User login
Advancements in nutritional management for critically ill patients
CRITICAL CARE NETWORK
Nonrespiratory Critical Care Section
Nutrition plays an important role in the management and recovery of critically ill patients admitted to the ICU. Major guidelines recommend that critically ill patients should receive 1.2 to 2.0 g/kg/day of protein, with an emphasis on early (within 48 hours of ICU admission) enteral nutrition.1-3
In a randomized controlled trial involving 173 critically ill patients who stayed in the ICU in Zhejiang, China, Wang and colleagues studied the impact of early high protein intake (1.5 g/kg/day vs 0.8 g/kg/day).4 The primary outcome of 28-day mortality was lower among the high protein intake group (8.14% vs 19.54%). Still, this intention-to-treat analysis did not reach a statistical significance (P = .051). However, a time-to-event analysis using the Cox proportional hazard model showed that the high protein intake group had a significantly lower 28-day mortality rate, shorter ICU stays, and improved nutritional status, particularly in patients with sepsis (P = .045).
In a systematic review and meta-analysis involving 19 randomized controlled trials and 1,731 patients, there was no definitive evidence that higher protein intake significantly reduces mortality. However, it may improve specific clinical outcomes like muscle mass retention and shorter duration of mechanical ventilation.5 Similarly, a post hoc analysis on the EFFORT Protein Trial focusing on critically ill patients with acute kidney injury (AKI) showed that higher protein intake did not significantly impact the duration of kidney replacement therapy but was associated with higher serum urea levels and slower time-to-discharge-alive among patients with AKI.6
For critically ill patients, increasing early protein intake to 1.5 g/kg/day is safe and may be beneficial. We still need more data to guide the best approach to determining the protein intake.
References
1. Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44(2):390-438. doi:10.1097/CCM.0000000000001525
2. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48-79. doi:10.1016/j.clnu.2018.08.037
3. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JJPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi:10.1177/0148607115621863
4. Wang Y, Ye Y, Xuan L, et al. Impact of early high protein intake in critically ill patients: a randomized controlled trial. Nutr Metab. 2024;21(1):39. doi.org/10.1186/s12986-024-00818-8
5. Lee ZY, Yap CSL, Hasan MS, et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260. doi.org/10.1186/s13054-021-03693-4
6. Stoppe C, Patel JJ, Zarbock A, et al. The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial. Crit Care. 2023;27(1):399. doi.org/10.1186/s13054-023-04663-8
CRITICAL CARE NETWORK
Nonrespiratory Critical Care Section
Nutrition plays an important role in the management and recovery of critically ill patients admitted to the ICU. Major guidelines recommend that critically ill patients should receive 1.2 to 2.0 g/kg/day of protein, with an emphasis on early (within 48 hours of ICU admission) enteral nutrition.1-3
In a randomized controlled trial involving 173 critically ill patients who stayed in the ICU in Zhejiang, China, Wang and colleagues studied the impact of early high protein intake (1.5 g/kg/day vs 0.8 g/kg/day).4 The primary outcome of 28-day mortality was lower among the high protein intake group (8.14% vs 19.54%). Still, this intention-to-treat analysis did not reach a statistical significance (P = .051). However, a time-to-event analysis using the Cox proportional hazard model showed that the high protein intake group had a significantly lower 28-day mortality rate, shorter ICU stays, and improved nutritional status, particularly in patients with sepsis (P = .045).
In a systematic review and meta-analysis involving 19 randomized controlled trials and 1,731 patients, there was no definitive evidence that higher protein intake significantly reduces mortality. However, it may improve specific clinical outcomes like muscle mass retention and shorter duration of mechanical ventilation.5 Similarly, a post hoc analysis on the EFFORT Protein Trial focusing on critically ill patients with acute kidney injury (AKI) showed that higher protein intake did not significantly impact the duration of kidney replacement therapy but was associated with higher serum urea levels and slower time-to-discharge-alive among patients with AKI.6
For critically ill patients, increasing early protein intake to 1.5 g/kg/day is safe and may be beneficial. We still need more data to guide the best approach to determining the protein intake.
References
1. Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44(2):390-438. doi:10.1097/CCM.0000000000001525
2. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48-79. doi:10.1016/j.clnu.2018.08.037
3. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JJPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi:10.1177/0148607115621863
4. Wang Y, Ye Y, Xuan L, et al. Impact of early high protein intake in critically ill patients: a randomized controlled trial. Nutr Metab. 2024;21(1):39. doi.org/10.1186/s12986-024-00818-8
5. Lee ZY, Yap CSL, Hasan MS, et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260. doi.org/10.1186/s13054-021-03693-4
6. Stoppe C, Patel JJ, Zarbock A, et al. The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial. Crit Care. 2023;27(1):399. doi.org/10.1186/s13054-023-04663-8
CRITICAL CARE NETWORK
Nonrespiratory Critical Care Section
Nutrition plays an important role in the management and recovery of critically ill patients admitted to the ICU. Major guidelines recommend that critically ill patients should receive 1.2 to 2.0 g/kg/day of protein, with an emphasis on early (within 48 hours of ICU admission) enteral nutrition.1-3
In a randomized controlled trial involving 173 critically ill patients who stayed in the ICU in Zhejiang, China, Wang and colleagues studied the impact of early high protein intake (1.5 g/kg/day vs 0.8 g/kg/day).4 The primary outcome of 28-day mortality was lower among the high protein intake group (8.14% vs 19.54%). Still, this intention-to-treat analysis did not reach a statistical significance (P = .051). However, a time-to-event analysis using the Cox proportional hazard model showed that the high protein intake group had a significantly lower 28-day mortality rate, shorter ICU stays, and improved nutritional status, particularly in patients with sepsis (P = .045).
In a systematic review and meta-analysis involving 19 randomized controlled trials and 1,731 patients, there was no definitive evidence that higher protein intake significantly reduces mortality. However, it may improve specific clinical outcomes like muscle mass retention and shorter duration of mechanical ventilation.5 Similarly, a post hoc analysis on the EFFORT Protein Trial focusing on critically ill patients with acute kidney injury (AKI) showed that higher protein intake did not significantly impact the duration of kidney replacement therapy but was associated with higher serum urea levels and slower time-to-discharge-alive among patients with AKI.6
For critically ill patients, increasing early protein intake to 1.5 g/kg/day is safe and may be beneficial. We still need more data to guide the best approach to determining the protein intake.
References
1. Taylor BE, McClave SA, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). Crit Care Med. 2016;44(2):390-438. doi:10.1097/CCM.0000000000001525
2. Singer P, Blaser AR, Berger MM, et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin Nutr. 2019;38(1):48-79. doi:10.1016/j.clnu.2018.08.037
3. McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN). JJPEN J Parenter Enteral Nutr. 2016;40(2):159-211. doi:10.1177/0148607115621863
4. Wang Y, Ye Y, Xuan L, et al. Impact of early high protein intake in critically ill patients: a randomized controlled trial. Nutr Metab. 2024;21(1):39. doi.org/10.1186/s12986-024-00818-8
5. Lee ZY, Yap CSL, Hasan MS, et al. The effect of higher versus lower protein delivery in critically ill patients: a systematic review and meta-analysis of randomized controlled trials. Crit Care. 2021;25(1):260. doi.org/10.1186/s13054-021-03693-4
6. Stoppe C, Patel JJ, Zarbock A, et al. The impact of higher protein dosing on outcomes in critically ill patients with acute kidney injury: a post hoc analysis of the EFFORT protein trial. Crit Care. 2023;27(1):399. doi.org/10.1186/s13054-023-04663-8
New developments on the forefront of intermediate-risk pulmonary embolism
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Cardiovascular Medicine and Surgery Section
Patients with intermediate-risk pulmonary embolism (IRPE), or those with right ventricular dysfunction without overt hemodynamic instability, represent a heterogenous population with short-term mortality ranging from 2% to 17%.1 While systemic anticoagulation is the mainstay therapy, select individuals may benefit from more immediate reperfusion. Unfortunately, only small, randomized trials exploring surrogate outcomes are available to guide modality and patient selection.2
To better define which patients with IRPE are best managed with which therapy, several large-scale randomized controlled trials are underway. PE-TRACT, a study funded by the National Institutes of Health, aims to randomize 500 patients with IRPE to anticoagulation alone vs one of several modalities of CBT with a focus on long-term functional outcomes, including peak oxygen consumption at 3 months and functional class at 1 year. Aspiration thrombectomy with the FlowTriever® device is being compared with anticoagulation alone in a study of 1,200 patients examining short-term composite end points.
While full-dose thrombolysis may decrease the composite outcome of death or hemodynamic deterioration in this population, the benefit is counterbalanced by the risk of significant bleeding. Whether reduced-dose thrombolysis is associated with improved outcomes has been questioned in several small studies. The PEITHO-3 trial plans to randomize 650 patients with IRPE to reduced-dose thrombolytics vs placebo, exploring several outcomes at 30 days. With multiple large trials ongoing, we anticipate important changes to the landscape of IRPE care over the coming years.
References
1. Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. 2015;148(1):211-218. doi:10.1378/chest.14-2551
2. Yuriditsky E, Horowitz JM. The role of the PERT in the management and therapeutic decision-making in pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11(9):693-694. doi:10.1093/ehjacc/zuac102
3. National Library of Medicine (US). A Randomized Trial of Ultrasound-facilitated, Catheter-directed, Thrombolysis Versus Anticoagulation for Acute Intermediate-high Risk Pulmonary Embolism: The Higher-risk Pulmonary Embolism Thrombolysis Study. Updated July 16, 2024. https://clinicaltrials.gov/study/NCT04790370
4. National Library of Medicine (US). PEERLESS II: RCT of FlowTriever vs. Anticoagulation Alone in Pulmonary Embolism. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT06055920
5. National Library of Medicine (US). A Reduced Dose of Thrombolytic Treatment for Patients With Intermediate High-risk Acute Pulmonary Embolism: a Randomized Controled Trial. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT04430569
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Cardiovascular Medicine and Surgery Section
Patients with intermediate-risk pulmonary embolism (IRPE), or those with right ventricular dysfunction without overt hemodynamic instability, represent a heterogenous population with short-term mortality ranging from 2% to 17%.1 While systemic anticoagulation is the mainstay therapy, select individuals may benefit from more immediate reperfusion. Unfortunately, only small, randomized trials exploring surrogate outcomes are available to guide modality and patient selection.2
To better define which patients with IRPE are best managed with which therapy, several large-scale randomized controlled trials are underway. PE-TRACT, a study funded by the National Institutes of Health, aims to randomize 500 patients with IRPE to anticoagulation alone vs one of several modalities of CBT with a focus on long-term functional outcomes, including peak oxygen consumption at 3 months and functional class at 1 year. Aspiration thrombectomy with the FlowTriever® device is being compared with anticoagulation alone in a study of 1,200 patients examining short-term composite end points.
While full-dose thrombolysis may decrease the composite outcome of death or hemodynamic deterioration in this population, the benefit is counterbalanced by the risk of significant bleeding. Whether reduced-dose thrombolysis is associated with improved outcomes has been questioned in several small studies. The PEITHO-3 trial plans to randomize 650 patients with IRPE to reduced-dose thrombolytics vs placebo, exploring several outcomes at 30 days. With multiple large trials ongoing, we anticipate important changes to the landscape of IRPE care over the coming years.
References
1. Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. 2015;148(1):211-218. doi:10.1378/chest.14-2551
2. Yuriditsky E, Horowitz JM. The role of the PERT in the management and therapeutic decision-making in pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11(9):693-694. doi:10.1093/ehjacc/zuac102
3. National Library of Medicine (US). A Randomized Trial of Ultrasound-facilitated, Catheter-directed, Thrombolysis Versus Anticoagulation for Acute Intermediate-high Risk Pulmonary Embolism: The Higher-risk Pulmonary Embolism Thrombolysis Study. Updated July 16, 2024. https://clinicaltrials.gov/study/NCT04790370
4. National Library of Medicine (US). PEERLESS II: RCT of FlowTriever vs. Anticoagulation Alone in Pulmonary Embolism. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT06055920
5. National Library of Medicine (US). A Reduced Dose of Thrombolytic Treatment for Patients With Intermediate High-risk Acute Pulmonary Embolism: a Randomized Controled Trial. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT04430569
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Cardiovascular Medicine and Surgery Section
Patients with intermediate-risk pulmonary embolism (IRPE), or those with right ventricular dysfunction without overt hemodynamic instability, represent a heterogenous population with short-term mortality ranging from 2% to 17%.1 While systemic anticoagulation is the mainstay therapy, select individuals may benefit from more immediate reperfusion. Unfortunately, only small, randomized trials exploring surrogate outcomes are available to guide modality and patient selection.2
To better define which patients with IRPE are best managed with which therapy, several large-scale randomized controlled trials are underway. PE-TRACT, a study funded by the National Institutes of Health, aims to randomize 500 patients with IRPE to anticoagulation alone vs one of several modalities of CBT with a focus on long-term functional outcomes, including peak oxygen consumption at 3 months and functional class at 1 year. Aspiration thrombectomy with the FlowTriever® device is being compared with anticoagulation alone in a study of 1,200 patients examining short-term composite end points.
While full-dose thrombolysis may decrease the composite outcome of death or hemodynamic deterioration in this population, the benefit is counterbalanced by the risk of significant bleeding. Whether reduced-dose thrombolysis is associated with improved outcomes has been questioned in several small studies. The PEITHO-3 trial plans to randomize 650 patients with IRPE to reduced-dose thrombolytics vs placebo, exploring several outcomes at 30 days. With multiple large trials ongoing, we anticipate important changes to the landscape of IRPE care over the coming years.
