User login
Abdominal myomectomy: Patient and surgical technique considerations
CASE Woman with fibroids seeks alternative to hysterectomy
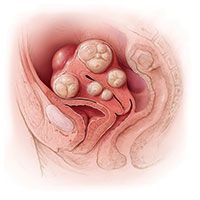
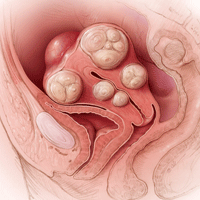
A 42-year-old woman (G2P2) presents to the office for evaluation of heavy menstrual bleeding and known uterine fibroids. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. She does not want to have any more children, but she wishes to avoid a hysterectomy.
Abdominal myomectomy: A good option for many women
Abdominal myomectomy is an underutilized procedure. With fibroids as the indication for surgery, 197,000 hysterectomies were performed in the United States in 2010, compared with approximately 40,000 myomectomies.1,2 Moreover, the rates of both laparoscopic and abdominal myomectomy have decreased following the controversial morcellation advisory issued by the US Food and Drug Administration.3
The differences in the hysterectomy and myomectomy rates might be explained by the many myths ascribed to myomectomy. Such myths include the beliefs that myomectomy, when compared with hysterectomy, is associated with greater risk of visceral injury, more blood loss, poor uterine healing, and high risk of fibroid recurrence, and that myomectomy is unlikely to improve patient symptoms.
Studies show, however, that these beliefs are wrong. The risk of needing treatment for new fibroid growth following myomectomy is low.4 Hysterectomy, compared with myomectomy for similar size uteri, is actually associated with a greater risk of injury to the bowel, bladder, and ureters and with a greater risk of operative hemorrhage. Furthermore, hysterectomy (without oophorectomy) can be associated with early menopause in approximately 10% of women, while myomectomy does not alter ovarian hormones. (See “7 Myomectomy myths debunked,” which appeared in the February 2017 issue of OBG
For women who have serious medical problems (severe anemia, ureteral obstruction) due to uterine fibroids, surgery usually is necessary. In addition, women may request surgery for fibroid-associated quality-of-life concerns, such as heavy menstrual bleeding, infertility, pelvic pressure, urinary frequency, or incontinence. In one prospective study, the authors found that when women were assessed 6 months after undergoing myomectomy, 75% reported experiencing a significant decrease in bothersome symptoms.7
Myomectomy may be considered even for women with large uterine fibroids who desire uterine conservation. In a systematic review of the perioperative morbidity associated with abdominal myomectomy compared with abdominal hysterectomy for fibroids, which included 1,520 women with uterine size up to 16 to 18 weeks, no difference was found in major morbidity rates.8 Investigators who studied 91 women with uterine size ranging from 16 to 36 weeks who underwent abdominal myomectomy reported 1 bowel injury, 1 bladder injury, and 1 reoperation for bowel obstruction; no women had conversion to hysterectomy.9
Since ObGyn residency training emphasizes hysterectomy techniques, many residents receive only limited exposure to myomectomy procedures. Increased exposure to and comfort with myomectomy surgical technique would encourage more gynecologists to offer this option to their patients who desire uterine conservation, including those who do not desire future childbearing.
Imaging techniques are essential in the preoperative evaluation
For women with fibroid-related symptoms who desire surgery with uterine preservation, determining the myomectomy approach (abdominal, laparoscopic/robotic, hysteroscopic) depends on accurate assessment of the size, number, and position of the fibroids. If abdominal myomectomy is planned because of uterine size, the presence of numerous fibroids, or patient choice, transvaginal/transabdominal ultrasonography usually is adequate for anticipating what will be found during surgery. Sonography is readily available and is the least costly imaging technique that can help differentiate fibroids from other pelvic pathology. Although small fibroids may not be seen on sonography, they can be palpated and removed at the time of open surgery.
If submucous fibroids need to be better defined, saline-infusion sonography can be performed. However, if laparoscopic/robotic myomectomy (which precludes accurate palpation during surgery) is being considered, magnetic resonance imaging (MRI) allows the best assessment of the size, number, and position of the fibroids.10 When adenomyosis is considered in the differential diagnosis, MRI is an accurate way to determine its presence and helps in planning the best surgical procedure and approach.
Correct anemia before surgery
Women with fibroids may have anemia requiring correction before surgery to reduce the need for intraoperative or postoperative blood transfusion. Mild iron deficiency anemia can be treated prior to surgery with oral elemental iron 150 to 200 mg per day. Vitamin C 1,000 mg per day helps to increase intestinal iron absorption. Three weeks of treatment with oral iron can increase hemoglobin concentration by 2 g/dL.
For more severe anemia or rapid correction of anemia, intravenous (IV) iron sucrose infusions, 200 mg infused over 2 hours and given 3 times per week for 3 weeks, can increase hemoglobin by 3 g/dL.11 In our ObGyn practice, hematologists manage iron infusions.
Read about abdominal incision technique
Abdominal incision technique
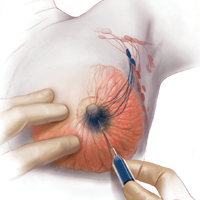
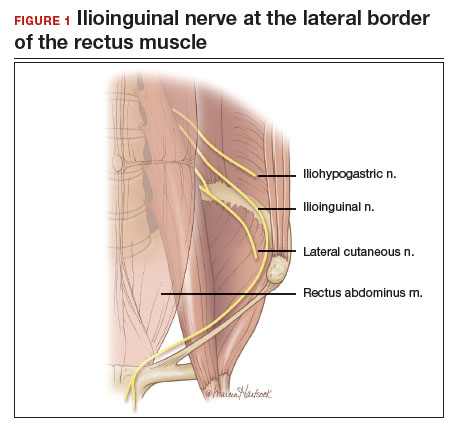
Even a large uterus with multiple fibroids usually can be managed through use of a transverse lower abdominal incision. Prior to reaching the lateral borders of the rectus abdominis, curve the fascial incision cephalad to avoid injury to the ileoinguinal nerves (FIGURE 1). Detaching the midline rectus fascia (linea alba) from the anterior abdominal wall, starting at the pubic symphysis and continuing up to the umbilicus, frees the rectus muscles and allows them to be easily separated (see VIDEO 1). Since fascia is not elastic, these 2 steps are important to allow more room to deliver the uterus through the incision.
Delivery of the uterus through the incision isolates the surgical field from the bowel, bladder, ureters, and pelvic nerves. Once the uterus is delivered, inspect and palpate it for fibroids. Identify the fundus and the position of the uterine cavity by locating both uterine cornua and imagining a straight line between them. It may be necessary to explore the endometrial cavity to look for and remove submucous fibroids. Then plan the necessary uterine incisions for removing all fibroids (see VIDEO 2).
Read about managing blood loss
4 approaches to managing intraoperative blood loss
In my practice, we employ misoprostol, tranexamic acid, vasopressin, and a uterine and ovarian vessel tourniquet to manage intraoperative blood loss.12 Although no data exist to show that using these methods together is advantageous, they have different mechanisms of action and no negative interactions.
Misoprostol 400 μg inserted vaginally 2 hours before surgery induces myometrial contraction and compression of the uterine vessels. This agent can reduce blood loss by 98 mL per case.12
Tranexamic acid, an antifibrinolytic, is given IV piggyback at the start of surgery at a dose of 10 mg/kg; it can reduce blood loss by 243 mL per case.12
Vasopressin 20 U in 100 mL normal saline, injected below the vascular pseudocapsule, causes vasoconstriction of capillaries and small arterioles and venules and can reduce blood loss by 246 mL per case.12 Intravascular injection should be avoided because rare cases of bradycardia and cardiovascular collapse have been reported.13 Using vasopressin to decrease blood loss during myomectomy is an off-label use of this drug.
Place a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. Tourniquet use is the most effective way to decrease blood loss during myomectomy, since it can reduce blood loss by 1,870 mL.12 For women who wish to preserve fertility, take care to ensure that the tourniquet does not compromise the tubes. For women who are certain they do not want to preserve fertility, discuss the possibility of performing bilateral salpingectomy to decrease the risk of subsequent tubal (“ovarian”) cancer.
Some surgeons incise the broad ligaments bilaterally and pass the tourniquet through the broad ligaments to avoid compromising blood flow to the ovaries. Occluding the utero- ovarian ligaments with bulldog clamps to control collateral blood flow from the ovarian artery has been described, but the clamps can tear these often enlarged and fragile uterine veins during manipulation of the uterus. Release the tourniquet every 15 to 30 minutes to allow reperfusion of the ovaries. In women with ovarian torsion lasting hours to days, the ovary has been found to resist hypoxia and recover function.14 Antral follicle counts of detorsed and contralateral normal ovaries following a mean of 13 hours of hypoxia are similar 3 months following detorsion.15
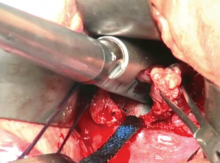
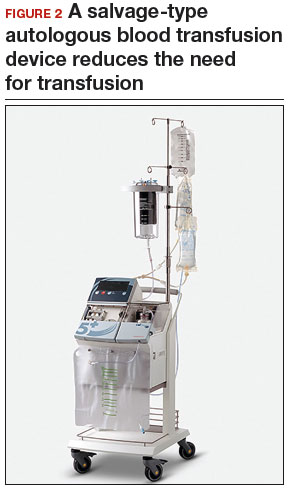
Consider blood salvage. For women with multiple or very large fibroids, consider using a salvage-type autologous blood transfusion device, which has been shown to reduce the need for heterologous blood transfusion.16 This device suctions blood from the operative field, mixes it with heparinized saline, and stores the blood in a canister (FIGURE 2). If the patient requires blood reinfusion, the stored blood is washed with saline, filtered, centrifuged, and given back to the patient intravenously. Blood salvage, or cell salvage, avoids the risks of infection and transfusion reaction, and the oxygen transport capacity of salvaged red blood cells is equal to or better than that of stored allogeneic red cells.
Additional surgical considerations
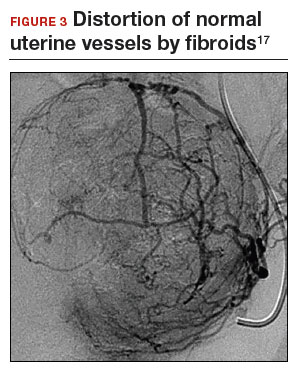
Previous teaching suggested that proper placement of the uterine incisions was an important factor in limiting blood loss. Some authors suggested that vertical uterine incisions would avoid injury to the ascending uterine vessels should inadvertent extension of the incision occur. Other authors proposed horizontal uterine incisions to avoid severing the arcuate vessels that branch off from the ascending uterine arteries and run transversely across the uterus. However, since fibroids distort the normal vascular architecture, it is not possible to entirely avoid severing vessels in the myometrium (FIGURE 3).17 Uterine incisions can therefore be made as needed based on the position of the fibroids and the need to avoid inadvertent extension to the ascending uterine vessels or cornua.17
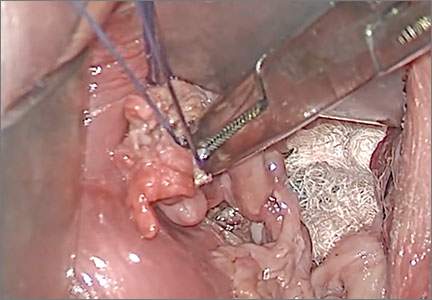
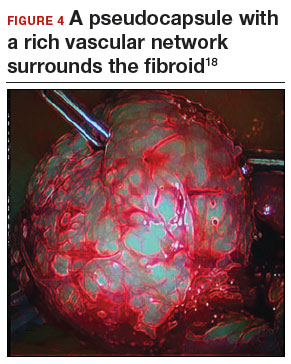
Fibroid anatomy and vascularity. Fibroids are entirely encased within the dense blood supply of a pseudocapsule (FIGURE 4),18 and no distinct “vascular pedicle” exists at the base of the fibroid.19 It is therefore important to extend the uterine incisions down through the entire pseudocapsule until the fibroid is clearly visible. This will identify a less vascular surgical plane, which is deeper than commonly recognized. Once the fibroid is reached, the pseudocapsule can be “wiped away” using a dry laparotomy sponge (see VIDEO 3). Staying under the pseudocapsule reduces bleeding and may preserve the tissue growth factors and neurotransmitters that are thought to promote wound healing.20
Adhesion prevention. Limiting the number of uterine incisions has been suggested as a way to reduce the risk of postoperative pelvic adhesions. To extract fibroids that are distant from an incision, however, tunnels must be created within the myometrium, and this makes hemostasis within these defects difficult. In that blood increases the risk of adhesion formation, tunneling may be counterproductive. If tunneling incisions are avoided and hemostasis is secured immediately, the risk of adhesion formation should be lessened.
Therefore, make incisions directly over the fibroids. Remove only easily accessed fibroids and promptly close the defects to secure hemostasis. Multiple uterine incisions may be needed; adhesion barriers may help limit adhesion formation.21
On final removal of the tourniquet, carefully inspect for bleeding and perform any necessary re-suturing. We place a pain pump (ON-Q* Pain Relief System, Halyard Health, Inc) for pain management and close the abdominal incision in the standard manner.
Postoperative care: Manage pain, restore function
The pain pump infuser, attached to one soaker catheter above and one below the fascia, provides continuous infusion of bupivacaine to the incision at 4 mL per hour for 4 days. The pain pump greatly reduces the need for postoperative opioids.22 Use of a patient-controlled analgesia pump, with its associated adverse effects (sedation, need for oxygen saturation monitoring, slowing of bowel function) can thus be avoided. The patient’s residual pain is controlled with oral oxycodone or hydrocodone and scheduled nonsteroidal anti-inflammatory drugs.
In my practice, we use an enhanced recovery after surgery (ERAS) protocol designed to reduce postoperative surgical stress and expedite a return to baseline physiologic body functions.23 Excellent well-researched, evidence-based studies support the effectiveness of ERAS in gynecologic and general surgery procedures.24
Pre-emptive, preoperative analgesia (gabapentin and celecoxib) and end-of-case IV acetaminophen are given to reduce the inflammatory response and the need for postoperative opioids. Once it is confirmed that the patient is hemodynamically stable, add ketorolac 30 mg IV every 6 hours on postoperative day 1. Nausea and vomiting prophylaxis includes ondansetron and dexamethasone at the end of surgery, avoidance of bowel edema with restriction of intraoperative and postoperative fluids (euvolemia), early oral feeding, and gum chewing. On the evening of surgery, the urinary catheter is removed to reduce the risk of bladder infection and facilitate ambulation. Encourage sitting at the bedside and early ambulation starting the evening of surgery to reduce risk of thromboembolism and to avoid skeletal muscle weakness and postoperative fatigue.
Most women are able to be discharged on postoperative day 2. They return to the office on postoperative day 5 for removal of the pain pump.
CASE Continued: Fibroids removed via abdominal myomectomy
We performed an abdominal myomectomy through a Pfannenstiel incision. Nine fibroids—3 of which were not seen on MRI—ranging in size from 1 to 7 cm were removed. Intravaginal misoprostol, IV tranexamic acid, subserosal vasopressin, and a uterine vessel tourniquet limited the intraoperative blood loss to 225 mL. After surgery, a pain pump and ERAS protocol allowed the patient to be discharged on postoperative day 2, and she returned to the office on day 5 for removal of the pain pump. Oral pain medication was continued on an as-needed basis.
Acknowledgement
The author would like to thank Stanley West, MD, for generously teaching him the surgical techniques for performing abdominal myomectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- Barrett ML, Weiss AJ, Stocks C, Steiner CA, Myers ER. Statistical brief 200. Procedures to treat benign uterine fibroids in hospital inpatient and hospital-based ambulatory surgery settings, 2013. Healthcare Cost and Utilization Project website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb200-Procedures-Treat-Uterine-Fibroids.jsp. Published January 2016. Accessed February 9, 2017.
- Stentz NC, Cooney L, Sammel MD, Shah DK. Impact of the Food and Drug Administration (FDA) safety communication on morcellation on surgical practice and perioperative morbidity following myomectomy [abstract p300]. Fertil Steril. 2016;106(3 suppl):e219.
- Hillis SD, Marchbanks PA, Peterson HB. Obstet Gynecol. 1996;87(4):539–543.
- Pritts E, Vanness D, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bogani G, Cliby WA, Aletti GD. Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol. 2015;137(1):167–172.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynaecol. 2013;33(7):655–662.
- West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85(1):36–39.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Kim YH, Chung HH, Kang SB, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014 Aug 15;(8):CD005355.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod. 2003;18(12):2599–2602.
- Yasa C, Dural O, Bastu E, Zorlu M, Demir O, Ugurlucan FG. Impact of laparoscopic ovarian detorsion on ovarian reserve. J Obstet Gynaecol Res. 2017;43(2):298–302.
- Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59(3):233–236.
- Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301–1303.
- Malavasi A, Cavalotti C, Nicolardi G, et al. The opioid neuropeptides in uterine fibroid pseudocapsules: a putative association with cervical integrity in human reproduction. Gynecol Endocrinol. 2013;29(11):982–988.
- Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18(5):1088–1093.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters [published online ahead of print March 22, 2016]. Curr Protein Pept Sci. doi:10.2174/1389203717666160322150338.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66(6):904–910.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203(6):914–932.
- Lassen K, Soop M, Nygren J, et al; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319–328.
CASE Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents to the office for evaluation of heavy menstrual bleeding and known uterine fibroids. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. She does not want to have any more children, but she wishes to avoid a hysterectomy.
Abdominal myomectomy: A good option for many women
Abdominal myomectomy is an underutilized procedure. With fibroids as the indication for surgery, 197,000 hysterectomies were performed in the United States in 2010, compared with approximately 40,000 myomectomies.1,2 Moreover, the rates of both laparoscopic and abdominal myomectomy have decreased following the controversial morcellation advisory issued by the US Food and Drug Administration.3
The differences in the hysterectomy and myomectomy rates might be explained by the many myths ascribed to myomectomy. Such myths include the beliefs that myomectomy, when compared with hysterectomy, is associated with greater risk of visceral injury, more blood loss, poor uterine healing, and high risk of fibroid recurrence, and that myomectomy is unlikely to improve patient symptoms.
Studies show, however, that these beliefs are wrong. The risk of needing treatment for new fibroid growth following myomectomy is low.4 Hysterectomy, compared with myomectomy for similar size uteri, is actually associated with a greater risk of injury to the bowel, bladder, and ureters and with a greater risk of operative hemorrhage. Furthermore, hysterectomy (without oophorectomy) can be associated with early menopause in approximately 10% of women, while myomectomy does not alter ovarian hormones. (See “7 Myomectomy myths debunked,” which appeared in the February 2017 issue of OBG
For women who have serious medical problems (severe anemia, ureteral obstruction) due to uterine fibroids, surgery usually is necessary. In addition, women may request surgery for fibroid-associated quality-of-life concerns, such as heavy menstrual bleeding, infertility, pelvic pressure, urinary frequency, or incontinence. In one prospective study, the authors found that when women were assessed 6 months after undergoing myomectomy, 75% reported experiencing a significant decrease in bothersome symptoms.7
Myomectomy may be considered even for women with large uterine fibroids who desire uterine conservation. In a systematic review of the perioperative morbidity associated with abdominal myomectomy compared with abdominal hysterectomy for fibroids, which included 1,520 women with uterine size up to 16 to 18 weeks, no difference was found in major morbidity rates.8 Investigators who studied 91 women with uterine size ranging from 16 to 36 weeks who underwent abdominal myomectomy reported 1 bowel injury, 1 bladder injury, and 1 reoperation for bowel obstruction; no women had conversion to hysterectomy.9
Since ObGyn residency training emphasizes hysterectomy techniques, many residents receive only limited exposure to myomectomy procedures. Increased exposure to and comfort with myomectomy surgical technique would encourage more gynecologists to offer this option to their patients who desire uterine conservation, including those who do not desire future childbearing.
Imaging techniques are essential in the preoperative evaluation
For women with fibroid-related symptoms who desire surgery with uterine preservation, determining the myomectomy approach (abdominal, laparoscopic/robotic, hysteroscopic) depends on accurate assessment of the size, number, and position of the fibroids. If abdominal myomectomy is planned because of uterine size, the presence of numerous fibroids, or patient choice, transvaginal/transabdominal ultrasonography usually is adequate for anticipating what will be found during surgery. Sonography is readily available and is the least costly imaging technique that can help differentiate fibroids from other pelvic pathology. Although small fibroids may not be seen on sonography, they can be palpated and removed at the time of open surgery.
If submucous fibroids need to be better defined, saline-infusion sonography can be performed. However, if laparoscopic/robotic myomectomy (which precludes accurate palpation during surgery) is being considered, magnetic resonance imaging (MRI) allows the best assessment of the size, number, and position of the fibroids.10 When adenomyosis is considered in the differential diagnosis, MRI is an accurate way to determine its presence and helps in planning the best surgical procedure and approach.
Correct anemia before surgery
Women with fibroids may have anemia requiring correction before surgery to reduce the need for intraoperative or postoperative blood transfusion. Mild iron deficiency anemia can be treated prior to surgery with oral elemental iron 150 to 200 mg per day. Vitamin C 1,000 mg per day helps to increase intestinal iron absorption. Three weeks of treatment with oral iron can increase hemoglobin concentration by 2 g/dL.
For more severe anemia or rapid correction of anemia, intravenous (IV) iron sucrose infusions, 200 mg infused over 2 hours and given 3 times per week for 3 weeks, can increase hemoglobin by 3 g/dL.11 In our ObGyn practice, hematologists manage iron infusions.
Read about abdominal incision technique
Abdominal incision technique
Even a large uterus with multiple fibroids usually can be managed through use of a transverse lower abdominal incision. Prior to reaching the lateral borders of the rectus abdominis, curve the fascial incision cephalad to avoid injury to the ileoinguinal nerves (FIGURE 1). Detaching the midline rectus fascia (linea alba) from the anterior abdominal wall, starting at the pubic symphysis and continuing up to the umbilicus, frees the rectus muscles and allows them to be easily separated (see VIDEO 1). Since fascia is not elastic, these 2 steps are important to allow more room to deliver the uterus through the incision.

Delivery of the uterus through the incision isolates the surgical field from the bowel, bladder, ureters, and pelvic nerves. Once the uterus is delivered, inspect and palpate it for fibroids. Identify the fundus and the position of the uterine cavity by locating both uterine cornua and imagining a straight line between them. It may be necessary to explore the endometrial cavity to look for and remove submucous fibroids. Then plan the necessary uterine incisions for removing all fibroids (see VIDEO 2).
Read about managing blood loss
4 approaches to managing intraoperative blood loss
In my practice, we employ misoprostol, tranexamic acid, vasopressin, and a uterine and ovarian vessel tourniquet to manage intraoperative blood loss.12 Although no data exist to show that using these methods together is advantageous, they have different mechanisms of action and no negative interactions.
Misoprostol 400 μg inserted vaginally 2 hours before surgery induces myometrial contraction and compression of the uterine vessels. This agent can reduce blood loss by 98 mL per case.12
Tranexamic acid, an antifibrinolytic, is given IV piggyback at the start of surgery at a dose of 10 mg/kg; it can reduce blood loss by 243 mL per case.12
Vasopressin 20 U in 100 mL normal saline, injected below the vascular pseudocapsule, causes vasoconstriction of capillaries and small arterioles and venules and can reduce blood loss by 246 mL per case.12 Intravascular injection should be avoided because rare cases of bradycardia and cardiovascular collapse have been reported.13 Using vasopressin to decrease blood loss during myomectomy is an off-label use of this drug.
Place a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. Tourniquet use is the most effective way to decrease blood loss during myomectomy, since it can reduce blood loss by 1,870 mL.12 For women who wish to preserve fertility, take care to ensure that the tourniquet does not compromise the tubes. For women who are certain they do not want to preserve fertility, discuss the possibility of performing bilateral salpingectomy to decrease the risk of subsequent tubal (“ovarian”) cancer.
Some surgeons incise the broad ligaments bilaterally and pass the tourniquet through the broad ligaments to avoid compromising blood flow to the ovaries. Occluding the utero- ovarian ligaments with bulldog clamps to control collateral blood flow from the ovarian artery has been described, but the clamps can tear these often enlarged and fragile uterine veins during manipulation of the uterus. Release the tourniquet every 15 to 30 minutes to allow reperfusion of the ovaries. In women with ovarian torsion lasting hours to days, the ovary has been found to resist hypoxia and recover function.14 Antral follicle counts of detorsed and contralateral normal ovaries following a mean of 13 hours of hypoxia are similar 3 months following detorsion.15
Consider blood salvage. For women with multiple or very large fibroids, consider using a salvage-type autologous blood transfusion device, which has been shown to reduce the need for heterologous blood transfusion.16 This device suctions blood from the operative field, mixes it with heparinized saline, and stores the blood in a canister (FIGURE 2). If the patient requires blood reinfusion, the stored blood is washed with saline, filtered, centrifuged, and given back to the patient intravenously. Blood salvage, or cell salvage, avoids the risks of infection and transfusion reaction, and the oxygen transport capacity of salvaged red blood cells is equal to or better than that of stored allogeneic red cells.

Additional surgical considerations
Previous teaching suggested that proper placement of the uterine incisions was an important factor in limiting blood loss. Some authors suggested that vertical uterine incisions would avoid injury to the ascending uterine vessels should inadvertent extension of the incision occur. Other authors proposed horizontal uterine incisions to avoid severing the arcuate vessels that branch off from the ascending uterine arteries and run transversely across the uterus. However, since fibroids distort the normal vascular architecture, it is not possible to entirely avoid severing vessels in the myometrium (FIGURE 3).17 Uterine incisions can therefore be made as needed based on the position of the fibroids and the need to avoid inadvertent extension to the ascending uterine vessels or cornua.17

Fibroid anatomy and vascularity. Fibroids are entirely encased within the dense blood supply of a pseudocapsule (FIGURE 4),18 and no distinct “vascular pedicle” exists at the base of the fibroid.19 It is therefore important to extend the uterine incisions down through the entire pseudocapsule until the fibroid is clearly visible. This will identify a less vascular surgical plane, which is deeper than commonly recognized. Once the fibroid is reached, the pseudocapsule can be “wiped away” using a dry laparotomy sponge (see VIDEO 3). Staying under the pseudocapsule reduces bleeding and may preserve the tissue growth factors and neurotransmitters that are thought to promote wound healing.20

Adhesion prevention. Limiting the number of uterine incisions has been suggested as a way to reduce the risk of postoperative pelvic adhesions. To extract fibroids that are distant from an incision, however, tunnels must be created within the myometrium, and this makes hemostasis within these defects difficult. In that blood increases the risk of adhesion formation, tunneling may be counterproductive. If tunneling incisions are avoided and hemostasis is secured immediately, the risk of adhesion formation should be lessened.
Therefore, make incisions directly over the fibroids. Remove only easily accessed fibroids and promptly close the defects to secure hemostasis. Multiple uterine incisions may be needed; adhesion barriers may help limit adhesion formation.21
On final removal of the tourniquet, carefully inspect for bleeding and perform any necessary re-suturing. We place a pain pump (ON-Q* Pain Relief System, Halyard Health, Inc) for pain management and close the abdominal incision in the standard manner.
Postoperative care: Manage pain, restore function
The pain pump infuser, attached to one soaker catheter above and one below the fascia, provides continuous infusion of bupivacaine to the incision at 4 mL per hour for 4 days. The pain pump greatly reduces the need for postoperative opioids.22 Use of a patient-controlled analgesia pump, with its associated adverse effects (sedation, need for oxygen saturation monitoring, slowing of bowel function) can thus be avoided. The patient’s residual pain is controlled with oral oxycodone or hydrocodone and scheduled nonsteroidal anti-inflammatory drugs.
In my practice, we use an enhanced recovery after surgery (ERAS) protocol designed to reduce postoperative surgical stress and expedite a return to baseline physiologic body functions.23 Excellent well-researched, evidence-based studies support the effectiveness of ERAS in gynecologic and general surgery procedures.24
Pre-emptive, preoperative analgesia (gabapentin and celecoxib) and end-of-case IV acetaminophen are given to reduce the inflammatory response and the need for postoperative opioids. Once it is confirmed that the patient is hemodynamically stable, add ketorolac 30 mg IV every 6 hours on postoperative day 1. Nausea and vomiting prophylaxis includes ondansetron and dexamethasone at the end of surgery, avoidance of bowel edema with restriction of intraoperative and postoperative fluids (euvolemia), early oral feeding, and gum chewing. On the evening of surgery, the urinary catheter is removed to reduce the risk of bladder infection and facilitate ambulation. Encourage sitting at the bedside and early ambulation starting the evening of surgery to reduce risk of thromboembolism and to avoid skeletal muscle weakness and postoperative fatigue.
Most women are able to be discharged on postoperative day 2. They return to the office on postoperative day 5 for removal of the pain pump.
CASE Continued: Fibroids removed via abdominal myomectomy
We performed an abdominal myomectomy through a Pfannenstiel incision. Nine fibroids—3 of which were not seen on MRI—ranging in size from 1 to 7 cm were removed. Intravaginal misoprostol, IV tranexamic acid, subserosal vasopressin, and a uterine vessel tourniquet limited the intraoperative blood loss to 225 mL. After surgery, a pain pump and ERAS protocol allowed the patient to be discharged on postoperative day 2, and she returned to the office on day 5 for removal of the pain pump. Oral pain medication was continued on an as-needed basis.
Acknowledgement
The author would like to thank Stanley West, MD, for generously teaching him the surgical techniques for performing abdominal myomectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
CASE Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents to the office for evaluation of heavy menstrual bleeding and known uterine fibroids. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. She does not want to have any more children, but she wishes to avoid a hysterectomy.
Abdominal myomectomy: A good option for many women
Abdominal myomectomy is an underutilized procedure. With fibroids as the indication for surgery, 197,000 hysterectomies were performed in the United States in 2010, compared with approximately 40,000 myomectomies.1,2 Moreover, the rates of both laparoscopic and abdominal myomectomy have decreased following the controversial morcellation advisory issued by the US Food and Drug Administration.3
The differences in the hysterectomy and myomectomy rates might be explained by the many myths ascribed to myomectomy. Such myths include the beliefs that myomectomy, when compared with hysterectomy, is associated with greater risk of visceral injury, more blood loss, poor uterine healing, and high risk of fibroid recurrence, and that myomectomy is unlikely to improve patient symptoms.
Studies show, however, that these beliefs are wrong. The risk of needing treatment for new fibroid growth following myomectomy is low.4 Hysterectomy, compared with myomectomy for similar size uteri, is actually associated with a greater risk of injury to the bowel, bladder, and ureters and with a greater risk of operative hemorrhage. Furthermore, hysterectomy (without oophorectomy) can be associated with early menopause in approximately 10% of women, while myomectomy does not alter ovarian hormones. (See “7 Myomectomy myths debunked,” which appeared in the February 2017 issue of OBG
For women who have serious medical problems (severe anemia, ureteral obstruction) due to uterine fibroids, surgery usually is necessary. In addition, women may request surgery for fibroid-associated quality-of-life concerns, such as heavy menstrual bleeding, infertility, pelvic pressure, urinary frequency, or incontinence. In one prospective study, the authors found that when women were assessed 6 months after undergoing myomectomy, 75% reported experiencing a significant decrease in bothersome symptoms.7
Myomectomy may be considered even for women with large uterine fibroids who desire uterine conservation. In a systematic review of the perioperative morbidity associated with abdominal myomectomy compared with abdominal hysterectomy for fibroids, which included 1,520 women with uterine size up to 16 to 18 weeks, no difference was found in major morbidity rates.8 Investigators who studied 91 women with uterine size ranging from 16 to 36 weeks who underwent abdominal myomectomy reported 1 bowel injury, 1 bladder injury, and 1 reoperation for bowel obstruction; no women had conversion to hysterectomy.9
Since ObGyn residency training emphasizes hysterectomy techniques, many residents receive only limited exposure to myomectomy procedures. Increased exposure to and comfort with myomectomy surgical technique would encourage more gynecologists to offer this option to their patients who desire uterine conservation, including those who do not desire future childbearing.
Imaging techniques are essential in the preoperative evaluation
For women with fibroid-related symptoms who desire surgery with uterine preservation, determining the myomectomy approach (abdominal, laparoscopic/robotic, hysteroscopic) depends on accurate assessment of the size, number, and position of the fibroids. If abdominal myomectomy is planned because of uterine size, the presence of numerous fibroids, or patient choice, transvaginal/transabdominal ultrasonography usually is adequate for anticipating what will be found during surgery. Sonography is readily available and is the least costly imaging technique that can help differentiate fibroids from other pelvic pathology. Although small fibroids may not be seen on sonography, they can be palpated and removed at the time of open surgery.
If submucous fibroids need to be better defined, saline-infusion sonography can be performed. However, if laparoscopic/robotic myomectomy (which precludes accurate palpation during surgery) is being considered, magnetic resonance imaging (MRI) allows the best assessment of the size, number, and position of the fibroids.10 When adenomyosis is considered in the differential diagnosis, MRI is an accurate way to determine its presence and helps in planning the best surgical procedure and approach.
Correct anemia before surgery
Women with fibroids may have anemia requiring correction before surgery to reduce the need for intraoperative or postoperative blood transfusion. Mild iron deficiency anemia can be treated prior to surgery with oral elemental iron 150 to 200 mg per day. Vitamin C 1,000 mg per day helps to increase intestinal iron absorption. Three weeks of treatment with oral iron can increase hemoglobin concentration by 2 g/dL.
For more severe anemia or rapid correction of anemia, intravenous (IV) iron sucrose infusions, 200 mg infused over 2 hours and given 3 times per week for 3 weeks, can increase hemoglobin by 3 g/dL.11 In our ObGyn practice, hematologists manage iron infusions.
Read about abdominal incision technique
Abdominal incision technique
Even a large uterus with multiple fibroids usually can be managed through use of a transverse lower abdominal incision. Prior to reaching the lateral borders of the rectus abdominis, curve the fascial incision cephalad to avoid injury to the ileoinguinal nerves (FIGURE 1). Detaching the midline rectus fascia (linea alba) from the anterior abdominal wall, starting at the pubic symphysis and continuing up to the umbilicus, frees the rectus muscles and allows them to be easily separated (see VIDEO 1). Since fascia is not elastic, these 2 steps are important to allow more room to deliver the uterus through the incision.

