User login
Gene therapy for hemophilia A gets fast-track review
The investigational gene therapy SPK-8011 for the treatment of hemophilia A is getting an accelerated review at the Food and Drug Administration, according to Spark Therapeutics.
The FDA granted breakthrough therapy designation to the gene therapy product in February and orphan drug status in January.
The investigational gene therapy SPK-8011 for the treatment of hemophilia A is getting an accelerated review at the Food and Drug Administration, according to Spark Therapeutics.
The FDA granted breakthrough therapy designation to the gene therapy product in February and orphan drug status in January.
The investigational gene therapy SPK-8011 for the treatment of hemophilia A is getting an accelerated review at the Food and Drug Administration, according to Spark Therapeutics.
The FDA granted breakthrough therapy designation to the gene therapy product in February and orphan drug status in January.
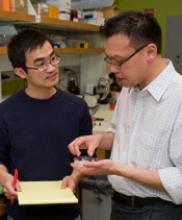
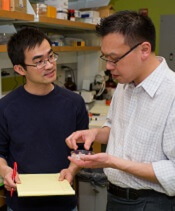
Team creates device to study hemostasis
Researchers have engineered a miniature model system for studying hemostasis.
They believe the device could serve as a drug discovery platform and potential diagnostic tool.
The team has already used the device to assess the effects of an antiplatelet agent and analyze blood from hemophilia A patients.
The researchers described this work in Nature Communications.
The team noted that hemostasis encompasses the interactions of platelets, coagulation factors, blood cells, endothelium, and hemodynamic forces.
“Current methods to study blood clotting require isolation of each of these components, which prevents us from seeing the big picture of what’s going with the patient’s blood clotting system,” said study author Wilbur Lam, MD, PhD, of Georgia Institute of Technology and Emory University in Atlanta, Georgia.
With this in mind, Dr Lam and his colleagues developed their device.
They believe it is the first system to reproduce all aspects of blood vessel injury seen in the microvasculature—blood loss due to trauma, clot formation by whole blood, and repair of the blood vessel lining. However, it does not reproduce aspects of larger blood vessels.
The researchers’ device has 3 layers. The top “vascular” layer consists of human endothelial cells cultured in a microchannel. The middle valve layer consists of a polydimethylsiloxane membrane, and the bottom is a “valve actuator” layer.
To assess hemostatic response, the researchers create a “wound” in this system. They exert both positive and negative pressure to create an opening about 130 micrometers across. Donated human blood can flow through this opening for testing.
The researchers said they used this system to demonstrate the importance of von Willebrand factor and endothelial phosphatidylserine in hemostasis.
The team used the device to test the antiplatelet agent eptifibatide as well. They found the drug leads to decreased clot contraction and a lower density of platelets within the hemostatic plug.
Finally, the researchers analyzed blood from hemophilia A patients and found that it “confers unstable hemostatic plug formation and altered fibrin architecture.”
Researchers have engineered a miniature model system for studying hemostasis.
They believe the device could serve as a drug discovery platform and potential diagnostic tool.
The team has already used the device to assess the effects of an antiplatelet agent and analyze blood from hemophilia A patients.
The researchers described this work in Nature Communications.
The team noted that hemostasis encompasses the interactions of platelets, coagulation factors, blood cells, endothelium, and hemodynamic forces.
“Current methods to study blood clotting require isolation of each of these components, which prevents us from seeing the big picture of what’s going with the patient’s blood clotting system,” said study author Wilbur Lam, MD, PhD, of Georgia Institute of Technology and Emory University in Atlanta, Georgia.
With this in mind, Dr Lam and his colleagues developed their device.
They believe it is the first system to reproduce all aspects of blood vessel injury seen in the microvasculature—blood loss due to trauma, clot formation by whole blood, and repair of the blood vessel lining. However, it does not reproduce aspects of larger blood vessels.
The researchers’ device has 3 layers. The top “vascular” layer consists of human endothelial cells cultured in a microchannel. The middle valve layer consists of a polydimethylsiloxane membrane, and the bottom is a “valve actuator” layer.
To assess hemostatic response, the researchers create a “wound” in this system. They exert both positive and negative pressure to create an opening about 130 micrometers across. Donated human blood can flow through this opening for testing.
The researchers said they used this system to demonstrate the importance of von Willebrand factor and endothelial phosphatidylserine in hemostasis.
The team used the device to test the antiplatelet agent eptifibatide as well. They found the drug leads to decreased clot contraction and a lower density of platelets within the hemostatic plug.
Finally, the researchers analyzed blood from hemophilia A patients and found that it “confers unstable hemostatic plug formation and altered fibrin architecture.”
Researchers have engineered a miniature model system for studying hemostasis.
They believe the device could serve as a drug discovery platform and potential diagnostic tool.
The team has already used the device to assess the effects of an antiplatelet agent and analyze blood from hemophilia A patients.
The researchers described this work in Nature Communications.
The team noted that hemostasis encompasses the interactions of platelets, coagulation factors, blood cells, endothelium, and hemodynamic forces.
“Current methods to study blood clotting require isolation of each of these components, which prevents us from seeing the big picture of what’s going with the patient’s blood clotting system,” said study author Wilbur Lam, MD, PhD, of Georgia Institute of Technology and Emory University in Atlanta, Georgia.
With this in mind, Dr Lam and his colleagues developed their device.
They believe it is the first system to reproduce all aspects of blood vessel injury seen in the microvasculature—blood loss due to trauma, clot formation by whole blood, and repair of the blood vessel lining. However, it does not reproduce aspects of larger blood vessels.
The researchers’ device has 3 layers. The top “vascular” layer consists of human endothelial cells cultured in a microchannel. The middle valve layer consists of a polydimethylsiloxane membrane, and the bottom is a “valve actuator” layer.
To assess hemostatic response, the researchers create a “wound” in this system. They exert both positive and negative pressure to create an opening about 130 micrometers across. Donated human blood can flow through this opening for testing.
The researchers said they used this system to demonstrate the importance of von Willebrand factor and endothelial phosphatidylserine in hemostasis.
The team used the device to test the antiplatelet agent eptifibatide as well. They found the drug leads to decreased clot contraction and a lower density of platelets within the hemostatic plug.
Finally, the researchers analyzed blood from hemophilia A patients and found that it “confers unstable hemostatic plug formation and altered fibrin architecture.”
Product seems effective for ITI in severe hemophilia A
Results of a retrospective study suggest a recombinant factor VIII Fc fusion protein (rFVIIIFc) can be effective for immune tolerance induction (ITI) in patients with severe hemophilia A and inhibitors.
Four of 7 first-time ITI patients achieved tolerization with rFVIIIFc at a median of 7.8 months.
Of 12 patients who had previously received treatment for ITI, 7 achieved a negative inhibitor level with rFVIIIFc. However, only 3 of these patients were still negative at the time of analysis.
In all, 16 of the 19 patients studied remained on rFVIIIFc for prophylaxis or ITI, and researchers said longer follow-up was needed.
No adverse events were reported in this study.
Manuel Carcao, MD, of The Hospital for Sick Children in Toronto, Ontario, Canada, and his colleagues conducted this study and published the results in Haemophilia.
The research was sponsored by Bioverativ Therapeutics, Inc. Bioverativ markets rFVIIIFc as Eloctate in some countries. Sobi markets rFVIIIFc (or efmoroctocog alfa) as Elocta in other countries.
The study included 19 patients—7 first-time ITI patients and 12 rescue patients—with severe hemophilia A and FVIII inhibitors.
First-timer results
Four of the 7 first-timers were tolerized and had transitioned to rFVIIIFc prophylaxis at the time of analysis.
Three patients met the definition of tolerization at 5 months, 7 months, and 9 months, respectively. All were all on a daily rFVIIIFc (85-200 IU/kg) regimen.
The fourth patient, who had been on a 3-times-per-week (50 IU/kg) ITI regimen, was considered tolerized at 14.8 months.
Of the 3 remaining patients, 2 had a decrease in Bethesda titer. The first patient had a decrease from 32 Bethesda units (BU) to 18 BU after 18 weeks of ITI. The second patient had a decrease from 378 BU to 23 BU after 58 weeks of ITI.
The third patient had an initial increase in titer from 3 BU to 16 BU after 15 weeks of rFVIIIFc. He went on to receive ITI with a different factor but did not respond. He resumed rFVIIIFc ITI after 27 weeks and had been receiving it for 7 weeks at the time of analysis. His most recent Bethesda titer had fallen. The researchers said this patient had been poorly compliant with ITI.
All 7 first-time ITI patients were still receiving rFVIIIFc—4 as prophylaxis and 3 for ITI—at the time of analysis.
Rescue results
Twelve patients had previously failed ITI. Seven of them achieved negative inhibitor levels with rFVIIIFc ITI. The median time to negativity was 14.1 weeks (range, 3 weeks to 67.6 weeks).
Three of the patients were still negative at the time of analysis. Two of them were still on rFVIIIFc ITI, and 1 was on rFVIIIFc prophylaxis.
Four patients who initially achieved negativity later developed a titer greater than 0.6 BU. Two of these patients were still on rFVIIIFc ITI at analysis, and 2 switched to other factors.
There were 5 patients who did not achieve negative inhibitor levels. One of these patients had a decrease in titer from 36 BU to 22 BU after 10 weeks. For the other 4 patients, titer was either unchanged or increased while on ITI.
Four of the 5 patients who did not achieve negative inhibitor levels remained on rFVIIIFc ITI at analysis, and 1 was placed on bypass therapy.
