User login
Elective laparoscopic appendectomy in gynecologic surgery: When, why, and how
Videos provided by Teresa Tam, MD, and Gerald Harkins, MD
CASE: Should appendectomy be included in total
laparoscopic hysterectomy?
A 39-year-old mother of two continues to experience severe dysmenorrhea and persistent menorrhagia despite undergoing endometrial ablation 2 years earlier. Her obstetric and gynecologic history is remarkable for a diagnosis of chronic pelvic pain, endometriosis, and failed endometrial ablation. Both her children were delivered by cesarean, and she has undergone tubal ligation. She requests hysterectomy to address the dysmenorrhea and menorrhagia once and for all.
A pelvic exam reveals an anteverted, 10-weeks’ size uterus with no adnexal masses or tenderness. After extensive discussion of the surgical procedure, the patient signs a consent for total laparoscopic hysterectomy.
Would you recommend appendectomy, too?
Prophylactic removal of the appendix during a benign gynecologic procedure is known as “elective incidental appendectomy.”1 Incidental appendectomy at the time of cesarean delivery was reported initially in 1959.2 Subsequent studies of removal of a normal-appearing appendix at the time of gynecologic surgery have met with considerable debate. Proponents argue that removal of the appendix at the time of abdominal hysterectomy does not increase operative time or postoperative morbidity. More important, it does prevent future appendicitis.3-5
Some surgeons disagree, citing an increase in operative time, hospital costs, and patient morbidity as reasonable concerns. They also note that appendectomy requires an additional surgical procedure, which could increase the risk of infection and other complications and lead to adhesion formation.
Advantages of incidental appendectomy include technical ease, low patient morbidity and mortality, and significant diagnostic and protective value.6 It also prevents conflicting diagnoses, especially in patients who have chronic pelvic pain, a ruptured ovarian cyst, or endometriosis. Other patients likely to benefit from elective incidental appendectomy are those who are undergoing abdominal radiation or chemotherapy, women unable to communicate health complaints, and those who are planning to undergo complex abdominal or pelvic procedures that are likely to cause extensive adhesions.1
In this article, we describe the rationale behind this procedure, as well as the technical steps involved.
The laparoscopic approach is preferred
Appendectomy is commonly performed laparoscopically. Semm first described this approach in 1983.7 Several studies since have reported that incidental laparoscopic appendectomy is safe, easy to perform, and should be offered to patients undergoing a concomitant gynecologic procedure.8-10 Laparoscopic removal of a normal appendix does not add morbidity or prolong hospitalization, compared with diagnostic laparoscopy. A large study drawing from the Nationwide Inpatient Sample (NIS) database found laparoscopic removal of the appendix to be associated with lower mortality, fewer complications, shorter hospitalization, and lower mean hospital charges, compared with open appendectomy.11 The same study found laparoscopic appendectomy to be the procedure of choice in both perforated and nonperforated appendicitis.
Overweight and obese patients also may benefit from the laparoscopic approach because it avoids problems associated with an open incision, such as the need for abdominal wall retraction, a longer hospital stay, and a risk of wound infection, compared with smaller incisions—especially in this high-risk population.12
Cost is another issue. Any prolonged surgical time and higher medical costs required for incidental appendectomy decrease as surgical proficiency and experience rise. The concomitant performance of endoscopic procedures can also reduce the risk associated with anesthesia for reoperations.
Endometriosis patients stand to benefit from appendectomy
There is compelling evidence that elective appendectomy is beneficial in patients who have endometriosis. Endometriosis of the bowel has been reported in 5.3% of all histologically proven endometriosis cases, with appendiceal endometriosis found in approximately 1% of women with endometriosis.13 Despite the low prevalence (2.8%) of appendiceal endometriosis,14 some studies reported a high incidence of appendiceal endometriosis when incidental appendectomy was performed. Patients who report right lower quadrant (RLQ) pain, chronic pelvic pain, and ovarian endometrioma had the highest incidence of abnormal histopathologic findings.15-17 Because most women with endometriosis present with these symptoms, it is prudent to counsel patients preoperatively about the incidence of appendiceal endometriosis and to visually examine the appendix during gynecologic surgery to identify incidental appendiceal pathology.
Age may influence the appendectomy decision
The incidence of acute appendicitis is highest among people aged 10 to 19 years. The estimated lifetime risk of appendicitis is 6.7%.18 The surgical dilemma is whether to perform incidental appendectomy in the nonadolescent population, which is at lower risk for appendicitis, as a preventive measure.
We lack randomized trials on the benefit of incidental appendectomy. A retrospective study of open procedures supported incidental appendectomy in patients younger than 35 years; for patients 35 to 50, the decision was left to the clinical judgment of the surgeon, based on the patient’s clinical condition.4 The same study failed to support incidental appendectomy in women older than 50 years.
When the appendix is not easily accessible, or the surgical complexity of the gynecologic procedure prevents the surgeon from safely performing an appendectomy, it is better to complete the planned gynecologic surgery and forgo the appendectomy. It is acceptable to make the decision to refrain from the appendectomy intraoperatively if the risk of complications outweighs the likely benefits. The practice of cautionary discretion demonstrates sound judgment and avoids compromising the safety of the patient.
1. Maintain at least three laparoscopic sites
- After the laparoscopic gynecologic procedure, maintain three trocar sites—preferably, two 5-mm trocars and one 12-mm trocar.
- The first 5-mm trocar, at the umbilical incision, accommodates the laparoscopic camera. The second 5-mm trocar serves as an accessory port for laparoscopic instruments and is inserted into the RLQ.
- The 12-mm trocar in the left lower quadrant (LLQ) is also used to insert endoscopic instruments. This trocar site will be used at the conclusion of the appendectomy to accommodate the mechanical stapling device and the specimen bag for removal of the excised organ.
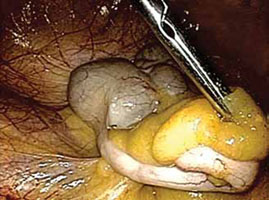
FIGURE 1: Visualize the appendix. Identify the cecum and ileocolic junction to locate the appendix.
2. Identify the appendix
- Perform a careful visual exploration of the abdominal contents to exclude other intra-abdominal pathology.
- Identify the cecum and ileocolic junction to locate the appendix (FIGURE 1).
- Visually inspect the appendix and identify any gross appendiceal pathology.
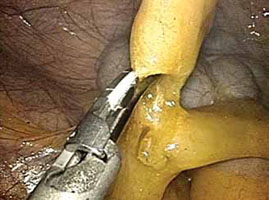
FIGURE 2: Divide the mesoappendix
Isolate, cauterize, and divide the mesoappendix using 5-mm ultrasonic shears.
3. Dissect the appendix (VIDEO 1)
- Insert an atraumatic forceps through the 5-mm right accessory trocar.
- Grasp the fatty tissue at the tip of the appendix and provide some traction.
- Elevate the appendix to facilitate visualization of the mesoappendix.
- Isolate the mesoappendix and cauterize and divide it using 5-mm ultrasonic shears inserted through the RLQ trocar (FIGURE 2).
- Release some of the upward tension from the specimen retraction by dropping the height of the instrument to prevent undue trauma and bleeding.
- Make a window between the mesentery and the base of the appendix to facilitate dissection.
- Skeletonize the mesoappendix at the junction of the appendiceal base and the cecum.
- During skeletonization, pay special attention to the appendiceal artery at the base of the mesoappendix.
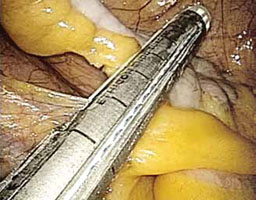
FIGURE 3: Apply the stapling device
Apply the stapling device across the base of the appendix.
4. Resect the appendix (VIDEO 2)
- Insert an automatic stapling device through the 12-mm port and apply it across the base of the appendix (FIGURE 3).
- Apply the mechanical stapling device for 15 seconds to crush the base of the appendix and empty its contents.
- Visualize both sides of the stapler to ensure that it is placed at the base of the appendix.
- Always check the tip of the device to ensure that the jaws of the stapling device fully compress the appendix and have not inadvertently grasped other abdominal contents.
- With the stapling device compressing the base of the appendix, release some of the upward tension on the specimen by dropping the height of the retraction.
- Activate the stapling device and completely excise the appendix from its base
(FIGURE 4). - Thoroughly inspect the appendiceal stump to ensure hemostasis (FIGURE 5).
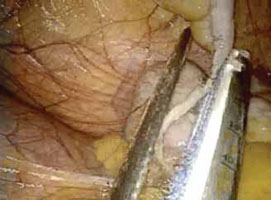
FIGURE 4: Excise the appendix
Activate the stapling device and completely excise the appendix from its base.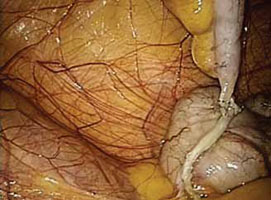
FIGURE 5: Ensure hemostasis
Thoroughly inspect the appendiceal stump and ensure hemostasis.
5. Remove the specimen (VIDEO 3)
- Remove the stapling device and replace it with a specimen retrieval bag, inserting it through the 12-mm port.
- Place the amputated appendix in the specimen retrieval bag to prevent abdominal contamination (FIGURE 6).
- Close the specimen retrieval bag inside the abdomen.
- Refrain from removing the specimen bag through the trocar or forcefully passing the appendix through a small incision. We usually withdraw the 12-mm trocar, then remove the cinched bag containing the resected appendix under direct visualization. We take all precautionary measures to prevent breakage of the bag, which would leak appendiceal contents into the abdomen.
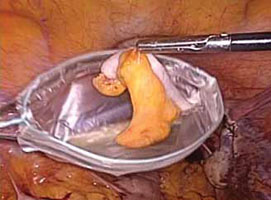
FIGURE 6: Remove the specimen
Place the amputated appendix in the specimen retrieval bag and remove it, intact, through the patient’s abdomen.
6. Perform a few last measures
- If the surgeon chooses, suction and irrigation can be performed at the completion of the appendectomy procedure.
- Send all surgical specimens to pathology for evaluation.
- Complete the operation in the usual laparoscopic fashion. Remove all instruments, and close the 12-mm trocar port site using the Carter Thomason fascial closure device. Close the remaining port sites using 2-0 interrupted suture (Monocryl). Apply skin adhesive to all laparoscopic incisions.
- For more on surgical technique of appendectomy, see Baggish,19 Jaffe and Berger,20 and Daniell and colleagues.21
Coding for appendectomy is fairly straightforward if you know the rules, but prophylactic removal of the appendix, whether performed at the time of a laparoscopic or open abdominal primary procedure, will usually lead to reimbursement difficulties for surgeons even though CPT codes exist to report the procedure. Knowing when and how to bill and document the circumstances for removal will go a long way in getting payment for the procedure. Note that these rules apply to a single surgeon who is performing the entire surgery. When an ObGyn is performing gyn procedures, but a general surgeon is the one who removes the appendix, that surgeon will not be subject to bundling rules, but will still have to make a case with the payer for removing an otherwise normal appendix.
There are 5 codes that can be used to report an appendectomy:
- 44950 Appendectomy;
- 44955 Appendectomy; when done for indicated purpose at time of other major procedure (not as separate procedure)
- 44960 Appendectomy; for ruptured appendix with abscess or generalized peritonitis
- 44970 Laparoscopy, surgical, appendectomy code
- 44979, Unlisted laparoscopy procedure, appendix.
Code 44950 represents either a stand-alone procedure or an incidental appendectomy when performed with other open abdominal procedures. Under CPT guidelines this code would only be reported 1) when this is the only procedure performed and the appendix is removed for a diagnosis other than rupture with abscess, or 2) with a modifier -52 added if the surgeon believes that an incidental appendectomy needs to be reported. Use of a modifier -52 will lead to review of the documentation by the payer, and it will be up to the surgeon to convince the payer that he should be paid for taking out an appendix that is found to be normal. Billing 44950 with other abdominal procedures without this modifier will lead to an outright denial due to bundling edits, which permanently bundle 44950 with all major abdominal procedures.
Code 44955 is the code to report when an appendectomy is performed for an indicated purpose at the time of other open abdominal procedures. For instance, the appendix may have been removed due to a finding of distention with fecalith or extensive adhesions binding the appendix to the abdominal wall. When this code is reported, no modifier is used because it is a CPT “add-on” code that can only be billed in conjunction with other procedures.
Code 44960 is only reported when no other open abdominal procedures are performed at the operative session and the reason for taking out the appendix is rupture with abscess. If rupture is found at the time of an abdominal procedure to remove a mass, for instance, code 44955 would be reported instead.
Code 44970 is the only laparoscopic approach code for an appendectomy, but it would only be reported when 1) the appendectomy was the only laparoscopic procedure performed, or 2) the appendectomy was incidental, but the surgeon felt it needed to be reported. There is no instruction about using a modifier -52 with 44970 to report an incidental appendectomy. According to the American Medical Association’s January 2012 issue of CPT Assistant, laparoscopic removal of the appendix for an indicated purpose at the time of another major laparoscopic procedure should be reported as 44979, Unlisted laparoscopy procedure, appendix.
Keep in mind that code 44970 is bundled into a long list of laparoscopic procedures, including codes for treating stress urinary incontinence and prolapse (CPT codes 51990–51992, 57425), sterilization procedures (CPT codes 58670–58671), hysterectomy procedures (CPT codes 58541–58544, 58548, 58550–58554, 58570–58573), myomectomy procedures (CPT code 58545–58546), as well as codes for lysis, removal of lesions and ovaries, or aspiration of lesions (CPT codes 49321–49322, 58660–58662). A modifier -59 (Distinct Procedural Service) can be reported to bypass these edits, but the payer will request documentation to ensure that the criteria for using this modifier apply. The CPT criteria include documentation of a different session, different procedure or surgery, different site or organ system, separate incision/excision, separate lesion, or separate injury (or area of injury in extensive injuries) which is not ordinarily encountered or performed on the same day by the same individual. Failure to discuss the reason for the removal in the body of the operative report will generally mean the payer will deny extra payment for the appendectomy.
—MELANIE WITT, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
Adequate training is essential
Surgical proficiency in laparoscopic appendectomy, like any surgical skill set, requires adequate training to ensure procedural familiarity and expertise and to encourage consistency. This training, preferably undertaken during residency, should be an essential part of obstetrics and gynecology education.
It is important to know which patient populations are at high risk for appendiceal pathology so that they can be assessed and counseled adequately prior to surgery. Among the components of patient counseling is a thorough and impartial discussion of procedural risks and benefits. Risk-benefit considerations should include the patient’s preferences so that she can be an active participant in her own health-care decisions.
Because the risks of appendectomy are minimal, and complications are rare, it is appropriate to offer elective laparoscopic appendectomy to patients scheduled to undergo benign gynecologic procedures, especially in the setting of chronic pelvic pain and endometriosis.
CASE: Resolved
The patient is counseled about the benefits and risks of laparoscopic incidental appendectomy, including the fact that it may be especially beneficial in women who have endometriosis. She consents to undergo the procedure at the time of her total laparoscopic hysterectomy.
Both procedures are performed safely, with no complications, and the patient’s immediate postoperative course is unremarkable. After one night of hospitalization, she is discharged home. The histopathologic report on the appendiceal specimen reveals endometriosis with fibrous obliteration of the lumen.
We want to hear from you! Tell us what you think.
CLICK HERE to access 8 Surgical Technique articles published in OBG Management
in 2012.
1. Elective coincidental appendectomy. ACOG Committee Opinion#323. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2005;106(5 Pt 1):1141-1142.
2. Davis ME. Gynecologic operations at cesarean section. Clin Obstet Gynecol. 1959;2:1095-1106.
3. Lynch CB, Sinha P, Jalloh S. Incidental appendectomy during gynecological surgery. Int J Gynecol Obstet. 1997;59(3):261-262.
4. Snyder TE, Selanders JR. Incidental appendectomy—yes or no? A retrospective case study and review of the literature. Infec Dis Obstet Gynecol. 1998;6(1):30-37.
5. Salom EM, Schey D, Penalver M, et al. The safety of incidental appendectomy at the time of abdominal hysterectomy. Am J Obstet Gynecol. 2003;189(6):1563-1568.
6. Lee JH, Choi JS, Jeon SW. Laparoscopic incidental appendectomy during laparoscopic surgery for ovarian endometrioma. Am J Obstet Gynecol. 2011;204(1):28.e1-5.
7. Semm K. Endoscopic appendectomy. Endoscopy. 1983;15(2):59-64.
8. O’Hanlan KA, Fisher DT, O’Holleran MS. 257 incidental appendectomies during total laparoscopic hysterectomy. JSLS. 2007;11(4):428-431.
9. Nezhat C, Nezhat F. Incidental appendectomy during videolaseroscopy. Am J Obstet Gynecol. 1991;165(3):559-564.
10. Song JY, Yordan E, Rotman C. Incidental appendectomy during endoscopic surgery. JSLS. 2009;13(3):376-383.
11. Masoomi H, Mills S, Dolich MO, et al. Comparison of outcomes of laparoscopic versus open appendectomy in adults: data from the Nationwide Inpatient Sample (NIS), 2006-2008. J Gastrointest Surg. 2011;15(12):2226-2231.
12. Jarnagin BK. The vermiform appendix in relation to gynecology. In: Rock JA Jones HW, eds. TeLinde’s Operative Gynecology. 10th ed. Chapter 42. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
13. Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69(5):727-730.
14. Gustofson RL, Kim N, Liu S, Stratton P. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86(2):298-303.
15. Berker B, Lashay N, Davarpanah R, Marziali M, Nezhat CH, Nezhat C. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12(3):206-209.
16. Harris RS, Foster WG, Surrey MW, Agarwal SK. Appendiceal disease in women with endometriosis and right lower quadrant pain. J Am Assoc Gynecol Laparosc. 2001;8(4):536-541.
17. Wie HJ, Lee JH, Kyung MS, Jung US, Choi JS. Is incidental appendectomy necessary in women with ovarian endometrioma? Aust NZ J Obstet Gyn. 2008;48(1):107-111.
18. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132(5):910-925.
19. Baggish MS. Appendectomy. In: Baggish MS Karram MM, eds. Atlas of Pelvic Anatomy and Gynecologic Surgery. 2nd ed. Chapter 100. Philadelphia, PA: Saunders; 2006.
20. Jaffe BM, Berger DH. The appendix. In: Brunicardi F Andersen D, Billiar T, et al, eds. Schwartz’s Principles of Surgery. 9th ed. Chapter 30. New York, NY: McGraw Hill Professional; 2010.
21. Daniell JF, Gurley LD, Kurtz BR, Chamber JF. The use of an automatic stapling device for laparoscopic appendectomy. Obstet Gynecol 1991;78(4):721-723.
Videos provided by Teresa Tam, MD, and Gerald Harkins, MD
CASE: Should appendectomy be included in total
laparoscopic hysterectomy?
A 39-year-old mother of two continues to experience severe dysmenorrhea and persistent menorrhagia despite undergoing endometrial ablation 2 years earlier. Her obstetric and gynecologic history is remarkable for a diagnosis of chronic pelvic pain, endometriosis, and failed endometrial ablation. Both her children were delivered by cesarean, and she has undergone tubal ligation. She requests hysterectomy to address the dysmenorrhea and menorrhagia once and for all.
A pelvic exam reveals an anteverted, 10-weeks’ size uterus with no adnexal masses or tenderness. After extensive discussion of the surgical procedure, the patient signs a consent for total laparoscopic hysterectomy.
Would you recommend appendectomy, too?
Prophylactic removal of the appendix during a benign gynecologic procedure is known as “elective incidental appendectomy.”1 Incidental appendectomy at the time of cesarean delivery was reported initially in 1959.2 Subsequent studies of removal of a normal-appearing appendix at the time of gynecologic surgery have met with considerable debate. Proponents argue that removal of the appendix at the time of abdominal hysterectomy does not increase operative time or postoperative morbidity. More important, it does prevent future appendicitis.3-5
Some surgeons disagree, citing an increase in operative time, hospital costs, and patient morbidity as reasonable concerns. They also note that appendectomy requires an additional surgical procedure, which could increase the risk of infection and other complications and lead to adhesion formation.
Advantages of incidental appendectomy include technical ease, low patient morbidity and mortality, and significant diagnostic and protective value.6 It also prevents conflicting diagnoses, especially in patients who have chronic pelvic pain, a ruptured ovarian cyst, or endometriosis. Other patients likely to benefit from elective incidental appendectomy are those who are undergoing abdominal radiation or chemotherapy, women unable to communicate health complaints, and those who are planning to undergo complex abdominal or pelvic procedures that are likely to cause extensive adhesions.1
In this article, we describe the rationale behind this procedure, as well as the technical steps involved.
The laparoscopic approach is preferred
Appendectomy is commonly performed laparoscopically. Semm first described this approach in 1983.7 Several studies since have reported that incidental laparoscopic appendectomy is safe, easy to perform, and should be offered to patients undergoing a concomitant gynecologic procedure.8-10 Laparoscopic removal of a normal appendix does not add morbidity or prolong hospitalization, compared with diagnostic laparoscopy. A large study drawing from the Nationwide Inpatient Sample (NIS) database found laparoscopic removal of the appendix to be associated with lower mortality, fewer complications, shorter hospitalization, and lower mean hospital charges, compared with open appendectomy.11 The same study found laparoscopic appendectomy to be the procedure of choice in both perforated and nonperforated appendicitis.
Overweight and obese patients also may benefit from the laparoscopic approach because it avoids problems associated with an open incision, such as the need for abdominal wall retraction, a longer hospital stay, and a risk of wound infection, compared with smaller incisions—especially in this high-risk population.12
Cost is another issue. Any prolonged surgical time and higher medical costs required for incidental appendectomy decrease as surgical proficiency and experience rise. The concomitant performance of endoscopic procedures can also reduce the risk associated with anesthesia for reoperations.
Endometriosis patients stand to benefit from appendectomy
There is compelling evidence that elective appendectomy is beneficial in patients who have endometriosis. Endometriosis of the bowel has been reported in 5.3% of all histologically proven endometriosis cases, with appendiceal endometriosis found in approximately 1% of women with endometriosis.13 Despite the low prevalence (2.8%) of appendiceal endometriosis,14 some studies reported a high incidence of appendiceal endometriosis when incidental appendectomy was performed. Patients who report right lower quadrant (RLQ) pain, chronic pelvic pain, and ovarian endometrioma had the highest incidence of abnormal histopathologic findings.15-17 Because most women with endometriosis present with these symptoms, it is prudent to counsel patients preoperatively about the incidence of appendiceal endometriosis and to visually examine the appendix during gynecologic surgery to identify incidental appendiceal pathology.
Age may influence the appendectomy decision
The incidence of acute appendicitis is highest among people aged 10 to 19 years. The estimated lifetime risk of appendicitis is 6.7%.18 The surgical dilemma is whether to perform incidental appendectomy in the nonadolescent population, which is at lower risk for appendicitis, as a preventive measure.
We lack randomized trials on the benefit of incidental appendectomy. A retrospective study of open procedures supported incidental appendectomy in patients younger than 35 years; for patients 35 to 50, the decision was left to the clinical judgment of the surgeon, based on the patient’s clinical condition.4 The same study failed to support incidental appendectomy in women older than 50 years.
When the appendix is not easily accessible, or the surgical complexity of the gynecologic procedure prevents the surgeon from safely performing an appendectomy, it is better to complete the planned gynecologic surgery and forgo the appendectomy. It is acceptable to make the decision to refrain from the appendectomy intraoperatively if the risk of complications outweighs the likely benefits. The practice of cautionary discretion demonstrates sound judgment and avoids compromising the safety of the patient.
1. Maintain at least three laparoscopic sites
- After the laparoscopic gynecologic procedure, maintain three trocar sites—preferably, two 5-mm trocars and one 12-mm trocar.
- The first 5-mm trocar, at the umbilical incision, accommodates the laparoscopic camera. The second 5-mm trocar serves as an accessory port for laparoscopic instruments and is inserted into the RLQ.
- The 12-mm trocar in the left lower quadrant (LLQ) is also used to insert endoscopic instruments. This trocar site will be used at the conclusion of the appendectomy to accommodate the mechanical stapling device and the specimen bag for removal of the excised organ.

FIGURE 1: Visualize the appendix. Identify the cecum and ileocolic junction to locate the appendix.
2. Identify the appendix
- Perform a careful visual exploration of the abdominal contents to exclude other intra-abdominal pathology.
- Identify the cecum and ileocolic junction to locate the appendix (FIGURE 1).
- Visually inspect the appendix and identify any gross appendiceal pathology.

FIGURE 2: Divide the mesoappendix
Isolate, cauterize, and divide the mesoappendix using 5-mm ultrasonic shears.
3. Dissect the appendix (VIDEO 1)
- Insert an atraumatic forceps through the 5-mm right accessory trocar.
- Grasp the fatty tissue at the tip of the appendix and provide some traction.
- Elevate the appendix to facilitate visualization of the mesoappendix.
- Isolate the mesoappendix and cauterize and divide it using 5-mm ultrasonic shears inserted through the RLQ trocar (FIGURE 2).
- Release some of the upward tension from the specimen retraction by dropping the height of the instrument to prevent undue trauma and bleeding.
- Make a window between the mesentery and the base of the appendix to facilitate dissection.
- Skeletonize the mesoappendix at the junction of the appendiceal base and the cecum.
- During skeletonization, pay special attention to the appendiceal artery at the base of the mesoappendix.

FIGURE 3: Apply the stapling device
Apply the stapling device across the base of the appendix.
4. Resect the appendix (VIDEO 2)
- Insert an automatic stapling device through the 12-mm port and apply it across the base of the appendix (FIGURE 3).
- Apply the mechanical stapling device for 15 seconds to crush the base of the appendix and empty its contents.
- Visualize both sides of the stapler to ensure that it is placed at the base of the appendix.
- Always check the tip of the device to ensure that the jaws of the stapling device fully compress the appendix and have not inadvertently grasped other abdominal contents.
- With the stapling device compressing the base of the appendix, release some of the upward tension on the specimen by dropping the height of the retraction.
- Activate the stapling device and completely excise the appendix from its base
(FIGURE 4). - Thoroughly inspect the appendiceal stump to ensure hemostasis (FIGURE 5).

FIGURE 4: Excise the appendix
Activate the stapling device and completely excise the appendix from its base.
FIGURE 5: Ensure hemostasis
Thoroughly inspect the appendiceal stump and ensure hemostasis.
5. Remove the specimen (VIDEO 3)
- Remove the stapling device and replace it with a specimen retrieval bag, inserting it through the 12-mm port.
- Place the amputated appendix in the specimen retrieval bag to prevent abdominal contamination (FIGURE 6).
- Close the specimen retrieval bag inside the abdomen.
- Refrain from removing the specimen bag through the trocar or forcefully passing the appendix through a small incision. We usually withdraw the 12-mm trocar, then remove the cinched bag containing the resected appendix under direct visualization. We take all precautionary measures to prevent breakage of the bag, which would leak appendiceal contents into the abdomen.

FIGURE 6: Remove the specimen
Place the amputated appendix in the specimen retrieval bag and remove it, intact, through the patient’s abdomen.
6. Perform a few last measures
- If the surgeon chooses, suction and irrigation can be performed at the completion of the appendectomy procedure.
- Send all surgical specimens to pathology for evaluation.
- Complete the operation in the usual laparoscopic fashion. Remove all instruments, and close the 12-mm trocar port site using the Carter Thomason fascial closure device. Close the remaining port sites using 2-0 interrupted suture (Monocryl). Apply skin adhesive to all laparoscopic incisions.
- For more on surgical technique of appendectomy, see Baggish,19 Jaffe and Berger,20 and Daniell and colleagues.21
Coding for appendectomy is fairly straightforward if you know the rules, but prophylactic removal of the appendix, whether performed at the time of a laparoscopic or open abdominal primary procedure, will usually lead to reimbursement difficulties for surgeons even though CPT codes exist to report the procedure. Knowing when and how to bill and document the circumstances for removal will go a long way in getting payment for the procedure. Note that these rules apply to a single surgeon who is performing the entire surgery. When an ObGyn is performing gyn procedures, but a general surgeon is the one who removes the appendix, that surgeon will not be subject to bundling rules, but will still have to make a case with the payer for removing an otherwise normal appendix.
There are 5 codes that can be used to report an appendectomy:
- 44950 Appendectomy;
- 44955 Appendectomy; when done for indicated purpose at time of other major procedure (not as separate procedure)
- 44960 Appendectomy; for ruptured appendix with abscess or generalized peritonitis
- 44970 Laparoscopy, surgical, appendectomy code
- 44979, Unlisted laparoscopy procedure, appendix.
Code 44950 represents either a stand-alone procedure or an incidental appendectomy when performed with other open abdominal procedures. Under CPT guidelines this code would only be reported 1) when this is the only procedure performed and the appendix is removed for a diagnosis other than rupture with abscess, or 2) with a modifier -52 added if the surgeon believes that an incidental appendectomy needs to be reported. Use of a modifier -52 will lead to review of the documentation by the payer, and it will be up to the surgeon to convince the payer that he should be paid for taking out an appendix that is found to be normal. Billing 44950 with other abdominal procedures without this modifier will lead to an outright denial due to bundling edits, which permanently bundle 44950 with all major abdominal procedures.
Code 44955 is the code to report when an appendectomy is performed for an indicated purpose at the time of other open abdominal procedures. For instance, the appendix may have been removed due to a finding of distention with fecalith or extensive adhesions binding the appendix to the abdominal wall. When this code is reported, no modifier is used because it is a CPT “add-on” code that can only be billed in conjunction with other procedures.
Code 44960 is only reported when no other open abdominal procedures are performed at the operative session and the reason for taking out the appendix is rupture with abscess. If rupture is found at the time of an abdominal procedure to remove a mass, for instance, code 44955 would be reported instead.
Code 44970 is the only laparoscopic approach code for an appendectomy, but it would only be reported when 1) the appendectomy was the only laparoscopic procedure performed, or 2) the appendectomy was incidental, but the surgeon felt it needed to be reported. There is no instruction about using a modifier -52 with 44970 to report an incidental appendectomy. According to the American Medical Association’s January 2012 issue of CPT Assistant, laparoscopic removal of the appendix for an indicated purpose at the time of another major laparoscopic procedure should be reported as 44979, Unlisted laparoscopy procedure, appendix.
Keep in mind that code 44970 is bundled into a long list of laparoscopic procedures, including codes for treating stress urinary incontinence and prolapse (CPT codes 51990–51992, 57425), sterilization procedures (CPT codes 58670–58671), hysterectomy procedures (CPT codes 58541–58544, 58548, 58550–58554, 58570–58573), myomectomy procedures (CPT code 58545–58546), as well as codes for lysis, removal of lesions and ovaries, or aspiration of lesions (CPT codes 49321–49322, 58660–58662). A modifier -59 (Distinct Procedural Service) can be reported to bypass these edits, but the payer will request documentation to ensure that the criteria for using this modifier apply. The CPT criteria include documentation of a different session, different procedure or surgery, different site or organ system, separate incision/excision, separate lesion, or separate injury (or area of injury in extensive injuries) which is not ordinarily encountered or performed on the same day by the same individual. Failure to discuss the reason for the removal in the body of the operative report will generally mean the payer will deny extra payment for the appendectomy.
—MELANIE WITT, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
Adequate training is essential
Surgical proficiency in laparoscopic appendectomy, like any surgical skill set, requires adequate training to ensure procedural familiarity and expertise and to encourage consistency. This training, preferably undertaken during residency, should be an essential part of obstetrics and gynecology education.
It is important to know which patient populations are at high risk for appendiceal pathology so that they can be assessed and counseled adequately prior to surgery. Among the components of patient counseling is a thorough and impartial discussion of procedural risks and benefits. Risk-benefit considerations should include the patient’s preferences so that she can be an active participant in her own health-care decisions.
Because the risks of appendectomy are minimal, and complications are rare, it is appropriate to offer elective laparoscopic appendectomy to patients scheduled to undergo benign gynecologic procedures, especially in the setting of chronic pelvic pain and endometriosis.
CASE: Resolved
The patient is counseled about the benefits and risks of laparoscopic incidental appendectomy, including the fact that it may be especially beneficial in women who have endometriosis. She consents to undergo the procedure at the time of her total laparoscopic hysterectomy.
Both procedures are performed safely, with no complications, and the patient’s immediate postoperative course is unremarkable. After one night of hospitalization, she is discharged home. The histopathologic report on the appendiceal specimen reveals endometriosis with fibrous obliteration of the lumen.
We want to hear from you! Tell us what you think.
CLICK HERE to access 8 Surgical Technique articles published in OBG Management
in 2012.
Videos provided by Teresa Tam, MD, and Gerald Harkins, MD
CASE: Should appendectomy be included in total
laparoscopic hysterectomy?
A 39-year-old mother of two continues to experience severe dysmenorrhea and persistent menorrhagia despite undergoing endometrial ablation 2 years earlier. Her obstetric and gynecologic history is remarkable for a diagnosis of chronic pelvic pain, endometriosis, and failed endometrial ablation. Both her children were delivered by cesarean, and she has undergone tubal ligation. She requests hysterectomy to address the dysmenorrhea and menorrhagia once and for all.
A pelvic exam reveals an anteverted, 10-weeks’ size uterus with no adnexal masses or tenderness. After extensive discussion of the surgical procedure, the patient signs a consent for total laparoscopic hysterectomy.
Would you recommend appendectomy, too?
Prophylactic removal of the appendix during a benign gynecologic procedure is known as “elective incidental appendectomy.”1 Incidental appendectomy at the time of cesarean delivery was reported initially in 1959.2 Subsequent studies of removal of a normal-appearing appendix at the time of gynecologic surgery have met with considerable debate. Proponents argue that removal of the appendix at the time of abdominal hysterectomy does not increase operative time or postoperative morbidity. More important, it does prevent future appendicitis.3-5
Some surgeons disagree, citing an increase in operative time, hospital costs, and patient morbidity as reasonable concerns. They also note that appendectomy requires an additional surgical procedure, which could increase the risk of infection and other complications and lead to adhesion formation.
Advantages of incidental appendectomy include technical ease, low patient morbidity and mortality, and significant diagnostic and protective value.6 It also prevents conflicting diagnoses, especially in patients who have chronic pelvic pain, a ruptured ovarian cyst, or endometriosis. Other patients likely to benefit from elective incidental appendectomy are those who are undergoing abdominal radiation or chemotherapy, women unable to communicate health complaints, and those who are planning to undergo complex abdominal or pelvic procedures that are likely to cause extensive adhesions.1
In this article, we describe the rationale behind this procedure, as well as the technical steps involved.
The laparoscopic approach is preferred
Appendectomy is commonly performed laparoscopically. Semm first described this approach in 1983.7 Several studies since have reported that incidental laparoscopic appendectomy is safe, easy to perform, and should be offered to patients undergoing a concomitant gynecologic procedure.8-10 Laparoscopic removal of a normal appendix does not add morbidity or prolong hospitalization, compared with diagnostic laparoscopy. A large study drawing from the Nationwide Inpatient Sample (NIS) database found laparoscopic removal of the appendix to be associated with lower mortality, fewer complications, shorter hospitalization, and lower mean hospital charges, compared with open appendectomy.11 The same study found laparoscopic appendectomy to be the procedure of choice in both perforated and nonperforated appendicitis.
Overweight and obese patients also may benefit from the laparoscopic approach because it avoids problems associated with an open incision, such as the need for abdominal wall retraction, a longer hospital stay, and a risk of wound infection, compared with smaller incisions—especially in this high-risk population.12
Cost is another issue. Any prolonged surgical time and higher medical costs required for incidental appendectomy decrease as surgical proficiency and experience rise. The concomitant performance of endoscopic procedures can also reduce the risk associated with anesthesia for reoperations.
Endometriosis patients stand to benefit from appendectomy
There is compelling evidence that elective appendectomy is beneficial in patients who have endometriosis. Endometriosis of the bowel has been reported in 5.3% of all histologically proven endometriosis cases, with appendiceal endometriosis found in approximately 1% of women with endometriosis.13 Despite the low prevalence (2.8%) of appendiceal endometriosis,14 some studies reported a high incidence of appendiceal endometriosis when incidental appendectomy was performed. Patients who report right lower quadrant (RLQ) pain, chronic pelvic pain, and ovarian endometrioma had the highest incidence of abnormal histopathologic findings.15-17 Because most women with endometriosis present with these symptoms, it is prudent to counsel patients preoperatively about the incidence of appendiceal endometriosis and to visually examine the appendix during gynecologic surgery to identify incidental appendiceal pathology.
Age may influence the appendectomy decision
The incidence of acute appendicitis is highest among people aged 10 to 19 years. The estimated lifetime risk of appendicitis is 6.7%.18 The surgical dilemma is whether to perform incidental appendectomy in the nonadolescent population, which is at lower risk for appendicitis, as a preventive measure.
We lack randomized trials on the benefit of incidental appendectomy. A retrospective study of open procedures supported incidental appendectomy in patients younger than 35 years; for patients 35 to 50, the decision was left to the clinical judgment of the surgeon, based on the patient’s clinical condition.4 The same study failed to support incidental appendectomy in women older than 50 years.
When the appendix is not easily accessible, or the surgical complexity of the gynecologic procedure prevents the surgeon from safely performing an appendectomy, it is better to complete the planned gynecologic surgery and forgo the appendectomy. It is acceptable to make the decision to refrain from the appendectomy intraoperatively if the risk of complications outweighs the likely benefits. The practice of cautionary discretion demonstrates sound judgment and avoids compromising the safety of the patient.
1. Maintain at least three laparoscopic sites
- After the laparoscopic gynecologic procedure, maintain three trocar sites—preferably, two 5-mm trocars and one 12-mm trocar.
- The first 5-mm trocar, at the umbilical incision, accommodates the laparoscopic camera. The second 5-mm trocar serves as an accessory port for laparoscopic instruments and is inserted into the RLQ.
- The 12-mm trocar in the left lower quadrant (LLQ) is also used to insert endoscopic instruments. This trocar site will be used at the conclusion of the appendectomy to accommodate the mechanical stapling device and the specimen bag for removal of the excised organ.

FIGURE 1: Visualize the appendix. Identify the cecum and ileocolic junction to locate the appendix.
2. Identify the appendix
- Perform a careful visual exploration of the abdominal contents to exclude other intra-abdominal pathology.
- Identify the cecum and ileocolic junction to locate the appendix (FIGURE 1).
- Visually inspect the appendix and identify any gross appendiceal pathology.

FIGURE 2: Divide the mesoappendix
Isolate, cauterize, and divide the mesoappendix using 5-mm ultrasonic shears.
3. Dissect the appendix (VIDEO 1)
- Insert an atraumatic forceps through the 5-mm right accessory trocar.
- Grasp the fatty tissue at the tip of the appendix and provide some traction.
- Elevate the appendix to facilitate visualization of the mesoappendix.
- Isolate the mesoappendix and cauterize and divide it using 5-mm ultrasonic shears inserted through the RLQ trocar (FIGURE 2).
- Release some of the upward tension from the specimen retraction by dropping the height of the instrument to prevent undue trauma and bleeding.
- Make a window between the mesentery and the base of the appendix to facilitate dissection.
- Skeletonize the mesoappendix at the junction of the appendiceal base and the cecum.
- During skeletonization, pay special attention to the appendiceal artery at the base of the mesoappendix.

FIGURE 3: Apply the stapling device
Apply the stapling device across the base of the appendix.
4. Resect the appendix (VIDEO 2)
- Insert an automatic stapling device through the 12-mm port and apply it across the base of the appendix (FIGURE 3).
- Apply the mechanical stapling device for 15 seconds to crush the base of the appendix and empty its contents.
- Visualize both sides of the stapler to ensure that it is placed at the base of the appendix.
- Always check the tip of the device to ensure that the jaws of the stapling device fully compress the appendix and have not inadvertently grasped other abdominal contents.
- With the stapling device compressing the base of the appendix, release some of the upward tension on the specimen by dropping the height of the retraction.
- Activate the stapling device and completely excise the appendix from its base
(FIGURE 4). - Thoroughly inspect the appendiceal stump to ensure hemostasis (FIGURE 5).

FIGURE 4: Excise the appendix
Activate the stapling device and completely excise the appendix from its base.
FIGURE 5: Ensure hemostasis
Thoroughly inspect the appendiceal stump and ensure hemostasis.
5. Remove the specimen (VIDEO 3)
- Remove the stapling device and replace it with a specimen retrieval bag, inserting it through the 12-mm port.
- Place the amputated appendix in the specimen retrieval bag to prevent abdominal contamination (FIGURE 6).
- Close the specimen retrieval bag inside the abdomen.
- Refrain from removing the specimen bag through the trocar or forcefully passing the appendix through a small incision. We usually withdraw the 12-mm trocar, then remove the cinched bag containing the resected appendix under direct visualization. We take all precautionary measures to prevent breakage of the bag, which would leak appendiceal contents into the abdomen.

