User login
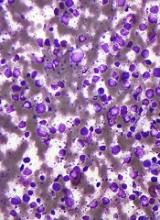
Researchers find drug target in anaplastic large-cell lymphoma
Preclinical research indicates that TYK2 inhibitors could be effective in treating anaplastic large-cell lymphoma (ALCL).
Researchers found evidence to suggest that TYK2 “is highly expressed in all cases of human ALCL.”
The team also discovered that TYK2 inhibition induces apoptosis in human ALCL cells, and it delays tumor onset, and prolongs survival in a mouse model of ALCL.
Olaf Merkel, PhD, of the Medical University of Vienna in Austria, and his colleagues detailed these findings in Leukemia.
The researchers said their analyses suggest TYK2 is expressed in all types of ALCL, regardless of ALK status, and TYK2 mediates the same anti-apoptotic response across ALCLs.
“Therefore, we could consider TYK2 signaling as the Achilles’ heel of ALCL, as, in all patients we have analyzed, the tumor cells relied on this activity to support the essential survival signal,” Dr. Merkel said in a statement.
He and his colleagues found that disrupting TYK2 – either via gene knockdown or with small-molecule TYK2 inhibitors – induced apoptosis in human ALCL cells in vitro.
In a mouse model of NPM-ALK-induced lymphoma, Tyk2 deletion slowed the rate of tumor growth and significantly prolonged survival. The median survival was 53.3 weeks in mice with Tyk2 deletion and 16.0 weeks in control mice (P less than .0001).
Additional experiments in human ALCL cell lines showed that “TYK2 is activated by autocrine production of IL-10 and IL-22 and by interaction with specific receptors expressed by the cells,” the researchers said.
They also found that “activated TYK2 leads to STAT1 and STAT3 phosphorylation, activated expression of MCL1, and aberrant ALCL cell survival.”
Taking these findings together, the researchers concluded that TYK2 inhibitors could be effective for treating ALCL.
“We are looking forward to TYK2 inhibitors becoming available,” said study coauthor Lukas Kenner, MD, of the Medical University of Vienna. “[I]n the more rare lymphomas, we urgently need better therapies.”
The researchers received grant funding from various organizations but reported having no conflicts of interest.
SOURCE: Prutsch N et al. Leukemia. 2018 Aug 21. doi: 10.1038/s41375-018-0239-1.
Preclinical research indicates that TYK2 inhibitors could be effective in treating anaplastic large-cell lymphoma (ALCL).
Researchers found evidence to suggest that TYK2 “is highly expressed in all cases of human ALCL.”
The team also discovered that TYK2 inhibition induces apoptosis in human ALCL cells, and it delays tumor onset, and prolongs survival in a mouse model of ALCL.
Olaf Merkel, PhD, of the Medical University of Vienna in Austria, and his colleagues detailed these findings in Leukemia.
The researchers said their analyses suggest TYK2 is expressed in all types of ALCL, regardless of ALK status, and TYK2 mediates the same anti-apoptotic response across ALCLs.
“Therefore, we could consider TYK2 signaling as the Achilles’ heel of ALCL, as, in all patients we have analyzed, the tumor cells relied on this activity to support the essential survival signal,” Dr. Merkel said in a statement.
He and his colleagues found that disrupting TYK2 – either via gene knockdown or with small-molecule TYK2 inhibitors – induced apoptosis in human ALCL cells in vitro.
In a mouse model of NPM-ALK-induced lymphoma, Tyk2 deletion slowed the rate of tumor growth and significantly prolonged survival. The median survival was 53.3 weeks in mice with Tyk2 deletion and 16.0 weeks in control mice (P less than .0001).
Additional experiments in human ALCL cell lines showed that “TYK2 is activated by autocrine production of IL-10 and IL-22 and by interaction with specific receptors expressed by the cells,” the researchers said.
They also found that “activated TYK2 leads to STAT1 and STAT3 phosphorylation, activated expression of MCL1, and aberrant ALCL cell survival.”
Taking these findings together, the researchers concluded that TYK2 inhibitors could be effective for treating ALCL.
“We are looking forward to TYK2 inhibitors becoming available,” said study coauthor Lukas Kenner, MD, of the Medical University of Vienna. “[I]n the more rare lymphomas, we urgently need better therapies.”
The researchers received grant funding from various organizations but reported having no conflicts of interest.
SOURCE: Prutsch N et al. Leukemia. 2018 Aug 21. doi: 10.1038/s41375-018-0239-1.
Preclinical research indicates that TYK2 inhibitors could be effective in treating anaplastic large-cell lymphoma (ALCL).
Researchers found evidence to suggest that TYK2 “is highly expressed in all cases of human ALCL.”
The team also discovered that TYK2 inhibition induces apoptosis in human ALCL cells, and it delays tumor onset, and prolongs survival in a mouse model of ALCL.
Olaf Merkel, PhD, of the Medical University of Vienna in Austria, and his colleagues detailed these findings in Leukemia.
The researchers said their analyses suggest TYK2 is expressed in all types of ALCL, regardless of ALK status, and TYK2 mediates the same anti-apoptotic response across ALCLs.
“Therefore, we could consider TYK2 signaling as the Achilles’ heel of ALCL, as, in all patients we have analyzed, the tumor cells relied on this activity to support the essential survival signal,” Dr. Merkel said in a statement.
He and his colleagues found that disrupting TYK2 – either via gene knockdown or with small-molecule TYK2 inhibitors – induced apoptosis in human ALCL cells in vitro.
In a mouse model of NPM-ALK-induced lymphoma, Tyk2 deletion slowed the rate of tumor growth and significantly prolonged survival. The median survival was 53.3 weeks in mice with Tyk2 deletion and 16.0 weeks in control mice (P less than .0001).
Additional experiments in human ALCL cell lines showed that “TYK2 is activated by autocrine production of IL-10 and IL-22 and by interaction with specific receptors expressed by the cells,” the researchers said.
They also found that “activated TYK2 leads to STAT1 and STAT3 phosphorylation, activated expression of MCL1, and aberrant ALCL cell survival.”
Taking these findings together, the researchers concluded that TYK2 inhibitors could be effective for treating ALCL.
“We are looking forward to TYK2 inhibitors becoming available,” said study coauthor Lukas Kenner, MD, of the Medical University of Vienna. “[I]n the more rare lymphomas, we urgently need better therapies.”
The researchers received grant funding from various organizations but reported having no conflicts of interest.
SOURCE: Prutsch N et al. Leukemia. 2018 Aug 21. doi: 10.1038/s41375-018-0239-1.
FROM LEUKEMIA
Key clinical point:
Major finding: TYK2 was expressed in all types of ALCL studied and mediated the same anti-apoptotic response across ALCLs.
Study details: A preclinical study of mouse and human ALCL cell lines.
Disclosures: The researchers received grant funding from various organizations but reported having no conflicts of interest.
Source: Prutsch N et al. Leukemia. 2018 Aug 21. doi: 10.1038/s41375-018-0239-1.
Adverse events outweigh promise of SGN-CD70A against NHL
An investigational antibody-drug conjugate labeled SGN-CD70A showed signs of efficacy against relapsed or refractory non-Hodgkin lymphomas in a phase 1 trial, but its future is clouded by a high incidence of treatment-associated thrombocytopenia, investigators reported.
Among 20 patients with diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma, and other histologies, SGN-CD70A was associated with one complete remission (CR) and three partial remissions (PR), two of which were ongoing at nearly 43 weeks of follow-up.
However, 15 of the 20 patients (75%) had treatment-related thrombocytopenias, and 13 of these adverse events (AEs) were grade 3 or greater in severity, reported Tycel Phillips, MD, of the University of Michigan, Ann Arbor, and his colleagues.
Notwithstanding the antibody-drug conjugate’s apparent efficacy in this early trial, “the applicability of SGN-CD70A is limited by the frequency and severity of thrombocytopenia, despite the long-term of response with limited drug exposure. Given that we are currently unable to mitigate this AE, the rationale for further investigation of SGN-CD70A remains limited and is, therefore, not planned,” they wrote in the journal Investigational New Drugs.
SGN-CD70A consists of an antibody directed against the plasma membrane protein CD70, a protease-cleavable linker, and a DNA-crosslinking pyrrolobenzodiazepine dimer drug. Its mechanism of action is via double-strand DNA breaks in CD70-positive cells that eventually cause programmed cell death.
