User login
Kidney Disease & “Bad Teeth”
Q)Someone at a conference I attended said kidney disease and bad teeth go hand in hand. Is this true? What does that mean for my patients?
“Bad teeth” can refer to periodontitis, a chronic inflammation of the tissue and structures around the teeth. The sixth most common disease in the world, periodontitis often leads to shrinkage of the gums, infection, and subsequent loosening or loss of teeth.3
Patients with chronic kidney disease (CKD) are predisposed to oral lesions and tooth decay related to dryness of the mouth; alterations in taste; malnutrition; and low albumin. Certain medications—such as ß-blockers, diuretics, anticholinergics, anticonvulsants, and serotonin reuptake inhibitors—can increase the risk for dry mouth and negatively affect oral structures.4
Compared with community-dwelling adults, those with CKD have higher rates of periodontitis, which increase with disease progression.5 A systematic review found that periodontitis increases the risk for CKD; evidence was inconclusive for the impact of periodontal treatment on estimated glomerular filtration rates (eGFR) but suggested positive improvements in eGFR.6
There is growing evidence of a multifaceted relationship between CKD, diabetes, periodontitis, and cardiovascular disease (CVD), the leading cause of mortality in patients with CKD.7 Studies have shown that periodontitis can contribute to systemic inflammation, inhibiting glycemic control and elevating the risk for conditions such as CVD.8-10
Diabetes, the most common cause of CKD, is associated with adverse dental outcomes and poor glycemic control. Vice versa, severe periodontitis increases risk for diabetes and worsening glucose control. Mechanical periodontal treatment has been shown to improve glycemic control.8
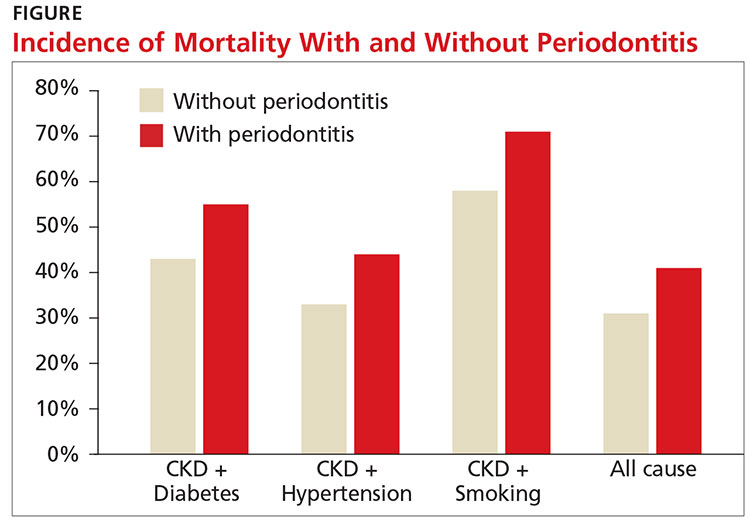
A recent study showed an increased risk for both CVD events and all-cause mortality in those with stage III to stage V CKD (eGFR < 60 mL/min/1.73 m2). The study also found that periodontitis increased 10-year all-cause mortality in this population (see Figure).11
Research is ongoing regarding the complex relationship between CKD and oral health. For patients with CKD at any stage, evidence promotes the benefits of good oral health habits. Encourage smoking cessation, daily flossing and tooth brushing, regular dental cleanings, and prompt evaluation and treatment of any oral issues.12 —CS
Cynthia Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants PLLC, South Charleston, West Virginia
3. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7):1387-1399.
4. Akar H, Akar GC, Carrero JJ, et al. Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol. 2011;6(1):218-226.
5. Borawski J, Wilczyn´ska-Borawska M, Stokowska W, Mys´liwiec M. The periodontal status of pre-dialysis chronic kidney disease and maintenance dialysis patients. Nephrol Dial Transplant. 2007;22(2):457-464.
6. Chambrone L, Foz AM, Guglielmetti MR, et al. Periodontitis and chronic kidney disease: a systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J Clin Periodontol. 2013;40(5):443-456.
7. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305.
8. Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045-1053.
9. Chapple IL, Genco R; Working Group 2 of Joint EFP/AAP Workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013; 40(14):S106-S112.
10. Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68(2):766-772.
11. Sharma P, Dietrich T, Ferro CJ, et al. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol. 2016;43(2):104-113.
12. Ariyamuthu VK, Nolph KD, Ringdahl BE. Periodontal disease in chronic kidney disease and end-stage renal disease patients: a review. Cardiorenal Med. 2013;3(1):71-78.
Q)Someone at a conference I attended said kidney disease and bad teeth go hand in hand. Is this true? What does that mean for my patients?
“Bad teeth” can refer to periodontitis, a chronic inflammation of the tissue and structures around the teeth. The sixth most common disease in the world, periodontitis often leads to shrinkage of the gums, infection, and subsequent loosening or loss of teeth.3
Patients with chronic kidney disease (CKD) are predisposed to oral lesions and tooth decay related to dryness of the mouth; alterations in taste; malnutrition; and low albumin. Certain medications—such as ß-blockers, diuretics, anticholinergics, anticonvulsants, and serotonin reuptake inhibitors—can increase the risk for dry mouth and negatively affect oral structures.4
Compared with community-dwelling adults, those with CKD have higher rates of periodontitis, which increase with disease progression.5 A systematic review found that periodontitis increases the risk for CKD; evidence was inconclusive for the impact of periodontal treatment on estimated glomerular filtration rates (eGFR) but suggested positive improvements in eGFR.6
There is growing evidence of a multifaceted relationship between CKD, diabetes, periodontitis, and cardiovascular disease (CVD), the leading cause of mortality in patients with CKD.7 Studies have shown that periodontitis can contribute to systemic inflammation, inhibiting glycemic control and elevating the risk for conditions such as CVD.8-10
Diabetes, the most common cause of CKD, is associated with adverse dental outcomes and poor glycemic control. Vice versa, severe periodontitis increases risk for diabetes and worsening glucose control. Mechanical periodontal treatment has been shown to improve glycemic control.8
A recent study showed an increased risk for both CVD events and all-cause mortality in those with stage III to stage V CKD (eGFR < 60 mL/min/1.73 m2). The study also found that periodontitis increased 10-year all-cause mortality in this population (see Figure).11

Research is ongoing regarding the complex relationship between CKD and oral health. For patients with CKD at any stage, evidence promotes the benefits of good oral health habits. Encourage smoking cessation, daily flossing and tooth brushing, regular dental cleanings, and prompt evaluation and treatment of any oral issues.12 —CS
Cynthia Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants PLLC, South Charleston, West Virginia
Q)Someone at a conference I attended said kidney disease and bad teeth go hand in hand. Is this true? What does that mean for my patients?
“Bad teeth” can refer to periodontitis, a chronic inflammation of the tissue and structures around the teeth. The sixth most common disease in the world, periodontitis often leads to shrinkage of the gums, infection, and subsequent loosening or loss of teeth.3
Patients with chronic kidney disease (CKD) are predisposed to oral lesions and tooth decay related to dryness of the mouth; alterations in taste; malnutrition; and low albumin. Certain medications—such as ß-blockers, diuretics, anticholinergics, anticonvulsants, and serotonin reuptake inhibitors—can increase the risk for dry mouth and negatively affect oral structures.4
Compared with community-dwelling adults, those with CKD have higher rates of periodontitis, which increase with disease progression.5 A systematic review found that periodontitis increases the risk for CKD; evidence was inconclusive for the impact of periodontal treatment on estimated glomerular filtration rates (eGFR) but suggested positive improvements in eGFR.6
There is growing evidence of a multifaceted relationship between CKD, diabetes, periodontitis, and cardiovascular disease (CVD), the leading cause of mortality in patients with CKD.7 Studies have shown that periodontitis can contribute to systemic inflammation, inhibiting glycemic control and elevating the risk for conditions such as CVD.8-10
Diabetes, the most common cause of CKD, is associated with adverse dental outcomes and poor glycemic control. Vice versa, severe periodontitis increases risk for diabetes and worsening glucose control. Mechanical periodontal treatment has been shown to improve glycemic control.8
A recent study showed an increased risk for both CVD events and all-cause mortality in those with stage III to stage V CKD (eGFR < 60 mL/min/1.73 m2). The study also found that periodontitis increased 10-year all-cause mortality in this population (see Figure).11

Research is ongoing regarding the complex relationship between CKD and oral health. For patients with CKD at any stage, evidence promotes the benefits of good oral health habits. Encourage smoking cessation, daily flossing and tooth brushing, regular dental cleanings, and prompt evaluation and treatment of any oral issues.12 —CS
Cynthia Smith, DNP, CNN-NP, FNP-BC, APRN
Renal Consultants PLLC, South Charleston, West Virginia
3. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7):1387-1399.
4. Akar H, Akar GC, Carrero JJ, et al. Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol. 2011;6(1):218-226.
5. Borawski J, Wilczyn´ska-Borawska M, Stokowska W, Mys´liwiec M. The periodontal status of pre-dialysis chronic kidney disease and maintenance dialysis patients. Nephrol Dial Transplant. 2007;22(2):457-464.
6. Chambrone L, Foz AM, Guglielmetti MR, et al. Periodontitis and chronic kidney disease: a systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J Clin Periodontol. 2013;40(5):443-456.
7. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305.
8. Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045-1053.
9. Chapple IL, Genco R; Working Group 2 of Joint EFP/AAP Workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013; 40(14):S106-S112.
10. Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68(2):766-772.
11. Sharma P, Dietrich T, Ferro CJ, et al. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol. 2016;43(2):104-113.
12. Ariyamuthu VK, Nolph KD, Ringdahl BE. Periodontal disease in chronic kidney disease and end-stage renal disease patients: a review. Cardiorenal Med. 2013;3(1):71-78.
3. Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78(7):1387-1399.
4. Akar H, Akar GC, Carrero JJ, et al. Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol. 2011;6(1):218-226.
5. Borawski J, Wilczyn´ska-Borawska M, Stokowska W, Mys´liwiec M. The periodontal status of pre-dialysis chronic kidney disease and maintenance dialysis patients. Nephrol Dial Transplant. 2007;22(2):457-464.
6. Chambrone L, Foz AM, Guglielmetti MR, et al. Periodontitis and chronic kidney disease: a systematic review of the association of diseases and the effect of periodontal treatment on estimated glomerular filtration rate. J Clin Periodontol. 2013;40(5):443-456.
7. Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296-1305.
8. Kassebaum NJ, Bernabé E, Dahiya M, et al. Global burden of severe periodontitis in 1990-2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045-1053.
9. Chapple IL, Genco R; Working Group 2 of Joint EFP/AAP Workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Clin Periodontol. 2013; 40(14):S106-S112.
10. Menon V, Greene T, Wang X, et al. C-reactive protein and albumin as predictors of all-cause and cardiovascular mortality in chronic kidney disease. Kidney Int. 2005;68(2):766-772.
11. Sharma P, Dietrich T, Ferro CJ, et al. Association between periodontitis and mortality in stages 3-5 chronic kidney disease: NHANES III and linked mortality study. J Clin Periodontol. 2016;43(2):104-113.
12. Ariyamuthu VK, Nolph KD, Ringdahl BE. Periodontal disease in chronic kidney disease and end-stage renal disease patients: a review. Cardiorenal Med. 2013;3(1):71-78.
Do Kidney Patients Know an App From a Nap?
Q)It seems that at every conference I attend, a tech/marketing rep stands up to rave about “this” app or “that” online program. My average patient is older than 60 (physiologically 80), has vision issues related to diabetes, hypertension, or cataracts, can’t afford a smartphone, wouldn’t know an app from a nap, and has trouble just managing to eat correctly. What makes these reps think that patients can use technology?
