User login
Checkpoint inhibitors look safe in rheumatology patients
People with rheumatologic diseases and cancer appear to be at no higher risk of having an adverse event or disease flare if they receive checkpoint inhibitor therapy, compared with the general population, experience from the Mayo Clinic suggests.
In a brief report published in Arthritis and Rheumatology, a team from the Mayo Clinic in Rochester, Minn., reported on 16 patients with rheumatologic diseases who received cancer immunotherapy. They found that only a minority experienced a flare of their disease or another immune-related event.
The rate of severe immune-related adverse effects (IRAEs) with a single immune checkpoint inhibitor (ICI) has been reported to be less than 2% among the average population. However, less is known about patients with underlying rheumatologic disease, largely because initial trials of ICIs had excluded patients with autoimmune diseases for fear the treatment would induce a disease flare, the researchers noted.
Small studies have suggested that people with inflammatory arthritis or connective tissue diseases have higher rates of IRAEs with immunotherapy, but it is unclear how often these events represented flares of their disease or new autoimmune events, and whether the events had any predictive significance for cancer survival.
In this study, researchers performed a retrospective review of medical records and identified 16 patients with rheumatologic diseases who had received checkpoint inhibitor therapy at the Mayo Clinic between 2011 and 2016.
The most common rheumatologic diseases among the 16 patients were rheumatoid arthritis, polymyalgia rheumatica, Sjögren’s syndrome, and systemic lupus erythematosus, and the most common cancers were malignant melanoma, pulmonary malignancies, and non-Hodgkin lymphoma. Seven of the patients were receiving immunosuppressive therapy or glucocorticoids for their rheumatologic disease upon initiation of a checkpoint inhibitor.
Ten patients had received a prior disease-modifying antirheumatic drug, but only two patients were still taking this at the time of ICI initiation.
Results showed that six of the patients (38%) had an IRAE or flare of their rheumatologic disease, two were graded as mild. All of the patients responded well to glucocorticoids and discontinuation of therapy. The most common event was colitis and just one patient had a flare of rheumatologic disease.
“This is consistent with what is currently known about the management of IRAEs,” the research team wrote. “This study adds further support to the emerging notion that the rate of IRAEs is not necessarily higher in this group compared to the general population.”
The type and severity of rheumatologic disease may play an important role in both the risk of disease flare and IRAEs, a factor that they were unable to assess in the current study, the researchers wrote.
“Further large, prospective studies are needed to address the link between the type, severity, and concurrent rheumatologic disease activity on the risk of flare and IRAE. It is possible that patients with more severe or active disease are at higher risk for these complications,” they wrote.
While patients in the study did not appear to have significantly increased incidence or severity of adverse effects, the research team advised that “treatment decisions must factor in clinical judgement.”
They noted that some studies had proposed predictive biomarkers, pretreatment workup, and monitoring, but this advice was based on a small body of evidence.
“Larger, prospective studies will be necessary to validate these findings and establish evidence-based guidelines for appropriate identification and rating of the rheumatologic IRAEs as well as their treatment, such that patients can continue to receive potentially life-saving cancer treatments,” they wrote.
One of the researchers reported advisory board membership with Bristol-Myers Squibb.
SOURCE: Richter M et al. Arthritis Rheumatol. 2018 Jan 24. doi: 10.1002/art.40397.
People with rheumatologic diseases and cancer appear to be at no higher risk of having an adverse event or disease flare if they receive checkpoint inhibitor therapy, compared with the general population, experience from the Mayo Clinic suggests.
In a brief report published in Arthritis and Rheumatology, a team from the Mayo Clinic in Rochester, Minn., reported on 16 patients with rheumatologic diseases who received cancer immunotherapy. They found that only a minority experienced a flare of their disease or another immune-related event.
The rate of severe immune-related adverse effects (IRAEs) with a single immune checkpoint inhibitor (ICI) has been reported to be less than 2% among the average population. However, less is known about patients with underlying rheumatologic disease, largely because initial trials of ICIs had excluded patients with autoimmune diseases for fear the treatment would induce a disease flare, the researchers noted.
Small studies have suggested that people with inflammatory arthritis or connective tissue diseases have higher rates of IRAEs with immunotherapy, but it is unclear how often these events represented flares of their disease or new autoimmune events, and whether the events had any predictive significance for cancer survival.
In this study, researchers performed a retrospective review of medical records and identified 16 patients with rheumatologic diseases who had received checkpoint inhibitor therapy at the Mayo Clinic between 2011 and 2016.
The most common rheumatologic diseases among the 16 patients were rheumatoid arthritis, polymyalgia rheumatica, Sjögren’s syndrome, and systemic lupus erythematosus, and the most common cancers were malignant melanoma, pulmonary malignancies, and non-Hodgkin lymphoma. Seven of the patients were receiving immunosuppressive therapy or glucocorticoids for their rheumatologic disease upon initiation of a checkpoint inhibitor.
Ten patients had received a prior disease-modifying antirheumatic drug, but only two patients were still taking this at the time of ICI initiation.
Results showed that six of the patients (38%) had an IRAE or flare of their rheumatologic disease, two were graded as mild. All of the patients responded well to glucocorticoids and discontinuation of therapy. The most common event was colitis and just one patient had a flare of rheumatologic disease.
“This is consistent with what is currently known about the management of IRAEs,” the research team wrote. “This study adds further support to the emerging notion that the rate of IRAEs is not necessarily higher in this group compared to the general population.”
The type and severity of rheumatologic disease may play an important role in both the risk of disease flare and IRAEs, a factor that they were unable to assess in the current study, the researchers wrote.
“Further large, prospective studies are needed to address the link between the type, severity, and concurrent rheumatologic disease activity on the risk of flare and IRAE. It is possible that patients with more severe or active disease are at higher risk for these complications,” they wrote.
While patients in the study did not appear to have significantly increased incidence or severity of adverse effects, the research team advised that “treatment decisions must factor in clinical judgement.”
They noted that some studies had proposed predictive biomarkers, pretreatment workup, and monitoring, but this advice was based on a small body of evidence.
“Larger, prospective studies will be necessary to validate these findings and establish evidence-based guidelines for appropriate identification and rating of the rheumatologic IRAEs as well as their treatment, such that patients can continue to receive potentially life-saving cancer treatments,” they wrote.
One of the researchers reported advisory board membership with Bristol-Myers Squibb.
SOURCE: Richter M et al. Arthritis Rheumatol. 2018 Jan 24. doi: 10.1002/art.40397.
People with rheumatologic diseases and cancer appear to be at no higher risk of having an adverse event or disease flare if they receive checkpoint inhibitor therapy, compared with the general population, experience from the Mayo Clinic suggests.
In a brief report published in Arthritis and Rheumatology, a team from the Mayo Clinic in Rochester, Minn., reported on 16 patients with rheumatologic diseases who received cancer immunotherapy. They found that only a minority experienced a flare of their disease or another immune-related event.
The rate of severe immune-related adverse effects (IRAEs) with a single immune checkpoint inhibitor (ICI) has been reported to be less than 2% among the average population. However, less is known about patients with underlying rheumatologic disease, largely because initial trials of ICIs had excluded patients with autoimmune diseases for fear the treatment would induce a disease flare, the researchers noted.
Small studies have suggested that people with inflammatory arthritis or connective tissue diseases have higher rates of IRAEs with immunotherapy, but it is unclear how often these events represented flares of their disease or new autoimmune events, and whether the events had any predictive significance for cancer survival.
In this study, researchers performed a retrospective review of medical records and identified 16 patients with rheumatologic diseases who had received checkpoint inhibitor therapy at the Mayo Clinic between 2011 and 2016.
The most common rheumatologic diseases among the 16 patients were rheumatoid arthritis, polymyalgia rheumatica, Sjögren’s syndrome, and systemic lupus erythematosus, and the most common cancers were malignant melanoma, pulmonary malignancies, and non-Hodgkin lymphoma. Seven of the patients were receiving immunosuppressive therapy or glucocorticoids for their rheumatologic disease upon initiation of a checkpoint inhibitor.
Ten patients had received a prior disease-modifying antirheumatic drug, but only two patients were still taking this at the time of ICI initiation.
Results showed that six of the patients (38%) had an IRAE or flare of their rheumatologic disease, two were graded as mild. All of the patients responded well to glucocorticoids and discontinuation of therapy. The most common event was colitis and just one patient had a flare of rheumatologic disease.
“This is consistent with what is currently known about the management of IRAEs,” the research team wrote. “This study adds further support to the emerging notion that the rate of IRAEs is not necessarily higher in this group compared to the general population.”
