User login
Acute antipsychotics do not worsen glycemia in psychiatric inpatients
CHICAGO – Treatment with antipsychotic agents in acute psychiatric inpatient units is not associated with increased blood glucose levels, according to a multicenter study.
"This finding is contrary to what many of us may have previously considered," Dr. Dawn D. Smiley observed in presenting the study findings at the annual scientific sessions of the American Diabetes Association.
A well-recognized association exists between the use of antipsychotic agents and an increased prevalence of diabetes and hyperglycemia, although it remains unclear whether the relationship is causal. The association is especially strong for the atypical or second-generation antipsychotics, which account for more than 90% of all antipsychotic use and are among the top-selling prescription drugs in the United States. Indeed, the Food and Drug Administration has for a decade required the product labeling for all atypical antipsychotics to include a warning about the risks of hyperglycemia and diabetes.
But most data on antipsychotic-associated diabetes come from long-term studies, which show that diabetes, when it occurs, most often does so within the first 6 months on the medication. Little is known about the acute impact of antipsychotic agents on blood glucose levels and clinical outcomes in patients admitted to acute psychiatric units for stays averaging a few weeks. This information gap provided the rationale for Dr. Smiley’s study.
She presented a chart review of 1,212 patients admitted to three acute inpatient psychiatric units in inner-city Atlanta during 2008. The mean age of the patients was 57 years, they had a mean body mass index of 27 kg/m2, and the mean daily blood glucose concentration was 122 mg/dL during their stay. Twenty-one percent of the patients had a history of diabetes, and 6% had new hyperglycemia at admission. The most common primary diagnoses on admission were suicidal ideation in 28% of patients, a primary psychotic disorder in 27%, and depression in 16%.
Thirty-seven percent of patients had no prior experience with antipsychotic agents but were newly started on an antipsychotic during their acute inpatient stay. An additional 38% had been on antipsychotic therapy prior to admission and continued on it while hospitalized. Twenty-five percent of patients had never been treated with antipsychotic agents and were not during their hospitalization. Fifty-two percent of patients were treated with atypical antipsychotic agents during their inpatient stay, 17% received both atypical and typical antipsychotics, and 6% got typical antipsychotics only, explained Dr. Smiley, an endocrinologist at Emory University, Atlanta.
She sought answers to three research questions:
• Does prior exposure to antipsychotic therapy affect inpatient blood glucose levels or clinical outcome measures? Mean psychiatric inpatient length of stay was significantly shorter for patients never exposed to antipsychotics, at 9.5 days, compared with 14.2 days in patients who continued on their previous antipsychotic regimen and 13.7 days in antipsychotic-naive patients started on an antipsychotic for the first time. The most likely explanation for the longer stays in patients on antipsychotic therapy was the need to monitor them for side effects, according to Dr. Smiley.
Importantly, mean daily blood glucose concentrations during psychiatric hospitalization did not differ between patients never exposed to antipsychotic agents, those started on an antipsychotic for the first time during their hospitalization, and patients continued on their previous antipsychotics.
• Does the type of antipsychotic agent used during the acute inpatient admission affect blood glucose levels or clinical outcomes?
Mean daily blood glucose levels were similar regardless of whether patients were on atypical antipsychotics, typical antipsychotics, or both. The mean hemoglobin A1c level was 7.3% in all three groups of antipsychotic-treated patients. However, the rate of medical complications during psychiatric hospitalization was significantly higher among patients on atypical agents, at 23%, compared with 17% in patients on typical antipsychotics and 13% in those on both.
Three percent of patients on atypical antipsychotics-only required transfer to a medical unit for treatment of a complication that arose during psychiatric hospitalization. That was also true for 3% of patients on combined atypical/typical antipsychotic therapy. In contrast, none of the relatively few patients on typical antipsychotics-only required transfer to a medical unit. It’s worth noting that all atypical antipsychotics are not alike in terms of the strength of their association with diabetes. Most antipsychotic-treated patients in this study got olanzapine (Zyprexa) or clozapine (Clozaril). Newer, less diabetogenic atypical agents were less frequently used in this largely indigent population.
• Does a history of diabetes or new hyperglycemia at admission affect outcome measures? Not unexpectedly, patients with a history of diabetes prior to admission had a significantly higher rate of medical complications during their psychiatric inpatient stay: a 28% rate, compared with 22% in patients with no history of diabetes but with hyperglycemia at admission and 20% in patients with neither metabolic abnormality. The most common complication in patients with a history of diabetes was urinary tract infection.
Again unsurprisingly, patients with a history of diabetes had a significantly higher rate of transfer to a medical unit because of medical complications: 5%, compared with no transfers in patients with new hyperglycemia at admission and a 1% transfer rate in patients with neither metabolic abnormality.
Dr. Smiley reported that her research funding came from the National Institutes of Health, Sanofi-Aventis, and Merck.
CHICAGO – Treatment with antipsychotic agents in acute psychiatric inpatient units is not associated with increased blood glucose levels, according to a multicenter study.
"This finding is contrary to what many of us may have previously considered," Dr. Dawn D. Smiley observed in presenting the study findings at the annual scientific sessions of the American Diabetes Association.
A well-recognized association exists between the use of antipsychotic agents and an increased prevalence of diabetes and hyperglycemia, although it remains unclear whether the relationship is causal. The association is especially strong for the atypical or second-generation antipsychotics, which account for more than 90% of all antipsychotic use and are among the top-selling prescription drugs in the United States. Indeed, the Food and Drug Administration has for a decade required the product labeling for all atypical antipsychotics to include a warning about the risks of hyperglycemia and diabetes.
But most data on antipsychotic-associated diabetes come from long-term studies, which show that diabetes, when it occurs, most often does so within the first 6 months on the medication. Little is known about the acute impact of antipsychotic agents on blood glucose levels and clinical outcomes in patients admitted to acute psychiatric units for stays averaging a few weeks. This information gap provided the rationale for Dr. Smiley’s study.
She presented a chart review of 1,212 patients admitted to three acute inpatient psychiatric units in inner-city Atlanta during 2008. The mean age of the patients was 57 years, they had a mean body mass index of 27 kg/m2, and the mean daily blood glucose concentration was 122 mg/dL during their stay. Twenty-one percent of the patients had a history of diabetes, and 6% had new hyperglycemia at admission. The most common primary diagnoses on admission were suicidal ideation in 28% of patients, a primary psychotic disorder in 27%, and depression in 16%.
Thirty-seven percent of patients had no prior experience with antipsychotic agents but were newly started on an antipsychotic during their acute inpatient stay. An additional 38% had been on antipsychotic therapy prior to admission and continued on it while hospitalized. Twenty-five percent of patients had never been treated with antipsychotic agents and were not during their hospitalization. Fifty-two percent of patients were treated with atypical antipsychotic agents during their inpatient stay, 17% received both atypical and typical antipsychotics, and 6% got typical antipsychotics only, explained Dr. Smiley, an endocrinologist at Emory University, Atlanta.
She sought answers to three research questions:
• Does prior exposure to antipsychotic therapy affect inpatient blood glucose levels or clinical outcome measures? Mean psychiatric inpatient length of stay was significantly shorter for patients never exposed to antipsychotics, at 9.5 days, compared with 14.2 days in patients who continued on their previous antipsychotic regimen and 13.7 days in antipsychotic-naive patients started on an antipsychotic for the first time. The most likely explanation for the longer stays in patients on antipsychotic therapy was the need to monitor them for side effects, according to Dr. Smiley.
Importantly, mean daily blood glucose concentrations during psychiatric hospitalization did not differ between patients never exposed to antipsychotic agents, those started on an antipsychotic for the first time during their hospitalization, and patients continued on their previous antipsychotics.
• Does the type of antipsychotic agent used during the acute inpatient admission affect blood glucose levels or clinical outcomes?
Mean daily blood glucose levels were similar regardless of whether patients were on atypical antipsychotics, typical antipsychotics, or both. The mean hemoglobin A1c level was 7.3% in all three groups of antipsychotic-treated patients. However, the rate of medical complications during psychiatric hospitalization was significantly higher among patients on atypical agents, at 23%, compared with 17% in patients on typical antipsychotics and 13% in those on both.
Three percent of patients on atypical antipsychotics-only required transfer to a medical unit for treatment of a complication that arose during psychiatric hospitalization. That was also true for 3% of patients on combined atypical/typical antipsychotic therapy. In contrast, none of the relatively few patients on typical antipsychotics-only required transfer to a medical unit. It’s worth noting that all atypical antipsychotics are not alike in terms of the strength of their association with diabetes. Most antipsychotic-treated patients in this study got olanzapine (Zyprexa) or clozapine (Clozaril). Newer, less diabetogenic atypical agents were less frequently used in this largely indigent population.
• Does a history of diabetes or new hyperglycemia at admission affect outcome measures? Not unexpectedly, patients with a history of diabetes prior to admission had a significantly higher rate of medical complications during their psychiatric inpatient stay: a 28% rate, compared with 22% in patients with no history of diabetes but with hyperglycemia at admission and 20% in patients with neither metabolic abnormality. The most common complication in patients with a history of diabetes was urinary tract infection.
Again unsurprisingly, patients with a history of diabetes had a significantly higher rate of transfer to a medical unit because of medical complications: 5%, compared with no transfers in patients with new hyperglycemia at admission and a 1% transfer rate in patients with neither metabolic abnormality.
Dr. Smiley reported that her research funding came from the National Institutes of Health, Sanofi-Aventis, and Merck.
CHICAGO – Treatment with antipsychotic agents in acute psychiatric inpatient units is not associated with increased blood glucose levels, according to a multicenter study.
"This finding is contrary to what many of us may have previously considered," Dr. Dawn D. Smiley observed in presenting the study findings at the annual scientific sessions of the American Diabetes Association.
A well-recognized association exists between the use of antipsychotic agents and an increased prevalence of diabetes and hyperglycemia, although it remains unclear whether the relationship is causal. The association is especially strong for the atypical or second-generation antipsychotics, which account for more than 90% of all antipsychotic use and are among the top-selling prescription drugs in the United States. Indeed, the Food and Drug Administration has for a decade required the product labeling for all atypical antipsychotics to include a warning about the risks of hyperglycemia and diabetes.
But most data on antipsychotic-associated diabetes come from long-term studies, which show that diabetes, when it occurs, most often does so within the first 6 months on the medication. Little is known about the acute impact of antipsychotic agents on blood glucose levels and clinical outcomes in patients admitted to acute psychiatric units for stays averaging a few weeks. This information gap provided the rationale for Dr. Smiley’s study.
She presented a chart review of 1,212 patients admitted to three acute inpatient psychiatric units in inner-city Atlanta during 2008. The mean age of the patients was 57 years, they had a mean body mass index of 27 kg/m2, and the mean daily blood glucose concentration was 122 mg/dL during their stay. Twenty-one percent of the patients had a history of diabetes, and 6% had new hyperglycemia at admission. The most common primary diagnoses on admission were suicidal ideation in 28% of patients, a primary psychotic disorder in 27%, and depression in 16%.
Thirty-seven percent of patients had no prior experience with antipsychotic agents but were newly started on an antipsychotic during their acute inpatient stay. An additional 38% had been on antipsychotic therapy prior to admission and continued on it while hospitalized. Twenty-five percent of patients had never been treated with antipsychotic agents and were not during their hospitalization. Fifty-two percent of patients were treated with atypical antipsychotic agents during their inpatient stay, 17% received both atypical and typical antipsychotics, and 6% got typical antipsychotics only, explained Dr. Smiley, an endocrinologist at Emory University, Atlanta.
She sought answers to three research questions:
• Does prior exposure to antipsychotic therapy affect inpatient blood glucose levels or clinical outcome measures? Mean psychiatric inpatient length of stay was significantly shorter for patients never exposed to antipsychotics, at 9.5 days, compared with 14.2 days in patients who continued on their previous antipsychotic regimen and 13.7 days in antipsychotic-naive patients started on an antipsychotic for the first time. The most likely explanation for the longer stays in patients on antipsychotic therapy was the need to monitor them for side effects, according to Dr. Smiley.
Importantly, mean daily blood glucose concentrations during psychiatric hospitalization did not differ between patients never exposed to antipsychotic agents, those started on an antipsychotic for the first time during their hospitalization, and patients continued on their previous antipsychotics.
• Does the type of antipsychotic agent used during the acute inpatient admission affect blood glucose levels or clinical outcomes?
Mean daily blood glucose levels were similar regardless of whether patients were on atypical antipsychotics, typical antipsychotics, or both. The mean hemoglobin A1c level was 7.3% in all three groups of antipsychotic-treated patients. However, the rate of medical complications during psychiatric hospitalization was significantly higher among patients on atypical agents, at 23%, compared with 17% in patients on typical antipsychotics and 13% in those on both.
Three percent of patients on atypical antipsychotics-only required transfer to a medical unit for treatment of a complication that arose during psychiatric hospitalization. That was also true for 3% of patients on combined atypical/typical antipsychotic therapy. In contrast, none of the relatively few patients on typical antipsychotics-only required transfer to a medical unit. It’s worth noting that all atypical antipsychotics are not alike in terms of the strength of their association with diabetes. Most antipsychotic-treated patients in this study got olanzapine (Zyprexa) or clozapine (Clozaril). Newer, less diabetogenic atypical agents were less frequently used in this largely indigent population.
• Does a history of diabetes or new hyperglycemia at admission affect outcome measures? Not unexpectedly, patients with a history of diabetes prior to admission had a significantly higher rate of medical complications during their psychiatric inpatient stay: a 28% rate, compared with 22% in patients with no history of diabetes but with hyperglycemia at admission and 20% in patients with neither metabolic abnormality. The most common complication in patients with a history of diabetes was urinary tract infection.
Again unsurprisingly, patients with a history of diabetes had a significantly higher rate of transfer to a medical unit because of medical complications: 5%, compared with no transfers in patients with new hyperglycemia at admission and a 1% transfer rate in patients with neither metabolic abnormality.
Dr. Smiley reported that her research funding came from the National Institutes of Health, Sanofi-Aventis, and Merck.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Major finding: Blood glucose levels during acute psychiatric hospitalizations did not differ between antipsychotic-naive patients newly placed on antipsychotic therapy during their inpatient stay, and patients never exposed to antipsychotic agents.
