User login
Managing geriatric bipolar disorder
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Recommendations for lab monitoring of atypical antipsychotics
Mr. H, age 31, is admitted to an acute psychiatric unit with major depressive disorder, substance dependence, insomnia, and generalized anxiety. In the past, he was treated unsuccessfully with sertraline, fluoxetine, clonazepam, venlafaxine, and lithium. The treatment team starts Mr. H on quetiapine, titrated to 150 mg at bedtime, to address suspected bipolar II disorder.
At baseline, Mr. H is 68 inches tall and slightly overweight at 176 lbs (body mass index [BMI] 26.8 kg/m2). The laboratory reports his glycated hemoglobin (HbA1c) at 5.4%; low-density lipoprotein (LDL), 60 mg/dL; total cholesterol, 122 mg/dL; triglycerides, 141 mg/dL; and high-density lipoprotein (HDL), 34 mg/dL.
Within 1 month, Mr. H experiences a 16% increase in body weight. HbA1c increases to 5.6%; LDL, to 93 mg/dL. These metabolic changes are not addressed, and he continues quetiapine for another 5 months. At the end of 6 months, Mr. H weighs 223.8 lbs (BMI 34 kg/m2)—a 27% increase from baseline. HbA1c is in the prediabetic range, at 5.9%, and LDL is 120 mg/dL.1 The treatment team discusses the risks of further metabolic effects, cardiovascular disease, and diabetes with Mr. H. He agrees to a change in therapy.
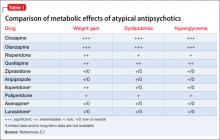
An increase in weight is thought to be associated with the actions of antipsychotics on H1and 5-HT2c receptors.7 Clozapine and olanzapine pose the highest risk of weight gain. Quetiapine and risperidone are considered of intermediate risk; aripiprazole and ziprasidone present the lowest risk(Table 1).5,7
Patients taking an atypical antipsychotic may experience an elevation of blood glucose, serum triglyceride, and LDL levels, and a decrease in the HDL level.2 These effects may be seen without an increase in BMI, and should be considered a direct effect of the antipsychotic.5 Although the mechanism by which dyslipidemia occurs is poorly understood, an increase in the blood glucose level is thought to be, in part, mediated by antagonism of M3 muscarinic receptors on pancreatic â-cells.7 Clozapine and olanzapine pose the highest risk of dyslipidemia. Quetiapine and risperidone are considered of intermediate risk; the risk associated with quetiapine is closer to that of olanzpine.8,9 Aripiprazole and ziprasidone present a lower risk of dyslipidemia and glucose elevations.5
Newer atypical antipsychotics, such as asenapine, iloperidone, paliperidone, and lurasidone, seem to have a lower metabolic risk profile, similar to those seen with aripiprazole and ziprasidone.5 Patients enrolled in initial clinical trials might not be antipsychotic naïve, however, and may have been taking a high metabolic risk antipsychotic. When these patients are switched to an antipsychotic that carries less of a metabolic risk, it might appear that they are experiencing a decrease in metabolic adverse events.
Metabolic data on newer atypical antipsychotics are limited; most have not been subject to long-term study. Routine monitoring of metabolic side effects is recommended for all atypical antipsychotics, regardless of risk profile.
Recommended monitoring
Because of the known metabolic side effects that occur in patients taking an atypical antipsychotic, baseline and periodic monitoring is recommended (Table 2).2,10 BMI and waist circumference should be recorded at baseline and tracked throughout treatment. Ideally, obtain measurements monthly for the first 3 months of therapy, or after any medication adjustments, then at 6 months, and annually thereafter. Encourage patients to track their own weight.
HbA1c and fasting plasma glucose levels should be measured at baseline and throughout the course of treatment. Obtain another set of measurements at 3 months, then annually thereafter, unless the patient develops type 2 diabetes mellitus.2
Obtaining a fasting lipid panel at baseline and periodically throughout the course of treatment is recommended. After baseline measurement, another panel should be taken at 3 months and annually thereafter. Guidelines of the American Diabetes Association recommend a fasting lipid panel every 5 years—however, good clinical practice dictates obtaining a lipid panel annually.
Managing metabolic side effects
Assess whether the patient can benefit from a lower dosage of current medication, switching to an antipsychotic with less of a risk of metabolic disturbance, or from discontinuation of therapy. In most cases, aim to use monotherapy because polypharmacy contributes to an increased risk of side effects.10
Weight management. Recommend nutrition counseling and physical activity for all patients who are overweight. Referral to a health care professional or to a program with expertise in weight management also might be beneficial.2 Include family members and significant others in the patient’s education when possible.
Impaired fasting glucose. Encourage a low-carbohydrate, high-protein diet with high intake of vegetables. Patients should obtain at least 30 minutes of physical activity, five times a week. Referral to a diabetes self-management class also is appropriate. Consider referral to a primary care physician or a clinician with expertise in diabetes.2
Impaired fasting lipids. Encourage your patients to adhere to a heart-healthy diet that is low in saturated fats and to get adequate physical activity. Referral to a dietician and primary care provider for medical management of dyslipidemia might be appropriate.2
Related Resources
- American Diabetes Association. Guide to living with diabetes. www.diabetes.org/living-with-diabetes.
- MOVE! Weight Management Program for Veterans. www. move.va.gov.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Clonazepam • Klonopin
Clozapine • Clozaril
Fluoxetine • Prozac
Iloperidone • Fanapt
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosure
The authors report no financial relationships with any of the manufacturers mentioned in this article or with manufacturers of competing products.
1. American Diabetes Association. Executive summary: standards of medical care in diabetes—2010. Diabetes Care. 2010;33:
S4-S10.
2. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and the North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
3. Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097.
4. Tarricone I, Ferrari Gozzi B, Serretti A, et al. Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychol Med. 2010;40(2):187-200.
5. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759.
6. De Hert M, Dobbelaere M, Sheridan EM, et al. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry. 2011;26(3):144-158.
7. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications. Oxford, United Kingdom: Cambridge University Press; 2008.
8. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):
1209-1223.
9. Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765-1773.
10. Gothefors D, Adolfsson R, Attvall S, et al; Swedish Psychiatric Association. Swedish clinical guidelines – prevention and management of metabolic risk in patients with severe psychiatric disorders. Nord J Psychiatry. 2010;64(5):294-302.
11. Schneiderhan ME, Batscha CL, Rosen C. Assessment of a point-of-care metabolic risk screening program in outpatients receiving antipsychotic agents. Pharmacotherapy. 2009;29(8): 975-987.
Mr. H, age 31, is admitted to an acute psychiatric unit with major depressive disorder, substance dependence, insomnia, and generalized anxiety. In the past, he was treated unsuccessfully with sertraline, fluoxetine, clonazepam, venlafaxine, and lithium. The treatment team starts Mr. H on quetiapine, titrated to 150 mg at bedtime, to address suspected bipolar II disorder.
At baseline, Mr. H is 68 inches tall and slightly overweight at 176 lbs (body mass index [BMI] 26.8 kg/m2). The laboratory reports his glycated hemoglobin (HbA1c) at 5.4%; low-density lipoprotein (LDL), 60 mg/dL; total cholesterol, 122 mg/dL; triglycerides, 141 mg/dL; and high-density lipoprotein (HDL), 34 mg/dL.
Within 1 month, Mr. H experiences a 16% increase in body weight. HbA1c increases to 5.6%; LDL, to 93 mg/dL. These metabolic changes are not addressed, and he continues quetiapine for another 5 months. At the end of 6 months, Mr. H weighs 223.8 lbs (BMI 34 kg/m2)—a 27% increase from baseline. HbA1c is in the prediabetic range, at 5.9%, and LDL is 120 mg/dL.1 The treatment team discusses the risks of further metabolic effects, cardiovascular disease, and diabetes with Mr. H. He agrees to a change in therapy.
An increase in weight is thought to be associated with the actions of antipsychotics on H1and 5-HT2c receptors.7 Clozapine and olanzapine pose the highest risk of weight gain. Quetiapine and risperidone are considered of intermediate risk; aripiprazole and ziprasidone present the lowest risk(Table 1).5,7
Patients taking an atypical antipsychotic may experience an elevation of blood glucose, serum triglyceride, and LDL levels, and a decrease in the HDL level.2 These effects may be seen without an increase in BMI, and should be considered a direct effect of the antipsychotic.5 Although the mechanism by which dyslipidemia occurs is poorly understood, an increase in the blood glucose level is thought to be, in part, mediated by antagonism of M3 muscarinic receptors on pancreatic â-cells.7 Clozapine and olanzapine pose the highest risk of dyslipidemia. Quetiapine and risperidone are considered of intermediate risk; the risk associated with quetiapine is closer to that of olanzpine.8,9 Aripiprazole and ziprasidone present a lower risk of dyslipidemia and glucose elevations.5
Newer atypical antipsychotics, such as asenapine, iloperidone, paliperidone, and lurasidone, seem to have a lower metabolic risk profile, similar to those seen with aripiprazole and ziprasidone.5 Patients enrolled in initial clinical trials might not be antipsychotic naïve, however, and may have been taking a high metabolic risk antipsychotic. When these patients are switched to an antipsychotic that carries less of a metabolic risk, it might appear that they are experiencing a decrease in metabolic adverse events.
Metabolic data on newer atypical antipsychotics are limited; most have not been subject to long-term study. Routine monitoring of metabolic side effects is recommended for all atypical antipsychotics, regardless of risk profile.
Recommended monitoring
Because of the known metabolic side effects that occur in patients taking an atypical antipsychotic, baseline and periodic monitoring is recommended (Table 2).2,10 BMI and waist circumference should be recorded at baseline and tracked throughout treatment. Ideally, obtain measurements monthly for the first 3 months of therapy, or after any medication adjustments, then at 6 months, and annually thereafter. Encourage patients to track their own weight.
HbA1c and fasting plasma glucose levels should be measured at baseline and throughout the course of treatment. Obtain another set of measurements at 3 months, then annually thereafter, unless the patient develops type 2 diabetes mellitus.2
Obtaining a fasting lipid panel at baseline and periodically throughout the course of treatment is recommended. After baseline measurement, another panel should be taken at 3 months and annually thereafter. Guidelines of the American Diabetes Association recommend a fasting lipid panel every 5 years—however, good clinical practice dictates obtaining a lipid panel annually.
Managing metabolic side effects
Assess whether the patient can benefit from a lower dosage of current medication, switching to an antipsychotic with less of a risk of metabolic disturbance, or from discontinuation of therapy. In most cases, aim to use monotherapy because polypharmacy contributes to an increased risk of side effects.10
Weight management. Recommend nutrition counseling and physical activity for all patients who are overweight. Referral to a health care professional or to a program with expertise in weight management also might be beneficial.2 Include family members and significant others in the patient’s education when possible.
Impaired fasting glucose. Encourage a low-carbohydrate, high-protein diet with high intake of vegetables. Patients should obtain at least 30 minutes of physical activity, five times a week. Referral to a diabetes self-management class also is appropriate. Consider referral to a primary care physician or a clinician with expertise in diabetes.2
Impaired fasting lipids. Encourage your patients to adhere to a heart-healthy diet that is low in saturated fats and to get adequate physical activity. Referral to a dietician and primary care provider for medical management of dyslipidemia might be appropriate.2
Related Resources
- American Diabetes Association. Guide to living with diabetes. www.diabetes.org/living-with-diabetes.
- MOVE! Weight Management Program for Veterans. www. move.va.gov.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Clonazepam • Klonopin
Clozapine • Clozaril
Fluoxetine • Prozac
Iloperidone • Fanapt
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosure
The authors report no financial relationships with any of the manufacturers mentioned in this article or with manufacturers of competing products.
Mr. H, age 31, is admitted to an acute psychiatric unit with major depressive disorder, substance dependence, insomnia, and generalized anxiety. In the past, he was treated unsuccessfully with sertraline, fluoxetine, clonazepam, venlafaxine, and lithium. The treatment team starts Mr. H on quetiapine, titrated to 150 mg at bedtime, to address suspected bipolar II disorder.
At baseline, Mr. H is 68 inches tall and slightly overweight at 176 lbs (body mass index [BMI] 26.8 kg/m2). The laboratory reports his glycated hemoglobin (HbA1c) at 5.4%; low-density lipoprotein (LDL), 60 mg/dL; total cholesterol, 122 mg/dL; triglycerides, 141 mg/dL; and high-density lipoprotein (HDL), 34 mg/dL.
Within 1 month, Mr. H experiences a 16% increase in body weight. HbA1c increases to 5.6%; LDL, to 93 mg/dL. These metabolic changes are not addressed, and he continues quetiapine for another 5 months. At the end of 6 months, Mr. H weighs 223.8 lbs (BMI 34 kg/m2)—a 27% increase from baseline. HbA1c is in the prediabetic range, at 5.9%, and LDL is 120 mg/dL.1 The treatment team discusses the risks of further metabolic effects, cardiovascular disease, and diabetes with Mr. H. He agrees to a change in therapy.
An increase in weight is thought to be associated with the actions of antipsychotics on H1and 5-HT2c receptors.7 Clozapine and olanzapine pose the highest risk of weight gain. Quetiapine and risperidone are considered of intermediate risk; aripiprazole and ziprasidone present the lowest risk(Table 1).5,7
Patients taking an atypical antipsychotic may experience an elevation of blood glucose, serum triglyceride, and LDL levels, and a decrease in the HDL level.2 These effects may be seen without an increase in BMI, and should be considered a direct effect of the antipsychotic.5 Although the mechanism by which dyslipidemia occurs is poorly understood, an increase in the blood glucose level is thought to be, in part, mediated by antagonism of M3 muscarinic receptors on pancreatic â-cells.7 Clozapine and olanzapine pose the highest risk of dyslipidemia. Quetiapine and risperidone are considered of intermediate risk; the risk associated with quetiapine is closer to that of olanzpine.8,9 Aripiprazole and ziprasidone present a lower risk of dyslipidemia and glucose elevations.5
Newer atypical antipsychotics, such as asenapine, iloperidone, paliperidone, and lurasidone, seem to have a lower metabolic risk profile, similar to those seen with aripiprazole and ziprasidone.5 Patients enrolled in initial clinical trials might not be antipsychotic naïve, however, and may have been taking a high metabolic risk antipsychotic. When these patients are switched to an antipsychotic that carries less of a metabolic risk, it might appear that they are experiencing a decrease in metabolic adverse events.
Metabolic data on newer atypical antipsychotics are limited; most have not been subject to long-term study. Routine monitoring of metabolic side effects is recommended for all atypical antipsychotics, regardless of risk profile.
Recommended monitoring
Because of the known metabolic side effects that occur in patients taking an atypical antipsychotic, baseline and periodic monitoring is recommended (Table 2).2,10 BMI and waist circumference should be recorded at baseline and tracked throughout treatment. Ideally, obtain measurements monthly for the first 3 months of therapy, or after any medication adjustments, then at 6 months, and annually thereafter. Encourage patients to track their own weight.
HbA1c and fasting plasma glucose levels should be measured at baseline and throughout the course of treatment. Obtain another set of measurements at 3 months, then annually thereafter, unless the patient develops type 2 diabetes mellitus.2
Obtaining a fasting lipid panel at baseline and periodically throughout the course of treatment is recommended. After baseline measurement, another panel should be taken at 3 months and annually thereafter. Guidelines of the American Diabetes Association recommend a fasting lipid panel every 5 years—however, good clinical practice dictates obtaining a lipid panel annually.
Managing metabolic side effects
Assess whether the patient can benefit from a lower dosage of current medication, switching to an antipsychotic with less of a risk of metabolic disturbance, or from discontinuation of therapy. In most cases, aim to use monotherapy because polypharmacy contributes to an increased risk of side effects.10
Weight management. Recommend nutrition counseling and physical activity for all patients who are overweight. Referral to a health care professional or to a program with expertise in weight management also might be beneficial.2 Include family members and significant others in the patient’s education when possible.
