User login
Minimally invasive ICH lysis safely helps when clot adequately shrinks
HONOLULU – A minimally invasive approach to lysing an intracerebral hemorrhage clot was safe but failed to produce a statistically significant improvement in long-term functional outcome when compared with usual medical management in a phase 3 randomized trial of 499 patients. However, the results also showed that when the procedure met its acute goal of cutting residual clot to a volume of 15 mL or less, it significantly boosted the percentage of patients with a modified Rankin Scale score of 0-3 when assessed a year after treatment, Daniel F. Hanley Jr., MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“Improved function and increased survival was produced by surgical [clot] reduction to 15 mL or less,” said Dr. Hanley, professor of neurology at Johns Hopkins University, Baltimore, and one of the organizers of the MISTIE III trial.
When assessed by another measure, treated patients showed significant, long-term functional improvement compared with controls when their clot burden dropped by at least 70% following the lytic procedure.
“This is the first description of specific thresholds of hematoma evacuation that impact functional outcomes in intracerebral hemorrhage surgery trials,” said Issam A. Awad, MD, professor of surgery and director of neurovascular surgery at the University of Chicago and coprincipal investigator of the trial.
The problem in the trial was that the surgeons who performed the interventions did not treat many patients aggressively enough to reach these thresholds. They achieved the prespecified goal of residual clot of 15 mL or less in 59% of patients, Dr. Hanley reported, even though the study protocol called for serial infusions of 1 mg of tissue plasminogen activator (Alteplase) into the clot via a placed catheter as many as nine times, administered at 8 hour intervals, with treatment to continue until patients reached the goal residual volume or until they had received all nine doses. In actual practice during the study, operators administered a median of four lytic doses.
“We showed that this goal was important, but not all sites embraced the goal,” Dr. Hanley said. Even though the participating clinicians had a specific interest in intracerebral hemorrhage patients and in this procedure, several nonetheless “had a poor understanding of the goal,” he said in an interview. He attributed the less-than-aggressive approach many operators took to the safety concern that further doses of the lytic drug could trigger recurrent hemorrhage.
“We showed that the goal was important. I think they will embrace the [hematoma evacuation] goal when they see these data,” Dr. Hanley predicted.
An as-treated analysis of the data that focused on the 145 of 246 patients who were treated with minimally invasive lysis and reached the target residual volume and who were then functionally assessed a year later, showed that the rate of patients with a modified Rankin Scale score of 0-3 was 53%, compared with 42% among the controls, an 11% difference.
This shows “a large treatment effect. This is a big, transformative treatment,” Dr. Hanley said. “Our data clearly show that more than half the patients had a positive outcome when their surgeons were more aggressive about clot removal.” He cautioned that the trial was not just about the volume of clot removed but was also about doing it in a gentle way, with a minimum of tissue trauma. Other approaches to reducing hematoma volume may be faster or more complete but they cannot now match the record of safety and efficacy documented in MISTIE III for minimally invasive clot lysis, Dr. Hanley noted.
MISTIE III (Minimally Invasive Surgery Plus Rt-PA for ICH Evacuation Phase III) enrolled patients at 78 centers in the United States and several other countries during 2013-2017. Patients had to enroll 12-72 hours after onset and present with a hematoma volume of at least 30 mL. Participating neurosurgeons used image-guided neuronavigation to place a 4- to 6-mm cannula through the clot, ideally straight through the hematoma’s long axis and with the tip placed within the largest clot segment. Among the 110 surgeons who performed this procedure during the study, 88% had never done it before, and operator and site experience linked with better performance. No surgeon who had already performed four minimally invasive lytic cases, and no center that had already performed seven cases, had a subsequent patient with a residual volume that exceeded 30 mL, Dr. Awad said. The surgical experience during the trial showed that catheter repositioning and using a second catheter were both safe ways to maximize evacuation of the hematoma, he added.
The trial’s primary endpoint, the rate of patients with a modified Rankin Scale score of 0-3 at 1 year after treatment in a modified intention-to-treat analysis that included all patients regardless of the amount of hematoma evacuation they received, showed a 45% rate among the patients who underwent minimally invasive lysis and a 41% rate among those in the control arm, a difference that was not statistically significant. Safety assessments showed that patients treated with the investigational approach had significantly lower mortality 7 days after treatment: 0.8% compared with 4.0%. By 1 year after treatment, mortality was cut by one-third in the minimally invasive patients, compared with the control patients, also a statistically significant difference. The rates of symptomatic bleeds and brain infections were similar in the two treatment groups, Dr. Hanley reported. Concurrently with his talk at the conference, a paper with the primary study results appeared online (Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736[19]30195-3).
MISTIE III was supported by the National Institute of Neurological Disorders and Stroke. The trial received no commercial support aside from free tissue plasminogen activator (Alteplase) supplied by Genentech. Dr. Hanley has been a consultant to BrainScope, Neurotrope, Portola, and Op2Lysis, and he has served as an expert witness on behalf of Medtronic. Dr. Awad had no disclosures.
SOURCE: Hanley DF et al. ISC 2019, Abstract LB4; Awad IS et al. ISC 2019, Abstract LB5.
The MISTIE III results showed that this approach to clot lysis is safe and feasible for surgeons to perform even if they have had limited experience with the procedure. I think that based on these findings, minimally-invasive clot lysis will become widely adopted. It’s pretty simple to perform in most patients. At my center in Houston, we already use it on a routine basis in patients like those enrolled in MISTIE III.
Some people may focus on the neutral primary endpoint result from the MISTIE III trial, but the study made two very important findings. First, the results showed that we have improved medical management of patients who have an intracerebral hemorrhage. The 1-year functional outcomes of patients in the control group of the study who had a 41% rate of scoring 0-3 on the modified Rankin Scale after 1 year was much better than we have seen in these patients in the past. Second, the results gave a clear signal that the more clot an operator can lyse to get the residual clot to 15 mL or less, the better patients do. Faster clot lysis might also be important.
It’s hard to call the minimally-invasive approach used in MISTIE III the new standard-of-care approach for these patients given the neutral primary endpoint of the study. On the other hand, if you have a treatment that poses little risk to patients and that you know could benefit them if it succeeds in minimizing residual clot volume, then it makes sense to try it. It’s a low-risk treatment with reasonable potential for benefit. Its demonstrated safety is very important.
Louise D. McCullough, MD, PhD , is a professor of neurology and chair of neurology at the University of Texas, Houston. She had no disclosures. She made these comments in an interview.
The MISTIE III results showed that this approach to clot lysis is safe and feasible for surgeons to perform even if they have had limited experience with the procedure. I think that based on these findings, minimally-invasive clot lysis will become widely adopted. It’s pretty simple to perform in most patients. At my center in Houston, we already use it on a routine basis in patients like those enrolled in MISTIE III.
Some people may focus on the neutral primary endpoint result from the MISTIE III trial, but the study made two very important findings. First, the results showed that we have improved medical management of patients who have an intracerebral hemorrhage. The 1-year functional outcomes of patients in the control group of the study who had a 41% rate of scoring 0-3 on the modified Rankin Scale after 1 year was much better than we have seen in these patients in the past. Second, the results gave a clear signal that the more clot an operator can lyse to get the residual clot to 15 mL or less, the better patients do. Faster clot lysis might also be important.
It’s hard to call the minimally-invasive approach used in MISTIE III the new standard-of-care approach for these patients given the neutral primary endpoint of the study. On the other hand, if you have a treatment that poses little risk to patients and that you know could benefit them if it succeeds in minimizing residual clot volume, then it makes sense to try it. It’s a low-risk treatment with reasonable potential for benefit. Its demonstrated safety is very important.
Louise D. McCullough, MD, PhD , is a professor of neurology and chair of neurology at the University of Texas, Houston. She had no disclosures. She made these comments in an interview.
The MISTIE III results showed that this approach to clot lysis is safe and feasible for surgeons to perform even if they have had limited experience with the procedure. I think that based on these findings, minimally-invasive clot lysis will become widely adopted. It’s pretty simple to perform in most patients. At my center in Houston, we already use it on a routine basis in patients like those enrolled in MISTIE III.
Some people may focus on the neutral primary endpoint result from the MISTIE III trial, but the study made two very important findings. First, the results showed that we have improved medical management of patients who have an intracerebral hemorrhage. The 1-year functional outcomes of patients in the control group of the study who had a 41% rate of scoring 0-3 on the modified Rankin Scale after 1 year was much better than we have seen in these patients in the past. Second, the results gave a clear signal that the more clot an operator can lyse to get the residual clot to 15 mL or less, the better patients do. Faster clot lysis might also be important.
It’s hard to call the minimally-invasive approach used in MISTIE III the new standard-of-care approach for these patients given the neutral primary endpoint of the study. On the other hand, if you have a treatment that poses little risk to patients and that you know could benefit them if it succeeds in minimizing residual clot volume, then it makes sense to try it. It’s a low-risk treatment with reasonable potential for benefit. Its demonstrated safety is very important.
Louise D. McCullough, MD, PhD , is a professor of neurology and chair of neurology at the University of Texas, Houston. She had no disclosures. She made these comments in an interview.
HONOLULU – A minimally invasive approach to lysing an intracerebral hemorrhage clot was safe but failed to produce a statistically significant improvement in long-term functional outcome when compared with usual medical management in a phase 3 randomized trial of 499 patients. However, the results also showed that when the procedure met its acute goal of cutting residual clot to a volume of 15 mL or less, it significantly boosted the percentage of patients with a modified Rankin Scale score of 0-3 when assessed a year after treatment, Daniel F. Hanley Jr., MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“Improved function and increased survival was produced by surgical [clot] reduction to 15 mL or less,” said Dr. Hanley, professor of neurology at Johns Hopkins University, Baltimore, and one of the organizers of the MISTIE III trial.
When assessed by another measure, treated patients showed significant, long-term functional improvement compared with controls when their clot burden dropped by at least 70% following the lytic procedure.
“This is the first description of specific thresholds of hematoma evacuation that impact functional outcomes in intracerebral hemorrhage surgery trials,” said Issam A. Awad, MD, professor of surgery and director of neurovascular surgery at the University of Chicago and coprincipal investigator of the trial.
The problem in the trial was that the surgeons who performed the interventions did not treat many patients aggressively enough to reach these thresholds. They achieved the prespecified goal of residual clot of 15 mL or less in 59% of patients, Dr. Hanley reported, even though the study protocol called for serial infusions of 1 mg of tissue plasminogen activator (Alteplase) into the clot via a placed catheter as many as nine times, administered at 8 hour intervals, with treatment to continue until patients reached the goal residual volume or until they had received all nine doses. In actual practice during the study, operators administered a median of four lytic doses.
“We showed that this goal was important, but not all sites embraced the goal,” Dr. Hanley said. Even though the participating clinicians had a specific interest in intracerebral hemorrhage patients and in this procedure, several nonetheless “had a poor understanding of the goal,” he said in an interview. He attributed the less-than-aggressive approach many operators took to the safety concern that further doses of the lytic drug could trigger recurrent hemorrhage.
“We showed that the goal was important. I think they will embrace the [hematoma evacuation] goal when they see these data,” Dr. Hanley predicted.
An as-treated analysis of the data that focused on the 145 of 246 patients who were treated with minimally invasive lysis and reached the target residual volume and who were then functionally assessed a year later, showed that the rate of patients with a modified Rankin Scale score of 0-3 was 53%, compared with 42% among the controls, an 11% difference.
This shows “a large treatment effect. This is a big, transformative treatment,” Dr. Hanley said. “Our data clearly show that more than half the patients had a positive outcome when their surgeons were more aggressive about clot removal.” He cautioned that the trial was not just about the volume of clot removed but was also about doing it in a gentle way, with a minimum of tissue trauma. Other approaches to reducing hematoma volume may be faster or more complete but they cannot now match the record of safety and efficacy documented in MISTIE III for minimally invasive clot lysis, Dr. Hanley noted.
MISTIE III (Minimally Invasive Surgery Plus Rt-PA for ICH Evacuation Phase III) enrolled patients at 78 centers in the United States and several other countries during 2013-2017. Patients had to enroll 12-72 hours after onset and present with a hematoma volume of at least 30 mL. Participating neurosurgeons used image-guided neuronavigation to place a 4- to 6-mm cannula through the clot, ideally straight through the hematoma’s long axis and with the tip placed within the largest clot segment. Among the 110 surgeons who performed this procedure during the study, 88% had never done it before, and operator and site experience linked with better performance. No surgeon who had already performed four minimally invasive lytic cases, and no center that had already performed seven cases, had a subsequent patient with a residual volume that exceeded 30 mL, Dr. Awad said. The surgical experience during the trial showed that catheter repositioning and using a second catheter were both safe ways to maximize evacuation of the hematoma, he added.
The trial’s primary endpoint, the rate of patients with a modified Rankin Scale score of 0-3 at 1 year after treatment in a modified intention-to-treat analysis that included all patients regardless of the amount of hematoma evacuation they received, showed a 45% rate among the patients who underwent minimally invasive lysis and a 41% rate among those in the control arm, a difference that was not statistically significant. Safety assessments showed that patients treated with the investigational approach had significantly lower mortality 7 days after treatment: 0.8% compared with 4.0%. By 1 year after treatment, mortality was cut by one-third in the minimally invasive patients, compared with the control patients, also a statistically significant difference. The rates of symptomatic bleeds and brain infections were similar in the two treatment groups, Dr. Hanley reported. Concurrently with his talk at the conference, a paper with the primary study results appeared online (Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736[19]30195-3).
MISTIE III was supported by the National Institute of Neurological Disorders and Stroke. The trial received no commercial support aside from free tissue plasminogen activator (Alteplase) supplied by Genentech. Dr. Hanley has been a consultant to BrainScope, Neurotrope, Portola, and Op2Lysis, and he has served as an expert witness on behalf of Medtronic. Dr. Awad had no disclosures.
SOURCE: Hanley DF et al. ISC 2019, Abstract LB4; Awad IS et al. ISC 2019, Abstract LB5.
HONOLULU – A minimally invasive approach to lysing an intracerebral hemorrhage clot was safe but failed to produce a statistically significant improvement in long-term functional outcome when compared with usual medical management in a phase 3 randomized trial of 499 patients. However, the results also showed that when the procedure met its acute goal of cutting residual clot to a volume of 15 mL or less, it significantly boosted the percentage of patients with a modified Rankin Scale score of 0-3 when assessed a year after treatment, Daniel F. Hanley Jr., MD, said at the International Stroke Conference, sponsored by the American Heart Association.
“Improved function and increased survival was produced by surgical [clot] reduction to 15 mL or less,” said Dr. Hanley, professor of neurology at Johns Hopkins University, Baltimore, and one of the organizers of the MISTIE III trial.
When assessed by another measure, treated patients showed significant, long-term functional improvement compared with controls when their clot burden dropped by at least 70% following the lytic procedure.
“This is the first description of specific thresholds of hematoma evacuation that impact functional outcomes in intracerebral hemorrhage surgery trials,” said Issam A. Awad, MD, professor of surgery and director of neurovascular surgery at the University of Chicago and coprincipal investigator of the trial.
The problem in the trial was that the surgeons who performed the interventions did not treat many patients aggressively enough to reach these thresholds. They achieved the prespecified goal of residual clot of 15 mL or less in 59% of patients, Dr. Hanley reported, even though the study protocol called for serial infusions of 1 mg of tissue plasminogen activator (Alteplase) into the clot via a placed catheter as many as nine times, administered at 8 hour intervals, with treatment to continue until patients reached the goal residual volume or until they had received all nine doses. In actual practice during the study, operators administered a median of four lytic doses.
“We showed that this goal was important, but not all sites embraced the goal,” Dr. Hanley said. Even though the participating clinicians had a specific interest in intracerebral hemorrhage patients and in this procedure, several nonetheless “had a poor understanding of the goal,” he said in an interview. He attributed the less-than-aggressive approach many operators took to the safety concern that further doses of the lytic drug could trigger recurrent hemorrhage.
“We showed that the goal was important. I think they will embrace the [hematoma evacuation] goal when they see these data,” Dr. Hanley predicted.
An as-treated analysis of the data that focused on the 145 of 246 patients who were treated with minimally invasive lysis and reached the target residual volume and who were then functionally assessed a year later, showed that the rate of patients with a modified Rankin Scale score of 0-3 was 53%, compared with 42% among the controls, an 11% difference.
This shows “a large treatment effect. This is a big, transformative treatment,” Dr. Hanley said. “Our data clearly show that more than half the patients had a positive outcome when their surgeons were more aggressive about clot removal.” He cautioned that the trial was not just about the volume of clot removed but was also about doing it in a gentle way, with a minimum of tissue trauma. Other approaches to reducing hematoma volume may be faster or more complete but they cannot now match the record of safety and efficacy documented in MISTIE III for minimally invasive clot lysis, Dr. Hanley noted.
MISTIE III (Minimally Invasive Surgery Plus Rt-PA for ICH Evacuation Phase III) enrolled patients at 78 centers in the United States and several other countries during 2013-2017. Patients had to enroll 12-72 hours after onset and present with a hematoma volume of at least 30 mL. Participating neurosurgeons used image-guided neuronavigation to place a 4- to 6-mm cannula through the clot, ideally straight through the hematoma’s long axis and with the tip placed within the largest clot segment. Among the 110 surgeons who performed this procedure during the study, 88% had never done it before, and operator and site experience linked with better performance. No surgeon who had already performed four minimally invasive lytic cases, and no center that had already performed seven cases, had a subsequent patient with a residual volume that exceeded 30 mL, Dr. Awad said. The surgical experience during the trial showed that catheter repositioning and using a second catheter were both safe ways to maximize evacuation of the hematoma, he added.
The trial’s primary endpoint, the rate of patients with a modified Rankin Scale score of 0-3 at 1 year after treatment in a modified intention-to-treat analysis that included all patients regardless of the amount of hematoma evacuation they received, showed a 45% rate among the patients who underwent minimally invasive lysis and a 41% rate among those in the control arm, a difference that was not statistically significant. Safety assessments showed that patients treated with the investigational approach had significantly lower mortality 7 days after treatment: 0.8% compared with 4.0%. By 1 year after treatment, mortality was cut by one-third in the minimally invasive patients, compared with the control patients, also a statistically significant difference. The rates of symptomatic bleeds and brain infections were similar in the two treatment groups, Dr. Hanley reported. Concurrently with his talk at the conference, a paper with the primary study results appeared online (Lancet. 2019 Feb 7. doi: 10.1016/S0140-6736[19]30195-3).
MISTIE III was supported by the National Institute of Neurological Disorders and Stroke. The trial received no commercial support aside from free tissue plasminogen activator (Alteplase) supplied by Genentech. Dr. Hanley has been a consultant to BrainScope, Neurotrope, Portola, and Op2Lysis, and he has served as an expert witness on behalf of Medtronic. Dr. Awad had no disclosures.
SOURCE: Hanley DF et al. ISC 2019, Abstract LB4; Awad IS et al. ISC 2019, Abstract LB5.
REPORTING FROM ISC 2019
Key clinical point: Minimally-invasive intracerebral clot lysis was safe and often effective when the residual clot shrank to 15 mL or less.
Major finding: One year after entry, 45% of MISTIE-treated patients and 41% of controls had a modified Rankin Scale score of 0-3.
Study details: MISTIE III, a multicenter, international, randomized trial of 499 patients.
Disclosures: MISTIE III was supported by the National Institute of Neurological Disorders and Stroke. The trial received no commercial support aside from free tissue plasminogen activator (Alteplase) supplied by Genentech. Dr. Hanley has been a consultant to BrainScope, Neurotrope, Portola, and Op2Lysis, and he has served as an expert witness on behalf of Medtronic. Dr. Awad had no disclosures.
Source: Hanley DF et al. ISC 2019, Abstract LB4; Awad IS et al. ISC 2019, Abstract LB5.
Acceptance and commitment therapy reduced IBD stress, depression
Eight weeks of a mindfulness intervention known as acceptance and commitment therapy (ACT) significantly improved stress and depression among patients with inflammatory bowel disease, and these improvements persisted for at least 12 weeks after therapy ended, according to the results of a randomized, controlled trial.
Source: The American Gastroenterological Association
In the intention-to-treat analysis, stress symptoms, as measured by the Depression Anxiety and Stress Scales (DASS-21), improved by 39% at week 8 and by 45% at week 20, reported Brona Wynne, PhD, of University College Dublin together with her associates. These improvements were highly significant compared with baseline and treatment as usual (P = .001 for both comparisons). “Post hoc analyses indicated that baseline stress levels were similar in control and treatment groups,” the researchers wrote in Gastroenterology. “The results of the per protocol analysis were comparable, with a 43% and 49% reduction in stress in the treatment group from baseline to 8 and 20 weeks.”
Multiple studies have documented high levels of stress and psychological dysfunction among patients with Crohn’s disease and ulcerative colitis. Studies of various mindfulness therapy, relaxation, stress management, cognitive-behavioral therapy, and hypnotherapy interventions often failed to collect key clinical data or were underpowered, uncontrolled, and unrandomized. Acceptance and commitment therapy uses mindfulness to identify adverse thoughts and experiences, accept these as part of life, and recommit to “move towards values that have been identified and adopted by the individual,” the investigators wrote. “This can be defined as the ability to contact the present moment more fully as a conscious human being and to change, or persist in, behavior when doing so serves valued ends.”
Their single-center study, which they said was the first to evaluate ACT in IBD patients, included 79 individuals with stable or mildly active Crohn’s disease (38 patients) or ulcerative colitis (41 patients) who were randomly assigned to ACT (37 patients) or control treatment as usual (42 patients). The two comparison groups were demographically and clinically similar. The ACT program involved eight 90-minute, weekly sessions of groups of 14-16 individuals, led by a single psychologist who tailored the course material toward IBD with a focus on lowering stress. An independent psychologist observed each session to assess adherence to protocol.
Not only did ACT meet the primary study endpoint, it also produced a 25% decrease in perceived stress (on a 1-10 scale) by week 8 and a 27% decrease in perceived stress by week 20 (P less than .001 versus treatment as usual). Depression scores in the ACT group also fell by 47% by week 8 and by 45% at week 20 (P = .01 versus treatment as usual). Anxiety levels decreased by 29% at week 8 and by 31% at week 20, but these improvements did not significantly differ from those in the control group (P = .39).
Interestingly, ACT did not significantly improve symptom burden, activities of daily living, disease-related worry, general well-being, C-reactive protein (CRP) levels, fecal calprotectin levels, or scores on the version used of the Clinical Assessment of Depression (CAD) or the short Mayo assessment. Hair cortisol levels showed an association with baseline stress and anxiety, but not with treatment response.
Care programs for IBD increasingly emphasize mental health services despite a lack of robust trials to support these interventions, the investigators noted. Thus, their findings highlight “the need for researchers and clinicians to further develop and optimize the content and delivery of psychological programs for IBD patients.”
Tillotts Pharma and Boston Scientific provided partial funding, but had no other role in the study. The researchers reported having no relevant conflicts of interest.
SOURCE: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
Factors that affect stress level and mood symptoms are vast when it comes to living with inflammatory bowel disease (IBD). Comorbid mood symptoms are common in patients with IBD, and psychological interventions are increasingly recommended as part of holistic, multidisciplinary treatment planning. Additionally, patients are open to GI-focused psychology treatments given the recognition that the complexities of living with IBD strongly influence emotional factors.
What must be acknowledged is the importance of long-term adherence to skills learned during the 8 weeks of ACT. Stress and mood symptoms tend to be more prevalent during times of flare. Given the relapsing and remitting nature of IBD, it must be conveyed that patients will need to continue the practice of this mindfulness-based intervention in the long term. Future studies are encouraged to look at longitudinal data assessing the manner in which these patients used their skill set during periods of flare or disease-related stress.
Factors that affect stress level and mood symptoms are vast when it comes to living with inflammatory bowel disease (IBD). Comorbid mood symptoms are common in patients with IBD, and psychological interventions are increasingly recommended as part of holistic, multidisciplinary treatment planning. Additionally, patients are open to GI-focused psychology treatments given the recognition that the complexities of living with IBD strongly influence emotional factors.
What must be acknowledged is the importance of long-term adherence to skills learned during the 8 weeks of ACT. Stress and mood symptoms tend to be more prevalent during times of flare. Given the relapsing and remitting nature of IBD, it must be conveyed that patients will need to continue the practice of this mindfulness-based intervention in the long term. Future studies are encouraged to look at longitudinal data assessing the manner in which these patients used their skill set during periods of flare or disease-related stress.
Factors that affect stress level and mood symptoms are vast when it comes to living with inflammatory bowel disease (IBD). Comorbid mood symptoms are common in patients with IBD, and psychological interventions are increasingly recommended as part of holistic, multidisciplinary treatment planning. Additionally, patients are open to GI-focused psychology treatments given the recognition that the complexities of living with IBD strongly influence emotional factors.
What must be acknowledged is the importance of long-term adherence to skills learned during the 8 weeks of ACT. Stress and mood symptoms tend to be more prevalent during times of flare. Given the relapsing and remitting nature of IBD, it must be conveyed that patients will need to continue the practice of this mindfulness-based intervention in the long term. Future studies are encouraged to look at longitudinal data assessing the manner in which these patients used their skill set during periods of flare or disease-related stress.
Eight weeks of a mindfulness intervention known as acceptance and commitment therapy (ACT) significantly improved stress and depression among patients with inflammatory bowel disease, and these improvements persisted for at least 12 weeks after therapy ended, according to the results of a randomized, controlled trial.
Source: The American Gastroenterological Association
In the intention-to-treat analysis, stress symptoms, as measured by the Depression Anxiety and Stress Scales (DASS-21), improved by 39% at week 8 and by 45% at week 20, reported Brona Wynne, PhD, of University College Dublin together with her associates. These improvements were highly significant compared with baseline and treatment as usual (P = .001 for both comparisons). “Post hoc analyses indicated that baseline stress levels were similar in control and treatment groups,” the researchers wrote in Gastroenterology. “The results of the per protocol analysis were comparable, with a 43% and 49% reduction in stress in the treatment group from baseline to 8 and 20 weeks.”
Multiple studies have documented high levels of stress and psychological dysfunction among patients with Crohn’s disease and ulcerative colitis. Studies of various mindfulness therapy, relaxation, stress management, cognitive-behavioral therapy, and hypnotherapy interventions often failed to collect key clinical data or were underpowered, uncontrolled, and unrandomized. Acceptance and commitment therapy uses mindfulness to identify adverse thoughts and experiences, accept these as part of life, and recommit to “move towards values that have been identified and adopted by the individual,” the investigators wrote. “This can be defined as the ability to contact the present moment more fully as a conscious human being and to change, or persist in, behavior when doing so serves valued ends.”
Their single-center study, which they said was the first to evaluate ACT in IBD patients, included 79 individuals with stable or mildly active Crohn’s disease (38 patients) or ulcerative colitis (41 patients) who were randomly assigned to ACT (37 patients) or control treatment as usual (42 patients). The two comparison groups were demographically and clinically similar. The ACT program involved eight 90-minute, weekly sessions of groups of 14-16 individuals, led by a single psychologist who tailored the course material toward IBD with a focus on lowering stress. An independent psychologist observed each session to assess adherence to protocol.
Not only did ACT meet the primary study endpoint, it also produced a 25% decrease in perceived stress (on a 1-10 scale) by week 8 and a 27% decrease in perceived stress by week 20 (P less than .001 versus treatment as usual). Depression scores in the ACT group also fell by 47% by week 8 and by 45% at week 20 (P = .01 versus treatment as usual). Anxiety levels decreased by 29% at week 8 and by 31% at week 20, but these improvements did not significantly differ from those in the control group (P = .39).
Interestingly, ACT did not significantly improve symptom burden, activities of daily living, disease-related worry, general well-being, C-reactive protein (CRP) levels, fecal calprotectin levels, or scores on the version used of the Clinical Assessment of Depression (CAD) or the short Mayo assessment. Hair cortisol levels showed an association with baseline stress and anxiety, but not with treatment response.
Care programs for IBD increasingly emphasize mental health services despite a lack of robust trials to support these interventions, the investigators noted. Thus, their findings highlight “the need for researchers and clinicians to further develop and optimize the content and delivery of psychological programs for IBD patients.”
Tillotts Pharma and Boston Scientific provided partial funding, but had no other role in the study. The researchers reported having no relevant conflicts of interest.
SOURCE: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
Eight weeks of a mindfulness intervention known as acceptance and commitment therapy (ACT) significantly improved stress and depression among patients with inflammatory bowel disease, and these improvements persisted for at least 12 weeks after therapy ended, according to the results of a randomized, controlled trial.
Source: The American Gastroenterological Association
In the intention-to-treat analysis, stress symptoms, as measured by the Depression Anxiety and Stress Scales (DASS-21), improved by 39% at week 8 and by 45% at week 20, reported Brona Wynne, PhD, of University College Dublin together with her associates. These improvements were highly significant compared with baseline and treatment as usual (P = .001 for both comparisons). “Post hoc analyses indicated that baseline stress levels were similar in control and treatment groups,” the researchers wrote in Gastroenterology. “The results of the per protocol analysis were comparable, with a 43% and 49% reduction in stress in the treatment group from baseline to 8 and 20 weeks.”
Multiple studies have documented high levels of stress and psychological dysfunction among patients with Crohn’s disease and ulcerative colitis. Studies of various mindfulness therapy, relaxation, stress management, cognitive-behavioral therapy, and hypnotherapy interventions often failed to collect key clinical data or were underpowered, uncontrolled, and unrandomized. Acceptance and commitment therapy uses mindfulness to identify adverse thoughts and experiences, accept these as part of life, and recommit to “move towards values that have been identified and adopted by the individual,” the investigators wrote. “This can be defined as the ability to contact the present moment more fully as a conscious human being and to change, or persist in, behavior when doing so serves valued ends.”
Their single-center study, which they said was the first to evaluate ACT in IBD patients, included 79 individuals with stable or mildly active Crohn’s disease (38 patients) or ulcerative colitis (41 patients) who were randomly assigned to ACT (37 patients) or control treatment as usual (42 patients). The two comparison groups were demographically and clinically similar. The ACT program involved eight 90-minute, weekly sessions of groups of 14-16 individuals, led by a single psychologist who tailored the course material toward IBD with a focus on lowering stress. An independent psychologist observed each session to assess adherence to protocol.
Not only did ACT meet the primary study endpoint, it also produced a 25% decrease in perceived stress (on a 1-10 scale) by week 8 and a 27% decrease in perceived stress by week 20 (P less than .001 versus treatment as usual). Depression scores in the ACT group also fell by 47% by week 8 and by 45% at week 20 (P = .01 versus treatment as usual). Anxiety levels decreased by 29% at week 8 and by 31% at week 20, but these improvements did not significantly differ from those in the control group (P = .39).
Interestingly, ACT did not significantly improve symptom burden, activities of daily living, disease-related worry, general well-being, C-reactive protein (CRP) levels, fecal calprotectin levels, or scores on the version used of the Clinical Assessment of Depression (CAD) or the short Mayo assessment. Hair cortisol levels showed an association with baseline stress and anxiety, but not with treatment response.
Care programs for IBD increasingly emphasize mental health services despite a lack of robust trials to support these interventions, the investigators noted. Thus, their findings highlight “the need for researchers and clinicians to further develop and optimize the content and delivery of psychological programs for IBD patients.”
Tillotts Pharma and Boston Scientific provided partial funding, but had no other role in the study. The researchers reported having no relevant conflicts of interest.
SOURCE: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
FROM GASTROENTEROLOGY
Key clinical point: An 8-week course of acceptance and commitment therapy improved stress and depression in patients with inflammatory bowel disease.
Major finding: Compared with controls, the intervention group experienced significant improvements in stress (P = .001) and depression (P = .01), but not anxiety.
Study details: Randomized controlled trial of 79 patients.
Disclosures: Tillotts Pharma and Boston Scientific provided partial funding but had no other role in the study. The researchers reported having no relevant conflicts of interest.
Source: Wynne B et al. Gastroenterology. 2018 Nov 16. doi: 10.1053/j.gastro.2018.11.030.
Increased risk of second cancers in mycosis fungoides
LA JOLLA, CALIF. – A retrospective study suggests patients with mycosis fungoides (MF) have an increased risk of developing hematologic and solid tumor malignancies.

