy.8—CAS
Cynthia A. Smith, DNP, APRN, FNP-BC
Renal Consultants PLLC, South Charleston, West Virginia
REFERENCES
1. Velazquez H, Perazella MA, Wright FS, Ellison DH. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:296-301.
2. Horn JR, Hansten PD. Trimethoprim and potassium-sparing drugs: a risk for hyperkalemia. www.pharmacytimes.com/publications/issue/2011/February2011/DrugInteractions-0211. Accessed August 24, 2015.
3. Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776-782.
4. Fralick M, Macdonald EM, Gomes T, et al. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ. 2014;349:g6196.
5. Gilbert SJ, Weiner DE, Gipson DS, et al. National Kidney Foundation’s Primer on Kidney Diseases. Philadelphia, PA: Elsevier; 2014.
6. Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
7. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
8. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrology. 2013;14:150.
Problematic Medications: Antibiotics in Renal Patients
Q) At a lecture I recently attended, the speaker said sulfamethoxazole/trimethoprim is a potentially dangerous medication. I use it all the time. Is there any data to support her comments? Where did she get her information?
Sulfamethoxazole/trimethoprim (SMX/TMP) is a combination of two antibiotics, each of which has the potential to interact with other substances.
It is well documented that sulfamethoxazole can inhibit the metabolism of cytochrome P450 2C9 substrates. Frequently prescribed medications that also use the cytochrome substrate include warfarin and oral antihypoglycemic agents.
Trimethoprim’s distinct properties also lead to drug interactions. Trimethoprim inhibits sodium uptake by the appropriate channels in the distal tubule of the kidney, preventing reabsorption and altering the electrical balance of the tubular cells. As a result, the amount of potassium excreted into the urine is reduced, yielding an accumulation of serum potassium.1
High serum potassium retention can manifest as hyperkalemia in patients with chronic kidney disease (CKD). Use of potassium-sparing drugs by patients with comorbidities, including CKD, can increase risk for hyperkalemia; concurrent use of these drugs with ACE inhibitors or angiotensin II receptor blockers (ARBs) compounds the risk.2 The first reports of hyperkalemia with trimethoprim use occurred in HIV patients treated with large doses for Pneumocystis carinii infection.3
In a population-based case-control study, the results of which were published in the British Medical Journal, Fralick and colleagues analyzed data on older patients (age 66 or older) who were taking either ACE inhibitors or ARBs in combination with an antibiotic.4 They found a significantly increased risk for sudden death within seven days of prescription of SMX/TMP, compared to amoxicillin; a secondary analysis also revealed an increased risk for sudden death within 14 days with SMX/TMP. The researchers speculated that this excess risk, which translated to 3 sudden deaths in 1,000 patients taking SMX/TMP versus 1 sudden death in 1,000 patients taking amoxicillin, “reflects unrecognized arrhythmic death due to hyperkalemia.”
Since more than 250 million prescriptions for ACE inhibitors/ARBs and 20 million prescriptions for SMX/TMP are written each year, there will be instances of overlap. The prudent clinician would prescribe a different antibiotic or, if avoidance is not possible, use the lowest effective dose and duration of SMX/TMP. Close monitoring of serum potassium levels is warranted in patients with comorbidities, especially CKD, who are taking ACE inhibitors or ARBs—and of course, in our geriatric population. —DLC
Debra L. Coplon, DNP, DCC
City of Memphis Wellness Clinic, Tennessee
REFERENCES
1. Velazquez H, Perazella MA, Wright FS, Ellison DH. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:296-301.
2. Horn JR, Hansten PD. Trimethoprim and potassium-sparing drugs: a risk for hyperkalemia. www.pharmacytimes.com/publications/issue/2011/February2011/DrugInteractions-0211. Accessed August 24, 2015.
3. Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776-782.
4. Fralick M, Macdonald EM, Gomes T, et al. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ. 2014;349:g6196.
5. Gilbert SJ, Weiner DE, Gipson DS, et al. National Kidney Foundation’s Primer on Kidney Diseases. Philadelphia, PA: Elsevier; 2014.
6. Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
7. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
8. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrology. 2013;14:150.
Q) At a lecture I recently attended, the speaker said sulfamethoxazole/trimethoprim is a potentially dangerous medication. I use it all the time. Is there any data to support her comments? Where did she get her information?
Sulfamethoxazole/trimethoprim (SMX/TMP) is a combination of two antibiotics, each of which has the potential to interact with other substances.
It is well documented that sulfamethoxazole can inhibit the metabolism of cytochrome P450 2C9 substrates. Frequently prescribed medications that also use the cytochrome substrate include warfarin and oral antihypoglycemic agents.
Trimethoprim’s distinct properties also lead to drug interactions. Trimethoprim inhibits sodium uptake by the appropriate channels in the distal tubule of the kidney, preventing reabsorption and altering the electrical balance of the tubular cells. As a result, the amount of potassium excreted into the urine is reduced, yielding an accumulation of serum potassium.1
High serum potassium retention can manifest as hyperkalemia in patients with chronic kidney disease (CKD). Use of potassium-sparing drugs by patients with comorbidities, including CKD, can increase risk for hyperkalemia; concurrent use of these drugs with ACE inhibitors or angiotensin II receptor blockers (ARBs) compounds the risk.2 The first reports of hyperkalemia with trimethoprim use occurred in HIV patients treated with large doses for Pneumocystis carinii infection.3
In a population-based case-control study, the results of which were published in the British Medical Journal, Fralick and colleagues analyzed data on older patients (age 66 or older) who were taking either ACE inhibitors or ARBs in combination with an antibiotic.4 They found a significantly increased risk for sudden death within seven days of prescription of SMX/TMP, compared to amoxicillin; a secondary analysis also revealed an increased risk for sudden death within 14 days with SMX/TMP. The researchers speculated that this excess risk, which translated to 3 sudden deaths in 1,000 patients taking SMX/TMP versus 1 sudden death in 1,000 patients taking amoxicillin, “reflects unrecognized arrhythmic death due to hyperkalemia.”
