User login
Atypical pulmonary cysts: Why to care
THORACIC ONCOLOGY AND CHEST PROCEDURES NETWORK
Lung Cancer Section
Since the American College of Radiology (ACR) updated its Lung CT Screening Reporting & Data System (Lung-RADS) to include atypical pulmonary cysts in 2022, there has been little discussion among chest physicians regarding the significance of pulmonary cysts and why these changes were made.
Lung-RADS 2022 defined atypical pulmonary cysts as single, unilocular cysts with a wall thickness greater than 2 mm or any multilocular cysts. These can be uniform, asymmetric, or have a focal nodularity. This change was prompted by data derived from multiple studies. First, a finding that 3.6% of lung cancers were associated with cysts at baseline.1 This was followed by a reanalysis of the NELSON trial’s missed cancers showing 22% of those overlooked during initial screening had findings of cystic disease, reaffirming the significance of atypical pulmonary cysts.2 Though the number is low, we now know 1.1% of all cancers present as an atypical cyst, with 4.7% of them being malignant.3
Based on these studies, cysts are a baseline Lung-RADS 4A—a finding that correlates to a higher risk and needs to be followed with a short-term CT scan in 3 months vs a PET. ACR does recommend reserving PET scans for wall thickness > 8 mm. If the repeat CT scan is stable, then the Lung-RADS designation is dropped to a 3 for follow-up.
References
1. Farooqi AO, Cham M, Zhang L, et al. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol. 2012;199(4):781-786.
2. Scholten ET, Horeweg N, Koning HJ, et al. Computed tomographic characteristics of interval and post screen carcinomas in lung cancer screening. Eur Radiol. 2015;25(1):81-88.
3. Mascalchi M, Attinà D, Bertelli E, et al. Lung cancer associated with cystic airspaces. J Comput Assist Tomogr. 2015;39(1):102-108.
THORACIC ONCOLOGY AND CHEST PROCEDURES NETWORK
Lung Cancer Section
Since the American College of Radiology (ACR) updated its Lung CT Screening Reporting & Data System (Lung-RADS) to include atypical pulmonary cysts in 2022, there has been little discussion among chest physicians regarding the significance of pulmonary cysts and why these changes were made.
Lung-RADS 2022 defined atypical pulmonary cysts as single, unilocular cysts with a wall thickness greater than 2 mm or any multilocular cysts. These can be uniform, asymmetric, or have a focal nodularity. This change was prompted by data derived from multiple studies. First, a finding that 3.6% of lung cancers were associated with cysts at baseline.1 This was followed by a reanalysis of the NELSON trial’s missed cancers showing 22% of those overlooked during initial screening had findings of cystic disease, reaffirming the significance of atypical pulmonary cysts.2 Though the number is low, we now know 1.1% of all cancers present as an atypical cyst, with 4.7% of them being malignant.3
Based on these studies, cysts are a baseline Lung-RADS 4A—a finding that correlates to a higher risk and needs to be followed with a short-term CT scan in 3 months vs a PET. ACR does recommend reserving PET scans for wall thickness > 8 mm. If the repeat CT scan is stable, then the Lung-RADS designation is dropped to a 3 for follow-up.
References
1. Farooqi AO, Cham M, Zhang L, et al. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol. 2012;199(4):781-786.
2. Scholten ET, Horeweg N, Koning HJ, et al. Computed tomographic characteristics of interval and post screen carcinomas in lung cancer screening. Eur Radiol. 2015;25(1):81-88.
3. Mascalchi M, Attinà D, Bertelli E, et al. Lung cancer associated with cystic airspaces. J Comput Assist Tomogr. 2015;39(1):102-108.
THORACIC ONCOLOGY AND CHEST PROCEDURES NETWORK
Lung Cancer Section
Since the American College of Radiology (ACR) updated its Lung CT Screening Reporting & Data System (Lung-RADS) to include atypical pulmonary cysts in 2022, there has been little discussion among chest physicians regarding the significance of pulmonary cysts and why these changes were made.
Lung-RADS 2022 defined atypical pulmonary cysts as single, unilocular cysts with a wall thickness greater than 2 mm or any multilocular cysts. These can be uniform, asymmetric, or have a focal nodularity. This change was prompted by data derived from multiple studies. First, a finding that 3.6% of lung cancers were associated with cysts at baseline.1 This was followed by a reanalysis of the NELSON trial’s missed cancers showing 22% of those overlooked during initial screening had findings of cystic disease, reaffirming the significance of atypical pulmonary cysts.2 Though the number is low, we now know 1.1% of all cancers present as an atypical cyst, with 4.7% of them being malignant.3
Based on these studies, cysts are a baseline Lung-RADS 4A—a finding that correlates to a higher risk and needs to be followed with a short-term CT scan in 3 months vs a PET. ACR does recommend reserving PET scans for wall thickness > 8 mm. If the repeat CT scan is stable, then the Lung-RADS designation is dropped to a 3 for follow-up.
References
1. Farooqi AO, Cham M, Zhang L, et al. Lung cancer associated with cystic airspaces. AJR Am J Roentgenol. 2012;199(4):781-786.
2. Scholten ET, Horeweg N, Koning HJ, et al. Computed tomographic characteristics of interval and post screen carcinomas in lung cancer screening. Eur Radiol. 2015;25(1):81-88.
3. Mascalchi M, Attinà D, Bertelli E, et al. Lung cancer associated with cystic airspaces. J Comput Assist Tomogr. 2015;39(1):102-108.
APPs and POCUS: Overcoming credentialing challenges
APP INTERSECTION
Advanced practice providers (APPs) play an integral role in the care and management of patients both in the ICU and across the spectrum of health care. Due to reduced residency hours and the coming physician shortage, APPs are playing, and will continue to play, a greater role in the care and management of critically ill patients.
Point of care ultrasound (POCUS) is a key diagnostic modality that promotes rapid diagnosis, shortens time to key interventions, and allows for interval reassessment. However, the benefit relies heavily on operator skill to obtain good quality images, interpret this within the context of the patient, and know what interventions, if any, to make. It is imperative that APPs, given their role within critical care, become not only familiar with POCUS but demonstrate proficiency in practice.
Before this can become standard, there are a number of challenges to overcome. Educational institutions are behind on integrating POCUS into the APP curriculum.1 Additionally, APPs have few opportunities to develop these skills as residencies and fellowships are not required prior to entering the workforce. Instead, for a majority of APPs, POCUS training relies on nonstandardized on-the-job training or an (typically expensive) off-site training, for which they may not receive reimbursement. Other barriers include cultural practices that limit POCUS use by APPs; inability to upload and document images obtained; lack of willing, skilled mentors with time to provide feedback and assess for clinical competence; and even accessibility of ultrasound machines.2
Despite these challenges, the clinical benefits of utilizing ultrasound in practice necessitates overcoming these barriers. Institutionally, when APPs perform POCUS, it can lead to more rapid diagnosis and triage of patients, provide billing opportunities, and may reduce overall costs of care. From an APP perspective, having formalized training and credentialing can lead to more consistent POCUS assessment between providers, comprehensive patient care, security in one’s skills and knowledge base, and a tangible way of communicating one’s credibility between institutions.
While some institutions have overcome these barriers and APPs are trained and credentialed in POCUS, this is not always the norm. For this to come to fruition, we need educators to incorporate this into curriculum, colleagues who are willing to mentor learners, cultural changes to allow APPs to develop and utilize necessary skills for practice, clear structure for obtaining and maintaining competency, physicians to champion these efforts, and institutions to credential skilled APPs. Until then, it is important that we as APPs seek to facilitate these institutional changes and continue to set and maintain high quality standards for ourselves.
CHEST 2024 is for APPs too
With more than 300 educational sessions on the CHEST 2024 program, it can be overwhelming to figure out which ones you should put on your schedule. That’s why CHEST asked Danielle McCamey, DNP, CRNP, ACNP-BC, FCCP, and LaDonna Brown, DNP, CRNA, of DNPs of Color (DOC), as well as Corinne Young, MSN, FNP-C, FCCP, of the Association of Pulmonary Advanced Practice Providers (APAPP), for their recommendations on sessions that advanced practice providers shouldn’t miss. Visit chestnet.org/annual-meeting-apps for all of their recommendations.
References
1. Rudy S, Widmar B, Wilbeck J. Ultrasound education for nurse practitioner students: strategies for curricular integration. J Nurse Pract. 2024;20(6):104986.
2. Resnyk J, Weichold A. Barriers to learning and performing point-of-care ultrasound (POCUS): An integrative review. J Prof Nursing. 2024;54:54-62.
APP INTERSECTION
Advanced practice providers (APPs) play an integral role in the care and management of patients both in the ICU and across the spectrum of health care. Due to reduced residency hours and the coming physician shortage, APPs are playing, and will continue to play, a greater role in the care and management of critically ill patients.
Point of care ultrasound (POCUS) is a key diagnostic modality that promotes rapid diagnosis, shortens time to key interventions, and allows for interval reassessment. However, the benefit relies heavily on operator skill to obtain good quality images, interpret this within the context of the patient, and know what interventions, if any, to make. It is imperative that APPs, given their role within critical care, become not only familiar with POCUS but demonstrate proficiency in practice.
Before this can become standard, there are a number of challenges to overcome. Educational institutions are behind on integrating POCUS into the APP curriculum.1 Additionally, APPs have few opportunities to develop these skills as residencies and fellowships are not required prior to entering the workforce. Instead, for a majority of APPs, POCUS training relies on nonstandardized on-the-job training or an (typically expensive) off-site training, for which they may not receive reimbursement. Other barriers include cultural practices that limit POCUS use by APPs; inability to upload and document images obtained; lack of willing, skilled mentors with time to provide feedback and assess for clinical competence; and even accessibility of ultrasound machines.2
Despite these challenges, the clinical benefits of utilizing ultrasound in practice necessitates overcoming these barriers. Institutionally, when APPs perform POCUS, it can lead to more rapid diagnosis and triage of patients, provide billing opportunities, and may reduce overall costs of care. From an APP perspective, having formalized training and credentialing can lead to more consistent POCUS assessment between providers, comprehensive patient care, security in one’s skills and knowledge base, and a tangible way of communicating one’s credibility between institutions.
While some institutions have overcome these barriers and APPs are trained and credentialed in POCUS, this is not always the norm. For this to come to fruition, we need educators to incorporate this into curriculum, colleagues who are willing to mentor learners, cultural changes to allow APPs to develop and utilize necessary skills for practice, clear structure for obtaining and maintaining competency, physicians to champion these efforts, and institutions to credential skilled APPs. Until then, it is important that we as APPs seek to facilitate these institutional changes and continue to set and maintain high quality standards for ourselves.
CHEST 2024 is for APPs too
With more than 300 educational sessions on the CHEST 2024 program, it can be overwhelming to figure out which ones you should put on your schedule. That’s why CHEST asked Danielle McCamey, DNP, CRNP, ACNP-BC, FCCP, and LaDonna Brown, DNP, CRNA, of DNPs of Color (DOC), as well as Corinne Young, MSN, FNP-C, FCCP, of the Association of Pulmonary Advanced Practice Providers (APAPP), for their recommendations on sessions that advanced practice providers shouldn’t miss. Visit chestnet.org/annual-meeting-apps for all of their recommendations.
