User login
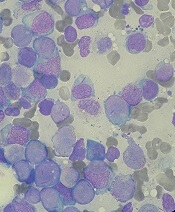
Beat AML trial delivers genomic results in 7 days
SAN DIEGO – Investigators demonstrated the feasibility of delivering genomic results in 7 days in a population of older, newly diagnosed patients with acute myeloid leukemia (AML).
The Beat AML Master Trial is an ongoing umbrella study that harnesses cytogenetic information and next generation sequencing to match patients with targeted therapies across a number of substudies or outside of the trial’s multicenter network.
The researchers chose AML for this precision-medicine study because of its rapid onset and lethal nature, its heterogeneity, and the availability of more-targeted therapies, said Amy Burd, PhD, of the Leukemia & Lymphoma Society, which is sponsoring the study.
Initial data from the trial showed that more than 95% of patients were assigned to treatment in 7 days or less, based on their personalized genomic information.
Overall, 285 patients had usable genomic screening data and were assigned to treatment. Of those patients, 273 were assigned to a treatment within 7 days, Dr. Burd reported at the annual meeting of the American Society of Hematology.
The speed of delivering these results is critical, said Joseph Mikhael, MD, chief medical officer for the International Myeloma Foundation in Phoenix, who moderated a media briefing on personalized medicine.
“One of the greatest challenges we faced in the concept of personalized medicine is by the time you’ve determined what is best for that patient ... the horse is already out of the barn,” Dr. Mikhael said. “You have to have started the patient on treatment already or else their disease could have progressed quite rapidly.”
In the past, genomic results might come back a month after the patient started therapy. “It was really almost academic,” he said.
In the Beat AML study, more than half (146 patients) were treated based on their AML subtype. The remaining patients (139) were not treated: 2.5% of patients died within 7 days, 7% of patients chose an alternative treatment prior to assignment, 20% chose standard of care, 9.1% chose an alternative trial after assignment, 8.1% chose palliative care, and the remainder had a reason that was not specified.
“The treatment decisions are made for what’s best for the patient even if that means a study outside of Beat AML,” Dr. Burd said.
Currently, there are 11 substudies offering treatment to trial participants across 13 clinical sites. There has been promising efficacy in many of the treatment arms, Dr. Burd said.
In the future, the researchers are looking to expand the substudies to look into novel drug combinations for certain AML subtypes, specifically isocitrate dehydrogenase 2–mutated groups.
Dr. Burd is an employee of the Leukemia & Lymphoma Society. Other coinvestigators reported financial relationships with the pharmaceutical industry. Dr. Mikhael reported research funding from AbbVie, Celgene, Onyx Pharmaceuticals, and Sanofi.
SOURCE: Burd A et al. ASH 2018, Abstract 559.
SAN DIEGO – Investigators demonstrated the feasibility of delivering genomic results in 7 days in a population of older, newly diagnosed patients with acute myeloid leukemia (AML).
The Beat AML Master Trial is an ongoing umbrella study that harnesses cytogenetic information and next generation sequencing to match patients with targeted therapies across a number of substudies or outside of the trial’s multicenter network.
The researchers chose AML for this precision-medicine study because of its rapid onset and lethal nature, its heterogeneity, and the availability of more-targeted therapies, said Amy Burd, PhD, of the Leukemia & Lymphoma Society, which is sponsoring the study.
Initial data from the trial showed that more than 95% of patients were assigned to treatment in 7 days or less, based on their personalized genomic information.
Overall, 285 patients had usable genomic screening data and were assigned to treatment. Of those patients, 273 were assigned to a treatment within 7 days, Dr. Burd reported at the annual meeting of the American Society of Hematology.
The speed of delivering these results is critical, said Joseph Mikhael, MD, chief medical officer for the International Myeloma Foundation in Phoenix, who moderated a media briefing on personalized medicine.
“One of the greatest challenges we faced in the concept of personalized medicine is by the time you’ve determined what is best for that patient ... the horse is already out of the barn,” Dr. Mikhael said. “You have to have started the patient on treatment already or else their disease could have progressed quite rapidly.”
In the past, genomic results might come back a month after the patient started therapy. “It was really almost academic,” he said.
In the Beat AML study, more than half (146 patients) were treated based on their AML subtype. The remaining patients (139) were not treated: 2.5% of patients died within 7 days, 7% of patients chose an alternative treatment prior to assignment, 20% chose standard of care, 9.1% chose an alternative trial after assignment, 8.1% chose palliative care, and the remainder had a reason that was not specified.
“The treatment decisions are made for what’s best for the patient even if that means a study outside of Beat AML,” Dr. Burd said.
Currently, there are 11 substudies offering treatment to trial participants across 13 clinical sites. There has been promising efficacy in many of the treatment arms, Dr. Burd said.
In the future, the researchers are looking to expand the substudies to look into novel drug combinations for certain AML subtypes, specifically isocitrate dehydrogenase 2–mutated groups.
Dr. Burd is an employee of the Leukemia & Lymphoma Society. Other coinvestigators reported financial relationships with the pharmaceutical industry. Dr. Mikhael reported research funding from AbbVie, Celgene, Onyx Pharmaceuticals, and Sanofi.
SOURCE: Burd A et al. ASH 2018, Abstract 559.
SAN DIEGO – Investigators demonstrated the feasibility of delivering genomic results in 7 days in a population of older, newly diagnosed patients with acute myeloid leukemia (AML).
The Beat AML Master Trial is an ongoing umbrella study that harnesses cytogenetic information and next generation sequencing to match patients with targeted therapies across a number of substudies or outside of the trial’s multicenter network.
The researchers chose AML for this precision-medicine study because of its rapid onset and lethal nature, its heterogeneity, and the availability of more-targeted therapies, said Amy Burd, PhD, of the Leukemia & Lymphoma Society, which is sponsoring the study.
Initial data from the trial showed that more than 95% of patients were assigned to treatment in 7 days or less, based on their personalized genomic information.
Overall, 285 patients had usable genomic screening data and were assigned to treatment. Of those patients, 273 were assigned to a treatment within 7 days, Dr. Burd reported at the annual meeting of the American Society of Hematology.
The speed of delivering these results is critical, said Joseph Mikhael, MD, chief medical officer for the International Myeloma Foundation in Phoenix, who moderated a media briefing on personalized medicine.
“One of the greatest challenges we faced in the concept of personalized medicine is by the time you’ve determined what is best for that patient ... the horse is already out of the barn,” Dr. Mikhael said. “You have to have started the patient on treatment already or else their disease could have progressed quite rapidly.”
In the past, genomic results might come back a month after the patient started therapy. “It was really almost academic,” he said.
In the Beat AML study, more than half (146 patients) were treated based on their AML subtype. The remaining patients (139) were not treated: 2.5% of patients died within 7 days, 7% of patients chose an alternative treatment prior to assignment, 20% chose standard of care, 9.1% chose an alternative trial after assignment, 8.1% chose palliative care, and the remainder had a reason that was not specified.
“The treatment decisions are made for what’s best for the patient even if that means a study outside of Beat AML,” Dr. Burd said.
Currently, there are 11 substudies offering treatment to trial participants across 13 clinical sites. There has been promising efficacy in many of the treatment arms, Dr. Burd said.
In the future, the researchers are looking to expand the substudies to look into novel drug combinations for certain AML subtypes, specifically isocitrate dehydrogenase 2–mutated groups.
Dr. Burd is an employee of the Leukemia & Lymphoma Society. Other coinvestigators reported financial relationships with the pharmaceutical industry. Dr. Mikhael reported research funding from AbbVie, Celgene, Onyx Pharmaceuticals, and Sanofi.
SOURCE: Burd A et al. ASH 2018, Abstract 559.
REPORTING FROM ASH 2018
Key clinical point:
Major finding: More than 95% of patients in the trial were assigned to treatment within 7 days based on results of their genomic screening.
Study details: An umbrella study of 285 patients aged 60 years and older with newly diagnosed acute myeloid leukemia.
Disclosures: The study is sponsored by the Leukemia & Lymphoma Society. Dr. Burd is an employee of the Society and other investigators reported funding from multiple pharmaceutical companies.
Source: Burd A et al. ASH 2018, Abstract 559.
FDA warns of serious side effect of AML treatment
The (Idhifa).
Enasidenib is FDA approved to treat adults with relapsed or refractory acute myeloid leukemia (AML) and an IDH2 mutation. The drug is known to be associated with differentiation syndrome, and the drug’s prescribing information contains a boxed warning about this life-threatening condition.
However, the FDA has found that patients and health care providers are missing the signs and symptoms of differentiation syndrome, and some patients are not receiving the necessary treatment in time.
The FDA also is warning that differentiation syndrome has been observed in AML patients taking the IDH1 inhibitor ivosidenib (Tibsovo).
However, the agency has not provided many details on cases related to this drug, which is FDA approved to treat adults with relapsed or refractory AML who have an IDH1 mutation.The FDA says differentiation syndrome may occur anywhere from 10 days to 5 months after starting enasidenib.
The agency is advising health care providers to describe the symptoms of differentiation syndrome to patients, both when starting them on enasidenib and at follow-up visits.
Symptoms of differentiation syndrome include:
- Acute respiratory distress represented by dyspnea and/or hypoxia and a need for supplemental oxygen.
- Pulmonary infiltrates and pleural effusion.
- Fever.
- Lymphadenopathy.
- Bone pain.
- Peripheral edema with rapid weight gain.
- Pericardial effusion.
- Hepatic, renal, and multiorgan dysfunction.
The FDA notes that differentiation syndrome may be mistaken for cardiogenic pulmonary edema, pneumonia, or sepsis.If health care providers suspect differentiation syndrome, they should promptly administer oral or intravenous corticosteroids, such as dexamethasone at 10 mg every 12 hours, according to the FDA. Providers also should monitor hemodynamics until improvement and provide supportive care as necessary.
If patients continue to experience renal dysfunction or severe pulmonary symptoms that require intubation or ventilator support for more than 48 hours after starting corticosteroids, enasidenib should be stopped until signs and symptoms of differentiation syndrome are no longer severe.
Corticosteroids should be tapered only after the symptoms resolve completely, as differentiation syndrome may recur if treatment is stopped too soon. The FDA notes that in the clinical trial that supported approval of enasidenib at least 14% of patients experienced differentiation syndrome.
The manufacturer’s latest safety report includes five deaths (from May 1, 2018, to July 31, 2018) associated with differentiation syndrome in patients taking enasidenib.
Differentiation syndrome was listed as the only cause of death in two cases. In the other cases, patients also had hemorrhagic stroke, pneumonia and sepsis, and sepsis alone.
One patient received systemic corticosteroids promptly but may have died of sepsis during hospitalization. In another patient, differentiation syndrome was not diagnosed or treated promptly.
Treatment details are not available for the remaining three patients who died, according to the FDA.
The FDA has also performed a systematic analysis of differentiation syndrome in 293 patients treated with enasidenib (n = 214) or ivosidenib (n = 179).
With both drugs, the incidence of differentiation syndrome was 19%. The condition was fatal in two of the ivosidenib-treated patients (6%) and two of the enasidenib-treated patients (5%).
Additional results from this analysis are scheduled to be presented at the annual meeting of the American Society of Hematology (Abstract 288).
The (Idhifa).
Enasidenib is FDA approved to treat adults with relapsed or refractory acute myeloid leukemia (AML) and an IDH2 mutation. The drug is known to be associated with differentiation syndrome, and the drug’s prescribing information contains a boxed warning about this life-threatening condition.
However, the FDA has found that patients and health care providers are missing the signs and symptoms of differentiation syndrome, and some patients are not receiving the necessary treatment in time.
The FDA also is warning that differentiation syndrome has been observed in AML patients taking the IDH1 inhibitor ivosidenib (Tibsovo).
However, the agency has not provided many details on cases related to this drug, which is FDA approved to treat adults with relapsed or refractory AML who have an IDH1 mutation.The FDA says differentiation syndrome may occur anywhere from 10 days to 5 months after starting enasidenib.
The agency is advising health care providers to describe the symptoms of differentiation syndrome to patients, both when starting them on enasidenib and at follow-up visits.
Symptoms of differentiation syndrome include:
- Acute respiratory distress represented by dyspnea and/or hypoxia and a need for supplemental oxygen.
- Pulmonary infiltrates and pleural effusion.
- Fever.
- Lymphadenopathy.
- Bone pain.
- Peripheral edema with rapid weight gain.