The type and severity of rheumatologic disease may play an important role in both the risk of disease flare and IRAEs, a factor that they were unable to assess in the current study, the researchers wrote.
“Further large, prospective studies are needed to address the link between the type, severity, and concurrent rheumatologic disease activity on the risk of flare and IRAE. It is possible that patients with more severe or active disease are at higher risk for these complications,” they wrote.
While patients in the study did not appear to have significantly increased incidence or severity of adverse effects, the research team advised that “treatment decisions must factor in clinical judgement.”
They noted that some studies had proposed predictive biomarkers, pretreatment workup, and monitoring, but this advice was based on a small body of evidence.
“Larger, prospective studies will be necessary to validate these findings and establish evidence-based guidelines for appropriate identification and rating of the rheumatologic IRAEs as well as their treatment, such that patients can continue to receive potentially life-saving cancer treatments,” they wrote.
One of the researchers reported advisory board membership with Bristol-Myers Squibb.
SOURCE: Richter M et al. Arthritis Rheumatol. 2018 Jan 24. doi: 10.1002/art.40397.
FROM ARTHRITIS AND RHEUMATOLOGY
Key clinical point:
Major finding: Six of 16 patients (38%) with rheumatologic disease and cancer had an IRAE or flare of their rheumatologic disease.
Study details: A single-center, retrospective records review to identify patients with rheumatologic diseases who had received checkpoint inhibitor therapy at Mayo Clinic between 2011 and 2016.
Disclosures: One of the authors reported advisory board membership with Bristol-Myers Squibb.
Source: Richter M et al. Arthritis Rheumatol. 2018 Jan 24. doi: 10.1002/art.40397.
Risks of MGUS persist beyond 30 years
A long-term study showed that patients with monoclonal gammopathy of undetermined significance (MGUS) were still at risk of progressing to other plasma-cell or lymphoid disorders after more than 30 years of follow-up.
The risk of developing such disorders was nearly 7 times higher in MGUS patients than in matched control subjects.
Patients with MGUS also had a significantly shorter median survival than controls.
Researchers reported these findings in NEJM.
“Monoclonal gammopathy of undetermined significance is present in more than 3% of the general population age 50 and older,” said study author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“In some cases, people with monoclonal gammopathy of undetermined significance go on to develop multiple myeloma.”
With this in mind, Dr Rajkumar and his colleagues studied 1384 patients—210 with IgM MGUS and 1129 with non-IgM MGUS. Patients were diagnosed with MGUS from 1960 through 1994, and their median age at diagnosis was 72.
The median follow-up was 34.1 years (range, 0.0 to 43.6), so there were 14,130 person-years of follow-up.
During that time, 147 patients progressed to another disorder, including:
- 97 to multiple myeloma
- 19 to non-Hodgkin lymphoma
- 14 to AL amyloidosis
- 13 to Waldenstrom’s macroglobulinemia
- 3 to chronic lymphocytic leukemia
- 1 to plasmacytoma.
The rate of progression in MGUS patients—11%—represented a risk of these disorders that was 6.5 times higher than the risk observed in an age- and sex-matched control population.
The risk of progression also increased over time for MGUS patients. Without accounting for death due to competing causes, the risk of progression was 10% at 10 years, 18% at 20 years, 28% at 30 years, and 36% at both 35 and 40 years.
“We also found that patients with monoclonal gammopathy of undetermined significance had shorter survival than comparable people without the condition, which raises the possibility there may be other disorders associated with monoclonal gammopathy of undetermined significance that still need further study,” Dr Rajkumar said.
The median survival was 8.1 years in MGUS patients and 12.4 years in controls (P<0.001).
Overall, 1300 MGUS patients (94%) had died at last follow-up. Of the 84 patients who were still alive, 5 had progressed. ![]()
A long-term study showed that patients with monoclonal gammopathy of undetermined significance (MGUS) were still at risk of progressing to other plasma-cell or lymphoid disorders after more than 30 years of follow-up.
The risk of developing such disorders was nearly 7 times higher in MGUS patients than in matched control subjects.
Patients with MGUS also had a significantly shorter median survival than controls.
Researchers reported these findings in NEJM.
“Monoclonal gammopathy of undetermined significance is present in more than 3% of the general population age 50 and older,” said study author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“In some cases, people with monoclonal gammopathy of undetermined significance go on to develop multiple myeloma.”
With this in mind, Dr Rajkumar and his colleagues studied 1384 patients—210 with IgM MGUS and 1129 with non-IgM MGUS. Patients were diagnosed with MGUS from 1960 through 1994, and their median age at diagnosis was 72.
The median follow-up was 34.1 years (range, 0.0 to 43.6), so there were 14,130 person-years of follow-up.
During that time, 147 patients progressed to another disorder, including:
- 97 to multiple myeloma
- 19 to non-Hodgkin lymphoma
- 14 to AL amyloidosis
- 13 to Waldenstrom’s macroglobulinemia
- 3 to chronic lymphocytic leukemia
- 1 to plasmacytoma.
The rate of progression in MGUS patients—11%—represented a risk of these disorders that was 6.5 times higher than the risk observed in an age- and sex-matched control population.
The risk of progression also increased over time for MGUS patients. Without accounting for death due to competing causes, the risk of progression was 10% at 10 years, 18% at 20 years, 28% at 30 years, and 36% at both 35 and 40 years.
“We also found that patients with monoclonal gammopathy of undetermined significance had shorter survival than comparable people without the condition, which raises the possibility there may be other disorders associated with monoclonal gammopathy of undetermined significance that still need further study,” Dr Rajkumar said.
The median survival was 8.1 years in MGUS patients and 12.4 years in controls (P<0.001).
Overall, 1300 MGUS patients (94%) had died at last follow-up. Of the 84 patients who were still alive, 5 had progressed. ![]()
A long-term study showed that patients with monoclonal gammopathy of undetermined significance (MGUS) were still at risk of progressing to other plasma-cell or lymphoid disorders after more than 30 years of follow-up.
The risk of developing such disorders was nearly 7 times higher in MGUS patients than in matched control subjects.
Patients with MGUS also had a significantly shorter median survival than controls.
Researchers reported these findings in NEJM.
“Monoclonal gammopathy of undetermined significance is present in more than 3% of the general population age 50 and older,” said study author S. Vincent Rajkumar, MD, of the Mayo Clinic in Rochester, Minnesota.
“In some cases, people with monoclonal gammopathy of undetermined significance go on to develop multiple myeloma.”
With this in mind, Dr Rajkumar and his colleagues studied 1384 patients—210 with IgM MGUS and 1129 with non-IgM MGUS. Patients were diagnosed with MGUS from 1960 through 1994, and their median age at diagnosis was 72.
The median follow-up was 34.1 years (range, 0.0 to 43.6), so there were 14,130 person-years of follow-up.
During that time, 147 patients progressed to another disorder, including:
- 97 to multiple myeloma
- 19 to non-Hodgkin lymphoma
- 14 to AL amyloidosis
- 13 to Waldenstrom’s macroglobulinemia
- 3 to chronic lymphocytic leukemia
- 1 to plasmacytoma.
The rate of progression in MGUS patients—11%—represented a risk of these disorders that was 6.5 times higher than the risk observed in an age- and sex-matched control population.
The risk of progression also increased over time for MGUS patients. Without accounting for death due to competing causes, the risk of progression was 10% at 10 years, 18% at 20 years, 28% at 30 years, and 36% at both 35 and 40 years.
“We also found that patients with monoclonal gammopathy of undetermined significance had shorter survival than comparable people without the condition, which raises the possibility there may be other disorders associated with monoclonal gammopathy of undetermined significance that still need further study,” Dr Rajkumar said.
The median survival was 8.1 years in MGUS patients and 12.4 years in controls (P<0.001).
Overall, 1300 MGUS patients (94%) had died at last follow-up. Of the 84 patients who were still alive, 5 had progressed. ![]()
CAR T-cell therapy on fast track in US, EU
The chimeric antigen receptor (CAR) T-cell therapy tisagenlecleucel (Kymriah, formerly CTL019) is getting fast-tracked in the United States (US) and European Union (EU).
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application (sBLA) for tisagenlecleucel for the treatment of adults with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who are ineligible for, or relapse after, autologous hematopoietic stem cell transplant (auto-HSCT).
Meanwhile, the European Medicines Agency (EMA) has granted accelerated assessment to the marketing authorization application (MAA) for tisagenlecleucel for the treatment of children and young adults with R/R B-cell acute lymphoblastic leukemia (ALL) and for adults with R/R DLBCL who are ineligible for auto-HSCT.