Dr. Phillips and his colleagues reported on the high-risk non-Hodgkin lymphoma cohort in the phase 1 trial. The cohort included nine patients with DLBCL, five with mantle cell lymphoma, two with transformed DLBCL, one with T- cell/histocyte–rich large B cell lymphoma, and three with unspecified NHL histologies.
The patients had undergone a median of 3.5 prior lines of systemic therapy, and all had relatively good performance status, with Eastern Cooperative Oncology Group scores of 0 or 1.
Patients were started on intravenous SGN-CD70A at a dose of 8 mcg/kg on day 1 of each 3-week cycle, with a planned dose escalation to 200 mcg/kg, The protocol was amended to dosing every 6 weeks, however, after the investigators observed prolonged thrombocytopenias in some patients. A total of 12 patients were treated every 3 weeks, and 8 were treated every 6 weeks.
The most common treatment-related AEs were thrombocytopenias, which occurred in three-quarters of all patients, and were largely grade 3 or greater in severity. Other treatment-related AEs of grade 3 or greater occurring in more than one patient include neutropenia in six patients; anemia in five patients; and congestive heart failure, Clostridium difficile infections, dyspnea, and decreased forced expiratory volume in two patients each.
Other common AEs were nausea and fatigue.
The investigators noted that the cause of the deep and durable thrombocytopenias could not be determined, despite assessment of known biomarkers for this complication.
The duration of the thrombocytopenia and the fact that some of the few responses that did occur were also durable after the end of treatment suggest that the dimer drug, the cytotoxic “payload” of the antibody-drug conjugate, was responsible for the effects they observed, the authors said.
The study was funded by Seattle Genetics. Dr. Phillips reported advisory board membership with the company, and four of the coauthors are employees of the company with equity interests.
SOURCE: Phillips T et al. Invest New Drugs. 2018 Aug 22. doi: 10.1007/s10637-018-0655-0.
An investigational antibody-drug conjugate labeled SGN-CD70A showed signs of efficacy against relapsed or refractory non-Hodgkin lymphomas in a phase 1 trial, but its future is clouded by a high incidence of treatment-associated thrombocytopenia, investigators reported.
Among 20 patients with diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma, and other histologies, SGN-CD70A was associated with one complete remission (CR) and three partial remissions (PR), two of which were ongoing at nearly 43 weeks of follow-up.
However, 15 of the 20 patients (75%) had treatment-related thrombocytopenias, and 13 of these adverse events (AEs) were grade 3 or greater in severity, reported Tycel Phillips, MD, of the University of Michigan, Ann Arbor, and his colleagues.
Notwithstanding the antibody-drug conjugate’s apparent efficacy in this early trial, “the applicability of SGN-CD70A is limited by the frequency and severity of thrombocytopenia, despite the long-term of response with limited drug exposure. Given that we are currently unable to mitigate this AE, the rationale for further investigation of SGN-CD70A remains limited and is, therefore, not planned,” they wrote in the journal Investigational New Drugs.
SGN-CD70A consists of an antibody directed against the plasma membrane protein CD70, a protease-cleavable linker, and a DNA-crosslinking pyrrolobenzodiazepine dimer drug. Its mechanism of action is via double-strand DNA breaks in CD70-positive cells that eventually cause programmed cell death.
Dr. Phillips and his colleagues reported on the high-risk non-Hodgkin lymphoma cohort in the phase 1 trial. The cohort included nine patients with DLBCL, five with mantle cell lymphoma, two with transformed DLBCL, one with T- cell/histocyte–rich large B cell lymphoma, and three with unspecified NHL histologies.
The patients had undergone a median of 3.5 prior lines of systemic therapy, and all had relatively good performance status, with Eastern Cooperative Oncology Group scores of 0 or 1.
Patients were started on intravenous SGN-CD70A at a dose of 8 mcg/kg on day 1 of each 3-week cycle, with a planned dose escalation to 200 mcg/kg, The protocol was amended to dosing every 6 weeks, however, after the investigators observed prolonged thrombocytopenias in some patients. A total of 12 patients were treated every 3 weeks, and 8 were treated every 6 weeks.
The most common treatment-related AEs were thrombocytopenias, which occurred in three-quarters of all patients, and were largely grade 3 or greater in severity. Other treatment-related AEs of grade 3 or greater occurring in more than one patient include neutropenia in six patients; anemia in five patients; and congestive heart failure, Clostridium difficile infections, dyspnea, and decreased forced expiratory volume in two patients each.
Other common AEs were nausea and fatigue.
The investigators noted that the cause of the deep and durable thrombocytopenias could not be determined, despite assessment of known biomarkers for this complication.
The duration of the thrombocytopenia and the fact that some of the few responses that did occur were also durable after the end of treatment suggest that the dimer drug, the cytotoxic “payload” of the antibody-drug conjugate, was responsible for the effects they observed, the authors said.
The study was funded by Seattle Genetics. Dr. Phillips reported advisory board membership with the company, and four of the coauthors are employees of the company with equity interests.
SOURCE: Phillips T et al. Invest New Drugs. 2018 Aug 22. doi: 10.1007/s10637-018-0655-0.
An investigational antibody-drug conjugate labeled SGN-CD70A showed signs of efficacy against relapsed or refractory non-Hodgkin lymphomas in a phase 1 trial, but its future is clouded by a high incidence of treatment-associated thrombocytopenia, investigators reported.
Among 20 patients with diffuse large B-cell lymphoma (DLBCL), mantle cell lymphoma, and other histologies, SGN-CD70A was associated with one complete remission (CR) and three partial remissions (PR), two of which were ongoing at nearly 43 weeks of follow-up.
However, 15 of the 20 patients (75%) had treatment-related thrombocytopenias, and 13 of these adverse events (AEs) were grade 3 or greater in severity, reported Tycel Phillips, MD, of the University of Michigan, Ann Arbor, and his colleagues.
Notwithstanding the antibody-drug conjugate’s apparent efficacy in this early trial, “the applicability of SGN-CD70A is limited by the frequency and severity of thrombocytopenia, despite the long-term of response with limited drug exposure. Given that we are currently unable to mitigate this AE, the rationale for further investigation of SGN-CD70A remains limited and is, therefore, not planned,” they wrote in the journal Investigational New Drugs.
SGN-CD70A consists of an antibody directed against the plasma membrane protein CD70, a protease-cleavable linker, and a DNA-crosslinking pyrrolobenzodiazepine dimer drug. Its mechanism of action is via double-strand DNA breaks in CD70-positive cells that eventually cause programmed cell death.
Dr. Phillips and his colleagues reported on the high-risk non-Hodgkin lymphoma cohort in the phase 1 trial. The cohort included nine patients with DLBCL, five with mantle cell lymphoma, two with transformed DLBCL, one with T- cell/histocyte–rich large B cell lymphoma, and three with unspecified NHL histologies.
The patients had undergone a median of 3.5 prior lines of systemic therapy, and all had relatively good performance status, with Eastern Cooperative Oncology Group scores of 0 or 1.
Patients were started on intravenous SGN-CD70A at a dose of 8 mcg/kg on day 1 of each 3-week cycle, with a planned dose escalation to 200 mcg/kg, The protocol was amended to dosing every 6 weeks, however, after the investigators observed prolonged thrombocytopenias in some patients. A total of 12 patients were treated every 3 weeks, and 8 were treated every 6 weeks.
The most common treatment-related AEs were thrombocytopenias, which occurred in three-quarters of all patients, and were largely grade 3 or greater in severity. Other treatment-related AEs of grade 3 or greater occurring in more than one patient include neutropenia in six patients; anemia in five patients; and congestive heart failure, Clostridium difficile infections, dyspnea, and decreased forced expiratory volume in two patients each.
Other common AEs were nausea and fatigue.
The investigators noted that the cause of the deep and durable thrombocytopenias could not be determined, despite assessment of known biomarkers for this complication.
The duration of the thrombocytopenia and the fact that some of the few responses that did occur were also durable after the end of treatment suggest that the dimer drug, the cytotoxic “payload” of the antibody-drug conjugate, was responsible for the effects they observed, the authors said.