We are often encouraged to incorporate technology into our daily interactions with patients. Meaningful use has us signing up 70-year-old patients for our practice’s patient portal and counting on them to write a message to us so we can receive credit. Our initial response is to groan and ask if the government knows what kind of patients we see.
However, a new article in the Clinical Journal of the American Society of Nephrology suggests that our patients may be more tech savvy than we think.1 The study found that patients with chronic kidney disease (CKD) not only know how to use a smartphone application but also find its implementation useful.
Patients included in the study were, on average, 59 and had stage IV to stage V CKD and an estimated glomerular filtration rate (eGFR) of ≥ 30 mL/min/1.73 m2. The study assessed knowledge of blood pressure, medications, CKD-related symptoms, and CKD-related laboratory tests.
Although 60% of the study cohort had never used a smartphone before, monthly adherence rates were higher than 80%. Outcomes included a statistically significant reduction in blood pressure, which was attributed to patients’ ability to better monitor their health and reduce their anxiety. The smartphone data sets also helped to identify cases of masked hypertension and more than 100 medication errors, 60% of which required intervention. Subsequent visits with providers were found to be more useful as a result, since both patients and providers had better quality information.
An accompanying editorial cautioned, however, that despite these positive findings, we must be mindful that smartphone ownership is less common among lower income patients. Fifty percent of those making less than $30,000 per year own a smartphone, compared with 84% of patients with an annual income of $75,000 or more.2 CKD patients are of varying socioeconomic status, with lower eGFR often corresponding to lower socioeconomic status.
So while medical apps have a future with CKD (and by implication, all) patients, they are not unlike much else in medicine: We must tailor our practice to meet the needs of our patient population. These findings are encouraging for use of smartphone technology, but it is not a “one size fits all” solution. —SM
Sherry Mathes, NP-C
Georgia Nephrology LLC, Lawrenceville, Georgia
1. Ong SW, Jassal SV, Miller JA, et al. Integrating a smartphone-based self-management system into usual care of advanced CKD. Clin J Am Soc Nephrol. 2016;11(6):1054-1062.
2. Desai T, Yee J, Soman S. Smartphone apps: a patient’s new best friend? Clin J Am Soc Nephrol. 2016;11(6):935-937.
Q)It seems that at every conference I attend, a tech/marketing rep stands up to rave about “this” app or “that” online program. My average patient is older than 60 (physiologically 80), has vision issues related to diabetes, hypertension, or cataracts, can’t afford a smartphone, wouldn’t know an app from a nap, and has trouble just managing to eat correctly. What makes these reps think that patients can use technology?
We are often encouraged to incorporate technology into our daily interactions with patients. Meaningful use has us signing up 70-year-old patients for our practice’s patient portal and counting on them to write a message to us so we can receive credit. Our initial response is to groan and ask if the government knows what kind of patients we see.
However, a new article in the Clinical Journal of the American Society of Nephrology suggests that our patients may be more tech savvy than we think.1 The study found that patients with chronic kidney disease (CKD) not only know how to use a smartphone application but also find its implementation useful.
Patients included in the study were, on average, 59 and had stage IV to stage V CKD and an estimated glomerular filtration rate (eGFR) of ≥ 30 mL/min/1.73 m2. The study assessed knowledge of blood pressure, medications, CKD-related symptoms, and CKD-related laboratory tests.
Although 60% of the study cohort had never used a smartphone before, monthly adherence rates were higher than 80%. Outcomes included a statistically significant reduction in blood pressure, which was attributed to patients’ ability to better monitor their health and reduce their anxiety. The smartphone data sets also helped to identify cases of masked hypertension and more than 100 medication errors, 60% of which required intervention. Subsequent visits with providers were found to be more useful as a result, since both patients and providers had better quality information.
An accompanying editorial cautioned, however, that despite these positive findings, we must be mindful that smartphone ownership is less common among lower income patients. Fifty percent of those making less than $30,000 per year own a smartphone, compared with 84% of patients with an annual income of $75,000 or more.2 CKD patients are of varying socioeconomic status, with lower eGFR often corresponding to lower socioeconomic status.
So while medical apps have a future with CKD (and by implication, all) patients, they are not unlike much else in medicine: We must tailor our practice to meet the needs of our patient population. These findings are encouraging for use of smartphone technology, but it is not a “one size fits all” solution. —SM
Sherry Mathes, NP-C
Georgia Nephrology LLC, Lawrenceville, Georgia
Q)It seems that at every conference I attend, a tech/marketing rep stands up to rave about “this” app or “that” online program. My average patient is older than 60 (physiologically 80), has vision issues related to diabetes, hypertension, or cataracts, can’t afford a smartphone, wouldn’t know an app from a nap, and has trouble just managing to eat correctly. What makes these reps think that patients can use technology?
We are often encouraged to incorporate technology into our daily interactions with patients. Meaningful use has us signing up 70-year-old patients for our practice’s patient portal and counting on them to write a message to us so we can receive credit. Our initial response is to groan and ask if the government knows what kind of patients we see.
However, a new article in the Clinical Journal of the American Society of Nephrology suggests that our patients may be more tech savvy than we think.1 The study found that patients with chronic kidney disease (CKD) not only know how to use a smartphone application but also find its implementation useful.
Patients included in the study were, on average, 59 and had stage IV to stage V CKD and an estimated glomerular filtration rate (eGFR) of ≥ 30 mL/min/1.73 m2. The study assessed knowledge of blood pressure, medications, CKD-related symptoms, and CKD-related laboratory tests.
Although 60% of the study cohort had never used a smartphone before, monthly adherence rates were higher than 80%. Outcomes included a statistically significant reduction in blood pressure, which was attributed to patients’ ability to better monitor their health and reduce their anxiety. The smartphone data sets also helped to identify cases of masked hypertension and more than 100 medication errors, 60% of which required intervention. Subsequent visits with providers were found to be more useful as a result, since both patients and providers had better quality information.
An accompanying editorial cautioned, however, that despite these positive findings, we must be mindful that smartphone ownership is less common among lower income patients. Fifty percent of those making less than $30,000 per year own a smartphone, compared with 84% of patients with an annual income of $75,000 or more.2 CKD patients are of varying socioeconomic status, with lower eGFR often corresponding to lower socioeconomic status.
So while medical apps have a future with CKD (and by implication, all) patients, they are not unlike much else in medicine: We must tailor our practice to meet the needs of our patient population. These findings are encouraging for use of smartphone technology, but it is not a “one size fits all” solution. —SM
Sherry Mathes, NP-C
Georgia Nephrology LLC, Lawrenceville, Georgia
1. Ong SW, Jassal SV, Miller JA, et al. Integrating a smartphone-based self-management system into usual care of advanced CKD. Clin J Am Soc Nephrol. 2016;11(6):1054-1062.
2. Desai T, Yee J, Soman S. Smartphone apps: a patient’s new best friend? Clin J Am Soc Nephrol. 2016;11(6):935-937.
1. Ong SW, Jassal SV, Miller JA, et al. Integrating a smartphone-based self-management system into usual care of advanced CKD. Clin J Am Soc Nephrol. 2016;11(6):1054-1062.
2. Desai T, Yee J, Soman S. Smartphone apps: a patient’s new best friend? Clin J Am Soc Nephrol. 2016;11(6):935-937.
Data-based Recommendations for Dialysis
Q) I work in a cardiology practice. Recently, a patient on dialysis mentioned that her nephrology practitioner recommended either home therapy or nocturnal dialysis. Why would someone recommend these, and what are the differences between home, nocturnal, and regular daytime dialysis?
Patients usually require dialysis when 90% or more of their renal function is lost.5 This can happen acutely or result from a chronic process. Dialysis performs many of the functions of a kidney, such as removing waste and fluid buildup that damaged kidneys cannot. It also helps maintain electrolyte balance.
There are several forms of hemodialysis including home, incenter, and nocturnal; the most frequently used is in-center hemodialysis.5 Patients on in-center hemodialysis visit the center three times a week, and their treatments last from three to five hours; the nationwide average is four hours. These patients have very restricted schedules and must maintain their appointments with limited flexibility. Food, drinks, and nonmedical personnel may not be allowed in the treatment area. Between treatments, patients must follow a diet that restricts fluid, sodium, and potassium intake.
Home dialysis has become a popular alternative, since it may be done in a location and at a time that is convenient for the patient. With more flexibility, many patients are able to continue working and feel like they have a more “normal” life. Types of home dialysis include home hemodialysis (HHD) or peritoneal dialysis (PD). A relative or friend may need to assist the patient during HHD, which is undergone more frequently (between five and seven days per week) and for a shorter duration of time than in-center dialysis. PD is done every day, either at night or multiple times throughout the day. Although no partner is needed for PD, a medical provider is available by phone to address any concerns that may arise during treatment.
Nocturnal hemodialysis is similar to daytime in-center hemodialysis, but it occurs while the patient is asleep. The treatment duration is longer (an average of eight hours per treatment). The slower blood flow allows for gentler dialysis. Patients who undergo nocturnal hemodialysis have higher survival and lower hospitalization rates, with better phosphorus control and blood pressure.6 This is attributed to the slower removal of excess fluid and more effective clearance of toxins.
So, why is your patient being encouraged to consider home or nocturnal dialysis? Studies have shown that for the cardiac patient, slower, gentler dialysis is preferable.7 The clinician who recommended it has the patient’s best interest in mind. —TAH
Tricia A. Howard, MHS, PA-C, DFAAPA
PA Program, South University, Savannah, Georgia
5. Gilbert S, Weiner DE. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier; 2014.
6. Lacson E, Wang W, Lester K, et al. Outcomes associated with in-center nocturnal hemodialysis from a large multicenter program. Clin J Am Soc Nephrol. 2010;5(2):220-226.
7. Lin J, Berns JS. Is hemodialysis bad for the heart? Semin Dial. 2012;25(1):86-87.
Q) I work in a cardiology practice. Recently, a patient on dialysis mentioned that her nephrology practitioner recommended either home therapy or nocturnal dialysis. Why would someone recommend these, and what are the differences between home, nocturnal, and regular daytime dialysis?
Patients usually require dialysis when 90% or more of their renal function is lost.5 This can happen acutely or result from a chronic process. Dialysis performs many of the functions of a kidney, such as removing waste and fluid buildup that damaged kidneys cannot. It also helps maintain electrolyte balance.
There are several forms of hemodialysis including home, incenter, and nocturnal; the most frequently used is in-center hemodialysis.5 Patients on in-center hemodialysis visit the center three times a week, and their treatments last from three to five hours; the nationwide average is four hours. These patients have very restricted schedules and must maintain their appointments with limited flexibility. Food, drinks, and nonmedical personnel may not be allowed in the treatment area. Between treatments, patients must follow a diet that restricts fluid, sodium, and potassium intake.
Home dialysis has become a popular alternative, since it may be done in a location and at a time that is convenient for the patient. With more flexibility, many patients are able to continue working and feel like they have a more “normal” life. Types of home dialysis include home hemodialysis (HHD) or peritoneal dialysis (PD). A relative or friend may need to assist the patient during HHD, which is undergone more frequently (between five and seven days per week) and for a shorter duration of time than in-center dialysis. PD is done every day, either at night or multiple times throughout the day. Although no partner is needed for PD, a medical provider is available by phone to address any concerns that may arise during treatment.
Nocturnal hemodialysis is similar to daytime in-center hemodialysis, but it occurs while the patient is asleep. The treatment duration is longer (an average of eight hours per treatment). The slower blood flow allows for gentler dialysis. Patients who undergo nocturnal hemodialysis have higher survival and lower hospitalization rates, with better phosphorus control and blood pressure.6 This is attributed to the slower removal of excess fluid and more effective clearance of toxins.
