User login
Post–FDA hearing: Will open power morcellation of uterine tissue remain an option during hysterectomy and myomectomy?
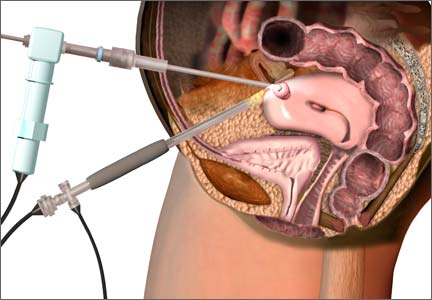
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In July 2014, the FDA convened a two-day hearing of the Obstetrics and Gynecology Devices Panel (one of the panels in its Medical Devices Advisory Committee) to consider whether power morcellation should remain an option and, if so, what restrictions or labeling might be recommended.
In advance of the FDA hearing, OBG Management invited two experts in women’s health to explore the options more deeply and address the future of minimally invasive surgery (MIS): Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
- options for tissue extraction going forward
- the importance of continuing to offer minimally invasive surgical approaches
- the need to educate surgeons about the safest approaches to tissue extraction.
Both surgeons believe that power morcellation should remain an option for selected cases, although neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be used whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?1
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. Our review is the most complete report to date, more comprehensive than the current reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL Task Force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. There are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
Related article: First large study on risk of cancer spread using power morcellation. Janelle Yates (News for your Practice; August 2014)
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction for individual patients undergoing uterine surgery.
AAGL recommendations on the use of power morcellationIn its position statement, the AAGL Tissue Extraction Task Force made the following main points, recommending that surgeons:
Further research also is needed to determine how best to diagnose sarcomas preoperatively, the task force noted. The full report is available on the AAGL Web site.1 —Ray A. Wertheim, MD |
How to manage tissue extraction going forward
OBG Management: Regardless of the FDA’s final decision, what should the gynecologic specialty be doing to avoid disseminating uterine tissue in the peritoneal cavity, particularly leiomyosarcoma?
Dr. Wertheim: MIS is a wonderful advancement in women’s health care. All surgical specialties are moving toward MIS. Our challenge is to perform it as safely as possible, given the data and instrumentation available.
In regard to leiomyosarcoma, because we lack the ability to accurately make the diagnosis preoperatively, we’ve identified risk factors that should be taken into consideration. They include advanced age, a history of radiation or tamoxifen use, black race, hereditary leiomyomatosis, renal cell carcinoma syndrome, and survival of childhood retinoblastoma.
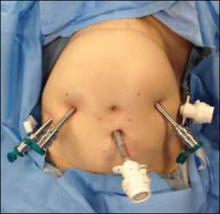
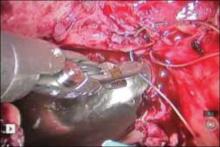
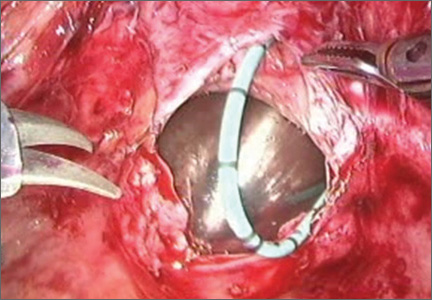
At this time, we have specimen-retrieval bags that can be used with power morcellation. However, it takes skill to be able to place a large specimen inside a bag without injuring surrounding organs due to limited visibility.
Education, at the hospital and national level, is in the works
OBG Management: How should we go about educating surgeons about MIS alternatives to open power morcellation?
Dr. Wertheim: In my hospital, we are mentoring surgeons to help them gain the new skills needed. In addition, I plan to give a grand rounds presentation on tissue extraction for hospitals in northern Virginia and also would like to offer a course in the near future. I’m also hoping that we’ll be able to offer courses around the country before the annual AAGL meeting this November.
At the annual AAGL meeting, the subject will be discussed at length, with an emphasis on identifying risk factors and conducting appropriate preoperative testing, with workshops likely to teach the skills needed to perform these surgeries as safely as possible.
Why a return to reliance on laparotomy would be unwise
OBG Management: Given all the concerns expressed recently about open power morcellation, do you think some surgeons will revert to abdominal hysterectomy rather than rely on MIS? Would such a move be safer than power morcellation?
Dr. Wertheim: That would be a disaster for women. Very reliable data have shown that MIS is safer than open surgery, with much quicker recovery. Almost all of my patients are discharged within 3 hours after surgery, and most no longer require pain medications other than nonsteroidal anti-inflammatory drugs by postoperative day 2. They’re usually back to work within 2 weeks.
We have worked long and hard to develop skills and instrumentation required to perform MIS safely—but nothing replaces good judgment. In some cases, laparotomy or conversion to a laparotomy may be indicated.
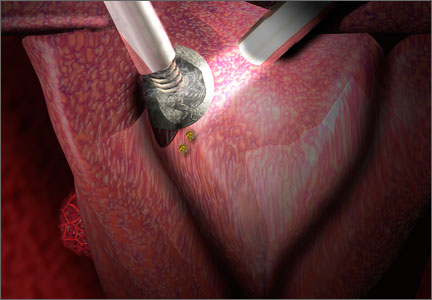
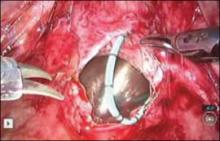
New instrumentation is needed and is being developed. In the meantime, my personal bias is to rule out risk factors for malignancy and continue to morcellate with a scalpel, preferably inside a bag. After all, we know that with open power morcellation, fragments and cells are usually left behind regardless of inspection and irrigation. These fragments may cause leiomyomatosis, endometriosis, bowel obstruction, sepsis, and possible dissemination of tumor fragments. Moreover, morcellation into small fragments complicates the pathologist’s ability to give an accurate report. The use of open power morcellation also subjects the patient to a risk of damage to surrounding organs—usually due to the surgeon’s inexperience.
As I have said before, our challenge is to perform these surgeries using the safest techniques possible, given the current data and instrumentation.
OBG Management: Dr. Reich, you have a unique perspective on this issue, because you pioneered laparoscopic hysterectomy. How has uterine tissue extraction evolved since then? Do you think open power morcellation should remain an option?
Dr. Reich: Uterine tissue extraction has not evolved. The terms “laparoscopic hysterectomy” and “total laparoscopic hysterectomy” imply vaginal extraction using a scalpel, not abdominal extraction using a morcellator. Unfortunately there is no substitute for hard work using a #10 blade on a long handle and special vaginal retraction tools.
In 1983, I made a decision to stop performing laparotomy for all gynecologic procedures, including hysterectomy, myomectomy, urology, oncology, abscesses, extensive adhesions, and rectovaginal endometriosis. I was an accomplished vaginal surgeon at that time, as well as a one-handed laparoscopic surgeon, operating while looking through the scope with one eye.
Interest in a laparoscopic approach to hysterectomy began with my presentations about laparoscopic hysterectomy in January 1988. At that time I had over 10 years of experience doing what is now called laparoscopic-assisted vaginal hysterectomy.
I wrote extensively about specimen removal using a scalpel before electronic power morcellators were available. Since then, I have asked those using power morcellators to stop calling their operation a laparoscopic hysterectomy, as it has more in common with an abdominal-extraction hysterectomy.
I have never advocated removing the uterus using power morcellators, and I still believe that most specimens can be removed vaginally without the spray of pieces of the specimen around the peritoneal cavity that occurs with power morcellation. This goes for hysterectomy involving a large uterus, myomectomy through a culdotomy incision, and removal of the uterine fundus after supracervical hysterectomy. (It is irresponsible to use expensive power morcellation to remove small supracervical hysterectomy specimens.) It is time to get back to learning and teaching vaginal morcellation, although I readily admit it is time consuming.
Nevertheless, I believe power morcellation should remain an option. Recent laparoscopic fellowship trainees know only this technique, which is still better than a return to mutilation by laparotomy.
Gynecology is a frustrating profession—30 years of MIS as a sideshow. General surgery has rapidly adopted a laparoscopic approach to most operations, after gynecologists taught them. Today most gynecologists do not do advanced laparoscopic surgery and would love to get back to open incision laparotomy for their operations. We cannot go back.
OBG Management: Dr. Wertheim and Dr. Reich, do your personal views of the morcellation issue differ at all from the official views of professional societies?
Dr. Wertheim: Yes. However, before I share them, I’d like to emphasize that the views I’m about to express are mine and mine only, not those of the AAGL or its task force.
The issue of uterine extraction is a highly emotional and political issue, about which there are few good data.
Abundant Level 1 data strongly support a vaginal or laparoscopic approach for benign hysterectomy when possible. ACOG and AAGL have issued position papers supporting these approaches for benign hysterectomies. Gynecologic surgeons and other surgical specialists have embraced MIS because it is safer, offers faster recovery, produces less postoperative pain, and has fewer complications than open surgery. However, AAGL has maintained for several years that morcellation is contraindicated in cases where uterine malignancy is either known or suspected.
The dilemma with open power morcellation is that even with our best diagnostic tools, the rare uterine sarcoma cannot always be definitively ruled out preoperatively. Endometrial cancer usually can be diagnosed before surgery. However, rare subtypes such as sarcomas are more difficult to reliably diagnose preoperatively, and risk factors for uterine sarcomas are not nearly as well understood as those for endometrial cancer.
I do agree with the FDA’s cautionary statement on April 17, which pointedly prohibits power morcellation for women with suspected precancer or known cancer of the gynecologic organs.2 However, the AAGL Task Force critically reviewed about 120 articles, including the studies assessed by the FDA. Concerns arose regarding the FDA’s interpretation of the data. Due to a number of deficiencies in these studies, some of the conclusions of the FDA may not be completely accurate. The studies analyzed by the FDA were not stratified by risk factors for sarcoma and were not necessarily performed in a setting of reproductive-aged women with presumed fibroids.
Dr. Reich: Here are my personal views about the sarcoma problem and I am sure they differ from the official views:
- Laparoscopic hysterectomy should always mean vaginal extraction unless a less disfiguring site can be discovered; power morcellation implies minilaparotomy and should be renamed to reflect that fact.
- Power morcellation must be differentiated from vaginal and minilaparotomy scalpel morcellation, especially in the media. Vaginal hysterectomy has entailed vaginal scalpel morcellation with successful outcomes for more than 100 years.
- Remember that most gynecologic cancers are approached using the laparoscope today. This certainly includes cervical and endometrial cancer and some ovarian cancers. (For example, one of my neighbors is a 25-year survivor of laparoscopically treated bilateral ovarian cancer who refused laparotomy!)
- I have removed sarcomas by vaginal morcellation during laparoscopic hysterectomy and laparoscopic myomectomy with no late sequelae. In fact, most cervical cancer surgery is done by laparoscopic surgery today. And even an open laparotomy hysterectomy can spread a sarcoma.
- The current morcellation debate arose when a single case of disseminated leiomyosarcoma became highly publicized. It involved a prominent physician whose leiomyosarcoma was unknown to her initial surgeon, and the malignancy was upstaged after the use of power morcellation during hysterectomy. After this case was covered in the media, other cases began to be reported in the lay press as well, some of which predated the publicized case. The truth is, regrettably, that sarcomas carry poor prognoses even when specimens are removed intact. And we don’t know much about the sarcoma that started this debate. Was it mild or aggressive? How many mitotic figures were there per high-powered field? And what was found macroscopicallyand microscopically at the subsequent laparotomy? We on the AAGL Task Force do not know the answers to these questions, although at least some of these variables are reported in other published cases. And because this case is likely to have a powerful effect on MIS in our country and the rest of the world, it is my opinion that we need to know these details.
What is your preferred surgical approach?
OBG Management: Do you perform open power morcellation in selected patients?
Dr. Wertheim: Even though I have performed morcellation with a scalpel transvaginally or through a mini-laparotomy incision for many years, I have never used open power morcellation because of the risk of leaving behind benign or malignant tissue fragments. Morcellation with a scalpel is easily learned and can be performed as quickly as power morcellation. Morcellation with a scalpel produces much larger pieces than with power morcellation. This probably markedly decreases the loss of fragments. I cannot make a definitive statement regarding cell loss, however. Until we have improved instrumentation and are better able to make a preoperative diagnosis of sarcoma, I’m going to rule out risk factors identified by the AAGL Task Force, do the appropriate work-up, and continue to morcellate with a scalpel, placing the specimen in a bag, if technically possible.
Dr. Reich: As I mentioned, I am a vaginal scalpel morcellator. I tried power morcellation when it first was developed but was never a fan. The same techniques used for vaginal extraction using a coring maneuver can be used abdominally through the umbilicus or a 1- or 2-cm trocar site.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. The Tissue Extraction Task Force, AAGL. AAGL Position Statement: Morcellation during uterine tissue extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed June 13, 2014.
2. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy. FDA Safety Communication. http://www.fda.gov
/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed June 13, 2014.
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In July 2014, the FDA convened a two-day hearing of the Obstetrics and Gynecology Devices Panel (one of the panels in its Medical Devices Advisory Committee) to consider whether power morcellation should remain an option and, if so, what restrictions or labeling might be recommended.
In advance of the FDA hearing, OBG Management invited two experts in women’s health to explore the options more deeply and address the future of minimally invasive surgery (MIS): Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
- options for tissue extraction going forward
- the importance of continuing to offer minimally invasive surgical approaches
- the need to educate surgeons about the safest approaches to tissue extraction.
Both surgeons believe that power morcellation should remain an option for selected cases, although neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be used whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?1
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. Our review is the most complete report to date, more comprehensive than the current reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL Task Force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. There are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
Related article: First large study on risk of cancer spread using power morcellation. Janelle Yates (News for your Practice; August 2014)
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction for individual patients undergoing uterine surgery.
AAGL recommendations on the use of power morcellationIn its position statement, the AAGL Tissue Extraction Task Force made the following main points, recommending that surgeons:
Further research also is needed to determine how best to diagnose sarcomas preoperatively, the task force noted. The full report is available on the AAGL Web site.1 —Ray A. Wertheim, MD |
How to manage tissue extraction going forward
OBG Management: Regardless of the FDA’s final decision, what should the gynecologic specialty be doing to avoid disseminating uterine tissue in the peritoneal cavity, particularly leiomyosarcoma?
Dr. Wertheim: MIS is a wonderful advancement in women’s health care. All surgical specialties are moving toward MIS. Our challenge is to perform it as safely as possible, given the data and instrumentation available.
In regard to leiomyosarcoma, because we lack the ability to accurately make the diagnosis preoperatively, we’ve identified risk factors that should be taken into consideration. They include advanced age, a history of radiation or tamoxifen use, black race, hereditary leiomyomatosis, renal cell carcinoma syndrome, and survival of childhood retinoblastoma.
At this time, we have specimen-retrieval bags that can be used with power morcellation. However, it takes skill to be able to place a large specimen inside a bag without injuring surrounding organs due to limited visibility.
Education, at the hospital and national level, is in the works
OBG Management: How should we go about educating surgeons about MIS alternatives to open power morcellation?
Dr. Wertheim: In my hospital, we are mentoring surgeons to help them gain the new skills needed. In addition, I plan to give a grand rounds presentation on tissue extraction for hospitals in northern Virginia and also would like to offer a course in the near future. I’m also hoping that we’ll be able to offer courses around the country before the annual AAGL meeting this November.
At the annual AAGL meeting, the subject will be discussed at length, with an emphasis on identifying risk factors and conducting appropriate preoperative testing, with workshops likely to teach the skills needed to perform these surgeries as safely as possible.
Why a return to reliance on laparotomy would be unwise
OBG Management: Given all the concerns expressed recently about open power morcellation, do you think some surgeons will revert to abdominal hysterectomy rather than rely on MIS? Would such a move be safer than power morcellation?
Dr. Wertheim: That would be a disaster for women. Very reliable data have shown that MIS is safer than open surgery, with much quicker recovery. Almost all of my patients are discharged within 3 hours after surgery, and most no longer require pain medications other than nonsteroidal anti-inflammatory drugs by postoperative day 2. They’re usually back to work within 2 weeks.
We have worked long and hard to develop skills and instrumentation required to perform MIS safely—but nothing replaces good judgment. In some cases, laparotomy or conversion to a laparotomy may be indicated.
New instrumentation is needed and is being developed. In the meantime, my personal bias is to rule out risk factors for malignancy and continue to morcellate with a scalpel, preferably inside a bag. After all, we know that with open power morcellation, fragments and cells are usually left behind regardless of inspection and irrigation. These fragments may cause leiomyomatosis, endometriosis, bowel obstruction, sepsis, and possible dissemination of tumor fragments. Moreover, morcellation into small fragments complicates the pathologist’s ability to give an accurate report. The use of open power morcellation also subjects the patient to a risk of damage to surrounding organs—usually due to the surgeon’s inexperience.
As I have said before, our challenge is to perform these surgeries using the safest techniques possible, given the current data and instrumentation.
OBG Management: Dr. Reich, you have a unique perspective on this issue, because you pioneered laparoscopic hysterectomy. How has uterine tissue extraction evolved since then? Do you think open power morcellation should remain an option?
Dr. Reich: Uterine tissue extraction has not evolved. The terms “laparoscopic hysterectomy” and “total laparoscopic hysterectomy” imply vaginal extraction using a scalpel, not abdominal extraction using a morcellator. Unfortunately there is no substitute for hard work using a #10 blade on a long handle and special vaginal retraction tools.
In 1983, I made a decision to stop performing laparotomy for all gynecologic procedures, including hysterectomy, myomectomy, urology, oncology, abscesses, extensive adhesions, and rectovaginal endometriosis. I was an accomplished vaginal surgeon at that time, as well as a one-handed laparoscopic surgeon, operating while looking through the scope with one eye.
Interest in a laparoscopic approach to hysterectomy began with my presentations about laparoscopic hysterectomy in January 1988. At that time I had over 10 years of experience doing what is now called laparoscopic-assisted vaginal hysterectomy.
I wrote extensively about specimen removal using a scalpel before electronic power morcellators were available. Since then, I have asked those using power morcellators to stop calling their operation a laparoscopic hysterectomy, as it has more in common with an abdominal-extraction hysterectomy.
I have never advocated removing the uterus using power morcellators, and I still believe that most specimens can be removed vaginally without the spray of pieces of the specimen around the peritoneal cavity that occurs with power morcellation. This goes for hysterectomy involving a large uterus, myomectomy through a culdotomy incision, and removal of the uterine fundus after supracervical hysterectomy. (It is irresponsible to use expensive power morcellation to remove small supracervical hysterectomy specimens.) It is time to get back to learning and teaching vaginal morcellation, although I readily admit it is time consuming.
Nevertheless, I believe power morcellation should remain an option. Recent laparoscopic fellowship trainees know only this technique, which is still better than a return to mutilation by laparotomy.
Gynecology is a frustrating profession—30 years of MIS as a sideshow. General surgery has rapidly adopted a laparoscopic approach to most operations, after gynecologists taught them. Today most gynecologists do not do advanced laparoscopic surgery and would love to get back to open incision laparotomy for their operations. We cannot go back.
OBG Management: Dr. Wertheim and Dr. Reich, do your personal views of the morcellation issue differ at all from the official views of professional societies?
Dr. Wertheim: Yes. However, before I share them, I’d like to emphasize that the views I’m about to express are mine and mine only, not those of the AAGL or its task force.
The issue of uterine extraction is a highly emotional and political issue, about which there are few good data.
Abundant Level 1 data strongly support a vaginal or laparoscopic approach for benign hysterectomy when possible. ACOG and AAGL have issued position papers supporting these approaches for benign hysterectomies. Gynecologic surgeons and other surgical specialists have embraced MIS because it is safer, offers faster recovery, produces less postoperative pain, and has fewer complications than open surgery. However, AAGL has maintained for several years that morcellation is contraindicated in cases where uterine malignancy is either known or suspected.
The dilemma with open power morcellation is that even with our best diagnostic tools, the rare uterine sarcoma cannot always be definitively ruled out preoperatively. Endometrial cancer usually can be diagnosed before surgery. However, rare subtypes such as sarcomas are more difficult to reliably diagnose preoperatively, and risk factors for uterine sarcomas are not nearly as well understood as those for endometrial cancer.
I do agree with the FDA’s cautionary statement on April 17, which pointedly prohibits power morcellation for women with suspected precancer or known cancer of the gynecologic organs.2 However, the AAGL Task Force critically reviewed about 120 articles, including the studies assessed by the FDA. Concerns arose regarding the FDA’s interpretation of the data. Due to a number of deficiencies in these studies, some of the conclusions of the FDA may not be completely accurate. The studies analyzed by the FDA were not stratified by risk factors for sarcoma and were not necessarily performed in a setting of reproductive-aged women with presumed fibroids.
Dr. Reich: Here are my personal views about the sarcoma problem and I am sure they differ from the official views:
- Laparoscopic hysterectomy should always mean vaginal extraction unless a less disfiguring site can be discovered; power morcellation implies minilaparotomy and should be renamed to reflect that fact.
- Power morcellation must be differentiated from vaginal and minilaparotomy scalpel morcellation, especially in the media. Vaginal hysterectomy has entailed vaginal scalpel morcellation with successful outcomes for more than 100 years.
- Remember that most gynecologic cancers are approached using the laparoscope today. This certainly includes cervical and endometrial cancer and some ovarian cancers. (For example, one of my neighbors is a 25-year survivor of laparoscopically treated bilateral ovarian cancer who refused laparotomy!)
- I have removed sarcomas by vaginal morcellation during laparoscopic hysterectomy and laparoscopic myomectomy with no late sequelae. In fact, most cervical cancer surgery is done by laparoscopic surgery today. And even an open laparotomy hysterectomy can spread a sarcoma.
- The current morcellation debate arose when a single case of disseminated leiomyosarcoma became highly publicized. It involved a prominent physician whose leiomyosarcoma was unknown to her initial surgeon, and the malignancy was upstaged after the use of power morcellation during hysterectomy. After this case was covered in the media, other cases began to be reported in the lay press as well, some of which predated the publicized case. The truth is, regrettably, that sarcomas carry poor prognoses even when specimens are removed intact. And we don’t know much about the sarcoma that started this debate. Was it mild or aggressive? How many mitotic figures were there per high-powered field? And what was found macroscopicallyand microscopically at the subsequent laparotomy? We on the AAGL Task Force do not know the answers to these questions, although at least some of these variables are reported in other published cases. And because this case is likely to have a powerful effect on MIS in our country and the rest of the world, it is my opinion that we need to know these details.
What is your preferred surgical approach?
OBG Management: Do you perform open power morcellation in selected patients?
Dr. Wertheim: Even though I have performed morcellation with a scalpel transvaginally or through a mini-laparotomy incision for many years, I have never used open power morcellation because of the risk of leaving behind benign or malignant tissue fragments. Morcellation with a scalpel is easily learned and can be performed as quickly as power morcellation. Morcellation with a scalpel produces much larger pieces than with power morcellation. This probably markedly decreases the loss of fragments. I cannot make a definitive statement regarding cell loss, however. Until we have improved instrumentation and are better able to make a preoperative diagnosis of sarcoma, I’m going to rule out risk factors identified by the AAGL Task Force, do the appropriate work-up, and continue to morcellate with a scalpel, placing the specimen in a bag, if technically possible.
Dr. Reich: As I mentioned, I am a vaginal scalpel morcellator. I tried power morcellation when it first was developed but was never a fan. The same techniques used for vaginal extraction using a coring maneuver can be used abdominally through the umbilicus or a 1- or 2-cm trocar site.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In July 2014, the FDA convened a two-day hearing of the Obstetrics and Gynecology Devices Panel (one of the panels in its Medical Devices Advisory Committee) to consider whether power morcellation should remain an option and, if so, what restrictions or labeling might be recommended.
In advance of the FDA hearing, OBG Management invited two experts in women’s health to explore the options more deeply and address the future of minimally invasive surgery (MIS): Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
- options for tissue extraction going forward
- the importance of continuing to offer minimally invasive surgical approaches
- the need to educate surgeons about the safest approaches to tissue extraction.
Both surgeons believe that power morcellation should remain an option for selected cases, although neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be used whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?1
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. Our review is the most complete report to date, more comprehensive than the current reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL Task Force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. There are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
Related article: First large study on risk of cancer spread using power morcellation. Janelle Yates (News for your Practice; August 2014)
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction for individual patients undergoing uterine surgery.
AAGL recommendations on the use of power morcellationIn its position statement, the AAGL Tissue Extraction Task Force made the following main points, recommending that surgeons:
Further research also is needed to determine how best to diagnose sarcomas preoperatively, the task force noted. The full report is available on the AAGL Web site.1 —Ray A. Wertheim, MD |
How to manage tissue extraction going forward
OBG Management: Regardless of the FDA’s final decision, what should the gynecologic specialty be doing to avoid disseminating uterine tissue in the peritoneal cavity, particularly leiomyosarcoma?
Dr. Wertheim: MIS is a wonderful advancement in women’s health care. All surgical specialties are moving toward MIS. Our challenge is to perform it as safely as possible, given the data and instrumentation available.
In regard to leiomyosarcoma, because we lack the ability to accurately make the diagnosis preoperatively, we’ve identified risk factors that should be taken into consideration. They include advanced age, a history of radiation or tamoxifen use, black race, hereditary leiomyomatosis, renal cell carcinoma syndrome, and survival of childhood retinoblastoma.
At this time, we have specimen-retrieval bags that can be used with power morcellation. However, it takes skill to be able to place a large specimen inside a bag without injuring surrounding organs due to limited visibility.
Education, at the hospital and national level, is in the works
OBG Management: How should we go about educating surgeons about MIS alternatives to open power morcellation?
Dr. Wertheim: In my hospital, we are mentoring surgeons to help them gain the new skills needed. In addition, I plan to give a grand rounds presentation on tissue extraction for hospitals in northern Virginia and also would like to offer a course in the near future. I’m also hoping that we’ll be able to offer courses around the country before the annual AAGL meeting this November.
At the annual AAGL meeting, the subject will be discussed at length, with an emphasis on identifying risk factors and conducting appropriate preoperative testing, with workshops likely to teach the skills needed to perform these surgeries as safely as possible.
Why a return to reliance on laparotomy would be unwise
OBG Management: Given all the concerns expressed recently about open power morcellation, do you think some surgeons will revert to abdominal hysterectomy rather than rely on MIS? Would such a move be safer than power morcellation?
Dr. Wertheim: That would be a disaster for women. Very reliable data have shown that MIS is safer than open surgery, with much quicker recovery. Almost all of my patients are discharged within 3 hours after surgery, and most no longer require pain medications other than nonsteroidal anti-inflammatory drugs by postoperative day 2. They’re usually back to work within 2 weeks.
We have worked long and hard to develop skills and instrumentation required to perform MIS safely—but nothing replaces good judgment. In some cases, laparotomy or conversion to a laparotomy may be indicated.
New instrumentation is needed and is being developed. In the meantime, my personal bias is to rule out risk factors for malignancy and continue to morcellate with a scalpel, preferably inside a bag. After all, we know that with open power morcellation, fragments and cells are usually left behind regardless of inspection and irrigation. These fragments may cause leiomyomatosis, endometriosis, bowel obstruction, sepsis, and possible dissemination of tumor fragments. Moreover, morcellation into small fragments complicates the pathologist’s ability to give an accurate report. The use of open power morcellation also subjects the patient to a risk of damage to surrounding organs—usually due to the surgeon’s inexperience.
As I have said before, our challenge is to perform these surgeries using the safest techniques possible, given the current data and instrumentation.
OBG Management: Dr. Reich, you have a unique perspective on this issue, because you pioneered laparoscopic hysterectomy. How has uterine tissue extraction evolved since then? Do you think open power morcellation should remain an option?
Dr. Reich: Uterine tissue extraction has not evolved. The terms “laparoscopic hysterectomy” and “total laparoscopic hysterectomy” imply vaginal extraction using a scalpel, not abdominal extraction using a morcellator. Unfortunately there is no substitute for hard work using a #10 blade on a long handle and special vaginal retraction tools.
In 1983, I made a decision to stop performing laparotomy for all gynecologic procedures, including hysterectomy, myomectomy, urology, oncology, abscesses, extensive adhesions, and rectovaginal endometriosis. I was an accomplished vaginal surgeon at that time, as well as a one-handed laparoscopic surgeon, operating while looking through the scope with one eye.
Interest in a laparoscopic approach to hysterectomy began with my presentations about laparoscopic hysterectomy in January 1988. At that time I had over 10 years of experience doing what is now called laparoscopic-assisted vaginal hysterectomy.
I wrote extensively about specimen removal using a scalpel before electronic power morcellators were available. Since then, I have asked those using power morcellators to stop calling their operation a laparoscopic hysterectomy, as it has more in common with an abdominal-extraction hysterectomy.
I have never advocated removing the uterus using power morcellators, and I still believe that most specimens can be removed vaginally without the spray of pieces of the specimen around the peritoneal cavity that occurs with power morcellation. This goes for hysterectomy involving a large uterus, myomectomy through a culdotomy incision, and removal of the uterine fundus after supracervical hysterectomy. (It is irresponsible to use expensive power morcellation to remove small supracervical hysterectomy specimens.) It is time to get back to learning and teaching vaginal morcellation, although I readily admit it is time consuming.
Nevertheless, I believe power morcellation should remain an option. Recent laparoscopic fellowship trainees know only this technique, which is still better than a return to mutilation by laparotomy.
Gynecology is a frustrating profession—30 years of MIS as a sideshow. General surgery has rapidly adopted a laparoscopic approach to most operations, after gynecologists taught them. Today most gynecologists do not do advanced laparoscopic surgery and would love to get back to open incision laparotomy for their operations. We cannot go back.
OBG Management: Dr. Wertheim and Dr. Reich, do your personal views of the morcellation issue differ at all from the official views of professional societies?
Dr. Wertheim: Yes. However, before I share them, I’d like to emphasize that the views I’m about to express are mine and mine only, not those of the AAGL or its task force.
The issue of uterine extraction is a highly emotional and political issue, about which there are few good data.
Abundant Level 1 data strongly support a vaginal or laparoscopic approach for benign hysterectomy when possible. ACOG and AAGL have issued position papers supporting these approaches for benign hysterectomies. Gynecologic surgeons and other surgical specialists have embraced MIS because it is safer, offers faster recovery, produces less postoperative pain, and has fewer complications than open surgery. However, AAGL has maintained for several years that morcellation is contraindicated in cases where uterine malignancy is either known or suspected.
The dilemma with open power morcellation is that even with our best diagnostic tools, the rare uterine sarcoma cannot always be definitively ruled out preoperatively. Endometrial cancer usually can be diagnosed before surgery. However, rare subtypes such as sarcomas are more difficult to reliably diagnose preoperatively, and risk factors for uterine sarcomas are not nearly as well understood as those for endometrial cancer.
I do agree with the FDA’s cautionary statement on April 17, which pointedly prohibits power morcellation for women with suspected precancer or known cancer of the gynecologic organs.2 However, the AAGL Task Force critically reviewed about 120 articles, including the studies assessed by the FDA. Concerns arose regarding the FDA’s interpretation of the data. Due to a number of deficiencies in these studies, some of the conclusions of the FDA may not be completely accurate. The studies analyzed by the FDA were not stratified by risk factors for sarcoma and were not necessarily performed in a setting of reproductive-aged women with presumed fibroids.
Dr. Reich: Here are my personal views about the sarcoma problem and I am sure they differ from the official views:
- Laparoscopic hysterectomy should always mean vaginal extraction unless a less disfiguring site can be discovered; power morcellation implies minilaparotomy and should be renamed to reflect that fact.
- Power morcellation must be differentiated from vaginal and minilaparotomy scalpel morcellation, especially in the media. Vaginal hysterectomy has entailed vaginal scalpel morcellation with successful outcomes for more than 100 years.
- Remember that most gynecologic cancers are approached using the laparoscope today. This certainly includes cervical and endometrial cancer and some ovarian cancers. (For example, one of my neighbors is a 25-year survivor of laparoscopically treated bilateral ovarian cancer who refused laparotomy!)
- I have removed sarcomas by vaginal morcellation during laparoscopic hysterectomy and laparoscopic myomectomy with no late sequelae. In fact, most cervical cancer surgery is done by laparoscopic surgery today. And even an open laparotomy hysterectomy can spread a sarcoma.
- The current morcellation debate arose when a single case of disseminated leiomyosarcoma became highly publicized. It involved a prominent physician whose leiomyosarcoma was unknown to her initial surgeon, and the malignancy was upstaged after the use of power morcellation during hysterectomy. After this case was covered in the media, other cases began to be reported in the lay press as well, some of which predated the publicized case. The truth is, regrettably, that sarcomas carry poor prognoses even when specimens are removed intact. And we don’t know much about the sarcoma that started this debate. Was it mild or aggressive? How many mitotic figures were there per high-powered field? And what was found macroscopicallyand microscopically at the subsequent laparotomy? We on the AAGL Task Force do not know the answers to these questions, although at least some of these variables are reported in other published cases. And because this case is likely to have a powerful effect on MIS in our country and the rest of the world, it is my opinion that we need to know these details.
What is your preferred surgical approach?
OBG Management: Do you perform open power morcellation in selected patients?
Dr. Wertheim: Even though I have performed morcellation with a scalpel transvaginally or through a mini-laparotomy incision for many years, I have never used open power morcellation because of the risk of leaving behind benign or malignant tissue fragments. Morcellation with a scalpel is easily learned and can be performed as quickly as power morcellation. Morcellation with a scalpel produces much larger pieces than with power morcellation. This probably markedly decreases the loss of fragments. I cannot make a definitive statement regarding cell loss, however. Until we have improved instrumentation and are better able to make a preoperative diagnosis of sarcoma, I’m going to rule out risk factors identified by the AAGL Task Force, do the appropriate work-up, and continue to morcellate with a scalpel, placing the specimen in a bag, if technically possible.
Dr. Reich: As I mentioned, I am a vaginal scalpel morcellator. I tried power morcellation when it first was developed but was never a fan. The same techniques used for vaginal extraction using a coring maneuver can be used abdominally through the umbilicus or a 1- or 2-cm trocar site.
WE WANT TO HEAR FROM YOU! Share your thoughts on this article. Send your Letter to the Editor to: [email protected]
1. The Tissue Extraction Task Force, AAGL. AAGL Position Statement: Morcellation during uterine tissue extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed June 13, 2014.
2. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy. FDA Safety Communication. http://www.fda.gov
/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed June 13, 2014.
1. The Tissue Extraction Task Force, AAGL. AAGL Position Statement: Morcellation during uterine tissue extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed June 13, 2014.
2. US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy. FDA Safety Communication. http://www.fda.gov
/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed June 13, 2014.
Visit the Morcellation Topic Collection Page for additional articles, videos, and audiocasts.
Will open power morcellation of uterine tissue remain an option during hysterectomy and myomectomy?
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In February 2014, Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s Hospital, wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
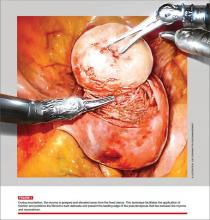
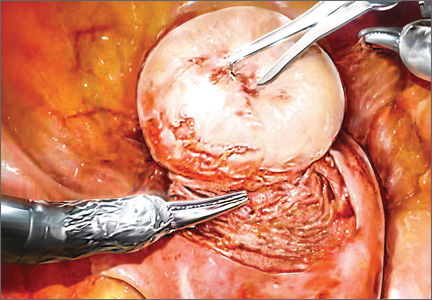
“When used to treat tumors presumed to be fibroids, open power morcellation is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
Not surprisingly, the numerous statements and warnings since then have led to some confusion in the specialty about the safest course of action for tissue extraction during hysterectomy and myomectomy in women with a large uterus.
To explore the options more deeply and address the future of minimally invasive surgery (MIS) in women’s health, OBG Management invited two experts to comment: Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
- options for tissue extraction going forward
- the importance of continuing to offer minimally invasive surgical approaches to patients
- the need to educate surgeons about the safest approaches to tissue extraction.
Both surgeons believe that power morcellation should remain an option for selected cases, though neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be utilized whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?2 How does it compare with the ACOG and FDA statements on the use of power morcellation?
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. I’ve been practicing for almost 40 years in academic and private settings, and I found this group to be the brightest, most caring and compassionate group with whom I’ve ever worked. Our review is the most complete report to date, more comprehensive than the reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL task force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. One of the problems we encountered was that there are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction in individual patients undergoing uterine surgery.
How to manage tissue extraction going forward
OBG Management: The FDA will convene another meeting on power morcellation July 10 and 11. Regardless of its final decision, what should the gynecologic specialty be doing to avoid disseminating uterine tissue in the peritoneal cavity, particularly leiomyosarcoma?
Dr. Wertheim: Yes, AAGL will be at the FDA’s July hearing because we are the experts. MIS is a wonderful advancement in women’s health care. All surgical specialties are moving toward MIS. Our challenge is to perform it as safely as possible, given the current data and instrumentation available.
In regard to leiomyosarcoma, because we lack the ability to accurately make the diagnosis preoperatively, we’ve identified risk factors that should be taken into consideration. Risk factors include advanced age, history of radiation or tamoxifen use, black race, hereditary leiomyomatosis, renal cell carcinoma syndrome, and survival of childhood retinoblastoma.
At this time, we have specimen-retrieval bags that can be used with power morcellation. However, it takes skill to be able to place a large specimen inside a bag without injuring surrounding organs due to limited visibility.
OBG Management: How should we go about educating surgeons about MIS alternatives to open power morcellation?
Education, at the hospital and national level, is in the works
Dr. Wertheim: In my hospital, we are mentoring surgeons to help them gain the new skills needed. In addition, Dr. Reich and I, along with Albert Steren, MD, a minimally invasive surgeon from Rockville, Maryland, are hosting an educational dinner meeting on tissue extraction on July 24 in northern Virginia. I plan to give a grand rounds presentation on tissue extraction for hospitals in northern Virginia and also would like to offer a course in the near future. I’m also hoping that we’ll be able to offer courses around the country before the annual AAGL meeting this November, since this is such a pressing issue.
At the annual AAGL meeting, the subject will be discussed at length, with an emphasis on identifying risk factors and conducting appropriate preoperative testing, with workshops likely to teach the skills needed to perform these surgeries as safely as possible.
Why a return to reliance on laparotomy would be unwise
OBG Management: Given all the concerns expressed recently about open power morcellation, do you think some surgeons will revert to abdominal hysterectomy rather than rely on MIS? Would such a move be safer than power morcellation?
Dr. Wertheim: That would be a disaster for women. Very reliable data have shown that MIS is safer than open surgery, with much quicker recovery. Almost all of my patients are discharged within 3 hours after surgery, and most no longer require pain medications other than nonsteroidal anti-inflammatory drugs (NSAIDs) by postoperative day 2. They’re usually back to work within 2 weeks.
We have worked long and hard to develop skills and instrumentation required to perform MIS safely—but nothing replaces good judgment. In some cases, laparotomy or conversion to a laparotomy may be indicated.
New instrumentation is needed and is being developed. In the meantime, my personal bias is to rule out risk factors for malignancy and continue to morcellate with a scalpel, preferably inside a bag. After all, we know that with open power morcellation, fragments and cells are usually left behind regardless of inspection and irrigation. These fragments may cause leiomyomatosis, endometriosis, bowel obstruction, sepsis, and possible dissemination of tumor fragments. Moreover, morcellation into small fragments complicates the pathologist’s ability to give an accurate report. The use of open power morcellation also subjects the patient to a risk of damage to surrounding organs—usually due to the surgeon’s inexperience.
As I have said before, our challenge is to perform these surgeries using the safest techniques possible, given the current data and instrumentation.
OBG Management: Dr. Reich, you have a unique perspective on this issue, since you pioneered laparoscopic hysterectomy. How has uterine tissue extraction evolved since then? Do you think open power morcellation should remain an option?
Dr. Reich: Uterine tissue extraction has not evolved. The terms “laparoscopic hysterectomy” and “total laparoscopic hysterectomy” imply vaginal extraction using a scalpel, not abdominal extraction using a morcellator. Unfortunately there is no substitute for hard work using a #10 blade on a long handle and special vaginal retraction tools.
In 1983, I made a decision to stop performing laparotomy for all gynecologic procedures, including hysterectomy, myomectomy, urology, oncology, abscesses, extensive adhesions, and rectovaginal endometriosis. I was an accomplished vaginal surgeon at that time, as well as a one-handed laparoscopic surgeon, operating while looking through the scope with one eye.
Interest in a laparoscopic approach to hysterectomy began with my presentations about laparoscopic hysterectomy in January 1988. At that time I had over 10 years of experience doing what is now called laparoscopic-assisted vaginal hysterectomy.
I wrote extensively about specimen removal using a scalpel before electronic power morcellators were available. Since then, I have asked those using power morcellators to stop calling their operation a laparoscopic hysterectomy, as it has more in common with an abdominal-extraction hysterectomy.
I have never advocated removing the uterus using power morcellators, and I still believe that most specimens can be removed vaginally without the spray of pieces of the specimen around the peritoneal cavity that occurs with power morcellation. This goes for hysterectomy involving a large uterus, myomectomy through a culdotomy incision, and removal of the uterine fundus after supracervical hysterectomy. (It is irresponsible to use expensive power morcellation to remove small supracervical hysterectomy specimens.) It is time to get back to learning and teaching vaginal morcellation, although I readily admit it is time consuming.
Nevertheless, I believe power morcellation should remain an option. Recent laparoscopic fellowship trainees know only this technique, which is still better than a return to mutilation by laparotomy.
Gynecology is a frustrating profession—30 years of MIS as a sideshow. General surgery has rapidly adopted a laparoscopic approach to most operations, after gynecologists taught them. Today the majority of gynecologists do not do advanced laparoscopic surgery and would love to get back to open incision laparotomy for their operations. We cannot go back.
OBG Management: Dr. Wertheim and Dr. Reich, do your personal views of the morcellation issue differ at all from the official views of professional societies?
Dr. Wertheim: Yes. However, before I share them, I’d like to emphasize that the views I’m about to express are mine and mine only, not those of the AAGL or its task force.
The issue of uterine extraction is a highly emotional and political issue, about which there are few good data.
Abundant Level 1 data strongly support a vaginal or laparoscopic approach for benign hysterectomy when possible. ACOG and AAGL have issued position papers supporting these approaches for benign hysterectomies. Gynecologic surgeons and other surgical specialists have embraced MIS because it is safer, offers faster recovery, produces less postoperative pain, and has fewer complications than open surgery. However, AAGL has maintained for several years that morcellation is contraindicated in cases where uterine malignancy is either known or suspected.
The dilemma with open power morcellation is that even with our best diagnostic tools, the rare uterine sarcoma cannot always be definitively ruled out preoperatively. Endometrial cancer usually can be diagnosed before surgery. However, rare subtypes such as sarcomas are more difficult to reliably diagnose preoperatively, and risk factors for uterine sarcomas are not nearly as well understood as those for endometrial cancer.
I do agree with the FDA’s cautionary statement, which pointedly prohibits power morcellation for women with suspected precancer or known cancer of the gynecologic organs.3 However, the AAGL task force critically reviewed about 120 articles, including the studies assessed by the FDA. Concerns arose regarding the FDA’s interpretation of the data. Due to a number of deficiencies in these studies, some of the conclusions of the FDA may not be completely accurate. The studies analyzed by the FDA were not stratified by risk factors for sarcoma and were not necessarily performed in a setting of reproductive-aged women with presumed fibroids.
Dr. Reich: Here are my personal views about the sarcoma problem and I am sure they differ from the official views of the professional societies:
- Laparoscopic hysterectomy should always mean vaginal extraction unless a less disfiguring site can be discovered; power morcellation implies minilaparotomy and should be renamed to reflect that fact.
- Power morcellation must be differentiated from vaginal and minilaparotomy scalpel morcellation, especially in the media. Vaginal hysterectomy has entailed vaginal scalpel morcellation with successful outcomes for more than 100 years.
- Remember that most gynecologic cancers are approached using the laparoscope today. This certainly includes cervical and endometrial cancer and some ovarian cancers. (For example, one of my neighbors is a 25-year survivor of laparoscopically treated bilateral ovarian cancer who refused laparotomy!)
- I have removed sarcomas by vaginal morcellation during laparoscopic hysterectomy and laparoscopic myomectomy with no late sequelae. In fact, most cervical cancer surgery is done by laparoscopic surgery today. And even an open laparotomy hysterectomy can spread a sarcoma.