Delivery of the uterus through the incision isolates the surgical field from the bowel, bladder, ureters, and pelvic nerves. Once the uterus is delivered, inspect and palpate it for fibroids. Identify the fundus and the position of the uterine cavity by locating both uterine cornua and imagining a straight line between them. It may be necessary to explore the endometrial cavity to look for and remove submucous fibroids. Then plan the necessary uterine incisions for removing all fibroids (see VIDEO 2).
Read about managing blood loss
4 approaches to managing intraoperative blood loss
In my practice, we employ misoprostol, tranexamic acid, vasopressin, and a uterine and ovarian vessel tourniquet to manage intraoperative blood loss.12 Although no data exist to show that using these methods together is advantageous, they have different mechanisms of action and no negative interactions.
Misoprostol 400 μg inserted vaginally 2 hours before surgery induces myometrial contraction and compression of the uterine vessels. This agent can reduce blood loss by 98 mL per case.12
Tranexamic acid, an antifibrinolytic, is given IV piggyback at the start of surgery at a dose of 10 mg/kg; it can reduce blood loss by 243 mL per case.12
Vasopressin 20 U in 100 mL normal saline, injected below the vascular pseudocapsule, causes vasoconstriction of capillaries and small arterioles and venules and can reduce blood loss by 246 mL per case.12 Intravascular injection should be avoided because rare cases of bradycardia and cardiovascular collapse have been reported.13 Using vasopressin to decrease blood loss during myomectomy is an off-label use of this drug.
Place a tourniquet around the lower uterine segment, including the infundibular pelvic ligaments. Tourniquet use is the most effective way to decrease blood loss during myomectomy, since it can reduce blood loss by 1,870 mL.12 For women who wish to preserve fertility, take care to ensure that the tourniquet does not compromise the tubes. For women who are certain they do not want to preserve fertility, discuss the possibility of performing bilateral salpingectomy to decrease the risk of subsequent tubal (“ovarian”) cancer.
Some surgeons incise the broad ligaments bilaterally and pass the tourniquet through the broad ligaments to avoid compromising blood flow to the ovaries. Occluding the utero- ovarian ligaments with bulldog clamps to control collateral blood flow from the ovarian artery has been described, but the clamps can tear these often enlarged and fragile uterine veins during manipulation of the uterus. Release the tourniquet every 15 to 30 minutes to allow reperfusion of the ovaries. In women with ovarian torsion lasting hours to days, the ovary has been found to resist hypoxia and recover function.14 Antral follicle counts of detorsed and contralateral normal ovaries following a mean of 13 hours of hypoxia are similar 3 months following detorsion.15
Consider blood salvage. For women with multiple or very large fibroids, consider using a salvage-type autologous blood transfusion device, which has been shown to reduce the need for heterologous blood transfusion.16 This device suctions blood from the operative field, mixes it with heparinized saline, and stores the blood in a canister (FIGURE 2). If the patient requires blood reinfusion, the stored blood is washed with saline, filtered, centrifuged, and given back to the patient intravenously. Blood salvage, or cell salvage, avoids the risks of infection and transfusion reaction, and the oxygen transport capacity of salvaged red blood cells is equal to or better than that of stored allogeneic red cells.

Additional surgical considerations
Previous teaching suggested that proper placement of the uterine incisions was an important factor in limiting blood loss. Some authors suggested that vertical uterine incisions would avoid injury to the ascending uterine vessels should inadvertent extension of the incision occur. Other authors proposed horizontal uterine incisions to avoid severing the arcuate vessels that branch off from the ascending uterine arteries and run transversely across the uterus. However, since fibroids distort the normal vascular architecture, it is not possible to entirely avoid severing vessels in the myometrium (FIGURE 3).17 Uterine incisions can therefore be made as needed based on the position of the fibroids and the need to avoid inadvertent extension to the ascending uterine vessels or cornua.17

Fibroid anatomy and vascularity. Fibroids are entirely encased within the dense blood supply of a pseudocapsule (FIGURE 4),18 and no distinct “vascular pedicle” exists at the base of the fibroid.19 It is therefore important to extend the uterine incisions down through the entire pseudocapsule until the fibroid is clearly visible. This will identify a less vascular surgical plane, which is deeper than commonly recognized. Once the fibroid is reached, the pseudocapsule can be “wiped away” using a dry laparotomy sponge (see VIDEO 3). Staying under the pseudocapsule reduces bleeding and may preserve the tissue growth factors and neurotransmitters that are thought to promote wound healing.20

Adhesion prevention. Limiting the number of uterine incisions has been suggested as a way to reduce the risk of postoperative pelvic adhesions. To extract fibroids that are distant from an incision, however, tunnels must be created within the myometrium, and this makes hemostasis within these defects difficult. In that blood increases the risk of adhesion formation, tunneling may be counterproductive. If tunneling incisions are avoided and hemostasis is secured immediately, the risk of adhesion formation should be lessened.
Therefore, make incisions directly over the fibroids. Remove only easily accessed fibroids and promptly close the defects to secure hemostasis. Multiple uterine incisions may be needed; adhesion barriers may help limit adhesion formation.21
On final removal of the tourniquet, carefully inspect for bleeding and perform any necessary re-suturing. We place a pain pump (ON-Q* Pain Relief System, Halyard Health, Inc) for pain management and close the abdominal incision in the standard manner.
Postoperative care: Manage pain, restore function
The pain pump infuser, attached to one soaker catheter above and one below the fascia, provides continuous infusion of bupivacaine to the incision at 4 mL per hour for 4 days. The pain pump greatly reduces the need for postoperative opioids.22 Use of a patient-controlled analgesia pump, with its associated adverse effects (sedation, need for oxygen saturation monitoring, slowing of bowel function) can thus be avoided. The patient’s residual pain is controlled with oral oxycodone or hydrocodone and scheduled nonsteroidal anti-inflammatory drugs.
In my practice, we use an enhanced recovery after surgery (ERAS) protocol designed to reduce postoperative surgical stress and expedite a return to baseline physiologic body functions.23 Excellent well-researched, evidence-based studies support the effectiveness of ERAS in gynecologic and general surgery procedures.24
Pre-emptive, preoperative analgesia (gabapentin and celecoxib) and end-of-case IV acetaminophen are given to reduce the inflammatory response and the need for postoperative opioids. Once it is confirmed that the patient is hemodynamically stable, add ketorolac 30 mg IV every 6 hours on postoperative day 1. Nausea and vomiting prophylaxis includes ondansetron and dexamethasone at the end of surgery, avoidance of bowel edema with restriction of intraoperative and postoperative fluids (euvolemia), early oral feeding, and gum chewing. On the evening of surgery, the urinary catheter is removed to reduce the risk of bladder infection and facilitate ambulation. Encourage sitting at the bedside and early ambulation starting the evening of surgery to reduce risk of thromboembolism and to avoid skeletal muscle weakness and postoperative fatigue.
Most women are able to be discharged on postoperative day 2. They return to the office on postoperative day 5 for removal of the pain pump.
CASE Continued: Fibroids removed via abdominal myomectomy
We performed an abdominal myomectomy through a Pfannenstiel incision. Nine fibroids—3 of which were not seen on MRI—ranging in size from 1 to 7 cm were removed. Intravaginal misoprostol, IV tranexamic acid, subserosal vasopressin, and a uterine vessel tourniquet limited the intraoperative blood loss to 225 mL. After surgery, a pain pump and ERAS protocol allowed the patient to be discharged on postoperative day 2, and she returned to the office on day 5 for removal of the pain pump. Oral pain medication was continued on an as-needed basis.
Acknowledgement
The author would like to thank Stanley West, MD, for generously teaching him the surgical techniques for performing abdominal myomectomy.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- Barrett ML, Weiss AJ, Stocks C, Steiner CA, Myers ER. Statistical brief 200. Procedures to treat benign uterine fibroids in hospital inpatient and hospital-based ambulatory surgery settings, 2013. Healthcare Cost and Utilization Project website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb200-Procedures-Treat-Uterine-Fibroids.jsp. Published January 2016. Accessed February 9, 2017.
- Stentz NC, Cooney L, Sammel MD, Shah DK. Impact of the Food and Drug Administration (FDA) safety communication on morcellation on surgical practice and perioperative morbidity following myomectomy [abstract p300]. Fertil Steril. 2016;106(3 suppl):e219.
- Hillis SD, Marchbanks PA, Peterson HB. Obstet Gynecol. 1996;87(4):539–543.
- Pritts E, Vanness D, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bogani G, Cliby WA, Aletti GD. Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol. 2015;137(1):167–172.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynaecol. 2013;33(7):655–662.
- West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85(1):36–39.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Kim YH, Chung HH, Kang SB, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014 Aug 15;(8):CD005355.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod. 2003;18(12):2599–2602.
- Yasa C, Dural O, Bastu E, Zorlu M, Demir O, Ugurlucan FG. Impact of laparoscopic ovarian detorsion on ovarian reserve. J Obstet Gynaecol Res. 2017;43(2):298–302.
- Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59(3):233–236.
- Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301–1303.
- Malavasi A, Cavalotti C, Nicolardi G, et al. The opioid neuropeptides in uterine fibroid pseudocapsules: a putative association with cervical integrity in human reproduction. Gynecol Endocrinol. 2013;29(11):982–988.
- Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18(5):1088–1093.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters [published online ahead of print March 22, 2016]. Curr Protein Pept Sci. doi:10.2174/1389203717666160322150338.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66(6):904–910.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203(6):914–932.
- Lassen K, Soop M, Nygren J, et al; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319–328.
- Wright JD, Herzog TJ, Tsui J, et al. Nationwide trends in the performance of inpatient hysterectomy in the United States. Obstet Gynecol. 2013;122(2 pt 1):233–241.
- Barrett ML, Weiss AJ, Stocks C, Steiner CA, Myers ER. Statistical brief 200. Procedures to treat benign uterine fibroids in hospital inpatient and hospital-based ambulatory surgery settings, 2013. Healthcare Cost and Utilization Project website. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb200-Procedures-Treat-Uterine-Fibroids.jsp. Published January 2016. Accessed February 9, 2017.
- Stentz NC, Cooney L, Sammel MD, Shah DK. Impact of the Food and Drug Administration (FDA) safety communication on morcellation on surgical practice and perioperative morbidity following myomectomy [abstract p300]. Fertil Steril. 2016;106(3 suppl):e219.
- Hillis SD, Marchbanks PA, Peterson HB. Obstet Gynecol. 1996;87(4):539–543.
- Pritts E, Vanness D, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12(3):165–177.
- Bogani G, Cliby WA, Aletti GD. Impact of morcellation on survival outcomes of patients with unexpected uterine leiomyosarcoma: a systematic review and meta-analysis. Gynecol Oncol. 2015;137(1):167–172.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynaecol. 2013;33(7):655–662.
- West S, Ruiz R, Parker WH. Abdominal myomectomy in women with very large uterine size. Fertil Steril. 2006;85(1):36–39.
- Dueholm M, Lundorf E, Hansen ES, Ledertoug S, Olesen F. Evaluation of the uterine cavity with magnetic resonance imaging, transvaginal sonography, hysterosonographic examination, and diagnostic hysteroscopy. Fertil Steril. 2001;76(2):350–357.
- Kim YH, Chung HH, Kang SB, Kim SC, Kim YT. Safety and usefulness of intravenous iron sucrose in the management of preoperative anemia in patients with menorrhagia: a phase IV, open-label, prospective, randomized study. Acta Haematol. 2009;121(1):37–41.
- Kongnyuy EJ, Wiysonge CS. Interventions to reduce haemorrhage during myomectomy for fibroids. Cochrane Database Syst Rev. 2014 Aug 15;(8):CD005355.
- Hobo R, Netsu S, Koyasu Y, Tsutsumi O. Bradycardia and cardiac arrest caused by intramyometrial injection of vasopressin during a laparoscopically assisted myomectomy. Obstet Gynecol. 2009;113(2 pt 2):484–486.
- Oelsner G, Cohen SB, Soriano D, Admon D, Mashiach S, Carp H. Minimal surgery for the twisted ischaemic adnexa can preserve ovarian function. Hum Reprod. 2003;18(12):2599–2602.
- Yasa C, Dural O, Bastu E, Zorlu M, Demir O, Ugurlucan FG. Impact of laparoscopic ovarian detorsion on ovarian reserve. J Obstet Gynaecol Res. 2017;43(2):298–302.
- Yamada T, Ikeda A, Okamoto Y, Okamoto Y, Kanda T, Ueki M. Intraoperative blood salvage in abdominal simple total hysterectomy for uterine myoma. Int J Gynaecol Obstet. 1997;59(3):233–236.
- Discepola F, Valenti DA, Reinhold C, Tulandi T. Analysis of arterial blood vessels surrounding the myoma: relevance to myomectomy. Obstet Gynecol. 2007;110(6):1301–1303.
- Malavasi A, Cavalotti C, Nicolardi G, et al. The opioid neuropeptides in uterine fibroid pseudocapsules: a putative association with cervical integrity in human reproduction. Gynecol Endocrinol. 2013;29(11):982–988.
- Walocha JA, Litwin JA, Miodonski AJ. Vascular system of intramural leiomyomata revealed by corrosion casting and scanning electron microscopy. Hum Reprod. 2003;18(5):1088–1093.
- Tinelli A, Mynbaev OA, Sparic R, et al. Angiogenesis and vascularization of uterine leiomyoma: clinical value of pseudocapsule containing peptides and neurotransmitters [published online ahead of print March 22, 2016]. Curr Protein Pept Sci. doi:10.2174/1389203717666160322150338.
- Diamond MP. Reduction of adhesions after uterine myomectomy by Seprafilm membrane (HAL-F): a blinded, prospective, randomized, multicenter clinical study. Seprafilm Adhesion Study Group. Fertil Steril. 1996;66(6):904–910.
- Liu SS, Richman JM, Thirlby RC, Wu CL. Efficacy of continuous wound catheters delivering local anesthetic for postoperative analgesia: a quantitative and qualitative systematic review of randomized controlled trials. J Am Coll Surg. 2006;203(6):914–932.
- Lassen K, Soop M, Nygren J, et al; Enhanced Recovery After Surgery (ERAS) Group. Consensus review of optimal perioperative care in colorectal surgery: Enhanced Recovery After Surgery (ERAS) Group recommendations. Arch Surg. 2009;144(10):961–969.
- Kalogera E, Bakkum-Gamez JN, Jankowski CJ, et al. Enhanced recovery in gynecologic surgery. Obstet Gynecol. 2013;122(2 pt 1):319–328.
7 Myomectomy myths debunked
Fibroids are extremely common and can be detected in 60% of African American women and 40% of white women by age 35. By age 50, more than 80% of African American women and almost 70% of white women have fibroids. Although most women with fibroids are relatively asymptomatic, women who have bothersome symptoms, such as heavy menstrual bleeding, urinary frequency, pelvic or abdominal pressure, or pain, account for nearly 30% of all gynecologic admissions in the United States. The cost of fibroid-related care, including surgery, hospital admissions, outpatient visits, and medications, is estimated at $4 to $9 billion per year.1 In addition, each woman seeking treatment for fibroid-related symptoms incurs an expense of $4,500 to $30,000 for lost work or disability every year.1
Many treatment options, including medical therapy and noninvasive procedures, are now available for women with symptomatic fibroids. For women who require surgical treatment, however, hysterectomy is often recommended. Fibroid-related hysterectomy currently accounts for 45% of all hysterectomies, or approximately 195,700 per year. Although the American College of Obstetricians and Gynecologists (ACOG) clinical management guidelines state that myomectomy is a safe and effective alternative to hysterectomy for treatment of women with symptomatic fibroids, only 30,000 myomectomies (abdominal, laparoscopic, and robotic-assisted approaches) are performed each year.2 Why is this? One reason may be that, although many women wish to have uterus-preserving treatment, they often feel that doctors are too quick to recommend hysterectomy as the first—and sometimes only—treatment option for fibroids.3
CASE: Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents for a third opinion regarding her heavy menstrual bleeding and known uterine fibroids. She does not want to have any more children, but she wishes to avoid a hysterectomy. Both her regular gynecologist and the second gynecologist she consulted recommended hysterectomy as the first, and only, treatment option. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. The patient’s other gynecologists advised her that a myomectomy would be a “bloody operation,” would leave her uterus looking like Swiss cheese, and is not appropriate for women who have completed childbearing.
The patient asks if myomectomy could be considered in her situation. How would you advise her regarding myomectomy as an alternative to hysterectomy?
Organ conservation is important
In 1931, prominent British gynecologic surgeon Victor Bonney said, “Since cure without deformity or loss of function must ever be surgery’s highest ideal, the general proposition that myomectomy is a greater surgical achievement is incontestable.”4 As current hysterectomy and myomectomy rates indicate, however, we are not attempting organ conservation very often.
Other specialties almost never remove an entire organ for benign growths. Using breast cancer surgery as an admirable paradigm, consider that in the early 20th century the standard treatment for breast cancer was a Halsted radical mastectomy with axial lymphadenectomy. By the 1930s, this disfiguring operation was replaced by simple mastectomy and radiation, and by the 1970s, by lumpectomy and lymphadenectomy. Currently, lumpectomy and sentinel node sampling is the standard of care for early stage breast cancer. This is an excellent example of “minimally invasive surgery,” a term fostered by gynecologists. And, these organ-preservingsurgeries are performed for women with cancer, not a benign condition like fibroids.
Although our approach to hysterectomy has evolved with the increasing use of laparoscopic or robotic assistance, removal of the entire uterus nevertheless remains the surgical goal. I think this narrow view of surgical options is a disservice to our patients.
Many of us were taught that myomectomy was associated with more complications and more blood loss than hysterectomy. We were taught that the uterus had no function other than childbearing and that removing the uterus had no adverse health effects. The dogma suggested that myomectomy preserved a uterus that looked like Swiss cheese and would not heal properly and that the risk of fibroid recurrence was high. These beliefs, however, are myths, which are discussed and debunked below. In second and third installments for this series on myomectomy, I present steps for successful abdominal and laparoscopic technique.
Read myths on hysterectomy, myomectomy, and fibroids
MYTH #1: Hysterectomy is safer than myomectomy
Myomectomy is performed within the confines of the uterus and myometrium, with only infrequent occasion to operate near the ureters, uterine vessels, bowel, or bladder. Therefore, it should not be surprising that studies show that fewer complications occur with myomectomy than with hysterectomy.
A retrospective review of 197 women who had myomectomy and 197 women who underwent hysterectomy with similar uterine size (14 vs 15 weeks) reported that 13% (n = 26) of women in the hysterectomy group experienced complications, including 1 bladder injury, 1 ureteral injury, and 3 bowel injuries; 8 women had an ileus and 6 women had a pelvic abscess.5 Only 5% (n = 11) of the myomectomy patients had complications, including 1 bladder injury; 2 women had reoperation for small bowel obstruction, and 6 women had an ileus. The risks of febrile morbidity, unintended surgical procedure, life-threatening events, and rehospitalization were similar for both groups.
Authors of a recent systematic review of 6 studies, which included 1,520 women with uterine size up to 18 weeks, found higher rates of visceral injury and longer hospital stays for women who had a hysterectomy compared with those who had a myomectomy (TABLE 1).6
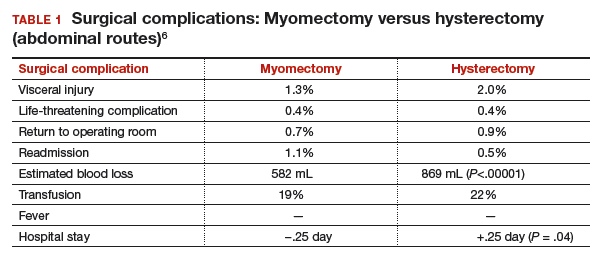
MYTH #2: Myomectomy is associated with more surgical blood loss than hysterectomy
In the previously cited study of 197 women treated with myomectomy and 197 women treated with hysterectomy, the estimated blood loss was greater in the hysterectomy group (484 mL) than in the myomectomy group (227 mL). When uterine size was corrected for, blood loss was no greater for myomectomy than for hysterectomy.5 The risk of hemorrhage (>500 mL blood loss) was greater in the hysterectomy group (14.2% vs 9.6%). Authors of the recent meta-analysis also found that the rate of transfusion was higher in the hysterectomy cohort. Tourniquets, misoprostol, vasopressin, and tranexamic acid all have been shown to significantly decrease surgical blood loss. (These treatments will be discussed in the next installment of this article series.)
MYTH #3: A uterus will look like Swiss cheese after a myomectomy
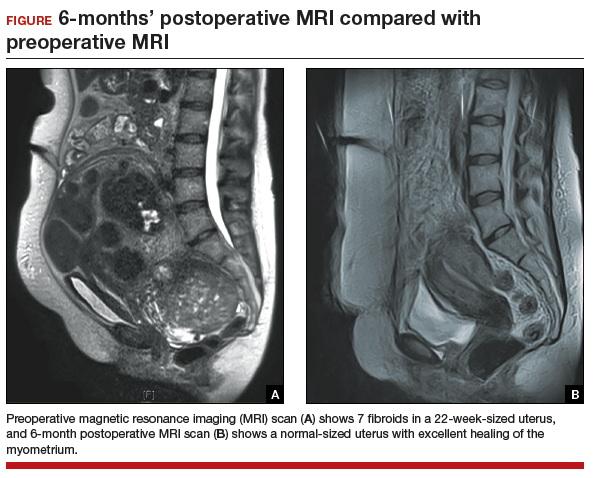
The uterus heals remarkably well after myomectomy. Three months following laparoscopic myomectomy, 3-dimensional Doppler ultrasonography demonstrated complete myometrial healing and normal blood flow to the uterus.7 In a study of women undergoing abdominal myomectomy, follow-up magnetic resonance imaging (MRI) with gadolinium showed complete healing of the myometrium and normal myometrial perfusion by 3 months.8 This study also found that, after removal of 65 g to 380 g of fibroids, the uterine volume 3 months after surgery was 65 mL, essentially equivalent to the normal volume of a uterus without fibroids (57 mL).8 See FIGURE for MRI scans of the uterus before and after myomectomy.
MYTH #4: Fibroids will just grow back after myomectomy
Once a fibroid is completely removed surgically, it does not grow back. The risk of new fibroid growth depends on the number of fibroids originally removed and the amount of time until menopause, when fibroids reduce in size and symptoms usually resolve. Given that the prevalence of fibroids is nearly 80% by age 50, studies measuring the detection of new fibroid growth of 1 cm on ultrasound imaging overstate the problem.9 What is likely a more important consideration for women is whether, following myomectomy, they will need another procedure for new fibroid-related symptoms.
Results of a meta-analysis of 872 women in 7 studies with 10- to 25-year follow-up indicated that 89% of women did not require another surgery.10 In another study, authors found that, over an average follow-up of 7.6 years, a second surgery occurred in 11% of the women who had 1 fibroid initially removed and for 26% of women who had multiple fibroids initially removed.11 In another study of 92 women who had either abdominal or laparoscopic myomectomy after age 45and who were followed for an average of 30 months, only 1 woman (1%) required a hysterectomy for fibroid-related symptoms.12 That patient had growth of a fibroid that was present but was not removed at her initial laparoscopic myomectomy.
Read myths 5–7 on ovarian conservation, fibroid growth, and symptom improvement
MYTH #5: Hysterectomy with ovarian conservation does not change hormone levels
Following hysterectomy with ovarian conservation, some women begin menopause earlier than age-matched women who have not undergone any surgery.13 Hysterectomy with ovarian conservation prior to age 50 has been associated with a significant increase in the risk of coronary heart disease, stroke, and heart failure.14 In a prospective longitudinal study, antimüllerian hormone (AMH) levels were persistently decreased following hysterectomy despite ovarian conservation.15 However, 3 months after myomectomy, no such changes in AMH levels were seen (TABLE 2).15