So, why is your patient being encouraged to consider home or nocturnal dialysis? Studies have shown that for the cardiac patient, slower, gentler dialysis is preferable.7 The clinician who recommended it has the patient’s best interest in mind. —TAH
Tricia A. Howard, MHS, PA-C, DFAAPA
PA Program, South University, Savannah, Georgia
Q) I work in a cardiology practice. Recently, a patient on dialysis mentioned that her nephrology practitioner recommended either home therapy or nocturnal dialysis. Why would someone recommend these, and what are the differences between home, nocturnal, and regular daytime dialysis?
Patients usually require dialysis when 90% or more of their renal function is lost.5 This can happen acutely or result from a chronic process. Dialysis performs many of the functions of a kidney, such as removing waste and fluid buildup that damaged kidneys cannot. It also helps maintain electrolyte balance.
There are several forms of hemodialysis including home, incenter, and nocturnal; the most frequently used is in-center hemodialysis.5 Patients on in-center hemodialysis visit the center three times a week, and their treatments last from three to five hours; the nationwide average is four hours. These patients have very restricted schedules and must maintain their appointments with limited flexibility. Food, drinks, and nonmedical personnel may not be allowed in the treatment area. Between treatments, patients must follow a diet that restricts fluid, sodium, and potassium intake.
Home dialysis has become a popular alternative, since it may be done in a location and at a time that is convenient for the patient. With more flexibility, many patients are able to continue working and feel like they have a more “normal” life. Types of home dialysis include home hemodialysis (HHD) or peritoneal dialysis (PD). A relative or friend may need to assist the patient during HHD, which is undergone more frequently (between five and seven days per week) and for a shorter duration of time than in-center dialysis. PD is done every day, either at night or multiple times throughout the day. Although no partner is needed for PD, a medical provider is available by phone to address any concerns that may arise during treatment.
Nocturnal hemodialysis is similar to daytime in-center hemodialysis, but it occurs while the patient is asleep. The treatment duration is longer (an average of eight hours per treatment). The slower blood flow allows for gentler dialysis. Patients who undergo nocturnal hemodialysis have higher survival and lower hospitalization rates, with better phosphorus control and blood pressure.6 This is attributed to the slower removal of excess fluid and more effective clearance of toxins.
So, why is your patient being encouraged to consider home or nocturnal dialysis? Studies have shown that for the cardiac patient, slower, gentler dialysis is preferable.7 The clinician who recommended it has the patient’s best interest in mind. —TAH
Tricia A. Howard, MHS, PA-C, DFAAPA
PA Program, South University, Savannah, Georgia
5. Gilbert S, Weiner DE. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier; 2014.
6. Lacson E, Wang W, Lester K, et al. Outcomes associated with in-center nocturnal hemodialysis from a large multicenter program. Clin J Am Soc Nephrol. 2010;5(2):220-226.
7. Lin J, Berns JS. Is hemodialysis bad for the heart? Semin Dial. 2012;25(1):86-87.
5. Gilbert S, Weiner DE. National Kidney Foundation Primer on Kidney Diseases. 6th ed. Philadelphia, PA: Elsevier; 2014.
6. Lacson E, Wang W, Lester K, et al. Outcomes associated with in-center nocturnal hemodialysis from a large multicenter program. Clin J Am Soc Nephrol. 2010;5(2):220-226.
7. Lin J, Berns JS. Is hemodialysis bad for the heart? Semin Dial. 2012;25(1):86-87.
Data-based Recommendations for CKD Screening
Q)
I’ve received mixed messages about whom to screen for chronic kidney disease (CKD). The US Preventive Services Task Force (USPSTF) recommends screening only patients at high risk, but kidney experts advise screening everyone. Who is right? What does the data show?
In 2012, the USPSTF stated that there was insufficient evidence to assess the benefit, or harm, of regularly screening asymptomatic adults for CKD.1 Other expert medical panels have come to this conclusion as well, and therefore only recommend screening highrisk patients.2
The National Kidney Foundation (NKF) encourages clinicians to assess all patients for risk factors of CKD. Diabetes and hypertension are strongly established risk factors for kidney disease; others include family history of kidney disease; cardiovascular disease; obesity; and older age.
If a patient is at risk for CKD, the NKF recommends testing serum creatinine levels to estimate glomerular filtration rate and testing urine for protein (microalbuminuria or macroalbuminuria). These tests are readily accessible in a primary care setting. It should be noted that one-time testing of serum creatinine and/or urine has not been studied for sensitivity or specificity in the diagnosis of CKD. Diagnosis should be based on decreased renal function or kidney damage occurring over a three-month span.3
In May 2016, Canadian researchers published results from the See Kidney Disease Targeted Screening Program for CKD, comparing CKD screening in the general population with a targeted, at-risk individual population.4 The study, which included more than 6,000 participants, revealed a higher rate of unrecognized CKD in the at-risk population than in the general population (21.9% and 14.7%, respectively).
These findings support the idea that screening at-risk patients identifies more cases of CKD than screening the general patient population does.4 Early diagnosis of CKD, through recognition of risk factors, provides an opportunity to decrease complications and manage conditions that contribute to the progression of renal disease.2,3 —RVR
Rebecca V. Rokosky, MSN, APRN, FNP
Renal Associates Clinical Advancement Center in San Antonio, Texas
1. Moyer VA. Screening for chronic kidney disease: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(8):567-570.
2. Vassalotti JA, Centor R, Turner BJ, et al. Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. 2016;129(2):153-162.
3. Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313(8):837-846.
Q)
I’ve received mixed messages about whom to screen for chronic kidney disease (CKD). The US Preventive Services Task Force (USPSTF) recommends screening only patients at high risk, but kidney experts advise screening everyone. Who is right? What does the data show?
In 2012, the USPSTF stated that there was insufficient evidence to assess the benefit, or harm, of regularly screening asymptomatic adults for CKD.1 Other expert medical panels have come to this conclusion as well, and therefore only recommend screening highrisk patients.2
The National Kidney Foundation (NKF) encourages clinicians to assess all patients for risk factors of CKD. Diabetes and hypertension are strongly established risk factors for kidney disease; others include family history of kidney disease; cardiovascular disease; obesity; and older age.
If a patient is at risk for CKD, the NKF recommends testing serum creatinine levels to estimate glomerular filtration rate and testing urine for protein (microalbuminuria or macroalbuminuria). These tests are readily accessible in a primary care setting. It should be noted that one-time testing of serum creatinine and/or urine has not been studied for sensitivity or specificity in the diagnosis of CKD. Diagnosis should be based on decreased renal function or kidney damage occurring over a three-month span.3
In May 2016, Canadian researchers published results from the See Kidney Disease Targeted Screening Program for CKD, comparing CKD screening in the general population with a targeted, at-risk individual population.4 The study, which included more than 6,000 participants, revealed a higher rate of unrecognized CKD in the at-risk population than in the general population (21.9% and 14.7%, respectively).
These findings support the idea that screening at-risk patients identifies more cases of CKD than screening the general patient population does.4 Early diagnosis of CKD, through recognition of risk factors, provides an opportunity to decrease complications and manage conditions that contribute to the progression of renal disease.2,3 —RVR
Rebecca V. Rokosky, MSN, APRN, FNP
Renal Associates Clinical Advancement Center in San Antonio, Texas
Q)
I’ve received mixed messages about whom to screen for chronic kidney disease (CKD). The US Preventive Services Task Force (USPSTF) recommends screening only patients at high risk, but kidney experts advise screening everyone. Who is right? What does the data show?
In 2012, the USPSTF stated that there was insufficient evidence to assess the benefit, or harm, of regularly screening asymptomatic adults for CKD.1 Other expert medical panels have come to this conclusion as well, and therefore only recommend screening highrisk patients.2
The National Kidney Foundation (NKF) encourages clinicians to assess all patients for risk factors of CKD. Diabetes and hypertension are strongly established risk factors for kidney disease; others include family history of kidney disease; cardiovascular disease; obesity; and older age.
If a patient is at risk for CKD, the NKF recommends testing serum creatinine levels to estimate glomerular filtration rate and testing urine for protein (microalbuminuria or macroalbuminuria). These tests are readily accessible in a primary care setting. It should be noted that one-time testing of serum creatinine and/or urine has not been studied for sensitivity or specificity in the diagnosis of CKD. Diagnosis should be based on decreased renal function or kidney damage occurring over a three-month span.3
In May 2016, Canadian researchers published results from the See Kidney Disease Targeted Screening Program for CKD, comparing CKD screening in the general population with a targeted, at-risk individual population.4 The study, which included more than 6,000 participants, revealed a higher rate of unrecognized CKD in the at-risk population than in the general population (21.9% and 14.7%, respectively).
These findings support the idea that screening at-risk patients identifies more cases of CKD than screening the general patient population does.4 Early diagnosis of CKD, through recognition of risk factors, provides an opportunity to decrease complications and manage conditions that contribute to the progression of renal disease.2,3 —RVR
Rebecca V. Rokosky, MSN, APRN, FNP
Renal Associates Clinical Advancement Center in San Antonio, Texas
1. Moyer VA. Screening for chronic kidney disease: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(8):567-570.
2. Vassalotti JA, Centor R, Turner BJ, et al. Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. 2016;129(2):153-162.
3. Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313(8):837-846.
1. Moyer VA. Screening for chronic kidney disease: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(8):567-570.
2. Vassalotti JA, Centor R, Turner BJ, et al. Practical approach to detection and management of chronic kidney disease for the primary care clinician. Am J Med. 2016;129(2):153-162.
3. Levey AS, Becker C, Inker LA. Glomerular filtration rate and albuminuria for detection and staging of acute and chronic kidney disease in adults: a systematic review. JAMA. 2015;313(8):837-846.
Expanding Treatment Options
Q) One of my diabetic patients read about finerenone in The New York Times. Apparently, it’s the “newest cure for albuminuria”! Is this just hype, or do the trials on this medication really show progress against kidney disease? Should I buy stock in the company?
Albuminuria (> 500 mg/d) associated with diabetic nephropathy and other glomerular diseases increases patient risk for chronic kidney disease (CKD) and its progression to end-stage renal disease (ESRD). Reduction of albuminuria has been shown to slow the progression of CKD.
Renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors or angiotensin receptor blockers, are considered firstline therapy to reduce albuminuria. Additional treatment modalities include diuretics, nondihydropyridine calcium channel blockers, ß-blockers, and aldosterone antagonist therapy. Limiting dietary sodium helps control blood pressure, thus slowing disease progression. In addition, some studies show that limiting phosphorus and protein (for the latter, intake of no more than 0.7 g/kg ideal body weight per day) may slow the progression of CKD. Unfortunately, despite these interventions, patients may still advance to ESRD.1
The aldosterone and steroidal mineralocorticoid receptor antagonists (MRA) spironolactone and eplerenone have been found to reduce albuminuria when used in conjunction with RAAS blockade. However, patients using this combination are up to eight times more likely to experience hyperkalemia—a serious, potentially life-threatening adverse condition—than those not using an MRA.2 The presence of hyperkalemia requires discontinuation of the RAAS blocker and the MRA, at least temporarily.
Finerenone, a nonsteroidal MRA with “greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro,” is in phase III trials for the treatment of systolic and diastolic dysfunction and reduction of morbidity and mortality associated with heart failure.2 One study has already demonstrated that finerenone (5 to 10 mg/d) is at least as effective as spironolactone (25 mg/d) for heart failure patients.3
The Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN) found that finerenone at 10 to 20 mg/d was superior to spironolactone and eplerenone, partly due to the decreased incidence of hyperkalemia. However, it should be noted that the lower incidence of hyperkalemia may be attributable to the fact that 66% of the study participants had an estimated glomerular filtration rate (eGFR) greater than 60 mL/min and that potential participants with a serum potassium level of more than 4.8 mEq/L were not included in the study.2
Additional research is needed to confirm superiority of finerenone over spironolactone and eplerenone, in conjunction with RAAS blockers, in the treatment of albuminuria and hyperkalemia. Including subjects with lower eGFR (such as patients with stage IV CKD who are at higher risk for hyperkalemia) would give a better indication of finerenone’s efficacy. In the meantime, it’s probably too soon to corner the market on this stock! —SEB
Susan E. Brown, MS, ARNP, ACNP-BC, CCRN
Great River Nephrology, West Burlington, Iowa
References
1. Parikh SV, Haddad NJ, Hebert LA. Retarding progression of kidney disease. In: Johnson RJ, Feehally J, Floege J, eds. Comprehensive Clinical Nephrology. 5th ed. Philadelphia, PA: Saunders; 2015:931-940.
2. Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884-894.
3. Kolkhof P, Delbeck M, Kretschmer A, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist, protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69-78.
Q) One of my diabetic patients read about finerenone in The New York Times. Apparently, it’s the “newest cure for albuminuria”! Is this just hype, or do the trials on this medication really show progress against kidney disease? Should I buy stock in the company?
Albuminuria (> 500 mg/d) associated with diabetic nephropathy and other glomerular diseases increases patient risk for chronic kidney disease (CKD) and its progression to end-stage renal disease (ESRD). Reduction of albuminuria has been shown to slow the progression of CKD.
Renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors or angiotensin receptor blockers, are considered firstline therapy to reduce albuminuria. Additional treatment modalities include diuretics, nondihydropyridine calcium channel blockers, ß-blockers, and aldosterone antagonist therapy. Limiting dietary sodium helps control blood pressure, thus slowing disease progression. In addition, some studies show that limiting phosphorus and protein (for the latter, intake of no more than 0.7 g/kg ideal body weight per day) may slow the progression of CKD. Unfortunately, despite these interventions, patients may still advance to ESRD.1
The aldosterone and steroidal mineralocorticoid receptor antagonists (MRA) spironolactone and eplerenone have been found to reduce albuminuria when used in conjunction with RAAS blockade. However, patients using this combination are up to eight times more likely to experience hyperkalemia—a serious, potentially life-threatening adverse condition—than those not using an MRA.2 The presence of hyperkalemia requires discontinuation of the RAAS blocker and the MRA, at least temporarily.
Finerenone, a nonsteroidal MRA with “greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro,” is in phase III trials for the treatment of systolic and diastolic dysfunction and reduction of morbidity and mortality associated with heart failure.2 One study has already demonstrated that finerenone (5 to 10 mg/d) is at least as effective as spironolactone (25 mg/d) for heart failure patients.3
The Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN) found that finerenone at 10 to 20 mg/d was superior to spironolactone and eplerenone, partly due to the decreased incidence of hyperkalemia. However, it should be noted that the lower incidence of hyperkalemia may be attributable to the fact that 66% of the study participants had an estimated glomerular filtration rate (eGFR) greater than 60 mL/min and that potential participants with a serum potassium level of more than 4.8 mEq/L were not included in the study.2
Additional research is needed to confirm superiority of finerenone over spironolactone and eplerenone, in conjunction with RAAS blockers, in the treatment of albuminuria and hyperkalemia. Including subjects with lower eGFR (such as patients with stage IV CKD who are at higher risk for hyperkalemia) would give a better indication of finerenone’s efficacy. In the meantime, it’s probably too soon to corner the market on this stock! —SEB
Susan E. Brown, MS, ARNP, ACNP-BC, CCRN
Great River Nephrology, West Burlington, Iowa
References
1. Parikh SV, Haddad NJ, Hebert LA. Retarding progression of kidney disease. In: Johnson RJ, Feehally J, Floege J, eds. Comprehensive Clinical Nephrology. 5th ed. Philadelphia, PA: Saunders; 2015:931-940.
2. Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884-894.
3. Kolkhof P, Delbeck M, Kretschmer A, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist, protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69-78.
Q) One of my diabetic patients read about finerenone in The New York Times. Apparently, it’s the “newest cure for albuminuria”! Is this just hype, or do the trials on this medication really show progress against kidney disease? Should I buy stock in the company?
Albuminuria (> 500 mg/d) associated with diabetic nephropathy and other glomerular diseases increases patient risk for chronic kidney disease (CKD) and its progression to end-stage renal disease (ESRD). Reduction of albuminuria has been shown to slow the progression of CKD.
Renin-angiotensin-aldosterone system (RAAS) blockers, such as ACE inhibitors or angiotensin receptor blockers, are considered firstline therapy to reduce albuminuria. Additional treatment modalities include diuretics, nondihydropyridine calcium channel blockers, ß-blockers, and aldosterone antagonist therapy. Limiting dietary sodium helps control blood pressure, thus slowing disease progression. In addition, some studies show that limiting phosphorus and protein (for the latter, intake of no more than 0.7 g/kg ideal body weight per day) may slow the progression of CKD. Unfortunately, despite these interventions, patients may still advance to ESRD.1
The aldosterone and steroidal mineralocorticoid receptor antagonists (MRA) spironolactone and eplerenone have been found to reduce albuminuria when used in conjunction with RAAS blockade. However, patients using this combination are up to eight times more likely to experience hyperkalemia—a serious, potentially life-threatening adverse condition—than those not using an MRA.2 The presence of hyperkalemia requires discontinuation of the RAAS blocker and the MRA, at least temporarily.
Finerenone, a nonsteroidal MRA with “greater receptor selectivity than spironolactone and better receptor affinity than eplerenone in vitro,” is in phase III trials for the treatment of systolic and diastolic dysfunction and reduction of morbidity and mortality associated with heart failure.2 One study has already demonstrated that finerenone (5 to 10 mg/d) is at least as effective as spironolactone (25 mg/d) for heart failure patients.3
The Mineralocorticoid Receptor Antagonist Tolerability Study-Diabetic Nephropathy (ARTS-DN) found that finerenone at 10 to 20 mg/d was superior to spironolactone and eplerenone, partly due to the decreased incidence of hyperkalemia. However, it should be noted that the lower incidence of hyperkalemia may be attributable to the fact that 66% of the study participants had an estimated glomerular filtration rate (eGFR) greater than 60 mL/min and that potential participants with a serum potassium level of more than 4.8 mEq/L were not included in the study.2
Additional research is needed to confirm superiority of finerenone over spironolactone and eplerenone, in conjunction with RAAS blockers, in the treatment of albuminuria and hyperkalemia. Including subjects with lower eGFR (such as patients with stage IV CKD who are at higher risk for hyperkalemia) would give a better indication of finerenone’s efficacy. In the meantime, it’s probably too soon to corner the market on this stock! —SEB
Susan E. Brown, MS, ARNP, ACNP-BC, CCRN
Great River Nephrology, West Burlington, Iowa
References
1. Parikh SV, Haddad NJ, Hebert LA. Retarding progression of kidney disease. In: Johnson RJ, Feehally J, Floege J, eds. Comprehensive Clinical Nephrology. 5th ed. Philadelphia, PA: Saunders; 2015:931-940.
2. Bakris GL, Agarwal R, Chan JC, et al. Effect of finerenone on albuminuria in patients with diabetic nephropathy: a randomized clinical trial. JAMA. 2015;314(9):884-894.
3. Kolkhof P, Delbeck M, Kretschmer A, et al. Finerenone, a novel selective nonsteroidal mineralocorticoid receptor antagonist, protects from rat cardiorenal injury. J Cardiovasc Pharmacol. 2014;64(1):69-78.
Nutrition Guidelines for CKD
Q) I see patients with diabetes, hypertension, chronic kidney disease (CKD), obesity ... often all within the same patient! I keep hearing that the DASH diet is best for these patients. Is this true? Do you have any suggestions (or handouts) for teaching good eating habits in a 15-minute office visit?
It is always nice to focus on what patients can do, rather than what they can’t. Patients with diabetes, kidney disease, heart disease, and obesity hear a lot of “can’t” messages, making “can” messages particularly important to emphasize.
Healthy diets for diabetes, heart, and kidney patients include foods low in trans and saturated fats and sodium. Not all CKD patients are required to follow a low-potassium diet; dietary restrictions are based on laboratory values, medications, and other factors. As we know, adding an ACE inhibitor or an angiotensin receptor blocker (ARB) to the treatment regimen can cause an elevation in serum potassium.
For adults with CKD, it is recommended that sodium intake be restricted to < 2,000 mg/d.4 And in this population, salt substitutes are not recommended, since they often contain large amounts of potassium chloride, which increases risk for hyperkalemia.5 Other spices (eg, garlic, pepper, lemon) are better substitutes for salt.
The late Paul Prudhomme, an award-winning chef from New Orleans, struggled with obesity and health issues for years. He developed wonderful, kidney-friendly spices free of salt and potassium. His line of spices, Magic Seasoning Blends, is sold in many grocery stores. You can recommend them without worry.
Studies have shown that the usual Western diet (which features an abundance of processed foods, fats, and sugars) contributes to kidney disease.6 The DASH (Dietary Approaches to Stop Hypertension) diet, developed by cardio experts, replaces these foods with healthier alternatives.
Recent research has shown that the DASH diet does, in fact, slow the progression of kidney disease.7 It also lowers blood pressure and decreases kidney stone formation, which are risk factors for kidney disease.
So, the DASH diet is protective for your patients (from both a kidney and a cardiac standpoint)—but how do you explain this in a 15-minute office visit?
Here are a few quick tips:
• Increase fruit and vegetable intake to include all colors on your plate (and no, tan is not really a color)
• If you eat meat, the cooking method should start with “B” (ie, bake, boil, broil, barbeque [without salty sauce]) ... Note that “fried” does not start with “B”!
• Use a smaller plate and you will not eat as much
• Use technology in your favor. There are great apps and downloads you can recommend (see Table). —CC
Christine Corbett, MSN, APRN, FNP-BC, CNN-NP
Kansas City Veterans Affairs, Kansas City, Missouri
References
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;(3):1-150.
5. National Kidney Disease Education Program. Potassium: tips for people with chronic kidney disease (CKD). www.niddk.nih.gov/health-information/health-communication-programs/nkdep/a-z/nutrition-potassium/Documents/nutrition-potassium-508.pdf. Accessed June 20, 2016.
6. Odermatt A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Renal Physiol. 2011;301(5):F919-F931.
7. Steiber A. DASH-style diet effective in preventing, delaying CKD progression. Renal and Urology News. 2012.
Q) I see patients with diabetes, hypertension, chronic kidney disease (CKD), obesity ... often all within the same patient! I keep hearing that the DASH diet is best for these patients. Is this true? Do you have any suggestions (or handouts) for teaching good eating habits in a 15-minute office visit?
It is always nice to focus on what patients can do, rather than what they can’t. Patients with diabetes, kidney disease, heart disease, and obesity hear a lot of “can’t” messages, making “can” messages particularly important to emphasize.
Healthy diets for diabetes, heart, and kidney patients include foods low in trans and saturated fats and sodium. Not all CKD patients are required to follow a low-potassium diet; dietary restrictions are based on laboratory values, medications, and other factors. As we know, adding an ACE inhibitor or an angiotensin receptor blocker (ARB) to the treatment regimen can cause an elevation in serum potassium.
For adults with CKD, it is recommended that sodium intake be restricted to < 2,000 mg/d.4 And in this population, salt substitutes are not recommended, since they often contain large amounts of potassium chloride, which increases risk for hyperkalemia.5 Other spices (eg, garlic, pepper, lemon) are better substitutes for salt.
The late Paul Prudhomme, an award-winning chef from New Orleans, struggled with obesity and health issues for years. He developed wonderful, kidney-friendly spices free of salt and potassium. His line of spices, Magic Seasoning Blends, is sold in many grocery stores. You can recommend them without worry.
