User login
External cephalic version: How to increase the chances for success
About 3% to 4% of all fetuses at term are in breech presentation. Since 2000, when Hannah and colleagues reported finding that vaginal delivery of breech-presenting babies was riskier than cesarean delivery,1 most breech-presenting neonates in the United States have been delivered abdominally2—despite subsequent questioning of some of that study’s conclusions.
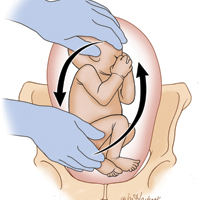
Each year in the United States, approximately 4 million babies are born, and fetal malpresentation accounts for 110,000 to 150,000 cesarean deliveries. In fact, about 15% of all cesarean deliveries in the United States are for breech presentation or transverse lie; in England the percentage is 10%.3 Fortunately, the repopularized technique of external cephalic version (ECV), in which the clinician externally rotates a breech- or transverse-lying fetus to a vertex position (FIGURE), along with the facilitating tools of tocolysis and neuraxial analgesia/anesthesia, is helping to reduce the number of breech presentations in fetuses at term and thus the number of cesarean deliveries and their sequelae—placenta accreta, prolonged recovery, and cesarean deliveries in subsequent pregnancies.
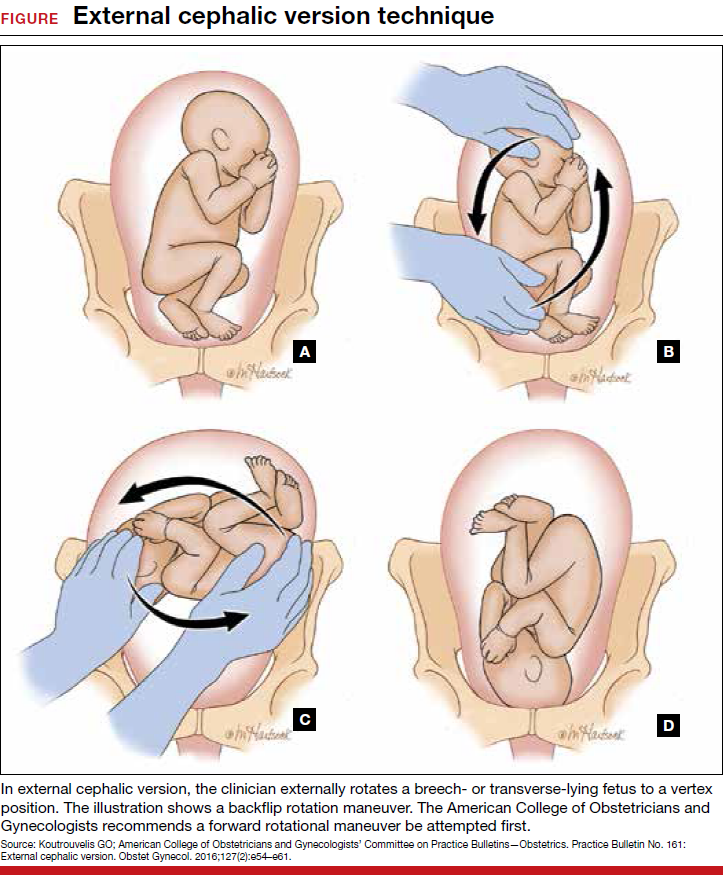
Reluctance to perform ECV is unfounded
In the United States, the practice of offering ECV to women who present with their fetus in breech presentation at term varies tremendously. It is routine at some institutions but not even offered at others.
Many ObGyns are reluctant to perform ECV. Cited reasons include the potential for injury to the fetus and mother (and related liability concerns), the ease of elective cesarean delivery, the variable success rate of ECV (35% to 86%),4 and the pain that women often have with the procedure. According to the literature, however, these concerns either are unfounded or can be mitigated with use of current techniques. Multiple studies have found that the risk of ECV to the fetus and mother is minimal, and that tocolysis and neuraxial anesthesia can facilitate the success of ECV and relieve the pain associated with the procedure.
Related article:
2017 Update on obstetrics
Indications for ECV
The indications for ECV include breech, oblique, or transverse lie presentation after 36 weeks’ gestation and the mother’s desire to avoid cesarean delivery. A clinician skilled in ECV and a facility where emergency cesarean delivery is possible are essential.
There are several instances in which ECV should not be attempted.
Contraindications include:
- concerns about fetal status, including nonreactive nonstress test, biophysical profile score <6/8, severe intrauterine growth restriction, decreased end-diastolic umbilical blood flow
- placenta previa
- multifetal gestation before delivery of first twin
- severe oligohydramnios
- severe preeclampsia
- significant fetal anomaly
- known malformation of uterus
- breech with hyperextended head or arms above shoulders, as seen on ultrasonography.
More controversial contraindications include prior uterine incision, maternal obesity (body mass index >40 kg/m2), ruptured membranes, and fetal macrosomia.
Read about timing, success rates, risk factors, alternate approaches for ECV
Optimal timing for the ECV procedure
Current practice is to wait until 36 to 37 weeks to perform ECV, as most fetuses spontaneously move into vertex presentation by 36 weeks’ gestation. This time frame has several advantages: Many unnecessary attempts at ECV are avoided; only 8% of fetuses in breech presentation after 36 weeks spontaneously change to vertex5; many fetuses revert to breech if ECV is performed too early; and prematurity generally is not an issue in the rare case that immediate delivery is required during or just after attempted ECV.
ECV during labor. Performing ECV during labor appears to pose no increased risk to mother or fetus if membranes are intact and there are no other contraindications to the procedure. Some clinicians perform ECV only during labor. The advantages are that the fetus has had every chance to move into vertex presentation on its own, the equipment used to continuously monitor the fetus during ECV is in place, and cesarean delivery and anesthesia are immediately available in the event ECV is unsuccessful.
The major disadvantage of waiting until labor is that the increased size of the fetus makes ECV more difficult. In addition, the membranes may have already ruptured, and the breech may have descended deeply into the pelvis.
Related article:
For the management of labor, patience is a virtue
Success rates in breech-to-vertex conversions
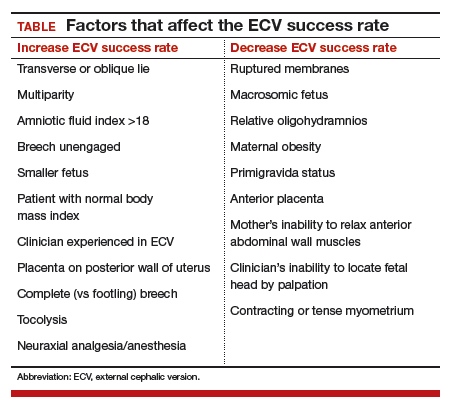
In 2016, the American College of Obstetricians and Gynecologists (ACOG) reported an average ECV success rate of 58% (range, 16% to 100%).6 ACOG noted that, with transverse lie, the success rate was significantly higher. Other studies have found a wide range of rates: 58% in 1,308 patients in a Cochrane review by Hofmeyr and colleagues7; 47% in a study by Beuckens and colleagues8; and 63.1% for primiparas and 82.7% for multiparas in a study by Tong Leung and colleagues.9 These rates were affected by whether ECV was performed with or without tocolysis, with or without intravenous analgesia, and with or without neuraxial analgesia/anesthesia (TABLE).
Likelihood of vaginal delivery after successful ECV
The rate of vaginal delivery after successful ECV is roughly half that of fetuses that were never in breech presentation.10 In successful ECV cases, dystocia and nonreassuring fetal heart rate patterns are the major indications for cesarean delivery. Some experts have speculated that the factors leading to near-term breech presentation—such as an unengaged presenting part or a mother’s smaller pelvis—also may be risk factors for dystocia in labor. Despite this, the rate of vaginal delivery of successfully verted babies has been reported to be as high as 80%.10
As might be expected, post-ECV vaginal deliveries are more common in multiparous than in primiparous women.
Although multiple problems may occur with ECV, generally they are rare and reversible. For instance, Grootscholten and colleagues found a stillbirth and placental abruption rate of only 0.25% in a large group of patients who underwent ECV.11 Similarly, the rate of emergency cesarean delivery was 0.35%. In addition, Hofmeyr and Kulier, in their Cochrane Data Review of 2015, found no significant differences in the Apgar scores and pH’s of babies in the ECV group compared with babies in breech presentation whose mothers did not undergo ECV.7 Results of other studies have confirmed the safety of ECV.12,13
One significant risk of ECV attempts is fetal-to-maternal blood transfer. Boucher and colleagues found that 2.4% of 1,244 women who underwent ECV had a positive Kleihauer-Betke test result, and, in one-third of the positive cases, more than 1 mL of fetal blood was found in maternal circulation.14 This risk can be minimized by administering Rho (D) immune globulin to all Rh-negative mothers after the procedure.
Even these small risks, however, should not be considered in isolation. The infrequent complications of ECV must be compared with what can occur with breech-presenting fetuses during labor or cesarean delivery: complications of breech vaginal delivery, cord prolapse, difficulties with cesarean delivery, and maternal operative complications related to present and future cesarean deliveries.
Alternative approaches to converting breech presentation of unproven efficacy
Over the years, attempts have been made to address breech presentations with measures short of ECV. There is little evidence that these measures work, or work consistently.
- Observation. After 36 weeks’ gestation, only 8% of fetuses in breech presentationspontaneously move into vertex presentation.5
- Maternal positioning. There is no good evidence that such maneuvers are effective in changing fetal presentation.15
- Moxibustion and acupuncture. Moxibustion is inhalation of smoke from burning herbal compounds. In formal studies using controls, these techniques did not consistently increase the rate of movement from breech to vertex presentation.16–18 Likewise, studies with the use of acupuncture have not shown consistent success in changing fetal presentation.19
Read about various methods to facilitate ECV success
Methods to facilitate ECV success
Two techniques that can facilitate ECV success are tocolysis, which relaxes the uterus, and neuraxial analgesia/anesthesia, which relaxes anterior abdominal wall muscles and reduces or relieves ECV-associated pain.
Tocolysis
In tocolysis, a medication is administered to reduce myometrial activity and to relax the uterine muscle so that it stretches more easily around the fetus during repositioning. Tocolytic medications originally were studied for their use in decreasing myometrial tone during preterm labor.
Tocolysis clearly is effective in increasing ECV success rates. Reviewing the results of 4 randomized trials, Cluver showed a 1.38 risk ratio for successful ECV when terbutaline was used versus when there was no tocolysis. The risk ratio for cesarean delivery was 0.82.20 Fernandez, in a study of 103 women divided into terbutaline versus placebo groups, had a 52% success rate for ECV with the terbutaline group versus only a 27% success rate with the placebo group.21
Tocolytic medications include terbutaline, nifedipine, and nitroglycerin.
Tocolysis most often involves the use of β2-adrenergic receptor agonists, particularly terbutaline (despite the boxed safety warning in its prescribing information). A 0.25-mg dose of terbutaline is given subcutaneously 15 to 30 minutes before ECV. Clinicians have successfully used β2-adrenergic receptor agonists in the treatment of patients in preterm labor, and there are more data on this class of medications than on other agents used to facilitate ECV.
Although nifedipine is as effective as terbutaline in the temporary treatment of preterm uterine contractions, several studies have found this calcium channel blocker less effective than terbutaline in facilitating ECV.22,23
The uterus-relaxing effect of nitroglycerin was once thought to make this medication appropriate for facilitating ECV, but multiple studies have found success rates unimproved. In some cases, the drug performed more poorly than placebo.24 Moreover, nitroglycerin is associated with a fairly high rate of adverse effects, such as headaches and blood pressure changes.
Neuraxial analgesia/anesthesia
Over the past 2 decades, there has been a resurgence in the use of neuraxial analgesia/anesthesia in ECV. This technique is more effective than others in improving ECV success rates, it reduces maternal discomfort, and it is very safe. Specifically, it relaxes the maternal abdominal wall muscles and thereby facilitates ECV. Another benefit is that the anesthesia is in place and available for use should emergency cesarean delivery be needed during or after attempted ECV. Neuraxial anesthesia, which includes spinal, epidural, and combined spinal-epidural techniques, is almost always used with tocolysis.
The major complications of neuraxial analgesia/anesthesia are maternal hypotension and fetal bradycardia. Each is dose related and usually transient.
In the past, there was concern that using regional anesthesia to control pain would reduce a patient’s natural warning symptoms and result in a clinician applying excessive force, thus increasing the chances of fetal and maternal injury and even fetal death. However, multiple studies have found that ECV complication rates are not increased with use of neuraxial methods.
Higher doses of neuraxial anesthesia produce higher ECV success rates. This dose-dependent relationship is almost surely attributable to the fact that, although lower dose neuraxial analgesia can relieve the pain associated with ECV, an anesthetic dose is needed to relax the abdominal wall muscles and facilitate fetus repositioning.
The literature is clear: ECV success rates are significantly increased with the use of neuraxial techniques, with anesthesia having higher success rates than analgesia. Reviewing the results of 6 controlled trials in which a total of 508 patients underwent ECV with tocolysis, Goetzinger and colleagues found that the chance of ECV success was almost 60% higher in the 253 patients who received regional anesthesia than in the 255 patients who received intravenous or no analgesia.25 Moreover, only 48.4% of the regional anesthesia patients as compared with 59.3% of patients who did not have regional anesthesia underwent cesarean delivery, roughly a 20% decrease. Pain scores were consistently lower in the regional anesthesia group. Multiple other studies have reported similar results.
Although the use of neuraxial anesthesia increases the ECV success rate, and decreases the cesarean delivery rate for breech presentation by 5% to 15%,25 some groups of obstetrics professionals, noting that the decreased cesarean delivery rate does not meet the formal criterion for statistical significance, have expressed reservations about recommending regional anesthesia for ECV. Thus, despite the positive results obtained with neuraxial anesthesia, neither the literature nor authoritative professional organizations definitively recommend the use of neuraxial anesthesia in facilitating ECV.
This lack of official recommendation, however, overlooks an important point: While the cesarean delivery percentage decrease that occurs with the use of neuraxial anesthesia may not be statistically significant, the promise of a pain-free procedure will encourage more women to undergo ECV. If the procedure population increases, then the average ECV success rate of roughly 60%6 applies to a larger base of patients, reducing the total number of cesarean deliveries for breech presentation. As only a small percentage of the 110,000 to 150,000 women with breech presentation at 36 weeks currently elects to undergo ECV, any increase in the number of women who proceed with attempts at fetal repositioning once procedural pain is no longer an issue will accordingly reduce the number of cesarean deliveries for the indication of malpresentation.
Related article:
Nitrous oxide for labor pain
Overarching goal: Reduce cesarean delivery rate and associated risks
In the United States, increasing the use of ECV in cases of breech-presenting fetuses would reduce the cesarean delivery rate by about 10%, thereby reducing recovery time for cesarean deliveries, minimizing the risks associated with these deliveries (current and future), and providing the health care system with a major cost savings.
Tocolysis and the use of neuraxial anesthesia each increases the ECV success rate and each is remarkably safe within the context of a well-defined protocol. Reducing the pain associated with ECV by administering neuraxial anesthesia will increase the number of women electing to undergo the procedure and ultimately will reduce the number of cesarean deliveries performed for the indication of breech presentation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned cesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet. 2000;356(9239):1375–1383.
- Weiniger CF, Lyell DJ, Tsen LC, et al. Maternal outcomes of term breech presentation delivery: impact of successful external cephalic version in a nationwide sample of delivery admissions in the United States. BMC Pregnancy Childbirth. 2016;16(1):150.
- Eller DP, Van Dorsten JP. Breech presentation. Curr Opin Obstet Gynecol.1993;5(5)664–668.
- Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 24th ed. New York, NY: McGraw Hill; 2014:570.
- Westgren M, Edvall H, Nordstrom L, Svalenius E, Ranstam J. Spontaneous cephalic version of breech presentation in the last trimester. Br J Obstet Gynaecol. 1985;92(1):19–22.
- External cephalic version. ACOG Practice Bulletin No. 161. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2016.
- Hofmeyr GJ, Kulier R, West HM. External cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2015;(4):CD000083.
- Beuckens A, Rijnders M, Verburgt-Doeleman GH, Rijninks-van Driel GC, Thorpe J, Hutton EK. An observational study of the success and complications of 2546 external cephalic versions in low-risk pregnant women performed by trained midwives. BJOG. 2016;123(3):415–423.
- Tong Leung VK, Suen SS, Singh Sahota D, Lau TK, Yeung Leung T. External cephalic version does not increase the risk of intra-uterine death: a 17-year experience and literature review. J Matern Fetal Neonatal Med. 2012;25(9):1774–1778.
- de Hundt M, Velzel J, de Groot CJ, Mol BW, Kok M. Mode of delivery after successful external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(6):1327–1334.
- Grootscholten K, Kok M, Oei SG, Mol BW, van der Post JA. External cephalic version–related risks: a meta-analysis. Obstet Gynecol. 2008;112(5):1143–1151.
- Collaris RJ, Oei SG. External cephalic version: a safe procedure? A systematic review of version-related risk. Acta Obstet Gynecol Scand. 2004;83(6):511–518.
- Khaw KS, Lee SW, Ngan Kee WD, et al. Randomized trial of anesthetic interventions in external cephalic version for breech presentation. Br J Anaesth. 2015;114(6):944–950.
- Boucher M, Marquette GP, Varin J, Champagne J, Bujold E. Fetomaternal hemorrhage during external cephalic version. Obstet Gynecol. 2008;112(1):79–84.
- Hofmeyr GJ, Kulier R. Cephalic version by postural management for breech presentation. Cochrane Database Syst Rev. 2012;(10):CD00051.
- Coulon C, Poleszczuk M, Paty-Montaigne MH, et al. Version of breech fetuses by moxibustion with acupuncture: a randomized controlled trial. Obstet Gynecol. 2014;124(1):32–39.
- Bue L, Lauszus FF. Moxibustion did not have an effect in a randomised clinical trial for version of breech position. Dan Med J. 2016;63(2):pii:A5199.
- Coyle ME, Smith CA, Peat B. Cephalic version by moxibustion for breech presentation. Cochrane Database Syst Rev. 2012;(5):CD003928.
- Sananes N, Roth GE, Aissi GA, et al. Acupuncture version of breech presentation: a randomized sham-controlled single-blinded trial. Eur J Obstet Gynecol Reprod Biol. 2016;204:24–30.
- Cluver C, Gyte GM, Sinclair M, Dowswell T, Hofmeyr G. Interventions for helping to turn breech babies to head first presentation when using external cephalic version. Cochrane Database Syst Rev. 2015;(2):CD000184.
- Fernandez CO, Bloom SL, Smulian JC, Ananth CV, Wendel GD Jr. A randomized placebo-controlled evaluation of terbutaline for external cephalic version. Obstet Gynecol. 1997;90(5):775–779.
- Mohamed Ismail NA, Ibrahim M, Mohd Naim N, Mahdy ZA, Jamil MA, Mohd Razi ZR. Nifedipine versus terbutaline for tocolysis in external cephalic version. Int J Gynaecol Obstet. 2008;102(3):263–266.
- Kok M, Bais J, van Lith J, et al. Nifedipine as a uterine relaxant for external cephalic version: a meta-analysis. Am J Obstet Gynecol. 2008;112(2 pt 1):271–276.
- Bujold E, Boucher M, Rinfred D, Berman S, Ferreira E, Marquette GP. Sublingual nitroglycerin versus placebo as a tocolytic for external cephalic version: a randomized controlled trial in parous women. Am J Obstet Gynecol. 2003;189(4):1070–1073.
- Goetzinger KR, Harper LM, Tuuli MG, Macones GA, Colditz GA. Effect of regional anesthesia on the success of external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1137–1144.
About 3% to 4% of all fetuses at term are in breech presentation. Since 2000, when Hannah and colleagues reported finding that vaginal delivery of breech-presenting babies was riskier than cesarean delivery,1 most breech-presenting neonates in the United States have been delivered abdominally2—despite subsequent questioning of some of that study’s conclusions.
Each year in the United States, approximately 4 million babies are born, and fetal malpresentation accounts for 110,000 to 150,000 cesarean deliveries. In fact, about 15% of all cesarean deliveries in the United States are for breech presentation or transverse lie; in England the percentage is 10%.3 Fortunately, the repopularized technique of external cephalic version (ECV), in which the clinician externally rotates a breech- or transverse-lying fetus to a vertex position (FIGURE), along with the facilitating tools of tocolysis and neuraxial analgesia/anesthesia, is helping to reduce the number of breech presentations in fetuses at term and thus the number of cesarean deliveries and their sequelae—placenta accreta, prolonged recovery, and cesarean deliveries in subsequent pregnancies.

