User login
From the Washington Office: Reporting global codes data in 2017
On July 1, 2017, the Centers for Medicare and Medicaid Services (CMS) will begin requiring practitioners in nine states who are part of groups of 10 or more to report data on the services that they provide for select 10- and 90-day global surgical codes. The data collected will be used to improve the accuracy of global codes starting in 2019.
Which states are impacted by the requirement to report global codes data?
What data must be reported?
Health care practitioners who meet claims-based data collection requirements will be required to report American Medical Association Current Procedure Terminology (CPT)* code 99024, Postoperative follow-up visit, normally included in the surgical package, to indicate that an evaluation and management service was performed during a postoperative period for a reason(s) related to the original procedure, for every postoperative visit they provide within the global period of a select list of 10- or 90-day global codes. CMS selected 293 services, which are provided to Medicare patients by more than 100 practitioners per year and are either furnished more than 10,000 times or have allowed charges of more than $10 million annually. The agency estimates that these 293 codes describe approximately 87 percent of all furnished 10- and 90-day global services, and approximately 77 percent of all Medicare expenditures for 10- and 90-day global services under the physician fee schedule.
Is claims-based data reporting mandatory? Is there a penalty for failure to report?
Reporting is mandatory. CMS’ goal is to gather data on postoperative visits as part of its effort to improve the accuracy of global code values starting in 2019. CMS has the authority to implement a 5 percent withhold in payment for global services for health care professionals who fail to report but has not implemented the withhold at this time. Although there is no penalty or withhold of payment for failure to report, ACS urges all surgeons required to report data to comply. Failure to report will result in incomplete data and should data analysis of both inpatient and outpatient postsurgical visits not reflect existing global code definitions, new assumptions may be created to redefine postoperative care.
Why is CMS requiring the reporting of global codes data?
For several years, CMS has communicated its concerns about the accuracy of the values assigned to 10- and 90-day global codes. In 2014, CMS proposed to transition all 10- and 90-day global codes to 0-day, with the requirement that postoperative visits would be reported separately. The ACS argued against this transition because it would have resulted in a reduction in surgeons’ reimbursement for 10- and 90-day global services.
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
On July 1, 2017, the Centers for Medicare and Medicaid Services (CMS) will begin requiring practitioners in nine states who are part of groups of 10 or more to report data on the services that they provide for select 10- and 90-day global surgical codes. The data collected will be used to improve the accuracy of global codes starting in 2019.
Which states are impacted by the requirement to report global codes data?
What data must be reported?
Health care practitioners who meet claims-based data collection requirements will be required to report American Medical Association Current Procedure Terminology (CPT)* code 99024, Postoperative follow-up visit, normally included in the surgical package, to indicate that an evaluation and management service was performed during a postoperative period for a reason(s) related to the original procedure, for every postoperative visit they provide within the global period of a select list of 10- or 90-day global codes. CMS selected 293 services, which are provided to Medicare patients by more than 100 practitioners per year and are either furnished more than 10,000 times or have allowed charges of more than $10 million annually. The agency estimates that these 293 codes describe approximately 87 percent of all furnished 10- and 90-day global services, and approximately 77 percent of all Medicare expenditures for 10- and 90-day global services under the physician fee schedule.
Is claims-based data reporting mandatory? Is there a penalty for failure to report?
Reporting is mandatory. CMS’ goal is to gather data on postoperative visits as part of its effort to improve the accuracy of global code values starting in 2019. CMS has the authority to implement a 5 percent withhold in payment for global services for health care professionals who fail to report but has not implemented the withhold at this time. Although there is no penalty or withhold of payment for failure to report, ACS urges all surgeons required to report data to comply. Failure to report will result in incomplete data and should data analysis of both inpatient and outpatient postsurgical visits not reflect existing global code definitions, new assumptions may be created to redefine postoperative care.
Why is CMS requiring the reporting of global codes data?
For several years, CMS has communicated its concerns about the accuracy of the values assigned to 10- and 90-day global codes. In 2014, CMS proposed to transition all 10- and 90-day global codes to 0-day, with the requirement that postoperative visits would be reported separately. The ACS argued against this transition because it would have resulted in a reduction in surgeons’ reimbursement for 10- and 90-day global services.
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
On July 1, 2017, the Centers for Medicare and Medicaid Services (CMS) will begin requiring practitioners in nine states who are part of groups of 10 or more to report data on the services that they provide for select 10- and 90-day global surgical codes. The data collected will be used to improve the accuracy of global codes starting in 2019.
Which states are impacted by the requirement to report global codes data?
What data must be reported?
Health care practitioners who meet claims-based data collection requirements will be required to report American Medical Association Current Procedure Terminology (CPT)* code 99024, Postoperative follow-up visit, normally included in the surgical package, to indicate that an evaluation and management service was performed during a postoperative period for a reason(s) related to the original procedure, for every postoperative visit they provide within the global period of a select list of 10- or 90-day global codes. CMS selected 293 services, which are provided to Medicare patients by more than 100 practitioners per year and are either furnished more than 10,000 times or have allowed charges of more than $10 million annually. The agency estimates that these 293 codes describe approximately 87 percent of all furnished 10- and 90-day global services, and approximately 77 percent of all Medicare expenditures for 10- and 90-day global services under the physician fee schedule.
Is claims-based data reporting mandatory? Is there a penalty for failure to report?
Reporting is mandatory. CMS’ goal is to gather data on postoperative visits as part of its effort to improve the accuracy of global code values starting in 2019. CMS has the authority to implement a 5 percent withhold in payment for global services for health care professionals who fail to report but has not implemented the withhold at this time. Although there is no penalty or withhold of payment for failure to report, ACS urges all surgeons required to report data to comply. Failure to report will result in incomplete data and should data analysis of both inpatient and outpatient postsurgical visits not reflect existing global code definitions, new assumptions may be created to redefine postoperative care.
Why is CMS requiring the reporting of global codes data?
For several years, CMS has communicated its concerns about the accuracy of the values assigned to 10- and 90-day global codes. In 2014, CMS proposed to transition all 10- and 90-day global codes to 0-day, with the requirement that postoperative visits would be reported separately. The ACS argued against this transition because it would have resulted in a reduction in surgeons’ reimbursement for 10- and 90-day global services.
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
From the Washington Office: Advocacy in Action
Fellows frequently ask how they can get more involved in the advocacy efforts of the ACS. Whether you are new to the arena of policy and advocacy or an experienced veteran of innumerable efforts directed at ensuring access to quality surgical care, I can think of no better way to learn new skills and exercise old ones than by attending the ACS’ annual Leadership and Advocacy Summit.
The 2017 Leadership and Advocacy Summit will take place May 6–9 at the Renaissance Washington, DC Downtown Hotel. More than 300 individuals have already registered and you can join them by registering via the link found here: https://www.facs.org/advocacy/participate/summit-2017/register
The Advocacy Summit portion of the meeting will kick-off on the evening of May 7 with a reception and dinner featuring bestselling author, MSNBC political analyst, and former Communications Chief for President George W. Bush, Nicolle Wallace as the Keynote Speaker.
A robust agenda is planned for Monday, May 8. The morning will lead off with a panel entitled, Perspectives on 2017 Health Care Reform, featuring health policy experts from the Georgetown University Law Center, the George Washington University Milken Institute School of Public Health, the American Enterprise Institute, and the Heritage Foundation. The Monday agenda will also feature a panel of senior staffers from Capitol Hill discussing issues of particular interest to Fellows, a Medicare physician payment panel, and an address from a leading authority on effective communications strategies designed to make your interaction with legislators and their staff more effective.
The luncheon speaker for Monday will be Fox News contributor and Washington Examiner columnist, Lisa Boothe. The afternoon agenda will conclude with a series of issue briefings from ACS staff (in preparation for the Hill visits to legislator’s offices scheduled for Tuesday, May 9) and remarks from several United States Senators. ACSPA-SurgeonsPAC will host a reception on Monday evening, May 8 for all 2017 PAC contributors and a guest.
On Tuesday morning, May 9, attendees will be transported to Capitol Hill to visit the offices of their individual Member of Congress, Senators and staff with visits concluding in time to make flights out of Washington that afternoon.
I encourage all Fellows who are able to set aside time for the event to do so as I believe all will find the program educational and the experience rewarding.
For information about the Leadership Summit, contact Connie Bura at [email protected], or 312-919-5290. For information about the Advocacy Summit, contact Michael Carmody at [email protected], or 202-672-1511.
Until next month ….
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
Fellows frequently ask how they can get more involved in the advocacy efforts of the ACS. Whether you are new to the arena of policy and advocacy or an experienced veteran of innumerable efforts directed at ensuring access to quality surgical care, I can think of no better way to learn new skills and exercise old ones than by attending the ACS’ annual Leadership and Advocacy Summit.
The 2017 Leadership and Advocacy Summit will take place May 6–9 at the Renaissance Washington, DC Downtown Hotel. More than 300 individuals have already registered and you can join them by registering via the link found here: https://www.facs.org/advocacy/participate/summit-2017/register
The Advocacy Summit portion of the meeting will kick-off on the evening of May 7 with a reception and dinner featuring bestselling author, MSNBC political analyst, and former Communications Chief for President George W. Bush, Nicolle Wallace as the Keynote Speaker.
A robust agenda is planned for Monday, May 8. The morning will lead off with a panel entitled, Perspectives on 2017 Health Care Reform, featuring health policy experts from the Georgetown University Law Center, the George Washington University Milken Institute School of Public Health, the American Enterprise Institute, and the Heritage Foundation. The Monday agenda will also feature a panel of senior staffers from Capitol Hill discussing issues of particular interest to Fellows, a Medicare physician payment panel, and an address from a leading authority on effective communications strategies designed to make your interaction with legislators and their staff more effective.
The luncheon speaker for Monday will be Fox News contributor and Washington Examiner columnist, Lisa Boothe. The afternoon agenda will conclude with a series of issue briefings from ACS staff (in preparation for the Hill visits to legislator’s offices scheduled for Tuesday, May 9) and remarks from several United States Senators. ACSPA-SurgeonsPAC will host a reception on Monday evening, May 8 for all 2017 PAC contributors and a guest.
On Tuesday morning, May 9, attendees will be transported to Capitol Hill to visit the offices of their individual Member of Congress, Senators and staff with visits concluding in time to make flights out of Washington that afternoon.
I encourage all Fellows who are able to set aside time for the event to do so as I believe all will find the program educational and the experience rewarding.
For information about the Leadership Summit, contact Connie Bura at [email protected], or 312-919-5290. For information about the Advocacy Summit, contact Michael Carmody at [email protected], or 202-672-1511.
Until next month ….
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
Fellows frequently ask how they can get more involved in the advocacy efforts of the ACS. Whether you are new to the arena of policy and advocacy or an experienced veteran of innumerable efforts directed at ensuring access to quality surgical care, I can think of no better way to learn new skills and exercise old ones than by attending the ACS’ annual Leadership and Advocacy Summit.
The 2017 Leadership and Advocacy Summit will take place May 6–9 at the Renaissance Washington, DC Downtown Hotel. More than 300 individuals have already registered and you can join them by registering via the link found here: https://www.facs.org/advocacy/participate/summit-2017/register
The Advocacy Summit portion of the meeting will kick-off on the evening of May 7 with a reception and dinner featuring bestselling author, MSNBC political analyst, and former Communications Chief for President George W. Bush, Nicolle Wallace as the Keynote Speaker.
A robust agenda is planned for Monday, May 8. The morning will lead off with a panel entitled, Perspectives on 2017 Health Care Reform, featuring health policy experts from the Georgetown University Law Center, the George Washington University Milken Institute School of Public Health, the American Enterprise Institute, and the Heritage Foundation. The Monday agenda will also feature a panel of senior staffers from Capitol Hill discussing issues of particular interest to Fellows, a Medicare physician payment panel, and an address from a leading authority on effective communications strategies designed to make your interaction with legislators and their staff more effective.
The luncheon speaker for Monday will be Fox News contributor and Washington Examiner columnist, Lisa Boothe. The afternoon agenda will conclude with a series of issue briefings from ACS staff (in preparation for the Hill visits to legislator’s offices scheduled for Tuesday, May 9) and remarks from several United States Senators. ACSPA-SurgeonsPAC will host a reception on Monday evening, May 8 for all 2017 PAC contributors and a guest.
On Tuesday morning, May 9, attendees will be transported to Capitol Hill to visit the offices of their individual Member of Congress, Senators and staff with visits concluding in time to make flights out of Washington that afternoon.
I encourage all Fellows who are able to set aside time for the event to do so as I believe all will find the program educational and the experience rewarding.
For information about the Leadership Summit, contact Connie Bura at [email protected], or 312-919-5290. For information about the Advocacy Summit, contact Michael Carmody at [email protected], or 202-672-1511.
Until next month ….
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, DC.
From the Washington Office: Navigating MIPS in 2017
2017 is here and the new Merit-based Incentive Payment System (MIPS) is now in effect. MIPS has taken a number of steps to streamline reporting and make it easier to avoid penalties and achieve positive updates. However, over time penalties for non-participation or poor performance will grow. Therefore, it is critically important that all surgeons make a plan for how they can best participate in order to succeed. Knowing what options are available is vital to navigating the new reporting requirements and achieving the best possible financial outcome.
Background on MIPS and its components
MIPS began measuring performance in January 2017. The data reported in 2017 will be used to adjust payments in 2019. MIPS took the Physician Quality Reporting System (PQRS), the Value Based Modifier (VM), and the EHR Incentive Program commonly referred to as Meaningful Use (EHR-MU), added a new component that provides credit for Improvement Activities and combined them to derive a composite MIPS Final Score. The components of the Final Score are known as Quality, (formerly PQRS), Cost, (formerly VM), Advancing Care Information (ACI), (formerly EHR-MU), and Improvement Activities. The weights for the individual components of the final score for the first year of the MIPS program are represented in the chart above.
Though CMS has chosen not to provide any weight to the Cost component during the first year of the program, those who report Quality data will receive feedback reports on their performance in the Cost component.
