User login
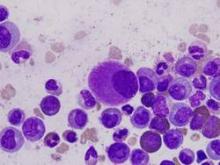
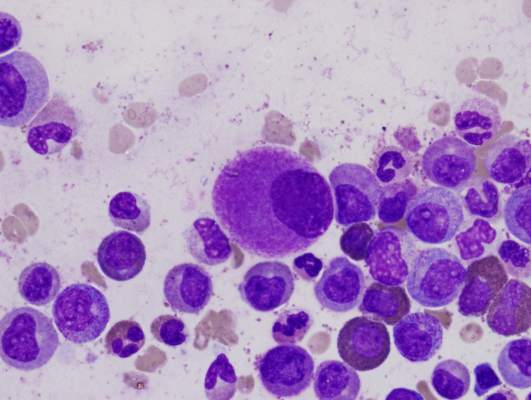
Combo may overcome TKI resistance in CML

Schürch, MD, PhD, (left)
and Adrian Ochsenbein, MD
Photo by Susi Bürki
Combining a tyrosine kinase inhibitor (TKI) with a monoclonal antibody (mAb) may circumvent TKI resistance in chronic myeloid leukemia (CML), according to preclinical research published in Science Translational Medicine.
To understand how TKI resistance develops, researchers analyzed the effect of these drugs on BCR-ABL1+ leukemia cell lines, cells from patients with newly diagnosed CML, and mouse models of CML.
The team found that TKIs induce CD70 expression in leukemic stem cells (LSCs) by downregulating microRNA-29. This results in reduced CD70 promoter DNA methylation and upregulation of the transcription factor specificity protein 1 (SP1).
The increase in CD70 triggers CD27 signaling and compensatory Wnt pathway activation. The researchers said this suggests LSCs evade TKIs by activating Wnt signaling through this route.
So the team hypothesized that combination treatment with a TKI and a mAb blocking the CD70/CD27 interaction would eradicate LSCs.
First, they tested an αCD27 mAb alone or in combination with a TKI in leukemia cell lines. Compared to either agent alone, αCD27/imatinib cotreatment significantly (P<0.001) reduced cell growth by inhibiting proliferation and enhancing apoptosis in SD-1 cells.
The researchers observed similar results when they tested the αCD27 mAb and nilotinib in SD-1 cells, as well as when they tested the αCD27 mAb with imatinib or ponatinib in KBM5 and KBM5r cells.
The team noted that αCD27/imatinib cotreatment inhibited Wnt pathway activation significantly stronger than either compound alone (P<0.001) but had little to no effect on Notch, Hedgehog, and MAP kinase pathways.
The researchers also conducted in vitro tests with an αCD70 mAb (clone 41D12-D) that was specifically designed to block the CD70/CD27 interaction without inducing effector functions such as antibody-dependent cell- or complement-mediated cytotoxicity and antibody-dependent cell-mediated phagocytosis.
They found that αCD70/imatinib cotreatment “potently reduced” CD34+ CML stem/progenitor cells in liquid cultures by inhibiting proliferation and increasing apoptosis. The combination also significantly impaired colony formation in semisolid cultures when compared to either agent alone (P<0.05).
In addition, αCD70/imatinib cotreatment eliminated human CD34+ CML stem/progenitor cells in murine xenografts. The LSCs were completely eradicated in 9 of 12 mice treated.
In a murine CML model, combination treatment with imatinib and an αCD70 mAb (clone FR70) significantly improved survival (P<0.001) compared to either agent alone. And 60% of mice (9 of 15) that received the combination were alive 90 days after transplantation.
The researchers said this suggests the LSCs were completely eradicated or at least effectively controlled long-term. ![]()

Schürch, MD, PhD, (left)
and Adrian Ochsenbein, MD
Photo by Susi Bürki
Combining a tyrosine kinase inhibitor (TKI) with a monoclonal antibody (mAb) may circumvent TKI resistance in chronic myeloid leukemia (CML), according to preclinical research published in Science Translational Medicine.
To understand how TKI resistance develops, researchers analyzed the effect of these drugs on BCR-ABL1+ leukemia cell lines, cells from patients with newly diagnosed CML, and mouse models of CML.
The team found that TKIs induce CD70 expression in leukemic stem cells (LSCs) by downregulating microRNA-29. This results in reduced CD70 promoter DNA methylation and upregulation of the transcription factor specificity protein 1 (SP1).
The increase in CD70 triggers CD27 signaling and compensatory Wnt pathway activation. The researchers said this suggests LSCs evade TKIs by activating Wnt signaling through this route.
So the team hypothesized that combination treatment with a TKI and a mAb blocking the CD70/CD27 interaction would eradicate LSCs.
First, they tested an αCD27 mAb alone or in combination with a TKI in leukemia cell lines. Compared to either agent alone, αCD27/imatinib cotreatment significantly (P<0.001) reduced cell growth by inhibiting proliferation and enhancing apoptosis in SD-1 cells.
The researchers observed similar results when they tested the αCD27 mAb and nilotinib in SD-1 cells, as well as when they tested the αCD27 mAb with imatinib or ponatinib in KBM5 and KBM5r cells.
The team noted that αCD27/imatinib cotreatment inhibited Wnt pathway activation significantly stronger than either compound alone (P<0.001) but had little to no effect on Notch, Hedgehog, and MAP kinase pathways.
The researchers also conducted in vitro tests with an αCD70 mAb (clone 41D12-D) that was specifically designed to block the CD70/CD27 interaction without inducing effector functions such as antibody-dependent cell- or complement-mediated cytotoxicity and antibody-dependent cell-mediated phagocytosis.
They found that αCD70/imatinib cotreatment “potently reduced” CD34+ CML stem/progenitor cells in liquid cultures by inhibiting proliferation and increasing apoptosis. The combination also significantly impaired colony formation in semisolid cultures when compared to either agent alone (P<0.05).
In addition, αCD70/imatinib cotreatment eliminated human CD34+ CML stem/progenitor cells in murine xenografts. The LSCs were completely eradicated in 9 of 12 mice treated.
In a murine CML model, combination treatment with imatinib and an αCD70 mAb (clone FR70) significantly improved survival (P<0.001) compared to either agent alone. And 60% of mice (9 of 15) that received the combination were alive 90 days after transplantation.
The researchers said this suggests the LSCs were completely eradicated or at least effectively controlled long-term. ![]()

Schürch, MD, PhD, (left)
and Adrian Ochsenbein, MD
Photo by Susi Bürki
Combining a tyrosine kinase inhibitor (TKI) with a monoclonal antibody (mAb) may circumvent TKI resistance in chronic myeloid leukemia (CML), according to preclinical research published in Science Translational Medicine.
To understand how TKI resistance develops, researchers analyzed the effect of these drugs on BCR-ABL1+ leukemia cell lines, cells from patients with newly diagnosed CML, and mouse models of CML.
The team found that TKIs induce CD70 expression in leukemic stem cells (LSCs) by downregulating microRNA-29. This results in reduced CD70 promoter DNA methylation and upregulation of the transcription factor specificity protein 1 (SP1).
The increase in CD70 triggers CD27 signaling and compensatory Wnt pathway activation. The researchers said this suggests LSCs evade TKIs by activating Wnt signaling through this route.
So the team hypothesized that combination treatment with a TKI and a mAb blocking the CD70/CD27 interaction would eradicate LSCs.
First, they tested an αCD27 mAb alone or in combination with a TKI in leukemia cell lines. Compared to either agent alone, αCD27/imatinib cotreatment significantly (P<0.001) reduced cell growth by inhibiting proliferation and enhancing apoptosis in SD-1 cells.
The researchers observed similar results when they tested the αCD27 mAb and nilotinib in SD-1 cells, as well as when they tested the αCD27 mAb with imatinib or ponatinib in KBM5 and KBM5r cells.
The team noted that αCD27/imatinib cotreatment inhibited Wnt pathway activation significantly stronger than either compound alone (P<0.001) but had little to no effect on Notch, Hedgehog, and MAP kinase pathways.
The researchers also conducted in vitro tests with an αCD70 mAb (clone 41D12-D) that was specifically designed to block the CD70/CD27 interaction without inducing effector functions such as antibody-dependent cell- or complement-mediated cytotoxicity and antibody-dependent cell-mediated phagocytosis.
They found that αCD70/imatinib cotreatment “potently reduced” CD34+ CML stem/progenitor cells in liquid cultures by inhibiting proliferation and increasing apoptosis. The combination also significantly impaired colony formation in semisolid cultures when compared to either agent alone (P<0.05).
In addition, αCD70/imatinib cotreatment eliminated human CD34+ CML stem/progenitor cells in murine xenografts. The LSCs were completely eradicated in 9 of 12 mice treated.
In a murine CML model, combination treatment with imatinib and an αCD70 mAb (clone FR70) significantly improved survival (P<0.001) compared to either agent alone. And 60% of mice (9 of 15) that received the combination were alive 90 days after transplantation.
The researchers said this suggests the LSCs were completely eradicated or at least effectively controlled long-term. ![]()
Inhibitor could treat range of hematologic disorders

A small molecule that targets the sonic Hedgehog signaling pathway has advanced to phase 2 trials in a range of hematologic disorders.
In a phase 1 study, the inhibitor, PF-04449913, exhibited activity in adults with leukemias, myelodysplastic syndromes (MDS), and myelofibrosis (MF).
Sixty percent of the patients studied experienced treatment-related adverse events (AEs), but there were no treatment-related deaths. Most deaths were disease-related.
Researchers detailed the results of this trial in The Lancet Haematology. The study was funded by Pfizer, the company developing PF-04449913, as well as the California Institute for Regenerative Medicine and European Leukemia Net.
Preclinical research showed that PF-04449913 forces dormant cancer stem cells in the bone marrow to begin differentiating and exit into the blood stream where they can be destroyed by chemotherapy agents targeting dividing cells.
“This drug gets that unwanted house guests to leave and never come back,” said Catriona Jamieson, MD, PhD, of University of California, San Diego School of Medicine.
“It’s a significant step forward in treating people with refractory or resistant myeloid leukemia, myelodysplastic syndrome, and myelofibrosis. It’s a bonus that the drug can be administered as easily as an aspirin, in a single, daily, oral tablet.”
For the first-in-human study, Dr Jamieson and her colleagues evaluated PF-04449913 in 47 adult patients. Twenty-eight of them had acute myeloid leukemia (AML), 6 had MDS, 5 had chronic myeloid leukemia (CML), 1 had chronic myelomonocytic leukemia (CMML), and 7 had MF.
Eighty-five percent of patients (n=40) had an ECOG performance status of 0-1. Eighty-one percent (n=38) had received previous systemic treatment, and 47% (n=22) had received 3 or more previous treatment regimens.
Patients received escalating daily doses of PF-04449913 in 28-day cycles. Treatment cycles were repeated until a patient experienced unacceptable AEs without evidence of clinical improvement. Patients who showed clinical activity without experiencing serious AEs received additional treatment cycles.
Dosing and AEs
Patients received PF-04449913 once daily at 5 mg (n=3), 10 mg (n=3), 20 mg (n=4), 40 mg (n=4), 80 mg (n=8), 120 mg (n=3), 180 mg (n=3), 270 mg (n=5), 400 mg (n=9), or 600 mg (n=5).
The researchers found the maximum-tolerated dose to be 400 mg once daily. The mean half-life was 23.9 hours in this dose group, and pharmacokinetics seemed to be dose-proportional.
Two patients experienced dose-limiting toxicities, 1 in the 80 mg group (grade 3 hypoxia and grade 3 pleural effusion), and 1 in the 600 mg group (grade 3 peripheral edema).
In all, 60% of patients (n=28) experienced treatment-related AEs. The most common were dysgeusia (28%), decreased appetite (19%), and alopecia (15%). There were 3 grade 4 AEs—1 case of neutropenia and 2 cases of thrombocytopenia.
There were 15 deaths, none of which were treatment-related. Eleven deaths were disease-related, and the remaining 4 were related to infection.
Clinical activity
The researchers said there was “some suggestion of clinical activity” in 23 patients (49%).
Of the 5 patients with CML (2 chronic phase and 3 blast phase), 1 patient with blast phase CML had a partial cytogenetic response to PF-04449913.
Of the 6 patients with MDS and 1 with CMML, 4 had stable disease after treatment. Two of these patients had hematologic improvement.
Two of the 7 patients with MF had clinical improvement.
Of the 28 patients with AML, 16 showed evidence of possible biological activity. One patient had a complete response and 4 had a partial response with incomplete hematologic recovery. Four AML patients had minor responses, and 7 had stable disease.
Given these results, PF-04449913 is now being investigated in 5 phase 2 trials of hematologic disorders, 4 of which are recruiting participants.
“Our hope is that this drug will enable more effective treatment to begin earlier and that, with earlier intervention, we can alter the course of disease and remove the need for, or improve the chances of success with, bone marrow transplantation,” Dr Jamieson said. “It’s all about reducing the burden of disease by intervening early.” ![]()