Studies have shown that the usual Western diet (which features an abundance of processed foods, fats, and sugars) contributes to kidney disease.6 The DASH (Dietary Approaches to Stop Hypertension) diet, developed by cardio experts, replaces these foods with healthier alternatives.
Recent research has shown that the DASH diet does, in fact, slow the progression of kidney disease.7 It also lowers blood pressure and decreases kidney stone formation, which are risk factors for kidney disease.
So, the DASH diet is protective for your patients (from both a kidney and a cardiac standpoint)—but how do you explain this in a 15-minute office visit?
Here are a few quick tips:
• Increase fruit and vegetable intake to include all colors on your plate (and no, tan is not really a color)
• If you eat meat, the cooking method should start with “B” (ie, bake, boil, broil, barbeque [without salty sauce]) ... Note that “fried” does not start with “B”!
• Use a smaller plate and you will not eat as much
• Use technology in your favor. There are great apps and downloads you can recommend (see Table). —CC
Christine Corbett, MSN, APRN, FNP-BC, CNN-NP
Kansas City Veterans Affairs, Kansas City, Missouri
References
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;(3):1-150.
5. National Kidney Disease Education Program. Potassium: tips for people with chronic kidney disease (CKD). www.niddk.nih.gov/health-information/health-communication-programs/nkdep/a-z/nutrition-potassium/Documents/nutrition-potassium-508.pdf. Accessed June 20, 2016.
6. Odermatt A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Renal Physiol. 2011;301(5):F919-F931.
7. Steiber A. DASH-style diet effective in preventing, delaying CKD progression. Renal and Urology News. 2012.
Q) I see patients with diabetes, hypertension, chronic kidney disease (CKD), obesity ... often all within the same patient! I keep hearing that the DASH diet is best for these patients. Is this true? Do you have any suggestions (or handouts) for teaching good eating habits in a 15-minute office visit?
It is always nice to focus on what patients can do, rather than what they can’t. Patients with diabetes, kidney disease, heart disease, and obesity hear a lot of “can’t” messages, making “can” messages particularly important to emphasize.
Healthy diets for diabetes, heart, and kidney patients include foods low in trans and saturated fats and sodium. Not all CKD patients are required to follow a low-potassium diet; dietary restrictions are based on laboratory values, medications, and other factors. As we know, adding an ACE inhibitor or an angiotensin receptor blocker (ARB) to the treatment regimen can cause an elevation in serum potassium.
For adults with CKD, it is recommended that sodium intake be restricted to < 2,000 mg/d.4 And in this population, salt substitutes are not recommended, since they often contain large amounts of potassium chloride, which increases risk for hyperkalemia.5 Other spices (eg, garlic, pepper, lemon) are better substitutes for salt.
The late Paul Prudhomme, an award-winning chef from New Orleans, struggled with obesity and health issues for years. He developed wonderful, kidney-friendly spices free of salt and potassium. His line of spices, Magic Seasoning Blends, is sold in many grocery stores. You can recommend them without worry.
Studies have shown that the usual Western diet (which features an abundance of processed foods, fats, and sugars) contributes to kidney disease.6 The DASH (Dietary Approaches to Stop Hypertension) diet, developed by cardio experts, replaces these foods with healthier alternatives.
Recent research has shown that the DASH diet does, in fact, slow the progression of kidney disease.7 It also lowers blood pressure and decreases kidney stone formation, which are risk factors for kidney disease.
So, the DASH diet is protective for your patients (from both a kidney and a cardiac standpoint)—but how do you explain this in a 15-minute office visit?
Here are a few quick tips:
• Increase fruit and vegetable intake to include all colors on your plate (and no, tan is not really a color)
• If you eat meat, the cooking method should start with “B” (ie, bake, boil, broil, barbeque [without salty sauce]) ... Note that “fried” does not start with “B”!
• Use a smaller plate and you will not eat as much
• Use technology in your favor. There are great apps and downloads you can recommend (see Table). —CC
Christine Corbett, MSN, APRN, FNP-BC, CNN-NP
Kansas City Veterans Affairs, Kansas City, Missouri
References
4. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;(3):1-150.
5. National Kidney Disease Education Program. Potassium: tips for people with chronic kidney disease (CKD). www.niddk.nih.gov/health-information/health-communication-programs/nkdep/a-z/nutrition-potassium/Documents/nutrition-potassium-508.pdf. Accessed June 20, 2016.
6. Odermatt A. The Western-style diet: a major risk factor for impaired kidney function and chronic kidney disease. Am J Physiol Renal Physiol. 2011;301(5):F919-F931.
7. Steiber A. DASH-style diet effective in preventing, delaying CKD progression. Renal and Urology News. 2012.
CKD: Latest on Screening
Q) suPAR, a new screening tool for chronic kidney disease, has gotten a lot of press recently. My practice is interested in implementing it, but we can’t find information on how to obtain it. Is it commercially available yet? How can we order it? And importantly for our patients, do insurance plans cover it?
Research has been ongoing regarding biomarkers that could identify those at risk for chronic kidney disease (CKD) long before loss of renal function is apparent. A recently published study suggests that the circulating protein, soluble urokinase-type plasminogen activator receptor (suPAR), may be such a biomarker.
In a study of 3,683 subjects (ages 20 to 90) undergoing cardiac catheterization, and a further evaluation of 347 subjects in the Women’s Interagency HIV Study, Hayek et al found that elevated levels of suPAR were independently associated with CKD and with accelerated loss of renal function. At five-year follow-up, 24% of the 1,335 subjects with an initial estimated glomerular filtration rate (eGFR) ≥ 60 mL/min/1.73 m2 had developed CKD. Risk for progression to CKD was about 41% in those with a baseline suPAR level ≥ 3,040 ng/mL, compared to 12% in those with lower baseline suPAR levels.1 Thus, the cutoff for high versus low risk appears to be 3,040 ng/mL.
Hayek and associates are not the first or the only investigators studying the connection between suPAR and kidney disease. Evolving research has suggested suPAR may be an initiating factor in the development of focal segmental glomerulosclerosis (FSGS).2 However, a recent study did not support this association.3
Currently, in the United States, laboratory testing for suPAR is available only for research purposes and has not been approved by the FDA for direct patient care.4 While more research is needed with different cohorts, there is much excitement in the field of nephrology regarding the potential role of suPAR as a biomarker for predicting CKD. —CS
Cindy Smith, DNP, APRN, CNN-NP, FNP-BC
Renal Consultants, PLLC, South Charleston, West Virgina
References
1. Hayek SS, Sever S, Ko Y-A, et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916-1925.
2. Spinale JM, Mariani LH, Kapoor S, et al. A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int. 2015;87(3):564-574.
3. Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis.Nature Med. 2011;17: 952-960.
4. Rush University Medical Center. Early warning found for chronic kidney disease: common protein in blood rises months or years before disease develops [news release]. November 5, 2015. www.rush.edu/news/press-releases/early-warning-found-chronic-kidney-disease. Accessed April 11, 2016.
Q) suPAR, a new screening tool for chronic kidney disease, has gotten a lot of press recently. My practice is interested in implementing it, but we can’t find information on how to obtain it. Is it commercially available yet? How can we order it? And importantly for our patients, do insurance plans cover it?
Research has been ongoing regarding biomarkers that could identify those at risk for chronic kidney disease (CKD) long before loss of renal function is apparent. A recently published study suggests that the circulating protein, soluble urokinase-type plasminogen activator receptor (suPAR), may be such a biomarker.
In a study of 3,683 subjects (ages 20 to 90) undergoing cardiac catheterization, and a further evaluation of 347 subjects in the Women’s Interagency HIV Study, Hayek et al found that elevated levels of suPAR were independently associated with CKD and with accelerated loss of renal function. At five-year follow-up, 24% of the 1,335 subjects with an initial estimated glomerular filtration rate (eGFR) ≥ 60 mL/min/1.73 m2 had developed CKD. Risk for progression to CKD was about 41% in those with a baseline suPAR level ≥ 3,040 ng/mL, compared to 12% in those with lower baseline suPAR levels.1 Thus, the cutoff for high versus low risk appears to be 3,040 ng/mL.
Hayek and associates are not the first or the only investigators studying the connection between suPAR and kidney disease. Evolving research has suggested suPAR may be an initiating factor in the development of focal segmental glomerulosclerosis (FSGS).2 However, a recent study did not support this association.3
Currently, in the United States, laboratory testing for suPAR is available only for research purposes and has not been approved by the FDA for direct patient care.4 While more research is needed with different cohorts, there is much excitement in the field of nephrology regarding the potential role of suPAR as a biomarker for predicting CKD. —CS
Cindy Smith, DNP, APRN, CNN-NP, FNP-BC
Renal Consultants, PLLC, South Charleston, West Virgina
References
1. Hayek SS, Sever S, Ko Y-A, et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916-1925.
2. Spinale JM, Mariani LH, Kapoor S, et al. A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int. 2015;87(3):564-574.
3. Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis.Nature Med. 2011;17: 952-960.
4. Rush University Medical Center. Early warning found for chronic kidney disease: common protein in blood rises months or years before disease develops [news release]. November 5, 2015. www.rush.edu/news/press-releases/early-warning-found-chronic-kidney-disease. Accessed April 11, 2016.
Q) suPAR, a new screening tool for chronic kidney disease, has gotten a lot of press recently. My practice is interested in implementing it, but we can’t find information on how to obtain it. Is it commercially available yet? How can we order it? And importantly for our patients, do insurance plans cover it?
Research has been ongoing regarding biomarkers that could identify those at risk for chronic kidney disease (CKD) long before loss of renal function is apparent. A recently published study suggests that the circulating protein, soluble urokinase-type plasminogen activator receptor (suPAR), may be such a biomarker.
In a study of 3,683 subjects (ages 20 to 90) undergoing cardiac catheterization, and a further evaluation of 347 subjects in the Women’s Interagency HIV Study, Hayek et al found that elevated levels of suPAR were independently associated with CKD and with accelerated loss of renal function. At five-year follow-up, 24% of the 1,335 subjects with an initial estimated glomerular filtration rate (eGFR) ≥ 60 mL/min/1.73 m2 had developed CKD. Risk for progression to CKD was about 41% in those with a baseline suPAR level ≥ 3,040 ng/mL, compared to 12% in those with lower baseline suPAR levels.1 Thus, the cutoff for high versus low risk appears to be 3,040 ng/mL.
Hayek and associates are not the first or the only investigators studying the connection between suPAR and kidney disease. Evolving research has suggested suPAR may be an initiating factor in the development of focal segmental glomerulosclerosis (FSGS).2 However, a recent study did not support this association.3
Currently, in the United States, laboratory testing for suPAR is available only for research purposes and has not been approved by the FDA for direct patient care.4 While more research is needed with different cohorts, there is much excitement in the field of nephrology regarding the potential role of suPAR as a biomarker for predicting CKD. —CS
Cindy Smith, DNP, APRN, CNN-NP, FNP-BC
Renal Consultants, PLLC, South Charleston, West Virgina
References
1. Hayek SS, Sever S, Ko Y-A, et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916-1925.
2. Spinale JM, Mariani LH, Kapoor S, et al. A reassessment of soluble urokinase-type plasminogen activator receptor in glomerular disease. Kidney Int. 2015;87(3):564-574.
3. Wei C, El Hindi S, Li J, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis.Nature Med. 2011;17: 952-960.
4. Rush University Medical Center. Early warning found for chronic kidney disease: common protein in blood rises months or years before disease develops [news release]. November 5, 2015. www.rush.edu/news/press-releases/early-warning-found-chronic-kidney-disease. Accessed April 11, 2016.
CKD: Latest on Management
Q) I have a patient with stage 3a chronic kidney disease (glomerular filtration rate, 45-60 mL/min/1.73 m2). I have her on a statin and an ACE inhibitor. Is there anything else I can do to slow the progression of kidney disease?
