User login
Fibroids: Is surgery the only management approach?
Two chronic gynecologic conditions notably affect a woman’s quality of life (QoL), including fertility – one is endometriosis, and the other is a fibroid uterus. For a benign tumor, fibroids have an impressive prevalence found in approximately 50%-60% of women during their reproductive years. By menopause, it is estimated that 70% of woman have a fibroid, yet the true incidence is unknown given that only 25% of women experience symptoms bothersome enough to warrant intervention. This month’s article reviews the burden of fibroids and the latest management options that may potentially avoid surgery.
Background
Fibroids are monoclonal tumors of uterine smooth muscle that originate from the myometrium. Risk factors include family history, being premenopausal, increasing time since last delivery, obesity, and hypertension (ACOG Practice Bulletin no. 228 Jun 2021: Obstet Gynecol. 2021 Jun 1;137[6]:e100-e15) but oral hormonal contraception, depot medroxyprogesterone acetate (MPA), and increased parity reduce the risk of fibroids. Compared with White women, Black women have a 2-3 times higher prevalence of fibroids, develop them at a younger age, and present with larger fibroids.
The FIGO leiomyoma classification is the agreed upon system for identifying fibroid location. Symptoms are all too familiar to gynecologists, with life-threatening hemorrhage with severe anemia being the most feared, particularly for FIGO types 1-5. Transvaginal ultrasound is the simplest imaging tool for evaluation.

Fibroids and fertility
Fibroids can impair fertility in several ways: alteration of local anatomy, including the detrimental effects of abnormal uterine bleeding; functional changes by increasing uterine contractions and impairing endometrium and myometrial blood supply; and changes to the local hormonal environment that could impair egg/sperm transport, or embryo implantation (Hum Reprod Update. 2017;22:665-86).
Prior to consideration of surgery, saline infusion sonogram can determine the degree of impact on the endometrium, which is most applicable to the infertility patient, but can also allow guidance toward the appropriate surgical approach.
Treatment options – medical
Management of fibroids is based on a woman’s age, desire for fertility, symptoms, and location of the fibroid(s). Expectant observation of a woman with fibroids may be a reasonable approach, provided the lack of symptoms impairing QoL and of anemia. Typically, there is no change in fibroid size during the short term, considered less than 1 year. Regarding fertility, studies are heterogeneous so there is no definitive conclusion that fibroids impair natural fertility (Reprod Biomed Online. 2021;43:100-10). Spontaneous regression, defined by a reduction in fibroid volume of greater than 20%, has been noted to occur in 7.0% of fibroids (Curr Obstet Gynecol Rep. 2018;7[3]:117-21).
When fertility is not desired, medical management of fibroids is the initial conservative approach. GnRH agonists have been utilized for temporary relief of menometrorrhagia because of fibroids and to reduce their volume, particularly preoperatively. However, extended treatment can induce bone mineral density loss. Add-back therapy (tibolone, raloxifene, estriol, and ipriflavone) is of value in reducing bone loss while MPA and tibolone may manage vasomotor symptoms. More recently, the use of a GnRH antagonist (elagolix) along with add-back therapy has been approved for up to 24 months by the Food and Drug Administration and has demonstrated a more than 50% amenorrhea rate at 12 months (Obstet Gynecol. 2020;135:1313-26).
Progesterone plays an important role in fibroid growth, but the mechanism is unclear. Although not FDA approved, selective progesterone receptor modulators (SPRM) act directly on fibroid size reduction at the level of the pituitary to induce amenorrhea through inhibition of ovulation. Also, more than one course of SPRMs can provide benefit for bleeding control and volume reduction. The SPRM ulipristal acetate for four courses of 3 months demonstrated 73.5% of patients experienced a fibroid volume reduction of greater than 25% and were amenorrheic (Fertil Steril. 2017;108:416-25). GnRH agonists or SPRMs may benefit women if the fibroid is larger than 3 cm or anemia exists, thereby precluding immediate surgery.
Other medication options include the levonorgestrel IUD, combined hormonal contraceptives, and tranexamic acid – all of which have limited data on effective results of treating abnormal uterine bleeding.
Treatment options – surgical
Fibroids are the most common reason for hysterectomy as they are the contributing indication in approximately one-third of surgeries. When future fertility is desired, current surgical options include hysteroscopic and laparoscopic (including robotic) myomectomy. Hysteroscopy is the standard approach for FIGO type 1 fibroids and can also manage some type 2 fibroids provided they are less than 3 cm and the latter is greater than 5 mm from the serosa. Type 2 fibroids may benefit from a “two-step” removal to allow the myometrium to contract and extrude the fibroid. In light of the risk of fluid overload with nonelectrolyte solutions that enable the use of monopolar cautery, many procedures are now performed with bipolar cautery or morcellators.
Laparoscopy (including robotic) has outcomes similar to those of laparotomy although the risk of uterine rupture with the former requires careful attention to thorough closure of the myometrial defect. Robotic myomectomy has outcomes similar to those of standard laparoscopy with less blood loss, but operating times may be prolonged (Best Pract Res Clin Obstet Gynaecol. 2018;46:113-9).
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women (Fertil Steril 2017;108;416-25). The rate of recurrence after myomectomy can be as great as 60% when patients are followed up to 5 years. Intramural fibroids greater than 2.85 cm and not distorting the uterine cavity may decrease in vitro fertilization (IVF) success (Fertil Steril 2014;101:716-21).
Noninvasive treatment modalities
Uterine artery embolization (UAE) is the most popular minimally invasive alternative to surgical myomectomy. Risks include postembolization syndrome (pain, fever, nausea, leukocytosis, and occasionally malaise), infection, and damage to fertility. Rarely, loss of ovarian function can occur, particularly in women above age 45. Because of the disruption of uterine blood flow, UAE increases the risk of accelerating ovarian aging and infertility as well as atrophic endometrium. In addition, pregnancy complications are increased including miscarriage, preterm labor, and postpartum hemorrhage. There is debate regarding the need for cesarean section at time of delivery given the potential for weakening of the uterine wall following UAE.
High-intensity focused ultrasound (HIFU) is guided by ultrasound or MRI and involves a high-energy-density ultrasound wave passing through the skin. The wave is absorbed and transformed into heat, causing the tissue protein to coagulate, and to be absorbed by the body. The procedure is scarless, carries a minimal risk of infection, and offers less pain compared with traditional approaches. However, HIFU is time consuming, and skin burns and unintentional tissue injury are a risk. A meta-analysis demonstrated improved symptoms of fibroids at 6 and 12 months (J Min Invasive Gynecol. 2021 in press).
Ultrasound-guided microwave ablation (MWA) uses an ablative electrode that is directly inserted into the target tissue via transcutaneous or transcervical approach via ultrasound guidance using microwave to produce heat for tissue coagulation necrosis. The advantages of MWA compared with HIFU and RFA are a higher tissue temperature, larger ablation volume, shorter operating time, less pain and no adverse major events (J Min Invasive Gynecol. 2021, in press).
Conclusion
The current literature cannot conclude that fibroids reduce the likelihood of achieving pregnancy with or without fertility treatment, based on a specific size, number, or location (not including submucosal or cavity-distorting intramural fibroids). Definitive evidence on the efficacy of myomectomy to improve fertility remains limited. Hysteroscopic myomectomy presumably improves pregnancy rates, but there is uncertainty as to its role in reducing miscarriage. Novel nonsurgical modalities are available and are expected to continue being developed but clarity on fertility outcomes is needed.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts of interests. Please contact him at [email protected].
Two chronic gynecologic conditions notably affect a woman’s quality of life (QoL), including fertility – one is endometriosis, and the other is a fibroid uterus. For a benign tumor, fibroids have an impressive prevalence found in approximately 50%-60% of women during their reproductive years. By menopause, it is estimated that 70% of woman have a fibroid, yet the true incidence is unknown given that only 25% of women experience symptoms bothersome enough to warrant intervention. This month’s article reviews the burden of fibroids and the latest management options that may potentially avoid surgery.
Background
Fibroids are monoclonal tumors of uterine smooth muscle that originate from the myometrium. Risk factors include family history, being premenopausal, increasing time since last delivery, obesity, and hypertension (ACOG Practice Bulletin no. 228 Jun 2021: Obstet Gynecol. 2021 Jun 1;137[6]:e100-e15) but oral hormonal contraception, depot medroxyprogesterone acetate (MPA), and increased parity reduce the risk of fibroids. Compared with White women, Black women have a 2-3 times higher prevalence of fibroids, develop them at a younger age, and present with larger fibroids.
The FIGO leiomyoma classification is the agreed upon system for identifying fibroid location. Symptoms are all too familiar to gynecologists, with life-threatening hemorrhage with severe anemia being the most feared, particularly for FIGO types 1-5. Transvaginal ultrasound is the simplest imaging tool for evaluation.

Fibroids and fertility
Fibroids can impair fertility in several ways: alteration of local anatomy, including the detrimental effects of abnormal uterine bleeding; functional changes by increasing uterine contractions and impairing endometrium and myometrial blood supply; and changes to the local hormonal environment that could impair egg/sperm transport, or embryo implantation (Hum Reprod Update. 2017;22:665-86).
Prior to consideration of surgery, saline infusion sonogram can determine the degree of impact on the endometrium, which is most applicable to the infertility patient, but can also allow guidance toward the appropriate surgical approach.
Treatment options – medical
Management of fibroids is based on a woman’s age, desire for fertility, symptoms, and location of the fibroid(s). Expectant observation of a woman with fibroids may be a reasonable approach, provided the lack of symptoms impairing QoL and of anemia. Typically, there is no change in fibroid size during the short term, considered less than 1 year. Regarding fertility, studies are heterogeneous so there is no definitive conclusion that fibroids impair natural fertility (Reprod Biomed Online. 2021;43:100-10). Spontaneous regression, defined by a reduction in fibroid volume of greater than 20%, has been noted to occur in 7.0% of fibroids (Curr Obstet Gynecol Rep. 2018;7[3]:117-21).
When fertility is not desired, medical management of fibroids is the initial conservative approach. GnRH agonists have been utilized for temporary relief of menometrorrhagia because of fibroids and to reduce their volume, particularly preoperatively. However, extended treatment can induce bone mineral density loss. Add-back therapy (tibolone, raloxifene, estriol, and ipriflavone) is of value in reducing bone loss while MPA and tibolone may manage vasomotor symptoms. More recently, the use of a GnRH antagonist (elagolix) along with add-back therapy has been approved for up to 24 months by the Food and Drug Administration and has demonstrated a more than 50% amenorrhea rate at 12 months (Obstet Gynecol. 2020;135:1313-26).