Reluctance to perform ECV is unfounded
In the United States, the practice of offering ECV to women who present with their fetus in breech presentation at term varies tremendously. It is routine at some institutions but not even offered at others.
Many ObGyns are reluctant to perform ECV. Cited reasons include the potential for injury to the fetus and mother (and related liability concerns), the ease of elective cesarean delivery, the variable success rate of ECV (35% to 86%),4 and the pain that women often have with the procedure. According to the literature, however, these concerns either are unfounded or can be mitigated with use of current techniques. Multiple studies have found that the risk of ECV to the fetus and mother is minimal, and that tocolysis and neuraxial anesthesia can facilitate the success of ECV and relieve the pain associated with the procedure.
Related article:
2017 Update on obstetrics
Indications for ECV
The indications for ECV include breech, oblique, or transverse lie presentation after 36 weeks’ gestation and the mother’s desire to avoid cesarean delivery. A clinician skilled in ECV and a facility where emergency cesarean delivery is possible are essential.
There are several instances in which ECV should not be attempted.
Contraindications include:
- concerns about fetal status, including nonreactive nonstress test, biophysical profile score <6/8, severe intrauterine growth restriction, decreased end-diastolic umbilical blood flow
- placenta previa
- multifetal gestation before delivery of first twin
- severe oligohydramnios
- severe preeclampsia
- significant fetal anomaly
- known malformation of uterus
- breech with hyperextended head or arms above shoulders, as seen on ultrasonography.
More controversial contraindications include prior uterine incision, maternal obesity (body mass index >40 kg/m2), ruptured membranes, and fetal macrosomia.
Read about timing, success rates, risk factors, alternate approaches for ECV
Optimal timing for the ECV procedure
Current practice is to wait until 36 to 37 weeks to perform ECV, as most fetuses spontaneously move into vertex presentation by 36 weeks’ gestation. This time frame has several advantages: Many unnecessary attempts at ECV are avoided; only 8% of fetuses in breech presentation after 36 weeks spontaneously change to vertex5; many fetuses revert to breech if ECV is performed too early; and prematurity generally is not an issue in the rare case that immediate delivery is required during or just after attempted ECV.
ECV during labor. Performing ECV during labor appears to pose no increased risk to mother or fetus if membranes are intact and there are no other contraindications to the procedure. Some clinicians perform ECV only during labor. The advantages are that the fetus has had every chance to move into vertex presentation on its own, the equipment used to continuously monitor the fetus during ECV is in place, and cesarean delivery and anesthesia are immediately available in the event ECV is unsuccessful.
The major disadvantage of waiting until labor is that the increased size of the fetus makes ECV more difficult. In addition, the membranes may have already ruptured, and the breech may have descended deeply into the pelvis.
Related article:
For the management of labor, patience is a virtue
Success rates in breech-to-vertex conversions
In 2016, the American College of Obstetricians and Gynecologists (ACOG) reported an average ECV success rate of 58% (range, 16% to 100%).6 ACOG noted that, with transverse lie, the success rate was significantly higher. Other studies have found a wide range of rates: 58% in 1,308 patients in a Cochrane review by Hofmeyr and colleagues7; 47% in a study by Beuckens and colleagues8; and 63.1% for primiparas and 82.7% for multiparas in a study by Tong Leung and colleagues.9 These rates were affected by whether ECV was performed with or without tocolysis, with or without intravenous analgesia, and with or without neuraxial analgesia/anesthesia (TABLE).

Likelihood of vaginal delivery after successful ECV
The rate of vaginal delivery after successful ECV is roughly half that of fetuses that were never in breech presentation.10 In successful ECV cases, dystocia and nonreassuring fetal heart rate patterns are the major indications for cesarean delivery. Some experts have speculated that the factors leading to near-term breech presentation—such as an unengaged presenting part or a mother’s smaller pelvis—also may be risk factors for dystocia in labor. Despite this, the rate of vaginal delivery of successfully verted babies has been reported to be as high as 80%.10
As might be expected, post-ECV vaginal deliveries are more common in multiparous than in primiparous women.
Although multiple problems may occur with ECV, generally they are rare and reversible. For instance, Grootscholten and colleagues found a stillbirth and placental abruption rate of only 0.25% in a large group of patients who underwent ECV.11 Similarly, the rate of emergency cesarean delivery was 0.35%. In addition, Hofmeyr and Kulier, in their Cochrane Data Review of 2015, found no significant differences in the Apgar scores and pH’s of babies in the ECV group compared with babies in breech presentation whose mothers did not undergo ECV.7 Results of other studies have confirmed the safety of ECV.12,13
One significant risk of ECV attempts is fetal-to-maternal blood transfer. Boucher and colleagues found that 2.4% of 1,244 women who underwent ECV had a positive Kleihauer-Betke test result, and, in one-third of the positive cases, more than 1 mL of fetal blood was found in maternal circulation.14 This risk can be minimized by administering Rho (D) immune globulin to all Rh-negative mothers after the procedure.
Even these small risks, however, should not be considered in isolation. The infrequent complications of ECV must be compared with what can occur with breech-presenting fetuses during labor or cesarean delivery: complications of breech vaginal delivery, cord prolapse, difficulties with cesarean delivery, and maternal operative complications related to present and future cesarean deliveries.
Alternative approaches to converting breech presentation of unproven efficacy
Over the years, attempts have been made to address breech presentations with measures short of ECV. There is little evidence that these measures work, or work consistently.
- Observation. After 36 weeks’ gestation, only 8% of fetuses in breech presentationspontaneously move into vertex presentation.5
- Maternal positioning. There is no good evidence that such maneuvers are effective in changing fetal presentation.15
- Moxibustion and acupuncture. Moxibustion is inhalation of smoke from burning herbal compounds. In formal studies using controls, these techniques did not consistently increase the rate of movement from breech to vertex presentation.16–18 Likewise, studies with the use of acupuncture have not shown consistent success in changing fetal presentation.19
Read about various methods to facilitate ECV success
Methods to facilitate ECV success
Two techniques that can facilitate ECV success are tocolysis, which relaxes the uterus, and neuraxial analgesia/anesthesia, which relaxes anterior abdominal wall muscles and reduces or relieves ECV-associated pain.
Tocolysis
In tocolysis, a medication is administered to reduce myometrial activity and to relax the uterine muscle so that it stretches more easily around the fetus during repositioning. Tocolytic medications originally were studied for their use in decreasing myometrial tone during preterm labor.
Tocolysis clearly is effective in increasing ECV success rates. Reviewing the results of 4 randomized trials, Cluver showed a 1.38 risk ratio for successful ECV when terbutaline was used versus when there was no tocolysis. The risk ratio for cesarean delivery was 0.82.20 Fernandez, in a study of 103 women divided into terbutaline versus placebo groups, had a 52% success rate for ECV with the terbutaline group versus only a 27% success rate with the placebo group.21
Tocolytic medications include terbutaline, nifedipine, and nitroglycerin.
Tocolysis most often involves the use of β2-adrenergic receptor agonists, particularly terbutaline (despite the boxed safety warning in its prescribing information). A 0.25-mg dose of terbutaline is given subcutaneously 15 to 30 minutes before ECV. Clinicians have successfully used β2-adrenergic receptor agonists in the treatment of patients in preterm labor, and there are more data on this class of medications than on other agents used to facilitate ECV.
Although nifedipine is as effective as terbutaline in the temporary treatment of preterm uterine contractions, several studies have found this calcium channel blocker less effective than terbutaline in facilitating ECV.22,23
The uterus-relaxing effect of nitroglycerin was once thought to make this medication appropriate for facilitating ECV, but multiple studies have found success rates unimproved. In some cases, the drug performed more poorly than placebo.24 Moreover, nitroglycerin is associated with a fairly high rate of adverse effects, such as headaches and blood pressure changes.
Neuraxial analgesia/anesthesia
Over the past 2 decades, there has been a resurgence in the use of neuraxial analgesia/anesthesia in ECV. This technique is more effective than others in improving ECV success rates, it reduces maternal discomfort, and it is very safe. Specifically, it relaxes the maternal abdominal wall muscles and thereby facilitates ECV. Another benefit is that the anesthesia is in place and available for use should emergency cesarean delivery be needed during or after attempted ECV. Neuraxial anesthesia, which includes spinal, epidural, and combined spinal-epidural techniques, is almost always used with tocolysis.
The major complications of neuraxial analgesia/anesthesia are maternal hypotension and fetal bradycardia. Each is dose related and usually transient.
In the past, there was concern that using regional anesthesia to control pain would reduce a patient’s natural warning symptoms and result in a clinician applying excessive force, thus increasing the chances of fetal and maternal injury and even fetal death. However, multiple studies have found that ECV complication rates are not increased with use of neuraxial methods.
Higher doses of neuraxial anesthesia produce higher ECV success rates. This dose-dependent relationship is almost surely attributable to the fact that, although lower dose neuraxial analgesia can relieve the pain associated with ECV, an anesthetic dose is needed to relax the abdominal wall muscles and facilitate fetus repositioning.
The literature is clear: ECV success rates are significantly increased with the use of neuraxial techniques, with anesthesia having higher success rates than analgesia. Reviewing the results of 6 controlled trials in which a total of 508 patients underwent ECV with tocolysis, Goetzinger and colleagues found that the chance of ECV success was almost 60% higher in the 253 patients who received regional anesthesia than in the 255 patients who received intravenous or no analgesia.25 Moreover, only 48.4% of the regional anesthesia patients as compared with 59.3% of patients who did not have regional anesthesia underwent cesarean delivery, roughly a 20% decrease. Pain scores were consistently lower in the regional anesthesia group. Multiple other studies have reported similar results.
Although the use of neuraxial anesthesia increases the ECV success rate, and decreases the cesarean delivery rate for breech presentation by 5% to 15%,25 some groups of obstetrics professionals, noting that the decreased cesarean delivery rate does not meet the formal criterion for statistical significance, have expressed reservations about recommending regional anesthesia for ECV. Thus, despite the positive results obtained with neuraxial anesthesia, neither the literature nor authoritative professional organizations definitively recommend the use of neuraxial anesthesia in facilitating ECV.
This lack of official recommendation, however, overlooks an important point: While the cesarean delivery percentage decrease that occurs with the use of neuraxial anesthesia may not be statistically significant, the promise of a pain-free procedure will encourage more women to undergo ECV. If the procedure population increases, then the average ECV success rate of roughly 60%6 applies to a larger base of patients, reducing the total number of cesarean deliveries for breech presentation. As only a small percentage of the 110,000 to 150,000 women with breech presentation at 36 weeks currently elects to undergo ECV, any increase in the number of women who proceed with attempts at fetal repositioning once procedural pain is no longer an issue will accordingly reduce the number of cesarean deliveries for the indication of malpresentation.
Related article:
Nitrous oxide for labor pain
Overarching goal: Reduce cesarean delivery rate and associated risks
In the United States, increasing the use of ECV in cases of breech-presenting fetuses would reduce the cesarean delivery rate by about 10%, thereby reducing recovery time for cesarean deliveries, minimizing the risks associated with these deliveries (current and future), and providing the health care system with a major cost savings.
Tocolysis and the use of neuraxial anesthesia each increases the ECV success rate and each is remarkably safe within the context of a well-defined protocol. Reducing the pain associated with ECV by administering neuraxial anesthesia will increase the number of women electing to undergo the procedure and ultimately will reduce the number of cesarean deliveries performed for the indication of breech presentation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
About 3% to 4% of all fetuses at term are in breech presentation. Since 2000, when Hannah and colleagues reported finding that vaginal delivery of breech-presenting babies was riskier than cesarean delivery,1 most breech-presenting neonates in the United States have been delivered abdominally2—despite subsequent questioning of some of that study’s conclusions.
Each year in the United States, approximately 4 million babies are born, and fetal malpresentation accounts for 110,000 to 150,000 cesarean deliveries. In fact, about 15% of all cesarean deliveries in the United States are for breech presentation or transverse lie; in England the percentage is 10%.3 Fortunately, the repopularized technique of external cephalic version (ECV), in which the clinician externally rotates a breech- or transverse-lying fetus to a vertex position (FIGURE), along with the facilitating tools of tocolysis and neuraxial analgesia/anesthesia, is helping to reduce the number of breech presentations in fetuses at term and thus the number of cesarean deliveries and their sequelae—placenta accreta, prolonged recovery, and cesarean deliveries in subsequent pregnancies.

Reluctance to perform ECV is unfounded
In the United States, the practice of offering ECV to women who present with their fetus in breech presentation at term varies tremendously. It is routine at some institutions but not even offered at others.
Many ObGyns are reluctant to perform ECV. Cited reasons include the potential for injury to the fetus and mother (and related liability concerns), the ease of elective cesarean delivery, the variable success rate of ECV (35% to 86%),4 and the pain that women often have with the procedure. According to the literature, however, these concerns either are unfounded or can be mitigated with use of current techniques. Multiple studies have found that the risk of ECV to the fetus and mother is minimal, and that tocolysis and neuraxial anesthesia can facilitate the success of ECV and relieve the pain associated with the procedure.
Related article:
2017 Update on obstetrics
Indications for ECV
The indications for ECV include breech, oblique, or transverse lie presentation after 36 weeks’ gestation and the mother’s desire to avoid cesarean delivery. A clinician skilled in ECV and a facility where emergency cesarean delivery is possible are essential.
There are several instances in which ECV should not be attempted.
Contraindications include:
- concerns about fetal status, including nonreactive nonstress test, biophysical profile score <6/8, severe intrauterine growth restriction, decreased end-diastolic umbilical blood flow
- placenta previa
- multifetal gestation before delivery of first twin
- severe oligohydramnios
- severe preeclampsia
- significant fetal anomaly
- known malformation of uterus
- breech with hyperextended head or arms above shoulders, as seen on ultrasonography.
More controversial contraindications include prior uterine incision, maternal obesity (body mass index >40 kg/m2), ruptured membranes, and fetal macrosomia.
Read about timing, success rates, risk factors, alternate approaches for ECV
Optimal timing for the ECV procedure
Current practice is to wait until 36 to 37 weeks to perform ECV, as most fetuses spontaneously move into vertex presentation by 36 weeks’ gestation. This time frame has several advantages: Many unnecessary attempts at ECV are avoided; only 8% of fetuses in breech presentation after 36 weeks spontaneously change to vertex5; many fetuses revert to breech if ECV is performed too early; and prematurity generally is not an issue in the rare case that immediate delivery is required during or just after attempted ECV.
ECV during labor. Performing ECV during labor appears to pose no increased risk to mother or fetus if membranes are intact and there are no other contraindications to the procedure. Some clinicians perform ECV only during labor. The advantages are that the fetus has had every chance to move into vertex presentation on its own, the equipment used to continuously monitor the fetus during ECV is in place, and cesarean delivery and anesthesia are immediately available in the event ECV is unsuccessful.
The major disadvantage of waiting until labor is that the increased size of the fetus makes ECV more difficult. In addition, the membranes may have already ruptured, and the breech may have descended deeply into the pelvis.
Related article:
For the management of labor, patience is a virtue
Success rates in breech-to-vertex conversions
In 2016, the American College of Obstetricians and Gynecologists (ACOG) reported an average ECV success rate of 58% (range, 16% to 100%).6 ACOG noted that, with transverse lie, the success rate was significantly higher. Other studies have found a wide range of rates: 58% in 1,308 patients in a Cochrane review by Hofmeyr and colleagues7; 47% in a study by Beuckens and colleagues8; and 63.1% for primiparas and 82.7% for multiparas in a study by Tong Leung and colleagues.9 These rates were affected by whether ECV was performed with or without tocolysis, with or without intravenous analgesia, and with or without neuraxial analgesia/anesthesia (TABLE).