Impaired fasting glucose. Encourage a low-carbohydrate, high-protein diet with high intake of vegetables. Patients should obtain at least 30 minutes of physical activity, five times a week. Referral to a diabetes self-management class also is appropriate. Consider referral to a primary care physician or a clinician with expertise in diabetes.2
Impaired fasting lipids. Encourage your patients to adhere to a heart-healthy diet that is low in saturated fats and to get adequate physical activity. Referral to a dietician and primary care provider for medical management of dyslipidemia might be appropriate.2
Related Resources
- American Diabetes Association. Guide to living with diabetes. www.diabetes.org/living-with-diabetes.
- MOVE! Weight Management Program for Veterans. www. move.va.gov.
Drug Brand Names
Aripiprazole • Abilify
Asenapine • Saphris
Clonazepam • Klonopin
Clozapine • Clozaril
Fluoxetine • Prozac
Iloperidone • Fanapt
Lithium • Eskalith, Lithobid
Lurasidone • Latuda
Olanzapine • Zyprexa
Paliperidone • Invega
Quetiapine • Seroquel
Risperidone • Risperdal
Sertraline • Zoloft
Venlafaxine • Effexor
Ziprasidone • Geodon
Disclosure
The authors report no financial relationships with any of the manufacturers mentioned in this article or with manufacturers of competing products.
1. American Diabetes Association. Executive summary: standards of medical care in diabetes—2010. Diabetes Care. 2010;33:
S4-S10.
2. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and the North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
3. Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097.
4. Tarricone I, Ferrari Gozzi B, Serretti A, et al. Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychol Med. 2010;40(2):187-200.
5. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759.
6. De Hert M, Dobbelaere M, Sheridan EM, et al. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry. 2011;26(3):144-158.
7. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications. Oxford, United Kingdom: Cambridge University Press; 2008.
8. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):
1209-1223.
9. Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765-1773.
10. Gothefors D, Adolfsson R, Attvall S, et al; Swedish Psychiatric Association. Swedish clinical guidelines – prevention and management of metabolic risk in patients with severe psychiatric disorders. Nord J Psychiatry. 2010;64(5):294-302.
11. Schneiderhan ME, Batscha CL, Rosen C. Assessment of a point-of-care metabolic risk screening program in outpatients receiving antipsychotic agents. Pharmacotherapy. 2009;29(8): 975-987.
1. American Diabetes Association. Executive summary: standards of medical care in diabetes—2010. Diabetes Care. 2010;33:
S4-S10.
2. American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, and the North American Association for the Study of Obesity. Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care. 2004;27(2):596-601.
3. Kahn RS, Fleischhacker WW, Boter H, et al; EUFEST study group. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085-1097.
4. Tarricone I, Ferrari Gozzi B, Serretti A, et al. Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychol Med. 2010;40(2):187-200.
5. De Hert M, Yu W, Detraux J, et al. Body weight and metabolic adverse effects of asenapine, iloperidone, lurasidone and paliperidone in the treatment of schizophrenia and bipolar disorder: a systematic review and exploratory meta-analysis. CNS Drugs. 2012;26(9):733-759.
6. De Hert M, Dobbelaere M, Sheridan EM, et al. Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: a systematic review of randomized, placebo controlled trials and guidelines for clinical practice. Eur Psychiatry. 2011;26(3):144-158.
7. Stahl SM. Stahl’s essential psychopharmacology, neuroscientific basis and practical applications. Oxford, United Kingdom: Cambridge University Press; 2008.
8. Lieberman JA, Stroup TS, McEvoy JP, et al; Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):
1209-1223.
9. Correll CU, Manu P, Olshanskiy V, et al. Cardiometabolic risk of second-generation antipsychotic medications during first-time use in children and adolescents. JAMA. 2009;302(16):1765-1773.
10. Gothefors D, Adolfsson R, Attvall S, et al; Swedish Psychiatric Association. Swedish clinical guidelines – prevention and management of metabolic risk in patients with severe psychiatric disorders. Nord J Psychiatry. 2010;64(5):294-302.
11. Schneiderhan ME, Batscha CL, Rosen C. Assessment of a point-of-care metabolic risk screening program in outpatients receiving antipsychotic agents. Pharmacotherapy. 2009;29(8): 975-987.
Eating disorder as an episode heralding in bipolar
The relationship between binge eating disorder and bipolar disorder is underappreciated in psychiatry. In fact, after many years of practice, I would submit that bipolar disorder can present as an episode of eating disorder. Failing to make this possible connection can have serious implications for our patients. If bipolar disorder is actually the diagnosis in these cases, treating them with selective serotonin reuptake inhibitors can lead to poor outcomes.
Several studies have explored the possible connection between bipolar disorder and eating disorder. One involving 717 patients with bipolar disorder who were participating in the Mayo Clinic Bipolar Biobank found that among patients with bipolar disorder, binge eating disorder and obesity are highly prevalent and correlated. The investigators went on to suggest that bipolar disorder and binge eating disorder "may represent a clinically important sub-phenotype" (J. Affect. Disord. 2013 June 3 [doi:10.1016/j.jad.2013.05.024]).
Another study of 875 outpatients with DSM-IV bipolar I or II found that more than 14% of them met the criteria for at least one comorbid lifetime eating disorder. The most common was binge eating disorder (J. Affect. Disord. 2011;128:191-8). However, these cases did not make the connection that eating disorder might be an episode heralding in bipolar disorder.
One of my own patients, whom I will call Miss G.,* fits one of those categories.
A complex presentation
I first saw Miss G. in spring 2010. She was aged 16 years and 6 months, stood at 5 feet, 8 inches tall, and was fairly built. She had acne on her face and looked more mature than her age.
The adolescent was an only child and lived with her parents. (Her father drove her to my office for almost all of her appointments.) She was in high school, and worked as a waitress and a cashier at a drugstore. She reported that she had good friends and denied any history of abuse. She had been called "chubby" and "fat," starting at the very early age of 7, but denied feeling sad or crying about being called fat – and never had to defend herself about it.
During the first visit, she described her problem this way: "I get anxious in the middle of the day (and) get hyped up at night." She said she had been diagnosed with eating disorder and had been getting treatment by a therapist for the past 4 years. She reported symptoms of excessive eating, bingeing, and then purging more than 2-10 times a day.
In fifth grade, her crash dieting had begun, which escalated into excessive eating, followed by purging, calorie counting, and excessive use of treadmills and other equipment at a gym in an effort to lose weight. At her lowest weight, she succeeded in getting down to 113 pounds. At her highest, she reached 150.
In her sophomore year, she said that the severity of her illness had led to fainting because of low potassium levels and hypotension requiring frequent visits and treatment in the emergency department to balance her electrolytes. She also reported having panic attacks, which had lessened over the last 2 years. She reported undergoing weekly blood tests for electrolytes and presently was within normal limits. She assured me that the problems leading to her fainting would never happen again. Although Miss G. had been under the treatment of a therapist, she was not under that therapist’s care when she came to see me. It seems that one day, Miss G. walked out of the therapist’s office in anger and was now feeling embarrassed about going back to her.
I continued to explore Miss G.’s symptoms further, which revealed a decreased focus and attention, with a dramatic drop in recent months in school performance, from straight As to Bs, and eventually, to Cs.
In subsequent sessions, the patient admitted to having gone days without sleeping at night and described having excessive energy, "craving for movement," stay(ing) awake, hyper, constant movement, action, happiness, cutting myself." At one point, she said, "I was giddy and buzzing on some weird high and had an impulse to throw up food."
Also during that year, Miss G. said she had "graffitied many parks, shoplifted food, eaten it, and then thrown it up, gotten caught dining and dashing at night." Then there were times when she would just "sit at home and cry – and have no motivation to go out."
In describing her symptoms after many months, she said: "I was depressed before anything started. I hated myself." She denied hearing any voices but admitted to hearing her own voice. "At times, it would start screaming," she said. The patient denied having ever made serious suicidal attempts but had constant thoughts of killing herself by hanging or cutting her wrists 4 months prior to her first visit with me.
Miss G. also had taken an overdose of aspirin, up to 7 grams in 15 hours, and was disappointed to learn that the dose was not lethal. She admitted to using multiple drugs, including alcohol, marijuana, heroin, cocaine, cigarettes, caffeine, and amphetamines to control her moods and behavior in the past. But she had never been treated with psychotropics.
Her family history proved significant. One of her great grandfathers had committed suicide, and a maternal aunt was in treatment with several psychotropics and was on disability.
My initial diagnoses
Initially, I diagnosed this patient with eating disorder, bulimic type; eating disorder not otherwise specified; and polysubstance dependence in early remission with multiple rule-outs, including anxiety disorder NOS; psychotic disorder, NOS; bipolar disorder NOS; and bipolar disorder with psychosis. I explained to her the diagnosis and my concern about the possibility of mood swings and the risk of being on SSRIs or other antidepressants that are commonly prescribed for eating disorders, and that can worsen what I suspected was underlying bipolar disorder, and alter the course and treatment outcome – and the overall clinical outcome. She really did not care about the diagnosis or the treatment and was willing to take any medication that would not cause weight gain. She agreed to take topiramate and adamantly decided against taking anything else.
On subsequent visits, she reported worsening of concentration and anger but insisted on continuing on the topiramate because it had lowered her appetite and her bingeing and purging behavior had become less frequent.
At this point, I confirmed the diagnosis as bipolar disorder and had her agree to take lamotrigine. She continued to experience anger and mood swings, although she was taking 300 mg of lamotrigine. Risperidone had no therapeutic response. Although it proved difficult to persuade her to take sodium valproate she agreed, because she understood the consequences of her anger. Miss G. knew that continuing to behave disrespectfully toward her teachers would jeopardize her education and her future.
To elaborate on the time frames, let me point out that Miss G. started on the topiramate on the first day of her treatment. The lamotrigine was started the following month. The sodium valproate was introduced about 7 months after that with improvement, but she continued to complain of weight gain and appetite, which was not controlled – even with an H-2 blocker. So I had to stop the sodium valproate 4 months after it was introduced. Her concentration continued to either decline or not improve with mood stabilization.
This is the point at which I introduced clonidine. Although Miss G. did experience some side effects, her concentration improved. Her mood remained fairly stable on lamotrigine and clonidine after I discontinued the sodium valproate.
My last session with Miss G. occurred about 1 year and 3.5 months after the first visit. On that day, she was casually but neatly dressed. She told me that she would be graduating from high school and attending college out of state.
When I asked her about some of the behaviors tied to her eating disorder, she said "not at all" but after further exploration she said "once or twice a week; it became a lifestyle and right now, it is not a lifestyle anymore," she said. Miss G. went on to describe her current weight of 145 as "ideal," but said she still struggled to see herself in a healthy way. "By being treated for my bipolar disorder, my eating disorder did not reach such a low point," she said. "I consider myself a recovered eating disorder patient."
Her mood was good and her affect appropriate. She said she had no thoughts of harming herself.
We must get this right
Most prior patients with a diagnosis of eating disorder come to my office on SSRIs with poor functioning and symptom control. Initially, they say, "You are the doctor; whatever you say," but in the end, they either failed to accept the diagnosis of bipolar disorder or to follow my treatment recommendations and left my practice. In each of these cases, I have been concerned that these patients with eating disorder diagnoses might indeed have bipolar disorder.
As I mentioned earlier, some studies have been conducted exploring the connections between eating disorder and bipolar disorder, but more are needed. Specifically, we need to determine the extent to which eating disorder and bipolar disorder are comorbidities – or whether eating disorder is an episode that leads to bipolar disorder. In addition, we must compare the treatment outcome and clinical course of patients who are treated with SSRIs for eating disorder with the treatment outcome and clinical course of patients who are treated with mood stabilizers – even if they have started episodes of eating disorder.
Finally, organized psychiatry must establish guidelines and develop tools for the proper diagnosis of bipolar disorder, even if eating disorder episodes are already under way.
*Miss G. enthusiastically gave her permission to publish these details about her treatment and even offered to allow me to use her full name, if doing so might help others get proper diagnosis and treatment.
Dr. Khoshnu is a general adult psychiatrist who is mostly in private practice in the West Caldwell and Somerset, N.J., areas. She also is affiliated with Overlook Hospital in Summit, N.J.
The relationship between binge eating disorder and bipolar disorder is underappreciated in psychiatry. In fact, after many years of practice, I would submit that bipolar disorder can present as an episode of eating disorder. Failing to make this possible connection can have serious implications for our patients. If bipolar disorder is actually the diagnosis in these cases, treating them with selective serotonin reuptake inhibitors can lead to poor outcomes.
Several studies have explored the possible connection between bipolar disorder and eating disorder. One involving 717 patients with bipolar disorder who were participating in the Mayo Clinic Bipolar Biobank found that among patients with bipolar disorder, binge eating disorder and obesity are highly prevalent and correlated. The investigators went on to suggest that bipolar disorder and binge eating disorder "may represent a clinically important sub-phenotype" (J. Affect. Disord. 2013 June 3 [doi:10.1016/j.jad.2013.05.024]).
Another study of 875 outpatients with DSM-IV bipolar I or II found that more than 14% of them met the criteria for at least one comorbid lifetime eating disorder. The most common was binge eating disorder (J. Affect. Disord. 2011;128:191-8). However, these cases did not make the connection that eating disorder might be an episode heralding in bipolar disorder.
One of my own patients, whom I will call Miss G.,* fits one of those categories.
A complex presentation
I first saw Miss G. in spring 2010. She was aged 16 years and 6 months, stood at 5 feet, 8 inches tall, and was fairly built. She had acne on her face and looked more mature than her age.
The adolescent was an only child and lived with her parents. (Her father drove her to my office for almost all of her appointments.) She was in high school, and worked as a waitress and a cashier at a drugstore. She reported that she had good friends and denied any history of abuse. She had been called "chubby" and "fat," starting at the very early age of 7, but denied feeling sad or crying about being called fat – and never had to defend herself about it.
During the first visit, she described her problem this way: "I get anxious in the middle of the day (and) get hyped up at night." She said she had been diagnosed with eating disorder and had been getting treatment by a therapist for the past 4 years. She reported symptoms of excessive eating, bingeing, and then purging more than 2-10 times a day.
In fifth grade, her crash dieting had begun, which escalated into excessive eating, followed by purging, calorie counting, and excessive use of treadmills and other equipment at a gym in an effort to lose weight. At her lowest weight, she succeeded in getting down to 113 pounds. At her highest, she reached 150.
In her sophomore year, she said that the severity of her illness had led to fainting because of low potassium levels and hypotension requiring frequent visits and treatment in the emergency department to balance her electrolytes. She also reported having panic attacks, which had lessened over the last 2 years. She reported undergoing weekly blood tests for electrolytes and presently was within normal limits. She assured me that the problems leading to her fainting would never happen again. Although Miss G. had been under the treatment of a therapist, she was not under that therapist’s care when she came to see me. It seems that one day, Miss G. walked out of the therapist’s office in anger and was now feeling embarrassed about going back to her.
I continued to explore Miss G.’s symptoms further, which revealed a decreased focus and attention, with a dramatic drop in recent months in school performance, from straight As to Bs, and eventually, to Cs.
In subsequent sessions, the patient admitted to having gone days without sleeping at night and described having excessive energy, "craving for movement," stay(ing) awake, hyper, constant movement, action, happiness, cutting myself." At one point, she said, "I was giddy and buzzing on some weird high and had an impulse to throw up food."
Also during that year, Miss G. said she had "graffitied many parks, shoplifted food, eaten it, and then thrown it up, gotten caught dining and dashing at night." Then there were times when she would just "sit at home and cry – and have no motivation to go out."
