User login
FDA clears instrument for blood typing, screening
The U.S. Food and Drug Administration has granted marketing clearance for the immunohematology instrument NEO Iris™.
NEO Iris is a fully automated blood bank instrument designed for the mid- to high-volume laboratory, according to Immucor, Inc., the company marketing the device.
Immucor says NEO Iris provides the highest type and screen throughput on the market—up to 60 types and screens per hour.
NEO Iris performs ABO/Rh D typing, weak D testing, donor confirmation, cytomegalovirus screening, immunoglobulin G direct antiglobulin testing and crossmatching, and antibody identification and screening.
The workflow management tool on Neo Iris has STAT priority and allows operators to run tests in any order at any time, according to Immucor.
The company says NEO Iris can hold up to 224 samples, and “modules can pipette, incubate, centrifuge, and read simultaneously.”
NEO Iris integrates with Immucor’s data management software, ImmuLINK®, to aggregate test results and produce reports with complete testing history.
Another feature of NEO Iris is blud_directSM, which provides technical support from an Immucor representative that can be accessed on the NEO Iris monitor.
For more information on the instrument, see the NEO Iris page on the Immucor website.
The U.S. Food and Drug Administration has granted marketing clearance for the immunohematology instrument NEO Iris™.
NEO Iris is a fully automated blood bank instrument designed for the mid- to high-volume laboratory, according to Immucor, Inc., the company marketing the device.
Immucor says NEO Iris provides the highest type and screen throughput on the market—up to 60 types and screens per hour.
NEO Iris performs ABO/Rh D typing, weak D testing, donor confirmation, cytomegalovirus screening, immunoglobulin G direct antiglobulin testing and crossmatching, and antibody identification and screening.
The workflow management tool on Neo Iris has STAT priority and allows operators to run tests in any order at any time, according to Immucor.
The company says NEO Iris can hold up to 224 samples, and “modules can pipette, incubate, centrifuge, and read simultaneously.”
NEO Iris integrates with Immucor’s data management software, ImmuLINK®, to aggregate test results and produce reports with complete testing history.
Another feature of NEO Iris is blud_directSM, which provides technical support from an Immucor representative that can be accessed on the NEO Iris monitor.
For more information on the instrument, see the NEO Iris page on the Immucor website.
The U.S. Food and Drug Administration has granted marketing clearance for the immunohematology instrument NEO Iris™.
NEO Iris is a fully automated blood bank instrument designed for the mid- to high-volume laboratory, according to Immucor, Inc., the company marketing the device.
Immucor says NEO Iris provides the highest type and screen throughput on the market—up to 60 types and screens per hour.
NEO Iris performs ABO/Rh D typing, weak D testing, donor confirmation, cytomegalovirus screening, immunoglobulin G direct antiglobulin testing and crossmatching, and antibody identification and screening.
The workflow management tool on Neo Iris has STAT priority and allows operators to run tests in any order at any time, according to Immucor.
The company says NEO Iris can hold up to 224 samples, and “modules can pipette, incubate, centrifuge, and read simultaneously.”
NEO Iris integrates with Immucor’s data management software, ImmuLINK®, to aggregate test results and produce reports with complete testing history.
Another feature of NEO Iris is blud_directSM, which provides technical support from an Immucor representative that can be accessed on the NEO Iris monitor.
For more information on the instrument, see the NEO Iris page on the Immucor website.
Breakthrough designation for pathogen-reduced cryoprecipitate
The U.S. Food and Drug Administration (FDA) has granted breakthrough device designation for pathogen-reduced cryoprecipitate manufactured from plasma treated with the INTERCEPT Blood System.
Cerus Corporation aims to seek FDA approval for this pathogen-reduced cryoprecipitate to treat massive hemorrhage.
“The proposed label indication for pathogen-reduced cryoprecipitate would be to control massive bleeding associated with fibrinogen deficiency,” said Laurence Corash, chief scientific officer of Cerus.
The proposed shelf life for pathogen-reduced cyroprecipitate is 5 days at room temperature.
The INTERCEPT Blood System is already FDA-approved for the inactivation of pathogens in platelets and plasma.
“Cryo[precipitate] is a natural extension to our current FDA-approved INTERCEPT Blood System for plasma,” said William “Obi” Greenman, president and chief executive officer of Cerus.
If the FDA granted approval, INTERCEPT-treated plasma could be manufactured into pathogen-reduced cryoprecipitate.
About breakthrough device designation
The FDA says it grants breakthrough designation to devices “that provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating human diseases or conditions.”
Breakthrough designation is intended to expedite the development, assessment, and review of medical devices. The breakthrough devices program supersedes the FDA’s expedited access pathway and priority review programs.
The U.S. Food and Drug Administration (FDA) has granted breakthrough device designation for pathogen-reduced cryoprecipitate manufactured from plasma treated with the INTERCEPT Blood System.
Cerus Corporation aims to seek FDA approval for this pathogen-reduced cryoprecipitate to treat massive hemorrhage.
“The proposed label indication for pathogen-reduced cryoprecipitate would be to control massive bleeding associated with fibrinogen deficiency,” said Laurence Corash, chief scientific officer of Cerus.
The proposed shelf life for pathogen-reduced cyroprecipitate is 5 days at room temperature.
The INTERCEPT Blood System is already FDA-approved for the inactivation of pathogens in platelets and plasma.
“Cryo[precipitate] is a natural extension to our current FDA-approved INTERCEPT Blood System for plasma,” said William “Obi” Greenman, president and chief executive officer of Cerus.
If the FDA granted approval, INTERCEPT-treated plasma could be manufactured into pathogen-reduced cryoprecipitate.
About breakthrough device designation
The FDA says it grants breakthrough designation to devices “that provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating human diseases or conditions.”
Breakthrough designation is intended to expedite the development, assessment, and review of medical devices. The breakthrough devices program supersedes the FDA’s expedited access pathway and priority review programs.
The U.S. Food and Drug Administration (FDA) has granted breakthrough device designation for pathogen-reduced cryoprecipitate manufactured from plasma treated with the INTERCEPT Blood System.
Cerus Corporation aims to seek FDA approval for this pathogen-reduced cryoprecipitate to treat massive hemorrhage.
“The proposed label indication for pathogen-reduced cryoprecipitate would be to control massive bleeding associated with fibrinogen deficiency,” said Laurence Corash, chief scientific officer of Cerus.
The proposed shelf life for pathogen-reduced cyroprecipitate is 5 days at room temperature.
The INTERCEPT Blood System is already FDA-approved for the inactivation of pathogens in platelets and plasma.
“Cryo[precipitate] is a natural extension to our current FDA-approved INTERCEPT Blood System for plasma,” said William “Obi” Greenman, president and chief executive officer of Cerus.
If the FDA granted approval, INTERCEPT-treated plasma could be manufactured into pathogen-reduced cryoprecipitate.
About breakthrough device designation
The FDA says it grants breakthrough designation to devices “that provide more effective treatment or diagnosis of life-threatening or irreversibly debilitating human diseases or conditions.”
Breakthrough designation is intended to expedite the development, assessment, and review of medical devices. The breakthrough devices program supersedes the FDA’s expedited access pathway and priority review programs.
Lower threshold for platelet transfusions appears safer
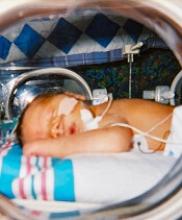
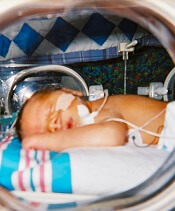
A lower threshold for platelet transfusions may be safer for preterm infants with severe thrombocytopenia, a new study suggests.
Researchers randomized preterm infants with severe thrombocytopenia to receive transfusions at platelet count thresholds of 50,000/mm3 or 25,000/mm3.
The team found that patients in the high-threshold group had a significantly higher risk of major bleeding or death.