References
1. Rudy S, Widmar B, Wilbeck J. Ultrasound education for nurse practitioner students: strategies for curricular integration. J Nurse Pract. 2024;20(6):104986.
2. Resnyk J, Weichold A. Barriers to learning and performing point-of-care ultrasound (POCUS): An integrative review. J Prof Nursing. 2024;54:54-62.
APP INTERSECTION
Advanced practice providers (APPs) play an integral role in the care and management of patients both in the ICU and across the spectrum of health care. Due to reduced residency hours and the coming physician shortage, APPs are playing, and will continue to play, a greater role in the care and management of critically ill patients.
Point of care ultrasound (POCUS) is a key diagnostic modality that promotes rapid diagnosis, shortens time to key interventions, and allows for interval reassessment. However, the benefit relies heavily on operator skill to obtain good quality images, interpret this within the context of the patient, and know what interventions, if any, to make. It is imperative that APPs, given their role within critical care, become not only familiar with POCUS but demonstrate proficiency in practice.
Before this can become standard, there are a number of challenges to overcome. Educational institutions are behind on integrating POCUS into the APP curriculum.1 Additionally, APPs have few opportunities to develop these skills as residencies and fellowships are not required prior to entering the workforce. Instead, for a majority of APPs, POCUS training relies on nonstandardized on-the-job training or an (typically expensive) off-site training, for which they may not receive reimbursement. Other barriers include cultural practices that limit POCUS use by APPs; inability to upload and document images obtained; lack of willing, skilled mentors with time to provide feedback and assess for clinical competence; and even accessibility of ultrasound machines.2
Despite these challenges, the clinical benefits of utilizing ultrasound in practice necessitates overcoming these barriers. Institutionally, when APPs perform POCUS, it can lead to more rapid diagnosis and triage of patients, provide billing opportunities, and may reduce overall costs of care. From an APP perspective, having formalized training and credentialing can lead to more consistent POCUS assessment between providers, comprehensive patient care, security in one’s skills and knowledge base, and a tangible way of communicating one’s credibility between institutions.
While some institutions have overcome these barriers and APPs are trained and credentialed in POCUS, this is not always the norm. For this to come to fruition, we need educators to incorporate this into curriculum, colleagues who are willing to mentor learners, cultural changes to allow APPs to develop and utilize necessary skills for practice, clear structure for obtaining and maintaining competency, physicians to champion these efforts, and institutions to credential skilled APPs. Until then, it is important that we as APPs seek to facilitate these institutional changes and continue to set and maintain high quality standards for ourselves.
CHEST 2024 is for APPs too
With more than 300 educational sessions on the CHEST 2024 program, it can be overwhelming to figure out which ones you should put on your schedule. That’s why CHEST asked Danielle McCamey, DNP, CRNP, ACNP-BC, FCCP, and LaDonna Brown, DNP, CRNA, of DNPs of Color (DOC), as well as Corinne Young, MSN, FNP-C, FCCP, of the Association of Pulmonary Advanced Practice Providers (APAPP), for their recommendations on sessions that advanced practice providers shouldn’t miss. Visit chestnet.org/annual-meeting-apps for all of their recommendations.
References
1. Rudy S, Widmar B, Wilbeck J. Ultrasound education for nurse practitioner students: strategies for curricular integration. J Nurse Pract. 2024;20(6):104986.
2. Resnyk J, Weichold A. Barriers to learning and performing point-of-care ultrasound (POCUS): An integrative review. J Prof Nursing. 2024;54:54-62.
Diagnostic yield reporting of bronchoscopic peripheral pulmonary nodule biopsies: A call for standardization
THORACIC ONCOLOGY AND CHEST PROCEDURES NETWORK
Interventional Procedures Section
More than 1.5 million Americans are diagnosed with an incidental CT scan-detected lung nodule annually. Advanced bronchoscopy, as a diagnostic tool for evaluation of these nodules, has evolved rapidly, incorporating a range of techniques and tools beyond CT scan-guided biopsies to assess peripheral lesions. The primary goal is to provide patients with accurate benign or malignant diagnoses. However, accurately determining the effectiveness of innovative technologies in providing a diagnosis remains challenging, in part due to limitations in study design and outcome reporting, along with the scarcity of comparative and randomized controlled studies.1,2 Current literature shows significant variability in diagnostic yield definition, lacking generalizability.
To address this issue, an official research statement by the American Thoracic Society and CHEST defines the diagnostic yield as “the proportion of all individuals undergoing the diagnostic procedure under evaluation in whom a specific malignant or benign diagnosis is established.”3 To achieve this measure, the numerator includes all patients with lung nodules in whom the result of a diagnostic procedure establishes a specific benign or malignant diagnosis that is readily sufficient to inform patient care without additional diagnostic workup, and the denominator should include all patients in whom the procedure was attempted or performed. This standardized definition is crucial for ensuring consistency across studies, allowing for comparison or pooling of results, enhancing the reliability of diagnostic yield data, and informing clinical decisions.
The adoption of standardized outcome definitions is essential to critically evaluate modern, minimally invasive procedures for peripheral lung nodules diagnosis and to guide patient-centered care while minimizing the downstream effects of nondiagnostic biopsies. Clear, transparent, and consistent reporting will enable physicians to choose the most appropriate diagnostic tools, improve patient outcomes by reducing unnecessary procedures, and expedite accurate diagnoses. This initiative is a crucial first step toward creating high-quality studies that can inform technology implementation decisions and promote equitable health care.
References
1. Tanner NT, Yarmus L, Chen A, et al. Standard bronchoscopy with fluoroscopy vs thin bronchoscopy and radial endobronchial ultrasound for biopsy of pulmonary lesions: a multicenter, prospective, randomized trial. Chest. 2018;154(5):1035-1043.
2. Ost DE, Ernst A, Lei X, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE Registry. Am J Resp Crit Care Med. 2016;193(1):68-77.
3. Gonzalez AV, Silvestri GA, Korevaar DA, et al. Assessment of advanced diagnostic bronchoscopy outcomes for peripheral lung lesions: a Delphi consensus definition of diagnostic yield and recommendations for patient-centered study designs. An official American Thoracic Society/American College of Chest Physicians research statement. Am J Respir Crit Care Med. 2024;209(6):634-646.
THORACIC ONCOLOGY AND CHEST PROCEDURES NETWORK
Interventional Procedures Section
More than 1.5 million Americans are diagnosed with an incidental CT scan-detected lung nodule annually. Advanced bronchoscopy, as a diagnostic tool for evaluation of these nodules, has evolved rapidly, incorporating a range of techniques and tools beyond CT scan-guided biopsies to assess peripheral lesions. The primary goal is to provide patients with accurate benign or malignant diagnoses. However, accurately determining the effectiveness of innovative technologies in providing a diagnosis remains challenging, in part due to limitations in study design and outcome reporting, along with the scarcity of comparative and randomized controlled studies.1,2 Current literature shows significant variability in diagnostic yield definition, lacking generalizability.
To address this issue, an official research statement by the American Thoracic Society and CHEST defines the diagnostic yield as “the proportion of all individuals undergoing the diagnostic procedure under evaluation in whom a specific malignant or benign diagnosis is established.”3 To achieve this measure, the numerator includes all patients with lung nodules in whom the result of a diagnostic procedure establishes a specific benign or malignant diagnosis that is readily sufficient to inform patient care without additional diagnostic workup, and the denominator should include all patients in whom the procedure was attempted or performed. This standardized definition is crucial for ensuring consistency across studies, allowing for comparison or pooling of results, enhancing the reliability of diagnostic yield data, and informing clinical decisions.
The adoption of standardized outcome definitions is essential to critically evaluate modern, minimally invasive procedures for peripheral lung nodules diagnosis and to guide patient-centered care while minimizing the downstream effects of nondiagnostic biopsies. Clear, transparent, and consistent reporting will enable physicians to choose the most appropriate diagnostic tools, improve patient outcomes by reducing unnecessary procedures, and expedite accurate diagnoses. This initiative is a crucial first step toward creating high-quality studies that can inform technology implementation decisions and promote equitable health care.
References
1. Tanner NT, Yarmus L, Chen A, et al. Standard bronchoscopy with fluoroscopy vs thin bronchoscopy and radial endobronchial ultrasound for biopsy of pulmonary lesions: a multicenter, prospective, randomized trial. Chest. 2018;154(5):1035-1043.
2. Ost DE, Ernst A, Lei X, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE Registry. Am J Resp Crit Care Med. 2016;193(1):68-77.
3. Gonzalez AV, Silvestri GA, Korevaar DA, et al. Assessment of advanced diagnostic bronchoscopy outcomes for peripheral lung lesions: a Delphi consensus definition of diagnostic yield and recommendations for patient-centered study designs. An official American Thoracic Society/American College of Chest Physicians research statement. Am J Respir Crit Care Med. 2024;209(6):634-646.
THORACIC ONCOLOGY AND CHEST PROCEDURES NETWORK
Interventional Procedures Section
More than 1.5 million Americans are diagnosed with an incidental CT scan-detected lung nodule annually. Advanced bronchoscopy, as a diagnostic tool for evaluation of these nodules, has evolved rapidly, incorporating a range of techniques and tools beyond CT scan-guided biopsies to assess peripheral lesions. The primary goal is to provide patients with accurate benign or malignant diagnoses. However, accurately determining the effectiveness of innovative technologies in providing a diagnosis remains challenging, in part due to limitations in study design and outcome reporting, along with the scarcity of comparative and randomized controlled studies.1,2 Current literature shows significant variability in diagnostic yield definition, lacking generalizability.
To address this issue, an official research statement by the American Thoracic Society and CHEST defines the diagnostic yield as “the proportion of all individuals undergoing the diagnostic procedure under evaluation in whom a specific malignant or benign diagnosis is established.”3 To achieve this measure, the numerator includes all patients with lung nodules in whom the result of a diagnostic procedure establishes a specific benign or malignant diagnosis that is readily sufficient to inform patient care without additional diagnostic workup, and the denominator should include all patients in whom the procedure was attempted or performed. This standardized definition is crucial for ensuring consistency across studies, allowing for comparison or pooling of results, enhancing the reliability of diagnostic yield data, and informing clinical decisions.
The adoption of standardized outcome definitions is essential to critically evaluate modern, minimally invasive procedures for peripheral lung nodules diagnosis and to guide patient-centered care while minimizing the downstream effects of nondiagnostic biopsies. Clear, transparent, and consistent reporting will enable physicians to choose the most appropriate diagnostic tools, improve patient outcomes by reducing unnecessary procedures, and expedite accurate diagnoses. This initiative is a crucial first step toward creating high-quality studies that can inform technology implementation decisions and promote equitable health care.
