User login
DLBCL survivors at greater risk of autoimmune, infectious diseases
ATLANTA—A population-based study indicates that, compared to other cancer survivors, patients who survive diffuse large B-cell lymphoma (DLBCL) have an increased risk of autoimmune and infectious diseases.
For example, investigators found the risk of being diagnosed with impaired humoral immunity was 16.2 times higher in female DLBCL survivors than in breast cancer survivors, 14.8 times higher in male DLBCL survivors than in prostate cancer survivors, and 12.5 times higher in all DLBCL survivors than in survivors of head and neck cancer.
“Most of the treatments that we give for lymphoma have profound effects on the immune system, either directly or indirectly, including many of the T-cell-directed therapies,” said Tanaya Shree, MD, PhD, of Stanford University Medical Center in California.
“There have been studies on many of the effects suffered by lymphoma survivors, but very little is known about their immune health.”
Dr Shree and her colleagues undertook this study to determine how the immune system fares in lymphoma survivors. The investigators limited their analysis to survivors of DLBCL.
Dr Shree presented the findings at the 2017 ASH Annual Meeting (abstract 198*).
Study design
Investigators pulled data from the California Cancer Registry for patients with DLBCL as their first primary cancer diagnosed between 1991 and 2012. Patients had to be 18 or older at diagnosis and have survived more than a year after diagnosis.
“Importantly, we counted only diagnoses [of autoimmune and infectious diseases] that first appeared between 1 and 10 years after cancer diagnosis,” Dr Shree explained. “So any diagnosis we saw that had also been seen prior to cancer diagnosis or even up to 1 year post-cancer diagnosis, we considered to be pre-existing and were excluded from the analysis in order to really focus on new incident cases during survivorship.”
Investigators used the same criteria for the comparator cohorts.
The survivor data was linked to statewide discharge databases, and investigators performed the incidence analysis based on ICD-9 codes.
Investigators used Poisson regression analysis to obtain incident ratios and adjusted the models for age, race, and year of diagnosis.
They graphed the incident rate ratios for all the diagnoses that were significantly different between the DLBCL cohort and the comparator cohorts.
“[W]e considered a P value of less than 0.0005 to be significant,” Dr Shree clarified.
Survivor characteristics
The cohorts comprised 802,255 survivors of DLBCL (n=21,690), breast cancer (n=337,591), prostate cancer (n=325,533), melanoma (n=73,196), and head and neck cancer (n=44,245).
“At least 75% of patients in each cohort were aged 40 to 79,” Dr Shree noted, “with a good representation of elderly patients.”
The median follow-up time was 6.1 years for DLBCL patients and ranged from 5.7 years for head and neck cancer survivors to 8.3 years for prostate cancer survivors.
About three-quarters of patients in each cohort had hospitalization data within 1 to 10 years from cancer diagnosis.
DLBCL vs breast cancer
“Interestingly, we found some familiar names amongst the top-scoring diagnoses,” Dr Shree said.
Deficiency of humoral immunity (16.2-fold), autoimmune hemolytic anemia (9.9-fold), Sicca syndrome (6.9-fold), and immune thrombocytopenia (3.1-fold) were higher in female DLBCL survivors than breast cancer survivors.
“All of these have known associations with lymphoma,” Dr Shree said. “But we also found, surprisingly, increased rates of fungal [6.0-fold] and viral pneumonia [3.3-fold], and many other codes associated with respiratory infections. We also found a 3-fold increased rate of meningitis.”
“The only diagnosis statistically more common amongst breast cancer patients was cervicitis and endocervicitis, and this likely relates to the fact that many of these patients are undergoing hormone therapy.”
DLBCL vs prostate cancer
“We saw some of the same diagnoses come up as top-scoring hits, including viral [4.5-fold] and fungal pneumonia [8.2-fold], and meningitis [3.9-fold], and, in this case, Staphylococcal meningitis [8.6-fold],” Dr Shree said.
Deficiency of humoral immunity (14.8-fold), autoimmune hemolytic anemia (8.9-fold), Sicca syndrome (8.6-fold), and immune thrombocytopenia (4.8-fold) were also higher in the male DLBCL survivors than in prostate cancer survivors.
“No diagnoses were statistically more common in the prostate cancer survivors [than in male DLBCL survivors],” Dr Shree noted.
DLBCL vs head and neck cancer
“Again, the top 4 hits were the same 4 diagnoses we have been seeing repeatedly,” Dr Shree said.
Deficiency of humoral immunity (12.5-fold), autoimmune hemolytic anemia (9.3-fold), Sicca syndrome (5.5-fold), and immune thrombocytopenia (4.5-fold) were increased for DLBCL survivors compared to survivors of head and neck cancer.
DLBCL survivors also had an increased risk of respiratory infections, especially viral (4.4-fold) and fungal pneumonias (4.0-fold), meningitis (3.0-fold), and chronic lymphocytic thyroiditis (2.8-fold), also known as Hashimoto’s thyroiditis.
On the other hand, bacterial pneumonias and skin infections were more common in the head and neck cancer survivors than in DLBCL survivors.
DLBCL vs melanoma
“Interestingly, we did not see an increased risk for immune thrombocytopenias [in DLBCL survivors] compared to melanoma survivors in this comparison, which we had in all the other comparisons,” Dr Shree noted.
“But we did see [an increased risk for] the other diagnoses that we had been tracking, including, again, fungal pneumonia [6.9-fold], viral pneumonia [4.7-fold], and miscellaneous viral infections [2.6-fold].”
The only diagnosis that was statistically more common among melanoma survivors than DLBCL survivors was vitiligo.
Risks persist over time
The investigators assessed whether the elevated risks were static over the 1- to 10-year analysis period.
They took the top diagnoses—humoral deficiency, autoimmune hemolytic anemia, Sicca syndrome, and immune thrombocytopenia—and reviewed them for all cohorts to determine the rate of new cases.
“[F]or 3 out of these 4 diagnoses [humoral deficiency, autoimmune hemolytic anemia, and Sicca syndrome], increased incident rates are highest in the first 1 to 3 years after diagnosis in the lymphoma patients,” Dr Shree said.
“But even at 5 to 10 years out, these patients continue to have increased incidence of these diagnoses compared to the other cohorts, suggesting that these risks really do remain elevated over some time.”
The investigators repeated the analysis using broader categories of diagnoses with each category encompassing many ICD-9 codes.
“[I]n 12 out of 18 broad categories that we looked at, we can still find statistically significant differences in the incident rates for these diagnoses, and they were all increased in the lymphoma patients compared to the other cohorts,” Dr Shree explained.
“[T]hese increases were seen across multiple comparisons, suggesting that this phenomenon seems to be really lymphoma-specific and not specific to any of the individual comparisons we had chosen to perform.”
The findings, she said, have a lot of implications.
“We are particularly interested in which features of patients’ treatment contribute most to these elevated risks,” Dr Shree said. “And, of course, we want to know what to be able to tell our patients and how to follow them during survivorship.”
The investigators are currently validating their findings with further analysis of the Stanford lymphoma survivors cohort of approximately 3500 patients. ![]()
*Data in the abstract differ from the presentation.
ATLANTA—A population-based study indicates that, compared to other cancer survivors, patients who survive diffuse large B-cell lymphoma (DLBCL) have an increased risk of autoimmune and infectious diseases.
For example, investigators found the risk of being diagnosed with impaired humoral immunity was 16.2 times higher in female DLBCL survivors than in breast cancer survivors, 14.8 times higher in male DLBCL survivors than in prostate cancer survivors, and 12.5 times higher in all DLBCL survivors than in survivors of head and neck cancer.
“Most of the treatments that we give for lymphoma have profound effects on the immune system, either directly or indirectly, including many of the T-cell-directed therapies,” said Tanaya Shree, MD, PhD, of Stanford University Medical Center in California.
“There have been studies on many of the effects suffered by lymphoma survivors, but very little is known about their immune health.”
Dr Shree and her colleagues undertook this study to determine how the immune system fares in lymphoma survivors. The investigators limited their analysis to survivors of DLBCL.
Dr Shree presented the findings at the 2017 ASH Annual Meeting (abstract 198*).
Study design
Investigators pulled data from the California Cancer Registry for patients with DLBCL as their first primary cancer diagnosed between 1991 and 2012. Patients had to be 18 or older at diagnosis and have survived more than a year after diagnosis.
“Importantly, we counted only diagnoses [of autoimmune and infectious diseases] that first appeared between 1 and 10 years after cancer diagnosis,” Dr Shree explained. “So any diagnosis we saw that had also been seen prior to cancer diagnosis or even up to 1 year post-cancer diagnosis, we considered to be pre-existing and were excluded from the analysis in order to really focus on new incident cases during survivorship.”
Investigators used the same criteria for the comparator cohorts.
The survivor data was linked to statewide discharge databases, and investigators performed the incidence analysis based on ICD-9 codes.
Investigators used Poisson regression analysis to obtain incident ratios and adjusted the models for age, race, and year of diagnosis.
They graphed the incident rate ratios for all the diagnoses that were significantly different between the DLBCL cohort and the comparator cohorts.
“[W]e considered a P value of less than 0.0005 to be significant,” Dr Shree clarified.
Survivor characteristics
The cohorts comprised 802,255 survivors of DLBCL (n=21,690), breast cancer (n=337,591), prostate cancer (n=325,533), melanoma (n=73,196), and head and neck cancer (n=44,245).
“At least 75% of patients in each cohort were aged 40 to 79,” Dr Shree noted, “with a good representation of elderly patients.”
The median follow-up time was 6.1 years for DLBCL patients and ranged from 5.7 years for head and neck cancer survivors to 8.3 years for prostate cancer survivors.
About three-quarters of patients in each cohort had hospitalization data within 1 to 10 years from cancer diagnosis.
DLBCL vs breast cancer
“Interestingly, we found some familiar names amongst the top-scoring diagnoses,” Dr Shree said.
Deficiency of humoral immunity (16.2-fold), autoimmune hemolytic anemia (9.9-fold), Sicca syndrome (6.9-fold), and immune thrombocytopenia (3.1-fold) were higher in female DLBCL survivors than breast cancer survivors.
“All of these have known associations with lymphoma,” Dr Shree said. “But we also found, surprisingly, increased rates of fungal [6.0-fold] and viral pneumonia [3.3-fold], and many other codes associated with respiratory infections. We also found a 3-fold increased rate of meningitis.”
“The only diagnosis statistically more common amongst breast cancer patients was cervicitis and endocervicitis, and this likely relates to the fact that many of these patients are undergoing hormone therapy.”
DLBCL vs prostate cancer
“We saw some of the same diagnoses come up as top-scoring hits, including viral [4.5-fold] and fungal pneumonia [8.2-fold], and meningitis [3.9-fold], and, in this case, Staphylococcal meningitis [8.6-fold],” Dr Shree said.
Deficiency of humoral immunity (14.8-fold), autoimmune hemolytic anemia (8.9-fold), Sicca syndrome (8.6-fold), and immune thrombocytopenia (4.8-fold) were also higher in the male DLBCL survivors than in prostate cancer survivors.
“No diagnoses were statistically more common in the prostate cancer survivors [than in male DLBCL survivors],” Dr Shree noted.
DLBCL vs head and neck cancer
“Again, the top 4 hits were the same 4 diagnoses we have been seeing repeatedly,” Dr Shree said.
Deficiency of humoral immunity (12.5-fold), autoimmune hemolytic anemia (9.3-fold), Sicca syndrome (5.5-fold), and immune thrombocytopenia (4.5-fold) were increased for DLBCL survivors compared to survivors of head and neck cancer.
DLBCL survivors also had an increased risk of respiratory infections, especially viral (4.4-fold) and fungal pneumonias (4.0-fold), meningitis (3.0-fold), and chronic lymphocytic thyroiditis (2.8-fold), also known as Hashimoto’s thyroiditis.
On the other hand, bacterial pneumonias and skin infections were more common in the head and neck cancer survivors than in DLBCL survivors.
DLBCL vs melanoma
“Interestingly, we did not see an increased risk for immune thrombocytopenias [in DLBCL survivors] compared to melanoma survivors in this comparison, which we had in all the other comparisons,” Dr Shree noted.
“But we did see [an increased risk for] the other diagnoses that we had been tracking, including, again, fungal pneumonia [6.9-fold], viral pneumonia [4.7-fold], and miscellaneous viral infections [2.6-fold].”
The only diagnosis that was statistically more common among melanoma survivors than DLBCL survivors was vitiligo.
Risks persist over time
The investigators assessed whether the elevated risks were static over the 1- to 10-year analysis period.
They took the top diagnoses—humoral deficiency, autoimmune hemolytic anemia, Sicca syndrome, and immune thrombocytopenia—and reviewed them for all cohorts to determine the rate of new cases.
“[F]or 3 out of these 4 diagnoses [humoral deficiency, autoimmune hemolytic anemia, and Sicca syndrome], increased incident rates are highest in the first 1 to 3 years after diagnosis in the lymphoma patients,” Dr Shree said.
“But even at 5 to 10 years out, these patients continue to have increased incidence of these diagnoses compared to the other cohorts, suggesting that these risks really do remain elevated over some time.”
The investigators repeated the analysis using broader categories of diagnoses with each category encompassing many ICD-9 codes.
“[I]n 12 out of 18 broad categories that we looked at, we can still find statistically significant differences in the incident rates for these diagnoses, and they were all increased in the lymphoma patients compared to the other cohorts,” Dr Shree explained.
“[T]hese increases were seen across multiple comparisons, suggesting that this phenomenon seems to be really lymphoma-specific and not specific to any of the individual comparisons we had chosen to perform.”
The findings, she said, have a lot of implications.
“We are particularly interested in which features of patients’ treatment contribute most to these elevated risks,” Dr Shree said. “And, of course, we want to know what to be able to tell our patients and how to follow them during survivorship.”
The investigators are currently validating their findings with further analysis of the Stanford lymphoma survivors cohort of approximately 3500 patients. ![]()
*Data in the abstract differ from the presentation.
ATLANTA—A population-based study indicates that, compared to other cancer survivors, patients who survive diffuse large B-cell lymphoma (DLBCL) have an increased risk of autoimmune and infectious diseases.
For example, investigators found the risk of being diagnosed with impaired humoral immunity was 16.2 times higher in female DLBCL survivors than in breast cancer survivors, 14.8 times higher in male DLBCL survivors than in prostate cancer survivors, and 12.5 times higher in all DLBCL survivors than in survivors of head and neck cancer.
“Most of the treatments that we give for lymphoma have profound effects on the immune system, either directly or indirectly, including many of the T-cell-directed therapies,” said Tanaya Shree, MD, PhD, of Stanford University Medical Center in California.
“There have been studies on many of the effects suffered by lymphoma survivors, but very little is known about their immune health.”
Dr Shree and her colleagues undertook this study to determine how the immune system fares in lymphoma survivors. The investigators limited their analysis to survivors of DLBCL.
Dr Shree presented the findings at the 2017 ASH Annual Meeting (abstract 198*).
Study design
Investigators pulled data from the California Cancer Registry for patients with DLBCL as their first primary cancer diagnosed between 1991 and 2012. Patients had to be 18 or older at diagnosis and have survived more than a year after diagnosis.
“Importantly, we counted only diagnoses [of autoimmune and infectious diseases] that first appeared between 1 and 10 years after cancer diagnosis,” Dr Shree explained. “So any diagnosis we saw that had also been seen prior to cancer diagnosis or even up to 1 year post-cancer diagnosis, we considered to be pre-existing and were excluded from the analysis in order to really focus on new incident cases during survivorship.”
Investigators used the same criteria for the comparator cohorts.
The survivor data was linked to statewide discharge databases, and investigators performed the incidence analysis based on ICD-9 codes.
Investigators used Poisson regression analysis to obtain incident ratios and adjusted the models for age, race, and year of diagnosis.
They graphed the incident rate ratios for all the diagnoses that were significantly different between the DLBCL cohort and the comparator cohorts.
“[W]e considered a P value of less than 0.0005 to be significant,” Dr Shree clarified.
Survivor characteristics
The cohorts comprised 802,255 survivors of DLBCL (n=21,690), breast cancer (n=337,591), prostate cancer (n=325,533), melanoma (n=73,196), and head and neck cancer (n=44,245).
“At least 75% of patients in each cohort were aged 40 to 79,” Dr Shree noted, “with a good representation of elderly patients.”