Anna Curley, MD, of the National Maternity Hospital in Dublin, Ireland, and her colleagues reported this finding in The New England Journal of Medicine.
The researchers studied 660 infants with a mean gestational age of 26.6 weeks. They were randomized to receive platelet transfusions at a high platelet-count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
Within 28 days of randomization, a new major bleeding episode or death occurred in 26% of the high-threshold group and 19% of the low-threshold group.
When the researchers adjusted for gestational age, intrauterine growth restriction, and trial site, the odds ratio (OR) for major bleeding or death was 1.57 (95% confidence interval [CI], 1.06-2.32; P=0.02).
The OR for death alone was 1.56 (95% CI, 0.95-2.55), and the hazard ratio for at least one major bleeding episode was 1.32 (95% CI, 1.00-1.74).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups—25% and 22%, respectively (OR=1.14; 95% CI, 0.78-1.67).
The researchers said the results of this trial suggest that reducing the transfusion threshold from 50,000/mm3 to 25,000/mm3 may prevent death or major bleeding in 7 of 100 preterm neonates with severe thrombocytopenia.
However, the team also acknowledged that it isn’t clear why reducing the threshold may reduce the risk of mortality or major bleeding in this patient group.
The researchers said a range of factors might play a role in adverse outcomes of platelet transfusion in preterm neonates, including inflammatory consequences, hemodynamic shifts, fragility of the germinal matrix, disturbances in organ and brain blood flow, preterm lungs with a large capillary bed and abundant immune cells, platelet-derived reactive oxygen species, proangiogenic factors, and vessel occlusion by platelet microthrombi.
This study was supported by the National Health Service Blood and Transplant Research and Development Committee, Sanquin Research, Addenbrooke’s Charitable Trust, the Neonatal Breath of Life Fund, and the National Institute for Health Research Clinical Research Network.
One study author reported consulting for Sanquin Research, and another declared travel funds from Cerus Corporation.
A lower threshold for platelet transfusions may be safer for preterm infants with severe thrombocytopenia, a new study suggests.
Researchers randomized preterm infants with severe thrombocytopenia to receive transfusions at platelet count thresholds of 50,000/mm3 or 25,000/mm3.
The team found that patients in the high-threshold group had a significantly higher risk of major bleeding or death.
Anna Curley, MD, of the National Maternity Hospital in Dublin, Ireland, and her colleagues reported this finding in The New England Journal of Medicine.
The researchers studied 660 infants with a mean gestational age of 26.6 weeks. They were randomized to receive platelet transfusions at a high platelet-count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
Within 28 days of randomization, a new major bleeding episode or death occurred in 26% of the high-threshold group and 19% of the low-threshold group.
When the researchers adjusted for gestational age, intrauterine growth restriction, and trial site, the odds ratio (OR) for major bleeding or death was 1.57 (95% confidence interval [CI], 1.06-2.32; P=0.02).
The OR for death alone was 1.56 (95% CI, 0.95-2.55), and the hazard ratio for at least one major bleeding episode was 1.32 (95% CI, 1.00-1.74).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups—25% and 22%, respectively (OR=1.14; 95% CI, 0.78-1.67).
The researchers said the results of this trial suggest that reducing the transfusion threshold from 50,000/mm3 to 25,000/mm3 may prevent death or major bleeding in 7 of 100 preterm neonates with severe thrombocytopenia.
However, the team also acknowledged that it isn’t clear why reducing the threshold may reduce the risk of mortality or major bleeding in this patient group.
The researchers said a range of factors might play a role in adverse outcomes of platelet transfusion in preterm neonates, including inflammatory consequences, hemodynamic shifts, fragility of the germinal matrix, disturbances in organ and brain blood flow, preterm lungs with a large capillary bed and abundant immune cells, platelet-derived reactive oxygen species, proangiogenic factors, and vessel occlusion by platelet microthrombi.
This study was supported by the National Health Service Blood and Transplant Research and Development Committee, Sanquin Research, Addenbrooke’s Charitable Trust, the Neonatal Breath of Life Fund, and the National Institute for Health Research Clinical Research Network.
One study author reported consulting for Sanquin Research, and another declared travel funds from Cerus Corporation.
A lower threshold for platelet transfusions may be safer for preterm infants with severe thrombocytopenia, a new study suggests.
Researchers randomized preterm infants with severe thrombocytopenia to receive transfusions at platelet count thresholds of 50,000/mm3 or 25,000/mm3.
The team found that patients in the high-threshold group had a significantly higher risk of major bleeding or death.
Anna Curley, MD, of the National Maternity Hospital in Dublin, Ireland, and her colleagues reported this finding in The New England Journal of Medicine.
The researchers studied 660 infants with a mean gestational age of 26.6 weeks. They were randomized to receive platelet transfusions at a high platelet-count threshold of 50,000/mm3 or a low threshold of 25,000/mm3.
Within 28 days of randomization, a new major bleeding episode or death occurred in 26% of the high-threshold group and 19% of the low-threshold group.
When the researchers adjusted for gestational age, intrauterine growth restriction, and trial site, the odds ratio (OR) for major bleeding or death was 1.57 (95% confidence interval [CI], 1.06-2.32; P=0.02).
The OR for death alone was 1.56 (95% CI, 0.95-2.55), and the hazard ratio for at least one major bleeding episode was 1.32 (95% CI, 1.00-1.74).
The rates of serious adverse events, not including major bleeding, were similar between the high- and low-threshold groups—25% and 22%, respectively (OR=1.14; 95% CI, 0.78-1.67).
The researchers said the results of this trial suggest that reducing the transfusion threshold from 50,000/mm3 to 25,000/mm3 may prevent death or major bleeding in 7 of 100 preterm neonates with severe thrombocytopenia.
However, the team also acknowledged that it isn’t clear why reducing the threshold may reduce the risk of mortality or major bleeding in this patient group.
The researchers said a range of factors might play a role in adverse outcomes of platelet transfusion in preterm neonates, including inflammatory consequences, hemodynamic shifts, fragility of the germinal matrix, disturbances in organ and brain blood flow, preterm lungs with a large capillary bed and abundant immune cells, platelet-derived reactive oxygen species, proangiogenic factors, and vessel occlusion by platelet microthrombi.
This study was supported by the National Health Service Blood and Transplant Research and Development Committee, Sanquin Research, Addenbrooke’s Charitable Trust, the Neonatal Breath of Life Fund, and the National Institute for Health Research Clinical Research Network.
One study author reported consulting for Sanquin Research, and another declared travel funds from Cerus Corporation.
Testing platelets reduces waste, cuts costs
BOSTON—Rapid bacterial testing of platelets in a hospital blood bank can result in both significant cost savings and reduced wastage of blood products, according to investigators.
In a single-center study, rapid bacterial testing of 6- or 7-day-old apheresis platelets resulted in projected annual cost savings of nearly $89,000 and cut the rate of platelet wastage from expiration by more than half.
Adam L. Booth, MD, of the University of Texas in Galveston, and his colleagues described this study in a poster presentation at AABB 2018 (abstract INV4).
Platelets typically have a shelf life of 5 days because longer storage increases the risk for bacterial growth and the potential for transfusion-transmitted infections, Dr. Booth and his colleagues noted.
In March 2016, the U.S. Food and Drug Administration (FDA) published a draft guidance proposing a change in regulations to allow for an extended shelf life if platelets are collected in an FDA-approved, 7-day storage container with labeling that requires testing every product with a bacterial detection device, or if the platelets are individually tested for bacterial detection using an approved device.
Dr. Booth and his colleagues wanted to see what effect the regulations, if implemented as expected, might have on acquisition costs and wastage of apheresis platelets.
The investigators reviewed their center’s platelet acquisition costs and wastage from expiration 12 months before and 6 months after implementation of a rapid bacterial testing protocol, with 6-month results projected out to 1 year for comparison purposes.
The team looked at data on bacterial testing of 6-day and 7-day-old apheresis platelets, and they excluded data on platelet units that were due to expire on day 5 because they were not stored in FDA-approved containers.