Progesterone plays an important role in fibroid growth, but the mechanism is unclear. Although not FDA approved, selective progesterone receptor modulators (SPRM) act directly on fibroid size reduction at the level of the pituitary to induce amenorrhea through inhibition of ovulation. Also, more than one course of SPRMs can provide benefit for bleeding control and volume reduction. The SPRM ulipristal acetate for four courses of 3 months demonstrated 73.5% of patients experienced a fibroid volume reduction of greater than 25% and were amenorrheic (Fertil Steril. 2017;108:416-25). GnRH agonists or SPRMs may benefit women if the fibroid is larger than 3 cm or anemia exists, thereby precluding immediate surgery.
Other medication options include the levonorgestrel IUD, combined hormonal contraceptives, and tranexamic acid – all of which have limited data on effective results of treating abnormal uterine bleeding.
Treatment options – surgical
Fibroids are the most common reason for hysterectomy as they are the contributing indication in approximately one-third of surgeries. When future fertility is desired, current surgical options include hysteroscopic and laparoscopic (including robotic) myomectomy. Hysteroscopy is the standard approach for FIGO type 1 fibroids and can also manage some type 2 fibroids provided they are less than 3 cm and the latter is greater than 5 mm from the serosa. Type 2 fibroids may benefit from a “two-step” removal to allow the myometrium to contract and extrude the fibroid. In light of the risk of fluid overload with nonelectrolyte solutions that enable the use of monopolar cautery, many procedures are now performed with bipolar cautery or morcellators.
Laparoscopy (including robotic) has outcomes similar to those of laparotomy although the risk of uterine rupture with the former requires careful attention to thorough closure of the myometrial defect. Robotic myomectomy has outcomes similar to those of standard laparoscopy with less blood loss, but operating times may be prolonged (Best Pract Res Clin Obstet Gynaecol. 2018;46:113-9).
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women (Fertil Steril 2017;108;416-25). The rate of recurrence after myomectomy can be as great as 60% when patients are followed up to 5 years. Intramural fibroids greater than 2.85 cm and not distorting the uterine cavity may decrease in vitro fertilization (IVF) success (Fertil Steril 2014;101:716-21).
Noninvasive treatment modalities
Uterine artery embolization (UAE) is the most popular minimally invasive alternative to surgical myomectomy. Risks include postembolization syndrome (pain, fever, nausea, leukocytosis, and occasionally malaise), infection, and damage to fertility. Rarely, loss of ovarian function can occur, particularly in women above age 45. Because of the disruption of uterine blood flow, UAE increases the risk of accelerating ovarian aging and infertility as well as atrophic endometrium. In addition, pregnancy complications are increased including miscarriage, preterm labor, and postpartum hemorrhage. There is debate regarding the need for cesarean section at time of delivery given the potential for weakening of the uterine wall following UAE.
High-intensity focused ultrasound (HIFU) is guided by ultrasound or MRI and involves a high-energy-density ultrasound wave passing through the skin. The wave is absorbed and transformed into heat, causing the tissue protein to coagulate, and to be absorbed by the body. The procedure is scarless, carries a minimal risk of infection, and offers less pain compared with traditional approaches. However, HIFU is time consuming, and skin burns and unintentional tissue injury are a risk. A meta-analysis demonstrated improved symptoms of fibroids at 6 and 12 months (J Min Invasive Gynecol. 2021 in press).
Ultrasound-guided microwave ablation (MWA) uses an ablative electrode that is directly inserted into the target tissue via transcutaneous or transcervical approach via ultrasound guidance using microwave to produce heat for tissue coagulation necrosis. The advantages of MWA compared with HIFU and RFA are a higher tissue temperature, larger ablation volume, shorter operating time, less pain and no adverse major events (J Min Invasive Gynecol. 2021, in press).
Conclusion
The current literature cannot conclude that fibroids reduce the likelihood of achieving pregnancy with or without fertility treatment, based on a specific size, number, or location (not including submucosal or cavity-distorting intramural fibroids). Definitive evidence on the efficacy of myomectomy to improve fertility remains limited. Hysteroscopic myomectomy presumably improves pregnancy rates, but there is uncertainty as to its role in reducing miscarriage. Novel nonsurgical modalities are available and are expected to continue being developed but clarity on fertility outcomes is needed.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts of interests. Please contact him at [email protected].
Two chronic gynecologic conditions notably affect a woman’s quality of life (QoL), including fertility – one is endometriosis, and the other is a fibroid uterus. For a benign tumor, fibroids have an impressive prevalence found in approximately 50%-60% of women during their reproductive years. By menopause, it is estimated that 70% of woman have a fibroid, yet the true incidence is unknown given that only 25% of women experience symptoms bothersome enough to warrant intervention. This month’s article reviews the burden of fibroids and the latest management options that may potentially avoid surgery.
Background
Fibroids are monoclonal tumors of uterine smooth muscle that originate from the myometrium. Risk factors include family history, being premenopausal, increasing time since last delivery, obesity, and hypertension (ACOG Practice Bulletin no. 228 Jun 2021: Obstet Gynecol. 2021 Jun 1;137[6]:e100-e15) but oral hormonal contraception, depot medroxyprogesterone acetate (MPA), and increased parity reduce the risk of fibroids. Compared with White women, Black women have a 2-3 times higher prevalence of fibroids, develop them at a younger age, and present with larger fibroids.
The FIGO leiomyoma classification is the agreed upon system for identifying fibroid location. Symptoms are all too familiar to gynecologists, with life-threatening hemorrhage with severe anemia being the most feared, particularly for FIGO types 1-5. Transvaginal ultrasound is the simplest imaging tool for evaluation.

Fibroids and fertility
Fibroids can impair fertility in several ways: alteration of local anatomy, including the detrimental effects of abnormal uterine bleeding; functional changes by increasing uterine contractions and impairing endometrium and myometrial blood supply; and changes to the local hormonal environment that could impair egg/sperm transport, or embryo implantation (Hum Reprod Update. 2017;22:665-86).
Prior to consideration of surgery, saline infusion sonogram can determine the degree of impact on the endometrium, which is most applicable to the infertility patient, but can also allow guidance toward the appropriate surgical approach.
Treatment options – medical
Management of fibroids is based on a woman’s age, desire for fertility, symptoms, and location of the fibroid(s). Expectant observation of a woman with fibroids may be a reasonable approach, provided the lack of symptoms impairing QoL and of anemia. Typically, there is no change in fibroid size during the short term, considered less than 1 year. Regarding fertility, studies are heterogeneous so there is no definitive conclusion that fibroids impair natural fertility (Reprod Biomed Online. 2021;43:100-10). Spontaneous regression, defined by a reduction in fibroid volume of greater than 20%, has been noted to occur in 7.0% of fibroids (Curr Obstet Gynecol Rep. 2018;7[3]:117-21).
When fertility is not desired, medical management of fibroids is the initial conservative approach. GnRH agonists have been utilized for temporary relief of menometrorrhagia because of fibroids and to reduce their volume, particularly preoperatively. However, extended treatment can induce bone mineral density loss. Add-back therapy (tibolone, raloxifene, estriol, and ipriflavone) is of value in reducing bone loss while MPA and tibolone may manage vasomotor symptoms. More recently, the use of a GnRH antagonist (elagolix) along with add-back therapy has been approved for up to 24 months by the Food and Drug Administration and has demonstrated a more than 50% amenorrhea rate at 12 months (Obstet Gynecol. 2020;135:1313-26).
Progesterone plays an important role in fibroid growth, but the mechanism is unclear. Although not FDA approved, selective progesterone receptor modulators (SPRM) act directly on fibroid size reduction at the level of the pituitary to induce amenorrhea through inhibition of ovulation. Also, more than one course of SPRMs can provide benefit for bleeding control and volume reduction. The SPRM ulipristal acetate for four courses of 3 months demonstrated 73.5% of patients experienced a fibroid volume reduction of greater than 25% and were amenorrheic (Fertil Steril. 2017;108:416-25). GnRH agonists or SPRMs may benefit women if the fibroid is larger than 3 cm or anemia exists, thereby precluding immediate surgery.
Other medication options include the levonorgestrel IUD, combined hormonal contraceptives, and tranexamic acid – all of which have limited data on effective results of treating abnormal uterine bleeding.
Treatment options – surgical
Fibroids are the most common reason for hysterectomy as they are the contributing indication in approximately one-third of surgeries. When future fertility is desired, current surgical options include hysteroscopic and laparoscopic (including robotic) myomectomy. Hysteroscopy is the standard approach for FIGO type 1 fibroids and can also manage some type 2 fibroids provided they are less than 3 cm and the latter is greater than 5 mm from the serosa. Type 2 fibroids may benefit from a “two-step” removal to allow the myometrium to contract and extrude the fibroid. In light of the risk of fluid overload with nonelectrolyte solutions that enable the use of monopolar cautery, many procedures are now performed with bipolar cautery or morcellators.
Laparoscopy (including robotic) has outcomes similar to those of laparotomy although the risk of uterine rupture with the former requires careful attention to thorough closure of the myometrial defect. Robotic myomectomy has outcomes similar to those of standard laparoscopy with less blood loss, but operating times may be prolonged (Best Pract Res Clin Obstet Gynaecol. 2018;46:113-9).
The rate of myomectomy is reported to be 9.2 per 10,000 woman-years in Black women and 1.3 per 10,000 woman years in White women (Fertil Steril 2017;108;416-25). The rate of recurrence after myomectomy can be as great as 60% when patients are followed up to 5 years. Intramural fibroids greater than 2.85 cm and not distorting the uterine cavity may decrease in vitro fertilization (IVF) success (Fertil Steril 2014;101:716-21).
Noninvasive treatment modalities
Uterine artery embolization (UAE) is the most popular minimally invasive alternative to surgical myomectomy. Risks include postembolization syndrome (pain, fever, nausea, leukocytosis, and occasionally malaise), infection, and damage to fertility. Rarely, loss of ovarian function can occur, particularly in women above age 45. Because of the disruption of uterine blood flow, UAE increases the risk of accelerating ovarian aging and infertility as well as atrophic endometrium. In addition, pregnancy complications are increased including miscarriage, preterm labor, and postpartum hemorrhage. There is debate regarding the need for cesarean section at time of delivery given the potential for weakening of the uterine wall following UAE.
High-intensity focused ultrasound (HIFU) is guided by ultrasound or MRI and involves a high-energy-density ultrasound wave passing through the skin. The wave is absorbed and transformed into heat, causing the tissue protein to coagulate, and to be absorbed by the body. The procedure is scarless, carries a minimal risk of infection, and offers less pain compared with traditional approaches. However, HIFU is time consuming, and skin burns and unintentional tissue injury are a risk. A meta-analysis demonstrated improved symptoms of fibroids at 6 and 12 months (J Min Invasive Gynecol. 2021 in press).