In describing her symptoms after many months, she said: "I was depressed before anything started. I hated myself." She denied hearing any voices but admitted to hearing her own voice. "At times, it would start screaming," she said. The patient denied having ever made serious suicidal attempts but had constant thoughts of killing herself by hanging or cutting her wrists 4 months prior to her first visit with me.
Miss G. also had taken an overdose of aspirin, up to 7 grams in 15 hours, and was disappointed to learn that the dose was not lethal. She admitted to using multiple drugs, including alcohol, marijuana, heroin, cocaine, cigarettes, caffeine, and amphetamines to control her moods and behavior in the past. But she had never been treated with psychotropics.
Her family history proved significant. One of her great grandfathers had committed suicide, and a maternal aunt was in treatment with several psychotropics and was on disability.
My initial diagnoses
Initially, I diagnosed this patient with eating disorder, bulimic type; eating disorder not otherwise specified; and polysubstance dependence in early remission with multiple rule-outs, including anxiety disorder NOS; psychotic disorder, NOS; bipolar disorder NOS; and bipolar disorder with psychosis. I explained to her the diagnosis and my concern about the possibility of mood swings and the risk of being on SSRIs or other antidepressants that are commonly prescribed for eating disorders, and that can worsen what I suspected was underlying bipolar disorder, and alter the course and treatment outcome – and the overall clinical outcome. She really did not care about the diagnosis or the treatment and was willing to take any medication that would not cause weight gain. She agreed to take topiramate and adamantly decided against taking anything else.
On subsequent visits, she reported worsening of concentration and anger but insisted on continuing on the topiramate because it had lowered her appetite and her bingeing and purging behavior had become less frequent.
At this point, I confirmed the diagnosis as bipolar disorder and had her agree to take lamotrigine. She continued to experience anger and mood swings, although she was taking 300 mg of lamotrigine. Risperidone had no therapeutic response. Although it proved difficult to persuade her to take sodium valproate she agreed, because she understood the consequences of her anger. Miss G. knew that continuing to behave disrespectfully toward her teachers would jeopardize her education and her future.
To elaborate on the time frames, let me point out that Miss G. started on the topiramate on the first day of her treatment. The lamotrigine was started the following month. The sodium valproate was introduced about 7 months after that with improvement, but she continued to complain of weight gain and appetite, which was not controlled – even with an H-2 blocker. So I had to stop the sodium valproate 4 months after it was introduced. Her concentration continued to either decline or not improve with mood stabilization.
This is the point at which I introduced clonidine. Although Miss G. did experience some side effects, her concentration improved. Her mood remained fairly stable on lamotrigine and clonidine after I discontinued the sodium valproate.
My last session with Miss G. occurred about 1 year and 3.5 months after the first visit. On that day, she was casually but neatly dressed. She told me that she would be graduating from high school and attending college out of state.
When I asked her about some of the behaviors tied to her eating disorder, she said "not at all" but after further exploration she said "once or twice a week; it became a lifestyle and right now, it is not a lifestyle anymore," she said. Miss G. went on to describe her current weight of 145 as "ideal," but said she still struggled to see herself in a healthy way. "By being treated for my bipolar disorder, my eating disorder did not reach such a low point," she said. "I consider myself a recovered eating disorder patient."
Her mood was good and her affect appropriate. She said she had no thoughts of harming herself.
We must get this right
Most prior patients with a diagnosis of eating disorder come to my office on SSRIs with poor functioning and symptom control. Initially, they say, "You are the doctor; whatever you say," but in the end, they either failed to accept the diagnosis of bipolar disorder or to follow my treatment recommendations and left my practice. In each of these cases, I have been concerned that these patients with eating disorder diagnoses might indeed have bipolar disorder.
As I mentioned earlier, some studies have been conducted exploring the connections between eating disorder and bipolar disorder, but more are needed. Specifically, we need to determine the extent to which eating disorder and bipolar disorder are comorbidities – or whether eating disorder is an episode that leads to bipolar disorder. In addition, we must compare the treatment outcome and clinical course of patients who are treated with SSRIs for eating disorder with the treatment outcome and clinical course of patients who are treated with mood stabilizers – even if they have started episodes of eating disorder.
Finally, organized psychiatry must establish guidelines and develop tools for the proper diagnosis of bipolar disorder, even if eating disorder episodes are already under way.
*Miss G. enthusiastically gave her permission to publish these details about her treatment and even offered to allow me to use her full name, if doing so might help others get proper diagnosis and treatment.
Dr. Khoshnu is a general adult psychiatrist who is mostly in private practice in the West Caldwell and Somerset, N.J., areas. She also is affiliated with Overlook Hospital in Summit, N.J.
The relationship between binge eating disorder and bipolar disorder is underappreciated in psychiatry. In fact, after many years of practice, I would submit that bipolar disorder can present as an episode of eating disorder. Failing to make this possible connection can have serious implications for our patients. If bipolar disorder is actually the diagnosis in these cases, treating them with selective serotonin reuptake inhibitors can lead to poor outcomes.
Several studies have explored the possible connection between bipolar disorder and eating disorder. One involving 717 patients with bipolar disorder who were participating in the Mayo Clinic Bipolar Biobank found that among patients with bipolar disorder, binge eating disorder and obesity are highly prevalent and correlated. The investigators went on to suggest that bipolar disorder and binge eating disorder "may represent a clinically important sub-phenotype" (J. Affect. Disord. 2013 June 3 [doi:10.1016/j.jad.2013.05.024]).
Another study of 875 outpatients with DSM-IV bipolar I or II found that more than 14% of them met the criteria for at least one comorbid lifetime eating disorder. The most common was binge eating disorder (J. Affect. Disord. 2011;128:191-8). However, these cases did not make the connection that eating disorder might be an episode heralding in bipolar disorder.
One of my own patients, whom I will call Miss G.,* fits one of those categories.
A complex presentation
I first saw Miss G. in spring 2010. She was aged 16 years and 6 months, stood at 5 feet, 8 inches tall, and was fairly built. She had acne on her face and looked more mature than her age.
The adolescent was an only child and lived with her parents. (Her father drove her to my office for almost all of her appointments.) She was in high school, and worked as a waitress and a cashier at a drugstore. She reported that she had good friends and denied any history of abuse. She had been called "chubby" and "fat," starting at the very early age of 7, but denied feeling sad or crying about being called fat – and never had to defend herself about it.
During the first visit, she described her problem this way: "I get anxious in the middle of the day (and) get hyped up at night." She said she had been diagnosed with eating disorder and had been getting treatment by a therapist for the past 4 years. She reported symptoms of excessive eating, bingeing, and then purging more than 2-10 times a day.
In fifth grade, her crash dieting had begun, which escalated into excessive eating, followed by purging, calorie counting, and excessive use of treadmills and other equipment at a gym in an effort to lose weight. At her lowest weight, she succeeded in getting down to 113 pounds. At her highest, she reached 150.
In her sophomore year, she said that the severity of her illness had led to fainting because of low potassium levels and hypotension requiring frequent visits and treatment in the emergency department to balance her electrolytes. She also reported having panic attacks, which had lessened over the last 2 years. She reported undergoing weekly blood tests for electrolytes and presently was within normal limits. She assured me that the problems leading to her fainting would never happen again. Although Miss G. had been under the treatment of a therapist, she was not under that therapist’s care when she came to see me. It seems that one day, Miss G. walked out of the therapist’s office in anger and was now feeling embarrassed about going back to her.
I continued to explore Miss G.’s symptoms further, which revealed a decreased focus and attention, with a dramatic drop in recent months in school performance, from straight As to Bs, and eventually, to Cs.
In subsequent sessions, the patient admitted to having gone days without sleeping at night and described having excessive energy, "craving for movement," stay(ing) awake, hyper, constant movement, action, happiness, cutting myself." At one point, she said, "I was giddy and buzzing on some weird high and had an impulse to throw up food."
Also during that year, Miss G. said she had "graffitied many parks, shoplifted food, eaten it, and then thrown it up, gotten caught dining and dashing at night." Then there were times when she would just "sit at home and cry – and have no motivation to go out."
In describing her symptoms after many months, she said: "I was depressed before anything started. I hated myself." She denied hearing any voices but admitted to hearing her own voice. "At times, it would start screaming," she said. The patient denied having ever made serious suicidal attempts but had constant thoughts of killing herself by hanging or cutting her wrists 4 months prior to her first visit with me.
Miss G. also had taken an overdose of aspirin, up to 7 grams in 15 hours, and was disappointed to learn that the dose was not lethal. She admitted to using multiple drugs, including alcohol, marijuana, heroin, cocaine, cigarettes, caffeine, and amphetamines to control her moods and behavior in the past. But she had never been treated with psychotropics.
Her family history proved significant. One of her great grandfathers had committed suicide, and a maternal aunt was in treatment with several psychotropics and was on disability.
My initial diagnoses
Initially, I diagnosed this patient with eating disorder, bulimic type; eating disorder not otherwise specified; and polysubstance dependence in early remission with multiple rule-outs, including anxiety disorder NOS; psychotic disorder, NOS; bipolar disorder NOS; and bipolar disorder with psychosis. I explained to her the diagnosis and my concern about the possibility of mood swings and the risk of being on SSRIs or other antidepressants that are commonly prescribed for eating disorders, and that can worsen what I suspected was underlying bipolar disorder, and alter the course and treatment outcome – and the overall clinical outcome. She really did not care about the diagnosis or the treatment and was willing to take any medication that would not cause weight gain. She agreed to take topiramate and adamantly decided against taking anything else.
On subsequent visits, she reported worsening of concentration and anger but insisted on continuing on the topiramate because it had lowered her appetite and her bingeing and purging behavior had become less frequent.
At this point, I confirmed the diagnosis as bipolar disorder and had her agree to take lamotrigine. She continued to experience anger and mood swings, although she was taking 300 mg of lamotrigine. Risperidone had no therapeutic response. Although it proved difficult to persuade her to take sodium valproate she agreed, because she understood the consequences of her anger. Miss G. knew that continuing to behave disrespectfully toward her teachers would jeopardize her education and her future.
To elaborate on the time frames, let me point out that Miss G. started on the topiramate on the first day of her treatment. The lamotrigine was started the following month. The sodium valproate was introduced about 7 months after that with improvement, but she continued to complain of weight gain and appetite, which was not controlled – even with an H-2 blocker. So I had to stop the sodium valproate 4 months after it was introduced. Her concentration continued to either decline or not improve with mood stabilization.
This is the point at which I introduced clonidine. Although Miss G. did experience some side effects, her concentration improved. Her mood remained fairly stable on lamotrigine and clonidine after I discontinued the sodium valproate.
My last session with Miss G. occurred about 1 year and 3.5 months after the first visit. On that day, she was casually but neatly dressed. She told me that she would be graduating from high school and attending college out of state.
When I asked her about some of the behaviors tied to her eating disorder, she said "not at all" but after further exploration she said "once or twice a week; it became a lifestyle and right now, it is not a lifestyle anymore," she said. Miss G. went on to describe her current weight of 145 as "ideal," but said she still struggled to see herself in a healthy way. "By being treated for my bipolar disorder, my eating disorder did not reach such a low point," she said. "I consider myself a recovered eating disorder patient."
Her mood was good and her affect appropriate. She said she had no thoughts of harming herself.
We must get this right
Most prior patients with a diagnosis of eating disorder come to my office on SSRIs with poor functioning and symptom control. Initially, they say, "You are the doctor; whatever you say," but in the end, they either failed to accept the diagnosis of bipolar disorder or to follow my treatment recommendations and left my practice. In each of these cases, I have been concerned that these patients with eating disorder diagnoses might indeed have bipolar disorder.
As I mentioned earlier, some studies have been conducted exploring the connections between eating disorder and bipolar disorder, but more are needed. Specifically, we need to determine the extent to which eating disorder and bipolar disorder are comorbidities – or whether eating disorder is an episode that leads to bipolar disorder. In addition, we must compare the treatment outcome and clinical course of patients who are treated with SSRIs for eating disorder with the treatment outcome and clinical course of patients who are treated with mood stabilizers – even if they have started episodes of eating disorder.
Finally, organized psychiatry must establish guidelines and develop tools for the proper diagnosis of bipolar disorder, even if eating disorder episodes are already under way.
*Miss G. enthusiastically gave her permission to publish these details about her treatment and even offered to allow me to use her full name, if doing so might help others get proper diagnosis and treatment.
Dr. Khoshnu is a general adult psychiatrist who is mostly in private practice in the West Caldwell and Somerset, N.J., areas. She also is affiliated with Overlook Hospital in Summit, N.J.
Caregiver support program decreases dementia emergency visits
BOSTON – A pilot program that supports the caregivers of Alzheimer’s disease patients decreased emergency admissions for those patients in San Francisco by more than 40% in 6 months, Elizabeth Edgerly, Ph.D., reported at the Alzheimer’s Association International Conference 2013.
The preliminary analysis also found that caregivers reported significant improvements in 7 out of 10 quality of life and quality of care measures, according to Dr. Edgerly, chief program officer of the Alzheimer’s Association Northern California and Northern Nevada Chapter.
The early results paint an encouraging picture of the future, she said in an interview.
"We are very excited about the emergency utilization data. We’re always confident about our capacity to impact efficacy, but affecting service utilization is a tough nut to crack. The beauty of this is, if we can improve quality of care while reducing utilization costs, it may actually be cost neutral to have this kind of a dementia support program."
The association created its Excellence in Dementia Care program in conjunction with Kaiser Permanente Northern California, the city and county of San Francisco, and the University of California, San Francisco. The Administration on Aging also provided funding.
San Francisco was the perfect city for the pilot project, Dr. Edgerly said. "It’s the only city in the United States with a strategic plan related to dementia. It also has an elderly, diverse population and many of the residents live alone."
The pilot program is part of the city plan’s goal of partnering with other institutions to improve quality of life and quality of care for people with dementia. It was designed to improve dementia care by both enhancing services and educating caregivers.
"Kaiser hired a full-time social worker for just dementia support and who only worked with the caregivers," Dr. Edgerly said. The social worker conducted initial evaluations and assessments and created an individualized dementia care program that was uploaded into the electronic medical record of each patient, making the individualized program available to everyone on the patient’s care team. The social worker also called caregivers proactively to make sure the caregivers’ needs were being met and that they could access community services.
The Alzheimer’s Association provided dementia care support experts manning a 24-hour help line, a MedicAlert + Alzheimer’s Association Safe Return bracelet for the patient, respite grants for day or evening supplemental care to give caregivers a break, and a support group where caregivers could meet to discusses their challenges.
The association also administered the educational portion of the program. The first component provided a primer on Alzheimer’s stage-by-stage effects on thinking, emotions, and behavior, and noted resources that were available for help. Caregivers also were informed about legal and financial planning and ways to keep a positive, safe, and compassionate home environment for as long as the patient could stay at home.
In surveys at baseline and after 6 months, caregivers rated their feelings about their abilities in 10 different areas: handling current patient problems in memory and behavior, handling future patient problems, dealing with their own frustrations, keeping the patient independent, caring for the patient as independently as possible, getting answers to patient problems, finding community organizations that provide answers, finding community organizations that provide services, getting answers to questions about services, independently arranging for services, and paying for services.
Overall, 92 of the 105 patient-caregiver dyads have completed the 6-month assessments, Dr. Edgerly said. The surveys indicated significant improvements on seven of the caregiver measures. Caregivers said they felt better able to handle concerns, to get information, and to obtain and pay for services.
Most importantly, the program led to about a 40% decrease in emergency department visits – a significant change, Dr. Edgerly said. There were also nonsignificant decreases in hospital length of stay, physician visits, and days spent in post–acute or long-term care facilities.
"Even the outcomes that didn’t improve significantly were all going in the right direction," Dr. Edgerly said, adding that she’s hoping for even better results when the data are analyzed in their entirety.