- The current morcellation debate arose when a single case of disseminated leiomyosarcoma became highly publicized. It involved a prominent physician whose leiomyosarcoma was unknown to her initial surgeon, and the malignancy was upstaged after the use of power morcellation during hysterectomy. After this case was covered in the media, other cases began to be reported in the lay press as well, some of which predated the publicized case. The truth is, regrettably, that sarcomas carry poor prognoses even when specimens are removed intact. And we don’t know much about the sarcoma that started this debate. Was it mild or aggressive? How many mitotic figures were there per high-powered field? And what was found macroscopically and microscopically at the subsequent laparotomy? We on the AAGL task force do not know the answers to these questions, although at least some of these variables are reported in other published cases. And because this case is likely to have a powerful effect on MIS in our country and the rest of the world, it is my opinion that we need to know these details.
What is your preferred surgical approach?
OBG Management: Do you perform open power morcellation in selected patients?
Dr. Wertheim: Even though I have performed morcellation with a scalpel transvaginally or through a mini-laparotomy incision for many years, I have never used open power morcellation because of the risk of leaving behind benign or malignant tissue fragments. Morcellation with a scalpel is easily learned and can be performed as quickly as power morcellation. Morcellation with a scalpel produces much larger pieces than with power morcellation. This probably markedly decreases the loss of fragments. I cannot make a definitive statement regarding cell loss, however. Until we have improved instrumentation and are better able to make a preoperative diagnosis of sarcoma, I’m going to rule out risk factors identified by the AAGL task force, do the appropriate work-up, and continue to morcellate with a scalpel, placing the specimen in a bag, if technically possible.
Dr. Reich: As I mentioned, I am a vaginal scalpel morcellator. I tried power morcellation when it first was developed but was never a fan. The same techniques used for vaginal extraction using a coring maneuver can be used abdominally through the umbilicus or a 1- or 2-cm trocar site.
What should the FDA’s next move be?
OBG Management: Care to make any predictions about the FDA’s final decision?
Dr. Wertheim: This has become a highly emotional and controversial issue with little good existing data. During the preoperative visit, this issue should be discussed with the patient using clear, lay-friendly language. Having said that, I also do not believe we should hide behind informed consent. The FDA has a responsibility to keep the public safe. If open power morcellation is allowed to continue, there will be another morcellated sarcoma or complications from retained benign tissue fragments. I doubt the FDA can live with this. I believe the risk factors identified by the AAGL task force should be ruled out, the appropriate workup done and then, if power morcellation is performed, it should be done inside a bag. In addition, I think the FDA should require that complications be reported and recorded in a registry.
Dr. Reich: I disagree. The FDA has to back off. It’s important to note that this is an American problem, as the rest of the world cannot afford power morcellators. The data are not in yet. The decision about what kind of hysterectomy is performed will be made by the “informed” patient, who undoubtedly will be very afraid to have MIS because of the surrounding negative publicity. We must do a better job of promoting the advantages of a minimally invasive approach.
OBG Management: Thank you both for your time and expertise.
Dr. Wertheim: Thank you for giving us the opportunity to express our opinions regarding this highly emotional and controversial issue.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Share your thoughts by sending a letter to [email protected]. Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
1. Barbieri RL. Benefits and pitfalls of open power morcellation of uterine fibroids. OBG Manag. 2014;26(2):10–15.
2. The Tissue Extraction Task Force, AAGL. AAGL Position Statement: Morcellation During Uterine Tissue Extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed June 13, 014.
3. US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy. FDA Safety Communication. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed June 13, 2014.
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In February 2014, Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s Hospital, wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
Not surprisingly, the numerous statements and warnings since then have led to some confusion in the specialty about the safest course of action for tissue extraction during hysterectomy and myomectomy in women with a large uterus.
To explore the options more deeply and address the future of minimally invasive surgery (MIS) in women’s health, OBG Management invited two experts to comment: Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
- options for tissue extraction going forward
- the importance of continuing to offer minimally invasive surgical approaches to patients
- the need to educate surgeons about the safest approaches to tissue extraction.
Both surgeons believe that power morcellation should remain an option for selected cases, though neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be utilized whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?2 How does it compare with the ACOG and FDA statements on the use of power morcellation?
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. I’ve been practicing for almost 40 years in academic and private settings, and I found this group to be the brightest, most caring and compassionate group with whom I’ve ever worked. Our review is the most complete report to date, more comprehensive than the reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL task force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. One of the problems we encountered was that there are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction in individual patients undergoing uterine surgery.
How to manage tissue extraction going forward
OBG Management: The FDA will convene another meeting on power morcellation July 10 and 11. Regardless of its final decision, what should the gynecologic specialty be doing to avoid disseminating uterine tissue in the peritoneal cavity, particularly leiomyosarcoma?
Dr. Wertheim: Yes, AAGL will be at the FDA’s July hearing because we are the experts. MIS is a wonderful advancement in women’s health care. All surgical specialties are moving toward MIS. Our challenge is to perform it as safely as possible, given the current data and instrumentation available.
In regard to leiomyosarcoma, because we lack the ability to accurately make the diagnosis preoperatively, we’ve identified risk factors that should be taken into consideration. Risk factors include advanced age, history of radiation or tamoxifen use, black race, hereditary leiomyomatosis, renal cell carcinoma syndrome, and survival of childhood retinoblastoma.
At this time, we have specimen-retrieval bags that can be used with power morcellation. However, it takes skill to be able to place a large specimen inside a bag without injuring surrounding organs due to limited visibility.
OBG Management: How should we go about educating surgeons about MIS alternatives to open power morcellation?
Education, at the hospital and national level, is in the works
Dr. Wertheim: In my hospital, we are mentoring surgeons to help them gain the new skills needed. In addition, Dr. Reich and I, along with Albert Steren, MD, a minimally invasive surgeon from Rockville, Maryland, are hosting an educational dinner meeting on tissue extraction on July 24 in northern Virginia. I plan to give a grand rounds presentation on tissue extraction for hospitals in northern Virginia and also would like to offer a course in the near future. I’m also hoping that we’ll be able to offer courses around the country before the annual AAGL meeting this November, since this is such a pressing issue.
At the annual AAGL meeting, the subject will be discussed at length, with an emphasis on identifying risk factors and conducting appropriate preoperative testing, with workshops likely to teach the skills needed to perform these surgeries as safely as possible.
Why a return to reliance on laparotomy would be unwise
OBG Management: Given all the concerns expressed recently about open power morcellation, do you think some surgeons will revert to abdominal hysterectomy rather than rely on MIS? Would such a move be safer than power morcellation?
Dr. Wertheim: That would be a disaster for women. Very reliable data have shown that MIS is safer than open surgery, with much quicker recovery. Almost all of my patients are discharged within 3 hours after surgery, and most no longer require pain medications other than nonsteroidal anti-inflammatory drugs (NSAIDs) by postoperative day 2. They’re usually back to work within 2 weeks.
We have worked long and hard to develop skills and instrumentation required to perform MIS safely—but nothing replaces good judgment. In some cases, laparotomy or conversion to a laparotomy may be indicated.
New instrumentation is needed and is being developed. In the meantime, my personal bias is to rule out risk factors for malignancy and continue to morcellate with a scalpel, preferably inside a bag. After all, we know that with open power morcellation, fragments and cells are usually left behind regardless of inspection and irrigation. These fragments may cause leiomyomatosis, endometriosis, bowel obstruction, sepsis, and possible dissemination of tumor fragments. Moreover, morcellation into small fragments complicates the pathologist’s ability to give an accurate report. The use of open power morcellation also subjects the patient to a risk of damage to surrounding organs—usually due to the surgeon’s inexperience.
As I have said before, our challenge is to perform these surgeries using the safest techniques possible, given the current data and instrumentation.
OBG Management: Dr. Reich, you have a unique perspective on this issue, since you pioneered laparoscopic hysterectomy. How has uterine tissue extraction evolved since then? Do you think open power morcellation should remain an option?
Dr. Reich: Uterine tissue extraction has not evolved. The terms “laparoscopic hysterectomy” and “total laparoscopic hysterectomy” imply vaginal extraction using a scalpel, not abdominal extraction using a morcellator. Unfortunately there is no substitute for hard work using a #10 blade on a long handle and special vaginal retraction tools.
In 1983, I made a decision to stop performing laparotomy for all gynecologic procedures, including hysterectomy, myomectomy, urology, oncology, abscesses, extensive adhesions, and rectovaginal endometriosis. I was an accomplished vaginal surgeon at that time, as well as a one-handed laparoscopic surgeon, operating while looking through the scope with one eye.
Interest in a laparoscopic approach to hysterectomy began with my presentations about laparoscopic hysterectomy in January 1988. At that time I had over 10 years of experience doing what is now called laparoscopic-assisted vaginal hysterectomy.
I wrote extensively about specimen removal using a scalpel before electronic power morcellators were available. Since then, I have asked those using power morcellators to stop calling their operation a laparoscopic hysterectomy, as it has more in common with an abdominal-extraction hysterectomy.
I have never advocated removing the uterus using power morcellators, and I still believe that most specimens can be removed vaginally without the spray of pieces of the specimen around the peritoneal cavity that occurs with power morcellation. This goes for hysterectomy involving a large uterus, myomectomy through a culdotomy incision, and removal of the uterine fundus after supracervical hysterectomy. (It is irresponsible to use expensive power morcellation to remove small supracervical hysterectomy specimens.) It is time to get back to learning and teaching vaginal morcellation, although I readily admit it is time consuming.
Nevertheless, I believe power morcellation should remain an option. Recent laparoscopic fellowship trainees know only this technique, which is still better than a return to mutilation by laparotomy.
Gynecology is a frustrating profession—30 years of MIS as a sideshow. General surgery has rapidly adopted a laparoscopic approach to most operations, after gynecologists taught them. Today the majority of gynecologists do not do advanced laparoscopic surgery and would love to get back to open incision laparotomy for their operations. We cannot go back.
OBG Management: Dr. Wertheim and Dr. Reich, do your personal views of the morcellation issue differ at all from the official views of professional societies?
Dr. Wertheim: Yes. However, before I share them, I’d like to emphasize that the views I’m about to express are mine and mine only, not those of the AAGL or its task force.
The issue of uterine extraction is a highly emotional and political issue, about which there are few good data.
Abundant Level 1 data strongly support a vaginal or laparoscopic approach for benign hysterectomy when possible. ACOG and AAGL have issued position papers supporting these approaches for benign hysterectomies. Gynecologic surgeons and other surgical specialists have embraced MIS because it is safer, offers faster recovery, produces less postoperative pain, and has fewer complications than open surgery. However, AAGL has maintained for several years that morcellation is contraindicated in cases where uterine malignancy is either known or suspected.
The dilemma with open power morcellation is that even with our best diagnostic tools, the rare uterine sarcoma cannot always be definitively ruled out preoperatively. Endometrial cancer usually can be diagnosed before surgery. However, rare subtypes such as sarcomas are more difficult to reliably diagnose preoperatively, and risk factors for uterine sarcomas are not nearly as well understood as those for endometrial cancer.
I do agree with the FDA’s cautionary statement, which pointedly prohibits power morcellation for women with suspected precancer or known cancer of the gynecologic organs.3 However, the AAGL task force critically reviewed about 120 articles, including the studies assessed by the FDA. Concerns arose regarding the FDA’s interpretation of the data. Due to a number of deficiencies in these studies, some of the conclusions of the FDA may not be completely accurate. The studies analyzed by the FDA were not stratified by risk factors for sarcoma and were not necessarily performed in a setting of reproductive-aged women with presumed fibroids.
Dr. Reich: Here are my personal views about the sarcoma problem and I am sure they differ from the official views of the professional societies:
- Laparoscopic hysterectomy should always mean vaginal extraction unless a less disfiguring site can be discovered; power morcellation implies minilaparotomy and should be renamed to reflect that fact.
- Power morcellation must be differentiated from vaginal and minilaparotomy scalpel morcellation, especially in the media. Vaginal hysterectomy has entailed vaginal scalpel morcellation with successful outcomes for more than 100 years.
- Remember that most gynecologic cancers are approached using the laparoscope today. This certainly includes cervical and endometrial cancer and some ovarian cancers. (For example, one of my neighbors is a 25-year survivor of laparoscopically treated bilateral ovarian cancer who refused laparotomy!)
- I have removed sarcomas by vaginal morcellation during laparoscopic hysterectomy and laparoscopic myomectomy with no late sequelae. In fact, most cervical cancer surgery is done by laparoscopic surgery today. And even an open laparotomy hysterectomy can spread a sarcoma.
- The current morcellation debate arose when a single case of disseminated leiomyosarcoma became highly publicized. It involved a prominent physician whose leiomyosarcoma was unknown to her initial surgeon, and the malignancy was upstaged after the use of power morcellation during hysterectomy. After this case was covered in the media, other cases began to be reported in the lay press as well, some of which predated the publicized case. The truth is, regrettably, that sarcomas carry poor prognoses even when specimens are removed intact. And we don’t know much about the sarcoma that started this debate. Was it mild or aggressive? How many mitotic figures were there per high-powered field? And what was found macroscopically and microscopically at the subsequent laparotomy? We on the AAGL task force do not know the answers to these questions, although at least some of these variables are reported in other published cases. And because this case is likely to have a powerful effect on MIS in our country and the rest of the world, it is my opinion that we need to know these details.
What is your preferred surgical approach?
OBG Management: Do you perform open power morcellation in selected patients?
Dr. Wertheim: Even though I have performed morcellation with a scalpel transvaginally or through a mini-laparotomy incision for many years, I have never used open power morcellation because of the risk of leaving behind benign or malignant tissue fragments. Morcellation with a scalpel is easily learned and can be performed as quickly as power morcellation. Morcellation with a scalpel produces much larger pieces than with power morcellation. This probably markedly decreases the loss of fragments. I cannot make a definitive statement regarding cell loss, however. Until we have improved instrumentation and are better able to make a preoperative diagnosis of sarcoma, I’m going to rule out risk factors identified by the AAGL task force, do the appropriate work-up, and continue to morcellate with a scalpel, placing the specimen in a bag, if technically possible.
Dr. Reich: As I mentioned, I am a vaginal scalpel morcellator. I tried power morcellation when it first was developed but was never a fan. The same techniques used for vaginal extraction using a coring maneuver can be used abdominally through the umbilicus or a 1- or 2-cm trocar site.
What should the FDA’s next move be?
OBG Management: Care to make any predictions about the FDA’s final decision?
Dr. Wertheim: This has become a highly emotional and controversial issue with little good existing data. During the preoperative visit, this issue should be discussed with the patient using clear, lay-friendly language. Having said that, I also do not believe we should hide behind informed consent. The FDA has a responsibility to keep the public safe. If open power morcellation is allowed to continue, there will be another morcellated sarcoma or complications from retained benign tissue fragments. I doubt the FDA can live with this. I believe the risk factors identified by the AAGL task force should be ruled out, the appropriate workup done and then, if power morcellation is performed, it should be done inside a bag. In addition, I think the FDA should require that complications be reported and recorded in a registry.
Dr. Reich: I disagree. The FDA has to back off. It’s important to note that this is an American problem, as the rest of the world cannot afford power morcellators. The data are not in yet. The decision about what kind of hysterectomy is performed will be made by the “informed” patient, who undoubtedly will be very afraid to have MIS because of the surrounding negative publicity. We must do a better job of promoting the advantages of a minimally invasive approach.
OBG Management: Thank you both for your time and expertise.
Dr. Wertheim: Thank you for giving us the opportunity to express our opinions regarding this highly emotional and controversial issue.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Share your thoughts by sending a letter to [email protected]. Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy has been in the limelight in 2014—particularly morcellation performed in an “open” fashion (without use of a protective bag). Concerns about the dispersion of tissue throughout the peritoneal cavity—including the risk of disseminating tissue from leiomyosarcoma, a rare but deadly cancer—have drawn statements from the American College of Obstetricians and Gynecologists (ACOG), the AAGL, the US Food and Drug Administration (FDA), and others, cautioning against the use of open power morcellation in women with a known or suspected malignancy.
In February 2014, Robert L. Barbieri, MD, chair of obstetrics and gynecology at Brigham and Women’s Hospital, wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
Not surprisingly, the numerous statements and warnings since then have led to some confusion in the specialty about the safest course of action for tissue extraction during hysterectomy and myomectomy in women with a large uterus.
To explore the options more deeply and address the future of minimally invasive surgery (MIS) in women’s health, OBG Management invited two experts to comment: Ray A. Wertheim, MD, Director of the AAGL Center of Excellence Minimally Invasive Gynecology Program at Inova Fair Oaks Hospital in Fairfax, Virginia, and Harry Reich, MD, widely known as the first surgeon to perform laparoscopic hysterectomy, among other achievements. Both Dr. Wertheim and Dr. Reich were members of the AAGL Tissue Extraction Task Force.
In this Q&A, Dr. Wertheim and Dr. Reich discuss:
- options for tissue extraction going forward
- the importance of continuing to offer minimally invasive surgical approaches to patients
- the need to educate surgeons about the safest approaches to tissue extraction.
Both surgeons believe that power morcellation should remain an option for selected cases, though neither performs the technique himself. Both surgeons also believe that minimally invasive approaches to hysterectomy and myomectomy are here to stay and should continue to be utilized whenever possible.
AAGL convened an impartial expert panel
OBG Management: Dr. Wertheim, could you tell us a little about the AAGL position statement on the use of power morcellation for uterine tissue extraction at hysterectomy or myomectomy, since you were on the task force that researched and wrote it?2 How does it compare with the ACOG and FDA statements on the use of power morcellation?
Dr. Wertheim: AAGL convened its task force to conduct a critical appraisal of the existing evidence related to the practice of uterine extraction in the setting of hysterectomy and myomectomy. Areas in need of further investigation also were identified.
The task force consisted of experts who had no conflicts, were not allowed to discuss or review findings with anyone, and were not reimbursed for their time. I’ve been practicing for almost 40 years in academic and private settings, and I found this group to be the brightest, most caring and compassionate group with whom I’ve ever worked. Our review is the most complete report to date, more comprehensive than the reports from the FDA, ACOG, the Society of Gynecologic Oncology (SGO), and the American Urogynecologic Society (AUGS).
Interestingly, AAGL, ACOG, SGO, and AUGS all reached the same conclusion: All existing methods of tissue extraction have benefits and risks that must be balanced.
OBG Management: How did the AAGL task force assess the evidence?
Dr. Wertheim: The quality of evidence and strength of recommendations were assessed using US Preventive Services Task Force guidelines. One of the problems we encountered was that there are very few good data on the issue of power morcellation for uterine tissue extraction, especially in regard to leiomyosarcoma. One needs to be careful making recommendations without good data.
At this time, we do not believe there is a single method of tissue extraction that can protect all patients. Therefore, all current methods should remain available. We believe that an understanding of the issues will allow surgeons, hospitals, and patients to make the appropriate informed choices regarding tissue extraction in individual patients undergoing uterine surgery.
How to manage tissue extraction going forward
OBG Management: The FDA will convene another meeting on power morcellation July 10 and 11. Regardless of its final decision, what should the gynecologic specialty be doing to avoid disseminating uterine tissue in the peritoneal cavity, particularly leiomyosarcoma?
Dr. Wertheim: Yes, AAGL will be at the FDA’s July hearing because we are the experts. MIS is a wonderful advancement in women’s health care. All surgical specialties are moving toward MIS. Our challenge is to perform it as safely as possible, given the current data and instrumentation available.
In regard to leiomyosarcoma, because we lack the ability to accurately make the diagnosis preoperatively, we’ve identified risk factors that should be taken into consideration. Risk factors include advanced age, history of radiation or tamoxifen use, black race, hereditary leiomyomatosis, renal cell carcinoma syndrome, and survival of childhood retinoblastoma.
At this time, we have specimen-retrieval bags that can be used with power morcellation. However, it takes skill to be able to place a large specimen inside a bag without injuring surrounding organs due to limited visibility.
OBG Management: How should we go about educating surgeons about MIS alternatives to open power morcellation?
Education, at the hospital and national level, is in the works
Dr. Wertheim: In my hospital, we are mentoring surgeons to help them gain the new skills needed. In addition, Dr. Reich and I, along with Albert Steren, MD, a minimally invasive surgeon from Rockville, Maryland, are hosting an educational dinner meeting on tissue extraction on July 24 in northern Virginia. I plan to give a grand rounds presentation on tissue extraction for hospitals in northern Virginia and also would like to offer a course in the near future. I’m also hoping that we’ll be able to offer courses around the country before the annual AAGL meeting this November, since this is such a pressing issue.
At the annual AAGL meeting, the subject will be discussed at length, with an emphasis on identifying risk factors and conducting appropriate preoperative testing, with workshops likely to teach the skills needed to perform these surgeries as safely as possible.
Why a return to reliance on laparotomy would be unwise
OBG Management: Given all the concerns expressed recently about open power morcellation, do you think some surgeons will revert to abdominal hysterectomy rather than rely on MIS? Would such a move be safer than power morcellation?
Dr. Wertheim: That would be a disaster for women. Very reliable data have shown that MIS is safer than open surgery, with much quicker recovery. Almost all of my patients are discharged within 3 hours after surgery, and most no longer require pain medications other than nonsteroidal anti-inflammatory drugs (NSAIDs) by postoperative day 2. They’re usually back to work within 2 weeks.
We have worked long and hard to develop skills and instrumentation required to perform MIS safely—but nothing replaces good judgment. In some cases, laparotomy or conversion to a laparotomy may be indicated.
New instrumentation is needed and is being developed. In the meantime, my personal bias is to rule out risk factors for malignancy and continue to morcellate with a scalpel, preferably inside a bag. After all, we know that with open power morcellation, fragments and cells are usually left behind regardless of inspection and irrigation. These fragments may cause leiomyomatosis, endometriosis, bowel obstruction, sepsis, and possible dissemination of tumor fragments. Moreover, morcellation into small fragments complicates the pathologist’s ability to give an accurate report. The use of open power morcellation also subjects the patient to a risk of damage to surrounding organs—usually due to the surgeon’s inexperience.
As I have said before, our challenge is to perform these surgeries using the safest techniques possible, given the current data and instrumentation.
OBG Management: Dr. Reich, you have a unique perspective on this issue, since you pioneered laparoscopic hysterectomy. How has uterine tissue extraction evolved since then? Do you think open power morcellation should remain an option?
Dr. Reich: Uterine tissue extraction has not evolved. The terms “laparoscopic hysterectomy” and “total laparoscopic hysterectomy” imply vaginal extraction using a scalpel, not abdominal extraction using a morcellator. Unfortunately there is no substitute for hard work using a #10 blade on a long handle and special vaginal retraction tools.
In 1983, I made a decision to stop performing laparotomy for all gynecologic procedures, including hysterectomy, myomectomy, urology, oncology, abscesses, extensive adhesions, and rectovaginal endometriosis. I was an accomplished vaginal surgeon at that time, as well as a one-handed laparoscopic surgeon, operating while looking through the scope with one eye.
Interest in a laparoscopic approach to hysterectomy began with my presentations about laparoscopic hysterectomy in January 1988. At that time I had over 10 years of experience doing what is now called laparoscopic-assisted vaginal hysterectomy.
I wrote extensively about specimen removal using a scalpel before electronic power morcellators were available. Since then, I have asked those using power morcellators to stop calling their operation a laparoscopic hysterectomy, as it has more in common with an abdominal-extraction hysterectomy.
I have never advocated removing the uterus using power morcellators, and I still believe that most specimens can be removed vaginally without the spray of pieces of the specimen around the peritoneal cavity that occurs with power morcellation. This goes for hysterectomy involving a large uterus, myomectomy through a culdotomy incision, and removal of the uterine fundus after supracervical hysterectomy. (It is irresponsible to use expensive power morcellation to remove small supracervical hysterectomy specimens.) It is time to get back to learning and teaching vaginal morcellation, although I readily admit it is time consuming.
Nevertheless, I believe power morcellation should remain an option. Recent laparoscopic fellowship trainees know only this technique, which is still better than a return to mutilation by laparotomy.
Gynecology is a frustrating profession—30 years of MIS as a sideshow. General surgery has rapidly adopted a laparoscopic approach to most operations, after gynecologists taught them. Today the majority of gynecologists do not do advanced laparoscopic surgery and would love to get back to open incision laparotomy for their operations. We cannot go back.
OBG Management: Dr. Wertheim and Dr. Reich, do your personal views of the morcellation issue differ at all from the official views of professional societies?
Dr. Wertheim: Yes. However, before I share them, I’d like to emphasize that the views I’m about to express are mine and mine only, not those of the AAGL or its task force.
The issue of uterine extraction is a highly emotional and political issue, about which there are few good data.
Abundant Level 1 data strongly support a vaginal or laparoscopic approach for benign hysterectomy when possible. ACOG and AAGL have issued position papers supporting these approaches for benign hysterectomies. Gynecologic surgeons and other surgical specialists have embraced MIS because it is safer, offers faster recovery, produces less postoperative pain, and has fewer complications than open surgery. However, AAGL has maintained for several years that morcellation is contraindicated in cases where uterine malignancy is either known or suspected.
The dilemma with open power morcellation is that even with our best diagnostic tools, the rare uterine sarcoma cannot always be definitively ruled out preoperatively. Endometrial cancer usually can be diagnosed before surgery. However, rare subtypes such as sarcomas are more difficult to reliably diagnose preoperatively, and risk factors for uterine sarcomas are not nearly as well understood as those for endometrial cancer.
I do agree with the FDA’s cautionary statement, which pointedly prohibits power morcellation for women with suspected precancer or known cancer of the gynecologic organs.3 However, the AAGL task force critically reviewed about 120 articles, including the studies assessed by the FDA. Concerns arose regarding the FDA’s interpretation of the data. Due to a number of deficiencies in these studies, some of the conclusions of the FDA may not be completely accurate. The studies analyzed by the FDA were not stratified by risk factors for sarcoma and were not necessarily performed in a setting of reproductive-aged women with presumed fibroids.
Dr. Reich: Here are my personal views about the sarcoma problem and I am sure they differ from the official views of the professional societies:
- Laparoscopic hysterectomy should always mean vaginal extraction unless a less disfiguring site can be discovered; power morcellation implies minilaparotomy and should be renamed to reflect that fact.
- Power morcellation must be differentiated from vaginal and minilaparotomy scalpel morcellation, especially in the media. Vaginal hysterectomy has entailed vaginal scalpel morcellation with successful outcomes for more than 100 years.
- Remember that most gynecologic cancers are approached using the laparoscope today. This certainly includes cervical and endometrial cancer and some ovarian cancers. (For example, one of my neighbors is a 25-year survivor of laparoscopically treated bilateral ovarian cancer who refused laparotomy!)
- I have removed sarcomas by vaginal morcellation during laparoscopic hysterectomy and laparoscopic myomectomy with no late sequelae. In fact, most cervical cancer surgery is done by laparoscopic surgery today. And even an open laparotomy hysterectomy can spread a sarcoma.
- The current morcellation debate arose when a single case of disseminated leiomyosarcoma became highly publicized. It involved a prominent physician whose leiomyosarcoma was unknown to her initial surgeon, and the malignancy was upstaged after the use of power morcellation during hysterectomy. After this case was covered in the media, other cases began to be reported in the lay press as well, some of which predated the publicized case. The truth is, regrettably, that sarcomas carry poor prognoses even when specimens are removed intact. And we don’t know much about the sarcoma that started this debate. Was it mild or aggressive? How many mitotic figures were there per high-powered field? And what was found macroscopically and microscopically at the subsequent laparotomy? We on the AAGL task force do not know the answers to these questions, although at least some of these variables are reported in other published cases. And because this case is likely to have a powerful effect on MIS in our country and the rest of the world, it is my opinion that we need to know these details.
What is your preferred surgical approach?
OBG Management: Do you perform open power morcellation in selected patients?
Dr. Wertheim: Even though I have performed morcellation with a scalpel transvaginally or through a mini-laparotomy incision for many years, I have never used open power morcellation because of the risk of leaving behind benign or malignant tissue fragments. Morcellation with a scalpel is easily learned and can be performed as quickly as power morcellation. Morcellation with a scalpel produces much larger pieces than with power morcellation. This probably markedly decreases the loss of fragments. I cannot make a definitive statement regarding cell loss, however. Until we have improved instrumentation and are better able to make a preoperative diagnosis of sarcoma, I’m going to rule out risk factors identified by the AAGL task force, do the appropriate work-up, and continue to morcellate with a scalpel, placing the specimen in a bag, if technically possible.
Dr. Reich: As I mentioned, I am a vaginal scalpel morcellator. I tried power morcellation when it first was developed but was never a fan. The same techniques used for vaginal extraction using a coring maneuver can be used abdominally through the umbilicus or a 1- or 2-cm trocar site.
What should the FDA’s next move be?
OBG Management: Care to make any predictions about the FDA’s final decision?
Dr. Wertheim: This has become a highly emotional and controversial issue with little good existing data. During the preoperative visit, this issue should be discussed with the patient using clear, lay-friendly language. Having said that, I also do not believe we should hide behind informed consent. The FDA has a responsibility to keep the public safe. If open power morcellation is allowed to continue, there will be another morcellated sarcoma or complications from retained benign tissue fragments. I doubt the FDA can live with this. I believe the risk factors identified by the AAGL task force should be ruled out, the appropriate workup done and then, if power morcellation is performed, it should be done inside a bag. In addition, I think the FDA should require that complications be reported and recorded in a registry.
Dr. Reich: I disagree. The FDA has to back off. It’s important to note that this is an American problem, as the rest of the world cannot afford power morcellators. The data are not in yet. The decision about what kind of hysterectomy is performed will be made by the “informed” patient, who undoubtedly will be very afraid to have MIS because of the surrounding negative publicity. We must do a better job of promoting the advantages of a minimally invasive approach.
OBG Management: Thank you both for your time and expertise.
Dr. Wertheim: Thank you for giving us the opportunity to express our opinions regarding this highly emotional and controversial issue.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Share your thoughts by sending a letter to [email protected]. Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
1. Barbieri RL. Benefits and pitfalls of open power morcellation of uterine fibroids. OBG Manag. 2014;26(2):10–15.
2. The Tissue Extraction Task Force, AAGL. AAGL Position Statement: Morcellation During Uterine Tissue Extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed June 13, 014.
3. US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy. FDA Safety Communication. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed June 13, 2014.
1. Barbieri RL. Benefits and pitfalls of open power morcellation of uterine fibroids. OBG Manag. 2014;26(2):10–15.
2. The Tissue Extraction Task Force, AAGL. AAGL Position Statement: Morcellation During Uterine Tissue Extraction. http://www.aagl.org/wp-content/uploads/2014/05/Tissue_Extraction_TFR.pdf. Accessed June 13, 014.
3. US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy. FDA Safety Communication. http://www.fda.gov/medicaldevices/safety/alertsandnotices/ucm393576.htm. Published April 17, 2014. Accessed June 13, 2014.
Visit the Morcellation Topic Collection Page for additional articles, videos, and audiocasts.
FDA, hospitals caution against laparoscopic power morcellation during hysterectomy and myomectomy
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy may be riskier than many have thought. That’s the conclusion reached by the US Food and Drug Administration (FDA) in a safety communication issued April 17, 2014. In its communication, the FDA “discouraged” use of power morcellation during hysterectomy and myomectomy. Shortly afterward, Brigham and Women’s and Massachusetts General hospitals in Boston banned power morcellation in all hysterectomy and myomectomy procedures. The hospitals may resume power morcellation at some future date using a containment system, pending guidance from the Institutional Review Board.
Robert L. Barbieri, MD, who is chair of obstetrics and gynecology at Brigham and Women’s Hospital, recently wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation [without a containment system] is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
The two Boston hospitals are not the only institutions reconsidering the use of power morcellation. Temple University Hospital in Philadelphia banned use of the procedure without a containment system in late February 2014.
And in December 2013, the Society of Gynecologic Oncology issued a position statement on the issue, which said, “power morcellation or other techniques that cut up the uterus in the abdomen have the potential to disseminate an otherwise contained malignancy throughout the abdominal cavity. For this reason, the Society of Gynecologic Oncology (SGO) asserts that it is generally contraindicated in the presence of documented or highly suspected malignancy, and may be inadvisable in premalignant conditions or risk-reducing surgery.”2
For its part, at the time of this writing, the AAGL, previously known as the American Association of Gynecologic Laparoscopists, “is reviewing the scientific evidence and best practices reported by our members,” stated an article in its Association News. “We recognize that, in rare cases, the use of power morcellators can lead to the dissemination of an occult malignancy of endometrial or myometrial origin, and also to dissemination of benign morcellated tissues. We encourage our members to fully research and understand the risks of power morcellation and to learn more about when alternative methods of tissue extraction may be appropriate.”3
FDA STOPS SHORT OF A BAN
In laying out its concerns, the FDA stopped short of an outright ban on power morcellation. Instead, it stated that, “based on currently available information, the FDA discourages the use of laparoscopic power morcellation during hysterectomy or myomectomy for uterine fibroids.”4
It also noted that approximately 1 in 350 women “undergoing hysterectomy or myomectomy for the treatment of fibroids is found to have an unsuspected uterine sarcoma.”4
Among its recommendations for health-care providers:
- avoid laparoscopic uterine power morcellation in women with suspected or known uterine cancer
- carefully consider all available treatment options for women with symptomatic uterine fibroids
- thoroughly discuss the benefits and risks of all treatments with patients.4
The FDA also noted that “some clinicians and medical institutions now advocate using a specimen ‘bag’ during morcellation in an attempt to contain the uterine tissue and minimize the risk of spread in the abdomen and pelvis.”4
ACOG HAS YET TO WEIGH IN
At the time of this writing, the most recent committee opinion on choosing a hysterectomy route from the American College of Obstetricians and Gynecologists (ACOG) to touch on the issue states that, “the decision to perform a hysterectomy via [minimally invasive surgery] (with or without morcellation) is based on a patient evaluation, including the patient’s history and general health, tests, and procedures, such as pre-surgery biopsies. The evaluation and diagnostic process also provides an opportunity to identify any cautions or contraindications, such as finding a gynecological cancer.”5
FILLING THE TECHNOLOGY GAP
Now that power morcellation appears to be receding as an option for minimally invasive gynecologic surgeons, what is the best approach?
In its position statement, the SGO recommends that, “Patients being considered for minimally invasive surgery performed by laparoscopic or robotic techniques who might require intracorporeal morcellation should be appropriately evaluated for the possibility of coexisting uterine or cervical malignancy. Other options to intracorporeal morcellation include removing the uterus through a mini-laparotomy or morcellating the uterus inside a laparoscopic bag.”2
K. Anthony Shibley, MD, a Minneapolis-area ObGyn, has developed a novel strategy to prevent tissue dissemination during open power morcellation, which is demonstrated in a video at obgmanagement.com. Similarly, Ceana Nezhat, MD, and Erica Dun, MD, demonstrate enclosed vaginal morcellation of a large uterus. Click here to access these and other features in the Morcellation Topic Collection.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, and the city and state in which you practice.
- Barbieri RL. Options for reducing the use of open power morcellation of uterine tumors. OBG Manag. 2014;26(3):10,11,20.
- Society of Gynecologic Oncology. Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation/. Published December 2013. Accessed April 8, 2014.
- AAGL Member Update: Disseminated Leiomyosarcoma with Power Morcellation. http://www.aagl.org/aaglnews/aagl-member-update-disseminated-leiomyosarcoma-with-power-morcellation/. Accessed April 11, 2014.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy may be riskier than many have thought. That’s the conclusion reached by the US Food and Drug Administration (FDA) in a safety communication issued April 17, 2014. In its communication, the FDA “discouraged” use of power morcellation during hysterectomy and myomectomy. Shortly afterward, Brigham and Women’s and Massachusetts General hospitals in Boston banned power morcellation in all hysterectomy and myomectomy procedures. The hospitals may resume power morcellation at some future date using a containment system, pending guidance from the Institutional Review Board.
Robert L. Barbieri, MD, who is chair of obstetrics and gynecology at Brigham and Women’s Hospital, recently wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation [without a containment system] is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
The two Boston hospitals are not the only institutions reconsidering the use of power morcellation. Temple University Hospital in Philadelphia banned use of the procedure without a containment system in late February 2014.
And in December 2013, the Society of Gynecologic Oncology issued a position statement on the issue, which said, “power morcellation or other techniques that cut up the uterus in the abdomen have the potential to disseminate an otherwise contained malignancy throughout the abdominal cavity. For this reason, the Society of Gynecologic Oncology (SGO) asserts that it is generally contraindicated in the presence of documented or highly suspected malignancy, and may be inadvisable in premalignant conditions or risk-reducing surgery.”2
For its part, at the time of this writing, the AAGL, previously known as the American Association of Gynecologic Laparoscopists, “is reviewing the scientific evidence and best practices reported by our members,” stated an article in its Association News. “We recognize that, in rare cases, the use of power morcellators can lead to the dissemination of an occult malignancy of endometrial or myometrial origin, and also to dissemination of benign morcellated tissues. We encourage our members to fully research and understand the risks of power morcellation and to learn more about when alternative methods of tissue extraction may be appropriate.”3
FDA STOPS SHORT OF A BAN
In laying out its concerns, the FDA stopped short of an outright ban on power morcellation. Instead, it stated that, “based on currently available information, the FDA discourages the use of laparoscopic power morcellation during hysterectomy or myomectomy for uterine fibroids.”4
It also noted that approximately 1 in 350 women “undergoing hysterectomy or myomectomy for the treatment of fibroids is found to have an unsuspected uterine sarcoma.”4
Among its recommendations for health-care providers:
- avoid laparoscopic uterine power morcellation in women with suspected or known uterine cancer
- carefully consider all available treatment options for women with symptomatic uterine fibroids
- thoroughly discuss the benefits and risks of all treatments with patients.4
The FDA also noted that “some clinicians and medical institutions now advocate using a specimen ‘bag’ during morcellation in an attempt to contain the uterine tissue and minimize the risk of spread in the abdomen and pelvis.”4
ACOG HAS YET TO WEIGH IN
At the time of this writing, the most recent committee opinion on choosing a hysterectomy route from the American College of Obstetricians and Gynecologists (ACOG) to touch on the issue states that, “the decision to perform a hysterectomy via [minimally invasive surgery] (with or without morcellation) is based on a patient evaluation, including the patient’s history and general health, tests, and procedures, such as pre-surgery biopsies. The evaluation and diagnostic process also provides an opportunity to identify any cautions or contraindications, such as finding a gynecological cancer.”5
FILLING THE TECHNOLOGY GAP
Now that power morcellation appears to be receding as an option for minimally invasive gynecologic surgeons, what is the best approach?
In its position statement, the SGO recommends that, “Patients being considered for minimally invasive surgery performed by laparoscopic or robotic techniques who might require intracorporeal morcellation should be appropriately evaluated for the possibility of coexisting uterine or cervical malignancy. Other options to intracorporeal morcellation include removing the uterus through a mini-laparotomy or morcellating the uterus inside a laparoscopic bag.”2
K. Anthony Shibley, MD, a Minneapolis-area ObGyn, has developed a novel strategy to prevent tissue dissemination during open power morcellation, which is demonstrated in a video at obgmanagement.com. Similarly, Ceana Nezhat, MD, and Erica Dun, MD, demonstrate enclosed vaginal morcellation of a large uterus. Click here to access these and other features in the Morcellation Topic Collection.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, and the city and state in which you practice.
The use of power morcellation to remove the uterus or uterine tumors during hysterectomy and myomectomy may be riskier than many have thought. That’s the conclusion reached by the US Food and Drug Administration (FDA) in a safety communication issued April 17, 2014. In its communication, the FDA “discouraged” use of power morcellation during hysterectomy and myomectomy. Shortly afterward, Brigham and Women’s and Massachusetts General hospitals in Boston banned power morcellation in all hysterectomy and myomectomy procedures. The hospitals may resume power morcellation at some future date using a containment system, pending guidance from the Institutional Review Board.
Robert L. Barbieri, MD, who is chair of obstetrics and gynecology at Brigham and Women’s Hospital, recently wrote about this concern for OBG Management in his capacity as editor in chief of the journal.
“When used to treat tumors presumed to be fibroids, open power morcellation [without a containment system] is associated with an increased risk of dispersing benign myoma tissue and occult malignant leiomyosarcoma tissue throughout the abdominal cavity,” he wrote.1 “Dispersion of benign myoma tissue may result in the growth of fibroids on the peritoneal surface, omentum, and bowel, causing abdominal and pelvic pain and necessitating reoperation. Dispersion of leiomyosarcoma tissue throughout the abdominal cavity may result in a Stage I cancer being upstaged to a Stage IV malignancy, requiring additional surgery and chemotherapy. In cases in which open power morcellation causes the upstaging of a leiomyosarcoma, the death rate is increased.”1
The two Boston hospitals are not the only institutions reconsidering the use of power morcellation. Temple University Hospital in Philadelphia banned use of the procedure without a containment system in late February 2014.
And in December 2013, the Society of Gynecologic Oncology issued a position statement on the issue, which said, “power morcellation or other techniques that cut up the uterus in the abdomen have the potential to disseminate an otherwise contained malignancy throughout the abdominal cavity. For this reason, the Society of Gynecologic Oncology (SGO) asserts that it is generally contraindicated in the presence of documented or highly suspected malignancy, and may be inadvisable in premalignant conditions or risk-reducing surgery.”2
For its part, at the time of this writing, the AAGL, previously known as the American Association of Gynecologic Laparoscopists, “is reviewing the scientific evidence and best practices reported by our members,” stated an article in its Association News. “We recognize that, in rare cases, the use of power morcellators can lead to the dissemination of an occult malignancy of endometrial or myometrial origin, and also to dissemination of benign morcellated tissues. We encourage our members to fully research and understand the risks of power morcellation and to learn more about when alternative methods of tissue extraction may be appropriate.”3
FDA STOPS SHORT OF A BAN
In laying out its concerns, the FDA stopped short of an outright ban on power morcellation. Instead, it stated that, “based on currently available information, the FDA discourages the use of laparoscopic power morcellation during hysterectomy or myomectomy for uterine fibroids.”4
It also noted that approximately 1 in 350 women “undergoing hysterectomy or myomectomy for the treatment of fibroids is found to have an unsuspected uterine sarcoma.”4
Among its recommendations for health-care providers:
- avoid laparoscopic uterine power morcellation in women with suspected or known uterine cancer
- carefully consider all available treatment options for women with symptomatic uterine fibroids
- thoroughly discuss the benefits and risks of all treatments with patients.4
The FDA also noted that “some clinicians and medical institutions now advocate using a specimen ‘bag’ during morcellation in an attempt to contain the uterine tissue and minimize the risk of spread in the abdomen and pelvis.”4
ACOG HAS YET TO WEIGH IN
At the time of this writing, the most recent committee opinion on choosing a hysterectomy route from the American College of Obstetricians and Gynecologists (ACOG) to touch on the issue states that, “the decision to perform a hysterectomy via [minimally invasive surgery] (with or without morcellation) is based on a patient evaluation, including the patient’s history and general health, tests, and procedures, such as pre-surgery biopsies. The evaluation and diagnostic process also provides an opportunity to identify any cautions or contraindications, such as finding a gynecological cancer.”5
FILLING THE TECHNOLOGY GAP
Now that power morcellation appears to be receding as an option for minimally invasive gynecologic surgeons, what is the best approach?
In its position statement, the SGO recommends that, “Patients being considered for minimally invasive surgery performed by laparoscopic or robotic techniques who might require intracorporeal morcellation should be appropriately evaluated for the possibility of coexisting uterine or cervical malignancy. Other options to intracorporeal morcellation include removing the uterus through a mini-laparotomy or morcellating the uterus inside a laparoscopic bag.”2
K. Anthony Shibley, MD, a Minneapolis-area ObGyn, has developed a novel strategy to prevent tissue dissemination during open power morcellation, which is demonstrated in a video at obgmanagement.com. Similarly, Ceana Nezhat, MD, and Erica Dun, MD, demonstrate enclosed vaginal morcellation of a large uterus. Click here to access these and other features in the Morcellation Topic Collection.
WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: [email protected] Please include your name, and the city and state in which you practice.
- Barbieri RL. Options for reducing the use of open power morcellation of uterine tumors. OBG Manag. 2014;26(3):10,11,20.
- Society of Gynecologic Oncology. Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation/. Published December 2013. Accessed April 8, 2014.
- AAGL Member Update: Disseminated Leiomyosarcoma with Power Morcellation. http://www.aagl.org/aaglnews/aagl-member-update-disseminated-leiomyosarcoma-with-power-morcellation/. Accessed April 11, 2014.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
- Barbieri RL. Options for reducing the use of open power morcellation of uterine tumors. OBG Manag. 2014;26(3):10,11,20.
- Society of Gynecologic Oncology. Position Statement: Morcellation. https://www.sgo.org/newsroom/position-statements-2/morcellation/. Published December 2013. Accessed April 8, 2014.
- AAGL Member Update: Disseminated Leiomyosarcoma with Power Morcellation. http://www.aagl.org/aaglnews/aagl-member-update-disseminated-leiomyosarcoma-with-power-morcellation/. Accessed April 11, 2014.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. April 17, 2014.