Likelihood of vaginal delivery after successful ECV
The rate of vaginal delivery after successful ECV is roughly half that of fetuses that were never in breech presentation.10 In successful ECV cases, dystocia and nonreassuring fetal heart rate patterns are the major indications for cesarean delivery. Some experts have speculated that the factors leading to near-term breech presentation—such as an unengaged presenting part or a mother’s smaller pelvis—also may be risk factors for dystocia in labor. Despite this, the rate of vaginal delivery of successfully verted babies has been reported to be as high as 80%.10
As might be expected, post-ECV vaginal deliveries are more common in multiparous than in primiparous women.
Although multiple problems may occur with ECV, generally they are rare and reversible. For instance, Grootscholten and colleagues found a stillbirth and placental abruption rate of only 0.25% in a large group of patients who underwent ECV.11 Similarly, the rate of emergency cesarean delivery was 0.35%. In addition, Hofmeyr and Kulier, in their Cochrane Data Review of 2015, found no significant differences in the Apgar scores and pH’s of babies in the ECV group compared with babies in breech presentation whose mothers did not undergo ECV.7 Results of other studies have confirmed the safety of ECV.12,13
One significant risk of ECV attempts is fetal-to-maternal blood transfer. Boucher and colleagues found that 2.4% of 1,244 women who underwent ECV had a positive Kleihauer-Betke test result, and, in one-third of the positive cases, more than 1 mL of fetal blood was found in maternal circulation.14 This risk can be minimized by administering Rho (D) immune globulin to all Rh-negative mothers after the procedure.
Even these small risks, however, should not be considered in isolation. The infrequent complications of ECV must be compared with what can occur with breech-presenting fetuses during labor or cesarean delivery: complications of breech vaginal delivery, cord prolapse, difficulties with cesarean delivery, and maternal operative complications related to present and future cesarean deliveries.
Alternative approaches to converting breech presentation of unproven efficacy
Over the years, attempts have been made to address breech presentations with measures short of ECV. There is little evidence that these measures work, or work consistently.
- Observation. After 36 weeks’ gestation, only 8% of fetuses in breech presentationspontaneously move into vertex presentation.5
- Maternal positioning. There is no good evidence that such maneuvers are effective in changing fetal presentation.15
- Moxibustion and acupuncture. Moxibustion is inhalation of smoke from burning herbal compounds. In formal studies using controls, these techniques did not consistently increase the rate of movement from breech to vertex presentation.16–18 Likewise, studies with the use of acupuncture have not shown consistent success in changing fetal presentation.19
Read about various methods to facilitate ECV success
Methods to facilitate ECV success
Two techniques that can facilitate ECV success are tocolysis, which relaxes the uterus, and neuraxial analgesia/anesthesia, which relaxes anterior abdominal wall muscles and reduces or relieves ECV-associated pain.
Tocolysis
In tocolysis, a medication is administered to reduce myometrial activity and to relax the uterine muscle so that it stretches more easily around the fetus during repositioning. Tocolytic medications originally were studied for their use in decreasing myometrial tone during preterm labor.
Tocolysis clearly is effective in increasing ECV success rates. Reviewing the results of 4 randomized trials, Cluver showed a 1.38 risk ratio for successful ECV when terbutaline was used versus when there was no tocolysis. The risk ratio for cesarean delivery was 0.82.20 Fernandez, in a study of 103 women divided into terbutaline versus placebo groups, had a 52% success rate for ECV with the terbutaline group versus only a 27% success rate with the placebo group.21
Tocolytic medications include terbutaline, nifedipine, and nitroglycerin.
Tocolysis most often involves the use of β2-adrenergic receptor agonists, particularly terbutaline (despite the boxed safety warning in its prescribing information). A 0.25-mg dose of terbutaline is given subcutaneously 15 to 30 minutes before ECV. Clinicians have successfully used β2-adrenergic receptor agonists in the treatment of patients in preterm labor, and there are more data on this class of medications than on other agents used to facilitate ECV.
Although nifedipine is as effective as terbutaline in the temporary treatment of preterm uterine contractions, several studies have found this calcium channel blocker less effective than terbutaline in facilitating ECV.22,23
The uterus-relaxing effect of nitroglycerin was once thought to make this medication appropriate for facilitating ECV, but multiple studies have found success rates unimproved. In some cases, the drug performed more poorly than placebo.24 Moreover, nitroglycerin is associated with a fairly high rate of adverse effects, such as headaches and blood pressure changes.
Neuraxial analgesia/anesthesia
Over the past 2 decades, there has been a resurgence in the use of neuraxial analgesia/anesthesia in ECV. This technique is more effective than others in improving ECV success rates, it reduces maternal discomfort, and it is very safe. Specifically, it relaxes the maternal abdominal wall muscles and thereby facilitates ECV. Another benefit is that the anesthesia is in place and available for use should emergency cesarean delivery be needed during or after attempted ECV. Neuraxial anesthesia, which includes spinal, epidural, and combined spinal-epidural techniques, is almost always used with tocolysis.
The major complications of neuraxial analgesia/anesthesia are maternal hypotension and fetal bradycardia. Each is dose related and usually transient.
In the past, there was concern that using regional anesthesia to control pain would reduce a patient’s natural warning symptoms and result in a clinician applying excessive force, thus increasing the chances of fetal and maternal injury and even fetal death. However, multiple studies have found that ECV complication rates are not increased with use of neuraxial methods.
Higher doses of neuraxial anesthesia produce higher ECV success rates. This dose-dependent relationship is almost surely attributable to the fact that, although lower dose neuraxial analgesia can relieve the pain associated with ECV, an anesthetic dose is needed to relax the abdominal wall muscles and facilitate fetus repositioning.
The literature is clear: ECV success rates are significantly increased with the use of neuraxial techniques, with anesthesia having higher success rates than analgesia. Reviewing the results of 6 controlled trials in which a total of 508 patients underwent ECV with tocolysis, Goetzinger and colleagues found that the chance of ECV success was almost 60% higher in the 253 patients who received regional anesthesia than in the 255 patients who received intravenous or no analgesia.25 Moreover, only 48.4% of the regional anesthesia patients as compared with 59.3% of patients who did not have regional anesthesia underwent cesarean delivery, roughly a 20% decrease. Pain scores were consistently lower in the regional anesthesia group. Multiple other studies have reported similar results.
Although the use of neuraxial anesthesia increases the ECV success rate, and decreases the cesarean delivery rate for breech presentation by 5% to 15%,25 some groups of obstetrics professionals, noting that the decreased cesarean delivery rate does not meet the formal criterion for statistical significance, have expressed reservations about recommending regional anesthesia for ECV. Thus, despite the positive results obtained with neuraxial anesthesia, neither the literature nor authoritative professional organizations definitively recommend the use of neuraxial anesthesia in facilitating ECV.
This lack of official recommendation, however, overlooks an important point: While the cesarean delivery percentage decrease that occurs with the use of neuraxial anesthesia may not be statistically significant, the promise of a pain-free procedure will encourage more women to undergo ECV. If the procedure population increases, then the average ECV success rate of roughly 60%6 applies to a larger base of patients, reducing the total number of cesarean deliveries for breech presentation. As only a small percentage of the 110,000 to 150,000 women with breech presentation at 36 weeks currently elects to undergo ECV, any increase in the number of women who proceed with attempts at fetal repositioning once procedural pain is no longer an issue will accordingly reduce the number of cesarean deliveries for the indication of malpresentation.
Related article:
Nitrous oxide for labor pain
Overarching goal: Reduce cesarean delivery rate and associated risks
In the United States, increasing the use of ECV in cases of breech-presenting fetuses would reduce the cesarean delivery rate by about 10%, thereby reducing recovery time for cesarean deliveries, minimizing the risks associated with these deliveries (current and future), and providing the health care system with a major cost savings.
Tocolysis and the use of neuraxial anesthesia each increases the ECV success rate and each is remarkably safe within the context of a well-defined protocol. Reducing the pain associated with ECV by administering neuraxial anesthesia will increase the number of women electing to undergo the procedure and ultimately will reduce the number of cesarean deliveries performed for the indication of breech presentation.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned cesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet. 2000;356(9239):1375–1383.
- Weiniger CF, Lyell DJ, Tsen LC, et al. Maternal outcomes of term breech presentation delivery: impact of successful external cephalic version in a nationwide sample of delivery admissions in the United States. BMC Pregnancy Childbirth. 2016;16(1):150.
- Eller DP, Van Dorsten JP. Breech presentation. Curr Opin Obstet Gynecol.1993;5(5)664–668.
- Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 24th ed. New York, NY: McGraw Hill; 2014:570.
- Westgren M, Edvall H, Nordstrom L, Svalenius E, Ranstam J. Spontaneous cephalic version of breech presentation in the last trimester. Br J Obstet Gynaecol. 1985;92(1):19–22.
- External cephalic version. ACOG Practice Bulletin No. 161. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2016.
- Hofmeyr GJ, Kulier R, West HM. External cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2015;(4):CD000083.
- Beuckens A, Rijnders M, Verburgt-Doeleman GH, Rijninks-van Driel GC, Thorpe J, Hutton EK. An observational study of the success and complications of 2546 external cephalic versions in low-risk pregnant women performed by trained midwives. BJOG. 2016;123(3):415–423.
- Tong Leung VK, Suen SS, Singh Sahota D, Lau TK, Yeung Leung T. External cephalic version does not increase the risk of intra-uterine death: a 17-year experience and literature review. J Matern Fetal Neonatal Med. 2012;25(9):1774–1778.
- de Hundt M, Velzel J, de Groot CJ, Mol BW, Kok M. Mode of delivery after successful external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(6):1327–1334.
- Grootscholten K, Kok M, Oei SG, Mol BW, van der Post JA. External cephalic version–related risks: a meta-analysis. Obstet Gynecol. 2008;112(5):1143–1151.
- Collaris RJ, Oei SG. External cephalic version: a safe procedure? A systematic review of version-related risk. Acta Obstet Gynecol Scand. 2004;83(6):511–518.
- Khaw KS, Lee SW, Ngan Kee WD, et al. Randomized trial of anesthetic interventions in external cephalic version for breech presentation. Br J Anaesth. 2015;114(6):944–950.
- Boucher M, Marquette GP, Varin J, Champagne J, Bujold E. Fetomaternal hemorrhage during external cephalic version. Obstet Gynecol. 2008;112(1):79–84.
- Hofmeyr GJ, Kulier R. Cephalic version by postural management for breech presentation. Cochrane Database Syst Rev. 2012;(10):CD00051.
- Coulon C, Poleszczuk M, Paty-Montaigne MH, et al. Version of breech fetuses by moxibustion with acupuncture: a randomized controlled trial. Obstet Gynecol. 2014;124(1):32–39.
- Bue L, Lauszus FF. Moxibustion did not have an effect in a randomised clinical trial for version of breech position. Dan Med J. 2016;63(2):pii:A5199.
- Coyle ME, Smith CA, Peat B. Cephalic version by moxibustion for breech presentation. Cochrane Database Syst Rev. 2012;(5):CD003928.
- Sananes N, Roth GE, Aissi GA, et al. Acupuncture version of breech presentation: a randomized sham-controlled single-blinded trial. Eur J Obstet Gynecol Reprod Biol. 2016;204:24–30.
- Cluver C, Gyte GM, Sinclair M, Dowswell T, Hofmeyr G. Interventions for helping to turn breech babies to head first presentation when using external cephalic version. Cochrane Database Syst Rev. 2015;(2):CD000184.
- Fernandez CO, Bloom SL, Smulian JC, Ananth CV, Wendel GD Jr. A randomized placebo-controlled evaluation of terbutaline for external cephalic version. Obstet Gynecol. 1997;90(5):775–779.
- Mohamed Ismail NA, Ibrahim M, Mohd Naim N, Mahdy ZA, Jamil MA, Mohd Razi ZR. Nifedipine versus terbutaline for tocolysis in external cephalic version. Int J Gynaecol Obstet. 2008;102(3):263–266.
- Kok M, Bais J, van Lith J, et al. Nifedipine as a uterine relaxant for external cephalic version: a meta-analysis. Am J Obstet Gynecol. 2008;112(2 pt 1):271–276.
- Bujold E, Boucher M, Rinfred D, Berman S, Ferreira E, Marquette GP. Sublingual nitroglycerin versus placebo as a tocolytic for external cephalic version: a randomized controlled trial in parous women. Am J Obstet Gynecol. 2003;189(4):1070–1073.
- Goetzinger KR, Harper LM, Tuuli MG, Macones GA, Colditz GA. Effect of regional anesthesia on the success of external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1137–1144.
- Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR. Planned cesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Term Breech Trial Collaborative Group. Lancet. 2000;356(9239):1375–1383.
- Weiniger CF, Lyell DJ, Tsen LC, et al. Maternal outcomes of term breech presentation delivery: impact of successful external cephalic version in a nationwide sample of delivery admissions in the United States. BMC Pregnancy Childbirth. 2016;16(1):150.
- Eller DP, Van Dorsten JP. Breech presentation. Curr Opin Obstet Gynecol.1993;5(5)664–668.
- Cunningham FG, Leveno KJ, Bloom SL, et al. Williams Obstetrics. 24th ed. New York, NY: McGraw Hill; 2014:570.
- Westgren M, Edvall H, Nordstrom L, Svalenius E, Ranstam J. Spontaneous cephalic version of breech presentation in the last trimester. Br J Obstet Gynaecol. 1985;92(1):19–22.
- External cephalic version. ACOG Practice Bulletin No. 161. American College of Obstetricians and Gynecologists. Washington, DC: ACOG; 2016.
- Hofmeyr GJ, Kulier R, West HM. External cephalic version for breech presentation at term. Cochrane Database Syst Rev. 2015;(4):CD000083.
- Beuckens A, Rijnders M, Verburgt-Doeleman GH, Rijninks-van Driel GC, Thorpe J, Hutton EK. An observational study of the success and complications of 2546 external cephalic versions in low-risk pregnant women performed by trained midwives. BJOG. 2016;123(3):415–423.
- Tong Leung VK, Suen SS, Singh Sahota D, Lau TK, Yeung Leung T. External cephalic version does not increase the risk of intra-uterine death: a 17-year experience and literature review. J Matern Fetal Neonatal Med. 2012;25(9):1774–1778.
- de Hundt M, Velzel J, de Groot CJ, Mol BW, Kok M. Mode of delivery after successful external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2014;123(6):1327–1334.
- Grootscholten K, Kok M, Oei SG, Mol BW, van der Post JA. External cephalic version–related risks: a meta-analysis. Obstet Gynecol. 2008;112(5):1143–1151.
- Collaris RJ, Oei SG. External cephalic version: a safe procedure? A systematic review of version-related risk. Acta Obstet Gynecol Scand. 2004;83(6):511–518.
- Khaw KS, Lee SW, Ngan Kee WD, et al. Randomized trial of anesthetic interventions in external cephalic version for breech presentation. Br J Anaesth. 2015;114(6):944–950.
- Boucher M, Marquette GP, Varin J, Champagne J, Bujold E. Fetomaternal hemorrhage during external cephalic version. Obstet Gynecol. 2008;112(1):79–84.
- Hofmeyr GJ, Kulier R. Cephalic version by postural management for breech presentation. Cochrane Database Syst Rev. 2012;(10):CD00051.
- Coulon C, Poleszczuk M, Paty-Montaigne MH, et al. Version of breech fetuses by moxibustion with acupuncture: a randomized controlled trial. Obstet Gynecol. 2014;124(1):32–39.
- Bue L, Lauszus FF. Moxibustion did not have an effect in a randomised clinical trial for version of breech position. Dan Med J. 2016;63(2):pii:A5199.
- Coyle ME, Smith CA, Peat B. Cephalic version by moxibustion for breech presentation. Cochrane Database Syst Rev. 2012;(5):CD003928.
- Sananes N, Roth GE, Aissi GA, et al. Acupuncture version of breech presentation: a randomized sham-controlled single-blinded trial. Eur J Obstet Gynecol Reprod Biol. 2016;204:24–30.
- Cluver C, Gyte GM, Sinclair M, Dowswell T, Hofmeyr G. Interventions for helping to turn breech babies to head first presentation when using external cephalic version. Cochrane Database Syst Rev. 2015;(2):CD000184.
- Fernandez CO, Bloom SL, Smulian JC, Ananth CV, Wendel GD Jr. A randomized placebo-controlled evaluation of terbutaline for external cephalic version. Obstet Gynecol. 1997;90(5):775–779.
- Mohamed Ismail NA, Ibrahim M, Mohd Naim N, Mahdy ZA, Jamil MA, Mohd Razi ZR. Nifedipine versus terbutaline for tocolysis in external cephalic version. Int J Gynaecol Obstet. 2008;102(3):263–266.
- Kok M, Bais J, van Lith J, et al. Nifedipine as a uterine relaxant for external cephalic version: a meta-analysis. Am J Obstet Gynecol. 2008;112(2 pt 1):271–276.
- Bujold E, Boucher M, Rinfred D, Berman S, Ferreira E, Marquette GP. Sublingual nitroglycerin versus placebo as a tocolytic for external cephalic version: a randomized controlled trial in parous women. Am J Obstet Gynecol. 2003;189(4):1070–1073.
- Goetzinger KR, Harper LM, Tuuli MG, Macones GA, Colditz GA. Effect of regional anesthesia on the success of external cephalic version: a systematic review and meta-analysis. Obstet Gynecol. 2011;118(5):1137–1144.
Fast Tracks
- Current practice is to wait until 36 to 37 weeks of gestation to perform ECV, since most fetuses spontaneously move into vertex presentation by 36 weeks
- Tocolysis, which relaxes the uterus, and neuraxial analgesia/anesthesia, which relaxes anterior abdominal wall muscles and reduces ECV-associated pain, can facilitate ECV success
- Several studies have found that nifedipine is less effective than terbutaline in facilitating ECV
- Higher doses of neuraxial anesthesia produce higher ECV success rates, possibly because the higher anesthetic dose relaxes the abdominal wall muscles and facilitates fetus repositioning
Endometriomas: Classification and surgical management
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Etiology
Endometriomas are extensively described in the literature, and their origin is the subject of several theories. In 1921, Sampson noted luteal membrane and ovarian epithelial tissues within endometriomas and was the first to indicate that endometriomas may result from the invasion of functional cysts by endometrial tissue.2,4,5 In 1979, Czernobilsky and Morris6 found endometrial and oviduct-like epithelium in ovarian endometriosis and concluded that ovarian tissue may be a common histologic precursor. Several other authors subsequently have reported finding different types of tissue within ovarian endometriomas, and not all of these chocolate cysts showed histologic evidence of endometriosis.4,7,8
Read about the classification of endometriomas
Disease classification
Our classification system identifies 2 types of endometriomas on the basis of their etiologies and characteristics. Type I, which arise from endometrial tissue implanted on the ovarian surface, are also called true endometriomas. Invagination of cortex and subsequent hemorrhage from endometrial tissue result in cyst formation. Endometrial tissue (endometrial stroma and glands) is histologically present in all type I endometriomas.1,4,9 These endometriomas usually are small (<5 cm in diameter) and have a densely adherent fibrous capsule.4 Often, there is no clear plane between cyst wall and ovarian stroma.3
Type II endometriomas arise from functional cysts involved in or invaded by cortical or pelvic side-wall endometrial implants or by type I endometriomas. Type II endometriomas are subclassified by the extent of endometrial implant involvement in the cyst wall. Type IIA endometriomas are hemorrhagic cysts with less than 10% of endometrial tissue within the cyst wall. Similar to the functional cysts from which they originate, type IIA endometriomas have a cyst wall that is separated easily from ovarian tissue during surgery.4,7,9 Although type II endometriomas tend to be larger than their type I counterparts, in some cases they are identified at an early stage of 2 to 5 cm. Endometriomas larger than 5 cm are almost always type II.4
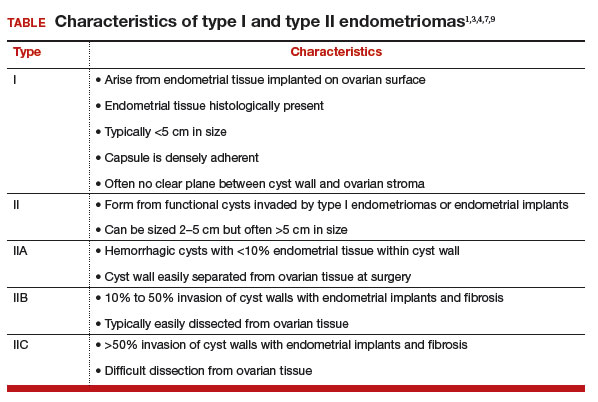
Type IIB and IIC endometriomas have endometrial implants and fibrosis within their cyst walls, with progressively more endometrial invasion in type IIC endometriomas (>50%) than in type IIB (10% to 50%). Consequently, type IIB cysts are relatively easy to dissect from ovarian tissue, except adjacent to an endometriotic area where the cyst densely adheres to the ovarian stroma. In type IIC, endometrial tissue more extensively penetrates the capsule, making dissection of diseased tissue from the ovarian stroma more difficult; in fact, separating type IIC cyst wall from ovarian stroma can be as challenging as excising a type I endometrioma.7 In most cases, a type IIC cyst is attached by adhesions and fibrosis to the pelvic side wall or uterus and ruptures during mobilization (TABLE).
Related article:
Imaging the endometrioma and mature cystic teratoma
Presentation and diagnosis
Almost all patients with an endometrioma concurrently have peritoneal endometriosis, which is characterized by dysmenorrhea, dyspareunia, chronic pelvic pain, infertility, and, in some cases, gastrointestinal or genitourinary dysfunction.1 Pelvic examination may reveal an adnexal mass that is an endometrioma, or an endometrioma may appear on imaging obtained in a pelvic pain or infertility work-up. Given its 73% sensitivity, 94% specificity, safety, and low cost, transvaginal ultrasonography is the preferred imaging modality for endometrioma.3 The characteristic ultrasonographic appearance is that of a round, homogeneous, fluid-filled mass with low-level echoes.1 Magnetic resonance imaging is appropriate when a more sensitive imaging modality is indicated, as for a patient with risk factors for malignancy.3,10–12
Read about the surgical management of endometriomas
Surgical management
Indications for surgical excision of endometriomas include pelvic pain, infertility, and prevention and diagnosis of malignancy. Endometriomas may be excised prior to use of assisted reproductive technology.13–15 Medical therapy, such as oral contraceptives, can be used to reduce the size of endometriomas but does not improve fertility.3 Certain ovarian cancers are more common in women with endometriosis, and ovarian tumors are thought to develop in about 1% of ovarian endometriosis cases.1,12 Therefore, endometrioma excision may reduce the risk of malignancy. As with other ovarian cysts, large endometriomas may be excised to reduce the risks of rupture and torsion.
Approach
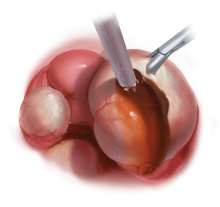
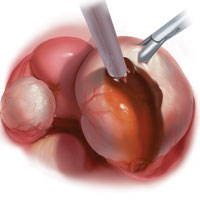
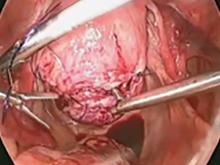
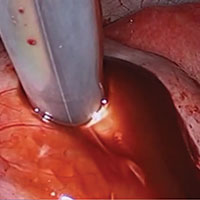
Laparoscopy is the preferred approach for endometrioma excision. Controversy exists regarding the ideal procedure: complete excision (with stripping of the cyst capsule) or drainage and ablation of the cyst wall. Compared with drainage and ablation, excision reduces recurrence of endometriomas; relieves dysmenorrhea, dyspareunia, pelvic pain, and other symptoms; and improves fertility.13,16 The recurrence rate may be as low as 5.8% with complete excision but is 90% with simple transvaginal aspiration.17,18 If not performed properly, however, cyst capsule stripping may damage nearby ovarian stroma and decrease the ovarian reserve.14 Some authors have advocated combining excision and ablation—performing cystectomy until there is no longer a clear plane between capsule and ovarian stroma and then ablating any remaining endometrial tissue.8
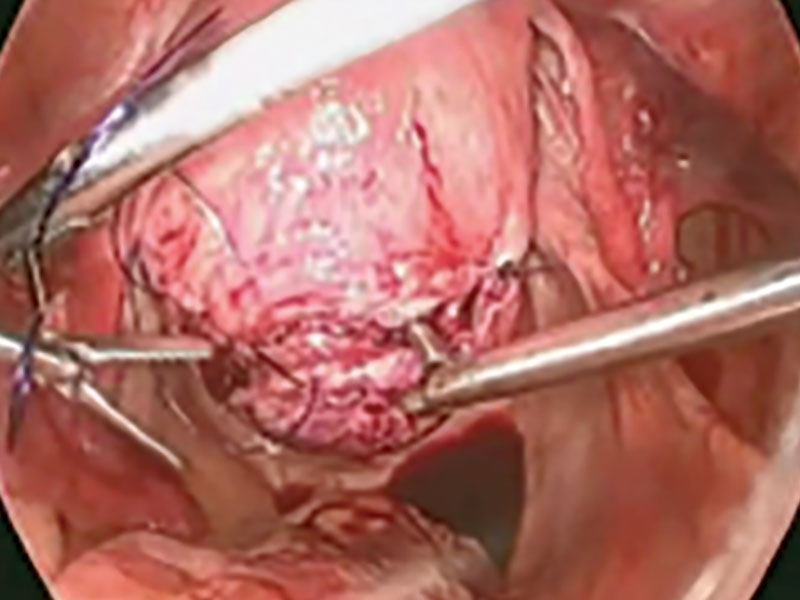
With type I and IIC endometriomas, we have seen the endometrial cyst wall infiltrating the ovarian stroma so deeply there is not always a definable plane. By contrast, type IIA and IIB endometriomas typically have a plane between the cyst wall and the ovarian cortex. In type II endometriomas, endometrial implants on the ovarian cortex infiltrate the plane of the cyst wall such that the juxtaposing lipomatous follicular cyst detaches with minimal intraoperative traction. Portions of type II endometriomas containing fibrosis and adhesions may become more difficult to peel off the cyst wall. For most endometriomas, at least 1 spot is difficult to peel off the ovary, and extra care must be taken at the hilum of ovary to avoid excising healthy ovarian cortex.4,5,7,8
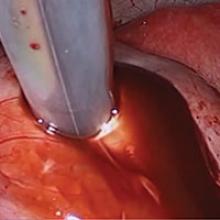
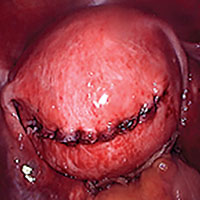
Our surgical approach accounts for the described variations in type I and II endometriomas. Endometrial contents often spill as the endometrioma is dissected off neighboring structures. When possible, endometriomas should be aspirated and irrigated prior to cystectomy to avoid seeding the pelvis and abdomen with spilled endometriotic contents. We use hydrodissection, the injection of dilute vasopressin with a laparoscopic needle, to create a plane between cyst wall and ovarian stroma and strip the cyst capsule with laparoscopic graspers. Type I endometriomas adhere densely to the ovary. Given the presence of fibrosis and adhesions, the cyst is excised in a piecemeal fashion. Care is taken to remove any endometrial implants from the ovary while preserving as much of the ovarian tissue as possible.1
Type II endometriomas are larger cysts originating from the invasion of endometrial implants or type I endometrioma into functional cysts. The difficulty of capsule excision varies according to the extent of endometrial invasion. Type IIA endometriomas contain less than 10% endometrial tissue within the cyst capsule. Thus, the standard ovarian cystectomy stripping technique is successful in removing more than 90% of the cyst capsule. Special care is taken in stripping the residual small portion that involves the endometrial glands and stroma and adheres densely to the ovary.
The larger proportion of endometrial tissue present in type IIB and IIC endometriomas degrades the plane between the cyst capsule and the ovarian stroma, making excision more difficult. Similar to the type I excision, a piecemeal approach is often necessary. If complete stripping of the cyst capsule would result in extensive loss of healthy ovarian tissue, then electrocautery, plasma energy, or laser ablation can be selectively used to destroy focal areas of endometrial invasion. Complete ablation may be difficult, as the endometrioma wall can be up to 5 mm thick.19 For these thick-walled endometriomas, we recommend excision (vs ablation), which lowers the risk of endometrioma recurrence.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
- Endometriomas are common adnexal masses in women affected by endometriosis and may exacerbate pelvic pain and impair fertility. Classification of endometriomas into type I and type II,depending on their etiology and characteristics, can guide minimally invasive surgical management.
- Type I endometriomas arise from invagination of endometrial implants on the ovarian cortex, resulting in dense fibrosis and adhesions. These lesions typically require piecemeal excision in order to completely remove the cyst capsule.
- Type II endometriomas result from invasion of endometrial tissue into preexisting functional cysts and are further subclassified by the proportion of cyst capsule containing endometrial tissue (IIA <10%, IIB 10% to 50%, IIC >50%).
- The difficulty of excising type II endometriomas correlates with the degree of endometrial invasion, with type IIA being relatively straightforward and type IIC being as challenging and piecemeal as type I.
- We generally favor complete excision rather than ablation of the cyst capsule, except for when excision would result in an unacceptable loss of healthy ovarian tissue.
Conclusion
Endometriomas, common adnexal masses in women affected by endometriosis, may exacerbate pelvic pain and impair fertility. Gynecologists should be prepared to excise endometriomas completely and exercise care in preserving as much of the ovarian stroma as possible. We classify endometriomas into 2 types: type I, which develop from invagination of endometrial implants in the ovarian cortex, and type II, which stem from invasion of functional cysts by endometrial implants or type I endometrioma. This distinction guides surgical management. We hope this article and its accompanying video will be helpful in guiding laparoscopic excision of type I and II endometriomas.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:265–302.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519.
- Keyhan S, Hughes C, Price T, Muasher S. An update on surgical versus expectant management of ovarian endometriomas in infertile women. Biomed Res Int. 2015;2015:204792.
- Nezhat F, Nezhat C, Allan CJ, Metzger DA, Sears DL. Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J Reprod Med. 1992;37(9):771–776.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:252–258.
- Czernobilsky B, Morris WJ. A histologic study of ovarian endometriosis with emphasis on hyperplastic and atypical changes. Obstet Gynecol. 1979;53(3):318–323.
- Nezhat F, Nezhat C, Nezhat C, Admon D. A fresh look at ovarian endometriomas. Contemp Ob Gyn. 1994;39(11):81–94.
- Donnez J, Lousse JC, Jadoul P, Donnez O, Squifflet J. Laparoscopic management of endometriomas using a combined technique of excisional (cystectomy) and ablative surgery. Fertil Steril. 2010;94(1):28–32.
- Nezhat C, Nezhat F, Nezhat C, Seidman DS. Classification of endometriosis. Improving the classification of endometriotic ovarian cysts. Hum Reprod. 1994;9(12):2212–2213.
- Nezhat FR, Pejovic T, Reis FM, Guo SW. The link between endometriosis and ovarian cancer: clinical implications. Int J Gynecol Cancer. 2014;24(4):623–628.
- Nezhat F, Apostol R, Mahmoud M, el Daouk M. Malignant transformation of endometriosis and its clinical significance. Fertil Steril. 2014;102(2):342–344.
- Nezhat FR, Apostal R, Nezhat C, Pejovic T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol. 2015;213(3):262–267.
- Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
- Yates J. Endometriosis and infertility: expert answers to 6 questions to help pinpoint the best route to pregnancy. OBG Manag. 2015;27(6):30–35.
- Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84(6):1574–1578.
- Exacoustos C, Zupi E, Amadio A, et al. Laparoscopic removal of endometriomas: sonographic evaluation of residual functioning ovarian tissue. Am J Obstet Gynecol. 2004;191(1):68–72.
- Gonçalves FC, Andres MP, Passman LJ, Gonçalves MO, Podgaec S. A systematic review of ultrasonography-guided transvaginal aspiration of recurrent ovarian endometrioma. Int J Gynaecol Obstet. 2016;134(1):3–7.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45(6):778–783.
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Etiology
Endometriomas are extensively described in the literature, and their origin is the subject of several theories. In 1921, Sampson noted luteal membrane and ovarian epithelial tissues within endometriomas and was the first to indicate that endometriomas may result from the invasion of functional cysts by endometrial tissue.2,4,5 In 1979, Czernobilsky and Morris6 found endometrial and oviduct-like epithelium in ovarian endometriosis and concluded that ovarian tissue may be a common histologic precursor. Several other authors subsequently have reported finding different types of tissue within ovarian endometriomas, and not all of these chocolate cysts showed histologic evidence of endometriosis.4,7,8
Read about the classification of endometriomas
Disease classification
Our classification system identifies 2 types of endometriomas on the basis of their etiologies and characteristics. Type I, which arise from endometrial tissue implanted on the ovarian surface, are also called true endometriomas. Invagination of cortex and subsequent hemorrhage from endometrial tissue result in cyst formation. Endometrial tissue (endometrial stroma and glands) is histologically present in all type I endometriomas.1,4,9 These endometriomas usually are small (<5 cm in diameter) and have a densely adherent fibrous capsule.4 Often, there is no clear plane between cyst wall and ovarian stroma.3
Type II endometriomas arise from functional cysts involved in or invaded by cortical or pelvic side-wall endometrial implants or by type I endometriomas. Type II endometriomas are subclassified by the extent of endometrial implant involvement in the cyst wall. Type IIA endometriomas are hemorrhagic cysts with less than 10% of endometrial tissue within the cyst wall. Similar to the functional cysts from which they originate, type IIA endometriomas have a cyst wall that is separated easily from ovarian tissue during surgery.4,7,9 Although type II endometriomas tend to be larger than their type I counterparts, in some cases they are identified at an early stage of 2 to 5 cm. Endometriomas larger than 5 cm are almost always type II.4
Type IIB and IIC endometriomas have endometrial implants and fibrosis within their cyst walls, with progressively more endometrial invasion in type IIC endometriomas (>50%) than in type IIB (10% to 50%). Consequently, type IIB cysts are relatively easy to dissect from ovarian tissue, except adjacent to an endometriotic area where the cyst densely adheres to the ovarian stroma. In type IIC, endometrial tissue more extensively penetrates the capsule, making dissection of diseased tissue from the ovarian stroma more difficult; in fact, separating type IIC cyst wall from ovarian stroma can be as challenging as excising a type I endometrioma.7 In most cases, a type IIC cyst is attached by adhesions and fibrosis to the pelvic side wall or uterus and ruptures during mobilization (TABLE).