In total, 9 of the 12 rescue patients were still on rFVIIIFc at analysis—9 for ITI and 1 as prophylaxis.
“The development of inhibitors is a tremendous challenge and significant burden for people with severe hemophilia A, and the goal of treatment should be eradication of inhibitors,” said Maha Radhakrishnan, MD, of Bioverativ.
“The results of this analysis are encouraging and support the need for additional and ongoing scientific research on [rFVIIIFc ] in ITI to determine whether an Fc-based recombinant factor VIII therapy can rapidly tolerize patients with inhibitors.”
Bioverativ and Sobi have initiated 2 prospective studies designed to further evaluate the use of rFVIIIFc for ITI in patients with severe hemophilia A and inhibitors (NCT03093480 and NCT03103542).
Results of a retrospective study suggest a recombinant factor VIII Fc fusion protein (rFVIIIFc) can be effective for immune tolerance induction (ITI) in patients with severe hemophilia A and inhibitors.
Four of 7 first-time ITI patients achieved tolerization with rFVIIIFc at a median of 7.8 months.
Of 12 patients who had previously received treatment for ITI, 7 achieved a negative inhibitor level with rFVIIIFc. However, only 3 of these patients were still negative at the time of analysis.
In all, 16 of the 19 patients studied remained on rFVIIIFc for prophylaxis or ITI, and researchers said longer follow-up was needed.
No adverse events were reported in this study.
Manuel Carcao, MD, of The Hospital for Sick Children in Toronto, Ontario, Canada, and his colleagues conducted this study and published the results in Haemophilia.
The research was sponsored by Bioverativ Therapeutics, Inc. Bioverativ markets rFVIIIFc as Eloctate in some countries. Sobi markets rFVIIIFc (or efmoroctocog alfa) as Elocta in other countries.
The study included 19 patients—7 first-time ITI patients and 12 rescue patients—with severe hemophilia A and FVIII inhibitors.
First-timer results
Four of the 7 first-timers were tolerized and had transitioned to rFVIIIFc prophylaxis at the time of analysis.
Three patients met the definition of tolerization at 5 months, 7 months, and 9 months, respectively. All were all on a daily rFVIIIFc (85-200 IU/kg) regimen.
The fourth patient, who had been on a 3-times-per-week (50 IU/kg) ITI regimen, was considered tolerized at 14.8 months.
Of the 3 remaining patients, 2 had a decrease in Bethesda titer. The first patient had a decrease from 32 Bethesda units (BU) to 18 BU after 18 weeks of ITI. The second patient had a decrease from 378 BU to 23 BU after 58 weeks of ITI.
The third patient had an initial increase in titer from 3 BU to 16 BU after 15 weeks of rFVIIIFc. He went on to receive ITI with a different factor but did not respond. He resumed rFVIIIFc ITI after 27 weeks and had been receiving it for 7 weeks at the time of analysis. His most recent Bethesda titer had fallen. The researchers said this patient had been poorly compliant with ITI.
All 7 first-time ITI patients were still receiving rFVIIIFc—4 as prophylaxis and 3 for ITI—at the time of analysis.
Rescue results
Twelve patients had previously failed ITI. Seven of them achieved negative inhibitor levels with rFVIIIFc ITI. The median time to negativity was 14.1 weeks (range, 3 weeks to 67.6 weeks).
Three of the patients were still negative at the time of analysis. Two of them were still on rFVIIIFc ITI, and 1 was on rFVIIIFc prophylaxis.
Four patients who initially achieved negativity later developed a titer greater than 0.6 BU. Two of these patients were still on rFVIIIFc ITI at analysis, and 2 switched to other factors.
There were 5 patients who did not achieve negative inhibitor levels. One of these patients had a decrease in titer from 36 BU to 22 BU after 10 weeks. For the other 4 patients, titer was either unchanged or increased while on ITI.
Four of the 5 patients who did not achieve negative inhibitor levels remained on rFVIIIFc ITI at analysis, and 1 was placed on bypass therapy.
In total, 9 of the 12 rescue patients were still on rFVIIIFc at analysis—9 for ITI and 1 as prophylaxis.
“The development of inhibitors is a tremendous challenge and significant burden for people with severe hemophilia A, and the goal of treatment should be eradication of inhibitors,” said Maha Radhakrishnan, MD, of Bioverativ.
“The results of this analysis are encouraging and support the need for additional and ongoing scientific research on [rFVIIIFc ] in ITI to determine whether an Fc-based recombinant factor VIII therapy can rapidly tolerize patients with inhibitors.”
Bioverativ and Sobi have initiated 2 prospective studies designed to further evaluate the use of rFVIIIFc for ITI in patients with severe hemophilia A and inhibitors (NCT03093480 and NCT03103542).
Results of a retrospective study suggest a recombinant factor VIII Fc fusion protein (rFVIIIFc) can be effective for immune tolerance induction (ITI) in patients with severe hemophilia A and inhibitors.
Four of 7 first-time ITI patients achieved tolerization with rFVIIIFc at a median of 7.8 months.
Of 12 patients who had previously received treatment for ITI, 7 achieved a negative inhibitor level with rFVIIIFc. However, only 3 of these patients were still negative at the time of analysis.
In all, 16 of the 19 patients studied remained on rFVIIIFc for prophylaxis or ITI, and researchers said longer follow-up was needed.
No adverse events were reported in this study.
Manuel Carcao, MD, of The Hospital for Sick Children in Toronto, Ontario, Canada, and his colleagues conducted this study and published the results in Haemophilia.
The research was sponsored by Bioverativ Therapeutics, Inc. Bioverativ markets rFVIIIFc as Eloctate in some countries. Sobi markets rFVIIIFc (or efmoroctocog alfa) as Elocta in other countries.
The study included 19 patients—7 first-time ITI patients and 12 rescue patients—with severe hemophilia A and FVIII inhibitors.
First-timer results
Four of the 7 first-timers were tolerized and had transitioned to rFVIIIFc prophylaxis at the time of analysis.
Three patients met the definition of tolerization at 5 months, 7 months, and 9 months, respectively. All were all on a daily rFVIIIFc (85-200 IU/kg) regimen.
The fourth patient, who had been on a 3-times-per-week (50 IU/kg) ITI regimen, was considered tolerized at 14.8 months.
Of the 3 remaining patients, 2 had a decrease in Bethesda titer. The first patient had a decrease from 32 Bethesda units (BU) to 18 BU after 18 weeks of ITI. The second patient had a decrease from 378 BU to 23 BU after 58 weeks of ITI.
The third patient had an initial increase in titer from 3 BU to 16 BU after 15 weeks of rFVIIIFc. He went on to receive ITI with a different factor but did not respond. He resumed rFVIIIFc ITI after 27 weeks and had been receiving it for 7 weeks at the time of analysis. His most recent Bethesda titer had fallen. The researchers said this patient had been poorly compliant with ITI.
All 7 first-time ITI patients were still receiving rFVIIIFc—4 as prophylaxis and 3 for ITI—at the time of analysis.
Rescue results
Twelve patients had previously failed ITI. Seven of them achieved negative inhibitor levels with rFVIIIFc ITI. The median time to negativity was 14.1 weeks (range, 3 weeks to 67.6 weeks).
Three of the patients were still negative at the time of analysis. Two of them were still on rFVIIIFc ITI, and 1 was on rFVIIIFc prophylaxis.
Four patients who initially achieved negativity later developed a titer greater than 0.6 BU. Two of these patients were still on rFVIIIFc ITI at analysis, and 2 switched to other factors.
There were 5 patients who did not achieve negative inhibitor levels. One of these patients had a decrease in titer from 36 BU to 22 BU after 10 weeks. For the other 4 patients, titer was either unchanged or increased while on ITI.
Four of the 5 patients who did not achieve negative inhibitor levels remained on rFVIIIFc ITI at analysis, and 1 was placed on bypass therapy.
In total, 9 of the 12 rescue patients were still on rFVIIIFc at analysis—9 for ITI and 1 as prophylaxis.
“The development of inhibitors is a tremendous challenge and significant burden for people with severe hemophilia A, and the goal of treatment should be eradication of inhibitors,” said Maha Radhakrishnan, MD, of Bioverativ.
“The results of this analysis are encouraging and support the need for additional and ongoing scientific research on [rFVIIIFc ] in ITI to determine whether an Fc-based recombinant factor VIII therapy can rapidly tolerize patients with inhibitors.”
Bioverativ and Sobi have initiated 2 prospective studies designed to further evaluate the use of rFVIIIFc for ITI in patients with severe hemophilia A and inhibitors (NCT03093480 and NCT03103542).
Product increases FIX levels in hemophilia B
MADRID—The recombinant factor IX (FIX) product CB 2679d/ISU304 can increase FIX levels in patients with severe hemophilia B, according to a phase 1/2 trial.
Results showed a continuous linear increase in FIX activity levels following daily subcutaneous (SQ) dosing of CB 2679d for 6 days.
Adverse events were mild to moderate, and none of the patients have developed inhibitors to CB 2679d or FIX.
Howard Levy, MB ChB, PhD, chief medical officer of Catalyst Biosciences, Inc., presented these results at the 11th Annual Congress of The European Association for Haemophilia and Allied Disorders (EAHAD).
The research was sponsored by Catalyst Biosciences, Inc.
This phase 1/2 trial was divided into 5 cohorts.
In cohort 1, researchers compared single doses of intravenous (IV) CB 2679d and IV BeneFIX (recombinant FIX). CB 2679d proved 22 times more potent than BeneFIX. The products’ half-lives were 27.0 hours and 21.0 hours, respectively.