The study was funded by Seattle Genetics. Dr. Phillips reported advisory board membership with the company, and four of the coauthors are employees of the company with equity interests.
SOURCE: Phillips T et al. Invest New Drugs. 2018 Aug 22. doi: 10.1007/s10637-018-0655-0.
FROM INVESTIGATIONAL NEW DRUGS
Key clinical point: A high incidence of unexplained
Major finding: In total, 15 of 20 patients had treatment-related thrombocytopenias; 13 of these adverse events were grade 3 or greater in severity.
Study details: A 20-patient NHL cohort of a phase 1 dose-finding, pharmacologic, safety, and preliminary efficacy trial of the antibody-drug conjugate SGN-CD70A.
Disclosures: The study was funded by Seattle Genetics. Dr. Phillips reported advisory board membership with the company, and four of the coauthors are employees of the company with equity interests.
Source: Phillips T et al. Invest New Drugs. 2018 Aug 22. doi: 10.1007/s10637-018-0655-0.
Predicting early outcomes in DLBCL
Measurement of circulating tumor DNA (ctDNA) could be a new and useful tool for predicting survival outcomes and response to therapy in patients with diffuse large B-cell lymphoma (DLBCL), according to researchers.
Pretreatment ctDNA levels predicted 24-month event-free survival as well as overall survival in a prospective study.
Changes in ctDNA during treatment were prognostic for outcomes as early as 21 days into therapy.
Ash A. Alizadeh, MD, PhD, of Stanford University in California, and his colleagues reported these findings in the Journal of Clinical Oncology.
ctDNA was detected in 98% of the 217 patients evaluated, which demonstrated the “potentially universal applicability” of this approach, the researchers wrote.
In an evaluation of pretreatment ctDNA levels, the researchers found a 2.5 log haploid genome equivalents per milliliter threshold stratified patient outcomes. Event-free survival was significantly inferior at 24 months in patients with ctDNA above that threshold, with hazard ratios of 2.6 (P=0.007) for frontline treatment and 2.9 (P=0.01) for salvage therapy.
On-treatment ctDNA levels were favorably prognostic for outcomes in patients receiving frontline therapy.
An early molecular response (EMR), defined as a 2-log decrease in ctDNA levels after one cycle of treatment, was associated with a 24-month event-free survival of 83% versus 50% for no EMR (P=0.0015).
Major molecular response (MMR), defined as a 2.5-log drop in ctDNA after two cycles of treatment, was associated with a 24-month event-free survival of 82% versus 46% for no MMR in patients on frontline therapy (P<0.001).
In one cohort of patients receiving salvage therapy, EMR also predicted superior 24-month event-free survival.
The EMR measure was also favorably prognostic for overall survival in both the frontline and salvage settings.
The prognostic value of measuring ctDNA was independent of International Prognostic Index and interim PET/CT studies, results of multivariable analyses showed.
Patients had “excellent outcomes” if they had both molecular response and favorable interim PET results, according to researchers. Conversely, patients were at “extremely high risk” for treatment failure if they had no molecular response and a positive PET scan.
“The identification of patients at exceptionally high risk (i.e., interim PET/CT positive and not achieving EMR/MMR) could provide an opportunity for early intervention with alternative treatments, including autologous bone marrow transplantation or chimeric antigen receptor T cells,” the researchers wrote.
Patients in the study were all treated with combination immunochemotherapy according to local standards.
Dr. Alizadeh reported disclosures related to CiberMed, Forty Seven, Janssen Oncology, Celgene, Roche/Genentech, and Gilead, as well as patent filings on ctDNA detection assigned to Stanford University.
Measurement of circulating tumor DNA (ctDNA) could be a new and useful tool for predicting survival outcomes and response to therapy in patients with diffuse large B-cell lymphoma (DLBCL), according to researchers.
Pretreatment ctDNA levels predicted 24-month event-free survival as well as overall survival in a prospective study.
Changes in ctDNA during treatment were prognostic for outcomes as early as 21 days into therapy.
Ash A. Alizadeh, MD, PhD, of Stanford University in California, and his colleagues reported these findings in the Journal of Clinical Oncology.
ctDNA was detected in 98% of the 217 patients evaluated, which demonstrated the “potentially universal applicability” of this approach, the researchers wrote.
In an evaluation of pretreatment ctDNA levels, the researchers found a 2.5 log haploid genome equivalents per milliliter threshold stratified patient outcomes. Event-free survival was significantly inferior at 24 months in patients with ctDNA above that threshold, with hazard ratios of 2.6 (P=0.007) for frontline treatment and 2.9 (P=0.01) for salvage therapy.
On-treatment ctDNA levels were favorably prognostic for outcomes in patients receiving frontline therapy.
An early molecular response (EMR), defined as a 2-log decrease in ctDNA levels after one cycle of treatment, was associated with a 24-month event-free survival of 83% versus 50% for no EMR (P=0.0015).
Major molecular response (MMR), defined as a 2.5-log drop in ctDNA after two cycles of treatment, was associated with a 24-month event-free survival of 82% versus 46% for no MMR in patients on frontline therapy (P<0.001).
In one cohort of patients receiving salvage therapy, EMR also predicted superior 24-month event-free survival.
The EMR measure was also favorably prognostic for overall survival in both the frontline and salvage settings.
The prognostic value of measuring ctDNA was independent of International Prognostic Index and interim PET/CT studies, results of multivariable analyses showed.
Patients had “excellent outcomes” if they had both molecular response and favorable interim PET results, according to researchers. Conversely, patients were at “extremely high risk” for treatment failure if they had no molecular response and a positive PET scan.
“The identification of patients at exceptionally high risk (i.e., interim PET/CT positive and not achieving EMR/MMR) could provide an opportunity for early intervention with alternative treatments, including autologous bone marrow transplantation or chimeric antigen receptor T cells,” the researchers wrote.
Patients in the study were all treated with combination immunochemotherapy according to local standards.
Dr. Alizadeh reported disclosures related to CiberMed, Forty Seven, Janssen Oncology, Celgene, Roche/Genentech, and Gilead, as well as patent filings on ctDNA detection assigned to Stanford University.
Measurement of circulating tumor DNA (ctDNA) could be a new and useful tool for predicting survival outcomes and response to therapy in patients with diffuse large B-cell lymphoma (DLBCL), according to researchers.
Pretreatment ctDNA levels predicted 24-month event-free survival as well as overall survival in a prospective study.
Changes in ctDNA during treatment were prognostic for outcomes as early as 21 days into therapy.
Ash A. Alizadeh, MD, PhD, of Stanford University in California, and his colleagues reported these findings in the Journal of Clinical Oncology.
ctDNA was detected in 98% of the 217 patients evaluated, which demonstrated the “potentially universal applicability” of this approach, the researchers wrote.
In an evaluation of pretreatment ctDNA levels, the researchers found a 2.5 log haploid genome equivalents per milliliter threshold stratified patient outcomes. Event-free survival was significantly inferior at 24 months in patients with ctDNA above that threshold, with hazard ratios of 2.6 (P=0.007) for frontline treatment and 2.9 (P=0.01) for salvage therapy.
On-treatment ctDNA levels were favorably prognostic for outcomes in patients receiving frontline therapy.
An early molecular response (EMR), defined as a 2-log decrease in ctDNA levels after one cycle of treatment, was associated with a 24-month event-free survival of 83% versus 50% for no EMR (P=0.0015).
Major molecular response (MMR), defined as a 2.5-log drop in ctDNA after two cycles of treatment, was associated with a 24-month event-free survival of 82% versus 46% for no MMR in patients on frontline therapy (P<0.001).
In one cohort of patients receiving salvage therapy, EMR also predicted superior 24-month event-free survival.
The EMR measure was also favorably prognostic for overall survival in both the frontline and salvage settings.
The prognostic value of measuring ctDNA was independent of International Prognostic Index and interim PET/CT studies, results of multivariable analyses showed.
Patients had “excellent outcomes” if they had both molecular response and favorable interim PET results, according to researchers. Conversely, patients were at “extremely high risk” for treatment failure if they had no molecular response and a positive PET scan.
“The identification of patients at exceptionally high risk (i.e., interim PET/CT positive and not achieving EMR/MMR) could provide an opportunity for early intervention with alternative treatments, including autologous bone marrow transplantation or chimeric antigen receptor T cells,” the researchers wrote.