A small molecule that targets the sonic Hedgehog signaling pathway has advanced to phase 2 trials in a range of hematologic disorders.
In a phase 1 study, the inhibitor, PF-04449913, exhibited activity in adults with leukemias, myelodysplastic syndromes (MDS), and myelofibrosis (MF).
Sixty percent of the patients studied experienced treatment-related adverse events (AEs), but there were no treatment-related deaths. Most deaths were disease-related.
Researchers detailed the results of this trial in The Lancet Haematology. The study was funded by Pfizer, the company developing PF-04449913, as well as the California Institute for Regenerative Medicine and European Leukemia Net.
Preclinical research showed that PF-04449913 forces dormant cancer stem cells in the bone marrow to begin differentiating and exit into the blood stream where they can be destroyed by chemotherapy agents targeting dividing cells.
“This drug gets that unwanted house guests to leave and never come back,” said Catriona Jamieson, MD, PhD, of University of California, San Diego School of Medicine.
“It’s a significant step forward in treating people with refractory or resistant myeloid leukemia, myelodysplastic syndrome, and myelofibrosis. It’s a bonus that the drug can be administered as easily as an aspirin, in a single, daily, oral tablet.”
For the first-in-human study, Dr Jamieson and her colleagues evaluated PF-04449913 in 47 adult patients. Twenty-eight of them had acute myeloid leukemia (AML), 6 had MDS, 5 had chronic myeloid leukemia (CML), 1 had chronic myelomonocytic leukemia (CMML), and 7 had MF.
Eighty-five percent of patients (n=40) had an ECOG performance status of 0-1. Eighty-one percent (n=38) had received previous systemic treatment, and 47% (n=22) had received 3 or more previous treatment regimens.
Patients received escalating daily doses of PF-04449913 in 28-day cycles. Treatment cycles were repeated until a patient experienced unacceptable AEs without evidence of clinical improvement. Patients who showed clinical activity without experiencing serious AEs received additional treatment cycles.
Dosing and AEs
Patients received PF-04449913 once daily at 5 mg (n=3), 10 mg (n=3), 20 mg (n=4), 40 mg (n=4), 80 mg (n=8), 120 mg (n=3), 180 mg (n=3), 270 mg (n=5), 400 mg (n=9), or 600 mg (n=5).
The researchers found the maximum-tolerated dose to be 400 mg once daily. The mean half-life was 23.9 hours in this dose group, and pharmacokinetics seemed to be dose-proportional.
Two patients experienced dose-limiting toxicities, 1 in the 80 mg group (grade 3 hypoxia and grade 3 pleural effusion), and 1 in the 600 mg group (grade 3 peripheral edema).
In all, 60% of patients (n=28) experienced treatment-related AEs. The most common were dysgeusia (28%), decreased appetite (19%), and alopecia (15%). There were 3 grade 4 AEs—1 case of neutropenia and 2 cases of thrombocytopenia.
There were 15 deaths, none of which were treatment-related. Eleven deaths were disease-related, and the remaining 4 were related to infection.
Clinical activity
The researchers said there was “some suggestion of clinical activity” in 23 patients (49%).
Of the 5 patients with CML (2 chronic phase and 3 blast phase), 1 patient with blast phase CML had a partial cytogenetic response to PF-04449913.
Of the 6 patients with MDS and 1 with CMML, 4 had stable disease after treatment. Two of these patients had hematologic improvement.
Two of the 7 patients with MF had clinical improvement.
Of the 28 patients with AML, 16 showed evidence of possible biological activity. One patient had a complete response and 4 had a partial response with incomplete hematologic recovery. Four AML patients had minor responses, and 7 had stable disease.
Given these results, PF-04449913 is now being investigated in 5 phase 2 trials of hematologic disorders, 4 of which are recruiting participants.
“Our hope is that this drug will enable more effective treatment to begin earlier and that, with earlier intervention, we can alter the course of disease and remove the need for, or improve the chances of success with, bone marrow transplantation,” Dr Jamieson said. “It’s all about reducing the burden of disease by intervening early.” ![]()

A small molecule that targets the sonic Hedgehog signaling pathway has advanced to phase 2 trials in a range of hematologic disorders.
In a phase 1 study, the inhibitor, PF-04449913, exhibited activity in adults with leukemias, myelodysplastic syndromes (MDS), and myelofibrosis (MF).
Sixty percent of the patients studied experienced treatment-related adverse events (AEs), but there were no treatment-related deaths. Most deaths were disease-related.
Researchers detailed the results of this trial in The Lancet Haematology. The study was funded by Pfizer, the company developing PF-04449913, as well as the California Institute for Regenerative Medicine and European Leukemia Net.
Preclinical research showed that PF-04449913 forces dormant cancer stem cells in the bone marrow to begin differentiating and exit into the blood stream where they can be destroyed by chemotherapy agents targeting dividing cells.
“This drug gets that unwanted house guests to leave and never come back,” said Catriona Jamieson, MD, PhD, of University of California, San Diego School of Medicine.
“It’s a significant step forward in treating people with refractory or resistant myeloid leukemia, myelodysplastic syndrome, and myelofibrosis. It’s a bonus that the drug can be administered as easily as an aspirin, in a single, daily, oral tablet.”
For the first-in-human study, Dr Jamieson and her colleagues evaluated PF-04449913 in 47 adult patients. Twenty-eight of them had acute myeloid leukemia (AML), 6 had MDS, 5 had chronic myeloid leukemia (CML), 1 had chronic myelomonocytic leukemia (CMML), and 7 had MF.
Eighty-five percent of patients (n=40) had an ECOG performance status of 0-1. Eighty-one percent (n=38) had received previous systemic treatment, and 47% (n=22) had received 3 or more previous treatment regimens.
Patients received escalating daily doses of PF-04449913 in 28-day cycles. Treatment cycles were repeated until a patient experienced unacceptable AEs without evidence of clinical improvement. Patients who showed clinical activity without experiencing serious AEs received additional treatment cycles.
Dosing and AEs
Patients received PF-04449913 once daily at 5 mg (n=3), 10 mg (n=3), 20 mg (n=4), 40 mg (n=4), 80 mg (n=8), 120 mg (n=3), 180 mg (n=3), 270 mg (n=5), 400 mg (n=9), or 600 mg (n=5).
The researchers found the maximum-tolerated dose to be 400 mg once daily. The mean half-life was 23.9 hours in this dose group, and pharmacokinetics seemed to be dose-proportional.
Two patients experienced dose-limiting toxicities, 1 in the 80 mg group (grade 3 hypoxia and grade 3 pleural effusion), and 1 in the 600 mg group (grade 3 peripheral edema).
In all, 60% of patients (n=28) experienced treatment-related AEs. The most common were dysgeusia (28%), decreased appetite (19%), and alopecia (15%). There were 3 grade 4 AEs—1 case of neutropenia and 2 cases of thrombocytopenia.
There were 15 deaths, none of which were treatment-related. Eleven deaths were disease-related, and the remaining 4 were related to infection.
Clinical activity
The researchers said there was “some suggestion of clinical activity” in 23 patients (49%).
Of the 5 patients with CML (2 chronic phase and 3 blast phase), 1 patient with blast phase CML had a partial cytogenetic response to PF-04449913.
Of the 6 patients with MDS and 1 with CMML, 4 had stable disease after treatment. Two of these patients had hematologic improvement.
Two of the 7 patients with MF had clinical improvement.
Of the 28 patients with AML, 16 showed evidence of possible biological activity. One patient had a complete response and 4 had a partial response with incomplete hematologic recovery. Four AML patients had minor responses, and 7 had stable disease.
Given these results, PF-04449913 is now being investigated in 5 phase 2 trials of hematologic disorders, 4 of which are recruiting participants.
“Our hope is that this drug will enable more effective treatment to begin earlier and that, with earlier intervention, we can alter the course of disease and remove the need for, or improve the chances of success with, bone marrow transplantation,” Dr Jamieson said. “It’s all about reducing the burden of disease by intervening early.” ![]()
CML patients die from comorbidities, not leukemia
Patients with chronic myeloid leukemia (CML) treated with imatinib are much more likely to die from their comorbid conditions than from the leukemia, according to a report published in Blood.
During the past decade, CML has been transformed from a routinely fatal disease to a chronic condition controlled by regular drug therapy using imatinib and newer tyrosine kinase inhibitors. The influence of comorbidities on survival outcomes has not been studied until now, said Dr. Susanne Saussele of Heidelberg University, Mannheim (Germany), and her associates.
They used data from a large German study of first-line imatinib therapy, focusing on 1,519 CML patients who were evaluable after a median follow-up of 68 months. Approximately 40% of these study participants had one or more of 511 evaluable comorbidities. The most common conditions relevant to CML and its treatment were diabetes, nonactive cancer other than CML, chronic pulmonary disease, renal insufficiency, MI, cerebrovascular disease, heart failure, and peripheral vascular disease.
Study participants were categorized by the number and severity of their comorbidities using the Charlson Comorbidity Index as CCI 2 (589 patients), CCI 3 or 4 (599 patients), CCI 5 or 6 (229 patients), or CCI 7 and above (102 patients), with higher levels indicating a greater burden of comorbidity. Overall 8-year survival probabilities directly correlated with CCI category, at 94% for CCI 2, 89% for CCI 3 or 4, 78% for CCI 5 or 6, and 46% for CCI 7 or above. In addition, CCI score was the most powerful predictor of overall survival, the researchers said (Blood 2015;126:42-9).
Comorbidities had no impact on the success of imatinib therapy. Even patients with multiple or severe comorbidities derived significant benefit from imatinib, and comorbidities had no negative effect on remission rates or disease progression. Taken together with comorbidities’ strong influence on mortality, this indicates that patients’ survival is determined more by their comorbidities than by CML itself, Dr. Saussele and her associates said.
Their findings also showed that overall survival alone is no longer an appropriate endpoint for assessing treatment efficacy in CML. Progression-free survival seems to be a more accurate measure of treatment effect, since it was not influenced by comorbidities in this study, they added.
Patients with chronic myeloid leukemia (CML) treated with imatinib are much more likely to die from their comorbid conditions than from the leukemia, according to a report published in Blood.
During the past decade, CML has been transformed from a routinely fatal disease to a chronic condition controlled by regular drug therapy using imatinib and newer tyrosine kinase inhibitors. The influence of comorbidities on survival outcomes has not been studied until now, said Dr. Susanne Saussele of Heidelberg University, Mannheim (Germany), and her associates.
They used data from a large German study of first-line imatinib therapy, focusing on 1,519 CML patients who were evaluable after a median follow-up of 68 months. Approximately 40% of these study participants had one or more of 511 evaluable comorbidities. The most common conditions relevant to CML and its treatment were diabetes, nonactive cancer other than CML, chronic pulmonary disease, renal insufficiency, MI, cerebrovascular disease, heart failure, and peripheral vascular disease.
Study participants were categorized by the number and severity of their comorbidities using the Charlson Comorbidity Index as CCI 2 (589 patients), CCI 3 or 4 (599 patients), CCI 5 or 6 (229 patients), or CCI 7 and above (102 patients), with higher levels indicating a greater burden of comorbidity. Overall 8-year survival probabilities directly correlated with CCI category, at 94% for CCI 2, 89% for CCI 3 or 4, 78% for CCI 5 or 6, and 46% for CCI 7 or above. In addition, CCI score was the most powerful predictor of overall survival, the researchers said (Blood 2015;126:42-9).
Comorbidities had no impact on the success of imatinib therapy. Even patients with multiple or severe comorbidities derived significant benefit from imatinib, and comorbidities had no negative effect on remission rates or disease progression. Taken together with comorbidities’ strong influence on mortality, this indicates that patients’ survival is determined more by their comorbidities than by CML itself, Dr. Saussele and her associates said.
Their findings also showed that overall survival alone is no longer an appropriate endpoint for assessing treatment efficacy in CML. Progression-free survival seems to be a more accurate measure of treatment effect, since it was not influenced by comorbidities in this study, they added.
Patients with chronic myeloid leukemia (CML) treated with imatinib are much more likely to die from their comorbid conditions than from the leukemia, according to a report published in Blood.
During the past decade, CML has been transformed from a routinely fatal disease to a chronic condition controlled by regular drug therapy using imatinib and newer tyrosine kinase inhibitors. The influence of comorbidities on survival outcomes has not been studied until now, said Dr. Susanne Saussele of Heidelberg University, Mannheim (Germany), and her associates.
They used data from a large German study of first-line imatinib therapy, focusing on 1,519 CML patients who were evaluable after a median follow-up of 68 months. Approximately 40% of these study participants had one or more of 511 evaluable comorbidities. The most common conditions relevant to CML and its treatment were diabetes, nonactive cancer other than CML, chronic pulmonary disease, renal insufficiency, MI, cerebrovascular disease, heart failure, and peripheral vascular disease.
Study participants were categorized by the number and severity of their comorbidities using the Charlson Comorbidity Index as CCI 2 (589 patients), CCI 3 or 4 (599 patients), CCI 5 or 6 (229 patients), or CCI 7 and above (102 patients), with higher levels indicating a greater burden of comorbidity. Overall 8-year survival probabilities directly correlated with CCI category, at 94% for CCI 2, 89% for CCI 3 or 4, 78% for CCI 5 or 6, and 46% for CCI 7 or above. In addition, CCI score was the most powerful predictor of overall survival, the researchers said (Blood 2015;126:42-9).
Comorbidities had no impact on the success of imatinib therapy. Even patients with multiple or severe comorbidities derived significant benefit from imatinib, and comorbidities had no negative effect on remission rates or disease progression. Taken together with comorbidities’ strong influence on mortality, this indicates that patients’ survival is determined more by their comorbidities than by CML itself, Dr. Saussele and her associates said.
Their findings also showed that overall survival alone is no longer an appropriate endpoint for assessing treatment efficacy in CML. Progression-free survival seems to be a more accurate measure of treatment effect, since it was not influenced by comorbidities in this study, they added.
FROM BLOOD
Key clinical point: Patients with chronic myeloid leukemia treated with imatinib are much more likely to die from comorbid conditions than from leukemia.
Major finding: Overall 8-year survival probabilities directly correlated with CCI category, at 94% for CCI 2, 89% for CCI 3 or 4, 78% for CCI 5 or 6, and 46% for CCI 7 or above.
Data source: A secondary analysis of data in a nationwide German study involving 1,519 patients treated with imatinib and followed for a median of 68 months.
Disclosures: This study was supported by the Deutsche Krebshilfe, Novartis, Kompetenznetz für Akute und Chronische Leukämien, Deutsche Jose-Carreras Leukämiestiftung, European LeukemiaNet, Roche, and Essex Pharma. Dr. Saussele reported honoraria and research funding from Pfizer, Novartis, and Bristol-Myers Squibb, and her associates reported ties to these companies and ARIAD.
Radiation increases risk of death from CML, other leukemias

