User login
Combo produces high response rate in CLL
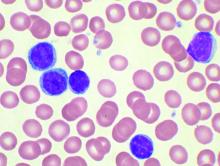
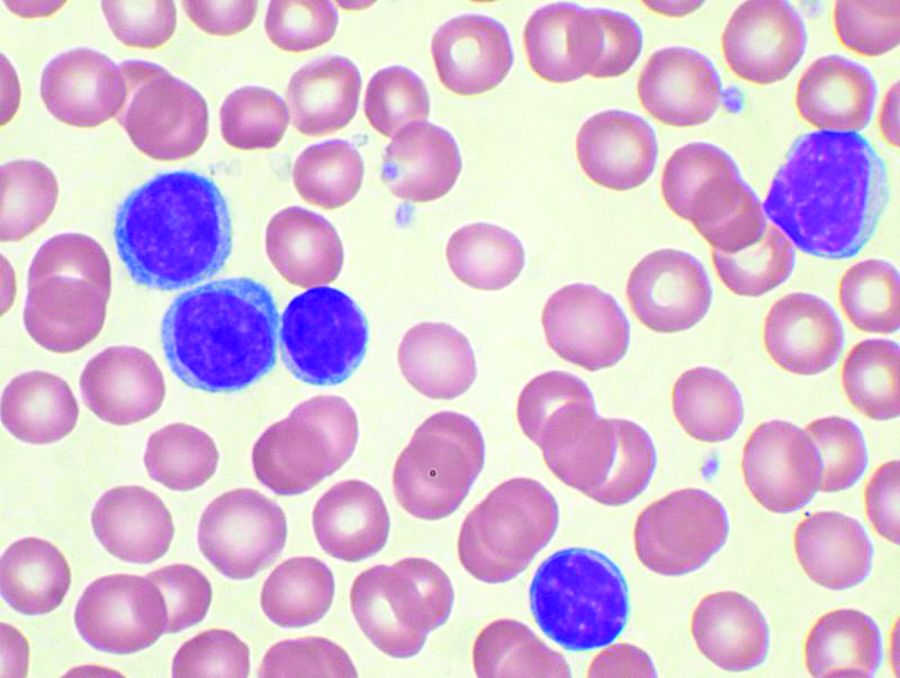
Bendamustine followed by obinutuzumab and venetoclax produces a high overall response rate in chronic lymphocytic leukemia (CLL), according to research published in The Lancet Oncology.
In an ongoing, phase 2 study, researchers examined the outcomes of this treatment in 66 patients with CLL.
Patients underwent initial debulking with two cycles of bendamustine, received six cycles of obinutuzumab and venetoclax for induction, and could receive up to 24 months of maintenance with obinutuzumab and venetoclax.
Efficacy
Of the 63 patients included in the efficacy analysis, 34 (54%) were treatment-naïve, and 29 (46%) had relapsed or refractory disease.
At the end of induction, the overall response rate was 95% (60/63), with responses observed in 100% (34/34) of treatment-naive patients and 90% (26/29) of relapsed/refractory patients.
Five patients (8%) achieved complete remission (CR)—3 who were treatment-naïve and 2 with relapsed/refractory disease.
Twenty patients (32%) had a clinical CR or CR with incomplete bone marrow recovery—14 who were treatment-naïve and 6 with relapsed/refractory disease.
Thirty-five patients (56%) had a partial response—17 who were treatment-naïve and 18 with relapsed/refractory disease.
By 15 months, both progression-free and overall survival were 100% among treatment-naive patients.
In the relapsed/refractory patients, progression-free survival was 83%, and overall survival was 90%.
Researchers observed minimal residual disease negativity in the peripheral blood of 91% (31/34) of treatment-naive patients and 83% (24/29) of relapsed/refractory patients. (Most patients did not have data for MRD in the bone marrow.)
Study author Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her colleagues described the efficacy of the combination as “encouraging.”
Safety
Safety data were available for all 66 patients. Of the 677 AEs, 427 (63%) were deemed related to study treatment, and 69 of these were serious AEs. Twelve patients had related, serious AEs during debulking, and 23 patients had related, serious AEs during induction.
The most common serious AEs were infections and cytopenias. There were four infections during debulking and 18 infections in 11 patients during induction. The most common infections were pneumonia and sepsis.
There were two cases of neutropenia during debulking and six cases in five patients during induction. There were four cases of thrombocytopenia in three patients during induction.
Other common treatment-related, serious AEs were:
- Infusion-related reactions—six cases during induction
- Coronary artery disorder—one case during debulking and three during induction
- Tumor lysis syndrome —one case during debulking and two during induction
- Neoplasms—two squamous cell carcinomas and one malignant melanoma during induction
- Increased creatinine—two cases during debulking.
Five patients in the relapsed/refractory group died—three of sepsis related to study treatment and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria, and other support from the pharmaceutical industry, including from the study sponsors.
Bendamustine followed by obinutuzumab and venetoclax produces a high overall response rate in chronic lymphocytic leukemia (CLL), according to research published in The Lancet Oncology.
In an ongoing, phase 2 study, researchers examined the outcomes of this treatment in 66 patients with CLL.
Patients underwent initial debulking with two cycles of bendamustine, received six cycles of obinutuzumab and venetoclax for induction, and could receive up to 24 months of maintenance with obinutuzumab and venetoclax.
Efficacy
Of the 63 patients included in the efficacy analysis, 34 (54%) were treatment-naïve, and 29 (46%) had relapsed or refractory disease.
At the end of induction, the overall response rate was 95% (60/63), with responses observed in 100% (34/34) of treatment-naive patients and 90% (26/29) of relapsed/refractory patients.
Five patients (8%) achieved complete remission (CR)—3 who were treatment-naïve and 2 with relapsed/refractory disease.
Twenty patients (32%) had a clinical CR or CR with incomplete bone marrow recovery—14 who were treatment-naïve and 6 with relapsed/refractory disease.
Thirty-five patients (56%) had a partial response—17 who were treatment-naïve and 18 with relapsed/refractory disease.
By 15 months, both progression-free and overall survival were 100% among treatment-naive patients.
In the relapsed/refractory patients, progression-free survival was 83%, and overall survival was 90%.
Researchers observed minimal residual disease negativity in the peripheral blood of 91% (31/34) of treatment-naive patients and 83% (24/29) of relapsed/refractory patients. (Most patients did not have data for MRD in the bone marrow.)
Study author Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her colleagues described the efficacy of the combination as “encouraging.”
Safety
Safety data were available for all 66 patients. Of the 677 AEs, 427 (63%) were deemed related to study treatment, and 69 of these were serious AEs. Twelve patients had related, serious AEs during debulking, and 23 patients had related, serious AEs during induction.
The most common serious AEs were infections and cytopenias. There were four infections during debulking and 18 infections in 11 patients during induction. The most common infections were pneumonia and sepsis.
There were two cases of neutropenia during debulking and six cases in five patients during induction. There were four cases of thrombocytopenia in three patients during induction.
Other common treatment-related, serious AEs were:
- Infusion-related reactions—six cases during induction
- Coronary artery disorder—one case during debulking and three during induction
- Tumor lysis syndrome —one case during debulking and two during induction
- Neoplasms—two squamous cell carcinomas and one malignant melanoma during induction
- Increased creatinine—two cases during debulking.
Five patients in the relapsed/refractory group died—three of sepsis related to study treatment and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria, and other support from the pharmaceutical industry, including from the study sponsors.
Bendamustine followed by obinutuzumab and venetoclax produces a high overall response rate in chronic lymphocytic leukemia (CLL), according to research published in The Lancet Oncology.
In an ongoing, phase 2 study, researchers examined the outcomes of this treatment in 66 patients with CLL.
Patients underwent initial debulking with two cycles of bendamustine, received six cycles of obinutuzumab and venetoclax for induction, and could receive up to 24 months of maintenance with obinutuzumab and venetoclax.
Efficacy
Of the 63 patients included in the efficacy analysis, 34 (54%) were treatment-naïve, and 29 (46%) had relapsed or refractory disease.
At the end of induction, the overall response rate was 95% (60/63), with responses observed in 100% (34/34) of treatment-naive patients and 90% (26/29) of relapsed/refractory patients.
Five patients (8%) achieved complete remission (CR)—3 who were treatment-naïve and 2 with relapsed/refractory disease.
Twenty patients (32%) had a clinical CR or CR with incomplete bone marrow recovery—14 who were treatment-naïve and 6 with relapsed/refractory disease.
Thirty-five patients (56%) had a partial response—17 who were treatment-naïve and 18 with relapsed/refractory disease.
By 15 months, both progression-free and overall survival were 100% among treatment-naive patients.
In the relapsed/refractory patients, progression-free survival was 83%, and overall survival was 90%.
Researchers observed minimal residual disease negativity in the peripheral blood of 91% (31/34) of treatment-naive patients and 83% (24/29) of relapsed/refractory patients. (Most patients did not have data for MRD in the bone marrow.)
Study author Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her colleagues described the efficacy of the combination as “encouraging.”
Safety
Safety data were available for all 66 patients. Of the 677 AEs, 427 (63%) were deemed related to study treatment, and 69 of these were serious AEs. Twelve patients had related, serious AEs during debulking, and 23 patients had related, serious AEs during induction.
The most common serious AEs were infections and cytopenias. There were four infections during debulking and 18 infections in 11 patients during induction. The most common infections were pneumonia and sepsis.
There were two cases of neutropenia during debulking and six cases in five patients during induction. There were four cases of thrombocytopenia in three patients during induction.
Other common treatment-related, serious AEs were:
- Infusion-related reactions—six cases during induction
- Coronary artery disorder—one case during debulking and three during induction
- Tumor lysis syndrome —one case during debulking and two during induction
- Neoplasms—two squamous cell carcinomas and one malignant melanoma during induction
- Increased creatinine—two cases during debulking.
Five patients in the relapsed/refractory group died—three of sepsis related to study treatment and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria, and other support from the pharmaceutical industry, including from the study sponsors.