- American College of Obstetricians and Gynecologists. Committee Opinion No. 444: Choosing the route of hysterectomy for benign disease. Obstet Gynecol. 2009;114(5):1156–1158.
To access articles, videos, and audiocasts in the Morcellation Topic Collection, click here.
Anatomy for the laparoscopic surgeon
CASE: OBESE PATIENT REQUESTS TOTAL LAPAROSCOPIC HYSTERECTOMY
A 45-year-old woman (G2P2), who delivered both children by cesarean section, schedules an office visit for a complaint of abnormal uterine bleeding. She is obese, with a body mass index (BMI) of 35 kg/m2, and has an enlarged uterus of approximately 14 weeks’ size with minimal descensus. An earlier trial of hormone therapy failed to provide relief. After you counsel her extensively about her treatment options, she elects to undergo total laparoscopic hysterectomy.
What anatomy would you review to help ensure the procedure’s success?
Although the vaginal route is preferred for hysterectomy, total laparoscopic hysterectomy is another minimally invasive option that offers lower morbidity and a shorter hospital stay than the abdominal approach.1 Perhaps more than any other variable, the key to safe, efficient, and effective laparoscopic surgery is a comprehensive knowledge of anatomy. For example, a thorough understanding of the anatomy of the anterior abdominal wall is critical to laparoscopic entry.2,3 Also, pelvic anatomy visualized two-dimensionally under magnification during traditional laparoscopy can look very different than it does during conventional surgery, due to the effects of the pneumoperitoneum, steep Trendelenburg position, and/or the use of uterine manipulators.3
The abdominal cavity is traditionally divided into nine regions. Regardless of the quadrants chosen for laparoscopic access, thorough knowledge of the relevant surface anatomy increases patient safety during surgery (FIGURE 1).
PRIMARY PORT PLACEMENT
Primary port placement, including insertion of the Veress needle, accounts for approximately 40% of laparoscopic complications.4 To help minimize complications, surgeons should ensure that the operating table remains level during placement. As the patient is moved into the Trendelenburg position, the great vessels are more in line with the 45-degree angle that most surgeons use when placing their Veress needle and primary trocar, which can lead to an increased risk of injury. Thus, proper positioning in relationship to anatomy is critical to successful laparoscopic surgery.
Veress or closed technique
Most gynecologists employ the closed method or Veress needle approach to establish pneumoperitoneum.5,6 an initial intraperitoneal pressure below 10 mm Hg, regardless of a woman’s body habitus, height, or age, indicates correct placement of the Veress needle.7,8 Vilos and colleagues demonstrated that Veress intraperitoneal pressure correlates positively with a woman’s weight and BMI and correlates negatively with her parity.8
Hasson or open technique
During the Hasson or open technique, many surgeons use the umbilical ring to gain entry into the abdominal cavity.9 Many view the umbilical ring as a window into the anterior abdominal wall, through which access to the peritoneal cavity can be achieved, but it can also be a site of hernia development.10 The shape of the umbilical ring can vary, appearing round or oval, but it also can be obliterated, slitted, or covered completely by a connecting band, which can result in more difficult laparoscopic entry.10
Palmer’s point
In the 1940s, the French gynecologist Raoul Palmer advocated placing the laparoscope at a point in the left midclavicular line, approximately 3 cm caudal to the costal margin, because visceral-parietal adhesions rarely were found there. Gynecologists still favor this entry site when intra-abdominal adhesions are likely, especially in patients with a history of significant adhesions or multiple previous pelvic surgeries.11 In a study published by Agarwala and colleagues, which included 504 patients with intra-abdominal adhesions, left upper quadrant entry was found to be safe with a complication rate as low as 0.39%.12
If supraumbilical or left upper quadrant port sites are used, the surface anatomy of the spleen and stomach become relevant. The portion of the stomach that is in contact with the abdominal wall is represented roughly by a triangular area extending between the tip of the 10th left costal cartilage, the tip of the ninth right cartilage, and the end of the eighth left costal cartilage.13 The size and shape of the stomach differs by position. Some authors recommend emptying the stomach using a nasogastric or oral gastric tube prior to port insertion to avoid injury.12
The spleen can be mapped using the 10th rib as representing its long axis; vertically, the spleen is situated between the upper border of the ninth and lower border of the 11th ribs.13 In patients without splenic enlargement, the spleen should not be found below the rib cage.
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (Surgical Technique, August 2013)
CASE CONTINUED
On the day of surgery, your patient is brought to the operating room. you use the Veress needle for insufflation. Your opening pressure is 5 mm Hg. You know that an opening pressure of less than 10 mm Hg indicates proper placement, so you continue on to place a 10-mm port. After inserting the primary umbilical port through the umbilicus, you decide to insert secondary ports through lower quadrants. Upon insertion, you note active bleeding at one of the secondary port sites.
How do you proceed?
VASCULAR ANATOMY OF THE ANTERIOR ABDOMINAL WALL
Understanding anterior abdominal wall anatomy and the course of the deep inferior and superficial epigastric vessels is essential to the safe placement of secondary laparoscopic ports. epigastric vessels are the most commonly injured vessels during laparoscopic surgery.14,15 The inferior epigastric vessels originate at the external iliac, immediately above the inguinal ligament. They course medially to the round ligament and travel beneath the lateral third of the rectus abdominis muscle. Using anterior abdominal wall landmarks, the inferior epigastric artery can be identified midway between the anterior superior iliac spine and the pubic symphysis as it travels toward the umbilicus. The inferior epigastric artery also serves as the lateral boundary of Hesselbach’s triangle; the other two boundaries are the lateral edge of the rectus abdominis and the medial aspect of the inguinal ligament (FIGURE 2).13
As the inferior epigastric vessels course cranially, the distance from the midline
decreases. the average distance from the midline at the pubis is approximately
7.5 cm. At the umbilicus, it is approximately 4.6 cm.16,17 The most efficient way to identify laparoscopically the inferior epigastric vessel is to first identify the round ligament. This can be done using a uterine manipulator to deviate the uterus to the contralateral side. Then observe the course of the inferior epigastric vessel just medial to the entry of the round ligament into the inguinal canal. The laparoscopic surgeon can then follow the course of the inferior epigastric vessels to determine the safest location for placement of secondary ports. Transillumination can identify the superficial epigastric vessels, which course within the subcutaneous tissue of the anterior abdominal wall, although it doesn’t identify the deep inferior epigastric vessels that are beneath the lateral third of the rectus muscle. The superficial epigastric vessels follow a course similar to that of the deep inferior epigastric vessels, however, and can serve as a surrogate to guide safe placement of accessory ports.17
Landmarks of the anterior abdominal wall during laparoscopic visualization can also guide placement of secondary ports. The median umbilical fold, which is the peritoneal covering of the umbilical ligament/urachus, travels between the bladder and umbilicus in the midline anterior abdominal wall. Immediately lateral is the medial umbilical fold, which is the peritoneal covering of the obliterated umbilical artery, a branch of the superior vesical artery that comes off the anterior trunk of the internal iliac artery.2 The lateral umbilical folds are lateral to the medial umbilical fold and are the peritoneal covering of the deep inferior epigastric vessels. Identification of these anterior abdominal wall landmarks can assist the surgeon in placing lateral ports so as to avoid injury to these vessels.
Related article: How to avoid major vessel injury during gynecologic laparoscopy Michael Baggish, MD (Surgical Technique, August 2012)
MAJOR RETROPERITONEAL VESSELS
Although major retroperitoneal vessel injury is uncommon, occurring in only 0.3% to 1.0% of laparoscopic surgeries, it has the potential to be catastrophic.18 Therefore, an understanding of the surface anatomy of the major vessels is essential for midline port placement.
The abdominal aorta begins about 4 cm above the transpyloric line and extends to
2 cm inferior and to the left of the umbilicus, or, more accurately, to a point 2 cm left of the middle line on a line that passes through the highest points of the iliac crests. The point of termination of the abdominal aorta corresponds to the level of the fourth lumbar vertebra; a line drawn from it to a point midway between the anterior superior iliac spine and the symphysis pubis indicates the common and external iliac arteries. The common iliac is represented by the upper third of this line and the external iliac, by the remaining two-thirds.13
Related article: How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy Michael Baggish, MD (Surgical Technique, October 2012)
OBESITY AND LAPAROSCOPIC SURGERY
Over two-thirds of the US adult population is now classified as overweight or obese.19 Research has shown that, compared with abdominal hysterectomy, laparoscopic surgery entails a shorter hospital stay, less blood loss, and fewer abdominal wall and wound infections, which are important advantages for this particular population.20
Laparoscopic entry can be particularly challenging in the obese patient. A study by Hurd and colleagues showed that the mean umbilical location was 2.4 cm caudal to the bifurcation of the aorta in the overweight population and 2.9 cm caudal in the obese population.21 Because the bifurcation of the aorta is more cephalad to the umbilicus in overweight and obese patients, the laparoscopic surgeon can introduce the Veress needle at a steeper angle and more perpendicular to the abdominal wall than for a thinner patient (FIGURE 3).
RELEVANT NERVES OF THE ANTERIOR ABDOMINAL WALL
The iliohypogastric and ilioinguinal nerves are also at risk for injury with laterally placed trocars through direct trauma or nerve entrapment. These nerves emerge from the T12 to L1 and L1 to L2 regions, respectively, and course through the muscles of the anterior abdominal wall. Specifically, the iliohypogastric nerve penetrates the fascia of the internal oblique muscle, and the ilioinguinal nerve penetrates the fascia of the transverse abdominus muscle.22 Fascial closure at lateral port sites can also increase the risk of injury to those nerves (FIGURE 4).23
CASE CONTINUED
As you continue your case, you have had to replace your right lower quadrant port several times. During the last insertion, you notice that you have an enlarging abdominal wall hematoma. You suspect that you have injured the inferior epigastric vessel.
How should it be repaired?
HOW TO PREVENT AND REPAIR INJURED DEEP INFERIOR EPIGASTRIC VESSELS
A thorough knowledge of anatomy is the most effective way to prevent these types of injuries. The use of bladeless radially expanding trocars and smooth conical-tip trocars that push the vessels away may result in fewer port-site bleeding complications and injuries than large pyramidal or cutting trocars.24–26 It is important to inspect all ports sites at the end of any laparoscopic procedure because the port itself can tamponade an injured anterior abdominal wall vessel and obscure an injury.
If an injury occurs, leave the trocars in place until a plan for repair is devised. First, start by compressing the bleeding point by moving the cannula against it. Because there are two bleeding ends, the vessels must be sutured cephalad and caudad to the site of injury. The use of electrosurgical desiccation is usually less successful.25 In obese patients we prefer to suture-ligate the bleeder intracorporeally or use a laparoscopic port closure device. In thin and pediatric patients, percutaneous suture ligation can be done easily.
Another option to control bleeding at the cannula site is placement of a Foley catheter to tamponade the vessel using a large balloon placed on tension.27 If abdominal loop sutures are used to control bleeding, the sutures typically are left intact for 8 hours prior to removal.25 If identification of the bleeding point is difficult, percutaneous placement of a suture ligature over a roll of gauze or using a Foley catheter to tamponade the bleeder can be helpful.
CASE RESOLVED
A laparoscopic port closure device is used to suture ligate the bleeding vessel. Hemostasis is achieved and the laparoscopic hysterectomy is completed without further complications.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Share your thoughts by sending a letter to [email protected]. Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- AAGL position statement: Route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1–3.
- Tokar B, Yucel F. Anatomical variations of medial umbilical ligament: clinical significance in laparoscopic exploration of children. Pediatr Surg Int. 2009;25(12):1077–1080.
- Nezhat CH, Nezhat F, Brill AI, Nezhat C. Normal variations of abdominal and pelvic anatomy evaluated at laparoscopy. Obstet Gynecol. 1999;94(2):238–242.
- Fuller J, Ashar BS, Carey-Corrado J. Trocar-associated injuries and fatalities: an analysis of 1,399 reports to the FDA. J Minim Invasive Gynecol. 2005;12(4):302–307.
- Jansen FW, Kolkman W, Bakkum EA, de Kroon CD, Trimbos-Kemper TC, Trimbos JB. Complications of laparoscopy: An inquiry about closed- versus open-entry technique. Am J Obstet Gynecol. 2004;190(3):634–638.
- Yuzpe AA. Pneumoperitoneum needle and trocar injuries in laparoscopy. A survey on possible contributing factors and prevention. J Reprod Med. 1990;35(5):485–490.
- Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10(3):415–420.
- Vilos AG, Vilos GA, Abu-Rafea B, Hollett-Caines J, Al-Omran M. Effect of body habitus and parity on the initial Veress intraperitoneal CO2 insufflation pressure during laparoscopic access in women. J Minim Invasive Gynecol. 2006;13(2):108–113.
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110(6):886–887.
- Oh CS, Won HS, Kwon CH, Chung IH. Morphologic variations of the umbilical ring, umbilical ligaments and ligamentum teres hepatis. Yonsei Med J. 2008;49(6):1004–1007.
- Chandler JG, Corson SL, Way LW. Three spectra of laparoscopic entry access injuries. J Am Coll Surg. 2001;192(4):478–491.
- Agarwala N, Liu CY. Safe entry techniques during laparoscopy: left upper quadrant entry using the ninth intercostal space—a review of 918 procedures. J Minim Invasive Gynecol. 2005;12(1):55–61.
- Williams PL, Warwick R, eds. Gray’s anatomy. 36th ed. Philadelphia, PA: Churchill Livingstone; 1980.
- Hurd WW, Pearl ML, DeLancey JO, Quint EH, Garnett B, Bude RO. Laparoscopic injury of abdominal wall blood vessels: A report of three cases. Obstet Gynecol. 1993;82(4 Pt 2 Suppl):673–676.
- Lin P, Grow DR. Complications of laparoscopy. Strategies for prevention and cure. Obstet Gynecol Clin North Am. 1999;26(1):23–38, v.
- Saber AA, Meslemani AM, Davis R, Pimentel R. Safety zones for anterior abdominal wall entry during laparoscopy: A CT scan mapping of epigastric vessels. Ann Surg. 2004;239(2):182–185.
- Hurd WW, Bude RO, DeLancey JO, Newman JS. The location of abdominal wall blood vessels in relationship to abdominal landmarks apparent at laparoscopy. Am J Obstet Gynecol. 1994;171(3):642–646.
- Sandadi S, Johannigman JA, Wong VL, Blebea J, Altose MD, Hurd WW. Recognition and management of major vessel injury during laparoscopy. J minim invasive gynecol. 2010;17(6):692–702.
- Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiol rev. 2007;29:6–28.
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane database syst rev. 2009;(3):CD003677.
- Hurd WW, Bude RO, DeLancey JO, Pearl ML. The relationship of the umbilicus to the aortic bifurcation: implications for laparoscopic technique. Obstet gynecol. 1992;80(1):48–51.
- Clarke-Pearson DL, Geller EJ. Complications of hysterectomy. Obstet gynecol. 2013;121(3):654–673.
- Shin JH, Howard FM. Abdominal wall nerve injury during laparoscopic gynecologic surgery: incidence, risk factors, and treatment outcomes. J minim invasive gynecol. 2012;19(4):448–453.
- Bhoyrul S, Payne J, Steffes B, Swanstrom L, Way LW. A randomized prospective study of radially expanding trocars in laparoscopic surgery. J gastrointest surg. 2000;4(4):392–397.
- Shirk GJ, Johns A, Redwine DB. Complications of laparoscopic surgery: How to avoid them and how to repair them. J minim invasive gynecol. 2006;13(4):352–361.
- Tews G, Arzt W, Bohaumilitzky T, Füreder R, Frölich H. Significant reduction of operational risk in laparoscopy through the use of a new blunt trocar. Surg gynecol obstet. 1991;173(1):67–68.
- Najmaldin A, Guillou P. A guide to laparoscopic surgery. 1st ed. Wiley-Blackwell; 1998.
CASE: OBESE PATIENT REQUESTS TOTAL LAPAROSCOPIC HYSTERECTOMY
A 45-year-old woman (G2P2), who delivered both children by cesarean section, schedules an office visit for a complaint of abnormal uterine bleeding. She is obese, with a body mass index (BMI) of 35 kg/m2, and has an enlarged uterus of approximately 14 weeks’ size with minimal descensus. An earlier trial of hormone therapy failed to provide relief. After you counsel her extensively about her treatment options, she elects to undergo total laparoscopic hysterectomy.
What anatomy would you review to help ensure the procedure’s success?
Although the vaginal route is preferred for hysterectomy, total laparoscopic hysterectomy is another minimally invasive option that offers lower morbidity and a shorter hospital stay than the abdominal approach.1 Perhaps more than any other variable, the key to safe, efficient, and effective laparoscopic surgery is a comprehensive knowledge of anatomy. For example, a thorough understanding of the anatomy of the anterior abdominal wall is critical to laparoscopic entry.2,3 Also, pelvic anatomy visualized two-dimensionally under magnification during traditional laparoscopy can look very different than it does during conventional surgery, due to the effects of the pneumoperitoneum, steep Trendelenburg position, and/or the use of uterine manipulators.3
The abdominal cavity is traditionally divided into nine regions. Regardless of the quadrants chosen for laparoscopic access, thorough knowledge of the relevant surface anatomy increases patient safety during surgery (FIGURE 1).
PRIMARY PORT PLACEMENT
Primary port placement, including insertion of the Veress needle, accounts for approximately 40% of laparoscopic complications.4 To help minimize complications, surgeons should ensure that the operating table remains level during placement. As the patient is moved into the Trendelenburg position, the great vessels are more in line with the 45-degree angle that most surgeons use when placing their Veress needle and primary trocar, which can lead to an increased risk of injury. Thus, proper positioning in relationship to anatomy is critical to successful laparoscopic surgery.
Veress or closed technique
Most gynecologists employ the closed method or Veress needle approach to establish pneumoperitoneum.5,6 an initial intraperitoneal pressure below 10 mm Hg, regardless of a woman’s body habitus, height, or age, indicates correct placement of the Veress needle.7,8 Vilos and colleagues demonstrated that Veress intraperitoneal pressure correlates positively with a woman’s weight and BMI and correlates negatively with her parity.8
Hasson or open technique
During the Hasson or open technique, many surgeons use the umbilical ring to gain entry into the abdominal cavity.9 Many view the umbilical ring as a window into the anterior abdominal wall, through which access to the peritoneal cavity can be achieved, but it can also be a site of hernia development.10 The shape of the umbilical ring can vary, appearing round or oval, but it also can be obliterated, slitted, or covered completely by a connecting band, which can result in more difficult laparoscopic entry.10
Palmer’s point
In the 1940s, the French gynecologist Raoul Palmer advocated placing the laparoscope at a point in the left midclavicular line, approximately 3 cm caudal to the costal margin, because visceral-parietal adhesions rarely were found there. Gynecologists still favor this entry site when intra-abdominal adhesions are likely, especially in patients with a history of significant adhesions or multiple previous pelvic surgeries.11 In a study published by Agarwala and colleagues, which included 504 patients with intra-abdominal adhesions, left upper quadrant entry was found to be safe with a complication rate as low as 0.39%.12
If supraumbilical or left upper quadrant port sites are used, the surface anatomy of the spleen and stomach become relevant. The portion of the stomach that is in contact with the abdominal wall is represented roughly by a triangular area extending between the tip of the 10th left costal cartilage, the tip of the ninth right cartilage, and the end of the eighth left costal cartilage.13 The size and shape of the stomach differs by position. Some authors recommend emptying the stomach using a nasogastric or oral gastric tube prior to port insertion to avoid injury.12
The spleen can be mapped using the 10th rib as representing its long axis; vertically, the spleen is situated between the upper border of the ninth and lower border of the 11th ribs.13 In patients without splenic enlargement, the spleen should not be found below the rib cage.
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (Surgical Technique, August 2013)
CASE CONTINUED
On the day of surgery, your patient is brought to the operating room. you use the Veress needle for insufflation. Your opening pressure is 5 mm Hg. You know that an opening pressure of less than 10 mm Hg indicates proper placement, so you continue on to place a 10-mm port. After inserting the primary umbilical port through the umbilicus, you decide to insert secondary ports through lower quadrants. Upon insertion, you note active bleeding at one of the secondary port sites.
How do you proceed?
VASCULAR ANATOMY OF THE ANTERIOR ABDOMINAL WALL
Understanding anterior abdominal wall anatomy and the course of the deep inferior and superficial epigastric vessels is essential to the safe placement of secondary laparoscopic ports. epigastric vessels are the most commonly injured vessels during laparoscopic surgery.14,15 The inferior epigastric vessels originate at the external iliac, immediately above the inguinal ligament. They course medially to the round ligament and travel beneath the lateral third of the rectus abdominis muscle. Using anterior abdominal wall landmarks, the inferior epigastric artery can be identified midway between the anterior superior iliac spine and the pubic symphysis as it travels toward the umbilicus. The inferior epigastric artery also serves as the lateral boundary of Hesselbach’s triangle; the other two boundaries are the lateral edge of the rectus abdominis and the medial aspect of the inguinal ligament (FIGURE 2).13
As the inferior epigastric vessels course cranially, the distance from the midline
decreases. the average distance from the midline at the pubis is approximately
7.5 cm. At the umbilicus, it is approximately 4.6 cm.16,17 The most efficient way to identify laparoscopically the inferior epigastric vessel is to first identify the round ligament. This can be done using a uterine manipulator to deviate the uterus to the contralateral side. Then observe the course of the inferior epigastric vessel just medial to the entry of the round ligament into the inguinal canal. The laparoscopic surgeon can then follow the course of the inferior epigastric vessels to determine the safest location for placement of secondary ports. Transillumination can identify the superficial epigastric vessels, which course within the subcutaneous tissue of the anterior abdominal wall, although it doesn’t identify the deep inferior epigastric vessels that are beneath the lateral third of the rectus muscle. The superficial epigastric vessels follow a course similar to that of the deep inferior epigastric vessels, however, and can serve as a surrogate to guide safe placement of accessory ports.17
Landmarks of the anterior abdominal wall during laparoscopic visualization can also guide placement of secondary ports. The median umbilical fold, which is the peritoneal covering of the umbilical ligament/urachus, travels between the bladder and umbilicus in the midline anterior abdominal wall. Immediately lateral is the medial umbilical fold, which is the peritoneal covering of the obliterated umbilical artery, a branch of the superior vesical artery that comes off the anterior trunk of the internal iliac artery.2 The lateral umbilical folds are lateral to the medial umbilical fold and are the peritoneal covering of the deep inferior epigastric vessels. Identification of these anterior abdominal wall landmarks can assist the surgeon in placing lateral ports so as to avoid injury to these vessels.
Related article: How to avoid major vessel injury during gynecologic laparoscopy Michael Baggish, MD (Surgical Technique, August 2012)
MAJOR RETROPERITONEAL VESSELS
Although major retroperitoneal vessel injury is uncommon, occurring in only 0.3% to 1.0% of laparoscopic surgeries, it has the potential to be catastrophic.18 Therefore, an understanding of the surface anatomy of the major vessels is essential for midline port placement.
The abdominal aorta begins about 4 cm above the transpyloric line and extends to
2 cm inferior and to the left of the umbilicus, or, more accurately, to a point 2 cm left of the middle line on a line that passes through the highest points of the iliac crests. The point of termination of the abdominal aorta corresponds to the level of the fourth lumbar vertebra; a line drawn from it to a point midway between the anterior superior iliac spine and the symphysis pubis indicates the common and external iliac arteries. The common iliac is represented by the upper third of this line and the external iliac, by the remaining two-thirds.13
Related article: How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy Michael Baggish, MD (Surgical Technique, October 2012)
OBESITY AND LAPAROSCOPIC SURGERY
Over two-thirds of the US adult population is now classified as overweight or obese.19 Research has shown that, compared with abdominal hysterectomy, laparoscopic surgery entails a shorter hospital stay, less blood loss, and fewer abdominal wall and wound infections, which are important advantages for this particular population.20
Laparoscopic entry can be particularly challenging in the obese patient. A study by Hurd and colleagues showed that the mean umbilical location was 2.4 cm caudal to the bifurcation of the aorta in the overweight population and 2.9 cm caudal in the obese population.21 Because the bifurcation of the aorta is more cephalad to the umbilicus in overweight and obese patients, the laparoscopic surgeon can introduce the Veress needle at a steeper angle and more perpendicular to the abdominal wall than for a thinner patient (FIGURE 3).
RELEVANT NERVES OF THE ANTERIOR ABDOMINAL WALL
The iliohypogastric and ilioinguinal nerves are also at risk for injury with laterally placed trocars through direct trauma or nerve entrapment. These nerves emerge from the T12 to L1 and L1 to L2 regions, respectively, and course through the muscles of the anterior abdominal wall. Specifically, the iliohypogastric nerve penetrates the fascia of the internal oblique muscle, and the ilioinguinal nerve penetrates the fascia of the transverse abdominus muscle.22 Fascial closure at lateral port sites can also increase the risk of injury to those nerves (FIGURE 4).23
CASE CONTINUED
As you continue your case, you have had to replace your right lower quadrant port several times. During the last insertion, you notice that you have an enlarging abdominal wall hematoma. You suspect that you have injured the inferior epigastric vessel.
How should it be repaired?
HOW TO PREVENT AND REPAIR INJURED DEEP INFERIOR EPIGASTRIC VESSELS
A thorough knowledge of anatomy is the most effective way to prevent these types of injuries. The use of bladeless radially expanding trocars and smooth conical-tip trocars that push the vessels away may result in fewer port-site bleeding complications and injuries than large pyramidal or cutting trocars.24–26 It is important to inspect all ports sites at the end of any laparoscopic procedure because the port itself can tamponade an injured anterior abdominal wall vessel and obscure an injury.
If an injury occurs, leave the trocars in place until a plan for repair is devised. First, start by compressing the bleeding point by moving the cannula against it. Because there are two bleeding ends, the vessels must be sutured cephalad and caudad to the site of injury. The use of electrosurgical desiccation is usually less successful.25 In obese patients we prefer to suture-ligate the bleeder intracorporeally or use a laparoscopic port closure device. In thin and pediatric patients, percutaneous suture ligation can be done easily.
Another option to control bleeding at the cannula site is placement of a Foley catheter to tamponade the vessel using a large balloon placed on tension.27 If abdominal loop sutures are used to control bleeding, the sutures typically are left intact for 8 hours prior to removal.25 If identification of the bleeding point is difficult, percutaneous placement of a suture ligature over a roll of gauze or using a Foley catheter to tamponade the bleeder can be helpful.
CASE RESOLVED
A laparoscopic port closure device is used to suture ligate the bleeding vessel. Hemostasis is achieved and the laparoscopic hysterectomy is completed without further complications.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Share your thoughts by sending a letter to [email protected]. Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
CASE: OBESE PATIENT REQUESTS TOTAL LAPAROSCOPIC HYSTERECTOMY
A 45-year-old woman (G2P2), who delivered both children by cesarean section, schedules an office visit for a complaint of abnormal uterine bleeding. She is obese, with a body mass index (BMI) of 35 kg/m2, and has an enlarged uterus of approximately 14 weeks’ size with minimal descensus. An earlier trial of hormone therapy failed to provide relief. After you counsel her extensively about her treatment options, she elects to undergo total laparoscopic hysterectomy.
What anatomy would you review to help ensure the procedure’s success?
Although the vaginal route is preferred for hysterectomy, total laparoscopic hysterectomy is another minimally invasive option that offers lower morbidity and a shorter hospital stay than the abdominal approach.1 Perhaps more than any other variable, the key to safe, efficient, and effective laparoscopic surgery is a comprehensive knowledge of anatomy. For example, a thorough understanding of the anatomy of the anterior abdominal wall is critical to laparoscopic entry.2,3 Also, pelvic anatomy visualized two-dimensionally under magnification during traditional laparoscopy can look very different than it does during conventional surgery, due to the effects of the pneumoperitoneum, steep Trendelenburg position, and/or the use of uterine manipulators.3
The abdominal cavity is traditionally divided into nine regions. Regardless of the quadrants chosen for laparoscopic access, thorough knowledge of the relevant surface anatomy increases patient safety during surgery (FIGURE 1).
PRIMARY PORT PLACEMENT
Primary port placement, including insertion of the Veress needle, accounts for approximately 40% of laparoscopic complications.4 To help minimize complications, surgeons should ensure that the operating table remains level during placement. As the patient is moved into the Trendelenburg position, the great vessels are more in line with the 45-degree angle that most surgeons use when placing their Veress needle and primary trocar, which can lead to an increased risk of injury. Thus, proper positioning in relationship to anatomy is critical to successful laparoscopic surgery.
Veress or closed technique
Most gynecologists employ the closed method or Veress needle approach to establish pneumoperitoneum.5,6 an initial intraperitoneal pressure below 10 mm Hg, regardless of a woman’s body habitus, height, or age, indicates correct placement of the Veress needle.7,8 Vilos and colleagues demonstrated that Veress intraperitoneal pressure correlates positively with a woman’s weight and BMI and correlates negatively with her parity.8
Hasson or open technique
During the Hasson or open technique, many surgeons use the umbilical ring to gain entry into the abdominal cavity.9 Many view the umbilical ring as a window into the anterior abdominal wall, through which access to the peritoneal cavity can be achieved, but it can also be a site of hernia development.10 The shape of the umbilical ring can vary, appearing round or oval, but it also can be obliterated, slitted, or covered completely by a connecting band, which can result in more difficult laparoscopic entry.10
Palmer’s point
In the 1940s, the French gynecologist Raoul Palmer advocated placing the laparoscope at a point in the left midclavicular line, approximately 3 cm caudal to the costal margin, because visceral-parietal adhesions rarely were found there. Gynecologists still favor this entry site when intra-abdominal adhesions are likely, especially in patients with a history of significant adhesions or multiple previous pelvic surgeries.11 In a study published by Agarwala and colleagues, which included 504 patients with intra-abdominal adhesions, left upper quadrant entry was found to be safe with a complication rate as low as 0.39%.12
If supraumbilical or left upper quadrant port sites are used, the surface anatomy of the spleen and stomach become relevant. The portion of the stomach that is in contact with the abdominal wall is represented roughly by a triangular area extending between the tip of the 10th left costal cartilage, the tip of the ninth right cartilage, and the end of the eighth left costal cartilage.13 The size and shape of the stomach differs by position. Some authors recommend emptying the stomach using a nasogastric or oral gastric tube prior to port insertion to avoid injury.12
The spleen can be mapped using the 10th rib as representing its long axis; vertically, the spleen is situated between the upper border of the ninth and lower border of the 11th ribs.13 In patients without splenic enlargement, the spleen should not be found below the rib cage.
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (Surgical Technique, August 2013)
CASE CONTINUED
On the day of surgery, your patient is brought to the operating room. you use the Veress needle for insufflation. Your opening pressure is 5 mm Hg. You know that an opening pressure of less than 10 mm Hg indicates proper placement, so you continue on to place a 10-mm port. After inserting the primary umbilical port through the umbilicus, you decide to insert secondary ports through lower quadrants. Upon insertion, you note active bleeding at one of the secondary port sites.
How do you proceed?
VASCULAR ANATOMY OF THE ANTERIOR ABDOMINAL WALL
Understanding anterior abdominal wall anatomy and the course of the deep inferior and superficial epigastric vessels is essential to the safe placement of secondary laparoscopic ports. epigastric vessels are the most commonly injured vessels during laparoscopic surgery.14,15 The inferior epigastric vessels originate at the external iliac, immediately above the inguinal ligament. They course medially to the round ligament and travel beneath the lateral third of the rectus abdominis muscle. Using anterior abdominal wall landmarks, the inferior epigastric artery can be identified midway between the anterior superior iliac spine and the pubic symphysis as it travels toward the umbilicus. The inferior epigastric artery also serves as the lateral boundary of Hesselbach’s triangle; the other two boundaries are the lateral edge of the rectus abdominis and the medial aspect of the inguinal ligament (FIGURE 2).13
As the inferior epigastric vessels course cranially, the distance from the midline
decreases. the average distance from the midline at the pubis is approximately
7.5 cm. At the umbilicus, it is approximately 4.6 cm.16,17 The most efficient way to identify laparoscopically the inferior epigastric vessel is to first identify the round ligament. This can be done using a uterine manipulator to deviate the uterus to the contralateral side. Then observe the course of the inferior epigastric vessel just medial to the entry of the round ligament into the inguinal canal. The laparoscopic surgeon can then follow the course of the inferior epigastric vessels to determine the safest location for placement of secondary ports. Transillumination can identify the superficial epigastric vessels, which course within the subcutaneous tissue of the anterior abdominal wall, although it doesn’t identify the deep inferior epigastric vessels that are beneath the lateral third of the rectus muscle. The superficial epigastric vessels follow a course similar to that of the deep inferior epigastric vessels, however, and can serve as a surrogate to guide safe placement of accessory ports.17
Landmarks of the anterior abdominal wall during laparoscopic visualization can also guide placement of secondary ports. The median umbilical fold, which is the peritoneal covering of the umbilical ligament/urachus, travels between the bladder and umbilicus in the midline anterior abdominal wall. Immediately lateral is the medial umbilical fold, which is the peritoneal covering of the obliterated umbilical artery, a branch of the superior vesical artery that comes off the anterior trunk of the internal iliac artery.2 The lateral umbilical folds are lateral to the medial umbilical fold and are the peritoneal covering of the deep inferior epigastric vessels. Identification of these anterior abdominal wall landmarks can assist the surgeon in placing lateral ports so as to avoid injury to these vessels.
Related article: How to avoid major vessel injury during gynecologic laparoscopy Michael Baggish, MD (Surgical Technique, August 2012)
MAJOR RETROPERITONEAL VESSELS
Although major retroperitoneal vessel injury is uncommon, occurring in only 0.3% to 1.0% of laparoscopic surgeries, it has the potential to be catastrophic.18 Therefore, an understanding of the surface anatomy of the major vessels is essential for midline port placement.
The abdominal aorta begins about 4 cm above the transpyloric line and extends to
2 cm inferior and to the left of the umbilicus, or, more accurately, to a point 2 cm left of the middle line on a line that passes through the highest points of the iliac crests. The point of termination of the abdominal aorta corresponds to the level of the fourth lumbar vertebra; a line drawn from it to a point midway between the anterior superior iliac spine and the symphysis pubis indicates the common and external iliac arteries. The common iliac is represented by the upper third of this line and the external iliac, by the remaining two-thirds.13
Related article: How to avoid intestinal and urinary tract injuries during gynecologic laparoscopy Michael Baggish, MD (Surgical Technique, October 2012)
OBESITY AND LAPAROSCOPIC SURGERY
Over two-thirds of the US adult population is now classified as overweight or obese.19 Research has shown that, compared with abdominal hysterectomy, laparoscopic surgery entails a shorter hospital stay, less blood loss, and fewer abdominal wall and wound infections, which are important advantages for this particular population.20
Laparoscopic entry can be particularly challenging in the obese patient. A study by Hurd and colleagues showed that the mean umbilical location was 2.4 cm caudal to the bifurcation of the aorta in the overweight population and 2.9 cm caudal in the obese population.21 Because the bifurcation of the aorta is more cephalad to the umbilicus in overweight and obese patients, the laparoscopic surgeon can introduce the Veress needle at a steeper angle and more perpendicular to the abdominal wall than for a thinner patient (FIGURE 3).
RELEVANT NERVES OF THE ANTERIOR ABDOMINAL WALL
The iliohypogastric and ilioinguinal nerves are also at risk for injury with laterally placed trocars through direct trauma or nerve entrapment. These nerves emerge from the T12 to L1 and L1 to L2 regions, respectively, and course through the muscles of the anterior abdominal wall. Specifically, the iliohypogastric nerve penetrates the fascia of the internal oblique muscle, and the ilioinguinal nerve penetrates the fascia of the transverse abdominus muscle.22 Fascial closure at lateral port sites can also increase the risk of injury to those nerves (FIGURE 4).23
CASE CONTINUED
As you continue your case, you have had to replace your right lower quadrant port several times. During the last insertion, you notice that you have an enlarging abdominal wall hematoma. You suspect that you have injured the inferior epigastric vessel.
How should it be repaired?
HOW TO PREVENT AND REPAIR INJURED DEEP INFERIOR EPIGASTRIC VESSELS
A thorough knowledge of anatomy is the most effective way to prevent these types of injuries. The use of bladeless radially expanding trocars and smooth conical-tip trocars that push the vessels away may result in fewer port-site bleeding complications and injuries than large pyramidal or cutting trocars.24–26 It is important to inspect all ports sites at the end of any laparoscopic procedure because the port itself can tamponade an injured anterior abdominal wall vessel and obscure an injury.
If an injury occurs, leave the trocars in place until a plan for repair is devised. First, start by compressing the bleeding point by moving the cannula against it. Because there are two bleeding ends, the vessels must be sutured cephalad and caudad to the site of injury. The use of electrosurgical desiccation is usually less successful.25 In obese patients we prefer to suture-ligate the bleeder intracorporeally or use a laparoscopic port closure device. In thin and pediatric patients, percutaneous suture ligation can be done easily.
Another option to control bleeding at the cannula site is placement of a Foley catheter to tamponade the vessel using a large balloon placed on tension.27 If abdominal loop sutures are used to control bleeding, the sutures typically are left intact for 8 hours prior to removal.25 If identification of the bleeding point is difficult, percutaneous placement of a suture ligature over a roll of gauze or using a Foley catheter to tamponade the bleeder can be helpful.
CASE RESOLVED
A laparoscopic port closure device is used to suture ligate the bleeding vessel. Hemostasis is achieved and the laparoscopic hysterectomy is completed without further complications.
Tell us what you think!
Drop us a line and let us know what you think about this or other current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Share your thoughts by sending a letter to [email protected]. Please include the city and state in which you practice. Stay in touch! Your feedback is important to us!
- AAGL position statement: Route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1–3.
- Tokar B, Yucel F. Anatomical variations of medial umbilical ligament: clinical significance in laparoscopic exploration of children. Pediatr Surg Int. 2009;25(12):1077–1080.
- Nezhat CH, Nezhat F, Brill AI, Nezhat C. Normal variations of abdominal and pelvic anatomy evaluated at laparoscopy. Obstet Gynecol. 1999;94(2):238–242.
- Fuller J, Ashar BS, Carey-Corrado J. Trocar-associated injuries and fatalities: an analysis of 1,399 reports to the FDA. J Minim Invasive Gynecol. 2005;12(4):302–307.
- Jansen FW, Kolkman W, Bakkum EA, de Kroon CD, Trimbos-Kemper TC, Trimbos JB. Complications of laparoscopy: An inquiry about closed- versus open-entry technique. Am J Obstet Gynecol. 2004;190(3):634–638.
- Yuzpe AA. Pneumoperitoneum needle and trocar injuries in laparoscopy. A survey on possible contributing factors and prevention. J Reprod Med. 1990;35(5):485–490.
- Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10(3):415–420.
- Vilos AG, Vilos GA, Abu-Rafea B, Hollett-Caines J, Al-Omran M. Effect of body habitus and parity on the initial Veress intraperitoneal CO2 insufflation pressure during laparoscopic access in women. J Minim Invasive Gynecol. 2006;13(2):108–113.
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110(6):886–887.
- Oh CS, Won HS, Kwon CH, Chung IH. Morphologic variations of the umbilical ring, umbilical ligaments and ligamentum teres hepatis. Yonsei Med J. 2008;49(6):1004–1007.
- Chandler JG, Corson SL, Way LW. Three spectra of laparoscopic entry access injuries. J Am Coll Surg. 2001;192(4):478–491.
- Agarwala N, Liu CY. Safe entry techniques during laparoscopy: left upper quadrant entry using the ninth intercostal space—a review of 918 procedures. J Minim Invasive Gynecol. 2005;12(1):55–61.
- Williams PL, Warwick R, eds. Gray’s anatomy. 36th ed. Philadelphia, PA: Churchill Livingstone; 1980.
- Hurd WW, Pearl ML, DeLancey JO, Quint EH, Garnett B, Bude RO. Laparoscopic injury of abdominal wall blood vessels: A report of three cases. Obstet Gynecol. 1993;82(4 Pt 2 Suppl):673–676.
- Lin P, Grow DR. Complications of laparoscopy. Strategies for prevention and cure. Obstet Gynecol Clin North Am. 1999;26(1):23–38, v.
- Saber AA, Meslemani AM, Davis R, Pimentel R. Safety zones for anterior abdominal wall entry during laparoscopy: A CT scan mapping of epigastric vessels. Ann Surg. 2004;239(2):182–185.
- Hurd WW, Bude RO, DeLancey JO, Newman JS. The location of abdominal wall blood vessels in relationship to abdominal landmarks apparent at laparoscopy. Am J Obstet Gynecol. 1994;171(3):642–646.
- Sandadi S, Johannigman JA, Wong VL, Blebea J, Altose MD, Hurd WW. Recognition and management of major vessel injury during laparoscopy. J minim invasive gynecol. 2010;17(6):692–702.
- Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiol rev. 2007;29:6–28.
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane database syst rev. 2009;(3):CD003677.
- Hurd WW, Bude RO, DeLancey JO, Pearl ML. The relationship of the umbilicus to the aortic bifurcation: implications for laparoscopic technique. Obstet gynecol. 1992;80(1):48–51.
- Clarke-Pearson DL, Geller EJ. Complications of hysterectomy. Obstet gynecol. 2013;121(3):654–673.
- Shin JH, Howard FM. Abdominal wall nerve injury during laparoscopic gynecologic surgery: incidence, risk factors, and treatment outcomes. J minim invasive gynecol. 2012;19(4):448–453.
- Bhoyrul S, Payne J, Steffes B, Swanstrom L, Way LW. A randomized prospective study of radially expanding trocars in laparoscopic surgery. J gastrointest surg. 2000;4(4):392–397.
- Shirk GJ, Johns A, Redwine DB. Complications of laparoscopic surgery: How to avoid them and how to repair them. J minim invasive gynecol. 2006;13(4):352–361.
- Tews G, Arzt W, Bohaumilitzky T, Füreder R, Frölich H. Significant reduction of operational risk in laparoscopy through the use of a new blunt trocar. Surg gynecol obstet. 1991;173(1):67–68.
- Najmaldin A, Guillou P. A guide to laparoscopic surgery. 1st ed. Wiley-Blackwell; 1998.
- AAGL position statement: Route of hysterectomy to treat benign uterine disease. J Minim Invasive Gynecol. 2011;18(1):1–3.
- Tokar B, Yucel F. Anatomical variations of medial umbilical ligament: clinical significance in laparoscopic exploration of children. Pediatr Surg Int. 2009;25(12):1077–1080.
- Nezhat CH, Nezhat F, Brill AI, Nezhat C. Normal variations of abdominal and pelvic anatomy evaluated at laparoscopy. Obstet Gynecol. 1999;94(2):238–242.
- Fuller J, Ashar BS, Carey-Corrado J. Trocar-associated injuries and fatalities: an analysis of 1,399 reports to the FDA. J Minim Invasive Gynecol. 2005;12(4):302–307.
- Jansen FW, Kolkman W, Bakkum EA, de Kroon CD, Trimbos-Kemper TC, Trimbos JB. Complications of laparoscopy: An inquiry about closed- versus open-entry technique. Am J Obstet Gynecol. 2004;190(3):634–638.
- Yuzpe AA. Pneumoperitoneum needle and trocar injuries in laparoscopy. A survey on possible contributing factors and prevention. J Reprod Med. 1990;35(5):485–490.
- Vilos GA, Vilos AG. Safe laparoscopic entry guided by Veress needle CO2 insufflation pressure. J Am Assoc Gynecol Laparosc. 2003;10(3):415–420.
- Vilos AG, Vilos GA, Abu-Rafea B, Hollett-Caines J, Al-Omran M. Effect of body habitus and parity on the initial Veress intraperitoneal CO2 insufflation pressure during laparoscopic access in women. J Minim Invasive Gynecol. 2006;13(2):108–113.
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110(6):886–887.
- Oh CS, Won HS, Kwon CH, Chung IH. Morphologic variations of the umbilical ring, umbilical ligaments and ligamentum teres hepatis. Yonsei Med J. 2008;49(6):1004–1007.
- Chandler JG, Corson SL, Way LW. Three spectra of laparoscopic entry access injuries. J Am Coll Surg. 2001;192(4):478–491.
- Agarwala N, Liu CY. Safe entry techniques during laparoscopy: left upper quadrant entry using the ninth intercostal space—a review of 918 procedures. J Minim Invasive Gynecol. 2005;12(1):55–61.
- Williams PL, Warwick R, eds. Gray’s anatomy. 36th ed. Philadelphia, PA: Churchill Livingstone; 1980.