Researchers found the risk of second malignancy was highest among MF patients aged 30 to 50 years and patients who had tumor stage or advanced stage MF.
The increased risk was present during the entire period after MF diagnosis, but it was greatest in the first 6 months after diagnosis and roughly a dozen years later.
Amrita Goyal, MD, of the University of Minnesota in Minneapolis, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The researchers first assessed the risk of second malignancy in 172 MF patients treated at UMN from 2005 to 2017, comparing this cohort to a control group of 172 patients with seborrheic dermatitis.
Second malignancies occurred in 24 MF patients and three controls, which was a significant difference (P = .0045). The most common second malignancies among the MF patients were melanoma (n = 4), prostate cancer (n = 3), and renal cell carcinoma (n = 3).
Further analyses revealed that MF patients were more likely to develop a second malignancy if they had tumor stage disease (P = .0024) or stage IIB or higher disease (P = .03).
To corroborate and expand upon these results, Dr. Goyal and her colleagues analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database on patients diagnosed with MF from 2000 to 2014.
Among the 6,196 MF patients in this cohort, there were 514 second cancers.
“We found that MF patients were, overall, 10 times more likely to develop a second malignancy [compared with the general population],” Dr. Goyal said.
Specifically, the standardized incidence ratio was 10.15 for all malignancies, 7.33 for solid tumors, and 41.72 for hematologic malignancies.
Standardized incidence ratios for individual malignancies were:
- 69.8 for Hodgkin lymphoma.
- 46.5 for non-Hodgkin lymphoma.
- 8.6 for leukemia.
- 7.2 for melanoma.
- 6.2 for lung cancer.
- 7.9 for female breast cancer.
- 5.2 for colon cancer.
- 4.1 for prostate cancer.
- 3.9 for renal cell carcinoma.
- 3.8 for pancreatic cancer.
- 3.6 for bladder cancer.
“We found there is an increased risk [of second malignancy] during the first 6 months after diagnosis of MF, likely related to patients being in contact with the health care system more,” Dr. Goyal said. “Over time, patients have about a 7- to 10-fold increased risk over baseline, until they reach about 12 or 13 years after diagnosis, at which point, there is an increase in risk.”
The researchers found the greatest risk of second malignancy was among patients aged 30 to 50 years, although there was an increased risk for all age groups.
“The reason we think patients are experiencing an increased risk of cancers is we believe this may be due to immune suppression secondary to the mycosis fungoides, although further studies need to be performed to determine if that’s accurate,” Dr. Goyal said.
To that end, she and her colleagues are planning gene expression studies in patients from the UMN cohort. The researchers plan to examine genes involved in the pathogenesis of second malignancies and MF progression in tissue samples from 36 MF patients, 12 who developed second malignancies and 24 who did not.
The current research was funded by the American Society of Hematology. Dr. Goyal reported having no relevant financial disclosures. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – A retrospective study suggests patients with mycosis fungoides (MF) have an increased risk of developing hematologic and solid tumor malignancies.

Researchers found the risk of second malignancy was highest among MF patients aged 30 to 50 years and patients who had tumor stage or advanced stage MF.
The increased risk was present during the entire period after MF diagnosis, but it was greatest in the first 6 months after diagnosis and roughly a dozen years later.
Amrita Goyal, MD, of the University of Minnesota in Minneapolis, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The researchers first assessed the risk of second malignancy in 172 MF patients treated at UMN from 2005 to 2017, comparing this cohort to a control group of 172 patients with seborrheic dermatitis.
Second malignancies occurred in 24 MF patients and three controls, which was a significant difference (P = .0045). The most common second malignancies among the MF patients were melanoma (n = 4), prostate cancer (n = 3), and renal cell carcinoma (n = 3).
Further analyses revealed that MF patients were more likely to develop a second malignancy if they had tumor stage disease (P = .0024) or stage IIB or higher disease (P = .03).
To corroborate and expand upon these results, Dr. Goyal and her colleagues analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database on patients diagnosed with MF from 2000 to 2014.
Among the 6,196 MF patients in this cohort, there were 514 second cancers.
“We found that MF patients were, overall, 10 times more likely to develop a second malignancy [compared with the general population],” Dr. Goyal said.
Specifically, the standardized incidence ratio was 10.15 for all malignancies, 7.33 for solid tumors, and 41.72 for hematologic malignancies.
Standardized incidence ratios for individual malignancies were:
- 69.8 for Hodgkin lymphoma.
- 46.5 for non-Hodgkin lymphoma.
- 8.6 for leukemia.
- 7.2 for melanoma.
- 6.2 for lung cancer.
- 7.9 for female breast cancer.
- 5.2 for colon cancer.
- 4.1 for prostate cancer.
- 3.9 for renal cell carcinoma.
- 3.8 for pancreatic cancer.
- 3.6 for bladder cancer.
“We found there is an increased risk [of second malignancy] during the first 6 months after diagnosis of MF, likely related to patients being in contact with the health care system more,” Dr. Goyal said. “Over time, patients have about a 7- to 10-fold increased risk over baseline, until they reach about 12 or 13 years after diagnosis, at which point, there is an increase in risk.”
The researchers found the greatest risk of second malignancy was among patients aged 30 to 50 years, although there was an increased risk for all age groups.
“The reason we think patients are experiencing an increased risk of cancers is we believe this may be due to immune suppression secondary to the mycosis fungoides, although further studies need to be performed to determine if that’s accurate,” Dr. Goyal said.
To that end, she and her colleagues are planning gene expression studies in patients from the UMN cohort. The researchers plan to examine genes involved in the pathogenesis of second malignancies and MF progression in tissue samples from 36 MF patients, 12 who developed second malignancies and 24 who did not.
The current research was funded by the American Society of Hematology. Dr. Goyal reported having no relevant financial disclosures. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – A retrospective study suggests patients with mycosis fungoides (MF) have an increased risk of developing hematologic and solid tumor malignancies.

Researchers found the risk of second malignancy was highest among MF patients aged 30 to 50 years and patients who had tumor stage or advanced stage MF.
The increased risk was present during the entire period after MF diagnosis, but it was greatest in the first 6 months after diagnosis and roughly a dozen years later.
Amrita Goyal, MD, of the University of Minnesota in Minneapolis, and her colleagues presented these findings at the annual T-cell Lymphoma Forum.
The researchers first assessed the risk of second malignancy in 172 MF patients treated at UMN from 2005 to 2017, comparing this cohort to a control group of 172 patients with seborrheic dermatitis.
Second malignancies occurred in 24 MF patients and three controls, which was a significant difference (P = .0045). The most common second malignancies among the MF patients were melanoma (n = 4), prostate cancer (n = 3), and renal cell carcinoma (n = 3).
Further analyses revealed that MF patients were more likely to develop a second malignancy if they had tumor stage disease (P = .0024) or stage IIB or higher disease (P = .03).
To corroborate and expand upon these results, Dr. Goyal and her colleagues analyzed data from the Surveillance, Epidemiology, and End Results (SEER) database on patients diagnosed with MF from 2000 to 2014.
Among the 6,196 MF patients in this cohort, there were 514 second cancers.
“We found that MF patients were, overall, 10 times more likely to develop a second malignancy [compared with the general population],” Dr. Goyal said.
Specifically, the standardized incidence ratio was 10.15 for all malignancies, 7.33 for solid tumors, and 41.72 for hematologic malignancies.
Standardized incidence ratios for individual malignancies were:
- 69.8 for Hodgkin lymphoma.
- 46.5 for non-Hodgkin lymphoma.
- 8.6 for leukemia.
- 7.2 for melanoma.
- 6.2 for lung cancer.
- 7.9 for female breast cancer.
- 5.2 for colon cancer.
- 4.1 for prostate cancer.
- 3.9 for renal cell carcinoma.
- 3.8 for pancreatic cancer.
- 3.6 for bladder cancer.
“We found there is an increased risk [of second malignancy] during the first 6 months after diagnosis of MF, likely related to patients being in contact with the health care system more,” Dr. Goyal said. “Over time, patients have about a 7- to 10-fold increased risk over baseline, until they reach about 12 or 13 years after diagnosis, at which point, there is an increase in risk.”
The researchers found the greatest risk of second malignancy was among patients aged 30 to 50 years, although there was an increased risk for all age groups.
“The reason we think patients are experiencing an increased risk of cancers is we believe this may be due to immune suppression secondary to the mycosis fungoides, although further studies need to be performed to determine if that’s accurate,” Dr. Goyal said.
To that end, she and her colleagues are planning gene expression studies in patients from the UMN cohort. The researchers plan to examine genes involved in the pathogenesis of second malignancies and MF progression in tissue samples from 36 MF patients, 12 who developed second malignancies and 24 who did not.
The current research was funded by the American Society of Hematology. Dr. Goyal reported having no relevant financial disclosures. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: In a cohort of MF patients from the SEER database, the standardized incidence ratio was 10.15 for all malignancies, 7.33 for solid tumors, and 41.72 for hematologic malignancies.
Study details: Retrospective study of 6,196 MF patients from the SEER database, and a single-center cohort of 172 MF patients who were matched to 172 patients with seborrheic dermatitis.
Disclosures: This research was funded by the American Society of Hematology. Dr. Goyal reported having no relevant financial disclosures.
Cobomarsen shows early promise for treating ATLL
LA JOLLA, CALIF. – Phase 1 results suggest cobomarsen is well tolerated and can maintain or improve responses in patients with previously treated adult T-cell leukemia/lymphoma (ATLL).
Five of eight ATLL patients studied experienced disease stabilization or improvement while receiving cobomarsen (MRG-106), an inhibitor of microRNA-155.
There were no grade 3/4 adverse events (AEs) or serious AEs related to cobomarsen in these patients.
Francine Foss, MD, of Yale Cancer Center in New Haven, Conn., and her colleagues presented these results at the annual T-cell Lymphoma Forum.
In this ongoing trial (NCT02580552), researchers are evaluating cobomarsen in patients with B- and T-cell lymphomas, including mycosis fungoides and ATLL.
Results are available for eight patients with previously treated ATLL. These patients had received a median of 4 (range, 1-10) prior systemic therapies, and they had a median age of 51 years (range, 40-68).
The patients received three loading doses of cobomarsen during the first week of cycle 1, followed by weekly dosing. All patients have received cobomarsen as a 600 mg intravenous infusion. They can remain on cobomarsen until they progress, experience clinically significant side effects, cannot tolerate the drug, or the trial is terminated.
The researchers have measured efficacy at least monthly by monitoring tumor cell burden in the peripheral blood and lymph nodes, as well as evaluating changes in skin involvement.
Stabilization and response
“Initially, we saw some very good responses in patients who had escalating disease. In other words, their disease was progressing after conventional chemotherapy,” Dr. Foss said. “They went on this microRNA, [and] their disease stabilized and then regressed. We saw, subsequently, in another three or four patients, the same pattern of activity.”
In all, five patients achieved or maintained a response while on cobomarsen. All five were still receiving the drug at the data cutoff on Dec. 13, 2018.
Two of these patients had acute disease and were in partial response (PR) at baseline. These patients had received cobomarsen for 87 days and 401 days as of the data cutoff.
The other three patients still receiving cobomarsen at the cutoff had lymphomatous disease. At baseline, two of the patients were in PR and one had stable disease.
The two patients in PR at baseline had received cobomarsen for 80 days and 366 days at the data cutoff. The patient with stable disease had received the drug for 161 days.
Progression and withdrawal
There were three patients who withdrew from the study because of disease progression. Two of these patients were relapsing with significant skin involvement at baseline.
One of the patients discontinued cobomarsen after 23 days of treatment. The other patient received cobomarsen for 91 days and left the study, then re-enrolled and received cobomarsen for another 42 days before withdrawing from the study again.
The third patient had relapsed lymphomatous disease at baseline. This patient had a mixed response to cobomarsen, with some nodes decreasing in size and others increasing. She discontinued cobomarsen after 9 days.
“It’s still early on in our experience with ATLL, so we don’t really know yet who the patient is that’s going to respond – what are the clinical features that would predict response in these patients,” Dr. Foss said. “And we’re still really trying to understand how we give the drug to these patients, for how long, and whether or not we can change the dosing interval. But, nevertheless, we have some very interesting data.”
Safety
There were no dose-limiting toxicities, AE-related discontinuations, treatment-related grade 3/4 AEs, or new opportunistic infections observed.
“[I] have to say, in using this drug now for over a year in two of my patients – and that’s with weekly administration – we really haven’t seen anything as far as adverse events,” Dr. Foss said.
She noted that one patient has reported transient diarrhea after dosing.
Two serious AEs – febrile neutropenia and pyrexia – occurred in one patient, but neither of these events were considered related to cobomarsen. The AEs occurred after the patient had stopped cobomarsen, and both events resolved.
There were no on-treatment deaths. One patient (the one who received cobomarsen for 9 days) died from disease progression approximately 2 months after stopping cobomarsen and while on a different therapy.
Dr. Foss said, based on their results, she and her colleagues are hoping to accrue more ATLL patients in this trial.
The trial is sponsored by miRagen Therapeutics. Dr. Foss is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Phase 1 results suggest cobomarsen is well tolerated and can maintain or improve responses in patients with previously treated adult T-cell leukemia/lymphoma (ATLL).
Five of eight ATLL patients studied experienced disease stabilization or improvement while receiving cobomarsen (MRG-106), an inhibitor of microRNA-155.
There were no grade 3/4 adverse events (AEs) or serious AEs related to cobomarsen in these patients.
Francine Foss, MD, of Yale Cancer Center in New Haven, Conn., and her colleagues presented these results at the annual T-cell Lymphoma Forum.
In this ongoing trial (NCT02580552), researchers are evaluating cobomarsen in patients with B- and T-cell lymphomas, including mycosis fungoides and ATLL.
Results are available for eight patients with previously treated ATLL. These patients had received a median of 4 (range, 1-10) prior systemic therapies, and they had a median age of 51 years (range, 40-68).
The patients received three loading doses of cobomarsen during the first week of cycle 1, followed by weekly dosing. All patients have received cobomarsen as a 600 mg intravenous infusion. They can remain on cobomarsen until they progress, experience clinically significant side effects, cannot tolerate the drug, or the trial is terminated.
The researchers have measured efficacy at least monthly by monitoring tumor cell burden in the peripheral blood and lymph nodes, as well as evaluating changes in skin involvement.
Stabilization and response
“Initially, we saw some very good responses in patients who had escalating disease. In other words, their disease was progressing after conventional chemotherapy,” Dr. Foss said. “They went on this microRNA, [and] their disease stabilized and then regressed. We saw, subsequently, in another three or four patients, the same pattern of activity.”
In all, five patients achieved or maintained a response while on cobomarsen. All five were still receiving the drug at the data cutoff on Dec. 13, 2018.
Two of these patients had acute disease and were in partial response (PR) at baseline. These patients had received cobomarsen for 87 days and 401 days as of the data cutoff.
The other three patients still receiving cobomarsen at the cutoff had lymphomatous disease. At baseline, two of the patients were in PR and one had stable disease.
The two patients in PR at baseline had received cobomarsen for 80 days and 366 days at the data cutoff. The patient with stable disease had received the drug for 161 days.
Progression and withdrawal
There were three patients who withdrew from the study because of disease progression. Two of these patients were relapsing with significant skin involvement at baseline.
One of the patients discontinued cobomarsen after 23 days of treatment. The other patient received cobomarsen for 91 days and left the study, then re-enrolled and received cobomarsen for another 42 days before withdrawing from the study again.
The third patient had relapsed lymphomatous disease at baseline. This patient had a mixed response to cobomarsen, with some nodes decreasing in size and others increasing. She discontinued cobomarsen after 9 days.
“It’s still early on in our experience with ATLL, so we don’t really know yet who the patient is that’s going to respond – what are the clinical features that would predict response in these patients,” Dr. Foss said. “And we’re still really trying to understand how we give the drug to these patients, for how long, and whether or not we can change the dosing interval. But, nevertheless, we have some very interesting data.”
Safety
There were no dose-limiting toxicities, AE-related discontinuations, treatment-related grade 3/4 AEs, or new opportunistic infections observed.
“[I] have to say, in using this drug now for over a year in two of my patients – and that’s with weekly administration – we really haven’t seen anything as far as adverse events,” Dr. Foss said.
She noted that one patient has reported transient diarrhea after dosing.
Two serious AEs – febrile neutropenia and pyrexia – occurred in one patient, but neither of these events were considered related to cobomarsen. The AEs occurred after the patient had stopped cobomarsen, and both events resolved.
There were no on-treatment deaths. One patient (the one who received cobomarsen for 9 days) died from disease progression approximately 2 months after stopping cobomarsen and while on a different therapy.
Dr. Foss said, based on their results, she and her colleagues are hoping to accrue more ATLL patients in this trial.
The trial is sponsored by miRagen Therapeutics. Dr. Foss is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Phase 1 results suggest cobomarsen is well tolerated and can maintain or improve responses in patients with previously treated adult T-cell leukemia/lymphoma (ATLL).
Five of eight ATLL patients studied experienced disease stabilization or improvement while receiving cobomarsen (MRG-106), an inhibitor of microRNA-155.
There were no grade 3/4 adverse events (AEs) or serious AEs related to cobomarsen in these patients.
Francine Foss, MD, of Yale Cancer Center in New Haven, Conn., and her colleagues presented these results at the annual T-cell Lymphoma Forum.
In this ongoing trial (NCT02580552), researchers are evaluating cobomarsen in patients with B- and T-cell lymphomas, including mycosis fungoides and ATLL.
Results are available for eight patients with previously treated ATLL. These patients had received a median of 4 (range, 1-10) prior systemic therapies, and they had a median age of 51 years (range, 40-68).
The patients received three loading doses of cobomarsen during the first week of cycle 1, followed by weekly dosing. All patients have received cobomarsen as a 600 mg intravenous infusion. They can remain on cobomarsen until they progress, experience clinically significant side effects, cannot tolerate the drug, or the trial is terminated.
The researchers have measured efficacy at least monthly by monitoring tumor cell burden in the peripheral blood and lymph nodes, as well as evaluating changes in skin involvement.
Stabilization and response
“Initially, we saw some very good responses in patients who had escalating disease. In other words, their disease was progressing after conventional chemotherapy,” Dr. Foss said. “They went on this microRNA, [and] their disease stabilized and then regressed. We saw, subsequently, in another three or four patients, the same pattern of activity.”
In all, five patients achieved or maintained a response while on cobomarsen. All five were still receiving the drug at the data cutoff on Dec. 13, 2018.
Two of these patients had acute disease and were in partial response (PR) at baseline. These patients had received cobomarsen for 87 days and 401 days as of the data cutoff.
The other three patients still receiving cobomarsen at the cutoff had lymphomatous disease. At baseline, two of the patients were in PR and one had stable disease.
The two patients in PR at baseline had received cobomarsen for 80 days and 366 days at the data cutoff. The patient with stable disease had received the drug for 161 days.
Progression and withdrawal
There were three patients who withdrew from the study because of disease progression. Two of these patients were relapsing with significant skin involvement at baseline.
One of the patients discontinued cobomarsen after 23 days of treatment. The other patient received cobomarsen for 91 days and left the study, then re-enrolled and received cobomarsen for another 42 days before withdrawing from the study again.
The third patient had relapsed lymphomatous disease at baseline. This patient had a mixed response to cobomarsen, with some nodes decreasing in size and others increasing. She discontinued cobomarsen after 9 days.
“It’s still early on in our experience with ATLL, so we don’t really know yet who the patient is that’s going to respond – what are the clinical features that would predict response in these patients,” Dr. Foss said. “And we’re still really trying to understand how we give the drug to these patients, for how long, and whether or not we can change the dosing interval. But, nevertheless, we have some very interesting data.”
Safety
There were no dose-limiting toxicities, AE-related discontinuations, treatment-related grade 3/4 AEs, or new opportunistic infections observed.
“[I] have to say, in using this drug now for over a year in two of my patients – and that’s with weekly administration – we really haven’t seen anything as far as adverse events,” Dr. Foss said.
She noted that one patient has reported transient diarrhea after dosing.
Two serious AEs – febrile neutropenia and pyrexia – occurred in one patient, but neither of these events were considered related to cobomarsen. The AEs occurred after the patient had stopped cobomarsen, and both events resolved.
There were no on-treatment deaths. One patient (the one who received cobomarsen for 9 days) died from disease progression approximately 2 months after stopping cobomarsen and while on a different therapy.
Dr. Foss said, based on their results, she and her colleagues are hoping to accrue more ATLL patients in this trial.
The trial is sponsored by miRagen Therapeutics. Dr. Foss is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: Five of eight ATLL patients studied experienced disease stabilization or improvement while receiving cobomarsen.
Study details: Phase 1 trial including eight ATLL patients.
Disclosures: The trial is sponsored by miRagen Therapeutics.
Cerdulatinib yields ‘encouraging’ results in CTCL, PTCL
LA JOLLA, CALIF. – The spleen tyrosine kinase/Janus kinase inhibitor cerdulatinib has demonstrated activity against relapsed and refractory T-cell lymphomas.