References
1. Fernández C, Bova C, Sanchez O, et al. Validation of a model for identification of patients at intermediate to high risk for complications associated with acute symptomatic pulmonary embolism. Chest. 2015;148(1):211-218. doi:10.1378/chest.14-2551
2. Yuriditsky E, Horowitz JM. The role of the PERT in the management and therapeutic decision-making in pulmonary embolism. Eur Heart J Acute Cardiovasc Care. 2022;11(9):693-694. doi:10.1093/ehjacc/zuac102
3. National Library of Medicine (US). A Randomized Trial of Ultrasound-facilitated, Catheter-directed, Thrombolysis Versus Anticoagulation for Acute Intermediate-high Risk Pulmonary Embolism: The Higher-risk Pulmonary Embolism Thrombolysis Study. Updated July 16, 2024. https://clinicaltrials.gov/study/NCT04790370
4. National Library of Medicine (US). PEERLESS II: RCT of FlowTriever vs. Anticoagulation Alone in Pulmonary Embolism. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT06055920
5. National Library of Medicine (US). A Reduced Dose of Thrombolytic Treatment for Patients With Intermediate High-risk Acute Pulmonary Embolism: a Randomized Controled Trial. Updated July 17, 2024. https://clinicaltrials.gov/study/NCT04430569
Bronchiectasis: A call to action
AIRWAYS DISORDERS NETWORK
Bronchiectasis Section
For years, the noncystic fibrosis (CF) bronchiectasis community has been trying to organize to provide better care for more than half a million adults with bronchiectasis in the United States. Internationally, the Europeans created the European Bronchiectasis Registry, which has been a powerful tool including nearly 20,000 patients, to answer important epidemiologic and management questions. We must do more for the bronchiectasis community.
Clinicaltrials.gov indicates that there are 8 international phase 3 or 4 clinical trials that are currently enrolling; 3 of those have enrollment sites in the United States. One such study from University of North Carolina at Chapel Hill is looking at the use of nebulized hypertonic saline in patients with non-CF bronchiectasis to understand the effect it has on mucociliary clearance. Emory University is looking at the use of elexacaftor/tezacaftor/ivacaftor (Trikafta) in patients with non-CF bronchiectasis; these patients have only 1 targetable mutation and a phenotype that resembles CF. This 8-week, open-label, single-center study aims to measure both clinical and biomarker outcomes after treatment with Trikafta. Finally, a phase 3 trial out of Florida, the ICoN-1 study, is examining the efficacy and safety of inhaled clofazimine in the treatment of nontuberculous mycobacteria (NTM). This double-blind, randomized trial will look at culture conversion and quality of life measures. Additionally, the COPD Foundation has created the Bronchiectasis and NTM Research Registry, an American cohort containing more than 5,000 patients and data from 22 different sites, to answer some of the most important questions for clinicians and patients.
We have made significant progress in bronchiectasis research; however, there is still much to learn. Together, we must make a concerted effort to enroll patients in clinical trials. Doing so will allow us to define our epidemiologic profile more precisely and explore new treatments and airway clearance techniques.
AIRWAYS DISORDERS NETWORK
Bronchiectasis Section
For years, the noncystic fibrosis (CF) bronchiectasis community has been trying to organize to provide better care for more than half a million adults with bronchiectasis in the United States. Internationally, the Europeans created the European Bronchiectasis Registry, which has been a powerful tool including nearly 20,000 patients, to answer important epidemiologic and management questions. We must do more for the bronchiectasis community.
Clinicaltrials.gov indicates that there are 8 international phase 3 or 4 clinical trials that are currently enrolling; 3 of those have enrollment sites in the United States. One such study from University of North Carolina at Chapel Hill is looking at the use of nebulized hypertonic saline in patients with non-CF bronchiectasis to understand the effect it has on mucociliary clearance. Emory University is looking at the use of elexacaftor/tezacaftor/ivacaftor (Trikafta) in patients with non-CF bronchiectasis; these patients have only 1 targetable mutation and a phenotype that resembles CF. This 8-week, open-label, single-center study aims to measure both clinical and biomarker outcomes after treatment with Trikafta. Finally, a phase 3 trial out of Florida, the ICoN-1 study, is examining the efficacy and safety of inhaled clofazimine in the treatment of nontuberculous mycobacteria (NTM). This double-blind, randomized trial will look at culture conversion and quality of life measures. Additionally, the COPD Foundation has created the Bronchiectasis and NTM Research Registry, an American cohort containing more than 5,000 patients and data from 22 different sites, to answer some of the most important questions for clinicians and patients.
We have made significant progress in bronchiectasis research; however, there is still much to learn. Together, we must make a concerted effort to enroll patients in clinical trials. Doing so will allow us to define our epidemiologic profile more precisely and explore new treatments and airway clearance techniques.
AIRWAYS DISORDERS NETWORK
Bronchiectasis Section
For years, the noncystic fibrosis (CF) bronchiectasis community has been trying to organize to provide better care for more than half a million adults with bronchiectasis in the United States. Internationally, the Europeans created the European Bronchiectasis Registry, which has been a powerful tool including nearly 20,000 patients, to answer important epidemiologic and management questions. We must do more for the bronchiectasis community.
Clinicaltrials.gov indicates that there are 8 international phase 3 or 4 clinical trials that are currently enrolling; 3 of those have enrollment sites in the United States. One such study from University of North Carolina at Chapel Hill is looking at the use of nebulized hypertonic saline in patients with non-CF bronchiectasis to understand the effect it has on mucociliary clearance. Emory University is looking at the use of elexacaftor/tezacaftor/ivacaftor (Trikafta) in patients with non-CF bronchiectasis; these patients have only 1 targetable mutation and a phenotype that resembles CF. This 8-week, open-label, single-center study aims to measure both clinical and biomarker outcomes after treatment with Trikafta. Finally, a phase 3 trial out of Florida, the ICoN-1 study, is examining the efficacy and safety of inhaled clofazimine in the treatment of nontuberculous mycobacteria (NTM). This double-blind, randomized trial will look at culture conversion and quality of life measures. Additionally, the COPD Foundation has created the Bronchiectasis and NTM Research Registry, an American cohort containing more than 5,000 patients and data from 22 different sites, to answer some of the most important questions for clinicians and patients.
We have made significant progress in bronchiectasis research; however, there is still much to learn. Together, we must make a concerted effort to enroll patients in clinical trials. Doing so will allow us to define our epidemiologic profile more precisely and explore new treatments and airway clearance techniques.
OSA in pregnancy: Who to test, how to screen, and does treatment help?
The increased prevalence in pregnancy can be explained by physiologic changes impacting the upper airway such as increases in maternal blood volume and reductions in oncotic pressure, as well as increases in circulating levels of estrogen and progesterone. OSA in pregnancy is associated with adverse perinatal outcomes such as hypertensive disorders of pregnancy, gestational diabetes, severe maternal morbidity abnormalities in fetal growth, preterm birth, and congenital abnormalities in the offspring.2,3 Despite this evidence, guidelines on the screening, diagnosis, and treatment of OSA in pregnancy have only recently been published and will be reviewed here.1
The obstetric subcommittee of the Society of Anesthesia and Sleep Medicine that produced these guidelines had expertise in obstetric anesthesiology, sleep medicine and sleep research, high-risk obstetrics, and obstetric medicine. The guideline aimed to answer 3 questions: 1) Who should be screened in pregnancy for OSA, 2) how to make a diagnosis of OSA in pregnancy and the postpartum period, and 3) what is the treatment for OSA in pregnancy and the postpartum period. Although the estimated number of annual pregnancies in the US declined between 2010 to 2019, these clinical questions remain critical considering the obesity epidemic, the ability to conceive despite advanced maternal age and chronic illnesses with the use of fertility treatments, and the crisis of severe maternal morbidity and mortality. As sleep disordered breathing (SDB) has been associated with many conditions linked to maternal mortality, better management of SDB in this population is key.
Screening for OSA in the pregnant population
The guideline does not support universal screening of all people who are pregnant, but rather suggests that people who are pregnant and at high risk for OSA, such as those with a body mass index (BMI) ≥30 kg/m2 and those with hypertensive disorders of pregnancy, or diabetes, in the index pregnancy or a prior pregnancy, be screened for OSA in the first or second trimester.1 Screening for OSA in pregnancy in limited populations is recommended due to the lower yield of universal screening and its potential burden on the health care system. Furthermore, screening for OSA in early pregnancy is suggested given the practical challenges of arranging testing, initiating, and allowing time for patients to become acclimated to therapy in later stages of pregnancy. However, even when timing of diagnosis may not allow for appropriate treatment of OSA during pregnancy, knowing a person’s OSA status before delivery is beneficial, particularly for patients at risk for Cesarean delivery who may require intubation and exposure to sedative medications, as well as those receiving epidural anesthesia, as OSA is a risk factor for respiratory depression.
Although screening was thought to be beneficial in specific populations, there is insufficient evidence to recommend any one screening tool. The guideline made recommendations against the use of the Berlin questionnaire, STOP-BANG questionnaire, Epworth Sleepiness Scale, or the ASA checklist.1 These screening tools were developed and validated in nonpregnant patient populations and their pooled sensitivity and specificity to detect OSA in pregnancy is low. Individual components of these screening tools, such as prepregnancy BMI, frequency and volume of snoring, hypertension, and neck circumference ≥16 inches have, however, been associated with OSA status.
Pregnancy-specific OSA screening tools have been proposed.4,5 The guideline suggests these pregnancy-specific tools may be considered for screening for OSA in pregnancy but still require external validation, especially in high-risk populations. The committee agreed that individuals with BMI >30kg/m2, hypertension, diabetes, and those with a history of difficult intubation or Mallampati score III or IV are considered at risk for OSA in pregnancy.
Diagnosis of OSA in the pregnant population
In the general population, in-laboratory polysomnogram (PSG) is the gold standard diagnostic test. However, for patients in whom uncomplicated OSA is suspected with a moderate to high pretest probability, unattended home sleep apnea testing (HSAT) is a reasonable initial study. On the other hand, in-lab PSG is recommended in mission-critical workers and when coexisting respiratory sleep disorders, or nonrespiratory sleep disorders, are suspected. For individuals who are pregnant and suspected of having OSA, the guideline suggests that HSAT is a reasonable diagnostic tool, as many level III devices have demonstrated good agreement between the respiratory disturbance index (RDI) and apnea-hypopnea index (AHI) measured by PSG.6 Notably, most studies have examined the performance of level III devices in late pregnancy in populations with obesity; hence, the performance of these devices in early pregnancy when risk for OSA is lower, or more subtle forms of SDB may be more common, is less clear but may be an acceptable first-line test.
The guideline did not provide recommendations for next steps following an inconclusive, technically inadequate, or negative HSAT. However, recommendations to proceed with in-lab PSG in individuals with clinical suspicion for OSA and a negative HSAT is a reasonable approach, keeping in mind the time restrictions of pregnancy. The more delayed the diagnosis, the less time there will be for initiation of and acclimation to therapy to maximize potential benefits during pregnancy. HSAT is especially practical and convenient for individuals with young families. The guideline does not recommend the use of overnight oximetry for diagnostic purposes.1
The postpartum period is usually associated with weight loss and reversal of pregnancy physiology. Generally, the decision to perform a repeat sleep study following weight loss is individualized, based on factors such as improved symptoms or sustained, significant weight loss. Though data show improvement in AHI following delivery, small studies show persistent OSA in nearly half of individuals diagnosed in pregnancy. Hence, as pregnancy increases the risk for OSA, and given that the postpartum status is not always associated with resolution of OSA, the guideline recommends considering repeat diagnostic testing in the postpartum period.1 The decision to repeat testing also depends on whether OSA or OSA symptoms predated pregnancy, on the persistence of symptoms, and the degree of weight loss with delivery and the postpartum body habitus.
Treatment of OSA in the pregnant population
The guideline recommends behavior modification in OSA similarly to individuals who are not pregnant (avoidance of sedatives, smoking, and alcohol).1 However, weight loss is not recommended in pregnancy due to the potential for harm to the fetus.
The gold standard treatment for people who are pregnant and have OSA is continuous positive airway pressure (CPAP). Treatment of OSA in pregnancy is complicated by the fact that very few women are referred to sleep practices due to time restrictions and logistical reasons, and that data demonstrating improved pregnancy outcomes with CPAP are scarce, limiting the prioritization of OSA management. However, expert consensus considers a theoretical benefit in the context of lack of current evidence of harm from treatment. Hence, at this point, the guideline recommends counseling around CPAP therapy be aimed at improvement in symptoms, AHI, and quality of life, rather than pregnancy-specific outcomes.1 This recommendation was based on observations from small case series that demonstrated improved breathing parameters during sleep and symptoms, and small randomized controlled trials (RCT), limited by short-term exposure to the intervention. However, since the publication of this guideline, a large RCT that randomized pregnant women with SDB to CPAP or usual care has demonstrated significantly lower diastolic blood pressure, an altered diastolic blood pressure trajectory, and a lower rate of preeclampsia in the group treated with CPAP compared with usual care.7
This guideline provides helpful insight on who to screen and how to manage OSA in pregnancy but additional research is needed to elucidate benefits of treatment and its effects on maternal and neonatal outcomes. Multidisciplinary collaborations between obstetric and sleep teams are necessary to ensure that screening and diagnostic strategies result in management change for improved outcomes.
References
1. Dominguez JE, Cantrell S, Habib AS, et al. Society of Anesthesia and Sleep Medicine and the Society for Obstetric Anesthesia and Perinatology Consensus Guideline on the screening, diagnosis and treatment of obstructive sleep apnea in pregnancy. Obstet Gynecol. 2023;142(2):403-423.
2. Bourjeily, G, Danilack C, Bublitz M, Muri J, Rosene-Montella K, Lipkind H. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med. 2000;66:233-240.