- Hurd WW, Pearl ML, DeLancey JO, Quint EH, Garnett B, Bude RO. Laparoscopic injury of abdominal wall blood vessels: A report of three cases. Obstet Gynecol. 1993;82(4 Pt 2 Suppl):673–676.
- Lin P, Grow DR. Complications of laparoscopy. Strategies for prevention and cure. Obstet Gynecol Clin North Am. 1999;26(1):23–38, v.
- Saber AA, Meslemani AM, Davis R, Pimentel R. Safety zones for anterior abdominal wall entry during laparoscopy: A CT scan mapping of epigastric vessels. Ann Surg. 2004;239(2):182–185.
- Hurd WW, Bude RO, DeLancey JO, Newman JS. The location of abdominal wall blood vessels in relationship to abdominal landmarks apparent at laparoscopy. Am J Obstet Gynecol. 1994;171(3):642–646.
- Sandadi S, Johannigman JA, Wong VL, Blebea J, Altose MD, Hurd WW. Recognition and management of major vessel injury during laparoscopy. J minim invasive gynecol. 2010;17(6):692–702.
- Wang Y, Beydoun MA. The obesity epidemic in the United States—gender, age, socioeconomic, racial/ethnic, and geographic characteristics: A systematic review and meta-regression analysis. Epidemiol rev. 2007;29:6–28.
- Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane database syst rev. 2009;(3):CD003677.
- Hurd WW, Bude RO, DeLancey JO, Pearl ML. The relationship of the umbilicus to the aortic bifurcation: implications for laparoscopic technique. Obstet gynecol. 1992;80(1):48–51.
- Clarke-Pearson DL, Geller EJ. Complications of hysterectomy. Obstet gynecol. 2013;121(3):654–673.
- Shin JH, Howard FM. Abdominal wall nerve injury during laparoscopic gynecologic surgery: incidence, risk factors, and treatment outcomes. J minim invasive gynecol. 2012;19(4):448–453.
- Bhoyrul S, Payne J, Steffes B, Swanstrom L, Way LW. A randomized prospective study of radially expanding trocars in laparoscopic surgery. J gastrointest surg. 2000;4(4):392–397.
- Shirk GJ, Johns A, Redwine DB. Complications of laparoscopic surgery: How to avoid them and how to repair them. J minim invasive gynecol. 2006;13(4):352–361.
- Tews G, Arzt W, Bohaumilitzky T, Füreder R, Frölich H. Significant reduction of operational risk in laparoscopy through the use of a new blunt trocar. Surg gynecol obstet. 1991;173(1):67–68.
- Najmaldin A, Guillou P. A guide to laparoscopic surgery. 1st ed. Wiley-Blackwell; 1998.
Pessaries for vaginal prolapse: Critical factors to successful fit and continued use
CASE 1. EARLY-STAGE PELVIC ORGAN PROLAPSE
AC is a 64-year-old white woman with early stage III anterior and apical pelvic organ prolapse (POP). The prolapse is now affecting her ability to do some of the things that she enjoys, such as gardening and golfing.
She has hypertension controlled with medication and no other significant medical issues except mild arthritic changes in her hands and hips. She reports being sexually active with her husband on roughly a weekly basis.
On examination, the leading edge of her prolapse is the anterior vaginal wall, protruding 1 cm beyond the introitus, and the cervix is at the hymenal ring. There is no significant posterior wall prolapse.
After she is counseled about all possible treatment approaches for her early-stage POP, the patient elects to try the vaginal pessary. Now, it is your job to determine the optimal pessary based on the extent of her condition and to educate her about the potential side effects and best practices for its ongoing use.
The vaginal pessary is an important component of a gynecologist’s armamentarium. It is a low-risk, cost-effective, nonsurgical treatment option for the management of POP and genuine stress urinary incontinence (SUI).1,2 It is unfortunate that training in North America typically provides clinicians with only a cursory experience with pessary selection and care, minimizing the device’s importance as a viable tool in a practitioner’s ongoing practice. In fact, most clinicians tend to view the pessary with a mixture of reluctance and disregard.
This is regrettable, as a majority (89%) of patients can be successfully fitted with a pessary,3 regardless of their stage or site of prolapse.4 Although high-stage prolapse does not predict failure, ring pessaries are used most successfully with stage II (100%) and stage III (71%) prolapse, while Gellhorn pessaries are most successful with stage IV (64%) prolapse.5
In this article we review the several pessary options available to clinicians, as well as how to insert them and the best scenarios for their use. We also discuss the key requirements for patient assessment and in-office fitting (meant to optimize the fit and, thereby, the success of use), the possible side effects of pessary use that patients need to be aware of, and appropriate follow-up.
WHEN IS A PESSARY YOUR BEST MANAGEMENT APPROACH?
There are several indications for pessary use,6 namely when:
- the patient has significant comorbid risk factors for surgery
- the patient prefers a nonsurgical alternative
- a goal is to avoid reoperation
- POP or cervical insufficiency is present during pregnancy
- the patient desires future fertility
- surgery must be delayed due to treatment of vaginal ulcerations
- the pessary will be used as a postoperative adjunct to mesh-based repair.
Pessaries have very few contraindications (TABLE). However, factors that do negatively affect successful fitting include:
- prior pelvic surgery
- multiparity
- obesity
- SUI
- short vaginal length (<7 cm)
- wide vaginal introitus (>4 fingerbreadths)
- significant posterior vaginal wall defect.5,7-9
There are two main categories of vaginal pessaries: support and space-filling. All pessaries come in different sizes and shapes. Most are made of medical-grade silicone, rendering them durable and autoclavable as well as resistant to absorption of vaginal discharge and odors. The ring pessary with support is the most commonly used support pessary. The Gellhorn pessary is the most commonly used space-filling pessary. It is used as a second-line treatment for patients unable to retain the ring-with-support pessary.
Related Article: Pessary and pelvic floor exercises for incontinence—are two better than one? G. Willy Davila, MD (Examining the Evidence, May 2010)
SUPPORT PESSARY OPTIONS
The support pessaries are used to treat SUI and POP. These pessaries typically are the easiest types for patients to use because they are more comfortable and simpler to remove and insert than space-filling pessaries. For example, a ring pessary is two-dimensional and lies perpendicular to the long axis of the vagina, allowing patients to have intercourse with it in place. Support-type pessaries include the ring, Gehrung, Shaatz, and lever.
Ring
This is the most commonly used pessary because it fits most women. There are four types of ring pessaries: the ring (FIGURE 1A), ring with support (FIGURE 1B), incontinence ring, and incontinence ring with support. The ring pessary is appropriate for all stages of POP. The ring with support has a diaphragm that is useful in women who have uterine prolapse with or without cystocele. The incontinence ring has a knob that is placed beneath the urethra to increase urethral pressure and is useful in cases of SUI.
Insertion. Fold the pessary by bringing the two small holes together, and lubricate the leading edge. Insert it past the introitus with the folded edge facing down. Allow the pessary to reopen, and direct it behind the cervix into the posterior fornix (FIGURE 2). Give it a slight twist with your index finger to prevent expulsion.
To see insertion demonstrated, watch Vaginal pessaries: An instructional video
Gehrung
This pessary is designed with an arch-shaped malleable rim with wires incorporated into the arms (FIGURE 3). Use of the Gehrung pessary is rare; it is most often used in women with cystocele or rectocele.
Insertion. Fold the pessary to insert it into the vagina. Upon insertion, keep both heels of the pessary parallel to the posterior vagina with the back arch pushed over the cervix in the anterior fornix and the front arch resting behind the symphysis pubis. The concave surface and diaphragm support the anterior vagina. Place the convex portion of the curve beneath the bulge. The two bases rest on the posterior vagina against the lateral levator muscles.
Shaatz
This support pessary has a circular base similar to the Gellhorn pessary but without the rigid stem (FIGURE 4).
Insertion. Because it is stiff, insert this pessary vertically and then turn it to a horizontal position once it is inside the vagina.
Lever
The Hodge, Smith, and Risser pessaries are collectively called the lever pessaries. They are used to manage uterine retroversion and POP. They are rarely used.
The Hodge pessary is beneficial to patients with a narrow vaginal introitus, mild cystocele, and cervical insufficiency. The anterior portion of a Hodge pessary is rectangular (FIGURE 5A).
The Smith pessary is useful for patients with well-defined pubic notches because the anterior portion is rounded (FIGURE 5B).
For patients with a very shallow pubic notch, the Risser pessary is useful. The Risser’s anterior portion is rectangular with indentation but wider than the Hodge pessary (FIGURE 5C).
Insertion. Fold the pessary and insert it into the vagina with the index finger on the posterior curved bar until the pessary rests behind the cervix and the anterior horizontal bar rests behind the symphysis pubis.
SPACE-OCCUPYING PESSARIES
The second pessary category is the space-filling pessary. These pessaries are used primarily to support severe POP, especially posthysterectomy vaginal vault prolapse. They have larger bases to support the vaginal apex or cervix; therefore, they are more difficult to insert and remove. When this pessary type is in place, sexual intercourse is not possible. Examples include the Gellhorn, donut, cube, and inflatable pessaries.
Gellhorn
The Gellhorn pessary is the most commonly used space-filling pessary. It has a broad base with a stem (FIGURE 6). The broad base supports the vaginal apex while the stem keeps the circular base from rotating and prevents pessary expulsion. The stem comes in long or short lengths. The concave base provides vaginal suction and keeps the pessary in place. The holes in the stem and base provide vaginal drainage. The Gellhorn pessary is useful for women with more advanced prolapse and less perineal support.
Insertion. Folding one side of the base to the stem, insert the Gellhorn pessary vertically inside the vagina. To facilitate insertion, separate the labia with the nondominant hand or depress the perineum with the index finger. Once the circular base is inside the vagina, push the pessary upward until the tip of the stem is just inside the vaginal introitus (FIGURE 7). Many medical illustrations inaccurately depict the Gellhorn pessary in a final placement that appears too high in the pelvis. This figure, which has the patient in a standing position, shows how low in the pelvis this space-filling pessary can sit in a patient with advanced prolapse.
Remove this pessary by gently pulling the stem while inserting the opposite hand beneath an edge of the pessary base to break the vaginal suction (Watch Vaginal pessaries: An instructional video).
Donut
The donut pessary is used for advanced prolapse because it fills a larger space. It is difficult to insert and remove because it is large, thick, and hollow (FIGURE 8).
Insertion. Insert it vertically and, once it is placed inside the vagina, rotate it to a horizontal position. A Kelly clamp can be used to grasp the pessary and facilitate removal.
Cube
The cube pessary supports third-degree uterine prolapse by holding the vaginal wall with suction (FIGURE 9). Because of the risk of vaginal erosion and lack of drainage in some designs, the cube pessary requires nightly removal and cleaning.
Insertion. Squeezing the pessary with the thumb, index, and middle fingers, insert the cube pessary at the vaginal apex.
Removal requires breaking the suction by placing a fingertip between the vaginal mucosa and the pessary and compressing the cube between the thumb and forefinger to remove. Gently tugging on the string also helps with removal.
Inflatable
This space-filling pessary is an air-filled ball that is inflated via an attached stem that also enables insertion and removal. The older Inflatoball pessary is made of latex, so its use is contraindicated in patients with latex allergy. Newer inflatable pessaries are silicone-based and consist of an air-filled donut, a stem with a valve, and an air pump (FIGURE 10). Some models also include a deflation key. The inflatable pessary comes in small, medium, large, and extra-large sizes. This pessary type must be removed and cleaned daily.
Insertion. Place the deflated pessary into the vagina. Move the ball-bearing valve within the stem (which controls the air flow) to a lateral projection on the side of the stem. To inflate, attach the inflation bulb. (Inflation typically requires 3 to 5 pumps of the bulb.) Move the ball bearing back into position to maintain the inflation, then detach the bulb. You can leave the stem outside the body or tuck it gently into the introitus (FIGURE 11).
INCONTINENCE PESSARIES
These devices are used specifically for SUI. The incontinence ring (FIGURE 12) and incontinence dish pessaries compress the urethra against the pubic symphysis. The knob is placed beneath the urethra, increasing the urethral closure pressure and thereby preventing urinary incontinence.
Related Article: Update on Urinary Incontinence Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (December 2011)
CASE 1 CONCLUDED
Given that AC has early-stage POP and is sexually active, a space-occupying pessary is not the optimal choice. Instead, a ring pessary with support is fitted for her trial.
What side effects might a patient anticipate with pessary use?
Vaginal discharge and slight odor are common. Pessary removal and cleaning are usually adequate to eliminate them. Temporary discontinuation of pessary use may be warranted until symptoms subside. If these maneuvers do not resolve the issue, then the patient should be examined to rule out other sources of infection.
Vaginal bleeding. Bleeding from vaginal abrasion and ulceration could be caused by trauma from pessary removal or vaginal impingement. Evaluation is warranted for any vaginal bleeding.
Changes in urinary function. Less commonly, women using a pessary may notice changes in their urinary function. Many women with anterior or apical prolapse will have altered urine streams with slow or trickling flow and possible hesitation upon initiation of voiding.
Alternatively, pessary placement may instigate stress-type incontinence akin to that seen after prolapse surgery. Changing pessary size may alleviate this condition. Otherwise, these side effects may reduce a patient’s willingness to continue pessary use.
How can a patient optimize her use of a pessary?
A patient can remove the pessary on a periodic basis or try to use it continuously. If she cannot or will not remove the pessary, then she will need to come back for scheduled visits, as described in the sidebar, “Essential components of a successfully fitted pessary.” If she is able to remove the pessary on her own, then she can use the device as needed or remove it for intercourse (though it is not necessary). She must remove it weekly, at a minimum, however, to both clean the pessary and give the vaginal walls a “rest,” which can minimize the potential for abrasions or erosions
ESSENTIAL COMPONENTS OF A SUCCESSFULLY FITTED PESSARY
Patient assessment
Accurate selection and placement of a pessary requires appropriate examination and fitting, beginning with determination of the patient’s stage of prolapse and introitus. Key steps include:
– Examine the patient with an empty bladder in the lithotomy position
– Perform bimanual pelvic and speculum examination using a Sims speculum (or bivalve speculum broken in half) with the patient in a supine position
– Administer the Pelvic Organ Prolapse Quantification (POP-Q) exam
– Perform digital examination
– Assess vaginal atrophy, vaginal introitus, and vaginal width and length
– Evaluate pelvic floor muscle strength (Kegel squeeze).
Next, gauge the correct pessary size by approximating the number of fingerbreadths accommodated across the vaginal width.
Another method of estimating pessary size is to insert two fingers inside the vagina and estimate the distance between the posterior fornix and the posterior pubic symphysis (Watch Vaginal pessaries: An instructional video). An easy reference is to start with a size 3 or 4 ring pessary if the vaginal introitus is 1 to 2 fingerbreadths in width and the prolapse is stage II to III. If the vagina accommodates 3 to 4 fingerbreadths, or there is stage IV prolapse, use a Gellhorn pessary.
Here are the different types of pessaries and the most common sizes available. (Pessary sizes change in quarter-inch increments.)
In-office trial
Insert the pessary into the vagina using the dominant hand. Using the nondominant hand, separate the introitus and depress the perineal body. Apply a small amount of lubricant to the leading edge of the pessary.
After insertion, ask the patient to strain and cough, ambulate in the office, and void. Reexamine the patient to ensure that the pessary is still in the correct position and that placement has not shifted. Perform the cough leak test with the patient in a standing position and the pessary in place. Re-examine the patient while she is in a standing position. Use the largest pessary that is comfortable for her. Advise her to bring the pessary back to the office if it gets expelled.
This is a trial-and-error process; advise the patient of this. It may require a trial of several styles and sizes to find the right pessary fit. Once you find the correct size, document the final pessary size.
Follow-up
Schedule a follow-up appointment 1 to 2 weeks after insertion. Ask the patient whether she has experienced any discomfort, malodorous discharge, or vaginal bleeding. Also inquire about any changes in urinary habits or bowel movements and related complaints.
Remove the pessary and clean it with mild soap and water. Examine the vagina for pressure points, abrasions, ulcerations, and erosions.
Teach the patient how to remove, clean, and reinsert the pessary, and advise her to perform these tasks on a weekly basis.
Schedule a follow-up visit in 1 to 2 months, and another visit 6 to 12 months after that.
CASE 2. ADVANCED-STAGE POP
BD is an 82-year-old widow (G5P4014) with stage IV vaginal prolapse. She has noticed some scant blood staining on her clothing. She frequently voids small amounts of urine but never feels complete relief. She defecates normally.
Her medical history is significant for coronary artery disease with prior myocardial infarction, with multiple stent placements over the years. She has hypertension, reduced ejection fraction, and diabetes. She is morbidly obese and suffers from degenerative joint disease. She had a vaginal hysterectomy several years ago for benign indications.
Upon examination, BD’s prolapse is large, with excoriations and hyperkeratosis of the skin over the prolapse. It is easily reduced in the office.
What is the best pessary for this patient, and how should she be followed and counseled regarding ongoing care?
Since the failure rate for pessary usage increases with advancing prolapse stage, a space-occupying pessary is most appropriate to try initially. A trial with a support pessary could be useful to allow the excoriations to heal and provide a healthier vaginal environment. A Gellhorn pessary is commonly used. An inflatable pessary could be an alternative if the Gellhorn fails to stay in place. The cube pessary, known to cause more abrasions and erosions than other pessaries, is a poor choice given the state of the patient’s vaginal tissues at baseline.
Space-occupying pessaries are more difficult to insert and remove and have a higher risk of pain or trauma. Start with shorter time intervals between visits, eventually spacing them out for the patient’s convenience. The usual interval for follow-up is 3 to 4 months; longer intervals could be offered if the patient is reliable, adherent, and reports no complaints with pessary use.
Related Article: Update on pelvic floor dysfunction: Focus on urinary incontinence Alexis A. Dieter, MD, and Cindy L. Amundsen, MD (November 2013)
OUTCOMES
Only short- and medium-term outcomes for pessary use have been described in the literature. Short-term (2 months) satisfaction and continued use, along with resolution of prolapse, occurred in 92% of patients.7 Previous hysterectomy or prolapse surgery may influence the short-term success of pessary use.10
More than half of sexually active women achieved long-term use (up to 2 years), regardless of prolapse severity. Brincat and colleagues found that long-term pessary use (1 to 2 years) approached 60% in 132 women with both urinary incontinence and prolapse. Women being treated for POP were more likely to continue pessary use than women being treated for SUI.11 Age, parity, estrogen use, and sexual activity were characteristics also studied in pessary fitting. Neither sexual activity nor stage of prolapse was a contraindication to use of a pessary; long-term use was found to be acceptable in sexually active women.11
Successful fitting of a vaginal pessary has been associated with improvement in voiding, urinary and fecal urgency, and incontinence. A vaginal pessary is a viable nonsurgical option for the management of POP and urinary incontinence and remains an optimal minimally invasive approach to such disorders.
CASE 2 CONCLUDED
The patient returns to the clinic 1 month after the original insertion. The pessary is removed, and the vagina is inspected, with no abrasions or ulcerations found. The vaginal cavity and pessary are cleaned with a mild soap-and-water mixture. The pessary is lubricated and reinserted. This process is repeated 2 months later, with subsequent follow-up intervals doubled (up to 6 months between visits) when the patient has no complaints of discharge or odor.
- Colmer, WM Jr. Use of the pessary. Am J Obstet Gynecol. 1953;65(1):170–174.
- Culligan PJ. Nonsurgical management to pelvic organ prolapse. Obstet Gynecol. 2012;119(4):852–860.
- Nygaard IE, Heit M. Stress urinary incontinence. Obstet Gynecol. 2004;104(3):607–620.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 85: Pelvic organ prolapse. Obstet Gynecol. 2007;110(3):717–729.
- Clemons JL, Aguilar VC, Tillinghast TA, Jackson ND, Myers DL. Risk factors associated with an unsuccessful pessary fitting trial in women with pelvic organ prolapse. Am J Obstet Gynecol. 2004;190(2):345–350.
- Clemons JL, Brubaker L, Falk SJ. Vaginal pessary treatment of prolapse and incontinence. UpToDate. http://www.uptodate.com/contents/vaginal-pessary-treatment-of-prolapse-and-incontinence. Updated February 8, 2013. Accessed November 7, 2013.
- Mutone MF, Terry C, Hale DS, Benson JT. Factors which influence the short-term success of pessary management of pelvic organ prolapse. Am J Obstet Gynecol. 2005;193(1):89–94.
- Fernando RJ, Thakar R, Sultan AH, Shah SM, Jones PW. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108(1):93–99.
- Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol. 2005;106(3):615–634.
- Donnelly MJ, Powell-Morgan S, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(5):302–307.
- Brincat C, Kenton K, Fitzgerald MP, et al. Sexual activity predicts continued pessary use. Am J Obstet Gynecol. 2004;191(1):198–200.
CASE 1. EARLY-STAGE PELVIC ORGAN PROLAPSE
AC is a 64-year-old white woman with early stage III anterior and apical pelvic organ prolapse (POP). The prolapse is now affecting her ability to do some of the things that she enjoys, such as gardening and golfing.
She has hypertension controlled with medication and no other significant medical issues except mild arthritic changes in her hands and hips. She reports being sexually active with her husband on roughly a weekly basis.
On examination, the leading edge of her prolapse is the anterior vaginal wall, protruding 1 cm beyond the introitus, and the cervix is at the hymenal ring. There is no significant posterior wall prolapse.
After she is counseled about all possible treatment approaches for her early-stage POP, the patient elects to try the vaginal pessary. Now, it is your job to determine the optimal pessary based on the extent of her condition and to educate her about the potential side effects and best practices for its ongoing use.
The vaginal pessary is an important component of a gynecologist’s armamentarium. It is a low-risk, cost-effective, nonsurgical treatment option for the management of POP and genuine stress urinary incontinence (SUI).1,2 It is unfortunate that training in North America typically provides clinicians with only a cursory experience with pessary selection and care, minimizing the device’s importance as a viable tool in a practitioner’s ongoing practice. In fact, most clinicians tend to view the pessary with a mixture of reluctance and disregard.
This is regrettable, as a majority (89%) of patients can be successfully fitted with a pessary,3 regardless of their stage or site of prolapse.4 Although high-stage prolapse does not predict failure, ring pessaries are used most successfully with stage II (100%) and stage III (71%) prolapse, while Gellhorn pessaries are most successful with stage IV (64%) prolapse.5
In this article we review the several pessary options available to clinicians, as well as how to insert them and the best scenarios for their use. We also discuss the key requirements for patient assessment and in-office fitting (meant to optimize the fit and, thereby, the success of use), the possible side effects of pessary use that patients need to be aware of, and appropriate follow-up.
WHEN IS A PESSARY YOUR BEST MANAGEMENT APPROACH?
There are several indications for pessary use,6 namely when:
- the patient has significant comorbid risk factors for surgery
- the patient prefers a nonsurgical alternative
- a goal is to avoid reoperation
- POP or cervical insufficiency is present during pregnancy
- the patient desires future fertility
- surgery must be delayed due to treatment of vaginal ulcerations
- the pessary will be used as a postoperative adjunct to mesh-based repair.
Pessaries have very few contraindications (TABLE). However, factors that do negatively affect successful fitting include:
- prior pelvic surgery
- multiparity
- obesity
- SUI
- short vaginal length (<7 cm)
- wide vaginal introitus (>4 fingerbreadths)
- significant posterior vaginal wall defect.5,7-9
There are two main categories of vaginal pessaries: support and space-filling. All pessaries come in different sizes and shapes. Most are made of medical-grade silicone, rendering them durable and autoclavable as well as resistant to absorption of vaginal discharge and odors. The ring pessary with support is the most commonly used support pessary. The Gellhorn pessary is the most commonly used space-filling pessary. It is used as a second-line treatment for patients unable to retain the ring-with-support pessary.
Related Article: Pessary and pelvic floor exercises for incontinence—are two better than one? G. Willy Davila, MD (Examining the Evidence, May 2010)
SUPPORT PESSARY OPTIONS
The support pessaries are used to treat SUI and POP. These pessaries typically are the easiest types for patients to use because they are more comfortable and simpler to remove and insert than space-filling pessaries. For example, a ring pessary is two-dimensional and lies perpendicular to the long axis of the vagina, allowing patients to have intercourse with it in place. Support-type pessaries include the ring, Gehrung, Shaatz, and lever.
Ring
This is the most commonly used pessary because it fits most women. There are four types of ring pessaries: the ring (FIGURE 1A), ring with support (FIGURE 1B), incontinence ring, and incontinence ring with support. The ring pessary is appropriate for all stages of POP. The ring with support has a diaphragm that is useful in women who have uterine prolapse with or without cystocele. The incontinence ring has a knob that is placed beneath the urethra to increase urethral pressure and is useful in cases of SUI.
Insertion. Fold the pessary by bringing the two small holes together, and lubricate the leading edge. Insert it past the introitus with the folded edge facing down. Allow the pessary to reopen, and direct it behind the cervix into the posterior fornix (FIGURE 2). Give it a slight twist with your index finger to prevent expulsion.
To see insertion demonstrated, watch Vaginal pessaries: An instructional video
Gehrung
This pessary is designed with an arch-shaped malleable rim with wires incorporated into the arms (FIGURE 3). Use of the Gehrung pessary is rare; it is most often used in women with cystocele or rectocele.
Insertion. Fold the pessary to insert it into the vagina. Upon insertion, keep both heels of the pessary parallel to the posterior vagina with the back arch pushed over the cervix in the anterior fornix and the front arch resting behind the symphysis pubis. The concave surface and diaphragm support the anterior vagina. Place the convex portion of the curve beneath the bulge. The two bases rest on the posterior vagina against the lateral levator muscles.
Shaatz
This support pessary has a circular base similar to the Gellhorn pessary but without the rigid stem (FIGURE 4).
Insertion. Because it is stiff, insert this pessary vertically and then turn it to a horizontal position once it is inside the vagina.
Lever
The Hodge, Smith, and Risser pessaries are collectively called the lever pessaries. They are used to manage uterine retroversion and POP. They are rarely used.
The Hodge pessary is beneficial to patients with a narrow vaginal introitus, mild cystocele, and cervical insufficiency. The anterior portion of a Hodge pessary is rectangular (FIGURE 5A).
The Smith pessary is useful for patients with well-defined pubic notches because the anterior portion is rounded (FIGURE 5B).
For patients with a very shallow pubic notch, the Risser pessary is useful. The Risser’s anterior portion is rectangular with indentation but wider than the Hodge pessary (FIGURE 5C).
Insertion. Fold the pessary and insert it into the vagina with the index finger on the posterior curved bar until the pessary rests behind the cervix and the anterior horizontal bar rests behind the symphysis pubis.
SPACE-OCCUPYING PESSARIES
The second pessary category is the space-filling pessary. These pessaries are used primarily to support severe POP, especially posthysterectomy vaginal vault prolapse. They have larger bases to support the vaginal apex or cervix; therefore, they are more difficult to insert and remove. When this pessary type is in place, sexual intercourse is not possible. Examples include the Gellhorn, donut, cube, and inflatable pessaries.
Gellhorn
The Gellhorn pessary is the most commonly used space-filling pessary. It has a broad base with a stem (FIGURE 6). The broad base supports the vaginal apex while the stem keeps the circular base from rotating and prevents pessary expulsion. The stem comes in long or short lengths. The concave base provides vaginal suction and keeps the pessary in place. The holes in the stem and base provide vaginal drainage. The Gellhorn pessary is useful for women with more advanced prolapse and less perineal support.
Insertion. Folding one side of the base to the stem, insert the Gellhorn pessary vertically inside the vagina. To facilitate insertion, separate the labia with the nondominant hand or depress the perineum with the index finger. Once the circular base is inside the vagina, push the pessary upward until the tip of the stem is just inside the vaginal introitus (FIGURE 7). Many medical illustrations inaccurately depict the Gellhorn pessary in a final placement that appears too high in the pelvis. This figure, which has the patient in a standing position, shows how low in the pelvis this space-filling pessary can sit in a patient with advanced prolapse.
Remove this pessary by gently pulling the stem while inserting the opposite hand beneath an edge of the pessary base to break the vaginal suction (Watch Vaginal pessaries: An instructional video).
Donut
The donut pessary is used for advanced prolapse because it fills a larger space. It is difficult to insert and remove because it is large, thick, and hollow (FIGURE 8).
Insertion. Insert it vertically and, once it is placed inside the vagina, rotate it to a horizontal position. A Kelly clamp can be used to grasp the pessary and facilitate removal.
Cube
The cube pessary supports third-degree uterine prolapse by holding the vaginal wall with suction (FIGURE 9). Because of the risk of vaginal erosion and lack of drainage in some designs, the cube pessary requires nightly removal and cleaning.
Insertion. Squeezing the pessary with the thumb, index, and middle fingers, insert the cube pessary at the vaginal apex.
Removal requires breaking the suction by placing a fingertip between the vaginal mucosa and the pessary and compressing the cube between the thumb and forefinger to remove. Gently tugging on the string also helps with removal.
Inflatable
This space-filling pessary is an air-filled ball that is inflated via an attached stem that also enables insertion and removal. The older Inflatoball pessary is made of latex, so its use is contraindicated in patients with latex allergy. Newer inflatable pessaries are silicone-based and consist of an air-filled donut, a stem with a valve, and an air pump (FIGURE 10). Some models also include a deflation key. The inflatable pessary comes in small, medium, large, and extra-large sizes. This pessary type must be removed and cleaned daily.
Insertion. Place the deflated pessary into the vagina. Move the ball-bearing valve within the stem (which controls the air flow) to a lateral projection on the side of the stem. To inflate, attach the inflation bulb. (Inflation typically requires 3 to 5 pumps of the bulb.) Move the ball bearing back into position to maintain the inflation, then detach the bulb. You can leave the stem outside the body or tuck it gently into the introitus (FIGURE 11).
INCONTINENCE PESSARIES
These devices are used specifically for SUI. The incontinence ring (FIGURE 12) and incontinence dish pessaries compress the urethra against the pubic symphysis. The knob is placed beneath the urethra, increasing the urethral closure pressure and thereby preventing urinary incontinence.
Related Article: Update on Urinary Incontinence Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (December 2011)
CASE 1 CONCLUDED
Given that AC has early-stage POP and is sexually active, a space-occupying pessary is not the optimal choice. Instead, a ring pessary with support is fitted for her trial.
What side effects might a patient anticipate with pessary use?
Vaginal discharge and slight odor are common. Pessary removal and cleaning are usually adequate to eliminate them. Temporary discontinuation of pessary use may be warranted until symptoms subside. If these maneuvers do not resolve the issue, then the patient should be examined to rule out other sources of infection.
Vaginal bleeding. Bleeding from vaginal abrasion and ulceration could be caused by trauma from pessary removal or vaginal impingement. Evaluation is warranted for any vaginal bleeding.
Changes in urinary function. Less commonly, women using a pessary may notice changes in their urinary function. Many women with anterior or apical prolapse will have altered urine streams with slow or trickling flow and possible hesitation upon initiation of voiding.
Alternatively, pessary placement may instigate stress-type incontinence akin to that seen after prolapse surgery. Changing pessary size may alleviate this condition. Otherwise, these side effects may reduce a patient’s willingness to continue pessary use.
How can a patient optimize her use of a pessary?
A patient can remove the pessary on a periodic basis or try to use it continuously. If she cannot or will not remove the pessary, then she will need to come back for scheduled visits, as described in the sidebar, “Essential components of a successfully fitted pessary.” If she is able to remove the pessary on her own, then she can use the device as needed or remove it for intercourse (though it is not necessary). She must remove it weekly, at a minimum, however, to both clean the pessary and give the vaginal walls a “rest,” which can minimize the potential for abrasions or erosions
ESSENTIAL COMPONENTS OF A SUCCESSFULLY FITTED PESSARY
Patient assessment
Accurate selection and placement of a pessary requires appropriate examination and fitting, beginning with determination of the patient’s stage of prolapse and introitus. Key steps include:
– Examine the patient with an empty bladder in the lithotomy position
– Perform bimanual pelvic and speculum examination using a Sims speculum (or bivalve speculum broken in half) with the patient in a supine position
– Administer the Pelvic Organ Prolapse Quantification (POP-Q) exam
– Perform digital examination
– Assess vaginal atrophy, vaginal introitus, and vaginal width and length
– Evaluate pelvic floor muscle strength (Kegel squeeze).
Next, gauge the correct pessary size by approximating the number of fingerbreadths accommodated across the vaginal width.
Another method of estimating pessary size is to insert two fingers inside the vagina and estimate the distance between the posterior fornix and the posterior pubic symphysis (Watch Vaginal pessaries: An instructional video). An easy reference is to start with a size 3 or 4 ring pessary if the vaginal introitus is 1 to 2 fingerbreadths in width and the prolapse is stage II to III. If the vagina accommodates 3 to 4 fingerbreadths, or there is stage IV prolapse, use a Gellhorn pessary.
Here are the different types of pessaries and the most common sizes available. (Pessary sizes change in quarter-inch increments.)
In-office trial
Insert the pessary into the vagina using the dominant hand. Using the nondominant hand, separate the introitus and depress the perineal body. Apply a small amount of lubricant to the leading edge of the pessary.
After insertion, ask the patient to strain and cough, ambulate in the office, and void. Reexamine the patient to ensure that the pessary is still in the correct position and that placement has not shifted. Perform the cough leak test with the patient in a standing position and the pessary in place. Re-examine the patient while she is in a standing position. Use the largest pessary that is comfortable for her. Advise her to bring the pessary back to the office if it gets expelled.
This is a trial-and-error process; advise the patient of this. It may require a trial of several styles and sizes to find the right pessary fit. Once you find the correct size, document the final pessary size.
Follow-up
Schedule a follow-up appointment 1 to 2 weeks after insertion. Ask the patient whether she has experienced any discomfort, malodorous discharge, or vaginal bleeding. Also inquire about any changes in urinary habits or bowel movements and related complaints.
Remove the pessary and clean it with mild soap and water. Examine the vagina for pressure points, abrasions, ulcerations, and erosions.
Teach the patient how to remove, clean, and reinsert the pessary, and advise her to perform these tasks on a weekly basis.
Schedule a follow-up visit in 1 to 2 months, and another visit 6 to 12 months after that.
CASE 2. ADVANCED-STAGE POP
BD is an 82-year-old widow (G5P4014) with stage IV vaginal prolapse. She has noticed some scant blood staining on her clothing. She frequently voids small amounts of urine but never feels complete relief. She defecates normally.
Her medical history is significant for coronary artery disease with prior myocardial infarction, with multiple stent placements over the years. She has hypertension, reduced ejection fraction, and diabetes. She is morbidly obese and suffers from degenerative joint disease. She had a vaginal hysterectomy several years ago for benign indications.
Upon examination, BD’s prolapse is large, with excoriations and hyperkeratosis of the skin over the prolapse. It is easily reduced in the office.
What is the best pessary for this patient, and how should she be followed and counseled regarding ongoing care?
Since the failure rate for pessary usage increases with advancing prolapse stage, a space-occupying pessary is most appropriate to try initially. A trial with a support pessary could be useful to allow the excoriations to heal and provide a healthier vaginal environment. A Gellhorn pessary is commonly used. An inflatable pessary could be an alternative if the Gellhorn fails to stay in place. The cube pessary, known to cause more abrasions and erosions than other pessaries, is a poor choice given the state of the patient’s vaginal tissues at baseline.
Space-occupying pessaries are more difficult to insert and remove and have a higher risk of pain or trauma. Start with shorter time intervals between visits, eventually spacing them out for the patient’s convenience. The usual interval for follow-up is 3 to 4 months; longer intervals could be offered if the patient is reliable, adherent, and reports no complaints with pessary use.
Related Article: Update on pelvic floor dysfunction: Focus on urinary incontinence Alexis A. Dieter, MD, and Cindy L. Amundsen, MD (November 2013)
OUTCOMES
Only short- and medium-term outcomes for pessary use have been described in the literature. Short-term (2 months) satisfaction and continued use, along with resolution of prolapse, occurred in 92% of patients.7 Previous hysterectomy or prolapse surgery may influence the short-term success of pessary use.10
More than half of sexually active women achieved long-term use (up to 2 years), regardless of prolapse severity. Brincat and colleagues found that long-term pessary use (1 to 2 years) approached 60% in 132 women with both urinary incontinence and prolapse. Women being treated for POP were more likely to continue pessary use than women being treated for SUI.11 Age, parity, estrogen use, and sexual activity were characteristics also studied in pessary fitting. Neither sexual activity nor stage of prolapse was a contraindication to use of a pessary; long-term use was found to be acceptable in sexually active women.11
Successful fitting of a vaginal pessary has been associated with improvement in voiding, urinary and fecal urgency, and incontinence. A vaginal pessary is a viable nonsurgical option for the management of POP and urinary incontinence and remains an optimal minimally invasive approach to such disorders.
CASE 2 CONCLUDED
The patient returns to the clinic 1 month after the original insertion. The pessary is removed, and the vagina is inspected, with no abrasions or ulcerations found. The vaginal cavity and pessary are cleaned with a mild soap-and-water mixture. The pessary is lubricated and reinserted. This process is repeated 2 months later, with subsequent follow-up intervals doubled (up to 6 months between visits) when the patient has no complaints of discharge or odor.
CASE 1. EARLY-STAGE PELVIC ORGAN PROLAPSE
AC is a 64-year-old white woman with early stage III anterior and apical pelvic organ prolapse (POP). The prolapse is now affecting her ability to do some of the things that she enjoys, such as gardening and golfing.
She has hypertension controlled with medication and no other significant medical issues except mild arthritic changes in her hands and hips. She reports being sexually active with her husband on roughly a weekly basis.
On examination, the leading edge of her prolapse is the anterior vaginal wall, protruding 1 cm beyond the introitus, and the cervix is at the hymenal ring. There is no significant posterior wall prolapse.
After she is counseled about all possible treatment approaches for her early-stage POP, the patient elects to try the vaginal pessary. Now, it is your job to determine the optimal pessary based on the extent of her condition and to educate her about the potential side effects and best practices for its ongoing use.
The vaginal pessary is an important component of a gynecologist’s armamentarium. It is a low-risk, cost-effective, nonsurgical treatment option for the management of POP and genuine stress urinary incontinence (SUI).1,2 It is unfortunate that training in North America typically provides clinicians with only a cursory experience with pessary selection and care, minimizing the device’s importance as a viable tool in a practitioner’s ongoing practice. In fact, most clinicians tend to view the pessary with a mixture of reluctance and disregard.
This is regrettable, as a majority (89%) of patients can be successfully fitted with a pessary,3 regardless of their stage or site of prolapse.4 Although high-stage prolapse does not predict failure, ring pessaries are used most successfully with stage II (100%) and stage III (71%) prolapse, while Gellhorn pessaries are most successful with stage IV (64%) prolapse.5
In this article we review the several pessary options available to clinicians, as well as how to insert them and the best scenarios for their use. We also discuss the key requirements for patient assessment and in-office fitting (meant to optimize the fit and, thereby, the success of use), the possible side effects of pessary use that patients need to be aware of, and appropriate follow-up.
WHEN IS A PESSARY YOUR BEST MANAGEMENT APPROACH?
There are several indications for pessary use,6 namely when:
- the patient has significant comorbid risk factors for surgery
- the patient prefers a nonsurgical alternative
- a goal is to avoid reoperation
- POP or cervical insufficiency is present during pregnancy
- the patient desires future fertility
- surgery must be delayed due to treatment of vaginal ulcerations
- the pessary will be used as a postoperative adjunct to mesh-based repair.
Pessaries have very few contraindications (TABLE). However, factors that do negatively affect successful fitting include:
- prior pelvic surgery
- multiparity
- obesity
- SUI
- short vaginal length (<7 cm)
- wide vaginal introitus (>4 fingerbreadths)
- significant posterior vaginal wall defect.5,7-9
There are two main categories of vaginal pessaries: support and space-filling. All pessaries come in different sizes and shapes. Most are made of medical-grade silicone, rendering them durable and autoclavable as well as resistant to absorption of vaginal discharge and odors. The ring pessary with support is the most commonly used support pessary. The Gellhorn pessary is the most commonly used space-filling pessary. It is used as a second-line treatment for patients unable to retain the ring-with-support pessary.
Related Article: Pessary and pelvic floor exercises for incontinence—are two better than one? G. Willy Davila, MD (Examining the Evidence, May 2010)
SUPPORT PESSARY OPTIONS
The support pessaries are used to treat SUI and POP. These pessaries typically are the easiest types for patients to use because they are more comfortable and simpler to remove and insert than space-filling pessaries. For example, a ring pessary is two-dimensional and lies perpendicular to the long axis of the vagina, allowing patients to have intercourse with it in place. Support-type pessaries include the ring, Gehrung, Shaatz, and lever.
Ring
This is the most commonly used pessary because it fits most women. There are four types of ring pessaries: the ring (FIGURE 1A), ring with support (FIGURE 1B), incontinence ring, and incontinence ring with support. The ring pessary is appropriate for all stages of POP. The ring with support has a diaphragm that is useful in women who have uterine prolapse with or without cystocele. The incontinence ring has a knob that is placed beneath the urethra to increase urethral pressure and is useful in cases of SUI.
Insertion. Fold the pessary by bringing the two small holes together, and lubricate the leading edge. Insert it past the introitus with the folded edge facing down. Allow the pessary to reopen, and direct it behind the cervix into the posterior fornix (FIGURE 2). Give it a slight twist with your index finger to prevent expulsion.
To see insertion demonstrated, watch Vaginal pessaries: An instructional video
Gehrung
This pessary is designed with an arch-shaped malleable rim with wires incorporated into the arms (FIGURE 3). Use of the Gehrung pessary is rare; it is most often used in women with cystocele or rectocele.
Insertion. Fold the pessary to insert it into the vagina. Upon insertion, keep both heels of the pessary parallel to the posterior vagina with the back arch pushed over the cervix in the anterior fornix and the front arch resting behind the symphysis pubis. The concave surface and diaphragm support the anterior vagina. Place the convex portion of the curve beneath the bulge. The two bases rest on the posterior vagina against the lateral levator muscles.
Shaatz
This support pessary has a circular base similar to the Gellhorn pessary but without the rigid stem (FIGURE 4).
Insertion. Because it is stiff, insert this pessary vertically and then turn it to a horizontal position once it is inside the vagina.
Lever
The Hodge, Smith, and Risser pessaries are collectively called the lever pessaries. They are used to manage uterine retroversion and POP. They are rarely used.
The Hodge pessary is beneficial to patients with a narrow vaginal introitus, mild cystocele, and cervical insufficiency. The anterior portion of a Hodge pessary is rectangular (FIGURE 5A).
The Smith pessary is useful for patients with well-defined pubic notches because the anterior portion is rounded (FIGURE 5B).
For patients with a very shallow pubic notch, the Risser pessary is useful. The Risser’s anterior portion is rectangular with indentation but wider than the Hodge pessary (FIGURE 5C).
Insertion. Fold the pessary and insert it into the vagina with the index finger on the posterior curved bar until the pessary rests behind the cervix and the anterior horizontal bar rests behind the symphysis pubis.
SPACE-OCCUPYING PESSARIES
The second pessary category is the space-filling pessary. These pessaries are used primarily to support severe POP, especially posthysterectomy vaginal vault prolapse. They have larger bases to support the vaginal apex or cervix; therefore, they are more difficult to insert and remove. When this pessary type is in place, sexual intercourse is not possible. Examples include the Gellhorn, donut, cube, and inflatable pessaries.
Gellhorn
The Gellhorn pessary is the most commonly used space-filling pessary. It has a broad base with a stem (FIGURE 6). The broad base supports the vaginal apex while the stem keeps the circular base from rotating and prevents pessary expulsion. The stem comes in long or short lengths. The concave base provides vaginal suction and keeps the pessary in place. The holes in the stem and base provide vaginal drainage. The Gellhorn pessary is useful for women with more advanced prolapse and less perineal support.
Insertion. Folding one side of the base to the stem, insert the Gellhorn pessary vertically inside the vagina. To facilitate insertion, separate the labia with the nondominant hand or depress the perineum with the index finger. Once the circular base is inside the vagina, push the pessary upward until the tip of the stem is just inside the vaginal introitus (FIGURE 7). Many medical illustrations inaccurately depict the Gellhorn pessary in a final placement that appears too high in the pelvis. This figure, which has the patient in a standing position, shows how low in the pelvis this space-filling pessary can sit in a patient with advanced prolapse.
Remove this pessary by gently pulling the stem while inserting the opposite hand beneath an edge of the pessary base to break the vaginal suction (Watch Vaginal pessaries: An instructional video).
Donut
The donut pessary is used for advanced prolapse because it fills a larger space. It is difficult to insert and remove because it is large, thick, and hollow (FIGURE 8).
Insertion. Insert it vertically and, once it is placed inside the vagina, rotate it to a horizontal position. A Kelly clamp can be used to grasp the pessary and facilitate removal.