Combo treatment yields MRD-negative remissions in CLL
The combination of the anti-CD20 antibody obinutuzumab and venetoclax in chronic lymphocytic leukemia shows a high overall response rate and compares favorably with established therapies, according to a new report.
The ongoing, open-label, phase 2 study examined the outcomes of six induction cycles, followed by up to 24 months of maintenance treatment with obinutuzumab and venetoclax, in 66 patients with chronic lymphocytic leukemia (CLL). Of the 63 patients included in the efficacy analysis, 34 (54%) had treatment-naive and 29 (46%) had relapsed or refractory disease.
After an initial debulking with two cycles of bendamustine, followed by the obinutuzumab and venetoclax treatment, researchers observed an overall response rate of 95%. By the end of the induction phase, all the treatment-naive patients responded, as did 90% of the relapsed or refractory patients. Five patients had achieved complete remission and 55 patients had a partial response, the researchers reported in Lancet Oncology.
By 15 months, both progression-free and overall survival was 100% among treatment-naive patients, while progression-free survival was 83% and overall survival was 90% among the relapsed or refractory patients at this point.
Researchers observed minimal residual disease (MRD) negativity in the peripheral blood of 91% of treatment-naive patients and 83% of relapsed or refractory patients.
The combination of venetoclax and obinutuzumab was chosen based on earlier trial data, which suggested a synergy between venetoclax and the less-potent anti-CD20 antibody rituximab.
Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her coauthors described the efficacy of the venetoclax and obinutuzumab combination as “encouraging.”
“The combination of venetoclax and obinutuzumab yields fast responses with MRD-negative remissions in most patients,” they wrote. “Based on the experience with venetoclax combined with rituximab in another trial and with venetoclax and obinutuzumab in this and another study, these deep, MRD-negative remissions seem to last for a substantial time after treatment termination.”
Of the 677 adverse events, 427 (63%) were deemed to be related to the study treatment, and 69 of these were serious adverse events.
The most common of these were infections, experienced by four patients during the debulking with bendamustine, and 18 cases in 11 patients during the induction treatment. This included pneumonia, sepsis and cytomegalovirus infection, as well as neutropenia and thrombocytopenia.
Six patients also experienced infusion-related reactions, four had coronary artery disorder – one during debulking and three during induction – and there were three cases of neoplasms.
Five patients in the relapsed or refractory group died; three of sepsis related to study treatment, and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria and other support from the pharmaceutical industry, including from the study sponsors.
SOURCE: Cramer P et al. Lancet Oncol. 2018 Aug 13. doi: 10.1016/S1470-2045(18)30414-5.
The combination of the anti-CD20 antibody obinutuzumab and venetoclax in chronic lymphocytic leukemia shows a high overall response rate and compares favorably with established therapies, according to a new report.
The ongoing, open-label, phase 2 study examined the outcomes of six induction cycles, followed by up to 24 months of maintenance treatment with obinutuzumab and venetoclax, in 66 patients with chronic lymphocytic leukemia (CLL). Of the 63 patients included in the efficacy analysis, 34 (54%) had treatment-naive and 29 (46%) had relapsed or refractory disease.
After an initial debulking with two cycles of bendamustine, followed by the obinutuzumab and venetoclax treatment, researchers observed an overall response rate of 95%. By the end of the induction phase, all the treatment-naive patients responded, as did 90% of the relapsed or refractory patients. Five patients had achieved complete remission and 55 patients had a partial response, the researchers reported in Lancet Oncology.
By 15 months, both progression-free and overall survival was 100% among treatment-naive patients, while progression-free survival was 83% and overall survival was 90% among the relapsed or refractory patients at this point.
Researchers observed minimal residual disease (MRD) negativity in the peripheral blood of 91% of treatment-naive patients and 83% of relapsed or refractory patients.
The combination of venetoclax and obinutuzumab was chosen based on earlier trial data, which suggested a synergy between venetoclax and the less-potent anti-CD20 antibody rituximab.
Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her coauthors described the efficacy of the venetoclax and obinutuzumab combination as “encouraging.”
“The combination of venetoclax and obinutuzumab yields fast responses with MRD-negative remissions in most patients,” they wrote. “Based on the experience with venetoclax combined with rituximab in another trial and with venetoclax and obinutuzumab in this and another study, these deep, MRD-negative remissions seem to last for a substantial time after treatment termination.”
Of the 677 adverse events, 427 (63%) were deemed to be related to the study treatment, and 69 of these were serious adverse events.
The most common of these were infections, experienced by four patients during the debulking with bendamustine, and 18 cases in 11 patients during the induction treatment. This included pneumonia, sepsis and cytomegalovirus infection, as well as neutropenia and thrombocytopenia.
Six patients also experienced infusion-related reactions, four had coronary artery disorder – one during debulking and three during induction – and there were three cases of neoplasms.
Five patients in the relapsed or refractory group died; three of sepsis related to study treatment, and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria and other support from the pharmaceutical industry, including from the study sponsors.
SOURCE: Cramer P et al. Lancet Oncol. 2018 Aug 13. doi: 10.1016/S1470-2045(18)30414-5.
The combination of the anti-CD20 antibody obinutuzumab and venetoclax in chronic lymphocytic leukemia shows a high overall response rate and compares favorably with established therapies, according to a new report.
The ongoing, open-label, phase 2 study examined the outcomes of six induction cycles, followed by up to 24 months of maintenance treatment with obinutuzumab and venetoclax, in 66 patients with chronic lymphocytic leukemia (CLL). Of the 63 patients included in the efficacy analysis, 34 (54%) had treatment-naive and 29 (46%) had relapsed or refractory disease.
After an initial debulking with two cycles of bendamustine, followed by the obinutuzumab and venetoclax treatment, researchers observed an overall response rate of 95%. By the end of the induction phase, all the treatment-naive patients responded, as did 90% of the relapsed or refractory patients. Five patients had achieved complete remission and 55 patients had a partial response, the researchers reported in Lancet Oncology.
By 15 months, both progression-free and overall survival was 100% among treatment-naive patients, while progression-free survival was 83% and overall survival was 90% among the relapsed or refractory patients at this point.
Researchers observed minimal residual disease (MRD) negativity in the peripheral blood of 91% of treatment-naive patients and 83% of relapsed or refractory patients.
The combination of venetoclax and obinutuzumab was chosen based on earlier trial data, which suggested a synergy between venetoclax and the less-potent anti-CD20 antibody rituximab.
Paula Cramer, MD, from the German CLL Study Group at University Hospital, Cologne, and her coauthors described the efficacy of the venetoclax and obinutuzumab combination as “encouraging.”
“The combination of venetoclax and obinutuzumab yields fast responses with MRD-negative remissions in most patients,” they wrote. “Based on the experience with venetoclax combined with rituximab in another trial and with venetoclax and obinutuzumab in this and another study, these deep, MRD-negative remissions seem to last for a substantial time after treatment termination.”
Of the 677 adverse events, 427 (63%) were deemed to be related to the study treatment, and 69 of these were serious adverse events.
The most common of these were infections, experienced by four patients during the debulking with bendamustine, and 18 cases in 11 patients during the induction treatment. This included pneumonia, sepsis and cytomegalovirus infection, as well as neutropenia and thrombocytopenia.
Six patients also experienced infusion-related reactions, four had coronary artery disorder – one during debulking and three during induction – and there were three cases of neoplasms.
Five patients in the relapsed or refractory group died; three of sepsis related to study treatment, and two from unrelated Richter’s transformation.
“With three deaths from sepsis in 66 enrolled patients, the treatment-related mortality seems high; however, in cases of low patient numbers, a few patients can have a substantial effect on the overall results,” the researchers wrote.
The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria and other support from the pharmaceutical industry, including from the study sponsors.
SOURCE: Cramer P et al. Lancet Oncol. 2018 Aug 13. doi: 10.1016/S1470-2045(18)30414-5.
FROM LANCET ONCOLOGY
Key clinical point:
Major finding: The overall response rate for obinutuzumab plus venetoclax in CLL was 95%.
Study details: An ongoing, phase 2, open-label trial in 66 patients with chronic lymphocytic leukemia.
Disclosures: The study was funded by F Hoffmann-La Roche and AbbVie. Several authors reported research funding, grants, honoraria, and other support from the pharmaceutical industry, including from the study sponsors.
Source: Cramer P et al. Lancet Oncol. 2018 Aug 13. doi: 10.1016/S1470-2045(18)30414-5.
Signal strength tied to potency of CAR T-cell therapy
Investigators found that chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model.
Intracellular signaling strength was a key determinant of T-cell fate in the study, which was published in Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
These findings suggest tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center in Seattle, Wash.
For this study, Salter and his colleagues used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T-cell signaling pathways, Salter said.
That overlap was somewhat surprising, according to Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor but the speed and strength of signaling, he added.
In particular, the CARs that evoked stronger signals also had increased T-cell dysfunction, decreasing their potency in the mouse model of lymphoma.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model.
By contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, Salter said.
These findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense.
“This is a modification that we think should be considered in future CAR design,” Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at efficacy, noted Stanley Riddell, MD, of Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said.
“But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics. He and Salter have filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
Investigators found that chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model.
Intracellular signaling strength was a key determinant of T-cell fate in the study, which was published in Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
These findings suggest tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center in Seattle, Wash.
For this study, Salter and his colleagues used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T-cell signaling pathways, Salter said.
That overlap was somewhat surprising, according to Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor but the speed and strength of signaling, he added.
In particular, the CARs that evoked stronger signals also had increased T-cell dysfunction, decreasing their potency in the mouse model of lymphoma.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model.
By contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, Salter said.
These findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense.
“This is a modification that we think should be considered in future CAR design,” Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at efficacy, noted Stanley Riddell, MD, of Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said.
“But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics. He and Salter have filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
Investigators found that chimeric antigen receptor (CAR) T cells with stronger signaling capabilities were less effective against lymphoma cells in a mouse model.
Intracellular signaling strength was a key determinant of T-cell fate in the study, which was published in Science Signaling.