FIGURE 6: Remove the specimen
Place the amputated appendix in the specimen retrieval bag and remove it, intact, through the patient’s abdomen.
6. Perform a few last measures
- If the surgeon chooses, suction and irrigation can be performed at the completion of the appendectomy procedure.
- Send all surgical specimens to pathology for evaluation.
- Complete the operation in the usual laparoscopic fashion. Remove all instruments, and close the 12-mm trocar port site using the Carter Thomason fascial closure device. Close the remaining port sites using 2-0 interrupted suture (Monocryl). Apply skin adhesive to all laparoscopic incisions.
- For more on surgical technique of appendectomy, see Baggish,19 Jaffe and Berger,20 and Daniell and colleagues.21
Coding for appendectomy is fairly straightforward if you know the rules, but prophylactic removal of the appendix, whether performed at the time of a laparoscopic or open abdominal primary procedure, will usually lead to reimbursement difficulties for surgeons even though CPT codes exist to report the procedure. Knowing when and how to bill and document the circumstances for removal will go a long way in getting payment for the procedure. Note that these rules apply to a single surgeon who is performing the entire surgery. When an ObGyn is performing gyn procedures, but a general surgeon is the one who removes the appendix, that surgeon will not be subject to bundling rules, but will still have to make a case with the payer for removing an otherwise normal appendix.
There are 5 codes that can be used to report an appendectomy:
- 44950 Appendectomy;
- 44955 Appendectomy; when done for indicated purpose at time of other major procedure (not as separate procedure)
- 44960 Appendectomy; for ruptured appendix with abscess or generalized peritonitis
- 44970 Laparoscopy, surgical, appendectomy code
- 44979, Unlisted laparoscopy procedure, appendix.
Code 44950 represents either a stand-alone procedure or an incidental appendectomy when performed with other open abdominal procedures. Under CPT guidelines this code would only be reported 1) when this is the only procedure performed and the appendix is removed for a diagnosis other than rupture with abscess, or 2) with a modifier -52 added if the surgeon believes that an incidental appendectomy needs to be reported. Use of a modifier -52 will lead to review of the documentation by the payer, and it will be up to the surgeon to convince the payer that he should be paid for taking out an appendix that is found to be normal. Billing 44950 with other abdominal procedures without this modifier will lead to an outright denial due to bundling edits, which permanently bundle 44950 with all major abdominal procedures.
Code 44955 is the code to report when an appendectomy is performed for an indicated purpose at the time of other open abdominal procedures. For instance, the appendix may have been removed due to a finding of distention with fecalith or extensive adhesions binding the appendix to the abdominal wall. When this code is reported, no modifier is used because it is a CPT “add-on” code that can only be billed in conjunction with other procedures.
Code 44960 is only reported when no other open abdominal procedures are performed at the operative session and the reason for taking out the appendix is rupture with abscess. If rupture is found at the time of an abdominal procedure to remove a mass, for instance, code 44955 would be reported instead.
Code 44970 is the only laparoscopic approach code for an appendectomy, but it would only be reported when 1) the appendectomy was the only laparoscopic procedure performed, or 2) the appendectomy was incidental, but the surgeon felt it needed to be reported. There is no instruction about using a modifier -52 with 44970 to report an incidental appendectomy. According to the American Medical Association’s January 2012 issue of CPT Assistant, laparoscopic removal of the appendix for an indicated purpose at the time of another major laparoscopic procedure should be reported as 44979, Unlisted laparoscopy procedure, appendix.
Keep in mind that code 44970 is bundled into a long list of laparoscopic procedures, including codes for treating stress urinary incontinence and prolapse (CPT codes 51990–51992, 57425), sterilization procedures (CPT codes 58670–58671), hysterectomy procedures (CPT codes 58541–58544, 58548, 58550–58554, 58570–58573), myomectomy procedures (CPT code 58545–58546), as well as codes for lysis, removal of lesions and ovaries, or aspiration of lesions (CPT codes 49321–49322, 58660–58662). A modifier -59 (Distinct Procedural Service) can be reported to bypass these edits, but the payer will request documentation to ensure that the criteria for using this modifier apply. The CPT criteria include documentation of a different session, different procedure or surgery, different site or organ system, separate incision/excision, separate lesion, or separate injury (or area of injury in extensive injuries) which is not ordinarily encountered or performed on the same day by the same individual. Failure to discuss the reason for the removal in the body of the operative report will generally mean the payer will deny extra payment for the appendectomy.
—MELANIE WITT, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
Adequate training is essential
Surgical proficiency in laparoscopic appendectomy, like any surgical skill set, requires adequate training to ensure procedural familiarity and expertise and to encourage consistency. This training, preferably undertaken during residency, should be an essential part of obstetrics and gynecology education.
It is important to know which patient populations are at high risk for appendiceal pathology so that they can be assessed and counseled adequately prior to surgery. Among the components of patient counseling is a thorough and impartial discussion of procedural risks and benefits. Risk-benefit considerations should include the patient’s preferences so that she can be an active participant in her own health-care decisions.
Because the risks of appendectomy are minimal, and complications are rare, it is appropriate to offer elective laparoscopic appendectomy to patients scheduled to undergo benign gynecologic procedures, especially in the setting of chronic pelvic pain and endometriosis.
CASE: Resolved
The patient is counseled about the benefits and risks of laparoscopic incidental appendectomy, including the fact that it may be especially beneficial in women who have endometriosis. She consents to undergo the procedure at the time of her total laparoscopic hysterectomy.
Both procedures are performed safely, with no complications, and the patient’s immediate postoperative course is unremarkable. After one night of hospitalization, she is discharged home. The histopathologic report on the appendiceal specimen reveals endometriosis with fibrous obliteration of the lumen.
We want to hear from you! Tell us what you think.
CLICK HERE to access 8 Surgical Technique articles published in OBG Management
in 2012.
1. Elective coincidental appendectomy. ACOG Committee Opinion#323. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2005;106(5 Pt 1):1141-1142.
2. Davis ME. Gynecologic operations at cesarean section. Clin Obstet Gynecol. 1959;2:1095-1106.
3. Lynch CB, Sinha P, Jalloh S. Incidental appendectomy during gynecological surgery. Int J Gynecol Obstet. 1997;59(3):261-262.
4. Snyder TE, Selanders JR. Incidental appendectomy—yes or no? A retrospective case study and review of the literature. Infec Dis Obstet Gynecol. 1998;6(1):30-37.
5. Salom EM, Schey D, Penalver M, et al. The safety of incidental appendectomy at the time of abdominal hysterectomy. Am J Obstet Gynecol. 2003;189(6):1563-1568.
6. Lee JH, Choi JS, Jeon SW. Laparoscopic incidental appendectomy during laparoscopic surgery for ovarian endometrioma. Am J Obstet Gynecol. 2011;204(1):28.e1-5.
7. Semm K. Endoscopic appendectomy. Endoscopy. 1983;15(2):59-64.
8. O’Hanlan KA, Fisher DT, O’Holleran MS. 257 incidental appendectomies during total laparoscopic hysterectomy. JSLS. 2007;11(4):428-431.
9. Nezhat C, Nezhat F. Incidental appendectomy during videolaseroscopy. Am J Obstet Gynecol. 1991;165(3):559-564.
10. Song JY, Yordan E, Rotman C. Incidental appendectomy during endoscopic surgery. JSLS. 2009;13(3):376-383.
11. Masoomi H, Mills S, Dolich MO, et al. Comparison of outcomes of laparoscopic versus open appendectomy in adults: data from the Nationwide Inpatient Sample (NIS), 2006-2008. J Gastrointest Surg. 2011;15(12):2226-2231.
12. Jarnagin BK. The vermiform appendix in relation to gynecology. In: Rock JA Jones HW, eds. TeLinde’s Operative Gynecology. 10th ed. Chapter 42. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
13. Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69(5):727-730.
14. Gustofson RL, Kim N, Liu S, Stratton P. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86(2):298-303.
15. Berker B, Lashay N, Davarpanah R, Marziali M, Nezhat CH, Nezhat C. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12(3):206-209.
16. Harris RS, Foster WG, Surrey MW, Agarwal SK. Appendiceal disease in women with endometriosis and right lower quadrant pain. J Am Assoc Gynecol Laparosc. 2001;8(4):536-541.
17. Wie HJ, Lee JH, Kyung MS, Jung US, Choi JS. Is incidental appendectomy necessary in women with ovarian endometrioma? Aust NZ J Obstet Gyn. 2008;48(1):107-111.
18. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132(5):910-925.
19. Baggish MS. Appendectomy. In: Baggish MS Karram MM, eds. Atlas of Pelvic Anatomy and Gynecologic Surgery. 2nd ed. Chapter 100. Philadelphia, PA: Saunders; 2006.
20. Jaffe BM, Berger DH. The appendix. In: Brunicardi F Andersen D, Billiar T, et al, eds. Schwartz’s Principles of Surgery. 9th ed. Chapter 30. New York, NY: McGraw Hill Professional; 2010.
21. Daniell JF, Gurley LD, Kurtz BR, Chamber JF. The use of an automatic stapling device for laparoscopic appendectomy. Obstet Gynecol 1991;78(4):721-723.
1. Elective coincidental appendectomy. ACOG Committee Opinion#323. American College of Obstetricians and Gynecologists. Obstet Gynecol. 2005;106(5 Pt 1):1141-1142.
2. Davis ME. Gynecologic operations at cesarean section. Clin Obstet Gynecol. 1959;2:1095-1106.
3. Lynch CB, Sinha P, Jalloh S. Incidental appendectomy during gynecological surgery. Int J Gynecol Obstet. 1997;59(3):261-262.
4. Snyder TE, Selanders JR. Incidental appendectomy—yes or no? A retrospective case study and review of the literature. Infec Dis Obstet Gynecol. 1998;6(1):30-37.
5. Salom EM, Schey D, Penalver M, et al. The safety of incidental appendectomy at the time of abdominal hysterectomy. Am J Obstet Gynecol. 2003;189(6):1563-1568.
6. Lee JH, Choi JS, Jeon SW. Laparoscopic incidental appendectomy during laparoscopic surgery for ovarian endometrioma. Am J Obstet Gynecol. 2011;204(1):28.e1-5.
7. Semm K. Endoscopic appendectomy. Endoscopy. 1983;15(2):59-64.
8. O’Hanlan KA, Fisher DT, O’Holleran MS. 257 incidental appendectomies during total laparoscopic hysterectomy. JSLS. 2007;11(4):428-431.
9. Nezhat C, Nezhat F. Incidental appendectomy during videolaseroscopy. Am J Obstet Gynecol. 1991;165(3):559-564.
10. Song JY, Yordan E, Rotman C. Incidental appendectomy during endoscopic surgery. JSLS. 2009;13(3):376-383.
11. Masoomi H, Mills S, Dolich MO, et al. Comparison of outcomes of laparoscopic versus open appendectomy in adults: data from the Nationwide Inpatient Sample (NIS), 2006-2008. J Gastrointest Surg. 2011;15(12):2226-2231.
12. Jarnagin BK. The vermiform appendix in relation to gynecology. In: Rock JA Jones HW, eds. TeLinde’s Operative Gynecology. 10th ed. Chapter 42. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
13. Weed JC, Ray JE. Endometriosis of the bowel. Obstet Gynecol. 1987;69(5):727-730.
14. Gustofson RL, Kim N, Liu S, Stratton P. Endometriosis and the appendix: a case series and comprehensive review of the literature. Fertil Steril. 2006;86(2):298-303.
15. Berker B, Lashay N, Davarpanah R, Marziali M, Nezhat CH, Nezhat C. Laparoscopic appendectomy in patients with endometriosis. J Minim Invasive Gynecol. 2005;12(3):206-209.
16. Harris RS, Foster WG, Surrey MW, Agarwal SK. Appendiceal disease in women with endometriosis and right lower quadrant pain. J Am Assoc Gynecol Laparosc. 2001;8(4):536-541.
17. Wie HJ, Lee JH, Kyung MS, Jung US, Choi JS. Is incidental appendectomy necessary in women with ovarian endometrioma? Aust NZ J Obstet Gyn. 2008;48(1):107-111.
18. Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132(5):910-925.
19. Baggish MS. Appendectomy. In: Baggish MS Karram MM, eds. Atlas of Pelvic Anatomy and Gynecologic Surgery. 2nd ed. Chapter 100. Philadelphia, PA: Saunders; 2006.
20. Jaffe BM, Berger DH. The appendix. In: Brunicardi F Andersen D, Billiar T, et al, eds. Schwartz’s Principles of Surgery. 9th ed. Chapter 30. New York, NY: McGraw Hill Professional; 2010.
21. Daniell JF, Gurley LD, Kurtz BR, Chamber JF. The use of an automatic stapling device for laparoscopic appendectomy. Obstet Gynecol 1991;78(4):721-723.
Top gynecologic surgeons gather for 2012 PAGS
Mark Walters, MD
![]()
Susan B. Levy, MD
![]()
![]()
Tomasso Falcone, MD
![]()
Amy Garcia, MD
More than 300 physicians attended the 15th annual Pelvic Anatomy and Gynecologic Surgery (PAGS) symposium December 13–15, 2012, in Las Vegas. One likely reason was an abundance of offerings, including:
- a laparoscopist’s view of pelvic and abdominal anatomy
- case-based discussion of the evaluation of female pelvic floor disorders
- a surgical video fest with expert discussion and audience participation
- an in-depth look at fibroid management
- a focus on hysterectomy, from the vaginal approach to single-port laparoscopy and robotics
- a panel discussion of pelvic pain and its management
- tips on avoiding and managing laparoscopic and other complications
- a breakout session on endometriosis surgery
- the latest on evaluation and management of fetal incontinence.
Here are a few additional highlights of the 2012 program:
Surgery for stress incontinence: Which sling is for which patient?
When it comes to slings, one size does not fit all. That point was emphasized by Mark Walters, MD, in a comprehensive session that described the surgical techniques behind various bladder-neck and midurethral sling procedures, as well as the associated cure rates, complications, and pros and cons. To watch a 7-minute video in which Dr. Walters elaborates on patient-selection criteria, CLICK HERE .
Surgical approach to prolapse—what I do and why I do it
“That’s something you have to come to grips with in your own practice—what’s best in your hands?” he said.
While showing videos of actual surgeries, he described specific techniques, pearls, and pitfalls, and emphasized the importance of cystoscopy to rule out bladder injury.
Keynote address: The economics of surgical gynecology
She also described the current payment environment, explained why the current trend in health-care spending is unsustainable, and stressed the need to find areas in surgical gynecologic practice that may benefit from improvements in health-care delivery. CLICK HERE for Dr. Levy’s overview of the issues on video.
After Dr. Levy’s keynote address on the economics of surgical gynecology, OBG Management gathered the opinions of four participants: Gary Bostrom, MD, of California; Richard Robinson, MD, of Georgia; Timothy Hall, MD, of North Carolina; and Todd Slater, MD, of Ohio. To hear their points of view, CLICK HERE .
Myomectomy: Open to robotic approaches
“Myomectomy is not a dying art by any stretch,” said PAGS Co-Chair Tommaso Falcone, MD, in opening this session. “In fact, it’s expected to increase,” he added, as more women seek to preserve their uterus.
He then proceeded to describe management approaches (including watchful waiting), indications for myomectomy, and surgical options, including data on both perioperative and reproductive outcomes.
CLICK HERE for a video summary of Dr. Falcone’s talk.
Laparoscopic supracervical hysterectomy
As more women seek to preserve their cervix at the time of hysterectomy, the supracervical approach is becoming increasingly common. Amy Garcia, MD, described the indications, technique, benefits, and risks associated with this procedure. CLICK HERE to hear Dr. Garcia highlight the key points of her talk.
Join me in Las Vegas for FUUS 2013!
“This is a unique meeting,” says Dr. Karram, “as it addresses both urologic and gynecologic issues related to female pelvic medicine and reconstructive surgery.” It’s also timely—with the first board exam for the subspecialty of female pelvic medicine and reconstructive surgery being held in June 2013. Prepare yourself to meet the demand for physicians who have the expertise to evaluate pelvic floor disorders.
“The meeting is attended by 50% gynecologists and 50% urologists, has many breakout sessions, and covers a variety of topics—everything from vaginal surgery for prolapse, voiding dysfunction, and types of reconstructive procedures with laparoscopic and robotic approaches,” says Dr. Karram, who is excited for this year’s special symposium by Karl J. Kreder, Jr, MD, on April 20 that addresses pelvic pain syndromes. For a complete agenda and registration details, visit www.fuus-cme.org.
Mark Walters, MD
![]()
Susan B. Levy, MD
![]()
![]()
Tomasso Falcone, MD
![]()
Amy Garcia, MD
More than 300 physicians attended the 15th annual Pelvic Anatomy and Gynecologic Surgery (PAGS) symposium December 13–15, 2012, in Las Vegas. One likely reason was an abundance of offerings, including:
- a laparoscopist’s view of pelvic and abdominal anatomy
- case-based discussion of the evaluation of female pelvic floor disorders
- a surgical video fest with expert discussion and audience participation
- an in-depth look at fibroid management
- a focus on hysterectomy, from the vaginal approach to single-port laparoscopy and robotics
- a panel discussion of pelvic pain and its management
- tips on avoiding and managing laparoscopic and other complications
- a breakout session on endometriosis surgery
- the latest on evaluation and management of fetal incontinence.
Here are a few additional highlights of the 2012 program:
Surgery for stress incontinence: Which sling is for which patient?
When it comes to slings, one size does not fit all. That point was emphasized by Mark Walters, MD, in a comprehensive session that described the surgical techniques behind various bladder-neck and midurethral sling procedures, as well as the associated cure rates, complications, and pros and cons. To watch a 7-minute video in which Dr. Walters elaborates on patient-selection criteria, CLICK HERE .
Surgical approach to prolapse—what I do and why I do it
“That’s something you have to come to grips with in your own practice—what’s best in your hands?” he said.
While showing videos of actual surgeries, he described specific techniques, pearls, and pitfalls, and emphasized the importance of cystoscopy to rule out bladder injury.
Keynote address: The economics of surgical gynecology
She also described the current payment environment, explained why the current trend in health-care spending is unsustainable, and stressed the need to find areas in surgical gynecologic practice that may benefit from improvements in health-care delivery. CLICK HERE for Dr. Levy’s overview of the issues on video.
After Dr. Levy’s keynote address on the economics of surgical gynecology, OBG Management gathered the opinions of four participants: Gary Bostrom, MD, of California; Richard Robinson, MD, of Georgia; Timothy Hall, MD, of North Carolina; and Todd Slater, MD, of Ohio. To hear their points of view, CLICK HERE .
Myomectomy: Open to robotic approaches
“Myomectomy is not a dying art by any stretch,” said PAGS Co-Chair Tommaso Falcone, MD, in opening this session. “In fact, it’s expected to increase,” he added, as more women seek to preserve their uterus.
He then proceeded to describe management approaches (including watchful waiting), indications for myomectomy, and surgical options, including data on both perioperative and reproductive outcomes.
CLICK HERE for a video summary of Dr. Falcone’s talk.
Laparoscopic supracervical hysterectomy
As more women seek to preserve their cervix at the time of hysterectomy, the supracervical approach is becoming increasingly common. Amy Garcia, MD, described the indications, technique, benefits, and risks associated with this procedure. CLICK HERE to hear Dr. Garcia highlight the key points of her talk.
Join me in Las Vegas for FUUS 2013!
“This is a unique meeting,” says Dr. Karram, “as it addresses both urologic and gynecologic issues related to female pelvic medicine and reconstructive surgery.” It’s also timely—with the first board exam for the subspecialty of female pelvic medicine and reconstructive surgery being held in June 2013. Prepare yourself to meet the demand for physicians who have the expertise to evaluate pelvic floor disorders.
“The meeting is attended by 50% gynecologists and 50% urologists, has many breakout sessions, and covers a variety of topics—everything from vaginal surgery for prolapse, voiding dysfunction, and types of reconstructive procedures with laparoscopic and robotic approaches,” says Dr. Karram, who is excited for this year’s special symposium by Karl J. Kreder, Jr, MD, on April 20 that addresses pelvic pain syndromes. For a complete agenda and registration details, visit www.fuus-cme.org.
Mark Walters, MD
![]()
Susan B. Levy, MD
![]()
![]()
Tomasso Falcone, MD
![]()
Amy Garcia, MD
More than 300 physicians attended the 15th annual Pelvic Anatomy and Gynecologic Surgery (PAGS) symposium December 13–15, 2012, in Las Vegas. One likely reason was an abundance of offerings, including:
- a laparoscopist’s view of pelvic and abdominal anatomy
- case-based discussion of the evaluation of female pelvic floor disorders
- a surgical video fest with expert discussion and audience participation
- an in-depth look at fibroid management
- a focus on hysterectomy, from the vaginal approach to single-port laparoscopy and robotics
- a panel discussion of pelvic pain and its management
- tips on avoiding and managing laparoscopic and other complications
- a breakout session on endometriosis surgery
- the latest on evaluation and management of fetal incontinence.
Here are a few additional highlights of the 2012 program:
Surgery for stress incontinence: Which sling is for which patient?
When it comes to slings, one size does not fit all. That point was emphasized by Mark Walters, MD, in a comprehensive session that described the surgical techniques behind various bladder-neck and midurethral sling procedures, as well as the associated cure rates, complications, and pros and cons. To watch a 7-minute video in which Dr. Walters elaborates on patient-selection criteria, CLICK HERE .
Surgical approach to prolapse—what I do and why I do it
“That’s something you have to come to grips with in your own practice—what’s best in your hands?” he said.
While showing videos of actual surgeries, he described specific techniques, pearls, and pitfalls, and emphasized the importance of cystoscopy to rule out bladder injury.
Keynote address: The economics of surgical gynecology
She also described the current payment environment, explained why the current trend in health-care spending is unsustainable, and stressed the need to find areas in surgical gynecologic practice that may benefit from improvements in health-care delivery. CLICK HERE for Dr. Levy’s overview of the issues on video.
After Dr. Levy’s keynote address on the economics of surgical gynecology, OBG Management gathered the opinions of four participants: Gary Bostrom, MD, of California; Richard Robinson, MD, of Georgia; Timothy Hall, MD, of North Carolina; and Todd Slater, MD, of Ohio. To hear their points of view, CLICK HERE .
Myomectomy: Open to robotic approaches
“Myomectomy is not a dying art by any stretch,” said PAGS Co-Chair Tommaso Falcone, MD, in opening this session. “In fact, it’s expected to increase,” he added, as more women seek to preserve their uterus.
He then proceeded to describe management approaches (including watchful waiting), indications for myomectomy, and surgical options, including data on both perioperative and reproductive outcomes.
CLICK HERE for a video summary of Dr. Falcone’s talk.
Laparoscopic supracervical hysterectomy
As more women seek to preserve their cervix at the time of hysterectomy, the supracervical approach is becoming increasingly common. Amy Garcia, MD, described the indications, technique, benefits, and risks associated with this procedure. CLICK HERE to hear Dr. Garcia highlight the key points of her talk.
Join me in Las Vegas for FUUS 2013!
“This is a unique meeting,” says Dr. Karram, “as it addresses both urologic and gynecologic issues related to female pelvic medicine and reconstructive surgery.” It’s also timely—with the first board exam for the subspecialty of female pelvic medicine and reconstructive surgery being held in June 2013. Prepare yourself to meet the demand for physicians who have the expertise to evaluate pelvic floor disorders.
“The meeting is attended by 50% gynecologists and 50% urologists, has many breakout sessions, and covers a variety of topics—everything from vaginal surgery for prolapse, voiding dysfunction, and types of reconstructive procedures with laparoscopic and robotic approaches,” says Dr. Karram, who is excited for this year’s special symposium by Karl J. Kreder, Jr, MD, on April 20 that addresses pelvic pain syndromes. For a complete agenda and registration details, visit www.fuus-cme.org.
When and how to place an autologous rectus fascia pubovaginal sling
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).
FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).
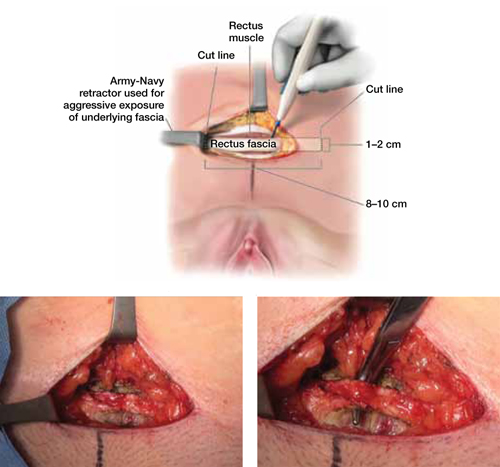
FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).
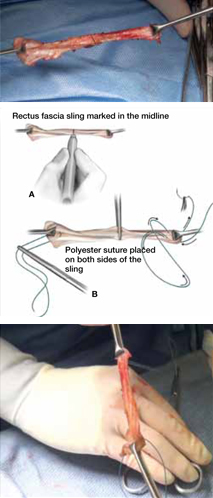
FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).
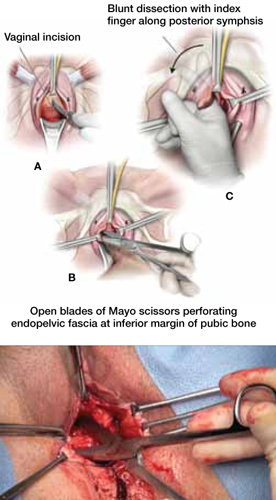
FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.
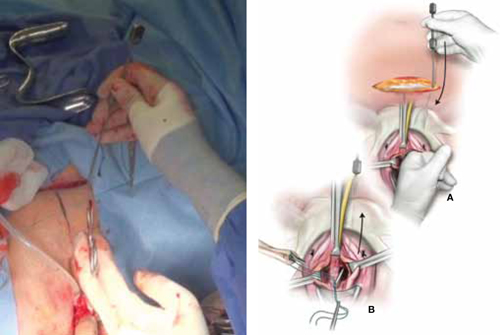
FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).
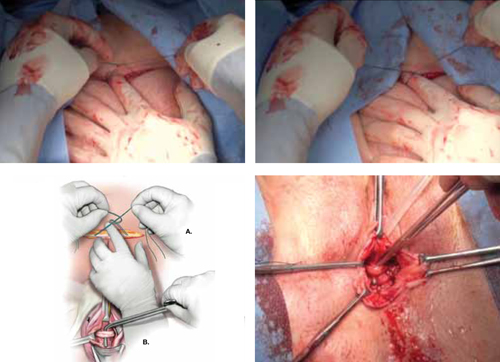
FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.

The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).

FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).

FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).

FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).

FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.

FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).

FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.
Watch 2 intraoperative videos
These videos were selected by Mickey Karram, MD, and presented courtesy of
International Academy of Pelvic Surgery
Developed in Partnership with International Academy of Pelvic Surgery
CASE 1: Recurrent SUI and mesh erosion
A 50-year-old woman reports urinary incontinence that is associated with activity and exertion—stress urinary incontinence (SUI)—and says it has worsened over the past year. She mentions that she underwent vaginal hysterectomy, with placement of a tension-free vaginal tape (TVT), about 2 years earlier.
During physical examination, the patient becomes incontinent when abdominal pressure is increased, with some urethral mobility (cotton-swab deflection to 25° from the horizontal). She is also noted to have erosion of the TVT tape into the vaginal lumen.
Urodynamic testing reveals easily demonstrable SUI at a volume of 150 mL when she is in the sitting position, with a Valsalva leak-point pressure of 55 cm H2O. Her bladder remains stable to a capacity of 520 mL. Cystoscopy yields unremarkable findings.
When she is offered surgical correction of her SUI, the patient expresses a preference for the use of her own tissues and says she does not want to have synthetic mesh placed.
Is this patient a candidate for a rectus fascia pubovaginal sling?
As more patients express reservations about the placement of synthetic mesh during sling procedures, the use of autologous rectus fascia pubovaginal slings has risen. The concept of using a patient’s own tissue as a sling to support the urethra dates to the early 20th century, but it was not until late in that century that the procedure gained widespread appreciation and evolved into its current form. Initially, the procedure entailed mobilizing a strip of abdominal muscle (either rectus or pyramidalis), freeing one end of the strip from its attachment, passing that end under the bladder neck, and reaffixing it to the abdominal muscle wall, forming a “U”-shaped sling around the bladder outlet. Subsequently, overlying abdominal fascia was included in the sling, eventually replacing the muscle altogether. The final innovation: An isolated strip of fascia was suspended by free sutures that were tied to the abdominal wall or attached on top of the abdominal rectus sheath.
The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during the transmission of increased abdominal pressure.
The sling is suspended on each end by free sutures that are attached directly to the abdominal wall musculature or, more commonly, tied to each other on the anterior surface of the abdominal wall.
Long-term success depends on healing and fibrotic processes, which occur primarily where the sling passes through the endopelvic fascia.
Although the pubovaginal sling procedure was pioneered as a surgical option for intrinsic sphincter deficiency (ISD), its indications have broadened to encompass all types of SUI. Its reliable results and durable outcomes make it one of the main standards of treatment, and the pubovaginal sling has been used extensively as primary therapy for:
- SUI related to ISD or urethral hypermobility
- as a salvage procedure for recurrent SUI
- as an adjunct to urethral and bladder reconstruction
- as a way to functionally close the urethra to abandon urethral access to the bladder.
In our opinion, the autologous pubovaginal sling is appropriate for patients with SUI who decline to have synthetic material implanted because of concerns related to long-term placement of synthetic mesh. Other good candidates are women who experience recurrent incontinence after placement of a synthetic sling or who develop a complication, such as vaginal erosion (VIDEO 1, Rectus fascia pubovaginal sling after an unsuccessful TVT), after placement of a synthetic sling. We also prefer to use an autologous sling in patients who have been radiated or who have sustained urethral injuries, as well as in patients who are undergoing simultaneous repair of urethrovaginal fistula or diverticulum—or those who have already undergone such repair.
What is the optimal sling material?
Rectus abdominis fascia versus fascia lata. The two most commonly used autologous tissues are rectus abdominus fascia and fascia lata. Both of these materials have been studied extensively and proven to be effective and reliable. Most surgeons prefer rectus fascia because it is easier and quicker to harvest.
Allogenic and xenogenic tissues. Allogenic (cadaveric) fascia lata and cadaveric dermis provide reasonable efficacy, but durability remains an issue, as high failure rates have been reported. Bovine and porcine dermis, as well as porcine small-intestine submucosa, are also effective for SUI, although durability remains a concern.
Synthetic materials. Synthetic graft materials of various designs and substances also have been used as sling material. Monofilament, large-pore weave grafts (Type 1 mesh) are recommended for implantation in the vagina. Although good efficacy can be achieved with synthetic mesh, the material also may increase the risk of serious complications, such as infection, vaginal extrusion, and genitourinary erosion, and is not recommended for use beneath the proximal urethra or bladder neck.

The autologous pubovaginal sling supports the proximal urethra and bladder neck to achieve continence by providing a direct compressive force on the urethra and bladder outlet, or by reestablishing a reinforcing platform or hammock against which the urethra is compressed during increased abdominal pressure.
How to harvest rectus fascia and create a sling
1. Choose anesthesia and perioperative antibiotics
Pubovaginal sling procedures are generally carried out under general anesthesia, but spinal or epidural anesthesia also is possible. Full-patient paralysis is not warranted but may facilitate closure of the rectus fascia after fascial harvesting.
Perioperative antibiotics usually are given to ensure appropriate coverage against skin and vaginal flora (for example, a cephalosporin or fluoroquinolone). In fact, perioperative antibiotics have become a mandated quality of care measure in the United States.
2. Position the patient for optimal access
Place the patient in the low lithotomy position with her legs in stirrups. The abdomen and perineum should be sterilely prepared and draped to provide access to the vagina and lower abdomen.
After the bladder is drained with a Foley catheter, place a weighted vaginal speculum and use either lateral labial retraction sutures or a self-retaining retractor system to facilitate vaginal exposure.
3. Make an abdominal incision
Make an 8- to 10-cm Pfannenstiel incision approximately 3 to 5 cm above the pubic bone, carry the dissection down to the level of the rectus fascia using a combination of electrocautery and blunt dissection, and sweep the fat and subcutaneous tissue clear of the rectus tissue (FIGURE 1).

FIGURE 1 Skin incision
Before initiating the operation, delineate the location of the transverse skin incision, which should measure 8 to 10 cm and be situated about 4 cm above the symphysis pubis. A vertical incision is also feasible, although it usually is less aesthetic.
4. Harvest the fascia
The rectus abdominis fascia can be harvested in a transverse or vertical orientation. A fascial segment at least 8 cm in length and 1.5 to 2 cm in width is recommended.
Delineate the fascial segment to be resected using a surgical marking pen or electrocautery, then incise the tissue sharply with a scalpel, scissors, or electrocautery along the drawn lines.
Virgin fascia is preferred, but the presence of fibrotic rectus fascia does not prohibit its use. If you are resecting the fascia close and parallel to the symphysis pubis, leave at least 0.5 to 1.0 cm attached to facilitate closure of the defect created in the fascia. Small Army/Navy retractors permit aggressive retraction of skin edges, making it possible to use a smaller skin incision (FIGURE 2).

FIGURE 2 Resect the fascial strip
After choosing the optimal location for excision, mark the area using electrocautery or a surgical marking pen. Then resect the strip using a scalpel or electrocautery. The strip should measure 8 to 10 cm in length and 1 to 2 cm in width. If the skin incision is small, Army/Navy retractors may enhance exposure.
5. Close the fascial defect
Use heavy-gauge (#1 or #0) delayed, absorbable suture in a running fashion. It may be necessary to mobilize the rectus abdominis fascial edges to ensure appropriate tension-free approximation. It is important that anesthesia be sufficient to ensure muscular relaxation and paralysis during closure.
6. Prepare the fascial sling
Affix a single #1 permanent (for example, polypropylene or polyester) suture to each end of the fascial segment by passing the needle through the undersurface of the sling and then back through the top of the sling. If necessary, defat the sling (FIGURE 3).

FIGURE 3 Attach suspensory sutures
A. Mark the midline of the fascial sling with a pen and gently grasp it using a hemostat. B. Attach a polyester suture to each end of the fascial sling after stripping it of any adipose tissue. Ensure that the initial entry and exit points of the polyester sutures are on the same side of the strip that originally abutted the rectus muscles.
7. Dissect the vagina
Use injectable-grade saline or a local analgesic, such as 1% lidocaine, to hydrodissect the subepithelial tissues of the distal portion of the anterior vaginal wall. Make a midline or inverted “U” incision into the vagina (FIGURE 4).
Create vaginal flaps that have sufficient mobility to ensure tension-free closure over the sling. Carry out dissection laterally and anteriorly until you encounter the endopelvic fascia, then incise the endopelvic fascia and dissect it from the posterior surface of the pubis to enter the retropubic space.
Although blunt dissection sometimes can be performed, sharp dissection with Mayo scissors is often required, especially in cases that involve recurrent stress incontinence (FIGURE 4).

FIGURE 4 Dissect the vagina
A. Use an inverted “U” or vertical incision on the vaginal mucosa overlying the midurethra and bladder. B. Carefully dissect the tissue to the pubic rami bilaterally until the urogenital diaphragm is identified, then sharply penetrate it using Mayo scissors. C. Enlarge the opening by repeating the procedure on the opposite side.
8. Pass retropubic needles
Pass Stamey needles or long clamps through the retropubic space from the open abdominal wound immediately posterior to the pubic bone, approximately 4 cm apart. You can maintain distal control of the needles by direct finger guidance through the vaginal incision. Be careful to advance the tip of the needle adjacent to the posterior surface of the pubic bone to avoid inadvertent bladder injury (FIGURE 5). Proper bladder drainage also helps to minimize injury to the bladder, which may be closely adherent to the pubis, especially if a prior retropubic procedure has been performed, as in Case 1.

FIGURE 5 Place the sling
A. Insert the Stamey needle through the rectus fascia and guide it into the vagina with the index finger placed against the tip of the needle. B. Thread both ends of the polyester suture into the eye of the Stamey needle and then retract the needle carefully until the suture ends are delivered abdominally at the level of the fascia.
9. Rule out bladder injury
Careful cystoscopic examination of the bladder is mandatory after passing the needles to rule out inadvertent injury. Injuries to the bladder typically occur at the 1 o’clock and 11 o’clock positions, so use a 70° lens, and fill the bladder completely to expand any mucosal redundancy. Wiggle the needles or clamps to help localize their position relative to the bladder wall.
10. Deploy the sling
Thread the free ends of the sutures affixed to the sling into the ends of the Stamey needles—or grasp them with clamps—and pull each suture up to the anterior abdominal wall through the retropubic space (FIGURE 5). Keep the sling centered and flat at the area of the bladder neck.
Some surgeons fix the sling in the midline to the underlying periurethral tissue using numerous delayed absorbable sutures. We prefer to leave the sling unattached to the underlying urethra and bladder neck.
11. Tension the sling
Various techniques are applicable. To ensure adequate “looseness,” we tie the sutures across the midline while holding a right-angle clamp between the sling material and the posterior urethral surface. The goal is for the sling to prevent the descent of the proximal urethra during increases in abdominal pressure without creating any outlet obstruction to the normal flow of urine (FIGURE 6).

FIGURE 6 Tension the sling
A. Tie the suspensory sutures abdominally above the fascial closure line. Tie the sutures across the assistant’s index finger to avoid excessive tension. B. Assess the tension using a right-angle clamp placed between the pubovaginal sling and the vagina.
12. Close the incisions
Close the abdominal skin incision using 3-0 and 4-0 absorbable sutures. Use 3-0 absorbable sutures to close the vaginal mucosa. We prefer to close the vagina after completion of the tensioning procedure, but some surgeons complete this step prior to tensioning.
13. Place a catheter, packing material
Place a bladder catheter and vaginal gauze packing. Both the catheter and gauze may be removed after 24 hours. If the patient is unable to void at that time, teach her intermittent self-catheterization, or place an indwelling Foley catheter for 1 week.
Outcomes show good efficacy
Pubovaginal slings are highly effective, with success rates between 50% and 75% after follow-up as long as 10 years.1 In 2011, Blaivas and Chaikin reported 4-year follow-up data, with improvement or cure in 100% of patients with uncomplicated SUI and in as many as 93% of patients in more complicated cases.2 Most failures were due to urge incontinence and occurred within the first 6 postoperative months; 3% of these urge patients were thought to have developed de novo urge incontinence.
Other studies have found de novo urgency and storage symptoms in as many as 23% of patients, with 11% of patients reporting voiding dysfunction and as many as 7.8% requiring long-term self-catheterization.1
Flawed methodology in the few randomized, controlled trials that have compared the pubovaginal sling with the tension-free vaginal tape (TVT) has cast doubt on their findings.3 Basok and colleagues found an increased rate of de novo urgency in the women treated with a pubovaginal sling, compared with those who underwent intravaginal slingplasty,4 whereas Sharifiaghdas and Mortazavi found equal efficacy between pubovaginal and retropubic midurethral synthetic slings.5 The most scientifically valid randomized, controlled trial found equal subjective cure rates and complication rates when a biologic pubovaginal sling was compared with the TVT.6 In that study, the pubovaginal sling was of porcine origin.
In a comparison of autologous and autograft slings, Flynn found equal control of SUI over 2 years, with reduced postoperative discomfort in the allograft group.7
When autologous pubovaginal slings were compared with Burch colposuspension in a randomized, controlled trial, fascial slings were better at controlling incontinence despite an increased morbidity profile.8
A meta-analysis found equal subjective cure rates and overall efficacy between pubovaginal and midurethral synthetic slings.9
Voiding dysfunction is the most common complication
Transient urinary retention may occur in as many as 20% of patients and requires intermittent self-catheterization until resolution (typically 2–4 weeks). Prolonged postoperative voiding dysfunction (lasting more than 4–6 weeks), including de novo urgency, urgency incontinence, and obstructive symptoms, may occur to some degree in as many as 25% of patients. However, fewer than 3% of women require subsequent urethrolysis for treatment of prolonged retention or obstructive voiding symptoms.
Synthetic full-length midurethral slings remain the standard of care
for SUI
Charles W. Nager, MD (November 2012)
Harvest the fascia first. Because substantial bleeding can occur during vaginal dissection, it is advisable to harvest the autologous fascia and prepare the sling by affixing sutures to it before dissecting the vagina. This facilitates timely insertion of the sling and minimal blood loss. Retropubic bleeding from high in the space that occurs during dissection almost always resolves upon placement of the sling. We recommend against prolonged attempts at hemostasis.
In urethral reconstruction, tension the sling after reconstruction. When placing an autologous pubovaginal sling in the setting of urethral reconstruction or as tissue interposition, harvest the fascia and prepare and deploy the sling (with passage of the retropubic sutures) before reconstructing the urethra—but refrain from tensioning until after the reconstruction is completed. Then affix the sling in the appropriate location and tension it. When the sling is placed after reconstruction, it can damage the reconstruction through traction or direct injury.
Don’t worry about surface orientation. During placement of the autologous sling material, surface orientation does not matter. Conventionally, however, the “body-side” or underside of the graft is placed on the body-side of the patient.
Tensioning varies between patients. For most women, sling tensioning can be accomplished by tying the sutures over one or two fingers placed across the fascia. In patients who have undergone multiple procedures and who have a nonmobile urethra, however, tension should be tighter and must be individualized, based on the patient’s anatomy, lower urinary tract function, and willingness to perform intermittent self-catheterization for a prolonged period of time.
CASE 1: Resolved
After you advise the patient of the risks and benefits of the rectus fascia pubovaginal sling, in comparison with a repeat synthetic midurethral sling, she continues to insist on the use of autologous tissue. She undergoes the pubovaginal sling operation with excision of eroded mesh without complication.
A 35-year-old woman reports continuous urinary leakage that is not associated with movement. She was previously told that she had an ectopic ureter implanted into a congenitally short urethra, and she underwent repair of the problem, including reimplantation of the ureter and placement of a cadaveric fascia lata sling. A congenital remnant—observed as a blind pouch via cystoscopy—was left attached to the urethra. Two years have passed since that operation.
Physical findings: A pelvic examination reveals complete loss of the posterior urethra. One possible explanation: The remnant became infected and caused a breakdown of the posterior urethra, with complete disappearance of the cadaveric fascia lata.
Recommended management: Complete urethral reconstruction, with transposition of a martius fat pad and repeat placement of a cadaveric fascia pubovaginal sling.
Technique: See Video 2, Urethral reconstruction.
We want to hear from you! Tell us what you think.
How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy
How to avoid major vessel injury during gynecologic laparoscopy
(August 2012)
CASE: Adhesions complicate multiple surgeries
In early 2007, a 37-year-old woman with a history of hysterectomy, adhesiolysis, bilateral partial salpingectomy, and cholecystectomy underwent an attempted laparoscopic bilateral salpingo-oophorectomy (BSO) for pelvic pain. The operation was converted to laparotomy because of severe adhesions and required several hours to complete.
After the BSO, the patient developed hydronephrosis in her left kidney secondary to an inflammatory cyst. In March 2007, a urologist placed a ureteral stent to relieve the obstruction. One month later, the patient was referred to a gynecologic oncologist for chronic pelvic pain.
On October 29, 2007, the patient underwent operative laparoscopy for adhesiolysis and appendectomy. No retroperitoneal exploration was attempted at the time. According to the operative note, the 10-mm port incision was enlarged to 3 cm to enable the surgeon to inspect the descending colon. Postoperatively, the patient reported persistent abdominal pain and fever and was admitted to the hospital for observation. Although she had a documented temperature of 102°F on October 31, with tachypnea, tachycardia, and a white blood cell (WBC) count of 2.9 x 103/μL, she was discharged home the same day.
The next morning, the patient returned to the hospital’s emergency room (ER) reporting worsening abdominal pain and shortness of breath. Her vital signs included a temperature of 95.8°F, heart rate of 135 bpm, respiration of 32 breaths/min, and blood pressure of 100/68 mm Hg. An examination revealed a tender, distended abdomen, and the patient exhibited guarding behavior upon palpation in all quadrants. Bowel sounds were hypoactive, and the WBC count was 4.2 x 103/μL. No differential count was ordered. A computed tomography (CT) scan showed free air in the abdomen, pneumomediastinum, and subcutaneous emphysema of the abdominal wall and chest wall.
The next day, a differential WBC count revealed bands elevated at a 25% level. A cardiac consultant diagnosed heart failure and remarked that pneumomediastinum should not occur after abdominal surgery. In the evening, the gynecologic oncologist performed a laparotomy and observed enteric contents in the abdominal cavity, as well as a defect of approximately 2 mm in the lower portion of the rectosigmoid colon. According to the operative note, the gynecologic oncologist stapled off the area below the defect and performed a descending loop colostomy.
Postoperatively, the patient remained septic, and vegetable matter was recovered from one of the drains, so a surgical consultant was called. On November 9, a general surgeon performed an exploratory laparotomy and found necrosis, hemorrhage, acute inflammation of the colostomy, separation of the colostomy from its sutured position on the anterior abdominal wall, and mucosa at the end of the Hartman pouch, necessitating resection of this segment of the colon back to the rectum. Numerous intra-abdominal abscesses were also drained.
Two days later, the patient returned to the OR for further abscess drainage and creation of a left end colostomy. She was discharged 1 month later.
On January 4, 2008, she went to the ER for nausea and abdominal pain. Five days later, a plastic surgeon performed extensive skin grafting on the chronically open abdominal wound. On March 12, the patient returned to the ER because of abdominal pain and was admitted for nasogastric drainage and intravenous (IV) fluids. She returned to the ER again on April 26, reporting pain. A CT scan revealed a cystic mass in the pelvis, which was drained under CT guidance. In June and July, the patient was seen in the ER three times for pain, nausea, and vomiting.
In January 2009, she underwent another laparotomy for takedown of the colostomy, lysis of adhesions, and excision of a left 4-cm pelvic cyst (pathology later revealed the cyst to be ovarian tissue). She also underwent a left-sided myocutaneous flap reconstruction of an abdominal wall defect, and a right-sided myocutaneous flap with placement of a 16 x 20–cm sheet of AlloDerm Tissue Matrix (LifeCell). She continues to experience abdominal pain and visits the ER for that reason. In March 2009, she underwent repeat drainage of a pelvic collection via CT imaging. No further follow-up is available.
Could this catastrophic course have been avoided? What might have prevented it?
Adhesions are likely after any abdominal procedure
The biggest risk factor for laparoscopy-related intestinal injury is the presence of pelvic or abdominal adhesions.1,2 Adhesions inevitably form after any intra-abdominal surgery, and new adhesions are likely with each successive intra-abdominal procedure. Even adhesiolysis leads to the formation of adhesions postoperatively.
Few reliable data suggest that adhesions cause pelvic pain, or that adhesiolysis relieves such pain.3 Furthermore, it may be impossible to predict with reasonable probability where adhesions may be located preoperatively or to know with certainty whether a portion of the intestine is adherent to the anterior abdominal wall directly below the usual subumbilical entry site. Because of the likelihood of adhesions in a patient who has undergone two or more laparotomies, it is risky to thrust a 10- to 12-mm trocar through the anterior abdominal wall below the navel.
A few variables influence the risk of injury
The trocar used in laparoscopic procedures plays a role in the risk of bowel injury. For example, relatively dull reusable devices may push nonfixed intestine away rather than penetrate the viscus. In contrast, razor-sharp disposable devices are more likely to cut into the underlying bowel.
Body habitus is also important. The obese woman is at greater risk for entry injuries, owing to physical aspects of the fatty anterior abdominal wall. When force is applied to the wall, it moves inward, toward the posterior wall, trapping intestine. In a thin woman, the abdominal wall is less elastic, so there is less excursion upon trocar entry.
Intestinal status is another variable to consider. A collapsed bowel is unlikely to be perforated by an entry trocar, whereas a thin, distended bowel is vulnerable to injury. Bowel status can be determined preoperatively using various modalities, including radiographic studies.
Careful surgical technique is imperative. Sharp dissection is always preferable to the blunt tearing of tissue, particularly in cases involving fibrous adhesions. Tearing a dense, unyielding adhesion is likely to remove a piece of intestinal wall because the tensile strength of the adhesion is typically greater than that of the viscus itself.
Thorough knowledge of pelvic anatomy is essential. It would be particularly egregious for a surgeon to mistake an adhesion for the normal peritoneal attachments of the left and sigmoid colon, or to resect the mesentery of the small bowel, believing it to be an adhesion.
Energy devices account for a significant number of intestinal injuries (FIGURE 1). Any surgeon who utilizes an energy device is obligated to protect the patient from a thermal injury—and the manufacturers of these instruments should provide reliable data on the safe use of the device, including information about the expected zone of conductive thermal spread based on power density and tissue type. As a general rule, avoid the use of monopolar electrosurgical devices for intra-abdominal dissection.
Adhesiolysis is a risky enterprise. Several studies have found a significant likelihood of bowel injury during lysis of adhesions.4-6 In two studies by Baggish, 94% of adhesiolysis-related injuries involved moderate or severe adhesions.5,6
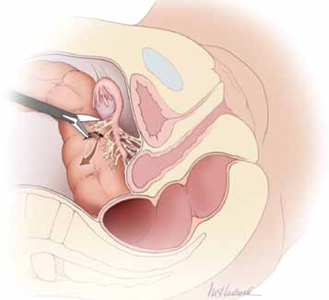
FIGURE 1 Use of energy devices is risky near bowel
Energy devices account for a significant number of intestinal injuries. In this figure, the arrow indicates leakage of fecal matter from the bowel defect.
Is laparoscopy the wisest approach?
It is important to weigh the risks of laparoscopy against the potential benefits for the patient. Surgical experience and skill are perhaps the most important variables to consider when deciding on an operative approach. A high volume of laparoscopic operations—performed by a gynecologic surgeon—should translate into a lower risk of injury to intra-abdominal structures.7 That is, the greater the number of cases performed, the lower the risk of injury.
Garry and colleagues conducted two parallel randomized trials comparing 1) laparoscopic and abdominal hysterectomy and 2) laparoscopic and vaginal hysterectomy as part of the eVALuate study.8 Laparoscopic hysterectomy was associated with a significantly higher rate of major complications than abdominal hysterectomy and took longer to perform. No major differences in the rate of complications were found between laparoscopic and vaginal hysterectomy.
In a review of laparoscopy-related bowel injuries, Brosens and colleagues found significant variations in the complication rate, depending on the experience of the surgeon—a 0.2% rate of access injuries for surgeons who had performed fewer than 100 procedures versus 0.06% for those who had performed more than 100 cases, and a 0.3% rate of operative injuries for surgeons who had performed fewer than 100 procedures versus 0.04% for more experienced surgeons.7
A few precautions can improve the safety of laparoscopy
If adhesions are known or suspected, primary laparoscopic entry should be planned for a site other than the infra-umbilical area. Options include:
- entry via the left hypochondrium in the midclavicular line
- an open procedure.
However, open laparoscopic entry does not always avert intestinal injury.9-11
If the anatomy is obscured once the abdomen has been entered safely, retroperitoneal dissection may be useful, particularly for exposure of the left colon. When it is unclear whether a structure to be incised is a loop of bowel or a distended, adherent oviduct, it is best to refrain from cutting it.
For adhesiolysis, traction and counter-traction are the techniques of choice. Dissection of intestine should always be parallel to the axis of the viscus. Remember, too, that the blood supply enters via the mesenteric margin of the intestine.
After any dissection involving the intestine, carefully inspect the bowel and describe that inspection in the operative report (FIGURE 2). If injury is suspected, consult a general surgeon and open the abdomen to permit thorough inspection of the intestines.
What the literature reveals about intestinal injury
Several published reports describe a large number of laparoscopic cases and the major attendant complications.12-16 A number of studies have focused on gastrointestinal (GI) complications associated with laparoscopic procedures, providing site-specific data.
Many injuries occur during entry
Vilos reported on 40 bowel injuries, of which 55% occurred during primary trocar entry (19 closed and three open entries).17
In a report on 62 GI injuries in 56 patients, Chapron and colleagues found that one-third occurred during the approach phase of the laparoscopy; they advocated creation of a pneumoperitoneum rather than direct trocar insertion.18
In a report from the Netherlands, 24 of 29 GI injuries occurred during the approach.2
In a review of 63 GI complications related to diagnostic and operative laparoscopy, 75% of injuries were associated with primary trocar insertion.19
Optical access trocars do not appear to be protective against bowel injury. One study of 79 complications associated with these devices found 24 bowel injuries.20
In addition, in two reports detailing 130 cases of small- and large-bowel perforations associated with laparoscopic procedures, Baggish found that 62 (77%) of small-bowel injuries and 20 (41%) of colonic injuries were entry-related.5,6
Energy devices can be problematic
In the study by Chapron and colleagues of 62 GI injuries, six were secondary to the use of electrosurgical devices, four of them involving monopolar instruments.18
In a study from Scotland, 27 of 117 (23%) of bowel injuries during laparoscopic procedures were attributable to a thermal event.21
Baggish found that 43% of operative injuries among 130 intestinal perforations were energy-related.5,6
Intraoperative diagnosis is optimal
Soderstrom reviewed 66 cases of laparoscopy-related bowel injuries and found three deaths attributable to a delay in diagnosis exceeding 72 hours.4
In a study by Vilos, the mean time for diagnosis of bowel injuries was 4 days (range, 0–23 days), with intraoperative diagnosis in only 35.7% of cases.17
In a Finnish nationwide analysis of laparoscopic complications, Harkki-Siren and Kurki found that small-bowel injuries were identified an average of 3.3 days after occurrence; when electrosurgery was involved in the injury, the average time to diagnosis was 4.8 days.22 As for large-bowel injuries, 44% were identified intraoperatively. In the remainder of cases, the average time from injury to diagnosis was 10.4 days for electrosurgical injuries and 1.3 days for injuries related to sharp dissection.
In the studies by Baggish, 82 of 130 (63%) intestinal injuries were diagnosed 48 hours or more after the operation.5,6
Baggish also made the following observations:
- The most common symptoms of intestinal injury were (in order of frequency) abdominal pain, bloating, nausea and vomiting, and fever or chills (or both). The most common signs were abdominal tenderness, abdominal distension, diminished bowel sounds, and elevated or subnormal temperature.
- Sepsis was apparent (due to the onset of systemic inflammatory response syndrome) in the majority of small-bowel perforations and virtually all colonic perforations. Findings of tachycardia, tachypnea, elevated leukocyte count, and bandemia suggested sepsis syndrome.
- Radiologically observed free air was often misinterpreted by the radiologist as being consistent with residual gas from the initial laparoscopy. In reality, most—if not all—CO2 gas is absorbed within 24 hours, particularly in obese women. Early CT imaging with oral contrast leads to the most expeditious, correct diagnosis, compared with flat and upright abdominal radiographs.
- Obese women did not exhibit rebound tenderness even though subsequent operative findings revealed extensive and severe peritonitis.
- When infection occurred, it usually was polymicrobial in nature. The most frequently cultured organisms include Escherichia coli, Enterococcus, alpha and beta Streptococcus, Staphylococcus, and Bacteroides.
Baggish concluded that earlier diagnosis could be achieved with careful inspection of the intestine at the conclusion of each operative procedure (FIGURE 2).
Similarly, Chapron and colleagues recommended meticulous inspection of all areas where bowel lysis has been performed. “When there is the slightest doubt, carry out tests for leakage (transanal injection of 200 mL methylene blue using a Foley catheter) in order not to overlook a rectosigmoid injury which would become apparent secondarily in a context of peritonitis,” they wrote. They also suggested that the patient be educated about the signs and symptoms of intestinal injury.18
Whenever a bowel injury is visualized intraoperatively, assume that it is transmural until it is proved otherwise.
FIGURE 2 Meticulous bowel inspection can identify perforation
It is vital to inspect the bowel after any dissection that involves the intestine, being especially alert for puncture wounds caused by a trocar and small tears associated with adhesiolysis.
SOURCE: Baggish MS, Karram MM. Atlas of Pelvic Anatomy and Gynecologic Surgery. 3rd ed. Philadelphia: Elsevier; 2011:1142.
How to avoid urinary tract injuries
Along with major vessel injury and intestinal perforation, bladder and ureteral injuries are the most common complications of laparoscopic surgery. Although urinary tract injuries are rarely fatal, they can cause a range of sequelae, including urinoma, vesicovaginal and ureterovaginal fistulas, hydroureter, hydronephrosis, renal damage, and kidney atrophy.
The incidence of ureteral injury during laparoscopy ranges from less than 0.1% to 1.0%, and the incidence of bladder injury ranges from less than 0.8% to 2.0%.23-26 Investigators in Singapore described eight urologic injuries among 485 laparoscopic hysterectomies and identified several risk factors:
- previous cesarean delivery
- multiple fibroids
- severe endometriosis.27
Another set of investigators found a history of laparotomy to be a risk factor for bladder injury during laparoscopic hysterectomy.28
Rooney and colleagues studied the effect of previous cesarean delivery on the risk of injury during hysterectomy.29 Among 5,092 hysterectomies—including 433 laparoscopic-assisted vaginal hysterectomies, 3,140 abdominal procedures, and 1,539 vaginal operations—the rate of bladder injury varied by approach. Cystotomy was observed in 0.76% of abdominal hysterectomies (33% had a previous cesarean delivery), 1.3% of vaginal procedures (21% had a previous cesarean), and 1.8% of laparoscopic operations (62.5% had a previous cesarean). The odds ratio for cystotomy during hysterectomy among women with a previous cesarean delivery was 1.26 for the abdominal approach, 3.00 for the vaginal route, and 7.50 for laparoscopic-assisted vaginal hysterectomy.29
Two studies highlight common aspects of injury
In a recent report of 75 urinary tract injuries associated with laparoscopic surgery, Baggish identified a total of 33 injuries involving the bladder and 42 of ureteral origin. Twelve of the bladder injuries were associated with the approach, and 21 were related to the surgery. In contrast, only one of the 42 ureteral injuries was related to the approach.30
Baggish also found that just under 50% of urinary tract injuries were related to the use of thermal energy, including all three vesicovaginal fistulas. Fourteen bladder lacerations occurred during separation of the bladder from the uterus during laparoscopic hysterectomy.30
Common sites of injury were at the infundibulopelvic ligament, between the infundibulopelvic ligament and the uterine vessels, and at or below the uterine vessels.30
None of the 42 ureteral injuries were diagnosed intraoperatively. In fact, 37 of these injuries were not correctly diagnosed until more than 48 hours after surgery. Two uterovaginal fistulas were also diagnosed in the late postoperative period.30
Bladder injuries were identified via cystoscopy or cystometrogram or by the instillation of methylene blue into the bladder, with observation from above for leakage. Ureteral injuries were identified by IV pyelogram, retrograde pyelogram, or attempted passage of a stent. Every ureteral injury showed up as hydroureter and hydronephrosis via pyelography.30
Grainger and colleagues reported five ureteral injuries associated with laparoscopic procedures.31 The principal symptoms were low back pain, abdominal pain, leukocytosis, and peritonitis. All five injuries were associated with endometriosis surgery, most commonly near the uterosacral ligaments.
Grainger and colleagues cited eight additional cases of injury. Three patients among the 13 total cases lost renal function, and two eventually required nephrectomy.31
How to prevent, identify, and manage urinary tract injuries
Thorough knowledge of anatomy and meticulous technique are imperative to prevent urinary tract injuries. Strategies include:
- Use sharp rather than blunt dissection.
- Know the risk factors for urinary tract injury, which include previous cesarean delivery or intra-abdominal surgery, presence of adhesions, and deep endometriosis.
- Be aware of the dangers posed by energy devices when they are used near the bladder and ureter. Even bipolar devices can cause thermal injury.
- Employ hydrodissection when there are bladder adhesions, and work nearer the uterus or vagina than the bladder, leaving a margin of tissue.
- When the ureter’s location is unclear relative to the operative site, do not hesitate to open the retroperitoneal space to observe the ureter. If necessary, dissect the ureter distally.
- Perform cystoscopy with IV indigo carmine injection at the conclusion of surgery to ensure that the ureter is not occluded.
- Be aware that peristalsis is not an indication of ureteral integrity. In fact, an obstructed ureter will pulsate more vigorously than a normal one.
- Consider preoperative ureteral catheterization, which may avert injury without increasing operative time, blood loss, and hospital stay,32 although the data are not definitive.33
- Be vigilant. Early identification of injuries reduces morbidity. In the case of ureteral obstruction, immediate stenting will usually obviate the need for ureteral implantation and nephrostomy if the obstruction is not complete.
- Intervene early to cut an obstructing suture or relieve ureteral bowing. Doing so may eliminate the obstruction altogether in many cases.
- If a laceration is found in the bladder trigone or its vicinity, always perform ureteral catheterization to help prevent the inadvertent suturing of the intravesical ureter into the repair.
- After repair of a bladder laceration, perform cystoscopy with IV injection of indigo carmine to ensure ureteral integrity.
- Use only absorbable suture in bladder repairs. I recommend 2-0 chromic catgut for the first layer, which should encompass muscularis and mucosa. Place a second layer of sutures using 3-0 polyglactin 910 (Vicryl), imbricating the first layer.
- After completion of a bladder repair, instill a solution of diluted methylene blue (1 part methylene blue to 100 parts sterile water or saline) to distend the bladder, and carefully inspect the closure to ensure that it is watertight. Then place a Foley catheter for a minimum of 2 weeks. Four to 6 weeks after repair, perform a cystogram to ensure that healing is complete, with no leakage.
- Call a urologist if you are not well-versed in bladder repair, or if the ureter is injured (or injury is suspected).
- Watch for fistula formation, an inevitable outcome of untreated bladder and ureteral injury, which may occur early or late in the postoperative course.
Choose an approach wisely
Laparoscopy is a learned skill. Supervised practice generally leads to greater levels of proficiency, and repetition of the same operations improves dexterity and execution. However, laparoscopy is also an art—some people have the touch and some do not.
Although laparoscopic techniques offer many advantages, they also have shortcomings. The complications described here, and the strategies I have offered for preventing and managing them, should help gynecologic surgeons determine whether laparoscopy is the optimal route of operation, based on surgical experience, characteristics of the individual patient, and other variables.
Update: Minimally Invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
We want to hear from you! Tell us what you think.
How to avoid major vessel injury during gynecologic laparoscopy
(August 2012)
CASE: Adhesions complicate multiple surgeries
In early 2007, a 37-year-old woman with a history of hysterectomy, adhesiolysis, bilateral partial salpingectomy, and cholecystectomy underwent an attempted laparoscopic bilateral salpingo-oophorectomy (BSO) for pelvic pain. The operation was converted to laparotomy because of severe adhesions and required several hours to complete.
After the BSO, the patient developed hydronephrosis in her left kidney secondary to an inflammatory cyst. In March 2007, a urologist placed a ureteral stent to relieve the obstruction. One month later, the patient was referred to a gynecologic oncologist for chronic pelvic pain.
On October 29, 2007, the patient underwent operative laparoscopy for adhesiolysis and appendectomy. No retroperitoneal exploration was attempted at the time. According to the operative note, the 10-mm port incision was enlarged to 3 cm to enable the surgeon to inspect the descending colon. Postoperatively, the patient reported persistent abdominal pain and fever and was admitted to the hospital for observation. Although she had a documented temperature of 102°F on October 31, with tachypnea, tachycardia, and a white blood cell (WBC) count of 2.9 x 103/μL, she was discharged home the same day.
The next morning, the patient returned to the hospital’s emergency room (ER) reporting worsening abdominal pain and shortness of breath. Her vital signs included a temperature of 95.8°F, heart rate of 135 bpm, respiration of 32 breaths/min, and blood pressure of 100/68 mm Hg. An examination revealed a tender, distended abdomen, and the patient exhibited guarding behavior upon palpation in all quadrants. Bowel sounds were hypoactive, and the WBC count was 4.2 x 103/μL. No differential count was ordered. A computed tomography (CT) scan showed free air in the abdomen, pneumomediastinum, and subcutaneous emphysema of the abdominal wall and chest wall.
The next day, a differential WBC count revealed bands elevated at a 25% level. A cardiac consultant diagnosed heart failure and remarked that pneumomediastinum should not occur after abdominal surgery. In the evening, the gynecologic oncologist performed a laparotomy and observed enteric contents in the abdominal cavity, as well as a defect of approximately 2 mm in the lower portion of the rectosigmoid colon. According to the operative note, the gynecologic oncologist stapled off the area below the defect and performed a descending loop colostomy.
Postoperatively, the patient remained septic, and vegetable matter was recovered from one of the drains, so a surgical consultant was called. On November 9, a general surgeon performed an exploratory laparotomy and found necrosis, hemorrhage, acute inflammation of the colostomy, separation of the colostomy from its sutured position on the anterior abdominal wall, and mucosa at the end of the Hartman pouch, necessitating resection of this segment of the colon back to the rectum. Numerous intra-abdominal abscesses were also drained.
Two days later, the patient returned to the OR for further abscess drainage and creation of a left end colostomy. She was discharged 1 month later.
On January 4, 2008, she went to the ER for nausea and abdominal pain. Five days later, a plastic surgeon performed extensive skin grafting on the chronically open abdominal wound. On March 12, the patient returned to the ER because of abdominal pain and was admitted for nasogastric drainage and intravenous (IV) fluids. She returned to the ER again on April 26, reporting pain. A CT scan revealed a cystic mass in the pelvis, which was drained under CT guidance. In June and July, the patient was seen in the ER three times for pain, nausea, and vomiting.
In January 2009, she underwent another laparotomy for takedown of the colostomy, lysis of adhesions, and excision of a left 4-cm pelvic cyst (pathology later revealed the cyst to be ovarian tissue). She also underwent a left-sided myocutaneous flap reconstruction of an abdominal wall defect, and a right-sided myocutaneous flap with placement of a 16 x 20–cm sheet of AlloDerm Tissue Matrix (LifeCell). She continues to experience abdominal pain and visits the ER for that reason. In March 2009, she underwent repeat drainage of a pelvic collection via CT imaging. No further follow-up is available.
Could this catastrophic course have been avoided? What might have prevented it?
Adhesions are likely after any abdominal procedure
The biggest risk factor for laparoscopy-related intestinal injury is the presence of pelvic or abdominal adhesions.1,2 Adhesions inevitably form after any intra-abdominal surgery, and new adhesions are likely with each successive intra-abdominal procedure. Even adhesiolysis leads to the formation of adhesions postoperatively.
Few reliable data suggest that adhesions cause pelvic pain, or that adhesiolysis relieves such pain.3 Furthermore, it may be impossible to predict with reasonable probability where adhesions may be located preoperatively or to know with certainty whether a portion of the intestine is adherent to the anterior abdominal wall directly below the usual subumbilical entry site. Because of the likelihood of adhesions in a patient who has undergone two or more laparotomies, it is risky to thrust a 10- to 12-mm trocar through the anterior abdominal wall below the navel.
A few variables influence the risk of injury
The trocar used in laparoscopic procedures plays a role in the risk of bowel injury. For example, relatively dull reusable devices may push nonfixed intestine away rather than penetrate the viscus. In contrast, razor-sharp disposable devices are more likely to cut into the underlying bowel.
Body habitus is also important. The obese woman is at greater risk for entry injuries, owing to physical aspects of the fatty anterior abdominal wall. When force is applied to the wall, it moves inward, toward the posterior wall, trapping intestine. In a thin woman, the abdominal wall is less elastic, so there is less excursion upon trocar entry.
Intestinal status is another variable to consider. A collapsed bowel is unlikely to be perforated by an entry trocar, whereas a thin, distended bowel is vulnerable to injury. Bowel status can be determined preoperatively using various modalities, including radiographic studies.
Careful surgical technique is imperative. Sharp dissection is always preferable to the blunt tearing of tissue, particularly in cases involving fibrous adhesions. Tearing a dense, unyielding adhesion is likely to remove a piece of intestinal wall because the tensile strength of the adhesion is typically greater than that of the viscus itself.
Thorough knowledge of pelvic anatomy is essential. It would be particularly egregious for a surgeon to mistake an adhesion for the normal peritoneal attachments of the left and sigmoid colon, or to resect the mesentery of the small bowel, believing it to be an adhesion.
Energy devices account for a significant number of intestinal injuries (FIGURE 1). Any surgeon who utilizes an energy device is obligated to protect the patient from a thermal injury—and the manufacturers of these instruments should provide reliable data on the safe use of the device, including information about the expected zone of conductive thermal spread based on power density and tissue type. As a general rule, avoid the use of monopolar electrosurgical devices for intra-abdominal dissection.
Adhesiolysis is a risky enterprise. Several studies have found a significant likelihood of bowel injury during lysis of adhesions.4-6 In two studies by Baggish, 94% of adhesiolysis-related injuries involved moderate or severe adhesions.5,6

