User login
CDC updates diagnostic guidelines for congenital Zika virus infection
All infants who either exhibit abnormal clinical or neuroimaging findings consistent with possible Zika infection, or who exhibit normal phenotypes but are born to mothers who are positive for Zika virus infection during pregnancy, should undergo laboratory testing for the virus, according to updated diagnostic guidance from the CDC.
All infants should undergo a comprehensive physical exam at birth, as well as a neurologic examination, postnatal head ultrasound, and standard hearing tests to determine any phenotypic signs of congenital Zika infections (MMWR. ePub: 2016 Aug 19. doi: 10.15585/mmwr.mm6533e2).
Laboratory samples should be collected within 2 days of birth. Molecular testing should be done via a real-time reverse transcription–polymerase chain reaction (rRT-PCR), while serologic testing should be carried out via IgM. If the former test is positive, then the infant is Zika positive; however, if the rRT-PCR is negative but the IgM is positive, then the conclusion can only be a “probable” congenital Zika infection.
For infants with laboratory-confirmed or probable congenital Zika infection, the CDC recommends outpatient management and follow-up. For those who are found negative for Zika despite having other symptoms consistent with infection, the CDC advises continued evaluation to determine the cause of any congenital anomalies.
If an infant has no overt symptoms of Zika virus but is found to have laboratory-confirmed or probable Zika, they should be given routine newborn care along with auditory brainstem response (ABR) testing and an opthalmology examination within 1 month of birth. Infants with no overt signs of Zika and a lab-confirmed negative result can resume standard newborn care with no additional monitoring.
Outpatient care should begin with clear establishment of a medical home, followed by monitoring the child’s growth and developmental screenings at every well child visit, according to the CDC. Vision screening should be repeated at all well child visits; ABR should be repeated 4-6 months after initial testing.
“Use a standardized, validated developmental screening tool at 9 months as currently recommended, or earlier for any parental or provider concerns,” according to lead author Kate Russell, MD, of the CDC’s Epidemic Intelligence Service, and her coauthors.
Cranial ultrasound should be performed on all infants, regardless of how normal any prenatal cranial ultrasounds were. Previously, the CDC advised that if a third trimester prenatal cranial ultrasound showed no abnormalities, a postnatal cranial ultrasound was not needed.
The CDC continues to advise that a multidisciplinary approach be taken to evaluation, diagnosis, and potential treatment of infants with congenital Zika virus infection.
“Because the types of services needed to care for infants with congenital Zika syndrome are complex, CDC recommends coordinated care through a multidisciplinary team and established medical home,” according to the guidance. “As a critical component of patient care and early identification of any delays, families should be empowered to be active participants in their child’s monitoring and care.”
All infants who either exhibit abnormal clinical or neuroimaging findings consistent with possible Zika infection, or who exhibit normal phenotypes but are born to mothers who are positive for Zika virus infection during pregnancy, should undergo laboratory testing for the virus, according to updated diagnostic guidance from the CDC.
All infants should undergo a comprehensive physical exam at birth, as well as a neurologic examination, postnatal head ultrasound, and standard hearing tests to determine any phenotypic signs of congenital Zika infections (MMWR. ePub: 2016 Aug 19. doi: 10.15585/mmwr.mm6533e2).
Laboratory samples should be collected within 2 days of birth. Molecular testing should be done via a real-time reverse transcription–polymerase chain reaction (rRT-PCR), while serologic testing should be carried out via IgM. If the former test is positive, then the infant is Zika positive; however, if the rRT-PCR is negative but the IgM is positive, then the conclusion can only be a “probable” congenital Zika infection.
For infants with laboratory-confirmed or probable congenital Zika infection, the CDC recommends outpatient management and follow-up. For those who are found negative for Zika despite having other symptoms consistent with infection, the CDC advises continued evaluation to determine the cause of any congenital anomalies.
If an infant has no overt symptoms of Zika virus but is found to have laboratory-confirmed or probable Zika, they should be given routine newborn care along with auditory brainstem response (ABR) testing and an opthalmology examination within 1 month of birth. Infants with no overt signs of Zika and a lab-confirmed negative result can resume standard newborn care with no additional monitoring.
Outpatient care should begin with clear establishment of a medical home, followed by monitoring the child’s growth and developmental screenings at every well child visit, according to the CDC. Vision screening should be repeated at all well child visits; ABR should be repeated 4-6 months after initial testing.
“Use a standardized, validated developmental screening tool at 9 months as currently recommended, or earlier for any parental or provider concerns,” according to lead author Kate Russell, MD, of the CDC’s Epidemic Intelligence Service, and her coauthors.
Cranial ultrasound should be performed on all infants, regardless of how normal any prenatal cranial ultrasounds were. Previously, the CDC advised that if a third trimester prenatal cranial ultrasound showed no abnormalities, a postnatal cranial ultrasound was not needed.
The CDC continues to advise that a multidisciplinary approach be taken to evaluation, diagnosis, and potential treatment of infants with congenital Zika virus infection.
“Because the types of services needed to care for infants with congenital Zika syndrome are complex, CDC recommends coordinated care through a multidisciplinary team and established medical home,” according to the guidance. “As a critical component of patient care and early identification of any delays, families should be empowered to be active participants in their child’s monitoring and care.”
All infants who either exhibit abnormal clinical or neuroimaging findings consistent with possible Zika infection, or who exhibit normal phenotypes but are born to mothers who are positive for Zika virus infection during pregnancy, should undergo laboratory testing for the virus, according to updated diagnostic guidance from the CDC.
All infants should undergo a comprehensive physical exam at birth, as well as a neurologic examination, postnatal head ultrasound, and standard hearing tests to determine any phenotypic signs of congenital Zika infections (MMWR. ePub: 2016 Aug 19. doi: 10.15585/mmwr.mm6533e2).
Laboratory samples should be collected within 2 days of birth. Molecular testing should be done via a real-time reverse transcription–polymerase chain reaction (rRT-PCR), while serologic testing should be carried out via IgM. If the former test is positive, then the infant is Zika positive; however, if the rRT-PCR is negative but the IgM is positive, then the conclusion can only be a “probable” congenital Zika infection.
For infants with laboratory-confirmed or probable congenital Zika infection, the CDC recommends outpatient management and follow-up. For those who are found negative for Zika despite having other symptoms consistent with infection, the CDC advises continued evaluation to determine the cause of any congenital anomalies.
If an infant has no overt symptoms of Zika virus but is found to have laboratory-confirmed or probable Zika, they should be given routine newborn care along with auditory brainstem response (ABR) testing and an opthalmology examination within 1 month of birth. Infants with no overt signs of Zika and a lab-confirmed negative result can resume standard newborn care with no additional monitoring.
Outpatient care should begin with clear establishment of a medical home, followed by monitoring the child’s growth and developmental screenings at every well child visit, according to the CDC. Vision screening should be repeated at all well child visits; ABR should be repeated 4-6 months after initial testing.
“Use a standardized, validated developmental screening tool at 9 months as currently recommended, or earlier for any parental or provider concerns,” according to lead author Kate Russell, MD, of the CDC’s Epidemic Intelligence Service, and her coauthors.
Cranial ultrasound should be performed on all infants, regardless of how normal any prenatal cranial ultrasounds were. Previously, the CDC advised that if a third trimester prenatal cranial ultrasound showed no abnormalities, a postnatal cranial ultrasound was not needed.
The CDC continues to advise that a multidisciplinary approach be taken to evaluation, diagnosis, and potential treatment of infants with congenital Zika virus infection.
“Because the types of services needed to care for infants with congenital Zika syndrome are complex, CDC recommends coordinated care through a multidisciplinary team and established medical home,” according to the guidance. “As a critical component of patient care and early identification of any delays, families should be empowered to be active participants in their child’s monitoring and care.”
FROM MMWR
Zika in pregnant women: CDC reports 189 new cases
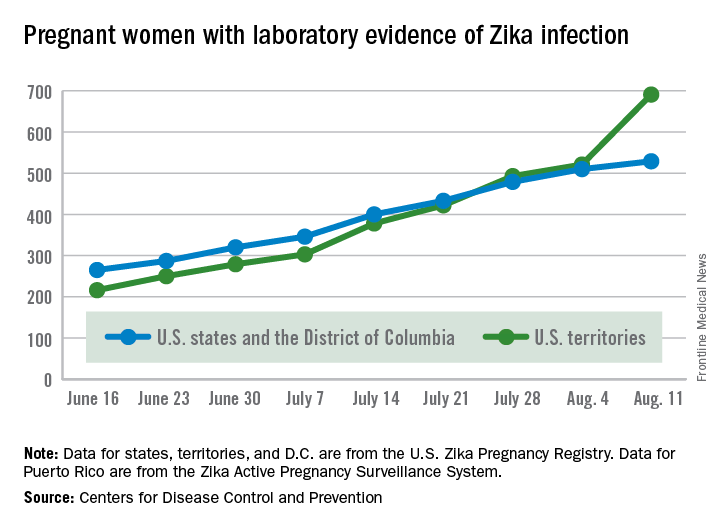
The number of pregnant women with laboratory evidence of Zika virus infection jumped by 189 during the week ending Aug. 11, with most of the increase coming in the U.S. territories, according to the Centers for Disease Control and Prevention.