3. Malhamé I, Bublitz MH, Wilson D, Sanapo L, Rochin E, Bourjeily G. Sleep disordered breathing and the risk of severe maternal morbidity in women with preeclampsia: a population-based study. Pregnancy Hypertens. 2022;30:215-220.
4. Izci-Balserak B, Zhu B, Gurubhagavatula I, Keenan BT, Pien GW. A screening algorithm for obstructive sleep apnea in pregnancy. Ann Am Thorac Soc. 2019;16(10):1286-1294.
5. Louis J, Koch MA, Reddy UM, et al. Predictors of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2018;218(5):521.e1.e12.
6. Sharkey K, Waters K, Millman R, Moore R, Martin SM, Bourjeily. Validation of the Apnea Risk Evaluation System (ARES) device against laboratory polysomnogram in pregnant women at risk for obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(5):497-502.
7. Tantrakul V, Ingsathit A, Liamsombut S, et al. Treatment of obstructive sleep apnea in high-risk pregnancy: a multicenter randomized controlled trial. Respir Res. 2023;24(1):171.
The increased prevalence in pregnancy can be explained by physiologic changes impacting the upper airway such as increases in maternal blood volume and reductions in oncotic pressure, as well as increases in circulating levels of estrogen and progesterone. OSA in pregnancy is associated with adverse perinatal outcomes such as hypertensive disorders of pregnancy, gestational diabetes, severe maternal morbidity abnormalities in fetal growth, preterm birth, and congenital abnormalities in the offspring.2,3 Despite this evidence, guidelines on the screening, diagnosis, and treatment of OSA in pregnancy have only recently been published and will be reviewed here.1
The obstetric subcommittee of the Society of Anesthesia and Sleep Medicine that produced these guidelines had expertise in obstetric anesthesiology, sleep medicine and sleep research, high-risk obstetrics, and obstetric medicine. The guideline aimed to answer 3 questions: 1) Who should be screened in pregnancy for OSA, 2) how to make a diagnosis of OSA in pregnancy and the postpartum period, and 3) what is the treatment for OSA in pregnancy and the postpartum period. Although the estimated number of annual pregnancies in the US declined between 2010 to 2019, these clinical questions remain critical considering the obesity epidemic, the ability to conceive despite advanced maternal age and chronic illnesses with the use of fertility treatments, and the crisis of severe maternal morbidity and mortality. As sleep disordered breathing (SDB) has been associated with many conditions linked to maternal mortality, better management of SDB in this population is key.
Screening for OSA in the pregnant population
The guideline does not support universal screening of all people who are pregnant, but rather suggests that people who are pregnant and at high risk for OSA, such as those with a body mass index (BMI) ≥30 kg/m2 and those with hypertensive disorders of pregnancy, or diabetes, in the index pregnancy or a prior pregnancy, be screened for OSA in the first or second trimester.1 Screening for OSA in pregnancy in limited populations is recommended due to the lower yield of universal screening and its potential burden on the health care system. Furthermore, screening for OSA in early pregnancy is suggested given the practical challenges of arranging testing, initiating, and allowing time for patients to become acclimated to therapy in later stages of pregnancy. However, even when timing of diagnosis may not allow for appropriate treatment of OSA during pregnancy, knowing a person’s OSA status before delivery is beneficial, particularly for patients at risk for Cesarean delivery who may require intubation and exposure to sedative medications, as well as those receiving epidural anesthesia, as OSA is a risk factor for respiratory depression.
Although screening was thought to be beneficial in specific populations, there is insufficient evidence to recommend any one screening tool. The guideline made recommendations against the use of the Berlin questionnaire, STOP-BANG questionnaire, Epworth Sleepiness Scale, or the ASA checklist.1 These screening tools were developed and validated in nonpregnant patient populations and their pooled sensitivity and specificity to detect OSA in pregnancy is low. Individual components of these screening tools, such as prepregnancy BMI, frequency and volume of snoring, hypertension, and neck circumference ≥16 inches have, however, been associated with OSA status.
Pregnancy-specific OSA screening tools have been proposed.4,5 The guideline suggests these pregnancy-specific tools may be considered for screening for OSA in pregnancy but still require external validation, especially in high-risk populations. The committee agreed that individuals with BMI >30kg/m2, hypertension, diabetes, and those with a history of difficult intubation or Mallampati score III or IV are considered at risk for OSA in pregnancy.
Diagnosis of OSA in the pregnant population
In the general population, in-laboratory polysomnogram (PSG) is the gold standard diagnostic test. However, for patients in whom uncomplicated OSA is suspected with a moderate to high pretest probability, unattended home sleep apnea testing (HSAT) is a reasonable initial study. On the other hand, in-lab PSG is recommended in mission-critical workers and when coexisting respiratory sleep disorders, or nonrespiratory sleep disorders, are suspected. For individuals who are pregnant and suspected of having OSA, the guideline suggests that HSAT is a reasonable diagnostic tool, as many level III devices have demonstrated good agreement between the respiratory disturbance index (RDI) and apnea-hypopnea index (AHI) measured by PSG.6 Notably, most studies have examined the performance of level III devices in late pregnancy in populations with obesity; hence, the performance of these devices in early pregnancy when risk for OSA is lower, or more subtle forms of SDB may be more common, is less clear but may be an acceptable first-line test.
The guideline did not provide recommendations for next steps following an inconclusive, technically inadequate, or negative HSAT. However, recommendations to proceed with in-lab PSG in individuals with clinical suspicion for OSA and a negative HSAT is a reasonable approach, keeping in mind the time restrictions of pregnancy. The more delayed the diagnosis, the less time there will be for initiation of and acclimation to therapy to maximize potential benefits during pregnancy. HSAT is especially practical and convenient for individuals with young families. The guideline does not recommend the use of overnight oximetry for diagnostic purposes.1
The postpartum period is usually associated with weight loss and reversal of pregnancy physiology. Generally, the decision to perform a repeat sleep study following weight loss is individualized, based on factors such as improved symptoms or sustained, significant weight loss. Though data show improvement in AHI following delivery, small studies show persistent OSA in nearly half of individuals diagnosed in pregnancy. Hence, as pregnancy increases the risk for OSA, and given that the postpartum status is not always associated with resolution of OSA, the guideline recommends considering repeat diagnostic testing in the postpartum period.1 The decision to repeat testing also depends on whether OSA or OSA symptoms predated pregnancy, on the persistence of symptoms, and the degree of weight loss with delivery and the postpartum body habitus.
Treatment of OSA in the pregnant population
The guideline recommends behavior modification in OSA similarly to individuals who are not pregnant (avoidance of sedatives, smoking, and alcohol).1 However, weight loss is not recommended in pregnancy due to the potential for harm to the fetus.
The gold standard treatment for people who are pregnant and have OSA is continuous positive airway pressure (CPAP). Treatment of OSA in pregnancy is complicated by the fact that very few women are referred to sleep practices due to time restrictions and logistical reasons, and that data demonstrating improved pregnancy outcomes with CPAP are scarce, limiting the prioritization of OSA management. However, expert consensus considers a theoretical benefit in the context of lack of current evidence of harm from treatment. Hence, at this point, the guideline recommends counseling around CPAP therapy be aimed at improvement in symptoms, AHI, and quality of life, rather than pregnancy-specific outcomes.1 This recommendation was based on observations from small case series that demonstrated improved breathing parameters during sleep and symptoms, and small randomized controlled trials (RCT), limited by short-term exposure to the intervention. However, since the publication of this guideline, a large RCT that randomized pregnant women with SDB to CPAP or usual care has demonstrated significantly lower diastolic blood pressure, an altered diastolic blood pressure trajectory, and a lower rate of preeclampsia in the group treated with CPAP compared with usual care.7
This guideline provides helpful insight on who to screen and how to manage OSA in pregnancy but additional research is needed to elucidate benefits of treatment and its effects on maternal and neonatal outcomes. Multidisciplinary collaborations between obstetric and sleep teams are necessary to ensure that screening and diagnostic strategies result in management change for improved outcomes.
References
1. Dominguez JE, Cantrell S, Habib AS, et al. Society of Anesthesia and Sleep Medicine and the Society for Obstetric Anesthesia and Perinatology Consensus Guideline on the screening, diagnosis and treatment of obstructive sleep apnea in pregnancy. Obstet Gynecol. 2023;142(2):403-423.
2. Bourjeily, G, Danilack C, Bublitz M, Muri J, Rosene-Montella K, Lipkind H. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med. 2000;66:233-240.
3. Malhamé I, Bublitz MH, Wilson D, Sanapo L, Rochin E, Bourjeily G. Sleep disordered breathing and the risk of severe maternal morbidity in women with preeclampsia: a population-based study. Pregnancy Hypertens. 2022;30:215-220.
4. Izci-Balserak B, Zhu B, Gurubhagavatula I, Keenan BT, Pien GW. A screening algorithm for obstructive sleep apnea in pregnancy. Ann Am Thorac Soc. 2019;16(10):1286-1294.
5. Louis J, Koch MA, Reddy UM, et al. Predictors of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2018;218(5):521.e1.e12.
6. Sharkey K, Waters K, Millman R, Moore R, Martin SM, Bourjeily. Validation of the Apnea Risk Evaluation System (ARES) device against laboratory polysomnogram in pregnant women at risk for obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(5):497-502.
7. Tantrakul V, Ingsathit A, Liamsombut S, et al. Treatment of obstructive sleep apnea in high-risk pregnancy: a multicenter randomized controlled trial. Respir Res. 2023;24(1):171.
The increased prevalence in pregnancy can be explained by physiologic changes impacting the upper airway such as increases in maternal blood volume and reductions in oncotic pressure, as well as increases in circulating levels of estrogen and progesterone. OSA in pregnancy is associated with adverse perinatal outcomes such as hypertensive disorders of pregnancy, gestational diabetes, severe maternal morbidity abnormalities in fetal growth, preterm birth, and congenital abnormalities in the offspring.2,3 Despite this evidence, guidelines on the screening, diagnosis, and treatment of OSA in pregnancy have only recently been published and will be reviewed here.1
The obstetric subcommittee of the Society of Anesthesia and Sleep Medicine that produced these guidelines had expertise in obstetric anesthesiology, sleep medicine and sleep research, high-risk obstetrics, and obstetric medicine. The guideline aimed to answer 3 questions: 1) Who should be screened in pregnancy for OSA, 2) how to make a diagnosis of OSA in pregnancy and the postpartum period, and 3) what is the treatment for OSA in pregnancy and the postpartum period. Although the estimated number of annual pregnancies in the US declined between 2010 to 2019, these clinical questions remain critical considering the obesity epidemic, the ability to conceive despite advanced maternal age and chronic illnesses with the use of fertility treatments, and the crisis of severe maternal morbidity and mortality. As sleep disordered breathing (SDB) has been associated with many conditions linked to maternal mortality, better management of SDB in this population is key.
Screening for OSA in the pregnant population
The guideline does not support universal screening of all people who are pregnant, but rather suggests that people who are pregnant and at high risk for OSA, such as those with a body mass index (BMI) ≥30 kg/m2 and those with hypertensive disorders of pregnancy, or diabetes, in the index pregnancy or a prior pregnancy, be screened for OSA in the first or second trimester.1 Screening for OSA in pregnancy in limited populations is recommended due to the lower yield of universal screening and its potential burden on the health care system. Furthermore, screening for OSA in early pregnancy is suggested given the practical challenges of arranging testing, initiating, and allowing time for patients to become acclimated to therapy in later stages of pregnancy. However, even when timing of diagnosis may not allow for appropriate treatment of OSA during pregnancy, knowing a person’s OSA status before delivery is beneficial, particularly for patients at risk for Cesarean delivery who may require intubation and exposure to sedative medications, as well as those receiving epidural anesthesia, as OSA is a risk factor for respiratory depression.
Although screening was thought to be beneficial in specific populations, there is insufficient evidence to recommend any one screening tool. The guideline made recommendations against the use of the Berlin questionnaire, STOP-BANG questionnaire, Epworth Sleepiness Scale, or the ASA checklist.1 These screening tools were developed and validated in nonpregnant patient populations and their pooled sensitivity and specificity to detect OSA in pregnancy is low. Individual components of these screening tools, such as prepregnancy BMI, frequency and volume of snoring, hypertension, and neck circumference ≥16 inches have, however, been associated with OSA status.
Pregnancy-specific OSA screening tools have been proposed.4,5 The guideline suggests these pregnancy-specific tools may be considered for screening for OSA in pregnancy but still require external validation, especially in high-risk populations. The committee agreed that individuals with BMI >30kg/m2, hypertension, diabetes, and those with a history of difficult intubation or Mallampati score III or IV are considered at risk for OSA in pregnancy.
Diagnosis of OSA in the pregnant population
In the general population, in-laboratory polysomnogram (PSG) is the gold standard diagnostic test. However, for patients in whom uncomplicated OSA is suspected with a moderate to high pretest probability, unattended home sleep apnea testing (HSAT) is a reasonable initial study. On the other hand, in-lab PSG is recommended in mission-critical workers and when coexisting respiratory sleep disorders, or nonrespiratory sleep disorders, are suspected. For individuals who are pregnant and suspected of having OSA, the guideline suggests that HSAT is a reasonable diagnostic tool, as many level III devices have demonstrated good agreement between the respiratory disturbance index (RDI) and apnea-hypopnea index (AHI) measured by PSG.6 Notably, most studies have examined the performance of level III devices in late pregnancy in populations with obesity; hence, the performance of these devices in early pregnancy when risk for OSA is lower, or more subtle forms of SDB may be more common, is less clear but may be an acceptable first-line test.