2017: The transition year
The Centers for Medicare & Medicaid Services (CMS) designated 2017 as a transition year and has provided a clear pathway to avoid penalties. In addition, CMS has reduced the reporting requirements in 2017 for those who wish to fully participate in preparation for the future or those practices whose goal is the achievement of a positive payment update. It is important to note that the funds available for positive payment updates are derived from the penalties assessed on those who choose NOT to participate. Accordingly, by making it easier to avoid penalties in the first year, CMS has also reduced the amount of funds available for positive incentives.
Participating to avoid penalties
For 2017, CMS instituted options to allow surgeons to “Pick Your Pace” for participation in MIPS. Those who choose not to participate at any level will receive the full negative payment adjustment of 4% in 2019. However, it is noteworthy that a 4% negative payment adjustment is less than half of the negative adjustments associated with the PQRS, VM, and Meaningful Use programs in 2016.
To avoid the 4% penalty, CMS only requires that surgeons test their ability to report data in any of three reporting components, namely Quality, ACI or Improvement Activities. Information for the Cost component is derived automatically and has no reporting requirement. To avoid a penalty, surgeons must simply report one Quality measure for a single patient, attest to participating in an approved Improvement Activity for at least 90 consecutive days or complete the Base score requirements for ACI.
Participating to prepare for future success
Those who wish to attempt to achieve a higher score must report data for 50% of all patients seen (for ALL payors) for any consecutive 90 day period. Accordingly, one could begin as late as October 2, 2017. How data is reported depends upon the circumstances of an individual’s practice as there are multiple methods (electronic health record, registry, or qualified clinical data registry) for submitting data to CMS. It should be noted that data can also be submitted either on an individual basis or as a group.
Reporting pathway toward potentially receiving a positive payment update: Reporting for Quality
To receive the full potential Quality score, data must be submitted for 50% of all patients seen (for ALL payors) for any consecutive 90 day period on a minimum of 6 measures including one Outcome measure. Alternatively, one can choose to use a specialty measure set to report on 50% of all patients seen (for ALL payors) for any consecutive 90 day period. Those who do meet the reporting requirement and perform well on the measures will receive up to 60 points toward their MIPS Final Score. For those who intend to simply avoid penalties for the first year of the MIPS program, reporting a single measure for a single patient will earn the 3 points necessary to meet the threshold prescribed by CMS to avoid a penalty.
Reporting for ACI
The ACI component is worth 25 percent of the MIPS Final Score. The assessment for ACI is a composite score composed of two parts, a Base score and a Performance score. To receive credit for the ACI component 2017, one must have either 2014 or 2015 Edition CEHRT.
The Base score is an “all-or-nothing” threshold and accounts for 50 percent of the total score for the ACI component. Achievement of the Base score is required before any score can be accrued for the Performance portion. Achieving the Base score is also one of the options prescribed by CMS sufficient to avoid MIPS penalties in the first year and if the Base Score is achieved, one will not receive a penalty for 2017. The ACI measures are intended to ensure that certified EHRs are being used for core tasks such as providing patients with online access to their medical records, exchanging health information with patients and other providers, electronic prescribing and protecting sensitive patient health information.
Once all of the measures for the Base score have been met, clinicians are eligible to receive credit for performance on both a subset of the Base score measures and on a set of additional optional measures. Bonus points are also available by reporting certain Improvement Activities via a certified EHR.
Reporting for Improvement Activities
While the Improvement Activities (IA) is a new category, surgeons are familiar with many of the activities including maintenance of certification, use of the ACS Surgical Risk Calculator, participation in a QCDR and registry with their state’s prescription drug monitoring program. Each activity is assigned a point value of either 20 points (high value) or 10 points (medium value). The reporting requirement for the IA is fulfilled by simple attestation via either a registry, qualified clinical data registry, or a portal on the CMS website. To receive full credit, most surgeons must select and attest to having completed between two and four activities for a total of 40 points. Some surgeons in rural or small practices will only need to complete one high value or two medium value activities to achieve full credit. Those who fulfill the requirement will receive 15 points toward the MIPS Final Score. For those whose goal is simply to avoid a penalty in the first reporting year of MIPS, reporting a single activity for 90 days is enough to avoid any MIPS penalties for 2017
For those seeking further information, the ACS website (www.facs.org/qpp) has additional fact sheets and informational videos on the MIPS program.
Until next month …
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
2017 is here and the new Merit-based Incentive Payment System (MIPS) is now in effect. MIPS has taken a number of steps to streamline reporting and make it easier to avoid penalties and achieve positive updates. However, over time penalties for non-participation or poor performance will grow. Therefore, it is critically important that all surgeons make a plan for how they can best participate in order to succeed. Knowing what options are available is vital to navigating the new reporting requirements and achieving the best possible financial outcome.
Background on MIPS and its components
MIPS began measuring performance in January 2017. The data reported in 2017 will be used to adjust payments in 2019. MIPS took the Physician Quality Reporting System (PQRS), the Value Based Modifier (VM), and the EHR Incentive Program commonly referred to as Meaningful Use (EHR-MU), added a new component that provides credit for Improvement Activities and combined them to derive a composite MIPS Final Score. The components of the Final Score are known as Quality, (formerly PQRS), Cost, (formerly VM), Advancing Care Information (ACI), (formerly EHR-MU), and Improvement Activities. The weights for the individual components of the final score for the first year of the MIPS program are represented in the chart above.
Though CMS has chosen not to provide any weight to the Cost component during the first year of the program, those who report Quality data will receive feedback reports on their performance in the Cost component.
2017: The transition year
The Centers for Medicare & Medicaid Services (CMS) designated 2017 as a transition year and has provided a clear pathway to avoid penalties. In addition, CMS has reduced the reporting requirements in 2017 for those who wish to fully participate in preparation for the future or those practices whose goal is the achievement of a positive payment update. It is important to note that the funds available for positive payment updates are derived from the penalties assessed on those who choose NOT to participate. Accordingly, by making it easier to avoid penalties in the first year, CMS has also reduced the amount of funds available for positive incentives.
Participating to avoid penalties
For 2017, CMS instituted options to allow surgeons to “Pick Your Pace” for participation in MIPS. Those who choose not to participate at any level will receive the full negative payment adjustment of 4% in 2019. However, it is noteworthy that a 4% negative payment adjustment is less than half of the negative adjustments associated with the PQRS, VM, and Meaningful Use programs in 2016.
To avoid the 4% penalty, CMS only requires that surgeons test their ability to report data in any of three reporting components, namely Quality, ACI or Improvement Activities. Information for the Cost component is derived automatically and has no reporting requirement. To avoid a penalty, surgeons must simply report one Quality measure for a single patient, attest to participating in an approved Improvement Activity for at least 90 consecutive days or complete the Base score requirements for ACI.
Participating to prepare for future success
Those who wish to attempt to achieve a higher score must report data for 50% of all patients seen (for ALL payors) for any consecutive 90 day period. Accordingly, one could begin as late as October 2, 2017. How data is reported depends upon the circumstances of an individual’s practice as there are multiple methods (electronic health record, registry, or qualified clinical data registry) for submitting data to CMS. It should be noted that data can also be submitted either on an individual basis or as a group.
Reporting pathway toward potentially receiving a positive payment update: Reporting for Quality
To receive the full potential Quality score, data must be submitted for 50% of all patients seen (for ALL payors) for any consecutive 90 day period on a minimum of 6 measures including one Outcome measure. Alternatively, one can choose to use a specialty measure set to report on 50% of all patients seen (for ALL payors) for any consecutive 90 day period. Those who do meet the reporting requirement and perform well on the measures will receive up to 60 points toward their MIPS Final Score. For those who intend to simply avoid penalties for the first year of the MIPS program, reporting a single measure for a single patient will earn the 3 points necessary to meet the threshold prescribed by CMS to avoid a penalty.
Reporting for ACI
The ACI component is worth 25 percent of the MIPS Final Score. The assessment for ACI is a composite score composed of two parts, a Base score and a Performance score. To receive credit for the ACI component 2017, one must have either 2014 or 2015 Edition CEHRT.
The Base score is an “all-or-nothing” threshold and accounts for 50 percent of the total score for the ACI component. Achievement of the Base score is required before any score can be accrued for the Performance portion. Achieving the Base score is also one of the options prescribed by CMS sufficient to avoid MIPS penalties in the first year and if the Base Score is achieved, one will not receive a penalty for 2017. The ACI measures are intended to ensure that certified EHRs are being used for core tasks such as providing patients with online access to their medical records, exchanging health information with patients and other providers, electronic prescribing and protecting sensitive patient health information.
Once all of the measures for the Base score have been met, clinicians are eligible to receive credit for performance on both a subset of the Base score measures and on a set of additional optional measures. Bonus points are also available by reporting certain Improvement Activities via a certified EHR.
Reporting for Improvement Activities
While the Improvement Activities (IA) is a new category, surgeons are familiar with many of the activities including maintenance of certification, use of the ACS Surgical Risk Calculator, participation in a QCDR and registry with their state’s prescription drug monitoring program. Each activity is assigned a point value of either 20 points (high value) or 10 points (medium value). The reporting requirement for the IA is fulfilled by simple attestation via either a registry, qualified clinical data registry, or a portal on the CMS website. To receive full credit, most surgeons must select and attest to having completed between two and four activities for a total of 40 points. Some surgeons in rural or small practices will only need to complete one high value or two medium value activities to achieve full credit. Those who fulfill the requirement will receive 15 points toward the MIPS Final Score. For those whose goal is simply to avoid a penalty in the first reporting year of MIPS, reporting a single activity for 90 days is enough to avoid any MIPS penalties for 2017
For those seeking further information, the ACS website (www.facs.org/qpp) has additional fact sheets and informational videos on the MIPS program.
Until next month …
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
2017 is here and the new Merit-based Incentive Payment System (MIPS) is now in effect. MIPS has taken a number of steps to streamline reporting and make it easier to avoid penalties and achieve positive updates. However, over time penalties for non-participation or poor performance will grow. Therefore, it is critically important that all surgeons make a plan for how they can best participate in order to succeed. Knowing what options are available is vital to navigating the new reporting requirements and achieving the best possible financial outcome.
Background on MIPS and its components
MIPS began measuring performance in January 2017. The data reported in 2017 will be used to adjust payments in 2019. MIPS took the Physician Quality Reporting System (PQRS), the Value Based Modifier (VM), and the EHR Incentive Program commonly referred to as Meaningful Use (EHR-MU), added a new component that provides credit for Improvement Activities and combined them to derive a composite MIPS Final Score. The components of the Final Score are known as Quality, (formerly PQRS), Cost, (formerly VM), Advancing Care Information (ACI), (formerly EHR-MU), and Improvement Activities. The weights for the individual components of the final score for the first year of the MIPS program are represented in the chart above.
Though CMS has chosen not to provide any weight to the Cost component during the first year of the program, those who report Quality data will receive feedback reports on their performance in the Cost component.
2017: The transition year
The Centers for Medicare & Medicaid Services (CMS) designated 2017 as a transition year and has provided a clear pathway to avoid penalties. In addition, CMS has reduced the reporting requirements in 2017 for those who wish to fully participate in preparation for the future or those practices whose goal is the achievement of a positive payment update. It is important to note that the funds available for positive payment updates are derived from the penalties assessed on those who choose NOT to participate. Accordingly, by making it easier to avoid penalties in the first year, CMS has also reduced the amount of funds available for positive incentives.
Participating to avoid penalties
For 2017, CMS instituted options to allow surgeons to “Pick Your Pace” for participation in MIPS. Those who choose not to participate at any level will receive the full negative payment adjustment of 4% in 2019. However, it is noteworthy that a 4% negative payment adjustment is less than half of the negative adjustments associated with the PQRS, VM, and Meaningful Use programs in 2016.
To avoid the 4% penalty, CMS only requires that surgeons test their ability to report data in any of three reporting components, namely Quality, ACI or Improvement Activities. Information for the Cost component is derived automatically and has no reporting requirement. To avoid a penalty, surgeons must simply report one Quality measure for a single patient, attest to participating in an approved Improvement Activity for at least 90 consecutive days or complete the Base score requirements for ACI.
Participating to prepare for future success
Those who wish to attempt to achieve a higher score must report data for 50% of all patients seen (for ALL payors) for any consecutive 90 day period. Accordingly, one could begin as late as October 2, 2017. How data is reported depends upon the circumstances of an individual’s practice as there are multiple methods (electronic health record, registry, or qualified clinical data registry) for submitting data to CMS. It should be noted that data can also be submitted either on an individual basis or as a group.
Reporting pathway toward potentially receiving a positive payment update: Reporting for Quality
To receive the full potential Quality score, data must be submitted for 50% of all patients seen (for ALL payors) for any consecutive 90 day period on a minimum of 6 measures including one Outcome measure. Alternatively, one can choose to use a specialty measure set to report on 50% of all patients seen (for ALL payors) for any consecutive 90 day period. Those who do meet the reporting requirement and perform well on the measures will receive up to 60 points toward their MIPS Final Score. For those who intend to simply avoid penalties for the first year of the MIPS program, reporting a single measure for a single patient will earn the 3 points necessary to meet the threshold prescribed by CMS to avoid a penalty.
Reporting for ACI
The ACI component is worth 25 percent of the MIPS Final Score. The assessment for ACI is a composite score composed of two parts, a Base score and a Performance score. To receive credit for the ACI component 2017, one must have either 2014 or 2015 Edition CEHRT.
The Base score is an “all-or-nothing” threshold and accounts for 50 percent of the total score for the ACI component. Achievement of the Base score is required before any score can be accrued for the Performance portion. Achieving the Base score is also one of the options prescribed by CMS sufficient to avoid MIPS penalties in the first year and if the Base Score is achieved, one will not receive a penalty for 2017. The ACI measures are intended to ensure that certified EHRs are being used for core tasks such as providing patients with online access to their medical records, exchanging health information with patients and other providers, electronic prescribing and protecting sensitive patient health information.