Prior to testing, the annual wastage rate was 24%, or 332 of 1,371 platelet units purchased. Using a mean per-unit cost of $516.96, the annual cost was more than $171,000.
After the start of testing, the annualized rate of wastage dropped to 10%, or 117 of 1,168 platelet units. So the annualized cost was more than $60,000.
The difference in cost—minus the cost of rapid bacterial testing (roughly $22,500)—resulted in an annual savings for the institution of nearly $89,000.
The number of units transfused and the associated costs of transfusions were similar between the time periods studied.
This study was internally funded. The authors reported having no conflicts of interest.
BOSTON—Rapid bacterial testing of platelets in a hospital blood bank can result in both significant cost savings and reduced wastage of blood products, according to investigators.
In a single-center study, rapid bacterial testing of 6- or 7-day-old apheresis platelets resulted in projected annual cost savings of nearly $89,000 and cut the rate of platelet wastage from expiration by more than half.
Adam L. Booth, MD, of the University of Texas in Galveston, and his colleagues described this study in a poster presentation at AABB 2018 (abstract INV4).
Platelets typically have a shelf life of 5 days because longer storage increases the risk for bacterial growth and the potential for transfusion-transmitted infections, Dr. Booth and his colleagues noted.
In March 2016, the U.S. Food and Drug Administration (FDA) published a draft guidance proposing a change in regulations to allow for an extended shelf life if platelets are collected in an FDA-approved, 7-day storage container with labeling that requires testing every product with a bacterial detection device, or if the platelets are individually tested for bacterial detection using an approved device.
Dr. Booth and his colleagues wanted to see what effect the regulations, if implemented as expected, might have on acquisition costs and wastage of apheresis platelets.
The investigators reviewed their center’s platelet acquisition costs and wastage from expiration 12 months before and 6 months after implementation of a rapid bacterial testing protocol, with 6-month results projected out to 1 year for comparison purposes.
The team looked at data on bacterial testing of 6-day and 7-day-old apheresis platelets, and they excluded data on platelet units that were due to expire on day 5 because they were not stored in FDA-approved containers.
Prior to testing, the annual wastage rate was 24%, or 332 of 1,371 platelet units purchased. Using a mean per-unit cost of $516.96, the annual cost was more than $171,000.
After the start of testing, the annualized rate of wastage dropped to 10%, or 117 of 1,168 platelet units. So the annualized cost was more than $60,000.
The difference in cost—minus the cost of rapid bacterial testing (roughly $22,500)—resulted in an annual savings for the institution of nearly $89,000.
The number of units transfused and the associated costs of transfusions were similar between the time periods studied.
This study was internally funded. The authors reported having no conflicts of interest.
BOSTON—Rapid bacterial testing of platelets in a hospital blood bank can result in both significant cost savings and reduced wastage of blood products, according to investigators.
In a single-center study, rapid bacterial testing of 6- or 7-day-old apheresis platelets resulted in projected annual cost savings of nearly $89,000 and cut the rate of platelet wastage from expiration by more than half.
Adam L. Booth, MD, of the University of Texas in Galveston, and his colleagues described this study in a poster presentation at AABB 2018 (abstract INV4).
Platelets typically have a shelf life of 5 days because longer storage increases the risk for bacterial growth and the potential for transfusion-transmitted infections, Dr. Booth and his colleagues noted.
In March 2016, the U.S. Food and Drug Administration (FDA) published a draft guidance proposing a change in regulations to allow for an extended shelf life if platelets are collected in an FDA-approved, 7-day storage container with labeling that requires testing every product with a bacterial detection device, or if the platelets are individually tested for bacterial detection using an approved device.
Dr. Booth and his colleagues wanted to see what effect the regulations, if implemented as expected, might have on acquisition costs and wastage of apheresis platelets.
The investigators reviewed their center’s platelet acquisition costs and wastage from expiration 12 months before and 6 months after implementation of a rapid bacterial testing protocol, with 6-month results projected out to 1 year for comparison purposes.
The team looked at data on bacterial testing of 6-day and 7-day-old apheresis platelets, and they excluded data on platelet units that were due to expire on day 5 because they were not stored in FDA-approved containers.
Prior to testing, the annual wastage rate was 24%, or 332 of 1,371 platelet units purchased. Using a mean per-unit cost of $516.96, the annual cost was more than $171,000.
After the start of testing, the annualized rate of wastage dropped to 10%, or 117 of 1,168 platelet units. So the annualized cost was more than $60,000.
The difference in cost—minus the cost of rapid bacterial testing (roughly $22,500)—resulted in an annual savings for the institution of nearly $89,000.
The number of units transfused and the associated costs of transfusions were similar between the time periods studied.
This study was internally funded. The authors reported having no conflicts of interest.
Blood donated after mass shootings may go to waste
Public calls for blood donations in response to mass shootings may be unnecessary and result in waste, according to researchers.
Mass shootings often trigger a sharp increase in blood donations for affected communities.
In response to the recent mass shooting at the Tree Of Life Synagogue in Pittsburgh, Pennsylvania, multiple news outlets called for blood donations, and local blood centers extended their hours to compensate for the increase in donations.
However, a new study suggests that blood products donated in response to mass shootings may go unused and have to be discarded.
This study was published in The Journal of Trauma and Acute Care Surgery.
The study authors focused on blood donated in response to the mass shooting that took place in Las Vegas on October 1, 2017. This shooting resulted in 58 deaths, 869 injuries, 220 hospital admissions, and at least 68 critical care admissions.
Three healthcare systems provided data for the study. In all, 519 shooting victims were treated within these systems, and 185 were admitted to the hospitals. During the first 24 hours, these patients received 499 blood components, or 2.7 units per admission.
“From our data, it is likely that the total 1-day blood component transfusions needed in Las Vegas were more than in any mass shooting on record,” said study author M. James Lozada, DO, of Vanderbilt University Medical Center in Nashville, Tennessee.
However, the blood donations made in response to the shooting surpassed the need.
A public call for blood donations was issued during a press conference in the early hours of October 2, and that call was amplified in news stories. Stories about blood donation in Las Vegas increased from a daily average of 10 to more than 100 on October 2.
From October 2 to 4, the American Red Cross saw a 53% increase in blood donations nationwide.
The Las Vegas blood bank, United Blood Services, reported receiving 791 donations right after the shooting.
Unfortunately, 137 of these donations (17%) went unused and were subsequently discarded, compared to an average of 26 wasted donations per month at the blood bank.
Therefore, Dr. Lozada and his colleagues concluded that a call for immediate blood donation was unnecessary.
“There is an emotional desire after these events on the part of the public to immediately donate blood, but that’s not always necessary, and it’s not always the best immediate response,” Dr. Lozada said. “The best thing you can do is donate blood year-round.”
“One of the things that we propose in the paper is for cities to develop some protocols for these kind of scenarios, where instead of issuing a blanket call for blood donation, you would do it in a systematic way. As one suggestion, you might do it by ZIP code.”
“Our findings are important to help us prepare for the next mass shooting in the United States. It shows us the amount of blood components we likely will need. It will also help first responders adequately prepare to save lives.”
There was no funding for this research, and the study authors declared no conflicts of interest.
Public calls for blood donations in response to mass shootings may be unnecessary and result in waste, according to researchers.
Mass shootings often trigger a sharp increase in blood donations for affected communities.
In response to the recent mass shooting at the Tree Of Life Synagogue in Pittsburgh, Pennsylvania, multiple news outlets called for blood donations, and local blood centers extended their hours to compensate for the increase in donations.
However, a new study suggests that blood products donated in response to mass shootings may go unused and have to be discarded.
This study was published in The Journal of Trauma and Acute Care Surgery.
The study authors focused on blood donated in response to the mass shooting that took place in Las Vegas on October 1, 2017. This shooting resulted in 58 deaths, 869 injuries, 220 hospital admissions, and at least 68 critical care admissions.