The guideline did not provide recommendations for next steps following an inconclusive, technically inadequate, or negative HSAT. However, recommendations to proceed with in-lab PSG in individuals with clinical suspicion for OSA and a negative HSAT is a reasonable approach, keeping in mind the time restrictions of pregnancy. The more delayed the diagnosis, the less time there will be for initiation of and acclimation to therapy to maximize potential benefits during pregnancy. HSAT is especially practical and convenient for individuals with young families. The guideline does not recommend the use of overnight oximetry for diagnostic purposes.1
The postpartum period is usually associated with weight loss and reversal of pregnancy physiology. Generally, the decision to perform a repeat sleep study following weight loss is individualized, based on factors such as improved symptoms or sustained, significant weight loss. Though data show improvement in AHI following delivery, small studies show persistent OSA in nearly half of individuals diagnosed in pregnancy. Hence, as pregnancy increases the risk for OSA, and given that the postpartum status is not always associated with resolution of OSA, the guideline recommends considering repeat diagnostic testing in the postpartum period.1 The decision to repeat testing also depends on whether OSA or OSA symptoms predated pregnancy, on the persistence of symptoms, and the degree of weight loss with delivery and the postpartum body habitus.
Treatment of OSA in the pregnant population
The guideline recommends behavior modification in OSA similarly to individuals who are not pregnant (avoidance of sedatives, smoking, and alcohol).1 However, weight loss is not recommended in pregnancy due to the potential for harm to the fetus.
The gold standard treatment for people who are pregnant and have OSA is continuous positive airway pressure (CPAP). Treatment of OSA in pregnancy is complicated by the fact that very few women are referred to sleep practices due to time restrictions and logistical reasons, and that data demonstrating improved pregnancy outcomes with CPAP are scarce, limiting the prioritization of OSA management. However, expert consensus considers a theoretical benefit in the context of lack of current evidence of harm from treatment. Hence, at this point, the guideline recommends counseling around CPAP therapy be aimed at improvement in symptoms, AHI, and quality of life, rather than pregnancy-specific outcomes.1 This recommendation was based on observations from small case series that demonstrated improved breathing parameters during sleep and symptoms, and small randomized controlled trials (RCT), limited by short-term exposure to the intervention. However, since the publication of this guideline, a large RCT that randomized pregnant women with SDB to CPAP or usual care has demonstrated significantly lower diastolic blood pressure, an altered diastolic blood pressure trajectory, and a lower rate of preeclampsia in the group treated with CPAP compared with usual care.7
This guideline provides helpful insight on who to screen and how to manage OSA in pregnancy but additional research is needed to elucidate benefits of treatment and its effects on maternal and neonatal outcomes. Multidisciplinary collaborations between obstetric and sleep teams are necessary to ensure that screening and diagnostic strategies result in management change for improved outcomes.
References
1. Dominguez JE, Cantrell S, Habib AS, et al. Society of Anesthesia and Sleep Medicine and the Society for Obstetric Anesthesia and Perinatology Consensus Guideline on the screening, diagnosis and treatment of obstructive sleep apnea in pregnancy. Obstet Gynecol. 2023;142(2):403-423.
2. Bourjeily, G, Danilack C, Bublitz M, Muri J, Rosene-Montella K, Lipkind H. Maternal obstructive sleep apnea and neonatal birth outcomes in a population based sample. Sleep Med. 2000;66:233-240.
3. Malhamé I, Bublitz MH, Wilson D, Sanapo L, Rochin E, Bourjeily G. Sleep disordered breathing and the risk of severe maternal morbidity in women with preeclampsia: a population-based study. Pregnancy Hypertens. 2022;30:215-220.
4. Izci-Balserak B, Zhu B, Gurubhagavatula I, Keenan BT, Pien GW. A screening algorithm for obstructive sleep apnea in pregnancy. Ann Am Thorac Soc. 2019;16(10):1286-1294.
5. Louis J, Koch MA, Reddy UM, et al. Predictors of sleep-disordered breathing in pregnancy. Am J Obstet Gynecol. 2018;218(5):521.e1.e12.
6. Sharkey K, Waters K, Millman R, Moore R, Martin SM, Bourjeily. Validation of the Apnea Risk Evaluation System (ARES) device against laboratory polysomnogram in pregnant women at risk for obstructive sleep apnea syndrome. J Clin Sleep Med. 2014;10(5):497-502.
7. Tantrakul V, Ingsathit A, Liamsombut S, et al. Treatment of obstructive sleep apnea in high-risk pregnancy: a multicenter randomized controlled trial. Respir Res. 2023;24(1):171.
The language of AI and its applications in health care
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.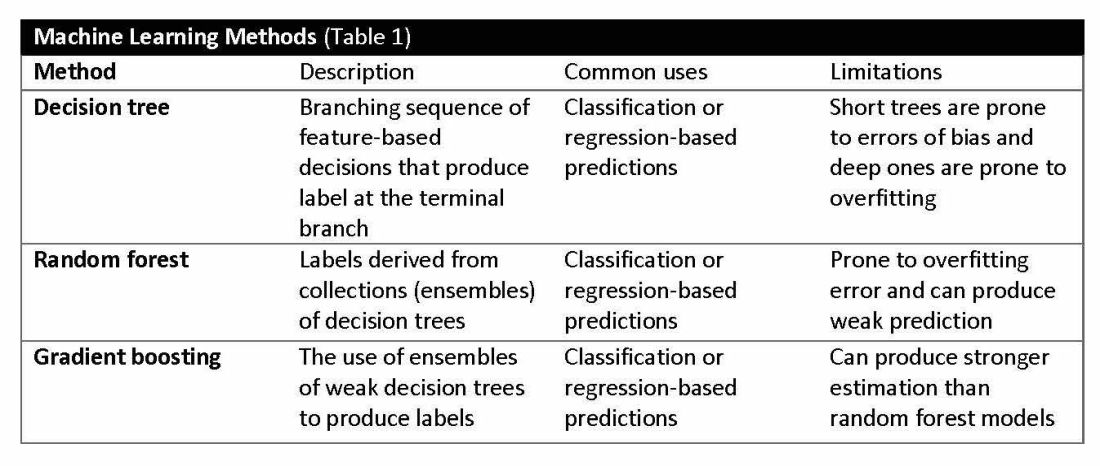
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
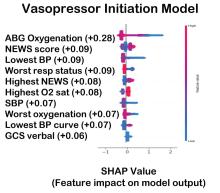
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
AI is a group of nonhuman techniques that utilize automated learning methods to extract information from datasets through generalization, classification, prediction, and association. In other words, AI is the simulation of human intelligence processes by machines. The branches of AI include natural language processing, speech recognition, machine vision, and expert systems. AI can make clinical care more efficient; however, many find its confusing terminology to be a barrier.1 This article provides concise definitions of AI terms and is intended to help physicians better understand how AI methods can be applied to clinical care. The clinical application of natural language processing and machine vision applications are more clinically intuitive than the roles of machine learning algorithms.
Machine learning and algorithms
Machine learning is a branch of AI that uses data and algorithms to mimic human reasoning through classification, pattern recognition, and prediction. Supervised and unsupervised machine-learning algorithms can analyze data and recognize undetected associations and relationships.
Supervised learning involves training models to make predictions using data sets that have correct outcome parameters called labels using predictive fields called features. Machine learning uses iterative analysis including random forest, decision tree, and gradient boosting methods that minimize predictive error metrics (see Table 1). This approach is widely used to improve diagnoses, predict disease progression or exacerbation, and personalize treatment plan modifications.
Supervised machine learning methods can be particularly effective for processing large volumes of medical information to identify patterns and make accurate predictions. In contrast, unsupervised learning techniques can analyze unlabeled data and help clinicians uncover hidden patterns or undetected groupings. Techniques including clustering, exploratory analysis, and anomaly detection are common applications. Both of these machine-learning approaches can be used to extract novel and helpful insights.
The utility of machine learning analyses depends on the size and accuracy of the available datasets. Small datasets can limit usability, while large datasets require substantial computational power. Predictive models are generated using training datasets and evaluated using separate evaluation datasets. Deep learning models, a subset of machine learning, can automatically readjust themselves to maintain or improve accuracy when analyzing new observations that include accurate labels.
Challenges of algorithms and calibration
Machine learning algorithms vary in complexity and accuracy. For example, a simple logistic regression model using time, date, latitude, and indoor/outdoor location can accurately recommend sunscreen application. This model identifies when solar radiation is high enough to warrant sunscreen use, avoiding unnecessary recommendations during nighttime hours or indoor locations. A more complex model might suffer from model overfitting and inappropriately suggest sunscreen before a tanning salon visit.
Complex machine learning models, like support vector machine (SVM) and decision tree methods, are useful when many features have predictive power. SVMs are useful for small but complex datasets. Features are manipulated in a multidimensional space to maximize the “margins” separating 2 groups. Decision tree analyses are useful when more than 2 groups are being analyzed. SVM and decision tree models can also lose accuracy by data overfitting.
Consider the development of an SVM analysis to predict whether an individual is a fellow or a senior faculty member. One could use high gray hair density feature values to identify senior faculty. When this algorithm is applied to an individual with alopecia, no amount of model adjustment can achieve high levels of discrimination because no hair is present. Rather than overfitting the model by adding more nonpredictive features, individuals with alopecia are analyzed by their own algorithm (tree) that uses the skin wrinkle/solar damage rather than the gray hair density feature.
Decision tree ensemble algorithms like random forest and gradient boosting use feature-based decision trees to process and classify data. Random forests are robust, scalable, and versatile, providing classifications and predictions while protecting against inaccurate data and outliers and have the advantage of being able to handle both categorical and continuous features. Gradient boosting, which uses an ensemble of weak decision trees, often outperforms random forests when individual trees perform only slightly better than random chance. This method incrementally builds the model by optimizing the residual errors of previous trees, leading to more accurate predictions.
In practice, gradient boosting can be used to fine-tune diagnostic models, improving their precision and reliability. A recent example of how gradient boosting of random forest predictions yielded highly accurate predictions for unplanned vasopressor initiation and intubation events 2 to 4 hours before an ICU adult became unstable.2
Assessing the accuracy of algorithms
The value of the data set is directly related to the accuracy of its labels. Traditional methods that measure model performance, such as sensitivity, specificity, and predictive values (PPV and NPV), have important limitations. They provide little insight into how a complex model made its prediction. Understanding which individual features drive model accuracy is key to fostering trust in model predictions. This can be done by comparing model output with and without including individual features. The results of all possible combinations are aggregated according to feature importance, which is summarized in the Shapley value for each model feature. Higher values indicate greater relative importance. SHAP plots help identify how much and how often specific features change the model output, presenting values of individual model estimates with and without a specific feature (see Figure 1).
Promoting AI use
AI and machine learning algorithms are coming to patient care. Understanding the language of AI helps caregivers integrate these tools into their practices. The science of AI faces serious challenges. Algorithms must be recalibrated to keep pace as therapies advance, disease prevalence changes, and our population ages. AI must address new challenges as they confront those suffering from respiratory diseases. This resource encourages clinicians with novel approaches by using AI methodologies to advance their development. We can better address future health care needs by promoting the equitable use of AI technologies, especially among socially disadvantaged developers.
References
1. Lilly CM, Soni AV, Dunlap D, et al. Advancing point of care testing by application of machine learning techniques and artificial intelligence. Chest. 2024 (in press).
2. Lilly CM, Kirk D, Pessach IM, et al. Application of machine learning models to biomedical and information system signals from critically ill adults. Chest. 2024;165(5):1139-1148.
CHEST releases new guideline on management of central airway obstruction
CHEST recently released a new clinical guideline on central airway obstruction (CAO).
“Central airway obstruction is associated with a poor prognosis, and the management of CAO is highly variable dependent on the provider expertise and local resources. By releasing this guideline, the panel hopes to standardize the definition of CAO and provide guidance for the management of patients to optimize care and improve outcomes,” said Kamran Mahmood, MD, MPH, FCCP, lead author on the guideline. “The guideline recommendations are developed using GRADE methodology and based on thorough evidence review and expert input. But the quality of overall evidence is very low, and the panel calls for well-designed studies and randomized controlled trials for the management of CAO.”
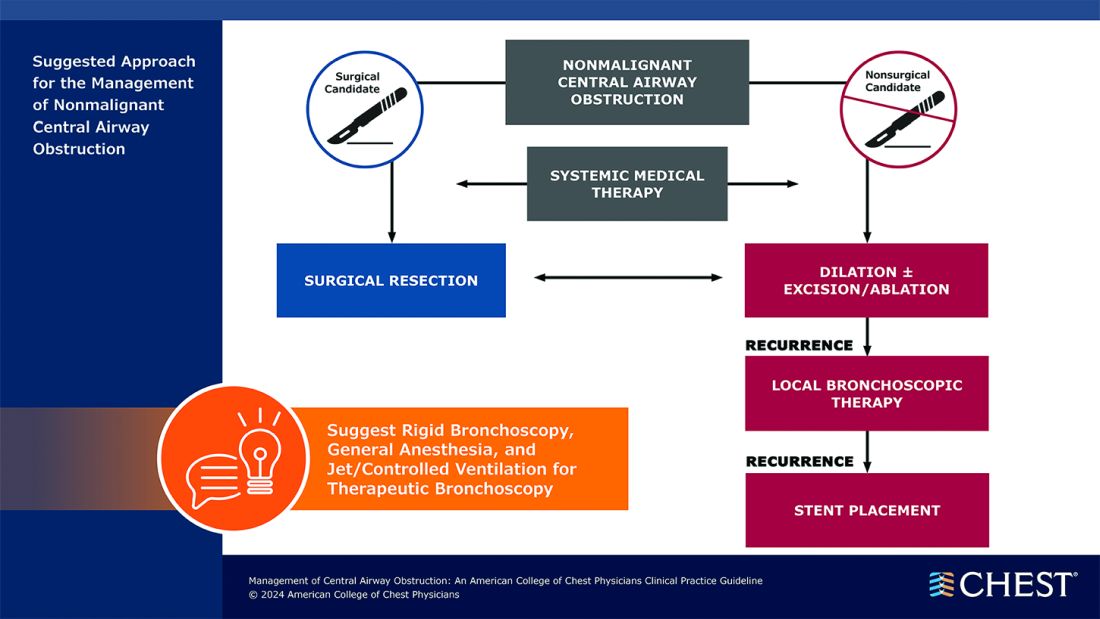
CAO can be caused by a variety of malignant and nonmalignant disorders, and multiple specialists may be involved in the care, including pulmonologists, interventional pulmonologists, radiologists, anesthesiologists, oncologists, radiation oncologists, thoracic surgeons and otolaryngologists, etc. The panel recommends shared decision-making with the patients and a multidisciplinary approach to manage CAO.