Once all of the measures for the Base score have been met, clinicians are eligible to receive credit for performance on both a subset of the Base score measures and on a set of additional optional measures. Bonus points are also available by reporting certain Improvement Activities via a certified EHR.
Reporting for Improvement Activities
While the Improvement Activities (IA) is a new category, surgeons are familiar with many of the activities including maintenance of certification, use of the ACS Surgical Risk Calculator, participation in a QCDR and registry with their state’s prescription drug monitoring program. Each activity is assigned a point value of either 20 points (high value) or 10 points (medium value). The reporting requirement for the IA is fulfilled by simple attestation via either a registry, qualified clinical data registry, or a portal on the CMS website. To receive full credit, most surgeons must select and attest to having completed between two and four activities for a total of 40 points. Some surgeons in rural or small practices will only need to complete one high value or two medium value activities to achieve full credit. Those who fulfill the requirement will receive 15 points toward the MIPS Final Score. For those whose goal is simply to avoid a penalty in the first reporting year of MIPS, reporting a single activity for 90 days is enough to avoid any MIPS penalties for 2017
For those seeking further information, the ACS website (www.facs.org/qpp) has additional fact sheets and informational videos on the MIPS program.
Until next month …
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
From the Washington Office: ACS Works to Establish Joint Trauma System in NDAA
I am frequently asked for examples of successes from the College’s advocacy efforts in DC. While many of our successes come in the form of legislation or regulation we are either able to significantly modify into more favorable form or to outright prevent from being enacted, this month’s topic provides an example of how our advocacy efforts are equally successful in obtaining specific provisions in legislation.
Over a year ago, staff of the Division of Advocacy and Health Policy were approached by members and staff of the Military Health System Strategic Partnership American College of Surgeons to assist them in their effort toward the establishment of both a Joint Trauma System (JTS) within the Defense Health Agency (to promote continuous improvement of trauma care provided to members of the Armed Forces) and a Joint Trauma Education and Training Directorate (JTETD) (to ensure military traumatologists maintain readiness with regard to critical surgical skills). I am pleased and proud to report that when the U.S. House of Representatives, on December 2, and the Senate, on December 8, passed the National Defense Authorization Act (NDAA), provisions for both the JTS and the JTETD, in nearly the precise wording as was proposed by ACS, were included in the legislation.
Our success in this effort was strongly supported by both Rep. Joe Heck, DO (R-Nev.), Chairman of the House Armed Services Subcommittee on Military Personnel, and Rep. Brad Wenstrup, DPM (R-Ohio). Rep. Heck, who is a Brigadier General in the Army Reserve, ensured that the language establishing the JTS within the U.S. Department of Defense and the JTETD were included in the House version of the NDAA. Rep. Wenstrup, who also serves in the Army Reserve, was key to securing language providing for review of the military trauma system under the JTS by a “non-government entity with subject matter experts.” This is an activity that the ACS Committee on Trauma Verification, Review, and Consultation Program conducts on a regular basis.
The Joint Trauma System will standardize trauma care in the military by establishing uniform standards for all military medical treatment facilities. The Joint Trauma Education and Training Directorate is charged with ensuring that trauma providers of the U.S. Armed Forces maintain a state of readiness. Under this provision, partnerships will be established with level one trauma centers in civilian academic medical centers and large metropolitan teaching hospitals where combat casualty care teams will embed to provide military surgeons with regular exposure to critically injured patients.
The Senate version of the NDAA did not contain language specifically outlining provisions for either the JTS or the JTETD. Because the House and Senate versions of the NDAA were different, a conference committee from both legislative bodies was appointed to settle the differences between the two versions of the legislation. Over the several months duration of the conference committee process, members of the ACS legislative affairs team met regularly with the offices of several key senators who serve on the Senate Armed Services Committee as well as with committee staff for both the Republican and Democrat members of the committee. During these meetings, we repeatedly “made the case” relative to the critically important nature of these provisions and were able to answer questions and address concerns relative to why ACS felt it was vitally important to include the House language in the final version of the bill. No doubt, these efforts were key in the decision of the Senate negotiators to recede their position and agree to the House language in the final version of the bill relative to these specific provisions.
Prior to the final House vote on the conference committee language of the NDAA, Rep. Wenstrup spoke on the House floor in support of the JTETD.
As I write, the legislation is awaiting signature by President Obama and it is expected he will do so in the coming days.
ACS’ successful efforts toward the establishment of the Joint Trauma System and the Joint Trauma Education and Training Directorate represent a significant achievement toward ensuring that our soldiers, sailors, airmen, Marines and guardsmen continue to receive the best of the best in trauma care while in the service of our nation.
Until next month ….
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
I am frequently asked for examples of successes from the College’s advocacy efforts in DC. While many of our successes come in the form of legislation or regulation we are either able to significantly modify into more favorable form or to outright prevent from being enacted, this month’s topic provides an example of how our advocacy efforts are equally successful in obtaining specific provisions in legislation.
Over a year ago, staff of the Division of Advocacy and Health Policy were approached by members and staff of the Military Health System Strategic Partnership American College of Surgeons to assist them in their effort toward the establishment of both a Joint Trauma System (JTS) within the Defense Health Agency (to promote continuous improvement of trauma care provided to members of the Armed Forces) and a Joint Trauma Education and Training Directorate (JTETD) (to ensure military traumatologists maintain readiness with regard to critical surgical skills). I am pleased and proud to report that when the U.S. House of Representatives, on December 2, and the Senate, on December 8, passed the National Defense Authorization Act (NDAA), provisions for both the JTS and the JTETD, in nearly the precise wording as was proposed by ACS, were included in the legislation.
Our success in this effort was strongly supported by both Rep. Joe Heck, DO (R-Nev.), Chairman of the House Armed Services Subcommittee on Military Personnel, and Rep. Brad Wenstrup, DPM (R-Ohio). Rep. Heck, who is a Brigadier General in the Army Reserve, ensured that the language establishing the JTS within the U.S. Department of Defense and the JTETD were included in the House version of the NDAA. Rep. Wenstrup, who also serves in the Army Reserve, was key to securing language providing for review of the military trauma system under the JTS by a “non-government entity with subject matter experts.” This is an activity that the ACS Committee on Trauma Verification, Review, and Consultation Program conducts on a regular basis.
The Joint Trauma System will standardize trauma care in the military by establishing uniform standards for all military medical treatment facilities. The Joint Trauma Education and Training Directorate is charged with ensuring that trauma providers of the U.S. Armed Forces maintain a state of readiness. Under this provision, partnerships will be established with level one trauma centers in civilian academic medical centers and large metropolitan teaching hospitals where combat casualty care teams will embed to provide military surgeons with regular exposure to critically injured patients.
The Senate version of the NDAA did not contain language specifically outlining provisions for either the JTS or the JTETD. Because the House and Senate versions of the NDAA were different, a conference committee from both legislative bodies was appointed to settle the differences between the two versions of the legislation. Over the several months duration of the conference committee process, members of the ACS legislative affairs team met regularly with the offices of several key senators who serve on the Senate Armed Services Committee as well as with committee staff for both the Republican and Democrat members of the committee. During these meetings, we repeatedly “made the case” relative to the critically important nature of these provisions and were able to answer questions and address concerns relative to why ACS felt it was vitally important to include the House language in the final version of the bill. No doubt, these efforts were key in the decision of the Senate negotiators to recede their position and agree to the House language in the final version of the bill relative to these specific provisions.
Prior to the final House vote on the conference committee language of the NDAA, Rep. Wenstrup spoke on the House floor in support of the JTETD.
As I write, the legislation is awaiting signature by President Obama and it is expected he will do so in the coming days.
ACS’ successful efforts toward the establishment of the Joint Trauma System and the Joint Trauma Education and Training Directorate represent a significant achievement toward ensuring that our soldiers, sailors, airmen, Marines and guardsmen continue to receive the best of the best in trauma care while in the service of our nation.
Until next month ….
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
I am frequently asked for examples of successes from the College’s advocacy efforts in DC. While many of our successes come in the form of legislation or regulation we are either able to significantly modify into more favorable form or to outright prevent from being enacted, this month’s topic provides an example of how our advocacy efforts are equally successful in obtaining specific provisions in legislation.
Over a year ago, staff of the Division of Advocacy and Health Policy were approached by members and staff of the Military Health System Strategic Partnership American College of Surgeons to assist them in their effort toward the establishment of both a Joint Trauma System (JTS) within the Defense Health Agency (to promote continuous improvement of trauma care provided to members of the Armed Forces) and a Joint Trauma Education and Training Directorate (JTETD) (to ensure military traumatologists maintain readiness with regard to critical surgical skills). I am pleased and proud to report that when the U.S. House of Representatives, on December 2, and the Senate, on December 8, passed the National Defense Authorization Act (NDAA), provisions for both the JTS and the JTETD, in nearly the precise wording as was proposed by ACS, were included in the legislation.
Our success in this effort was strongly supported by both Rep. Joe Heck, DO (R-Nev.), Chairman of the House Armed Services Subcommittee on Military Personnel, and Rep. Brad Wenstrup, DPM (R-Ohio). Rep. Heck, who is a Brigadier General in the Army Reserve, ensured that the language establishing the JTS within the U.S. Department of Defense and the JTETD were included in the House version of the NDAA. Rep. Wenstrup, who also serves in the Army Reserve, was key to securing language providing for review of the military trauma system under the JTS by a “non-government entity with subject matter experts.” This is an activity that the ACS Committee on Trauma Verification, Review, and Consultation Program conducts on a regular basis.
The Joint Trauma System will standardize trauma care in the military by establishing uniform standards for all military medical treatment facilities. The Joint Trauma Education and Training Directorate is charged with ensuring that trauma providers of the U.S. Armed Forces maintain a state of readiness. Under this provision, partnerships will be established with level one trauma centers in civilian academic medical centers and large metropolitan teaching hospitals where combat casualty care teams will embed to provide military surgeons with regular exposure to critically injured patients.
The Senate version of the NDAA did not contain language specifically outlining provisions for either the JTS or the JTETD. Because the House and Senate versions of the NDAA were different, a conference committee from both legislative bodies was appointed to settle the differences between the two versions of the legislation. Over the several months duration of the conference committee process, members of the ACS legislative affairs team met regularly with the offices of several key senators who serve on the Senate Armed Services Committee as well as with committee staff for both the Republican and Democrat members of the committee. During these meetings, we repeatedly “made the case” relative to the critically important nature of these provisions and were able to answer questions and address concerns relative to why ACS felt it was vitally important to include the House language in the final version of the bill. No doubt, these efforts were key in the decision of the Senate negotiators to recede their position and agree to the House language in the final version of the bill relative to these specific provisions.
Prior to the final House vote on the conference committee language of the NDAA, Rep. Wenstrup spoke on the House floor in support of the JTETD.
As I write, the legislation is awaiting signature by President Obama and it is expected he will do so in the coming days.
ACS’ successful efforts toward the establishment of the Joint Trauma System and the Joint Trauma Education and Training Directorate represent a significant achievement toward ensuring that our soldiers, sailors, airmen, Marines and guardsmen continue to receive the best of the best in trauma care while in the service of our nation.
Until next month ….
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
Clinical Practice Improvement Activities: The New Reporting Requirement
As was summarized last month, Centers for Medicare and Medicaid Services (CMS) released the final rule pertaining to the Medicare Access and CHIP Reauthorization Act (MACRA) on October 14, 2016. In the ensuing weeks, Division of Advocacy and Health Policy staff had the opportunity to read and further analyze the rule. In general, the initial favorable impression held up under more careful scrutiny. Based on the provisions in the final rule, we continue to make adjustments and modifications to the resources available to Fellows to assist them to prepare for 2017 on the website found at www.facs.org/qpp. By the time this column is printed/released, I anticipate that the video series found on the website will have been updated and expanded upon.
Because change is unsettling, one of the most frequent topics of conversation and question concerning MACRA is the new reporting requirement known as Improvement Activities. Previously, in the proposed rule, this component was known as the Clinical Practice Improvement Activities. In the final rule, the nomenclature was shortened to Improvement Activities.
The reporting mechanism specified by CMS for 2017 is simple attestation. That attestation may be accomplished via any traditional reporting mechanism other than by claims. Accordingly, the use of CMS approved “traditional” registries (registry reporting option) or qualified clinical data registries, such as the ACS’ Surgeon Specific Registry (SSR), will be valid modes by which one may report. Discussions are underway to determine how best to incorporate the Improvement Activities into the SSR. Alternatively, CMS plans to make available a portal on its website where providers will be able to attest to their having satisfied the Improvement Activity requirement.
In reviewing the list of 93 activities, examples of such that likely would or could be applicable to surgeons include:
• Use of a QCDR (qualified clinical data registry) to generate regular performance feedback (20 points)
• Participation in a QCDR, clinical data registries, or other registries run by other government agencies or private entities such as a hospital or medical or surgical society (10 points)
• Provision of episodic care management, including management across transitions and referrals that could include routine and timely follow-up to hospitalizations and ED visits and/or managing care intensively through new diagnoses, injuries and exacerbations of illness (10 points)
• Provision of specialist reports back to referring providers to close the referral loop (10 points)
• Timely communication of test results defined as timely identification of abnormal test results with timely follow-up (10 points)
• Participation in a QCDR, demonstrating performance of activities that promote use of standard practices, tools and processes for quality improvement (10 points)
• Bilateral exchange of necessary patient information to guide patient care that could include participation in a health information exchange or use of structured referral notes (10 points)
• Participation in a QCDR, demonstrating performance of activities that promote implementation of shared clinical decision making capabilities (10 points)
• Use of evidence-based decision aids to support shared decision-making (10 points)
• Participation in Maintenance of Certification Part IV (10 points)
• Annual registration by eligible clinician or group in the prescription drug monitoring program of the state where they practice (10 points)
• Consultation of prescription drug monitoring program prior to the issuance of a Controlled Substance Schedule II opioid prescription that lasts for longer than 3 days (20 points)
• Use of tools that assist specialty practices in tracking specific measures that are meaningful to their practice, such as use of the Surgical Risk Calculator (10 points)
• Seeing new and follow-up Medicaid patients in a timely manner, including individuals dually eligible for Medicaid and Medicare (20 points)
Based upon the list above (and others not included), and because the requirement specified for reporting the Improvement Activities is simple attestation, I am confident that all surgeons will be able to meet the requirement with minimal effort and achieve full credit for this component of the MIPS Composite Performance score. In these last weeks of 2016, I would encourage all Fellows to visit the ACS QPP website at www.facs.org/qpp to map out their overall strategy for success with the new Medicare physician payment system that will become effective beginning in January of 2017.