power plant in Germany
Protracted exposure to ionizing radiation, even at low doses, can increase a person’s risk of dying from certain leukemias, according to research published in The Lancet Haematology.
The study showed that protracted radiation exposure was associated with an excess risk of leukemia mortality, particularly for chronic myeloid leukemia (CML).
However, there was no excess mortality risk for chronic lymphocytic leukemia (CLL).
Investigators also observed an association between ionizing radiation exposure and death from multiple myeloma or lymphoma, but they said the evidence for these associations was not strong.
“To date, this study provides the most precise evaluation of the risk of developing leukemia linked to the protracted low doses of radiation received by nuclear workers throughout their careers,” said study author Ausrele Kesminiene, MD, of the International Agency for Research on Cancer, the specialized cancer agency of the World Health Organization.
“It shows that the nuclear workers we studied have a small increase in the risk of dying from leukemia as their exposure to radiation increases.”
This study, known as INWORKS, included 308,297 workers who were monitored for exposure to radiation.
Subjects were employed for at least 1 year by the Atomic Energy Commission, AREVA Nuclear Cycle, or the National Electricity Company in France or the Departments of Energy and Defense in the US. The study also included nuclear industry employers in the National Registry for Radiation Workers in the UK.
Investigators assessed the risk of death from hematologic malignancies among these subjects. The team used Poisson regression to quantify associations between the estimated radiation dose in the red bone marrow and mortality from malignancy.
The mean follow-up was 27 years, and nearly 22% of workers died during that time. The mean cumulative radiation dose was 16 mGy, the median was 2.1 mGy, and the mean yearly dose was 1.1 mGy.
Quantifying the risk
The investigators found “strong evidence” for a positive association between exposure to ionizing radiation and the risk of death from leukemias, excluding CLL. Specifically, the excess relative risk of mortality per Gy of radiation was 2.96 (90% CI 1.17-5.21).
Even low doses of radiation posed a risk. Fifty-three percent of deaths from leukemia (excluding CLL) occurred in workers who had accrued less than 5 mGy of radiation.
However, the relative risk of death from leukemia (excluding CLL) increased with the radiation dose. The relative risk was 1.00 for 0-5 mGy, 1.01 for 5-50 mGy, 1.30 for 50-100 mGy, 1.19 for 100-200 mGy, 2.30 for 200-300 mGy, and 1.70 for more than 300 mGy.
The data also showed the risk of cancer mortality associated with radiation exposure varied according to the type of leukemia.
The excess relative risk of mortality was 10.45 for CML, 1.29 for acute myeloid leukemia, and 5.80 for acute lymphoblastic leukemia. For CLL, the excess relative risk was -1.06.
The investigators also found positive associations between radiation exposure and mortality from Hodgkin lymphoma, non-Hodgkin lymphoma, and multiple myeloma. However, the findings were “highly imprecise,” with confidence intervals that spanned 0. ![]()

power plant in Germany
Protracted exposure to ionizing radiation, even at low doses, can increase a person’s risk of dying from certain leukemias, according to research published in The Lancet Haematology.
The study showed that protracted radiation exposure was associated with an excess risk of leukemia mortality, particularly for chronic myeloid leukemia (CML).
However, there was no excess mortality risk for chronic lymphocytic leukemia (CLL).
Investigators also observed an association between ionizing radiation exposure and death from multiple myeloma or lymphoma, but they said the evidence for these associations was not strong.
“To date, this study provides the most precise evaluation of the risk of developing leukemia linked to the protracted low doses of radiation received by nuclear workers throughout their careers,” said study author Ausrele Kesminiene, MD, of the International Agency for Research on Cancer, the specialized cancer agency of the World Health Organization.
“It shows that the nuclear workers we studied have a small increase in the risk of dying from leukemia as their exposure to radiation increases.”
This study, known as INWORKS, included 308,297 workers who were monitored for exposure to radiation.
Subjects were employed for at least 1 year by the Atomic Energy Commission, AREVA Nuclear Cycle, or the National Electricity Company in France or the Departments of Energy and Defense in the US. The study also included nuclear industry employers in the National Registry for Radiation Workers in the UK.
Investigators assessed the risk of death from hematologic malignancies among these subjects. The team used Poisson regression to quantify associations between the estimated radiation dose in the red bone marrow and mortality from malignancy.
The mean follow-up was 27 years, and nearly 22% of workers died during that time. The mean cumulative radiation dose was 16 mGy, the median was 2.1 mGy, and the mean yearly dose was 1.1 mGy.
Quantifying the risk
The investigators found “strong evidence” for a positive association between exposure to ionizing radiation and the risk of death from leukemias, excluding CLL. Specifically, the excess relative risk of mortality per Gy of radiation was 2.96 (90% CI 1.17-5.21).
Even low doses of radiation posed a risk. Fifty-three percent of deaths from leukemia (excluding CLL) occurred in workers who had accrued less than 5 mGy of radiation.
However, the relative risk of death from leukemia (excluding CLL) increased with the radiation dose. The relative risk was 1.00 for 0-5 mGy, 1.01 for 5-50 mGy, 1.30 for 50-100 mGy, 1.19 for 100-200 mGy, 2.30 for 200-300 mGy, and 1.70 for more than 300 mGy.
The data also showed the risk of cancer mortality associated with radiation exposure varied according to the type of leukemia.
The excess relative risk of mortality was 10.45 for CML, 1.29 for acute myeloid leukemia, and 5.80 for acute lymphoblastic leukemia. For CLL, the excess relative risk was -1.06.
The investigators also found positive associations between radiation exposure and mortality from Hodgkin lymphoma, non-Hodgkin lymphoma, and multiple myeloma. However, the findings were “highly imprecise,” with confidence intervals that spanned 0. ![]()

power plant in Germany
Protracted exposure to ionizing radiation, even at low doses, can increase a person’s risk of dying from certain leukemias, according to research published in The Lancet Haematology.
The study showed that protracted radiation exposure was associated with an excess risk of leukemia mortality, particularly for chronic myeloid leukemia (CML).
However, there was no excess mortality risk for chronic lymphocytic leukemia (CLL).
Investigators also observed an association between ionizing radiation exposure and death from multiple myeloma or lymphoma, but they said the evidence for these associations was not strong.
“To date, this study provides the most precise evaluation of the risk of developing leukemia linked to the protracted low doses of radiation received by nuclear workers throughout their careers,” said study author Ausrele Kesminiene, MD, of the International Agency for Research on Cancer, the specialized cancer agency of the World Health Organization.
“It shows that the nuclear workers we studied have a small increase in the risk of dying from leukemia as their exposure to radiation increases.”
This study, known as INWORKS, included 308,297 workers who were monitored for exposure to radiation.
Subjects were employed for at least 1 year by the Atomic Energy Commission, AREVA Nuclear Cycle, or the National Electricity Company in France or the Departments of Energy and Defense in the US. The study also included nuclear industry employers in the National Registry for Radiation Workers in the UK.
Investigators assessed the risk of death from hematologic malignancies among these subjects. The team used Poisson regression to quantify associations between the estimated radiation dose in the red bone marrow and mortality from malignancy.
The mean follow-up was 27 years, and nearly 22% of workers died during that time. The mean cumulative radiation dose was 16 mGy, the median was 2.1 mGy, and the mean yearly dose was 1.1 mGy.
Quantifying the risk
The investigators found “strong evidence” for a positive association between exposure to ionizing radiation and the risk of death from leukemias, excluding CLL. Specifically, the excess relative risk of mortality per Gy of radiation was 2.96 (90% CI 1.17-5.21).
Even low doses of radiation posed a risk. Fifty-three percent of deaths from leukemia (excluding CLL) occurred in workers who had accrued less than 5 mGy of radiation.
However, the relative risk of death from leukemia (excluding CLL) increased with the radiation dose. The relative risk was 1.00 for 0-5 mGy, 1.01 for 5-50 mGy, 1.30 for 50-100 mGy, 1.19 for 100-200 mGy, 2.30 for 200-300 mGy, and 1.70 for more than 300 mGy.
The data also showed the risk of cancer mortality associated with radiation exposure varied according to the type of leukemia.
The excess relative risk of mortality was 10.45 for CML, 1.29 for acute myeloid leukemia, and 5.80 for acute lymphoblastic leukemia. For CLL, the excess relative risk was -1.06.
The investigators also found positive associations between radiation exposure and mortality from Hodgkin lymphoma, non-Hodgkin lymphoma, and multiple myeloma. However, the findings were “highly imprecise,” with confidence intervals that spanned 0. ![]()
Nivolumab produces ‘dramatic’ responses in HL

Photo courtesy of UCLA
LUGANO—The PD-1 checkpoint inhibitor nivolumab produces rapid, durable, and, in some cases, “dramatic” responses in Hodgkin lymphoma (HL), according to a speaker at the 13th International Congress on Malignant Lymphoma.
The drug has also produced durable responses in follicular lymphoma (FL), cutaneous T-cell lymphoma (CTCL), and peripheral T-cell lymphoma (PTCL), although patient numbers for these malignancies are small.
John Timmerman, MD, of the University of California, Los Angeles, presented these results from a phase 1 study of patients with relapsed or refractory lymphoid malignancies and chronic HL (abstract 010).
Bristol-Myers Squibb and Ono Pharmaceutical Company are sponsors of the trial.
Original results of the study, with a data cutoff of June 2014, were reported at ASH 2014, with 40 weeks of median follow-up.
The update presented at 13-ICML, with a data lock in April 2015, includes an additional 10 months of data, for a median follow-up of 76 weeks.
Investigators enrolled 105 patients in this dose-escalation study to receive nivolumab at 1 mg/kg, then 3 mg/kg, every 2 weeks for 2 years.
Twenty-three patients had HL. Thirty-one had B-cell non-Hodgkin lymphoma (NHL), including 11 with FL and 10 with diffuse large B-cell lymphoma (DLBCL).
Twenty-three patients had T-cell NHL, including 5 with PTCL and 13 with CTCL/mycosis fungoides (MF). Twenty-seven patients had multiple myeloma (MM), and 1 had chronic myeloid leukemia.
Patients were heavily pretreated. Seventy-eight percent of HL patients and 26% of T-NHL patients had prior brentuximab vedotin. And 78% (HL), 14% (B-NHL), 9% (T-NHL), and 56% (MM) of patients had a prior autologous transplant.
The median number of prior therapies was 5 (range, 2-15) for HL patients and ranged from 1 to 16 for all patients.
The study’s primary endpoint was safety and tolerability, and the secondary endpoint was efficacy.
Safety and tolerability
Ninety-seven percent of patients had an adverse event, 69% of them related to study treatment and 21% of them treatment-related grade 3-4 events.
Fifteen patients (14%) discontinued treatment due to a related adverse event, including 3 with pneumonitis and 1 each with enteritis, stomatitis, pancreatitis, rash, conjunctivitis, sepsis, diplopia, myositis, neutropenia, myelodysplastic syndrome, increased creatinine phosphokinase, and peripheral neuropathy.
“Immune-related adverse events were generally seen early on and generally of low grade,” Dr Timmerman said. “However, it is notable that there were several grade 3 immune-related adverse events that can be seen as far as 6 months out after the start of therapy.”
These included skin, gastrointestinal, and pulmonary events. Most immune-related adverse events (83%) were resolved using protocol-prescribed procedures.
Efficacy
The overall response rate was 87% for HL, 36% for DLBCL, 40% for FL, 15% for CTCL/MF, 40% for PTCL, and 4% for MM.
Dr Timmerman pointed out that, since ASH, 2 additional conversions from partial response (PR) to complete response (CR) occurred in patients with HL. To date, 6 of 23 HL patients have achieved a CR and 14 a PR.
In B-cell NHL, there were additional conversions from PR to CR in DLBCL, while responses remained the same in FL and in the 4 responders with T-cell lymphomas.
“Intriguingly, there has been 1 late CR in the multiple myeloma cohort, which previously had shown no responses,” Dr Timmerman said.
Durability of response
This study suggests PD-1 blockade can produce durable responses in hematologic malignancies, as it does in melanoma and renal cell carcinoma.
In HL, the median response duration at a median follow-up of 86 weeks has not yet been reached, and half (n=10) of the responses are still ongoing.
In FL, CTCL, and PTCL, the median response duration has not been reached at a median follow-up of 81, 43, and 31 weeks, respectively. Of note, there are ongoing responses in at least half of patients in these tumor types.
In HL, none of the 6 patients in CR has progressed, although there have been some progressions in the PR group.
The rapidity of responses is also notable, Dr Timmerman said.
“[I]t’s very interesting that some patients have resolution of symptoms and improvement of symptoms within even 1 day of starting nivolumab therapy,” he said.
And responses to nivolumab in HL “can be very dramatic,” he added, as illustrated in the following case from the Mayo Clinic.
A patient with multiple sites of bulky FDG-avid tumors was scheduled to enter hospice. But first, he entered the nivolumab trial. Within 6 weeks of initiating treatment, he had achieved a near-CR. This response has been maintained for 2 years.
“The occurrence of very durable responses in the PR and CR groups has led us to question whether patients should go on to allogeneic stem cell transplantation after achieving responses with nivolumab or, rather, continue on nivolumab as long as their response remains,” Dr Timmerman said.
He added that an international, phase 2 trial in HL is underway and is accruing briskly.
Nivolumab was awarded breakthrough designation by the US Food and Drug Administration last year. Breakthrough designation is intended to expedite the development and review of drugs for serious or life-threatening conditions. ![]()