The median follow-up time was 6.1 years for DLBCL patients and ranged from 5.7 years for head and neck cancer survivors to 8.3 years for prostate cancer survivors.
About three-quarters of patients in each cohort had hospitalization data within 1 to 10 years from cancer diagnosis.
DLBCL vs breast cancer
“Interestingly, we found some familiar names amongst the top-scoring diagnoses,” Dr Shree said.
Deficiency of humoral immunity (16.2-fold), autoimmune hemolytic anemia (9.9-fold), Sicca syndrome (6.9-fold), and immune thrombocytopenia (3.1-fold) were higher in female DLBCL survivors than breast cancer survivors.
“All of these have known associations with lymphoma,” Dr Shree said. “But we also found, surprisingly, increased rates of fungal [6.0-fold] and viral pneumonia [3.3-fold], and many other codes associated with respiratory infections. We also found a 3-fold increased rate of meningitis.”
“The only diagnosis statistically more common amongst breast cancer patients was cervicitis and endocervicitis, and this likely relates to the fact that many of these patients are undergoing hormone therapy.”
DLBCL vs prostate cancer
“We saw some of the same diagnoses come up as top-scoring hits, including viral [4.5-fold] and fungal pneumonia [8.2-fold], and meningitis [3.9-fold], and, in this case, Staphylococcal meningitis [8.6-fold],” Dr Shree said.
Deficiency of humoral immunity (14.8-fold), autoimmune hemolytic anemia (8.9-fold), Sicca syndrome (8.6-fold), and immune thrombocytopenia (4.8-fold) were also higher in the male DLBCL survivors than in prostate cancer survivors.
“No diagnoses were statistically more common in the prostate cancer survivors [than in male DLBCL survivors],” Dr Shree noted.
DLBCL vs head and neck cancer
“Again, the top 4 hits were the same 4 diagnoses we have been seeing repeatedly,” Dr Shree said.
Deficiency of humoral immunity (12.5-fold), autoimmune hemolytic anemia (9.3-fold), Sicca syndrome (5.5-fold), and immune thrombocytopenia (4.5-fold) were increased for DLBCL survivors compared to survivors of head and neck cancer.
DLBCL survivors also had an increased risk of respiratory infections, especially viral (4.4-fold) and fungal pneumonias (4.0-fold), meningitis (3.0-fold), and chronic lymphocytic thyroiditis (2.8-fold), also known as Hashimoto’s thyroiditis.
On the other hand, bacterial pneumonias and skin infections were more common in the head and neck cancer survivors than in DLBCL survivors.
DLBCL vs melanoma
“Interestingly, we did not see an increased risk for immune thrombocytopenias [in DLBCL survivors] compared to melanoma survivors in this comparison, which we had in all the other comparisons,” Dr Shree noted.
“But we did see [an increased risk for] the other diagnoses that we had been tracking, including, again, fungal pneumonia [6.9-fold], viral pneumonia [4.7-fold], and miscellaneous viral infections [2.6-fold].”
The only diagnosis that was statistically more common among melanoma survivors than DLBCL survivors was vitiligo.
Risks persist over time
The investigators assessed whether the elevated risks were static over the 1- to 10-year analysis period.
They took the top diagnoses—humoral deficiency, autoimmune hemolytic anemia, Sicca syndrome, and immune thrombocytopenia—and reviewed them for all cohorts to determine the rate of new cases.
“[F]or 3 out of these 4 diagnoses [humoral deficiency, autoimmune hemolytic anemia, and Sicca syndrome], increased incident rates are highest in the first 1 to 3 years after diagnosis in the lymphoma patients,” Dr Shree said.
“But even at 5 to 10 years out, these patients continue to have increased incidence of these diagnoses compared to the other cohorts, suggesting that these risks really do remain elevated over some time.”
The investigators repeated the analysis using broader categories of diagnoses with each category encompassing many ICD-9 codes.
“[I]n 12 out of 18 broad categories that we looked at, we can still find statistically significant differences in the incident rates for these diagnoses, and they were all increased in the lymphoma patients compared to the other cohorts,” Dr Shree explained.
“[T]hese increases were seen across multiple comparisons, suggesting that this phenomenon seems to be really lymphoma-specific and not specific to any of the individual comparisons we had chosen to perform.”
The findings, she said, have a lot of implications.
“We are particularly interested in which features of patients’ treatment contribute most to these elevated risks,” Dr Shree said. “And, of course, we want to know what to be able to tell our patients and how to follow them during survivorship.”
The investigators are currently validating their findings with further analysis of the Stanford lymphoma survivors cohort of approximately 3500 patients. ![]()
*Data in the abstract differ from the presentation.
Brentuximab vedotin sBLA receives priority review
The US Food and Drug Administration (FDA) has accepted for priority review a supplemental biologics license application (sBLA) for brentuximab vedotin (ADCETRIS).
With this sBLA, Seattle Genetics, Inc., is seeking approval for brentuximab vedotin in combination with chemotherapy for frontline treatment of patients with advanced classical Hodgkin lymphoma (HL).
The FDA expects to make a decision on the sBLA by May 1, 2018.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The priority review for this sBLA is based on positive results from the phase 3 ECHELON-1 trial.
The FDA previously granted brentuximab vedotin breakthrough therapy designation based on ECHELON-1 results.
Breakthrough therapy designation is intended to expedite the development and review of promising drug candidates for serious or life-threatening conditions. It is based upon clinical evidence of substantial improvement over existing therapies in one or more clinically significant endpoints.
ECHELON-1
Result from ECHELON-1 were presented at the 2017 ASH Annual Meeting and simultaneously published in NEJM.
In this trial, researchers compared brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for 1334 patients with advanced HL.
The study’s primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review facility, A+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm.
There was no significant difference between the treatment arms when it came to response rates or overall survival.
The objective response rate was 86% in the A+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The interim 2-year overall survival rate was 97% in the A+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
The overall incidence of adverse events (AEs) was 99% in the A+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with A+AVD, while pulmonary toxicity was more common with ABVD.
About brentuximab vedotin
Brentuximab vedotin is already FDA-approved to treat adults with:
- Classical HL who have failed autologous hematopoietic stem cell transplant (auto-HSCT) or, in those who are not auto-HSCT candidates, have failed at least 2 prior multi-agent chemotherapy regimens.
- Classical HL at high risk of relapse or progression as post-auto-HSCT consolidation.
- Primary cutaneous anaplastic large-cell lymphoma (ALCL) or CD30-expressing mycosis fungoides who have received prior systemic therapy.
- Systemic ALCL who have failed at least 1 prior multi-agent chemotherapy regimen. (The drug has accelerated approval for this indication, based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.)

The US Food and Drug Administration (FDA) has accepted for priority review a supplemental biologics license application (sBLA) for brentuximab vedotin (ADCETRIS).
With this sBLA, Seattle Genetics, Inc., is seeking approval for brentuximab vedotin in combination with chemotherapy for frontline treatment of patients with advanced classical Hodgkin lymphoma (HL).
The FDA expects to make a decision on the sBLA by May 1, 2018.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The priority review for this sBLA is based on positive results from the phase 3 ECHELON-1 trial.
The FDA previously granted brentuximab vedotin breakthrough therapy designation based on ECHELON-1 results.
Breakthrough therapy designation is intended to expedite the development and review of promising drug candidates for serious or life-threatening conditions. It is based upon clinical evidence of substantial improvement over existing therapies in one or more clinically significant endpoints.
ECHELON-1
Result from ECHELON-1 were presented at the 2017 ASH Annual Meeting and simultaneously published in NEJM.
In this trial, researchers compared brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for 1334 patients with advanced HL.
The study’s primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review facility, A+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm.
There was no significant difference between the treatment arms when it came to response rates or overall survival.
The objective response rate was 86% in the A+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The interim 2-year overall survival rate was 97% in the A+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
The overall incidence of adverse events (AEs) was 99% in the A+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with A+AVD, while pulmonary toxicity was more common with ABVD.
About brentuximab vedotin
Brentuximab vedotin is already FDA-approved to treat adults with:
- Classical HL who have failed autologous hematopoietic stem cell transplant (auto-HSCT) or, in those who are not auto-HSCT candidates, have failed at least 2 prior multi-agent chemotherapy regimens.
- Classical HL at high risk of relapse or progression as post-auto-HSCT consolidation.
- Primary cutaneous anaplastic large-cell lymphoma (ALCL) or CD30-expressing mycosis fungoides who have received prior systemic therapy.
- Systemic ALCL who have failed at least 1 prior multi-agent chemotherapy regimen. (The drug has accelerated approval for this indication, based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.)

The US Food and Drug Administration (FDA) has accepted for priority review a supplemental biologics license application (sBLA) for brentuximab vedotin (ADCETRIS).
With this sBLA, Seattle Genetics, Inc., is seeking approval for brentuximab vedotin in combination with chemotherapy for frontline treatment of patients with advanced classical Hodgkin lymphoma (HL).
The FDA expects to make a decision on the sBLA by May 1, 2018.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The priority review for this sBLA is based on positive results from the phase 3 ECHELON-1 trial.
The FDA previously granted brentuximab vedotin breakthrough therapy designation based on ECHELON-1 results.
Breakthrough therapy designation is intended to expedite the development and review of promising drug candidates for serious or life-threatening conditions. It is based upon clinical evidence of substantial improvement over existing therapies in one or more clinically significant endpoints.
ECHELON-1
Result from ECHELON-1 were presented at the 2017 ASH Annual Meeting and simultaneously published in NEJM.
In this trial, researchers compared brentuximab vedotin, doxorubicin, vinblastine, and dacarbazine (A+AVD) to doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) as frontline treatment for 1334 patients with advanced HL.
The study’s primary endpoint was modified progression-free survival (PFS), which was defined as time to progression, death, or evidence of non-complete response after completion of frontline therapy followed by subsequent anticancer therapy.
According to an independent review facility, A+AVD provided a significant improvement in modified PFS compared to ABVD. The hazard ratio was 0.77 (P=0.035), which corresponds to a 23% reduction in the risk of progression, death, or the need for additional anticancer therapy.
The 2-year modified PFS rate was 82.1% in the A+AVD arm and 77.2% in the ABVD arm.
There was no significant difference between the treatment arms when it came to response rates or overall survival.
The objective response rate was 86% in the A+AVD arm and 83% in the ABVD arm (P=0.12). The complete response rate was 73% and 70%, respectively (P=0.22).
The interim 2-year overall survival rate was 97% in the A+AVD arm and 95% in the ABVD arm (hazard ratio=0.72; P=0.19).
The overall incidence of adverse events (AEs) was 99% in the A+AVD arm and 98% in the ABVD arm. The incidence of grade 3 or higher AEs was 83% and 66%, respectively, and the incidence of serious AEs was 43% and 27%, respectively.
Neutropenia, febrile neutropenia, and peripheral neuropathy were more common with A+AVD, while pulmonary toxicity was more common with ABVD.
About brentuximab vedotin
Brentuximab vedotin is already FDA-approved to treat adults with:
- Classical HL who have failed autologous hematopoietic stem cell transplant (auto-HSCT) or, in those who are not auto-HSCT candidates, have failed at least 2 prior multi-agent chemotherapy regimens.
- Classical HL at high risk of relapse or progression as post-auto-HSCT consolidation.
- Primary cutaneous anaplastic large-cell lymphoma (ALCL) or CD30-expressing mycosis fungoides who have received prior systemic therapy.
- Systemic ALCL who have failed at least 1 prior multi-agent chemotherapy regimen. (The drug has accelerated approval for this indication, based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.)

BTK inhibitor zanubrutinib active in non-Hodgkin lymphomas
ATLANTA – , according to data presented at the annual meeting of the American Society of Hematology.
Response rates ranged from 31% to 88% depending on the lymphoma subtype. Overall, approximately 10% of patients discontinued the drug because of adverse events, reported Constantine S. Tam, MBBS, MD, of Peter MacCallum Cancer Centre & St. Vincent’s Hospital, Melbourne.
“There was encouraging activity against all the spectrum of indolent and aggressive NHL subtypes … and durable responses were observed across a variety of histologies,” Dr. Tam said.
Zanubrutinib is a second-generation BTK inhibitor that, based on biochemical assays, has higher selectivity against BTK than ibrutinib, Dr. Tam said.
He presented results of an open-label, multicenter, phase 1b study of daily or twice-daily zanubrutinib in patients with B-cell malignancies, most of them relapsed or refractory to prior therapies. The lymphoma subtypes he presented included diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL).
For 34 patients with indolent lymphomas (FL and MZL), the most frequent adverse events were petechiae/purpura/contusion and upper respiratory tract infection. Eleven grade 3-5 adverse events were reported, including neutropenia, infection, nausea, urinary tract infection, and abdominal pain.
Atrial fibrillation was observed in two patients in the aggressive NHL cohort, for an overall AF rate of approximately 2%, Dr. Tam said.
For 65 patients with aggressive lymphomas (DLBCL and MCL), the most frequent adverse events were petechiae/purpura/contusion and diarrhea; 27 grade 3-5 adverse events were reported, including neutropenia, pneumonia, and anemia.
The highest overall response rate reported was for MCL, at 88% (28 of 32 patients) followed by MZL at 78% (7 of 9 patients), FL at 41% (7 of 17 patients), and DLBCL 31% (8 of 26 patients).
The recommended phase 2 dose for zanubrutinib is either 320 mg/day once daily or a split dose of 160 mg twice daily, Dr. Tam said.
Based on this experience, investigators started a registration trial of zanubrutinib in combination with obinutuzumab for FL, and additional trials are planned, according to Dr. Tam.
There are also registration trials in Waldenstrom macroglobulinemia and chronic lymphocytic leukemia based on other data suggesting activity of zanubrutinib in those disease types, he added.
Zanubrutinib is a product of BeiGene. Dr. Tam reported disclosures related to Roche, Janssen Cilag, Abbvie, Celgene, Pharmacyclics, Onyx, and Amgen.
SOURCE: Tam C et al, ASH 2017, Abstract 152
ATLANTA – , according to data presented at the annual meeting of the American Society of Hematology.
Response rates ranged from 31% to 88% depending on the lymphoma subtype. Overall, approximately 10% of patients discontinued the drug because of adverse events, reported Constantine S. Tam, MBBS, MD, of Peter MacCallum Cancer Centre & St. Vincent’s Hospital, Melbourne.
“There was encouraging activity against all the spectrum of indolent and aggressive NHL subtypes … and durable responses were observed across a variety of histologies,” Dr. Tam said.
Zanubrutinib is a second-generation BTK inhibitor that, based on biochemical assays, has higher selectivity against BTK than ibrutinib, Dr. Tam said.
He presented results of an open-label, multicenter, phase 1b study of daily or twice-daily zanubrutinib in patients with B-cell malignancies, most of them relapsed or refractory to prior therapies. The lymphoma subtypes he presented included diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL).
For 34 patients with indolent lymphomas (FL and MZL), the most frequent adverse events were petechiae/purpura/contusion and upper respiratory tract infection. Eleven grade 3-5 adverse events were reported, including neutropenia, infection, nausea, urinary tract infection, and abdominal pain.
Atrial fibrillation was observed in two patients in the aggressive NHL cohort, for an overall AF rate of approximately 2%, Dr. Tam said.
For 65 patients with aggressive lymphomas (DLBCL and MCL), the most frequent adverse events were petechiae/purpura/contusion and diarrhea; 27 grade 3-5 adverse events were reported, including neutropenia, pneumonia, and anemia.
The highest overall response rate reported was for MCL, at 88% (28 of 32 patients) followed by MZL at 78% (7 of 9 patients), FL at 41% (7 of 17 patients), and DLBCL 31% (8 of 26 patients).
The recommended phase 2 dose for zanubrutinib is either 320 mg/day once daily or a split dose of 160 mg twice daily, Dr. Tam said.
Based on this experience, investigators started a registration trial of zanubrutinib in combination with obinutuzumab for FL, and additional trials are planned, according to Dr. Tam.
There are also registration trials in Waldenstrom macroglobulinemia and chronic lymphocytic leukemia based on other data suggesting activity of zanubrutinib in those disease types, he added.
Zanubrutinib is a product of BeiGene. Dr. Tam reported disclosures related to Roche, Janssen Cilag, Abbvie, Celgene, Pharmacyclics, Onyx, and Amgen.
SOURCE: Tam C et al, ASH 2017, Abstract 152
ATLANTA – , according to data presented at the annual meeting of the American Society of Hematology.