FIGURE 1 Use of energy devices is risky near bowel
Energy devices account for a significant number of intestinal injuries. In this figure, the arrow indicates leakage of fecal matter from the bowel defect.
Is laparoscopy the wisest approach?
It is important to weigh the risks of laparoscopy against the potential benefits for the patient. Surgical experience and skill are perhaps the most important variables to consider when deciding on an operative approach. A high volume of laparoscopic operations—performed by a gynecologic surgeon—should translate into a lower risk of injury to intra-abdominal structures.7 That is, the greater the number of cases performed, the lower the risk of injury.
Garry and colleagues conducted two parallel randomized trials comparing 1) laparoscopic and abdominal hysterectomy and 2) laparoscopic and vaginal hysterectomy as part of the eVALuate study.8 Laparoscopic hysterectomy was associated with a significantly higher rate of major complications than abdominal hysterectomy and took longer to perform. No major differences in the rate of complications were found between laparoscopic and vaginal hysterectomy.
In a review of laparoscopy-related bowel injuries, Brosens and colleagues found significant variations in the complication rate, depending on the experience of the surgeon—a 0.2% rate of access injuries for surgeons who had performed fewer than 100 procedures versus 0.06% for those who had performed more than 100 cases, and a 0.3% rate of operative injuries for surgeons who had performed fewer than 100 procedures versus 0.04% for more experienced surgeons.7
A few precautions can improve the safety of laparoscopy
If adhesions are known or suspected, primary laparoscopic entry should be planned for a site other than the infra-umbilical area. Options include:
- entry via the left hypochondrium in the midclavicular line
- an open procedure.
However, open laparoscopic entry does not always avert intestinal injury.9-11
If the anatomy is obscured once the abdomen has been entered safely, retroperitoneal dissection may be useful, particularly for exposure of the left colon. When it is unclear whether a structure to be incised is a loop of bowel or a distended, adherent oviduct, it is best to refrain from cutting it.
For adhesiolysis, traction and counter-traction are the techniques of choice. Dissection of intestine should always be parallel to the axis of the viscus. Remember, too, that the blood supply enters via the mesenteric margin of the intestine.
After any dissection involving the intestine, carefully inspect the bowel and describe that inspection in the operative report (FIGURE 2). If injury is suspected, consult a general surgeon and open the abdomen to permit thorough inspection of the intestines.
What the literature reveals about intestinal injury
Several published reports describe a large number of laparoscopic cases and the major attendant complications.12-16 A number of studies have focused on gastrointestinal (GI) complications associated with laparoscopic procedures, providing site-specific data.
Many injuries occur during entry
Vilos reported on 40 bowel injuries, of which 55% occurred during primary trocar entry (19 closed and three open entries).17
In a report on 62 GI injuries in 56 patients, Chapron and colleagues found that one-third occurred during the approach phase of the laparoscopy; they advocated creation of a pneumoperitoneum rather than direct trocar insertion.18
In a report from the Netherlands, 24 of 29 GI injuries occurred during the approach.2
In a review of 63 GI complications related to diagnostic and operative laparoscopy, 75% of injuries were associated with primary trocar insertion.19
Optical access trocars do not appear to be protective against bowel injury. One study of 79 complications associated with these devices found 24 bowel injuries.20
In addition, in two reports detailing 130 cases of small- and large-bowel perforations associated with laparoscopic procedures, Baggish found that 62 (77%) of small-bowel injuries and 20 (41%) of colonic injuries were entry-related.5,6
Energy devices can be problematic
In the study by Chapron and colleagues of 62 GI injuries, six were secondary to the use of electrosurgical devices, four of them involving monopolar instruments.18
In a study from Scotland, 27 of 117 (23%) of bowel injuries during laparoscopic procedures were attributable to a thermal event.21
Baggish found that 43% of operative injuries among 130 intestinal perforations were energy-related.5,6
Intraoperative diagnosis is optimal
Soderstrom reviewed 66 cases of laparoscopy-related bowel injuries and found three deaths attributable to a delay in diagnosis exceeding 72 hours.4
In a study by Vilos, the mean time for diagnosis of bowel injuries was 4 days (range, 0–23 days), with intraoperative diagnosis in only 35.7% of cases.17
In a Finnish nationwide analysis of laparoscopic complications, Harkki-Siren and Kurki found that small-bowel injuries were identified an average of 3.3 days after occurrence; when electrosurgery was involved in the injury, the average time to diagnosis was 4.8 days.22 As for large-bowel injuries, 44% were identified intraoperatively. In the remainder of cases, the average time from injury to diagnosis was 10.4 days for electrosurgical injuries and 1.3 days for injuries related to sharp dissection.
In the studies by Baggish, 82 of 130 (63%) intestinal injuries were diagnosed 48 hours or more after the operation.5,6
Baggish also made the following observations:
- The most common symptoms of intestinal injury were (in order of frequency) abdominal pain, bloating, nausea and vomiting, and fever or chills (or both). The most common signs were abdominal tenderness, abdominal distension, diminished bowel sounds, and elevated or subnormal temperature.
- Sepsis was apparent (due to the onset of systemic inflammatory response syndrome) in the majority of small-bowel perforations and virtually all colonic perforations. Findings of tachycardia, tachypnea, elevated leukocyte count, and bandemia suggested sepsis syndrome.
- Radiologically observed free air was often misinterpreted by the radiologist as being consistent with residual gas from the initial laparoscopy. In reality, most—if not all—CO2 gas is absorbed within 24 hours, particularly in obese women. Early CT imaging with oral contrast leads to the most expeditious, correct diagnosis, compared with flat and upright abdominal radiographs.
- Obese women did not exhibit rebound tenderness even though subsequent operative findings revealed extensive and severe peritonitis.
- When infection occurred, it usually was polymicrobial in nature. The most frequently cultured organisms include Escherichia coli, Enterococcus, alpha and beta Streptococcus, Staphylococcus, and Bacteroides.
Baggish concluded that earlier diagnosis could be achieved with careful inspection of the intestine at the conclusion of each operative procedure (FIGURE 2).
Similarly, Chapron and colleagues recommended meticulous inspection of all areas where bowel lysis has been performed. “When there is the slightest doubt, carry out tests for leakage (transanal injection of 200 mL methylene blue using a Foley catheter) in order not to overlook a rectosigmoid injury which would become apparent secondarily in a context of peritonitis,” they wrote. They also suggested that the patient be educated about the signs and symptoms of intestinal injury.18
Whenever a bowel injury is visualized intraoperatively, assume that it is transmural until it is proved otherwise.

FIGURE 2 Meticulous bowel inspection can identify perforation
It is vital to inspect the bowel after any dissection that involves the intestine, being especially alert for puncture wounds caused by a trocar and small tears associated with adhesiolysis.
SOURCE: Baggish MS, Karram MM. Atlas of Pelvic Anatomy and Gynecologic Surgery. 3rd ed. Philadelphia: Elsevier; 2011:1142.
How to avoid urinary tract injuries
Along with major vessel injury and intestinal perforation, bladder and ureteral injuries are the most common complications of laparoscopic surgery. Although urinary tract injuries are rarely fatal, they can cause a range of sequelae, including urinoma, vesicovaginal and ureterovaginal fistulas, hydroureter, hydronephrosis, renal damage, and kidney atrophy.
The incidence of ureteral injury during laparoscopy ranges from less than 0.1% to 1.0%, and the incidence of bladder injury ranges from less than 0.8% to 2.0%.23-26 Investigators in Singapore described eight urologic injuries among 485 laparoscopic hysterectomies and identified several risk factors:
- previous cesarean delivery
- multiple fibroids
- severe endometriosis.27
Another set of investigators found a history of laparotomy to be a risk factor for bladder injury during laparoscopic hysterectomy.28
Rooney and colleagues studied the effect of previous cesarean delivery on the risk of injury during hysterectomy.29 Among 5,092 hysterectomies—including 433 laparoscopic-assisted vaginal hysterectomies, 3,140 abdominal procedures, and 1,539 vaginal operations—the rate of bladder injury varied by approach. Cystotomy was observed in 0.76% of abdominal hysterectomies (33% had a previous cesarean delivery), 1.3% of vaginal procedures (21% had a previous cesarean), and 1.8% of laparoscopic operations (62.5% had a previous cesarean). The odds ratio for cystotomy during hysterectomy among women with a previous cesarean delivery was 1.26 for the abdominal approach, 3.00 for the vaginal route, and 7.50 for laparoscopic-assisted vaginal hysterectomy.29
Two studies highlight common aspects of injury
In a recent report of 75 urinary tract injuries associated with laparoscopic surgery, Baggish identified a total of 33 injuries involving the bladder and 42 of ureteral origin. Twelve of the bladder injuries were associated with the approach, and 21 were related to the surgery. In contrast, only one of the 42 ureteral injuries was related to the approach.30
Baggish also found that just under 50% of urinary tract injuries were related to the use of thermal energy, including all three vesicovaginal fistulas. Fourteen bladder lacerations occurred during separation of the bladder from the uterus during laparoscopic hysterectomy.30
Common sites of injury were at the infundibulopelvic ligament, between the infundibulopelvic ligament and the uterine vessels, and at or below the uterine vessels.30
None of the 42 ureteral injuries were diagnosed intraoperatively. In fact, 37 of these injuries were not correctly diagnosed until more than 48 hours after surgery. Two uterovaginal fistulas were also diagnosed in the late postoperative period.30
Bladder injuries were identified via cystoscopy or cystometrogram or by the instillation of methylene blue into the bladder, with observation from above for leakage. Ureteral injuries were identified by IV pyelogram, retrograde pyelogram, or attempted passage of a stent. Every ureteral injury showed up as hydroureter and hydronephrosis via pyelography.30
Grainger and colleagues reported five ureteral injuries associated with laparoscopic procedures.31 The principal symptoms were low back pain, abdominal pain, leukocytosis, and peritonitis. All five injuries were associated with endometriosis surgery, most commonly near the uterosacral ligaments.
Grainger and colleagues cited eight additional cases of injury. Three patients among the 13 total cases lost renal function, and two eventually required nephrectomy.31
How to prevent, identify, and manage urinary tract injuries
Thorough knowledge of anatomy and meticulous technique are imperative to prevent urinary tract injuries. Strategies include:
- Use sharp rather than blunt dissection.
- Know the risk factors for urinary tract injury, which include previous cesarean delivery or intra-abdominal surgery, presence of adhesions, and deep endometriosis.
- Be aware of the dangers posed by energy devices when they are used near the bladder and ureter. Even bipolar devices can cause thermal injury.
- Employ hydrodissection when there are bladder adhesions, and work nearer the uterus or vagina than the bladder, leaving a margin of tissue.
- When the ureter’s location is unclear relative to the operative site, do not hesitate to open the retroperitoneal space to observe the ureter. If necessary, dissect the ureter distally.
- Perform cystoscopy with IV indigo carmine injection at the conclusion of surgery to ensure that the ureter is not occluded.
- Be aware that peristalsis is not an indication of ureteral integrity. In fact, an obstructed ureter will pulsate more vigorously than a normal one.
- Consider preoperative ureteral catheterization, which may avert injury without increasing operative time, blood loss, and hospital stay,32 although the data are not definitive.33
- Be vigilant. Early identification of injuries reduces morbidity. In the case of ureteral obstruction, immediate stenting will usually obviate the need for ureteral implantation and nephrostomy if the obstruction is not complete.
- Intervene early to cut an obstructing suture or relieve ureteral bowing. Doing so may eliminate the obstruction altogether in many cases.
- If a laceration is found in the bladder trigone or its vicinity, always perform ureteral catheterization to help prevent the inadvertent suturing of the intravesical ureter into the repair.
- After repair of a bladder laceration, perform cystoscopy with IV injection of indigo carmine to ensure ureteral integrity.
- Use only absorbable suture in bladder repairs. I recommend 2-0 chromic catgut for the first layer, which should encompass muscularis and mucosa. Place a second layer of sutures using 3-0 polyglactin 910 (Vicryl), imbricating the first layer.
- After completion of a bladder repair, instill a solution of diluted methylene blue (1 part methylene blue to 100 parts sterile water or saline) to distend the bladder, and carefully inspect the closure to ensure that it is watertight. Then place a Foley catheter for a minimum of 2 weeks. Four to 6 weeks after repair, perform a cystogram to ensure that healing is complete, with no leakage.
- Call a urologist if you are not well-versed in bladder repair, or if the ureter is injured (or injury is suspected).
- Watch for fistula formation, an inevitable outcome of untreated bladder and ureteral injury, which may occur early or late in the postoperative course.
Choose an approach wisely
Laparoscopy is a learned skill. Supervised practice generally leads to greater levels of proficiency, and repetition of the same operations improves dexterity and execution. However, laparoscopy is also an art—some people have the touch and some do not.
Although laparoscopic techniques offer many advantages, they also have shortcomings. The complications described here, and the strategies I have offered for preventing and managing them, should help gynecologic surgeons determine whether laparoscopy is the optimal route of operation, based on surgical experience, characteristics of the individual patient, and other variables.
Update: Minimally Invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
We want to hear from you! Tell us what you think.
How to avoid major vessel injury during gynecologic laparoscopy
(August 2012)
CASE: Adhesions complicate multiple surgeries
In early 2007, a 37-year-old woman with a history of hysterectomy, adhesiolysis, bilateral partial salpingectomy, and cholecystectomy underwent an attempted laparoscopic bilateral salpingo-oophorectomy (BSO) for pelvic pain. The operation was converted to laparotomy because of severe adhesions and required several hours to complete.
After the BSO, the patient developed hydronephrosis in her left kidney secondary to an inflammatory cyst. In March 2007, a urologist placed a ureteral stent to relieve the obstruction. One month later, the patient was referred to a gynecologic oncologist for chronic pelvic pain.
On October 29, 2007, the patient underwent operative laparoscopy for adhesiolysis and appendectomy. No retroperitoneal exploration was attempted at the time. According to the operative note, the 10-mm port incision was enlarged to 3 cm to enable the surgeon to inspect the descending colon. Postoperatively, the patient reported persistent abdominal pain and fever and was admitted to the hospital for observation. Although she had a documented temperature of 102°F on October 31, with tachypnea, tachycardia, and a white blood cell (WBC) count of 2.9 x 103/μL, she was discharged home the same day.
The next morning, the patient returned to the hospital’s emergency room (ER) reporting worsening abdominal pain and shortness of breath. Her vital signs included a temperature of 95.8°F, heart rate of 135 bpm, respiration of 32 breaths/min, and blood pressure of 100/68 mm Hg. An examination revealed a tender, distended abdomen, and the patient exhibited guarding behavior upon palpation in all quadrants. Bowel sounds were hypoactive, and the WBC count was 4.2 x 103/μL. No differential count was ordered. A computed tomography (CT) scan showed free air in the abdomen, pneumomediastinum, and subcutaneous emphysema of the abdominal wall and chest wall.
The next day, a differential WBC count revealed bands elevated at a 25% level. A cardiac consultant diagnosed heart failure and remarked that pneumomediastinum should not occur after abdominal surgery. In the evening, the gynecologic oncologist performed a laparotomy and observed enteric contents in the abdominal cavity, as well as a defect of approximately 2 mm in the lower portion of the rectosigmoid colon. According to the operative note, the gynecologic oncologist stapled off the area below the defect and performed a descending loop colostomy.
Postoperatively, the patient remained septic, and vegetable matter was recovered from one of the drains, so a surgical consultant was called. On November 9, a general surgeon performed an exploratory laparotomy and found necrosis, hemorrhage, acute inflammation of the colostomy, separation of the colostomy from its sutured position on the anterior abdominal wall, and mucosa at the end of the Hartman pouch, necessitating resection of this segment of the colon back to the rectum. Numerous intra-abdominal abscesses were also drained.
Two days later, the patient returned to the OR for further abscess drainage and creation of a left end colostomy. She was discharged 1 month later.
On January 4, 2008, she went to the ER for nausea and abdominal pain. Five days later, a plastic surgeon performed extensive skin grafting on the chronically open abdominal wound. On March 12, the patient returned to the ER because of abdominal pain and was admitted for nasogastric drainage and intravenous (IV) fluids. She returned to the ER again on April 26, reporting pain. A CT scan revealed a cystic mass in the pelvis, which was drained under CT guidance. In June and July, the patient was seen in the ER three times for pain, nausea, and vomiting.
In January 2009, she underwent another laparotomy for takedown of the colostomy, lysis of adhesions, and excision of a left 4-cm pelvic cyst (pathology later revealed the cyst to be ovarian tissue). She also underwent a left-sided myocutaneous flap reconstruction of an abdominal wall defect, and a right-sided myocutaneous flap with placement of a 16 x 20–cm sheet of AlloDerm Tissue Matrix (LifeCell). She continues to experience abdominal pain and visits the ER for that reason. In March 2009, she underwent repeat drainage of a pelvic collection via CT imaging. No further follow-up is available.
Could this catastrophic course have been avoided? What might have prevented it?
Adhesions are likely after any abdominal procedure
The biggest risk factor for laparoscopy-related intestinal injury is the presence of pelvic or abdominal adhesions.1,2 Adhesions inevitably form after any intra-abdominal surgery, and new adhesions are likely with each successive intra-abdominal procedure. Even adhesiolysis leads to the formation of adhesions postoperatively.
Few reliable data suggest that adhesions cause pelvic pain, or that adhesiolysis relieves such pain.3 Furthermore, it may be impossible to predict with reasonable probability where adhesions may be located preoperatively or to know with certainty whether a portion of the intestine is adherent to the anterior abdominal wall directly below the usual subumbilical entry site. Because of the likelihood of adhesions in a patient who has undergone two or more laparotomies, it is risky to thrust a 10- to 12-mm trocar through the anterior abdominal wall below the navel.
A few variables influence the risk of injury
The trocar used in laparoscopic procedures plays a role in the risk of bowel injury. For example, relatively dull reusable devices may push nonfixed intestine away rather than penetrate the viscus. In contrast, razor-sharp disposable devices are more likely to cut into the underlying bowel.
Body habitus is also important. The obese woman is at greater risk for entry injuries, owing to physical aspects of the fatty anterior abdominal wall. When force is applied to the wall, it moves inward, toward the posterior wall, trapping intestine. In a thin woman, the abdominal wall is less elastic, so there is less excursion upon trocar entry.
Intestinal status is another variable to consider. A collapsed bowel is unlikely to be perforated by an entry trocar, whereas a thin, distended bowel is vulnerable to injury. Bowel status can be determined preoperatively using various modalities, including radiographic studies.
Careful surgical technique is imperative. Sharp dissection is always preferable to the blunt tearing of tissue, particularly in cases involving fibrous adhesions. Tearing a dense, unyielding adhesion is likely to remove a piece of intestinal wall because the tensile strength of the adhesion is typically greater than that of the viscus itself.
Thorough knowledge of pelvic anatomy is essential. It would be particularly egregious for a surgeon to mistake an adhesion for the normal peritoneal attachments of the left and sigmoid colon, or to resect the mesentery of the small bowel, believing it to be an adhesion.
Energy devices account for a significant number of intestinal injuries (FIGURE 1). Any surgeon who utilizes an energy device is obligated to protect the patient from a thermal injury—and the manufacturers of these instruments should provide reliable data on the safe use of the device, including information about the expected zone of conductive thermal spread based on power density and tissue type. As a general rule, avoid the use of monopolar electrosurgical devices for intra-abdominal dissection.
Adhesiolysis is a risky enterprise. Several studies have found a significant likelihood of bowel injury during lysis of adhesions.4-6 In two studies by Baggish, 94% of adhesiolysis-related injuries involved moderate or severe adhesions.5,6

FIGURE 1 Use of energy devices is risky near bowel
Energy devices account for a significant number of intestinal injuries. In this figure, the arrow indicates leakage of fecal matter from the bowel defect.
Is laparoscopy the wisest approach?
It is important to weigh the risks of laparoscopy against the potential benefits for the patient. Surgical experience and skill are perhaps the most important variables to consider when deciding on an operative approach. A high volume of laparoscopic operations—performed by a gynecologic surgeon—should translate into a lower risk of injury to intra-abdominal structures.7 That is, the greater the number of cases performed, the lower the risk of injury.
Garry and colleagues conducted two parallel randomized trials comparing 1) laparoscopic and abdominal hysterectomy and 2) laparoscopic and vaginal hysterectomy as part of the eVALuate study.8 Laparoscopic hysterectomy was associated with a significantly higher rate of major complications than abdominal hysterectomy and took longer to perform. No major differences in the rate of complications were found between laparoscopic and vaginal hysterectomy.
In a review of laparoscopy-related bowel injuries, Brosens and colleagues found significant variations in the complication rate, depending on the experience of the surgeon—a 0.2% rate of access injuries for surgeons who had performed fewer than 100 procedures versus 0.06% for those who had performed more than 100 cases, and a 0.3% rate of operative injuries for surgeons who had performed fewer than 100 procedures versus 0.04% for more experienced surgeons.7
A few precautions can improve the safety of laparoscopy
If adhesions are known or suspected, primary laparoscopic entry should be planned for a site other than the infra-umbilical area. Options include:
- entry via the left hypochondrium in the midclavicular line
- an open procedure.
However, open laparoscopic entry does not always avert intestinal injury.9-11
If the anatomy is obscured once the abdomen has been entered safely, retroperitoneal dissection may be useful, particularly for exposure of the left colon. When it is unclear whether a structure to be incised is a loop of bowel or a distended, adherent oviduct, it is best to refrain from cutting it.
For adhesiolysis, traction and counter-traction are the techniques of choice. Dissection of intestine should always be parallel to the axis of the viscus. Remember, too, that the blood supply enters via the mesenteric margin of the intestine.
After any dissection involving the intestine, carefully inspect the bowel and describe that inspection in the operative report (FIGURE 2). If injury is suspected, consult a general surgeon and open the abdomen to permit thorough inspection of the intestines.
What the literature reveals about intestinal injury
Several published reports describe a large number of laparoscopic cases and the major attendant complications.12-16 A number of studies have focused on gastrointestinal (GI) complications associated with laparoscopic procedures, providing site-specific data.
Many injuries occur during entry
Vilos reported on 40 bowel injuries, of which 55% occurred during primary trocar entry (19 closed and three open entries).17
In a report on 62 GI injuries in 56 patients, Chapron and colleagues found that one-third occurred during the approach phase of the laparoscopy; they advocated creation of a pneumoperitoneum rather than direct trocar insertion.18
In a report from the Netherlands, 24 of 29 GI injuries occurred during the approach.2
In a review of 63 GI complications related to diagnostic and operative laparoscopy, 75% of injuries were associated with primary trocar insertion.19
Optical access trocars do not appear to be protective against bowel injury. One study of 79 complications associated with these devices found 24 bowel injuries.20
In addition, in two reports detailing 130 cases of small- and large-bowel perforations associated with laparoscopic procedures, Baggish found that 62 (77%) of small-bowel injuries and 20 (41%) of colonic injuries were entry-related.5,6
Energy devices can be problematic
In the study by Chapron and colleagues of 62 GI injuries, six were secondary to the use of electrosurgical devices, four of them involving monopolar instruments.18
In a study from Scotland, 27 of 117 (23%) of bowel injuries during laparoscopic procedures were attributable to a thermal event.21
Baggish found that 43% of operative injuries among 130 intestinal perforations were energy-related.5,6
Intraoperative diagnosis is optimal
Soderstrom reviewed 66 cases of laparoscopy-related bowel injuries and found three deaths attributable to a delay in diagnosis exceeding 72 hours.4
In a study by Vilos, the mean time for diagnosis of bowel injuries was 4 days (range, 0–23 days), with intraoperative diagnosis in only 35.7% of cases.17
In a Finnish nationwide analysis of laparoscopic complications, Harkki-Siren and Kurki found that small-bowel injuries were identified an average of 3.3 days after occurrence; when electrosurgery was involved in the injury, the average time to diagnosis was 4.8 days.22 As for large-bowel injuries, 44% were identified intraoperatively. In the remainder of cases, the average time from injury to diagnosis was 10.4 days for electrosurgical injuries and 1.3 days for injuries related to sharp dissection.
In the studies by Baggish, 82 of 130 (63%) intestinal injuries were diagnosed 48 hours or more after the operation.5,6
Baggish also made the following observations:
- The most common symptoms of intestinal injury were (in order of frequency) abdominal pain, bloating, nausea and vomiting, and fever or chills (or both). The most common signs were abdominal tenderness, abdominal distension, diminished bowel sounds, and elevated or subnormal temperature.
- Sepsis was apparent (due to the onset of systemic inflammatory response syndrome) in the majority of small-bowel perforations and virtually all colonic perforations. Findings of tachycardia, tachypnea, elevated leukocyte count, and bandemia suggested sepsis syndrome.
- Radiologically observed free air was often misinterpreted by the radiologist as being consistent with residual gas from the initial laparoscopy. In reality, most—if not all—CO2 gas is absorbed within 24 hours, particularly in obese women. Early CT imaging with oral contrast leads to the most expeditious, correct diagnosis, compared with flat and upright abdominal radiographs.
- Obese women did not exhibit rebound tenderness even though subsequent operative findings revealed extensive and severe peritonitis.
- When infection occurred, it usually was polymicrobial in nature. The most frequently cultured organisms include Escherichia coli, Enterococcus, alpha and beta Streptococcus, Staphylococcus, and Bacteroides.
Baggish concluded that earlier diagnosis could be achieved with careful inspection of the intestine at the conclusion of each operative procedure (FIGURE 2).
Similarly, Chapron and colleagues recommended meticulous inspection of all areas where bowel lysis has been performed. “When there is the slightest doubt, carry out tests for leakage (transanal injection of 200 mL methylene blue using a Foley catheter) in order not to overlook a rectosigmoid injury which would become apparent secondarily in a context of peritonitis,” they wrote. They also suggested that the patient be educated about the signs and symptoms of intestinal injury.18
Whenever a bowel injury is visualized intraoperatively, assume that it is transmural until it is proved otherwise.

FIGURE 2 Meticulous bowel inspection can identify perforation
It is vital to inspect the bowel after any dissection that involves the intestine, being especially alert for puncture wounds caused by a trocar and small tears associated with adhesiolysis.
SOURCE: Baggish MS, Karram MM. Atlas of Pelvic Anatomy and Gynecologic Surgery. 3rd ed. Philadelphia: Elsevier; 2011:1142.
How to avoid urinary tract injuries
Along with major vessel injury and intestinal perforation, bladder and ureteral injuries are the most common complications of laparoscopic surgery. Although urinary tract injuries are rarely fatal, they can cause a range of sequelae, including urinoma, vesicovaginal and ureterovaginal fistulas, hydroureter, hydronephrosis, renal damage, and kidney atrophy.
The incidence of ureteral injury during laparoscopy ranges from less than 0.1% to 1.0%, and the incidence of bladder injury ranges from less than 0.8% to 2.0%.23-26 Investigators in Singapore described eight urologic injuries among 485 laparoscopic hysterectomies and identified several risk factors:
- previous cesarean delivery
- multiple fibroids
- severe endometriosis.27
Another set of investigators found a history of laparotomy to be a risk factor for bladder injury during laparoscopic hysterectomy.28
Rooney and colleagues studied the effect of previous cesarean delivery on the risk of injury during hysterectomy.29 Among 5,092 hysterectomies—including 433 laparoscopic-assisted vaginal hysterectomies, 3,140 abdominal procedures, and 1,539 vaginal operations—the rate of bladder injury varied by approach. Cystotomy was observed in 0.76% of abdominal hysterectomies (33% had a previous cesarean delivery), 1.3% of vaginal procedures (21% had a previous cesarean), and 1.8% of laparoscopic operations (62.5% had a previous cesarean). The odds ratio for cystotomy during hysterectomy among women with a previous cesarean delivery was 1.26 for the abdominal approach, 3.00 for the vaginal route, and 7.50 for laparoscopic-assisted vaginal hysterectomy.29
Two studies highlight common aspects of injury
In a recent report of 75 urinary tract injuries associated with laparoscopic surgery, Baggish identified a total of 33 injuries involving the bladder and 42 of ureteral origin. Twelve of the bladder injuries were associated with the approach, and 21 were related to the surgery. In contrast, only one of the 42 ureteral injuries was related to the approach.30
Baggish also found that just under 50% of urinary tract injuries were related to the use of thermal energy, including all three vesicovaginal fistulas. Fourteen bladder lacerations occurred during separation of the bladder from the uterus during laparoscopic hysterectomy.30
Common sites of injury were at the infundibulopelvic ligament, between the infundibulopelvic ligament and the uterine vessels, and at or below the uterine vessels.30
None of the 42 ureteral injuries were diagnosed intraoperatively. In fact, 37 of these injuries were not correctly diagnosed until more than 48 hours after surgery. Two uterovaginal fistulas were also diagnosed in the late postoperative period.30
Bladder injuries were identified via cystoscopy or cystometrogram or by the instillation of methylene blue into the bladder, with observation from above for leakage. Ureteral injuries were identified by IV pyelogram, retrograde pyelogram, or attempted passage of a stent. Every ureteral injury showed up as hydroureter and hydronephrosis via pyelography.30
Grainger and colleagues reported five ureteral injuries associated with laparoscopic procedures.31 The principal symptoms were low back pain, abdominal pain, leukocytosis, and peritonitis. All five injuries were associated with endometriosis surgery, most commonly near the uterosacral ligaments.
Grainger and colleagues cited eight additional cases of injury. Three patients among the 13 total cases lost renal function, and two eventually required nephrectomy.31
How to prevent, identify, and manage urinary tract injuries
Thorough knowledge of anatomy and meticulous technique are imperative to prevent urinary tract injuries. Strategies include:
- Use sharp rather than blunt dissection.
- Know the risk factors for urinary tract injury, which include previous cesarean delivery or intra-abdominal surgery, presence of adhesions, and deep endometriosis.
- Be aware of the dangers posed by energy devices when they are used near the bladder and ureter. Even bipolar devices can cause thermal injury.
- Employ hydrodissection when there are bladder adhesions, and work nearer the uterus or vagina than the bladder, leaving a margin of tissue.
- When the ureter’s location is unclear relative to the operative site, do not hesitate to open the retroperitoneal space to observe the ureter. If necessary, dissect the ureter distally.
- Perform cystoscopy with IV indigo carmine injection at the conclusion of surgery to ensure that the ureter is not occluded.
- Be aware that peristalsis is not an indication of ureteral integrity. In fact, an obstructed ureter will pulsate more vigorously than a normal one.
- Consider preoperative ureteral catheterization, which may avert injury without increasing operative time, blood loss, and hospital stay,32 although the data are not definitive.33
- Be vigilant. Early identification of injuries reduces morbidity. In the case of ureteral obstruction, immediate stenting will usually obviate the need for ureteral implantation and nephrostomy if the obstruction is not complete.
- Intervene early to cut an obstructing suture or relieve ureteral bowing. Doing so may eliminate the obstruction altogether in many cases.
- If a laceration is found in the bladder trigone or its vicinity, always perform ureteral catheterization to help prevent the inadvertent suturing of the intravesical ureter into the repair.
- After repair of a bladder laceration, perform cystoscopy with IV injection of indigo carmine to ensure ureteral integrity.
- Use only absorbable suture in bladder repairs. I recommend 2-0 chromic catgut for the first layer, which should encompass muscularis and mucosa. Place a second layer of sutures using 3-0 polyglactin 910 (Vicryl), imbricating the first layer.
- After completion of a bladder repair, instill a solution of diluted methylene blue (1 part methylene blue to 100 parts sterile water or saline) to distend the bladder, and carefully inspect the closure to ensure that it is watertight. Then place a Foley catheter for a minimum of 2 weeks. Four to 6 weeks after repair, perform a cystogram to ensure that healing is complete, with no leakage.
- Call a urologist if you are not well-versed in bladder repair, or if the ureter is injured (or injury is suspected).
- Watch for fistula formation, an inevitable outcome of untreated bladder and ureteral injury, which may occur early or late in the postoperative course.
Choose an approach wisely
Laparoscopy is a learned skill. Supervised practice generally leads to greater levels of proficiency, and repetition of the same operations improves dexterity and execution. However, laparoscopy is also an art—some people have the touch and some do not.
Although laparoscopic techniques offer many advantages, they also have shortcomings. The complications described here, and the strategies I have offered for preventing and managing them, should help gynecologic surgeons determine whether laparoscopy is the optimal route of operation, based on surgical experience, characteristics of the individual patient, and other variables.
Update: Minimally Invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
We want to hear from you! Tell us what you think.
Ins and outs of straight-stick laparoscopic myomectomy
Watch 3 videos illustrating laparoscopic myomectomy
These videos were provided by Gaby Moawad, MD, and James Robinson, MD, MS.
By age 50, almost 70% of white women and more than 80% of black women will have a uterine leiomyoma.1 These benign, hormone-sensitive neoplasms1 are asymptomatic in the majority of women, but they can cause infertility, abnormal uterine bleeding, and bulk symptoms.2
When symptomatic, fibroids are amenable to multiple management options, ranging from expectant management to medical therapy to uterine artery embolization to myomectomy to hysterectomy. Myomectomy remains the surgical option of choice for women with symptomatic fibroids who wish to retain their fertility. It is also an option for some women who may not desire fertility retention but who do wish to maintain their uterus.
Compared with traditional myomectomy by laparotomy, laparoscopic myomectomy offers the advantages of:
- less blood loss
- less postoperative pain
- less postoperative adhesions formation
- faster recovery
- better cosmesis.3,4
Current technology makes performing laparoscopic myomectomy by either “straight-stick” or robotic assistance a viable option for most women.
In this article, we describe our technique in performing straight-stick laparoscopic myomectomy.
Preoperative evaluation: The first key to success
Laparoscopic myomectomy is an advanced, delicate, and challenging surgery. Preoperative evaluation is integral to its planning and a successful outcome.
Imaging
We recommend magnetic resonance imaging (MRI) as a standard order whenever a laparoscopic approach to myomectomy is being considered, for several reasons. First, MRI of the abdomen and pelvis with contrast allows for a precise map of the location of fibroids in relation to the myometrium and the uterine cavity. Reviewing the MRI results with the patient preoperatively gives both you and the patient a clearer picture of the challenges ahead. Patients tell us they appreciate these easier-to-understand images of their anatomy and, in cases when the decision is made to proceed abdominally, it is more clear to the patient why the decision is being made.
Surgically, the MRI helps compensate for the lack of tactile feedback when faced with deep intramural fibroids laparoscopically. The MRI also helps avoid operative surprises. Experience teaches us that, when relying on transvaginal ultrasound alone, adenomyotic regions can be identified mistakenly as fibroids. Preoperative MRI can help you avoid this discovery at the time of surgery.
A flexible office hysteroscopy serves as an adjunct to MRI for precise preoperative cavitary evaluation, especially when fibroids are present in close proximity to the endometrial cavity or the patient reports menorrhagia as a component of her symptomatology. When small submucosal fibroids exist in addition to larger fibroids, we frequently perform a combined hysteroscopic and laparoscopic approach to myomectomy.
Although bowel preparation does not diminish complications from bowel surgery or improve outcome,5 we generally use laxative suppositories the night prior to surgery to improve access to the posterior cul-de-sac and reduce bulk resulting from a sigmoid full of feces.
Preoperative laboratory evaluation should always include complete blood count, beta hCG, blood type testing, and antibody screen. In patients with known anemia or large intramural fibroids, we typically match the patient for 2 units of packed red blood cells. Additionally, if significant blood loss is anticipated, cell saver technology can be modified to accommodate a laparoscopic suction tip, allowing the patient’s own blood to be collected and readministered.
Aside from the standard risks of surgery, including bleeding, transfusion, infection, and injury to adjacent organs, myomectomy has its own unique risks that need to be made clear to patients preoperatively.
Surgery timing. Initially, women with symptomatic fibroids are at significant risk for developing more fibroids in the future. In fact, 25% of women who undergo myomectomy will require a second surgery at some point in their lives to address recurrent symptoms.1 If women are young, not yet ready to conceive, and are still relatively asymptomatic, waiting to perform myomectomy may be the most prudent course.
Future uterine rupture. There are no good myomectomy data to guide us with respect to the risk of uterine rupture at future pregnancy. When we perform deep intramural myomectomy (regardless of endometrial disruption), we extrapolate from classical cesarean section data and counsel our patients to have planned cesarean sections for all future pregnancies. Patients are also counseled that uterine rupture has been well described after laparoscopic myomectomy prior to the onset of labor so any sudden onset of pain or bleeding during the late second or third trimester of pregnancy has to be regarded as a medical emergency.
Pregnancy. Again, no good data exist to guide us with respect to postoperative timing of future pregnancy. We typically suggest patients refrain from conceiving following myomectomy for at least 6 months. We are aware that other well-respected surgeons have different thresholds.
Reference
1. Andiani GB, Fedele L, Parazzini F, Villa L. Risk of recurrence after myomectomy. Br J Obstet Gynaecol. 1991;98(4):385-389.
How to minimize blood loss
Blood loss during laparoscopic myomectomy generally is less than during laparotomy due to venous compression from pneumoperitoneum. However, blood loss remains a chief concern when performing laparoscopic myomectomy. A variety of techniques have been described to minimize blood loss, including injection of dilute vasopressin6 and tourniquet placement around uterine vessels.7
Injection. To temporarily minimize bleeding in the surgical field, we routinely utilize subserosal injection of dilute vasopressin (20 IU in 100 mL of normal saline) until visible vessels blanch. This practice is more effective than deep myoma or myometrial injection.
Tourniquet. We selectively use a laparoscopically placed tourniquet to compress uterine arteries at the mid-cervix during surgery. One approach is as follows (see VIDEO 1):
- Open windows in bilateral broad ligaments lateral to the uterine pedicles and medial to the ureters.
- Pass the end of a 14-16 French red rubber catheter through one of the low lateral port sites with the port removed.
- Tag the trailing end of the tourniquet outside the abdomen and replace the port alongside the catheter.
- Pass the end of the catheter down through the ipsilateral broad ligament window and under the posterior cervix.
- Pass the end of the catheter up through the contralateral broad ligament window and over the anterior cervix.
- Pass the end of the catheter through each of the broad ligament windows a second time and then out through the contralateral port site.
- Pull the tourniquet tight from both port sites (which will occlude the uterine arteries). Place Kelly clamps on the catheter ends where they exit the port sites to maintain tension until the end of the uterine repair.
Lateral ports can still be utilized with the tourniquet in place.
Permanent occlusion. In women undergoing laparoscopic myomectomy who have completed child bearing, we advocate permanent uterine artery occlusion at the origin of the uterine arteries retroperitoneally. This can be performed in a number of ways— utilizing clips, suture, or transection. Uterine artery occlusion not only leads to less operative blood loss but preliminary studies also suggest it decreases the risk of fibroid recurrence.8
After the patient is prepped and draped in low lithotomy position with her arms tucked at her sides, drain the bladder with an indwelling catheter. Insert a uterine manipulator, such as the VCare (Conmed Corporation), into the uterus.
Obtain umbilical entry for a 30° optic scope, and place the patient in steep Trendelenburg position. We use two 5-mm lateral ports and one 12-mm suprapubic port. The level of placement of the lateral ports is tailored to the size of the fibroids; it can be anywhere from the level of the anterior iliac spine to the level of the umbilicus for fibroids contained in the pelvis or in the abdomen, respectively.
Uterine incision
After vasopressin injection, tourniquet placement, or permanent uterine artery occlusion is performed as described above, we advocate a transverse uterine incision. We do so mainly because:
- The transverse incision runs parallel to the arcuate vessels of the myometrium, leading to less bleeding.
- We suture from the lateral ports so the transverse incision facilitates a more ergonomic repair.
Perform the uterine incision (we use the Harmonic Ace [Ethicon Endo-Surgery, Inc]) through the uterine serosa deep toward the myoma. Incision size should be appropriate to the diameter of the fibroid; smaller incisions result in unnecessary struggling during enucleation of the fibroid. Incision depth should reach the fibroid capsule, and this incision should be developed over the entire fibroid. Tunneling in the myometrium is undesirable and should be avoided because it increases myometrial injury as well as the risk of hematoma.
Fibroid enucleation
Once the initial uterine incision is complete, enucleate the fibroid using a combination of traction, countertraction, sharp, and blunt dissection (see VIDEO 2). Pearls to successful enucleation include:
- Maintain traction and countertraction when cutting tissue. This helps to identify appropriate planes and allows tissue to separate quickly, minimizing thermal energy spread.
- Replace the tenaculum or myoma screw regularly at the border of the myoma and myometrium. The ultrasonic scalpel blade can be drilled into the myoma in order to create traction on the myoma.
- Bluntly peel tissue from the myoma outward. Ideally all myometrium and vessels should stay with the uterus. A properly enucleated fibroid will be pearly in appearance and avascular.
- Be particularly careful when in contact with the endometrium. Even submucous fibroids can be enucleated regularly without entering the endometrial cavity.
Uterine repair
If the endometrial cavity is inadvertently entered, close the defect (we use a 2-0 monocryl or Vicryl suture). Next, imbricate the endometrium over with successive layers, taking special care not to pass a needle into the endometrial cavity. In cases in which significant endometrial disruption cannot be avoided, use a postoperative intrauterine balloon stent. This is placed postoperatively and left in place for 2 weeks. To stimulate endometrial proliferation, we prescribe oral estradiol 1 mg twice per day for 4 weeks. Following endometrial stimulation, we prescribe a 10-day progestin withdrawal and ask the patient to return to the office for a flexible diagnostic hysteroscopy following her first menses to ensure cavitary integrity.
Once fibroid enucleation is complete, perform a multilayer closure of the defect using an absorbable, unidirectional barbed suture (V-Loc, Covidien). Eliminate all dead space in the closure. The last two throws of each barbed suture should be in the direction of the prior two throws to secure the suture. Finally, cut the suture at the tissue edge without leaving any trailing tail or knot.
Why use a barbed suture? The advantages of using absorbable barbed suture include:
- elimination of knot tying
- shorter closure time
- better tension distribution throughout the wound.
Close the seromuscularis layer in a hemostatic baseball stitch fashion to minimize suture exposure and subsequent adhesions (FIGURE). Use of the suprapubic port for the needle retrieval device facilitates placement of the alternating “inside-out” baseball stitch (see VIDEO 3).

FIGURE Use of a hemostatic baseball stitch (with absorbable, unidirectional barbed suture) to close the seromuscularis layer of the uterus, thereby minimizing suture exposure and subsequent adhesions.
Morcellation
Mechanically morcellate the fibroids through the suprapubic port site. We use an electrical morcellator (Karl Storz Endoscopy). In cases of massive fibroids (>15 cm) we utilize cold- knife morcellation with a # 10 scalpel through an extended 3-cm suprapubic incision with a vertical fascial incision protected by a self-retaining wound retractor. It is important to be vigilant to remove all fibroid pieces as postoperative disseminated leiomyomatosis is well described.
Adhesion prevention
Myomectomy is notorious for creating dense and challenging postoperative adhesions. Given the high rate of repeat surgery for patients undergoing the procedure, anything you can do to limit the adhesion load will be appreciated by both your patient and her next surgeon. Without exception, the most important adhesion-prevention strategy is meticulous attention to tissue handling and hemostasis. In general, laparoscopy leads to fewer adhesions than laparotomy, but a bloody field and raw uterine serosa will create an environment ripe for adhesions regardless of surgical approach. If the operative field is dry, use commercial adhesion prevention aids according to manufacturers’ recommendations.
- Use preoperative MRI to tailor your surgical approach
- When menorrhagia is a presenting symptom, assess the endometrial cavity preoperatively and consider combining the laparoscopic myomectomy with hysteroscopic myomectomy
- Minimize blood loss with vasopressin or a laparoscopic tourniquet
- Utilize a transverse uterine incision and a lateral suturing technique
- Use delayed absorbable barbed suture to close the myometrial defect
- Bury the seromuscular closure suture by utilizing an “inside-out” baseball stitch
- If the risk for postoperative cavitary adhesions is high, consider postoperative balloon placement with close postoperative follow-up
- Advise patients to wait 6 months prior to attempting to conceive and have a low threshold for scheduled cesarean delivery to minimize the risk of uterine rupture
Concluding thoughts, from experience
Laparoscopic myomectomy is a challenging yet rewarding procedure. For essential points to our approach, see “Laparoscopic myomectomy: Key takeaways” on this page.
Other important things to keep in mind:
- Fibroid presentation is as varied as the women who have them—meticulous preoperative preparation is an absolute must.
- Utilize well-established approaches to preventing blood loss, removing fibroids, and repairing the uterine defects. The accomplished gynecologic laparoscopist will be successful in the majority of cases.
- Practice suturing in a box-trainer setting before taking on initial cases. Early cases should focus on straightforward subserosal fibroids and, as skills progress, more and more difficult cases will become reasonable.
- Do not place any hard and fast limit on either the number or size of fibroids you are willing to remove laparoscopically. Rather, rely on sound surgical judgment, an honest assessment of your limitations, and a healthy dose of caution as you approach every new patient. Never sacrifice the quality of your repair for a less invasive approach to surgery.
Laparoscopic myomectomy: 8 pearls
Jon I. Einarsson, MD, MPH (March 2010)
Give vasopressin to reduce bleeding in gynecologic surgery
Robert L. Barbieri, MD (Editorial, March 2010)
Barbed suture, now in the toolbox of minimally invasive gyn surgery
Jon I. Einarsson, MD, MPH; James A. Greenberg, MD (September 2009)
When necessity calls for treating uterine fibroids
William H. Parker, MD (Surgical Techniques, June 2008)
Advising your patients–Uterine fibroids: Childbearing, cancer, and hormone effects
William H. Parker, MD (May 2008)
We want to hear from you! Tell us what you think.
1. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100-107.
2. Wallach EE, Vlahos NF. Uterine myomas: an overview of development clinical features, and management. Obstet Gynecol. 2004;104(2):393-406.
3. Stringer NH, Walker JC, Meyer PM. Comparison of 49 laparoscopic myomec- tomies and 49 open myomectomies. J Am Assoc Gynecol Laparosc. 1997;4(4):457-464.
4. Bulletti C, Polli V, Negrini V, Giacomucci E, Flamigni C. Adhesion formation after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1996;3(4):533-536.
5. Ram E, Sherman Y, Weil R, Vishne T, Kravarusic D, Dreznik Z. Is mechanical bowel preparation mandatory for elective colon surgery? A prospective randomized study. Arch Surg. 2005;140(3):285-288.
6. Frederick J, Fletcher H, Simeon D, Mullings A, Hardie M. Intramyometrial vasopressin as a haemostatic agent during myomectomy. Br J Obstet Gynaecol. 1994;101(5):435-437.
7. Taylor A, Sharma M, Tsirkas P, Di Spiezio Sardo A, Setchell M, Magos A. Reducing blood loss at open myomectomy using triple tourniquets: a randomised controlled trial. BJOG. 2005;112(3):340-345.
8. Burbank F, Hutchins FL, Jr. Uterine artery occlusion by embolization or surgery for the treatment of fibroids: A unifying hypothesis-transient uterine ischemia. J Am Assoc Gynecol Laparosc. 2000;7(suppl 4):S1-S49.
Watch 3 videos illustrating laparoscopic myomectomy
These videos were provided by Gaby Moawad, MD, and James Robinson, MD, MS.
By age 50, almost 70% of white women and more than 80% of black women will have a uterine leiomyoma.1 These benign, hormone-sensitive neoplasms1 are asymptomatic in the majority of women, but they can cause infertility, abnormal uterine bleeding, and bulk symptoms.2
When symptomatic, fibroids are amenable to multiple management options, ranging from expectant management to medical therapy to uterine artery embolization to myomectomy to hysterectomy. Myomectomy remains the surgical option of choice for women with symptomatic fibroids who wish to retain their fertility. It is also an option for some women who may not desire fertility retention but who do wish to maintain their uterus.
Compared with traditional myomectomy by laparotomy, laparoscopic myomectomy offers the advantages of:
- less blood loss
- less postoperative pain
- less postoperative adhesions formation
- faster recovery
- better cosmesis.3,4
Current technology makes performing laparoscopic myomectomy by either “straight-stick” or robotic assistance a viable option for most women.
In this article, we describe our technique in performing straight-stick laparoscopic myomectomy.
Preoperative evaluation: The first key to success
Laparoscopic myomectomy is an advanced, delicate, and challenging surgery. Preoperative evaluation is integral to its planning and a successful outcome.
Imaging
We recommend magnetic resonance imaging (MRI) as a standard order whenever a laparoscopic approach to myomectomy is being considered, for several reasons. First, MRI of the abdomen and pelvis with contrast allows for a precise map of the location of fibroids in relation to the myometrium and the uterine cavity. Reviewing the MRI results with the patient preoperatively gives both you and the patient a clearer picture of the challenges ahead. Patients tell us they appreciate these easier-to-understand images of their anatomy and, in cases when the decision is made to proceed abdominally, it is more clear to the patient why the decision is being made.
Surgically, the MRI helps compensate for the lack of tactile feedback when faced with deep intramural fibroids laparoscopically. The MRI also helps avoid operative surprises. Experience teaches us that, when relying on transvaginal ultrasound alone, adenomyotic regions can be identified mistakenly as fibroids. Preoperative MRI can help you avoid this discovery at the time of surgery.
A flexible office hysteroscopy serves as an adjunct to MRI for precise preoperative cavitary evaluation, especially when fibroids are present in close proximity to the endometrial cavity or the patient reports menorrhagia as a component of her symptomatology. When small submucosal fibroids exist in addition to larger fibroids, we frequently perform a combined hysteroscopic and laparoscopic approach to myomectomy.
Although bowel preparation does not diminish complications from bowel surgery or improve outcome,5 we generally use laxative suppositories the night prior to surgery to improve access to the posterior cul-de-sac and reduce bulk resulting from a sigmoid full of feces.
Preoperative laboratory evaluation should always include complete blood count, beta hCG, blood type testing, and antibody screen. In patients with known anemia or large intramural fibroids, we typically match the patient for 2 units of packed red blood cells. Additionally, if significant blood loss is anticipated, cell saver technology can be modified to accommodate a laparoscopic suction tip, allowing the patient’s own blood to be collected and readministered.
Aside from the standard risks of surgery, including bleeding, transfusion, infection, and injury to adjacent organs, myomectomy has its own unique risks that need to be made clear to patients preoperatively.
Surgery timing. Initially, women with symptomatic fibroids are at significant risk for developing more fibroids in the future. In fact, 25% of women who undergo myomectomy will require a second surgery at some point in their lives to address recurrent symptoms.1 If women are young, not yet ready to conceive, and are still relatively asymptomatic, waiting to perform myomectomy may be the most prudent course.
Future uterine rupture. There are no good myomectomy data to guide us with respect to the risk of uterine rupture at future pregnancy. When we perform deep intramural myomectomy (regardless of endometrial disruption), we extrapolate from classical cesarean section data and counsel our patients to have planned cesarean sections for all future pregnancies. Patients are also counseled that uterine rupture has been well described after laparoscopic myomectomy prior to the onset of labor so any sudden onset of pain or bleeding during the late second or third trimester of pregnancy has to be regarded as a medical emergency.
Pregnancy. Again, no good data exist to guide us with respect to postoperative timing of future pregnancy. We typically suggest patients refrain from conceiving following myomectomy for at least 6 months. We are aware that other well-respected surgeons have different thresholds.
Reference
1. Andiani GB, Fedele L, Parazzini F, Villa L. Risk of recurrence after myomectomy. Br J Obstet Gynaecol. 1991;98(4):385-389.
How to minimize blood loss
Blood loss during laparoscopic myomectomy generally is less than during laparotomy due to venous compression from pneumoperitoneum. However, blood loss remains a chief concern when performing laparoscopic myomectomy. A variety of techniques have been described to minimize blood loss, including injection of dilute vasopressin6 and tourniquet placement around uterine vessels.7
Injection. To temporarily minimize bleeding in the surgical field, we routinely utilize subserosal injection of dilute vasopressin (20 IU in 100 mL of normal saline) until visible vessels blanch. This practice is more effective than deep myoma or myometrial injection.
Tourniquet. We selectively use a laparoscopically placed tourniquet to compress uterine arteries at the mid-cervix during surgery. One approach is as follows (see VIDEO 1):
- Open windows in bilateral broad ligaments lateral to the uterine pedicles and medial to the ureters.
- Pass the end of a 14-16 French red rubber catheter through one of the low lateral port sites with the port removed.
- Tag the trailing end of the tourniquet outside the abdomen and replace the port alongside the catheter.
- Pass the end of the catheter down through the ipsilateral broad ligament window and under the posterior cervix.
- Pass the end of the catheter up through the contralateral broad ligament window and over the anterior cervix.
- Pass the end of the catheter through each of the broad ligament windows a second time and then out through the contralateral port site.
- Pull the tourniquet tight from both port sites (which will occlude the uterine arteries). Place Kelly clamps on the catheter ends where they exit the port sites to maintain tension until the end of the uterine repair.
Lateral ports can still be utilized with the tourniquet in place.
Permanent occlusion. In women undergoing laparoscopic myomectomy who have completed child bearing, we advocate permanent uterine artery occlusion at the origin of the uterine arteries retroperitoneally. This can be performed in a number of ways— utilizing clips, suture, or transection. Uterine artery occlusion not only leads to less operative blood loss but preliminary studies also suggest it decreases the risk of fibroid recurrence.8
After the patient is prepped and draped in low lithotomy position with her arms tucked at her sides, drain the bladder with an indwelling catheter. Insert a uterine manipulator, such as the VCare (Conmed Corporation), into the uterus.
Obtain umbilical entry for a 30° optic scope, and place the patient in steep Trendelenburg position. We use two 5-mm lateral ports and one 12-mm suprapubic port. The level of placement of the lateral ports is tailored to the size of the fibroids; it can be anywhere from the level of the anterior iliac spine to the level of the umbilicus for fibroids contained in the pelvis or in the abdomen, respectively.
Uterine incision
After vasopressin injection, tourniquet placement, or permanent uterine artery occlusion is performed as described above, we advocate a transverse uterine incision. We do so mainly because:
- The transverse incision runs parallel to the arcuate vessels of the myometrium, leading to less bleeding.
- We suture from the lateral ports so the transverse incision facilitates a more ergonomic repair.
Perform the uterine incision (we use the Harmonic Ace [Ethicon Endo-Surgery, Inc]) through the uterine serosa deep toward the myoma. Incision size should be appropriate to the diameter of the fibroid; smaller incisions result in unnecessary struggling during enucleation of the fibroid. Incision depth should reach the fibroid capsule, and this incision should be developed over the entire fibroid. Tunneling in the myometrium is undesirable and should be avoided because it increases myometrial injury as well as the risk of hematoma.
Fibroid enucleation
Once the initial uterine incision is complete, enucleate the fibroid using a combination of traction, countertraction, sharp, and blunt dissection (see VIDEO 2). Pearls to successful enucleation include:
- Maintain traction and countertraction when cutting tissue. This helps to identify appropriate planes and allows tissue to separate quickly, minimizing thermal energy spread.
- Replace the tenaculum or myoma screw regularly at the border of the myoma and myometrium. The ultrasonic scalpel blade can be drilled into the myoma in order to create traction on the myoma.
- Bluntly peel tissue from the myoma outward. Ideally all myometrium and vessels should stay with the uterus. A properly enucleated fibroid will be pearly in appearance and avascular.
- Be particularly careful when in contact with the endometrium. Even submucous fibroids can be enucleated regularly without entering the endometrial cavity.
Uterine repair
If the endometrial cavity is inadvertently entered, close the defect (we use a 2-0 monocryl or Vicryl suture). Next, imbricate the endometrium over with successive layers, taking special care not to pass a needle into the endometrial cavity. In cases in which significant endometrial disruption cannot be avoided, use a postoperative intrauterine balloon stent. This is placed postoperatively and left in place for 2 weeks. To stimulate endometrial proliferation, we prescribe oral estradiol 1 mg twice per day for 4 weeks. Following endometrial stimulation, we prescribe a 10-day progestin withdrawal and ask the patient to return to the office for a flexible diagnostic hysteroscopy following her first menses to ensure cavitary integrity.
Once fibroid enucleation is complete, perform a multilayer closure of the defect using an absorbable, unidirectional barbed suture (V-Loc, Covidien). Eliminate all dead space in the closure. The last two throws of each barbed suture should be in the direction of the prior two throws to secure the suture. Finally, cut the suture at the tissue edge without leaving any trailing tail or knot.
Why use a barbed suture? The advantages of using absorbable barbed suture include:
- elimination of knot tying
- shorter closure time
- better tension distribution throughout the wound.
Close the seromuscularis layer in a hemostatic baseball stitch fashion to minimize suture exposure and subsequent adhesions (FIGURE). Use of the suprapubic port for the needle retrieval device facilitates placement of the alternating “inside-out” baseball stitch (see VIDEO 3).