Early natural menopause has been associated with an increase in cardiovascular disease and death, and bilateral oophorectomy has been associated with increased risks of cardiovascular disease, all-cause mortality, lung cancer, colon cancer, anxiety, and depression. Although taking estrogen might obviate these adverse health effects, the majority of women who receive a prescription for estrogen following surgery are no longer taking it 5 years later.
MYTH #6: Fibroid growth in a premenopausal patient means cancer may be present
While most fibroids grow slowly, rapid growth of benign fibroids is very common. Using computerized analysis of a group of 72 women having serial MRI scans, investigators found that 34% of benign fibroids increased more than 20% in volume over 6 months.16 In premenopausal women, “rapid uterine growth” almost never indicates presence of uterine sarcoma. One study reported only 1 sarcoma among 371 women operated on for rapid growth of presumed fibroids.17 Using current criteria from the World Health Organization to determine the pathologic diagnosis, however, that 1 woman was determined to have had an atypical leiomyoma. Therefore, the prevalence of leiomyosarcoma in that study approached zero. In addition, in the 198 women who had a 6-week increase in uterine size over 1 year (one published definition of rapid growth), no sarcomas were found.17
Because of recent concern about leiomyosarcoma and morcellation of fibroids, some gynecologists have reverted to advising women that growing fibroids might be cancer and that hysterectomy is recommended. However, there is no evidence that fibroid growth is a sign of leiomyosarcoma in premenopausal women. Leiomyosarcoma should strongly be considered in a postmenopausal woman on no hormone therapy who has growth of a presumed fibroid.
MYTH #7: Myomectomy will not improve symptoms
Fibroid-related symptoms can be significant; women who undergo hysterectomy because of fibroid-related symptoms have significantly worse scores on the 36-Item Short-Form Survey (SF-36) quality-of-life questionnaire than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis.18
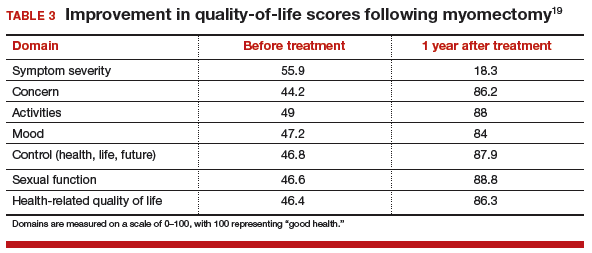
For women with fibroid-related symptoms, myomectomy has been shown to improve quality of life. A study of 72 women showed that SF-36 scores improved significantly following myomectomy (TABLE 3, page 48).19 In another study that used the European Quality of Life Five-Dimension Scale and Visual Analog Scale, 95 women had significant improvement in quality of life (P<.001) following laparoscopic myomectomy.20
For some women, hysterectomy may have an impact on emotional quality of life. Some women report decreased sexual desire after hysterectomy. They worry that partners will see them as “not whole” and less desirable. Some women expect that hysterectomy will lead to depression, crying, lack of sexual desire, and vaginal dryness.21 No such changes have been reported for women having myomectomy.
CASE Continued: Third consult leads patient to schedule surgical procedure
After reviewing the patient’s symptoms, examination, and ultrasound results, we advise the patient that abdominal myomectomy is indeed appropriate and feasible in her case. She schedules surgery for the following month.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1–e9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387–400.
- Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–e20.
- Bonney V. The technique and results of myomectomy. Lancet. 1931;217(5604):171-177.
- Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183(6):1448–1455.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynecol. 2013;33(7):655–662.
- Chang WC, Chang DY, Huang SC, et al. Use of three-dimensional ultrasonography in the evaluation of uterine perfusion and healing after laparoscopic myomectomy. Fertil Steril. 2009;92(3):1110–1115.
- Tsuji S, Takahashi K, Imaoka I, Sugimura K, Miyazaki K, Noda Y. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006;61(2):106–110.
- Sudik R, Husch K, Steller J, Daume E. Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):209–214.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Malone, LJ. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34(2):200–203.
- Kim DH, Kim ML, Song T, Kim MK, Yoon BS, Seong SJ. Is myomectomy in women aged 45 years and older an effective option? Eur J Obstet Gynecol Reprod Biol. 2014;177:57–60.
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112(7):956–962.
- Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci. 2008;105(50):19887–19892.
- Parker W, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93(6):915–921.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Radosa JC, Radosa CG, Mavrova R, et al. Postoperative quality of life and sexual function in premenopausal women undergoing laparoscopic myomectomy for symptomatic fibroids: a prospective observational cohort study. PLoS One. 2016;29;11(11):e0166659.
- Groff JY, Mullen PD, Byrd T, Shelton AJ, Lees E, Goode J. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9(suppl 2):39S–50S.
Fibroids are extremely common and can be detected in 60% of African American women and 40% of white women by age 35. By age 50, more than 80% of African American women and almost 70% of white women have fibroids. Although most women with fibroids are relatively asymptomatic, women who have bothersome symptoms, such as heavy menstrual bleeding, urinary frequency, pelvic or abdominal pressure, or pain, account for nearly 30% of all gynecologic admissions in the United States. The cost of fibroid-related care, including surgery, hospital admissions, outpatient visits, and medications, is estimated at $4 to $9 billion per year.1 In addition, each woman seeking treatment for fibroid-related symptoms incurs an expense of $4,500 to $30,000 for lost work or disability every year.1
Many treatment options, including medical therapy and noninvasive procedures, are now available for women with symptomatic fibroids. For women who require surgical treatment, however, hysterectomy is often recommended. Fibroid-related hysterectomy currently accounts for 45% of all hysterectomies, or approximately 195,700 per year. Although the American College of Obstetricians and Gynecologists (ACOG) clinical management guidelines state that myomectomy is a safe and effective alternative to hysterectomy for treatment of women with symptomatic fibroids, only 30,000 myomectomies (abdominal, laparoscopic, and robotic-assisted approaches) are performed each year.2 Why is this? One reason may be that, although many women wish to have uterus-preserving treatment, they often feel that doctors are too quick to recommend hysterectomy as the first—and sometimes only—treatment option for fibroids.3
CASE: Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents for a third opinion regarding her heavy menstrual bleeding and known uterine fibroids. She does not want to have any more children, but she wishes to avoid a hysterectomy. Both her regular gynecologist and the second gynecologist she consulted recommended hysterectomy as the first, and only, treatment option. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. The patient’s other gynecologists advised her that a myomectomy would be a “bloody operation,” would leave her uterus looking like Swiss cheese, and is not appropriate for women who have completed childbearing.
The patient asks if myomectomy could be considered in her situation. How would you advise her regarding myomectomy as an alternative to hysterectomy?
Organ conservation is important
In 1931, prominent British gynecologic surgeon Victor Bonney said, “Since cure without deformity or loss of function must ever be surgery’s highest ideal, the general proposition that myomectomy is a greater surgical achievement is incontestable.”4 As current hysterectomy and myomectomy rates indicate, however, we are not attempting organ conservation very often.
Other specialties almost never remove an entire organ for benign growths. Using breast cancer surgery as an admirable paradigm, consider that in the early 20th century the standard treatment for breast cancer was a Halsted radical mastectomy with axial lymphadenectomy. By the 1930s, this disfiguring operation was replaced by simple mastectomy and radiation, and by the 1970s, by lumpectomy and lymphadenectomy. Currently, lumpectomy and sentinel node sampling is the standard of care for early stage breast cancer. This is an excellent example of “minimally invasive surgery,” a term fostered by gynecologists. And, these organ-preservingsurgeries are performed for women with cancer, not a benign condition like fibroids.
Although our approach to hysterectomy has evolved with the increasing use of laparoscopic or robotic assistance, removal of the entire uterus nevertheless remains the surgical goal. I think this narrow view of surgical options is a disservice to our patients.
Many of us were taught that myomectomy was associated with more complications and more blood loss than hysterectomy. We were taught that the uterus had no function other than childbearing and that removing the uterus had no adverse health effects. The dogma suggested that myomectomy preserved a uterus that looked like Swiss cheese and would not heal properly and that the risk of fibroid recurrence was high. These beliefs, however, are myths, which are discussed and debunked below. In second and third installments for this series on myomectomy, I present steps for successful abdominal and laparoscopic technique.
Read myths on hysterectomy, myomectomy, and fibroids
MYTH #1: Hysterectomy is safer than myomectomy
Myomectomy is performed within the confines of the uterus and myometrium, with only infrequent occasion to operate near the ureters, uterine vessels, bowel, or bladder. Therefore, it should not be surprising that studies show that fewer complications occur with myomectomy than with hysterectomy.
A retrospective review of 197 women who had myomectomy and 197 women who underwent hysterectomy with similar uterine size (14 vs 15 weeks) reported that 13% (n = 26) of women in the hysterectomy group experienced complications, including 1 bladder injury, 1 ureteral injury, and 3 bowel injuries; 8 women had an ileus and 6 women had a pelvic abscess.5 Only 5% (n = 11) of the myomectomy patients had complications, including 1 bladder injury; 2 women had reoperation for small bowel obstruction, and 6 women had an ileus. The risks of febrile morbidity, unintended surgical procedure, life-threatening events, and rehospitalization were similar for both groups.
Authors of a recent systematic review of 6 studies, which included 1,520 women with uterine size up to 18 weeks, found higher rates of visceral injury and longer hospital stays for women who had a hysterectomy compared with those who had a myomectomy (TABLE 1).6

MYTH #2: Myomectomy is associated with more surgical blood loss than hysterectomy
In the previously cited study of 197 women treated with myomectomy and 197 women treated with hysterectomy, the estimated blood loss was greater in the hysterectomy group (484 mL) than in the myomectomy group (227 mL). When uterine size was corrected for, blood loss was no greater for myomectomy than for hysterectomy.5 The risk of hemorrhage (>500 mL blood loss) was greater in the hysterectomy group (14.2% vs 9.6%). Authors of the recent meta-analysis also found that the rate of transfusion was higher in the hysterectomy cohort. Tourniquets, misoprostol, vasopressin, and tranexamic acid all have been shown to significantly decrease surgical blood loss. (These treatments will be discussed in the next installment of this article series.)
MYTH #3: A uterus will look like Swiss cheese after a myomectomy
The uterus heals remarkably well after myomectomy. Three months following laparoscopic myomectomy, 3-dimensional Doppler ultrasonography demonstrated complete myometrial healing and normal blood flow to the uterus.7 In a study of women undergoing abdominal myomectomy, follow-up magnetic resonance imaging (MRI) with gadolinium showed complete healing of the myometrium and normal myometrial perfusion by 3 months.8 This study also found that, after removal of 65 g to 380 g of fibroids, the uterine volume 3 months after surgery was 65 mL, essentially equivalent to the normal volume of a uterus without fibroids (57 mL).8 See FIGURE for MRI scans of the uterus before and after myomectomy.

MYTH #4: Fibroids will just grow back after myomectomy
Once a fibroid is completely removed surgically, it does not grow back. The risk of new fibroid growth depends on the number of fibroids originally removed and the amount of time until menopause, when fibroids reduce in size and symptoms usually resolve. Given that the prevalence of fibroids is nearly 80% by age 50, studies measuring the detection of new fibroid growth of 1 cm on ultrasound imaging overstate the problem.9 What is likely a more important consideration for women is whether, following myomectomy, they will need another procedure for new fibroid-related symptoms.
Results of a meta-analysis of 872 women in 7 studies with 10- to 25-year follow-up indicated that 89% of women did not require another surgery.10 In another study, authors found that, over an average follow-up of 7.6 years, a second surgery occurred in 11% of the women who had 1 fibroid initially removed and for 26% of women who had multiple fibroids initially removed.11 In another study of 92 women who had either abdominal or laparoscopic myomectomy after age 45and who were followed for an average of 30 months, only 1 woman (1%) required a hysterectomy for fibroid-related symptoms.12 That patient had growth of a fibroid that was present but was not removed at her initial laparoscopic myomectomy.
Read myths 5–7 on ovarian conservation, fibroid growth, and symptom improvement
MYTH #5: Hysterectomy with ovarian conservation does not change hormone levels
Following hysterectomy with ovarian conservation, some women begin menopause earlier than age-matched women who have not undergone any surgery.13 Hysterectomy with ovarian conservation prior to age 50 has been associated with a significant increase in the risk of coronary heart disease, stroke, and heart failure.14 In a prospective longitudinal study, antimüllerian hormone (AMH) levels were persistently decreased following hysterectomy despite ovarian conservation.15 However, 3 months after myomectomy, no such changes in AMH levels were seen (TABLE 2).15

Early natural menopause has been associated with an increase in cardiovascular disease and death, and bilateral oophorectomy has been associated with increased risks of cardiovascular disease, all-cause mortality, lung cancer, colon cancer, anxiety, and depression. Although taking estrogen might obviate these adverse health effects, the majority of women who receive a prescription for estrogen following surgery are no longer taking it 5 years later.
MYTH #6: Fibroid growth in a premenopausal patient means cancer may be present
While most fibroids grow slowly, rapid growth of benign fibroids is very common. Using computerized analysis of a group of 72 women having serial MRI scans, investigators found that 34% of benign fibroids increased more than 20% in volume over 6 months.16 In premenopausal women, “rapid uterine growth” almost never indicates presence of uterine sarcoma. One study reported only 1 sarcoma among 371 women operated on for rapid growth of presumed fibroids.17 Using current criteria from the World Health Organization to determine the pathologic diagnosis, however, that 1 woman was determined to have had an atypical leiomyoma. Therefore, the prevalence of leiomyosarcoma in that study approached zero. In addition, in the 198 women who had a 6-week increase in uterine size over 1 year (one published definition of rapid growth), no sarcomas were found.17
Because of recent concern about leiomyosarcoma and morcellation of fibroids, some gynecologists have reverted to advising women that growing fibroids might be cancer and that hysterectomy is recommended. However, there is no evidence that fibroid growth is a sign of leiomyosarcoma in premenopausal women. Leiomyosarcoma should strongly be considered in a postmenopausal woman on no hormone therapy who has growth of a presumed fibroid.
MYTH #7: Myomectomy will not improve symptoms
Fibroid-related symptoms can be significant; women who undergo hysterectomy because of fibroid-related symptoms have significantly worse scores on the 36-Item Short-Form Survey (SF-36) quality-of-life questionnaire than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis.18
For women with fibroid-related symptoms, myomectomy has been shown to improve quality of life. A study of 72 women showed that SF-36 scores improved significantly following myomectomy (TABLE 3, page 48).19 In another study that used the European Quality of Life Five-Dimension Scale and Visual Analog Scale, 95 women had significant improvement in quality of life (P<.001) following laparoscopic myomectomy.20

For some women, hysterectomy may have an impact on emotional quality of life. Some women report decreased sexual desire after hysterectomy. They worry that partners will see them as “not whole” and less desirable. Some women expect that hysterectomy will lead to depression, crying, lack of sexual desire, and vaginal dryness.21 No such changes have been reported for women having myomectomy.
CASE Continued: Third consult leads patient to schedule surgical procedure
After reviewing the patient’s symptoms, examination, and ultrasound results, we advise the patient that abdominal myomectomy is indeed appropriate and feasible in her case. She schedules surgery for the following month.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Fibroids are extremely common and can be detected in 60% of African American women and 40% of white women by age 35. By age 50, more than 80% of African American women and almost 70% of white women have fibroids. Although most women with fibroids are relatively asymptomatic, women who have bothersome symptoms, such as heavy menstrual bleeding, urinary frequency, pelvic or abdominal pressure, or pain, account for nearly 30% of all gynecologic admissions in the United States. The cost of fibroid-related care, including surgery, hospital admissions, outpatient visits, and medications, is estimated at $4 to $9 billion per year.1 In addition, each woman seeking treatment for fibroid-related symptoms incurs an expense of $4,500 to $30,000 for lost work or disability every year.1
Many treatment options, including medical therapy and noninvasive procedures, are now available for women with symptomatic fibroids. For women who require surgical treatment, however, hysterectomy is often recommended. Fibroid-related hysterectomy currently accounts for 45% of all hysterectomies, or approximately 195,700 per year. Although the American College of Obstetricians and Gynecologists (ACOG) clinical management guidelines state that myomectomy is a safe and effective alternative to hysterectomy for treatment of women with symptomatic fibroids, only 30,000 myomectomies (abdominal, laparoscopic, and robotic-assisted approaches) are performed each year.2 Why is this? One reason may be that, although many women wish to have uterus-preserving treatment, they often feel that doctors are too quick to recommend hysterectomy as the first—and sometimes only—treatment option for fibroids.3
CASE: Woman with fibroids seeks alternative to hysterectomy
A 42-year-old woman (G2P2) presents for a third opinion regarding her heavy menstrual bleeding and known uterine fibroids. She does not want to have any more children, but she wishes to avoid a hysterectomy. Both her regular gynecologist and the second gynecologist she consulted recommended hysterectomy as the first, and only, treatment option. Physical examination reveals a 16-week-sized uterus, and ultrasonography shows at least 6 fibroids, 2 of which impinge on the uterine cavity. The patient’s other gynecologists advised her that a myomectomy would be a “bloody operation,” would leave her uterus looking like Swiss cheese, and is not appropriate for women who have completed childbearing.
The patient asks if myomectomy could be considered in her situation. How would you advise her regarding myomectomy as an alternative to hysterectomy?
Organ conservation is important
In 1931, prominent British gynecologic surgeon Victor Bonney said, “Since cure without deformity or loss of function must ever be surgery’s highest ideal, the general proposition that myomectomy is a greater surgical achievement is incontestable.”4 As current hysterectomy and myomectomy rates indicate, however, we are not attempting organ conservation very often.
Other specialties almost never remove an entire organ for benign growths. Using breast cancer surgery as an admirable paradigm, consider that in the early 20th century the standard treatment for breast cancer was a Halsted radical mastectomy with axial lymphadenectomy. By the 1930s, this disfiguring operation was replaced by simple mastectomy and radiation, and by the 1970s, by lumpectomy and lymphadenectomy. Currently, lumpectomy and sentinel node sampling is the standard of care for early stage breast cancer. This is an excellent example of “minimally invasive surgery,” a term fostered by gynecologists. And, these organ-preservingsurgeries are performed for women with cancer, not a benign condition like fibroids.
Although our approach to hysterectomy has evolved with the increasing use of laparoscopic or robotic assistance, removal of the entire uterus nevertheless remains the surgical goal. I think this narrow view of surgical options is a disservice to our patients.
Many of us were taught that myomectomy was associated with more complications and more blood loss than hysterectomy. We were taught that the uterus had no function other than childbearing and that removing the uterus had no adverse health effects. The dogma suggested that myomectomy preserved a uterus that looked like Swiss cheese and would not heal properly and that the risk of fibroid recurrence was high. These beliefs, however, are myths, which are discussed and debunked below. In second and third installments for this series on myomectomy, I present steps for successful abdominal and laparoscopic technique.
Read myths on hysterectomy, myomectomy, and fibroids
MYTH #1: Hysterectomy is safer than myomectomy
Myomectomy is performed within the confines of the uterus and myometrium, with only infrequent occasion to operate near the ureters, uterine vessels, bowel, or bladder. Therefore, it should not be surprising that studies show that fewer complications occur with myomectomy than with hysterectomy.
A retrospective review of 197 women who had myomectomy and 197 women who underwent hysterectomy with similar uterine size (14 vs 15 weeks) reported that 13% (n = 26) of women in the hysterectomy group experienced complications, including 1 bladder injury, 1 ureteral injury, and 3 bowel injuries; 8 women had an ileus and 6 women had a pelvic abscess.5 Only 5% (n = 11) of the myomectomy patients had complications, including 1 bladder injury; 2 women had reoperation for small bowel obstruction, and 6 women had an ileus. The risks of febrile morbidity, unintended surgical procedure, life-threatening events, and rehospitalization were similar for both groups.
Authors of a recent systematic review of 6 studies, which included 1,520 women with uterine size up to 18 weeks, found higher rates of visceral injury and longer hospital stays for women who had a hysterectomy compared with those who had a myomectomy (TABLE 1).6

MYTH #2: Myomectomy is associated with more surgical blood loss than hysterectomy
In the previously cited study of 197 women treated with myomectomy and 197 women treated with hysterectomy, the estimated blood loss was greater in the hysterectomy group (484 mL) than in the myomectomy group (227 mL). When uterine size was corrected for, blood loss was no greater for myomectomy than for hysterectomy.5 The risk of hemorrhage (>500 mL blood loss) was greater in the hysterectomy group (14.2% vs 9.6%). Authors of the recent meta-analysis also found that the rate of transfusion was higher in the hysterectomy cohort. Tourniquets, misoprostol, vasopressin, and tranexamic acid all have been shown to significantly decrease surgical blood loss. (These treatments will be discussed in the next installment of this article series.)
MYTH #3: A uterus will look like Swiss cheese after a myomectomy
The uterus heals remarkably well after myomectomy. Three months following laparoscopic myomectomy, 3-dimensional Doppler ultrasonography demonstrated complete myometrial healing and normal blood flow to the uterus.7 In a study of women undergoing abdominal myomectomy, follow-up magnetic resonance imaging (MRI) with gadolinium showed complete healing of the myometrium and normal myometrial perfusion by 3 months.8 This study also found that, after removal of 65 g to 380 g of fibroids, the uterine volume 3 months after surgery was 65 mL, essentially equivalent to the normal volume of a uterus without fibroids (57 mL).8 See FIGURE for MRI scans of the uterus before and after myomectomy.

MYTH #4: Fibroids will just grow back after myomectomy
Once a fibroid is completely removed surgically, it does not grow back. The risk of new fibroid growth depends on the number of fibroids originally removed and the amount of time until menopause, when fibroids reduce in size and symptoms usually resolve. Given that the prevalence of fibroids is nearly 80% by age 50, studies measuring the detection of new fibroid growth of 1 cm on ultrasound imaging overstate the problem.9 What is likely a more important consideration for women is whether, following myomectomy, they will need another procedure for new fibroid-related symptoms.
Results of a meta-analysis of 872 women in 7 studies with 10- to 25-year follow-up indicated that 89% of women did not require another surgery.10 In another study, authors found that, over an average follow-up of 7.6 years, a second surgery occurred in 11% of the women who had 1 fibroid initially removed and for 26% of women who had multiple fibroids initially removed.11 In another study of 92 women who had either abdominal or laparoscopic myomectomy after age 45and who were followed for an average of 30 months, only 1 woman (1%) required a hysterectomy for fibroid-related symptoms.12 That patient had growth of a fibroid that was present but was not removed at her initial laparoscopic myomectomy.
Read myths 5–7 on ovarian conservation, fibroid growth, and symptom improvement
MYTH #5: Hysterectomy with ovarian conservation does not change hormone levels
Following hysterectomy with ovarian conservation, some women begin menopause earlier than age-matched women who have not undergone any surgery.13 Hysterectomy with ovarian conservation prior to age 50 has been associated with a significant increase in the risk of coronary heart disease, stroke, and heart failure.14 In a prospective longitudinal study, antimüllerian hormone (AMH) levels were persistently decreased following hysterectomy despite ovarian conservation.15 However, 3 months after myomectomy, no such changes in AMH levels were seen (TABLE 2).15

Early natural menopause has been associated with an increase in cardiovascular disease and death, and bilateral oophorectomy has been associated with increased risks of cardiovascular disease, all-cause mortality, lung cancer, colon cancer, anxiety, and depression. Although taking estrogen might obviate these adverse health effects, the majority of women who receive a prescription for estrogen following surgery are no longer taking it 5 years later.
MYTH #6: Fibroid growth in a premenopausal patient means cancer may be present
While most fibroids grow slowly, rapid growth of benign fibroids is very common. Using computerized analysis of a group of 72 women having serial MRI scans, investigators found that 34% of benign fibroids increased more than 20% in volume over 6 months.16 In premenopausal women, “rapid uterine growth” almost never indicates presence of uterine sarcoma. One study reported only 1 sarcoma among 371 women operated on for rapid growth of presumed fibroids.17 Using current criteria from the World Health Organization to determine the pathologic diagnosis, however, that 1 woman was determined to have had an atypical leiomyoma. Therefore, the prevalence of leiomyosarcoma in that study approached zero. In addition, in the 198 women who had a 6-week increase in uterine size over 1 year (one published definition of rapid growth), no sarcomas were found.17
Because of recent concern about leiomyosarcoma and morcellation of fibroids, some gynecologists have reverted to advising women that growing fibroids might be cancer and that hysterectomy is recommended. However, there is no evidence that fibroid growth is a sign of leiomyosarcoma in premenopausal women. Leiomyosarcoma should strongly be considered in a postmenopausal woman on no hormone therapy who has growth of a presumed fibroid.
MYTH #7: Myomectomy will not improve symptoms
Fibroid-related symptoms can be significant; women who undergo hysterectomy because of fibroid-related symptoms have significantly worse scores on the 36-Item Short-Form Survey (SF-36) quality-of-life questionnaire than women diagnosed with hypertension, heart disease, chronic lung disease, or arthritis.18
For women with fibroid-related symptoms, myomectomy has been shown to improve quality of life. A study of 72 women showed that SF-36 scores improved significantly following myomectomy (TABLE 3, page 48).19 In another study that used the European Quality of Life Five-Dimension Scale and Visual Analog Scale, 95 women had significant improvement in quality of life (P<.001) following laparoscopic myomectomy.20