Three healthcare systems provided data for the study. In all, 519 shooting victims were treated within these systems, and 185 were admitted to the hospitals. During the first 24 hours, these patients received 499 blood components, or 2.7 units per admission.
“From our data, it is likely that the total 1-day blood component transfusions needed in Las Vegas were more than in any mass shooting on record,” said study author M. James Lozada, DO, of Vanderbilt University Medical Center in Nashville, Tennessee.
However, the blood donations made in response to the shooting surpassed the need.
A public call for blood donations was issued during a press conference in the early hours of October 2, and that call was amplified in news stories. Stories about blood donation in Las Vegas increased from a daily average of 10 to more than 100 on October 2.
From October 2 to 4, the American Red Cross saw a 53% increase in blood donations nationwide.
The Las Vegas blood bank, United Blood Services, reported receiving 791 donations right after the shooting.
Unfortunately, 137 of these donations (17%) went unused and were subsequently discarded, compared to an average of 26 wasted donations per month at the blood bank.
Therefore, Dr. Lozada and his colleagues concluded that a call for immediate blood donation was unnecessary.
“There is an emotional desire after these events on the part of the public to immediately donate blood, but that’s not always necessary, and it’s not always the best immediate response,” Dr. Lozada said. “The best thing you can do is donate blood year-round.”
“One of the things that we propose in the paper is for cities to develop some protocols for these kind of scenarios, where instead of issuing a blanket call for blood donation, you would do it in a systematic way. As one suggestion, you might do it by ZIP code.”
“Our findings are important to help us prepare for the next mass shooting in the United States. It shows us the amount of blood components we likely will need. It will also help first responders adequately prepare to save lives.”
There was no funding for this research, and the study authors declared no conflicts of interest.
Public calls for blood donations in response to mass shootings may be unnecessary and result in waste, according to researchers.
Mass shootings often trigger a sharp increase in blood donations for affected communities.
In response to the recent mass shooting at the Tree Of Life Synagogue in Pittsburgh, Pennsylvania, multiple news outlets called for blood donations, and local blood centers extended their hours to compensate for the increase in donations.
However, a new study suggests that blood products donated in response to mass shootings may go unused and have to be discarded.
This study was published in The Journal of Trauma and Acute Care Surgery.
The study authors focused on blood donated in response to the mass shooting that took place in Las Vegas on October 1, 2017. This shooting resulted in 58 deaths, 869 injuries, 220 hospital admissions, and at least 68 critical care admissions.
Three healthcare systems provided data for the study. In all, 519 shooting victims were treated within these systems, and 185 were admitted to the hospitals. During the first 24 hours, these patients received 499 blood components, or 2.7 units per admission.
“From our data, it is likely that the total 1-day blood component transfusions needed in Las Vegas were more than in any mass shooting on record,” said study author M. James Lozada, DO, of Vanderbilt University Medical Center in Nashville, Tennessee.
However, the blood donations made in response to the shooting surpassed the need.
A public call for blood donations was issued during a press conference in the early hours of October 2, and that call was amplified in news stories. Stories about blood donation in Las Vegas increased from a daily average of 10 to more than 100 on October 2.
From October 2 to 4, the American Red Cross saw a 53% increase in blood donations nationwide.
The Las Vegas blood bank, United Blood Services, reported receiving 791 donations right after the shooting.
Unfortunately, 137 of these donations (17%) went unused and were subsequently discarded, compared to an average of 26 wasted donations per month at the blood bank.
Therefore, Dr. Lozada and his colleagues concluded that a call for immediate blood donation was unnecessary.
“There is an emotional desire after these events on the part of the public to immediately donate blood, but that’s not always necessary, and it’s not always the best immediate response,” Dr. Lozada said. “The best thing you can do is donate blood year-round.”
“One of the things that we propose in the paper is for cities to develop some protocols for these kind of scenarios, where instead of issuing a blanket call for blood donation, you would do it in a systematic way. As one suggestion, you might do it by ZIP code.”
“Our findings are important to help us prepare for the next mass shooting in the United States. It shows us the amount of blood components we likely will need. It will also help first responders adequately prepare to save lives.”
There was no funding for this research, and the study authors declared no conflicts of interest.
Transfusion errors more common in kids than adults, study suggests
BOSTON—Even the most vigilant hospitals experience transfusion errors and problems with blood storage, according to researchers.
A review of data from 32 U.S. hospitals showed that pediatric transfusions were associated with a higher rate of safety problems than adult transfusions, with errors differing by age group.
The most common error in the pediatric population was failure to follow protocol, and the most common error in the adult population was that scheduled transfusions were not performed.
Sarah Vossoughi, MD, of Columbia University and New York–Presbyterian Hospital in New York, described these findings at AABB 2018 (abstract QT4).
To evaluate patient safety events related to blood transfusions, Dr. Vossoughi and her colleagues reviewed data on events reported by three children’s hospitals and 29 adult hospitals. Events were reported to either the AABB Center for Patient Safety or the medical center’s own adverse event reporting system from January 2010 through September 2017.
The researchers identified a total of 1,806 reports associated with approximately 1,088,884 transfusions.
There were 249 reports associated with 99,064 pediatric transfusions and 1,577 reports associated with 989,820 adult transfusions. So the reporting rate was 251 per 100,000 transfusions for the pediatric population and 157 per 100,000 transfusions for the adult population (P<0.001).
The most common error for pediatric patients—failure to follow the transfusion protocol—made up 31% of the pediatric errors.
“In a lot of the pediatric hospitals, it’s kind of like the Wild West,” Dr. Vossoughi said. “People say, ‘Well, I know it’s the hospital policy, but this child is special, so I’m going to do it this way, this time.’ That seems to be a culture in pediatrics, whereas, on the adult side, [clinicians] seem to be much less likely to just deviate from the protocol.”
Among adults, the most common error was “transfusion not performed,” which accounted for 43% of the adult errors. Dr. Vossoughi said transfusions may be skipped due to a bungled patient hand-off during a shift change or when a patient is being moved from one unit to another.
“The next day, they’ll check the patient’s CBC [complete blood count] and realize that the patient didn’t respond to the infusion that it turned out they never got, and then the product will be found on the floor, expired,” Dr. Vossoughi said.
She and her colleagues also found that 20% of pediatric errors and 24% of adult errors were associated with incorrect storage of blood products on the patient floor.
“It’s very common for blood banks to find platelets in the refrigerator,” Dr. Vossoughi said. “It doesn’t matter how old you are or what type of hospital you’re at. Everyone’s putting platelets in the fridge.”
Dr. Vossoughi and her colleagues believe these findings could help inpatient blood management programs target education and interventions to providers who commit similar errors.
“If you know that a particular provider group has problems following the protocol, maybe you can make the protocol a little simpler to follow or make the checklist less cumbersome, and then maybe they’ll follow them more often,” Dr. Vossoughi said.
This research was supported by the AABB Center for Patient Safety and University of Vermont Medical Center. The researchers reported no conflicts of interest.
BOSTON—Even the most vigilant hospitals experience transfusion errors and problems with blood storage, according to researchers.
A review of data from 32 U.S. hospitals showed that pediatric transfusions were associated with a higher rate of safety problems than adult transfusions, with errors differing by age group.
The most common error in the pediatric population was failure to follow protocol, and the most common error in the adult population was that scheduled transfusions were not performed.
Sarah Vossoughi, MD, of Columbia University and New York–Presbyterian Hospital in New York, described these findings at AABB 2018 (abstract QT4).
To evaluate patient safety events related to blood transfusions, Dr. Vossoughi and her colleagues reviewed data on events reported by three children’s hospitals and 29 adult hospitals. Events were reported to either the AABB Center for Patient Safety or the medical center’s own adverse event reporting system from January 2010 through September 2017.
The researchers identified a total of 1,806 reports associated with approximately 1,088,884 transfusions.