Patients in the study were all treated with combination immunochemotherapy according to local standards.
Dr. Alizadeh reported disclosures related to CiberMed, Forty Seven, Janssen Oncology, Celgene, Roche/Genentech, and Gilead, as well as patent filings on ctDNA detection assigned to Stanford University.
New BTK inhibitor under review in China
for the treatment of relapsed/refractory mantle cell lymphoma (MCL).
The U.S. Food and Drug Administration recently granted the drug fast track designation for the treatment of patients with Waldenström’s macroglobulinemia.
The application in China is supported by results from a phase 2, single-arm trial of 86 patients with relapsed/refractory MCL who received 160 mg zanubrutinib orally twice daily. The overall response rate was 84%, which included 59% of patients with a complete response. At 8.3 months of follow-up, the median duration of response had not been reached, according to the drug’s sponsor BeiGene.
Zanubrutinib is being studied in several ongoing trials, including for the treatment of untreated chronic lymphocytic leukemia (CLL), for relapsed/refractory follicular lymphoma in combination with obinutuzumab, and comparing it to ibrutinib in Waldenström’s macroglobulinemia and CLL/small lymphocytic lymphoma.
for the treatment of relapsed/refractory mantle cell lymphoma (MCL).
The U.S. Food and Drug Administration recently granted the drug fast track designation for the treatment of patients with Waldenström’s macroglobulinemia.
The application in China is supported by results from a phase 2, single-arm trial of 86 patients with relapsed/refractory MCL who received 160 mg zanubrutinib orally twice daily. The overall response rate was 84%, which included 59% of patients with a complete response. At 8.3 months of follow-up, the median duration of response had not been reached, according to the drug’s sponsor BeiGene.
Zanubrutinib is being studied in several ongoing trials, including for the treatment of untreated chronic lymphocytic leukemia (CLL), for relapsed/refractory follicular lymphoma in combination with obinutuzumab, and comparing it to ibrutinib in Waldenström’s macroglobulinemia and CLL/small lymphocytic lymphoma.
for the treatment of relapsed/refractory mantle cell lymphoma (MCL).
The U.S. Food and Drug Administration recently granted the drug fast track designation for the treatment of patients with Waldenström’s macroglobulinemia.
The application in China is supported by results from a phase 2, single-arm trial of 86 patients with relapsed/refractory MCL who received 160 mg zanubrutinib orally twice daily. The overall response rate was 84%, which included 59% of patients with a complete response. At 8.3 months of follow-up, the median duration of response had not been reached, according to the drug’s sponsor BeiGene.
Zanubrutinib is being studied in several ongoing trials, including for the treatment of untreated chronic lymphocytic leukemia (CLL), for relapsed/refractory follicular lymphoma in combination with obinutuzumab, and comparing it to ibrutinib in Waldenström’s macroglobulinemia and CLL/small lymphocytic lymphoma.
NICE says CAR T-cell therapy isn’t cost-effective
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance recommending against the use of axicabtagene ciloleucel (Yescarta) in England.
Axicabtagene ciloleucel is a chimeric antigen receptor (CAR) T-cell therapy that was just approved by the European Commission to treat patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) or primary mediastinal B-cell lymphoma (PMBCL) who have received two or more lines of systemic therapy.
However, NICE has said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy.
Additionally, the cost of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of National Health Service (NHS) resources.
NICE’s draft guidance points out that there is no standard treatment for patients with relapsed or refractory DLBCL or PMBCL who have received two or more systemic therapies. These patients receive best supportive care, which usually includes salvage chemotherapy.
Results from the ZUMA-1 trial suggest the majority of DLBCL/PMBCL patients given axicabtagene ciloleucel do respond to treatment.
However, there is no direct data comparing axicabtagene ciloleucel with salvage chemotherapy, so the benefit of the CAR T-cell therapy over chemotherapy is unknown.
The draft guidance also notes that axicabtagene ciloleucel meets NICE’s criteria to be considered a life-extending treatment at the end of life.
However, the CAR T-cell therapy cannot be considered a cost-effective use of NHS resources. The cost-effectiveness estimates for axicabtagene ciloleucel, compared with salvage chemotherapy, were above £50,000 per year of quality adjusted life gained, the upper limit of the specially extended range of cost-effectiveness for cancer treatments.
Furthermore, axicabtagene ciloleucel does not meet the criteria for inclusion in the Cancer Drugs Fund. NICE said axicabtagene ciloleucel does not have the plausible potential to be cost effective, which would be necessary for inclusion in the fund while further evidence of the treatment’s longer-term benefits is collected.
“Although promising, there is still much more we need to know about CAR-T, and, unfortunately, in this case, we are not able to recommend axicabtagene ciloleucel for use in the NHS in England at the cost per patient set by Kite Pharma,” said Meindert Boysen, director of the centre for health technology evaluation at NICE.
The consultation period for the draft guidance runs until September 18, 2018.
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance recommending against the use of axicabtagene ciloleucel (Yescarta) in England.
Axicabtagene ciloleucel is a chimeric antigen receptor (CAR) T-cell therapy that was just approved by the European Commission to treat patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) or primary mediastinal B-cell lymphoma (PMBCL) who have received two or more lines of systemic therapy.
However, NICE has said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy.
Additionally, the cost of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of National Health Service (NHS) resources.
NICE’s draft guidance points out that there is no standard treatment for patients with relapsed or refractory DLBCL or PMBCL who have received two or more systemic therapies. These patients receive best supportive care, which usually includes salvage chemotherapy.
Results from the ZUMA-1 trial suggest the majority of DLBCL/PMBCL patients given axicabtagene ciloleucel do respond to treatment.
However, there is no direct data comparing axicabtagene ciloleucel with salvage chemotherapy, so the benefit of the CAR T-cell therapy over chemotherapy is unknown.
The draft guidance also notes that axicabtagene ciloleucel meets NICE’s criteria to be considered a life-extending treatment at the end of life.
However, the CAR T-cell therapy cannot be considered a cost-effective use of NHS resources. The cost-effectiveness estimates for axicabtagene ciloleucel, compared with salvage chemotherapy, were above £50,000 per year of quality adjusted life gained, the upper limit of the specially extended range of cost-effectiveness for cancer treatments.
Furthermore, axicabtagene ciloleucel does not meet the criteria for inclusion in the Cancer Drugs Fund. NICE said axicabtagene ciloleucel does not have the plausible potential to be cost effective, which would be necessary for inclusion in the fund while further evidence of the treatment’s longer-term benefits is collected.
“Although promising, there is still much more we need to know about CAR-T, and, unfortunately, in this case, we are not able to recommend axicabtagene ciloleucel for use in the NHS in England at the cost per patient set by Kite Pharma,” said Meindert Boysen, director of the centre for health technology evaluation at NICE.
The consultation period for the draft guidance runs until September 18, 2018.
The National Institute for Health and Care Excellence (NICE) has issued a draft guidance recommending against the use of axicabtagene ciloleucel (Yescarta) in England.
Axicabtagene ciloleucel is a chimeric antigen receptor (CAR) T-cell therapy that was just approved by the European Commission to treat patients with relapsed/refractory diffuse large B-cell lymphoma (DLBCL) or primary mediastinal B-cell lymphoma (PMBCL) who have received two or more lines of systemic therapy.
However, NICE has said it isn’t clear how much of a benefit axicabtagene ciloleucel may provide over salvage chemotherapy.
Additionally, the cost of axicabtagene ciloleucel is too high for the therapy to be considered a cost-effective use of National Health Service (NHS) resources.
NICE’s draft guidance points out that there is no standard treatment for patients with relapsed or refractory DLBCL or PMBCL who have received two or more systemic therapies. These patients receive best supportive care, which usually includes salvage chemotherapy.
Results from the ZUMA-1 trial suggest the majority of DLBCL/PMBCL patients given axicabtagene ciloleucel do respond to treatment.
However, there is no direct data comparing axicabtagene ciloleucel with salvage chemotherapy, so the benefit of the CAR T-cell therapy over chemotherapy is unknown.
The draft guidance also notes that axicabtagene ciloleucel meets NICE’s criteria to be considered a life-extending treatment at the end of life.