Cube
The cube pessary supports third-degree uterine prolapse by holding the vaginal wall with suction (FIGURE 9). Because of the risk of vaginal erosion and lack of drainage in some designs, the cube pessary requires nightly removal and cleaning.
Insertion. Squeezing the pessary with the thumb, index, and middle fingers, insert the cube pessary at the vaginal apex.
Removal requires breaking the suction by placing a fingertip between the vaginal mucosa and the pessary and compressing the cube between the thumb and forefinger to remove. Gently tugging on the string also helps with removal.
Inflatable
This space-filling pessary is an air-filled ball that is inflated via an attached stem that also enables insertion and removal. The older Inflatoball pessary is made of latex, so its use is contraindicated in patients with latex allergy. Newer inflatable pessaries are silicone-based and consist of an air-filled donut, a stem with a valve, and an air pump (FIGURE 10). Some models also include a deflation key. The inflatable pessary comes in small, medium, large, and extra-large sizes. This pessary type must be removed and cleaned daily.
Insertion. Place the deflated pessary into the vagina. Move the ball-bearing valve within the stem (which controls the air flow) to a lateral projection on the side of the stem. To inflate, attach the inflation bulb. (Inflation typically requires 3 to 5 pumps of the bulb.) Move the ball bearing back into position to maintain the inflation, then detach the bulb. You can leave the stem outside the body or tuck it gently into the introitus (FIGURE 11).
INCONTINENCE PESSARIES
These devices are used specifically for SUI. The incontinence ring (FIGURE 12) and incontinence dish pessaries compress the urethra against the pubic symphysis. The knob is placed beneath the urethra, increasing the urethral closure pressure and thereby preventing urinary incontinence.
Related Article: Update on Urinary Incontinence Karen L. Noblett, MD, MAS, and Stephanie A. Jacobs, MD (December 2011)
CASE 1 CONCLUDED
Given that AC has early-stage POP and is sexually active, a space-occupying pessary is not the optimal choice. Instead, a ring pessary with support is fitted for her trial.
What side effects might a patient anticipate with pessary use?
Vaginal discharge and slight odor are common. Pessary removal and cleaning are usually adequate to eliminate them. Temporary discontinuation of pessary use may be warranted until symptoms subside. If these maneuvers do not resolve the issue, then the patient should be examined to rule out other sources of infection.
Vaginal bleeding. Bleeding from vaginal abrasion and ulceration could be caused by trauma from pessary removal or vaginal impingement. Evaluation is warranted for any vaginal bleeding.
Changes in urinary function. Less commonly, women using a pessary may notice changes in their urinary function. Many women with anterior or apical prolapse will have altered urine streams with slow or trickling flow and possible hesitation upon initiation of voiding.
Alternatively, pessary placement may instigate stress-type incontinence akin to that seen after prolapse surgery. Changing pessary size may alleviate this condition. Otherwise, these side effects may reduce a patient’s willingness to continue pessary use.
How can a patient optimize her use of a pessary?
A patient can remove the pessary on a periodic basis or try to use it continuously. If she cannot or will not remove the pessary, then she will need to come back for scheduled visits, as described in the sidebar, “Essential components of a successfully fitted pessary.” If she is able to remove the pessary on her own, then she can use the device as needed or remove it for intercourse (though it is not necessary). She must remove it weekly, at a minimum, however, to both clean the pessary and give the vaginal walls a “rest,” which can minimize the potential for abrasions or erosions
ESSENTIAL COMPONENTS OF A SUCCESSFULLY FITTED PESSARY
Patient assessment
Accurate selection and placement of a pessary requires appropriate examination and fitting, beginning with determination of the patient’s stage of prolapse and introitus. Key steps include:
– Examine the patient with an empty bladder in the lithotomy position
– Perform bimanual pelvic and speculum examination using a Sims speculum (or bivalve speculum broken in half) with the patient in a supine position
– Administer the Pelvic Organ Prolapse Quantification (POP-Q) exam
– Perform digital examination
– Assess vaginal atrophy, vaginal introitus, and vaginal width and length
– Evaluate pelvic floor muscle strength (Kegel squeeze).
Next, gauge the correct pessary size by approximating the number of fingerbreadths accommodated across the vaginal width.
Another method of estimating pessary size is to insert two fingers inside the vagina and estimate the distance between the posterior fornix and the posterior pubic symphysis (Watch Vaginal pessaries: An instructional video). An easy reference is to start with a size 3 or 4 ring pessary if the vaginal introitus is 1 to 2 fingerbreadths in width and the prolapse is stage II to III. If the vagina accommodates 3 to 4 fingerbreadths, or there is stage IV prolapse, use a Gellhorn pessary.
Here are the different types of pessaries and the most common sizes available. (Pessary sizes change in quarter-inch increments.)
In-office trial
Insert the pessary into the vagina using the dominant hand. Using the nondominant hand, separate the introitus and depress the perineal body. Apply a small amount of lubricant to the leading edge of the pessary.
After insertion, ask the patient to strain and cough, ambulate in the office, and void. Reexamine the patient to ensure that the pessary is still in the correct position and that placement has not shifted. Perform the cough leak test with the patient in a standing position and the pessary in place. Re-examine the patient while she is in a standing position. Use the largest pessary that is comfortable for her. Advise her to bring the pessary back to the office if it gets expelled.
This is a trial-and-error process; advise the patient of this. It may require a trial of several styles and sizes to find the right pessary fit. Once you find the correct size, document the final pessary size.
Follow-up
Schedule a follow-up appointment 1 to 2 weeks after insertion. Ask the patient whether she has experienced any discomfort, malodorous discharge, or vaginal bleeding. Also inquire about any changes in urinary habits or bowel movements and related complaints.
Remove the pessary and clean it with mild soap and water. Examine the vagina for pressure points, abrasions, ulcerations, and erosions.
Teach the patient how to remove, clean, and reinsert the pessary, and advise her to perform these tasks on a weekly basis.
Schedule a follow-up visit in 1 to 2 months, and another visit 6 to 12 months after that.
CASE 2. ADVANCED-STAGE POP
BD is an 82-year-old widow (G5P4014) with stage IV vaginal prolapse. She has noticed some scant blood staining on her clothing. She frequently voids small amounts of urine but never feels complete relief. She defecates normally.
Her medical history is significant for coronary artery disease with prior myocardial infarction, with multiple stent placements over the years. She has hypertension, reduced ejection fraction, and diabetes. She is morbidly obese and suffers from degenerative joint disease. She had a vaginal hysterectomy several years ago for benign indications.
Upon examination, BD’s prolapse is large, with excoriations and hyperkeratosis of the skin over the prolapse. It is easily reduced in the office.
What is the best pessary for this patient, and how should she be followed and counseled regarding ongoing care?
Since the failure rate for pessary usage increases with advancing prolapse stage, a space-occupying pessary is most appropriate to try initially. A trial with a support pessary could be useful to allow the excoriations to heal and provide a healthier vaginal environment. A Gellhorn pessary is commonly used. An inflatable pessary could be an alternative if the Gellhorn fails to stay in place. The cube pessary, known to cause more abrasions and erosions than other pessaries, is a poor choice given the state of the patient’s vaginal tissues at baseline.
Space-occupying pessaries are more difficult to insert and remove and have a higher risk of pain or trauma. Start with shorter time intervals between visits, eventually spacing them out for the patient’s convenience. The usual interval for follow-up is 3 to 4 months; longer intervals could be offered if the patient is reliable, adherent, and reports no complaints with pessary use.
Related Article: Update on pelvic floor dysfunction: Focus on urinary incontinence Alexis A. Dieter, MD, and Cindy L. Amundsen, MD (November 2013)
OUTCOMES
Only short- and medium-term outcomes for pessary use have been described in the literature. Short-term (2 months) satisfaction and continued use, along with resolution of prolapse, occurred in 92% of patients.7 Previous hysterectomy or prolapse surgery may influence the short-term success of pessary use.10
More than half of sexually active women achieved long-term use (up to 2 years), regardless of prolapse severity. Brincat and colleagues found that long-term pessary use (1 to 2 years) approached 60% in 132 women with both urinary incontinence and prolapse. Women being treated for POP were more likely to continue pessary use than women being treated for SUI.11 Age, parity, estrogen use, and sexual activity were characteristics also studied in pessary fitting. Neither sexual activity nor stage of prolapse was a contraindication to use of a pessary; long-term use was found to be acceptable in sexually active women.11
Successful fitting of a vaginal pessary has been associated with improvement in voiding, urinary and fecal urgency, and incontinence. A vaginal pessary is a viable nonsurgical option for the management of POP and urinary incontinence and remains an optimal minimally invasive approach to such disorders.
CASE 2 CONCLUDED
The patient returns to the clinic 1 month after the original insertion. The pessary is removed, and the vagina is inspected, with no abrasions or ulcerations found. The vaginal cavity and pessary are cleaned with a mild soap-and-water mixture. The pessary is lubricated and reinserted. This process is repeated 2 months later, with subsequent follow-up intervals doubled (up to 6 months between visits) when the patient has no complaints of discharge or odor.
- Colmer, WM Jr. Use of the pessary. Am J Obstet Gynecol. 1953;65(1):170–174.
- Culligan PJ. Nonsurgical management to pelvic organ prolapse. Obstet Gynecol. 2012;119(4):852–860.
- Nygaard IE, Heit M. Stress urinary incontinence. Obstet Gynecol. 2004;104(3):607–620.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 85: Pelvic organ prolapse. Obstet Gynecol. 2007;110(3):717–729.
- Clemons JL, Aguilar VC, Tillinghast TA, Jackson ND, Myers DL. Risk factors associated with an unsuccessful pessary fitting trial in women with pelvic organ prolapse. Am J Obstet Gynecol. 2004;190(2):345–350.
- Clemons JL, Brubaker L, Falk SJ. Vaginal pessary treatment of prolapse and incontinence. UpToDate. http://www.uptodate.com/contents/vaginal-pessary-treatment-of-prolapse-and-incontinence. Updated February 8, 2013. Accessed November 7, 2013.
- Mutone MF, Terry C, Hale DS, Benson JT. Factors which influence the short-term success of pessary management of pelvic organ prolapse. Am J Obstet Gynecol. 2005;193(1):89–94.
- Fernando RJ, Thakar R, Sultan AH, Shah SM, Jones PW. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108(1):93–99.
- Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol. 2005;106(3):615–634.
- Donnelly MJ, Powell-Morgan S, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(5):302–307.
- Brincat C, Kenton K, Fitzgerald MP, et al. Sexual activity predicts continued pessary use. Am J Obstet Gynecol. 2004;191(1):198–200.
- Colmer, WM Jr. Use of the pessary. Am J Obstet Gynecol. 1953;65(1):170–174.
- Culligan PJ. Nonsurgical management to pelvic organ prolapse. Obstet Gynecol. 2012;119(4):852–860.
- Nygaard IE, Heit M. Stress urinary incontinence. Obstet Gynecol. 2004;104(3):607–620.
- ACOG Committee on Practice Bulletins—Gynecology. ACOG Practice Bulletin No. 85: Pelvic organ prolapse. Obstet Gynecol. 2007;110(3):717–729.
- Clemons JL, Aguilar VC, Tillinghast TA, Jackson ND, Myers DL. Risk factors associated with an unsuccessful pessary fitting trial in women with pelvic organ prolapse. Am J Obstet Gynecol. 2004;190(2):345–350.
- Clemons JL, Brubaker L, Falk SJ. Vaginal pessary treatment of prolapse and incontinence. UpToDate. http://www.uptodate.com/contents/vaginal-pessary-treatment-of-prolapse-and-incontinence. Updated February 8, 2013. Accessed November 7, 2013.
- Mutone MF, Terry C, Hale DS, Benson JT. Factors which influence the short-term success of pessary management of pelvic organ prolapse. Am J Obstet Gynecol. 2005;193(1):89–94.
- Fernando RJ, Thakar R, Sultan AH, Shah SM, Jones PW. Effect of vaginal pessaries on symptoms associated with pelvic organ prolapse. Obstet Gynecol. 2006;108(1):93–99.
- Weber AM, Richter HE. Pelvic organ prolapse. Obstet Gynecol. 2005;106(3):615–634.
- Donnelly MJ, Powell-Morgan S, Olsen AL, et al. Vaginal pessaries for the management of stress and mixed incontinence. Int Urogynecol J Pelvic Floor Dysfunct. 2004;15(5):302–307.
- Brincat C, Kenton K, Fitzgerald MP, et al. Sexual activity predicts continued pessary use. Am J Obstet Gynecol. 2004;191(1):198–200.
![]()
Teresa Tam, MD
In this 15-minute video Dr. Tam demonstrates insertion and removal of the ring and Gellhorn pessaries and illustrates proper technique for estimating pessary size.
Cost-conscious choices for minimally invasive gynecologic surgery
CASE: COST-CONSCIOUS LAPAROSCOPIC HYSTERECTOMY
A 43-year-old woman undergoes laparoscopic hysterectomy for treatment of uterine fibroids and menorrhagia. Once she is prepped with ChloraPrep, a RUMI II uterine manipulator is placed. Laparoscopic ports include a Structural Balloon Trocar, a VersaStep Plus trocar, and two Versaport trocars. The surgeon uses an Olympus Thunderbeat device—a combination of ultrasonic and bipolar energy—to perform the majority of the procedure. The uterus is morcellated using the disposable Gynecare Morcellex, and the vaginal cuff is closed using a series of 2-0 PDS II sutures. The skin incisions are closed using Dermabond skin adhesive.
The total cost of the products used in this case: $1,705.60.
Could different product choices have reduced this figure?
As health-care costs continue to rise faster than inflation, with total health-care expenditures accounting for about 18% of the US gross domestic product, we face increasing pressure to take cost into account in the management of our patients.1 Not surprisingly, cost-effectiveness and outcome quality have become measures in many clinical trials that compare standard and alternative therapies. The field of women’s health—and, specifically, minimally invasive gynecologic surgery—is not immune to such comparisons.
Overall, conventional laparoscopic gynecologic procedures tend to have lower costs than laparotomy, due to shorter hospital stays, faster recovery, and fewer complications.2–4 What is not fully appreciated is how the choice of laparoscopic instrumentation and associated products affects surgical costs. In this article, we review these costs with the goal of raising awareness among minimally invasive gynecologic surgeons.
In the sections that follow, we highlight several aspects of laparoscopic gynecologic surgery that may affect your selection of instruments and products, describing the difference in cost as well as some unique characteristics of the products. Please note that our comparison focuses solely on cost, not ease of utility, effectiveness, surgical technique, risk of complications, or any other assessment. We’d also like to point out that numerous other instruments and devices are commercially available besides those listed in this article.
A few variables to keep in mind
Even when taking cost into consideration, tailor the selection of instruments and supplies to your capabilities and comfort, as well as characteristics particular to the patient and the planned procedure. Also keep in mind that your institution may have arrangements with companies that supply minimally invasive instruments, and such arrangements may limit your options to some degree. Be aware that reprocessed ports and instruments are now available at a reduced cost.
We believe it is crucial for surgeons to be cognizant of all products potentially available to them prior to attending a surgical case.
Related article: Update on Technology Barbara S. Levy, MD (September 2013)
Skin preparation and other preoperative considerations
Multiple preoperative skin preparations are available (TABLE 1). Traditionally, a povidone-iodine topical antiseptic such as Betadine has been used for skin and vaginal preparation prior to gynecologic surgery. Hibaclens and ChloraPrep are different combinations of chlorhexidine gluconate and isopropyl alcohol that act as broad-spectrum antiseptics. ChloraPrep is applied with a wand-like applicator and contains a much higher concentration of isopropyl alcohol than Hibaclens (70% vs 4%), rendering it more flammable. It also requires more drying time before the case is started. Clear and tinted ChloraPrep formulations are available.
It makes good sense to have instruments and devices readily available in the operating room (OR) at the start of a case, to avoid unnecessary surgical delays, but we recommend that you refrain from opening these tools until they are required intraoperatively. It is possible that the case will require conversion to laparotomy or that, after direct visualization of the pathology, different ports or instruments will be required.
Uterine manipulators
Cannulation of the cervical canal allows for uterine manipulation, increasing intraoperative traction and exposure and visualization of the adnexae and peritoneal surfaces.
The Hulka and Pelosi reusable uterine manipulators are fairly standard and easy to apply. Specialized, single-use manipulators also are available, including the VCare uterine manipulator/elevator, which consists of two opposing cups. One cup (available in four sizes, from small to extra-large) fits around the cervix and defines the site for colpotomy, and the other cup helps maintain pneumoperitoneum once a colpotomy is created.
The Zinnanti Uterine Manipulator Injector (ZUMI) is a rigid, curved shaft with an intrauterine balloon to help prevent expulsion. It also has an integrated injection channel to allow for intraoperative chromotubation.
The RUMI System fits individual patient anatomy with various tip lengths and colpotomy cup sizes (TABLE 2).
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Entry style and ports
The peritoneal cavity can be entered using either a closed (Veress needle) or open (Hasson) technique.5 Closed entry may allow for quicker access to the peritoneal cavity. A recent Cochrane review of 28 randomized, controlled trials, including 4,860 patients undergoing laparoscopy, compared outcomes between laparoscopic entry techniques.6 It found no difference in major vascular or visceral injury between closed and open techniques at the umbilicus. However, open entry was associated with greater successful entry into the peritoneal cavity, as well as less extraperitoneal insufflation and a lower omental injury rate, compared with closed entry.6
Left-upper-quadrant entry is another option when adhesions are anticipated or abnormal anatomy is encountered at the umbilicus.
In general, complications related to laparoscopic entry are quite rare in gynecologic surgery, ranging from 0.18% to 0.5%.7,8 A minimally invasive surgeon may prefer one entry technique over the other, but should be able to perform both methods competently and recognize when a particular technique is warranted.
Choosing a port
Laparoscopic ports usually range from 5 mm to 12 mm and can be fixed or variable in size. The primary port, usually placed through the umbilicus, can be a standard, blunt, 10-mm (Hasson) port, or it can be specialized to ease entry of the port or stabilize the port once it is introduced through the skin incision.
Optical trocars have a transparent tip that allows the surgeon to visualize the abdominal-wall entry layer by layer using a 0° laparoscope, usually after pneumoperitoneum is created with a Veress needle. Other specialized ports include those that have balloons or foam collars, or both, to secure the port without traditional stay sutures on the fascia and minimize leakage of pneumoperitoneum.
Accessory ports
When choosing an accessory port type and size, it is important to anticipate what instruments and devices, such as an Endo Catch bag, suture, needle, or morcellator, will need to pass through it. Also know whether 5-mm and 10-mm laparoscopes are available, and anticipate whether a second port with insufflation capabilities will be required.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
Pediport trocars are user-friendly 5-mm bladed ports that deploy a mushroom-shaped stabilizer to prevent dislodgement. A Versaport bladed trocar has a spring-loaded entry shield, which slides over to protect the blade once the peritoneal cavity is entered.
VersaStep bladeless trocars are introduced after a Step insufflation needle has been inserted. These trocars create a smaller fascial defect than conventional bladed trocars for an equivalent cannula size (TABLE 3).
Cutting and coagulating
Both monopolar and bipolar electrosurgical techniques are commonly employed in gynecologic laparoscopy. A wide variety of disposable and reusable instruments are available for monopolar energy, such as scissors, a hook, and a spatula.
Bipolar devices also can be disposable or reusable. Although bipolar electrosurgery minimizes injury to surrounding tissues by containing the current within the jaws of the forceps, it cannot cut or seal large vessels. As a result, several advanced bipolar devices with sealing and transecting capabilities have emerged (LigaSure, ENSEAL). Ultrasonic devices also can coagulate and cut at lower temperatures by converting electrical energy to mechanical energy (TABLE 4).
Sutures
Aspects of minimally invasive gynecologic surgery that require the use of suture include, but are not limited to, closure of the vaginal cuff, oophoropexy, and reapproximation of the ovarian cortex after cystectomy. Syntheticand delayed absorbable sutures, such as PDS II, are used frequently.
Recently, a new class of suture emerged and continues to gain popularity: barbed suture. This type of suture anchors to the tissue without the need for intra- or extracorporeal knots (TABLE 5).
Related article: Ins and outs of straight-stick laparoscopic myomectomy James Robinson, MD, MS, and Gaby Moawad, MD (September 2012)
Tissue removal
Adnexae and pathologic tissue, such as dermoid cysts, can be removed intact from the peritoneal cavity using an Endo Catch Single-Use Specimen Pouch, a polyurethane sac. Careful use, with placement of the ovary with the cyst into the pouch prior to cystectomy, can prevent spillage beyond the bag.
Large uteri that cannot be extracted through a colpotomy can be morcellated into smaller pieces to ease removal through a small laparoscopic incision or the colpotomy. Both reusable and disposable morcellator hand pieces are available (TABLE 6).
Skin closure
Final subcuticular closure can be accomplished using sutures or skin adhesive. Sutures may be synthetic, absorbable monofilament (eg, Caprosyn), or synthetic, absorbable, braided multifilament (eg, PolySorb).
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery Baha M. Sibai, MD (March 2012)
Skin adhesives close incisions quickly, avoid inflammation related to foreign bodies, and ease patient concerns that sometimes arise when absorbable suture persists postoperatively (TABLE 5).
Take-home points
As health-care third-party payers and hospitals continue to evaluate surgeons individually and compare procedures between surgeons, reimbursements may be stratified, favoring physicians who demonstrate both quality outcomes and cost-containment.
There are many ways a minimally invasive surgeon can implement cost-conscious choices that have little to no impact on the quality of the outcome.
Surgeons who are familiar with the instruments and models available for use at their institution are better prepared to make wise cost-conscious decisions.
Cost is not the only indicator of value, however. The surgeon must know how to apply tools correctly and be familiar with their limitations, and should choose instruments and products for their safety and ease of use. More often than not, a surgeon’s training and personal experience define—and sometimes restrict—the choice of devices.
CASE: SAME OUTCOME, LOWER COST
The patient undergoes laparoscopic hysterectomy for treatment of uterine fibroids and menorrhagia. However, in this scenario, the surgeon makes the following product choices: The patient is prepped with Betadine, and a reusable Pelosi uterine manipulator is placed. Laparoscopic ports include a Kii Balloon Blunt Tip, a reprocessed VersaStep Plus trocar, and two Pediport trocars. The surgeon uses the PKS Lyons Dissecting Forceps and reprocessed EndoShears to perform the hysterectomy, incorporating the Karl Storz Rotocut G1 Morcellator, a reusable system with single-use blades, to morcellate the uterus. The vaginal cuff is closed using V-Loc barbed suture, and skin incisions are closed with 4-0 Polysorb, a polyglycolic acid, absorbable suture.
The cost of these products: $1,005.68, or roughly $700 less than the set-up described at the beginning of this article (TABLE 7).
Acknowledgment
The authors thank Susan Wykoop, BSN, RN, and Anna Emery, MSN, RN, for their assistance in gathering specific cost-related information.
We want to hear from you! Tell us what you think.
- Health expenditure, total (% of GDP). The World Bank. http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Accessed October 17, 2013.
- Vilos GA, Alshimmiri MM. Cost-benefit analysis of laparoscopic versus laparotomy salpingo-oophorectomy for benign tubo-ovarian disease. J Am Assoc Gynecol Laparosc. 1995;2(3):299–303.
- Gray DT, Thorburn J, Lundorff P, et al. A cost-effectiveness study of a randomized trial of laparoscopy versus laparotomy for ectopic pregnancy. Lancet. 1995;345(8958):1139–1143.
- Chapron C, Fauconnier A, Goffinet F, et al. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Results of a meta-analysis. Hum Reprod. 2002;17(5):1334–1342.
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110(6):886–887.
- Ahmad G, O’Flynn H, Duffy JM, Phillips K, Watson A. Laparoscopic entry techniques. Cochrane Database Syst Rev. 2012;2:CD006583.
- Hasson HM, Rotman C, Rana N, Kumari NA. Open laparoscopy: 29-year experience. Obstet Gynecol. 2000;96(5 Pt 1):763–766.
- Schafer M, Lauper M, Krahenbuhl L. Trocar and Veress needle injuries during laparoscopy. Surg Endosc. 2001;15(3):275–280.
CASE: COST-CONSCIOUS LAPAROSCOPIC HYSTERECTOMY
A 43-year-old woman undergoes laparoscopic hysterectomy for treatment of uterine fibroids and menorrhagia. Once she is prepped with ChloraPrep, a RUMI II uterine manipulator is placed. Laparoscopic ports include a Structural Balloon Trocar, a VersaStep Plus trocar, and two Versaport trocars. The surgeon uses an Olympus Thunderbeat device—a combination of ultrasonic and bipolar energy—to perform the majority of the procedure. The uterus is morcellated using the disposable Gynecare Morcellex, and the vaginal cuff is closed using a series of 2-0 PDS II sutures. The skin incisions are closed using Dermabond skin adhesive.
The total cost of the products used in this case: $1,705.60.
Could different product choices have reduced this figure?
As health-care costs continue to rise faster than inflation, with total health-care expenditures accounting for about 18% of the US gross domestic product, we face increasing pressure to take cost into account in the management of our patients.1 Not surprisingly, cost-effectiveness and outcome quality have become measures in many clinical trials that compare standard and alternative therapies. The field of women’s health—and, specifically, minimally invasive gynecologic surgery—is not immune to such comparisons.
Overall, conventional laparoscopic gynecologic procedures tend to have lower costs than laparotomy, due to shorter hospital stays, faster recovery, and fewer complications.2–4 What is not fully appreciated is how the choice of laparoscopic instrumentation and associated products affects surgical costs. In this article, we review these costs with the goal of raising awareness among minimally invasive gynecologic surgeons.
In the sections that follow, we highlight several aspects of laparoscopic gynecologic surgery that may affect your selection of instruments and products, describing the difference in cost as well as some unique characteristics of the products. Please note that our comparison focuses solely on cost, not ease of utility, effectiveness, surgical technique, risk of complications, or any other assessment. We’d also like to point out that numerous other instruments and devices are commercially available besides those listed in this article.
A few variables to keep in mind
Even when taking cost into consideration, tailor the selection of instruments and supplies to your capabilities and comfort, as well as characteristics particular to the patient and the planned procedure. Also keep in mind that your institution may have arrangements with companies that supply minimally invasive instruments, and such arrangements may limit your options to some degree. Be aware that reprocessed ports and instruments are now available at a reduced cost.
We believe it is crucial for surgeons to be cognizant of all products potentially available to them prior to attending a surgical case.
Related article: Update on Technology Barbara S. Levy, MD (September 2013)
Skin preparation and other preoperative considerations
Multiple preoperative skin preparations are available (TABLE 1). Traditionally, a povidone-iodine topical antiseptic such as Betadine has been used for skin and vaginal preparation prior to gynecologic surgery. Hibaclens and ChloraPrep are different combinations of chlorhexidine gluconate and isopropyl alcohol that act as broad-spectrum antiseptics. ChloraPrep is applied with a wand-like applicator and contains a much higher concentration of isopropyl alcohol than Hibaclens (70% vs 4%), rendering it more flammable. It also requires more drying time before the case is started. Clear and tinted ChloraPrep formulations are available.
It makes good sense to have instruments and devices readily available in the operating room (OR) at the start of a case, to avoid unnecessary surgical delays, but we recommend that you refrain from opening these tools until they are required intraoperatively. It is possible that the case will require conversion to laparotomy or that, after direct visualization of the pathology, different ports or instruments will be required.
Uterine manipulators
Cannulation of the cervical canal allows for uterine manipulation, increasing intraoperative traction and exposure and visualization of the adnexae and peritoneal surfaces.
The Hulka and Pelosi reusable uterine manipulators are fairly standard and easy to apply. Specialized, single-use manipulators also are available, including the VCare uterine manipulator/elevator, which consists of two opposing cups. One cup (available in four sizes, from small to extra-large) fits around the cervix and defines the site for colpotomy, and the other cup helps maintain pneumoperitoneum once a colpotomy is created.
The Zinnanti Uterine Manipulator Injector (ZUMI) is a rigid, curved shaft with an intrauterine balloon to help prevent expulsion. It also has an integrated injection channel to allow for intraoperative chromotubation.
The RUMI System fits individual patient anatomy with various tip lengths and colpotomy cup sizes (TABLE 2).
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Entry style and ports
The peritoneal cavity can be entered using either a closed (Veress needle) or open (Hasson) technique.5 Closed entry may allow for quicker access to the peritoneal cavity. A recent Cochrane review of 28 randomized, controlled trials, including 4,860 patients undergoing laparoscopy, compared outcomes between laparoscopic entry techniques.6 It found no difference in major vascular or visceral injury between closed and open techniques at the umbilicus. However, open entry was associated with greater successful entry into the peritoneal cavity, as well as less extraperitoneal insufflation and a lower omental injury rate, compared with closed entry.6
Left-upper-quadrant entry is another option when adhesions are anticipated or abnormal anatomy is encountered at the umbilicus.
In general, complications related to laparoscopic entry are quite rare in gynecologic surgery, ranging from 0.18% to 0.5%.7,8 A minimally invasive surgeon may prefer one entry technique over the other, but should be able to perform both methods competently and recognize when a particular technique is warranted.
Choosing a port
Laparoscopic ports usually range from 5 mm to 12 mm and can be fixed or variable in size. The primary port, usually placed through the umbilicus, can be a standard, blunt, 10-mm (Hasson) port, or it can be specialized to ease entry of the port or stabilize the port once it is introduced through the skin incision.
Optical trocars have a transparent tip that allows the surgeon to visualize the abdominal-wall entry layer by layer using a 0° laparoscope, usually after pneumoperitoneum is created with a Veress needle. Other specialized ports include those that have balloons or foam collars, or both, to secure the port without traditional stay sutures on the fascia and minimize leakage of pneumoperitoneum.
Accessory ports
When choosing an accessory port type and size, it is important to anticipate what instruments and devices, such as an Endo Catch bag, suture, needle, or morcellator, will need to pass through it. Also know whether 5-mm and 10-mm laparoscopes are available, and anticipate whether a second port with insufflation capabilities will be required.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
Pediport trocars are user-friendly 5-mm bladed ports that deploy a mushroom-shaped stabilizer to prevent dislodgement. A Versaport bladed trocar has a spring-loaded entry shield, which slides over to protect the blade once the peritoneal cavity is entered.
VersaStep bladeless trocars are introduced after a Step insufflation needle has been inserted. These trocars create a smaller fascial defect than conventional bladed trocars for an equivalent cannula size (TABLE 3).
Cutting and coagulating
Both monopolar and bipolar electrosurgical techniques are commonly employed in gynecologic laparoscopy. A wide variety of disposable and reusable instruments are available for monopolar energy, such as scissors, a hook, and a spatula.
Bipolar devices also can be disposable or reusable. Although bipolar electrosurgery minimizes injury to surrounding tissues by containing the current within the jaws of the forceps, it cannot cut or seal large vessels. As a result, several advanced bipolar devices with sealing and transecting capabilities have emerged (LigaSure, ENSEAL). Ultrasonic devices also can coagulate and cut at lower temperatures by converting electrical energy to mechanical energy (TABLE 4).
Sutures
Aspects of minimally invasive gynecologic surgery that require the use of suture include, but are not limited to, closure of the vaginal cuff, oophoropexy, and reapproximation of the ovarian cortex after cystectomy. Syntheticand delayed absorbable sutures, such as PDS II, are used frequently.
Recently, a new class of suture emerged and continues to gain popularity: barbed suture. This type of suture anchors to the tissue without the need for intra- or extracorporeal knots (TABLE 5).
Related article: Ins and outs of straight-stick laparoscopic myomectomy James Robinson, MD, MS, and Gaby Moawad, MD (September 2012)
Tissue removal
Adnexae and pathologic tissue, such as dermoid cysts, can be removed intact from the peritoneal cavity using an Endo Catch Single-Use Specimen Pouch, a polyurethane sac. Careful use, with placement of the ovary with the cyst into the pouch prior to cystectomy, can prevent spillage beyond the bag.
Large uteri that cannot be extracted through a colpotomy can be morcellated into smaller pieces to ease removal through a small laparoscopic incision or the colpotomy. Both reusable and disposable morcellator hand pieces are available (TABLE 6).
Skin closure
Final subcuticular closure can be accomplished using sutures or skin adhesive. Sutures may be synthetic, absorbable monofilament (eg, Caprosyn), or synthetic, absorbable, braided multifilament (eg, PolySorb).
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery Baha M. Sibai, MD (March 2012)
Skin adhesives close incisions quickly, avoid inflammation related to foreign bodies, and ease patient concerns that sometimes arise when absorbable suture persists postoperatively (TABLE 5).
Take-home points
As health-care third-party payers and hospitals continue to evaluate surgeons individually and compare procedures between surgeons, reimbursements may be stratified, favoring physicians who demonstrate both quality outcomes and cost-containment.
There are many ways a minimally invasive surgeon can implement cost-conscious choices that have little to no impact on the quality of the outcome.
Surgeons who are familiar with the instruments and models available for use at their institution are better prepared to make wise cost-conscious decisions.
Cost is not the only indicator of value, however. The surgeon must know how to apply tools correctly and be familiar with their limitations, and should choose instruments and products for their safety and ease of use. More often than not, a surgeon’s training and personal experience define—and sometimes restrict—the choice of devices.
CASE: SAME OUTCOME, LOWER COST
The patient undergoes laparoscopic hysterectomy for treatment of uterine fibroids and menorrhagia. However, in this scenario, the surgeon makes the following product choices: The patient is prepped with Betadine, and a reusable Pelosi uterine manipulator is placed. Laparoscopic ports include a Kii Balloon Blunt Tip, a reprocessed VersaStep Plus trocar, and two Pediport trocars. The surgeon uses the PKS Lyons Dissecting Forceps and reprocessed EndoShears to perform the hysterectomy, incorporating the Karl Storz Rotocut G1 Morcellator, a reusable system with single-use blades, to morcellate the uterus. The vaginal cuff is closed using V-Loc barbed suture, and skin incisions are closed with 4-0 Polysorb, a polyglycolic acid, absorbable suture.
The cost of these products: $1,005.68, or roughly $700 less than the set-up described at the beginning of this article (TABLE 7).
Acknowledgment
The authors thank Susan Wykoop, BSN, RN, and Anna Emery, MSN, RN, for their assistance in gathering specific cost-related information.
We want to hear from you! Tell us what you think.
CASE: COST-CONSCIOUS LAPAROSCOPIC HYSTERECTOMY
A 43-year-old woman undergoes laparoscopic hysterectomy for treatment of uterine fibroids and menorrhagia. Once she is prepped with ChloraPrep, a RUMI II uterine manipulator is placed. Laparoscopic ports include a Structural Balloon Trocar, a VersaStep Plus trocar, and two Versaport trocars. The surgeon uses an Olympus Thunderbeat device—a combination of ultrasonic and bipolar energy—to perform the majority of the procedure. The uterus is morcellated using the disposable Gynecare Morcellex, and the vaginal cuff is closed using a series of 2-0 PDS II sutures. The skin incisions are closed using Dermabond skin adhesive.
The total cost of the products used in this case: $1,705.60.
Could different product choices have reduced this figure?
As health-care costs continue to rise faster than inflation, with total health-care expenditures accounting for about 18% of the US gross domestic product, we face increasing pressure to take cost into account in the management of our patients.1 Not surprisingly, cost-effectiveness and outcome quality have become measures in many clinical trials that compare standard and alternative therapies. The field of women’s health—and, specifically, minimally invasive gynecologic surgery—is not immune to such comparisons.
Overall, conventional laparoscopic gynecologic procedures tend to have lower costs than laparotomy, due to shorter hospital stays, faster recovery, and fewer complications.2–4 What is not fully appreciated is how the choice of laparoscopic instrumentation and associated products affects surgical costs. In this article, we review these costs with the goal of raising awareness among minimally invasive gynecologic surgeons.
In the sections that follow, we highlight several aspects of laparoscopic gynecologic surgery that may affect your selection of instruments and products, describing the difference in cost as well as some unique characteristics of the products. Please note that our comparison focuses solely on cost, not ease of utility, effectiveness, surgical technique, risk of complications, or any other assessment. We’d also like to point out that numerous other instruments and devices are commercially available besides those listed in this article.
A few variables to keep in mind
Even when taking cost into consideration, tailor the selection of instruments and supplies to your capabilities and comfort, as well as characteristics particular to the patient and the planned procedure. Also keep in mind that your institution may have arrangements with companies that supply minimally invasive instruments, and such arrangements may limit your options to some degree. Be aware that reprocessed ports and instruments are now available at a reduced cost.
We believe it is crucial for surgeons to be cognizant of all products potentially available to them prior to attending a surgical case.
Related article: Update on Technology Barbara S. Levy, MD (September 2013)
Skin preparation and other preoperative considerations
Multiple preoperative skin preparations are available (TABLE 1). Traditionally, a povidone-iodine topical antiseptic such as Betadine has been used for skin and vaginal preparation prior to gynecologic surgery. Hibaclens and ChloraPrep are different combinations of chlorhexidine gluconate and isopropyl alcohol that act as broad-spectrum antiseptics. ChloraPrep is applied with a wand-like applicator and contains a much higher concentration of isopropyl alcohol than Hibaclens (70% vs 4%), rendering it more flammable. It also requires more drying time before the case is started. Clear and tinted ChloraPrep formulations are available.
It makes good sense to have instruments and devices readily available in the operating room (OR) at the start of a case, to avoid unnecessary surgical delays, but we recommend that you refrain from opening these tools until they are required intraoperatively. It is possible that the case will require conversion to laparotomy or that, after direct visualization of the pathology, different ports or instruments will be required.
Uterine manipulators
Cannulation of the cervical canal allows for uterine manipulation, increasing intraoperative traction and exposure and visualization of the adnexae and peritoneal surfaces.
The Hulka and Pelosi reusable uterine manipulators are fairly standard and easy to apply. Specialized, single-use manipulators also are available, including the VCare uterine manipulator/elevator, which consists of two opposing cups. One cup (available in four sizes, from small to extra-large) fits around the cervix and defines the site for colpotomy, and the other cup helps maintain pneumoperitoneum once a colpotomy is created.
The Zinnanti Uterine Manipulator Injector (ZUMI) is a rigid, curved shaft with an intrauterine balloon to help prevent expulsion. It also has an integrated injection channel to allow for intraoperative chromotubation.
The RUMI System fits individual patient anatomy with various tip lengths and colpotomy cup sizes (TABLE 2).
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Entry style and ports
The peritoneal cavity can be entered using either a closed (Veress needle) or open (Hasson) technique.5 Closed entry may allow for quicker access to the peritoneal cavity. A recent Cochrane review of 28 randomized, controlled trials, including 4,860 patients undergoing laparoscopy, compared outcomes between laparoscopic entry techniques.6 It found no difference in major vascular or visceral injury between closed and open techniques at the umbilicus. However, open entry was associated with greater successful entry into the peritoneal cavity, as well as less extraperitoneal insufflation and a lower omental injury rate, compared with closed entry.6
Left-upper-quadrant entry is another option when adhesions are anticipated or abnormal anatomy is encountered at the umbilicus.
In general, complications related to laparoscopic entry are quite rare in gynecologic surgery, ranging from 0.18% to 0.5%.7,8 A minimally invasive surgeon may prefer one entry technique over the other, but should be able to perform both methods competently and recognize when a particular technique is warranted.
Choosing a port
Laparoscopic ports usually range from 5 mm to 12 mm and can be fixed or variable in size. The primary port, usually placed through the umbilicus, can be a standard, blunt, 10-mm (Hasson) port, or it can be specialized to ease entry of the port or stabilize the port once it is introduced through the skin incision.
Optical trocars have a transparent tip that allows the surgeon to visualize the abdominal-wall entry layer by layer using a 0° laparoscope, usually after pneumoperitoneum is created with a Veress needle. Other specialized ports include those that have balloons or foam collars, or both, to secure the port without traditional stay sutures on the fascia and minimize leakage of pneumoperitoneum.
Accessory ports
When choosing an accessory port type and size, it is important to anticipate what instruments and devices, such as an Endo Catch bag, suture, needle, or morcellator, will need to pass through it. Also know whether 5-mm and 10-mm laparoscopes are available, and anticipate whether a second port with insufflation capabilities will be required.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
Pediport trocars are user-friendly 5-mm bladed ports that deploy a mushroom-shaped stabilizer to prevent dislodgement. A Versaport bladed trocar has a spring-loaded entry shield, which slides over to protect the blade once the peritoneal cavity is entered.
VersaStep bladeless trocars are introduced after a Step insufflation needle has been inserted. These trocars create a smaller fascial defect than conventional bladed trocars for an equivalent cannula size (TABLE 3).
Cutting and coagulating
Both monopolar and bipolar electrosurgical techniques are commonly employed in gynecologic laparoscopy. A wide variety of disposable and reusable instruments are available for monopolar energy, such as scissors, a hook, and a spatula.
Bipolar devices also can be disposable or reusable. Although bipolar electrosurgery minimizes injury to surrounding tissues by containing the current within the jaws of the forceps, it cannot cut or seal large vessels. As a result, several advanced bipolar devices with sealing and transecting capabilities have emerged (LigaSure, ENSEAL). Ultrasonic devices also can coagulate and cut at lower temperatures by converting electrical energy to mechanical energy (TABLE 4).
Sutures
Aspects of minimally invasive gynecologic surgery that require the use of suture include, but are not limited to, closure of the vaginal cuff, oophoropexy, and reapproximation of the ovarian cortex after cystectomy. Syntheticand delayed absorbable sutures, such as PDS II, are used frequently.
Recently, a new class of suture emerged and continues to gain popularity: barbed suture. This type of suture anchors to the tissue without the need for intra- or extracorporeal knots (TABLE 5).
Related article: Ins and outs of straight-stick laparoscopic myomectomy James Robinson, MD, MS, and Gaby Moawad, MD (September 2012)
Tissue removal
Adnexae and pathologic tissue, such as dermoid cysts, can be removed intact from the peritoneal cavity using an Endo Catch Single-Use Specimen Pouch, a polyurethane sac. Careful use, with placement of the ovary with the cyst into the pouch prior to cystectomy, can prevent spillage beyond the bag.
Large uteri that cannot be extracted through a colpotomy can be morcellated into smaller pieces to ease removal through a small laparoscopic incision or the colpotomy. Both reusable and disposable morcellator hand pieces are available (TABLE 6).
Skin closure
Final subcuticular closure can be accomplished using sutures or skin adhesive. Sutures may be synthetic, absorbable monofilament (eg, Caprosyn), or synthetic, absorbable, braided multifilament (eg, PolySorb).
Related article: 10 practical, evidence-based recommendations for improving maternal outcomes of cesarean delivery Baha M. Sibai, MD (March 2012)
Skin adhesives close incisions quickly, avoid inflammation related to foreign bodies, and ease patient concerns that sometimes arise when absorbable suture persists postoperatively (TABLE 5).
Take-home points
As health-care third-party payers and hospitals continue to evaluate surgeons individually and compare procedures between surgeons, reimbursements may be stratified, favoring physicians who demonstrate both quality outcomes and cost-containment.
There are many ways a minimally invasive surgeon can implement cost-conscious choices that have little to no impact on the quality of the outcome.
Surgeons who are familiar with the instruments and models available for use at their institution are better prepared to make wise cost-conscious decisions.
Cost is not the only indicator of value, however. The surgeon must know how to apply tools correctly and be familiar with their limitations, and should choose instruments and products for their safety and ease of use. More often than not, a surgeon’s training and personal experience define—and sometimes restrict—the choice of devices.
CASE: SAME OUTCOME, LOWER COST
The patient undergoes laparoscopic hysterectomy for treatment of uterine fibroids and menorrhagia. However, in this scenario, the surgeon makes the following product choices: The patient is prepped with Betadine, and a reusable Pelosi uterine manipulator is placed. Laparoscopic ports include a Kii Balloon Blunt Tip, a reprocessed VersaStep Plus trocar, and two Pediport trocars. The surgeon uses the PKS Lyons Dissecting Forceps and reprocessed EndoShears to perform the hysterectomy, incorporating the Karl Storz Rotocut G1 Morcellator, a reusable system with single-use blades, to morcellate the uterus. The vaginal cuff is closed using V-Loc barbed suture, and skin incisions are closed with 4-0 Polysorb, a polyglycolic acid, absorbable suture.
The cost of these products: $1,005.68, or roughly $700 less than the set-up described at the beginning of this article (TABLE 7).
Acknowledgment
The authors thank Susan Wykoop, BSN, RN, and Anna Emery, MSN, RN, for their assistance in gathering specific cost-related information.
We want to hear from you! Tell us what you think.
- Health expenditure, total (% of GDP). The World Bank. http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Accessed October 17, 2013.