Data source: This was a chart review of 1,212 patients admitted to three acute inpatient psychiatric units located in Atlanta.
Disclosures: Dr. Smiley reported that her research funding came from the National Institutes of Health, Sanofi-Aventis, and Merck.
Lurasidone shows efficacy in bipolar depression
HOLLYWOOD, FLA. – Lurasidone has hit all of its primary and secondary efficacy endpoints in a phase III clinical trial for the treatment of bipolar I depression.
As a result, the drug, already marketed as Latuda for the treatment of schizophrenia, has been approved by the Food and Drug Administration for a requested expanded indication in treating bipolar I depression. The approval, which came July 1, was made for lurasidone as monotherapy and adjunctive therapy with lithium or valproate.
The phase III PREVAIL 2 (Program to Evaluate the Antidepressant Impact of Lurasidone) study was a 6-week, double-blind, placebo-controlled, multicenter clinical trial involving 505 patients with bipolar I depression. They were randomized to once-daily, flexibly dosed lurasidone at either 20-60 mg/day or 80-120 mg/day, or to placebo.
The primary efficacy endpoint was change from baseline through week 6 in scores on MADRS (the Montgomery-Åsberg Depression Rating Scale). From a mean baseline score of 30, both the lower- and higher-dose lurasidone groups averaged identical 15.4-point reductions, a significantly greater improvement than the 10.7-point decrease in placebo-treated controls, Dr. Antony D. Loebel reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
This pattern of closely similar efficacy in the lower- and higher-dose lurasidone groups, whose mean modal doses were 34.9 and 92.3 mg/day, respectively, was repeated for the other study endpoints, observed Dr. Loebel, executive vice president and chief medical officer at Sunovion Pharmaceuticals, Fort Lee, N.J.
For example, the Clinical Global Impression, Bipolar Severity depression score decreased from a baseline of 4.5 by a mean of 1.8 points in the lower-dose lurasidone group and 1.7 points in the higher-dose arm, significantly greater than the 1.1-point decline with placebo.
Similarly, 53% and 51% of the lower- and higher-dose lurasidone groups, respectively, were deemed treatment responders based upon at least a 50% reduction in MADRS scores, compared with 30% of controls.
The remission rate, defined as a final MADRS score of 12 or less, was 42% in the lower-dose lurasidone arm, 40% in patients on higher-dose therapy, and 25% with placebo.
All three patient groups showed similarly modest decreases over time in the Young Mania Rating Scale: a mean 1-point drop in the lower-dose lurasidone arm, a 0.7-point reduction with higher-dose therapy, and a 0.9-point reduction with placebo. That’s an important and reassuring finding, because attempts to treat bipolar mania using conventional antidepressants can result in a switch to mania. In this study, treatment-emergent mania occurred in just 1% of subjects on lower-dose lurasidone, 0% on higher-dose therapy, and 1% on placebo, Dr. Loebel continued.
Numerous other studies have established that roughly 90% of patients with bipolar depression experience severe functional impairment. Of note, PREVAIL 2 patients in the lower-dose lurasidone arm displayed a highly significant mean 9.5-point reduction from a baseline score of 20 on the Sheehan Disability Scale, and the higher-dose lurasidone group showed a 9.8-point decrease. In contrast, scores did not change over time in the control group.
In a similar vein, scores on the Quick Inventory of Depressive Symptomatology-Self-Report improved from a baseline of 33.5 by 19.3 and 19.8 points, respectively, in the lower- and higher-dose lurasidone arms, significantly better than the 12.8-point improvement with placebo, reported Dr. Loebel also of the department of psychiatry at New York University.
A total of 6%-7% of subjects in each study arm discontinued the trial because of adverse events. Nausea and akathisia were the two adverse events that were seen more frequently with lurasidone than placebo. No significant changes in lipids, body weight, or glycemic control were observed.
Lurasidone’s efficacy in bipolar depression is attributed to the drug’s unique pharmacodynamic profile. It is a more potent blocker of the serotonin 5HT7 receptor than are other atypical antipsychotics. It also is a strong antagonist of the dopamine D2 and 5-HT2A receptors, a moderate partial agonist at the 5HT1a receptor, and has a moderate antagonist effect at the alpha-2c receptor.
Before the approval, quetiapine (Seroquel) was the only drug approved as monotherapy for bipolar depression.
Sunovion Pharmaceuticals sponsored the phase III trial. Dr. Loebel is a company employee.
*This story was updated 7/3/2013.
HOLLYWOOD, FLA. – Lurasidone has hit all of its primary and secondary efficacy endpoints in a phase III clinical trial for the treatment of bipolar I depression.
As a result, the drug, already marketed as Latuda for the treatment of schizophrenia, has been approved by the Food and Drug Administration for a requested expanded indication in treating bipolar I depression. The approval, which came July 1, was made for lurasidone as monotherapy and adjunctive therapy with lithium or valproate.
The phase III PREVAIL 2 (Program to Evaluate the Antidepressant Impact of Lurasidone) study was a 6-week, double-blind, placebo-controlled, multicenter clinical trial involving 505 patients with bipolar I depression. They were randomized to once-daily, flexibly dosed lurasidone at either 20-60 mg/day or 80-120 mg/day, or to placebo.
The primary efficacy endpoint was change from baseline through week 6 in scores on MADRS (the Montgomery-Åsberg Depression Rating Scale). From a mean baseline score of 30, both the lower- and higher-dose lurasidone groups averaged identical 15.4-point reductions, a significantly greater improvement than the 10.7-point decrease in placebo-treated controls, Dr. Antony D. Loebel reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
This pattern of closely similar efficacy in the lower- and higher-dose lurasidone groups, whose mean modal doses were 34.9 and 92.3 mg/day, respectively, was repeated for the other study endpoints, observed Dr. Loebel, executive vice president and chief medical officer at Sunovion Pharmaceuticals, Fort Lee, N.J.
For example, the Clinical Global Impression, Bipolar Severity depression score decreased from a baseline of 4.5 by a mean of 1.8 points in the lower-dose lurasidone group and 1.7 points in the higher-dose arm, significantly greater than the 1.1-point decline with placebo.
Similarly, 53% and 51% of the lower- and higher-dose lurasidone groups, respectively, were deemed treatment responders based upon at least a 50% reduction in MADRS scores, compared with 30% of controls.
The remission rate, defined as a final MADRS score of 12 or less, was 42% in the lower-dose lurasidone arm, 40% in patients on higher-dose therapy, and 25% with placebo.
All three patient groups showed similarly modest decreases over time in the Young Mania Rating Scale: a mean 1-point drop in the lower-dose lurasidone arm, a 0.7-point reduction with higher-dose therapy, and a 0.9-point reduction with placebo. That’s an important and reassuring finding, because attempts to treat bipolar mania using conventional antidepressants can result in a switch to mania. In this study, treatment-emergent mania occurred in just 1% of subjects on lower-dose lurasidone, 0% on higher-dose therapy, and 1% on placebo, Dr. Loebel continued.
Numerous other studies have established that roughly 90% of patients with bipolar depression experience severe functional impairment. Of note, PREVAIL 2 patients in the lower-dose lurasidone arm displayed a highly significant mean 9.5-point reduction from a baseline score of 20 on the Sheehan Disability Scale, and the higher-dose lurasidone group showed a 9.8-point decrease. In contrast, scores did not change over time in the control group.
In a similar vein, scores on the Quick Inventory of Depressive Symptomatology-Self-Report improved from a baseline of 33.5 by 19.3 and 19.8 points, respectively, in the lower- and higher-dose lurasidone arms, significantly better than the 12.8-point improvement with placebo, reported Dr. Loebel also of the department of psychiatry at New York University.
A total of 6%-7% of subjects in each study arm discontinued the trial because of adverse events. Nausea and akathisia were the two adverse events that were seen more frequently with lurasidone than placebo. No significant changes in lipids, body weight, or glycemic control were observed.
Lurasidone’s efficacy in bipolar depression is attributed to the drug’s unique pharmacodynamic profile. It is a more potent blocker of the serotonin 5HT7 receptor than are other atypical antipsychotics. It also is a strong antagonist of the dopamine D2 and 5-HT2A receptors, a moderate partial agonist at the 5HT1a receptor, and has a moderate antagonist effect at the alpha-2c receptor.
Before the approval, quetiapine (Seroquel) was the only drug approved as monotherapy for bipolar depression.
Sunovion Pharmaceuticals sponsored the phase III trial. Dr. Loebel is a company employee.
*This story was updated 7/3/2013.
HOLLYWOOD, FLA. – Lurasidone has hit all of its primary and secondary efficacy endpoints in a phase III clinical trial for the treatment of bipolar I depression.
As a result, the drug, already marketed as Latuda for the treatment of schizophrenia, has been approved by the Food and Drug Administration for a requested expanded indication in treating bipolar I depression. The approval, which came July 1, was made for lurasidone as monotherapy and adjunctive therapy with lithium or valproate.
The phase III PREVAIL 2 (Program to Evaluate the Antidepressant Impact of Lurasidone) study was a 6-week, double-blind, placebo-controlled, multicenter clinical trial involving 505 patients with bipolar I depression. They were randomized to once-daily, flexibly dosed lurasidone at either 20-60 mg/day or 80-120 mg/day, or to placebo.
The primary efficacy endpoint was change from baseline through week 6 in scores on MADRS (the Montgomery-Åsberg Depression Rating Scale). From a mean baseline score of 30, both the lower- and higher-dose lurasidone groups averaged identical 15.4-point reductions, a significantly greater improvement than the 10.7-point decrease in placebo-treated controls, Dr. Antony D. Loebel reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
This pattern of closely similar efficacy in the lower- and higher-dose lurasidone groups, whose mean modal doses were 34.9 and 92.3 mg/day, respectively, was repeated for the other study endpoints, observed Dr. Loebel, executive vice president and chief medical officer at Sunovion Pharmaceuticals, Fort Lee, N.J.
For example, the Clinical Global Impression, Bipolar Severity depression score decreased from a baseline of 4.5 by a mean of 1.8 points in the lower-dose lurasidone group and 1.7 points in the higher-dose arm, significantly greater than the 1.1-point decline with placebo.
Similarly, 53% and 51% of the lower- and higher-dose lurasidone groups, respectively, were deemed treatment responders based upon at least a 50% reduction in MADRS scores, compared with 30% of controls.
The remission rate, defined as a final MADRS score of 12 or less, was 42% in the lower-dose lurasidone arm, 40% in patients on higher-dose therapy, and 25% with placebo.
All three patient groups showed similarly modest decreases over time in the Young Mania Rating Scale: a mean 1-point drop in the lower-dose lurasidone arm, a 0.7-point reduction with higher-dose therapy, and a 0.9-point reduction with placebo. That’s an important and reassuring finding, because attempts to treat bipolar mania using conventional antidepressants can result in a switch to mania. In this study, treatment-emergent mania occurred in just 1% of subjects on lower-dose lurasidone, 0% on higher-dose therapy, and 1% on placebo, Dr. Loebel continued.
Numerous other studies have established that roughly 90% of patients with bipolar depression experience severe functional impairment. Of note, PREVAIL 2 patients in the lower-dose lurasidone arm displayed a highly significant mean 9.5-point reduction from a baseline score of 20 on the Sheehan Disability Scale, and the higher-dose lurasidone group showed a 9.8-point decrease. In contrast, scores did not change over time in the control group.
In a similar vein, scores on the Quick Inventory of Depressive Symptomatology-Self-Report improved from a baseline of 33.5 by 19.3 and 19.8 points, respectively, in the lower- and higher-dose lurasidone arms, significantly better than the 12.8-point improvement with placebo, reported Dr. Loebel also of the department of psychiatry at New York University.
A total of 6%-7% of subjects in each study arm discontinued the trial because of adverse events. Nausea and akathisia were the two adverse events that were seen more frequently with lurasidone than placebo. No significant changes in lipids, body weight, or glycemic control were observed.
Lurasidone’s efficacy in bipolar depression is attributed to the drug’s unique pharmacodynamic profile. It is a more potent blocker of the serotonin 5HT7 receptor than are other atypical antipsychotics. It also is a strong antagonist of the dopamine D2 and 5-HT2A receptors, a moderate partial agonist at the 5HT1a receptor, and has a moderate antagonist effect at the alpha-2c receptor.
Before the approval, quetiapine (Seroquel) was the only drug approved as monotherapy for bipolar depression.
Sunovion Pharmaceuticals sponsored the phase III trial. Dr. Loebel is a company employee.
*This story was updated 7/3/2013.
AT THE NCDEU MEETING
Major finding: Patients with bipolar I depression responded to 6 weeks of lurasidone at either a lower or higher dose range with identical 15.4-point improvements from a baseline score of 30 on the Montgomery-Åsberg Depression Rating Scale, significantly better than the 10.7-point improvement with placebo.
Data source: PREVAIL 2 was a phase III, double-blind, 6-week randomized trial involving 505 patients with bipolar I depression.
Disclosures: The study was sponsored by Sunovion. The presenter is a company employee.
New diagnosis fits third of bipolar teens
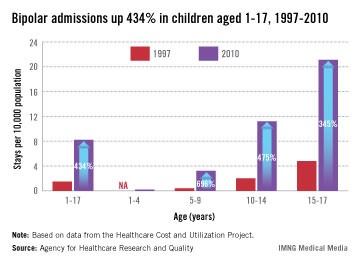
SAN FRANCISCO – Thirty-seven percent of 175 hospitalized adolescents diagnosed with bipolar disorder met criteria for a new disorder listed in the DSM-5 – disruptive mood dysregulation disorder.
Nearly all of the patients (96%) had been diagnosed with bipolar I disorder "not otherwise specified" (NOS) at the time of admission, a retrospective study found. Three other bipolar diagnoses were applied to two patients each: bipolar depression, bipolar mania, or mixed-episode bipolar disorder, David L. Pogge, Ph.D., reported at the annual meeting of the American Psychiatric Association.
The findings suggest that a substantial proportion of adolescent inpatients diagnosed with bipolar disorder may instead meet criteria for disruptive mood dysregulation disorder, and that clinicians should be more careful in diagnosing bipolar disorder, especially bipolar NOS, said Dr. Pogge of the department of psychology and counseling at Fairleigh Dickinson University, Teaneck, N.J. He also serves as director of psychology at Four Winds Hospital, which operates four campuses in New York state.