Response rates ranged from 31% to 88% depending on the lymphoma subtype. Overall, approximately 10% of patients discontinued the drug because of adverse events, reported Constantine S. Tam, MBBS, MD, of Peter MacCallum Cancer Centre & St. Vincent’s Hospital, Melbourne.
“There was encouraging activity against all the spectrum of indolent and aggressive NHL subtypes … and durable responses were observed across a variety of histologies,” Dr. Tam said.
Zanubrutinib is a second-generation BTK inhibitor that, based on biochemical assays, has higher selectivity against BTK than ibrutinib, Dr. Tam said.
He presented results of an open-label, multicenter, phase 1b study of daily or twice-daily zanubrutinib in patients with B-cell malignancies, most of them relapsed or refractory to prior therapies. The lymphoma subtypes he presented included diffuse large B-cell lymphoma (DLBCL), follicular lymphoma (FL), mantle cell lymphoma (MCL), and marginal zone lymphoma (MZL).
For 34 patients with indolent lymphomas (FL and MZL), the most frequent adverse events were petechiae/purpura/contusion and upper respiratory tract infection. Eleven grade 3-5 adverse events were reported, including neutropenia, infection, nausea, urinary tract infection, and abdominal pain.
Atrial fibrillation was observed in two patients in the aggressive NHL cohort, for an overall AF rate of approximately 2%, Dr. Tam said.
For 65 patients with aggressive lymphomas (DLBCL and MCL), the most frequent adverse events were petechiae/purpura/contusion and diarrhea; 27 grade 3-5 adverse events were reported, including neutropenia, pneumonia, and anemia.
The highest overall response rate reported was for MCL, at 88% (28 of 32 patients) followed by MZL at 78% (7 of 9 patients), FL at 41% (7 of 17 patients), and DLBCL 31% (8 of 26 patients).
The recommended phase 2 dose for zanubrutinib is either 320 mg/day once daily or a split dose of 160 mg twice daily, Dr. Tam said.
Based on this experience, investigators started a registration trial of zanubrutinib in combination with obinutuzumab for FL, and additional trials are planned, according to Dr. Tam.
There are also registration trials in Waldenstrom macroglobulinemia and chronic lymphocytic leukemia based on other data suggesting activity of zanubrutinib in those disease types, he added.
Zanubrutinib is a product of BeiGene. Dr. Tam reported disclosures related to Roche, Janssen Cilag, Abbvie, Celgene, Pharmacyclics, Onyx, and Amgen.
SOURCE: Tam C et al, ASH 2017, Abstract 152
REPORTING FROM ASH 2017
Key clinical point: Monotherapy with the BTK inhibitor zanubrutinib (BGB-3111) was active and well tolerated in patients with a variety of non-Hodgkin lymphoma (NHL) subtypes.
Major finding: Response rates ranged from 31% to 88% depending on the lymphoma subtype.
Data source: Preliminary results of an open-label, multicenter, phase 1b study including 99 patients with relapsed or refractory diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma, or marginal zone lymphoma.
Disclosures: Zanubrutinib is a product of BeiGene. Constantine S. Tam, MBBS, MD, reported disclosures related to Roche, Janssen Cilag, Abbvie, Celgene, Pharmacyclics, Onyx, and Amgen.
Source: Tam C et al. ASH 2017, Abstract 152.
Update reveals ongoing responses in ZUMA-1
ATLANTA—The chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (axi-cel; KTE-C19) is showing consistent, ongoing responses more than a year after infusion.
An updated analysis of the phase 1/2 ZUMA-1 trial showed that 42% of patients who received axi-cel maintained an objective response at a median follow-up of 15.4 months.
Forty percent of patients have maintained a complete response (CR).
This compares with a 44% objective response rate and a 39% CR rate in the primary analysis of phase 2 ZUMA-1 data, when the median follow-up was 8.7 months.
Sattva S. Neelapu, MD, of MD Anderson Cancer Center in Houston, Texas, reported the long-term results from ZUMA-1 at the 2017 ASH Annual Meeting (abstract 578). The findings were published simultaneously in NEJM.
The primary phase 2 analysis was previously presented at the AACR Annual Meeting 2017.
At ASH 2017, Dr Neelapu disclosed that he has received research funding and served as a consultant for Kite Pharma, the developer of axi-cel. Kite Pharma and the Leukemia & Lymphoma Society Therapy Acceleration Program supported ZUMA-1.
Study schema and patient characteristics
Phase 1 of ZUMA-1 enrolled 7 patients with diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL).
In phase 2, 101 patients were grouped into 2 cohorts—77 with refractory DLBCL and 24 with refractory PMBCL/TFL.
A total of 108 patients were treated in phases 1 and 2 and were included in the long-term pooled analysis.
Patients received a conditioning regimen of cyclophosphamide and fludarabine and, 2 days later, a fixed dose of axi-cel at 2 x 106 CAR T cells/kg.
“Importantly, the product could be manufactured for 99% of enrolled patients,” Dr Neelapu said. “Moreover, 91% of the enrolled patients were dosed with axi-cel, and there were no patients lost to follow-up.”
Patients in the pooled analysis were a median age of 58 (range, 23–76), and 27 (25%) were 65 or older.
Seventy-three patients (68%) were male, 62 (57%) had an ECOG status of 1, 90 (83%) had stage III or IV disease, and 48 (44%) had an IPI score of 3 to 4.
Seventy-six patients (70%) had received 3 or more prior therapies.
Eighty patients (74%) were refractory to their second or later line of therapy, and 70 (65%) had progressive disease as their best response to their last prior therapy. Twenty-five patients (23%) had relapsed after autologous stem cell transplant.
Response
The data cutoff for the long-term analysis was August 11, 2017.
In addition to the ongoing responses mentioned above, the best objective response was 82% in both the phase 2 primary analysis and the long-term analysis for phases 1 and 2.
CR as the best objective response increased from 54% in the primary analysis to 58% at the longer follow-up.
“We did observe deepening of the responses over time,” Dr Neelapu said. “At the time of the first tumor assessment, 60 patients had either partial remission or stable disease. But 23 of those 60 eventually achieved a complete remission up to 15 months post-infusion without any additional therapy.”
The median time to conversion from partial response to CR was 64 days (range, 49–242).
“The durability of these responses was observed consistently across key covariates,” Dr Neelapu added, “including the refractory subgroups, the disease stage groups, IPI risk groups. The CD19 status at baseline did not matter, nor did the cell of origin, or the CD4/CD8 ratio of the product.”
Furthermore, the investigators observed no differences in patients who received tocilizumab or corticosteroids.
The median duration of response for all patients was 11.1 months. For those who achieved CR, the median duration of response has not yet been reached.
Three of the 7 patients (43%) in the phase 1 part of the trial had an ongoing CR at 24 months.
At the median follow-up of 15.4 months, 42% of patients were progression-free, and 56% were alive.
The median overall survival has not been reached. Investigators estimated the 18-month overall survival to be 52%.
Safety
Adverse events (AEs) of grade 3 or higher occurred in 97% of patients, and serious AEs of grade 3 or higher occurred in 46% of patients in the updated analysis.
No new axi-cel-related AEs of cytokine release syndrome, neurologic events, or grade 5 AEs have arisen since the primary analysis.
There were four grade 5 events, 2 of which were related to axi-cel.
“All these four grade 5 events were previously reported—three in the phase 2 and one in the phase 1 trial,” Dr Neelapu said.
Most patients experienced hypogammaglobulinemia and B-cell aplasia. Eight percent of patients had IVIG support during the study.
Infections, such as pneumonia, influenza, and viral infection, were the most common new-onset treatment-emergent serious AEs occurring after 6 months in 10 patients. All were manageable and resolved prior to the data cut-off.
Persistence and resistance
“We observed long-term persistence of the CAR T cells,” Dr Neelapu said.
CAR T cells persisted in 71% of patients still responding at 1 year. And durable responses were observed in patients with and without detectable CAR T cells.
A central review committee analyzed biopsies of 21 evaluable patients at progression to try to determine the mechanism of resistance.
Fourteen of 21 (67%) biopsies were CD19-positive. Of these, 9 were PD-L1-positive, 4 were PD-L1-negative, and 1 was not evaluable.
Seven patients (33%) were CD19-negative compared to baseline. Of these, 4 were PD-L1-positive, 2 were PD-L1-negative, and 1 was not evaluable.
“This PD-L1 expression was observed in both CD19-positive relapses and CD19-negative relapses,” Dr Neelapu emphasized.
Of the 21 patients, 62% were PD-L1-positive.
Investigators hypothesize that 2 potential mechanisms could contribute to relapse: loss of CD19 and expression of PD-L1.
Axi-cel (Yescarta™) was approved by the US Food and Drug Administration in October for the treatment of adults with relapsed or refractory large B-cell lymphoma. ![]()
ATLANTA—The chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (axi-cel; KTE-C19) is showing consistent, ongoing responses more than a year after infusion.
An updated analysis of the phase 1/2 ZUMA-1 trial showed that 42% of patients who received axi-cel maintained an objective response at a median follow-up of 15.4 months.
Forty percent of patients have maintained a complete response (CR).
This compares with a 44% objective response rate and a 39% CR rate in the primary analysis of phase 2 ZUMA-1 data, when the median follow-up was 8.7 months.
Sattva S. Neelapu, MD, of MD Anderson Cancer Center in Houston, Texas, reported the long-term results from ZUMA-1 at the 2017 ASH Annual Meeting (abstract 578). The findings were published simultaneously in NEJM.
The primary phase 2 analysis was previously presented at the AACR Annual Meeting 2017.
At ASH 2017, Dr Neelapu disclosed that he has received research funding and served as a consultant for Kite Pharma, the developer of axi-cel. Kite Pharma and the Leukemia & Lymphoma Society Therapy Acceleration Program supported ZUMA-1.
Study schema and patient characteristics
Phase 1 of ZUMA-1 enrolled 7 patients with diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL).
In phase 2, 101 patients were grouped into 2 cohorts—77 with refractory DLBCL and 24 with refractory PMBCL/TFL.
A total of 108 patients were treated in phases 1 and 2 and were included in the long-term pooled analysis.
Patients received a conditioning regimen of cyclophosphamide and fludarabine and, 2 days later, a fixed dose of axi-cel at 2 x 106 CAR T cells/kg.
“Importantly, the product could be manufactured for 99% of enrolled patients,” Dr Neelapu said. “Moreover, 91% of the enrolled patients were dosed with axi-cel, and there were no patients lost to follow-up.”
Patients in the pooled analysis were a median age of 58 (range, 23–76), and 27 (25%) were 65 or older.
Seventy-three patients (68%) were male, 62 (57%) had an ECOG status of 1, 90 (83%) had stage III or IV disease, and 48 (44%) had an IPI score of 3 to 4.
Seventy-six patients (70%) had received 3 or more prior therapies.
Eighty patients (74%) were refractory to their second or later line of therapy, and 70 (65%) had progressive disease as their best response to their last prior therapy. Twenty-five patients (23%) had relapsed after autologous stem cell transplant.
Response
The data cutoff for the long-term analysis was August 11, 2017.
In addition to the ongoing responses mentioned above, the best objective response was 82% in both the phase 2 primary analysis and the long-term analysis for phases 1 and 2.
CR as the best objective response increased from 54% in the primary analysis to 58% at the longer follow-up.
“We did observe deepening of the responses over time,” Dr Neelapu said. “At the time of the first tumor assessment, 60 patients had either partial remission or stable disease. But 23 of those 60 eventually achieved a complete remission up to 15 months post-infusion without any additional therapy.”
The median time to conversion from partial response to CR was 64 days (range, 49–242).
“The durability of these responses was observed consistently across key covariates,” Dr Neelapu added, “including the refractory subgroups, the disease stage groups, IPI risk groups. The CD19 status at baseline did not matter, nor did the cell of origin, or the CD4/CD8 ratio of the product.”
Furthermore, the investigators observed no differences in patients who received tocilizumab or corticosteroids.
The median duration of response for all patients was 11.1 months. For those who achieved CR, the median duration of response has not yet been reached.
Three of the 7 patients (43%) in the phase 1 part of the trial had an ongoing CR at 24 months.
At the median follow-up of 15.4 months, 42% of patients were progression-free, and 56% were alive.
The median overall survival has not been reached. Investigators estimated the 18-month overall survival to be 52%.
Safety
Adverse events (AEs) of grade 3 or higher occurred in 97% of patients, and serious AEs of grade 3 or higher occurred in 46% of patients in the updated analysis.
No new axi-cel-related AEs of cytokine release syndrome, neurologic events, or grade 5 AEs have arisen since the primary analysis.
There were four grade 5 events, 2 of which were related to axi-cel.
“All these four grade 5 events were previously reported—three in the phase 2 and one in the phase 1 trial,” Dr Neelapu said.
Most patients experienced hypogammaglobulinemia and B-cell aplasia. Eight percent of patients had IVIG support during the study.
Infections, such as pneumonia, influenza, and viral infection, were the most common new-onset treatment-emergent serious AEs occurring after 6 months in 10 patients. All were manageable and resolved prior to the data cut-off.
Persistence and resistance
“We observed long-term persistence of the CAR T cells,” Dr Neelapu said.
CAR T cells persisted in 71% of patients still responding at 1 year. And durable responses were observed in patients with and without detectable CAR T cells.
A central review committee analyzed biopsies of 21 evaluable patients at progression to try to determine the mechanism of resistance.
Fourteen of 21 (67%) biopsies were CD19-positive. Of these, 9 were PD-L1-positive, 4 were PD-L1-negative, and 1 was not evaluable.
Seven patients (33%) were CD19-negative compared to baseline. Of these, 4 were PD-L1-positive, 2 were PD-L1-negative, and 1 was not evaluable.
“This PD-L1 expression was observed in both CD19-positive relapses and CD19-negative relapses,” Dr Neelapu emphasized.
Of the 21 patients, 62% were PD-L1-positive.
Investigators hypothesize that 2 potential mechanisms could contribute to relapse: loss of CD19 and expression of PD-L1.
Axi-cel (Yescarta™) was approved by the US Food and Drug Administration in October for the treatment of adults with relapsed or refractory large B-cell lymphoma. ![]()
ATLANTA—The chimeric antigen receptor (CAR) T-cell therapy axicabtagene ciloleucel (axi-cel; KTE-C19) is showing consistent, ongoing responses more than a year after infusion.
An updated analysis of the phase 1/2 ZUMA-1 trial showed that 42% of patients who received axi-cel maintained an objective response at a median follow-up of 15.4 months.
Forty percent of patients have maintained a complete response (CR).
This compares with a 44% objective response rate and a 39% CR rate in the primary analysis of phase 2 ZUMA-1 data, when the median follow-up was 8.7 months.
Sattva S. Neelapu, MD, of MD Anderson Cancer Center in Houston, Texas, reported the long-term results from ZUMA-1 at the 2017 ASH Annual Meeting (abstract 578). The findings were published simultaneously in NEJM.
The primary phase 2 analysis was previously presented at the AACR Annual Meeting 2017.
At ASH 2017, Dr Neelapu disclosed that he has received research funding and served as a consultant for Kite Pharma, the developer of axi-cel. Kite Pharma and the Leukemia & Lymphoma Society Therapy Acceleration Program supported ZUMA-1.
Study schema and patient characteristics
Phase 1 of ZUMA-1 enrolled 7 patients with diffuse large B-cell lymphoma (DLBCL), primary mediastinal B-cell lymphoma (PMBCL), or transformed follicular lymphoma (TFL).
In phase 2, 101 patients were grouped into 2 cohorts—77 with refractory DLBCL and 24 with refractory PMBCL/TFL.
A total of 108 patients were treated in phases 1 and 2 and were included in the long-term pooled analysis.
Patients received a conditioning regimen of cyclophosphamide and fludarabine and, 2 days later, a fixed dose of axi-cel at 2 x 106 CAR T cells/kg.
“Importantly, the product could be manufactured for 99% of enrolled patients,” Dr Neelapu said. “Moreover, 91% of the enrolled patients were dosed with axi-cel, and there were no patients lost to follow-up.”
Patients in the pooled analysis were a median age of 58 (range, 23–76), and 27 (25%) were 65 or older.
Seventy-three patients (68%) were male, 62 (57%) had an ECOG status of 1, 90 (83%) had stage III or IV disease, and 48 (44%) had an IPI score of 3 to 4.
Seventy-six patients (70%) had received 3 or more prior therapies.