There were 249 reports associated with 99,064 pediatric transfusions and 1,577 reports associated with 989,820 adult transfusions. So the reporting rate was 251 per 100,000 transfusions for the pediatric population and 157 per 100,000 transfusions for the adult population (P<0.001).
The most common error for pediatric patients—failure to follow the transfusion protocol—made up 31% of the pediatric errors.
“In a lot of the pediatric hospitals, it’s kind of like the Wild West,” Dr. Vossoughi said. “People say, ‘Well, I know it’s the hospital policy, but this child is special, so I’m going to do it this way, this time.’ That seems to be a culture in pediatrics, whereas, on the adult side, [clinicians] seem to be much less likely to just deviate from the protocol.”
Among adults, the most common error was “transfusion not performed,” which accounted for 43% of the adult errors. Dr. Vossoughi said transfusions may be skipped due to a bungled patient hand-off during a shift change or when a patient is being moved from one unit to another.
“The next day, they’ll check the patient’s CBC [complete blood count] and realize that the patient didn’t respond to the infusion that it turned out they never got, and then the product will be found on the floor, expired,” Dr. Vossoughi said.
She and her colleagues also found that 20% of pediatric errors and 24% of adult errors were associated with incorrect storage of blood products on the patient floor.
“It’s very common for blood banks to find platelets in the refrigerator,” Dr. Vossoughi said. “It doesn’t matter how old you are or what type of hospital you’re at. Everyone’s putting platelets in the fridge.”
Dr. Vossoughi and her colleagues believe these findings could help inpatient blood management programs target education and interventions to providers who commit similar errors.
“If you know that a particular provider group has problems following the protocol, maybe you can make the protocol a little simpler to follow or make the checklist less cumbersome, and then maybe they’ll follow them more often,” Dr. Vossoughi said.
This research was supported by the AABB Center for Patient Safety and University of Vermont Medical Center. The researchers reported no conflicts of interest.
BOSTON—Even the most vigilant hospitals experience transfusion errors and problems with blood storage, according to researchers.
A review of data from 32 U.S. hospitals showed that pediatric transfusions were associated with a higher rate of safety problems than adult transfusions, with errors differing by age group.
The most common error in the pediatric population was failure to follow protocol, and the most common error in the adult population was that scheduled transfusions were not performed.
Sarah Vossoughi, MD, of Columbia University and New York–Presbyterian Hospital in New York, described these findings at AABB 2018 (abstract QT4).
To evaluate patient safety events related to blood transfusions, Dr. Vossoughi and her colleagues reviewed data on events reported by three children’s hospitals and 29 adult hospitals. Events were reported to either the AABB Center for Patient Safety or the medical center’s own adverse event reporting system from January 2010 through September 2017.
The researchers identified a total of 1,806 reports associated with approximately 1,088,884 transfusions.
There were 249 reports associated with 99,064 pediatric transfusions and 1,577 reports associated with 989,820 adult transfusions. So the reporting rate was 251 per 100,000 transfusions for the pediatric population and 157 per 100,000 transfusions for the adult population (P<0.001).
The most common error for pediatric patients—failure to follow the transfusion protocol—made up 31% of the pediatric errors.
“In a lot of the pediatric hospitals, it’s kind of like the Wild West,” Dr. Vossoughi said. “People say, ‘Well, I know it’s the hospital policy, but this child is special, so I’m going to do it this way, this time.’ That seems to be a culture in pediatrics, whereas, on the adult side, [clinicians] seem to be much less likely to just deviate from the protocol.”
Among adults, the most common error was “transfusion not performed,” which accounted for 43% of the adult errors. Dr. Vossoughi said transfusions may be skipped due to a bungled patient hand-off during a shift change or when a patient is being moved from one unit to another.
“The next day, they’ll check the patient’s CBC [complete blood count] and realize that the patient didn’t respond to the infusion that it turned out they never got, and then the product will be found on the floor, expired,” Dr. Vossoughi said.
She and her colleagues also found that 20% of pediatric errors and 24% of adult errors were associated with incorrect storage of blood products on the patient floor.
“It’s very common for blood banks to find platelets in the refrigerator,” Dr. Vossoughi said. “It doesn’t matter how old you are or what type of hospital you’re at. Everyone’s putting platelets in the fridge.”
Dr. Vossoughi and her colleagues believe these findings could help inpatient blood management programs target education and interventions to providers who commit similar errors.
“If you know that a particular provider group has problems following the protocol, maybe you can make the protocol a little simpler to follow or make the checklist less cumbersome, and then maybe they’ll follow them more often,” Dr. Vossoughi said.
This research was supported by the AABB Center for Patient Safety and University of Vermont Medical Center. The researchers reported no conflicts of interest.
Team reports success with mobile platelet collection
BOSTON—If donors can’t get to the apheresis center, bring the apheresis center to the donors.
Researchers have found that apheresis platelet collection in the field is practical with proper planning and support.
A team at the University of California at Los Angeles (UCLA) Blood and Platelet Center explored adding mobile apheresis units to their existing community blood drives.
They found that, with careful planning and coordination, they could augment their supply of blood products and introduce potential new donors to the idea of apheresis donations at the hospital.
The researchers reported these findings in a poster presentation at AABB 2018 (poster BBC 135).
It all started with a needs drive for an oncology patient at UCLA, explained David Anthony, manager of the UCLA Blood and Platelet Center.
“She wanted to bring in donors and had her whole community behind her,” Anthony said. “And we thought, well, she’s an oncology patient, and she uses platelets, and we had talked about doing platelets out in the field rather than just at fixed sites, and we thought that this would be a good chance to try it.”
Until the mobile unit was established, apheresis platelet collections for the hospital-based donor center were limited to two fixed collection sites, with mobile units used only for collection of whole blood.
To see whether concurrent whole blood and platelet community drives were practical, the center’s blood donor field recruiter requested to schedule a community drive in a region of the county where potential donors had expressed a high level of interest in apheresis platelet donations.
Operations staff visited the site to assess its suitability, including appropriate space for donor registration and history taking, separate areas for whole blood and apheresis donations, and a donor recovery area. The assessment included ensuring there were suitable electrical outlets, space, and support for apheresis machines.
“Over about 2 weeks, we discussed with our medical directors, [infusion technicians], and our mobile people what we would need to do it,” Anthony said. “The recruiter out in the field was able to go to a high school drive out in that area, recruit donors, and get [platelet] precounts from them so that we could find out who was a good candidate.”
Once they had platelet counts from potential apheresis donors, 10 donors were prescreened based on their eligibility to donate multiple products, history of donations and red blood cell loss, and, for women who had previously had more than one pregnancy, favorable human leukocyte antigen test results.
Four of the prescreened donors were scheduled to donate platelets, and the time slot also included two backup donors, one of whom ultimately donated platelets.
The first drive resulted in the collection of seven platelet products, including three double products and one single product.
The donated products resulted in about a $3,000 cost savings by obviating the need for purchasing products from an outside supplier and bolstered the blood bank’s inventory on a normally low collection day, the researchers reported.
“We’ve had two more apheresis drives since then, and we’ll have another one in 3 weeks,” Anthony said.
He acknowledged that it is more challenging to recruit, educate, and retain donors in the field than in the brick-and-mortar hospital setting.
“We have to make sure that they’re going to show up if we’re going to make the effort to take a machine out there, whereas, at our centers, we have regular donors who come in every 2 weeks,” Anthony said. “It’s easy for them to make an appointment, and they know where we are.”
The center plans to continue concurrent monthly whole blood and platelet collection drives, he added.
This pilot program was internally funded. The researchers reported having no relevant conflicts of interest.
BOSTON—If donors can’t get to the apheresis center, bring the apheresis center to the donors.
Researchers have found that apheresis platelet collection in the field is practical with proper planning and support.
A team at the University of California at Los Angeles (UCLA) Blood and Platelet Center explored adding mobile apheresis units to their existing community blood drives.