- Pericardial effusion.
- Hepatic, renal, and multiorgan dysfunction.
The FDA notes that differentiation syndrome may be mistaken for cardiogenic pulmonary edema, pneumonia, or sepsis.If health care providers suspect differentiation syndrome, they should promptly administer oral or intravenous corticosteroids, such as dexamethasone at 10 mg every 12 hours, according to the FDA. Providers also should monitor hemodynamics until improvement and provide supportive care as necessary.
If patients continue to experience renal dysfunction or severe pulmonary symptoms that require intubation or ventilator support for more than 48 hours after starting corticosteroids, enasidenib should be stopped until signs and symptoms of differentiation syndrome are no longer severe.
Corticosteroids should be tapered only after the symptoms resolve completely, as differentiation syndrome may recur if treatment is stopped too soon. The FDA notes that in the clinical trial that supported approval of enasidenib at least 14% of patients experienced differentiation syndrome.
The manufacturer’s latest safety report includes five deaths (from May 1, 2018, to July 31, 2018) associated with differentiation syndrome in patients taking enasidenib.
Differentiation syndrome was listed as the only cause of death in two cases. In the other cases, patients also had hemorrhagic stroke, pneumonia and sepsis, and sepsis alone.
One patient received systemic corticosteroids promptly but may have died of sepsis during hospitalization. In another patient, differentiation syndrome was not diagnosed or treated promptly.
Treatment details are not available for the remaining three patients who died, according to the FDA.
The FDA has also performed a systematic analysis of differentiation syndrome in 293 patients treated with enasidenib (n = 214) or ivosidenib (n = 179).
With both drugs, the incidence of differentiation syndrome was 19%. The condition was fatal in two of the ivosidenib-treated patients (6%) and two of the enasidenib-treated patients (5%).
Additional results from this analysis are scheduled to be presented at the annual meeting of the American Society of Hematology (Abstract 288).
The (Idhifa).
Enasidenib is FDA approved to treat adults with relapsed or refractory acute myeloid leukemia (AML) and an IDH2 mutation. The drug is known to be associated with differentiation syndrome, and the drug’s prescribing information contains a boxed warning about this life-threatening condition.
However, the FDA has found that patients and health care providers are missing the signs and symptoms of differentiation syndrome, and some patients are not receiving the necessary treatment in time.
The FDA also is warning that differentiation syndrome has been observed in AML patients taking the IDH1 inhibitor ivosidenib (Tibsovo).
However, the agency has not provided many details on cases related to this drug, which is FDA approved to treat adults with relapsed or refractory AML who have an IDH1 mutation.The FDA says differentiation syndrome may occur anywhere from 10 days to 5 months after starting enasidenib.
The agency is advising health care providers to describe the symptoms of differentiation syndrome to patients, both when starting them on enasidenib and at follow-up visits.
Symptoms of differentiation syndrome include:
- Acute respiratory distress represented by dyspnea and/or hypoxia and a need for supplemental oxygen.
- Pulmonary infiltrates and pleural effusion.
- Fever.
- Lymphadenopathy.
- Bone pain.
- Peripheral edema with rapid weight gain.
- Pericardial effusion.
- Hepatic, renal, and multiorgan dysfunction.
The FDA notes that differentiation syndrome may be mistaken for cardiogenic pulmonary edema, pneumonia, or sepsis.If health care providers suspect differentiation syndrome, they should promptly administer oral or intravenous corticosteroids, such as dexamethasone at 10 mg every 12 hours, according to the FDA. Providers also should monitor hemodynamics until improvement and provide supportive care as necessary.
If patients continue to experience renal dysfunction or severe pulmonary symptoms that require intubation or ventilator support for more than 48 hours after starting corticosteroids, enasidenib should be stopped until signs and symptoms of differentiation syndrome are no longer severe.
Corticosteroids should be tapered only after the symptoms resolve completely, as differentiation syndrome may recur if treatment is stopped too soon. The FDA notes that in the clinical trial that supported approval of enasidenib at least 14% of patients experienced differentiation syndrome.
The manufacturer’s latest safety report includes five deaths (from May 1, 2018, to July 31, 2018) associated with differentiation syndrome in patients taking enasidenib.
Differentiation syndrome was listed as the only cause of death in two cases. In the other cases, patients also had hemorrhagic stroke, pneumonia and sepsis, and sepsis alone.
One patient received systemic corticosteroids promptly but may have died of sepsis during hospitalization. In another patient, differentiation syndrome was not diagnosed or treated promptly.
Treatment details are not available for the remaining three patients who died, according to the FDA.
The FDA has also performed a systematic analysis of differentiation syndrome in 293 patients treated with enasidenib (n = 214) or ivosidenib (n = 179).
With both drugs, the incidence of differentiation syndrome was 19%. The condition was fatal in two of the ivosidenib-treated patients (6%) and two of the enasidenib-treated patients (5%).
Additional results from this analysis are scheduled to be presented at the annual meeting of the American Society of Hematology (Abstract 288).
Serious side effect of AML treatment going unnoticed, FDA warns
The U.S. Food and Drug Administration (FDA) has released a safety communication warning that cases of differentiation syndrome are going unnoticed in patients treated with the IDH2 inhibitor enasidenib (Idhifa).
Enasidenib is FDA-approved to treat adults with relapsed or refractory acute myeloid leukemia (AML) and an IDH2 mutation.
The drug is known to be associated with differentiation syndrome, and the prescribing information contains a boxed warning about this life-threatening condition.
However, the FDA has found that patients and healthcare providers are missing the signs and symptoms of differentiation syndrome, and some patients are not receiving the necessary treatment in time.
The FDA is also warning that differentiation syndrome has been observed in AML patients taking the IDH1 inhibitor ivosidenib (Tibsovo).
However, the agency has not provided many details on cases related to this drug, which is FDA-approved to treat adults with relapsed or refractory AML who have an IDH1 mutation.
Recognizing differentiation syndrome
The FDA says differentiation syndrome may occur anywhere from 10 days to 5 months after starting enasidenib.
The agency is advising healthcare providers to describe the symptoms of differentiation syndrome to patients, both when starting them on enasidenib and at follow-up visits.
Symptoms of differentiation syndrome include:
- Acute respiratory distress represented by dyspnea and/or hypoxia and a need for supplemental oxygen
- Pulmonary infiltrates and pleural effusion
- Fever
- Lymphadenopathy
- Bone pain
- Peripheral edema with rapid weight gain
- Pericardial effusion
- Hepatic, renal, and multiorgan dysfunction.
The FDA notes that differentiation syndrome may be mistaken for cardiogenic pulmonary edema, pneumonia, or sepsis.
Treatment
If healthcare providers suspect differentiation syndrome, they should promptly administer oral or intravenous corticosteroids, such as dexamethasone at 10 mg every 12 hours, according to the FDA.
Providers should also monitor hemodynamics until improvement and provide supportive care as necessary.
If patients continue to experience renal dysfunction or severe pulmonary symptoms requiring intubation or ventilator support for more than 48 hours after starting corticosteroids, enasidenib should be stopped until signs and symptoms of differentiation syndrome are no longer severe.
Corticosteroids should be tapered only after the symptoms resolve completely, as differentiation syndrome may recur if treatment is stopped too soon.
Cases of differentiation syndrome
The FDA notes that, in the phase 1/2 trial that supported the U.S. approval of enasidenib, at least 14% of patients experienced differentiation syndrome.
The manufacturer’s latest safety report includes 5 deaths (from May 1, 2018, to July 31, 2018) associated with differentiation syndrome in patients taking enasidenib.
Differentiation syndrome was listed as the only cause of death in two cases. In the remaining three cases, patients also had hemorrhagic stroke, pneumonia and sepsis, and sepsis alone.
One patient received systemic corticosteroids promptly but may have died of sepsis during hospitalization. In another patient, differentiation syndrome was not diagnosed or treated promptly. Treatment details are not available for the remaining three patients, according to the FDA.
The FDA has also performed a systematic analysis of differentiation syndrome in 293 patients treated with enasidenib (n=214) or ivosidenib (n=179).
With both drugs, the incidence of differentiation syndrome was 19%. The condition was fatal in 6% (n=2) of ivosidenib-treated patients and 5% (n=2) of enasidenib-treated patients.
Additional results from this analysis are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 288).
The U.S. Food and Drug Administration (FDA) has released a safety communication warning that cases of differentiation syndrome are going unnoticed in patients treated with the IDH2 inhibitor enasidenib (Idhifa).
Enasidenib is FDA-approved to treat adults with relapsed or refractory acute myeloid leukemia (AML) and an IDH2 mutation.
The drug is known to be associated with differentiation syndrome, and the prescribing information contains a boxed warning about this life-threatening condition.
However, the FDA has found that patients and healthcare providers are missing the signs and symptoms of differentiation syndrome, and some patients are not receiving the necessary treatment in time.
The FDA is also warning that differentiation syndrome has been observed in AML patients taking the IDH1 inhibitor ivosidenib (Tibsovo).
However, the agency has not provided many details on cases related to this drug, which is FDA-approved to treat adults with relapsed or refractory AML who have an IDH1 mutation.
Recognizing differentiation syndrome
The FDA says differentiation syndrome may occur anywhere from 10 days to 5 months after starting enasidenib.
The agency is advising healthcare providers to describe the symptoms of differentiation syndrome to patients, both when starting them on enasidenib and at follow-up visits.
Symptoms of differentiation syndrome include:
- Acute respiratory distress represented by dyspnea and/or hypoxia and a need for supplemental oxygen
- Pulmonary infiltrates and pleural effusion
- Fever
- Lymphadenopathy
- Bone pain
- Peripheral edema with rapid weight gain
- Pericardial effusion
- Hepatic, renal, and multiorgan dysfunction.
The FDA notes that differentiation syndrome may be mistaken for cardiogenic pulmonary edema, pneumonia, or sepsis.
Treatment
If healthcare providers suspect differentiation syndrome, they should promptly administer oral or intravenous corticosteroids, such as dexamethasone at 10 mg every 12 hours, according to the FDA.
Providers should also monitor hemodynamics until improvement and provide supportive care as necessary.
If patients continue to experience renal dysfunction or severe pulmonary symptoms requiring intubation or ventilator support for more than 48 hours after starting corticosteroids, enasidenib should be stopped until signs and symptoms of differentiation syndrome are no longer severe.
Corticosteroids should be tapered only after the symptoms resolve completely, as differentiation syndrome may recur if treatment is stopped too soon.
Cases of differentiation syndrome
The FDA notes that, in the phase 1/2 trial that supported the U.S. approval of enasidenib, at least 14% of patients experienced differentiation syndrome.
The manufacturer’s latest safety report includes 5 deaths (from May 1, 2018, to July 31, 2018) associated with differentiation syndrome in patients taking enasidenib.
Differentiation syndrome was listed as the only cause of death in two cases. In the remaining three cases, patients also had hemorrhagic stroke, pneumonia and sepsis, and sepsis alone.
One patient received systemic corticosteroids promptly but may have died of sepsis during hospitalization. In another patient, differentiation syndrome was not diagnosed or treated promptly. Treatment details are not available for the remaining three patients, according to the FDA.
The FDA has also performed a systematic analysis of differentiation syndrome in 293 patients treated with enasidenib (n=214) or ivosidenib (n=179).
With both drugs, the incidence of differentiation syndrome was 19%. The condition was fatal in 6% (n=2) of ivosidenib-treated patients and 5% (n=2) of enasidenib-treated patients.
Additional results from this analysis are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 288).
The U.S. Food and Drug Administration (FDA) has released a safety communication warning that cases of differentiation syndrome are going unnoticed in patients treated with the IDH2 inhibitor enasidenib (Idhifa).
Enasidenib is FDA-approved to treat adults with relapsed or refractory acute myeloid leukemia (AML) and an IDH2 mutation.
The drug is known to be associated with differentiation syndrome, and the prescribing information contains a boxed warning about this life-threatening condition.
However, the FDA has found that patients and healthcare providers are missing the signs and symptoms of differentiation syndrome, and some patients are not receiving the necessary treatment in time.
The FDA is also warning that differentiation syndrome has been observed in AML patients taking the IDH1 inhibitor ivosidenib (Tibsovo).
However, the agency has not provided many details on cases related to this drug, which is FDA-approved to treat adults with relapsed or refractory AML who have an IDH1 mutation.