Ultrasound-guided microwave ablation (MWA) uses an ablative electrode that is directly inserted into the target tissue via transcutaneous or transcervical approach via ultrasound guidance using microwave to produce heat for tissue coagulation necrosis. The advantages of MWA compared with HIFU and RFA are a higher tissue temperature, larger ablation volume, shorter operating time, less pain and no adverse major events (J Min Invasive Gynecol. 2021, in press).
Conclusion
The current literature cannot conclude that fibroids reduce the likelihood of achieving pregnancy with or without fertility treatment, based on a specific size, number, or location (not including submucosal or cavity-distorting intramural fibroids). Definitive evidence on the efficacy of myomectomy to improve fertility remains limited. Hysteroscopic myomectomy presumably improves pregnancy rates, but there is uncertainty as to its role in reducing miscarriage. Novel nonsurgical modalities are available and are expected to continue being developed but clarity on fertility outcomes is needed.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts of interests. Please contact him at [email protected].
Polycystic ovary syndrome: It’s not just about fertility
Polycystic ovary syndrome, the most common endocrinopathy and most common cause of female infertility, affects 8%-13% of reproductive-aged women. PCOS has a profound impact on a woman’s life yet its diagnosis and management remain confusing despite being first described nearly a century ago by Stein and Leventhal.
To illustrate, in a global survey of 1,385 women with PCOS, one-third or more reported a delay of greater than 2 years and nearly half required evaluation by at least three health professionals before a diagnosis was established (J Clin Endocrinol Metab. 2017;102[2]:604-12). A vital health problem that urgently requires a gap analysis and needs assessment, PCOS is not “just about fertility” but has extensive gynecologic and metabolic consequences that require a personalized approach to care coordinated among the fields of internal medicine, pediatrics, dermatology, and, of course, gynecology.
Diagnosis in adults and adolescence
Normal menstrual intervals do not always equate with ovulation. Up to 40% of hirsute women with monthly cycles may not ovulate regularly. The Rotterdam criteria are used to confirm PCOS and require two of the following three: 1) ovulation dysfunction (cycle interval > 35 d or < 8 cycles/year); 2) hyperandrogenism (i.e., elevated total or free testosterone, DHEAS, or signs of hirsutism or acne with Ferriman-Gallwey score greater than 6); 3) polycystic ovaries on ultrasound (20 or more 2- to 9-mm follicles on at least one ovary, and/or increased ovarian volume (> 10 mL) – all at the exclusion of other etiologies including hyperprolactinemia, thyroid dysfunction, androgen-secreting tumors including Cushing’s syndrome, and nonclassic adrenal hyperplasia mostly easily screened by obtaining 17-hydroxyprogesterone.
For adolescents, by age 14 most will have adult androgen levels. Ovarian ultrasound should not be used as a criterion in this age group given the frequency of this appearance. Due to frequent menstrual irregularity, it is recommended to wait at least 2 years post menarche before consideration of a diagnosis.
Antimüllerian hormone is two- to threefold higher in women with PCOS but this hormone level has not yet been accepted as a diagnostic criterion.
The metabolic connection
A multisystem disorder whose name misdirects its morbidity, PCOS affects the metabolic, reproductive, and psychological system through vicious cycles of distorted feedback signals. Without a consensus of its origin, there appears to be a hypersensitivity of pituitary luteinizing hormone (LH) to hypothalamic gonadotrophin-releasing hormone. Consequently, elevated LH stimulates ovarian theca cells to increase androgens with resultant hyperandrogenic consequences. Parenthetically, the tonic elevation in LH explains the false-positive surges PCOS women experience when testing their urine during ovulation induction.
Elevations in insulin from unexplained damage to the insulin receptor acts synergistically with LH to increase ovarian androgens and inhibit ovulation. Hyperinsulinemia and abdominal fat deposition contribute to impaired glucose tolerance which is threefold higher with PCOS.
The metabolic syndrome, an association of disorders including hypertension, impaired glucose tolerance, dyslipidemia, and obesity, occurs at an increased overall prevalence rate of 43%-47% in women with PCOS, which is twice as high as in women without PCOS. PCOS is associated with low-grade chronic inflammation, which places these women at increased risk of nonalcoholic fatty liver disease. Dyslipidemia is the most common metabolic disorder in PCOS. These metabolic consequences, including obstructive sleep apnea, are worsened by hyperandrogenemia and an elevated BMI.
A genetic link
Multigenetic in origin, PCOS has a fivefold higher risk of inheritance from mothers with PCOS to daughters influenced by prenatal androgen exposure in utero. Genetic studies suggest a causal relationship between PCOS with body mass index, insulin resistance, onset of menopause, depression, and male-pattern balding (PLoS Genet 2018;14[12]:e10007813).
Fifteen genetic risk areas in the human genome seem to predispose to PCOS. New results suggest that altering the gut microbiome via prebiotic or probiotic therapies may be a potential treatment option.
Reproductive and gynecologic management
Due to chronic anovulation, unopposed estrogen can result in abnormal endometrial bleeding, endometrial hyperplasia, and a fourfold risk of endometrial cancer. This underscores the importance of regular progestin withdrawal, combined oral contraception (COC), or a progestin intrauterine device.
PCOS is a leading cause of infertility and is associated with abnormal bleeding, miscarriage, gestational diabetes, and gestational hypertension, all of which are higher based on a hyperandrogenic phenotype.
The rate of infertility in women with PCOS is 70%-80%, with ovulation dysfunction being the dominant cause. For years, the mainstay for ovulation induction was clomiphene citrate; however, letrozole has shown higher pregnancy success rates, particularly in women who have a BMI greater than 30 kg/m2. (N Engl J Med. 2014;371:119-29). Despite multiple studies demonstrating its efficacy and safety, letrozole remains without Food and Drug Administration approval for ovulation induction.
Metformin has been recommended in women with prediabetes or a BMI above 30, and it may improve menstrual regularity but has not been shown to improve live birth rates nor reduce the pregnancy complications of miscarriage or gestational diabetes. Inositol, the ubiquitous endogenous carbohydrate, has not demonstrated clear improvement in reproduction.
Laparoscopic ovarian diathermy (LOD) is a second-line treatment option, as is the use of gonadotropins, to overcome unsuccessful conservative attempts at ovulation induction. LOD is more invasive but outcomes are equivalent to gonadotropin usage while providing a dramatic reduction in multiple gestation, ovarian hyperstimulation syndrome, and cost (not including the surgical procedure). Ultimately, in vitro fertilization is an option for continued infertility in women with PCOS.
Metabolic/gynecologic management
Given the multisystem effect of PCOS, health care providers caring for these women should be vigilant and aggressive at ensuring appropriate monitoring and management. For women with PCOS with an elevated BMI, lifestyle modification is the first line of management. Weight loss alone of only 2%-5% may restore ovulation function.
The combination of dyslipidemia, elevated BMI, and impaired glucose tolerance would presumably predict the risk of cardiovascular events, yet the impact is not proven. Despite an increase in carotid intima media thickness, there are data that suggest only an increase in stroke or myocardial infarction (J Clin Endocrinol Metab. 2019;104[4]:1221-31).
Hyperandrogenism is cosmetically and psychologically disrupting to PCOS patients. The topical application of eflornithine hydrochloride may be of value for mild to moderate facial hair growth. Spironolactone is the preferred first-line agent. (Caution: effective contraception is necessary to avoid feminization of a male fetus). Women with PCOS have a higher risk of disordered eating and body image distress as well as a fivefold higher rate of mental distress such as anxiety and depression.
No specific diet has been determined as part of treatment, yet healthy food selection and caloric intake combined with exercise has been shown to improve metabolic and psychological well-being.
Conclusion
PCOS is a ubiquitous, frustrating, and life-altering disease. Health care providers, particularly those in women’s health, must ensure appropriate counseling and education with evidence-based medicine to empower patients toward improved health.
Dr. Trolice is director of Fertility CARE - The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts of interest. Please contact him at [email protected].
Polycystic ovary syndrome, the most common endocrinopathy and most common cause of female infertility, affects 8%-13% of reproductive-aged women. PCOS has a profound impact on a woman’s life yet its diagnosis and management remain confusing despite being first described nearly a century ago by Stein and Leventhal.
To illustrate, in a global survey of 1,385 women with PCOS, one-third or more reported a delay of greater than 2 years and nearly half required evaluation by at least three health professionals before a diagnosis was established (J Clin Endocrinol Metab. 2017;102[2]:604-12). A vital health problem that urgently requires a gap analysis and needs assessment, PCOS is not “just about fertility” but has extensive gynecologic and metabolic consequences that require a personalized approach to care coordinated among the fields of internal medicine, pediatrics, dermatology, and, of course, gynecology.
Diagnosis in adults and adolescence
Normal menstrual intervals do not always equate with ovulation. Up to 40% of hirsute women with monthly cycles may not ovulate regularly. The Rotterdam criteria are used to confirm PCOS and require two of the following three: 1) ovulation dysfunction (cycle interval > 35 d or < 8 cycles/year); 2) hyperandrogenism (i.e., elevated total or free testosterone, DHEAS, or signs of hirsutism or acne with Ferriman-Gallwey score greater than 6); 3) polycystic ovaries on ultrasound (20 or more 2- to 9-mm follicles on at least one ovary, and/or increased ovarian volume (> 10 mL) – all at the exclusion of other etiologies including hyperprolactinemia, thyroid dysfunction, androgen-secreting tumors including Cushing’s syndrome, and nonclassic adrenal hyperplasia mostly easily screened by obtaining 17-hydroxyprogesterone.
For adolescents, by age 14 most will have adult androgen levels. Ovarian ultrasound should not be used as a criterion in this age group given the frequency of this appearance. Due to frequent menstrual irregularity, it is recommended to wait at least 2 years post menarche before consideration of a diagnosis.
Antimüllerian hormone is two- to threefold higher in women with PCOS but this hormone level has not yet been accepted as a diagnostic criterion.
The metabolic connection
A multisystem disorder whose name misdirects its morbidity, PCOS affects the metabolic, reproductive, and psychological system through vicious cycles of distorted feedback signals. Without a consensus of its origin, there appears to be a hypersensitivity of pituitary luteinizing hormone (LH) to hypothalamic gonadotrophin-releasing hormone. Consequently, elevated LH stimulates ovarian theca cells to increase androgens with resultant hyperandrogenic consequences. Parenthetically, the tonic elevation in LH explains the false-positive surges PCOS women experience when testing their urine during ovulation induction.
Elevations in insulin from unexplained damage to the insulin receptor acts synergistically with LH to increase ovarian androgens and inhibit ovulation. Hyperinsulinemia and abdominal fat deposition contribute to impaired glucose tolerance which is threefold higher with PCOS.