The program’s positive impact on health care resources could be enough to attract the interest of other insurers, she said. "If this intervention reduced expenses associated with hospital or emergency department visits, an insurer might see that as good business sense."
The project was funded by the Alzheimer’s Association of Northern California and Northern Nevada, Kaiser Permanente Northern California, and the National Institute on Aging.
On Twitter @Alz_Gal
BOSTON – A pilot program that supports the caregivers of Alzheimer’s disease patients decreased emergency admissions for those patients in San Francisco by more than 40% in 6 months, Elizabeth Edgerly, Ph.D., reported at the Alzheimer’s Association International Conference 2013.
The preliminary analysis also found that caregivers reported significant improvements in 7 out of 10 quality of life and quality of care measures, according to Dr. Edgerly, chief program officer of the Alzheimer’s Association Northern California and Northern Nevada Chapter.
The early results paint an encouraging picture of the future, she said in an interview.
"We are very excited about the emergency utilization data. We’re always confident about our capacity to impact efficacy, but affecting service utilization is a tough nut to crack. The beauty of this is, if we can improve quality of care while reducing utilization costs, it may actually be cost neutral to have this kind of a dementia support program."
The association created its Excellence in Dementia Care program in conjunction with Kaiser Permanente Northern California, the city and county of San Francisco, and the University of California, San Francisco. The Administration on Aging also provided funding.
San Francisco was the perfect city for the pilot project, Dr. Edgerly said. "It’s the only city in the United States with a strategic plan related to dementia. It also has an elderly, diverse population and many of the residents live alone."
The pilot program is part of the city plan’s goal of partnering with other institutions to improve quality of life and quality of care for people with dementia. It was designed to improve dementia care by both enhancing services and educating caregivers.
"Kaiser hired a full-time social worker for just dementia support and who only worked with the caregivers," Dr. Edgerly said. The social worker conducted initial evaluations and assessments and created an individualized dementia care program that was uploaded into the electronic medical record of each patient, making the individualized program available to everyone on the patient’s care team. The social worker also called caregivers proactively to make sure the caregivers’ needs were being met and that they could access community services.
The Alzheimer’s Association provided dementia care support experts manning a 24-hour help line, a MedicAlert + Alzheimer’s Association Safe Return bracelet for the patient, respite grants for day or evening supplemental care to give caregivers a break, and a support group where caregivers could meet to discusses their challenges.
The association also administered the educational portion of the program. The first component provided a primer on Alzheimer’s stage-by-stage effects on thinking, emotions, and behavior, and noted resources that were available for help. Caregivers also were informed about legal and financial planning and ways to keep a positive, safe, and compassionate home environment for as long as the patient could stay at home.
In surveys at baseline and after 6 months, caregivers rated their feelings about their abilities in 10 different areas: handling current patient problems in memory and behavior, handling future patient problems, dealing with their own frustrations, keeping the patient independent, caring for the patient as independently as possible, getting answers to patient problems, finding community organizations that provide answers, finding community organizations that provide services, getting answers to questions about services, independently arranging for services, and paying for services.
Overall, 92 of the 105 patient-caregiver dyads have completed the 6-month assessments, Dr. Edgerly said. The surveys indicated significant improvements on seven of the caregiver measures. Caregivers said they felt better able to handle concerns, to get information, and to obtain and pay for services.
Most importantly, the program led to about a 40% decrease in emergency department visits – a significant change, Dr. Edgerly said. There were also nonsignificant decreases in hospital length of stay, physician visits, and days spent in post–acute or long-term care facilities.
"Even the outcomes that didn’t improve significantly were all going in the right direction," Dr. Edgerly said, adding that she’s hoping for even better results when the data are analyzed in their entirety.
The program’s positive impact on health care resources could be enough to attract the interest of other insurers, she said. "If this intervention reduced expenses associated with hospital or emergency department visits, an insurer might see that as good business sense."
The project was funded by the Alzheimer’s Association of Northern California and Northern Nevada, Kaiser Permanente Northern California, and the National Institute on Aging.
On Twitter @Alz_Gal
BOSTON – A pilot program that supports the caregivers of Alzheimer’s disease patients decreased emergency admissions for those patients in San Francisco by more than 40% in 6 months, Elizabeth Edgerly, Ph.D., reported at the Alzheimer’s Association International Conference 2013.
The preliminary analysis also found that caregivers reported significant improvements in 7 out of 10 quality of life and quality of care measures, according to Dr. Edgerly, chief program officer of the Alzheimer’s Association Northern California and Northern Nevada Chapter.
The early results paint an encouraging picture of the future, she said in an interview.
"We are very excited about the emergency utilization data. We’re always confident about our capacity to impact efficacy, but affecting service utilization is a tough nut to crack. The beauty of this is, if we can improve quality of care while reducing utilization costs, it may actually be cost neutral to have this kind of a dementia support program."
The association created its Excellence in Dementia Care program in conjunction with Kaiser Permanente Northern California, the city and county of San Francisco, and the University of California, San Francisco. The Administration on Aging also provided funding.
San Francisco was the perfect city for the pilot project, Dr. Edgerly said. "It’s the only city in the United States with a strategic plan related to dementia. It also has an elderly, diverse population and many of the residents live alone."
The pilot program is part of the city plan’s goal of partnering with other institutions to improve quality of life and quality of care for people with dementia. It was designed to improve dementia care by both enhancing services and educating caregivers.
"Kaiser hired a full-time social worker for just dementia support and who only worked with the caregivers," Dr. Edgerly said. The social worker conducted initial evaluations and assessments and created an individualized dementia care program that was uploaded into the electronic medical record of each patient, making the individualized program available to everyone on the patient’s care team. The social worker also called caregivers proactively to make sure the caregivers’ needs were being met and that they could access community services.
The Alzheimer’s Association provided dementia care support experts manning a 24-hour help line, a MedicAlert + Alzheimer’s Association Safe Return bracelet for the patient, respite grants for day or evening supplemental care to give caregivers a break, and a support group where caregivers could meet to discusses their challenges.
The association also administered the educational portion of the program. The first component provided a primer on Alzheimer’s stage-by-stage effects on thinking, emotions, and behavior, and noted resources that were available for help. Caregivers also were informed about legal and financial planning and ways to keep a positive, safe, and compassionate home environment for as long as the patient could stay at home.
In surveys at baseline and after 6 months, caregivers rated their feelings about their abilities in 10 different areas: handling current patient problems in memory and behavior, handling future patient problems, dealing with their own frustrations, keeping the patient independent, caring for the patient as independently as possible, getting answers to patient problems, finding community organizations that provide answers, finding community organizations that provide services, getting answers to questions about services, independently arranging for services, and paying for services.
Overall, 92 of the 105 patient-caregiver dyads have completed the 6-month assessments, Dr. Edgerly said. The surveys indicated significant improvements on seven of the caregiver measures. Caregivers said they felt better able to handle concerns, to get information, and to obtain and pay for services.
Most importantly, the program led to about a 40% decrease in emergency department visits – a significant change, Dr. Edgerly said. There were also nonsignificant decreases in hospital length of stay, physician visits, and days spent in post–acute or long-term care facilities.
"Even the outcomes that didn’t improve significantly were all going in the right direction," Dr. Edgerly said, adding that she’s hoping for even better results when the data are analyzed in their entirety.
The program’s positive impact on health care resources could be enough to attract the interest of other insurers, she said. "If this intervention reduced expenses associated with hospital or emergency department visits, an insurer might see that as good business sense."
The project was funded by the Alzheimer’s Association of Northern California and Northern Nevada, Kaiser Permanente Northern California, and the National Institute on Aging.
On Twitter @Alz_Gal
AT AAIC 2013
Major finding: A program designed to support the caregivers of Alzheimer’s disease patients decreased emergency room visits by 40% over a 6-month period.
Data source: A program that integrated medical and social care and enrolled 105 caregiver-patient pairs.
Disclosures: The project was funded by the Alzheimer’s Association of Northern California and Northern Nevada, Kaiser Permanente Northern California, and the National Institute on Aging.
CRTC1 polymorphisms affect BMI, novel study finds
Polymorphisms of the CREB-regulated transcription coactivator 1 gene contribute to the genetics of human obesity in psychiatric patients and in the general population, results from a novel study demonstrated.
Specifically, the CRTC1 nonsynonymous polymorphism rs3746266A>G was associated with body mass index in three independent psychiatric samples in which lower BMI values were measured in carriers of the G allele compared with noncarriers, while the protective effect of the T allele of rs6510997C>T (a proxy of rs3746266A>G) against fat accumulation also was observed in a large population-based sample.
"Psychiatric, psychological, sociodemographic, and behavioral factors, as well as heritability, have been shown to influence individual susceptibility to overweight or obesity, both in the general population and in psychiatric patients before and after treatment with potentially weight gain–inducing psychotropic drugs," researchers led by Eva Choong, Pharm.D., Ph.D., reported online Aug. 7 in JAMA Psychiatry. "Genome-wide association studies conducted to date only explain a small fraction of body mass index (BMI) heritability, and more obesity susceptibility genes remain to be discovered."
For the study, which is thought to be the first of its kind, researchers from two university hospitals and a private clinic in Switzerland evaluated the effect of three CRTC1 polymorphisms on BMI and/or fat mass in a cohort of 152 patients taking weight gain–inducing psychotropic drugs (sample 1). The CRTC1 variant that was significantly associated with BMI was then replicated in two independent psychiatric samples, which consisted of 174 patients in sample 2 and 118 patients in sample 3, and in two white population-based samples, which consisted of 5,338 patients in sample 4 and 123,865 patients in sample 5 (JAMA Psychiatry 2013 Aug. 7 [doi: 10.1001/jamapsychiatry.2013.187]).
The researchers found that in the three psychiatric samples, carriers of the CRTC1 rs3746266A>G allele had a lower BMI than did noncarriers (P = .001 in sample 1, P = .05 in sample 2, and P = .0003 in sample 3). In a combined analysis that excluded patients taking other weight gain–inducing drugs, G-allele carriers had a 1.81-kg/m2 lower BMI, compared with noncarriers (P less than .0001). The strongest association was seen in women age 45; in this subset of patients, G-allele carriers had a 3.87-kg/m2 lower BMI, compared with noncarriers (P less than .0001).
In the analysis of population-based samples, the T allele of rs651099C>T, which is a proxy of the G allele, was associated with lower BMI (P = .01 in sample 5) and fat mass (P = .03 in sample 4).
The investigators acknowledged several limitations of their study. For example, most patients were not drug naive and had already developed weight gain because of previous treatments. "It was therefore not possible to determine with certainty whether the strong association of CRTC1 genotypes with BMI and fat mass in psychiatric populations was due to the psychiatric illness and/or to the pharmacological treatment," they wrote. "Extensive hormonal measurements were not available for our samples, so the role of sex hormones on the association of CRTC1 variants with adiposity could not be explored."
In addition, the racial and ethnic makeup of the patients sampled means that the results are not generalizable.
Despite those limitations, Dr. Choong expressed optimism about where these results might lead. "Our results suggest that CRTC1 plays an important role in the high prevalence of overweight and obesity observed in psychiatric patients," she and her colleagues concluded. "Besides, CRTC1 could play a role in the genetics of obesity in the general population, thereby increasing our understanding of the multiple mechanisms influencing obesity.
"Finally, the strong associations of CRTC1 variants with adiposity in women younger than 45 years support further research on the interrelationship between adiposity and the reproductive function."
The study was funded in part by the Swiss National Research Foundation and by the National Center of Competence in Research, which is funded by the Swiss National Science Foundation. Dr. Choong had no disclosures; several of her colleagues disclosed that they have received grants or honoraria from numerous pharmaceutical companies.
Polymorphisms of the CREB-regulated transcription coactivator 1 gene contribute to the genetics of human obesity in psychiatric patients and in the general population, results from a novel study demonstrated.
Specifically, the CRTC1 nonsynonymous polymorphism rs3746266A>G was associated with body mass index in three independent psychiatric samples in which lower BMI values were measured in carriers of the G allele compared with noncarriers, while the protective effect of the T allele of rs6510997C>T (a proxy of rs3746266A>G) against fat accumulation also was observed in a large population-based sample.
"Psychiatric, psychological, sociodemographic, and behavioral factors, as well as heritability, have been shown to influence individual susceptibility to overweight or obesity, both in the general population and in psychiatric patients before and after treatment with potentially weight gain–inducing psychotropic drugs," researchers led by Eva Choong, Pharm.D., Ph.D., reported online Aug. 7 in JAMA Psychiatry. "Genome-wide association studies conducted to date only explain a small fraction of body mass index (BMI) heritability, and more obesity susceptibility genes remain to be discovered."
For the study, which is thought to be the first of its kind, researchers from two university hospitals and a private clinic in Switzerland evaluated the effect of three CRTC1 polymorphisms on BMI and/or fat mass in a cohort of 152 patients taking weight gain–inducing psychotropic drugs (sample 1). The CRTC1 variant that was significantly associated with BMI was then replicated in two independent psychiatric samples, which consisted of 174 patients in sample 2 and 118 patients in sample 3, and in two white population-based samples, which consisted of 5,338 patients in sample 4 and 123,865 patients in sample 5 (JAMA Psychiatry 2013 Aug. 7 [doi: 10.1001/jamapsychiatry.2013.187]).
The researchers found that in the three psychiatric samples, carriers of the CRTC1 rs3746266A>G allele had a lower BMI than did noncarriers (P = .001 in sample 1, P = .05 in sample 2, and P = .0003 in sample 3). In a combined analysis that excluded patients taking other weight gain–inducing drugs, G-allele carriers had a 1.81-kg/m2 lower BMI, compared with noncarriers (P less than .0001). The strongest association was seen in women age 45; in this subset of patients, G-allele carriers had a 3.87-kg/m2 lower BMI, compared with noncarriers (P less than .0001).
In the analysis of population-based samples, the T allele of rs651099C>T, which is a proxy of the G allele, was associated with lower BMI (P = .01 in sample 5) and fat mass (P = .03 in sample 4).
The investigators acknowledged several limitations of their study. For example, most patients were not drug naive and had already developed weight gain because of previous treatments. "It was therefore not possible to determine with certainty whether the strong association of CRTC1 genotypes with BMI and fat mass in psychiatric populations was due to the psychiatric illness and/or to the pharmacological treatment," they wrote. "Extensive hormonal measurements were not available for our samples, so the role of sex hormones on the association of CRTC1 variants with adiposity could not be explored."
In addition, the racial and ethnic makeup of the patients sampled means that the results are not generalizable.
Despite those limitations, Dr. Choong expressed optimism about where these results might lead. "Our results suggest that CRTC1 plays an important role in the high prevalence of overweight and obesity observed in psychiatric patients," she and her colleagues concluded. "Besides, CRTC1 could play a role in the genetics of obesity in the general population, thereby increasing our understanding of the multiple mechanisms influencing obesity.
"Finally, the strong associations of CRTC1 variants with adiposity in women younger than 45 years support further research on the interrelationship between adiposity and the reproductive function."
The study was funded in part by the Swiss National Research Foundation and by the National Center of Competence in Research, which is funded by the Swiss National Science Foundation. Dr. Choong had no disclosures; several of her colleagues disclosed that they have received grants or honoraria from numerous pharmaceutical companies.
Polymorphisms of the CREB-regulated transcription coactivator 1 gene contribute to the genetics of human obesity in psychiatric patients and in the general population, results from a novel study demonstrated.
Specifically, the CRTC1 nonsynonymous polymorphism rs3746266A>G was associated with body mass index in three independent psychiatric samples in which lower BMI values were measured in carriers of the G allele compared with noncarriers, while the protective effect of the T allele of rs6510997C>T (a proxy of rs3746266A>G) against fat accumulation also was observed in a large population-based sample.