Recognizing differentiation syndrome
The FDA says differentiation syndrome may occur anywhere from 10 days to 5 months after starting enasidenib.
The agency is advising healthcare providers to describe the symptoms of differentiation syndrome to patients, both when starting them on enasidenib and at follow-up visits.
Symptoms of differentiation syndrome include:
- Acute respiratory distress represented by dyspnea and/or hypoxia and a need for supplemental oxygen
- Pulmonary infiltrates and pleural effusion
- Fever
- Lymphadenopathy
- Bone pain
- Peripheral edema with rapid weight gain
- Pericardial effusion
- Hepatic, renal, and multiorgan dysfunction.
The FDA notes that differentiation syndrome may be mistaken for cardiogenic pulmonary edema, pneumonia, or sepsis.
Treatment
If healthcare providers suspect differentiation syndrome, they should promptly administer oral or intravenous corticosteroids, such as dexamethasone at 10 mg every 12 hours, according to the FDA.
Providers should also monitor hemodynamics until improvement and provide supportive care as necessary.
If patients continue to experience renal dysfunction or severe pulmonary symptoms requiring intubation or ventilator support for more than 48 hours after starting corticosteroids, enasidenib should be stopped until signs and symptoms of differentiation syndrome are no longer severe.
Corticosteroids should be tapered only after the symptoms resolve completely, as differentiation syndrome may recur if treatment is stopped too soon.
Cases of differentiation syndrome
The FDA notes that, in the phase 1/2 trial that supported the U.S. approval of enasidenib, at least 14% of patients experienced differentiation syndrome.
The manufacturer’s latest safety report includes 5 deaths (from May 1, 2018, to July 31, 2018) associated with differentiation syndrome in patients taking enasidenib.
Differentiation syndrome was listed as the only cause of death in two cases. In the remaining three cases, patients also had hemorrhagic stroke, pneumonia and sepsis, and sepsis alone.
One patient received systemic corticosteroids promptly but may have died of sepsis during hospitalization. In another patient, differentiation syndrome was not diagnosed or treated promptly. Treatment details are not available for the remaining three patients, according to the FDA.
The FDA has also performed a systematic analysis of differentiation syndrome in 293 patients treated with enasidenib (n=214) or ivosidenib (n=179).
With both drugs, the incidence of differentiation syndrome was 19%. The condition was fatal in 6% (n=2) of ivosidenib-treated patients and 5% (n=2) of enasidenib-treated patients.
Additional results from this analysis are scheduled to be presented at the 2018 ASH Annual Meeting (abstract 288).
Your guide to ASH 2018: Abstracts to watch
With more than 3,000 scientific abstracts at the 2018 annual meeting of the American Society of Hematology, it can be tough to figure out what research is most relevant to practice. But the editorial advisory board of Hematology News is making it easier this year with their picks for what to watch and why.
Lymphomas
Brian T. Hill, MD, of the Cleveland Clinic, offered his top picks in lymphoma research. Results of the phase 3 international Alliance North American Intergroup Study A041202 will be presented during the ASH plenary session at 2 p.m. PT on Sunday, Dec. 2 in Hall AB of the San Diego Convention Center (Abstract 6). The study compared bendamustine plus rituximab with ibrutinib and the combination of ibrutinib plus rituximab to see if the ibrutinib-containing therapies would have superior progression-free survival (PFS) in chronic lymphocytic leukemia (CLL), compared with chemoimmunotherapy. Results indicate that ibrutinib had superior PFS in older patients with CLL and could be a standard of care in this population.
The study is worth watching because it is the first report of a head-to-head trial of chemotherapy versus ibrutinib for first-line treatment of CLL, Dr. Hill said.
Two more studies offer important reports of “real world” experiences with chimeric antigen receptor (CAR) T-cell therapy.
In one multicenter retrospective study, researchers evaluated the outcomes of axicabtagene ciloleucel (axi-cel) CAR T-cell therapy for relapsed/refractory aggressive B-cell lymphoma when it is used a standard care. The researchers will report that 30-day responses in the real-world setting were comparable to the best responses seen in the ZUMA-1 trial. The full results will be reported at 9:30 a.m. PT on Saturday, Dec. 1 in Pacific Ballroom 20 of the Marriott Marquis San Diego Marina (Abstract 91).
Another retrospective analysis looked at the use of axi-cell and revealed some critical differences from ZUMA-1, specifically the overall response rate (ORR) and complete response (CR) rate were lower than those reported in the pivotal clinical trial. The findings will be reported at 9:45 a.m. PT on Saturday, Dec. 1 in Pacific Ballroom 20 of the Marriott Marquis San Diego Marina (Abstract 92).
Researchers will also present the unblinded results from the ECHELON-2 study, which compared the efficacy and safety of brentuximab vedotin in combination with cyclophosphamide, doxorubicin, and prednisone (CHP) versus standard CHOP for the treatment of patients with peripheral T-cell lymphoma. The results will be presented at 6:15 p.m. PT on Monday, Dec. 3 in room 6F of the San Diego convention center (Abstract 997).
Previously reported blinded pooled data showed that the treatment was well tolerated with 3-year PFS of 53% and OS of 73%.
“This should be a new standard of care for T-cell lymphomas,” Dr. Hill said.
CAR T-cell therapy
There are a number of abstracts featuring the latest results on CAR T-cell therapy. Helen Heslop, MD, of Baylor College of Medicine, Houston, recommended an updated analysis from the ELIANA study, which looked at the efficacy and safety of tisagenlecleucel in for children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL).
“Longer-term follow-up of the ELIANA study shows encouraging remission-duration data in pediatric and young adults with ALL without additional therapy,” Dr. Heslop said.
The findings will be presented at 4:30 p.m. PT on Monday, Dec. 3 in room 6A at the San Diego Convention Center (Abstract 895).
Another notable presentation will feature results from a phase 1B/2 trial evaluating infusion of CAR T cells targeting the CD30 molecule and encoding the CD28 endodomain (CD30.CAR-Ts) after lymphodepleting chemotherapy in patients with relapsed or refractory CD30+ Hodgkin lymphoma and non-Hodgkin lymphoma.
The researchers will report that there was a significant PFS advances for who received the highest dose level of the CAR T treatment, combined with bendamustine and fludarabine.
The study will be presented at 11 a.m. PT on Monday, Dec. 3 in room 6F at the San Diego Convention Center (Abstract 681).
Dr. Heslop also recommends another study being presented in the same session, which also shows encouraging results with CD30.CAR-Ts. Dr. Heslop is one of the co-investigators on the phase 1 RELY-30 trial, which is evaluating the efficacy of CD30.CAR-Ts after lymphodepleting chemotherapy. Preliminary results suggest a substantial improvement in efficacy. The findings will be presented at 10:45 a.m. PT on Monday, Dec. 3 in room 6F of the San Diego Convention Center (Abstract 680).
MDS/MPN
Vikas Gupta, MD, of Princess Margaret Cancer Center in Toronto, highlighted three abstracts to watch in the areas of myelodysplastic syndrome (MDS) and myeloproliferative neoplasms (MPN).
The phase 3 Medalist trial is a randomized double-blind placebo controlled study of luspatercept to treatment anemia in patients with MDS with ring sideroblasts who require red blood cell transfusion. The researchers will report significantly reduced transfusion burdens for luspatercept, compared with placebo.
“This is a practice-changing, pivotal trial in the field of MDS for the treatment of anemia,” Dr. Gupta said.
The findings will be presented at 2 p.m. PT on Sunday, Dec. 2 during the plenary session in Hall AB in the San Diego Convention Center (Abstract 1).
Also during the Sunday plenary session is a presentation on MPN therapy (Abstract 4). Researchers will present data on secreted mutant calreticulins as rogue cytokines trigger thrombopoietin receptor (TpoR) activation, specifically in CALR-mutated cells.
“This study investigates in to the mechanistic oncogenetic aspects of mutant calreticulin, and has potential for therapeutic approaches in the future,” Dr. Gupta said.
The ASH meeting will also feature the final analysis of the MPN-RC 112 consortium trial of pegylated interferon alfa-2a versus hydroxyurea for the treatment of high-risk polycythemia vera (PV) and essential thrombocythemia (ET). The researchers will report that the CR rates at 12 and 24 months were similar in patients treated with pegylated interferon alfa-2a and hydroxyurea, but pegylated interferon alfa-2a was associated with a higher rate of serious toxicities.
“There is a continuous debate on optimal first-line cytoreductive therapy for high risk PV/ET, and this is one of the first randomized study to answer this question,” Dr. Gupta said.
The findings will be presented at 7 a.m. PT on Monday, Dec. 3 in Grand Hall D at the Manchester Grand Hyatt San Diego (Abstract 577).
AML
For attendees interested in the latest developments in acute myeloid leukemia, Thomas Fischer, MD, of Otto-von-Guericke-University Magdeburg (Germany), highlighted three don’t-miss sessions.
In an analysis of a large cohort of FLT3-ITD mutated AML patients in the RATIFY trial, researchers looked at the prognostic impact of ITD insertion site.
“Interestingly, in this large cohort of 452 FLT3-ITD mutated AML, the negative prognostic impact of beta1-sheet insertion site of FLT3-ITD could be confirmed,” Dr. Fischer said. “Further analysis of a potential predictive effect on outcome of midostaurin treatment is ongoing and will be very interesting.”
The findings will be presented at 5 p.m. PT on Sunday, Dec. 2 in Seaport Ballroom F at the Manchester Grand Hyatt San Diego (Abstract 435).
Another notable presentation features results from the phase 2 RADIUS trial, a randomized study comparing standard of care, with and without midostaurin, after allogeneic stem cell transplant in FLT3-ITD–mutated AML.
“Here, efficacy and toxicity of midostaurin was investigated in a [minimal residual disease] situation post-alloSCT,” Dr. Fischer said. “Interestingly, adding midostaurin to standard of care reduced the risk of relapse at 18 months post-alloSCT by 46%.”
The complete findings will be presented at 10:45 a.m. PT on Monday, Dec. 3 in Seaport Ballroom F at the Manchester Grand Hyatt San Diego (Abstract 662).
Dr. Fischer singled out another study looking at the efficacy and safety of single-agent quizartinib in patients with FLT3-ITD mutated AML. In this large, randomized trial the researchers noted a significant improvement in CR rates and survival benefit with the single agent FLT3 inhibitors, compared with salvage chemotherapy for patients with relapsed/refractory mutated AML.
The findings will be presented at 8 a.m. on Monday, Dec. 3 in Seaport Ballroom F at the Manchester Grand Hyatt San Diego (Abstract 563).
Notable posters
Iberia Romina Sosa, MD, PhD, of Baylor College of Medicine in Houston, suggested several posters worth visiting in the areas of thrombosis and bleeding.
Poster 1134 looks at the TNF-alpha driven inflammation and mitochondrial dysfunction in the platelet hyperreactivity of aging and MPN.
How do you know if your therapy for thrombotic thrombocytopenic purpura is working? Poster 3736 examines the measurement of cell-derived microparticles as a possible tool to monitor response to therapy.
You don’t have to be taking aspirin to have a bleeding profile characteristic with consumption of a cyclooxygenase inhibitor. Poster 1156 provides a first report of a platelet function disorder caused by autosomal recessive inheritance of PTGS1.
Poster 2477 takes a closer look at fitusiran, an antithrombin inhibitor, which improves thrombin generation in patients with hemophilia A or B. Protocol amendments for safety monitoring move fitusiran to phase 3 trials, Dr. Sosa said.
With more than 3,000 scientific abstracts at the 2018 annual meeting of the American Society of Hematology, it can be tough to figure out what research is most relevant to practice. But the editorial advisory board of Hematology News is making it easier this year with their picks for what to watch and why.
Lymphomas
Brian T. Hill, MD, of the Cleveland Clinic, offered his top picks in lymphoma research. Results of the phase 3 international Alliance North American Intergroup Study A041202 will be presented during the ASH plenary session at 2 p.m. PT on Sunday, Dec. 2 in Hall AB of the San Diego Convention Center (Abstract 6). The study compared bendamustine plus rituximab with ibrutinib and the combination of ibrutinib plus rituximab to see if the ibrutinib-containing therapies would have superior progression-free survival (PFS) in chronic lymphocytic leukemia (CLL), compared with chemoimmunotherapy. Results indicate that ibrutinib had superior PFS in older patients with CLL and could be a standard of care in this population.
