User login
Medical groups urge emergency funding to combat Zika
A group of 68 organizations, including a number of medical societies, have called on Congress to pass an emergency spending bill requested by President Obama to fund activities to combat the spread of Zika virus.
In letters sent to House and Senate leaders, the groups called on Congress to “provide new funding rather than repurpose money from other high-priority programs at the Centers for Disease Control and Prevention and other federal agencies to ensure our health security and public health preparedness.”
On Feb. 22, President Obama asked Congress to consider $1.9 billion in supplemental spending related to the Zika virus. Congress has not acted on the request.
“According to the World Health Organization, the virus is spreading explosively, and scientists predict there will be $3 million to $4 million new infections in the Americas this year,” Dr. Hal C. Lawrence III, executive vice president and CEO of the American Congress of Obstetricians and Gynecologists, said during an April 5 press conference. “Zika is expected to infect 1 in 5 – 20% – of Puerto Ricans by the end of 2016 and as summer draws near, it may spread to the continental United States.”
There is “great concern” among ob.gyns. who may treat pregnant women exposed to Zika virus, Dr. Lawrence said. “These physicians strive to provide the best possible care for their patients. However, as health care providers, we just don’t have enough information at this time, nor enough treatment options.”
He emphasized that there are no treatment options or preventions guidelines “other than avoid being bitten by mosquitoes and using contraception if you don’t want to get pregnant.”
The lack of information and uncertainty makes it difficult for women “to make decisions about planning their pregnancies and causes pregnant women to suffer anxiety and distress,” he said. “In order to do more, we must know more. That’s why we are pleading with Congress to pass the emergency supplemental spending bill that President Obama has requested.”
Joining ACOG on the letter are American Academy of Family Physicians, American Academy of Pediatrics, American Medical Association, Infectious Diseases Society of America, HIV Medicine Association, March of Dimes, and others. Pharmaceutical manufacturers Johnson & Johnson and Novavax are signatories as well.
“If we take immediate action, we may be able to dramatically slow the spread of Zika, giving scientists time to develop and test a vaccine,” according to the letter. “Without action, we fear the number of newborns born with debilitating birth defects will only continue to rise.”
A group of 68 organizations, including a number of medical societies, have called on Congress to pass an emergency spending bill requested by President Obama to fund activities to combat the spread of Zika virus.
In letters sent to House and Senate leaders, the groups called on Congress to “provide new funding rather than repurpose money from other high-priority programs at the Centers for Disease Control and Prevention and other federal agencies to ensure our health security and public health preparedness.”
On Feb. 22, President Obama asked Congress to consider $1.9 billion in supplemental spending related to the Zika virus. Congress has not acted on the request.
“According to the World Health Organization, the virus is spreading explosively, and scientists predict there will be $3 million to $4 million new infections in the Americas this year,” Dr. Hal C. Lawrence III, executive vice president and CEO of the American Congress of Obstetricians and Gynecologists, said during an April 5 press conference. “Zika is expected to infect 1 in 5 – 20% – of Puerto Ricans by the end of 2016 and as summer draws near, it may spread to the continental United States.”
There is “great concern” among ob.gyns. who may treat pregnant women exposed to Zika virus, Dr. Lawrence said. “These physicians strive to provide the best possible care for their patients. However, as health care providers, we just don’t have enough information at this time, nor enough treatment options.”
He emphasized that there are no treatment options or preventions guidelines “other than avoid being bitten by mosquitoes and using contraception if you don’t want to get pregnant.”
The lack of information and uncertainty makes it difficult for women “to make decisions about planning their pregnancies and causes pregnant women to suffer anxiety and distress,” he said. “In order to do more, we must know more. That’s why we are pleading with Congress to pass the emergency supplemental spending bill that President Obama has requested.”
Joining ACOG on the letter are American Academy of Family Physicians, American Academy of Pediatrics, American Medical Association, Infectious Diseases Society of America, HIV Medicine Association, March of Dimes, and others. Pharmaceutical manufacturers Johnson & Johnson and Novavax are signatories as well.
“If we take immediate action, we may be able to dramatically slow the spread of Zika, giving scientists time to develop and test a vaccine,” according to the letter. “Without action, we fear the number of newborns born with debilitating birth defects will only continue to rise.”
A group of 68 organizations, including a number of medical societies, have called on Congress to pass an emergency spending bill requested by President Obama to fund activities to combat the spread of Zika virus.
In letters sent to House and Senate leaders, the groups called on Congress to “provide new funding rather than repurpose money from other high-priority programs at the Centers for Disease Control and Prevention and other federal agencies to ensure our health security and public health preparedness.”
On Feb. 22, President Obama asked Congress to consider $1.9 billion in supplemental spending related to the Zika virus. Congress has not acted on the request.
“According to the World Health Organization, the virus is spreading explosively, and scientists predict there will be $3 million to $4 million new infections in the Americas this year,” Dr. Hal C. Lawrence III, executive vice president and CEO of the American Congress of Obstetricians and Gynecologists, said during an April 5 press conference. “Zika is expected to infect 1 in 5 – 20% – of Puerto Ricans by the end of 2016 and as summer draws near, it may spread to the continental United States.”
There is “great concern” among ob.gyns. who may treat pregnant women exposed to Zika virus, Dr. Lawrence said. “These physicians strive to provide the best possible care for their patients. However, as health care providers, we just don’t have enough information at this time, nor enough treatment options.”
He emphasized that there are no treatment options or preventions guidelines “other than avoid being bitten by mosquitoes and using contraception if you don’t want to get pregnant.”
The lack of information and uncertainty makes it difficult for women “to make decisions about planning their pregnancies and causes pregnant women to suffer anxiety and distress,” he said. “In order to do more, we must know more. That’s why we are pleading with Congress to pass the emergency supplemental spending bill that President Obama has requested.”
Joining ACOG on the letter are American Academy of Family Physicians, American Academy of Pediatrics, American Medical Association, Infectious Diseases Society of America, HIV Medicine Association, March of Dimes, and others. Pharmaceutical manufacturers Johnson & Johnson and Novavax are signatories as well.
“If we take immediate action, we may be able to dramatically slow the spread of Zika, giving scientists time to develop and test a vaccine,” according to the letter. “Without action, we fear the number of newborns born with debilitating birth defects will only continue to rise.”
CDC urges states to prepare for Zika
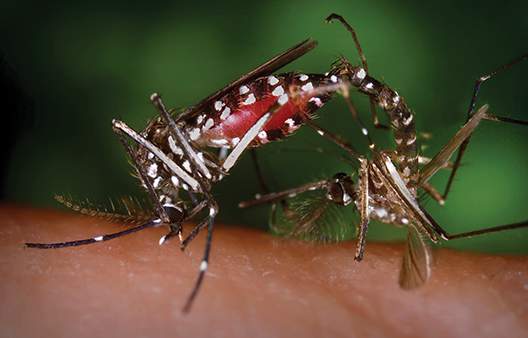
As warmer weather raises the possibility of local Zika virus transmission in the continental United States, federal health officials are pushing state and local governments to devise plans aimed at protecting pregnant women from infection, to increase access to contraception, and to ensure better coordination by mosquito control districts.
On April 1, the U.S. Centers for Disease Prevention and Control hosted a day-long seminar on Zika attended by some 300 state and local public health professionals. Zika virus, which is increasingly linked to adverse fetal outcomes including microcephaly, is now spreading in Puerto Rico, the U.S. Virgin Islands, and American Samoa.
Though most of the U.S. response effort is currently concentrated in Puerto Rico, and no local transmission has yet been reported in the continental U.S., a CDC report issued concurrently with the conference highlighted the potential for Zika transmission within the U.S. The entire southern half of the continental U.S., and much of its east coast, is home to the Aedes aegypti mosquitoes that can carry Zika virus.
In the same report, CDC also stressed that pregnant women should avoid travel to areas where Zika is being rapidly transmitted, and avoid sexual contact or consistently use condoms with partners who “reside in or have traveled to areas with active Zika virus transmission.”
“The key here is to reduce the risk to pregnant women,” Dr. Tom Frieden, CDC director, said at a news conference during the meeting. “There is an urgent need for all of us to learn more and do more,” he noted, acknowledging that officials had concerns about securing sufficient federal funding, getting rapid screening tools commercially developed in the absence of such funding, and improving the capabilities of U.S. mosquito control districts in the states most likely to be affected.
As local clusters of dengue virus disease have occurred in Florida, Texas, and Hawaii, Dr. Frieden said these states need to be particularly responsive to the Zika threat. However, Zika transmission could follow a different pattern, he said.
While Dr. Frieden described some local mosquito control districts and their capabilities as robust, others are considerably less so. Many districts in vulnerable states are not contiguous, leading to potential gaps in vector control. Dr. Frieden noted that even where control is most intensive, such as in Puerto Rico, vector resistance to common pesticides is a problem.
Dr. Frieden also stressed the importance of widening local access to contraception. He clarified that the CDC was not advising couples to avoid or delay pregnancy, even in Puerto Rico. However, he said, “if a woman and her partner choose not to become pregnant [there should be] ready access to effective contraception,” particularly the long-term reversible methods likely to be most effective.
Amy Pope, deputy homeland security advisor in the Obama administration, underscored the concern about Zika-specific funding. In February, the administration requested some $1.9 billion from Congress to combat Zika, including by improving access to contraception, developing vaccines and diagnostics, and other efforts. This funding has yet to be approved, Ms. Pope noted, adding that some members of Congress proposed redirecting funding that had been earmarked to combat Ebola.
“Congress is asking the American people to choose which disease it wants the most protection from,” Ms. Pope said, and Dr. Frieden reminded the conference that the Ebola crisis was not over. A new case from Liberia was announced April 1, he noted, and an ongoing cluster of transmission is occurring in Guinea.
As warmer weather raises the possibility of local Zika virus transmission in the continental United States, federal health officials are pushing state and local governments to devise plans aimed at protecting pregnant women from infection, to increase access to contraception, and to ensure better coordination by mosquito control districts.
On April 1, the U.S. Centers for Disease Prevention and Control hosted a day-long seminar on Zika attended by some 300 state and local public health professionals. Zika virus, which is increasingly linked to adverse fetal outcomes including microcephaly, is now spreading in Puerto Rico, the U.S. Virgin Islands, and American Samoa.
Though most of the U.S. response effort is currently concentrated in Puerto Rico, and no local transmission has yet been reported in the continental U.S., a CDC report issued concurrently with the conference highlighted the potential for Zika transmission within the U.S. The entire southern half of the continental U.S., and much of its east coast, is home to the Aedes aegypti mosquitoes that can carry Zika virus.
In the same report, CDC also stressed that pregnant women should avoid travel to areas where Zika is being rapidly transmitted, and avoid sexual contact or consistently use condoms with partners who “reside in or have traveled to areas with active Zika virus transmission.”
“The key here is to reduce the risk to pregnant women,” Dr. Tom Frieden, CDC director, said at a news conference during the meeting. “There is an urgent need for all of us to learn more and do more,” he noted, acknowledging that officials had concerns about securing sufficient federal funding, getting rapid screening tools commercially developed in the absence of such funding, and improving the capabilities of U.S. mosquito control districts in the states most likely to be affected.
As local clusters of dengue virus disease have occurred in Florida, Texas, and Hawaii, Dr. Frieden said these states need to be particularly responsive to the Zika threat. However, Zika transmission could follow a different pattern, he said.
While Dr. Frieden described some local mosquito control districts and their capabilities as robust, others are considerably less so. Many districts in vulnerable states are not contiguous, leading to potential gaps in vector control. Dr. Frieden noted that even where control is most intensive, such as in Puerto Rico, vector resistance to common pesticides is a problem.
Dr. Frieden also stressed the importance of widening local access to contraception. He clarified that the CDC was not advising couples to avoid or delay pregnancy, even in Puerto Rico. However, he said, “if a woman and her partner choose not to become pregnant [there should be] ready access to effective contraception,” particularly the long-term reversible methods likely to be most effective.
Amy Pope, deputy homeland security advisor in the Obama administration, underscored the concern about Zika-specific funding. In February, the administration requested some $1.9 billion from Congress to combat Zika, including by improving access to contraception, developing vaccines and diagnostics, and other efforts. This funding has yet to be approved, Ms. Pope noted, adding that some members of Congress proposed redirecting funding that had been earmarked to combat Ebola.
“Congress is asking the American people to choose which disease it wants the most protection from,” Ms. Pope said, and Dr. Frieden reminded the conference that the Ebola crisis was not over. A new case from Liberia was announced April 1, he noted, and an ongoing cluster of transmission is occurring in Guinea.
As warmer weather raises the possibility of local Zika virus transmission in the continental United States, federal health officials are pushing state and local governments to devise plans aimed at protecting pregnant women from infection, to increase access to contraception, and to ensure better coordination by mosquito control districts.
On April 1, the U.S. Centers for Disease Prevention and Control hosted a day-long seminar on Zika attended by some 300 state and local public health professionals. Zika virus, which is increasingly linked to adverse fetal outcomes including microcephaly, is now spreading in Puerto Rico, the U.S. Virgin Islands, and American Samoa.
Though most of the U.S. response effort is currently concentrated in Puerto Rico, and no local transmission has yet been reported in the continental U.S., a CDC report issued concurrently with the conference highlighted the potential for Zika transmission within the U.S. The entire southern half of the continental U.S., and much of its east coast, is home to the Aedes aegypti mosquitoes that can carry Zika virus.