FIGURE Use of a hemostatic baseball stitch (with absorbable, unidirectional barbed suture) to close the seromuscularis layer of the uterus, thereby minimizing suture exposure and subsequent adhesions.
Morcellation
Mechanically morcellate the fibroids through the suprapubic port site. We use an electrical morcellator (Karl Storz Endoscopy). In cases of massive fibroids (>15 cm) we utilize cold- knife morcellation with a # 10 scalpel through an extended 3-cm suprapubic incision with a vertical fascial incision protected by a self-retaining wound retractor. It is important to be vigilant to remove all fibroid pieces as postoperative disseminated leiomyomatosis is well described.
Adhesion prevention
Myomectomy is notorious for creating dense and challenging postoperative adhesions. Given the high rate of repeat surgery for patients undergoing the procedure, anything you can do to limit the adhesion load will be appreciated by both your patient and her next surgeon. Without exception, the most important adhesion-prevention strategy is meticulous attention to tissue handling and hemostasis. In general, laparoscopy leads to fewer adhesions than laparotomy, but a bloody field and raw uterine serosa will create an environment ripe for adhesions regardless of surgical approach. If the operative field is dry, use commercial adhesion prevention aids according to manufacturers’ recommendations.
- Use preoperative MRI to tailor your surgical approach
- When menorrhagia is a presenting symptom, assess the endometrial cavity preoperatively and consider combining the laparoscopic myomectomy with hysteroscopic myomectomy
- Minimize blood loss with vasopressin or a laparoscopic tourniquet
- Utilize a transverse uterine incision and a lateral suturing technique
- Use delayed absorbable barbed suture to close the myometrial defect
- Bury the seromuscular closure suture by utilizing an “inside-out” baseball stitch
- If the risk for postoperative cavitary adhesions is high, consider postoperative balloon placement with close postoperative follow-up
- Advise patients to wait 6 months prior to attempting to conceive and have a low threshold for scheduled cesarean delivery to minimize the risk of uterine rupture
Concluding thoughts, from experience
Laparoscopic myomectomy is a challenging yet rewarding procedure. For essential points to our approach, see “Laparoscopic myomectomy: Key takeaways” on this page.
Other important things to keep in mind:
- Fibroid presentation is as varied as the women who have them—meticulous preoperative preparation is an absolute must.
- Utilize well-established approaches to preventing blood loss, removing fibroids, and repairing the uterine defects. The accomplished gynecologic laparoscopist will be successful in the majority of cases.
- Practice suturing in a box-trainer setting before taking on initial cases. Early cases should focus on straightforward subserosal fibroids and, as skills progress, more and more difficult cases will become reasonable.
- Do not place any hard and fast limit on either the number or size of fibroids you are willing to remove laparoscopically. Rather, rely on sound surgical judgment, an honest assessment of your limitations, and a healthy dose of caution as you approach every new patient. Never sacrifice the quality of your repair for a less invasive approach to surgery.
Laparoscopic myomectomy: 8 pearls
Jon I. Einarsson, MD, MPH (March 2010)
Give vasopressin to reduce bleeding in gynecologic surgery
Robert L. Barbieri, MD (Editorial, March 2010)
Barbed suture, now in the toolbox of minimally invasive gyn surgery
Jon I. Einarsson, MD, MPH; James A. Greenberg, MD (September 2009)
When necessity calls for treating uterine fibroids
William H. Parker, MD (Surgical Techniques, June 2008)
Advising your patients–Uterine fibroids: Childbearing, cancer, and hormone effects
William H. Parker, MD (May 2008)
We want to hear from you! Tell us what you think.
Watch 3 videos illustrating laparoscopic myomectomy
These videos were provided by Gaby Moawad, MD, and James Robinson, MD, MS.
By age 50, almost 70% of white women and more than 80% of black women will have a uterine leiomyoma.1 These benign, hormone-sensitive neoplasms1 are asymptomatic in the majority of women, but they can cause infertility, abnormal uterine bleeding, and bulk symptoms.2
When symptomatic, fibroids are amenable to multiple management options, ranging from expectant management to medical therapy to uterine artery embolization to myomectomy to hysterectomy. Myomectomy remains the surgical option of choice for women with symptomatic fibroids who wish to retain their fertility. It is also an option for some women who may not desire fertility retention but who do wish to maintain their uterus.
Compared with traditional myomectomy by laparotomy, laparoscopic myomectomy offers the advantages of:
- less blood loss
- less postoperative pain
- less postoperative adhesions formation
- faster recovery
- better cosmesis.3,4
Current technology makes performing laparoscopic myomectomy by either “straight-stick” or robotic assistance a viable option for most women.
In this article, we describe our technique in performing straight-stick laparoscopic myomectomy.
Preoperative evaluation: The first key to success
Laparoscopic myomectomy is an advanced, delicate, and challenging surgery. Preoperative evaluation is integral to its planning and a successful outcome.
Imaging
We recommend magnetic resonance imaging (MRI) as a standard order whenever a laparoscopic approach to myomectomy is being considered, for several reasons. First, MRI of the abdomen and pelvis with contrast allows for a precise map of the location of fibroids in relation to the myometrium and the uterine cavity. Reviewing the MRI results with the patient preoperatively gives both you and the patient a clearer picture of the challenges ahead. Patients tell us they appreciate these easier-to-understand images of their anatomy and, in cases when the decision is made to proceed abdominally, it is more clear to the patient why the decision is being made.
Surgically, the MRI helps compensate for the lack of tactile feedback when faced with deep intramural fibroids laparoscopically. The MRI also helps avoid operative surprises. Experience teaches us that, when relying on transvaginal ultrasound alone, adenomyotic regions can be identified mistakenly as fibroids. Preoperative MRI can help you avoid this discovery at the time of surgery.
A flexible office hysteroscopy serves as an adjunct to MRI for precise preoperative cavitary evaluation, especially when fibroids are present in close proximity to the endometrial cavity or the patient reports menorrhagia as a component of her symptomatology. When small submucosal fibroids exist in addition to larger fibroids, we frequently perform a combined hysteroscopic and laparoscopic approach to myomectomy.
Although bowel preparation does not diminish complications from bowel surgery or improve outcome,5 we generally use laxative suppositories the night prior to surgery to improve access to the posterior cul-de-sac and reduce bulk resulting from a sigmoid full of feces.
Preoperative laboratory evaluation should always include complete blood count, beta hCG, blood type testing, and antibody screen. In patients with known anemia or large intramural fibroids, we typically match the patient for 2 units of packed red blood cells. Additionally, if significant blood loss is anticipated, cell saver technology can be modified to accommodate a laparoscopic suction tip, allowing the patient’s own blood to be collected and readministered.
Aside from the standard risks of surgery, including bleeding, transfusion, infection, and injury to adjacent organs, myomectomy has its own unique risks that need to be made clear to patients preoperatively.
Surgery timing. Initially, women with symptomatic fibroids are at significant risk for developing more fibroids in the future. In fact, 25% of women who undergo myomectomy will require a second surgery at some point in their lives to address recurrent symptoms.1 If women are young, not yet ready to conceive, and are still relatively asymptomatic, waiting to perform myomectomy may be the most prudent course.
Future uterine rupture. There are no good myomectomy data to guide us with respect to the risk of uterine rupture at future pregnancy. When we perform deep intramural myomectomy (regardless of endometrial disruption), we extrapolate from classical cesarean section data and counsel our patients to have planned cesarean sections for all future pregnancies. Patients are also counseled that uterine rupture has been well described after laparoscopic myomectomy prior to the onset of labor so any sudden onset of pain or bleeding during the late second or third trimester of pregnancy has to be regarded as a medical emergency.
Pregnancy. Again, no good data exist to guide us with respect to postoperative timing of future pregnancy. We typically suggest patients refrain from conceiving following myomectomy for at least 6 months. We are aware that other well-respected surgeons have different thresholds.
Reference
1. Andiani GB, Fedele L, Parazzini F, Villa L. Risk of recurrence after myomectomy. Br J Obstet Gynaecol. 1991;98(4):385-389.
How to minimize blood loss
Blood loss during laparoscopic myomectomy generally is less than during laparotomy due to venous compression from pneumoperitoneum. However, blood loss remains a chief concern when performing laparoscopic myomectomy. A variety of techniques have been described to minimize blood loss, including injection of dilute vasopressin6 and tourniquet placement around uterine vessels.7
Injection. To temporarily minimize bleeding in the surgical field, we routinely utilize subserosal injection of dilute vasopressin (20 IU in 100 mL of normal saline) until visible vessels blanch. This practice is more effective than deep myoma or myometrial injection.
Tourniquet. We selectively use a laparoscopically placed tourniquet to compress uterine arteries at the mid-cervix during surgery. One approach is as follows (see VIDEO 1):
- Open windows in bilateral broad ligaments lateral to the uterine pedicles and medial to the ureters.
- Pass the end of a 14-16 French red rubber catheter through one of the low lateral port sites with the port removed.
- Tag the trailing end of the tourniquet outside the abdomen and replace the port alongside the catheter.
- Pass the end of the catheter down through the ipsilateral broad ligament window and under the posterior cervix.
- Pass the end of the catheter up through the contralateral broad ligament window and over the anterior cervix.
- Pass the end of the catheter through each of the broad ligament windows a second time and then out through the contralateral port site.
- Pull the tourniquet tight from both port sites (which will occlude the uterine arteries). Place Kelly clamps on the catheter ends where they exit the port sites to maintain tension until the end of the uterine repair.
Lateral ports can still be utilized with the tourniquet in place.
Permanent occlusion. In women undergoing laparoscopic myomectomy who have completed child bearing, we advocate permanent uterine artery occlusion at the origin of the uterine arteries retroperitoneally. This can be performed in a number of ways— utilizing clips, suture, or transection. Uterine artery occlusion not only leads to less operative blood loss but preliminary studies also suggest it decreases the risk of fibroid recurrence.8
After the patient is prepped and draped in low lithotomy position with her arms tucked at her sides, drain the bladder with an indwelling catheter. Insert a uterine manipulator, such as the VCare (Conmed Corporation), into the uterus.
Obtain umbilical entry for a 30° optic scope, and place the patient in steep Trendelenburg position. We use two 5-mm lateral ports and one 12-mm suprapubic port. The level of placement of the lateral ports is tailored to the size of the fibroids; it can be anywhere from the level of the anterior iliac spine to the level of the umbilicus for fibroids contained in the pelvis or in the abdomen, respectively.
Uterine incision
After vasopressin injection, tourniquet placement, or permanent uterine artery occlusion is performed as described above, we advocate a transverse uterine incision. We do so mainly because:
- The transverse incision runs parallel to the arcuate vessels of the myometrium, leading to less bleeding.
- We suture from the lateral ports so the transverse incision facilitates a more ergonomic repair.
Perform the uterine incision (we use the Harmonic Ace [Ethicon Endo-Surgery, Inc]) through the uterine serosa deep toward the myoma. Incision size should be appropriate to the diameter of the fibroid; smaller incisions result in unnecessary struggling during enucleation of the fibroid. Incision depth should reach the fibroid capsule, and this incision should be developed over the entire fibroid. Tunneling in the myometrium is undesirable and should be avoided because it increases myometrial injury as well as the risk of hematoma.
Fibroid enucleation
Once the initial uterine incision is complete, enucleate the fibroid using a combination of traction, countertraction, sharp, and blunt dissection (see VIDEO 2). Pearls to successful enucleation include:
- Maintain traction and countertraction when cutting tissue. This helps to identify appropriate planes and allows tissue to separate quickly, minimizing thermal energy spread.
- Replace the tenaculum or myoma screw regularly at the border of the myoma and myometrium. The ultrasonic scalpel blade can be drilled into the myoma in order to create traction on the myoma.
- Bluntly peel tissue from the myoma outward. Ideally all myometrium and vessels should stay with the uterus. A properly enucleated fibroid will be pearly in appearance and avascular.
- Be particularly careful when in contact with the endometrium. Even submucous fibroids can be enucleated regularly without entering the endometrial cavity.
Uterine repair
If the endometrial cavity is inadvertently entered, close the defect (we use a 2-0 monocryl or Vicryl suture). Next, imbricate the endometrium over with successive layers, taking special care not to pass a needle into the endometrial cavity. In cases in which significant endometrial disruption cannot be avoided, use a postoperative intrauterine balloon stent. This is placed postoperatively and left in place for 2 weeks. To stimulate endometrial proliferation, we prescribe oral estradiol 1 mg twice per day for 4 weeks. Following endometrial stimulation, we prescribe a 10-day progestin withdrawal and ask the patient to return to the office for a flexible diagnostic hysteroscopy following her first menses to ensure cavitary integrity.
Once fibroid enucleation is complete, perform a multilayer closure of the defect using an absorbable, unidirectional barbed suture (V-Loc, Covidien). Eliminate all dead space in the closure. The last two throws of each barbed suture should be in the direction of the prior two throws to secure the suture. Finally, cut the suture at the tissue edge without leaving any trailing tail or knot.
Why use a barbed suture? The advantages of using absorbable barbed suture include:
- elimination of knot tying
- shorter closure time
- better tension distribution throughout the wound.
Close the seromuscularis layer in a hemostatic baseball stitch fashion to minimize suture exposure and subsequent adhesions (FIGURE). Use of the suprapubic port for the needle retrieval device facilitates placement of the alternating “inside-out” baseball stitch (see VIDEO 3).

FIGURE Use of a hemostatic baseball stitch (with absorbable, unidirectional barbed suture) to close the seromuscularis layer of the uterus, thereby minimizing suture exposure and subsequent adhesions.
Morcellation
Mechanically morcellate the fibroids through the suprapubic port site. We use an electrical morcellator (Karl Storz Endoscopy). In cases of massive fibroids (>15 cm) we utilize cold- knife morcellation with a # 10 scalpel through an extended 3-cm suprapubic incision with a vertical fascial incision protected by a self-retaining wound retractor. It is important to be vigilant to remove all fibroid pieces as postoperative disseminated leiomyomatosis is well described.
Adhesion prevention
Myomectomy is notorious for creating dense and challenging postoperative adhesions. Given the high rate of repeat surgery for patients undergoing the procedure, anything you can do to limit the adhesion load will be appreciated by both your patient and her next surgeon. Without exception, the most important adhesion-prevention strategy is meticulous attention to tissue handling and hemostasis. In general, laparoscopy leads to fewer adhesions than laparotomy, but a bloody field and raw uterine serosa will create an environment ripe for adhesions regardless of surgical approach. If the operative field is dry, use commercial adhesion prevention aids according to manufacturers’ recommendations.
- Use preoperative MRI to tailor your surgical approach
- When menorrhagia is a presenting symptom, assess the endometrial cavity preoperatively and consider combining the laparoscopic myomectomy with hysteroscopic myomectomy
- Minimize blood loss with vasopressin or a laparoscopic tourniquet
- Utilize a transverse uterine incision and a lateral suturing technique
- Use delayed absorbable barbed suture to close the myometrial defect
- Bury the seromuscular closure suture by utilizing an “inside-out” baseball stitch
- If the risk for postoperative cavitary adhesions is high, consider postoperative balloon placement with close postoperative follow-up
- Advise patients to wait 6 months prior to attempting to conceive and have a low threshold for scheduled cesarean delivery to minimize the risk of uterine rupture
Concluding thoughts, from experience
Laparoscopic myomectomy is a challenging yet rewarding procedure. For essential points to our approach, see “Laparoscopic myomectomy: Key takeaways” on this page.
Other important things to keep in mind:
- Fibroid presentation is as varied as the women who have them—meticulous preoperative preparation is an absolute must.
- Utilize well-established approaches to preventing blood loss, removing fibroids, and repairing the uterine defects. The accomplished gynecologic laparoscopist will be successful in the majority of cases.
- Practice suturing in a box-trainer setting before taking on initial cases. Early cases should focus on straightforward subserosal fibroids and, as skills progress, more and more difficult cases will become reasonable.
- Do not place any hard and fast limit on either the number or size of fibroids you are willing to remove laparoscopically. Rather, rely on sound surgical judgment, an honest assessment of your limitations, and a healthy dose of caution as you approach every new patient. Never sacrifice the quality of your repair for a less invasive approach to surgery.
Laparoscopic myomectomy: 8 pearls
Jon I. Einarsson, MD, MPH (March 2010)
Give vasopressin to reduce bleeding in gynecologic surgery
Robert L. Barbieri, MD (Editorial, March 2010)
Barbed suture, now in the toolbox of minimally invasive gyn surgery
Jon I. Einarsson, MD, MPH; James A. Greenberg, MD (September 2009)
When necessity calls for treating uterine fibroids
William H. Parker, MD (Surgical Techniques, June 2008)
Advising your patients–Uterine fibroids: Childbearing, cancer, and hormone effects
William H. Parker, MD (May 2008)
We want to hear from you! Tell us what you think.
1. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100-107.
2. Wallach EE, Vlahos NF. Uterine myomas: an overview of development clinical features, and management. Obstet Gynecol. 2004;104(2):393-406.
3. Stringer NH, Walker JC, Meyer PM. Comparison of 49 laparoscopic myomec- tomies and 49 open myomectomies. J Am Assoc Gynecol Laparosc. 1997;4(4):457-464.
4. Bulletti C, Polli V, Negrini V, Giacomucci E, Flamigni C. Adhesion formation after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1996;3(4):533-536.
5. Ram E, Sherman Y, Weil R, Vishne T, Kravarusic D, Dreznik Z. Is mechanical bowel preparation mandatory for elective colon surgery? A prospective randomized study. Arch Surg. 2005;140(3):285-288.
6. Frederick J, Fletcher H, Simeon D, Mullings A, Hardie M. Intramyometrial vasopressin as a haemostatic agent during myomectomy. Br J Obstet Gynaecol. 1994;101(5):435-437.
7. Taylor A, Sharma M, Tsirkas P, Di Spiezio Sardo A, Setchell M, Magos A. Reducing blood loss at open myomectomy using triple tourniquets: a randomised controlled trial. BJOG. 2005;112(3):340-345.
8. Burbank F, Hutchins FL, Jr. Uterine artery occlusion by embolization or surgery for the treatment of fibroids: A unifying hypothesis-transient uterine ischemia. J Am Assoc Gynecol Laparosc. 2000;7(suppl 4):S1-S49.
1. Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100-107.
2. Wallach EE, Vlahos NF. Uterine myomas: an overview of development clinical features, and management. Obstet Gynecol. 2004;104(2):393-406.
3. Stringer NH, Walker JC, Meyer PM. Comparison of 49 laparoscopic myomec- tomies and 49 open myomectomies. J Am Assoc Gynecol Laparosc. 1997;4(4):457-464.
4. Bulletti C, Polli V, Negrini V, Giacomucci E, Flamigni C. Adhesion formation after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc. 1996;3(4):533-536.
5. Ram E, Sherman Y, Weil R, Vishne T, Kravarusic D, Dreznik Z. Is mechanical bowel preparation mandatory for elective colon surgery? A prospective randomized study. Arch Surg. 2005;140(3):285-288.
6. Frederick J, Fletcher H, Simeon D, Mullings A, Hardie M. Intramyometrial vasopressin as a haemostatic agent during myomectomy. Br J Obstet Gynaecol. 1994;101(5):435-437.
7. Taylor A, Sharma M, Tsirkas P, Di Spiezio Sardo A, Setchell M, Magos A. Reducing blood loss at open myomectomy using triple tourniquets: a randomised controlled trial. BJOG. 2005;112(3):340-345.
8. Burbank F, Hutchins FL, Jr. Uterine artery occlusion by embolization or surgery for the treatment of fibroids: A unifying hypothesis-transient uterine ischemia. J Am Assoc Gynecol Laparosc. 2000;7(suppl 4):S1-S49.
How to avoid major vessel injury during gynecologic laparoscopy
Update: Minimally invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
CASE: Abdominal entry leads to life-threatening injury
A 50-year-old woman with a BMI of 25 kg/m2, a strong family history of breast and ovarian cancer, and a confirmed BRCA mutation was scheduled for prophylactic bilateral salpingo-oophorectomy via robotic laparoscopy on November 26, 2009. At the time of the procedure, the gynecologic surgeon selected a site for the camera trocar that was several centimeters above the umbilicus. After making a transverse incision, he inserted a Veress needle and insufflated the abdomen with CO2 gas until intra-abdominal pressure reached 17 mm Hg. He then thrust an 11-inch disposable trocar through the anterior abdominal wall, attached the camera to the laparoscope, confirmed proper intraperitoneal placement, and inserted two additional trocars under direct vision.
Shortly after these actions, the anesthesiologist reported that the patient’s blood pressure had dropped precipitously, along with end tidal CO2. The surgeon examined the peritoneal cavity and discovered blood in the right paracolic gutter. The anesthesiologist advised the surgeon that he could no longer detect the patient’s blood pressure; electrocardiography revealed pulseless electrical activity.
The surgical team began chest compressions, evacuated the pneumoperitoneum, and removed all trocars. Blood was noted on the camera trocar, and the device was secured by the OR staff. The surgeon performed an emergent laparotomy, making the incision within 4 minutes of the beginning of CPR. Exploration revealed a large retroperitoneal hematoma above the area of the aortic bifurcation and inferior vena cava.
General and vascular surgeons were called. The general surgeon opened the retroperitoneum and found an extreme amount of clotted and unclotted blood. The vascular surgeon described the initial injury as a 1.5-cm laceration of the distal aorta, just above the bifurcation. A cell saver was requested and recorded blood loss of 12,000 mL.
The vascular surgeon clamped the aorta proximally; he also clamped both common iliac arteries. He then repaired the lacerations on the aorta using 5-0 Prolene suture (Ethicon). The aorta was significantly narrowed, however, so the surgeon decided to replace the distal aorta, which he then resected and repaired using a 14-mm Dacron graft (DuPont).
Further inspection revealed continuing retroperitoneal bleeding. The vascular surgeon found and repaired a laceration of the inferior mesenteric vein. He also clipped multiple small veins to stop bleeding.
When a hole in the transverse colon was identified, the general surgeon—who had left the operating table—rescrubbed to repair it. He also discovered an injury to the mesentery of the transverse colon and repaired both wounds, resecting the perforated segment. The divided, stapled colon was dropped back into the abdomen because the bowel was dusky. Despite an epinephrine drip, the patient was hypotensive and coagulopathic. The abdomen was packed and covered with sterile cassette film, with towels covering the open wound.
The patient was taken to the postanesthesia care unit in guarded condition and was subsequently transferred to the ICU, where her blood pressure dropped again. She was returned to the OR, where the packs were removed and a bleeding right common iliac artery was repaired using 5-0 Prolene suture. The next day, she underwent bilateral salpingo-oophorectomy with a transverse colon colostomy.
Because of the colon injury, the vascular surgeon believed that the Dacron graft had been contaminated. On December 1, the graft was taken down, a left femoral-vein autograft was harvested, and a reconstructive conduit was created for the terminal aorta. The patient underwent three additional procedures to place mesh into the abdominal wall. When the mesh became infected, it was removed.
The patient remained in the hospital for 1 month, after which she was transferred to a long-term care facility. She suffered permanent neurologic injuries because of prolonged hypoxia and continues to require supportive care.
How could this catastrophe have been avoided?
Traumatic injury to the great retroperitoneal vessels is an emergent and life-threatening event. During gynecologic laparoscopy, it is most likely to occur during entry into the anterior abdominal wall.
Most laparoscopic procedures require entry into the anterior abdominal wall for placement of a trocar and a sleeve that serves as a portal for insertion of the endoscope. Secondary ports provide entry points for manipulative and operative tools.
The most critical entry point is primary placement of the viewing device. Secondary trocars are always inserted under direct visualization; therefore, they carry a lower risk of inflicting injury to underlying viscera and vessels.
Practice safe entry
In the early days of laparoscopy, only one method of entry existed. Over time, however, several other techniques have been devised.
The initial method—still widely utilized—is known as the closed or blind technique. The surgeon creates a pneumoperitoneum with the use of a needle that is 18 gauge to 2.5 mm in diameter; the needle is placed through a subumbilical incision. Once intraperitoneal placement is confirmed, CO2 gas is infused into the peritoneal cavity until the abdomen is tympanic to percussion (usually at pressures of 14 to 18 mm Hg).
Next, the surgeon aims the trocar toward the uterus at a 45° angle, maintaining the device in the midline. Entry is confirmed by opening the trocar’s trap-door valve and witnessing a rush of CO2 gas.
Another entry technique—the open technique—is used almost universally by general surgeons. The procedure is a type of microlaparotomy. After making the subumbilical incision, the fascia of the abdominal wall is pierced and the peritoneum is grasped and opened bluntly or sharply. Once the edges of the peritoneum are secured, a blunt trocar (Hasson trocar) is inserted. Then the trocar is removed, leaving the sleeve in place to accept the laparoscope.
Another entry variation, called direct entry, employs no pneumoperitoneum. In this approach, the surgeon grabs the anterior abdominal wall, sharply elevating it, and directly thrusts the reusable or disposable trocar into the abdominal cavity.
An extensive review of entry techniques has been published elsewhere.1
Many complications arise from entry techniques and devices
A survey of Australian gynecologists about entry techniques found that 73% of respondents used a Veress needle and pneumoperitoneum for entry and that 83% used a location other than the infraumbilical site when periumbilical adhesions were suspected. Twenty-one percent had experienced a major retroperitoneal vascular injury, but 33% lacked a plan to manage such injuries.2
In their review of entry techniques, Vilos and colleagues asserted that Veress-needle insertion should be accompanied by pneumoperitoneal pressures of 20 to 30 mm Hg rather than a predetermined volume of CO2 gas.1 They also recommended insertion in the left upper quadrant when periumbilical adhesions are suspected or when insufflation at the umbilicus fails three times.
Newer entry devices include the optical-view trocar and the radially expanding trocar. The first consists of a plastic, conically tipped instrument that is optically clear. At least hypothetically, this device permits the surgeon to view each layer of the abdominal wall as he or she thrusts the device under “direct vision” into the abdominal cavity.
The radially expanding trocar is inserted over a Veress needle into the abdominal cavity. Its initial diameter is only 3 mm; once the instrument is in place, however, a blunt plastic trocar and sleeve are pushed into the mesh-like, radially expanding tube until it reaches 11 to 12 mm in diameter. The blunt trocar is then removed, leaving the plastic sheath and mesh material in place to accept the laparoscope. One key advantage of this device is the mesh component, which resists slippage or movement as the laparoscope is moved in and out of the sheath.
Vilos and colleagues concluded that open entry was neither superior nor inferior to other entry techniques and that direct entry without pneumoperitoneum may be as safe as Veress-needle techniques and associated with a lower risk of gas embolism. They also reported that shielded trocars are not associated with fewer visceral or vascular injuries and that visual-entry trocars lack superiority, compared with other devices, for the prevention of visceral or vascular injuries.1
Other review articles about entry techniques similarly found no objective evidence that any single technique is superior.3 However, data are conflicting on the safety of the optical trocar, compared with other trocars, with some data showing marginal advantages and others demonstrating no difference.4-6
Follow a few key entry guidelines
In 1990, Yuzpe reported a mail-in survey of 800 practicing ObGyns in Canada on the topic of pneumoperitoneum and trocar injuries.7 Of the 407 physicians who responded, 16.7% reported that the pneumoperitoneum needle caused a visceral or major vessel injury, and 16.5% attributed the injury to the primary trocar. Among 109 vessel injuries, 31 were caused by the pneumoperitoneum needle, and 28 of 104 injuries were caused by the primary trocar.
To be safe, Veress needle and primary trocar entry require critical attention to the angle and direction of the thrust relative to the abdominal cavity (FIGURE, page 24). For example, if the Veress needle or the sharp tip of the trocar deviate to the right or left of the midline during entry into the abdomen, injury to the iliac vessels is a clear risk.
Most laparoscopic surgeons stand on the left-hand side of the patient and face her feet. Trocar deviation for a right-handed person tends to vector to the right, especially when a twisting motion is utilized. Correct alignment of the primary trocar is straight down the middle of the lower abdomen on a virtual or real line drawn from the center of the navel to the center of the symphysis.
An entry angle of 45° to 60° will carry the needle or trocar toward the bladder or uterus and away from the aorta and left common iliac vein. In contrast, a 90° thrust will aim the device dangerously toward the great vessels. A slightly upward and right-sided deviation from the subumbilical entry will place the needle and trocar in the direction of the inferior vena cava and right common iliac vessels. A 90° entry with deviation to the left will position the entry device at the inferior mesenteric vessels and the left common iliac vessels.
Primary abdominal entry
An entry angle of 45° to 60°, regardless of whether a needle or trocar is used, will carry the device toward the bladder or uterus and away from the aorta and left common iliac vein.In a review of access complications associated with laparoscopy, including major vascular injuries, Philips and Amaral listed variables responsible for large-vessel injury; they also documented the incidence of such injuries associated with laparoscopic cholecystectomy.8 They recommended that the patient be placed in the Trendelenburg position and that the needle or trocar be inserted at a 45° angle that stays within the midline; they also concluded that the trocar should be placed when pneumoperitoneal pressures exceed 20 mm Hg. They advised against direct insertion in patients with a history of pelvic surgery as well as in thin patients.
Place secondary trocars under direct visualization
Secondary trocars should always be placed under direct, visually controlled entry and, at least hypothetically, should never injure any great vessel. Nevertheless, secondary trocars do sometimes cause injury, most often as a result of extreme lateral entry near the inguinal ligament. The vessels at risk are the external iliac artery and vein.
Injuries are also invariably associated with adhesiolysis and anatomic problems. Precise knowledge of pelvic anatomy is not only a requisite for pelvic surgery in general but also for laparoscopic surgery, in which the operative view is less clear than it is in open procedures.
Know the risks associated with operative tools
Suturing and knot tying are not easy maneuvers during laparoscopic procedures and add significant operative time. Although they are performed more easily when robotics is utilized, few gynecologists are skilled practitioners. As a result, accessory instruments have been developed to prevent and control bleeding during laparoscopic operations. These devices include monopolar and bipolar instruments, lasers, ultrasonic tools, and stapling devices.
Avoid monopolar electrosurgery
This modality should be avoided whenever possible because the risk of injury is significantly higher than with bipolar electrosurgery. The key disadvantages of monopolar energy are high-frequency leaks; low-frequency currents; direct, indirect, and capacitative coupling; and return-electrode failures. None of these problems are common with bipolar techniques.
However, all electrosurgical devices carry a risk of thermal injury through direct tissue contact and conduction of heat to neighboring tissues and structures.
A full discussion of the physics and tissue actions of electrosurgical devices may be found elsewhere.9
CO2 is the safest laser
A variety of lasers have been used in laparoscopic surgery. The neodymium–YAG, KTP-532, and CO2 lasers have been used most frequently for gynecologic operations.
Because of its wavelength, the CO2 laser is the safest device for intra-abdominal use. Advantages include precision and control. In addition, the CO2 laser is absorbed by water very effectively. As a result, hydrodissection techniques can facilitate effective backstopping of the laser beam in strategic locations, thereby preventing injury to surrounding structures.
Laser energy is not conducted through tissue in the same way that electrosurgical energy is conducted. Therefore, the laser is ideal for vaporizing endometrial implants and cutting adhesions.
Beware of heat generated by the ultrasonic shears
This device, known more commonly as the Harmonic Scalpel (Ethicon), employs high-frequency sound waves to shear and coagulate tissue and prevent bleeding. It does not require conduction through tissues but does require contact with tissues. Because friction produces heat, these devices can become hot enough to inflict unintended burns on tissues that are inadvertently touched by the hot tip or by heat transmitted from the operative site by thermal conduction.
Stapler may inadvertently involve adjacent structures
This laparoscopic device has the advantage of not requiring or emitting energy other than the mechanical force of the operator’s hand. Disadvantages associated with the stapler center on the inadvertent inclusion of other structures within the jaws of the instrument. In addition, the staplers themselves tend to be large and somewhat unwieldy in close quarters, adding to the risk of stapling nearby viscera.
Further information on the physics and actions of lasers, ultrasonic shears, and staplers is available.10,11
Obesity may increase the risk of major vessel injury
A recent study by Baggish found obesity to be a high-risk circumstance for major vessel injury.12 In the study, 22 of 31 women who sustained injury were overweight or obese, with a BMI ranging from 26 to 30 kg/m2.
Obesity increases the risk of major vessel injury because of the greater elasticity of the anterior abdominal wall. As force secondary to the downward thrust of the trocar is placed on the abdominal wall, it is pushed inward in the direction of the posterior wall. In contrast, thin women have rigid abdominal walls with minimal elasticity, so the force of the trocar thrust does not create significant displacement.
Baggish also found that disposable trocars accounted for 90% of major vascular injuries and that use of long trocars accounted for 43% of deaths.12
Injury and death are rare but real risks
In a multicenter study in France over 9 years, investigators reviewed 29,966 diagnostic and operative laparoscopic procedures and found a mortality rate of 3.33 deaths for every 100,000 laparoscopies and an overall complication rate of 4.64 complications for every 1,000 procedures.13 They found the complication rate to be significantly correlated with the complexity of the procedure (P = .0001). One in three complications (34.1%; n = 43) occurred during set-up, and one in four (28.6%) were not identified intraoperatively.13
The risk of great vessel injury associated with laparoscopy most frequently quoted is 0.5 injury for every 1,000 procedures.14 A multicenter study reported the prevalence of this complication to be 1.05 injuries per 1,000 procedures.15
The mortality rate associated with major vessel injury has been reported in several studies to range from 8% to 17%.14-17
Two articles measured the distance from various points on the anterior abdominal wall to the great retroperitoneal vessels during laparoscopic operations; they also measured the force required for the trocar to penetrate the abdominal wall.18,19 They found significant differences in the distance from the site of primary trocar insertion to the aorta and iliac vessels, depending on the BMI of the patient. In women with a BMI below 25 kg/m2, the mean distance to the aorta was 11.21 cm. In women with a BMI of 25 to 30, it was 14.14 cm, and in women with a BMI over 30, it was 15.14 cm. They also found variations in the mean thickness of the abdominal wall, which was 3.48 cm, 3.85 cm, and 5.05 cm in women with a BMI of less than 25, 25–30, and more than 30, respectively.
As for the force required for entry, investigators found that disposable cutting trocars can traverse the anterior abdominal wall with less force and less time, compared with reusable trocars and optical viewing devices.18,19
Another study measured the thickness of the abdominal wall and the distance to the great vessels by magnetic resonance imaging or computed tomography.20 However, this study was not performed during laparoscopy with pneumoperitoneum in place.
As previously mentioned, Baggish reported on 31 cases of major-vessel injury associated with laparoscopic operations involving 49 major-vessel injuries. Twenty-eight injuries occurred as a result of entry techniques: 26 occurred during primary trocar insertion, and two were related to secondary trocar thrusts.12 Four injuries and three deaths were associated with use of an 11-inch disposable trocar.
Of the injuries associated with primary trocar insertion, 10 occurred during direct insertion and 26 after creation of pneumoperitoneum. Open laparoscopy was performed in two cases.12 The TABLE details the number of vessels injured and the sites of injury in this study.
Seven women (23%) died as a direct result of venous injury. Collateral injury to other structures was observed in 16 cases. Blood loss ranged from 1,000 mL to 7,000 mL.12
Sites of major vessel injury in one study of gynecologic laparoscopy
| Site | Number of vascular injuries |
|---|---|
| Right iliac artery | 14 |
| Right iliac vein | 12 |
| Left iliac artery | 3 |
| Left iliac vein | 9 |
| Aorta | 4 |
| Vena cava | 2 |
| Mesenteric | 2 |
| Interior epigastric* | 2 |
| Other | 1 |
| Total injuries | 49 |
| Source: Baggish12 | |
Avoid these common errors
The most common errors in gynecologic laparoscopy include:
- delayed diagnosis
- failure to act on a visible retroperitoneal hematoma
- failure to cross-match adequate supplies of blood and blood products
- failure to adequately transfuse blood and blood products
- clamping the large damaged vessel
- opening the abdomen via Pfannenstiel incision
- failure to call for a vascular surgeon in a timely manner.
When a major vascular injury occurs, a well-informed surgeon will take the following measures:
- call for a vascular surgeon immediately. (Baggish found that there was a substantial delay in getting a vascular surgeon to the operating table in four of 31 cases.12)
- open the abdomen via a midline incision
- use a sponge stick to apply direct pressure to the bleeding vessel
- obtain an emergency type and cross-match and order a minimum of 6 U of blood plus fresh frozen plasma
- obtain a baseline complete blood count, platelet count, fibrinogen level, and test for fibrin-split products
- advise the anesthesiologist to seek additional help
- call for additional OR nursing personnel
- assign one circulator to run stats and record critical data.21-33
Prevention is the best strategy
As the opening case demonstrates, major vessel injury can occur without warning and cause cascading problems that can lead to permanent disability—even death. Because most serious vessel injuries occur during entry into the anterior abdominal wall, careful attention to entry techniques and the patient’s unique circumstances (obesity, presence of adhesions) can go a long way toward averting injury. Vigilance for the possibility of injury is also important throughout the procedure. When injury does occur, it is critical to call for help as soon as possible and to have safeguards in place to manage it.
Tune in again in October 2012 for Part 2 of this series, which offers insight into gastrointestinal and urinary tract injuries during laparoscopy and offers valuable guidance on avoiding and managing related complications.
We want to hear from you! Tell us what you think.
Update: Minimally invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
CASE: Abdominal entry leads to life-threatening injury
A 50-year-old woman with a BMI of 25 kg/m2, a strong family history of breast and ovarian cancer, and a confirmed BRCA mutation was scheduled for prophylactic bilateral salpingo-oophorectomy via robotic laparoscopy on November 26, 2009. At the time of the procedure, the gynecologic surgeon selected a site for the camera trocar that was several centimeters above the umbilicus. After making a transverse incision, he inserted a Veress needle and insufflated the abdomen with CO2 gas until intra-abdominal pressure reached 17 mm Hg. He then thrust an 11-inch disposable trocar through the anterior abdominal wall, attached the camera to the laparoscope, confirmed proper intraperitoneal placement, and inserted two additional trocars under direct vision.
Shortly after these actions, the anesthesiologist reported that the patient’s blood pressure had dropped precipitously, along with end tidal CO2. The surgeon examined the peritoneal cavity and discovered blood in the right paracolic gutter. The anesthesiologist advised the surgeon that he could no longer detect the patient’s blood pressure; electrocardiography revealed pulseless electrical activity.
The surgical team began chest compressions, evacuated the pneumoperitoneum, and removed all trocars. Blood was noted on the camera trocar, and the device was secured by the OR staff. The surgeon performed an emergent laparotomy, making the incision within 4 minutes of the beginning of CPR. Exploration revealed a large retroperitoneal hematoma above the area of the aortic bifurcation and inferior vena cava.
General and vascular surgeons were called. The general surgeon opened the retroperitoneum and found an extreme amount of clotted and unclotted blood. The vascular surgeon described the initial injury as a 1.5-cm laceration of the distal aorta, just above the bifurcation. A cell saver was requested and recorded blood loss of 12,000 mL.
The vascular surgeon clamped the aorta proximally; he also clamped both common iliac arteries. He then repaired the lacerations on the aorta using 5-0 Prolene suture (Ethicon). The aorta was significantly narrowed, however, so the surgeon decided to replace the distal aorta, which he then resected and repaired using a 14-mm Dacron graft (DuPont).
Further inspection revealed continuing retroperitoneal bleeding. The vascular surgeon found and repaired a laceration of the inferior mesenteric vein. He also clipped multiple small veins to stop bleeding.
When a hole in the transverse colon was identified, the general surgeon—who had left the operating table—rescrubbed to repair it. He also discovered an injury to the mesentery of the transverse colon and repaired both wounds, resecting the perforated segment. The divided, stapled colon was dropped back into the abdomen because the bowel was dusky. Despite an epinephrine drip, the patient was hypotensive and coagulopathic. The abdomen was packed and covered with sterile cassette film, with towels covering the open wound.
The patient was taken to the postanesthesia care unit in guarded condition and was subsequently transferred to the ICU, where her blood pressure dropped again. She was returned to the OR, where the packs were removed and a bleeding right common iliac artery was repaired using 5-0 Prolene suture. The next day, she underwent bilateral salpingo-oophorectomy with a transverse colon colostomy.
Because of the colon injury, the vascular surgeon believed that the Dacron graft had been contaminated. On December 1, the graft was taken down, a left femoral-vein autograft was harvested, and a reconstructive conduit was created for the terminal aorta. The patient underwent three additional procedures to place mesh into the abdominal wall. When the mesh became infected, it was removed.
The patient remained in the hospital for 1 month, after which she was transferred to a long-term care facility. She suffered permanent neurologic injuries because of prolonged hypoxia and continues to require supportive care.
How could this catastrophe have been avoided?
Traumatic injury to the great retroperitoneal vessels is an emergent and life-threatening event. During gynecologic laparoscopy, it is most likely to occur during entry into the anterior abdominal wall.
Most laparoscopic procedures require entry into the anterior abdominal wall for placement of a trocar and a sleeve that serves as a portal for insertion of the endoscope. Secondary ports provide entry points for manipulative and operative tools.
The most critical entry point is primary placement of the viewing device. Secondary trocars are always inserted under direct visualization; therefore, they carry a lower risk of inflicting injury to underlying viscera and vessels.
Practice safe entry
In the early days of laparoscopy, only one method of entry existed. Over time, however, several other techniques have been devised.
The initial method—still widely utilized—is known as the closed or blind technique. The surgeon creates a pneumoperitoneum with the use of a needle that is 18 gauge to 2.5 mm in diameter; the needle is placed through a subumbilical incision. Once intraperitoneal placement is confirmed, CO2 gas is infused into the peritoneal cavity until the abdomen is tympanic to percussion (usually at pressures of 14 to 18 mm Hg).
Next, the surgeon aims the trocar toward the uterus at a 45° angle, maintaining the device in the midline. Entry is confirmed by opening the trocar’s trap-door valve and witnessing a rush of CO2 gas.
Another entry technique—the open technique—is used almost universally by general surgeons. The procedure is a type of microlaparotomy. After making the subumbilical incision, the fascia of the abdominal wall is pierced and the peritoneum is grasped and opened bluntly or sharply. Once the edges of the peritoneum are secured, a blunt trocar (Hasson trocar) is inserted. Then the trocar is removed, leaving the sleeve in place to accept the laparoscope.
Another entry variation, called direct entry, employs no pneumoperitoneum. In this approach, the surgeon grabs the anterior abdominal wall, sharply elevating it, and directly thrusts the reusable or disposable trocar into the abdominal cavity.
An extensive review of entry techniques has been published elsewhere.1
Many complications arise from entry techniques and devices
A survey of Australian gynecologists about entry techniques found that 73% of respondents used a Veress needle and pneumoperitoneum for entry and that 83% used a location other than the infraumbilical site when periumbilical adhesions were suspected. Twenty-one percent had experienced a major retroperitoneal vascular injury, but 33% lacked a plan to manage such injuries.2
In their review of entry techniques, Vilos and colleagues asserted that Veress-needle insertion should be accompanied by pneumoperitoneal pressures of 20 to 30 mm Hg rather than a predetermined volume of CO2 gas.1 They also recommended insertion in the left upper quadrant when periumbilical adhesions are suspected or when insufflation at the umbilicus fails three times.
Newer entry devices include the optical-view trocar and the radially expanding trocar. The first consists of a plastic, conically tipped instrument that is optically clear. At least hypothetically, this device permits the surgeon to view each layer of the abdominal wall as he or she thrusts the device under “direct vision” into the abdominal cavity.
The radially expanding trocar is inserted over a Veress needle into the abdominal cavity. Its initial diameter is only 3 mm; once the instrument is in place, however, a blunt plastic trocar and sleeve are pushed into the mesh-like, radially expanding tube until it reaches 11 to 12 mm in diameter. The blunt trocar is then removed, leaving the plastic sheath and mesh material in place to accept the laparoscope. One key advantage of this device is the mesh component, which resists slippage or movement as the laparoscope is moved in and out of the sheath.
Vilos and colleagues concluded that open entry was neither superior nor inferior to other entry techniques and that direct entry without pneumoperitoneum may be as safe as Veress-needle techniques and associated with a lower risk of gas embolism. They also reported that shielded trocars are not associated with fewer visceral or vascular injuries and that visual-entry trocars lack superiority, compared with other devices, for the prevention of visceral or vascular injuries.1
Other review articles about entry techniques similarly found no objective evidence that any single technique is superior.3 However, data are conflicting on the safety of the optical trocar, compared with other trocars, with some data showing marginal advantages and others demonstrating no difference.4-6
Follow a few key entry guidelines
In 1990, Yuzpe reported a mail-in survey of 800 practicing ObGyns in Canada on the topic of pneumoperitoneum and trocar injuries.7 Of the 407 physicians who responded, 16.7% reported that the pneumoperitoneum needle caused a visceral or major vessel injury, and 16.5% attributed the injury to the primary trocar. Among 109 vessel injuries, 31 were caused by the pneumoperitoneum needle, and 28 of 104 injuries were caused by the primary trocar.
To be safe, Veress needle and primary trocar entry require critical attention to the angle and direction of the thrust relative to the abdominal cavity (FIGURE, page 24). For example, if the Veress needle or the sharp tip of the trocar deviate to the right or left of the midline during entry into the abdomen, injury to the iliac vessels is a clear risk.
Most laparoscopic surgeons stand on the left-hand side of the patient and face her feet. Trocar deviation for a right-handed person tends to vector to the right, especially when a twisting motion is utilized. Correct alignment of the primary trocar is straight down the middle of the lower abdomen on a virtual or real line drawn from the center of the navel to the center of the symphysis.
An entry angle of 45° to 60° will carry the needle or trocar toward the bladder or uterus and away from the aorta and left common iliac vein. In contrast, a 90° thrust will aim the device dangerously toward the great vessels. A slightly upward and right-sided deviation from the subumbilical entry will place the needle and trocar in the direction of the inferior vena cava and right common iliac vessels. A 90° entry with deviation to the left will position the entry device at the inferior mesenteric vessels and the left common iliac vessels.

