User login
No Link Between PPI Use and Risk for Cardiovascular Events
TOPLINE:
There is no significant association between the use of proton pump inhibitors (PPIs) and risk for cardiovascular events, a meta-analysis shows. However, patients with gastroesophageal reflux disease (GERD) do experience a slight increase in cardiovascular events with PPI use.
METHODOLOGY:
- PPIs are commonly used gastric acid suppressants; however, they have pleiotropic effects, some of which have been hypothesized to augment cardiovascular disorders.
- Researchers conducted a meta-analysis of randomized clinical trials with at least 100 patients and treatment durations > 30 days, which compared groups receiving PPIs to those on placebo or other active treatments.
- The primary outcome was a composite of nonfatal myocardial infarctions, nonfatal strokes, fatal cardiovascular adverse events, coronary revascularizations, and hospitalizations for unstable angina.
TAKEAWAY:
- Researchers included data from 52 placebo-controlled trials, with 14,988 patients and 8323 patients randomized to receive a PPI or placebo, respectively; the mean treatment duration was 0.45 person-years for those treated with PPIs and 0.32 person-years for those treated with placebo.
- Among placebo-controlled trials, 24 were conducted in patients with GERD.
- Researchers also included 61 active-controlled trials that compared PPIs with histamine-2 receptor antagonists (51 trials) or other active treatments.
- The incidence rate ratio for the primary outcome was 0.72 when comparing PPI to placebo, indicating no significant association between PPI and cardiovascular events.
- Among patients with GERD, cardiovascular events occurred only in those treated with PPIs, leading to approximately one excess cardiovascular event per 100 person-years of PPI treatment relative to placebo.
- Researchers found no association between PPI treatment and the risk for cardiovascular events in trials comparing PPIs with other active treatments.
IN PRACTICE:
“We found no association of cardiovascular events with PPI treatment,” the authors wrote. “Cardiovascular events appeared more frequent with PPI treatment in GERD trials, but results from this subgroup should be interpreted with the limitations of the analysis in mind.”
SOURCE:
The study, led by Andrew D. Mosholder, MD, MPH, Division of Epidemiology, US Food and Drug Administration Center for Drug Evaluation and Research, Silver Spring, Maryland, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
This study lacked individual patient data, which precluded a time-to-event analysis or an analysis accounting for patient characteristics such as age or sex. The mean duration of PPI treatment in these trials was a few months, limiting the assessment of cardiovascular risk with extended use. The risk estimates were influenced the most by data on omeprazole and esomeprazole.
DISCLOSURES:
This study did not receive any funding. The authors declared no conflicts of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
There is no significant association between the use of proton pump inhibitors (PPIs) and risk for cardiovascular events, a meta-analysis shows. However, patients with gastroesophageal reflux disease (GERD) do experience a slight increase in cardiovascular events with PPI use.
METHODOLOGY:
- PPIs are commonly used gastric acid suppressants; however, they have pleiotropic effects, some of which have been hypothesized to augment cardiovascular disorders.
- Researchers conducted a meta-analysis of randomized clinical trials with at least 100 patients and treatment durations > 30 days, which compared groups receiving PPIs to those on placebo or other active treatments.
- The primary outcome was a composite of nonfatal myocardial infarctions, nonfatal strokes, fatal cardiovascular adverse events, coronary revascularizations, and hospitalizations for unstable angina.
TAKEAWAY:
- Researchers included data from 52 placebo-controlled trials, with 14,988 patients and 8323 patients randomized to receive a PPI or placebo, respectively; the mean treatment duration was 0.45 person-years for those treated with PPIs and 0.32 person-years for those treated with placebo.
- Among placebo-controlled trials, 24 were conducted in patients with GERD.
- Researchers also included 61 active-controlled trials that compared PPIs with histamine-2 receptor antagonists (51 trials) or other active treatments.
- The incidence rate ratio for the primary outcome was 0.72 when comparing PPI to placebo, indicating no significant association between PPI and cardiovascular events.