In the same report, CDC also stressed that pregnant women should avoid travel to areas where Zika is being rapidly transmitted, and avoid sexual contact or consistently use condoms with partners who “reside in or have traveled to areas with active Zika virus transmission.”
“The key here is to reduce the risk to pregnant women,” Dr. Tom Frieden, CDC director, said at a news conference during the meeting. “There is an urgent need for all of us to learn more and do more,” he noted, acknowledging that officials had concerns about securing sufficient federal funding, getting rapid screening tools commercially developed in the absence of such funding, and improving the capabilities of U.S. mosquito control districts in the states most likely to be affected.
As local clusters of dengue virus disease have occurred in Florida, Texas, and Hawaii, Dr. Frieden said these states need to be particularly responsive to the Zika threat. However, Zika transmission could follow a different pattern, he said.
While Dr. Frieden described some local mosquito control districts and their capabilities as robust, others are considerably less so. Many districts in vulnerable states are not contiguous, leading to potential gaps in vector control. Dr. Frieden noted that even where control is most intensive, such as in Puerto Rico, vector resistance to common pesticides is a problem.
Dr. Frieden also stressed the importance of widening local access to contraception. He clarified that the CDC was not advising couples to avoid or delay pregnancy, even in Puerto Rico. However, he said, “if a woman and her partner choose not to become pregnant [there should be] ready access to effective contraception,” particularly the long-term reversible methods likely to be most effective.
Amy Pope, deputy homeland security advisor in the Obama administration, underscored the concern about Zika-specific funding. In February, the administration requested some $1.9 billion from Congress to combat Zika, including by improving access to contraception, developing vaccines and diagnostics, and other efforts. This funding has yet to be approved, Ms. Pope noted, adding that some members of Congress proposed redirecting funding that had been earmarked to combat Ebola.
“Congress is asking the American people to choose which disease it wants the most protection from,” Ms. Pope said, and Dr. Frieden reminded the conference that the Ebola crisis was not over. A new case from Liberia was announced April 1, he noted, and an ongoing cluster of transmission is occurring in Guinea.
New Zika case study fills some research gaps
A new case study adds yet more evidence to the link between congenital Zika virus infection and fetal brain damage while offering insights into how the virus affects brain development at different stages.
“Our study highlights the possible importance of [Zika virus] RNA testing of serum obtained from pregnant women beyond the first week after symptom onset, as well as a more detailed evaluation of the fetal intracranial anatomy by means of serial fetal ultrasonography or fetal brain MRI,” wrote Dr. Rita W. Driggers of Johns Hopkins University, Baltimore, and her associates (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMoa1601824).
The study also continues to help fill in the gaps in research highlighted by Dr. Lyle R. Petersen and his associates at the Centers for Disease Control and Prevention, in an overview of the virus published in the same issue (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMra1602113).
“These include a complete understanding of the frequency and full spectrum of clinical outcomes resulting from fetal Zika virus infection and of the environmental factors that influence emergence, as well as the development of discriminating diagnostic tools for flaviruses, animal models for fetal developmental effects due to viral infection, new vector control products and strategies, effective therapeutics, and vaccines to protect humans against the disease,” the CDC authors wrote.
In the case study, a 33-year-old Finnish woman developed an infection from Zika virus in her 11th week of pregnancy while on vacation in Mexico, Guatemala, and Belize. She experienced the common Zika virus symptoms of a mild fever, eye pain, rash, and muscle pain for 5 days and had evidence of Zika virus RNA in her blood between 16 and 21 weeks’ gestation.
Although the fetal head size remained within the normal range during the 16th and 17th weeks of pregnancy, fetal head circumference dropped from the 47th percentile at 16 weeks gestation to the 24th percentile at 20 weeks’ gestation. Ultrasound and MRI imagery found fetal brain abnormalities, including a thin cerebral mantle and potential agenesis of the corpus callosum at 19 and 20 weeks’ gestation, but neither microcephaly nor calcifications in the brain were seen.
“We suspect these reductions in brain growth would have eventually met the criteria for microcephaly,” the researchers wrote. “As this case shows, the latency period between Zika virus infection of the fetal brain and the detection of microcephaly and intracranial calcifications on ultrasonography is likely to be prolonged.”
Negative findings during this time could be “falsely reassuring and might delay critical time-sensitive decision making,” they added.
The woman chose to terminate the pregnancy at 21 weeks, and high viral loads of Zika were found in the fetal brain during a postmortem exam. The fetus also had lower amounts of Zika RNA in the muscle, liver, lung, and spleen, as did the mother’s amniotic fluid.
“Although the evidence of the association between the presence of Zika virus in pregnant women and fetal brain abnormalities continues to grow, the timing of infection during fetal development and other factors that may have an effect on viral pathogenesis and their effects on the appearance of the brain abnormalities are poorly understood,” the case study researchers wrote.
The CDC authors also note the challenges of differentiating Zika virus infections from dengue or other flavivirus infections. “Reliable testing regimens for the diagnosis of prenatal and antenatal Zika virus infection have not been established,” they wrote.
The CDC authors also predict millions more Zika cases in the Americas, given the incidence of dengue and chikungunya cases previously, but the burden of long-term effects is harder to predict. “The long-term outlook with regard to the current Zika outbreak in the Americas is uncertain,” they wrote. “Herd immunity sufficient to slow further transmission will undoubtedly occur, although this will not obviate the need for immediate and long-term prevention and control strategies.”
Researchers from the CDC and those who reported the case study reported having no financial disclosures.
We need research to clarify the best way to provide protection and to prevent serious consequences of Zika virus and other flaviviruses that were previously unknown. Until recently, Zika virus was believed to cause only mild disease, which it still does in the majority of cases. The main concern today is the growing body of evidence that Zika virus infection results in severe neurologic complications – Guillain-Barré syndrome in infected patients and microcephaly in unborn babies – combined with the very rapid spread of the virus.
Although vaccines may come too late for countries currently affected by the Zika virus epidemic, the development of a vaccine that can, above all, protect pregnant women and their babies remains an imperative for countries where the epidemic is expected to arrive in the foreseeable future. The goal would be to allow for medium- to long-term control of Zika virus analogous in some ways to the control of rubella. It is critical that we collaborate rather than compete to find answers to the questions that worry millions of women of child-bearing age in areas where Zika virus is spreading rapidly and may become endemic.
Dr. Charlotte J. Haug is an international correspondent for the New England Journal of Medicine; Marie-Paule Kieny, Ph.D., is assistant director-general for health systems and innovation at the World Health Organization; and Dr. Bernadette Murgue is the project manager of the WHO’s R&D Blueprint. They reported having no financial disclosures. These comments are adapted from an editorial (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMp1603734).
We need research to clarify the best way to provide protection and to prevent serious consequences of Zika virus and other flaviviruses that were previously unknown. Until recently, Zika virus was believed to cause only mild disease, which it still does in the majority of cases. The main concern today is the growing body of evidence that Zika virus infection results in severe neurologic complications – Guillain-Barré syndrome in infected patients and microcephaly in unborn babies – combined with the very rapid spread of the virus.
Although vaccines may come too late for countries currently affected by the Zika virus epidemic, the development of a vaccine that can, above all, protect pregnant women and their babies remains an imperative for countries where the epidemic is expected to arrive in the foreseeable future. The goal would be to allow for medium- to long-term control of Zika virus analogous in some ways to the control of rubella. It is critical that we collaborate rather than compete to find answers to the questions that worry millions of women of child-bearing age in areas where Zika virus is spreading rapidly and may become endemic.
Dr. Charlotte J. Haug is an international correspondent for the New England Journal of Medicine; Marie-Paule Kieny, Ph.D., is assistant director-general for health systems and innovation at the World Health Organization; and Dr. Bernadette Murgue is the project manager of the WHO’s R&D Blueprint. They reported having no financial disclosures. These comments are adapted from an editorial (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMp1603734).
We need research to clarify the best way to provide protection and to prevent serious consequences of Zika virus and other flaviviruses that were previously unknown. Until recently, Zika virus was believed to cause only mild disease, which it still does in the majority of cases. The main concern today is the growing body of evidence that Zika virus infection results in severe neurologic complications – Guillain-Barré syndrome in infected patients and microcephaly in unborn babies – combined with the very rapid spread of the virus.
Although vaccines may come too late for countries currently affected by the Zika virus epidemic, the development of a vaccine that can, above all, protect pregnant women and their babies remains an imperative for countries where the epidemic is expected to arrive in the foreseeable future. The goal would be to allow for medium- to long-term control of Zika virus analogous in some ways to the control of rubella. It is critical that we collaborate rather than compete to find answers to the questions that worry millions of women of child-bearing age in areas where Zika virus is spreading rapidly and may become endemic.
Dr. Charlotte J. Haug is an international correspondent for the New England Journal of Medicine; Marie-Paule Kieny, Ph.D., is assistant director-general for health systems and innovation at the World Health Organization; and Dr. Bernadette Murgue is the project manager of the WHO’s R&D Blueprint. They reported having no financial disclosures. These comments are adapted from an editorial (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMp1603734).
A new case study adds yet more evidence to the link between congenital Zika virus infection and fetal brain damage while offering insights into how the virus affects brain development at different stages.
“Our study highlights the possible importance of [Zika virus] RNA testing of serum obtained from pregnant women beyond the first week after symptom onset, as well as a more detailed evaluation of the fetal intracranial anatomy by means of serial fetal ultrasonography or fetal brain MRI,” wrote Dr. Rita W. Driggers of Johns Hopkins University, Baltimore, and her associates (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMoa1601824).
The study also continues to help fill in the gaps in research highlighted by Dr. Lyle R. Petersen and his associates at the Centers for Disease Control and Prevention, in an overview of the virus published in the same issue (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMra1602113).
“These include a complete understanding of the frequency and full spectrum of clinical outcomes resulting from fetal Zika virus infection and of the environmental factors that influence emergence, as well as the development of discriminating diagnostic tools for flaviruses, animal models for fetal developmental effects due to viral infection, new vector control products and strategies, effective therapeutics, and vaccines to protect humans against the disease,” the CDC authors wrote.
In the case study, a 33-year-old Finnish woman developed an infection from Zika virus in her 11th week of pregnancy while on vacation in Mexico, Guatemala, and Belize. She experienced the common Zika virus symptoms of a mild fever, eye pain, rash, and muscle pain for 5 days and had evidence of Zika virus RNA in her blood between 16 and 21 weeks’ gestation.
Although the fetal head size remained within the normal range during the 16th and 17th weeks of pregnancy, fetal head circumference dropped from the 47th percentile at 16 weeks gestation to the 24th percentile at 20 weeks’ gestation. Ultrasound and MRI imagery found fetal brain abnormalities, including a thin cerebral mantle and potential agenesis of the corpus callosum at 19 and 20 weeks’ gestation, but neither microcephaly nor calcifications in the brain were seen.
“We suspect these reductions in brain growth would have eventually met the criteria for microcephaly,” the researchers wrote. “As this case shows, the latency period between Zika virus infection of the fetal brain and the detection of microcephaly and intracranial calcifications on ultrasonography is likely to be prolonged.”
Negative findings during this time could be “falsely reassuring and might delay critical time-sensitive decision making,” they added.
The woman chose to terminate the pregnancy at 21 weeks, and high viral loads of Zika were found in the fetal brain during a postmortem exam. The fetus also had lower amounts of Zika RNA in the muscle, liver, lung, and spleen, as did the mother’s amniotic fluid.
“Although the evidence of the association between the presence of Zika virus in pregnant women and fetal brain abnormalities continues to grow, the timing of infection during fetal development and other factors that may have an effect on viral pathogenesis and their effects on the appearance of the brain abnormalities are poorly understood,” the case study researchers wrote.
The CDC authors also note the challenges of differentiating Zika virus infections from dengue or other flavivirus infections. “Reliable testing regimens for the diagnosis of prenatal and antenatal Zika virus infection have not been established,” they wrote.
The CDC authors also predict millions more Zika cases in the Americas, given the incidence of dengue and chikungunya cases previously, but the burden of long-term effects is harder to predict. “The long-term outlook with regard to the current Zika outbreak in the Americas is uncertain,” they wrote. “Herd immunity sufficient to slow further transmission will undoubtedly occur, although this will not obviate the need for immediate and long-term prevention and control strategies.”
Researchers from the CDC and those who reported the case study reported having no financial disclosures.
A new case study adds yet more evidence to the link between congenital Zika virus infection and fetal brain damage while offering insights into how the virus affects brain development at different stages.
“Our study highlights the possible importance of [Zika virus] RNA testing of serum obtained from pregnant women beyond the first week after symptom onset, as well as a more detailed evaluation of the fetal intracranial anatomy by means of serial fetal ultrasonography or fetal brain MRI,” wrote Dr. Rita W. Driggers of Johns Hopkins University, Baltimore, and her associates (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMoa1601824).
The study also continues to help fill in the gaps in research highlighted by Dr. Lyle R. Petersen and his associates at the Centers for Disease Control and Prevention, in an overview of the virus published in the same issue (N Engl J Med. 2016 March 30. doi: 10.1056/NEJMra1602113).