The study is worth watching because it is the first report of a head-to-head trial of chemotherapy versus ibrutinib for first-line treatment of CLL, Dr. Hill said.
Two more studies offer important reports of “real world” experiences with chimeric antigen receptor (CAR) T-cell therapy.
In one multicenter retrospective study, researchers evaluated the outcomes of axicabtagene ciloleucel (axi-cel) CAR T-cell therapy for relapsed/refractory aggressive B-cell lymphoma when it is used a standard care. The researchers will report that 30-day responses in the real-world setting were comparable to the best responses seen in the ZUMA-1 trial. The full results will be reported at 9:30 a.m. PT on Saturday, Dec. 1 in Pacific Ballroom 20 of the Marriott Marquis San Diego Marina (Abstract 91).
Another retrospective analysis looked at the use of axi-cell and revealed some critical differences from ZUMA-1, specifically the overall response rate (ORR) and complete response (CR) rate were lower than those reported in the pivotal clinical trial. The findings will be reported at 9:45 a.m. PT on Saturday, Dec. 1 in Pacific Ballroom 20 of the Marriott Marquis San Diego Marina (Abstract 92).
Researchers will also present the unblinded results from the ECHELON-2 study, which compared the efficacy and safety of brentuximab vedotin in combination with cyclophosphamide, doxorubicin, and prednisone (CHP) versus standard CHOP for the treatment of patients with peripheral T-cell lymphoma. The results will be presented at 6:15 p.m. PT on Monday, Dec. 3 in room 6F of the San Diego convention center (Abstract 997).
Previously reported blinded pooled data showed that the treatment was well tolerated with 3-year PFS of 53% and OS of 73%.
“This should be a new standard of care for T-cell lymphomas,” Dr. Hill said.
CAR T-cell therapy
There are a number of abstracts featuring the latest results on CAR T-cell therapy. Helen Heslop, MD, of Baylor College of Medicine, Houston, recommended an updated analysis from the ELIANA study, which looked at the efficacy and safety of tisagenlecleucel in for children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL).
“Longer-term follow-up of the ELIANA study shows encouraging remission-duration data in pediatric and young adults with ALL without additional therapy,” Dr. Heslop said.
The findings will be presented at 4:30 p.m. PT on Monday, Dec. 3 in room 6A at the San Diego Convention Center (Abstract 895).
Another notable presentation will feature results from a phase 1B/2 trial evaluating infusion of CAR T cells targeting the CD30 molecule and encoding the CD28 endodomain (CD30.CAR-Ts) after lymphodepleting chemotherapy in patients with relapsed or refractory CD30+ Hodgkin lymphoma and non-Hodgkin lymphoma.
The researchers will report that there was a significant PFS advances for who received the highest dose level of the CAR T treatment, combined with bendamustine and fludarabine.
The study will be presented at 11 a.m. PT on Monday, Dec. 3 in room 6F at the San Diego Convention Center (Abstract 681).
Dr. Heslop also recommends another study being presented in the same session, which also shows encouraging results with CD30.CAR-Ts. Dr. Heslop is one of the co-investigators on the phase 1 RELY-30 trial, which is evaluating the efficacy of CD30.CAR-Ts after lymphodepleting chemotherapy. Preliminary results suggest a substantial improvement in efficacy. The findings will be presented at 10:45 a.m. PT on Monday, Dec. 3 in room 6F of the San Diego Convention Center (Abstract 680).
MDS/MPN
Vikas Gupta, MD, of Princess Margaret Cancer Center in Toronto, highlighted three abstracts to watch in the areas of myelodysplastic syndrome (MDS) and myeloproliferative neoplasms (MPN).
The phase 3 Medalist trial is a randomized double-blind placebo controlled study of luspatercept to treatment anemia in patients with MDS with ring sideroblasts who require red blood cell transfusion. The researchers will report significantly reduced transfusion burdens for luspatercept, compared with placebo.
“This is a practice-changing, pivotal trial in the field of MDS for the treatment of anemia,” Dr. Gupta said.
The findings will be presented at 2 p.m. PT on Sunday, Dec. 2 during the plenary session in Hall AB in the San Diego Convention Center (Abstract 1).
Also during the Sunday plenary session is a presentation on MPN therapy (Abstract 4). Researchers will present data on secreted mutant calreticulins as rogue cytokines trigger thrombopoietin receptor (TpoR) activation, specifically in CALR-mutated cells.
“This study investigates in to the mechanistic oncogenetic aspects of mutant calreticulin, and has potential for therapeutic approaches in the future,” Dr. Gupta said.
The ASH meeting will also feature the final analysis of the MPN-RC 112 consortium trial of pegylated interferon alfa-2a versus hydroxyurea for the treatment of high-risk polycythemia vera (PV) and essential thrombocythemia (ET). The researchers will report that the CR rates at 12 and 24 months were similar in patients treated with pegylated interferon alfa-2a and hydroxyurea, but pegylated interferon alfa-2a was associated with a higher rate of serious toxicities.
“There is a continuous debate on optimal first-line cytoreductive therapy for high risk PV/ET, and this is one of the first randomized study to answer this question,” Dr. Gupta said.
The findings will be presented at 7 a.m. PT on Monday, Dec. 3 in Grand Hall D at the Manchester Grand Hyatt San Diego (Abstract 577).
AML
For attendees interested in the latest developments in acute myeloid leukemia, Thomas Fischer, MD, of Otto-von-Guericke-University Magdeburg (Germany), highlighted three don’t-miss sessions.
In an analysis of a large cohort of FLT3-ITD mutated AML patients in the RATIFY trial, researchers looked at the prognostic impact of ITD insertion site.
“Interestingly, in this large cohort of 452 FLT3-ITD mutated AML, the negative prognostic impact of beta1-sheet insertion site of FLT3-ITD could be confirmed,” Dr. Fischer said. “Further analysis of a potential predictive effect on outcome of midostaurin treatment is ongoing and will be very interesting.”
The findings will be presented at 5 p.m. PT on Sunday, Dec. 2 in Seaport Ballroom F at the Manchester Grand Hyatt San Diego (Abstract 435).
Another notable presentation features results from the phase 2 RADIUS trial, a randomized study comparing standard of care, with and without midostaurin, after allogeneic stem cell transplant in FLT3-ITD–mutated AML.
“Here, efficacy and toxicity of midostaurin was investigated in a [minimal residual disease] situation post-alloSCT,” Dr. Fischer said. “Interestingly, adding midostaurin to standard of care reduced the risk of relapse at 18 months post-alloSCT by 46%.”
The complete findings will be presented at 10:45 a.m. PT on Monday, Dec. 3 in Seaport Ballroom F at the Manchester Grand Hyatt San Diego (Abstract 662).
Dr. Fischer singled out another study looking at the efficacy and safety of single-agent quizartinib in patients with FLT3-ITD mutated AML. In this large, randomized trial the researchers noted a significant improvement in CR rates and survival benefit with the single agent FLT3 inhibitors, compared with salvage chemotherapy for patients with relapsed/refractory mutated AML.
The findings will be presented at 8 a.m. on Monday, Dec. 3 in Seaport Ballroom F at the Manchester Grand Hyatt San Diego (Abstract 563).
Notable posters
Iberia Romina Sosa, MD, PhD, of Baylor College of Medicine in Houston, suggested several posters worth visiting in the areas of thrombosis and bleeding.
Poster 1134 looks at the TNF-alpha driven inflammation and mitochondrial dysfunction in the platelet hyperreactivity of aging and MPN.
How do you know if your therapy for thrombotic thrombocytopenic purpura is working? Poster 3736 examines the measurement of cell-derived microparticles as a possible tool to monitor response to therapy.
You don’t have to be taking aspirin to have a bleeding profile characteristic with consumption of a cyclooxygenase inhibitor. Poster 1156 provides a first report of a platelet function disorder caused by autosomal recessive inheritance of PTGS1.
Poster 2477 takes a closer look at fitusiran, an antithrombin inhibitor, which improves thrombin generation in patients with hemophilia A or B. Protocol amendments for safety monitoring move fitusiran to phase 3 trials, Dr. Sosa said.
With more than 3,000 scientific abstracts at the 2018 annual meeting of the American Society of Hematology, it can be tough to figure out what research is most relevant to practice. But the editorial advisory board of Hematology News is making it easier this year with their picks for what to watch and why.
Lymphomas
Brian T. Hill, MD, of the Cleveland Clinic, offered his top picks in lymphoma research. Results of the phase 3 international Alliance North American Intergroup Study A041202 will be presented during the ASH plenary session at 2 p.m. PT on Sunday, Dec. 2 in Hall AB of the San Diego Convention Center (Abstract 6). The study compared bendamustine plus rituximab with ibrutinib and the combination of ibrutinib plus rituximab to see if the ibrutinib-containing therapies would have superior progression-free survival (PFS) in chronic lymphocytic leukemia (CLL), compared with chemoimmunotherapy. Results indicate that ibrutinib had superior PFS in older patients with CLL and could be a standard of care in this population.
The study is worth watching because it is the first report of a head-to-head trial of chemotherapy versus ibrutinib for first-line treatment of CLL, Dr. Hill said.
Two more studies offer important reports of “real world” experiences with chimeric antigen receptor (CAR) T-cell therapy.
In one multicenter retrospective study, researchers evaluated the outcomes of axicabtagene ciloleucel (axi-cel) CAR T-cell therapy for relapsed/refractory aggressive B-cell lymphoma when it is used a standard care. The researchers will report that 30-day responses in the real-world setting were comparable to the best responses seen in the ZUMA-1 trial. The full results will be reported at 9:30 a.m. PT on Saturday, Dec. 1 in Pacific Ballroom 20 of the Marriott Marquis San Diego Marina (Abstract 91).
Another retrospective analysis looked at the use of axi-cell and revealed some critical differences from ZUMA-1, specifically the overall response rate (ORR) and complete response (CR) rate were lower than those reported in the pivotal clinical trial. The findings will be reported at 9:45 a.m. PT on Saturday, Dec. 1 in Pacific Ballroom 20 of the Marriott Marquis San Diego Marina (Abstract 92).
Researchers will also present the unblinded results from the ECHELON-2 study, which compared the efficacy and safety of brentuximab vedotin in combination with cyclophosphamide, doxorubicin, and prednisone (CHP) versus standard CHOP for the treatment of patients with peripheral T-cell lymphoma. The results will be presented at 6:15 p.m. PT on Monday, Dec. 3 in room 6F of the San Diego convention center (Abstract 997).
Previously reported blinded pooled data showed that the treatment was well tolerated with 3-year PFS of 53% and OS of 73%.
“This should be a new standard of care for T-cell lymphomas,” Dr. Hill said.
CAR T-cell therapy
There are a number of abstracts featuring the latest results on CAR T-cell therapy. Helen Heslop, MD, of Baylor College of Medicine, Houston, recommended an updated analysis from the ELIANA study, which looked at the efficacy and safety of tisagenlecleucel in for children and young adults with relapsed/refractory acute lymphoblastic leukemia (ALL).
“Longer-term follow-up of the ELIANA study shows encouraging remission-duration data in pediatric and young adults with ALL without additional therapy,” Dr. Heslop said.
The findings will be presented at 4:30 p.m. PT on Monday, Dec. 3 in room 6A at the San Diego Convention Center (Abstract 895).
Another notable presentation will feature results from a phase 1B/2 trial evaluating infusion of CAR T cells targeting the CD30 molecule and encoding the CD28 endodomain (CD30.CAR-Ts) after lymphodepleting chemotherapy in patients with relapsed or refractory CD30+ Hodgkin lymphoma and non-Hodgkin lymphoma.
The researchers will report that there was a significant PFS advances for who received the highest dose level of the CAR T treatment, combined with bendamustine and fludarabine.
The study will be presented at 11 a.m. PT on Monday, Dec. 3 in room 6F at the San Diego Convention Center (Abstract 681).
Dr. Heslop also recommends another study being presented in the same session, which also shows encouraging results with CD30.CAR-Ts. Dr. Heslop is one of the co-investigators on the phase 1 RELY-30 trial, which is evaluating the efficacy of CD30.CAR-Ts after lymphodepleting chemotherapy. Preliminary results suggest a substantial improvement in efficacy. The findings will be presented at 10:45 a.m. PT on Monday, Dec. 3 in room 6F of the San Diego Convention Center (Abstract 680).
MDS/MPN
Vikas Gupta, MD, of Princess Margaret Cancer Center in Toronto, highlighted three abstracts to watch in the areas of myelodysplastic syndrome (MDS) and myeloproliferative neoplasms (MPN).