Since more than 250 million prescriptions for ACE inhibitors/ARBs and 20 million prescriptions for SMX/TMP are written each year, there will be instances of overlap. The prudent clinician would prescribe a different antibiotic or, if avoidance is not possible, use the lowest effective dose and duration of SMX/TMP. Close monitoring of serum potassium levels is warranted in patients with comorbidities, especially CKD, who are taking ACE inhibitors or ARBs—and of course, in our geriatric population. —DLC
Debra L. Coplon, DNP, DCC
City of Memphis Wellness Clinic, Tennessee
REFERENCES
1. Velazquez H, Perazella MA, Wright FS, Ellison DH. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:296-301.
2. Horn JR, Hansten PD. Trimethoprim and potassium-sparing drugs: a risk for hyperkalemia. www.pharmacytimes.com/publications/issue/2011/February2011/DrugInteractions-0211. Accessed August 24, 2015.
3. Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776-782.
4. Fralick M, Macdonald EM, Gomes T, et al. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ. 2014;349:g6196.
5. Gilbert SJ, Weiner DE, Gipson DS, et al. National Kidney Foundation’s Primer on Kidney Diseases. Philadelphia, PA: Elsevier; 2014.
6. Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
7. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
8. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrology. 2013;14:150.
Q) At a lecture I recently attended, the speaker said sulfamethoxazole/trimethoprim is a potentially dangerous medication. I use it all the time. Is there any data to support her comments? Where did she get her information?
Sulfamethoxazole/trimethoprim (SMX/TMP) is a combination of two antibiotics, each of which has the potential to interact with other substances.
It is well documented that sulfamethoxazole can inhibit the metabolism of cytochrome P450 2C9 substrates. Frequently prescribed medications that also use the cytochrome substrate include warfarin and oral antihypoglycemic agents.
Trimethoprim’s distinct properties also lead to drug interactions. Trimethoprim inhibits sodium uptake by the appropriate channels in the distal tubule of the kidney, preventing reabsorption and altering the electrical balance of the tubular cells. As a result, the amount of potassium excreted into the urine is reduced, yielding an accumulation of serum potassium.1
High serum potassium retention can manifest as hyperkalemia in patients with chronic kidney disease (CKD). Use of potassium-sparing drugs by patients with comorbidities, including CKD, can increase risk for hyperkalemia; concurrent use of these drugs with ACE inhibitors or angiotensin II receptor blockers (ARBs) compounds the risk.2 The first reports of hyperkalemia with trimethoprim use occurred in HIV patients treated with large doses for Pneumocystis carinii infection.3
In a population-based case-control study, the results of which were published in the British Medical Journal, Fralick and colleagues analyzed data on older patients (age 66 or older) who were taking either ACE inhibitors or ARBs in combination with an antibiotic.4 They found a significantly increased risk for sudden death within seven days of prescription of SMX/TMP, compared to amoxicillin; a secondary analysis also revealed an increased risk for sudden death within 14 days with SMX/TMP. The researchers speculated that this excess risk, which translated to 3 sudden deaths in 1,000 patients taking SMX/TMP versus 1 sudden death in 1,000 patients taking amoxicillin, “reflects unrecognized arrhythmic death due to hyperkalemia.”
Since more than 250 million prescriptions for ACE inhibitors/ARBs and 20 million prescriptions for SMX/TMP are written each year, there will be instances of overlap. The prudent clinician would prescribe a different antibiotic or, if avoidance is not possible, use the lowest effective dose and duration of SMX/TMP. Close monitoring of serum potassium levels is warranted in patients with comorbidities, especially CKD, who are taking ACE inhibitors or ARBs—and of course, in our geriatric population. —DLC
Debra L. Coplon, DNP, DCC
City of Memphis Wellness Clinic, Tennessee
REFERENCES
1. Velazquez H, Perazella MA, Wright FS, Ellison DH. Renal mechanism of trimethoprim-induced hyperkalemia. Ann Intern Med. 1993;119:296-301.
2. Horn JR, Hansten PD. Trimethoprim and potassium-sparing drugs: a risk for hyperkalemia. www.pharmacytimes.com/publications/issue/2011/February2011/DrugInteractions-0211. Accessed August 24, 2015.
3. Medina I, Mills J, Leoung G, et al. Oral therapy for Pneumocystis carinii pneumonia in the acquired immunodeficiency syndrome: a controlled trial of trimethoprim-sulfamethoxazole versus trimethoprim-dapsone. N Engl J Med. 1990;323:776-782.
4. Fralick M, Macdonald EM, Gomes T, et al. Co-trimoxazole and sudden death in patients receiving inhibitors of renin-angiotensin system: population based study. BMJ. 2014;349:g6196.
5. Gilbert SJ, Weiner DE, Gipson DS, et al. National Kidney Foundation’s Primer on Kidney Diseases. Philadelphia, PA: Elsevier; 2014.
6. Muriithi AK, Leung N, Valeri AM, et al. Biopsy-proven acute interstitial nephritis, 1993-2011: a case series. Am J Kidney Dis. 2014;64(4):558-566.
7. Blank ML, Parkin L, Paul C, Herbison P. A nationwide nested case-control study indicates an increased risk of acute interstitial nephritis with proton pump inhibitor use. Kidney Int. 2014;86(4):837-844.
8. Klepser DG, Collier DS, Cochran GL. Proton pump inhibitors and acute kidney injury: a nested case-control study. BMC Nephrology. 2013;14:150.
Understanding Hematuria: Causes
Q) I have been treating a 60-year-old man with a long history of microscopic hematuria and waxing/waning proteinuria. What could be the cause of his hematuria?