In a phase 2 trial, cerdulatinib produced responses in 34% of patients with peripheral T-cell lymphoma (PTCL) and 26% of those with cutaneous T-cell lymphoma (CTCL).
The best responders were patients with angioimmunoblastic T-cell lymphoma, half of whom achieved a complete response (CR).
The most common grade 3 or higher adverse events (AEs) were amylase increase and lipase increase. However, these increases resolved with dose reduction or interruption, and there were no cases of clinical pancreatitis.
“The data is very encouraging,” said Tatyana Feldman, MD, of the John Theurer Cancer Center in Hackensack, N.J.
Dr. Feldman and her colleagues previously presented results from the phase 2 trial of cerdulatinib (NCT01994382) at the 2018 annual congress of the European Hematology Association.
Dr. Feldman and her colleagues presented data from expansion cohorts of the ongoing trial at the annual T-cell Lymphoma Forum. The cohorts included patients with PTCL or CTCL who had received at least one prior systemic therapy.
PTCL cohort
The 45 PTCL patients had a median age of 65 years (range, 21-84). They had received a median of 3 (range, 1-12) prior therapeutic regimens, 51% were refractory to their last therapy, and 27% had undergone stem cell transplant (SCT).
The patients received cerdulatinib at 30 mg orally twice a day until progression or intolerance, and 41 patients were evaluable for response.
The overall response rate was 34% (n = 14). Eleven patients had a CR, three had a partial response (PR), and nine had stable disease.
Responses according to subtype were as follows:
- 7 CRs and 1 PR in angioimmunoblastic T-cell lymphoma.
- 2 CRs in PTCL not otherwise specified.
- 1 CR in gamma-delta T-cell lymphoma.
- 1 PR in ALK-negative anaplastic large-cell lymphoma.
- 1 CR and 1 PR in adult T-cell leukemia/lymphoma.
Eight responders have remained on cerdulatinib for anywhere from 3 months to more than 12 months. Five patients have had a response lasting at least 6 months. One patient went on to SCT after achieving a CR.
The most common grade 3 or higher AEs observed in PTCL patients were amylase increase (n = 8), lipase increase (n = 6), pneumonia/lung infection (n = 5), neutropenia (n = 4), diarrhea (n = 4), febrile neutropenia (n = 4), abdominal pain (n = 4), sepsis/bacteremia (n = 3), anemia (n = 3), fatigue (n = 2), and pain (n = 1).
There were two grade 5 AEs – acute respiratory distress syndrome and pneumonia.
CTCL cohort
The 29 CTCL patients had a median age of 62 years (range, 24-79). They had received a median of 4 (range, 1-13) prior therapies, 55% were refractory to their last therapy, and 3% had undergone SCT.
The patients received cerdulatinib at 30 mg orally twice a day until progression or intolerance, and 27 were evaluable for response.
The overall response rate was 26% (n = 7). Two patients achieved a CR, five achieved a PR, and nine had stable disease. Responses occurred in mycosis fungoides and Sézary syndrome.
Eleven of 23 patients (48%) achieved at least a 50% reduction in skin lesions, and the researchers observed rapid improvements in pruritus.
“I saw patients who would take the first pill, and they would call me and say, ‘I no longer itch,’ ” Dr. Feldman said.
The most common grade 3 or higher AEs in CTCL patients were lipase increase (n = 11), amylase increase (n = 5), sepsis/bacteremia (n = 3), pain (n = 2), fatigue (n = 1), neutropenia (n = 1), and diarrhea (n = 1).
“It’s a very well-tolerated drug,” Dr. Feldman said, adding that there were “really no severe side effects which would prohibit the use of the drug.”
She noted that cerdulatinib’s “favorable” side effect profile might make it a promising candidate for use in combination regimens.
“I think it will be possible to combine it with other drugs in development in T-cell lymphoma. … immunological checkpoint inhibitors, epigenetic modulators such as HDAC [histone deacetylase] inhibitors, methylating agents, and PI3 kinase inhibitors,” Dr. Feldman said.
She reported having no disclosures relevant to this study. The trial is sponsored by Portola Pharmaceuticals.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The spleen tyrosine kinase/Janus kinase inhibitor cerdulatinib has demonstrated activity against relapsed and refractory T-cell lymphomas.

In a phase 2 trial, cerdulatinib produced responses in 34% of patients with peripheral T-cell lymphoma (PTCL) and 26% of those with cutaneous T-cell lymphoma (CTCL).
The best responders were patients with angioimmunoblastic T-cell lymphoma, half of whom achieved a complete response (CR).
The most common grade 3 or higher adverse events (AEs) were amylase increase and lipase increase. However, these increases resolved with dose reduction or interruption, and there were no cases of clinical pancreatitis.
“The data is very encouraging,” said Tatyana Feldman, MD, of the John Theurer Cancer Center in Hackensack, N.J.
Dr. Feldman and her colleagues previously presented results from the phase 2 trial of cerdulatinib (NCT01994382) at the 2018 annual congress of the European Hematology Association.
Dr. Feldman and her colleagues presented data from expansion cohorts of the ongoing trial at the annual T-cell Lymphoma Forum. The cohorts included patients with PTCL or CTCL who had received at least one prior systemic therapy.
PTCL cohort
The 45 PTCL patients had a median age of 65 years (range, 21-84). They had received a median of 3 (range, 1-12) prior therapeutic regimens, 51% were refractory to their last therapy, and 27% had undergone stem cell transplant (SCT).
The patients received cerdulatinib at 30 mg orally twice a day until progression or intolerance, and 41 patients were evaluable for response.
The overall response rate was 34% (n = 14). Eleven patients had a CR, three had a partial response (PR), and nine had stable disease.
Responses according to subtype were as follows:
- 7 CRs and 1 PR in angioimmunoblastic T-cell lymphoma.
- 2 CRs in PTCL not otherwise specified.
- 1 CR in gamma-delta T-cell lymphoma.
- 1 PR in ALK-negative anaplastic large-cell lymphoma.
- 1 CR and 1 PR in adult T-cell leukemia/lymphoma.
Eight responders have remained on cerdulatinib for anywhere from 3 months to more than 12 months. Five patients have had a response lasting at least 6 months. One patient went on to SCT after achieving a CR.
The most common grade 3 or higher AEs observed in PTCL patients were amylase increase (n = 8), lipase increase (n = 6), pneumonia/lung infection (n = 5), neutropenia (n = 4), diarrhea (n = 4), febrile neutropenia (n = 4), abdominal pain (n = 4), sepsis/bacteremia (n = 3), anemia (n = 3), fatigue (n = 2), and pain (n = 1).
There were two grade 5 AEs – acute respiratory distress syndrome and pneumonia.
CTCL cohort
The 29 CTCL patients had a median age of 62 years (range, 24-79). They had received a median of 4 (range, 1-13) prior therapies, 55% were refractory to their last therapy, and 3% had undergone SCT.
The patients received cerdulatinib at 30 mg orally twice a day until progression or intolerance, and 27 were evaluable for response.
The overall response rate was 26% (n = 7). Two patients achieved a CR, five achieved a PR, and nine had stable disease. Responses occurred in mycosis fungoides and Sézary syndrome.
Eleven of 23 patients (48%) achieved at least a 50% reduction in skin lesions, and the researchers observed rapid improvements in pruritus.
“I saw patients who would take the first pill, and they would call me and say, ‘I no longer itch,’ ” Dr. Feldman said.
The most common grade 3 or higher AEs in CTCL patients were lipase increase (n = 11), amylase increase (n = 5), sepsis/bacteremia (n = 3), pain (n = 2), fatigue (n = 1), neutropenia (n = 1), and diarrhea (n = 1).
“It’s a very well-tolerated drug,” Dr. Feldman said, adding that there were “really no severe side effects which would prohibit the use of the drug.”
She noted that cerdulatinib’s “favorable” side effect profile might make it a promising candidate for use in combination regimens.
“I think it will be possible to combine it with other drugs in development in T-cell lymphoma. … immunological checkpoint inhibitors, epigenetic modulators such as HDAC [histone deacetylase] inhibitors, methylating agents, and PI3 kinase inhibitors,” Dr. Feldman said.
She reported having no disclosures relevant to this study. The trial is sponsored by Portola Pharmaceuticals.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The spleen tyrosine kinase/Janus kinase inhibitor cerdulatinib has demonstrated activity against relapsed and refractory T-cell lymphomas.

In a phase 2 trial, cerdulatinib produced responses in 34% of patients with peripheral T-cell lymphoma (PTCL) and 26% of those with cutaneous T-cell lymphoma (CTCL).
The best responders were patients with angioimmunoblastic T-cell lymphoma, half of whom achieved a complete response (CR).
The most common grade 3 or higher adverse events (AEs) were amylase increase and lipase increase. However, these increases resolved with dose reduction or interruption, and there were no cases of clinical pancreatitis.
“The data is very encouraging,” said Tatyana Feldman, MD, of the John Theurer Cancer Center in Hackensack, N.J.
Dr. Feldman and her colleagues previously presented results from the phase 2 trial of cerdulatinib (NCT01994382) at the 2018 annual congress of the European Hematology Association.
Dr. Feldman and her colleagues presented data from expansion cohorts of the ongoing trial at the annual T-cell Lymphoma Forum. The cohorts included patients with PTCL or CTCL who had received at least one prior systemic therapy.
PTCL cohort
The 45 PTCL patients had a median age of 65 years (range, 21-84). They had received a median of 3 (range, 1-12) prior therapeutic regimens, 51% were refractory to their last therapy, and 27% had undergone stem cell transplant (SCT).
The patients received cerdulatinib at 30 mg orally twice a day until progression or intolerance, and 41 patients were evaluable for response.
The overall response rate was 34% (n = 14). Eleven patients had a CR, three had a partial response (PR), and nine had stable disease.
Responses according to subtype were as follows:
- 7 CRs and 1 PR in angioimmunoblastic T-cell lymphoma.
- 2 CRs in PTCL not otherwise specified.
- 1 CR in gamma-delta T-cell lymphoma.
- 1 PR in ALK-negative anaplastic large-cell lymphoma.
- 1 CR and 1 PR in adult T-cell leukemia/lymphoma.
Eight responders have remained on cerdulatinib for anywhere from 3 months to more than 12 months. Five patients have had a response lasting at least 6 months. One patient went on to SCT after achieving a CR.
The most common grade 3 or higher AEs observed in PTCL patients were amylase increase (n = 8), lipase increase (n = 6), pneumonia/lung infection (n = 5), neutropenia (n = 4), diarrhea (n = 4), febrile neutropenia (n = 4), abdominal pain (n = 4), sepsis/bacteremia (n = 3), anemia (n = 3), fatigue (n = 2), and pain (n = 1).
There were two grade 5 AEs – acute respiratory distress syndrome and pneumonia.
CTCL cohort
The 29 CTCL patients had a median age of 62 years (range, 24-79). They had received a median of 4 (range, 1-13) prior therapies, 55% were refractory to their last therapy, and 3% had undergone SCT.
The patients received cerdulatinib at 30 mg orally twice a day until progression or intolerance, and 27 were evaluable for response.
The overall response rate was 26% (n = 7). Two patients achieved a CR, five achieved a PR, and nine had stable disease. Responses occurred in mycosis fungoides and Sézary syndrome.
Eleven of 23 patients (48%) achieved at least a 50% reduction in skin lesions, and the researchers observed rapid improvements in pruritus.
“I saw patients who would take the first pill, and they would call me and say, ‘I no longer itch,’ ” Dr. Feldman said.
The most common grade 3 or higher AEs in CTCL patients were lipase increase (n = 11), amylase increase (n = 5), sepsis/bacteremia (n = 3), pain (n = 2), fatigue (n = 1), neutropenia (n = 1), and diarrhea (n = 1).
“It’s a very well-tolerated drug,” Dr. Feldman said, adding that there were “really no severe side effects which would prohibit the use of the drug.”
She noted that cerdulatinib’s “favorable” side effect profile might make it a promising candidate for use in combination regimens.
“I think it will be possible to combine it with other drugs in development in T-cell lymphoma. … immunological checkpoint inhibitors, epigenetic modulators such as HDAC [histone deacetylase] inhibitors, methylating agents, and PI3 kinase inhibitors,” Dr. Feldman said.
She reported having no disclosures relevant to this study. The trial is sponsored by Portola Pharmaceuticals.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: The overall response rate was 34% in patients with peripheral T-cell lymphoma (PTCL) and 26% in patients with cutaneous T-cell lymphoma (CTCL).
Study details: Expansion cohorts of a phase 2 trial including 45 PTCL patients and 29 CTCL patients
Disclosures: The study was funded by Portola Pharmaceuticals. The investigator reported having no relevant conflicts.
Combo emerges as bridge to transplant in rel/ref PTCL
LA JOLLA, CALIF. – The combination of duvelisib and romidepsin is active and can provide a bridge to transplant in relapsed or refractory peripheral T-cell lymphoma (PTCL), according to researchers.

In a phase 1 trial, duvelisib plus romidepsin produced an overall response rate (ORR) of 59% in patients with PTCL. Sixteen patients achieved a response, nine had a complete response (CR), and six complete responders went on to transplant.
“So we think that you can achieve remission deep enough to then move on to a potentially curative approach,” said study investigator Neha Mehta-Shah, MD, of Washington University in St. Louis.
She and her colleagues evaluated romidepsin plus duvelisib, as well as bortezomib plus duvelisib, in a phase 1 trial (NCT02783625) of patients with relapsed or refractory PTCL or cutaneous T-cell lymphoma (CTCL).
Dr. Mehta-Shah presented the results at the annual T-cell Lymphoma Forum.
She reported results in 80 patients – 51 with PTCL and 29 with CTCL. The patients’ median age was 64 years (range, 28-83), and 57% of the study population were men. Patients had received a median of 3 (range, 1-16) prior therapies, and 16% had received a prior transplant.
Treatment
Dr. Mehta-Shah noted that patients and providers could choose whether patients would receive romidepsin or bortezomib.
Patients in the romidepsin arm received romidepsin at 10 mg/m2 on days 1, 8, and 15 of each 28-day cycle. Patients in the bortezomib arm received bortezomib at 1 mg/m2 on days 1, 4, 8, and 11 of each cycle.
Duvelisib dosing was escalated, so patients received duvelisib at 25 mg, 50 mg, or 75 mg twice daily.
In the bortezomib arm, there was one dose-limiting toxicity – grade 3 neutropenia – in a patient who received duvelisib at the 25-mg dose. There were no dose-limiting toxicities in the romidepsin arm.
The researchers determined that the maximum tolerated dose (MTD) of duvelisib was 75 mg twice daily in the romidepsin arm and 25 mg twice daily in the bortezomib arm.
Lead-in phase
The study also had a lead-in phase during which patients could receive single-agent duvelisib.
“Because the original phase 1 study of duvelisib did not collect as many prospective tumor biopsies or on-treatment biopsies, we built into this study a lead-in phase so that we could characterize on-treatment biopsies to better understand mechanisms of response or resistance,” Dr. Mehta-Shah said.
Patients and providers could choose to be part of the lead-in phase, she noted. Patients who did not achieve a CR during this phase went on to receive either combination therapy, which was predetermined before the monotherapy began.
There were 14 patients who received duvelisib monotherapy at 75 mg twice daily. Four of them achieved a CR, and three had a partial response (PR). Ten patients went on to receive romidepsin as well. One of them achieved a CR, and three had a PR.
There were 12 patients who received duvelisib monotherapy at 25 mg twice daily. Three of them achieved a CR, and two had a PR. Nine patients went on to receive bortezomib as well. This combination produced one CR and two PRs.
Efficacy with romidepsin
Among all evaluable PTCL patients in the romidepsin arm, the ORR was 59% (16/27), and the CR rate was 33% (9/27).
Responses occurred in seven patients with PTCL not otherwise specified (NOS), six with angioimmunoblastic T-cell lymphoma (AITL), one with hepatosplenic T-cell lymphoma, one with aggressive epidermotropic CD8+ T-cell lymphoma, and one with primary cutaneous PTCL.
CRs occurred in five patients with AITL and four with PTCL-NOS. Six patients who achieved a CR went on to transplant.
Among evaluable CTCL patients in the romidepsin arm, the ORR was 45% (5/11), and there were no CRs. Responses occurred in three patients with mycosis fungoides and two with Sézary syndrome.
The median progression-free survival was 5.41 months in CTCL patients and 6.72 months in PTCL patients.
Efficacy with bortezomib
Among evaluable PTCL patients in the bortezomib arm, the ORR was 44% (7/16), and the CR rate was 25% (4/16).
Responses occurred in three patients with AITL and four with PTCL-NOS. CRs occurred in two patients with each subtype.
Among evaluable CTCL patients in the bortezomib arm, the ORR was 27% (4/15), and there were no CRs. Responses occurred in one patient with mycosis fungoides and three with Sézary syndrome. One CTCL patient went on to transplant.
The median progression-free survival was 4.56 months among CTCL patients and 4.39 months in PTCL patients.
Safety
Dr. Mehta-Shah said both combinations were considered safe and well tolerated. However, there was a grade 5 adverse event (AE) – Stevens-Johnson syndrome – that occurred in the bortezomib arm and was considered possibly related to treatment.
Grade 3/4 AEs observed in the 31 patients treated at the MTD in the romidepsin arm were transaminase increase (n = 7), diarrhea (n = 6), hyponatremia (n = 4), neutrophil count decrease (n = 10), and platelet count decrease (n = 3).
Grade 3/4 AEs observed in the 23 patients treated at the MTD in the bortezomib arm were transaminase increase (n = 2) and neutrophil count decrease (n = 5).
Grade 3/4 transaminitis seemed to be more common among patients who received duvelisib alone during the lead-in phase, Dr. Mehta-Shah said.
Among patients treated at the MTD in the romidepsin arm, grade 3/4 transaminitis occurred in four patients treated during the lead-in phase and three who began receiving romidepsin and duvelisib together. In the bortezomib arm, grade 3/4 transaminitis occurred in two patients treated at the MTD, both of whom received duvelisib alone during the lead-in phase.
Based on these results, Dr. Mehta-Shah and her colleagues are planning to expand the romidepsin arm to an additional 25 patients. By testing the combination in more patients, the researchers hope to better understand the occurrence of transaminitis and assess the durability of response.
This study is supported by Verastem. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The combination of duvelisib and romidepsin is active and can provide a bridge to transplant in relapsed or refractory peripheral T-cell lymphoma (PTCL), according to researchers.