The metabolic syndrome, an association of disorders including hypertension, impaired glucose tolerance, dyslipidemia, and obesity, occurs at an increased overall prevalence rate of 43%-47% in women with PCOS, which is twice as high as in women without PCOS. PCOS is associated with low-grade chronic inflammation, which places these women at increased risk of nonalcoholic fatty liver disease. Dyslipidemia is the most common metabolic disorder in PCOS. These metabolic consequences, including obstructive sleep apnea, are worsened by hyperandrogenemia and an elevated BMI.
A genetic link
Multigenetic in origin, PCOS has a fivefold higher risk of inheritance from mothers with PCOS to daughters influenced by prenatal androgen exposure in utero. Genetic studies suggest a causal relationship between PCOS with body mass index, insulin resistance, onset of menopause, depression, and male-pattern balding (PLoS Genet 2018;14[12]:e10007813).
Fifteen genetic risk areas in the human genome seem to predispose to PCOS. New results suggest that altering the gut microbiome via prebiotic or probiotic therapies may be a potential treatment option.
Reproductive and gynecologic management
Due to chronic anovulation, unopposed estrogen can result in abnormal endometrial bleeding, endometrial hyperplasia, and a fourfold risk of endometrial cancer. This underscores the importance of regular progestin withdrawal, combined oral contraception (COC), or a progestin intrauterine device.
PCOS is a leading cause of infertility and is associated with abnormal bleeding, miscarriage, gestational diabetes, and gestational hypertension, all of which are higher based on a hyperandrogenic phenotype.
The rate of infertility in women with PCOS is 70%-80%, with ovulation dysfunction being the dominant cause. For years, the mainstay for ovulation induction was clomiphene citrate; however, letrozole has shown higher pregnancy success rates, particularly in women who have a BMI greater than 30 kg/m2. (N Engl J Med. 2014;371:119-29). Despite multiple studies demonstrating its efficacy and safety, letrozole remains without Food and Drug Administration approval for ovulation induction.
Metformin has been recommended in women with prediabetes or a BMI above 30, and it may improve menstrual regularity but has not been shown to improve live birth rates nor reduce the pregnancy complications of miscarriage or gestational diabetes. Inositol, the ubiquitous endogenous carbohydrate, has not demonstrated clear improvement in reproduction.
Laparoscopic ovarian diathermy (LOD) is a second-line treatment option, as is the use of gonadotropins, to overcome unsuccessful conservative attempts at ovulation induction. LOD is more invasive but outcomes are equivalent to gonadotropin usage while providing a dramatic reduction in multiple gestation, ovarian hyperstimulation syndrome, and cost (not including the surgical procedure). Ultimately, in vitro fertilization is an option for continued infertility in women with PCOS.
Metabolic/gynecologic management
Given the multisystem effect of PCOS, health care providers caring for these women should be vigilant and aggressive at ensuring appropriate monitoring and management. For women with PCOS with an elevated BMI, lifestyle modification is the first line of management. Weight loss alone of only 2%-5% may restore ovulation function.
The combination of dyslipidemia, elevated BMI, and impaired glucose tolerance would presumably predict the risk of cardiovascular events, yet the impact is not proven. Despite an increase in carotid intima media thickness, there are data that suggest only an increase in stroke or myocardial infarction (J Clin Endocrinol Metab. 2019;104[4]:1221-31).
Hyperandrogenism is cosmetically and psychologically disrupting to PCOS patients. The topical application of eflornithine hydrochloride may be of value for mild to moderate facial hair growth. Spironolactone is the preferred first-line agent. (Caution: effective contraception is necessary to avoid feminization of a male fetus). Women with PCOS have a higher risk of disordered eating and body image distress as well as a fivefold higher rate of mental distress such as anxiety and depression.
No specific diet has been determined as part of treatment, yet healthy food selection and caloric intake combined with exercise has been shown to improve metabolic and psychological well-being.
Conclusion
PCOS is a ubiquitous, frustrating, and life-altering disease. Health care providers, particularly those in women’s health, must ensure appropriate counseling and education with evidence-based medicine to empower patients toward improved health.
Dr. Trolice is director of Fertility CARE - The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts of interest. Please contact him at [email protected].
Polycystic ovary syndrome, the most common endocrinopathy and most common cause of female infertility, affects 8%-13% of reproductive-aged women. PCOS has a profound impact on a woman’s life yet its diagnosis and management remain confusing despite being first described nearly a century ago by Stein and Leventhal.
To illustrate, in a global survey of 1,385 women with PCOS, one-third or more reported a delay of greater than 2 years and nearly half required evaluation by at least three health professionals before a diagnosis was established (J Clin Endocrinol Metab. 2017;102[2]:604-12). A vital health problem that urgently requires a gap analysis and needs assessment, PCOS is not “just about fertility” but has extensive gynecologic and metabolic consequences that require a personalized approach to care coordinated among the fields of internal medicine, pediatrics, dermatology, and, of course, gynecology.
Diagnosis in adults and adolescence
Normal menstrual intervals do not always equate with ovulation. Up to 40% of hirsute women with monthly cycles may not ovulate regularly. The Rotterdam criteria are used to confirm PCOS and require two of the following three: 1) ovulation dysfunction (cycle interval > 35 d or < 8 cycles/year); 2) hyperandrogenism (i.e., elevated total or free testosterone, DHEAS, or signs of hirsutism or acne with Ferriman-Gallwey score greater than 6); 3) polycystic ovaries on ultrasound (20 or more 2- to 9-mm follicles on at least one ovary, and/or increased ovarian volume (> 10 mL) – all at the exclusion of other etiologies including hyperprolactinemia, thyroid dysfunction, androgen-secreting tumors including Cushing’s syndrome, and nonclassic adrenal hyperplasia mostly easily screened by obtaining 17-hydroxyprogesterone.
For adolescents, by age 14 most will have adult androgen levels. Ovarian ultrasound should not be used as a criterion in this age group given the frequency of this appearance. Due to frequent menstrual irregularity, it is recommended to wait at least 2 years post menarche before consideration of a diagnosis.
Antimüllerian hormone is two- to threefold higher in women with PCOS but this hormone level has not yet been accepted as a diagnostic criterion.
The metabolic connection
A multisystem disorder whose name misdirects its morbidity, PCOS affects the metabolic, reproductive, and psychological system through vicious cycles of distorted feedback signals. Without a consensus of its origin, there appears to be a hypersensitivity of pituitary luteinizing hormone (LH) to hypothalamic gonadotrophin-releasing hormone. Consequently, elevated LH stimulates ovarian theca cells to increase androgens with resultant hyperandrogenic consequences. Parenthetically, the tonic elevation in LH explains the false-positive surges PCOS women experience when testing their urine during ovulation induction.
Elevations in insulin from unexplained damage to the insulin receptor acts synergistically with LH to increase ovarian androgens and inhibit ovulation. Hyperinsulinemia and abdominal fat deposition contribute to impaired glucose tolerance which is threefold higher with PCOS.
The metabolic syndrome, an association of disorders including hypertension, impaired glucose tolerance, dyslipidemia, and obesity, occurs at an increased overall prevalence rate of 43%-47% in women with PCOS, which is twice as high as in women without PCOS. PCOS is associated with low-grade chronic inflammation, which places these women at increased risk of nonalcoholic fatty liver disease. Dyslipidemia is the most common metabolic disorder in PCOS. These metabolic consequences, including obstructive sleep apnea, are worsened by hyperandrogenemia and an elevated BMI.
A genetic link
Multigenetic in origin, PCOS has a fivefold higher risk of inheritance from mothers with PCOS to daughters influenced by prenatal androgen exposure in utero. Genetic studies suggest a causal relationship between PCOS with body mass index, insulin resistance, onset of menopause, depression, and male-pattern balding (PLoS Genet 2018;14[12]:e10007813).
Fifteen genetic risk areas in the human genome seem to predispose to PCOS. New results suggest that altering the gut microbiome via prebiotic or probiotic therapies may be a potential treatment option.
Reproductive and gynecologic management
Due to chronic anovulation, unopposed estrogen can result in abnormal endometrial bleeding, endometrial hyperplasia, and a fourfold risk of endometrial cancer. This underscores the importance of regular progestin withdrawal, combined oral contraception (COC), or a progestin intrauterine device.
PCOS is a leading cause of infertility and is associated with abnormal bleeding, miscarriage, gestational diabetes, and gestational hypertension, all of which are higher based on a hyperandrogenic phenotype.
The rate of infertility in women with PCOS is 70%-80%, with ovulation dysfunction being the dominant cause. For years, the mainstay for ovulation induction was clomiphene citrate; however, letrozole has shown higher pregnancy success rates, particularly in women who have a BMI greater than 30 kg/m2. (N Engl J Med. 2014;371:119-29). Despite multiple studies demonstrating its efficacy and safety, letrozole remains without Food and Drug Administration approval for ovulation induction.
Metformin has been recommended in women with prediabetes or a BMI above 30, and it may improve menstrual regularity but has not been shown to improve live birth rates nor reduce the pregnancy complications of miscarriage or gestational diabetes. Inositol, the ubiquitous endogenous carbohydrate, has not demonstrated clear improvement in reproduction.
Laparoscopic ovarian diathermy (LOD) is a second-line treatment option, as is the use of gonadotropins, to overcome unsuccessful conservative attempts at ovulation induction. LOD is more invasive but outcomes are equivalent to gonadotropin usage while providing a dramatic reduction in multiple gestation, ovarian hyperstimulation syndrome, and cost (not including the surgical procedure). Ultimately, in vitro fertilization is an option for continued infertility in women with PCOS.
Metabolic/gynecologic management
Given the multisystem effect of PCOS, health care providers caring for these women should be vigilant and aggressive at ensuring appropriate monitoring and management. For women with PCOS with an elevated BMI, lifestyle modification is the first line of management. Weight loss alone of only 2%-5% may restore ovulation function.
The combination of dyslipidemia, elevated BMI, and impaired glucose tolerance would presumably predict the risk of cardiovascular events, yet the impact is not proven. Despite an increase in carotid intima media thickness, there are data that suggest only an increase in stroke or myocardial infarction (J Clin Endocrinol Metab. 2019;104[4]:1221-31).
Hyperandrogenism is cosmetically and psychologically disrupting to PCOS patients. The topical application of eflornithine hydrochloride may be of value for mild to moderate facial hair growth. Spironolactone is the preferred first-line agent. (Caution: effective contraception is necessary to avoid feminization of a male fetus). Women with PCOS have a higher risk of disordered eating and body image distress as well as a fivefold higher rate of mental distress such as anxiety and depression.
No specific diet has been determined as part of treatment, yet healthy food selection and caloric intake combined with exercise has been shown to improve metabolic and psychological well-being.
Conclusion
PCOS is a ubiquitous, frustrating, and life-altering disease. Health care providers, particularly those in women’s health, must ensure appropriate counseling and education with evidence-based medicine to empower patients toward improved health.