“These include a complete understanding of the frequency and full spectrum of clinical outcomes resulting from fetal Zika virus infection and of the environmental factors that influence emergence, as well as the development of discriminating diagnostic tools for flaviruses, animal models for fetal developmental effects due to viral infection, new vector control products and strategies, effective therapeutics, and vaccines to protect humans against the disease,” the CDC authors wrote.
In the case study, a 33-year-old Finnish woman developed an infection from Zika virus in her 11th week of pregnancy while on vacation in Mexico, Guatemala, and Belize. She experienced the common Zika virus symptoms of a mild fever, eye pain, rash, and muscle pain for 5 days and had evidence of Zika virus RNA in her blood between 16 and 21 weeks’ gestation.
Although the fetal head size remained within the normal range during the 16th and 17th weeks of pregnancy, fetal head circumference dropped from the 47th percentile at 16 weeks gestation to the 24th percentile at 20 weeks’ gestation. Ultrasound and MRI imagery found fetal brain abnormalities, including a thin cerebral mantle and potential agenesis of the corpus callosum at 19 and 20 weeks’ gestation, but neither microcephaly nor calcifications in the brain were seen.
“We suspect these reductions in brain growth would have eventually met the criteria for microcephaly,” the researchers wrote. “As this case shows, the latency period between Zika virus infection of the fetal brain and the detection of microcephaly and intracranial calcifications on ultrasonography is likely to be prolonged.”
Negative findings during this time could be “falsely reassuring and might delay critical time-sensitive decision making,” they added.
The woman chose to terminate the pregnancy at 21 weeks, and high viral loads of Zika were found in the fetal brain during a postmortem exam. The fetus also had lower amounts of Zika RNA in the muscle, liver, lung, and spleen, as did the mother’s amniotic fluid.
“Although the evidence of the association between the presence of Zika virus in pregnant women and fetal brain abnormalities continues to grow, the timing of infection during fetal development and other factors that may have an effect on viral pathogenesis and their effects on the appearance of the brain abnormalities are poorly understood,” the case study researchers wrote.
The CDC authors also note the challenges of differentiating Zika virus infections from dengue or other flavivirus infections. “Reliable testing regimens for the diagnosis of prenatal and antenatal Zika virus infection have not been established,” they wrote.
The CDC authors also predict millions more Zika cases in the Americas, given the incidence of dengue and chikungunya cases previously, but the burden of long-term effects is harder to predict. “The long-term outlook with regard to the current Zika outbreak in the Americas is uncertain,” they wrote. “Herd immunity sufficient to slow further transmission will undoubtedly occur, although this will not obviate the need for immediate and long-term prevention and control strategies.”
Researchers from the CDC and those who reported the case study reported having no financial disclosures.
FROM THE NEW ENGLAND JOURNAL OF MEDICINE
CDC issues interim guidance for care of Ebola survivors
The Centers for Disease Control and Prevention has issued interim guidance for U.S. health care providers to safely care for survivors of Ebola virus disease (EVD).
The guidance includes information about sequelae of EVD as well as data on Ebola virus persistence in EVD survivors, and infection prevention and control recommendations for U.S. health care providers when evaluating a patient who is an EVD survivor. Of the eleven patients with Ebola virus disease who were managed in U.S. health care facilities during 2014-2015, nine survived. The CDC notes in the guidance that it is also possible that some EVD survivors from West Africa could seek medical care in the United States.
In most cases, the CDC notes, persons who have completely recovered from EVD do not experience a relapse of Ebola virus associated with systemic illness, although survivors can experience complications after surviving acute EVD. Reported complications among EVD survivors include nonspecific fatigue, joint pain, muscle aches, headaches, suppurative parotitis, pericarditis, orchitis, sexual dysfunction, hair loss, vision loss (including uveitis and permanent blindness), hearing loss, tinnitus, paresthesia or dysesthesia, memory loss, insomnia, depression, anxiety, and posttraumatic stress disorder.
The risk of infectivity from patients with persistent infection is unknown, the CDC guidance states, but “appears to be low and is likely to decrease over time.” The guidance includes infection control practices that should be used to “ensure that health care personnel do not contract infections from patients, whether or not they are known to be infectious,” and that personnel do not spread infectious material to other patients during routine medical care.
Medical professionals can view the complete care guidance on the CDC website. The CDC has also published additional resources to supplement the guidance. These include:
Messages for the Care of Survivors of Ebola Virus Disease. Topics in this resource include health problems EVD survivors may experience, guidance to use standard precautions for all patient care, and recommendations for when extra precautions may be needed.
FAQ on Screening for Ebola Virus Disease for Providers, Healthcare Facilities, and Health Departments. FAQs include information on adjusting screening practices for acutely ill patients while reiterating that a thorough travel history for all patients should be obtained to ensure proper infection control measures are in place. Additionally, information on hospital management recommendations for evaluating ill travelers from West Africa are included.
On Twitter @richpizzi
The Centers for Disease Control and Prevention has issued interim guidance for U.S. health care providers to safely care for survivors of Ebola virus disease (EVD).
The guidance includes information about sequelae of EVD as well as data on Ebola virus persistence in EVD survivors, and infection prevention and control recommendations for U.S. health care providers when evaluating a patient who is an EVD survivor. Of the eleven patients with Ebola virus disease who were managed in U.S. health care facilities during 2014-2015, nine survived. The CDC notes in the guidance that it is also possible that some EVD survivors from West Africa could seek medical care in the United States.
In most cases, the CDC notes, persons who have completely recovered from EVD do not experience a relapse of Ebola virus associated with systemic illness, although survivors can experience complications after surviving acute EVD. Reported complications among EVD survivors include nonspecific fatigue, joint pain, muscle aches, headaches, suppurative parotitis, pericarditis, orchitis, sexual dysfunction, hair loss, vision loss (including uveitis and permanent blindness), hearing loss, tinnitus, paresthesia or dysesthesia, memory loss, insomnia, depression, anxiety, and posttraumatic stress disorder.
The risk of infectivity from patients with persistent infection is unknown, the CDC guidance states, but “appears to be low and is likely to decrease over time.” The guidance includes infection control practices that should be used to “ensure that health care personnel do not contract infections from patients, whether or not they are known to be infectious,” and that personnel do not spread infectious material to other patients during routine medical care.
Medical professionals can view the complete care guidance on the CDC website. The CDC has also published additional resources to supplement the guidance. These include:
Messages for the Care of Survivors of Ebola Virus Disease. Topics in this resource include health problems EVD survivors may experience, guidance to use standard precautions for all patient care, and recommendations for when extra precautions may be needed.
FAQ on Screening for Ebola Virus Disease for Providers, Healthcare Facilities, and Health Departments. FAQs include information on adjusting screening practices for acutely ill patients while reiterating that a thorough travel history for all patients should be obtained to ensure proper infection control measures are in place. Additionally, information on hospital management recommendations for evaluating ill travelers from West Africa are included.
On Twitter @richpizzi
The Centers for Disease Control and Prevention has issued interim guidance for U.S. health care providers to safely care for survivors of Ebola virus disease (EVD).
The guidance includes information about sequelae of EVD as well as data on Ebola virus persistence in EVD survivors, and infection prevention and control recommendations for U.S. health care providers when evaluating a patient who is an EVD survivor. Of the eleven patients with Ebola virus disease who were managed in U.S. health care facilities during 2014-2015, nine survived. The CDC notes in the guidance that it is also possible that some EVD survivors from West Africa could seek medical care in the United States.
In most cases, the CDC notes, persons who have completely recovered from EVD do not experience a relapse of Ebola virus associated with systemic illness, although survivors can experience complications after surviving acute EVD. Reported complications among EVD survivors include nonspecific fatigue, joint pain, muscle aches, headaches, suppurative parotitis, pericarditis, orchitis, sexual dysfunction, hair loss, vision loss (including uveitis and permanent blindness), hearing loss, tinnitus, paresthesia or dysesthesia, memory loss, insomnia, depression, anxiety, and posttraumatic stress disorder.
The risk of infectivity from patients with persistent infection is unknown, the CDC guidance states, but “appears to be low and is likely to decrease over time.” The guidance includes infection control practices that should be used to “ensure that health care personnel do not contract infections from patients, whether or not they are known to be infectious,” and that personnel do not spread infectious material to other patients during routine medical care.
Medical professionals can view the complete care guidance on the CDC website. The CDC has also published additional resources to supplement the guidance. These include:
Messages for the Care of Survivors of Ebola Virus Disease. Topics in this resource include health problems EVD survivors may experience, guidance to use standard precautions for all patient care, and recommendations for when extra precautions may be needed.
FAQ on Screening for Ebola Virus Disease for Providers, Healthcare Facilities, and Health Departments. FAQs include information on adjusting screening practices for acutely ill patients while reiterating that a thorough travel history for all patients should be obtained to ensure proper infection control measures are in place. Additionally, information on hospital management recommendations for evaluating ill travelers from West Africa are included.
On Twitter @richpizzi
CDC updates advice on preventing sexual transmission of Zika virus
Men potentially exposed to Zika virus should use a condom during all sex or abstain from sex for at least 8 weeks, according to new recommendations from the Centers for Disease Control and Prevention on reducing the risk of sexual transmission of the virus.
Men with confirmed infections or clinical symptoms of Zika should similarly abstain or use a condom for at least 6 months, the CDC recommends in the Morbidity and Mortality Weekly Report released on March 25 (MMWR 2016. Mar 25. doi: http://dx.doi.org/10.15585/mmwr.mm6512e3er).
These recommendations update and replace those issued by the CDC on Feb. 5 and include new guidance for men who live in, or have traveled to, an area with active Zika virus transmission. The recommendations apply to all types of sexual activity involving the penis, including vaginal intercourse, anal intercourse, or fellatio.
“The previous recommendations focused on women who were already pregnant,” Dr. Denise J. Jamieson, co-lead of the Pregnancy and Birth Defects Team of the CDC Zika Virus Response Team, said during a press briefing. “What’s new is that we are now concerned about the periconceptional period, around the time the woman conceives.”
For men with pregnant sex partners, the agency recommends consistent and accurate use of condoms during any type of sex, or abstinence during the length of the pregnancy.
“This course is the best way to avoid even a minimal risk of sexual transmission of Zika virus, which could have adverse fetal effects when contracted during pregnancy,” the CDC report states, adding that pregnant women should ask their male sex partners about recent travel to areas with currently circulating Zika virus.
For couples not expecting a child, but concerned about sexual transmission of Zika, men with a confirmed Zika infection or clinical symptoms of Zika infection should consider using condoms or abstaining from sex for at least 6 months after their symptoms appear. This recommendation is based on tripling 62 days – the longest time interval after infection during which the virus was successfully isolated from semen.
If men have traveled to areas with active Zika transmission but have not developed symptoms, the CDC recommends condom use or abstinence for at least 8 weeks after leaving the area. Those living in areas with active transmission should also consider condom use during sex or abstaining from sex until active transmission has ceased.
These recommendations come as more evidence points to a link between Zika infection and fetal abnormalities, including microcephaly and fetal mortality.
“I think we’re learning more every day, and I think the evidence of a link between Zika and a range of poor pregnancy outcomes is becoming stronger and stronger,” Dr. Jamieson said. “At this point, we’re not using causal language, but the evidence is mounting.”
The CDC also released two other reports focusing on the need to increase access to contraception for residents of Puerto Rico and interim guidance for health care providers of women of childbearing age who have been potentially exposed to Zika virus.
As of March 25, the CDC has reported 273 U.S. cases of Zika virus infections from 35 states and Washington, D.C. All of these – except six sexually transmitted cases – are travel related.
Additionally, Puerto Rico’s most recent case total is 261, all locally transmitted by mosquitoes, except for three travel-associated cases. American Samoa has 14 cases, and the U.S. Virgin Islands have 11 cases, all thought to be locally transmitted.
“Long-acting contraception methods are not readily available in Puerto Rico, and from our health care provider colleagues in Puerto Rico, there is a desire to provide a more broad range of contraception options to women in Puerto Rico,” Dr. Jamieson said.
She said the CDC is developing a plan to make long-acting contraceptive methods more available in Puerto Rico.
When advising couples who wish to become pregnant after the man has had confirmed or suspected Zika infection, the CDC recommends waiting at least 6 months after the man’s onset of Zika symptoms or confirmed infection before attempting to conceive.
Although no evidence suggests that Zika virus will cause congenital infections in pregnancies conceived after a woman’s infection has resolved, data on the virus’s incubation period is limited, according to the CDC.
“Women with Zika virus disease should wait until at least 8 weeks after symptom onset before attempting conception,” wrote Dr. Emily E. Petersen and her colleagues in the guidance on caring for women of reproductive age with possible Zika virus exposure. “No data are available regarding the risk for congenital infection among pregnant women with asymptomatic infection.”
Similarly, asymptomatic women potentially exposed to Zika virus should also wait at least 8 weeks after the possible exposure date before trying to conceive.
Men potentially exposed to Zika virus should use a condom during all sex or abstain from sex for at least 8 weeks, according to new recommendations from the Centers for Disease Control and Prevention on reducing the risk of sexual transmission of the virus.
Men with confirmed infections or clinical symptoms of Zika should similarly abstain or use a condom for at least 6 months, the CDC recommends in the Morbidity and Mortality Weekly Report released on March 25 (MMWR 2016. Mar 25. doi: http://dx.doi.org/10.15585/mmwr.mm6512e3er).