In cohorts 2 and 3, researchers compared single, ascending doses of IV CB 2679d to SQ CB 2679d. The bioavailability of SQ CB 2679d was 18.5%, and the half-life was 98.7 hours.
Cohort 4 was dropped, as it was another comparison of IV and SQ CB 2679d, which was considered unnecessary.
Cohort 5 included 5 patients who received daily doses of SQ CB 2679d. For 6 days, patients received CB 2679d at a dose of 140 IU/kg SQ.
The patients’ FIX activity levels increased from a median of <1% at baseline to a median of 15.7% (interquartile range, 14.9% to 16.6%) after all 6 doses.
The increase in FIX activity levels after the daily dosing was linear, indicating that continued SQ dosing may increase FIX activity further.
The median half-life was 63.2 hours (interquartile range, 60.2 to 64 hours), with the result that activity levels were still at 4% to 6.4% five days after the last dose.
No inhibitors to CB 2679d or FIX were observed in any of the cohorts in this trial.
In cohort 5, adverse events included pain, erythema, redness, and bruising after injections. Bruising occurred only with initial injections, and the severity of pain, erythema, and redness decreased over time (from moderate to mild).
“Existing IV therapies have FIX trough levels that can drop as low as 1% to 3% before repeat dosing,” Dr Levey said. “Daily subcutaneous dosing of CB 2679d has the potential to minimize the variability in FIX activity levels observed between IV doses and maintain individuals in the mild or even normal hemophilia range. This study demonstrates that, even after just 6 days of treatment, CB 2679d compares favorably to currently approved therapies for hemophilia B, all of which are infused IV.” ![]()
MADRID—The recombinant factor IX (FIX) product CB 2679d/ISU304 can increase FIX levels in patients with severe hemophilia B, according to a phase 1/2 trial.
Results showed a continuous linear increase in FIX activity levels following daily subcutaneous (SQ) dosing of CB 2679d for 6 days.
Adverse events were mild to moderate, and none of the patients have developed inhibitors to CB 2679d or FIX.
Howard Levy, MB ChB, PhD, chief medical officer of Catalyst Biosciences, Inc., presented these results at the 11th Annual Congress of The European Association for Haemophilia and Allied Disorders (EAHAD).
The research was sponsored by Catalyst Biosciences, Inc.
This phase 1/2 trial was divided into 5 cohorts.
In cohort 1, researchers compared single doses of intravenous (IV) CB 2679d and IV BeneFIX (recombinant FIX). CB 2679d proved 22 times more potent than BeneFIX. The products’ half-lives were 27.0 hours and 21.0 hours, respectively.
In cohorts 2 and 3, researchers compared single, ascending doses of IV CB 2679d to SQ CB 2679d. The bioavailability of SQ CB 2679d was 18.5%, and the half-life was 98.7 hours.
Cohort 4 was dropped, as it was another comparison of IV and SQ CB 2679d, which was considered unnecessary.
Cohort 5 included 5 patients who received daily doses of SQ CB 2679d. For 6 days, patients received CB 2679d at a dose of 140 IU/kg SQ.
The patients’ FIX activity levels increased from a median of <1% at baseline to a median of 15.7% (interquartile range, 14.9% to 16.6%) after all 6 doses.
The increase in FIX activity levels after the daily dosing was linear, indicating that continued SQ dosing may increase FIX activity further.
The median half-life was 63.2 hours (interquartile range, 60.2 to 64 hours), with the result that activity levels were still at 4% to 6.4% five days after the last dose.
No inhibitors to CB 2679d or FIX were observed in any of the cohorts in this trial.
In cohort 5, adverse events included pain, erythema, redness, and bruising after injections. Bruising occurred only with initial injections, and the severity of pain, erythema, and redness decreased over time (from moderate to mild).
“Existing IV therapies have FIX trough levels that can drop as low as 1% to 3% before repeat dosing,” Dr Levey said. “Daily subcutaneous dosing of CB 2679d has the potential to minimize the variability in FIX activity levels observed between IV doses and maintain individuals in the mild or even normal hemophilia range. This study demonstrates that, even after just 6 days of treatment, CB 2679d compares favorably to currently approved therapies for hemophilia B, all of which are infused IV.” ![]()
MADRID—The recombinant factor IX (FIX) product CB 2679d/ISU304 can increase FIX levels in patients with severe hemophilia B, according to a phase 1/2 trial.
Results showed a continuous linear increase in FIX activity levels following daily subcutaneous (SQ) dosing of CB 2679d for 6 days.
Adverse events were mild to moderate, and none of the patients have developed inhibitors to CB 2679d or FIX.
Howard Levy, MB ChB, PhD, chief medical officer of Catalyst Biosciences, Inc., presented these results at the 11th Annual Congress of The European Association for Haemophilia and Allied Disorders (EAHAD).
The research was sponsored by Catalyst Biosciences, Inc.
This phase 1/2 trial was divided into 5 cohorts.
In cohort 1, researchers compared single doses of intravenous (IV) CB 2679d and IV BeneFIX (recombinant FIX). CB 2679d proved 22 times more potent than BeneFIX. The products’ half-lives were 27.0 hours and 21.0 hours, respectively.
In cohorts 2 and 3, researchers compared single, ascending doses of IV CB 2679d to SQ CB 2679d. The bioavailability of SQ CB 2679d was 18.5%, and the half-life was 98.7 hours.
Cohort 4 was dropped, as it was another comparison of IV and SQ CB 2679d, which was considered unnecessary.
Cohort 5 included 5 patients who received daily doses of SQ CB 2679d. For 6 days, patients received CB 2679d at a dose of 140 IU/kg SQ.
The patients’ FIX activity levels increased from a median of <1% at baseline to a median of 15.7% (interquartile range, 14.9% to 16.6%) after all 6 doses.
The increase in FIX activity levels after the daily dosing was linear, indicating that continued SQ dosing may increase FIX activity further.
The median half-life was 63.2 hours (interquartile range, 60.2 to 64 hours), with the result that activity levels were still at 4% to 6.4% five days after the last dose.
No inhibitors to CB 2679d or FIX were observed in any of the cohorts in this trial.
In cohort 5, adverse events included pain, erythema, redness, and bruising after injections. Bruising occurred only with initial injections, and the severity of pain, erythema, and redness decreased over time (from moderate to mild).
“Existing IV therapies have FIX trough levels that can drop as low as 1% to 3% before repeat dosing,” Dr Levey said. “Daily subcutaneous dosing of CB 2679d has the potential to minimize the variability in FIX activity levels observed between IV doses and maintain individuals in the mild or even normal hemophilia range. This study demonstrates that, even after just 6 days of treatment, CB 2679d compares favorably to currently approved therapies for hemophilia B, all of which are infused IV.” ![]()
Factor IX product launched in US
The recombinant, GlycoPEGylated coagulation factor IX product Rebinyn® is now available in the US for the treatment of patients with hemophilia B.
Last May, Rebinyn was approved by the US Food and Drug Administration for on-demand treatment and control of bleeding episodes as well as perioperative management of bleeding in adults and children with hemophilia B.
The product is not approved for routine prophylaxis or immune tolerance induction in hemophilia B patients.
The full prescribing information is available at www.Rebinyn.com.
Rebinyn is also approved for use in the European Union, where it is known as nonacog beta pegol or by the brand name Refixia.
There, the product is approved for use as prophylaxis, for on-demand treatment of bleeding, and for control of bleeding related to surgical procedures in adolescents (older than 12 years of age) and adults with hemophilia B.
Trial results
The US and European approvals of nonacog beta pegol (N9-GP) were based on results from the paradigm™ clinical trials. Results from the paradigm 4 trial were published in Thrombosis Research in May 2016.
Paradigm 4 was an extension trial enrolling patients who had participated in the phase 3 trials paradigm 2 and paradigm 3.
In paradigm 2, researchers assessed N9-GP as treatment and prophylaxis in previously treated patients with hemophilia B. In paradigm 3, researchers assessed N9-GP in hemophilia B patients undergoing surgical procedures.
Paradigm 4 included 71 patients (ages 13 to 70) who continued to receive N9-GP as on-demand treatment (40 IU/kg for mild/moderate bleeds and 80 IU/kg for severe bleeds) or prophylaxis (10 IU/kg or 40 IU/kg once-weekly). Sixty-five patients completed treatment.
Safety
None of the patients developed factor IX inhibitors. Two patients had transient binding antibodies to N9-GP, but there was no sign that these antibodies had an inhibitory effect.
Four patients developed anti-CHO antibodies, but only 2 of these patients were still positive for these antibodies at the end of the trial.
There were a total of 155 adverse events. However, only 4 of these (in 3 patients) were considered possibly or probably related to N9-GP.
These events consisted of an injection site rash in 1 patient, 2 overdoses in 1 patient, and neutropenia in 1 patient. The rash and neutropenia resolved, and the patient who overdosed recovered without complications.
Efficacy
The researchers said the success rate for the treatment of reported bleeds was 94.6%. Most bleeds (87.9%) were resolved with a single injection of N9-GP, but 9.2% required 2 injections, and 2.9% required 3 or 4 injections.
The median annualized bleeding rate for patients on prophylaxis was 1.05 (interquartile range [IQR], 0.00–2.20) overall. It was 1.36 (IQR, 0.00-2.23) for the 10 IU/kg arm and 1.00 (IQR, 0.00-2.03) for the 40 IU/kg arm.
There were 14 patients on prophylaxis who underwent 23 minor surgical procedures.