By contrast, CAR signaling pathways could not be predicted solely by the costimulatory domains used to construct the receptor, investigators said.
These findings suggest tailoring CAR design based on signal strength might improve the efficacy and reduce the toxicity of CAR T-cell therapy, according to Alexander Salter, an MD/PhD student at Fred Hutchinson Cancer Research Center in Seattle, Wash.
For this study, Salter and his colleagues used mass spectrometry to evaluate CARs encoding CD28 or 4-1BB costimulatory domains in primary human T cells.
While CARs with CD28 domains elicited more robust intracellular signaling than those with 4-1BB domains, there was considerable overlap in activation of T-cell signaling pathways, Salter said.
That overlap was somewhat surprising, according to Salter, since researchers have generally assumed that CARs with CD28 and 4-1BB costimulatory domains will primarily signal through those respective pathways.
“No matter what costimulatory domain was encoded by the receptor, both CARs… activated both CD28 and 41BB signaling pathways,” Salter said.
The major determinant of efficacy in the study turned out to be not the domain used to construct the receptor but the speed and strength of signaling, he added.
In particular, the CARs that evoked stronger signals also had increased T-cell dysfunction, decreasing their potency in the mouse model of lymphoma.
The T cells with a CD28 CAR had very strong initial antitumor function that quickly waned in the mouse model.
By contrast, the “slower burning” 4-1BB CAR signal led to T cells that better retained their function in vivo and were associated with longer median survival in the model, Salter said.
These findings suggest tailoring CAR design based on signal strength may improve clinical efficacy and reduce toxicity.
As part of the study, Salter and his co-investigators were able to modify the CAR CD28 domain to make the signaling of the CD28 CARs less intense.
“This is a modification that we think should be considered in future CAR design,” Salter said.
While the alterations in the CD28 signaling domain were able to reduce levels of cytokines produced by T cells, the study was primarily designed to look at efficacy, noted Stanley Riddell, MD, of Fred Hutchinson Cancer Research Center.
“Our models were not set up to address the question of toxicity, so we can’t directly say this would translate to what we would see in patients,” Dr. Riddell said.
“But I think we gleaned a lot of insights as to why cytokines are produced at greater or lesser levels with various CAR designs, and insights as to how to redesign these receptors to lower the levels of cytokines they make without compromising their ability to kill.”
Dr. Riddell is a founder, shareholder, and scientific advisor of Juno Therapeutics. He and Salter have filed a patent application on the use of mutant CD28 CARs for cellular therapy. Co-author Raphael Gottardo, PhD, also with Fred Hutchinson Cancer Research Center, is a consultant for Juno Therapeutics. No other competing interests were reported.
Role of SES in childhood cancer survival disparities
Socioeconomic status (SES) may explain some racial/ethnic disparities in childhood cancer survival, according to new research.
The study showed that whites had a significant survival advantage over blacks and Hispanics for several childhood cancers.
SES significantly mediated the association between race/ethnicity and survival for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), neuroblastoma, and non-Hodgkin lymphoma (NHL).
Rebecca Kehm, PhD, of Columbia University in New York, New York, and her colleagues reported these findings in Cancer alongside a related editorial.
The researchers examined population-based cancer survival data from the Surveillance, Epidemiology, and End Results database.
The team collected information on 31,866 patients, ages 0 to 19, who were diagnosed with cancer between 2000 and 2011.
Survival differences by race/ethnicity
The researchers found that whites had a significant survival advantage over blacks for the cancers listed in the following table.
| Survival—black vs white | |||
| Cancer | Mortality hazard ratio | 95% confidence interval | P value |
| ALL | 1.43 | 1.15-1.77 | <0.01 |
| AML | 1.68 | 1.36-2.07 | <0.001 |
| Neuroblastoma | 1.38 | 1.08-1.75 | 0.01 |
| NHL | 1.53 | 1.14-2.07 | 0.01 |
| Hodgkin lymphoma | 1.66 | 1.06-2.60 | 0.03 |
| Astrocytoma | 1.95 | 1.57-2.43 | <0.001 |
| Non-astrocytoma CNS tumor | 1.53 | 1.25-1.88 | <0.001 |
| Non-rhabdomyosarcoma STS | 1.40 | 1.06-1.84 | 0.02 |
| Rhabdomyosarcoma | 1.44 | 1.10-1.88 | 0.01 |
In addition, whites had a significant survival advantage over Hispanics for the following cancers.
| Survival—Hispanic vs white | |||
| Cancer | Mortality hazard ratio | 95% confidence interval | P value |
| ALL | 1.63 | 1.43-1.86 | <0.001 |
| Neuroblastoma | 1.31 | 1.04-1.65 | 0.02 |
| NHL | 1.65 | 1.29-2.12 | <0.001 |
| Astrocytoma | 1.34 | 1.10-1.64 | <0.01 |
| Wilms tumor | 1.60 | 1.04-2.45 | 0.03 |
| Germ cell tumor | 1.63 | 1.19-2.24 | <0.01 |
Impact of SES
SES significantly mediated the association between race/ethnicity and survival for ALL, AML, neuroblastoma, and NHL but not for Hodgkin lymphoma or other cancers.
For black versus white patients, SES reduced the original association between race/ethnicity and survival by:
- 44% for ALL
- 28% for AML
- 49% for neuroblastoma
- 34% for NHL.
For Hispanics versus whites, SES reduced the original association between race/ethnicity and survival by:
- 31% for ALL
- 73% for AML
- 48% for neuroblastoma
- 28% for NHL.
“These findings provide insight for future intervention efforts aimed at closing the survival gap,” Dr Kehm said.
“For cancers in which socioeconomic status is a key factor in explaining racial and ethnic survival disparities, behavioral and supportive interventions that address social and economic barriers to effective care are warranted. However, for cancers in which survival is less influenced by socioeconomic status, more research is needed on underlying differences in tumor biology and drug processing.”
This research was supported by a grant from the National Institutes of Health, and the study’s authors made no disclosures.
Socioeconomic status (SES) may explain some racial/ethnic disparities in childhood cancer survival, according to new research.
The study showed that whites had a significant survival advantage over blacks and Hispanics for several childhood cancers.
SES significantly mediated the association between race/ethnicity and survival for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), neuroblastoma, and non-Hodgkin lymphoma (NHL).
Rebecca Kehm, PhD, of Columbia University in New York, New York, and her colleagues reported these findings in Cancer alongside a related editorial.
The researchers examined population-based cancer survival data from the Surveillance, Epidemiology, and End Results database.
The team collected information on 31,866 patients, ages 0 to 19, who were diagnosed with cancer between 2000 and 2011.
Survival differences by race/ethnicity
The researchers found that whites had a significant survival advantage over blacks for the cancers listed in the following table.
| Survival—black vs white | |||
| Cancer | Mortality hazard ratio | 95% confidence interval | P value |
| ALL | 1.43 | 1.15-1.77 | <0.01 |
| AML | 1.68 | 1.36-2.07 | <0.001 |
| Neuroblastoma | 1.38 | 1.08-1.75 | 0.01 |
| NHL | 1.53 | 1.14-2.07 | 0.01 |
| Hodgkin lymphoma | 1.66 | 1.06-2.60 | 0.03 |
| Astrocytoma | 1.95 | 1.57-2.43 | <0.001 |
| Non-astrocytoma CNS tumor | 1.53 | 1.25-1.88 | <0.001 |
| Non-rhabdomyosarcoma STS | 1.40 | 1.06-1.84 | 0.02 |
| Rhabdomyosarcoma | 1.44 | 1.10-1.88 | 0.01 |
In addition, whites had a significant survival advantage over Hispanics for the following cancers.
| Survival—Hispanic vs white | |||
| Cancer | Mortality hazard ratio | 95% confidence interval | P value |
| ALL | 1.63 | 1.43-1.86 | <0.001 |
| Neuroblastoma | 1.31 | 1.04-1.65 | 0.02 |
| NHL | 1.65 | 1.29-2.12 | <0.001 |
| Astrocytoma | 1.34 | 1.10-1.64 | <0.01 |
| Wilms tumor | 1.60 | 1.04-2.45 | 0.03 |
| Germ cell tumor | 1.63 | 1.19-2.24 | <0.01 |
Impact of SES
SES significantly mediated the association between race/ethnicity and survival for ALL, AML, neuroblastoma, and NHL but not for Hodgkin lymphoma or other cancers.
For black versus white patients, SES reduced the original association between race/ethnicity and survival by:
- 44% for ALL
- 28% for AML
- 49% for neuroblastoma
- 34% for NHL.
For Hispanics versus whites, SES reduced the original association between race/ethnicity and survival by:
- 31% for ALL
- 73% for AML
- 48% for neuroblastoma
- 28% for NHL.
“These findings provide insight for future intervention efforts aimed at closing the survival gap,” Dr Kehm said.
“For cancers in which socioeconomic status is a key factor in explaining racial and ethnic survival disparities, behavioral and supportive interventions that address social and economic barriers to effective care are warranted. However, for cancers in which survival is less influenced by socioeconomic status, more research is needed on underlying differences in tumor biology and drug processing.”
This research was supported by a grant from the National Institutes of Health, and the study’s authors made no disclosures.
Socioeconomic status (SES) may explain some racial/ethnic disparities in childhood cancer survival, according to new research.
The study showed that whites had a significant survival advantage over blacks and Hispanics for several childhood cancers.
SES significantly mediated the association between race/ethnicity and survival for acute lymphoblastic leukemia (ALL), acute myeloid leukemia (AML), neuroblastoma, and non-Hodgkin lymphoma (NHL).
Rebecca Kehm, PhD, of Columbia University in New York, New York, and her colleagues reported these findings in Cancer alongside a related editorial.
The researchers examined population-based cancer survival data from the Surveillance, Epidemiology, and End Results database.
The team collected information on 31,866 patients, ages 0 to 19, who were diagnosed with cancer between 2000 and 2011.