"Psychiatric, psychological, sociodemographic, and behavioral factors, as well as heritability, have been shown to influence individual susceptibility to overweight or obesity, both in the general population and in psychiatric patients before and after treatment with potentially weight gain–inducing psychotropic drugs," researchers led by Eva Choong, Pharm.D., Ph.D., reported online Aug. 7 in JAMA Psychiatry. "Genome-wide association studies conducted to date only explain a small fraction of body mass index (BMI) heritability, and more obesity susceptibility genes remain to be discovered."
For the study, which is thought to be the first of its kind, researchers from two university hospitals and a private clinic in Switzerland evaluated the effect of three CRTC1 polymorphisms on BMI and/or fat mass in a cohort of 152 patients taking weight gain–inducing psychotropic drugs (sample 1). The CRTC1 variant that was significantly associated with BMI was then replicated in two independent psychiatric samples, which consisted of 174 patients in sample 2 and 118 patients in sample 3, and in two white population-based samples, which consisted of 5,338 patients in sample 4 and 123,865 patients in sample 5 (JAMA Psychiatry 2013 Aug. 7 [doi: 10.1001/jamapsychiatry.2013.187]).
The researchers found that in the three psychiatric samples, carriers of the CRTC1 rs3746266A>G allele had a lower BMI than did noncarriers (P = .001 in sample 1, P = .05 in sample 2, and P = .0003 in sample 3). In a combined analysis that excluded patients taking other weight gain–inducing drugs, G-allele carriers had a 1.81-kg/m2 lower BMI, compared with noncarriers (P less than .0001). The strongest association was seen in women age 45; in this subset of patients, G-allele carriers had a 3.87-kg/m2 lower BMI, compared with noncarriers (P less than .0001).
In the analysis of population-based samples, the T allele of rs651099C>T, which is a proxy of the G allele, was associated with lower BMI (P = .01 in sample 5) and fat mass (P = .03 in sample 4).
The investigators acknowledged several limitations of their study. For example, most patients were not drug naive and had already developed weight gain because of previous treatments. "It was therefore not possible to determine with certainty whether the strong association of CRTC1 genotypes with BMI and fat mass in psychiatric populations was due to the psychiatric illness and/or to the pharmacological treatment," they wrote. "Extensive hormonal measurements were not available for our samples, so the role of sex hormones on the association of CRTC1 variants with adiposity could not be explored."
In addition, the racial and ethnic makeup of the patients sampled means that the results are not generalizable.
Despite those limitations, Dr. Choong expressed optimism about where these results might lead. "Our results suggest that CRTC1 plays an important role in the high prevalence of overweight and obesity observed in psychiatric patients," she and her colleagues concluded. "Besides, CRTC1 could play a role in the genetics of obesity in the general population, thereby increasing our understanding of the multiple mechanisms influencing obesity.
"Finally, the strong associations of CRTC1 variants with adiposity in women younger than 45 years support further research on the interrelationship between adiposity and the reproductive function."
The study was funded in part by the Swiss National Research Foundation and by the National Center of Competence in Research, which is funded by the Swiss National Science Foundation. Dr. Choong had no disclosures; several of her colleagues disclosed that they have received grants or honoraria from numerous pharmaceutical companies.
FROM JAMA PSYCHIATRY
Major finding: In three samples of psychiatric patients, carriers of the CREB-regulated transcription coactivator 1 gene polymorphism rs3746266A>G allele had a lower BMI than did noncarriers (P = .001 in sample 1, P = .05 in sample 2, and P = .0003 in sample 3). In an analysis of two separate population-based samples, the T allele of rs651099C>T, which is a proxy of the G allele, was associated with lower BMI (P = .01 in sample 5) and fat mass (P = .03 in sample 4).
Data source: An analysis of five different samples of patients from two university hospitals and a private clinic in Switzerland.
Disclosures: The study was funded in part by the Swiss National Research Foundation and by the National Center of Competence in Research, which is funded by the Swiss National Science Foundation. Dr. Choong had no disclosures; several of her colleagues disclosed that they have received grants or honoraria from numerous pharmaceutical companies.
No laughing matter: Laughter is good psychiatric medicine
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
CASE REPORT: Laughter as therapy
Mrs. A is a 56-year-old married woman who has bipolar disorder. She has survived several suicide attempts. Her family history is positive for bipolar disorder and completed suicides.
After her most recent suicide attempt and a course of electroconvulsive therapy, Mrs. A recovered sufficiently to begin a spiritual journey that led her to practice a technique known as Laughter Yoga (Box) and, eventually, to become a Laughter Yoga instructor.
Mrs. A begins Laughter Yoga sessions by talking openly with students about her illness and the beneficial effects that laughter therapy has had on its course: She once had at least two major bipolar episodes a year, she explains, but has been in full remission for several years despite severe psychosocial stressors. In addition to practicing Laughter Yoga, Mrs. A is now maintained on a mood stabilizer that failed in the past to control her mood cycles.
Does laughter have a place in your practice?
It is said that laughter is good medicine—but is it good psychiatric medicine? Where might humor and laughter fit in the psychiatrist’s armamentarium? Is laughter physiologically beneficial to psychiatric patients? And are there adverse effects or contraindications to laughter in psychiatry? This article:
• reviews studies that have examined the anatomy, physiology, and psychology of humor and laughtera
• offers answers to the questions posed above (Table).
“Gelotology,” from the Greek “gelos,” laughter, is the science of laughter. The three components of humor and laughter are:
• the emotional component, which triggers emotions produced by a humorous situation
• the cognitive component, in which a person “gets it”
• the movement of facial, respiratory, and abdominal muscles.
Furthermore, tension and surprise are needed for laughter.
Theories about humor are varied
Philosophers since Plato have proposed theories of humor; modern theories of humor can be traced to Freud’s work.1 The psychoanalytic literature on humor focuses on the role of humor in sublimation of feelings of anger and hostility, while releasing affect in an economical way.
Erikson also wrote about the role of humor in a child’s developing superego, which helps resolve the conflict with maternal authority.2
In a comprehensive review of theories of humor, Krichtafovitch explains that cognitive theories address the role of incongruity and contrast in the induction of laughter, whereas social theories explore the roles of aggression, hostility, superiority, triumph, derision, and disparagement in humor and laughter. The effect of humor, Krichtafovitch explains, is to elevate the social status of the joker while the listener’s social status is lifted through his (her) ability to “get it.” Thus, humor plays a meaningful role in creating a bond between speaker and listener.3
The neuroanatomy of laughter
Here is some of what we have learned about mapping the brain to the basis of laughter:
• Consider a 16-year-old girl who underwent neurosurgery for intractable seizures. During surgery, various parts of the brain were stimulated to test for the focus of the seizures. She laughed every time the left frontal superior gyrus was stimulated. According to the report, she apparently laughed first, then made up a story that was funny to her.4
• Pseudobulbar affect—excessive, usually incongruent laughter, secondary to neurologic disease or traumatic brain injury—is an example of the biologic basis of laughter.
• Many functional brain imaging studies of laughter have been published.5 These studies show involvement of various regions of the brain in laughter, including the amygdala, hypothalamus, and temporal and cerebellar regions.
• Sex differences also have been noted in the neuroanatomy of laughter. Females activate the left prefrontal cortex more than males do, suggesting a greater degree of executive processing and language-based decoding. Females also exhibit greater activation of mesolimbic regions, including the nucleus accumbens, implying a greater reward network response.6
• Wild et al7 reported that separate cortical regions are responsible for the production of facial expressions that are emotionally driven (through laughter) and voluntary.
The physiology of laughter
Humans begin to laugh at approximately 4 months of age. Children laugh, on average, 400 times a day; adults do so an average of only 5 times a day.8 In addition:
• Tickling a baby induces her (him) to laugh, which, in turn, makes the parent laugh; a social bond develops during this playful exercise. This response is probably mediated by 5-HT1A receptors, which, when stimulated, induces the release of oxytocin, which facilitates social bonding.9
• Potent stimulation of 5-HT1A receptors through ingestion of 3,4-methylenedioxy-N-methylamphetamine (Ecstasy) leads to uncontrollable laughter and mirth.10
• Lower species are also known to enjoy humor. Mice emit a chirping sound when tickled, and laughter is contagious among monkeys.11
• Berk et al12,13 reported that, when 52 healthy men watched a funny video for 30 minutes, they had significantly higher activity of natural killer (NK) cells and higher levels of IgG, IgA, and IgM compared with men who watched an emotionally neutral documentary.
• Bennett et al14 showed that, in 33 healthy women, the harder the laughter, the higher the NK activity.
• Sugawara et al15 showed improved cardiovascular function in 17 healthy persons (age 23 to 42) who watched a 30-minute comedy video, compared with their cardiovascular function when they watched a documentary video of equal length.
• Svebak et al16 examined the effect of humor as measured by the Sense of Humor Survey on the survival rate of more then 53,000 adults in one county in Norway. They concluded that the higher the sense of humor score, the higher the odds ratio of surviving 7 years, compared with subjects who had a lower sense of humor.
Clinical studies of laughter
The Coping Humor Scale (CHS) and the Humor Response Scale (HRS) are the two most widely used tools to measure a person’s innate sense of humor (the CHS) and the ability to respond to a humorous situation (the HRS).17 Several studies about the effects of laughter on illness are notable:
• Laughter increased NK cell activity, lowered prorenin gene expression, and lowered the postprandial glucose level in 34 patients with diabetes, compared with 16 matched controls.18-21
• Clark et al studied the sense of humor of 150 patients with cardiac disease compared with 150 controls. They found that “people with heart disease responded less humorously to everyday life situations.” They generally laughed less, even in positive situations, and displayed more anger and hostility.22
• In his work on the salutatory effect of laughter on the experience of pain, Cousins described how he dealt with his painful arthritis by watching Marx Brothers movies23:
I made the joyous discovery that 10 minutes of genuine belly laughter had an anesthetic effect and would give me at least two hours of pain-free sleep… When the pain-killing effect of the laughter wore off, we would switch on the motion picture projector again and not infrequently, it would lead to another pain-free interval.
• Hearty laughter leads to pain relief, probably through the release of endorphins. Dunbar et al24 tested this hypothesis in a series of six experimental studies in the laboratory (watching videos) and in a naturalistic context (watching stage performances), using a change in pain threshold as an indirect measure of endorphin release. The results show that the pain threshold is significantly higher after laughter than in the control condition. This pain-tolerance effect is caused by the laughter itself, not simply because of a change in positive affect.
Laughter therapy for depression
Three studies have demonstrated the benefit of laughter therapy in depression:
• When Ko and Youn25 studied 48 geriatric depressed patients and 61 age-matched controls, they found a significantly lower Geriatric Depression Scale score and a better Pittsburgh Sleep Quality Index score in patients who had been exposed to four weekly laughter groups, compared with persons who had been exposed to a control group.
• Shahidi et al26 randomly assigned 60 community-dwelling female, geriatric, depressed patients to a laughter yoga group, an exercise group, and a control group. Laughter yoga and exercise were equally effective, and both were significantly superior to the control condition. The laughter yoga group scored significantly better than the other two groups on the Life Satisfaction Scale. The researchers concluded that, in addition to improved mood, patients who laugh experience increased life satisfaction.
• Fonzi et al27 summarized data on the neurophysiology of laughter and the effect of laughter on the hypothalamus-pituitary-adrenal axis. They noted that depression reduces the frequency of laughter and, inversely, laughter reduces the severity of depression. Laughter, they reported, also increases the connectivity of patients with people in their life, which further alleviates symptoms of depression.
Other therapeutic uses of laughter
Humor can strengthen the bond of the therapeutic relationship. Patients who laugh with their physicians are more likely to feel connected with them, follow their advice, and feel more satisfied with their encounter. One study found that primary care physicians who gave positive statements, spent more time with patients, and included humor or laughter during their visits lowered their risk of being sued for malpractice.28
Consider also the use of laughter in altering family dynamics in a therapeutic setting: Mr. and Mrs. B attend therapy in my practice to address a difficult situation with their adult children. One of them enables their children socially and financially; the other continually complains about this enabling. When the tension was high and the couple had reached an impasse during a visit, the therapist offered an anecdote from the 2006 motion picture Failure to Launch (in which a man lives in the security of his parents’ home even though he is in his 30s), that dissipated the hostility they had shown toward each other and toward their children. The couple was then able to proceed to conflict resolution.
Recommendations, caveats
If you are considering incorporating laughter into therapy, keep in mind that:
• you should ensure that the patient does not perceive humor as minimizing the seriousness of their problems
• humor can be a minefield if not used judiciously, or if used at all, around certain sensitive topics, such as race, ethnicity, religion, political affiliation, and sexual orientation
• the timing of humor is particularly essential for it to succeed in the context of a therapeutic relationship
• from a medical perspective, laughter in patients who are recovering from abdominal or other major surgery might compromise wound healing because of increased intra-abdominal pressure associated with laughing
• patients who have asthma, especially exercise-induced asthma, might be at risk of developing an acute asthmatic attack when they laugh very hard. Lebowitz et al29 demonstrated that laughter can have a negative effect on patients with chronic obstructive pulmonary disease.
It is advisable in some situations to avoid humor in psychotherapy, such as when the patient or family is hostile—because, as noted, they might perceive laughter and humor as an attempt to minimize the seriousness of their discontent.
Bottom Line
Humor and laughter are underutilized and underreported in therapy, in part because it is a nascent field of research. Laughter has social and physiologic benefits that can be used in the context of a therapeutic relationship to help patients with a variety of ailments, including depression, anxiety, and pain.
Related Resources
- Association for Applied and Therapeutic Humor. www.aath.org.
- Mora-Ripoll R. The therapeutic value of laughter in medicine. Altern Ther Health Med. 2010;16:56-64.
- Strean WB. Laughter prescription. Can Fam Physician. 2009;55:965-967.
Disclosure
Dr. Nasr reports no financial relationship with manufacturers of any products mentioned in this article or with manufacturers of competing products.
Acknowledgements
The author acknowledges the assistance of Francois E. Alouf, MD, for suggestions on topics to include in the article; John W. Crayton, MD, for reviewing the manuscript; and Burdette Wendt for assistance with the references.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
1. Freud S, Strachey J, trans., ed. Jokes and their relation to the unconscious. New York, NY: W. W. Norton & Company; 1990.
2. Capps D. Mother, melancholia, and humor in Erik H. Erikson’s earliest writings. J Relig Health. 2008;47:415-432.
3. Krichtafovitch I. Humor theory. Parker, CO: Outskirts Press; 2006.
4. Fried I, Wilson CL, MacDonald KA, et al. Electric current stimulates laughter. Nature. 1998;12;391:650.
5. Bartolo A, Benuzzi F, Nocetti L, et al. Humor comprehension and appreciation: an FMRI study. J Cogn Neurosci. 2006;18:1789-1798.
6. Azim E, Mobbs D, Jo B, et al. Sex differences in brain activation elicited by humor. Proc Natl Acad Sci U S A. 2005;102:16496-16501.
7. Wild B, Rodden FA, Rapp A, et al. Humor and smiling: cortical regions selective for cognitive, affective, and volitional components. Neurology. 2006;66:887-893.
8. Freedman LW. Mosby’s complementary and alternative medicine. A research-based approach. St. Louis, MO: Mosby; 2004:24.
9. Lukas M, Toth I, Reber SO, et al. The neuropeptide oxytocin facilitates pro-social behavior and prevents social avoidance in rats and mice. Neuropsychopharmacology. 2011;36:
2159-2168.
10. Thompson MR, Callaghan PD, Hunt GE, et al. A role for oxytocin and 5-HT(1A) receptors in the prosocial effects of 3,4 methylenedioxymethamphetamine (“ecstasy”). Neuroscience. 2007;146:509-514.