References
1. Tanner NT, Yarmus L, Chen A, et al. Standard bronchoscopy with fluoroscopy vs thin bronchoscopy and radial endobronchial ultrasound for biopsy of pulmonary lesions: a multicenter, prospective, randomized trial. Chest. 2018;154(5):1035-1043.
2. Ost DE, Ernst A, Lei X, et al. Diagnostic yield and complications of bronchoscopy for peripheral lung lesions. Results of the AQuIRE Registry. Am J Resp Crit Care Med. 2016;193(1):68-77.
3. Gonzalez AV, Silvestri GA, Korevaar DA, et al. Assessment of advanced diagnostic bronchoscopy outcomes for peripheral lung lesions: a Delphi consensus definition of diagnostic yield and recommendations for patient-centered study designs. An official American Thoracic Society/American College of Chest Physicians research statement. Am J Respir Crit Care Med. 2024;209(6):634-646.
Post–intensive care syndrome and insomnia
SLEEP MEDICINE NETWORK
Nonrespiratory Sleep Section
There has been a recent interest in post–intensive care syndrome (PICS), as an increasing number of patients are surviving critical illness. PICS is defined as “new onset or worsening of impairments in physical, cognitive, and/or mental health that arises after an ICU stay and persists beyond hospital discharge.1 We know that poor sleep is a common occurrence in the ICU, which can contribute to cognitive impairment and could be due to various risk factors, including age, individual comorbidities, reason for admission, and ICU interventions.2 Sleep impairment after hospital discharge is highly prevalent for up to 1 year after hospitalization.
The most common sleep impairment described after hospital discharge from the ICU is insomnia, which coexists with anxiety, depression, and posttraumatic stress disorder.3 When patients are seen in a post-ICU clinic, a multimodal strategy is needed for the treatment of insomnia, which includes practicing good sleep hygiene, cognitive behavioral therapy for insomnia (CBT-I), and pharmacotherapy if indicated.
Since the American Academy of Sleep Medicine (AASM) 2021 clinical practice guideline on behavioral and psychological treatments for chronic insomnia, which made a strong recommendation for CBT-I, we continue to face barriers to incorporating CBT-I into our own clinical practice.4 This is due to limited access to CBT-I psychotherapists and patients’ lack of knowledge or treatment beliefs, among other reasons. However, there are numerous digital CBT-I platforms that patients can freely access from their mobile phone and are listed in the AASM article, “Digital cognitive behavioral therapy for insomnia: Platforms and characteristics,” which can help with treatment of insomnia.
For patients who are seen in post-ICU clinics, the first step in treating insomnia is discussing good sleep hygiene, providing resources for CBT-I (digital or in person), and treating coexistent psychiatric conditions.
References
1. Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Int Med. 2017;5(2):90-92.
2. Zampieri FG, et al. Ann Am Thorac Soc. 2023;20(11):1558-1560.
3. Altman MT, Knauert MP, Pisani MA. Sleep disturbance after hospitalization and critical illness: a systematic review. Ann Am Thorac Soc. 2017;14(9):1457-1468.
4. Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255-262.
SLEEP MEDICINE NETWORK
Nonrespiratory Sleep Section
There has been a recent interest in post–intensive care syndrome (PICS), as an increasing number of patients are surviving critical illness. PICS is defined as “new onset or worsening of impairments in physical, cognitive, and/or mental health that arises after an ICU stay and persists beyond hospital discharge.1 We know that poor sleep is a common occurrence in the ICU, which can contribute to cognitive impairment and could be due to various risk factors, including age, individual comorbidities, reason for admission, and ICU interventions.2 Sleep impairment after hospital discharge is highly prevalent for up to 1 year after hospitalization.
The most common sleep impairment described after hospital discharge from the ICU is insomnia, which coexists with anxiety, depression, and posttraumatic stress disorder.3 When patients are seen in a post-ICU clinic, a multimodal strategy is needed for the treatment of insomnia, which includes practicing good sleep hygiene, cognitive behavioral therapy for insomnia (CBT-I), and pharmacotherapy if indicated.
Since the American Academy of Sleep Medicine (AASM) 2021 clinical practice guideline on behavioral and psychological treatments for chronic insomnia, which made a strong recommendation for CBT-I, we continue to face barriers to incorporating CBT-I into our own clinical practice.4 This is due to limited access to CBT-I psychotherapists and patients’ lack of knowledge or treatment beliefs, among other reasons. However, there are numerous digital CBT-I platforms that patients can freely access from their mobile phone and are listed in the AASM article, “Digital cognitive behavioral therapy for insomnia: Platforms and characteristics,” which can help with treatment of insomnia.
For patients who are seen in post-ICU clinics, the first step in treating insomnia is discussing good sleep hygiene, providing resources for CBT-I (digital or in person), and treating coexistent psychiatric conditions.
References
1. Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Int Med. 2017;5(2):90-92.
2. Zampieri FG, et al. Ann Am Thorac Soc. 2023;20(11):1558-1560.
3. Altman MT, Knauert MP, Pisani MA. Sleep disturbance after hospitalization and critical illness: a systematic review. Ann Am Thorac Soc. 2017;14(9):1457-1468.
4. Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255-262.
SLEEP MEDICINE NETWORK
Nonrespiratory Sleep Section
There has been a recent interest in post–intensive care syndrome (PICS), as an increasing number of patients are surviving critical illness. PICS is defined as “new onset or worsening of impairments in physical, cognitive, and/or mental health that arises after an ICU stay and persists beyond hospital discharge.1 We know that poor sleep is a common occurrence in the ICU, which can contribute to cognitive impairment and could be due to various risk factors, including age, individual comorbidities, reason for admission, and ICU interventions.2 Sleep impairment after hospital discharge is highly prevalent for up to 1 year after hospitalization.
The most common sleep impairment described after hospital discharge from the ICU is insomnia, which coexists with anxiety, depression, and posttraumatic stress disorder.3 When patients are seen in a post-ICU clinic, a multimodal strategy is needed for the treatment of insomnia, which includes practicing good sleep hygiene, cognitive behavioral therapy for insomnia (CBT-I), and pharmacotherapy if indicated.
Since the American Academy of Sleep Medicine (AASM) 2021 clinical practice guideline on behavioral and psychological treatments for chronic insomnia, which made a strong recommendation for CBT-I, we continue to face barriers to incorporating CBT-I into our own clinical practice.4 This is due to limited access to CBT-I psychotherapists and patients’ lack of knowledge or treatment beliefs, among other reasons. However, there are numerous digital CBT-I platforms that patients can freely access from their mobile phone and are listed in the AASM article, “Digital cognitive behavioral therapy for insomnia: Platforms and characteristics,” which can help with treatment of insomnia.
For patients who are seen in post-ICU clinics, the first step in treating insomnia is discussing good sleep hygiene, providing resources for CBT-I (digital or in person), and treating coexistent psychiatric conditions.
References
1. Rawal G, Yadav S, Kumar R. Post-intensive care syndrome: an overview. J Transl Int Med. 2017;5(2):90-92.
2. Zampieri FG, et al. Ann Am Thorac Soc. 2023;20(11):1558-1560.
3. Altman MT, Knauert MP, Pisani MA. Sleep disturbance after hospitalization and critical illness: a systematic review. Ann Am Thorac Soc. 2017;14(9):1457-1468.
4. Edinger JD, Arnedt JT, Bertisch SM, et al. Behavioral and psychological treatments for chronic insomnia disorder in adults: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(2):255-262.
Short telomere length and immunosuppression: Updates in nonidiopathic pulmonary fibrosis, interstitial lung disease
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Interstitial Lung Disease Section
Interstitial lung diseases (ILDs) are a diverse group of relentlessly progressive fibroinflammatory disorders. Pharmacotherapy includes antifibrotics and immunosuppressants as foundational strategies to mitigate loss of lung function. There has been a growing interest in telomere length and its response to immunosuppression in the ILD community.
Telomeres are repetitive nucleotide sequences that “cap” chromosomes and protect against chromosomal shortening during cell replication. Genetic and environmental factors can lead to premature shortening of telomeres. Once a critical length is reached, the cell enters senescence. Short telomere length has been linked to rapid progression, worse outcomes, and poor response to immunosuppressants in idiopathic pulmonary fibrosis (IPF).
Data in patients with non-IPF ILD (which is arguably more difficult to diagnose and manage) were lacking until a recent retrospective cohort study of patients from five centers across the US demonstrated that immunosuppressant exposure in patients with age-adjusted telomere length <10th percentile was associated with a reduced 2-year transplant-free survival in fibrotic hypersensitivity pneumonitis and unclassifiable ILD subgroups.1 This study was underpowered to detect associations in the connective tissue disease-ILD group. Interestingly, authors noted that immunosuppressant exposure was not associated with lung function decline in the short telomere group, suggesting that worse outcomes may be attributable to unmasking extrapulmonary manifestations of short telomeres, such as bone marrow failure and impaired adaptive immunity. Studies like these are essential to guide decision-making in the age of personalized medicine and underscore the necessity for prospective studies to validate these findings.
References
1. Zhang D, Adegunsoye A, Oldham JM, et al. Telomere length and immunosuppression in non-idiopathic pulmonary fibrosis interstitial lung disease. Eur Respir J. 2023;62(5):2300441.
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Interstitial Lung Disease Section
Interstitial lung diseases (ILDs) are a diverse group of relentlessly progressive fibroinflammatory disorders. Pharmacotherapy includes antifibrotics and immunosuppressants as foundational strategies to mitigate loss of lung function. There has been a growing interest in telomere length and its response to immunosuppression in the ILD community.
Telomeres are repetitive nucleotide sequences that “cap” chromosomes and protect against chromosomal shortening during cell replication. Genetic and environmental factors can lead to premature shortening of telomeres. Once a critical length is reached, the cell enters senescence. Short telomere length has been linked to rapid progression, worse outcomes, and poor response to immunosuppressants in idiopathic pulmonary fibrosis (IPF).
Data in patients with non-IPF ILD (which is arguably more difficult to diagnose and manage) were lacking until a recent retrospective cohort study of patients from five centers across the US demonstrated that immunosuppressant exposure in patients with age-adjusted telomere length <10th percentile was associated with a reduced 2-year transplant-free survival in fibrotic hypersensitivity pneumonitis and unclassifiable ILD subgroups.1 This study was underpowered to detect associations in the connective tissue disease-ILD group. Interestingly, authors noted that immunosuppressant exposure was not associated with lung function decline in the short telomere group, suggesting that worse outcomes may be attributable to unmasking extrapulmonary manifestations of short telomeres, such as bone marrow failure and impaired adaptive immunity. Studies like these are essential to guide decision-making in the age of personalized medicine and underscore the necessity for prospective studies to validate these findings.
References
1. Zhang D, Adegunsoye A, Oldham JM, et al. Telomere length and immunosuppression in non-idiopathic pulmonary fibrosis interstitial lung disease. Eur Respir J. 2023;62(5):2300441.