Hematuria is a consequence of erythrocytes, or red blood cells (RBCs), in the urine. This can cause a visible change in color, considered gross or macroscopic hematuria; or the blood may only be visible under microscopy or by urine dipstick (referred to as microscopic hematuria).
Both findings are followed up with urinalysis to quantify erythrocytes, protein, and presence of casts and to review RBC morphology. This information will assist in determining if the hematuria is glomerular or nonglomerular in origin.1
The examination and treatment plan for nonglomerular hematuria will focus on urinary tract diseases. If the patient is found to have glomerular hematuria, the focus will be on diseases of the kidney. A thorough history and physical should be performed in addition to urinalysis.
Glomerular disease is suggested in those with micro- or macroscopic proteinuria, proteinuria > 1 g/24h, or an absence of casts. Our index patient has microscopic hematuria and “waxing/waning” (unquantified) proteinuria, suggesting glomerular origin.
There are a number of renal causes for glomerular bleeding, including primary glomerulonephritis, multisystem autoimmune disease, and hereditary or infective glomerulonephritis.2 Renal biopsy is recommended for patients who have hypertension, proteinuria, and hematuria, to determine the cause and thus determine the appropriate treatment.
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
Q) I have been treating a 60-year-old man with a long history of microscopic hematuria and waxing/waning proteinuria. What could be the cause of his hematuria?
Hematuria is a consequence of erythrocytes, or red blood cells (RBCs), in the urine. This can cause a visible change in color, considered gross or macroscopic hematuria; or the blood may only be visible under microscopy or by urine dipstick (referred to as microscopic hematuria).
Both findings are followed up with urinalysis to quantify erythrocytes, protein, and presence of casts and to review RBC morphology. This information will assist in determining if the hematuria is glomerular or nonglomerular in origin.1
The examination and treatment plan for nonglomerular hematuria will focus on urinary tract diseases. If the patient is found to have glomerular hematuria, the focus will be on diseases of the kidney. A thorough history and physical should be performed in addition to urinalysis.
Glomerular disease is suggested in those with micro- or macroscopic proteinuria, proteinuria > 1 g/24h, or an absence of casts. Our index patient has microscopic hematuria and “waxing/waning” (unquantified) proteinuria, suggesting glomerular origin.
There are a number of renal causes for glomerular bleeding, including primary glomerulonephritis, multisystem autoimmune disease, and hereditary or infective glomerulonephritis.2 Renal biopsy is recommended for patients who have hypertension, proteinuria, and hematuria, to determine the cause and thus determine the appropriate treatment.
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
Q) I have been treating a 60-year-old man with a long history of microscopic hematuria and waxing/waning proteinuria. What could be the cause of his hematuria?
Hematuria is a consequence of erythrocytes, or red blood cells (RBCs), in the urine. This can cause a visible change in color, considered gross or macroscopic hematuria; or the blood may only be visible under microscopy or by urine dipstick (referred to as microscopic hematuria).
Both findings are followed up with urinalysis to quantify erythrocytes, protein, and presence of casts and to review RBC morphology. This information will assist in determining if the hematuria is glomerular or nonglomerular in origin.1
The examination and treatment plan for nonglomerular hematuria will focus on urinary tract diseases. If the patient is found to have glomerular hematuria, the focus will be on diseases of the kidney. A thorough history and physical should be performed in addition to urinalysis.
Glomerular disease is suggested in those with micro- or macroscopic proteinuria, proteinuria > 1 g/24h, or an absence of casts. Our index patient has microscopic hematuria and “waxing/waning” (unquantified) proteinuria, suggesting glomerular origin.
There are a number of renal causes for glomerular bleeding, including primary glomerulonephritis, multisystem autoimmune disease, and hereditary or infective glomerulonephritis.2 Renal biopsy is recommended for patients who have hypertension, proteinuria, and hematuria, to determine the cause and thus determine the appropriate treatment.
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
Understanding Hematuria: IgA Nephropathy
Q) My hematuria patient had more significant proteinuria recently, so the nephrologist sent him for kidney biopsy. It was read as IgA nephropathy: “classic mesangial staining on IF with moderate-advanced chronic injury (15/32 gloms globally sclerosed, 40% IFTA, mild arteriosclerosis).” What exactly does this mean, and what is IgA nephropathy?
Immunoglobulin A (IgA) nephropathy is the most common type of glomerulonephritis; up to 40% of patients with IgA nephropathy develop end-stage renal disease within 20 years of diagnosis. More common in men, IgA nephropathy is usually diagnosed in people in their second or third decades of life.2,3
Considered an autoimmune disease, IgA nephropathy typically presents with macroscopic or gross hematuria that occurs within 24 hours of the onset of an upper respiratory infection (URI). The hematuria typically resolves quickly, in one to three days. An individual bacterial or viral element has not yet been identified.
IgA nephropathy is an immune response to the URI. IgA is secreted from mucosal surfaces at the back of the mouth and then deposited in the glomerular mesangium, a “stalk of cells” associated with the capillaries of the renal glomerulus.1 It is speculated that genetics, environment, and/or hypersensitivity to food antigens may play a part in IgA nephropathy. Results from biopsies of blood relatives of patients with confirmed IgA nephropathy suggest a familial role.1
IgA nephropathy is prevalent in persons who live in the Pacific Rim and Southern Europe. However, this association may be the result of a sampling error due to investigation of all microscopic hematuria in these areas. In all, 90% of IgA is sporadic.4 It is often asymptomatic, aside from occasional back and flank pain secondary to inflammation of the renal capsule. Unfortunately, many patients develop renal impairment and hypertension by the time they are diagnosed.