However, the CAR T-cell therapy cannot be considered a cost-effective use of NHS resources. The cost-effectiveness estimates for axicabtagene ciloleucel, compared with salvage chemotherapy, were above £50,000 per year of quality adjusted life gained, the upper limit of the specially extended range of cost-effectiveness for cancer treatments.
Furthermore, axicabtagene ciloleucel does not meet the criteria for inclusion in the Cancer Drugs Fund. NICE said axicabtagene ciloleucel does not have the plausible potential to be cost effective, which would be necessary for inclusion in the fund while further evidence of the treatment’s longer-term benefits is collected.
“Although promising, there is still much more we need to know about CAR-T, and, unfortunately, in this case, we are not able to recommend axicabtagene ciloleucel for use in the NHS in England at the cost per patient set by Kite Pharma,” said Meindert Boysen, director of the centre for health technology evaluation at NICE.
The consultation period for the draft guidance runs until September 18, 2018.
European Commission approves first CAR T-cell therapies
The , two chimeric antigen receptor (CAR) T-cell therapies.
Tisagenlecleucel is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
Axicabtagene ciloleucel is approved for adults with relapsed/refractory DLBCL and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy. The treatment is marketed by Kite, a Gilead company.
The axicabtagene ciloleucel approval is based on results from the single arm, ZUMA-1 trial. During the study of 101 patients who received a single infusion, 72% responded to therapy and 51% achieved a complete response. At 1 year, median overall survival had not been reached.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
Updated results from JULIET were presented at the annual congress of the European Hematology Association in June 2018. The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so because of disease progression or clinical deterioration.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
Updated results from ELIANA were published in New England Journal of Medicine (2018;378:439-48).
The trial included 75 children and young adults with relapsed/refractory ALL. The overall remission rate was 81% (61/75), with 60% of patients (n = 45) achieving a complete remission (CR) and 21% (n = 16) achieving a CR with incomplete hematologic recovery (CRi).
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The , two chimeric antigen receptor (CAR) T-cell therapies.
Tisagenlecleucel is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
Axicabtagene ciloleucel is approved for adults with relapsed/refractory DLBCL and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy. The treatment is marketed by Kite, a Gilead company.
The axicabtagene ciloleucel approval is based on results from the single arm, ZUMA-1 trial. During the study of 101 patients who received a single infusion, 72% responded to therapy and 51% achieved a complete response. At 1 year, median overall survival had not been reached.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
Updated results from JULIET were presented at the annual congress of the European Hematology Association in June 2018. The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so because of disease progression or clinical deterioration.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
Updated results from ELIANA were published in New England Journal of Medicine (2018;378:439-48).
The trial included 75 children and young adults with relapsed/refractory ALL. The overall remission rate was 81% (61/75), with 60% of patients (n = 45) achieving a complete remission (CR) and 21% (n = 16) achieving a CR with incomplete hematologic recovery (CRi).
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
The , two chimeric antigen receptor (CAR) T-cell therapies.
Tisagenlecleucel is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
Axicabtagene ciloleucel is approved for adults with relapsed/refractory DLBCL and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy. The treatment is marketed by Kite, a Gilead company.
The axicabtagene ciloleucel approval is based on results from the single arm, ZUMA-1 trial. During the study of 101 patients who received a single infusion, 72% responded to therapy and 51% achieved a complete response. At 1 year, median overall survival had not been reached.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
Updated results from JULIET were presented at the annual congress of the European Hematology Association in June 2018. The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so because of disease progression or clinical deterioration.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
Updated results from ELIANA were published in New England Journal of Medicine (2018;378:439-48).
The trial included 75 children and young adults with relapsed/refractory ALL. The overall remission rate was 81% (61/75), with 60% of patients (n = 45) achieving a complete remission (CR) and 21% (n = 16) achieving a CR with incomplete hematologic recovery (CRi).
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
EC approves CAR T-cell therapy for DLBCL, PMBCL
The European Commission (EC) has approved the chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (Yescarta®) to treat two types of lymphoma.
Axicabtagene ciloleucel is now approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy.
The approval extends to all member countries of the European Union, as well as Norway, Iceland, and Liechtenstein.
The EC’s approval of axicabtagene ciloleucel is supported by data from the ZUMA-1 trial.
Results from this phase 2 trial were presented at the 2017 ASH Annual Meeting and published simultaneously in NEJM.
The trial enrolled 111 patients with relapsed/refractory B-cell lymphomas. There were 101 patients who received axicabtagene ciloleucel—77 with DLBCL, 8 with PMBCL, and 16 with transformed follicular lymphoma (TFL).
Patients received conditioning with low-dose cyclophosphamide and fludarabine, followed by axicabtagene ciloleucel.
The objective response rate (ORR) was 82% (n=83), and the complete response (CR) rate was 54% (n=55).
Among the DLBCL patients, the ORR was 82% (63/77), and the CR rate was 49% (38/77). In the patients with PMBCL or TFL, the ORR was 83% (20/24), and the CR rate was 71% (17/24).
With a median follow-up of 15.4 months, 42% of patients retained their response, and 40% retained a CR.
At 18 months, the overall survival rate was 52%. Most deaths were due to disease progression.
However, 2 patients died of adverse events related to axicabtagene ciloleucel, both cytokine release syndrome.
The most common grade 3 or higher adverse events were neutropenia (78%), anemia (43%), thrombocytopenia (38%), and febrile neutropenia (31%).
Grade 3 or higher cytokine release syndrome occurred in 13% of patients, and grade 3 or higher neurologic events occurred in 28%.
The European Commission (EC) has approved the chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (Yescarta®) to treat two types of lymphoma.
Axicabtagene ciloleucel is now approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy.
The approval extends to all member countries of the European Union, as well as Norway, Iceland, and Liechtenstein.
The EC’s approval of axicabtagene ciloleucel is supported by data from the ZUMA-1 trial.
Results from this phase 2 trial were presented at the 2017 ASH Annual Meeting and published simultaneously in NEJM.
The trial enrolled 111 patients with relapsed/refractory B-cell lymphomas. There were 101 patients who received axicabtagene ciloleucel—77 with DLBCL, 8 with PMBCL, and 16 with transformed follicular lymphoma (TFL).
Patients received conditioning with low-dose cyclophosphamide and fludarabine, followed by axicabtagene ciloleucel.
The objective response rate (ORR) was 82% (n=83), and the complete response (CR) rate was 54% (n=55).
Among the DLBCL patients, the ORR was 82% (63/77), and the CR rate was 49% (38/77). In the patients with PMBCL or TFL, the ORR was 83% (20/24), and the CR rate was 71% (17/24).
With a median follow-up of 15.4 months, 42% of patients retained their response, and 40% retained a CR.
At 18 months, the overall survival rate was 52%. Most deaths were due to disease progression.
However, 2 patients died of adverse events related to axicabtagene ciloleucel, both cytokine release syndrome.
The most common grade 3 or higher adverse events were neutropenia (78%), anemia (43%), thrombocytopenia (38%), and febrile neutropenia (31%).
Grade 3 or higher cytokine release syndrome occurred in 13% of patients, and grade 3 or higher neurologic events occurred in 28%.
The European Commission (EC) has approved the chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (Yescarta®) to treat two types of lymphoma.
Axicabtagene ciloleucel is now approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) and primary mediastinal large B-cell lymphoma (PMBCL) after two or more lines of systemic therapy.
The approval extends to all member countries of the European Union, as well as Norway, Iceland, and Liechtenstein.
The EC’s approval of axicabtagene ciloleucel is supported by data from the ZUMA-1 trial.
Results from this phase 2 trial were presented at the 2017 ASH Annual Meeting and published simultaneously in NEJM.
The trial enrolled 111 patients with relapsed/refractory B-cell lymphomas. There were 101 patients who received axicabtagene ciloleucel—77 with DLBCL, 8 with PMBCL, and 16 with transformed follicular lymphoma (TFL).
Patients received conditioning with low-dose cyclophosphamide and fludarabine, followed by axicabtagene ciloleucel.
The objective response rate (ORR) was 82% (n=83), and the complete response (CR) rate was 54% (n=55).
Among the DLBCL patients, the ORR was 82% (63/77), and the CR rate was 49% (38/77). In the patients with PMBCL or TFL, the ORR was 83% (20/24), and the CR rate was 71% (17/24).