If the sBLA and MAA are approved, tisagenlecleucel will be the first CAR T-cell therapy available for 2 distinct indications in non-Hodgkin lymphoma and B-cell ALL.
Tisagenlecleucel became the first CAR T-cell therapy to receive regulatory approval when it was approved by the FDA in August 2017 for use in patients up to 25 years of age who have B-cell precursor ALL that is refractory or in second or later relapse.
Supporting data
The regulatory applications for tisagenlecleucel in the US and EU are supported by data from the Novartis-sponsored global clinical trial program in children and young adults with R/R B-cell ALL and adults with R/R DLBCL.
Results from the phase 2 JULIET trial served as the basis of the sBLA and MAA for tisagenlecleucel in adults with R/R DLCBL. Data from this trial were presented at the 2017 ASH Annual Meeting in December.
Results from the phase 2 ELIANA study were submitted as part of the MAA for tisagenlecleucel in children and young adults with R/R B-cell ALL. Data from this trial were presented at the 2017 EHA Congress last June.
About priority review, accelerated assessment
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The EMA grants accelerated assessment when a product is expected to be of major public health interest, particularly from the point of view of therapeutic innovation.
Accelerated assessment shortens the review period from 210 days to 150 days. ![]()
The chimeric antigen receptor (CAR) T-cell therapy tisagenlecleucel (Kymriah, formerly CTL019) is getting fast-tracked in the United States (US) and European Union (EU).
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application (sBLA) for tisagenlecleucel for the treatment of adults with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who are ineligible for, or relapse after, autologous hematopoietic stem cell transplant (auto-HSCT).
Meanwhile, the European Medicines Agency (EMA) has granted accelerated assessment to the marketing authorization application (MAA) for tisagenlecleucel for the treatment of children and young adults with R/R B-cell acute lymphoblastic leukemia (ALL) and for adults with R/R DLBCL who are ineligible for auto-HSCT.
If the sBLA and MAA are approved, tisagenlecleucel will be the first CAR T-cell therapy available for 2 distinct indications in non-Hodgkin lymphoma and B-cell ALL.
Tisagenlecleucel became the first CAR T-cell therapy to receive regulatory approval when it was approved by the FDA in August 2017 for use in patients up to 25 years of age who have B-cell precursor ALL that is refractory or in second or later relapse.
Supporting data
The regulatory applications for tisagenlecleucel in the US and EU are supported by data from the Novartis-sponsored global clinical trial program in children and young adults with R/R B-cell ALL and adults with R/R DLBCL.
Results from the phase 2 JULIET trial served as the basis of the sBLA and MAA for tisagenlecleucel in adults with R/R DLCBL. Data from this trial were presented at the 2017 ASH Annual Meeting in December.
Results from the phase 2 ELIANA study were submitted as part of the MAA for tisagenlecleucel in children and young adults with R/R B-cell ALL. Data from this trial were presented at the 2017 EHA Congress last June.
About priority review, accelerated assessment
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The EMA grants accelerated assessment when a product is expected to be of major public health interest, particularly from the point of view of therapeutic innovation.
Accelerated assessment shortens the review period from 210 days to 150 days. ![]()
The chimeric antigen receptor (CAR) T-cell therapy tisagenlecleucel (Kymriah, formerly CTL019) is getting fast-tracked in the United States (US) and European Union (EU).
The US Food and Drug Administration (FDA) has accepted for priority review the supplemental biologics license application (sBLA) for tisagenlecleucel for the treatment of adults with relapsed or refractory (R/R) diffuse large B-cell lymphoma (DLBCL) who are ineligible for, or relapse after, autologous hematopoietic stem cell transplant (auto-HSCT).
Meanwhile, the European Medicines Agency (EMA) has granted accelerated assessment to the marketing authorization application (MAA) for tisagenlecleucel for the treatment of children and young adults with R/R B-cell acute lymphoblastic leukemia (ALL) and for adults with R/R DLBCL who are ineligible for auto-HSCT.
If the sBLA and MAA are approved, tisagenlecleucel will be the first CAR T-cell therapy available for 2 distinct indications in non-Hodgkin lymphoma and B-cell ALL.
Tisagenlecleucel became the first CAR T-cell therapy to receive regulatory approval when it was approved by the FDA in August 2017 for use in patients up to 25 years of age who have B-cell precursor ALL that is refractory or in second or later relapse.
Supporting data
The regulatory applications for tisagenlecleucel in the US and EU are supported by data from the Novartis-sponsored global clinical trial program in children and young adults with R/R B-cell ALL and adults with R/R DLBCL.
Results from the phase 2 JULIET trial served as the basis of the sBLA and MAA for tisagenlecleucel in adults with R/R DLCBL. Data from this trial were presented at the 2017 ASH Annual Meeting in December.
Results from the phase 2 ELIANA study were submitted as part of the MAA for tisagenlecleucel in children and young adults with R/R B-cell ALL. Data from this trial were presented at the 2017 EHA Congress last June.
About priority review, accelerated assessment
The FDA grants priority review to applications for products that may provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA’s goal is to take action on a priority review application within 6 months of receiving it, rather than the standard 10 months.
The EMA grants accelerated assessment when a product is expected to be of major public health interest, particularly from the point of view of therapeutic innovation.
Accelerated assessment shortens the review period from 210 days to 150 days. ![]()
FDA grants priority review to CAR T-cell therapy for DLBCL
The Food and Drug Administration has granted a priority review for the CAR T-cell therapy tisagenlecleucel suspension, formerly CTL019, for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma who are ineligible for or relapsed after autologous stem cell transplant.
The current application is based on a 6-month primary analysis from the single-arm, phase 2 JULIET clinical trial in adult patients with relapsed or refractory diffuse large B-cell lymphoma. According to results presented at ASH 2017, among 81 patients followed for at least 3 months before data cutoff, best overall response rate was 53%, and 40% had a complete response. Cytokine release syndrome (all grades) occurred in 58% of infused patients. Other grade 3 or 4 adverse events included neurologic toxicities, cytopenias lasting more than 28 days, infections, and febrile neutropenia.
Tisagenlecleucel suspension is marketed as Kymriah by Novartis.
The Food and Drug Administration has granted a priority review for the CAR T-cell therapy tisagenlecleucel suspension, formerly CTL019, for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma who are ineligible for or relapsed after autologous stem cell transplant.
The current application is based on a 6-month primary analysis from the single-arm, phase 2 JULIET clinical trial in adult patients with relapsed or refractory diffuse large B-cell lymphoma. According to results presented at ASH 2017, among 81 patients followed for at least 3 months before data cutoff, best overall response rate was 53%, and 40% had a complete response. Cytokine release syndrome (all grades) occurred in 58% of infused patients. Other grade 3 or 4 adverse events included neurologic toxicities, cytopenias lasting more than 28 days, infections, and febrile neutropenia.
Tisagenlecleucel suspension is marketed as Kymriah by Novartis.
The Food and Drug Administration has granted a priority review for the CAR T-cell therapy tisagenlecleucel suspension, formerly CTL019, for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma who are ineligible for or relapsed after autologous stem cell transplant.
The current application is based on a 6-month primary analysis from the single-arm, phase 2 JULIET clinical trial in adult patients with relapsed or refractory diffuse large B-cell lymphoma. According to results presented at ASH 2017, among 81 patients followed for at least 3 months before data cutoff, best overall response rate was 53%, and 40% had a complete response. Cytokine release syndrome (all grades) occurred in 58% of infused patients. Other grade 3 or 4 adverse events included neurologic toxicities, cytopenias lasting more than 28 days, infections, and febrile neutropenia.
Tisagenlecleucel suspension is marketed as Kymriah by Novartis.
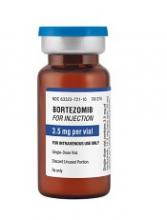
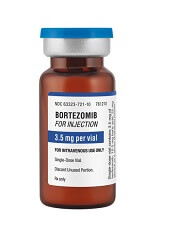
Generic bortezomib available in US
Fresenius Kabi has introduced its generic version of Velcade, Bortezomib for Injection, to the US market.
This is the first intravenous alternative to Velcade available in the US.
Bortezomib for Injection is available as a single dose vial containing 3.5 mg of lyophilized powder.
The product is approved to treat patients with multiple myeloma and patients with mantle cell lymphoma who have received at least 1 prior therapy.
For details, see the prescribing information for Bortezomib for Injection.
Velcade is a registered trademark of Millennium Pharmaceuticals, Inc. ![]()
Fresenius Kabi has introduced its generic version of Velcade, Bortezomib for Injection, to the US market.
This is the first intravenous alternative to Velcade available in the US.