Photo courtesy of UCLA
LUGANO—The PD-1 checkpoint inhibitor nivolumab produces rapid, durable, and, in some cases, “dramatic” responses in Hodgkin lymphoma (HL), according to a speaker at the 13th International Congress on Malignant Lymphoma.
The drug has also produced durable responses in follicular lymphoma (FL), cutaneous T-cell lymphoma (CTCL), and peripheral T-cell lymphoma (PTCL), although patient numbers for these malignancies are small.
John Timmerman, MD, of the University of California, Los Angeles, presented these results from a phase 1 study of patients with relapsed or refractory lymphoid malignancies and chronic HL (abstract 010).
Bristol-Myers Squibb and Ono Pharmaceutical Company are sponsors of the trial.
Original results of the study, with a data cutoff of June 2014, were reported at ASH 2014, with 40 weeks of median follow-up.
The update presented at 13-ICML, with a data lock in April 2015, includes an additional 10 months of data, for a median follow-up of 76 weeks.
Investigators enrolled 105 patients in this dose-escalation study to receive nivolumab at 1 mg/kg, then 3 mg/kg, every 2 weeks for 2 years.
Twenty-three patients had HL. Thirty-one had B-cell non-Hodgkin lymphoma (NHL), including 11 with FL and 10 with diffuse large B-cell lymphoma (DLBCL).
Twenty-three patients had T-cell NHL, including 5 with PTCL and 13 with CTCL/mycosis fungoides (MF). Twenty-seven patients had multiple myeloma (MM), and 1 had chronic myeloid leukemia.
Patients were heavily pretreated. Seventy-eight percent of HL patients and 26% of T-NHL patients had prior brentuximab vedotin. And 78% (HL), 14% (B-NHL), 9% (T-NHL), and 56% (MM) of patients had a prior autologous transplant.
The median number of prior therapies was 5 (range, 2-15) for HL patients and ranged from 1 to 16 for all patients.
The study’s primary endpoint was safety and tolerability, and the secondary endpoint was efficacy.
Safety and tolerability
Ninety-seven percent of patients had an adverse event, 69% of them related to study treatment and 21% of them treatment-related grade 3-4 events.
Fifteen patients (14%) discontinued treatment due to a related adverse event, including 3 with pneumonitis and 1 each with enteritis, stomatitis, pancreatitis, rash, conjunctivitis, sepsis, diplopia, myositis, neutropenia, myelodysplastic syndrome, increased creatinine phosphokinase, and peripheral neuropathy.
“Immune-related adverse events were generally seen early on and generally of low grade,” Dr Timmerman said. “However, it is notable that there were several grade 3 immune-related adverse events that can be seen as far as 6 months out after the start of therapy.”
These included skin, gastrointestinal, and pulmonary events. Most immune-related adverse events (83%) were resolved using protocol-prescribed procedures.
Efficacy
The overall response rate was 87% for HL, 36% for DLBCL, 40% for FL, 15% for CTCL/MF, 40% for PTCL, and 4% for MM.
Dr Timmerman pointed out that, since ASH, 2 additional conversions from partial response (PR) to complete response (CR) occurred in patients with HL. To date, 6 of 23 HL patients have achieved a CR and 14 a PR.
In B-cell NHL, there were additional conversions from PR to CR in DLBCL, while responses remained the same in FL and in the 4 responders with T-cell lymphomas.
“Intriguingly, there has been 1 late CR in the multiple myeloma cohort, which previously had shown no responses,” Dr Timmerman said.
Durability of response
This study suggests PD-1 blockade can produce durable responses in hematologic malignancies, as it does in melanoma and renal cell carcinoma.
In HL, the median response duration at a median follow-up of 86 weeks has not yet been reached, and half (n=10) of the responses are still ongoing.
In FL, CTCL, and PTCL, the median response duration has not been reached at a median follow-up of 81, 43, and 31 weeks, respectively. Of note, there are ongoing responses in at least half of patients in these tumor types.
In HL, none of the 6 patients in CR has progressed, although there have been some progressions in the PR group.
The rapidity of responses is also notable, Dr Timmerman said.
“[I]t’s very interesting that some patients have resolution of symptoms and improvement of symptoms within even 1 day of starting nivolumab therapy,” he said.
And responses to nivolumab in HL “can be very dramatic,” he added, as illustrated in the following case from the Mayo Clinic.
A patient with multiple sites of bulky FDG-avid tumors was scheduled to enter hospice. But first, he entered the nivolumab trial. Within 6 weeks of initiating treatment, he had achieved a near-CR. This response has been maintained for 2 years.
“The occurrence of very durable responses in the PR and CR groups has led us to question whether patients should go on to allogeneic stem cell transplantation after achieving responses with nivolumab or, rather, continue on nivolumab as long as their response remains,” Dr Timmerman said.
He added that an international, phase 2 trial in HL is underway and is accruing briskly.
Nivolumab was awarded breakthrough designation by the US Food and Drug Administration last year. Breakthrough designation is intended to expedite the development and review of drugs for serious or life-threatening conditions. ![]()

Photo courtesy of UCLA
LUGANO—The PD-1 checkpoint inhibitor nivolumab produces rapid, durable, and, in some cases, “dramatic” responses in Hodgkin lymphoma (HL), according to a speaker at the 13th International Congress on Malignant Lymphoma.
The drug has also produced durable responses in follicular lymphoma (FL), cutaneous T-cell lymphoma (CTCL), and peripheral T-cell lymphoma (PTCL), although patient numbers for these malignancies are small.
John Timmerman, MD, of the University of California, Los Angeles, presented these results from a phase 1 study of patients with relapsed or refractory lymphoid malignancies and chronic HL (abstract 010).
Bristol-Myers Squibb and Ono Pharmaceutical Company are sponsors of the trial.
Original results of the study, with a data cutoff of June 2014, were reported at ASH 2014, with 40 weeks of median follow-up.
The update presented at 13-ICML, with a data lock in April 2015, includes an additional 10 months of data, for a median follow-up of 76 weeks.
Investigators enrolled 105 patients in this dose-escalation study to receive nivolumab at 1 mg/kg, then 3 mg/kg, every 2 weeks for 2 years.
Twenty-three patients had HL. Thirty-one had B-cell non-Hodgkin lymphoma (NHL), including 11 with FL and 10 with diffuse large B-cell lymphoma (DLBCL).
Twenty-three patients had T-cell NHL, including 5 with PTCL and 13 with CTCL/mycosis fungoides (MF). Twenty-seven patients had multiple myeloma (MM), and 1 had chronic myeloid leukemia.
Patients were heavily pretreated. Seventy-eight percent of HL patients and 26% of T-NHL patients had prior brentuximab vedotin. And 78% (HL), 14% (B-NHL), 9% (T-NHL), and 56% (MM) of patients had a prior autologous transplant.
The median number of prior therapies was 5 (range, 2-15) for HL patients and ranged from 1 to 16 for all patients.
The study’s primary endpoint was safety and tolerability, and the secondary endpoint was efficacy.
Safety and tolerability
Ninety-seven percent of patients had an adverse event, 69% of them related to study treatment and 21% of them treatment-related grade 3-4 events.
Fifteen patients (14%) discontinued treatment due to a related adverse event, including 3 with pneumonitis and 1 each with enteritis, stomatitis, pancreatitis, rash, conjunctivitis, sepsis, diplopia, myositis, neutropenia, myelodysplastic syndrome, increased creatinine phosphokinase, and peripheral neuropathy.
“Immune-related adverse events were generally seen early on and generally of low grade,” Dr Timmerman said. “However, it is notable that there were several grade 3 immune-related adverse events that can be seen as far as 6 months out after the start of therapy.”
These included skin, gastrointestinal, and pulmonary events. Most immune-related adverse events (83%) were resolved using protocol-prescribed procedures.
Efficacy
The overall response rate was 87% for HL, 36% for DLBCL, 40% for FL, 15% for CTCL/MF, 40% for PTCL, and 4% for MM.
Dr Timmerman pointed out that, since ASH, 2 additional conversions from partial response (PR) to complete response (CR) occurred in patients with HL. To date, 6 of 23 HL patients have achieved a CR and 14 a PR.
In B-cell NHL, there were additional conversions from PR to CR in DLBCL, while responses remained the same in FL and in the 4 responders with T-cell lymphomas.
“Intriguingly, there has been 1 late CR in the multiple myeloma cohort, which previously had shown no responses,” Dr Timmerman said.
Durability of response
This study suggests PD-1 blockade can produce durable responses in hematologic malignancies, as it does in melanoma and renal cell carcinoma.
In HL, the median response duration at a median follow-up of 86 weeks has not yet been reached, and half (n=10) of the responses are still ongoing.
In FL, CTCL, and PTCL, the median response duration has not been reached at a median follow-up of 81, 43, and 31 weeks, respectively. Of note, there are ongoing responses in at least half of patients in these tumor types.
In HL, none of the 6 patients in CR has progressed, although there have been some progressions in the PR group.
The rapidity of responses is also notable, Dr Timmerman said.
“[I]t’s very interesting that some patients have resolution of symptoms and improvement of symptoms within even 1 day of starting nivolumab therapy,” he said.
And responses to nivolumab in HL “can be very dramatic,” he added, as illustrated in the following case from the Mayo Clinic.
A patient with multiple sites of bulky FDG-avid tumors was scheduled to enter hospice. But first, he entered the nivolumab trial. Within 6 weeks of initiating treatment, he had achieved a near-CR. This response has been maintained for 2 years.
“The occurrence of very durable responses in the PR and CR groups has led us to question whether patients should go on to allogeneic stem cell transplantation after achieving responses with nivolumab or, rather, continue on nivolumab as long as their response remains,” Dr Timmerman said.
He added that an international, phase 2 trial in HL is underway and is accruing briskly.
Nivolumab was awarded breakthrough designation by the US Food and Drug Administration last year. Breakthrough designation is intended to expedite the development and review of drugs for serious or life-threatening conditions. ![]()
Dose reductions make ponatinib safer for CP-CML