For patients with stage 3a chronic kidney disease (CKD), ongoing evaluation of risk factors and management can impact the rate of disease progression. The cornerstones of CKD care include identification and treatment of the cause; management of hypertension, albuminuria, and diabetes (if applicable); reduction of cardiovascular (CV) risk; and correction of metabolic abnormalities.5
When considering factors that can contribute to kidney injury, clinicians should consider possible pre-, intra-, and post-renal processes that could potentially cause injury.
Prerenal: Approximately 20% of cardiac output is directed to the kidneys. Reduced left ventricular function, diastolic dysfunction, and pulmonary hypertension can all contribute to a reduction in renal blood flow and subsequent kidney injury.6
Intrarenal: Exploration of possible intra-renal processes begins with a thorough history of any familial disease, hematuria, stones, proteinuria, and exposure to nephrotoxins. The nephrotoxicity profile of all medications should be examined, and patients should be educated about products, particularly OTC medications (eg, NSAIDs, common cold preparations, and herbal or weight-loss products), that can be harmful to the kidneys. Patients should also be made aware of the risk for contrast-induced renal injury, especially when considering imaging or cardiac testing. Since diabetes is a leading cause of kidney disease, good diabetic control can reduce nephropathy and slow disease progression.
Postrenal: Benign prostatic hypertrophy, kidney stones, and neurogenic bladder can all cause injury. These warrant further evaluation and treatment.
CKD often worsens existing hypertension, which is an independent risk factor for kidney failure.7 Goal blood pressure (BP) for all patients without significant albuminuria should be < 140/90 mm Hg; for those with urinary albumin ≥ 30 mg/24 h, the goal is < 130/80 mm Hg.8 Choice of antihypertensive agents can be tailored to other comorbidities, but an ACE inhibitor or angiotensin receptor blocker should be considered firstline treatment. Nocturnal hypertension is common in patients with CKD and an independent marker of CV risk. By dosing antihypertensive medications at bedtime, the clinician supports CV risk reduction.9
CKD is an independent risk factor for CV disease, thus risk factor modification should be aggressively pursued. Regardless of the cause of CKD, cigarette smoking has been associated with a more rapid decline in renal function. Patients should be counseled on the risks and offered interventions to assist in smoking cessation.10 There is also emerging evidence that exercise likely benefits the vascular health of the kidneys and appears to slow the rate of kidney decline.11,12 Overall, lifestyle interventions that help mitigate CV risk may directly benefit preservation of kidney function as well.
Metabolic abnormalities increase with CKD progression. Maintaining proper bone health through control of phosphate/acidosis and calcium equilibrium reduces morbidity as it relates to vascular and soft-tissue calcification. This can often be effectively managed through dietary modifications in early to moderate CKD. As the number of functioning nephrons decrease in CKD, so does the ability of the kidney to maintain proper acid/base balance. Persistent metabolic acidosis is related to CKD progression. Acid buffering with oral bicarbonate may be needed to achieve a goal CO2 of 22 to 32 mEq/L.8
Through adoption of a comprehensive approach—one that is inclusive of the patient—optimal outcomes can be achieved for this rapidly growing and often underrecognized population. —CJ, AH-B, IS, BB
Crystal Johnson, PA-C
Angela Harker-Bacchus, FNP-BC
Irina Sadovskaya, PA-C
Beverly Benmoussa, FNP-BC
Transplant Nephrology Extra-Renal CKD Clinic, University of Michigan
References
5. Murphree DD, Thelen SM. Chronic kidney disease in primary care. J Am Board Fam Med. 2010;23(4):542-550.
6. Coppolino G, Presta P, Saturno L, Fuiano G. Acute kidney injury in patients undergoing cardiac surgery. J Nephrol. 2013;26(1):32-40.
7. Ravera M, Re M, Defarri L, et al. Importance of blood pressure control in chronic kidney disease. J Am Soc Nephrol. 2006;17(4 suppl 2):S98-S103.
8. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3(suppl):1-150.
9. Hermida RC, Ayala DE, Mojón A, Fernández JR. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22(12):2313-2321.
10. Ricardo AC, Anderson CA, Yang W, et al. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2015;65(3):412-424.
11. Gould DW, Graham-Brown MPM, Watson EL, et al. Physiological benefits of exercise in pre-dialysis chronic kidney disease. Nephrology (Carlton). 2014;19(9):519-527.
12. Robinson-Cohen C, Littman AJ, Duncan GE, et al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol. 2014;25(2):399-406.
Q) I have a patient with stage 3a chronic kidney disease (glomerular filtration rate, 45-60 mL/min/1.73 m2). I have her on a statin and an ACE inhibitor. Is there anything else I can do to slow the progression of kidney disease?
For patients with stage 3a chronic kidney disease (CKD), ongoing evaluation of risk factors and management can impact the rate of disease progression. The cornerstones of CKD care include identification and treatment of the cause; management of hypertension, albuminuria, and diabetes (if applicable); reduction of cardiovascular (CV) risk; and correction of metabolic abnormalities.5
When considering factors that can contribute to kidney injury, clinicians should consider possible pre-, intra-, and post-renal processes that could potentially cause injury.
Prerenal: Approximately 20% of cardiac output is directed to the kidneys. Reduced left ventricular function, diastolic dysfunction, and pulmonary hypertension can all contribute to a reduction in renal blood flow and subsequent kidney injury.6
Intrarenal: Exploration of possible intra-renal processes begins with a thorough history of any familial disease, hematuria, stones, proteinuria, and exposure to nephrotoxins. The nephrotoxicity profile of all medications should be examined, and patients should be educated about products, particularly OTC medications (eg, NSAIDs, common cold preparations, and herbal or weight-loss products), that can be harmful to the kidneys. Patients should also be made aware of the risk for contrast-induced renal injury, especially when considering imaging or cardiac testing. Since diabetes is a leading cause of kidney disease, good diabetic control can reduce nephropathy and slow disease progression.
Postrenal: Benign prostatic hypertrophy, kidney stones, and neurogenic bladder can all cause injury. These warrant further evaluation and treatment.
CKD often worsens existing hypertension, which is an independent risk factor for kidney failure.7 Goal blood pressure (BP) for all patients without significant albuminuria should be < 140/90 mm Hg; for those with urinary albumin ≥ 30 mg/24 h, the goal is < 130/80 mm Hg.8 Choice of antihypertensive agents can be tailored to other comorbidities, but an ACE inhibitor or angiotensin receptor blocker should be considered firstline treatment. Nocturnal hypertension is common in patients with CKD and an independent marker of CV risk. By dosing antihypertensive medications at bedtime, the clinician supports CV risk reduction.9
CKD is an independent risk factor for CV disease, thus risk factor modification should be aggressively pursued. Regardless of the cause of CKD, cigarette smoking has been associated with a more rapid decline in renal function. Patients should be counseled on the risks and offered interventions to assist in smoking cessation.10 There is also emerging evidence that exercise likely benefits the vascular health of the kidneys and appears to slow the rate of kidney decline.11,12 Overall, lifestyle interventions that help mitigate CV risk may directly benefit preservation of kidney function as well.
Metabolic abnormalities increase with CKD progression. Maintaining proper bone health through control of phosphate/acidosis and calcium equilibrium reduces morbidity as it relates to vascular and soft-tissue calcification. This can often be effectively managed through dietary modifications in early to moderate CKD. As the number of functioning nephrons decrease in CKD, so does the ability of the kidney to maintain proper acid/base balance. Persistent metabolic acidosis is related to CKD progression. Acid buffering with oral bicarbonate may be needed to achieve a goal CO2 of 22 to 32 mEq/L.8
Through adoption of a comprehensive approach—one that is inclusive of the patient—optimal outcomes can be achieved for this rapidly growing and often underrecognized population. —CJ, AH-B, IS, BB
Crystal Johnson, PA-C
Angela Harker-Bacchus, FNP-BC
Irina Sadovskaya, PA-C
Beverly Benmoussa, FNP-BC
Transplant Nephrology Extra-Renal CKD Clinic, University of Michigan
References
5. Murphree DD, Thelen SM. Chronic kidney disease in primary care. J Am Board Fam Med. 2010;23(4):542-550.
6. Coppolino G, Presta P, Saturno L, Fuiano G. Acute kidney injury in patients undergoing cardiac surgery. J Nephrol. 2013;26(1):32-40.
7. Ravera M, Re M, Defarri L, et al. Importance of blood pressure control in chronic kidney disease. J Am Soc Nephrol. 2006;17(4 suppl 2):S98-S103.
8. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3(suppl):1-150.
9. Hermida RC, Ayala DE, Mojón A, Fernández JR. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22(12):2313-2321.
10. Ricardo AC, Anderson CA, Yang W, et al. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2015;65(3):412-424.
11. Gould DW, Graham-Brown MPM, Watson EL, et al. Physiological benefits of exercise in pre-dialysis chronic kidney disease. Nephrology (Carlton). 2014;19(9):519-527.
12. Robinson-Cohen C, Littman AJ, Duncan GE, et al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol. 2014;25(2):399-406.
Q) I have a patient with stage 3a chronic kidney disease (glomerular filtration rate, 45-60 mL/min/1.73 m2). I have her on a statin and an ACE inhibitor. Is there anything else I can do to slow the progression of kidney disease?
For patients with stage 3a chronic kidney disease (CKD), ongoing evaluation of risk factors and management can impact the rate of disease progression. The cornerstones of CKD care include identification and treatment of the cause; management of hypertension, albuminuria, and diabetes (if applicable); reduction of cardiovascular (CV) risk; and correction of metabolic abnormalities.5
When considering factors that can contribute to kidney injury, clinicians should consider possible pre-, intra-, and post-renal processes that could potentially cause injury.
Prerenal: Approximately 20% of cardiac output is directed to the kidneys. Reduced left ventricular function, diastolic dysfunction, and pulmonary hypertension can all contribute to a reduction in renal blood flow and subsequent kidney injury.6
Intrarenal: Exploration of possible intra-renal processes begins with a thorough history of any familial disease, hematuria, stones, proteinuria, and exposure to nephrotoxins. The nephrotoxicity profile of all medications should be examined, and patients should be educated about products, particularly OTC medications (eg, NSAIDs, common cold preparations, and herbal or weight-loss products), that can be harmful to the kidneys. Patients should also be made aware of the risk for contrast-induced renal injury, especially when considering imaging or cardiac testing. Since diabetes is a leading cause of kidney disease, good diabetic control can reduce nephropathy and slow disease progression.
Postrenal: Benign prostatic hypertrophy, kidney stones, and neurogenic bladder can all cause injury. These warrant further evaluation and treatment.
CKD often worsens existing hypertension, which is an independent risk factor for kidney failure.7 Goal blood pressure (BP) for all patients without significant albuminuria should be < 140/90 mm Hg; for those with urinary albumin ≥ 30 mg/24 h, the goal is < 130/80 mm Hg.8 Choice of antihypertensive agents can be tailored to other comorbidities, but an ACE inhibitor or angiotensin receptor blocker should be considered firstline treatment. Nocturnal hypertension is common in patients with CKD and an independent marker of CV risk. By dosing antihypertensive medications at bedtime, the clinician supports CV risk reduction.9
CKD is an independent risk factor for CV disease, thus risk factor modification should be aggressively pursued. Regardless of the cause of CKD, cigarette smoking has been associated with a more rapid decline in renal function. Patients should be counseled on the risks and offered interventions to assist in smoking cessation.10 There is also emerging evidence that exercise likely benefits the vascular health of the kidneys and appears to slow the rate of kidney decline.11,12 Overall, lifestyle interventions that help mitigate CV risk may directly benefit preservation of kidney function as well.