11. Ross MD, Owren MJ, Zimmermann E. The evolution of laughter in great apes and humans. Commun Integr Biol. 2010;3(2):191-194.
12. Berk LS, Tan SA, Fry WF, et al. Neuroendocrine and stress hormone changes during mirthful laughter. Am J Med Sci. 1989;298:390-396.
13. Berk LS, Felten DL, Tan SA, et al. Modulation of neuroimmune parameters during the eustress of humor-associated mirthful laughter. Altern Ther Health Med. 2001; 7:62-72,74-76.
14. Bennett MP, Zeller JM, Rosenberg L, et al. The effect of mirthful laughter on stress and natural killer cell activity. Altern Ther Health Med. 2003;9:38-45.
15. Sugawara J, Tarumi T, Tanaka H. Effect of mirthful laughter on vascular function. Am J Cardiol. 2010;106:856-859.
16. Svebak S, Romundstad S, Holmen J. A 7-year prospective study of sense of humor and mortality in an adult county population: the HUNT-2 study. Int J Psychiatry Med. 2010;40:125-146.
17. Martin RA. The Situational Humor Response Questionnaire (SHRQ) and Coping Humor Scale (CHS): a decade of research findings. Humor: International Journal of Humor Research. 1996;9(3-4):251-272.
18. Hayashi T, Urayama O, Hori M, et al. Laughter modulates prorenin receptor gene expression in patients with type 2 diabetes. J Psychosom Res. 2007;62:703-706.
19. Hayashi T, Murakami K. The effects of laughter on post-prandial glucose levels and gene expression in type 2 diabetic patients. Life Sci. 2009;85:185-187.
20. Takahashi K, Iwase M, Yamashita K, et al. The elevation of natural killer cell activity induced by laughter in a crossover designed study. Int J Mol Med. 2001;8:645-650.
21. Nasir UM, Iwanaga S, Nabi AH, et al. Laughter therapy modulates the parameters of renin-angiotensin system in patients with type 2 diabetes. Int J Mol Med. 2005;16:1077-1081.
22. Clark A, Seidler A, Miller M. Inverse association between sense of humor and coronary heart disease. Int J Cardiol. 2001;80:87-88.
23. Cousins N. The anatomy of an illness as perceived by the patient: reflections on healing and regeneration. New York, NY: Norton; 1979:39.
24. Dunbar RI, Baron R, Frangou A, et al. Social laughter is correlated with an elevated pain threshold. Proc Biol Sci. 2012;279(1731):1161-1167.
25. Ko HJ, Youn CH. Effects of laughter therapy on depression, cognition and sleep among the community-dwelling elderly. Geriatr Gerontol Int. 2011;11:267-274.
26. Shahidi M, Mojtahed A, Modabbernia A, et al. Laughter yoga versus group exercise program in elderly depressed women: a randomized controlled trial. Int J Geriatr Psychiatry. 2011;26:322-327.
27. Fonzi L, Matteucci G, Bersani G. Laughter and depression: hypothesis of pathogenic and therapeutic correlation. Riv Psichiatr. 2010;45:1-6.
28. Levinson W, Roter DL, Mullooly JP, et al. Physician-patient communication: the relationship with malpractice claims among primary care physicians and surgeons. JAMA. 1997;277:553-559.
29. Lebowitz KR, Suh S, Diaz PT, et al. Effects of humor and laughter on psychological functioning, quality of life, health status, and pulmonary functioning among patients with chronic obstructive pulmonary disease: a preliminary investigation. Heart Lung. 2011;40:310-319.
Mixed state bipolar episodes reconceptualized
A new study has proposed a more nuanced approach for the diagnosis and treatment of mixed episodes in bipolar I patients.
The study, conducted by Dr. Isabella Pacchiarotti and her colleagues implemented a "factor structure" approach toward acute mood episodes, with the goal of deconstructing mixed episodes in particular.
Researchers studied 187 bipolar I patients hospitalized for an acute episode – manic, depressive, or mixed – and diagnosed using DSM-IV-TR criteria. Patients were evaluated for manic, mixed, and depressive symptoms using the Brief Psychiatric Rating Scale (BPRS 4.0), the Hamilton Depression Rating Scale (HDRS-21), and the Young Mania Rating Scale (YMRS). Patients also completed a self-reported temperament evaluation after discharge, reported Dr. Pacchiarotti of the Bipolar Disorders Program at the Institute of Clinical Neuroscience, University of Barcelona.
A principal component factor analysis performed on the BPRS found five clinically relevant factors: psychosis (factor 1), euphoric mania (factor 2), "mixity" (factor 3), dysphoria (factor 4), and inhibited depression (factor 5).
Psychosis was characterized by positive loading for bizarre behavior, unusual thought content, hallucinations, disorientation, conceptual disorganization, mannerisms and posturing, distractibility, and self-neglect. Euphoric mania was characterized by elevated mood and grandiosity, with negative loading for depression, guilt, suicidality, somatic concern, tension, and anxiety. "Mixity" was defined by suicidality, excitement, motor hyperactivity, tension, and anxiety, with negative loading for motor retardation. Dysphoria had positive loading for hostility, uncooperativeness, and suspiciousness. Lastly, inhibited depression was defined by depression, guilt, motor retardation, emotional withdrawal, and blunted affect.
The investigators also performed an analysis to determine which factors were associated with depressive, manic, and mixed mood. No association was found between depressive episodes and any of the five factors. However, manic episodes were a predictor of psychosis and euphoric mania (factors 1 and 2), and mixed episodes were associated with mixity (factor 3). None of the episode types were predictors of dysphoria or inhibited depression (factors 3 and 4).
An important result of this study is the discovery of the mixity factor, which provides a more comprehensive profile of mixed episodes than does the conventional combination of manic and depressive symptoms. "Most mixed state scholars feel DSM-IV criteria for mixed states to be too restrictive," the authors wrote in their report. This study endorses the existence of two subtypes of mixed episodes: one defined by factor 3 and characterized by an anxious-agitated dimension, and the other defined by factor 4, characterized by irritability and dysphoria.
In addition, the authors provided recommendations for diagnostic improvements based on the findings of this paper. First, the DSM-IV excludes anxiety and includes psychomotor retardation in the diagnosis of mixed states, though the current research has found at least one group of bipolar I patients who presented with anxiety and did not have psychomotor retardation. The researchers suggested expanding the current diagnostic criteria to include this subset of bipolar patients.
The authors listed a few limitations to this study. First, the sample size was rather small. Second, this study analyzed only bipolar I patients, and did not assess individuals who were bipolar hypomanic or depressed with subsyndromal symptoms of opposite polarity. Third, this study did not evaluate patients over all phases of illness, only when they were admitted to the hospital. Fourth, most patients in this study were on medication when they were hospitalized, which might have affected results, particularly in factor 3. Lastly, further research is needed to generate more psychometric measures of mixed states, as few are currently available.
The authors of this study expressed hope that these new insights into the factor structure of bipolar episodes will result in improved diagnostic and treatment options in the future.
The study was funded by the several entities, including the Spanish Ministry of Economy and Competitiveness, and the Instituto de Salud Carlos III, Madrid.
A new study has proposed a more nuanced approach for the diagnosis and treatment of mixed episodes in bipolar I patients.
The study, conducted by Dr. Isabella Pacchiarotti and her colleagues implemented a "factor structure" approach toward acute mood episodes, with the goal of deconstructing mixed episodes in particular.
Researchers studied 187 bipolar I patients hospitalized for an acute episode – manic, depressive, or mixed – and diagnosed using DSM-IV-TR criteria. Patients were evaluated for manic, mixed, and depressive symptoms using the Brief Psychiatric Rating Scale (BPRS 4.0), the Hamilton Depression Rating Scale (HDRS-21), and the Young Mania Rating Scale (YMRS). Patients also completed a self-reported temperament evaluation after discharge, reported Dr. Pacchiarotti of the Bipolar Disorders Program at the Institute of Clinical Neuroscience, University of Barcelona.
A principal component factor analysis performed on the BPRS found five clinically relevant factors: psychosis (factor 1), euphoric mania (factor 2), "mixity" (factor 3), dysphoria (factor 4), and inhibited depression (factor 5).
Psychosis was characterized by positive loading for bizarre behavior, unusual thought content, hallucinations, disorientation, conceptual disorganization, mannerisms and posturing, distractibility, and self-neglect. Euphoric mania was characterized by elevated mood and grandiosity, with negative loading for depression, guilt, suicidality, somatic concern, tension, and anxiety. "Mixity" was defined by suicidality, excitement, motor hyperactivity, tension, and anxiety, with negative loading for motor retardation. Dysphoria had positive loading for hostility, uncooperativeness, and suspiciousness. Lastly, inhibited depression was defined by depression, guilt, motor retardation, emotional withdrawal, and blunted affect.
The investigators also performed an analysis to determine which factors were associated with depressive, manic, and mixed mood. No association was found between depressive episodes and any of the five factors. However, manic episodes were a predictor of psychosis and euphoric mania (factors 1 and 2), and mixed episodes were associated with mixity (factor 3). None of the episode types were predictors of dysphoria or inhibited depression (factors 3 and 4).
An important result of this study is the discovery of the mixity factor, which provides a more comprehensive profile of mixed episodes than does the conventional combination of manic and depressive symptoms. "Most mixed state scholars feel DSM-IV criteria for mixed states to be too restrictive," the authors wrote in their report. This study endorses the existence of two subtypes of mixed episodes: one defined by factor 3 and characterized by an anxious-agitated dimension, and the other defined by factor 4, characterized by irritability and dysphoria.
In addition, the authors provided recommendations for diagnostic improvements based on the findings of this paper. First, the DSM-IV excludes anxiety and includes psychomotor retardation in the diagnosis of mixed states, though the current research has found at least one group of bipolar I patients who presented with anxiety and did not have psychomotor retardation. The researchers suggested expanding the current diagnostic criteria to include this subset of bipolar patients.
The authors listed a few limitations to this study. First, the sample size was rather small. Second, this study analyzed only bipolar I patients, and did not assess individuals who were bipolar hypomanic or depressed with subsyndromal symptoms of opposite polarity. Third, this study did not evaluate patients over all phases of illness, only when they were admitted to the hospital. Fourth, most patients in this study were on medication when they were hospitalized, which might have affected results, particularly in factor 3. Lastly, further research is needed to generate more psychometric measures of mixed states, as few are currently available.
The authors of this study expressed hope that these new insights into the factor structure of bipolar episodes will result in improved diagnostic and treatment options in the future.
The study was funded by the several entities, including the Spanish Ministry of Economy and Competitiveness, and the Instituto de Salud Carlos III, Madrid.
A new study has proposed a more nuanced approach for the diagnosis and treatment of mixed episodes in bipolar I patients.
The study, conducted by Dr. Isabella Pacchiarotti and her colleagues implemented a "factor structure" approach toward acute mood episodes, with the goal of deconstructing mixed episodes in particular.
Researchers studied 187 bipolar I patients hospitalized for an acute episode – manic, depressive, or mixed – and diagnosed using DSM-IV-TR criteria. Patients were evaluated for manic, mixed, and depressive symptoms using the Brief Psychiatric Rating Scale (BPRS 4.0), the Hamilton Depression Rating Scale (HDRS-21), and the Young Mania Rating Scale (YMRS). Patients also completed a self-reported temperament evaluation after discharge, reported Dr. Pacchiarotti of the Bipolar Disorders Program at the Institute of Clinical Neuroscience, University of Barcelona.
A principal component factor analysis performed on the BPRS found five clinically relevant factors: psychosis (factor 1), euphoric mania (factor 2), "mixity" (factor 3), dysphoria (factor 4), and inhibited depression (factor 5).
Psychosis was characterized by positive loading for bizarre behavior, unusual thought content, hallucinations, disorientation, conceptual disorganization, mannerisms and posturing, distractibility, and self-neglect. Euphoric mania was characterized by elevated mood and grandiosity, with negative loading for depression, guilt, suicidality, somatic concern, tension, and anxiety. "Mixity" was defined by suicidality, excitement, motor hyperactivity, tension, and anxiety, with negative loading for motor retardation. Dysphoria had positive loading for hostility, uncooperativeness, and suspiciousness. Lastly, inhibited depression was defined by depression, guilt, motor retardation, emotional withdrawal, and blunted affect.
The investigators also performed an analysis to determine which factors were associated with depressive, manic, and mixed mood. No association was found between depressive episodes and any of the five factors. However, manic episodes were a predictor of psychosis and euphoric mania (factors 1 and 2), and mixed episodes were associated with mixity (factor 3). None of the episode types were predictors of dysphoria or inhibited depression (factors 3 and 4).
An important result of this study is the discovery of the mixity factor, which provides a more comprehensive profile of mixed episodes than does the conventional combination of manic and depressive symptoms. "Most mixed state scholars feel DSM-IV criteria for mixed states to be too restrictive," the authors wrote in their report. This study endorses the existence of two subtypes of mixed episodes: one defined by factor 3 and characterized by an anxious-agitated dimension, and the other defined by factor 4, characterized by irritability and dysphoria.
In addition, the authors provided recommendations for diagnostic improvements based on the findings of this paper. First, the DSM-IV excludes anxiety and includes psychomotor retardation in the diagnosis of mixed states, though the current research has found at least one group of bipolar I patients who presented with anxiety and did not have psychomotor retardation. The researchers suggested expanding the current diagnostic criteria to include this subset of bipolar patients.
The authors listed a few limitations to this study. First, the sample size was rather small. Second, this study analyzed only bipolar I patients, and did not assess individuals who were bipolar hypomanic or depressed with subsyndromal symptoms of opposite polarity. Third, this study did not evaluate patients over all phases of illness, only when they were admitted to the hospital. Fourth, most patients in this study were on medication when they were hospitalized, which might have affected results, particularly in factor 3. Lastly, further research is needed to generate more psychometric measures of mixed states, as few are currently available.
The authors of this study expressed hope that these new insights into the factor structure of bipolar episodes will result in improved diagnostic and treatment options in the future.
The study was funded by the several entities, including the Spanish Ministry of Economy and Competitiveness, and the Instituto de Salud Carlos III, Madrid.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Major finding: This study uncovered five factors of bipolar episodes: psychosis, euphoric mania, mixity, dysphoria, and inhibited depression.
Data source: An assessment of 187 DSM-IV-TR-diagnosed bipolar I patients using the Brief Psychiatric Rating Scale (BPRS 4.0)
Disclosures: The study was funded by several entities, including the Spanish Ministry of Economy and Competitiveness, and the Instituto de Salud Carlos III, Madrid.
Depression more troublesome than mania for youth with bipolar disorder
Among youths with bipolar spectrum disorder, depressive symptoms more adversely affected their psychological functioning and quality of life than did manic symptoms, results from a small study showed.
"We hypothesized that the impact of bipolar depression in youth would be significant, but the lopsided nature of the results was more striking than expected," researchers led by Anna R. Van Meter reported online in the Journal of Affective Disorders. "Across numerous measures, depression was a significant predictor of negative outcomes, mania was not.
"This is not to say that mania is not impairing, our sample included only youth with bipolar disorder, so we cannot comment on the degree to which mania and/or depression caused problems for youth with bipolar disorder, relative to youth without mood disturbance. Still, at the very least, these findings suggest that the collective focus on mania, often it seems at the exclusion of depression, may be misguided."
For the study, Ms. Van Meter and her associates recruited 54 youths aged 7-13 years old who met DSM-IV-TR criteria for bipolar spectrum disorders from a clinic in a large Midwestern city (J. Affect. Dis. 2013 June 12 [doi:10.1016/j.jad.2013.05.039]).