VIENNA—Administering ponatinib at lower doses can reduce the risk of arterial occlusive events (AOE) without hindering responses in patients with chronic-phase chronic myeloid leukemia (CP-CML), data from the PACE trial suggest.
When earlier results of this phase 2 study showed that ponatinib can cause AOEs, trials of the drug were put on partial clinical hold. Enrollment was stalled temporarily, and investigators began reducing ponatinib doses.
Now, updated data from the PACE trial suggest ponatinib can be administered safely and effectively in certain patients with CP-CML.
At a median follow-up of about 3.5 years, 95% of CP-CML patients who underwent dose reductions maintained a major cytogenetic response (MCyR). And AOEs occurred in 7% of patients who underwent dose reductions, compared to 13% of patients who did not.
“These continued responses . . . in such a heavily pretreated patient population are very encouraging,” said study investigator Jorge E. Cortes, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“Careful assessment of the benefit and risk of initiating ponatinib therapy, particularly in patients who may be at increased risk for arterial occlusive events, can help identify patients with refractory, Ph+ leukemias who can benefit most from this treatment.”
Dr Cortes and his colleagues presented data from the PACE trial at the 20th Congress of the European Hematology Association as abstract P234*. The study was sponsored by Ariad Pharmaceuticals, the company developing ponatinib.
Updated results
The trial included patients with resistant or intolerant CML or Philadelphia chromosome-positive acute lymphoblastic leukemia. A total of 449 patients received ponatinib at a starting dose of 45 mg/day.
Ninety-three percent of patients had previously received 2 or more approved tyrosine kinase inhibitors (TKI), and 55% had previously received 3 or more approved TKIs.
Dr Cortes and his colleagues presented data on 270 CP-CML patients. At a median follow-up of 42.3 months (data as of February 2, 2015), 114 patients (42%) continue to receive ponatinib.
Eighteen percent of patients discontinued treatment due to adverse events (AEs), 10% due to disease progression, 3% due to death, and 27% for other reasons.
Fifty-nine percent of CP-CML patients achieved an MCyR at any time during the study, and 83% of responders are estimated to remain in MCyR at 3 years. Thirty-nine percent of patients achieved a major molecular response (MMR).
The estimated progression-free survival at 3 years is 60%, and the estimated overall survival is 81%.
Twenty-three percent of CP-CML patients experienced an AOE designated a serious AE, and 28% experienced any AOE. The median time to onset for AOEs was 14.1 months (range, 0.3–44.0).
Four percent of CP-CML patients experienced a venous thromboembolism (VTE) that was considered an serious AE, and 5% experienced any VTE.
The most common all-grade, treatment-emergent AEs occurring in at least 40% of CP-CML patients were abdominal pain (46%), rash (46%), thrombocytopenia (45%), headache (43%), constipation (41%), and dry skin (41%).
Outcomes after dose reductions
On October 10, 2013, Ariad provided dose-reduction recommendations to investigators for patients remaining on the PACE trial. The following dose reductions were recommended, unless the benefit-risk analysis warranted treatment with a higher dose:
- CP-CML patients who already achieved an MCyR should have their ponatinib dose reduced to 15 mg/day
- CP-CML patients who had not already achieved an MCyR should have their dose reduced to 30 mg/day
- Advanced-phase patients should have their dose reduced to 30 mg/day.
As of February 2015, with 1.3 years (16 months) of follow-up after these recommendations, 95% of CP-CML patients maintained their response, whether or not they underwent prospective dose reductions.
Of the patients who were in MCyR as of October 10, 2013, and had a prospective dose reduction, 95% (61/64) maintained their response at 1.3 years. Of the patients who were in MMR as of October 10, 2013, and had a prospective dose reduction, 94% (44/47) maintained their response at 1.3 years.
Forty-two patients in MCyR did not undergo prospective dose reductions (the majority of which were already at a reduced dose of 30 mg or 15 mg as of October 10, 2013). Of these patients, 93% (n=39) maintained an MCyR after 1.3 more years of ponatinib treatment.
Twenty-four patients in MMR did not undergo prospective dose reductions, and 96% of these patients (n=22) maintained their response at 1.3 years.
Seven percent (5/71) of patients without prior AOEs who underwent dose reductions had a new AOE during the 1.3-year interval after dose reduction.
Thirteen percent (9/67) of patients without prior AOEs who did not undergo dose reductions had a new AOE in the same time interval. ![]()
*Information in the abstract differs from that presented at the meeting.

VIENNA—Administering ponatinib at lower doses can reduce the risk of arterial occlusive events (AOE) without hindering responses in patients with chronic-phase chronic myeloid leukemia (CP-CML), data from the PACE trial suggest.
When earlier results of this phase 2 study showed that ponatinib can cause AOEs, trials of the drug were put on partial clinical hold. Enrollment was stalled temporarily, and investigators began reducing ponatinib doses.
Now, updated data from the PACE trial suggest ponatinib can be administered safely and effectively in certain patients with CP-CML.
At a median follow-up of about 3.5 years, 95% of CP-CML patients who underwent dose reductions maintained a major cytogenetic response (MCyR). And AOEs occurred in 7% of patients who underwent dose reductions, compared to 13% of patients who did not.
“These continued responses . . . in such a heavily pretreated patient population are very encouraging,” said study investigator Jorge E. Cortes, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“Careful assessment of the benefit and risk of initiating ponatinib therapy, particularly in patients who may be at increased risk for arterial occlusive events, can help identify patients with refractory, Ph+ leukemias who can benefit most from this treatment.”
Dr Cortes and his colleagues presented data from the PACE trial at the 20th Congress of the European Hematology Association as abstract P234*. The study was sponsored by Ariad Pharmaceuticals, the company developing ponatinib.
Updated results
The trial included patients with resistant or intolerant CML or Philadelphia chromosome-positive acute lymphoblastic leukemia. A total of 449 patients received ponatinib at a starting dose of 45 mg/day.
Ninety-three percent of patients had previously received 2 or more approved tyrosine kinase inhibitors (TKI), and 55% had previously received 3 or more approved TKIs.
Dr Cortes and his colleagues presented data on 270 CP-CML patients. At a median follow-up of 42.3 months (data as of February 2, 2015), 114 patients (42%) continue to receive ponatinib.
Eighteen percent of patients discontinued treatment due to adverse events (AEs), 10% due to disease progression, 3% due to death, and 27% for other reasons.
Fifty-nine percent of CP-CML patients achieved an MCyR at any time during the study, and 83% of responders are estimated to remain in MCyR at 3 years. Thirty-nine percent of patients achieved a major molecular response (MMR).
The estimated progression-free survival at 3 years is 60%, and the estimated overall survival is 81%.
Twenty-three percent of CP-CML patients experienced an AOE designated a serious AE, and 28% experienced any AOE. The median time to onset for AOEs was 14.1 months (range, 0.3–44.0).
Four percent of CP-CML patients experienced a venous thromboembolism (VTE) that was considered an serious AE, and 5% experienced any VTE.
The most common all-grade, treatment-emergent AEs occurring in at least 40% of CP-CML patients were abdominal pain (46%), rash (46%), thrombocytopenia (45%), headache (43%), constipation (41%), and dry skin (41%).
Outcomes after dose reductions
On October 10, 2013, Ariad provided dose-reduction recommendations to investigators for patients remaining on the PACE trial. The following dose reductions were recommended, unless the benefit-risk analysis warranted treatment with a higher dose:
- CP-CML patients who already achieved an MCyR should have their ponatinib dose reduced to 15 mg/day
- CP-CML patients who had not already achieved an MCyR should have their dose reduced to 30 mg/day
- Advanced-phase patients should have their dose reduced to 30 mg/day.
As of February 2015, with 1.3 years (16 months) of follow-up after these recommendations, 95% of CP-CML patients maintained their response, whether or not they underwent prospective dose reductions.
Of the patients who were in MCyR as of October 10, 2013, and had a prospective dose reduction, 95% (61/64) maintained their response at 1.3 years. Of the patients who were in MMR as of October 10, 2013, and had a prospective dose reduction, 94% (44/47) maintained their response at 1.3 years.
Forty-two patients in MCyR did not undergo prospective dose reductions (the majority of which were already at a reduced dose of 30 mg or 15 mg as of October 10, 2013). Of these patients, 93% (n=39) maintained an MCyR after 1.3 more years of ponatinib treatment.
Twenty-four patients in MMR did not undergo prospective dose reductions, and 96% of these patients (n=22) maintained their response at 1.3 years.
Seven percent (5/71) of patients without prior AOEs who underwent dose reductions had a new AOE during the 1.3-year interval after dose reduction.
Thirteen percent (9/67) of patients without prior AOEs who did not undergo dose reductions had a new AOE in the same time interval. ![]()
*Information in the abstract differs from that presented at the meeting.