Related article:
Imaging the endometrioma and mature cystic teratoma
Presentation and diagnosis
Almost all patients with an endometrioma concurrently have peritoneal endometriosis, which is characterized by dysmenorrhea, dyspareunia, chronic pelvic pain, infertility, and, in some cases, gastrointestinal or genitourinary dysfunction.1 Pelvic examination may reveal an adnexal mass that is an endometrioma, or an endometrioma may appear on imaging obtained in a pelvic pain or infertility work-up. Given its 73% sensitivity, 94% specificity, safety, and low cost, transvaginal ultrasonography is the preferred imaging modality for endometrioma.3 The characteristic ultrasonographic appearance is that of a round, homogeneous, fluid-filled mass with low-level echoes.1 Magnetic resonance imaging is appropriate when a more sensitive imaging modality is indicated, as for a patient with risk factors for malignancy.3,10–12
Read about the surgical management of endometriomas
Surgical management
Indications for surgical excision of endometriomas include pelvic pain, infertility, and prevention and diagnosis of malignancy. Endometriomas may be excised prior to use of assisted reproductive technology.13–15 Medical therapy, such as oral contraceptives, can be used to reduce the size of endometriomas but does not improve fertility.3 Certain ovarian cancers are more common in women with endometriosis, and ovarian tumors are thought to develop in about 1% of ovarian endometriosis cases.1,12 Therefore, endometrioma excision may reduce the risk of malignancy. As with other ovarian cysts, large endometriomas may be excised to reduce the risks of rupture and torsion.
Approach
Laparoscopy is the preferred approach for endometrioma excision. Controversy exists regarding the ideal procedure: complete excision (with stripping of the cyst capsule) or drainage and ablation of the cyst wall. Compared with drainage and ablation, excision reduces recurrence of endometriomas; relieves dysmenorrhea, dyspareunia, pelvic pain, and other symptoms; and improves fertility.13,16 The recurrence rate may be as low as 5.8% with complete excision but is 90% with simple transvaginal aspiration.17,18 If not performed properly, however, cyst capsule stripping may damage nearby ovarian stroma and decrease the ovarian reserve.14 Some authors have advocated combining excision and ablation—performing cystectomy until there is no longer a clear plane between capsule and ovarian stroma and then ablating any remaining endometrial tissue.8
With type I and IIC endometriomas, we have seen the endometrial cyst wall infiltrating the ovarian stroma so deeply there is not always a definable plane. By contrast, type IIA and IIB endometriomas typically have a plane between the cyst wall and the ovarian cortex. In type II endometriomas, endometrial implants on the ovarian cortex infiltrate the plane of the cyst wall such that the juxtaposing lipomatous follicular cyst detaches with minimal intraoperative traction. Portions of type II endometriomas containing fibrosis and adhesions may become more difficult to peel off the cyst wall. For most endometriomas, at least 1 spot is difficult to peel off the ovary, and extra care must be taken at the hilum of ovary to avoid excising healthy ovarian cortex.4,5,7,8
Our surgical approach accounts for the described variations in type I and II endometriomas. Endometrial contents often spill as the endometrioma is dissected off neighboring structures. When possible, endometriomas should be aspirated and irrigated prior to cystectomy to avoid seeding the pelvis and abdomen with spilled endometriotic contents. We use hydrodissection, the injection of dilute vasopressin with a laparoscopic needle, to create a plane between cyst wall and ovarian stroma and strip the cyst capsule with laparoscopic graspers. Type I endometriomas adhere densely to the ovary. Given the presence of fibrosis and adhesions, the cyst is excised in a piecemeal fashion. Care is taken to remove any endometrial implants from the ovary while preserving as much of the ovarian tissue as possible.1
Type II endometriomas are larger cysts originating from the invasion of endometrial implants or type I endometrioma into functional cysts. The difficulty of capsule excision varies according to the extent of endometrial invasion. Type IIA endometriomas contain less than 10% endometrial tissue within the cyst capsule. Thus, the standard ovarian cystectomy stripping technique is successful in removing more than 90% of the cyst capsule. Special care is taken in stripping the residual small portion that involves the endometrial glands and stroma and adheres densely to the ovary.
The larger proportion of endometrial tissue present in type IIB and IIC endometriomas degrades the plane between the cyst capsule and the ovarian stroma, making excision more difficult. Similar to the type I excision, a piecemeal approach is often necessary. If complete stripping of the cyst capsule would result in extensive loss of healthy ovarian tissue, then electrocautery, plasma energy, or laser ablation can be selectively used to destroy focal areas of endometrial invasion. Complete ablation may be difficult, as the endometrioma wall can be up to 5 mm thick.19 For these thick-walled endometriomas, we recommend excision (vs ablation), which lowers the risk of endometrioma recurrence.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
- Endometriomas are common adnexal masses in women affected by endometriosis and may exacerbate pelvic pain and impair fertility. Classification of endometriomas into type I and type II,depending on their etiology and characteristics, can guide minimally invasive surgical management.
- Type I endometriomas arise from invagination of endometrial implants on the ovarian cortex, resulting in dense fibrosis and adhesions. These lesions typically require piecemeal excision in order to completely remove the cyst capsule.
- Type II endometriomas result from invasion of endometrial tissue into preexisting functional cysts and are further subclassified by the proportion of cyst capsule containing endometrial tissue (IIA <10%, IIB 10% to 50%, IIC >50%).
- The difficulty of excising type II endometriomas correlates with the degree of endometrial invasion, with type IIA being relatively straightforward and type IIC being as challenging and piecemeal as type I.
- We generally favor complete excision rather than ablation of the cyst capsule, except for when excision would result in an unacceptable loss of healthy ovarian tissue.
Conclusion
Endometriomas, common adnexal masses in women affected by endometriosis, may exacerbate pelvic pain and impair fertility. Gynecologists should be prepared to excise endometriomas completely and exercise care in preserving as much of the ovarian stroma as possible. We classify endometriomas into 2 types: type I, which develop from invagination of endometrial implants in the ovarian cortex, and type II, which stem from invasion of functional cysts by endometrial implants or type I endometrioma. This distinction guides surgical management. We hope this article and its accompanying video will be helpful in guiding laparoscopic excision of type I and II endometriomas.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Related article:
Endometriosis: Expert answers to 7 crucial questions on diagnosis
Etiology
Endometriomas are extensively described in the literature, and their origin is the subject of several theories. In 1921, Sampson noted luteal membrane and ovarian epithelial tissues within endometriomas and was the first to indicate that endometriomas may result from the invasion of functional cysts by endometrial tissue.2,4,5 In 1979, Czernobilsky and Morris6 found endometrial and oviduct-like epithelium in ovarian endometriosis and concluded that ovarian tissue may be a common histologic precursor. Several other authors subsequently have reported finding different types of tissue within ovarian endometriomas, and not all of these chocolate cysts showed histologic evidence of endometriosis.4,7,8
Read about the classification of endometriomas
Disease classification
Our classification system identifies 2 types of endometriomas on the basis of their etiologies and characteristics. Type I, which arise from endometrial tissue implanted on the ovarian surface, are also called true endometriomas. Invagination of cortex and subsequent hemorrhage from endometrial tissue result in cyst formation. Endometrial tissue (endometrial stroma and glands) is histologically present in all type I endometriomas.1,4,9 These endometriomas usually are small (<5 cm in diameter) and have a densely adherent fibrous capsule.4 Often, there is no clear plane between cyst wall and ovarian stroma.3
Type II endometriomas arise from functional cysts involved in or invaded by cortical or pelvic side-wall endometrial implants or by type I endometriomas. Type II endometriomas are subclassified by the extent of endometrial implant involvement in the cyst wall. Type IIA endometriomas are hemorrhagic cysts with less than 10% of endometrial tissue within the cyst wall. Similar to the functional cysts from which they originate, type IIA endometriomas have a cyst wall that is separated easily from ovarian tissue during surgery.4,7,9 Although type II endometriomas tend to be larger than their type I counterparts, in some cases they are identified at an early stage of 2 to 5 cm. Endometriomas larger than 5 cm are almost always type II.4
Type IIB and IIC endometriomas have endometrial implants and fibrosis within their cyst walls, with progressively more endometrial invasion in type IIC endometriomas (>50%) than in type IIB (10% to 50%). Consequently, type IIB cysts are relatively easy to dissect from ovarian tissue, except adjacent to an endometriotic area where the cyst densely adheres to the ovarian stroma. In type IIC, endometrial tissue more extensively penetrates the capsule, making dissection of diseased tissue from the ovarian stroma more difficult; in fact, separating type IIC cyst wall from ovarian stroma can be as challenging as excising a type I endometrioma.7 In most cases, a type IIC cyst is attached by adhesions and fibrosis to the pelvic side wall or uterus and ruptures during mobilization (TABLE).