For some women, hysterectomy may have an impact on emotional quality of life. Some women report decreased sexual desire after hysterectomy. They worry that partners will see them as “not whole” and less desirable. Some women expect that hysterectomy will lead to depression, crying, lack of sexual desire, and vaginal dryness.21 No such changes have been reported for women having myomectomy.
CASE Continued: Third consult leads patient to schedule surgical procedure
After reviewing the patient’s symptoms, examination, and ultrasound results, we advise the patient that abdominal myomectomy is indeed appropriate and feasible in her case. She schedules surgery for the following month.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1–e9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387–400.
- Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–e20.
- Bonney V. The technique and results of myomectomy. Lancet. 1931;217(5604):171-177.
- Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183(6):1448–1455.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynecol. 2013;33(7):655–662.
- Chang WC, Chang DY, Huang SC, et al. Use of three-dimensional ultrasonography in the evaluation of uterine perfusion and healing after laparoscopic myomectomy. Fertil Steril. 2009;92(3):1110–1115.
- Tsuji S, Takahashi K, Imaoka I, Sugimura K, Miyazaki K, Noda Y. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006;61(2):106–110.
- Sudik R, Husch K, Steller J, Daume E. Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):209–214.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Malone, LJ. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34(2):200–203.
- Kim DH, Kim ML, Song T, Kim MK, Yoon BS, Seong SJ. Is myomectomy in women aged 45 years and older an effective option? Eur J Obstet Gynecol Reprod Biol. 2014;177:57–60.
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112(7):956–962.
- Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci. 2008;105(50):19887–19892.
- Parker W, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93(6):915–921.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Radosa JC, Radosa CG, Mavrova R, et al. Postoperative quality of life and sexual function in premenopausal women undergoing laparoscopic myomectomy for symptomatic fibroids: a prospective observational cohort study. PLoS One. 2016;29;11(11):e0166659.
- Groff JY, Mullen PD, Byrd T, Shelton AJ, Lees E, Goode J. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9(suppl 2):39S–50S.
- Cardozo ER, Clark AD, Banks NK, Henne MB, Stegmann BJ, Segars JH. The estimated annual cost of leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(3):211.e1–e9.
- American College of Obstetricians and Gynecologists Committee on Practice Bulletins–Gynecology. ACOG Practice Bulletin No. 96: alternatives to hysterectomy in the management of leiomyomas. Obstet Gynecol. 2008;112(2 pt 1):387–400.
- Borah BJ, Nicholson WK, Bradley L, Stewart EA. The impact of uterine leiomyomas: a national survey of affected women. Am J Obstet Gynecol. 2013;209(4):319.e1–e20.
- Bonney V. The technique and results of myomectomy. Lancet. 1931;217(5604):171-177.
- Sawin SW, Pilevsky ND, Berlin JA, Barnhart KT. Comparability of perioperative morbidity between abdominal myomectomy and hysterectomy for women with uterine leiomyomas. Am J Obstet Gynecol. 2000;183(6):1448–1455.
- Pundir J, Walawalkar R, Seshadri S, Khalaf Y, El-Toukhy T. Perioperative morbidity associated with abdominal myomectomy compared with total abdominal hysterectomy for uterine fibroids. J Obstet Gynecol. 2013;33(7):655–662.
- Chang WC, Chang DY, Huang SC, et al. Use of three-dimensional ultrasonography in the evaluation of uterine perfusion and healing after laparoscopic myomectomy. Fertil Steril. 2009;92(3):1110–1115.
- Tsuji S, Takahashi K, Imaoka I, Sugimura K, Miyazaki K, Noda Y. MRI evaluation of the uterine structure after myomectomy. Gynecol Obstet Invest. 2006;61(2):106–110.
- Sudik R, Husch K, Steller J, Daume E. Fertility and pregnancy outcome after myomectomy in sterility patients. Eur J Obstet Gynecol Reprod Biol. 1996;65(2):209–214.
- Fauconnier A, Chapron C, Babaki-Fard K, Dubuisson JB. Recurrence of leiomyomata after myomectomy. Hum Reprod Update. 2000;6(6):595–602.
- Malone, LJ. Myomectomy: recurrence after removal of solitary and multiple myomas. Obstet Gynecol. 1969;34(2):200–203.
- Kim DH, Kim ML, Song T, Kim MK, Yoon BS, Seong SJ. Is myomectomy in women aged 45 years and older an effective option? Eur J Obstet Gynecol Reprod Biol. 2014;177:57–60.
- Farquhar CM, Sadler L, Harvey SA, Stewart AW. The association of hysterectomy and menopause: a prospective cohort study. BJOG. 2005;112(7):956–962.
- Ingelsson E, Lundholm C, Johansson AL, Altman D. Hysterectomy and risk of cardiovascular disease: a population-based cohort study. Eur Heart J. 2011;32(6):745–750.
- Wang HY, Quan S, Zhang RL, et al. Comparison of serum anti-Mullerian hormone levels following hysterectomy and myomectomy for benign gynaecological conditions. Eur J Obstet Gynecol Reprod Biol. 2013;171(2):368–371.
- Peddada SD, Laughlin SK, Miner K, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci. 2008;105(50):19887–19892.
- Parker W, Fu YS, Berek JS. Uterine sarcoma in patients operated on for presumed leiomyoma and rapidly growing leiomyoma. Obstet Gynecol. 1994;83(3):414–418.
- Rowe MK, Kanouse DE, Mittman BS, Bernstein SJ. Quality of life among women undergoing hysterectomies. Obstet Gynecol. 1999;93(6):915–921.
- Dilek S, Ertunc D, Tok EC, Cimen R, Doruk A. The effect of myomectomy on health-related quality of life of women with myoma uteri. J Obstet Gynaecol Res. 2010;36(2):364–369.
- Radosa JC, Radosa CG, Mavrova R, et al. Postoperative quality of life and sexual function in premenopausal women undergoing laparoscopic myomectomy for symptomatic fibroids: a prospective observational cohort study. PLoS One. 2016;29;11(11):e0166659.
- Groff JY, Mullen PD, Byrd T, Shelton AJ, Lees E, Goode J. Decision making, beliefs, and attitudes toward hysterectomy: a focus group study with medically underserved women in Texas. J Womens Health Gend Based Med. 2000;9(suppl 2):39S–50S.
Modern breast surgery: What you should know
In a striking trend, the rate of contralateral prophylactic mastectomy (CPM) has risen by 30% over the last 10 years in the United States.1 Many women undergo CPM because of the fear and anxiety of cancer recurrence and their perceived risk of contralateral breast cancer; however, few women have a medical condition that necessitates removal of the contralateral breast. The medical indications for CPM include having a pathogenic genetic mutation (eg, BRCA1 and BRCA2), a strong family history of breast cancer, or prior mediastina chest radiation.
The actual risk of contralateral breast cancer is much lower than perceived. In women without a genetic mutation, the 10-year risk of contralateral breast cancer is only 3% to 5%.1 Also, CPM does not prevent the development of metastatic disease and offers no survival benefit over breast conservation or unilateral mastectomy.2 Furthermore, compared with unilateral therapeutic mastectomy, the “upgrade” to a CPM carries a 2.7-fold risk of a major surgical complication.3 It is therefore important that patients receive appropriate counseling regarding CPM, and that this counseling include cancer stage at diagnosis, family history and genetic risk, and cancer versus surgical risk (see “Counseling patients on contralateral prophylactic mastectomy” for key points to cover in patient discussions).
Counseling patients on contralateral prophylactic mastectomy
Commonly, patients diagnosed with breast cancer consider having their contralateral healthy breast removed as part of a bilateral mastectomy. They often experience severe anxiety about the cancer coming back and believe that removing both breasts will enable them to live longer. Keep the following key facts in mind when discussing treatment options with breast cancer patients.
Cancer stage at diagnosis. How long a patient lives from the time of her breast cancer diagnosis depends on the stage of the cancer at diagnosis, not the type of surgery performed. A woman with early stage I or stage II breast cancer has an 80% to 90% chance of being cancer free in 5 years.1 The chance of cancer recurring in the bones, liver, or lungs (metastatic breast cancer) will not be changed by removing the healthy breast. The risk of metastatic recurrence can be reduced, however, with chemotherapy and/or with hormone-blocker therapy.
Family history and genetic risk. Few women have a strong family history of breast and/or ovarian and other cancers, and this issue should be addressed with genetic counseling and testing prior to surgery. Those who carry a cancer-causing gene, such as BRCA1 or BRCA2, are at increased risk (40% to 60%) for a second or third breast cancer, especially if they are diagnosed at a young age (<50 years).2,3 In women who have a genetic mutation, removing both breasts and sometimes the ovaries can prevent development of another breast cancer. But this will not prevent spread of the cancer that is already present. Only chemotherapy and hormone blockers can prevent the spread of cancer.
Cancer risk versus surgical risk. For women with no family history of breast cancer, no genetic mutation, and no prior chest wall radiation, the risk of developing a new breast cancer in their other breast is only 3% to 5% every 10 years.3,4 This means that they have a 95% chance of not developing a new breast cancer in their healthy breast. Notably, removing the healthy breast can double the risk of postsurgical complications, including bleeding, infection, and loss of tissue and implant. The mastectomy site will be numb and the skin and nipple areola will not have any function other than cosmetic. Finally, wound complications from surgery could delay the start of important cancer treatment, such as chemotherapy or radiation.
The bottom line. Unless a woman has a strong family history of breast cancer, is diagnosed at a very young age, or has a genetic cancer-causing mutation, removing the contralateral healthy breast is not medically necessary and is not routinely recommended.
References
- Hennigs A, Riedel F, Gondos A, et al. Prognosis of breast cancer molecular subtypes in routine clinical care: a large prospective cohort study. BMC Cancer. 2016;16(1):734.
- Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27(35):5887–5992.
- Curtis RE, Ron E, Hankey BF, Hoover RN. New malignancies following breast cancer. In: Curtis RE, Freedman DM, Ron E, et al, eds. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: National Cancer Institute. NIH Publ. No. 05-5302. 2006:181–205. http://seer.cancer.gov/archive/publications/mpmono. Accessed September 18, 2016.
- Nichols HB, Berrington de Gonzalez A, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29(12):1564–1569.
Women should be made aware that there are alternatives to mastectomy that have similar, or even better, outcomes with improved quality of life. Furthermore, a multi‑disciplinary, team-oriented approach with emphasis on minimally invasive biopsy and better cosmetic outcomes has enhanced quality of care. Knowledge of this team approach and of modern breast cancer treatments is essential for general ObGyns as this understanding improves the overall care and guidance—specifically regarding referral to expert, high-volume breast surgeons—provided to those women most in need.
Expanded treatment options for breast cancer
Advancements in breast surgery, better imaging, and targeted therapies are changing the paradigm of breast cancer treatment.
Image-guided biopsy is key in decision making
When an abnormality is found in the breast, surgical excision of an undiagnosed breast lesion is no longer considered an appropriate first step. Use of image-guided biopsy or minimally invasive core needle biopsy allows for accurate diagnosis of a breast lesion while avoiding a potentially breast deforming and expensive surgical operation. It is always better to go into the operating room (OR) with a diagnosis and do the right operation the first time.
A core needle biopsy, results of which demonstrate a benign lesion, helps avoid breast surgery in women who do not need it. If cancer is diagnosed on biopsy, the extent of disease can be better evaluated and decision making can be more informed, with a multidisciplinary approach used to consider the various options, including genetic counseling, plastic surgery consultation, or neoadjuvant therapy. Some lesions, such as those too close to the skin, chest wall, or an implant, may not be amenable to core needle biopsy and therefore require surgical excision for diagnosis.
Benefits of a multidisciplinary tumor conference
It is important for a multidisciplinary group of cancer specialists to review a patient’s case and discuss the ideal treatment plan prior to surgery. Some breast cancer subtypes (such as human epidermal growth factor receptor 2 [HER2]–overamplified breast cancer and many triple-negative breast cancers) are very sensitive to chemotherapy, and patients with these tumor types may benefit from receiving neoadjuvant chemotherapy prior to surgery. New types of chemotherapy may allow up to 60% of some breast cancers to diminish almost completely, with subsequent improved cosmetic results of breast surgery.4 It may also allow time for genetic counseling and testing prior to surgery. (See “How to code for a multidisciplinary tumor conference” for appropriate coding procedure.)
How to code for a multidisciplinary tumor conference
Melanie Witt, RN, MA
There are two coding choices for team conferences involving physician participation. If the patient and/or family is present, the CPT instruction is to bill a problem E/M service code (99201-99215) based on the time spent during this coordination of care/counseling. Documentation would include details about the conference decisions and implications for care, rather than history or examination.
If the patient is not present, report 99367 (Medical team conference with interdisciplinary team of health care professionals, patient and/or family not present, 30 minutes or more; participation by physician), but note that this code was developed under the assumption that the conference would be performed in a facility setting. Diagnostic coding would be breast cancer.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
This is an excerpt from a companion coding resource for breast cancer–related procedures by Ms. Witt. To read the companion article, “Coding for breast cancer–related procedures: A how-to guide,” in its entirety, click here.
Image-guided lumpectomy
Advances in breast imaging have led to increased identification of nonpalpable breast cancers. Surgical excision of nonpalpable breast lesions requires image guidance, which can be done using a variety of techniques.
Wire-guided localization (WGL) has been used in practice for the past 40 years. The procedure involves placement of a hooked wire under local anesthesia using either mammographic or ultrasound guidance. This procedure is mostly done in the radiology department on the same day as the surgery and requires that the radiologist coordinate with the OR schedule. Besides scheduling conflicts and delays in surgery, this procedure can be complicated by wires becoming dislodged, transected, or migrated, and limits the surgeon’s ability to cosmetically hide the scar in relation to position of the wire. It is uncomfortable for the patient, who must be transported from the radiology department to the OR with a wire extruding from her breast.
An alternative localization technique is placement of a radioactive source within the tumor, which can then be identified in the OR with a gamma probe.
Iodine I 125 Radioactive seed localization (RSL) involves placing a 4-mm titanium radiolabeled seed into the breast lesion under mammographic or ultrasound guidance (FIGURES 1 and 2). The procedure can be performed a few days before surgery in the radiology department, and there is less chance for the seed to become displaced or dislodged. This technique provides scheduling flexibility for the radiologist and reduces OR delays. The surgeon uses the same gamma probe for sentinel node biopsy to find the lesion in the breast, using the setting specific for iodine I 125. Incisions can be tailored anywhere in the breast, and the seed is detected by a focal gamma signal. Once the lumpectomy is performed, the specimen is probed and radiographed to confirm removal of the seed and adequate margins.
Limitations of this procedure include potential loss of the seed during the operation and radiation safety issues regarding handling and disposal of the radioactive isotope. Once the seed has been placed in the patient’s body, it must be removed surgically, as the half-life of iodine I 125 is long (60 days).5 Care must therefore be taken to optimize medical clearance prior to seed placement and to avoid surgery cancellations.
Intraoperative ultrasound (IOUS) allows the surgeon to identify the lesion under general anesthesia in the OR, which is more comfortable for the patient. The surgical incision can be tailored cosmetically and the lumpectomy can be performed with real-time ultrasound visualization of the tumor during dissection. This technique eliminates the need for a separate preoperative seed or wire localization in radiology. However, it can be used only for lesions or clips that are visible by ultrasound. The excised specimen can be evaluated for confirmation of tumor removal and adequate margins via ultrasound and re-excision of close margins can be accomplished immediately if needed.
Results of a meta-analysis of WGL versus IOUS demonstrated a significant reduction of positive margins with the use of IOUS.6 Results of the COBALT trial, in which patients were assigned randomly to excision of palpable breast cancers with either IOUS or palpation, demonstrated a 14% reduction in positive margins in favor of IOUS.7 Surgeon-performed breast ultrasound requires advanced training and accreditation in breast ultrasound through a rigorous certification process offered by the American Society of Breast Surgeons (www.breastsurgeons.org).
Oncoplastic lumpectomy
This approach to lumpectomy combines adequate oncologist resection of the breast tumor with plastic surgery techniques to achieve superior cosmesis. This approach allows complete removal of the tumor with negative margins, yet maintains the normal shape and contour of the breast. Two techniques have been described: volume displacement and volume replacement.
With the volume displacement technique, the surgeon uses adjacent tissue advancement to fill the lumpectomy cavity with the patient’s own surrounding breast tissue (FIGURE 3). The volume replacement technique requires the transposition of autologous tissue from elsewhere in the body.
Oncoplastic lumpectomy allows more women with larger tumors to undergo breast conservation with better cosmetic results. It reduces the number of mastectomies performed without compromising local control and avoids the need for extensive plastic surgery reconstruction and implants. Special effort and attention must be paid to ensure adequate margins utilizing intraoperative specimen radiograph and pathology evaluation.
This procedure requires that the surgeon acquire specialized skills and knowledge of oncologic and plastic surgery techniques, and it is best performed with the collaboration of a multidisciplinary team. Compared with conventional lumpectomy or mastectomy, oncoplastic breast conservation has been shown to reduce re-excision rates, and it has similar rates of local and distant recurrence and similar disease-free survival and overall survival.8,9
Total skin- and nipple-sparing mastectomy
Some patients do not have the option of breast conservation. Women with multicentric breast cancer (more than 1 tumor in different quadrants of the breast) are better served with mastectomy. Surgical techniques for mastectomy have improved and provide women with various options. One option is skin- and nipple-sparing mastectomy, which preserves the skin envelope overlying the breast (including the skin of the nipple and areola) while removing the glandular elements of the breast and the majority of ductal tissue beneath the nipple-areola complex (FIGURE 4). This surgery can be performed via hidden scars at the inframammary crease or periareolar and is combined with immediate reconstruction, which provides an excellent cosmetic result.
Surgical considerations include removing glandular breast tissue within its anatomic boundaries while maintaining the blood supply to the skin and nipple-areola complex. Furthermore, there must be close dissection of ductal tissue beneath the nipple-areola complex and intraoperative frozen section of the nipple margin in cancer cases. Nipple-sparing mastectomy is oncologically safe in carefully selected patients who do not have cancer near or within the skin or nipple (eg, Paget disease).10 It is also safe as a prophylactic procedure for patients with genetic mutations, such as BRCA1 and BRCA2.11 The procedure is not ideal for smokers or patients with large, pendulous breasts. There is a 3% risk of breast cancer recurrence at the nipple or in the skin or muscle.10 Surgical complications include a 10% to 20% risk of skin or nipple necrosis.12
How do we manage the lymph nodes: Axillary dissection vs sentinel node biopsy?
Evaluation of the axillary nodes is currently part of breast cancer staging and can help the clinician determine the need for adjuvant chemotherapy. It also may assist in assessing the need for extending the radiation field beyond the breast to include the regional lymph nodes. Patients with early stage (stage I and II) breast cancer who do not have abnormal palpable lymph nodes or biopsy-proven metastasis to axillary nodes qualify for sentinel lymph node (SLN) biopsy.
Sentinel node biopsy = less morbidity with no loss of accuracy. Compared with axillary lymph node dissection (ALND; removing all the level I and II nodes in the axilla), SLN biopsy has a 98% accuracy and is associated with less morbidity from lymphedema. The procedure involves injecting the breast with 2 tracers: a radioactive isotope, injected into the breast within 24 hours of the operation, and isosulfan blue dye, injected into the breast in the OR at the time of surgery (see illustration). Both tracers travel through the breast lymphatics and concentrate in the first few lymph nodes that drain the breast. The surgery is performed through a separate axillary incision, and the blue and radioactive lymph nodes are individually dissected and removed for pathologic evaluation. On average, 2 to 4 sentinel nodes are removed, including any suspicious palpable nodes. In experienced hands, this procedure has a false-negative rate of less than 5% to 10%.13
Axillary node dissection no longer standard of care. The indication for a completion ALND has changed based on the results of the randomized trial, ACOSOG Z0011.14 In this trial, patients with early stage breast cancer and 1 to 2 positive SLNs who were undergoing breast conservation therapy with radiation and adjuvant systemic therapy were randomly assigned to ALND or no ALND. (The trial did not include patients who were undergoing mastectomy, neoadjuvant chemotherapy, or who had more than 2 metastatic lymph nodes.) The investigators found no difference in overall or disease-free survival or local-regional recurrence between the 2 treatment groups over 9.2 years of follow up.14
Based on this practice-changing trial result, guidelines of the National Comprehensive Cancer Network no longer recommend completion ALND for patients who meet the ACOSOG Z0011 criteria. For patients who do not meet ACOSOG Z0011 criteria, we do intraoperative pathologic lymph node assessment with either frozen section or imprint cytology, and we perform immediate ALND when results are positive.
Indications for SLN biopsy include:
- invasive breast cancer with clinically negative axillary nodes
- ductal carcinoma in situ (DCIS) with microinvasion or extensive enough to require mastectomy
- clinically negative axillary nodes after neoadjuvant chemotherapy.
Contraindications for SLN biopsy include:
- bulky palpable lymphadenopathy
- pregnancy, as the safety of radioactive isotope and blue dye is not well studied; in isotope mapping the radiation dose is small and within safety limits for pregnant patients
- inflammatory breast cancer.
Complications of any axillary surgery may include risk of lymphedema (5% with SLN biopsy and 30% to 40% with ALND).15 Other complications include neuropathy of the affected arm with chronic pain and numbness of the skin.
Positive trends: Improved patient outcomes, specialized clinician training
Management of breast cancer has changed dramatically over the past several decades. More women are surviving breast cancer thanks to improvements in early detection, an individualized treatment approach with less aggressive surgery, and more effective targeted systemic therapies. A multidisciplinary, team-oriented approach with emphasis on minimally invasive biopsy and better cosmetic outcomes has enhanced quality of care.
Complexity in breast disease management has led to the development of formal fellowship training in breast surgical oncology. Studies have demonstrated that patients treated by high-volume breast surgeons are more satisfied with their care and have improved cancer outcomes.16,17 Women should be aware that they have different options for their breast cancer care, and surgeons with advanced specialization in this field may provide optimal results and better quality of care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Nichols HB, Berrington de Gonzalez A, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29(12):1564–1569.
- Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer [published online ahead of print March 8, 2016]. Ann Surg. doi:10.1097/SLA.0000000000001698.
- Miller ME, Czechura T, Martz B, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Ann Surg Oncol. 2013;20(13):4113–4120.
- Zhang X, Zhang XJ, Zhang TY, et al. Effect and safety of dual anti-human epidermal growth factor receptor 2 therapy compared to monotherapy in patients with human epidermal growth factor receptor 2-positive breast cancer: a systematic review. BMC Cancer. 2014;14:625.
- Ahmed M, Rubio IT, Klaase JM, Douek M. Surgical treatment of nonpalpable primary invasive and in situ breast cancer. Nat Rev Clin Oncol. 2015;12(11):645–663.
- Ahmed M, Douek M. Intra-operative ultrasound versus wire-guided localization in the surgical management of non-palpable breast cancers: systemic review and meta-analysis. Breast Cancer Res Treat. 2013;140(3):435–446.
- Krekel NM, Haloua MH, Lopes Cardozo AM, et al. Intraoperative ultrasound guidance for palpable breast cancer excision (COBALT trial): a multicentre, randomised controlled trial. Lancet Oncol. 2013;14(1):48–54.
- Chakravorty A, Shrestha AK, Sanmugalingam N, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol. 2012;38(5):395–398.
- De Lorenzi F, Hubner G, Rotmensz N, et al. Oncological results of oncoplastic breast-conserving surgery: long term follow-up of a large series at a single institution: a matched-cohort analysis. Eur J Surg Oncol. 2016;42(1):71–77.
- De La Cruz L, Moody AM, Tappy EE, Blankenship AA, Hecht EM. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systematic review. Ann Surg Oncol. 2015;22(10):3241–3249.
- Yao K, Liederbach E, Tang R, et al. Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and review of the literature. Ann Surg Oncol. 2015;22(2):370–376.
- Fortunato L, Loreti A, Andrich R, et al. When mastectomy is needed: is the nipple-sparing procedure a new standard with very few contraindications? J Surg Oncol. 2013;108(4):207–212.
- Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251(4):595–600.
- Giuliano AE, Hunt K, Ballman KV, et al. Ten-year survival results of ACOSOG Z0011: a randomized trial of axillary node dissection in women with clinical T1-2 N0 M0 breast cancer who have a positive sentinel node (Alliance). In: 2016 ASCO Annual Meeting; June 3-7, 2016. J Clin Oncol. 2016;34(15; May 20 suppl): Abstract 1007.
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515.
- Skinner KA, Helsper JT, Deapen D, Ye W, Sposto R. Breast cancer: do specialists make a difference? Ann Surg Oncol. 2003;10(6):606–615.
- Waljee JF, Hawley S, Alderman AK, Morrow M, Katz SJ. Patient satisfaction with treatment of breast cancer: does surgeon specialization matter? J Clin Oncol. 2007;25(24):3694–3698.
In a striking trend, the rate of contralateral prophylactic mastectomy (CPM) has risen by 30% over the last 10 years in the United States.1 Many women undergo CPM because of the fear and anxiety of cancer recurrence and their perceived risk of contralateral breast cancer; however, few women have a medical condition that necessitates removal of the contralateral breast. The medical indications for CPM include having a pathogenic genetic mutation (eg, BRCA1 and BRCA2), a strong family history of breast cancer, or prior mediastina chest radiation.
The actual risk of contralateral breast cancer is much lower than perceived. In women without a genetic mutation, the 10-year risk of contralateral breast cancer is only 3% to 5%.1 Also, CPM does not prevent the development of metastatic disease and offers no survival benefit over breast conservation or unilateral mastectomy.2 Furthermore, compared with unilateral therapeutic mastectomy, the “upgrade” to a CPM carries a 2.7-fold risk of a major surgical complication.3 It is therefore important that patients receive appropriate counseling regarding CPM, and that this counseling include cancer stage at diagnosis, family history and genetic risk, and cancer versus surgical risk (see “Counseling patients on contralateral prophylactic mastectomy” for key points to cover in patient discussions).
Counseling patients on contralateral prophylactic mastectomy
Commonly, patients diagnosed with breast cancer consider having their contralateral healthy breast removed as part of a bilateral mastectomy. They often experience severe anxiety about the cancer coming back and believe that removing both breasts will enable them to live longer. Keep the following key facts in mind when discussing treatment options with breast cancer patients.
Cancer stage at diagnosis. How long a patient lives from the time of her breast cancer diagnosis depends on the stage of the cancer at diagnosis, not the type of surgery performed. A woman with early stage I or stage II breast cancer has an 80% to 90% chance of being cancer free in 5 years.1 The chance of cancer recurring in the bones, liver, or lungs (metastatic breast cancer) will not be changed by removing the healthy breast. The risk of metastatic recurrence can be reduced, however, with chemotherapy and/or with hormone-blocker therapy.
Family history and genetic risk. Few women have a strong family history of breast and/or ovarian and other cancers, and this issue should be addressed with genetic counseling and testing prior to surgery. Those who carry a cancer-causing gene, such as BRCA1 or BRCA2, are at increased risk (40% to 60%) for a second or third breast cancer, especially if they are diagnosed at a young age (<50 years).2,3 In women who have a genetic mutation, removing both breasts and sometimes the ovaries can prevent development of another breast cancer. But this will not prevent spread of the cancer that is already present. Only chemotherapy and hormone blockers can prevent the spread of cancer.
Cancer risk versus surgical risk. For women with no family history of breast cancer, no genetic mutation, and no prior chest wall radiation, the risk of developing a new breast cancer in their other breast is only 3% to 5% every 10 years.3,4 This means that they have a 95% chance of not developing a new breast cancer in their healthy breast. Notably, removing the healthy breast can double the risk of postsurgical complications, including bleeding, infection, and loss of tissue and implant. The mastectomy site will be numb and the skin and nipple areola will not have any function other than cosmetic. Finally, wound complications from surgery could delay the start of important cancer treatment, such as chemotherapy or radiation.
The bottom line. Unless a woman has a strong family history of breast cancer, is diagnosed at a very young age, or has a genetic cancer-causing mutation, removing the contralateral healthy breast is not medically necessary and is not routinely recommended.
References
- Hennigs A, Riedel F, Gondos A, et al. Prognosis of breast cancer molecular subtypes in routine clinical care: a large prospective cohort study. BMC Cancer. 2016;16(1):734.
- Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27(35):5887–5992.
- Curtis RE, Ron E, Hankey BF, Hoover RN. New malignancies following breast cancer. In: Curtis RE, Freedman DM, Ron E, et al, eds. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: National Cancer Institute. NIH Publ. No. 05-5302. 2006:181–205. http://seer.cancer.gov/archive/publications/mpmono. Accessed September 18, 2016.
- Nichols HB, Berrington de Gonzalez A, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29(12):1564–1569.
Women should be made aware that there are alternatives to mastectomy that have similar, or even better, outcomes with improved quality of life. Furthermore, a multi‑disciplinary, team-oriented approach with emphasis on minimally invasive biopsy and better cosmetic outcomes has enhanced quality of care. Knowledge of this team approach and of modern breast cancer treatments is essential for general ObGyns as this understanding improves the overall care and guidance—specifically regarding referral to expert, high-volume breast surgeons—provided to those women most in need.
Expanded treatment options for breast cancer
Advancements in breast surgery, better imaging, and targeted therapies are changing the paradigm of breast cancer treatment.
Image-guided biopsy is key in decision making
When an abnormality is found in the breast, surgical excision of an undiagnosed breast lesion is no longer considered an appropriate first step. Use of image-guided biopsy or minimally invasive core needle biopsy allows for accurate diagnosis of a breast lesion while avoiding a potentially breast deforming and expensive surgical operation. It is always better to go into the operating room (OR) with a diagnosis and do the right operation the first time.
A core needle biopsy, results of which demonstrate a benign lesion, helps avoid breast surgery in women who do not need it. If cancer is diagnosed on biopsy, the extent of disease can be better evaluated and decision making can be more informed, with a multidisciplinary approach used to consider the various options, including genetic counseling, plastic surgery consultation, or neoadjuvant therapy. Some lesions, such as those too close to the skin, chest wall, or an implant, may not be amenable to core needle biopsy and therefore require surgical excision for diagnosis.
Benefits of a multidisciplinary tumor conference
It is important for a multidisciplinary group of cancer specialists to review a patient’s case and discuss the ideal treatment plan prior to surgery. Some breast cancer subtypes (such as human epidermal growth factor receptor 2 [HER2]–overamplified breast cancer and many triple-negative breast cancers) are very sensitive to chemotherapy, and patients with these tumor types may benefit from receiving neoadjuvant chemotherapy prior to surgery. New types of chemotherapy may allow up to 60% of some breast cancers to diminish almost completely, with subsequent improved cosmetic results of breast surgery.4 It may also allow time for genetic counseling and testing prior to surgery. (See “How to code for a multidisciplinary tumor conference” for appropriate coding procedure.)
How to code for a multidisciplinary tumor conference
Melanie Witt, RN, MA
There are two coding choices for team conferences involving physician participation. If the patient and/or family is present, the CPT instruction is to bill a problem E/M service code (99201-99215) based on the time spent during this coordination of care/counseling. Documentation would include details about the conference decisions and implications for care, rather than history or examination.
If the patient is not present, report 99367 (Medical team conference with interdisciplinary team of health care professionals, patient and/or family not present, 30 minutes or more; participation by physician), but note that this code was developed under the assumption that the conference would be performed in a facility setting. Diagnostic coding would be breast cancer.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
This is an excerpt from a companion coding resource for breast cancer–related procedures by Ms. Witt. To read the companion article, “Coding for breast cancer–related procedures: A how-to guide,” in its entirety, click here.
Image-guided lumpectomy
Advances in breast imaging have led to increased identification of nonpalpable breast cancers. Surgical excision of nonpalpable breast lesions requires image guidance, which can be done using a variety of techniques.
Wire-guided localization (WGL) has been used in practice for the past 40 years. The procedure involves placement of a hooked wire under local anesthesia using either mammographic or ultrasound guidance. This procedure is mostly done in the radiology department on the same day as the surgery and requires that the radiologist coordinate with the OR schedule. Besides scheduling conflicts and delays in surgery, this procedure can be complicated by wires becoming dislodged, transected, or migrated, and limits the surgeon’s ability to cosmetically hide the scar in relation to position of the wire. It is uncomfortable for the patient, who must be transported from the radiology department to the OR with a wire extruding from her breast.
An alternative localization technique is placement of a radioactive source within the tumor, which can then be identified in the OR with a gamma probe.
Iodine I 125 Radioactive seed localization (RSL) involves placing a 4-mm titanium radiolabeled seed into the breast lesion under mammographic or ultrasound guidance (FIGURES 1 and 2). The procedure can be performed a few days before surgery in the radiology department, and there is less chance for the seed to become displaced or dislodged. This technique provides scheduling flexibility for the radiologist and reduces OR delays. The surgeon uses the same gamma probe for sentinel node biopsy to find the lesion in the breast, using the setting specific for iodine I 125. Incisions can be tailored anywhere in the breast, and the seed is detected by a focal gamma signal. Once the lumpectomy is performed, the specimen is probed and radiographed to confirm removal of the seed and adequate margins.


Limitations of this procedure include potential loss of the seed during the operation and radiation safety issues regarding handling and disposal of the radioactive isotope. Once the seed has been placed in the patient’s body, it must be removed surgically, as the half-life of iodine I 125 is long (60 days).5 Care must therefore be taken to optimize medical clearance prior to seed placement and to avoid surgery cancellations.
Intraoperative ultrasound (IOUS) allows the surgeon to identify the lesion under general anesthesia in the OR, which is more comfortable for the patient. The surgical incision can be tailored cosmetically and the lumpectomy can be performed with real-time ultrasound visualization of the tumor during dissection. This technique eliminates the need for a separate preoperative seed or wire localization in radiology. However, it can be used only for lesions or clips that are visible by ultrasound. The excised specimen can be evaluated for confirmation of tumor removal and adequate margins via ultrasound and re-excision of close margins can be accomplished immediately if needed.
Results of a meta-analysis of WGL versus IOUS demonstrated a significant reduction of positive margins with the use of IOUS.6 Results of the COBALT trial, in which patients were assigned randomly to excision of palpable breast cancers with either IOUS or palpation, demonstrated a 14% reduction in positive margins in favor of IOUS.7 Surgeon-performed breast ultrasound requires advanced training and accreditation in breast ultrasound through a rigorous certification process offered by the American Society of Breast Surgeons (www.breastsurgeons.org).
Oncoplastic lumpectomy
This approach to lumpectomy combines adequate oncologist resection of the breast tumor with plastic surgery techniques to achieve superior cosmesis. This approach allows complete removal of the tumor with negative margins, yet maintains the normal shape and contour of the breast. Two techniques have been described: volume displacement and volume replacement.
With the volume displacement technique, the surgeon uses adjacent tissue advancement to fill the lumpectomy cavity with the patient’s own surrounding breast tissue (FIGURE 3). The volume replacement technique requires the transposition of autologous tissue from elsewhere in the body.

Oncoplastic lumpectomy allows more women with larger tumors to undergo breast conservation with better cosmetic results. It reduces the number of mastectomies performed without compromising local control and avoids the need for extensive plastic surgery reconstruction and implants. Special effort and attention must be paid to ensure adequate margins utilizing intraoperative specimen radiograph and pathology evaluation.
This procedure requires that the surgeon acquire specialized skills and knowledge of oncologic and plastic surgery techniques, and it is best performed with the collaboration of a multidisciplinary team. Compared with conventional lumpectomy or mastectomy, oncoplastic breast conservation has been shown to reduce re-excision rates, and it has similar rates of local and distant recurrence and similar disease-free survival and overall survival.8,9
Total skin- and nipple-sparing mastectomy
Some patients do not have the option of breast conservation. Women with multicentric breast cancer (more than 1 tumor in different quadrants of the breast) are better served with mastectomy. Surgical techniques for mastectomy have improved and provide women with various options. One option is skin- and nipple-sparing mastectomy, which preserves the skin envelope overlying the breast (including the skin of the nipple and areola) while removing the glandular elements of the breast and the majority of ductal tissue beneath the nipple-areola complex (FIGURE 4). This surgery can be performed via hidden scars at the inframammary crease or periareolar and is combined with immediate reconstruction, which provides an excellent cosmetic result.