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Interstitial Lung Disease Section
Interstitial lung diseases (ILDs) are a diverse group of relentlessly progressive fibroinflammatory disorders. Pharmacotherapy includes antifibrotics and immunosuppressants as foundational strategies to mitigate loss of lung function. There has been a growing interest in telomere length and its response to immunosuppression in the ILD community.
Telomeres are repetitive nucleotide sequences that “cap” chromosomes and protect against chromosomal shortening during cell replication. Genetic and environmental factors can lead to premature shortening of telomeres. Once a critical length is reached, the cell enters senescence. Short telomere length has been linked to rapid progression, worse outcomes, and poor response to immunosuppressants in idiopathic pulmonary fibrosis (IPF).
Data in patients with non-IPF ILD (which is arguably more difficult to diagnose and manage) were lacking until a recent retrospective cohort study of patients from five centers across the US demonstrated that immunosuppressant exposure in patients with age-adjusted telomere length <10th percentile was associated with a reduced 2-year transplant-free survival in fibrotic hypersensitivity pneumonitis and unclassifiable ILD subgroups.1 This study was underpowered to detect associations in the connective tissue disease-ILD group. Interestingly, authors noted that immunosuppressant exposure was not associated with lung function decline in the short telomere group, suggesting that worse outcomes may be attributable to unmasking extrapulmonary manifestations of short telomeres, such as bone marrow failure and impaired adaptive immunity. Studies like these are essential to guide decision-making in the age of personalized medicine and underscore the necessity for prospective studies to validate these findings.
References
1. Zhang D, Adegunsoye A, Oldham JM, et al. Telomere length and immunosuppression in non-idiopathic pulmonary fibrosis interstitial lung disease. Eur Respir J. 2023;62(5):2300441.
Expanding recommendations for RSV vaccination
AIRWAYS DISORDERS NETWORK
Asthma and COPD Section
Respiratory syncytial virus (RSV) has been increasingly recognized as a prevalent cause of lower respiratory tract infection (LRTI) among adults in the United States. The risk of hospitalization and mortality from RSV-associated respiratory failure is higher in those with chronic lung disease. In adults aged 65 years or older, RSV has shown to cause up to 160,000 hospitalizations and 10,000 deaths annually.
RSV has been well established as a major cause of LRTI and morbidity among infants. Maternal vaccination with RSVPreF in patients who are pregnant is suggested between 32 0/7 and 36 6/7 weeks of gestation if the date of delivery falls during RSV season to prevent severe illness in young infants in their first months of life. At present, there are no data supporting vaccine administration to patients who are pregnant delivering outside of the RSV season.
What about the rest of the patients? A phase 3b clinical trial to assess the safety and immunogenicity of the RSVPreF3 vaccine in individuals 18 to 49 years of age at increased risk for RSV LRTI, including those with chronic respiratory diseases, is currently underway with projected completion in April 2025 (clinical trials.gov; ID NCT06389487). Additional studies examining safety and immunogenicity combining RSV vaccines with PCV20, influenza, COVID, or Tdap vaccines are also underway. These outcomes will be significant for future recommendations to further lower the risk of developing LRTI, hospitalization, and death among patients less than the age of 60 with chronic lung diseases.
Resources
1. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices - United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793-801.
2. Healthcare Providers: RSV Vaccination for Adults 60 Years of Age and Over. Centers for Disease Control and Prevention. Updated March 1, 2024. https://www.cdc.gov/vaccines/vpd/rsv/hcp/older-adults.html
3. Ault KA, Hughes BL, Riley LE. Maternal Respiratory Syncytial Virus Vaccination. The American College of Obstetricians and Gynecologists. Updated December 11, 2023. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2023/09/maternal-respiratory-syncytial-virus-vaccination
AIRWAYS DISORDERS NETWORK
Asthma and COPD Section
Respiratory syncytial virus (RSV) has been increasingly recognized as a prevalent cause of lower respiratory tract infection (LRTI) among adults in the United States. The risk of hospitalization and mortality from RSV-associated respiratory failure is higher in those with chronic lung disease. In adults aged 65 years or older, RSV has shown to cause up to 160,000 hospitalizations and 10,000 deaths annually.
RSV has been well established as a major cause of LRTI and morbidity among infants. Maternal vaccination with RSVPreF in patients who are pregnant is suggested between 32 0/7 and 36 6/7 weeks of gestation if the date of delivery falls during RSV season to prevent severe illness in young infants in their first months of life. At present, there are no data supporting vaccine administration to patients who are pregnant delivering outside of the RSV season.
What about the rest of the patients? A phase 3b clinical trial to assess the safety and immunogenicity of the RSVPreF3 vaccine in individuals 18 to 49 years of age at increased risk for RSV LRTI, including those with chronic respiratory diseases, is currently underway with projected completion in April 2025 (clinical trials.gov; ID NCT06389487). Additional studies examining safety and immunogenicity combining RSV vaccines with PCV20, influenza, COVID, or Tdap vaccines are also underway. These outcomes will be significant for future recommendations to further lower the risk of developing LRTI, hospitalization, and death among patients less than the age of 60 with chronic lung diseases.
Resources
1. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices - United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793-801.
2. Healthcare Providers: RSV Vaccination for Adults 60 Years of Age and Over. Centers for Disease Control and Prevention. Updated March 1, 2024. https://www.cdc.gov/vaccines/vpd/rsv/hcp/older-adults.html
3. Ault KA, Hughes BL, Riley LE. Maternal Respiratory Syncytial Virus Vaccination. The American College of Obstetricians and Gynecologists. Updated December 11, 2023. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2023/09/maternal-respiratory-syncytial-virus-vaccination
AIRWAYS DISORDERS NETWORK
Asthma and COPD Section
Respiratory syncytial virus (RSV) has been increasingly recognized as a prevalent cause of lower respiratory tract infection (LRTI) among adults in the United States. The risk of hospitalization and mortality from RSV-associated respiratory failure is higher in those with chronic lung disease. In adults aged 65 years or older, RSV has shown to cause up to 160,000 hospitalizations and 10,000 deaths annually.
RSV has been well established as a major cause of LRTI and morbidity among infants. Maternal vaccination with RSVPreF in patients who are pregnant is suggested between 32 0/7 and 36 6/7 weeks of gestation if the date of delivery falls during RSV season to prevent severe illness in young infants in their first months of life. At present, there are no data supporting vaccine administration to patients who are pregnant delivering outside of the RSV season.
What about the rest of the patients? A phase 3b clinical trial to assess the safety and immunogenicity of the RSVPreF3 vaccine in individuals 18 to 49 years of age at increased risk for RSV LRTI, including those with chronic respiratory diseases, is currently underway with projected completion in April 2025 (clinical trials.gov; ID NCT06389487). Additional studies examining safety and immunogenicity combining RSV vaccines with PCV20, influenza, COVID, or Tdap vaccines are also underway. These outcomes will be significant for future recommendations to further lower the risk of developing LRTI, hospitalization, and death among patients less than the age of 60 with chronic lung diseases.
Resources
1. Melgar M, Britton A, Roper LE, et al. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices - United States, 2023. MMWR Morb Mortal Wkly Rep. 2023;72(29):793-801.
2. Healthcare Providers: RSV Vaccination for Adults 60 Years of Age and Over. Centers for Disease Control and Prevention. Updated March 1, 2024. https://www.cdc.gov/vaccines/vpd/rsv/hcp/older-adults.html
3. Ault KA, Hughes BL, Riley LE. Maternal Respiratory Syncytial Virus Vaccination. The American College of Obstetricians and Gynecologists. Updated December 11, 2023. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2023/09/maternal-respiratory-syncytial-virus-vaccination
Coding & billing: A look into G2211 for visit complexities
This add-on code is for new (99202-99205) and established (99212-99215) office visits. CMS created this add-on code to address the additional costs and resources associated with providing longitudinal care.
G2211 – Visit complexity inherent to evaluation and management (E/M) associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious condition, or a complex condition (Add-on code; list separately in addition to office/outpatient (O/O) E/M visit, new or established)
The documentation should demonstrate the intent and need for ongoing care. Otherwise, no additional documentation is required. CMS pays $16.04 for each service (wRVU = 0.33). It may be reported each time the patient is seen, and there is currently no limit to how often it may be used. Also, there is no additional copay requirement for patients.
Do’s and don’ts
Do report in the following situations when longitudinal care is provided:
- The provider has or intends to have a long-term, ongoing relationship with the patient (ie, G2211 can be used for a new patient visit)
- Audio/video virtual visits
- May be reported with Prolonged Care Services G2212
- When advanced practice providers or physician colleagues in the same specialty practice see the patient (ie, if you see the patient for an urgent visit, but the patient is usually followed by your partner, you can still use G2211)
- When working with graduate medical education trainees (along with the -GC modifier), and as long as the conditions described in the description of G2211 are met
Do NOT report in the following situations:
- If modifier -25 is appended to the E/M service when another service is provided on the same day (eg, pulmonary function tests, 6-minute walk tests, immunization)
- Audio-only virtual visits, hospital, skilled nursing facility, or long-term acute care hospital
- If the patient is not expected to return for ongoing care
- If the reason for longitudinal care does not include a “single, serious condition or a complex condition” (eg, annual visits for a stable 6 mm lung nodule)
CMS expects that this will be billed with 38% of all E/M services initially and potentially up to 54% over time. We feel this is reimbursement for the work being done to care for our patients with single, serious, or complex conditions. Both Medicare and Medicare Advantage plans are expected to reimburse for this service. Whether other payers will do the same is unclear, but it will become clear with time and further negotiation at the local level. In the meantime, members are encouraged to report this code for all appropriate patient encounters.
Questions and answers — G2211
Question: What private insurances cover G2211?
Answer: As of March 1, 2024, four national payers have confirmed coverage of G2211:
- Cigna (Medicare Advantage only),
- Humana (commercial and Medicare Advantage),
- United Healthcare (commercial and Medicare Advantage), and
- Aetna (Medicare Advantage).
Question: What needs to be documented for G2211?
Answer: CMS states, “You must document the reason for billing the office and outpatient (O/O) and evaluation and management (E/M). The visits themselves would need to be medically reasonable and necessary for the practitioner to report G2211. In addition, the documentation would need to illustrate medical necessity of the O/O E/M visit. We [CMS] haven’t required additional documentation.”
American Thoracic Society (ATS) and CHEST also recommend including a detailed assessment and plan for the visit, as well as any follow-up. The complexity of the visit should be clear in your documentation to support the medical necessity for reporting the G2211.
Question: How can a provider show that a new patient visit (99202-99205) is part of continuing care?
Answer: The treating practitioner should make sure their documentation supports their intent to provide ongoing care to the patient. Establishing such intent goes beyond a statement that the provider plans to provide ongoing care or schedule a follow-up visit. The circumstances of the visit should support the extra work involved in becoming the focal point of the patient’s care or providing ongoing care for a serious or complex condition.
Question: Dr. Red works at a primary care practice, is the focal point for a patient’s care, and has reported G2211. If Dr. Yellow, who is in the same specialty, or Mr. Green, a nurse practitioner, is covering for Dr. Red, and the patient comes in for a visit, can they report G2211 for that visit?