These recommendations update and replace those issued by the CDC on Feb. 5 and include new guidance for men who live in, or have traveled to, an area with active Zika virus transmission. The recommendations apply to all types of sexual activity involving the penis, including vaginal intercourse, anal intercourse, or fellatio.
“The previous recommendations focused on women who were already pregnant,” Dr. Denise J. Jamieson, co-lead of the Pregnancy and Birth Defects Team of the CDC Zika Virus Response Team, said during a press briefing. “What’s new is that we are now concerned about the periconceptional period, around the time the woman conceives.”
For men with pregnant sex partners, the agency recommends consistent and accurate use of condoms during any type of sex, or abstinence during the length of the pregnancy.
“This course is the best way to avoid even a minimal risk of sexual transmission of Zika virus, which could have adverse fetal effects when contracted during pregnancy,” the CDC report states, adding that pregnant women should ask their male sex partners about recent travel to areas with currently circulating Zika virus.
For couples not expecting a child, but concerned about sexual transmission of Zika, men with a confirmed Zika infection or clinical symptoms of Zika infection should consider using condoms or abstaining from sex for at least 6 months after their symptoms appear. This recommendation is based on tripling 62 days – the longest time interval after infection during which the virus was successfully isolated from semen.
If men have traveled to areas with active Zika transmission but have not developed symptoms, the CDC recommends condom use or abstinence for at least 8 weeks after leaving the area. Those living in areas with active transmission should also consider condom use during sex or abstaining from sex until active transmission has ceased.
These recommendations come as more evidence points to a link between Zika infection and fetal abnormalities, including microcephaly and fetal mortality.
“I think we’re learning more every day, and I think the evidence of a link between Zika and a range of poor pregnancy outcomes is becoming stronger and stronger,” Dr. Jamieson said. “At this point, we’re not using causal language, but the evidence is mounting.”
The CDC also released two other reports focusing on the need to increase access to contraception for residents of Puerto Rico and interim guidance for health care providers of women of childbearing age who have been potentially exposed to Zika virus.
As of March 25, the CDC has reported 273 U.S. cases of Zika virus infections from 35 states and Washington, D.C. All of these – except six sexually transmitted cases – are travel related.
Additionally, Puerto Rico’s most recent case total is 261, all locally transmitted by mosquitoes, except for three travel-associated cases. American Samoa has 14 cases, and the U.S. Virgin Islands have 11 cases, all thought to be locally transmitted.
“Long-acting contraception methods are not readily available in Puerto Rico, and from our health care provider colleagues in Puerto Rico, there is a desire to provide a more broad range of contraception options to women in Puerto Rico,” Dr. Jamieson said.
She said the CDC is developing a plan to make long-acting contraceptive methods more available in Puerto Rico.
When advising couples who wish to become pregnant after the man has had confirmed or suspected Zika infection, the CDC recommends waiting at least 6 months after the man’s onset of Zika symptoms or confirmed infection before attempting to conceive.
Although no evidence suggests that Zika virus will cause congenital infections in pregnancies conceived after a woman’s infection has resolved, data on the virus’s incubation period is limited, according to the CDC.
“Women with Zika virus disease should wait until at least 8 weeks after symptom onset before attempting conception,” wrote Dr. Emily E. Petersen and her colleagues in the guidance on caring for women of reproductive age with possible Zika virus exposure. “No data are available regarding the risk for congenital infection among pregnant women with asymptomatic infection.”
Similarly, asymptomatic women potentially exposed to Zika virus should also wait at least 8 weeks after the possible exposure date before trying to conceive.
Men potentially exposed to Zika virus should use a condom during all sex or abstain from sex for at least 8 weeks, according to new recommendations from the Centers for Disease Control and Prevention on reducing the risk of sexual transmission of the virus.
Men with confirmed infections or clinical symptoms of Zika should similarly abstain or use a condom for at least 6 months, the CDC recommends in the Morbidity and Mortality Weekly Report released on March 25 (MMWR 2016. Mar 25. doi: http://dx.doi.org/10.15585/mmwr.mm6512e3er).
These recommendations update and replace those issued by the CDC on Feb. 5 and include new guidance for men who live in, or have traveled to, an area with active Zika virus transmission. The recommendations apply to all types of sexual activity involving the penis, including vaginal intercourse, anal intercourse, or fellatio.
“The previous recommendations focused on women who were already pregnant,” Dr. Denise J. Jamieson, co-lead of the Pregnancy and Birth Defects Team of the CDC Zika Virus Response Team, said during a press briefing. “What’s new is that we are now concerned about the periconceptional period, around the time the woman conceives.”
For men with pregnant sex partners, the agency recommends consistent and accurate use of condoms during any type of sex, or abstinence during the length of the pregnancy.
“This course is the best way to avoid even a minimal risk of sexual transmission of Zika virus, which could have adverse fetal effects when contracted during pregnancy,” the CDC report states, adding that pregnant women should ask their male sex partners about recent travel to areas with currently circulating Zika virus.
For couples not expecting a child, but concerned about sexual transmission of Zika, men with a confirmed Zika infection or clinical symptoms of Zika infection should consider using condoms or abstaining from sex for at least 6 months after their symptoms appear. This recommendation is based on tripling 62 days – the longest time interval after infection during which the virus was successfully isolated from semen.
If men have traveled to areas with active Zika transmission but have not developed symptoms, the CDC recommends condom use or abstinence for at least 8 weeks after leaving the area. Those living in areas with active transmission should also consider condom use during sex or abstaining from sex until active transmission has ceased.
These recommendations come as more evidence points to a link between Zika infection and fetal abnormalities, including microcephaly and fetal mortality.
“I think we’re learning more every day, and I think the evidence of a link between Zika and a range of poor pregnancy outcomes is becoming stronger and stronger,” Dr. Jamieson said. “At this point, we’re not using causal language, but the evidence is mounting.”
The CDC also released two other reports focusing on the need to increase access to contraception for residents of Puerto Rico and interim guidance for health care providers of women of childbearing age who have been potentially exposed to Zika virus.
As of March 25, the CDC has reported 273 U.S. cases of Zika virus infections from 35 states and Washington, D.C. All of these – except six sexually transmitted cases – are travel related.
Additionally, Puerto Rico’s most recent case total is 261, all locally transmitted by mosquitoes, except for three travel-associated cases. American Samoa has 14 cases, and the U.S. Virgin Islands have 11 cases, all thought to be locally transmitted.
“Long-acting contraception methods are not readily available in Puerto Rico, and from our health care provider colleagues in Puerto Rico, there is a desire to provide a more broad range of contraception options to women in Puerto Rico,” Dr. Jamieson said.
She said the CDC is developing a plan to make long-acting contraceptive methods more available in Puerto Rico.
When advising couples who wish to become pregnant after the man has had confirmed or suspected Zika infection, the CDC recommends waiting at least 6 months after the man’s onset of Zika symptoms or confirmed infection before attempting to conceive.
Although no evidence suggests that Zika virus will cause congenital infections in pregnancies conceived after a woman’s infection has resolved, data on the virus’s incubation period is limited, according to the CDC.
“Women with Zika virus disease should wait until at least 8 weeks after symptom onset before attempting conception,” wrote Dr. Emily E. Petersen and her colleagues in the guidance on caring for women of reproductive age with possible Zika virus exposure. “No data are available regarding the risk for congenital infection among pregnant women with asymptomatic infection.”
Similarly, asymptomatic women potentially exposed to Zika virus should also wait at least 8 weeks after the possible exposure date before trying to conceive.
ECCMID 2016: Antimicrobial resistance, the microbiome and systems vaccinology
The global infectious disease and clinical microbiology community meets every year at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), the world’s largest congress on infectious diseases and medical microbiology, to present and discuss recent research results and to offer solutions to the most pressing infection problems.
The 2016 ECCMID annual conference, organized by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), will take place April 9-12 in Amsterdam. Discussions at this event not only help translate research findings into diagnostic tools, guidelines, best practices, and international policies; they also raise awareness of emerging health care challenges.
At ECCMID 2016, researchers will present more than 3,000 abstracts with the latest findings and recommendations to help improve diagnosis, prevention, and the clinical care given to patients. The Congress offers more than 150 oral presentations, including keynote lectures, symposia, oral sessions, educational workshops, and meet-the-experts sessions, as well as more than 2,000 poster presentations.
The main topics this year are strategies to detect and tackle antimicrobial resistance in various settings, approaches for prevention involving vaccines and infection control, as well as descriptions of novel diagnostic technologies. Always among the most popular sessions are lectures by winners of the ESCMID Award for Excellence and the Young Investigator Awards, as well as oral presentations on groundbreaking research, and late-breaking abstracts.
Also included will be mini oral “e-poster” presentations. Printed posters will be presented, but they will also be available at e-poster viewing stations, where visitors can scroll through abstracts of paper presentations.
Keynote speeches this year will feature innovative approaches to vaccines; microbiome and tuberculosis therapies; lectures on how nonhuman antibiotics affect public health; and an economic perspective on antimicrobial resistance.
Special topics
This year, the ECCMID Program Committee has decided to offer two special tracks for the late-breaking abstract sessions, focused on topics requiring a coordinated response from infection specialists across all disciplines.
The first topic is refugee and migrant health. The thousands of people who are currently migrating challenge public health systems in transition and the host countries. Clinicians and public health specialists need to develop strategies for the screening, prevention, and treatment of infectious diseases that were largely eradicated in Europe but are now gradually being reintroduced.
The second focus of the late-breaking abstracts is on emerging colistin resistance. Reports about the emergence of plasmid-borne resistance to this last-resort antibiotic have come from China, Canada, the United Kingdom, and most countries in continental Europe. Colistin resistance can spread easily between different types of bacteria, says Dr. Murat Akova, current ESCMID president and professor of medicine at Hacettepe University in Ankara, Turkey, and the world needs to wake up and take note.
In terms of viral infections, experts at the Congress will evaluate HIV and hepatitis C treatments in several interesting sessions. Researchers will also present results on emerging infections, including those caused by the Zika virus. Dr. Jean Paul Stahl, vice chairman of the ESCMID Study Group for Infectious Diseases of the Brain and professor of infectious diseases at University Hospital in Grenoble, France, says the current Zika virus epidemic is an important example of the great need we have for new evidence-based approaches on how to best manage emerging infections.
The outbreaks of Zika and Ebola in the last few years have seen the international community mobilize on infectious disease issues in a more collaborative manner than ever before, which should help reduce the severity of future outbreaks. But viral infections extend far beyond the recent outbreaks of unusual pathologies, and there are a number of important developments taking place among some of the more common viruses.
For more information on ECCMID 2016, visit http://www.eccmid.org/.
Dr. Winfried V. Kern is professor of medicine at the Albert Ludwigs University of Freiburg and head of the division of infectious diseases, department of medicine, and Centre for Infectious Diseases and Travel Medicine, University Hospital, Freiburg, Germany. His professional interests include bacterial multidrug resistance mechanisms and epidemiology, hospital antibiotic stewardship programs, health care–associated infections including infections in the immunocompromised host.
The global infectious disease and clinical microbiology community meets every year at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), the world’s largest congress on infectious diseases and medical microbiology, to present and discuss recent research results and to offer solutions to the most pressing infection problems.
The 2016 ECCMID annual conference, organized by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), will take place April 9-12 in Amsterdam. Discussions at this event not only help translate research findings into diagnostic tools, guidelines, best practices, and international policies; they also raise awareness of emerging health care challenges.
At ECCMID 2016, researchers will present more than 3,000 abstracts with the latest findings and recommendations to help improve diagnosis, prevention, and the clinical care given to patients. The Congress offers more than 150 oral presentations, including keynote lectures, symposia, oral sessions, educational workshops, and meet-the-experts sessions, as well as more than 2,000 poster presentations.
The main topics this year are strategies to detect and tackle antimicrobial resistance in various settings, approaches for prevention involving vaccines and infection control, as well as descriptions of novel diagnostic technologies. Always among the most popular sessions are lectures by winners of the ESCMID Award for Excellence and the Young Investigator Awards, as well as oral presentations on groundbreaking research, and late-breaking abstracts.
Also included will be mini oral “e-poster” presentations. Printed posters will be presented, but they will also be available at e-poster viewing stations, where visitors can scroll through abstracts of paper presentations.
Keynote speeches this year will feature innovative approaches to vaccines; microbiome and tuberculosis therapies; lectures on how nonhuman antibiotics affect public health; and an economic perspective on antimicrobial resistance.
Special topics
This year, the ECCMID Program Committee has decided to offer two special tracks for the late-breaking abstract sessions, focused on topics requiring a coordinated response from infection specialists across all disciplines.
The first topic is refugee and migrant health. The thousands of people who are currently migrating challenge public health systems in transition and the host countries. Clinicians and public health specialists need to develop strategies for the screening, prevention, and treatment of infectious diseases that were largely eradicated in Europe but are now gradually being reintroduced.
The second focus of the late-breaking abstracts is on emerging colistin resistance. Reports about the emergence of plasmid-borne resistance to this last-resort antibiotic have come from China, Canada, the United Kingdom, and most countries in continental Europe. Colistin resistance can spread easily between different types of bacteria, says Dr. Murat Akova, current ESCMID president and professor of medicine at Hacettepe University in Ankara, Turkey, and the world needs to wake up and take note.