With a median follow-up of 15.4 months, 42% of patients retained their response, and 40% retained a CR.
At 18 months, the overall survival rate was 52%. Most deaths were due to disease progression.
However, 2 patients died of adverse events related to axicabtagene ciloleucel, both cytokine release syndrome.
The most common grade 3 or higher adverse events were neutropenia (78%), anemia (43%), thrombocytopenia (38%), and febrile neutropenia (31%).
Grade 3 or higher cytokine release syndrome occurred in 13% of patients, and grade 3 or higher neurologic events occurred in 28%.
ctDNA predicts early outcomes in DLBCL
Measurement of circulating tumor DNA (ctDNA) could be a new and useful tool for predicting survival outcomes and response to therapy in patients with diffuse large B-cell lymphoma (DLBCL), according to authors of a recent prospective study.
Pretreatment ctDNA levels predicted 24-month event-free survival – an important disease milestone in DLBCL – as well as overall survival in the study, which included more than 200 patients at six institutions in North America and Europe.
Changes in ctDNA during treatment were prognostic for outcomes as early as 21 days into therapy, according to corresponding author Ash A. Alizadeh, MD, PhD, of Stanford (Calif.) University and his coinvestigators.
“Our data suggest that both pretreatment and dynamic assessments of ctDNA are feasible and can add to established risk factors,” Dr. Alizadeh and his coauthors reported in the Journal of Clinical Oncology.
ctDNA was detected in 98% of the 217 patients evaluated, which demonstrated the “potentially universal applicability” of this approach, they wrote in the report.
In an evaluation of pretreatment ctDNA levels, investigators found a 2.5 log haploid genome equivalents per milliliter (hGE/mL) threshold stratified patient outcomes. Event-free survival was significantly inferior at 24 months in patients with ctDNA above that threshold, with hazard ratios of 2.6 (P = .007) for frontline treatment and 2.9 (P = 0.01) for salvage.
On-treatment ctDNA levels were favorably prognostic for outcomes in patients receiving frontline therapy, according to investigators. An early molecular response (EMR), defined as a 2-log decrease in ctDNA levels after one cycle of treatment, was associated with a 24-month event-free survival of 83% versus 50% for no EMR (P = .0015).
Major molecular response (MMR), defined as a 2.5-log drop in ctDNA after two cycles of treatment, was associated with a 24-month event-free survival of 82% versus 46% for no MMR in patients on frontline therapy (P less than .001).
In one cohort of patients receiving salvage therapy, EMR also predicted superior 24-month event-free survival, according to investigators.
The EMR measure was also favorably prognostic for overall survival in both the frontline and salvage settings.
The prognostic value of measuring ctDNA was independent of International Prognostic Index (IPI) and interim PET/CT studies, results of multivariable analysis showed.
Patients had “excellent outcomes” if they had both molecular response and favorable interim PET results, and conversely, patients were at “extremely high risk” for treatment failure if they had no molecular response and a positive PET scan.
“The identification of patients at exceptionally high risk (i.e., interim PET/CT positive and not achieving EMR/MMR) could provide an opportunity for early intervention with alternative treatments, including autologous bone marrow transplantation or chimeric antigen receptor T cells,” the researchers wrote.
Patients in the study were all treated with combination immunochemotherapy according to local standards.
Dr. Alizadeh reported disclosures related to CiberMed, Forty Seven, Janssen Oncology, Celgene, Roche/Genentech, and Gilead, as well as patent filings on ctDNA detection assigned to Stanford University.
SOURCE: Kurtz DM et al. J Clin Oncol. 2018 Aug 20. doi: 10.1200/JCO.2018.78.5246.
Measurement of circulating tumor DNA (ctDNA) could be a new and useful tool for predicting survival outcomes and response to therapy in patients with diffuse large B-cell lymphoma (DLBCL), according to authors of a recent prospective study.
Pretreatment ctDNA levels predicted 24-month event-free survival – an important disease milestone in DLBCL – as well as overall survival in the study, which included more than 200 patients at six institutions in North America and Europe.
Changes in ctDNA during treatment were prognostic for outcomes as early as 21 days into therapy, according to corresponding author Ash A. Alizadeh, MD, PhD, of Stanford (Calif.) University and his coinvestigators.
“Our data suggest that both pretreatment and dynamic assessments of ctDNA are feasible and can add to established risk factors,” Dr. Alizadeh and his coauthors reported in the Journal of Clinical Oncology.
ctDNA was detected in 98% of the 217 patients evaluated, which demonstrated the “potentially universal applicability” of this approach, they wrote in the report.
In an evaluation of pretreatment ctDNA levels, investigators found a 2.5 log haploid genome equivalents per milliliter (hGE/mL) threshold stratified patient outcomes. Event-free survival was significantly inferior at 24 months in patients with ctDNA above that threshold, with hazard ratios of 2.6 (P = .007) for frontline treatment and 2.9 (P = 0.01) for salvage.
On-treatment ctDNA levels were favorably prognostic for outcomes in patients receiving frontline therapy, according to investigators. An early molecular response (EMR), defined as a 2-log decrease in ctDNA levels after one cycle of treatment, was associated with a 24-month event-free survival of 83% versus 50% for no EMR (P = .0015).
Major molecular response (MMR), defined as a 2.5-log drop in ctDNA after two cycles of treatment, was associated with a 24-month event-free survival of 82% versus 46% for no MMR in patients on frontline therapy (P less than .001).
In one cohort of patients receiving salvage therapy, EMR also predicted superior 24-month event-free survival, according to investigators.
The EMR measure was also favorably prognostic for overall survival in both the frontline and salvage settings.
The prognostic value of measuring ctDNA was independent of International Prognostic Index (IPI) and interim PET/CT studies, results of multivariable analysis showed.
Patients had “excellent outcomes” if they had both molecular response and favorable interim PET results, and conversely, patients were at “extremely high risk” for treatment failure if they had no molecular response and a positive PET scan.
“The identification of patients at exceptionally high risk (i.e., interim PET/CT positive and not achieving EMR/MMR) could provide an opportunity for early intervention with alternative treatments, including autologous bone marrow transplantation or chimeric antigen receptor T cells,” the researchers wrote.
Patients in the study were all treated with combination immunochemotherapy according to local standards.
Dr. Alizadeh reported disclosures related to CiberMed, Forty Seven, Janssen Oncology, Celgene, Roche/Genentech, and Gilead, as well as patent filings on ctDNA detection assigned to Stanford University.
SOURCE: Kurtz DM et al. J Clin Oncol. 2018 Aug 20. doi: 10.1200/JCO.2018.78.5246.
Measurement of circulating tumor DNA (ctDNA) could be a new and useful tool for predicting survival outcomes and response to therapy in patients with diffuse large B-cell lymphoma (DLBCL), according to authors of a recent prospective study.
Pretreatment ctDNA levels predicted 24-month event-free survival – an important disease milestone in DLBCL – as well as overall survival in the study, which included more than 200 patients at six institutions in North America and Europe.
Changes in ctDNA during treatment were prognostic for outcomes as early as 21 days into therapy, according to corresponding author Ash A. Alizadeh, MD, PhD, of Stanford (Calif.) University and his coinvestigators.
“Our data suggest that both pretreatment and dynamic assessments of ctDNA are feasible and can add to established risk factors,” Dr. Alizadeh and his coauthors reported in the Journal of Clinical Oncology.
ctDNA was detected in 98% of the 217 patients evaluated, which demonstrated the “potentially universal applicability” of this approach, they wrote in the report.
In an evaluation of pretreatment ctDNA levels, investigators found a 2.5 log haploid genome equivalents per milliliter (hGE/mL) threshold stratified patient outcomes. Event-free survival was significantly inferior at 24 months in patients with ctDNA above that threshold, with hazard ratios of 2.6 (P = .007) for frontline treatment and 2.9 (P = 0.01) for salvage.
On-treatment ctDNA levels were favorably prognostic for outcomes in patients receiving frontline therapy, according to investigators. An early molecular response (EMR), defined as a 2-log decrease in ctDNA levels after one cycle of treatment, was associated with a 24-month event-free survival of 83% versus 50% for no EMR (P = .0015).