Answer: Yes. The same specialty/same provider rules would apply in this situation. But remember that Dr. Yellow’s or Mr. Green’s documentation for that encounter must support the code.
Question: Can a resident report G2211 under the primary care exemption?
Answer: Yes, according to CMS staff, so long as the service and the documentation meet all the requirements for the exemption and the visit complexity code. For example, the resident can only report low-level E/M codes, and the resident must be “the focal point for that person’s care.”
Question: Are there frequency limits for how often we can report G2211, either for a single patient in a given time period or by a provider or a practice?
Answer: Not at this time, but make sure your providers are following the rules for reporting the code. “There’s got to be documentation that suggests why the practitioner believes they are treating the patient on this long-standing, longitudinal trajectory, and we’ll be able to see how that interaction is happening,” senior CMS staff said. CMS staff further issued a subtle warning to providers by reminding them that CMS has a very strong integrity program. Your practice can avoid problems with thorough training, frequent chart review, and encouraging the team to ask questions until you feel that everyone is comfortable with the code.
Question: Are there any limits on the specialties that can report the code? Is it just for primary care providers?
Answer: No. Remember that a provider who is managing a single serious or complex condition can also report the code. But CMS expects the documentation to support the ongoing nature of the treatment. If a patient sees a provider as a one-off encounter, perhaps to manage an acute problem, that visit wouldn’t qualify. But if the provider clearly documents that they are actively managing the patient’s condition, the encounters could qualify.
Question: Will CMS issue a list of conditions that meet the code’s serious or complex condition requirement?
Answer: CMS has included the examples of HIV and sickle cell anemia in existing guidance, and it plans to issue a few more examples “that help folks understand what is expected.” However, it won’t be a complete list of every condition that might qualify.
Originally published in the May 2023 issue of the American Thoracic Society’s ATS Coding & Billing Quarterly. Republished with permission from the American Thoracic Society.
This add-on code is for new (99202-99205) and established (99212-99215) office visits. CMS created this add-on code to address the additional costs and resources associated with providing longitudinal care.
G2211 – Visit complexity inherent to evaluation and management (E/M) associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious condition, or a complex condition (Add-on code; list separately in addition to office/outpatient (O/O) E/M visit, new or established)
The documentation should demonstrate the intent and need for ongoing care. Otherwise, no additional documentation is required. CMS pays $16.04 for each service (wRVU = 0.33). It may be reported each time the patient is seen, and there is currently no limit to how often it may be used. Also, there is no additional copay requirement for patients.
Do’s and don’ts
Do report in the following situations when longitudinal care is provided:
- The provider has or intends to have a long-term, ongoing relationship with the patient (ie, G2211 can be used for a new patient visit)
- Audio/video virtual visits
- May be reported with Prolonged Care Services G2212
- When advanced practice providers or physician colleagues in the same specialty practice see the patient (ie, if you see the patient for an urgent visit, but the patient is usually followed by your partner, you can still use G2211)
- When working with graduate medical education trainees (along with the -GC modifier), and as long as the conditions described in the description of G2211 are met
Do NOT report in the following situations:
- If modifier -25 is appended to the E/M service when another service is provided on the same day (eg, pulmonary function tests, 6-minute walk tests, immunization)
- Audio-only virtual visits, hospital, skilled nursing facility, or long-term acute care hospital
- If the patient is not expected to return for ongoing care
- If the reason for longitudinal care does not include a “single, serious condition or a complex condition” (eg, annual visits for a stable 6 mm lung nodule)
CMS expects that this will be billed with 38% of all E/M services initially and potentially up to 54% over time. We feel this is reimbursement for the work being done to care for our patients with single, serious, or complex conditions. Both Medicare and Medicare Advantage plans are expected to reimburse for this service. Whether other payers will do the same is unclear, but it will become clear with time and further negotiation at the local level. In the meantime, members are encouraged to report this code for all appropriate patient encounters.
Questions and answers — G2211
Question: What private insurances cover G2211?
Answer: As of March 1, 2024, four national payers have confirmed coverage of G2211:
- Cigna (Medicare Advantage only),
- Humana (commercial and Medicare Advantage),
- United Healthcare (commercial and Medicare Advantage), and
- Aetna (Medicare Advantage).
Question: What needs to be documented for G2211?
Answer: CMS states, “You must document the reason for billing the office and outpatient (O/O) and evaluation and management (E/M). The visits themselves would need to be medically reasonable and necessary for the practitioner to report G2211. In addition, the documentation would need to illustrate medical necessity of the O/O E/M visit. We [CMS] haven’t required additional documentation.”
American Thoracic Society (ATS) and CHEST also recommend including a detailed assessment and plan for the visit, as well as any follow-up. The complexity of the visit should be clear in your documentation to support the medical necessity for reporting the G2211.
Question: How can a provider show that a new patient visit (99202-99205) is part of continuing care?
Answer: The treating practitioner should make sure their documentation supports their intent to provide ongoing care to the patient. Establishing such intent goes beyond a statement that the provider plans to provide ongoing care or schedule a follow-up visit. The circumstances of the visit should support the extra work involved in becoming the focal point of the patient’s care or providing ongoing care for a serious or complex condition.
Question: Dr. Red works at a primary care practice, is the focal point for a patient’s care, and has reported G2211. If Dr. Yellow, who is in the same specialty, or Mr. Green, a nurse practitioner, is covering for Dr. Red, and the patient comes in for a visit, can they report G2211 for that visit?
Answer: Yes. The same specialty/same provider rules would apply in this situation. But remember that Dr. Yellow’s or Mr. Green’s documentation for that encounter must support the code.
Question: Can a resident report G2211 under the primary care exemption?
Answer: Yes, according to CMS staff, so long as the service and the documentation meet all the requirements for the exemption and the visit complexity code. For example, the resident can only report low-level E/M codes, and the resident must be “the focal point for that person’s care.”
Question: Are there frequency limits for how often we can report G2211, either for a single patient in a given time period or by a provider or a practice?
Answer: Not at this time, but make sure your providers are following the rules for reporting the code. “There’s got to be documentation that suggests why the practitioner believes they are treating the patient on this long-standing, longitudinal trajectory, and we’ll be able to see how that interaction is happening,” senior CMS staff said. CMS staff further issued a subtle warning to providers by reminding them that CMS has a very strong integrity program. Your practice can avoid problems with thorough training, frequent chart review, and encouraging the team to ask questions until you feel that everyone is comfortable with the code.
Question: Are there any limits on the specialties that can report the code? Is it just for primary care providers?
Answer: No. Remember that a provider who is managing a single serious or complex condition can also report the code. But CMS expects the documentation to support the ongoing nature of the treatment. If a patient sees a provider as a one-off encounter, perhaps to manage an acute problem, that visit wouldn’t qualify. But if the provider clearly documents that they are actively managing the patient’s condition, the encounters could qualify.
Question: Will CMS issue a list of conditions that meet the code’s serious or complex condition requirement?
Answer: CMS has included the examples of HIV and sickle cell anemia in existing guidance, and it plans to issue a few more examples “that help folks understand what is expected.” However, it won’t be a complete list of every condition that might qualify.
Originally published in the May 2023 issue of the American Thoracic Society’s ATS Coding & Billing Quarterly. Republished with permission from the American Thoracic Society.
This add-on code is for new (99202-99205) and established (99212-99215) office visits. CMS created this add-on code to address the additional costs and resources associated with providing longitudinal care.
G2211 – Visit complexity inherent to evaluation and management (E/M) associated with medical care services that serve as the continuing focal point for all needed health care services and/or with medical care services that are part of ongoing care related to a patient’s single, serious condition, or a complex condition (Add-on code; list separately in addition to office/outpatient (O/O) E/M visit, new or established)
The documentation should demonstrate the intent and need for ongoing care. Otherwise, no additional documentation is required. CMS pays $16.04 for each service (wRVU = 0.33). It may be reported each time the patient is seen, and there is currently no limit to how often it may be used. Also, there is no additional copay requirement for patients.
Do’s and don’ts
Do report in the following situations when longitudinal care is provided:
- The provider has or intends to have a long-term, ongoing relationship with the patient (ie, G2211 can be used for a new patient visit)
- Audio/video virtual visits
- May be reported with Prolonged Care Services G2212
- When advanced practice providers or physician colleagues in the same specialty practice see the patient (ie, if you see the patient for an urgent visit, but the patient is usually followed by your partner, you can still use G2211)
- When working with graduate medical education trainees (along with the -GC modifier), and as long as the conditions described in the description of G2211 are met
Do NOT report in the following situations:
- If modifier -25 is appended to the E/M service when another service is provided on the same day (eg, pulmonary function tests, 6-minute walk tests, immunization)
- Audio-only virtual visits, hospital, skilled nursing facility, or long-term acute care hospital
- If the patient is not expected to return for ongoing care
- If the reason for longitudinal care does not include a “single, serious condition or a complex condition” (eg, annual visits for a stable 6 mm lung nodule)
CMS expects that this will be billed with 38% of all E/M services initially and potentially up to 54% over time. We feel this is reimbursement for the work being done to care for our patients with single, serious, or complex conditions. Both Medicare and Medicare Advantage plans are expected to reimburse for this service. Whether other payers will do the same is unclear, but it will become clear with time and further negotiation at the local level. In the meantime, members are encouraged to report this code for all appropriate patient encounters.
Questions and answers — G2211
Question: What private insurances cover G2211?
Answer: As of March 1, 2024, four national payers have confirmed coverage of G2211:
- Cigna (Medicare Advantage only),
- Humana (commercial and Medicare Advantage),
- United Healthcare (commercial and Medicare Advantage), and
- Aetna (Medicare Advantage).
Question: What needs to be documented for G2211?
Answer: CMS states, “You must document the reason for billing the office and outpatient (O/O) and evaluation and management (E/M). The visits themselves would need to be medically reasonable and necessary for the practitioner to report G2211. In addition, the documentation would need to illustrate medical necessity of the O/O E/M visit. We [CMS] haven’t required additional documentation.”
American Thoracic Society (ATS) and CHEST also recommend including a detailed assessment and plan for the visit, as well as any follow-up. The complexity of the visit should be clear in your documentation to support the medical necessity for reporting the G2211.
Question: How can a provider show that a new patient visit (99202-99205) is part of continuing care?
Answer: The treating practitioner should make sure their documentation supports their intent to provide ongoing care to the patient. Establishing such intent goes beyond a statement that the provider plans to provide ongoing care or schedule a follow-up visit. The circumstances of the visit should support the extra work involved in becoming the focal point of the patient’s care or providing ongoing care for a serious or complex condition.
Question: Dr. Red works at a primary care practice, is the focal point for a patient’s care, and has reported G2211. If Dr. Yellow, who is in the same specialty, or Mr. Green, a nurse practitioner, is covering for Dr. Red, and the patient comes in for a visit, can they report G2211 for that visit?
Answer: Yes. The same specialty/same provider rules would apply in this situation. But remember that Dr. Yellow’s or Mr. Green’s documentation for that encounter must support the code.