Surgical considerations include removing glandular breast tissue within its anatomic boundaries while maintaining the blood supply to the skin and nipple-areola complex. Furthermore, there must be close dissection of ductal tissue beneath the nipple-areola complex and intraoperative frozen section of the nipple margin in cancer cases. Nipple-sparing mastectomy is oncologically safe in carefully selected patients who do not have cancer near or within the skin or nipple (eg, Paget disease).10 It is also safe as a prophylactic procedure for patients with genetic mutations, such as BRCA1 and BRCA2.11 The procedure is not ideal for smokers or patients with large, pendulous breasts. There is a 3% risk of breast cancer recurrence at the nipple or in the skin or muscle.10 Surgical complications include a 10% to 20% risk of skin or nipple necrosis.12
How do we manage the lymph nodes: Axillary dissection vs sentinel node biopsy?
Evaluation of the axillary nodes is currently part of breast cancer staging and can help the clinician determine the need for adjuvant chemotherapy. It also may assist in assessing the need for extending the radiation field beyond the breast to include the regional lymph nodes. Patients with early stage (stage I and II) breast cancer who do not have abnormal palpable lymph nodes or biopsy-proven metastasis to axillary nodes qualify for sentinel lymph node (SLN) biopsy.
Sentinel node biopsy = less morbidity with no loss of accuracy. Compared with axillary lymph node dissection (ALND; removing all the level I and II nodes in the axilla), SLN biopsy has a 98% accuracy and is associated with less morbidity from lymphedema. The procedure involves injecting the breast with 2 tracers: a radioactive isotope, injected into the breast within 24 hours of the operation, and isosulfan blue dye, injected into the breast in the OR at the time of surgery (see illustration). Both tracers travel through the breast lymphatics and concentrate in the first few lymph nodes that drain the breast. The surgery is performed through a separate axillary incision, and the blue and radioactive lymph nodes are individually dissected and removed for pathologic evaluation. On average, 2 to 4 sentinel nodes are removed, including any suspicious palpable nodes. In experienced hands, this procedure has a false-negative rate of less than 5% to 10%.13

Axillary node dissection no longer standard of care. The indication for a completion ALND has changed based on the results of the randomized trial, ACOSOG Z0011.14 In this trial, patients with early stage breast cancer and 1 to 2 positive SLNs who were undergoing breast conservation therapy with radiation and adjuvant systemic therapy were randomly assigned to ALND or no ALND. (The trial did not include patients who were undergoing mastectomy, neoadjuvant chemotherapy, or who had more than 2 metastatic lymph nodes.) The investigators found no difference in overall or disease-free survival or local-regional recurrence between the 2 treatment groups over 9.2 years of follow up.14
Based on this practice-changing trial result, guidelines of the National Comprehensive Cancer Network no longer recommend completion ALND for patients who meet the ACOSOG Z0011 criteria. For patients who do not meet ACOSOG Z0011 criteria, we do intraoperative pathologic lymph node assessment with either frozen section or imprint cytology, and we perform immediate ALND when results are positive.
Indications for SLN biopsy include:
- invasive breast cancer with clinically negative axillary nodes
- ductal carcinoma in situ (DCIS) with microinvasion or extensive enough to require mastectomy
- clinically negative axillary nodes after neoadjuvant chemotherapy.
Contraindications for SLN biopsy include:
- bulky palpable lymphadenopathy
- pregnancy, as the safety of radioactive isotope and blue dye is not well studied; in isotope mapping the radiation dose is small and within safety limits for pregnant patients
- inflammatory breast cancer.
Complications of any axillary surgery may include risk of lymphedema (5% with SLN biopsy and 30% to 40% with ALND).15 Other complications include neuropathy of the affected arm with chronic pain and numbness of the skin.
Positive trends: Improved patient outcomes, specialized clinician training
Management of breast cancer has changed dramatically over the past several decades. More women are surviving breast cancer thanks to improvements in early detection, an individualized treatment approach with less aggressive surgery, and more effective targeted systemic therapies. A multidisciplinary, team-oriented approach with emphasis on minimally invasive biopsy and better cosmetic outcomes has enhanced quality of care.
Complexity in breast disease management has led to the development of formal fellowship training in breast surgical oncology. Studies have demonstrated that patients treated by high-volume breast surgeons are more satisfied with their care and have improved cancer outcomes.16,17 Women should be aware that they have different options for their breast cancer care, and surgeons with advanced specialization in this field may provide optimal results and better quality of care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In a striking trend, the rate of contralateral prophylactic mastectomy (CPM) has risen by 30% over the last 10 years in the United States.1 Many women undergo CPM because of the fear and anxiety of cancer recurrence and their perceived risk of contralateral breast cancer; however, few women have a medical condition that necessitates removal of the contralateral breast. The medical indications for CPM include having a pathogenic genetic mutation (eg, BRCA1 and BRCA2), a strong family history of breast cancer, or prior mediastina chest radiation.
The actual risk of contralateral breast cancer is much lower than perceived. In women without a genetic mutation, the 10-year risk of contralateral breast cancer is only 3% to 5%.1 Also, CPM does not prevent the development of metastatic disease and offers no survival benefit over breast conservation or unilateral mastectomy.2 Furthermore, compared with unilateral therapeutic mastectomy, the “upgrade” to a CPM carries a 2.7-fold risk of a major surgical complication.3 It is therefore important that patients receive appropriate counseling regarding CPM, and that this counseling include cancer stage at diagnosis, family history and genetic risk, and cancer versus surgical risk (see “Counseling patients on contralateral prophylactic mastectomy” for key points to cover in patient discussions).
Counseling patients on contralateral prophylactic mastectomy
Commonly, patients diagnosed with breast cancer consider having their contralateral healthy breast removed as part of a bilateral mastectomy. They often experience severe anxiety about the cancer coming back and believe that removing both breasts will enable them to live longer. Keep the following key facts in mind when discussing treatment options with breast cancer patients.
Cancer stage at diagnosis. How long a patient lives from the time of her breast cancer diagnosis depends on the stage of the cancer at diagnosis, not the type of surgery performed. A woman with early stage I or stage II breast cancer has an 80% to 90% chance of being cancer free in 5 years.1 The chance of cancer recurring in the bones, liver, or lungs (metastatic breast cancer) will not be changed by removing the healthy breast. The risk of metastatic recurrence can be reduced, however, with chemotherapy and/or with hormone-blocker therapy.
Family history and genetic risk. Few women have a strong family history of breast and/or ovarian and other cancers, and this issue should be addressed with genetic counseling and testing prior to surgery. Those who carry a cancer-causing gene, such as BRCA1 or BRCA2, are at increased risk (40% to 60%) for a second or third breast cancer, especially if they are diagnosed at a young age (<50 years).2,3 In women who have a genetic mutation, removing both breasts and sometimes the ovaries can prevent development of another breast cancer. But this will not prevent spread of the cancer that is already present. Only chemotherapy and hormone blockers can prevent the spread of cancer.
Cancer risk versus surgical risk. For women with no family history of breast cancer, no genetic mutation, and no prior chest wall radiation, the risk of developing a new breast cancer in their other breast is only 3% to 5% every 10 years.3,4 This means that they have a 95% chance of not developing a new breast cancer in their healthy breast. Notably, removing the healthy breast can double the risk of postsurgical complications, including bleeding, infection, and loss of tissue and implant. The mastectomy site will be numb and the skin and nipple areola will not have any function other than cosmetic. Finally, wound complications from surgery could delay the start of important cancer treatment, such as chemotherapy or radiation.
The bottom line. Unless a woman has a strong family history of breast cancer, is diagnosed at a very young age, or has a genetic cancer-causing mutation, removing the contralateral healthy breast is not medically necessary and is not routinely recommended.
References
- Hennigs A, Riedel F, Gondos A, et al. Prognosis of breast cancer molecular subtypes in routine clinical care: a large prospective cohort study. BMC Cancer. 2016;16(1):734.
- Graeser MK, Engel C, Rhiem K, et al. Contralateral breast cancer risk in BRCA1 and BRCA2 mutation carriers. J Clin Oncol. 2009;27(35):5887–5992.
- Curtis RE, Ron E, Hankey BF, Hoover RN. New malignancies following breast cancer. In: Curtis RE, Freedman DM, Ron E, et al, eds. New Malignancies Among Cancer Survivors: SEER Cancer Registries, 1973-2000. Bethesda, MD: National Cancer Institute. NIH Publ. No. 05-5302. 2006:181–205. http://seer.cancer.gov/archive/publications/mpmono. Accessed September 18, 2016.
- Nichols HB, Berrington de Gonzalez A, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29(12):1564–1569.
Women should be made aware that there are alternatives to mastectomy that have similar, or even better, outcomes with improved quality of life. Furthermore, a multi‑disciplinary, team-oriented approach with emphasis on minimally invasive biopsy and better cosmetic outcomes has enhanced quality of care. Knowledge of this team approach and of modern breast cancer treatments is essential for general ObGyns as this understanding improves the overall care and guidance—specifically regarding referral to expert, high-volume breast surgeons—provided to those women most in need.
Expanded treatment options for breast cancer
Advancements in breast surgery, better imaging, and targeted therapies are changing the paradigm of breast cancer treatment.
Image-guided biopsy is key in decision making
When an abnormality is found in the breast, surgical excision of an undiagnosed breast lesion is no longer considered an appropriate first step. Use of image-guided biopsy or minimally invasive core needle biopsy allows for accurate diagnosis of a breast lesion while avoiding a potentially breast deforming and expensive surgical operation. It is always better to go into the operating room (OR) with a diagnosis and do the right operation the first time.
A core needle biopsy, results of which demonstrate a benign lesion, helps avoid breast surgery in women who do not need it. If cancer is diagnosed on biopsy, the extent of disease can be better evaluated and decision making can be more informed, with a multidisciplinary approach used to consider the various options, including genetic counseling, plastic surgery consultation, or neoadjuvant therapy. Some lesions, such as those too close to the skin, chest wall, or an implant, may not be amenable to core needle biopsy and therefore require surgical excision for diagnosis.
Benefits of a multidisciplinary tumor conference
It is important for a multidisciplinary group of cancer specialists to review a patient’s case and discuss the ideal treatment plan prior to surgery. Some breast cancer subtypes (such as human epidermal growth factor receptor 2 [HER2]–overamplified breast cancer and many triple-negative breast cancers) are very sensitive to chemotherapy, and patients with these tumor types may benefit from receiving neoadjuvant chemotherapy prior to surgery. New types of chemotherapy may allow up to 60% of some breast cancers to diminish almost completely, with subsequent improved cosmetic results of breast surgery.4 It may also allow time for genetic counseling and testing prior to surgery. (See “How to code for a multidisciplinary tumor conference” for appropriate coding procedure.)
How to code for a multidisciplinary tumor conference
Melanie Witt, RN, MA
There are two coding choices for team conferences involving physician participation. If the patient and/or family is present, the CPT instruction is to bill a problem E/M service code (99201-99215) based on the time spent during this coordination of care/counseling. Documentation would include details about the conference decisions and implications for care, rather than history or examination.
If the patient is not present, report 99367 (Medical team conference with interdisciplinary team of health care professionals, patient and/or family not present, 30 minutes or more; participation by physician), but note that this code was developed under the assumption that the conference would be performed in a facility setting. Diagnostic coding would be breast cancer.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
This is an excerpt from a companion coding resource for breast cancer–related procedures by Ms. Witt. To read the companion article, “Coding for breast cancer–related procedures: A how-to guide,” in its entirety, click here.
Image-guided lumpectomy
Advances in breast imaging have led to increased identification of nonpalpable breast cancers. Surgical excision of nonpalpable breast lesions requires image guidance, which can be done using a variety of techniques.
Wire-guided localization (WGL) has been used in practice for the past 40 years. The procedure involves placement of a hooked wire under local anesthesia using either mammographic or ultrasound guidance. This procedure is mostly done in the radiology department on the same day as the surgery and requires that the radiologist coordinate with the OR schedule. Besides scheduling conflicts and delays in surgery, this procedure can be complicated by wires becoming dislodged, transected, or migrated, and limits the surgeon’s ability to cosmetically hide the scar in relation to position of the wire. It is uncomfortable for the patient, who must be transported from the radiology department to the OR with a wire extruding from her breast.
An alternative localization technique is placement of a radioactive source within the tumor, which can then be identified in the OR with a gamma probe.
Iodine I 125 Radioactive seed localization (RSL) involves placing a 4-mm titanium radiolabeled seed into the breast lesion under mammographic or ultrasound guidance (FIGURES 1 and 2). The procedure can be performed a few days before surgery in the radiology department, and there is less chance for the seed to become displaced or dislodged. This technique provides scheduling flexibility for the radiologist and reduces OR delays. The surgeon uses the same gamma probe for sentinel node biopsy to find the lesion in the breast, using the setting specific for iodine I 125. Incisions can be tailored anywhere in the breast, and the seed is detected by a focal gamma signal. Once the lumpectomy is performed, the specimen is probed and radiographed to confirm removal of the seed and adequate margins.


Limitations of this procedure include potential loss of the seed during the operation and radiation safety issues regarding handling and disposal of the radioactive isotope. Once the seed has been placed in the patient’s body, it must be removed surgically, as the half-life of iodine I 125 is long (60 days).5 Care must therefore be taken to optimize medical clearance prior to seed placement and to avoid surgery cancellations.
Intraoperative ultrasound (IOUS) allows the surgeon to identify the lesion under general anesthesia in the OR, which is more comfortable for the patient. The surgical incision can be tailored cosmetically and the lumpectomy can be performed with real-time ultrasound visualization of the tumor during dissection. This technique eliminates the need for a separate preoperative seed or wire localization in radiology. However, it can be used only for lesions or clips that are visible by ultrasound. The excised specimen can be evaluated for confirmation of tumor removal and adequate margins via ultrasound and re-excision of close margins can be accomplished immediately if needed.
Results of a meta-analysis of WGL versus IOUS demonstrated a significant reduction of positive margins with the use of IOUS.6 Results of the COBALT trial, in which patients were assigned randomly to excision of palpable breast cancers with either IOUS or palpation, demonstrated a 14% reduction in positive margins in favor of IOUS.7 Surgeon-performed breast ultrasound requires advanced training and accreditation in breast ultrasound through a rigorous certification process offered by the American Society of Breast Surgeons (www.breastsurgeons.org).
Oncoplastic lumpectomy
This approach to lumpectomy combines adequate oncologist resection of the breast tumor with plastic surgery techniques to achieve superior cosmesis. This approach allows complete removal of the tumor with negative margins, yet maintains the normal shape and contour of the breast. Two techniques have been described: volume displacement and volume replacement.
With the volume displacement technique, the surgeon uses adjacent tissue advancement to fill the lumpectomy cavity with the patient’s own surrounding breast tissue (FIGURE 3). The volume replacement technique requires the transposition of autologous tissue from elsewhere in the body.

Oncoplastic lumpectomy allows more women with larger tumors to undergo breast conservation with better cosmetic results. It reduces the number of mastectomies performed without compromising local control and avoids the need for extensive plastic surgery reconstruction and implants. Special effort and attention must be paid to ensure adequate margins utilizing intraoperative specimen radiograph and pathology evaluation.
This procedure requires that the surgeon acquire specialized skills and knowledge of oncologic and plastic surgery techniques, and it is best performed with the collaboration of a multidisciplinary team. Compared with conventional lumpectomy or mastectomy, oncoplastic breast conservation has been shown to reduce re-excision rates, and it has similar rates of local and distant recurrence and similar disease-free survival and overall survival.8,9
Total skin- and nipple-sparing mastectomy
Some patients do not have the option of breast conservation. Women with multicentric breast cancer (more than 1 tumor in different quadrants of the breast) are better served with mastectomy. Surgical techniques for mastectomy have improved and provide women with various options. One option is skin- and nipple-sparing mastectomy, which preserves the skin envelope overlying the breast (including the skin of the nipple and areola) while removing the glandular elements of the breast and the majority of ductal tissue beneath the nipple-areola complex (FIGURE 4). This surgery can be performed via hidden scars at the inframammary crease or periareolar and is combined with immediate reconstruction, which provides an excellent cosmetic result.

Surgical considerations include removing glandular breast tissue within its anatomic boundaries while maintaining the blood supply to the skin and nipple-areola complex. Furthermore, there must be close dissection of ductal tissue beneath the nipple-areola complex and intraoperative frozen section of the nipple margin in cancer cases. Nipple-sparing mastectomy is oncologically safe in carefully selected patients who do not have cancer near or within the skin or nipple (eg, Paget disease).10 It is also safe as a prophylactic procedure for patients with genetic mutations, such as BRCA1 and BRCA2.11 The procedure is not ideal for smokers or patients with large, pendulous breasts. There is a 3% risk of breast cancer recurrence at the nipple or in the skin or muscle.10 Surgical complications include a 10% to 20% risk of skin or nipple necrosis.12
How do we manage the lymph nodes: Axillary dissection vs sentinel node biopsy?
Evaluation of the axillary nodes is currently part of breast cancer staging and can help the clinician determine the need for adjuvant chemotherapy. It also may assist in assessing the need for extending the radiation field beyond the breast to include the regional lymph nodes. Patients with early stage (stage I and II) breast cancer who do not have abnormal palpable lymph nodes or biopsy-proven metastasis to axillary nodes qualify for sentinel lymph node (SLN) biopsy.
Sentinel node biopsy = less morbidity with no loss of accuracy. Compared with axillary lymph node dissection (ALND; removing all the level I and II nodes in the axilla), SLN biopsy has a 98% accuracy and is associated with less morbidity from lymphedema. The procedure involves injecting the breast with 2 tracers: a radioactive isotope, injected into the breast within 24 hours of the operation, and isosulfan blue dye, injected into the breast in the OR at the time of surgery (see illustration). Both tracers travel through the breast lymphatics and concentrate in the first few lymph nodes that drain the breast. The surgery is performed through a separate axillary incision, and the blue and radioactive lymph nodes are individually dissected and removed for pathologic evaluation. On average, 2 to 4 sentinel nodes are removed, including any suspicious palpable nodes. In experienced hands, this procedure has a false-negative rate of less than 5% to 10%.13

Axillary node dissection no longer standard of care. The indication for a completion ALND has changed based on the results of the randomized trial, ACOSOG Z0011.14 In this trial, patients with early stage breast cancer and 1 to 2 positive SLNs who were undergoing breast conservation therapy with radiation and adjuvant systemic therapy were randomly assigned to ALND or no ALND. (The trial did not include patients who were undergoing mastectomy, neoadjuvant chemotherapy, or who had more than 2 metastatic lymph nodes.) The investigators found no difference in overall or disease-free survival or local-regional recurrence between the 2 treatment groups over 9.2 years of follow up.14
Based on this practice-changing trial result, guidelines of the National Comprehensive Cancer Network no longer recommend completion ALND for patients who meet the ACOSOG Z0011 criteria. For patients who do not meet ACOSOG Z0011 criteria, we do intraoperative pathologic lymph node assessment with either frozen section or imprint cytology, and we perform immediate ALND when results are positive.
Indications for SLN biopsy include:
- invasive breast cancer with clinically negative axillary nodes
- ductal carcinoma in situ (DCIS) with microinvasion or extensive enough to require mastectomy
- clinically negative axillary nodes after neoadjuvant chemotherapy.
Contraindications for SLN biopsy include:
- bulky palpable lymphadenopathy
- pregnancy, as the safety of radioactive isotope and blue dye is not well studied; in isotope mapping the radiation dose is small and within safety limits for pregnant patients
- inflammatory breast cancer.
Complications of any axillary surgery may include risk of lymphedema (5% with SLN biopsy and 30% to 40% with ALND).15 Other complications include neuropathy of the affected arm with chronic pain and numbness of the skin.
Positive trends: Improved patient outcomes, specialized clinician training
Management of breast cancer has changed dramatically over the past several decades. More women are surviving breast cancer thanks to improvements in early detection, an individualized treatment approach with less aggressive surgery, and more effective targeted systemic therapies. A multidisciplinary, team-oriented approach with emphasis on minimally invasive biopsy and better cosmetic outcomes has enhanced quality of care.
Complexity in breast disease management has led to the development of formal fellowship training in breast surgical oncology. Studies have demonstrated that patients treated by high-volume breast surgeons are more satisfied with their care and have improved cancer outcomes.16,17 Women should be aware that they have different options for their breast cancer care, and surgeons with advanced specialization in this field may provide optimal results and better quality of care.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
- Nichols HB, Berrington de Gonzalez A, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29(12):1564–1569.
- Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer [published online ahead of print March 8, 2016]. Ann Surg. doi:10.1097/SLA.0000000000001698.
- Miller ME, Czechura T, Martz B, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Ann Surg Oncol. 2013;20(13):4113–4120.
- Zhang X, Zhang XJ, Zhang TY, et al. Effect and safety of dual anti-human epidermal growth factor receptor 2 therapy compared to monotherapy in patients with human epidermal growth factor receptor 2-positive breast cancer: a systematic review. BMC Cancer. 2014;14:625.
- Ahmed M, Rubio IT, Klaase JM, Douek M. Surgical treatment of nonpalpable primary invasive and in situ breast cancer. Nat Rev Clin Oncol. 2015;12(11):645–663.
- Ahmed M, Douek M. Intra-operative ultrasound versus wire-guided localization in the surgical management of non-palpable breast cancers: systemic review and meta-analysis. Breast Cancer Res Treat. 2013;140(3):435–446.
- Krekel NM, Haloua MH, Lopes Cardozo AM, et al. Intraoperative ultrasound guidance for palpable breast cancer excision (COBALT trial): a multicentre, randomised controlled trial. Lancet Oncol. 2013;14(1):48–54.
- Chakravorty A, Shrestha AK, Sanmugalingam N, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol. 2012;38(5):395–398.
- De Lorenzi F, Hubner G, Rotmensz N, et al. Oncological results of oncoplastic breast-conserving surgery: long term follow-up of a large series at a single institution: a matched-cohort analysis. Eur J Surg Oncol. 2016;42(1):71–77.
- De La Cruz L, Moody AM, Tappy EE, Blankenship AA, Hecht EM. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systematic review. Ann Surg Oncol. 2015;22(10):3241–3249.
- Yao K, Liederbach E, Tang R, et al. Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and review of the literature. Ann Surg Oncol. 2015;22(2):370–376.
- Fortunato L, Loreti A, Andrich R, et al. When mastectomy is needed: is the nipple-sparing procedure a new standard with very few contraindications? J Surg Oncol. 2013;108(4):207–212.
- Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251(4):595–600.
- Giuliano AE, Hunt K, Ballman KV, et al. Ten-year survival results of ACOSOG Z0011: a randomized trial of axillary node dissection in women with clinical T1-2 N0 M0 breast cancer who have a positive sentinel node (Alliance). In: 2016 ASCO Annual Meeting; June 3-7, 2016. J Clin Oncol. 2016;34(15; May 20 suppl): Abstract 1007.
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515.
- Skinner KA, Helsper JT, Deapen D, Ye W, Sposto R. Breast cancer: do specialists make a difference? Ann Surg Oncol. 2003;10(6):606–615.
- Waljee JF, Hawley S, Alderman AK, Morrow M, Katz SJ. Patient satisfaction with treatment of breast cancer: does surgeon specialization matter? J Clin Oncol. 2007;25(24):3694–3698.
- Nichols HB, Berrington de Gonzalez A, Lacey JV Jr, Rosenberg PS, Anderson WF. Declining incidence of contralateral breast cancer in the United States from 1975 to 2006. J Clin Oncol. 2011;29(12):1564–1569.
- Wong SM, Freedman RA, Sagara Y, Aydogan F, Barry WT, Golshan M. Growing use of contralateral prophylactic mastectomy despite no improvement in long-term survival for invasive breast cancer [published online ahead of print March 8, 2016]. Ann Surg. doi:10.1097/SLA.0000000000001698.
- Miller ME, Czechura T, Martz B, et al. Operative risks associated with contralateral prophylactic mastectomy: a single institution experience. Ann Surg Oncol. 2013;20(13):4113–4120.
- Zhang X, Zhang XJ, Zhang TY, et al. Effect and safety of dual anti-human epidermal growth factor receptor 2 therapy compared to monotherapy in patients with human epidermal growth factor receptor 2-positive breast cancer: a systematic review. BMC Cancer. 2014;14:625.
- Ahmed M, Rubio IT, Klaase JM, Douek M. Surgical treatment of nonpalpable primary invasive and in situ breast cancer. Nat Rev Clin Oncol. 2015;12(11):645–663.
- Ahmed M, Douek M. Intra-operative ultrasound versus wire-guided localization in the surgical management of non-palpable breast cancers: systemic review and meta-analysis. Breast Cancer Res Treat. 2013;140(3):435–446.
- Krekel NM, Haloua MH, Lopes Cardozo AM, et al. Intraoperative ultrasound guidance for palpable breast cancer excision (COBALT trial): a multicentre, randomised controlled trial. Lancet Oncol. 2013;14(1):48–54.
- Chakravorty A, Shrestha AK, Sanmugalingam N, et al. How safe is oncoplastic breast conservation? Comparative analysis with standard breast conserving surgery. Eur J Surg Oncol. 2012;38(5):395–398.
- De Lorenzi F, Hubner G, Rotmensz N, et al. Oncological results of oncoplastic breast-conserving surgery: long term follow-up of a large series at a single institution: a matched-cohort analysis. Eur J Surg Oncol. 2016;42(1):71–77.
- De La Cruz L, Moody AM, Tappy EE, Blankenship AA, Hecht EM. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systematic review. Ann Surg Oncol. 2015;22(10):3241–3249.
- Yao K, Liederbach E, Tang R, et al. Nipple-sparing mastectomy in BRCA1/2 mutation carriers: an interim analysis and review of the literature. Ann Surg Oncol. 2015;22(2):370–376.
- Fortunato L, Loreti A, Andrich R, et al. When mastectomy is needed: is the nipple-sparing procedure a new standard with very few contraindications? J Surg Oncol. 2013;108(4):207–212.
- Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251(4):595–600.
- Giuliano AE, Hunt K, Ballman KV, et al. Ten-year survival results of ACOSOG Z0011: a randomized trial of axillary node dissection in women with clinical T1-2 N0 M0 breast cancer who have a positive sentinel node (Alliance). In: 2016 ASCO Annual Meeting; June 3-7, 2016. J Clin Oncol. 2016;34(15; May 20 suppl): Abstract 1007.
- DiSipio T, Rye S, Newman B, Hayes S. Incidence of unilateral arm lymphedema after breast cancer: a systematic review and meta-analysis. Lancet Oncol. 2013;14(6):500–515.
- Skinner KA, Helsper JT, Deapen D, Ye W, Sposto R. Breast cancer: do specialists make a difference? Ann Surg Oncol. 2003;10(6):606–615.
- Waljee JF, Hawley S, Alderman AK, Morrow M, Katz SJ. Patient satisfaction with treatment of breast cancer: does surgeon specialization matter? J Clin Oncol. 2007;25(24):3694–3698.
In this Article
- Counseling patients on CPM
- Oncoplastic lumpectomy approach
- How to manage the lymph nodes
Cesarean scar defect: What is it and how should it be treated?
Cesarean delivery is one of the most common surgical procedures in women, with rates of 30% or more in the United States.1 As a result, the rate is rising for cesarean scar defect—the presence of a “niche” at the site of cesarean delivery scar—with the reported prevalence between 24% and 70% in a random population of women with at least one cesarean delivery.2 Other terms for cesarean scar defect include a niche, isthmocele, uteroperitoneal fistula, and diverticulum.1–9
Formation of cesarean scar defect
Cesarean scar defect forms after cesarean delivery, at the site of hysterotomy, on the anterior wall of the uterine isthmus (FIGURE 1). While this is the typical location, the defect has also been found at the endocervical canal and mid-uterine body. Improper healing of the cesarean incision leads to thinning of the anterior uterine wall, which creates an indentation and fluid-filled pouch at the cesarean scar site. The exact reason why a niche develops has not yet been determined; however, there are several hypotheses, broken down by pregnancy-related and patient-related factors. Surgical techniques that may increase the chance of niche development include low (cervical) hysterotomy, single-layer uterine wall closure, use of locking sutures, closure of hysterotomy with endometrial-sparing technique, and multiple cesarean deliveries.3,4 Patients with medical conditions that may impact wound healing (such as diabetes and smoking) may be at increased risk for niche formation.