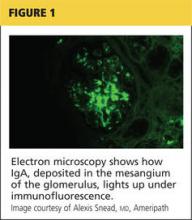
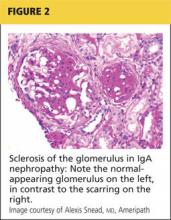
Renal biopsy is used to confirm/diagnose IgA nephropathy. IgA, deposited in the mesangium of the glomerulus, lights up under immunofluorescence (IF; see Figure 1). In some patients, this mesangial deposition results in sclerosis, scarring, and/or inflammation of the glomerulus (see Figure 2).
An international panel of experts created guidelines (the Oxford classification system) for reporting IgA kidney biopsies. Six adverse pathologic features have been identified:
• Mesangial cellularity score
• Percentage of segmental sclerosis
• Endocapillary hypercellularity
• Cellular and/or fibrocellular crescents
• Percentage of interstitial fibrosis/tubular atrophy (IFTA)
• Arteriosclerosis score5,6
Interstitial fibrosis, crescents, and as little as 25% glomerular sclerosis found on biopsy increases the likelihood of disease progression.5 Clinically, hypertension, a reduced glomerular filtration rate, increasing age, and proteinuria of > 1g/24h have been identified as risk factors for progression of IgA nephropathy. Up to 30% of patients diagnosed will require renal replacement therapy within 20 years.1
The case patient’s findings include the typical IF staining of IgA in the glomerulus. The biopsy report also indicates that 40% of the glomeruli (less than half) have interstitial fibrosis and that the structural integrity of the tubules has been affected secondary to IgA accumulation in the mesangium. These findings are suggestive of progressive disease.
There is no known way to stop IgA deposition in the mesangium. Tonsillectomy to reduce mucosal IgA release has been suggested but is controversial.
Treatment of IgA nephropathy focuses on preserving renal function by reducing proteinuria through the use of ACE inhibitors and/or angiotensin receptor blockers. Aggressive blood pressure management is achieved by blocking the renin-angiotensin-aldosterone system (RAAS).
Other methods for decreasing progression of renal disease are directed at reducing the immune and inflammatory response via immunosuppressant medications.3 The use of immunosuppressive agents, though controversial, is recommended for those who have progressive disease and/or proteinuria despite achieving target blood pressure with full RAAS blockade.1
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
3. Barratt J, Feehally J. Treatment of IgA nephropathy. Kidney Int. 2006;69(11):1934-1938.
4. Johnson R, Feehally J. Comprehensive Clinical Nephrology. 2nd ed. London: Mosby; 2000.
5. Walsh M, Sar A, Lee D, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010; 5(3):425-430.
6. Roberts I. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009; 76(5):546-556.
Q) My hematuria patient had more significant proteinuria recently, so the nephrologist sent him for kidney biopsy. It was read as IgA nephropathy: “classic mesangial staining on IF with moderate-advanced chronic injury (15/32 gloms globally sclerosed, 40% IFTA, mild arteriosclerosis).” What exactly does this mean, and what is IgA nephropathy?
Immunoglobulin A (IgA) nephropathy is the most common type of glomerulonephritis; up to 40% of patients with IgA nephropathy develop end-stage renal disease within 20 years of diagnosis. More common in men, IgA nephropathy is usually diagnosed in people in their second or third decades of life.2,3
Considered an autoimmune disease, IgA nephropathy typically presents with macroscopic or gross hematuria that occurs within 24 hours of the onset of an upper respiratory infection (URI). The hematuria typically resolves quickly, in one to three days. An individual bacterial or viral element has not yet been identified.
IgA nephropathy is an immune response to the URI. IgA is secreted from mucosal surfaces at the back of the mouth and then deposited in the glomerular mesangium, a “stalk of cells” associated with the capillaries of the renal glomerulus.1 It is speculated that genetics, environment, and/or hypersensitivity to food antigens may play a part in IgA nephropathy. Results from biopsies of blood relatives of patients with confirmed IgA nephropathy suggest a familial role.1
IgA nephropathy is prevalent in persons who live in the Pacific Rim and Southern Europe. However, this association may be the result of a sampling error due to investigation of all microscopic hematuria in these areas. In all, 90% of IgA is sporadic.4 It is often asymptomatic, aside from occasional back and flank pain secondary to inflammation of the renal capsule. Unfortunately, many patients develop renal impairment and hypertension by the time they are diagnosed.
Renal biopsy is used to confirm/diagnose IgA nephropathy. IgA, deposited in the mesangium of the glomerulus, lights up under immunofluorescence (IF; see Figure 1). In some patients, this mesangial deposition results in sclerosis, scarring, and/or inflammation of the glomerulus (see Figure 2).
An international panel of experts created guidelines (the Oxford classification system) for reporting IgA kidney biopsies. Six adverse pathologic features have been identified:
• Mesangial cellularity score
• Percentage of segmental sclerosis
• Endocapillary hypercellularity
• Cellular and/or fibrocellular crescents
• Percentage of interstitial fibrosis/tubular atrophy (IFTA)
• Arteriosclerosis score5,6
Interstitial fibrosis, crescents, and as little as 25% glomerular sclerosis found on biopsy increases the likelihood of disease progression.5 Clinically, hypertension, a reduced glomerular filtration rate, increasing age, and proteinuria of > 1g/24h have been identified as risk factors for progression of IgA nephropathy. Up to 30% of patients diagnosed will require renal replacement therapy within 20 years.1
The case patient’s findings include the typical IF staining of IgA in the glomerulus. The biopsy report also indicates that 40% of the glomeruli (less than half) have interstitial fibrosis and that the structural integrity of the tubules has been affected secondary to IgA accumulation in the mesangium. These findings are suggestive of progressive disease.
There is no known way to stop IgA deposition in the mesangium. Tonsillectomy to reduce mucosal IgA release has been suggested but is controversial.
Treatment of IgA nephropathy focuses on preserving renal function by reducing proteinuria through the use of ACE inhibitors and/or angiotensin receptor blockers. Aggressive blood pressure management is achieved by blocking the renin-angiotensin-aldosterone system (RAAS).