The study included records for all 1,505 patients aged 13-17 years who were admitted to a private psychiatric hospital over a 2-year period. At the time of admission, clinicians rated 1,351 patients as having at least moderate depression and 368 as also having severe symptoms of hostility and explosiveness. They diagnosed bipolar disorder in 259 cases. The investigators analyzed records for 174 patients with complete records or who had at least moderate depression and severe symptoms of hostility and explosiveness but no signs of elation or euphoria at the time of admission.
Disruptive mood dysregulation disorder is marked by intense temper outbursts superimposed on a background of persistent depressed or irritable mood. Temper outbursts and aggression are common reasons for inpatient admissions of children and adolescents, Dr. Pogge noted in his poster presentation.
Compared with the 63% of patients who did not meet criteria for disruptive mood dysregulation disorder (DMDD), patients who met the DMDD criteria were significantly more likely to experience restraint or seclusion while hospitalized (30% vs. 20%), receive a significantly higher number of restraints or seclusions (2.2 vs. 0.8), and remain hospitalized significantly longer (25 days vs. 21 days), he reported. At the time of discharge, clinicians’ ratings on the Global Assessment of Functioning (GAF) scale indicated significantly greater global psychopathology in patients with DMDD (a mean GAF score of 44), compared with patients who did not meet DMDD criteria (a mean GAF score of 50).
The two groups did not differ significantly by age, clinician ratings of depression severity, or clinical ratings of global psychopathology at admission.
The study identified a subgroup of adolescent inpatients diagnosed with bipolar disorder without euphoric symptoms who exhibited explosiveness, hostility, and concurrent depression, comprising roughly a third of bipolar disorder diagnoses in the cohort. The findings suggest that these patients who lack signs of elevated mood and meet DMDD criteria routinely get diagnosed with bipolar I disorder, have a more problematic hospital stay, and have more symptoms at discharge, Dr. Pogge and his coinvestigators concluded.
The bipolar diagnoses might be incorrect, or there might be a substantial rate of comorbidity between DMDD and bipolar disease, he said.
The results also suggest that DMDD might be a common reason for psychiatric hospitalization of adolescents.
The study excluded patients whose records suggested other confounding factors or were missing any data on outcome measures.
Dr. Pogge reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Thirty-seven percent of 175 hospitalized adolescents diagnosed with bipolar disorder met criteria for a new disorder listed in the DSM-5 – disruptive mood dysregulation disorder.
Nearly all of the patients (96%) had been diagnosed with bipolar I disorder "not otherwise specified" (NOS) at the time of admission, a retrospective study found. Three other bipolar diagnoses were applied to two patients each: bipolar depression, bipolar mania, or mixed-episode bipolar disorder, David L. Pogge, Ph.D., reported at the annual meeting of the American Psychiatric Association.
The findings suggest that a substantial proportion of adolescent inpatients diagnosed with bipolar disorder may instead meet criteria for disruptive mood dysregulation disorder, and that clinicians should be more careful in diagnosing bipolar disorder, especially bipolar NOS, said Dr. Pogge of the department of psychology and counseling at Fairleigh Dickinson University, Teaneck, N.J. He also serves as director of psychology at Four Winds Hospital, which operates four campuses in New York state.
The study included records for all 1,505 patients aged 13-17 years who were admitted to a private psychiatric hospital over a 2-year period. At the time of admission, clinicians rated 1,351 patients as having at least moderate depression and 368 as also having severe symptoms of hostility and explosiveness. They diagnosed bipolar disorder in 259 cases. The investigators analyzed records for 174 patients with complete records or who had at least moderate depression and severe symptoms of hostility and explosiveness but no signs of elation or euphoria at the time of admission.
Disruptive mood dysregulation disorder is marked by intense temper outbursts superimposed on a background of persistent depressed or irritable mood. Temper outbursts and aggression are common reasons for inpatient admissions of children and adolescents, Dr. Pogge noted in his poster presentation.
Compared with the 63% of patients who did not meet criteria for disruptive mood dysregulation disorder (DMDD), patients who met the DMDD criteria were significantly more likely to experience restraint or seclusion while hospitalized (30% vs. 20%), receive a significantly higher number of restraints or seclusions (2.2 vs. 0.8), and remain hospitalized significantly longer (25 days vs. 21 days), he reported. At the time of discharge, clinicians’ ratings on the Global Assessment of Functioning (GAF) scale indicated significantly greater global psychopathology in patients with DMDD (a mean GAF score of 44), compared with patients who did not meet DMDD criteria (a mean GAF score of 50).
The two groups did not differ significantly by age, clinician ratings of depression severity, or clinical ratings of global psychopathology at admission.
The study identified a subgroup of adolescent inpatients diagnosed with bipolar disorder without euphoric symptoms who exhibited explosiveness, hostility, and concurrent depression, comprising roughly a third of bipolar disorder diagnoses in the cohort. The findings suggest that these patients who lack signs of elevated mood and meet DMDD criteria routinely get diagnosed with bipolar I disorder, have a more problematic hospital stay, and have more symptoms at discharge, Dr. Pogge and his coinvestigators concluded.
The bipolar diagnoses might be incorrect, or there might be a substantial rate of comorbidity between DMDD and bipolar disease, he said.
The results also suggest that DMDD might be a common reason for psychiatric hospitalization of adolescents.
The study excluded patients whose records suggested other confounding factors or were missing any data on outcome measures.
Dr. Pogge reported having no relevant financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Thirty-seven percent of 175 hospitalized adolescents diagnosed with bipolar disorder met criteria for a new disorder listed in the DSM-5 – disruptive mood dysregulation disorder.
Nearly all of the patients (96%) had been diagnosed with bipolar I disorder "not otherwise specified" (NOS) at the time of admission, a retrospective study found. Three other bipolar diagnoses were applied to two patients each: bipolar depression, bipolar mania, or mixed-episode bipolar disorder, David L. Pogge, Ph.D., reported at the annual meeting of the American Psychiatric Association.
The findings suggest that a substantial proportion of adolescent inpatients diagnosed with bipolar disorder may instead meet criteria for disruptive mood dysregulation disorder, and that clinicians should be more careful in diagnosing bipolar disorder, especially bipolar NOS, said Dr. Pogge of the department of psychology and counseling at Fairleigh Dickinson University, Teaneck, N.J. He also serves as director of psychology at Four Winds Hospital, which operates four campuses in New York state.
The study included records for all 1,505 patients aged 13-17 years who were admitted to a private psychiatric hospital over a 2-year period. At the time of admission, clinicians rated 1,351 patients as having at least moderate depression and 368 as also having severe symptoms of hostility and explosiveness. They diagnosed bipolar disorder in 259 cases. The investigators analyzed records for 174 patients with complete records or who had at least moderate depression and severe symptoms of hostility and explosiveness but no signs of elation or euphoria at the time of admission.
Disruptive mood dysregulation disorder is marked by intense temper outbursts superimposed on a background of persistent depressed or irritable mood. Temper outbursts and aggression are common reasons for inpatient admissions of children and adolescents, Dr. Pogge noted in his poster presentation.
Compared with the 63% of patients who did not meet criteria for disruptive mood dysregulation disorder (DMDD), patients who met the DMDD criteria were significantly more likely to experience restraint or seclusion while hospitalized (30% vs. 20%), receive a significantly higher number of restraints or seclusions (2.2 vs. 0.8), and remain hospitalized significantly longer (25 days vs. 21 days), he reported. At the time of discharge, clinicians’ ratings on the Global Assessment of Functioning (GAF) scale indicated significantly greater global psychopathology in patients with DMDD (a mean GAF score of 44), compared with patients who did not meet DMDD criteria (a mean GAF score of 50).
The two groups did not differ significantly by age, clinician ratings of depression severity, or clinical ratings of global psychopathology at admission.
The study identified a subgroup of adolescent inpatients diagnosed with bipolar disorder without euphoric symptoms who exhibited explosiveness, hostility, and concurrent depression, comprising roughly a third of bipolar disorder diagnoses in the cohort. The findings suggest that these patients who lack signs of elevated mood and meet DMDD criteria routinely get diagnosed with bipolar I disorder, have a more problematic hospital stay, and have more symptoms at discharge, Dr. Pogge and his coinvestigators concluded.
The bipolar diagnoses might be incorrect, or there might be a substantial rate of comorbidity between DMDD and bipolar disease, he said.
The results also suggest that DMDD might be a common reason for psychiatric hospitalization of adolescents.
The study excluded patients whose records suggested other confounding factors or were missing any data on outcome measures.
Dr. Pogge reported having no relevant financial disclosures.
On Twitter @sherryboschert
AT APA ANNUAL MEETING
Major finding: A putative diagnosis of disruptive mood dysregulation disorder fit 37% of 174 adolescent inpatients diagnosed with bipolar disorder.
Data source: Retrospective study of records for 2 years of admissions at one private psychiatric hospital.
Disclosures: Dr. Pogge reported having no relevant financial disclosures.
TMS may bring remission in bipolar depression
HOLLYWOOD, FLA. – Transcranial magnetic stimulation shows promise in highly treatment-resistant bipolar depression, a small observational study suggested.
Most studies of transcranial magnetic stimulation (TMS) have focused on treatment of major depressive disorder, the indication for which this noninvasive device therapy has Food and Drug Administration approval. TMS also is under study for the treatment of headaches, as well as for improvement of the negative symptoms of schizophrenia.
Yet little work has been done on TMS for bipolar depression, even though a pressing need exists for new treatment options for this often highly disruptive mood disorder. Antidepressant medications are often ineffective or can trigger a switch to a manic or hypomanic episode, Dr. William S. Gilmer noted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
He reported on 10 patients with bipolar II disorder and 4 who met criteria for bipolar disorder not otherwise specified. All had complicated nonpsychotic depression. All were refractory to and/or intolerant of multiple antidepressant agents during their current episode; indeed, the patients had previously been on a mean of 6.4 antidepressant drugs during this episode, which had already lasted more than 18 months in 9 of 14 cases. Four patients were previous nonresponders to ECT. None of the subjects had bipolar activation symptoms such as grandiosity, nocturnal alertness, or agitation at baseline, according to Dr. Gilmer, associate professor of clinical psychiatry and behavioral sciences at Northwestern University, Chicago.
After an extended period during which he attempted to optimize the participants’ medications, including reducing benzodiazepines and increasing mood-stabilizing drugs if necessary, all patients underwent high-frequency TMS at 10-Hz over the left dorsolateral prefrontal cortex 5 days per week according to the standard procedure using the NeuroStar device marketed by Neuronetics.
Nine of 14 patients achieved a clinically meaningful antidepressant response to TMS, as defined by at least a 50% reduction in scores on the Quick Inventory of Depressive Symptomatology (QIDS-16 SR) from an initial mean baseline of 18.9. A mean of 24.6 sessions was required. Four patients achieved and maintained remission, as defined by a QIDS-16 SR score below 6; this required a mean of 24.8 TMS sessions, followed by a taper phase.
Two of the four previous nonresponders to ECT achieved full remission on TMS, and a third had a significant response.
Although there were no study dropouts, seven patients experienced bipolar activation symptoms during TMS therapy that required drug therapy. Of note, four of five TMS nonresponders experienced clinically significant activation symptoms, compared with just three of nine TMS responders. Thus, the emergence of activation symptoms during TMS may turn out to be a predictor of poor outcome. This is a possibility warranting further study in controlled trials aimed at establishing optimal TMS treatment parameters in bipolar depression, the psychiatrist suggested.
Dr. Gilmer is on the speakers’ bureau for Neuronetics.
HOLLYWOOD, FLA. – Transcranial magnetic stimulation shows promise in highly treatment-resistant bipolar depression, a small observational study suggested.
Most studies of transcranial magnetic stimulation (TMS) have focused on treatment of major depressive disorder, the indication for which this noninvasive device therapy has Food and Drug Administration approval. TMS also is under study for the treatment of headaches, as well as for improvement of the negative symptoms of schizophrenia.
Yet little work has been done on TMS for bipolar depression, even though a pressing need exists for new treatment options for this often highly disruptive mood disorder. Antidepressant medications are often ineffective or can trigger a switch to a manic or hypomanic episode, Dr. William S. Gilmer noted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
He reported on 10 patients with bipolar II disorder and 4 who met criteria for bipolar disorder not otherwise specified. All had complicated nonpsychotic depression. All were refractory to and/or intolerant of multiple antidepressant agents during their current episode; indeed, the patients had previously been on a mean of 6.4 antidepressant drugs during this episode, which had already lasted more than 18 months in 9 of 14 cases. Four patients were previous nonresponders to ECT. None of the subjects had bipolar activation symptoms such as grandiosity, nocturnal alertness, or agitation at baseline, according to Dr. Gilmer, associate professor of clinical psychiatry and behavioral sciences at Northwestern University, Chicago.
After an extended period during which he attempted to optimize the participants’ medications, including reducing benzodiazepines and increasing mood-stabilizing drugs if necessary, all patients underwent high-frequency TMS at 10-Hz over the left dorsolateral prefrontal cortex 5 days per week according to the standard procedure using the NeuroStar device marketed by Neuronetics.
Nine of 14 patients achieved a clinically meaningful antidepressant response to TMS, as defined by at least a 50% reduction in scores on the Quick Inventory of Depressive Symptomatology (QIDS-16 SR) from an initial mean baseline of 18.9. A mean of 24.6 sessions was required. Four patients achieved and maintained remission, as defined by a QIDS-16 SR score below 6; this required a mean of 24.8 TMS sessions, followed by a taper phase.
Two of the four previous nonresponders to ECT achieved full remission on TMS, and a third had a significant response.
Although there were no study dropouts, seven patients experienced bipolar activation symptoms during TMS therapy that required drug therapy. Of note, four of five TMS nonresponders experienced clinically significant activation symptoms, compared with just three of nine TMS responders. Thus, the emergence of activation symptoms during TMS may turn out to be a predictor of poor outcome. This is a possibility warranting further study in controlled trials aimed at establishing optimal TMS treatment parameters in bipolar depression, the psychiatrist suggested.
Dr. Gilmer is on the speakers’ bureau for Neuronetics.
HOLLYWOOD, FLA. – Transcranial magnetic stimulation shows promise in highly treatment-resistant bipolar depression, a small observational study suggested.