Until next month ….
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
As was summarized last month, Centers for Medicare and Medicaid Services (CMS) released the final rule pertaining to the Medicare Access and CHIP Reauthorization Act (MACRA) on October 14, 2016. In the ensuing weeks, Division of Advocacy and Health Policy staff had the opportunity to read and further analyze the rule. In general, the initial favorable impression held up under more careful scrutiny. Based on the provisions in the final rule, we continue to make adjustments and modifications to the resources available to Fellows to assist them to prepare for 2017 on the website found at www.facs.org/qpp. By the time this column is printed/released, I anticipate that the video series found on the website will have been updated and expanded upon.
Because change is unsettling, one of the most frequent topics of conversation and question concerning MACRA is the new reporting requirement known as Improvement Activities. Previously, in the proposed rule, this component was known as the Clinical Practice Improvement Activities. In the final rule, the nomenclature was shortened to Improvement Activities.
The reporting mechanism specified by CMS for 2017 is simple attestation. That attestation may be accomplished via any traditional reporting mechanism other than by claims. Accordingly, the use of CMS approved “traditional” registries (registry reporting option) or qualified clinical data registries, such as the ACS’ Surgeon Specific Registry (SSR), will be valid modes by which one may report. Discussions are underway to determine how best to incorporate the Improvement Activities into the SSR. Alternatively, CMS plans to make available a portal on its website where providers will be able to attest to their having satisfied the Improvement Activity requirement.
In reviewing the list of 93 activities, examples of such that likely would or could be applicable to surgeons include:
• Use of a QCDR (qualified clinical data registry) to generate regular performance feedback (20 points)
• Participation in a QCDR, clinical data registries, or other registries run by other government agencies or private entities such as a hospital or medical or surgical society (10 points)
• Provision of episodic care management, including management across transitions and referrals that could include routine and timely follow-up to hospitalizations and ED visits and/or managing care intensively through new diagnoses, injuries and exacerbations of illness (10 points)
• Provision of specialist reports back to referring providers to close the referral loop (10 points)
• Timely communication of test results defined as timely identification of abnormal test results with timely follow-up (10 points)
• Participation in a QCDR, demonstrating performance of activities that promote use of standard practices, tools and processes for quality improvement (10 points)
• Bilateral exchange of necessary patient information to guide patient care that could include participation in a health information exchange or use of structured referral notes (10 points)
• Participation in a QCDR, demonstrating performance of activities that promote implementation of shared clinical decision making capabilities (10 points)
• Use of evidence-based decision aids to support shared decision-making (10 points)
• Participation in Maintenance of Certification Part IV (10 points)
• Annual registration by eligible clinician or group in the prescription drug monitoring program of the state where they practice (10 points)
• Consultation of prescription drug monitoring program prior to the issuance of a Controlled Substance Schedule II opioid prescription that lasts for longer than 3 days (20 points)
• Use of tools that assist specialty practices in tracking specific measures that are meaningful to their practice, such as use of the Surgical Risk Calculator (10 points)
• Seeing new and follow-up Medicaid patients in a timely manner, including individuals dually eligible for Medicaid and Medicare (20 points)
Based upon the list above (and others not included), and because the requirement specified for reporting the Improvement Activities is simple attestation, I am confident that all surgeons will be able to meet the requirement with minimal effort and achieve full credit for this component of the MIPS Composite Performance score. In these last weeks of 2016, I would encourage all Fellows to visit the ACS QPP website at www.facs.org/qpp to map out their overall strategy for success with the new Medicare physician payment system that will become effective beginning in January of 2017.
Until next month ….
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
As was summarized last month, Centers for Medicare and Medicaid Services (CMS) released the final rule pertaining to the Medicare Access and CHIP Reauthorization Act (MACRA) on October 14, 2016. In the ensuing weeks, Division of Advocacy and Health Policy staff had the opportunity to read and further analyze the rule. In general, the initial favorable impression held up under more careful scrutiny. Based on the provisions in the final rule, we continue to make adjustments and modifications to the resources available to Fellows to assist them to prepare for 2017 on the website found at www.facs.org/qpp. By the time this column is printed/released, I anticipate that the video series found on the website will have been updated and expanded upon.
Because change is unsettling, one of the most frequent topics of conversation and question concerning MACRA is the new reporting requirement known as Improvement Activities. Previously, in the proposed rule, this component was known as the Clinical Practice Improvement Activities. In the final rule, the nomenclature was shortened to Improvement Activities.
The reporting mechanism specified by CMS for 2017 is simple attestation. That attestation may be accomplished via any traditional reporting mechanism other than by claims. Accordingly, the use of CMS approved “traditional” registries (registry reporting option) or qualified clinical data registries, such as the ACS’ Surgeon Specific Registry (SSR), will be valid modes by which one may report. Discussions are underway to determine how best to incorporate the Improvement Activities into the SSR. Alternatively, CMS plans to make available a portal on its website where providers will be able to attest to their having satisfied the Improvement Activity requirement.
In reviewing the list of 93 activities, examples of such that likely would or could be applicable to surgeons include:
• Use of a QCDR (qualified clinical data registry) to generate regular performance feedback (20 points)
• Participation in a QCDR, clinical data registries, or other registries run by other government agencies or private entities such as a hospital or medical or surgical society (10 points)
• Provision of episodic care management, including management across transitions and referrals that could include routine and timely follow-up to hospitalizations and ED visits and/or managing care intensively through new diagnoses, injuries and exacerbations of illness (10 points)
• Provision of specialist reports back to referring providers to close the referral loop (10 points)
• Timely communication of test results defined as timely identification of abnormal test results with timely follow-up (10 points)
• Participation in a QCDR, demonstrating performance of activities that promote use of standard practices, tools and processes for quality improvement (10 points)
• Bilateral exchange of necessary patient information to guide patient care that could include participation in a health information exchange or use of structured referral notes (10 points)
• Participation in a QCDR, demonstrating performance of activities that promote implementation of shared clinical decision making capabilities (10 points)
• Use of evidence-based decision aids to support shared decision-making (10 points)
• Participation in Maintenance of Certification Part IV (10 points)
• Annual registration by eligible clinician or group in the prescription drug monitoring program of the state where they practice (10 points)
• Consultation of prescription drug monitoring program prior to the issuance of a Controlled Substance Schedule II opioid prescription that lasts for longer than 3 days (20 points)
• Use of tools that assist specialty practices in tracking specific measures that are meaningful to their practice, such as use of the Surgical Risk Calculator (10 points)
• Seeing new and follow-up Medicaid patients in a timely manner, including individuals dually eligible for Medicaid and Medicare (20 points)
Based upon the list above (and others not included), and because the requirement specified for reporting the Improvement Activities is simple attestation, I am confident that all surgeons will be able to meet the requirement with minimal effort and achieve full credit for this component of the MIPS Composite Performance score. In these last weeks of 2016, I would encourage all Fellows to visit the ACS QPP website at www.facs.org/qpp to map out their overall strategy for success with the new Medicare physician payment system that will become effective beginning in January of 2017.
Until next month ….
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
Release of the MACRA Final Rule
On October 14, 2016, the Centers for Medicare and Medicaid Services (CMS) released the final rule pertaining to the Medicare Access and CHIP Reauthorization Act (MACRA). As I write, almost three weeks later, Division of Advocacy and Health Policy staff are generally pleased with the contents of the rule as there were no big “negative” surprises and stakeholder input in response to the proposed rule seems to have been broadly taken to heart by the administration at CMS.
As Fellows prepare for 2017, they should take note of several changes that were made to the original proposed rule. Some key changes are summarized below.
With regard to what was previously referred to as the Clinical Practice Improvement Activities (CPIA), the nomenclature as well as the associated requirement have been shortened and simplified. Now called simply Improvement Activities, to achieve full credit most physicians will need to report on between two to four of the nearly 100 possible activities as opposed to up to the six activities needed to meet the requirements as outlined in the proposed rule. Fortunately, the reporting requirement for the Improvement Activities component remains the simple attestation that one has participated in the selected activities for a period of 90 continuous days during the 2017 reporting period. Improvement Activities continues to comprise 15% of the Composite Score.
With release of the final rule, we now have a more concrete definition of what CMS Acting Administrator Andrew Slavitt meant by “Pick Your Pace” which was the topic of last month’s column (October 2016, p. 15). CMS is looking at the 2017 reporting period as a transition year with which it hopes to engage physicians in participation in its new Medicare physician payment plan. As such, surgeons and other physicians will NOT receive a negative assessment on their 2019 Medicare payment if they simply report on one Quality measure for 90 days, OR one Improvement Activity for 90 days (again by simple attestation) OR four required Advancing Care Information measures utilizing a certified electronic health record (EHR). Accordingly, it is entirely possible for ALL to avoid the 4% penalty prescribed for those who report nothing for 2017.
ACS has developed numerous resources to assist surgeons in preparing for the 2017 reporting period. In addition to articles published in ACS Surgery News and other ACS publications, a website has been launched at www.facs.org/qpp. The website contains a series of videos based on the requirements outlined in the proposed rule, downloadable Power Point presentations, a glossary of terms and acronyms and perhaps, most importantly, a list of activities that surgeons can undertake now in order to best prepare themselves for the changes outlined in the final rule for January 2017.
In the coming weeks, plans are in place to revise the slide presentations and videos to reflect the modifications of requirements found in the final rule, publish a series of fact sheets designed for surgeons in various practice circumstances (employed surgeons, private practice surgeons, surgeons in small and/or rural practice, surgeons in large group practice), revise and republish the booklet entitled Resources for the New Medicare Physician Payment System, first made available to attendees at Clinical Congress in Washington in October, as well as the recording of an instructional webinar.
Based on the requirements outlined in the MACRA final rule, I am very confident that with minimal effort surgeons will be able to avoid a negative payment adjustment in 2019 based on their performance in the 2017 reporting period. Further, for those surgeons who are already participating in quality reporting and/or are well familiar with the requirements of the electronic health record program, it is entirely possible they will receive a positive update. ACS staff continue to endeavor to provide resources to Fellows to ensure their success.
Until next month…
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
On October 14, 2016, the Centers for Medicare and Medicaid Services (CMS) released the final rule pertaining to the Medicare Access and CHIP Reauthorization Act (MACRA). As I write, almost three weeks later, Division of Advocacy and Health Policy staff are generally pleased with the contents of the rule as there were no big “negative” surprises and stakeholder input in response to the proposed rule seems to have been broadly taken to heart by the administration at CMS.
As Fellows prepare for 2017, they should take note of several changes that were made to the original proposed rule. Some key changes are summarized below.
With regard to what was previously referred to as the Clinical Practice Improvement Activities (CPIA), the nomenclature as well as the associated requirement have been shortened and simplified. Now called simply Improvement Activities, to achieve full credit most physicians will need to report on between two to four of the nearly 100 possible activities as opposed to up to the six activities needed to meet the requirements as outlined in the proposed rule. Fortunately, the reporting requirement for the Improvement Activities component remains the simple attestation that one has participated in the selected activities for a period of 90 continuous days during the 2017 reporting period. Improvement Activities continues to comprise 15% of the Composite Score.
With release of the final rule, we now have a more concrete definition of what CMS Acting Administrator Andrew Slavitt meant by “Pick Your Pace” which was the topic of last month’s column (October 2016, p. 15). CMS is looking at the 2017 reporting period as a transition year with which it hopes to engage physicians in participation in its new Medicare physician payment plan. As such, surgeons and other physicians will NOT receive a negative assessment on their 2019 Medicare payment if they simply report on one Quality measure for 90 days, OR one Improvement Activity for 90 days (again by simple attestation) OR four required Advancing Care Information measures utilizing a certified electronic health record (EHR). Accordingly, it is entirely possible for ALL to avoid the 4% penalty prescribed for those who report nothing for 2017.
ACS has developed numerous resources to assist surgeons in preparing for the 2017 reporting period. In addition to articles published in ACS Surgery News and other ACS publications, a website has been launched at www.facs.org/qpp. The website contains a series of videos based on the requirements outlined in the proposed rule, downloadable Power Point presentations, a glossary of terms and acronyms and perhaps, most importantly, a list of activities that surgeons can undertake now in order to best prepare themselves for the changes outlined in the final rule for January 2017.
In the coming weeks, plans are in place to revise the slide presentations and videos to reflect the modifications of requirements found in the final rule, publish a series of fact sheets designed for surgeons in various practice circumstances (employed surgeons, private practice surgeons, surgeons in small and/or rural practice, surgeons in large group practice), revise and republish the booklet entitled Resources for the New Medicare Physician Payment System, first made available to attendees at Clinical Congress in Washington in October, as well as the recording of an instructional webinar.
Based on the requirements outlined in the MACRA final rule, I am very confident that with minimal effort surgeons will be able to avoid a negative payment adjustment in 2019 based on their performance in the 2017 reporting period. Further, for those surgeons who are already participating in quality reporting and/or are well familiar with the requirements of the electronic health record program, it is entirely possible they will receive a positive update. ACS staff continue to endeavor to provide resources to Fellows to ensure their success.
Until next month…
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
On October 14, 2016, the Centers for Medicare and Medicaid Services (CMS) released the final rule pertaining to the Medicare Access and CHIP Reauthorization Act (MACRA). As I write, almost three weeks later, Division of Advocacy and Health Policy staff are generally pleased with the contents of the rule as there were no big “negative” surprises and stakeholder input in response to the proposed rule seems to have been broadly taken to heart by the administration at CMS.