The territories reported 170 new cases of Zika infection for that week, while the 50 states and the District of Columbia had 19 new cases in pregnant women. There have been 1,220 cases in pregnant women in the United States so far: 691 in the territories and 529 in the states and D.C. Among all Americans, there have been 10,295 cases of Zika: 8,035 in the territories and 2,260 in the states/D.C., the CDC reported.

The number of Zika-related poor outcomes did not change during the week ending Aug. 11. The number of liveborn infants born with birth defects stayed at 16 in the states/D.C. and 1 in the territories, and the number of pregnancy losses with birth defects held at 5 in the states/D.C. and 1 in the territories, the CDC said. State- or territorial-level data are not being reported to protect the privacy of affected women and children.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika virus–related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The number of pregnant women with laboratory evidence of Zika virus infection jumped by 189 during the week ending Aug. 11, with most of the increase coming in the U.S. territories, according to the Centers for Disease Control and Prevention.
The territories reported 170 new cases of Zika infection for that week, while the 50 states and the District of Columbia had 19 new cases in pregnant women. There have been 1,220 cases in pregnant women in the United States so far: 691 in the territories and 529 in the states and D.C. Among all Americans, there have been 10,295 cases of Zika: 8,035 in the territories and 2,260 in the states/D.C., the CDC reported.

The number of Zika-related poor outcomes did not change during the week ending Aug. 11. The number of liveborn infants born with birth defects stayed at 16 in the states/D.C. and 1 in the territories, and the number of pregnancy losses with birth defects held at 5 in the states/D.C. and 1 in the territories, the CDC said. State- or territorial-level data are not being reported to protect the privacy of affected women and children.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika virus–related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The number of pregnant women with laboratory evidence of Zika virus infection jumped by 189 during the week ending Aug. 11, with most of the increase coming in the U.S. territories, according to the Centers for Disease Control and Prevention.
The territories reported 170 new cases of Zika infection for that week, while the 50 states and the District of Columbia had 19 new cases in pregnant women. There have been 1,220 cases in pregnant women in the United States so far: 691 in the territories and 529 in the states and D.C. Among all Americans, there have been 10,295 cases of Zika: 8,035 in the territories and 2,260 in the states/D.C., the CDC reported.

The number of Zika-related poor outcomes did not change during the week ending Aug. 11. The number of liveborn infants born with birth defects stayed at 16 in the states/D.C. and 1 in the territories, and the number of pregnancy losses with birth defects held at 5 in the states/D.C. and 1 in the territories, the CDC said. State- or territorial-level data are not being reported to protect the privacy of affected women and children.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika virus–related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
Candida auris in Venezuela outbreak is triazole-resistant, opportunistic
BOSTON – An investigation into 18 nosocomial Candida auris infections at a tertiary care center in Venezuela showed that isolates of the emerging fungal pathogen obtained during the outbreak were resistant to fluconazole and voriconazole. However, the isolates were intermediately susceptible to amphotericin B and susceptible to 5-fluorocitosine, and demonstrated high susceptibility to the candin antifungal anidulafungin.
Dr. Belinda Calvo, an infectious disease specialist at the University of Maracaibo, Venezuela, and her collaborators reported these findings, related to a 2012-2013 C. auris outbreak at the hospital. Dr. Calvo and her coinvestigators noted that other invasive C. auris outbreaks have been reported in India, Korea, and South Africa, but that “the real prevalence of this organism may be underestimated,” since common rapid microbial identification techniques may misidentify the species.
In a poster session at the annual meeting of the American Society of Microbiology, Dr. Calvo and her collaborators reported that the 18 patients involved in the Venezuelan outbreak were critically ill, of whom 11 were pediatric, and all had central venous catheter placement. All but two of the pediatric patients were neonates, and all had serious underlying morbidities; several had significant congenital anomalies. The median patient age was 26 days (range, 2 days to 72 years), reflecting the high number of neonates affected. One of the adult patients had esophageal carcinoma. Overall, 10/18 patients (56%) had undergone surgical procedures, and all had received antibiotics.
As has been reported in other C. auris outbreaks, isolates from blood cultures of affected individuals were initially reported as C. haemulonii by the Vitek 2 C automated microbial identification system. Molecular identification was completed by sequencing the internal transcribed spacer (ITS) of the rDNA gene, with analysis aided by the National Institutes of Health’s GenBank and the Netherland’s CBS Fungal Diversity Centre , in order to confirm the identity of the fungal isolates as C. auris. Dr. Calvo and her associates were able to generate a dendrogram of the 18 isolates, showing high clonality, a trait shared with other nosocomial C. auris outbreaks.
Susceptibility testing of the C. auris cultured from blood samples of the affected patients showed that fluconazole had a minimum inhibitory concentration to inhibit the growth of 50% of the organisms (MIC50) of greater than 64 mcg/mL. For fluconazole, the MIC90, range, and geometric mean were all also above 64 mcg/mL, indicating a high level of resistance. For voriconazole, the MICs, range, and mean were all 4 mcg/mL. For amphotericin B, the MIC50 was 1 mcg/mL, the MIC90 was 2 mcg/mL, the range was 1-2, and the geometric mean was 1.414 mcg/mL.
The high number of pediatric patients affected, as well as early pathogen identification with speedy and appropriate antifungal therapy and prompt removal of central venous catheters, likely contributed to the relatively low 30-day crude mortality rate of 28%, said Dr. Calvo and her coauthors.
“C. auris should be considered an emergent multiresistant species,” wrote Dr. Calbo and her collaborators, noting that the opportunistic pathogen has a “high potential for nosocomial horizontal transmission.”
In June 2016, the Centers for Disease Control issued a clinical alert to U.S. healthcare facilities regarding the global emergence of invasive infections caused by C. auris.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – An investigation into 18 nosocomial Candida auris infections at a tertiary care center in Venezuela showed that isolates of the emerging fungal pathogen obtained during the outbreak were resistant to fluconazole and voriconazole. However, the isolates were intermediately susceptible to amphotericin B and susceptible to 5-fluorocitosine, and demonstrated high susceptibility to the candin antifungal anidulafungin.
Dr. Belinda Calvo, an infectious disease specialist at the University of Maracaibo, Venezuela, and her collaborators reported these findings, related to a 2012-2013 C. auris outbreak at the hospital. Dr. Calvo and her coinvestigators noted that other invasive C. auris outbreaks have been reported in India, Korea, and South Africa, but that “the real prevalence of this organism may be underestimated,” since common rapid microbial identification techniques may misidentify the species.
In a poster session at the annual meeting of the American Society of Microbiology, Dr. Calvo and her collaborators reported that the 18 patients involved in the Venezuelan outbreak were critically ill, of whom 11 were pediatric, and all had central venous catheter placement. All but two of the pediatric patients were neonates, and all had serious underlying morbidities; several had significant congenital anomalies. The median patient age was 26 days (range, 2 days to 72 years), reflecting the high number of neonates affected. One of the adult patients had esophageal carcinoma. Overall, 10/18 patients (56%) had undergone surgical procedures, and all had received antibiotics.
As has been reported in other C. auris outbreaks, isolates from blood cultures of affected individuals were initially reported as C. haemulonii by the Vitek 2 C automated microbial identification system. Molecular identification was completed by sequencing the internal transcribed spacer (ITS) of the rDNA gene, with analysis aided by the National Institutes of Health’s GenBank and the Netherland’s CBS Fungal Diversity Centre , in order to confirm the identity of the fungal isolates as C. auris. Dr. Calvo and her associates were able to generate a dendrogram of the 18 isolates, showing high clonality, a trait shared with other nosocomial C. auris outbreaks.
Susceptibility testing of the C. auris cultured from blood samples of the affected patients showed that fluconazole had a minimum inhibitory concentration to inhibit the growth of 50% of the organisms (MIC50) of greater than 64 mcg/mL. For fluconazole, the MIC90, range, and geometric mean were all also above 64 mcg/mL, indicating a high level of resistance. For voriconazole, the MICs, range, and mean were all 4 mcg/mL. For amphotericin B, the MIC50 was 1 mcg/mL, the MIC90 was 2 mcg/mL, the range was 1-2, and the geometric mean was 1.414 mcg/mL.