Metabolic abnormalities increase with CKD progression. Maintaining proper bone health through control of phosphate/acidosis and calcium equilibrium reduces morbidity as it relates to vascular and soft-tissue calcification. This can often be effectively managed through dietary modifications in early to moderate CKD. As the number of functioning nephrons decrease in CKD, so does the ability of the kidney to maintain proper acid/base balance. Persistent metabolic acidosis is related to CKD progression. Acid buffering with oral bicarbonate may be needed to achieve a goal CO2 of 22 to 32 mEq/L.8
Through adoption of a comprehensive approach—one that is inclusive of the patient—optimal outcomes can be achieved for this rapidly growing and often underrecognized population. —CJ, AH-B, IS, BB
Crystal Johnson, PA-C
Angela Harker-Bacchus, FNP-BC
Irina Sadovskaya, PA-C
Beverly Benmoussa, FNP-BC
Transplant Nephrology Extra-Renal CKD Clinic, University of Michigan
References
5. Murphree DD, Thelen SM. Chronic kidney disease in primary care. J Am Board Fam Med. 2010;23(4):542-550.
6. Coppolino G, Presta P, Saturno L, Fuiano G. Acute kidney injury in patients undergoing cardiac surgery. J Nephrol. 2013;26(1):32-40.
7. Ravera M, Re M, Defarri L, et al. Importance of blood pressure control in chronic kidney disease. J Am Soc Nephrol. 2006;17(4 suppl 2):S98-S103.
8. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2013;3(suppl):1-150.
9. Hermida RC, Ayala DE, Mojón A, Fernández JR. Bedtime dosing of antihypertensive medications reduces cardiovascular risk in CKD. J Am Soc Nephrol. 2011;22(12):2313-2321.
10. Ricardo AC, Anderson CA, Yang W, et al. Healthy lifestyle and risk of kidney disease progression, atherosclerotic events, and death in CKD: findings from the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis. 2015;65(3):412-424.
11. Gould DW, Graham-Brown MPM, Watson EL, et al. Physiological benefits of exercise in pre-dialysis chronic kidney disease. Nephrology (Carlton). 2014;19(9):519-527.
12. Robinson-Cohen C, Littman AJ, Duncan GE, et al. Physical activity and change in estimated GFR among persons with CKD. J Am Soc Nephrol. 2014;25(2):399-406.
Kidney Disease: Surprising Patients
Q) Recently, I have seen four or five Asian-American patients with really bad kidney function. All of them were thin but had diabetes, hypertension, and a serum creatinine > 2 mg/dL. The kidney disease was a shock to them (and me). Am I missing something here?
Diabetes and hypertension are the most common causes of chronic kidney disease (CKD), with diabetes slightly edging out hypertension for the number 1 slot.1 Although Asian Americans have a tendency toward a lower body mass index (BMI) than the general population, this does not exclude them from developing diabetes or hypertension.
About 20% (1 in 5) of Asian-American adults have both diabetes and hypertension. In fact, Asian Americans with a BMI ≤ 25 often develop type 2 diabetes (T2DM), which is a direct contrast to other racial and ethnic groups in whom T2DM is more prevalent at higher BMIs. The current thinking is that Asian Americans have a higher percentage of body fat at lower BMIs.2 Among racial and ethnic subgroups, Asian Americans have the highest prevalence of undiagnosed diabetes (close to 50%).2
In 2004, after adjusting for lower BMI, McNeely and Boyko found that the incidence of diabetes in Asian Americans was 60% higher than in the Hispanic population.3 In 2015, this influenced the American Diabetes Association (ADA) to change its recommendation for diabetes screening in Asian Americans, lowering the threshold to a BMI of 23.4
Since abdominal or visceral fat is a risk factor for heart disease, hypertension, and diabetes, and it appears that the Asian-American population carries excess fat centrally, this population is also at risk for cardiac disease.5 For that reason, in this population, the American Heart Association recommends measuring waist circumference to screen for hidden abdominal adiposity.6
Thus, the trend you are seeing in your patient population is really only the tip of the iceberg. The Asian-American population is the fastest-growing ethnic group in the United States.3 It’s time to update your diabetes screening protocols. —SWM
Shushanne Wynter-Minott, DNP, FNP-BC
Memorial Healthcare System, Hollywood, Florida
References
1. CDC. National Chronic Kidney Disease Fact Sheet, 2014. www.cdc.gov/diabetes/pubs/pdf/kidney_Factsheet.pdf. Accessed February 3, 2016.
2. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029.
3. McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care. 2004;27(1):66-69.
4. American Diabetes Association. Standards of medical care in diabetes—2015: summary of revisions. Diabetes Care. 2015;38(suppl):S4.
5. Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9(7):381-387.
6. Rao G, Powell-Wiley TM, Ancheta I, et al; American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health. Identification of obesity and cardiovascular risk in ethnically and racially diverse populations: a scientific statement from the American Heart Association. Circulation. 2015;132(5):457-472.
Q) Recently, I have seen four or five Asian-American patients with really bad kidney function. All of them were thin but had diabetes, hypertension, and a serum creatinine > 2 mg/dL. The kidney disease was a shock to them (and me). Am I missing something here?
Diabetes and hypertension are the most common causes of chronic kidney disease (CKD), with diabetes slightly edging out hypertension for the number 1 slot.1 Although Asian Americans have a tendency toward a lower body mass index (BMI) than the general population, this does not exclude them from developing diabetes or hypertension.
About 20% (1 in 5) of Asian-American adults have both diabetes and hypertension. In fact, Asian Americans with a BMI ≤ 25 often develop type 2 diabetes (T2DM), which is a direct contrast to other racial and ethnic groups in whom T2DM is more prevalent at higher BMIs. The current thinking is that Asian Americans have a higher percentage of body fat at lower BMIs.2 Among racial and ethnic subgroups, Asian Americans have the highest prevalence of undiagnosed diabetes (close to 50%).2
In 2004, after adjusting for lower BMI, McNeely and Boyko found that the incidence of diabetes in Asian Americans was 60% higher than in the Hispanic population.3 In 2015, this influenced the American Diabetes Association (ADA) to change its recommendation for diabetes screening in Asian Americans, lowering the threshold to a BMI of 23.4
Since abdominal or visceral fat is a risk factor for heart disease, hypertension, and diabetes, and it appears that the Asian-American population carries excess fat centrally, this population is also at risk for cardiac disease.5 For that reason, in this population, the American Heart Association recommends measuring waist circumference to screen for hidden abdominal adiposity.6
Thus, the trend you are seeing in your patient population is really only the tip of the iceberg. The Asian-American population is the fastest-growing ethnic group in the United States.3 It’s time to update your diabetes screening protocols. —SWM
Shushanne Wynter-Minott, DNP, FNP-BC
Memorial Healthcare System, Hollywood, Florida
References
1. CDC. National Chronic Kidney Disease Fact Sheet, 2014. www.cdc.gov/diabetes/pubs/pdf/kidney_Factsheet.pdf. Accessed February 3, 2016.
2. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029.
3. McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care. 2004;27(1):66-69.
4. American Diabetes Association. Standards of medical care in diabetes—2015: summary of revisions. Diabetes Care. 2015;38(suppl):S4.
5. Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9(7):381-387.
6. Rao G, Powell-Wiley TM, Ancheta I, et al; American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health. Identification of obesity and cardiovascular risk in ethnically and racially diverse populations: a scientific statement from the American Heart Association. Circulation. 2015;132(5):457-472.
Q) Recently, I have seen four or five Asian-American patients with really bad kidney function. All of them were thin but had diabetes, hypertension, and a serum creatinine > 2 mg/dL. The kidney disease was a shock to them (and me). Am I missing something here?
Diabetes and hypertension are the most common causes of chronic kidney disease (CKD), with diabetes slightly edging out hypertension for the number 1 slot.1 Although Asian Americans have a tendency toward a lower body mass index (BMI) than the general population, this does not exclude them from developing diabetes or hypertension.
About 20% (1 in 5) of Asian-American adults have both diabetes and hypertension. In fact, Asian Americans with a BMI ≤ 25 often develop type 2 diabetes (T2DM), which is a direct contrast to other racial and ethnic groups in whom T2DM is more prevalent at higher BMIs. The current thinking is that Asian Americans have a higher percentage of body fat at lower BMIs.2 Among racial and ethnic subgroups, Asian Americans have the highest prevalence of undiagnosed diabetes (close to 50%).2
In 2004, after adjusting for lower BMI, McNeely and Boyko found that the incidence of diabetes in Asian Americans was 60% higher than in the Hispanic population.3 In 2015, this influenced the American Diabetes Association (ADA) to change its recommendation for diabetes screening in Asian Americans, lowering the threshold to a BMI of 23.4
Since abdominal or visceral fat is a risk factor for heart disease, hypertension, and diabetes, and it appears that the Asian-American population carries excess fat centrally, this population is also at risk for cardiac disease.5 For that reason, in this population, the American Heart Association recommends measuring waist circumference to screen for hidden abdominal adiposity.6
Thus, the trend you are seeing in your patient population is really only the tip of the iceberg. The Asian-American population is the fastest-growing ethnic group in the United States.3 It’s time to update your diabetes screening protocols. —SWM
Shushanne Wynter-Minott, DNP, FNP-BC
Memorial Healthcare System, Hollywood, Florida
References
1. CDC. National Chronic Kidney Disease Fact Sheet, 2014. www.cdc.gov/diabetes/pubs/pdf/kidney_Factsheet.pdf. Accessed February 3, 2016.
2. Menke A, Casagrande S, Geiss L, Cowie CC. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314(10):1021-1029.
3. McNeely MJ, Boyko EJ. Type 2 diabetes prevalence in Asian Americans: results of a national health survey. Diabetes Care. 2004;27(1):66-69.
4. American Diabetes Association. Standards of medical care in diabetes—2015: summary of revisions. Diabetes Care. 2015;38(suppl):S4.
5. Park YW, Allison DB, Heymsfield SB, Gallagher D. Larger amounts of visceral adipose tissue in Asian Americans. Obes Res. 2001;9(7):381-387.
6. Rao G, Powell-Wiley TM, Ancheta I, et al; American Heart Association Obesity Committee of the Council on Lifestyle and Cardiometabolic Health. Identification of obesity and cardiovascular risk in ethnically and racially diverse populations: a scientific statement from the American Heart Association. Circulation. 2015;132(5):457-472.
Kidney Disease: Unexpected Consequences
Q) We were operating on a 58-year-old woman for a subcapital fracture of her right hip. The orthopedist mentioned that the patient had kidney disease and that it probably caused her hip fracture. I didn’t know kidney disease causes hip fractures. Is this true?