Primary abdominal entry
An entry angle of 45° to 60°, regardless of whether a needle or trocar is used, will carry the device toward the bladder or uterus and away from the aorta and left common iliac vein.In a review of access complications associated with laparoscopy, including major vascular injuries, Philips and Amaral listed variables responsible for large-vessel injury; they also documented the incidence of such injuries associated with laparoscopic cholecystectomy.8 They recommended that the patient be placed in the Trendelenburg position and that the needle or trocar be inserted at a 45° angle that stays within the midline; they also concluded that the trocar should be placed when pneumoperitoneal pressures exceed 20 mm Hg. They advised against direct insertion in patients with a history of pelvic surgery as well as in thin patients.
Place secondary trocars under direct visualization
Secondary trocars should always be placed under direct, visually controlled entry and, at least hypothetically, should never injure any great vessel. Nevertheless, secondary trocars do sometimes cause injury, most often as a result of extreme lateral entry near the inguinal ligament. The vessels at risk are the external iliac artery and vein.
Injuries are also invariably associated with adhesiolysis and anatomic problems. Precise knowledge of pelvic anatomy is not only a requisite for pelvic surgery in general but also for laparoscopic surgery, in which the operative view is less clear than it is in open procedures.
Know the risks associated with operative tools
Suturing and knot tying are not easy maneuvers during laparoscopic procedures and add significant operative time. Although they are performed more easily when robotics is utilized, few gynecologists are skilled practitioners. As a result, accessory instruments have been developed to prevent and control bleeding during laparoscopic operations. These devices include monopolar and bipolar instruments, lasers, ultrasonic tools, and stapling devices.
Avoid monopolar electrosurgery
This modality should be avoided whenever possible because the risk of injury is significantly higher than with bipolar electrosurgery. The key disadvantages of monopolar energy are high-frequency leaks; low-frequency currents; direct, indirect, and capacitative coupling; and return-electrode failures. None of these problems are common with bipolar techniques.
However, all electrosurgical devices carry a risk of thermal injury through direct tissue contact and conduction of heat to neighboring tissues and structures.
A full discussion of the physics and tissue actions of electrosurgical devices may be found elsewhere.9
CO2 is the safest laser
A variety of lasers have been used in laparoscopic surgery. The neodymium–YAG, KTP-532, and CO2 lasers have been used most frequently for gynecologic operations.
Because of its wavelength, the CO2 laser is the safest device for intra-abdominal use. Advantages include precision and control. In addition, the CO2 laser is absorbed by water very effectively. As a result, hydrodissection techniques can facilitate effective backstopping of the laser beam in strategic locations, thereby preventing injury to surrounding structures.
Laser energy is not conducted through tissue in the same way that electrosurgical energy is conducted. Therefore, the laser is ideal for vaporizing endometrial implants and cutting adhesions.
Beware of heat generated by the ultrasonic shears
This device, known more commonly as the Harmonic Scalpel (Ethicon), employs high-frequency sound waves to shear and coagulate tissue and prevent bleeding. It does not require conduction through tissues but does require contact with tissues. Because friction produces heat, these devices can become hot enough to inflict unintended burns on tissues that are inadvertently touched by the hot tip or by heat transmitted from the operative site by thermal conduction.
Stapler may inadvertently involve adjacent structures
This laparoscopic device has the advantage of not requiring or emitting energy other than the mechanical force of the operator’s hand. Disadvantages associated with the stapler center on the inadvertent inclusion of other structures within the jaws of the instrument. In addition, the staplers themselves tend to be large and somewhat unwieldy in close quarters, adding to the risk of stapling nearby viscera.
Further information on the physics and actions of lasers, ultrasonic shears, and staplers is available.10,11
Obesity may increase the risk of major vessel injury
A recent study by Baggish found obesity to be a high-risk circumstance for major vessel injury.12 In the study, 22 of 31 women who sustained injury were overweight or obese, with a BMI ranging from 26 to 30 kg/m2.
Obesity increases the risk of major vessel injury because of the greater elasticity of the anterior abdominal wall. As force secondary to the downward thrust of the trocar is placed on the abdominal wall, it is pushed inward in the direction of the posterior wall. In contrast, thin women have rigid abdominal walls with minimal elasticity, so the force of the trocar thrust does not create significant displacement.
Baggish also found that disposable trocars accounted for 90% of major vascular injuries and that use of long trocars accounted for 43% of deaths.12
Injury and death are rare but real risks
In a multicenter study in France over 9 years, investigators reviewed 29,966 diagnostic and operative laparoscopic procedures and found a mortality rate of 3.33 deaths for every 100,000 laparoscopies and an overall complication rate of 4.64 complications for every 1,000 procedures.13 They found the complication rate to be significantly correlated with the complexity of the procedure (P = .0001). One in three complications (34.1%; n = 43) occurred during set-up, and one in four (28.6%) were not identified intraoperatively.13
The risk of great vessel injury associated with laparoscopy most frequently quoted is 0.5 injury for every 1,000 procedures.14 A multicenter study reported the prevalence of this complication to be 1.05 injuries per 1,000 procedures.15
The mortality rate associated with major vessel injury has been reported in several studies to range from 8% to 17%.14-17
Two articles measured the distance from various points on the anterior abdominal wall to the great retroperitoneal vessels during laparoscopic operations; they also measured the force required for the trocar to penetrate the abdominal wall.18,19 They found significant differences in the distance from the site of primary trocar insertion to the aorta and iliac vessels, depending on the BMI of the patient. In women with a BMI below 25 kg/m2, the mean distance to the aorta was 11.21 cm. In women with a BMI of 25 to 30, it was 14.14 cm, and in women with a BMI over 30, it was 15.14 cm. They also found variations in the mean thickness of the abdominal wall, which was 3.48 cm, 3.85 cm, and 5.05 cm in women with a BMI of less than 25, 25–30, and more than 30, respectively.
As for the force required for entry, investigators found that disposable cutting trocars can traverse the anterior abdominal wall with less force and less time, compared with reusable trocars and optical viewing devices.18,19
Another study measured the thickness of the abdominal wall and the distance to the great vessels by magnetic resonance imaging or computed tomography.20 However, this study was not performed during laparoscopy with pneumoperitoneum in place.
As previously mentioned, Baggish reported on 31 cases of major-vessel injury associated with laparoscopic operations involving 49 major-vessel injuries. Twenty-eight injuries occurred as a result of entry techniques: 26 occurred during primary trocar insertion, and two were related to secondary trocar thrusts.12 Four injuries and three deaths were associated with use of an 11-inch disposable trocar.
Of the injuries associated with primary trocar insertion, 10 occurred during direct insertion and 26 after creation of pneumoperitoneum. Open laparoscopy was performed in two cases.12 The TABLE details the number of vessels injured and the sites of injury in this study.
Seven women (23%) died as a direct result of venous injury. Collateral injury to other structures was observed in 16 cases. Blood loss ranged from 1,000 mL to 7,000 mL.12
Sites of major vessel injury in one study of gynecologic laparoscopy
| Site | Number of vascular injuries |
|---|---|
| Right iliac artery | 14 |
| Right iliac vein | 12 |
| Left iliac artery | 3 |
| Left iliac vein | 9 |
| Aorta | 4 |
| Vena cava | 2 |
| Mesenteric | 2 |
| Interior epigastric* | 2 |
| Other | 1 |
| Total injuries | 49 |
| Source: Baggish12 | |
Avoid these common errors
The most common errors in gynecologic laparoscopy include:
- delayed diagnosis
- failure to act on a visible retroperitoneal hematoma
- failure to cross-match adequate supplies of blood and blood products
- failure to adequately transfuse blood and blood products
- clamping the large damaged vessel
- opening the abdomen via Pfannenstiel incision
- failure to call for a vascular surgeon in a timely manner.
When a major vascular injury occurs, a well-informed surgeon will take the following measures:
- call for a vascular surgeon immediately. (Baggish found that there was a substantial delay in getting a vascular surgeon to the operating table in four of 31 cases.12)
- open the abdomen via a midline incision
- use a sponge stick to apply direct pressure to the bleeding vessel
- obtain an emergency type and cross-match and order a minimum of 6 U of blood plus fresh frozen plasma
- obtain a baseline complete blood count, platelet count, fibrinogen level, and test for fibrin-split products
- advise the anesthesiologist to seek additional help
- call for additional OR nursing personnel
- assign one circulator to run stats and record critical data.21-33
Prevention is the best strategy
As the opening case demonstrates, major vessel injury can occur without warning and cause cascading problems that can lead to permanent disability—even death. Because most serious vessel injuries occur during entry into the anterior abdominal wall, careful attention to entry techniques and the patient’s unique circumstances (obesity, presence of adhesions) can go a long way toward averting injury. Vigilance for the possibility of injury is also important throughout the procedure. When injury does occur, it is critical to call for help as soon as possible and to have safeguards in place to manage it.
Tune in again in October 2012 for Part 2 of this series, which offers insight into gastrointestinal and urinary tract injuries during laparoscopy and offers valuable guidance on avoiding and managing related complications.
We want to hear from you! Tell us what you think.
Update: Minimally invasive Surgery
Amy Garcia, MD (April 2012)
10 practical, evidence-based suggestions to improve your minimally invasive surgical skills now
Catherine A. Matthews, MD (April 2011)
CASE: Abdominal entry leads to life-threatening injury
A 50-year-old woman with a BMI of 25 kg/m2, a strong family history of breast and ovarian cancer, and a confirmed BRCA mutation was scheduled for prophylactic bilateral salpingo-oophorectomy via robotic laparoscopy on November 26, 2009. At the time of the procedure, the gynecologic surgeon selected a site for the camera trocar that was several centimeters above the umbilicus. After making a transverse incision, he inserted a Veress needle and insufflated the abdomen with CO2 gas until intra-abdominal pressure reached 17 mm Hg. He then thrust an 11-inch disposable trocar through the anterior abdominal wall, attached the camera to the laparoscope, confirmed proper intraperitoneal placement, and inserted two additional trocars under direct vision.
Shortly after these actions, the anesthesiologist reported that the patient’s blood pressure had dropped precipitously, along with end tidal CO2. The surgeon examined the peritoneal cavity and discovered blood in the right paracolic gutter. The anesthesiologist advised the surgeon that he could no longer detect the patient’s blood pressure; electrocardiography revealed pulseless electrical activity.
The surgical team began chest compressions, evacuated the pneumoperitoneum, and removed all trocars. Blood was noted on the camera trocar, and the device was secured by the OR staff. The surgeon performed an emergent laparotomy, making the incision within 4 minutes of the beginning of CPR. Exploration revealed a large retroperitoneal hematoma above the area of the aortic bifurcation and inferior vena cava.
General and vascular surgeons were called. The general surgeon opened the retroperitoneum and found an extreme amount of clotted and unclotted blood. The vascular surgeon described the initial injury as a 1.5-cm laceration of the distal aorta, just above the bifurcation. A cell saver was requested and recorded blood loss of 12,000 mL.
The vascular surgeon clamped the aorta proximally; he also clamped both common iliac arteries. He then repaired the lacerations on the aorta using 5-0 Prolene suture (Ethicon). The aorta was significantly narrowed, however, so the surgeon decided to replace the distal aorta, which he then resected and repaired using a 14-mm Dacron graft (DuPont).
Further inspection revealed continuing retroperitoneal bleeding. The vascular surgeon found and repaired a laceration of the inferior mesenteric vein. He also clipped multiple small veins to stop bleeding.
When a hole in the transverse colon was identified, the general surgeon—who had left the operating table—rescrubbed to repair it. He also discovered an injury to the mesentery of the transverse colon and repaired both wounds, resecting the perforated segment. The divided, stapled colon was dropped back into the abdomen because the bowel was dusky. Despite an epinephrine drip, the patient was hypotensive and coagulopathic. The abdomen was packed and covered with sterile cassette film, with towels covering the open wound.
The patient was taken to the postanesthesia care unit in guarded condition and was subsequently transferred to the ICU, where her blood pressure dropped again. She was returned to the OR, where the packs were removed and a bleeding right common iliac artery was repaired using 5-0 Prolene suture. The next day, she underwent bilateral salpingo-oophorectomy with a transverse colon colostomy.
Because of the colon injury, the vascular surgeon believed that the Dacron graft had been contaminated. On December 1, the graft was taken down, a left femoral-vein autograft was harvested, and a reconstructive conduit was created for the terminal aorta. The patient underwent three additional procedures to place mesh into the abdominal wall. When the mesh became infected, it was removed.
The patient remained in the hospital for 1 month, after which she was transferred to a long-term care facility. She suffered permanent neurologic injuries because of prolonged hypoxia and continues to require supportive care.
How could this catastrophe have been avoided?
Traumatic injury to the great retroperitoneal vessels is an emergent and life-threatening event. During gynecologic laparoscopy, it is most likely to occur during entry into the anterior abdominal wall.
Most laparoscopic procedures require entry into the anterior abdominal wall for placement of a trocar and a sleeve that serves as a portal for insertion of the endoscope. Secondary ports provide entry points for manipulative and operative tools.
The most critical entry point is primary placement of the viewing device. Secondary trocars are always inserted under direct visualization; therefore, they carry a lower risk of inflicting injury to underlying viscera and vessels.
Practice safe entry
In the early days of laparoscopy, only one method of entry existed. Over time, however, several other techniques have been devised.
The initial method—still widely utilized—is known as the closed or blind technique. The surgeon creates a pneumoperitoneum with the use of a needle that is 18 gauge to 2.5 mm in diameter; the needle is placed through a subumbilical incision. Once intraperitoneal placement is confirmed, CO2 gas is infused into the peritoneal cavity until the abdomen is tympanic to percussion (usually at pressures of 14 to 18 mm Hg).
Next, the surgeon aims the trocar toward the uterus at a 45° angle, maintaining the device in the midline. Entry is confirmed by opening the trocar’s trap-door valve and witnessing a rush of CO2 gas.
Another entry technique—the open technique—is used almost universally by general surgeons. The procedure is a type of microlaparotomy. After making the subumbilical incision, the fascia of the abdominal wall is pierced and the peritoneum is grasped and opened bluntly or sharply. Once the edges of the peritoneum are secured, a blunt trocar (Hasson trocar) is inserted. Then the trocar is removed, leaving the sleeve in place to accept the laparoscope.
Another entry variation, called direct entry, employs no pneumoperitoneum. In this approach, the surgeon grabs the anterior abdominal wall, sharply elevating it, and directly thrusts the reusable or disposable trocar into the abdominal cavity.
An extensive review of entry techniques has been published elsewhere.1
Many complications arise from entry techniques and devices
A survey of Australian gynecologists about entry techniques found that 73% of respondents used a Veress needle and pneumoperitoneum for entry and that 83% used a location other than the infraumbilical site when periumbilical adhesions were suspected. Twenty-one percent had experienced a major retroperitoneal vascular injury, but 33% lacked a plan to manage such injuries.2
In their review of entry techniques, Vilos and colleagues asserted that Veress-needle insertion should be accompanied by pneumoperitoneal pressures of 20 to 30 mm Hg rather than a predetermined volume of CO2 gas.1 They also recommended insertion in the left upper quadrant when periumbilical adhesions are suspected or when insufflation at the umbilicus fails three times.
Newer entry devices include the optical-view trocar and the radially expanding trocar. The first consists of a plastic, conically tipped instrument that is optically clear. At least hypothetically, this device permits the surgeon to view each layer of the abdominal wall as he or she thrusts the device under “direct vision” into the abdominal cavity.
The radially expanding trocar is inserted over a Veress needle into the abdominal cavity. Its initial diameter is only 3 mm; once the instrument is in place, however, a blunt plastic trocar and sleeve are pushed into the mesh-like, radially expanding tube until it reaches 11 to 12 mm in diameter. The blunt trocar is then removed, leaving the plastic sheath and mesh material in place to accept the laparoscope. One key advantage of this device is the mesh component, which resists slippage or movement as the laparoscope is moved in and out of the sheath.
Vilos and colleagues concluded that open entry was neither superior nor inferior to other entry techniques and that direct entry without pneumoperitoneum may be as safe as Veress-needle techniques and associated with a lower risk of gas embolism. They also reported that shielded trocars are not associated with fewer visceral or vascular injuries and that visual-entry trocars lack superiority, compared with other devices, for the prevention of visceral or vascular injuries.1
Other review articles about entry techniques similarly found no objective evidence that any single technique is superior.3 However, data are conflicting on the safety of the optical trocar, compared with other trocars, with some data showing marginal advantages and others demonstrating no difference.4-6
Follow a few key entry guidelines
In 1990, Yuzpe reported a mail-in survey of 800 practicing ObGyns in Canada on the topic of pneumoperitoneum and trocar injuries.7 Of the 407 physicians who responded, 16.7% reported that the pneumoperitoneum needle caused a visceral or major vessel injury, and 16.5% attributed the injury to the primary trocar. Among 109 vessel injuries, 31 were caused by the pneumoperitoneum needle, and 28 of 104 injuries were caused by the primary trocar.
To be safe, Veress needle and primary trocar entry require critical attention to the angle and direction of the thrust relative to the abdominal cavity (FIGURE, page 24). For example, if the Veress needle or the sharp tip of the trocar deviate to the right or left of the midline during entry into the abdomen, injury to the iliac vessels is a clear risk.
Most laparoscopic surgeons stand on the left-hand side of the patient and face her feet. Trocar deviation for a right-handed person tends to vector to the right, especially when a twisting motion is utilized. Correct alignment of the primary trocar is straight down the middle of the lower abdomen on a virtual or real line drawn from the center of the navel to the center of the symphysis.
An entry angle of 45° to 60° will carry the needle or trocar toward the bladder or uterus and away from the aorta and left common iliac vein. In contrast, a 90° thrust will aim the device dangerously toward the great vessels. A slightly upward and right-sided deviation from the subumbilical entry will place the needle and trocar in the direction of the inferior vena cava and right common iliac vessels. A 90° entry with deviation to the left will position the entry device at the inferior mesenteric vessels and the left common iliac vessels.

Primary abdominal entry
An entry angle of 45° to 60°, regardless of whether a needle or trocar is used, will carry the device toward the bladder or uterus and away from the aorta and left common iliac vein.In a review of access complications associated with laparoscopy, including major vascular injuries, Philips and Amaral listed variables responsible for large-vessel injury; they also documented the incidence of such injuries associated with laparoscopic cholecystectomy.8 They recommended that the patient be placed in the Trendelenburg position and that the needle or trocar be inserted at a 45° angle that stays within the midline; they also concluded that the trocar should be placed when pneumoperitoneal pressures exceed 20 mm Hg. They advised against direct insertion in patients with a history of pelvic surgery as well as in thin patients.
Place secondary trocars under direct visualization
Secondary trocars should always be placed under direct, visually controlled entry and, at least hypothetically, should never injure any great vessel. Nevertheless, secondary trocars do sometimes cause injury, most often as a result of extreme lateral entry near the inguinal ligament. The vessels at risk are the external iliac artery and vein.
Injuries are also invariably associated with adhesiolysis and anatomic problems. Precise knowledge of pelvic anatomy is not only a requisite for pelvic surgery in general but also for laparoscopic surgery, in which the operative view is less clear than it is in open procedures.
Know the risks associated with operative tools
Suturing and knot tying are not easy maneuvers during laparoscopic procedures and add significant operative time. Although they are performed more easily when robotics is utilized, few gynecologists are skilled practitioners. As a result, accessory instruments have been developed to prevent and control bleeding during laparoscopic operations. These devices include monopolar and bipolar instruments, lasers, ultrasonic tools, and stapling devices.
Avoid monopolar electrosurgery
This modality should be avoided whenever possible because the risk of injury is significantly higher than with bipolar electrosurgery. The key disadvantages of monopolar energy are high-frequency leaks; low-frequency currents; direct, indirect, and capacitative coupling; and return-electrode failures. None of these problems are common with bipolar techniques.
However, all electrosurgical devices carry a risk of thermal injury through direct tissue contact and conduction of heat to neighboring tissues and structures.
A full discussion of the physics and tissue actions of electrosurgical devices may be found elsewhere.9
CO2 is the safest laser
A variety of lasers have been used in laparoscopic surgery. The neodymium–YAG, KTP-532, and CO2 lasers have been used most frequently for gynecologic operations.
Because of its wavelength, the CO2 laser is the safest device for intra-abdominal use. Advantages include precision and control. In addition, the CO2 laser is absorbed by water very effectively. As a result, hydrodissection techniques can facilitate effective backstopping of the laser beam in strategic locations, thereby preventing injury to surrounding structures.
Laser energy is not conducted through tissue in the same way that electrosurgical energy is conducted. Therefore, the laser is ideal for vaporizing endometrial implants and cutting adhesions.
Beware of heat generated by the ultrasonic shears
This device, known more commonly as the Harmonic Scalpel (Ethicon), employs high-frequency sound waves to shear and coagulate tissue and prevent bleeding. It does not require conduction through tissues but does require contact with tissues. Because friction produces heat, these devices can become hot enough to inflict unintended burns on tissues that are inadvertently touched by the hot tip or by heat transmitted from the operative site by thermal conduction.
Stapler may inadvertently involve adjacent structures
This laparoscopic device has the advantage of not requiring or emitting energy other than the mechanical force of the operator’s hand. Disadvantages associated with the stapler center on the inadvertent inclusion of other structures within the jaws of the instrument. In addition, the staplers themselves tend to be large and somewhat unwieldy in close quarters, adding to the risk of stapling nearby viscera.
Further information on the physics and actions of lasers, ultrasonic shears, and staplers is available.10,11
Obesity may increase the risk of major vessel injury
A recent study by Baggish found obesity to be a high-risk circumstance for major vessel injury.12 In the study, 22 of 31 women who sustained injury were overweight or obese, with a BMI ranging from 26 to 30 kg/m2.
Obesity increases the risk of major vessel injury because of the greater elasticity of the anterior abdominal wall. As force secondary to the downward thrust of the trocar is placed on the abdominal wall, it is pushed inward in the direction of the posterior wall. In contrast, thin women have rigid abdominal walls with minimal elasticity, so the force of the trocar thrust does not create significant displacement.
Baggish also found that disposable trocars accounted for 90% of major vascular injuries and that use of long trocars accounted for 43% of deaths.12
Injury and death are rare but real risks
In a multicenter study in France over 9 years, investigators reviewed 29,966 diagnostic and operative laparoscopic procedures and found a mortality rate of 3.33 deaths for every 100,000 laparoscopies and an overall complication rate of 4.64 complications for every 1,000 procedures.13 They found the complication rate to be significantly correlated with the complexity of the procedure (P = .0001). One in three complications (34.1%; n = 43) occurred during set-up, and one in four (28.6%) were not identified intraoperatively.13
The risk of great vessel injury associated with laparoscopy most frequently quoted is 0.5 injury for every 1,000 procedures.14 A multicenter study reported the prevalence of this complication to be 1.05 injuries per 1,000 procedures.15
The mortality rate associated with major vessel injury has been reported in several studies to range from 8% to 17%.14-17
Two articles measured the distance from various points on the anterior abdominal wall to the great retroperitoneal vessels during laparoscopic operations; they also measured the force required for the trocar to penetrate the abdominal wall.18,19 They found significant differences in the distance from the site of primary trocar insertion to the aorta and iliac vessels, depending on the BMI of the patient. In women with a BMI below 25 kg/m2, the mean distance to the aorta was 11.21 cm. In women with a BMI of 25 to 30, it was 14.14 cm, and in women with a BMI over 30, it was 15.14 cm. They also found variations in the mean thickness of the abdominal wall, which was 3.48 cm, 3.85 cm, and 5.05 cm in women with a BMI of less than 25, 25–30, and more than 30, respectively.
As for the force required for entry, investigators found that disposable cutting trocars can traverse the anterior abdominal wall with less force and less time, compared with reusable trocars and optical viewing devices.18,19
Another study measured the thickness of the abdominal wall and the distance to the great vessels by magnetic resonance imaging or computed tomography.20 However, this study was not performed during laparoscopy with pneumoperitoneum in place.
As previously mentioned, Baggish reported on 31 cases of major-vessel injury associated with laparoscopic operations involving 49 major-vessel injuries. Twenty-eight injuries occurred as a result of entry techniques: 26 occurred during primary trocar insertion, and two were related to secondary trocar thrusts.12 Four injuries and three deaths were associated with use of an 11-inch disposable trocar.
Of the injuries associated with primary trocar insertion, 10 occurred during direct insertion and 26 after creation of pneumoperitoneum. Open laparoscopy was performed in two cases.12 The TABLE details the number of vessels injured and the sites of injury in this study.
Seven women (23%) died as a direct result of venous injury. Collateral injury to other structures was observed in 16 cases. Blood loss ranged from 1,000 mL to 7,000 mL.12
Sites of major vessel injury in one study of gynecologic laparoscopy
| Site | Number of vascular injuries |
|---|---|
| Right iliac artery | 14 |
| Right iliac vein | 12 |
| Left iliac artery | 3 |
| Left iliac vein | 9 |
| Aorta | 4 |
| Vena cava | 2 |
| Mesenteric | 2 |
| Interior epigastric* | 2 |
| Other | 1 |
| Total injuries | 49 |
| Source: Baggish12 | |
Avoid these common errors
The most common errors in gynecologic laparoscopy include:
- delayed diagnosis
- failure to act on a visible retroperitoneal hematoma
- failure to cross-match adequate supplies of blood and blood products
- failure to adequately transfuse blood and blood products
- clamping the large damaged vessel
- opening the abdomen via Pfannenstiel incision
- failure to call for a vascular surgeon in a timely manner.
When a major vascular injury occurs, a well-informed surgeon will take the following measures:
- call for a vascular surgeon immediately. (Baggish found that there was a substantial delay in getting a vascular surgeon to the operating table in four of 31 cases.12)
- open the abdomen via a midline incision
- use a sponge stick to apply direct pressure to the bleeding vessel
- obtain an emergency type and cross-match and order a minimum of 6 U of blood plus fresh frozen plasma
- obtain a baseline complete blood count, platelet count, fibrinogen level, and test for fibrin-split products
- advise the anesthesiologist to seek additional help
- call for additional OR nursing personnel
- assign one circulator to run stats and record critical data.21-33
Prevention is the best strategy
As the opening case demonstrates, major vessel injury can occur without warning and cause cascading problems that can lead to permanent disability—even death. Because most serious vessel injuries occur during entry into the anterior abdominal wall, careful attention to entry techniques and the patient’s unique circumstances (obesity, presence of adhesions) can go a long way toward averting injury. Vigilance for the possibility of injury is also important throughout the procedure. When injury does occur, it is critical to call for help as soon as possible and to have safeguards in place to manage it.
Tune in again in October 2012 for Part 2 of this series, which offers insight into gastrointestinal and urinary tract injuries during laparoscopy and offers valuable guidance on avoiding and managing related complications.
We want to hear from you! Tell us what you think.
A stepwise approach to cervical cerclage
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (Examining the Evidence, April 2012)
Update on obstetrics
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
Placement of a suture around an incompetent cervix to prevent premature pregnancy loss was first described more than 50 years ago1,2—but the few randomized studies that have been published (all of them in the past decade) devote very little attention to technique.3-7
Are we to assume, then, that over more than 50 years, no modifications to technique have been devised?
Are we to assume as well that in the two largest randomized studies to date, which involved 266 cerclages inserted in 27 different medical centers over more than 4 years,5,7 all cerclages were inserted in an identical manner using the same technique originated more than half a century ago?
In this article, we lay out five principles to achieve effective cerclage and describe a stepwise approach to technique. This technique is based on our experience with approximately 2,000 cerclages performed in a single medical center. We emphasize anatomic landmarks and surgical principles that are based on the published literature as well as our personal experience.
Five principles of effective cerclage
Place the cerclage as high as possible
In the original paper on cerclage, McDonald emphasized the need to place the suture as high as possible to be as close as possible to the level of the internal cervical os.2
Zilianti and colleagues elegantly described how—in the absence of cerclage—cervical tissue begins to change at the level of the internal os, forming a funnel that advances downward in the shape of the letters “Y,” “V,” and “U.”8 If we accept this notion, then the only way to prevent further shortening from the top down is by placing a high cerclage.
Studies have demonstrated improved pregnancy outcomes after placement of a high cervico-isthmic cerclage following failure of a “conventional” low cerclage.9,10
Place the cerclage adjacent to the cervical stroma
Macroscopic and microscopic visualization of the cervix reveals the following main layers:
- epithelium/mucosa, which covers the deeper connective tissue known as cervical stroma
- cervical stroma, which may be divided into two zones: 1) a superficial, subepithelial zone that appears histologically as loose stromal bands and 2) a deeper, dense collagen layer.
It is the dense collagen layer of the cervical stroma that affords most of the resistance to forces of deformation, whereas the loose stromal layer and the epithelium above it slide easily over the deeper stroma. Including too great a proportion of these “slippery” components within the cerclage could increase the risk of displacement and failure.11,12
Shirodkar was the first to suggest that the mucosa and submucosa be excluded from cerclage,1 and a detailed submucosal cerclage insertion was described by Fahmy more than 30 years ago.13 We support his recommendation that cerclage placement be as close to the inner cervical stroma as possible and that it include as little as possible of the surrounding tissue.
Take three encircling cervical “bites”
In his original publication, McDonald described “five or six bites with the needle” to encircle the cervix.2 Later authors usually described four encircling steps, but no reliable study has challenged the original dogma.
Although we lack science to favor one approach over another, common sense suggests that three bites (versus four or five) offer the following advantages. They:
- produce less penetrating injury
- require less manipulation of the cervix
- are simpler and quicker to perform
- offer less opportunity for the cerclage tape to get twisted (a flat tape provides for better distribution of the load)
- permit a small gap between the two final exit points of the tape (at 5 o’clock and 7 o’clock), which allows for easier cinching and tightening of the cerclage (FIGURE 1).
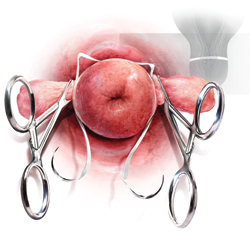
FIGURE 1 A small gap between the ends of the cerclage tape, which exit at 5 o’clock and 7 o’clock, allows for easier cinching and tightening.
Both Shirodkar1 and McDonald2 described placement of the knot (or approximation of the “ends”) at 12 o’clock, anteriorly. However, this approach can complicate removal if strong pressure is applied to the cerclage or if it is covered by tissue. Attempts to remove the cerclage can result in bladder injury.
For these reasons, we prefer the approach described by Caspi and colleagues, who placed the knot in the back of the cervix at 6 o’clock.14 In that location, the surgeon can reach as high as desired, and there is no risk of organ injury during removal.
That said, it should be noted that removal of a cerclage with a knot at 6 o’clock is more difficult than removal of one with a knot at 12 o’clock—but the convenience of the operator should be secondary to safety and efficacy of the cerclage.
Place a figure of 8 around the cerclage knot
Because of the proximity of the bladder anteriorly, there is a limit to how high one can place the cerclage anteriorly. However, in the back of the cervix, one can place the cerclage much higher without risk of injury. This approach sometimes will result in downward forces on the posterior part (where the knot is) and occasionally may cover the knot with tissue from the posterior vaginal fornix.
For these reasons, we propose securing the knot of the cerclage tape to the posterior surface of the cervical “core” by placing a figure of 8 using bright blue Prolene #1 (Ethicon) to prevent slippage and help call attention to the knot when the time for removal comes.
1. Use a weighted speculum to retract the posterior-inferior vaginal wall. Have an assistant hold one or two right-angle retractors to retract the other aspects of the vaginal wall, including the bladder anteriorly, as needed.
2. Clamp the anterior and posterior lips of the cervix and tug them lightly—at all times—in an outward direction (FIGURE 2).
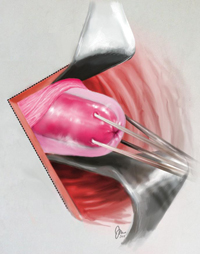
FIGURE 2 Clamping of the cervix
Clamp the anterior and posterior lips of the cervix and tug them lightly and steadily in an outward direction.
3. Retract and release the bladder several times using a right-angle retractor for more accurate identification of the cervico-vesical fold (FIGURE 3 and FIGURE 4). Note the distance from the external os to the cervico-vesical fold; it should be 2 cm or farther. (If it is less than 2 cm, another type of cerclage may be preferable.)
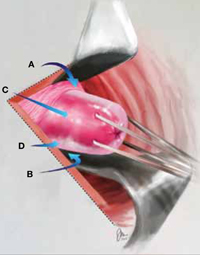
FIGURE 3 Anatomic landmarks
Cerclage is facilitated by orientation to the following landmarks; A. cervico-vesical fold; B. posterior fornix; C. cervical stroma; D. cervical mucosa.
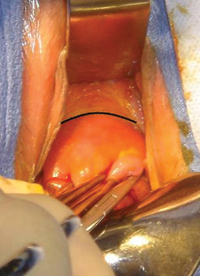
FIGURE 4 Cervico-vesical fold
The black line indicates the location of the fold.
4. Identify the roof of the posterior fornix (FIGURE 3 and FIGURE 5).
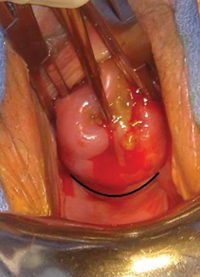
FIGURE 5 Roof of the posterior fornix
This landmark is delineated in black.
5. Using Allis clamps bilaterally, clamp the soft tissue covering the cervical core (stroma), between the cervico-vesical junction anteriorly and the superior point of the posterior fornix posteriorly. This is a cardinal step because it separates the core from the mucosal/ submucosal elements (FIGURE 6). (Helpful hint: To achieve optimal placement of the lateral Allis clamps, place the open clamp ever so slightly to one side of the middle of the cervix. As you close the instrument, let the clamp slide off the cervical core until it is locked adjacent to it. This takes the soft tissue and supporting blood vessels out of the operative field.)
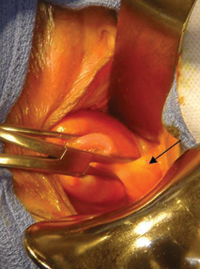
FIGURE 6 Anterolateral view
The pericervical mucosa (black arrow) after application of an Allis clamp.
6. Take three bites, 1 mm in depth, through the cervical core using a 5-mm Mersilene tape and a blunt-tipped needle (RS21; Ethicon). One bite should encompass 12:30 to 11:30 anteriorly. Another bite should go in at 3 o’clock and out at 5 o’clock, and another bite should go in at 9 o’clock and out at 7 o’clock (FIGURE 1). (Helpful hint: Ensure that the direction of the pull always is a direct extension of the passage through tissue in small steps and not an outward direction toward the operator. An instrument such as a curved Mayo clamp should be placed at the point of the needle’s exit to reduce the risk of injury. At the conclusion of the three bites, the Mersilene tape should be the same length on each side, exiting at 5 o’clock and 7 o’clock, as stated earlier [FIGURE 7].)
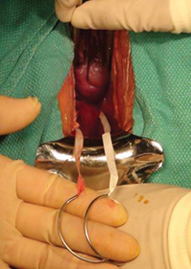
FIGURE 7 Ensure equal distribution of the tape
After taking three bites of tissue, ensure that the ends of the Mersilene tape are of equal length on each side.
7. Once the tape is of equal length on both sides, closely encircling three sides of the cervical core, empty the bladder with a catheter to ensure the presence of clear urine. Bloody urine could be an indication for cystoscopy to rule out bladder injury.
8. Cut the needles off of the tape and tie the cerclage in three ties, the first one being a surgical tie. After tying the first tie, ensure proper tension by pressing gently with the index finger up and down on the knot; if it is properly tensioned, it will not be displaced by this movement. (Helpful hint: There is no clear indication of how tight a cerclage should be tied. We suggest making the first tie as close as possible to the cervical core to create a visible, and palpable, depression in the soft tissue at the area of the knot [FIGURE 8]).

FIGURE 8 Tying the cerclage
Make the first tie as close as possible to the cervical core so that it creates a visible, and palpable, depression in the soft tissue at the area of the knot. Inset: Cerclage tie secured by a figure of 8.
9. Trim the ends of the tape to 3 cm to facilitate easy identification and manipulation at the time of removal. Place a figure of 8, using bright blue Prolene #1 (Ethicon), around the knot, securing it to the posterior surface of the cervical core (FIGURE 9). The tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography in FIGURE 10. (Helpful hint: Place surgical gauze under pressure around the cervix to support hemostasis after removal of the clamps. Remove the gauze approximately 30 minutes after the procedure.)
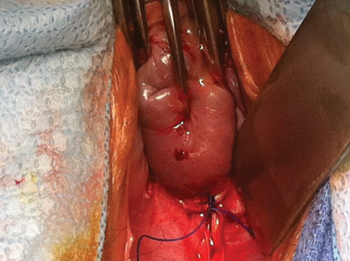
FIGURE 9 Mark the cerclage
Place a figure of 8, using bright blue Prolene #1, around the knot of the cerclage, securing it to the posterior surface of the cervical core.
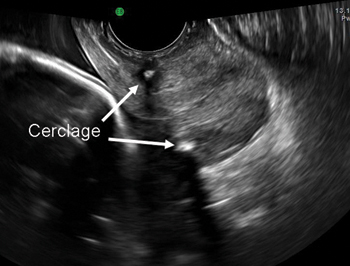
FIGURE 10 Final placement
The cerclage tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography.
Technique is applicable to most cerclage procedures
One potential limitation of this technique is the fact that it is based on surgical experience in a single center, although it includes more than 2,000 operations performed at that center. Therefore, we lack data on the ease of teaching and reproducing this technique. Nevertheless, our approach incorporates various elements that previously were proposed to enhance the effectiveness of cerclage. It likely will be applicable to most cerclage procedures, with the exception of a few unique cases. These unique cases—most of them involving the failure of conventional cerclage—may require more elaborate technique.
As for data on the location of biomechanical stresses on cervical tissue during pregnancy, the literature indicates that the forces of maximum deformation begin internally at the level of the cervico-uterine junction.12 If not successfully resisted, these forces will proceed down along the cervical canal and could lead to premature pregnancy loss.
Although the superiority of a high cerclage has not yet been proven clinically, it appears to be more effective than low placement because it is more likely to provide support at the right location.
As pregnancy progresses, the challenge to the cervix increases—not only because of increasing uterine volume but also because of greater uterine activity. Both raise the risk of cerclage slippage and displacement.15 To address these issues, several investigators proposed an approach that excludes the slippery mucosal layer.1,13,14
The original Shirodkar cerclage and its modifications—but not the McDonald cerclage and its subsequent modifications—included an “anchor” suture attaching the cerclage band to the firm cervical stromal layer as a means to prevent downward slippage and displacement.1 It remains to be seen whether this addition of a figure of 8 using nonabsorbable suture, as proposed here, is indeed effective.
We believe that, if a standardized way to perform effective cerclage can be agreed upon, we also might devise a better way to compare results based on proper patient selection.
We want to hear from you! Tell us what you think.
1. Shirodkar VN. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic. 1955;52:299.-
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynaecol Br Emp. 1957;64(3):346-350.
3. Rust OA, Atlas RO, Jones KJ, Benham BN, Balducci J. A randomized trial of cerclage versus no cerclage among patients with ultrasonographically detected second-trimester preterm dilatation of the internal os. Am J Obstet Gynecol. 2000;183(4):830-835.
4. Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final Results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185(5):1106-1112.
5. To MS, Alfirevic Z, Heath VC, et al. Fetal Medicine Foundation Second Trimester Screening Group. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363(9424):1849-1853.
6. Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191(4):1311-1317.
7. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
8. Zilianti M, Azuaga A, Calderon F, Pagés G, Mendoza G. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14(10):719-724.
9. Herron MA, Parer JT. Transabdominal cerclage for fetal wastage due to cervical incompetence. Obstet Gynecol. 1988;71(6 Pt 1):865-868.
10. Katz M, Abrahams C. Transvaginal placement of cervicoisthmic cerclage: Report on pregnancy outcome. Am J Obstet Gynecol. 2005;192(6):1989-1992.
11. Ferenczy A. Ultrastructure of the uterine cervix. In: Huszar G ed. The Physiology and Biochemistry of the Uterus in Pregnancy and Labor. Boca Raton, FL: CRC Press; 2000: 239–260.
12. Heaps RH, House M, Socrate S, Leppert P, Strauss JF, III. Matrix biology and preterm birth. In: Petraglia F Strauss JF III, Gabbe SG, Weiss G, eds. Preterm Birth: Mechanisms, Mediators, Prediction, Prevention, and Interventions. United Kingdom: Informa; 2007:71–93.
13. Fahmy K. A closed submucous cervical suture for the incompetent cervix. Int Surg. 1978;63(2):77-80.
14. Caspi E, Schneider DF, Mor Z, Langer R, Weinraub Z, Bukovsky I. Cervical internal os cerclage: description of a new technique and comparison with Shirodkar operation. Am J Perinatol. 1990;7(4):347-349.
15. Harger JH. Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol. 2002;100:1313-1327.
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (Examining the Evidence, April 2012)
Update on obstetrics
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
Placement of a suture around an incompetent cervix to prevent premature pregnancy loss was first described more than 50 years ago1,2—but the few randomized studies that have been published (all of them in the past decade) devote very little attention to technique.3-7
Are we to assume, then, that over more than 50 years, no modifications to technique have been devised?
Are we to assume as well that in the two largest randomized studies to date, which involved 266 cerclages inserted in 27 different medical centers over more than 4 years,5,7 all cerclages were inserted in an identical manner using the same technique originated more than half a century ago?
In this article, we lay out five principles to achieve effective cerclage and describe a stepwise approach to technique. This technique is based on our experience with approximately 2,000 cerclages performed in a single medical center. We emphasize anatomic landmarks and surgical principles that are based on the published literature as well as our personal experience.
Five principles of effective cerclage
Place the cerclage as high as possible
In the original paper on cerclage, McDonald emphasized the need to place the suture as high as possible to be as close as possible to the level of the internal cervical os.2
Zilianti and colleagues elegantly described how—in the absence of cerclage—cervical tissue begins to change at the level of the internal os, forming a funnel that advances downward in the shape of the letters “Y,” “V,” and “U.”8 If we accept this notion, then the only way to prevent further shortening from the top down is by placing a high cerclage.
Studies have demonstrated improved pregnancy outcomes after placement of a high cervico-isthmic cerclage following failure of a “conventional” low cerclage.9,10
Place the cerclage adjacent to the cervical stroma
Macroscopic and microscopic visualization of the cervix reveals the following main layers:
- epithelium/mucosa, which covers the deeper connective tissue known as cervical stroma
- cervical stroma, which may be divided into two zones: 1) a superficial, subepithelial zone that appears histologically as loose stromal bands and 2) a deeper, dense collagen layer.
It is the dense collagen layer of the cervical stroma that affords most of the resistance to forces of deformation, whereas the loose stromal layer and the epithelium above it slide easily over the deeper stroma. Including too great a proportion of these “slippery” components within the cerclage could increase the risk of displacement and failure.11,12
Shirodkar was the first to suggest that the mucosa and submucosa be excluded from cerclage,1 and a detailed submucosal cerclage insertion was described by Fahmy more than 30 years ago.13 We support his recommendation that cerclage placement be as close to the inner cervical stroma as possible and that it include as little as possible of the surrounding tissue.
Take three encircling cervical “bites”
In his original publication, McDonald described “five or six bites with the needle” to encircle the cervix.2 Later authors usually described four encircling steps, but no reliable study has challenged the original dogma.
Although we lack science to favor one approach over another, common sense suggests that three bites (versus four or five) offer the following advantages. They:
- produce less penetrating injury
- require less manipulation of the cervix
- are simpler and quicker to perform
- offer less opportunity for the cerclage tape to get twisted (a flat tape provides for better distribution of the load)
- permit a small gap between the two final exit points of the tape (at 5 o’clock and 7 o’clock), which allows for easier cinching and tightening of the cerclage (FIGURE 1).

FIGURE 1 A small gap between the ends of the cerclage tape, which exit at 5 o’clock and 7 o’clock, allows for easier cinching and tightening.
Both Shirodkar1 and McDonald2 described placement of the knot (or approximation of the “ends”) at 12 o’clock, anteriorly. However, this approach can complicate removal if strong pressure is applied to the cerclage or if it is covered by tissue. Attempts to remove the cerclage can result in bladder injury.
For these reasons, we prefer the approach described by Caspi and colleagues, who placed the knot in the back of the cervix at 6 o’clock.14 In that location, the surgeon can reach as high as desired, and there is no risk of organ injury during removal.
That said, it should be noted that removal of a cerclage with a knot at 6 o’clock is more difficult than removal of one with a knot at 12 o’clock—but the convenience of the operator should be secondary to safety and efficacy of the cerclage.
Place a figure of 8 around the cerclage knot
Because of the proximity of the bladder anteriorly, there is a limit to how high one can place the cerclage anteriorly. However, in the back of the cervix, one can place the cerclage much higher without risk of injury. This approach sometimes will result in downward forces on the posterior part (where the knot is) and occasionally may cover the knot with tissue from the posterior vaginal fornix.
For these reasons, we propose securing the knot of the cerclage tape to the posterior surface of the cervical “core” by placing a figure of 8 using bright blue Prolene #1 (Ethicon) to prevent slippage and help call attention to the knot when the time for removal comes.
1. Use a weighted speculum to retract the posterior-inferior vaginal wall. Have an assistant hold one or two right-angle retractors to retract the other aspects of the vaginal wall, including the bladder anteriorly, as needed.
2. Clamp the anterior and posterior lips of the cervix and tug them lightly—at all times—in an outward direction (FIGURE 2).

FIGURE 2 Clamping of the cervix
Clamp the anterior and posterior lips of the cervix and tug them lightly and steadily in an outward direction.
3. Retract and release the bladder several times using a right-angle retractor for more accurate identification of the cervico-vesical fold (FIGURE 3 and FIGURE 4). Note the distance from the external os to the cervico-vesical fold; it should be 2 cm or farther. (If it is less than 2 cm, another type of cerclage may be preferable.)

FIGURE 3 Anatomic landmarks
Cerclage is facilitated by orientation to the following landmarks; A. cervico-vesical fold; B. posterior fornix; C. cervical stroma; D. cervical mucosa.

FIGURE 4 Cervico-vesical fold
The black line indicates the location of the fold.
4. Identify the roof of the posterior fornix (FIGURE 3 and FIGURE 5).

FIGURE 5 Roof of the posterior fornix
This landmark is delineated in black.
5. Using Allis clamps bilaterally, clamp the soft tissue covering the cervical core (stroma), between the cervico-vesical junction anteriorly and the superior point of the posterior fornix posteriorly. This is a cardinal step because it separates the core from the mucosal/ submucosal elements (FIGURE 6). (Helpful hint: To achieve optimal placement of the lateral Allis clamps, place the open clamp ever so slightly to one side of the middle of the cervix. As you close the instrument, let the clamp slide off the cervical core until it is locked adjacent to it. This takes the soft tissue and supporting blood vessels out of the operative field.)

FIGURE 6 Anterolateral view
The pericervical mucosa (black arrow) after application of an Allis clamp.
6. Take three bites, 1 mm in depth, through the cervical core using a 5-mm Mersilene tape and a blunt-tipped needle (RS21; Ethicon). One bite should encompass 12:30 to 11:30 anteriorly. Another bite should go in at 3 o’clock and out at 5 o’clock, and another bite should go in at 9 o’clock and out at 7 o’clock (FIGURE 1). (Helpful hint: Ensure that the direction of the pull always is a direct extension of the passage through tissue in small steps and not an outward direction toward the operator. An instrument such as a curved Mayo clamp should be placed at the point of the needle’s exit to reduce the risk of injury. At the conclusion of the three bites, the Mersilene tape should be the same length on each side, exiting at 5 o’clock and 7 o’clock, as stated earlier [FIGURE 7].)

FIGURE 7 Ensure equal distribution of the tape
After taking three bites of tissue, ensure that the ends of the Mersilene tape are of equal length on each side.
7. Once the tape is of equal length on both sides, closely encircling three sides of the cervical core, empty the bladder with a catheter to ensure the presence of clear urine. Bloody urine could be an indication for cystoscopy to rule out bladder injury.
8. Cut the needles off of the tape and tie the cerclage in three ties, the first one being a surgical tie. After tying the first tie, ensure proper tension by pressing gently with the index finger up and down on the knot; if it is properly tensioned, it will not be displaced by this movement. (Helpful hint: There is no clear indication of how tight a cerclage should be tied. We suggest making the first tie as close as possible to the cervical core to create a visible, and palpable, depression in the soft tissue at the area of the knot [FIGURE 8]).

FIGURE 8 Tying the cerclage
Make the first tie as close as possible to the cervical core so that it creates a visible, and palpable, depression in the soft tissue at the area of the knot. Inset: Cerclage tie secured by a figure of 8.
9. Trim the ends of the tape to 3 cm to facilitate easy identification and manipulation at the time of removal. Place a figure of 8, using bright blue Prolene #1 (Ethicon), around the knot, securing it to the posterior surface of the cervical core (FIGURE 9). The tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography in FIGURE 10. (Helpful hint: Place surgical gauze under pressure around the cervix to support hemostasis after removal of the clamps. Remove the gauze approximately 30 minutes after the procedure.)

FIGURE 9 Mark the cerclage
Place a figure of 8, using bright blue Prolene #1, around the knot of the cerclage, securing it to the posterior surface of the cervical core.

FIGURE 10 Final placement
The cerclage tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography.
Technique is applicable to most cerclage procedures
One potential limitation of this technique is the fact that it is based on surgical experience in a single center, although it includes more than 2,000 operations performed at that center. Therefore, we lack data on the ease of teaching and reproducing this technique. Nevertheless, our approach incorporates various elements that previously were proposed to enhance the effectiveness of cerclage. It likely will be applicable to most cerclage procedures, with the exception of a few unique cases. These unique cases—most of them involving the failure of conventional cerclage—may require more elaborate technique.
As for data on the location of biomechanical stresses on cervical tissue during pregnancy, the literature indicates that the forces of maximum deformation begin internally at the level of the cervico-uterine junction.12 If not successfully resisted, these forces will proceed down along the cervical canal and could lead to premature pregnancy loss.
Although the superiority of a high cerclage has not yet been proven clinically, it appears to be more effective than low placement because it is more likely to provide support at the right location.
As pregnancy progresses, the challenge to the cervix increases—not only because of increasing uterine volume but also because of greater uterine activity. Both raise the risk of cerclage slippage and displacement.15 To address these issues, several investigators proposed an approach that excludes the slippery mucosal layer.1,13,14
The original Shirodkar cerclage and its modifications—but not the McDonald cerclage and its subsequent modifications—included an “anchor” suture attaching the cerclage band to the firm cervical stromal layer as a means to prevent downward slippage and displacement.1 It remains to be seen whether this addition of a figure of 8 using nonabsorbable suture, as proposed here, is indeed effective.
We believe that, if a standardized way to perform effective cerclage can be agreed upon, we also might devise a better way to compare results based on proper patient selection.
We want to hear from you! Tell us what you think.
Does vaginal progesterone reduce preterm delivery among asymptomatic women who have a short cervix in the midtrimester?
John T. Repke, MD (Examining the Evidence, April 2012)
Update on obstetrics
John T. Repke, MD, and Jaimey M. Pauli, MD (January 2012)
Placement of a suture around an incompetent cervix to prevent premature pregnancy loss was first described more than 50 years ago1,2—but the few randomized studies that have been published (all of them in the past decade) devote very little attention to technique.3-7
Are we to assume, then, that over more than 50 years, no modifications to technique have been devised?
Are we to assume as well that in the two largest randomized studies to date, which involved 266 cerclages inserted in 27 different medical centers over more than 4 years,5,7 all cerclages were inserted in an identical manner using the same technique originated more than half a century ago?
In this article, we lay out five principles to achieve effective cerclage and describe a stepwise approach to technique. This technique is based on our experience with approximately 2,000 cerclages performed in a single medical center. We emphasize anatomic landmarks and surgical principles that are based on the published literature as well as our personal experience.
Five principles of effective cerclage
Place the cerclage as high as possible
In the original paper on cerclage, McDonald emphasized the need to place the suture as high as possible to be as close as possible to the level of the internal cervical os.2
Zilianti and colleagues elegantly described how—in the absence of cerclage—cervical tissue begins to change at the level of the internal os, forming a funnel that advances downward in the shape of the letters “Y,” “V,” and “U.”8 If we accept this notion, then the only way to prevent further shortening from the top down is by placing a high cerclage.
Studies have demonstrated improved pregnancy outcomes after placement of a high cervico-isthmic cerclage following failure of a “conventional” low cerclage.9,10
Place the cerclage adjacent to the cervical stroma
Macroscopic and microscopic visualization of the cervix reveals the following main layers:
- epithelium/mucosa, which covers the deeper connective tissue known as cervical stroma
- cervical stroma, which may be divided into two zones: 1) a superficial, subepithelial zone that appears histologically as loose stromal bands and 2) a deeper, dense collagen layer.
It is the dense collagen layer of the cervical stroma that affords most of the resistance to forces of deformation, whereas the loose stromal layer and the epithelium above it slide easily over the deeper stroma. Including too great a proportion of these “slippery” components within the cerclage could increase the risk of displacement and failure.11,12
Shirodkar was the first to suggest that the mucosa and submucosa be excluded from cerclage,1 and a detailed submucosal cerclage insertion was described by Fahmy more than 30 years ago.13 We support his recommendation that cerclage placement be as close to the inner cervical stroma as possible and that it include as little as possible of the surrounding tissue.
Take three encircling cervical “bites”
In his original publication, McDonald described “five or six bites with the needle” to encircle the cervix.2 Later authors usually described four encircling steps, but no reliable study has challenged the original dogma.
Although we lack science to favor one approach over another, common sense suggests that three bites (versus four or five) offer the following advantages. They:
- produce less penetrating injury
- require less manipulation of the cervix
- are simpler and quicker to perform
- offer less opportunity for the cerclage tape to get twisted (a flat tape provides for better distribution of the load)
- permit a small gap between the two final exit points of the tape (at 5 o’clock and 7 o’clock), which allows for easier cinching and tightening of the cerclage (FIGURE 1).

FIGURE 1 A small gap between the ends of the cerclage tape, which exit at 5 o’clock and 7 o’clock, allows for easier cinching and tightening.
Both Shirodkar1 and McDonald2 described placement of the knot (or approximation of the “ends”) at 12 o’clock, anteriorly. However, this approach can complicate removal if strong pressure is applied to the cerclage or if it is covered by tissue. Attempts to remove the cerclage can result in bladder injury.
For these reasons, we prefer the approach described by Caspi and colleagues, who placed the knot in the back of the cervix at 6 o’clock.14 In that location, the surgeon can reach as high as desired, and there is no risk of organ injury during removal.
That said, it should be noted that removal of a cerclage with a knot at 6 o’clock is more difficult than removal of one with a knot at 12 o’clock—but the convenience of the operator should be secondary to safety and efficacy of the cerclage.
Place a figure of 8 around the cerclage knot
Because of the proximity of the bladder anteriorly, there is a limit to how high one can place the cerclage anteriorly. However, in the back of the cervix, one can place the cerclage much higher without risk of injury. This approach sometimes will result in downward forces on the posterior part (where the knot is) and occasionally may cover the knot with tissue from the posterior vaginal fornix.
For these reasons, we propose securing the knot of the cerclage tape to the posterior surface of the cervical “core” by placing a figure of 8 using bright blue Prolene #1 (Ethicon) to prevent slippage and help call attention to the knot when the time for removal comes.
1. Use a weighted speculum to retract the posterior-inferior vaginal wall. Have an assistant hold one or two right-angle retractors to retract the other aspects of the vaginal wall, including the bladder anteriorly, as needed.
2. Clamp the anterior and posterior lips of the cervix and tug them lightly—at all times—in an outward direction (FIGURE 2).

FIGURE 2 Clamping of the cervix
Clamp the anterior and posterior lips of the cervix and tug them lightly and steadily in an outward direction.
3. Retract and release the bladder several times using a right-angle retractor for more accurate identification of the cervico-vesical fold (FIGURE 3 and FIGURE 4). Note the distance from the external os to the cervico-vesical fold; it should be 2 cm or farther. (If it is less than 2 cm, another type of cerclage may be preferable.)

FIGURE 3 Anatomic landmarks
Cerclage is facilitated by orientation to the following landmarks; A. cervico-vesical fold; B. posterior fornix; C. cervical stroma; D. cervical mucosa.

FIGURE 4 Cervico-vesical fold
The black line indicates the location of the fold.
4. Identify the roof of the posterior fornix (FIGURE 3 and FIGURE 5).