Most studies of transcranial magnetic stimulation (TMS) have focused on treatment of major depressive disorder, the indication for which this noninvasive device therapy has Food and Drug Administration approval. TMS also is under study for the treatment of headaches, as well as for improvement of the negative symptoms of schizophrenia.
Yet little work has been done on TMS for bipolar depression, even though a pressing need exists for new treatment options for this often highly disruptive mood disorder. Antidepressant medications are often ineffective or can trigger a switch to a manic or hypomanic episode, Dr. William S. Gilmer noted at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
He reported on 10 patients with bipolar II disorder and 4 who met criteria for bipolar disorder not otherwise specified. All had complicated nonpsychotic depression. All were refractory to and/or intolerant of multiple antidepressant agents during their current episode; indeed, the patients had previously been on a mean of 6.4 antidepressant drugs during this episode, which had already lasted more than 18 months in 9 of 14 cases. Four patients were previous nonresponders to ECT. None of the subjects had bipolar activation symptoms such as grandiosity, nocturnal alertness, or agitation at baseline, according to Dr. Gilmer, associate professor of clinical psychiatry and behavioral sciences at Northwestern University, Chicago.
After an extended period during which he attempted to optimize the participants’ medications, including reducing benzodiazepines and increasing mood-stabilizing drugs if necessary, all patients underwent high-frequency TMS at 10-Hz over the left dorsolateral prefrontal cortex 5 days per week according to the standard procedure using the NeuroStar device marketed by Neuronetics.
Nine of 14 patients achieved a clinically meaningful antidepressant response to TMS, as defined by at least a 50% reduction in scores on the Quick Inventory of Depressive Symptomatology (QIDS-16 SR) from an initial mean baseline of 18.9. A mean of 24.6 sessions was required. Four patients achieved and maintained remission, as defined by a QIDS-16 SR score below 6; this required a mean of 24.8 TMS sessions, followed by a taper phase.
Two of the four previous nonresponders to ECT achieved full remission on TMS, and a third had a significant response.
Although there were no study dropouts, seven patients experienced bipolar activation symptoms during TMS therapy that required drug therapy. Of note, four of five TMS nonresponders experienced clinically significant activation symptoms, compared with just three of nine TMS responders. Thus, the emergence of activation symptoms during TMS may turn out to be a predictor of poor outcome. This is a possibility warranting further study in controlled trials aimed at establishing optimal TMS treatment parameters in bipolar depression, the psychiatrist suggested.
Dr. Gilmer is on the speakers’ bureau for Neuronetics.
AT THE NCDEU MEETING
Major finding: Nine of 14 patients with highly treatment-resistant bipolar depression achieved a clinically meaningful antidepressant response to transcranial magnetic stimulation, including 4 who reached and maintained remission. Two of the four in remission were prior nonresponders to ECT.
Data source: This observational study was a naturalistic case series with no control group. Patients had been unresponsive to and/or intolerant of a mean of 6.4 antidepressant medications during their current episode of bipolar depression, which had been ongoing for more than 18 months in nine cases.
Disclosures: Dr. Gilmer is on the speakers’ bureau for Neuronetics, which markets the transcranial magnetic stimulation device used in the investigation.
Valacyclovir improves cognition in bipolar patients
HOLLYWOOD, FLA. – A 4-month course of the oral antiviral agent valacyclovir boosted cognition in herpes simplex virus-1–seropositive patients with bipolar disorder and cognitive impairment in a randomized, double-blind placebo-controlled clinical trial.
In this 60-patient study, 53% of participants assigned to valacyclovir (Valtrex) exhibited a clinically meaningful improvement in cognitive function, defined as at least a 10-point gain over baseline on the Repeatable Battery for the Assessment of Neurological Status (RBANS), compared with 14% in the placebo arm, Dr. Jennifer L. Payne reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
The impressive improvement in response to valacyclovir documented in this study lends support to the viral hypothesis of mental illness, she said. This hypothesis posits that infection with one of the herpes viruses can in genetically vulnerable individuals lead to major mental illness, including schizophrenia, bipolar disorder, and perhaps Alzheimer’s disease.
"The idea behind this is that episodic reactivation of the virus in the [central nervous system] by stress or other stimuli could trigger mood, cognitive, or psychotic symptoms. If you treat patients you see this all the time: A patient comes under some kind of stress and becomes psychiatrically ill. One of the thoughts is that [herpes simplex virus] could underlie some of that psychopathology," according to Dr. Payne, a psychiatrist and director of the women’s mood disorders center at Johns Hopkins University, Baltimore.
She credited Faith B. Dickerson, Ph.D., of the Sheppard Pratt Institute in Baltimore with earlier groundbreaking work identifying a link between herpes simplex virus type 1 (HSV-1) and cognitive dysfunction in bipolar disorder. Dr. Dickerson and her colleagues reported a roughly 20-fold increased risk for cognitive impairment on the RBANS in HSV-1–seropositive, compared with seronegative patients with bipolar disorder (Biol. Psychiatry 2004;55:588-93).
The RBANS is a paper and pencil test that takes roughly 25 minutes. The mean score in the general population is 100. One standard deviation below the mean is a score of 85. Upon retaking the test, 16% of the general population are able to improve their score by 10 points or more, a rate close to the placebo group’s performance in Dr. Payne’s bipolar disorder study. The RBANS has individual sections for attention, immediate memory, delayed memory, language, and visuospatial construction.
HSV-1 typically causes oral herpes lesions. It’s an extremely common infection. By middle age, roughly 70% of Americans have serologic antibody titers to HSV-1. The virus infects the CNS and often remains in a latent state for many years, punctuated by symptomatic recurrences.
Dr. Payne screened 106 bipolar disorder patients; 84 proved HSV-1 seropositive. The mean baseline RBANS score in the seropositive patients was 75, compared with 92 in the seronegative cohort. The observed association between HSV-1 serologic status and RBANS score remained significant in a multivariate logistic regression analysis after investigators controlled for education level.
The 60 study participants were bipolar disorder outpatients, with an average age of 43. Thirty-seven percent met diagnostic criteria for bipolar I disorder; the rest, for bipolar II disorder. All were required to have baseline cognitive impairment as reflected by an RBANS score of 85 or less. Their average baseline Montgomery-Asberg Depression Rating Scale (MADRS) score was 24, with a Young Mania Rating Scale (YMRS) score of 8. Patients remained on their usual psychiatric medications during the study.
Participants in the 4-month trial were evaluated every 2 weeks for a change in mood symptoms using the MADRS and YMRS. The results came as a surprise.
"Our hypothesis had been that cognitive improvement with valacyclovir would be associated with improvement in depression, but the MADRS scores didn’t change over time," according to Dr. Payne.
As is typical in months-long clinical trials conducted in patients with bipolar disorder, there was a high dropout rate. Mean RBANS scores in the 19 patients in the valacyclovir group who completed the study improved from a baseline of 67.6 to 77.7 at 4 months. The 22 study completers in the control group showed no significant change in scores over time.
Dr. Payne said that if these results are confirmed in another clinical trial – and she plans to conduct one including seropositive bipolar disorder patients who are not cognitively impaired – it would be practice changing.
"If these findings hold up, it would indicate that as clinicians, we need to be testing for HSV-1 and treating it in our patients," she said.
Her future plans also include studying HSV-1 antibody status, cognition, and the possible impact of antiviral therapy in patients with major depressive disorder.
Patients with bipolar disorder often complain and exhibit symptoms of cognitive dysfunction, particularly in the domains of attention, memory, and executive function. The dysfunction typically worsens during manic or depressive episodes, but it’s often still present when bipolar disorder patients are affectively neutral.
The randomized trial was funded by the Stanley Medical Research Institute. Dr. Payne reported serving as a consultant to Pfizer and AstraZeneca.
HOLLYWOOD, FLA. – A 4-month course of the oral antiviral agent valacyclovir boosted cognition in herpes simplex virus-1–seropositive patients with bipolar disorder and cognitive impairment in a randomized, double-blind placebo-controlled clinical trial.
In this 60-patient study, 53% of participants assigned to valacyclovir (Valtrex) exhibited a clinically meaningful improvement in cognitive function, defined as at least a 10-point gain over baseline on the Repeatable Battery for the Assessment of Neurological Status (RBANS), compared with 14% in the placebo arm, Dr. Jennifer L. Payne reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
The impressive improvement in response to valacyclovir documented in this study lends support to the viral hypothesis of mental illness, she said. This hypothesis posits that infection with one of the herpes viruses can in genetically vulnerable individuals lead to major mental illness, including schizophrenia, bipolar disorder, and perhaps Alzheimer’s disease.
"The idea behind this is that episodic reactivation of the virus in the [central nervous system] by stress or other stimuli could trigger mood, cognitive, or psychotic symptoms. If you treat patients you see this all the time: A patient comes under some kind of stress and becomes psychiatrically ill. One of the thoughts is that [herpes simplex virus] could underlie some of that psychopathology," according to Dr. Payne, a psychiatrist and director of the women’s mood disorders center at Johns Hopkins University, Baltimore.
She credited Faith B. Dickerson, Ph.D., of the Sheppard Pratt Institute in Baltimore with earlier groundbreaking work identifying a link between herpes simplex virus type 1 (HSV-1) and cognitive dysfunction in bipolar disorder. Dr. Dickerson and her colleagues reported a roughly 20-fold increased risk for cognitive impairment on the RBANS in HSV-1–seropositive, compared with seronegative patients with bipolar disorder (Biol. Psychiatry 2004;55:588-93).
The RBANS is a paper and pencil test that takes roughly 25 minutes. The mean score in the general population is 100. One standard deviation below the mean is a score of 85. Upon retaking the test, 16% of the general population are able to improve their score by 10 points or more, a rate close to the placebo group’s performance in Dr. Payne’s bipolar disorder study. The RBANS has individual sections for attention, immediate memory, delayed memory, language, and visuospatial construction.
HSV-1 typically causes oral herpes lesions. It’s an extremely common infection. By middle age, roughly 70% of Americans have serologic antibody titers to HSV-1. The virus infects the CNS and often remains in a latent state for many years, punctuated by symptomatic recurrences.
Dr. Payne screened 106 bipolar disorder patients; 84 proved HSV-1 seropositive. The mean baseline RBANS score in the seropositive patients was 75, compared with 92 in the seronegative cohort. The observed association between HSV-1 serologic status and RBANS score remained significant in a multivariate logistic regression analysis after investigators controlled for education level.
The 60 study participants were bipolar disorder outpatients, with an average age of 43. Thirty-seven percent met diagnostic criteria for bipolar I disorder; the rest, for bipolar II disorder. All were required to have baseline cognitive impairment as reflected by an RBANS score of 85 or less. Their average baseline Montgomery-Asberg Depression Rating Scale (MADRS) score was 24, with a Young Mania Rating Scale (YMRS) score of 8. Patients remained on their usual psychiatric medications during the study.
Participants in the 4-month trial were evaluated every 2 weeks for a change in mood symptoms using the MADRS and YMRS. The results came as a surprise.
"Our hypothesis had been that cognitive improvement with valacyclovir would be associated with improvement in depression, but the MADRS scores didn’t change over time," according to Dr. Payne.
As is typical in months-long clinical trials conducted in patients with bipolar disorder, there was a high dropout rate. Mean RBANS scores in the 19 patients in the valacyclovir group who completed the study improved from a baseline of 67.6 to 77.7 at 4 months. The 22 study completers in the control group showed no significant change in scores over time.
Dr. Payne said that if these results are confirmed in another clinical trial – and she plans to conduct one including seropositive bipolar disorder patients who are not cognitively impaired – it would be practice changing.
"If these findings hold up, it would indicate that as clinicians, we need to be testing for HSV-1 and treating it in our patients," she said.
Her future plans also include studying HSV-1 antibody status, cognition, and the possible impact of antiviral therapy in patients with major depressive disorder.
Patients with bipolar disorder often complain and exhibit symptoms of cognitive dysfunction, particularly in the domains of attention, memory, and executive function. The dysfunction typically worsens during manic or depressive episodes, but it’s often still present when bipolar disorder patients are affectively neutral.
The randomized trial was funded by the Stanley Medical Research Institute. Dr. Payne reported serving as a consultant to Pfizer and AstraZeneca.
HOLLYWOOD, FLA. – A 4-month course of the oral antiviral agent valacyclovir boosted cognition in herpes simplex virus-1–seropositive patients with bipolar disorder and cognitive impairment in a randomized, double-blind placebo-controlled clinical trial.
In this 60-patient study, 53% of participants assigned to valacyclovir (Valtrex) exhibited a clinically meaningful improvement in cognitive function, defined as at least a 10-point gain over baseline on the Repeatable Battery for the Assessment of Neurological Status (RBANS), compared with 14% in the placebo arm, Dr. Jennifer L. Payne reported at a meeting of the New Clinical Drug Evaluation Unit sponsored by the National Institute of Mental Health.
The impressive improvement in response to valacyclovir documented in this study lends support to the viral hypothesis of mental illness, she said. This hypothesis posits that infection with one of the herpes viruses can in genetically vulnerable individuals lead to major mental illness, including schizophrenia, bipolar disorder, and perhaps Alzheimer’s disease.
"The idea behind this is that episodic reactivation of the virus in the [central nervous system] by stress or other stimuli could trigger mood, cognitive, or psychotic symptoms. If you treat patients you see this all the time: A patient comes under some kind of stress and becomes psychiatrically ill. One of the thoughts is that [herpes simplex virus] could underlie some of that psychopathology," according to Dr. Payne, a psychiatrist and director of the women’s mood disorders center at Johns Hopkins University, Baltimore.
She credited Faith B. Dickerson, Ph.D., of the Sheppard Pratt Institute in Baltimore with earlier groundbreaking work identifying a link between herpes simplex virus type 1 (HSV-1) and cognitive dysfunction in bipolar disorder. Dr. Dickerson and her colleagues reported a roughly 20-fold increased risk for cognitive impairment on the RBANS in HSV-1–seropositive, compared with seronegative patients with bipolar disorder (Biol. Psychiatry 2004;55:588-93).
The RBANS is a paper and pencil test that takes roughly 25 minutes. The mean score in the general population is 100. One standard deviation below the mean is a score of 85. Upon retaking the test, 16% of the general population are able to improve their score by 10 points or more, a rate close to the placebo group’s performance in Dr. Payne’s bipolar disorder study. The RBANS has individual sections for attention, immediate memory, delayed memory, language, and visuospatial construction.