In terms of viral infections, experts at the Congress will evaluate HIV and hepatitis C treatments in several interesting sessions. Researchers will also present results on emerging infections, including those caused by the Zika virus. Dr. Jean Paul Stahl, vice chairman of the ESCMID Study Group for Infectious Diseases of the Brain and professor of infectious diseases at University Hospital in Grenoble, France, says the current Zika virus epidemic is an important example of the great need we have for new evidence-based approaches on how to best manage emerging infections.
The outbreaks of Zika and Ebola in the last few years have seen the international community mobilize on infectious disease issues in a more collaborative manner than ever before, which should help reduce the severity of future outbreaks. But viral infections extend far beyond the recent outbreaks of unusual pathologies, and there are a number of important developments taking place among some of the more common viruses.
For more information on ECCMID 2016, visit http://www.eccmid.org/.
Dr. Winfried V. Kern is professor of medicine at the Albert Ludwigs University of Freiburg and head of the division of infectious diseases, department of medicine, and Centre for Infectious Diseases and Travel Medicine, University Hospital, Freiburg, Germany. His professional interests include bacterial multidrug resistance mechanisms and epidemiology, hospital antibiotic stewardship programs, health care–associated infections including infections in the immunocompromised host.
The global infectious disease and clinical microbiology community meets every year at the European Congress of Clinical Microbiology and Infectious Diseases (ECCMID), the world’s largest congress on infectious diseases and medical microbiology, to present and discuss recent research results and to offer solutions to the most pressing infection problems.
The 2016 ECCMID annual conference, organized by the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), will take place April 9-12 in Amsterdam. Discussions at this event not only help translate research findings into diagnostic tools, guidelines, best practices, and international policies; they also raise awareness of emerging health care challenges.
At ECCMID 2016, researchers will present more than 3,000 abstracts with the latest findings and recommendations to help improve diagnosis, prevention, and the clinical care given to patients. The Congress offers more than 150 oral presentations, including keynote lectures, symposia, oral sessions, educational workshops, and meet-the-experts sessions, as well as more than 2,000 poster presentations.
The main topics this year are strategies to detect and tackle antimicrobial resistance in various settings, approaches for prevention involving vaccines and infection control, as well as descriptions of novel diagnostic technologies. Always among the most popular sessions are lectures by winners of the ESCMID Award for Excellence and the Young Investigator Awards, as well as oral presentations on groundbreaking research, and late-breaking abstracts.
Also included will be mini oral “e-poster” presentations. Printed posters will be presented, but they will also be available at e-poster viewing stations, where visitors can scroll through abstracts of paper presentations.
Keynote speeches this year will feature innovative approaches to vaccines; microbiome and tuberculosis therapies; lectures on how nonhuman antibiotics affect public health; and an economic perspective on antimicrobial resistance.
Special topics
This year, the ECCMID Program Committee has decided to offer two special tracks for the late-breaking abstract sessions, focused on topics requiring a coordinated response from infection specialists across all disciplines.
The first topic is refugee and migrant health. The thousands of people who are currently migrating challenge public health systems in transition and the host countries. Clinicians and public health specialists need to develop strategies for the screening, prevention, and treatment of infectious diseases that were largely eradicated in Europe but are now gradually being reintroduced.
The second focus of the late-breaking abstracts is on emerging colistin resistance. Reports about the emergence of plasmid-borne resistance to this last-resort antibiotic have come from China, Canada, the United Kingdom, and most countries in continental Europe. Colistin resistance can spread easily between different types of bacteria, says Dr. Murat Akova, current ESCMID president and professor of medicine at Hacettepe University in Ankara, Turkey, and the world needs to wake up and take note.
In terms of viral infections, experts at the Congress will evaluate HIV and hepatitis C treatments in several interesting sessions. Researchers will also present results on emerging infections, including those caused by the Zika virus. Dr. Jean Paul Stahl, vice chairman of the ESCMID Study Group for Infectious Diseases of the Brain and professor of infectious diseases at University Hospital in Grenoble, France, says the current Zika virus epidemic is an important example of the great need we have for new evidence-based approaches on how to best manage emerging infections.
The outbreaks of Zika and Ebola in the last few years have seen the international community mobilize on infectious disease issues in a more collaborative manner than ever before, which should help reduce the severity of future outbreaks. But viral infections extend far beyond the recent outbreaks of unusual pathologies, and there are a number of important developments taking place among some of the more common viruses.
For more information on ECCMID 2016, visit http://www.eccmid.org/.
Dr. Winfried V. Kern is professor of medicine at the Albert Ludwigs University of Freiburg and head of the division of infectious diseases, department of medicine, and Centre for Infectious Diseases and Travel Medicine, University Hospital, Freiburg, Germany. His professional interests include bacterial multidrug resistance mechanisms and epidemiology, hospital antibiotic stewardship programs, health care–associated infections including infections in the immunocompromised host.
CDC urges precautions during L&D to prevent Zika transmission
Health care providers working in labor and delivery rooms should employ the standard precautions for infection control to reduce the theoretical risk of Zika transmission, according to recommendations from the Centers for Disease Control and Prevention.
“Because of the potential for exposure to large volumes of body fluids during the labor and delivery process and the sometimes unpredictable and fast-paced nature of obstetrical care, the use of Standard Precautions in these settings is essential to prevent possible transmission of Zika virus from patients to health care personnel,” Dr. Christine K. Olson and her colleagues at the CDC wrote in the Morbidity and Mortality Weekly Report.
No cases of occupational transmission of Zika via bodily fluids have been reported so far, but sexual transmission has occurred and the virus’s RNA has been found in blood, urine, saliva, and amniotic fluid. The risk of occupational exposure therefore theoretically exists, the authors wrote (MMWR. 2016 Mar 22. doi: 10.15585/mmwr.mm6511e3er).
The standard precautions are aimed at preventing transmission of any infectious agent present in blood, body fluids, secretions, nonperspiration excretions, nonintact skin, and mucous membranes. They include five main elements: hand hygiene, use of personal protection equipment (PPE), respiratory hygiene and cough etiquette, safe injection practices, and safe handling of potentially contaminated equipment or surfaces in the patient environment.
Standard PPE recommendations in labor and delivery rooms include eye protection during deliveries to prevent contamination from blood and bodily fluids and the use of double-gloving since the outer layer often contains perforations.
Health care personnel should already be following these standard precautions in all health care settings, but the CDC report will likely remind providers of the importance of these infection control procedures and improve compliance, said Dr. Aaron Caughey, chair of the department of obstetrics and gynecology and associate dean for Women’s Health Research and Policy at Oregon Health and Science University, Portland.
“Those are the standard recommendations on every labor floor for delivery currently, so absolutely it’s feasible,” he said in an interview. However, “for a low-risk woman doing a vaginal delivery without complications, I know that some people skimp on eyewear, and I know there are occasionally people who will wear just one pair of gloves,” he said.
The CDC report also noted varying levels of adherence to the standard precautions.
“Numerous barriers to the appropriate use of PPE have been cited, including the perception that PPE is uncomfortable and limits dexterity, fogging of goggles or face masks, the misperception that prescription eyeglasses provide adequate eye protection, lack of available PPE, forgetting to use PPE, lack of time in urgent clinical situations to don appropriate PPE, the perception that the patient poses minimal risk, and concerns about interference with patient care,” Dr. Olson and her colleagues wrote.
Dr. Caughey drew parallels to the late 1980s and early 1990s, when compliance with eye safety and glove safety precautions increased dramatically alongside the HIV epidemic.
“With these recommendations, it will probably get a little more heightened, particularly if there are parts of the country where Zika becomes endemic,” he said. “The big difference with Zika is if a man contracts Zika or a woman who’s not pregnant contracts Zika, the risks to them are very, very low. It’s really about pregnant women and the risk to the fetus.”
The CDC authors specifically noted that the theoretical risk for Zika transmission is most relevant for female health care personnel who may be pregnant, or for male or female health care personnel attempting to conceive. They recommended that personnel determine the most appropriate PPE based on the likelihood of body fluid exposure for each type of procedure or activity.
An amniotomy or placement of an intrauterine pressure catheter may require mask, eye protection, gloves and an impermeable gown, for example, but vaginal exams of pregnant women with minimal cervical dilation and intact membranes likely only call for the use of gloves. Anesthesia providers should wear sterile gloves and a surgical mask when they place catheters or administer intrathecal injections, and all providers should wear double gloves while handling sharps.
“When performing procedures including vaginal deliveries, manual placenta removal, bimanual uterine massage, and repair of vaginal lacerations, PPE should include (in addition to mucous membrane and skin protection) impermeable gowns and knee-high impermeable shoe covers,” they wrote.
Health care providers working in labor and delivery rooms should employ the standard precautions for infection control to reduce the theoretical risk of Zika transmission, according to recommendations from the Centers for Disease Control and Prevention.
“Because of the potential for exposure to large volumes of body fluids during the labor and delivery process and the sometimes unpredictable and fast-paced nature of obstetrical care, the use of Standard Precautions in these settings is essential to prevent possible transmission of Zika virus from patients to health care personnel,” Dr. Christine K. Olson and her colleagues at the CDC wrote in the Morbidity and Mortality Weekly Report.
No cases of occupational transmission of Zika via bodily fluids have been reported so far, but sexual transmission has occurred and the virus’s RNA has been found in blood, urine, saliva, and amniotic fluid. The risk of occupational exposure therefore theoretically exists, the authors wrote (MMWR. 2016 Mar 22. doi: 10.15585/mmwr.mm6511e3er).
The standard precautions are aimed at preventing transmission of any infectious agent present in blood, body fluids, secretions, nonperspiration excretions, nonintact skin, and mucous membranes. They include five main elements: hand hygiene, use of personal protection equipment (PPE), respiratory hygiene and cough etiquette, safe injection practices, and safe handling of potentially contaminated equipment or surfaces in the patient environment.
Standard PPE recommendations in labor and delivery rooms include eye protection during deliveries to prevent contamination from blood and bodily fluids and the use of double-gloving since the outer layer often contains perforations.
Health care personnel should already be following these standard precautions in all health care settings, but the CDC report will likely remind providers of the importance of these infection control procedures and improve compliance, said Dr. Aaron Caughey, chair of the department of obstetrics and gynecology and associate dean for Women’s Health Research and Policy at Oregon Health and Science University, Portland.
“Those are the standard recommendations on every labor floor for delivery currently, so absolutely it’s feasible,” he said in an interview. However, “for a low-risk woman doing a vaginal delivery without complications, I know that some people skimp on eyewear, and I know there are occasionally people who will wear just one pair of gloves,” he said.
The CDC report also noted varying levels of adherence to the standard precautions.
“Numerous barriers to the appropriate use of PPE have been cited, including the perception that PPE is uncomfortable and limits dexterity, fogging of goggles or face masks, the misperception that prescription eyeglasses provide adequate eye protection, lack of available PPE, forgetting to use PPE, lack of time in urgent clinical situations to don appropriate PPE, the perception that the patient poses minimal risk, and concerns about interference with patient care,” Dr. Olson and her colleagues wrote.
Dr. Caughey drew parallels to the late 1980s and early 1990s, when compliance with eye safety and glove safety precautions increased dramatically alongside the HIV epidemic.
“With these recommendations, it will probably get a little more heightened, particularly if there are parts of the country where Zika becomes endemic,” he said. “The big difference with Zika is if a man contracts Zika or a woman who’s not pregnant contracts Zika, the risks to them are very, very low. It’s really about pregnant women and the risk to the fetus.”
The CDC authors specifically noted that the theoretical risk for Zika transmission is most relevant for female health care personnel who may be pregnant, or for male or female health care personnel attempting to conceive. They recommended that personnel determine the most appropriate PPE based on the likelihood of body fluid exposure for each type of procedure or activity.
An amniotomy or placement of an intrauterine pressure catheter may require mask, eye protection, gloves and an impermeable gown, for example, but vaginal exams of pregnant women with minimal cervical dilation and intact membranes likely only call for the use of gloves. Anesthesia providers should wear sterile gloves and a surgical mask when they place catheters or administer intrathecal injections, and all providers should wear double gloves while handling sharps.
“When performing procedures including vaginal deliveries, manual placenta removal, bimanual uterine massage, and repair of vaginal lacerations, PPE should include (in addition to mucous membrane and skin protection) impermeable gowns and knee-high impermeable shoe covers,” they wrote.
Health care providers working in labor and delivery rooms should employ the standard precautions for infection control to reduce the theoretical risk of Zika transmission, according to recommendations from the Centers for Disease Control and Prevention.
“Because of the potential for exposure to large volumes of body fluids during the labor and delivery process and the sometimes unpredictable and fast-paced nature of obstetrical care, the use of Standard Precautions in these settings is essential to prevent possible transmission of Zika virus from patients to health care personnel,” Dr. Christine K. Olson and her colleagues at the CDC wrote in the Morbidity and Mortality Weekly Report.
No cases of occupational transmission of Zika via bodily fluids have been reported so far, but sexual transmission has occurred and the virus’s RNA has been found in blood, urine, saliva, and amniotic fluid. The risk of occupational exposure therefore theoretically exists, the authors wrote (MMWR. 2016 Mar 22. doi: 10.15585/mmwr.mm6511e3er).