The hemostatic response was considered “excellent” (better than expected/predicted for the procedure in question) in 19 procedures and “good” (as expected) in 2 procedures. In the remaining 2 procedures, hemostatic responses were not determined. ![]()
The recombinant, GlycoPEGylated coagulation factor IX product Rebinyn® is now available in the US for the treatment of patients with hemophilia B.
Last May, Rebinyn was approved by the US Food and Drug Administration for on-demand treatment and control of bleeding episodes as well as perioperative management of bleeding in adults and children with hemophilia B.
The product is not approved for routine prophylaxis or immune tolerance induction in hemophilia B patients.
The full prescribing information is available at www.Rebinyn.com.
Rebinyn is also approved for use in the European Union, where it is known as nonacog beta pegol or by the brand name Refixia.
There, the product is approved for use as prophylaxis, for on-demand treatment of bleeding, and for control of bleeding related to surgical procedures in adolescents (older than 12 years of age) and adults with hemophilia B.
Trial results
The US and European approvals of nonacog beta pegol (N9-GP) were based on results from the paradigm™ clinical trials. Results from the paradigm 4 trial were published in Thrombosis Research in May 2016.
Paradigm 4 was an extension trial enrolling patients who had participated in the phase 3 trials paradigm 2 and paradigm 3.
In paradigm 2, researchers assessed N9-GP as treatment and prophylaxis in previously treated patients with hemophilia B. In paradigm 3, researchers assessed N9-GP in hemophilia B patients undergoing surgical procedures.
Paradigm 4 included 71 patients (ages 13 to 70) who continued to receive N9-GP as on-demand treatment (40 IU/kg for mild/moderate bleeds and 80 IU/kg for severe bleeds) or prophylaxis (10 IU/kg or 40 IU/kg once-weekly). Sixty-five patients completed treatment.
Safety
None of the patients developed factor IX inhibitors. Two patients had transient binding antibodies to N9-GP, but there was no sign that these antibodies had an inhibitory effect.
Four patients developed anti-CHO antibodies, but only 2 of these patients were still positive for these antibodies at the end of the trial.
There were a total of 155 adverse events. However, only 4 of these (in 3 patients) were considered possibly or probably related to N9-GP.
These events consisted of an injection site rash in 1 patient, 2 overdoses in 1 patient, and neutropenia in 1 patient. The rash and neutropenia resolved, and the patient who overdosed recovered without complications.
Efficacy
The researchers said the success rate for the treatment of reported bleeds was 94.6%. Most bleeds (87.9%) were resolved with a single injection of N9-GP, but 9.2% required 2 injections, and 2.9% required 3 or 4 injections.
The median annualized bleeding rate for patients on prophylaxis was 1.05 (interquartile range [IQR], 0.00–2.20) overall. It was 1.36 (IQR, 0.00-2.23) for the 10 IU/kg arm and 1.00 (IQR, 0.00-2.03) for the 40 IU/kg arm.
There were 14 patients on prophylaxis who underwent 23 minor surgical procedures.
The hemostatic response was considered “excellent” (better than expected/predicted for the procedure in question) in 19 procedures and “good” (as expected) in 2 procedures. In the remaining 2 procedures, hemostatic responses were not determined. ![]()
The recombinant, GlycoPEGylated coagulation factor IX product Rebinyn® is now available in the US for the treatment of patients with hemophilia B.
Last May, Rebinyn was approved by the US Food and Drug Administration for on-demand treatment and control of bleeding episodes as well as perioperative management of bleeding in adults and children with hemophilia B.
The product is not approved for routine prophylaxis or immune tolerance induction in hemophilia B patients.
The full prescribing information is available at www.Rebinyn.com.
Rebinyn is also approved for use in the European Union, where it is known as nonacog beta pegol or by the brand name Refixia.
There, the product is approved for use as prophylaxis, for on-demand treatment of bleeding, and for control of bleeding related to surgical procedures in adolescents (older than 12 years of age) and adults with hemophilia B.
Trial results
The US and European approvals of nonacog beta pegol (N9-GP) were based on results from the paradigm™ clinical trials. Results from the paradigm 4 trial were published in Thrombosis Research in May 2016.
Paradigm 4 was an extension trial enrolling patients who had participated in the phase 3 trials paradigm 2 and paradigm 3.
In paradigm 2, researchers assessed N9-GP as treatment and prophylaxis in previously treated patients with hemophilia B. In paradigm 3, researchers assessed N9-GP in hemophilia B patients undergoing surgical procedures.
Paradigm 4 included 71 patients (ages 13 to 70) who continued to receive N9-GP as on-demand treatment (40 IU/kg for mild/moderate bleeds and 80 IU/kg for severe bleeds) or prophylaxis (10 IU/kg or 40 IU/kg once-weekly). Sixty-five patients completed treatment.
Safety
None of the patients developed factor IX inhibitors. Two patients had transient binding antibodies to N9-GP, but there was no sign that these antibodies had an inhibitory effect.
Four patients developed anti-CHO antibodies, but only 2 of these patients were still positive for these antibodies at the end of the trial.
There were a total of 155 adverse events. However, only 4 of these (in 3 patients) were considered possibly or probably related to N9-GP.
These events consisted of an injection site rash in 1 patient, 2 overdoses in 1 patient, and neutropenia in 1 patient. The rash and neutropenia resolved, and the patient who overdosed recovered without complications.
Efficacy
The researchers said the success rate for the treatment of reported bleeds was 94.6%. Most bleeds (87.9%) were resolved with a single injection of N9-GP, but 9.2% required 2 injections, and 2.9% required 3 or 4 injections.
The median annualized bleeding rate for patients on prophylaxis was 1.05 (interquartile range [IQR], 0.00–2.20) overall. It was 1.36 (IQR, 0.00-2.23) for the 10 IU/kg arm and 1.00 (IQR, 0.00-2.03) for the 40 IU/kg arm.
There were 14 patients on prophylaxis who underwent 23 minor surgical procedures.
The hemostatic response was considered “excellent” (better than expected/predicted for the procedure in question) in 19 procedures and “good” (as expected) in 2 procedures. In the remaining 2 procedures, hemostatic responses were not determined. ![]()
Hemophilia A drug heads toward approval in Europe
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to the drug in January 2018, according to a statement. The recommendation will now be considered by the European Commission.
Emicizumab, which is marketed in the United States as Hemlibra, was approved by the Food and Drug Administration for adult and pediatric patients with hemophilia A with Factor VIII inhibitors in November. It is the first monoclonal antibody to be recommended for use in patients with hemophilia A with inhibitors, says the statement.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to the drug in January 2018, according to a statement. The recommendation will now be considered by the European Commission.
Emicizumab, which is marketed in the United States as Hemlibra, was approved by the Food and Drug Administration for adult and pediatric patients with hemophilia A with Factor VIII inhibitors in November. It is the first monoclonal antibody to be recommended for use in patients with hemophilia A with inhibitors, says the statement.
The European Medicines Agency’s Committee for Medicinal Products for Human Use recommended granting marketing authorization to the drug in January 2018, according to a statement. The recommendation will now be considered by the European Commission.
Emicizumab, which is marketed in the United States as Hemlibra, was approved by the Food and Drug Administration for adult and pediatric patients with hemophilia A with Factor VIII inhibitors in November. It is the first monoclonal antibody to be recommended for use in patients with hemophilia A with inhibitors, says the statement.
IV bevacizumab improves severe bleeding in HHT
Intravenous (IV) bevacizumab “dramatically” improves severe bleeding associated with hereditary hemorrhagic telangiectasia (HHT), according to researchers.
In a retrospective study, HHT patients with severe bleeding had a substantial reduction in nose bleeds and gastrointestinal (GI) bleeding after treatment with IV bevacizumab.
In addition, patients were able to stop or considerably reduce red blood cell (RBC) transfusions.
The researchers therefore believe that IV bevacizumab should be considered as a first-line therapy for the treatment of refractory bleeding in patients with severe HHT.
The team detailed this research and in Mayo Clinic Proceedings alongside a related editorial.
“Some HHT patients suffer from severe epistaxis and gastrointestinal bleeding, which can result in severe anemia and years of blood transfusions,” said study author Vivek N. Iyer, MD, of the Mayo Clinic in Rochester, Minnesota.
“Both problems also appear to sometimes worsen with age. In some patients, both epistaxis and GI bleeding can become refractory/resistant to existing treatment options, leaving patients severely anemic and dependent on iron infusions or blood transfusions. Quality of life is very poor in these cases.”
With this in mind, Dr Iyer and his colleagues analyzed the records of 34 patients who were treated with IV bevacizumab for severe HHT-related bleeding from June 2013 through January 2017.
Patient characteristics
Patients had a median age of 63 (range, 57 to 72). Sixty-two percent of patients were female.
The primary source of bleeding was epistaxis in 15 patients, GI bleeding in 4 patients, and combined epistaxis and GI bleeding in 15 patients.
Prior epistaxis treatments included potassium-titanyl-phosphate/other laser procedures (62%), sclerotherapy (26%), endovascular angiographic embolization (21%), septodermoplasty (24%), subcutaneous bevacizumab injections (21%), and bevacizumab nasal spray (29%).
Seventy-one percent of patients underwent upper endoscopy (100% of these with telangiectasias), and 53% underwent colonoscopy (56% of these with telangiectasias).
Forty-one percent of patients had IV iron supplementation in the past 6 months.
Twenty-eight patients had received RBC transfusions. Sixteen patients were transfusion-dependent and had received a median of 75 transfusions before starting treatment with bevacizumab. The median duration of transfusion dependence was 6 years.