Dr. Trolice is director of Fertility CARE - The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. He has no conflicts of interest. Please contact him at [email protected].
Preimplantation genetic testing for aneuploidy
Why does the debate linger after 30 years?
The holy grail of assisted reproductive technology (ART) is the delivery of a healthy child. From the world’s first successful ART cycle of in vitro fertilization in 1978 (3 years later in the United States), the goal of every cycle is to provide the woman with an embryo that has the highest potential for implantation and, ultimately, a single live birth.
Embryo aneuploidy is a major factor in the success of human reproduction. As women age, aneuploidy is reported in less than 30% of women aged younger than 35 years but rises to 90% for those in their mid-40s. Intuitively and through randomized, controlled trials, chromosome testing of embryos is a reasonable approach toward improved cycle outcomes and allows for the transfer of a single euploid embryo.
Recently, the phrase “add-ons” has entered the vernacular of editorials on IVF. These additional procedures are offered to patients with the expectation of improving results, yet many have not been supported by rigorous scientifically controlled research trials, e.g., endometrial scratch, embryo glue, and time-lapse imaging of embryos. Where does preimplantation genetic testing (PGT) belong in the IVF armamentarium and why, after 30 years, are there two diametrically opposed views on its benefit? (We will not address testing for single gene defects or chromosome structural rearrangements.)
How did we get here?
The first iteration of PGT used fluorescence in situ hybridization to not only identify X-linked recessive diseases (Hum Genet. 1992;89:18-22) but also the most common chromosome disorders (13, 18, 21, X, Y) by removing one to two blastomere cells from a day 3 embryo (six- to eight-cell stage). Despite wide enthusiasm, the technique was eventually determined to reduce implantation by nearly 40% and was abandoned; presumably impairing the embryo by removing up to one-third of its make-up.
Because of extended embryo culture to the blastocyst stage along with the improved cryopreservation process of vitrification, the next generation of embryo analysis surfaced, what we now refer to as PGT 2.0. Currently, approximately five to six cells from the outer embryo trophectoderm are removed and sent to a specialized laboratory for 24-chromosome screening while the biopsied embryos are cryopreserved. Outcome data (aneuploidy rates, mosaicism) have been influenced by the evolution of genetic platforms – from array comparative genome hybridization to single-nucleotide polymorphism array, to quantitative polymerase chain reaction, to next-generation sequencing (NGS). The newest platform, NGS with high resolution, provides the most extensive degree of analysis by detecting unbalanced translocations and a low cut-off percentage for mosaicism (20%). The clinical error rate is approximately 1%-2%, improved from the 2%-4% of earlier techniques.
The phenomenon of mosaicism describes two distinct cell lines in one embryo (typically one normal and one abnormal) and is defined based on the percentage of mosaicism – currently, the lower limit is 20%. Embryos with less than 20%-30% mosaicism are considered euploid and those greater than 70%-80% are aneuploid. Of note, clinics that do not request the reporting of mosaicism can result in the potential discarding of embryos labeled as aneuploid that would otherwise have potentially resulted in a live birth. The higher the cut-off value for designating mosaicism, the lower the false-positive rate (declaring an embryo aneuploid when euploid). While there is no safe degree of mosaicism, most transfers have resulted in chromosomally normal infants despite a lower implantation rate and higher miscarriage rate.
Current status
The greatest advantage of PGT for aneuploidy (PGT-A) is its increase in promoting a single embryo transfer. Medical evidence supports pregnancy outcomes equivalent from a single euploid embryo transfer versus a double “untested” embryo transfer.
Only a handful of randomized, controlled trials have evaluated the efficacy of PGT-A. Outcomes have favored improved live birth rates; however, criticism exists for enrolling only good prognosis patients given their high likelihood of developing blastocyst embryos to biopsy. The only trial that used an “intention to treat” protocol (rather than randomization at the time of biopsy) did not demonstrate any difference in live birth or miscarriage comparing embryo selection by PGT-A versus embryo morphology alone. However, post hoc analysis did show a benefit with PGT-A in the 35- to 40-year-old age group, not in the less than 35-year-old group. All other trials demonstrated a reduction in miscarriage with PGT-A but only as a secondary outcome.
The medical literature does not support PGT-A to manage patients with recurrent pregnancy loss and there is no evidence for improvement in women aged less than 35 years or egg donors (F&S Reports. 2021;2:36-42). PGT-A has been effective in patients wishing family balancing.
Controversy
Enthusiasm for PGT-A is countered by lingering concerns. Trophectoderm cells are not in 100% concordance with the inner cell mass, which presumably explains the reports of chromosomally normal live births from the transfer of aneuploid embryos. Biopsy techniques among embryologists are not standardized. As a result, damage to the embryo has been raised as a possible explanation for equivalent pregnancy rates in studies showing no superiority of PGT-A in pregnancy outcome, although this point has recently been refuted.
PGT-A also embraces the “blast-or-bust” credo whereby no embryo transfer occurs unless a blastocyst embryo develops. This continues to beg the unanswerable question – would a woman who did not develop a blastocyst embryo for potential biopsy still conceive if she underwent a day 3 cleavage stage embryo transfer?
Future
Exciting iterations are encroaching for PGT 3.0. One method is blastocyst fluid aspiration to obtain DNA suitable for analysis by molecular genetic methods. Another is noninvasive PGT whereby spent media from the embryo is analyzed using cell-free DNA. Concordance with inner cell mass is reasonably good (approximately 85%) but needs to improve. A major advantage is the biopsy skill set among embryologists is eliminated. A criticism of noninvasive PGT is the risk of false-positive results from contamination of aneuploid cell secretion by physiologic apoptotic cells. Confined placental mosaicism can also increase aneuploidy in cell-free DNA thereby contributing to false positives.
Conclusion
PGT-A is robust technology that appears to benefit women aged above 35 years but not the general infertile population. Error rates must be consistent among laboratories and be lowered. Regarding mosaic embryos, the American Society for Reproductive Medicine guidelines recommend offering another egg retrieval if only mosaic embryos are available and to only consider mosaic embryo transfer following extensive genetic counseling. Long-term effects of PGT-A on children are lacking. The Cochrane Database concluded there was insufficient evidence to make PGT-A routine.
So, the debate is clear and ongoing – universal versus discretionary use of PGT-A? As in all things of life, one size does not fit all, and PGT-A is no exception.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Contact him at [email protected].
Why does the debate linger after 30 years?
Why does the debate linger after 30 years?
The holy grail of assisted reproductive technology (ART) is the delivery of a healthy child. From the world’s first successful ART cycle of in vitro fertilization in 1978 (3 years later in the United States), the goal of every cycle is to provide the woman with an embryo that has the highest potential for implantation and, ultimately, a single live birth.
Embryo aneuploidy is a major factor in the success of human reproduction. As women age, aneuploidy is reported in less than 30% of women aged younger than 35 years but rises to 90% for those in their mid-40s. Intuitively and through randomized, controlled trials, chromosome testing of embryos is a reasonable approach toward improved cycle outcomes and allows for the transfer of a single euploid embryo.
Recently, the phrase “add-ons” has entered the vernacular of editorials on IVF. These additional procedures are offered to patients with the expectation of improving results, yet many have not been supported by rigorous scientifically controlled research trials, e.g., endometrial scratch, embryo glue, and time-lapse imaging of embryos. Where does preimplantation genetic testing (PGT) belong in the IVF armamentarium and why, after 30 years, are there two diametrically opposed views on its benefit? (We will not address testing for single gene defects or chromosome structural rearrangements.)
How did we get here?
The first iteration of PGT used fluorescence in situ hybridization to not only identify X-linked recessive diseases (Hum Genet. 1992;89:18-22) but also the most common chromosome disorders (13, 18, 21, X, Y) by removing one to two blastomere cells from a day 3 embryo (six- to eight-cell stage). Despite wide enthusiasm, the technique was eventually determined to reduce implantation by nearly 40% and was abandoned; presumably impairing the embryo by removing up to one-third of its make-up.
Because of extended embryo culture to the blastocyst stage along with the improved cryopreservation process of vitrification, the next generation of embryo analysis surfaced, what we now refer to as PGT 2.0. Currently, approximately five to six cells from the outer embryo trophectoderm are removed and sent to a specialized laboratory for 24-chromosome screening while the biopsied embryos are cryopreserved. Outcome data (aneuploidy rates, mosaicism) have been influenced by the evolution of genetic platforms – from array comparative genome hybridization to single-nucleotide polymorphism array, to quantitative polymerase chain reaction, to next-generation sequencing (NGS). The newest platform, NGS with high resolution, provides the most extensive degree of analysis by detecting unbalanced translocations and a low cut-off percentage for mosaicism (20%). The clinical error rate is approximately 1%-2%, improved from the 2%-4% of earlier techniques.
The phenomenon of mosaicism describes two distinct cell lines in one embryo (typically one normal and one abnormal) and is defined based on the percentage of mosaicism – currently, the lower limit is 20%. Embryos with less than 20%-30% mosaicism are considered euploid and those greater than 70%-80% are aneuploid. Of note, clinics that do not request the reporting of mosaicism can result in the potential discarding of embryos labeled as aneuploid that would otherwise have potentially resulted in a live birth. The higher the cut-off value for designating mosaicism, the lower the false-positive rate (declaring an embryo aneuploid when euploid). While there is no safe degree of mosaicism, most transfers have resulted in chromosomally normal infants despite a lower implantation rate and higher miscarriage rate.
Current status
The greatest advantage of PGT for aneuploidy (PGT-A) is its increase in promoting a single embryo transfer. Medical evidence supports pregnancy outcomes equivalent from a single euploid embryo transfer versus a double “untested” embryo transfer.
Only a handful of randomized, controlled trials have evaluated the efficacy of PGT-A. Outcomes have favored improved live birth rates; however, criticism exists for enrolling only good prognosis patients given their high likelihood of developing blastocyst embryos to biopsy. The only trial that used an “intention to treat” protocol (rather than randomization at the time of biopsy) did not demonstrate any difference in live birth or miscarriage comparing embryo selection by PGT-A versus embryo morphology alone. However, post hoc analysis did show a benefit with PGT-A in the 35- to 40-year-old age group, not in the less than 35-year-old group. All other trials demonstrated a reduction in miscarriage with PGT-A but only as a secondary outcome.
The medical literature does not support PGT-A to manage patients with recurrent pregnancy loss and there is no evidence for improvement in women aged less than 35 years or egg donors (F&S Reports. 2021;2:36-42). PGT-A has been effective in patients wishing family balancing.
Controversy
Enthusiasm for PGT-A is countered by lingering concerns. Trophectoderm cells are not in 100% concordance with the inner cell mass, which presumably explains the reports of chromosomally normal live births from the transfer of aneuploid embryos. Biopsy techniques among embryologists are not standardized. As a result, damage to the embryo has been raised as a possible explanation for equivalent pregnancy rates in studies showing no superiority of PGT-A in pregnancy outcome, although this point has recently been refuted.