Eighty patients (74%) were refractory to their second or later line of therapy, and 70 (65%) had progressive disease as their best response to their last prior therapy. Twenty-five patients (23%) had relapsed after autologous stem cell transplant.
Response
The data cutoff for the long-term analysis was August 11, 2017.
In addition to the ongoing responses mentioned above, the best objective response was 82% in both the phase 2 primary analysis and the long-term analysis for phases 1 and 2.
CR as the best objective response increased from 54% in the primary analysis to 58% at the longer follow-up.
“We did observe deepening of the responses over time,” Dr Neelapu said. “At the time of the first tumor assessment, 60 patients had either partial remission or stable disease. But 23 of those 60 eventually achieved a complete remission up to 15 months post-infusion without any additional therapy.”
The median time to conversion from partial response to CR was 64 days (range, 49–242).
“The durability of these responses was observed consistently across key covariates,” Dr Neelapu added, “including the refractory subgroups, the disease stage groups, IPI risk groups. The CD19 status at baseline did not matter, nor did the cell of origin, or the CD4/CD8 ratio of the product.”
Furthermore, the investigators observed no differences in patients who received tocilizumab or corticosteroids.
The median duration of response for all patients was 11.1 months. For those who achieved CR, the median duration of response has not yet been reached.
Three of the 7 patients (43%) in the phase 1 part of the trial had an ongoing CR at 24 months.
At the median follow-up of 15.4 months, 42% of patients were progression-free, and 56% were alive.
The median overall survival has not been reached. Investigators estimated the 18-month overall survival to be 52%.
Safety
Adverse events (AEs) of grade 3 or higher occurred in 97% of patients, and serious AEs of grade 3 or higher occurred in 46% of patients in the updated analysis.
No new axi-cel-related AEs of cytokine release syndrome, neurologic events, or grade 5 AEs have arisen since the primary analysis.
There were four grade 5 events, 2 of which were related to axi-cel.
“All these four grade 5 events were previously reported—three in the phase 2 and one in the phase 1 trial,” Dr Neelapu said.
Most patients experienced hypogammaglobulinemia and B-cell aplasia. Eight percent of patients had IVIG support during the study.
Infections, such as pneumonia, influenza, and viral infection, were the most common new-onset treatment-emergent serious AEs occurring after 6 months in 10 patients. All were manageable and resolved prior to the data cut-off.
Persistence and resistance
“We observed long-term persistence of the CAR T cells,” Dr Neelapu said.
CAR T cells persisted in 71% of patients still responding at 1 year. And durable responses were observed in patients with and without detectable CAR T cells.
A central review committee analyzed biopsies of 21 evaluable patients at progression to try to determine the mechanism of resistance.
Fourteen of 21 (67%) biopsies were CD19-positive. Of these, 9 were PD-L1-positive, 4 were PD-L1-negative, and 1 was not evaluable.
Seven patients (33%) were CD19-negative compared to baseline. Of these, 4 were PD-L1-positive, 2 were PD-L1-negative, and 1 was not evaluable.
“This PD-L1 expression was observed in both CD19-positive relapses and CD19-negative relapses,” Dr Neelapu emphasized.
Of the 21 patients, 62% were PD-L1-positive.
Investigators hypothesize that 2 potential mechanisms could contribute to relapse: loss of CD19 and expression of PD-L1.
Axi-cel (Yescarta™) was approved by the US Food and Drug Administration in October for the treatment of adults with relapsed or refractory large B-cell lymphoma. ![]()
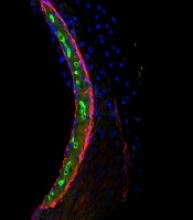
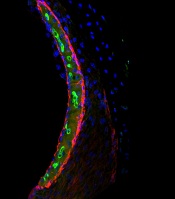
Research explains why cisplatin causes hearing loss
Researchers have gained new insight into hearing loss caused by cisplatin.
By measuring and mapping cisplatin retention in mouse and human inner ear tissues, the researchers found that cisplatin builds up in the inner ear and can remain there for years.
The team also found that a region in the inner ear called the stria vascularis could be targeted to prevent hearing loss resulting from cisplatin.
Lisa L. Cunningham, PhD, of the National Institute on Deafness and other Communications Disorders (NIDCD) in Bethesda, Maryland, and her colleagues reported these findings in Nature Communications.
The researchers noted that cisplatin can cause permanent hearing loss in 40% to 80% of treated patients. The team’s new findings help explain why.
The researchers found that, in most areas of the body, cisplatin is eliminated within days or weeks of treatment, but, in the inner ear, the drug remains much longer.
The team developed a mouse model that represents cisplatin-induced hearing loss seen in human patients.
By looking at inner ear tissue of mice after the first, second, and third cisplatin treatment, the researchers saw that cisplatin remained in the mouse inner ear much longer than in most other body tissues, and the drug builds up with each successive treatment.
The team also studied inner ear tissue donated by deceased adults who had been treated with cisplatin and found the drug is retained in the inner ear months or years after treatment.
When the researchers examined inner ear tissue from a child, they found cisplatin buildup that was even higher than that seen in adults.
Taken together, these results suggest the inner ear readily takes up cisplatin but has limited ability to remove the drug.
In mice and human tissues, the researchers saw the highest buildup of cisplatin in a part of the inner ear called the stria vascularis, which helps maintain the positive electrical charge in inner ear fluid that certain cells need to detect sound.
The team found the accumulation of cisplatin in the stria vascularis contributed to cisplatin-related hearing loss.
“Our findings suggest that if we can prevent cisplatin from entering the stria vascularis in the inner ear during treatment, we may be able to protect cancer patients from developing cisplatin-induced hearing loss,” Dr Cunningham said. ![]()
Researchers have gained new insight into hearing loss caused by cisplatin.
By measuring and mapping cisplatin retention in mouse and human inner ear tissues, the researchers found that cisplatin builds up in the inner ear and can remain there for years.
The team also found that a region in the inner ear called the stria vascularis could be targeted to prevent hearing loss resulting from cisplatin.
Lisa L. Cunningham, PhD, of the National Institute on Deafness and other Communications Disorders (NIDCD) in Bethesda, Maryland, and her colleagues reported these findings in Nature Communications.
The researchers noted that cisplatin can cause permanent hearing loss in 40% to 80% of treated patients. The team’s new findings help explain why.
The researchers found that, in most areas of the body, cisplatin is eliminated within days or weeks of treatment, but, in the inner ear, the drug remains much longer.
The team developed a mouse model that represents cisplatin-induced hearing loss seen in human patients.
By looking at inner ear tissue of mice after the first, second, and third cisplatin treatment, the researchers saw that cisplatin remained in the mouse inner ear much longer than in most other body tissues, and the drug builds up with each successive treatment.
The team also studied inner ear tissue donated by deceased adults who had been treated with cisplatin and found the drug is retained in the inner ear months or years after treatment.
When the researchers examined inner ear tissue from a child, they found cisplatin buildup that was even higher than that seen in adults.
Taken together, these results suggest the inner ear readily takes up cisplatin but has limited ability to remove the drug.
In mice and human tissues, the researchers saw the highest buildup of cisplatin in a part of the inner ear called the stria vascularis, which helps maintain the positive electrical charge in inner ear fluid that certain cells need to detect sound.
The team found the accumulation of cisplatin in the stria vascularis contributed to cisplatin-related hearing loss.
“Our findings suggest that if we can prevent cisplatin from entering the stria vascularis in the inner ear during treatment, we may be able to protect cancer patients from developing cisplatin-induced hearing loss,” Dr Cunningham said. ![]()
Researchers have gained new insight into hearing loss caused by cisplatin.
By measuring and mapping cisplatin retention in mouse and human inner ear tissues, the researchers found that cisplatin builds up in the inner ear and can remain there for years.
The team also found that a region in the inner ear called the stria vascularis could be targeted to prevent hearing loss resulting from cisplatin.
Lisa L. Cunningham, PhD, of the National Institute on Deafness and other Communications Disorders (NIDCD) in Bethesda, Maryland, and her colleagues reported these findings in Nature Communications.
The researchers noted that cisplatin can cause permanent hearing loss in 40% to 80% of treated patients. The team’s new findings help explain why.
The researchers found that, in most areas of the body, cisplatin is eliminated within days or weeks of treatment, but, in the inner ear, the drug remains much longer.
The team developed a mouse model that represents cisplatin-induced hearing loss seen in human patients.
By looking at inner ear tissue of mice after the first, second, and third cisplatin treatment, the researchers saw that cisplatin remained in the mouse inner ear much longer than in most other body tissues, and the drug builds up with each successive treatment.
The team also studied inner ear tissue donated by deceased adults who had been treated with cisplatin and found the drug is retained in the inner ear months or years after treatment.
When the researchers examined inner ear tissue from a child, they found cisplatin buildup that was even higher than that seen in adults.
Taken together, these results suggest the inner ear readily takes up cisplatin but has limited ability to remove the drug.
In mice and human tissues, the researchers saw the highest buildup of cisplatin in a part of the inner ear called the stria vascularis, which helps maintain the positive electrical charge in inner ear fluid that certain cells need to detect sound.
The team found the accumulation of cisplatin in the stria vascularis contributed to cisplatin-related hearing loss.
“Our findings suggest that if we can prevent cisplatin from entering the stria vascularis in the inner ear during treatment, we may be able to protect cancer patients from developing cisplatin-induced hearing loss,” Dr Cunningham said. ![]()
Chemo-free combo should be option for rel/ref CLL, doc says
ATLANTA—The combination of venetoclax and rituximab (VR) should be a standard treatment option for adults with relapsed/refractory chronic lymphocytic leukemia (CLL), according to a speaker at the 2017 ASH Annual Meeting.
Data from the phase 3 MURANO study showed that patients with relapsed/refractory CLL who received VR had significantly longer progression-free survival (PFS) than those who received bendamustine and rituximab (BR).
In addition, “secondary endpoints were consistently in favor of venetoclax-rituximab,” said study investigator John F. Seymour, MBBS, PhD, of Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
Adverse events (AEs) were largely consistent with the known safety profiles of the drugs studied, but tumor lysis syndrome (TLS) was infrequent and occurred at a similar frequency in both treatment arms.
“Thus, overall, I believe venetoclax and rituximab should be considered as a suitable standard therapeutic option in patients with relapsed/refractory CLL,” Dr Seymour said.
It is important to note, however, that patients in the VR arm of this study could receive venetoclax for up to 2 years, whereas patients in the BR arm received study treatment for a maximum of six 28-day cycles.
Dr Seymour presented results from MURANO as a late-breaking abstract at ASH (LBA-2). The study was sponsored by Hoffman-La Roche and AbbVie.
MURANO enrolled 389 CLL patients who had received 1 to 3 prior therapies. Patients were randomized to receive VR (n=194) or BR (n=195). Baseline characteristics were similar between the treatment arms.
In both arms, patients received a single monthly dose of rituximab for 6 cycles. The first dose was 375 mg/m2, and all subsequent doses were 500 mg/m2.
In the VR arm, patients received a 4-week or 5-week dose ramp-up of venetoclax from 20 mg to 400 mg daily. This was intended to mitigate the risk of TLS, which has been observed in previous studies of venetoclax.
Patients in the VR arm continued with daily venetoclax at 400 mg for a maximum of 2 years or until disease progression or cessation due to toxicity. They started receiving rituximab after the ramp-up period (at week 6).
In the BR arm, patients received bendamustine at 70 mg/m2 on days 1 and 2 of each 28-day cycle for 6 cycles. Patients could proceed to subsequent therapy if they progressed.
The median follow-up was 23.8 months (range, 0-37.4 months).
Twenty-five percent of patients in the VR arm and 17% in the BR arm discontinued treatment ahead of schedule. Reasons for discontinuation (in the VR and BR arms, respectively) were disease progression (5% and 3%), AEs (12% and 6%), death (1% and 2%), and “other” (6% and 7%).
Survival
The study’s primary endpoint was investigator-assessed PFS. PFS according to an independent review committee (IRC) was a secondary endpoint.
According to investigators, the median PFS was not reached in the VR arm and was 17.0 months in the BR arm (hazard ratio [HR]=0.17, P<0.0001). According to the IRC, the median PFS was not reached in the VR arm and was 18.1 months in the BR arm (HR=0.17, P<0.0001).
According to investigators, the estimated PFS at 24 months was 84.9% in the VR arm and 36.3% in the BR arm. According to the IRC, the 24-month PFS was 82.8% and 37.4%, respectively.
The benefit with VR was consistent across subgroups. Patients had a PFS benefit regardless of their number of prior therapies, deletion 17p status, TP53 mutational status, baseline IGHV mutational status, and whether they had relapsed or refractory disease.
Dr Seymour acknowledged that the differences in treatment duration between the BR and VR arms may have affected the interpretation of these results.
“[T]he treatment duration differed, although, of course, the capacity to deliver more than 6 cycles of bendamustine-rituximab would have been problematic,” he said. “There is some data that antibody treatment may prolong progression-free survival. However, when this study was designed, in 2013, that data was certainly not available. And I believe, currently, maintenance antibody is not an accepted standard of treatment.”
The median overall survival (OS) was not reached in either treatment arm. The 1-year OS rate was 95.9% in the VR arm and 91.1% in the BR arm. The 2-year OS rate was 91.9% and 86.6%, respectively (HR=0.48, P=0.0186).
“[W]ith median follow-up of just on 2 years, there is already a clinically meaningful difference [in OS between the treatment arms],” Dr Seymour said.
“This is not attributable to any difference in availability of novel therapies. Of the 54 patients who received subsequent therapy after progression on the bendamustine-rituximab arm, 40 of those received novel targeted agents.”
Response and MRD
According to investigators, the overall response rate was 93.3% (181/194) in the VR arm and 67.7% (312/195) in the BR arm (P<0.0001). According to the IRC, the overall response rate was 92.3% (179/194) and 72.3% (141/195), respectively (P<0.0001).
According to investigators, the rate of complete response (CR) or CR with incomplete marrow recovery (CRi) was 26.8% (n=52) in the VR arm and 8.2% (n=16) in the BR arm. According to the IRC, the CR/CRi rate was 8.2% (n=16) and 3.6% (n=7), respectively.
Dr Seymour acknowledged the differences in CR/CRi between investigator and IRC assessments. He said 28 of the 42 discrepancies in the VR arm “were attributable to residual CT scan nodal abnormalities in the 16- to 30-mm size.” However, he also noted that 88% of these patients were negative for minimal residual disease (MRD) in the peripheral blood at that time point.
MRD was assessed every 3 months. Patients were counted as MRD-positive if they were positive by either allele-specific oligonucleotide polymerase chain reaction or multicolor flow cytometry. Patients were also counted as MRD-positive if there was a failure to collect a sample.
The proportion of patients who were MRD-negative in the VR and BR arms, respectively, was:
- 45% and 6% at 4 months
- 62% and 13% at 9 months
- 60% and 10% at 12 months
- 57% and 9% at 15 months
- 60% and 5% at 18 months.
Dr Seymour pointed out that 65 patients in the VR arm surpassed the maximum treatment duration for venetoclax (2 years) and therefore stopped receiving the drug, but only 12 of these patients have follow-up beyond 3 months.
“So information about the durability of response after cessation remains immature at the moment,” he said.
Safety
All patients in the VR arm and 98% in the BR arm had at least 1 AE. The rate of serious AEs was 46% and 43%, respectively. The rate of grade 3/4 AEs was 82% and 70%, respectively.
Grade 3/4 AEs with at least a 2% difference in incidence between the treatment arms (in the VR and BR arms, respectively) were neutropenia (58% and 39%), anemia (11% and 14%), thrombocytopenia (6% and 10%), febrile neutropenia (4% and 10%), pneumonia (5% and 8%), infusion-related reactions (2% and 5%), TLS (3% and 1%), hypotension (0% and 3%), hyperglycemia (2% and 0%), and hypogammaglobulinemia (2% and 0%).
The rate of grade 5 AEs was 5% in the VR arm and 6% in the BR arm.
Grade 5 AEs in the VR arm were pneumonia (n=3), sepsis (n=1), cardiac failure (n=1), myocardial infarction (n=1), sudden cardiac death (n=1), colorectal cancer (n=1), status epilepticus (n=1), and acute respiratory failure (n=1).