The standard precautions are aimed at preventing transmission of any infectious agent present in blood, body fluids, secretions, nonperspiration excretions, nonintact skin, and mucous membranes. They include five main elements: hand hygiene, use of personal protection equipment (PPE), respiratory hygiene and cough etiquette, safe injection practices, and safe handling of potentially contaminated equipment or surfaces in the patient environment.
Standard PPE recommendations in labor and delivery rooms include eye protection during deliveries to prevent contamination from blood and bodily fluids and the use of double-gloving since the outer layer often contains perforations.
Health care personnel should already be following these standard precautions in all health care settings, but the CDC report will likely remind providers of the importance of these infection control procedures and improve compliance, said Dr. Aaron Caughey, chair of the department of obstetrics and gynecology and associate dean for Women’s Health Research and Policy at Oregon Health and Science University, Portland.
“Those are the standard recommendations on every labor floor for delivery currently, so absolutely it’s feasible,” he said in an interview. However, “for a low-risk woman doing a vaginal delivery without complications, I know that some people skimp on eyewear, and I know there are occasionally people who will wear just one pair of gloves,” he said.
The CDC report also noted varying levels of adherence to the standard precautions.
“Numerous barriers to the appropriate use of PPE have been cited, including the perception that PPE is uncomfortable and limits dexterity, fogging of goggles or face masks, the misperception that prescription eyeglasses provide adequate eye protection, lack of available PPE, forgetting to use PPE, lack of time in urgent clinical situations to don appropriate PPE, the perception that the patient poses minimal risk, and concerns about interference with patient care,” Dr. Olson and her colleagues wrote.
Dr. Caughey drew parallels to the late 1980s and early 1990s, when compliance with eye safety and glove safety precautions increased dramatically alongside the HIV epidemic.
“With these recommendations, it will probably get a little more heightened, particularly if there are parts of the country where Zika becomes endemic,” he said. “The big difference with Zika is if a man contracts Zika or a woman who’s not pregnant contracts Zika, the risks to them are very, very low. It’s really about pregnant women and the risk to the fetus.”
The CDC authors specifically noted that the theoretical risk for Zika transmission is most relevant for female health care personnel who may be pregnant, or for male or female health care personnel attempting to conceive. They recommended that personnel determine the most appropriate PPE based on the likelihood of body fluid exposure for each type of procedure or activity.
An amniotomy or placement of an intrauterine pressure catheter may require mask, eye protection, gloves and an impermeable gown, for example, but vaginal exams of pregnant women with minimal cervical dilation and intact membranes likely only call for the use of gloves. Anesthesia providers should wear sterile gloves and a surgical mask when they place catheters or administer intrathecal injections, and all providers should wear double gloves while handling sharps.
“When performing procedures including vaginal deliveries, manual placenta removal, bimanual uterine massage, and repair of vaginal lacerations, PPE should include (in addition to mucous membrane and skin protection) impermeable gowns and knee-high impermeable shoe covers,” they wrote.
FROM MMWR
Zika virus: More questions than answers?
With spring break in full swing and summer vacations right around the corner, pediatricians are increasingly fielding questions from families about Zika virus.
“There are a lot of resources available online, but they’re constantly being updated, and it’s difficult to stay current,” a friend and fellow pediatrician confided. “It seems like there’s new information every day, but still as many questions as answers.”
A quick PubMed search validated her concern: More than 200 articles have been published about Zika virus since the beginning of the year. The Centers for Disease Control and Prevention and the World Health Organization post new information to their Zika websites regularly, if not daily, and the WHO has released a Zika app for clinicians. Understanding that the busy pediatrician may not always have time to peruse these authoritative references during the course of a day in the office, I’ve compiled some common questions and answers.
“Is Zika really as serious as the media portrays it?” asked the mother of two children as she contemplated Caribbean vacation plans. In truth, most healthy people infected with Zika virus never develop symptoms. Illness, when it occurs, is most often mild and includes low-grade fever, headache, arthralgia, myalgia, nonpurulent conjunctivitis, and a maculopapular rash. Unlike dengue, another Flavivirus carried by Aedes mosquitoes, Zika does not cause hemorrhagic fever, and death appears to be rare.
An understanding of Zika infection and neurologic complications is a work in progress. A 20-fold increase in the incidence of Guillain-Barré (GBS) cases was noted in French Polynesia during a 2013-2014 outbreak of Zika virus.
In a case-control study involving 42 patients hospitalized with GBS, 98% had anti–Zika virus IgM or IgG, and all had neutralizing antibodies against Zika virus, compared with 56% of 98 control patients (P less than .0001 ) (Lancet. 2016 Feb 29. doi: 10.1016/S0140-6736(16)00562-6).
To date, 10 countries or territories have reported GBS cases with confirmed Zika virus infection. According to the World Health Organization, “Zika virus is highly likely to be a cause of the elevated incidence of GBS in countries and territories in the Western Pacific and Americas,” but further research is needed. Zika has recently been associated with other neurologic disorders, including myelitis, and the full spectrum of disease is likely not yet known.
Most Zika virus infections are transmitted from the bite of an Aedes mosquito. What we know about Zika transmission among humans continues to evolve. Viremia can persist for 14 or more days after the onset of symptoms, during which time blood is a potential source of infection. Two possible cases of transfusion-related viral transmission are under investigation in Brazil, and during the French Polynesia outbreak, 3% of samples from asymptomatic blood donors contained detectable Zika RNA. The U.S. Food and Drug Administration has recommended that individuals who have lived in or traveled to an area with active Zika virus transmission defer blood donation for 4 weeks after departure from the area .
Zika virus also has been detected in the urine and saliva of infected individuals, but these fluids have not been linked to transmission. Sexual transmission from infected men to their partners is well documented, but the period of risk remains undefined. The virus can persist in the semen long after viremia clears, and in one individual, Zika virus was detected in the semen 62 days after symptom onset.
Maternal-fetal transmission can occur as early as the first trimester and as late as at the time of delivery. Zika virus has been recovered from both amniotic fluid and placentas. The consequences of maternal-fetal transmission are less certain. Coincident with an epidemic of Zika in Brazil, that country has observed a marked increase in the incidence of microcephaly. Between Oct. 22, 2015, and March 12, 2016, 6,480 cases of microcephaly and/or central nervous system malformation were reported in Brazil, contrasting sharply with the average of 163 cases reported annually from 2001 to 2014. Zika virus has been linked to 863 cases of microcephaly investigated thus far. Proving causality takes time, but the World Health Organization says the link between microcephaly and Zika infection is “strongly suspected.”
Because of the association between Zika virus and birth defects, including abnormal brain development, eye abnormalities, and hearing deficits, the CDC currently recommends that pregnant women not travel to areas with Zika transmission, while men who have lived in or traveled to an area with Zika and who have a pregnant partner should either use condoms or not have sex for the duration of the pregnancy.
The good news for nonpregnant women who contract Zika infection is that the infection is not thought to pose any risk to future pregnancies. Currently, there is no evidence that a fetus conceived after maternal viremia has resolved would be at risk for infection. Still, many unanswered questions remain about Zika infection during pregnancy. For example, it’s currently unknown how often infection is transmitted from an infected mother to her fetus, or if infection is more severe at a particular point in gestation.
Although Zika virus has been isolated from breast milk, no infections have been linked to breastfeeding, and mothers are encouraged to continue to nurse, even in areas with widespread transmission. Infection with Zika at the time of birth or later in childhood has not been linked to microcephaly. Beyond that, the long-term health outcomes of infants and children with Zika virus infection are unknown.
“How far north do you think the virus will spread?” one mom asked me. “Do I need to be worried?”
For public health officials, that’s the sixty-four thousand dollar question. To date, there have been no cases acquired as a result of a mosquito bite in the United States, but the edge of the outbreak continues to creep north. Local transmission of the virus was reported in Cuba on March 14.
As of March 16, 2016, 258 travel-associated Zika virus cases have been diagnosed in the United States, including 18 in pregnant women. Six of these were sexually transmitted. Theoretically, “onward transmission” from one of these cases could occur if the right kind of mosquito bites an infected person during the period of active viremia and then bites someone else, transferring a tiny amount of the virus-contaminated blood.
According to CDC experts, “Texas, Florida, and Hawaii are likely to be the U.S. states with the highest risk of experiencing local transmission of Zika virus by mosquitoes.” Although this estimate is based on prior experience with similar viruses, the principal vector of Zika, Aedes aegypti, has been identified as far west as California and in a number of states across the South, including my home state of Kentucky. Aedes albopictus mosquitoes also have been proven competent vectors for Zika virus transmission and are more widely distributed throughout the continental United States.
In a thoughtful review published in JAMA Pediatrics, “What Pediatricians and Other Clinicians Should Know About Zika Virus,” Dr. Mark W. Kline and Dr. Gordon E. Schutze noted that up to two-thirds of the U.S. population live in an area where Aedes mosquitoes are present at least part of the year (JAMA Pediatr. 2016 Feb 18. doi: 10.1001/jamapediatrics.2016.0429). Fortunately, transmission of dengue and chikungunya, two other viruses carried by the same insect, is still very uncommon. Public health experts are urging individuals with Zika virus infection to avoid mosquito bites during the first week of illness, to protect others.
We should start now counseling our patients and families to avoid mosquito bites at home and abroad. Besides Zika virus, mosquitoes transmit several pathogens in the United States each year, including West Nile virus, LaCrosse encephalitis virus, St. Louis encephalitis virus, and dengue.
Any collections of standing water should be eliminated, as these can be mosquito breeding grounds. These include flower pots, buckets, barrels, and discarded tires. The water in bird baths and pet dishes should be changed at least weekly, and children’s wading pools should be drained and stored on their side after use.
To the extent practical, exposed skin should be covered with long-sleeved shirts, long pants, and socks when individuals are in areas with mosquito activity. To enhance protection, clothing can be treated with permethrin, or pretreated clothing can be worn. An FDA-registered insect repellent should be applied to exposed skin, especially during hours of highest mosquito activity. Zika-carrying mosquitoes bite during the day, or dawn to dusk. Effective repellents include DEET, picaridin, IR3535, and oil of lemon eucalyptus, although families should read labels carefully as instructions for use vary, as does the recommended time period of reapplication. Combination sunscreen/insect repellent products are not recommended as repellent usually does not need to be reapplied as often as sunscreen. Parents also should be reminded not to use oil of lemon eucalyptus–containing products on children under 3 years of age.
“We’re going to get a lot more questions as the weather turns warmer,” said a colleague of mine. “I’m just waiting for the first call about a child who develops fever and a rash after a mosquito bite. Parents will wonder if it could be Zika.”
It is going to be an interesting summer. Stay tuned.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
With spring break in full swing and summer vacations right around the corner, pediatricians are increasingly fielding questions from families about Zika virus.
“There are a lot of resources available online, but they’re constantly being updated, and it’s difficult to stay current,” a friend and fellow pediatrician confided. “It seems like there’s new information every day, but still as many questions as answers.”
A quick PubMed search validated her concern: More than 200 articles have been published about Zika virus since the beginning of the year. The Centers for Disease Control and Prevention and the World Health Organization post new information to their Zika websites regularly, if not daily, and the WHO has released a Zika app for clinicians. Understanding that the busy pediatrician may not always have time to peruse these authoritative references during the course of a day in the office, I’ve compiled some common questions and answers.
“Is Zika really as serious as the media portrays it?” asked the mother of two children as she contemplated Caribbean vacation plans. In truth, most healthy people infected with Zika virus never develop symptoms. Illness, when it occurs, is most often mild and includes low-grade fever, headache, arthralgia, myalgia, nonpurulent conjunctivitis, and a maculopapular rash. Unlike dengue, another Flavivirus carried by Aedes mosquitoes, Zika does not cause hemorrhagic fever, and death appears to be rare.
An understanding of Zika infection and neurologic complications is a work in progress. A 20-fold increase in the incidence of Guillain-Barré (GBS) cases was noted in French Polynesia during a 2013-2014 outbreak of Zika virus.
In a case-control study involving 42 patients hospitalized with GBS, 98% had anti–Zika virus IgM or IgG, and all had neutralizing antibodies against Zika virus, compared with 56% of 98 control patients (P less than .0001 ) (Lancet. 2016 Feb 29. doi: 10.1016/S0140-6736(16)00562-6).
To date, 10 countries or territories have reported GBS cases with confirmed Zika virus infection. According to the World Health Organization, “Zika virus is highly likely to be a cause of the elevated incidence of GBS in countries and territories in the Western Pacific and Americas,” but further research is needed. Zika has recently been associated with other neurologic disorders, including myelitis, and the full spectrum of disease is likely not yet known.
Most Zika virus infections are transmitted from the bite of an Aedes mosquito. What we know about Zika transmission among humans continues to evolve. Viremia can persist for 14 or more days after the onset of symptoms, during which time blood is a potential source of infection. Two possible cases of transfusion-related viral transmission are under investigation in Brazil, and during the French Polynesia outbreak, 3% of samples from asymptomatic blood donors contained detectable Zika RNA. The U.S. Food and Drug Administration has recommended that individuals who have lived in or traveled to an area with active Zika virus transmission defer blood donation for 4 weeks after departure from the area .