In a phase 1 trial, duvelisib plus romidepsin produced an overall response rate (ORR) of 59% in patients with PTCL. Sixteen patients achieved a response, nine had a complete response (CR), and six complete responders went on to transplant.
“So we think that you can achieve remission deep enough to then move on to a potentially curative approach,” said study investigator Neha Mehta-Shah, MD, of Washington University in St. Louis.
She and her colleagues evaluated romidepsin plus duvelisib, as well as bortezomib plus duvelisib, in a phase 1 trial (NCT02783625) of patients with relapsed or refractory PTCL or cutaneous T-cell lymphoma (CTCL).
Dr. Mehta-Shah presented the results at the annual T-cell Lymphoma Forum.
She reported results in 80 patients – 51 with PTCL and 29 with CTCL. The patients’ median age was 64 years (range, 28-83), and 57% of the study population were men. Patients had received a median of 3 (range, 1-16) prior therapies, and 16% had received a prior transplant.
Treatment
Dr. Mehta-Shah noted that patients and providers could choose whether patients would receive romidepsin or bortezomib.
Patients in the romidepsin arm received romidepsin at 10 mg/m2 on days 1, 8, and 15 of each 28-day cycle. Patients in the bortezomib arm received bortezomib at 1 mg/m2 on days 1, 4, 8, and 11 of each cycle.
Duvelisib dosing was escalated, so patients received duvelisib at 25 mg, 50 mg, or 75 mg twice daily.
In the bortezomib arm, there was one dose-limiting toxicity – grade 3 neutropenia – in a patient who received duvelisib at the 25-mg dose. There were no dose-limiting toxicities in the romidepsin arm.
The researchers determined that the maximum tolerated dose (MTD) of duvelisib was 75 mg twice daily in the romidepsin arm and 25 mg twice daily in the bortezomib arm.
Lead-in phase
The study also had a lead-in phase during which patients could receive single-agent duvelisib.
“Because the original phase 1 study of duvelisib did not collect as many prospective tumor biopsies or on-treatment biopsies, we built into this study a lead-in phase so that we could characterize on-treatment biopsies to better understand mechanisms of response or resistance,” Dr. Mehta-Shah said.
Patients and providers could choose to be part of the lead-in phase, she noted. Patients who did not achieve a CR during this phase went on to receive either combination therapy, which was predetermined before the monotherapy began.
There were 14 patients who received duvelisib monotherapy at 75 mg twice daily. Four of them achieved a CR, and three had a partial response (PR). Ten patients went on to receive romidepsin as well. One of them achieved a CR, and three had a PR.
There were 12 patients who received duvelisib monotherapy at 25 mg twice daily. Three of them achieved a CR, and two had a PR. Nine patients went on to receive bortezomib as well. This combination produced one CR and two PRs.
Efficacy with romidepsin
Among all evaluable PTCL patients in the romidepsin arm, the ORR was 59% (16/27), and the CR rate was 33% (9/27).
Responses occurred in seven patients with PTCL not otherwise specified (NOS), six with angioimmunoblastic T-cell lymphoma (AITL), one with hepatosplenic T-cell lymphoma, one with aggressive epidermotropic CD8+ T-cell lymphoma, and one with primary cutaneous PTCL.
CRs occurred in five patients with AITL and four with PTCL-NOS. Six patients who achieved a CR went on to transplant.
Among evaluable CTCL patients in the romidepsin arm, the ORR was 45% (5/11), and there were no CRs. Responses occurred in three patients with mycosis fungoides and two with Sézary syndrome.
The median progression-free survival was 5.41 months in CTCL patients and 6.72 months in PTCL patients.
Efficacy with bortezomib
Among evaluable PTCL patients in the bortezomib arm, the ORR was 44% (7/16), and the CR rate was 25% (4/16).
Responses occurred in three patients with AITL and four with PTCL-NOS. CRs occurred in two patients with each subtype.
Among evaluable CTCL patients in the bortezomib arm, the ORR was 27% (4/15), and there were no CRs. Responses occurred in one patient with mycosis fungoides and three with Sézary syndrome. One CTCL patient went on to transplant.
The median progression-free survival was 4.56 months among CTCL patients and 4.39 months in PTCL patients.
Safety
Dr. Mehta-Shah said both combinations were considered safe and well tolerated. However, there was a grade 5 adverse event (AE) – Stevens-Johnson syndrome – that occurred in the bortezomib arm and was considered possibly related to treatment.
Grade 3/4 AEs observed in the 31 patients treated at the MTD in the romidepsin arm were transaminase increase (n = 7), diarrhea (n = 6), hyponatremia (n = 4), neutrophil count decrease (n = 10), and platelet count decrease (n = 3).
Grade 3/4 AEs observed in the 23 patients treated at the MTD in the bortezomib arm were transaminase increase (n = 2) and neutrophil count decrease (n = 5).
Grade 3/4 transaminitis seemed to be more common among patients who received duvelisib alone during the lead-in phase, Dr. Mehta-Shah said.
Among patients treated at the MTD in the romidepsin arm, grade 3/4 transaminitis occurred in four patients treated during the lead-in phase and three who began receiving romidepsin and duvelisib together. In the bortezomib arm, grade 3/4 transaminitis occurred in two patients treated at the MTD, both of whom received duvelisib alone during the lead-in phase.
Based on these results, Dr. Mehta-Shah and her colleagues are planning to expand the romidepsin arm to an additional 25 patients. By testing the combination in more patients, the researchers hope to better understand the occurrence of transaminitis and assess the durability of response.
This study is supported by Verastem. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – The combination of duvelisib and romidepsin is active and can provide a bridge to transplant in relapsed or refractory peripheral T-cell lymphoma (PTCL), according to researchers.

In a phase 1 trial, duvelisib plus romidepsin produced an overall response rate (ORR) of 59% in patients with PTCL. Sixteen patients achieved a response, nine had a complete response (CR), and six complete responders went on to transplant.
“So we think that you can achieve remission deep enough to then move on to a potentially curative approach,” said study investigator Neha Mehta-Shah, MD, of Washington University in St. Louis.
She and her colleagues evaluated romidepsin plus duvelisib, as well as bortezomib plus duvelisib, in a phase 1 trial (NCT02783625) of patients with relapsed or refractory PTCL or cutaneous T-cell lymphoma (CTCL).
Dr. Mehta-Shah presented the results at the annual T-cell Lymphoma Forum.
She reported results in 80 patients – 51 with PTCL and 29 with CTCL. The patients’ median age was 64 years (range, 28-83), and 57% of the study population were men. Patients had received a median of 3 (range, 1-16) prior therapies, and 16% had received a prior transplant.
Treatment
Dr. Mehta-Shah noted that patients and providers could choose whether patients would receive romidepsin or bortezomib.
Patients in the romidepsin arm received romidepsin at 10 mg/m2 on days 1, 8, and 15 of each 28-day cycle. Patients in the bortezomib arm received bortezomib at 1 mg/m2 on days 1, 4, 8, and 11 of each cycle.
Duvelisib dosing was escalated, so patients received duvelisib at 25 mg, 50 mg, or 75 mg twice daily.
In the bortezomib arm, there was one dose-limiting toxicity – grade 3 neutropenia – in a patient who received duvelisib at the 25-mg dose. There were no dose-limiting toxicities in the romidepsin arm.
The researchers determined that the maximum tolerated dose (MTD) of duvelisib was 75 mg twice daily in the romidepsin arm and 25 mg twice daily in the bortezomib arm.
Lead-in phase
The study also had a lead-in phase during which patients could receive single-agent duvelisib.
“Because the original phase 1 study of duvelisib did not collect as many prospective tumor biopsies or on-treatment biopsies, we built into this study a lead-in phase so that we could characterize on-treatment biopsies to better understand mechanisms of response or resistance,” Dr. Mehta-Shah said.
Patients and providers could choose to be part of the lead-in phase, she noted. Patients who did not achieve a CR during this phase went on to receive either combination therapy, which was predetermined before the monotherapy began.
There were 14 patients who received duvelisib monotherapy at 75 mg twice daily. Four of them achieved a CR, and three had a partial response (PR). Ten patients went on to receive romidepsin as well. One of them achieved a CR, and three had a PR.
There were 12 patients who received duvelisib monotherapy at 25 mg twice daily. Three of them achieved a CR, and two had a PR. Nine patients went on to receive bortezomib as well. This combination produced one CR and two PRs.
Efficacy with romidepsin
Among all evaluable PTCL patients in the romidepsin arm, the ORR was 59% (16/27), and the CR rate was 33% (9/27).
Responses occurred in seven patients with PTCL not otherwise specified (NOS), six with angioimmunoblastic T-cell lymphoma (AITL), one with hepatosplenic T-cell lymphoma, one with aggressive epidermotropic CD8+ T-cell lymphoma, and one with primary cutaneous PTCL.
CRs occurred in five patients with AITL and four with PTCL-NOS. Six patients who achieved a CR went on to transplant.
Among evaluable CTCL patients in the romidepsin arm, the ORR was 45% (5/11), and there were no CRs. Responses occurred in three patients with mycosis fungoides and two with Sézary syndrome.
The median progression-free survival was 5.41 months in CTCL patients and 6.72 months in PTCL patients.
Efficacy with bortezomib
Among evaluable PTCL patients in the bortezomib arm, the ORR was 44% (7/16), and the CR rate was 25% (4/16).
Responses occurred in three patients with AITL and four with PTCL-NOS. CRs occurred in two patients with each subtype.
Among evaluable CTCL patients in the bortezomib arm, the ORR was 27% (4/15), and there were no CRs. Responses occurred in one patient with mycosis fungoides and three with Sézary syndrome. One CTCL patient went on to transplant.
The median progression-free survival was 4.56 months among CTCL patients and 4.39 months in PTCL patients.
Safety
Dr. Mehta-Shah said both combinations were considered safe and well tolerated. However, there was a grade 5 adverse event (AE) – Stevens-Johnson syndrome – that occurred in the bortezomib arm and was considered possibly related to treatment.
Grade 3/4 AEs observed in the 31 patients treated at the MTD in the romidepsin arm were transaminase increase (n = 7), diarrhea (n = 6), hyponatremia (n = 4), neutrophil count decrease (n = 10), and platelet count decrease (n = 3).
Grade 3/4 AEs observed in the 23 patients treated at the MTD in the bortezomib arm were transaminase increase (n = 2) and neutrophil count decrease (n = 5).
Grade 3/4 transaminitis seemed to be more common among patients who received duvelisib alone during the lead-in phase, Dr. Mehta-Shah said.
Among patients treated at the MTD in the romidepsin arm, grade 3/4 transaminitis occurred in four patients treated during the lead-in phase and three who began receiving romidepsin and duvelisib together. In the bortezomib arm, grade 3/4 transaminitis occurred in two patients treated at the MTD, both of whom received duvelisib alone during the lead-in phase.
Based on these results, Dr. Mehta-Shah and her colleagues are planning to expand the romidepsin arm to an additional 25 patients. By testing the combination in more patients, the researchers hope to better understand the occurrence of transaminitis and assess the durability of response.
This study is supported by Verastem. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is held by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: The overall response rate was 59%, and six of nine complete responders went on to transplant.
Study details: Phase 1 trial of 80 patients that included 27 evaluable PTCL patients who received romidepsin and duvelisib.
Disclosures: The study is supported by Verastem. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
New concepts in the management of acute pancreatitis
Introduction
Acute pancreatitis (AP) is a major clinical and financial burden in the United States. Several major clinical guidelines provide evidence-based recommendations for the clinical management decisions in AP, including those from the American College of Gastroenterology (ACG; 2013),1 and the International Association of Pancreatology (IAP; 2013).2 More recently, the American Gastroenterological Association (AGA) released their own set of guidelines.3,4 In this update on AP, we review these guidelines and reference recent literature focused on epidemiology, risk factors, etiology, diagnosis, risk stratification, and recent advances in the early medical management of AP. Regarding the latter, we review six treatment interventions (pain management, intravenous fluid resuscitation, feeding, prophylactic antibiotics, probiotics, and timing of endoscopic retrograde cholangiopancreatography (ERCP) in acute biliary pancreatitis) and four preventive interventions (alcohol and smoking cessation, same-admission cholecystectomy for acute biliary pancreatitis, and chemoprevention and fluid administration for post-ERCP pancreatitis [PEP]). Updates on multidisciplinary management of (infected) pancreatic necrosis is beyond the scope of this review. Table 1 summarizes the concepts discussed in this article.

Recent advances in epidemiology and evaluation of AP
Epidemiology
AP is the third most common cause of gastrointestinal-related hospitalizations and fourth most common cause of readmission in 2014.5 Recent epidemiologic studies show conflicting trends for the incidence of AP, both increasing6 and decreasing,7 likely attributable to significant differences in study designs. Importantly, multiple studies have demonstrated that hospital length of stay, costs, and mortality have declined since 2009.6,8-10
Persistent organ failure (POF), defined as organ failure lasting more than 48 hours, is the major cause of death in AP. POF, if only a single organ during AP, is associated with 27%-36% mortality; if it is multiorgan, it is associated with 47% mortality.1,11 Other factors associated with increased hospital mortality include infected pancreatic necrosis,12-14 diabetes mellitus,15 hospital-acquired infection,16 advanced age (70 years and older),17 and obesity.18 Predictive factors of 1-year mortality include readmission within 30 days, higher Charlson Comorbidity Index, and longer hospitalization.19
Risk factors
We briefly highlight recent insights into risk factors for AP (Table 1) and refer to a recent review for further discussion.20 Current and former tobacco use are independent risk factors for AP.21 The dose-response relationship of alcohol to the risk of pancreatitis is complex,22 but five standard drinks per day for 5 years is a commonly used cut-off.1,23 New evidence suggests that the relationship between the dose of alcohol and risk of AP differs by sex, linearly in men but nonlinearly (J-shaped) in women.24 Risk of AP in women was decreased with alcohol consumption of up to 40 g/day (one standard drink contains 14 g of alcohol) and increased above this amount. Cannabis is a possible risk factor for toxin-induced AP and abstinence appears to abolish risk of recurrent attacks.25
Patients with inflammatory bowel disease (IBD) have a 2.9-fold higher risk for AP versus non-IBD cohorts26 with the most common etiologies are from gallstones and medications.27 In patients with end-stage renal disease (ESRD), the risk of AP is higher in those who receive peritoneal dialysis, compared with hemodialysis28-33 and who are women, older, or have cholelithiasis or liver disease.34As recently reviewed,35 pancreatic cancer appears to be associated with first-attack pancreatitis with few exceptions.36 In this setting, the overall incidence of pancreatic cancer is low (1.5%). The risk is greatest within the first year of the attack of AP, negligible below age 40 years but steadily rising through the fifth to eighth decades.37 Pancreatic cancer screening is a conditional recommendation of the ACG guidelines in patients with unexplained AP, particularly those aged 40 years or older.1
Etiology and diagnosis
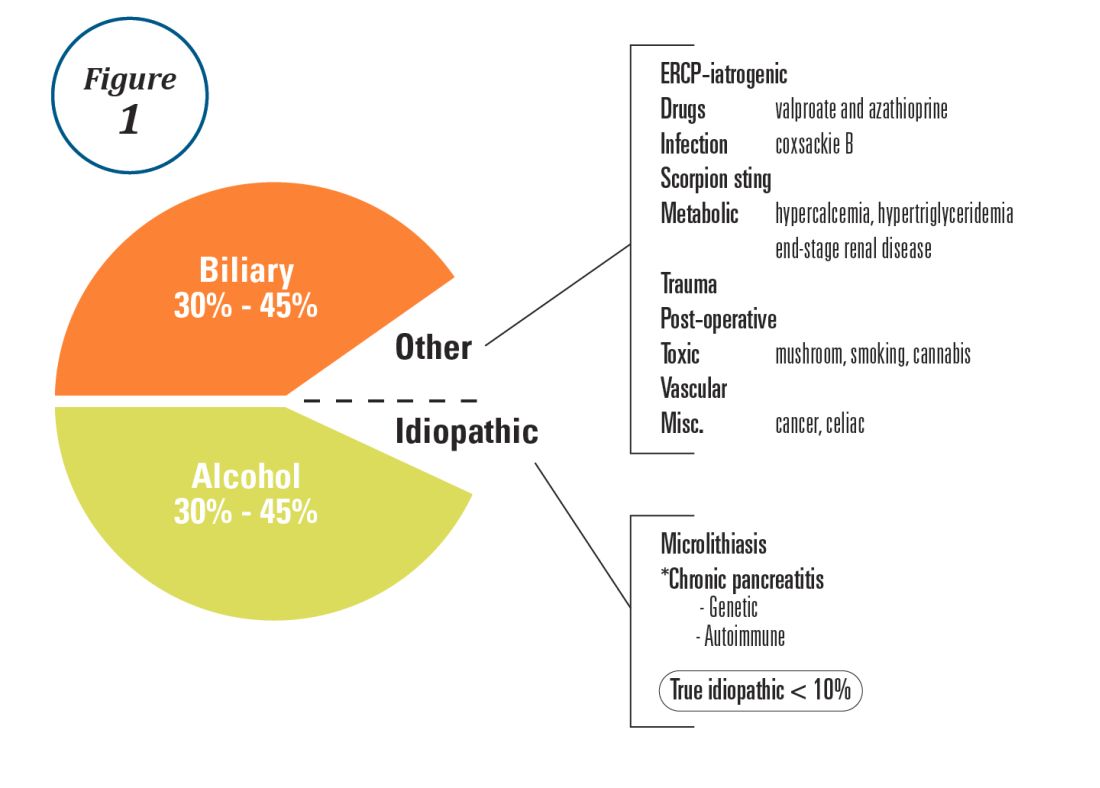
Alcohol and gallstones remain the most prevalent etiologies for AP.1 While hypertriglyceridemia accounted for 9% of AP in a systematic review of acute pancreatitis in 15 different countries,38 it is the second most common cause of acute pancreatitis in Asia (especially China).39 Figure 1 provides a breakdown of the etiologies and risk factors of pancreatitis. Importantly, it remains challenging to assign several toxic-metabolic etiologies as either a cause or risk factor for AP, particularly with regards to alcohol, smoking, and cannabis to name a few.
Guidelines and recent studies of AP raise questions about the threshold above which hypertriglyceridemia causes or poses as an important cofactor for AP. American and European societies define the threshold for triglycerides at 885-1,000 mg/dL.1,42,43 Pedersen et al. provide evidence of a graded risk of AP with hypertriglyceridemia: In multivariable analysis, adjusted hazard ratios for AP were much higher with nonfasting mild to moderately elevated plasma triglycerides (177-885 mg/dL), compared with normal values (below 89 mg/dL).44 Moreover, the risk of severe AP (developing POF) increases in proportion to triglyceride value, independent of the underlying cause of AP.45