Grade 5 AEs in the BR arm included sepsis (n=2), lung cancer (n=2), Listeria sepsis (n=1), Scedosporium infection (n=1), lymphoma (n=1), hemorrhagic stroke (n=1), pulmonary embolism (n=1), acute myeloid leukemia (n=1), and sudden death (n=1). ![]()
ATLANTA—The combination of venetoclax and rituximab (VR) should be a standard treatment option for adults with relapsed/refractory chronic lymphocytic leukemia (CLL), according to a speaker at the 2017 ASH Annual Meeting.
Data from the phase 3 MURANO study showed that patients with relapsed/refractory CLL who received VR had significantly longer progression-free survival (PFS) than those who received bendamustine and rituximab (BR).
In addition, “secondary endpoints were consistently in favor of venetoclax-rituximab,” said study investigator John F. Seymour, MBBS, PhD, of Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
Adverse events (AEs) were largely consistent with the known safety profiles of the drugs studied, but tumor lysis syndrome (TLS) was infrequent and occurred at a similar frequency in both treatment arms.
“Thus, overall, I believe venetoclax and rituximab should be considered as a suitable standard therapeutic option in patients with relapsed/refractory CLL,” Dr Seymour said.
It is important to note, however, that patients in the VR arm of this study could receive venetoclax for up to 2 years, whereas patients in the BR arm received study treatment for a maximum of six 28-day cycles.
Dr Seymour presented results from MURANO as a late-breaking abstract at ASH (LBA-2). The study was sponsored by Hoffman-La Roche and AbbVie.
MURANO enrolled 389 CLL patients who had received 1 to 3 prior therapies. Patients were randomized to receive VR (n=194) or BR (n=195). Baseline characteristics were similar between the treatment arms.
In both arms, patients received a single monthly dose of rituximab for 6 cycles. The first dose was 375 mg/m2, and all subsequent doses were 500 mg/m2.
In the VR arm, patients received a 4-week or 5-week dose ramp-up of venetoclax from 20 mg to 400 mg daily. This was intended to mitigate the risk of TLS, which has been observed in previous studies of venetoclax.
Patients in the VR arm continued with daily venetoclax at 400 mg for a maximum of 2 years or until disease progression or cessation due to toxicity. They started receiving rituximab after the ramp-up period (at week 6).
In the BR arm, patients received bendamustine at 70 mg/m2 on days 1 and 2 of each 28-day cycle for 6 cycles. Patients could proceed to subsequent therapy if they progressed.
The median follow-up was 23.8 months (range, 0-37.4 months).
Twenty-five percent of patients in the VR arm and 17% in the BR arm discontinued treatment ahead of schedule. Reasons for discontinuation (in the VR and BR arms, respectively) were disease progression (5% and 3%), AEs (12% and 6%), death (1% and 2%), and “other” (6% and 7%).
Survival
The study’s primary endpoint was investigator-assessed PFS. PFS according to an independent review committee (IRC) was a secondary endpoint.
According to investigators, the median PFS was not reached in the VR arm and was 17.0 months in the BR arm (hazard ratio [HR]=0.17, P<0.0001). According to the IRC, the median PFS was not reached in the VR arm and was 18.1 months in the BR arm (HR=0.17, P<0.0001).
According to investigators, the estimated PFS at 24 months was 84.9% in the VR arm and 36.3% in the BR arm. According to the IRC, the 24-month PFS was 82.8% and 37.4%, respectively.
The benefit with VR was consistent across subgroups. Patients had a PFS benefit regardless of their number of prior therapies, deletion 17p status, TP53 mutational status, baseline IGHV mutational status, and whether they had relapsed or refractory disease.
Dr Seymour acknowledged that the differences in treatment duration between the BR and VR arms may have affected the interpretation of these results.
“[T]he treatment duration differed, although, of course, the capacity to deliver more than 6 cycles of bendamustine-rituximab would have been problematic,” he said. “There is some data that antibody treatment may prolong progression-free survival. However, when this study was designed, in 2013, that data was certainly not available. And I believe, currently, maintenance antibody is not an accepted standard of treatment.”
The median overall survival (OS) was not reached in either treatment arm. The 1-year OS rate was 95.9% in the VR arm and 91.1% in the BR arm. The 2-year OS rate was 91.9% and 86.6%, respectively (HR=0.48, P=0.0186).
“[W]ith median follow-up of just on 2 years, there is already a clinically meaningful difference [in OS between the treatment arms],” Dr Seymour said.
“This is not attributable to any difference in availability of novel therapies. Of the 54 patients who received subsequent therapy after progression on the bendamustine-rituximab arm, 40 of those received novel targeted agents.”
Response and MRD
According to investigators, the overall response rate was 93.3% (181/194) in the VR arm and 67.7% (312/195) in the BR arm (P<0.0001). According to the IRC, the overall response rate was 92.3% (179/194) and 72.3% (141/195), respectively (P<0.0001).
According to investigators, the rate of complete response (CR) or CR with incomplete marrow recovery (CRi) was 26.8% (n=52) in the VR arm and 8.2% (n=16) in the BR arm. According to the IRC, the CR/CRi rate was 8.2% (n=16) and 3.6% (n=7), respectively.
Dr Seymour acknowledged the differences in CR/CRi between investigator and IRC assessments. He said 28 of the 42 discrepancies in the VR arm “were attributable to residual CT scan nodal abnormalities in the 16- to 30-mm size.” However, he also noted that 88% of these patients were negative for minimal residual disease (MRD) in the peripheral blood at that time point.
MRD was assessed every 3 months. Patients were counted as MRD-positive if they were positive by either allele-specific oligonucleotide polymerase chain reaction or multicolor flow cytometry. Patients were also counted as MRD-positive if there was a failure to collect a sample.
The proportion of patients who were MRD-negative in the VR and BR arms, respectively, was:
- 45% and 6% at 4 months
- 62% and 13% at 9 months
- 60% and 10% at 12 months
- 57% and 9% at 15 months
- 60% and 5% at 18 months.
Dr Seymour pointed out that 65 patients in the VR arm surpassed the maximum treatment duration for venetoclax (2 years) and therefore stopped receiving the drug, but only 12 of these patients have follow-up beyond 3 months.
“So information about the durability of response after cessation remains immature at the moment,” he said.
Safety
All patients in the VR arm and 98% in the BR arm had at least 1 AE. The rate of serious AEs was 46% and 43%, respectively. The rate of grade 3/4 AEs was 82% and 70%, respectively.
Grade 3/4 AEs with at least a 2% difference in incidence between the treatment arms (in the VR and BR arms, respectively) were neutropenia (58% and 39%), anemia (11% and 14%), thrombocytopenia (6% and 10%), febrile neutropenia (4% and 10%), pneumonia (5% and 8%), infusion-related reactions (2% and 5%), TLS (3% and 1%), hypotension (0% and 3%), hyperglycemia (2% and 0%), and hypogammaglobulinemia (2% and 0%).
The rate of grade 5 AEs was 5% in the VR arm and 6% in the BR arm.
Grade 5 AEs in the VR arm were pneumonia (n=3), sepsis (n=1), cardiac failure (n=1), myocardial infarction (n=1), sudden cardiac death (n=1), colorectal cancer (n=1), status epilepticus (n=1), and acute respiratory failure (n=1).
Grade 5 AEs in the BR arm included sepsis (n=2), lung cancer (n=2), Listeria sepsis (n=1), Scedosporium infection (n=1), lymphoma (n=1), hemorrhagic stroke (n=1), pulmonary embolism (n=1), acute myeloid leukemia (n=1), and sudden death (n=1). ![]()
ATLANTA—The combination of venetoclax and rituximab (VR) should be a standard treatment option for adults with relapsed/refractory chronic lymphocytic leukemia (CLL), according to a speaker at the 2017 ASH Annual Meeting.
Data from the phase 3 MURANO study showed that patients with relapsed/refractory CLL who received VR had significantly longer progression-free survival (PFS) than those who received bendamustine and rituximab (BR).
In addition, “secondary endpoints were consistently in favor of venetoclax-rituximab,” said study investigator John F. Seymour, MBBS, PhD, of Peter MacCallum Cancer Centre in Melbourne, Victoria, Australia.
Adverse events (AEs) were largely consistent with the known safety profiles of the drugs studied, but tumor lysis syndrome (TLS) was infrequent and occurred at a similar frequency in both treatment arms.
“Thus, overall, I believe venetoclax and rituximab should be considered as a suitable standard therapeutic option in patients with relapsed/refractory CLL,” Dr Seymour said.
It is important to note, however, that patients in the VR arm of this study could receive venetoclax for up to 2 years, whereas patients in the BR arm received study treatment for a maximum of six 28-day cycles.
Dr Seymour presented results from MURANO as a late-breaking abstract at ASH (LBA-2). The study was sponsored by Hoffman-La Roche and AbbVie.
MURANO enrolled 389 CLL patients who had received 1 to 3 prior therapies. Patients were randomized to receive VR (n=194) or BR (n=195). Baseline characteristics were similar between the treatment arms.
In both arms, patients received a single monthly dose of rituximab for 6 cycles. The first dose was 375 mg/m2, and all subsequent doses were 500 mg/m2.
In the VR arm, patients received a 4-week or 5-week dose ramp-up of venetoclax from 20 mg to 400 mg daily. This was intended to mitigate the risk of TLS, which has been observed in previous studies of venetoclax.
Patients in the VR arm continued with daily venetoclax at 400 mg for a maximum of 2 years or until disease progression or cessation due to toxicity. They started receiving rituximab after the ramp-up period (at week 6).
In the BR arm, patients received bendamustine at 70 mg/m2 on days 1 and 2 of each 28-day cycle for 6 cycles. Patients could proceed to subsequent therapy if they progressed.
The median follow-up was 23.8 months (range, 0-37.4 months).
Twenty-five percent of patients in the VR arm and 17% in the BR arm discontinued treatment ahead of schedule. Reasons for discontinuation (in the VR and BR arms, respectively) were disease progression (5% and 3%), AEs (12% and 6%), death (1% and 2%), and “other” (6% and 7%).
Survival
The study’s primary endpoint was investigator-assessed PFS. PFS according to an independent review committee (IRC) was a secondary endpoint.
According to investigators, the median PFS was not reached in the VR arm and was 17.0 months in the BR arm (hazard ratio [HR]=0.17, P<0.0001). According to the IRC, the median PFS was not reached in the VR arm and was 18.1 months in the BR arm (HR=0.17, P<0.0001).
According to investigators, the estimated PFS at 24 months was 84.9% in the VR arm and 36.3% in the BR arm. According to the IRC, the 24-month PFS was 82.8% and 37.4%, respectively.
The benefit with VR was consistent across subgroups. Patients had a PFS benefit regardless of their number of prior therapies, deletion 17p status, TP53 mutational status, baseline IGHV mutational status, and whether they had relapsed or refractory disease.
Dr Seymour acknowledged that the differences in treatment duration between the BR and VR arms may have affected the interpretation of these results.
“[T]he treatment duration differed, although, of course, the capacity to deliver more than 6 cycles of bendamustine-rituximab would have been problematic,” he said. “There is some data that antibody treatment may prolong progression-free survival. However, when this study was designed, in 2013, that data was certainly not available. And I believe, currently, maintenance antibody is not an accepted standard of treatment.”
The median overall survival (OS) was not reached in either treatment arm. The 1-year OS rate was 95.9% in the VR arm and 91.1% in the BR arm. The 2-year OS rate was 91.9% and 86.6%, respectively (HR=0.48, P=0.0186).
“[W]ith median follow-up of just on 2 years, there is already a clinically meaningful difference [in OS between the treatment arms],” Dr Seymour said.
“This is not attributable to any difference in availability of novel therapies. Of the 54 patients who received subsequent therapy after progression on the bendamustine-rituximab arm, 40 of those received novel targeted agents.”
Response and MRD
According to investigators, the overall response rate was 93.3% (181/194) in the VR arm and 67.7% (312/195) in the BR arm (P<0.0001). According to the IRC, the overall response rate was 92.3% (179/194) and 72.3% (141/195), respectively (P<0.0001).
According to investigators, the rate of complete response (CR) or CR with incomplete marrow recovery (CRi) was 26.8% (n=52) in the VR arm and 8.2% (n=16) in the BR arm. According to the IRC, the CR/CRi rate was 8.2% (n=16) and 3.6% (n=7), respectively.
Dr Seymour acknowledged the differences in CR/CRi between investigator and IRC assessments. He said 28 of the 42 discrepancies in the VR arm “were attributable to residual CT scan nodal abnormalities in the 16- to 30-mm size.” However, he also noted that 88% of these patients were negative for minimal residual disease (MRD) in the peripheral blood at that time point.
MRD was assessed every 3 months. Patients were counted as MRD-positive if they were positive by either allele-specific oligonucleotide polymerase chain reaction or multicolor flow cytometry. Patients were also counted as MRD-positive if there was a failure to collect a sample.
The proportion of patients who were MRD-negative in the VR and BR arms, respectively, was:
- 45% and 6% at 4 months
- 62% and 13% at 9 months
- 60% and 10% at 12 months
- 57% and 9% at 15 months
- 60% and 5% at 18 months.
Dr Seymour pointed out that 65 patients in the VR arm surpassed the maximum treatment duration for venetoclax (2 years) and therefore stopped receiving the drug, but only 12 of these patients have follow-up beyond 3 months.
“So information about the durability of response after cessation remains immature at the moment,” he said.
Safety
All patients in the VR arm and 98% in the BR arm had at least 1 AE. The rate of serious AEs was 46% and 43%, respectively. The rate of grade 3/4 AEs was 82% and 70%, respectively.
Grade 3/4 AEs with at least a 2% difference in incidence between the treatment arms (in the VR and BR arms, respectively) were neutropenia (58% and 39%), anemia (11% and 14%), thrombocytopenia (6% and 10%), febrile neutropenia (4% and 10%), pneumonia (5% and 8%), infusion-related reactions (2% and 5%), TLS (3% and 1%), hypotension (0% and 3%), hyperglycemia (2% and 0%), and hypogammaglobulinemia (2% and 0%).
The rate of grade 5 AEs was 5% in the VR arm and 6% in the BR arm.
Grade 5 AEs in the VR arm were pneumonia (n=3), sepsis (n=1), cardiac failure (n=1), myocardial infarction (n=1), sudden cardiac death (n=1), colorectal cancer (n=1), status epilepticus (n=1), and acute respiratory failure (n=1).
Grade 5 AEs in the BR arm included sepsis (n=2), lung cancer (n=2), Listeria sepsis (n=1), Scedosporium infection (n=1), lymphoma (n=1), hemorrhagic stroke (n=1), pulmonary embolism (n=1), acute myeloid leukemia (n=1), and sudden death (n=1). ![]()
Risk stratification may be possible with JCAR017
ATLANTA—Data suggest a therapeutic window may exist for chimeric antigen receptor (CAR) T-cell expansion with JCAR017, according to a preliminary model.
In a core set of 67 patients with diffuse large B-cell lymphoma (DLBCL) who had received JCAR017 in the TRANSCEND NHL 001 trial, investigators observed that baseline high tumor burden and inflammatory biomarkers were associated with high CAR T-cell expansion and increased rates of cytokine release syndrome (CRS) and neurotoxicity.
If the model holds up, researchers say they could potentially identify patients at risk for low or high T-cell expansion levels and develop a strategy to enhance or limit the expansion.
TRANSCEND NHL 001 (NCT02631044) is a multicenter, phase 1 trial in relapsed or refractory non-Hodgkin lymphoma evaluating 2 dose levels of JCAR017, also known as lisocabtagene maraleucel, or liso-cel for short.
Liso-cel is a CD19-directed 4-1BB CAR T cell administered at precise doses of CD4+ and CD8+ CAR T cells. It had previously demonstrated high complete remission (CR) rates and low incidences of CRS and neurotoxicity.
Tanya Saddiqi, MD, of City of Hope National Medical Center in Duarte, California, presented data from the dose-finding and expansion cohorts at the 2017 ASH Annual Meeting (abstract 193*).
Study design
Patients with DLBCL after 2 lines of prior therapy or mantle cell lymphoma after 1 prior line of therapy were eligible to enroll in TRANSCEND NHL 001.
Patients with de novo DLBCL, those who transformed from follicular lymphoma, or those with high-grade B-cell lymphoma made up the pivotal or core population. All DLBCL patients enrolled on the trial comprised the full population.
Patients were screened, enrolled, and underwent apheresis. Bridging therapy was permitted while their CAR T cells were being manufactured.
Patients then had a PET scan and lab tests prior to lymphodepletion.