As Fellows prepare for 2017, they should take note of several changes that were made to the original proposed rule. Some key changes are summarized below.
With regard to what was previously referred to as the Clinical Practice Improvement Activities (CPIA), the nomenclature as well as the associated requirement have been shortened and simplified. Now called simply Improvement Activities, to achieve full credit most physicians will need to report on between two to four of the nearly 100 possible activities as opposed to up to the six activities needed to meet the requirements as outlined in the proposed rule. Fortunately, the reporting requirement for the Improvement Activities component remains the simple attestation that one has participated in the selected activities for a period of 90 continuous days during the 2017 reporting period. Improvement Activities continues to comprise 15% of the Composite Score.
With release of the final rule, we now have a more concrete definition of what CMS Acting Administrator Andrew Slavitt meant by “Pick Your Pace” which was the topic of last month’s column (October 2016, p. 15). CMS is looking at the 2017 reporting period as a transition year with which it hopes to engage physicians in participation in its new Medicare physician payment plan. As such, surgeons and other physicians will NOT receive a negative assessment on their 2019 Medicare payment if they simply report on one Quality measure for 90 days, OR one Improvement Activity for 90 days (again by simple attestation) OR four required Advancing Care Information measures utilizing a certified electronic health record (EHR). Accordingly, it is entirely possible for ALL to avoid the 4% penalty prescribed for those who report nothing for 2017.
ACS has developed numerous resources to assist surgeons in preparing for the 2017 reporting period. In addition to articles published in ACS Surgery News and other ACS publications, a website has been launched at www.facs.org/qpp. The website contains a series of videos based on the requirements outlined in the proposed rule, downloadable Power Point presentations, a glossary of terms and acronyms and perhaps, most importantly, a list of activities that surgeons can undertake now in order to best prepare themselves for the changes outlined in the final rule for January 2017.
In the coming weeks, plans are in place to revise the slide presentations and videos to reflect the modifications of requirements found in the final rule, publish a series of fact sheets designed for surgeons in various practice circumstances (employed surgeons, private practice surgeons, surgeons in small and/or rural practice, surgeons in large group practice), revise and republish the booklet entitled Resources for the New Medicare Physician Payment System, first made available to attendees at Clinical Congress in Washington in October, as well as the recording of an instructional webinar.
Based on the requirements outlined in the MACRA final rule, I am very confident that with minimal effort surgeons will be able to avoid a negative payment adjustment in 2019 based on their performance in the 2017 reporting period. Further, for those surgeons who are already participating in quality reporting and/or are well familiar with the requirements of the electronic health record program, it is entirely possible they will receive a positive update. ACS staff continue to endeavor to provide resources to Fellows to ensure their success.
Until next month…
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
Coming your way ... MIPS
In this column, I will return to the topic of the Quality Payment Program (QPP), Centers for Medicare & Medicaid Services’ new payment program, currently scheduled to go into effect January 2017. The QPP has two tracks, the Merit-Based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs). Again, it is widely expected that for the first several years of the program, the vast majority of surgeons will participate in the QPP via the MIPS track.
To briefly review from the July edition of this column, MIPS consists of four components:
1) Quality.
2) Resource Use.
3) Advancing Care Information (ACI).
4) Clinical Practice Improvement Activities (CPIA).
The Quality component replaces the Physician Quality Reporting System (PQRS), the Resource Use component replaces the Value-Based Modifier (VBM), the Advancing Care Information (ACI) component replaces the Electronic Health Record Meaningful Use (EHR-MU) program and is proposed to simplify the program by modifying requirements. The fourth component of MIPS, the Clinical Practice Improvement Activities (CPIA), is new.
MIPS participants will be assigned a Composite Performance Score based on their performance in all four components. As proposed for 2017, 50% of the MIPS Composite Performance Score will be based on performance in the Quality component, 25% will be based on the ACI component, 15% will be based on the CPIA, and 10% will be based on Resource Use. Thus, 75% of one’s score will be determined by performance in the Quality and ACI components. With that in mind, the remainder of this month’s column will be dedicated to the ACI with next month’s column focusing on the Quality component.
The Advancing Care Component (ACI)
As discussed above, the ACI component modifies and replaces the Electronic Health Record Meaningful Use (EHR-MU) program. As currently proposed, (and subject to change with release of the final rule in early November 2016), the score for ACI will be derived in two parts: a base score (50 points) and a performance score.
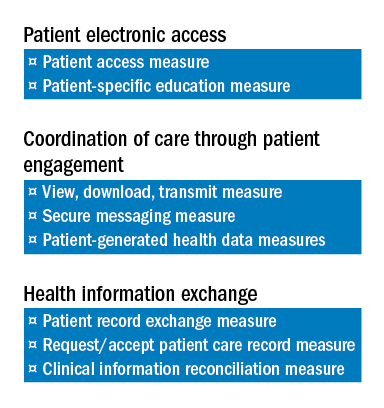
The threshold for achieving the base score remains an “all or nothing” proposition. Achieving the threshold is based upon reporting a yes/no statement OR the appropriate numerator and denominator for each measure, as required, for the following six objectives:
1) Protect Patient Health Information.
2) Electronic Prescribing.
3) Patient Electronic Access to Health Information.
4) Coordination of Care through Patient Engagement.
5) Health Information Exchange.
6) Public Health and Clinical Data Reporting.
Only after meeting the requirements for the base score is one eligible to receive additional performance score credit based on the level of performance on a subset of three of the objectives required to achieve the base score. Those three are:
1) Patient Electronic Access to Health Information.
2) Coordination of Care through Patient Engagement.
3) Health Information Exchange
There are a total of eight measures for these three objectives.
The two measures specific to the Patient Electronic Access objective are:
a) Patient Access Measure.
b) Patient-Specific Education Measure.
Coordination of Care through Patient Engagement is assessed based upon the following three measures:
a) View, Download, Transmit Measure.
b) Secure Messaging Measure.
c) Patient-Generated Health Data Measures.
For the Health Information Exchange objective, assessment is also based on three measures. They are:
a) Patient Record Exchange Measure.
b) Request/Accept Patient Care Record Measure.
c) Clinical Information Reconciliation Measure.
Scoring for each of these eight measures is based upon reporting both a numerator and denominator, thus facilitating the calculation of percentage score to which a point value is assigned. For example, a performance rate of 85 on one of the listed measures would earn 8.5 points toward the performance score. The sum of the points accumulated for these eight measures is added to Threshold Score to determine the final score for ACI. Again, 25% of one’s MIPS Composite Score is derived from performance in the ACI component.
It warrants repeating and re-emphasis that the above information is subject to modification pending the release of the MIPS Final Rule, expected in early November.
As mentioned above, the Quality component of MIPS replaces the PQRS. Quality assessment will comprise 50% of the MIPS Composite Score. In next month’s column, I will provide an outline of the changes CMS is proposing for the Quality assessment. As a preview, some good news can be found in the fact that the current MIPS Quality component proposal will require providers to report on a total of six measures as opposed to the previous PQRS requirement to report on nine.
Until next month …
In this column, I will return to the topic of the Quality Payment Program (QPP), Centers for Medicare & Medicaid Services’ new payment program, currently scheduled to go into effect January 2017. The QPP has two tracks, the Merit-Based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs). Again, it is widely expected that for the first several years of the program, the vast majority of surgeons will participate in the QPP via the MIPS track.
To briefly review from the July edition of this column, MIPS consists of four components:
1) Quality.
2) Resource Use.
3) Advancing Care Information (ACI).
4) Clinical Practice Improvement Activities (CPIA).
The Quality component replaces the Physician Quality Reporting System (PQRS), the Resource Use component replaces the Value-Based Modifier (VBM), the Advancing Care Information (ACI) component replaces the Electronic Health Record Meaningful Use (EHR-MU) program and is proposed to simplify the program by modifying requirements. The fourth component of MIPS, the Clinical Practice Improvement Activities (CPIA), is new.
MIPS participants will be assigned a Composite Performance Score based on their performance in all four components. As proposed for 2017, 50% of the MIPS Composite Performance Score will be based on performance in the Quality component, 25% will be based on the ACI component, 15% will be based on the CPIA, and 10% will be based on Resource Use. Thus, 75% of one’s score will be determined by performance in the Quality and ACI components. With that in mind, the remainder of this month’s column will be dedicated to the ACI with next month’s column focusing on the Quality component.
The Advancing Care Component (ACI)
As discussed above, the ACI component modifies and replaces the Electronic Health Record Meaningful Use (EHR-MU) program. As currently proposed, (and subject to change with release of the final rule in early November 2016), the score for ACI will be derived in two parts: a base score (50 points) and a performance score.

The threshold for achieving the base score remains an “all or nothing” proposition. Achieving the threshold is based upon reporting a yes/no statement OR the appropriate numerator and denominator for each measure, as required, for the following six objectives:
1) Protect Patient Health Information.
2) Electronic Prescribing.
3) Patient Electronic Access to Health Information.
4) Coordination of Care through Patient Engagement.
5) Health Information Exchange.
6) Public Health and Clinical Data Reporting.
Only after meeting the requirements for the base score is one eligible to receive additional performance score credit based on the level of performance on a subset of three of the objectives required to achieve the base score. Those three are:
1) Patient Electronic Access to Health Information.
2) Coordination of Care through Patient Engagement.
3) Health Information Exchange
There are a total of eight measures for these three objectives.
The two measures specific to the Patient Electronic Access objective are:
a) Patient Access Measure.
b) Patient-Specific Education Measure.
Coordination of Care through Patient Engagement is assessed based upon the following three measures:
a) View, Download, Transmit Measure.
b) Secure Messaging Measure.
c) Patient-Generated Health Data Measures.
For the Health Information Exchange objective, assessment is also based on three measures. They are:
a) Patient Record Exchange Measure.
b) Request/Accept Patient Care Record Measure.
c) Clinical Information Reconciliation Measure.
Scoring for each of these eight measures is based upon reporting both a numerator and denominator, thus facilitating the calculation of percentage score to which a point value is assigned. For example, a performance rate of 85 on one of the listed measures would earn 8.5 points toward the performance score. The sum of the points accumulated for these eight measures is added to Threshold Score to determine the final score for ACI. Again, 25% of one’s MIPS Composite Score is derived from performance in the ACI component.
It warrants repeating and re-emphasis that the above information is subject to modification pending the release of the MIPS Final Rule, expected in early November.
As mentioned above, the Quality component of MIPS replaces the PQRS. Quality assessment will comprise 50% of the MIPS Composite Score. In next month’s column, I will provide an outline of the changes CMS is proposing for the Quality assessment. As a preview, some good news can be found in the fact that the current MIPS Quality component proposal will require providers to report on a total of six measures as opposed to the previous PQRS requirement to report on nine.
Until next month …
In this column, I will return to the topic of the Quality Payment Program (QPP), Centers for Medicare & Medicaid Services’ new payment program, currently scheduled to go into effect January 2017. The QPP has two tracks, the Merit-Based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs). Again, it is widely expected that for the first several years of the program, the vast majority of surgeons will participate in the QPP via the MIPS track.
To briefly review from the July edition of this column, MIPS consists of four components:
1) Quality.
2) Resource Use.
3) Advancing Care Information (ACI).
4) Clinical Practice Improvement Activities (CPIA).
The Quality component replaces the Physician Quality Reporting System (PQRS), the Resource Use component replaces the Value-Based Modifier (VBM), the Advancing Care Information (ACI) component replaces the Electronic Health Record Meaningful Use (EHR-MU) program and is proposed to simplify the program by modifying requirements. The fourth component of MIPS, the Clinical Practice Improvement Activities (CPIA), is new.
MIPS participants will be assigned a Composite Performance Score based on their performance in all four components. As proposed for 2017, 50% of the MIPS Composite Performance Score will be based on performance in the Quality component, 25% will be based on the ACI component, 15% will be based on the CPIA, and 10% will be based on Resource Use. Thus, 75% of one’s score will be determined by performance in the Quality and ACI components. With that in mind, the remainder of this month’s column will be dedicated to the ACI with next month’s column focusing on the Quality component.
The Advancing Care Component (ACI)
As discussed above, the ACI component modifies and replaces the Electronic Health Record Meaningful Use (EHR-MU) program. As currently proposed, (and subject to change with release of the final rule in early November 2016), the score for ACI will be derived in two parts: a base score (50 points) and a performance score.

The threshold for achieving the base score remains an “all or nothing” proposition. Achieving the threshold is based upon reporting a yes/no statement OR the appropriate numerator and denominator for each measure, as required, for the following six objectives:
1) Protect Patient Health Information.
2) Electronic Prescribing.
3) Patient Electronic Access to Health Information.
4) Coordination of Care through Patient Engagement.
5) Health Information Exchange.
6) Public Health and Clinical Data Reporting.
Only after meeting the requirements for the base score is one eligible to receive additional performance score credit based on the level of performance on a subset of three of the objectives required to achieve the base score. Those three are:
1) Patient Electronic Access to Health Information.
2) Coordination of Care through Patient Engagement.
3) Health Information Exchange
There are a total of eight measures for these three objectives.
The two measures specific to the Patient Electronic Access objective are:
a) Patient Access Measure.
b) Patient-Specific Education Measure.
Coordination of Care through Patient Engagement is assessed based upon the following three measures:
a) View, Download, Transmit Measure.
b) Secure Messaging Measure.
c) Patient-Generated Health Data Measures.
For the Health Information Exchange objective, assessment is also based on three measures. They are:
a) Patient Record Exchange Measure.
b) Request/Accept Patient Care Record Measure.
c) Clinical Information Reconciliation Measure.
Scoring for each of these eight measures is based upon reporting both a numerator and denominator, thus facilitating the calculation of percentage score to which a point value is assigned. For example, a performance rate of 85 on one of the listed measures would earn 8.5 points toward the performance score. The sum of the points accumulated for these eight measures is added to Threshold Score to determine the final score for ACI. Again, 25% of one’s MIPS Composite Score is derived from performance in the ACI component.