The phase 3 Medalist trial is a randomized double-blind placebo controlled study of luspatercept to treatment anemia in patients with MDS with ring sideroblasts who require red blood cell transfusion. The researchers will report significantly reduced transfusion burdens for luspatercept, compared with placebo.
“This is a practice-changing, pivotal trial in the field of MDS for the treatment of anemia,” Dr. Gupta said.
The findings will be presented at 2 p.m. PT on Sunday, Dec. 2 during the plenary session in Hall AB in the San Diego Convention Center (Abstract 1).
Also during the Sunday plenary session is a presentation on MPN therapy (Abstract 4). Researchers will present data on secreted mutant calreticulins as rogue cytokines trigger thrombopoietin receptor (TpoR) activation, specifically in CALR-mutated cells.
“This study investigates in to the mechanistic oncogenetic aspects of mutant calreticulin, and has potential for therapeutic approaches in the future,” Dr. Gupta said.
The ASH meeting will also feature the final analysis of the MPN-RC 112 consortium trial of pegylated interferon alfa-2a versus hydroxyurea for the treatment of high-risk polycythemia vera (PV) and essential thrombocythemia (ET). The researchers will report that the CR rates at 12 and 24 months were similar in patients treated with pegylated interferon alfa-2a and hydroxyurea, but pegylated interferon alfa-2a was associated with a higher rate of serious toxicities.
“There is a continuous debate on optimal first-line cytoreductive therapy for high risk PV/ET, and this is one of the first randomized study to answer this question,” Dr. Gupta said.
The findings will be presented at 7 a.m. PT on Monday, Dec. 3 in Grand Hall D at the Manchester Grand Hyatt San Diego (Abstract 577).
AML
For attendees interested in the latest developments in acute myeloid leukemia, Thomas Fischer, MD, of Otto-von-Guericke-University Magdeburg (Germany), highlighted three don’t-miss sessions.
In an analysis of a large cohort of FLT3-ITD mutated AML patients in the RATIFY trial, researchers looked at the prognostic impact of ITD insertion site.
“Interestingly, in this large cohort of 452 FLT3-ITD mutated AML, the negative prognostic impact of beta1-sheet insertion site of FLT3-ITD could be confirmed,” Dr. Fischer said. “Further analysis of a potential predictive effect on outcome of midostaurin treatment is ongoing and will be very interesting.”
The findings will be presented at 5 p.m. PT on Sunday, Dec. 2 in Seaport Ballroom F at the Manchester Grand Hyatt San Diego (Abstract 435).
Another notable presentation features results from the phase 2 RADIUS trial, a randomized study comparing standard of care, with and without midostaurin, after allogeneic stem cell transplant in FLT3-ITD–mutated AML.
“Here, efficacy and toxicity of midostaurin was investigated in a [minimal residual disease] situation post-alloSCT,” Dr. Fischer said. “Interestingly, adding midostaurin to standard of care reduced the risk of relapse at 18 months post-alloSCT by 46%.”
The complete findings will be presented at 10:45 a.m. PT on Monday, Dec. 3 in Seaport Ballroom F at the Manchester Grand Hyatt San Diego (Abstract 662).
Dr. Fischer singled out another study looking at the efficacy and safety of single-agent quizartinib in patients with FLT3-ITD mutated AML. In this large, randomized trial the researchers noted a significant improvement in CR rates and survival benefit with the single agent FLT3 inhibitors, compared with salvage chemotherapy for patients with relapsed/refractory mutated AML.
The findings will be presented at 8 a.m. on Monday, Dec. 3 in Seaport Ballroom F at the Manchester Grand Hyatt San Diego (Abstract 563).
Notable posters
Iberia Romina Sosa, MD, PhD, of Baylor College of Medicine in Houston, suggested several posters worth visiting in the areas of thrombosis and bleeding.
Poster 1134 looks at the TNF-alpha driven inflammation and mitochondrial dysfunction in the platelet hyperreactivity of aging and MPN.
How do you know if your therapy for thrombotic thrombocytopenic purpura is working? Poster 3736 examines the measurement of cell-derived microparticles as a possible tool to monitor response to therapy.
You don’t have to be taking aspirin to have a bleeding profile characteristic with consumption of a cyclooxygenase inhibitor. Poster 1156 provides a first report of a platelet function disorder caused by autosomal recessive inheritance of PTGS1.
Poster 2477 takes a closer look at fitusiran, an antithrombin inhibitor, which improves thrombin generation in patients with hemophilia A or B. Protocol amendments for safety monitoring move fitusiran to phase 3 trials, Dr. Sosa said.
FDA approves gilteritinib for relapsed/refractory AML
The U.S. Food and Drug Administration (FDA) has approved gilteritinib (Xospata) for use in adults who have relapsed or refractory acute myeloid leukemia (AML) with a FLT3 mutation, as detected by an FDA-approved test.
The FDA also expanded the approved indication for the LeukoStrat CDx FLT3 Mutation Assay to include use with gilteritinib.
The LeukoStrat CDx FLT3 Mutation Assay, developed by Invivoscribe Technologies, Inc., is used to detect FLT3 mutations in patients with AML.
Gilteritinib, developed by Astellas Pharma, has demonstrated inhibitory activity against FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain.
The FDA’s approval of gilteritinib was based on an interim analysis of the ADMIRAL trial (NCT02421939).
The trial enrolled adults with relapsed or refractory AML who had a FLT3 ITD, D835, or I836 mutation, according to the LeukoStrat CDx FLT3 Mutation Assay.
Patients received gilteritinib at 120 mg daily until they developed unacceptable toxicity or did not show a clinical benefit.
Efficacy results are available for 138 patients, with a median follow-up of 4.6 months (range, 2.8 to 15.8).
The complete response (CR) rate was 11.6% (16/138), the rate of CR with partial hematologic recovery (CRh) was 9.4% (13/138), and the rate of CR/CRh was 21% (29/138).
The median duration of CR/CRh was 4.6 months.
There were 106 patients who were transfusion-dependent at baseline, and 33 of these patients (31.1%) became transfusion-independent during the post-baseline period.
Seventeen of the 32 patients (53.1%) who were transfusion-independent at baseline remained transfusion-independent.
Safety results are available for 292 patients. The median duration of exposure to gilteritinib in this group was 3 months (range, 0.1 to 42.8).
The most common adverse events were myalgia/arthralgia (42%), transaminase increase (41%), fatigue/malaise (40%), fever (35%), noninfectious diarrhea (34%), dyspnea (34%), edema (34%), rash (30%), pneumonia (30%), nausea (27%), constipation (27%), stomatitis (26%), cough (25%), headache (21%), hypotension (21%), dizziness (20%), and vomiting (20%).
Eight percent of patients (n=22) discontinued gilteritinib due to adverse events. The most common were pneumonia (2%), sepsis (2%), and dyspnea (1%).
For more details on the ADMIRAL trial and gilteritinib, see the full prescribing information.
The U.S. Food and Drug Administration (FDA) has approved gilteritinib (Xospata) for use in adults who have relapsed or refractory acute myeloid leukemia (AML) with a FLT3 mutation, as detected by an FDA-approved test.
The FDA also expanded the approved indication for the LeukoStrat CDx FLT3 Mutation Assay to include use with gilteritinib.
The LeukoStrat CDx FLT3 Mutation Assay, developed by Invivoscribe Technologies, Inc., is used to detect FLT3 mutations in patients with AML.
Gilteritinib, developed by Astellas Pharma, has demonstrated inhibitory activity against FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain.
The FDA’s approval of gilteritinib was based on an interim analysis of the ADMIRAL trial (NCT02421939).
The trial enrolled adults with relapsed or refractory AML who had a FLT3 ITD, D835, or I836 mutation, according to the LeukoStrat CDx FLT3 Mutation Assay.
Patients received gilteritinib at 120 mg daily until they developed unacceptable toxicity or did not show a clinical benefit.
Efficacy results are available for 138 patients, with a median follow-up of 4.6 months (range, 2.8 to 15.8).
The complete response (CR) rate was 11.6% (16/138), the rate of CR with partial hematologic recovery (CRh) was 9.4% (13/138), and the rate of CR/CRh was 21% (29/138).
The median duration of CR/CRh was 4.6 months.
There were 106 patients who were transfusion-dependent at baseline, and 33 of these patients (31.1%) became transfusion-independent during the post-baseline period.
Seventeen of the 32 patients (53.1%) who were transfusion-independent at baseline remained transfusion-independent.
Safety results are available for 292 patients. The median duration of exposure to gilteritinib in this group was 3 months (range, 0.1 to 42.8).
The most common adverse events were myalgia/arthralgia (42%), transaminase increase (41%), fatigue/malaise (40%), fever (35%), noninfectious diarrhea (34%), dyspnea (34%), edema (34%), rash (30%), pneumonia (30%), nausea (27%), constipation (27%), stomatitis (26%), cough (25%), headache (21%), hypotension (21%), dizziness (20%), and vomiting (20%).
Eight percent of patients (n=22) discontinued gilteritinib due to adverse events. The most common were pneumonia (2%), sepsis (2%), and dyspnea (1%).
For more details on the ADMIRAL trial and gilteritinib, see the full prescribing information.
The U.S. Food and Drug Administration (FDA) has approved gilteritinib (Xospata) for use in adults who have relapsed or refractory acute myeloid leukemia (AML) with a FLT3 mutation, as detected by an FDA-approved test.
The FDA also expanded the approved indication for the LeukoStrat CDx FLT3 Mutation Assay to include use with gilteritinib.
The LeukoStrat CDx FLT3 Mutation Assay, developed by Invivoscribe Technologies, Inc., is used to detect FLT3 mutations in patients with AML.
Gilteritinib, developed by Astellas Pharma, has demonstrated inhibitory activity against FLT3 internal tandem duplication (ITD) and FLT3 tyrosine kinase domain.
The FDA’s approval of gilteritinib was based on an interim analysis of the ADMIRAL trial (NCT02421939).
The trial enrolled adults with relapsed or refractory AML who had a FLT3 ITD, D835, or I836 mutation, according to the LeukoStrat CDx FLT3 Mutation Assay.
Patients received gilteritinib at 120 mg daily until they developed unacceptable toxicity or did not show a clinical benefit.
Efficacy results are available for 138 patients, with a median follow-up of 4.6 months (range, 2.8 to 15.8).
The complete response (CR) rate was 11.6% (16/138), the rate of CR with partial hematologic recovery (CRh) was 9.4% (13/138), and the rate of CR/CRh was 21% (29/138).
The median duration of CR/CRh was 4.6 months.
There were 106 patients who were transfusion-dependent at baseline, and 33 of these patients (31.1%) became transfusion-independent during the post-baseline period.
Seventeen of the 32 patients (53.1%) who were transfusion-independent at baseline remained transfusion-independent.
Safety results are available for 292 patients. The median duration of exposure to gilteritinib in this group was 3 months (range, 0.1 to 42.8).
The most common adverse events were myalgia/arthralgia (42%), transaminase increase (41%), fatigue/malaise (40%), fever (35%), noninfectious diarrhea (34%), dyspnea (34%), edema (34%), rash (30%), pneumonia (30%), nausea (27%), constipation (27%), stomatitis (26%), cough (25%), headache (21%), hypotension (21%), dizziness (20%), and vomiting (20%).
Eight percent of patients (n=22) discontinued gilteritinib due to adverse events. The most common were pneumonia (2%), sepsis (2%), and dyspnea (1%).
For more details on the ADMIRAL trial and gilteritinib, see the full prescribing information.
FDA approves gilteritinib for AML with FLT3 mutation
as detected by an FDA-approved test.
The FDA also expanded the approved indication for the LeukoStrat CDx FLT3 Mutation Assay to include use with gilteritinib. The LeukoStrat CDx FLT3 Mutation Assay, developed by Invivoscribe, is used to detect the FLT3 mutation in patients with AML.
Gilteritinib, developed by Astellas Pharma, has demonstrated inhibitory activity against FLT3 internal tandem duplication and FLT3 tyrosine kinase domain.
The FDA’s approval of gilteritinib was based on an interim analysis of the ADMIRAL trial, which enrolled adults with relapsed or refractory AML who had a FLT3 ITD, D835 or I836 mutation, according to the LeukoStrat CDx FLT3 Mutation Assay.