They used regression analyses to evaluate clinician and parent reports of manic and depressive symptoms from numerous survey instruments in an effort to determine how each set of symptoms affected child functioning. Measures included the Washington University Schedule for Affective Disorders, the Children’s Global Assessment Scale, the Young Mania Rating Scale, and the Child Bipolar Depression Rating Scale.
The mean age of the 54 study participants was 9 years, 42% were female, and more than half (60%) were of white European descent. Diagnoses included bipolar disorder not otherwise specified (57%), bipolar I disorder (41%), and bipolar II disorder (2%).
Parent-rated child depression symptoms were associated with problem behaviors (P less than .05) and lower quality of life (P less than .001), while clinician-rated child depression was associated with greater psychiatric illness (P less than .05), lower child self-concept (P less than .001), lower quality of life (P less than .05), hopelessness (P less than .05), and suicidal ideation (P less than .05), reported Ms. Van Meter of the department of psychology at the University of North Carolina, Chapel Hill, and her associates.
At the same time, parent-rated mania was associated with better self-esteem (P less than 0.05) and physical well-being (P less than .05), while clinician-rated mania was associated with greater psychiatric illness (P less than .05) and physical well-being (P less than .05).
"Which specific aspects of bipolar depression cause decreased quality of life and through what mechanisms mania provides protection against functional impairment remain important areas for study," the researchers concluded. "In general, the identification of how different symptom constellations in pediatric bipolar disorder relate to functional impairment should be an important mission for our research agenda. Thus, this study introduces new questions to guide researchers in developing a more in-depth understanding of this complex disorder and raises awareness of the debilitating effects of bipolar depression in youth."
They acknowledged certain limitations of the study. One is that specific outcomes predicted by parent- and clinician-rated symptoms vary.
The study was supported by the National Institute of Mental Health. The researchers stated that they had no relevant financial conflicts to disclose.
Among youths with bipolar spectrum disorder, depressive symptoms more adversely affected their psychological functioning and quality of life than did manic symptoms, results from a small study showed.
"We hypothesized that the impact of bipolar depression in youth would be significant, but the lopsided nature of the results was more striking than expected," researchers led by Anna R. Van Meter reported online in the Journal of Affective Disorders. "Across numerous measures, depression was a significant predictor of negative outcomes, mania was not.
"This is not to say that mania is not impairing, our sample included only youth with bipolar disorder, so we cannot comment on the degree to which mania and/or depression caused problems for youth with bipolar disorder, relative to youth without mood disturbance. Still, at the very least, these findings suggest that the collective focus on mania, often it seems at the exclusion of depression, may be misguided."
For the study, Ms. Van Meter and her associates recruited 54 youths aged 7-13 years old who met DSM-IV-TR criteria for bipolar spectrum disorders from a clinic in a large Midwestern city (J. Affect. Dis. 2013 June 12 [doi:10.1016/j.jad.2013.05.039]).
They used regression analyses to evaluate clinician and parent reports of manic and depressive symptoms from numerous survey instruments in an effort to determine how each set of symptoms affected child functioning. Measures included the Washington University Schedule for Affective Disorders, the Children’s Global Assessment Scale, the Young Mania Rating Scale, and the Child Bipolar Depression Rating Scale.
The mean age of the 54 study participants was 9 years, 42% were female, and more than half (60%) were of white European descent. Diagnoses included bipolar disorder not otherwise specified (57%), bipolar I disorder (41%), and bipolar II disorder (2%).
Parent-rated child depression symptoms were associated with problem behaviors (P less than .05) and lower quality of life (P less than .001), while clinician-rated child depression was associated with greater psychiatric illness (P less than .05), lower child self-concept (P less than .001), lower quality of life (P less than .05), hopelessness (P less than .05), and suicidal ideation (P less than .05), reported Ms. Van Meter of the department of psychology at the University of North Carolina, Chapel Hill, and her associates.
At the same time, parent-rated mania was associated with better self-esteem (P less than 0.05) and physical well-being (P less than .05), while clinician-rated mania was associated with greater psychiatric illness (P less than .05) and physical well-being (P less than .05).
"Which specific aspects of bipolar depression cause decreased quality of life and through what mechanisms mania provides protection against functional impairment remain important areas for study," the researchers concluded. "In general, the identification of how different symptom constellations in pediatric bipolar disorder relate to functional impairment should be an important mission for our research agenda. Thus, this study introduces new questions to guide researchers in developing a more in-depth understanding of this complex disorder and raises awareness of the debilitating effects of bipolar depression in youth."
They acknowledged certain limitations of the study. One is that specific outcomes predicted by parent- and clinician-rated symptoms vary.
The study was supported by the National Institute of Mental Health. The researchers stated that they had no relevant financial conflicts to disclose.
Among youths with bipolar spectrum disorder, depressive symptoms more adversely affected their psychological functioning and quality of life than did manic symptoms, results from a small study showed.
"We hypothesized that the impact of bipolar depression in youth would be significant, but the lopsided nature of the results was more striking than expected," researchers led by Anna R. Van Meter reported online in the Journal of Affective Disorders. "Across numerous measures, depression was a significant predictor of negative outcomes, mania was not.
"This is not to say that mania is not impairing, our sample included only youth with bipolar disorder, so we cannot comment on the degree to which mania and/or depression caused problems for youth with bipolar disorder, relative to youth without mood disturbance. Still, at the very least, these findings suggest that the collective focus on mania, often it seems at the exclusion of depression, may be misguided."
For the study, Ms. Van Meter and her associates recruited 54 youths aged 7-13 years old who met DSM-IV-TR criteria for bipolar spectrum disorders from a clinic in a large Midwestern city (J. Affect. Dis. 2013 June 12 [doi:10.1016/j.jad.2013.05.039]).
They used regression analyses to evaluate clinician and parent reports of manic and depressive symptoms from numerous survey instruments in an effort to determine how each set of symptoms affected child functioning. Measures included the Washington University Schedule for Affective Disorders, the Children’s Global Assessment Scale, the Young Mania Rating Scale, and the Child Bipolar Depression Rating Scale.
The mean age of the 54 study participants was 9 years, 42% were female, and more than half (60%) were of white European descent. Diagnoses included bipolar disorder not otherwise specified (57%), bipolar I disorder (41%), and bipolar II disorder (2%).
Parent-rated child depression symptoms were associated with problem behaviors (P less than .05) and lower quality of life (P less than .001), while clinician-rated child depression was associated with greater psychiatric illness (P less than .05), lower child self-concept (P less than .001), lower quality of life (P less than .05), hopelessness (P less than .05), and suicidal ideation (P less than .05), reported Ms. Van Meter of the department of psychology at the University of North Carolina, Chapel Hill, and her associates.
At the same time, parent-rated mania was associated with better self-esteem (P less than 0.05) and physical well-being (P less than .05), while clinician-rated mania was associated with greater psychiatric illness (P less than .05) and physical well-being (P less than .05).
"Which specific aspects of bipolar depression cause decreased quality of life and through what mechanisms mania provides protection against functional impairment remain important areas for study," the researchers concluded. "In general, the identification of how different symptom constellations in pediatric bipolar disorder relate to functional impairment should be an important mission for our research agenda. Thus, this study introduces new questions to guide researchers in developing a more in-depth understanding of this complex disorder and raises awareness of the debilitating effects of bipolar depression in youth."
They acknowledged certain limitations of the study. One is that specific outcomes predicted by parent- and clinician-rated symptoms vary.
The study was supported by the National Institute of Mental Health. The researchers stated that they had no relevant financial conflicts to disclose.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Major finding: Among youth with bipolar disorder, parent-rated child depression symptoms were associated with problem behaviors (P less than .05) and lower quality of life (P less than .001), yet parent-rated mania was associated with better self-esteem and physical well-being (both P less than .05). At the same time, clinician-rated child depression was associated with greater psychiatric illness (P less than .05) and lower child self-concept (P less than 0.001), while clinician-rated mania was associated with greater psychiatric illness and physical well-being (both P less than 0.05).
Data source: A study of 54 youths aged 7-13 years old who met DSM-IV-TR criteria for bipolar spectrum disorders.
Disclosures: The study was supported by the National Institute of Mental Health. The researchers stated that they had no relevant financial conflicts to disclose.
Bipolar disorder strongly tied to premature death
Women and men with bipolar disorder were more likely to die prematurely than were those without bipolar disorder, according to results from a Swedish national cohort study involving nearly 6.6 million adults.
After adjustment for age, marital status, educational level, employment status, and income, all-cause mortality among the 6,618 adults with bipolar disorder in the cohort was increased twofold for both women (adjusted hazard ratio, 2.34) and men (AHR, 2.03), who died an average of 9.0 and 8.5 years earlier, respectively, did than those without bipolar disorder, according to Dr. Casey Crump of Stanford (Calif.) University, and his colleagues.
Those with bipolar disorder died prematurely from various causes, including cardiovascular disease, diabetes, chronic obstructive pulmonary disease (COPD), influenza or pneumonia, unintentional injuries, and suicide. Among women, stroke and cancer (particularly colon cancer) were also among the causes of premature death. Suicide was a particular risk for both women and men, who had 10-fold and 8-fold increases in risk, respectively (AHRs, 10.37 and 8.09), but the life expectancy differences were not fully explained by unnatural deaths, the investigators reported July 17 online in JAMA Psychiatry.
The most significant causes of death were influenza or pneumonia (3.7- and 4.4-fold increased risk for women and men, respectively), diabetes (3.6- and 2.6-fold increased risk, respectively), and COPD (2.9- and 2.6-fold increased risk).
In a separate model, the potential mediating effect of substance use disorders also was evaluated, and the effect was found to be modest, the investigators noted.
The associations between the various conditions and premature death were weakest for chronic diseases in those with a prior diagnosis, compared with those without a prior diagnosis (AHRs, 1.40 vs. 2.38), suggesting that earlier medical diagnosis and treatment might attenuate the increased mortality risk among affected individuals, they said (JAMA Psychiatry 2013 July 17 [doi: 10.1001/jamapsychiatry.2013.1394]).
"More complete provision of primary, preventive medical care among bipolar disorder patients is needed to reduce early mortality in this vulnerable population," they said, noting that multiple underlying mechanisms, including lifestyle factors, pathophysiologic mechanisms, genetic factors, and certain treatments for bipolar disorder, contribute to the disparities.
"The current study found evidence of modestly increased mortality among bipolar disorder patients who used carbamazepine, risperidone, or valproic acid or who solely used olanzapine, whereas users of aripiprazole, quetiapine, or lamotrigine had modestly reduced mortality compared with those who solely used lithium," they said.
However, consistent with prior research, those who used none of these medications had even higher rates of all-cause mortality – and twice the suicide risk – of those who used medication.
Study participants were 3,918 women and 2,700 men aged 20 years or older who lived in Sweden for at least 2 years as of Jan. 1, 2003. They were followed up to assess for physical comorbidities and mortality for 7 years. Bipolar disorder in the cohort was identified by any diagnosis during the preceding 2 years, and by the use of specific medications commonly used for bipolar disorder maintenance treatment.
The findings of this study, which is among the first to examine the association between bipolar disorder and mortality using complete diagnoses for a national population, adds to the increasing knowledge about factors that contribute to premature mortality in patients with bipolar disorder, but it is unclear to what extent the findings can be generalized to other health care systems, the investigators said.
"The substantial health disparities we found between bipolar disorder patients and the rest of the Swedish population may be even larger in other countries without universal health care," they noted.
This study was supported by a grant from the National Institute on Drug Abuse and an Agreement on Medical Training and Research (Lund, Sweden) project grant. The authors reported having no disclosures.
Women and men with bipolar disorder were more likely to die prematurely than were those without bipolar disorder, according to results from a Swedish national cohort study involving nearly 6.6 million adults.
After adjustment for age, marital status, educational level, employment status, and income, all-cause mortality among the 6,618 adults with bipolar disorder in the cohort was increased twofold for both women (adjusted hazard ratio, 2.34) and men (AHR, 2.03), who died an average of 9.0 and 8.5 years earlier, respectively, did than those without bipolar disorder, according to Dr. Casey Crump of Stanford (Calif.) University, and his colleagues.
Those with bipolar disorder died prematurely from various causes, including cardiovascular disease, diabetes, chronic obstructive pulmonary disease (COPD), influenza or pneumonia, unintentional injuries, and suicide. Among women, stroke and cancer (particularly colon cancer) were also among the causes of premature death. Suicide was a particular risk for both women and men, who had 10-fold and 8-fold increases in risk, respectively (AHRs, 10.37 and 8.09), but the life expectancy differences were not fully explained by unnatural deaths, the investigators reported July 17 online in JAMA Psychiatry.
The most significant causes of death were influenza or pneumonia (3.7- and 4.4-fold increased risk for women and men, respectively), diabetes (3.6- and 2.6-fold increased risk, respectively), and COPD (2.9- and 2.6-fold increased risk).
In a separate model, the potential mediating effect of substance use disorders also was evaluated, and the effect was found to be modest, the investigators noted.
The associations between the various conditions and premature death were weakest for chronic diseases in those with a prior diagnosis, compared with those without a prior diagnosis (AHRs, 1.40 vs. 2.38), suggesting that earlier medical diagnosis and treatment might attenuate the increased mortality risk among affected individuals, they said (JAMA Psychiatry 2013 July 17 [doi: 10.1001/jamapsychiatry.2013.1394]).
"More complete provision of primary, preventive medical care among bipolar disorder patients is needed to reduce early mortality in this vulnerable population," they said, noting that multiple underlying mechanisms, including lifestyle factors, pathophysiologic mechanisms, genetic factors, and certain treatments for bipolar disorder, contribute to the disparities.
"The current study found evidence of modestly increased mortality among bipolar disorder patients who used carbamazepine, risperidone, or valproic acid or who solely used olanzapine, whereas users of aripiprazole, quetiapine, or lamotrigine had modestly reduced mortality compared with those who solely used lithium," they said.
However, consistent with prior research, those who used none of these medications had even higher rates of all-cause mortality – and twice the suicide risk – of those who used medication.
Study participants were 3,918 women and 2,700 men aged 20 years or older who lived in Sweden for at least 2 years as of Jan. 1, 2003. They were followed up to assess for physical comorbidities and mortality for 7 years. Bipolar disorder in the cohort was identified by any diagnosis during the preceding 2 years, and by the use of specific medications commonly used for bipolar disorder maintenance treatment.
The findings of this study, which is among the first to examine the association between bipolar disorder and mortality using complete diagnoses for a national population, adds to the increasing knowledge about factors that contribute to premature mortality in patients with bipolar disorder, but it is unclear to what extent the findings can be generalized to other health care systems, the investigators said.
"The substantial health disparities we found between bipolar disorder patients and the rest of the Swedish population may be even larger in other countries without universal health care," they noted.
This study was supported by a grant from the National Institute on Drug Abuse and an Agreement on Medical Training and Research (Lund, Sweden) project grant. The authors reported having no disclosures.
Women and men with bipolar disorder were more likely to die prematurely than were those without bipolar disorder, according to results from a Swedish national cohort study involving nearly 6.6 million adults.
After adjustment for age, marital status, educational level, employment status, and income, all-cause mortality among the 6,618 adults with bipolar disorder in the cohort was increased twofold for both women (adjusted hazard ratio, 2.34) and men (AHR, 2.03), who died an average of 9.0 and 8.5 years earlier, respectively, did than those without bipolar disorder, according to Dr. Casey Crump of Stanford (Calif.) University, and his colleagues.
Those with bipolar disorder died prematurely from various causes, including cardiovascular disease, diabetes, chronic obstructive pulmonary disease (COPD), influenza or pneumonia, unintentional injuries, and suicide. Among women, stroke and cancer (particularly colon cancer) were also among the causes of premature death. Suicide was a particular risk for both women and men, who had 10-fold and 8-fold increases in risk, respectively (AHRs, 10.37 and 8.09), but the life expectancy differences were not fully explained by unnatural deaths, the investigators reported July 17 online in JAMA Psychiatry.