Related article:
Imaging the endometrioma and mature cystic teratoma
Presentation and diagnosis
Almost all patients with an endometrioma concurrently have peritoneal endometriosis, which is characterized by dysmenorrhea, dyspareunia, chronic pelvic pain, infertility, and, in some cases, gastrointestinal or genitourinary dysfunction.1 Pelvic examination may reveal an adnexal mass that is an endometrioma, or an endometrioma may appear on imaging obtained in a pelvic pain or infertility work-up. Given its 73% sensitivity, 94% specificity, safety, and low cost, transvaginal ultrasonography is the preferred imaging modality for endometrioma.3 The characteristic ultrasonographic appearance is that of a round, homogeneous, fluid-filled mass with low-level echoes.1 Magnetic resonance imaging is appropriate when a more sensitive imaging modality is indicated, as for a patient with risk factors for malignancy.3,10–12
Read about the surgical management of endometriomas
Surgical management
Indications for surgical excision of endometriomas include pelvic pain, infertility, and prevention and diagnosis of malignancy. Endometriomas may be excised prior to use of assisted reproductive technology.13–15 Medical therapy, such as oral contraceptives, can be used to reduce the size of endometriomas but does not improve fertility.3 Certain ovarian cancers are more common in women with endometriosis, and ovarian tumors are thought to develop in about 1% of ovarian endometriosis cases.1,12 Therefore, endometrioma excision may reduce the risk of malignancy. As with other ovarian cysts, large endometriomas may be excised to reduce the risks of rupture and torsion.
Approach
Laparoscopy is the preferred approach for endometrioma excision. Controversy exists regarding the ideal procedure: complete excision (with stripping of the cyst capsule) or drainage and ablation of the cyst wall. Compared with drainage and ablation, excision reduces recurrence of endometriomas; relieves dysmenorrhea, dyspareunia, pelvic pain, and other symptoms; and improves fertility.13,16 The recurrence rate may be as low as 5.8% with complete excision but is 90% with simple transvaginal aspiration.17,18 If not performed properly, however, cyst capsule stripping may damage nearby ovarian stroma and decrease the ovarian reserve.14 Some authors have advocated combining excision and ablation—performing cystectomy until there is no longer a clear plane between capsule and ovarian stroma and then ablating any remaining endometrial tissue.8
With type I and IIC endometriomas, we have seen the endometrial cyst wall infiltrating the ovarian stroma so deeply there is not always a definable plane. By contrast, type IIA and IIB endometriomas typically have a plane between the cyst wall and the ovarian cortex. In type II endometriomas, endometrial implants on the ovarian cortex infiltrate the plane of the cyst wall such that the juxtaposing lipomatous follicular cyst detaches with minimal intraoperative traction. Portions of type II endometriomas containing fibrosis and adhesions may become more difficult to peel off the cyst wall. For most endometriomas, at least 1 spot is difficult to peel off the ovary, and extra care must be taken at the hilum of ovary to avoid excising healthy ovarian cortex.4,5,7,8
Our surgical approach accounts for the described variations in type I and II endometriomas. Endometrial contents often spill as the endometrioma is dissected off neighboring structures. When possible, endometriomas should be aspirated and irrigated prior to cystectomy to avoid seeding the pelvis and abdomen with spilled endometriotic contents. We use hydrodissection, the injection of dilute vasopressin with a laparoscopic needle, to create a plane between cyst wall and ovarian stroma and strip the cyst capsule with laparoscopic graspers. Type I endometriomas adhere densely to the ovary. Given the presence of fibrosis and adhesions, the cyst is excised in a piecemeal fashion. Care is taken to remove any endometrial implants from the ovary while preserving as much of the ovarian tissue as possible.1
Type II endometriomas are larger cysts originating from the invasion of endometrial implants or type I endometrioma into functional cysts. The difficulty of capsule excision varies according to the extent of endometrial invasion. Type IIA endometriomas contain less than 10% endometrial tissue within the cyst capsule. Thus, the standard ovarian cystectomy stripping technique is successful in removing more than 90% of the cyst capsule. Special care is taken in stripping the residual small portion that involves the endometrial glands and stroma and adheres densely to the ovary.
The larger proportion of endometrial tissue present in type IIB and IIC endometriomas degrades the plane between the cyst capsule and the ovarian stroma, making excision more difficult. Similar to the type I excision, a piecemeal approach is often necessary. If complete stripping of the cyst capsule would result in extensive loss of healthy ovarian tissue, then electrocautery, plasma energy, or laser ablation can be selectively used to destroy focal areas of endometrial invasion. Complete ablation may be difficult, as the endometrioma wall can be up to 5 mm thick.19 For these thick-walled endometriomas, we recommend excision (vs ablation), which lowers the risk of endometrioma recurrence.
Related article:
Endometriosis and pain: Expert answers to 6 questions targeting your management options
- Endometriomas are common adnexal masses in women affected by endometriosis and may exacerbate pelvic pain and impair fertility. Classification of endometriomas into type I and type II,depending on their etiology and characteristics, can guide minimally invasive surgical management.
- Type I endometriomas arise from invagination of endometrial implants on the ovarian cortex, resulting in dense fibrosis and adhesions. These lesions typically require piecemeal excision in order to completely remove the cyst capsule.
- Type II endometriomas result from invasion of endometrial tissue into preexisting functional cysts and are further subclassified by the proportion of cyst capsule containing endometrial tissue (IIA <10%, IIB 10% to 50%, IIC >50%).
- The difficulty of excising type II endometriomas correlates with the degree of endometrial invasion, with type IIA being relatively straightforward and type IIC being as challenging and piecemeal as type I.
- We generally favor complete excision rather than ablation of the cyst capsule, except for when excision would result in an unacceptable loss of healthy ovarian tissue.
Conclusion
Endometriomas, common adnexal masses in women affected by endometriosis, may exacerbate pelvic pain and impair fertility. Gynecologists should be prepared to excise endometriomas completely and exercise care in preserving as much of the ovarian stroma as possible. We classify endometriomas into 2 types: type I, which develop from invagination of endometrial implants in the ovarian cortex, and type II, which stem from invasion of functional cysts by endometrial implants or type I endometrioma. This distinction guides surgical management. We hope this article and its accompanying video will be helpful in guiding laparoscopic excision of type I and II endometriomas.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:265–302.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519.
- Keyhan S, Hughes C, Price T, Muasher S. An update on surgical versus expectant management of ovarian endometriomas in infertile women. Biomed Res Int. 2015;2015:204792.
- Nezhat F, Nezhat C, Allan CJ, Metzger DA, Sears DL. Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J Reprod Med. 1992;37(9):771–776.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:252–258.
- Czernobilsky B, Morris WJ. A histologic study of ovarian endometriosis with emphasis on hyperplastic and atypical changes. Obstet Gynecol. 1979;53(3):318–323.
- Nezhat F, Nezhat C, Nezhat C, Admon D. A fresh look at ovarian endometriomas. Contemp Ob Gyn. 1994;39(11):81–94.
- Donnez J, Lousse JC, Jadoul P, Donnez O, Squifflet J. Laparoscopic management of endometriomas using a combined technique of excisional (cystectomy) and ablative surgery. Fertil Steril. 2010;94(1):28–32.
- Nezhat C, Nezhat F, Nezhat C, Seidman DS. Classification of endometriosis. Improving the classification of endometriotic ovarian cysts. Hum Reprod. 1994;9(12):2212–2213.
- Nezhat FR, Pejovic T, Reis FM, Guo SW. The link between endometriosis and ovarian cancer: clinical implications. Int J Gynecol Cancer. 2014;24(4):623–628.
- Nezhat F, Apostol R, Mahmoud M, el Daouk M. Malignant transformation of endometriosis and its clinical significance. Fertil Steril. 2014;102(2):342–344.
- Nezhat FR, Apostal R, Nezhat C, Pejovic T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol. 2015;213(3):262–267.
- Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
- Yates J. Endometriosis and infertility: expert answers to 6 questions to help pinpoint the best route to pregnancy. OBG Manag. 2015;27(6):30–35.
- Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84(6):1574–1578.
- Exacoustos C, Zupi E, Amadio A, et al. Laparoscopic removal of endometriomas: sonographic evaluation of residual functioning ovarian tissue. Am J Obstet Gynecol. 2004;191(1):68–72.
- Gonçalves FC, Andres MP, Passman LJ, Gonçalves MO, Podgaec S. A systematic review of ultrasonography-guided transvaginal aspiration of recurrent ovarian endometrioma. Int J Gynaecol Obstet. 2016;134(1):3–7.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45(6):778–783.
- Nezhat C, Buescher E, Paka C, et al. Video-assisted laparoscopic treatment of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:265–302.
- Burney RO, Giudice LC. Pathogenesis and pathophysiology of endometriosis. Fertil Steril. 2012;98(3):511–519.
- Keyhan S, Hughes C, Price T, Muasher S. An update on surgical versus expectant management of ovarian endometriomas in infertile women. Biomed Res Int. 2015;2015:204792.
- Nezhat F, Nezhat C, Allan CJ, Metzger DA, Sears DL. Clinical and histologic classification of endometriomas. Implications for a mechanism of pathogenesis. J Reprod Med. 1992;37(9):771–776.
- Burney RO, Giudice LC. The pathogenesis of endometriosis. In: Nezhat C, Nezhat F, Nezhat C, eds. Nezhat’s Video-Assisted and Robotic-Assisted Laparoscopy and Hysteroscopy. 4th ed. New York, NY: Cambridge University Press; 2013:252–258.
- Czernobilsky B, Morris WJ. A histologic study of ovarian endometriosis with emphasis on hyperplastic and atypical changes. Obstet Gynecol. 1979;53(3):318–323.
- Nezhat F, Nezhat C, Nezhat C, Admon D. A fresh look at ovarian endometriomas. Contemp Ob Gyn. 1994;39(11):81–94.
- Donnez J, Lousse JC, Jadoul P, Donnez O, Squifflet J. Laparoscopic management of endometriomas using a combined technique of excisional (cystectomy) and ablative surgery. Fertil Steril. 2010;94(1):28–32.
- Nezhat C, Nezhat F, Nezhat C, Seidman DS. Classification of endometriosis. Improving the classification of endometriotic ovarian cysts. Hum Reprod. 1994;9(12):2212–2213.
- Nezhat FR, Pejovic T, Reis FM, Guo SW. The link between endometriosis and ovarian cancer: clinical implications. Int J Gynecol Cancer. 2014;24(4):623–628.
- Nezhat F, Apostol R, Mahmoud M, el Daouk M. Malignant transformation of endometriosis and its clinical significance. Fertil Steril. 2014;102(2):342–344.
- Nezhat FR, Apostal R, Nezhat C, Pejovic T. New insights in the pathophysiology of ovarian cancer and implications for screening and prevention. Am J Obstet Gynecol. 2015;213(3):262–267.
- Hart RJ, Hickey M, Maouris P, Buckett W. Excisional surgery versus ablative surgery for ovarian endometriomata. Cochrane Database Syst Rev. 2008;(2):CD004992.
- Yates J. Endometriosis and infertility: expert answers to 6 questions to help pinpoint the best route to pregnancy. OBG Manag. 2015;27(6):30–35.
- Littman E, Giudice L, Lathi R, Berker B, Milki A, Nezhat C. Role of laparoscopic treatment of endometriosis in patients with failed in vitro fertilization cycles. Fertil Steril. 2005;84(6):1574–1578.
- Exacoustos C, Zupi E, Amadio A, et al. Laparoscopic removal of endometriomas: sonographic evaluation of residual functioning ovarian tissue. Am J Obstet Gynecol. 2004;191(1):68–72.
- Gonçalves FC, Andres MP, Passman LJ, Gonçalves MO, Podgaec S. A systematic review of ultrasonography-guided transvaginal aspiration of recurrent ovarian endometrioma. Int J Gynaecol Obstet. 2016;134(1):3–7.
- Alborzi S, Momtahan M, Parsanezhad ME, Dehbashi S, Zolghadri J, Alborzi S. A prospective, randomized study comparing laparoscopic ovarian cystectomy versus fenestration and coagulation in patients with endometriomas. Fertil Steril. 2004;82(6):1633–1637.
- Nezhat C, Crowgey SR, Garrison CP. Surgical treatment of endometriosis via laser laparoscopy. Fertil Steril. 1986;45(6):778–783.
IN THIS ARTICLE
Laparoscopic myomectomy: Tips for patient selection and technique
CASE Patient wants minimally invasive surgery for her fibroids, and no hysterectomy
A 44-year-old G1P1 woman comes to the office to discuss her uterine fibroids, heavy menstrual bleeding, and urinary frequency. Treatment with oral contraceptives has not been effective in reducing the bleeding. She now wants surgical treatment without a hysterectomy (the hysterectomy was recommended by her previous gynecologist). On examination, a 14-week-size irregular uterus is felt. Myomectomy is discussed, and the patient asks if minimally invasive surgery (MIS) is possible. Complete blood cell count testing shows a hemoglobin level of 9.4 g/dL. Pelvic magnetic resonance imaging (MRI) shows a 6-cm type 2 posterior fundal fibroid and a 6-cm type 5 posterior lower-uterine-segment fibroid (FIGURE 1). These 2 fibroids have regular contours, and enhancement is not increased with contrast, consistent with benign fibroids.
Determining that laparoscopic myomectomy is a good option
Fibroids may affect quality of life—they may cause heavy menstrual bleeding, pelvic pain or pressure, or urinary frequency or incontinence. For many women who want large or numerous fibroids removed but the uterus preserved, abdominal myomectomy is required. Smaller and less numerous fibroids usually can be managed laparoscopically or with robotic assistance.
A systematic review of 6 randomized, controlled trials comparing laparoscopic and open myomectomy in 576 patients found that, although laparoscopic myomectomy was associated with longer operative time (approximately 13 minutes), it was also linked to less operative blood loss, fewer overall complications, reduced postoperative pain, and faster recovery.1 However, wide application of the laparoscopic approach may be limited by the size and number of fibroids that can be reasonably removed and by the surgical skill needed for fibroid excision and laparoscopic suturing.
Four imaging modalities can be used for fibroids: transvaginal sonography (TVS), saline-infusion sonography (SIS), hysteroscopy, and MRI. TVS is the most readily available and least costly modality used to differentiate fibroids from other pelvic pathology; SIS provides contrast for the endometrial cavity and better defines submucous fibroids; and hysteroscopy detects visually apparent distortion of the cavity. MRI, however, provides the most complete evaluation of size, position, and number of fibroids.
A study comparing TVS, SIS, hysteroscopy, and MRI found that number and position of fibroids were best identified with MRI.2 In addition, with MRI, the proximity of the fibroids and uterus to the bladder, rectum, and iliac bones can be evaluated. As tactility in laparoscopic and robot-assisted surgery is very limited, surgeons who use MRI to accurately assess fibroids preoperatively may be able to avoid missing them during the procedure.3 MRI also can be used reliably to diagnose adenomyosis and may be able to help identify uterine sarcoma.
Tip. For all women considering laparoscopic or robot-assisted myomectomy, I order pelvic MRI with and without contrast. Having the radiologist limit the number of MRI sequences may reduce the cost and make it comparable to that of other imaging modalities. I request T2-weighted MRI scans in the coronal, sagittal, and axial planes; in addition, to determine distortion of the uterine cavity by submucous fibroids, I request scans in the planes parallel with and perpendicular to the uterine axis. One gadolinium-enhanced T1-weighted MRI scan is needed to evaluate perfusion.
Although radiologists are experts in image interpretation, they are unfamiliar with the treatments and surgical issues that gynecologists must consider. Reading MRI scans for fibroids is straightforward, and gynecologists who regularly treat women with fibroids should consider viewing images with a radiologist until they become proficient.
Related article:
Surgical management of broad ligament fibroids
Surgeons who have the experience and skill and know the size, number, and position of fibroids are able to select the appropriate candidates for laparoscopic myomectomy. Authors of a study of 2,050 laparoscopic myomectomies found that fibroids larger than 5 cm, removal of more than 3 fibroids, and broad ligament fibroids were more likely to be associated with major complications, including visceral injury, conversion to laparotomy, and bleeding requiring blood transfusion.4
In laparoscopic myomectomy, uterus reconstruction requires laparoscopic suturing. Although robot-assisted myomectomy may make laparoscopic suturing easier, the added cost, longer operative time, and unimproved outcomes must be considered too.
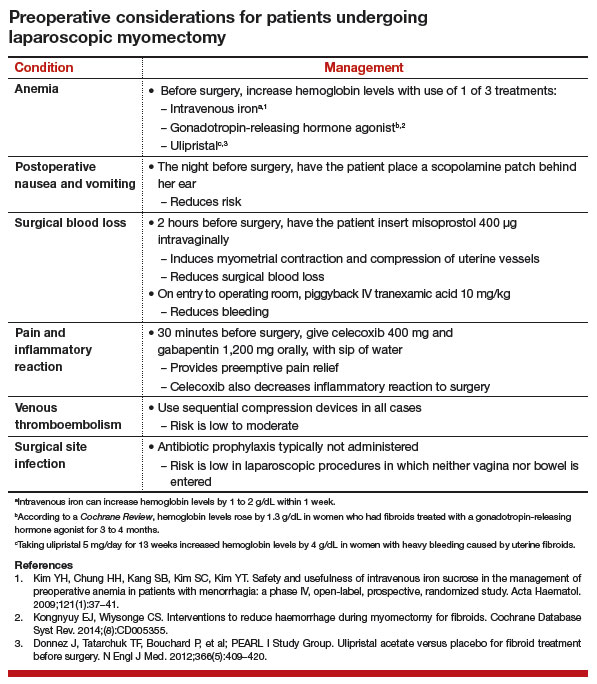
Read about trocar placement and managing blood loss
Trocar placement
Place the patient in the dorsal lithotomy position.
Tip. For most women, I do not use a uterine manipulator, as my assistant can manipulate the uterus with laparoscopic graspers.
Port placement should be based on the position and size of the fibroids to be removed. Laparoscopic suturing is more ergonomic with 2 ports placed on one side of the patient (FIGURE 2). For suture access, a 12-mm port is placed about 2 cm medial to the iliac crest and a 5-mm port is placed medial to the 12-mm port, near the level of the umbilicus. Lateral trocars should be placed high, above the superior aspect of the uterus, to make it easier to access the fibroids, and lateral to the inferior epigastric vessels, to avoid injuring those vessels. If the uterus is near or above the umbilicus, a left upper quadrant approach may be used, with the access ports placed above the umbilicus.
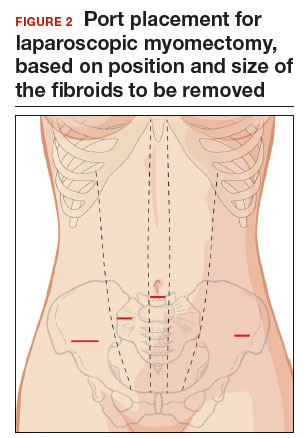
Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Managing intraoperative blood loss
I use a combination of 3 agents to reduce intraoperative blood loss during laparoscopic myomectomy: preoperative misoprostol and tranexamic acid and intraoperative vasopressin. Although there are no data showing an advantage in using these drugs together, the agents have different mechanisms of action and no negative interactions.
Injected below the vascular pseudocapsule, 20 units of vasopressin in 100 mL of normal saline causes vasoconstriction of capillaries, small arterioles, and venules. Avoid intravascular injection given that bradycardia and cardiovascular collapse have been reported (rare cases). Loss of peripheral pulses, bradycardia, unmeasurable blood pressure, and cardiac complications have been reported after myometrial injection of ≥5 units of vasopressin.5
Although vasopressin is a powerful vasoconstrictor, these clinical findings are often interpreted as severe hypotension. However, evaluation of peripheral arterial blood flow by Doppler ultrasonography has revealed severe vasospasm and increased proximal blood pressure.5 Keep this potential reaction in mind to avoid misinterpreting findings and treating a patient with vasopressors. Presence of palpable carotid pulses and maintenance of normal partial pressure of end-tidal carbon dioxide can help differentiate peripheral vasospasm from global hypotension.
Use of vasopressin to reduce blood loss during myomectomy is off-label. On occasion, I apply a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. I use a red Robinson catheter, throw 1 tie in front of the uterus, pull with graspers on both ends until it is tight, and then clamp the half-knot with a locking grasper.
Tip. Although a salvage-type autologous blood transfusion device may be used during laparoscopic or robot-assisted myomectomy, cases in which this device is considered for very large or multiple fibroids might be better managed with abdominal myomectomy.
Surgical technique
After injecting vasopressin, I use a high-frequency mechanical vibration scalpel to incise the myometrium directly over a prominent fibroid and carry the incision deeply until fibroid tissue is definite. Alternatively, a monopolar laparoscopic needle can be used in cut mode—which also limits damage to the myometrium.
Tip. The course of vessels over a fibroid is unpredictable, and we cannot be certain that any uterine incision will avoid bleeding. Therefore, I make transverse incisions, which allow more ergonomic laparoscopic suturing.
It is important to incise completely through the myometrium and through the pink-red pseudocapsule containing the vascular network surrounding the fibroid. This plane is often deeper than usually recognized and can be identified just over the white fibroid.
The fibroid is grasped with a tenaculum for traction, and countertraction is applied with a grasper on the myometrial edges. Once the fibroid is reached, graspers and the mechanical vibration scalpel are used to tease the pseudocapsule away from the fibroid (VIDEO).
Tip. Staying under the pseudocapsule reduces bleeding and may preserve the tissue’s growth factors and neurotransmitters, which are thought to promote wound healing.6
Dissection with the mechanical vibration scalpel (or monopolar needle) should be performed under visual control to identify the tissue adhering to the fibroid, which is desiccated and then divided. The fibroid is dissected until free of the myometrium and is placed in the right lower abdomen. Small fibroids can be strung together on a long suture so none will be lost. Using bipolar paddles, desiccate large bleeding vessels in the myometrial defect sparingly, with care taken to avoid devascularizing the myometrium, which might compromise wound healing. Myometrial repair should be performed in accordance with the accepted surgical technique used in laparotomy.
Place delayed absorbable sutures in 2 or 3 layers, as needed, to reapproximate the myometrium and secure hemostasis.
Tip: I use 0 polydioxanone interrupted figure-of-8 sutures, but continuous running sutures with or without barbs also can be used. For the serosa, I use a continuous barbed suture in a baseball stitch, which buries both the raw edges of the serosa and the barbs for smooth closure (FIGURE 3). These closure methods have not been compared to see which provides superior wound healing or subsequent wound strength.
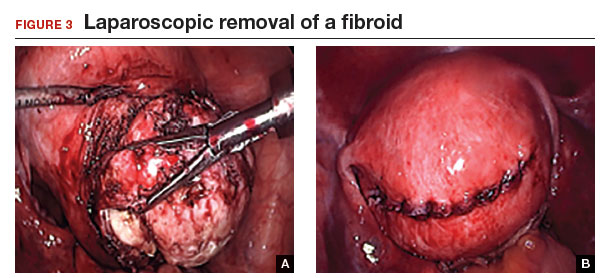
The fibroid can be morcellated with an electromechanical morcellator or a scalpel (hand morcellation). Either instrument can be used in contained or uncontained fashion. I insert an electromechanical morcellator through the right lower quadrant incision and morcellate tissue in the anterior midpelvis. Safety requires careful control of the rotating blade and scrutiny of the bowel, bladder, and major vessels. Our operating room has 4 rules for morcellator use:
- The blade is activated only under direct visualization.
- Both the surgeon and the assistant must say “ready” before the blade is activated.
- The hand holding the morcellator must remain still while tissue is being drawn into the device.
- Any undue resistance from the tissue is cause to stop the blade. This precaution is taken because there is a tendency to drop the blade in an attempt to overcome the resistance.
Tip: I limit rotational forces and scattering of tissue by “pulsing” the blade on and off when morcellating softer tissue.
Various methods of contained morcellation (morcellation in a containment bag) have been described.7 In one method, tissue is placed in a bag, the neck of the bag is brought through an enlarged umbilical incision, and the tissue is cut into small pieces until it is entirely removed. Another method is to use an electromechanical morcellator with a specially designed containment bag inside the abdomen. The bag is introduced through a 12-mm port and unfurled inside the abdomen; the specimen is placed in the bag; the neck of the bag is brought out through the port; the bag is insufflated with carbon dioxide; the laparoscope, a 5-mm grasper, and the morcellator tip are passed into the bag; and morcellation is performed. Early studies of contained morcellation reported longer operating times, leaking bags, and visceral injuries. In 2016, the US Food and Drug Administration (FDA) cleared the PneumoLiner containment system but required that its manufacturer (Advanced Surgical Concepts) warn patients and health care providers that its bag has not been proved to reduce the risk of spreading cancer during morcellation procedures.8
During laparoscopic myomectomy, fibroid removal by myometrial dissection disperses tissue fragments, and the unprotected fibroid is usually stored in the abdomen until hemostasis is secured and suturing completed. Limiting the rotational forces that lead to further dispersement and irrigating copiously to remove tissue fragments help eliminate residual tissue.
The pelvis and the abdomen are irrigated with normal saline (approximately 3 L) and suctioned multiple times.
Tip. Alternating between the Trendelenburg and reverse Trendelenburg positions allows fluid to wash tissue down to the pelvis, where it is more easily seen and removed.
Careful inspection for tissue fragments and copious irrigation and suctioning are important in reducing the risk that tissue fragments will remain in the peritoneal cavity and parasitic fibroids will develop. In cases of occult leiomyosarcoma (LMS), this step may be particularly important.
I place a knitted fabric of modified cellulose over the hysterotomy suture lines to reduce the incidence of adhesion formation. Once the procedure is complete, the local anesthetic bupivicaine is injected deep into the incision sites. Injecting anesthetic before making the incisions does not provide better pain relief; injecting after the procedure provides pain relief for 6 hours.9
Related article:
Robot-assisted laparoscopic myomectomy
Morcellation and risk of leiomyosarcoma
Given the need to prevent laparoscopic morcellators from inadvertently spreading tissue within the peritoneal cavity of women with occult LMS, the FDA issued a safety communication in 2014 warning against their use in the majority of women who undergo myomectomy or hysterectomy for fibroids.10 However, Pritts and colleagues estimated the prevalence of LMS in women who had surgery for presumed uterine fibroids at about 1 in 2,000 (0.05%), significantly lower than the FDA’s estimate of 1 in 350.10,11 In 2015, a large population-based prospective registry study found 2 cases of occult LMS in 8,720 fibroid surgery patients (0.02%).12
Related article:
The FDA’s review of the data on open power morcellation was “inadequate, irresponsible” and a “disservice to women”
Since LMS metastasizes through the bloodstream, there is no reliable evidence that morcellation influences survival or that electromechanical morcellation is inferior to vaginal or mini-laparotomy morcellation with a scalpel. According to recent publications, compared with MIS, open abdominal surgery is associated with more morbidity and mortality in women.13 Since the FDA advisory was issued, the number of abdominal surgeries has increased, as has the number of related complications.13
I use electromechanical morcellation techniques for women who want MIS. All surgical procedures have potential risks, and patients’ and physicians’ understanding of risks forms the foundation of medical decision making. The possibility of occult LMS should be considered by women and their gynecologists, and proper informed consent, noting both the LMS risk and the increased risks of abdominal surgery, should be obtained.
Related article:
Tissue extraction: Can the pendulum change direction?
Risk of uterine rupture after laparoscopic myomectomy
After abdominal myomectomy, uterine rupture during pregnancy or delivery is rare, according to reviews of delivery records of many thousands of women.14 Operative techniques, instruments, and energy sources used during laparoscopic or robot-assisted myomectomy may differ from those used during laparotomy, and anecdotal communications suggest that uterine rupture may be more common after laparoscopic or robot-assisted myomectomy. A meta-analysis of 56 articles (3,685 pregnancies) published between 1970 and 2013 found 29 cases of uterine rupture after myomectomy, with no statistical difference in rupture risk between laparoscopic and abdominal myomectomy.15 As most reports are case studies or small case series, the incidence of rupture cannot be reliably calculated.
There is no consensus regarding the factors that may increase the risk of uterine rupture after laparoscopic myomectomy. Three factors are postulated to interfere with myometrial wound healing and increase uterine rupture risk: failure to adequately suture myometrial defects, excessive use of monopolar or bipolar electrosurgery with devascularization of the myometrium, and lack of hemostasis with subsequent hematoma formation.16 It seems prudent that surgeons should adhere to time-tested techniques for abdominal myomectomy. Even with use of ideal surgical techniques, however, individual wound-healing characteristics may predispose to uterine rupture.
CASE Resolved
After giving proper informed consent, the patient underwent laparoscopic myomectomy and electromechanical morcellation. Her 2 fibroids were removed, with a blood loss of 200 mL, and that afternoon she was discharged from the surgery center with written postoperative instructions and oral pain medication. A telephone call the next day found her comfortable, with no nausea or vomiting, and happy to be fibroid free. Pathologic inspection of the morcellated tissue confirmed that the fibroids were benign. At 2-week follow-up, the patient was no longer taking pain medication and was ready to return to work and normal activity. Her fatigue persisted, though, and she arranged to take time to rest during the day.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):14–21.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Parker WH. The utility of MRI for the surgical treatment of women with uterine fibroid tumors. Am J Obstet Gynecol. 2012;206(1):31–36.
- Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453–462.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci. 2016;18(2):129–139.
- Taylan E, Sahin C, Zeybek B, Akdemir A. Contained morcellation: review of current methods and future directions. Front Surg. 2017;4:15.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Published April 7, 2016. Accessed June 9, 2017.
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;(3):CD007049.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed June 9, 2017.
- Pritts EA, Vanness DJ, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bojahr B, De Wilde RL, Tchartchian G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet. 2015;292(3):665–672.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1–e13.
- Palerme GR, Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol. 1966;94(4):571–576.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
CASE Patient wants minimally invasive surgery for her fibroids, and no hysterectomy
A 44-year-old G1P1 woman comes to the office to discuss her uterine fibroids, heavy menstrual bleeding, and urinary frequency. Treatment with oral contraceptives has not been effective in reducing the bleeding. She now wants surgical treatment without a hysterectomy (the hysterectomy was recommended by her previous gynecologist). On examination, a 14-week-size irregular uterus is felt. Myomectomy is discussed, and the patient asks if minimally invasive surgery (MIS) is possible. Complete blood cell count testing shows a hemoglobin level of 9.4 g/dL. Pelvic magnetic resonance imaging (MRI) shows a 6-cm type 2 posterior fundal fibroid and a 6-cm type 5 posterior lower-uterine-segment fibroid (FIGURE 1). These 2 fibroids have regular contours, and enhancement is not increased with contrast, consistent with benign fibroids.