Major molecular response (MMR), defined as a 2.5-log drop in ctDNA after two cycles of treatment, was associated with a 24-month event-free survival of 82% versus 46% for no MMR in patients on frontline therapy (P less than .001).
In one cohort of patients receiving salvage therapy, EMR also predicted superior 24-month event-free survival, according to investigators.
The EMR measure was also favorably prognostic for overall survival in both the frontline and salvage settings.
The prognostic value of measuring ctDNA was independent of International Prognostic Index (IPI) and interim PET/CT studies, results of multivariable analysis showed.
Patients had “excellent outcomes” if they had both molecular response and favorable interim PET results, and conversely, patients were at “extremely high risk” for treatment failure if they had no molecular response and a positive PET scan.
“The identification of patients at exceptionally high risk (i.e., interim PET/CT positive and not achieving EMR/MMR) could provide an opportunity for early intervention with alternative treatments, including autologous bone marrow transplantation or chimeric antigen receptor T cells,” the researchers wrote.
Patients in the study were all treated with combination immunochemotherapy according to local standards.
Dr. Alizadeh reported disclosures related to CiberMed, Forty Seven, Janssen Oncology, Celgene, Roche/Genentech, and Gilead, as well as patent filings on ctDNA detection assigned to Stanford University.
SOURCE: Kurtz DM et al. J Clin Oncol. 2018 Aug 20. doi: 10.1200/JCO.2018.78.5246.
FROM THE JOURNAL OF CLINICAL ONCOLOGY
Key clinical point:
Major finding: Early molecular response (a 2-log decrease in ctDNA levels after one treatment cycle) was associated with a 24-month event-free survival of 83% versus 50% for no early molecular response (P = .0015).
Study details: Prospective analysis of pretreatment and dynamic on-treatment ctDNA levels in patients with DLBCL who received standard immunochemotherapy.
Disclosures: Study authors reported disclosures related to CiberMed, Forty Seven, Janssen Oncology, Celgene, Roche/Genentech, and Gilead, among others.
Source: Kurtz DM et al. J Clin Oncol. 2018 Aug 20. doi: 10.1200/JCO.2018.78.5246.
EC approves CAR T-cell therapy for ALL, DLBCL
The European Commission (EC) has granted approval for tisagenlecleucel (Kymriah®), a chimeric antigen receptor (CAR) T-cell therapy targeting CD19.
Tisagenlecleucel (formerly CTL019) is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
The EC’s approval extends to all member countries of the European Union, as well as Norway, Iceland, and Liechtenstein.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
JULIET trial
Updated results from JULIET were presented at the 23rd Annual Congress of the European Hematology Association in June (abstract S799).
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. The median duration of response was not reached.
At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least one AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The European Commission (EC) has granted approval for tisagenlecleucel (Kymriah®), a chimeric antigen receptor (CAR) T-cell therapy targeting CD19.
Tisagenlecleucel (formerly CTL019) is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
The EC’s approval extends to all member countries of the European Union, as well as Norway, Iceland, and Liechtenstein.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
JULIET trial
Updated results from JULIET were presented at the 23rd Annual Congress of the European Hematology Association in June (abstract S799).
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. The median duration of response was not reached.
At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least one AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
The European Commission (EC) has granted approval for tisagenlecleucel (Kymriah®), a chimeric antigen receptor (CAR) T-cell therapy targeting CD19.
Tisagenlecleucel (formerly CTL019) is now approved for use in pediatric and young adult patients up to 25 years of age with B-cell acute lymphoblastic leukemia (ALL) that is refractory, in relapse post-transplant, or in second or later relapse.
Tisagenlecleucel is also approved to treat adults with relapsed or refractory diffuse large B-cell lymphoma (DLBCL) who have received two or more lines of systemic therapy.
The EC’s approval extends to all member countries of the European Union, as well as Norway, Iceland, and Liechtenstein.
Novartis expects to launch tisagenlecleucel initially for pediatric ALL. The company said timing for tisagenlecleucel availability in each country will depend on multiple factors, including the onboarding of qualified treatment centers for the appropriate indications, as well as the completion of national reimbursement procedures.
The EC’s approval of tisagenlecleucel is based on results from the phase 2 JULIET and ELIANA trials.
JULIET trial
Updated results from JULIET were presented at the 23rd Annual Congress of the European Hematology Association in June (abstract S799).
The trial enrolled 165 adults with relapsed/refractory DLBCL, and 111 of them received a single infusion of tisagenlecleucel. Most of the patients who discontinued before dosing did so due to disease progression or clinical deterioration. The patients’ median age at baseline was 56 (range, 22-76).
Ninety-two percent of patients received bridging therapy, and 93% received lymphodepleting chemotherapy prior to tisagenlecleucel.
The median time from infusion to data cutoff was 13.9 months.
The overall response rate was 52%, and the complete response (CR) rate was 40%. The median duration of response was not reached.
At the time of data cutoff, none of the responders had gone on to receive a stem cell transplant.
For all infused patients (n=111), the 12-month overall survival (OS) rate was 49%, and the median OS was 11.7 months. The median OS was not reached for patients in CR.
Within 8 weeks of tisagenlecleucel infusion, 22% of patients had developed grade 3/4 cytokine release syndrome (CRS). Fifteen percent of patients received tocilizumab for CRS, including 3% of patients with grade 2 CRS and 50% of patients with grade 3 CRS.
Other adverse events (AEs) of interest included grade 3/4 neurologic events (12%), grade 3/4 cytopenias lasting more than 28 days (32%), grade 3/4 infections (20%), and grade 3/4 febrile neutropenia (15%).
ELIANA trial
Updated results from ELIANA were published in NEJM in February.
The trial included 75 children and young adults with relapsed/refractory ALL. The patients’ median age was 11 (range, 3 to 23).
All 75 patients received a single infusion of tisagenlecleucel, and 72 received lymphodepleting chemotherapy.
The median duration of follow-up was 13.1 months. The study’s primary endpoint was overall remission rate, which was defined as the rate of a best overall response of either CR or CR with incomplete hematologic recovery (CRi) within 3 months.
The overall remission rate was 81% (61/75), with 60% of patients (n=45) achieving a CR and 21% (n=16) achieving a CRi.
All patients whose best response was CR/CRi were negative for minimal residual disease. The median duration of response was not met.
Eight patients proceeded to transplant while in remission. At last follow-up, 4 were still in remission, and 4 had unknown disease status.
At 6 months, the event-free survival rate was 73%, and the OS rate was 90%. At 12 months, the rates were 50% and 76%, respectively.
All patients experienced at least one AE, and 95% had AEs thought to be related to tisagenlecleucel. The rate of grade 3/4 AEs was 88%, and the rate of related grade 3/4 AEs was 73%.
AEs of special interest included CRS (77%), neurologic events (40%), infections (43%), febrile neutropenia (35%), cytopenias not resolved by day 28 (37%), and tumor lysis syndrome (4%).
FDA approves new use for ibrutinib in WM
The US Food and Drug Administration (FDA) has approved ibrutinib (Imbruvica®) for use in combination with rituximab to treat adults with Waldenström’s macroglobulinemia (WM).
This is the ninth FDA approval for ibrutinib and the second approval for the drug in WM.
The latest approval was supported by the phase 3 iNNOVATE trial, in which researchers compared ibrutinib plus rituximab to rituximab alone in patients with previously untreated or relapsed/refractory WM.
Results from iNNOVATE were presented at the 2018 ASCO Annual Meeting (abstract 8003) and simultaneously published in NEJM.
The trial enrolled 150 patients. They received rituximab at 375 mg/m2 with weekly infusions at weeks 1 to 4 and 17 to 20. They also received either ibrutinib (420 mg) or placebo once daily continuously until criteria for permanent discontinuation were met.
Overall response rates were significantly higher in the ibrutinib arm than the placebo arm—92% and 47%, respectively (P<0.0001). Complete response rates were 3% and 1%, respectively.
The median time to next treatment was not reached for the ibrutinib arm and was 18 months for the placebo arm (hazard ratio=0.096; P<0.0001). Of the patients randomized to ibrutinib plus rituximab, 75% continued on treatment at last follow-up.
The 30-month progression-free survival rates were 82% in the ibrutinib arm and 28% in the placebo arm. The median progression-free survival was not reached in the ibrutinib arm and was 20.3 months in the placebo arm (hazard ratio=0.20; P<0.0001).