Zika virus also has been detected in the urine and saliva of infected individuals, but these fluids have not been linked to transmission. Sexual transmission from infected men to their partners is well documented, but the period of risk remains undefined. The virus can persist in the semen long after viremia clears, and in one individual, Zika virus was detected in the semen 62 days after symptom onset.
Maternal-fetal transmission can occur as early as the first trimester and as late as at the time of delivery. Zika virus has been recovered from both amniotic fluid and placentas. The consequences of maternal-fetal transmission are less certain. Coincident with an epidemic of Zika in Brazil, that country has observed a marked increase in the incidence of microcephaly. Between Oct. 22, 2015, and March 12, 2016, 6,480 cases of microcephaly and/or central nervous system malformation were reported in Brazil, contrasting sharply with the average of 163 cases reported annually from 2001 to 2014. Zika virus has been linked to 863 cases of microcephaly investigated thus far. Proving causality takes time, but the World Health Organization says the link between microcephaly and Zika infection is “strongly suspected.”
Because of the association between Zika virus and birth defects, including abnormal brain development, eye abnormalities, and hearing deficits, the CDC currently recommends that pregnant women not travel to areas with Zika transmission, while men who have lived in or traveled to an area with Zika and who have a pregnant partner should either use condoms or not have sex for the duration of the pregnancy.
The good news for nonpregnant women who contract Zika infection is that the infection is not thought to pose any risk to future pregnancies. Currently, there is no evidence that a fetus conceived after maternal viremia has resolved would be at risk for infection. Still, many unanswered questions remain about Zika infection during pregnancy. For example, it’s currently unknown how often infection is transmitted from an infected mother to her fetus, or if infection is more severe at a particular point in gestation.
Although Zika virus has been isolated from breast milk, no infections have been linked to breastfeeding, and mothers are encouraged to continue to nurse, even in areas with widespread transmission. Infection with Zika at the time of birth or later in childhood has not been linked to microcephaly. Beyond that, the long-term health outcomes of infants and children with Zika virus infection are unknown.
“How far north do you think the virus will spread?” one mom asked me. “Do I need to be worried?”
For public health officials, that’s the sixty-four thousand dollar question. To date, there have been no cases acquired as a result of a mosquito bite in the United States, but the edge of the outbreak continues to creep north. Local transmission of the virus was reported in Cuba on March 14.
As of March 16, 2016, 258 travel-associated Zika virus cases have been diagnosed in the United States, including 18 in pregnant women. Six of these were sexually transmitted. Theoretically, “onward transmission” from one of these cases could occur if the right kind of mosquito bites an infected person during the period of active viremia and then bites someone else, transferring a tiny amount of the virus-contaminated blood.
According to CDC experts, “Texas, Florida, and Hawaii are likely to be the U.S. states with the highest risk of experiencing local transmission of Zika virus by mosquitoes.” Although this estimate is based on prior experience with similar viruses, the principal vector of Zika, Aedes aegypti, has been identified as far west as California and in a number of states across the South, including my home state of Kentucky. Aedes albopictus mosquitoes also have been proven competent vectors for Zika virus transmission and are more widely distributed throughout the continental United States.
In a thoughtful review published in JAMA Pediatrics, “What Pediatricians and Other Clinicians Should Know About Zika Virus,” Dr. Mark W. Kline and Dr. Gordon E. Schutze noted that up to two-thirds of the U.S. population live in an area where Aedes mosquitoes are present at least part of the year (JAMA Pediatr. 2016 Feb 18. doi: 10.1001/jamapediatrics.2016.0429). Fortunately, transmission of dengue and chikungunya, two other viruses carried by the same insect, is still very uncommon. Public health experts are urging individuals with Zika virus infection to avoid mosquito bites during the first week of illness, to protect others.
We should start now counseling our patients and families to avoid mosquito bites at home and abroad. Besides Zika virus, mosquitoes transmit several pathogens in the United States each year, including West Nile virus, LaCrosse encephalitis virus, St. Louis encephalitis virus, and dengue.
Any collections of standing water should be eliminated, as these can be mosquito breeding grounds. These include flower pots, buckets, barrels, and discarded tires. The water in bird baths and pet dishes should be changed at least weekly, and children’s wading pools should be drained and stored on their side after use.
To the extent practical, exposed skin should be covered with long-sleeved shirts, long pants, and socks when individuals are in areas with mosquito activity. To enhance protection, clothing can be treated with permethrin, or pretreated clothing can be worn. An FDA-registered insect repellent should be applied to exposed skin, especially during hours of highest mosquito activity. Zika-carrying mosquitoes bite during the day, or dawn to dusk. Effective repellents include DEET, picaridin, IR3535, and oil of lemon eucalyptus, although families should read labels carefully as instructions for use vary, as does the recommended time period of reapplication. Combination sunscreen/insect repellent products are not recommended as repellent usually does not need to be reapplied as often as sunscreen. Parents also should be reminded not to use oil of lemon eucalyptus–containing products on children under 3 years of age.
“We’re going to get a lot more questions as the weather turns warmer,” said a colleague of mine. “I’m just waiting for the first call about a child who develops fever and a rash after a mosquito bite. Parents will wonder if it could be Zika.”
It is going to be an interesting summer. Stay tuned.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
With spring break in full swing and summer vacations right around the corner, pediatricians are increasingly fielding questions from families about Zika virus.
“There are a lot of resources available online, but they’re constantly being updated, and it’s difficult to stay current,” a friend and fellow pediatrician confided. “It seems like there’s new information every day, but still as many questions as answers.”
A quick PubMed search validated her concern: More than 200 articles have been published about Zika virus since the beginning of the year. The Centers for Disease Control and Prevention and the World Health Organization post new information to their Zika websites regularly, if not daily, and the WHO has released a Zika app for clinicians. Understanding that the busy pediatrician may not always have time to peruse these authoritative references during the course of a day in the office, I’ve compiled some common questions and answers.
“Is Zika really as serious as the media portrays it?” asked the mother of two children as she contemplated Caribbean vacation plans. In truth, most healthy people infected with Zika virus never develop symptoms. Illness, when it occurs, is most often mild and includes low-grade fever, headache, arthralgia, myalgia, nonpurulent conjunctivitis, and a maculopapular rash. Unlike dengue, another Flavivirus carried by Aedes mosquitoes, Zika does not cause hemorrhagic fever, and death appears to be rare.
An understanding of Zika infection and neurologic complications is a work in progress. A 20-fold increase in the incidence of Guillain-Barré (GBS) cases was noted in French Polynesia during a 2013-2014 outbreak of Zika virus.
In a case-control study involving 42 patients hospitalized with GBS, 98% had anti–Zika virus IgM or IgG, and all had neutralizing antibodies against Zika virus, compared with 56% of 98 control patients (P less than .0001 ) (Lancet. 2016 Feb 29. doi: 10.1016/S0140-6736(16)00562-6).
To date, 10 countries or territories have reported GBS cases with confirmed Zika virus infection. According to the World Health Organization, “Zika virus is highly likely to be a cause of the elevated incidence of GBS in countries and territories in the Western Pacific and Americas,” but further research is needed. Zika has recently been associated with other neurologic disorders, including myelitis, and the full spectrum of disease is likely not yet known.
Most Zika virus infections are transmitted from the bite of an Aedes mosquito. What we know about Zika transmission among humans continues to evolve. Viremia can persist for 14 or more days after the onset of symptoms, during which time blood is a potential source of infection. Two possible cases of transfusion-related viral transmission are under investigation in Brazil, and during the French Polynesia outbreak, 3% of samples from asymptomatic blood donors contained detectable Zika RNA. The U.S. Food and Drug Administration has recommended that individuals who have lived in or traveled to an area with active Zika virus transmission defer blood donation for 4 weeks after departure from the area .
Zika virus also has been detected in the urine and saliva of infected individuals, but these fluids have not been linked to transmission. Sexual transmission from infected men to their partners is well documented, but the period of risk remains undefined. The virus can persist in the semen long after viremia clears, and in one individual, Zika virus was detected in the semen 62 days after symptom onset.
Maternal-fetal transmission can occur as early as the first trimester and as late as at the time of delivery. Zika virus has been recovered from both amniotic fluid and placentas. The consequences of maternal-fetal transmission are less certain. Coincident with an epidemic of Zika in Brazil, that country has observed a marked increase in the incidence of microcephaly. Between Oct. 22, 2015, and March 12, 2016, 6,480 cases of microcephaly and/or central nervous system malformation were reported in Brazil, contrasting sharply with the average of 163 cases reported annually from 2001 to 2014. Zika virus has been linked to 863 cases of microcephaly investigated thus far. Proving causality takes time, but the World Health Organization says the link between microcephaly and Zika infection is “strongly suspected.”
Because of the association between Zika virus and birth defects, including abnormal brain development, eye abnormalities, and hearing deficits, the CDC currently recommends that pregnant women not travel to areas with Zika transmission, while men who have lived in or traveled to an area with Zika and who have a pregnant partner should either use condoms or not have sex for the duration of the pregnancy.
The good news for nonpregnant women who contract Zika infection is that the infection is not thought to pose any risk to future pregnancies. Currently, there is no evidence that a fetus conceived after maternal viremia has resolved would be at risk for infection. Still, many unanswered questions remain about Zika infection during pregnancy. For example, it’s currently unknown how often infection is transmitted from an infected mother to her fetus, or if infection is more severe at a particular point in gestation.
Although Zika virus has been isolated from breast milk, no infections have been linked to breastfeeding, and mothers are encouraged to continue to nurse, even in areas with widespread transmission. Infection with Zika at the time of birth or later in childhood has not been linked to microcephaly. Beyond that, the long-term health outcomes of infants and children with Zika virus infection are unknown.
“How far north do you think the virus will spread?” one mom asked me. “Do I need to be worried?”
For public health officials, that’s the sixty-four thousand dollar question. To date, there have been no cases acquired as a result of a mosquito bite in the United States, but the edge of the outbreak continues to creep north. Local transmission of the virus was reported in Cuba on March 14.
As of March 16, 2016, 258 travel-associated Zika virus cases have been diagnosed in the United States, including 18 in pregnant women. Six of these were sexually transmitted. Theoretically, “onward transmission” from one of these cases could occur if the right kind of mosquito bites an infected person during the period of active viremia and then bites someone else, transferring a tiny amount of the virus-contaminated blood.
According to CDC experts, “Texas, Florida, and Hawaii are likely to be the U.S. states with the highest risk of experiencing local transmission of Zika virus by mosquitoes.” Although this estimate is based on prior experience with similar viruses, the principal vector of Zika, Aedes aegypti, has been identified as far west as California and in a number of states across the South, including my home state of Kentucky. Aedes albopictus mosquitoes also have been proven competent vectors for Zika virus transmission and are more widely distributed throughout the continental United States.
In a thoughtful review published in JAMA Pediatrics, “What Pediatricians and Other Clinicians Should Know About Zika Virus,” Dr. Mark W. Kline and Dr. Gordon E. Schutze noted that up to two-thirds of the U.S. population live in an area where Aedes mosquitoes are present at least part of the year (JAMA Pediatr. 2016 Feb 18. doi: 10.1001/jamapediatrics.2016.0429). Fortunately, transmission of dengue and chikungunya, two other viruses carried by the same insect, is still very uncommon. Public health experts are urging individuals with Zika virus infection to avoid mosquito bites during the first week of illness, to protect others.
We should start now counseling our patients and families to avoid mosquito bites at home and abroad. Besides Zika virus, mosquitoes transmit several pathogens in the United States each year, including West Nile virus, LaCrosse encephalitis virus, St. Louis encephalitis virus, and dengue.
Any collections of standing water should be eliminated, as these can be mosquito breeding grounds. These include flower pots, buckets, barrels, and discarded tires. The water in bird baths and pet dishes should be changed at least weekly, and children’s wading pools should be drained and stored on their side after use.
To the extent practical, exposed skin should be covered with long-sleeved shirts, long pants, and socks when individuals are in areas with mosquito activity. To enhance protection, clothing can be treated with permethrin, or pretreated clothing can be worn. An FDA-registered insect repellent should be applied to exposed skin, especially during hours of highest mosquito activity. Zika-carrying mosquitoes bite during the day, or dawn to dusk. Effective repellents include DEET, picaridin, IR3535, and oil of lemon eucalyptus, although families should read labels carefully as instructions for use vary, as does the recommended time period of reapplication. Combination sunscreen/insect repellent products are not recommended as repellent usually does not need to be reapplied as often as sunscreen. Parents also should be reminded not to use oil of lemon eucalyptus–containing products on children under 3 years of age.
“We’re going to get a lot more questions as the weather turns warmer,” said a colleague of mine. “I’m just waiting for the first call about a child who develops fever and a rash after a mosquito bite. Parents will wonder if it could be Zika.”
It is going to be an interesting summer. Stay tuned.
Dr. Bryant is a pediatrician specializing in infectious diseases at the University of Louisville (Ky.) and Kosair Children’s Hospital, also in Louisville. She had no relevant financial disclosures.
FDA approves new anthrax treatment
The Food and Drug Administration has approved Anthim (obiltoxaximab) injection to treat inhalational anthrax, in combination with appropriate antibacterial drugs.