Below, you’ll find flowcharts outlining the suggested treatments for patients with a malignant or nonmalignant CAO.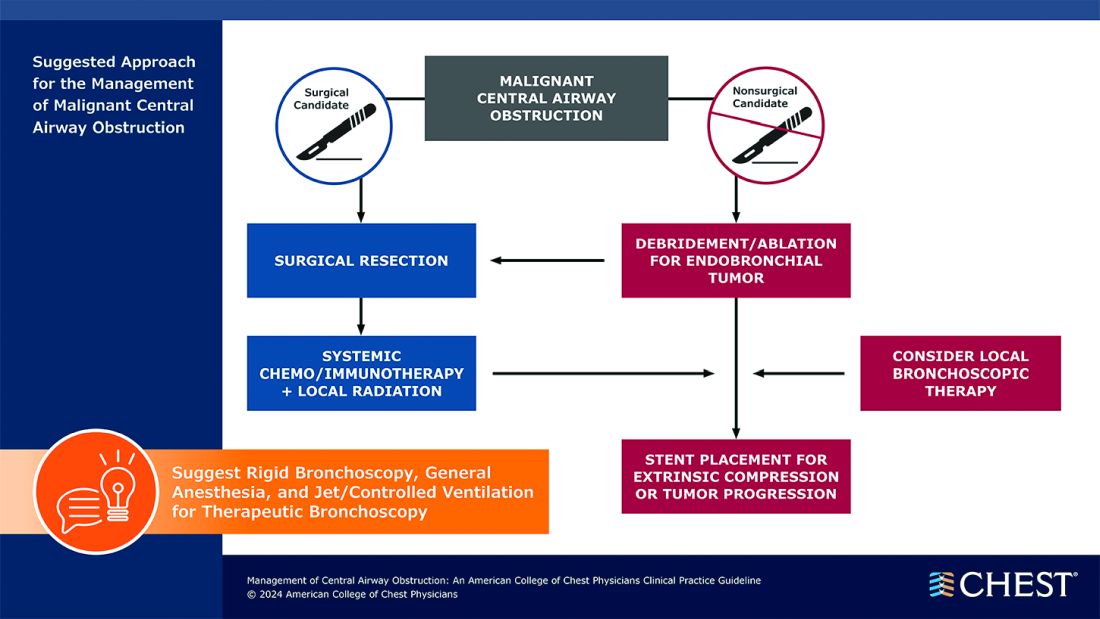
Visit www.chestnet.org/CAO-guideline to download the flowcharts and access the complete guideline.
CHEST recently released a new clinical guideline on central airway obstruction (CAO).
“Central airway obstruction is associated with a poor prognosis, and the management of CAO is highly variable dependent on the provider expertise and local resources. By releasing this guideline, the panel hopes to standardize the definition of CAO and provide guidance for the management of patients to optimize care and improve outcomes,” said Kamran Mahmood, MD, MPH, FCCP, lead author on the guideline. “The guideline recommendations are developed using GRADE methodology and based on thorough evidence review and expert input. But the quality of overall evidence is very low, and the panel calls for well-designed studies and randomized controlled trials for the management of CAO.”
CAO can be caused by a variety of malignant and nonmalignant disorders, and multiple specialists may be involved in the care, including pulmonologists, interventional pulmonologists, radiologists, anesthesiologists, oncologists, radiation oncologists, thoracic surgeons and otolaryngologists, etc. The panel recommends shared decision-making with the patients and a multidisciplinary approach to manage CAO.
Below, you’ll find flowcharts outlining the suggested treatments for patients with a malignant or nonmalignant CAO.

Visit www.chestnet.org/CAO-guideline to download the flowcharts and access the complete guideline.
CHEST recently released a new clinical guideline on central airway obstruction (CAO).
“Central airway obstruction is associated with a poor prognosis, and the management of CAO is highly variable dependent on the provider expertise and local resources. By releasing this guideline, the panel hopes to standardize the definition of CAO and provide guidance for the management of patients to optimize care and improve outcomes,” said Kamran Mahmood, MD, MPH, FCCP, lead author on the guideline. “The guideline recommendations are developed using GRADE methodology and based on thorough evidence review and expert input. But the quality of overall evidence is very low, and the panel calls for well-designed studies and randomized controlled trials for the management of CAO.”
CAO can be caused by a variety of malignant and nonmalignant disorders, and multiple specialists may be involved in the care, including pulmonologists, interventional pulmonologists, radiologists, anesthesiologists, oncologists, radiation oncologists, thoracic surgeons and otolaryngologists, etc. The panel recommends shared decision-making with the patients and a multidisciplinary approach to manage CAO.
Below, you’ll find flowcharts outlining the suggested treatments for patients with a malignant or nonmalignant CAO.

Visit www.chestnet.org/CAO-guideline to download the flowcharts and access the complete guideline.
Changing the tumor board conversation: Immunotherapy in resectable NSCLC
Without a doubt, immunotherapy has transformed the treatment landscape of non-small cell lung cancer (NSCLC) and enhanced survival rates across the different stages of disease. High recurrence rates following complete surgical resection prompted the study of immune checkpoint inhibitors (ICI) in earlier, operable stages of disease. This shift toward early application of ICI reflects the larger trend toward merging precision oncology with lung cancer staging. The resulting complexity in treatment and decision making creates systemic and logistical challenges that will require health care systems to adapt and improve.
Adjuvant immunotherapy for NSCLC
Prior to recent approvals for adjuvant immunotherapy, it was standard to give chemotherapy following resection of stage IB-IIIA disease, which offered a statistically nonsignificant survival gain. Recurrence in these patients is believed to be related to postsurgical micrometastasis. The utilization of alternative mechanisms to prevent recurrence is increasingly more common.
Atezolizumab, a PD-L1 inhibitor, is currently approved as first-line adjuvant treatment following chemotherapy in post-NSCLC resection patients with PD-L1 scores ≥1%. This category one recommendation by the National Comprehensive Cancer Network (NCCN) is based on results from the IMpower010 trial, which randomized patients to Atezolizumab vs best supportive care. All were early-stage NSCLC, stage IB-IIIA, who underwent resection followed by platinum-based chemotherapy. Statistically significant benefits were found in disease-free survival (DFS) with a trend toward overall survival.1
The PEARLS/KEYNOTE-091 trial evaluated another PD-L1 inhibitor, Pembrolizumab, as adjuvant therapy. Its design largely mirrored the IMPower010 study, but it differed in that the ICI was administered with or without chemotherapy following resection in patients with stage IB-IIIA NSCLC. Improvements in DFS were found in the overall population, leading to FDA approval for adjuvant therapy in 2023.2
These approvals require changes to the management of operable NSCLC. Until recently, it was not routine to send surgical specimens for additional testing because adjuvant treatment meant chemotherapy only. However, it is now essential that all surgically resected malignant tissue be sent for genomic sequencing and PD-L1 testing. Selecting the next form of therapy, whether it is an ICI or targeted drug therapy, depends on it.
From a surgical perspective, quality surgery with accurate nodal staging is crucial. The surgical findings can determine and identify those who are candidates for adjuvant immunotherapy. For these same reasons, it is helpful to advise surgeons preoperatively that targeted adjuvant therapy is being considered after resection.
Neoadjuvant immunotherapy for NSCLC
ICIs have also been used as neoadjuvant treatment for operable NSCLC. In 2021, the Checkmate-816 trial evaluated Nivolumab with platinum doublet chemotherapy prior to resection of stage IB-IIIa NSCLC. When compared with chemotherapy alone, there were significant improvements in EFS, MPR, and time to death or distant metastasis (TTDM) out to 3 years. At a median follow-up time of 41.4 months, only 28% in the nivolumab group had recurrence postsurgery compared with 42% in the chemotherapy-alone group.3 As a result, certain patients who are likely to receive adjuvant chemotherapy may additionally receive neoadjuvant immunotherapy with chemotherapy before surgical resection. In 2023, the KEYNOTE-671 study demonstrated that neoadjuvant Pembrolizumab and chemotherapy in patients with resectable stage II-IIIb (N2 stage) NSCLC improved EFS. At a median follow-up of 25.2 months, the EFS was 62.4% in the Pembrolizumab group vs 40.6% in the placebo group (P < .001).4
Such changes in treatment options mean patients should be discussed first and simultaneous referrals to oncology and surgery should occur in early-stage NSCLC. Up-front genomic phenotyping and PD-L1 testing may assist in decision making. High PD-L1 levels correlate better with response.
When an ICI-chemotherapy combination is given up front for newly diagnosed NSCLC, there is the potential for large reductions in tumor size and lymph node burden. Although the NCCN does not recommend ICIs to induce resectability, a patient originally deemed inoperable could theoretically become a surgical candidate with neoadjuvant ICI treatment. There is also the potential for toxicity, which could increase the risk of surgery when it does occur. Such scenarios will require frequent tumor board discussions so plans can be adjusted in real time to optimize outcomes as clinical circumstances change.
Perioperative immunotherapy for NSCLC
It is clear that both neoadjuvant and adjuvant immunotherapy can improve outcomes for patients with resectable NSCLC. The combination of neoadjuvant with adjuvant immunotherapy/chemotherapy is currently being studied. Two recent phase III clinical trials, NEOTORCH and AEGAEN, have found statistical improvements in EFS and MPR with this approach.5,6 These studies have not found their way into the NCCN guidelines yet but are sure to be considered in future iterations. Once adopted, the tumor board at each institution will have more options to choose from but many more decisions to make.
References
1. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357. [Published correction appears in Lancet. 2021 Nov 6;398(10312):1686.]
2. O’Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286.
3. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985.
4. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491-503.
5. Lu S, Zhang W, Wu L, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. 2024;331(3):201-211.
6. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684.
Without a doubt, immunotherapy has transformed the treatment landscape of non-small cell lung cancer (NSCLC) and enhanced survival rates across the different stages of disease. High recurrence rates following complete surgical resection prompted the study of immune checkpoint inhibitors (ICI) in earlier, operable stages of disease. This shift toward early application of ICI reflects the larger trend toward merging precision oncology with lung cancer staging. The resulting complexity in treatment and decision making creates systemic and logistical challenges that will require health care systems to adapt and improve.
Adjuvant immunotherapy for NSCLC
Prior to recent approvals for adjuvant immunotherapy, it was standard to give chemotherapy following resection of stage IB-IIIA disease, which offered a statistically nonsignificant survival gain. Recurrence in these patients is believed to be related to postsurgical micrometastasis. The utilization of alternative mechanisms to prevent recurrence is increasingly more common.
Atezolizumab, a PD-L1 inhibitor, is currently approved as first-line adjuvant treatment following chemotherapy in post-NSCLC resection patients with PD-L1 scores ≥1%. This category one recommendation by the National Comprehensive Cancer Network (NCCN) is based on results from the IMpower010 trial, which randomized patients to Atezolizumab vs best supportive care. All were early-stage NSCLC, stage IB-IIIA, who underwent resection followed by platinum-based chemotherapy. Statistically significant benefits were found in disease-free survival (DFS) with a trend toward overall survival.1
The PEARLS/KEYNOTE-091 trial evaluated another PD-L1 inhibitor, Pembrolizumab, as adjuvant therapy. Its design largely mirrored the IMPower010 study, but it differed in that the ICI was administered with or without chemotherapy following resection in patients with stage IB-IIIA NSCLC. Improvements in DFS were found in the overall population, leading to FDA approval for adjuvant therapy in 2023.2
These approvals require changes to the management of operable NSCLC. Until recently, it was not routine to send surgical specimens for additional testing because adjuvant treatment meant chemotherapy only. However, it is now essential that all surgically resected malignant tissue be sent for genomic sequencing and PD-L1 testing. Selecting the next form of therapy, whether it is an ICI or targeted drug therapy, depends on it.
From a surgical perspective, quality surgery with accurate nodal staging is crucial. The surgical findings can determine and identify those who are candidates for adjuvant immunotherapy. For these same reasons, it is helpful to advise surgeons preoperatively that targeted adjuvant therapy is being considered after resection.
Neoadjuvant immunotherapy for NSCLC
ICIs have also been used as neoadjuvant treatment for operable NSCLC. In 2021, the Checkmate-816 trial evaluated Nivolumab with platinum doublet chemotherapy prior to resection of stage IB-IIIa NSCLC. When compared with chemotherapy alone, there were significant improvements in EFS, MPR, and time to death or distant metastasis (TTDM) out to 3 years. At a median follow-up time of 41.4 months, only 28% in the nivolumab group had recurrence postsurgery compared with 42% in the chemotherapy-alone group.3 As a result, certain patients who are likely to receive adjuvant chemotherapy may additionally receive neoadjuvant immunotherapy with chemotherapy before surgical resection. In 2023, the KEYNOTE-671 study demonstrated that neoadjuvant Pembrolizumab and chemotherapy in patients with resectable stage II-IIIb (N2 stage) NSCLC improved EFS. At a median follow-up of 25.2 months, the EFS was 62.4% in the Pembrolizumab group vs 40.6% in the placebo group (P < .001).4

Such changes in treatment options mean patients should be discussed first and simultaneous referrals to oncology and surgery should occur in early-stage NSCLC. Up-front genomic phenotyping and PD-L1 testing may assist in decision making. High PD-L1 levels correlate better with response.