Bortezomib for Injection is available as a single dose vial containing 3.5 mg of lyophilized powder.
The product is approved to treat patients with multiple myeloma and patients with mantle cell lymphoma who have received at least 1 prior therapy.
For details, see the prescribing information for Bortezomib for Injection.
Velcade is a registered trademark of Millennium Pharmaceuticals, Inc. ![]()
Fresenius Kabi has introduced its generic version of Velcade, Bortezomib for Injection, to the US market.
This is the first intravenous alternative to Velcade available in the US.
Bortezomib for Injection is available as a single dose vial containing 3.5 mg of lyophilized powder.
The product is approved to treat patients with multiple myeloma and patients with mantle cell lymphoma who have received at least 1 prior therapy.
For details, see the prescribing information for Bortezomib for Injection.
Velcade is a registered trademark of Millennium Pharmaceuticals, Inc. ![]()
AUDIO: Immunotherapy’s role in NHL
ATLANTA – The use of immune checkpoint blockade is increasingly becoming standard therapy in Hodgkin lymphoma, but this approach has so far garnered mixed results in non-Hodgkin lymphoma, Stephen Ansell, MD, PhD, said at the annual meeting of the American Society of Hematology.
In an interview, Dr. Ansell, professor of medicine and chair of the lymphoma group at the Mayo Clinic, Rochester, Minn., said responses have been variable with promising results from immune checkpoint inhibitors in primary mediastinal large B-cell lymphoma, some NK/T-cell lymphomas, and primary CNS lymphoma. However, responses have been modest in low-grade lymphoma.
Dr. Ansell, who chaired a session at ASH 2017 on immunotherapy’s expanding role in non-Hodgkin lymphoma, said one of the major challenges of using immune checkpoint blockade in non-Hodgkin lymphoma is the complicated biology. For example, there are a lot of regulatory T cells that actually inhibit the immune response, and many of the T cells that are present within the tumor have an exhausted phenotype and are poorly functioning. Additionally, some of the cytokines that would seem to be stimulating the immune system can, over time, slowly produce T-cell exhaustion.
“Sort of like too much of a good thing ends up being a bad thing,” he said.
These are the issues that are fueling research today, Dr. Ansell said. Going forward he said he expects to see more combination approaches to therapy, such as using an agonistic positive signal plus the blocking of an inhibitory signal with chemotherapy.
Dr. Ansell reported that Mayo Clinic receives clinical trial support from Merck, Bristol-Myers Squibb, Seattle Genetics, Trillium, and Affimed.
ATLANTA – The use of immune checkpoint blockade is increasingly becoming standard therapy in Hodgkin lymphoma, but this approach has so far garnered mixed results in non-Hodgkin lymphoma, Stephen Ansell, MD, PhD, said at the annual meeting of the American Society of Hematology.
In an interview, Dr. Ansell, professor of medicine and chair of the lymphoma group at the Mayo Clinic, Rochester, Minn., said responses have been variable with promising results from immune checkpoint inhibitors in primary mediastinal large B-cell lymphoma, some NK/T-cell lymphomas, and primary CNS lymphoma. However, responses have been modest in low-grade lymphoma.
Dr. Ansell, who chaired a session at ASH 2017 on immunotherapy’s expanding role in non-Hodgkin lymphoma, said one of the major challenges of using immune checkpoint blockade in non-Hodgkin lymphoma is the complicated biology. For example, there are a lot of regulatory T cells that actually inhibit the immune response, and many of the T cells that are present within the tumor have an exhausted phenotype and are poorly functioning. Additionally, some of the cytokines that would seem to be stimulating the immune system can, over time, slowly produce T-cell exhaustion.
“Sort of like too much of a good thing ends up being a bad thing,” he said.
These are the issues that are fueling research today, Dr. Ansell said. Going forward he said he expects to see more combination approaches to therapy, such as using an agonistic positive signal plus the blocking of an inhibitory signal with chemotherapy.
Dr. Ansell reported that Mayo Clinic receives clinical trial support from Merck, Bristol-Myers Squibb, Seattle Genetics, Trillium, and Affimed.
ATLANTA – The use of immune checkpoint blockade is increasingly becoming standard therapy in Hodgkin lymphoma, but this approach has so far garnered mixed results in non-Hodgkin lymphoma, Stephen Ansell, MD, PhD, said at the annual meeting of the American Society of Hematology.
In an interview, Dr. Ansell, professor of medicine and chair of the lymphoma group at the Mayo Clinic, Rochester, Minn., said responses have been variable with promising results from immune checkpoint inhibitors in primary mediastinal large B-cell lymphoma, some NK/T-cell lymphomas, and primary CNS lymphoma. However, responses have been modest in low-grade lymphoma.
Dr. Ansell, who chaired a session at ASH 2017 on immunotherapy’s expanding role in non-Hodgkin lymphoma, said one of the major challenges of using immune checkpoint blockade in non-Hodgkin lymphoma is the complicated biology. For example, there are a lot of regulatory T cells that actually inhibit the immune response, and many of the T cells that are present within the tumor have an exhausted phenotype and are poorly functioning. Additionally, some of the cytokines that would seem to be stimulating the immune system can, over time, slowly produce T-cell exhaustion.
“Sort of like too much of a good thing ends up being a bad thing,” he said.
These are the issues that are fueling research today, Dr. Ansell said. Going forward he said he expects to see more combination approaches to therapy, such as using an agonistic positive signal plus the blocking of an inhibitory signal with chemotherapy.
Dr. Ansell reported that Mayo Clinic receives clinical trial support from Merck, Bristol-Myers Squibb, Seattle Genetics, Trillium, and Affimed.
EXPERT ANALYSIS FROM ASH 2017
VcR-CVAD yields high responses, ‘excellent’ survival in MCL
Adding rituximab and bortezomib to a moderate-intensity chemotherapy regimen and following it up with maintenance rituximab produced high response rates and “excellent” survival outcomes for adults with previously untreated mantle cell lymphoma (MCL), investigators reported in long-term follow-up of a small study.
The objective response rate (ORR) among 30 patients with MCL treated with VcR-CVAD – bortezomib (Velcade), rituximab, and hyperCVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) followed by rituximab maintenance – was 90%, including a high proportion of complete responses (CR) or unconfirmed complete responses.
After a median follow-up of 7.8 years, the rates of 6-year progression-free and overall survival (PFS and OS) were 53% and 70%, respectively, with patients older and younger than 60 years having equally good outcomes, according to Julie E. Chang, MD, of the Wisconsin Institute of Medical Research in Madison, and her colleagues.
VcR-CVAD is a moderate-intensity regimen with a favorable toxicity profile that allowed tolerability even in an older population, the investigators noted. “An important lesson illustrated by VcR-CVAD is that long-term remissions are achievable in some patients without intensive inductions or consolidation,” they wrote in Clinical Lymphoma, Myeloma & Leukemia.
The investigators previously reported that after a median follow-up of 42 months, the 3-year PFS and OS were 63% and 86%, respectively, and that these outcomes were comparable to those reported with more intensive regimens (Br J Haematol. 2011 Oct;155[2]:190-7).
As noted, the ORR was 90%, including 77% CR/unconfirmed CR, 6-year PFS was 53%, and 6-year OS was 70%.
A univariate analysis showed a significant interaction between lactate dehydrogenase levels and age for PFS, and a trend, albeit not significant, toward an interaction with LDH levels and age for OS.
In multivariate analysis, worse Eastern Cooperative Oncology Group (ECOG) performance status at baseline showed a nonsignificant trend toward worse OS. In contrast, an increase of one in the number of extranodal disease sites was associated with better OS (relative risk 0.66, 95% confidence interval 0.01-0.66).
The investigators noted that the advent of new agents with activity against MCL and the use of prognostic information, such as minimal residual disease measurements, could help clinicians develop induction and maintenance strategies with better efficacy and lower toxicity than VcR-CVAD.
The study was supported by the National Institutes of Health, Millennium Pharmaceuticals, and the University of Wisconsin Forward Lymphoma Research Fund. Dr. Chang reported research funding from Genentech. One coauthor disclosed consulting work for Genentech and Millennium and research funding from Genentech.
SOURCE: Chang J et al. Clin Lymphoma Myeloma Leuk. 2018 Jan;18(1):e61-e67. doi: 10.1016/j.clml.2017.10.006.
Adding rituximab and bortezomib to a moderate-intensity chemotherapy regimen and following it up with maintenance rituximab produced high response rates and “excellent” survival outcomes for adults with previously untreated mantle cell lymphoma (MCL), investigators reported in long-term follow-up of a small study.