They found that, with careful planning and coordination, they could augment their supply of blood products and introduce potential new donors to the idea of apheresis donations at the hospital.
The researchers reported these findings in a poster presentation at AABB 2018 (poster BBC 135).
It all started with a needs drive for an oncology patient at UCLA, explained David Anthony, manager of the UCLA Blood and Platelet Center.
“She wanted to bring in donors and had her whole community behind her,” Anthony said. “And we thought, well, she’s an oncology patient, and she uses platelets, and we had talked about doing platelets out in the field rather than just at fixed sites, and we thought that this would be a good chance to try it.”
Until the mobile unit was established, apheresis platelet collections for the hospital-based donor center were limited to two fixed collection sites, with mobile units used only for collection of whole blood.
To see whether concurrent whole blood and platelet community drives were practical, the center’s blood donor field recruiter requested to schedule a community drive in a region of the county where potential donors had expressed a high level of interest in apheresis platelet donations.
Operations staff visited the site to assess its suitability, including appropriate space for donor registration and history taking, separate areas for whole blood and apheresis donations, and a donor recovery area. The assessment included ensuring there were suitable electrical outlets, space, and support for apheresis machines.
“Over about 2 weeks, we discussed with our medical directors, [infusion technicians], and our mobile people what we would need to do it,” Anthony said. “The recruiter out in the field was able to go to a high school drive out in that area, recruit donors, and get [platelet] precounts from them so that we could find out who was a good candidate.”
Once they had platelet counts from potential apheresis donors, 10 donors were prescreened based on their eligibility to donate multiple products, history of donations and red blood cell loss, and, for women who had previously had more than one pregnancy, favorable human leukocyte antigen test results.
Four of the prescreened donors were scheduled to donate platelets, and the time slot also included two backup donors, one of whom ultimately donated platelets.
The first drive resulted in the collection of seven platelet products, including three double products and one single product.
The donated products resulted in about a $3,000 cost savings by obviating the need for purchasing products from an outside supplier and bolstered the blood bank’s inventory on a normally low collection day, the researchers reported.
“We’ve had two more apheresis drives since then, and we’ll have another one in 3 weeks,” Anthony said.
He acknowledged that it is more challenging to recruit, educate, and retain donors in the field than in the brick-and-mortar hospital setting.
“We have to make sure that they’re going to show up if we’re going to make the effort to take a machine out there, whereas, at our centers, we have regular donors who come in every 2 weeks,” Anthony said. “It’s easy for them to make an appointment, and they know where we are.”
The center plans to continue concurrent monthly whole blood and platelet collection drives, he added.
This pilot program was internally funded. The researchers reported having no relevant conflicts of interest.
BOSTON—If donors can’t get to the apheresis center, bring the apheresis center to the donors.
Researchers have found that apheresis platelet collection in the field is practical with proper planning and support.
A team at the University of California at Los Angeles (UCLA) Blood and Platelet Center explored adding mobile apheresis units to their existing community blood drives.
They found that, with careful planning and coordination, they could augment their supply of blood products and introduce potential new donors to the idea of apheresis donations at the hospital.
The researchers reported these findings in a poster presentation at AABB 2018 (poster BBC 135).
It all started with a needs drive for an oncology patient at UCLA, explained David Anthony, manager of the UCLA Blood and Platelet Center.
“She wanted to bring in donors and had her whole community behind her,” Anthony said. “And we thought, well, she’s an oncology patient, and she uses platelets, and we had talked about doing platelets out in the field rather than just at fixed sites, and we thought that this would be a good chance to try it.”
Until the mobile unit was established, apheresis platelet collections for the hospital-based donor center were limited to two fixed collection sites, with mobile units used only for collection of whole blood.
To see whether concurrent whole blood and platelet community drives were practical, the center’s blood donor field recruiter requested to schedule a community drive in a region of the county where potential donors had expressed a high level of interest in apheresis platelet donations.
Operations staff visited the site to assess its suitability, including appropriate space for donor registration and history taking, separate areas for whole blood and apheresis donations, and a donor recovery area. The assessment included ensuring there were suitable electrical outlets, space, and support for apheresis machines.
“Over about 2 weeks, we discussed with our medical directors, [infusion technicians], and our mobile people what we would need to do it,” Anthony said. “The recruiter out in the field was able to go to a high school drive out in that area, recruit donors, and get [platelet] precounts from them so that we could find out who was a good candidate.”
Once they had platelet counts from potential apheresis donors, 10 donors were prescreened based on their eligibility to donate multiple products, history of donations and red blood cell loss, and, for women who had previously had more than one pregnancy, favorable human leukocyte antigen test results.
Four of the prescreened donors were scheduled to donate platelets, and the time slot also included two backup donors, one of whom ultimately donated platelets.
The first drive resulted in the collection of seven platelet products, including three double products and one single product.
The donated products resulted in about a $3,000 cost savings by obviating the need for purchasing products from an outside supplier and bolstered the blood bank’s inventory on a normally low collection day, the researchers reported.
“We’ve had two more apheresis drives since then, and we’ll have another one in 3 weeks,” Anthony said.
He acknowledged that it is more challenging to recruit, educate, and retain donors in the field than in the brick-and-mortar hospital setting.
“We have to make sure that they’re going to show up if we’re going to make the effort to take a machine out there, whereas, at our centers, we have regular donors who come in every 2 weeks,” Anthony said. “It’s easy for them to make an appointment, and they know where we are.”
The center plans to continue concurrent monthly whole blood and platelet collection drives, he added.
This pilot program was internally funded. The researchers reported having no relevant conflicts of interest.
Drones can deliver blood products, but hurdles remain
BOSTON—Drone-delivered blood products may be coming soon to a hospital near you, experts said at AABB 2018.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries,” Kenney said. “That’s almost 20,000 units of blood.”
One-third of all deliveries are needed for urgent, life-saving interventions, he added.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, according to Kenney.
How it works
Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section.
The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products, which can be done by text message or via WhatsApp, a free messaging and voiceover IP calling service.
After the order was placed, Zipline was able to deliver blood products using multiple drone launches over the course of 90 minutes. The deliveries consisted of 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15 to 25 minutes by air, Kenney said.
The drones—more formally known as “unmanned aerial vehicles”—fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters, and drop the package, which has a parachute attached.
Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Kenney said.
“The whole process is 100% autonomous,” he noted. “The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package.”
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are significantly cheaper than ground-based services for local delivery, according to Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold cited statistics suggesting the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more. However, drone delivery could be as cheap as 5 cents per mile.
Barriers to drone delivery
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, according to Dr. Eastvold.
He described the hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters in the United States.
Dr. Eastvold said the barriers to adoption of drone-based delivery systems include differences in state laws about when, where, and how drones can be used and who can operate them as well as Federal Aviation Administration (FAA) airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation for most drone operators, meaning the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery.
In 2015, Johns Hopkins researchers reported that transporting blood samples on hobby-sized drones did not affect the results of common and routine blood tests.
In 2016, the researchers showed that large bags of blood products can maintain temperature and cellular integrity when transported by drones.
In 2017, the researchers demonstrated that a drone could deliver blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Kenney said his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
BOSTON—Drone-delivered blood products may be coming soon to a hospital near you, experts said at AABB 2018.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries,” Kenney said. “That’s almost 20,000 units of blood.”
One-third of all deliveries are needed for urgent, life-saving interventions, he added.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, according to Kenney.
How it works
Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section.
The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products, which can be done by text message or via WhatsApp, a free messaging and voiceover IP calling service.
After the order was placed, Zipline was able to deliver blood products using multiple drone launches over the course of 90 minutes. The deliveries consisted of 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15 to 25 minutes by air, Kenney said.
The drones—more formally known as “unmanned aerial vehicles”—fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters, and drop the package, which has a parachute attached.
Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Kenney said.
“The whole process is 100% autonomous,” he noted. “The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package.”