The high number of pediatric patients affected, as well as early pathogen identification with speedy and appropriate antifungal therapy and prompt removal of central venous catheters, likely contributed to the relatively low 30-day crude mortality rate of 28%, said Dr. Calvo and her coauthors.
“C. auris should be considered an emergent multiresistant species,” wrote Dr. Calbo and her collaborators, noting that the opportunistic pathogen has a “high potential for nosocomial horizontal transmission.”
In June 2016, the Centers for Disease Control issued a clinical alert to U.S. healthcare facilities regarding the global emergence of invasive infections caused by C. auris.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
BOSTON – An investigation into 18 nosocomial Candida auris infections at a tertiary care center in Venezuela showed that isolates of the emerging fungal pathogen obtained during the outbreak were resistant to fluconazole and voriconazole. However, the isolates were intermediately susceptible to amphotericin B and susceptible to 5-fluorocitosine, and demonstrated high susceptibility to the candin antifungal anidulafungin.
Dr. Belinda Calvo, an infectious disease specialist at the University of Maracaibo, Venezuela, and her collaborators reported these findings, related to a 2012-2013 C. auris outbreak at the hospital. Dr. Calvo and her coinvestigators noted that other invasive C. auris outbreaks have been reported in India, Korea, and South Africa, but that “the real prevalence of this organism may be underestimated,” since common rapid microbial identification techniques may misidentify the species.
In a poster session at the annual meeting of the American Society of Microbiology, Dr. Calvo and her collaborators reported that the 18 patients involved in the Venezuelan outbreak were critically ill, of whom 11 were pediatric, and all had central venous catheter placement. All but two of the pediatric patients were neonates, and all had serious underlying morbidities; several had significant congenital anomalies. The median patient age was 26 days (range, 2 days to 72 years), reflecting the high number of neonates affected. One of the adult patients had esophageal carcinoma. Overall, 10/18 patients (56%) had undergone surgical procedures, and all had received antibiotics.
As has been reported in other C. auris outbreaks, isolates from blood cultures of affected individuals were initially reported as C. haemulonii by the Vitek 2 C automated microbial identification system. Molecular identification was completed by sequencing the internal transcribed spacer (ITS) of the rDNA gene, with analysis aided by the National Institutes of Health’s GenBank and the Netherland’s CBS Fungal Diversity Centre , in order to confirm the identity of the fungal isolates as C. auris. Dr. Calvo and her associates were able to generate a dendrogram of the 18 isolates, showing high clonality, a trait shared with other nosocomial C. auris outbreaks.
Susceptibility testing of the C. auris cultured from blood samples of the affected patients showed that fluconazole had a minimum inhibitory concentration to inhibit the growth of 50% of the organisms (MIC50) of greater than 64 mcg/mL. For fluconazole, the MIC90, range, and geometric mean were all also above 64 mcg/mL, indicating a high level of resistance. For voriconazole, the MICs, range, and mean were all 4 mcg/mL. For amphotericin B, the MIC50 was 1 mcg/mL, the MIC90 was 2 mcg/mL, the range was 1-2, and the geometric mean was 1.414 mcg/mL.
The high number of pediatric patients affected, as well as early pathogen identification with speedy and appropriate antifungal therapy and prompt removal of central venous catheters, likely contributed to the relatively low 30-day crude mortality rate of 28%, said Dr. Calvo and her coauthors.
“C. auris should be considered an emergent multiresistant species,” wrote Dr. Calbo and her collaborators, noting that the opportunistic pathogen has a “high potential for nosocomial horizontal transmission.”
In June 2016, the Centers for Disease Control issued a clinical alert to U.S. healthcare facilities regarding the global emergence of invasive infections caused by C. auris.
The study authors reported no external sources of funding and no conflicts of interest.
On Twitter @karioakes
AT ASM 2016
Key clinical point: Isolates in an outbreak of nosocomially acquired Candida auris were fluconazole-resistant.
Major finding: All C. auris isolates were resistant to fluconazole, with geometric mean minimum inhibitory concentrations greater than 64 mcg/mL.
Data source: Retrospective, single-center study of 18 pediatric and adult patients with C. auris infections at a tertiary care center in Venezuela.
Disclosures: The study investigators reported no outside sources of funding and no disclosures.
CDC reports two new cases of Zika-related birth defects
Two live-born infants with Zika virus–related birth defects were reported the week ending Aug. 4, 2016, bringing the U.S. total to 17, the Centers for Disease Control and Prevention reported.
One of the two infants was born in the 50 states and the District of Columbia, and the other was the first live-born infant with Zika-related birth defects born in the U.S. territories. State- or territorial-level data are not being reported to protect the privacy of affected women and children, the CDC said.
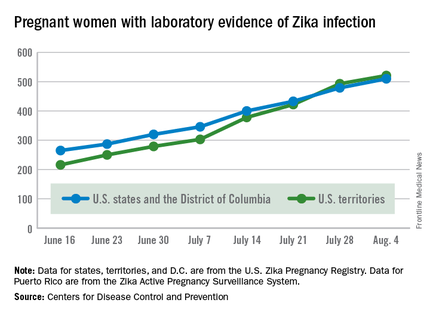
There were no new pregnancy losses among Zika-infected women in the territories, keeping the total at one for the year, but the CDC seems to have adjusted the number of pregnancy losses for the states and D.C., as the most recent total for the year is now five, after six were reported the previous week.
A total of 59 new cases of Zika infection in pregnant women were reported for the week ending Aug. 4: 31 new cases in the states/D.C. and 28 in the territories. For the year, there have been 510 cases of pregnant women with laboratory evidence of Zika infection in the states/D.C. and 521 in the territories, for a U.S. total of 1,031, the CDC reported.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika virus–related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
Two live-born infants with Zika virus–related birth defects were reported the week ending Aug. 4, 2016, bringing the U.S. total to 17, the Centers for Disease Control and Prevention reported.
One of the two infants was born in the 50 states and the District of Columbia, and the other was the first live-born infant with Zika-related birth defects born in the U.S. territories. State- or territorial-level data are not being reported to protect the privacy of affected women and children, the CDC said.

There were no new pregnancy losses among Zika-infected women in the territories, keeping the total at one for the year, but the CDC seems to have adjusted the number of pregnancy losses for the states and D.C., as the most recent total for the year is now five, after six were reported the previous week.
A total of 59 new cases of Zika infection in pregnant women were reported for the week ending Aug. 4: 31 new cases in the states/D.C. and 28 in the territories. For the year, there have been 510 cases of pregnant women with laboratory evidence of Zika infection in the states/D.C. and 521 in the territories, for a U.S. total of 1,031, the CDC reported.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika virus–related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
Two live-born infants with Zika virus–related birth defects were reported the week ending Aug. 4, 2016, bringing the U.S. total to 17, the Centers for Disease Control and Prevention reported.
One of the two infants was born in the 50 states and the District of Columbia, and the other was the first live-born infant with Zika-related birth defects born in the U.S. territories. State- or territorial-level data are not being reported to protect the privacy of affected women and children, the CDC said.

There were no new pregnancy losses among Zika-infected women in the territories, keeping the total at one for the year, but the CDC seems to have adjusted the number of pregnancy losses for the states and D.C., as the most recent total for the year is now five, after six were reported the previous week.
A total of 59 new cases of Zika infection in pregnant women were reported for the week ending Aug. 4: 31 new cases in the states/D.C. and 28 in the territories. For the year, there have been 510 cases of pregnant women with laboratory evidence of Zika infection in the states/D.C. and 521 in the territories, for a U.S. total of 1,031, the CDC reported.
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika virus–related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
West Nile testing underutilized in endemic areas
Testing for West Nile virus (WNV) is underutilized in areas where the disease is endemic, according to Jakapat Vanichanan, MD, and his associates.
In a sample of 751 patients admitted to Houston hospitals for meningitis or encephalitis, 390 patients experienced onset of symptoms during the WNV peak season between June and October, but only 281 of the 751 patients received WNV testing. Of the 281 patients tested for WNV, 32 were diagnosed with acute infection.
Patients tested for WNV were more likely to have acute focal neurologic deficits, be of older age, require an MRI, be prescribed antiviral therapy, have worse clinical outcomes, and experience concomitant testing for mycobacterial, fungal, or other viral infections. Of the 32 patients diagnosed with WNV, 16 were admitted with meningitis and 16 were admitted with encephalitis.