The objective response rate (ORR) among 30 patients with MCL treated with VcR-CVAD – bortezomib (Velcade), rituximab, and hyperCVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) followed by rituximab maintenance – was 90%, including a high proportion of complete responses (CR) or unconfirmed complete responses.
After a median follow-up of 7.8 years, the rates of 6-year progression-free and overall survival (PFS and OS) were 53% and 70%, respectively, with patients older and younger than 60 years having equally good outcomes, according to Julie E. Chang, MD, of the Wisconsin Institute of Medical Research in Madison, and her colleagues.
VcR-CVAD is a moderate-intensity regimen with a favorable toxicity profile that allowed tolerability even in an older population, the investigators noted. “An important lesson illustrated by VcR-CVAD is that long-term remissions are achievable in some patients without intensive inductions or consolidation,” they wrote in Clinical Lymphoma, Myeloma & Leukemia.
The investigators previously reported that after a median follow-up of 42 months, the 3-year PFS and OS were 63% and 86%, respectively, and that these outcomes were comparable to those reported with more intensive regimens (Br J Haematol. 2011 Oct;155[2]:190-7).
As noted, the ORR was 90%, including 77% CR/unconfirmed CR, 6-year PFS was 53%, and 6-year OS was 70%.
A univariate analysis showed a significant interaction between lactate dehydrogenase levels and age for PFS, and a trend, albeit not significant, toward an interaction with LDH levels and age for OS.
In multivariate analysis, worse Eastern Cooperative Oncology Group (ECOG) performance status at baseline showed a nonsignificant trend toward worse OS. In contrast, an increase of one in the number of extranodal disease sites was associated with better OS (relative risk 0.66, 95% confidence interval 0.01-0.66).
The investigators noted that the advent of new agents with activity against MCL and the use of prognostic information, such as minimal residual disease measurements, could help clinicians develop induction and maintenance strategies with better efficacy and lower toxicity than VcR-CVAD.
The study was supported by the National Institutes of Health, Millennium Pharmaceuticals, and the University of Wisconsin Forward Lymphoma Research Fund. Dr. Chang reported research funding from Genentech. One coauthor disclosed consulting work for Genentech and Millennium and research funding from Genentech.
SOURCE: Chang J et al. Clin Lymphoma Myeloma Leuk. 2018 Jan;18(1):e61-e67. doi: 10.1016/j.clml.2017.10.006.
Adding rituximab and bortezomib to a moderate-intensity chemotherapy regimen and following it up with maintenance rituximab produced high response rates and “excellent” survival outcomes for adults with previously untreated mantle cell lymphoma (MCL), investigators reported in long-term follow-up of a small study.
The objective response rate (ORR) among 30 patients with MCL treated with VcR-CVAD – bortezomib (Velcade), rituximab, and hyperCVAD (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) followed by rituximab maintenance – was 90%, including a high proportion of complete responses (CR) or unconfirmed complete responses.
After a median follow-up of 7.8 years, the rates of 6-year progression-free and overall survival (PFS and OS) were 53% and 70%, respectively, with patients older and younger than 60 years having equally good outcomes, according to Julie E. Chang, MD, of the Wisconsin Institute of Medical Research in Madison, and her colleagues.
VcR-CVAD is a moderate-intensity regimen with a favorable toxicity profile that allowed tolerability even in an older population, the investigators noted. “An important lesson illustrated by VcR-CVAD is that long-term remissions are achievable in some patients without intensive inductions or consolidation,” they wrote in Clinical Lymphoma, Myeloma & Leukemia.
The investigators previously reported that after a median follow-up of 42 months, the 3-year PFS and OS were 63% and 86%, respectively, and that these outcomes were comparable to those reported with more intensive regimens (Br J Haematol. 2011 Oct;155[2]:190-7).
As noted, the ORR was 90%, including 77% CR/unconfirmed CR, 6-year PFS was 53%, and 6-year OS was 70%.
A univariate analysis showed a significant interaction between lactate dehydrogenase levels and age for PFS, and a trend, albeit not significant, toward an interaction with LDH levels and age for OS.
In multivariate analysis, worse Eastern Cooperative Oncology Group (ECOG) performance status at baseline showed a nonsignificant trend toward worse OS. In contrast, an increase of one in the number of extranodal disease sites was associated with better OS (relative risk 0.66, 95% confidence interval 0.01-0.66).
The investigators noted that the advent of new agents with activity against MCL and the use of prognostic information, such as minimal residual disease measurements, could help clinicians develop induction and maintenance strategies with better efficacy and lower toxicity than VcR-CVAD.
The study was supported by the National Institutes of Health, Millennium Pharmaceuticals, and the University of Wisconsin Forward Lymphoma Research Fund. Dr. Chang reported research funding from Genentech. One coauthor disclosed consulting work for Genentech and Millennium and research funding from Genentech.
SOURCE: Chang J et al. Clin Lymphoma Myeloma Leuk. 2018 Jan;18(1):e61-e67. doi: 10.1016/j.clml.2017.10.006.
FROM CLINICAL LYMPHOMA, MYELOMA & LEUKEMIA
Key clinical point:
Major finding: The objective response rate was 90%, including 77% complete or unconfirmed complete responses.
Study details: Open-label study of 30 patients with previously untreated MCL.
Disclosures: The study was supported by the National Institutes of Health, Millennium Pharmaceuticals, and the University of Wisconsin Forward Lymphoma Research Fund. Dr. Chang reported research funding from Genentech. A coauthor reported consulting work for Genentech and Millennium and research funding from Genentech.
Source: Chang J et al. Clin Lymphoma Myeloma Leuk. 2018 Jan;18(1):e61-e67. doi: 10.1016/j.clml.2017.10.006.
Survival differences among AYAs with blood cancers
A new report has revealed differences in survival among adolescents and young adults (AYAs) with hematologic malignancies.
The report includes information on AYAs—ages 15 to 39—living in Los Angeles County who were diagnosed with common cancers between 1988 and 2014.
The data showed differences in 5-year survival rates according to sex, race, age, and socioeconomic status (SES).
For example, lymphoma survival rates were lower for males, African Americans (AAs), older AYAs, and patients with low socioeconomic status (SES).
For AYAs with leukemias, there was no survival difference according to sex, but AAs had worse survival than patients of other races. And the impact of age and SES varied according to leukemia type.
“Cancer survival data are poorly understood for 15- to 39-year-olds,” noted Amie Hwang, PhD, of the University of Southern California Keck School of Medicine in Los Angeles.
That is why she and her colleagues created the report, “Cancer in Los Angeles County: Survival Among Adolescents and Young Adults 1988-2014.”
According to the authors, this is the first report to break down cancer survival rates for AYAs into segments on race/ethnicity, sex, age group, SES, and cancer stage.
Survival data for patients with hematologic malignancies were as follows.
Acute lymphoblastic leukemia
There were 1137 cases of acute lymphoblastic leukemia in the AYA population in Los Angeles County during the period studied. This included 752 males and 385 females.
Five-year survival was similar between males (43%) and females (41%).
Younger AYAs had better survival than older AYAs (48% for ages 15-24, 35% for ages 25-34, and 32% for ages 35-39).
Survival was highest among non-Latino whites (NLWs, 56%), followed by Asian/Pacific Islanders (APIs, 52%), patients of other/unknown races (51%), Latino whites (LWs, 38%), and AAs (29%).
Survival declined with SES (55% for high, 42% for middle, and 36% for low SES).
Acute myeloid leukemia
There were 1195 cases of acute myeloid leukemia—641 males and 554 females.
Five-year survival was similar for males (40%) and females (43%) as well as for the different age groups (45% for ages 15-24 vs 40% for the older age groups).
Survival was highest among NLWs (44%), followed by LWs (43%), APIs (40%), other/unknown (33%), and AAs (25%).
Survival declined somewhat with SES (49% for high, 39% for middle, and 41% for low SES).
Chronic myeloid leukemia
There were 655 cases of chronic myeloid leukemia—408 males and 247 females.
Five-year survival was similar for males (70%) and females (71%), but it was slightly higher for older AYAs (69% for ages 15-24, 68% for ages 25-34, and 76% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (76%), followed by LWs (73%), NLWs/APIs (both 72%), and AAs (57%).
Survival declined somewhat with SES (76% for high, 67% for middle, and 68% for low SES).
Hodgkin lymphoma
There were 2993 AYAs diagnosed with Hodgkin lymphoma—1553 males and 1440 females.
The 5-year survival rate was higher in females (93%) than males (86%) and in younger AYAs (93% for ages 15-24, 89% for ages 25-34, and 85% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (96%), followed by APIs/NLWs (both 91%), LWs (88%), and AAs (83%).