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are significantly cheaper than ground-based services for local delivery, according to Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold cited statistics suggesting the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more. However, drone delivery could be as cheap as 5 cents per mile.
Barriers to drone delivery
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, according to Dr. Eastvold.
He described the hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters in the United States.
Dr. Eastvold said the barriers to adoption of drone-based delivery systems include differences in state laws about when, where, and how drones can be used and who can operate them as well as Federal Aviation Administration (FAA) airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation for most drone operators, meaning the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery.
In 2015, Johns Hopkins researchers reported that transporting blood samples on hobby-sized drones did not affect the results of common and routine blood tests.
In 2016, the researchers showed that large bags of blood products can maintain temperature and cellular integrity when transported by drones.
In 2017, the researchers demonstrated that a drone could deliver blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Kenney said his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
BOSTON—Drone-delivered blood products may be coming soon to a hospital near you, experts said at AABB 2018.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries,” Kenney said. “That’s almost 20,000 units of blood.”
One-third of all deliveries are needed for urgent, life-saving interventions, he added.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, according to Kenney.
How it works
Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section.
The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products, which can be done by text message or via WhatsApp, a free messaging and voiceover IP calling service.
After the order was placed, Zipline was able to deliver blood products using multiple drone launches over the course of 90 minutes. The deliveries consisted of 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15 to 25 minutes by air, Kenney said.
The drones—more formally known as “unmanned aerial vehicles”—fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters, and drop the package, which has a parachute attached.
Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Kenney said.
“The whole process is 100% autonomous,” he noted. “The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package.”
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are significantly cheaper than ground-based services for local delivery, according to Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold cited statistics suggesting the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more. However, drone delivery could be as cheap as 5 cents per mile.
Barriers to drone delivery
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, according to Dr. Eastvold.
He described the hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters in the United States.
Dr. Eastvold said the barriers to adoption of drone-based delivery systems include differences in state laws about when, where, and how drones can be used and who can operate them as well as Federal Aviation Administration (FAA) airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation for most drone operators, meaning the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery.
In 2015, Johns Hopkins researchers reported that transporting blood samples on hobby-sized drones did not affect the results of common and routine blood tests.
In 2016, the researchers showed that large bags of blood products can maintain temperature and cellular integrity when transported by drones.
In 2017, the researchers demonstrated that a drone could deliver blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Kenney said his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
Need blood STAT? Call for a drone
BOSTON – While Amazon and other retailers are experimenting with drones to deliver toasters and toilet seats to your doorstep, drone-delivered platelets and fresh frozen plasma may be coming soon to a hospital near you, experts said at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries – that’s almost 20,000 units of blood,” he said.
One-third of all deliveries are needed for urgent, life-saving interventions, he said.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, Mr. Kenney said.
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, however, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, said Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold, who is also a private pilot, described the regulatory hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters are currently. He added, however, “I can guarantee you that in the future this is going to be an applicable technology to our industry in one way, shape, or another.”
Fast and cheap
Speed and cost are two of the most compelling arguments for blood banks to use drones. Mr. Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section. The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products – ordering can be done by text message or via WhatsApp, a free, cross-platform messaging and voiceover IP calling service – and over the course of 90 minutes Zipline was able to deliver, using multiple drone launches, 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15-25 minutes by air, Mr. Kenney said.
The drones – more formally known as “unmanned aerial vehicles” (UAVs) – fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters and drop the package, which has a parachute attached. Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Mr. Kenney said.
“The whole process is 100% autonomous. The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package,” he explained.
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are also significantly cheaper than ground-based services for local delivery, Dr. Eastvold noted. He cited a study showing that the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more, drones could be as cheap as 5 cents per mile with delivery within about 30 minutes, he said.
The fly in the ointment
Dr. Eastvold outlined the significant barriers to adoption of drone-based delivery systems in the United States, ranging from differences in state laws about when, where, and how drones can be used and who can operate them, to Federal Aviation Administration airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation only for most drone operators, meaning that the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 years old or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery. In 2017, they demonstrated that a drone could successfully deliver human blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Mr. Kenney said that his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
BOSTON – While Amazon and other retailers are experimenting with drones to deliver toasters and toilet seats to your doorstep, drone-delivered platelets and fresh frozen plasma may be coming soon to a hospital near you, experts said at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries – that’s almost 20,000 units of blood,” he said.
One-third of all deliveries are needed for urgent, life-saving interventions, he said.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, Mr. Kenney said.
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, however, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, said Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold, who is also a private pilot, described the regulatory hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters are currently. He added, however, “I can guarantee you that in the future this is going to be an applicable technology to our industry in one way, shape, or another.”
Fast and cheap
Speed and cost are two of the most compelling arguments for blood banks to use drones. Mr. Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section. The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products – ordering can be done by text message or via WhatsApp, a free, cross-platform messaging and voiceover IP calling service – and over the course of 90 minutes Zipline was able to deliver, using multiple drone launches, 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15-25 minutes by air, Mr. Kenney said.
The drones – more formally known as “unmanned aerial vehicles” (UAVs) – fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters and drop the package, which has a parachute attached. Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Mr. Kenney said.
“The whole process is 100% autonomous. The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package,” he explained.
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are also significantly cheaper than ground-based services for local delivery, Dr. Eastvold noted. He cited a study showing that the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more, drones could be as cheap as 5 cents per mile with delivery within about 30 minutes, he said.
The fly in the ointment
Dr. Eastvold outlined the significant barriers to adoption of drone-based delivery systems in the United States, ranging from differences in state laws about when, where, and how drones can be used and who can operate them, to Federal Aviation Administration airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation only for most drone operators, meaning that the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 years old or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery. In 2017, they demonstrated that a drone could successfully deliver human blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Mr. Kenney said that his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
BOSTON – While Amazon and other retailers are experimenting with drones to deliver toasters and toilet seats to your doorstep, drone-delivered platelets and fresh frozen plasma may be coming soon to a hospital near you, experts said at AABB 2018, the annual meeting of the group formerly known as the American Association of Blood Banks.
Using a system of completely autonomous delivery drones launched from a central location, U.S.-based Zipline International delivers blood products to treat postpartum hemorrhage, trauma, malaria, and other life-threatening conditions to patients in rural Rwanda, according to company spokesman Chris Kenney.
“In less than 2 years in Rwanda, we’ve made almost 10,000 deliveries – that’s almost 20,000 units of blood,” he said.
One-third of all deliveries are needed for urgent, life-saving interventions, he said.
The system, which delivers 30% of all blood products used in Rwanda outside the capital Kigali, has resulted in 100% availability of blood products when needed, a 98% reduction in waste (i.e., when unused blood products are discarded because of age), and a 175% increase in the use of platelets and fresh frozen plasma, Mr. Kenney said.
Setting up an airborne delivery network in the largely unregulated and uncrowded Rwandan airspace was a relatively simple process, however, compared with the myriad challenges of establishing a similar system for deliveries to urban medical centers in Boston, Chicago, New York, or Los Angeles, said Paul Eastvold, MD, chief medical officer at Vitalant, a nonprofit network of community blood banks headquartered in Spokane, Wash.
Dr. Eastvold, who is also a private pilot, described the regulatory hurdles that will need to be surmounted before blood-delivery drones are as common a sight as traffic helicopters are currently. He added, however, “I can guarantee you that in the future this is going to be an applicable technology to our industry in one way, shape, or another.”
Fast and cheap
Speed and cost are two of the most compelling arguments for blood banks to use drones. Mr. Kenney described the case of a 24-year-old Rwandan woman who had uncontrolled bleeding from complications following a cesarean section. The clinicians treating her opted to give her an immediate red blood cell transfusion, but she continued to bleed, and the hospital ran out of red blood cells in about 15 minutes.
They placed an order for more blood products – ordering can be done by text message or via WhatsApp, a free, cross-platform messaging and voiceover IP calling service – and over the course of 90 minutes Zipline was able to deliver, using multiple drone launches, 7 units of red blood cells, 4 units of plasma, and 2 units of platelets, all of which were transfused into the patient and allowed her condition to stabilize.