- Vilos GA, Alshimmiri MM. Cost-benefit analysis of laparoscopic versus laparotomy salpingo-oophorectomy for benign tubo-ovarian disease. J Am Assoc Gynecol Laparosc. 1995;2(3):299–303.
- Gray DT, Thorburn J, Lundorff P, et al. A cost-effectiveness study of a randomized trial of laparoscopy versus laparotomy for ectopic pregnancy. Lancet. 1995;345(8958):1139–1143.
- Chapron C, Fauconnier A, Goffinet F, et al. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Results of a meta-analysis. Hum Reprod. 2002;17(5):1334–1342.
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110(6):886–887.
- Ahmad G, O’Flynn H, Duffy JM, Phillips K, Watson A. Laparoscopic entry techniques. Cochrane Database Syst Rev. 2012;2:CD006583.
- Hasson HM, Rotman C, Rana N, Kumari NA. Open laparoscopy: 29-year experience. Obstet Gynecol. 2000;96(5 Pt 1):763–766.
- Schafer M, Lauper M, Krahenbuhl L. Trocar and Veress needle injuries during laparoscopy. Surg Endosc. 2001;15(3):275–280.
- Health expenditure, total (% of GDP). The World Bank. http://data.worldbank.org/indicator/SH.XPD.TOTL.ZS. Accessed October 17, 2013.
- Vilos GA, Alshimmiri MM. Cost-benefit analysis of laparoscopic versus laparotomy salpingo-oophorectomy for benign tubo-ovarian disease. J Am Assoc Gynecol Laparosc. 1995;2(3):299–303.
- Gray DT, Thorburn J, Lundorff P, et al. A cost-effectiveness study of a randomized trial of laparoscopy versus laparotomy for ectopic pregnancy. Lancet. 1995;345(8958):1139–1143.
- Chapron C, Fauconnier A, Goffinet F, et al. Laparoscopic surgery is not inherently dangerous for patients presenting with benign gynaecologic pathology. Results of a meta-analysis. Hum Reprod. 2002;17(5):1334–1342.
- Hasson HM. A modified instrument and method for laparoscopy. Am J Obstet Gynecol. 1971;110(6):886–887.
- Ahmad G, O’Flynn H, Duffy JM, Phillips K, Watson A. Laparoscopic entry techniques. Cochrane Database Syst Rev. 2012;2:CD006583.
- Hasson HM, Rotman C, Rana N, Kumari NA. Open laparoscopy: 29-year experience. Obstet Gynecol. 2000;96(5 Pt 1):763–766.
- Schafer M, Lauper M, Krahenbuhl L. Trocar and Veress needle injuries during laparoscopy. Surg Endosc. 2001;15(3):275–280.
For uterine-sparing fibroid treatment, consider laparoscopic ultrasound-guided radiofrequency ablation
CASE: WOMAN WITH HEAVY BLEEDING HOPES TO AVOID HYSTERECTOMY
L.W. is a 44-year-old woman (G2P2) with a 2-year history of menorrhagia and severe dysmenorrhea but no intermenstrual spotting or bleeding. She reports that she has tried to control her symptoms using hormonal methods, without success.
Examination reveals that she has an irregular, nontender uterus 8 weeks in size. Transvaginal ultrasonography shows two deep, prominent, intramural fibroids. The first is 2 cm by 3 cm in size in the left lateral uterus, adjacent to the endometrial stripe. The second fibroid is 3 cm by 4 cm in the fundal region. Sonohysterography reveals no intracavitary fibroids, although the left lateral myoma has distorted the endometrial cavity.
The patient is seeking removal of her fibroids but would like to preserve her uterus, if at all possible.
What options would you offer her?
Uterine fibroids are common in premenopausal women, affecting 70% to 80% of the population, especially women of African descent.1 Although the majority of women with fibroids are asymptomatic, approximately 30% report pelvic pressure, dyspareunia, dysmenorrhea, or abnormal uterine bleeding.2
Our patients are increasingly aware of uterine-sparing treatments for symptomatic fibroids.3,4 Women seek conservative procedures to avoid the risks and extended recovery times commonly associated with major surgery. Current laparoscopic techniques for removal of uterine fibroids can be complex and require advanced surgical skills. Deep intramural fibroids may not always be visible or readily accessible, making laparoscopic removal a more invasive and challenging approach for many gynecologic surgeons.
A significant recent advancement in minimally invasive gynecologic treatment of fibroids is the Acessa System (Halt Medical, Brentwood, California). This laparoscopic system involves the outpatient use of ultrasound-guided radiofrequency volumetric thermal ablation, or RFVTA.
In this article, I outline the development of this option and step you through its technique. I also review the outcomes data that have been published to date.
How the procedure evolved
In the 1980s, Donnez and Nisolle developed a method of laparoscopic Nd:YAG laser treatment of uterine fibroids, commonly referred to as myolysis.5 Later, Goldfarb developed a laparoscopic bipolar needle technique that coagulated and occluded blood vessels at the periphery of the fibroid.6 However, myolysis led to the formation of significant adhesions to the small bowel or omentum, or both, and was abandoned by the surgical community.
Other fibroid treatments have been developed, such as laparoscopic myomectomy, uterine artery ligation, uterine artery embolization (alone or as adjuvant treatment), and high-intensity focused ultrasound. Although these procedures have made a significant contribution to the minimally invasive treatment of fibroids, the need for reintervention can be substantial, as high as 29% at 1 to 10 years of follow-up in some reports.7–17
Related article: Ins and outs of straight-stick myomectomy James Robinson, MD, MD, and Gaby Moawad, MD (September 2012)
Radiofrequency needle ablation has been used successfully in the treatment and destruction of liver and kidney tumors for years. Lee envisioned use of the technology to treat uterine fibroids. He developed a technique using a retractable multiarray radiofrequency needle (Starburst XL, RITA Medical Systems, Fremont, California) that is inserted directly into the fibroid. In 2002, he reported the first use of intraperitoneal ultrasound for needle guidance in the laparoscopic ablation of 197 myomas in 52 symptomatic women who had declined hysterectomy and myomectomy.18 He found that a significant proportion of women experienced resolution of their symptoms, including heavy menstrual bleeding, dysmenorrhea, dyspareunia, and pelvic pressure, by 3 months, with continued improvement at 12 months.
Because he was unhappy with aspects of the RITA needle that prevented accurate and consistent ablation of fibroids, he developed other devices that would lead to RFVTA and the Acessa System. This system allows the gynecologic surgeon to target and treat deep intramural fibroids that may not be readily visible via conventional laparoscopy.
Between the time of Lee’s first report and his subsequent refinement of the device and procedure, several international fibroid radiofrequency ablation procedures emerged, including those of Bergamini, Milic, Ghezzi, and Carrafiello.19–22 These investigators published results in small cohorts and described significant improvement in patient-reported Uterine Fibroid Symptom and Quality-of-Life (UFS-QOL) scores as well as a reduction in myoma volume.23 Their studies provided evidence that RFA is a safe and effective uterine-sparing treatment of symptomatic uterine fibroids for selected patients.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
How it works
RFVTA uses laparoscopy and laparoscopic ultrasound to guide placement of a needle electrode with a deployable array. Earlier international studies of radiofrequency ablation18–22 and ongoing studies of RFVTA23–26 have demonstrated the procedure’s therapeutic potential. Acessa was recently cleared (November 2012) by the US Food and Drug Administration (FDA).
RFVTA technique, step by step
1. Begin with a standard 5-mm laparoscopic infraumbilical port for the camera and video laparoscope (See VIDEO).
2. Place a 12-mm port in the midline, suprapubically at the level of the uterus, for insertion of the laparoscopic ultrasound probe. Now it is possible to map the uterus and plan an approach to destroy the fibroids.
3. Once you have determined your approach, insert the handpiece containing the radiofrequency needle through the abdominal wall under laparoscopic visualization.
4. Place the needle into the targeted fibroid using both laparoscopic and ultrasound guidance. Depending on the size of the fibroid, the needle array can be deployed to the maximum diameter necessary to effect destruction.
5. Engage the radiofrequency generator (FIGURE 1), utilizing the timing function for optimal destruction of the fibroid, based on the appropriate algorithm. The fibroid then will be ablated and destroyed without damage to the surrounding healthy myometrium.
The Acessa radiofrequency generator and accessories are designed to deliver monopolar radiofrequency energy to tissue through the handheld electrical probe. The procedure is monitored by real-time feedback to and from the generator via each of the thermocouples at the tips of the seven-needle array (FIGURE 1). In addition, laparoscopic ultrasound-guided visualization permits monitoring of needle placement within the fibroid capsule (FIGURES 2A and 2B) and confirmation of hemostasis upon removal of the probe tip (FIGURE 2C).
RFVTA treats a wide range of fibroids
The Acessa System is used to treat fibroids of any location and type, with the exception of pedunculated or Type 0 myomas, in which case laparoscopic or hysteroscopic myomectomy is recommended. Calcified fibroids pose no challenges. A single radiofrequency needle generally is used to treat multiple fibroids, with ablation zones ranging in diameter from 1.0 to 6.7 cm. Large, irregularly shaped fibroids may require multiple ablation zones during the same procedure.
Final steps
6. Once the treatment is complete, as confirmed by informatics transmitted by the generator, retract the needle array, set the generator to coagulation mode to coagulate the needle track during withdrawal of the probe, and confirm hemostasis (FIGURE 2C).
7. After completion of all fibroid destruction, remove the disposable radiofrequency probe and close the laparoscopic and ultrasound port sites using standard procedures.
The patient can be discharged the same day and generally is able to return to normal activities within 3 to 5 days.
Postoperative analgesia usually consists solely of nonsteroidal anti-inflammatory agents.
A look at outcomes data
Garza and colleagues published the first feasibility report in 2011.24 They described the results of laparoscopic, ultrasound-guided RFVTA for the treatment of symptomatic uterine fibroids in Mexico using an Acessa predecessor. The 31 women enrolled in the study desired uterine preservation. Of these participants, 19 had completed 12 months of follow-up by the time the report was submitted for publication. Their responses to the UFS-QOL questionnaire indicated that their symptoms had decreased significantly in severity from baseline to 12 months, and their quality-of-life scores also improved significantly (P <.001). Mean uterine volume also declined significantly.
Robles and colleagues set out to confirm these findings using the investigational Halt Ablation System. They conducted a prospective, single-center, open-label clinical trial in Guatemala.25 They found dramatic improvement in symptom severity and health-related quality of life from baseline to 12 months. Mean uterine volume also decreased by 23.6% during this period. In addition, the severity and duration of menses declined.
Acessa is currently not approved for women seeking future childbearing. However, several successful pregnancies have been reported following the procedure.24,26–28 Theoretically, because it causes less damage to and disruption of healthy myometrium, Acessa may prove to be preferable to myomectomy. Head-to-head studies to evaluate this premise are ongoing.
Latest findings confirm widespread improvement of symptoms
The most recent and detailed reports of safety and efficacy of laparoscopic, ultrasound-guided RFVTA at 12 and 24 months were published by teams led by Chudnoff26 and Guido27 earlier this year. These investigations involved 135 women who reported moderate to severe heavy menstrual bleeding (160 mL–500 mL by alkaline hematin analysis). Clinically significant reduction in menstrual blood loss was achieved in 67.7% of participants at 12 months of follow-up.29
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Galen and colleagues performed a retrospective analysis of these same patients, demonstrating that RFVTA of intramural fibroids without submucosal components leads to a clinically and statistically significant reduction in menstrual blood loss.30
Patients generally began these trials with high symptom-severity scores and low health-related quality of life, yet all achieved significant improvement over time, as their UFS-QOL scores indicate (TABLE). The majority of patients (96%) were treated on an outpatient basis. As measured by contrast-enhanced magnetic resonance imaging, total mean uterine volume decreased by 24.3% at 12 months, and total mean fibroid volume decreased by 45.1% during the same interval. Ninety-four percent of the women reported that the treatment was effective in eliminating their symptoms at 12 months, and 98% said they would recommend the procedure to a friend having the same health problems.
Complication rates were low
Complications included a postoperative pelvic abscess and four other minor events. The latter were treated at the time of RFVTA or resolved without additional procedures. The reintervention rate was 0.7% (1/135 patients).26
Guido and colleagues reported 24-month outcomes for the 124 women entering the second year of the Halt study and affirmed the findings of Chudnoff’s team.27 The 112 patient-reported UFS-QOL scores at 24 months were virtually identical to those reported at 12 months. Six surgical reinterventions (6 of 124 patients, or 4.8%) for fibroid-related bleeding were reported between 12 and 24 months.
280 women have been treated so far
As of September 2013, 280 women have been treated under five international study protocols using the Halt Acessa System, and approximately two-thirds have been followed for more than 12 months, with excellent results. Two of the five clinical trials are under way in Germany and Canada:
- The Laparoscopic Uterine-Sparing Techniques Outcomes and Reinterventions (LUSTOR) trial in Germany is a prospective, randomized, single-center study comparing outcomes of RFVTA and laparoscopic myomectomy. Fifty women were enrolled and treated and will be followed for as long as 60 months.
- The Treatment Results of Uterine-Sparing Technologies (TRUST) trial is a prospective, multicenter, randomized study in Canada that compares direct and indirect costs associated with RFVTA, laparoscopic and abdominal myomectomy, and uterine artery embolization. Some US sites also will participate. As many as 200 women will be treated and followed for as long as 60 months.
RFVTA offers several benefits
Although hysterectomy provides a definitive “cure” to fibroid-related symptoms, it is not preferred by many patients and generally is more costly, with an increased risk of complications, a longer hospital stay, longer recovery (6–8 weeks), longer disability and more time off from work, and potential long-term sequelae.
The Acessa procedure not only is an outpatient, minimally invasive, uterine-sparing fibroid treatment option, it permits access to and ablation of almost all fibroids (with the exception of penduculated or Type 0 myomas). The data to date suggest that RFVTA provides many benefits, including a decline in symptom severity and improved health-related quality of life, as well as good cosmesis, quick recovery, and a rapid return to full activity.
There is a learning curve for RFVTA with Acessa—as with any new procedure. If physicians already perform ultrasound imaging, the learning process is shorter. Physicians interested in Acessa and furthering their education and training in laparoscopic RFVTA should consult the manufacturer’s professional education division through the company Web site at http://www.haltmedical.com.
CASE: RESOLVED
After she is counseled about the various uterine-sparing options for treatment, L.W. chooses to undergo laparoscopic, ultrasound-guided RFVTA using the Acessa System. The procedure is performed successfully, with the two fibroids destroyed using the appropriate algorithms, which took into account the size of the fibroids and the deployed needle array. Two additional 1-cm fibroids were found during the procedure and ablated. The patient was discharged home shortly after the procedure, with a nonsteroidal anti-inflammatory drug given for pain relief.
We want to hear from you! Tell us what you think.
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107.
- Katz VL, Lentz G, Lobo RA, Gershenson D, eds. Comprehensive Gynecology. 5th ed. Philadelphia, PA: Elsevier Mosby Saunders; 2007.
- Rosen P. The endangered uterus. More Magazine Web site. http://www.more.com/health/wellness/endangered-uterus. December 2008/January 2009. Accessed October 16, 2013.
- Tulandi T, Levy BS. So You’re Having a Hysterectomy? Hoboken, NJ: John Wiley & Sons; 2004.
- Donnez J, Squifflet J, Polet R, Nisolle M. Laparoscopic myolysis. Human Reprod Update. 2000;6(6):609–613.
- Goldfarb HA. Bipolar laparoscopic needles for myoma coagulation. J Am Assoc Gynecol Laparosc. 1995;2(2):175–179.
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881.
- Goodwin SC, Spies JB, Worthington-Kirsch R, et al; Fibroid Registry for Outcomes Data (FIBROID) Registry Steering Committee and Core Site Investigators. Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008;111(1):22–33.
- Yoo EH, Lee PI, Huh C-Y, et al. Predictors of leiomyoma recurrence after laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(6):690–697.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356(4):360–370.
- Huang JY, Kafy S, Dugas A, Valenti D, Tulandi T. Failure of uterine fibroid embolization. Fertil Steril. 2006;85(1):30–35.
- Kroncke TJ, Gauruder-Burmester A, Scheurig C, et al. Transarterial embolization for uterine fibroids: clinical success rate and results of magnetic resonance imaging [in German]. Rofo. 2005;177(1):89–98.
- Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31(1):73–85.
- Munoz JL, Jimenez JS, Hernandez C, et al. Hysteroscopic myomectomy: our experience and review. JSLS. 2003;7(1):39–48.
- Reed SD, Newton KM, Thompson LB, McCrummen BA, Warolin AK. The incidence of repeat uterine surgery following myomectomy. J Womens Health (Larchmt). 2006;15(9):1046–1052.
- Spies JB, Bruno J, Czeyda-Pommersheim F, et al. Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol. 2005;106(5 Pt 1):933–939.
- Lee BB. Radiofrequency ablation of uterine leiomyomata: a new minimally invasive hysterectomy alternative. Obstet Gynecol. 2002;99(4Suppl):9S.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192(3):768–773.
- Milic A, Asch MR, Hawrylyshyn PA, et al. Laparoscopic ultrasound-guided radiofrequency ablation of uterine fibroids. Cardiovasc Intervent Radiol. 2006;29(4):694–698.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21(11):2081–2085.
- Carrafiello G, Recaldini C, Fontana F, et al. Ultrasound-guided radiofrequency thermal ablation of uterine fibroids: medium-term follow-up. Cardiovasc Intervent Radiol. 2010;33(1):113–119.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomymata. Obstet Gynecol. 2002;99(2):290–300.
- Garza Leal JG, Hernandez Leon I, Castillo Saenz L, Lee BB. Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 ablation system. J Minim Invasive Gynecol. 2011;18(3):364–371.
- Robles R, Aguirre VA, Argueta AI, Guerrero MR. Laparoscopic radiofrequency volumetric ablation of uterine myomas with 12 months of follow-up. Int J Gynecol Obstet. 2013;120(1):65–69.
- Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013;121(5):1075–1082.
- Guido RS, Macer JA, Abbott K, Falls JL, Tilley IB, Chudnoff SG. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Quality Life Outcomes. 2013;11(139):1–8.
- Berman JM, Puscheck EE, Diamond MP. Full-term vaginal live birth after laparoscopic radiofrequency ablation of a large, symptomatic intramural fibroid. J Reprod Med. 2012;57:159–163.
- Galen DI, Isaacson KB, Lee BB. Does menstrual bleeding decrease after ablation of intramural myomas? A retrospective study [published online ahead of print September 6, 2013]. J Minim Invasive Gynecol. doi: 10.1016/j.jmig.2013.05.007.
- Lukes AS, Muse K, Richter HE, Moore KA, Patrick DL. Estimating a meaningful reduction in menstrual blood loss for women with heavy bleeding. Curr Med Res Opinion. 2010;26(11):2673–2678.
CASE: WOMAN WITH HEAVY BLEEDING HOPES TO AVOID HYSTERECTOMY
L.W. is a 44-year-old woman (G2P2) with a 2-year history of menorrhagia and severe dysmenorrhea but no intermenstrual spotting or bleeding. She reports that she has tried to control her symptoms using hormonal methods, without success.
Examination reveals that she has an irregular, nontender uterus 8 weeks in size. Transvaginal ultrasonography shows two deep, prominent, intramural fibroids. The first is 2 cm by 3 cm in size in the left lateral uterus, adjacent to the endometrial stripe. The second fibroid is 3 cm by 4 cm in the fundal region. Sonohysterography reveals no intracavitary fibroids, although the left lateral myoma has distorted the endometrial cavity.
The patient is seeking removal of her fibroids but would like to preserve her uterus, if at all possible.
What options would you offer her?
Uterine fibroids are common in premenopausal women, affecting 70% to 80% of the population, especially women of African descent.1 Although the majority of women with fibroids are asymptomatic, approximately 30% report pelvic pressure, dyspareunia, dysmenorrhea, or abnormal uterine bleeding.2
Our patients are increasingly aware of uterine-sparing treatments for symptomatic fibroids.3,4 Women seek conservative procedures to avoid the risks and extended recovery times commonly associated with major surgery. Current laparoscopic techniques for removal of uterine fibroids can be complex and require advanced surgical skills. Deep intramural fibroids may not always be visible or readily accessible, making laparoscopic removal a more invasive and challenging approach for many gynecologic surgeons.
A significant recent advancement in minimally invasive gynecologic treatment of fibroids is the Acessa System (Halt Medical, Brentwood, California). This laparoscopic system involves the outpatient use of ultrasound-guided radiofrequency volumetric thermal ablation, or RFVTA.
In this article, I outline the development of this option and step you through its technique. I also review the outcomes data that have been published to date.
How the procedure evolved
In the 1980s, Donnez and Nisolle developed a method of laparoscopic Nd:YAG laser treatment of uterine fibroids, commonly referred to as myolysis.5 Later, Goldfarb developed a laparoscopic bipolar needle technique that coagulated and occluded blood vessels at the periphery of the fibroid.6 However, myolysis led to the formation of significant adhesions to the small bowel or omentum, or both, and was abandoned by the surgical community.
Other fibroid treatments have been developed, such as laparoscopic myomectomy, uterine artery ligation, uterine artery embolization (alone or as adjuvant treatment), and high-intensity focused ultrasound. Although these procedures have made a significant contribution to the minimally invasive treatment of fibroids, the need for reintervention can be substantial, as high as 29% at 1 to 10 years of follow-up in some reports.7–17
Related article: Ins and outs of straight-stick myomectomy James Robinson, MD, MD, and Gaby Moawad, MD (September 2012)
Radiofrequency needle ablation has been used successfully in the treatment and destruction of liver and kidney tumors for years. Lee envisioned use of the technology to treat uterine fibroids. He developed a technique using a retractable multiarray radiofrequency needle (Starburst XL, RITA Medical Systems, Fremont, California) that is inserted directly into the fibroid. In 2002, he reported the first use of intraperitoneal ultrasound for needle guidance in the laparoscopic ablation of 197 myomas in 52 symptomatic women who had declined hysterectomy and myomectomy.18 He found that a significant proportion of women experienced resolution of their symptoms, including heavy menstrual bleeding, dysmenorrhea, dyspareunia, and pelvic pressure, by 3 months, with continued improvement at 12 months.
Because he was unhappy with aspects of the RITA needle that prevented accurate and consistent ablation of fibroids, he developed other devices that would lead to RFVTA and the Acessa System. This system allows the gynecologic surgeon to target and treat deep intramural fibroids that may not be readily visible via conventional laparoscopy.
Between the time of Lee’s first report and his subsequent refinement of the device and procedure, several international fibroid radiofrequency ablation procedures emerged, including those of Bergamini, Milic, Ghezzi, and Carrafiello.19–22 These investigators published results in small cohorts and described significant improvement in patient-reported Uterine Fibroid Symptom and Quality-of-Life (UFS-QOL) scores as well as a reduction in myoma volume.23 Their studies provided evidence that RFA is a safe and effective uterine-sparing treatment of symptomatic uterine fibroids for selected patients.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
How it works
RFVTA uses laparoscopy and laparoscopic ultrasound to guide placement of a needle electrode with a deployable array. Earlier international studies of radiofrequency ablation18–22 and ongoing studies of RFVTA23–26 have demonstrated the procedure’s therapeutic potential. Acessa was recently cleared (November 2012) by the US Food and Drug Administration (FDA).
RFVTA technique, step by step
1. Begin with a standard 5-mm laparoscopic infraumbilical port for the camera and video laparoscope (See VIDEO).
2. Place a 12-mm port in the midline, suprapubically at the level of the uterus, for insertion of the laparoscopic ultrasound probe. Now it is possible to map the uterus and plan an approach to destroy the fibroids.
3. Once you have determined your approach, insert the handpiece containing the radiofrequency needle through the abdominal wall under laparoscopic visualization.
4. Place the needle into the targeted fibroid using both laparoscopic and ultrasound guidance. Depending on the size of the fibroid, the needle array can be deployed to the maximum diameter necessary to effect destruction.
5. Engage the radiofrequency generator (FIGURE 1), utilizing the timing function for optimal destruction of the fibroid, based on the appropriate algorithm. The fibroid then will be ablated and destroyed without damage to the surrounding healthy myometrium.
The Acessa radiofrequency generator and accessories are designed to deliver monopolar radiofrequency energy to tissue through the handheld electrical probe. The procedure is monitored by real-time feedback to and from the generator via each of the thermocouples at the tips of the seven-needle array (FIGURE 1). In addition, laparoscopic ultrasound-guided visualization permits monitoring of needle placement within the fibroid capsule (FIGURES 2A and 2B) and confirmation of hemostasis upon removal of the probe tip (FIGURE 2C).
RFVTA treats a wide range of fibroids
The Acessa System is used to treat fibroids of any location and type, with the exception of pedunculated or Type 0 myomas, in which case laparoscopic or hysteroscopic myomectomy is recommended. Calcified fibroids pose no challenges. A single radiofrequency needle generally is used to treat multiple fibroids, with ablation zones ranging in diameter from 1.0 to 6.7 cm. Large, irregularly shaped fibroids may require multiple ablation zones during the same procedure.
Final steps
6. Once the treatment is complete, as confirmed by informatics transmitted by the generator, retract the needle array, set the generator to coagulation mode to coagulate the needle track during withdrawal of the probe, and confirm hemostasis (FIGURE 2C).
7. After completion of all fibroid destruction, remove the disposable radiofrequency probe and close the laparoscopic and ultrasound port sites using standard procedures.
The patient can be discharged the same day and generally is able to return to normal activities within 3 to 5 days.
Postoperative analgesia usually consists solely of nonsteroidal anti-inflammatory agents.
A look at outcomes data
Garza and colleagues published the first feasibility report in 2011.24 They described the results of laparoscopic, ultrasound-guided RFVTA for the treatment of symptomatic uterine fibroids in Mexico using an Acessa predecessor. The 31 women enrolled in the study desired uterine preservation. Of these participants, 19 had completed 12 months of follow-up by the time the report was submitted for publication. Their responses to the UFS-QOL questionnaire indicated that their symptoms had decreased significantly in severity from baseline to 12 months, and their quality-of-life scores also improved significantly (P <.001). Mean uterine volume also declined significantly.
Robles and colleagues set out to confirm these findings using the investigational Halt Ablation System. They conducted a prospective, single-center, open-label clinical trial in Guatemala.25 They found dramatic improvement in symptom severity and health-related quality of life from baseline to 12 months. Mean uterine volume also decreased by 23.6% during this period. In addition, the severity and duration of menses declined.
Acessa is currently not approved for women seeking future childbearing. However, several successful pregnancies have been reported following the procedure.24,26–28 Theoretically, because it causes less damage to and disruption of healthy myometrium, Acessa may prove to be preferable to myomectomy. Head-to-head studies to evaluate this premise are ongoing.
Latest findings confirm widespread improvement of symptoms
The most recent and detailed reports of safety and efficacy of laparoscopic, ultrasound-guided RFVTA at 12 and 24 months were published by teams led by Chudnoff26 and Guido27 earlier this year. These investigations involved 135 women who reported moderate to severe heavy menstrual bleeding (160 mL–500 mL by alkaline hematin analysis). Clinically significant reduction in menstrual blood loss was achieved in 67.7% of participants at 12 months of follow-up.29
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Galen and colleagues performed a retrospective analysis of these same patients, demonstrating that RFVTA of intramural fibroids without submucosal components leads to a clinically and statistically significant reduction in menstrual blood loss.30
Patients generally began these trials with high symptom-severity scores and low health-related quality of life, yet all achieved significant improvement over time, as their UFS-QOL scores indicate (TABLE). The majority of patients (96%) were treated on an outpatient basis. As measured by contrast-enhanced magnetic resonance imaging, total mean uterine volume decreased by 24.3% at 12 months, and total mean fibroid volume decreased by 45.1% during the same interval. Ninety-four percent of the women reported that the treatment was effective in eliminating their symptoms at 12 months, and 98% said they would recommend the procedure to a friend having the same health problems.
Complication rates were low
Complications included a postoperative pelvic abscess and four other minor events. The latter were treated at the time of RFVTA or resolved without additional procedures. The reintervention rate was 0.7% (1/135 patients).26
Guido and colleagues reported 24-month outcomes for the 124 women entering the second year of the Halt study and affirmed the findings of Chudnoff’s team.27 The 112 patient-reported UFS-QOL scores at 24 months were virtually identical to those reported at 12 months. Six surgical reinterventions (6 of 124 patients, or 4.8%) for fibroid-related bleeding were reported between 12 and 24 months.
280 women have been treated so far
As of September 2013, 280 women have been treated under five international study protocols using the Halt Acessa System, and approximately two-thirds have been followed for more than 12 months, with excellent results. Two of the five clinical trials are under way in Germany and Canada:
- The Laparoscopic Uterine-Sparing Techniques Outcomes and Reinterventions (LUSTOR) trial in Germany is a prospective, randomized, single-center study comparing outcomes of RFVTA and laparoscopic myomectomy. Fifty women were enrolled and treated and will be followed for as long as 60 months.
- The Treatment Results of Uterine-Sparing Technologies (TRUST) trial is a prospective, multicenter, randomized study in Canada that compares direct and indirect costs associated with RFVTA, laparoscopic and abdominal myomectomy, and uterine artery embolization. Some US sites also will participate. As many as 200 women will be treated and followed for as long as 60 months.
RFVTA offers several benefits
Although hysterectomy provides a definitive “cure” to fibroid-related symptoms, it is not preferred by many patients and generally is more costly, with an increased risk of complications, a longer hospital stay, longer recovery (6–8 weeks), longer disability and more time off from work, and potential long-term sequelae.
The Acessa procedure not only is an outpatient, minimally invasive, uterine-sparing fibroid treatment option, it permits access to and ablation of almost all fibroids (with the exception of penduculated or Type 0 myomas). The data to date suggest that RFVTA provides many benefits, including a decline in symptom severity and improved health-related quality of life, as well as good cosmesis, quick recovery, and a rapid return to full activity.
There is a learning curve for RFVTA with Acessa—as with any new procedure. If physicians already perform ultrasound imaging, the learning process is shorter. Physicians interested in Acessa and furthering their education and training in laparoscopic RFVTA should consult the manufacturer’s professional education division through the company Web site at http://www.haltmedical.com.
CASE: RESOLVED
After she is counseled about the various uterine-sparing options for treatment, L.W. chooses to undergo laparoscopic, ultrasound-guided RFVTA using the Acessa System. The procedure is performed successfully, with the two fibroids destroyed using the appropriate algorithms, which took into account the size of the fibroids and the deployed needle array. Two additional 1-cm fibroids were found during the procedure and ablated. The patient was discharged home shortly after the procedure, with a nonsteroidal anti-inflammatory drug given for pain relief.
We want to hear from you! Tell us what you think.
CASE: WOMAN WITH HEAVY BLEEDING HOPES TO AVOID HYSTERECTOMY
L.W. is a 44-year-old woman (G2P2) with a 2-year history of menorrhagia and severe dysmenorrhea but no intermenstrual spotting or bleeding. She reports that she has tried to control her symptoms using hormonal methods, without success.
Examination reveals that she has an irregular, nontender uterus 8 weeks in size. Transvaginal ultrasonography shows two deep, prominent, intramural fibroids. The first is 2 cm by 3 cm in size in the left lateral uterus, adjacent to the endometrial stripe. The second fibroid is 3 cm by 4 cm in the fundal region. Sonohysterography reveals no intracavitary fibroids, although the left lateral myoma has distorted the endometrial cavity.
The patient is seeking removal of her fibroids but would like to preserve her uterus, if at all possible.
What options would you offer her?
Uterine fibroids are common in premenopausal women, affecting 70% to 80% of the population, especially women of African descent.1 Although the majority of women with fibroids are asymptomatic, approximately 30% report pelvic pressure, dyspareunia, dysmenorrhea, or abnormal uterine bleeding.2
Our patients are increasingly aware of uterine-sparing treatments for symptomatic fibroids.3,4 Women seek conservative procedures to avoid the risks and extended recovery times commonly associated with major surgery. Current laparoscopic techniques for removal of uterine fibroids can be complex and require advanced surgical skills. Deep intramural fibroids may not always be visible or readily accessible, making laparoscopic removal a more invasive and challenging approach for many gynecologic surgeons.
A significant recent advancement in minimally invasive gynecologic treatment of fibroids is the Acessa System (Halt Medical, Brentwood, California). This laparoscopic system involves the outpatient use of ultrasound-guided radiofrequency volumetric thermal ablation, or RFVTA.
In this article, I outline the development of this option and step you through its technique. I also review the outcomes data that have been published to date.
How the procedure evolved
In the 1980s, Donnez and Nisolle developed a method of laparoscopic Nd:YAG laser treatment of uterine fibroids, commonly referred to as myolysis.5 Later, Goldfarb developed a laparoscopic bipolar needle technique that coagulated and occluded blood vessels at the periphery of the fibroid.6 However, myolysis led to the formation of significant adhesions to the small bowel or omentum, or both, and was abandoned by the surgical community.
Other fibroid treatments have been developed, such as laparoscopic myomectomy, uterine artery ligation, uterine artery embolization (alone or as adjuvant treatment), and high-intensity focused ultrasound. Although these procedures have made a significant contribution to the minimally invasive treatment of fibroids, the need for reintervention can be substantial, as high as 29% at 1 to 10 years of follow-up in some reports.7–17
Related article: Ins and outs of straight-stick myomectomy James Robinson, MD, MD, and Gaby Moawad, MD (September 2012)
Radiofrequency needle ablation has been used successfully in the treatment and destruction of liver and kidney tumors for years. Lee envisioned use of the technology to treat uterine fibroids. He developed a technique using a retractable multiarray radiofrequency needle (Starburst XL, RITA Medical Systems, Fremont, California) that is inserted directly into the fibroid. In 2002, he reported the first use of intraperitoneal ultrasound for needle guidance in the laparoscopic ablation of 197 myomas in 52 symptomatic women who had declined hysterectomy and myomectomy.18 He found that a significant proportion of women experienced resolution of their symptoms, including heavy menstrual bleeding, dysmenorrhea, dyspareunia, and pelvic pressure, by 3 months, with continued improvement at 12 months.
Because he was unhappy with aspects of the RITA needle that prevented accurate and consistent ablation of fibroids, he developed other devices that would lead to RFVTA and the Acessa System. This system allows the gynecologic surgeon to target and treat deep intramural fibroids that may not be readily visible via conventional laparoscopy.
Between the time of Lee’s first report and his subsequent refinement of the device and procedure, several international fibroid radiofrequency ablation procedures emerged, including those of Bergamini, Milic, Ghezzi, and Carrafiello.19–22 These investigators published results in small cohorts and described significant improvement in patient-reported Uterine Fibroid Symptom and Quality-of-Life (UFS-QOL) scores as well as a reduction in myoma volume.23 Their studies provided evidence that RFA is a safe and effective uterine-sparing treatment of symptomatic uterine fibroids for selected patients.
Related article: Update on Minimally Invasive Surgery Amy Garcia, MD (April 2011)
How it works
RFVTA uses laparoscopy and laparoscopic ultrasound to guide placement of a needle electrode with a deployable array. Earlier international studies of radiofrequency ablation18–22 and ongoing studies of RFVTA23–26 have demonstrated the procedure’s therapeutic potential. Acessa was recently cleared (November 2012) by the US Food and Drug Administration (FDA).
RFVTA technique, step by step
1. Begin with a standard 5-mm laparoscopic infraumbilical port for the camera and video laparoscope (See VIDEO).
2. Place a 12-mm port in the midline, suprapubically at the level of the uterus, for insertion of the laparoscopic ultrasound probe. Now it is possible to map the uterus and plan an approach to destroy the fibroids.
3. Once you have determined your approach, insert the handpiece containing the radiofrequency needle through the abdominal wall under laparoscopic visualization.
4. Place the needle into the targeted fibroid using both laparoscopic and ultrasound guidance. Depending on the size of the fibroid, the needle array can be deployed to the maximum diameter necessary to effect destruction.
5. Engage the radiofrequency generator (FIGURE 1), utilizing the timing function for optimal destruction of the fibroid, based on the appropriate algorithm. The fibroid then will be ablated and destroyed without damage to the surrounding healthy myometrium.
The Acessa radiofrequency generator and accessories are designed to deliver monopolar radiofrequency energy to tissue through the handheld electrical probe. The procedure is monitored by real-time feedback to and from the generator via each of the thermocouples at the tips of the seven-needle array (FIGURE 1). In addition, laparoscopic ultrasound-guided visualization permits monitoring of needle placement within the fibroid capsule (FIGURES 2A and 2B) and confirmation of hemostasis upon removal of the probe tip (FIGURE 2C).
RFVTA treats a wide range of fibroids
The Acessa System is used to treat fibroids of any location and type, with the exception of pedunculated or Type 0 myomas, in which case laparoscopic or hysteroscopic myomectomy is recommended. Calcified fibroids pose no challenges. A single radiofrequency needle generally is used to treat multiple fibroids, with ablation zones ranging in diameter from 1.0 to 6.7 cm. Large, irregularly shaped fibroids may require multiple ablation zones during the same procedure.
Final steps
6. Once the treatment is complete, as confirmed by informatics transmitted by the generator, retract the needle array, set the generator to coagulation mode to coagulate the needle track during withdrawal of the probe, and confirm hemostasis (FIGURE 2C).
7. After completion of all fibroid destruction, remove the disposable radiofrequency probe and close the laparoscopic and ultrasound port sites using standard procedures.
The patient can be discharged the same day and generally is able to return to normal activities within 3 to 5 days.
Postoperative analgesia usually consists solely of nonsteroidal anti-inflammatory agents.
A look at outcomes data
Garza and colleagues published the first feasibility report in 2011.24 They described the results of laparoscopic, ultrasound-guided RFVTA for the treatment of symptomatic uterine fibroids in Mexico using an Acessa predecessor. The 31 women enrolled in the study desired uterine preservation. Of these participants, 19 had completed 12 months of follow-up by the time the report was submitted for publication. Their responses to the UFS-QOL questionnaire indicated that their symptoms had decreased significantly in severity from baseline to 12 months, and their quality-of-life scores also improved significantly (P <.001). Mean uterine volume also declined significantly.
Robles and colleagues set out to confirm these findings using the investigational Halt Ablation System. They conducted a prospective, single-center, open-label clinical trial in Guatemala.25 They found dramatic improvement in symptom severity and health-related quality of life from baseline to 12 months. Mean uterine volume also decreased by 23.6% during this period. In addition, the severity and duration of menses declined.
Acessa is currently not approved for women seeking future childbearing. However, several successful pregnancies have been reported following the procedure.24,26–28 Theoretically, because it causes less damage to and disruption of healthy myometrium, Acessa may prove to be preferable to myomectomy. Head-to-head studies to evaluate this premise are ongoing.
Latest findings confirm widespread improvement of symptoms
The most recent and detailed reports of safety and efficacy of laparoscopic, ultrasound-guided RFVTA at 12 and 24 months were published by teams led by Chudnoff26 and Guido27 earlier this year. These investigations involved 135 women who reported moderate to severe heavy menstrual bleeding (160 mL–500 mL by alkaline hematin analysis). Clinically significant reduction in menstrual blood loss was achieved in 67.7% of participants at 12 months of follow-up.29
Related article: Tips and techniques for robot-assisted laparoscopic myomectomy Arnold P. Advincula, MD, and Bich-Van Tran, MD (August 2013)
Galen and colleagues performed a retrospective analysis of these same patients, demonstrating that RFVTA of intramural fibroids without submucosal components leads to a clinically and statistically significant reduction in menstrual blood loss.30
Patients generally began these trials with high symptom-severity scores and low health-related quality of life, yet all achieved significant improvement over time, as their UFS-QOL scores indicate (TABLE). The majority of patients (96%) were treated on an outpatient basis. As measured by contrast-enhanced magnetic resonance imaging, total mean uterine volume decreased by 24.3% at 12 months, and total mean fibroid volume decreased by 45.1% during the same interval. Ninety-four percent of the women reported that the treatment was effective in eliminating their symptoms at 12 months, and 98% said they would recommend the procedure to a friend having the same health problems.
Complication rates were low
Complications included a postoperative pelvic abscess and four other minor events. The latter were treated at the time of RFVTA or resolved without additional procedures. The reintervention rate was 0.7% (1/135 patients).26
Guido and colleagues reported 24-month outcomes for the 124 women entering the second year of the Halt study and affirmed the findings of Chudnoff’s team.27 The 112 patient-reported UFS-QOL scores at 24 months were virtually identical to those reported at 12 months. Six surgical reinterventions (6 of 124 patients, or 4.8%) for fibroid-related bleeding were reported between 12 and 24 months.
280 women have been treated so far
As of September 2013, 280 women have been treated under five international study protocols using the Halt Acessa System, and approximately two-thirds have been followed for more than 12 months, with excellent results. Two of the five clinical trials are under way in Germany and Canada:
- The Laparoscopic Uterine-Sparing Techniques Outcomes and Reinterventions (LUSTOR) trial in Germany is a prospective, randomized, single-center study comparing outcomes of RFVTA and laparoscopic myomectomy. Fifty women were enrolled and treated and will be followed for as long as 60 months.
- The Treatment Results of Uterine-Sparing Technologies (TRUST) trial is a prospective, multicenter, randomized study in Canada that compares direct and indirect costs associated with RFVTA, laparoscopic and abdominal myomectomy, and uterine artery embolization. Some US sites also will participate. As many as 200 women will be treated and followed for as long as 60 months.
RFVTA offers several benefits
Although hysterectomy provides a definitive “cure” to fibroid-related symptoms, it is not preferred by many patients and generally is more costly, with an increased risk of complications, a longer hospital stay, longer recovery (6–8 weeks), longer disability and more time off from work, and potential long-term sequelae.
The Acessa procedure not only is an outpatient, minimally invasive, uterine-sparing fibroid treatment option, it permits access to and ablation of almost all fibroids (with the exception of penduculated or Type 0 myomas). The data to date suggest that RFVTA provides many benefits, including a decline in symptom severity and improved health-related quality of life, as well as good cosmesis, quick recovery, and a rapid return to full activity.
There is a learning curve for RFVTA with Acessa—as with any new procedure. If physicians already perform ultrasound imaging, the learning process is shorter. Physicians interested in Acessa and furthering their education and training in laparoscopic RFVTA should consult the manufacturer’s professional education division through the company Web site at http://www.haltmedical.com.
CASE: RESOLVED
After she is counseled about the various uterine-sparing options for treatment, L.W. chooses to undergo laparoscopic, ultrasound-guided RFVTA using the Acessa System. The procedure is performed successfully, with the two fibroids destroyed using the appropriate algorithms, which took into account the size of the fibroids and the deployed needle array. Two additional 1-cm fibroids were found during the procedure and ablated. The patient was discharged home shortly after the procedure, with a nonsteroidal anti-inflammatory drug given for pain relief.
We want to hear from you! Tell us what you think.
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107.
- Katz VL, Lentz G, Lobo RA, Gershenson D, eds. Comprehensive Gynecology. 5th ed. Philadelphia, PA: Elsevier Mosby Saunders; 2007.
- Rosen P. The endangered uterus. More Magazine Web site. http://www.more.com/health/wellness/endangered-uterus. December 2008/January 2009. Accessed October 16, 2013.
- Tulandi T, Levy BS. So You’re Having a Hysterectomy? Hoboken, NJ: John Wiley & Sons; 2004.
- Donnez J, Squifflet J, Polet R, Nisolle M. Laparoscopic myolysis. Human Reprod Update. 2000;6(6):609–613.
- Goldfarb HA. Bipolar laparoscopic needles for myoma coagulation. J Am Assoc Gynecol Laparosc. 1995;2(2):175–179.
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881.
- Goodwin SC, Spies JB, Worthington-Kirsch R, et al; Fibroid Registry for Outcomes Data (FIBROID) Registry Steering Committee and Core Site Investigators. Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008;111(1):22–33.
- Yoo EH, Lee PI, Huh C-Y, et al. Predictors of leiomyoma recurrence after laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(6):690–697.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356(4):360–370.
- Huang JY, Kafy S, Dugas A, Valenti D, Tulandi T. Failure of uterine fibroid embolization. Fertil Steril. 2006;85(1):30–35.
- Kroncke TJ, Gauruder-Burmester A, Scheurig C, et al. Transarterial embolization for uterine fibroids: clinical success rate and results of magnetic resonance imaging [in German]. Rofo. 2005;177(1):89–98.
- Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31(1):73–85.
- Munoz JL, Jimenez JS, Hernandez C, et al. Hysteroscopic myomectomy: our experience and review. JSLS. 2003;7(1):39–48.
- Reed SD, Newton KM, Thompson LB, McCrummen BA, Warolin AK. The incidence of repeat uterine surgery following myomectomy. J Womens Health (Larchmt). 2006;15(9):1046–1052.