The most significant causes of death were influenza or pneumonia (3.7- and 4.4-fold increased risk for women and men, respectively), diabetes (3.6- and 2.6-fold increased risk, respectively), and COPD (2.9- and 2.6-fold increased risk).
In a separate model, the potential mediating effect of substance use disorders also was evaluated, and the effect was found to be modest, the investigators noted.
The associations between the various conditions and premature death were weakest for chronic diseases in those with a prior diagnosis, compared with those without a prior diagnosis (AHRs, 1.40 vs. 2.38), suggesting that earlier medical diagnosis and treatment might attenuate the increased mortality risk among affected individuals, they said (JAMA Psychiatry 2013 July 17 [doi: 10.1001/jamapsychiatry.2013.1394]).
"More complete provision of primary, preventive medical care among bipolar disorder patients is needed to reduce early mortality in this vulnerable population," they said, noting that multiple underlying mechanisms, including lifestyle factors, pathophysiologic mechanisms, genetic factors, and certain treatments for bipolar disorder, contribute to the disparities.
"The current study found evidence of modestly increased mortality among bipolar disorder patients who used carbamazepine, risperidone, or valproic acid or who solely used olanzapine, whereas users of aripiprazole, quetiapine, or lamotrigine had modestly reduced mortality compared with those who solely used lithium," they said.
However, consistent with prior research, those who used none of these medications had even higher rates of all-cause mortality – and twice the suicide risk – of those who used medication.
Study participants were 3,918 women and 2,700 men aged 20 years or older who lived in Sweden for at least 2 years as of Jan. 1, 2003. They were followed up to assess for physical comorbidities and mortality for 7 years. Bipolar disorder in the cohort was identified by any diagnosis during the preceding 2 years, and by the use of specific medications commonly used for bipolar disorder maintenance treatment.
The findings of this study, which is among the first to examine the association between bipolar disorder and mortality using complete diagnoses for a national population, adds to the increasing knowledge about factors that contribute to premature mortality in patients with bipolar disorder, but it is unclear to what extent the findings can be generalized to other health care systems, the investigators said.
"The substantial health disparities we found between bipolar disorder patients and the rest of the Swedish population may be even larger in other countries without universal health care," they noted.
This study was supported by a grant from the National Institute on Drug Abuse and an Agreement on Medical Training and Research (Lund, Sweden) project grant. The authors reported having no disclosures.
FROM JAMA PSYCHIATRY
Major finding: All-cause mortality in adults with bipolar disorder in the cohort was increased twofold for both women and men (adjusted hazard ratios, 2.34 and 2.03, respectively).
Data source: A Swedish national cohort study involving nearly 6.6 million adults.
Disclosures: This study was supported by a grant from the National Institute on Drug Abuse, and by an Agreement on Medical Training and Research (Lund, Sweden) project grant. The authors reported having no disclosures.
Affective processing may differ in bipolar I patients
The underlying neural mechanisms for processing affective stimuli appear to differ in patients with bipolar disorder II, compared with those of healthy controls, a study has shown.
Both functional MRI scans during patients’ exposure to facial expressions and an overt task of identifying fearful or happy faces revealed differences in bipolar patients’ processing of emotional information relative to healthy controls, reported Kelly A. Sagar and her associates at McLean Hospital, Belmont, Mass., in the Journal of Affective Disorders (2013 May 30 [doi:10.1016/j.jad.2013.05.019]).
Ms. Sagar’s team wrote that their findings suggest that bipolar I patients "have difficulties with the identification of certain emotional expressions," potentially resulting "in an inability to appropriately read social cues, which often leads to miscommunication, misinterpretation, and compromised interpersonal relationships."
The researchers assessed the affective processing of 23 bipolar I patients and 18 healthy controls in two separate tasks. The bipolar I patients, with a mean age of 26.65 (plus or minus 6.65) and a mean bipolar disorder onset age of 16.5 (plus or minus 3.65) were primarily euthymic at the time of the study, and their pharmacotherapeutic regimens had been stable for at least 12 weeks before the study began.
Four bipolar patients were unmedicated at the time of the study, four were taking antidepressants, four were taking benzodiazepines, 11 were taking antipsychotics, and 17 were taking mood stabilizers. The healthy controls tended to be younger, with a mean age of 23.11 (plus or minus 3.15), but the controls’ years of education (15.53 plus or minus 21.22 ) were similar to those of the bipolar participants (14.57 plus or minus 1.68).
During the functional MRI task, the participants completed a backward-masked affect paradigm in which they viewed black and white photographs of male and female faces with different expressions, shown for 30 milliseconds each. The two affective conditions were fearful and happy, alternated with neutral faces and with neutral masks shown between each face for 170 milliseconds.
Ms. Sagar’s team reported results for both single sample analyses and contrast analyses (subtracting one group map from the other for each face condition).
In the single sample analyses, the patients with bipolar disorder showed altered activation in the amygdala and increased activation in the anterior cingulate and dorsolateral prefrontal cortex during the fear condition, compared with the controls. The bipolar patients’ activation patterns in these three regions were more diffuse during the happy condition than in the controls.
In the contrast analyses, relative to controls, bipolar patients showed increased activation in the anterior cingulate cortex, bilateral amygdala, and dorsolateral prefrontal cortex during the fear condition and higher activation in the subgenual anterior cingulate, right dorsolateral prefrontal cortex, and left amygdala during the happy condition. The controls showed higher activation in the midcingulate and left dorsolateral prefrontal cortex during the happy condition, compared with the participants with bipolar disorder.
After the functional MRI scan, participants completed the computerized Facial Expression of Emotion Stimuli and Test, in which they had to identify the most closely represented emotion (anger, disgust, fear, happiness, sadness, or surprise) for 60 faces shown for 5 seconds each.
The bipolar participants identified fewer of the fearful faces, with an average 68.91% accuracy (standard deviation = 21.74), compared with 80% accuracy (SD = 14.14) among the controls. Identification of the happy faces, however, was comparable between the groups, with 97.39% accuracy (SD = 7.51) among the bipolar participants and 98.89% accuracy (SD = 3.23) among the controls.
The researchers noted that the functional MRI scan results revealed that the differences in emotional processing between bipolar patients and healthy controls occurred when the stimuli were shown "below the level of conscious awareness, suggesting a disruption early in the neural circuit responsible for affective processing." These findings, along with the greater difficulty bipolar patients had in identifying fearful faces during the overt task, corroborate similar findings in other studies, including a meta-analysis finding impairments among bipolar patients in recognizing facial emotions.
"Given the behavioral alterations and difficulty in inhibiting inappropriate responses often seen in patients with [bipolar disorder], these findings may have implications for reading cues in social situations, which may result in negative consequences," the authors wrote.
The study was limited by the moderate number of participants, the statistically significant but likely not biologically significant, age differences between the two groups, and the inability to be certain whether medication status could have affected the results.
The study was funded by the Jim and Pat Poitras Foundation and by a National Institute on Drug Abuse grant. The authors reported that they had no relevant financial disclosures.
The underlying neural mechanisms for processing affective stimuli appear to differ in patients with bipolar disorder II, compared with those of healthy controls, a study has shown.
Both functional MRI scans during patients’ exposure to facial expressions and an overt task of identifying fearful or happy faces revealed differences in bipolar patients’ processing of emotional information relative to healthy controls, reported Kelly A. Sagar and her associates at McLean Hospital, Belmont, Mass., in the Journal of Affective Disorders (2013 May 30 [doi:10.1016/j.jad.2013.05.019]).
Ms. Sagar’s team wrote that their findings suggest that bipolar I patients "have difficulties with the identification of certain emotional expressions," potentially resulting "in an inability to appropriately read social cues, which often leads to miscommunication, misinterpretation, and compromised interpersonal relationships."
The researchers assessed the affective processing of 23 bipolar I patients and 18 healthy controls in two separate tasks. The bipolar I patients, with a mean age of 26.65 (plus or minus 6.65) and a mean bipolar disorder onset age of 16.5 (plus or minus 3.65) were primarily euthymic at the time of the study, and their pharmacotherapeutic regimens had been stable for at least 12 weeks before the study began.
Four bipolar patients were unmedicated at the time of the study, four were taking antidepressants, four were taking benzodiazepines, 11 were taking antipsychotics, and 17 were taking mood stabilizers. The healthy controls tended to be younger, with a mean age of 23.11 (plus or minus 3.15), but the controls’ years of education (15.53 plus or minus 21.22 ) were similar to those of the bipolar participants (14.57 plus or minus 1.68).
During the functional MRI task, the participants completed a backward-masked affect paradigm in which they viewed black and white photographs of male and female faces with different expressions, shown for 30 milliseconds each. The two affective conditions were fearful and happy, alternated with neutral faces and with neutral masks shown between each face for 170 milliseconds.
Ms. Sagar’s team reported results for both single sample analyses and contrast analyses (subtracting one group map from the other for each face condition).
In the single sample analyses, the patients with bipolar disorder showed altered activation in the amygdala and increased activation in the anterior cingulate and dorsolateral prefrontal cortex during the fear condition, compared with the controls. The bipolar patients’ activation patterns in these three regions were more diffuse during the happy condition than in the controls.
In the contrast analyses, relative to controls, bipolar patients showed increased activation in the anterior cingulate cortex, bilateral amygdala, and dorsolateral prefrontal cortex during the fear condition and higher activation in the subgenual anterior cingulate, right dorsolateral prefrontal cortex, and left amygdala during the happy condition. The controls showed higher activation in the midcingulate and left dorsolateral prefrontal cortex during the happy condition, compared with the participants with bipolar disorder.
After the functional MRI scan, participants completed the computerized Facial Expression of Emotion Stimuli and Test, in which they had to identify the most closely represented emotion (anger, disgust, fear, happiness, sadness, or surprise) for 60 faces shown for 5 seconds each.
The bipolar participants identified fewer of the fearful faces, with an average 68.91% accuracy (standard deviation = 21.74), compared with 80% accuracy (SD = 14.14) among the controls. Identification of the happy faces, however, was comparable between the groups, with 97.39% accuracy (SD = 7.51) among the bipolar participants and 98.89% accuracy (SD = 3.23) among the controls.
The researchers noted that the functional MRI scan results revealed that the differences in emotional processing between bipolar patients and healthy controls occurred when the stimuli were shown "below the level of conscious awareness, suggesting a disruption early in the neural circuit responsible for affective processing." These findings, along with the greater difficulty bipolar patients had in identifying fearful faces during the overt task, corroborate similar findings in other studies, including a meta-analysis finding impairments among bipolar patients in recognizing facial emotions.
"Given the behavioral alterations and difficulty in inhibiting inappropriate responses often seen in patients with [bipolar disorder], these findings may have implications for reading cues in social situations, which may result in negative consequences," the authors wrote.
The study was limited by the moderate number of participants, the statistically significant but likely not biologically significant, age differences between the two groups, and the inability to be certain whether medication status could have affected the results.
The study was funded by the Jim and Pat Poitras Foundation and by a National Institute on Drug Abuse grant. The authors reported that they had no relevant financial disclosures.
The underlying neural mechanisms for processing affective stimuli appear to differ in patients with bipolar disorder II, compared with those of healthy controls, a study has shown.
Both functional MRI scans during patients’ exposure to facial expressions and an overt task of identifying fearful or happy faces revealed differences in bipolar patients’ processing of emotional information relative to healthy controls, reported Kelly A. Sagar and her associates at McLean Hospital, Belmont, Mass., in the Journal of Affective Disorders (2013 May 30 [doi:10.1016/j.jad.2013.05.019]).
Ms. Sagar’s team wrote that their findings suggest that bipolar I patients "have difficulties with the identification of certain emotional expressions," potentially resulting "in an inability to appropriately read social cues, which often leads to miscommunication, misinterpretation, and compromised interpersonal relationships."
The researchers assessed the affective processing of 23 bipolar I patients and 18 healthy controls in two separate tasks. The bipolar I patients, with a mean age of 26.65 (plus or minus 6.65) and a mean bipolar disorder onset age of 16.5 (plus or minus 3.65) were primarily euthymic at the time of the study, and their pharmacotherapeutic regimens had been stable for at least 12 weeks before the study began.
Four bipolar patients were unmedicated at the time of the study, four were taking antidepressants, four were taking benzodiazepines, 11 were taking antipsychotics, and 17 were taking mood stabilizers. The healthy controls tended to be younger, with a mean age of 23.11 (plus or minus 3.15), but the controls’ years of education (15.53 plus or minus 21.22 ) were similar to those of the bipolar participants (14.57 plus or minus 1.68).
During the functional MRI task, the participants completed a backward-masked affect paradigm in which they viewed black and white photographs of male and female faces with different expressions, shown for 30 milliseconds each. The two affective conditions were fearful and happy, alternated with neutral faces and with neutral masks shown between each face for 170 milliseconds.
Ms. Sagar’s team reported results for both single sample analyses and contrast analyses (subtracting one group map from the other for each face condition).
In the single sample analyses, the patients with bipolar disorder showed altered activation in the amygdala and increased activation in the anterior cingulate and dorsolateral prefrontal cortex during the fear condition, compared with the controls. The bipolar patients’ activation patterns in these three regions were more diffuse during the happy condition than in the controls.
In the contrast analyses, relative to controls, bipolar patients showed increased activation in the anterior cingulate cortex, bilateral amygdala, and dorsolateral prefrontal cortex during the fear condition and higher activation in the subgenual anterior cingulate, right dorsolateral prefrontal cortex, and left amygdala during the happy condition. The controls showed higher activation in the midcingulate and left dorsolateral prefrontal cortex during the happy condition, compared with the participants with bipolar disorder.
After the functional MRI scan, participants completed the computerized Facial Expression of Emotion Stimuli and Test, in which they had to identify the most closely represented emotion (anger, disgust, fear, happiness, sadness, or surprise) for 60 faces shown for 5 seconds each.
The bipolar participants identified fewer of the fearful faces, with an average 68.91% accuracy (standard deviation = 21.74), compared with 80% accuracy (SD = 14.14) among the controls. Identification of the happy faces, however, was comparable between the groups, with 97.39% accuracy (SD = 7.51) among the bipolar participants and 98.89% accuracy (SD = 3.23) among the controls.
The researchers noted that the functional MRI scan results revealed that the differences in emotional processing between bipolar patients and healthy controls occurred when the stimuli were shown "below the level of conscious awareness, suggesting a disruption early in the neural circuit responsible for affective processing." These findings, along with the greater difficulty bipolar patients had in identifying fearful faces during the overt task, corroborate similar findings in other studies, including a meta-analysis finding impairments among bipolar patients in recognizing facial emotions.
"Given the behavioral alterations and difficulty in inhibiting inappropriate responses often seen in patients with [bipolar disorder], these findings may have implications for reading cues in social situations, which may result in negative consequences," the authors wrote.
The study was limited by the moderate number of participants, the statistically significant but likely not biologically significant, age differences between the two groups, and the inability to be certain whether medication status could have affected the results.
The study was funded by the Jim and Pat Poitras Foundation and by a National Institute on Drug Abuse grant. The authors reported that they had no relevant financial disclosures.
FROM THE JOURNAL OF AFFECTIVE DISORDERS
Major finding: Functional MRI scans revealed that bipolar patients less accurately identified fearful facial expressions (68.91% accuracy vs. 80%) but identified happy ones at comparable rates to controls in a separate task (97.39% vs. 98.89%).
Data source: The findings are based on an analysis of fMRI scans and results from the Facial Expression of Emotion Stimuli and Test for 23 bipolar I participants and 18 healthy controls.
Disclosures: The study was funded by the Jim and Pat Poitras Foundation and by a National Institute on Drug Abuse grant. The authors reported that they had no relevant financial disclosures.