Other methods for decreasing progression of renal disease are directed at reducing the immune and inflammatory response via immunosuppressant medications.3 The use of immunosuppressive agents, though controversial, is recommended for those who have progressive disease and/or proteinuria despite achieving target blood pressure with full RAAS blockade.1
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
3. Barratt J, Feehally J. Treatment of IgA nephropathy. Kidney Int. 2006;69(11):1934-1938.
4. Johnson R, Feehally J. Comprehensive Clinical Nephrology. 2nd ed. London: Mosby; 2000.
5. Walsh M, Sar A, Lee D, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010; 5(3):425-430.
6. Roberts I. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009; 76(5):546-556.
Q) My hematuria patient had more significant proteinuria recently, so the nephrologist sent him for kidney biopsy. It was read as IgA nephropathy: “classic mesangial staining on IF with moderate-advanced chronic injury (15/32 gloms globally sclerosed, 40% IFTA, mild arteriosclerosis).” What exactly does this mean, and what is IgA nephropathy?
Immunoglobulin A (IgA) nephropathy is the most common type of glomerulonephritis; up to 40% of patients with IgA nephropathy develop end-stage renal disease within 20 years of diagnosis. More common in men, IgA nephropathy is usually diagnosed in people in their second or third decades of life.2,3
Considered an autoimmune disease, IgA nephropathy typically presents with macroscopic or gross hematuria that occurs within 24 hours of the onset of an upper respiratory infection (URI). The hematuria typically resolves quickly, in one to three days. An individual bacterial or viral element has not yet been identified.
IgA nephropathy is an immune response to the URI. IgA is secreted from mucosal surfaces at the back of the mouth and then deposited in the glomerular mesangium, a “stalk of cells” associated with the capillaries of the renal glomerulus.1 It is speculated that genetics, environment, and/or hypersensitivity to food antigens may play a part in IgA nephropathy. Results from biopsies of blood relatives of patients with confirmed IgA nephropathy suggest a familial role.1
IgA nephropathy is prevalent in persons who live in the Pacific Rim and Southern Europe. However, this association may be the result of a sampling error due to investigation of all microscopic hematuria in these areas. In all, 90% of IgA is sporadic.4 It is often asymptomatic, aside from occasional back and flank pain secondary to inflammation of the renal capsule. Unfortunately, many patients develop renal impairment and hypertension by the time they are diagnosed.
Renal biopsy is used to confirm/diagnose IgA nephropathy. IgA, deposited in the mesangium of the glomerulus, lights up under immunofluorescence (IF; see Figure 1). In some patients, this mesangial deposition results in sclerosis, scarring, and/or inflammation of the glomerulus (see Figure 2).
An international panel of experts created guidelines (the Oxford classification system) for reporting IgA kidney biopsies. Six adverse pathologic features have been identified:
• Mesangial cellularity score
• Percentage of segmental sclerosis
• Endocapillary hypercellularity
• Cellular and/or fibrocellular crescents
• Percentage of interstitial fibrosis/tubular atrophy (IFTA)
• Arteriosclerosis score5,6
Interstitial fibrosis, crescents, and as little as 25% glomerular sclerosis found on biopsy increases the likelihood of disease progression.5 Clinically, hypertension, a reduced glomerular filtration rate, increasing age, and proteinuria of > 1g/24h have been identified as risk factors for progression of IgA nephropathy. Up to 30% of patients diagnosed will require renal replacement therapy within 20 years.1
The case patient’s findings include the typical IF staining of IgA in the glomerulus. The biopsy report also indicates that 40% of the glomeruli (less than half) have interstitial fibrosis and that the structural integrity of the tubules has been affected secondary to IgA accumulation in the mesangium. These findings are suggestive of progressive disease.
There is no known way to stop IgA deposition in the mesangium. Tonsillectomy to reduce mucosal IgA release has been suggested but is controversial.
Treatment of IgA nephropathy focuses on preserving renal function by reducing proteinuria through the use of ACE inhibitors and/or angiotensin receptor blockers. Aggressive blood pressure management is achieved by blocking the renin-angiotensin-aldosterone system (RAAS).
Other methods for decreasing progression of renal disease are directed at reducing the immune and inflammatory response via immunosuppressant medications.3 The use of immunosuppressive agents, though controversial, is recommended for those who have progressive disease and/or proteinuria despite achieving target blood pressure with full RAAS blockade.1
Amy L. Hazel, RN, MSN, CNP
Kidney & Hypertension Consultants, Canton, Ohio
REFERENCES
1. Greenberg A. Primer on Kidney Diseases. 5th ed. Philadelphia, PA: Elsevier Saunders; 2005.
2. Barratt J, Feehally J. IgA nephropathy [disease of the month]. J Am Soc Nephrol. 2005;16(7): 2088-2097.
3. Barratt J, Feehally J. Treatment of IgA nephropathy. Kidney Int. 2006;69(11):1934-1938.
4. Johnson R, Feehally J. Comprehensive Clinical Nephrology. 2nd ed. London: Mosby; 2000.
5. Walsh M, Sar A, Lee D, et al. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin J Am Soc Nephrol. 2010; 5(3):425-430.
6. Roberts I. The Oxford classification of IgA nephropathy: pathology definitions, correlations, and reproducibility. Kidney Int. 2009; 76(5):546-556.
Acute Kidney Injury: Prevalent in Sugarcane Harvesters
Q) I’ve heard a lot of talk about all the kidney problems that the sugarcane workers in Central America have. Does anyone know why this is happening?