Diagnosis of AP is derived from the revised Atlanta classification.46 The recommended timing and indications for offering cross-sectional imaging are after 48-72 hours in patients with no improvement to initial care.1 Endoscopic ultrasonography (EUS) has better diagnostic accuracy and sensitivity, compared with magnetic resonance cholangiopancreatography (MRCP) for choledocholithiasis, and has comparable specificity.47,48 Among noninvasive imaging modalities, MRCP is more sensitive than computed tomography (CT) for diagnosing choledocholithiasis.49 Despite guideline recommendations for more selective use of pancreatic imaging in the early assessment of AP, utilization of early CT or MRCP imaging (within the first 24 hours of care) remained high during 2014-2015, compared with 2006-2007.50
ERCP is not recommended as a pure diagnostic tool, owing to the availability of other diagnostic tests and a complication rate of 5%-10% with risks involving PEP, cholangitis, perforation, and hemorrhage.51 A recent systematic review of EUS and ERCP in acute biliary pancreatitis concluded that EUS had lower failure rates and had no complications, and the use of EUS avoided ERCP in 71.2% of cases.52
Risk stratification
The goals of using risk stratification tools in AP are to identify patients at risk for developing major outcomes, including POF, infected pancreatic necrosis, and death, and to ensure timely triaging of patients to an appropriate level of care. Existing prediction models have only moderate predictive value.53,54 Examples include simple risk stratification tools such as blood urea nitrogen (BUN) and hemoconcentration,55,56 disease-modifying patient variables (age, obesity, etc.), biomarkers (i.e., angiopoietin 2),57 and more complex clinical scoring systems such as Acute Physiology and Chronic Health Evaluation II (APACHE II), BISAP (BUN, impaired mental status, SIRS criteria, age, pleural effusion) score, early warning system (EWS), Glasgow-Imrie score, Japanese severity score, and recently the Pancreatitis Activity Scoring System (PASS).58 Two recent guidelines affirmed the importance of predicting the severity of AP, using one or more predictive tools.1,2 The recent 2018 AGA technical review does not debate this commonsense approach, but does highlight that there is no published observational study or randomized, controlled trial (RCT) investigating whether prediction tools affect clinical outcomes.4
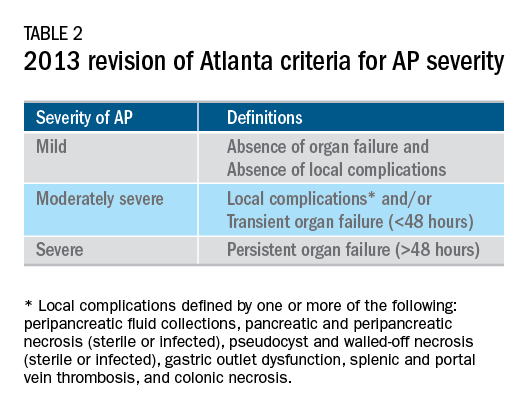
Recent advances in early treatment of AP
Literature review and definitions
The AP literature contains heterogeneous definitions of severe AP and of what constitutes a major outcome in AP. Based on definitions of the 2013 revised Atlanta Criteria, the 2018 AGA technical review and clinical guidelines emphasized precise definitions of primary outcomes of clinical importance in AP, including death, persistent single organ failure, or persistent multiple organ failure, each requiring a duration of more than 48 hours, and infected pancreatic or peripancreatic necrosis or both (Table 2).3,4
Pain management
Management of pain in AP is complex and requires a detailed discussion beyond the scope of this review, but recent clinical and translational studies raise questions about the current practice of using opioids for pain management in AP. A provocative, multicenter, retrospective cohort study reported lower 30-day mortality among critically ill patients who received epidural analgesia versus standard care without epidural analgesia.59 The possible mechanism of protection and the drugs administered are unclear. An interesting hypothesis is that the epidural cohort may have received lower exposure to morphine, which may increase gut permeability, the risk of infectious complications, and severity of AP, based on a translational study in mice.60
Intravenous fluid administration
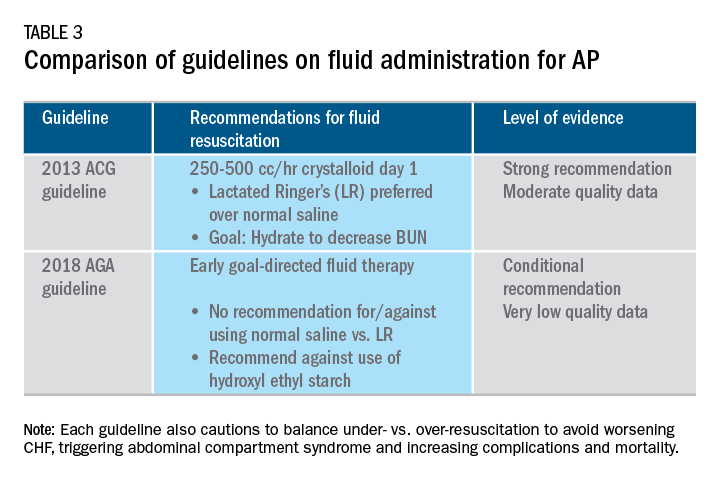
Supportive care with the use of IV fluid hydration is a mainstay of treatment for AP in the first 12-24 hours. Table 3 summarizes the guidelines in regards to IV fluid administration as delineated by the ACG and AGA guidelines on the management of pancreatitis.1,3 Guidelines advocate for early fluid resuscitation to correct intravascular depletion in order to reduce morbidity and mortality associated with AP.1,2,4 The 2018 AGA guidelines endorse a conditional recommendation for using goal-directed therapy for initial fluid management,3 do not recommend for or against normal saline versus lactated Ringer’s (LR), but do advise against the use of hydroxyethyl starch fluids.3 Consistent with these recommendations, two recent RCTs published subsequent to the prespecified time periods of the AGA technical review and guideline, observed no significant differences between LR and normal saline on clinically meaningful outcomes.61,62 The AGA guidelines acknowledge that evidence was of very-low quality in support of goal-directed therapy,3,4 which has not been shown to have a significant reduction in persistent multiple organ failure, mortality, or pancreatic necrosis, compared with usual care. As the authors noted, interpretation of the data was limited by the absence of other critical outcomes in these trials (infected pancreatic necrosis), lack of uniformity of specific outcomes and definitions of transient and POF, few trials, and risk of bias. There is a clear need for a large RCT to provide evidence to guide decision making with fluid resuscitation in AP, particularly in regard to fluid type, volume, rate, duration, endpoints, and clinical outcomes.
Feeding
More recently, the focus of nutrition in the management of AP has shifted away from patients remaining nil per os (NPO). Current guidelines advocate for early oral feeding (within 24 hours) in mild AP,3,4 in order to protect the gut-mucosal barrier. Remaining NPO when compared with early oral feeding has a 2.5-fold higher risk for interventions for necrosis.4 The recently published AGA technical review identified no significant impact on outcomes of early versus delayed oral feeding, which is consistent with observations of a landmark Dutch PYTHON trial entitled “Early versus on-demand nasoenteric tube feeding in acute pancreatitis.”4,63 There is no clear cutoff point for initiating feeding for those with severe AP. A suggested practical approach is to initiate feeding within 24-72 hours and offer enteral nutrition for those intolerant to oral feeds. In severe AP and moderately severe AP, enteral nutrition is recommended over parenteral nutrition.3,4 Enteral nutrition significantly reduces the risk of infected peripancreatic necrosis, single organ failure, and multiorgan failure.4 Finally, the AGA guidelines provide a conditional recommendation for providing enteral nutrition support through either the nasogastric or nasoenteric route.3 Further studies are required to determine the optimal timing, rate, and formulation of enteral nutrition in severe AP.
Antibiotics and probiotics
Current guidelines do not support the use of prophylactic antibiotics to prevent infection in necrotizing AP and severe AP.1-3 The AGA technical review reported that prophylactic antibiotics did not reduce infected pancreatic or peripancreatic necrosis, persistent single organ failure, or mortality.4 Guidelines advocate against the use of probiotics for severe AP, because of increased mortality risk.1
Timing of ERCP in acute biliary pancreatitis
There is universal agreement for offering urgent ERCP (within 24 hours) in biliary AP complicated by cholangitis.1-3,64 Figure 2 demonstrates an example of a cholangiogram completed within 24 hours of presentation of biliary AP complicated by cholangitis.
In the absence of cholangitis, the timing of ERCP for AP with persistent biliary obstruction is less clear.1-3 In line with recent guidelines, the 2018 AGA guidelines advocate against routine use of urgent ERCP for biliary AP without cholangitis,3 a conditional recommendation with overall low quality of data.4 The AGA technical review found that urgent ERCP, compared with conservative management in acute biliary pancreatitis without cholangitis had no significant effect on mortality, organ failure, infected pancreatic necrosis, and total necrotizing pancreatitis, but did significantly shorten hospital length of stay.4 There are limited data to guide decision making of when nonurgent ERCP should be performed in hospitalized patients with biliary AP with persistent obstruction and no cholangitis.3,64
Alcohol and smoking cessation
The AGA technical review advocates for brief alcohol intervention during hospitalization for alcohol-induced AP on the basis of one RCT that addresses the impact of alcohol counseling on recurrent bouts of AP4 plus evidence from a Cochrane review of alcohol-reduction strategies in primary care populations.65 Cessation of smoking – an established independent risk factor of AP – recurrent AP and chronic pancreatitis, should also be recommended as part of the management of AP.
Cholecystectomy
Evidence supports same-admission cholecystectomy for mild gallstone AP, a strong recommendation of published AGA guidelines.3 When compared with delayed cholecystectomy, same-admission cholecystectomy significantly reduced gallstone-related complications, readmissions for recurrent pancreatitis, and pancreaticobiliary complications, without having a significant impact on mortality during a 6-month follow-up period.66 Delaying cholecystectomy 6 weeks in patients with moderate-severe gallstone AP appears to reduce morbidity, including the development of infected collections, and mortality.4 An ongoing RCT, the APEC trial, aims to determine whether early ERCP with biliary sphincterotomy reduces major complications or death when compared with no intervention for biliary AP in patients at high risk of complications.67
Chemoprevention and IV fluid management of post-ERCP pancreatitis
Accumulating data support the effectiveness of chemoprevention, pancreatic stent placement, and fluid administration to prevent post-ERCP pancreatitis. Multiple RCTs, meta-analyses, and systematic reviews indicate that rectal NSAIDs) reduce post-ERCP pancreatitis onset68-71 and moderate-severe post-ERCP pancreatitis. Additionally, placement of a pancreatic duct stent may decrease the risk of severe post-ERCP pancreatitis in high-risk patients.3 Guidelines do not comment on fluid administrations for prevention of post-ERCP pancreatitis, but studies have shown that greater periprocedural IV fluid was an independent protective factor against moderate to severe PEP72 and was associated with shorter hospital length of stay.73 Recent meta-analyses and RCTs support using LR prior to ERCP to prevent PEP.74-77 Interestingly, a recent RCT shows that the combination of rectal indomethacin and LR, compared with combination placebo and normal saline reduced the risk of PEP in high-risk patients.78
Two ongoing multicenter RCTs will clarify the role of combination therapy. The Dutch FLUYT RCT aims to determine the optimal combination of rectal NSAIDs and periprocedural infusion of IV fluids to reduce the incidence of PEP and moderate-severe PEP79 and the Stent vs. Indomethacin (SVI) trial aims to determine the whether combination pancreatic stent placement plus rectal indomethacin is superior to monotherapy indomethacin for preventing post-ERCP pancreatitis in high-risk cases.80
Implications for clinical practice
The diagnosis and optimal management of AP require a systematic approach with multidisciplinary decision making. Morbidity and mortality in AP are driven by early or late POF, and the latter often is triggered by infected necrosis. Risk stratification of these patients at the point of contact is a commonsense approach to enable triaging of patients to the appropriate level of care. Regardless of pancreatitis severity, recommended treatment interventions include goal-directed IV fluid resuscitation, early feeding by mouth or enteral tube when necessary, avoidance of prophylactic antibiotics, avoidance of probiotics, and urgent ERCP for patients with acute biliary pancreatitis complicated by cholangitis. Key measures for preventing hospital readmission and pancreatitis include same-admission cholecystectomy for acute biliary pancreatitis and alcohol and smoking cessation. Preventive measures for post-ERCP pancreatitis in patients undergoing ERCP include rectal indomethacin, prophylactic pancreatic duct stent placement, and periprocedural fluid resuscitation.
Dr. Mandalia is a fellow, gastroenterology, department of internal medicine, division of gastroenterology, Michigan Medicine, Ann Arbor; Dr. DiMagno is associate professor of medicine, director, comprehensive pancreas program, department of internal medicine, division of gastroenterology, University of Michigan, Ann Arbor. Dr. Mandalia reports no conflicts of interest.
References
1. Tenner S et al. Am J Gastroenterol. 2013;108:1400.
2. Besseline M et al. Pancreatology. 2013;13(4, Supplement 2):e1-15.
3. Crockett SD et al. Gastroenterology. 2018;154(4):1096-101.
4. Vege SS et al. Gastroenterology. 2018;154(4):1103-39.
5. Peery AF et al. Gastroenterology. 2019 Jan;156(1):254-72.e11.
6. Krishna SG et al. Pancreas. 2017;46(4):482-8.
7. Sellers ZM et al. Gastroenterology. 2018;155(2):469-78.e1.
8. Brown A et al. JOP. 2008;9(4):408-14.
9. Fagenholz PJ et al. Ann Epidemiol. 2007;17(7):491.e1-.e8.
10. McNabb-Baltar J et al. Pancreas. 2014;43(5):687-91.
11. Johnson CD et al. Gut. 2004;53(9):1340-4.
12. Dellinger EP et al. Ann Surg. 2012;256(6):875-80.
13. Petrov MS et al. Gastroenterology. 2010;139(3):813-20.
14. Sternby H et al. Ann Surg. Apr 18. doi: 10.1097/SLA.0000000000002766.
15. Huh JH et al. J Clin Gastroenterol. 2018;52(2):178-83.
16. Wu BU et al. Gastroenterology. 2008;135(3):816-20.
17. Gardner TB et al. Clin Gastroenterol Hepatol. 2008;6(10):1070-6.
18. Krishna SG et al. Am J Gastroenterol. 2015;110(11):1608-19.
19. Lee PJ et al. Pancreas. 2016;45(4):561-4.
20. Mandalia A et al. F1000Research. 2018 Jun 28;7.
21. Majumder S et al. Pancreas. 2015;44(4):540-6.
22. DiMagno MJ. Clin Gastroenterol Hepatol. 2011;9(11):920-2.
23. Yadav D, Whitcomb DC. Nature Rev Gastroenterol Hepatol. 2010;7(3):131-45.
24. Samokhvalov AV et al. EBioMedicine. 2015;2(12):1996-2002.
25. Barkin JA et al. Pancreas. 2017;46(8):1035-8.
26. Chen Y-T et al. J Gastroenterol Hepatol. 2016;31(4):782-7.
27. Ramos LR et al. J Crohns Colitis. 2016;10(1):95-104.
28. Avram MM. Nephron. 1977;18(1):68-71.
29. Lankisch PG et al. Nephrol Dial Transplant. 2008;23(4):1401-5.
30. Owyang C et al. Mayo Clin Proc. 1979;54(12):769-73.
31. Owyang Cet al. Gut. 1982;23(5):357-61.
32. Quraishi ER et al. Am J Gastroenterol. 2005;100:2288.
33. Vaziri ND et al. Nephron. 1987;46(4):347-9.
34. Chen HJ et al. Nephrol Dial Transplant. 2017;32(10):1731-6.
35. Kirkegard J et al. Gastroenterology. 2018;May;154(6):1729-36.
36. Karlson BM, et al. Gastroenterology. 1997;113(2):587-92.
37. Munigala S et al. Clin Gastroenterol Hepatol. 2014;12(7):1143-50.e1.
38. Carr RA et al. Pancreatology. 2016;16(4):469-76.
39. Li X et al. BMC Gastroenterol. 2018;18(1):89.
40. Ahmed AU et al. Clin Gastroenterol Hepatol. 2016;14(5):738-46.
41. Sankaran SJ et al. Gastroenterology. 2015;149(6):1490-500.e1.
42. Berglund L et al. J Clin Endocrinol Metab. 2012;97(9):2969-89.
43. Catapano AL et al. Atherosclerosis. 2011;217(1):3-46.
44. Pedersen SB et al. JAMA Intern Med. 2016;176(12):1834-42.
45. Nawaz H et al. Am J Gastroenterol. 2015;110(10):1497-503.
46. Banks PA et al. Gut. 2013;62(1):102-11.
47. Kondo S et al. Eur J Radiol. 2005;54(2):271-5.
48. Meeralam Y et al. Gastrointest Endosc. 2017;86(6):986-93.
49. Stimac D et al. Am J Gastroenterol. 2007;102(5):997-1004.
50. Jin DX et al. Dig Dis Sci. 2017;62(10):2894-9.
51. Freeman ML. Gastrointest Endosc Clin N Am. 2012;22(3):567-86.
52. De Lisi S et al. Eur J Gastroenterol Hepatol. 2011;23(5):367-74.
53. Di MY et al. Ann Int Med. 2016;165(7):482-90.
54. Mounzer R et al. Gastroenterology. 2012;142(7):1476-82; quiz e15-6.
55. Koutroumpakis E et al. Am J Gastroenterol. 2015;110(12):1707-16.
56. Wu BU et al. Gastroenterology. 2009;137(1):129-35.
57. Buddingh KT et al. J Am Coll Surg. 2014;218(1):26-32.
58. Buxbaum J et al. Am J Gastroenterol. 2018;113(5):755-64.
59. Jabaudon M et al. Crit Car Med. 2018;46(3):e198-e205.
60. Barlass U et al. Gut. 2018;67(4):600-2.
61. Buxbaum JL et al. Am J Gastroenterol. 2017;112(5):797-803.
62. de-Madaria E et al. United Eur Gastroenterol J. 2018;6(1):63-72.
63. Bakker OJ et al. N Engl J Med. 2014;371(21):1983-93.
64. Tse F et al. Cochrane Database Syst Rev. 2012(5):Cd009779.
65. Kaner EFS et al. Cochrane Database Syst Rev. 2007(2):Cd004148.
66. da Costa DW et al. Lancet. 2015;386(10000):1261-8.
67. Schepers NJ et al. Trials. 2016;17:5.
68. Vadala di Prampero SF et al. Eur J Gastroenterol Hepatol. 2016;28(12):1415-24.
69. Kubiliun NM et al. Clin Gastroenterol Hepatol. 2015;13(7):1231-9; quiz e70-1.
70. Wan J et al. BMC Gastroenterol. 2017;17(1):43.
71. Yang C et al. Pancreatology. 2017;17(5):681-8.
72. DiMagno MJ et al. Pancreas. 2014;43(4):642-7.
73. Sagi SV et al. J Gastroenterol Hepatol. 2014;29(6):1316-20.
74. Choi JH et al. Clin Gastroenterol Hepatol. 2017;15(1):86-92.e1.
75. Wu D et al. J Clin Gastroenterol. 2017;51(8):e68-e76.
76. Zhang ZF et al. J Clin Gastroenterol. 2017;51(3):e17-e26.
77. Park CH et al. Endoscopy 2018 Apr;50(4):378-85.
78. Mok SRS et al. Gastrointest Endosc. 2017;85(5):1005-13.
79. Smeets XJN et al. Trials. 2018;19(1):207.
80. Elmunzer BJ et al. Trials. 2016;17(1):120.
Introduction
Acute pancreatitis (AP) is a major clinical and financial burden in the United States. Several major clinical guidelines provide evidence-based recommendations for the clinical management decisions in AP, including those from the American College of Gastroenterology (ACG; 2013),1 and the International Association of Pancreatology (IAP; 2013).2 More recently, the American Gastroenterological Association (AGA) released their own set of guidelines.3,4 In this update on AP, we review these guidelines and reference recent literature focused on epidemiology, risk factors, etiology, diagnosis, risk stratification, and recent advances in the early medical management of AP. Regarding the latter, we review six treatment interventions (pain management, intravenous fluid resuscitation, feeding, prophylactic antibiotics, probiotics, and timing of endoscopic retrograde cholangiopancreatography (ERCP) in acute biliary pancreatitis) and four preventive interventions (alcohol and smoking cessation, same-admission cholecystectomy for acute biliary pancreatitis, and chemoprevention and fluid administration for post-ERCP pancreatitis [PEP]). Updates on multidisciplinary management of (infected) pancreatic necrosis is beyond the scope of this review. Table 1 summarizes the concepts discussed in this article.

Recent advances in epidemiology and evaluation of AP
Epidemiology
AP is the third most common cause of gastrointestinal-related hospitalizations and fourth most common cause of readmission in 2014.5 Recent epidemiologic studies show conflicting trends for the incidence of AP, both increasing6 and decreasing,7 likely attributable to significant differences in study designs. Importantly, multiple studies have demonstrated that hospital length of stay, costs, and mortality have declined since 2009.6,8-10
Persistent organ failure (POF), defined as organ failure lasting more than 48 hours, is the major cause of death in AP. POF, if only a single organ during AP, is associated with 27%-36% mortality; if it is multiorgan, it is associated with 47% mortality.1,11 Other factors associated with increased hospital mortality include infected pancreatic necrosis,12-14 diabetes mellitus,15 hospital-acquired infection,16 advanced age (70 years and older),17 and obesity.18 Predictive factors of 1-year mortality include readmission within 30 days, higher Charlson Comorbidity Index, and longer hospitalization.19
Risk factors
We briefly highlight recent insights into risk factors for AP (Table 1) and refer to a recent review for further discussion.20 Current and former tobacco use are independent risk factors for AP.21 The dose-response relationship of alcohol to the risk of pancreatitis is complex,22 but five standard drinks per day for 5 years is a commonly used cut-off.1,23 New evidence suggests that the relationship between the dose of alcohol and risk of AP differs by sex, linearly in men but nonlinearly (J-shaped) in women.24 Risk of AP in women was decreased with alcohol consumption of up to 40 g/day (one standard drink contains 14 g of alcohol) and increased above this amount. Cannabis is a possible risk factor for toxin-induced AP and abstinence appears to abolish risk of recurrent attacks.25
Patients with inflammatory bowel disease (IBD) have a 2.9-fold higher risk for AP versus non-IBD cohorts26 with the most common etiologies are from gallstones and medications.27 In patients with end-stage renal disease (ESRD), the risk of AP is higher in those who receive peritoneal dialysis, compared with hemodialysis28-33 and who are women, older, or have cholelithiasis or liver disease.34As recently reviewed,35 pancreatic cancer appears to be associated with first-attack pancreatitis with few exceptions.36 In this setting, the overall incidence of pancreatic cancer is low (1.5%). The risk is greatest within the first year of the attack of AP, negligible below age 40 years but steadily rising through the fifth to eighth decades.37 Pancreatic cancer screening is a conditional recommendation of the ACG guidelines in patients with unexplained AP, particularly those aged 40 years or older.1
Etiology and diagnosis

Alcohol and gallstones remain the most prevalent etiologies for AP.1 While hypertriglyceridemia accounted for 9% of AP in a systematic review of acute pancreatitis in 15 different countries,38 it is the second most common cause of acute pancreatitis in Asia (especially China).39 Figure 1 provides a breakdown of the etiologies and risk factors of pancreatitis. Importantly, it remains challenging to assign several toxic-metabolic etiologies as either a cause or risk factor for AP, particularly with regards to alcohol, smoking, and cannabis to name a few.
Guidelines and recent studies of AP raise questions about the threshold above which hypertriglyceridemia causes or poses as an important cofactor for AP. American and European societies define the threshold for triglycerides at 885-1,000 mg/dL.1,42,43 Pedersen et al. provide evidence of a graded risk of AP with hypertriglyceridemia: In multivariable analysis, adjusted hazard ratios for AP were much higher with nonfasting mild to moderately elevated plasma triglycerides (177-885 mg/dL), compared with normal values (below 89 mg/dL).44 Moreover, the risk of severe AP (developing POF) increases in proportion to triglyceride value, independent of the underlying cause of AP.45

Diagnosis of AP is derived from the revised Atlanta classification.46 The recommended timing and indications for offering cross-sectional imaging are after 48-72 hours in patients with no improvement to initial care.1 Endoscopic ultrasonography (EUS) has better diagnostic accuracy and sensitivity, compared with magnetic resonance cholangiopancreatography (MRCP) for choledocholithiasis, and has comparable specificity.47,48 Among noninvasive imaging modalities, MRCP is more sensitive than computed tomography (CT) for diagnosing choledocholithiasis.49 Despite guideline recommendations for more selective use of pancreatic imaging in the early assessment of AP, utilization of early CT or MRCP imaging (within the first 24 hours of care) remained high during 2014-2015, compared with 2006-2007.50
ERCP is not recommended as a pure diagnostic tool, owing to the availability of other diagnostic tests and a complication rate of 5%-10% with risks involving PEP, cholangitis, perforation, and hemorrhage.51 A recent systematic review of EUS and ERCP in acute biliary pancreatitis concluded that EUS had lower failure rates and had no complications, and the use of EUS avoided ERCP in 71.2% of cases.52
Risk stratification
The goals of using risk stratification tools in AP are to identify patients at risk for developing major outcomes, including POF, infected pancreatic necrosis, and death, and to ensure timely triaging of patients to an appropriate level of care. Existing prediction models have only moderate predictive value.53,54 Examples include simple risk stratification tools such as blood urea nitrogen (BUN) and hemoconcentration,55,56 disease-modifying patient variables (age, obesity, etc.), biomarkers (i.e., angiopoietin 2),57 and more complex clinical scoring systems such as Acute Physiology and Chronic Health Evaluation II (APACHE II), BISAP (BUN, impaired mental status, SIRS criteria, age, pleural effusion) score, early warning system (EWS), Glasgow-Imrie score, Japanese severity score, and recently the Pancreatitis Activity Scoring System (PASS).58 Two recent guidelines affirmed the importance of predicting the severity of AP, using one or more predictive tools.1,2 The recent 2018 AGA technical review does not debate this commonsense approach, but does highlight that there is no published observational study or randomized, controlled trial (RCT) investigating whether prediction tools affect clinical outcomes.4

Recent advances in early treatment of AP
Literature review and definitions
The AP literature contains heterogeneous definitions of severe AP and of what constitutes a major outcome in AP. Based on definitions of the 2013 revised Atlanta Criteria, the 2018 AGA technical review and clinical guidelines emphasized precise definitions of primary outcomes of clinical importance in AP, including death, persistent single organ failure, or persistent multiple organ failure, each requiring a duration of more than 48 hours, and infected pancreatic or peripancreatic necrosis or both (Table 2).3,4
Pain management
Management of pain in AP is complex and requires a detailed discussion beyond the scope of this review, but recent clinical and translational studies raise questions about the current practice of using opioids for pain management in AP. A provocative, multicenter, retrospective cohort study reported lower 30-day mortality among critically ill patients who received epidural analgesia versus standard care without epidural analgesia.59 The possible mechanism of protection and the drugs administered are unclear. An interesting hypothesis is that the epidural cohort may have received lower exposure to morphine, which may increase gut permeability, the risk of infectious complications, and severity of AP, based on a translational study in mice.60
Intravenous fluid administration