- Spies JB, Bruno J, Czeyda-Pommersheim F, et al. Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol. 2005;106(5 Pt 1):933–939.
- Lee BB. Radiofrequency ablation of uterine leiomyomata: a new minimally invasive hysterectomy alternative. Obstet Gynecol. 2002;99(4Suppl):9S.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192(3):768–773.
- Milic A, Asch MR, Hawrylyshyn PA, et al. Laparoscopic ultrasound-guided radiofrequency ablation of uterine fibroids. Cardiovasc Intervent Radiol. 2006;29(4):694–698.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21(11):2081–2085.
- Carrafiello G, Recaldini C, Fontana F, et al. Ultrasound-guided radiofrequency thermal ablation of uterine fibroids: medium-term follow-up. Cardiovasc Intervent Radiol. 2010;33(1):113–119.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomymata. Obstet Gynecol. 2002;99(2):290–300.
- Garza Leal JG, Hernandez Leon I, Castillo Saenz L, Lee BB. Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 ablation system. J Minim Invasive Gynecol. 2011;18(3):364–371.
- Robles R, Aguirre VA, Argueta AI, Guerrero MR. Laparoscopic radiofrequency volumetric ablation of uterine myomas with 12 months of follow-up. Int J Gynecol Obstet. 2013;120(1):65–69.
- Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013;121(5):1075–1082.
- Guido RS, Macer JA, Abbott K, Falls JL, Tilley IB, Chudnoff SG. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Quality Life Outcomes. 2013;11(139):1–8.
- Berman JM, Puscheck EE, Diamond MP. Full-term vaginal live birth after laparoscopic radiofrequency ablation of a large, symptomatic intramural fibroid. J Reprod Med. 2012;57:159–163.
- Galen DI, Isaacson KB, Lee BB. Does menstrual bleeding decrease after ablation of intramural myomas? A retrospective study [published online ahead of print September 6, 2013]. J Minim Invasive Gynecol. doi: 10.1016/j.jmig.2013.05.007.
- Lukes AS, Muse K, Richter HE, Moore KA, Patrick DL. Estimating a meaningful reduction in menstrual blood loss for women with heavy bleeding. Curr Med Res Opinion. 2010;26(11):2673–2678.
- Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188(1):100–107.
- Katz VL, Lentz G, Lobo RA, Gershenson D, eds. Comprehensive Gynecology. 5th ed. Philadelphia, PA: Elsevier Mosby Saunders; 2007.
- Rosen P. The endangered uterus. More Magazine Web site. http://www.more.com/health/wellness/endangered-uterus. December 2008/January 2009. Accessed October 16, 2013.
- Tulandi T, Levy BS. So You’re Having a Hysterectomy? Hoboken, NJ: John Wiley & Sons; 2004.
- Donnez J, Squifflet J, Polet R, Nisolle M. Laparoscopic myolysis. Human Reprod Update. 2000;6(6):609–613.
- Goldfarb HA. Bipolar laparoscopic needles for myoma coagulation. J Am Assoc Gynecol Laparosc. 1995;2(2):175–179.
- Hanafi M. Predictors of leiomyoma recurrence after myomectomy. Obstet Gynecol. 2005;105(4):877–881.
- Goodwin SC, Spies JB, Worthington-Kirsch R, et al; Fibroid Registry for Outcomes Data (FIBROID) Registry Steering Committee and Core Site Investigators. Uterine artery embolization for treatment of leiomyomata: long-term outcomes from the FIBROID Registry. Obstet Gynecol. 2008;111(1):22–33.
- Yoo EH, Lee PI, Huh C-Y, et al. Predictors of leiomyoma recurrence after laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(6):690–697.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Edwards RD, Moss JG, Lumsden MA, et al; Committee of the Randomized Trial of Embolization versus Surgical Treatment for Fibroids. Uterine-artery embolization versus surgery for symptomatic uterine fibroids. N Engl J Med. 2007;356(4):360–370.
- Huang JY, Kafy S, Dugas A, Valenti D, Tulandi T. Failure of uterine fibroid embolization. Fertil Steril. 2006;85(1):30–35.
- Kroncke TJ, Gauruder-Burmester A, Scheurig C, et al. Transarterial embolization for uterine fibroids: clinical success rate and results of magnetic resonance imaging [in German]. Rofo. 2005;177(1):89–98.
- Mara M, Maskova J, Fucikova Z, et al. Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol. 2008;31(1):73–85.
- Munoz JL, Jimenez JS, Hernandez C, et al. Hysteroscopic myomectomy: our experience and review. JSLS. 2003;7(1):39–48.
- Reed SD, Newton KM, Thompson LB, McCrummen BA, Warolin AK. The incidence of repeat uterine surgery following myomectomy. J Womens Health (Larchmt). 2006;15(9):1046–1052.
- Spies JB, Bruno J, Czeyda-Pommersheim F, et al. Long-term outcome of uterine artery embolization of leiomyomata. Obstet Gynecol. 2005;106(5 Pt 1):933–939.
- Lee BB. Radiofrequency ablation of uterine leiomyomata: a new minimally invasive hysterectomy alternative. Obstet Gynecol. 2002;99(4Suppl):9S.
- Bergamini V, Ghezzi F, Cromi A, et al. Laparoscopic radiofrequency thermal ablation: a new approach to symptomatic uterine myomas. Am J Obstet Gynecol. 2005;192(3):768–773.
- Milic A, Asch MR, Hawrylyshyn PA, et al. Laparoscopic ultrasound-guided radiofrequency ablation of uterine fibroids. Cardiovasc Intervent Radiol. 2006;29(4):694–698.
- Ghezzi F, Cromi A, Bergamini V, et al. Midterm outcome of radiofrequency thermal ablation for symptomatic uterine myomas. Surg Endosc. 2007;21(11):2081–2085.
- Carrafiello G, Recaldini C, Fontana F, et al. Ultrasound-guided radiofrequency thermal ablation of uterine fibroids: medium-term follow-up. Cardiovasc Intervent Radiol. 2010;33(1):113–119.
- Spies JB, Coyne K, Guaou Guaou N, et al. The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomymata. Obstet Gynecol. 2002;99(2):290–300.
- Garza Leal JG, Hernandez Leon I, Castillo Saenz L, Lee BB. Laparoscopic ultrasound-guided radiofrequency volumetric thermal ablation of symptomatic uterine leiomyomas: feasibility study using the Halt 2000 ablation system. J Minim Invasive Gynecol. 2011;18(3):364–371.
- Robles R, Aguirre VA, Argueta AI, Guerrero MR. Laparoscopic radiofrequency volumetric ablation of uterine myomas with 12 months of follow-up. Int J Gynecol Obstet. 2013;120(1):65–69.
- Chudnoff SG, Berman JM, Levine DJ, Harris M, Guido RS, Banks E. Outpatient procedure for the treatment and relief of symptomatic uterine myomas. Obstet Gynecol. 2013;121(5):1075–1082.
- Guido RS, Macer JA, Abbott K, Falls JL, Tilley IB, Chudnoff SG. Radiofrequency volumetric thermal ablation of fibroids: a prospective, clinical analysis of two years’ outcome from the Halt trial. Health Quality Life Outcomes. 2013;11(139):1–8.
- Berman JM, Puscheck EE, Diamond MP. Full-term vaginal live birth after laparoscopic radiofrequency ablation of a large, symptomatic intramural fibroid. J Reprod Med. 2012;57:159–163.
- Galen DI, Isaacson KB, Lee BB. Does menstrual bleeding decrease after ablation of intramural myomas? A retrospective study [published online ahead of print September 6, 2013]. J Minim Invasive Gynecol. doi: 10.1016/j.jmig.2013.05.007.
- Lukes AS, Muse K, Richter HE, Moore KA, Patrick DL. Estimating a meaningful reduction in menstrual blood loss for women with heavy bleeding. Curr Med Res Opinion. 2010;26(11):2673–2678.
Tips and techniques for robot-assisted laparoscopic myomectomy
CASE: IS OPEN MYOMECTOMY THE BEST OPTION FOR THIS PATIENT?
In her only pregnancy, a 34-year-old patient experienced a spontaneous first-trimester loss and underwent dilation and curettage. She had noted an increase in her abdominal girth, as well as pelvic pressure, but had attributed both to the pregnancy. Three months after the pregnancy loss, however, neither had resolved. Because she hopes to conceive again and deliver a healthy infant, the patient consulted a gynecologist. After ultrasonography revealed multiple fibroids, that physician recommended open myomectomy. The patient, a Jehovah’s Witness, comes to your office for a second opinion.
On physical examination, she has a 16-weeks’ sized irregular uterus with the cervix displaced behind the pubic symphysis. T2 weighted scans from magnetic resonance imaging (MRI) of the pelvis in the sagittal view reveal multiple subserosal and intramural fibroids that displace, but do not involve, the uterine cavity (FIGURE 2). The MRI results confirm that the uterus extends beyond the pelvis above the sacral promontory, the fundus lies a few centimeters below the umbilicus, and there is no evidence of adenomyosis. The patient’s hemoglobin level is normal (12.2 g/dL).
What surgical approach would you recommend?
Endometrial ablation, uterine artery embolization, MRI-guided focused ultrasound, hysterectomy, and myomectomy are all treatments for symptomatic uterine fibroids. For women desiring uterine preservation and future fertility, however, myomectomy is the preferred option of many experts.
Myomectomy traditionally has been performed via an open laparotomy approach. With the rise of minimally invasive surgery in gynecology, safe endoscopic surgical approaches and techniques have evolved.
The EndoWrist technology from the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, California) provides increased instrument range of motion, enabling the surgeon to mimic open surgical technique, thereby simplifying the technical challenges of conventional laparoscopic suturing and knot-tying. However, this technology does not minimize or simplify the challenges that leiomyomas can pose, including enucleation (FIGURE 1). Although it has facilitated the progression and adoption of endoscopic myomectomy, the da Vinci system requires an experienced gynecologic endoscopic surgeon.
In this article, we outline the essential steps and offer some clinical surgical pearls to make robot-assisted laparoscopic myomectomy a systematic, safe, and efficient procedure.
Benefits of the robotic approach
Compared with open abdominal myomectomy, the robot-assisted laparoscopic approach is associated with less blood loss, lower complication rates, and shorter hospitalization.1 A retrospective case study from the Cleveland Clinic confirmed these findings when investigators compared surgical outcomes between the robot-assisted laparoscopic approach, standard laparoscopy, and open myomectomy.2 In an assessment of 575 cases (393 open, 93 laparoscopic, and 89 robot-assisted laparoscopic), they found the robot-assisted laparoscopic approach to be associated with the removal of significantly larger myomas (vs standard laparoscopy), as well as lower blood loss and shorter hospitalization (vs open myomectomy).2
Related Article: The robot is gaining ground in gynecologic surgery. Should you be using it?
Comprehensive preoperative assessment is critical
Careful patient selection and thorough preoperative assessment are the cornerstones of successful robot-assisted laparoscopic myomectomy. Among the variables that should be considered in selecting patients are uterine size, the patient’s body habitus, and the quantity, size, consistency, type, and location of fibroids.
Size of the uterus, body habitus, and laxity of the abdominal wall all influence the surgeon’s ability to create the necessary operating space. Intraperitoneal space is required during myomectomy because of the need to apply traction and countertraction during enucleation of fibroids. If the necessary space cannot be obtained, a minilaparotomy technique is one alternative. This technique, described by Glasser, limits the skin incision to 3 to 6 cm in myomectomies for large fibroids that can be accessed easily anteriorly.3
Number and location of fibroids. Women with a solitary fibroid, a few dominant fibroids, or multiple pedunculated fibroids are excellent candidates for an endoscopic approach. Although there are no limits on the number of fibroids that can be removed, women with what we have termed “miliary fibroids,” or multiple fibroids disseminated throughout the entire myometrium, with very little normal myometrium, are poor surgical candidates. Not only does the presence of these fibroids leave some concern about the functional ability of the remaining myometrium in pregnancy, but it may be technically difficult to adequately resect all of the critical fibroids and reapproximate the myometrial defects.
The consistency of fibroids also affects the ease of the enucleation process during myomectomy. Due to the soft, spongy nature of degenerating fibroids and their tendency to fragment and shred when manipulated, these cases are more challenging and should not be attempted without a solid foundation of surgical experience.
MRI serves several purposes
Fibroids can be localized and identified as pedunculated, subserosal, intramural, or submucosal via MRI. Measurements and spatial orientation of the fibroids within the uterus can be formulated using T2 weighted coronal, axial, and sagittal images.
The risk of entering the uterine cavity, as well as the risk of synechiae, may be significantly greater if leiomyomas abut and distort the cavity. Surgical strategies, such as planning the location of the hysterotomy or the inclusion of other procedures (eg, hysteroscopic resection for type 0 or type 1 submucosal fibroids), can be formulated with the information provided by MRI. In cases involving multiple fibroids or intramural fibroids, in particular, MRI serves as a surgical “treasure map” or “GPS.” Preoperative MRI is also one way to offset the lack of haptic feedback during surgery to locate the myomas for removal. As we mentioned earlier, important characteristics, such as degeneration or calcification, also can be readily observed on MRI.
Most important, MRI can distinguish adenomyosis from leiomyomas. Adenomyosis can mimic leiomyomas—both clinically and on sonographic imaging—particularly when it is focal in nature. MRI can make the distinction between these two entities so that patients can be counseled appropriately.
SURGICAL TECHNIQUES
Use a uterine manipulator
This device will facilitate the enucleation process, providing another focal point for traction and countertraction. A variety of uterine manipulators are available. We use the Advincula Arch (Cooper Surgical, Trumball, Connecticut) in conjunction with the Uterine Positioning System (Cooper Surgical). The latter attaches to the operating table and to the Advincula Arch to secure the uterus in a steady position throughout the procedure.
During enucleation, the manipulator is crucial to hold the uterus within the pelvis and the field of vision and to act as countertraction as traction is applied to the fibroid.
Individualize port placement
Rather than premeasure port placement on the abdomen, we individualize it, based on a variety of characteristics, including body habitus and uterine pathology (FIGURE 3). However, we follow some basic principles:
- We insert a Veress needle through the umbilicus to achieve pneumoperitoneum
- After insufflation, we use an upper quadrant entry (right or left, depending on which side the robot patient side cart is docked) under direct visualization using a 5-mm laparoscope and optical trocar. This entry will serve as the assistant port for surgery.
- Before placing the rest of the ports under direct visualization, relative to uterine pathology, we elevate the uterus out of the pelvis. This step ensures that enough distance is placed between the camera and the instrument arms to adequately visualize and perform the surgery.
- In patients with a uterus larger than approximately 14- to 16-weeks’ size, a supraumbilical camera port often is necessary.
- We generally employ a four-arm technique using a 12-mm Xcel trocar (Ethicon Endo-Surgery, Blue Ash, Ohio) that is 150 mm in length for the camera port, three 8-mm telerobotic trocar ports, and a 5-mm Airseal trocar (SurgiQuest, Orange, Connecticut) for the assistant port. There should be at least one hand’s breadth between the ports to minimize arm collision and maximize range of motion.
- Although the 12-mm Xcel trocar comes in a variety of lengths (75–150 mm), we strongly recommend, and exclusively use, the longest length for the camera. Once the camera is docked high on the neck of the longer trocar, more space is created between the setup joints of the robotic arms, enabling greater range of motion and fewer instrument and arm collisions.
- We generally use the following wristed robotic instruments to perform myomectomy: tenaculum, monopolar scissors, and PlasmaKinetic (PK) bipolar forceps.
Inject vasopressin into the myometrium
Vasopressin causes vasospasm and uterine muscle contraction and decreases blood loss during myomectomy. It should be diluted (20 U in 50–200 mL of normal saline), introduced with a 7-inch, 22-gauge spinal needle through the anterior abdominal wall, and injected into the myometrium and serosa overlying the fibroid (VIDEO 1 and VIDEO 2).
Perform this step with care, with aspiration prior to injection, to avoid intravascular injection. Although vasopressin is safe overall, serious complications and rare cases of life-threatening hypotension, pulmonary edema, bradycardia, and cardiac arrest have been reported after the injection of as little as 3 U into the myometrium.4–7
Relative contraindications to vasopressin, such as hypertension, should be discussed with anesthesia prior to use of the drug during surgery.
Enucleate the fibroid
Although either a vertical or a horizontal-transverse incision can be made overlying the uterus, a transverse incision allows for technical optimization of wristed movements for suturing and efficient closure. Whenever possible, therefore, we favor a transverse hysterotomy.
During enucleation, keep the use of thermal energy to a minimum. The same holds true for the uterine incision, although its length can be extended as needed.
Using the wristed robotic tenaculum (or an assistant using a laparoscopic tenaculum or corkscrew), grasp and elevate the myoma away from the fixed uterus (FIGURE 1). This step is not intended to enucleate the myoma through force, but to apply traction and position the fibroid to best delineate and present the leading edge of the pseudocapsule that lies between the myoma and the myometrium. Dissection then can proceed using a “push and spread” technique, bluntly separating the natural plane between the fibroid and the myometrium. Occasionally, fibrous attachments of the pseudocapsule can be transected sharply using the bipolar forceps and monopolar scissors.
Again, we encourage the intermittent use of minimal thermal energy to facilitate this process and achieve temporary hemostasis. As the dissection progresses, the fibroid can be regrasped closer to its leading edge, causing the myoma to be rolled out (VIDEO 3 and VIDEO 4).
Close the myometrium We advocate multilayer closure with reapproximation of the myometrium and serosal edges to achieve hemostasis and prevent hematoma (VIDEO 5).
The half-life of vasopressin ranges from 20 to 40 minutes. By this point of the procedure, assuming that the use of thermal energy has been minimal, the myometrial edges should be bleeding slightly, demonstrating adequate reperfusion. The myometrial defect then can be repaired using delayed absorbable suture, such as 2-0 V-Loc 180 (Covidien, Mansfield, Massachusetts).
Barbed suture has revolutionized laparoscopy and minimally invasive surgery, eliminating the need for endoscopic knot-tying. Quill suture (Angiotech, Vancouver, British Columbia, Canada) and V-Loc suture are used safely throughout gynecology, myomectomy included.8,9 However, when the endometrial cavity has been entered, avoid using barbed suture to reapproximate this initial layer to prevent synechiae.
No closure technique has been shown to prevent uterine rupture. Uterine rupture during pregnancy is one of the most serious potential complications following myomectomy. The precise risk of rupture after laparoscopic or robot-assisted laparoscopic myomectomy has not been determined.
Parker and colleagues evaluated case reports of uterine rupture after laparoscopic myomectomy in an attempt to identify a common causal risk factor. In their review of 19 uterine ruptures, however, they were unable to identify a single plausible risk factor. Uterine rupture has occurred in cases involving three-layer closure, removal of pedunculated fibroids, removal of fibroids as small as 1 cm, and in cases where no thermal energy was used.10
Pregnancy rates and outcomes have not been well-established because of confounding variables, such as a high prevalence of infertility and difficulty with long-term follow-up. One of the largest retrospective case studies on this topic involved 872 women who underwent robot-assisted myomectomy.11 Preterm delivery was correlated with the number of myomas removed and an anterior location of the largest incision.11
Undock the robot for morcellation
We strongly recommend that fibroids be morcellated using the 5-mm laparoscope while the robot is undocked, for several reasons. First, we advocate use of the robotic camera port site for morcellation. In the umbilicus, or midline, patients generally experience less pain. And with insufflation, the camera port site is the highest point on the abdomen, allowing greater distance between the morcellator and the iliac vessels and other major structures.
Second, the 12-mm robotic camera is heavy and cumbersome, easily causing fatigue when held separately. The robotic arms and patient side cart are bulky and can be limiting, physically impeding the range of motion necessary to morcellate safely, effectively, and efficiently.
After undocking the robot, remove the midline camera port to introduce the morcellator with the aid of a 5-mm laparoscope through a lateral port. We recommend taking the patient out of a steep Trendelenberg position and placing her in minimal Trendelenberg during morcellation to keep the specimen and fragments from falling to the upper abdomen.
Perfect the art of morcellation
A number of morcellators use electrical or mechanical energy. Blades ranging in diameter from 12 to 20 mm also are available. We favor the reusable MOREsolution tissue-extraction system (Blue Endo, Lenexa, Kansas) with a disposable 20-mm blade, particularly for large or multiple myomas.
The art of morcellation can be learned (VIDEO 6 and VIDEO 7). We recommend the following strategies:
- Slower morcellation speeds cause less fragmentation but may prolong the surgery significantly when the myomas are large. For such myomas, as well as cases that involve significant calcification, we recommend morcellation speeds of at least 600 rpm.
- A beveled trocar is preferred because it allows for longer continuous morcellation along the surface of the myoma and less fragmentation and coring.
- As morcellation nears completion and the specimen begins to fragment more, use short bursts of activation with increased traction, and ask the assistant to help stabilize the end pieces. This approach will help minimize the dissemination of fragments throughout the entire abdominal and pelvic cavity.
- Reapproximate the fascia for all trocar sites larger than 10 mm to prevent incisional hernias. When you exchange the robotic camera port with the morcellator, only one port site will require fascial closure because all other trocar sites typically are 8 mm or smaller.
It is critical that you inspect and remove all fragments and debris after morcellation to prevent iatrogenic multiple peritoneal parasitic myomas. First described in 2006,12 this unusual complication, leiomyomatosis peritonealis disseminate, has been reported with greater frequency as minimally invasive surgery and morcellation have become more common. This complication is thought to arise from small fragments left behind after morcellation of a uterus or myoma. Although spontaneous cases can occur, they are rare.
Place an adhesion barrier
Myomectomy can induce the formation of significant adhesions. For that reason, as the final step before fascial closure, we recommend that an adhesion barrier be placed over any hysterotomy sites. Although they are indicated and FDA-approved only for laparotomy, we typically place Interceed (Ethicon, Cornelia, Georgia) or Seprafilm (Genzyme, Framingham, Massachusetts) over hysterotomy sites.
CODING FOR ROBOT-ASSISTED MYOMECTOMY: ADDITIONAL REIMBURSEMENT MAY NOT BE FORTHCOMING
Robot-assisted surgery is an emerging technology. As such, many health insurance companies, the American Congress of Obstetricians and Gynecologists (ACOG), and Current Procedural Terminology (CPT) editorial staff have weighed in on it. In essence, many payers have indicated that they will not provide the physician with additional reimbursement for performing a surgical procedure using robotic assistance. That is not to say that all payers will rule out additional reimbursement, although most of the larger payers have indicated that additional reimbursement is not going to happen.
Both ACOG and CPT officials have indicated that robot-assisted surgical procedures should be reported using existing CPT codes, based on the procedure and the surgical approach used, rather than coding them as an unlisted procedure. These organizations also have indicated that use of the modifier –22 on the basic laparoscopic procedure would be inappropriate because robotic assistance does not represent an unusual procedure, based on the patient’s condition.
However, if there is a chance that you can gain additional reimbursement for robotic surgery, how can you inform the payer that it was performed? The only currently accepted way to do so is to report code S2900, Surgical techniques requiring use of robotic surgical system (list separately in addition to the code for the primary procedure), in addition to the basic code for the laparoscopic approach. Code S2900 was added by CPT to the national code set in 2005 at the request of Blue Cross/Blue Shield so that the payer could track the incidence of robotic surgery. Because it is not a “regular” CPT code, S2900 was never assigned a relative value, so it is up to the surgeon to set a surgical charge for use of the robot. In doing so, the surgeon must be able to provide supporting documentation as to why additional reimbursement is being requested and on what basis the charge was calculated.
Therefore, if a robot-assisted laparoscopic myomectomy is performed, the first CPT code listed on the claim should be 58545, Laparoscopy, surgical, myomectomy, excision; 1 to 4 intramural myomas with total weight of 250 g or less and/or removal of surface myomas. An alternative would be code 58546, Laparoscopy, surgical, myomectomy, excision; 5 or more intramural myomas and/or intramural myomas with total weight greater than 250 g.
Code S2900 then would be listed second. No modifiers (such as modifier –59 [distinct procedure] or –51 [multiple procedures]) should be added to S2900 because this code does not represent either a distinct or multiple surgical procedure.
—MELANIE WITT, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
CASE: RESOLVED
Because of the patient’s religious beliefs, minimal blood loss is an important goal for any surgery she undergoes. Consequently, you recommend robot-assisted laparoscopic myomectomy, and the operation is completed without complications.
TAKE-HOME MESSAGE
The success of minimally invasive myomectomy requires a careful preoperative work-up and thorough understanding of surgical dissection and suturing techniques. In combination with this knowledge, advanced surgical technology, such as robotics and barbed suture, can truly transform the way myomectomy is performed, providing both patients and physicians with additional options for the conservative management of symptomatic uterine fibroids.
KEY POINTS FOR SUCCESS WITH THE ROBOT
Select patients with care for robot-assisted laparoscopic myomectomy, and perform thorough preoperative assessment. When planning a surgical approach, keep in mind the patient’s uterine size and body habitus and the quantity, size, consistency, type, and location of fibroids.
Use preoperative magnetic resonance imaging to characterize and locate fibroids and differentiate adenomyosis from leiomyomas.
In patients with a uterus larger than approximately 14- to 16-weeks’ size, consider a supraumbilical camera port.
Although the 12-mm Xcel trocar comes in a variety of lengths (75–150 mm), use the 150-mm length for the camera port. Once the camera is docked high on the neck of the longer trocar, more space is created between the setup joints of the robotic arms, enabling greater range of motion and fewer instrument and arm collisions.
Whenever possible, perform a transverse hysterotomy, keeping the length of the incision as short as possible and minimizing use of thermal energy during enucleation of fibroids.
Do not enucleate myomas through force, but apply traction and position each fibroid in order to best delineate and pre-sent the leading edge between the myoma and the myometrium.
Use multilayer closure with reapproximation of the myometrium and serosal edges to achieve hemostasis and prevent hematoma.
Perform morcellation through a 5-mm laparoscope with the robot undocked, using the camera port site for morcellation.
Take the patient out of a steep Trendelenberg position and place her in minimal Trendelenberg during morcellation to optimize ergonomics and prevent fragments from falling into the upper
abdomen.
Inspect the abdomen and remove all fragments and debris after morcellation to help prevent leiomyomatosis peritonealis disseminate.
- Advincula AP, Xu X, Goudeau S 4th, Ransom SB. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14(6):698–705.
- Barakat EE, Bedaiwy MA, Zimberg S, Nutter B, Nosseir M, Falcone T. Robotic-assisted, laparoscopic, and abdominal myomectomy: a comparison of surgical outcomes. Obstet Gynecol. 2011;117(2 Pt 1):256–265.
- Glasser MH. Minilaparotomy myomectomy: a minimally invasive alternative for the large fibroid uterus. J Minim Invasive Gynecol. 2005;12(3):275–283.
- Hung MH, Wang YM, Chia YY, Liu K. Intramyometrial injection of vasopressin causes bradycardia and cardiac arrest—report of two cases. Acta Anaesthesiol Taiwan. 2006;44(4):243–247.
- Tulandi T, Beique F, Kimia M. Pulmonary edema: a complication of local injection of. Fertil Steril. 1996;66(3):478–480.
- Nezhat F, Admon D Nezhat CH, Dicorpo JE, Nezhat C. Life-threatening hypotension after vasopressin injection during operative laparoscopy, followed by uneventful repeat laparoscopy. J Am Assoc Gynecol Laparosc. 1994;2(1):83–86.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: Severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Einarsson JI, Chavan NR, Suzuki Y, Jonsdottir G, Vellinga TT, Greenberg JA. Use of bidirectional barbed suture in laparoscopic myomectomy: evaluation of perioperative outcomes, safety and efficacy. J Minim Invasive Gynecol. 2011;18(1):92–95.
- Angioli R, Plotti F, Montera R, et al. A new type of absorbable barbed suture for use in laparoscopic myomectomy. Int J Gynaecol Obstet. 2012;117(3):220–223.
- Parker WH, Einarsson J, Istre O, Dubuisson J. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
- Pitter MC, Gargiulo AR, Bonaventura LM, Lehman JS, Srouji SS. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28(1):99–108.
- Paul PG, Koshy AK. Multiple peritoneal parasitic myomas after laparoscopic myomectomy and morcellation. Fertil Steril. 2006;85(2):492–493.
CASE: IS OPEN MYOMECTOMY THE BEST OPTION FOR THIS PATIENT?
In her only pregnancy, a 34-year-old patient experienced a spontaneous first-trimester loss and underwent dilation and curettage. She had noted an increase in her abdominal girth, as well as pelvic pressure, but had attributed both to the pregnancy. Three months after the pregnancy loss, however, neither had resolved. Because she hopes to conceive again and deliver a healthy infant, the patient consulted a gynecologist. After ultrasonography revealed multiple fibroids, that physician recommended open myomectomy. The patient, a Jehovah’s Witness, comes to your office for a second opinion.
On physical examination, she has a 16-weeks’ sized irregular uterus with the cervix displaced behind the pubic symphysis. T2 weighted scans from magnetic resonance imaging (MRI) of the pelvis in the sagittal view reveal multiple subserosal and intramural fibroids that displace, but do not involve, the uterine cavity (FIGURE 2). The MRI results confirm that the uterus extends beyond the pelvis above the sacral promontory, the fundus lies a few centimeters below the umbilicus, and there is no evidence of adenomyosis. The patient’s hemoglobin level is normal (12.2 g/dL).
What surgical approach would you recommend?
Endometrial ablation, uterine artery embolization, MRI-guided focused ultrasound, hysterectomy, and myomectomy are all treatments for symptomatic uterine fibroids. For women desiring uterine preservation and future fertility, however, myomectomy is the preferred option of many experts.
Myomectomy traditionally has been performed via an open laparotomy approach. With the rise of minimally invasive surgery in gynecology, safe endoscopic surgical approaches and techniques have evolved.
The EndoWrist technology from the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, California) provides increased instrument range of motion, enabling the surgeon to mimic open surgical technique, thereby simplifying the technical challenges of conventional laparoscopic suturing and knot-tying. However, this technology does not minimize or simplify the challenges that leiomyomas can pose, including enucleation (FIGURE 1). Although it has facilitated the progression and adoption of endoscopic myomectomy, the da Vinci system requires an experienced gynecologic endoscopic surgeon.
In this article, we outline the essential steps and offer some clinical surgical pearls to make robot-assisted laparoscopic myomectomy a systematic, safe, and efficient procedure.
Benefits of the robotic approach
Compared with open abdominal myomectomy, the robot-assisted laparoscopic approach is associated with less blood loss, lower complication rates, and shorter hospitalization.1 A retrospective case study from the Cleveland Clinic confirmed these findings when investigators compared surgical outcomes between the robot-assisted laparoscopic approach, standard laparoscopy, and open myomectomy.2 In an assessment of 575 cases (393 open, 93 laparoscopic, and 89 robot-assisted laparoscopic), they found the robot-assisted laparoscopic approach to be associated with the removal of significantly larger myomas (vs standard laparoscopy), as well as lower blood loss and shorter hospitalization (vs open myomectomy).2
Related Article: The robot is gaining ground in gynecologic surgery. Should you be using it?
Comprehensive preoperative assessment is critical
Careful patient selection and thorough preoperative assessment are the cornerstones of successful robot-assisted laparoscopic myomectomy. Among the variables that should be considered in selecting patients are uterine size, the patient’s body habitus, and the quantity, size, consistency, type, and location of fibroids.
Size of the uterus, body habitus, and laxity of the abdominal wall all influence the surgeon’s ability to create the necessary operating space. Intraperitoneal space is required during myomectomy because of the need to apply traction and countertraction during enucleation of fibroids. If the necessary space cannot be obtained, a minilaparotomy technique is one alternative. This technique, described by Glasser, limits the skin incision to 3 to 6 cm in myomectomies for large fibroids that can be accessed easily anteriorly.3
Number and location of fibroids. Women with a solitary fibroid, a few dominant fibroids, or multiple pedunculated fibroids are excellent candidates for an endoscopic approach. Although there are no limits on the number of fibroids that can be removed, women with what we have termed “miliary fibroids,” or multiple fibroids disseminated throughout the entire myometrium, with very little normal myometrium, are poor surgical candidates. Not only does the presence of these fibroids leave some concern about the functional ability of the remaining myometrium in pregnancy, but it may be technically difficult to adequately resect all of the critical fibroids and reapproximate the myometrial defects.
The consistency of fibroids also affects the ease of the enucleation process during myomectomy. Due to the soft, spongy nature of degenerating fibroids and their tendency to fragment and shred when manipulated, these cases are more challenging and should not be attempted without a solid foundation of surgical experience.
MRI serves several purposes
Fibroids can be localized and identified as pedunculated, subserosal, intramural, or submucosal via MRI. Measurements and spatial orientation of the fibroids within the uterus can be formulated using T2 weighted coronal, axial, and sagittal images.
The risk of entering the uterine cavity, as well as the risk of synechiae, may be significantly greater if leiomyomas abut and distort the cavity. Surgical strategies, such as planning the location of the hysterotomy or the inclusion of other procedures (eg, hysteroscopic resection for type 0 or type 1 submucosal fibroids), can be formulated with the information provided by MRI. In cases involving multiple fibroids or intramural fibroids, in particular, MRI serves as a surgical “treasure map” or “GPS.” Preoperative MRI is also one way to offset the lack of haptic feedback during surgery to locate the myomas for removal. As we mentioned earlier, important characteristics, such as degeneration or calcification, also can be readily observed on MRI.
Most important, MRI can distinguish adenomyosis from leiomyomas. Adenomyosis can mimic leiomyomas—both clinically and on sonographic imaging—particularly when it is focal in nature. MRI can make the distinction between these two entities so that patients can be counseled appropriately.
SURGICAL TECHNIQUES
Use a uterine manipulator
This device will facilitate the enucleation process, providing another focal point for traction and countertraction. A variety of uterine manipulators are available. We use the Advincula Arch (Cooper Surgical, Trumball, Connecticut) in conjunction with the Uterine Positioning System (Cooper Surgical). The latter attaches to the operating table and to the Advincula Arch to secure the uterus in a steady position throughout the procedure.
During enucleation, the manipulator is crucial to hold the uterus within the pelvis and the field of vision and to act as countertraction as traction is applied to the fibroid.
Individualize port placement
Rather than premeasure port placement on the abdomen, we individualize it, based on a variety of characteristics, including body habitus and uterine pathology (FIGURE 3). However, we follow some basic principles:
- We insert a Veress needle through the umbilicus to achieve pneumoperitoneum
- After insufflation, we use an upper quadrant entry (right or left, depending on which side the robot patient side cart is docked) under direct visualization using a 5-mm laparoscope and optical trocar. This entry will serve as the assistant port for surgery.
- Before placing the rest of the ports under direct visualization, relative to uterine pathology, we elevate the uterus out of the pelvis. This step ensures that enough distance is placed between the camera and the instrument arms to adequately visualize and perform the surgery.
- In patients with a uterus larger than approximately 14- to 16-weeks’ size, a supraumbilical camera port often is necessary.
- We generally employ a four-arm technique using a 12-mm Xcel trocar (Ethicon Endo-Surgery, Blue Ash, Ohio) that is 150 mm in length for the camera port, three 8-mm telerobotic trocar ports, and a 5-mm Airseal trocar (SurgiQuest, Orange, Connecticut) for the assistant port. There should be at least one hand’s breadth between the ports to minimize arm collision and maximize range of motion.
- Although the 12-mm Xcel trocar comes in a variety of lengths (75–150 mm), we strongly recommend, and exclusively use, the longest length for the camera. Once the camera is docked high on the neck of the longer trocar, more space is created between the setup joints of the robotic arms, enabling greater range of motion and fewer instrument and arm collisions.
- We generally use the following wristed robotic instruments to perform myomectomy: tenaculum, monopolar scissors, and PlasmaKinetic (PK) bipolar forceps.
Inject vasopressin into the myometrium
Vasopressin causes vasospasm and uterine muscle contraction and decreases blood loss during myomectomy. It should be diluted (20 U in 50–200 mL of normal saline), introduced with a 7-inch, 22-gauge spinal needle through the anterior abdominal wall, and injected into the myometrium and serosa overlying the fibroid (VIDEO 1 and VIDEO 2).
Perform this step with care, with aspiration prior to injection, to avoid intravascular injection. Although vasopressin is safe overall, serious complications and rare cases of life-threatening hypotension, pulmonary edema, bradycardia, and cardiac arrest have been reported after the injection of as little as 3 U into the myometrium.4–7
Relative contraindications to vasopressin, such as hypertension, should be discussed with anesthesia prior to use of the drug during surgery.
Enucleate the fibroid
Although either a vertical or a horizontal-transverse incision can be made overlying the uterus, a transverse incision allows for technical optimization of wristed movements for suturing and efficient closure. Whenever possible, therefore, we favor a transverse hysterotomy.
During enucleation, keep the use of thermal energy to a minimum. The same holds true for the uterine incision, although its length can be extended as needed.
Using the wristed robotic tenaculum (or an assistant using a laparoscopic tenaculum or corkscrew), grasp and elevate the myoma away from the fixed uterus (FIGURE 1). This step is not intended to enucleate the myoma through force, but to apply traction and position the fibroid to best delineate and present the leading edge of the pseudocapsule that lies between the myoma and the myometrium. Dissection then can proceed using a “push and spread” technique, bluntly separating the natural plane between the fibroid and the myometrium. Occasionally, fibrous attachments of the pseudocapsule can be transected sharply using the bipolar forceps and monopolar scissors.
Again, we encourage the intermittent use of minimal thermal energy to facilitate this process and achieve temporary hemostasis. As the dissection progresses, the fibroid can be regrasped closer to its leading edge, causing the myoma to be rolled out (VIDEO 3 and VIDEO 4).
Close the myometrium We advocate multilayer closure with reapproximation of the myometrium and serosal edges to achieve hemostasis and prevent hematoma (VIDEO 5).
The half-life of vasopressin ranges from 20 to 40 minutes. By this point of the procedure, assuming that the use of thermal energy has been minimal, the myometrial edges should be bleeding slightly, demonstrating adequate reperfusion. The myometrial defect then can be repaired using delayed absorbable suture, such as 2-0 V-Loc 180 (Covidien, Mansfield, Massachusetts).
Barbed suture has revolutionized laparoscopy and minimally invasive surgery, eliminating the need for endoscopic knot-tying. Quill suture (Angiotech, Vancouver, British Columbia, Canada) and V-Loc suture are used safely throughout gynecology, myomectomy included.8,9 However, when the endometrial cavity has been entered, avoid using barbed suture to reapproximate this initial layer to prevent synechiae.
No closure technique has been shown to prevent uterine rupture. Uterine rupture during pregnancy is one of the most serious potential complications following myomectomy. The precise risk of rupture after laparoscopic or robot-assisted laparoscopic myomectomy has not been determined.
Parker and colleagues evaluated case reports of uterine rupture after laparoscopic myomectomy in an attempt to identify a common causal risk factor. In their review of 19 uterine ruptures, however, they were unable to identify a single plausible risk factor. Uterine rupture has occurred in cases involving three-layer closure, removal of pedunculated fibroids, removal of fibroids as small as 1 cm, and in cases where no thermal energy was used.10
Pregnancy rates and outcomes have not been well-established because of confounding variables, such as a high prevalence of infertility and difficulty with long-term follow-up. One of the largest retrospective case studies on this topic involved 872 women who underwent robot-assisted myomectomy.11 Preterm delivery was correlated with the number of myomas removed and an anterior location of the largest incision.11
Undock the robot for morcellation
We strongly recommend that fibroids be morcellated using the 5-mm laparoscope while the robot is undocked, for several reasons. First, we advocate use of the robotic camera port site for morcellation. In the umbilicus, or midline, patients generally experience less pain. And with insufflation, the camera port site is the highest point on the abdomen, allowing greater distance between the morcellator and the iliac vessels and other major structures.
Second, the 12-mm robotic camera is heavy and cumbersome, easily causing fatigue when held separately. The robotic arms and patient side cart are bulky and can be limiting, physically impeding the range of motion necessary to morcellate safely, effectively, and efficiently.
After undocking the robot, remove the midline camera port to introduce the morcellator with the aid of a 5-mm laparoscope through a lateral port. We recommend taking the patient out of a steep Trendelenberg position and placing her in minimal Trendelenberg during morcellation to keep the specimen and fragments from falling to the upper abdomen.
Perfect the art of morcellation
A number of morcellators use electrical or mechanical energy. Blades ranging in diameter from 12 to 20 mm also are available. We favor the reusable MOREsolution tissue-extraction system (Blue Endo, Lenexa, Kansas) with a disposable 20-mm blade, particularly for large or multiple myomas.
The art of morcellation can be learned (VIDEO 6 and VIDEO 7). We recommend the following strategies:
- Slower morcellation speeds cause less fragmentation but may prolong the surgery significantly when the myomas are large. For such myomas, as well as cases that involve significant calcification, we recommend morcellation speeds of at least 600 rpm.
- A beveled trocar is preferred because it allows for longer continuous morcellation along the surface of the myoma and less fragmentation and coring.
- As morcellation nears completion and the specimen begins to fragment more, use short bursts of activation with increased traction, and ask the assistant to help stabilize the end pieces. This approach will help minimize the dissemination of fragments throughout the entire abdominal and pelvic cavity.
- Reapproximate the fascia for all trocar sites larger than 10 mm to prevent incisional hernias. When you exchange the robotic camera port with the morcellator, only one port site will require fascial closure because all other trocar sites typically are 8 mm or smaller.
It is critical that you inspect and remove all fragments and debris after morcellation to prevent iatrogenic multiple peritoneal parasitic myomas. First described in 2006,12 this unusual complication, leiomyomatosis peritonealis disseminate, has been reported with greater frequency as minimally invasive surgery and morcellation have become more common. This complication is thought to arise from small fragments left behind after morcellation of a uterus or myoma. Although spontaneous cases can occur, they are rare.
Place an adhesion barrier
Myomectomy can induce the formation of significant adhesions. For that reason, as the final step before fascial closure, we recommend that an adhesion barrier be placed over any hysterotomy sites. Although they are indicated and FDA-approved only for laparotomy, we typically place Interceed (Ethicon, Cornelia, Georgia) or Seprafilm (Genzyme, Framingham, Massachusetts) over hysterotomy sites.
CODING FOR ROBOT-ASSISTED MYOMECTOMY: ADDITIONAL REIMBURSEMENT MAY NOT BE FORTHCOMING
Robot-assisted surgery is an emerging technology. As such, many health insurance companies, the American Congress of Obstetricians and Gynecologists (ACOG), and Current Procedural Terminology (CPT) editorial staff have weighed in on it. In essence, many payers have indicated that they will not provide the physician with additional reimbursement for performing a surgical procedure using robotic assistance. That is not to say that all payers will rule out additional reimbursement, although most of the larger payers have indicated that additional reimbursement is not going to happen.
Both ACOG and CPT officials have indicated that robot-assisted surgical procedures should be reported using existing CPT codes, based on the procedure and the surgical approach used, rather than coding them as an unlisted procedure. These organizations also have indicated that use of the modifier –22 on the basic laparoscopic procedure would be inappropriate because robotic assistance does not represent an unusual procedure, based on the patient’s condition.
However, if there is a chance that you can gain additional reimbursement for robotic surgery, how can you inform the payer that it was performed? The only currently accepted way to do so is to report code S2900, Surgical techniques requiring use of robotic surgical system (list separately in addition to the code for the primary procedure), in addition to the basic code for the laparoscopic approach. Code S2900 was added by CPT to the national code set in 2005 at the request of Blue Cross/Blue Shield so that the payer could track the incidence of robotic surgery. Because it is not a “regular” CPT code, S2900 was never assigned a relative value, so it is up to the surgeon to set a surgical charge for use of the robot. In doing so, the surgeon must be able to provide supporting documentation as to why additional reimbursement is being requested and on what basis the charge was calculated.
Therefore, if a robot-assisted laparoscopic myomectomy is performed, the first CPT code listed on the claim should be 58545, Laparoscopy, surgical, myomectomy, excision; 1 to 4 intramural myomas with total weight of 250 g or less and/or removal of surface myomas. An alternative would be code 58546, Laparoscopy, surgical, myomectomy, excision; 5 or more intramural myomas and/or intramural myomas with total weight greater than 250 g.
Code S2900 then would be listed second. No modifiers (such as modifier –59 [distinct procedure] or –51 [multiple procedures]) should be added to S2900 because this code does not represent either a distinct or multiple surgical procedure.
—MELANIE WITT, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
CASE: RESOLVED
Because of the patient’s religious beliefs, minimal blood loss is an important goal for any surgery she undergoes. Consequently, you recommend robot-assisted laparoscopic myomectomy, and the operation is completed without complications.