“Although supportive treatment remains the standard of care for patients with WNND [West Nile neuroinvasive diseases], performing appropriate WNV testing may yield several benefits. An accurate diagnosis more precisely defines disease burden and epidemiology, an ongoing surveillance deficiency. Moreover, identifying WNV may lead to early detection of long-term neurologic and neurocognitive sequelae after WNND and thus enable earlier intervention,” the investigators said.
Find the full study in Emerging Infectious Diseases (doi: 10.3201/eid2209.152050).
Testing for West Nile virus (WNV) is underutilized in areas where the disease is endemic, according to Jakapat Vanichanan, MD, and his associates.
In a sample of 751 patients admitted to Houston hospitals for meningitis or encephalitis, 390 patients experienced onset of symptoms during the WNV peak season between June and October, but only 281 of the 751 patients received WNV testing. Of the 281 patients tested for WNV, 32 were diagnosed with acute infection.
Patients tested for WNV were more likely to have acute focal neurologic deficits, be of older age, require an MRI, be prescribed antiviral therapy, have worse clinical outcomes, and experience concomitant testing for mycobacterial, fungal, or other viral infections. Of the 32 patients diagnosed with WNV, 16 were admitted with meningitis and 16 were admitted with encephalitis.
“Although supportive treatment remains the standard of care for patients with WNND [West Nile neuroinvasive diseases], performing appropriate WNV testing may yield several benefits. An accurate diagnosis more precisely defines disease burden and epidemiology, an ongoing surveillance deficiency. Moreover, identifying WNV may lead to early detection of long-term neurologic and neurocognitive sequelae after WNND and thus enable earlier intervention,” the investigators said.
Find the full study in Emerging Infectious Diseases (doi: 10.3201/eid2209.152050).
Testing for West Nile virus (WNV) is underutilized in areas where the disease is endemic, according to Jakapat Vanichanan, MD, and his associates.
In a sample of 751 patients admitted to Houston hospitals for meningitis or encephalitis, 390 patients experienced onset of symptoms during the WNV peak season between June and October, but only 281 of the 751 patients received WNV testing. Of the 281 patients tested for WNV, 32 were diagnosed with acute infection.
Patients tested for WNV were more likely to have acute focal neurologic deficits, be of older age, require an MRI, be prescribed antiviral therapy, have worse clinical outcomes, and experience concomitant testing for mycobacterial, fungal, or other viral infections. Of the 32 patients diagnosed with WNV, 16 were admitted with meningitis and 16 were admitted with encephalitis.
“Although supportive treatment remains the standard of care for patients with WNND [West Nile neuroinvasive diseases], performing appropriate WNV testing may yield several benefits. An accurate diagnosis more precisely defines disease burden and epidemiology, an ongoing surveillance deficiency. Moreover, identifying WNV may lead to early detection of long-term neurologic and neurocognitive sequelae after WNND and thus enable earlier intervention,” the investigators said.
Find the full study in Emerging Infectious Diseases (doi: 10.3201/eid2209.152050).
FROM EMERGING INFECTIOUS DISEASES
Study evaluates Zika syndrome with joint contractures
Congenital Zika syndrome should be added to the differential diagnosis of congenital infections and arthrogryposis (joint contractures), results from a case series study in Brazil suggest.
“Brain impairment in the presence of microcephaly is the main characteristic of a congenital Zika virus syndrome,” researchers led by Vanessa van der Linden, MD, wrote in a study published online Aug. 9 in the BMJ. “However, little is still known about this condition and its clinical spectrum, which also concerns newborns with a normal head circumference. Two studies have described the association between arthrogryposis and microcephaly in newborns presumed to have congenital Zika virus infection [See Morb Mortal Wkly. Rep. 2016;65:59-62 and Ultrasound Obstet Gynecol. 2016;47:6-7].” The authors went on to note that while arthrogryposis might be considered more of a sign than a specific disease, “it might be associated with several disorders. However, there are no reports in the literature about other congenital infections in humans associated with arthrogryposis.”
Dr. van der Linden, a pediatric neurologist with the Association for Assistance of Disabled Children, Recife, Brazil, and her associates retrospectively evaluated the medical records of seven patients with arthrogryposis associated with congenital infection believed to be caused by Zika virus, during the Brazilian microcephaly epidemic (BMJ. 2016;354:i3899). The main outcomes of interest were clinical, radiologic, and electromyographic findings, and likely collaboration between clinical and primary neurological abnormalities.
The researchers reported that brain images of all seven children revealed characteristics of congenital infection and arthrogryposis. Two children (29%) tested positive for IgM antibody for Zika virus in the cerebrospinal fluid, while arthrogryposis was present in the arms and legs of six children (86%) and in the legs of one child (14%). In addition, hip x-rays showed bilateral dislocation in all seven children and subluxation of the knee associated with genu valgus in three (43%). No evidence of abnormalities was seen on high-definition ultrasonography of the joints, but moderate signs of remodeling of the motor units and a reduced recruitment pattern were found on needle electromyography. Results from brain computed tomography conducted in all seven patients and magnetic resonance imaging conducted in five revealed malformations of cortical development, calcifications predominantly in the cortex and subcortical white matter, reduction in brain volume, ventriculomegaly, and hypoplasia of the brainstem and cerebellum. Spinal MRI conducted in four children showed apparent thinning of the cord and reduced ventral roots.
“Further research is needed with a larger number of cases to study the neurological abnormalities behind arthrogryposis, including histopathology of autopsy samples or tissues from stillborn babies,” the researchers concluded. “As we do not know the potential implications of congenital Zika virus infection as it evolves, children must receive orthopedic follow-up, even those with a standard first orthopedic evaluation, because they could develop musculoskeletal deformities secondary to neurological impairment, central or peripheral, or both, as these occur in patients with cerebral palsy and other chronic encephalopathies.”
The researchers reported having no financial disclosures.
Congenital Zika syndrome should be added to the differential diagnosis of congenital infections and arthrogryposis (joint contractures), results from a case series study in Brazil suggest.
“Brain impairment in the presence of microcephaly is the main characteristic of a congenital Zika virus syndrome,” researchers led by Vanessa van der Linden, MD, wrote in a study published online Aug. 9 in the BMJ. “However, little is still known about this condition and its clinical spectrum, which also concerns newborns with a normal head circumference. Two studies have described the association between arthrogryposis and microcephaly in newborns presumed to have congenital Zika virus infection [See Morb Mortal Wkly. Rep. 2016;65:59-62 and Ultrasound Obstet Gynecol. 2016;47:6-7].” The authors went on to note that while arthrogryposis might be considered more of a sign than a specific disease, “it might be associated with several disorders. However, there are no reports in the literature about other congenital infections in humans associated with arthrogryposis.”
Dr. van der Linden, a pediatric neurologist with the Association for Assistance of Disabled Children, Recife, Brazil, and her associates retrospectively evaluated the medical records of seven patients with arthrogryposis associated with congenital infection believed to be caused by Zika virus, during the Brazilian microcephaly epidemic (BMJ. 2016;354:i3899). The main outcomes of interest were clinical, radiologic, and electromyographic findings, and likely collaboration between clinical and primary neurological abnormalities.
The researchers reported that brain images of all seven children revealed characteristics of congenital infection and arthrogryposis. Two children (29%) tested positive for IgM antibody for Zika virus in the cerebrospinal fluid, while arthrogryposis was present in the arms and legs of six children (86%) and in the legs of one child (14%). In addition, hip x-rays showed bilateral dislocation in all seven children and subluxation of the knee associated with genu valgus in three (43%). No evidence of abnormalities was seen on high-definition ultrasonography of the joints, but moderate signs of remodeling of the motor units and a reduced recruitment pattern were found on needle electromyography. Results from brain computed tomography conducted in all seven patients and magnetic resonance imaging conducted in five revealed malformations of cortical development, calcifications predominantly in the cortex and subcortical white matter, reduction in brain volume, ventriculomegaly, and hypoplasia of the brainstem and cerebellum. Spinal MRI conducted in four children showed apparent thinning of the cord and reduced ventral roots.
“Further research is needed with a larger number of cases to study the neurological abnormalities behind arthrogryposis, including histopathology of autopsy samples or tissues from stillborn babies,” the researchers concluded. “As we do not know the potential implications of congenital Zika virus infection as it evolves, children must receive orthopedic follow-up, even those with a standard first orthopedic evaluation, because they could develop musculoskeletal deformities secondary to neurological impairment, central or peripheral, or both, as these occur in patients with cerebral palsy and other chronic encephalopathies.”
The researchers reported having no financial disclosures.
Congenital Zika syndrome should be added to the differential diagnosis of congenital infections and arthrogryposis (joint contractures), results from a case series study in Brazil suggest.