Supportive care with the use of IV fluid hydration is a mainstay of treatment for AP in the first 12-24 hours. Table 3 summarizes the guidelines in regards to IV fluid administration as delineated by the ACG and AGA guidelines on the management of pancreatitis.1,3 Guidelines advocate for early fluid resuscitation to correct intravascular depletion in order to reduce morbidity and mortality associated with AP.1,2,4 The 2018 AGA guidelines endorse a conditional recommendation for using goal-directed therapy for initial fluid management,3 do not recommend for or against normal saline versus lactated Ringer’s (LR), but do advise against the use of hydroxyethyl starch fluids.3 Consistent with these recommendations, two recent RCTs published subsequent to the prespecified time periods of the AGA technical review and guideline, observed no significant differences between LR and normal saline on clinically meaningful outcomes.61,62 The AGA guidelines acknowledge that evidence was of very-low quality in support of goal-directed therapy,3,4 which has not been shown to have a significant reduction in persistent multiple organ failure, mortality, or pancreatic necrosis, compared with usual care. As the authors noted, interpretation of the data was limited by the absence of other critical outcomes in these trials (infected pancreatic necrosis), lack of uniformity of specific outcomes and definitions of transient and POF, few trials, and risk of bias. There is a clear need for a large RCT to provide evidence to guide decision making with fluid resuscitation in AP, particularly in regard to fluid type, volume, rate, duration, endpoints, and clinical outcomes.
Feeding
More recently, the focus of nutrition in the management of AP has shifted away from patients remaining nil per os (NPO). Current guidelines advocate for early oral feeding (within 24 hours) in mild AP,3,4 in order to protect the gut-mucosal barrier. Remaining NPO when compared with early oral feeding has a 2.5-fold higher risk for interventions for necrosis.4 The recently published AGA technical review identified no significant impact on outcomes of early versus delayed oral feeding, which is consistent with observations of a landmark Dutch PYTHON trial entitled “Early versus on-demand nasoenteric tube feeding in acute pancreatitis.”4,63 There is no clear cutoff point for initiating feeding for those with severe AP. A suggested practical approach is to initiate feeding within 24-72 hours and offer enteral nutrition for those intolerant to oral feeds. In severe AP and moderately severe AP, enteral nutrition is recommended over parenteral nutrition.3,4 Enteral nutrition significantly reduces the risk of infected peripancreatic necrosis, single organ failure, and multiorgan failure.4 Finally, the AGA guidelines provide a conditional recommendation for providing enteral nutrition support through either the nasogastric or nasoenteric route.3 Further studies are required to determine the optimal timing, rate, and formulation of enteral nutrition in severe AP.
Antibiotics and probiotics
Current guidelines do not support the use of prophylactic antibiotics to prevent infection in necrotizing AP and severe AP.1-3 The AGA technical review reported that prophylactic antibiotics did not reduce infected pancreatic or peripancreatic necrosis, persistent single organ failure, or mortality.4 Guidelines advocate against the use of probiotics for severe AP, because of increased mortality risk.1
Timing of ERCP in acute biliary pancreatitis
There is universal agreement for offering urgent ERCP (within 24 hours) in biliary AP complicated by cholangitis.1-3,64 Figure 2 demonstrates an example of a cholangiogram completed within 24 hours of presentation of biliary AP complicated by cholangitis.
In the absence of cholangitis, the timing of ERCP for AP with persistent biliary obstruction is less clear.1-3 In line with recent guidelines, the 2018 AGA guidelines advocate against routine use of urgent ERCP for biliary AP without cholangitis,3 a conditional recommendation with overall low quality of data.4 The AGA technical review found that urgent ERCP, compared with conservative management in acute biliary pancreatitis without cholangitis had no significant effect on mortality, organ failure, infected pancreatic necrosis, and total necrotizing pancreatitis, but did significantly shorten hospital length of stay.4 There are limited data to guide decision making of when nonurgent ERCP should be performed in hospitalized patients with biliary AP with persistent obstruction and no cholangitis.3,64
Alcohol and smoking cessation
The AGA technical review advocates for brief alcohol intervention during hospitalization for alcohol-induced AP on the basis of one RCT that addresses the impact of alcohol counseling on recurrent bouts of AP4 plus evidence from a Cochrane review of alcohol-reduction strategies in primary care populations.65 Cessation of smoking – an established independent risk factor of AP – recurrent AP and chronic pancreatitis, should also be recommended as part of the management of AP.
Cholecystectomy
Evidence supports same-admission cholecystectomy for mild gallstone AP, a strong recommendation of published AGA guidelines.3 When compared with delayed cholecystectomy, same-admission cholecystectomy significantly reduced gallstone-related complications, readmissions for recurrent pancreatitis, and pancreaticobiliary complications, without having a significant impact on mortality during a 6-month follow-up period.66 Delaying cholecystectomy 6 weeks in patients with moderate-severe gallstone AP appears to reduce morbidity, including the development of infected collections, and mortality.4 An ongoing RCT, the APEC trial, aims to determine whether early ERCP with biliary sphincterotomy reduces major complications or death when compared with no intervention for biliary AP in patients at high risk of complications.67
Chemoprevention and IV fluid management of post-ERCP pancreatitis
Accumulating data support the effectiveness of chemoprevention, pancreatic stent placement, and fluid administration to prevent post-ERCP pancreatitis. Multiple RCTs, meta-analyses, and systematic reviews indicate that rectal NSAIDs) reduce post-ERCP pancreatitis onset68-71 and moderate-severe post-ERCP pancreatitis. Additionally, placement of a pancreatic duct stent may decrease the risk of severe post-ERCP pancreatitis in high-risk patients.3 Guidelines do not comment on fluid administrations for prevention of post-ERCP pancreatitis, but studies have shown that greater periprocedural IV fluid was an independent protective factor against moderate to severe PEP72 and was associated with shorter hospital length of stay.73 Recent meta-analyses and RCTs support using LR prior to ERCP to prevent PEP.74-77 Interestingly, a recent RCT shows that the combination of rectal indomethacin and LR, compared with combination placebo and normal saline reduced the risk of PEP in high-risk patients.78
Two ongoing multicenter RCTs will clarify the role of combination therapy. The Dutch FLUYT RCT aims to determine the optimal combination of rectal NSAIDs and periprocedural infusion of IV fluids to reduce the incidence of PEP and moderate-severe PEP79 and the Stent vs. Indomethacin (SVI) trial aims to determine the whether combination pancreatic stent placement plus rectal indomethacin is superior to monotherapy indomethacin for preventing post-ERCP pancreatitis in high-risk cases.80
Implications for clinical practice
The diagnosis and optimal management of AP require a systematic approach with multidisciplinary decision making. Morbidity and mortality in AP are driven by early or late POF, and the latter often is triggered by infected necrosis. Risk stratification of these patients at the point of contact is a commonsense approach to enable triaging of patients to the appropriate level of care. Regardless of pancreatitis severity, recommended treatment interventions include goal-directed IV fluid resuscitation, early feeding by mouth or enteral tube when necessary, avoidance of prophylactic antibiotics, avoidance of probiotics, and urgent ERCP for patients with acute biliary pancreatitis complicated by cholangitis. Key measures for preventing hospital readmission and pancreatitis include same-admission cholecystectomy for acute biliary pancreatitis and alcohol and smoking cessation. Preventive measures for post-ERCP pancreatitis in patients undergoing ERCP include rectal indomethacin, prophylactic pancreatic duct stent placement, and periprocedural fluid resuscitation.
Dr. Mandalia is a fellow, gastroenterology, department of internal medicine, division of gastroenterology, Michigan Medicine, Ann Arbor; Dr. DiMagno is associate professor of medicine, director, comprehensive pancreas program, department of internal medicine, division of gastroenterology, University of Michigan, Ann Arbor. Dr. Mandalia reports no conflicts of interest.
References
1. Tenner S et al. Am J Gastroenterol. 2013;108:1400.
2. Besseline M et al. Pancreatology. 2013;13(4, Supplement 2):e1-15.
3. Crockett SD et al. Gastroenterology. 2018;154(4):1096-101.
4. Vege SS et al. Gastroenterology. 2018;154(4):1103-39.
5. Peery AF et al. Gastroenterology. 2019 Jan;156(1):254-72.e11.
6. Krishna SG et al. Pancreas. 2017;46(4):482-8.
7. Sellers ZM et al. Gastroenterology. 2018;155(2):469-78.e1.
8. Brown A et al. JOP. 2008;9(4):408-14.
9. Fagenholz PJ et al. Ann Epidemiol. 2007;17(7):491.e1-.e8.
10. McNabb-Baltar J et al. Pancreas. 2014;43(5):687-91.
11. Johnson CD et al. Gut. 2004;53(9):1340-4.
12. Dellinger EP et al. Ann Surg. 2012;256(6):875-80.
13. Petrov MS et al. Gastroenterology. 2010;139(3):813-20.
14. Sternby H et al. Ann Surg. Apr 18. doi: 10.1097/SLA.0000000000002766.
15. Huh JH et al. J Clin Gastroenterol. 2018;52(2):178-83.
16. Wu BU et al. Gastroenterology. 2008;135(3):816-20.
17. Gardner TB et al. Clin Gastroenterol Hepatol. 2008;6(10):1070-6.
18. Krishna SG et al. Am J Gastroenterol. 2015;110(11):1608-19.
19. Lee PJ et al. Pancreas. 2016;45(4):561-4.
20. Mandalia A et al. F1000Research. 2018 Jun 28;7.
21. Majumder S et al. Pancreas. 2015;44(4):540-6.
22. DiMagno MJ. Clin Gastroenterol Hepatol. 2011;9(11):920-2.
23. Yadav D, Whitcomb DC. Nature Rev Gastroenterol Hepatol. 2010;7(3):131-45.
24. Samokhvalov AV et al. EBioMedicine. 2015;2(12):1996-2002.
25. Barkin JA et al. Pancreas. 2017;46(8):1035-8.
26. Chen Y-T et al. J Gastroenterol Hepatol. 2016;31(4):782-7.
27. Ramos LR et al. J Crohns Colitis. 2016;10(1):95-104.
28. Avram MM. Nephron. 1977;18(1):68-71.
29. Lankisch PG et al. Nephrol Dial Transplant. 2008;23(4):1401-5.
30. Owyang C et al. Mayo Clin Proc. 1979;54(12):769-73.
31. Owyang Cet al. Gut. 1982;23(5):357-61.
32. Quraishi ER et al. Am J Gastroenterol. 2005;100:2288.
33. Vaziri ND et al. Nephron. 1987;46(4):347-9.
34. Chen HJ et al. Nephrol Dial Transplant. 2017;32(10):1731-6.
35. Kirkegard J et al. Gastroenterology. 2018;May;154(6):1729-36.
36. Karlson BM, et al. Gastroenterology. 1997;113(2):587-92.
37. Munigala S et al. Clin Gastroenterol Hepatol. 2014;12(7):1143-50.e1.
38. Carr RA et al. Pancreatology. 2016;16(4):469-76.
39. Li X et al. BMC Gastroenterol. 2018;18(1):89.
40. Ahmed AU et al. Clin Gastroenterol Hepatol. 2016;14(5):738-46.
41. Sankaran SJ et al. Gastroenterology. 2015;149(6):1490-500.e1.
42. Berglund L et al. J Clin Endocrinol Metab. 2012;97(9):2969-89.
43. Catapano AL et al. Atherosclerosis. 2011;217(1):3-46.
44. Pedersen SB et al. JAMA Intern Med. 2016;176(12):1834-42.
45. Nawaz H et al. Am J Gastroenterol. 2015;110(10):1497-503.
46. Banks PA et al. Gut. 2013;62(1):102-11.
47. Kondo S et al. Eur J Radiol. 2005;54(2):271-5.
48. Meeralam Y et al. Gastrointest Endosc. 2017;86(6):986-93.
49. Stimac D et al. Am J Gastroenterol. 2007;102(5):997-1004.
50. Jin DX et al. Dig Dis Sci. 2017;62(10):2894-9.
51. Freeman ML. Gastrointest Endosc Clin N Am. 2012;22(3):567-86.
52. De Lisi S et al. Eur J Gastroenterol Hepatol. 2011;23(5):367-74.
53. Di MY et al. Ann Int Med. 2016;165(7):482-90.
54. Mounzer R et al. Gastroenterology. 2012;142(7):1476-82; quiz e15-6.
55. Koutroumpakis E et al. Am J Gastroenterol. 2015;110(12):1707-16.
56. Wu BU et al. Gastroenterology. 2009;137(1):129-35.
57. Buddingh KT et al. J Am Coll Surg. 2014;218(1):26-32.
58. Buxbaum J et al. Am J Gastroenterol. 2018;113(5):755-64.
59. Jabaudon M et al. Crit Car Med. 2018;46(3):e198-e205.
60. Barlass U et al. Gut. 2018;67(4):600-2.
61. Buxbaum JL et al. Am J Gastroenterol. 2017;112(5):797-803.
62. de-Madaria E et al. United Eur Gastroenterol J. 2018;6(1):63-72.
63. Bakker OJ et al. N Engl J Med. 2014;371(21):1983-93.
64. Tse F et al. Cochrane Database Syst Rev. 2012(5):Cd009779.
65. Kaner EFS et al. Cochrane Database Syst Rev. 2007(2):Cd004148.
66. da Costa DW et al. Lancet. 2015;386(10000):1261-8.
67. Schepers NJ et al. Trials. 2016;17:5.
68. Vadala di Prampero SF et al. Eur J Gastroenterol Hepatol. 2016;28(12):1415-24.
69. Kubiliun NM et al. Clin Gastroenterol Hepatol. 2015;13(7):1231-9; quiz e70-1.
70. Wan J et al. BMC Gastroenterol. 2017;17(1):43.
71. Yang C et al. Pancreatology. 2017;17(5):681-8.
72. DiMagno MJ et al. Pancreas. 2014;43(4):642-7.
73. Sagi SV et al. J Gastroenterol Hepatol. 2014;29(6):1316-20.
74. Choi JH et al. Clin Gastroenterol Hepatol. 2017;15(1):86-92.e1.
75. Wu D et al. J Clin Gastroenterol. 2017;51(8):e68-e76.
76. Zhang ZF et al. J Clin Gastroenterol. 2017;51(3):e17-e26.
77. Park CH et al. Endoscopy 2018 Apr;50(4):378-85.
78. Mok SRS et al. Gastrointest Endosc. 2017;85(5):1005-13.
79. Smeets XJN et al. Trials. 2018;19(1):207.
80. Elmunzer BJ et al. Trials. 2016;17(1):120.
Introduction
Acute pancreatitis (AP) is a major clinical and financial burden in the United States. Several major clinical guidelines provide evidence-based recommendations for the clinical management decisions in AP, including those from the American College of Gastroenterology (ACG; 2013),1 and the International Association of Pancreatology (IAP; 2013).2 More recently, the American Gastroenterological Association (AGA) released their own set of guidelines.3,4 In this update on AP, we review these guidelines and reference recent literature focused on epidemiology, risk factors, etiology, diagnosis, risk stratification, and recent advances in the early medical management of AP. Regarding the latter, we review six treatment interventions (pain management, intravenous fluid resuscitation, feeding, prophylactic antibiotics, probiotics, and timing of endoscopic retrograde cholangiopancreatography (ERCP) in acute biliary pancreatitis) and four preventive interventions (alcohol and smoking cessation, same-admission cholecystectomy for acute biliary pancreatitis, and chemoprevention and fluid administration for post-ERCP pancreatitis [PEP]). Updates on multidisciplinary management of (infected) pancreatic necrosis is beyond the scope of this review. Table 1 summarizes the concepts discussed in this article.

Recent advances in epidemiology and evaluation of AP
Epidemiology
AP is the third most common cause of gastrointestinal-related hospitalizations and fourth most common cause of readmission in 2014.5 Recent epidemiologic studies show conflicting trends for the incidence of AP, both increasing6 and decreasing,7 likely attributable to significant differences in study designs. Importantly, multiple studies have demonstrated that hospital length of stay, costs, and mortality have declined since 2009.6,8-10
Persistent organ failure (POF), defined as organ failure lasting more than 48 hours, is the major cause of death in AP. POF, if only a single organ during AP, is associated with 27%-36% mortality; if it is multiorgan, it is associated with 47% mortality.1,11 Other factors associated with increased hospital mortality include infected pancreatic necrosis,12-14 diabetes mellitus,15 hospital-acquired infection,16 advanced age (70 years and older),17 and obesity.18 Predictive factors of 1-year mortality include readmission within 30 days, higher Charlson Comorbidity Index, and longer hospitalization.19
Risk factors
We briefly highlight recent insights into risk factors for AP (Table 1) and refer to a recent review for further discussion.20 Current and former tobacco use are independent risk factors for AP.21 The dose-response relationship of alcohol to the risk of pancreatitis is complex,22 but five standard drinks per day for 5 years is a commonly used cut-off.1,23 New evidence suggests that the relationship between the dose of alcohol and risk of AP differs by sex, linearly in men but nonlinearly (J-shaped) in women.24 Risk of AP in women was decreased with alcohol consumption of up to 40 g/day (one standard drink contains 14 g of alcohol) and increased above this amount. Cannabis is a possible risk factor for toxin-induced AP and abstinence appears to abolish risk of recurrent attacks.25
Patients with inflammatory bowel disease (IBD) have a 2.9-fold higher risk for AP versus non-IBD cohorts26 with the most common etiologies are from gallstones and medications.27 In patients with end-stage renal disease (ESRD), the risk of AP is higher in those who receive peritoneal dialysis, compared with hemodialysis28-33 and who are women, older, or have cholelithiasis or liver disease.34As recently reviewed,35 pancreatic cancer appears to be associated with first-attack pancreatitis with few exceptions.36 In this setting, the overall incidence of pancreatic cancer is low (1.5%). The risk is greatest within the first year of the attack of AP, negligible below age 40 years but steadily rising through the fifth to eighth decades.37 Pancreatic cancer screening is a conditional recommendation of the ACG guidelines in patients with unexplained AP, particularly those aged 40 years or older.1
Etiology and diagnosis

Alcohol and gallstones remain the most prevalent etiologies for AP.1 While hypertriglyceridemia accounted for 9% of AP in a systematic review of acute pancreatitis in 15 different countries,38 it is the second most common cause of acute pancreatitis in Asia (especially China).39 Figure 1 provides a breakdown of the etiologies and risk factors of pancreatitis. Importantly, it remains challenging to assign several toxic-metabolic etiologies as either a cause or risk factor for AP, particularly with regards to alcohol, smoking, and cannabis to name a few.
Guidelines and recent studies of AP raise questions about the threshold above which hypertriglyceridemia causes or poses as an important cofactor for AP. American and European societies define the threshold for triglycerides at 885-1,000 mg/dL.1,42,43 Pedersen et al. provide evidence of a graded risk of AP with hypertriglyceridemia: In multivariable analysis, adjusted hazard ratios for AP were much higher with nonfasting mild to moderately elevated plasma triglycerides (177-885 mg/dL), compared with normal values (below 89 mg/dL).44 Moreover, the risk of severe AP (developing POF) increases in proportion to triglyceride value, independent of the underlying cause of AP.45

Diagnosis of AP is derived from the revised Atlanta classification.46 The recommended timing and indications for offering cross-sectional imaging are after 48-72 hours in patients with no improvement to initial care.1 Endoscopic ultrasonography (EUS) has better diagnostic accuracy and sensitivity, compared with magnetic resonance cholangiopancreatography (MRCP) for choledocholithiasis, and has comparable specificity.47,48 Among noninvasive imaging modalities, MRCP is more sensitive than computed tomography (CT) for diagnosing choledocholithiasis.49 Despite guideline recommendations for more selective use of pancreatic imaging in the early assessment of AP, utilization of early CT or MRCP imaging (within the first 24 hours of care) remained high during 2014-2015, compared with 2006-2007.50
ERCP is not recommended as a pure diagnostic tool, owing to the availability of other diagnostic tests and a complication rate of 5%-10% with risks involving PEP, cholangitis, perforation, and hemorrhage.51 A recent systematic review of EUS and ERCP in acute biliary pancreatitis concluded that EUS had lower failure rates and had no complications, and the use of EUS avoided ERCP in 71.2% of cases.52
Risk stratification
The goals of using risk stratification tools in AP are to identify patients at risk for developing major outcomes, including POF, infected pancreatic necrosis, and death, and to ensure timely triaging of patients to an appropriate level of care. Existing prediction models have only moderate predictive value.53,54 Examples include simple risk stratification tools such as blood urea nitrogen (BUN) and hemoconcentration,55,56 disease-modifying patient variables (age, obesity, etc.), biomarkers (i.e., angiopoietin 2),57 and more complex clinical scoring systems such as Acute Physiology and Chronic Health Evaluation II (APACHE II), BISAP (BUN, impaired mental status, SIRS criteria, age, pleural effusion) score, early warning system (EWS), Glasgow-Imrie score, Japanese severity score, and recently the Pancreatitis Activity Scoring System (PASS).58 Two recent guidelines affirmed the importance of predicting the severity of AP, using one or more predictive tools.1,2 The recent 2018 AGA technical review does not debate this commonsense approach, but does highlight that there is no published observational study or randomized, controlled trial (RCT) investigating whether prediction tools affect clinical outcomes.4

Recent advances in early treatment of AP
Literature review and definitions
The AP literature contains heterogeneous definitions of severe AP and of what constitutes a major outcome in AP. Based on definitions of the 2013 revised Atlanta Criteria, the 2018 AGA technical review and clinical guidelines emphasized precise definitions of primary outcomes of clinical importance in AP, including death, persistent single organ failure, or persistent multiple organ failure, each requiring a duration of more than 48 hours, and infected pancreatic or peripancreatic necrosis or both (Table 2).3,4
Pain management
Management of pain in AP is complex and requires a detailed discussion beyond the scope of this review, but recent clinical and translational studies raise questions about the current practice of using opioids for pain management in AP. A provocative, multicenter, retrospective cohort study reported lower 30-day mortality among critically ill patients who received epidural analgesia versus standard care without epidural analgesia.59 The possible mechanism of protection and the drugs administered are unclear. An interesting hypothesis is that the epidural cohort may have received lower exposure to morphine, which may increase gut permeability, the risk of infectious complications, and severity of AP, based on a translational study in mice.60
Intravenous fluid administration