Deliveries that would take a minimum of 3 hours by road can be accomplished in about 15-25 minutes by air, Mr. Kenney said.
The drones – more formally known as “unmanned aerial vehicles” (UAVs) – fly a loop starting at the distribution center, find their target, descend to a height of about 10 meters and drop the package, which has a parachute attached. Packages can be delivered within a drop zone the size of two parking spaces, even in gale-force winds, Mr. Kenney said.
“The whole process is 100% autonomous. The aircraft knows where it’s going, it knows what conditions [are], it knows what its payload characteristics are and flies to the delivery point and drops its package,” he explained.
As drones return to the distribution center, they are snared from the air with a wire that catches a small tail hook on the fuselage.
Airborne deliveries are also significantly cheaper than ground-based services for local delivery, Dr. Eastvold noted. He cited a study showing that the cost of ground shipping from a local warehouse by carriers such as UPS or FedEx could be $6 or more, drones could be as cheap as 5 cents per mile with delivery within about 30 minutes, he said.
The fly in the ointment
Dr. Eastvold outlined the significant barriers to adoption of drone-based delivery systems in the United States, ranging from differences in state laws about when, where, and how drones can be used and who can operate them, to Federal Aviation Administration airspace restrictions and regulations.
For example, the FAA currently requires “line-of-sight” operation only for most drone operators, meaning that the operator must have visual contact with the drone at all times. The FAA will, however, grant waivers to individual operators for specified flying conditions on a case-by-case basis, if compelling need or extenuating circumstances can be satisfactorily explained.
In addition, federal regulations require commercial drone pilots to be 16 years old or older, be fluent in English, be in a physical and mental condition that would not interfere with safe operation of a drone, pass an aeronautical knowledge exam at an FAA-approved testing center, and undergo a Transportation Safety Administration background security screening.
Despite these challenges, at least one U.S. medical center, Johns Hopkins University, is testing the use of drones for blood delivery. In 2017, they demonstrated that a drone could successfully deliver human blood samples in temperature-controlled conditions across 161 miles of Arizona desert, in a flight lasting 3 hours.
Mr. Kenney said that his company is developing a second distribution center in Rwanda that will expand coverage to the entire country and is also working with the FAA, federal regulators, and the state of North Carolina to develop a drone-based blood delivery system in the United States.
AT AABB 2018
FDA approves test to determine blood compatibility
The U.S. Food and Drug Administration (FDA) has approved ID CORE XT, a molecular-based assay used to determine blood donor and recipient compatibility.
ID CORE XT is a qualitative, polymerase chain reaction-based and hybridization-based genotyping test.
It is used for the simultaneous identification of multiple alleles encoding human erythrocyte antigens in genomic DNA extracted from whole blood specimens collected in ethylenediaminetetraacetic acid.
The test genotypes 29 polymorphisms determining 37 human erythrocyte antigen phenotypes of 10 blood group systems—Rh, Kell, Kidd, Duffy, MNS, Diego, Dombrock, Colton, Cartwright, and Lutheran.
ID CORE XT is the second molecular assay approved by the FDA for use in transfusion medicine and the first to report genotypes as final results.
The approval of ID CORE XT was granted to Progenika Biopharma S.A., a Grifols company.
According to Progenika, ID CORE XT will benefit patients who require ongoing transfusions, such as individuals with hemoglobinopathies.
ID CORE XT can also be used for cancer patients who require more thorough blood typing, patients with warm autoimmune hemolytic anemia, and patients taking daratumumab.
“The approval of the ID CORE XT test can streamline blood compatibility testing and provides an additional alternative to testing blood with antisera,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research.
In a study published in Blood Transfusion this year, typing results with ID CORE XT were similar to results obtained with serology and molecular methods.
Researchers said there was 100% agreement between the positive results predicted by ID CORE XT and results obtained via serology (100% sensitivity).
For negative results, there was one discrepancy for E antigen (99.9% agreement) and 33 discrepancies for Fyb antigen (95.5% agreement). However, additional testing suggested that serology produced 34 false-negatives.
Both positive and negative results with ID CORE XT were in full agreement with results obtained via molecular methods (100% sensitivity and specificity).
The U.S. Food and Drug Administration (FDA) has approved ID CORE XT, a molecular-based assay used to determine blood donor and recipient compatibility.
ID CORE XT is a qualitative, polymerase chain reaction-based and hybridization-based genotyping test.
It is used for the simultaneous identification of multiple alleles encoding human erythrocyte antigens in genomic DNA extracted from whole blood specimens collected in ethylenediaminetetraacetic acid.
The test genotypes 29 polymorphisms determining 37 human erythrocyte antigen phenotypes of 10 blood group systems—Rh, Kell, Kidd, Duffy, MNS, Diego, Dombrock, Colton, Cartwright, and Lutheran.
ID CORE XT is the second molecular assay approved by the FDA for use in transfusion medicine and the first to report genotypes as final results.
The approval of ID CORE XT was granted to Progenika Biopharma S.A., a Grifols company.
According to Progenika, ID CORE XT will benefit patients who require ongoing transfusions, such as individuals with hemoglobinopathies.
ID CORE XT can also be used for cancer patients who require more thorough blood typing, patients with warm autoimmune hemolytic anemia, and patients taking daratumumab.
“The approval of the ID CORE XT test can streamline blood compatibility testing and provides an additional alternative to testing blood with antisera,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research.
In a study published in Blood Transfusion this year, typing results with ID CORE XT were similar to results obtained with serology and molecular methods.
Researchers said there was 100% agreement between the positive results predicted by ID CORE XT and results obtained via serology (100% sensitivity).
For negative results, there was one discrepancy for E antigen (99.9% agreement) and 33 discrepancies for Fyb antigen (95.5% agreement). However, additional testing suggested that serology produced 34 false-negatives.
Both positive and negative results with ID CORE XT were in full agreement with results obtained via molecular methods (100% sensitivity and specificity).
The U.S. Food and Drug Administration (FDA) has approved ID CORE XT, a molecular-based assay used to determine blood donor and recipient compatibility.
ID CORE XT is a qualitative, polymerase chain reaction-based and hybridization-based genotyping test.
It is used for the simultaneous identification of multiple alleles encoding human erythrocyte antigens in genomic DNA extracted from whole blood specimens collected in ethylenediaminetetraacetic acid.
The test genotypes 29 polymorphisms determining 37 human erythrocyte antigen phenotypes of 10 blood group systems—Rh, Kell, Kidd, Duffy, MNS, Diego, Dombrock, Colton, Cartwright, and Lutheran.
ID CORE XT is the second molecular assay approved by the FDA for use in transfusion medicine and the first to report genotypes as final results.
The approval of ID CORE XT was granted to Progenika Biopharma S.A., a Grifols company.
According to Progenika, ID CORE XT will benefit patients who require ongoing transfusions, such as individuals with hemoglobinopathies.
ID CORE XT can also be used for cancer patients who require more thorough blood typing, patients with warm autoimmune hemolytic anemia, and patients taking daratumumab.
“The approval of the ID CORE XT test can streamline blood compatibility testing and provides an additional alternative to testing blood with antisera,” said Peter Marks, MD, PhD, director of the FDA’s Center for Biologics Evaluation and Research.
In a study published in Blood Transfusion this year, typing results with ID CORE XT were similar to results obtained with serology and molecular methods.
Researchers said there was 100% agreement between the positive results predicted by ID CORE XT and results obtained via serology (100% sensitivity).
For negative results, there was one discrepancy for E antigen (99.9% agreement) and 33 discrepancies for Fyb antigen (95.5% agreement). However, additional testing suggested that serology produced 34 false-negatives.
Both positive and negative results with ID CORE XT were in full agreement with results obtained via molecular methods (100% sensitivity and specificity).