The unusually high rates of chronic kidney disease (CKD) among sugarcane workers in Central America have been a subject of great interest since National Public Radio (NPR) aired a special on this topic.3 There has been a rising epidemic of CKD in otherwise healthy male farm workers (ages 20 to 50), particularly those who harvest sugarcane.4,5 It has been hypothesized that recurrent episodes of acute kidney injury (AKI)—related to dehydration, volume depletion, pollutants, and rhabdomyolysis with inflammatory stress—are the underlying cause.5
Sugarcane harvesters typically work nine-hour days, six days per week, in extremely high temperatures and while wearing heavy, hot clothing. Each worker cuts approximately 10 tons of sugarcane daily, since they are paid based on cutting volume. Workers drink between five and 10 L of water during their shifts.
Santos et al designed a study to prospectively examine the effects of burnt sugarcane harvesting on renal function in healthy male farm workers. Twenty-eight men (ages 19 to 39) with no CKD risk factors (diabetes, smoking, obesity, hypertension, illicit drug or alcohol use) were followed for eight months from preharvest to postharvest. Blood samples were collected at the beginning and at the end of the workday and preharvest and postharvest season.5
Preseason lab values were normal in all 28 men. But postseason, all workers had elevated creatinine levels, with five meeting the criteria for AKI (see Table at left).5,6
Santos and colleagues identified potential causes for AKI in this population. These included
• Dehydration and volume depletion (episodes of tachycardia, increased urine density, lower urinary/serum sodium, higher hematocrit)
• Rhabdomyolysis (increased creatine kinase at the end of each workday)
• Systemic inflammation (increased white blood count, neutrophils, lymphocytes, and monocytes during the workday—possibly indicative of an inflammatory burst)
• Other factors (burning of the sugarcane releasing unknown nephrotoxic substances; unreported NSAIDs use)5
Compared to workers who showed early signs of CKD, those who developed frank AKI were more likely to have hyponatremia. Recommendations to reduce the problem include consumption of water/salt hydrating drinks, use of appropriate clothing, work-hour limitations, and changes to payment structures (ie, from a volume system to an hourly or daily system). Furthermore, education on the need to avoid alcohol, illicit drugs, and NSAIDs during the harvest season should help to decrease incidence of AKI among these workers.
Elizabeth C. Evans, RN, MSN, CNP, DNP
Renal Medicine Associates, Albuquerque, New Mexico
REFERENCES
1. Ayuk J, Gittoes N. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem. 2014;51(Pt 2):179-188.
2. Firouzi A, Maadani M, Kiani R, et al. Intravenous magnesium sulfate: new method in prevention of contrast-induced nephropathy in primary percutaneous coronary intervention. Int Urol Nephrol. 2015;47(3):521-525.
3. Beaubien J. Mysterious kidney disease slays farm workers in central America. National Public Radio; 2014. www.npr.org/blogs/health/2014/04/30/306907097/mysterious-kidney-disease-slays-farmworkers-in-central-america. Accessed April 1, 2015.
4. Almaguer M, Herrera R, Orantes CM. Chronic kidney disease of unknown etiology in agricultural communities. MEDICC Rev. 2014;16(2):9-15.
5. Santos UP, Zanetta DMT, Burdmann EA. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int. 2015;87(4):792-799.
6. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138.
Q) I’ve heard a lot of talk about all the kidney problems that the sugarcane workers in Central America have. Does anyone know why this is happening?
The unusually high rates of chronic kidney disease (CKD) among sugarcane workers in Central America have been a subject of great interest since National Public Radio (NPR) aired a special on this topic.3 There has been a rising epidemic of CKD in otherwise healthy male farm workers (ages 20 to 50), particularly those who harvest sugarcane.4,5 It has been hypothesized that recurrent episodes of acute kidney injury (AKI)—related to dehydration, volume depletion, pollutants, and rhabdomyolysis with inflammatory stress—are the underlying cause.5
Sugarcane harvesters typically work nine-hour days, six days per week, in extremely high temperatures and while wearing heavy, hot clothing. Each worker cuts approximately 10 tons of sugarcane daily, since they are paid based on cutting volume. Workers drink between five and 10 L of water during their shifts.
Santos et al designed a study to prospectively examine the effects of burnt sugarcane harvesting on renal function in healthy male farm workers. Twenty-eight men (ages 19 to 39) with no CKD risk factors (diabetes, smoking, obesity, hypertension, illicit drug or alcohol use) were followed for eight months from preharvest to postharvest. Blood samples were collected at the beginning and at the end of the workday and preharvest and postharvest season.5
Preseason lab values were normal in all 28 men. But postseason, all workers had elevated creatinine levels, with five meeting the criteria for AKI (see Table at left).5,6
Santos and colleagues identified potential causes for AKI in this population. These included
• Dehydration and volume depletion (episodes of tachycardia, increased urine density, lower urinary/serum sodium, higher hematocrit)
• Rhabdomyolysis (increased creatine kinase at the end of each workday)
• Systemic inflammation (increased white blood count, neutrophils, lymphocytes, and monocytes during the workday—possibly indicative of an inflammatory burst)
• Other factors (burning of the sugarcane releasing unknown nephrotoxic substances; unreported NSAIDs use)5
Compared to workers who showed early signs of CKD, those who developed frank AKI were more likely to have hyponatremia. Recommendations to reduce the problem include consumption of water/salt hydrating drinks, use of appropriate clothing, work-hour limitations, and changes to payment structures (ie, from a volume system to an hourly or daily system). Furthermore, education on the need to avoid alcohol, illicit drugs, and NSAIDs during the harvest season should help to decrease incidence of AKI among these workers.
Elizabeth C. Evans, RN, MSN, CNP, DNP
Renal Medicine Associates, Albuquerque, New Mexico
REFERENCES
1. Ayuk J, Gittoes N. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem. 2014;51(Pt 2):179-188.
2. Firouzi A, Maadani M, Kiani R, et al. Intravenous magnesium sulfate: new method in prevention of contrast-induced nephropathy in primary percutaneous coronary intervention. Int Urol Nephrol. 2015;47(3):521-525.