HSV-1 typically causes oral herpes lesions. It’s an extremely common infection. By middle age, roughly 70% of Americans have serologic antibody titers to HSV-1. The virus infects the CNS and often remains in a latent state for many years, punctuated by symptomatic recurrences.
Dr. Payne screened 106 bipolar disorder patients; 84 proved HSV-1 seropositive. The mean baseline RBANS score in the seropositive patients was 75, compared with 92 in the seronegative cohort. The observed association between HSV-1 serologic status and RBANS score remained significant in a multivariate logistic regression analysis after investigators controlled for education level.
The 60 study participants were bipolar disorder outpatients, with an average age of 43. Thirty-seven percent met diagnostic criteria for bipolar I disorder; the rest, for bipolar II disorder. All were required to have baseline cognitive impairment as reflected by an RBANS score of 85 or less. Their average baseline Montgomery-Asberg Depression Rating Scale (MADRS) score was 24, with a Young Mania Rating Scale (YMRS) score of 8. Patients remained on their usual psychiatric medications during the study.
Participants in the 4-month trial were evaluated every 2 weeks for a change in mood symptoms using the MADRS and YMRS. The results came as a surprise.
"Our hypothesis had been that cognitive improvement with valacyclovir would be associated with improvement in depression, but the MADRS scores didn’t change over time," according to Dr. Payne.
As is typical in months-long clinical trials conducted in patients with bipolar disorder, there was a high dropout rate. Mean RBANS scores in the 19 patients in the valacyclovir group who completed the study improved from a baseline of 67.6 to 77.7 at 4 months. The 22 study completers in the control group showed no significant change in scores over time.
Dr. Payne said that if these results are confirmed in another clinical trial – and she plans to conduct one including seropositive bipolar disorder patients who are not cognitively impaired – it would be practice changing.
"If these findings hold up, it would indicate that as clinicians, we need to be testing for HSV-1 and treating it in our patients," she said.
Her future plans also include studying HSV-1 antibody status, cognition, and the possible impact of antiviral therapy in patients with major depressive disorder.
Patients with bipolar disorder often complain and exhibit symptoms of cognitive dysfunction, particularly in the domains of attention, memory, and executive function. The dysfunction typically worsens during manic or depressive episodes, but it’s often still present when bipolar disorder patients are affectively neutral.
The randomized trial was funded by the Stanley Medical Research Institute. Dr. Payne reported serving as a consultant to Pfizer and AstraZeneca.
AT THE NCDEU MEETING
Major finding: Fifty-three percent of herpes simplex virus-1–seropositive patients with bipolar disorder and cognitive impairment exhibited objective cognitive improvement after 4 months on valacyclovir, as did a mere 14% on placebo.
Data source: A 60-patient randomized, double-blind, placebo-controlled clinical trial.
Disclosures: The study was sponsored by the Stanley Medical Research Institute. Dr. Payne reported serving as a consultant to Pfizer and AstraZeneca.
ED psychiatrists perform nuanced assessments of traumatized patients
Since Freud, the fields of psychiatry, psychology, and psychoanalysis have grappled with trauma and its role in psychopathology. In modern times, these fields remain uncertain about the relative influence that biology, character, and environment play in disordering the lives of traumatized patients. Modern theories try to make sense of early attachment and its impact on development and resiliency. Most bluntly put, why do some soldiers go to war and return seemingly well adapted, while others can not reenter civilian life because the psychic scars are too constricting?
In the psychiatric emergency room, psychiatrists quickly take in multiple, complex facets of a patient’s life in an attempt to assess and judge the patient’s ability to withstand their predicament in the community. Our raw purpose is to assess safety and decide whether the patient needs be committed to a psychiatric inpatient facility. In that assessment, the doctor quickly tries to take in the history, biology, character, and the social milieu of the patient, and judge the degree of risk that patients pose to themselves, their families, and their communities.
The task of the psychiatrist in this job is immense. It is profoundly complicated. It is rich and riddled with considerations. It is not seen so by many in our field, and not by our patients. To patients and the public, we are at worst jailers. To our field, we are at worst crude physicians in a souped-up triage unit.
But think about what we really do. We are surveyors and judges of trauma and character.
Freud knew that sexual abuse and death on the battlefield occurred in his time. He was profoundly struck by both. He created theories to explain these dark experiences. Freud also knew that our deep human psyches contain capacities beyond immediate experience and that one could project and layer fantasy into lived experience. He grappled with the role that fantasy plays in our interpretations of experience. He angered feminists and others because his theoretical changes undermined the role of real trauma and highlighted the role the individual mind plays in repeating trauma.
In the psychiatric ER, we witness the real traumas that weave through patient lives. We often experience these traumas, even to the extent of avoiding certain patients whose realities strike too close to home. This is especially potent for psychiatrists with young children working with patients whose stories and affects stir our worst fears for our own children and families.
We also deal with patients and personalities who seem to seek repeated suffering in the form of both micro- and macrotrauma. We sympathize with the involuntary, victim-bound suffering, and we cringe at and speculate about the conscious and unconscious degrees of self-sabotage on display.
There are several forms of social, political, and historical trauma that are close to home for me and my contemporary American peers that repeatedly come up in patient narratives in the emergency room: racism, sexism, homophobia, and transphobia. In my 41 years of life, I have encountered or experienced all of these forms of oppression, trauma, and microaggressions. I know that they are "real." Many psychiatric ER psychiatrists know that they are real. But in the psych ER, where profound decisions weigh on individual assessment, what is real and what that means becomes relative.
It may be obvious upon arrival that a patient has just suffered a serious physical injury, sexual assault, or severe and obvious abandonment by a loved one. But when patients report histories of such events and a seeming pattern of repeated abuse, we naturally wonder about their perception and their own role in creating self-destructive experiences. Because we have experience and understanding of the human compulsion to repeat even painful life experiences, we are cautiously skeptical when a narrative is full of catastrophe, especially when presented as accidental or without agency.
For instance, even though we know about the depth of racism in our history and its profoundly traumatogenic potential (though this gets little or no attention in formal training!), we must be curious about the meaning an individual attaches to it, and how that meaning may serve or defeat his or her psychological existence and progression across life development.
The same goes for women, gay people, and transgender people who are so exposed and vulnerable to real and perceived physical and emotional trauma and aggression on a daily basis.
How does a person suffer in the moment after an infliction of trauma or aggression? How does a person encode that experience in her character, early in life and later in life? How does it color her experience and interactions? In the comprehensive psychiatric emergency program (CPEP), we must try to answer all these complex questions in what seems like a blink of the eye!
We only see a slice of a person’s life. We focus on the patient’s "history of present illness," the narrative history told in that moment, and the mental status exam. We rely heavily on collateral information to corroborate as much as possible. We use this to measure accuracy and distortion, always holding a skeptical lens against our patient!
While we want to believe our patients and take their histories at face value, we can’t fully do so because we know, instinctively or through training, that fantasy permeates the human mind and transforms meaning. Yet, at the same time, we assess that very idiosyncratic meaning for what it is, because that meaning stays with patients as they move from our ER to the street or the unit. And it is that meaning, embedded in an individual’s coping strategies and character that partly predicts what and how the person will do. It is an inherent consideration of risk and resilience, and we instinctively factor that into our decision making. This is the art of psychiatry at its best.
The CPEP is the frontline of psychiatry. Some residents dislike it because it vibrates with anxiety and responsibility. It is a place in which clinicians tend to come unglued behind the scenes, joking as if at a party, talking loudly and blurting out inane and obscene lines from TV shows and real life. In the back room, the burdened frontline psychiatrists and staff attempt to regain control and wrestle back their own meaning in life, in the face of withstanding traumas, distortions, and psychosis – the delinking of meaning – while rendering verdicts of risk and resilience based in rapid assessments of social, cultural, characterological, and biological factors that make up patient lives.
We swiftly analyze our patients and decide their immediate fate – street, home, unit, extended observation, needles, blood, medications, even visitors and babysitters. We hold them, or we jail them, depending on your view. But we do it with benign and perhaps grandiose intentions to protect, comfort, and quickly "know" them in order to progress them to the next most right place. And we do this almost without knowing that and how we are navigating a delicate and profoundly intricate path of evaluation and decision-making that is uniquely human in its intellectual and emotional nuance.
It is a job full of sharp edges and soft curves, a job that makes a bouncer an analyst and an analyst a bouncer. It is a job never to be reduced to algorithm or computation.
Dr. Pula is a psychiatrist at New York–Presbyterian/Columbia University Medical Center. He also is in private practice and is a psychoanalytic candidate at Columbia.
Since Freud, the fields of psychiatry, psychology, and psychoanalysis have grappled with trauma and its role in psychopathology. In modern times, these fields remain uncertain about the relative influence that biology, character, and environment play in disordering the lives of traumatized patients. Modern theories try to make sense of early attachment and its impact on development and resiliency. Most bluntly put, why do some soldiers go to war and return seemingly well adapted, while others can not reenter civilian life because the psychic scars are too constricting?
In the psychiatric emergency room, psychiatrists quickly take in multiple, complex facets of a patient’s life in an attempt to assess and judge the patient’s ability to withstand their predicament in the community. Our raw purpose is to assess safety and decide whether the patient needs be committed to a psychiatric inpatient facility. In that assessment, the doctor quickly tries to take in the history, biology, character, and the social milieu of the patient, and judge the degree of risk that patients pose to themselves, their families, and their communities.
The task of the psychiatrist in this job is immense. It is profoundly complicated. It is rich and riddled with considerations. It is not seen so by many in our field, and not by our patients. To patients and the public, we are at worst jailers. To our field, we are at worst crude physicians in a souped-up triage unit.
But think about what we really do. We are surveyors and judges of trauma and character.
Freud knew that sexual abuse and death on the battlefield occurred in his time. He was profoundly struck by both. He created theories to explain these dark experiences. Freud also knew that our deep human psyches contain capacities beyond immediate experience and that one could project and layer fantasy into lived experience. He grappled with the role that fantasy plays in our interpretations of experience. He angered feminists and others because his theoretical changes undermined the role of real trauma and highlighted the role the individual mind plays in repeating trauma.
In the psychiatric ER, we witness the real traumas that weave through patient lives. We often experience these traumas, even to the extent of avoiding certain patients whose realities strike too close to home. This is especially potent for psychiatrists with young children working with patients whose stories and affects stir our worst fears for our own children and families.
We also deal with patients and personalities who seem to seek repeated suffering in the form of both micro- and macrotrauma. We sympathize with the involuntary, victim-bound suffering, and we cringe at and speculate about the conscious and unconscious degrees of self-sabotage on display.
There are several forms of social, political, and historical trauma that are close to home for me and my contemporary American peers that repeatedly come up in patient narratives in the emergency room: racism, sexism, homophobia, and transphobia. In my 41 years of life, I have encountered or experienced all of these forms of oppression, trauma, and microaggressions. I know that they are "real." Many psychiatric ER psychiatrists know that they are real. But in the psych ER, where profound decisions weigh on individual assessment, what is real and what that means becomes relative.
It may be obvious upon arrival that a patient has just suffered a serious physical injury, sexual assault, or severe and obvious abandonment by a loved one. But when patients report histories of such events and a seeming pattern of repeated abuse, we naturally wonder about their perception and their own role in creating self-destructive experiences. Because we have experience and understanding of the human compulsion to repeat even painful life experiences, we are cautiously skeptical when a narrative is full of catastrophe, especially when presented as accidental or without agency.
For instance, even though we know about the depth of racism in our history and its profoundly traumatogenic potential (though this gets little or no attention in formal training!), we must be curious about the meaning an individual attaches to it, and how that meaning may serve or defeat his or her psychological existence and progression across life development.
The same goes for women, gay people, and transgender people who are so exposed and vulnerable to real and perceived physical and emotional trauma and aggression on a daily basis.
How does a person suffer in the moment after an infliction of trauma or aggression? How does a person encode that experience in her character, early in life and later in life? How does it color her experience and interactions? In the comprehensive psychiatric emergency program (CPEP), we must try to answer all these complex questions in what seems like a blink of the eye!
We only see a slice of a person’s life. We focus on the patient’s "history of present illness," the narrative history told in that moment, and the mental status exam. We rely heavily on collateral information to corroborate as much as possible. We use this to measure accuracy and distortion, always holding a skeptical lens against our patient!
While we want to believe our patients and take their histories at face value, we can’t fully do so because we know, instinctively or through training, that fantasy permeates the human mind and transforms meaning. Yet, at the same time, we assess that very idiosyncratic meaning for what it is, because that meaning stays with patients as they move from our ER to the street or the unit. And it is that meaning, embedded in an individual’s coping strategies and character that partly predicts what and how the person will do. It is an inherent consideration of risk and resilience, and we instinctively factor that into our decision making. This is the art of psychiatry at its best.
The CPEP is the frontline of psychiatry. Some residents dislike it because it vibrates with anxiety and responsibility. It is a place in which clinicians tend to come unglued behind the scenes, joking as if at a party, talking loudly and blurting out inane and obscene lines from TV shows and real life. In the back room, the burdened frontline psychiatrists and staff attempt to regain control and wrestle back their own meaning in life, in the face of withstanding traumas, distortions, and psychosis – the delinking of meaning – while rendering verdicts of risk and resilience based in rapid assessments of social, cultural, characterological, and biological factors that make up patient lives.
We swiftly analyze our patients and decide their immediate fate – street, home, unit, extended observation, needles, blood, medications, even visitors and babysitters. We hold them, or we jail them, depending on your view. But we do it with benign and perhaps grandiose intentions to protect, comfort, and quickly "know" them in order to progress them to the next most right place. And we do this almost without knowing that and how we are navigating a delicate and profoundly intricate path of evaluation and decision-making that is uniquely human in its intellectual and emotional nuance.
It is a job full of sharp edges and soft curves, a job that makes a bouncer an analyst and an analyst a bouncer. It is a job never to be reduced to algorithm or computation.
Dr. Pula is a psychiatrist at New York–Presbyterian/Columbia University Medical Center. He also is in private practice and is a psychoanalytic candidate at Columbia.