When an ICI-chemotherapy combination is given up front for newly diagnosed NSCLC, there is the potential for large reductions in tumor size and lymph node burden. Although the NCCN does not recommend ICIs to induce resectability, a patient originally deemed inoperable could theoretically become a surgical candidate with neoadjuvant ICI treatment. There is also the potential for toxicity, which could increase the risk of surgery when it does occur. Such scenarios will require frequent tumor board discussions so plans can be adjusted in real time to optimize outcomes as clinical circumstances change.
Perioperative immunotherapy for NSCLC
It is clear that both neoadjuvant and adjuvant immunotherapy can improve outcomes for patients with resectable NSCLC. The combination of neoadjuvant with adjuvant immunotherapy/chemotherapy is currently being studied. Two recent phase III clinical trials, NEOTORCH and AEGAEN, have found statistical improvements in EFS and MPR with this approach.5,6 These studies have not found their way into the NCCN guidelines yet but are sure to be considered in future iterations. Once adopted, the tumor board at each institution will have more options to choose from but many more decisions to make.
References
1. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357. [Published correction appears in Lancet. 2021 Nov 6;398(10312):1686.]
2. O’Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286.
3. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985.
4. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491-503.
5. Lu S, Zhang W, Wu L, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. 2024;331(3):201-211.
6. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684.
Without a doubt, immunotherapy has transformed the treatment landscape of non-small cell lung cancer (NSCLC) and enhanced survival rates across the different stages of disease. High recurrence rates following complete surgical resection prompted the study of immune checkpoint inhibitors (ICI) in earlier, operable stages of disease. This shift toward early application of ICI reflects the larger trend toward merging precision oncology with lung cancer staging. The resulting complexity in treatment and decision making creates systemic and logistical challenges that will require health care systems to adapt and improve.
Adjuvant immunotherapy for NSCLC
Prior to recent approvals for adjuvant immunotherapy, it was standard to give chemotherapy following resection of stage IB-IIIA disease, which offered a statistically nonsignificant survival gain. Recurrence in these patients is believed to be related to postsurgical micrometastasis. The utilization of alternative mechanisms to prevent recurrence is increasingly more common.
Atezolizumab, a PD-L1 inhibitor, is currently approved as first-line adjuvant treatment following chemotherapy in post-NSCLC resection patients with PD-L1 scores ≥1%. This category one recommendation by the National Comprehensive Cancer Network (NCCN) is based on results from the IMpower010 trial, which randomized patients to Atezolizumab vs best supportive care. All were early-stage NSCLC, stage IB-IIIA, who underwent resection followed by platinum-based chemotherapy. Statistically significant benefits were found in disease-free survival (DFS) with a trend toward overall survival.1
The PEARLS/KEYNOTE-091 trial evaluated another PD-L1 inhibitor, Pembrolizumab, as adjuvant therapy. Its design largely mirrored the IMPower010 study, but it differed in that the ICI was administered with or without chemotherapy following resection in patients with stage IB-IIIA NSCLC. Improvements in DFS were found in the overall population, leading to FDA approval for adjuvant therapy in 2023.2
These approvals require changes to the management of operable NSCLC. Until recently, it was not routine to send surgical specimens for additional testing because adjuvant treatment meant chemotherapy only. However, it is now essential that all surgically resected malignant tissue be sent for genomic sequencing and PD-L1 testing. Selecting the next form of therapy, whether it is an ICI or targeted drug therapy, depends on it.
From a surgical perspective, quality surgery with accurate nodal staging is crucial. The surgical findings can determine and identify those who are candidates for adjuvant immunotherapy. For these same reasons, it is helpful to advise surgeons preoperatively that targeted adjuvant therapy is being considered after resection.
Neoadjuvant immunotherapy for NSCLC
ICIs have also been used as neoadjuvant treatment for operable NSCLC. In 2021, the Checkmate-816 trial evaluated Nivolumab with platinum doublet chemotherapy prior to resection of stage IB-IIIa NSCLC. When compared with chemotherapy alone, there were significant improvements in EFS, MPR, and time to death or distant metastasis (TTDM) out to 3 years. At a median follow-up time of 41.4 months, only 28% in the nivolumab group had recurrence postsurgery compared with 42% in the chemotherapy-alone group.3 As a result, certain patients who are likely to receive adjuvant chemotherapy may additionally receive neoadjuvant immunotherapy with chemotherapy before surgical resection. In 2023, the KEYNOTE-671 study demonstrated that neoadjuvant Pembrolizumab and chemotherapy in patients with resectable stage II-IIIb (N2 stage) NSCLC improved EFS. At a median follow-up of 25.2 months, the EFS was 62.4% in the Pembrolizumab group vs 40.6% in the placebo group (P < .001).4

Such changes in treatment options mean patients should be discussed first and simultaneous referrals to oncology and surgery should occur in early-stage NSCLC. Up-front genomic phenotyping and PD-L1 testing may assist in decision making. High PD-L1 levels correlate better with response.
When an ICI-chemotherapy combination is given up front for newly diagnosed NSCLC, there is the potential for large reductions in tumor size and lymph node burden. Although the NCCN does not recommend ICIs to induce resectability, a patient originally deemed inoperable could theoretically become a surgical candidate with neoadjuvant ICI treatment. There is also the potential for toxicity, which could increase the risk of surgery when it does occur. Such scenarios will require frequent tumor board discussions so plans can be adjusted in real time to optimize outcomes as clinical circumstances change.
Perioperative immunotherapy for NSCLC
It is clear that both neoadjuvant and adjuvant immunotherapy can improve outcomes for patients with resectable NSCLC. The combination of neoadjuvant with adjuvant immunotherapy/chemotherapy is currently being studied. Two recent phase III clinical trials, NEOTORCH and AEGAEN, have found statistical improvements in EFS and MPR with this approach.5,6 These studies have not found their way into the NCCN guidelines yet but are sure to be considered in future iterations. Once adopted, the tumor board at each institution will have more options to choose from but many more decisions to make.
References
1. Felip E, Altorki N, Zhou C, et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344-1357. [Published correction appears in Lancet. 2021 Nov 6;398(10312):1686.]
2. O’Brien M, Paz-Ares L, Marreaud S, et al. Pembrolizumab versus placebo as adjuvant therapy for completely resected stage IB-IIIA non-small-cell lung cancer (PEARLS/KEYNOTE-091): an interim analysis of a randomised, triple-blind, phase 3 trial. Lancet Oncol. 2022;23(10):1274-1286.
3. Forde PM, Spicer J, Lu S, et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N Engl J Med. 2022;386(21):1973-1985.
4. Wakelee H, Liberman M, Kato T, et al. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N Engl J Med. 2023;389(6):491-503.
5. Lu S, Zhang W, Wu L, et al. Perioperative toripalimab plus chemotherapy for patients with resectable non-small cell lung cancer: the neotorch randomized clinical trial. JAMA. 2024;331(3):201-211.
6. Heymach JV, Harpole D, Mitsudomi T, et al. Perioperative durvalumab for resectable non-small-cell lung cancer. N Engl J Med. 2023;389(18):1672-1684.
Trauma epidemiology and the organization of trauma care in the US
CHEST INFECTIONS AND DISASTER RESPONSE NETWORK
Disaster Response and Global Health Section
Patients who are injured do better when treated at trauma centers.
During CHEST 2023 in Honolulu last year, the Disaster Response and Global Health Section hosted a presentation to a packed audience highlighting the history of the trauma model system in America. Attendees learned about the emergence of trauma systems in the US and the survival advantages that trauma centers offer to patients who are injured.
Inception of the first “trauma manual” was during President Lincoln’s term to address the need for a process to care for patients who were injured during the Civil War. By the time World War II occurred, hospitals had established the idea of emergency departments, and the foundations for an emergency medical system (EMS) emerged on the world scene. Eventually, the Hill-Burton Act of 1946 required hospitals to have emergency departments by incentivizing them with federal funding.
The Committee on Trauma was founded in the 1950s as an adaptation of the American College of Surgeons’ 1922 Committee on Fractures. Then, in 1966, the National Highway Traffic Safety Administration rolled out a federal initiative for all states to develop EMS programs. Additionally, the National Academy of Sciences published Accidental Death and Disability: The Neglected Disease of Modern Society, paving the way for the Emergency Medical Services Systems Act of 1973, along with the Wedworth-Townsend Paramedic Act in California.
The concept of today’s trauma centers started in 1966 at Cook County Hospital in Chicago and Shock Trauma Center in Baltimore.
CHEST INFECTIONS AND DISASTER RESPONSE NETWORK
Disaster Response and Global Health Section
Patients who are injured do better when treated at trauma centers.
During CHEST 2023 in Honolulu last year, the Disaster Response and Global Health Section hosted a presentation to a packed audience highlighting the history of the trauma model system in America. Attendees learned about the emergence of trauma systems in the US and the survival advantages that trauma centers offer to patients who are injured.
Inception of the first “trauma manual” was during President Lincoln’s term to address the need for a process to care for patients who were injured during the Civil War. By the time World War II occurred, hospitals had established the idea of emergency departments, and the foundations for an emergency medical system (EMS) emerged on the world scene. Eventually, the Hill-Burton Act of 1946 required hospitals to have emergency departments by incentivizing them with federal funding.
The Committee on Trauma was founded in the 1950s as an adaptation of the American College of Surgeons’ 1922 Committee on Fractures. Then, in 1966, the National Highway Traffic Safety Administration rolled out a federal initiative for all states to develop EMS programs. Additionally, the National Academy of Sciences published Accidental Death and Disability: The Neglected Disease of Modern Society, paving the way for the Emergency Medical Services Systems Act of 1973, along with the Wedworth-Townsend Paramedic Act in California.
The concept of today’s trauma centers started in 1966 at Cook County Hospital in Chicago and Shock Trauma Center in Baltimore.
CHEST INFECTIONS AND DISASTER RESPONSE NETWORK
Disaster Response and Global Health Section
Patients who are injured do better when treated at trauma centers.
During CHEST 2023 in Honolulu last year, the Disaster Response and Global Health Section hosted a presentation to a packed audience highlighting the history of the trauma model system in America. Attendees learned about the emergence of trauma systems in the US and the survival advantages that trauma centers offer to patients who are injured.
Inception of the first “trauma manual” was during President Lincoln’s term to address the need for a process to care for patients who were injured during the Civil War. By the time World War II occurred, hospitals had established the idea of emergency departments, and the foundations for an emergency medical system (EMS) emerged on the world scene. Eventually, the Hill-Burton Act of 1946 required hospitals to have emergency departments by incentivizing them with federal funding.
The Committee on Trauma was founded in the 1950s as an adaptation of the American College of Surgeons’ 1922 Committee on Fractures. Then, in 1966, the National Highway Traffic Safety Administration rolled out a federal initiative for all states to develop EMS programs. Additionally, the National Academy of Sciences published Accidental Death and Disability: The Neglected Disease of Modern Society, paving the way for the Emergency Medical Services Systems Act of 1973, along with the Wedworth-Townsend Paramedic Act in California.
The concept of today’s trauma centers started in 1966 at Cook County Hospital in Chicago and Shock Trauma Center in Baltimore.
On 5 Ps: PSG, PM, PPG, PulseOx, and PAT
OSA is a very prevalent condition in the general population, but still many patients remain undiagnosed and untreated. Prolonged, untreated OSA is an independent risk factor for major cardiovascular morbidity and mortality. Therefore, timely diagnosis and treatment are required.
Polysomnography (PSG) remains to this day the gold standard for diagnosing sleep apnea. A standard PSG (type I) is performed in a sleep laboratory in the presence of specialized sleep technicians and utilizes EEG, electrooculogram (EOG), and electromyogram (EMG) to determine sleep stages, oronasal thermal and pressure transducer sensors to monitor airflow, respiratory inductance plethysmography to record respiratory effort, EMG for limb movements, pulse oximetry (PulseOx), ECG, and video or body sensor devices to confirm body position. Rising rates of sleep testing have created demand for an alternative to cumbersome, costly, and resource-intensive in-lab PSGs. As such, home sleep apnea testing (HSAT) has emerged as a simpler, more accessible, and cost-effective alternative diagnostic tool.
In 2007, the American Academy of Sleep Medicine (AASM) endorsed Portable Monitoring (PM) as an alternative to standard PSG, with the caveat that it should be used only in patients with a high pretest probability of sleep apnea, without respiratory or cardiovascular disorders and comorbid sleep disorders. All HSAT devices (type II-IV) are required to have a minimum of an oronasal thermal sensor/nasal pressure transducer, respiratory inductance plethysmography, and PulseOx. A major limitation of most HSAT devices is the lack of EEG, preventing detection of cortical arousals and wake time, forcing the use of total recording time as a surrogate for total sleep time.
Peripheral arterial tonometry (PAT)-based HSAT devices are unique in this respect, as their proprietary algorithms allow estimates of total sleep time by monitoring changes in peripheral vascular tone. Anyone who has seen a PAT-based HSAT may have noticed very different outputs from traditional HSATs.