FIGURE 5 Roof of the posterior fornix
This landmark is delineated in black.
5. Using Allis clamps bilaterally, clamp the soft tissue covering the cervical core (stroma), between the cervico-vesical junction anteriorly and the superior point of the posterior fornix posteriorly. This is a cardinal step because it separates the core from the mucosal/ submucosal elements (FIGURE 6). (Helpful hint: To achieve optimal placement of the lateral Allis clamps, place the open clamp ever so slightly to one side of the middle of the cervix. As you close the instrument, let the clamp slide off the cervical core until it is locked adjacent to it. This takes the soft tissue and supporting blood vessels out of the operative field.)

FIGURE 6 Anterolateral view
The pericervical mucosa (black arrow) after application of an Allis clamp.
6. Take three bites, 1 mm in depth, through the cervical core using a 5-mm Mersilene tape and a blunt-tipped needle (RS21; Ethicon). One bite should encompass 12:30 to 11:30 anteriorly. Another bite should go in at 3 o’clock and out at 5 o’clock, and another bite should go in at 9 o’clock and out at 7 o’clock (FIGURE 1). (Helpful hint: Ensure that the direction of the pull always is a direct extension of the passage through tissue in small steps and not an outward direction toward the operator. An instrument such as a curved Mayo clamp should be placed at the point of the needle’s exit to reduce the risk of injury. At the conclusion of the three bites, the Mersilene tape should be the same length on each side, exiting at 5 o’clock and 7 o’clock, as stated earlier [FIGURE 7].)

FIGURE 7 Ensure equal distribution of the tape
After taking three bites of tissue, ensure that the ends of the Mersilene tape are of equal length on each side.
7. Once the tape is of equal length on both sides, closely encircling three sides of the cervical core, empty the bladder with a catheter to ensure the presence of clear urine. Bloody urine could be an indication for cystoscopy to rule out bladder injury.
8. Cut the needles off of the tape and tie the cerclage in three ties, the first one being a surgical tie. After tying the first tie, ensure proper tension by pressing gently with the index finger up and down on the knot; if it is properly tensioned, it will not be displaced by this movement. (Helpful hint: There is no clear indication of how tight a cerclage should be tied. We suggest making the first tie as close as possible to the cervical core to create a visible, and palpable, depression in the soft tissue at the area of the knot [FIGURE 8]).

FIGURE 8 Tying the cerclage
Make the first tie as close as possible to the cervical core so that it creates a visible, and palpable, depression in the soft tissue at the area of the knot. Inset: Cerclage tie secured by a figure of 8.
9. Trim the ends of the tape to 3 cm to facilitate easy identification and manipulation at the time of removal. Place a figure of 8, using bright blue Prolene #1 (Ethicon), around the knot, securing it to the posterior surface of the cervical core (FIGURE 9). The tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography in FIGURE 10. (Helpful hint: Place surgical gauze under pressure around the cervix to support hemostasis after removal of the clamps. Remove the gauze approximately 30 minutes after the procedure.)

FIGURE 9 Mark the cerclage
Place a figure of 8, using bright blue Prolene #1, around the knot of the cerclage, securing it to the posterior surface of the cervical core.

FIGURE 10 Final placement
The cerclage tape should encircle the firm part of the cervix near the internal os, as shown by transvaginal ultrasonography.
Technique is applicable to most cerclage procedures
One potential limitation of this technique is the fact that it is based on surgical experience in a single center, although it includes more than 2,000 operations performed at that center. Therefore, we lack data on the ease of teaching and reproducing this technique. Nevertheless, our approach incorporates various elements that previously were proposed to enhance the effectiveness of cerclage. It likely will be applicable to most cerclage procedures, with the exception of a few unique cases. These unique cases—most of them involving the failure of conventional cerclage—may require more elaborate technique.
As for data on the location of biomechanical stresses on cervical tissue during pregnancy, the literature indicates that the forces of maximum deformation begin internally at the level of the cervico-uterine junction.12 If not successfully resisted, these forces will proceed down along the cervical canal and could lead to premature pregnancy loss.
Although the superiority of a high cerclage has not yet been proven clinically, it appears to be more effective than low placement because it is more likely to provide support at the right location.
As pregnancy progresses, the challenge to the cervix increases—not only because of increasing uterine volume but also because of greater uterine activity. Both raise the risk of cerclage slippage and displacement.15 To address these issues, several investigators proposed an approach that excludes the slippery mucosal layer.1,13,14
The original Shirodkar cerclage and its modifications—but not the McDonald cerclage and its subsequent modifications—included an “anchor” suture attaching the cerclage band to the firm cervical stromal layer as a means to prevent downward slippage and displacement.1 It remains to be seen whether this addition of a figure of 8 using nonabsorbable suture, as proposed here, is indeed effective.
We believe that, if a standardized way to perform effective cerclage can be agreed upon, we also might devise a better way to compare results based on proper patient selection.
We want to hear from you! Tell us what you think.
1. Shirodkar VN. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic. 1955;52:299.-
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynaecol Br Emp. 1957;64(3):346-350.
3. Rust OA, Atlas RO, Jones KJ, Benham BN, Balducci J. A randomized trial of cerclage versus no cerclage among patients with ultrasonographically detected second-trimester preterm dilatation of the internal os. Am J Obstet Gynecol. 2000;183(4):830-835.
4. Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final Results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185(5):1106-1112.
5. To MS, Alfirevic Z, Heath VC, et al. Fetal Medicine Foundation Second Trimester Screening Group. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363(9424):1849-1853.
6. Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191(4):1311-1317.
7. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
8. Zilianti M, Azuaga A, Calderon F, Pagés G, Mendoza G. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14(10):719-724.
9. Herron MA, Parer JT. Transabdominal cerclage for fetal wastage due to cervical incompetence. Obstet Gynecol. 1988;71(6 Pt 1):865-868.
10. Katz M, Abrahams C. Transvaginal placement of cervicoisthmic cerclage: Report on pregnancy outcome. Am J Obstet Gynecol. 2005;192(6):1989-1992.
11. Ferenczy A. Ultrastructure of the uterine cervix. In: Huszar G ed. The Physiology and Biochemistry of the Uterus in Pregnancy and Labor. Boca Raton, FL: CRC Press; 2000: 239–260.
12. Heaps RH, House M, Socrate S, Leppert P, Strauss JF, III. Matrix biology and preterm birth. In: Petraglia F Strauss JF III, Gabbe SG, Weiss G, eds. Preterm Birth: Mechanisms, Mediators, Prediction, Prevention, and Interventions. United Kingdom: Informa; 2007:71–93.
13. Fahmy K. A closed submucous cervical suture for the incompetent cervix. Int Surg. 1978;63(2):77-80.
14. Caspi E, Schneider DF, Mor Z, Langer R, Weinraub Z, Bukovsky I. Cervical internal os cerclage: description of a new technique and comparison with Shirodkar operation. Am J Perinatol. 1990;7(4):347-349.
15. Harger JH. Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol. 2002;100:1313-1327.
1. Shirodkar VN. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic. 1955;52:299.-
2. McDonald IA. Suture of the cervix for inevitable miscarriage. J Obstet Gynaecol Br Emp. 1957;64(3):346-350.
3. Rust OA, Atlas RO, Jones KJ, Benham BN, Balducci J. A randomized trial of cerclage versus no cerclage among patients with ultrasonographically detected second-trimester preterm dilatation of the internal os. Am J Obstet Gynecol. 2000;183(4):830-835.
4. Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, van Geijn HP. Final Results of the Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT): therapeutic cerclage with bed rest versus bed rest alone. Am J Obstet Gynecol. 2001;185(5):1106-1112.
5. To MS, Alfirevic Z, Heath VC, et al. Fetal Medicine Foundation Second Trimester Screening Group. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet. 2004;363(9424):1849-1853.
6. Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. Am J Obstet Gynecol. 2004;191(4):1311-1317.
7. Owen J, Hankins G, Iams JD, et al. Multicenter randomized trial of cerclage for preterm birth in high-risk women with shortened midtrimester cervical length. Am J Obstet Gynecol. 2009;201(4):375.e1-8.
8. Zilianti M, Azuaga A, Calderon F, Pagés G, Mendoza G. Monitoring the effacement of the uterine cervix by transperineal sonography: a new perspective. J Ultrasound Med. 1995;14(10):719-724.
9. Herron MA, Parer JT. Transabdominal cerclage for fetal wastage due to cervical incompetence. Obstet Gynecol. 1988;71(6 Pt 1):865-868.
10. Katz M, Abrahams C. Transvaginal placement of cervicoisthmic cerclage: Report on pregnancy outcome. Am J Obstet Gynecol. 2005;192(6):1989-1992.
11. Ferenczy A. Ultrastructure of the uterine cervix. In: Huszar G ed. The Physiology and Biochemistry of the Uterus in Pregnancy and Labor. Boca Raton, FL: CRC Press; 2000: 239–260.
12. Heaps RH, House M, Socrate S, Leppert P, Strauss JF, III. Matrix biology and preterm birth. In: Petraglia F Strauss JF III, Gabbe SG, Weiss G, eds. Preterm Birth: Mechanisms, Mediators, Prediction, Prevention, and Interventions. United Kingdom: Informa; 2007:71–93.
13. Fahmy K. A closed submucous cervical suture for the incompetent cervix. Int Surg. 1978;63(2):77-80.
14. Caspi E, Schneider DF, Mor Z, Langer R, Weinraub Z, Bukovsky I. Cervical internal os cerclage: description of a new technique and comparison with Shirodkar operation. Am J Perinatol. 1990;7(4):347-349.
15. Harger JH. Cerclage and cervical insufficiency: an evidence-based analysis. Obstet Gynecol. 2002;100:1313-1327.
Your surgical toolbox should include topical hemostatic agents—here is why
Vessel-sealing devices and hemostatic adjuvants are expanding the surgical armamentarium. These products provide a spectrum of alternatives that can serve you and your surgical patient well when traditional techniques for obtaining hemostasis fail to provide a satisfactory result. (Keep in mind, however, that technology is no substitute for excellent technique!)
In this article, we highlight three common scenarios in which topical hemostatic agents may be useful during gynecologic surgery. In addition, in the sidebar, five surgeons describe the hemostatic products they rely on most often—and tell why.

Following hysterectomy, persistent oozing along the anterior vaginal margin, distal to the cuff and adjacent to the site of bladder mobilization, may be managed with the aid of a topical hemostatic agent—in this case, a fibrin sealant.
When the site of bleeding is difficult to reach
CASE 1: Oozing at the site of bladder mobilization
You perform total hysterectomy in a 44-year-old woman who has uterine fibroids. After the procedure, you notice persistent oozing along the anterior vaginal margin, distal to the cuff and adjacent to where the bladder was mobilized.
How do you manage the oozing?
Wide mobilization of the bladder is a vital step in the safe performance of hysterectomy. Adhesions may complicate the process if the patient has had previous abdominal surgery, infection, or inflammation. Following mobilization of the bladder and removal of the uterus, bleeding may be visible along the adventitia of the posterior bladder wall or along the anterior surface of the vagina, distal to the cuff, as it is in this case (see the illustration).
Judicious application of an energy source is an option, but thermal injury to the bladder is a concern. A good alternative is proper placement of a hemostatic suture, but it can sometimes be difficult to avoid incorporating the bladder or injuring or obstructing the nearby ureter.
In this case, the location of the bleeding deep in the operative field poses a challenge, because of limited exposure and the proximity of the bladder and ureters. Virtually any hemostatic agent would work well in this circumstance (TABLE). For example, a flowable agent or fibrin sealant could be thoroughly applied to the area during a minimally invasive or open procedure and would naturally conform to the irregularities in the tissue, particularly the junction between the vagina and bladder flap.
A pliable product such as Surgicel Nu-Knit or Fibrillar would also work well in these circumstances, although successful application during laparoscopy may depend on the size of the trocar. For example, Nu-Knit would require trimming to a size suitable for passage through a trocar, made easier by moistening with saline. The weave of Fibrillar makes it more challenging to pass, intact, through a trocar; rolling the material into a cylindrical shape may reduce its diameter and allow it to pass more easily.
CASE 1: Resolved
You apply a fibrin sealant to the site of bleeding, and the oozing abates. Once complete hemostasis is ensured, you conclude the surgery and transfer the patient to recovery, where she does well.
Profiles in hemostasis: Strengths and weaknesses of topical agent
| Agent (brands) | Composition | Forms available | Mechanism of action | Advantages | Caveats | Duration | Relative cost* |
|---|---|---|---|---|---|---|---|
| Physical agents | |||||||
| Gelatin matrix (Gelfoam, Gelfilm, Surgifoam) | Porcine- derived collagen | Sponge, film, powder | Provides physical matrix for clot formation | Non-antigenic; neutral pH; may be used with thrombin | Material expansion may cause compression; Not for use in closed spaces or near nerve structures | 4–6 weeks | $ |
| Oxidized regenerated cellulose (Surgicel Fibrillar, Surgicel Nu-Knit) | Wood pulp | Mesh or packed fibers | Provides physical matrix for clot formation; acidic pH causes hemolysis and local clot formation | Pliable, easy to place through laparoscope; acidic pH has antimicrobial effect | Works best in a dry field. Acidic pH inactivates biologic agents, such as thrombin, and may increase inflammation. Avoid using excess material. | 2–4 weeks | $ |
| Microfibrillar collagen (Avitene, Instat, Helitene Helistat) | Bovine-derived collagen | Powder, non-woven sheet, sponge | Absorbable acid salt. Provides physical scaffold for platelet activation and clot initiation. | Sheet form may be passed through laparoscope; minimal expansion | Rare allergic reactions reported; may contribute to granuloma formation | 8–12 weeks | $$ |
| Biologically active agents | |||||||
| Topical thrombin (Thrombin-JMI, Recothrom, Evithrom, rh Thrombin) | Bovine, human, or recombinant | Liquid | Promotes conversion of fibrinogen to fibrin | May be combined effectively with physical agents of neutral pH; recombinant human thrombin will be available in the near future | Risk of blood-borne infection with non-recombinant human thrombin; risk of anaphylaxis and antibody formation with bovine thrombin | N/A | $$ |
| Hemostatic matrix (Floseal, Surgiflo) | Thrombin plus gelatin | Foam | Gelatin granules provide expansion and compression while thrombin initiates clot formation | May be used in areas of small arterial bleeding | Requires contact with blood | 6–8 weeks | $$$ |
| Fibrin sealants (Evicel, Tisseel, Crosseal) | Human | Liquid | Combination of fibrinogen and thrombin causes cleavage of fibrinogen to fibrin and resultant clot initiation | Fast-acting; hemostatic and adhesive properties; works well for diffusely oozing surfaces | Contraindicated in patients who have a history of anaphylactic reaction to serum-derived products or IgA deficiency | 10–14 days | $$$ |
| * Median cost for use in one case Key: $=inexpensive; $$=moderately expensive; $$$=expensive | |||||||
Controlling bleeding without injuring underlying tissue
CASE 2: After adhesiolysis, bleeding at multiple sites
You perform adnexectomy on a 47-year-old woman who has a large (7 to 8 cm), benign ovarian mass. As you operate, you discover that the lesion is adherent to the sigmoid mesentery and the posterior aspect of the uterus; it is also adherent to the pelvic sidewall, directly along the course of the ureter. Although you are able to release the various adhesive attachments, persistent bleeding is noted at multiple pinpoint areas along the mesentery, uterine serosa, and pelvic sidewall, even after the application of direct pressure.
What do you do next?
Although cautery can be used liberally on the uterus, its application to mesentery carries a risk of injury to the mesenteric vessels and bowel wall. Caution is advised when you are attempting to control bleeding on the peritoneum overlying the ureter, whether you are using suture ligature or an energy source. Ideally, you should identify the ureter using a retroperitoneal approach and mobilize it laterally before employing any of these techniques.
There are several potential approaches to the bleeding described in Case 2, all of them involving hemostatic adjuvants. The first decision you need to make, however, is whether to address each region separately or all sites in unison. If you opt to address them together—either during an open procedure or laparoscopy—a fibrin sealant (e.g., Evicel, Tisseel) is one option. It can be applied using a dripping technique or aerosolization, either of which allows for broad application of a thin film of the agent. The limitation of this approach is the volume of agent required to resolve the bleeding, with a potential need for multiple doses to completely coat the area.
Because fibrin sealants function independently of the patient’s coagulation cascade, they are particularly useful in the presence of disseminated intravascular coagulation (DIC) and other coagulopathies that might limit the effectiveness of preparations that require the patient’s own serum.
An alternative approach to Case 2 is to apply an oxidized regenerated cellulose (ORC) derivative directly to the affected areas. Various forms are available (e.g., Surgicel Fibrillar, Surgicel Nu-Knit). These ORC products can be cut and customized to the area in need of hemostasis, allowing each site to be addressed individually. These agents typically remain adherent after they are applied due to the nature of the interaction between the product, blood, and tissue.
A liquid or foam hemostatic agent (e.g., Surgiflo, Floseal, topical thrombin) could also be employed in this case, but application can be a challenge on a large area with a heterogeneous topography because of the tendency of such agents to migrate under the force of gravity, pooling away from the source of bleeding.
Is combining agents a good idea?
Although they are not typically approved for use in combination, sequential application of hemostatic agents may be considered when bleeding persists.
All hemostatic agents work best in combination with the application of pressure. It usually is advisable to use moist gauze for this purpose because it can be lifted away without significant adherence to the underlying hemostatic complex, avoiding clot
disruption.
CASE 2: Resolved
You opt to use an ORC product, customizing it to fit each bleeding site, and apply direct pressure. When hemostasis has been achieved at all sites, you complete the operation. The patient has an uneventful postoperative course.
Protect structures along the pelvic sidewall
CASE 3: When the application of pressure isn’t enough
While performing a left salpingo-oophorectomy for a 12-cm ovarian lesion, you use a retroperitoneal approach to identify the structures along the pelvic sidewall. During identification of the ureter, you encounter bleeding from a small vessel in the adjacent fatty areolar tissue. After a period of observation, during which you apply pressure to the area of concern, bleeding persists.
What hemostatic agent do you employ to stop it?
The careful application of steady pressure is often enough to safely control bleeding in the area of the pelvic sidewall. In the event that pressure alone fails to resolve the bleeding, however, it is critical to choose a remedy that avoids injuring the ureter, iliac vessels, and infundibulopelvic ligament. Wide exposure of the space may allow for direct identification of the point of bleeding and precise application of cautery, a hemoclip, or a tie. When this approach is not feasible, other solutions must be sought.
When traditional hemostatic techniques fail in delicate anatomic sites, such as the periureteral area, hemostatic agents are an effective option that can minimize the risk of injury to surrounding vital structures. The contour of the space calls for a product that can intercalate, such as a foam, sealant, or Surgicel Fibrillar. Direct, precise application to the point of bleeding is critical, and the “bunching up” of a more rigid and bulky agent may limit its application to the area of concern. Use of a moist gauze to apply direct pressure after application of the agent will increase the likelihood of success.
CASE 3: Resolved
You decide to apply a foam hemostatic agent because of its ability to conform to the irregular space. You also continue to apply gentle pressure to the point of bleeding, using a moist gauze. Within minutes, hemostasis is achieved. You are then able to finish the operation.
Other variables to consider
As these three cases illustrate, the use of hemostatic agents to control surgical bleeding requires an individualized approach. The site and amount of bleeding, as well as the patient’s hemodynamic and coagulation status, are key variables to be considered when selecting an agent.
For instance, because of their components, fibrin sealants can function independently of the patient’s coagulation status. ORC products provide a matrix that facilitates platelet aggregration and may be less effective when anti-platelet agents have been used.
It is also appropriate for the surgeon to be familiar with the relative cost of the agents available at his or her institution. In particular, when several agents may be equally effective in a particular set of circumstances, cost may be the determining factor.
Availability of these agents varies from one institution to the next; as a result, it can be challenging to maintain familiarity with all of the products in the marketplace. Having access to a diverse, readily available set of “go to” agents is critical to ensure rapid application in a clinical setting.
The surgeon’s preference also is important, particularly in regard to the ease of preparation and handling. Some agents may not be as suitable for minimally invasive procedures (see TABLE). For others, special laparoscopic applicators are available.
When using a hemostatic agent, it pays to consider the duration of its effect in the surgical site. Both the quantity of the agent that is applied and characteristics of the local operative site influence how quickly the agent degrades. Keep this in mind when imaging studies are planned for the early postoperative period. An ORC preparation, for example, may appear with small pockets of air that resemble an abscess. Effective communication with the radiology team is critical to avoid the misinterpretation of findings.
Curious to discover the preferences and practices of surgeons likely to utilize topical hemostatic agents, OBG Management polled several experienced and expert surgeons, including members of the journal’s Board of Editors and Virtual Board of Editors. Their diverse responses offer a snapshot of gynecologic surgical practice in 2012—but all agree that hemostatic products are no substitute for sound surgical technique.
JANELLE YATES, SENIOR EDITOR





We want to hear from you! Tell us what you think.
Recommended reading
Achneck HE, Sileshi B, Jamiolkowski RM, et al. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg. 2010;251(2):217-228.
Chapman WC, Singla N, Genyk Y, et al. A phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis. J Am Coll Surg. 2007;205(2):256-265.
Holub Z, Jabor A. Laparoscopic management of bleeding after laparoscopic or vaginal hysterectomy. JSLS. 2004;8(3):235-238.
Sharma JB, Malhotra M. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83(3):271-275.
Sharma JB, Malhotra M. Topical oxidized cellulose for tubal hemorrhage hemostasis during laparoscopic sterilization. Int J Gynaecol Obstet. 2003;82(2):221-222.
Vessel-sealing devices and hemostatic adjuvants are expanding the surgical armamentarium. These products provide a spectrum of alternatives that can serve you and your surgical patient well when traditional techniques for obtaining hemostasis fail to provide a satisfactory result. (Keep in mind, however, that technology is no substitute for excellent technique!)
In this article, we highlight three common scenarios in which topical hemostatic agents may be useful during gynecologic surgery. In addition, in the sidebar, five surgeons describe the hemostatic products they rely on most often—and tell why.

Following hysterectomy, persistent oozing along the anterior vaginal margin, distal to the cuff and adjacent to the site of bladder mobilization, may be managed with the aid of a topical hemostatic agent—in this case, a fibrin sealant.
When the site of bleeding is difficult to reach
CASE 1: Oozing at the site of bladder mobilization
You perform total hysterectomy in a 44-year-old woman who has uterine fibroids. After the procedure, you notice persistent oozing along the anterior vaginal margin, distal to the cuff and adjacent to where the bladder was mobilized.
How do you manage the oozing?
Wide mobilization of the bladder is a vital step in the safe performance of hysterectomy. Adhesions may complicate the process if the patient has had previous abdominal surgery, infection, or inflammation. Following mobilization of the bladder and removal of the uterus, bleeding may be visible along the adventitia of the posterior bladder wall or along the anterior surface of the vagina, distal to the cuff, as it is in this case (see the illustration).
Judicious application of an energy source is an option, but thermal injury to the bladder is a concern. A good alternative is proper placement of a hemostatic suture, but it can sometimes be difficult to avoid incorporating the bladder or injuring or obstructing the nearby ureter.
In this case, the location of the bleeding deep in the operative field poses a challenge, because of limited exposure and the proximity of the bladder and ureters. Virtually any hemostatic agent would work well in this circumstance (TABLE). For example, a flowable agent or fibrin sealant could be thoroughly applied to the area during a minimally invasive or open procedure and would naturally conform to the irregularities in the tissue, particularly the junction between the vagina and bladder flap.
A pliable product such as Surgicel Nu-Knit or Fibrillar would also work well in these circumstances, although successful application during laparoscopy may depend on the size of the trocar. For example, Nu-Knit would require trimming to a size suitable for passage through a trocar, made easier by moistening with saline. The weave of Fibrillar makes it more challenging to pass, intact, through a trocar; rolling the material into a cylindrical shape may reduce its diameter and allow it to pass more easily.
CASE 1: Resolved
You apply a fibrin sealant to the site of bleeding, and the oozing abates. Once complete hemostasis is ensured, you conclude the surgery and transfer the patient to recovery, where she does well.
Profiles in hemostasis: Strengths and weaknesses of topical agent
| Agent (brands) | Composition | Forms available | Mechanism of action | Advantages | Caveats | Duration | Relative cost* |
|---|---|---|---|---|---|---|---|
| Physical agents | |||||||
| Gelatin matrix (Gelfoam, Gelfilm, Surgifoam) | Porcine- derived collagen | Sponge, film, powder | Provides physical matrix for clot formation | Non-antigenic; neutral pH; may be used with thrombin | Material expansion may cause compression; Not for use in closed spaces or near nerve structures | 4–6 weeks | $ |
| Oxidized regenerated cellulose (Surgicel Fibrillar, Surgicel Nu-Knit) | Wood pulp | Mesh or packed fibers | Provides physical matrix for clot formation; acidic pH causes hemolysis and local clot formation | Pliable, easy to place through laparoscope; acidic pH has antimicrobial effect | Works best in a dry field. Acidic pH inactivates biologic agents, such as thrombin, and may increase inflammation. Avoid using excess material. | 2–4 weeks | $ |
| Microfibrillar collagen (Avitene, Instat, Helitene Helistat) | Bovine-derived collagen | Powder, non-woven sheet, sponge | Absorbable acid salt. Provides physical scaffold for platelet activation and clot initiation. | Sheet form may be passed through laparoscope; minimal expansion | Rare allergic reactions reported; may contribute to granuloma formation | 8–12 weeks | $$ |
| Biologically active agents | |||||||
| Topical thrombin (Thrombin-JMI, Recothrom, Evithrom, rh Thrombin) | Bovine, human, or recombinant | Liquid | Promotes conversion of fibrinogen to fibrin | May be combined effectively with physical agents of neutral pH; recombinant human thrombin will be available in the near future | Risk of blood-borne infection with non-recombinant human thrombin; risk of anaphylaxis and antibody formation with bovine thrombin | N/A | $$ |
| Hemostatic matrix (Floseal, Surgiflo) | Thrombin plus gelatin | Foam | Gelatin granules provide expansion and compression while thrombin initiates clot formation | May be used in areas of small arterial bleeding | Requires contact with blood | 6–8 weeks | $$$ |
| Fibrin sealants (Evicel, Tisseel, Crosseal) | Human | Liquid | Combination of fibrinogen and thrombin causes cleavage of fibrinogen to fibrin and resultant clot initiation | Fast-acting; hemostatic and adhesive properties; works well for diffusely oozing surfaces | Contraindicated in patients who have a history of anaphylactic reaction to serum-derived products or IgA deficiency | 10–14 days | $$$ |
| * Median cost for use in one case Key: $=inexpensive; $$=moderately expensive; $$$=expensive | |||||||
Controlling bleeding without injuring underlying tissue
CASE 2: After adhesiolysis, bleeding at multiple sites
You perform adnexectomy on a 47-year-old woman who has a large (7 to 8 cm), benign ovarian mass. As you operate, you discover that the lesion is adherent to the sigmoid mesentery and the posterior aspect of the uterus; it is also adherent to the pelvic sidewall, directly along the course of the ureter. Although you are able to release the various adhesive attachments, persistent bleeding is noted at multiple pinpoint areas along the mesentery, uterine serosa, and pelvic sidewall, even after the application of direct pressure.
What do you do next?
Although cautery can be used liberally on the uterus, its application to mesentery carries a risk of injury to the mesenteric vessels and bowel wall. Caution is advised when you are attempting to control bleeding on the peritoneum overlying the ureter, whether you are using suture ligature or an energy source. Ideally, you should identify the ureter using a retroperitoneal approach and mobilize it laterally before employing any of these techniques.
There are several potential approaches to the bleeding described in Case 2, all of them involving hemostatic adjuvants. The first decision you need to make, however, is whether to address each region separately or all sites in unison. If you opt to address them together—either during an open procedure or laparoscopy—a fibrin sealant (e.g., Evicel, Tisseel) is one option. It can be applied using a dripping technique or aerosolization, either of which allows for broad application of a thin film of the agent. The limitation of this approach is the volume of agent required to resolve the bleeding, with a potential need for multiple doses to completely coat the area.
Because fibrin sealants function independently of the patient’s coagulation cascade, they are particularly useful in the presence of disseminated intravascular coagulation (DIC) and other coagulopathies that might limit the effectiveness of preparations that require the patient’s own serum.
An alternative approach to Case 2 is to apply an oxidized regenerated cellulose (ORC) derivative directly to the affected areas. Various forms are available (e.g., Surgicel Fibrillar, Surgicel Nu-Knit). These ORC products can be cut and customized to the area in need of hemostasis, allowing each site to be addressed individually. These agents typically remain adherent after they are applied due to the nature of the interaction between the product, blood, and tissue.
A liquid or foam hemostatic agent (e.g., Surgiflo, Floseal, topical thrombin) could also be employed in this case, but application can be a challenge on a large area with a heterogeneous topography because of the tendency of such agents to migrate under the force of gravity, pooling away from the source of bleeding.
Is combining agents a good idea?
Although they are not typically approved for use in combination, sequential application of hemostatic agents may be considered when bleeding persists.
All hemostatic agents work best in combination with the application of pressure. It usually is advisable to use moist gauze for this purpose because it can be lifted away without significant adherence to the underlying hemostatic complex, avoiding clot
disruption.
CASE 2: Resolved
You opt to use an ORC product, customizing it to fit each bleeding site, and apply direct pressure. When hemostasis has been achieved at all sites, you complete the operation. The patient has an uneventful postoperative course.
Protect structures along the pelvic sidewall
CASE 3: When the application of pressure isn’t enough
While performing a left salpingo-oophorectomy for a 12-cm ovarian lesion, you use a retroperitoneal approach to identify the structures along the pelvic sidewall. During identification of the ureter, you encounter bleeding from a small vessel in the adjacent fatty areolar tissue. After a period of observation, during which you apply pressure to the area of concern, bleeding persists.
What hemostatic agent do you employ to stop it?
The careful application of steady pressure is often enough to safely control bleeding in the area of the pelvic sidewall. In the event that pressure alone fails to resolve the bleeding, however, it is critical to choose a remedy that avoids injuring the ureter, iliac vessels, and infundibulopelvic ligament. Wide exposure of the space may allow for direct identification of the point of bleeding and precise application of cautery, a hemoclip, or a tie. When this approach is not feasible, other solutions must be sought.
When traditional hemostatic techniques fail in delicate anatomic sites, such as the periureteral area, hemostatic agents are an effective option that can minimize the risk of injury to surrounding vital structures. The contour of the space calls for a product that can intercalate, such as a foam, sealant, or Surgicel Fibrillar. Direct, precise application to the point of bleeding is critical, and the “bunching up” of a more rigid and bulky agent may limit its application to the area of concern. Use of a moist gauze to apply direct pressure after application of the agent will increase the likelihood of success.
CASE 3: Resolved
You decide to apply a foam hemostatic agent because of its ability to conform to the irregular space. You also continue to apply gentle pressure to the point of bleeding, using a moist gauze. Within minutes, hemostasis is achieved. You are then able to finish the operation.
Other variables to consider
As these three cases illustrate, the use of hemostatic agents to control surgical bleeding requires an individualized approach. The site and amount of bleeding, as well as the patient’s hemodynamic and coagulation status, are key variables to be considered when selecting an agent.
For instance, because of their components, fibrin sealants can function independently of the patient’s coagulation status. ORC products provide a matrix that facilitates platelet aggregration and may be less effective when anti-platelet agents have been used.
It is also appropriate for the surgeon to be familiar with the relative cost of the agents available at his or her institution. In particular, when several agents may be equally effective in a particular set of circumstances, cost may be the determining factor.
Availability of these agents varies from one institution to the next; as a result, it can be challenging to maintain familiarity with all of the products in the marketplace. Having access to a diverse, readily available set of “go to” agents is critical to ensure rapid application in a clinical setting.
The surgeon’s preference also is important, particularly in regard to the ease of preparation and handling. Some agents may not be as suitable for minimally invasive procedures (see TABLE). For others, special laparoscopic applicators are available.
When using a hemostatic agent, it pays to consider the duration of its effect in the surgical site. Both the quantity of the agent that is applied and characteristics of the local operative site influence how quickly the agent degrades. Keep this in mind when imaging studies are planned for the early postoperative period. An ORC preparation, for example, may appear with small pockets of air that resemble an abscess. Effective communication with the radiology team is critical to avoid the misinterpretation of findings.
Curious to discover the preferences and practices of surgeons likely to utilize topical hemostatic agents, OBG Management polled several experienced and expert surgeons, including members of the journal’s Board of Editors and Virtual Board of Editors. Their diverse responses offer a snapshot of gynecologic surgical practice in 2012—but all agree that hemostatic products are no substitute for sound surgical technique.
JANELLE YATES, SENIOR EDITOR





We want to hear from you! Tell us what you think.
Vessel-sealing devices and hemostatic adjuvants are expanding the surgical armamentarium. These products provide a spectrum of alternatives that can serve you and your surgical patient well when traditional techniques for obtaining hemostasis fail to provide a satisfactory result. (Keep in mind, however, that technology is no substitute for excellent technique!)
In this article, we highlight three common scenarios in which topical hemostatic agents may be useful during gynecologic surgery. In addition, in the sidebar, five surgeons describe the hemostatic products they rely on most often—and tell why.

Following hysterectomy, persistent oozing along the anterior vaginal margin, distal to the cuff and adjacent to the site of bladder mobilization, may be managed with the aid of a topical hemostatic agent—in this case, a fibrin sealant.
When the site of bleeding is difficult to reach
CASE 1: Oozing at the site of bladder mobilization
You perform total hysterectomy in a 44-year-old woman who has uterine fibroids. After the procedure, you notice persistent oozing along the anterior vaginal margin, distal to the cuff and adjacent to where the bladder was mobilized.
How do you manage the oozing?
Wide mobilization of the bladder is a vital step in the safe performance of hysterectomy. Adhesions may complicate the process if the patient has had previous abdominal surgery, infection, or inflammation. Following mobilization of the bladder and removal of the uterus, bleeding may be visible along the adventitia of the posterior bladder wall or along the anterior surface of the vagina, distal to the cuff, as it is in this case (see the illustration).
Judicious application of an energy source is an option, but thermal injury to the bladder is a concern. A good alternative is proper placement of a hemostatic suture, but it can sometimes be difficult to avoid incorporating the bladder or injuring or obstructing the nearby ureter.
In this case, the location of the bleeding deep in the operative field poses a challenge, because of limited exposure and the proximity of the bladder and ureters. Virtually any hemostatic agent would work well in this circumstance (TABLE). For example, a flowable agent or fibrin sealant could be thoroughly applied to the area during a minimally invasive or open procedure and would naturally conform to the irregularities in the tissue, particularly the junction between the vagina and bladder flap.
A pliable product such as Surgicel Nu-Knit or Fibrillar would also work well in these circumstances, although successful application during laparoscopy may depend on the size of the trocar. For example, Nu-Knit would require trimming to a size suitable for passage through a trocar, made easier by moistening with saline. The weave of Fibrillar makes it more challenging to pass, intact, through a trocar; rolling the material into a cylindrical shape may reduce its diameter and allow it to pass more easily.
CASE 1: Resolved
You apply a fibrin sealant to the site of bleeding, and the oozing abates. Once complete hemostasis is ensured, you conclude the surgery and transfer the patient to recovery, where she does well.
Profiles in hemostasis: Strengths and weaknesses of topical agent
| Agent (brands) | Composition | Forms available | Mechanism of action | Advantages | Caveats | Duration | Relative cost* |
|---|---|---|---|---|---|---|---|
| Physical agents | |||||||
| Gelatin matrix (Gelfoam, Gelfilm, Surgifoam) | Porcine- derived collagen | Sponge, film, powder | Provides physical matrix for clot formation | Non-antigenic; neutral pH; may be used with thrombin | Material expansion may cause compression; Not for use in closed spaces or near nerve structures | 4–6 weeks | $ |
| Oxidized regenerated cellulose (Surgicel Fibrillar, Surgicel Nu-Knit) | Wood pulp | Mesh or packed fibers | Provides physical matrix for clot formation; acidic pH causes hemolysis and local clot formation | Pliable, easy to place through laparoscope; acidic pH has antimicrobial effect | Works best in a dry field. Acidic pH inactivates biologic agents, such as thrombin, and may increase inflammation. Avoid using excess material. | 2–4 weeks | $ |
| Microfibrillar collagen (Avitene, Instat, Helitene Helistat) | Bovine-derived collagen | Powder, non-woven sheet, sponge | Absorbable acid salt. Provides physical scaffold for platelet activation and clot initiation. | Sheet form may be passed through laparoscope; minimal expansion | Rare allergic reactions reported; may contribute to granuloma formation | 8–12 weeks | $$ |
| Biologically active agents | |||||||
| Topical thrombin (Thrombin-JMI, Recothrom, Evithrom, rh Thrombin) | Bovine, human, or recombinant | Liquid | Promotes conversion of fibrinogen to fibrin | May be combined effectively with physical agents of neutral pH; recombinant human thrombin will be available in the near future | Risk of blood-borne infection with non-recombinant human thrombin; risk of anaphylaxis and antibody formation with bovine thrombin | N/A | $$ |
| Hemostatic matrix (Floseal, Surgiflo) | Thrombin plus gelatin | Foam | Gelatin granules provide expansion and compression while thrombin initiates clot formation | May be used in areas of small arterial bleeding | Requires contact with blood | 6–8 weeks | $$$ |
| Fibrin sealants (Evicel, Tisseel, Crosseal) | Human | Liquid | Combination of fibrinogen and thrombin causes cleavage of fibrinogen to fibrin and resultant clot initiation | Fast-acting; hemostatic and adhesive properties; works well for diffusely oozing surfaces | Contraindicated in patients who have a history of anaphylactic reaction to serum-derived products or IgA deficiency | 10–14 days | $$$ |
| * Median cost for use in one case Key: $=inexpensive; $$=moderately expensive; $$$=expensive | |||||||
Controlling bleeding without injuring underlying tissue
CASE 2: After adhesiolysis, bleeding at multiple sites
You perform adnexectomy on a 47-year-old woman who has a large (7 to 8 cm), benign ovarian mass. As you operate, you discover that the lesion is adherent to the sigmoid mesentery and the posterior aspect of the uterus; it is also adherent to the pelvic sidewall, directly along the course of the ureter. Although you are able to release the various adhesive attachments, persistent bleeding is noted at multiple pinpoint areas along the mesentery, uterine serosa, and pelvic sidewall, even after the application of direct pressure.
What do you do next?
Although cautery can be used liberally on the uterus, its application to mesentery carries a risk of injury to the mesenteric vessels and bowel wall. Caution is advised when you are attempting to control bleeding on the peritoneum overlying the ureter, whether you are using suture ligature or an energy source. Ideally, you should identify the ureter using a retroperitoneal approach and mobilize it laterally before employing any of these techniques.
There are several potential approaches to the bleeding described in Case 2, all of them involving hemostatic adjuvants. The first decision you need to make, however, is whether to address each region separately or all sites in unison. If you opt to address them together—either during an open procedure or laparoscopy—a fibrin sealant (e.g., Evicel, Tisseel) is one option. It can be applied using a dripping technique or aerosolization, either of which allows for broad application of a thin film of the agent. The limitation of this approach is the volume of agent required to resolve the bleeding, with a potential need for multiple doses to completely coat the area.
Because fibrin sealants function independently of the patient’s coagulation cascade, they are particularly useful in the presence of disseminated intravascular coagulation (DIC) and other coagulopathies that might limit the effectiveness of preparations that require the patient’s own serum.
An alternative approach to Case 2 is to apply an oxidized regenerated cellulose (ORC) derivative directly to the affected areas. Various forms are available (e.g., Surgicel Fibrillar, Surgicel Nu-Knit). These ORC products can be cut and customized to the area in need of hemostasis, allowing each site to be addressed individually. These agents typically remain adherent after they are applied due to the nature of the interaction between the product, blood, and tissue.
A liquid or foam hemostatic agent (e.g., Surgiflo, Floseal, topical thrombin) could also be employed in this case, but application can be a challenge on a large area with a heterogeneous topography because of the tendency of such agents to migrate under the force of gravity, pooling away from the source of bleeding.
Is combining agents a good idea?
Although they are not typically approved for use in combination, sequential application of hemostatic agents may be considered when bleeding persists.
All hemostatic agents work best in combination with the application of pressure. It usually is advisable to use moist gauze for this purpose because it can be lifted away without significant adherence to the underlying hemostatic complex, avoiding clot
disruption.
CASE 2: Resolved
You opt to use an ORC product, customizing it to fit each bleeding site, and apply direct pressure. When hemostasis has been achieved at all sites, you complete the operation. The patient has an uneventful postoperative course.
Protect structures along the pelvic sidewall
CASE 3: When the application of pressure isn’t enough
While performing a left salpingo-oophorectomy for a 12-cm ovarian lesion, you use a retroperitoneal approach to identify the structures along the pelvic sidewall. During identification of the ureter, you encounter bleeding from a small vessel in the adjacent fatty areolar tissue. After a period of observation, during which you apply pressure to the area of concern, bleeding persists.
What hemostatic agent do you employ to stop it?
The careful application of steady pressure is often enough to safely control bleeding in the area of the pelvic sidewall. In the event that pressure alone fails to resolve the bleeding, however, it is critical to choose a remedy that avoids injuring the ureter, iliac vessels, and infundibulopelvic ligament. Wide exposure of the space may allow for direct identification of the point of bleeding and precise application of cautery, a hemoclip, or a tie. When this approach is not feasible, other solutions must be sought.
When traditional hemostatic techniques fail in delicate anatomic sites, such as the periureteral area, hemostatic agents are an effective option that can minimize the risk of injury to surrounding vital structures. The contour of the space calls for a product that can intercalate, such as a foam, sealant, or Surgicel Fibrillar. Direct, precise application to the point of bleeding is critical, and the “bunching up” of a more rigid and bulky agent may limit its application to the area of concern. Use of a moist gauze to apply direct pressure after application of the agent will increase the likelihood of success.
CASE 3: Resolved
You decide to apply a foam hemostatic agent because of its ability to conform to the irregular space. You also continue to apply gentle pressure to the point of bleeding, using a moist gauze. Within minutes, hemostasis is achieved. You are then able to finish the operation.
Other variables to consider
As these three cases illustrate, the use of hemostatic agents to control surgical bleeding requires an individualized approach. The site and amount of bleeding, as well as the patient’s hemodynamic and coagulation status, are key variables to be considered when selecting an agent.
For instance, because of their components, fibrin sealants can function independently of the patient’s coagulation status. ORC products provide a matrix that facilitates platelet aggregration and may be less effective when anti-platelet agents have been used.
It is also appropriate for the surgeon to be familiar with the relative cost of the agents available at his or her institution. In particular, when several agents may be equally effective in a particular set of circumstances, cost may be the determining factor.
Availability of these agents varies from one institution to the next; as a result, it can be challenging to maintain familiarity with all of the products in the marketplace. Having access to a diverse, readily available set of “go to” agents is critical to ensure rapid application in a clinical setting.
The surgeon’s preference also is important, particularly in regard to the ease of preparation and handling. Some agents may not be as suitable for minimally invasive procedures (see TABLE). For others, special laparoscopic applicators are available.
When using a hemostatic agent, it pays to consider the duration of its effect in the surgical site. Both the quantity of the agent that is applied and characteristics of the local operative site influence how quickly the agent degrades. Keep this in mind when imaging studies are planned for the early postoperative period. An ORC preparation, for example, may appear with small pockets of air that resemble an abscess. Effective communication with the radiology team is critical to avoid the misinterpretation of findings.
Curious to discover the preferences and practices of surgeons likely to utilize topical hemostatic agents, OBG Management polled several experienced and expert surgeons, including members of the journal’s Board of Editors and Virtual Board of Editors. Their diverse responses offer a snapshot of gynecologic surgical practice in 2012—but all agree that hemostatic products are no substitute for sound surgical technique.
JANELLE YATES, SENIOR EDITOR





We want to hear from you! Tell us what you think.
Recommended reading
Achneck HE, Sileshi B, Jamiolkowski RM, et al. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg. 2010;251(2):217-228.
Chapman WC, Singla N, Genyk Y, et al. A phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis. J Am Coll Surg. 2007;205(2):256-265.
Holub Z, Jabor A. Laparoscopic management of bleeding after laparoscopic or vaginal hysterectomy. JSLS. 2004;8(3):235-238.
Sharma JB, Malhotra M. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83(3):271-275.
Sharma JB, Malhotra M. Topical oxidized cellulose for tubal hemorrhage hemostasis during laparoscopic sterilization. Int J Gynaecol Obstet. 2003;82(2):221-222.
Recommended reading
Achneck HE, Sileshi B, Jamiolkowski RM, et al. A comprehensive review of topical hemostatic agents: efficacy and recommendations for use. Ann Surg. 2010;251(2):217-228.
Chapman WC, Singla N, Genyk Y, et al. A phase 3, randomized, double-blind comparative study of the efficacy and safety of topical recombinant human thrombin and bovine thrombin in surgical hemostasis. J Am Coll Surg. 2007;205(2):256-265.
Holub Z, Jabor A. Laparoscopic management of bleeding after laparoscopic or vaginal hysterectomy. JSLS. 2004;8(3):235-238.
Sharma JB, Malhotra M. Laparoscopic oxidized cellulose (Surgicel) application for small uterine perforations. Int J Gynaecol Obstet. 2003;83(3):271-275.
Sharma JB, Malhotra M. Topical oxidized cellulose for tubal hemorrhage hemostasis during laparoscopic sterilization. Int J Gynaecol Obstet. 2003;82(2):221-222.
Step by step: Obliterating the vaginal canal to correct pelvic organ prolapse
- LeFort partial colpocleisis
- Colpectomy and colpocleisis
- Colpectomy and colpocleisis after two previously failed obliterative procedures
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
As women live longer, on average, pelvic floor disorders are, as a whole, becoming more prevalent and a greater health and social problem. Many women entering the eighth and ninth decades of life display symptomatic pelvic organ prolapse (POP)—often after an unsuccessful trial of a pessary or even surgery.
These elderly patients often have other concomitant medical issues and are not sexually active, making extensive surgery for them less than an ideal solution. Instead, surgical procedures that obliterate the vaginal canal can alleviate their symptoms of POP.
In this article, we provide a step-by-step description of:
- LeFort partial colpocleisis in a woman who still has her uterus in place
- partial or complete colpectomy and colpocleisis in a woman who has post-hysterectomy prolapse
- levator plication and perineorrhaphy, as essential concluding steps in these procedures.
LeFort partial colpocleisis
An obliterative procedure in the form of a LeFort partial colpocleisis is an option when a patient 1) has her uterus and 2) is no longer sexually active. Because the uterus is retained in this procedure, however, keep in mind that it will be difficult to evaluate any uterine bleeding or cervical pathology in the future. Endovaginal ultrasonography or an endometrial biopsy, and a Pap smear, must be done before LeFort surgery.
The ideal candidate for LeFort partial colpocleisis is a woman who has complete uterine prolapse, or procidentia (FIGURE 1), which is characterized by symmetric eversion of the anterior and posterior vaginal walls.

FIGURE 1 Pelvic organ prolapse, preoperatively
Top: Uterine procidentia. A patient who has this condition is an ideal candidate for LeFort partial colpocleisis. Bottom: Asymmetric anterior vaginal prolapse.
LeFort partial colpocleisis: Key step by key step
![]()
![]()
![]()
![]()
![]()
![]()
![]()
FIGURE 2 shows key steps in performing LeFort partial colpocleisis. See Video #1 at www.obgmanagement.com for demonstrations of how to perform LeFort partial colpocleisis.
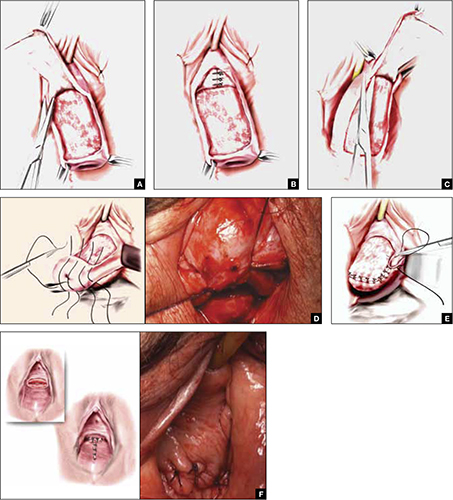
FIGURE 2 Steps: LeFort partial colpocleisis
A. Denude the anterior vaginal epithelium. B. Plicate the neck of the bladder. C. Next, denude the posterior vaginal epithelium. D. Approximate most proximal surfaces. E. Place lateral sutures to allow for drainage canals. F. The uterus has been replaced and most of the distal incisions closed.
Total colpectomy and colpocleisis: Key step by key step
In a patient who has post-hysterectomy prolapse and is not interested in continued sexual function, total colpectomy and colpocleisis provide a highly minimally invasive, durable option to correct her prolapse.
If there is complete eversion of the vagina then, truly, total colpectomy and colpocleisis is the procedure of choice. If there is significant prolapse of only one segment of the pelvic floor, however—for example, the anterior vaginal wall (FIGURE 1)—then aggressive repair of this variant with a narrowing down of the genital hiatus accomplishes the same result without requiring complete removal of what appears to be fairly well supported vaginal mucosa.
Here are key steps for performing partial or complete colpectomy and colpocleisis.
![]()
![]()
Completely remove the vaginal epithelium (FIGURES 3A and 3B); your goal is to leave most of the muscularis of the vaginal wall on the prolapse.
Avoid the peritoneal cavity if at all possible; when the main portion of the prolapse is secondary to an enterocele and the vaginal epithelium is very thin, however, formal excision of the enterocele sac, with closing of the defect, may be required.
![]()
If at all possible, avoid the peritoneum and the wall of the viscera, whether bladder or bowel. Invert the apex of the soft tissue, using the tip of forceps, as each purse-string suture is tied.
There is a variation of this procedure: Perform a separate anterior and posterior colporrhaphy, with two purse-string sutures used to approximate the anterior and posterior segments, thus obliterating any dead space.
![]()
![]()
See Video #2 and Video #3 for a demonstration of how to perform a complete colpectomy and colpocleisis. FIGURE 3D shows the completed colpocleisis.
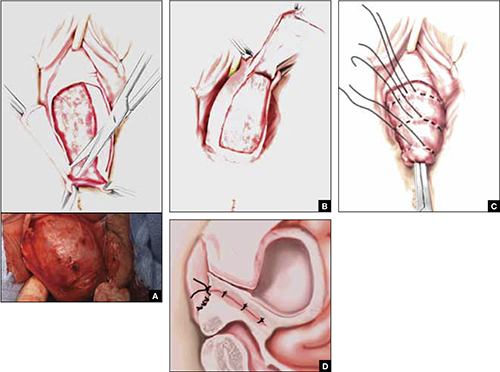
FIGURE 3 Steps: Total colpectomy and colpocleisis
Denude the anterior vaginal epithelium (A) and then the posterior epithelium (B). C. Place sequential purse-string sutures. D. The completed colpocleisis, in cross-section.
Distal levatoroplasty with high perineorrhaphy: Key step by key step
![]()
![]()
![]()
![]()
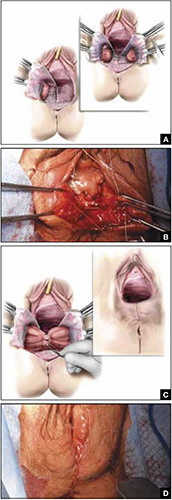
FIGURE 4 Steps: Distal levatoroplasty with high perineorrhaphy
A. Lateral dissection to the levator ani muscles. Inset: levator ani plicated with sequential sutures. B. Place three sutures to plicate the levator ani. C. Secure the plication sutures. Inset C, and D: Completed levatoroplasty.
Our experience
We are often asked questions about the procedures that we’ve just described, including patients’ satisfaction with the outcome, complications, and the risk that prolapse will recur. In the accompanying box, “Questions we’re asked (and answers we give) about obliterative surgery,” opposite, we give our responses to eight common inquiries.
about obliterative surgery
Q1 How satisfied are women with the outcome of these procedures—do many regret having their vaginal canal obliterated?
A Overall, studies indicate that 85% to 100% of patients are “satisfied” or “very satisfied” with the outcomes of obliterative procedures.1 There are rare reports of regret after colpocleisis over loss of coital ability; in one study of a series of procedures,2 5% of subjects expressed regret postoperatively.
Q2 Why is levatoroplasty and perineorrhaphy such an important part of both the LeFort partial colpocleisis and colpectomy and colpocleisis?
A The aim of both these procedures is to reduce prolapsed tissue. The true durability of repair comes from significantly decreasing the caliber of the genital hiatus, with the hope of closing off the bulk of the distal vaginal canal. This can really only be accomplished by utilizing an aggressive levatoroplasty and perineorrhaphy, described in the text.
Q3 How often do patients develop de novo stress incontinence or significant voiding dysfunction, or both, after an obliterative procedure?
A The risk of developing urinary incontinence after an obliterative procedure is difficult to ascertain. In general, patients who had retention or a high postvoid residual volume preoperatively have a good outcome in regard to correcting their voiding dysfunction. This is because, in most cases, the voiding dysfunction is directly related to the anatomic distortion created by the prolapse.
Q4 What is the rate of prolapse recurrence after these procedures, and how is a recurrence managed?
A Multiple studies have documented an excellent anatomic outcome after these procedures, with a prolapse recurrence rate of only 1% to 8%.3 Very little has been written about how to best manage recurrent prolapse after an obliterative procedure. Most surgeons would, most likely, recommend repeat colpocleisis or aggressive levatoroplasty and perineorrhaphy. (Note: The patient whose colpectomy and colpocleisis is shown in Video #3 failed two previous colpectomy and colpocleisis procedures.)
Q5 Can these procedures be performed under local anesthesia, with some intravenous sedation, or under regional anesthesia—thereby avoiding intubation?
A Yes. We have utilized IV sedation and bilateral block successfully to perform these procedures. (Note: Video #3 of LeFort partial colpocleisis shows the procedure performed under local anesthesia.)
Q6 What does the literature say about common complications after these procedures?
A Postoperative morbidity and mortality in the elderly surgical population is a considerable concern. Significant postoperative complications occur in approximately 5% of patients in modern series4—often attributed to the effects of age and to the frail condition of patients who are commonly selected for colpocleisis.
Specifically, approximately 5% of patients experience a postoperative cardiac, thromboembolic, pulmonary, or cerebrovascular event. Transfusion is the most commonly reported major complication related to the procedure itself. Other complications include:
- fever and its associated morbidity
- pneumonia
- ongoing vaginal bleeding
- pyelonephritis
- hematoma
- cystotomy
- ureteral occlusion.
Minor surgical complications occur at a rate of approximately 15%. Surgical mortality is about 1 in 400 cases.
Q7 Do you routinely undertake urodynamic study of patients who are scheduled to undergo an obliterative procedure?
A At minimum, a lower urinary tract evaluation should include a postvoid residual volume study and, we believe, some kind of a filling study and stress test, with reduction of the prolapse. Beyond that, we recommend that you conduct more detailed urodynamic tests on a patient-by-patient basis, when you think that the findings will add to the clinical picture.
Q8 Would you ever perform a vaginal hysterectomy and then proceed with a colpectomy and colpocleisis?
A The principal rationale for performing hysterectomy at the time of colpocleisis is to eliminate the risk of endometrial or cervical carcinoma. Hysterectomy also eliminates the risk of pyometra, a rare but serious complication that can occur when the lateral canals become obstructed after a LeFort procedure.
A recent study5 looked at 1) concomitant hysterectomy in conjunction with colpectomy and colpocleisis and 2) traditional LeFort partial colpocleisis. In this retrospective review, objective and subjective success rates were high, but patients who underwent hysterectomy had a statistically significantly greater decline in postoperative hematocrit and a significant increase in the need for transfusion, compared with patients who did not undergo hysterectomy (35% vs. 13%).
References
1. Fitzgerald MP, Richter HE, Bradley CS, et al. Pelvic support, pelvic symptoms and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1603-1609.
2. Hullfish K, Bobbjerg B, Steers W. Colpocleisis for pelvic organ prolapse Patient Goals Quality of life and Satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
3. Fitzgerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189(5):1241-1244.
4. von Pechmann WS, Muton M, Fyffe J, Hale DS. Total colpocleisis with high levator placation for the treatment of advanced organ prolapse. Am J Obstet Gynecol. 2003;189(1):121-126.
5. Kohli NE, Sze E, Karram M. Pyometra following LeFort colpocleisis. Int Urogyn J. 1996;7(5):264-266.
We want to hear from you! Tell us what you think.
- LeFort partial colpocleisis
- Colpectomy and colpocleisis
- Colpectomy and colpocleisis after two previously failed obliterative procedures
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
As women live longer, on average, pelvic floor disorders are, as a whole, becoming more prevalent and a greater health and social problem. Many women entering the eighth and ninth decades of life display symptomatic pelvic organ prolapse (POP)—often after an unsuccessful trial of a pessary or even surgery.
These elderly patients often have other concomitant medical issues and are not sexually active, making extensive surgery for them less than an ideal solution. Instead, surgical procedures that obliterate the vaginal canal can alleviate their symptoms of POP.
In this article, we provide a step-by-step description of:
- LeFort partial colpocleisis in a woman who still has her uterus in place
- partial or complete colpectomy and colpocleisis in a woman who has post-hysterectomy prolapse
- levator plication and perineorrhaphy, as essential concluding steps in these procedures.
LeFort partial colpocleisis
An obliterative procedure in the form of a LeFort partial colpocleisis is an option when a patient 1) has her uterus and 2) is no longer sexually active. Because the uterus is retained in this procedure, however, keep in mind that it will be difficult to evaluate any uterine bleeding or cervical pathology in the future. Endovaginal ultrasonography or an endometrial biopsy, and a Pap smear, must be done before LeFort surgery.
The ideal candidate for LeFort partial colpocleisis is a woman who has complete uterine prolapse, or procidentia (FIGURE 1), which is characterized by symmetric eversion of the anterior and posterior vaginal walls.