Viewed hysteroscopically, the defect appears as a concave shape in the anterior uterine wall; to the inexperienced eye, it may resemble a second cavity (FIGURE 2).
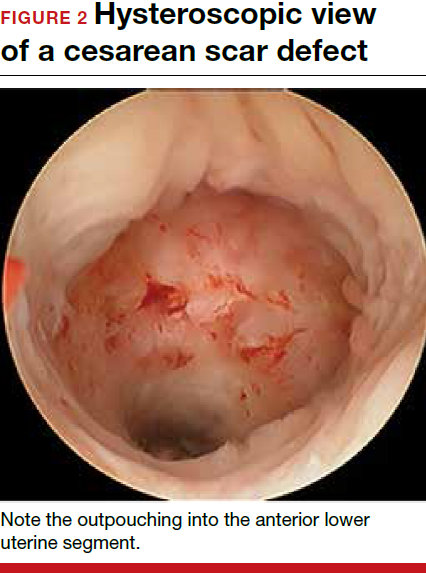
Pelvic pain and other serious consequences
The presence of fibrotic tissue in the niche acts like a valve, leading to the accumulation of blood in this reservoir-like area. A niche thus can cause delayed menstruation through the cervix, resulting in abnormal bleeding, pelvic pain, vaginal discharge, dysmenorrhea, dyspareunia, and infertility. Accumulated blood in this area can ultimately degrade cervical mucus and sperm quality, as well as inhibit sperm transport, a proposed mechanism of infertility.5,6 Women with a niche who conceive are at potential risk for cesarean scar ectopic pregnancy, with the embryo implanting in the pouch and subsequently growing and developing improperly.
Read about evaluation and treatment.
Evaluation and treatment
Patients presenting with the symptoms de-scribed above who have had a prior cesarean delivery should be evaluated for a cesarean scar defect.9 The best time to assess for the abnormality is after the patient’s menstrual cycle, when the endometrial lining is at its thinnest and recently menstruated blood has collected in the defect (this can highlight the niche on imaging). Transvaginal ultrasonography (FIGURE 3) or saline-infusion sonohysterogram serve as a first-line test for in-office diagnosis.7 Magnetic resonance imaging (MRI), 3-D ultrasonography, and hysteroscopy are additional useful imaging modalities that can aid in the diagnosis.
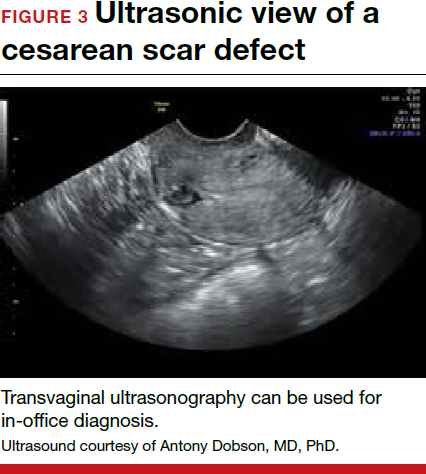
Treatments for cesarean scar defect vary dramatically and include hormonal therapy, hysteroscopic resection, vaginal or laparoscopic repair, and hysterectomy. Nonsurgical treatment should be reserved for women who desire a noninvasive approach, as the evidence for symptom resolution is limited.8
To promote fertility and decrease symptoms, the abnormal, fibrotic tissue must be removed. In our experience, since 2003, we have found that use of a laparoscopic approach is best for women desiring future fertility and that hysteroscopic resection is best for women whose childbearing is completed.9 Our management is dictated by the patient’s fertility plans, since there is concern that cesarean scar defect in a gravid uterus presents a risk for uterine rupture. The laparoscopic approach allows the defect to be repaired and the integrity of the myometrium restored.9
What are the coding options for cesarean scar defect repair?
Melanie Witt, RN, CPC, COBGC, MA
As the accompanying article discusses, the primary treatment for a cesarean scar defect depends on whether the patient wishes to preserve fertility, but assigning a procedure code for either surgical option will entail reporting an unlisted procedure code.
Under Current Procedural Terminology (CPT) guidelines (which are developed and copyrighted by the American Medical Association), procedure code selected must accurately describe the service/procedure performed rather than just approximate the service. This means that when a procedure-specific code does not exist, an unlisted procedure code that represents the type of surgery, the approach, and the anatomic site needs to be selected.
When an unlisted CPT code is reported, payment is based on the complexity of the surgery, and one way to communicate this to a payer is to provide additional documentation that not only includes the operative report but also suggests one or more existing CPT codes that have a published relative value unit (RVU) that approximates the work involved for the unlisted procedure.
The coding options for hysteroscopic and laparoscopic treatment options are listed below. The comparison codes offered will give the surgeon a range to look at, but the ultimate decision to use one of those suggested, or to choose an entirely different comparison code, is entirely within the control of the physician.
ICD-10-CM diagnostic coding
While the cesarean scar defect is a sequela of cesarean delivery, which is always reported as a secondary code, the choice of a primary diagnosis code can be either a gynecologic and/or an obstetric complication code. The choice may be determined by payer policy, as the use of an obstetric complication may not be accepted with a gynecologic procedure code. From a coding perspective, however, use of all 3 of these codes from the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) paints the most accurate description of the defect and its cause:
- N85.8 Other specified noninflammatory disorders of uterus versus
- O34.21 Maternal care for scar from previous cesarean delivery plus
- O94 Sequelae of complication of pregnancy, childbirth, and the puerperium.
Hysteroscopic resection codes:
- 58579 Unlisted hysteroscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58561 (Hysteroscopy, surgical; with removal of leiomyomata) with 15.48 RVUs or 58560 (Hysteroscopy, surgical; with division or resection of intrauterine septum [any method]) with 10.92 RVUs.
Laparoscopic repair codes:
- 58578 Unlisted laparoscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58520 (Hysterorrhaphy, repair of ruptured uterus [nonobstetrical] 24.25 RVUs or 58662 (Laparoscopy, surgical; with fulguration or excision of lesions of the ovary, pelvic viscera, or peritoneal surface by any method) with 20.14 RVUs.
You may also want to report a diagnostic hysteroscopy (code 58555), but keep in mind that payment will depend on documentation that clearly indicates that the use of the hysteroscope was for diagnostic purposes. Use of the hysteroscope to simply identify the surgical site to be repaired via the laparoscope will usually not be reimbursed separately.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
Read about techniques for repair.
Techniques for repairing cesarean scar defect
For hysteroscopic resection of a niche, the uterus is distended and the intrauterine defect is visualized hysteroscopically, as seen in FIGURE 2. Using a bipolar or unipolar resectoscope, resect the fibrotic tissue of the defect and endometrial-like glands present within the niche. The goal of this relatively quick procedure is to open up the reservoir and facilitate the complete drainage of menstrual blood, thus alleviating the patient’s symptoms.Postoperatively, follow the patient for symptom resolution, and evaluate for defect resolution with transvaginal ultrasonography.
For a laparoscopic repair, first identify the niche hysteroscopically. At the same time as hysteroscopic examination of the cavity, the defect can be evaluated laparoscopically (FIGURE 4). The light from the hysteroscope can be visualized easily laparoscopically because of the thinned myometrium in the area of the defect. Map out the niche by transvaginally passing a cervical dilator into the defect in the uterine cavity (FIGURE 5). Again, given the thinning of this segment of the uterus, the dilator can be easily visualized laparoscopically. Be cautious when placing this dilator, as there is often overlying bladder. Prevent incidental cystotomy by gently advancing the dilator into the defect only until the niche can be adequately detected.9At this point, develop a bladder flap by opening the vesicovaginal and vesicocervical space, mobilizing the bladder inferiorly (FIGURE 6). With the guide of the dilator mapping out the defect (FIGURE 7), excise the fibrotic edges of the niche with thermal energy (monopolar cautery or CO2 laser) or sharp dissection (FIGURE 8). This leaves healthy myometrial tissue margins. Reapproximate these margins with absorbable suture (2-0 polyglactin 910 [Vicryl]) in an interrupted or running fashion, in 2 layers9 (FIGURE 9). Following the laparoscopic repair, perform hysteroscopic evaluation of the uterine cavity to assure complete resolution of the defect (FIGURE 10). With the hysteroscope in place, perform concurrent laparoscopic assessment of the repair. Check for impermeability by assuring no hysteroscopic fluid escapes at the site of repaired hysterotomy.9
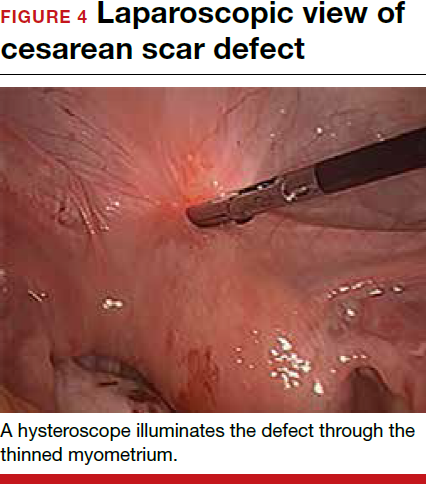
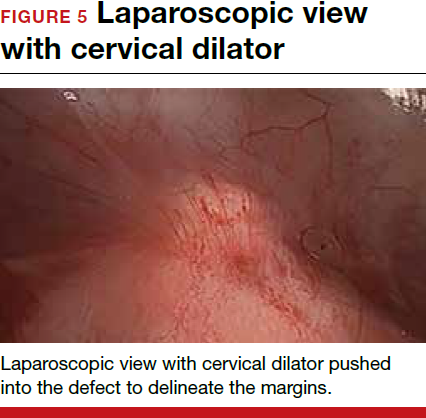
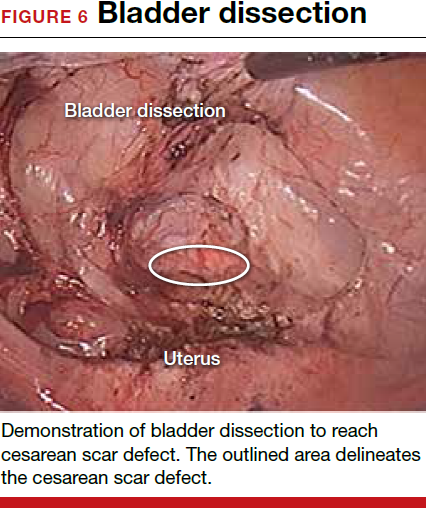
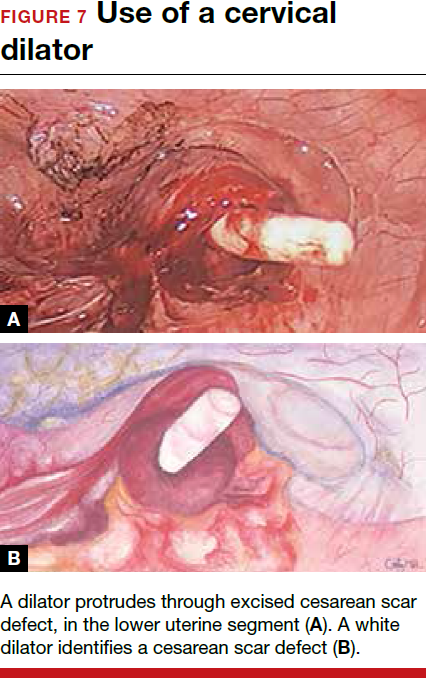
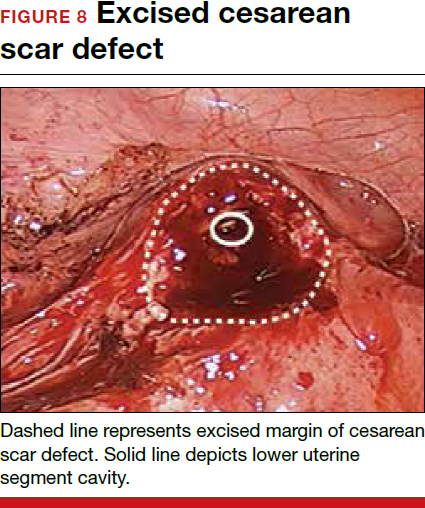
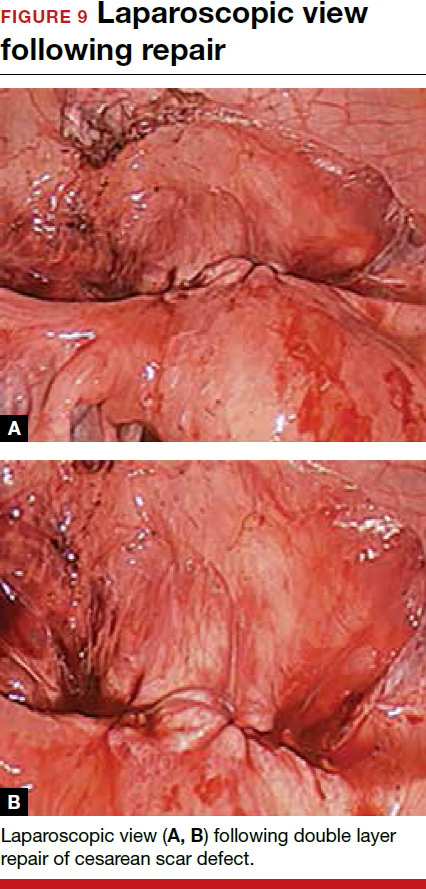
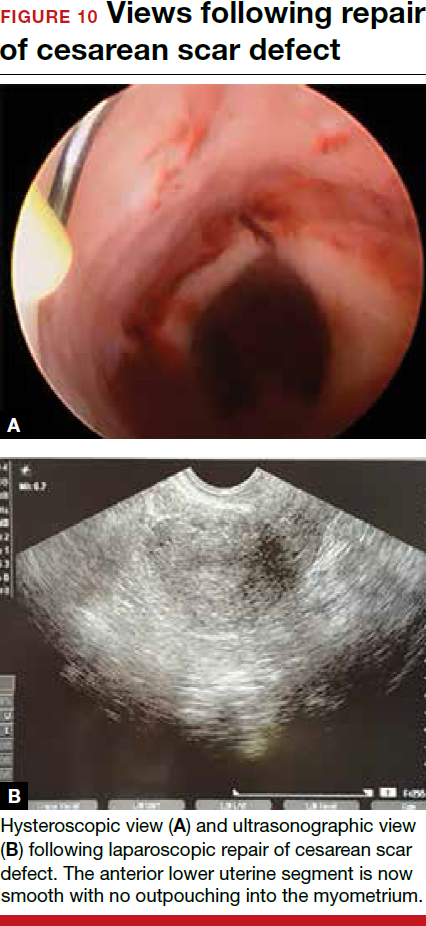
Postoperative care requires following the patient for symptom resolution and counseling regarding future fertility plans. We recommend that patients wait 6 months following the procedure before attempting conception.
When it comes to recommendations regarding preventing cesarean scar defects, additional randomized controlled trials need to be performed to evaluate various surgical techniques. At this time, there is no conclusive evidence that one method of hysterotomy closure is superior to another in preventing cesarean scar defect.
Symptoms often resolve with repair
When a patient with a prior cesarean delivery presents with symptoms of abnormal uterine bleeding, vaginal discharge, dysmenorrhea, dyspareunia, pelvic pain, or infertility that remain unexplained, consider cesarean scar defect as the culprit. Once a diagnosis of niche has been confirmed, the treatment approach should be dictated by the patient’s plans for future fertility. Hysteroscopic resection has been reported to have a 92% to 100% success rate for resolving symptoms of pain and bleeding, while 75% of patients undergoing laparoscopic niche repair for infertility achieved pregnancy.10,11 In our practice, a majority of patients experience symptom relief and go on to carry healthy pregnancies.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Cesarean delivery is one of the most common surgical procedures in women, with rates of 30% or more in the United States.1 As a result, the rate is rising for cesarean scar defect—the presence of a “niche” at the site of cesarean delivery scar—with the reported prevalence between 24% and 70% in a random population of women with at least one cesarean delivery.2 Other terms for cesarean scar defect include a niche, isthmocele, uteroperitoneal fistula, and diverticulum.1–9
Formation of cesarean scar defect
Cesarean scar defect forms after cesarean delivery, at the site of hysterotomy, on the anterior wall of the uterine isthmus (FIGURE 1). While this is the typical location, the defect has also been found at the endocervical canal and mid-uterine body. Improper healing of the cesarean incision leads to thinning of the anterior uterine wall, which creates an indentation and fluid-filled pouch at the cesarean scar site. The exact reason why a niche develops has not yet been determined; however, there are several hypotheses, broken down by pregnancy-related and patient-related factors. Surgical techniques that may increase the chance of niche development include low (cervical) hysterotomy, single-layer uterine wall closure, use of locking sutures, closure of hysterotomy with endometrial-sparing technique, and multiple cesarean deliveries.3,4 Patients with medical conditions that may impact wound healing (such as diabetes and smoking) may be at increased risk for niche formation.

Viewed hysteroscopically, the defect appears as a concave shape in the anterior uterine wall; to the inexperienced eye, it may resemble a second cavity (FIGURE 2).

Pelvic pain and other serious consequences
The presence of fibrotic tissue in the niche acts like a valve, leading to the accumulation of blood in this reservoir-like area. A niche thus can cause delayed menstruation through the cervix, resulting in abnormal bleeding, pelvic pain, vaginal discharge, dysmenorrhea, dyspareunia, and infertility. Accumulated blood in this area can ultimately degrade cervical mucus and sperm quality, as well as inhibit sperm transport, a proposed mechanism of infertility.5,6 Women with a niche who conceive are at potential risk for cesarean scar ectopic pregnancy, with the embryo implanting in the pouch and subsequently growing and developing improperly.
Read about evaluation and treatment.
Evaluation and treatment
Patients presenting with the symptoms de-scribed above who have had a prior cesarean delivery should be evaluated for a cesarean scar defect.9 The best time to assess for the abnormality is after the patient’s menstrual cycle, when the endometrial lining is at its thinnest and recently menstruated blood has collected in the defect (this can highlight the niche on imaging). Transvaginal ultrasonography (FIGURE 3) or saline-infusion sonohysterogram serve as a first-line test for in-office diagnosis.7 Magnetic resonance imaging (MRI), 3-D ultrasonography, and hysteroscopy are additional useful imaging modalities that can aid in the diagnosis.

Treatments for cesarean scar defect vary dramatically and include hormonal therapy, hysteroscopic resection, vaginal or laparoscopic repair, and hysterectomy. Nonsurgical treatment should be reserved for women who desire a noninvasive approach, as the evidence for symptom resolution is limited.8
To promote fertility and decrease symptoms, the abnormal, fibrotic tissue must be removed. In our experience, since 2003, we have found that use of a laparoscopic approach is best for women desiring future fertility and that hysteroscopic resection is best for women whose childbearing is completed.9 Our management is dictated by the patient’s fertility plans, since there is concern that cesarean scar defect in a gravid uterus presents a risk for uterine rupture. The laparoscopic approach allows the defect to be repaired and the integrity of the myometrium restored.9
What are the coding options for cesarean scar defect repair?
Melanie Witt, RN, CPC, COBGC, MA
As the accompanying article discusses, the primary treatment for a cesarean scar defect depends on whether the patient wishes to preserve fertility, but assigning a procedure code for either surgical option will entail reporting an unlisted procedure code.
Under Current Procedural Terminology (CPT) guidelines (which are developed and copyrighted by the American Medical Association), procedure code selected must accurately describe the service/procedure performed rather than just approximate the service. This means that when a procedure-specific code does not exist, an unlisted procedure code that represents the type of surgery, the approach, and the anatomic site needs to be selected.
When an unlisted CPT code is reported, payment is based on the complexity of the surgery, and one way to communicate this to a payer is to provide additional documentation that not only includes the operative report but also suggests one or more existing CPT codes that have a published relative value unit (RVU) that approximates the work involved for the unlisted procedure.
The coding options for hysteroscopic and laparoscopic treatment options are listed below. The comparison codes offered will give the surgeon a range to look at, but the ultimate decision to use one of those suggested, or to choose an entirely different comparison code, is entirely within the control of the physician.
ICD-10-CM diagnostic coding
While the cesarean scar defect is a sequela of cesarean delivery, which is always reported as a secondary code, the choice of a primary diagnosis code can be either a gynecologic and/or an obstetric complication code. The choice may be determined by payer policy, as the use of an obstetric complication may not be accepted with a gynecologic procedure code. From a coding perspective, however, use of all 3 of these codes from the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) paints the most accurate description of the defect and its cause:
- N85.8 Other specified noninflammatory disorders of uterus versus
- O34.21 Maternal care for scar from previous cesarean delivery plus
- O94 Sequelae of complication of pregnancy, childbirth, and the puerperium.
Hysteroscopic resection codes:
- 58579 Unlisted hysteroscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58561 (Hysteroscopy, surgical; with removal of leiomyomata) with 15.48 RVUs or 58560 (Hysteroscopy, surgical; with division or resection of intrauterine septum [any method]) with 10.92 RVUs.
Laparoscopic repair codes:
- 58578 Unlisted laparoscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58520 (Hysterorrhaphy, repair of ruptured uterus [nonobstetrical] 24.25 RVUs or 58662 (Laparoscopy, surgical; with fulguration or excision of lesions of the ovary, pelvic viscera, or peritoneal surface by any method) with 20.14 RVUs.
You may also want to report a diagnostic hysteroscopy (code 58555), but keep in mind that payment will depend on documentation that clearly indicates that the use of the hysteroscope was for diagnostic purposes. Use of the hysteroscope to simply identify the surgical site to be repaired via the laparoscope will usually not be reimbursed separately.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
Read about techniques for repair.
Techniques for repairing cesarean scar defect
For hysteroscopic resection of a niche, the uterus is distended and the intrauterine defect is visualized hysteroscopically, as seen in FIGURE 2. Using a bipolar or unipolar resectoscope, resect the fibrotic tissue of the defect and endometrial-like glands present within the niche. The goal of this relatively quick procedure is to open up the reservoir and facilitate the complete drainage of menstrual blood, thus alleviating the patient’s symptoms.Postoperatively, follow the patient for symptom resolution, and evaluate for defect resolution with transvaginal ultrasonography.
For a laparoscopic repair, first identify the niche hysteroscopically. At the same time as hysteroscopic examination of the cavity, the defect can be evaluated laparoscopically (FIGURE 4). The light from the hysteroscope can be visualized easily laparoscopically because of the thinned myometrium in the area of the defect. Map out the niche by transvaginally passing a cervical dilator into the defect in the uterine cavity (FIGURE 5). Again, given the thinning of this segment of the uterus, the dilator can be easily visualized laparoscopically. Be cautious when placing this dilator, as there is often overlying bladder. Prevent incidental cystotomy by gently advancing the dilator into the defect only until the niche can be adequately detected.9At this point, develop a bladder flap by opening the vesicovaginal and vesicocervical space, mobilizing the bladder inferiorly (FIGURE 6). With the guide of the dilator mapping out the defect (FIGURE 7), excise the fibrotic edges of the niche with thermal energy (monopolar cautery or CO2 laser) or sharp dissection (FIGURE 8). This leaves healthy myometrial tissue margins. Reapproximate these margins with absorbable suture (2-0 polyglactin 910 [Vicryl]) in an interrupted or running fashion, in 2 layers9 (FIGURE 9). Following the laparoscopic repair, perform hysteroscopic evaluation of the uterine cavity to assure complete resolution of the defect (FIGURE 10). With the hysteroscope in place, perform concurrent laparoscopic assessment of the repair. Check for impermeability by assuring no hysteroscopic fluid escapes at the site of repaired hysterotomy.9







Postoperative care requires following the patient for symptom resolution and counseling regarding future fertility plans. We recommend that patients wait 6 months following the procedure before attempting conception.
When it comes to recommendations regarding preventing cesarean scar defects, additional randomized controlled trials need to be performed to evaluate various surgical techniques. At this time, there is no conclusive evidence that one method of hysterotomy closure is superior to another in preventing cesarean scar defect.
Symptoms often resolve with repair
When a patient with a prior cesarean delivery presents with symptoms of abnormal uterine bleeding, vaginal discharge, dysmenorrhea, dyspareunia, pelvic pain, or infertility that remain unexplained, consider cesarean scar defect as the culprit. Once a diagnosis of niche has been confirmed, the treatment approach should be dictated by the patient’s plans for future fertility. Hysteroscopic resection has been reported to have a 92% to 100% success rate for resolving symptoms of pain and bleeding, while 75% of patients undergoing laparoscopic niche repair for infertility achieved pregnancy.10,11 In our practice, a majority of patients experience symptom relief and go on to carry healthy pregnancies.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Cesarean delivery is one of the most common surgical procedures in women, with rates of 30% or more in the United States.1 As a result, the rate is rising for cesarean scar defect—the presence of a “niche” at the site of cesarean delivery scar—with the reported prevalence between 24% and 70% in a random population of women with at least one cesarean delivery.2 Other terms for cesarean scar defect include a niche, isthmocele, uteroperitoneal fistula, and diverticulum.1–9
Formation of cesarean scar defect
Cesarean scar defect forms after cesarean delivery, at the site of hysterotomy, on the anterior wall of the uterine isthmus (FIGURE 1). While this is the typical location, the defect has also been found at the endocervical canal and mid-uterine body. Improper healing of the cesarean incision leads to thinning of the anterior uterine wall, which creates an indentation and fluid-filled pouch at the cesarean scar site. The exact reason why a niche develops has not yet been determined; however, there are several hypotheses, broken down by pregnancy-related and patient-related factors. Surgical techniques that may increase the chance of niche development include low (cervical) hysterotomy, single-layer uterine wall closure, use of locking sutures, closure of hysterotomy with endometrial-sparing technique, and multiple cesarean deliveries.3,4 Patients with medical conditions that may impact wound healing (such as diabetes and smoking) may be at increased risk for niche formation.

Viewed hysteroscopically, the defect appears as a concave shape in the anterior uterine wall; to the inexperienced eye, it may resemble a second cavity (FIGURE 2).

Pelvic pain and other serious consequences
The presence of fibrotic tissue in the niche acts like a valve, leading to the accumulation of blood in this reservoir-like area. A niche thus can cause delayed menstruation through the cervix, resulting in abnormal bleeding, pelvic pain, vaginal discharge, dysmenorrhea, dyspareunia, and infertility. Accumulated blood in this area can ultimately degrade cervical mucus and sperm quality, as well as inhibit sperm transport, a proposed mechanism of infertility.5,6 Women with a niche who conceive are at potential risk for cesarean scar ectopic pregnancy, with the embryo implanting in the pouch and subsequently growing and developing improperly.
Read about evaluation and treatment.
Evaluation and treatment
Patients presenting with the symptoms de-scribed above who have had a prior cesarean delivery should be evaluated for a cesarean scar defect.9 The best time to assess for the abnormality is after the patient’s menstrual cycle, when the endometrial lining is at its thinnest and recently menstruated blood has collected in the defect (this can highlight the niche on imaging). Transvaginal ultrasonography (FIGURE 3) or saline-infusion sonohysterogram serve as a first-line test for in-office diagnosis.7 Magnetic resonance imaging (MRI), 3-D ultrasonography, and hysteroscopy are additional useful imaging modalities that can aid in the diagnosis.

Treatments for cesarean scar defect vary dramatically and include hormonal therapy, hysteroscopic resection, vaginal or laparoscopic repair, and hysterectomy. Nonsurgical treatment should be reserved for women who desire a noninvasive approach, as the evidence for symptom resolution is limited.8
To promote fertility and decrease symptoms, the abnormal, fibrotic tissue must be removed. In our experience, since 2003, we have found that use of a laparoscopic approach is best for women desiring future fertility and that hysteroscopic resection is best for women whose childbearing is completed.9 Our management is dictated by the patient’s fertility plans, since there is concern that cesarean scar defect in a gravid uterus presents a risk for uterine rupture. The laparoscopic approach allows the defect to be repaired and the integrity of the myometrium restored.9
What are the coding options for cesarean scar defect repair?
Melanie Witt, RN, CPC, COBGC, MA
As the accompanying article discusses, the primary treatment for a cesarean scar defect depends on whether the patient wishes to preserve fertility, but assigning a procedure code for either surgical option will entail reporting an unlisted procedure code.
Under Current Procedural Terminology (CPT) guidelines (which are developed and copyrighted by the American Medical Association), procedure code selected must accurately describe the service/procedure performed rather than just approximate the service. This means that when a procedure-specific code does not exist, an unlisted procedure code that represents the type of surgery, the approach, and the anatomic site needs to be selected.
When an unlisted CPT code is reported, payment is based on the complexity of the surgery, and one way to communicate this to a payer is to provide additional documentation that not only includes the operative report but also suggests one or more existing CPT codes that have a published relative value unit (RVU) that approximates the work involved for the unlisted procedure.
The coding options for hysteroscopic and laparoscopic treatment options are listed below. The comparison codes offered will give the surgeon a range to look at, but the ultimate decision to use one of those suggested, or to choose an entirely different comparison code, is entirely within the control of the physician.
ICD-10-CM diagnostic coding
While the cesarean scar defect is a sequela of cesarean delivery, which is always reported as a secondary code, the choice of a primary diagnosis code can be either a gynecologic and/or an obstetric complication code. The choice may be determined by payer policy, as the use of an obstetric complication may not be accepted with a gynecologic procedure code. From a coding perspective, however, use of all 3 of these codes from the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) paints the most accurate description of the defect and its cause:
- N85.8 Other specified noninflammatory disorders of uterus versus
- O34.21 Maternal care for scar from previous cesarean delivery plus
- O94 Sequelae of complication of pregnancy, childbirth, and the puerperium.
Hysteroscopic resection codes:
- 58579 Unlisted hysteroscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58561 (Hysteroscopy, surgical; with removal of leiomyomata) with 15.48 RVUs or 58560 (Hysteroscopy, surgical; with division or resection of intrauterine septum [any method]) with 10.92 RVUs.
Laparoscopic repair codes:
- 58578 Unlisted laparoscopy procedure, uterus
- The codes that may most closely approximate the physician work include 58520 (Hysterorrhaphy, repair of ruptured uterus [nonobstetrical] 24.25 RVUs or 58662 (Laparoscopy, surgical; with fulguration or excision of lesions of the ovary, pelvic viscera, or peritoneal surface by any method) with 20.14 RVUs.
You may also want to report a diagnostic hysteroscopy (code 58555), but keep in mind that payment will depend on documentation that clearly indicates that the use of the hysteroscope was for diagnostic purposes. Use of the hysteroscope to simply identify the surgical site to be repaired via the laparoscope will usually not be reimbursed separately.
Ms. Witt is an independent coding and documentation consultant and former program manager, department of coding and nomenclature, American Congress of Obstetricians and Gynecologists.
The author reports no financial relationships relevant to this article.
Read about techniques for repair.
Techniques for repairing cesarean scar defect
For hysteroscopic resection of a niche, the uterus is distended and the intrauterine defect is visualized hysteroscopically, as seen in FIGURE 2. Using a bipolar or unipolar resectoscope, resect the fibrotic tissue of the defect and endometrial-like glands present within the niche. The goal of this relatively quick procedure is to open up the reservoir and facilitate the complete drainage of menstrual blood, thus alleviating the patient’s symptoms.Postoperatively, follow the patient for symptom resolution, and evaluate for defect resolution with transvaginal ultrasonography.
For a laparoscopic repair, first identify the niche hysteroscopically. At the same time as hysteroscopic examination of the cavity, the defect can be evaluated laparoscopically (FIGURE 4). The light from the hysteroscope can be visualized easily laparoscopically because of the thinned myometrium in the area of the defect. Map out the niche by transvaginally passing a cervical dilator into the defect in the uterine cavity (FIGURE 5). Again, given the thinning of this segment of the uterus, the dilator can be easily visualized laparoscopically. Be cautious when placing this dilator, as there is often overlying bladder. Prevent incidental cystotomy by gently advancing the dilator into the defect only until the niche can be adequately detected.9At this point, develop a bladder flap by opening the vesicovaginal and vesicocervical space, mobilizing the bladder inferiorly (FIGURE 6). With the guide of the dilator mapping out the defect (FIGURE 7), excise the fibrotic edges of the niche with thermal energy (monopolar cautery or CO2 laser) or sharp dissection (FIGURE 8). This leaves healthy myometrial tissue margins. Reapproximate these margins with absorbable suture (2-0 polyglactin 910 [Vicryl]) in an interrupted or running fashion, in 2 layers9 (FIGURE 9). Following the laparoscopic repair, perform hysteroscopic evaluation of the uterine cavity to assure complete resolution of the defect (FIGURE 10). With the hysteroscope in place, perform concurrent laparoscopic assessment of the repair. Check for impermeability by assuring no hysteroscopic fluid escapes at the site of repaired hysterotomy.9