It warrants repeating and re-emphasis that the above information is subject to modification pending the release of the MIPS Final Rule, expected in early November.
As mentioned above, the Quality component of MIPS replaces the PQRS. Quality assessment will comprise 50% of the MIPS Composite Score. In next month’s column, I will provide an outline of the changes CMS is proposing for the Quality assessment. As a preview, some good news can be found in the fact that the current MIPS Quality component proposal will require providers to report on a total of six measures as opposed to the previous PQRS requirement to report on nine.
Until next month …
From the Washington Office: Globals … again
Regular readers of this column may remember the March 2015 edition devoted to the topic of the CMS’s proposal to transition all 10-day and 90-day global codes to 0-day global codes in 2017 and 2018, respectively. As a result of a coordinated advocacy effort of the American College of Surgeons and a coalition of 24 other surgical and medical groups including the American Medical Association, the American Academy of Dermatology, and the American College of Cardiology, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) included a provision that required that the Centers for Medicare & Medicaid Services (CMS) instead collect data from a representative sample of providers to facilitate the accurate valuation of surgical services before proposing any changes to the global payment structure.
Fast forward to July 7, 2016, and the release of the 2017 Physician Fee Schedule (PFS) proposed rule. In that proposed rule, the CMS disregards the specific legislative language from Congress and proposes to collect data from all physicians who provide 10- and 90-day global services. This would obviously create yet another huge administrative burden AND also coincide with the time physicians and practices are engaged in efforts to implement the changes required by the new Quality Payment Program (QPP) mandated by MACRA. Specifically, if the proposed PFS rule is finalized, all surgeons would be required to submit data in 10-minute increments for all 10- and 90-day global code services.
Obviously, this is in direct conflict with the language in MACRA that directs the CMS to collect these data from a “representative sample” of practitioners.
Upon discovering the CMS’s plan in the proposed rule, the legislative team in ACS’s Division of Advocacy and Health Policy contacted the congressional sponsors of the original effort directed at the global codes, Rep. Larry Bucshon, MD, FACS (R-IN), and Rep. Ami Bera, MD (D-CA). Dr. Bucshon and Dr. Bera began circulating a letter, addressed to Health and Human Services Secretary Sylvia Burwell and CMS Acting Administrator Andrew Slavitt, urging the CMS to abandon the proposed policy outlined in the 2017 PFS proposed rule regarding the arduous data collection requirements for global codes.
In the week leading up to the summer congressional recess, the ACS sent the letter to all 435 offices in the House of Representatives urging other members to sign on to the letter. The ACS lobbyists and those from the coalition of groups previously involved in the efforts relative to global codes are currently engaged in individual follow-up with offices as well. The goal is to make a strong showing to the CMS with a large number of signatures from members of Congress in the hope that the CMS will modify the final rule in accordance with the legislative language found in MACRA.
This is where we need your help!
By the time you receive this issue of ACS Surgery News, all Fellows will have received an email requesting that they respond by contacting their individual members of Congress to urge them to sign on to the letter. This may be accomplished either by placing a call or by sending an email communication.
Those choosing to call may use the ACS Legislative Hotline at 877-996-4464. Follow the instructions to be connected to the office of your member of Congress. Once connected, please inform them that you are a constituent, and then deliver the following message:
“As a surgeon and a constituent, I urge Rep. _____ to join Rep Dr. Larry Bucshon and Rep. Dr. Ami Bera in supporting the bipartisan sign-on letter to the CMS in order to stop the administratively burdensome data entry changes proposed by the CMS relative to 10- and 90-day global codes.
“The proposed changes would mandate that all practitioners who perform global code services enter data in 10-minute intervals for every patient billed under global codes rather than adhering to the direction of Congress to obtain the necessary information from a ‘representative sample’ as was mandated in the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA).”
For those wishing further information on this matter or for those who would prefer to contact their representative by email, an ACTION Alert can be found on the SurgeonsVoice website (www.surgeonsvoice.com – click on the Take Action tab on the right side of the page). The alert addressing the global codes issue is at the top of the list and includes a fact sheet that outlines the issue and provides background information along with a link to facilitate transmittal of your message urging your representative to sign on to the Bucshon-Bera letter. Because Congress has adjourned for their summer recess and will not return until Sept. 6, 2016, we have ample time to gather the overwhelming support we need to initiate action precluding the inclusion of this flawed proposal in the final rule, which is expected to be released the first week of November 2016.
I respectfully request that ALL Fellows do their part and contact their member of Congress via one of the two methods provided. There can be no argument that the minimal time required to invest in our collective advocacy efforts relative to this matter pales in comparison to the time required to comply with the proposed CMS policy we seek to prevent being published in the final PFS rule.
Until next month …
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
Regular readers of this column may remember the March 2015 edition devoted to the topic of the CMS’s proposal to transition all 10-day and 90-day global codes to 0-day global codes in 2017 and 2018, respectively. As a result of a coordinated advocacy effort of the American College of Surgeons and a coalition of 24 other surgical and medical groups including the American Medical Association, the American Academy of Dermatology, and the American College of Cardiology, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) included a provision that required that the Centers for Medicare & Medicaid Services (CMS) instead collect data from a representative sample of providers to facilitate the accurate valuation of surgical services before proposing any changes to the global payment structure.
Fast forward to July 7, 2016, and the release of the 2017 Physician Fee Schedule (PFS) proposed rule. In that proposed rule, the CMS disregards the specific legislative language from Congress and proposes to collect data from all physicians who provide 10- and 90-day global services. This would obviously create yet another huge administrative burden AND also coincide with the time physicians and practices are engaged in efforts to implement the changes required by the new Quality Payment Program (QPP) mandated by MACRA. Specifically, if the proposed PFS rule is finalized, all surgeons would be required to submit data in 10-minute increments for all 10- and 90-day global code services.
Obviously, this is in direct conflict with the language in MACRA that directs the CMS to collect these data from a “representative sample” of practitioners.
Upon discovering the CMS’s plan in the proposed rule, the legislative team in ACS’s Division of Advocacy and Health Policy contacted the congressional sponsors of the original effort directed at the global codes, Rep. Larry Bucshon, MD, FACS (R-IN), and Rep. Ami Bera, MD (D-CA). Dr. Bucshon and Dr. Bera began circulating a letter, addressed to Health and Human Services Secretary Sylvia Burwell and CMS Acting Administrator Andrew Slavitt, urging the CMS to abandon the proposed policy outlined in the 2017 PFS proposed rule regarding the arduous data collection requirements for global codes.
In the week leading up to the summer congressional recess, the ACS sent the letter to all 435 offices in the House of Representatives urging other members to sign on to the letter. The ACS lobbyists and those from the coalition of groups previously involved in the efforts relative to global codes are currently engaged in individual follow-up with offices as well. The goal is to make a strong showing to the CMS with a large number of signatures from members of Congress in the hope that the CMS will modify the final rule in accordance with the legislative language found in MACRA.
This is where we need your help!
By the time you receive this issue of ACS Surgery News, all Fellows will have received an email requesting that they respond by contacting their individual members of Congress to urge them to sign on to the letter. This may be accomplished either by placing a call or by sending an email communication.
Those choosing to call may use the ACS Legislative Hotline at 877-996-4464. Follow the instructions to be connected to the office of your member of Congress. Once connected, please inform them that you are a constituent, and then deliver the following message:
“As a surgeon and a constituent, I urge Rep. _____ to join Rep Dr. Larry Bucshon and Rep. Dr. Ami Bera in supporting the bipartisan sign-on letter to the CMS in order to stop the administratively burdensome data entry changes proposed by the CMS relative to 10- and 90-day global codes.
“The proposed changes would mandate that all practitioners who perform global code services enter data in 10-minute intervals for every patient billed under global codes rather than adhering to the direction of Congress to obtain the necessary information from a ‘representative sample’ as was mandated in the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA).”
For those wishing further information on this matter or for those who would prefer to contact their representative by email, an ACTION Alert can be found on the SurgeonsVoice website (www.surgeonsvoice.com – click on the Take Action tab on the right side of the page). The alert addressing the global codes issue is at the top of the list and includes a fact sheet that outlines the issue and provides background information along with a link to facilitate transmittal of your message urging your representative to sign on to the Bucshon-Bera letter. Because Congress has adjourned for their summer recess and will not return until Sept. 6, 2016, we have ample time to gather the overwhelming support we need to initiate action precluding the inclusion of this flawed proposal in the final rule, which is expected to be released the first week of November 2016.
I respectfully request that ALL Fellows do their part and contact their member of Congress via one of the two methods provided. There can be no argument that the minimal time required to invest in our collective advocacy efforts relative to this matter pales in comparison to the time required to comply with the proposed CMS policy we seek to prevent being published in the final PFS rule.
Until next month …
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
Regular readers of this column may remember the March 2015 edition devoted to the topic of the CMS’s proposal to transition all 10-day and 90-day global codes to 0-day global codes in 2017 and 2018, respectively. As a result of a coordinated advocacy effort of the American College of Surgeons and a coalition of 24 other surgical and medical groups including the American Medical Association, the American Academy of Dermatology, and the American College of Cardiology, the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA) included a provision that required that the Centers for Medicare & Medicaid Services (CMS) instead collect data from a representative sample of providers to facilitate the accurate valuation of surgical services before proposing any changes to the global payment structure.
Fast forward to July 7, 2016, and the release of the 2017 Physician Fee Schedule (PFS) proposed rule. In that proposed rule, the CMS disregards the specific legislative language from Congress and proposes to collect data from all physicians who provide 10- and 90-day global services. This would obviously create yet another huge administrative burden AND also coincide with the time physicians and practices are engaged in efforts to implement the changes required by the new Quality Payment Program (QPP) mandated by MACRA. Specifically, if the proposed PFS rule is finalized, all surgeons would be required to submit data in 10-minute increments for all 10- and 90-day global code services.
Obviously, this is in direct conflict with the language in MACRA that directs the CMS to collect these data from a “representative sample” of practitioners.
Upon discovering the CMS’s plan in the proposed rule, the legislative team in ACS’s Division of Advocacy and Health Policy contacted the congressional sponsors of the original effort directed at the global codes, Rep. Larry Bucshon, MD, FACS (R-IN), and Rep. Ami Bera, MD (D-CA). Dr. Bucshon and Dr. Bera began circulating a letter, addressed to Health and Human Services Secretary Sylvia Burwell and CMS Acting Administrator Andrew Slavitt, urging the CMS to abandon the proposed policy outlined in the 2017 PFS proposed rule regarding the arduous data collection requirements for global codes.
In the week leading up to the summer congressional recess, the ACS sent the letter to all 435 offices in the House of Representatives urging other members to sign on to the letter. The ACS lobbyists and those from the coalition of groups previously involved in the efforts relative to global codes are currently engaged in individual follow-up with offices as well. The goal is to make a strong showing to the CMS with a large number of signatures from members of Congress in the hope that the CMS will modify the final rule in accordance with the legislative language found in MACRA.
This is where we need your help!
By the time you receive this issue of ACS Surgery News, all Fellows will have received an email requesting that they respond by contacting their individual members of Congress to urge them to sign on to the letter. This may be accomplished either by placing a call or by sending an email communication.
Those choosing to call may use the ACS Legislative Hotline at 877-996-4464. Follow the instructions to be connected to the office of your member of Congress. Once connected, please inform them that you are a constituent, and then deliver the following message:
“As a surgeon and a constituent, I urge Rep. _____ to join Rep Dr. Larry Bucshon and Rep. Dr. Ami Bera in supporting the bipartisan sign-on letter to the CMS in order to stop the administratively burdensome data entry changes proposed by the CMS relative to 10- and 90-day global codes.
“The proposed changes would mandate that all practitioners who perform global code services enter data in 10-minute intervals for every patient billed under global codes rather than adhering to the direction of Congress to obtain the necessary information from a ‘representative sample’ as was mandated in the Medicare Access and CHIP Reauthorization Act of 2015 (MACRA).”
For those wishing further information on this matter or for those who would prefer to contact their representative by email, an ACTION Alert can be found on the SurgeonsVoice website (www.surgeonsvoice.com – click on the Take Action tab on the right side of the page). The alert addressing the global codes issue is at the top of the list and includes a fact sheet that outlines the issue and provides background information along with a link to facilitate transmittal of your message urging your representative to sign on to the Bucshon-Bera letter. Because Congress has adjourned for their summer recess and will not return until Sept. 6, 2016, we have ample time to gather the overwhelming support we need to initiate action precluding the inclusion of this flawed proposal in the final rule, which is expected to be released the first week of November 2016.
I respectfully request that ALL Fellows do their part and contact their member of Congress via one of the two methods provided. There can be no argument that the minimal time required to invest in our collective advocacy efforts relative to this matter pales in comparison to the time required to comply with the proposed CMS policy we seek to prevent being published in the final PFS rule.
Until next month …
Dr. Bailey is a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy in the ACS offices in Washington, D.C.
From the Washington Office: The operationalization of MACRA
On April 27, 2016, the Centers for Medicare and Medicaid Services (CMS) released its proposed rule on the Medicare Access and CHIP Reauthorization Act (MACRA). Fellows will remember that the MACRA legislation, passed in April of 2015, permanently repealed the Sustainable Growth Rate (SGR) formula and thus, represents the greatest sea change in Medicare physician payment since the establishment of the RBRVS (Resource-Based Relative Value Scale) in 1992.
In broadest policy terms, the law continues to advance the CMS policy goal of basing payment on quality and value over volume. From a granular perspective, the law combines Medicare’s three current quality programs into one new system. CMS published this 982-page proposed rule after reviewing the comments submitted by ACS and other interested parties in response to its request for information last fall. As I write, staff of the Division of Advocacy and Health Policy are in the process of crafting the ACS response to the proposed rule. Comments were due on June 27, 2016. It is anticipated that CMS will publish the final rule later this year, likely in late October or early November. Accordingly, the following description of the implementation of MACRA is based on the current understanding from the proposed rule, and is likely to change in some respects with the final rule.