PGT-A also embraces the “blast-or-bust” credo whereby no embryo transfer occurs unless a blastocyst embryo develops. This continues to beg the unanswerable question – would a woman who did not develop a blastocyst embryo for potential biopsy still conceive if she underwent a day 3 cleavage stage embryo transfer?
Future
Exciting iterations are encroaching for PGT 3.0. One method is blastocyst fluid aspiration to obtain DNA suitable for analysis by molecular genetic methods. Another is noninvasive PGT whereby spent media from the embryo is analyzed using cell-free DNA. Concordance with inner cell mass is reasonably good (approximately 85%) but needs to improve. A major advantage is the biopsy skill set among embryologists is eliminated. A criticism of noninvasive PGT is the risk of false-positive results from contamination of aneuploid cell secretion by physiologic apoptotic cells. Confined placental mosaicism can also increase aneuploidy in cell-free DNA thereby contributing to false positives.
Conclusion
PGT-A is robust technology that appears to benefit women aged above 35 years but not the general infertile population. Error rates must be consistent among laboratories and be lowered. Regarding mosaic embryos, the American Society for Reproductive Medicine guidelines recommend offering another egg retrieval if only mosaic embryos are available and to only consider mosaic embryo transfer following extensive genetic counseling. Long-term effects of PGT-A on children are lacking. The Cochrane Database concluded there was insufficient evidence to make PGT-A routine.
So, the debate is clear and ongoing – universal versus discretionary use of PGT-A? As in all things of life, one size does not fit all, and PGT-A is no exception.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Contact him at [email protected].
The holy grail of assisted reproductive technology (ART) is the delivery of a healthy child. From the world’s first successful ART cycle of in vitro fertilization in 1978 (3 years later in the United States), the goal of every cycle is to provide the woman with an embryo that has the highest potential for implantation and, ultimately, a single live birth.
Embryo aneuploidy is a major factor in the success of human reproduction. As women age, aneuploidy is reported in less than 30% of women aged younger than 35 years but rises to 90% for those in their mid-40s. Intuitively and through randomized, controlled trials, chromosome testing of embryos is a reasonable approach toward improved cycle outcomes and allows for the transfer of a single euploid embryo.
Recently, the phrase “add-ons” has entered the vernacular of editorials on IVF. These additional procedures are offered to patients with the expectation of improving results, yet many have not been supported by rigorous scientifically controlled research trials, e.g., endometrial scratch, embryo glue, and time-lapse imaging of embryos. Where does preimplantation genetic testing (PGT) belong in the IVF armamentarium and why, after 30 years, are there two diametrically opposed views on its benefit? (We will not address testing for single gene defects or chromosome structural rearrangements.)
How did we get here?
The first iteration of PGT used fluorescence in situ hybridization to not only identify X-linked recessive diseases (Hum Genet. 1992;89:18-22) but also the most common chromosome disorders (13, 18, 21, X, Y) by removing one to two blastomere cells from a day 3 embryo (six- to eight-cell stage). Despite wide enthusiasm, the technique was eventually determined to reduce implantation by nearly 40% and was abandoned; presumably impairing the embryo by removing up to one-third of its make-up.
Because of extended embryo culture to the blastocyst stage along with the improved cryopreservation process of vitrification, the next generation of embryo analysis surfaced, what we now refer to as PGT 2.0. Currently, approximately five to six cells from the outer embryo trophectoderm are removed and sent to a specialized laboratory for 24-chromosome screening while the biopsied embryos are cryopreserved. Outcome data (aneuploidy rates, mosaicism) have been influenced by the evolution of genetic platforms – from array comparative genome hybridization to single-nucleotide polymorphism array, to quantitative polymerase chain reaction, to next-generation sequencing (NGS). The newest platform, NGS with high resolution, provides the most extensive degree of analysis by detecting unbalanced translocations and a low cut-off percentage for mosaicism (20%). The clinical error rate is approximately 1%-2%, improved from the 2%-4% of earlier techniques.
The phenomenon of mosaicism describes two distinct cell lines in one embryo (typically one normal and one abnormal) and is defined based on the percentage of mosaicism – currently, the lower limit is 20%. Embryos with less than 20%-30% mosaicism are considered euploid and those greater than 70%-80% are aneuploid. Of note, clinics that do not request the reporting of mosaicism can result in the potential discarding of embryos labeled as aneuploid that would otherwise have potentially resulted in a live birth. The higher the cut-off value for designating mosaicism, the lower the false-positive rate (declaring an embryo aneuploid when euploid). While there is no safe degree of mosaicism, most transfers have resulted in chromosomally normal infants despite a lower implantation rate and higher miscarriage rate.
Current status
The greatest advantage of PGT for aneuploidy (PGT-A) is its increase in promoting a single embryo transfer. Medical evidence supports pregnancy outcomes equivalent from a single euploid embryo transfer versus a double “untested” embryo transfer.
Only a handful of randomized, controlled trials have evaluated the efficacy of PGT-A. Outcomes have favored improved live birth rates; however, criticism exists for enrolling only good prognosis patients given their high likelihood of developing blastocyst embryos to biopsy. The only trial that used an “intention to treat” protocol (rather than randomization at the time of biopsy) did not demonstrate any difference in live birth or miscarriage comparing embryo selection by PGT-A versus embryo morphology alone. However, post hoc analysis did show a benefit with PGT-A in the 35- to 40-year-old age group, not in the less than 35-year-old group. All other trials demonstrated a reduction in miscarriage with PGT-A but only as a secondary outcome.
The medical literature does not support PGT-A to manage patients with recurrent pregnancy loss and there is no evidence for improvement in women aged less than 35 years or egg donors (F&S Reports. 2021;2:36-42). PGT-A has been effective in patients wishing family balancing.
Controversy
Enthusiasm for PGT-A is countered by lingering concerns. Trophectoderm cells are not in 100% concordance with the inner cell mass, which presumably explains the reports of chromosomally normal live births from the transfer of aneuploid embryos. Biopsy techniques among embryologists are not standardized. As a result, damage to the embryo has been raised as a possible explanation for equivalent pregnancy rates in studies showing no superiority of PGT-A in pregnancy outcome, although this point has recently been refuted.
PGT-A also embraces the “blast-or-bust” credo whereby no embryo transfer occurs unless a blastocyst embryo develops. This continues to beg the unanswerable question – would a woman who did not develop a blastocyst embryo for potential biopsy still conceive if she underwent a day 3 cleavage stage embryo transfer?
Future
Exciting iterations are encroaching for PGT 3.0. One method is blastocyst fluid aspiration to obtain DNA suitable for analysis by molecular genetic methods. Another is noninvasive PGT whereby spent media from the embryo is analyzed using cell-free DNA. Concordance with inner cell mass is reasonably good (approximately 85%) but needs to improve. A major advantage is the biopsy skill set among embryologists is eliminated. A criticism of noninvasive PGT is the risk of false-positive results from contamination of aneuploid cell secretion by physiologic apoptotic cells. Confined placental mosaicism can also increase aneuploidy in cell-free DNA thereby contributing to false positives.
Conclusion
PGT-A is robust technology that appears to benefit women aged above 35 years but not the general infertile population. Error rates must be consistent among laboratories and be lowered. Regarding mosaic embryos, the American Society for Reproductive Medicine guidelines recommend offering another egg retrieval if only mosaic embryos are available and to only consider mosaic embryo transfer following extensive genetic counseling. Long-term effects of PGT-A on children are lacking. The Cochrane Database concluded there was insufficient evidence to make PGT-A routine.
So, the debate is clear and ongoing – universal versus discretionary use of PGT-A? As in all things of life, one size does not fit all, and PGT-A is no exception.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando. Contact him at [email protected].
Recurrent miscarriage: What’s the evidence-based evaluation and management?
A pregnancy loss at any gestational age is devastating. Women and/or couples may, unfairly, self-blame as they desperately seek substantive answers. Their support systems, including health care providers, offer some, albeit fleeting, comfort. Conception is merely the start of an emotionally arduous first trimester that often results in a learned helplessness. This month, we focus on the comprehensive evaluation and the medical evidence–based approach to recurrent pregnancy loss (RPL).
RPL is defined by the American Society for Reproductive Medicine as two or more clinical pregnancy losses of less than 20 weeks’ gestation with a prevalence of approximately 5%. Embryo aneuploidy is the most common reason for a spontaneous miscarriage, occurring in 50%-70% of losses. The risk of spontaneous miscarriage during the reproductive years follows a J-shaped pattern. The lowest percentage is in women aged 25-29 years (9.8%), with a nadir at age 27 (9.5%), then an increasingly steep rise after age 35 to a peak at age 45 and over (53.6%). The loss rate is closer to 50% of all fertilizations since many spontaneous miscarriages occur at 2-4 weeks, before a pregnancy can be clinically diagnosed. The frequency of embryo aneuploidy significantly decreases and embryo euploidy increases with successive numbers of spontaneous miscarriages.
After three or more spontaneous miscarriages, nulliparous women appear to have a higher rate of subsequent pregnancy loss, compared with parous women (BMJ. 2000;320:1708). We recommend an evaluation following two losses given the lack of evidence for a difference in diagnostic yield following two versus three miscarriages and particularly because of the emotional effects of impact of RPL.