Postoperative care requires following the patient for symptom resolution and counseling regarding future fertility plans. We recommend that patients wait 6 months following the procedure before attempting conception.
When it comes to recommendations regarding preventing cesarean scar defects, additional randomized controlled trials need to be performed to evaluate various surgical techniques. At this time, there is no conclusive evidence that one method of hysterotomy closure is superior to another in preventing cesarean scar defect.
Symptoms often resolve with repair
When a patient with a prior cesarean delivery presents with symptoms of abnormal uterine bleeding, vaginal discharge, dysmenorrhea, dyspareunia, pelvic pain, or infertility that remain unexplained, consider cesarean scar defect as the culprit. Once a diagnosis of niche has been confirmed, the treatment approach should be dictated by the patient’s plans for future fertility. Hysteroscopic resection has been reported to have a 92% to 100% success rate for resolving symptoms of pain and bleeding, while 75% of patients undergoing laparoscopic niche repair for infertility achieved pregnancy.10,11 In our practice, a majority of patients experience symptom relief and go on to carry healthy pregnancies.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Article
Managing complications at the time of vaginal hysterectomy
Careful attention to technique at the time of vaginal hysterectomy is vital. Equally important is prior consideration of potential complications and the best ways to address them. Four trouble spots include:
- uterine tissue extraction (Although this is not a complication of vaginal hysterectomy, tissue extraction aids in debulking and removal of a large uterus.)
- protection of the ureters (It is important to palpate these structures before placing cardinal pedicle clamps, to protect ureteral integrity.)
- repair of inadvertent cystotomy
- control of bleeding in the setting of adnexectomy.
I focus on optimal approaches to these 4 scenarios in this article.
For a review of vaginal hysterectomy technique, see “Vaginal hysterectomy with basic instrumentation,” by Barbara S. Levy, MD, which appeared in the October 2015 issue of OBG Management. For salpingectomy and salpingo-oophorectomy technique, see my article entitled “Salpingectomy after vaginal hysterectomy: Technique, tips, and pearls,” which appeared in the November issue of this journal.
Both articles are available in the archive at obgmanagement.com and, like this one, are based on the AAGL-produced Online Master Class on Vaginal Hysterectomy, a Web-based program cosponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. That program is available at https://www.aagl.org/vaghystwebinar/.
A step toward success: Begin morcellation by splitting the uterus
Manual morcellation to reduce uterine size and ease transvaginal removal is a useful technique to know. Five aspects of manual morcellation warrant emphasis:
1. Anterior and posterior entry into the cul-de-sacs is essential before attempting morcellation.
2. The blood supply on both sides of the uterus must be controlled.
3. During resection, take care to cut only tissue that can be visualized. Avoid resection beyond what you can easily see.
4. Once morcellation is completed, always go back and check the pedicles for hemostasis. During morcellation, these pedicles tend to get stretched, and bleeding may arise that wasn’t present originally.
5. Morcellation should be performed only after malignancy has been ruled out—it is a technique intended for benign uteri only.
By bivalving the uterus it is possible to follow the endocervical canal up into the uterine cavity (FIGURE 1). Our technique at the Mayo Clinic is to place tenacula at the 3 and 9 o’clock positions prior to bivalving. A small amount of bleeding may occur because of collateral blood supply from the gonadal pedicles, but it should be minimal, as the uterine vessels have been secured.
FIGURE 1 Bivalve the uterus
|
To begin morcellation, split the uterus down the midline, with tenacula placed at the 3- and 9-o’clock positions, then follow the endocervical canal into the uterine cavity (A). Use a knife blade to take portions of myomas and other tissue to debulk the uterus (B). |
Proceed with morcellation once the uterus is bivalved. Use a Jacobs tenaculum to grasp the serosal portion of the uterus. Apply downward traction with your nondominant hand, and use the knife blade to resect portions of the uterus so that it can be debulked.
When a large myoma is encountered during morcellation, it often is possible to “finger-fracture” some of the filmy adhesions holding it in place, or to follow the pseudo-capsule of the fibroid in order to shell it out. In many cases, fibroids can be removed intact using these methods. If intact removal is not possible, debulk the fibroid by taking individual “bites.”
Tip. When the uterus is greatly enlarged, grasp it with a tenaculum so that it does not retract when you incise it. When large myomas are anticipated, keep an extra tenaculum on hand, as well as extra knife blades, as blades dull quickly when used to cut through calcified tissue. Continue to apply traction with your nondominant hand to allow each piece of tissue to be more readily developed (FIGURE 2).
Tip. When managing the round-ligament complex on each side, stay between the round ligaments (your “goal posts”) to avoid getting too lateral. Keep the cervix intact for orientation purposes. Focus on diminishing the bulk of the uterus so that you can get around the utero-ovarian pedicles.
To control the utero-ovarian pedicle on the patient’s right side, place a finger underneath it, with traction applied. Place a Heaney clamp from the top down. Repeat this action on the patient’s left side, but place the Heaney clamp from the bottom up.
Manual morcellation of tissue is useful in small uteri that are tough to access, but the procedure is very helpful in large uteri in order to remove them transvaginally.
Protect the ureters: Palpate them before clamping the pedicles
Palpating the ureters at the time of hysterectomy can protect their integrity during the procedure. The following technique has been used at the Mayo Clinic for many years and allows for location of the ureter so a cardinal pedicle clamp can be placed without injury.
Enter the anterior cul-de-sac so that you can insert a finger and palpate the ureter before you place the cardinal pedicle clamp on each side. Place Deaver retractors at the 12 o’clock and 2- to 3-o’clock positions. Insert your nondominant index finger into the anterior cul-de-sac and palpate the ureter against the Deaver clamp in the 2- to 3-o’clock position (FIGURE 3). (The ureter can be felt between your index finger and the Deaver retractor.) The ureter will have the most descent in a uterus that has some prolapse, compared with a nonprolapsed uterus.
Tip. One common error is mistaking the edge of the vaginal cuff for the ureter. Be certain that you insert your finger deeply into the cul-de-sac so that it is the ureter you feel and not the cuff edge.
Successful cystotomy repair technique
Inadvertent cystotomy is a common fear for surgeons at the time of vaginal hysterectomy. I prefer to empty the bladder before beginning the hysterectomy because it reduces the target zone that a distended bladder presents. Some surgeons prefer to maintain a bit of fluid in the bladder so that, if they cut into the bladder, a small urine stream results. The approach is a matter of preference.
Cystotomy is most common during anterior dissection. If it occurs, recognize it and mark the defect with suture. Do not attempt to repair the hole at this point, but opt to finish the hysterectomy.
Cystoscopy is an important element of cystotomy repair. Once the hysterectomy is completed, look inside the bladder and determine where the defect is in relationship to the ureteral orifices. Typically, it will be beyond the interureteric ridge, along the posterior portion of the bladder, usually in the midline.
As critical as the repair itself is management of bladder drainage afterward. If you repair the hole thoroughly and drain the bladder adequately for 14 days, the defect should heal fully.
Technique for entry into anterior cul-de-sac
One way to avert bladder injury is to enter the anterior cul-de-sac very carefully. Begin by ensuring that the bladder is empty and placing a Deaver retractor at the 12 o’clock position. Also place tenacula anteriorly and posteriorly to help direct traction. This will allow good visualization of the bladder reflection.
Tip. One common mistake is making the incision too low or too near the cervix, which makes dissection more difficult and increases the likelihood that you will enter the wrong plane. Be sure you know where the bladder is, and make an adequate incision that is not too distal. Otherwise, dissection will be harder to carry out.
I prefer to make one clean incision with the knife, rather than multiple incisions, because multiple cuts increase the likelihood that you will inadvertently injure the wrong tissue. Use good traction and countertraction, and hug the uterus. Work low on the uterus, but not in the uterus. If you cut into muscle, you will get more bleeding and may end up digging a hole.
After you make the incision, put your finger through it to help develop that space further. You can confirm entry into the peritoneum by noting the characteristic slippery feel of the peritoneal lining. After you insert a Deaver retractor anteriorly, reinsert your finger and mobilize the area further. Then you can easily reach in and tent the peritoneum to cut it.
Technique for cystotomy repair
Two-layer closure is a minimum. On occasion, a third layer may be beneficial. Begin with running closure of the first layer using 2-0 chromic suture—a good suture choice in the urinary tract. This suture is inflammatory, which will help seal the wound, but it also dissolves quickly, preventing stone formation.
Use through-and-through closure on the first layer, followed by a second imbricating layer. If desired, use the peritoneum as a third layer.
Horizontal repair is typical, although vertical closure may be necessary if the defect is near a ureteral orifice and horizontal closure might compromise that side. That decision must be made intraoperatively.
When vertical repair is necessary, begin your repair just above the defect, placing the suture through and through. The hole should be visible. There is no need to be extramucosal in needle placement. Simply get a good bite of the tissue and run the repair down the bladder wall.
Next, stop and apply traction to the repair to check for any small defects that may have been overlooked. By placing a little traction on that first suture tag, any such defects will become apparent. Then go back and close them in a secondary imbricating layer.
After 2-layer closure, fill the bladder retrograde. I prefer to use a couple of drops of methylene blue in normal saline and place a clean white piece of packing material beneath the wound. If the packing material remains unstained by blue, the repair is watertight.
Incorporate the peritoneum as another layer of repair of the defect. I imbricate 2 layers in the bladder. Then, if necessary, I use that peritoneum as an additional layer (FIGURE 4).
Strategies to control bleeding at adnexectomy
Be vigilant for bleeding when removing the tubes and/or ovaries. At salpingectomy, be extremely gentle with the mesosalpinx because it can be avulsed easily off of surrounding tissue. If bleeding occurs, oversewing, or even ovary removal, could end up being the only options.
Good visualization is essential during vaginal procedures. Retractors, lighted suction irrigators, a headlamp, good overhead lighting, and appropriate instrumentation are critical for success.
Heaney clamp technique for vaginal oophorectomy
Begin by placing an Allis clamp on the utero-ovarian pedicle. Then clamp the ovary and tube with a second Allis clamp. Next, insert a Heaney clamp through the small window between the cardinal pedicle and the utero-ovarian pedicle (FIGURE 5). Clamp the tissue and place a free tie around it.
Because this is a major vascular pedicle, doubly ligate it. As you tie the first suture, have an assistant flash the clamp open and closed, then excise the specimen. There is no need to worry about losing the pedicle because it already has been ligated once. Next, stick-tie it, placing the needle distal to the free tie to avoid piercing the gonadal vessels beyond.
The technique is standard. Be gentle, and ensure good hemostasis when finished.
Tip. In my experience, any bleeding runs down from the pedicle rather than out toward me. So be sure to look down and below the pedicle to ensure hemostasis.
Additional pearls
- When performing vaginal hysterectomy, the ovaries are almost always removable transvaginally. There is no need to begin the case laparoscopically to remove the tubes and/or ovaries and then perform the hysterectomy vaginally.
- Deaver retractors offer good exposure; visualization is critical.
- Make sure the tissue is dry before you cut the last suture.
- If you prefer to use a laparoscopic stapler to secure the pedicles, proceed as before: Place an Allis clamp on the pedicle. Place a second clamp on the ovary and tube. Now you can insert the stapler into the created window, as with the Heaney clamp (FIGURE 6).
- Use a 60-mm stapler to cut the pedicle in one try. If using a 45-mm device, the stapler may need to be fired twice. This makes the procedure more expensive and risks more bleeding.
- When closing the stapler jaws, avoid clamping small bowel or packing material. Ensure stapler tip visibility well before firing.
The round ligament technique
When transecting the round ligament, it is critical to stay just beneath it to avoid bleeding and venturing into the mesosalpinx. Gently hug the tissue inferior to the round ligament and let it retract (FIGURE 7). This will allow isolation of the gonadal vessels nicely, especially if an adnexal mass is present. Then isolate the specimen and remove it, stick-tying the pedicle afterward to secure it.
When tying the pedicle, place the suture around the distal aspect to ensure that the back of the pedicle is enclosed, and do not lose it when you release the clamp. A slightly different technique is to use an endoloop to cross the gonadal vessels and control them. Use a suction irrigator and good lighting to get good exposure.
Next, place the clamp, making sure you don’t inadvertently grasp the packing material. Visualize both tips of the clamp before incising. Trim the specimen flush with the clamp. Then you can thread an endoloop over the top of the clamp. This is an inexpensive technique that allows a higher reach into the pelvic cavity. Finally, cinch down the endoloop to control the vessels.
When performing bilateral salpingo-oophorectomy, a long, fine clamp, such as the M.D. Anderson clamp, can help you reach up to control the gonadal vessels in the event that you lose your initial grip on those vessels (FIGURE 8).
Be prepared
Have a plan in place to manage any complications that arise during surgery. Just as obstetricians plan ahead to prepare for shoulder dystocia and other emergencies, gynecologic surgeons must prepare for surgical complications. Tissue extraction strategies can aid in the debulking and removal of large uteri, and the proper tools, lighting, and assistance are critical to success.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Careful attention to technique at the time of vaginal hysterectomy is vital. Equally important is prior consideration of potential complications and the best ways to address them. Four trouble spots include:
- uterine tissue extraction (Although this is not a complication of vaginal hysterectomy, tissue extraction aids in debulking and removal of a large uterus.)
- protection of the ureters (It is important to palpate these structures before placing cardinal pedicle clamps, to protect ureteral integrity.)
- repair of inadvertent cystotomy
- control of bleeding in the setting of adnexectomy.
I focus on optimal approaches to these 4 scenarios in this article.
For a review of vaginal hysterectomy technique, see “Vaginal hysterectomy with basic instrumentation,” by Barbara S. Levy, MD, which appeared in the October 2015 issue of OBG Management. For salpingectomy and salpingo-oophorectomy technique, see my article entitled “Salpingectomy after vaginal hysterectomy: Technique, tips, and pearls,” which appeared in the November issue of this journal.
Both articles are available in the archive at obgmanagement.com and, like this one, are based on the AAGL-produced Online Master Class on Vaginal Hysterectomy, a Web-based program cosponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. That program is available at https://www.aagl.org/vaghystwebinar/.
A step toward success: Begin morcellation by splitting the uterus
Manual morcellation to reduce uterine size and ease transvaginal removal is a useful technique to know. Five aspects of manual morcellation warrant emphasis:
1. Anterior and posterior entry into the cul-de-sacs is essential before attempting morcellation.
2. The blood supply on both sides of the uterus must be controlled.
3. During resection, take care to cut only tissue that can be visualized. Avoid resection beyond what you can easily see.
4. Once morcellation is completed, always go back and check the pedicles for hemostasis. During morcellation, these pedicles tend to get stretched, and bleeding may arise that wasn’t present originally.
5. Morcellation should be performed only after malignancy has been ruled out—it is a technique intended for benign uteri only.
By bivalving the uterus it is possible to follow the endocervical canal up into the uterine cavity (FIGURE 1). Our technique at the Mayo Clinic is to place tenacula at the 3 and 9 o’clock positions prior to bivalving. A small amount of bleeding may occur because of collateral blood supply from the gonadal pedicles, but it should be minimal, as the uterine vessels have been secured.
FIGURE 1 Bivalve the uterus
|
To begin morcellation, split the uterus down the midline, with tenacula placed at the 3- and 9-o’clock positions, then follow the endocervical canal into the uterine cavity (A). Use a knife blade to take portions of myomas and other tissue to debulk the uterus (B). |
Proceed with morcellation once the uterus is bivalved. Use a Jacobs tenaculum to grasp the serosal portion of the uterus. Apply downward traction with your nondominant hand, and use the knife blade to resect portions of the uterus so that it can be debulked.
When a large myoma is encountered during morcellation, it often is possible to “finger-fracture” some of the filmy adhesions holding it in place, or to follow the pseudo-capsule of the fibroid in order to shell it out. In many cases, fibroids can be removed intact using these methods. If intact removal is not possible, debulk the fibroid by taking individual “bites.”
Tip. When the uterus is greatly enlarged, grasp it with a tenaculum so that it does not retract when you incise it. When large myomas are anticipated, keep an extra tenaculum on hand, as well as extra knife blades, as blades dull quickly when used to cut through calcified tissue. Continue to apply traction with your nondominant hand to allow each piece of tissue to be more readily developed (FIGURE 2).
Tip. When managing the round-ligament complex on each side, stay between the round ligaments (your “goal posts”) to avoid getting too lateral. Keep the cervix intact for orientation purposes. Focus on diminishing the bulk of the uterus so that you can get around the utero-ovarian pedicles.
To control the utero-ovarian pedicle on the patient’s right side, place a finger underneath it, with traction applied. Place a Heaney clamp from the top down. Repeat this action on the patient’s left side, but place the Heaney clamp from the bottom up.
Manual morcellation of tissue is useful in small uteri that are tough to access, but the procedure is very helpful in large uteri in order to remove them transvaginally.
Protect the ureters: Palpate them before clamping the pedicles
Palpating the ureters at the time of hysterectomy can protect their integrity during the procedure. The following technique has been used at the Mayo Clinic for many years and allows for location of the ureter so a cardinal pedicle clamp can be placed without injury.
Enter the anterior cul-de-sac so that you can insert a finger and palpate the ureter before you place the cardinal pedicle clamp on each side. Place Deaver retractors at the 12 o’clock and 2- to 3-o’clock positions. Insert your nondominant index finger into the anterior cul-de-sac and palpate the ureter against the Deaver clamp in the 2- to 3-o’clock position (FIGURE 3). (The ureter can be felt between your index finger and the Deaver retractor.) The ureter will have the most descent in a uterus that has some prolapse, compared with a nonprolapsed uterus.
Tip. One common error is mistaking the edge of the vaginal cuff for the ureter. Be certain that you insert your finger deeply into the cul-de-sac so that it is the ureter you feel and not the cuff edge.
Successful cystotomy repair technique
Inadvertent cystotomy is a common fear for surgeons at the time of vaginal hysterectomy. I prefer to empty the bladder before beginning the hysterectomy because it reduces the target zone that a distended bladder presents. Some surgeons prefer to maintain a bit of fluid in the bladder so that, if they cut into the bladder, a small urine stream results. The approach is a matter of preference.
Cystotomy is most common during anterior dissection. If it occurs, recognize it and mark the defect with suture. Do not attempt to repair the hole at this point, but opt to finish the hysterectomy.
Cystoscopy is an important element of cystotomy repair. Once the hysterectomy is completed, look inside the bladder and determine where the defect is in relationship to the ureteral orifices. Typically, it will be beyond the interureteric ridge, along the posterior portion of the bladder, usually in the midline.
As critical as the repair itself is management of bladder drainage afterward. If you repair the hole thoroughly and drain the bladder adequately for 14 days, the defect should heal fully.
Technique for entry into anterior cul-de-sac
One way to avert bladder injury is to enter the anterior cul-de-sac very carefully. Begin by ensuring that the bladder is empty and placing a Deaver retractor at the 12 o’clock position. Also place tenacula anteriorly and posteriorly to help direct traction. This will allow good visualization of the bladder reflection.
Tip. One common mistake is making the incision too low or too near the cervix, which makes dissection more difficult and increases the likelihood that you will enter the wrong plane. Be sure you know where the bladder is, and make an adequate incision that is not too distal. Otherwise, dissection will be harder to carry out.
I prefer to make one clean incision with the knife, rather than multiple incisions, because multiple cuts increase the likelihood that you will inadvertently injure the wrong tissue. Use good traction and countertraction, and hug the uterus. Work low on the uterus, but not in the uterus. If you cut into muscle, you will get more bleeding and may end up digging a hole.
After you make the incision, put your finger through it to help develop that space further. You can confirm entry into the peritoneum by noting the characteristic slippery feel of the peritoneal lining. After you insert a Deaver retractor anteriorly, reinsert your finger and mobilize the area further. Then you can easily reach in and tent the peritoneum to cut it.
Technique for cystotomy repair
Two-layer closure is a minimum. On occasion, a third layer may be beneficial. Begin with running closure of the first layer using 2-0 chromic suture—a good suture choice in the urinary tract. This suture is inflammatory, which will help seal the wound, but it also dissolves quickly, preventing stone formation.
Use through-and-through closure on the first layer, followed by a second imbricating layer. If desired, use the peritoneum as a third layer.
Horizontal repair is typical, although vertical closure may be necessary if the defect is near a ureteral orifice and horizontal closure might compromise that side. That decision must be made intraoperatively.
When vertical repair is necessary, begin your repair just above the defect, placing the suture through and through. The hole should be visible. There is no need to be extramucosal in needle placement. Simply get a good bite of the tissue and run the repair down the bladder wall.
Next, stop and apply traction to the repair to check for any small defects that may have been overlooked. By placing a little traction on that first suture tag, any such defects will become apparent. Then go back and close them in a secondary imbricating layer.
After 2-layer closure, fill the bladder retrograde. I prefer to use a couple of drops of methylene blue in normal saline and place a clean white piece of packing material beneath the wound. If the packing material remains unstained by blue, the repair is watertight.
Incorporate the peritoneum as another layer of repair of the defect. I imbricate 2 layers in the bladder. Then, if necessary, I use that peritoneum as an additional layer (FIGURE 4).
Strategies to control bleeding at adnexectomy
Be vigilant for bleeding when removing the tubes and/or ovaries. At salpingectomy, be extremely gentle with the mesosalpinx because it can be avulsed easily off of surrounding tissue. If bleeding occurs, oversewing, or even ovary removal, could end up being the only options.
Good visualization is essential during vaginal procedures. Retractors, lighted suction irrigators, a headlamp, good overhead lighting, and appropriate instrumentation are critical for success.
Heaney clamp technique for vaginal oophorectomy
Begin by placing an Allis clamp on the utero-ovarian pedicle. Then clamp the ovary and tube with a second Allis clamp. Next, insert a Heaney clamp through the small window between the cardinal pedicle and the utero-ovarian pedicle (FIGURE 5). Clamp the tissue and place a free tie around it.
Because this is a major vascular pedicle, doubly ligate it. As you tie the first suture, have an assistant flash the clamp open and closed, then excise the specimen. There is no need to worry about losing the pedicle because it already has been ligated once. Next, stick-tie it, placing the needle distal to the free tie to avoid piercing the gonadal vessels beyond.
The technique is standard. Be gentle, and ensure good hemostasis when finished.
Tip. In my experience, any bleeding runs down from the pedicle rather than out toward me. So be sure to look down and below the pedicle to ensure hemostasis.
Additional pearls
- When performing vaginal hysterectomy, the ovaries are almost always removable transvaginally. There is no need to begin the case laparoscopically to remove the tubes and/or ovaries and then perform the hysterectomy vaginally.
- Deaver retractors offer good exposure; visualization is critical.
- Make sure the tissue is dry before you cut the last suture.
- If you prefer to use a laparoscopic stapler to secure the pedicles, proceed as before: Place an Allis clamp on the pedicle. Place a second clamp on the ovary and tube. Now you can insert the stapler into the created window, as with the Heaney clamp (FIGURE 6).
- Use a 60-mm stapler to cut the pedicle in one try. If using a 45-mm device, the stapler may need to be fired twice. This makes the procedure more expensive and risks more bleeding.
- When closing the stapler jaws, avoid clamping small bowel or packing material. Ensure stapler tip visibility well before firing.
The round ligament technique
When transecting the round ligament, it is critical to stay just beneath it to avoid bleeding and venturing into the mesosalpinx. Gently hug the tissue inferior to the round ligament and let it retract (FIGURE 7). This will allow isolation of the gonadal vessels nicely, especially if an adnexal mass is present. Then isolate the specimen and remove it, stick-tying the pedicle afterward to secure it.
When tying the pedicle, place the suture around the distal aspect to ensure that the back of the pedicle is enclosed, and do not lose it when you release the clamp. A slightly different technique is to use an endoloop to cross the gonadal vessels and control them. Use a suction irrigator and good lighting to get good exposure.
Next, place the clamp, making sure you don’t inadvertently grasp the packing material. Visualize both tips of the clamp before incising. Trim the specimen flush with the clamp. Then you can thread an endoloop over the top of the clamp. This is an inexpensive technique that allows a higher reach into the pelvic cavity. Finally, cinch down the endoloop to control the vessels.
When performing bilateral salpingo-oophorectomy, a long, fine clamp, such as the M.D. Anderson clamp, can help you reach up to control the gonadal vessels in the event that you lose your initial grip on those vessels (FIGURE 8).
Be prepared
Have a plan in place to manage any complications that arise during surgery. Just as obstetricians plan ahead to prepare for shoulder dystocia and other emergencies, gynecologic surgeons must prepare for surgical complications. Tissue extraction strategies can aid in the debulking and removal of large uteri, and the proper tools, lighting, and assistance are critical to success.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
Careful attention to technique at the time of vaginal hysterectomy is vital. Equally important is prior consideration of potential complications and the best ways to address them. Four trouble spots include:
- uterine tissue extraction (Although this is not a complication of vaginal hysterectomy, tissue extraction aids in debulking and removal of a large uterus.)
- protection of the ureters (It is important to palpate these structures before placing cardinal pedicle clamps, to protect ureteral integrity.)
- repair of inadvertent cystotomy
- control of bleeding in the setting of adnexectomy.
I focus on optimal approaches to these 4 scenarios in this article.
For a review of vaginal hysterectomy technique, see “Vaginal hysterectomy with basic instrumentation,” by Barbara S. Levy, MD, which appeared in the October 2015 issue of OBG Management. For salpingectomy and salpingo-oophorectomy technique, see my article entitled “Salpingectomy after vaginal hysterectomy: Technique, tips, and pearls,” which appeared in the November issue of this journal.
Both articles are available in the archive at obgmanagement.com and, like this one, are based on the AAGL-produced Online Master Class on Vaginal Hysterectomy, a Web-based program cosponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. That program is available at https://www.aagl.org/vaghystwebinar/.
A step toward success: Begin morcellation by splitting the uterus
Manual morcellation to reduce uterine size and ease transvaginal removal is a useful technique to know. Five aspects of manual morcellation warrant emphasis:
1. Anterior and posterior entry into the cul-de-sacs is essential before attempting morcellation.
2. The blood supply on both sides of the uterus must be controlled.
3. During resection, take care to cut only tissue that can be visualized. Avoid resection beyond what you can easily see.
4. Once morcellation is completed, always go back and check the pedicles for hemostasis. During morcellation, these pedicles tend to get stretched, and bleeding may arise that wasn’t present originally.
5. Morcellation should be performed only after malignancy has been ruled out—it is a technique intended for benign uteri only.
By bivalving the uterus it is possible to follow the endocervical canal up into the uterine cavity (FIGURE 1). Our technique at the Mayo Clinic is to place tenacula at the 3 and 9 o’clock positions prior to bivalving. A small amount of bleeding may occur because of collateral blood supply from the gonadal pedicles, but it should be minimal, as the uterine vessels have been secured.
FIGURE 1 Bivalve the uterus
|
To begin morcellation, split the uterus down the midline, with tenacula placed at the 3- and 9-o’clock positions, then follow the endocervical canal into the uterine cavity (A). Use a knife blade to take portions of myomas and other tissue to debulk the uterus (B). |
Proceed with morcellation once the uterus is bivalved. Use a Jacobs tenaculum to grasp the serosal portion of the uterus. Apply downward traction with your nondominant hand, and use the knife blade to resect portions of the uterus so that it can be debulked.
When a large myoma is encountered during morcellation, it often is possible to “finger-fracture” some of the filmy adhesions holding it in place, or to follow the pseudo-capsule of the fibroid in order to shell it out. In many cases, fibroids can be removed intact using these methods. If intact removal is not possible, debulk the fibroid by taking individual “bites.”
Tip. When the uterus is greatly enlarged, grasp it with a tenaculum so that it does not retract when you incise it. When large myomas are anticipated, keep an extra tenaculum on hand, as well as extra knife blades, as blades dull quickly when used to cut through calcified tissue. Continue to apply traction with your nondominant hand to allow each piece of tissue to be more readily developed (FIGURE 2).
Tip. When managing the round-ligament complex on each side, stay between the round ligaments (your “goal posts”) to avoid getting too lateral. Keep the cervix intact for orientation purposes. Focus on diminishing the bulk of the uterus so that you can get around the utero-ovarian pedicles.
To control the utero-ovarian pedicle on the patient’s right side, place a finger underneath it, with traction applied. Place a Heaney clamp from the top down. Repeat this action on the patient’s left side, but place the Heaney clamp from the bottom up.
Manual morcellation of tissue is useful in small uteri that are tough to access, but the procedure is very helpful in large uteri in order to remove them transvaginally.
Protect the ureters: Palpate them before clamping the pedicles
Palpating the ureters at the time of hysterectomy can protect their integrity during the procedure. The following technique has been used at the Mayo Clinic for many years and allows for location of the ureter so a cardinal pedicle clamp can be placed without injury.
Enter the anterior cul-de-sac so that you can insert a finger and palpate the ureter before you place the cardinal pedicle clamp on each side. Place Deaver retractors at the 12 o’clock and 2- to 3-o’clock positions. Insert your nondominant index finger into the anterior cul-de-sac and palpate the ureter against the Deaver clamp in the 2- to 3-o’clock position (FIGURE 3). (The ureter can be felt between your index finger and the Deaver retractor.) The ureter will have the most descent in a uterus that has some prolapse, compared with a nonprolapsed uterus.
Tip. One common error is mistaking the edge of the vaginal cuff for the ureter. Be certain that you insert your finger deeply into the cul-de-sac so that it is the ureter you feel and not the cuff edge.
Successful cystotomy repair technique
Inadvertent cystotomy is a common fear for surgeons at the time of vaginal hysterectomy. I prefer to empty the bladder before beginning the hysterectomy because it reduces the target zone that a distended bladder presents. Some surgeons prefer to maintain a bit of fluid in the bladder so that, if they cut into the bladder, a small urine stream results. The approach is a matter of preference.
Cystotomy is most common during anterior dissection. If it occurs, recognize it and mark the defect with suture. Do not attempt to repair the hole at this point, but opt to finish the hysterectomy.
Cystoscopy is an important element of cystotomy repair. Once the hysterectomy is completed, look inside the bladder and determine where the defect is in relationship to the ureteral orifices. Typically, it will be beyond the interureteric ridge, along the posterior portion of the bladder, usually in the midline.
As critical as the repair itself is management of bladder drainage afterward. If you repair the hole thoroughly and drain the bladder adequately for 14 days, the defect should heal fully.
Technique for entry into anterior cul-de-sac
One way to avert bladder injury is to enter the anterior cul-de-sac very carefully. Begin by ensuring that the bladder is empty and placing a Deaver retractor at the 12 o’clock position. Also place tenacula anteriorly and posteriorly to help direct traction. This will allow good visualization of the bladder reflection.
Tip. One common mistake is making the incision too low or too near the cervix, which makes dissection more difficult and increases the likelihood that you will enter the wrong plane. Be sure you know where the bladder is, and make an adequate incision that is not too distal. Otherwise, dissection will be harder to carry out.
I prefer to make one clean incision with the knife, rather than multiple incisions, because multiple cuts increase the likelihood that you will inadvertently injure the wrong tissue. Use good traction and countertraction, and hug the uterus. Work low on the uterus, but not in the uterus. If you cut into muscle, you will get more bleeding and may end up digging a hole.
After you make the incision, put your finger through it to help develop that space further. You can confirm entry into the peritoneum by noting the characteristic slippery feel of the peritoneal lining. After you insert a Deaver retractor anteriorly, reinsert your finger and mobilize the area further. Then you can easily reach in and tent the peritoneum to cut it.
Technique for cystotomy repair
Two-layer closure is a minimum. On occasion, a third layer may be beneficial. Begin with running closure of the first layer using 2-0 chromic suture—a good suture choice in the urinary tract. This suture is inflammatory, which will help seal the wound, but it also dissolves quickly, preventing stone formation.
Use through-and-through closure on the first layer, followed by a second imbricating layer. If desired, use the peritoneum as a third layer.
Horizontal repair is typical, although vertical closure may be necessary if the defect is near a ureteral orifice and horizontal closure might compromise that side. That decision must be made intraoperatively.
When vertical repair is necessary, begin your repair just above the defect, placing the suture through and through. The hole should be visible. There is no need to be extramucosal in needle placement. Simply get a good bite of the tissue and run the repair down the bladder wall.
Next, stop and apply traction to the repair to check for any small defects that may have been overlooked. By placing a little traction on that first suture tag, any such defects will become apparent. Then go back and close them in a secondary imbricating layer.
After 2-layer closure, fill the bladder retrograde. I prefer to use a couple of drops of methylene blue in normal saline and place a clean white piece of packing material beneath the wound. If the packing material remains unstained by blue, the repair is watertight.
Incorporate the peritoneum as another layer of repair of the defect. I imbricate 2 layers in the bladder. Then, if necessary, I use that peritoneum as an additional layer (FIGURE 4).
Strategies to control bleeding at adnexectomy
Be vigilant for bleeding when removing the tubes and/or ovaries. At salpingectomy, be extremely gentle with the mesosalpinx because it can be avulsed easily off of surrounding tissue. If bleeding occurs, oversewing, or even ovary removal, could end up being the only options.
Good visualization is essential during vaginal procedures. Retractors, lighted suction irrigators, a headlamp, good overhead lighting, and appropriate instrumentation are critical for success.
Heaney clamp technique for vaginal oophorectomy
Begin by placing an Allis clamp on the utero-ovarian pedicle. Then clamp the ovary and tube with a second Allis clamp. Next, insert a Heaney clamp through the small window between the cardinal pedicle and the utero-ovarian pedicle (FIGURE 5). Clamp the tissue and place a free tie around it.
Because this is a major vascular pedicle, doubly ligate it. As you tie the first suture, have an assistant flash the clamp open and closed, then excise the specimen. There is no need to worry about losing the pedicle because it already has been ligated once. Next, stick-tie it, placing the needle distal to the free tie to avoid piercing the gonadal vessels beyond.
The technique is standard. Be gentle, and ensure good hemostasis when finished.
Tip. In my experience, any bleeding runs down from the pedicle rather than out toward me. So be sure to look down and below the pedicle to ensure hemostasis.
Additional pearls
- When performing vaginal hysterectomy, the ovaries are almost always removable transvaginally. There is no need to begin the case laparoscopically to remove the tubes and/or ovaries and then perform the hysterectomy vaginally.
- Deaver retractors offer good exposure; visualization is critical.
- Make sure the tissue is dry before you cut the last suture.
- If you prefer to use a laparoscopic stapler to secure the pedicles, proceed as before: Place an Allis clamp on the pedicle. Place a second clamp on the ovary and tube. Now you can insert the stapler into the created window, as with the Heaney clamp (FIGURE 6).
- Use a 60-mm stapler to cut the pedicle in one try. If using a 45-mm device, the stapler may need to be fired twice. This makes the procedure more expensive and risks more bleeding.
- When closing the stapler jaws, avoid clamping small bowel or packing material. Ensure stapler tip visibility well before firing.
The round ligament technique
When transecting the round ligament, it is critical to stay just beneath it to avoid bleeding and venturing into the mesosalpinx. Gently hug the tissue inferior to the round ligament and let it retract (FIGURE 7). This will allow isolation of the gonadal vessels nicely, especially if an adnexal mass is present. Then isolate the specimen and remove it, stick-tying the pedicle afterward to secure it.
When tying the pedicle, place the suture around the distal aspect to ensure that the back of the pedicle is enclosed, and do not lose it when you release the clamp. A slightly different technique is to use an endoloop to cross the gonadal vessels and control them. Use a suction irrigator and good lighting to get good exposure.
Next, place the clamp, making sure you don’t inadvertently grasp the packing material. Visualize both tips of the clamp before incising. Trim the specimen flush with the clamp. Then you can thread an endoloop over the top of the clamp. This is an inexpensive technique that allows a higher reach into the pelvic cavity. Finally, cinch down the endoloop to control the vessels.
When performing bilateral salpingo-oophorectomy, a long, fine clamp, such as the M.D. Anderson clamp, can help you reach up to control the gonadal vessels in the event that you lose your initial grip on those vessels (FIGURE 8).
Be prepared
Have a plan in place to manage any complications that arise during surgery. Just as obstetricians plan ahead to prepare for shoulder dystocia and other emergencies, gynecologic surgeons must prepare for surgical complications. Tissue extraction strategies can aid in the debulking and removal of large uteri, and the proper tools, lighting, and assistance are critical to success.
Share your thoughts on this article! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Article
- Ensuring ureter protection
- Cystotomy repair
- Bleeding control strategies
This article is based on the AAGL-produced and ACOG/SGS cosponsored Online Master Class on Vaginal Hysterectomy
Salpingectomy after vaginal hysterectomy: Technique, tips, and pearls
In this article, I describe my technique for a vaginal approach to right salpingectomy with ovarian preservation, as well as right salpingo-oophorectomy, in a patient lacking a left tube and ovary. This technique is fully illustrated on a cadaver in the Web-based master course in vaginal hysterectomy produced by the AAGL and co-sponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. That course is available online at https://www.aagl.org/vaghystwebinar.
For a detailed description of vaginal hysterectomy technique, see the article entitled “Vaginal hysterectomy using basic instrumentation,” by Barbara S. Levy, MD, which appeared in the October 2015 issue of OBG Management. Next month, in the December 2015 issue of the journal, I will detail my strategies for managing complications associated with vaginal hysterectomy, salpingectomy, and salpingo-oophorectomy.
Right salpingectomy
FIGURE 1 Locate the tube |
The fallopian tube will almost always be found on top of the ovary. |
FIGURE 2 Isolate the tube 
|
| Grasp the tube and bring it to the midline. |
Start with light traction
Begin by placing an instrument on the round ligament, tube, and uterine-ovarian pedicle, exerting light traction. Note that the tube will always be found on top of the ovary
(FIGURE 1). Take care during placement of packing material to avoid sweeping the fimbriae of the tube up and out of the surgical field. You may need to play with the packing a bit until you are able to deliver the tube.
Once you identify the tube, isolate it by bringing it down to the midline (FIGURE 2). One thing to note if you’re accustomed to performing bilateral salpingo-oophorectomy: The gonadal pedicle is fairly substantive and can sustain a bit of tugging. However, if you’re performing salpingectomy with ovarian preservation, you need to be much more careful in your handling of the tube because the mesosalpinx is extremely delicate.
After you bring the tube to the midline, grasp it using a Heaney or Shallcross clamp. You could use energy to take this pedicle or clamp and tie it.
Make sure that the packing material is out of the way and that you have most of the tube nicely isolated. Don’t take the tube too far up in the surgical field because, if you lose it, it can be hard to control the bleeding. Ensure that you have grasped the fimbriated end of the tube.
In some cases you can leave a portion of the tube right next to the round ligament (FIGURE 3). You can go back and take that portion later, if you desire. But when it comes to the potential for the fallopian tube to generate carcinoma, most of the concern involves the mid to distal end of the tube rather than the cornual portion.
Once the Shallcross clamp has a good purchase on the pedicle, bring the suture around the clamp and then pass it under the tube so that you encircle the mesosalpinx pedicle (FIGURE 4). It is extremely important during salpingectomy to tie this suture down gently but tightly. In the process, have your assistant flash the Shallcross clamp open when you tie the suture. Otherwise, the suture will tend to tear through the mesosalpinx. Be very careful in your handling of the specimen at this point. Next, cut right along the edge of the clamp to remove the tube.
FIGURE 3 Focus on the distal tube
| FIGURE 4 Clamp and tie the pedicle 
| |
| The cornual portion of the tube (proximal to the round ligament) can be left behind, if desired. The propensity for cancer centers on the distal end of the tube. | Bring the suture around the clamp and then pass it under the tube so that you encircle the mesosalpinx pedicle. |
If you prefer, you can stick-tie the remaining portion again, but usually one tie will suffice because there is such a small pedicle there. The distal portion of the pedicle eventually will necrose close to the tie. The next step is ensuring hemostasis.
On occasion, if you lose the pedicle high in the surgical field, you can try to oversew it. A 2-0 Vicryl suture may be used to place a figure-eight stitch to control bleeding around the mesosalpinx. Alternatively, an energy device may be used for hemostasis. Rarely, if you encounter bleeding that does not respond to the previous suggestions, you may need to remove the ovary to control bleeding if the tissue tears.
Transvaginal technique for salpingo-oophorectomy
Once the hysterectomy is completed, grasp the round ligament, tube, and uterine-ovarian pedicle, placing slight tension on the pedicle, and free the right round ligament to ease isolation of the gonadal vessels. Using electrocautery, carefully transect the round ligament. It is critical when isolating the round ligament to transect only the ligament and not to get deep into the underlying tissue or bleeding will ensue. If you “hug” just the round ligament, you will open into the broad ligament and easily be able to isolate the gonadal pedicle.
Once the pedicle is nicely isolated, readjust your retractors or lighting to improve visualization. Now the gonadal vessels can be isolated up high much more easily (FIGURE 1).
Next, use a Heaney clamp to grab the pedicle, making sure that the ovary is medial to the clamp (FIGURE 2).