Question: Can a resident report G2211 under the primary care exemption?
Answer: Yes, according to CMS staff, so long as the service and the documentation meet all the requirements for the exemption and the visit complexity code. For example, the resident can only report low-level E/M codes, and the resident must be “the focal point for that person’s care.”
Question: Are there frequency limits for how often we can report G2211, either for a single patient in a given time period or by a provider or a practice?
Answer: Not at this time, but make sure your providers are following the rules for reporting the code. “There’s got to be documentation that suggests why the practitioner believes they are treating the patient on this long-standing, longitudinal trajectory, and we’ll be able to see how that interaction is happening,” senior CMS staff said. CMS staff further issued a subtle warning to providers by reminding them that CMS has a very strong integrity program. Your practice can avoid problems with thorough training, frequent chart review, and encouraging the team to ask questions until you feel that everyone is comfortable with the code.
Question: Are there any limits on the specialties that can report the code? Is it just for primary care providers?
Answer: No. Remember that a provider who is managing a single serious or complex condition can also report the code. But CMS expects the documentation to support the ongoing nature of the treatment. If a patient sees a provider as a one-off encounter, perhaps to manage an acute problem, that visit wouldn’t qualify. But if the provider clearly documents that they are actively managing the patient’s condition, the encounters could qualify.
Question: Will CMS issue a list of conditions that meet the code’s serious or complex condition requirement?
Answer: CMS has included the examples of HIV and sickle cell anemia in existing guidance, and it plans to issue a few more examples “that help folks understand what is expected.” However, it won’t be a complete list of every condition that might qualify.
Originally published in the May 2023 issue of the American Thoracic Society’s ATS Coding & Billing Quarterly. Republished with permission from the American Thoracic Society.
Use of albumin in critically ill patients
Intravenous albumin is a human-derived blood product studied widely in a variety of patient populations. Despite its frequent use in critical care, few high-quality studies have demonstrated improvements in patient-important outcomes. Compared with crystalloids, albumin increases the risk of fluid overload and bleeding and infections in patients undergoing cardiac surgery.1,2 In addition, albumin is costly, and its production is fraught with donor supply chain ethical concerns (the majority of albumin is derived from paid plasma donors).
Albumin use is highly variable between countries, hospitals, and even clinicians within the same specialty due to several factors, including the perception of minimal risk with albumin, concerns regarding insufficient short-term hemodynamic response to crystalloid, and lack of high-quality evidence to inform clinical practice. We will discuss when intensivists should consider albumin use (with prescription personalized to patient context) and when it should be avoided due to the concerns for patient harm.
An intensivist might consider albumin as a reasonable treatment option in patients with cirrhosis undergoing large volume paracentesis to prevent paracentesis-induced circulatory dysfunction, and in patients with cirrhosis and spontaneous bacterial peritonitis (SBP), as data suggests use in this setting leads to a reduction in mortality.3 Clinicians should be aware that even for these widely accepted albumin indications, which are supported by published guidelines, the certainty of evidence is low, recommendations are weak (conditional), and, therefore, albumin should always be personalized to the patient based on volume of paracentesis fluid removed, prior history of hypotension after procedures, and degree of renal dysfunction.4
There are also several conditions for which an intensivist might consider albumin and for which albumin is commonly administered but lacks high-quality studies to support its use either as a frontline or rescue fluid therapy. One such condition is type 1 hepatorenal syndrome (HRS), for which albumin is widely used; however, there are no randomized controlled trials that have compared albumin with placebo.
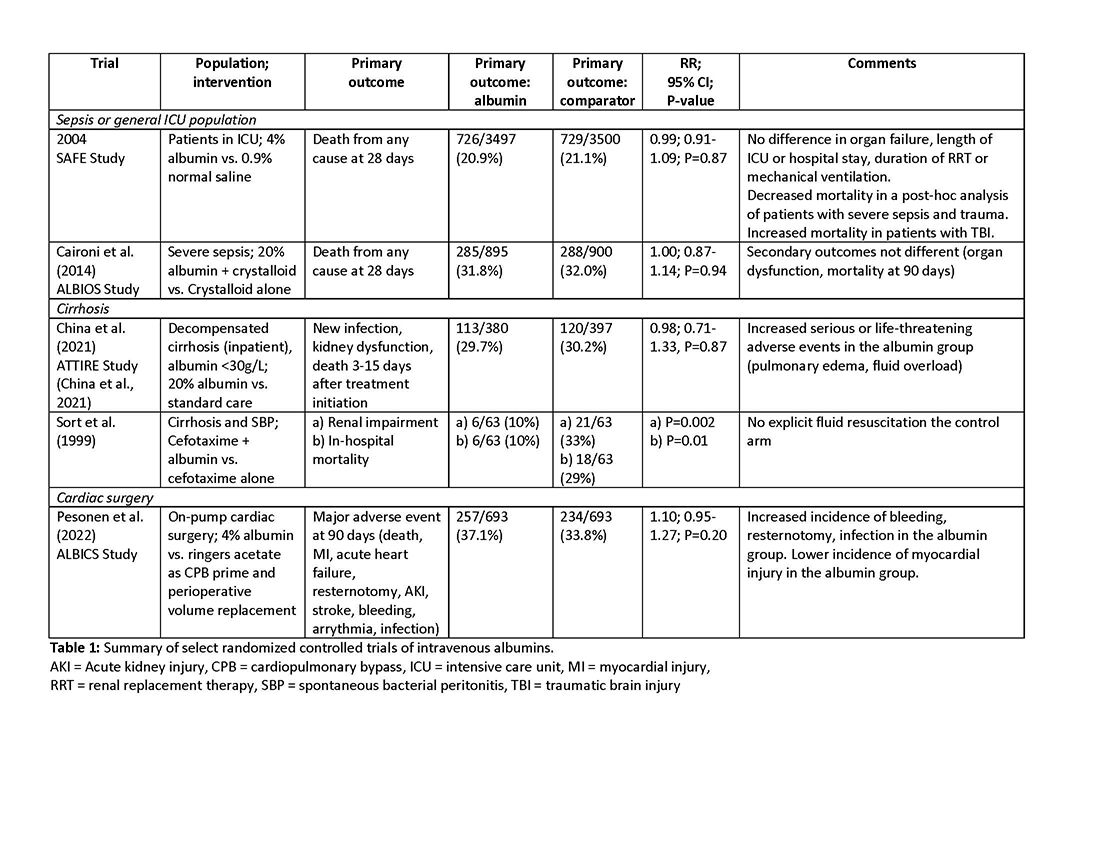
As with any intervention, the use of albumin is associated with risks. In patients undergoing on-pump cardiac surgery, the ALBICS study showed that albumin did not reduce the risk of major adverse events and, instead, increased risk of bleeding, resternotomy, and infection.2 The ATTIRE trial showed that in patients hospitalized with decompensated cirrhosis and serum albumin <30 g/L, albumin failed to reduce infection, renal impairment, or mortality while increasing life-threatening adverse events, including pulmonary edema and fluid overload.1 Similarly, in patients with cirrhosis and extraperitoneal infections, albumin showed no benefit in reducing renal impairment or mortality, and its use was associated with higher rates of pulmonary edema.6 Lastly, critically ill patients with traumatic brain injury (TBI) who received fluid resuscitation with albumin have been shown to experience higher mortality compared with saline.7 Thus, based on current evidence, intravenous albumin is not recommended for patients undergoing cardiac surgery (priming of the bypass circuit or volume replacement), patients hospitalized with decompensated cirrhosis and hypoalbuminemia, patients hospitalized with cirrhosis and extraperitoneal infections, and critically ill patients with TBI.4
Overall, intravenous albumin prescription in critical care patients requires a personalized approach informed by current best evidence and is not without potential harm.
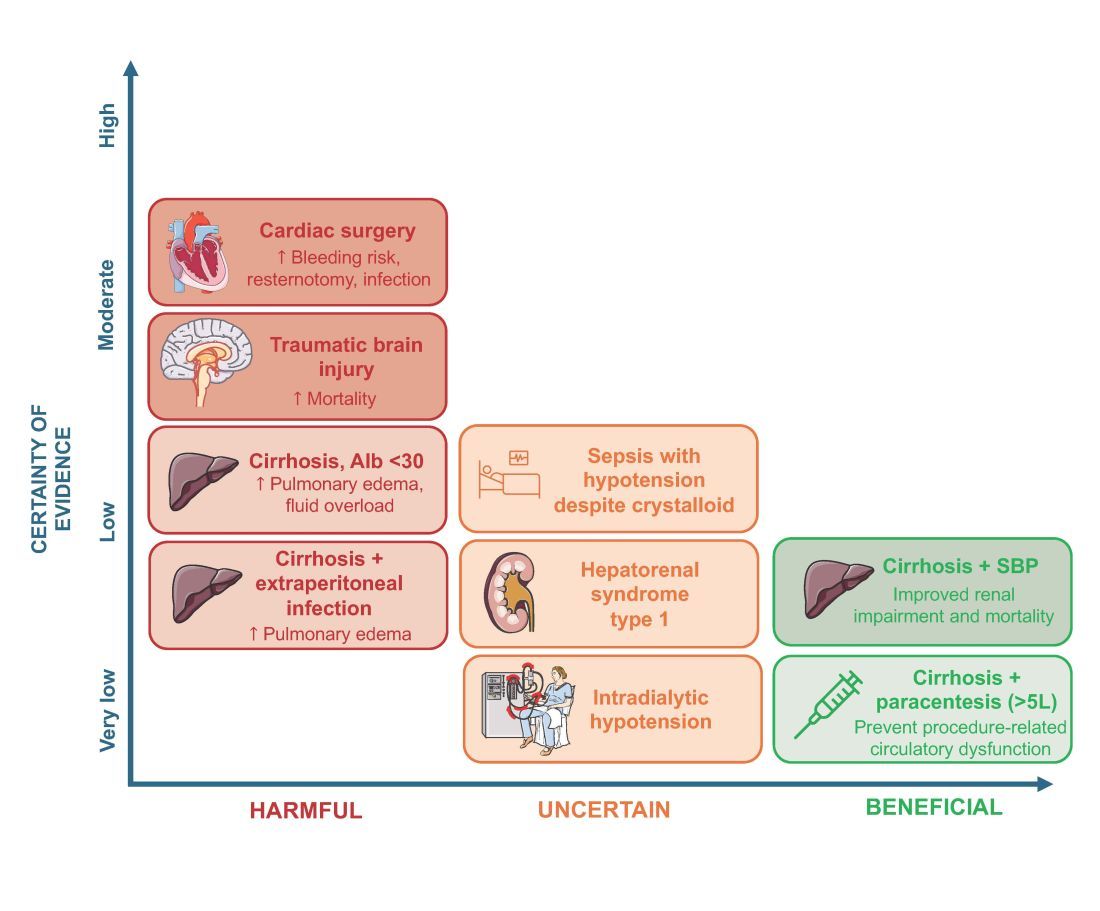
High-quality evidence is currently lacking in many clinical settings, and large randomized controlled trials are underway to provide further insights into the utility of albumin. These trials will address albumin use in the following: acute kidney injury requiring renal replacement therapy (ALTER-AKI, NCT04705896), inpatients with community-acquired pneumonia (NCT04071041), high-risk cardiac surgery (ACTRN1261900135516703), and septic shock (NCT03869385).