CMS has designated the payment program operationalizing the MACRA law as the Quality Payment Program (QPP). The QPP has two tracks, the Merit-based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs). For the first several years of the program, it is widely expected that the vast majority of physicians will participate in the QPP via the MIPS track. As such, I will direct the remainder of the text this month to the MIPS program.
MIPS: Merit-Based Incentive Payment System
The Merit-based Incentive Payment System, MIPS, consists of four components. They are: Quality, Resource Use, Advancing Care Information (ACI) and the Clinical Practice Improvement Activities (CPIA). Though the names have changed, Fellows are familiar with the substance of three of the components. For example, the Quality component replaces the Physician Quality Reporting System (PQRS); the Resource Use component replaces the Value-Based Modifier (VBM); and the Advancing Care Information component modifies and replaces the Electronic Health Record Meaningful Use (EHR-MU) program. The fourth component of MIPS is the new Clinical Practice Improvement Activities, which the legislation designates as intended to provide “credit for work to improve practice and facilitates future participation in alternative payment models.”
Composite Performance Score: MIPS participants will be assigned a Composite Performance Score based on their performance in all four components. For 2017, the first year for assessment under the QPP, 50 percent of the score will be based on performance in the Quality component, 10 percent will be based on the Resource Use component, 25 percent will be based on the Advancing Care Information component, and 15 percent will be based on the Clinical Practice Improvement Activities. In future years, there will be a gradual increase in the relative value of the Resource Use component with an equal and accompanying decrease in the value of the Quality component. As proposed, by the third assessment year (2019), the Quality and Resource Use components are expected to each account for 30 percent of the Composite Performance Score.
MIPS Quality Component: Though the Quality component of MIPS replaces the PQRS, CMS is proposing some changes that Fellows will welcome. As opposed to the previous PQRS requirement to report nine measures, the MIPS Quality component requires providers to report only six measures. One of these six must be an “Outcome” measure and another must be a “Cross-cutting” measure. While the reporting threshold for the percentage of patients on which reports will be required is proposed to increase substantially, ACS and other physician groups will be advocating that the required percentage published in the final rule be close to the 50 percent level found in current law.
Resources Use Component: There is also some good news relative to the Resource Use component in that there are NO reporting requirements. CMS will calculate this component from Medicare claims data and base its assessment of individual provider performance on the resource measures currently used for the Value-Based Modifier. Namely, those are the VBM Total per Capita Cost measure and the VBM Medicare Spending per Beneficiary measure. In addition, CMS will also be taking into account measures that specifically focus on episodes of care, something for which the College has previously advocated. Beginning in 2018, CMS plans to also take into consideration factors of patient condition and patient relation in order to address physician concerns about risk adjustment and attribution.
Advancing Care Information Component: This modifies and replaces the Electronic Health Record Meaningful Use program. The score for this component is derived in two parts, a Base score (50 percent) and a Performance score (up to an additional 50 percent). The threshold for achieving the Base score remains “all or nothing.” Only after meeting the requirements for the Base score is one eligible to receive additional Performance score credit based on the level of performance on a subset of the same measures required to achieve the Base score. Assessment in 2017 will be based on the EHR-MU requirements published in the 2015 Final Rule for the EHR-MU program.
Clinical Practice Improvement Component: The fourth component of MIPS is the Clinical Practice Improvement Activities component. As mentioned previously, this is a new requirement with no prior analogous program requirement. As such, it is very much in evolution. In the first year of MIPS assessment (2017), achieving full credit for the CPIA component should not pose much additional administrative burden as reporting will be by simple attestation. Physicians will chose from a list of 94 activities assigned two different weighted values. In order to receive full credit for the CPIA component, most providers will need to attest that they have participated in a minimum of three and a maximum of six of the 94 activities, depending on the weight of the activities selected, for 90 days.
As outlined above, the final rule on the Quality Payment Program is expected to be released in late October or early November. As proposed, assessment under the provisions of that final rule would begin in January of 2017. This leaves all providers with a very short time window in which to become familiar with the program that will impact their Medicare payment beginning in 2019.
Accordingly, in the next several editions of this column, I will provide more specific information about each of the four MIPS components, the scoring mechanism for MIPS assessment and the aforementioned alternate track to MIPS, the Alternative Payment Models. While it is easy to understand how many Fellows could initially find this change daunting and overwhelming, I am confident that with a minimal investment of time all can develop adequate working knowledge of the MIPS and APMs to participate successfully in the QPP.
Until next month...
Dr. Bailey is an ACS Fellow, a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy, in the ACS offices in Washington.
On April 27, 2016, the Centers for Medicare and Medicaid Services (CMS) released its proposed rule on the Medicare Access and CHIP Reauthorization Act (MACRA). Fellows will remember that the MACRA legislation, passed in April of 2015, permanently repealed the Sustainable Growth Rate (SGR) formula and thus, represents the greatest sea change in Medicare physician payment since the establishment of the RBRVS (Resource-Based Relative Value Scale) in 1992.
In broadest policy terms, the law continues to advance the CMS policy goal of basing payment on quality and value over volume. From a granular perspective, the law combines Medicare’s three current quality programs into one new system. CMS published this 982-page proposed rule after reviewing the comments submitted by ACS and other interested parties in response to its request for information last fall. As I write, staff of the Division of Advocacy and Health Policy are in the process of crafting the ACS response to the proposed rule. Comments were due on June 27, 2016. It is anticipated that CMS will publish the final rule later this year, likely in late October or early November. Accordingly, the following description of the implementation of MACRA is based on the current understanding from the proposed rule, and is likely to change in some respects with the final rule.
CMS has designated the payment program operationalizing the MACRA law as the Quality Payment Program (QPP). The QPP has two tracks, the Merit-based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs). For the first several years of the program, it is widely expected that the vast majority of physicians will participate in the QPP via the MIPS track. As such, I will direct the remainder of the text this month to the MIPS program.
MIPS: Merit-Based Incentive Payment System
The Merit-based Incentive Payment System, MIPS, consists of four components. They are: Quality, Resource Use, Advancing Care Information (ACI) and the Clinical Practice Improvement Activities (CPIA). Though the names have changed, Fellows are familiar with the substance of three of the components. For example, the Quality component replaces the Physician Quality Reporting System (PQRS); the Resource Use component replaces the Value-Based Modifier (VBM); and the Advancing Care Information component modifies and replaces the Electronic Health Record Meaningful Use (EHR-MU) program. The fourth component of MIPS is the new Clinical Practice Improvement Activities, which the legislation designates as intended to provide “credit for work to improve practice and facilitates future participation in alternative payment models.”
Composite Performance Score: MIPS participants will be assigned a Composite Performance Score based on their performance in all four components. For 2017, the first year for assessment under the QPP, 50 percent of the score will be based on performance in the Quality component, 10 percent will be based on the Resource Use component, 25 percent will be based on the Advancing Care Information component, and 15 percent will be based on the Clinical Practice Improvement Activities. In future years, there will be a gradual increase in the relative value of the Resource Use component with an equal and accompanying decrease in the value of the Quality component. As proposed, by the third assessment year (2019), the Quality and Resource Use components are expected to each account for 30 percent of the Composite Performance Score.
MIPS Quality Component: Though the Quality component of MIPS replaces the PQRS, CMS is proposing some changes that Fellows will welcome. As opposed to the previous PQRS requirement to report nine measures, the MIPS Quality component requires providers to report only six measures. One of these six must be an “Outcome” measure and another must be a “Cross-cutting” measure. While the reporting threshold for the percentage of patients on which reports will be required is proposed to increase substantially, ACS and other physician groups will be advocating that the required percentage published in the final rule be close to the 50 percent level found in current law.
Resources Use Component: There is also some good news relative to the Resource Use component in that there are NO reporting requirements. CMS will calculate this component from Medicare claims data and base its assessment of individual provider performance on the resource measures currently used for the Value-Based Modifier. Namely, those are the VBM Total per Capita Cost measure and the VBM Medicare Spending per Beneficiary measure. In addition, CMS will also be taking into account measures that specifically focus on episodes of care, something for which the College has previously advocated. Beginning in 2018, CMS plans to also take into consideration factors of patient condition and patient relation in order to address physician concerns about risk adjustment and attribution.
Advancing Care Information Component: This modifies and replaces the Electronic Health Record Meaningful Use program. The score for this component is derived in two parts, a Base score (50 percent) and a Performance score (up to an additional 50 percent). The threshold for achieving the Base score remains “all or nothing.” Only after meeting the requirements for the Base score is one eligible to receive additional Performance score credit based on the level of performance on a subset of the same measures required to achieve the Base score. Assessment in 2017 will be based on the EHR-MU requirements published in the 2015 Final Rule for the EHR-MU program.
Clinical Practice Improvement Component: The fourth component of MIPS is the Clinical Practice Improvement Activities component. As mentioned previously, this is a new requirement with no prior analogous program requirement. As such, it is very much in evolution. In the first year of MIPS assessment (2017), achieving full credit for the CPIA component should not pose much additional administrative burden as reporting will be by simple attestation. Physicians will chose from a list of 94 activities assigned two different weighted values. In order to receive full credit for the CPIA component, most providers will need to attest that they have participated in a minimum of three and a maximum of six of the 94 activities, depending on the weight of the activities selected, for 90 days.
As outlined above, the final rule on the Quality Payment Program is expected to be released in late October or early November. As proposed, assessment under the provisions of that final rule would begin in January of 2017. This leaves all providers with a very short time window in which to become familiar with the program that will impact their Medicare payment beginning in 2019.
Accordingly, in the next several editions of this column, I will provide more specific information about each of the four MIPS components, the scoring mechanism for MIPS assessment and the aforementioned alternate track to MIPS, the Alternative Payment Models. While it is easy to understand how many Fellows could initially find this change daunting and overwhelming, I am confident that with a minimal investment of time all can develop adequate working knowledge of the MIPS and APMs to participate successfully in the QPP.
Until next month...
Dr. Bailey is an ACS Fellow, a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy, in the ACS offices in Washington.
On April 27, 2016, the Centers for Medicare and Medicaid Services (CMS) released its proposed rule on the Medicare Access and CHIP Reauthorization Act (MACRA). Fellows will remember that the MACRA legislation, passed in April of 2015, permanently repealed the Sustainable Growth Rate (SGR) formula and thus, represents the greatest sea change in Medicare physician payment since the establishment of the RBRVS (Resource-Based Relative Value Scale) in 1992.
In broadest policy terms, the law continues to advance the CMS policy goal of basing payment on quality and value over volume. From a granular perspective, the law combines Medicare’s three current quality programs into one new system. CMS published this 982-page proposed rule after reviewing the comments submitted by ACS and other interested parties in response to its request for information last fall. As I write, staff of the Division of Advocacy and Health Policy are in the process of crafting the ACS response to the proposed rule. Comments were due on June 27, 2016. It is anticipated that CMS will publish the final rule later this year, likely in late October or early November. Accordingly, the following description of the implementation of MACRA is based on the current understanding from the proposed rule, and is likely to change in some respects with the final rule.
CMS has designated the payment program operationalizing the MACRA law as the Quality Payment Program (QPP). The QPP has two tracks, the Merit-based Incentive Payment System (MIPS) and advanced Alternative Payment Models (APMs). For the first several years of the program, it is widely expected that the vast majority of physicians will participate in the QPP via the MIPS track. As such, I will direct the remainder of the text this month to the MIPS program.
MIPS: Merit-Based Incentive Payment System
The Merit-based Incentive Payment System, MIPS, consists of four components. They are: Quality, Resource Use, Advancing Care Information (ACI) and the Clinical Practice Improvement Activities (CPIA). Though the names have changed, Fellows are familiar with the substance of three of the components. For example, the Quality component replaces the Physician Quality Reporting System (PQRS); the Resource Use component replaces the Value-Based Modifier (VBM); and the Advancing Care Information component modifies and replaces the Electronic Health Record Meaningful Use (EHR-MU) program. The fourth component of MIPS is the new Clinical Practice Improvement Activities, which the legislation designates as intended to provide “credit for work to improve practice and facilitates future participation in alternative payment models.”
Composite Performance Score: MIPS participants will be assigned a Composite Performance Score based on their performance in all four components. For 2017, the first year for assessment under the QPP, 50 percent of the score will be based on performance in the Quality component, 10 percent will be based on the Resource Use component, 25 percent will be based on the Advancing Care Information component, and 15 percent will be based on the Clinical Practice Improvement Activities. In future years, there will be a gradual increase in the relative value of the Resource Use component with an equal and accompanying decrease in the value of the Quality component. As proposed, by the third assessment year (2019), the Quality and Resource Use components are expected to each account for 30 percent of the Composite Performance Score.
MIPS Quality Component: Though the Quality component of MIPS replaces the PQRS, CMS is proposing some changes that Fellows will welcome. As opposed to the previous PQRS requirement to report nine measures, the MIPS Quality component requires providers to report only six measures. One of these six must be an “Outcome” measure and another must be a “Cross-cutting” measure. While the reporting threshold for the percentage of patients on which reports will be required is proposed to increase substantially, ACS and other physician groups will be advocating that the required percentage published in the final rule be close to the 50 percent level found in current law.
Resources Use Component: There is also some good news relative to the Resource Use component in that there are NO reporting requirements. CMS will calculate this component from Medicare claims data and base its assessment of individual provider performance on the resource measures currently used for the Value-Based Modifier. Namely, those are the VBM Total per Capita Cost measure and the VBM Medicare Spending per Beneficiary measure. In addition, CMS will also be taking into account measures that specifically focus on episodes of care, something for which the College has previously advocated. Beginning in 2018, CMS plans to also take into consideration factors of patient condition and patient relation in order to address physician concerns about risk adjustment and attribution.