PAT is based on the concept that airflow obstruction may lead to a surge in sympathetic tone, causing vasoconstriction and reduced blood volume in the peripheral vascular bed. A PAT-based device measures relative changes in blood volume and combines this information with actigraphy signals, PulseOx, and heart rate to diagnose the presence of respiratory events. Sleep apnea severity stratification is accomplished by the use of pAHI or pRDI (PAT-based apnea-hypopnea index and respiratory disturbance index, respectively).
PAT-based technology was first approved by the FDA in 2001 as a diagnostic tool for sleep apnea. The 2 best-known medical devices are WatchPAT® and NightOwl®, both of which have been FDA-approved and studied against PSG. To obtain an accurate and sustainable PAT signal, WatchPAT has a pneumo-optic finger probe designed to generate a uniform, subdiastolic pressure on the finger that minimizes venous blood pooling, prevents uncontrolled venous backflow, and effectively unloads the arterial wall tension without blocking digital arterial flow. NightOwl is a smaller device, with a single fingertip sensor that acquires actigraphy and PPG data to measure heart rate, Pulse Ox, and PAT.
The physiological basis of PAT relies on photoplethysmography (PPG), a noninvasive optical monitoring technique that generates a waveform, which ultimately correlates with the circulatory volume of the respective tissue.1 The PPG technology relies on the fact that when a specific tissue is exposed to light signal of a specific wavelength, its absorbance by tissue fluctuates with arterial pulsations. Pulse oximetry represents the most used application of PPG. Recent advances in PPG signal analysis have fueled its use in clinical and consumer sleep technologies and allowed new capabilities, including capturing heart rate and rhythm, pulse rate variability, arterial stiffness, and even—with somewhat less accuracy—energy expenditure, maximum O2 consumption, and blood pressure. Combining actigraphy monitoring with PPG technology took both consumer and medical-grade sleep technologies further, allowing the estimation of parameters such as sleep stages, sleep times, and respiratory events. With myriad new sleep trackers claiming to assess total sleep time, wake time, light or deep sleep, and even respiratory events, the obvious clinical question is centered on their comparative accuracy, as well against more traditional PM and the gold standard PSG.
Numerous studies have evaluated the efficacy of PAT-based devices for diagnosing sleep apnea, with variable findings. Many have shown good correlations between mean AHI and pAHI. Others have highlighted significant discrepancies in the measurements between PSG and PAT, questioning the reliability of PAT-based devices in the diagnosis and severity stratification of OSA. One meta-analysis of 14 studies showed a high degree of correlation between pAHI and AHI.2 Another study reported a concordance of 80% between PAT-based testing and consecutive PSG, with an increase to 86% at a higher AHI (>15/h).3 A subsequent meta-analysis showed that PAT was significantly less sensitive for diagnosing OSA than PSG, particularly for mild or moderate severity disease, emphasizing the need for further confirmation with PSG when faced with inconclusive or negative results.4 A large sleep clinic-based cohort study of 500 patients with OSA showed that WatchPAT devices misclassify OSA in a sizeable proportion of patients (30%-50%), leading to both over- and under-estimation of severity.5 Van Pee, et al, found that their PAT-based HSAT NightOwl performed better, using both the 3% and 4% hypopnea scoring rules and a novel near-border zone labeling.6
Some of the discordance in AHI between PAT and PSG appears to be related to age and sex. In our large sample comparing PAT to PSG, we found that using PAT-based data in concert with demographic (age, gender) and anthropometric (neck circumference, body mass index) variables improved the diagnostic accuracy of PAT-based testing.7 Another study concluded that manual scoring of WatchPAT automated results improved concordance with PSG, particularly in older participants and women. Several studies on WatchPAT recordings have demonstrated significant artifacts and inaccuracies in the PulseOx data. Although WatchPAT employs automated algorithms to remove erroneous data, a thorough visual inspection and manual correction of study data is still essential to derive accurate results.
Recent studies have found that PAT-based tests can also differentiate between central and obstructive respiratory events by using pulse signal upstroke variations caused by changes in intrathoracic pressure and respiratory/chest wall movement recorded by body position sensors, but large-scale studies are needed to confirm these findings. Korkalainen, et al, recently employed a deep-learning model to perform sleep staging on the PPG PulseOx signals from nearly 900 PSGs in patients with suspected OSA.8 The deep learning approach enabled the differentiation of sleep stages and accurate estimation of the total sleep time. Going forward, this could easily enhance the diagnostic yield of PM recordings and enable cost-efficient, long-term monitoring of sleep.
Although PAT-based home sleep tests have emerged as a simple and convenient option for the evaluation of sleep apnea, several studies have highlighted their limited sensitivity as a screening tool for mild and moderate cases of sleep apnea. Furthermore, the scope of these tests remains limited, rendering them rather unsuitable for assessment of more complex sleep disorders like narcolepsy or restless leg syndrome. Therefore, when OSA is suspected, the PAT-based sleep study is a good screening tool, but negative tests should not preclude further investigation. Where a high probability of sleep apnea exists but PAT-based testing shows no or mild OSA, an in-lab sleep study should be performed.
References
1. Ryals S, Chiang A, Schutte-Rodin S, et al. Photoplethysmography -- new applications for an old technology: a sleep technology review. J Clin Sleep Med. 2023;19(1):189-195.
2. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343-1350.
3. Röcken J, Schumann DM, Herrmann MJ, et al. Peripheral arterial tonometry versus polysomnography in suspected obstructive sleep apnoea. Eur J Med Res. 2023;28(1):251.
4. Iftikhar IH, Finch CE, Shah AS, Augunstein CA, Ioachimescu OC. A meta-analysis of diagnostic test performance of peripheral arterial tonometry studies. J Clin Sleep Med. 2022;18(4):1093-1102.
5. Ioachimescu OC, Allam JS, Samarghandi A, et al. Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J Clin Sleep Med. 2020;16(10):1663-1674.
6. Van Pee B, Massie F, Vits S, et al. A multicentric validation study of a novel home sleep apnea test based on peripheral arterial tonometry. Sleep. 2022;45(5).
7. Ioachimescu OC, Dholakia SA, Venkateshiah SB, et al. Improving the performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea. J Investig Med. 2020;68(8):1370-1378.
8. Korkalainen H, Aakko J, Duce B, et al. Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43(11).
OSA is a very prevalent condition in the general population, but still many patients remain undiagnosed and untreated. Prolonged, untreated OSA is an independent risk factor for major cardiovascular morbidity and mortality. Therefore, timely diagnosis and treatment are required.
Polysomnography (PSG) remains to this day the gold standard for diagnosing sleep apnea. A standard PSG (type I) is performed in a sleep laboratory in the presence of specialized sleep technicians and utilizes EEG, electrooculogram (EOG), and electromyogram (EMG) to determine sleep stages, oronasal thermal and pressure transducer sensors to monitor airflow, respiratory inductance plethysmography to record respiratory effort, EMG for limb movements, pulse oximetry (PulseOx), ECG, and video or body sensor devices to confirm body position. Rising rates of sleep testing have created demand for an alternative to cumbersome, costly, and resource-intensive in-lab PSGs. As such, home sleep apnea testing (HSAT) has emerged as a simpler, more accessible, and cost-effective alternative diagnostic tool.
In 2007, the American Academy of Sleep Medicine (AASM) endorsed Portable Monitoring (PM) as an alternative to standard PSG, with the caveat that it should be used only in patients with a high pretest probability of sleep apnea, without respiratory or cardiovascular disorders and comorbid sleep disorders. All HSAT devices (type II-IV) are required to have a minimum of an oronasal thermal sensor/nasal pressure transducer, respiratory inductance plethysmography, and PulseOx. A major limitation of most HSAT devices is the lack of EEG, preventing detection of cortical arousals and wake time, forcing the use of total recording time as a surrogate for total sleep time.
Peripheral arterial tonometry (PAT)-based HSAT devices are unique in this respect, as their proprietary algorithms allow estimates of total sleep time by monitoring changes in peripheral vascular tone. Anyone who has seen a PAT-based HSAT may have noticed very different outputs from traditional HSATs.
PAT is based on the concept that airflow obstruction may lead to a surge in sympathetic tone, causing vasoconstriction and reduced blood volume in the peripheral vascular bed. A PAT-based device measures relative changes in blood volume and combines this information with actigraphy signals, PulseOx, and heart rate to diagnose the presence of respiratory events. Sleep apnea severity stratification is accomplished by the use of pAHI or pRDI (PAT-based apnea-hypopnea index and respiratory disturbance index, respectively).
PAT-based technology was first approved by the FDA in 2001 as a diagnostic tool for sleep apnea. The 2 best-known medical devices are WatchPAT® and NightOwl®, both of which have been FDA-approved and studied against PSG. To obtain an accurate and sustainable PAT signal, WatchPAT has a pneumo-optic finger probe designed to generate a uniform, subdiastolic pressure on the finger that minimizes venous blood pooling, prevents uncontrolled venous backflow, and effectively unloads the arterial wall tension without blocking digital arterial flow. NightOwl is a smaller device, with a single fingertip sensor that acquires actigraphy and PPG data to measure heart rate, Pulse Ox, and PAT.
The physiological basis of PAT relies on photoplethysmography (PPG), a noninvasive optical monitoring technique that generates a waveform, which ultimately correlates with the circulatory volume of the respective tissue.1 The PPG technology relies on the fact that when a specific tissue is exposed to light signal of a specific wavelength, its absorbance by tissue fluctuates with arterial pulsations. Pulse oximetry represents the most used application of PPG. Recent advances in PPG signal analysis have fueled its use in clinical and consumer sleep technologies and allowed new capabilities, including capturing heart rate and rhythm, pulse rate variability, arterial stiffness, and even—with somewhat less accuracy—energy expenditure, maximum O2 consumption, and blood pressure. Combining actigraphy monitoring with PPG technology took both consumer and medical-grade sleep technologies further, allowing the estimation of parameters such as sleep stages, sleep times, and respiratory events. With myriad new sleep trackers claiming to assess total sleep time, wake time, light or deep sleep, and even respiratory events, the obvious clinical question is centered on their comparative accuracy, as well against more traditional PM and the gold standard PSG.
Numerous studies have evaluated the efficacy of PAT-based devices for diagnosing sleep apnea, with variable findings. Many have shown good correlations between mean AHI and pAHI. Others have highlighted significant discrepancies in the measurements between PSG and PAT, questioning the reliability of PAT-based devices in the diagnosis and severity stratification of OSA. One meta-analysis of 14 studies showed a high degree of correlation between pAHI and AHI.2 Another study reported a concordance of 80% between PAT-based testing and consecutive PSG, with an increase to 86% at a higher AHI (>15/h).3 A subsequent meta-analysis showed that PAT was significantly less sensitive for diagnosing OSA than PSG, particularly for mild or moderate severity disease, emphasizing the need for further confirmation with PSG when faced with inconclusive or negative results.4 A large sleep clinic-based cohort study of 500 patients with OSA showed that WatchPAT devices misclassify OSA in a sizeable proportion of patients (30%-50%), leading to both over- and under-estimation of severity.5 Van Pee, et al, found that their PAT-based HSAT NightOwl performed better, using both the 3% and 4% hypopnea scoring rules and a novel near-border zone labeling.6
Some of the discordance in AHI between PAT and PSG appears to be related to age and sex. In our large sample comparing PAT to PSG, we found that using PAT-based data in concert with demographic (age, gender) and anthropometric (neck circumference, body mass index) variables improved the diagnostic accuracy of PAT-based testing.7 Another study concluded that manual scoring of WatchPAT automated results improved concordance with PSG, particularly in older participants and women. Several studies on WatchPAT recordings have demonstrated significant artifacts and inaccuracies in the PulseOx data. Although WatchPAT employs automated algorithms to remove erroneous data, a thorough visual inspection and manual correction of study data is still essential to derive accurate results.
Recent studies have found that PAT-based tests can also differentiate between central and obstructive respiratory events by using pulse signal upstroke variations caused by changes in intrathoracic pressure and respiratory/chest wall movement recorded by body position sensors, but large-scale studies are needed to confirm these findings. Korkalainen, et al, recently employed a deep-learning model to perform sleep staging on the PPG PulseOx signals from nearly 900 PSGs in patients with suspected OSA.8 The deep learning approach enabled the differentiation of sleep stages and accurate estimation of the total sleep time. Going forward, this could easily enhance the diagnostic yield of PM recordings and enable cost-efficient, long-term monitoring of sleep.
Although PAT-based home sleep tests have emerged as a simple and convenient option for the evaluation of sleep apnea, several studies have highlighted their limited sensitivity as a screening tool for mild and moderate cases of sleep apnea. Furthermore, the scope of these tests remains limited, rendering them rather unsuitable for assessment of more complex sleep disorders like narcolepsy or restless leg syndrome. Therefore, when OSA is suspected, the PAT-based sleep study is a good screening tool, but negative tests should not preclude further investigation. Where a high probability of sleep apnea exists but PAT-based testing shows no or mild OSA, an in-lab sleep study should be performed.
References
1. Ryals S, Chiang A, Schutte-Rodin S, et al. Photoplethysmography -- new applications for an old technology: a sleep technology review. J Clin Sleep Med. 2023;19(1):189-195.
2. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343-1350.
3. Röcken J, Schumann DM, Herrmann MJ, et al. Peripheral arterial tonometry versus polysomnography in suspected obstructive sleep apnoea. Eur J Med Res. 2023;28(1):251.
4. Iftikhar IH, Finch CE, Shah AS, Augunstein CA, Ioachimescu OC. A meta-analysis of diagnostic test performance of peripheral arterial tonometry studies. J Clin Sleep Med. 2022;18(4):1093-1102.
5. Ioachimescu OC, Allam JS, Samarghandi A, et al. Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J Clin Sleep Med. 2020;16(10):1663-1674.