| 
| |
| FIGURE 1: ISOLATE THE GONADAL VESSELS Once optimal visualization is achieved, the gonadal vessels can be isolated easily. | FIGURE 2: KEEP THE OVARY MEDIAL TO THE CLAMP Use a Heaney clamp to grab the pedicle, keeping the ovary medial to the clamp. |
In this setting, there are a number of techniques you can use to complete the salpingo-oophorectomy. I tend to doubly ligate the pedicle. To begin, cut the tagging suture to get it out of the way. Then place a free tie lateral to the clamp, bringing it down and underneath to fully encircle the pedicle. Ligate the pedicle then cut the free tie. Follow by cutting the pedicle beside the Heaney clamp and removing the specimen. Stick-tie the remaining pedicle.
Locate the free tie, which is easily identified. Place your needle between that free tie and the clamp so that you do not pierce the vessels proximal to the tie with that needle. Then doubly ligate the pedicle.
Check for hemostasis and, once confirmed, cut the pedicle tie. Because this patient does not have a left tube and ovary, the procedure is now completed.
Conclusion
The tubes are usually readily accessible for removal at the time of vaginal hysterectomy. There is evolving evidence that the tube may play a role in malignancy of the female genital tract. Thus, removal may be preventive. In addition, if there are paratubal cysts or hydrosalpinx from prior tubal ligation, it makes sense to remove the tube. There is little evidence to suggest that removal of the tubes accelerates the menopausal transition due to compromise of the blood supply to the ovaries.
You must be very gentle when handling and removing just the tubes. The mesosalpinx is delicate and easily torn or traumatized. A careful and deliberate approach is warranted.
Bilateral salpingectomy: Key take-aways
Locate the tube. The fallopian tube always lies on top of the ovary and should be found there. On occasion, the abdominal packing used to move the bowel out of the pelvis will “hide” the tube; readjusting this packing often solves the problem.
Be gentle with the mesosalpinx as it is very delicate and can easily avulse. It is very important to “flash the clamp” (open the clamp and then close it) as you free-tie the mesosalpinx to avoid cutting through the delicate pedicle.
Remove as much tube as possible. The fimbriae end of the tube usually is free and easy to identify. Try to remove as much of the tube as possible. Often, a bit of the proximal tube is left in the utero-ovarian pedicle tie.
Clean up. You will often find peritubal cysts or “tubal clips” from a sterilization procedure. I recommend that you remove any of these you encounter to avoid problems down the road. Often, these cysts and clip-like devices are removed as part of the specimen.
Dry up. Always confirm hemostasis before concluding the procedure. If there is bleeding, be sure to assess the mesosalpinx. Occasionally, the pedicle can be torn higher up, near the gonadal vessels. Investigate this region if bleeding seems to be an issue.
Transvaginal salpingo-oophorectomy: Key take-aways
Perfect a technique. There are many approaches to transvaginal removal of the adnexae; pick one and perfect it. The better you are, the fewer complications you will have. Recognize that a different approach (use of a stapler or energy sealing device, for example) may prove useful in some settings. Be surgically versatile and recognize situations that might call for something other than your usual approach.
Optimize visualization. The tubes and ovaries are usually very accessible vaginally. Use an abdominal pack to move the bowel out of the pelvis. Adequate retraction and use of a lighted retractor or suction irrigator will facilitate exposure.
Ligate the gonadal vessels. Retraction of the tube and ovary complex medially away from the pelvic sidewall will allow you to place a clamp (or stapler or energy device) lateral to secure the gonadal vessels and ensure complete removal of the adnexae.
Release the round ligament. Although this step is usually not required, it will allow you to isolate the adnexae more precisely, especially when dealing with an adnexal mass transvaginally. It is critical that you “hug” the round ligament and refrain from penetrating deeply into the underlying tissues, or bleeding will occur. Once the round ligament is released, the tube and ovary are isolated on the gonadal pedicle and can be completely excised with this technique.
Manage bleeding. If suturing, I prefer to doubly ligate the gonadal vessels. Once I clamp the pedicle, I “free-tie” the gonadal vessels with an initial suture. This suture secures the vascular pedicle and prevents retraction. The adnexae can then be removed, followed by placement of a “stick-tie” to re-ligate the pedicle. Although this vascular pedicle is more robust than the mesosalpinx, it, too, can be avulsed, so it is important to proceed with caution. I recommend having a long clamp (uterine packing forceps or MD Anderson clamp) available in your instrument pan to facilitate specific isolation of the gonadal vessels along the pelvic sidewall in the event avulsion does occur.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this article, I describe my technique for a vaginal approach to right salpingectomy with ovarian preservation, as well as right salpingo-oophorectomy, in a patient lacking a left tube and ovary. This technique is fully illustrated on a cadaver in the Web-based master course in vaginal hysterectomy produced by the AAGL and co-sponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. That course is available online at https://www.aagl.org/vaghystwebinar.
For a detailed description of vaginal hysterectomy technique, see the article entitled “Vaginal hysterectomy using basic instrumentation,” by Barbara S. Levy, MD, which appeared in the October 2015 issue of OBG Management. Next month, in the December 2015 issue of the journal, I will detail my strategies for managing complications associated with vaginal hysterectomy, salpingectomy, and salpingo-oophorectomy.
Right salpingectomy
FIGURE 1 Locate the tube |
The fallopian tube will almost always be found on top of the ovary. |
FIGURE 2 Isolate the tube 
|
| Grasp the tube and bring it to the midline. |
Start with light traction
Begin by placing an instrument on the round ligament, tube, and uterine-ovarian pedicle, exerting light traction. Note that the tube will always be found on top of the ovary
(FIGURE 1). Take care during placement of packing material to avoid sweeping the fimbriae of the tube up and out of the surgical field. You may need to play with the packing a bit until you are able to deliver the tube.
Once you identify the tube, isolate it by bringing it down to the midline (FIGURE 2). One thing to note if you’re accustomed to performing bilateral salpingo-oophorectomy: The gonadal pedicle is fairly substantive and can sustain a bit of tugging. However, if you’re performing salpingectomy with ovarian preservation, you need to be much more careful in your handling of the tube because the mesosalpinx is extremely delicate.
After you bring the tube to the midline, grasp it using a Heaney or Shallcross clamp. You could use energy to take this pedicle or clamp and tie it.
Make sure that the packing material is out of the way and that you have most of the tube nicely isolated. Don’t take the tube too far up in the surgical field because, if you lose it, it can be hard to control the bleeding. Ensure that you have grasped the fimbriated end of the tube.
In some cases you can leave a portion of the tube right next to the round ligament (FIGURE 3). You can go back and take that portion later, if you desire. But when it comes to the potential for the fallopian tube to generate carcinoma, most of the concern involves the mid to distal end of the tube rather than the cornual portion.
Once the Shallcross clamp has a good purchase on the pedicle, bring the suture around the clamp and then pass it under the tube so that you encircle the mesosalpinx pedicle (FIGURE 4). It is extremely important during salpingectomy to tie this suture down gently but tightly. In the process, have your assistant flash the Shallcross clamp open when you tie the suture. Otherwise, the suture will tend to tear through the mesosalpinx. Be very careful in your handling of the specimen at this point. Next, cut right along the edge of the clamp to remove the tube.
FIGURE 3 Focus on the distal tube
| FIGURE 4 Clamp and tie the pedicle 
| |
| The cornual portion of the tube (proximal to the round ligament) can be left behind, if desired. The propensity for cancer centers on the distal end of the tube. | Bring the suture around the clamp and then pass it under the tube so that you encircle the mesosalpinx pedicle. |
If you prefer, you can stick-tie the remaining portion again, but usually one tie will suffice because there is such a small pedicle there. The distal portion of the pedicle eventually will necrose close to the tie. The next step is ensuring hemostasis.
On occasion, if you lose the pedicle high in the surgical field, you can try to oversew it. A 2-0 Vicryl suture may be used to place a figure-eight stitch to control bleeding around the mesosalpinx. Alternatively, an energy device may be used for hemostasis. Rarely, if you encounter bleeding that does not respond to the previous suggestions, you may need to remove the ovary to control bleeding if the tissue tears.
Transvaginal technique for salpingo-oophorectomy
Once the hysterectomy is completed, grasp the round ligament, tube, and uterine-ovarian pedicle, placing slight tension on the pedicle, and free the right round ligament to ease isolation of the gonadal vessels. Using electrocautery, carefully transect the round ligament. It is critical when isolating the round ligament to transect only the ligament and not to get deep into the underlying tissue or bleeding will ensue. If you “hug” just the round ligament, you will open into the broad ligament and easily be able to isolate the gonadal pedicle.
Once the pedicle is nicely isolated, readjust your retractors or lighting to improve visualization. Now the gonadal vessels can be isolated up high much more easily (FIGURE 1).
Next, use a Heaney clamp to grab the pedicle, making sure that the ovary is medial to the clamp (FIGURE 2).

| 
| |
| FIGURE 1: ISOLATE THE GONADAL VESSELS Once optimal visualization is achieved, the gonadal vessels can be isolated easily. | FIGURE 2: KEEP THE OVARY MEDIAL TO THE CLAMP Use a Heaney clamp to grab the pedicle, keeping the ovary medial to the clamp. |
In this setting, there are a number of techniques you can use to complete the salpingo-oophorectomy. I tend to doubly ligate the pedicle. To begin, cut the tagging suture to get it out of the way. Then place a free tie lateral to the clamp, bringing it down and underneath to fully encircle the pedicle. Ligate the pedicle then cut the free tie. Follow by cutting the pedicle beside the Heaney clamp and removing the specimen. Stick-tie the remaining pedicle.
Locate the free tie, which is easily identified. Place your needle between that free tie and the clamp so that you do not pierce the vessels proximal to the tie with that needle. Then doubly ligate the pedicle.
Check for hemostasis and, once confirmed, cut the pedicle tie. Because this patient does not have a left tube and ovary, the procedure is now completed.
Conclusion
The tubes are usually readily accessible for removal at the time of vaginal hysterectomy. There is evolving evidence that the tube may play a role in malignancy of the female genital tract. Thus, removal may be preventive. In addition, if there are paratubal cysts or hydrosalpinx from prior tubal ligation, it makes sense to remove the tube. There is little evidence to suggest that removal of the tubes accelerates the menopausal transition due to compromise of the blood supply to the ovaries.
You must be very gentle when handling and removing just the tubes. The mesosalpinx is delicate and easily torn or traumatized. A careful and deliberate approach is warranted.
Bilateral salpingectomy: Key take-aways
Locate the tube. The fallopian tube always lies on top of the ovary and should be found there. On occasion, the abdominal packing used to move the bowel out of the pelvis will “hide” the tube; readjusting this packing often solves the problem.
Be gentle with the mesosalpinx as it is very delicate and can easily avulse. It is very important to “flash the clamp” (open the clamp and then close it) as you free-tie the mesosalpinx to avoid cutting through the delicate pedicle.
Remove as much tube as possible. The fimbriae end of the tube usually is free and easy to identify. Try to remove as much of the tube as possible. Often, a bit of the proximal tube is left in the utero-ovarian pedicle tie.
Clean up. You will often find peritubal cysts or “tubal clips” from a sterilization procedure. I recommend that you remove any of these you encounter to avoid problems down the road. Often, these cysts and clip-like devices are removed as part of the specimen.
Dry up. Always confirm hemostasis before concluding the procedure. If there is bleeding, be sure to assess the mesosalpinx. Occasionally, the pedicle can be torn higher up, near the gonadal vessels. Investigate this region if bleeding seems to be an issue.
Transvaginal salpingo-oophorectomy: Key take-aways
Perfect a technique. There are many approaches to transvaginal removal of the adnexae; pick one and perfect it. The better you are, the fewer complications you will have. Recognize that a different approach (use of a stapler or energy sealing device, for example) may prove useful in some settings. Be surgically versatile and recognize situations that might call for something other than your usual approach.
Optimize visualization. The tubes and ovaries are usually very accessible vaginally. Use an abdominal pack to move the bowel out of the pelvis. Adequate retraction and use of a lighted retractor or suction irrigator will facilitate exposure.
Ligate the gonadal vessels. Retraction of the tube and ovary complex medially away from the pelvic sidewall will allow you to place a clamp (or stapler or energy device) lateral to secure the gonadal vessels and ensure complete removal of the adnexae.
Release the round ligament. Although this step is usually not required, it will allow you to isolate the adnexae more precisely, especially when dealing with an adnexal mass transvaginally. It is critical that you “hug” the round ligament and refrain from penetrating deeply into the underlying tissues, or bleeding will occur. Once the round ligament is released, the tube and ovary are isolated on the gonadal pedicle and can be completely excised with this technique.
Manage bleeding. If suturing, I prefer to doubly ligate the gonadal vessels. Once I clamp the pedicle, I “free-tie” the gonadal vessels with an initial suture. This suture secures the vascular pedicle and prevents retraction. The adnexae can then be removed, followed by placement of a “stick-tie” to re-ligate the pedicle. Although this vascular pedicle is more robust than the mesosalpinx, it, too, can be avulsed, so it is important to proceed with caution. I recommend having a long clamp (uterine packing forceps or MD Anderson clamp) available in your instrument pan to facilitate specific isolation of the gonadal vessels along the pelvic sidewall in the event avulsion does occur.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this article, I describe my technique for a vaginal approach to right salpingectomy with ovarian preservation, as well as right salpingo-oophorectomy, in a patient lacking a left tube and ovary. This technique is fully illustrated on a cadaver in the Web-based master course in vaginal hysterectomy produced by the AAGL and co-sponsored by the American College of Obstetricians and Gynecologists and the Society of Gynecologic Surgeons. That course is available online at https://www.aagl.org/vaghystwebinar.
For a detailed description of vaginal hysterectomy technique, see the article entitled “Vaginal hysterectomy using basic instrumentation,” by Barbara S. Levy, MD, which appeared in the October 2015 issue of OBG Management. Next month, in the December 2015 issue of the journal, I will detail my strategies for managing complications associated with vaginal hysterectomy, salpingectomy, and salpingo-oophorectomy.
Right salpingectomy
FIGURE 1 Locate the tube |
The fallopian tube will almost always be found on top of the ovary. |
FIGURE 2 Isolate the tube 
|
| Grasp the tube and bring it to the midline. |
Start with light traction
Begin by placing an instrument on the round ligament, tube, and uterine-ovarian pedicle, exerting light traction. Note that the tube will always be found on top of the ovary
(FIGURE 1). Take care during placement of packing material to avoid sweeping the fimbriae of the tube up and out of the surgical field. You may need to play with the packing a bit until you are able to deliver the tube.
Once you identify the tube, isolate it by bringing it down to the midline (FIGURE 2). One thing to note if you’re accustomed to performing bilateral salpingo-oophorectomy: The gonadal pedicle is fairly substantive and can sustain a bit of tugging. However, if you’re performing salpingectomy with ovarian preservation, you need to be much more careful in your handling of the tube because the mesosalpinx is extremely delicate.
After you bring the tube to the midline, grasp it using a Heaney or Shallcross clamp. You could use energy to take this pedicle or clamp and tie it.
Make sure that the packing material is out of the way and that you have most of the tube nicely isolated. Don’t take the tube too far up in the surgical field because, if you lose it, it can be hard to control the bleeding. Ensure that you have grasped the fimbriated end of the tube.
In some cases you can leave a portion of the tube right next to the round ligament (FIGURE 3). You can go back and take that portion later, if you desire. But when it comes to the potential for the fallopian tube to generate carcinoma, most of the concern involves the mid to distal end of the tube rather than the cornual portion.
Once the Shallcross clamp has a good purchase on the pedicle, bring the suture around the clamp and then pass it under the tube so that you encircle the mesosalpinx pedicle (FIGURE 4). It is extremely important during salpingectomy to tie this suture down gently but tightly. In the process, have your assistant flash the Shallcross clamp open when you tie the suture. Otherwise, the suture will tend to tear through the mesosalpinx. Be very careful in your handling of the specimen at this point. Next, cut right along the edge of the clamp to remove the tube.
FIGURE 3 Focus on the distal tube
| FIGURE 4 Clamp and tie the pedicle 
| |
| The cornual portion of the tube (proximal to the round ligament) can be left behind, if desired. The propensity for cancer centers on the distal end of the tube. | Bring the suture around the clamp and then pass it under the tube so that you encircle the mesosalpinx pedicle. |
If you prefer, you can stick-tie the remaining portion again, but usually one tie will suffice because there is such a small pedicle there. The distal portion of the pedicle eventually will necrose close to the tie. The next step is ensuring hemostasis.
On occasion, if you lose the pedicle high in the surgical field, you can try to oversew it. A 2-0 Vicryl suture may be used to place a figure-eight stitch to control bleeding around the mesosalpinx. Alternatively, an energy device may be used for hemostasis. Rarely, if you encounter bleeding that does not respond to the previous suggestions, you may need to remove the ovary to control bleeding if the tissue tears.
Transvaginal technique for salpingo-oophorectomy
Once the hysterectomy is completed, grasp the round ligament, tube, and uterine-ovarian pedicle, placing slight tension on the pedicle, and free the right round ligament to ease isolation of the gonadal vessels. Using electrocautery, carefully transect the round ligament. It is critical when isolating the round ligament to transect only the ligament and not to get deep into the underlying tissue or bleeding will ensue. If you “hug” just the round ligament, you will open into the broad ligament and easily be able to isolate the gonadal pedicle.
Once the pedicle is nicely isolated, readjust your retractors or lighting to improve visualization. Now the gonadal vessels can be isolated up high much more easily (FIGURE 1).
Next, use a Heaney clamp to grab the pedicle, making sure that the ovary is medial to the clamp (FIGURE 2).

| 
| |
| FIGURE 1: ISOLATE THE GONADAL VESSELS Once optimal visualization is achieved, the gonadal vessels can be isolated easily. | FIGURE 2: KEEP THE OVARY MEDIAL TO THE CLAMP Use a Heaney clamp to grab the pedicle, keeping the ovary medial to the clamp. |
In this setting, there are a number of techniques you can use to complete the salpingo-oophorectomy. I tend to doubly ligate the pedicle. To begin, cut the tagging suture to get it out of the way. Then place a free tie lateral to the clamp, bringing it down and underneath to fully encircle the pedicle. Ligate the pedicle then cut the free tie. Follow by cutting the pedicle beside the Heaney clamp and removing the specimen. Stick-tie the remaining pedicle.
Locate the free tie, which is easily identified. Place your needle between that free tie and the clamp so that you do not pierce the vessels proximal to the tie with that needle. Then doubly ligate the pedicle.
Check for hemostasis and, once confirmed, cut the pedicle tie. Because this patient does not have a left tube and ovary, the procedure is now completed.
Conclusion
The tubes are usually readily accessible for removal at the time of vaginal hysterectomy. There is evolving evidence that the tube may play a role in malignancy of the female genital tract. Thus, removal may be preventive. In addition, if there are paratubal cysts or hydrosalpinx from prior tubal ligation, it makes sense to remove the tube. There is little evidence to suggest that removal of the tubes accelerates the menopausal transition due to compromise of the blood supply to the ovaries.
You must be very gentle when handling and removing just the tubes. The mesosalpinx is delicate and easily torn or traumatized. A careful and deliberate approach is warranted.
Bilateral salpingectomy: Key take-aways
Locate the tube. The fallopian tube always lies on top of the ovary and should be found there. On occasion, the abdominal packing used to move the bowel out of the pelvis will “hide” the tube; readjusting this packing often solves the problem.
Be gentle with the mesosalpinx as it is very delicate and can easily avulse. It is very important to “flash the clamp” (open the clamp and then close it) as you free-tie the mesosalpinx to avoid cutting through the delicate pedicle.
Remove as much tube as possible. The fimbriae end of the tube usually is free and easy to identify. Try to remove as much of the tube as possible. Often, a bit of the proximal tube is left in the utero-ovarian pedicle tie.
Clean up. You will often find peritubal cysts or “tubal clips” from a sterilization procedure. I recommend that you remove any of these you encounter to avoid problems down the road. Often, these cysts and clip-like devices are removed as part of the specimen.
Dry up. Always confirm hemostasis before concluding the procedure. If there is bleeding, be sure to assess the mesosalpinx. Occasionally, the pedicle can be torn higher up, near the gonadal vessels. Investigate this region if bleeding seems to be an issue.
Transvaginal salpingo-oophorectomy: Key take-aways
Perfect a technique. There are many approaches to transvaginal removal of the adnexae; pick one and perfect it. The better you are, the fewer complications you will have. Recognize that a different approach (use of a stapler or energy sealing device, for example) may prove useful in some settings. Be surgically versatile and recognize situations that might call for something other than your usual approach.
Optimize visualization. The tubes and ovaries are usually very accessible vaginally. Use an abdominal pack to move the bowel out of the pelvis. Adequate retraction and use of a lighted retractor or suction irrigator will facilitate exposure.
Ligate the gonadal vessels. Retraction of the tube and ovary complex medially away from the pelvic sidewall will allow you to place a clamp (or stapler or energy device) lateral to secure the gonadal vessels and ensure complete removal of the adnexae.
Release the round ligament. Although this step is usually not required, it will allow you to isolate the adnexae more precisely, especially when dealing with an adnexal mass transvaginally. It is critical that you “hug” the round ligament and refrain from penetrating deeply into the underlying tissues, or bleeding will occur. Once the round ligament is released, the tube and ovary are isolated on the gonadal pedicle and can be completely excised with this technique.
Manage bleeding. If suturing, I prefer to doubly ligate the gonadal vessels. Once I clamp the pedicle, I “free-tie” the gonadal vessels with an initial suture. This suture secures the vascular pedicle and prevents retraction. The adnexae can then be removed, followed by placement of a “stick-tie” to re-ligate the pedicle. Although this vascular pedicle is more robust than the mesosalpinx, it, too, can be avulsed, so it is important to proceed with caution. I recommend having a long clamp (uterine packing forceps or MD Anderson clamp) available in your instrument pan to facilitate specific isolation of the gonadal vessels along the pelvic sidewall in the event avulsion does occur.
Share your thoughts! Send your Letter to the Editor to [email protected]. Please include your name and the city and state in which you practice.
In this Article
- Technique for salpingo-oophorectomy
- Salpingectomy: Key take-aways
- Pointers for salpingo- oophorectomy