Determining that laparoscopic myomectomy is a good option
Fibroids may affect quality of life—they may cause heavy menstrual bleeding, pelvic pain or pressure, or urinary frequency or incontinence. For many women who want large or numerous fibroids removed but the uterus preserved, abdominal myomectomy is required. Smaller and less numerous fibroids usually can be managed laparoscopically or with robotic assistance.
A systematic review of 6 randomized, controlled trials comparing laparoscopic and open myomectomy in 576 patients found that, although laparoscopic myomectomy was associated with longer operative time (approximately 13 minutes), it was also linked to less operative blood loss, fewer overall complications, reduced postoperative pain, and faster recovery.1 However, wide application of the laparoscopic approach may be limited by the size and number of fibroids that can be reasonably removed and by the surgical skill needed for fibroid excision and laparoscopic suturing.
Four imaging modalities can be used for fibroids: transvaginal sonography (TVS), saline-infusion sonography (SIS), hysteroscopy, and MRI. TVS is the most readily available and least costly modality used to differentiate fibroids from other pelvic pathology; SIS provides contrast for the endometrial cavity and better defines submucous fibroids; and hysteroscopy detects visually apparent distortion of the cavity. MRI, however, provides the most complete evaluation of size, position, and number of fibroids.
A study comparing TVS, SIS, hysteroscopy, and MRI found that number and position of fibroids were best identified with MRI.2 In addition, with MRI, the proximity of the fibroids and uterus to the bladder, rectum, and iliac bones can be evaluated. As tactility in laparoscopic and robot-assisted surgery is very limited, surgeons who use MRI to accurately assess fibroids preoperatively may be able to avoid missing them during the procedure.3 MRI also can be used reliably to diagnose adenomyosis and may be able to help identify uterine sarcoma.
Tip. For all women considering laparoscopic or robot-assisted myomectomy, I order pelvic MRI with and without contrast. Having the radiologist limit the number of MRI sequences may reduce the cost and make it comparable to that of other imaging modalities. I request T2-weighted MRI scans in the coronal, sagittal, and axial planes; in addition, to determine distortion of the uterine cavity by submucous fibroids, I request scans in the planes parallel with and perpendicular to the uterine axis. One gadolinium-enhanced T1-weighted MRI scan is needed to evaluate perfusion.
Although radiologists are experts in image interpretation, they are unfamiliar with the treatments and surgical issues that gynecologists must consider. Reading MRI scans for fibroids is straightforward, and gynecologists who regularly treat women with fibroids should consider viewing images with a radiologist until they become proficient.
Related article:
Surgical management of broad ligament fibroids
Surgeons who have the experience and skill and know the size, number, and position of fibroids are able to select the appropriate candidates for laparoscopic myomectomy. Authors of a study of 2,050 laparoscopic myomectomies found that fibroids larger than 5 cm, removal of more than 3 fibroids, and broad ligament fibroids were more likely to be associated with major complications, including visceral injury, conversion to laparotomy, and bleeding requiring blood transfusion.4
In laparoscopic myomectomy, uterus reconstruction requires laparoscopic suturing. Although robot-assisted myomectomy may make laparoscopic suturing easier, the added cost, longer operative time, and unimproved outcomes must be considered too.