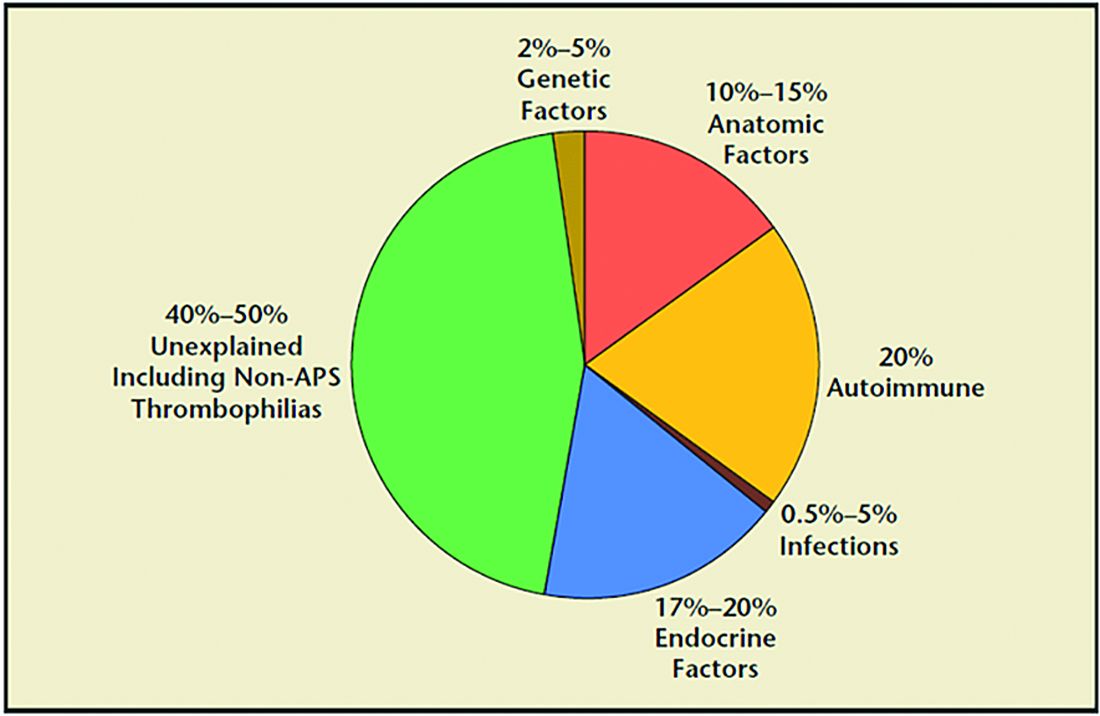
RPL causes, percentages of contribution, and evaluation
1. Genetic (2%-5%). Because of the risk of an embryo with an unbalanced chromosomal rearrangement inherited from a translocation present in either of the couple, a blood karyotype of the couple is essential despite a history of one or more successful live births. While in vitro fertilization (IVF) with preimplantation genetic testing for structural rearrangements (PGT-SR) can successfully diagnose affected embryos to avoid their intrauterine transfer, overall live birth rates are similar when comparing natural conception attempts with PGT-SR, although the latter may reduce miscarriages.
2. Anatomic (10%-15%). Hysteroscopy, hysterosalpingogram, or saline ultrasound can be used to image the uterine cavity to evaluate for polyps, fibroids, scarring, or a congenital septum – all of which can be surgically corrected. Chronic endometritis has been found in 27% of patients with recurrent miscarriage (and in 14% with recurrent implantation failure), therefore testing by biopsy is reasonable. An elevated level of homocysteine has been reported to impair DNA methylation and gene expression, causing defective chorionic villous vascularization in spontaneous miscarriage tissues. We recommend folic acid supplementation and the avoidance of testing for MTHFR (methylenetetrahydrofolate reductase). Of note, the recent TRUST study showed no significant benefit from metroplasty in comparison with expectant management in 12 months of observation resulting in a live birth rate of 31% versus 35%, respectively.
3. Acquired thrombophilias (20%). Medical evidence supports testing for the antiphospholipid antibody syndrome (APS), i.e., RPL with either the presence of lupus anticoagulant (LAC), anticardiolipin antibodies, or anti-beta2 glycoprotein for IgG and IgM. Persistent LAC or elevations of antibodies greater than 40 GPL or greater than the 99th percentile for more than 12 weeks justifies the use of low-molecular-weight heparin (LMWH). APS has been shown to cause RPL, thrombosis, and/or autoimmune thrombocytopenia. There is no definitive evidence to support testing for MTHFR or any other thrombophilias for first trimester RPL. APS has up to a 90% fetal loss rate without therapeutic intervention. Treatment includes low-dose aspirin (81 mg daily) and LMWH. These medications are thought to help prevent thrombosis in the placenta, helping to maintain pregnancies.
4. Hormonal (17%-20%). The most common hormonal disorders increasing the risk for miscarriage is thyroid dysfunction (both hyper- and hypothyroid), prolactin elevations, and lack of glucose control. While the concern for a luteal phase (LPD) prevails, there is no accepted definition or treatment. There is recent evidence that antibodies to thyroid peroxidase may increase miscarriage and that low-dose thyroid replacement may reduce this risk. One other important area is the polycystic ovarian syndrome (PCOS). This hormonal abnormality affects 6%-20% of all reproductive aged women and may increase miscarriage.
5. Unexplained (40%-50%). The most frustrating but most common reason for RPL. Nevertheless, close monitoring and supportive care throughout the first trimester has been demonstrated in medical studies to improve outcome.
Seven surprising facts about recurrent miscarriage
1. Folic acid 4 mg daily may decrease embryo chromosomal abnormalities and miscarriage.
Folic acid in doses of at least 0.4 mg daily have long been advocated to reduce spina bifida and neural tube defects. It is optimal to begin folic acid for several months prior to conception attempts. There is evidence it may help treat RPL by reducing the chance for chromosomal errors.
2. A randomized trial did not demonstrate an improved live birth rate using progesterone in the first trimester. However, women enrolled may not have begun progesterone until 6 weeks of pregnancy, begging the question if earlier progesterone would have demonstrated improvement.
Dydrogesterone, a progestogen that is highly selective for the progesterone receptor, lacks estrogenic, androgenic, anabolic, and corticoid properties. Although not available in the United States, dydrogesterone appears to reduce the rate of idiopathic recurrent miscarriage (two or more losses). Also, progesterone support has been shown to reduce loss in threatened miscarriage – 17 OHPC 500 mg IM weekly in the first trimester.
3. No benefit of aspirin and/or heparin to treat unexplained RM.
The use of aspirin and/or heparin-like medication has convincingly been shown to not improve live birth rates in RPL.
4. Inherited thrombophilias are NOT associated with RM and should not be tested.
Screening for factor V (Leiden mutation), factor II (Prothrombin G20210A), and MTHFR have not been shown to cause RM and no treatment, such as aspirin and/or heparin-like medications, improves the live birth rate.
5. Close monitoring and empathetic care improves outcomes.
For unknown reasons, clinics providing close monitoring, emotional support, and education to patients with unexplained RM report higher live birth rates, compared with patients not receiving this level of care.
6. Behavior changes reduce miscarriage.
Elevations in body mass index (BMI) and cigarette smoking both increase the risk of miscarriage. As a result, a healthy BMI and eliminating tobacco use reduce the risk of pregnancy loss. Excessive caffeine use (more than two equivalent cups of caffeine in coffee per day) also may increase spontaneous miscarriage.
7. Fertility medications, intrauterine insemination, in vitro fertilization, or preimplantation genetic testing for aneuploidy (PGT-A) do not improve outcomes.
While patients and, often, health care providers, feel compelled to proceed with fertility treatment, ovulation induction medications, intrauterine insemination, in vitro fertilization, or PGT-A have not been shown to improve the chance for a live birth. PGT-A did not reduce the risk of miscarriage in women with recurrent pregnancy loss.
In summary, following two or more pregnancy losses, I recommend obtaining chromosomal testing of the couple, viewing the uterine cavity, blood testing for thyroid, prolactin, and glucose control, and acquired thrombophilias (as above). Fortunately, when the cause is unexplained, the woman has a 70%-80% chance of a spontaneous live birth over the next 10 years from diagnosis. By further understanding, knowing how to diagnose, and, finally, treating the cause of RPL we can hopefully prevent the heartbreak women and couples endure.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A pregnancy loss at any gestational age is devastating. Women and/or couples may, unfairly, self-blame as they desperately seek substantive answers. Their support systems, including health care providers, offer some, albeit fleeting, comfort. Conception is merely the start of an emotionally arduous first trimester that often results in a learned helplessness. This month, we focus on the comprehensive evaluation and the medical evidence–based approach to recurrent pregnancy loss (RPL).
RPL is defined by the American Society for Reproductive Medicine as two or more clinical pregnancy losses of less than 20 weeks’ gestation with a prevalence of approximately 5%. Embryo aneuploidy is the most common reason for a spontaneous miscarriage, occurring in 50%-70% of losses. The risk of spontaneous miscarriage during the reproductive years follows a J-shaped pattern. The lowest percentage is in women aged 25-29 years (9.8%), with a nadir at age 27 (9.5%), then an increasingly steep rise after age 35 to a peak at age 45 and over (53.6%). The loss rate is closer to 50% of all fertilizations since many spontaneous miscarriages occur at 2-4 weeks, before a pregnancy can be clinically diagnosed. The frequency of embryo aneuploidy significantly decreases and embryo euploidy increases with successive numbers of spontaneous miscarriages.
After three or more spontaneous miscarriages, nulliparous women appear to have a higher rate of subsequent pregnancy loss, compared with parous women (BMJ. 2000;320:1708). We recommend an evaluation following two losses given the lack of evidence for a difference in diagnostic yield following two versus three miscarriages and particularly because of the emotional effects of impact of RPL.
RPL causes, percentages of contribution, and evaluation
1. Genetic (2%-5%). Because of the risk of an embryo with an unbalanced chromosomal rearrangement inherited from a translocation present in either of the couple, a blood karyotype of the couple is essential despite a history of one or more successful live births. While in vitro fertilization (IVF) with preimplantation genetic testing for structural rearrangements (PGT-SR) can successfully diagnose affected embryos to avoid their intrauterine transfer, overall live birth rates are similar when comparing natural conception attempts with PGT-SR, although the latter may reduce miscarriages.
2. Anatomic (10%-15%). Hysteroscopy, hysterosalpingogram, or saline ultrasound can be used to image the uterine cavity to evaluate for polyps, fibroids, scarring, or a congenital septum – all of which can be surgically corrected. Chronic endometritis has been found in 27% of patients with recurrent miscarriage (and in 14% with recurrent implantation failure), therefore testing by biopsy is reasonable. An elevated level of homocysteine has been reported to impair DNA methylation and gene expression, causing defective chorionic villous vascularization in spontaneous miscarriage tissues. We recommend folic acid supplementation and the avoidance of testing for MTHFR (methylenetetrahydrofolate reductase). Of note, the recent TRUST study showed no significant benefit from metroplasty in comparison with expectant management in 12 months of observation resulting in a live birth rate of 31% versus 35%, respectively.
3. Acquired thrombophilias (20%). Medical evidence supports testing for the antiphospholipid antibody syndrome (APS), i.e., RPL with either the presence of lupus anticoagulant (LAC), anticardiolipin antibodies, or anti-beta2 glycoprotein for IgG and IgM. Persistent LAC or elevations of antibodies greater than 40 GPL or greater than the 99th percentile for more than 12 weeks justifies the use of low-molecular-weight heparin (LMWH). APS has been shown to cause RPL, thrombosis, and/or autoimmune thrombocytopenia. There is no definitive evidence to support testing for MTHFR or any other thrombophilias for first trimester RPL. APS has up to a 90% fetal loss rate without therapeutic intervention. Treatment includes low-dose aspirin (81 mg daily) and LMWH. These medications are thought to help prevent thrombosis in the placenta, helping to maintain pregnancies.