Evolving evidence suggests an association between diminishing renal function and increased risk for fracture. Here’s a look at the available data:
Atherosclerosis Risk in Communities (ARIC) Study. During a median 13 years’ follow-up of 10,955 community-based older adults, investigators identified higher albuminuria level and decreased creatinine-based estimated glomerular filtration rate (eGFR) as significant risk factors for fracture. Other risk factors included older age, race (Caucasians had the highest incidence), and sex (women were more likely than men to sustain a fracture). A nonlinear relationship was observed between eGFR and fracture diagnosis, with a graded association between fracture and albuminuria level.7
Cardiovascular Health Study. In this study of 4,699 older community-based adults, kidney function was assessed by measurement of serum cystatin C. During a mean follow-up of 7.1 years, higher cystatin C levels correlated to a higher risk for hip fracture in both sexes. In women, there was a significant association between diminishing renal function and hip fracture status: Those with lower eGFRs had a higher incidence of fractures. There was a similar magnitude of association among men, but it was not significant.8
Health, Aging and Body Composite Study. In 2,754 older adults, an association was noted between decreased femoral neck bone mineral density (BMD) and increased risk for fracture in those with and without CKD stage 3 to 5. With a concurrent diagnosis of osteoporosis, there was a 110% increased risk for nonspinal fracture in those with CKD and a 63% increased risk for those without CKD.9 In a study of 485 adult hemodialysis patients, decreased total hip and femoral neck BMD was associated with an increased risk for fractures in women with parathyroid hormone levels on the lower range of acceptable in this population (intact parathyroid hormone level [IPTH] < 204 pg/mL) and for spinal fractures in both genders.10
Bone changes associated with deterioration of renal function are complex and multifactorial. Human bone is a composite of protein fused to mineral crystals, primarily calcium and phosphate. Bone is dynamic, being broken down and rebuilt throughout adulthood, with the skeleton almost completely rebuilt every 10 years.11
CKD–mineral and bone disorder (CKD–MBD) is a systemic disorder seen in those with kidney disease that affects bone and mineral metabolism. Its manifestations include abnormalities in the bone, calcifications of vascular and/or soft tissues, abnormal vitamin D metabolism, and disruptions in the phosphorus, calcium, and parathyroid hormone levels. These components, and the severity of the condition, vary by stage of CKD. One component of CKD–MBD, renal osteodystrophy, is associated with changes in bone morphology and is definitively diagnosed by bone biopsy.12
Care of these patients is complex and can be compounded by osteoporosis and/or loss of bone strength. Osteoporosis, like CKD, increases in incidence with age and is associated with fracture risk.11
While useful for diagnosing osteoporosis and predicting fracture risk in the general population, dual-energy X-ray densitometry (DXA) has not been recommended in those with CKD due to the type of bone changes that occur with diminished renal function.12 However, evolving evidence regarding use of DXA in these patients prompted a Kidney Disease: Improving Global Outcomes (KDIGO) “controversies” conference to recommend reexamination of the evidence regarding this recommendation.13 KDIGO’s 2009 clinical practice guideline on CKD–MBD (http://kdigo.org/home/mineral-bone-disorder/) can be of benefit in the assessment and care of affected patients. —CS
Cindy Smith, DNP, APRN, CNN-NP, FNP-BC
Renal Consultants, PLLC, South Charleston, West Virgina
References
7. Daya NR, Voskertchian A, Schneider ALC, et al. Kidney function and fracture risk: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2016;67(2):218-226.
8. Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol. 2007;18:282-286.
9. Yenchek RH, Ix JH, Shlipak MG, et al. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7(7):1130-1136.
10. Iimori S, Mori Y, Akita W, et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—a single-center cohort study. Nephrol Dial Transplant. 2012;27:345-351.
11. Office of the Surgeon General (US). Bone Health and Osteoporosis: a Report of the Surgeon General. Rockville, MD: Office of the Surgeon General; 2004.
12. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1-S130.
13. Ketteler M, Elder GJ, Evenepoel P, et al. Revisiting KDIGO clinical practice guideline on chronic kidney disease-mineral and bone disorder: a commentary from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int. 2015;87(3):502-528.
Q) We were operating on a 58-year-old woman for a subcapital fracture of her right hip. The orthopedist mentioned that the patient had kidney disease and that it probably caused her hip fracture. I didn’t know kidney disease causes hip fractures. Is this true?
Evolving evidence suggests an association between diminishing renal function and increased risk for fracture. Here’s a look at the available data:
Atherosclerosis Risk in Communities (ARIC) Study. During a median 13 years’ follow-up of 10,955 community-based older adults, investigators identified higher albuminuria level and decreased creatinine-based estimated glomerular filtration rate (eGFR) as significant risk factors for fracture. Other risk factors included older age, race (Caucasians had the highest incidence), and sex (women were more likely than men to sustain a fracture). A nonlinear relationship was observed between eGFR and fracture diagnosis, with a graded association between fracture and albuminuria level.7
Cardiovascular Health Study. In this study of 4,699 older community-based adults, kidney function was assessed by measurement of serum cystatin C. During a mean follow-up of 7.1 years, higher cystatin C levels correlated to a higher risk for hip fracture in both sexes. In women, there was a significant association between diminishing renal function and hip fracture status: Those with lower eGFRs had a higher incidence of fractures. There was a similar magnitude of association among men, but it was not significant.8
Health, Aging and Body Composite Study. In 2,754 older adults, an association was noted between decreased femoral neck bone mineral density (BMD) and increased risk for fracture in those with and without CKD stage 3 to 5. With a concurrent diagnosis of osteoporosis, there was a 110% increased risk for nonspinal fracture in those with CKD and a 63% increased risk for those without CKD.9 In a study of 485 adult hemodialysis patients, decreased total hip and femoral neck BMD was associated with an increased risk for fractures in women with parathyroid hormone levels on the lower range of acceptable in this population (intact parathyroid hormone level [IPTH] < 204 pg/mL) and for spinal fractures in both genders.10
Bone changes associated with deterioration of renal function are complex and multifactorial. Human bone is a composite of protein fused to mineral crystals, primarily calcium and phosphate. Bone is dynamic, being broken down and rebuilt throughout adulthood, with the skeleton almost completely rebuilt every 10 years.11
CKD–mineral and bone disorder (CKD–MBD) is a systemic disorder seen in those with kidney disease that affects bone and mineral metabolism. Its manifestations include abnormalities in the bone, calcifications of vascular and/or soft tissues, abnormal vitamin D metabolism, and disruptions in the phosphorus, calcium, and parathyroid hormone levels. These components, and the severity of the condition, vary by stage of CKD. One component of CKD–MBD, renal osteodystrophy, is associated with changes in bone morphology and is definitively diagnosed by bone biopsy.12
Care of these patients is complex and can be compounded by osteoporosis and/or loss of bone strength. Osteoporosis, like CKD, increases in incidence with age and is associated with fracture risk.11
While useful for diagnosing osteoporosis and predicting fracture risk in the general population, dual-energy X-ray densitometry (DXA) has not been recommended in those with CKD due to the type of bone changes that occur with diminished renal function.12 However, evolving evidence regarding use of DXA in these patients prompted a Kidney Disease: Improving Global Outcomes (KDIGO) “controversies” conference to recommend reexamination of the evidence regarding this recommendation.13 KDIGO’s 2009 clinical practice guideline on CKD–MBD (http://kdigo.org/home/mineral-bone-disorder/) can be of benefit in the assessment and care of affected patients. —CS
Cindy Smith, DNP, APRN, CNN-NP, FNP-BC
Renal Consultants, PLLC, South Charleston, West Virgina
References
7. Daya NR, Voskertchian A, Schneider ALC, et al. Kidney function and fracture risk: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2016;67(2):218-226.
8. Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol. 2007;18:282-286.
9. Yenchek RH, Ix JH, Shlipak MG, et al. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7(7):1130-1136.
10. Iimori S, Mori Y, Akita W, et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—a single-center cohort study. Nephrol Dial Transplant. 2012;27:345-351.
11. Office of the Surgeon General (US). Bone Health and Osteoporosis: a Report of the Surgeon General. Rockville, MD: Office of the Surgeon General; 2004.
12. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1-S130.
13. Ketteler M, Elder GJ, Evenepoel P, et al. Revisiting KDIGO clinical practice guideline on chronic kidney disease-mineral and bone disorder: a commentary from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int. 2015;87(3):502-528.
Q) We were operating on a 58-year-old woman for a subcapital fracture of her right hip. The orthopedist mentioned that the patient had kidney disease and that it probably caused her hip fracture. I didn’t know kidney disease causes hip fractures. Is this true?
Evolving evidence suggests an association between diminishing renal function and increased risk for fracture. Here’s a look at the available data:
Atherosclerosis Risk in Communities (ARIC) Study. During a median 13 years’ follow-up of 10,955 community-based older adults, investigators identified higher albuminuria level and decreased creatinine-based estimated glomerular filtration rate (eGFR) as significant risk factors for fracture. Other risk factors included older age, race (Caucasians had the highest incidence), and sex (women were more likely than men to sustain a fracture). A nonlinear relationship was observed between eGFR and fracture diagnosis, with a graded association between fracture and albuminuria level.7
Cardiovascular Health Study. In this study of 4,699 older community-based adults, kidney function was assessed by measurement of serum cystatin C. During a mean follow-up of 7.1 years, higher cystatin C levels correlated to a higher risk for hip fracture in both sexes. In women, there was a significant association between diminishing renal function and hip fracture status: Those with lower eGFRs had a higher incidence of fractures. There was a similar magnitude of association among men, but it was not significant.8
Health, Aging and Body Composite Study. In 2,754 older adults, an association was noted between decreased femoral neck bone mineral density (BMD) and increased risk for fracture in those with and without CKD stage 3 to 5. With a concurrent diagnosis of osteoporosis, there was a 110% increased risk for nonspinal fracture in those with CKD and a 63% increased risk for those without CKD.9 In a study of 485 adult hemodialysis patients, decreased total hip and femoral neck BMD was associated with an increased risk for fractures in women with parathyroid hormone levels on the lower range of acceptable in this population (intact parathyroid hormone level [IPTH] < 204 pg/mL) and for spinal fractures in both genders.10
Bone changes associated with deterioration of renal function are complex and multifactorial. Human bone is a composite of protein fused to mineral crystals, primarily calcium and phosphate. Bone is dynamic, being broken down and rebuilt throughout adulthood, with the skeleton almost completely rebuilt every 10 years.11
CKD–mineral and bone disorder (CKD–MBD) is a systemic disorder seen in those with kidney disease that affects bone and mineral metabolism. Its manifestations include abnormalities in the bone, calcifications of vascular and/or soft tissues, abnormal vitamin D metabolism, and disruptions in the phosphorus, calcium, and parathyroid hormone levels. These components, and the severity of the condition, vary by stage of CKD. One component of CKD–MBD, renal osteodystrophy, is associated with changes in bone morphology and is definitively diagnosed by bone biopsy.12
Care of these patients is complex and can be compounded by osteoporosis and/or loss of bone strength. Osteoporosis, like CKD, increases in incidence with age and is associated with fracture risk.11
While useful for diagnosing osteoporosis and predicting fracture risk in the general population, dual-energy X-ray densitometry (DXA) has not been recommended in those with CKD due to the type of bone changes that occur with diminished renal function.12 However, evolving evidence regarding use of DXA in these patients prompted a Kidney Disease: Improving Global Outcomes (KDIGO) “controversies” conference to recommend reexamination of the evidence regarding this recommendation.13 KDIGO’s 2009 clinical practice guideline on CKD–MBD (http://kdigo.org/home/mineral-bone-disorder/) can be of benefit in the assessment and care of affected patients. —CS
Cindy Smith, DNP, APRN, CNN-NP, FNP-BC
Renal Consultants, PLLC, South Charleston, West Virgina
References
7. Daya NR, Voskertchian A, Schneider ALC, et al. Kidney function and fracture risk: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis. 2016;67(2):218-226.
8. Fried LF, Biggs ML, Shlipak MG, et al. Association of kidney function with incident hip fracture in older adults. J Am Soc Nephrol. 2007;18:282-286.
9. Yenchek RH, Ix JH, Shlipak MG, et al. Bone mineral density and fracture risk in older individuals with CKD. Clin J Am Soc Nephrol. 2012;7(7):1130-1136.
10. Iimori S, Mori Y, Akita W, et al. Diagnostic usefulness of bone mineral density and biochemical markers of bone turnover in predicting fracture in CKD stage 5D patients—a single-center cohort study. Nephrol Dial Transplant. 2012;27:345-351.
11. Office of the Surgeon General (US). Bone Health and Osteoporosis: a Report of the Surgeon General. Rockville, MD: Office of the Surgeon General; 2004.
12. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1-S130.
13. Ketteler M, Elder GJ, Evenepoel P, et al. Revisiting KDIGO clinical practice guideline on chronic kidney disease-mineral and bone disorder: a commentary from a Kidney Disease: Improving Global Outcomes controversies conference. Kidney Int. 2015;87(3):502-528.