6. Van Pee B, Massie F, Vits S, et al. A multicentric validation study of a novel home sleep apnea test based on peripheral arterial tonometry. Sleep. 2022;45(5).
7. Ioachimescu OC, Dholakia SA, Venkateshiah SB, et al. Improving the performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea. J Investig Med. 2020;68(8):1370-1378.
8. Korkalainen H, Aakko J, Duce B, et al. Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43(11).
OSA is a very prevalent condition in the general population, but still many patients remain undiagnosed and untreated. Prolonged, untreated OSA is an independent risk factor for major cardiovascular morbidity and mortality. Therefore, timely diagnosis and treatment are required.
Polysomnography (PSG) remains to this day the gold standard for diagnosing sleep apnea. A standard PSG (type I) is performed in a sleep laboratory in the presence of specialized sleep technicians and utilizes EEG, electrooculogram (EOG), and electromyogram (EMG) to determine sleep stages, oronasal thermal and pressure transducer sensors to monitor airflow, respiratory inductance plethysmography to record respiratory effort, EMG for limb movements, pulse oximetry (PulseOx), ECG, and video or body sensor devices to confirm body position. Rising rates of sleep testing have created demand for an alternative to cumbersome, costly, and resource-intensive in-lab PSGs. As such, home sleep apnea testing (HSAT) has emerged as a simpler, more accessible, and cost-effective alternative diagnostic tool.
In 2007, the American Academy of Sleep Medicine (AASM) endorsed Portable Monitoring (PM) as an alternative to standard PSG, with the caveat that it should be used only in patients with a high pretest probability of sleep apnea, without respiratory or cardiovascular disorders and comorbid sleep disorders. All HSAT devices (type II-IV) are required to have a minimum of an oronasal thermal sensor/nasal pressure transducer, respiratory inductance plethysmography, and PulseOx. A major limitation of most HSAT devices is the lack of EEG, preventing detection of cortical arousals and wake time, forcing the use of total recording time as a surrogate for total sleep time.
Peripheral arterial tonometry (PAT)-based HSAT devices are unique in this respect, as their proprietary algorithms allow estimates of total sleep time by monitoring changes in peripheral vascular tone. Anyone who has seen a PAT-based HSAT may have noticed very different outputs from traditional HSATs.
PAT is based on the concept that airflow obstruction may lead to a surge in sympathetic tone, causing vasoconstriction and reduced blood volume in the peripheral vascular bed. A PAT-based device measures relative changes in blood volume and combines this information with actigraphy signals, PulseOx, and heart rate to diagnose the presence of respiratory events. Sleep apnea severity stratification is accomplished by the use of pAHI or pRDI (PAT-based apnea-hypopnea index and respiratory disturbance index, respectively).
PAT-based technology was first approved by the FDA in 2001 as a diagnostic tool for sleep apnea. The 2 best-known medical devices are WatchPAT® and NightOwl®, both of which have been FDA-approved and studied against PSG. To obtain an accurate and sustainable PAT signal, WatchPAT has a pneumo-optic finger probe designed to generate a uniform, subdiastolic pressure on the finger that minimizes venous blood pooling, prevents uncontrolled venous backflow, and effectively unloads the arterial wall tension without blocking digital arterial flow. NightOwl is a smaller device, with a single fingertip sensor that acquires actigraphy and PPG data to measure heart rate, Pulse Ox, and PAT.
The physiological basis of PAT relies on photoplethysmography (PPG), a noninvasive optical monitoring technique that generates a waveform, which ultimately correlates with the circulatory volume of the respective tissue.1 The PPG technology relies on the fact that when a specific tissue is exposed to light signal of a specific wavelength, its absorbance by tissue fluctuates with arterial pulsations. Pulse oximetry represents the most used application of PPG. Recent advances in PPG signal analysis have fueled its use in clinical and consumer sleep technologies and allowed new capabilities, including capturing heart rate and rhythm, pulse rate variability, arterial stiffness, and even—with somewhat less accuracy—energy expenditure, maximum O2 consumption, and blood pressure. Combining actigraphy monitoring with PPG technology took both consumer and medical-grade sleep technologies further, allowing the estimation of parameters such as sleep stages, sleep times, and respiratory events. With myriad new sleep trackers claiming to assess total sleep time, wake time, light or deep sleep, and even respiratory events, the obvious clinical question is centered on their comparative accuracy, as well against more traditional PM and the gold standard PSG.
Numerous studies have evaluated the efficacy of PAT-based devices for diagnosing sleep apnea, with variable findings. Many have shown good correlations between mean AHI and pAHI. Others have highlighted significant discrepancies in the measurements between PSG and PAT, questioning the reliability of PAT-based devices in the diagnosis and severity stratification of OSA. One meta-analysis of 14 studies showed a high degree of correlation between pAHI and AHI.2 Another study reported a concordance of 80% between PAT-based testing and consecutive PSG, with an increase to 86% at a higher AHI (>15/h).3 A subsequent meta-analysis showed that PAT was significantly less sensitive for diagnosing OSA than PSG, particularly for mild or moderate severity disease, emphasizing the need for further confirmation with PSG when faced with inconclusive or negative results.4 A large sleep clinic-based cohort study of 500 patients with OSA showed that WatchPAT devices misclassify OSA in a sizeable proportion of patients (30%-50%), leading to both over- and under-estimation of severity.5 Van Pee, et al, found that their PAT-based HSAT NightOwl performed better, using both the 3% and 4% hypopnea scoring rules and a novel near-border zone labeling.6
Some of the discordance in AHI between PAT and PSG appears to be related to age and sex. In our large sample comparing PAT to PSG, we found that using PAT-based data in concert with demographic (age, gender) and anthropometric (neck circumference, body mass index) variables improved the diagnostic accuracy of PAT-based testing.7 Another study concluded that manual scoring of WatchPAT automated results improved concordance with PSG, particularly in older participants and women. Several studies on WatchPAT recordings have demonstrated significant artifacts and inaccuracies in the PulseOx data. Although WatchPAT employs automated algorithms to remove erroneous data, a thorough visual inspection and manual correction of study data is still essential to derive accurate results.
Recent studies have found that PAT-based tests can also differentiate between central and obstructive respiratory events by using pulse signal upstroke variations caused by changes in intrathoracic pressure and respiratory/chest wall movement recorded by body position sensors, but large-scale studies are needed to confirm these findings. Korkalainen, et al, recently employed a deep-learning model to perform sleep staging on the PPG PulseOx signals from nearly 900 PSGs in patients with suspected OSA.8 The deep learning approach enabled the differentiation of sleep stages and accurate estimation of the total sleep time. Going forward, this could easily enhance the diagnostic yield of PM recordings and enable cost-efficient, long-term monitoring of sleep.
Although PAT-based home sleep tests have emerged as a simple and convenient option for the evaluation of sleep apnea, several studies have highlighted their limited sensitivity as a screening tool for mild and moderate cases of sleep apnea. Furthermore, the scope of these tests remains limited, rendering them rather unsuitable for assessment of more complex sleep disorders like narcolepsy or restless leg syndrome. Therefore, when OSA is suspected, the PAT-based sleep study is a good screening tool, but negative tests should not preclude further investigation. Where a high probability of sleep apnea exists but PAT-based testing shows no or mild OSA, an in-lab sleep study should be performed.
References
1. Ryals S, Chiang A, Schutte-Rodin S, et al. Photoplethysmography -- new applications for an old technology: a sleep technology review. J Clin Sleep Med. 2023;19(1):189-195.
2. Yalamanchali S, Farajian V, Hamilton C, Pott TR, Samuelson CG, Friedman M. Diagnosis of obstructive sleep apnea by peripheral arterial tonometry: meta-analysis. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1343-1350.
3. Röcken J, Schumann DM, Herrmann MJ, et al. Peripheral arterial tonometry versus polysomnography in suspected obstructive sleep apnoea. Eur J Med Res. 2023;28(1):251.
4. Iftikhar IH, Finch CE, Shah AS, Augunstein CA, Ioachimescu OC. A meta-analysis of diagnostic test performance of peripheral arterial tonometry studies. J Clin Sleep Med. 2022;18(4):1093-1102.
5. Ioachimescu OC, Allam JS, Samarghandi A, et al. Performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea in a large sleep clinic cohort. J Clin Sleep Med. 2020;16(10):1663-1674.
6. Van Pee B, Massie F, Vits S, et al. A multicentric validation study of a novel home sleep apnea test based on peripheral arterial tonometry. Sleep. 2022;45(5).
7. Ioachimescu OC, Dholakia SA, Venkateshiah SB, et al. Improving the performance of peripheral arterial tonometry-based testing for the diagnosis of obstructive sleep apnea. J Investig Med. 2020;68(8):1370-1378.
8. Korkalainen H, Aakko J, Duce B, et al. Deep learning enables sleep staging from photoplethysmogram for patients with suspected sleep apnea. Sleep. 2020;43(11).
On thoughtful selection of medications in the acute critical care setting
CRITICAL CARE NETWORK
Palliative and End-of-Life Care Section
As critical care medicine continues to advance understanding of ICU survivorship, thoughtful selection of medications in the acute setting can potentially mitigate long-term cognitive, physical, and affective effects.
As of yet, no significant studies have linked opioid use in critical care to new diagnoses of opioid use disorder, but the opioid epidemic has taught us that profligate use of opioids can have devastating effects despite best intentions. Continuous infusions of full agonist opioids for sedation remain an important tool in management of sedation. For acute pain, buprenorphine represents an attractive alternative for patients who are both intubated and nonintubated. It provides equal pain relief as full agonist opioids while causing less respiratory depression, less delirium, less nausea, less constipation, less euphoria, and less misuse potential. Its unique partial mu-opioid agonism is responsible for the improved nausea, constipation, and respiratory depression, while the kappa and delta receptor antagonisms are responsible for antidepressant effects as well as lessened opioid craving, sedation, and dysphoria. Given the variety of doses and routes for buprenorphine, palliative medicine consults can help navigate preventing precipitated withdrawal in patients who are opioid-tolerant and the variety of available dosing and routes.
It is a testament to the growth of critical care medicine that we now have the privilege and responsibility to account for long-term sequelae of our lifesaving interventions, rather than the old model of “prevent death at all costs.” Continued integration of high-quality symptom management into critical care offers an opportunity to better balance life-prolonging treatment and optimize quality of life.
References
1. Neale KJ, Weimer MB, Davis MP, et al. Top ten tips palliative care clinicians should know about buprenorphine. J Palliat Med. 2023;26(1):120-130. doi: 10.1089/jpm.2022.0399
CRITICAL CARE NETWORK
Palliative and End-of-Life Care Section
As critical care medicine continues to advance understanding of ICU survivorship, thoughtful selection of medications in the acute setting can potentially mitigate long-term cognitive, physical, and affective effects.
As of yet, no significant studies have linked opioid use in critical care to new diagnoses of opioid use disorder, but the opioid epidemic has taught us that profligate use of opioids can have devastating effects despite best intentions. Continuous infusions of full agonist opioids for sedation remain an important tool in management of sedation. For acute pain, buprenorphine represents an attractive alternative for patients who are both intubated and nonintubated. It provides equal pain relief as full agonist opioids while causing less respiratory depression, less delirium, less nausea, less constipation, less euphoria, and less misuse potential. Its unique partial mu-opioid agonism is responsible for the improved nausea, constipation, and respiratory depression, while the kappa and delta receptor antagonisms are responsible for antidepressant effects as well as lessened opioid craving, sedation, and dysphoria. Given the variety of doses and routes for buprenorphine, palliative medicine consults can help navigate preventing precipitated withdrawal in patients who are opioid-tolerant and the variety of available dosing and routes.
It is a testament to the growth of critical care medicine that we now have the privilege and responsibility to account for long-term sequelae of our lifesaving interventions, rather than the old model of “prevent death at all costs.” Continued integration of high-quality symptom management into critical care offers an opportunity to better balance life-prolonging treatment and optimize quality of life.
References
1. Neale KJ, Weimer MB, Davis MP, et al. Top ten tips palliative care clinicians should know about buprenorphine. J Palliat Med. 2023;26(1):120-130. doi: 10.1089/jpm.2022.0399
CRITICAL CARE NETWORK
Palliative and End-of-Life Care Section
As critical care medicine continues to advance understanding of ICU survivorship, thoughtful selection of medications in the acute setting can potentially mitigate long-term cognitive, physical, and affective effects.
As of yet, no significant studies have linked opioid use in critical care to new diagnoses of opioid use disorder, but the opioid epidemic has taught us that profligate use of opioids can have devastating effects despite best intentions. Continuous infusions of full agonist opioids for sedation remain an important tool in management of sedation. For acute pain, buprenorphine represents an attractive alternative for patients who are both intubated and nonintubated. It provides equal pain relief as full agonist opioids while causing less respiratory depression, less delirium, less nausea, less constipation, less euphoria, and less misuse potential. Its unique partial mu-opioid agonism is responsible for the improved nausea, constipation, and respiratory depression, while the kappa and delta receptor antagonisms are responsible for antidepressant effects as well as lessened opioid craving, sedation, and dysphoria. Given the variety of doses and routes for buprenorphine, palliative medicine consults can help navigate preventing precipitated withdrawal in patients who are opioid-tolerant and the variety of available dosing and routes.
It is a testament to the growth of critical care medicine that we now have the privilege and responsibility to account for long-term sequelae of our lifesaving interventions, rather than the old model of “prevent death at all costs.” Continued integration of high-quality symptom management into critical care offers an opportunity to better balance life-prolonging treatment and optimize quality of life.
References
1. Neale KJ, Weimer MB, Davis MP, et al. Top ten tips palliative care clinicians should know about buprenorphine. J Palliat Med. 2023;26(1):120-130. doi: 10.1089/jpm.2022.0399