“Brain impairment in the presence of microcephaly is the main characteristic of a congenital Zika virus syndrome,” researchers led by Vanessa van der Linden, MD, wrote in a study published online Aug. 9 in the BMJ. “However, little is still known about this condition and its clinical spectrum, which also concerns newborns with a normal head circumference. Two studies have described the association between arthrogryposis and microcephaly in newborns presumed to have congenital Zika virus infection [See Morb Mortal Wkly. Rep. 2016;65:59-62 and Ultrasound Obstet Gynecol. 2016;47:6-7].” The authors went on to note that while arthrogryposis might be considered more of a sign than a specific disease, “it might be associated with several disorders. However, there are no reports in the literature about other congenital infections in humans associated with arthrogryposis.”
Dr. van der Linden, a pediatric neurologist with the Association for Assistance of Disabled Children, Recife, Brazil, and her associates retrospectively evaluated the medical records of seven patients with arthrogryposis associated with congenital infection believed to be caused by Zika virus, during the Brazilian microcephaly epidemic (BMJ. 2016;354:i3899). The main outcomes of interest were clinical, radiologic, and electromyographic findings, and likely collaboration between clinical and primary neurological abnormalities.
The researchers reported that brain images of all seven children revealed characteristics of congenital infection and arthrogryposis. Two children (29%) tested positive for IgM antibody for Zika virus in the cerebrospinal fluid, while arthrogryposis was present in the arms and legs of six children (86%) and in the legs of one child (14%). In addition, hip x-rays showed bilateral dislocation in all seven children and subluxation of the knee associated with genu valgus in three (43%). No evidence of abnormalities was seen on high-definition ultrasonography of the joints, but moderate signs of remodeling of the motor units and a reduced recruitment pattern were found on needle electromyography. Results from brain computed tomography conducted in all seven patients and magnetic resonance imaging conducted in five revealed malformations of cortical development, calcifications predominantly in the cortex and subcortical white matter, reduction in brain volume, ventriculomegaly, and hypoplasia of the brainstem and cerebellum. Spinal MRI conducted in four children showed apparent thinning of the cord and reduced ventral roots.
“Further research is needed with a larger number of cases to study the neurological abnormalities behind arthrogryposis, including histopathology of autopsy samples or tissues from stillborn babies,” the researchers concluded. “As we do not know the potential implications of congenital Zika virus infection as it evolves, children must receive orthopedic follow-up, even those with a standard first orthopedic evaluation, because they could develop musculoskeletal deformities secondary to neurological impairment, central or peripheral, or both, as these occur in patients with cerebral palsy and other chronic encephalopathies.”
The researchers reported having no financial disclosures.
FROM BMJ
Key clinical point: The differential diagnosis of congenital infections and arthrogryposis should include congenital Zika syndrome.
Major finding: All seven children revealed characteristics of congenital infection and arthrogryposis. Two children (29%) tested positive for IgM antibody for Zika virus in the cerebrospinal fluid, while arthrogryposis was present in the arms and legs of six children (86%) and in the legs of one child (14%).
Data source: A retrospective case series study of seven children with arthrogryposis associated with congenital infection believed to be caused by Zika virus.
Disclosures: The researchers reported having no financial disclosures.
Blood viral RNA may indicate severity of MERS coronavirus clinical course
The presence of blood viral RNA in patients presenting with possible Middle East respiratory syndrome coronavirus (MERS-CoV) may be a very reliable indicator of the severity of the infection’s clinical course, according to a new study published in Emerging Infectious Diseases.
“Our study aimed to evaluate the diagnostic utility of blood specimens for MERS-CoV infection by using large numbers of patients with a single viral origin and to determine the relationship between blood viral detection and clinical characteristics,” wrote the authors, led by So Yeon Kim, MD, of the National Medical Center in Seoul, South Korea.
The investigators recruited 21 MERS-CoV patients within South Korea, all of whom had been previously diagnosed by the Korea Centers for Disease Control and Prevention via respiratory samples and were of “a single viral origin.” All subjects contributed ethylenediaminetetraacetic acid (EDTA)-treated whole blood and serum specimens, from which viral RNA was extracted (Emerg Infect Dis. 2016 Oct 15;22[10]. doi: 10.3201/eid2210.160218).
Viral RNA was detected in 6 of 21 whole blood samples and 6 of 21 serum samples at hospital admission. However, because two patients were viral positive in either specimen subtype of EDTA-treated whole blood or serum, the overall detection rate for the population was 7 of 21 (33%). Being positive for blood viral RNA at admission was found to be associated with a fever of higher than 37.5 °C (99.5 °F) on the date of sample collection (P = .007), being placed on mechanical ventilation at some point during the clinical course (P = .003), extracorporeal membrane oxygenation (P = .025), and death (P = .025).
However, “between the blood viral RNA-positive and -negative groups, we found no differences in age, duration from symptom onset to diagnosis of MERS-CoV infection, or an invasive procedure before the specimens were obtained,” the investigators noted.
The takeaway, the authors underscore, is that although early blood viral RNA presence may not be a useful diagnostic tool, it “might be a good prognostic indicator of severe outcome” due to its high association with worse clinical course.
The research was funded by the National Medical Center Research Institute.
The presence of blood viral RNA in patients presenting with possible Middle East respiratory syndrome coronavirus (MERS-CoV) may be a very reliable indicator of the severity of the infection’s clinical course, according to a new study published in Emerging Infectious Diseases.
“Our study aimed to evaluate the diagnostic utility of blood specimens for MERS-CoV infection by using large numbers of patients with a single viral origin and to determine the relationship between blood viral detection and clinical characteristics,” wrote the authors, led by So Yeon Kim, MD, of the National Medical Center in Seoul, South Korea.
The investigators recruited 21 MERS-CoV patients within South Korea, all of whom had been previously diagnosed by the Korea Centers for Disease Control and Prevention via respiratory samples and were of “a single viral origin.” All subjects contributed ethylenediaminetetraacetic acid (EDTA)-treated whole blood and serum specimens, from which viral RNA was extracted (Emerg Infect Dis. 2016 Oct 15;22[10]. doi: 10.3201/eid2210.160218).
Viral RNA was detected in 6 of 21 whole blood samples and 6 of 21 serum samples at hospital admission. However, because two patients were viral positive in either specimen subtype of EDTA-treated whole blood or serum, the overall detection rate for the population was 7 of 21 (33%). Being positive for blood viral RNA at admission was found to be associated with a fever of higher than 37.5 °C (99.5 °F) on the date of sample collection (P = .007), being placed on mechanical ventilation at some point during the clinical course (P = .003), extracorporeal membrane oxygenation (P = .025), and death (P = .025).
However, “between the blood viral RNA-positive and -negative groups, we found no differences in age, duration from symptom onset to diagnosis of MERS-CoV infection, or an invasive procedure before the specimens were obtained,” the investigators noted.
The takeaway, the authors underscore, is that although early blood viral RNA presence may not be a useful diagnostic tool, it “might be a good prognostic indicator of severe outcome” due to its high association with worse clinical course.
The research was funded by the National Medical Center Research Institute.
The presence of blood viral RNA in patients presenting with possible Middle East respiratory syndrome coronavirus (MERS-CoV) may be a very reliable indicator of the severity of the infection’s clinical course, according to a new study published in Emerging Infectious Diseases.
“Our study aimed to evaluate the diagnostic utility of blood specimens for MERS-CoV infection by using large numbers of patients with a single viral origin and to determine the relationship between blood viral detection and clinical characteristics,” wrote the authors, led by So Yeon Kim, MD, of the National Medical Center in Seoul, South Korea.
The investigators recruited 21 MERS-CoV patients within South Korea, all of whom had been previously diagnosed by the Korea Centers for Disease Control and Prevention via respiratory samples and were of “a single viral origin.” All subjects contributed ethylenediaminetetraacetic acid (EDTA)-treated whole blood and serum specimens, from which viral RNA was extracted (Emerg Infect Dis. 2016 Oct 15;22[10]. doi: 10.3201/eid2210.160218).
Viral RNA was detected in 6 of 21 whole blood samples and 6 of 21 serum samples at hospital admission. However, because two patients were viral positive in either specimen subtype of EDTA-treated whole blood or serum, the overall detection rate for the population was 7 of 21 (33%). Being positive for blood viral RNA at admission was found to be associated with a fever of higher than 37.5 °C (99.5 °F) on the date of sample collection (P = .007), being placed on mechanical ventilation at some point during the clinical course (P = .003), extracorporeal membrane oxygenation (P = .025), and death (P = .025).