FIGURE 1 Pelvic organ prolapse, preoperatively
Top: Uterine procidentia. A patient who has this condition is an ideal candidate for LeFort partial colpocleisis. Bottom: Asymmetric anterior vaginal prolapse.
LeFort partial colpocleisis: Key step by key step
![]()
![]()
![]()
![]()
![]()
![]()
![]()
FIGURE 2 shows key steps in performing LeFort partial colpocleisis. See Video #1 at www.obgmanagement.com for demonstrations of how to perform LeFort partial colpocleisis.

FIGURE 2 Steps: LeFort partial colpocleisis
A. Denude the anterior vaginal epithelium. B. Plicate the neck of the bladder. C. Next, denude the posterior vaginal epithelium. D. Approximate most proximal surfaces. E. Place lateral sutures to allow for drainage canals. F. The uterus has been replaced and most of the distal incisions closed.
Total colpectomy and colpocleisis: Key step by key step
In a patient who has post-hysterectomy prolapse and is not interested in continued sexual function, total colpectomy and colpocleisis provide a highly minimally invasive, durable option to correct her prolapse.
If there is complete eversion of the vagina then, truly, total colpectomy and colpocleisis is the procedure of choice. If there is significant prolapse of only one segment of the pelvic floor, however—for example, the anterior vaginal wall (FIGURE 1)—then aggressive repair of this variant with a narrowing down of the genital hiatus accomplishes the same result without requiring complete removal of what appears to be fairly well supported vaginal mucosa.
Here are key steps for performing partial or complete colpectomy and colpocleisis.
![]()
![]()
Completely remove the vaginal epithelium (FIGURES 3A and 3B); your goal is to leave most of the muscularis of the vaginal wall on the prolapse.
Avoid the peritoneal cavity if at all possible; when the main portion of the prolapse is secondary to an enterocele and the vaginal epithelium is very thin, however, formal excision of the enterocele sac, with closing of the defect, may be required.
![]()
If at all possible, avoid the peritoneum and the wall of the viscera, whether bladder or bowel. Invert the apex of the soft tissue, using the tip of forceps, as each purse-string suture is tied.
There is a variation of this procedure: Perform a separate anterior and posterior colporrhaphy, with two purse-string sutures used to approximate the anterior and posterior segments, thus obliterating any dead space.
![]()
![]()
See Video #2 and Video #3 for a demonstration of how to perform a complete colpectomy and colpocleisis. FIGURE 3D shows the completed colpocleisis.

FIGURE 3 Steps: Total colpectomy and colpocleisis
Denude the anterior vaginal epithelium (A) and then the posterior epithelium (B). C. Place sequential purse-string sutures. D. The completed colpocleisis, in cross-section.
Distal levatoroplasty with high perineorrhaphy: Key step by key step
![]()
![]()
![]()
![]()

FIGURE 4 Steps: Distal levatoroplasty with high perineorrhaphy
A. Lateral dissection to the levator ani muscles. Inset: levator ani plicated with sequential sutures. B. Place three sutures to plicate the levator ani. C. Secure the plication sutures. Inset C, and D: Completed levatoroplasty.
Our experience
We are often asked questions about the procedures that we’ve just described, including patients’ satisfaction with the outcome, complications, and the risk that prolapse will recur. In the accompanying box, “Questions we’re asked (and answers we give) about obliterative surgery,” opposite, we give our responses to eight common inquiries.
about obliterative surgery
Q1 How satisfied are women with the outcome of these procedures—do many regret having their vaginal canal obliterated?
A Overall, studies indicate that 85% to 100% of patients are “satisfied” or “very satisfied” with the outcomes of obliterative procedures.1 There are rare reports of regret after colpocleisis over loss of coital ability; in one study of a series of procedures,2 5% of subjects expressed regret postoperatively.
Q2 Why is levatoroplasty and perineorrhaphy such an important part of both the LeFort partial colpocleisis and colpectomy and colpocleisis?
A The aim of both these procedures is to reduce prolapsed tissue. The true durability of repair comes from significantly decreasing the caliber of the genital hiatus, with the hope of closing off the bulk of the distal vaginal canal. This can really only be accomplished by utilizing an aggressive levatoroplasty and perineorrhaphy, described in the text.
Q3 How often do patients develop de novo stress incontinence or significant voiding dysfunction, or both, after an obliterative procedure?
A The risk of developing urinary incontinence after an obliterative procedure is difficult to ascertain. In general, patients who had retention or a high postvoid residual volume preoperatively have a good outcome in regard to correcting their voiding dysfunction. This is because, in most cases, the voiding dysfunction is directly related to the anatomic distortion created by the prolapse.
Q4 What is the rate of prolapse recurrence after these procedures, and how is a recurrence managed?
A Multiple studies have documented an excellent anatomic outcome after these procedures, with a prolapse recurrence rate of only 1% to 8%.3 Very little has been written about how to best manage recurrent prolapse after an obliterative procedure. Most surgeons would, most likely, recommend repeat colpocleisis or aggressive levatoroplasty and perineorrhaphy. (Note: The patient whose colpectomy and colpocleisis is shown in Video #3 failed two previous colpectomy and colpocleisis procedures.)
Q5 Can these procedures be performed under local anesthesia, with some intravenous sedation, or under regional anesthesia—thereby avoiding intubation?
A Yes. We have utilized IV sedation and bilateral block successfully to perform these procedures. (Note: Video #3 of LeFort partial colpocleisis shows the procedure performed under local anesthesia.)
Q6 What does the literature say about common complications after these procedures?
A Postoperative morbidity and mortality in the elderly surgical population is a considerable concern. Significant postoperative complications occur in approximately 5% of patients in modern series4—often attributed to the effects of age and to the frail condition of patients who are commonly selected for colpocleisis.
Specifically, approximately 5% of patients experience a postoperative cardiac, thromboembolic, pulmonary, or cerebrovascular event. Transfusion is the most commonly reported major complication related to the procedure itself. Other complications include:
- fever and its associated morbidity
- pneumonia
- ongoing vaginal bleeding
- pyelonephritis
- hematoma
- cystotomy
- ureteral occlusion.
Minor surgical complications occur at a rate of approximately 15%. Surgical mortality is about 1 in 400 cases.
Q7 Do you routinely undertake urodynamic study of patients who are scheduled to undergo an obliterative procedure?
A At minimum, a lower urinary tract evaluation should include a postvoid residual volume study and, we believe, some kind of a filling study and stress test, with reduction of the prolapse. Beyond that, we recommend that you conduct more detailed urodynamic tests on a patient-by-patient basis, when you think that the findings will add to the clinical picture.
Q8 Would you ever perform a vaginal hysterectomy and then proceed with a colpectomy and colpocleisis?
A The principal rationale for performing hysterectomy at the time of colpocleisis is to eliminate the risk of endometrial or cervical carcinoma. Hysterectomy also eliminates the risk of pyometra, a rare but serious complication that can occur when the lateral canals become obstructed after a LeFort procedure.
A recent study5 looked at 1) concomitant hysterectomy in conjunction with colpectomy and colpocleisis and 2) traditional LeFort partial colpocleisis. In this retrospective review, objective and subjective success rates were high, but patients who underwent hysterectomy had a statistically significantly greater decline in postoperative hematocrit and a significant increase in the need for transfusion, compared with patients who did not undergo hysterectomy (35% vs. 13%).
References
1. Fitzgerald MP, Richter HE, Bradley CS, et al. Pelvic support, pelvic symptoms and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1603-1609.
2. Hullfish K, Bobbjerg B, Steers W. Colpocleisis for pelvic organ prolapse Patient Goals Quality of life and Satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
3. Fitzgerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189(5):1241-1244.
4. von Pechmann WS, Muton M, Fyffe J, Hale DS. Total colpocleisis with high levator placation for the treatment of advanced organ prolapse. Am J Obstet Gynecol. 2003;189(1):121-126.
5. Kohli NE, Sze E, Karram M. Pyometra following LeFort colpocleisis. Int Urogyn J. 1996;7(5):264-266.
We want to hear from you! Tell us what you think.
- LeFort partial colpocleisis
- Colpectomy and colpocleisis
- Colpectomy and colpocleisis after two previously failed obliterative procedures
These videos were selected by Mickey Karram, MD, and are presented courtesy of the International Academy of Pelvic Surgery (IAPS)
This article, with accompanying video footage, is presented with the support of the International Academy of Pelvic Surgery.
As women live longer, on average, pelvic floor disorders are, as a whole, becoming more prevalent and a greater health and social problem. Many women entering the eighth and ninth decades of life display symptomatic pelvic organ prolapse (POP)—often after an unsuccessful trial of a pessary or even surgery.
These elderly patients often have other concomitant medical issues and are not sexually active, making extensive surgery for them less than an ideal solution. Instead, surgical procedures that obliterate the vaginal canal can alleviate their symptoms of POP.
In this article, we provide a step-by-step description of:
- LeFort partial colpocleisis in a woman who still has her uterus in place
- partial or complete colpectomy and colpocleisis in a woman who has post-hysterectomy prolapse
- levator plication and perineorrhaphy, as essential concluding steps in these procedures.
LeFort partial colpocleisis
An obliterative procedure in the form of a LeFort partial colpocleisis is an option when a patient 1) has her uterus and 2) is no longer sexually active. Because the uterus is retained in this procedure, however, keep in mind that it will be difficult to evaluate any uterine bleeding or cervical pathology in the future. Endovaginal ultrasonography or an endometrial biopsy, and a Pap smear, must be done before LeFort surgery.
The ideal candidate for LeFort partial colpocleisis is a woman who has complete uterine prolapse, or procidentia (FIGURE 1), which is characterized by symmetric eversion of the anterior and posterior vaginal walls.

FIGURE 1 Pelvic organ prolapse, preoperatively
Top: Uterine procidentia. A patient who has this condition is an ideal candidate for LeFort partial colpocleisis. Bottom: Asymmetric anterior vaginal prolapse.
LeFort partial colpocleisis: Key step by key step
![]()
![]()
![]()
![]()
![]()
![]()
![]()
FIGURE 2 shows key steps in performing LeFort partial colpocleisis. See Video #1 at www.obgmanagement.com for demonstrations of how to perform LeFort partial colpocleisis.

FIGURE 2 Steps: LeFort partial colpocleisis
A. Denude the anterior vaginal epithelium. B. Plicate the neck of the bladder. C. Next, denude the posterior vaginal epithelium. D. Approximate most proximal surfaces. E. Place lateral sutures to allow for drainage canals. F. The uterus has been replaced and most of the distal incisions closed.
Total colpectomy and colpocleisis: Key step by key step
In a patient who has post-hysterectomy prolapse and is not interested in continued sexual function, total colpectomy and colpocleisis provide a highly minimally invasive, durable option to correct her prolapse.
If there is complete eversion of the vagina then, truly, total colpectomy and colpocleisis is the procedure of choice. If there is significant prolapse of only one segment of the pelvic floor, however—for example, the anterior vaginal wall (FIGURE 1)—then aggressive repair of this variant with a narrowing down of the genital hiatus accomplishes the same result without requiring complete removal of what appears to be fairly well supported vaginal mucosa.
Here are key steps for performing partial or complete colpectomy and colpocleisis.
![]()
![]()
Completely remove the vaginal epithelium (FIGURES 3A and 3B); your goal is to leave most of the muscularis of the vaginal wall on the prolapse.
Avoid the peritoneal cavity if at all possible; when the main portion of the prolapse is secondary to an enterocele and the vaginal epithelium is very thin, however, formal excision of the enterocele sac, with closing of the defect, may be required.
![]()
If at all possible, avoid the peritoneum and the wall of the viscera, whether bladder or bowel. Invert the apex of the soft tissue, using the tip of forceps, as each purse-string suture is tied.
There is a variation of this procedure: Perform a separate anterior and posterior colporrhaphy, with two purse-string sutures used to approximate the anterior and posterior segments, thus obliterating any dead space.
![]()
![]()
See Video #2 and Video #3 for a demonstration of how to perform a complete colpectomy and colpocleisis. FIGURE 3D shows the completed colpocleisis.

FIGURE 3 Steps: Total colpectomy and colpocleisis
Denude the anterior vaginal epithelium (A) and then the posterior epithelium (B). C. Place sequential purse-string sutures. D. The completed colpocleisis, in cross-section.
Distal levatoroplasty with high perineorrhaphy: Key step by key step
![]()
![]()
![]()
![]()

FIGURE 4 Steps: Distal levatoroplasty with high perineorrhaphy
A. Lateral dissection to the levator ani muscles. Inset: levator ani plicated with sequential sutures. B. Place three sutures to plicate the levator ani. C. Secure the plication sutures. Inset C, and D: Completed levatoroplasty.
Our experience
We are often asked questions about the procedures that we’ve just described, including patients’ satisfaction with the outcome, complications, and the risk that prolapse will recur. In the accompanying box, “Questions we’re asked (and answers we give) about obliterative surgery,” opposite, we give our responses to eight common inquiries.
about obliterative surgery
Q1 How satisfied are women with the outcome of these procedures—do many regret having their vaginal canal obliterated?
A Overall, studies indicate that 85% to 100% of patients are “satisfied” or “very satisfied” with the outcomes of obliterative procedures.1 There are rare reports of regret after colpocleisis over loss of coital ability; in one study of a series of procedures,2 5% of subjects expressed regret postoperatively.
Q2 Why is levatoroplasty and perineorrhaphy such an important part of both the LeFort partial colpocleisis and colpectomy and colpocleisis?
A The aim of both these procedures is to reduce prolapsed tissue. The true durability of repair comes from significantly decreasing the caliber of the genital hiatus, with the hope of closing off the bulk of the distal vaginal canal. This can really only be accomplished by utilizing an aggressive levatoroplasty and perineorrhaphy, described in the text.
Q3 How often do patients develop de novo stress incontinence or significant voiding dysfunction, or both, after an obliterative procedure?
A The risk of developing urinary incontinence after an obliterative procedure is difficult to ascertain. In general, patients who had retention or a high postvoid residual volume preoperatively have a good outcome in regard to correcting their voiding dysfunction. This is because, in most cases, the voiding dysfunction is directly related to the anatomic distortion created by the prolapse.
Q4 What is the rate of prolapse recurrence after these procedures, and how is a recurrence managed?
A Multiple studies have documented an excellent anatomic outcome after these procedures, with a prolapse recurrence rate of only 1% to 8%.3 Very little has been written about how to best manage recurrent prolapse after an obliterative procedure. Most surgeons would, most likely, recommend repeat colpocleisis or aggressive levatoroplasty and perineorrhaphy. (Note: The patient whose colpectomy and colpocleisis is shown in Video #3 failed two previous colpectomy and colpocleisis procedures.)
Q5 Can these procedures be performed under local anesthesia, with some intravenous sedation, or under regional anesthesia—thereby avoiding intubation?
A Yes. We have utilized IV sedation and bilateral block successfully to perform these procedures. (Note: Video #3 of LeFort partial colpocleisis shows the procedure performed under local anesthesia.)
Q6 What does the literature say about common complications after these procedures?
A Postoperative morbidity and mortality in the elderly surgical population is a considerable concern. Significant postoperative complications occur in approximately 5% of patients in modern series4—often attributed to the effects of age and to the frail condition of patients who are commonly selected for colpocleisis.
Specifically, approximately 5% of patients experience a postoperative cardiac, thromboembolic, pulmonary, or cerebrovascular event. Transfusion is the most commonly reported major complication related to the procedure itself. Other complications include:
- fever and its associated morbidity
- pneumonia
- ongoing vaginal bleeding
- pyelonephritis
- hematoma
- cystotomy
- ureteral occlusion.
Minor surgical complications occur at a rate of approximately 15%. Surgical mortality is about 1 in 400 cases.
Q7 Do you routinely undertake urodynamic study of patients who are scheduled to undergo an obliterative procedure?
A At minimum, a lower urinary tract evaluation should include a postvoid residual volume study and, we believe, some kind of a filling study and stress test, with reduction of the prolapse. Beyond that, we recommend that you conduct more detailed urodynamic tests on a patient-by-patient basis, when you think that the findings will add to the clinical picture.
Q8 Would you ever perform a vaginal hysterectomy and then proceed with a colpectomy and colpocleisis?
A The principal rationale for performing hysterectomy at the time of colpocleisis is to eliminate the risk of endometrial or cervical carcinoma. Hysterectomy also eliminates the risk of pyometra, a rare but serious complication that can occur when the lateral canals become obstructed after a LeFort procedure.
A recent study5 looked at 1) concomitant hysterectomy in conjunction with colpectomy and colpocleisis and 2) traditional LeFort partial colpocleisis. In this retrospective review, objective and subjective success rates were high, but patients who underwent hysterectomy had a statistically significantly greater decline in postoperative hematocrit and a significant increase in the need for transfusion, compared with patients who did not undergo hysterectomy (35% vs. 13%).
References
1. Fitzgerald MP, Richter HE, Bradley CS, et al. Pelvic support, pelvic symptoms and patient satisfaction after colpocleisis. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19(12):1603-1609.
2. Hullfish K, Bobbjerg B, Steers W. Colpocleisis for pelvic organ prolapse Patient Goals Quality of life and Satisfaction. Obstet Gynecol. 2007;110(2 Pt 1):341-345.
3. Fitzgerald MP, Brubaker L. Colpocleisis and urinary incontinence. Am J Obstet Gynecol. 2003;189(5):1241-1244.
4. von Pechmann WS, Muton M, Fyffe J, Hale DS. Total colpocleisis with high levator placation for the treatment of advanced organ prolapse. Am J Obstet Gynecol. 2003;189(1):121-126.
5. Kohli NE, Sze E, Karram M. Pyometra following LeFort colpocleisis. Int Urogyn J. 1996;7(5):264-266.
We want to hear from you! Tell us what you think.
Strategies and steps for the surgical management of endometriosis
- Resection of an endometrioma in severe disease, using a “stripping” technique
- Ovarian cystectomy
- Resection of endometriosis from the left ligament
- Resection of endometriosis on the bladder
These videos were provided by Anthony Luciano, MD.
Endometriosis affects 7% to 10% of women in the United States, mostly during reproductive years.1 The estimated annual cost for managing the approximately 10 million affected women? More than $17 billion.2 The added cost of this chronic disease, with recurrences of pain and infertility, comes in the form of serious life disruption, emotional suffering, marital and social dysfunction, and diminished productivity.
Although the prevalence of endometriosis is highest during the third and fourth decades of life, the disease is also common in adolescent girls. Indeed, 45% of adolescents who have chronic pelvic pain are found to have endometriosis; if their pain does not respond to an oral contraceptive (OC) or a non-steroidal anti-inflammatory drug, 70% are subsequently found at laparoscopy to have endometriosis.3
What is it?
Endometriosis is the presence of functional endometrial tissue outside the uterus, such as eutopic endometrium. The disease responds to effects of cyclic ovarian hormones, proliferating and bleeding with each menstrual cycle, which often leads to diffuse inflammation, adhesions, and growth of endometriotic nodules or cysts (FIGURE 1).
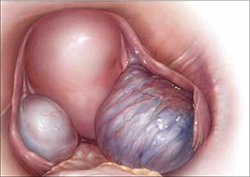
FIGURE 1 Drainage will not suffice
Surgical management of ovarian endometriomas must go beyond simple drainage, which has little therapeutic value because symptoms recur and endometriomas re-form quickly after simple drainage in almost all patients.Symptoms tend to reflect affected organs:
- Because the pelvic organs are most often involved, the classic symptom triad of the disease comprises dysmenorrhea, dyspareunia, and infertility.
- Urinary urgency, dysuria, dyschezia, and tenesmus are frequent complaints when the bladder or rectosigmoid is involved.
- When distant organs are affected, such as the upper abdomen, diaphragm, lungs, and bowel, the patient may complain of respiratory symptoms, hemoptysis, pneumothorax, shoulder pain, upper abdominal pain, and episodic gastrointestinal dysfunction.
The hallmark of endometriosis is catamenial symptoms, which are usually cyclic and most severe around the time of menses. Clinical signs include palpable tender nodules and fibrosis on the anterior and posterior cul de sac, fixed retroverted or anteverted uterus, and adnexal cystic masses.
Because none of these symptoms or signs is specific for endometriosis, diagnosis relies on laparoscopy, which allows the surgeon to:
- visualize it in its various appearances and locations (FIGURE 2)
- confirm the diagnosis histologically with directed excisional biopsy
- treat it surgically with either excision or ablation.
In this article, we describe various surgical techniques for the management of endometriosis. Beyond resection or ablation of lesions, however, your care should also be directed to postoperative measures to prevent its recurrence and to avoid repeated surgical interventions—which, regrettably, are much too common in women who are afflicted by this enigmatic disease.
FIGURE 2 Endometriosis: A disease of varying appearance
Lesions of endometriosis can be pink, dark, clear, or white on the pelvic sidewall (A), bowel (B), and diaphragm (C); under the rib cage (D); and on the ureter (E) (left ureter shown here).
CASE Severe disease in a young woman
S. D. is a 22-year-old unmarried nulligravida who came to the emergency service complaining of acute onset of severe low abdominal pain, which developed while she was running. She was afebrile and in obvious distress, with diffuse lower abdominal tenderness and guarding, especially on the left side.
Ultrasonography revealed a 7-cm adnexal cystic mass suggestive of endometrioma (FIGURE 3).
Two years before this episode, S. D. underwent laparoscopic resection of a 5-cm endometrioma on the right ovary. Subsequently, she was treated with a cyclic OC, which she discontinued after 1 year because she was not sexually active.
The family history is positive for endometriosis in her mother, who had undergone multiple laparoscopic investigations and, eventually, total hysterectomy with bilateral salpingo-oophorectomy at 40 years of age.
S. D. was treated on the emergency service with analgesics and referred to you for surgical management.
S. D. has severe disease that requires aggressive surgical resection and a lifelong management plan. That plan includes liberal use of medical therapy to prevent recurrence of symptoms and avoid repeated surgical procedures—including the total hysterectomy with bilateral salpingo-oophorectomy that her mother underwent.
What is the best immediate treatment plan? Should you:
- drain the cyst?
- drain it and coagulate or ablate its wall?
- resect the wall of the cyst?
- perform salpingo-oophorectomy?
You also ask yourself: What is the risk of recurrence of endometrioma and its symptoms after each of those treatments? And how can I reduce those risks?
FIGURE 3 Endometrioma
Endometrioma on ultrasonography (A), with its characteristic homogeneous, echogenic appearance and “ground glass” pattern, and through the laparoscope (B). These images are from the patient whose case is described in the text.
Focal point: Ovary
The ovary is the most common organ affected by endometriosis. The presence of ovarian endometriomas, in 17% to 44% of patients who have this disease,4 is often associated with an advanced stage of disease.
In a population of 1,785 patients who were surgically treated for ovarian endometriosis, Redwine reported that only 1% had exclusively ovarian involvement; 99% also had diffuse pelvic disease,5 suggesting that ovarian endometrioma is a marker of extensive disease, which often requires a gynecologic surgeon who has advanced skills and experience in the surgical management of severe endometriosis.
Simple drainage is inadequate
Surgical management of ovarian endometrioma must go beyond simple drainage, which has little therapeutic value because symptoms recur and endometriomas re-form quickly after simple drainage in almost all patients.6 The currently accepted surgical management of endometrioma involves either 1) coagulation and ablation of the wall of the cyst with electrosurgery or laser or 2) removal of the cyst wall from the ovary with blunt and sharp dissection.
Several studies have compared these two techniques, but only two7,8 were prospectively randomized.
Study #1. Beretta and co-workers7 studied 64 patients who had ovarian endometriomas larger than 3 cm and randomized them to cystectomy by complete stripping of the cyst wall or to drainage of fluid followed by electrocoagulation to ablate the endometriosis lesions within the cyst wall. The two groups were followed for 2 years to assess the recurrence of symptoms and the pregnancy rate in the patients who were infertile.
Recurrence of symptoms and the need for medical or surgical intervention occurred with less frequency and much later in the resection group than in the ablation group: 19 months, compared to 9.5 months, postoperatively. The cumulative pregnancy rate 24 months postoperatively was also much higher in the resection group (66.7%) than in the ablative group (23.5%).
Study #2. In a later study,8 Alborzi and colleagues randomized 100 patients who had endometrioma to cystectomy or to drainage and coagulation of the cyst wall. The mean recurrence rate, 2 years postoperatively, was much lower in the excision group (15.8%) than in the ablative group (56.7%). The cumulative pregnancy rate at 12 months was higher in the excision group (54.9%, compared to 23.3%). Furthermore, the reoperation rate at 24 months was much lower in the excision group (5.8%) than in the ablative group (22.9%).
These favorable results for cystectomy over ablation were validated by a Cochrane Review, which concluded that excision of endometriomas is the preferred approach because it provides 1) a more favorable outcome than drainage and ablation, 2) lower rates of recurrence of endometriomas and symptoms, and 3) a much higher spontaneous pregnancy rate in infertile women.9
Although resection of the cyst wall is technically more challenging and takes longer to perform than drainage and ablation, we exclusively perform resection rather than ablation of endometriomas because we believe that more lasting therapeutic effects and reduced recurrence of symptoms and disease justify the extra effort and a longer procedure.
Drawback of cystectomy
A potential risk of cystectomy is that it can diminish ovarian reserve and, in rare cases, induce premature menopause, which can be devastating for women whose main purpose for having surgery is to restore or improve their fertility.
The impact of laparoscopic ovarian cystectomy on ovarian reserve was prospectively studied by Chang and co-workers,10 who measured preoperative and postoperative levels of anti-müllerian hormone (AMH) in 13 women who had endometrioma, 6 who had mature teratoma, and 1 who had mucinous cystadenoma. One week postoperatively, the AMH level decreased significantly overall in all groups. At 4 and 12 weeks postoperatively, however, the AMH level returned to preoperative levels among subjects in the non-endometrioma group but not among subjects who had endometrioma; rather, their level remained statistically lower than the preoperative level during the entire 3 months of follow-up.
Stripping the wall of an endometrioma cyst is more difficult than it is for other benign cysts, such as cystic teratoma or cystadenoma, in which there usually is a well-defined dissection plane between the wall of the cyst and surrounding stromal tissue—allowing for easy and clean separation of the wall. The cyst wall of an endometrioma, on the other hand, is intimately attached to underlying ovarian stroma; lack of a clear cleavage plane between cyst and ovarian stroma often results in unintentional removal of layers of ovarian cortex with underlying follicles, which, in turn, may lead to a reduction in ovarian reserve.
Histologic analyses of resected endometrioma cyst walls have reported follicle-containing ovarian tissue attached to the stripped cyst wall in 54% of cases.11,12 That observation explains why, and how, ovarian reserve can be compromised after resection of endometrioma.
Further risk: Ovarian failure
In rare cases, excision of endometriomas results in complete ovarian failure, described by Busacca and colleagues, who reported three cases of ovarian failure (2.4%) after resection of bilateral endometriomas in 126 patients.13 They attributed ovarian failure to excessive cauterization that compromised vascularization, as well as to excessive removal of ovarian tissue.
It is important, therefore, to strip the thinnest layer of the cyst capsule and to reduce the amount of electrocoagulation of ovarian stroma as much as possible to safeguard functional ovarian tissue.
CASE continued
S. D. was scheduled for laparoscopy to remove the endometrioma and other concurrent pelvic and peritoneal pathology, such as endometriosis and pelvic adhesions. You also scheduled her for hysteroscopy to evaluate the endometrial cavity for potential pathology, such as endometrial polyps and uterine septum, which appear to be more common in women who have endometriosis.
Nawroth and co-workers14 found a much higher incidence of endometriosis in patients who had a septate uterus. Metalliotakis and co-workers15 found congenital uterine malformations to be more common in patients who had endometriosis, compared with controls; uterine septum was, by far, the most common anomaly.
CASE continued
Hysteroscopy revealed a small and broad septum, which was resected sharply with hysteroscopic scissors (FIGURE 4). Laparoscopy revealed a 7-cm endometrioma on the left ovary, with adhesions to the posterior broad ligament and pelvic sidewall. S. D. also had deep implants of endometriosis on the left pelvic sidewall, the posterior cul de sac, the right pelvic sidewall, and the right ovary, which was cohesively adherent to the ovarian fossa.
As you expected, S. D. has stage-IV disease, according to the revised American Fertility Society Classification.
Following adhesiolysis, the endometrioma was resected (see VIDEO 1). Because of the large ovarian defect, the edges of the ovary were approximated with imbricating running 3-0 Vicryl suture. Deep endometriosis was also resected. Superficial endometriosis was peeled off or coagulated using bipolar forceps.
Note: Alternatively, and with comparable results, resection may be performed with a laser or other energy source. We prefer resection, rather than ablation, of deep endometriosis, but no data exists to support one technique over the other.
FIGURE 4 Septate uterus with deep cornua
Through the hysteroscope, a shallow septum is visible at the fundus of the uterus, dividing the upper endometrial cavity into two chambers (A), with deep cornua on the left (B) and right (C). Normal fundal anatomy is restored by septolysis along the avascular plane (D).
Technique: How we resect endometrioma
In removing endometrioma (see VIDEO 2), it is important to grasp the thinnest part of the cyst wall and progressively strip it, to avoid removing excess ovarian tissue and to reduce the risk of compromising ovarian reserve.
After draining the endometrioma of its chocolate-colored fluid, we irrigate and drain the cyst several times with warm lactated Ringers’ solution to promote separation of the cyst wall from underlying stroma and better identify the dissection plane. The cyst wall is inspected by introducing the laparoscope into the cyst to examine its surface, which is often laden with implants of deep and superficial endometriosis.
If we cannot easily identify the plane of dissection along the edges, we may evert the cyst and make an incision at its base to create a wedge between the wall of the cyst and underlying stroma. The edge of the incised wall is then grasped and retracted to create a space between the wall and the underlying stroma, from which it is progressively stripped from the ovary.
Traction and counter-traction are the hallmarks of dissection here; sometimes, we use laparoscopic scissors to sharply resect the ovarian stromal attachments that adhere cohesively to the cyst wall. This technique is continued until the entire cyst wall is removed. When follicle-containing ovarian tissue remains attached to the cyst wall, we introduce the closed tips of the Dolphin forceps between the cyst wall and adjacent follicle-containing stroma, spread the tips apart, and recover the true plane of dissection between the thin wall of the cyst and stroma.
After the wall of the cyst is removed, the ovarian crater invariably bleeds because blood vessels supplying the wall have been separated and opened. Utilizing warm lactated Ringers’ solution, we copiously irrigate the bleeding ovarian stroma to identify each bleeding vessel and, by placing the tips of the micro-bipolar forceps on either side of the bleeder, individually coagulate each vessel, thus inflicting minimal thermal damage to the surrounding stroma.
Pearl. Avoid using Kleppinger forceps to indiscriminately coagulate the bloody stroma in the crater created after the cystectomy, because doing so can result in excessive destruction of ovarian tissue or inadvertent coagulation of hylar vessels that would interrupt the blood supply to the ovary, compromising its function.16
Suturing. Some surgeons find that fenestration, drainage, and coagulation of the cyst wall is acceptable, but we have concerns not only about incomplete ablation of the endometriosis on the cyst wall, which may be responsible for the higher recurrence rate of disease, but also about the risk of thermal injury to underlying follicles, which may compromise ovarian reserve.16
Hemostasis. Once complete hemostasis has been achieved, the decision to approximate (or not) the edges, preferably with fine absorbable suture, is based on how large the defect is and whether or not the edges of the crater spontaneously come together. For large craters, we usually close the ovary with a 3-0 or 4-0 Vicryl continuous suture, imbricating the edges to expose as little suture material as possible to reduce postoperative formation of adhesions, which is common after ovarian surgery.17
Last, we ensure that hemostasis is present. Often we apply an anti-adhesion solution, such as icodextrin 4% (Adept). This agent has been shown to reduce postoperative adhesion formation, especially after laparoscopic surgery for endometriosis.18
A high level of skill is needed
Ovarian endometriomas signal advanced disease; advanced surgical skills are required to treat them adequately. Simple drainage is of little therapeutic value and should seldom be considered a treatment option. Although drainage plus ablation of the cyst wall ameliorates symptoms, excision of endometriomas should be considered preferable because it provides a more favorable outcome, a lower risk of recurrence of endometriomas and symptoms, and a higher rate of spontaneous pregnancy in previously infertile women.7-9
To recap, we advise the surgeon to:
- Manage ovarian endometriomas with resection of the entire cyst wall, grasping and stripping the thinnest layer of the cyst wall without removing underlying functional ovarian stroma.
- Avoid excessive cauterization of the underlying ovarian stroma by utilizing micro-bipolar forceps and applying energy only around bleeding vessels.
- Close stromal defects, when the crater is large and its edges do not spontaneously come together, by approximating the edges with an imbricating resorbable suture.
CASE continued
As in most cases of advanced endometriosis, S. D. also had diffuse implants of deep and superficial endometriosis on the peritoneum of the pelvic sidewalls and on the anterior and posterior cul de sac.
Should you ablate or resect these lesions? Are there advantages to either approach?
Ablation of endometriosis implants may involve either electrocoagulation of the lesion with bipolar energy or laser vaporization/coagulation, which destroys or devitalizes active endometriosis but does not actually remove the lesion. Ablation destroys the lesion without getting a specimen for histologic diagnosis.
Resection of endometriosis implants involves complete removal of the lesion from its epithelial surface to the depth of its base. Resection can be performed with scissors, laser, or monopolar electrosurgery. Resection removes the lesion in its entirety, yielding a histologic diagnosis and allowing you to determine whether, indeed, the entire specimen has been removed.
The question of what is more effective—ablating or resecting endometriosis implants?—was addressed in a prospective study in which 141 patients with endometriosis-related pain were randomized at laparoscopic surgery to either excision or ablation/coagulation of endometriosis lesions.19 Six months postoperatively, the pain score decreased by, on average, 11.2 points in the excision group and 8.7 points in the coagulation/ablative group.
Because the difference in those average pain scores was not statistically significant, however, investigators concluded that the techniques are comparable, with similar efficacy. That interpretation has been criticized because the study was underpowered and included only patients who had mild endometriosis—leaving open the possibility that deep endometriosis may not be adequately treated by electrocoagulation or ablation.
In contrast to superficial endometriosis, which may respond similarly to ablation or resection, deep endometriosis is difficult to ablate either with electrosurgery or a laser because the energy cannot reach deeper layers and active disease is therefore likely to be left behind. Moreover, when endometriosis overlies vital structures, such as the ureter or bowel, ablation of the lesion may cause thermal damage to the underlying organ, and such damage may not manifest until several days later, when the patient experiences, say, urinary leakage in the peritoneum or symptoms of bowel perforation.
FIGURE 5 illustrates a case in which CO2 laser ablation of endometriosis that had been causing deep dyspareunia did not alleviate symptoms. Because those symptoms persisted, the patient was referred to our center, where a second laparoscopy revealed deep nodules of endometriosis, 1 to 2 cm in diameter, extending from the right and the left uterosacral ligaments deep into the perirectal space, bilaterally.
As the bottom panel of FIGURE 5 shows, excised nodules were deep and large; neither laser nor electrosurgery would have been able to ablate or devitalize the deep endometriosis at the base of these 2-cm nodules.
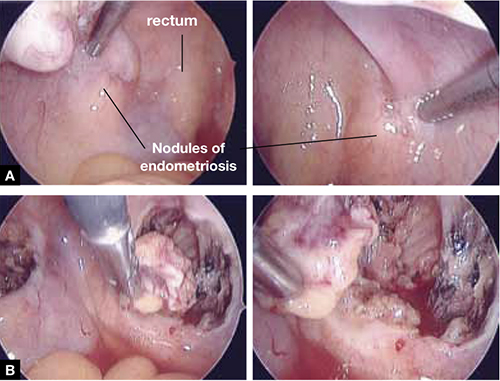
FIGURE 5 Deep nodules present a surgical challenge
These nodules of endometriosis on the right and left uterosacral ligaments (panel A) did not respond to CO2 laser ablation. Upon progressive resection, the implants were found to be deep, extending into the perirectal space (panel B). (See also VIDEO 3, resection of endometriosis from the left uterosacal ligament, close to the ureter.) FIGURE 6, illustrates endometriosis overlying the bladder and left ureter (see also VIDEO 4). Ablation of endometriosis in these areas may be inadequate if it is not deep enough, and dangerous if it goes too deep. As FIGURE 6 shows, excision assures the surgeon that the entire lesion has been removed and that underlying vital structures have been safeguarded.
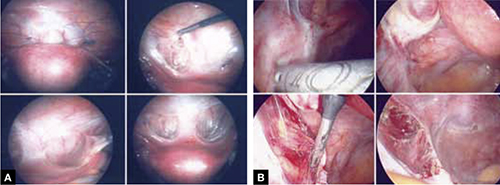
FIGURE 6 Urinary tract involvement
Endometriosis overlying the bladder is grasped, retracted, and resected (panel A). Endometriosis compresses the left ureter (panel B). The peritoneum above the lesion is entered, the ureter is displaced laterally, and the lesion is safely resected.
What we do, and recommend
When endometriosis is superficial and does not overlie vital organs, such as the bladder, ureter, and bowel, ablation and resection may be equally safe and effective. When endometriosis is deep and overlying vital organs, however, complete resection—with careful dissection of the lesion off underlying structures—offers a more complete and a safer surgical approach.
CASE continued
Now that S. D. has been treated surgically by complete excision of endometriosis, adhesions, and endometriomas, you must consider a management plan that will reduce the risks 1) of recurrence of symptoms and disease and 2) that further surgery will be necessary in the future—a risk that, in her case, exceeds 50% because of her young age, nulliparity, and the severity of her disease.20,21 Indeed, you are aware that, had preventive measures been implemented after her initial surgery 2 years earlier, it is unlikely that S. D. would have developed the second endometrioma and most likely that she would not have needed the second surgery.
Prevention of recurrence is necessary—and doable
The importance of implementing preventive measures to reduce the risk of recurrence of endometriosis and its symptoms has been suggested by several studies. It was underscored recently in a prospective, randomized study conducted by Serracchioli and colleagues,22 in which 239 women who had undergone laparoscopic resection of endometriomas were randomly assigned to expectant management (control group), a cyclic oral contraceptive (OC), or a continuous oral contraceptives for 24 months, and evaluated every 6 months.
At the end of the study, recurrence of symptoms occurred in 30% of controls; 15% of subjects taking a cyclic OC; and 7.5% of the subjects taking a continuous OC. The recurrence rate of endometrioma in this study was reduced by 50% (cyclic OC) and 75% (continuous OC).22
Similar results were reported in a case-controlled study by Vercellini and co-workers,23 who found that the risk of recurrence of endometrioma was reduced by 60% when postoperative OCs were used long-term and by 30% when used for a duration of less than 12 months.
These studies suggest that, by suppressing ovulation and inducing a state of hypomenorrhea or amenorrhea, the risk of recurrence of endometriosis and its symptoms can be significantly reduced.
The importance of amenorrhea in reducing the postoperative recurrence of endometriosis and symptoms has been underscored by two important studies that evaluated the role of postoperative endometrial ablation or postoperative insertion of the levonorgestrel intrauterine system (LNG-IUS; Mirena), neither of which suppresses ovulation but both of which induce a state of hypomenorrhea or amenorrhea.24,25
In a prospective randomized study by Bulletti and co-workers,24 28 patients who had symptomatic endometriosis underwent laparoscopic conservative surgery. Endometrial ablation was performed in 14 of the 28. Two years later, all patients underwent second-look laparoscopy; recurrence of endometriosis was found in 9 of the 14 non-ablation patients but in none in the ablation group. Resolution or significant improvement of symptoms were reported in 13 of 14 women in the ablation group but only in 3 of 14 in the non-ablation group—supporting the premise that amenorrhea or hypomenorrhea by itself, without suppressing ovulation, significantly reduces the risk that endometriosis will recur.
Similar beneficial results from hypomenorrhea/amenorrhea on the risk of recurrence of symptoms have been reported when the LNG-IUS is inserted following conservative surgery for endometriosis. In a prospective study by Vercellini and colleagues,25 40 symptomatic patients who had stage-III or stage-IV disease were randomized to either insertion of the LNG-IUS or a control group after conservative laparoscopic surgery. Recurrence of pain was significantly (P = .012) reduced in the LNG-IUS group (45%), compared with the control group (10%). Control subjects were also much less satisfied with their treatment than those who were treated with the LNG-IUS.
The importance of inducing a state of amenorrhea to reduce the risk of disease recurrence was further underscored by a recent study. Shakiba and colleagues26 reported on the recurrence of endometriosis that required further surgery as long as 7 years after the subjects had been surgically treated for symptomatic endometriosis. The need for subsequent surgery was 8% after hysterectomy and bilateral salpingo-oophorectomy; 12% after hysterectomy alone; and 60% after conservative laparoscopy with preservation of both uterus and ovaries.
Taken together, these data show that, unless the patient is rendered amenorrheic or hypomenorrheic, her risk of recurrence exceeds 50%.
It is important, therefore, to consider conservative surgical management of endometriosis as only the beginning of a lifelong management plan. That plan begins with complete resection of all visible endometriosis and adhesions, resection of endometriomas, and restoration of normal anatomy as much as possible.
When endometriosis cannot be completely resected—as when it involves small bowel or the diaphragm, or is diffusely on the large bowel—we recommend medical suppressive therapy. Our preference is depot leuprolide acetate (Lupron Depot), always with add-back therapy to minimize side effects, which include vasomotor symptoms, vaginal dryness, and bone loss,27 until the patient is significantly asymptomatic, which may take 6 to 9 months.
CASE Concluded, with long-term intervention
You counsel S. D. to remain on a low-dose hormonal OC continuously, until such time that she wants to conceive. If a patient does not want to conceive for at least 5 years, the LNG-IUS may be inserted at surgery to induce hypomenorrhea and reduce the risk of recurrence for the next 5 years.
When hormonal contraceptives are inadequate to control symptoms, adding the aromatase enzyme inhibitor letrozole (Femara), 2.5 mg daily for 6 to 9 months, usually alleviates symptoms with minimal side effects, as long as the patient keeps taking a hormonal contraceptive. Using letrozole without hormonal contraception has not been studied; doing so may lead to formation of ovarian cysts, and is therefore not recommended for managing symptomatic endometriosis.
If the patient wants to become pregnant, encourage her to actively undertake fertility treatment as soon as possible after surgery, thereby minimizing the risk of recurrence of symptoms and disease. The best option may be to employ assisted reproductive technology, but patients cannot always afford it; when that is the case, consider controlled ovarian stimulation and intrauterine insemination.
We want to hear from you! Tell us what you think.
1. Bulun S E. Endometriosis. N Engl J Med. 2009;360(3):268-279.
2. Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86(6):1561-1572.
3. Laufer MR, Goitein L, Bush M, Cramer DW, Emans SJ. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding conventional therapy. J Pediatr Adolesc Gynecol. 1997;10(4):199-202.
4. Gruppo Italiano per lo studio dell’endometriosi. Prevalence and anatomic distribution of endometriosis in women with selected gynaecological conditions: results from a multicenter Italian study. Hum Reprod. 1994;9(6):1158-1162.
5. Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72(2):319-315.
6. Muzii L, Marana R, Caruana P, Catalano GF, Mancuso S. Laparoscopic findings after transvaginal ultrasound-guided aspiration of ovarian endometriomas. Hum Reprod. 1995;10(11):2902-2903
7. Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatment of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176-1180
8. Alborzi S, Momtahan M, Paranezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633-1637
9. Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomas. Cochrane Database Syst Rev. 2005;(3):CD004992.-
10. Chang HJ, Sang HH, Jung RL, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril. 2010;94(1):343-349.
11. Muzii L. Bianchi A Crocè C, Manci N, Panici PB. Laparoscopic excision of ovarian cysts: is the stripping technique a tissue sparing procedure? Fertil Steril. 2002;77(3):609-614.
12. Hachisuga T, Kawarabyashi T. Histopathological analysis of laparoscopically treated ovarian endometriotic cysts with special reference to loss of follicles. Hum Reprod. 2002;17(2):432-435.
13. Busacca M, Riparini J Somigliana E, et al. Postsurgical ovarian failure after laparoscopic excision of bilateral endometriomas. Am J Obstet Gynecol. 2006;195(2):421-425.
14. Nawroth F, Rahimi G, Nawroth C, Foth D, Ludwig M, Schmidt T. Is there an association between septate uterus and endometriosis? Hum Reprod. 2006;21(2):542-546.
15. Matalliotakis IM, Goumenou AG, Matalliotakis M, Arici A. Uterine anomalies in women with endometriosis. J Endometriosis. 2010;2(4):213-217.
16. Li CZ, Liu B, Wen ZQ, Sun Q. The impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cyst: a prospective clinical study of 191 patients. Fertil Steril. 2009;92(4):1428-1435.
17. Luciano DE, Roy G, Luciano AA. Adhesion reformation after laparoscopic adhesiolysis: where what type, and in whom are they most likely to recur. J Minim Invasive Gynecol. 2008;15(1):44-48.
18. Colin CB, Luciano AA, Martin D, et al. Adept (icodextrin 4% solution) reduces adhesions after laparoscopic surgery for adhesiolysis: a double-blind, randomized, controlled study. Fertil Steril. 2007;88(5):1413-1426.
19. Wright J, Lotfallah H, Jones K, Lovell D. A randomized study of excision vs ablation for mild endometriosis. Fertil Steril. 2004;83(6):1830-1836.
20. Cheong Y, Tay P, Luk F, Gan HC, Li TC, Cooke I. Laparoscopic surgery for endometriosis: How often do we need to re-operate? J Obstet Gynaecol. 2008;28(1):82-85.
21. Liu X, Yuan L, Shen F, Zhu Z, Jiang H, Guo SW. Patterns of and risk factors for recurrence in women with ovarian endometriomas. Obstet Gynecol. 2007;109(6):1411-1120.
22. Seracchioli R, Mabrouk M, Frasca C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometriomas recurrence: a randomized controlled trial. Fertil Steril. 2010;93(1):52-56.
23. Vercellini P, Somigliana E, Daguati R, Vigano P, Meroni F, Crosignani PG. Postoperative oral contraceptive exposure and risk of endometrioma recurrence. Am J Obstet Gynecol. 2008;198(5):504.e1-5.
24. Bulletti C, DeZiegler D, Stefanetti M, Cicinelli E, Pelosi E, Flamigni C. Endometriosis: absence of recurrence in patients after endometrial ablation. Hum Reprod. 2001;16(12):2676-2679.
25. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80(2):305-309.
26. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285-1292.
27. Surrey ES, Hornstein MD. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: a long-term follow-up Obstet Gynecol. 2002;99(5 Pt 1):709-719.
- Resection of an endometrioma in severe disease, using a “stripping” technique
- Ovarian cystectomy
- Resection of endometriosis from the left ligament
- Resection of endometriosis on the bladder
These videos were provided by Anthony Luciano, MD.
Endometriosis affects 7% to 10% of women in the United States, mostly during reproductive years.1 The estimated annual cost for managing the approximately 10 million affected women? More than $17 billion.2 The added cost of this chronic disease, with recurrences of pain and infertility, comes in the form of serious life disruption, emotional suffering, marital and social dysfunction, and diminished productivity.
Although the prevalence of endometriosis is highest during the third and fourth decades of life, the disease is also common in adolescent girls. Indeed, 45% of adolescents who have chronic pelvic pain are found to have endometriosis; if their pain does not respond to an oral contraceptive (OC) or a non-steroidal anti-inflammatory drug, 70% are subsequently found at laparoscopy to have endometriosis.3
What is it?
Endometriosis is the presence of functional endometrial tissue outside the uterus, such as eutopic endometrium. The disease responds to effects of cyclic ovarian hormones, proliferating and bleeding with each menstrual cycle, which often leads to diffuse inflammation, adhesions, and growth of endometriotic nodules or cysts (FIGURE 1).

FIGURE 1 Drainage will not suffice
Surgical management of ovarian endometriomas must go beyond simple drainage, which has little therapeutic value because symptoms recur and endometriomas re-form quickly after simple drainage in almost all patients.Symptoms tend to reflect affected organs:
- Because the pelvic organs are most often involved, the classic symptom triad of the disease comprises dysmenorrhea, dyspareunia, and infertility.
- Urinary urgency, dysuria, dyschezia, and tenesmus are frequent complaints when the bladder or rectosigmoid is involved.
- When distant organs are affected, such as the upper abdomen, diaphragm, lungs, and bowel, the patient may complain of respiratory symptoms, hemoptysis, pneumothorax, shoulder pain, upper abdominal pain, and episodic gastrointestinal dysfunction.
The hallmark of endometriosis is catamenial symptoms, which are usually cyclic and most severe around the time of menses. Clinical signs include palpable tender nodules and fibrosis on the anterior and posterior cul de sac, fixed retroverted or anteverted uterus, and adnexal cystic masses.
Because none of these symptoms or signs is specific for endometriosis, diagnosis relies on laparoscopy, which allows the surgeon to:
- visualize it in its various appearances and locations (FIGURE 2)
- confirm the diagnosis histologically with directed excisional biopsy
- treat it surgically with either excision or ablation.
In this article, we describe various surgical techniques for the management of endometriosis. Beyond resection or ablation of lesions, however, your care should also be directed to postoperative measures to prevent its recurrence and to avoid repeated surgical interventions—which, regrettably, are much too common in women who are afflicted by this enigmatic disease.