Since Freud, the fields of psychiatry, psychology, and psychoanalysis have grappled with trauma and its role in psychopathology. In modern times, these fields remain uncertain about the relative influence that biology, character, and environment play in disordering the lives of traumatized patients. Modern theories try to make sense of early attachment and its impact on development and resiliency. Most bluntly put, why do some soldiers go to war and return seemingly well adapted, while others can not reenter civilian life because the psychic scars are too constricting?
In the psychiatric emergency room, psychiatrists quickly take in multiple, complex facets of a patient’s life in an attempt to assess and judge the patient’s ability to withstand their predicament in the community. Our raw purpose is to assess safety and decide whether the patient needs be committed to a psychiatric inpatient facility. In that assessment, the doctor quickly tries to take in the history, biology, character, and the social milieu of the patient, and judge the degree of risk that patients pose to themselves, their families, and their communities.
The task of the psychiatrist in this job is immense. It is profoundly complicated. It is rich and riddled with considerations. It is not seen so by many in our field, and not by our patients. To patients and the public, we are at worst jailers. To our field, we are at worst crude physicians in a souped-up triage unit.
But think about what we really do. We are surveyors and judges of trauma and character.
Freud knew that sexual abuse and death on the battlefield occurred in his time. He was profoundly struck by both. He created theories to explain these dark experiences. Freud also knew that our deep human psyches contain capacities beyond immediate experience and that one could project and layer fantasy into lived experience. He grappled with the role that fantasy plays in our interpretations of experience. He angered feminists and others because his theoretical changes undermined the role of real trauma and highlighted the role the individual mind plays in repeating trauma.
In the psychiatric ER, we witness the real traumas that weave through patient lives. We often experience these traumas, even to the extent of avoiding certain patients whose realities strike too close to home. This is especially potent for psychiatrists with young children working with patients whose stories and affects stir our worst fears for our own children and families.
We also deal with patients and personalities who seem to seek repeated suffering in the form of both micro- and macrotrauma. We sympathize with the involuntary, victim-bound suffering, and we cringe at and speculate about the conscious and unconscious degrees of self-sabotage on display.
There are several forms of social, political, and historical trauma that are close to home for me and my contemporary American peers that repeatedly come up in patient narratives in the emergency room: racism, sexism, homophobia, and transphobia. In my 41 years of life, I have encountered or experienced all of these forms of oppression, trauma, and microaggressions. I know that they are "real." Many psychiatric ER psychiatrists know that they are real. But in the psych ER, where profound decisions weigh on individual assessment, what is real and what that means becomes relative.
It may be obvious upon arrival that a patient has just suffered a serious physical injury, sexual assault, or severe and obvious abandonment by a loved one. But when patients report histories of such events and a seeming pattern of repeated abuse, we naturally wonder about their perception and their own role in creating self-destructive experiences. Because we have experience and understanding of the human compulsion to repeat even painful life experiences, we are cautiously skeptical when a narrative is full of catastrophe, especially when presented as accidental or without agency.
For instance, even though we know about the depth of racism in our history and its profoundly traumatogenic potential (though this gets little or no attention in formal training!), we must be curious about the meaning an individual attaches to it, and how that meaning may serve or defeat his or her psychological existence and progression across life development.
The same goes for women, gay people, and transgender people who are so exposed and vulnerable to real and perceived physical and emotional trauma and aggression on a daily basis.
How does a person suffer in the moment after an infliction of trauma or aggression? How does a person encode that experience in her character, early in life and later in life? How does it color her experience and interactions? In the comprehensive psychiatric emergency program (CPEP), we must try to answer all these complex questions in what seems like a blink of the eye!
We only see a slice of a person’s life. We focus on the patient’s "history of present illness," the narrative history told in that moment, and the mental status exam. We rely heavily on collateral information to corroborate as much as possible. We use this to measure accuracy and distortion, always holding a skeptical lens against our patient!
While we want to believe our patients and take their histories at face value, we can’t fully do so because we know, instinctively or through training, that fantasy permeates the human mind and transforms meaning. Yet, at the same time, we assess that very idiosyncratic meaning for what it is, because that meaning stays with patients as they move from our ER to the street or the unit. And it is that meaning, embedded in an individual’s coping strategies and character that partly predicts what and how the person will do. It is an inherent consideration of risk and resilience, and we instinctively factor that into our decision making. This is the art of psychiatry at its best.
The CPEP is the frontline of psychiatry. Some residents dislike it because it vibrates with anxiety and responsibility. It is a place in which clinicians tend to come unglued behind the scenes, joking as if at a party, talking loudly and blurting out inane and obscene lines from TV shows and real life. In the back room, the burdened frontline psychiatrists and staff attempt to regain control and wrestle back their own meaning in life, in the face of withstanding traumas, distortions, and psychosis – the delinking of meaning – while rendering verdicts of risk and resilience based in rapid assessments of social, cultural, characterological, and biological factors that make up patient lives.
We swiftly analyze our patients and decide their immediate fate – street, home, unit, extended observation, needles, blood, medications, even visitors and babysitters. We hold them, or we jail them, depending on your view. But we do it with benign and perhaps grandiose intentions to protect, comfort, and quickly "know" them in order to progress them to the next most right place. And we do this almost without knowing that and how we are navigating a delicate and profoundly intricate path of evaluation and decision-making that is uniquely human in its intellectual and emotional nuance.
It is a job full of sharp edges and soft curves, a job that makes a bouncer an analyst and an analyst a bouncer. It is a job never to be reduced to algorithm or computation.
Dr. Pula is a psychiatrist at New York–Presbyterian/Columbia University Medical Center. He also is in private practice and is a psychoanalytic candidate at Columbia.
Childhood neglect affects adult close-relationship capacity
SAN FRANCISCO – Childhood neglect correlated with impaired capacity for close social relationships as an adult in a study of 114 nonpsychotic psychiatric inpatients.
The difficulty for patients with a history of childhood neglect centered more in maintaining than in starting close social relationships as adults, Thachell Tanis and Lisa J. Cohen, Ph.D., reported in a press briefing and a poster presentation at the annual meeting of the American Psychiatric Association.
The study used separate clinical self-report surveys to assess patients’ childhood histories and adult relational capacity. The Multidimensional Neglectful Behavior Scale assessed emotional, physical, cognitive, and supervisory neglect in the patients’ past. Patients also completed the relational domain of the Severity Indices of Personality Problems to assess capacity for intimacy or enduring relationships and the ability to feel recognized in relationships.
Each type of neglect significantly and negatively affected each facet in the relational domain. "Everything correlated with everything," said Ms. Tanis, a doctoral student at City College of New York.
The deficits were most striking in patients’ capacity for enduring relationships, said Dr. Cohen, professor of clinical psychiatry and behavioral sciences at Albert Einstein College of Medicine, New York. Childhood neglect as a whole, for example, correlated with an 81% greater negative effect on adult capacity for enduring relationships, compared with the negative effect on capacity for intimacy.
The cohort was 57% female, with a mean age of 39 years. Psychiatric diagnoses included depression in 55%, substance use disorder in 20%, bipolar disorder in 12%, anxiety in 10%, psychosis in 1%, and other diagnoses in 3%. The cohort was 44% white, 28% Hispanic, 16% black, 6% multiracial, 5% Asian, and 2% other ethnicities. The percentages total more than 100% because of rounding.
Prior studies have well documented the adverse effects of more dramatic forms of childhood maltreatment. Physical and sexual abuses in childhood, for example, have been associated with adult depression, eating disorders, and personality disorders, Dr. Cohen said. Only in recent years have mental health providers recognized the importance of less dramatic forms of childhood maltreatment, such as emotional abuse and neglect, and begun to study those issues.
The topic deserves further exploration, she added, because identifying these impairments in people with histories of childhood neglect might lead to better case conceptualization and possibly better treatment.
"You really do want to pay attention to your patients’ history of childhood neglect," Dr. Cohen said. "There may be more subtle types of maltreatment that have pernicious effects over time." If there is a history of neglect in childhood, pay attention not only to the patient’s ability to engage in relationships but the ability to maintain such relationships over time, she added.
The study was limited by using retrospective, self-report measures and its focus on inpatients, which might restrict the generalizability of the results.
The investigators reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Childhood neglect correlated with impaired capacity for close social relationships as an adult in a study of 114 nonpsychotic psychiatric inpatients.
The difficulty for patients with a history of childhood neglect centered more in maintaining than in starting close social relationships as adults, Thachell Tanis and Lisa J. Cohen, Ph.D., reported in a press briefing and a poster presentation at the annual meeting of the American Psychiatric Association.
The study used separate clinical self-report surveys to assess patients’ childhood histories and adult relational capacity. The Multidimensional Neglectful Behavior Scale assessed emotional, physical, cognitive, and supervisory neglect in the patients’ past. Patients also completed the relational domain of the Severity Indices of Personality Problems to assess capacity for intimacy or enduring relationships and the ability to feel recognized in relationships.
Each type of neglect significantly and negatively affected each facet in the relational domain. "Everything correlated with everything," said Ms. Tanis, a doctoral student at City College of New York.
The deficits were most striking in patients’ capacity for enduring relationships, said Dr. Cohen, professor of clinical psychiatry and behavioral sciences at Albert Einstein College of Medicine, New York. Childhood neglect as a whole, for example, correlated with an 81% greater negative effect on adult capacity for enduring relationships, compared with the negative effect on capacity for intimacy.
The cohort was 57% female, with a mean age of 39 years. Psychiatric diagnoses included depression in 55%, substance use disorder in 20%, bipolar disorder in 12%, anxiety in 10%, psychosis in 1%, and other diagnoses in 3%. The cohort was 44% white, 28% Hispanic, 16% black, 6% multiracial, 5% Asian, and 2% other ethnicities. The percentages total more than 100% because of rounding.
Prior studies have well documented the adverse effects of more dramatic forms of childhood maltreatment. Physical and sexual abuses in childhood, for example, have been associated with adult depression, eating disorders, and personality disorders, Dr. Cohen said. Only in recent years have mental health providers recognized the importance of less dramatic forms of childhood maltreatment, such as emotional abuse and neglect, and begun to study those issues.
The topic deserves further exploration, she added, because identifying these impairments in people with histories of childhood neglect might lead to better case conceptualization and possibly better treatment.
"You really do want to pay attention to your patients’ history of childhood neglect," Dr. Cohen said. "There may be more subtle types of maltreatment that have pernicious effects over time." If there is a history of neglect in childhood, pay attention not only to the patient’s ability to engage in relationships but the ability to maintain such relationships over time, she added.
The study was limited by using retrospective, self-report measures and its focus on inpatients, which might restrict the generalizability of the results.
The investigators reported having no financial disclosures.
On Twitter @sherryboschert
SAN FRANCISCO – Childhood neglect correlated with impaired capacity for close social relationships as an adult in a study of 114 nonpsychotic psychiatric inpatients.
The difficulty for patients with a history of childhood neglect centered more in maintaining than in starting close social relationships as adults, Thachell Tanis and Lisa J. Cohen, Ph.D., reported in a press briefing and a poster presentation at the annual meeting of the American Psychiatric Association.
The study used separate clinical self-report surveys to assess patients’ childhood histories and adult relational capacity. The Multidimensional Neglectful Behavior Scale assessed emotional, physical, cognitive, and supervisory neglect in the patients’ past. Patients also completed the relational domain of the Severity Indices of Personality Problems to assess capacity for intimacy or enduring relationships and the ability to feel recognized in relationships.
Each type of neglect significantly and negatively affected each facet in the relational domain. "Everything correlated with everything," said Ms. Tanis, a doctoral student at City College of New York.
The deficits were most striking in patients’ capacity for enduring relationships, said Dr. Cohen, professor of clinical psychiatry and behavioral sciences at Albert Einstein College of Medicine, New York. Childhood neglect as a whole, for example, correlated with an 81% greater negative effect on adult capacity for enduring relationships, compared with the negative effect on capacity for intimacy.
The cohort was 57% female, with a mean age of 39 years. Psychiatric diagnoses included depression in 55%, substance use disorder in 20%, bipolar disorder in 12%, anxiety in 10%, psychosis in 1%, and other diagnoses in 3%. The cohort was 44% white, 28% Hispanic, 16% black, 6% multiracial, 5% Asian, and 2% other ethnicities. The percentages total more than 100% because of rounding.
Prior studies have well documented the adverse effects of more dramatic forms of childhood maltreatment. Physical and sexual abuses in childhood, for example, have been associated with adult depression, eating disorders, and personality disorders, Dr. Cohen said. Only in recent years have mental health providers recognized the importance of less dramatic forms of childhood maltreatment, such as emotional abuse and neglect, and begun to study those issues.
The topic deserves further exploration, she added, because identifying these impairments in people with histories of childhood neglect might lead to better case conceptualization and possibly better treatment.
"You really do want to pay attention to your patients’ history of childhood neglect," Dr. Cohen said. "There may be more subtle types of maltreatment that have pernicious effects over time." If there is a history of neglect in childhood, pay attention not only to the patient’s ability to engage in relationships but the ability to maintain such relationships over time, she added.
The study was limited by using retrospective, self-report measures and its focus on inpatients, which might restrict the generalizability of the results.
The investigators reported having no financial disclosures.
On Twitter @sherryboschert
AT APA ANNUAL MEETING
Major finding: A history of childhood neglect correlated with impaired adult capacity for close relationships, with an 81% greater negative effect on the capacity to maintain relationships compared with starting them.
Data source: Survey assessments of 114 nonpsychotic psychiatric inpatients.
Disclosures: The investigators reported having no financial disclosures.
Behavioral weight-loss intervention works for patients with serious mental illness
A behavioral weight-loss intervention significantly reduced excess weight in adults who had serious mental illness, according to a study published online March 21 in the New England Journal of Medicine.
This intervention, which was specifically tailored to the needs of people with mental illness, allowed steady weight loss that progressed over the 18-month duration of the study. In contrast, most studies of lifestyle interventions in the general population show an early peak in weight loss, often followed by weight regain.
These findings suggest that "despite substantial challenges, persons with serious mental illness are able to lose weight with a tailored intervention," said Dr. Gail L. Daumit of the Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University, Baltimore, and her associates.
The prevalence of obesity is nearly twice as high among people with serious mental illness as among the general population. Mortality is two to three times higher, and cardiovascular disease is the primary cause of death in the seriously mentally ill.