Survival differences by race/ethnicity
The researchers found that whites had a significant survival advantage over blacks for the cancers listed in the following table.
| Survival—black vs white | |||
| Cancer | Mortality hazard ratio | 95% confidence interval | P value |
| ALL | 1.43 | 1.15-1.77 | <0.01 |
| AML | 1.68 | 1.36-2.07 | <0.001 |
| Neuroblastoma | 1.38 | 1.08-1.75 | 0.01 |
| NHL | 1.53 | 1.14-2.07 | 0.01 |
| Hodgkin lymphoma | 1.66 | 1.06-2.60 | 0.03 |
| Astrocytoma | 1.95 | 1.57-2.43 | <0.001 |
| Non-astrocytoma CNS tumor | 1.53 | 1.25-1.88 | <0.001 |
| Non-rhabdomyosarcoma STS | 1.40 | 1.06-1.84 | 0.02 |
| Rhabdomyosarcoma | 1.44 | 1.10-1.88 | 0.01 |
In addition, whites had a significant survival advantage over Hispanics for the following cancers.
| Survival—Hispanic vs white | |||
| Cancer | Mortality hazard ratio | 95% confidence interval | P value |
| ALL | 1.63 | 1.43-1.86 | <0.001 |
| Neuroblastoma | 1.31 | 1.04-1.65 | 0.02 |
| NHL | 1.65 | 1.29-2.12 | <0.001 |
| Astrocytoma | 1.34 | 1.10-1.64 | <0.01 |
| Wilms tumor | 1.60 | 1.04-2.45 | 0.03 |
| Germ cell tumor | 1.63 | 1.19-2.24 | <0.01 |
Impact of SES
SES significantly mediated the association between race/ethnicity and survival for ALL, AML, neuroblastoma, and NHL but not for Hodgkin lymphoma or other cancers.
For black versus white patients, SES reduced the original association between race/ethnicity and survival by:
- 44% for ALL
- 28% for AML
- 49% for neuroblastoma
- 34% for NHL.
For Hispanics versus whites, SES reduced the original association between race/ethnicity and survival by:
- 31% for ALL
- 73% for AML
- 48% for neuroblastoma
- 28% for NHL.
“These findings provide insight for future intervention efforts aimed at closing the survival gap,” Dr Kehm said.
“For cancers in which socioeconomic status is a key factor in explaining racial and ethnic survival disparities, behavioral and supportive interventions that address social and economic barriers to effective care are warranted. However, for cancers in which survival is less influenced by socioeconomic status, more research is needed on underlying differences in tumor biology and drug processing.”
This research was supported by a grant from the National Institutes of Health, and the study’s authors made no disclosures.
Access to care drives disparity between urban, rural cancer patients
New research suggests that better access to quality care may reduce disparities in survival between cancer patients living in rural areas of the US and those living in urban areas.
The study showed that urban and rural cancer patients had similar survival outcomes when they were enrolled in clinical trials.
These results, published in JAMA Network Open, cast new light on decades of research showing that cancer patients living in rural areas don’t live as long as urban cancer patients.
“These findings were a surprise, since we thought we might find the same disparities others had found,” said study author Joseph Unger, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
“But clinical trials are a key difference here. In trials, patients are uniformly assessed, treated, and followed under a strict, guideline-driven protocol. This suggests that giving people with cancer access to uniform treatment strategies could help resolve the disparities in outcomes that we see between rural and urban patients.”
Dr Unger and his colleagues studied data on 36,995 patients who were enrolled in 44 phase 3 or phase 2/3 SWOG trials from 1986 through 2012. All 50 states were represented.
Patients had 17 different cancer types, including acute myeloid leukemia (AML), non-Hodgkin lymphoma (NHL), and multiple myeloma (MM).
Using US Department of Agriculture population classifications known as Rural-Urban Continuum Codes, the researchers categorized the patients as either rural or urban and analyzed their outcomes.
A minority of patients (19.4%, n=7184) were from rural locations. They were significantly more likely than urban patients to be 65 or older (P<0.001) and significantly less likely to be black (vs all other races; P<0.001).
However, there was no significant between-group difference in sex (P=0.53), and all major US geographic regions (West, Midwest, South, and Northeast) were represented.
Results
The researchers limited their analysis of survival to the first 5 years after trial enrollment to emphasize outcomes related to cancer and its treatment. They looked at overall survival (OS) as well as cancer-specific survival.
The team found no meaningful difference in OS or cancer-specific survival between rural and urban patients for 16 of the 17 cancer types.
The exception was estrogen receptor-negative, progesterone receptor-negative breast cancer. Rural patients with this cancer didn’t live as long as their urban counterparts. The hazard ratio (HR) was 1.27 (95% CI, 1.06-1.51; P=0.008) for OS and 1.26 (95% CI, 1.04-1.52; P=0.02) for cancer-specific survival.
The researchers believe this finding could be attributed to a few factors, including timely access to follow-up chemotherapy after patients’ first round of cancer treatment.
Although there were no significant survival differences for patients with hematologic malignancies, rural patients had slightly better OS if they had advanced indolent NHL or AML but slightly worse OS if they had MM or advanced aggressive NHL. The HRs were as follows:
- Advanced indolent NHL—HR=0.91 (95% CI, 0.64-1.29; P=0.60)
- AML—HR=0.94 (95% CI, 0.83-1.06; P=0.29)
- MM—HR=1.05 (95% CI, 0.93-1.18, P=0.46)
- Advanced aggressive NHL—HR=1.05 (95% CI, 0.87-1.27; P=0.60).
Rural patients had slightly better cancer-specific survival if they had advanced indolent NHL but slightly worse cancer-specific survival if they had AML, MM, or advanced aggressive NHL. The HRs were as follows:
- Advanced indolent NHL—HR=0.98 (95% CI, 0.66-1.45; P=0.90)
- AML—HR=1.01 (95% CI, 0.86-1.20; P=0.87)
- MM—HR=1.04 (95% CI, 0.90-1.20; P=0.60)
- Advanced aggressive NHL—HR=1.08 (95% CI, 0.87-1.34; P=0.50).
The researchers said these findings suggest it is access to care, and not other characteristics, that drive the survival disparities typically observed between urban and rural cancer patients.
“If people diagnosed with cancer, regardless of where they live, receive similar care and have similar outcomes, then a reasonable inference is that the best way to improve outcomes for rural patients is to improve their access to quality care,” Dr Unger said.
This research was supported by the National Cancer Institute and the HOPE Foundation. The researchers reported financial relationships with various pharmaceutical companies.
New research suggests that better access to quality care may reduce disparities in survival between cancer patients living in rural areas of the US and those living in urban areas.
The study showed that urban and rural cancer patients had similar survival outcomes when they were enrolled in clinical trials.
These results, published in JAMA Network Open, cast new light on decades of research showing that cancer patients living in rural areas don’t live as long as urban cancer patients.
“These findings were a surprise, since we thought we might find the same disparities others had found,” said study author Joseph Unger, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
“But clinical trials are a key difference here. In trials, patients are uniformly assessed, treated, and followed under a strict, guideline-driven protocol. This suggests that giving people with cancer access to uniform treatment strategies could help resolve the disparities in outcomes that we see between rural and urban patients.”
Dr Unger and his colleagues studied data on 36,995 patients who were enrolled in 44 phase 3 or phase 2/3 SWOG trials from 1986 through 2012. All 50 states were represented.
Patients had 17 different cancer types, including acute myeloid leukemia (AML), non-Hodgkin lymphoma (NHL), and multiple myeloma (MM).
Using US Department of Agriculture population classifications known as Rural-Urban Continuum Codes, the researchers categorized the patients as either rural or urban and analyzed their outcomes.
A minority of patients (19.4%, n=7184) were from rural locations. They were significantly more likely than urban patients to be 65 or older (P<0.001) and significantly less likely to be black (vs all other races; P<0.001).
However, there was no significant between-group difference in sex (P=0.53), and all major US geographic regions (West, Midwest, South, and Northeast) were represented.
Results
The researchers limited their analysis of survival to the first 5 years after trial enrollment to emphasize outcomes related to cancer and its treatment. They looked at overall survival (OS) as well as cancer-specific survival.
The team found no meaningful difference in OS or cancer-specific survival between rural and urban patients for 16 of the 17 cancer types.
The exception was estrogen receptor-negative, progesterone receptor-negative breast cancer. Rural patients with this cancer didn’t live as long as their urban counterparts. The hazard ratio (HR) was 1.27 (95% CI, 1.06-1.51; P=0.008) for OS and 1.26 (95% CI, 1.04-1.52; P=0.02) for cancer-specific survival.
The researchers believe this finding could be attributed to a few factors, including timely access to follow-up chemotherapy after patients’ first round of cancer treatment.
Although there were no significant survival differences for patients with hematologic malignancies, rural patients had slightly better OS if they had advanced indolent NHL or AML but slightly worse OS if they had MM or advanced aggressive NHL. The HRs were as follows:
- Advanced indolent NHL—HR=0.91 (95% CI, 0.64-1.29; P=0.60)
- AML—HR=0.94 (95% CI, 0.83-1.06; P=0.29)
- MM—HR=1.05 (95% CI, 0.93-1.18, P=0.46)
- Advanced aggressive NHL—HR=1.05 (95% CI, 0.87-1.27; P=0.60).
Rural patients had slightly better cancer-specific survival if they had advanced indolent NHL but slightly worse cancer-specific survival if they had AML, MM, or advanced aggressive NHL. The HRs were as follows:
- Advanced indolent NHL—HR=0.98 (95% CI, 0.66-1.45; P=0.90)
- AML—HR=1.01 (95% CI, 0.86-1.20; P=0.87)
- MM—HR=1.04 (95% CI, 0.90-1.20; P=0.60)
- Advanced aggressive NHL—HR=1.08 (95% CI, 0.87-1.34; P=0.50).
The researchers said these findings suggest it is access to care, and not other characteristics, that drive the survival disparities typically observed between urban and rural cancer patients.
“If people diagnosed with cancer, regardless of where they live, receive similar care and have similar outcomes, then a reasonable inference is that the best way to improve outcomes for rural patients is to improve their access to quality care,” Dr Unger said.