However, “between the blood viral RNA-positive and -negative groups, we found no differences in age, duration from symptom onset to diagnosis of MERS-CoV infection, or an invasive procedure before the specimens were obtained,” the investigators noted.
The takeaway, the authors underscore, is that although early blood viral RNA presence may not be a useful diagnostic tool, it “might be a good prognostic indicator of severe outcome” due to its high association with worse clinical course.
The research was funded by the National Medical Center Research Institute.
FROM EMERGING INFECTIOUS DISEASES
Key clinical point: Checking for blood viral RNA at hospital admission may be a reliable indicator of the severity of MERS coronavirus infection clinical course.
Major finding: Blood viral RNA positivity at admission was associated with fever higher than 37.5 °C on the sampling date (P = .007), requirement for mechanical ventilation during the following clinical course (P = .003), extracorporeal membrane oxygenation (P = .025), and patient death (P = .025).
Data source: Prospective analysis of 21 patients with Middle East respiratory syndrome coronavirus (MERS-CoV).
Disclosures: The research was funded by the National Medical Center Research Institute.
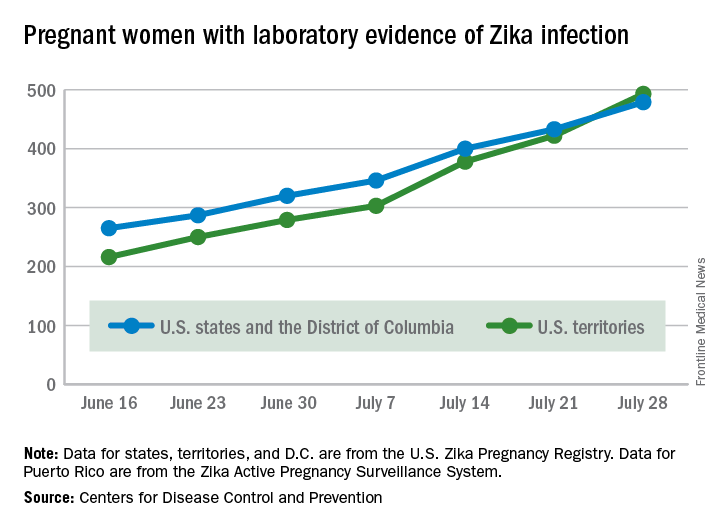
Territories now have U.S. majority of pregnant women with Zika
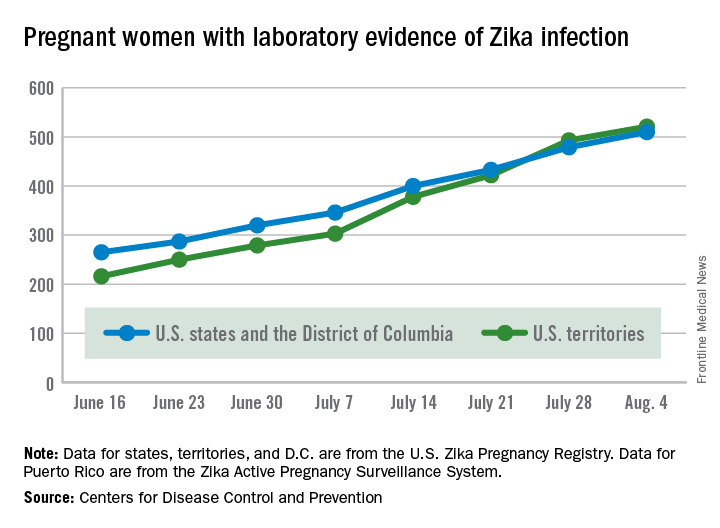
The total number of pregnant women with evidence of Zika virus infection reported in the U.S. territories surpassed that of the 50 states and the District of Columbia during the week ending July 28, 2016, according to the Centers for Disease Control and Prevention.
There were 71 new cases of Zika in pregnant women reported in U.S. territories that week, bringing the total for the year to 493. The states and D.C. reported 46 new cases, for a total of 479 for the year, which puts the United States as a whole at 972 cases of confirmed Zika virus infection in pregnant women for 2016, the CDC reported Aug. 4.
Among the territories, the overwhelming majority of Zika cases are in Puerto Rico, which has reported 5,482 cases so far, compared with 44 in American Samoa and 22 in the U.S. Virgin Islands. In all, there have been 1,825 cases reported in the states and D.C., the CDC reported.
The territories, so far, have mostly avoided Zika-related pregnancy losses and birth defects, with only one case of pregnancy loss and no infants born with birth defects in 2016. Two more cases of infants born with birth defects were reported, however, in the states and D.C. for the week ending July 28, bringing the state/D.C. total to 15 for the year, but no new pregnancy losses with Zika-related birth defects were added to the six reported so far, the CDC announced.
“These outcomes occurred in pregnancies with laboratory evidence of Zika virus infection,” the CDC noted, and it is not known “whether they were caused by Zika virus infection or other factors.”
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The total number of pregnant women with evidence of Zika virus infection reported in the U.S. territories surpassed that of the 50 states and the District of Columbia during the week ending July 28, 2016, according to the Centers for Disease Control and Prevention.
There were 71 new cases of Zika in pregnant women reported in U.S. territories that week, bringing the total for the year to 493. The states and D.C. reported 46 new cases, for a total of 479 for the year, which puts the United States as a whole at 972 cases of confirmed Zika virus infection in pregnant women for 2016, the CDC reported Aug. 4.
Among the territories, the overwhelming majority of Zika cases are in Puerto Rico, which has reported 5,482 cases so far, compared with 44 in American Samoa and 22 in the U.S. Virgin Islands. In all, there have been 1,825 cases reported in the states and D.C., the CDC reported.
The territories, so far, have mostly avoided Zika-related pregnancy losses and birth defects, with only one case of pregnancy loss and no infants born with birth defects in 2016. Two more cases of infants born with birth defects were reported, however, in the states and D.C. for the week ending July 28, bringing the state/D.C. total to 15 for the year, but no new pregnancy losses with Zika-related birth defects were added to the six reported so far, the CDC announced.
“These outcomes occurred in pregnancies with laboratory evidence of Zika virus infection,” the CDC noted, and it is not known “whether they were caused by Zika virus infection or other factors.”
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
The total number of pregnant women with evidence of Zika virus infection reported in the U.S. territories surpassed that of the 50 states and the District of Columbia during the week ending July 28, 2016, according to the Centers for Disease Control and Prevention.
There were 71 new cases of Zika in pregnant women reported in U.S. territories that week, bringing the total for the year to 493. The states and D.C. reported 46 new cases, for a total of 479 for the year, which puts the United States as a whole at 972 cases of confirmed Zika virus infection in pregnant women for 2016, the CDC reported Aug. 4.
Among the territories, the overwhelming majority of Zika cases are in Puerto Rico, which has reported 5,482 cases so far, compared with 44 in American Samoa and 22 in the U.S. Virgin Islands. In all, there have been 1,825 cases reported in the states and D.C., the CDC reported.
The territories, so far, have mostly avoided Zika-related pregnancy losses and birth defects, with only one case of pregnancy loss and no infants born with birth defects in 2016. Two more cases of infants born with birth defects were reported, however, in the states and D.C. for the week ending July 28, bringing the state/D.C. total to 15 for the year, but no new pregnancy losses with Zika-related birth defects were added to the six reported so far, the CDC announced.
“These outcomes occurred in pregnancies with laboratory evidence of Zika virus infection,” the CDC noted, and it is not known “whether they were caused by Zika virus infection or other factors.”
The figures for states, territories, and D.C. reflect reporting to the U.S. Zika Pregnancy Registry; data for Puerto Rico are reported to the U.S. Zika Active Pregnancy Surveillance System.
Zika-related birth defects recorded by the CDC could include microcephaly, calcium deposits in the brain indicating possible brain damage, excess fluid in the brain cavities and surrounding the brain, absent or poorly formed brain structures, abnormal eye development, or other problems resulting from brain damage that affect nerves, muscles, and bones. The pregnancy losses encompass any miscarriage, stillbirth, and termination with evidence of birth defects.
NIH launches trial of Zika vaccine candidate
A clinical trial to evaluate a candidate vaccine for Zika virus is underway, with preliminary results from the multisite phase I trial expected by the end of 2016.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, announced the trial and gave an update about other Zika virus vaccine development efforts during an Aug. 3 telephone briefing with reporters. The announcement came just days after the continental United States saw its first cases of local transmission of Zika virus in Florida.
The phase I clinical trial began on Aug. 2 and will evaluate the safety and immunogenicity of an investigational DNA vaccine for Zika virus. This vaccine is similar to one that early-stage trials have shown to be safe and immunogenic for West Nile virus, a flavivirus closely related to Zika. The Zika virus vaccine had promising preclinical results, Dr. Fauci said. “Preliminary immune responses that we’ve seen in a variety of animal models have been rather robust.”