Advancing Care Information Component: This modifies and replaces the Electronic Health Record Meaningful Use program. The score for this component is derived in two parts, a Base score (50 percent) and a Performance score (up to an additional 50 percent). The threshold for achieving the Base score remains “all or nothing.” Only after meeting the requirements for the Base score is one eligible to receive additional Performance score credit based on the level of performance on a subset of the same measures required to achieve the Base score. Assessment in 2017 will be based on the EHR-MU requirements published in the 2015 Final Rule for the EHR-MU program.
Clinical Practice Improvement Component: The fourth component of MIPS is the Clinical Practice Improvement Activities component. As mentioned previously, this is a new requirement with no prior analogous program requirement. As such, it is very much in evolution. In the first year of MIPS assessment (2017), achieving full credit for the CPIA component should not pose much additional administrative burden as reporting will be by simple attestation. Physicians will chose from a list of 94 activities assigned two different weighted values. In order to receive full credit for the CPIA component, most providers will need to attest that they have participated in a minimum of three and a maximum of six of the 94 activities, depending on the weight of the activities selected, for 90 days.
As outlined above, the final rule on the Quality Payment Program is expected to be released in late October or early November. As proposed, assessment under the provisions of that final rule would begin in January of 2017. This leaves all providers with a very short time window in which to become familiar with the program that will impact their Medicare payment beginning in 2019.
Accordingly, in the next several editions of this column, I will provide more specific information about each of the four MIPS components, the scoring mechanism for MIPS assessment and the aforementioned alternate track to MIPS, the Alternative Payment Models. While it is easy to understand how many Fellows could initially find this change daunting and overwhelming, I am confident that with a minimal investment of time all can develop adequate working knowledge of the MIPS and APMs to participate successfully in the QPP.
Until next month...
Dr. Bailey is an ACS Fellow, a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy, in the ACS offices in Washington.
Surgical workforce shortages in rural areas
This month I write about one of the College’s current advocacy efforts directed at ensuring an adequate surgical workforce in underserved and rural areas. Evidence indicates a current and growing shortage of surgeons available to serve the needs of populations in certain parts of the country. A shortage of general surgeons is a clear component to the crisis in health care workforce. Accordingly, the American College of Surgeons (ACS) is urging policy makers to recognize that only surgeons are uniquely qualified to provide certain necessary, lifesaving procedures, which other health professionals are neither trained nor competent to provide.
To determine where these areas of shortage are located and where access to surgical care is thus potentially a challenge, the ACS is strongly supporting the efforts of Representatives Larry Bucshon, MD, FACS (R-Ind.) and Ami Bera, MD (D-Calif.) who recently introduced H.R. 4959, the Ensuring Access to General Surgery Act of 2016. This legislation serves to direct the Secretary of the Department of Health and Human Services (HHS) to conduct a study on the designation of surgical Health Professional Shortage Areas (HPSA).
A variety of federal programs use the HPSA designation to improve access to health care by focusing aid and assistance on specific geographic areas and populations with the greatest unmet needs. The division of HHS known as the Health Resources and Services Administration (HRSA) has developed criteria used to determine whether certain geographic areas, population groups, or facilities may be designated as a HPSA. HPSA designation may be applied to urban or rural geographic areas, specific population groups, medical provider groups, or other public health care facilities. Currently, HRSA limits HPSA designations to shortages in primary care services, dental services, or mental health services.
HRSA has never designated an entity as a HPSA purely based upon a shortage of surgical services. In light of the available evidence relative to the shortage of surgical providers in certain parts of the country, ACS believes that research is necessary to determine exactly what constitutes a surgical shortage area, e.g., establish definitional criteria, with subsequent application of those criteria to determine where areas so defined are located. Such would provide HRSA with a valuable tool to utilize in efforts directed at increasing patient access to surgical care. Ultimately, offering incentives to surgeons to locate or remain in HPSA communities could become critical in guaranteeing all Medicare beneficiaries, regardless of geographic location, have access to quality surgical care. Determining what constitutes a surgical shortage area will serve to help HRSA to appropriately focus its resources.
Accordingly, we need your help and urge you to take action today.
Using the information below, please call your representatives today and urge them to join their colleagues and cosponsor H.R. 4959, the Ensuring Access to General Surgery Act of 2016.
Instructions
Call toll-free: 1-877-996-4464
You will be connected to your representative‘s office. Once you are connected, provide your name and indicate that you are a constituent. You should also be prepared to provide additional contact information for follow-up purposes.
Next, we suggest you use the following message:
• As a surgeon and as your constituent, I urge you to join your colleagues and cosponsor H.R. 4959, the Ensuring Access to General Surgery Act of 2016, which would direct the Secretary of Department of Health and Human Services (HHS) to conduct a study to designate General Surgery Health Professional Shortage Areas (HPSA).
• The division of HHS known as the Health Resources and Services Administration (HRSA) has developed designation criteria in order to determine whether certain geographic areas, population groups, or facilities may be designated as a HPSA.
• HRSA has never designated an entity as a HPSA purely based upon a shortage of surgical services.
• In light of evidence relative to a shortage of surgeons, ACS believes that research is necessary to determine exactly what constitutes a surgical shortage area and subsequently where these areas exist.
Alternatively, for those who were seeking a topic on which to initiate a personal in-district meeting with representatives and their staff as was discussed in last month’s edition of this column, H.R. 4959 presents a prime subject for such in order to have a focused meeting with a specific ask on a “white hat” issue that will surely resonate with members of Congress. Currently, in-district work periods are scheduled for the last week of June, the last two weeks of July, and the entire month of August.
As always, those with questions or concerns, or those who need assistance in setting up an in-district meeting may contact staff of the Division of Advocacy and Health Policy by phone at 202-337-2701 or via e-mail at [email protected].
Thank you for taking the time to engage and take action on this critical issue.
Please encourage your colleagues to do likewise.
Until next month ...
Dr. Patrick V. Bailey is an ACS Fellow, a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy, in the ACS offices in Washington, DC.
This month I write about one of the College’s current advocacy efforts directed at ensuring an adequate surgical workforce in underserved and rural areas. Evidence indicates a current and growing shortage of surgeons available to serve the needs of populations in certain parts of the country. A shortage of general surgeons is a clear component to the crisis in health care workforce. Accordingly, the American College of Surgeons (ACS) is urging policy makers to recognize that only surgeons are uniquely qualified to provide certain necessary, lifesaving procedures, which other health professionals are neither trained nor competent to provide.
To determine where these areas of shortage are located and where access to surgical care is thus potentially a challenge, the ACS is strongly supporting the efforts of Representatives Larry Bucshon, MD, FACS (R-Ind.) and Ami Bera, MD (D-Calif.) who recently introduced H.R. 4959, the Ensuring Access to General Surgery Act of 2016. This legislation serves to direct the Secretary of the Department of Health and Human Services (HHS) to conduct a study on the designation of surgical Health Professional Shortage Areas (HPSA).
A variety of federal programs use the HPSA designation to improve access to health care by focusing aid and assistance on specific geographic areas and populations with the greatest unmet needs. The division of HHS known as the Health Resources and Services Administration (HRSA) has developed criteria used to determine whether certain geographic areas, population groups, or facilities may be designated as a HPSA. HPSA designation may be applied to urban or rural geographic areas, specific population groups, medical provider groups, or other public health care facilities. Currently, HRSA limits HPSA designations to shortages in primary care services, dental services, or mental health services.
HRSA has never designated an entity as a HPSA purely based upon a shortage of surgical services. In light of the available evidence relative to the shortage of surgical providers in certain parts of the country, ACS believes that research is necessary to determine exactly what constitutes a surgical shortage area, e.g., establish definitional criteria, with subsequent application of those criteria to determine where areas so defined are located. Such would provide HRSA with a valuable tool to utilize in efforts directed at increasing patient access to surgical care. Ultimately, offering incentives to surgeons to locate or remain in HPSA communities could become critical in guaranteeing all Medicare beneficiaries, regardless of geographic location, have access to quality surgical care. Determining what constitutes a surgical shortage area will serve to help HRSA to appropriately focus its resources.
Accordingly, we need your help and urge you to take action today.
Using the information below, please call your representatives today and urge them to join their colleagues and cosponsor H.R. 4959, the Ensuring Access to General Surgery Act of 2016.
Instructions
Call toll-free: 1-877-996-4464
You will be connected to your representative‘s office. Once you are connected, provide your name and indicate that you are a constituent. You should also be prepared to provide additional contact information for follow-up purposes.
Next, we suggest you use the following message:
• As a surgeon and as your constituent, I urge you to join your colleagues and cosponsor H.R. 4959, the Ensuring Access to General Surgery Act of 2016, which would direct the Secretary of Department of Health and Human Services (HHS) to conduct a study to designate General Surgery Health Professional Shortage Areas (HPSA).
• The division of HHS known as the Health Resources and Services Administration (HRSA) has developed designation criteria in order to determine whether certain geographic areas, population groups, or facilities may be designated as a HPSA.
• HRSA has never designated an entity as a HPSA purely based upon a shortage of surgical services.
• In light of evidence relative to a shortage of surgeons, ACS believes that research is necessary to determine exactly what constitutes a surgical shortage area and subsequently where these areas exist.
Alternatively, for those who were seeking a topic on which to initiate a personal in-district meeting with representatives and their staff as was discussed in last month’s edition of this column, H.R. 4959 presents a prime subject for such in order to have a focused meeting with a specific ask on a “white hat” issue that will surely resonate with members of Congress. Currently, in-district work periods are scheduled for the last week of June, the last two weeks of July, and the entire month of August.
As always, those with questions or concerns, or those who need assistance in setting up an in-district meeting may contact staff of the Division of Advocacy and Health Policy by phone at 202-337-2701 or via e-mail at [email protected].
Thank you for taking the time to engage and take action on this critical issue.
Please encourage your colleagues to do likewise.
Until next month ...
Dr. Patrick V. Bailey is an ACS Fellow, a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy, in the ACS offices in Washington, DC.
This month I write about one of the College’s current advocacy efforts directed at ensuring an adequate surgical workforce in underserved and rural areas. Evidence indicates a current and growing shortage of surgeons available to serve the needs of populations in certain parts of the country. A shortage of general surgeons is a clear component to the crisis in health care workforce. Accordingly, the American College of Surgeons (ACS) is urging policy makers to recognize that only surgeons are uniquely qualified to provide certain necessary, lifesaving procedures, which other health professionals are neither trained nor competent to provide.
To determine where these areas of shortage are located and where access to surgical care is thus potentially a challenge, the ACS is strongly supporting the efforts of Representatives Larry Bucshon, MD, FACS (R-Ind.) and Ami Bera, MD (D-Calif.) who recently introduced H.R. 4959, the Ensuring Access to General Surgery Act of 2016. This legislation serves to direct the Secretary of the Department of Health and Human Services (HHS) to conduct a study on the designation of surgical Health Professional Shortage Areas (HPSA).
A variety of federal programs use the HPSA designation to improve access to health care by focusing aid and assistance on specific geographic areas and populations with the greatest unmet needs. The division of HHS known as the Health Resources and Services Administration (HRSA) has developed criteria used to determine whether certain geographic areas, population groups, or facilities may be designated as a HPSA. HPSA designation may be applied to urban or rural geographic areas, specific population groups, medical provider groups, or other public health care facilities. Currently, HRSA limits HPSA designations to shortages in primary care services, dental services, or mental health services.
HRSA has never designated an entity as a HPSA purely based upon a shortage of surgical services. In light of the available evidence relative to the shortage of surgical providers in certain parts of the country, ACS believes that research is necessary to determine exactly what constitutes a surgical shortage area, e.g., establish definitional criteria, with subsequent application of those criteria to determine where areas so defined are located. Such would provide HRSA with a valuable tool to utilize in efforts directed at increasing patient access to surgical care. Ultimately, offering incentives to surgeons to locate or remain in HPSA communities could become critical in guaranteeing all Medicare beneficiaries, regardless of geographic location, have access to quality surgical care. Determining what constitutes a surgical shortage area will serve to help HRSA to appropriately focus its resources.
Accordingly, we need your help and urge you to take action today.
Using the information below, please call your representatives today and urge them to join their colleagues and cosponsor H.R. 4959, the Ensuring Access to General Surgery Act of 2016.
Instructions
Call toll-free: 1-877-996-4464
You will be connected to your representative‘s office. Once you are connected, provide your name and indicate that you are a constituent. You should also be prepared to provide additional contact information for follow-up purposes.
Next, we suggest you use the following message:
• As a surgeon and as your constituent, I urge you to join your colleagues and cosponsor H.R. 4959, the Ensuring Access to General Surgery Act of 2016, which would direct the Secretary of Department of Health and Human Services (HHS) to conduct a study to designate General Surgery Health Professional Shortage Areas (HPSA).
• The division of HHS known as the Health Resources and Services Administration (HRSA) has developed designation criteria in order to determine whether certain geographic areas, population groups, or facilities may be designated as a HPSA.
• HRSA has never designated an entity as a HPSA purely based upon a shortage of surgical services.
• In light of evidence relative to a shortage of surgeons, ACS believes that research is necessary to determine exactly what constitutes a surgical shortage area and subsequently where these areas exist.
Alternatively, for those who were seeking a topic on which to initiate a personal in-district meeting with representatives and their staff as was discussed in last month’s edition of this column, H.R. 4959 presents a prime subject for such in order to have a focused meeting with a specific ask on a “white hat” issue that will surely resonate with members of Congress. Currently, in-district work periods are scheduled for the last week of June, the last two weeks of July, and the entire month of August.
As always, those with questions or concerns, or those who need assistance in setting up an in-district meeting may contact staff of the Division of Advocacy and Health Policy by phone at 202-337-2701 or via e-mail at [email protected].
Thank you for taking the time to engage and take action on this critical issue.
Please encourage your colleagues to do likewise.
Until next month ...
Dr. Patrick V. Bailey is an ACS Fellow, a pediatric surgeon, and Medical Director, Advocacy, for the Division of Advocacy and Health Policy, in the ACS offices in Washington, DC.