Read about trocar placement and managing blood loss
Trocar placement
Place the patient in the dorsal lithotomy position.
Tip. For most women, I do not use a uterine manipulator, as my assistant can manipulate the uterus with laparoscopic graspers.
Port placement should be based on the position and size of the fibroids to be removed. Laparoscopic suturing is more ergonomic with 2 ports placed on one side of the patient (FIGURE 2). For suture access, a 12-mm port is placed about 2 cm medial to the iliac crest and a 5-mm port is placed medial to the 12-mm port, near the level of the umbilicus. Lateral trocars should be placed high, above the superior aspect of the uterus, to make it easier to access the fibroids, and lateral to the inferior epigastric vessels, to avoid injuring those vessels. If the uterus is near or above the umbilicus, a left upper quadrant approach may be used, with the access ports placed above the umbilicus.

Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Managing intraoperative blood loss
I use a combination of 3 agents to reduce intraoperative blood loss during laparoscopic myomectomy: preoperative misoprostol and tranexamic acid and intraoperative vasopressin. Although there are no data showing an advantage in using these drugs together, the agents have different mechanisms of action and no negative interactions.
Injected below the vascular pseudocapsule, 20 units of vasopressin in 100 mL of normal saline causes vasoconstriction of capillaries, small arterioles, and venules. Avoid intravascular injection given that bradycardia and cardiovascular collapse have been reported (rare cases). Loss of peripheral pulses, bradycardia, unmeasurable blood pressure, and cardiac complications have been reported after myometrial injection of ≥5 units of vasopressin.5
Although vasopressin is a powerful vasoconstrictor, these clinical findings are often interpreted as severe hypotension. However, evaluation of peripheral arterial blood flow by Doppler ultrasonography has revealed severe vasospasm and increased proximal blood pressure.5 Keep this potential reaction in mind to avoid misinterpreting findings and treating a patient with vasopressors. Presence of palpable carotid pulses and maintenance of normal partial pressure of end-tidal carbon dioxide can help differentiate peripheral vasospasm from global hypotension.
Use of vasopressin to reduce blood loss during myomectomy is off-label. On occasion, I apply a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. I use a red Robinson catheter, throw 1 tie in front of the uterus, pull with graspers on both ends until it is tight, and then clamp the half-knot with a locking grasper.
Tip. Although a salvage-type autologous blood transfusion device may be used during laparoscopic or robot-assisted myomectomy, cases in which this device is considered for very large or multiple fibroids might be better managed with abdominal myomectomy.
Surgical technique
After injecting vasopressin, I use a high-frequency mechanical vibration scalpel to incise the myometrium directly over a prominent fibroid and carry the incision deeply until fibroid tissue is definite. Alternatively, a monopolar laparoscopic needle can be used in cut mode—which also limits damage to the myometrium.
Tip. The course of vessels over a fibroid is unpredictable, and we cannot be certain that any uterine incision will avoid bleeding. Therefore, I make transverse incisions, which allow more ergonomic laparoscopic suturing.
It is important to incise completely through the myometrium and through the pink-red pseudocapsule containing the vascular network surrounding the fibroid. This plane is often deeper than usually recognized and can be identified just over the white fibroid.
The fibroid is grasped with a tenaculum for traction, and countertraction is applied with a grasper on the myometrial edges. Once the fibroid is reached, graspers and the mechanical vibration scalpel are used to tease the pseudocapsule away from the fibroid (VIDEO).
Tip. Staying under the pseudocapsule reduces bleeding and may preserve the tissue’s growth factors and neurotransmitters, which are thought to promote wound healing.6
Dissection with the mechanical vibration scalpel (or monopolar needle) should be performed under visual control to identify the tissue adhering to the fibroid, which is desiccated and then divided. The fibroid is dissected until free of the myometrium and is placed in the right lower abdomen. Small fibroids can be strung together on a long suture so none will be lost. Using bipolar paddles, desiccate large bleeding vessels in the myometrial defect sparingly, with care taken to avoid devascularizing the myometrium, which might compromise wound healing. Myometrial repair should be performed in accordance with the accepted surgical technique used in laparotomy.
Place delayed absorbable sutures in 2 or 3 layers, as needed, to reapproximate the myometrium and secure hemostasis.
Tip: I use 0 polydioxanone interrupted figure-of-8 sutures, but continuous running sutures with or without barbs also can be used. For the serosa, I use a continuous barbed suture in a baseball stitch, which buries both the raw edges of the serosa and the barbs for smooth closure (FIGURE 3). These closure methods have not been compared to see which provides superior wound healing or subsequent wound strength.

The fibroid can be morcellated with an electromechanical morcellator or a scalpel (hand morcellation). Either instrument can be used in contained or uncontained fashion. I insert an electromechanical morcellator through the right lower quadrant incision and morcellate tissue in the anterior midpelvis. Safety requires careful control of the rotating blade and scrutiny of the bowel, bladder, and major vessels. Our operating room has 4 rules for morcellator use:
- The blade is activated only under direct visualization.
- Both the surgeon and the assistant must say “ready” before the blade is activated.
- The hand holding the morcellator must remain still while tissue is being drawn into the device.
- Any undue resistance from the tissue is cause to stop the blade. This precaution is taken because there is a tendency to drop the blade in an attempt to overcome the resistance.
Tip: I limit rotational forces and scattering of tissue by “pulsing” the blade on and off when morcellating softer tissue.
Various methods of contained morcellation (morcellation in a containment bag) have been described.7 In one method, tissue is placed in a bag, the neck of the bag is brought through an enlarged umbilical incision, and the tissue is cut into small pieces until it is entirely removed. Another method is to use an electromechanical morcellator with a specially designed containment bag inside the abdomen. The bag is introduced through a 12-mm port and unfurled inside the abdomen; the specimen is placed in the bag; the neck of the bag is brought out through the port; the bag is insufflated with carbon dioxide; the laparoscope, a 5-mm grasper, and the morcellator tip are passed into the bag; and morcellation is performed. Early studies of contained morcellation reported longer operating times, leaking bags, and visceral injuries. In 2016, the US Food and Drug Administration (FDA) cleared the PneumoLiner containment system but required that its manufacturer (Advanced Surgical Concepts) warn patients and health care providers that its bag has not been proved to reduce the risk of spreading cancer during morcellation procedures.8
During laparoscopic myomectomy, fibroid removal by myometrial dissection disperses tissue fragments, and the unprotected fibroid is usually stored in the abdomen until hemostasis is secured and suturing completed. Limiting the rotational forces that lead to further dispersement and irrigating copiously to remove tissue fragments help eliminate residual tissue.
The pelvis and the abdomen are irrigated with normal saline (approximately 3 L) and suctioned multiple times.
Tip. Alternating between the Trendelenburg and reverse Trendelenburg positions allows fluid to wash tissue down to the pelvis, where it is more easily seen and removed.
Careful inspection for tissue fragments and copious irrigation and suctioning are important in reducing the risk that tissue fragments will remain in the peritoneal cavity and parasitic fibroids will develop. In cases of occult leiomyosarcoma (LMS), this step may be particularly important.
I place a knitted fabric of modified cellulose over the hysterotomy suture lines to reduce the incidence of adhesion formation. Once the procedure is complete, the local anesthetic bupivicaine is injected deep into the incision sites. Injecting anesthetic before making the incisions does not provide better pain relief; injecting after the procedure provides pain relief for 6 hours.9
Related article:
Robot-assisted laparoscopic myomectomy
Morcellation and risk of leiomyosarcoma
Given the need to prevent laparoscopic morcellators from inadvertently spreading tissue within the peritoneal cavity of women with occult LMS, the FDA issued a safety communication in 2014 warning against their use in the majority of women who undergo myomectomy or hysterectomy for fibroids.10 However, Pritts and colleagues estimated the prevalence of LMS in women who had surgery for presumed uterine fibroids at about 1 in 2,000 (0.05%), significantly lower than the FDA’s estimate of 1 in 350.10,11 In 2015, a large population-based prospective registry study found 2 cases of occult LMS in 8,720 fibroid surgery patients (0.02%).12
Related article:
The FDA’s review of the data on open power morcellation was “inadequate, irresponsible” and a “disservice to women”
Since LMS metastasizes through the bloodstream, there is no reliable evidence that morcellation influences survival or that electromechanical morcellation is inferior to vaginal or mini-laparotomy morcellation with a scalpel. According to recent publications, compared with MIS, open abdominal surgery is associated with more morbidity and mortality in women.13 Since the FDA advisory was issued, the number of abdominal surgeries has increased, as has the number of related complications.13
I use electromechanical morcellation techniques for women who want MIS. All surgical procedures have potential risks, and patients’ and physicians’ understanding of risks forms the foundation of medical decision making. The possibility of occult LMS should be considered by women and their gynecologists, and proper informed consent, noting both the LMS risk and the increased risks of abdominal surgery, should be obtained.
Related article:
Tissue extraction: Can the pendulum change direction?
Risk of uterine rupture after laparoscopic myomectomy
After abdominal myomectomy, uterine rupture during pregnancy or delivery is rare, according to reviews of delivery records of many thousands of women.14 Operative techniques, instruments, and energy sources used during laparoscopic or robot-assisted myomectomy may differ from those used during laparotomy, and anecdotal communications suggest that uterine rupture may be more common after laparoscopic or robot-assisted myomectomy. A meta-analysis of 56 articles (3,685 pregnancies) published between 1970 and 2013 found 29 cases of uterine rupture after myomectomy, with no statistical difference in rupture risk between laparoscopic and abdominal myomectomy.15 As most reports are case studies or small case series, the incidence of rupture cannot be reliably calculated.
There is no consensus regarding the factors that may increase the risk of uterine rupture after laparoscopic myomectomy. Three factors are postulated to interfere with myometrial wound healing and increase uterine rupture risk: failure to adequately suture myometrial defects, excessive use of monopolar or bipolar electrosurgery with devascularization of the myometrium, and lack of hemostasis with subsequent hematoma formation.16 It seems prudent that surgeons should adhere to time-tested techniques for abdominal myomectomy. Even with use of ideal surgical techniques, however, individual wound-healing characteristics may predispose to uterine rupture.
CASE Resolved
After giving proper informed consent, the patient underwent laparoscopic myomectomy and electromechanical morcellation. Her 2 fibroids were removed, with a blood loss of 200 mL, and that afternoon she was discharged from the surgery center with written postoperative instructions and oral pain medication. A telephone call the next day found her comfortable, with no nausea or vomiting, and happy to be fibroid free. Pathologic inspection of the morcellated tissue confirmed that the fibroids were benign. At 2-week follow-up, the patient was no longer taking pain medication and was ready to return to work and normal activity. Her fatigue persisted, though, and she arranged to take time to rest during the day.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Patient wants minimally invasive surgery for her fibroids, and no hysterectomy
A 44-year-old G1P1 woman comes to the office to discuss her uterine fibroids, heavy menstrual bleeding, and urinary frequency. Treatment with oral contraceptives has not been effective in reducing the bleeding. She now wants surgical treatment without a hysterectomy (the hysterectomy was recommended by her previous gynecologist). On examination, a 14-week-size irregular uterus is felt. Myomectomy is discussed, and the patient asks if minimally invasive surgery (MIS) is possible. Complete blood cell count testing shows a hemoglobin level of 9.4 g/dL. Pelvic magnetic resonance imaging (MRI) shows a 6-cm type 2 posterior fundal fibroid and a 6-cm type 5 posterior lower-uterine-segment fibroid (FIGURE 1). These 2 fibroids have regular contours, and enhancement is not increased with contrast, consistent with benign fibroids.