VIENNA—Administering ponatinib at lower doses can reduce the risk of arterial occlusive events (AOE) without hindering responses in patients with chronic-phase chronic myeloid leukemia (CP-CML), data from the PACE trial suggest.
When earlier results of this phase 2 study showed that ponatinib can cause AOEs, trials of the drug were put on partial clinical hold. Enrollment was stalled temporarily, and investigators began reducing ponatinib doses.
Now, updated data from the PACE trial suggest ponatinib can be administered safely and effectively in certain patients with CP-CML.
At a median follow-up of about 3.5 years, 95% of CP-CML patients who underwent dose reductions maintained a major cytogenetic response (MCyR). And AOEs occurred in 7% of patients who underwent dose reductions, compared to 13% of patients who did not.
“These continued responses . . . in such a heavily pretreated patient population are very encouraging,” said study investigator Jorge E. Cortes, MD, of The University of Texas MD Anderson Cancer Center in Houston.
“Careful assessment of the benefit and risk of initiating ponatinib therapy, particularly in patients who may be at increased risk for arterial occlusive events, can help identify patients with refractory, Ph+ leukemias who can benefit most from this treatment.”
Dr Cortes and his colleagues presented data from the PACE trial at the 20th Congress of the European Hematology Association as abstract P234*. The study was sponsored by Ariad Pharmaceuticals, the company developing ponatinib.
Updated results
The trial included patients with resistant or intolerant CML or Philadelphia chromosome-positive acute lymphoblastic leukemia. A total of 449 patients received ponatinib at a starting dose of 45 mg/day.
Ninety-three percent of patients had previously received 2 or more approved tyrosine kinase inhibitors (TKI), and 55% had previously received 3 or more approved TKIs.
Dr Cortes and his colleagues presented data on 270 CP-CML patients. At a median follow-up of 42.3 months (data as of February 2, 2015), 114 patients (42%) continue to receive ponatinib.
Eighteen percent of patients discontinued treatment due to adverse events (AEs), 10% due to disease progression, 3% due to death, and 27% for other reasons.
Fifty-nine percent of CP-CML patients achieved an MCyR at any time during the study, and 83% of responders are estimated to remain in MCyR at 3 years. Thirty-nine percent of patients achieved a major molecular response (MMR).
The estimated progression-free survival at 3 years is 60%, and the estimated overall survival is 81%.
Twenty-three percent of CP-CML patients experienced an AOE designated a serious AE, and 28% experienced any AOE. The median time to onset for AOEs was 14.1 months (range, 0.3–44.0).
Four percent of CP-CML patients experienced a venous thromboembolism (VTE) that was considered an serious AE, and 5% experienced any VTE.
The most common all-grade, treatment-emergent AEs occurring in at least 40% of CP-CML patients were abdominal pain (46%), rash (46%), thrombocytopenia (45%), headache (43%), constipation (41%), and dry skin (41%).
Outcomes after dose reductions
On October 10, 2013, Ariad provided dose-reduction recommendations to investigators for patients remaining on the PACE trial. The following dose reductions were recommended, unless the benefit-risk analysis warranted treatment with a higher dose:
- CP-CML patients who already achieved an MCyR should have their ponatinib dose reduced to 15 mg/day
- CP-CML patients who had not already achieved an MCyR should have their dose reduced to 30 mg/day
- Advanced-phase patients should have their dose reduced to 30 mg/day.
As of February 2015, with 1.3 years (16 months) of follow-up after these recommendations, 95% of CP-CML patients maintained their response, whether or not they underwent prospective dose reductions.
Of the patients who were in MCyR as of October 10, 2013, and had a prospective dose reduction, 95% (61/64) maintained their response at 1.3 years. Of the patients who were in MMR as of October 10, 2013, and had a prospective dose reduction, 94% (44/47) maintained their response at 1.3 years.
Forty-two patients in MCyR did not undergo prospective dose reductions (the majority of which were already at a reduced dose of 30 mg or 15 mg as of October 10, 2013). Of these patients, 93% (n=39) maintained an MCyR after 1.3 more years of ponatinib treatment.
Twenty-four patients in MMR did not undergo prospective dose reductions, and 96% of these patients (n=22) maintained their response at 1.3 years.
Seven percent (5/71) of patients without prior AOEs who underwent dose reductions had a new AOE during the 1.3-year interval after dose reduction.
Thirteen percent (9/67) of patients without prior AOEs who did not undergo dose reductions had a new AOE in the same time interval. ![]()
*Information in the abstract differs from that presented at the meeting.
EHA: Dasatinib gets early edge over imatinib in CML
VIENNA – Patients with chronic-phase chronic myeloid leukemia treated with first-line dasatinib achieved significantly more molecular responses at 2 years than those treated with imatinib in the SPIRIT 2 trial.
So far there is no difference, however, in disease progression or overall survival in the ongoing phase III trial, Dr. Stephen O’Brien reported at the annual congress of the European Hematology Association.
With 814 patients, SPIRIT 2 is the largest randomized trial of dasatinib (Sprycel) vs. imatinib (Gleevec).
Its design is similar to the ongoing 519-patient DASISION trial, which reported higher response rates with dasatinib than imatinib in the same setting, but similar progression-free and overall survival rates at 3-year follow-up.
The primary endpoint of SPIRIT 2 is event-free survival at 5 years and will be available in March 2018, he said. Patients at 172 hospitals in the United Kingdom were evenly randomized to imatinib 400 mg daily or dasatinib 100 mg daily. One patient in each group was excluded due to protocol violation or withdrawal of consent. Median follow-up is 42.4 months.
At 24 months, 60.6% of imatinib patients (246/406) and 71.4% of dasatinib patients (290/406) remained on treatment.
Significantly more patients treated with dasatinib than imatinib achieved a complete cytogenetic response at 12 months (53.3% vs. 42%; P = .003), but the difference was diminished at 24 months (33.7% vs. 27.5%; P = .189). These results should be interpreted with caution, however, because the data were incomplete, Dr. O’Brien, of Newcastle University Medical School, Newcastle upon Tyne, England, said.
He noted that the molecular data are more reliable and were calculated based on samples drawn within a 6-week window on either side of the 24-month time point. Values had to be imputed for 22 patients who had no 24-month sample taken, although this imputation should not impact survival outcomes, he said. Major molecular response was defined as a 3-log reduction in the BCR-ABL/ABL ratio, relative to baseline, with data also captured for patients achieving a 4-log reduction.
Significantly more patients on dasatinib than imatinib achieved an MR3 response (57.5% vs. 46%; P < .001) and MR4.5 response (20.2% vs. 14.3%; P = .026).
More patients stopped imatinib than dasatinib due to investigator and/or patient concerns about inadequate response (10.8% vs. 1.3%), whereas nonhematologic toxicities drove more patients to abandon dasatinib (22% vs. 12%), according to Dr. O’Brien.
Pleural effusion, a known toxicity with dasatinib, occurred in 24.1% of patients given the drug vs. 1.2% given imatinib, requiring drainage in 22 cases vs. 1 case, respectively. There was also a “difficult-to-explain” signal for breathlessness with no obvious cause (15.5% vs. 8%). Hypertension was confirmed in only one of these cases and symptoms resolved in others when the drug was withdrawn, he said.
Serious cardiac adverse events were reported in 2.2% of patients in the imatinib arm and 4.2% in the dasatinib arm. Again, the results should be interpreted with caution because trials set up at the time of SPIRIT2 in 2008 were not designed to look carefully at this outcome, Dr. O’Brien observed.
In all, 38 patients have died; 19 in each group.
*Correction 6/18/2015: The headline for an earlier version of this article misstated the type of cancer treated in this study.
VIENNA – Patients with chronic-phase chronic myeloid leukemia treated with first-line dasatinib achieved significantly more molecular responses at 2 years than those treated with imatinib in the SPIRIT 2 trial.
So far there is no difference, however, in disease progression or overall survival in the ongoing phase III trial, Dr. Stephen O’Brien reported at the annual congress of the European Hematology Association.
With 814 patients, SPIRIT 2 is the largest randomized trial of dasatinib (Sprycel) vs. imatinib (Gleevec).
Its design is similar to the ongoing 519-patient DASISION trial, which reported higher response rates with dasatinib than imatinib in the same setting, but similar progression-free and overall survival rates at 3-year follow-up.
The primary endpoint of SPIRIT 2 is event-free survival at 5 years and will be available in March 2018, he said. Patients at 172 hospitals in the United Kingdom were evenly randomized to imatinib 400 mg daily or dasatinib 100 mg daily. One patient in each group was excluded due to protocol violation or withdrawal of consent. Median follow-up is 42.4 months.
At 24 months, 60.6% of imatinib patients (246/406) and 71.4% of dasatinib patients (290/406) remained on treatment.
Significantly more patients treated with dasatinib than imatinib achieved a complete cytogenetic response at 12 months (53.3% vs. 42%; P = .003), but the difference was diminished at 24 months (33.7% vs. 27.5%; P = .189). These results should be interpreted with caution, however, because the data were incomplete, Dr. O’Brien, of Newcastle University Medical School, Newcastle upon Tyne, England, said.
He noted that the molecular data are more reliable and were calculated based on samples drawn within a 6-week window on either side of the 24-month time point. Values had to be imputed for 22 patients who had no 24-month sample taken, although this imputation should not impact survival outcomes, he said. Major molecular response was defined as a 3-log reduction in the BCR-ABL/ABL ratio, relative to baseline, with data also captured for patients achieving a 4-log reduction.
Significantly more patients on dasatinib than imatinib achieved an MR3 response (57.5% vs. 46%; P < .001) and MR4.5 response (20.2% vs. 14.3%; P = .026).
More patients stopped imatinib than dasatinib due to investigator and/or patient concerns about inadequate response (10.8% vs. 1.3%), whereas nonhematologic toxicities drove more patients to abandon dasatinib (22% vs. 12%), according to Dr. O’Brien.
Pleural effusion, a known toxicity with dasatinib, occurred in 24.1% of patients given the drug vs. 1.2% given imatinib, requiring drainage in 22 cases vs. 1 case, respectively. There was also a “difficult-to-explain” signal for breathlessness with no obvious cause (15.5% vs. 8%). Hypertension was confirmed in only one of these cases and symptoms resolved in others when the drug was withdrawn, he said.
Serious cardiac adverse events were reported in 2.2% of patients in the imatinib arm and 4.2% in the dasatinib arm. Again, the results should be interpreted with caution because trials set up at the time of SPIRIT2 in 2008 were not designed to look carefully at this outcome, Dr. O’Brien observed.
In all, 38 patients have died; 19 in each group.
*Correction 6/18/2015: The headline for an earlier version of this article misstated the type of cancer treated in this study.
VIENNA – Patients with chronic-phase chronic myeloid leukemia treated with first-line dasatinib achieved significantly more molecular responses at 2 years than those treated with imatinib in the SPIRIT 2 trial.
So far there is no difference, however, in disease progression or overall survival in the ongoing phase III trial, Dr. Stephen O’Brien reported at the annual congress of the European Hematology Association.
With 814 patients, SPIRIT 2 is the largest randomized trial of dasatinib (Sprycel) vs. imatinib (Gleevec).
Its design is similar to the ongoing 519-patient DASISION trial, which reported higher response rates with dasatinib than imatinib in the same setting, but similar progression-free and overall survival rates at 3-year follow-up.
The primary endpoint of SPIRIT 2 is event-free survival at 5 years and will be available in March 2018, he said. Patients at 172 hospitals in the United Kingdom were evenly randomized to imatinib 400 mg daily or dasatinib 100 mg daily. One patient in each group was excluded due to protocol violation or withdrawal of consent. Median follow-up is 42.4 months.
At 24 months, 60.6% of imatinib patients (246/406) and 71.4% of dasatinib patients (290/406) remained on treatment.
Significantly more patients treated with dasatinib than imatinib achieved a complete cytogenetic response at 12 months (53.3% vs. 42%; P = .003), but the difference was diminished at 24 months (33.7% vs. 27.5%; P = .189). These results should be interpreted with caution, however, because the data were incomplete, Dr. O’Brien, of Newcastle University Medical School, Newcastle upon Tyne, England, said.
He noted that the molecular data are more reliable and were calculated based on samples drawn within a 6-week window on either side of the 24-month time point. Values had to be imputed for 22 patients who had no 24-month sample taken, although this imputation should not impact survival outcomes, he said. Major molecular response was defined as a 3-log reduction in the BCR-ABL/ABL ratio, relative to baseline, with data also captured for patients achieving a 4-log reduction.
Significantly more patients on dasatinib than imatinib achieved an MR3 response (57.5% vs. 46%; P < .001) and MR4.5 response (20.2% vs. 14.3%; P = .026).
More patients stopped imatinib than dasatinib due to investigator and/or patient concerns about inadequate response (10.8% vs. 1.3%), whereas nonhematologic toxicities drove more patients to abandon dasatinib (22% vs. 12%), according to Dr. O’Brien.
Pleural effusion, a known toxicity with dasatinib, occurred in 24.1% of patients given the drug vs. 1.2% given imatinib, requiring drainage in 22 cases vs. 1 case, respectively. There was also a “difficult-to-explain” signal for breathlessness with no obvious cause (15.5% vs. 8%). Hypertension was confirmed in only one of these cases and symptoms resolved in others when the drug was withdrawn, he said.
Serious cardiac adverse events were reported in 2.2% of patients in the imatinib arm and 4.2% in the dasatinib arm. Again, the results should be interpreted with caution because trials set up at the time of SPIRIT2 in 2008 were not designed to look carefully at this outcome, Dr. O’Brien observed.
In all, 38 patients have died; 19 in each group.
*Correction 6/18/2015: The headline for an earlier version of this article misstated the type of cancer treated in this study.
AT THE EHA CONGRESS
Key clinical point: Dasatinib provides more molecular responses than imatinib, but no survival advantage at 2 years in the first-line treatment of chronic-phase chronic myeloid leukemia.
Major finding: More patients receiving dasatinib than imatinib achieved an MR3 response (57.5% vs. 46%; P < .001) and MR4.5 response (20.2% vs. 6%; P = .02).
Data source: Randomized, phase III trial in 814 patients with newly diagnosed chronic myeloid leukemia in chronic phase.
Disclosures: Bristol-Myers Squibb sponsored the study. Dr. O’Brien reported honoraria and research funding from Ariad Pharmaceuticals, Bristol-Myers Squibb, Novartis, and Pfizer.
MKIs can overcome resistance in CML

PHILADELPHIA—Two multikinase inhibitors (MKIs) can treat chronic myeloid leukemia (CML) that is resistant to other inhibitors, according to preclinical research.
A series of in vitro experiments showed that the MKIs, sorafenib and axitinib, can overcome treatment resistance mediated by hyperactivation of the Src kinase Lyn, overexpression of the docking protein Gab2, and the presence of the Bcr-Abl T315I mutation.
Sebastian Halbach, of the University of Freiburg in Germany, and his colleagues presented these findings in a poster at the AACR Annual Meeting 2015 (abstract 2708).
Mechanisms of resistance
Halbach noted that CML is driven by the hyperactive fusion kinase Bcr-Abl, which builds up its own signaling network with various proteins, such as Gab2 and Lyn.
Resistance to tyrosine kinase inhibitors (TKIs) and MKIs can be caused by mutations in the Bcr-Abl oncogene, such as T315I, or by aberrant activity of components of the Bcr-Abl signaling network.
“We have previously shown in our lab that overexpression of the docking protein Gab2 . . . confers resistance against imatinib and dasatinib,” Halbach said. “And another mechanism of resistance is hyperactivation of the Src kinase Lyn.”
For the current study, Halbach and his colleagues investigated the role of Lyn by introducing imatinib, dasatinib, or DMSO to K562 cells (blast-phase CML), Lyn-transformed K562 cells, and Lyn-Y508F-transformed K562 cells.
They also compared imatinib and DMSO in Ba/F3 cells (a murine pro-B cell line), Lyn-transformed Ba/F3 cells, and Lyn-Y508F-transformed Ba/F3 cells.
The results of these experiments showed that hyperactive Lyn confers resistance to imatinib but not dasatinib.
“That’s not that surprising because dasatinib is a multikinase inhibitor which targets Src kinases,” Halbach noted. “Therefore, the hyperactivity of Lyn is directly targeted.”
Identifying new drugs
Having established that TKI and MKI resistance in CML can be mediated by Lyn and Gab2, as well as T315I, Halbach and his colleagues wanted to find drugs that would overcome this problem. They screened a panel of inhibitors and identified sorafenib and axitinib.
The researchers first evaluated the effects of sorafenib and axitinib against the T315I mutation. They tested the 2 MKIs—as well as imatinib, dasatinib, nilotinib, ponatinib, and DMSO—in the KBM5 cell line (blast-phase CML) and the KBM5-T315I cell line (imatinib-resistant CML).
Sorafenib and axitinib killed KBM5-T315I cells more effectively than any of the other inhibitors. The 2 MKIs also decreased the metabolic activity of T315I-positive cells more effectively than imatinib, dasatinib, or nilotinib, but not ponatinib, which produced similar results.
Next, Halbach and his colleagues tested sorafenib, axitinib, and the aforementioned inhibitors in K562 cells overexpressing Gab2. Overexpression of Gab2 conferred resistance to imatinib, dasatinib, nilotinib, and ponatinib, but not sorafenib and axitinib.
Both sorafenib and axitinib decreased the metabolic activity of Gab2-overexpressing cells more effectively than any of the other inhibitors.
Lastly, the researchers tested all of the inhibitors in Lyn-transformed K562 cells, Lyn-Y508F-transformed K562 cells, and K562 cells. They found that sorafenib and axitinib both overcame Lyn-Y508F-mediated resistance.
Sorafenib and axitinib killed K562 cells and Lyn-transformed K562 cells more effectively than any of the other inhibitors. The 2 MKIs also killed Lyn-Y508F-transformed K562 cells more effectively than imatinib and nilotinib, but not ponatinib or dasatinib.
Sorafenib decreased the metabolic activity of Lyn-Y508F-transformed K562 cells more effectively than all of the other inhibitors. But axitinib only proved more effective than imatinib in this regard.
Halbach said he hopes sorafenib and axitinib can one day serve as alternatives to ponatinib for CML patients, especially those with T315I mutations or high Gab2 levels.
For now, his team’s next step is to further analyze the influence of axitinib and sorafenib on the Bcr-Abl—Gab2 signaling complex. ![]()