Survival declined with SES (95% for high, 89% for middle, and 83% for low SES).
And survival was lower for patients with advanced-stage disease (93% localized, 94% regional, and 83% distant).
Non-Hodkgin lymphoma
There were 4485 AYAs diagnosed with non-Hodgkin lymphoma during the study period—3064 males and 1421 females.
The 5-year survival rate was higher in females (75%) than males (46%) and in younger AYAs (69% for ages 15-24, 51% for ages 25-34, and 52% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (88%), followed by APIs (68%), LWs/NLWs (both 53%), and AAs (50%).
Survival declined with SES (68% for high, 54% for middle, and 45% for low SES).
And survival was lower for patients with advanced-stage disease (61% localized, 66% regional, and 46% distant).
“Adolescents and young adults go to the doctor less often because they have this superhero mentality, like they’re invincible,” said author Dennis Deapen, DrPH, of the University of Southern California Keck School of Medicine.
“Once they do go to a health professional, their cancer diagnosis can be delayed because cancer isn’t the first concern doctors have for this age group. It comes as no surprise that patients diagnosed with late-stage cancer have reduced survival rates.” ![]()
A new report has revealed differences in survival among adolescents and young adults (AYAs) with hematologic malignancies.
The report includes information on AYAs—ages 15 to 39—living in Los Angeles County who were diagnosed with common cancers between 1988 and 2014.
The data showed differences in 5-year survival rates according to sex, race, age, and socioeconomic status (SES).
For example, lymphoma survival rates were lower for males, African Americans (AAs), older AYAs, and patients with low socioeconomic status (SES).
For AYAs with leukemias, there was no survival difference according to sex, but AAs had worse survival than patients of other races. And the impact of age and SES varied according to leukemia type.
“Cancer survival data are poorly understood for 15- to 39-year-olds,” noted Amie Hwang, PhD, of the University of Southern California Keck School of Medicine in Los Angeles.
That is why she and her colleagues created the report, “Cancer in Los Angeles County: Survival Among Adolescents and Young Adults 1988-2014.”
According to the authors, this is the first report to break down cancer survival rates for AYAs into segments on race/ethnicity, sex, age group, SES, and cancer stage.
Survival data for patients with hematologic malignancies were as follows.
Acute lymphoblastic leukemia
There were 1137 cases of acute lymphoblastic leukemia in the AYA population in Los Angeles County during the period studied. This included 752 males and 385 females.
Five-year survival was similar between males (43%) and females (41%).
Younger AYAs had better survival than older AYAs (48% for ages 15-24, 35% for ages 25-34, and 32% for ages 35-39).
Survival was highest among non-Latino whites (NLWs, 56%), followed by Asian/Pacific Islanders (APIs, 52%), patients of other/unknown races (51%), Latino whites (LWs, 38%), and AAs (29%).
Survival declined with SES (55% for high, 42% for middle, and 36% for low SES).
Acute myeloid leukemia
There were 1195 cases of acute myeloid leukemia—641 males and 554 females.
Five-year survival was similar for males (40%) and females (43%) as well as for the different age groups (45% for ages 15-24 vs 40% for the older age groups).
Survival was highest among NLWs (44%), followed by LWs (43%), APIs (40%), other/unknown (33%), and AAs (25%).
Survival declined somewhat with SES (49% for high, 39% for middle, and 41% for low SES).
Chronic myeloid leukemia
There were 655 cases of chronic myeloid leukemia—408 males and 247 females.
Five-year survival was similar for males (70%) and females (71%), but it was slightly higher for older AYAs (69% for ages 15-24, 68% for ages 25-34, and 76% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (76%), followed by LWs (73%), NLWs/APIs (both 72%), and AAs (57%).
Survival declined somewhat with SES (76% for high, 67% for middle, and 68% for low SES).
Hodgkin lymphoma
There were 2993 AYAs diagnosed with Hodgkin lymphoma—1553 males and 1440 females.
The 5-year survival rate was higher in females (93%) than males (86%) and in younger AYAs (93% for ages 15-24, 89% for ages 25-34, and 85% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (96%), followed by APIs/NLWs (both 91%), LWs (88%), and AAs (83%).
Survival declined with SES (95% for high, 89% for middle, and 83% for low SES).
And survival was lower for patients with advanced-stage disease (93% localized, 94% regional, and 83% distant).
Non-Hodkgin lymphoma
There were 4485 AYAs diagnosed with non-Hodgkin lymphoma during the study period—3064 males and 1421 females.
The 5-year survival rate was higher in females (75%) than males (46%) and in younger AYAs (69% for ages 15-24, 51% for ages 25-34, and 52% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (88%), followed by APIs (68%), LWs/NLWs (both 53%), and AAs (50%).
Survival declined with SES (68% for high, 54% for middle, and 45% for low SES).
And survival was lower for patients with advanced-stage disease (61% localized, 66% regional, and 46% distant).
“Adolescents and young adults go to the doctor less often because they have this superhero mentality, like they’re invincible,” said author Dennis Deapen, DrPH, of the University of Southern California Keck School of Medicine.
“Once they do go to a health professional, their cancer diagnosis can be delayed because cancer isn’t the first concern doctors have for this age group. It comes as no surprise that patients diagnosed with late-stage cancer have reduced survival rates.” ![]()
A new report has revealed differences in survival among adolescents and young adults (AYAs) with hematologic malignancies.
The report includes information on AYAs—ages 15 to 39—living in Los Angeles County who were diagnosed with common cancers between 1988 and 2014.
The data showed differences in 5-year survival rates according to sex, race, age, and socioeconomic status (SES).
For example, lymphoma survival rates were lower for males, African Americans (AAs), older AYAs, and patients with low socioeconomic status (SES).
For AYAs with leukemias, there was no survival difference according to sex, but AAs had worse survival than patients of other races. And the impact of age and SES varied according to leukemia type.
“Cancer survival data are poorly understood for 15- to 39-year-olds,” noted Amie Hwang, PhD, of the University of Southern California Keck School of Medicine in Los Angeles.
That is why she and her colleagues created the report, “Cancer in Los Angeles County: Survival Among Adolescents and Young Adults 1988-2014.”
According to the authors, this is the first report to break down cancer survival rates for AYAs into segments on race/ethnicity, sex, age group, SES, and cancer stage.
Survival data for patients with hematologic malignancies were as follows.
Acute lymphoblastic leukemia
There were 1137 cases of acute lymphoblastic leukemia in the AYA population in Los Angeles County during the period studied. This included 752 males and 385 females.
Five-year survival was similar between males (43%) and females (41%).
Younger AYAs had better survival than older AYAs (48% for ages 15-24, 35% for ages 25-34, and 32% for ages 35-39).
Survival was highest among non-Latino whites (NLWs, 56%), followed by Asian/Pacific Islanders (APIs, 52%), patients of other/unknown races (51%), Latino whites (LWs, 38%), and AAs (29%).
Survival declined with SES (55% for high, 42% for middle, and 36% for low SES).
Acute myeloid leukemia
There were 1195 cases of acute myeloid leukemia—641 males and 554 females.
Five-year survival was similar for males (40%) and females (43%) as well as for the different age groups (45% for ages 15-24 vs 40% for the older age groups).
Survival was highest among NLWs (44%), followed by LWs (43%), APIs (40%), other/unknown (33%), and AAs (25%).
Survival declined somewhat with SES (49% for high, 39% for middle, and 41% for low SES).
Chronic myeloid leukemia
There were 655 cases of chronic myeloid leukemia—408 males and 247 females.
Five-year survival was similar for males (70%) and females (71%), but it was slightly higher for older AYAs (69% for ages 15-24, 68% for ages 25-34, and 76% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (76%), followed by LWs (73%), NLWs/APIs (both 72%), and AAs (57%).
Survival declined somewhat with SES (76% for high, 67% for middle, and 68% for low SES).
Hodgkin lymphoma
There were 2993 AYAs diagnosed with Hodgkin lymphoma—1553 males and 1440 females.
The 5-year survival rate was higher in females (93%) than males (86%) and in younger AYAs (93% for ages 15-24, 89% for ages 25-34, and 85% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (96%), followed by APIs/NLWs (both 91%), LWs (88%), and AAs (83%).
Survival declined with SES (95% for high, 89% for middle, and 83% for low SES).
And survival was lower for patients with advanced-stage disease (93% localized, 94% regional, and 83% distant).
Non-Hodkgin lymphoma
There were 4485 AYAs diagnosed with non-Hodgkin lymphoma during the study period—3064 males and 1421 females.