Determining that laparoscopic myomectomy is a good option
Fibroids may affect quality of life—they may cause heavy menstrual bleeding, pelvic pain or pressure, or urinary frequency or incontinence. For many women who want large or numerous fibroids removed but the uterus preserved, abdominal myomectomy is required. Smaller and less numerous fibroids usually can be managed laparoscopically or with robotic assistance.
A systematic review of 6 randomized, controlled trials comparing laparoscopic and open myomectomy in 576 patients found that, although laparoscopic myomectomy was associated with longer operative time (approximately 13 minutes), it was also linked to less operative blood loss, fewer overall complications, reduced postoperative pain, and faster recovery.1 However, wide application of the laparoscopic approach may be limited by the size and number of fibroids that can be reasonably removed and by the surgical skill needed for fibroid excision and laparoscopic suturing.
Four imaging modalities can be used for fibroids: transvaginal sonography (TVS), saline-infusion sonography (SIS), hysteroscopy, and MRI. TVS is the most readily available and least costly modality used to differentiate fibroids from other pelvic pathology; SIS provides contrast for the endometrial cavity and better defines submucous fibroids; and hysteroscopy detects visually apparent distortion of the cavity. MRI, however, provides the most complete evaluation of size, position, and number of fibroids.
A study comparing TVS, SIS, hysteroscopy, and MRI found that number and position of fibroids were best identified with MRI.2 In addition, with MRI, the proximity of the fibroids and uterus to the bladder, rectum, and iliac bones can be evaluated. As tactility in laparoscopic and robot-assisted surgery is very limited, surgeons who use MRI to accurately assess fibroids preoperatively may be able to avoid missing them during the procedure.3 MRI also can be used reliably to diagnose adenomyosis and may be able to help identify uterine sarcoma.
Tip. For all women considering laparoscopic or robot-assisted myomectomy, I order pelvic MRI with and without contrast. Having the radiologist limit the number of MRI sequences may reduce the cost and make it comparable to that of other imaging modalities. I request T2-weighted MRI scans in the coronal, sagittal, and axial planes; in addition, to determine distortion of the uterine cavity by submucous fibroids, I request scans in the planes parallel with and perpendicular to the uterine axis. One gadolinium-enhanced T1-weighted MRI scan is needed to evaluate perfusion.
Although radiologists are experts in image interpretation, they are unfamiliar with the treatments and surgical issues that gynecologists must consider. Reading MRI scans for fibroids is straightforward, and gynecologists who regularly treat women with fibroids should consider viewing images with a radiologist until they become proficient.
Related article:
Surgical management of broad ligament fibroids
Surgeons who have the experience and skill and know the size, number, and position of fibroids are able to select the appropriate candidates for laparoscopic myomectomy. Authors of a study of 2,050 laparoscopic myomectomies found that fibroids larger than 5 cm, removal of more than 3 fibroids, and broad ligament fibroids were more likely to be associated with major complications, including visceral injury, conversion to laparotomy, and bleeding requiring blood transfusion.4
In laparoscopic myomectomy, uterus reconstruction requires laparoscopic suturing. Although robot-assisted myomectomy may make laparoscopic suturing easier, the added cost, longer operative time, and unimproved outcomes must be considered too.

Read about trocar placement and managing blood loss
Trocar placement
Place the patient in the dorsal lithotomy position.
Tip. For most women, I do not use a uterine manipulator, as my assistant can manipulate the uterus with laparoscopic graspers.
Port placement should be based on the position and size of the fibroids to be removed. Laparoscopic suturing is more ergonomic with 2 ports placed on one side of the patient (FIGURE 2). For suture access, a 12-mm port is placed about 2 cm medial to the iliac crest and a 5-mm port is placed medial to the 12-mm port, near the level of the umbilicus. Lateral trocars should be placed high, above the superior aspect of the uterus, to make it easier to access the fibroids, and lateral to the inferior epigastric vessels, to avoid injuring those vessels. If the uterus is near or above the umbilicus, a left upper quadrant approach may be used, with the access ports placed above the umbilicus.

Related article:
How to avoid major vessel injury during gynecologic laparoscopy
Managing intraoperative blood loss
I use a combination of 3 agents to reduce intraoperative blood loss during laparoscopic myomectomy: preoperative misoprostol and tranexamic acid and intraoperative vasopressin. Although there are no data showing an advantage in using these drugs together, the agents have different mechanisms of action and no negative interactions.
Injected below the vascular pseudocapsule, 20 units of vasopressin in 100 mL of normal saline causes vasoconstriction of capillaries, small arterioles, and venules. Avoid intravascular injection given that bradycardia and cardiovascular collapse have been reported (rare cases). Loss of peripheral pulses, bradycardia, unmeasurable blood pressure, and cardiac complications have been reported after myometrial injection of ≥5 units of vasopressin.5
Although vasopressin is a powerful vasoconstrictor, these clinical findings are often interpreted as severe hypotension. However, evaluation of peripheral arterial blood flow by Doppler ultrasonography has revealed severe vasospasm and increased proximal blood pressure.5 Keep this potential reaction in mind to avoid misinterpreting findings and treating a patient with vasopressors. Presence of palpable carotid pulses and maintenance of normal partial pressure of end-tidal carbon dioxide can help differentiate peripheral vasospasm from global hypotension.
Use of vasopressin to reduce blood loss during myomectomy is off-label. On occasion, I apply a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. I use a red Robinson catheter, throw 1 tie in front of the uterus, pull with graspers on both ends until it is tight, and then clamp the half-knot with a locking grasper.
Tip. Although a salvage-type autologous blood transfusion device may be used during laparoscopic or robot-assisted myomectomy, cases in which this device is considered for very large or multiple fibroids might be better managed with abdominal myomectomy.
Surgical technique
After injecting vasopressin, I use a high-frequency mechanical vibration scalpel to incise the myometrium directly over a prominent fibroid and carry the incision deeply until fibroid tissue is definite. Alternatively, a monopolar laparoscopic needle can be used in cut mode—which also limits damage to the myometrium.
Tip. The course of vessels over a fibroid is unpredictable, and we cannot be certain that any uterine incision will avoid bleeding. Therefore, I make transverse incisions, which allow more ergonomic laparoscopic suturing.
It is important to incise completely through the myometrium and through the pink-red pseudocapsule containing the vascular network surrounding the fibroid. This plane is often deeper than usually recognized and can be identified just over the white fibroid.
The fibroid is grasped with a tenaculum for traction, and countertraction is applied with a grasper on the myometrial edges. Once the fibroid is reached, graspers and the mechanical vibration scalpel are used to tease the pseudocapsule away from the fibroid (VIDEO).
Tip. Staying under the pseudocapsule reduces bleeding and may preserve the tissue’s growth factors and neurotransmitters, which are thought to promote wound healing.6
Dissection with the mechanical vibration scalpel (or monopolar needle) should be performed under visual control to identify the tissue adhering to the fibroid, which is desiccated and then divided. The fibroid is dissected until free of the myometrium and is placed in the right lower abdomen. Small fibroids can be strung together on a long suture so none will be lost. Using bipolar paddles, desiccate large bleeding vessels in the myometrial defect sparingly, with care taken to avoid devascularizing the myometrium, which might compromise wound healing. Myometrial repair should be performed in accordance with the accepted surgical technique used in laparotomy.
Place delayed absorbable sutures in 2 or 3 layers, as needed, to reapproximate the myometrium and secure hemostasis.
Tip: I use 0 polydioxanone interrupted figure-of-8 sutures, but continuous running sutures with or without barbs also can be used. For the serosa, I use a continuous barbed suture in a baseball stitch, which buries both the raw edges of the serosa and the barbs for smooth closure (FIGURE 3). These closure methods have not been compared to see which provides superior wound healing or subsequent wound strength.

The fibroid can be morcellated with an electromechanical morcellator or a scalpel (hand morcellation). Either instrument can be used in contained or uncontained fashion. I insert an electromechanical morcellator through the right lower quadrant incision and morcellate tissue in the anterior midpelvis. Safety requires careful control of the rotating blade and scrutiny of the bowel, bladder, and major vessels. Our operating room has 4 rules for morcellator use:
- The blade is activated only under direct visualization.
- Both the surgeon and the assistant must say “ready” before the blade is activated.
- The hand holding the morcellator must remain still while tissue is being drawn into the device.
- Any undue resistance from the tissue is cause to stop the blade. This precaution is taken because there is a tendency to drop the blade in an attempt to overcome the resistance.
Tip: I limit rotational forces and scattering of tissue by “pulsing” the blade on and off when morcellating softer tissue.
Various methods of contained morcellation (morcellation in a containment bag) have been described.7 In one method, tissue is placed in a bag, the neck of the bag is brought through an enlarged umbilical incision, and the tissue is cut into small pieces until it is entirely removed. Another method is to use an electromechanical morcellator with a specially designed containment bag inside the abdomen. The bag is introduced through a 12-mm port and unfurled inside the abdomen; the specimen is placed in the bag; the neck of the bag is brought out through the port; the bag is insufflated with carbon dioxide; the laparoscope, a 5-mm grasper, and the morcellator tip are passed into the bag; and morcellation is performed. Early studies of contained morcellation reported longer operating times, leaking bags, and visceral injuries. In 2016, the US Food and Drug Administration (FDA) cleared the PneumoLiner containment system but required that its manufacturer (Advanced Surgical Concepts) warn patients and health care providers that its bag has not been proved to reduce the risk of spreading cancer during morcellation procedures.8
During laparoscopic myomectomy, fibroid removal by myometrial dissection disperses tissue fragments, and the unprotected fibroid is usually stored in the abdomen until hemostasis is secured and suturing completed. Limiting the rotational forces that lead to further dispersement and irrigating copiously to remove tissue fragments help eliminate residual tissue.
The pelvis and the abdomen are irrigated with normal saline (approximately 3 L) and suctioned multiple times.
Tip. Alternating between the Trendelenburg and reverse Trendelenburg positions allows fluid to wash tissue down to the pelvis, where it is more easily seen and removed.
Careful inspection for tissue fragments and copious irrigation and suctioning are important in reducing the risk that tissue fragments will remain in the peritoneal cavity and parasitic fibroids will develop. In cases of occult leiomyosarcoma (LMS), this step may be particularly important.
I place a knitted fabric of modified cellulose over the hysterotomy suture lines to reduce the incidence of adhesion formation. Once the procedure is complete, the local anesthetic bupivicaine is injected deep into the incision sites. Injecting anesthetic before making the incisions does not provide better pain relief; injecting after the procedure provides pain relief for 6 hours.9
Related article:
Robot-assisted laparoscopic myomectomy
Morcellation and risk of leiomyosarcoma
Given the need to prevent laparoscopic morcellators from inadvertently spreading tissue within the peritoneal cavity of women with occult LMS, the FDA issued a safety communication in 2014 warning against their use in the majority of women who undergo myomectomy or hysterectomy for fibroids.10 However, Pritts and colleagues estimated the prevalence of LMS in women who had surgery for presumed uterine fibroids at about 1 in 2,000 (0.05%), significantly lower than the FDA’s estimate of 1 in 350.10,11 In 2015, a large population-based prospective registry study found 2 cases of occult LMS in 8,720 fibroid surgery patients (0.02%).12
Related article:
The FDA’s review of the data on open power morcellation was “inadequate, irresponsible” and a “disservice to women”
Since LMS metastasizes through the bloodstream, there is no reliable evidence that morcellation influences survival or that electromechanical morcellation is inferior to vaginal or mini-laparotomy morcellation with a scalpel. According to recent publications, compared with MIS, open abdominal surgery is associated with more morbidity and mortality in women.13 Since the FDA advisory was issued, the number of abdominal surgeries has increased, as has the number of related complications.13
I use electromechanical morcellation techniques for women who want MIS. All surgical procedures have potential risks, and patients’ and physicians’ understanding of risks forms the foundation of medical decision making. The possibility of occult LMS should be considered by women and their gynecologists, and proper informed consent, noting both the LMS risk and the increased risks of abdominal surgery, should be obtained.
Related article:
Tissue extraction: Can the pendulum change direction?
Risk of uterine rupture after laparoscopic myomectomy
After abdominal myomectomy, uterine rupture during pregnancy or delivery is rare, according to reviews of delivery records of many thousands of women.14 Operative techniques, instruments, and energy sources used during laparoscopic or robot-assisted myomectomy may differ from those used during laparotomy, and anecdotal communications suggest that uterine rupture may be more common after laparoscopic or robot-assisted myomectomy. A meta-analysis of 56 articles (3,685 pregnancies) published between 1970 and 2013 found 29 cases of uterine rupture after myomectomy, with no statistical difference in rupture risk between laparoscopic and abdominal myomectomy.15 As most reports are case studies or small case series, the incidence of rupture cannot be reliably calculated.
There is no consensus regarding the factors that may increase the risk of uterine rupture after laparoscopic myomectomy. Three factors are postulated to interfere with myometrial wound healing and increase uterine rupture risk: failure to adequately suture myometrial defects, excessive use of monopolar or bipolar electrosurgery with devascularization of the myometrium, and lack of hemostasis with subsequent hematoma formation.16 It seems prudent that surgeons should adhere to time-tested techniques for abdominal myomectomy. Even with use of ideal surgical techniques, however, individual wound-healing characteristics may predispose to uterine rupture.
CASE Resolved
After giving proper informed consent, the patient underwent laparoscopic myomectomy and electromechanical morcellation. Her 2 fibroids were removed, with a blood loss of 200 mL, and that afternoon she was discharged from the surgery center with written postoperative instructions and oral pain medication. A telephone call the next day found her comfortable, with no nausea or vomiting, and happy to be fibroid free. Pathologic inspection of the morcellated tissue confirmed that the fibroids were benign. At 2-week follow-up, the patient was no longer taking pain medication and was ready to return to work and normal activity. Her fatigue persisted, though, and she arranged to take time to rest during the day.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):14–21.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Parker WH. The utility of MRI for the surgical treatment of women with uterine fibroid tumors. Am J Obstet Gynecol. 2012;206(1):31–36.
- Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453–462.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci. 2016;18(2):129–139.
- Taylan E, Sahin C, Zeybek B, Akdemir A. Contained morcellation: review of current methods and future directions. Front Surg. 2017;4:15.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Published April 7, 2016. Accessed June 9, 2017.
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;(3):CD007049.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed June 9, 2017.
- Pritts EA, Vanness DJ, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bojahr B, De Wilde RL, Tchartchian G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet. 2015;292(3):665–672.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1–e13.
- Palerme GR, Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol. 1966;94(4):571–576.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
- Jin C, Hu Y, Chen XC, et al. Laparoscopic versus open myomectomy—a meta-analysis of randomized controlled trials. Eur J Obstet Gynecol Reprod Biol. 2009;145(1):14–21.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Parker WH. The utility of MRI for the surgical treatment of women with uterine fibroid tumors. Am J Obstet Gynecol. 2012;206(1):31–36.
- Sizzi O, Rossetti A, Malzoni M, et al. Italian multicenter study on complications of laparoscopic myomectomy. J Minim Invasive Gynecol. 2007;14(4):453–462.
- Riess ML, Ulrichs JG, Pagel PS, Woehlck HJ. Case report: severe vasospasm mimics hypotension after high-dose intrauterine vasopressin. Anesth Analg. 2011;113(5):1103–1105.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters. Curr Protein Pept Sci. 2016;18(2):129–139.
- Taylan E, Sahin C, Zeybek B, Akdemir A. Contained morcellation: review of current methods and future directions. Front Surg. 2017;4:15.
- US Food and Drug Administration. FDA allows marketing of first-of-kind tissue containment system for use with certain laparoscopic power morcellators in select patients. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm494650.htm. Published April 7, 2016. Accessed June 9, 2017.
- Loizides S, Gurusamy KS, Nagendran M, Rossi M, Guerrini GP, Davidson BR. Wound infiltration with local anesthetic agents for laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;(3):CD007049.
- US Food and Drug Administration. Laparoscopic uterine power morcellation in hysterectomy and myomectomy: FDA safety communication. http://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm393576.htm. Published April 17, 2014. Accessed June 9, 2017.
- Pritts EA, Vanness DJ, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bojahr B, De Wilde RL, Tchartchian G. Malignancy rate of 10,731 uteri morcellated during laparoscopic supracervical hysterectomy (LASH). Arch Gynecol Obstet. 2015;292(3):665–672.
- Harris JA, Swenson CW, Uppal S, et al. Practice patterns and postoperative complications before and after US Food and Drug Administration safety communication on power morcellation. Am J Obstet Gynecol. 2016;214(1):98.e1–e13.
- Palerme GR, Friedman EA. Rupture of the gravid uterus in the third trimester. Am J Obstet Gynecol. 1966;94(4):571–576.
- Claeys J, Hellendoorn I, Hamerlynck T, Bosteels J, Weyers S. The risk of uterine rupture after myomectomy: a systematic review of the literature and meta-analysis. Gynecol Surg. 2014;11(3):197–206.
- Parker WH, Einarsson J, Istre O, Dubuisson JB. Risk factors for uterine rupture after laparoscopic myomectomy. J Minim Invasive Gynecol. 2010;17(5):551–554.
Laparoscopic excision of type I and type II endometriomas

Read the accompanying article: “Endometriomas: Classification and surgical management”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Read the accompanying article: “Endometriomas: Classification and surgical management”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.

Read the accompanying article: “Endometriomas: Classification and surgical management”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Laparoscopic myomectomy technique
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Read the accompanying article by Dr. Parker: “Laparoscopic myomectomy: Tips for patient selection and technique”
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.