FIGURE 2 Endometriosis: A disease of varying appearance
Lesions of endometriosis can be pink, dark, clear, or white on the pelvic sidewall (A), bowel (B), and diaphragm (C); under the rib cage (D); and on the ureter (E) (left ureter shown here).
CASE Severe disease in a young woman
S. D. is a 22-year-old unmarried nulligravida who came to the emergency service complaining of acute onset of severe low abdominal pain, which developed while she was running. She was afebrile and in obvious distress, with diffuse lower abdominal tenderness and guarding, especially on the left side.
Ultrasonography revealed a 7-cm adnexal cystic mass suggestive of endometrioma (FIGURE 3).
Two years before this episode, S. D. underwent laparoscopic resection of a 5-cm endometrioma on the right ovary. Subsequently, she was treated with a cyclic OC, which she discontinued after 1 year because she was not sexually active.
The family history is positive for endometriosis in her mother, who had undergone multiple laparoscopic investigations and, eventually, total hysterectomy with bilateral salpingo-oophorectomy at 40 years of age.
S. D. was treated on the emergency service with analgesics and referred to you for surgical management.
S. D. has severe disease that requires aggressive surgical resection and a lifelong management plan. That plan includes liberal use of medical therapy to prevent recurrence of symptoms and avoid repeated surgical procedures—including the total hysterectomy with bilateral salpingo-oophorectomy that her mother underwent.
What is the best immediate treatment plan? Should you:
- drain the cyst?
- drain it and coagulate or ablate its wall?
- resect the wall of the cyst?
- perform salpingo-oophorectomy?
You also ask yourself: What is the risk of recurrence of endometrioma and its symptoms after each of those treatments? And how can I reduce those risks?

FIGURE 3 Endometrioma
Endometrioma on ultrasonography (A), with its characteristic homogeneous, echogenic appearance and “ground glass” pattern, and through the laparoscope (B). These images are from the patient whose case is described in the text.
Focal point: Ovary
The ovary is the most common organ affected by endometriosis. The presence of ovarian endometriomas, in 17% to 44% of patients who have this disease,4 is often associated with an advanced stage of disease.
In a population of 1,785 patients who were surgically treated for ovarian endometriosis, Redwine reported that only 1% had exclusively ovarian involvement; 99% also had diffuse pelvic disease,5 suggesting that ovarian endometrioma is a marker of extensive disease, which often requires a gynecologic surgeon who has advanced skills and experience in the surgical management of severe endometriosis.
Simple drainage is inadequate
Surgical management of ovarian endometrioma must go beyond simple drainage, which has little therapeutic value because symptoms recur and endometriomas re-form quickly after simple drainage in almost all patients.6 The currently accepted surgical management of endometrioma involves either 1) coagulation and ablation of the wall of the cyst with electrosurgery or laser or 2) removal of the cyst wall from the ovary with blunt and sharp dissection.
Several studies have compared these two techniques, but only two7,8 were prospectively randomized.
Study #1. Beretta and co-workers7 studied 64 patients who had ovarian endometriomas larger than 3 cm and randomized them to cystectomy by complete stripping of the cyst wall or to drainage of fluid followed by electrocoagulation to ablate the endometriosis lesions within the cyst wall. The two groups were followed for 2 years to assess the recurrence of symptoms and the pregnancy rate in the patients who were infertile.
Recurrence of symptoms and the need for medical or surgical intervention occurred with less frequency and much later in the resection group than in the ablation group: 19 months, compared to 9.5 months, postoperatively. The cumulative pregnancy rate 24 months postoperatively was also much higher in the resection group (66.7%) than in the ablative group (23.5%).
Study #2. In a later study,8 Alborzi and colleagues randomized 100 patients who had endometrioma to cystectomy or to drainage and coagulation of the cyst wall. The mean recurrence rate, 2 years postoperatively, was much lower in the excision group (15.8%) than in the ablative group (56.7%). The cumulative pregnancy rate at 12 months was higher in the excision group (54.9%, compared to 23.3%). Furthermore, the reoperation rate at 24 months was much lower in the excision group (5.8%) than in the ablative group (22.9%).
These favorable results for cystectomy over ablation were validated by a Cochrane Review, which concluded that excision of endometriomas is the preferred approach because it provides 1) a more favorable outcome than drainage and ablation, 2) lower rates of recurrence of endometriomas and symptoms, and 3) a much higher spontaneous pregnancy rate in infertile women.9
Although resection of the cyst wall is technically more challenging and takes longer to perform than drainage and ablation, we exclusively perform resection rather than ablation of endometriomas because we believe that more lasting therapeutic effects and reduced recurrence of symptoms and disease justify the extra effort and a longer procedure.
Drawback of cystectomy
A potential risk of cystectomy is that it can diminish ovarian reserve and, in rare cases, induce premature menopause, which can be devastating for women whose main purpose for having surgery is to restore or improve their fertility.
The impact of laparoscopic ovarian cystectomy on ovarian reserve was prospectively studied by Chang and co-workers,10 who measured preoperative and postoperative levels of anti-müllerian hormone (AMH) in 13 women who had endometrioma, 6 who had mature teratoma, and 1 who had mucinous cystadenoma. One week postoperatively, the AMH level decreased significantly overall in all groups. At 4 and 12 weeks postoperatively, however, the AMH level returned to preoperative levels among subjects in the non-endometrioma group but not among subjects who had endometrioma; rather, their level remained statistically lower than the preoperative level during the entire 3 months of follow-up.
Stripping the wall of an endometrioma cyst is more difficult than it is for other benign cysts, such as cystic teratoma or cystadenoma, in which there usually is a well-defined dissection plane between the wall of the cyst and surrounding stromal tissue—allowing for easy and clean separation of the wall. The cyst wall of an endometrioma, on the other hand, is intimately attached to underlying ovarian stroma; lack of a clear cleavage plane between cyst and ovarian stroma often results in unintentional removal of layers of ovarian cortex with underlying follicles, which, in turn, may lead to a reduction in ovarian reserve.
Histologic analyses of resected endometrioma cyst walls have reported follicle-containing ovarian tissue attached to the stripped cyst wall in 54% of cases.11,12 That observation explains why, and how, ovarian reserve can be compromised after resection of endometrioma.
Further risk: Ovarian failure
In rare cases, excision of endometriomas results in complete ovarian failure, described by Busacca and colleagues, who reported three cases of ovarian failure (2.4%) after resection of bilateral endometriomas in 126 patients.13 They attributed ovarian failure to excessive cauterization that compromised vascularization, as well as to excessive removal of ovarian tissue.
It is important, therefore, to strip the thinnest layer of the cyst capsule and to reduce the amount of electrocoagulation of ovarian stroma as much as possible to safeguard functional ovarian tissue.
CASE continued
S. D. was scheduled for laparoscopy to remove the endometrioma and other concurrent pelvic and peritoneal pathology, such as endometriosis and pelvic adhesions. You also scheduled her for hysteroscopy to evaluate the endometrial cavity for potential pathology, such as endometrial polyps and uterine septum, which appear to be more common in women who have endometriosis.
Nawroth and co-workers14 found a much higher incidence of endometriosis in patients who had a septate uterus. Metalliotakis and co-workers15 found congenital uterine malformations to be more common in patients who had endometriosis, compared with controls; uterine septum was, by far, the most common anomaly.
CASE continued
Hysteroscopy revealed a small and broad septum, which was resected sharply with hysteroscopic scissors (FIGURE 4). Laparoscopy revealed a 7-cm endometrioma on the left ovary, with adhesions to the posterior broad ligament and pelvic sidewall. S. D. also had deep implants of endometriosis on the left pelvic sidewall, the posterior cul de sac, the right pelvic sidewall, and the right ovary, which was cohesively adherent to the ovarian fossa.
As you expected, S. D. has stage-IV disease, according to the revised American Fertility Society Classification.
Following adhesiolysis, the endometrioma was resected (see VIDEO 1). Because of the large ovarian defect, the edges of the ovary were approximated with imbricating running 3-0 Vicryl suture. Deep endometriosis was also resected. Superficial endometriosis was peeled off or coagulated using bipolar forceps.
Note: Alternatively, and with comparable results, resection may be performed with a laser or other energy source. We prefer resection, rather than ablation, of deep endometriosis, but no data exists to support one technique over the other.

FIGURE 4 Septate uterus with deep cornua
Through the hysteroscope, a shallow septum is visible at the fundus of the uterus, dividing the upper endometrial cavity into two chambers (A), with deep cornua on the left (B) and right (C). Normal fundal anatomy is restored by septolysis along the avascular plane (D).
Technique: How we resect endometrioma
In removing endometrioma (see VIDEO 2), it is important to grasp the thinnest part of the cyst wall and progressively strip it, to avoid removing excess ovarian tissue and to reduce the risk of compromising ovarian reserve.
After draining the endometrioma of its chocolate-colored fluid, we irrigate and drain the cyst several times with warm lactated Ringers’ solution to promote separation of the cyst wall from underlying stroma and better identify the dissection plane. The cyst wall is inspected by introducing the laparoscope into the cyst to examine its surface, which is often laden with implants of deep and superficial endometriosis.
If we cannot easily identify the plane of dissection along the edges, we may evert the cyst and make an incision at its base to create a wedge between the wall of the cyst and underlying stroma. The edge of the incised wall is then grasped and retracted to create a space between the wall and the underlying stroma, from which it is progressively stripped from the ovary.
Traction and counter-traction are the hallmarks of dissection here; sometimes, we use laparoscopic scissors to sharply resect the ovarian stromal attachments that adhere cohesively to the cyst wall. This technique is continued until the entire cyst wall is removed. When follicle-containing ovarian tissue remains attached to the cyst wall, we introduce the closed tips of the Dolphin forceps between the cyst wall and adjacent follicle-containing stroma, spread the tips apart, and recover the true plane of dissection between the thin wall of the cyst and stroma.
After the wall of the cyst is removed, the ovarian crater invariably bleeds because blood vessels supplying the wall have been separated and opened. Utilizing warm lactated Ringers’ solution, we copiously irrigate the bleeding ovarian stroma to identify each bleeding vessel and, by placing the tips of the micro-bipolar forceps on either side of the bleeder, individually coagulate each vessel, thus inflicting minimal thermal damage to the surrounding stroma.
Pearl. Avoid using Kleppinger forceps to indiscriminately coagulate the bloody stroma in the crater created after the cystectomy, because doing so can result in excessive destruction of ovarian tissue or inadvertent coagulation of hylar vessels that would interrupt the blood supply to the ovary, compromising its function.16
Suturing. Some surgeons find that fenestration, drainage, and coagulation of the cyst wall is acceptable, but we have concerns not only about incomplete ablation of the endometriosis on the cyst wall, which may be responsible for the higher recurrence rate of disease, but also about the risk of thermal injury to underlying follicles, which may compromise ovarian reserve.16
Hemostasis. Once complete hemostasis has been achieved, the decision to approximate (or not) the edges, preferably with fine absorbable suture, is based on how large the defect is and whether or not the edges of the crater spontaneously come together. For large craters, we usually close the ovary with a 3-0 or 4-0 Vicryl continuous suture, imbricating the edges to expose as little suture material as possible to reduce postoperative formation of adhesions, which is common after ovarian surgery.17
Last, we ensure that hemostasis is present. Often we apply an anti-adhesion solution, such as icodextrin 4% (Adept). This agent has been shown to reduce postoperative adhesion formation, especially after laparoscopic surgery for endometriosis.18
A high level of skill is needed
Ovarian endometriomas signal advanced disease; advanced surgical skills are required to treat them adequately. Simple drainage is of little therapeutic value and should seldom be considered a treatment option. Although drainage plus ablation of the cyst wall ameliorates symptoms, excision of endometriomas should be considered preferable because it provides a more favorable outcome, a lower risk of recurrence of endometriomas and symptoms, and a higher rate of spontaneous pregnancy in previously infertile women.7-9
To recap, we advise the surgeon to:
- Manage ovarian endometriomas with resection of the entire cyst wall, grasping and stripping the thinnest layer of the cyst wall without removing underlying functional ovarian stroma.
- Avoid excessive cauterization of the underlying ovarian stroma by utilizing micro-bipolar forceps and applying energy only around bleeding vessels.
- Close stromal defects, when the crater is large and its edges do not spontaneously come together, by approximating the edges with an imbricating resorbable suture.
CASE continued
As in most cases of advanced endometriosis, S. D. also had diffuse implants of deep and superficial endometriosis on the peritoneum of the pelvic sidewalls and on the anterior and posterior cul de sac.
Should you ablate or resect these lesions? Are there advantages to either approach?
Ablation of endometriosis implants may involve either electrocoagulation of the lesion with bipolar energy or laser vaporization/coagulation, which destroys or devitalizes active endometriosis but does not actually remove the lesion. Ablation destroys the lesion without getting a specimen for histologic diagnosis.
Resection of endometriosis implants involves complete removal of the lesion from its epithelial surface to the depth of its base. Resection can be performed with scissors, laser, or monopolar electrosurgery. Resection removes the lesion in its entirety, yielding a histologic diagnosis and allowing you to determine whether, indeed, the entire specimen has been removed.
The question of what is more effective—ablating or resecting endometriosis implants?—was addressed in a prospective study in which 141 patients with endometriosis-related pain were randomized at laparoscopic surgery to either excision or ablation/coagulation of endometriosis lesions.19 Six months postoperatively, the pain score decreased by, on average, 11.2 points in the excision group and 8.7 points in the coagulation/ablative group.
Because the difference in those average pain scores was not statistically significant, however, investigators concluded that the techniques are comparable, with similar efficacy. That interpretation has been criticized because the study was underpowered and included only patients who had mild endometriosis—leaving open the possibility that deep endometriosis may not be adequately treated by electrocoagulation or ablation.
In contrast to superficial endometriosis, which may respond similarly to ablation or resection, deep endometriosis is difficult to ablate either with electrosurgery or a laser because the energy cannot reach deeper layers and active disease is therefore likely to be left behind. Moreover, when endometriosis overlies vital structures, such as the ureter or bowel, ablation of the lesion may cause thermal damage to the underlying organ, and such damage may not manifest until several days later, when the patient experiences, say, urinary leakage in the peritoneum or symptoms of bowel perforation.
FIGURE 5 illustrates a case in which CO2 laser ablation of endometriosis that had been causing deep dyspareunia did not alleviate symptoms. Because those symptoms persisted, the patient was referred to our center, where a second laparoscopy revealed deep nodules of endometriosis, 1 to 2 cm in diameter, extending from the right and the left uterosacral ligaments deep into the perirectal space, bilaterally.
As the bottom panel of FIGURE 5 shows, excised nodules were deep and large; neither laser nor electrosurgery would have been able to ablate or devitalize the deep endometriosis at the base of these 2-cm nodules.

FIGURE 5 Deep nodules present a surgical challenge
These nodules of endometriosis on the right and left uterosacral ligaments (panel A) did not respond to CO2 laser ablation. Upon progressive resection, the implants were found to be deep, extending into the perirectal space (panel B). (See also VIDEO 3, resection of endometriosis from the left uterosacal ligament, close to the ureter.) FIGURE 6, illustrates endometriosis overlying the bladder and left ureter (see also VIDEO 4). Ablation of endometriosis in these areas may be inadequate if it is not deep enough, and dangerous if it goes too deep. As FIGURE 6 shows, excision assures the surgeon that the entire lesion has been removed and that underlying vital structures have been safeguarded.

FIGURE 6 Urinary tract involvement
Endometriosis overlying the bladder is grasped, retracted, and resected (panel A). Endometriosis compresses the left ureter (panel B). The peritoneum above the lesion is entered, the ureter is displaced laterally, and the lesion is safely resected.
What we do, and recommend
When endometriosis is superficial and does not overlie vital organs, such as the bladder, ureter, and bowel, ablation and resection may be equally safe and effective. When endometriosis is deep and overlying vital organs, however, complete resection—with careful dissection of the lesion off underlying structures—offers a more complete and a safer surgical approach.
CASE continued
Now that S. D. has been treated surgically by complete excision of endometriosis, adhesions, and endometriomas, you must consider a management plan that will reduce the risks 1) of recurrence of symptoms and disease and 2) that further surgery will be necessary in the future—a risk that, in her case, exceeds 50% because of her young age, nulliparity, and the severity of her disease.20,21 Indeed, you are aware that, had preventive measures been implemented after her initial surgery 2 years earlier, it is unlikely that S. D. would have developed the second endometrioma and most likely that she would not have needed the second surgery.
Prevention of recurrence is necessary—and doable
The importance of implementing preventive measures to reduce the risk of recurrence of endometriosis and its symptoms has been suggested by several studies. It was underscored recently in a prospective, randomized study conducted by Serracchioli and colleagues,22 in which 239 women who had undergone laparoscopic resection of endometriomas were randomly assigned to expectant management (control group), a cyclic oral contraceptive (OC), or a continuous oral contraceptives for 24 months, and evaluated every 6 months.
At the end of the study, recurrence of symptoms occurred in 30% of controls; 15% of subjects taking a cyclic OC; and 7.5% of the subjects taking a continuous OC. The recurrence rate of endometrioma in this study was reduced by 50% (cyclic OC) and 75% (continuous OC).22
Similar results were reported in a case-controlled study by Vercellini and co-workers,23 who found that the risk of recurrence of endometrioma was reduced by 60% when postoperative OCs were used long-term and by 30% when used for a duration of less than 12 months.
These studies suggest that, by suppressing ovulation and inducing a state of hypomenorrhea or amenorrhea, the risk of recurrence of endometriosis and its symptoms can be significantly reduced.
The importance of amenorrhea in reducing the postoperative recurrence of endometriosis and symptoms has been underscored by two important studies that evaluated the role of postoperative endometrial ablation or postoperative insertion of the levonorgestrel intrauterine system (LNG-IUS; Mirena), neither of which suppresses ovulation but both of which induce a state of hypomenorrhea or amenorrhea.24,25
In a prospective randomized study by Bulletti and co-workers,24 28 patients who had symptomatic endometriosis underwent laparoscopic conservative surgery. Endometrial ablation was performed in 14 of the 28. Two years later, all patients underwent second-look laparoscopy; recurrence of endometriosis was found in 9 of the 14 non-ablation patients but in none in the ablation group. Resolution or significant improvement of symptoms were reported in 13 of 14 women in the ablation group but only in 3 of 14 in the non-ablation group—supporting the premise that amenorrhea or hypomenorrhea by itself, without suppressing ovulation, significantly reduces the risk that endometriosis will recur.
Similar beneficial results from hypomenorrhea/amenorrhea on the risk of recurrence of symptoms have been reported when the LNG-IUS is inserted following conservative surgery for endometriosis. In a prospective study by Vercellini and colleagues,25 40 symptomatic patients who had stage-III or stage-IV disease were randomized to either insertion of the LNG-IUS or a control group after conservative laparoscopic surgery. Recurrence of pain was significantly (P = .012) reduced in the LNG-IUS group (45%), compared with the control group (10%). Control subjects were also much less satisfied with their treatment than those who were treated with the LNG-IUS.
The importance of inducing a state of amenorrhea to reduce the risk of disease recurrence was further underscored by a recent study. Shakiba and colleagues26 reported on the recurrence of endometriosis that required further surgery as long as 7 years after the subjects had been surgically treated for symptomatic endometriosis. The need for subsequent surgery was 8% after hysterectomy and bilateral salpingo-oophorectomy; 12% after hysterectomy alone; and 60% after conservative laparoscopy with preservation of both uterus and ovaries.
Taken together, these data show that, unless the patient is rendered amenorrheic or hypomenorrheic, her risk of recurrence exceeds 50%.
It is important, therefore, to consider conservative surgical management of endometriosis as only the beginning of a lifelong management plan. That plan begins with complete resection of all visible endometriosis and adhesions, resection of endometriomas, and restoration of normal anatomy as much as possible.
When endometriosis cannot be completely resected—as when it involves small bowel or the diaphragm, or is diffusely on the large bowel—we recommend medical suppressive therapy. Our preference is depot leuprolide acetate (Lupron Depot), always with add-back therapy to minimize side effects, which include vasomotor symptoms, vaginal dryness, and bone loss,27 until the patient is significantly asymptomatic, which may take 6 to 9 months.
CASE Concluded, with long-term intervention
You counsel S. D. to remain on a low-dose hormonal OC continuously, until such time that she wants to conceive. If a patient does not want to conceive for at least 5 years, the LNG-IUS may be inserted at surgery to induce hypomenorrhea and reduce the risk of recurrence for the next 5 years.
When hormonal contraceptives are inadequate to control symptoms, adding the aromatase enzyme inhibitor letrozole (Femara), 2.5 mg daily for 6 to 9 months, usually alleviates symptoms with minimal side effects, as long as the patient keeps taking a hormonal contraceptive. Using letrozole without hormonal contraception has not been studied; doing so may lead to formation of ovarian cysts, and is therefore not recommended for managing symptomatic endometriosis.
If the patient wants to become pregnant, encourage her to actively undertake fertility treatment as soon as possible after surgery, thereby minimizing the risk of recurrence of symptoms and disease. The best option may be to employ assisted reproductive technology, but patients cannot always afford it; when that is the case, consider controlled ovarian stimulation and intrauterine insemination.
We want to hear from you! Tell us what you think.
- Resection of an endometrioma in severe disease, using a “stripping” technique
- Ovarian cystectomy
- Resection of endometriosis from the left ligament
- Resection of endometriosis on the bladder
These videos were provided by Anthony Luciano, MD.
Endometriosis affects 7% to 10% of women in the United States, mostly during reproductive years.1 The estimated annual cost for managing the approximately 10 million affected women? More than $17 billion.2 The added cost of this chronic disease, with recurrences of pain and infertility, comes in the form of serious life disruption, emotional suffering, marital and social dysfunction, and diminished productivity.
Although the prevalence of endometriosis is highest during the third and fourth decades of life, the disease is also common in adolescent girls. Indeed, 45% of adolescents who have chronic pelvic pain are found to have endometriosis; if their pain does not respond to an oral contraceptive (OC) or a non-steroidal anti-inflammatory drug, 70% are subsequently found at laparoscopy to have endometriosis.3
What is it?
Endometriosis is the presence of functional endometrial tissue outside the uterus, such as eutopic endometrium. The disease responds to effects of cyclic ovarian hormones, proliferating and bleeding with each menstrual cycle, which often leads to diffuse inflammation, adhesions, and growth of endometriotic nodules or cysts (FIGURE 1).

FIGURE 1 Drainage will not suffice
Surgical management of ovarian endometriomas must go beyond simple drainage, which has little therapeutic value because symptoms recur and endometriomas re-form quickly after simple drainage in almost all patients.Symptoms tend to reflect affected organs:
- Because the pelvic organs are most often involved, the classic symptom triad of the disease comprises dysmenorrhea, dyspareunia, and infertility.
- Urinary urgency, dysuria, dyschezia, and tenesmus are frequent complaints when the bladder or rectosigmoid is involved.
- When distant organs are affected, such as the upper abdomen, diaphragm, lungs, and bowel, the patient may complain of respiratory symptoms, hemoptysis, pneumothorax, shoulder pain, upper abdominal pain, and episodic gastrointestinal dysfunction.
The hallmark of endometriosis is catamenial symptoms, which are usually cyclic and most severe around the time of menses. Clinical signs include palpable tender nodules and fibrosis on the anterior and posterior cul de sac, fixed retroverted or anteverted uterus, and adnexal cystic masses.
Because none of these symptoms or signs is specific for endometriosis, diagnosis relies on laparoscopy, which allows the surgeon to:
- visualize it in its various appearances and locations (FIGURE 2)
- confirm the diagnosis histologically with directed excisional biopsy
- treat it surgically with either excision or ablation.
In this article, we describe various surgical techniques for the management of endometriosis. Beyond resection or ablation of lesions, however, your care should also be directed to postoperative measures to prevent its recurrence and to avoid repeated surgical interventions—which, regrettably, are much too common in women who are afflicted by this enigmatic disease.

FIGURE 2 Endometriosis: A disease of varying appearance
Lesions of endometriosis can be pink, dark, clear, or white on the pelvic sidewall (A), bowel (B), and diaphragm (C); under the rib cage (D); and on the ureter (E) (left ureter shown here).
CASE Severe disease in a young woman
S. D. is a 22-year-old unmarried nulligravida who came to the emergency service complaining of acute onset of severe low abdominal pain, which developed while she was running. She was afebrile and in obvious distress, with diffuse lower abdominal tenderness and guarding, especially on the left side.
Ultrasonography revealed a 7-cm adnexal cystic mass suggestive of endometrioma (FIGURE 3).
Two years before this episode, S. D. underwent laparoscopic resection of a 5-cm endometrioma on the right ovary. Subsequently, she was treated with a cyclic OC, which she discontinued after 1 year because she was not sexually active.
The family history is positive for endometriosis in her mother, who had undergone multiple laparoscopic investigations and, eventually, total hysterectomy with bilateral salpingo-oophorectomy at 40 years of age.
S. D. was treated on the emergency service with analgesics and referred to you for surgical management.
S. D. has severe disease that requires aggressive surgical resection and a lifelong management plan. That plan includes liberal use of medical therapy to prevent recurrence of symptoms and avoid repeated surgical procedures—including the total hysterectomy with bilateral salpingo-oophorectomy that her mother underwent.
What is the best immediate treatment plan? Should you:
- drain the cyst?
- drain it and coagulate or ablate its wall?
- resect the wall of the cyst?
- perform salpingo-oophorectomy?
You also ask yourself: What is the risk of recurrence of endometrioma and its symptoms after each of those treatments? And how can I reduce those risks?

FIGURE 3 Endometrioma
Endometrioma on ultrasonography (A), with its characteristic homogeneous, echogenic appearance and “ground glass” pattern, and through the laparoscope (B). These images are from the patient whose case is described in the text.
Focal point: Ovary
The ovary is the most common organ affected by endometriosis. The presence of ovarian endometriomas, in 17% to 44% of patients who have this disease,4 is often associated with an advanced stage of disease.
In a population of 1,785 patients who were surgically treated for ovarian endometriosis, Redwine reported that only 1% had exclusively ovarian involvement; 99% also had diffuse pelvic disease,5 suggesting that ovarian endometrioma is a marker of extensive disease, which often requires a gynecologic surgeon who has advanced skills and experience in the surgical management of severe endometriosis.
Simple drainage is inadequate
Surgical management of ovarian endometrioma must go beyond simple drainage, which has little therapeutic value because symptoms recur and endometriomas re-form quickly after simple drainage in almost all patients.6 The currently accepted surgical management of endometrioma involves either 1) coagulation and ablation of the wall of the cyst with electrosurgery or laser or 2) removal of the cyst wall from the ovary with blunt and sharp dissection.
Several studies have compared these two techniques, but only two7,8 were prospectively randomized.
Study #1. Beretta and co-workers7 studied 64 patients who had ovarian endometriomas larger than 3 cm and randomized them to cystectomy by complete stripping of the cyst wall or to drainage of fluid followed by electrocoagulation to ablate the endometriosis lesions within the cyst wall. The two groups were followed for 2 years to assess the recurrence of symptoms and the pregnancy rate in the patients who were infertile.
Recurrence of symptoms and the need for medical or surgical intervention occurred with less frequency and much later in the resection group than in the ablation group: 19 months, compared to 9.5 months, postoperatively. The cumulative pregnancy rate 24 months postoperatively was also much higher in the resection group (66.7%) than in the ablative group (23.5%).
Study #2. In a later study,8 Alborzi and colleagues randomized 100 patients who had endometrioma to cystectomy or to drainage and coagulation of the cyst wall. The mean recurrence rate, 2 years postoperatively, was much lower in the excision group (15.8%) than in the ablative group (56.7%). The cumulative pregnancy rate at 12 months was higher in the excision group (54.9%, compared to 23.3%). Furthermore, the reoperation rate at 24 months was much lower in the excision group (5.8%) than in the ablative group (22.9%).
These favorable results for cystectomy over ablation were validated by a Cochrane Review, which concluded that excision of endometriomas is the preferred approach because it provides 1) a more favorable outcome than drainage and ablation, 2) lower rates of recurrence of endometriomas and symptoms, and 3) a much higher spontaneous pregnancy rate in infertile women.9
Although resection of the cyst wall is technically more challenging and takes longer to perform than drainage and ablation, we exclusively perform resection rather than ablation of endometriomas because we believe that more lasting therapeutic effects and reduced recurrence of symptoms and disease justify the extra effort and a longer procedure.
Drawback of cystectomy
A potential risk of cystectomy is that it can diminish ovarian reserve and, in rare cases, induce premature menopause, which can be devastating for women whose main purpose for having surgery is to restore or improve their fertility.
The impact of laparoscopic ovarian cystectomy on ovarian reserve was prospectively studied by Chang and co-workers,10 who measured preoperative and postoperative levels of anti-müllerian hormone (AMH) in 13 women who had endometrioma, 6 who had mature teratoma, and 1 who had mucinous cystadenoma. One week postoperatively, the AMH level decreased significantly overall in all groups. At 4 and 12 weeks postoperatively, however, the AMH level returned to preoperative levels among subjects in the non-endometrioma group but not among subjects who had endometrioma; rather, their level remained statistically lower than the preoperative level during the entire 3 months of follow-up.
Stripping the wall of an endometrioma cyst is more difficult than it is for other benign cysts, such as cystic teratoma or cystadenoma, in which there usually is a well-defined dissection plane between the wall of the cyst and surrounding stromal tissue—allowing for easy and clean separation of the wall. The cyst wall of an endometrioma, on the other hand, is intimately attached to underlying ovarian stroma; lack of a clear cleavage plane between cyst and ovarian stroma often results in unintentional removal of layers of ovarian cortex with underlying follicles, which, in turn, may lead to a reduction in ovarian reserve.
Histologic analyses of resected endometrioma cyst walls have reported follicle-containing ovarian tissue attached to the stripped cyst wall in 54% of cases.11,12 That observation explains why, and how, ovarian reserve can be compromised after resection of endometrioma.
Further risk: Ovarian failure
In rare cases, excision of endometriomas results in complete ovarian failure, described by Busacca and colleagues, who reported three cases of ovarian failure (2.4%) after resection of bilateral endometriomas in 126 patients.13 They attributed ovarian failure to excessive cauterization that compromised vascularization, as well as to excessive removal of ovarian tissue.
It is important, therefore, to strip the thinnest layer of the cyst capsule and to reduce the amount of electrocoagulation of ovarian stroma as much as possible to safeguard functional ovarian tissue.
CASE continued
S. D. was scheduled for laparoscopy to remove the endometrioma and other concurrent pelvic and peritoneal pathology, such as endometriosis and pelvic adhesions. You also scheduled her for hysteroscopy to evaluate the endometrial cavity for potential pathology, such as endometrial polyps and uterine septum, which appear to be more common in women who have endometriosis.
Nawroth and co-workers14 found a much higher incidence of endometriosis in patients who had a septate uterus. Metalliotakis and co-workers15 found congenital uterine malformations to be more common in patients who had endometriosis, compared with controls; uterine septum was, by far, the most common anomaly.
CASE continued
Hysteroscopy revealed a small and broad septum, which was resected sharply with hysteroscopic scissors (FIGURE 4). Laparoscopy revealed a 7-cm endometrioma on the left ovary, with adhesions to the posterior broad ligament and pelvic sidewall. S. D. also had deep implants of endometriosis on the left pelvic sidewall, the posterior cul de sac, the right pelvic sidewall, and the right ovary, which was cohesively adherent to the ovarian fossa.
As you expected, S. D. has stage-IV disease, according to the revised American Fertility Society Classification.
Following adhesiolysis, the endometrioma was resected (see VIDEO 1). Because of the large ovarian defect, the edges of the ovary were approximated with imbricating running 3-0 Vicryl suture. Deep endometriosis was also resected. Superficial endometriosis was peeled off or coagulated using bipolar forceps.
Note: Alternatively, and with comparable results, resection may be performed with a laser or other energy source. We prefer resection, rather than ablation, of deep endometriosis, but no data exists to support one technique over the other.

FIGURE 4 Septate uterus with deep cornua
Through the hysteroscope, a shallow septum is visible at the fundus of the uterus, dividing the upper endometrial cavity into two chambers (A), with deep cornua on the left (B) and right (C). Normal fundal anatomy is restored by septolysis along the avascular plane (D).
Technique: How we resect endometrioma
In removing endometrioma (see VIDEO 2), it is important to grasp the thinnest part of the cyst wall and progressively strip it, to avoid removing excess ovarian tissue and to reduce the risk of compromising ovarian reserve.
After draining the endometrioma of its chocolate-colored fluid, we irrigate and drain the cyst several times with warm lactated Ringers’ solution to promote separation of the cyst wall from underlying stroma and better identify the dissection plane. The cyst wall is inspected by introducing the laparoscope into the cyst to examine its surface, which is often laden with implants of deep and superficial endometriosis.
If we cannot easily identify the plane of dissection along the edges, we may evert the cyst and make an incision at its base to create a wedge between the wall of the cyst and underlying stroma. The edge of the incised wall is then grasped and retracted to create a space between the wall and the underlying stroma, from which it is progressively stripped from the ovary.
Traction and counter-traction are the hallmarks of dissection here; sometimes, we use laparoscopic scissors to sharply resect the ovarian stromal attachments that adhere cohesively to the cyst wall. This technique is continued until the entire cyst wall is removed. When follicle-containing ovarian tissue remains attached to the cyst wall, we introduce the closed tips of the Dolphin forceps between the cyst wall and adjacent follicle-containing stroma, spread the tips apart, and recover the true plane of dissection between the thin wall of the cyst and stroma.
After the wall of the cyst is removed, the ovarian crater invariably bleeds because blood vessels supplying the wall have been separated and opened. Utilizing warm lactated Ringers’ solution, we copiously irrigate the bleeding ovarian stroma to identify each bleeding vessel and, by placing the tips of the micro-bipolar forceps on either side of the bleeder, individually coagulate each vessel, thus inflicting minimal thermal damage to the surrounding stroma.
Pearl. Avoid using Kleppinger forceps to indiscriminately coagulate the bloody stroma in the crater created after the cystectomy, because doing so can result in excessive destruction of ovarian tissue or inadvertent coagulation of hylar vessels that would interrupt the blood supply to the ovary, compromising its function.16
Suturing. Some surgeons find that fenestration, drainage, and coagulation of the cyst wall is acceptable, but we have concerns not only about incomplete ablation of the endometriosis on the cyst wall, which may be responsible for the higher recurrence rate of disease, but also about the risk of thermal injury to underlying follicles, which may compromise ovarian reserve.16
Hemostasis. Once complete hemostasis has been achieved, the decision to approximate (or not) the edges, preferably with fine absorbable suture, is based on how large the defect is and whether or not the edges of the crater spontaneously come together. For large craters, we usually close the ovary with a 3-0 or 4-0 Vicryl continuous suture, imbricating the edges to expose as little suture material as possible to reduce postoperative formation of adhesions, which is common after ovarian surgery.17
Last, we ensure that hemostasis is present. Often we apply an anti-adhesion solution, such as icodextrin 4% (Adept). This agent has been shown to reduce postoperative adhesion formation, especially after laparoscopic surgery for endometriosis.18
A high level of skill is needed
Ovarian endometriomas signal advanced disease; advanced surgical skills are required to treat them adequately. Simple drainage is of little therapeutic value and should seldom be considered a treatment option. Although drainage plus ablation of the cyst wall ameliorates symptoms, excision of endometriomas should be considered preferable because it provides a more favorable outcome, a lower risk of recurrence of endometriomas and symptoms, and a higher rate of spontaneous pregnancy in previously infertile women.7-9
To recap, we advise the surgeon to:
- Manage ovarian endometriomas with resection of the entire cyst wall, grasping and stripping the thinnest layer of the cyst wall without removing underlying functional ovarian stroma.
- Avoid excessive cauterization of the underlying ovarian stroma by utilizing micro-bipolar forceps and applying energy only around bleeding vessels.
- Close stromal defects, when the crater is large and its edges do not spontaneously come together, by approximating the edges with an imbricating resorbable suture.
CASE continued
As in most cases of advanced endometriosis, S. D. also had diffuse implants of deep and superficial endometriosis on the peritoneum of the pelvic sidewalls and on the anterior and posterior cul de sac.
Should you ablate or resect these lesions? Are there advantages to either approach?
Ablation of endometriosis implants may involve either electrocoagulation of the lesion with bipolar energy or laser vaporization/coagulation, which destroys or devitalizes active endometriosis but does not actually remove the lesion. Ablation destroys the lesion without getting a specimen for histologic diagnosis.
Resection of endometriosis implants involves complete removal of the lesion from its epithelial surface to the depth of its base. Resection can be performed with scissors, laser, or monopolar electrosurgery. Resection removes the lesion in its entirety, yielding a histologic diagnosis and allowing you to determine whether, indeed, the entire specimen has been removed.
The question of what is more effective—ablating or resecting endometriosis implants?—was addressed in a prospective study in which 141 patients with endometriosis-related pain were randomized at laparoscopic surgery to either excision or ablation/coagulation of endometriosis lesions.19 Six months postoperatively, the pain score decreased by, on average, 11.2 points in the excision group and 8.7 points in the coagulation/ablative group.
Because the difference in those average pain scores was not statistically significant, however, investigators concluded that the techniques are comparable, with similar efficacy. That interpretation has been criticized because the study was underpowered and included only patients who had mild endometriosis—leaving open the possibility that deep endometriosis may not be adequately treated by electrocoagulation or ablation.
In contrast to superficial endometriosis, which may respond similarly to ablation or resection, deep endometriosis is difficult to ablate either with electrosurgery or a laser because the energy cannot reach deeper layers and active disease is therefore likely to be left behind. Moreover, when endometriosis overlies vital structures, such as the ureter or bowel, ablation of the lesion may cause thermal damage to the underlying organ, and such damage may not manifest until several days later, when the patient experiences, say, urinary leakage in the peritoneum or symptoms of bowel perforation.
FIGURE 5 illustrates a case in which CO2 laser ablation of endometriosis that had been causing deep dyspareunia did not alleviate symptoms. Because those symptoms persisted, the patient was referred to our center, where a second laparoscopy revealed deep nodules of endometriosis, 1 to 2 cm in diameter, extending from the right and the left uterosacral ligaments deep into the perirectal space, bilaterally.
As the bottom panel of FIGURE 5 shows, excised nodules were deep and large; neither laser nor electrosurgery would have been able to ablate or devitalize the deep endometriosis at the base of these 2-cm nodules.

FIGURE 5 Deep nodules present a surgical challenge
These nodules of endometriosis on the right and left uterosacral ligaments (panel A) did not respond to CO2 laser ablation. Upon progressive resection, the implants were found to be deep, extending into the perirectal space (panel B). (See also VIDEO 3, resection of endometriosis from the left uterosacal ligament, close to the ureter.) FIGURE 6, illustrates endometriosis overlying the bladder and left ureter (see also VIDEO 4). Ablation of endometriosis in these areas may be inadequate if it is not deep enough, and dangerous if it goes too deep. As FIGURE 6 shows, excision assures the surgeon that the entire lesion has been removed and that underlying vital structures have been safeguarded.

FIGURE 6 Urinary tract involvement
Endometriosis overlying the bladder is grasped, retracted, and resected (panel A). Endometriosis compresses the left ureter (panel B). The peritoneum above the lesion is entered, the ureter is displaced laterally, and the lesion is safely resected.
What we do, and recommend
When endometriosis is superficial and does not overlie vital organs, such as the bladder, ureter, and bowel, ablation and resection may be equally safe and effective. When endometriosis is deep and overlying vital organs, however, complete resection—with careful dissection of the lesion off underlying structures—offers a more complete and a safer surgical approach.
CASE continued
Now that S. D. has been treated surgically by complete excision of endometriosis, adhesions, and endometriomas, you must consider a management plan that will reduce the risks 1) of recurrence of symptoms and disease and 2) that further surgery will be necessary in the future—a risk that, in her case, exceeds 50% because of her young age, nulliparity, and the severity of her disease.20,21 Indeed, you are aware that, had preventive measures been implemented after her initial surgery 2 years earlier, it is unlikely that S. D. would have developed the second endometrioma and most likely that she would not have needed the second surgery.
Prevention of recurrence is necessary—and doable
The importance of implementing preventive measures to reduce the risk of recurrence of endometriosis and its symptoms has been suggested by several studies. It was underscored recently in a prospective, randomized study conducted by Serracchioli and colleagues,22 in which 239 women who had undergone laparoscopic resection of endometriomas were randomly assigned to expectant management (control group), a cyclic oral contraceptive (OC), or a continuous oral contraceptives for 24 months, and evaluated every 6 months.
At the end of the study, recurrence of symptoms occurred in 30% of controls; 15% of subjects taking a cyclic OC; and 7.5% of the subjects taking a continuous OC. The recurrence rate of endometrioma in this study was reduced by 50% (cyclic OC) and 75% (continuous OC).22
Similar results were reported in a case-controlled study by Vercellini and co-workers,23 who found that the risk of recurrence of endometrioma was reduced by 60% when postoperative OCs were used long-term and by 30% when used for a duration of less than 12 months.
These studies suggest that, by suppressing ovulation and inducing a state of hypomenorrhea or amenorrhea, the risk of recurrence of endometriosis and its symptoms can be significantly reduced.
The importance of amenorrhea in reducing the postoperative recurrence of endometriosis and symptoms has been underscored by two important studies that evaluated the role of postoperative endometrial ablation or postoperative insertion of the levonorgestrel intrauterine system (LNG-IUS; Mirena), neither of which suppresses ovulation but both of which induce a state of hypomenorrhea or amenorrhea.24,25
In a prospective randomized study by Bulletti and co-workers,24 28 patients who had symptomatic endometriosis underwent laparoscopic conservative surgery. Endometrial ablation was performed in 14 of the 28. Two years later, all patients underwent second-look laparoscopy; recurrence of endometriosis was found in 9 of the 14 non-ablation patients but in none in the ablation group. Resolution or significant improvement of symptoms were reported in 13 of 14 women in the ablation group but only in 3 of 14 in the non-ablation group—supporting the premise that amenorrhea or hypomenorrhea by itself, without suppressing ovulation, significantly reduces the risk that endometriosis will recur.
Similar beneficial results from hypomenorrhea/amenorrhea on the risk of recurrence of symptoms have been reported when the LNG-IUS is inserted following conservative surgery for endometriosis. In a prospective study by Vercellini and colleagues,25 40 symptomatic patients who had stage-III or stage-IV disease were randomized to either insertion of the LNG-IUS or a control group after conservative laparoscopic surgery. Recurrence of pain was significantly (P = .012) reduced in the LNG-IUS group (45%), compared with the control group (10%). Control subjects were also much less satisfied with their treatment than those who were treated with the LNG-IUS.
The importance of inducing a state of amenorrhea to reduce the risk of disease recurrence was further underscored by a recent study. Shakiba and colleagues26 reported on the recurrence of endometriosis that required further surgery as long as 7 years after the subjects had been surgically treated for symptomatic endometriosis. The need for subsequent surgery was 8% after hysterectomy and bilateral salpingo-oophorectomy; 12% after hysterectomy alone; and 60% after conservative laparoscopy with preservation of both uterus and ovaries.
Taken together, these data show that, unless the patient is rendered amenorrheic or hypomenorrheic, her risk of recurrence exceeds 50%.
It is important, therefore, to consider conservative surgical management of endometriosis as only the beginning of a lifelong management plan. That plan begins with complete resection of all visible endometriosis and adhesions, resection of endometriomas, and restoration of normal anatomy as much as possible.
When endometriosis cannot be completely resected—as when it involves small bowel or the diaphragm, or is diffusely on the large bowel—we recommend medical suppressive therapy. Our preference is depot leuprolide acetate (Lupron Depot), always with add-back therapy to minimize side effects, which include vasomotor symptoms, vaginal dryness, and bone loss,27 until the patient is significantly asymptomatic, which may take 6 to 9 months.
CASE Concluded, with long-term intervention
You counsel S. D. to remain on a low-dose hormonal OC continuously, until such time that she wants to conceive. If a patient does not want to conceive for at least 5 years, the LNG-IUS may be inserted at surgery to induce hypomenorrhea and reduce the risk of recurrence for the next 5 years.
When hormonal contraceptives are inadequate to control symptoms, adding the aromatase enzyme inhibitor letrozole (Femara), 2.5 mg daily for 6 to 9 months, usually alleviates symptoms with minimal side effects, as long as the patient keeps taking a hormonal contraceptive. Using letrozole without hormonal contraception has not been studied; doing so may lead to formation of ovarian cysts, and is therefore not recommended for managing symptomatic endometriosis.
If the patient wants to become pregnant, encourage her to actively undertake fertility treatment as soon as possible after surgery, thereby minimizing the risk of recurrence of symptoms and disease. The best option may be to employ assisted reproductive technology, but patients cannot always afford it; when that is the case, consider controlled ovarian stimulation and intrauterine insemination.
We want to hear from you! Tell us what you think.
1. Bulun S E. Endometriosis. N Engl J Med. 2009;360(3):268-279.
2. Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86(6):1561-1572.
3. Laufer MR, Goitein L, Bush M, Cramer DW, Emans SJ. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding conventional therapy. J Pediatr Adolesc Gynecol. 1997;10(4):199-202.
4. Gruppo Italiano per lo studio dell’endometriosi. Prevalence and anatomic distribution of endometriosis in women with selected gynaecological conditions: results from a multicenter Italian study. Hum Reprod. 1994;9(6):1158-1162.
5. Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72(2):319-315.
6. Muzii L, Marana R, Caruana P, Catalano GF, Mancuso S. Laparoscopic findings after transvaginal ultrasound-guided aspiration of ovarian endometriomas. Hum Reprod. 1995;10(11):2902-2903
7. Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatment of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176-1180
8. Alborzi S, Momtahan M, Paranezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633-1637
9. Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomas. Cochrane Database Syst Rev. 2005;(3):CD004992.-
10. Chang HJ, Sang HH, Jung RL, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril. 2010;94(1):343-349.
11. Muzii L. Bianchi A Crocè C, Manci N, Panici PB. Laparoscopic excision of ovarian cysts: is the stripping technique a tissue sparing procedure? Fertil Steril. 2002;77(3):609-614.
12. Hachisuga T, Kawarabyashi T. Histopathological analysis of laparoscopically treated ovarian endometriotic cysts with special reference to loss of follicles. Hum Reprod. 2002;17(2):432-435.
13. Busacca M, Riparini J Somigliana E, et al. Postsurgical ovarian failure after laparoscopic excision of bilateral endometriomas. Am J Obstet Gynecol. 2006;195(2):421-425.
14. Nawroth F, Rahimi G, Nawroth C, Foth D, Ludwig M, Schmidt T. Is there an association between septate uterus and endometriosis? Hum Reprod. 2006;21(2):542-546.
15. Matalliotakis IM, Goumenou AG, Matalliotakis M, Arici A. Uterine anomalies in women with endometriosis. J Endometriosis. 2010;2(4):213-217.
16. Li CZ, Liu B, Wen ZQ, Sun Q. The impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cyst: a prospective clinical study of 191 patients. Fertil Steril. 2009;92(4):1428-1435.
17. Luciano DE, Roy G, Luciano AA. Adhesion reformation after laparoscopic adhesiolysis: where what type, and in whom are they most likely to recur. J Minim Invasive Gynecol. 2008;15(1):44-48.
18. Colin CB, Luciano AA, Martin D, et al. Adept (icodextrin 4% solution) reduces adhesions after laparoscopic surgery for adhesiolysis: a double-blind, randomized, controlled study. Fertil Steril. 2007;88(5):1413-1426.
19. Wright J, Lotfallah H, Jones K, Lovell D. A randomized study of excision vs ablation for mild endometriosis. Fertil Steril. 2004;83(6):1830-1836.
20. Cheong Y, Tay P, Luk F, Gan HC, Li TC, Cooke I. Laparoscopic surgery for endometriosis: How often do we need to re-operate? J Obstet Gynaecol. 2008;28(1):82-85.
21. Liu X, Yuan L, Shen F, Zhu Z, Jiang H, Guo SW. Patterns of and risk factors for recurrence in women with ovarian endometriomas. Obstet Gynecol. 2007;109(6):1411-1120.
22. Seracchioli R, Mabrouk M, Frasca C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometriomas recurrence: a randomized controlled trial. Fertil Steril. 2010;93(1):52-56.
23. Vercellini P, Somigliana E, Daguati R, Vigano P, Meroni F, Crosignani PG. Postoperative oral contraceptive exposure and risk of endometrioma recurrence. Am J Obstet Gynecol. 2008;198(5):504.e1-5.
24. Bulletti C, DeZiegler D, Stefanetti M, Cicinelli E, Pelosi E, Flamigni C. Endometriosis: absence of recurrence in patients after endometrial ablation. Hum Reprod. 2001;16(12):2676-2679.
25. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80(2):305-309.
26. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285-1292.
27. Surrey ES, Hornstein MD. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: a long-term follow-up Obstet Gynecol. 2002;99(5 Pt 1):709-719.
1. Bulun S E. Endometriosis. N Engl J Med. 2009;360(3):268-279.
2. Gao X, Outley J, Botteman M, Spalding J, Simon JA, Pashos CL. Economic burden of endometriosis. Fertil Steril. 2006;86(6):1561-1572.
3. Laufer MR, Goitein L, Bush M, Cramer DW, Emans SJ. Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding conventional therapy. J Pediatr Adolesc Gynecol. 1997;10(4):199-202.
4. Gruppo Italiano per lo studio dell’endometriosi. Prevalence and anatomic distribution of endometriosis in women with selected gynaecological conditions: results from a multicenter Italian study. Hum Reprod. 1994;9(6):1158-1162.
5. Redwine DB. Ovarian endometriosis: a marker for more extensive pelvic and intestinal disease. Fertil Steril. 1999;72(2):319-315.
6. Muzii L, Marana R, Caruana P, Catalano GF, Mancuso S. Laparoscopic findings after transvaginal ultrasound-guided aspiration of ovarian endometriomas. Hum Reprod. 1995;10(11):2902-2903
7. Beretta P, Franchi M, Ghezzi F, Busacca M, Zupi E, Bolis P. Randomized clinical trial of two laparoscopic treatment of endometriomas: cystectomy versus drainage and coagulation. Fertil Steril. 1998;70(6):1176-1180
8. Alborzi S, Momtahan M, Paranezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633-1637
9. Hart RJ, Hickey M, Maouris P, Buckett W, Garry R. Excisional surgery versus ablative surgery for ovarian endometriomas. Cochrane Database Syst Rev. 2005;(3):CD004992.-
10. Chang HJ, Sang HH, Jung RL, et al. Impact of laparoscopic cystectomy on ovarian reserve: serial changes of serum anti-Müllerian hormone levels. Fertil Steril. 2010;94(1):343-349.
11. Muzii L. Bianchi A Crocè C, Manci N, Panici PB. Laparoscopic excision of ovarian cysts: is the stripping technique a tissue sparing procedure? Fertil Steril. 2002;77(3):609-614.
12. Hachisuga T, Kawarabyashi T. Histopathological analysis of laparoscopically treated ovarian endometriotic cysts with special reference to loss of follicles. Hum Reprod. 2002;17(2):432-435.
13. Busacca M, Riparini J Somigliana E, et al. Postsurgical ovarian failure after laparoscopic excision of bilateral endometriomas. Am J Obstet Gynecol. 2006;195(2):421-425.
14. Nawroth F, Rahimi G, Nawroth C, Foth D, Ludwig M, Schmidt T. Is there an association between septate uterus and endometriosis? Hum Reprod. 2006;21(2):542-546.
15. Matalliotakis IM, Goumenou AG, Matalliotakis M, Arici A. Uterine anomalies in women with endometriosis. J Endometriosis. 2010;2(4):213-217.
16. Li CZ, Liu B, Wen ZQ, Sun Q. The impact of electrocoagulation on ovarian reserve after laparoscopic excision of ovarian cyst: a prospective clinical study of 191 patients. Fertil Steril. 2009;92(4):1428-1435.
17. Luciano DE, Roy G, Luciano AA. Adhesion reformation after laparoscopic adhesiolysis: where what type, and in whom are they most likely to recur. J Minim Invasive Gynecol. 2008;15(1):44-48.
18. Colin CB, Luciano AA, Martin D, et al. Adept (icodextrin 4% solution) reduces adhesions after laparoscopic surgery for adhesiolysis: a double-blind, randomized, controlled study. Fertil Steril. 2007;88(5):1413-1426.
19. Wright J, Lotfallah H, Jones K, Lovell D. A randomized study of excision vs ablation for mild endometriosis. Fertil Steril. 2004;83(6):1830-1836.
20. Cheong Y, Tay P, Luk F, Gan HC, Li TC, Cooke I. Laparoscopic surgery for endometriosis: How often do we need to re-operate? J Obstet Gynaecol. 2008;28(1):82-85.
21. Liu X, Yuan L, Shen F, Zhu Z, Jiang H, Guo SW. Patterns of and risk factors for recurrence in women with ovarian endometriomas. Obstet Gynecol. 2007;109(6):1411-1120.
22. Seracchioli R, Mabrouk M, Frasca C, et al. Long-term cyclic and continuous oral contraceptive therapy and endometriomas recurrence: a randomized controlled trial. Fertil Steril. 2010;93(1):52-56.
23. Vercellini P, Somigliana E, Daguati R, Vigano P, Meroni F, Crosignani PG. Postoperative oral contraceptive exposure and risk of endometrioma recurrence. Am J Obstet Gynecol. 2008;198(5):504.e1-5.
24. Bulletti C, DeZiegler D, Stefanetti M, Cicinelli E, Pelosi E, Flamigni C. Endometriosis: absence of recurrence in patients after endometrial ablation. Hum Reprod. 2001;16(12):2676-2679.
25. Vercellini P, Frontino G, De Giorgi O, Aimi G, Zaina B, Crosignani PG. Comparison of levonorgestrel-releasing intrauterine device versus expectant management after conservative surgery for symptomatic endometriosis: a pilot study. Fertil Steril. 2003;80(2):305-309.
26. Shakiba K, Bena JF, McGill KM, Minger J, Falcone T. Surgical treatment of endometriosis: a 7-year follow-up on the requirement for further surgery. Obstet Gynecol. 2008;111(6):1285-1292.
27. Surrey ES, Hornstein MD. Prolonged GnRH agonist and add-back therapy for symptomatic endometriosis: a long-term follow-up Obstet Gynecol. 2002;99(5 Pt 1):709-719.