3. Beaubien J. Mysterious kidney disease slays farm workers in central America. National Public Radio; 2014. www.npr.org/blogs/health/2014/04/30/306907097/mysterious-kidney-disease-slays-farmworkers-in-central-america. Accessed April 1, 2015.
4. Almaguer M, Herrera R, Orantes CM. Chronic kidney disease of unknown etiology in agricultural communities. MEDICC Rev. 2014;16(2):9-15.
5. Santos UP, Zanetta DMT, Burdmann EA. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int. 2015;87(4):792-799.
6. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138.
Q) I’ve heard a lot of talk about all the kidney problems that the sugarcane workers in Central America have. Does anyone know why this is happening?
The unusually high rates of chronic kidney disease (CKD) among sugarcane workers in Central America have been a subject of great interest since National Public Radio (NPR) aired a special on this topic.3 There has been a rising epidemic of CKD in otherwise healthy male farm workers (ages 20 to 50), particularly those who harvest sugarcane.4,5 It has been hypothesized that recurrent episodes of acute kidney injury (AKI)—related to dehydration, volume depletion, pollutants, and rhabdomyolysis with inflammatory stress—are the underlying cause.5
Sugarcane harvesters typically work nine-hour days, six days per week, in extremely high temperatures and while wearing heavy, hot clothing. Each worker cuts approximately 10 tons of sugarcane daily, since they are paid based on cutting volume. Workers drink between five and 10 L of water during their shifts.
Santos et al designed a study to prospectively examine the effects of burnt sugarcane harvesting on renal function in healthy male farm workers. Twenty-eight men (ages 19 to 39) with no CKD risk factors (diabetes, smoking, obesity, hypertension, illicit drug or alcohol use) were followed for eight months from preharvest to postharvest. Blood samples were collected at the beginning and at the end of the workday and preharvest and postharvest season.5
Preseason lab values were normal in all 28 men. But postseason, all workers had elevated creatinine levels, with five meeting the criteria for AKI (see Table at left).5,6
Santos and colleagues identified potential causes for AKI in this population. These included
• Dehydration and volume depletion (episodes of tachycardia, increased urine density, lower urinary/serum sodium, higher hematocrit)
• Rhabdomyolysis (increased creatine kinase at the end of each workday)
• Systemic inflammation (increased white blood count, neutrophils, lymphocytes, and monocytes during the workday—possibly indicative of an inflammatory burst)
• Other factors (burning of the sugarcane releasing unknown nephrotoxic substances; unreported NSAIDs use)5
Compared to workers who showed early signs of CKD, those who developed frank AKI were more likely to have hyponatremia. Recommendations to reduce the problem include consumption of water/salt hydrating drinks, use of appropriate clothing, work-hour limitations, and changes to payment structures (ie, from a volume system to an hourly or daily system). Furthermore, education on the need to avoid alcohol, illicit drugs, and NSAIDs during the harvest season should help to decrease incidence of AKI among these workers.
Elizabeth C. Evans, RN, MSN, CNP, DNP
Renal Medicine Associates, Albuquerque, New Mexico
REFERENCES
1. Ayuk J, Gittoes N. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem. 2014;51(Pt 2):179-188.
2. Firouzi A, Maadani M, Kiani R, et al. Intravenous magnesium sulfate: new method in prevention of contrast-induced nephropathy in primary percutaneous coronary intervention. Int Urol Nephrol. 2015;47(3):521-525.
3. Beaubien J. Mysterious kidney disease slays farm workers in central America. National Public Radio; 2014. www.npr.org/blogs/health/2014/04/30/306907097/mysterious-kidney-disease-slays-farmworkers-in-central-america. Accessed April 1, 2015.
4. Almaguer M, Herrera R, Orantes CM. Chronic kidney disease of unknown etiology in agricultural communities. MEDICC Rev. 2014;16(2):9-15.
5. Santos UP, Zanetta DMT, Burdmann EA. Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int. 2015;87(4):792-799.
6. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney Int Suppl. 2012;2:1-138.
Acute Kidney Injury: Magnesium for Protection
Q) Our radiology department is discussing use of IV magnesium for diabetic patients to “protect them from kidney injury.” Is this a standard of care now?
Magnesium, the fourth most abundant cation in the body, plays an important physiologic role. Balance is maintained by renal regulation of magnesium reabsorption, and deficiency occurs when there is increased renal excretion initiated by osmotic diuresis. Clinical manifestations of deficiency include cardiac arrhythmias, neuromuscular hyperexcitability, and biochemical abnormalities of hypocalcaemia and hypokalemia.
Diabetes is one of the leading causes of magnesium deficiency, with incidence ranging from 25% to 39%.1 Fluctuations in serum magnesium concentrations are directly correlated with fasting blood glucose, A1C levels, albumin excretion, and the duration of diabetes. It has been postulated that magnesium depletion, via its effect on inositol transport, is pathogenic in the progression of diabetic complications.
Contrast-induced acute kidney injury (CI-AKI) is a potentially adverse consequence of percutaneous coronary interventions (PCI), particularly in diabetic patients. It results in significant morbidity and mortality and adds to the costs of diagnostic and interventional cardiology procedures. Intravenous (IV) agents used during radiologic imaging are notorious for causing acute kidney injury in diabetic patients. Preprocedural hydration and discontinuation of all nephrotoxic medications have proven beneficial in protecting these patients from CI-AKI.
A recent prospective, randomized, open-label clinical trial looked at the effect of administering IV magnesium prior to PCI.2 The control group underwent standard preprocedural hydration and discontinuation of nephrotoxic medications. The study group added IV magnesium to the standard protocol.
In this single-center study, 26.6% of patients in the control group and 14.5% in the study group sustained CI-AKI, a statistically significant result (P = .01). Neither group experienced mortality or required dialysis.