Patients received gilteritinib at 120 mg daily until they developed unacceptable toxicity or did not show a clinical benefit. Efficacy results are available for 138 patients, with a median follow-up of 4.6 months.
The complete response (CR) rate was 11.6% (16/138), the CR rate with partial hematologic recovery (CRh) was 9.4% (13/138), and the CR/CRh rate was 21% (29/138). The median duration of CR/CRh was 4.6 months.
There were 106 patients who were transfusion dependent at baseline, and 33 of these patients (31.1%) became transfusion independent during the post-baseline period.
Seventeen of the 32 patients (53.1%) who were transfusion independent at baseline remained transfusion independent.
Safety results are available for 292 patients. The median duration of exposure to gilteritinib in this group was 3 months.
The most common adverse events were myalgia/arthralgia, transaminase increase, fatigue/malaise, fever, noninfectious diarrhea, dyspnea, edema, rash, pneumonia, nausea, constipation, stomatitis, cough, headache, hypotension, dizziness, and vomiting.
A total of 8% of patients (n = 22) discontinued gilteritinib because of adverse events, the most common of which were pneumonia (2%), sepsis (2%), and dyspnea (1%).
as detected by an FDA-approved test.
The FDA also expanded the approved indication for the LeukoStrat CDx FLT3 Mutation Assay to include use with gilteritinib. The LeukoStrat CDx FLT3 Mutation Assay, developed by Invivoscribe, is used to detect the FLT3 mutation in patients with AML.
Gilteritinib, developed by Astellas Pharma, has demonstrated inhibitory activity against FLT3 internal tandem duplication and FLT3 tyrosine kinase domain.
The FDA’s approval of gilteritinib was based on an interim analysis of the ADMIRAL trial, which enrolled adults with relapsed or refractory AML who had a FLT3 ITD, D835 or I836 mutation, according to the LeukoStrat CDx FLT3 Mutation Assay.
Patients received gilteritinib at 120 mg daily until they developed unacceptable toxicity or did not show a clinical benefit. Efficacy results are available for 138 patients, with a median follow-up of 4.6 months.
The complete response (CR) rate was 11.6% (16/138), the CR rate with partial hematologic recovery (CRh) was 9.4% (13/138), and the CR/CRh rate was 21% (29/138). The median duration of CR/CRh was 4.6 months.
There were 106 patients who were transfusion dependent at baseline, and 33 of these patients (31.1%) became transfusion independent during the post-baseline period.
Seventeen of the 32 patients (53.1%) who were transfusion independent at baseline remained transfusion independent.
Safety results are available for 292 patients. The median duration of exposure to gilteritinib in this group was 3 months.
The most common adverse events were myalgia/arthralgia, transaminase increase, fatigue/malaise, fever, noninfectious diarrhea, dyspnea, edema, rash, pneumonia, nausea, constipation, stomatitis, cough, headache, hypotension, dizziness, and vomiting.
A total of 8% of patients (n = 22) discontinued gilteritinib because of adverse events, the most common of which were pneumonia (2%), sepsis (2%), and dyspnea (1%).
as detected by an FDA-approved test.
The FDA also expanded the approved indication for the LeukoStrat CDx FLT3 Mutation Assay to include use with gilteritinib. The LeukoStrat CDx FLT3 Mutation Assay, developed by Invivoscribe, is used to detect the FLT3 mutation in patients with AML.
Gilteritinib, developed by Astellas Pharma, has demonstrated inhibitory activity against FLT3 internal tandem duplication and FLT3 tyrosine kinase domain.
The FDA’s approval of gilteritinib was based on an interim analysis of the ADMIRAL trial, which enrolled adults with relapsed or refractory AML who had a FLT3 ITD, D835 or I836 mutation, according to the LeukoStrat CDx FLT3 Mutation Assay.
Patients received gilteritinib at 120 mg daily until they developed unacceptable toxicity or did not show a clinical benefit. Efficacy results are available for 138 patients, with a median follow-up of 4.6 months.
The complete response (CR) rate was 11.6% (16/138), the CR rate with partial hematologic recovery (CRh) was 9.4% (13/138), and the CR/CRh rate was 21% (29/138). The median duration of CR/CRh was 4.6 months.
There were 106 patients who were transfusion dependent at baseline, and 33 of these patients (31.1%) became transfusion independent during the post-baseline period.
Seventeen of the 32 patients (53.1%) who were transfusion independent at baseline remained transfusion independent.
Safety results are available for 292 patients. The median duration of exposure to gilteritinib in this group was 3 months.
The most common adverse events were myalgia/arthralgia, transaminase increase, fatigue/malaise, fever, noninfectious diarrhea, dyspnea, edema, rash, pneumonia, nausea, constipation, stomatitis, cough, headache, hypotension, dizziness, and vomiting.
A total of 8% of patients (n = 22) discontinued gilteritinib because of adverse events, the most common of which were pneumonia (2%), sepsis (2%), and dyspnea (1%).
FDA grants priority review to quizartinib
The U.S. Food and Drug Administration (FDA) has accepted for priority review a new drug application (NDA) for the FLT3 inhibitor quizartinib.
With this NDA, Daiichi Sankyo is seeking approval for quizartinib to treat adults with relapsed/refractory FLT3-ITD acute myeloid leukemia (AML).
The FDA grants priority review to applications for products that are expected to provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA aims to take action on a priority review application within 6 months rather than the standard 10 months.
The FDA is expected to make a decision on the quizartinib NDA by May 25, 2019.
In addition to priority review, quizartinib has breakthrough therapy designation and fast track designation from the FDA.
Trial results
The NDA for quizartinib is supported by results from the phase 3 QuANTUM-R study. Topline results from this study were presented at the 23rd Congress of the European Hematology Association in June, and new analyses are set to be presented at the 2018 ASH Annual Meeting in December (abstract 563).
QuANTUM-R enrolled adults with FLT3-ITD AML (at least 3% FLT3-ITD allelic ratio) who had refractory disease or had relapsed within 6 months of their first complete response (CR).
Patients were randomized to receive once-daily treatment with quizartinib (n=245) or a salvage chemotherapy regimen (n=122)—low-dose cytarabine (LoDAC, n=29); combination mitoxantrone, etoposide, and cytarabine (MEC, n=40); or combination fludarabine, cytarabine, and idarubicin (FLAG-IDA, n=53).
Patients who responded to treatment could proceed to hematopoietic stem cell transplant (HSCT), and those in the quizartinib arm could resume quizartinib after HSCT.
In all, 241 patients received quizartinib, and 94 received salvage chemotherapy—LoDAC (n=22), MEC (n=25), and FLAG-IDA (n=47). Of the 28 patients in the chemotherapy group who were not treated, most withdrew consent.
Thirty-two percent of quizartinib-treated patients and 12% of the chemotherapy group went on to HSCT.
Efficacy
The median follow-up was 23.5 months. The efficacy results include all randomized patients.
The overall response rate was 69% in the quizartinib arm and 30% in the chemotherapy arm. The composite CR rate was 48% in the quizartinib arm and 27% in the chemotherapy arm. This includes:
- The CR rate (4% and 1%, respectively)
- The rate of CR with incomplete platelet recovery (4% and 0%, respectively)
- The rate of CR with incomplete hematologic recovery (40% and 26%, respectively).
The median event-free survival was 6.0 weeks in the quizartinib arm and 3.7 weeks in the chemotherapy arm (hazard ratio=0.90, P=0.1071).
The median overall survival was 6.2 months in the quizartinib arm and 4.7 months in the chemotherapy arm (hazard ratio=0.76, P=0.0177). The 1-year overall survival rate was 27% and 20%, respectively.
Safety
The safety results include only patients who received their assigned treatment.
Grade 3 or higher hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Thrombocytopenia (35% and 34%)
- Anemia (30% and 29%)
- Neutropenia (32% and 25%)
- Febrile neutropenia (31% and 21%)
- Leukopenia (17% and 16%).
Grade 3 or higher non-hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Sepsis/septic shock (16% and 18%)
- Hypokalemia (12% and 9%)
- Pneumonia (12% and 9%)
- Fatigue (8% and 1%)
- Dyspnea (5% for both)
- Hypophosphatemia (5% for both).
The U.S. Food and Drug Administration (FDA) has accepted for priority review a new drug application (NDA) for the FLT3 inhibitor quizartinib.
With this NDA, Daiichi Sankyo is seeking approval for quizartinib to treat adults with relapsed/refractory FLT3-ITD acute myeloid leukemia (AML).
The FDA grants priority review to applications for products that are expected to provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA aims to take action on a priority review application within 6 months rather than the standard 10 months.
The FDA is expected to make a decision on the quizartinib NDA by May 25, 2019.
In addition to priority review, quizartinib has breakthrough therapy designation and fast track designation from the FDA.
Trial results
The NDA for quizartinib is supported by results from the phase 3 QuANTUM-R study. Topline results from this study were presented at the 23rd Congress of the European Hematology Association in June, and new analyses are set to be presented at the 2018 ASH Annual Meeting in December (abstract 563).
QuANTUM-R enrolled adults with FLT3-ITD AML (at least 3% FLT3-ITD allelic ratio) who had refractory disease or had relapsed within 6 months of their first complete response (CR).
Patients were randomized to receive once-daily treatment with quizartinib (n=245) or a salvage chemotherapy regimen (n=122)—low-dose cytarabine (LoDAC, n=29); combination mitoxantrone, etoposide, and cytarabine (MEC, n=40); or combination fludarabine, cytarabine, and idarubicin (FLAG-IDA, n=53).
Patients who responded to treatment could proceed to hematopoietic stem cell transplant (HSCT), and those in the quizartinib arm could resume quizartinib after HSCT.
In all, 241 patients received quizartinib, and 94 received salvage chemotherapy—LoDAC (n=22), MEC (n=25), and FLAG-IDA (n=47). Of the 28 patients in the chemotherapy group who were not treated, most withdrew consent.
Thirty-two percent of quizartinib-treated patients and 12% of the chemotherapy group went on to HSCT.
Efficacy
The median follow-up was 23.5 months. The efficacy results include all randomized patients.
The overall response rate was 69% in the quizartinib arm and 30% in the chemotherapy arm. The composite CR rate was 48% in the quizartinib arm and 27% in the chemotherapy arm. This includes:
- The CR rate (4% and 1%, respectively)
- The rate of CR with incomplete platelet recovery (4% and 0%, respectively)
- The rate of CR with incomplete hematologic recovery (40% and 26%, respectively).
The median event-free survival was 6.0 weeks in the quizartinib arm and 3.7 weeks in the chemotherapy arm (hazard ratio=0.90, P=0.1071).
The median overall survival was 6.2 months in the quizartinib arm and 4.7 months in the chemotherapy arm (hazard ratio=0.76, P=0.0177). The 1-year overall survival rate was 27% and 20%, respectively.
Safety
The safety results include only patients who received their assigned treatment.
Grade 3 or higher hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Thrombocytopenia (35% and 34%)
- Anemia (30% and 29%)
- Neutropenia (32% and 25%)
- Febrile neutropenia (31% and 21%)
- Leukopenia (17% and 16%).
Grade 3 or higher non-hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Sepsis/septic shock (16% and 18%)
- Hypokalemia (12% and 9%)
- Pneumonia (12% and 9%)
- Fatigue (8% and 1%)
- Dyspnea (5% for both)
- Hypophosphatemia (5% for both).
The U.S. Food and Drug Administration (FDA) has accepted for priority review a new drug application (NDA) for the FLT3 inhibitor quizartinib.
With this NDA, Daiichi Sankyo is seeking approval for quizartinib to treat adults with relapsed/refractory FLT3-ITD acute myeloid leukemia (AML).
The FDA grants priority review to applications for products that are expected to provide significant improvements in the treatment, diagnosis, or prevention of serious conditions.
The FDA aims to take action on a priority review application within 6 months rather than the standard 10 months.
The FDA is expected to make a decision on the quizartinib NDA by May 25, 2019.
In addition to priority review, quizartinib has breakthrough therapy designation and fast track designation from the FDA.
Trial results
The NDA for quizartinib is supported by results from the phase 3 QuANTUM-R study. Topline results from this study were presented at the 23rd Congress of the European Hematology Association in June, and new analyses are set to be presented at the 2018 ASH Annual Meeting in December (abstract 563).
QuANTUM-R enrolled adults with FLT3-ITD AML (at least 3% FLT3-ITD allelic ratio) who had refractory disease or had relapsed within 6 months of their first complete response (CR).