The DNA vaccine uses a plasmid to deliver genes that code for specific Zika virus proteins. “When the plasmid expresses the envelope protein from the Zika virus, it does so in a way that mimics the virus,” provoking a host immune response that includes neutralizing antibodies and T cells, according to lead trial investigator Julie Ledgerwood, DO, chief of the clinical trials program at the NIAID’s Vaccine Research Center.
The phase I clinical trial will enroll 80 healthy volunteers, aged 18-35 years. All participants will receive the investigational Zika virus vaccine at their first visit, with vaccine delivery via a needle-free system that injects the vaccine directly into the deltoid muscle.
Forty participants will receive one additional dose of the vaccine, with half of those people receiving the additional dose at 8 weeks after the first vaccine, and the other half receiving the additional dose at 12 weeks after the first dose. The other 40 participants will receive two additional doses, with half receiving them at 4 and 8 weeks after the first vaccination, and the other half receiving the extra doses at 4 and 20 weeks. All participants will receive the same dose at each vaccination.
Participants will be asked to monitor their temperature daily for a week after each vaccine dose is received; they will also report any adverse events. The trial design provides for several follow-up visits to track safety and to measure immune response in 44 weeks of short-term follow-up. Additionally, two follow-up visits at 18 months and 2 years post vaccination will measure the durability of the immune response.
The clinical trial will be run at the National Institutes of Health Clinical Center in Bethesda, Md., at Emory University in Atlanta, and at the University of Maryland’s Center for Vaccine Development in Baltimore.
Initial safety and immune response data should be available by the end of 2016, Dr. Fauci said. If these data are promising, a phase II clinical trial will be launched in early 2017 in several Zika-endemic countries. The NIH has the in-house capability to manufacture the 2,500-5,000 doses of vaccine that the phase II trial is expected to require.
However, said Dr. Fauci, without congressional approval of additional funding for Zika virus vaccine efforts, the transition to a phase II clinical trial is far from certain. Interruption in funding would be “effectively impeding our smooth process on the vaccine development front,” he said. “When I say we are going to run out of money soon, I mean really soon.”
Dr. Fauci, in response to questions, said that he continues to be bullish on such platform-based approaches to vaccines. The DNA strategy, in particular, represents “a very convenient and easily scalable vaccine,” he said. “We are moving much more toward platform-based vaccines … because of their ease and convenience, and scaling up and rapidity.”
The scope of a vaccination program will depend on the endemicity of the virus in a given area, said Dr. Fauci. In an endemic area, “Ultimately, you want to get women of childbearing age, as well as their sexual partners,” he said. This means that target populations will be as young as possible, to make sure women and their partners are vaccinated by the time pregnancy becomes a possibility.
Other Zika virus vaccine development efforts underway at NIAID include a strategy that uses a live attenuated virus, an approach used for a dengue virus vaccine that is currently in a phase 3 clinical trial in Brazil, according to the agency’s website. Dengue and Zika are closely related viruses. Another investigational Zika virus vaccine uses genetic engineering to create a vaccine derived from vesicular stomatitis virus, which infects cattle. This strategy, also used in one approach to Ebola vaccination, is in the pre-clinical stage.
On Twitter @karioakes
A clinical trial to evaluate a candidate vaccine for Zika virus is underway, with preliminary results from the multisite phase I trial expected by the end of 2016.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, announced the trial and gave an update about other Zika virus vaccine development efforts during an Aug. 3 telephone briefing with reporters. The announcement came just days after the continental United States saw its first cases of local transmission of Zika virus in Florida.
The phase I clinical trial began on Aug. 2 and will evaluate the safety and immunogenicity of an investigational DNA vaccine for Zika virus. This vaccine is similar to one that early-stage trials have shown to be safe and immunogenic for West Nile virus, a flavivirus closely related to Zika. The Zika virus vaccine had promising preclinical results, Dr. Fauci said. “Preliminary immune responses that we’ve seen in a variety of animal models have been rather robust.”
The DNA vaccine uses a plasmid to deliver genes that code for specific Zika virus proteins. “When the plasmid expresses the envelope protein from the Zika virus, it does so in a way that mimics the virus,” provoking a host immune response that includes neutralizing antibodies and T cells, according to lead trial investigator Julie Ledgerwood, DO, chief of the clinical trials program at the NIAID’s Vaccine Research Center.
The phase I clinical trial will enroll 80 healthy volunteers, aged 18-35 years. All participants will receive the investigational Zika virus vaccine at their first visit, with vaccine delivery via a needle-free system that injects the vaccine directly into the deltoid muscle.
Forty participants will receive one additional dose of the vaccine, with half of those people receiving the additional dose at 8 weeks after the first vaccine, and the other half receiving the additional dose at 12 weeks after the first dose. The other 40 participants will receive two additional doses, with half receiving them at 4 and 8 weeks after the first vaccination, and the other half receiving the extra doses at 4 and 20 weeks. All participants will receive the same dose at each vaccination.
Participants will be asked to monitor their temperature daily for a week after each vaccine dose is received; they will also report any adverse events. The trial design provides for several follow-up visits to track safety and to measure immune response in 44 weeks of short-term follow-up. Additionally, two follow-up visits at 18 months and 2 years post vaccination will measure the durability of the immune response.
The clinical trial will be run at the National Institutes of Health Clinical Center in Bethesda, Md., at Emory University in Atlanta, and at the University of Maryland’s Center for Vaccine Development in Baltimore.
Initial safety and immune response data should be available by the end of 2016, Dr. Fauci said. If these data are promising, a phase II clinical trial will be launched in early 2017 in several Zika-endemic countries. The NIH has the in-house capability to manufacture the 2,500-5,000 doses of vaccine that the phase II trial is expected to require.
However, said Dr. Fauci, without congressional approval of additional funding for Zika virus vaccine efforts, the transition to a phase II clinical trial is far from certain. Interruption in funding would be “effectively impeding our smooth process on the vaccine development front,” he said. “When I say we are going to run out of money soon, I mean really soon.”
Dr. Fauci, in response to questions, said that he continues to be bullish on such platform-based approaches to vaccines. The DNA strategy, in particular, represents “a very convenient and easily scalable vaccine,” he said. “We are moving much more toward platform-based vaccines … because of their ease and convenience, and scaling up and rapidity.”
The scope of a vaccination program will depend on the endemicity of the virus in a given area, said Dr. Fauci. In an endemic area, “Ultimately, you want to get women of childbearing age, as well as their sexual partners,” he said. This means that target populations will be as young as possible, to make sure women and their partners are vaccinated by the time pregnancy becomes a possibility.
Other Zika virus vaccine development efforts underway at NIAID include a strategy that uses a live attenuated virus, an approach used for a dengue virus vaccine that is currently in a phase 3 clinical trial in Brazil, according to the agency’s website. Dengue and Zika are closely related viruses. Another investigational Zika virus vaccine uses genetic engineering to create a vaccine derived from vesicular stomatitis virus, which infects cattle. This strategy, also used in one approach to Ebola vaccination, is in the pre-clinical stage.
On Twitter @karioakes
A clinical trial to evaluate a candidate vaccine for Zika virus is underway, with preliminary results from the multisite phase I trial expected by the end of 2016.
Anthony S. Fauci, MD, director of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health, announced the trial and gave an update about other Zika virus vaccine development efforts during an Aug. 3 telephone briefing with reporters. The announcement came just days after the continental United States saw its first cases of local transmission of Zika virus in Florida.
The phase I clinical trial began on Aug. 2 and will evaluate the safety and immunogenicity of an investigational DNA vaccine for Zika virus. This vaccine is similar to one that early-stage trials have shown to be safe and immunogenic for West Nile virus, a flavivirus closely related to Zika. The Zika virus vaccine had promising preclinical results, Dr. Fauci said. “Preliminary immune responses that we’ve seen in a variety of animal models have been rather robust.”
The DNA vaccine uses a plasmid to deliver genes that code for specific Zika virus proteins. “When the plasmid expresses the envelope protein from the Zika virus, it does so in a way that mimics the virus,” provoking a host immune response that includes neutralizing antibodies and T cells, according to lead trial investigator Julie Ledgerwood, DO, chief of the clinical trials program at the NIAID’s Vaccine Research Center.
The phase I clinical trial will enroll 80 healthy volunteers, aged 18-35 years. All participants will receive the investigational Zika virus vaccine at their first visit, with vaccine delivery via a needle-free system that injects the vaccine directly into the deltoid muscle.