This research was supported by the National Cancer Institute and the HOPE Foundation. The researchers reported financial relationships with various pharmaceutical companies.
New research suggests that better access to quality care may reduce disparities in survival between cancer patients living in rural areas of the US and those living in urban areas.
The study showed that urban and rural cancer patients had similar survival outcomes when they were enrolled in clinical trials.
These results, published in JAMA Network Open, cast new light on decades of research showing that cancer patients living in rural areas don’t live as long as urban cancer patients.
“These findings were a surprise, since we thought we might find the same disparities others had found,” said study author Joseph Unger, PhD, of Fred Hutchinson Cancer Research Center in Seattle, Washington.
“But clinical trials are a key difference here. In trials, patients are uniformly assessed, treated, and followed under a strict, guideline-driven protocol. This suggests that giving people with cancer access to uniform treatment strategies could help resolve the disparities in outcomes that we see between rural and urban patients.”
Dr Unger and his colleagues studied data on 36,995 patients who were enrolled in 44 phase 3 or phase 2/3 SWOG trials from 1986 through 2012. All 50 states were represented.
Patients had 17 different cancer types, including acute myeloid leukemia (AML), non-Hodgkin lymphoma (NHL), and multiple myeloma (MM).
Using US Department of Agriculture population classifications known as Rural-Urban Continuum Codes, the researchers categorized the patients as either rural or urban and analyzed their outcomes.
A minority of patients (19.4%, n=7184) were from rural locations. They were significantly more likely than urban patients to be 65 or older (P<0.001) and significantly less likely to be black (vs all other races; P<0.001).
However, there was no significant between-group difference in sex (P=0.53), and all major US geographic regions (West, Midwest, South, and Northeast) were represented.
Results
The researchers limited their analysis of survival to the first 5 years after trial enrollment to emphasize outcomes related to cancer and its treatment. They looked at overall survival (OS) as well as cancer-specific survival.
The team found no meaningful difference in OS or cancer-specific survival between rural and urban patients for 16 of the 17 cancer types.
The exception was estrogen receptor-negative, progesterone receptor-negative breast cancer. Rural patients with this cancer didn’t live as long as their urban counterparts. The hazard ratio (HR) was 1.27 (95% CI, 1.06-1.51; P=0.008) for OS and 1.26 (95% CI, 1.04-1.52; P=0.02) for cancer-specific survival.
The researchers believe this finding could be attributed to a few factors, including timely access to follow-up chemotherapy after patients’ first round of cancer treatment.
Although there were no significant survival differences for patients with hematologic malignancies, rural patients had slightly better OS if they had advanced indolent NHL or AML but slightly worse OS if they had MM or advanced aggressive NHL. The HRs were as follows:
- Advanced indolent NHL—HR=0.91 (95% CI, 0.64-1.29; P=0.60)
- AML—HR=0.94 (95% CI, 0.83-1.06; P=0.29)
- MM—HR=1.05 (95% CI, 0.93-1.18, P=0.46)
- Advanced aggressive NHL—HR=1.05 (95% CI, 0.87-1.27; P=0.60).
Rural patients had slightly better cancer-specific survival if they had advanced indolent NHL but slightly worse cancer-specific survival if they had AML, MM, or advanced aggressive NHL. The HRs were as follows:
- Advanced indolent NHL—HR=0.98 (95% CI, 0.66-1.45; P=0.90)
- AML—HR=1.01 (95% CI, 0.86-1.20; P=0.87)
- MM—HR=1.04 (95% CI, 0.90-1.20; P=0.60)
- Advanced aggressive NHL—HR=1.08 (95% CI, 0.87-1.34; P=0.50).
The researchers said these findings suggest it is access to care, and not other characteristics, that drive the survival disparities typically observed between urban and rural cancer patients.
“If people diagnosed with cancer, regardless of where they live, receive similar care and have similar outcomes, then a reasonable inference is that the best way to improve outcomes for rural patients is to improve their access to quality care,” Dr Unger said.
This research was supported by the National Cancer Institute and the HOPE Foundation. The researchers reported financial relationships with various pharmaceutical companies.
Meta-analysis supports rituximab maintenance in MCL
albeit with the trade-off of higher risk of neutropenia, according to results of a meta-analysis reported in HemaSphere.
Investigators led by Liat Vidal, MD, of Tel-Aviv University, analyzed data from six randomized controlled trials of maintenance therapy including 858 patients with MCL who had a complete or partial response to induction therapy. The maintenance therapy was rituximab in five trials and bortezomib (Velcade) in one trial. The median duration of follow-up was 26-59 months across trials.
Main results showed that, compared with patients who were simply observed or given maintenance interferon-alfa, those given maintenance rituximab had a significantly reduced risk of progression or death (pooled hazard ratio, 0.58; 95% confidence interval, 0.45-0.73) and a nonsignificantly reduced risk of death (pHR, 0.79; 95% CI, 0.58-1.06).
Rituximab maintenance therapy was associated with a doubling of the risk of grade 3 or 4 neutropenia (risk ratio, 2.02; 95% CI, 1.50-2.73). However, there was no significant difference between groups with respect to risks of infection, or grade 3 or 4 anemia or thrombocythemia.
None of the included trials reported on quality of life outcomes.
The lone trial of bortezomib maintenance did not find any significant event-free survival or overall survival benefit.
“Based on our results, rituximab maintenance is recommended after immunochemotherapy with R-CHOP or cytarabine-containing induction in the front-line setting for transplant-eligible and -ineligible patients, and after R-CHOP in the relapse setting. It is unclear if maintenance is of benefit after different induction chemotherapy such as bendamustine or fludarabine,” Dr. Vidal and coauthors conclude. “By contrast, current data does not support improved outcomes with bortezomib maintenance for MCL patients.”
Dr. Vidal disclosed that she is an employee of Syneos Health. The study received no funding.
SOURCE: Vidal L et al. HemaSphere. 2018 Aug;2(4):e136.
albeit with the trade-off of higher risk of neutropenia, according to results of a meta-analysis reported in HemaSphere.
Investigators led by Liat Vidal, MD, of Tel-Aviv University, analyzed data from six randomized controlled trials of maintenance therapy including 858 patients with MCL who had a complete or partial response to induction therapy. The maintenance therapy was rituximab in five trials and bortezomib (Velcade) in one trial. The median duration of follow-up was 26-59 months across trials.
Main results showed that, compared with patients who were simply observed or given maintenance interferon-alfa, those given maintenance rituximab had a significantly reduced risk of progression or death (pooled hazard ratio, 0.58; 95% confidence interval, 0.45-0.73) and a nonsignificantly reduced risk of death (pHR, 0.79; 95% CI, 0.58-1.06).
Rituximab maintenance therapy was associated with a doubling of the risk of grade 3 or 4 neutropenia (risk ratio, 2.02; 95% CI, 1.50-2.73). However, there was no significant difference between groups with respect to risks of infection, or grade 3 or 4 anemia or thrombocythemia.
None of the included trials reported on quality of life outcomes.
The lone trial of bortezomib maintenance did not find any significant event-free survival or overall survival benefit.
“Based on our results, rituximab maintenance is recommended after immunochemotherapy with R-CHOP or cytarabine-containing induction in the front-line setting for transplant-eligible and -ineligible patients, and after R-CHOP in the relapse setting. It is unclear if maintenance is of benefit after different induction chemotherapy such as bendamustine or fludarabine,” Dr. Vidal and coauthors conclude. “By contrast, current data does not support improved outcomes with bortezomib maintenance for MCL patients.”
Dr. Vidal disclosed that she is an employee of Syneos Health. The study received no funding.
SOURCE: Vidal L et al. HemaSphere. 2018 Aug;2(4):e136.
albeit with the trade-off of higher risk of neutropenia, according to results of a meta-analysis reported in HemaSphere.
Investigators led by Liat Vidal, MD, of Tel-Aviv University, analyzed data from six randomized controlled trials of maintenance therapy including 858 patients with MCL who had a complete or partial response to induction therapy. The maintenance therapy was rituximab in five trials and bortezomib (Velcade) in one trial. The median duration of follow-up was 26-59 months across trials.
Main results showed that, compared with patients who were simply observed or given maintenance interferon-alfa, those given maintenance rituximab had a significantly reduced risk of progression or death (pooled hazard ratio, 0.58; 95% confidence interval, 0.45-0.73) and a nonsignificantly reduced risk of death (pHR, 0.79; 95% CI, 0.58-1.06).
Rituximab maintenance therapy was associated with a doubling of the risk of grade 3 or 4 neutropenia (risk ratio, 2.02; 95% CI, 1.50-2.73). However, there was no significant difference between groups with respect to risks of infection, or grade 3 or 4 anemia or thrombocythemia.
None of the included trials reported on quality of life outcomes.
The lone trial of bortezomib maintenance did not find any significant event-free survival or overall survival benefit.
“Based on our results, rituximab maintenance is recommended after immunochemotherapy with R-CHOP or cytarabine-containing induction in the front-line setting for transplant-eligible and -ineligible patients, and after R-CHOP in the relapse setting. It is unclear if maintenance is of benefit after different induction chemotherapy such as bendamustine or fludarabine,” Dr. Vidal and coauthors conclude. “By contrast, current data does not support improved outcomes with bortezomib maintenance for MCL patients.”
Dr. Vidal disclosed that she is an employee of Syneos Health. The study received no funding.
SOURCE: Vidal L et al. HemaSphere. 2018 Aug;2(4):e136.
FROM HEMASPHERE
Key clinical point: Rituximab maintenance therapy improves outcomes in patients with MCL.
Major finding: Compared with observation or maintenance interferon-alfa, maintenance rituximab was associated with reduced risk of progression-free survival events (HR, 0.58) and increased risk of grade 3 or 4 neutropenia (RR, 2.02).
Study details: A meta-analysis of six randomized controlled trials including 858 patients with MCL who had a response to induction therapy.
Disclosures: Dr. Vidal disclosed that she is an employee of Syneos Health. The study received no funding.
Source: Vidal L et al. HemaSphere. 2018 Aug;2(4):e136.