Patients were randomized to receive once-daily treatment with quizartinib (n=245) or a salvage chemotherapy regimen (n=122)—low-dose cytarabine (LoDAC, n=29); combination mitoxantrone, etoposide, and cytarabine (MEC, n=40); or combination fludarabine, cytarabine, and idarubicin (FLAG-IDA, n=53).
Patients who responded to treatment could proceed to hematopoietic stem cell transplant (HSCT), and those in the quizartinib arm could resume quizartinib after HSCT.
In all, 241 patients received quizartinib, and 94 received salvage chemotherapy—LoDAC (n=22), MEC (n=25), and FLAG-IDA (n=47). Of the 28 patients in the chemotherapy group who were not treated, most withdrew consent.
Thirty-two percent of quizartinib-treated patients and 12% of the chemotherapy group went on to HSCT.
Efficacy
The median follow-up was 23.5 months. The efficacy results include all randomized patients.
The overall response rate was 69% in the quizartinib arm and 30% in the chemotherapy arm. The composite CR rate was 48% in the quizartinib arm and 27% in the chemotherapy arm. This includes:
- The CR rate (4% and 1%, respectively)
- The rate of CR with incomplete platelet recovery (4% and 0%, respectively)
- The rate of CR with incomplete hematologic recovery (40% and 26%, respectively).
The median event-free survival was 6.0 weeks in the quizartinib arm and 3.7 weeks in the chemotherapy arm (hazard ratio=0.90, P=0.1071).
The median overall survival was 6.2 months in the quizartinib arm and 4.7 months in the chemotherapy arm (hazard ratio=0.76, P=0.0177). The 1-year overall survival rate was 27% and 20%, respectively.
Safety
The safety results include only patients who received their assigned treatment.
Grade 3 or higher hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Thrombocytopenia (35% and 34%)
- Anemia (30% and 29%)
- Neutropenia (32% and 25%)
- Febrile neutropenia (31% and 21%)
- Leukopenia (17% and 16%).
Grade 3 or higher non-hematologic treatment-emergent adverse events occurring in at least 5% of patients (in the quizartinib and chemotherapy groups, respectively) included:
- Sepsis/septic shock (16% and 18%)
- Hypokalemia (12% and 9%)
- Pneumonia (12% and 9%)
- Fatigue (8% and 1%)
- Dyspnea (5% for both)
- Hypophosphatemia (5% for both).
FDA approves glasdegib for AML
The U.S. Food and Drug Administration has approved the hedgehog pathway inhibitor glasdegib (Daurismo) for use in combination with low-dose cytarabine (LDAC) to treat adults with newly diagnosed acute myeloid leukemia who are aged 75 years and older or who are ineligible for intensive chemotherapy.
The prescribing information for glasdegib includes a boxed warning detailing the risk of embryo-fetal death or severe birth defects associated with the drug.
The FDA’s approval of glasdegib is based on results from the phase 2 BRIGHT AML 1003 trial (NCT01546038). This trial included 111 adults with newly diagnosed AML and 14 patients with other conditions. The patients were randomized to receive glasdegib (at 100 mg daily) in combination with LDAC (n = 84) or LDAC alone (n = 41).
The complete response rate among the AML patients was 18.2% (14/77) in the glasdegib-LDAC arm and 2.6% (1/38) in the LDAC-only arm. The median overall survival was 8.3 months in the glasdegib-LDAC arm and 4.3 months in the LDAC-only arm (hazard ratio, 0.46; P = .0002).
The most common adverse events in the first 90 days of treatment, occurring in at least 30% of patients in either arm (glasdegib-LDAC and LDAC alone, respectively, were anemia (43% and 42%), fatigue (36% and 32%), hemorrhage (36% and 42%), febrile neutropenia (31% and 22%), musculoskeletal pain (30% and 17%), edema (30% and 20%), and thrombocytopenia (30% and 27%).
The incidence of serious adverse events was 79% in the glasdegib arm, and the most common events were febrile neutropenia (29%), pneumonia (23%), hemorrhage (12%), anemia (7%) and sepsis (7%).
Additional data from this trial are included in the prescribing information for glasdegib.
Glasdegib is a product of Pfizer.
The U.S. Food and Drug Administration has approved the hedgehog pathway inhibitor glasdegib (Daurismo) for use in combination with low-dose cytarabine (LDAC) to treat adults with newly diagnosed acute myeloid leukemia who are aged 75 years and older or who are ineligible for intensive chemotherapy.
The prescribing information for glasdegib includes a boxed warning detailing the risk of embryo-fetal death or severe birth defects associated with the drug.
The FDA’s approval of glasdegib is based on results from the phase 2 BRIGHT AML 1003 trial (NCT01546038). This trial included 111 adults with newly diagnosed AML and 14 patients with other conditions. The patients were randomized to receive glasdegib (at 100 mg daily) in combination with LDAC (n = 84) or LDAC alone (n = 41).
The complete response rate among the AML patients was 18.2% (14/77) in the glasdegib-LDAC arm and 2.6% (1/38) in the LDAC-only arm. The median overall survival was 8.3 months in the glasdegib-LDAC arm and 4.3 months in the LDAC-only arm (hazard ratio, 0.46; P = .0002).
The most common adverse events in the first 90 days of treatment, occurring in at least 30% of patients in either arm (glasdegib-LDAC and LDAC alone, respectively, were anemia (43% and 42%), fatigue (36% and 32%), hemorrhage (36% and 42%), febrile neutropenia (31% and 22%), musculoskeletal pain (30% and 17%), edema (30% and 20%), and thrombocytopenia (30% and 27%).
The incidence of serious adverse events was 79% in the glasdegib arm, and the most common events were febrile neutropenia (29%), pneumonia (23%), hemorrhage (12%), anemia (7%) and sepsis (7%).
Additional data from this trial are included in the prescribing information for glasdegib.
Glasdegib is a product of Pfizer.
The U.S. Food and Drug Administration has approved the hedgehog pathway inhibitor glasdegib (Daurismo) for use in combination with low-dose cytarabine (LDAC) to treat adults with newly diagnosed acute myeloid leukemia who are aged 75 years and older or who are ineligible for intensive chemotherapy.
The prescribing information for glasdegib includes a boxed warning detailing the risk of embryo-fetal death or severe birth defects associated with the drug.
The FDA’s approval of glasdegib is based on results from the phase 2 BRIGHT AML 1003 trial (NCT01546038). This trial included 111 adults with newly diagnosed AML and 14 patients with other conditions. The patients were randomized to receive glasdegib (at 100 mg daily) in combination with LDAC (n = 84) or LDAC alone (n = 41).
The complete response rate among the AML patients was 18.2% (14/77) in the glasdegib-LDAC arm and 2.6% (1/38) in the LDAC-only arm. The median overall survival was 8.3 months in the glasdegib-LDAC arm and 4.3 months in the LDAC-only arm (hazard ratio, 0.46; P = .0002).
The most common adverse events in the first 90 days of treatment, occurring in at least 30% of patients in either arm (glasdegib-LDAC and LDAC alone, respectively, were anemia (43% and 42%), fatigue (36% and 32%), hemorrhage (36% and 42%), febrile neutropenia (31% and 22%), musculoskeletal pain (30% and 17%), edema (30% and 20%), and thrombocytopenia (30% and 27%).
The incidence of serious adverse events was 79% in the glasdegib arm, and the most common events were febrile neutropenia (29%), pneumonia (23%), hemorrhage (12%), anemia (7%) and sepsis (7%).
Additional data from this trial are included in the prescribing information for glasdegib.
Glasdegib is a product of Pfizer.
FDA approves venetoclax for AML
The U.S. Food and Drug Administration (FDA) has granted accelerated approval to venetoclax (Venclexta®) for use in acute myeloid leukemia (AML).
The BCL-2 inhibitor is now approved for use in combination with azacitidine, decitabine, or low-dose cytarabine to treat adults with newly diagnosed AML who are age 75 and older or who are ineligible for intensive chemotherapy.
The FDA grants accelerated approval based on a surrogate or intermediate endpoint that is reasonably likely to predict clinical benefit.
Therefore, continued approval of venetoclax in AML may be contingent upon verification of clinical benefit in confirmatory trials.
The approval is based on data from two studies—the phase 1 b M14-358 trial (NCT02203773) and the phase 1/2 M14-387 trial (NCT02287233).
M14-358 trial
In M14-358, newly diagnosed AML patients received venetoclax in combination with azacitidine (n=84) or decitabine (n=31). There were 67 patients in the azacitidine arm and 13 in the decitabine arm who were 75 or older or were ineligible for intensive induction chemotherapy.
Patients received venetoclax via a daily ramp-up to a final dose of 400 mg once daily. They received prophylaxis for tumor lysis syndrome and were hospitalized for monitoring during the ramp-up.
They received azacitidine at 75 mg/m2 on days 1-7 of each 28-day cycle or decitabine at 20 mg/m2 on days 1-5 of each cycle. Patients continued treatment until disease progression or unacceptable toxicity.
The median follow-up was 7.9 months for the azacitidine arm and 11 months for the decitabine arm.
The complete response (CR) rate was 37% (25/67) in the azacitidine arm and 54% (7/13) in the decitabine arm. The rates of CR with partial hematologic recovery were 24% (16/67) and 7.7% (1/13), respectively.
The most common adverse events (AEs)—occurring in at least 30% of patients in both arms—were nausea, diarrhea, constipation, neutropenia, thrombocytopenia, hemorrhage, peripheral edema, vomiting, fatigue, febrile neutropenia, rash, and anemia.
The incidence of serious AEs was 75% overall. The most frequent serious AEs (occurring in at least 5% of patients) were febrile neutropenia, pneumonia (excluding fungal), sepsis (excluding fungal), respiratory failure, and multiple organ dysfunction syndrome.
The incidence of fatal AEs was 1.5% within 30 days of treatment initiation.
M14-387 trial
The M14-387 trial included 82 AML patients who received venetoclax plus low-dose cytarabine. Patients were newly diagnosed with AML, but some had previous exposure to a hypomethylating agent for an antecedent hematologic disorder.
There were 61 patients who were 75 or older or were ineligible for intensive induction chemotherapy.
Patients received venetoclax via daily ramp-up to a final dose of 600 mg once daily. They received prophylaxis for tumor lysis syndrome and were hospitalized for monitoring during the ramp-up.
Cytarabine was given at 20 mg/m2 on days 1-10 of each 28-day cycle. Patients continued to receive treatment until disease progression or unacceptable toxicity.
At a median follow-up of 6.5 months, the CR rate was 21% (13/61), and the rate of CR with partial hematologic recovery was 21% (13/61).
The most common AEs (occurring in at least 30% of patients) were nausea, thrombocytopenia, hemorrhage, febrile neutropenia, neutropenia, diarrhea, fatigue, constipation, and dyspnea.
The incidence of serious AEs was 95%. The most frequent serious AEs (occurring in at least 5% of patients) were febrile neutropenia, sepsis (excluding fungal), hemorrhage, pneumonia (excluding fungal), and device-related infection.
The incidence of fatal AEs was 4.9% within 30 days of treatment initiation.
Additional details from the M14-358 and M14-387 trials are available in the prescribing information for venetoclax.
Venetoclax is being developed by AbbVie and Roche. It is jointly commercialized by AbbVie and Genentech, a member of the Roche Group, in the United States and by AbbVie elsewhere.
The U.S. Food and Drug Administration (FDA) has granted accelerated approval to venetoclax (Venclexta®) for use in acute myeloid leukemia (AML).
The BCL-2 inhibitor is now approved for use in combination with azacitidine, decitabine, or low-dose cytarabine to treat adults with newly diagnosed AML who are age 75 and older or who are ineligible for intensive chemotherapy.
The FDA grants accelerated approval based on a surrogate or intermediate endpoint that is reasonably likely to predict clinical benefit.
Therefore, continued approval of venetoclax in AML may be contingent upon verification of clinical benefit in confirmatory trials.
The approval is based on data from two studies—the phase 1 b M14-358 trial (NCT02203773) and the phase 1/2 M14-387 trial (NCT02287233).
M14-358 trial
In M14-358, newly diagnosed AML patients received venetoclax in combination with azacitidine (n=84) or decitabine (n=31). There were 67 patients in the azacitidine arm and 13 in the decitabine arm who were 75 or older or were ineligible for intensive induction chemotherapy.