Treatment
The typical initial dosing cycle of bevacizumab consisted of 8 doses (4 doses each administered 2 weeks apart, followed by 4 doses each administered 1 month apart) over a period of around 22 weeks.
Further maintenance doses after the initial dosing cycle were individualized in each patient.
At last follow-up, 3 patients were still receiving bevacizumab from the initial dosing protocol. For the 31 patients who had completed the first dosing cycle, the median duration of follow-up was 13.6 months.
Eighteen patients required at least 1 top-up dose of IV bevacizumab because of worsening bleeding and/or anemia.
Efficacy
The median follow-up was 17.6 months (range, 3 to 42.5 months).
An Epistaxis Severity Score (ESS) questionnaire was used to assess the severity of nose bleeds both at the beginning of the study and after starting bevacizumab.
One month after starting treatment, there was a significant reduction in ESS scores (P<0.001). This improvement was maintained after patients completed the initial treatment cycle.
The median ESS score was 6.5 at baseline, 3.3 at 1 month, 4.0 at 3 months, 2.3 at the end of the first cycle, 2.0 at 1 to 3 months after the first cycle, 3.2 at 4 to 6 months, and 2.8 at 7 to 12 months.
GI bleeding also improved, with resolution or improvement of anemia in all 19 patients with this condition.
There was a reduction in RBC transfusions as well. The proportion of patients receiving transfusions was 53% in the 6 months before IV bevacizumab, 15% in the 1 to 3 months after starting IV bevacizumab, 14% at 4 to 6 months, 8% at 7 to 9 months, and 9% at 9 to 12 months.
Eighty-eight percent (n=14) of the transfusion-dependent patients had received a transfusion in the 6 months prior to starting IV bevacizumab. This compares to 31% (n=5) of patients in the 1 to 3 months after the start of treatment, 29% (n=4) at 4 to 6 months, 14% (n=2) at 7 to 9 months, and 8% (n=1) at 9 to 12 months.
Safety
Four patients had hypertension (HTN). One had pre-existing HTN and had to double the daily dose of lisinopril from 10 mg to 20 mg.
Two HTN patients had to start antihypertensive medications. The fourth patient experienced hypertensive urgency with a temporary decline in renal function. However, the patient was able to resume bevacizumab.
Two patients had infusion-related chills and fever, but premedication with acetaminophen and diphenhydramine prevented these events from recurring.
Three patients died during follow-up. Causes of death were stroke, infective endocarditis (methicillin-sensitive Staphylococcus aureus) with multiple cerebral infarcts, and postoperative respiratory failure (after left atrial appendectomy for paroxysmal atrial fibrillation).
None of these deaths were directly linked to bevacizumab.
“This study provides good-quality evidence for the excellent efficacy and safety of intravenous bevacizumab in the treatment of these patients,” Dr Iyer said. “Intravenous bevacizumab should be considered as a standard, first-line treatment option for HHT patients with severe bleeding and transfusion-dependent anemia.” ![]()
Intravenous (IV) bevacizumab “dramatically” improves severe bleeding associated with hereditary hemorrhagic telangiectasia (HHT), according to researchers.
In a retrospective study, HHT patients with severe bleeding had a substantial reduction in nose bleeds and gastrointestinal (GI) bleeding after treatment with IV bevacizumab.
In addition, patients were able to stop or considerably reduce red blood cell (RBC) transfusions.
The researchers therefore believe that IV bevacizumab should be considered as a first-line therapy for the treatment of refractory bleeding in patients with severe HHT.
The team detailed this research and in Mayo Clinic Proceedings alongside a related editorial.
“Some HHT patients suffer from severe epistaxis and gastrointestinal bleeding, which can result in severe anemia and years of blood transfusions,” said study author Vivek N. Iyer, MD, of the Mayo Clinic in Rochester, Minnesota.
“Both problems also appear to sometimes worsen with age. In some patients, both epistaxis and GI bleeding can become refractory/resistant to existing treatment options, leaving patients severely anemic and dependent on iron infusions or blood transfusions. Quality of life is very poor in these cases.”
With this in mind, Dr Iyer and his colleagues analyzed the records of 34 patients who were treated with IV bevacizumab for severe HHT-related bleeding from June 2013 through January 2017.
Patient characteristics
Patients had a median age of 63 (range, 57 to 72). Sixty-two percent of patients were female.
The primary source of bleeding was epistaxis in 15 patients, GI bleeding in 4 patients, and combined epistaxis and GI bleeding in 15 patients.
Prior epistaxis treatments included potassium-titanyl-phosphate/other laser procedures (62%), sclerotherapy (26%), endovascular angiographic embolization (21%), septodermoplasty (24%), subcutaneous bevacizumab injections (21%), and bevacizumab nasal spray (29%).
Seventy-one percent of patients underwent upper endoscopy (100% of these with telangiectasias), and 53% underwent colonoscopy (56% of these with telangiectasias).
Forty-one percent of patients had IV iron supplementation in the past 6 months.
Twenty-eight patients had received RBC transfusions. Sixteen patients were transfusion-dependent and had received a median of 75 transfusions before starting treatment with bevacizumab. The median duration of transfusion dependence was 6 years.
Treatment
The typical initial dosing cycle of bevacizumab consisted of 8 doses (4 doses each administered 2 weeks apart, followed by 4 doses each administered 1 month apart) over a period of around 22 weeks.
Further maintenance doses after the initial dosing cycle were individualized in each patient.
At last follow-up, 3 patients were still receiving bevacizumab from the initial dosing protocol. For the 31 patients who had completed the first dosing cycle, the median duration of follow-up was 13.6 months.
Eighteen patients required at least 1 top-up dose of IV bevacizumab because of worsening bleeding and/or anemia.
Efficacy
The median follow-up was 17.6 months (range, 3 to 42.5 months).
An Epistaxis Severity Score (ESS) questionnaire was used to assess the severity of nose bleeds both at the beginning of the study and after starting bevacizumab.
One month after starting treatment, there was a significant reduction in ESS scores (P<0.001). This improvement was maintained after patients completed the initial treatment cycle.
The median ESS score was 6.5 at baseline, 3.3 at 1 month, 4.0 at 3 months, 2.3 at the end of the first cycle, 2.0 at 1 to 3 months after the first cycle, 3.2 at 4 to 6 months, and 2.8 at 7 to 12 months.
GI bleeding also improved, with resolution or improvement of anemia in all 19 patients with this condition.
There was a reduction in RBC transfusions as well. The proportion of patients receiving transfusions was 53% in the 6 months before IV bevacizumab, 15% in the 1 to 3 months after starting IV bevacizumab, 14% at 4 to 6 months, 8% at 7 to 9 months, and 9% at 9 to 12 months.
Eighty-eight percent (n=14) of the transfusion-dependent patients had received a transfusion in the 6 months prior to starting IV bevacizumab. This compares to 31% (n=5) of patients in the 1 to 3 months after the start of treatment, 29% (n=4) at 4 to 6 months, 14% (n=2) at 7 to 9 months, and 8% (n=1) at 9 to 12 months.
Safety
Four patients had hypertension (HTN). One had pre-existing HTN and had to double the daily dose of lisinopril from 10 mg to 20 mg.
Two HTN patients had to start antihypertensive medications. The fourth patient experienced hypertensive urgency with a temporary decline in renal function. However, the patient was able to resume bevacizumab.
Two patients had infusion-related chills and fever, but premedication with acetaminophen and diphenhydramine prevented these events from recurring.
Three patients died during follow-up. Causes of death were stroke, infective endocarditis (methicillin-sensitive Staphylococcus aureus) with multiple cerebral infarcts, and postoperative respiratory failure (after left atrial appendectomy for paroxysmal atrial fibrillation).
None of these deaths were directly linked to bevacizumab.
“This study provides good-quality evidence for the excellent efficacy and safety of intravenous bevacizumab in the treatment of these patients,” Dr Iyer said. “Intravenous bevacizumab should be considered as a standard, first-line treatment option for HHT patients with severe bleeding and transfusion-dependent anemia.” ![]()
Intravenous (IV) bevacizumab “dramatically” improves severe bleeding associated with hereditary hemorrhagic telangiectasia (HHT), according to researchers.
In a retrospective study, HHT patients with severe bleeding had a substantial reduction in nose bleeds and gastrointestinal (GI) bleeding after treatment with IV bevacizumab.
In addition, patients were able to stop or considerably reduce red blood cell (RBC) transfusions.
The researchers therefore believe that IV bevacizumab should be considered as a first-line therapy for the treatment of refractory bleeding in patients with severe HHT.
The team detailed this research and in Mayo Clinic Proceedings alongside a related editorial.
“Some HHT patients suffer from severe epistaxis and gastrointestinal bleeding, which can result in severe anemia and years of blood transfusions,” said study author Vivek N. Iyer, MD, of the Mayo Clinic in Rochester, Minnesota.
“Both problems also appear to sometimes worsen with age. In some patients, both epistaxis and GI bleeding can become refractory/resistant to existing treatment options, leaving patients severely anemic and dependent on iron infusions or blood transfusions. Quality of life is very poor in these cases.”
With this in mind, Dr Iyer and his colleagues analyzed the records of 34 patients who were treated with IV bevacizumab for severe HHT-related bleeding from June 2013 through January 2017.
Patient characteristics
Patients had a median age of 63 (range, 57 to 72). Sixty-two percent of patients were female.
The primary source of bleeding was epistaxis in 15 patients, GI bleeding in 4 patients, and combined epistaxis and GI bleeding in 15 patients.