Real-world bleeding risk with ibrutinib
The Bruton tyrosine kinase inhibitor ibrutinib has been linked to a 20-fold increased risk of major bleeding in blood cancer patients taking concomitant antiplatelet and anticoagulation therapy in a clinical setting.
Caution should be used when weighing the risks and benefits of ibrutinib for patients already taking antiplatelet or anticoagulation therapy, or both, wrote Joseph Mock, MD, of the University of Virginia Health System in Charlottesville, and his colleagues.
Their report was published in Clinical Lymphoma, Myeloma & Leukemia.
Ibrutinib had been associated with an increased risk of bleeding, albeit low, in the clinical trial setting, but the authors suggested this rate could be higher in everyday clinical practice.
“Much of the information [from clinical trials] on the bleeding risk with ibrutinib, included pooled analyses, was from patients exclusively treated in clinical trials with specific exclusion criteria,” the researchers wrote. “These criteria have generally excluded patients with significant comorbidities. However, these patients are seen in clinical practice.”
The researchers conducted a review of patients treated within the University of Virginia Health System between January 2012 and May 2016.
The team identified 70 patients, with an average age of 72, who were taking ibrutinib for chronic lymphocytic leukemia (64%), mantle cell lymphoma (27%), diffuse large B-cell lymphoma (4%), lymphoblastic lymphoma (3%), and Waldenström’s macroglobulinemia (1%).
Bleeding of any grade occurred in 56% of patients, mostly grade 1-2 bruising and epistaxis.
However, major bleeding, defined as grade 3 or higher, occurred in 19% of patients (n=13). Seven of these patients were taking combined antiplatelet and anticoagulant therapy, 4 were taking antiplatelet agents alone, 1 was taking an anticoagulant agent alone, and 1 was taking only ibrutinib.
Univariate analysis showed that the factors associated with an increased risk of major bleeding were antiplatelet or anticoagulant medication, the combination of the 2 medications, interacting medications, anemia (hemoglobin less than 12 g/dL), and an elevated international normalized ratio (INR, > 1.5).
In a multivariate analysis, only the following factors were associated with an increased risk of major bleeding:
- Concomitant antiplatelet and anticoagulant use—hazard ratio=20.0 (95% CI, 2.1-200.0; P=0.0005) vs no antiplatelet/anticoagulant therapy
- Elevated INR—hazard ratio=4.6 (95% CI, 1.1-19.6; P=0.0409).
The researchers said the risk of major bleeding in patients taking both antiplatelet and anticoagulant therapy was “unacceptably high” and “medications other than ibrutinib should be considered” in this patient population.
Overall, the team said their findings confirm “the increasingly recognized risk of major bleeding complications with ibrutinib compared with what was originally reported in the clinical trial setting.”
They noted that this study was limited by the relatively small population size. Their finding that platelet count was not associated with bleeding risk was also “counterintuitive.”
The Bruton tyrosine kinase inhibitor ibrutinib has been linked to a 20-fold increased risk of major bleeding in blood cancer patients taking concomitant antiplatelet and anticoagulation therapy in a clinical setting.
Caution should be used when weighing the risks and benefits of ibrutinib for patients already taking antiplatelet or anticoagulation therapy, or both, wrote Joseph Mock, MD, of the University of Virginia Health System in Charlottesville, and his colleagues.
Their report was published in Clinical Lymphoma, Myeloma & Leukemia.
Ibrutinib had been associated with an increased risk of bleeding, albeit low, in the clinical trial setting, but the authors suggested this rate could be higher in everyday clinical practice.
“Much of the information [from clinical trials] on the bleeding risk with ibrutinib, included pooled analyses, was from patients exclusively treated in clinical trials with specific exclusion criteria,” the researchers wrote. “These criteria have generally excluded patients with significant comorbidities. However, these patients are seen in clinical practice.”
The researchers conducted a review of patients treated within the University of Virginia Health System between January 2012 and May 2016.
The team identified 70 patients, with an average age of 72, who were taking ibrutinib for chronic lymphocytic leukemia (64%), mantle cell lymphoma (27%), diffuse large B-cell lymphoma (4%), lymphoblastic lymphoma (3%), and Waldenström’s macroglobulinemia (1%).
Bleeding of any grade occurred in 56% of patients, mostly grade 1-2 bruising and epistaxis.
However, major bleeding, defined as grade 3 or higher, occurred in 19% of patients (n=13). Seven of these patients were taking combined antiplatelet and anticoagulant therapy, 4 were taking antiplatelet agents alone, 1 was taking an anticoagulant agent alone, and 1 was taking only ibrutinib.
Univariate analysis showed that the factors associated with an increased risk of major bleeding were antiplatelet or anticoagulant medication, the combination of the 2 medications, interacting medications, anemia (hemoglobin less than 12 g/dL), and an elevated international normalized ratio (INR, > 1.5).
In a multivariate analysis, only the following factors were associated with an increased risk of major bleeding:
- Concomitant antiplatelet and anticoagulant use—hazard ratio=20.0 (95% CI, 2.1-200.0; P=0.0005) vs no antiplatelet/anticoagulant therapy
- Elevated INR—hazard ratio=4.6 (95% CI, 1.1-19.6; P=0.0409).
The researchers said the risk of major bleeding in patients taking both antiplatelet and anticoagulant therapy was “unacceptably high” and “medications other than ibrutinib should be considered” in this patient population.
Overall, the team said their findings confirm “the increasingly recognized risk of major bleeding complications with ibrutinib compared with what was originally reported in the clinical trial setting.”
They noted that this study was limited by the relatively small population size. Their finding that platelet count was not associated with bleeding risk was also “counterintuitive.”
The Bruton tyrosine kinase inhibitor ibrutinib has been linked to a 20-fold increased risk of major bleeding in blood cancer patients taking concomitant antiplatelet and anticoagulation therapy in a clinical setting.
Caution should be used when weighing the risks and benefits of ibrutinib for patients already taking antiplatelet or anticoagulation therapy, or both, wrote Joseph Mock, MD, of the University of Virginia Health System in Charlottesville, and his colleagues.
Their report was published in Clinical Lymphoma, Myeloma & Leukemia.
Ibrutinib had been associated with an increased risk of bleeding, albeit low, in the clinical trial setting, but the authors suggested this rate could be higher in everyday clinical practice.
“Much of the information [from clinical trials] on the bleeding risk with ibrutinib, included pooled analyses, was from patients exclusively treated in clinical trials with specific exclusion criteria,” the researchers wrote. “These criteria have generally excluded patients with significant comorbidities. However, these patients are seen in clinical practice.”
The researchers conducted a review of patients treated within the University of Virginia Health System between January 2012 and May 2016.
The team identified 70 patients, with an average age of 72, who were taking ibrutinib for chronic lymphocytic leukemia (64%), mantle cell lymphoma (27%), diffuse large B-cell lymphoma (4%), lymphoblastic lymphoma (3%), and Waldenström’s macroglobulinemia (1%).
Bleeding of any grade occurred in 56% of patients, mostly grade 1-2 bruising and epistaxis.
However, major bleeding, defined as grade 3 or higher, occurred in 19% of patients (n=13). Seven of these patients were taking combined antiplatelet and anticoagulant therapy, 4 were taking antiplatelet agents alone, 1 was taking an anticoagulant agent alone, and 1 was taking only ibrutinib.
Univariate analysis showed that the factors associated with an increased risk of major bleeding were antiplatelet or anticoagulant medication, the combination of the 2 medications, interacting medications, anemia (hemoglobin less than 12 g/dL), and an elevated international normalized ratio (INR, > 1.5).
In a multivariate analysis, only the following factors were associated with an increased risk of major bleeding:
- Concomitant antiplatelet and anticoagulant use—hazard ratio=20.0 (95% CI, 2.1-200.0; P=0.0005) vs no antiplatelet/anticoagulant therapy
- Elevated INR—hazard ratio=4.6 (95% CI, 1.1-19.6; P=0.0409).
The researchers said the risk of major bleeding in patients taking both antiplatelet and anticoagulant therapy was “unacceptably high” and “medications other than ibrutinib should be considered” in this patient population.
Overall, the team said their findings confirm “the increasingly recognized risk of major bleeding complications with ibrutinib compared with what was originally reported in the clinical trial setting.”
They noted that this study was limited by the relatively small population size. Their finding that platelet count was not associated with bleeding risk was also “counterintuitive.”
Phase 1 CAR T trial for NHL launches in Cleveland
University Hospitals Seidman Cancer Center in Cleveland has launched a phase 1 clinical trial to study the safety of CAR T therapy for non-Hodgkin lymphoma.
The trial will enroll 12-15 adult patients with non-Hodgkin lymphoma who have not responded to standard therapies, according to a statement from University Hospitals Seidman Cancer Center.
The principal investigator for the trial will be Paolo Caimi, MD, of UH Seidman and Case Western Reserve University.
UH Seidman, affiliated with Case Western Reserve University, is one of a handful of centers that has the ability to manufacture the CAR T cells from the patient’s own genetically modified T cells on site in the shared Case Western Reserve University National Center for Regenerative Medicine and the UH Seidman Cellular Therapy Laboratory, saving time for patients.
“Having the ability to make cells on-site means there will be a shorter turnaround time in having the cells available for the patient, compared to shipping them off-site,” said Dr. Caimi in the press statement.
University Hospitals Seidman Cancer Center in Cleveland has launched a phase 1 clinical trial to study the safety of CAR T therapy for non-Hodgkin lymphoma.
The trial will enroll 12-15 adult patients with non-Hodgkin lymphoma who have not responded to standard therapies, according to a statement from University Hospitals Seidman Cancer Center.
The principal investigator for the trial will be Paolo Caimi, MD, of UH Seidman and Case Western Reserve University.
UH Seidman, affiliated with Case Western Reserve University, is one of a handful of centers that has the ability to manufacture the CAR T cells from the patient’s own genetically modified T cells on site in the shared Case Western Reserve University National Center for Regenerative Medicine and the UH Seidman Cellular Therapy Laboratory, saving time for patients.