The 5-year survival rate was higher in females (75%) than males (46%) and in younger AYAs (69% for ages 15-24, 51% for ages 25-34, and 52% for ages 35-39).
Survival was highest among patients in the “other/unknown” race category (88%), followed by APIs (68%), LWs/NLWs (both 53%), and AAs (50%).
Survival declined with SES (68% for high, 54% for middle, and 45% for low SES).
And survival was lower for patients with advanced-stage disease (61% localized, 66% regional, and 46% distant).
“Adolescents and young adults go to the doctor less often because they have this superhero mentality, like they’re invincible,” said author Dennis Deapen, DrPH, of the University of Southern California Keck School of Medicine.
“Once they do go to a health professional, their cancer diagnosis can be delayed because cancer isn’t the first concern doctors have for this age group. It comes as no surprise that patients diagnosed with late-stage cancer have reduced survival rates.” ![]()
Marine animals aid development of cytotoxicity assay
Researchers have looked to deep-sea creatures with the goal of creating a better cytotoxicity assay.
The team harnessed the power of enzymes responsible for marine animal bioluminescence to create the “Matador assay,” which can be used to determine whether cellular and immune-therapeutic agents are actually killing target cells.
The researchers said the Matador assay is quick and simple as well as “highly sensitive,” with the ability to detect cytotoxicity induced by several types of therapies.
Preet M. Chaudhary, MD, PhD, of the University of Southern California Keck School of Medicine in Los Angeles, and his colleagues described the assay in Scientific Reports.
“One of the most promising areas in cancer research is immunotherapy. . .,” Dr Chaudhary said. “It is also one of the most difficult because the methods for testing immunotherapies are not ideal.”
“Radioactive chromium release assay is the gold standard for testing whether an immunotherapy kills cancer cells. This method is expensive, complicated, and requires special disposal practices. Other available methods also suffer from limitations and don’t allow scientists to rapidly screen immunotherapeutic agents to find the best candidates.”
Dr Chaudhary and his colleagues set out to develop a simple, precise, and inexpensive cytotoxicity assay based on marine animal luciferases, the enzymes responsible for bioluminescence.
The team used a group of small crustaceans and deep-sea shrimp, which were selected for their bright bioluminescence. Their luciferases became the basis of the Matador assay.
Engineered to get trapped inside cells, the luciferases leak out of cells when they die, causing a visible glow. The level of luminescence can then be measured with a luminometer.
To test the Matador assay’s effectiveness at measuring cell death, the researchers used several types of cancer cells, including chronic myelogenous leukemia, acute myelogenous leukemia, Burkitt lymphoma, and solid tumor cells.
The team treated these cells with a variety of therapies, including chimeric antigen receptor (CAR) T cells, bispecific T-cell engagers, monoclonal antibodies, and natural killer cells.
Results showed the Matador assay could detect the death of a single cell, a level of sensitivity superior to that of existing cytotoxicity assays.
The researchers also pointed out that the Matador assay is fast, inexpensive, and can be performed in a 384-well plate format, saving time and reagents.
“In our hands, the Matador assay can detect cell death in as little as 30 minutes, which can ultimately translate to more expedient treatments for patients getting cellular immunotherapies such as CAR T cells,” Dr Chaudhary said.
In fact, Dr Chaudhary’s lab has developed more than 75 cancer cell lines expressing the marine luciferases and used them with the Matador assay to develop next-generation CAR T cells.
Dr Chaudhary believes the Matador assay has many potential applications in biomedical research and cellular therapy manufacturing.
“It could potentially play a role in screening other types of anticancer agents or even measuring environmental toxins,” he said. ![]()
Researchers have looked to deep-sea creatures with the goal of creating a better cytotoxicity assay.
The team harnessed the power of enzymes responsible for marine animal bioluminescence to create the “Matador assay,” which can be used to determine whether cellular and immune-therapeutic agents are actually killing target cells.
The researchers said the Matador assay is quick and simple as well as “highly sensitive,” with the ability to detect cytotoxicity induced by several types of therapies.
Preet M. Chaudhary, MD, PhD, of the University of Southern California Keck School of Medicine in Los Angeles, and his colleagues described the assay in Scientific Reports.
“One of the most promising areas in cancer research is immunotherapy. . .,” Dr Chaudhary said. “It is also one of the most difficult because the methods for testing immunotherapies are not ideal.”
“Radioactive chromium release assay is the gold standard for testing whether an immunotherapy kills cancer cells. This method is expensive, complicated, and requires special disposal practices. Other available methods also suffer from limitations and don’t allow scientists to rapidly screen immunotherapeutic agents to find the best candidates.”
Dr Chaudhary and his colleagues set out to develop a simple, precise, and inexpensive cytotoxicity assay based on marine animal luciferases, the enzymes responsible for bioluminescence.
The team used a group of small crustaceans and deep-sea shrimp, which were selected for their bright bioluminescence. Their luciferases became the basis of the Matador assay.
Engineered to get trapped inside cells, the luciferases leak out of cells when they die, causing a visible glow. The level of luminescence can then be measured with a luminometer.
To test the Matador assay’s effectiveness at measuring cell death, the researchers used several types of cancer cells, including chronic myelogenous leukemia, acute myelogenous leukemia, Burkitt lymphoma, and solid tumor cells.
The team treated these cells with a variety of therapies, including chimeric antigen receptor (CAR) T cells, bispecific T-cell engagers, monoclonal antibodies, and natural killer cells.
Results showed the Matador assay could detect the death of a single cell, a level of sensitivity superior to that of existing cytotoxicity assays.
The researchers also pointed out that the Matador assay is fast, inexpensive, and can be performed in a 384-well plate format, saving time and reagents.
“In our hands, the Matador assay can detect cell death in as little as 30 minutes, which can ultimately translate to more expedient treatments for patients getting cellular immunotherapies such as CAR T cells,” Dr Chaudhary said.
In fact, Dr Chaudhary’s lab has developed more than 75 cancer cell lines expressing the marine luciferases and used them with the Matador assay to develop next-generation CAR T cells.
Dr Chaudhary believes the Matador assay has many potential applications in biomedical research and cellular therapy manufacturing.
“It could potentially play a role in screening other types of anticancer agents or even measuring environmental toxins,” he said. ![]()
Researchers have looked to deep-sea creatures with the goal of creating a better cytotoxicity assay.
The team harnessed the power of enzymes responsible for marine animal bioluminescence to create the “Matador assay,” which can be used to determine whether cellular and immune-therapeutic agents are actually killing target cells.
The researchers said the Matador assay is quick and simple as well as “highly sensitive,” with the ability to detect cytotoxicity induced by several types of therapies.
Preet M. Chaudhary, MD, PhD, of the University of Southern California Keck School of Medicine in Los Angeles, and his colleagues described the assay in Scientific Reports.
“One of the most promising areas in cancer research is immunotherapy. . .,” Dr Chaudhary said. “It is also one of the most difficult because the methods for testing immunotherapies are not ideal.”
“Radioactive chromium release assay is the gold standard for testing whether an immunotherapy kills cancer cells. This method is expensive, complicated, and requires special disposal practices. Other available methods also suffer from limitations and don’t allow scientists to rapidly screen immunotherapeutic agents to find the best candidates.”
Dr Chaudhary and his colleagues set out to develop a simple, precise, and inexpensive cytotoxicity assay based on marine animal luciferases, the enzymes responsible for bioluminescence.
The team used a group of small crustaceans and deep-sea shrimp, which were selected for their bright bioluminescence. Their luciferases became the basis of the Matador assay.
Engineered to get trapped inside cells, the luciferases leak out of cells when they die, causing a visible glow. The level of luminescence can then be measured with a luminometer.
To test the Matador assay’s effectiveness at measuring cell death, the researchers used several types of cancer cells, including chronic myelogenous leukemia, acute myelogenous leukemia, Burkitt lymphoma, and solid tumor cells.
The team treated these cells with a variety of therapies, including chimeric antigen receptor (CAR) T cells, bispecific T-cell engagers, monoclonal antibodies, and natural killer cells.
Results showed the Matador assay could detect the death of a single cell, a level of sensitivity superior to that of existing cytotoxicity assays.
The researchers also pointed out that the Matador assay is fast, inexpensive, and can be performed in a 384-well plate format, saving time and reagents.
“In our hands, the Matador assay can detect cell death in as little as 30 minutes, which can ultimately translate to more expedient treatments for patients getting cellular immunotherapies such as CAR T cells,” Dr Chaudhary said.
In fact, Dr Chaudhary’s lab has developed more than 75 cancer cell lines expressing the marine luciferases and used them with the Matador assay to develop next-generation CAR T cells.