Anthim is a monoclonal antibody that neutralizes toxins produced by the bacterium Bacillus anthracis. Anthim was approved under the FDA’s Animal Rule, which allows efficacy findings from adequate and well-controlled animal studies to support FDA approval when it is not feasible or ethical to conduct efficacy trials in humans. Anthim is also approved to prevent inhalational anthrax when alternative therapies are not available or not appropriate.
The drug was developed by Elusys Therapeutics Inc. of Pine Brook, N.J., in conjunction with the U.S. Department of Health and Human Services’ Biomedical Advanced Research and Development Authority.
Inhalational anthrax is a rare disease that can occur after exposure to infected animals or contaminated animal products, or as a result of an intentional release of anthrax spores. When inhaled, the anthrax bacteria replicate in the body and produce toxins that can cause massive and irreversible tissue injury and death.
Anthim’s effectiveness was demonstrated in studies conducted in animals based on survival at the end of the studies. More animals treated with Anthim lived, compared with animals treated with placebo. Anthim administered in combination with antibacterial drugs resulted in higher survival outcomes than antibacterial therapy alone.
The safety of Anthim was evaluated in 320 healthy human volunteers. The most frequently reported side effects were headache, itching (pruritus), upper respiratory tract infections, cough, nasal congestion, hives, and bruising, swelling and pain at the infusion site.
For more information, see the FDA’s approval announcement.
On Twitter @richpizzi
The Food and Drug Administration has approved Anthim (obiltoxaximab) injection to treat inhalational anthrax, in combination with appropriate antibacterial drugs.
Anthim is a monoclonal antibody that neutralizes toxins produced by the bacterium Bacillus anthracis. Anthim was approved under the FDA’s Animal Rule, which allows efficacy findings from adequate and well-controlled animal studies to support FDA approval when it is not feasible or ethical to conduct efficacy trials in humans. Anthim is also approved to prevent inhalational anthrax when alternative therapies are not available or not appropriate.
The drug was developed by Elusys Therapeutics Inc. of Pine Brook, N.J., in conjunction with the U.S. Department of Health and Human Services’ Biomedical Advanced Research and Development Authority.
Inhalational anthrax is a rare disease that can occur after exposure to infected animals or contaminated animal products, or as a result of an intentional release of anthrax spores. When inhaled, the anthrax bacteria replicate in the body and produce toxins that can cause massive and irreversible tissue injury and death.
Anthim’s effectiveness was demonstrated in studies conducted in animals based on survival at the end of the studies. More animals treated with Anthim lived, compared with animals treated with placebo. Anthim administered in combination with antibacterial drugs resulted in higher survival outcomes than antibacterial therapy alone.
The safety of Anthim was evaluated in 320 healthy human volunteers. The most frequently reported side effects were headache, itching (pruritus), upper respiratory tract infections, cough, nasal congestion, hives, and bruising, swelling and pain at the infusion site.
For more information, see the FDA’s approval announcement.
On Twitter @richpizzi
The Food and Drug Administration has approved Anthim (obiltoxaximab) injection to treat inhalational anthrax, in combination with appropriate antibacterial drugs.
Anthim is a monoclonal antibody that neutralizes toxins produced by the bacterium Bacillus anthracis. Anthim was approved under the FDA’s Animal Rule, which allows efficacy findings from adequate and well-controlled animal studies to support FDA approval when it is not feasible or ethical to conduct efficacy trials in humans. Anthim is also approved to prevent inhalational anthrax when alternative therapies are not available or not appropriate.
The drug was developed by Elusys Therapeutics Inc. of Pine Brook, N.J., in conjunction with the U.S. Department of Health and Human Services’ Biomedical Advanced Research and Development Authority.
Inhalational anthrax is a rare disease that can occur after exposure to infected animals or contaminated animal products, or as a result of an intentional release of anthrax spores. When inhaled, the anthrax bacteria replicate in the body and produce toxins that can cause massive and irreversible tissue injury and death.
Anthim’s effectiveness was demonstrated in studies conducted in animals based on survival at the end of the studies. More animals treated with Anthim lived, compared with animals treated with placebo. Anthim administered in combination with antibacterial drugs resulted in higher survival outcomes than antibacterial therapy alone.
The safety of Anthim was evaluated in 320 healthy human volunteers. The most frequently reported side effects were headache, itching (pruritus), upper respiratory tract infections, cough, nasal congestion, hives, and bruising, swelling and pain at the infusion site.
For more information, see the FDA’s approval announcement.
On Twitter @richpizzi
FDA approves Zika test as CDC confirmed cases rises to 116
A faster diagnostic tool for Zika virus infection than those currently used received approval by the Food and Drug Administration under an Emergency Use Authorization, the agency reported Friday, the same day the Centers for Disease Control and Prevention reported that 116 U.S. residents have tested positive for Zika as of Feb. 26.
The Trioplex Realtime RT-PCR Assay requires only a single test to differentiate current chikungunya, dengue or Zika infections instead of three separate tests. The CDC will start distributing the test to qualified domestic and international labs over the next two weeks, but U.S. hospitals and similar primary care settings will not have the test.
Nearly a quarter (24%) of the 116 cases reported by the CDC were diagnosed using reverse transcription-polymerase chain reaction (RT-PCR) to detect Zika RNA, and nearly all the remaining (76%) relied on detection of anti-Zika antibodies with ELISA serologic testing. Two cases were determined based on epidemiological links to a confirmed case and serologic evidence of an unspecified flavivirus infection. These cases do not include additional onesdiagnosed and reported from state and territorial departments with local lab testing.
“Zika virus disease should be considered in patients with acute onset of fever, rash, arthralgia or conjunctivitis who traveled to areas with ongoing transmission or had unprotected sex with someone who traveled to those areas and developed compatible symptoms within two weeks of returning,” said Dr. Paige Armstrong, of the Epidemic Intelligence Service at the CDC in Atlanta, and her associates (MMWR 2016 March 18).
The researchers reported on all U.S. residents who received positive lab tests for Zika infection from the CDC between January 1, 2015, and February 26, 2016. Among the cases, from 33 states and Washington, D.C., one was an infant with microcephaly who contracted the virus congenitally, five had recently had sexual contact with someone who had traveled to an area where Zika was actively circulating, and 110 had traveled to a region active with Zika. Haiti, El Salvador, Colombia, Honduras and Guatemala comprised the most commonly visited countries among the cases.
Most infections of Zika, transmitted primarily by the Aedes aegypti mosquito, remain asymptomatic, and even those with the characteristic symptoms of rash, fever, arthralgia and nonpurulent conjunctivitis tend to have a mild illness. Nearly all (96%) of the 116 cases had at least two of these symptoms, and 65% had three or more. Among all the cases, 98% experienced a rash, 82% a fever, 66% joint pain, 57% headache, 55% myalgia and 37% conjunctivitis. Four were hospitalized, and none died.
“Until more is known about the effects of Zika virus infection on the developing fetus, pregnant women should postpone travel to areas where Zika virus transmission is ongoing,” Dr. Armstrong and associates wrote. “Pregnant women who develop a clinically compatible illness during or within two weeks of returning from an area with Zika virus transmission should be tested for Zika virus infection; testing may also be offered to asymptomatic pregnant women 2–12 weeks after travel to an area with active Zika transmission.”
The study was funded by the CDC, and the authors reported no disclosures.
A faster diagnostic tool for Zika virus infection than those currently used received approval by the Food and Drug Administration under an Emergency Use Authorization, the agency reported Friday, the same day the Centers for Disease Control and Prevention reported that 116 U.S. residents have tested positive for Zika as of Feb. 26.
The Trioplex Realtime RT-PCR Assay requires only a single test to differentiate current chikungunya, dengue or Zika infections instead of three separate tests. The CDC will start distributing the test to qualified domestic and international labs over the next two weeks, but U.S. hospitals and similar primary care settings will not have the test.
Nearly a quarter (24%) of the 116 cases reported by the CDC were diagnosed using reverse transcription-polymerase chain reaction (RT-PCR) to detect Zika RNA, and nearly all the remaining (76%) relied on detection of anti-Zika antibodies with ELISA serologic testing. Two cases were determined based on epidemiological links to a confirmed case and serologic evidence of an unspecified flavivirus infection. These cases do not include additional onesdiagnosed and reported from state and territorial departments with local lab testing.
“Zika virus disease should be considered in patients with acute onset of fever, rash, arthralgia or conjunctivitis who traveled to areas with ongoing transmission or had unprotected sex with someone who traveled to those areas and developed compatible symptoms within two weeks of returning,” said Dr. Paige Armstrong, of the Epidemic Intelligence Service at the CDC in Atlanta, and her associates (MMWR 2016 March 18).
The researchers reported on all U.S. residents who received positive lab tests for Zika infection from the CDC between January 1, 2015, and February 26, 2016. Among the cases, from 33 states and Washington, D.C., one was an infant with microcephaly who contracted the virus congenitally, five had recently had sexual contact with someone who had traveled to an area where Zika was actively circulating, and 110 had traveled to a region active with Zika. Haiti, El Salvador, Colombia, Honduras and Guatemala comprised the most commonly visited countries among the cases.
Most infections of Zika, transmitted primarily by the Aedes aegypti mosquito, remain asymptomatic, and even those with the characteristic symptoms of rash, fever, arthralgia and nonpurulent conjunctivitis tend to have a mild illness. Nearly all (96%) of the 116 cases had at least two of these symptoms, and 65% had three or more. Among all the cases, 98% experienced a rash, 82% a fever, 66% joint pain, 57% headache, 55% myalgia and 37% conjunctivitis. Four were hospitalized, and none died.
“Until more is known about the effects of Zika virus infection on the developing fetus, pregnant women should postpone travel to areas where Zika virus transmission is ongoing,” Dr. Armstrong and associates wrote. “Pregnant women who develop a clinically compatible illness during or within two weeks of returning from an area with Zika virus transmission should be tested for Zika virus infection; testing may also be offered to asymptomatic pregnant women 2–12 weeks after travel to an area with active Zika transmission.”
The study was funded by the CDC, and the authors reported no disclosures.
A faster diagnostic tool for Zika virus infection than those currently used received approval by the Food and Drug Administration under an Emergency Use Authorization, the agency reported Friday, the same day the Centers for Disease Control and Prevention reported that 116 U.S. residents have tested positive for Zika as of Feb. 26.
The Trioplex Realtime RT-PCR Assay requires only a single test to differentiate current chikungunya, dengue or Zika infections instead of three separate tests. The CDC will start distributing the test to qualified domestic and international labs over the next two weeks, but U.S. hospitals and similar primary care settings will not have the test.
Nearly a quarter (24%) of the 116 cases reported by the CDC were diagnosed using reverse transcription-polymerase chain reaction (RT-PCR) to detect Zika RNA, and nearly all the remaining (76%) relied on detection of anti-Zika antibodies with ELISA serologic testing. Two cases were determined based on epidemiological links to a confirmed case and serologic evidence of an unspecified flavivirus infection. These cases do not include additional onesdiagnosed and reported from state and territorial departments with local lab testing.
“Zika virus disease should be considered in patients with acute onset of fever, rash, arthralgia or conjunctivitis who traveled to areas with ongoing transmission or had unprotected sex with someone who traveled to those areas and developed compatible symptoms within two weeks of returning,” said Dr. Paige Armstrong, of the Epidemic Intelligence Service at the CDC in Atlanta, and her associates (MMWR 2016 March 18).
The researchers reported on all U.S. residents who received positive lab tests for Zika infection from the CDC between January 1, 2015, and February 26, 2016. Among the cases, from 33 states and Washington, D.C., one was an infant with microcephaly who contracted the virus congenitally, five had recently had sexual contact with someone who had traveled to an area where Zika was actively circulating, and 110 had traveled to a region active with Zika. Haiti, El Salvador, Colombia, Honduras and Guatemala comprised the most commonly visited countries among the cases.
Most infections of Zika, transmitted primarily by the Aedes aegypti mosquito, remain asymptomatic, and even those with the characteristic symptoms of rash, fever, arthralgia and nonpurulent conjunctivitis tend to have a mild illness. Nearly all (96%) of the 116 cases had at least two of these symptoms, and 65% had three or more. Among all the cases, 98% experienced a rash, 82% a fever, 66% joint pain, 57% headache, 55% myalgia and 37% conjunctivitis. Four were hospitalized, and none died.
“Until more is known about the effects of Zika virus infection on the developing fetus, pregnant women should postpone travel to areas where Zika virus transmission is ongoing,” Dr. Armstrong and associates wrote. “Pregnant women who develop a clinically compatible illness during or within two weeks of returning from an area with Zika virus transmission should be tested for Zika virus infection; testing may also be offered to asymptomatic pregnant women 2–12 weeks after travel to an area with active Zika transmission.”
The study was funded by the CDC, and the authors reported no disclosures.
MORBIDITY AND MORTALITY WEEKLY REPORT
Key clinical point: CDC lab-confirmed Zika infections in U.S. residents rises to 116.
Major finding: 110 had traveled to active Zika regions and 5 contracted the virus sexually; 96% experienced at least two of the four characteristic symptoms.
Data source: The findings are based on all cases of lab-positive Zika infection among U.S. residents whose testing occurred at the Centers for Disease Control and Prevention between January 1, 2015, and February 26, 2016.
Disclosures: The study was funded by the CDC. The authors reported no disclosures.