Supportive care with the use of IV fluid hydration is a mainstay of treatment for AP in the first 12-24 hours. Table 3 summarizes the guidelines in regards to IV fluid administration as delineated by the ACG and AGA guidelines on the management of pancreatitis.1,3 Guidelines advocate for early fluid resuscitation to correct intravascular depletion in order to reduce morbidity and mortality associated with AP.1,2,4 The 2018 AGA guidelines endorse a conditional recommendation for using goal-directed therapy for initial fluid management,3 do not recommend for or against normal saline versus lactated Ringer’s (LR), but do advise against the use of hydroxyethyl starch fluids.3 Consistent with these recommendations, two recent RCTs published subsequent to the prespecified time periods of the AGA technical review and guideline, observed no significant differences between LR and normal saline on clinically meaningful outcomes.61,62 The AGA guidelines acknowledge that evidence was of very-low quality in support of goal-directed therapy,3,4 which has not been shown to have a significant reduction in persistent multiple organ failure, mortality, or pancreatic necrosis, compared with usual care. As the authors noted, interpretation of the data was limited by the absence of other critical outcomes in these trials (infected pancreatic necrosis), lack of uniformity of specific outcomes and definitions of transient and POF, few trials, and risk of bias. There is a clear need for a large RCT to provide evidence to guide decision making with fluid resuscitation in AP, particularly in regard to fluid type, volume, rate, duration, endpoints, and clinical outcomes.
Feeding
More recently, the focus of nutrition in the management of AP has shifted away from patients remaining nil per os (NPO). Current guidelines advocate for early oral feeding (within 24 hours) in mild AP,3,4 in order to protect the gut-mucosal barrier. Remaining NPO when compared with early oral feeding has a 2.5-fold higher risk for interventions for necrosis.4 The recently published AGA technical review identified no significant impact on outcomes of early versus delayed oral feeding, which is consistent with observations of a landmark Dutch PYTHON trial entitled “Early versus on-demand nasoenteric tube feeding in acute pancreatitis.”4,63 There is no clear cutoff point for initiating feeding for those with severe AP. A suggested practical approach is to initiate feeding within 24-72 hours and offer enteral nutrition for those intolerant to oral feeds. In severe AP and moderately severe AP, enteral nutrition is recommended over parenteral nutrition.3,4 Enteral nutrition significantly reduces the risk of infected peripancreatic necrosis, single organ failure, and multiorgan failure.4 Finally, the AGA guidelines provide a conditional recommendation for providing enteral nutrition support through either the nasogastric or nasoenteric route.3 Further studies are required to determine the optimal timing, rate, and formulation of enteral nutrition in severe AP.
Antibiotics and probiotics
Current guidelines do not support the use of prophylactic antibiotics to prevent infection in necrotizing AP and severe AP.1-3 The AGA technical review reported that prophylactic antibiotics did not reduce infected pancreatic or peripancreatic necrosis, persistent single organ failure, or mortality.4 Guidelines advocate against the use of probiotics for severe AP, because of increased mortality risk.1
Timing of ERCP in acute biliary pancreatitis
There is universal agreement for offering urgent ERCP (within 24 hours) in biliary AP complicated by cholangitis.1-3,64 Figure 2 demonstrates an example of a cholangiogram completed within 24 hours of presentation of biliary AP complicated by cholangitis.
In the absence of cholangitis, the timing of ERCP for AP with persistent biliary obstruction is less clear.1-3 In line with recent guidelines, the 2018 AGA guidelines advocate against routine use of urgent ERCP for biliary AP without cholangitis,3 a conditional recommendation with overall low quality of data.4 The AGA technical review found that urgent ERCP, compared with conservative management in acute biliary pancreatitis without cholangitis had no significant effect on mortality, organ failure, infected pancreatic necrosis, and total necrotizing pancreatitis, but did significantly shorten hospital length of stay.4 There are limited data to guide decision making of when nonurgent ERCP should be performed in hospitalized patients with biliary AP with persistent obstruction and no cholangitis.3,64
Alcohol and smoking cessation
The AGA technical review advocates for brief alcohol intervention during hospitalization for alcohol-induced AP on the basis of one RCT that addresses the impact of alcohol counseling on recurrent bouts of AP4 plus evidence from a Cochrane review of alcohol-reduction strategies in primary care populations.65 Cessation of smoking – an established independent risk factor of AP – recurrent AP and chronic pancreatitis, should also be recommended as part of the management of AP.
Cholecystectomy
Evidence supports same-admission cholecystectomy for mild gallstone AP, a strong recommendation of published AGA guidelines.3 When compared with delayed cholecystectomy, same-admission cholecystectomy significantly reduced gallstone-related complications, readmissions for recurrent pancreatitis, and pancreaticobiliary complications, without having a significant impact on mortality during a 6-month follow-up period.66 Delaying cholecystectomy 6 weeks in patients with moderate-severe gallstone AP appears to reduce morbidity, including the development of infected collections, and mortality.4 An ongoing RCT, the APEC trial, aims to determine whether early ERCP with biliary sphincterotomy reduces major complications or death when compared with no intervention for biliary AP in patients at high risk of complications.67
Chemoprevention and IV fluid management of post-ERCP pancreatitis
Accumulating data support the effectiveness of chemoprevention, pancreatic stent placement, and fluid administration to prevent post-ERCP pancreatitis. Multiple RCTs, meta-analyses, and systematic reviews indicate that rectal NSAIDs) reduce post-ERCP pancreatitis onset68-71 and moderate-severe post-ERCP pancreatitis. Additionally, placement of a pancreatic duct stent may decrease the risk of severe post-ERCP pancreatitis in high-risk patients.3 Guidelines do not comment on fluid administrations for prevention of post-ERCP pancreatitis, but studies have shown that greater periprocedural IV fluid was an independent protective factor against moderate to severe PEP72 and was associated with shorter hospital length of stay.73 Recent meta-analyses and RCTs support using LR prior to ERCP to prevent PEP.74-77 Interestingly, a recent RCT shows that the combination of rectal indomethacin and LR, compared with combination placebo and normal saline reduced the risk of PEP in high-risk patients.78
Two ongoing multicenter RCTs will clarify the role of combination therapy. The Dutch FLUYT RCT aims to determine the optimal combination of rectal NSAIDs and periprocedural infusion of IV fluids to reduce the incidence of PEP and moderate-severe PEP79 and the Stent vs. Indomethacin (SVI) trial aims to determine the whether combination pancreatic stent placement plus rectal indomethacin is superior to monotherapy indomethacin for preventing post-ERCP pancreatitis in high-risk cases.80
Implications for clinical practice
The diagnosis and optimal management of AP require a systematic approach with multidisciplinary decision making. Morbidity and mortality in AP are driven by early or late POF, and the latter often is triggered by infected necrosis. Risk stratification of these patients at the point of contact is a commonsense approach to enable triaging of patients to the appropriate level of care. Regardless of pancreatitis severity, recommended treatment interventions include goal-directed IV fluid resuscitation, early feeding by mouth or enteral tube when necessary, avoidance of prophylactic antibiotics, avoidance of probiotics, and urgent ERCP for patients with acute biliary pancreatitis complicated by cholangitis. Key measures for preventing hospital readmission and pancreatitis include same-admission cholecystectomy for acute biliary pancreatitis and alcohol and smoking cessation. Preventive measures for post-ERCP pancreatitis in patients undergoing ERCP include rectal indomethacin, prophylactic pancreatic duct stent placement, and periprocedural fluid resuscitation.
Dr. Mandalia is a fellow, gastroenterology, department of internal medicine, division of gastroenterology, Michigan Medicine, Ann Arbor; Dr. DiMagno is associate professor of medicine, director, comprehensive pancreas program, department of internal medicine, division of gastroenterology, University of Michigan, Ann Arbor. Dr. Mandalia reports no conflicts of interest.
References
1. Tenner S et al. Am J Gastroenterol. 2013;108:1400.
2. Besseline M et al. Pancreatology. 2013;13(4, Supplement 2):e1-15.
3. Crockett SD et al. Gastroenterology. 2018;154(4):1096-101.
4. Vege SS et al. Gastroenterology. 2018;154(4):1103-39.
5. Peery AF et al. Gastroenterology. 2019 Jan;156(1):254-72.e11.
6. Krishna SG et al. Pancreas. 2017;46(4):482-8.
7. Sellers ZM et al. Gastroenterology. 2018;155(2):469-78.e1.
8. Brown A et al. JOP. 2008;9(4):408-14.
9. Fagenholz PJ et al. Ann Epidemiol. 2007;17(7):491.e1-.e8.
10. McNabb-Baltar J et al. Pancreas. 2014;43(5):687-91.
11. Johnson CD et al. Gut. 2004;53(9):1340-4.
12. Dellinger EP et al. Ann Surg. 2012;256(6):875-80.
13. Petrov MS et al. Gastroenterology. 2010;139(3):813-20.
14. Sternby H et al. Ann Surg. Apr 18. doi: 10.1097/SLA.0000000000002766.
15. Huh JH et al. J Clin Gastroenterol. 2018;52(2):178-83.
16. Wu BU et al. Gastroenterology. 2008;135(3):816-20.
17. Gardner TB et al. Clin Gastroenterol Hepatol. 2008;6(10):1070-6.
18. Krishna SG et al. Am J Gastroenterol. 2015;110(11):1608-19.
19. Lee PJ et al. Pancreas. 2016;45(4):561-4.
20. Mandalia A et al. F1000Research. 2018 Jun 28;7.
21. Majumder S et al. Pancreas. 2015;44(4):540-6.
22. DiMagno MJ. Clin Gastroenterol Hepatol. 2011;9(11):920-2.
23. Yadav D, Whitcomb DC. Nature Rev Gastroenterol Hepatol. 2010;7(3):131-45.
24. Samokhvalov AV et al. EBioMedicine. 2015;2(12):1996-2002.
25. Barkin JA et al. Pancreas. 2017;46(8):1035-8.
26. Chen Y-T et al. J Gastroenterol Hepatol. 2016;31(4):782-7.
27. Ramos LR et al. J Crohns Colitis. 2016;10(1):95-104.
28. Avram MM. Nephron. 1977;18(1):68-71.
29. Lankisch PG et al. Nephrol Dial Transplant. 2008;23(4):1401-5.
30. Owyang C et al. Mayo Clin Proc. 1979;54(12):769-73.
31. Owyang Cet al. Gut. 1982;23(5):357-61.
32. Quraishi ER et al. Am J Gastroenterol. 2005;100:2288.
33. Vaziri ND et al. Nephron. 1987;46(4):347-9.
34. Chen HJ et al. Nephrol Dial Transplant. 2017;32(10):1731-6.
35. Kirkegard J et al. Gastroenterology. 2018;May;154(6):1729-36.
36. Karlson BM, et al. Gastroenterology. 1997;113(2):587-92.
37. Munigala S et al. Clin Gastroenterol Hepatol. 2014;12(7):1143-50.e1.
38. Carr RA et al. Pancreatology. 2016;16(4):469-76.
39. Li X et al. BMC Gastroenterol. 2018;18(1):89.
40. Ahmed AU et al. Clin Gastroenterol Hepatol. 2016;14(5):738-46.
41. Sankaran SJ et al. Gastroenterology. 2015;149(6):1490-500.e1.
42. Berglund L et al. J Clin Endocrinol Metab. 2012;97(9):2969-89.
43. Catapano AL et al. Atherosclerosis. 2011;217(1):3-46.
44. Pedersen SB et al. JAMA Intern Med. 2016;176(12):1834-42.
45. Nawaz H et al. Am J Gastroenterol. 2015;110(10):1497-503.
46. Banks PA et al. Gut. 2013;62(1):102-11.
47. Kondo S et al. Eur J Radiol. 2005;54(2):271-5.
48. Meeralam Y et al. Gastrointest Endosc. 2017;86(6):986-93.
49. Stimac D et al. Am J Gastroenterol. 2007;102(5):997-1004.
50. Jin DX et al. Dig Dis Sci. 2017;62(10):2894-9.
51. Freeman ML. Gastrointest Endosc Clin N Am. 2012;22(3):567-86.
52. De Lisi S et al. Eur J Gastroenterol Hepatol. 2011;23(5):367-74.
53. Di MY et al. Ann Int Med. 2016;165(7):482-90.
54. Mounzer R et al. Gastroenterology. 2012;142(7):1476-82; quiz e15-6.
55. Koutroumpakis E et al. Am J Gastroenterol. 2015;110(12):1707-16.
56. Wu BU et al. Gastroenterology. 2009;137(1):129-35.
57. Buddingh KT et al. J Am Coll Surg. 2014;218(1):26-32.
58. Buxbaum J et al. Am J Gastroenterol. 2018;113(5):755-64.
59. Jabaudon M et al. Crit Car Med. 2018;46(3):e198-e205.
60. Barlass U et al. Gut. 2018;67(4):600-2.
61. Buxbaum JL et al. Am J Gastroenterol. 2017;112(5):797-803.
62. de-Madaria E et al. United Eur Gastroenterol J. 2018;6(1):63-72.
63. Bakker OJ et al. N Engl J Med. 2014;371(21):1983-93.
64. Tse F et al. Cochrane Database Syst Rev. 2012(5):Cd009779.
65. Kaner EFS et al. Cochrane Database Syst Rev. 2007(2):Cd004148.
66. da Costa DW et al. Lancet. 2015;386(10000):1261-8.
67. Schepers NJ et al. Trials. 2016;17:5.
68. Vadala di Prampero SF et al. Eur J Gastroenterol Hepatol. 2016;28(12):1415-24.
69. Kubiliun NM et al. Clin Gastroenterol Hepatol. 2015;13(7):1231-9; quiz e70-1.
70. Wan J et al. BMC Gastroenterol. 2017;17(1):43.
71. Yang C et al. Pancreatology. 2017;17(5):681-8.
72. DiMagno MJ et al. Pancreas. 2014;43(4):642-7.
73. Sagi SV et al. J Gastroenterol Hepatol. 2014;29(6):1316-20.
74. Choi JH et al. Clin Gastroenterol Hepatol. 2017;15(1):86-92.e1.
75. Wu D et al. J Clin Gastroenterol. 2017;51(8):e68-e76.
76. Zhang ZF et al. J Clin Gastroenterol. 2017;51(3):e17-e26.
77. Park CH et al. Endoscopy 2018 Apr;50(4):378-85.
78. Mok SRS et al. Gastrointest Endosc. 2017;85(5):1005-13.
79. Smeets XJN et al. Trials. 2018;19(1):207.
80. Elmunzer BJ et al. Trials. 2016;17(1):120.
Applying ECHELON-2 results to clinical practice
LA JOLLA, CALIF. – Results from the ECHELON-2 trial led to the U.S. approval of brentuximab vedotin (BV) in combination with cyclophosphamide, doxorubicin, and prednisone (CHP), but there are still questions about how to apply the trial results to practice.

At the annual T-cell Lymphoma Forum, trial investigators and other physicians debated the best use of this combination.
BV-CHP is approved to treat patients with previously untreated systemic anaplastic large-cell lymphoma (sALCL) or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma (AITL) and PTCL not otherwise specified (NOS).
Patients who received BV-CHP in ECHELON-2 had superior progression-free survival (PFS) and overall survival (OS) compared to patients who received cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
These results were initially presented at the 2018 annual meeting of the American Society of Hematology and simultaneously published in The Lancet (2019 Jan 19;393[10168]:229-40).
ECHELON-2 investigator Owen O’Connor, MD, PhD, of Columbia University Medical Center in New York, also presented details on the trial at the T-cell Lymphoma Forum. His presentation was followed by a discussion with meeting attendees about applying the trial results to clinical practice.
CD30 expression
One of the issues discussed was the importance of CD30 expression in deciding which patients should receive BV.
For a patient to be eligible for ECHELON-2, the diagnostic biopsy had to show at least 10% of the neoplastic cells were CD30-positive. However, the Food and Drug Administration (FDA) has not made a similar requirement for prescribing BV. PTCL patients with any level of CD30 expression are eligible for treatment with BV-CHP, according to the FDA.
“[I]t’s still a matter of great debate and controversy as to whether we have good enough data to suggest that there’s a threshold effect with regard to the expression of CD30 and responsiveness or sensitivity to brentuximab vedotin,” Dr. O’Connor said.
“This has been an issue from the very first day with this drug, which is, ‘Just how much CD30 do you need to get a response?’ I can’t speak on behalf of the FDA, but I think they are not absolutely convinced that there’s a threshold. They take [CD30-] positive as ‘good enough’ across the board.”
“The FDA has said, ‘The data we’ve seen says there’s a lot of heterogeneity [with biopsies].’ You may do a biopsy and find 30% [of cells are CD30-positive], and you may do another biopsy [in the same patient] and find less than 10%. I don’t think the regulatory agencies are convinced that a single biopsy looking at CD30 ... is representative of the entire tumor burden.”
Andrei Shustov, MD, an ECHELON-2 investigator from the University of Washington in Seattle, questioned whether CD30 expression should be considered when deciding on the use of BV in PTCL.
“Is CD30 staining relevant at all, or should we default back to studies, say, in colon cancer where we didn’t even care about EGFR because we might be missing it by current techniques?” Dr. Shustov asked. “Should we even worry about CD30 expression ... because we cannot reliably detect low levels of CD30?”
Some attendees echoed this sentiment, questioning the utility of assessing CD30 expression. Other attendees said they would defer to the trial data and only treat patients with BV-CHP if they had at least 10% CD30.
PTCL subtypes
Meeting attendees also discussed the value of BV in different PTCL subtypes.
At the request of European regulatory agencies, ECHELON-2 was largely focused on patients with sALCL. They made up 70% of the total trial population, while 16% of patients had PTCL-NOS, 12% had AITL, and a small number of patients had other subtypes. These numbers meant ECHELON-2 was not powered to determine differences in OS or PFS in non-sALCL subtypes.
As a result, some attendees expressed concerns about using BV-CHP to treat PTCL-NOS or AITL. They argued that it wasn’t clear whether patients with these subtypes would derive more benefit from BV-CHP, CHOP, or CHOP plus etoposide (CHOEP).
Other attendees said they would feel comfortable using BV-CHP in patients with PTCL-NOS or AITL based on ECHELON-2 results.
CHOP vs. CHOEP
The use of CHOP in ECHELON-2 was another point of discussion. Some attendees said CHOEP should have been used as the comparator instead.
A few individuals mentioned retrospective data suggesting CHOEP may confer a benefit over CHOP in PTCL (Blood. 2010 Nov 4;116[18]:3418-25).
Marek Trneny, MD, of Charles University General Hospital in Prague, referenced new data from the Czech National Lymphoma Registry, which showed that patients newly diagnosed with PTCL had superior PFS and OS when they received CHOEP rather than CHOP.
Based on these findings, Dr. Trneny said he would consider treating CD30-positive PTCL patients with CHOEP plus BV rather than BV-CHP.
However, most other attendees said they would not consider adding BV to CHOEP due to the absence of clinical trial data supporting this approach.
Some attendees did say they would use CHOEP instead of BV-CHP, particularly in patients with PTCL-NOS or AITL and in patients with CD30 expression below 10%.
ECHELON-2 was funded by Seattle Genetics and Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical Company.
Dr. O’Connor and Dr. Shustov were investigators on ECHELON-2. Dr. O’Connor is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Results from the ECHELON-2 trial led to the U.S. approval of brentuximab vedotin (BV) in combination with cyclophosphamide, doxorubicin, and prednisone (CHP), but there are still questions about how to apply the trial results to practice.

At the annual T-cell Lymphoma Forum, trial investigators and other physicians debated the best use of this combination.
BV-CHP is approved to treat patients with previously untreated systemic anaplastic large-cell lymphoma (sALCL) or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma (AITL) and PTCL not otherwise specified (NOS).
Patients who received BV-CHP in ECHELON-2 had superior progression-free survival (PFS) and overall survival (OS) compared to patients who received cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
These results were initially presented at the 2018 annual meeting of the American Society of Hematology and simultaneously published in The Lancet (2019 Jan 19;393[10168]:229-40).
ECHELON-2 investigator Owen O’Connor, MD, PhD, of Columbia University Medical Center in New York, also presented details on the trial at the T-cell Lymphoma Forum. His presentation was followed by a discussion with meeting attendees about applying the trial results to clinical practice.
CD30 expression
One of the issues discussed was the importance of CD30 expression in deciding which patients should receive BV.
For a patient to be eligible for ECHELON-2, the diagnostic biopsy had to show at least 10% of the neoplastic cells were CD30-positive. However, the Food and Drug Administration (FDA) has not made a similar requirement for prescribing BV. PTCL patients with any level of CD30 expression are eligible for treatment with BV-CHP, according to the FDA.
“[I]t’s still a matter of great debate and controversy as to whether we have good enough data to suggest that there’s a threshold effect with regard to the expression of CD30 and responsiveness or sensitivity to brentuximab vedotin,” Dr. O’Connor said.
“This has been an issue from the very first day with this drug, which is, ‘Just how much CD30 do you need to get a response?’ I can’t speak on behalf of the FDA, but I think they are not absolutely convinced that there’s a threshold. They take [CD30-] positive as ‘good enough’ across the board.”
“The FDA has said, ‘The data we’ve seen says there’s a lot of heterogeneity [with biopsies].’ You may do a biopsy and find 30% [of cells are CD30-positive], and you may do another biopsy [in the same patient] and find less than 10%. I don’t think the regulatory agencies are convinced that a single biopsy looking at CD30 ... is representative of the entire tumor burden.”
Andrei Shustov, MD, an ECHELON-2 investigator from the University of Washington in Seattle, questioned whether CD30 expression should be considered when deciding on the use of BV in PTCL.
“Is CD30 staining relevant at all, or should we default back to studies, say, in colon cancer where we didn’t even care about EGFR because we might be missing it by current techniques?” Dr. Shustov asked. “Should we even worry about CD30 expression ... because we cannot reliably detect low levels of CD30?”
Some attendees echoed this sentiment, questioning the utility of assessing CD30 expression. Other attendees said they would defer to the trial data and only treat patients with BV-CHP if they had at least 10% CD30.
PTCL subtypes
Meeting attendees also discussed the value of BV in different PTCL subtypes.
At the request of European regulatory agencies, ECHELON-2 was largely focused on patients with sALCL. They made up 70% of the total trial population, while 16% of patients had PTCL-NOS, 12% had AITL, and a small number of patients had other subtypes. These numbers meant ECHELON-2 was not powered to determine differences in OS or PFS in non-sALCL subtypes.
As a result, some attendees expressed concerns about using BV-CHP to treat PTCL-NOS or AITL. They argued that it wasn’t clear whether patients with these subtypes would derive more benefit from BV-CHP, CHOP, or CHOP plus etoposide (CHOEP).
Other attendees said they would feel comfortable using BV-CHP in patients with PTCL-NOS or AITL based on ECHELON-2 results.
CHOP vs. CHOEP
The use of CHOP in ECHELON-2 was another point of discussion. Some attendees said CHOEP should have been used as the comparator instead.
A few individuals mentioned retrospective data suggesting CHOEP may confer a benefit over CHOP in PTCL (Blood. 2010 Nov 4;116[18]:3418-25).
Marek Trneny, MD, of Charles University General Hospital in Prague, referenced new data from the Czech National Lymphoma Registry, which showed that patients newly diagnosed with PTCL had superior PFS and OS when they received CHOEP rather than CHOP.
Based on these findings, Dr. Trneny said he would consider treating CD30-positive PTCL patients with CHOEP plus BV rather than BV-CHP.
However, most other attendees said they would not consider adding BV to CHOEP due to the absence of clinical trial data supporting this approach.
Some attendees did say they would use CHOEP instead of BV-CHP, particularly in patients with PTCL-NOS or AITL and in patients with CD30 expression below 10%.
ECHELON-2 was funded by Seattle Genetics and Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical Company.
Dr. O’Connor and Dr. Shustov were investigators on ECHELON-2. Dr. O’Connor is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. – Results from the ECHELON-2 trial led to the U.S. approval of brentuximab vedotin (BV) in combination with cyclophosphamide, doxorubicin, and prednisone (CHP), but there are still questions about how to apply the trial results to practice.

At the annual T-cell Lymphoma Forum, trial investigators and other physicians debated the best use of this combination.
BV-CHP is approved to treat patients with previously untreated systemic anaplastic large-cell lymphoma (sALCL) or other CD30-expressing peripheral T-cell lymphomas (PTCLs), including angioimmunoblastic T-cell lymphoma (AITL) and PTCL not otherwise specified (NOS).
Patients who received BV-CHP in ECHELON-2 had superior progression-free survival (PFS) and overall survival (OS) compared to patients who received cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP).
These results were initially presented at the 2018 annual meeting of the American Society of Hematology and simultaneously published in The Lancet (2019 Jan 19;393[10168]:229-40).
ECHELON-2 investigator Owen O’Connor, MD, PhD, of Columbia University Medical Center in New York, also presented details on the trial at the T-cell Lymphoma Forum. His presentation was followed by a discussion with meeting attendees about applying the trial results to clinical practice.
CD30 expression
One of the issues discussed was the importance of CD30 expression in deciding which patients should receive BV.
For a patient to be eligible for ECHELON-2, the diagnostic biopsy had to show at least 10% of the neoplastic cells were CD30-positive. However, the Food and Drug Administration (FDA) has not made a similar requirement for prescribing BV. PTCL patients with any level of CD30 expression are eligible for treatment with BV-CHP, according to the FDA.
“[I]t’s still a matter of great debate and controversy as to whether we have good enough data to suggest that there’s a threshold effect with regard to the expression of CD30 and responsiveness or sensitivity to brentuximab vedotin,” Dr. O’Connor said.
“This has been an issue from the very first day with this drug, which is, ‘Just how much CD30 do you need to get a response?’ I can’t speak on behalf of the FDA, but I think they are not absolutely convinced that there’s a threshold. They take [CD30-] positive as ‘good enough’ across the board.”
“The FDA has said, ‘The data we’ve seen says there’s a lot of heterogeneity [with biopsies].’ You may do a biopsy and find 30% [of cells are CD30-positive], and you may do another biopsy [in the same patient] and find less than 10%. I don’t think the regulatory agencies are convinced that a single biopsy looking at CD30 ... is representative of the entire tumor burden.”
Andrei Shustov, MD, an ECHELON-2 investigator from the University of Washington in Seattle, questioned whether CD30 expression should be considered when deciding on the use of BV in PTCL.
“Is CD30 staining relevant at all, or should we default back to studies, say, in colon cancer where we didn’t even care about EGFR because we might be missing it by current techniques?” Dr. Shustov asked. “Should we even worry about CD30 expression ... because we cannot reliably detect low levels of CD30?”
Some attendees echoed this sentiment, questioning the utility of assessing CD30 expression. Other attendees said they would defer to the trial data and only treat patients with BV-CHP if they had at least 10% CD30.
PTCL subtypes
Meeting attendees also discussed the value of BV in different PTCL subtypes.
At the request of European regulatory agencies, ECHELON-2 was largely focused on patients with sALCL. They made up 70% of the total trial population, while 16% of patients had PTCL-NOS, 12% had AITL, and a small number of patients had other subtypes. These numbers meant ECHELON-2 was not powered to determine differences in OS or PFS in non-sALCL subtypes.
As a result, some attendees expressed concerns about using BV-CHP to treat PTCL-NOS or AITL. They argued that it wasn’t clear whether patients with these subtypes would derive more benefit from BV-CHP, CHOP, or CHOP plus etoposide (CHOEP).
Other attendees said they would feel comfortable using BV-CHP in patients with PTCL-NOS or AITL based on ECHELON-2 results.
CHOP vs. CHOEP
The use of CHOP in ECHELON-2 was another point of discussion. Some attendees said CHOEP should have been used as the comparator instead.
A few individuals mentioned retrospective data suggesting CHOEP may confer a benefit over CHOP in PTCL (Blood. 2010 Nov 4;116[18]:3418-25).
Marek Trneny, MD, of Charles University General Hospital in Prague, referenced new data from the Czech National Lymphoma Registry, which showed that patients newly diagnosed with PTCL had superior PFS and OS when they received CHOEP rather than CHOP.
Based on these findings, Dr. Trneny said he would consider treating CD30-positive PTCL patients with CHOEP plus BV rather than BV-CHP.
However, most other attendees said they would not consider adding BV to CHOEP due to the absence of clinical trial data supporting this approach.
Some attendees did say they would use CHOEP instead of BV-CHP, particularly in patients with PTCL-NOS or AITL and in patients with CD30 expression below 10%.
ECHELON-2 was funded by Seattle Genetics and Millennium Pharmaceuticals, a wholly owned subsidiary of Takeda Pharmaceutical Company.
Dr. O’Connor and Dr. Shustov were investigators on ECHELON-2. Dr. O’Connor is a cochair of the T-cell Lymphoma Forum. The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
EXPERT ANALYSIS FROM TCLF 2019
Combo treatment may improve quality of life in CTCL
LA JOLLA, CALIF. — Treatment with brentuximab vedotin (BV) and lenalidomide (len) may improve quality of life (QOL) for patients with cutaneous T-cell lymphoma (CTCL), according to the principal investigator of a phase 2 trial.