Forty participants will receive one additional dose of the vaccine, with half of those people receiving the additional dose at 8 weeks after the first vaccine, and the other half receiving the additional dose at 12 weeks after the first dose. The other 40 participants will receive two additional doses, with half receiving them at 4 and 8 weeks after the first vaccination, and the other half receiving the extra doses at 4 and 20 weeks. All participants will receive the same dose at each vaccination.
Participants will be asked to monitor their temperature daily for a week after each vaccine dose is received; they will also report any adverse events. The trial design provides for several follow-up visits to track safety and to measure immune response in 44 weeks of short-term follow-up. Additionally, two follow-up visits at 18 months and 2 years post vaccination will measure the durability of the immune response.
The clinical trial will be run at the National Institutes of Health Clinical Center in Bethesda, Md., at Emory University in Atlanta, and at the University of Maryland’s Center for Vaccine Development in Baltimore.
Initial safety and immune response data should be available by the end of 2016, Dr. Fauci said. If these data are promising, a phase II clinical trial will be launched in early 2017 in several Zika-endemic countries. The NIH has the in-house capability to manufacture the 2,500-5,000 doses of vaccine that the phase II trial is expected to require.
However, said Dr. Fauci, without congressional approval of additional funding for Zika virus vaccine efforts, the transition to a phase II clinical trial is far from certain. Interruption in funding would be “effectively impeding our smooth process on the vaccine development front,” he said. “When I say we are going to run out of money soon, I mean really soon.”
Dr. Fauci, in response to questions, said that he continues to be bullish on such platform-based approaches to vaccines. The DNA strategy, in particular, represents “a very convenient and easily scalable vaccine,” he said. “We are moving much more toward platform-based vaccines … because of their ease and convenience, and scaling up and rapidity.”
The scope of a vaccination program will depend on the endemicity of the virus in a given area, said Dr. Fauci. In an endemic area, “Ultimately, you want to get women of childbearing age, as well as their sexual partners,” he said. This means that target populations will be as young as possible, to make sure women and their partners are vaccinated by the time pregnancy becomes a possibility.
Other Zika virus vaccine development efforts underway at NIAID include a strategy that uses a live attenuated virus, an approach used for a dengue virus vaccine that is currently in a phase 3 clinical trial in Brazil, according to the agency’s website. Dengue and Zika are closely related viruses. Another investigational Zika virus vaccine uses genetic engineering to create a vaccine derived from vesicular stomatitis virus, which infects cattle. This strategy, also used in one approach to Ebola vaccination, is in the pre-clinical stage.
On Twitter @karioakes
Zika virus RNA detected in serum beyond previously estimated time frame
Zika virus RNA was detected in the serum of five pregnant women beyond previously estimated time frames, according to a new case series study.
“This report adds to the existing evidence that Zika virus RNA in serum may be detected longer than previously expected, an observation now reported among at least eight pregnant women,” reported Dr. Dana Meaney-Delman of the Centers for Disease Control & Prevention, and her colleagues wrote. (Obstet Gynecol. 2016. doi: 10.1097/AOG.0000000000001625).
Five pregnant women who had traveled to or lived in one or more countries with active Zika virus transmission and had prolonged detection of Zika virus RNA in serum were reported to the U.S. Zika Pregnancy Registry, an enhanced surveillance initiative developed by the CDC to collect information on maternal exposure history, clinical presentation, laboratory testing, prenatal imaging, pregnancy screening and complications, fetal and neonatal outcomes, and infant development through the first year of life.
Prolonged detection was defined as the presence of Zika virus RNA detected in serum by real-time reverse transcription-polymerase chain reaction at 14 or more days after symptom onset for symptomatic pregnant women or 21 or more days after last possible exposure to Zika virus for asymptomatic pregnant women. A previous study reported a mean Zika viral RNA duration of 9.9 days, with 14 days being the longest duration of Zika virus RNA detection in a nonpregnant person.
Among the four symptomatic pregnant women, Zika virus RNA was detected in the serum at 17, 23, 44, and 46 days following symptom onset. In the one asymptomatic pregnant woman, Zika virus RNA was detected in serum at 53 days after her travel from an area with active Zika transmission.
Among the five pregnancies, one is ongoing, one was aborted and the fetus tested positive for fetal Zika virus infection, and three resulted in live births of healthy neonates with no reported abnormalities.
“Several questions remain regarding the findings of prolonged detection of Zika virus RNA. Most notably, the duration of Zika virus RNA in serum requires further investigation to determine whether there is a correlation between prolonged viral RNA detection and the presence of infectious virus,” the researchers wrote.
Read the study results here.
On Twitter @jessnicolecraig
Zika virus RNA was detected in the serum of five pregnant women beyond previously estimated time frames, according to a new case series study.
“This report adds to the existing evidence that Zika virus RNA in serum may be detected longer than previously expected, an observation now reported among at least eight pregnant women,” reported Dr. Dana Meaney-Delman of the Centers for Disease Control & Prevention, and her colleagues wrote. (Obstet Gynecol. 2016. doi: 10.1097/AOG.0000000000001625).
Five pregnant women who had traveled to or lived in one or more countries with active Zika virus transmission and had prolonged detection of Zika virus RNA in serum were reported to the U.S. Zika Pregnancy Registry, an enhanced surveillance initiative developed by the CDC to collect information on maternal exposure history, clinical presentation, laboratory testing, prenatal imaging, pregnancy screening and complications, fetal and neonatal outcomes, and infant development through the first year of life.
Prolonged detection was defined as the presence of Zika virus RNA detected in serum by real-time reverse transcription-polymerase chain reaction at 14 or more days after symptom onset for symptomatic pregnant women or 21 or more days after last possible exposure to Zika virus for asymptomatic pregnant women. A previous study reported a mean Zika viral RNA duration of 9.9 days, with 14 days being the longest duration of Zika virus RNA detection in a nonpregnant person.
Among the four symptomatic pregnant women, Zika virus RNA was detected in the serum at 17, 23, 44, and 46 days following symptom onset. In the one asymptomatic pregnant woman, Zika virus RNA was detected in serum at 53 days after her travel from an area with active Zika transmission.
Among the five pregnancies, one is ongoing, one was aborted and the fetus tested positive for fetal Zika virus infection, and three resulted in live births of healthy neonates with no reported abnormalities.
“Several questions remain regarding the findings of prolonged detection of Zika virus RNA. Most notably, the duration of Zika virus RNA in serum requires further investigation to determine whether there is a correlation between prolonged viral RNA detection and the presence of infectious virus,” the researchers wrote.
Read the study results here.
On Twitter @jessnicolecraig
Zika virus RNA was detected in the serum of five pregnant women beyond previously estimated time frames, according to a new case series study.
“This report adds to the existing evidence that Zika virus RNA in serum may be detected longer than previously expected, an observation now reported among at least eight pregnant women,” reported Dr. Dana Meaney-Delman of the Centers for Disease Control & Prevention, and her colleagues wrote. (Obstet Gynecol. 2016. doi: 10.1097/AOG.0000000000001625).
Five pregnant women who had traveled to or lived in one or more countries with active Zika virus transmission and had prolonged detection of Zika virus RNA in serum were reported to the U.S. Zika Pregnancy Registry, an enhanced surveillance initiative developed by the CDC to collect information on maternal exposure history, clinical presentation, laboratory testing, prenatal imaging, pregnancy screening and complications, fetal and neonatal outcomes, and infant development through the first year of life.
Prolonged detection was defined as the presence of Zika virus RNA detected in serum by real-time reverse transcription-polymerase chain reaction at 14 or more days after symptom onset for symptomatic pregnant women or 21 or more days after last possible exposure to Zika virus for asymptomatic pregnant women. A previous study reported a mean Zika viral RNA duration of 9.9 days, with 14 days being the longest duration of Zika virus RNA detection in a nonpregnant person.
Among the four symptomatic pregnant women, Zika virus RNA was detected in the serum at 17, 23, 44, and 46 days following symptom onset. In the one asymptomatic pregnant woman, Zika virus RNA was detected in serum at 53 days after her travel from an area with active Zika transmission.
Among the five pregnancies, one is ongoing, one was aborted and the fetus tested positive for fetal Zika virus infection, and three resulted in live births of healthy neonates with no reported abnormalities.
“Several questions remain regarding the findings of prolonged detection of Zika virus RNA. Most notably, the duration of Zika virus RNA in serum requires further investigation to determine whether there is a correlation between prolonged viral RNA detection and the presence of infectious virus,” the researchers wrote.
Read the study results here.
On Twitter @jessnicolecraig
FROM OBSTETRICS & GYNECOLOGY