Dr Chaudhary believes the Matador assay has many potential applications in biomedical research and cellular therapy manufacturing.
“It could potentially play a role in screening other types of anticancer agents or even measuring environmental toxins,” he said. ![]()
Overcoming resistance to ibrutinib in CLL
New research appears to explain why ibrutinib may be less effective in certain patients with chronic lymphocytic leukemia (CLL).
It seems the Bruton’s tyrosine kinase (BTK) inhibitor has a diminished capacity to delocalize and kill tumor cells expressing an adhesive protein called CD49d.
But combining ibrutinib with drugs that block CD49d activation could prevent CLL cells from sheltering in lymphoid organs.
Valter Gattei, MD, of CRO Aviano National Cancer Institute in Aviano, Italy, and his colleagues reported these findings in the Journal of Experimental Medicine.
The team noted that CD49d, the α chain of the CD49d/CD29 integrin heterodimer very late antigen 4 (VLA-4), is expressed in about 40% of CLL cases.
These patients tend to have poorer outcomes than patients who do not express CD49d, but the role of VLA-4 in CLL was unclear.
With this study, researchers found that B-cell receptor (BCR) signaling can activate VLA-4 in CD49d-expressing CLL cells, thereby enhancing the cells’ adhesiveness.
Even though ibrutinib treatment impaired BCR signaling in these cells, it was unable to fully prevent the pathway from activating VLA-4 and enhancing cell adhesion.
The researchers analyzed 3 cohorts of CLL patients and found that patients expressing higher levels of CD49d had reduced responses to ibrutinib.
The BTK inhibitor appeared less able to displace tumor cells from lymph nodes into the blood, resulting in decreased lymph node shrinkage and shorter progression-free survival times.
“Our results suggest that VLA-4-expressing CLL cells residing in the secondary lymphoid organs can receive BCR-mediated stimuli that can activate VLA-4 even in the presence of ibrutinib,” said study author Antonella Zucchetto, ScD, also of CRO Aviano National Cancer Institute.
“This activation leads to enhanced retention of VLA-4-positive CLL cells in tissue sites, thereby affecting patient outcome.”
Fortunately, the researchers found a way around this obstacle. Inhibiting BTK and phosphatidylinositide 3-kinase (PI3K) simultaneously completely blocked VLA-4 activation in CLL cells.
The researchers treated CLL cells with ibrutinib, the PI3K inhibitor idelalisib, or a combination of both.
Neither drug alone was able to fully block anti-IgM-induced VLA-4 activation. However, the team found that simultaneous inhibition of BTK and PI3K “completely abolished the integrin response to BCR triggering.”
The researchers also added idelalisib to ibrutinib-treated CLL cells (collected from patients at day 30 on ibrutinib) and observed a complete upset of anti-IgM–induced VLA-4 activation.
“Our data suggest that evaluation of CD49d expression in patients initiating ibrutinib therapy may identify those cases that would benefit from combination therapy approaches designed to completely block VLA-4 activation and VLA-4-mediated retention of leukemic cells in protective tissue compartments,” Dr Gattei said. ![]()
New research appears to explain why ibrutinib may be less effective in certain patients with chronic lymphocytic leukemia (CLL).
It seems the Bruton’s tyrosine kinase (BTK) inhibitor has a diminished capacity to delocalize and kill tumor cells expressing an adhesive protein called CD49d.
But combining ibrutinib with drugs that block CD49d activation could prevent CLL cells from sheltering in lymphoid organs.
Valter Gattei, MD, of CRO Aviano National Cancer Institute in Aviano, Italy, and his colleagues reported these findings in the Journal of Experimental Medicine.
The team noted that CD49d, the α chain of the CD49d/CD29 integrin heterodimer very late antigen 4 (VLA-4), is expressed in about 40% of CLL cases.
These patients tend to have poorer outcomes than patients who do not express CD49d, but the role of VLA-4 in CLL was unclear.
With this study, researchers found that B-cell receptor (BCR) signaling can activate VLA-4 in CD49d-expressing CLL cells, thereby enhancing the cells’ adhesiveness.
Even though ibrutinib treatment impaired BCR signaling in these cells, it was unable to fully prevent the pathway from activating VLA-4 and enhancing cell adhesion.
The researchers analyzed 3 cohorts of CLL patients and found that patients expressing higher levels of CD49d had reduced responses to ibrutinib.
The BTK inhibitor appeared less able to displace tumor cells from lymph nodes into the blood, resulting in decreased lymph node shrinkage and shorter progression-free survival times.
“Our results suggest that VLA-4-expressing CLL cells residing in the secondary lymphoid organs can receive BCR-mediated stimuli that can activate VLA-4 even in the presence of ibrutinib,” said study author Antonella Zucchetto, ScD, also of CRO Aviano National Cancer Institute.
“This activation leads to enhanced retention of VLA-4-positive CLL cells in tissue sites, thereby affecting patient outcome.”
Fortunately, the researchers found a way around this obstacle. Inhibiting BTK and phosphatidylinositide 3-kinase (PI3K) simultaneously completely blocked VLA-4 activation in CLL cells.
The researchers treated CLL cells with ibrutinib, the PI3K inhibitor idelalisib, or a combination of both.
Neither drug alone was able to fully block anti-IgM-induced VLA-4 activation. However, the team found that simultaneous inhibition of BTK and PI3K “completely abolished the integrin response to BCR triggering.”
The researchers also added idelalisib to ibrutinib-treated CLL cells (collected from patients at day 30 on ibrutinib) and observed a complete upset of anti-IgM–induced VLA-4 activation.
“Our data suggest that evaluation of CD49d expression in patients initiating ibrutinib therapy may identify those cases that would benefit from combination therapy approaches designed to completely block VLA-4 activation and VLA-4-mediated retention of leukemic cells in protective tissue compartments,” Dr Gattei said. ![]()
New research appears to explain why ibrutinib may be less effective in certain patients with chronic lymphocytic leukemia (CLL).
It seems the Bruton’s tyrosine kinase (BTK) inhibitor has a diminished capacity to delocalize and kill tumor cells expressing an adhesive protein called CD49d.
But combining ibrutinib with drugs that block CD49d activation could prevent CLL cells from sheltering in lymphoid organs.
Valter Gattei, MD, of CRO Aviano National Cancer Institute in Aviano, Italy, and his colleagues reported these findings in the Journal of Experimental Medicine.
The team noted that CD49d, the α chain of the CD49d/CD29 integrin heterodimer very late antigen 4 (VLA-4), is expressed in about 40% of CLL cases.
These patients tend to have poorer outcomes than patients who do not express CD49d, but the role of VLA-4 in CLL was unclear.
With this study, researchers found that B-cell receptor (BCR) signaling can activate VLA-4 in CD49d-expressing CLL cells, thereby enhancing the cells’ adhesiveness.
Even though ibrutinib treatment impaired BCR signaling in these cells, it was unable to fully prevent the pathway from activating VLA-4 and enhancing cell adhesion.
The researchers analyzed 3 cohorts of CLL patients and found that patients expressing higher levels of CD49d had reduced responses to ibrutinib.
The BTK inhibitor appeared less able to displace tumor cells from lymph nodes into the blood, resulting in decreased lymph node shrinkage and shorter progression-free survival times.
“Our results suggest that VLA-4-expressing CLL cells residing in the secondary lymphoid organs can receive BCR-mediated stimuli that can activate VLA-4 even in the presence of ibrutinib,” said study author Antonella Zucchetto, ScD, also of CRO Aviano National Cancer Institute.
“This activation leads to enhanced retention of VLA-4-positive CLL cells in tissue sites, thereby affecting patient outcome.”
Fortunately, the researchers found a way around this obstacle. Inhibiting BTK and phosphatidylinositide 3-kinase (PI3K) simultaneously completely blocked VLA-4 activation in CLL cells.
The researchers treated CLL cells with ibrutinib, the PI3K inhibitor idelalisib, or a combination of both.
Neither drug alone was able to fully block anti-IgM-induced VLA-4 activation. However, the team found that simultaneous inhibition of BTK and PI3K “completely abolished the integrin response to BCR triggering.”
The researchers also added idelalisib to ibrutinib-treated CLL cells (collected from patients at day 30 on ibrutinib) and observed a complete upset of anti-IgM–induced VLA-4 activation.
“Our data suggest that evaluation of CD49d expression in patients initiating ibrutinib therapy may identify those cases that would benefit from combination therapy approaches designed to completely block VLA-4 activation and VLA-4-mediated retention of leukemic cells in protective tissue compartments,” Dr Gattei said. ![]()