In this small trial, most CTCL patients experienced relief from pruritus after one cycle of treatment with BV-len.
Investigators also observed durable responses to the combination, although two patients experienced tumor flare prior to response.
“Because of the tumor flare, we decreased the dose of lenalidomide ... and, since then, it has not been a major problem,” said Basem M. William, MD, principal investigator of the trial and a professor at Ohio State University in Columbus.
“We’re trying to be more reassuring to patients that, if they experience a little bit of tumor flare, as long as it’s not dangerous or life-threatening, if they can hold on with the treatment, this might translate to a later durable response.”
Dr. William and his colleagues presented results from this ongoing, phase 2 trial (NCT03409432) at the annual T-cell Lymphoma Forum.
Thus far, the investigators have treated 12 patients with relapsed or refractory CTCL or peripheral T-cell lymphoma (PTCL). The CTCL patients had received at least two lines of skin-directed therapy or one line of systemic therapy, and the PTCL patients had received at least one line of systemic therapy.
Dr. William and his colleagues reported results for 10 patients. Six patients had mycosis fungoides (MF), two had Sézary syndrome (SS), one had CD30+ lymphoproliferative disorder, and one had systemic anaplastic large-cell lymphoma (ALCL).
The patients’ median age was 59 (range, 49-74), there were nine males, and patients had received a median of 2 (range, 1-10) prior therapies.
The first seven patients received BV at 1.2 mg/kg and len at 20 mg daily every 3 weeks. However, after the investigators observed tumor flare in two patients, the dose of len was lowered to 10 mg.
Safety
The investigators said all adverse events (AEs) were reversible by stopping therapy, there were no grade 4 AEs, and none of the patients had grade 3 or higher neuropathy.
“We have not seen an excess of neuropathy, which is very important because both brentuximab and lenalidomide are known to cause neuropathy,” Dr. William said. “So we were fairly concerned that there would be a synergistic neurotoxic effect, which we don’t want, but we haven’t seen that.”
The most common treatment-related AE was neutropenia. Grade 3 neutropenia occurred in four patients.
Other grade 3 AEs, which occurred in patients on the 20 mg dose of len, were thrombocytopenia (n = 1), dyspnea (n = 1), vertigo (n = 1), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome (n = 1), and tumor flare (n = 1).
Three patients discontinued treatment because of AEs — thrombocytopenia, tumor flare, and DRESS syndrome.
Tumor flare and response
“We did see tumor flare in two initial patients treated with the higher dose of lenalidomide, and we had to remove them from the study for their safety,” Dr. William said. “One of them had a full-blown DRESS syndrome. For their safety, we did have to remove them, but both did experience durable remissions after.”
One of the patients with tumor flare, who had MF, didn’t require treatment for 6 months after going off study. The other patient, who had SS, cleared the clone from his blood but developed DRESS syndrome.
In all, three patients achieved a response to treatment. The ALCL patient had a complete response, and two MF patients achieved a partial response.
Two MF patients and one SS patient had stable disease. The remaining four patients — two with MF, one with SS, and one with lymphoproliferative disorder — progressed.
QOL
The investigators used the Skindex-16 to assess the effect of treatment on QOL.
Five of six evaluable patients with CTCL had a 50% or greater reduction in their Skindex-16 scores after two cycles of treatment. In fact, most patients had relief from pruritus after one cycle, Dr. William said.
“Patients with cutaneous T-cell lymphoma, their biggest problem is with the symptom burden, with pruritus,” he said. “They’re really miserable from all the itching they have. They cannot sleep at night. So we’re fairly excited that most of the patients we’ve treated so far had relief from pruritus just after one cycle.”
Dr. William said he and his colleagues are excited about the overall results they have observed with BV-len, although it’s “still pretty early” in the trial. The investigators are planning to enroll a total of 42 patients and may open the trial at a second center.
The study is sponsored by Ohio State University and the lenalidomide is provided by Celgene. Dr. William reported relationships with miRagen Therapeutics, GuidePoint, Kyowa Kirin, and Celgene.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Treatment with brentuximab vedotin (BV) and lenalidomide (len) may improve quality of life (QOL) for patients with cutaneous T-cell lymphoma (CTCL), according to the principal investigator of a phase 2 trial.

In this small trial, most CTCL patients experienced relief from pruritus after one cycle of treatment with BV-len.
Investigators also observed durable responses to the combination, although two patients experienced tumor flare prior to response.
“Because of the tumor flare, we decreased the dose of lenalidomide ... and, since then, it has not been a major problem,” said Basem M. William, MD, principal investigator of the trial and a professor at Ohio State University in Columbus.
“We’re trying to be more reassuring to patients that, if they experience a little bit of tumor flare, as long as it’s not dangerous or life-threatening, if they can hold on with the treatment, this might translate to a later durable response.”
Dr. William and his colleagues presented results from this ongoing, phase 2 trial (NCT03409432) at the annual T-cell Lymphoma Forum.
Thus far, the investigators have treated 12 patients with relapsed or refractory CTCL or peripheral T-cell lymphoma (PTCL). The CTCL patients had received at least two lines of skin-directed therapy or one line of systemic therapy, and the PTCL patients had received at least one line of systemic therapy.
Dr. William and his colleagues reported results for 10 patients. Six patients had mycosis fungoides (MF), two had Sézary syndrome (SS), one had CD30+ lymphoproliferative disorder, and one had systemic anaplastic large-cell lymphoma (ALCL).
The patients’ median age was 59 (range, 49-74), there were nine males, and patients had received a median of 2 (range, 1-10) prior therapies.
The first seven patients received BV at 1.2 mg/kg and len at 20 mg daily every 3 weeks. However, after the investigators observed tumor flare in two patients, the dose of len was lowered to 10 mg.
Safety
The investigators said all adverse events (AEs) were reversible by stopping therapy, there were no grade 4 AEs, and none of the patients had grade 3 or higher neuropathy.
“We have not seen an excess of neuropathy, which is very important because both brentuximab and lenalidomide are known to cause neuropathy,” Dr. William said. “So we were fairly concerned that there would be a synergistic neurotoxic effect, which we don’t want, but we haven’t seen that.”
The most common treatment-related AE was neutropenia. Grade 3 neutropenia occurred in four patients.
Other grade 3 AEs, which occurred in patients on the 20 mg dose of len, were thrombocytopenia (n = 1), dyspnea (n = 1), vertigo (n = 1), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome (n = 1), and tumor flare (n = 1).
Three patients discontinued treatment because of AEs — thrombocytopenia, tumor flare, and DRESS syndrome.
Tumor flare and response
“We did see tumor flare in two initial patients treated with the higher dose of lenalidomide, and we had to remove them from the study for their safety,” Dr. William said. “One of them had a full-blown DRESS syndrome. For their safety, we did have to remove them, but both did experience durable remissions after.”
One of the patients with tumor flare, who had MF, didn’t require treatment for 6 months after going off study. The other patient, who had SS, cleared the clone from his blood but developed DRESS syndrome.
In all, three patients achieved a response to treatment. The ALCL patient had a complete response, and two MF patients achieved a partial response.
Two MF patients and one SS patient had stable disease. The remaining four patients — two with MF, one with SS, and one with lymphoproliferative disorder — progressed.
QOL
The investigators used the Skindex-16 to assess the effect of treatment on QOL.
Five of six evaluable patients with CTCL had a 50% or greater reduction in their Skindex-16 scores after two cycles of treatment. In fact, most patients had relief from pruritus after one cycle, Dr. William said.
“Patients with cutaneous T-cell lymphoma, their biggest problem is with the symptom burden, with pruritus,” he said. “They’re really miserable from all the itching they have. They cannot sleep at night. So we’re fairly excited that most of the patients we’ve treated so far had relief from pruritus just after one cycle.”
Dr. William said he and his colleagues are excited about the overall results they have observed with BV-len, although it’s “still pretty early” in the trial. The investigators are planning to enroll a total of 42 patients and may open the trial at a second center.
The study is sponsored by Ohio State University and the lenalidomide is provided by Celgene. Dr. William reported relationships with miRagen Therapeutics, GuidePoint, Kyowa Kirin, and Celgene.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Treatment with brentuximab vedotin (BV) and lenalidomide (len) may improve quality of life (QOL) for patients with cutaneous T-cell lymphoma (CTCL), according to the principal investigator of a phase 2 trial.

In this small trial, most CTCL patients experienced relief from pruritus after one cycle of treatment with BV-len.
Investigators also observed durable responses to the combination, although two patients experienced tumor flare prior to response.
“Because of the tumor flare, we decreased the dose of lenalidomide ... and, since then, it has not been a major problem,” said Basem M. William, MD, principal investigator of the trial and a professor at Ohio State University in Columbus.
“We’re trying to be more reassuring to patients that, if they experience a little bit of tumor flare, as long as it’s not dangerous or life-threatening, if they can hold on with the treatment, this might translate to a later durable response.”
Dr. William and his colleagues presented results from this ongoing, phase 2 trial (NCT03409432) at the annual T-cell Lymphoma Forum.
Thus far, the investigators have treated 12 patients with relapsed or refractory CTCL or peripheral T-cell lymphoma (PTCL). The CTCL patients had received at least two lines of skin-directed therapy or one line of systemic therapy, and the PTCL patients had received at least one line of systemic therapy.
Dr. William and his colleagues reported results for 10 patients. Six patients had mycosis fungoides (MF), two had Sézary syndrome (SS), one had CD30+ lymphoproliferative disorder, and one had systemic anaplastic large-cell lymphoma (ALCL).
The patients’ median age was 59 (range, 49-74), there were nine males, and patients had received a median of 2 (range, 1-10) prior therapies.
The first seven patients received BV at 1.2 mg/kg and len at 20 mg daily every 3 weeks. However, after the investigators observed tumor flare in two patients, the dose of len was lowered to 10 mg.
Safety
The investigators said all adverse events (AEs) were reversible by stopping therapy, there were no grade 4 AEs, and none of the patients had grade 3 or higher neuropathy.
“We have not seen an excess of neuropathy, which is very important because both brentuximab and lenalidomide are known to cause neuropathy,” Dr. William said. “So we were fairly concerned that there would be a synergistic neurotoxic effect, which we don’t want, but we haven’t seen that.”
The most common treatment-related AE was neutropenia. Grade 3 neutropenia occurred in four patients.
Other grade 3 AEs, which occurred in patients on the 20 mg dose of len, were thrombocytopenia (n = 1), dyspnea (n = 1), vertigo (n = 1), drug rash with eosinophilia and systemic symptoms (DRESS) syndrome (n = 1), and tumor flare (n = 1).
Three patients discontinued treatment because of AEs — thrombocytopenia, tumor flare, and DRESS syndrome.
Tumor flare and response
“We did see tumor flare in two initial patients treated with the higher dose of lenalidomide, and we had to remove them from the study for their safety,” Dr. William said. “One of them had a full-blown DRESS syndrome. For their safety, we did have to remove them, but both did experience durable remissions after.”
One of the patients with tumor flare, who had MF, didn’t require treatment for 6 months after going off study. The other patient, who had SS, cleared the clone from his blood but developed DRESS syndrome.
In all, three patients achieved a response to treatment. The ALCL patient had a complete response, and two MF patients achieved a partial response.
Two MF patients and one SS patient had stable disease. The remaining four patients — two with MF, one with SS, and one with lymphoproliferative disorder — progressed.
QOL
The investigators used the Skindex-16 to assess the effect of treatment on QOL.
Five of six evaluable patients with CTCL had a 50% or greater reduction in their Skindex-16 scores after two cycles of treatment. In fact, most patients had relief from pruritus after one cycle, Dr. William said.
“Patients with cutaneous T-cell lymphoma, their biggest problem is with the symptom burden, with pruritus,” he said. “They’re really miserable from all the itching they have. They cannot sleep at night. So we’re fairly excited that most of the patients we’ve treated so far had relief from pruritus just after one cycle.”
Dr. William said he and his colleagues are excited about the overall results they have observed with BV-len, although it’s “still pretty early” in the trial. The investigators are planning to enroll a total of 42 patients and may open the trial at a second center.
The study is sponsored by Ohio State University and the lenalidomide is provided by Celgene. Dr. William reported relationships with miRagen Therapeutics, GuidePoint, Kyowa Kirin, and Celgene.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: Five of six evaluable CTCL patients had a 50% or greater reduction in their Skindex-16 scores after two cycles of treatment.
Study details: A phase 2 study with results reported for 10 patients.
Disclosures: The study is sponsored by Ohio State University and the lenalidomide is provided by Celgene. The principal investigator reported relationships with miRagen Therapeutics, GuidePoint, Kyowa Kirin, and Celgene.
Four-drug combo shows durable responses in relapsed/refractory lymphomas
LA JOLLA, CALIF. — Results of a phase 1 trial suggest a four-drug combination can produce durable responses in patients with relapsed or refractory T- and B-cell lymphomas.
Seven of 15 patients responded to treatment with romidepsin, gemcitabine, oxaliplatin, and dexamethasone, including six patients who achieved a complete response (CR).
The median duration of response was 8.5 months, and three patients had responses lasting more than 24 months.
Patients with angioimmunoblastic T-cell lymphoma (AITL) in particular responded well to the combination.
Neha Mehta-Shah, MD, of Washington University in St. Louis, and her colleagues presented these results in a poster at the annual T-cell Lymphoma Forum.
“[I]t was thought that the addition of histone deacetylase inhibitors to traditional platinum-based chemotherapies, which tend to cause DNA damage, would increase the response of platinum-based therapies,” Dr. Shah said.
With that in mind, she and her colleagues added romidepsin to gemcitabine, oxaliplatin, and dexamethasone and evaluated this combination in patients with relapsed/refractory lymphomas.
The trial (NCT02181218) enrolled 15 patients — 6 with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 6 with diffuse large B-cell lymphoma (DLBCL), and 3 with AITL.
The patients’ median age was 66 (range, 55-83), and they had received a median of 2 (range, 1-4) prior therapies.
The researchers tested three dose levels of romidepsin — 8 mg/m2, 10 mg/m2, and 12 mg/m2 — given on day 2 of a 21-day cycle. The study originally included romidepsin on day 8 as well. However, the researchers discontinued the day 8 dose after patients developed grade 4 thrombocytopenia.
Patients also received gemcitabine at 1,000 mg/m2 (day 1), oxaliplatin at 100 mg/m2 (day 1), and dexamethasone at 20 mg (days 1-4). All patients received pegfilgrastim at 6 mg (day 3) as well.
The patients could receive up to eight cycles of treatment if they had stable disease or better and did not experience significant toxicity.
Safety
There was one dose-limiting toxicity (DLT) — pneumonia — at the 8 mg/m2 dose of romidepsin (given on days 2 and 8). There was one DLT — bleeding — at the 10 mg/m2 dose (day 2 only).
Two patients experienced DLTs — neutropenic fever and grade 4 thrombocytopenia — at the 12 mg/m2 dose (day 2 only).
Based on these events, 10 mg/m2 was considered the maximum-tolerated dose of romidepsin.
The most common adverse events (AEs) in this trial were thrombocytopenia (n = 13), electrolyte abnormalities (n = 12), liver function abnormalities (n = 10), anemia (n = 9), neutropenia (n = 8), fatigue (n = 7), nausea (n = 7), and creatinine increase (n = 5).
Grade 3/4 AEs included thrombocytopenia (n = 13), neutropenia (n = 5), anemia (n = 3), hyperglycemia (n = 2), hyperuricemia (n = 2), febrile neutropenia (n = 1), tumor lysis syndrome (n = 1), vomiting (n = 1), peripheral sensory neuropathy (n = 1), pneumonia (n = 1), sepsis (n = 1), bleeding (n = 1), and elevated troponin (n = 1).
Serious AEs requiring hospitalization included pneumonia (n = 1), nausea and vomiting (n = 1), tumor lysis syndrome (n = 1), and complications of disease progression (n = 4).
Efficacy
The overall response rate was 47% (7/15). CRs occurred in all three patients with AITL and two patients with DLBCL. One patient with PTCL-NOS had a CR, and one had a partial response.
The median duration of response was 8.5 months (range, 1.2-36.6 months). Four patients remain in CR — two with AITL, one with PTCL-NOS, and one with DLBCL.
Dr. Shah noted that the CRs in the AITL patients “have been quite prolonged.” One patient had a CR lasting 27 months, and another had a CR lasting 29 months.
Dr. Shah said these results are particularly exciting because patients discontinued study treatment after eight cycles or two cycles after they achieved a CR.
“[S]ome of these patients remained in remission for 2 years without any therapy thereafter, which is quite impressive in a population where the median survival — for patients with relapsed/refractory AITL — is thought to be 6-10 months,” Dr. Shah said.
She noted that this study is ongoing with an expansion cohort of patients with T-cell lymphomas.
This research was supported by Celgene. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Results of a phase 1 trial suggest a four-drug combination can produce durable responses in patients with relapsed or refractory T- and B-cell lymphomas.
Seven of 15 patients responded to treatment with romidepsin, gemcitabine, oxaliplatin, and dexamethasone, including six patients who achieved a complete response (CR).
The median duration of response was 8.5 months, and three patients had responses lasting more than 24 months.
Patients with angioimmunoblastic T-cell lymphoma (AITL) in particular responded well to the combination.
Neha Mehta-Shah, MD, of Washington University in St. Louis, and her colleagues presented these results in a poster at the annual T-cell Lymphoma Forum.
“[I]t was thought that the addition of histone deacetylase inhibitors to traditional platinum-based chemotherapies, which tend to cause DNA damage, would increase the response of platinum-based therapies,” Dr. Shah said.
With that in mind, she and her colleagues added romidepsin to gemcitabine, oxaliplatin, and dexamethasone and evaluated this combination in patients with relapsed/refractory lymphomas.
The trial (NCT02181218) enrolled 15 patients — 6 with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 6 with diffuse large B-cell lymphoma (DLBCL), and 3 with AITL.
The patients’ median age was 66 (range, 55-83), and they had received a median of 2 (range, 1-4) prior therapies.
The researchers tested three dose levels of romidepsin — 8 mg/m2, 10 mg/m2, and 12 mg/m2 — given on day 2 of a 21-day cycle. The study originally included romidepsin on day 8 as well. However, the researchers discontinued the day 8 dose after patients developed grade 4 thrombocytopenia.
Patients also received gemcitabine at 1,000 mg/m2 (day 1), oxaliplatin at 100 mg/m2 (day 1), and dexamethasone at 20 mg (days 1-4). All patients received pegfilgrastim at 6 mg (day 3) as well.
The patients could receive up to eight cycles of treatment if they had stable disease or better and did not experience significant toxicity.
Safety
There was one dose-limiting toxicity (DLT) — pneumonia — at the 8 mg/m2 dose of romidepsin (given on days 2 and 8). There was one DLT — bleeding — at the 10 mg/m2 dose (day 2 only).
Two patients experienced DLTs — neutropenic fever and grade 4 thrombocytopenia — at the 12 mg/m2 dose (day 2 only).
Based on these events, 10 mg/m2 was considered the maximum-tolerated dose of romidepsin.
The most common adverse events (AEs) in this trial were thrombocytopenia (n = 13), electrolyte abnormalities (n = 12), liver function abnormalities (n = 10), anemia (n = 9), neutropenia (n = 8), fatigue (n = 7), nausea (n = 7), and creatinine increase (n = 5).
Grade 3/4 AEs included thrombocytopenia (n = 13), neutropenia (n = 5), anemia (n = 3), hyperglycemia (n = 2), hyperuricemia (n = 2), febrile neutropenia (n = 1), tumor lysis syndrome (n = 1), vomiting (n = 1), peripheral sensory neuropathy (n = 1), pneumonia (n = 1), sepsis (n = 1), bleeding (n = 1), and elevated troponin (n = 1).
Serious AEs requiring hospitalization included pneumonia (n = 1), nausea and vomiting (n = 1), tumor lysis syndrome (n = 1), and complications of disease progression (n = 4).
Efficacy
The overall response rate was 47% (7/15). CRs occurred in all three patients with AITL and two patients with DLBCL. One patient with PTCL-NOS had a CR, and one had a partial response.
The median duration of response was 8.5 months (range, 1.2-36.6 months). Four patients remain in CR — two with AITL, one with PTCL-NOS, and one with DLBCL.
Dr. Shah noted that the CRs in the AITL patients “have been quite prolonged.” One patient had a CR lasting 27 months, and another had a CR lasting 29 months.
Dr. Shah said these results are particularly exciting because patients discontinued study treatment after eight cycles or two cycles after they achieved a CR.
“[S]ome of these patients remained in remission for 2 years without any therapy thereafter, which is quite impressive in a population where the median survival — for patients with relapsed/refractory AITL — is thought to be 6-10 months,” Dr. Shah said.
She noted that this study is ongoing with an expansion cohort of patients with T-cell lymphomas.
This research was supported by Celgene. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
LA JOLLA, CALIF. — Results of a phase 1 trial suggest a four-drug combination can produce durable responses in patients with relapsed or refractory T- and B-cell lymphomas.
Seven of 15 patients responded to treatment with romidepsin, gemcitabine, oxaliplatin, and dexamethasone, including six patients who achieved a complete response (CR).
The median duration of response was 8.5 months, and three patients had responses lasting more than 24 months.
Patients with angioimmunoblastic T-cell lymphoma (AITL) in particular responded well to the combination.
Neha Mehta-Shah, MD, of Washington University in St. Louis, and her colleagues presented these results in a poster at the annual T-cell Lymphoma Forum.
“[I]t was thought that the addition of histone deacetylase inhibitors to traditional platinum-based chemotherapies, which tend to cause DNA damage, would increase the response of platinum-based therapies,” Dr. Shah said.
With that in mind, she and her colleagues added romidepsin to gemcitabine, oxaliplatin, and dexamethasone and evaluated this combination in patients with relapsed/refractory lymphomas.
The trial (NCT02181218) enrolled 15 patients — 6 with peripheral T-cell lymphoma not otherwise specified (PTCL-NOS), 6 with diffuse large B-cell lymphoma (DLBCL), and 3 with AITL.
The patients’ median age was 66 (range, 55-83), and they had received a median of 2 (range, 1-4) prior therapies.
The researchers tested three dose levels of romidepsin — 8 mg/m2, 10 mg/m2, and 12 mg/m2 — given on day 2 of a 21-day cycle. The study originally included romidepsin on day 8 as well. However, the researchers discontinued the day 8 dose after patients developed grade 4 thrombocytopenia.
Patients also received gemcitabine at 1,000 mg/m2 (day 1), oxaliplatin at 100 mg/m2 (day 1), and dexamethasone at 20 mg (days 1-4). All patients received pegfilgrastim at 6 mg (day 3) as well.
The patients could receive up to eight cycles of treatment if they had stable disease or better and did not experience significant toxicity.
Safety
There was one dose-limiting toxicity (DLT) — pneumonia — at the 8 mg/m2 dose of romidepsin (given on days 2 and 8). There was one DLT — bleeding — at the 10 mg/m2 dose (day 2 only).
Two patients experienced DLTs — neutropenic fever and grade 4 thrombocytopenia — at the 12 mg/m2 dose (day 2 only).
Based on these events, 10 mg/m2 was considered the maximum-tolerated dose of romidepsin.
The most common adverse events (AEs) in this trial were thrombocytopenia (n = 13), electrolyte abnormalities (n = 12), liver function abnormalities (n = 10), anemia (n = 9), neutropenia (n = 8), fatigue (n = 7), nausea (n = 7), and creatinine increase (n = 5).
Grade 3/4 AEs included thrombocytopenia (n = 13), neutropenia (n = 5), anemia (n = 3), hyperglycemia (n = 2), hyperuricemia (n = 2), febrile neutropenia (n = 1), tumor lysis syndrome (n = 1), vomiting (n = 1), peripheral sensory neuropathy (n = 1), pneumonia (n = 1), sepsis (n = 1), bleeding (n = 1), and elevated troponin (n = 1).
Serious AEs requiring hospitalization included pneumonia (n = 1), nausea and vomiting (n = 1), tumor lysis syndrome (n = 1), and complications of disease progression (n = 4).
Efficacy
The overall response rate was 47% (7/15). CRs occurred in all three patients with AITL and two patients with DLBCL. One patient with PTCL-NOS had a CR, and one had a partial response.
The median duration of response was 8.5 months (range, 1.2-36.6 months). Four patients remain in CR — two with AITL, one with PTCL-NOS, and one with DLBCL.
Dr. Shah noted that the CRs in the AITL patients “have been quite prolonged.” One patient had a CR lasting 27 months, and another had a CR lasting 29 months.
Dr. Shah said these results are particularly exciting because patients discontinued study treatment after eight cycles or two cycles after they achieved a CR.
“[S]ome of these patients remained in remission for 2 years without any therapy thereafter, which is quite impressive in a population where the median survival — for patients with relapsed/refractory AITL — is thought to be 6-10 months,” Dr. Shah said.
She noted that this study is ongoing with an expansion cohort of patients with T-cell lymphomas.
This research was supported by Celgene. Dr. Shah reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.
The T-cell Lymphoma Forum is organized by Jonathan Wood & Associates, which is owned by the same company as this news organization.
REPORTING FROM TCLF 2019
Key clinical point:
Major finding: Seven patients responded, and three patients had responses lasting more than 24 months.
Study details: Phase 1 trial of 15 patients.
Disclosures: This research was supported by Celgene. The presenter reported relationships with Celgene, Kyowa Kirin, Bristol-Myers Squibb, Verastem, and Genentech.