Several factors contribute to this high prevalence. Unhealthy diets and physical inactivity are common in this patient population, and many psychotropic medications cause weight gain. Given their often low socioeconomic status, many people with serious mental illness also have reduced access to healthier, more-expensive foods and to safe, affordable places to exercise.
In addition, the stigma associated with mental illness also might make patients reluctant to participate in mainstream physical activities. And many have impairments in memory and executive function as well as psychiatric symptoms that "impede learning and the adoption of new behaviors," said Dr. Daumit, who also is affiliated with Johns Hopkins Bloomberg School of Public Health, and her colleagues.
They tested the effectiveness of an intervention that addressed these needs in the ACHIEVE (Achieving Healthy Lifestyles in Psychiatric Rehabilitation) clinical trial. The trial involved 291 overweight or obese adults who were participating in seven community outpatient psychiatric rehabilitation programs.
Such programs typically offer vocational and skills training, case management, and other services to the mentally ill; they usually have commercial kitchens that provide meals and snacks, as well as communal spaces suitable for group exercise.
"Program enrollees often attend these programs multiple times each week, which facilitates the delivery of lifestyle interventions that involve frequent contact," the researchers said (N. Engl. J. Med. 2013 March 21 [doi:10.1056/NEJMoa1214530]).
A total of 144 study subjects were randomly assigned to receive the intervention and 147 to a control group that received no intervention. The mean subject age was 45 years. Approximately half of the subjects were men, and 38% were black.
Nearly 80% of the study population was unable to work. A total of 58% had schizophrenia or schizoaffective disorder, 22% had bipolar disorder, and 12% had major depression. Patients were taking a mean of three psychotropic medications each.
The intervention addressed deficits in memory and executive function by dividing information into small components and by frequently repeating practice of skills.
Group and individual weight-management sessions focused on cutting caloric intake, especially by avoiding sugary drinks and junk food; consuming five servings of fruits and vegetables every day; controlling portion size; and eating healthy snacks.
Group exercise sessions focused on moderate-intensity aerobic exercise that gradually increased in duration and intensity.
The mean net weight loss increased over time only for patients who participated in the intervention. This loss was 1.8 kg at 6 months, and 3.4 kg at the completion of the trial. Although modest, this amount "compares favorably with weight loss in lifestyle-intervention trials in the general population" and has been associated with benefits such as reducing the risk of cardiovascular disease, Dr. Daumit and her associates said.
Participants in the intervention group continued to lose weight after 6 months and did not regain any weight, even when they attended fewer weight-management and exercise sessions over time. "One possible explanation is that persons with serious mental illness take longer than those without serious mental illness to engage in an intervention and make requisite behavioral changes," the investigators said.
"Our results show that overweight and obese adults with serious mental illness can make substantial lifestyle changes despite the myriad challenges they face," and that similar interventions should be implemented for this high-risk population, they added.
The study was funded by the National Institute of Mental Health. No conflicts of interest were reported.
A behavioral weight-loss intervention significantly reduced excess weight in adults who had serious mental illness, according to a study published online March 21 in the New England Journal of Medicine.
This intervention, which was specifically tailored to the needs of people with mental illness, allowed steady weight loss that progressed over the 18-month duration of the study. In contrast, most studies of lifestyle interventions in the general population show an early peak in weight loss, often followed by weight regain.
These findings suggest that "despite substantial challenges, persons with serious mental illness are able to lose weight with a tailored intervention," said Dr. Gail L. Daumit of the Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University, Baltimore, and her associates.
The prevalence of obesity is nearly twice as high among people with serious mental illness as among the general population. Mortality is two to three times higher, and cardiovascular disease is the primary cause of death in the seriously mentally ill.
Several factors contribute to this high prevalence. Unhealthy diets and physical inactivity are common in this patient population, and many psychotropic medications cause weight gain. Given their often low socioeconomic status, many people with serious mental illness also have reduced access to healthier, more-expensive foods and to safe, affordable places to exercise.
In addition, the stigma associated with mental illness also might make patients reluctant to participate in mainstream physical activities. And many have impairments in memory and executive function as well as psychiatric symptoms that "impede learning and the adoption of new behaviors," said Dr. Daumit, who also is affiliated with Johns Hopkins Bloomberg School of Public Health, and her colleagues.
They tested the effectiveness of an intervention that addressed these needs in the ACHIEVE (Achieving Healthy Lifestyles in Psychiatric Rehabilitation) clinical trial. The trial involved 291 overweight or obese adults who were participating in seven community outpatient psychiatric rehabilitation programs.
Such programs typically offer vocational and skills training, case management, and other services to the mentally ill; they usually have commercial kitchens that provide meals and snacks, as well as communal spaces suitable for group exercise.
"Program enrollees often attend these programs multiple times each week, which facilitates the delivery of lifestyle interventions that involve frequent contact," the researchers said (N. Engl. J. Med. 2013 March 21 [doi:10.1056/NEJMoa1214530]).
A total of 144 study subjects were randomly assigned to receive the intervention and 147 to a control group that received no intervention. The mean subject age was 45 years. Approximately half of the subjects were men, and 38% were black.
Nearly 80% of the study population was unable to work. A total of 58% had schizophrenia or schizoaffective disorder, 22% had bipolar disorder, and 12% had major depression. Patients were taking a mean of three psychotropic medications each.
The intervention addressed deficits in memory and executive function by dividing information into small components and by frequently repeating practice of skills.
Group and individual weight-management sessions focused on cutting caloric intake, especially by avoiding sugary drinks and junk food; consuming five servings of fruits and vegetables every day; controlling portion size; and eating healthy snacks.
Group exercise sessions focused on moderate-intensity aerobic exercise that gradually increased in duration and intensity.
The mean net weight loss increased over time only for patients who participated in the intervention. This loss was 1.8 kg at 6 months, and 3.4 kg at the completion of the trial. Although modest, this amount "compares favorably with weight loss in lifestyle-intervention trials in the general population" and has been associated with benefits such as reducing the risk of cardiovascular disease, Dr. Daumit and her associates said.
Participants in the intervention group continued to lose weight after 6 months and did not regain any weight, even when they attended fewer weight-management and exercise sessions over time. "One possible explanation is that persons with serious mental illness take longer than those without serious mental illness to engage in an intervention and make requisite behavioral changes," the investigators said.
"Our results show that overweight and obese adults with serious mental illness can make substantial lifestyle changes despite the myriad challenges they face," and that similar interventions should be implemented for this high-risk population, they added.
The study was funded by the National Institute of Mental Health. No conflicts of interest were reported.
A behavioral weight-loss intervention significantly reduced excess weight in adults who had serious mental illness, according to a study published online March 21 in the New England Journal of Medicine.
This intervention, which was specifically tailored to the needs of people with mental illness, allowed steady weight loss that progressed over the 18-month duration of the study. In contrast, most studies of lifestyle interventions in the general population show an early peak in weight loss, often followed by weight regain.
These findings suggest that "despite substantial challenges, persons with serious mental illness are able to lose weight with a tailored intervention," said Dr. Gail L. Daumit of the Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins University, Baltimore, and her associates.
The prevalence of obesity is nearly twice as high among people with serious mental illness as among the general population. Mortality is two to three times higher, and cardiovascular disease is the primary cause of death in the seriously mentally ill.
Several factors contribute to this high prevalence. Unhealthy diets and physical inactivity are common in this patient population, and many psychotropic medications cause weight gain. Given their often low socioeconomic status, many people with serious mental illness also have reduced access to healthier, more-expensive foods and to safe, affordable places to exercise.
In addition, the stigma associated with mental illness also might make patients reluctant to participate in mainstream physical activities. And many have impairments in memory and executive function as well as psychiatric symptoms that "impede learning and the adoption of new behaviors," said Dr. Daumit, who also is affiliated with Johns Hopkins Bloomberg School of Public Health, and her colleagues.
They tested the effectiveness of an intervention that addressed these needs in the ACHIEVE (Achieving Healthy Lifestyles in Psychiatric Rehabilitation) clinical trial. The trial involved 291 overweight or obese adults who were participating in seven community outpatient psychiatric rehabilitation programs.
Such programs typically offer vocational and skills training, case management, and other services to the mentally ill; they usually have commercial kitchens that provide meals and snacks, as well as communal spaces suitable for group exercise.
"Program enrollees often attend these programs multiple times each week, which facilitates the delivery of lifestyle interventions that involve frequent contact," the researchers said (N. Engl. J. Med. 2013 March 21 [doi:10.1056/NEJMoa1214530]).
A total of 144 study subjects were randomly assigned to receive the intervention and 147 to a control group that received no intervention. The mean subject age was 45 years. Approximately half of the subjects were men, and 38% were black.
Nearly 80% of the study population was unable to work. A total of 58% had schizophrenia or schizoaffective disorder, 22% had bipolar disorder, and 12% had major depression. Patients were taking a mean of three psychotropic medications each.
The intervention addressed deficits in memory and executive function by dividing information into small components and by frequently repeating practice of skills.
Group and individual weight-management sessions focused on cutting caloric intake, especially by avoiding sugary drinks and junk food; consuming five servings of fruits and vegetables every day; controlling portion size; and eating healthy snacks.
Group exercise sessions focused on moderate-intensity aerobic exercise that gradually increased in duration and intensity.
The mean net weight loss increased over time only for patients who participated in the intervention. This loss was 1.8 kg at 6 months, and 3.4 kg at the completion of the trial. Although modest, this amount "compares favorably with weight loss in lifestyle-intervention trials in the general population" and has been associated with benefits such as reducing the risk of cardiovascular disease, Dr. Daumit and her associates said.
Participants in the intervention group continued to lose weight after 6 months and did not regain any weight, even when they attended fewer weight-management and exercise sessions over time. "One possible explanation is that persons with serious mental illness take longer than those without serious mental illness to engage in an intervention and make requisite behavioral changes," the investigators said.
"Our results show that overweight and obese adults with serious mental illness can make substantial lifestyle changes despite the myriad challenges they face," and that similar interventions should be implemented for this high-risk population, they added.
The study was funded by the National Institute of Mental Health. No conflicts of interest were reported.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
Major Finding: Adults with serious mental illnesses who participated in a behavioral weight-loss intervention lost a mean of 1.8 kg at 6 months and 3.4 kg at the completion of the trial.
Data Source: An analysis of outcomes from an 18-month behavioral weight-loss intervention involving 291 overweight or obese people with schizophrenia, bipolar disorder, or major depression.
Disclosures: This study was funded by the National Institute of Mental Health. No conflicts of interest were reported.
Bipolar disorder hospitalization rates for children soar
Hospital stays for bipolar disorder among children aged 1-17 years increased 434% from 1997 to 2010, the Agency for Healthcare Research and Quality reported.
In 2010, the rate of bipolar stays in children under age 18 was 8.2 per 10,000 population, compared with 1.5 per 10,000 in 1997, with the increase occurring in all ages, the AHRQ said.
The admission rate in children aged 5-9 years went from 0.4 to 3.2 per 10,000, an increase of 696%. Among children 10-14 years old, the rate was 2.0/10,000 in 1997 and 11.2/10,000 in 2010, an increase of 475%. The bipolar admission rate for those aged 15-17 years rose 345%, going from 4.8/10,000 in 1997 to 21.1/10,000 in 2010, according to the report.
In 2010, bipolar disorder accounted for 48% of hospital stays for mood disorders in children, compared with 16% in 1997, the AHRQ said.
Hospital stays for bipolar disorder among children aged 1-17 years increased 434% from 1997 to 2010, the Agency for Healthcare Research and Quality reported.
In 2010, the rate of bipolar stays in children under age 18 was 8.2 per 10,000 population, compared with 1.5 per 10,000 in 1997, with the increase occurring in all ages, the AHRQ said.
The admission rate in children aged 5-9 years went from 0.4 to 3.2 per 10,000, an increase of 696%. Among children 10-14 years old, the rate was 2.0/10,000 in 1997 and 11.2/10,000 in 2010, an increase of 475%. The bipolar admission rate for those aged 15-17 years rose 345%, going from 4.8/10,000 in 1997 to 21.1/10,000 in 2010, according to the report.
In 2010, bipolar disorder accounted for 48% of hospital stays for mood disorders in children, compared with 16% in 1997, the AHRQ said.
Hospital stays for bipolar disorder among children aged 1-17 years increased 434% from 1997 to 2010, the Agency for Healthcare Research and Quality reported.
In 2010, the rate of bipolar stays in children under age 18 was 8.2 per 10,000 population, compared with 1.5 per 10,000 in 1997, with the increase occurring in all ages, the AHRQ said.
The admission rate in children aged 5-9 years went from 0.4 to 3.2 per 10,000, an increase of 696%. Among children 10-14 years old, the rate was 2.0/10,000 in 1997 and 11.2/10,000 in 2010, an increase of 475%. The bipolar admission rate for those aged 15-17 years rose 345%, going from 4.8/10,000 in 1997 to 21.1/10,000 in 2010, according to the report.
In 2010, bipolar disorder accounted for 48% of hospital stays for mood disorders in children, compared with 16% in 1997, the AHRQ said.
Brain and mind assessment in psychiatry
A mountain of evidence indicates that psychosis and bipolar disorder (BD) are brain disorders with an array of thought, mood, cognition, and behavioral aberrations.
Yet the clinical assessment of those neuropsychiatric disorders predominantly is restricted to evaluating mental and behavioral signs and symptoms. It’s time we comprehensively assess our psychiatric patients’ brains, not just describe their minds. This is the only way we can eventually identify the roots of serious mental illness and develop accurate and effective therapeutic interventions and preventions.
Consider the following brain probes, measures, and assessments that are rarely done in patients with first-episode schizophrenia, BD, or major depression. These clinical and technological cerebral evaluation methods all are available and feasible and are being routinely exploited in neurology and other medical specialties. Not using them represents missed opportunities to advance the scientific underpinnings of psychiatric diagnosis and treatment.
Complete neurologic examination, including cranial nerves, motor functions, sensory status, reflexes (including primitive reflexes), and soft neurologic signs. Psychiatrists rarely perform such examinations, although they can easily relearn and incorporate them in their critical initial assessment of severe psychiatric episodes. Researchers have identified many neurologic findings in drug-naïve psychotic patients before they receive medications in whom adverse effects may mask or add to motor or sensory abnormalities.