4. Hormonal (17%-20%). The most common hormonal disorders increasing the risk for miscarriage is thyroid dysfunction (both hyper- and hypothyroid), prolactin elevations, and lack of glucose control. While the concern for a luteal phase (LPD) prevails, there is no accepted definition or treatment. There is recent evidence that antibodies to thyroid peroxidase may increase miscarriage and that low-dose thyroid replacement may reduce this risk. One other important area is the polycystic ovarian syndrome (PCOS). This hormonal abnormality affects 6%-20% of all reproductive aged women and may increase miscarriage.
5. Unexplained (40%-50%). The most frustrating but most common reason for RPL. Nevertheless, close monitoring and supportive care throughout the first trimester has been demonstrated in medical studies to improve outcome.

Seven surprising facts about recurrent miscarriage
1. Folic acid 4 mg daily may decrease embryo chromosomal abnormalities and miscarriage.
Folic acid in doses of at least 0.4 mg daily have long been advocated to reduce spina bifida and neural tube defects. It is optimal to begin folic acid for several months prior to conception attempts. There is evidence it may help treat RPL by reducing the chance for chromosomal errors.
2. A randomized trial did not demonstrate an improved live birth rate using progesterone in the first trimester. However, women enrolled may not have begun progesterone until 6 weeks of pregnancy, begging the question if earlier progesterone would have demonstrated improvement.
Dydrogesterone, a progestogen that is highly selective for the progesterone receptor, lacks estrogenic, androgenic, anabolic, and corticoid properties. Although not available in the United States, dydrogesterone appears to reduce the rate of idiopathic recurrent miscarriage (two or more losses). Also, progesterone support has been shown to reduce loss in threatened miscarriage – 17 OHPC 500 mg IM weekly in the first trimester.
3. No benefit of aspirin and/or heparin to treat unexplained RM.
The use of aspirin and/or heparin-like medication has convincingly been shown to not improve live birth rates in RPL.
4. Inherited thrombophilias are NOT associated with RM and should not be tested.
Screening for factor V (Leiden mutation), factor II (Prothrombin G20210A), and MTHFR have not been shown to cause RM and no treatment, such as aspirin and/or heparin-like medications, improves the live birth rate.
5. Close monitoring and empathetic care improves outcomes.
For unknown reasons, clinics providing close monitoring, emotional support, and education to patients with unexplained RM report higher live birth rates, compared with patients not receiving this level of care.
6. Behavior changes reduce miscarriage.
Elevations in body mass index (BMI) and cigarette smoking both increase the risk of miscarriage. As a result, a healthy BMI and eliminating tobacco use reduce the risk of pregnancy loss. Excessive caffeine use (more than two equivalent cups of caffeine in coffee per day) also may increase spontaneous miscarriage.
7. Fertility medications, intrauterine insemination, in vitro fertilization, or preimplantation genetic testing for aneuploidy (PGT-A) do not improve outcomes.
While patients and, often, health care providers, feel compelled to proceed with fertility treatment, ovulation induction medications, intrauterine insemination, in vitro fertilization, or PGT-A have not been shown to improve the chance for a live birth. PGT-A did not reduce the risk of miscarriage in women with recurrent pregnancy loss.
In summary, following two or more pregnancy losses, I recommend obtaining chromosomal testing of the couple, viewing the uterine cavity, blood testing for thyroid, prolactin, and glucose control, and acquired thrombophilias (as above). Fortunately, when the cause is unexplained, the woman has a 70%-80% chance of a spontaneous live birth over the next 10 years from diagnosis. By further understanding, knowing how to diagnose, and, finally, treating the cause of RPL we can hopefully prevent the heartbreak women and couples endure.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.
A pregnancy loss at any gestational age is devastating. Women and/or couples may, unfairly, self-blame as they desperately seek substantive answers. Their support systems, including health care providers, offer some, albeit fleeting, comfort. Conception is merely the start of an emotionally arduous first trimester that often results in a learned helplessness. This month, we focus on the comprehensive evaluation and the medical evidence–based approach to recurrent pregnancy loss (RPL).
RPL is defined by the American Society for Reproductive Medicine as two or more clinical pregnancy losses of less than 20 weeks’ gestation with a prevalence of approximately 5%. Embryo aneuploidy is the most common reason for a spontaneous miscarriage, occurring in 50%-70% of losses. The risk of spontaneous miscarriage during the reproductive years follows a J-shaped pattern. The lowest percentage is in women aged 25-29 years (9.8%), with a nadir at age 27 (9.5%), then an increasingly steep rise after age 35 to a peak at age 45 and over (53.6%). The loss rate is closer to 50% of all fertilizations since many spontaneous miscarriages occur at 2-4 weeks, before a pregnancy can be clinically diagnosed. The frequency of embryo aneuploidy significantly decreases and embryo euploidy increases with successive numbers of spontaneous miscarriages.
After three or more spontaneous miscarriages, nulliparous women appear to have a higher rate of subsequent pregnancy loss, compared with parous women (BMJ. 2000;320:1708). We recommend an evaluation following two losses given the lack of evidence for a difference in diagnostic yield following two versus three miscarriages and particularly because of the emotional effects of impact of RPL.
RPL causes, percentages of contribution, and evaluation
1. Genetic (2%-5%). Because of the risk of an embryo with an unbalanced chromosomal rearrangement inherited from a translocation present in either of the couple, a blood karyotype of the couple is essential despite a history of one or more successful live births. While in vitro fertilization (IVF) with preimplantation genetic testing for structural rearrangements (PGT-SR) can successfully diagnose affected embryos to avoid their intrauterine transfer, overall live birth rates are similar when comparing natural conception attempts with PGT-SR, although the latter may reduce miscarriages.
2. Anatomic (10%-15%). Hysteroscopy, hysterosalpingogram, or saline ultrasound can be used to image the uterine cavity to evaluate for polyps, fibroids, scarring, or a congenital septum – all of which can be surgically corrected. Chronic endometritis has been found in 27% of patients with recurrent miscarriage (and in 14% with recurrent implantation failure), therefore testing by biopsy is reasonable. An elevated level of homocysteine has been reported to impair DNA methylation and gene expression, causing defective chorionic villous vascularization in spontaneous miscarriage tissues. We recommend folic acid supplementation and the avoidance of testing for MTHFR (methylenetetrahydrofolate reductase). Of note, the recent TRUST study showed no significant benefit from metroplasty in comparison with expectant management in 12 months of observation resulting in a live birth rate of 31% versus 35%, respectively.
3. Acquired thrombophilias (20%). Medical evidence supports testing for the antiphospholipid antibody syndrome (APS), i.e., RPL with either the presence of lupus anticoagulant (LAC), anticardiolipin antibodies, or anti-beta2 glycoprotein for IgG and IgM. Persistent LAC or elevations of antibodies greater than 40 GPL or greater than the 99th percentile for more than 12 weeks justifies the use of low-molecular-weight heparin (LMWH). APS has been shown to cause RPL, thrombosis, and/or autoimmune thrombocytopenia. There is no definitive evidence to support testing for MTHFR or any other thrombophilias for first trimester RPL. APS has up to a 90% fetal loss rate without therapeutic intervention. Treatment includes low-dose aspirin (81 mg daily) and LMWH. These medications are thought to help prevent thrombosis in the placenta, helping to maintain pregnancies.
4. Hormonal (17%-20%). The most common hormonal disorders increasing the risk for miscarriage is thyroid dysfunction (both hyper- and hypothyroid), prolactin elevations, and lack of glucose control. While the concern for a luteal phase (LPD) prevails, there is no accepted definition or treatment. There is recent evidence that antibodies to thyroid peroxidase may increase miscarriage and that low-dose thyroid replacement may reduce this risk. One other important area is the polycystic ovarian syndrome (PCOS). This hormonal abnormality affects 6%-20% of all reproductive aged women and may increase miscarriage.
5. Unexplained (40%-50%). The most frustrating but most common reason for RPL. Nevertheless, close monitoring and supportive care throughout the first trimester has been demonstrated in medical studies to improve outcome.

Seven surprising facts about recurrent miscarriage
1. Folic acid 4 mg daily may decrease embryo chromosomal abnormalities and miscarriage.
Folic acid in doses of at least 0.4 mg daily have long been advocated to reduce spina bifida and neural tube defects. It is optimal to begin folic acid for several months prior to conception attempts. There is evidence it may help treat RPL by reducing the chance for chromosomal errors.
2. A randomized trial did not demonstrate an improved live birth rate using progesterone in the first trimester. However, women enrolled may not have begun progesterone until 6 weeks of pregnancy, begging the question if earlier progesterone would have demonstrated improvement.
Dydrogesterone, a progestogen that is highly selective for the progesterone receptor, lacks estrogenic, androgenic, anabolic, and corticoid properties. Although not available in the United States, dydrogesterone appears to reduce the rate of idiopathic recurrent miscarriage (two or more losses). Also, progesterone support has been shown to reduce loss in threatened miscarriage – 17 OHPC 500 mg IM weekly in the first trimester.
3. No benefit of aspirin and/or heparin to treat unexplained RM.
The use of aspirin and/or heparin-like medication has convincingly been shown to not improve live birth rates in RPL.
4. Inherited thrombophilias are NOT associated with RM and should not be tested.
Screening for factor V (Leiden mutation), factor II (Prothrombin G20210A), and MTHFR have not been shown to cause RM and no treatment, such as aspirin and/or heparin-like medications, improves the live birth rate.
5. Close monitoring and empathetic care improves outcomes.
For unknown reasons, clinics providing close monitoring, emotional support, and education to patients with unexplained RM report higher live birth rates, compared with patients not receiving this level of care.
6. Behavior changes reduce miscarriage.
Elevations in body mass index (BMI) and cigarette smoking both increase the risk of miscarriage. As a result, a healthy BMI and eliminating tobacco use reduce the risk of pregnancy loss. Excessive caffeine use (more than two equivalent cups of caffeine in coffee per day) also may increase spontaneous miscarriage.
7. Fertility medications, intrauterine insemination, in vitro fertilization, or preimplantation genetic testing for aneuploidy (PGT-A) do not improve outcomes.
While patients and, often, health care providers, feel compelled to proceed with fertility treatment, ovulation induction medications, intrauterine insemination, in vitro fertilization, or PGT-A have not been shown to improve the chance for a live birth. PGT-A did not reduce the risk of miscarriage in women with recurrent pregnancy loss.
In summary, following two or more pregnancy losses, I recommend obtaining chromosomal testing of the couple, viewing the uterine cavity, blood testing for thyroid, prolactin, and glucose control, and acquired thrombophilias (as above). Fortunately, when the cause is unexplained, the woman has a 70%-80% chance of a spontaneous live birth over the next 10 years from diagnosis. By further understanding, knowing how to diagnose, and, finally, treating the cause of RPL we can hopefully prevent the heartbreak women and couples endure.
Dr. Trolice is director of Fertility CARE – The IVF Center in Winter Park, Fla., and professor of obstetrics and gynecology at the University of Central Florida, Orlando.