Prior epistaxis treatments included potassium-titanyl-phosphate/other laser procedures (62%), sclerotherapy (26%), endovascular angiographic embolization (21%), septodermoplasty (24%), subcutaneous bevacizumab injections (21%), and bevacizumab nasal spray (29%).
Seventy-one percent of patients underwent upper endoscopy (100% of these with telangiectasias), and 53% underwent colonoscopy (56% of these with telangiectasias).
Forty-one percent of patients had IV iron supplementation in the past 6 months.
Twenty-eight patients had received RBC transfusions. Sixteen patients were transfusion-dependent and had received a median of 75 transfusions before starting treatment with bevacizumab. The median duration of transfusion dependence was 6 years.
Treatment
The typical initial dosing cycle of bevacizumab consisted of 8 doses (4 doses each administered 2 weeks apart, followed by 4 doses each administered 1 month apart) over a period of around 22 weeks.
Further maintenance doses after the initial dosing cycle were individualized in each patient.
At last follow-up, 3 patients were still receiving bevacizumab from the initial dosing protocol. For the 31 patients who had completed the first dosing cycle, the median duration of follow-up was 13.6 months.
Eighteen patients required at least 1 top-up dose of IV bevacizumab because of worsening bleeding and/or anemia.
Efficacy
The median follow-up was 17.6 months (range, 3 to 42.5 months).
An Epistaxis Severity Score (ESS) questionnaire was used to assess the severity of nose bleeds both at the beginning of the study and after starting bevacizumab.
One month after starting treatment, there was a significant reduction in ESS scores (P<0.001). This improvement was maintained after patients completed the initial treatment cycle.
The median ESS score was 6.5 at baseline, 3.3 at 1 month, 4.0 at 3 months, 2.3 at the end of the first cycle, 2.0 at 1 to 3 months after the first cycle, 3.2 at 4 to 6 months, and 2.8 at 7 to 12 months.
GI bleeding also improved, with resolution or improvement of anemia in all 19 patients with this condition.
There was a reduction in RBC transfusions as well. The proportion of patients receiving transfusions was 53% in the 6 months before IV bevacizumab, 15% in the 1 to 3 months after starting IV bevacizumab, 14% at 4 to 6 months, 8% at 7 to 9 months, and 9% at 9 to 12 months.
Eighty-eight percent (n=14) of the transfusion-dependent patients had received a transfusion in the 6 months prior to starting IV bevacizumab. This compares to 31% (n=5) of patients in the 1 to 3 months after the start of treatment, 29% (n=4) at 4 to 6 months, 14% (n=2) at 7 to 9 months, and 8% (n=1) at 9 to 12 months.
Safety
Four patients had hypertension (HTN). One had pre-existing HTN and had to double the daily dose of lisinopril from 10 mg to 20 mg.
Two HTN patients had to start antihypertensive medications. The fourth patient experienced hypertensive urgency with a temporary decline in renal function. However, the patient was able to resume bevacizumab.
Two patients had infusion-related chills and fever, but premedication with acetaminophen and diphenhydramine prevented these events from recurring.
Three patients died during follow-up. Causes of death were stroke, infective endocarditis (methicillin-sensitive Staphylococcus aureus) with multiple cerebral infarcts, and postoperative respiratory failure (after left atrial appendectomy for paroxysmal atrial fibrillation).
None of these deaths were directly linked to bevacizumab.
“This study provides good-quality evidence for the excellent efficacy and safety of intravenous bevacizumab in the treatment of these patients,” Dr Iyer said. “Intravenous bevacizumab should be considered as a standard, first-line treatment option for HHT patients with severe bleeding and transfusion-dependent anemia.” ![]()
CHMP recommends approval of emicizumab
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval of emicizumab (Hemlibra®), a bispecific factor IXa- and factor X-directed antibody.
The recommendation is for emicizumab to be used as routine prophylaxis in patients of all ages who have hemophilia A with factor VIII inhibitors.
The marketing authorization application for emicizumab is being reviewed under accelerated assessment, a procedure granted to medicines the CHMP believes are of major interest for public health and therapeutic innovation.
The CHMP’s opinion on emicizumab will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation for emicizumab is based on results from 2 phase 3 trials—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July 2017. Updated results from HAVEN 2 were presented at the 2017 ASH Annual Meeting.
HAVEN 1
The study enrolled 109 patients (age 12 and older) with hemophilia A and FVIII inhibitors who were previously treated with bypassing agents (BPAs) on-demand or as prophylaxis.
The patients were randomized to receive emicizumab prophylaxis or no prophylaxis. On-demand treatment of breakthrough bleeds with BPAs was allowed.
There was a significant reduction in treated bleeds of 87% with emicizumab prophylaxis compared to no prophylaxis (95% CI: 72.3; 94.3, P<0.0001). And there was an 80% reduction in all bleeds with emicizumab (95% CI: 62.5; 89.8, P<0.0001).
Adverse events (AEs) occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced thromboembolic events (TEs), and 3 had thrombotic microangiopathy (TMA) while receiving emicizumab prophylaxis and more than 100 u/kg/day of activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
In this single-arm trial, researchers evaluated emicizumab prophylaxis in 60 patients, ages 1 to 17, who had hemophilia A with FVIII inhibitors.
The efficacy analysis included 57 patients who were younger than 12. The 3 older patients were only included in the safety analysis.
Of the 57 patients, 64.9% had 0 bleeds, 94.7% had 0 treated bleeds, and 98.2% had 0 treated spontaneous bleeds and 0 treated joint bleeds. None of the patients had treated target joint bleeds.
Forty patients had a total of 201 AEs. The most common of these were viral upper respiratory tract infections (16.7%) and injection site reactions (16.7%).
There were no TEs or TMA events, and none of the patients tested positive for anti-drug antibodies. None of the 7 serious AEs in this trial were considered treatment-related. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval of emicizumab (Hemlibra®), a bispecific factor IXa- and factor X-directed antibody.
The recommendation is for emicizumab to be used as routine prophylaxis in patients of all ages who have hemophilia A with factor VIII inhibitors.
The marketing authorization application for emicizumab is being reviewed under accelerated assessment, a procedure granted to medicines the CHMP believes are of major interest for public health and therapeutic innovation.
The CHMP’s opinion on emicizumab will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation for emicizumab is based on results from 2 phase 3 trials—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July 2017. Updated results from HAVEN 2 were presented at the 2017 ASH Annual Meeting.
HAVEN 1
The study enrolled 109 patients (age 12 and older) with hemophilia A and FVIII inhibitors who were previously treated with bypassing agents (BPAs) on-demand or as prophylaxis.
The patients were randomized to receive emicizumab prophylaxis or no prophylaxis. On-demand treatment of breakthrough bleeds with BPAs was allowed.
There was a significant reduction in treated bleeds of 87% with emicizumab prophylaxis compared to no prophylaxis (95% CI: 72.3; 94.3, P<0.0001). And there was an 80% reduction in all bleeds with emicizumab (95% CI: 62.5; 89.8, P<0.0001).
Adverse events (AEs) occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced thromboembolic events (TEs), and 3 had thrombotic microangiopathy (TMA) while receiving emicizumab prophylaxis and more than 100 u/kg/day of activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
In this single-arm trial, researchers evaluated emicizumab prophylaxis in 60 patients, ages 1 to 17, who had hemophilia A with FVIII inhibitors.
The efficacy analysis included 57 patients who were younger than 12. The 3 older patients were only included in the safety analysis.
Of the 57 patients, 64.9% had 0 bleeds, 94.7% had 0 treated bleeds, and 98.2% had 0 treated spontaneous bleeds and 0 treated joint bleeds. None of the patients had treated target joint bleeds.
Forty patients had a total of 201 AEs. The most common of these were viral upper respiratory tract infections (16.7%) and injection site reactions (16.7%).
There were no TEs or TMA events, and none of the patients tested positive for anti-drug antibodies. None of the 7 serious AEs in this trial were considered treatment-related. ![]()
The European Medicines Agency’s Committee for Medicinal Products for Human Use (CHMP) has recommended approval of emicizumab (Hemlibra®), a bispecific factor IXa- and factor X-directed antibody.
The recommendation is for emicizumab to be used as routine prophylaxis in patients of all ages who have hemophilia A with factor VIII inhibitors.
The marketing authorization application for emicizumab is being reviewed under accelerated assessment, a procedure granted to medicines the CHMP believes are of major interest for public health and therapeutic innovation.
The CHMP’s opinion on emicizumab will be reviewed by the European Commission (EC).
If the EC agrees with the CHMP, the commission will grant a centralized marketing authorization that will be valid in the European Union. Norway, Iceland, and Liechtenstein will make corresponding decisions on the basis of the EC’s decision.
The EC typically makes a decision within 67 days of the CHMP’s recommendation.
The CHMP’s recommendation for emicizumab is based on results from 2 phase 3 trials—HAVEN 1 and HAVEN 2.
Results from HAVEN 1 were published in NEJM and presented at the 26th ISTH Congress in July 2017. Updated results from HAVEN 2 were presented at the 2017 ASH Annual Meeting.
HAVEN 1
The study enrolled 109 patients (age 12 and older) with hemophilia A and FVIII inhibitors who were previously treated with bypassing agents (BPAs) on-demand or as prophylaxis.
The patients were randomized to receive emicizumab prophylaxis or no prophylaxis. On-demand treatment of breakthrough bleeds with BPAs was allowed.
There was a significant reduction in treated bleeds of 87% with emicizumab prophylaxis compared to no prophylaxis (95% CI: 72.3; 94.3, P<0.0001). And there was an 80% reduction in all bleeds with emicizumab (95% CI: 62.5; 89.8, P<0.0001).