“Having the ability to make cells on-site means there will be a shorter turnaround time in having the cells available for the patient, compared to shipping them off-site,” said Dr. Caimi in the press statement.
University Hospitals Seidman Cancer Center in Cleveland has launched a phase 1 clinical trial to study the safety of CAR T therapy for non-Hodgkin lymphoma.
The trial will enroll 12-15 adult patients with non-Hodgkin lymphoma who have not responded to standard therapies, according to a statement from University Hospitals Seidman Cancer Center.
The principal investigator for the trial will be Paolo Caimi, MD, of UH Seidman and Case Western Reserve University.
UH Seidman, affiliated with Case Western Reserve University, is one of a handful of centers that has the ability to manufacture the CAR T cells from the patient’s own genetically modified T cells on site in the shared Case Western Reserve University National Center for Regenerative Medicine and the UH Seidman Cellular Therapy Laboratory, saving time for patients.
“Having the ability to make cells on-site means there will be a shorter turnaround time in having the cells available for the patient, compared to shipping them off-site,” said Dr. Caimi in the press statement.
Key clinical point: A phase 1 trial of CAR T therapy is enrolling adult patients with NHL who have not responded to standard therapies.
Major finding: The trial site has the ability to manufacture the cells on site, saving patients time.
Study details: A phase 1 trial to evaluate safety.
Disclosures: The study will be funded by University Hospitals Seidman Cancer Center.
Caution urged over real-world bleeding risk with ibrutinib
The Bruton tyrosine kinase inhibitor ibrutinib has been linked to an almost 20-fold increased risk of major bleeding in blood cancer patients taking concomitant antiplatelet and anticoagulation therapy in a clinical setting.
Caution should be used when weighing the risks and benefits of ibrutinib for patients already taking antiplatelet or anticoagulation therapy, or both, wrote Paul R. Kunk, MD, of University of Virginia, Charlottesville, and his colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
Ibrutinib had been associated with an increased risk of bleeding, albeit low, in the clinical trial setting but the authors suggested that this rate could be higher in everyday clinical practice.
“Much of the information [from clinical trials] on the bleeding risk with ibrutinib, included pooled analyses, was from patients exclusively treated in clinical trials with specific exclusion criteria. These criteria have generally excluded patients with significant comorbidities. However, these patients are seen in clinical practice,” the researchers wrote.
They conducted a review of patients attending their center and associated regional clinics between January 2012 and May 2016. They identified 70 patients, average age 72, who were taking ibrutinib for chronic lymphocytic leukemia (64%) and mantle cell lymphoma (27%), diffuse large B-cell lymphoma (4%), lymphoblastic lymphoma (3%), and Waldenström macroglobulinemia (1%).
The analysis showed that bleeding of any grade occurred in 56% of patients, mostly grade 1-2 bruising and epistaxis. However, major bleeding, defined as grade 3, occurred in 13 patients (19%), a figure that the authors noted was greater than the rate of around 7% reported by clinical trials.
Of these patients, seven were taking combined antiplatelet and anticoagulant therapy, four were taking antiplatelets alone, one was taking an anticoagulant agent alone, and one was taking only ibrutinib.
Univariate analysis showed that the factors associated with an increased risk of major bleeding included antiplatelet or anticoagulant medication, the combination of the two medications or interacting medications, anemia (hemoglobin less than 12 g/dL) and an elevated international normalized ratio (greater than 1.5).
However, in a multivariate analysis, only combined antiplatelet and anticoagulant use (hazard ratio, 20.0; 95% confidence interval, 2.1-200.0; P less than .01) and an elevated INR (HR, 4.6; 95% CI, 1.1-19.6; P less than .01) remained statistically significant.
The researchers said the risk of major bleeding in patients taking both antiplatelet and anticoagulant therapy was “unacceptably high” and “medications other than ibrutinib should be considered” in this patient population.
Overall, they said their findings confirmed “the increasingly recognized risk of major bleeding complications with ibrutinib compared with what was originally reported in the clinical trial setting.
“As ibrutinib increases in use, it is paramount to increase awareness of the known adverse events. This is especially important given the association of ibrutinib use with atrial fibrillation,” they wrote.
They noted that their trial was limited by the relatively small population size. Their finding that platelet count was not associated with bleeding risk was also “counterintuitive,” they noted.
SOURCE: Kunk PR et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 15. doi: 10.1016/j.clml.2018.07.287.
The Bruton tyrosine kinase inhibitor ibrutinib has been linked to an almost 20-fold increased risk of major bleeding in blood cancer patients taking concomitant antiplatelet and anticoagulation therapy in a clinical setting.
Caution should be used when weighing the risks and benefits of ibrutinib for patients already taking antiplatelet or anticoagulation therapy, or both, wrote Paul R. Kunk, MD, of University of Virginia, Charlottesville, and his colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
Ibrutinib had been associated with an increased risk of bleeding, albeit low, in the clinical trial setting but the authors suggested that this rate could be higher in everyday clinical practice.
“Much of the information [from clinical trials] on the bleeding risk with ibrutinib, included pooled analyses, was from patients exclusively treated in clinical trials with specific exclusion criteria. These criteria have generally excluded patients with significant comorbidities. However, these patients are seen in clinical practice,” the researchers wrote.
They conducted a review of patients attending their center and associated regional clinics between January 2012 and May 2016. They identified 70 patients, average age 72, who were taking ibrutinib for chronic lymphocytic leukemia (64%) and mantle cell lymphoma (27%), diffuse large B-cell lymphoma (4%), lymphoblastic lymphoma (3%), and Waldenström macroglobulinemia (1%).
The analysis showed that bleeding of any grade occurred in 56% of patients, mostly grade 1-2 bruising and epistaxis. However, major bleeding, defined as grade 3, occurred in 13 patients (19%), a figure that the authors noted was greater than the rate of around 7% reported by clinical trials.
Of these patients, seven were taking combined antiplatelet and anticoagulant therapy, four were taking antiplatelets alone, one was taking an anticoagulant agent alone, and one was taking only ibrutinib.
Univariate analysis showed that the factors associated with an increased risk of major bleeding included antiplatelet or anticoagulant medication, the combination of the two medications or interacting medications, anemia (hemoglobin less than 12 g/dL) and an elevated international normalized ratio (greater than 1.5).
However, in a multivariate analysis, only combined antiplatelet and anticoagulant use (hazard ratio, 20.0; 95% confidence interval, 2.1-200.0; P less than .01) and an elevated INR (HR, 4.6; 95% CI, 1.1-19.6; P less than .01) remained statistically significant.
The researchers said the risk of major bleeding in patients taking both antiplatelet and anticoagulant therapy was “unacceptably high” and “medications other than ibrutinib should be considered” in this patient population.
Overall, they said their findings confirmed “the increasingly recognized risk of major bleeding complications with ibrutinib compared with what was originally reported in the clinical trial setting.
“As ibrutinib increases in use, it is paramount to increase awareness of the known adverse events. This is especially important given the association of ibrutinib use with atrial fibrillation,” they wrote.
They noted that their trial was limited by the relatively small population size. Their finding that platelet count was not associated with bleeding risk was also “counterintuitive,” they noted.
SOURCE: Kunk PR et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 15. doi: 10.1016/j.clml.2018.07.287.
The Bruton tyrosine kinase inhibitor ibrutinib has been linked to an almost 20-fold increased risk of major bleeding in blood cancer patients taking concomitant antiplatelet and anticoagulation therapy in a clinical setting.
Caution should be used when weighing the risks and benefits of ibrutinib for patients already taking antiplatelet or anticoagulation therapy, or both, wrote Paul R. Kunk, MD, of University of Virginia, Charlottesville, and his colleagues. Their report is in Clinical Lymphoma, Myeloma & Leukemia.
Ibrutinib had been associated with an increased risk of bleeding, albeit low, in the clinical trial setting but the authors suggested that this rate could be higher in everyday clinical practice.
“Much of the information [from clinical trials] on the bleeding risk with ibrutinib, included pooled analyses, was from patients exclusively treated in clinical trials with specific exclusion criteria. These criteria have generally excluded patients with significant comorbidities. However, these patients are seen in clinical practice,” the researchers wrote.
They conducted a review of patients attending their center and associated regional clinics between January 2012 and May 2016. They identified 70 patients, average age 72, who were taking ibrutinib for chronic lymphocytic leukemia (64%) and mantle cell lymphoma (27%), diffuse large B-cell lymphoma (4%), lymphoblastic lymphoma (3%), and Waldenström macroglobulinemia (1%).
The analysis showed that bleeding of any grade occurred in 56% of patients, mostly grade 1-2 bruising and epistaxis. However, major bleeding, defined as grade 3, occurred in 13 patients (19%), a figure that the authors noted was greater than the rate of around 7% reported by clinical trials.
Of these patients, seven were taking combined antiplatelet and anticoagulant therapy, four were taking antiplatelets alone, one was taking an anticoagulant agent alone, and one was taking only ibrutinib.
Univariate analysis showed that the factors associated with an increased risk of major bleeding included antiplatelet or anticoagulant medication, the combination of the two medications or interacting medications, anemia (hemoglobin less than 12 g/dL) and an elevated international normalized ratio (greater than 1.5).
However, in a multivariate analysis, only combined antiplatelet and anticoagulant use (hazard ratio, 20.0; 95% confidence interval, 2.1-200.0; P less than .01) and an elevated INR (HR, 4.6; 95% CI, 1.1-19.6; P less than .01) remained statistically significant.
The researchers said the risk of major bleeding in patients taking both antiplatelet and anticoagulant therapy was “unacceptably high” and “medications other than ibrutinib should be considered” in this patient population.
Overall, they said their findings confirmed “the increasingly recognized risk of major bleeding complications with ibrutinib compared with what was originally reported in the clinical trial setting.
“As ibrutinib increases in use, it is paramount to increase awareness of the known adverse events. This is especially important given the association of ibrutinib use with atrial fibrillation,” they wrote.