“This is the time point of our interest,” Dr Saddiqi said, “to see if there are any patient characteristics or biomarkers that we can identify . . . that could help us figure out which patients are at higher risk of toxicity, potentially.”
Lymphodepletion consisted of fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2 for 3 days).
Patients received the JCAR017 infusion, and, at specific time points thereafter, cytokine, pharmacokinetic (PK), and clinical lab evaluations were conducted. PK evaluation and scans were performed every 3 months for the first year after JCAR017 infusion, and safety and viral vector follow-up for 15 years.
Dose levels were 5 x 107 cells as a single or double dose (DL1S) and 1 x 108 cells as a single dose (DL2S). Dose level 2 was chosen for further study, and double dosing was discontinued.
“Double dosing was actually not pursued further,” Dr Saddiqi explained, “because it did not seem to add any benefit over single dosing.”
At the time of the presentation, 91 total patients were treated, 67 of whom were the core population.
Results
Dr Saddiqi reported that patients treated with JCAR017 achieved a relatively high best overall response rate (ORR) and high durable CR rates.
“And this seems to be especially true for the core set of patients and particularly for patients at dose level 2,” she added.
At all dose levels, the core patients had a best ORR of 84% (41/49) and a CR rate of 61% (30/49).
At follow-up of 3 months or longer, the core group had an ORR of 65% (26/40) for all dose levels, 52% (11/21) for dose level 1, and 80% (12/15) for dose level 2.
The 3-month CR rate was 53% (21/40) for all dose levels in the core group, 33% (7/21) in dose level 1, and 73% (11/15) in dose level 2.
Dr Saddiqi noted that CRS and neurotoxicity did not differ by dose level or schedule, and there were no grade 5 events of CRS or neurotoxicity.
“Among the core group, dose level change did not add to their toxicity,” she said. “And so the question is: Is it patient factors, is it tumor factors? What is it that is actually causing the toxicities in these patients?”
Dr Saddiqi focused the presentation on patient factors.
Patient factors
The data showed that tumor burden and lactose dehydrogenase (LDH) levels were higher in patients with CRS and neurotoxicity.
Univariate analysis revealed that CRS and neurotoxicity were associated with a shorter time since diagnosis.
However, prior number of therapies, patient weight, and disease stage were not associated with CRS or neurotoxicity.
Investigators were able to identify preliminary risk boundaries. Core patients with high LDH levels (≥ 500 U/L) and sum of the products of diameters (SPD) ≥ 50 cm2 at baseline had an 8-fold increase in risk of CRS and neurotoxicity.
“Inversely, if these patients did not meet the cutoff for LDH or SPD,” Dr Saddiqi pointed out, “if they were lower than that, they have significantly lower CRS and neurotoxicity events.”
Investigators also observed that baseline markers of inflammation and inflammatory cytokines trended higher in patients with CRS and neurotoxicity. For CRS, this includes ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNFα, and MIP-1β. For neurotoxicity, this includes ferritin, CRP, d-Dimer, IL-6, IL-15, TNFα, and MIP-1α.
The team also observed that tumor burden, baseline markers of inflammation, and inflammatory cytokines trended lower in core patients with durable responses.
“Interestingly, it’s inversely true that patients who did have these higher levels [of inflammation markers], and higher tumor burden, and higher LDH, actually were the ones that were either showing no response at 3 months or had lost their response by the 3-month assessment point,” Dr Saddiqi explained.
And in patients with higher baseline tumor burden and inflammatory cytokine levels, JCAR017 T-cell expansion trended higher.
“Some were deemed to be super expanders because their CAR T-cell levels were very high in their blood,” she added.
The investigators created a preliminary logistic model based on the data that suggests a therapeutic window might be able to limit toxicity and optimize efficacy.
The model indicates that patients with higher tumor burden, higher LDH, and higher inflammatory state at baseline seem to be the ones who are having more CRS and more neurotoxicity after CAR T-cell infusion.
“They are expanding their cells much more, yet their responses at 3 months seem to be affected adversely by this entire situation,” Dr Saddiqi said.
"One explanation, potentially, could be that these CAR T cells are seeing a lot of antigen when they go into the body. They have the perfect cytokine milieu to grow, expand, and go crazy in the body, if you will, and very quickly peter out as well because there’s T-cell exhaustion that happens rather rapidly and clinical responses are then then lost.”
The investigators believe that if they can identify those patients ahead of time who may be at risk of too high expansion or too low expansion of their CAR T cells, they may be able to find strategies to push expansion into the “sweet spot of CAR T-cell expansion and ultimately get the holy grail of having durable responses for all with minimal toxicity,” Dr Saddiqi concluded.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics, Inc. Dr Saddiqi has served on a steering committee for JCAR017. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Data suggest a therapeutic window may exist for chimeric antigen receptor (CAR) T-cell expansion with JCAR017, according to a preliminary model.
In a core set of 67 patients with diffuse large B-cell lymphoma (DLBCL) who had received JCAR017 in the TRANSCEND NHL 001 trial, investigators observed that baseline high tumor burden and inflammatory biomarkers were associated with high CAR T-cell expansion and increased rates of cytokine release syndrome (CRS) and neurotoxicity.
If the model holds up, researchers say they could potentially identify patients at risk for low or high T-cell expansion levels and develop a strategy to enhance or limit the expansion.
TRANSCEND NHL 001 (NCT02631044) is a multicenter, phase 1 trial in relapsed or refractory non-Hodgkin lymphoma evaluating 2 dose levels of JCAR017, also known as lisocabtagene maraleucel, or liso-cel for short.
Liso-cel is a CD19-directed 4-1BB CAR T cell administered at precise doses of CD4+ and CD8+ CAR T cells. It had previously demonstrated high complete remission (CR) rates and low incidences of CRS and neurotoxicity.
Tanya Saddiqi, MD, of City of Hope National Medical Center in Duarte, California, presented data from the dose-finding and expansion cohorts at the 2017 ASH Annual Meeting (abstract 193*).
Study design
Patients with DLBCL after 2 lines of prior therapy or mantle cell lymphoma after 1 prior line of therapy were eligible to enroll in TRANSCEND NHL 001.
Patients with de novo DLBCL, those who transformed from follicular lymphoma, or those with high-grade B-cell lymphoma made up the pivotal or core population. All DLBCL patients enrolled on the trial comprised the full population.
Patients were screened, enrolled, and underwent apheresis. Bridging therapy was permitted while their CAR T cells were being manufactured.
Patients then had a PET scan and lab tests prior to lymphodepletion.
“This is the time point of our interest,” Dr Saddiqi said, “to see if there are any patient characteristics or biomarkers that we can identify . . . that could help us figure out which patients are at higher risk of toxicity, potentially.”
Lymphodepletion consisted of fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2 for 3 days).
Patients received the JCAR017 infusion, and, at specific time points thereafter, cytokine, pharmacokinetic (PK), and clinical lab evaluations were conducted. PK evaluation and scans were performed every 3 months for the first year after JCAR017 infusion, and safety and viral vector follow-up for 15 years.
Dose levels were 5 x 107 cells as a single or double dose (DL1S) and 1 x 108 cells as a single dose (DL2S). Dose level 2 was chosen for further study, and double dosing was discontinued.
“Double dosing was actually not pursued further,” Dr Saddiqi explained, “because it did not seem to add any benefit over single dosing.”
At the time of the presentation, 91 total patients were treated, 67 of whom were the core population.
Results
Dr Saddiqi reported that patients treated with JCAR017 achieved a relatively high best overall response rate (ORR) and high durable CR rates.
“And this seems to be especially true for the core set of patients and particularly for patients at dose level 2,” she added.
At all dose levels, the core patients had a best ORR of 84% (41/49) and a CR rate of 61% (30/49).
At follow-up of 3 months or longer, the core group had an ORR of 65% (26/40) for all dose levels, 52% (11/21) for dose level 1, and 80% (12/15) for dose level 2.
The 3-month CR rate was 53% (21/40) for all dose levels in the core group, 33% (7/21) in dose level 1, and 73% (11/15) in dose level 2.
Dr Saddiqi noted that CRS and neurotoxicity did not differ by dose level or schedule, and there were no grade 5 events of CRS or neurotoxicity.
“Among the core group, dose level change did not add to their toxicity,” she said. “And so the question is: Is it patient factors, is it tumor factors? What is it that is actually causing the toxicities in these patients?”
Dr Saddiqi focused the presentation on patient factors.
Patient factors
The data showed that tumor burden and lactose dehydrogenase (LDH) levels were higher in patients with CRS and neurotoxicity.
Univariate analysis revealed that CRS and neurotoxicity were associated with a shorter time since diagnosis.
However, prior number of therapies, patient weight, and disease stage were not associated with CRS or neurotoxicity.
Investigators were able to identify preliminary risk boundaries. Core patients with high LDH levels (≥ 500 U/L) and sum of the products of diameters (SPD) ≥ 50 cm2 at baseline had an 8-fold increase in risk of CRS and neurotoxicity.
“Inversely, if these patients did not meet the cutoff for LDH or SPD,” Dr Saddiqi pointed out, “if they were lower than that, they have significantly lower CRS and neurotoxicity events.”
Investigators also observed that baseline markers of inflammation and inflammatory cytokines trended higher in patients with CRS and neurotoxicity. For CRS, this includes ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNFα, and MIP-1β. For neurotoxicity, this includes ferritin, CRP, d-Dimer, IL-6, IL-15, TNFα, and MIP-1α.
The team also observed that tumor burden, baseline markers of inflammation, and inflammatory cytokines trended lower in core patients with durable responses.
“Interestingly, it’s inversely true that patients who did have these higher levels [of inflammation markers], and higher tumor burden, and higher LDH, actually were the ones that were either showing no response at 3 months or had lost their response by the 3-month assessment point,” Dr Saddiqi explained.
And in patients with higher baseline tumor burden and inflammatory cytokine levels, JCAR017 T-cell expansion trended higher.
“Some were deemed to be super expanders because their CAR T-cell levels were very high in their blood,” she added.
The investigators created a preliminary logistic model based on the data that suggests a therapeutic window might be able to limit toxicity and optimize efficacy.
The model indicates that patients with higher tumor burden, higher LDH, and higher inflammatory state at baseline seem to be the ones who are having more CRS and more neurotoxicity after CAR T-cell infusion.
“They are expanding their cells much more, yet their responses at 3 months seem to be affected adversely by this entire situation,” Dr Saddiqi said.
"One explanation, potentially, could be that these CAR T cells are seeing a lot of antigen when they go into the body. They have the perfect cytokine milieu to grow, expand, and go crazy in the body, if you will, and very quickly peter out as well because there’s T-cell exhaustion that happens rather rapidly and clinical responses are then then lost.”
The investigators believe that if they can identify those patients ahead of time who may be at risk of too high expansion or too low expansion of their CAR T cells, they may be able to find strategies to push expansion into the “sweet spot of CAR T-cell expansion and ultimately get the holy grail of having durable responses for all with minimal toxicity,” Dr Saddiqi concluded.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics, Inc. Dr Saddiqi has served on a steering committee for JCAR017. ![]()
*Data in the presentation differ from the abstract.
ATLANTA—Data suggest a therapeutic window may exist for chimeric antigen receptor (CAR) T-cell expansion with JCAR017, according to a preliminary model.
In a core set of 67 patients with diffuse large B-cell lymphoma (DLBCL) who had received JCAR017 in the TRANSCEND NHL 001 trial, investigators observed that baseline high tumor burden and inflammatory biomarkers were associated with high CAR T-cell expansion and increased rates of cytokine release syndrome (CRS) and neurotoxicity.
If the model holds up, researchers say they could potentially identify patients at risk for low or high T-cell expansion levels and develop a strategy to enhance or limit the expansion.
TRANSCEND NHL 001 (NCT02631044) is a multicenter, phase 1 trial in relapsed or refractory non-Hodgkin lymphoma evaluating 2 dose levels of JCAR017, also known as lisocabtagene maraleucel, or liso-cel for short.
Liso-cel is a CD19-directed 4-1BB CAR T cell administered at precise doses of CD4+ and CD8+ CAR T cells. It had previously demonstrated high complete remission (CR) rates and low incidences of CRS and neurotoxicity.
Tanya Saddiqi, MD, of City of Hope National Medical Center in Duarte, California, presented data from the dose-finding and expansion cohorts at the 2017 ASH Annual Meeting (abstract 193*).
Study design
Patients with DLBCL after 2 lines of prior therapy or mantle cell lymphoma after 1 prior line of therapy were eligible to enroll in TRANSCEND NHL 001.
Patients with de novo DLBCL, those who transformed from follicular lymphoma, or those with high-grade B-cell lymphoma made up the pivotal or core population. All DLBCL patients enrolled on the trial comprised the full population.
Patients were screened, enrolled, and underwent apheresis. Bridging therapy was permitted while their CAR T cells were being manufactured.
Patients then had a PET scan and lab tests prior to lymphodepletion.
“This is the time point of our interest,” Dr Saddiqi said, “to see if there are any patient characteristics or biomarkers that we can identify . . . that could help us figure out which patients are at higher risk of toxicity, potentially.”
Lymphodepletion consisted of fludarabine (30 mg/m2) and cyclophosphamide (300 mg/m2 for 3 days).
Patients received the JCAR017 infusion, and, at specific time points thereafter, cytokine, pharmacokinetic (PK), and clinical lab evaluations were conducted. PK evaluation and scans were performed every 3 months for the first year after JCAR017 infusion, and safety and viral vector follow-up for 15 years.
Dose levels were 5 x 107 cells as a single or double dose (DL1S) and 1 x 108 cells as a single dose (DL2S). Dose level 2 was chosen for further study, and double dosing was discontinued.
“Double dosing was actually not pursued further,” Dr Saddiqi explained, “because it did not seem to add any benefit over single dosing.”
At the time of the presentation, 91 total patients were treated, 67 of whom were the core population.
Results
Dr Saddiqi reported that patients treated with JCAR017 achieved a relatively high best overall response rate (ORR) and high durable CR rates.
“And this seems to be especially true for the core set of patients and particularly for patients at dose level 2,” she added.
At all dose levels, the core patients had a best ORR of 84% (41/49) and a CR rate of 61% (30/49).
At follow-up of 3 months or longer, the core group had an ORR of 65% (26/40) for all dose levels, 52% (11/21) for dose level 1, and 80% (12/15) for dose level 2.
The 3-month CR rate was 53% (21/40) for all dose levels in the core group, 33% (7/21) in dose level 1, and 73% (11/15) in dose level 2.
Dr Saddiqi noted that CRS and neurotoxicity did not differ by dose level or schedule, and there were no grade 5 events of CRS or neurotoxicity.
“Among the core group, dose level change did not add to their toxicity,” she said. “And so the question is: Is it patient factors, is it tumor factors? What is it that is actually causing the toxicities in these patients?”
Dr Saddiqi focused the presentation on patient factors.
Patient factors
The data showed that tumor burden and lactose dehydrogenase (LDH) levels were higher in patients with CRS and neurotoxicity.
Univariate analysis revealed that CRS and neurotoxicity were associated with a shorter time since diagnosis.
However, prior number of therapies, patient weight, and disease stage were not associated with CRS or neurotoxicity.
Investigators were able to identify preliminary risk boundaries. Core patients with high LDH levels (≥ 500 U/L) and sum of the products of diameters (SPD) ≥ 50 cm2 at baseline had an 8-fold increase in risk of CRS and neurotoxicity.
“Inversely, if these patients did not meet the cutoff for LDH or SPD,” Dr Saddiqi pointed out, “if they were lower than that, they have significantly lower CRS and neurotoxicity events.”
Investigators also observed that baseline markers of inflammation and inflammatory cytokines trended higher in patients with CRS and neurotoxicity. For CRS, this includes ferritin, C-reactive protein (CRP), IL-10, IL-15, IL-16, TNFα, and MIP-1β. For neurotoxicity, this includes ferritin, CRP, d-Dimer, IL-6, IL-15, TNFα, and MIP-1α.
The team also observed that tumor burden, baseline markers of inflammation, and inflammatory cytokines trended lower in core patients with durable responses.
“Interestingly, it’s inversely true that patients who did have these higher levels [of inflammation markers], and higher tumor burden, and higher LDH, actually were the ones that were either showing no response at 3 months or had lost their response by the 3-month assessment point,” Dr Saddiqi explained.
And in patients with higher baseline tumor burden and inflammatory cytokine levels, JCAR017 T-cell expansion trended higher.