Adverse events (AEs) occurring in at least 5% of patients treated with emicizumab were local injection site reactions, headache, fatigue, upper respiratory tract infection, and arthralgia.
Two patients experienced thromboembolic events (TEs), and 3 had thrombotic microangiopathy (TMA) while receiving emicizumab prophylaxis and more than 100 u/kg/day of activated prothrombin complex concentrate, on average, for 24 hours or more before the event. Two of these patients had also received recombinant factor VIIa.
Neither TE required anticoagulation therapy, and 1 patient restarted emicizumab. The cases of TMA observed were transient, and 1 patient restarted emicizumab.
HAVEN 2
In this single-arm trial, researchers evaluated emicizumab prophylaxis in 60 patients, ages 1 to 17, who had hemophilia A with FVIII inhibitors.
The efficacy analysis included 57 patients who were younger than 12. The 3 older patients were only included in the safety analysis.
Of the 57 patients, 64.9% had 0 bleeds, 94.7% had 0 treated bleeds, and 98.2% had 0 treated spontaneous bleeds and 0 treated joint bleeds. None of the patients had treated target joint bleeds.
Forty patients had a total of 201 AEs. The most common of these were viral upper respiratory tract infections (16.7%) and injection site reactions (16.7%).
There were no TEs or TMA events, and none of the patients tested positive for anti-drug antibodies. None of the 7 serious AEs in this trial were considered treatment-related. ![]()
Gene therapy moves from promise to reality
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
After decades of hype, dashed hopes, and setbacks, gene therapy has finally arrived and is poised to transform the treatment paradigm for many diseases, according to Cynthia E. Dunbar, MD, senior investigator at the Hematology Branch of the National Heart, Lung, and Blood Institute (NHLBI), part of the National Institutes of Health.
Hematologists can expect more developments that build on current successes with chimeric antigen receptor (CAR) T-cell therapy and gene therapy advances for hemophilia, as well as emerging advances in gene editing techniques including the CRISPR/Cas9 approach, Dr. Dunbar said in an interview.
That’s on top of a small number of regulatory approvals in the United States and Europe, she said. “Along with that, there’s a lot of interest and now involvement from biotechnology companies and even large pharmaceutical companies. I think all those factors really have to come together to create this kind of acceleration, and I’ve never seen anything like this previously.”
Dr. Dunbar – a former editor in chief of the journal Blood – and her colleagues recently published a review of current developments and emerging gene therapy technologies in the journal Science (2018 Jan 12. doi: 10.1126/science.aan4672).
“We really felt it was the right time to write the article,” she said.
Milestones
A new approach to cancer treatment was ushered in on Aug. 30, 2017, with the Food and Drug Administration approval of tisagenlecleucel, the first-ever gene therapy available in the United States. The CD19-directed CAR T-cell therapy is indicated for treatment of certain pediatric or young adult patients with B-cell precursor acute lymphoblastic leukemia that is refractory or in second or later relapse.
Soon afterward, FDA approved another CD19-directed CAR T-cell therapy, axicabtagene ciloleucel, for adult patients with large B-cell lymphoma after two or more lines of systemic therapy.
“It’s a very interesting time for immunotherapies in general,” Dr. Dunbar said. “There’s a huge number of options in terms of PD-1 inhibitors and other pharmacologics or antibodies that allow the patient’s own immune system to attack tumors. CAR T-cell therapy is an obvious step beyond that, in terms of arming your own T cells to very specifically target tumor cells.”
But randomized trials or meta-analyses may be necessary to determine the place of CAR T-cell therapy in the treatment armamentarium for acute lymphoblastic leukemia and large B-cell lymphoma given their cost and the availability of other therapeutic options, Dr. Dunbar suggested.
“Gene therapies have a large upfront cost, but if they’re truly curative and a one-time treatment, then they may in the long run be much cheaper than doing failed multiple transplants or needing monoclonal antibody infusion every 2 weeks for the rest of your life,” she said.
Another major success story still in the works, according to Dr. Dunbar, is the treatment of hemophilia A and B with gene therapy approaches. The positive data include a recent report showing that transgene-derived factor IX coagulant activity allowed for the termination of baseline prophylaxis, and the near elimination of bleeding and factor use, in patients with hemophilia B (N Engl J Med. 2017 Dec 7;377[23]:2215-27).
While gene therapy for hemophilia A has been more challenging, another recent report nevertheless demonstrated sustained normalization of factor VIII activity level with a single intravenous infusion of adeno-associated virus serotype 5 vector encoding a B-domain–deleted human factor VIII (N Engl J Med. 2017 Dec 28;377[26]:2519-30).
“The proof-of-principle was already there in hemophilia B,” Dr. Dunbar said. “It really was just a question of figuring out a way to package and deliver a Factor VIII that would work in the constraints of an AAV [adeno-associated virus] vector.”
Meanwhile, myeloma trials of CAR T-cell therapy seem very promising so far, but the challenge in that disease could be finding a place for gene therapy in a “much more diverse treatment landscape” that includes multiple effective regimens, according to Dr. Dunbar.
Future trends, challenges
Looking forward, she said.
Notably, genome editing approaches to treat sickle cell anemia are likely to move forward in the near future, according to Dr. Dunbar, following reports validating an erythroid enhancer of human BCL11A as a target for reinduction of fetal hemoglobin (Nature. 2015 Nov 12;527[7577]:192-7).
But all of this gene therapy development creates an educational challenge for frontline clinicians, even if the administration of CAR T-cell therapy and other advanced treatments is limited to highly specialized centers.
“There’s a lot of training that needs to go on with hematologists, oncologists, and other doctors about how to care for these patients after these treatments, in terms of what to look for and how to intervene early to prevent, for instance, severe toxicity from cytokine release syndrome,” Dr. Dunbar said.
Dr. Dunbar reported having no relevant financial disclosures.
Sotatercept promising for treatment of anemia in MDS
A novel agent holds promise as a treatment option for anemia in patients with lower-risk myelodysplastic syndromes who are not helped by erythropoiesis-stimulating agents (ESAs), according to results from a phase 2 trial.
Sotatercept (ACE-011) is a first-in-class novel recombinant fusion protein, and was found to be effective and well tolerated, increasing hemoglobin concentrations and decreasing the transfusion burden in this patient population.
Nearly half (29, 47%) of 62 patients with a high transfusion burden achieved hematologic improvement–erythroid (HI-E), which for them was a reduction in red blood cell transfusion from baseline of 4 U or more for at least 56 days. Additionally, 7 of 12 patients (58%) with a low transfusion burden also achieved HI-E, defined as an increase in hemoglobin of 1.5 g/dL or more that was sustained for at least 56 days without a transfusion.
“Taken together, these findings provide proof of principle that the recombinant protein sotatercept can restore ineffective erythropoiesis in patients with lower-risk myelodysplastic syndromes, with an acceptable safety profile,” Rami Komrokji, MD, of Moffitt Cancer Center and Research Institute, Tampa, and his colleagues, wrote in the Lancet Haematology.
There are few effective treatment options available for patients with lower-risk myelodysplastic syndromes who have anemia, especially after they fail primary or secondary treatment with ESAs, or for those who are not likely to benefit from ESA therapy.
In this phase 2 trial, the researchers sought to establish a safe and effective dose of sotatercept in a cohort of 74 patients. Of this group, 7 received 0.1 mg/kg sotatercept, 6 got 0.3 mg/kg, 21 received 0.5 mg/kg, 35 got 1.0 mg/kg, and 5 patients received doses up to 2.0 mg/kg. The primary efficacy endpoint of the study was the proportion of patients who achieved HI-E.
All of the patients were pretreated, having received prior therapy for myelodysplastic syndromes, including ESAs, hypomethylating agents (azacitidine or decitabine), lenalidomide, and other agents including corticosteroids and immunomodulators.
Within this cohort, 36 patients (49%; 95% confidence intervaI, 38-60) achieved HI-E while 20 patients (27%; 95% CI, 18-38) achieved independence from transfusion for at least 56 days.
Fatigue (26%) and peripheral edema (24%) were the most common adverse events reported, while grade 3-4 treatment-emergent adverse events (TEAEs) were reported in 34% of patients. Of these, 4 patients had grade 3-4 TEAEs that were probably related to the treatment. The most common grade 3-4 TEAEs were lipase increase and anemia, and each was reported in three patients. Additionally, 17 patients (23%) experienced at least one serious TEAE, including a death from a treatment-emergent subdural hematoma (which caused the patient to fall).
The study was funded by the Celgene. Dr. Komrokji reported financial relationships with Celgene and Novartis. Other study authors reported relationships with various pharmaceutical companies.
SOURCE: Komrokji R et al. Lancet Haematol. 2018 Jan 10. doi: 10.1016/S2352-3026(18)30002-4.
Sotatercept appears to have promise in treating anemia in patients with lower-risk myelodysplastic syndromes, and has also demonstrated an acceptable safety profile, according to Valeria Santini, MD.
“Ameliorating anemia in myelodysplastic syndromes by reversing ineffective erythropoiesis secondary to aberrant TGF [transforming growth factor]-beta stimulation is indeed an interesting new therapeutic avenue for these patients,” she wrote.
Dr. Santini also pointed out that the “most intriguing aspect of sotatercept” is its unique mechanism of action. The current study demonstrated the agent’s erythroid-stimulating and antiosteoporotic activity, which should encourage continuing research into the mutifaceted and extremely complex TGF-beta pathway.