They noted that their trial was limited by the relatively small population size. Their finding that platelet count was not associated with bleeding risk was also “counterintuitive,” they noted.
SOURCE: Kunk PR et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 15. doi: 10.1016/j.clml.2018.07.287.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point: Clinicians should exercise caution when prescribing antiplatelet and anticoagulant medications in people taking the Bruton tyrosine kinase inhibitor ibrutinib.
Major finding: The use of both antiplatelet and anticoagulant therapy significantly increased the risk of a major bleed event (HR, 19.2; 95% CI, 2.3-166.7; P less than .01) in patients also taking ibrutinib.
Study details: A retrospective analysis of prescription data from 70 patients seen at a single U.S. cancer center and its regional clinics between January 2012 and May 2016.
Disclosures: Two of the authors reported receiving clinical trial support from Acerta and Abbvie.
Source: Kunk PR et al. Clin Lymphoma Myeloma Leuk. 2018 Jul 15. doi: 10.1016/j.clml.2018.07.287.
Auto-HSCT linked to higher AML, MDS risk
Patients undergoing autologous hematopoietic stem cell transplant (auto-HSCT) for lymphoma or myeloma have an increased risk of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), according to a retrospective study.
The study suggested these patients have 10 to 100 times the risk of AML or MDS as the general population.
The elevated risk also exceeds that of similar lymphoma and myeloma patients largely untreated with auto-HSCT.
Tomas Radivoyevitch, PhD, of the Cleveland Clinic Foundation in Ohio, and his colleagues reported these findings in Leukemia Research.
The investigators noted that exposure to DNA-damaging drugs and ionizing radiation—both used in auto-HSCT—is known to increase the risk of AML and MDS.
With this in mind, the team analyzed data on auto-HSCT recipients reported to the Center for International Blood and Marrow Transplant Research (CIBMTR).
Analyses were based on 9028 patients undergoing auto-HSCT from 1995 to 2010 for Hodgkin lymphoma (n=916), non-Hodgkin lymphoma (NHL, n=3546), or plasma cell myeloma (n=4566). Their median duration of follow-up was 90 months, 110 months, and 97 months, respectively.
Overall, 3.7% of the cohort developed AML or MDS after their transplant.
More aggressive transplant protocols increased the likelihood of this outcome. The risk of developing AML or MDS was higher for:
- Hodgkin lymphoma patients who received conditioning with total body radiation versus chemotherapy alone (hazard ratio [HR], 4.0)
- NHL patients who received conditioning with total body radiation (HR, 1.7) or with busulfan and melphalan or cyclophosphamide (HR, 1.8) versus the BEAM regimen (bischloroethylnitrosourea, etoposide, cytarabine, and melphalan)
- NHL or myeloma patients who received 3 or more lines of chemotherapy versus 1 line (HR, 1.9 for NHL and 1.8 for myeloma)
- NHL patients who underwent transplant in 2005 to 2010 versus 1995 to 1999 (HR, 2.1).
Patients reported to the Surveillance, Epidemiology and End Results database with the same lymphoma and myeloma diagnoses, few of whom underwent auto-HSCT, had risks of AML and MDS that were 5 to 10 times higher than the background level in the population.
However, the study auto-HSCT cohort had a risk of AML that was 10 to 50 times higher and a relative risk of MDS that was roughly 100 times higher than the background level.
“These increases may be related to exposure to high doses of DNA-damaging drugs given for [auto-HSCT], but this hypothesis can only be tested in a prospective study,” Dr Radivoyevitch and his coinvestigators wrote.
The reason for the greater elevation of MDS risk, compared with AML risk, is unknown.
“One possible explanation is that many cases of MDS evolve to AML, and that earlier diagnosis from increased post-transplant surveillance resulted in a deficiency of AML,” the investigators wrote. “A second is based on steeper MDS versus AML incidences versus age . . . and the possibility that transplantation recipient marrow ages (ie, marrow biological ages) are perhaps decades older than calendar ages.”
The study authors said they had no relevant conflicts of interest. The CIBMTR is supported by several US government agencies and numerous pharmaceutical companies.
Patients undergoing autologous hematopoietic stem cell transplant (auto-HSCT) for lymphoma or myeloma have an increased risk of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), according to a retrospective study.
The study suggested these patients have 10 to 100 times the risk of AML or MDS as the general population.
The elevated risk also exceeds that of similar lymphoma and myeloma patients largely untreated with auto-HSCT.
Tomas Radivoyevitch, PhD, of the Cleveland Clinic Foundation in Ohio, and his colleagues reported these findings in Leukemia Research.
The investigators noted that exposure to DNA-damaging drugs and ionizing radiation—both used in auto-HSCT—is known to increase the risk of AML and MDS.
With this in mind, the team analyzed data on auto-HSCT recipients reported to the Center for International Blood and Marrow Transplant Research (CIBMTR).
Analyses were based on 9028 patients undergoing auto-HSCT from 1995 to 2010 for Hodgkin lymphoma (n=916), non-Hodgkin lymphoma (NHL, n=3546), or plasma cell myeloma (n=4566). Their median duration of follow-up was 90 months, 110 months, and 97 months, respectively.
Overall, 3.7% of the cohort developed AML or MDS after their transplant.
More aggressive transplant protocols increased the likelihood of this outcome. The risk of developing AML or MDS was higher for:
- Hodgkin lymphoma patients who received conditioning with total body radiation versus chemotherapy alone (hazard ratio [HR], 4.0)
- NHL patients who received conditioning with total body radiation (HR, 1.7) or with busulfan and melphalan or cyclophosphamide (HR, 1.8) versus the BEAM regimen (bischloroethylnitrosourea, etoposide, cytarabine, and melphalan)
- NHL or myeloma patients who received 3 or more lines of chemotherapy versus 1 line (HR, 1.9 for NHL and 1.8 for myeloma)
- NHL patients who underwent transplant in 2005 to 2010 versus 1995 to 1999 (HR, 2.1).
Patients reported to the Surveillance, Epidemiology and End Results database with the same lymphoma and myeloma diagnoses, few of whom underwent auto-HSCT, had risks of AML and MDS that were 5 to 10 times higher than the background level in the population.
However, the study auto-HSCT cohort had a risk of AML that was 10 to 50 times higher and a relative risk of MDS that was roughly 100 times higher than the background level.
“These increases may be related to exposure to high doses of DNA-damaging drugs given for [auto-HSCT], but this hypothesis can only be tested in a prospective study,” Dr Radivoyevitch and his coinvestigators wrote.
The reason for the greater elevation of MDS risk, compared with AML risk, is unknown.
“One possible explanation is that many cases of MDS evolve to AML, and that earlier diagnosis from increased post-transplant surveillance resulted in a deficiency of AML,” the investigators wrote. “A second is based on steeper MDS versus AML incidences versus age . . . and the possibility that transplantation recipient marrow ages (ie, marrow biological ages) are perhaps decades older than calendar ages.”
The study authors said they had no relevant conflicts of interest. The CIBMTR is supported by several US government agencies and numerous pharmaceutical companies.
Patients undergoing autologous hematopoietic stem cell transplant (auto-HSCT) for lymphoma or myeloma have an increased risk of acute myeloid leukemia (AML) and myelodysplastic syndromes (MDS), according to a retrospective study.
The study suggested these patients have 10 to 100 times the risk of AML or MDS as the general population.
The elevated risk also exceeds that of similar lymphoma and myeloma patients largely untreated with auto-HSCT.
Tomas Radivoyevitch, PhD, of the Cleveland Clinic Foundation in Ohio, and his colleagues reported these findings in Leukemia Research.
The investigators noted that exposure to DNA-damaging drugs and ionizing radiation—both used in auto-HSCT—is known to increase the risk of AML and MDS.
With this in mind, the team analyzed data on auto-HSCT recipients reported to the Center for International Blood and Marrow Transplant Research (CIBMTR).
Analyses were based on 9028 patients undergoing auto-HSCT from 1995 to 2010 for Hodgkin lymphoma (n=916), non-Hodgkin lymphoma (NHL, n=3546), or plasma cell myeloma (n=4566). Their median duration of follow-up was 90 months, 110 months, and 97 months, respectively.
Overall, 3.7% of the cohort developed AML or MDS after their transplant.
More aggressive transplant protocols increased the likelihood of this outcome. The risk of developing AML or MDS was higher for:
- Hodgkin lymphoma patients who received conditioning with total body radiation versus chemotherapy alone (hazard ratio [HR], 4.0)
- NHL patients who received conditioning with total body radiation (HR, 1.7) or with busulfan and melphalan or cyclophosphamide (HR, 1.8) versus the BEAM regimen (bischloroethylnitrosourea, etoposide, cytarabine, and melphalan)
- NHL or myeloma patients who received 3 or more lines of chemotherapy versus 1 line (HR, 1.9 for NHL and 1.8 for myeloma)
- NHL patients who underwent transplant in 2005 to 2010 versus 1995 to 1999 (HR, 2.1).
Patients reported to the Surveillance, Epidemiology and End Results database with the same lymphoma and myeloma diagnoses, few of whom underwent auto-HSCT, had risks of AML and MDS that were 5 to 10 times higher than the background level in the population.
However, the study auto-HSCT cohort had a risk of AML that was 10 to 50 times higher and a relative risk of MDS that was roughly 100 times higher than the background level.
“These increases may be related to exposure to high doses of DNA-damaging drugs given for [auto-HSCT], but this hypothesis can only be tested in a prospective study,” Dr Radivoyevitch and his coinvestigators wrote.
The reason for the greater elevation of MDS risk, compared with AML risk, is unknown.
“One possible explanation is that many cases of MDS evolve to AML, and that earlier diagnosis from increased post-transplant surveillance resulted in a deficiency of AML,” the investigators wrote. “A second is based on steeper MDS versus AML incidences versus age . . . and the possibility that transplantation recipient marrow ages (ie, marrow biological ages) are perhaps decades older than calendar ages.”
The study authors said they had no relevant conflicts of interest. The CIBMTR is supported by several US government agencies and numerous pharmaceutical companies.