TAKE-HOME MESSAGE
The success of minimally invasive myomectomy requires a careful preoperative work-up and thorough understanding of surgical dissection and suturing techniques. In combination with this knowledge, advanced surgical technology, such as robotics and barbed suture, can truly transform the way myomectomy is performed, providing both patients and physicians with additional options for the conservative management of symptomatic uterine fibroids.
KEY POINTS FOR SUCCESS WITH THE ROBOT
Select patients with care for robot-assisted laparoscopic myomectomy, and perform thorough preoperative assessment. When planning a surgical approach, keep in mind the patient’s uterine size and body habitus and the quantity, size, consistency, type, and location of fibroids.
Use preoperative magnetic resonance imaging to characterize and locate fibroids and differentiate adenomyosis from leiomyomas.
In patients with a uterus larger than approximately 14- to 16-weeks’ size, consider a supraumbilical camera port.
Although the 12-mm Xcel trocar comes in a variety of lengths (75–150 mm), use the 150-mm length for the camera port. Once the camera is docked high on the neck of the longer trocar, more space is created between the setup joints of the robotic arms, enabling greater range of motion and fewer instrument and arm collisions.
Whenever possible, perform a transverse hysterotomy, keeping the length of the incision as short as possible and minimizing use of thermal energy during enucleation of fibroids.
Do not enucleate myomas through force, but apply traction and position each fibroid in order to best delineate and pre-sent the leading edge between the myoma and the myometrium.
Use multilayer closure with reapproximation of the myometrium and serosal edges to achieve hemostasis and prevent hematoma.
Perform morcellation through a 5-mm laparoscope with the robot undocked, using the camera port site for morcellation.
Take the patient out of a steep Trendelenberg position and place her in minimal Trendelenberg during morcellation to optimize ergonomics and prevent fragments from falling into the upper
abdomen.
Inspect the abdomen and remove all fragments and debris after morcellation to help prevent leiomyomatosis peritonealis disseminate.
CASE: IS OPEN MYOMECTOMY THE BEST OPTION FOR THIS PATIENT?
In her only pregnancy, a 34-year-old patient experienced a spontaneous first-trimester loss and underwent dilation and curettage. She had noted an increase in her abdominal girth, as well as pelvic pressure, but had attributed both to the pregnancy. Three months after the pregnancy loss, however, neither had resolved. Because she hopes to conceive again and deliver a healthy infant, the patient consulted a gynecologist. After ultrasonography revealed multiple fibroids, that physician recommended open myomectomy. The patient, a Jehovah’s Witness, comes to your office for a second opinion.
On physical examination, she has a 16-weeks’ sized irregular uterus with the cervix displaced behind the pubic symphysis. T2 weighted scans from magnetic resonance imaging (MRI) of the pelvis in the sagittal view reveal multiple subserosal and intramural fibroids that displace, but do not involve, the uterine cavity (FIGURE 2). The MRI results confirm that the uterus extends beyond the pelvis above the sacral promontory, the fundus lies a few centimeters below the umbilicus, and there is no evidence of adenomyosis. The patient’s hemoglobin level is normal (12.2 g/dL).
What surgical approach would you recommend?
Endometrial ablation, uterine artery embolization, MRI-guided focused ultrasound, hysterectomy, and myomectomy are all treatments for symptomatic uterine fibroids. For women desiring uterine preservation and future fertility, however, myomectomy is the preferred option of many experts.
Myomectomy traditionally has been performed via an open laparotomy approach. With the rise of minimally invasive surgery in gynecology, safe endoscopic surgical approaches and techniques have evolved.
The EndoWrist technology from the da Vinci Surgical System (Intuitive Surgical, Sunnyvale, California) provides increased instrument range of motion, enabling the surgeon to mimic open surgical technique, thereby simplifying the technical challenges of conventional laparoscopic suturing and knot-tying. However, this technology does not minimize or simplify the challenges that leiomyomas can pose, including enucleation (FIGURE 1). Although it has facilitated the progression and adoption of endoscopic myomectomy, the da Vinci system requires an experienced gynecologic endoscopic surgeon.
In this article, we outline the essential steps and offer some clinical surgical pearls to make robot-assisted laparoscopic myomectomy a systematic, safe, and efficient procedure.
Benefits of the robotic approach
Compared with open abdominal myomectomy, the robot-assisted laparoscopic approach is associated with less blood loss, lower complication rates, and shorter hospitalization.1 A retrospective case study from the Cleveland Clinic confirmed these findings when investigators compared surgical outcomes between the robot-assisted laparoscopic approach, standard laparoscopy, and open myomectomy.2 In an assessment of 575 cases (393 open, 93 laparoscopic, and 89 robot-assisted laparoscopic), they found the robot-assisted laparoscopic approach to be associated with the removal of significantly larger myomas (vs standard laparoscopy), as well as lower blood loss and shorter hospitalization (vs open myomectomy).2
Related Article: The robot is gaining ground in gynecologic surgery. Should you be using it?
Comprehensive preoperative assessment is critical
Careful patient selection and thorough preoperative assessment are the cornerstones of successful robot-assisted laparoscopic myomectomy. Among the variables that should be considered in selecting patients are uterine size, the patient’s body habitus, and the quantity, size, consistency, type, and location of fibroids.
Size of the uterus, body habitus, and laxity of the abdominal wall all influence the surgeon’s ability to create the necessary operating space. Intraperitoneal space is required during myomectomy because of the need to apply traction and countertraction during enucleation of fibroids. If the necessary space cannot be obtained, a minilaparotomy technique is one alternative. This technique, described by Glasser, limits the skin incision to 3 to 6 cm in myomectomies for large fibroids that can be accessed easily anteriorly.3
Number and location of fibroids. Women with a solitary fibroid, a few dominant fibroids, or multiple pedunculated fibroids are excellent candidates for an endoscopic approach. Although there are no limits on the number of fibroids that can be removed, women with what we have termed “miliary fibroids,” or multiple fibroids disseminated throughout the entire myometrium, with very little normal myometrium, are poor surgical candidates. Not only does the presence of these fibroids leave some concern about the functional ability of the remaining myometrium in pregnancy, but it may be technically difficult to adequately resect all of the critical fibroids and reapproximate the myometrial defects.
The consistency of fibroids also affects the ease of the enucleation process during myomectomy. Due to the soft, spongy nature of degenerating fibroids and their tendency to fragment and shred when manipulated, these cases are more challenging and should not be attempted without a solid foundation of surgical experience.
MRI serves several purposes
Fibroids can be localized and identified as pedunculated, subserosal, intramural, or submucosal via MRI. Measurements and spatial orientation of the fibroids within the uterus can be formulated using T2 weighted coronal, axial, and sagittal images.
The risk of entering the uterine cavity, as well as the risk of synechiae, may be significantly greater if leiomyomas abut and distort the cavity. Surgical strategies, such as planning the location of the hysterotomy or the inclusion of other procedures (eg, hysteroscopic resection for type 0 or type 1 submucosal fibroids), can be formulated with the information provided by MRI. In cases involving multiple fibroids or intramural fibroids, in particular, MRI serves as a surgical “treasure map” or “GPS.” Preoperative MRI is also one way to offset the lack of haptic feedback during surgery to locate the myomas for removal. As we mentioned earlier, important characteristics, such as degeneration or calcification, also can be readily observed on MRI.
Most important, MRI can distinguish adenomyosis from leiomyomas. Adenomyosis can mimic leiomyomas—both clinically and on sonographic imaging—particularly when it is focal in nature. MRI can make the distinction between these two entities so that patients can be counseled appropriately.
SURGICAL TECHNIQUES
Use a uterine manipulator
This device will facilitate the enucleation process, providing another focal point for traction and countertraction. A variety of uterine manipulators are available. We use the Advincula Arch (Cooper Surgical, Trumball, Connecticut) in conjunction with the Uterine Positioning System (Cooper Surgical). The latter attaches to the operating table and to the Advincula Arch to secure the uterus in a steady position throughout the procedure.
During enucleation, the manipulator is crucial to hold the uterus within the pelvis and the field of vision and to act as countertraction as traction is applied to the fibroid.
Individualize port placement
Rather than premeasure port placement on the abdomen, we individualize it, based on a variety of characteristics, including body habitus and uterine pathology (FIGURE 3). However, we follow some basic principles:
- We insert a Veress needle through the umbilicus to achieve pneumoperitoneum
- After insufflation, we use an upper quadrant entry (right or left, depending on which side the robot patient side cart is docked) under direct visualization using a 5-mm laparoscope and optical trocar. This entry will serve as the assistant port for surgery.
- Before placing the rest of the ports under direct visualization, relative to uterine pathology, we elevate the uterus out of the pelvis. This step ensures that enough distance is placed between the camera and the instrument arms to adequately visualize and perform the surgery.
- In patients with a uterus larger than approximately 14- to 16-weeks’ size, a supraumbilical camera port often is necessary.
- We generally employ a four-arm technique using a 12-mm Xcel trocar (Ethicon Endo-Surgery, Blue Ash, Ohio) that is 150 mm in length for the camera port, three 8-mm telerobotic trocar ports, and a 5-mm Airseal trocar (SurgiQuest, Orange, Connecticut) for the assistant port. There should be at least one hand’s breadth between the ports to minimize arm collision and maximize range of motion.
- Although the 12-mm Xcel trocar comes in a variety of lengths (75–150 mm), we strongly recommend, and exclusively use, the longest length for the camera. Once the camera is docked high on the neck of the longer trocar, more space is created between the setup joints of the robotic arms, enabling greater range of motion and fewer instrument and arm collisions.
- We generally use the following wristed robotic instruments to perform myomectomy: tenaculum, monopolar scissors, and PlasmaKinetic (PK) bipolar forceps.
Inject vasopressin into the myometrium
Vasopressin causes vasospasm and uterine muscle contraction and decreases blood loss during myomectomy. It should be diluted (20 U in 50–200 mL of normal saline), introduced with a 7-inch, 22-gauge spinal needle through the anterior abdominal wall, and injected into the myometrium and serosa overlying the fibroid (VIDEO 1 and VIDEO 2).
Perform this step with care, with aspiration prior to injection, to avoid intravascular injection. Although vasopressin is safe overall, serious complications and rare cases of life-threatening hypotension, pulmonary edema, bradycardia, and cardiac arrest have been reported after the injection of as little as 3 U into the myometrium.4–7
Relative contraindications to vasopressin, such as hypertension, should be discussed with anesthesia prior to use of the drug during surgery.
Enucleate the fibroid
Although either a vertical or a horizontal-transverse incision can be made overlying the uterus, a transverse incision allows for technical optimization of wristed movements for suturing and efficient closure. Whenever possible, therefore, we favor a transverse hysterotomy.
During enucleation, keep the use of thermal energy to a minimum. The same holds true for the uterine incision, although its length can be extended as needed.
Using the wristed robotic tenaculum (or an assistant using a laparoscopic tenaculum or corkscrew), grasp and elevate the myoma away from the fixed uterus (FIGURE 1). This step is not intended to enucleate the myoma through force, but to apply traction and position the fibroid to best delineate and present the leading edge of the pseudocapsule that lies between the myoma and the myometrium. Dissection then can proceed using a “push and spread” technique, bluntly separating the natural plane between the fibroid and the myometrium. Occasionally, fibrous attachments of the pseudocapsule can be transected sharply using the bipolar forceps and monopolar scissors.
Again, we encourage the intermittent use of minimal thermal energy to facilitate this process and achieve temporary hemostasis. As the dissection progresses, the fibroid can be regrasped closer to its leading edge, causing the myoma to be rolled out (VIDEO 3 and VIDEO 4).
Close the myometrium We advocate multilayer closure with reapproximation of the myometrium and serosal edges to achieve hemostasis and prevent hematoma (VIDEO 5).
The half-life of vasopressin ranges from 20 to 40 minutes. By this point of the procedure, assuming that the use of thermal energy has been minimal, the myometrial edges should be bleeding slightly, demonstrating adequate reperfusion. The myometrial defect then can be repaired using delayed absorbable suture, such as 2-0 V-Loc 180 (Covidien, Mansfield, Massachusetts).
Barbed suture has revolutionized laparoscopy and minimally invasive surgery, eliminating the need for endoscopic knot-tying. Quill suture (Angiotech, Vancouver, British Columbia, Canada) and V-Loc suture are used safely throughout gynecology, myomectomy included.8,9 However, when the endometrial cavity has been entered, avoid using barbed suture to reapproximate this initial layer to prevent synechiae.
No closure technique has been shown to prevent uterine rupture. Uterine rupture during pregnancy is one of the most serious potential complications following myomectomy. The precise risk of rupture after laparoscopic or robot-assisted laparoscopic myomectomy has not been determined.
Parker and colleagues evaluated case reports of uterine rupture after laparoscopic myomectomy in an attempt to identify a common causal risk factor. In their review of 19 uterine ruptures, however, they were unable to identify a single plausible risk factor. Uterine rupture has occurred in cases involving three-layer closure, removal of pedunculated fibroids, removal of fibroids as small as 1 cm, and in cases where no thermal energy was used.10
Pregnancy rates and outcomes have not been well-established because of confounding variables, such as a high prevalence of infertility and difficulty with long-term follow-up. One of the largest retrospective case studies on this topic involved 872 women who underwent robot-assisted myomectomy.11 Preterm delivery was correlated with the number of myomas removed and an anterior location of the largest incision.11
Undock the robot for morcellation
We strongly recommend that fibroids be morcellated using the 5-mm laparoscope while the robot is undocked, for several reasons. First, we advocate use of the robotic camera port site for morcellation. In the umbilicus, or midline, patients generally experience less pain. And with insufflation, the camera port site is the highest point on the abdomen, allowing greater distance between the morcellator and the iliac vessels and other major structures.
Second, the 12-mm robotic camera is heavy and cumbersome, easily causing fatigue when held separately. The robotic arms and patient side cart are bulky and can be limiting, physically impeding the range of motion necessary to morcellate safely, effectively, and efficiently.
After undocking the robot, remove the midline camera port to introduce the morcellator with the aid of a 5-mm laparoscope through a lateral port. We recommend taking the patient out of a steep Trendelenberg position and placing her in minimal Trendelenberg during morcellation to keep the specimen and fragments from falling to the upper abdomen.
Perfect the art of morcellation
A number of morcellators use electrical or mechanical energy. Blades ranging in diameter from 12 to 20 mm also are available. We favor the reusable MOREsolution tissue-extraction system (Blue Endo, Lenexa, Kansas) with a disposable 20-mm blade, particularly for large or multiple myomas.
The art of morcellation can be learned (VIDEO 6 and VIDEO 7). We recommend the following strategies:
- Slower morcellation speeds cause less fragmentation but may prolong the surgery significantly when the myomas are large. For such myomas, as well as cases that involve significant calcification, we recommend morcellation speeds of at least 600 rpm.
- A beveled trocar is preferred because it allows for longer continuous morcellation along the surface of the myoma and less fragmentation and coring.
- As morcellation nears completion and the specimen begins to fragment more, use short bursts of activation with increased traction, and ask the assistant to help stabilize the end pieces. This approach will help minimize the dissemination of fragments throughout the entire abdominal and pelvic cavity.
- Reapproximate the fascia for all trocar sites larger than 10 mm to prevent incisional hernias. When you exchange the robotic camera port with the morcellator, only one port site will require fascial closure because all other trocar sites typically are 8 mm or smaller.
It is critical that you inspect and remove all fragments and debris after morcellation to prevent iatrogenic multiple peritoneal parasitic myomas. First described in 2006,12 this unusual complication, leiomyomatosis peritonealis disseminate, has been reported with greater frequency as minimally invasive surgery and morcellation have become more common. This complication is thought to arise from small fragments left behind after morcellation of a uterus or myoma. Although spontaneous cases can occur, they are rare.
Place an adhesion barrier
Myomectomy can induce the formation of significant adhesions. For that reason, as the final step before fascial closure, we recommend that an adhesion barrier be placed over any hysterotomy sites. Although they are indicated and FDA-approved only for laparotomy, we typically place Interceed (Ethicon, Cornelia, Georgia) or Seprafilm (Genzyme, Framingham, Massachusetts) over hysterotomy sites.
CODING FOR ROBOT-ASSISTED MYOMECTOMY: ADDITIONAL REIMBURSEMENT MAY NOT BE FORTHCOMING
Robot-assisted surgery is an emerging technology. As such, many health insurance companies, the American Congress of Obstetricians and Gynecologists (ACOG), and Current Procedural Terminology (CPT) editorial staff have weighed in on it. In essence, many payers have indicated that they will not provide the physician with additional reimbursement for performing a surgical procedure using robotic assistance. That is not to say that all payers will rule out additional reimbursement, although most of the larger payers have indicated that additional reimbursement is not going to happen.
Both ACOG and CPT officials have indicated that robot-assisted surgical procedures should be reported using existing CPT codes, based on the procedure and the surgical approach used, rather than coding them as an unlisted procedure. These organizations also have indicated that use of the modifier –22 on the basic laparoscopic procedure would be inappropriate because robotic assistance does not represent an unusual procedure, based on the patient’s condition.
However, if there is a chance that you can gain additional reimbursement for robotic surgery, how can you inform the payer that it was performed? The only currently accepted way to do so is to report code S2900, Surgical techniques requiring use of robotic surgical system (list separately in addition to the code for the primary procedure), in addition to the basic code for the laparoscopic approach. Code S2900 was added by CPT to the national code set in 2005 at the request of Blue Cross/Blue Shield so that the payer could track the incidence of robotic surgery. Because it is not a “regular” CPT code, S2900 was never assigned a relative value, so it is up to the surgeon to set a surgical charge for use of the robot. In doing so, the surgeon must be able to provide supporting documentation as to why additional reimbursement is being requested and on what basis the charge was calculated.
Therefore, if a robot-assisted laparoscopic myomectomy is performed, the first CPT code listed on the claim should be 58545, Laparoscopy, surgical, myomectomy, excision; 1 to 4 intramural myomas with total weight of 250 g or less and/or removal of surface myomas. An alternative would be code 58546, Laparoscopy, surgical, myomectomy, excision; 5 or more intramural myomas and/or intramural myomas with total weight greater than 250 g.
Code S2900 then would be listed second. No modifiers (such as modifier –59 [distinct procedure] or –51 [multiple procedures]) should be added to S2900 because this code does not represent either a distinct or multiple surgical procedure.
—MELANIE WITT, RN, CPC, COBGC, MA
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
CASE: RESOLVED
Because of the patient’s religious beliefs, minimal blood loss is an important goal for any surgery she undergoes. Consequently, you recommend robot-assisted laparoscopic myomectomy, and the operation is completed without complications.
TAKE-HOME MESSAGE
The success of minimally invasive myomectomy requires a careful preoperative work-up and thorough understanding of surgical dissection and suturing techniques. In combination with this knowledge, advanced surgical technology, such as robotics and barbed suture, can truly transform the way myomectomy is performed, providing both patients and physicians with additional options for the conservative management of symptomatic uterine fibroids.
KEY POINTS FOR SUCCESS WITH THE ROBOT
Select patients with care for robot-assisted laparoscopic myomectomy, and perform thorough preoperative assessment. When planning a surgical approach, keep in mind the patient’s uterine size and body habitus and the quantity, size, consistency, type, and location of fibroids.
Use preoperative magnetic resonance imaging to characterize and locate fibroids and differentiate adenomyosis from leiomyomas.
In patients with a uterus larger than approximately 14- to 16-weeks’ size, consider a supraumbilical camera port.
Although the 12-mm Xcel trocar comes in a variety of lengths (75–150 mm), use the 150-mm length for the camera port. Once the camera is docked high on the neck of the longer trocar, more space is created between the setup joints of the robotic arms, enabling greater range of motion and fewer instrument and arm collisions.
Whenever possible, perform a transverse hysterotomy, keeping the length of the incision as short as possible and minimizing use of thermal energy during enucleation of fibroids.
Do not enucleate myomas through force, but apply traction and position each fibroid in order to best delineate and pre-sent the leading edge between the myoma and the myometrium.
Use multilayer closure with reapproximation of the myometrium and serosal edges to achieve hemostasis and prevent hematoma.
Perform morcellation through a 5-mm laparoscope with the robot undocked, using the camera port site for morcellation.
Take the patient out of a steep Trendelenberg position and place her in minimal Trendelenberg during morcellation to optimize ergonomics and prevent fragments from falling into the upper
abdomen.
Inspect the abdomen and remove all fragments and debris after morcellation to help prevent leiomyomatosis peritonealis disseminate.
- Advincula AP, Xu X, Goudeau S 4th, Ransom SB. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14(6):698–705.
- Barakat EE, Bedaiwy MA, Zimberg S, Nutter B, Nosseir M, Falcone T. Robotic-assisted, laparoscopic, and abdominal myomectomy: a comparison of surgical outcomes. Obstet Gynecol. 2011;117(2 Pt 1):256–265.
- Glasser MH. Minilaparotomy myomectomy: a minimally invasive alternative for the large fibroid uterus. J Minim Invasive Gynecol. 2005;12(3):275–283.
- Hung MH, Wang YM, Chia YY, Liu K. Intramyometrial injection of vasopressin causes bradycardia and cardiac arrest—report of two cases. Acta Anaesthesiol Taiwan. 2006;44(4):243–247.
- Tulandi T, Beique F, Kimia M. Pulmonary edema: a complication of local injection of. Fertil Steril. 1996;66(3):478–480.
- Nezhat F, Admon D Nezhat CH, Dicorpo JE, Nezhat C. Life-threatening hypotension after vasopressin injection during operative laparoscopy, followed by uneventful repeat laparoscopy. J Am Assoc Gynecol Laparosc. 1994;2(1):83–86.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: Severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Einarsson JI, Chavan NR, Suzuki Y, Jonsdottir G, Vellinga TT, Greenberg JA. Use of bidirectional barbed suture in laparoscopic myomectomy: evaluation of perioperative outcomes, safety and efficacy. J Minim Invasive Gynecol. 2011;18(1):92–95.
- Angioli R, Plotti F, Montera R, et al. A new type of absorbable barbed suture for use in laparoscopic myomectomy. Int J Gynaecol Obstet. 2012;117(3):220–223.
- Parker WH, Einarsson J, Istre O, Dubuisson J. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
- Pitter MC, Gargiulo AR, Bonaventura LM, Lehman JS, Srouji SS. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28(1):99–108.
- Paul PG, Koshy AK. Multiple peritoneal parasitic myomas after laparoscopic myomectomy and morcellation. Fertil Steril. 2006;85(2):492–493.
- Advincula AP, Xu X, Goudeau S 4th, Ransom SB. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: a comparison of short-term surgical outcomes and immediate costs. J Minim Invasive Gynecol. 2007;14(6):698–705.
- Barakat EE, Bedaiwy MA, Zimberg S, Nutter B, Nosseir M, Falcone T. Robotic-assisted, laparoscopic, and abdominal myomectomy: a comparison of surgical outcomes. Obstet Gynecol. 2011;117(2 Pt 1):256–265.
- Glasser MH. Minilaparotomy myomectomy: a minimally invasive alternative for the large fibroid uterus. J Minim Invasive Gynecol. 2005;12(3):275–283.
- Hung MH, Wang YM, Chia YY, Liu K. Intramyometrial injection of vasopressin causes bradycardia and cardiac arrest—report of two cases. Acta Anaesthesiol Taiwan. 2006;44(4):243–247.
- Tulandi T, Beique F, Kimia M. Pulmonary edema: a complication of local injection of. Fertil Steril. 1996;66(3):478–480.
- Nezhat F, Admon D Nezhat CH, Dicorpo JE, Nezhat C. Life-threatening hypotension after vasopressin injection during operative laparoscopy, followed by uneventful repeat laparoscopy. J Am Assoc Gynecol Laparosc. 1994;2(1):83–86.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: Severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Einarsson JI, Chavan NR, Suzuki Y, Jonsdottir G, Vellinga TT, Greenberg JA. Use of bidirectional barbed suture in laparoscopic myomectomy: evaluation of perioperative outcomes, safety and efficacy. J Minim Invasive Gynecol. 2011;18(1):92–95.
- Angioli R, Plotti F, Montera R, et al. A new type of absorbable barbed suture for use in laparoscopic myomectomy. Int J Gynaecol Obstet. 2012;117(3):220–223.
- Parker WH, Einarsson J, Istre O, Dubuisson J. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
- Pitter MC, Gargiulo AR, Bonaventura LM, Lehman JS, Srouji SS. Pregnancy outcomes following robot-assisted myomectomy. Hum Reprod. 2013;28(1):99–108.
- Paul PG, Koshy AK. Multiple peritoneal parasitic myomas after laparoscopic myomectomy and morcellation. Fertil Steril. 2006;85(2):492–493.
Robotic vesicovaginal fistula repair: A systematic, endoscopic approach
In modern times in the United States, the vesicovaginal fistula (VVF) arises chiefly as a sequela of gynecologic surgery, usually hysterectomy. The injury most likely occurs at the time of dissection of the bladder flap off of the lower uterine segment and upper vagina.1 With increasing use of endoscopy and electrosurgery at the time of hysterectomy,2 the occurrence of VVF is likely to increase. Because of this, fistulas stemming from benign gynecologic surgical activity tend to occur above the trigone near the vaginal cuff.
Current technique of fistula repair involves either a vaginal approach, with the Latsko procedure,3 or an abdominal approach, involving a laparotomy; laparoscopy also is used with increasing frequency.4
Vaginal versus endoscopic approach. The vaginal approach can be straightforward, such as in cases of vault prolapse or a distally located fistula, or more difficult if the fistula is apical in location, especially if the apex is well suspended and the vagina is of normal length. I have found the abdominal approach to be optimal if the fistula is near the cuff and the vagina is of normal length and well suspended.
Classical teaching tells us that the first repair of the VVF is likely to be the most successful, with successively lower cure rates as the number of repair attempts increases. For this reason, I advocate the abdominal approach in most cases of apically placed VVFs.
Surgical approach
Why endoscopic, why robotic? Often, repair of the VVF is complicated by:
-
the challenge of locating the defect in the bladder
-
the technical difficulty in oversewing the bladder, which often must be done on the underside of the bladder, between the vaginal and bladder walls.
To tackle these challenges, an endoscopic approach promises improved visualization, and the robotic approach allows for surgical closure with improved visibility characteristic of endoscopy, while preserving the manual dexterity characteristic of open surgery.
Timing. It is believed that, in order to improve chances of successful surgical repair, the fistula should be approached either immediately (ie, within 1 to 2 weeks of the insult) or delayed by 8 to 12 or more weeks after the causative surgery.5
Preparation. Vesicovaginal fistulas can rarely involve the ureters, and this ureteric involvement needs to be ruled out. Accordingly, the workup of the VVF should begin with a thorough cystoscopic evaluation of the bladder, with retrograde pyelography to evaluate the integrity of the ureters bilaterally.
During this procedure, the location of the fistulous tract should be meticulously mapped. Care should be taken to document the location and extent of the fistula, as well as to identify the presence of multiple or separate tracts. If these tracts are present, they also need also to be catalogued. In my practice, the cystoscopy/retrograde pyelogram is performed as a separate surgical encounter.
Surgical technique
After the fistula is mapped and ureteric integrity is confirmed, the definitive surgical repair is performed. The steps to the surgical approach are straightforward.
1. Insert stents into the fistula and bilaterally into the ureters
Stenting the fistula permits rapid identification of the fistula tract without the need to enter the bladder separately. The ureteric stents you use should be one color, and the fistula stent should be a second color. I use 5 French yellow stents to cannulate the ureters and a 6 French blue stent for the fistula itself. Insert the fistula stent from the bladder side of the fistula. It should exit through the vagina (FIGURES 1A and 1B). In addition, place a 3-way Foley catheter for drainage and irrigate the bladder when indicated.
Figure 1. Stent insertion
Cystoscopically place a 6 French blue stent into the bladder side of the fistula. (A) The stent as seen from inside the bladder. (B) The stent as seen from the vaginal side.
2. Place the ports for optimal access
A 0° camera is adequate for visualization. Port placement is similar to that used for robotic sacral colpopexy; I use a supraumbilically placed camera port and three 8-mm robot ports (two on the left and one on the right of the umbilicus). Each port should be separated by about 10 cm. An assistant port is placed to the far right, for passing and retrieving sutures (FIGURE 2). An atraumatic grasper, monopolar scissors, and bipolar Maryland-type forceps are placed within the ports to begin the surgical procedure.
Figure 2. Ideal port placement
Place the camera port supraunbilically, with two 8-mm robot ports
3. Place a vaginal stent to aid dissection
The stent should be a sterile cylinder and have a diameter of 2 cm to 5 cm (to match the vaginal caliber). The tip should be rounded and flattened, with an extended handle available for manipulating the stent. The handle can be held by an assistant or attached to an external uterine positioning system (FIGURE 3); I use the Uterine Positioning SystemTM (Cooper Surgical, Trumbull, Connecticut).
4. Incise the vaginal cuff
Transversely incise the vaginal cuff with the monopolar scissors (VIDEO 1).
This allows entry into the vagina at the apex. The blue stent in the fistula should be visible at the anterior vaginal wall, as demonstrated in FIGURE 4.
5. Dissect the vaginal wall
Dissect the anterior vaginal wall down to the fistula, and dissect the bladder off of the vaginal wall for about 1.5 cm to 2 cm around the fistula tract (FIGURE 5 and VIDEO 2).
6. Cut the stent
Cut the stent passing thru the fistula, to move it out of the way.
7. Close the bladder
Stitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture (FIGURE 6 and Video 3 . I prefer polyglactin 910 (Vicryl; Ethicon, Somerville, New Jersey) because it is easier to handle.
FIGURE 6: Close the bladderStitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture. Keep the closure line free of tension.
8. Close the vaginal side of the fistula
Stitch the vaginal side of the fistula in a running fashion with 2-0 absorbable synthetic suture.
9. Verify closure
Check for watertight closure by retrofilling the bladder with 100 mL of sterile milk (obtained from the labor/delivery suite). Observe the suture line for any evidence of milk leakage. (Sterile milk does not stain the tissues, and this preserves tissue visibility. For this reason, milk is preferable to indigo carmine or methylene blue.)
10. Remove the stents from the bladder
Cystoscopically remove all stents.
11. Close the laparoscopic ports
12. Follow up to ensure surgical success
Leave the indwelling Foley catheter in place for 2 to 3 weeks. After such time, remove the catheter and perform voiding cystourethrogram to document bladder wall integrity.
Discussion
I have described a systematic approach to robotic VVF repair. The robotic portion of the procedure should require about 60 to 90 minutes in the absence of significant adhesions. The technique is amenable to a laparoscopic approach, when performed by an appropriately skilled operator.
Final takeaways. Important takeaways to this repair include:
- Stent the fistula to make it easy to find intraoperatively.
- Enter the vagina from above to rapidly locate the fistula tract.
- Use sterile milk to fill the bladder to look for leaks. This works without staining the tissues.
- Minimize tension on the bladder suture line.
In modern times in the United States, the vesicovaginal fistula (VVF) arises chiefly as a sequela of gynecologic surgery, usually hysterectomy. The injury most likely occurs at the time of dissection of the bladder flap off of the lower uterine segment and upper vagina.1 With increasing use of endoscopy and electrosurgery at the time of hysterectomy,2 the occurrence of VVF is likely to increase. Because of this, fistulas stemming from benign gynecologic surgical activity tend to occur above the trigone near the vaginal cuff.
Current technique of fistula repair involves either a vaginal approach, with the Latsko procedure,3 or an abdominal approach, involving a laparotomy; laparoscopy also is used with increasing frequency.4
Vaginal versus endoscopic approach. The vaginal approach can be straightforward, such as in cases of vault prolapse or a distally located fistula, or more difficult if the fistula is apical in location, especially if the apex is well suspended and the vagina is of normal length. I have found the abdominal approach to be optimal if the fistula is near the cuff and the vagina is of normal length and well suspended.
Classical teaching tells us that the first repair of the VVF is likely to be the most successful, with successively lower cure rates as the number of repair attempts increases. For this reason, I advocate the abdominal approach in most cases of apically placed VVFs.
Surgical approach
Why endoscopic, why robotic? Often, repair of the VVF is complicated by:
-
the challenge of locating the defect in the bladder
-
the technical difficulty in oversewing the bladder, which often must be done on the underside of the bladder, between the vaginal and bladder walls.
To tackle these challenges, an endoscopic approach promises improved visualization, and the robotic approach allows for surgical closure with improved visibility characteristic of endoscopy, while preserving the manual dexterity characteristic of open surgery.
Timing. It is believed that, in order to improve chances of successful surgical repair, the fistula should be approached either immediately (ie, within 1 to 2 weeks of the insult) or delayed by 8 to 12 or more weeks after the causative surgery.5
Preparation. Vesicovaginal fistulas can rarely involve the ureters, and this ureteric involvement needs to be ruled out. Accordingly, the workup of the VVF should begin with a thorough cystoscopic evaluation of the bladder, with retrograde pyelography to evaluate the integrity of the ureters bilaterally.
During this procedure, the location of the fistulous tract should be meticulously mapped. Care should be taken to document the location and extent of the fistula, as well as to identify the presence of multiple or separate tracts. If these tracts are present, they also need also to be catalogued. In my practice, the cystoscopy/retrograde pyelogram is performed as a separate surgical encounter.
Surgical technique
After the fistula is mapped and ureteric integrity is confirmed, the definitive surgical repair is performed. The steps to the surgical approach are straightforward.
1. Insert stents into the fistula and bilaterally into the ureters
Stenting the fistula permits rapid identification of the fistula tract without the need to enter the bladder separately. The ureteric stents you use should be one color, and the fistula stent should be a second color. I use 5 French yellow stents to cannulate the ureters and a 6 French blue stent for the fistula itself. Insert the fistula stent from the bladder side of the fistula. It should exit through the vagina (FIGURES 1A and 1B). In addition, place a 3-way Foley catheter for drainage and irrigate the bladder when indicated.
Figure 1. Stent insertion
Cystoscopically place a 6 French blue stent into the bladder side of the fistula. (A) The stent as seen from inside the bladder. (B) The stent as seen from the vaginal side.
2. Place the ports for optimal access
A 0° camera is adequate for visualization. Port placement is similar to that used for robotic sacral colpopexy; I use a supraumbilically placed camera port and three 8-mm robot ports (two on the left and one on the right of the umbilicus). Each port should be separated by about 10 cm. An assistant port is placed to the far right, for passing and retrieving sutures (FIGURE 2). An atraumatic grasper, monopolar scissors, and bipolar Maryland-type forceps are placed within the ports to begin the surgical procedure.
Figure 2. Ideal port placement
Place the camera port supraunbilically, with two 8-mm robot ports
3. Place a vaginal stent to aid dissection
The stent should be a sterile cylinder and have a diameter of 2 cm to 5 cm (to match the vaginal caliber). The tip should be rounded and flattened, with an extended handle available for manipulating the stent. The handle can be held by an assistant or attached to an external uterine positioning system (FIGURE 3); I use the Uterine Positioning SystemTM (Cooper Surgical, Trumbull, Connecticut).
4. Incise the vaginal cuff
Transversely incise the vaginal cuff with the monopolar scissors (VIDEO 1).
This allows entry into the vagina at the apex. The blue stent in the fistula should be visible at the anterior vaginal wall, as demonstrated in FIGURE 4.
5. Dissect the vaginal wall
Dissect the anterior vaginal wall down to the fistula, and dissect the bladder off of the vaginal wall for about 1.5 cm to 2 cm around the fistula tract (FIGURE 5 and VIDEO 2).
6. Cut the stent
Cut the stent passing thru the fistula, to move it out of the way.
7. Close the bladder
Stitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture (FIGURE 6 and Video 3 . I prefer polyglactin 910 (Vicryl; Ethicon, Somerville, New Jersey) because it is easier to handle.
FIGURE 6: Close the bladderStitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture. Keep the closure line free of tension.
8. Close the vaginal side of the fistula
Stitch the vaginal side of the fistula in a running fashion with 2-0 absorbable synthetic suture.
9. Verify closure
Check for watertight closure by retrofilling the bladder with 100 mL of sterile milk (obtained from the labor/delivery suite). Observe the suture line for any evidence of milk leakage. (Sterile milk does not stain the tissues, and this preserves tissue visibility. For this reason, milk is preferable to indigo carmine or methylene blue.)
10. Remove the stents from the bladder
Cystoscopically remove all stents.
11. Close the laparoscopic ports
12. Follow up to ensure surgical success
Leave the indwelling Foley catheter in place for 2 to 3 weeks. After such time, remove the catheter and perform voiding cystourethrogram to document bladder wall integrity.
Discussion
I have described a systematic approach to robotic VVF repair. The robotic portion of the procedure should require about 60 to 90 minutes in the absence of significant adhesions. The technique is amenable to a laparoscopic approach, when performed by an appropriately skilled operator.
Final takeaways. Important takeaways to this repair include:
- Stent the fistula to make it easy to find intraoperatively.
- Enter the vagina from above to rapidly locate the fistula tract.
- Use sterile milk to fill the bladder to look for leaks. This works without staining the tissues.
- Minimize tension on the bladder suture line.
In modern times in the United States, the vesicovaginal fistula (VVF) arises chiefly as a sequela of gynecologic surgery, usually hysterectomy. The injury most likely occurs at the time of dissection of the bladder flap off of the lower uterine segment and upper vagina.1 With increasing use of endoscopy and electrosurgery at the time of hysterectomy,2 the occurrence of VVF is likely to increase. Because of this, fistulas stemming from benign gynecologic surgical activity tend to occur above the trigone near the vaginal cuff.
Current technique of fistula repair involves either a vaginal approach, with the Latsko procedure,3 or an abdominal approach, involving a laparotomy; laparoscopy also is used with increasing frequency.4
Vaginal versus endoscopic approach. The vaginal approach can be straightforward, such as in cases of vault prolapse or a distally located fistula, or more difficult if the fistula is apical in location, especially if the apex is well suspended and the vagina is of normal length. I have found the abdominal approach to be optimal if the fistula is near the cuff and the vagina is of normal length and well suspended.
Classical teaching tells us that the first repair of the VVF is likely to be the most successful, with successively lower cure rates as the number of repair attempts increases. For this reason, I advocate the abdominal approach in most cases of apically placed VVFs.
Surgical approach
Why endoscopic, why robotic? Often, repair of the VVF is complicated by:
-
the challenge of locating the defect in the bladder
-
the technical difficulty in oversewing the bladder, which often must be done on the underside of the bladder, between the vaginal and bladder walls.
To tackle these challenges, an endoscopic approach promises improved visualization, and the robotic approach allows for surgical closure with improved visibility characteristic of endoscopy, while preserving the manual dexterity characteristic of open surgery.
Timing. It is believed that, in order to improve chances of successful surgical repair, the fistula should be approached either immediately (ie, within 1 to 2 weeks of the insult) or delayed by 8 to 12 or more weeks after the causative surgery.5
Preparation. Vesicovaginal fistulas can rarely involve the ureters, and this ureteric involvement needs to be ruled out. Accordingly, the workup of the VVF should begin with a thorough cystoscopic evaluation of the bladder, with retrograde pyelography to evaluate the integrity of the ureters bilaterally.
During this procedure, the location of the fistulous tract should be meticulously mapped. Care should be taken to document the location and extent of the fistula, as well as to identify the presence of multiple or separate tracts. If these tracts are present, they also need also to be catalogued. In my practice, the cystoscopy/retrograde pyelogram is performed as a separate surgical encounter.
Surgical technique
After the fistula is mapped and ureteric integrity is confirmed, the definitive surgical repair is performed. The steps to the surgical approach are straightforward.
1. Insert stents into the fistula and bilaterally into the ureters
Stenting the fistula permits rapid identification of the fistula tract without the need to enter the bladder separately. The ureteric stents you use should be one color, and the fistula stent should be a second color. I use 5 French yellow stents to cannulate the ureters and a 6 French blue stent for the fistula itself. Insert the fistula stent from the bladder side of the fistula. It should exit through the vagina (FIGURES 1A and 1B). In addition, place a 3-way Foley catheter for drainage and irrigate the bladder when indicated.
Figure 1. Stent insertion
Cystoscopically place a 6 French blue stent into the bladder side of the fistula. (A) The stent as seen from inside the bladder. (B) The stent as seen from the vaginal side.
2. Place the ports for optimal access
A 0° camera is adequate for visualization. Port placement is similar to that used for robotic sacral colpopexy; I use a supraumbilically placed camera port and three 8-mm robot ports (two on the left and one on the right of the umbilicus). Each port should be separated by about 10 cm. An assistant port is placed to the far right, for passing and retrieving sutures (FIGURE 2). An atraumatic grasper, monopolar scissors, and bipolar Maryland-type forceps are placed within the ports to begin the surgical procedure.
Figure 2. Ideal port placement
Place the camera port supraunbilically, with two 8-mm robot ports
3. Place a vaginal stent to aid dissection
The stent should be a sterile cylinder and have a diameter of 2 cm to 5 cm (to match the vaginal caliber). The tip should be rounded and flattened, with an extended handle available for manipulating the stent. The handle can be held by an assistant or attached to an external uterine positioning system (FIGURE 3); I use the Uterine Positioning SystemTM (Cooper Surgical, Trumbull, Connecticut).
4. Incise the vaginal cuff
Transversely incise the vaginal cuff with the monopolar scissors (VIDEO 1).
This allows entry into the vagina at the apex. The blue stent in the fistula should be visible at the anterior vaginal wall, as demonstrated in FIGURE 4.
5. Dissect the vaginal wall
Dissect the anterior vaginal wall down to the fistula, and dissect the bladder off of the vaginal wall for about 1.5 cm to 2 cm around the fistula tract (FIGURE 5 and VIDEO 2).
6. Cut the stent
Cut the stent passing thru the fistula, to move it out of the way.
7. Close the bladder
Stitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture (FIGURE 6 and Video 3 . I prefer polyglactin 910 (Vicryl; Ethicon, Somerville, New Jersey) because it is easier to handle.
FIGURE 6: Close the bladderStitch the bladder in a running fashion using three layers of 3-0 rapid absorbable synthetic suture. Keep the closure line free of tension.
8. Close the vaginal side of the fistula
Stitch the vaginal side of the fistula in a running fashion with 2-0 absorbable synthetic suture.
9. Verify closure
Check for watertight closure by retrofilling the bladder with 100 mL of sterile milk (obtained from the labor/delivery suite). Observe the suture line for any evidence of milk leakage. (Sterile milk does not stain the tissues, and this preserves tissue visibility. For this reason, milk is preferable to indigo carmine or methylene blue.)
10. Remove the stents from the bladder
Cystoscopically remove all stents.
11. Close the laparoscopic ports
12. Follow up to ensure surgical success
Leave the indwelling Foley catheter in place for 2 to 3 weeks. After such time, remove the catheter and perform voiding cystourethrogram to document bladder wall integrity.
Discussion
I have described a systematic approach to robotic VVF repair. The robotic portion of the procedure should require about 60 to 90 minutes in the absence of significant adhesions. The technique is amenable to a laparoscopic approach, when performed by an appropriately skilled operator.
Final takeaways. Important takeaways to this repair include:
- Stent the fistula to make it easy to find intraoperatively.
- Enter the vagina from above to rapidly locate the fistula tract.
- Use sterile milk to fill the bladder to look for leaks. This works without staining the tissues.
- Minimize tension on the bladder suture line.
Pros and cons of robotics in endometriosis surgery
"We need to be responsible individuals with how we shepherd in this new technology." From the Endometriosis Foundation of America's 4th Annual Medical Symposium: The American Perspective, March 11, 2013
Related article: The robot is gaining ground in gynecologic surgery. Should you be using it?
A roundtable discussion with:
Arnold P. Advincula, MD
Cheryl B. Iglesia, MD
Rosanne M. Kho, MD
Jamal Mourad, DO
Marie Fidela R. Paraiso, MD
Jason D. Wright, MD
"We need to be responsible individuals with how we shepherd in this new technology." From the Endometriosis Foundation of America's 4th Annual Medical Symposium: The American Perspective, March 11, 2013
Related article: The robot is gaining ground in gynecologic surgery. Should you be using it?
A roundtable discussion with:
Arnold P. Advincula, MD
Cheryl B. Iglesia, MD
Rosanne M. Kho, MD
Jamal Mourad, DO
Marie Fidela R. Paraiso, MD
Jason D. Wright, MD
"We need to be responsible individuals with how we shepherd in this new technology." From the Endometriosis Foundation of America's 4th Annual Medical Symposium: The American Perspective, March 11, 2013
Related article: The robot is gaining ground in gynecologic surgery. Should you be using it?
A roundtable discussion with:
Arnold P. Advincula, MD
Cheryl B. Iglesia, MD
Rosanne M. Kho, MD
Jamal Mourad, DO
Marie Fidela R. Paraiso, MD
Jason D. Wright, MD