While important results were demonstrated in this study, several questions remain, Dr. Santini noted. For example, what are the clinical characteristics of the patients who were sensitive to and responded to treatment with sotatercept? Are these patients different from those who responded to a different agent, luspatercept?
Dr. Santini is with department of hematology at the University of Florence (Italy). She reported giving lectures in supported symposia for Celgene, Janssen, and Novartis and serving on the advisory boards for Abbvie, Otsuka, and Janssen. Her remarks were adapted from an accompanying editorial (Lancet Haematol. 2018 Jan 10. doi: 10.1016/S2352-3026[18]30003-6).
Sotatercept appears to have promise in treating anemia in patients with lower-risk myelodysplastic syndromes, and has also demonstrated an acceptable safety profile, according to Valeria Santini, MD.
“Ameliorating anemia in myelodysplastic syndromes by reversing ineffective erythropoiesis secondary to aberrant TGF [transforming growth factor]-beta stimulation is indeed an interesting new therapeutic avenue for these patients,” she wrote.
Dr. Santini also pointed out that the “most intriguing aspect of sotatercept” is its unique mechanism of action. The current study demonstrated the agent’s erythroid-stimulating and antiosteoporotic activity, which should encourage continuing research into the mutifaceted and extremely complex TGF-beta pathway.
While important results were demonstrated in this study, several questions remain, Dr. Santini noted. For example, what are the clinical characteristics of the patients who were sensitive to and responded to treatment with sotatercept? Are these patients different from those who responded to a different agent, luspatercept?
Dr. Santini is with department of hematology at the University of Florence (Italy). She reported giving lectures in supported symposia for Celgene, Janssen, and Novartis and serving on the advisory boards for Abbvie, Otsuka, and Janssen. Her remarks were adapted from an accompanying editorial (Lancet Haematol. 2018 Jan 10. doi: 10.1016/S2352-3026[18]30003-6).
Sotatercept appears to have promise in treating anemia in patients with lower-risk myelodysplastic syndromes, and has also demonstrated an acceptable safety profile, according to Valeria Santini, MD.
“Ameliorating anemia in myelodysplastic syndromes by reversing ineffective erythropoiesis secondary to aberrant TGF [transforming growth factor]-beta stimulation is indeed an interesting new therapeutic avenue for these patients,” she wrote.
Dr. Santini also pointed out that the “most intriguing aspect of sotatercept” is its unique mechanism of action. The current study demonstrated the agent’s erythroid-stimulating and antiosteoporotic activity, which should encourage continuing research into the mutifaceted and extremely complex TGF-beta pathway.
While important results were demonstrated in this study, several questions remain, Dr. Santini noted. For example, what are the clinical characteristics of the patients who were sensitive to and responded to treatment with sotatercept? Are these patients different from those who responded to a different agent, luspatercept?
Dr. Santini is with department of hematology at the University of Florence (Italy). She reported giving lectures in supported symposia for Celgene, Janssen, and Novartis and serving on the advisory boards for Abbvie, Otsuka, and Janssen. Her remarks were adapted from an accompanying editorial (Lancet Haematol. 2018 Jan 10. doi: 10.1016/S2352-3026[18]30003-6).
A novel agent holds promise as a treatment option for anemia in patients with lower-risk myelodysplastic syndromes who are not helped by erythropoiesis-stimulating agents (ESAs), according to results from a phase 2 trial.
Sotatercept (ACE-011) is a first-in-class novel recombinant fusion protein, and was found to be effective and well tolerated, increasing hemoglobin concentrations and decreasing the transfusion burden in this patient population.
Nearly half (29, 47%) of 62 patients with a high transfusion burden achieved hematologic improvement–erythroid (HI-E), which for them was a reduction in red blood cell transfusion from baseline of 4 U or more for at least 56 days. Additionally, 7 of 12 patients (58%) with a low transfusion burden also achieved HI-E, defined as an increase in hemoglobin of 1.5 g/dL or more that was sustained for at least 56 days without a transfusion.
“Taken together, these findings provide proof of principle that the recombinant protein sotatercept can restore ineffective erythropoiesis in patients with lower-risk myelodysplastic syndromes, with an acceptable safety profile,” Rami Komrokji, MD, of Moffitt Cancer Center and Research Institute, Tampa, and his colleagues, wrote in the Lancet Haematology.
There are few effective treatment options available for patients with lower-risk myelodysplastic syndromes who have anemia, especially after they fail primary or secondary treatment with ESAs, or for those who are not likely to benefit from ESA therapy.
In this phase 2 trial, the researchers sought to establish a safe and effective dose of sotatercept in a cohort of 74 patients. Of this group, 7 received 0.1 mg/kg sotatercept, 6 got 0.3 mg/kg, 21 received 0.5 mg/kg, 35 got 1.0 mg/kg, and 5 patients received doses up to 2.0 mg/kg. The primary efficacy endpoint of the study was the proportion of patients who achieved HI-E.
All of the patients were pretreated, having received prior therapy for myelodysplastic syndromes, including ESAs, hypomethylating agents (azacitidine or decitabine), lenalidomide, and other agents including corticosteroids and immunomodulators.
Within this cohort, 36 patients (49%; 95% confidence intervaI, 38-60) achieved HI-E while 20 patients (27%; 95% CI, 18-38) achieved independence from transfusion for at least 56 days.
Fatigue (26%) and peripheral edema (24%) were the most common adverse events reported, while grade 3-4 treatment-emergent adverse events (TEAEs) were reported in 34% of patients. Of these, 4 patients had grade 3-4 TEAEs that were probably related to the treatment. The most common grade 3-4 TEAEs were lipase increase and anemia, and each was reported in three patients. Additionally, 17 patients (23%) experienced at least one serious TEAE, including a death from a treatment-emergent subdural hematoma (which caused the patient to fall).
The study was funded by the Celgene. Dr. Komrokji reported financial relationships with Celgene and Novartis. Other study authors reported relationships with various pharmaceutical companies.
SOURCE: Komrokji R et al. Lancet Haematol. 2018 Jan 10. doi: 10.1016/S2352-3026(18)30002-4.
A novel agent holds promise as a treatment option for anemia in patients with lower-risk myelodysplastic syndromes who are not helped by erythropoiesis-stimulating agents (ESAs), according to results from a phase 2 trial.
Sotatercept (ACE-011) is a first-in-class novel recombinant fusion protein, and was found to be effective and well tolerated, increasing hemoglobin concentrations and decreasing the transfusion burden in this patient population.
Nearly half (29, 47%) of 62 patients with a high transfusion burden achieved hematologic improvement–erythroid (HI-E), which for them was a reduction in red blood cell transfusion from baseline of 4 U or more for at least 56 days. Additionally, 7 of 12 patients (58%) with a low transfusion burden also achieved HI-E, defined as an increase in hemoglobin of 1.5 g/dL or more that was sustained for at least 56 days without a transfusion.
“Taken together, these findings provide proof of principle that the recombinant protein sotatercept can restore ineffective erythropoiesis in patients with lower-risk myelodysplastic syndromes, with an acceptable safety profile,” Rami Komrokji, MD, of Moffitt Cancer Center and Research Institute, Tampa, and his colleagues, wrote in the Lancet Haematology.
There are few effective treatment options available for patients with lower-risk myelodysplastic syndromes who have anemia, especially after they fail primary or secondary treatment with ESAs, or for those who are not likely to benefit from ESA therapy.
In this phase 2 trial, the researchers sought to establish a safe and effective dose of sotatercept in a cohort of 74 patients. Of this group, 7 received 0.1 mg/kg sotatercept, 6 got 0.3 mg/kg, 21 received 0.5 mg/kg, 35 got 1.0 mg/kg, and 5 patients received doses up to 2.0 mg/kg. The primary efficacy endpoint of the study was the proportion of patients who achieved HI-E.
All of the patients were pretreated, having received prior therapy for myelodysplastic syndromes, including ESAs, hypomethylating agents (azacitidine or decitabine), lenalidomide, and other agents including corticosteroids and immunomodulators.
Within this cohort, 36 patients (49%; 95% confidence intervaI, 38-60) achieved HI-E while 20 patients (27%; 95% CI, 18-38) achieved independence from transfusion for at least 56 days.
Fatigue (26%) and peripheral edema (24%) were the most common adverse events reported, while grade 3-4 treatment-emergent adverse events (TEAEs) were reported in 34% of patients. Of these, 4 patients had grade 3-4 TEAEs that were probably related to the treatment. The most common grade 3-4 TEAEs were lipase increase and anemia, and each was reported in three patients. Additionally, 17 patients (23%) experienced at least one serious TEAE, including a death from a treatment-emergent subdural hematoma (which caused the patient to fall).
The study was funded by the Celgene. Dr. Komrokji reported financial relationships with Celgene and Novartis. Other study authors reported relationships with various pharmaceutical companies.
SOURCE: Komrokji R et al. Lancet Haematol. 2018 Jan 10. doi: 10.1016/S2352-3026(18)30002-4.
FROM LANCET HAEMATOLOGY
Key clinical point:
Major finding: In all, 36 patients (49%) achieved hematologic improvement–erythroid and 20 patients (27%) achieved independence from transfusion for at least 56 days.
Data source: A phase 2 trial that included 74 patients with lower-risk myelodysplastic syndromes who did not respond to erythropoiesis-stimulating agents.
Disclosures: Celgene funded the study. Dr. Komrokji reported financial relationships with Celgene and Novartis. Other study authors reported relationships with various pharmaceutical companies.
Source: Komrokji R et al. Lancet Haematol. 2018 Jan 10. doi: 10.1016/S2352-3026(18)30002-4.