PHILADELPHIA—Two multikinase inhibitors (MKIs) can treat chronic myeloid leukemia (CML) that is resistant to other inhibitors, according to preclinical research.
A series of in vitro experiments showed that the MKIs, sorafenib and axitinib, can overcome treatment resistance mediated by hyperactivation of the Src kinase Lyn, overexpression of the docking protein Gab2, and the presence of the Bcr-Abl T315I mutation.
Sebastian Halbach, of the University of Freiburg in Germany, and his colleagues presented these findings in a poster at the AACR Annual Meeting 2015 (abstract 2708).
Mechanisms of resistance
Halbach noted that CML is driven by the hyperactive fusion kinase Bcr-Abl, which builds up its own signaling network with various proteins, such as Gab2 and Lyn.
Resistance to tyrosine kinase inhibitors (TKIs) and MKIs can be caused by mutations in the Bcr-Abl oncogene, such as T315I, or by aberrant activity of components of the Bcr-Abl signaling network.
“We have previously shown in our lab that overexpression of the docking protein Gab2 . . . confers resistance against imatinib and dasatinib,” Halbach said. “And another mechanism of resistance is hyperactivation of the Src kinase Lyn.”
For the current study, Halbach and his colleagues investigated the role of Lyn by introducing imatinib, dasatinib, or DMSO to K562 cells (blast-phase CML), Lyn-transformed K562 cells, and Lyn-Y508F-transformed K562 cells.
They also compared imatinib and DMSO in Ba/F3 cells (a murine pro-B cell line), Lyn-transformed Ba/F3 cells, and Lyn-Y508F-transformed Ba/F3 cells.
The results of these experiments showed that hyperactive Lyn confers resistance to imatinib but not dasatinib.
“That’s not that surprising because dasatinib is a multikinase inhibitor which targets Src kinases,” Halbach noted. “Therefore, the hyperactivity of Lyn is directly targeted.”
Identifying new drugs
Having established that TKI and MKI resistance in CML can be mediated by Lyn and Gab2, as well as T315I, Halbach and his colleagues wanted to find drugs that would overcome this problem. They screened a panel of inhibitors and identified sorafenib and axitinib.
The researchers first evaluated the effects of sorafenib and axitinib against the T315I mutation. They tested the 2 MKIs—as well as imatinib, dasatinib, nilotinib, ponatinib, and DMSO—in the KBM5 cell line (blast-phase CML) and the KBM5-T315I cell line (imatinib-resistant CML).
Sorafenib and axitinib killed KBM5-T315I cells more effectively than any of the other inhibitors. The 2 MKIs also decreased the metabolic activity of T315I-positive cells more effectively than imatinib, dasatinib, or nilotinib, but not ponatinib, which produced similar results.
Next, Halbach and his colleagues tested sorafenib, axitinib, and the aforementioned inhibitors in K562 cells overexpressing Gab2. Overexpression of Gab2 conferred resistance to imatinib, dasatinib, nilotinib, and ponatinib, but not sorafenib and axitinib.
Both sorafenib and axitinib decreased the metabolic activity of Gab2-overexpressing cells more effectively than any of the other inhibitors.
Lastly, the researchers tested all of the inhibitors in Lyn-transformed K562 cells, Lyn-Y508F-transformed K562 cells, and K562 cells. They found that sorafenib and axitinib both overcame Lyn-Y508F-mediated resistance.
Sorafenib and axitinib killed K562 cells and Lyn-transformed K562 cells more effectively than any of the other inhibitors. The 2 MKIs also killed Lyn-Y508F-transformed K562 cells more effectively than imatinib and nilotinib, but not ponatinib or dasatinib.
Sorafenib decreased the metabolic activity of Lyn-Y508F-transformed K562 cells more effectively than all of the other inhibitors. But axitinib only proved more effective than imatinib in this regard.
Halbach said he hopes sorafenib and axitinib can one day serve as alternatives to ponatinib for CML patients, especially those with T315I mutations or high Gab2 levels.
For now, his team’s next step is to further analyze the influence of axitinib and sorafenib on the Bcr-Abl—Gab2 signaling complex. ![]()

PHILADELPHIA—Two multikinase inhibitors (MKIs) can treat chronic myeloid leukemia (CML) that is resistant to other inhibitors, according to preclinical research.
A series of in vitro experiments showed that the MKIs, sorafenib and axitinib, can overcome treatment resistance mediated by hyperactivation of the Src kinase Lyn, overexpression of the docking protein Gab2, and the presence of the Bcr-Abl T315I mutation.
Sebastian Halbach, of the University of Freiburg in Germany, and his colleagues presented these findings in a poster at the AACR Annual Meeting 2015 (abstract 2708).
Mechanisms of resistance
Halbach noted that CML is driven by the hyperactive fusion kinase Bcr-Abl, which builds up its own signaling network with various proteins, such as Gab2 and Lyn.
Resistance to tyrosine kinase inhibitors (TKIs) and MKIs can be caused by mutations in the Bcr-Abl oncogene, such as T315I, or by aberrant activity of components of the Bcr-Abl signaling network.
“We have previously shown in our lab that overexpression of the docking protein Gab2 . . . confers resistance against imatinib and dasatinib,” Halbach said. “And another mechanism of resistance is hyperactivation of the Src kinase Lyn.”
For the current study, Halbach and his colleagues investigated the role of Lyn by introducing imatinib, dasatinib, or DMSO to K562 cells (blast-phase CML), Lyn-transformed K562 cells, and Lyn-Y508F-transformed K562 cells.
They also compared imatinib and DMSO in Ba/F3 cells (a murine pro-B cell line), Lyn-transformed Ba/F3 cells, and Lyn-Y508F-transformed Ba/F3 cells.
The results of these experiments showed that hyperactive Lyn confers resistance to imatinib but not dasatinib.
“That’s not that surprising because dasatinib is a multikinase inhibitor which targets Src kinases,” Halbach noted. “Therefore, the hyperactivity of Lyn is directly targeted.”
Identifying new drugs
Having established that TKI and MKI resistance in CML can be mediated by Lyn and Gab2, as well as T315I, Halbach and his colleagues wanted to find drugs that would overcome this problem. They screened a panel of inhibitors and identified sorafenib and axitinib.
The researchers first evaluated the effects of sorafenib and axitinib against the T315I mutation. They tested the 2 MKIs—as well as imatinib, dasatinib, nilotinib, ponatinib, and DMSO—in the KBM5 cell line (blast-phase CML) and the KBM5-T315I cell line (imatinib-resistant CML).
Sorafenib and axitinib killed KBM5-T315I cells more effectively than any of the other inhibitors. The 2 MKIs also decreased the metabolic activity of T315I-positive cells more effectively than imatinib, dasatinib, or nilotinib, but not ponatinib, which produced similar results.
Next, Halbach and his colleagues tested sorafenib, axitinib, and the aforementioned inhibitors in K562 cells overexpressing Gab2. Overexpression of Gab2 conferred resistance to imatinib, dasatinib, nilotinib, and ponatinib, but not sorafenib and axitinib.
Both sorafenib and axitinib decreased the metabolic activity of Gab2-overexpressing cells more effectively than any of the other inhibitors.
Lastly, the researchers tested all of the inhibitors in Lyn-transformed K562 cells, Lyn-Y508F-transformed K562 cells, and K562 cells. They found that sorafenib and axitinib both overcame Lyn-Y508F-mediated resistance.
Sorafenib and axitinib killed K562 cells and Lyn-transformed K562 cells more effectively than any of the other inhibitors. The 2 MKIs also killed Lyn-Y508F-transformed K562 cells more effectively than imatinib and nilotinib, but not ponatinib or dasatinib.
Sorafenib decreased the metabolic activity of Lyn-Y508F-transformed K562 cells more effectively than all of the other inhibitors. But axitinib only proved more effective than imatinib in this regard.
Halbach said he hopes sorafenib and axitinib can one day serve as alternatives to ponatinib for CML patients, especially those with T315I mutations or high Gab2 levels.
For now, his team’s next step is to further analyze the influence of axitinib and sorafenib on the Bcr-Abl—Gab2 signaling complex. ![]()
Drug approved to treat CML, ALL in Canada

Photo courtesy of the FDA
Health Canada has approved ponatinib hydrochloride (Iclusig) to treat adults with any phase of chronic myeloid leukemia (CML) or Philadelphia
chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) for whom other tyrosine kinase inhibitor (TKI) therapy is not appropriate, including CML or Ph+ ALL patients with the T315I mutation and those who have exhibited prior TKI resistance or intolerance.
Ponatinib is approved under the Notice of Compliance with Conditions policy based on promising evidence of clinical effectiveness.
Products approved under this policy are intended for the treatment, prevention, or diagnosis of a serious, life-threatening, or severely debilitating illness. The products must have demonstrated promising benefit, be of high quality, and possess an acceptable safety profile based on a benefit/risk assessment.
These products either respond to a serious unmet medical need in Canada or have demonstrated a significant improvement in the benefit/risk profile over existing therapies.
Ponatinib will be made available in Canada through a controlled distribution program. Prescribers who have completed the certification procedure will be able to prescribe the drug. Trained pharmacies will verify the prescriber’s certified status prior to dispensing ponatinib to the patient.
Health Canada’s decision to approve ponatinib was based on 2-year data from the phase 2 PACE trial.
A trial set to begin in mid-2015 will serve as the confirmatory trial for the Health Canada approval. Investigators will evaluate 3 starting doses of ponatinib in patients with refractory, chronic-phase CML who are resistant to at least 2 approved TKIs.
PACE trial
Researchers conducted this trial in patients with CML or Ph+ ALL who were resistant or intolerant to prior TKI therapy, or who had the T315I mutation.
Ponatinib demonstrated anti-leukemic activity in these patients, prompting a major cytogenetic response (MCyR) in 56% of chronic-phase CML patients and in 70% of patients with the T315I mutation. MCyR within the first 12 months of treatment was the primary endpoint for chronic-phase patients.
In patients with advanced disease, 57% of accelerated-phase CML patients and 31% of blast-phase CML patients achieved a major hematologic response (MaHR). MaHR within the first 6 months was the primary endpoint for patients with advanced disease. In patients with Ph+ ALL, 41% achieved MaHR.
Common non-hematologic adverse events included rash (38%), abdominal pain (38%), headache (35%), dry skin (35%), constipation (34%), fatigue (27%), pyrexia (27%), nausea (26%), arthralgia (25%), hypertension (21%), increased lipase (19%), and increased amylase (7%).
Hematologic events of any grade included thrombocytopenia (42%), neutropenia (24%), and anemia (20%). Serious adverse events of arterial thromboembolism, including arterial stenosis, occurred in patients with cardiovascular risk factors.
Extended follow-up data from the PACE trial, collected in 2013, suggested ponatinib can increase the risk of thrombotic events. When these data came to light, officials in the European Union and the US, where ponatinib had already been approved, began to investigate the drug.
Ponatinib was pulled from the US market for a little over 2 months, and trials of the drug were placed on partial hold while the Food and Drug Administration evaluated the drug’s safety. Ponatinib went back on the market in January 2014, with new safety measures in place.
The drug was not pulled from the market in the European Union, but the European Medicine’s Agency released recommendations for safer use of ponatinib. The Committee for Medicinal Products for Human Use reviewed data on ponatinib and decided the drug’s benefits outweigh its risks. ![]()