The 30-month overall survival rates were 94% in the ibrutinib arm and 92% in the placebo arm.
Grade 3 or higher treatment-emergent adverse events (AEs) occurred in 60% of patients in the ibrutinib arm and 61% in the placebo arm. Serious AEs occurred in 43% and 33%, respectively.
There were no fatal AEs in the ibrutinib arm and 3 in the rituximab arm.
Grade 3 or higher AEs that occurred more frequently in the ibrutinib arm than the placebo arm included atrial fibrillation (12% vs 1%) and hypertension (13% vs 4%).
AEs that occurred less frequently in the ibrutinib arm than the placebo arm included grade 3 or higher infusion reactions (1% vs 16%) and any-grade IgM flare (8% vs 47%).
About ibrutinib
Ibrutinib is a Bruton’s tyrosine kinase inhibitor that is FDA-approved to treat chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), chronic graft-versus-host disease (cGVHD), and WM.
In November 2013, ibrutinib was approved to treat adults with MCL who have received at least one prior therapy.
In February 2014, ibrutinib was approved to treat adults with CLL who have received at least one prior therapy. In July 2014, ibrutinib received approval for adult CLL patients with 17p deletion, and, in March 2016, ibrutinib was approved as a frontline CLL treatment.
Ibrutinib was approved for use as a single agent in adults with WM in January 2015.
In May 2016, ibrutinib was approved in combination with bendamustine and rituximab for adults with previously treated CLL/SLL.
In January 2017, ibrutinib was approved for adults with MZL who require systemic therapy and have received at least one prior anti-CD20-based therapy.
In August 2017, ibrutinib was approved to treat adults with cGVHD that did not respond to one or more lines of systemic therapy.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
The US Food and Drug Administration (FDA) has approved ibrutinib (Imbruvica®) for use in combination with rituximab to treat adults with Waldenström’s macroglobulinemia (WM).
This is the ninth FDA approval for ibrutinib and the second approval for the drug in WM.
The latest approval was supported by the phase 3 iNNOVATE trial, in which researchers compared ibrutinib plus rituximab to rituximab alone in patients with previously untreated or relapsed/refractory WM.
Results from iNNOVATE were presented at the 2018 ASCO Annual Meeting (abstract 8003) and simultaneously published in NEJM.
The trial enrolled 150 patients. They received rituximab at 375 mg/m2 with weekly infusions at weeks 1 to 4 and 17 to 20. They also received either ibrutinib (420 mg) or placebo once daily continuously until criteria for permanent discontinuation were met.
Overall response rates were significantly higher in the ibrutinib arm than the placebo arm—92% and 47%, respectively (P<0.0001). Complete response rates were 3% and 1%, respectively.
The median time to next treatment was not reached for the ibrutinib arm and was 18 months for the placebo arm (hazard ratio=0.096; P<0.0001). Of the patients randomized to ibrutinib plus rituximab, 75% continued on treatment at last follow-up.
The 30-month progression-free survival rates were 82% in the ibrutinib arm and 28% in the placebo arm. The median progression-free survival was not reached in the ibrutinib arm and was 20.3 months in the placebo arm (hazard ratio=0.20; P<0.0001).
The 30-month overall survival rates were 94% in the ibrutinib arm and 92% in the placebo arm.
Grade 3 or higher treatment-emergent adverse events (AEs) occurred in 60% of patients in the ibrutinib arm and 61% in the placebo arm. Serious AEs occurred in 43% and 33%, respectively.
There were no fatal AEs in the ibrutinib arm and 3 in the rituximab arm.
Grade 3 or higher AEs that occurred more frequently in the ibrutinib arm than the placebo arm included atrial fibrillation (12% vs 1%) and hypertension (13% vs 4%).
AEs that occurred less frequently in the ibrutinib arm than the placebo arm included grade 3 or higher infusion reactions (1% vs 16%) and any-grade IgM flare (8% vs 47%).
About ibrutinib
Ibrutinib is a Bruton’s tyrosine kinase inhibitor that is FDA-approved to treat chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), chronic graft-versus-host disease (cGVHD), and WM.
In November 2013, ibrutinib was approved to treat adults with MCL who have received at least one prior therapy.
In February 2014, ibrutinib was approved to treat adults with CLL who have received at least one prior therapy. In July 2014, ibrutinib received approval for adult CLL patients with 17p deletion, and, in March 2016, ibrutinib was approved as a frontline CLL treatment.
Ibrutinib was approved for use as a single agent in adults with WM in January 2015.
In May 2016, ibrutinib was approved in combination with bendamustine and rituximab for adults with previously treated CLL/SLL.
In January 2017, ibrutinib was approved for adults with MZL who require systemic therapy and have received at least one prior anti-CD20-based therapy.
In August 2017, ibrutinib was approved to treat adults with cGVHD that did not respond to one or more lines of systemic therapy.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.
The US Food and Drug Administration (FDA) has approved ibrutinib (Imbruvica®) for use in combination with rituximab to treat adults with Waldenström’s macroglobulinemia (WM).
This is the ninth FDA approval for ibrutinib and the second approval for the drug in WM.
The latest approval was supported by the phase 3 iNNOVATE trial, in which researchers compared ibrutinib plus rituximab to rituximab alone in patients with previously untreated or relapsed/refractory WM.
Results from iNNOVATE were presented at the 2018 ASCO Annual Meeting (abstract 8003) and simultaneously published in NEJM.
The trial enrolled 150 patients. They received rituximab at 375 mg/m2 with weekly infusions at weeks 1 to 4 and 17 to 20. They also received either ibrutinib (420 mg) or placebo once daily continuously until criteria for permanent discontinuation were met.
Overall response rates were significantly higher in the ibrutinib arm than the placebo arm—92% and 47%, respectively (P<0.0001). Complete response rates were 3% and 1%, respectively.
The median time to next treatment was not reached for the ibrutinib arm and was 18 months for the placebo arm (hazard ratio=0.096; P<0.0001). Of the patients randomized to ibrutinib plus rituximab, 75% continued on treatment at last follow-up.
The 30-month progression-free survival rates were 82% in the ibrutinib arm and 28% in the placebo arm. The median progression-free survival was not reached in the ibrutinib arm and was 20.3 months in the placebo arm (hazard ratio=0.20; P<0.0001).
The 30-month overall survival rates were 94% in the ibrutinib arm and 92% in the placebo arm.
Grade 3 or higher treatment-emergent adverse events (AEs) occurred in 60% of patients in the ibrutinib arm and 61% in the placebo arm. Serious AEs occurred in 43% and 33%, respectively.
There were no fatal AEs in the ibrutinib arm and 3 in the rituximab arm.
Grade 3 or higher AEs that occurred more frequently in the ibrutinib arm than the placebo arm included atrial fibrillation (12% vs 1%) and hypertension (13% vs 4%).
AEs that occurred less frequently in the ibrutinib arm than the placebo arm included grade 3 or higher infusion reactions (1% vs 16%) and any-grade IgM flare (8% vs 47%).
About ibrutinib
Ibrutinib is a Bruton’s tyrosine kinase inhibitor that is FDA-approved to treat chronic lymphocytic leukemia (CLL), small lymphocytic lymphoma (SLL), mantle cell lymphoma (MCL), marginal zone lymphoma (MZL), chronic graft-versus-host disease (cGVHD), and WM.
In November 2013, ibrutinib was approved to treat adults with MCL who have received at least one prior therapy.
In February 2014, ibrutinib was approved to treat adults with CLL who have received at least one prior therapy. In July 2014, ibrutinib received approval for adult CLL patients with 17p deletion, and, in March 2016, ibrutinib was approved as a frontline CLL treatment.
Ibrutinib was approved for use as a single agent in adults with WM in January 2015.
In May 2016, ibrutinib was approved in combination with bendamustine and rituximab for adults with previously treated CLL/SLL.
In January 2017, ibrutinib was approved for adults with MZL who require systemic therapy and have received at least one prior anti-CD20-based therapy.
In August 2017, ibrutinib was approved to treat adults with cGVHD that did not respond to one or more lines of systemic therapy.
Ibrutinib is jointly developed and commercialized by Pharmacyclics LLC, an AbbVie company, and Janssen Biotech, Inc.