Financial/nonfinancial disclosures
Nicole Relke: None. Mark Hewitt: None. Bram Rochwerg: None. Jeannie Callum: Research support from Canadian Blood Services and Octapharma.
References
1. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. doi:10.1056/NEJMoa2022166
2. Pesonen E, Vlasov H, Suojaranta R, et al. Effect of 4% albumin solution vs ringer acetate on major adverse events in patients undergoing cardiac surgery with cardiopulmonary bypass: a randomized clinical trial. JAMA. 2022;328(3):251-258. doi:10.1001/jama.2022.10461
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. NEJM. 1999;341:403-409.
4. Callum J, Skubas NJ, Bathla A, et al. Use of intravenous albumin: a guideline from the international collaboration for transfusion medicine guidelines. Chest. 2024:S0012-3692(24)00285-X. doi:10.1016/j.chest.2024.02.049
5. Torp N. High doses of albumin increases mortality and complications in terlipressin treated patients with cirrhosis: insights from the ATTIRE trial. Paper presented at the AASLD; 2023; San Diego, CA. https://www.aasld.org/the-liver-meeting/high-doses-albumin-increases-mortality-and-complications-terlipressin-treated
6. Wong YJ, Qiu TY, Tam YC, Mohan BP, Gallegos-Orozco JF, Adler DG. Efficacy and safety of IV albumin for non-spontaneous bacterial peritonitis infection among patients with cirrhosis: a systematic review and meta-analysis. Dig Liver Dis. 2020;52(10):1137-1142. doi:10.1016/j.dld.2020.05.047
7. Myburgh J, Cooper JD, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874-884.
Intravenous albumin is a human-derived blood product studied widely in a variety of patient populations. Despite its frequent use in critical care, few high-quality studies have demonstrated improvements in patient-important outcomes. Compared with crystalloids, albumin increases the risk of fluid overload and bleeding and infections in patients undergoing cardiac surgery.1,2 In addition, albumin is costly, and its production is fraught with donor supply chain ethical concerns (the majority of albumin is derived from paid plasma donors).
Albumin use is highly variable between countries, hospitals, and even clinicians within the same specialty due to several factors, including the perception of minimal risk with albumin, concerns regarding insufficient short-term hemodynamic response to crystalloid, and lack of high-quality evidence to inform clinical practice. We will discuss when intensivists should consider albumin use (with prescription personalized to patient context) and when it should be avoided due to the concerns for patient harm.
An intensivist might consider albumin as a reasonable treatment option in patients with cirrhosis undergoing large volume paracentesis to prevent paracentesis-induced circulatory dysfunction, and in patients with cirrhosis and spontaneous bacterial peritonitis (SBP), as data suggests use in this setting leads to a reduction in mortality.3 Clinicians should be aware that even for these widely accepted albumin indications, which are supported by published guidelines, the certainty of evidence is low, recommendations are weak (conditional), and, therefore, albumin should always be personalized to the patient based on volume of paracentesis fluid removed, prior history of hypotension after procedures, and degree of renal dysfunction.4
There are also several conditions for which an intensivist might consider albumin and for which albumin is commonly administered but lacks high-quality studies to support its use either as a frontline or rescue fluid therapy. One such condition is type 1 hepatorenal syndrome (HRS), for which albumin is widely used; however, there are no randomized controlled trials that have compared albumin with placebo.

As with any intervention, the use of albumin is associated with risks. In patients undergoing on-pump cardiac surgery, the ALBICS study showed that albumin did not reduce the risk of major adverse events and, instead, increased risk of bleeding, resternotomy, and infection.2 The ATTIRE trial showed that in patients hospitalized with decompensated cirrhosis and serum albumin <30 g/L, albumin failed to reduce infection, renal impairment, or mortality while increasing life-threatening adverse events, including pulmonary edema and fluid overload.1 Similarly, in patients with cirrhosis and extraperitoneal infections, albumin showed no benefit in reducing renal impairment or mortality, and its use was associated with higher rates of pulmonary edema.6 Lastly, critically ill patients with traumatic brain injury (TBI) who received fluid resuscitation with albumin have been shown to experience higher mortality compared with saline.7 Thus, based on current evidence, intravenous albumin is not recommended for patients undergoing cardiac surgery (priming of the bypass circuit or volume replacement), patients hospitalized with decompensated cirrhosis and hypoalbuminemia, patients hospitalized with cirrhosis and extraperitoneal infections, and critically ill patients with TBI.4
Overall, intravenous albumin prescription in critical care patients requires a personalized approach informed by current best evidence and is not without potential harm.

High-quality evidence is currently lacking in many clinical settings, and large randomized controlled trials are underway to provide further insights into the utility of albumin. These trials will address albumin use in the following: acute kidney injury requiring renal replacement therapy (ALTER-AKI, NCT04705896), inpatients with community-acquired pneumonia (NCT04071041), high-risk cardiac surgery (ACTRN1261900135516703), and septic shock (NCT03869385).
Financial/nonfinancial disclosures
Nicole Relke: None. Mark Hewitt: None. Bram Rochwerg: None. Jeannie Callum: Research support from Canadian Blood Services and Octapharma.
References
1. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. doi:10.1056/NEJMoa2022166
2. Pesonen E, Vlasov H, Suojaranta R, et al. Effect of 4% albumin solution vs ringer acetate on major adverse events in patients undergoing cardiac surgery with cardiopulmonary bypass: a randomized clinical trial. JAMA. 2022;328(3):251-258. doi:10.1001/jama.2022.10461
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. NEJM. 1999;341:403-409.
4. Callum J, Skubas NJ, Bathla A, et al. Use of intravenous albumin: a guideline from the international collaboration for transfusion medicine guidelines. Chest. 2024:S0012-3692(24)00285-X. doi:10.1016/j.chest.2024.02.049
5. Torp N. High doses of albumin increases mortality and complications in terlipressin treated patients with cirrhosis: insights from the ATTIRE trial. Paper presented at the AASLD; 2023; San Diego, CA. https://www.aasld.org/the-liver-meeting/high-doses-albumin-increases-mortality-and-complications-terlipressin-treated
6. Wong YJ, Qiu TY, Tam YC, Mohan BP, Gallegos-Orozco JF, Adler DG. Efficacy and safety of IV albumin for non-spontaneous bacterial peritonitis infection among patients with cirrhosis: a systematic review and meta-analysis. Dig Liver Dis. 2020;52(10):1137-1142. doi:10.1016/j.dld.2020.05.047
7. Myburgh J, Cooper JD, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874-884.
Intravenous albumin is a human-derived blood product studied widely in a variety of patient populations. Despite its frequent use in critical care, few high-quality studies have demonstrated improvements in patient-important outcomes. Compared with crystalloids, albumin increases the risk of fluid overload and bleeding and infections in patients undergoing cardiac surgery.1,2 In addition, albumin is costly, and its production is fraught with donor supply chain ethical concerns (the majority of albumin is derived from paid plasma donors).
Albumin use is highly variable between countries, hospitals, and even clinicians within the same specialty due to several factors, including the perception of minimal risk with albumin, concerns regarding insufficient short-term hemodynamic response to crystalloid, and lack of high-quality evidence to inform clinical practice. We will discuss when intensivists should consider albumin use (with prescription personalized to patient context) and when it should be avoided due to the concerns for patient harm.
An intensivist might consider albumin as a reasonable treatment option in patients with cirrhosis undergoing large volume paracentesis to prevent paracentesis-induced circulatory dysfunction, and in patients with cirrhosis and spontaneous bacterial peritonitis (SBP), as data suggests use in this setting leads to a reduction in mortality.3 Clinicians should be aware that even for these widely accepted albumin indications, which are supported by published guidelines, the certainty of evidence is low, recommendations are weak (conditional), and, therefore, albumin should always be personalized to the patient based on volume of paracentesis fluid removed, prior history of hypotension after procedures, and degree of renal dysfunction.4
There are also several conditions for which an intensivist might consider albumin and for which albumin is commonly administered but lacks high-quality studies to support its use either as a frontline or rescue fluid therapy. One such condition is type 1 hepatorenal syndrome (HRS), for which albumin is widely used; however, there are no randomized controlled trials that have compared albumin with placebo.

As with any intervention, the use of albumin is associated with risks. In patients undergoing on-pump cardiac surgery, the ALBICS study showed that albumin did not reduce the risk of major adverse events and, instead, increased risk of bleeding, resternotomy, and infection.2 The ATTIRE trial showed that in patients hospitalized with decompensated cirrhosis and serum albumin <30 g/L, albumin failed to reduce infection, renal impairment, or mortality while increasing life-threatening adverse events, including pulmonary edema and fluid overload.1 Similarly, in patients with cirrhosis and extraperitoneal infections, albumin showed no benefit in reducing renal impairment or mortality, and its use was associated with higher rates of pulmonary edema.6 Lastly, critically ill patients with traumatic brain injury (TBI) who received fluid resuscitation with albumin have been shown to experience higher mortality compared with saline.7 Thus, based on current evidence, intravenous albumin is not recommended for patients undergoing cardiac surgery (priming of the bypass circuit or volume replacement), patients hospitalized with decompensated cirrhosis and hypoalbuminemia, patients hospitalized with cirrhosis and extraperitoneal infections, and critically ill patients with TBI.4
Overall, intravenous albumin prescription in critical care patients requires a personalized approach informed by current best evidence and is not without potential harm.

High-quality evidence is currently lacking in many clinical settings, and large randomized controlled trials are underway to provide further insights into the utility of albumin. These trials will address albumin use in the following: acute kidney injury requiring renal replacement therapy (ALTER-AKI, NCT04705896), inpatients with community-acquired pneumonia (NCT04071041), high-risk cardiac surgery (ACTRN1261900135516703), and septic shock (NCT03869385).
Financial/nonfinancial disclosures
Nicole Relke: None. Mark Hewitt: None. Bram Rochwerg: None. Jeannie Callum: Research support from Canadian Blood Services and Octapharma.
References
1. China L, Freemantle N, Forrest E, et al. A randomized trial of albumin infusions in hospitalized patients with cirrhosis. N Engl J Med. 2021;384(9):808-817. doi:10.1056/NEJMoa2022166
2. Pesonen E, Vlasov H, Suojaranta R, et al. Effect of 4% albumin solution vs ringer acetate on major adverse events in patients undergoing cardiac surgery with cardiopulmonary bypass: a randomized clinical trial. JAMA. 2022;328(3):251-258. doi:10.1001/jama.2022.10461
3. Sort P, Navasa M, Arroyo V, et al. Effect of intravenous albumin on renal impairment and mortality in patients with cirrhosis and spontaneous bacterial peritonitis. NEJM. 1999;341:403-409.