Neurocognitive testing. An extensive body of literature has definitively demonstrated severe cognitive deficits across multiple domains in schizophrenia, BD, and major depression. Yet, inexplicably, few first-episode patients are assessed with a standard battery of tests for memory, attention, visuospatial skills, or executive functions in clinical practice. Cognitive deficits are a product of abnormal neural pathways and neurocognitive tests can provide tremendous insight into regional and overall brain functions and provide clues for etiopathology and a road map for rehabilitation.
Neuroimaging. Multiple sophisticated techniques to assess brain structure and function are used in research but rarely in clinical practice. These include:
Morphological MRI,, which can provide exquisitely detailed anatomical information about cortical and subcortical structures. This can help identify lesions that cause mania, schizophrenia-like disorders, or depression secondary to a brain pathology. Even if no lesion is found, the pattern of atrophy, hypertrophy, ectopic gray matter, or hyperplasia can help identify subtypes of heterogeneous psychotic and mood disorders, and may lead to a specific diagnosis and treatment.
Magnetic resonance spectroscopy (MRS) is essentially a living biopsy of the brain in any region, detailing the spectrum and amount of various neurochemical substances (such as glutamine, γ-aminobutyric acid, creatine, N-acetylaspartic acid, or lactate) using proton spectroscopy, high energy phosphates such as adenosine diphosphate (ADP) or adenosine triphosphate (ATP) or membrane breakdown products (such as phosphomonoester and phosphodiester) using phosphorous MRS. Researchers are gradually “mapping” the regional chemistry of the brain in health and disease, which may provide profound insights for understanding the neurobiology of serious mental disorders.
Functional MRI, which can display the underactivation or overactivation of various brain regions at rest or while experiencing severe symptoms such as hallucinations or melancholia or while performing a cognitive task. Significant insights about brain pathways can be gleaned from this test.
Diffusion tensor imaging (DTI), which can assess myelin integrity and provide critical data about white matter tract pathology and intra- and inter-hemispheric disconnectivity. Pathological myelin findings in psychotic and mood disorders already are prompting novel treatments for these disabling brain illnesses.
Cerebrospinal fluid (CSF) examination. Psychiatrists rarely perform lumbar punctures (LP) in first-episode patients, although psychotic or bipolar disorders are as severe and disabling as multiple sclerosis or meningitis, where an LP is routine. This longstanding omission is the result of the antiquated notion that CSF in psychiatric patients is not abnormal and uninformative. But the fact is that CSF in patients with psychotic or mood disorders may contain many recently discovered biomarkers that shed light on the tremendous neurochemical changes during an acute psychotic, manic, or depressive episode. So the focus in psychiatry is not simply on red blood cells, white blood cells, glucose levels, or proteins, as in a routine LP, but on the emerging biomarkers of brain pathologies that have been implicated in the psychotic and mood disorders, including:
- inflammatory signaling and biomarkers (such as cytokines, interleukins, TNF-α)
- apoptotic (such as caspase-3, Fas, ARTS) and anti-apoptotic proteins (Bcl-2)
- neurotropic (growth) factor (such as BDNF, NGF, VEGF)
- oxidative stress biomarkers (such as TBARS, TRAP, PCC, SOD, and TAOP)
- myelin byproducts (such as S100B, oligodendrocytic proteins)
- glutamate/glutamine abnormalities
- lipodomic aberrations
- metabolomic profiles
- mitochondrial deficits (such as low glutathione and GPX)
- immunoglobulins (such as IgG, IgM).
If CSF analysis is done routinely, unprecedented discoveries can be made about the nature of brain pathologies and potential diagnostic biomarkers in various subtypes of serious psychiatric disorders, leading to specific and personalized treatments.
It’s time that we go beyond the current descriptive approach that includes a brief mental status exam. We must conduct a comprehensive investigation of our patients’ abnormal brains, which are responsible for their anomalous minds and impaired functioning. It’s time to capitalize on the amazing neuroscience advances to understand our patients’ brains. It’s time that we employ translational psychiatry to guide our diagnosis and treatment of severe mental disorders.
A mountain of evidence indicates that psychosis and bipolar disorder (BD) are brain disorders with an array of thought, mood, cognition, and behavioral aberrations.
Yet the clinical assessment of those neuropsychiatric disorders predominantly is restricted to evaluating mental and behavioral signs and symptoms. It’s time we comprehensively assess our psychiatric patients’ brains, not just describe their minds. This is the only way we can eventually identify the roots of serious mental illness and develop accurate and effective therapeutic interventions and preventions.
Consider the following brain probes, measures, and assessments that are rarely done in patients with first-episode schizophrenia, BD, or major depression. These clinical and technological cerebral evaluation methods all are available and feasible and are being routinely exploited in neurology and other medical specialties. Not using them represents missed opportunities to advance the scientific underpinnings of psychiatric diagnosis and treatment.
Complete neurologic examination, including cranial nerves, motor functions, sensory status, reflexes (including primitive reflexes), and soft neurologic signs. Psychiatrists rarely perform such examinations, although they can easily relearn and incorporate them in their critical initial assessment of severe psychiatric episodes. Researchers have identified many neurologic findings in drug-naïve psychotic patients before they receive medications in whom adverse effects may mask or add to motor or sensory abnormalities.
Neurocognitive testing. An extensive body of literature has definitively demonstrated severe cognitive deficits across multiple domains in schizophrenia, BD, and major depression. Yet, inexplicably, few first-episode patients are assessed with a standard battery of tests for memory, attention, visuospatial skills, or executive functions in clinical practice. Cognitive deficits are a product of abnormal neural pathways and neurocognitive tests can provide tremendous insight into regional and overall brain functions and provide clues for etiopathology and a road map for rehabilitation.
Neuroimaging. Multiple sophisticated techniques to assess brain structure and function are used in research but rarely in clinical practice. These include:
Morphological MRI,, which can provide exquisitely detailed anatomical information about cortical and subcortical structures. This can help identify lesions that cause mania, schizophrenia-like disorders, or depression secondary to a brain pathology. Even if no lesion is found, the pattern of atrophy, hypertrophy, ectopic gray matter, or hyperplasia can help identify subtypes of heterogeneous psychotic and mood disorders, and may lead to a specific diagnosis and treatment.
Magnetic resonance spectroscopy (MRS) is essentially a living biopsy of the brain in any region, detailing the spectrum and amount of various neurochemical substances (such as glutamine, γ-aminobutyric acid, creatine, N-acetylaspartic acid, or lactate) using proton spectroscopy, high energy phosphates such as adenosine diphosphate (ADP) or adenosine triphosphate (ATP) or membrane breakdown products (such as phosphomonoester and phosphodiester) using phosphorous MRS. Researchers are gradually “mapping” the regional chemistry of the brain in health and disease, which may provide profound insights for understanding the neurobiology of serious mental disorders.
Functional MRI, which can display the underactivation or overactivation of various brain regions at rest or while experiencing severe symptoms such as hallucinations or melancholia or while performing a cognitive task. Significant insights about brain pathways can be gleaned from this test.
Diffusion tensor imaging (DTI), which can assess myelin integrity and provide critical data about white matter tract pathology and intra- and inter-hemispheric disconnectivity. Pathological myelin findings in psychotic and mood disorders already are prompting novel treatments for these disabling brain illnesses.
Cerebrospinal fluid (CSF) examination. Psychiatrists rarely perform lumbar punctures (LP) in first-episode patients, although psychotic or bipolar disorders are as severe and disabling as multiple sclerosis or meningitis, where an LP is routine. This longstanding omission is the result of the antiquated notion that CSF in psychiatric patients is not abnormal and uninformative. But the fact is that CSF in patients with psychotic or mood disorders may contain many recently discovered biomarkers that shed light on the tremendous neurochemical changes during an acute psychotic, manic, or depressive episode. So the focus in psychiatry is not simply on red blood cells, white blood cells, glucose levels, or proteins, as in a routine LP, but on the emerging biomarkers of brain pathologies that have been implicated in the psychotic and mood disorders, including:
- inflammatory signaling and biomarkers (such as cytokines, interleukins, TNF-α)
- apoptotic (such as caspase-3, Fas, ARTS) and anti-apoptotic proteins (Bcl-2)
- neurotropic (growth) factor (such as BDNF, NGF, VEGF)
- oxidative stress biomarkers (such as TBARS, TRAP, PCC, SOD, and TAOP)
- myelin byproducts (such as S100B, oligodendrocytic proteins)
- glutamate/glutamine abnormalities
- lipodomic aberrations
- metabolomic profiles
- mitochondrial deficits (such as low glutathione and GPX)
- immunoglobulins (such as IgG, IgM).
If CSF analysis is done routinely, unprecedented discoveries can be made about the nature of brain pathologies and potential diagnostic biomarkers in various subtypes of serious psychiatric disorders, leading to specific and personalized treatments.
It’s time that we go beyond the current descriptive approach that includes a brief mental status exam. We must conduct a comprehensive investigation of our patients’ abnormal brains, which are responsible for their anomalous minds and impaired functioning. It’s time to capitalize on the amazing neuroscience advances to understand our patients’ brains. It’s time that we employ translational psychiatry to guide our diagnosis and treatment of severe mental disorders.
A mountain of evidence indicates that psychosis and bipolar disorder (BD) are brain disorders with an array of thought, mood, cognition, and behavioral aberrations.
Yet the clinical assessment of those neuropsychiatric disorders predominantly is restricted to evaluating mental and behavioral signs and symptoms. It’s time we comprehensively assess our psychiatric patients’ brains, not just describe their minds. This is the only way we can eventually identify the roots of serious mental illness and develop accurate and effective therapeutic interventions and preventions.
Consider the following brain probes, measures, and assessments that are rarely done in patients with first-episode schizophrenia, BD, or major depression. These clinical and technological cerebral evaluation methods all are available and feasible and are being routinely exploited in neurology and other medical specialties. Not using them represents missed opportunities to advance the scientific underpinnings of psychiatric diagnosis and treatment.
Complete neurologic examination, including cranial nerves, motor functions, sensory status, reflexes (including primitive reflexes), and soft neurologic signs. Psychiatrists rarely perform such examinations, although they can easily relearn and incorporate them in their critical initial assessment of severe psychiatric episodes. Researchers have identified many neurologic findings in drug-naïve psychotic patients before they receive medications in whom adverse effects may mask or add to motor or sensory abnormalities.
Neurocognitive testing. An extensive body of literature has definitively demonstrated severe cognitive deficits across multiple domains in schizophrenia, BD, and major depression. Yet, inexplicably, few first-episode patients are assessed with a standard battery of tests for memory, attention, visuospatial skills, or executive functions in clinical practice. Cognitive deficits are a product of abnormal neural pathways and neurocognitive tests can provide tremendous insight into regional and overall brain functions and provide clues for etiopathology and a road map for rehabilitation.
Neuroimaging. Multiple sophisticated techniques to assess brain structure and function are used in research but rarely in clinical practice. These include:
Morphological MRI,, which can provide exquisitely detailed anatomical information about cortical and subcortical structures. This can help identify lesions that cause mania, schizophrenia-like disorders, or depression secondary to a brain pathology. Even if no lesion is found, the pattern of atrophy, hypertrophy, ectopic gray matter, or hyperplasia can help identify subtypes of heterogeneous psychotic and mood disorders, and may lead to a specific diagnosis and treatment.
Magnetic resonance spectroscopy (MRS) is essentially a living biopsy of the brain in any region, detailing the spectrum and amount of various neurochemical substances (such as glutamine, γ-aminobutyric acid, creatine, N-acetylaspartic acid, or lactate) using proton spectroscopy, high energy phosphates such as adenosine diphosphate (ADP) or adenosine triphosphate (ATP) or membrane breakdown products (such as phosphomonoester and phosphodiester) using phosphorous MRS. Researchers are gradually “mapping” the regional chemistry of the brain in health and disease, which may provide profound insights for understanding the neurobiology of serious mental disorders.
Functional MRI, which can display the underactivation or overactivation of various brain regions at rest or while experiencing severe symptoms such as hallucinations or melancholia or while performing a cognitive task. Significant insights about brain pathways can be gleaned from this test.
Diffusion tensor imaging (DTI), which can assess myelin integrity and provide critical data about white matter tract pathology and intra- and inter-hemispheric disconnectivity. Pathological myelin findings in psychotic and mood disorders already are prompting novel treatments for these disabling brain illnesses.
Cerebrospinal fluid (CSF) examination. Psychiatrists rarely perform lumbar punctures (LP) in first-episode patients, although psychotic or bipolar disorders are as severe and disabling as multiple sclerosis or meningitis, where an LP is routine. This longstanding omission is the result of the antiquated notion that CSF in psychiatric patients is not abnormal and uninformative. But the fact is that CSF in patients with psychotic or mood disorders may contain many recently discovered biomarkers that shed light on the tremendous neurochemical changes during an acute psychotic, manic, or depressive episode. So the focus in psychiatry is not simply on red blood cells, white blood cells, glucose levels, or proteins, as in a routine LP, but on the emerging biomarkers of brain pathologies that have been implicated in the psychotic and mood disorders, including:
- inflammatory signaling and biomarkers (such as cytokines, interleukins, TNF-α)
- apoptotic (such as caspase-3, Fas, ARTS) and anti-apoptotic proteins (Bcl-2)
- neurotropic (growth) factor (such as BDNF, NGF, VEGF)
- oxidative stress biomarkers (such as TBARS, TRAP, PCC, SOD, and TAOP)
- myelin byproducts (such as S100B, oligodendrocytic proteins)
- glutamate/glutamine abnormalities
- lipodomic aberrations
- metabolomic profiles
- mitochondrial deficits (such as low glutathione and GPX)
- immunoglobulins (such as IgG, IgM).
If CSF analysis is done routinely, unprecedented discoveries can be made about the nature of brain pathologies and potential diagnostic biomarkers in various subtypes of serious psychiatric disorders, leading to specific and personalized treatments.
It’s time that we go beyond the current descriptive approach that includes a brief mental status exam. We must conduct a comprehensive investigation of our patients’ abnormal brains, which are responsible for their anomalous minds and impaired functioning. It’s time to capitalize on the amazing neuroscience advances to understand our patients’ brains. It’s time that we employ translational psychiatry to guide our diagnosis and treatment of severe mental disorders.