Photo courtesy of the FDA
Health Canada has approved ponatinib hydrochloride (Iclusig) to treat adults with any phase of chronic myeloid leukemia (CML) or Philadelphia
chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) for whom other tyrosine kinase inhibitor (TKI) therapy is not appropriate, including CML or Ph+ ALL patients with the T315I mutation and those who have exhibited prior TKI resistance or intolerance.
Ponatinib is approved under the Notice of Compliance with Conditions policy based on promising evidence of clinical effectiveness.
Products approved under this policy are intended for the treatment, prevention, or diagnosis of a serious, life-threatening, or severely debilitating illness. The products must have demonstrated promising benefit, be of high quality, and possess an acceptable safety profile based on a benefit/risk assessment.
These products either respond to a serious unmet medical need in Canada or have demonstrated a significant improvement in the benefit/risk profile over existing therapies.
Ponatinib will be made available in Canada through a controlled distribution program. Prescribers who have completed the certification procedure will be able to prescribe the drug. Trained pharmacies will verify the prescriber’s certified status prior to dispensing ponatinib to the patient.
Health Canada’s decision to approve ponatinib was based on 2-year data from the phase 2 PACE trial.
A trial set to begin in mid-2015 will serve as the confirmatory trial for the Health Canada approval. Investigators will evaluate 3 starting doses of ponatinib in patients with refractory, chronic-phase CML who are resistant to at least 2 approved TKIs.
PACE trial
Researchers conducted this trial in patients with CML or Ph+ ALL who were resistant or intolerant to prior TKI therapy, or who had the T315I mutation.
Ponatinib demonstrated anti-leukemic activity in these patients, prompting a major cytogenetic response (MCyR) in 56% of chronic-phase CML patients and in 70% of patients with the T315I mutation. MCyR within the first 12 months of treatment was the primary endpoint for chronic-phase patients.
In patients with advanced disease, 57% of accelerated-phase CML patients and 31% of blast-phase CML patients achieved a major hematologic response (MaHR). MaHR within the first 6 months was the primary endpoint for patients with advanced disease. In patients with Ph+ ALL, 41% achieved MaHR.
Common non-hematologic adverse events included rash (38%), abdominal pain (38%), headache (35%), dry skin (35%), constipation (34%), fatigue (27%), pyrexia (27%), nausea (26%), arthralgia (25%), hypertension (21%), increased lipase (19%), and increased amylase (7%).
Hematologic events of any grade included thrombocytopenia (42%), neutropenia (24%), and anemia (20%). Serious adverse events of arterial thromboembolism, including arterial stenosis, occurred in patients with cardiovascular risk factors.
Extended follow-up data from the PACE trial, collected in 2013, suggested ponatinib can increase the risk of thrombotic events. When these data came to light, officials in the European Union and the US, where ponatinib had already been approved, began to investigate the drug.
Ponatinib was pulled from the US market for a little over 2 months, and trials of the drug were placed on partial hold while the Food and Drug Administration evaluated the drug’s safety. Ponatinib went back on the market in January 2014, with new safety measures in place.
The drug was not pulled from the market in the European Union, but the European Medicine’s Agency released recommendations for safer use of ponatinib. The Committee for Medicinal Products for Human Use reviewed data on ponatinib and decided the drug’s benefits outweigh its risks. ![]()

Photo courtesy of the FDA
Health Canada has approved ponatinib hydrochloride (Iclusig) to treat adults with any phase of chronic myeloid leukemia (CML) or Philadelphia
chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) for whom other tyrosine kinase inhibitor (TKI) therapy is not appropriate, including CML or Ph+ ALL patients with the T315I mutation and those who have exhibited prior TKI resistance or intolerance.
Ponatinib is approved under the Notice of Compliance with Conditions policy based on promising evidence of clinical effectiveness.
Products approved under this policy are intended for the treatment, prevention, or diagnosis of a serious, life-threatening, or severely debilitating illness. The products must have demonstrated promising benefit, be of high quality, and possess an acceptable safety profile based on a benefit/risk assessment.
These products either respond to a serious unmet medical need in Canada or have demonstrated a significant improvement in the benefit/risk profile over existing therapies.
Ponatinib will be made available in Canada through a controlled distribution program. Prescribers who have completed the certification procedure will be able to prescribe the drug. Trained pharmacies will verify the prescriber’s certified status prior to dispensing ponatinib to the patient.
Health Canada’s decision to approve ponatinib was based on 2-year data from the phase 2 PACE trial.
A trial set to begin in mid-2015 will serve as the confirmatory trial for the Health Canada approval. Investigators will evaluate 3 starting doses of ponatinib in patients with refractory, chronic-phase CML who are resistant to at least 2 approved TKIs.
PACE trial
Researchers conducted this trial in patients with CML or Ph+ ALL who were resistant or intolerant to prior TKI therapy, or who had the T315I mutation.
Ponatinib demonstrated anti-leukemic activity in these patients, prompting a major cytogenetic response (MCyR) in 56% of chronic-phase CML patients and in 70% of patients with the T315I mutation. MCyR within the first 12 months of treatment was the primary endpoint for chronic-phase patients.
In patients with advanced disease, 57% of accelerated-phase CML patients and 31% of blast-phase CML patients achieved a major hematologic response (MaHR). MaHR within the first 6 months was the primary endpoint for patients with advanced disease. In patients with Ph+ ALL, 41% achieved MaHR.
Common non-hematologic adverse events included rash (38%), abdominal pain (38%), headache (35%), dry skin (35%), constipation (34%), fatigue (27%), pyrexia (27%), nausea (26%), arthralgia (25%), hypertension (21%), increased lipase (19%), and increased amylase (7%).
Hematologic events of any grade included thrombocytopenia (42%), neutropenia (24%), and anemia (20%). Serious adverse events of arterial thromboembolism, including arterial stenosis, occurred in patients with cardiovascular risk factors.
Extended follow-up data from the PACE trial, collected in 2013, suggested ponatinib can increase the risk of thrombotic events. When these data came to light, officials in the European Union and the US, where ponatinib had already been approved, began to investigate the drug.
Ponatinib was pulled from the US market for a little over 2 months, and trials of the drug were placed on partial hold while the Food and Drug Administration evaluated the drug’s safety. Ponatinib went back on the market in January 2014, with new safety measures in place.
The drug was not pulled from the market in the European Union, but the European Medicine’s Agency released recommendations for safer use of ponatinib. The Committee for Medicinal Products for Human Use reviewed data on ponatinib and decided the drug’s benefits outweigh its risks.
Study reveals potential strategy for treating CML

Image courtesy of UCSD
New discoveries concerning a well-known tumor suppressor protein could help advance the diagnosis and treatment of chronic myeloid leukemia (CML), according to researchers.
They found that levels of the protein, BRCA1, are significantly decreased in advanced phases of CML, the expression of BCR-ABL1 correlates with decreased levels of BRCA1, and this downregulation of BRCA1 is caused by the inhibition of BRCA1 messenger RNA (mRNA) translation.
These discoveries explain the mechanism that supports CML development and uncover its weakness, the investigators said. They reported their findings in Cell Cycle.
“Our data demonstrated that BRCA1 synthesis is diminished in [the] advanced stage[s] of CML,” said study author Paulina Podszywałow-Bartnicka, PhD, of the Nencki Institute in Warsaw, Poland.
“The gene coding for BRCA1 protein is not mutated. However, BRCA1 mRNA, which is necessary for the protein production, is aggregated and stored in protein complexes [and], thus, not available for the protein synthesis.”
To gain more insight into this phenomenon, the investigators looked at 2 mRNA-binding proteins, HuR and TIAR. They found that BCR-ABL1 promoted cytosolic localization of TIAR and HuR, the proteins’ binding to BRCA1 mRNA, and formation of the TIAR-HuR complex.
The researchers also found that HuR positively regulated BRCA1 mRNA stability and translation, while TIAR negatively regulated BRCA1 translation.
TIAR-dependent downregulation of BRCA1 was a result of endoplasmic reticulum stress, which is activated in BCR-ABL1 expressing cells. And experiments showed that silencing TIAR in CML cells elevated BRCA1 levels.
This suggests that TIAR-mediated repression of BRCA1 mRNA translation is responsible for the downregulation of BRCA1 observed in BCR-ABL1-positive leukemia cells.
The investigators said this research indicates that BRCA1 deficiency, which supports CML, can be also used as a weapon against the disease.
“When a cell has damaged one signaling pathway or one gene, it may function properly due to alternative pathways . . . ,” explained study author Tomasz Skorski, MD, PhD, of Temple University School of Medicine in Philadelphia, Pennsylvania.
“Only when this alternative pathway is inhibited [do cells] lose the ability to survive. As we know that one of the [DNA double-strand break] repair pathways which depend on BRCA1 is blocked in leukemia cells, we can try to find the alternative, parallel pathway and inhibit it as well.”
This will induce apoptosis via synthetic lethality, but only in leukemia cells, because healthy cells still have functional BRCA1-dependent signaling. Dr Skorski noted that therapies based on BRCA1 deficiency are currently under investigation in clinical trials.

Image courtesy of UCSD
New discoveries concerning a well-known tumor suppressor protein could help advance the diagnosis and treatment of chronic myeloid leukemia (CML), according to researchers.
They found that levels of the protein, BRCA1, are significantly decreased in advanced phases of CML, the expression of BCR-ABL1 correlates with decreased levels of BRCA1, and this downregulation of BRCA1 is caused by the inhibition of BRCA1 messenger RNA (mRNA) translation.
These discoveries explain the mechanism that supports CML development and uncover its weakness, the investigators said. They reported their findings in Cell Cycle.
“Our data demonstrated that BRCA1 synthesis is diminished in [the] advanced stage[s] of CML,” said study author Paulina Podszywałow-Bartnicka, PhD, of the Nencki Institute in Warsaw, Poland.
“The gene coding for BRCA1 protein is not mutated. However, BRCA1 mRNA, which is necessary for the protein production, is aggregated and stored in protein complexes [and], thus, not available for the protein synthesis.”
To gain more insight into this phenomenon, the investigators looked at 2 mRNA-binding proteins, HuR and TIAR. They found that BCR-ABL1 promoted cytosolic localization of TIAR and HuR, the proteins’ binding to BRCA1 mRNA, and formation of the TIAR-HuR complex.
The researchers also found that HuR positively regulated BRCA1 mRNA stability and translation, while TIAR negatively regulated BRCA1 translation.
TIAR-dependent downregulation of BRCA1 was a result of endoplasmic reticulum stress, which is activated in BCR-ABL1 expressing cells. And experiments showed that silencing TIAR in CML cells elevated BRCA1 levels.
This suggests that TIAR-mediated repression of BRCA1 mRNA translation is responsible for the downregulation of BRCA1 observed in BCR-ABL1-positive leukemia cells.
The investigators said this research indicates that BRCA1 deficiency, which supports CML, can be also used as a weapon against the disease.
“When a cell has damaged one signaling pathway or one gene, it may function properly due to alternative pathways . . . ,” explained study author Tomasz Skorski, MD, PhD, of Temple University School of Medicine in Philadelphia, Pennsylvania.
“Only when this alternative pathway is inhibited [do cells] lose the ability to survive. As we know that one of the [DNA double-strand break] repair pathways which depend on BRCA1 is blocked in leukemia cells, we can try to find the alternative, parallel pathway and inhibit it as well.”
This will induce apoptosis via synthetic lethality, but only in leukemia cells, because healthy cells still have functional BRCA1-dependent signaling. Dr Skorski noted that therapies based on BRCA1 deficiency are currently under investigation in clinical trials.

Image courtesy of UCSD
New discoveries concerning a well-known tumor suppressor protein could help advance the diagnosis and treatment of chronic myeloid leukemia (CML), according to researchers.
They found that levels of the protein, BRCA1, are significantly decreased in advanced phases of CML, the expression of BCR-ABL1 correlates with decreased levels of BRCA1, and this downregulation of BRCA1 is caused by the inhibition of BRCA1 messenger RNA (mRNA) translation.
These discoveries explain the mechanism that supports CML development and uncover its weakness, the investigators said. They reported their findings in Cell Cycle.
“Our data demonstrated that BRCA1 synthesis is diminished in [the] advanced stage[s] of CML,” said study author Paulina Podszywałow-Bartnicka, PhD, of the Nencki Institute in Warsaw, Poland.
“The gene coding for BRCA1 protein is not mutated. However, BRCA1 mRNA, which is necessary for the protein production, is aggregated and stored in protein complexes [and], thus, not available for the protein synthesis.”
To gain more insight into this phenomenon, the investigators looked at 2 mRNA-binding proteins, HuR and TIAR. They found that BCR-ABL1 promoted cytosolic localization of TIAR and HuR, the proteins’ binding to BRCA1 mRNA, and formation of the TIAR-HuR complex.
The researchers also found that HuR positively regulated BRCA1 mRNA stability and translation, while TIAR negatively regulated BRCA1 translation.
TIAR-dependent downregulation of BRCA1 was a result of endoplasmic reticulum stress, which is activated in BCR-ABL1 expressing cells. And experiments showed that silencing TIAR in CML cells elevated BRCA1 levels.
This suggests that TIAR-mediated repression of BRCA1 mRNA translation is responsible for the downregulation of BRCA1 observed in BCR-ABL1-positive leukemia cells.
The investigators said this research indicates that BRCA1 deficiency, which supports CML, can be also used as a weapon against the disease.
“When a cell has damaged one signaling pathway or one gene, it may function properly due to alternative pathways . . . ,” explained study author Tomasz Skorski, MD, PhD, of Temple University School of Medicine in Philadelphia, Pennsylvania.
“Only when this alternative pathway is inhibited [do cells] lose the ability to survive. As we know that one of the [DNA double-strand break] repair pathways which depend on BRCA1 is blocked in leukemia cells, we can try to find the alternative, parallel pathway and inhibit it as well.”
This will induce apoptosis via synthetic lethality, but only in leukemia cells, because healthy cells still have functional BRCA1-dependent signaling. Dr Skorski noted that therapies based on BRCA1 deficiency are currently under investigation in clinical trials.