Patients received venetoclax via a daily ramp-up to a final dose of 400 mg once daily. They received prophylaxis for tumor lysis syndrome and were hospitalized for monitoring during the ramp-up.
They received azacitidine at 75 mg/m2 on days 1-7 of each 28-day cycle or decitabine at 20 mg/m2 on days 1-5 of each cycle. Patients continued treatment until disease progression or unacceptable toxicity.
The median follow-up was 7.9 months for the azacitidine arm and 11 months for the decitabine arm.
The complete response (CR) rate was 37% (25/67) in the azacitidine arm and 54% (7/13) in the decitabine arm. The rates of CR with partial hematologic recovery were 24% (16/67) and 7.7% (1/13), respectively.
The most common adverse events (AEs)—occurring in at least 30% of patients in both arms—were nausea, diarrhea, constipation, neutropenia, thrombocytopenia, hemorrhage, peripheral edema, vomiting, fatigue, febrile neutropenia, rash, and anemia.
The incidence of serious AEs was 75% overall. The most frequent serious AEs (occurring in at least 5% of patients) were febrile neutropenia, pneumonia (excluding fungal), sepsis (excluding fungal), respiratory failure, and multiple organ dysfunction syndrome.
The incidence of fatal AEs was 1.5% within 30 days of treatment initiation.
M14-387 trial
The M14-387 trial included 82 AML patients who received venetoclax plus low-dose cytarabine. Patients were newly diagnosed with AML, but some had previous exposure to a hypomethylating agent for an antecedent hematologic disorder.
There were 61 patients who were 75 or older or were ineligible for intensive induction chemotherapy.
Patients received venetoclax via daily ramp-up to a final dose of 600 mg once daily. They received prophylaxis for tumor lysis syndrome and were hospitalized for monitoring during the ramp-up.
Cytarabine was given at 20 mg/m2 on days 1-10 of each 28-day cycle. Patients continued to receive treatment until disease progression or unacceptable toxicity.
At a median follow-up of 6.5 months, the CR rate was 21% (13/61), and the rate of CR with partial hematologic recovery was 21% (13/61).
The most common AEs (occurring in at least 30% of patients) were nausea, thrombocytopenia, hemorrhage, febrile neutropenia, neutropenia, diarrhea, fatigue, constipation, and dyspnea.
The incidence of serious AEs was 95%. The most frequent serious AEs (occurring in at least 5% of patients) were febrile neutropenia, sepsis (excluding fungal), hemorrhage, pneumonia (excluding fungal), and device-related infection.
The incidence of fatal AEs was 4.9% within 30 days of treatment initiation.
Additional details from the M14-358 and M14-387 trials are available in the prescribing information for venetoclax.
Venetoclax is being developed by AbbVie and Roche. It is jointly commercialized by AbbVie and Genentech, a member of the Roche Group, in the United States and by AbbVie elsewhere.
The U.S. Food and Drug Administration (FDA) has granted accelerated approval to venetoclax (Venclexta®) for use in acute myeloid leukemia (AML).
The BCL-2 inhibitor is now approved for use in combination with azacitidine, decitabine, or low-dose cytarabine to treat adults with newly diagnosed AML who are age 75 and older or who are ineligible for intensive chemotherapy.
The FDA grants accelerated approval based on a surrogate or intermediate endpoint that is reasonably likely to predict clinical benefit.
Therefore, continued approval of venetoclax in AML may be contingent upon verification of clinical benefit in confirmatory trials.
The approval is based on data from two studies—the phase 1 b M14-358 trial (NCT02203773) and the phase 1/2 M14-387 trial (NCT02287233).
M14-358 trial
In M14-358, newly diagnosed AML patients received venetoclax in combination with azacitidine (n=84) or decitabine (n=31). There were 67 patients in the azacitidine arm and 13 in the decitabine arm who were 75 or older or were ineligible for intensive induction chemotherapy.
Patients received venetoclax via a daily ramp-up to a final dose of 400 mg once daily. They received prophylaxis for tumor lysis syndrome and were hospitalized for monitoring during the ramp-up.
They received azacitidine at 75 mg/m2 on days 1-7 of each 28-day cycle or decitabine at 20 mg/m2 on days 1-5 of each cycle. Patients continued treatment until disease progression or unacceptable toxicity.
The median follow-up was 7.9 months for the azacitidine arm and 11 months for the decitabine arm.
The complete response (CR) rate was 37% (25/67) in the azacitidine arm and 54% (7/13) in the decitabine arm. The rates of CR with partial hematologic recovery were 24% (16/67) and 7.7% (1/13), respectively.
The most common adverse events (AEs)—occurring in at least 30% of patients in both arms—were nausea, diarrhea, constipation, neutropenia, thrombocytopenia, hemorrhage, peripheral edema, vomiting, fatigue, febrile neutropenia, rash, and anemia.
The incidence of serious AEs was 75% overall. The most frequent serious AEs (occurring in at least 5% of patients) were febrile neutropenia, pneumonia (excluding fungal), sepsis (excluding fungal), respiratory failure, and multiple organ dysfunction syndrome.
The incidence of fatal AEs was 1.5% within 30 days of treatment initiation.
M14-387 trial
The M14-387 trial included 82 AML patients who received venetoclax plus low-dose cytarabine. Patients were newly diagnosed with AML, but some had previous exposure to a hypomethylating agent for an antecedent hematologic disorder.
There were 61 patients who were 75 or older or were ineligible for intensive induction chemotherapy.
Patients received venetoclax via daily ramp-up to a final dose of 600 mg once daily. They received prophylaxis for tumor lysis syndrome and were hospitalized for monitoring during the ramp-up.
Cytarabine was given at 20 mg/m2 on days 1-10 of each 28-day cycle. Patients continued to receive treatment until disease progression or unacceptable toxicity.
At a median follow-up of 6.5 months, the CR rate was 21% (13/61), and the rate of CR with partial hematologic recovery was 21% (13/61).
The most common AEs (occurring in at least 30% of patients) were nausea, thrombocytopenia, hemorrhage, febrile neutropenia, neutropenia, diarrhea, fatigue, constipation, and dyspnea.
The incidence of serious AEs was 95%. The most frequent serious AEs (occurring in at least 5% of patients) were febrile neutropenia, sepsis (excluding fungal), hemorrhage, pneumonia (excluding fungal), and device-related infection.
The incidence of fatal AEs was 4.9% within 30 days of treatment initiation.
Additional details from the M14-358 and M14-387 trials are available in the prescribing information for venetoclax.
Venetoclax is being developed by AbbVie and Roche. It is jointly commercialized by AbbVie and Genentech, a member of the Roche Group, in the United States and by AbbVie elsewhere.
FDA approves glasdegib for AML
The U.S. Food and Drug Administration (FDA) has approved the hedgehog pathway inhibitor glasdegib (Daurismo™) to treat certain patients with acute myeloid leukemia (AML).
Glasdegib is approved for use in combination with low-dose cytarabine (LDAC) to treat adults with newly diagnosed AML who are age 75 and older or who are ineligible for intensive chemotherapy.
Glasdegib was approved under priority review and also received orphan drug designation from the FDA.
The prescribing information for glasdegib includes a boxed warning detailing the risk of embryo-fetal death or severe birth defects associated with the drug.
The FDA’s approval of glasdegib is based on results from the phase 2 BRIGHT AML 1003 trial (NCT01546038).
This trial included 111 adults with newly diagnosed AML and 14 patients with other conditions.
The patients were randomized to receive glasdegib (at 100 mg daily) in combination with LDAC (n=84) or LDAC alone (n=41).
The complete response rate among the AML patients was 18.2% (14/77) in the glasdegib arm and 2.6% (1/38) in the LDAC arm.
The median overall survival was 8.3 months in the glasdegib arm and 4.3 months in the LDAC arm (hazard ratio=0.46; P=0.0002).
The most common adverse events (AEs) in the first 90 days of treatment—occurring in at least 30% of patients in either arm (glasdegib-LDAC and LDAC alone, respectively)—were:
- Anemia (43% and 42%)
- Fatigue (36% and 32%)
- Hemorrhage (36% and 42%)
- Febrile neutropenia (31% and 22%)
- Musculoskeletal pain (30% and 17%)
- Edema (30% and 20%)
- Thrombocytopenia (30% and 27%).
The incidence of serious AEs was 79% in the glasdegib arm. The most common serious AEs occurring in at least 5% of patients in this arm were febrile neutropenia (29%), pneumonia (23%), hemorrhage (12%), anemia (7%), and sepsis (7%).
Additional data from this trial are included in the prescribing information for glasdegib. Glasdegib is a product of Pfizer.
The U.S. Food and Drug Administration (FDA) has approved the hedgehog pathway inhibitor glasdegib (Daurismo™) to treat certain patients with acute myeloid leukemia (AML).
Glasdegib is approved for use in combination with low-dose cytarabine (LDAC) to treat adults with newly diagnosed AML who are age 75 and older or who are ineligible for intensive chemotherapy.
Glasdegib was approved under priority review and also received orphan drug designation from the FDA.
The prescribing information for glasdegib includes a boxed warning detailing the risk of embryo-fetal death or severe birth defects associated with the drug.
The FDA’s approval of glasdegib is based on results from the phase 2 BRIGHT AML 1003 trial (NCT01546038).
This trial included 111 adults with newly diagnosed AML and 14 patients with other conditions.
The patients were randomized to receive glasdegib (at 100 mg daily) in combination with LDAC (n=84) or LDAC alone (n=41).
The complete response rate among the AML patients was 18.2% (14/77) in the glasdegib arm and 2.6% (1/38) in the LDAC arm.
The median overall survival was 8.3 months in the glasdegib arm and 4.3 months in the LDAC arm (hazard ratio=0.46; P=0.0002).
The most common adverse events (AEs) in the first 90 days of treatment—occurring in at least 30% of patients in either arm (glasdegib-LDAC and LDAC alone, respectively)—were:
- Anemia (43% and 42%)
- Fatigue (36% and 32%)
- Hemorrhage (36% and 42%)
- Febrile neutropenia (31% and 22%)
- Musculoskeletal pain (30% and 17%)
- Edema (30% and 20%)
- Thrombocytopenia (30% and 27%).
The incidence of serious AEs was 79% in the glasdegib arm. The most common serious AEs occurring in at least 5% of patients in this arm were febrile neutropenia (29%), pneumonia (23%), hemorrhage (12%), anemia (7%), and sepsis (7%).
Additional data from this trial are included in the prescribing information for glasdegib. Glasdegib is a product of Pfizer.
The U.S. Food and Drug Administration (FDA) has approved the hedgehog pathway inhibitor glasdegib (Daurismo™) to treat certain patients with acute myeloid leukemia (AML).
Glasdegib is approved for use in combination with low-dose cytarabine (LDAC) to treat adults with newly diagnosed AML who are age 75 and older or who are ineligible for intensive chemotherapy.
Glasdegib was approved under priority review and also received orphan drug designation from the FDA.
The prescribing information for glasdegib includes a boxed warning detailing the risk of embryo-fetal death or severe birth defects associated with the drug.
The FDA’s approval of glasdegib is based on results from the phase 2 BRIGHT AML 1003 trial (NCT01546038).
This trial included 111 adults with newly diagnosed AML and 14 patients with other conditions.
The patients were randomized to receive glasdegib (at 100 mg daily) in combination with LDAC (n=84) or LDAC alone (n=41).
The complete response rate among the AML patients was 18.2% (14/77) in the glasdegib arm and 2.6% (1/38) in the LDAC arm.
The median overall survival was 8.3 months in the glasdegib arm and 4.3 months in the LDAC arm (hazard ratio=0.46; P=0.0002).
The most common adverse events (AEs) in the first 90 days of treatment—occurring in at least 30% of patients in either arm (glasdegib-LDAC and LDAC alone, respectively)—were:
- Anemia (43% and 42%)
- Fatigue (36% and 32%)
- Hemorrhage (36% and 42%)
- Febrile neutropenia (31% and 22%)
- Musculoskeletal pain (30% and 17%)
- Edema (30% and 20%)
- Thrombocytopenia (30% and 27%).
The incidence of serious AEs was 79% in the glasdegib arm. The most common serious AEs occurring in at least 5% of patients in this arm were febrile neutropenia (29%), pneumonia (23%), hemorrhage (12%), anemia (7%), and sepsis (7%).
Additional data from this trial are included in the prescribing information for glasdegib. Glasdegib is a product of Pfizer.