Although not considered standard of care at this time, prophylactic use of IV magnesium (pending pre-op labs), along with the recognized benefit of preprocedural hydration and discontinuation of nephrotoxic medications, can be supported in primary PCI patients. Your radiology department is on the cutting edge of protecting these very high-risk patients.
Debra L. Coplon, DNP, DCC
City of Memphis Wellness Clinic, Tennessee
REFERENCES
1. Ayuk J, Gittoes N. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem. 2014;51(Pt 2):179-188.
2. Firouzi A, Maadani M, Kiani R, et al. Intravenous magnesium sulfate: new method in prevention of contrast-induced nephropathy in primary percutaneous coronary intervention. Int Urol Nephrol. 2015;47(3):521-525.
Q) Our radiology department is discussing use of IV magnesium for diabetic patients to “protect them from kidney injury.” Is this a standard of care now?
Magnesium, the fourth most abundant cation in the body, plays an important physiologic role. Balance is maintained by renal regulation of magnesium reabsorption, and deficiency occurs when there is increased renal excretion initiated by osmotic diuresis. Clinical manifestations of deficiency include cardiac arrhythmias, neuromuscular hyperexcitability, and biochemical abnormalities of hypocalcaemia and hypokalemia.
Diabetes is one of the leading causes of magnesium deficiency, with incidence ranging from 25% to 39%.1 Fluctuations in serum magnesium concentrations are directly correlated with fasting blood glucose, A1C levels, albumin excretion, and the duration of diabetes. It has been postulated that magnesium depletion, via its effect on inositol transport, is pathogenic in the progression of diabetic complications.
Contrast-induced acute kidney injury (CI-AKI) is a potentially adverse consequence of percutaneous coronary interventions (PCI), particularly in diabetic patients. It results in significant morbidity and mortality and adds to the costs of diagnostic and interventional cardiology procedures. Intravenous (IV) agents used during radiologic imaging are notorious for causing acute kidney injury in diabetic patients. Preprocedural hydration and discontinuation of all nephrotoxic medications have proven beneficial in protecting these patients from CI-AKI.
A recent prospective, randomized, open-label clinical trial looked at the effect of administering IV magnesium prior to PCI.2 The control group underwent standard preprocedural hydration and discontinuation of nephrotoxic medications. The study group added IV magnesium to the standard protocol.
In this single-center study, 26.6% of patients in the control group and 14.5% in the study group sustained CI-AKI, a statistically significant result (P = .01). Neither group experienced mortality or required dialysis.
Although not considered standard of care at this time, prophylactic use of IV magnesium (pending pre-op labs), along with the recognized benefit of preprocedural hydration and discontinuation of nephrotoxic medications, can be supported in primary PCI patients. Your radiology department is on the cutting edge of protecting these very high-risk patients.
Debra L. Coplon, DNP, DCC
City of Memphis Wellness Clinic, Tennessee
REFERENCES
1. Ayuk J, Gittoes N. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem. 2014;51(Pt 2):179-188.
2. Firouzi A, Maadani M, Kiani R, et al. Intravenous magnesium sulfate: new method in prevention of contrast-induced nephropathy in primary percutaneous coronary intervention. Int Urol Nephrol. 2015;47(3):521-525.
Q) Our radiology department is discussing use of IV magnesium for diabetic patients to “protect them from kidney injury.” Is this a standard of care now?
Magnesium, the fourth most abundant cation in the body, plays an important physiologic role. Balance is maintained by renal regulation of magnesium reabsorption, and deficiency occurs when there is increased renal excretion initiated by osmotic diuresis. Clinical manifestations of deficiency include cardiac arrhythmias, neuromuscular hyperexcitability, and biochemical abnormalities of hypocalcaemia and hypokalemia.
Diabetes is one of the leading causes of magnesium deficiency, with incidence ranging from 25% to 39%.1 Fluctuations in serum magnesium concentrations are directly correlated with fasting blood glucose, A1C levels, albumin excretion, and the duration of diabetes. It has been postulated that magnesium depletion, via its effect on inositol transport, is pathogenic in the progression of diabetic complications.
Contrast-induced acute kidney injury (CI-AKI) is a potentially adverse consequence of percutaneous coronary interventions (PCI), particularly in diabetic patients. It results in significant morbidity and mortality and adds to the costs of diagnostic and interventional cardiology procedures. Intravenous (IV) agents used during radiologic imaging are notorious for causing acute kidney injury in diabetic patients. Preprocedural hydration and discontinuation of all nephrotoxic medications have proven beneficial in protecting these patients from CI-AKI.
A recent prospective, randomized, open-label clinical trial looked at the effect of administering IV magnesium prior to PCI.2 The control group underwent standard preprocedural hydration and discontinuation of nephrotoxic medications. The study group added IV magnesium to the standard protocol.
In this single-center study, 26.6% of patients in the control group and 14.5% in the study group sustained CI-AKI, a statistically significant result (P = .01). Neither group experienced mortality or required dialysis.
Although not considered standard of care at this time, prophylactic use of IV magnesium (pending pre-op labs), along with the recognized benefit of preprocedural hydration and discontinuation of nephrotoxic medications, can be supported in primary PCI patients. Your radiology department is on the cutting edge of protecting these very high-risk patients.
Debra L. Coplon, DNP, DCC
City of Memphis Wellness Clinic, Tennessee
REFERENCES
1. Ayuk J, Gittoes N. Contemporary view of the clinical relevance of magnesium homeostasis. Ann Clin Biochem. 2014;51(Pt 2):179-188.
2. Firouzi A, Maadani M, Kiani R, et al. Intravenous magnesium sulfate: new method in prevention of contrast-induced nephropathy in primary percutaneous coronary intervention. Int Urol Nephrol. 2015;47(3):521-525.