“Some were deemed to be super expanders because their CAR T-cell levels were very high in their blood,” she added.
The investigators created a preliminary logistic model based on the data that suggests a therapeutic window might be able to limit toxicity and optimize efficacy.
The model indicates that patients with higher tumor burden, higher LDH, and higher inflammatory state at baseline seem to be the ones who are having more CRS and more neurotoxicity after CAR T-cell infusion.
“They are expanding their cells much more, yet their responses at 3 months seem to be affected adversely by this entire situation,” Dr Saddiqi said.
"One explanation, potentially, could be that these CAR T cells are seeing a lot of antigen when they go into the body. They have the perfect cytokine milieu to grow, expand, and go crazy in the body, if you will, and very quickly peter out as well because there’s T-cell exhaustion that happens rather rapidly and clinical responses are then then lost.”
The investigators believe that if they can identify those patients ahead of time who may be at risk of too high expansion or too low expansion of their CAR T cells, they may be able to find strategies to push expansion into the “sweet spot of CAR T-cell expansion and ultimately get the holy grail of having durable responses for all with minimal toxicity,” Dr Saddiqi concluded.
TRANSCEND NHL 001 is sponsored by Juno Therapeutics, Inc. Dr Saddiqi has served on a steering committee for JCAR017. ![]()
*Data in the presentation differ from the abstract.
Pembrolizumab sBLA receives priority review
The US Food and Drug Administration (FDA) has granted priority review to a supplemental biologics license application (sBLA) for the anti-PD-1 therapy pembrolizumab (KEYTRUDA).
With this sBLA, Merck is seeking approval for pembrolizumab to treat adult and pediatric patients with refractory primary mediastinal B-cell lymphoma (PMBCL) or patients with PMBCL who have relapsed after 2 or more prior lines of therapy.
The FDA expects to make a decision on the sBLA by April 3, 2018.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Pembrolizumab is currently FDA-approved to treat classical Hodgkin lymphoma, melanoma, lung cancer, head and neck cancer, urothelial carcinoma, microsatellite instability-high cancer, and gastric cancer.
The sBLA for pembrolizumab as a treatment for PMBCL is supported by the phase 2 KEYNOTE-170 trial. Results from this trial were presented at the 2017 ASH Annual Meeting (abstract 2833).
KEYNOTE-170 is an ongoing study in which researchers are evaluating pembrolizumab (given at a 200 mg fixed dose every 3 weeks) in patients with relapsed/refractory PMBCL or relapsed/refractory Richter syndrome.
The PMBCL cohort enrolled patients who relapsed after autologous stem cell transplant (ASCT), were refractory to ASCT, or were ineligible for ASCT. Patients ineligible for ASCT had to have received 2 or more lines of prior therapy.
The median duration of follow-up was 10.5 months (range, 0.1-17.7).
In the efficacy population (n=29), the overall response rate was 41% (n=12), and the complete response rate was 24% (n=7).
The median time to response was 2.8 months (range, 2.4-5.5), and the median duration of response was not reached (range, 1.1+ to 13.6+ months).
Of the 53 patients evaluated for safety, 57% (n=30) experienced treatment-related adverse events (TRAEs), including 21% (n=11) who experienced grade 3-4 TRAEs.
The most common TRAEs (occurring in at least 5% of patients) were neutropenia (n=11), hypothyroidism (n=4), asthenia (n=3), and pyrexia (n=3).
Immune-mediated adverse events of all grades occurred in 11% (n=6) of patients. These include hypothyroidism (n=4), hyperthyroidism (n=2), pneumonitis (n=1), and thyroiditis (n=1). There were no treatment-related deaths. ![]()
*Data in the abstract differ from the presentation.
The US Food and Drug Administration (FDA) has granted priority review to a supplemental biologics license application (sBLA) for the anti-PD-1 therapy pembrolizumab (KEYTRUDA).
With this sBLA, Merck is seeking approval for pembrolizumab to treat adult and pediatric patients with refractory primary mediastinal B-cell lymphoma (PMBCL) or patients with PMBCL who have relapsed after 2 or more prior lines of therapy.
The FDA expects to make a decision on the sBLA by April 3, 2018.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Pembrolizumab is currently FDA-approved to treat classical Hodgkin lymphoma, melanoma, lung cancer, head and neck cancer, urothelial carcinoma, microsatellite instability-high cancer, and gastric cancer.
The sBLA for pembrolizumab as a treatment for PMBCL is supported by the phase 2 KEYNOTE-170 trial. Results from this trial were presented at the 2017 ASH Annual Meeting (abstract 2833).
KEYNOTE-170 is an ongoing study in which researchers are evaluating pembrolizumab (given at a 200 mg fixed dose every 3 weeks) in patients with relapsed/refractory PMBCL or relapsed/refractory Richter syndrome.
The PMBCL cohort enrolled patients who relapsed after autologous stem cell transplant (ASCT), were refractory to ASCT, or were ineligible for ASCT. Patients ineligible for ASCT had to have received 2 or more lines of prior therapy.
The median duration of follow-up was 10.5 months (range, 0.1-17.7).
In the efficacy population (n=29), the overall response rate was 41% (n=12), and the complete response rate was 24% (n=7).
The median time to response was 2.8 months (range, 2.4-5.5), and the median duration of response was not reached (range, 1.1+ to 13.6+ months).
Of the 53 patients evaluated for safety, 57% (n=30) experienced treatment-related adverse events (TRAEs), including 21% (n=11) who experienced grade 3-4 TRAEs.
The most common TRAEs (occurring in at least 5% of patients) were neutropenia (n=11), hypothyroidism (n=4), asthenia (n=3), and pyrexia (n=3).
Immune-mediated adverse events of all grades occurred in 11% (n=6) of patients. These include hypothyroidism (n=4), hyperthyroidism (n=2), pneumonitis (n=1), and thyroiditis (n=1). There were no treatment-related deaths. ![]()
*Data in the abstract differ from the presentation.
The US Food and Drug Administration (FDA) has granted priority review to a supplemental biologics license application (sBLA) for the anti-PD-1 therapy pembrolizumab (KEYTRUDA).
With this sBLA, Merck is seeking approval for pembrolizumab to treat adult and pediatric patients with refractory primary mediastinal B-cell lymphoma (PMBCL) or patients with PMBCL who have relapsed after 2 or more prior lines of therapy.
The FDA expects to make a decision on the sBLA by April 3, 2018.
The agency’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
Pembrolizumab is currently FDA-approved to treat classical Hodgkin lymphoma, melanoma, lung cancer, head and neck cancer, urothelial carcinoma, microsatellite instability-high cancer, and gastric cancer.
The sBLA for pembrolizumab as a treatment for PMBCL is supported by the phase 2 KEYNOTE-170 trial. Results from this trial were presented at the 2017 ASH Annual Meeting (abstract 2833).
KEYNOTE-170 is an ongoing study in which researchers are evaluating pembrolizumab (given at a 200 mg fixed dose every 3 weeks) in patients with relapsed/refractory PMBCL or relapsed/refractory Richter syndrome.
The PMBCL cohort enrolled patients who relapsed after autologous stem cell transplant (ASCT), were refractory to ASCT, or were ineligible for ASCT. Patients ineligible for ASCT had to have received 2 or more lines of prior therapy.
The median duration of follow-up was 10.5 months (range, 0.1-17.7).
In the efficacy population (n=29), the overall response rate was 41% (n=12), and the complete response rate was 24% (n=7).
The median time to response was 2.8 months (range, 2.4-5.5), and the median duration of response was not reached (range, 1.1+ to 13.6+ months).
Of the 53 patients evaluated for safety, 57% (n=30) experienced treatment-related adverse events (TRAEs), including 21% (n=11) who experienced grade 3-4 TRAEs.
The most common TRAEs (occurring in at least 5% of patients) were neutropenia (n=11), hypothyroidism (n=4), asthenia (n=3), and pyrexia (n=3).
Immune-mediated adverse events of all grades occurred in 11% (n=6) of patients. These include hypothyroidism (n=4), hyperthyroidism (n=2), pneumonitis (n=1), and thyroiditis (n=1). There were no treatment-related deaths.
*Data in the abstract differ from the presentation.
Primary analysis confirms interim findings of CTL019 in DLBCL
ATLANTA—The first chimeric antigen receptor (CAR) T-cell therapy approved in the US to treat children and young adults with leukemia is also producing high response rates in lymphoma, according to investigators of the JULIET trial.
They reported that tisagenlecleucel (formerly CTL019) produced an overall response rate (ORR) of 53% and a complete response (CR) rate of 40% in patients with diffuse large B-cell lymphoma (DLBCL).
Additionally, researchers say the stability in the response rate at 3 and 6 months—38% and 37%, respectively—indicates the durability of the therapy.
At 3 months, 32% of patients who achieved CR remained in CR. At 6 months, 30% remained in CR.
Researchers believe these results confirm the durable clinical benefit reported previously.
Stephen J. Schuster, MD, of the University of Pennsylvania in Philadelphia, presented the JULIET data at the 2017 ASH Annual Meeting (abstract 577).
“Only about half of relapsed diffuse large B-cell lymphoma patients are eligible for transplant,” Dr Schuster said. “[O]f those patients, only about a half respond to salvage chemotherapy, and a significant number of patients relapse post-transplant. So there is really a large unmet need for these patients, and CAR T-cell therapy is a potential agent [for them].”
The JULIET trial was a global, single-arm, phase 2 trial evaluating tisagenlecleucel in DLBCL patients. Tisagenlecleucel (Kymriah™) consists of CAR T cells with a CD19 antigen-binding domain, a 4-1BB costimulatory domain, and a CD3-zeta signaling domain.
The trial was conducted at 27 sites in 10 countries across North America, Europe, Australia, and Asia. There were 2 centralized manufacturing sites, one in Europe and one in the US.
Patients had to be 18 years or older, have had 2 or more prior lines of therapy for DLBCL, and have progressive disease or be ineligible for autologous stem cell transplant (auto-SCT). They could not have had any prior anti-CD19 therapy, and they could not have any central nervous system involvement.
The primary endpoint was best ORR using Lugano criteria with assessment by an independent review committee. Secondary endpoints included duration of response, overall survival (OS), and safety.
Study design and enrollment
Patients were screened and underwent apheresis with cryopreservation of their leukapheresis products during screening, which “allowed for enrollment of all eligible patients,” Dr Schuster said.
Patients could receive bridging chemotherapy while they awaited the manufacture of the CAR T cells.
“What’s important to note is that, early on in the trial, there was a shortage of manufacturing capacity, and this led to a longer-than-anticipated interval between enrollment and treatment,” Dr Schuster said. “This interval decreased as manufacturing capacity improved throughout the trial.”
When their CAR T cells were ready, patients were restaged, lymphodepleted, and received the tisagenlecleucel infusion. The dose ranged from 0.6 x 108 to 6.0 x 108 CAR-positive T cells.
The infusion could be conducted on an inpatient or outpatient basis at the investigator’s discretion, Dr Schuster said.
As of the data cutoff in March 2017, investigators enrolled 147 patients and infused 99 with tisagenlecleucel.
Forty-three patients discontinued before infusion, 9 because of an inability to manufacture the T-cell product and 34 due to death (n=16), physician decision (n=12), patient decision (n=3), adverse event (n=2), and protocol deviation (n=1). Five patients were pending infusion.
There were 81 patients with at least 3 months of follow-up or earlier disease progression evaluable for response.
Patient characteristics
Patients were a median age of 56 (range, 22–76), and 23% were 65 or older. All had an ECOG performance status of 0 or 1, 80% had DLBCL, and 19% had transformed follicular lymphoma.
Fifteen percent had double or triple hits in CMYC, BCL2, and BCL6 genes, and 52% had germinal center B-cell type disease.
Forty-four percent had 2 prior lines of therapy, 31% had 3 prior lines of therapy, and 19% had 4 to 6 prior lines of therapy. All were either refractory to or relapsed from their last therapy.
Forty-seven percent had undergone prior auto-SCT.
Eighty-nine of the 99 patients infused with tisagenlecleucel received bridging therapy, and 92 received lymphodepleting therapy.
Twenty-six patients were infused as outpatients, and 20 remained as outpatients for 3 or more days after the infusion.
Efficacy
The trial met its primary endpoint with an ORR of 53% tested against the null hypothesis of 20% or less. Forty percent of patients achieved a CR, and 14% had a partial response.
The ORR was consistent across all subgroups, including age, sex, lines of prior antineoplastic therapy, cell of origin, and rearranged MYC/BCL2/BCL6.
“The durability of response, however, which is really the message, is shown by the stability between 3- and 6-month response rates, 38% and 37%, respectively,” Dr Schuster said. “The response rate at 3 months is really indicative of the long-term benefit of this treatment approach.”
The investigators observed no apparent relationship between tumor response at month 3 and dose. And they observed responses at all dose levels.
The very early response may be due, to a certain extent, to the chemotherapy, according to Dr Schuster.
“The effect of the T cells becomes evident as you follow these patients over time,” he said.
The median duration of response and overall response have not been reached. And 74% of patients were relapse-free at 6 months.
“Importantly, almost all the complete responders at month 3 remained in complete response,” Dr Schuster said.
Safety
Adverse events of special interest that occurred within 8 weeks of the infusion included:
- Cytokine release syndrome (CRS)—58% all grades, 15% grade 3, 8% grade 4
- Neurologic events—21% all grades, 8% grade 3, 4% grade 4
- Prolonged cytopenia—36% all grades, 15% grade 3, 12% grade 4
- Infections—34% all grades, 18% grade 3, 2% grade 4
- Febrile neutropenia—13% all grades, 11% grade 3, 2% grade 4
No deaths occurred due to tisagenlecleucel, CRS, or cerebral edema.
Fifty-seven patients developed CRS. The median time to onset of CRS was 3 days (range, 1–9), and the median duration of CRS was 7 days (range, 2–30).
Twenty-eight percent of patients developed hypotension that required intervention, 6% requiring high-dose vasopressors. Eight percent were intubated, and 16% received anticytokine therapy—15% with tocilizumab and 11% with corticosteroids.
Investigators did not observe a relationship between dose and neurological events. However, they did detect a higher probability of CRS with the higher doses of tisagenlecleucel.
They also noted that dose and exposure were independent.
Dr Schuster indicated that these data are the basis for global regulatory submissions.
Manufacture of tisagenlecleucel was centralized, and investigators believe the trial shows the feasibility of global distribution of CAR T-cell therapy using cryopreserved apheresis and centralized manufacturing.
Novartis Pharmaceuticals, the sponsor of the trial, is now able to commercially manufacture the CAR T cells in 22 days.
Dr Schuster disclosed research funding and consulting fees from Novartis and Celgene.
ATLANTA—The first chimeric antigen receptor (CAR) T-cell therapy approved in the US to treat children and young adults with leukemia is also producing high response rates in lymphoma, according to investigators of the JULIET trial.
They reported that tisagenlecleucel (formerly CTL019) produced an overall response rate (ORR) of 53% and a complete response (CR) rate of 40% in patients with diffuse large B-cell lymphoma (DLBCL).
Additionally, researchers say the stability in the response rate at 3 and 6 months—38% and 37%, respectively—indicates the durability of the therapy.
At 3 months, 32% of patients who achieved CR remained in CR. At 6 months, 30% remained in CR.
Researchers believe these results confirm the durable clinical benefit reported previously.
Stephen J. Schuster, MD, of the University of Pennsylvania in Philadelphia, presented the JULIET data at the 2017 ASH Annual Meeting (abstract 577).
“Only about half of relapsed diffuse large B-cell lymphoma patients are eligible for transplant,” Dr Schuster said. “[O]f those patients, only about a half respond to salvage chemotherapy, and a significant number of patients relapse post-transplant. So there is really a large unmet need for these patients, and CAR T-cell therapy is a potential agent [for them].”
The JULIET trial was a global, single-arm, phase 2 trial evaluating tisagenlecleucel in DLBCL patients. Tisagenlecleucel (Kymriah™) consists of CAR T cells with a CD19 antigen-binding domain, a 4-1BB costimulatory domain, and a CD3-zeta signaling domain.
The trial was conducted at 27 sites in 10 countries across North America, Europe, Australia, and Asia. There were 2 centralized manufacturing sites, one in Europe and one in the US.
Patients had to be 18 years or older, have had 2 or more prior lines of therapy for DLBCL, and have progressive disease or be ineligible for autologous stem cell transplant (auto-SCT). They could not have had any prior anti-CD19 therapy, and they could not have any central nervous system involvement.