4. Callum J, Skubas NJ, Bathla A, et al. Use of intravenous albumin: a guideline from the international collaboration for transfusion medicine guidelines. Chest. 2024:S0012-3692(24)00285-X. doi:10.1016/j.chest.2024.02.049
5. Torp N. High doses of albumin increases mortality and complications in terlipressin treated patients with cirrhosis: insights from the ATTIRE trial. Paper presented at the AASLD; 2023; San Diego, CA. https://www.aasld.org/the-liver-meeting/high-doses-albumin-increases-mortality-and-complications-terlipressin-treated
6. Wong YJ, Qiu TY, Tam YC, Mohan BP, Gallegos-Orozco JF, Adler DG. Efficacy and safety of IV albumin for non-spontaneous bacterial peritonitis infection among patients with cirrhosis: a systematic review and meta-analysis. Dig Liver Dis. 2020;52(10):1137-1142. doi:10.1016/j.dld.2020.05.047
7. Myburgh J, Cooper JD, Finfer S, et al. Saline or albumin for fluid resuscitation in patients with traumatic brain injury. N Engl J Med. 2007;357(9):874-884.
Right heart catheterization practice patterns in pulmonary hypertension in the US
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Pulmonary Vascular Disease Section
While these cutoffs are straightforward, a gap in practical application is evidenced by considerable variability in how PH providers perform and interpret RHC hemodynamic information.
A recent survey of 145 PH providers conducted by CHEST’s Pulmonary Vascular Disease Section shed light on the current RHC practices in the US.2 Regarding the respondents’ characteristics, 85% were in the 30-60 age range, 68% were males, and 71% were pulmonologists.
About half of the providers perform the RHC themselves. Most review the hemodynamic tracings, but up to 21% rely on the final report alone. Regarding PCWP, most (86%) obtain it during end-expiration, but only 42% routinely measure a PCWP saturation for confirmation. When faced with PVR discrepancies between thermodilution and indirect Fick (IFick), up to 30% chose either IFick or didn’t know which one to trust. Nearly 20% repeat the RHC at least annually, and 80% whenever the patient declines.
This study provides the largest reported data on real-world RHC practices by PH physicians in the US. We found significant variability in hemodynamic interpretation. Standardization of RHC performance and hemodynamic evaluation is crucial to ensure appropriate PH management.
– Abubakr A. Bajwa, MBBS, FCCP
Member-at-Large
– Samantha Pettigrew, MD
Fellow-in-Training
– Francisco J. Soto, MD, MS, FCCP
Section Vice Chair
References
1. Simonneau et al. Eur Resp J. 2019;53(1):1801913
2. Soto et al. CHEST. 2023;164(4):Supplement A5832-A5834
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Pulmonary Vascular Disease Section
While these cutoffs are straightforward, a gap in practical application is evidenced by considerable variability in how PH providers perform and interpret RHC hemodynamic information.
A recent survey of 145 PH providers conducted by CHEST’s Pulmonary Vascular Disease Section shed light on the current RHC practices in the US.2 Regarding the respondents’ characteristics, 85% were in the 30-60 age range, 68% were males, and 71% were pulmonologists.
About half of the providers perform the RHC themselves. Most review the hemodynamic tracings, but up to 21% rely on the final report alone. Regarding PCWP, most (86%) obtain it during end-expiration, but only 42% routinely measure a PCWP saturation for confirmation. When faced with PVR discrepancies between thermodilution and indirect Fick (IFick), up to 30% chose either IFick or didn’t know which one to trust. Nearly 20% repeat the RHC at least annually, and 80% whenever the patient declines.
This study provides the largest reported data on real-world RHC practices by PH physicians in the US. We found significant variability in hemodynamic interpretation. Standardization of RHC performance and hemodynamic evaluation is crucial to ensure appropriate PH management.
– Abubakr A. Bajwa, MBBS, FCCP
Member-at-Large
– Samantha Pettigrew, MD
Fellow-in-Training
– Francisco J. Soto, MD, MS, FCCP
Section Vice Chair
References
1. Simonneau et al. Eur Resp J. 2019;53(1):1801913
2. Soto et al. CHEST. 2023;164(4):Supplement A5832-A5834
PULMONARY VASCULAR AND CARDIOVASCULAR NETWORK
Pulmonary Vascular Disease Section
While these cutoffs are straightforward, a gap in practical application is evidenced by considerable variability in how PH providers perform and interpret RHC hemodynamic information.
A recent survey of 145 PH providers conducted by CHEST’s Pulmonary Vascular Disease Section shed light on the current RHC practices in the US.2 Regarding the respondents’ characteristics, 85% were in the 30-60 age range, 68% were males, and 71% were pulmonologists.
About half of the providers perform the RHC themselves. Most review the hemodynamic tracings, but up to 21% rely on the final report alone. Regarding PCWP, most (86%) obtain it during end-expiration, but only 42% routinely measure a PCWP saturation for confirmation. When faced with PVR discrepancies between thermodilution and indirect Fick (IFick), up to 30% chose either IFick or didn’t know which one to trust. Nearly 20% repeat the RHC at least annually, and 80% whenever the patient declines.
This study provides the largest reported data on real-world RHC practices by PH physicians in the US. We found significant variability in hemodynamic interpretation. Standardization of RHC performance and hemodynamic evaluation is crucial to ensure appropriate PH management.
– Abubakr A. Bajwa, MBBS, FCCP
Member-at-Large
– Samantha Pettigrew, MD
Fellow-in-Training
– Francisco J. Soto, MD, MS, FCCP
Section Vice Chair
References
1. Simonneau et al. Eur Resp J. 2019;53(1):1801913
2. Soto et al. CHEST. 2023;164(4):Supplement A5832-A5834
Machine learning meets cardiopulmonary exercise testing
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Pulmonary Physiology and Rehabilitation Section
Several studies have explored automation of CPET interpretation, the most notable of which utilized machine learning.1
Recently, Schwendinger et al. investigated the ability of machine learning algorithms to not only categorize (pulmonary-vascular, mechanical-ventilatory, cardiocirculatory, and muscular), but also assign severity scores (0-6) to exercise limitations found in a group of 200 CPETs performed on adult patients referred to a lung clinic in Germany.2 Decision trees were constructed for each of the limitation categories by identifying variables with the lowest Root Mean Square Error (RMSE), which were comparable to agreement within expert interpretations. Combining decision trees allowed for a more comprehensive analysis with identification of multiple abnormalities in the same test.
A major limitation to the study is limited applicability to general patient populations without suspected lung disease. This bias is reflected in the decision tree for cardiovascular limitation that relied on VO2 peak and FEV1 alone. The authors were unable to construct a decision tree for muscular limitations due to a lack of identified cases.
Overall, these results suggest that refinement of machine learning algorithms built with larger heterogeneous data sets and expert interpretation can make CPETs accessible to the nonexpert clinician as long as test quality can be replicated across centers.
–Joseph Russo, MD
Fellow-in-Training
– Fatima Zeba, MD
Member-at-Large
References
1. Portella JJ, Andonian BJ, Brown DE, et al. Using machine learning to identify organ system specific limitations to exercise via cardiopulmonary exercise testing. IEEE J Biomed Health Inform. 2022;26(8):4228-4237.
2. Schwendinger F, Biehler AK, Nagy-Huber M, et al. Using machine learning-based algorithms to identify and quantify exercise limitations in clinical practice: are we there yet? Med Sci Sports Exerc. 2024;56(2):159-169.
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Pulmonary Physiology and Rehabilitation Section
Several studies have explored automation of CPET interpretation, the most notable of which utilized machine learning.1
Recently, Schwendinger et al. investigated the ability of machine learning algorithms to not only categorize (pulmonary-vascular, mechanical-ventilatory, cardiocirculatory, and muscular), but also assign severity scores (0-6) to exercise limitations found in a group of 200 CPETs performed on adult patients referred to a lung clinic in Germany.2 Decision trees were constructed for each of the limitation categories by identifying variables with the lowest Root Mean Square Error (RMSE), which were comparable to agreement within expert interpretations. Combining decision trees allowed for a more comprehensive analysis with identification of multiple abnormalities in the same test.
A major limitation to the study is limited applicability to general patient populations without suspected lung disease. This bias is reflected in the decision tree for cardiovascular limitation that relied on VO2 peak and FEV1 alone. The authors were unable to construct a decision tree for muscular limitations due to a lack of identified cases.
Overall, these results suggest that refinement of machine learning algorithms built with larger heterogeneous data sets and expert interpretation can make CPETs accessible to the nonexpert clinician as long as test quality can be replicated across centers.
–Joseph Russo, MD
Fellow-in-Training
– Fatima Zeba, MD
Member-at-Large
References
1. Portella JJ, Andonian BJ, Brown DE, et al. Using machine learning to identify organ system specific limitations to exercise via cardiopulmonary exercise testing. IEEE J Biomed Health Inform. 2022;26(8):4228-4237.
2. Schwendinger F, Biehler AK, Nagy-Huber M, et al. Using machine learning-based algorithms to identify and quantify exercise limitations in clinical practice: are we there yet? Med Sci Sports Exerc. 2024;56(2):159-169.
DIFFUSE LUNG DISEASE AND LUNG TRANSPLANT NETWORK
Pulmonary Physiology and Rehabilitation Section
Several studies have explored automation of CPET interpretation, the most notable of which utilized machine learning.1
Recently, Schwendinger et al. investigated the ability of machine learning algorithms to not only categorize (pulmonary-vascular, mechanical-ventilatory, cardiocirculatory, and muscular), but also assign severity scores (0-6) to exercise limitations found in a group of 200 CPETs performed on adult patients referred to a lung clinic in Germany.2 Decision trees were constructed for each of the limitation categories by identifying variables with the lowest Root Mean Square Error (RMSE), which were comparable to agreement within expert interpretations. Combining decision trees allowed for a more comprehensive analysis with identification of multiple abnormalities in the same test.
A major limitation to the study is limited applicability to general patient populations without suspected lung disease. This bias is reflected in the decision tree for cardiovascular limitation that relied on VO2 peak and FEV1 alone. The authors were unable to construct a decision tree for muscular limitations due to a lack of identified cases.
Overall, these results suggest that refinement of machine learning algorithms built with larger heterogeneous data sets and expert interpretation can make CPETs accessible to the nonexpert clinician as long as test quality can be replicated across centers.
–Joseph Russo, MD
Fellow-in-Training
– Fatima Zeba, MD
Member-at-Large
References
1. Portella JJ, Andonian BJ, Brown DE, et al. Using machine learning to identify organ system specific limitations to exercise via cardiopulmonary exercise testing. IEEE J Biomed Health Inform. 2022;26(8):4228-4237.
2. Schwendinger F, Biehler AK, Nagy-Huber M, et al. Using machine learning-based algorithms to identify and quantify exercise limitations in clinical practice: are we there yet? Med Sci Sports Exerc. 2024;56(2):159-169.



