The primary endpoint was best ORR using Lugano criteria with assessment by an independent review committee. Secondary endpoints included duration of response, overall survival (OS), and safety.
Study design and enrollment
Patients were screened and underwent apheresis with cryopreservation of their leukapheresis products during screening, which “allowed for enrollment of all eligible patients,” Dr Schuster said.
Patients could receive bridging chemotherapy while they awaited the manufacture of the CAR T cells.
“What’s important to note is that, early on in the trial, there was a shortage of manufacturing capacity, and this led to a longer-than-anticipated interval between enrollment and treatment,” Dr Schuster said. “This interval decreased as manufacturing capacity improved throughout the trial.”
When their CAR T cells were ready, patients were restaged, lymphodepleted, and received the tisagenlecleucel infusion. The dose ranged from 0.6 x 108 to 6.0 x 108 CAR-positive T cells.
The infusion could be conducted on an inpatient or outpatient basis at the investigator’s discretion, Dr Schuster said.
As of the data cutoff in March 2017, investigators enrolled 147 patients and infused 99 with tisagenlecleucel.
Forty-three patients discontinued before infusion, 9 because of an inability to manufacture the T-cell product and 34 due to death (n=16), physician decision (n=12), patient decision (n=3), adverse event (n=2), and protocol deviation (n=1). Five patients were pending infusion.
There were 81 patients with at least 3 months of follow-up or earlier disease progression evaluable for response.
Patient characteristics
Patients were a median age of 56 (range, 22–76), and 23% were 65 or older. All had an ECOG performance status of 0 or 1, 80% had DLBCL, and 19% had transformed follicular lymphoma.
Fifteen percent had double or triple hits in CMYC, BCL2, and BCL6 genes, and 52% had germinal center B-cell type disease.
Forty-four percent had 2 prior lines of therapy, 31% had 3 prior lines of therapy, and 19% had 4 to 6 prior lines of therapy. All were either refractory to or relapsed from their last therapy.
Forty-seven percent had undergone prior auto-SCT.
Eighty-nine of the 99 patients infused with tisagenlecleucel received bridging therapy, and 92 received lymphodepleting therapy.
Twenty-six patients were infused as outpatients, and 20 remained as outpatients for 3 or more days after the infusion.
Efficacy
The trial met its primary endpoint with an ORR of 53% tested against the null hypothesis of 20% or less. Forty percent of patients achieved a CR, and 14% had a partial response.
The ORR was consistent across all subgroups, including age, sex, lines of prior antineoplastic therapy, cell of origin, and rearranged MYC/BCL2/BCL6.
“The durability of response, however, which is really the message, is shown by the stability between 3- and 6-month response rates, 38% and 37%, respectively,” Dr Schuster said. “The response rate at 3 months is really indicative of the long-term benefit of this treatment approach.”
The investigators observed no apparent relationship between tumor response at month 3 and dose. And they observed responses at all dose levels.
The very early response may be due, to a certain extent, to the chemotherapy, according to Dr Schuster.
“The effect of the T cells becomes evident as you follow these patients over time,” he said.
The median duration of response and overall response have not been reached. And 74% of patients were relapse-free at 6 months.
“Importantly, almost all the complete responders at month 3 remained in complete response,” Dr Schuster said.
Safety
Adverse events of special interest that occurred within 8 weeks of the infusion included:
- Cytokine release syndrome (CRS)—58% all grades, 15% grade 3, 8% grade 4
- Neurologic events—21% all grades, 8% grade 3, 4% grade 4
- Prolonged cytopenia—36% all grades, 15% grade 3, 12% grade 4
- Infections—34% all grades, 18% grade 3, 2% grade 4
- Febrile neutropenia—13% all grades, 11% grade 3, 2% grade 4
No deaths occurred due to tisagenlecleucel, CRS, or cerebral edema.
Fifty-seven patients developed CRS. The median time to onset of CRS was 3 days (range, 1–9), and the median duration of CRS was 7 days (range, 2–30).
Twenty-eight percent of patients developed hypotension that required intervention, 6% requiring high-dose vasopressors. Eight percent were intubated, and 16% received anticytokine therapy—15% with tocilizumab and 11% with corticosteroids.
Investigators did not observe a relationship between dose and neurological events. However, they did detect a higher probability of CRS with the higher doses of tisagenlecleucel.
They also noted that dose and exposure were independent.
Dr Schuster indicated that these data are the basis for global regulatory submissions.
Manufacture of tisagenlecleucel was centralized, and investigators believe the trial shows the feasibility of global distribution of CAR T-cell therapy using cryopreserved apheresis and centralized manufacturing.
Novartis Pharmaceuticals, the sponsor of the trial, is now able to commercially manufacture the CAR T cells in 22 days.
Dr Schuster disclosed research funding and consulting fees from Novartis and Celgene.
ATLANTA—The first chimeric antigen receptor (CAR) T-cell therapy approved in the US to treat children and young adults with leukemia is also producing high response rates in lymphoma, according to investigators of the JULIET trial.
They reported that tisagenlecleucel (formerly CTL019) produced an overall response rate (ORR) of 53% and a complete response (CR) rate of 40% in patients with diffuse large B-cell lymphoma (DLBCL).
Additionally, researchers say the stability in the response rate at 3 and 6 months—38% and 37%, respectively—indicates the durability of the therapy.
At 3 months, 32% of patients who achieved CR remained in CR. At 6 months, 30% remained in CR.
Researchers believe these results confirm the durable clinical benefit reported previously.
Stephen J. Schuster, MD, of the University of Pennsylvania in Philadelphia, presented the JULIET data at the 2017 ASH Annual Meeting (abstract 577).
“Only about half of relapsed diffuse large B-cell lymphoma patients are eligible for transplant,” Dr Schuster said. “[O]f those patients, only about a half respond to salvage chemotherapy, and a significant number of patients relapse post-transplant. So there is really a large unmet need for these patients, and CAR T-cell therapy is a potential agent [for them].”
The JULIET trial was a global, single-arm, phase 2 trial evaluating tisagenlecleucel in DLBCL patients. Tisagenlecleucel (Kymriah™) consists of CAR T cells with a CD19 antigen-binding domain, a 4-1BB costimulatory domain, and a CD3-zeta signaling domain.
The trial was conducted at 27 sites in 10 countries across North America, Europe, Australia, and Asia. There were 2 centralized manufacturing sites, one in Europe and one in the US.
Patients had to be 18 years or older, have had 2 or more prior lines of therapy for DLBCL, and have progressive disease or be ineligible for autologous stem cell transplant (auto-SCT). They could not have had any prior anti-CD19 therapy, and they could not have any central nervous system involvement.
The primary endpoint was best ORR using Lugano criteria with assessment by an independent review committee. Secondary endpoints included duration of response, overall survival (OS), and safety.
Study design and enrollment
Patients were screened and underwent apheresis with cryopreservation of their leukapheresis products during screening, which “allowed for enrollment of all eligible patients,” Dr Schuster said.
Patients could receive bridging chemotherapy while they awaited the manufacture of the CAR T cells.
“What’s important to note is that, early on in the trial, there was a shortage of manufacturing capacity, and this led to a longer-than-anticipated interval between enrollment and treatment,” Dr Schuster said. “This interval decreased as manufacturing capacity improved throughout the trial.”
When their CAR T cells were ready, patients were restaged, lymphodepleted, and received the tisagenlecleucel infusion. The dose ranged from 0.6 x 108 to 6.0 x 108 CAR-positive T cells.
The infusion could be conducted on an inpatient or outpatient basis at the investigator’s discretion, Dr Schuster said.
As of the data cutoff in March 2017, investigators enrolled 147 patients and infused 99 with tisagenlecleucel.
Forty-three patients discontinued before infusion, 9 because of an inability to manufacture the T-cell product and 34 due to death (n=16), physician decision (n=12), patient decision (n=3), adverse event (n=2), and protocol deviation (n=1). Five patients were pending infusion.
There were 81 patients with at least 3 months of follow-up or earlier disease progression evaluable for response.
Patient characteristics
Patients were a median age of 56 (range, 22–76), and 23% were 65 or older. All had an ECOG performance status of 0 or 1, 80% had DLBCL, and 19% had transformed follicular lymphoma.
Fifteen percent had double or triple hits in CMYC, BCL2, and BCL6 genes, and 52% had germinal center B-cell type disease.
Forty-four percent had 2 prior lines of therapy, 31% had 3 prior lines of therapy, and 19% had 4 to 6 prior lines of therapy. All were either refractory to or relapsed from their last therapy.
Forty-seven percent had undergone prior auto-SCT.
Eighty-nine of the 99 patients infused with tisagenlecleucel received bridging therapy, and 92 received lymphodepleting therapy.
Twenty-six patients were infused as outpatients, and 20 remained as outpatients for 3 or more days after the infusion.
Efficacy
The trial met its primary endpoint with an ORR of 53% tested against the null hypothesis of 20% or less. Forty percent of patients achieved a CR, and 14% had a partial response.
The ORR was consistent across all subgroups, including age, sex, lines of prior antineoplastic therapy, cell of origin, and rearranged MYC/BCL2/BCL6.
“The durability of response, however, which is really the message, is shown by the stability between 3- and 6-month response rates, 38% and 37%, respectively,” Dr Schuster said. “The response rate at 3 months is really indicative of the long-term benefit of this treatment approach.”
The investigators observed no apparent relationship between tumor response at month 3 and dose. And they observed responses at all dose levels.
The very early response may be due, to a certain extent, to the chemotherapy, according to Dr Schuster.
“The effect of the T cells becomes evident as you follow these patients over time,” he said.
The median duration of response and overall response have not been reached. And 74% of patients were relapse-free at 6 months.
“Importantly, almost all the complete responders at month 3 remained in complete response,” Dr Schuster said.
Safety
Adverse events of special interest that occurred within 8 weeks of the infusion included:
- Cytokine release syndrome (CRS)—58% all grades, 15% grade 3, 8% grade 4
- Neurologic events—21% all grades, 8% grade 3, 4% grade 4
- Prolonged cytopenia—36% all grades, 15% grade 3, 12% grade 4
- Infections—34% all grades, 18% grade 3, 2% grade 4
- Febrile neutropenia—13% all grades, 11% grade 3, 2% grade 4
No deaths occurred due to tisagenlecleucel, CRS, or cerebral edema.
Fifty-seven patients developed CRS. The median time to onset of CRS was 3 days (range, 1–9), and the median duration of CRS was 7 days (range, 2–30).
Twenty-eight percent of patients developed hypotension that required intervention, 6% requiring high-dose vasopressors. Eight percent were intubated, and 16% received anticytokine therapy—15% with tocilizumab and 11% with corticosteroids.
Investigators did not observe a relationship between dose and neurological events. However, they did detect a higher probability of CRS with the higher doses of tisagenlecleucel.
They also noted that dose and exposure were independent.
Dr Schuster indicated that these data are the basis for global regulatory submissions.
Manufacture of tisagenlecleucel was centralized, and investigators believe the trial shows the feasibility of global distribution of CAR T-cell therapy using cryopreserved apheresis and centralized manufacturing.
Novartis Pharmaceuticals, the sponsor of the trial, is now able to commercially manufacture the CAR T cells in 22 days.
Dr Schuster disclosed research funding and consulting fees from Novartis and Celgene.
VIDEO - New lymphoma drug approvals: Clinical use, future directions
ATLANTA – 2017 was a banner year for the approval of new drugs to treat hematologic disorders.
At a special interest session at the annual meeting of American Society of Hematology, representatives from the Food and Drug Administration joined forces with clinicians to discuss the use of the newly approved treatments in the real-world setting.
In this video interview,
“This is extremely exciting,” she said regarding the pace of new approvals for hematologic malignancies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Axicabtagene ciloleucel, a CAR T-cell product approved in October for the treatment of relapsed/refractory large B-cell lymphoma in adults, is particularly interesting, she said.
“The data shows that if you look at a population of diffuse large B-cell lymphoma patients, that historically have a very poor outcome, there is definitely an impressive response rate and improved survival, compared to the natural history cohort,” said Dr. Heslop of Baylor College of Medicine, Houston.
However, while the findings are encouraging, only 30%-40% are having a durable response, she added.
“So I think there’ll be lots of efforts to try and improve the response rate by combination with other agents such as checkpoint inhibitors or other immunomodulators,” she said.
With respect to the second-generation Bruton’s tyrosine kinase inhibitor acalabrutinib, which was approved in October for adults with mantle cell lymphoma who have been treated with at least one prior therapy, she discussed the potential for improved outcomes and the importance of looking further into its use in patients who have failed ibrutinib therapy, as well as its use in combination with other agents, such as bendamustine and rituximab early in the course of disease.
Copanlisib, a PI3 kinase inhibitor approved in September, is an addition to the armamentarium for adult patients with relapsed follicular lymphoma after two lines of previous therapy.
“It still does have some side effects, as do other drugs in this class, so I think it’s place will still need to be defined,” Dr. Heslop said.
She reported having no relevant financial disclosures.
ATLANTA – 2017 was a banner year for the approval of new drugs to treat hematologic disorders.
At a special interest session at the annual meeting of American Society of Hematology, representatives from the Food and Drug Administration joined forces with clinicians to discuss the use of the newly approved treatments in the real-world setting.
In this video interview,
“This is extremely exciting,” she said regarding the pace of new approvals for hematologic malignancies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Axicabtagene ciloleucel, a CAR T-cell product approved in October for the treatment of relapsed/refractory large B-cell lymphoma in adults, is particularly interesting, she said.
“The data shows that if you look at a population of diffuse large B-cell lymphoma patients, that historically have a very poor outcome, there is definitely an impressive response rate and improved survival, compared to the natural history cohort,” said Dr. Heslop of Baylor College of Medicine, Houston.
However, while the findings are encouraging, only 30%-40% are having a durable response, she added.
“So I think there’ll be lots of efforts to try and improve the response rate by combination with other agents such as checkpoint inhibitors or other immunomodulators,” she said.
With respect to the second-generation Bruton’s tyrosine kinase inhibitor acalabrutinib, which was approved in October for adults with mantle cell lymphoma who have been treated with at least one prior therapy, she discussed the potential for improved outcomes and the importance of looking further into its use in patients who have failed ibrutinib therapy, as well as its use in combination with other agents, such as bendamustine and rituximab early in the course of disease.
Copanlisib, a PI3 kinase inhibitor approved in September, is an addition to the armamentarium for adult patients with relapsed follicular lymphoma after two lines of previous therapy.
“It still does have some side effects, as do other drugs in this class, so I think it’s place will still need to be defined,” Dr. Heslop said.
She reported having no relevant financial disclosures.
ATLANTA – 2017 was a banner year for the approval of new drugs to treat hematologic disorders.
At a special interest session at the annual meeting of American Society of Hematology, representatives from the Food and Drug Administration joined forces with clinicians to discuss the use of the newly approved treatments in the real-world setting.
In this video interview,
“This is extremely exciting,” she said regarding the pace of new approvals for hematologic malignancies.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Axicabtagene ciloleucel, a CAR T-cell product approved in October for the treatment of relapsed/refractory large B-cell lymphoma in adults, is particularly interesting, she said.
“The data shows that if you look at a population of diffuse large B-cell lymphoma patients, that historically have a very poor outcome, there is definitely an impressive response rate and improved survival, compared to the natural history cohort,” said Dr. Heslop of Baylor College of Medicine, Houston.
However, while the findings are encouraging, only 30%-40% are having a durable response, she added.
“So I think there’ll be lots of efforts to try and improve the response rate by combination with other agents such as checkpoint inhibitors or other immunomodulators,” she said.
With respect to the second-generation Bruton’s tyrosine kinase inhibitor acalabrutinib, which was approved in October for adults with mantle cell lymphoma who have been treated with at least one prior therapy, she discussed the potential for improved outcomes and the importance of looking further into its use in patients who have failed ibrutinib therapy, as well as its use in combination with other agents, such as bendamustine and rituximab early in the course of disease.
Copanlisib, a PI3 kinase inhibitor approved in September, is an addition to the armamentarium for adult patients with relapsed follicular lymphoma after two lines of previous therapy.
“It still does have some side effects, as do other drugs in this class, so I think it’s place will still need to be defined,” Dr. Heslop said.
She reported having no relevant financial disclosures.
REPORTING FROM ASH 2017