- Among patients with GERD, cardiovascular events occurred only in those treated with PPIs, leading to approximately one excess cardiovascular event per 100 person-years of PPI treatment relative to placebo.
- Researchers found no association between PPI treatment and the risk for cardiovascular events in trials comparing PPIs with other active treatments.
IN PRACTICE:
“We found no association of cardiovascular events with PPI treatment,” the authors wrote. “Cardiovascular events appeared more frequent with PPI treatment in GERD trials, but results from this subgroup should be interpreted with the limitations of the analysis in mind.”
SOURCE:
The study, led by Andrew D. Mosholder, MD, MPH, Division of Epidemiology, US Food and Drug Administration Center for Drug Evaluation and Research, Silver Spring, Maryland, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
This study lacked individual patient data, which precluded a time-to-event analysis or an analysis accounting for patient characteristics such as age or sex. The mean duration of PPI treatment in these trials was a few months, limiting the assessment of cardiovascular risk with extended use. The risk estimates were influenced the most by data on omeprazole and esomeprazole.
DISCLOSURES:
This study did not receive any funding. The authors declared no conflicts of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
TOPLINE:
There is no significant association between the use of proton pump inhibitors (PPIs) and risk for cardiovascular events, a meta-analysis shows. However, patients with gastroesophageal reflux disease (GERD) do experience a slight increase in cardiovascular events with PPI use.
METHODOLOGY:
- PPIs are commonly used gastric acid suppressants; however, they have pleiotropic effects, some of which have been hypothesized to augment cardiovascular disorders.
- Researchers conducted a meta-analysis of randomized clinical trials with at least 100 patients and treatment durations > 30 days, which compared groups receiving PPIs to those on placebo or other active treatments.
- The primary outcome was a composite of nonfatal myocardial infarctions, nonfatal strokes, fatal cardiovascular adverse events, coronary revascularizations, and hospitalizations for unstable angina.
TAKEAWAY:
- Researchers included data from 52 placebo-controlled trials, with 14,988 patients and 8323 patients randomized to receive a PPI or placebo, respectively; the mean treatment duration was 0.45 person-years for those treated with PPIs and 0.32 person-years for those treated with placebo.
- Among placebo-controlled trials, 24 were conducted in patients with GERD.
- Researchers also included 61 active-controlled trials that compared PPIs with histamine-2 receptor antagonists (51 trials) or other active treatments.
- The incidence rate ratio for the primary outcome was 0.72 when comparing PPI to placebo, indicating no significant association between PPI and cardiovascular events.
- Among patients with GERD, cardiovascular events occurred only in those treated with PPIs, leading to approximately one excess cardiovascular event per 100 person-years of PPI treatment relative to placebo.
- Researchers found no association between PPI treatment and the risk for cardiovascular events in trials comparing PPIs with other active treatments.
IN PRACTICE:
“We found no association of cardiovascular events with PPI treatment,” the authors wrote. “Cardiovascular events appeared more frequent with PPI treatment in GERD trials, but results from this subgroup should be interpreted with the limitations of the analysis in mind.”
SOURCE:
The study, led by Andrew D. Mosholder, MD, MPH, Division of Epidemiology, US Food and Drug Administration Center for Drug Evaluation and Research, Silver Spring, Maryland, was published online in The American Journal of Gastroenterology.
LIMITATIONS:
This study lacked individual patient data, which precluded a time-to-event analysis or an analysis accounting for patient characteristics such as age or sex. The mean duration of PPI treatment in these trials was a few months, limiting the assessment of cardiovascular risk with extended use. The risk estimates were influenced the most by data on omeprazole and esomeprazole.
DISCLOSURES:
This study did not receive any funding. The authors declared no conflicts of interests.
This article was created using several editorial tools, including AI, as part of the process. Human editors reviewed this content before publication. A version of this article first appeared on Medscape.com.
AI-Assisted Colonoscopy Linked to Higher Rate of Benign Lesion Removal
PHILADELPHIA — according to a study presented at the annual meeting of the American College of Gastroenterology (ACG).
In particular, AIAC led to a statistically and clinically significant increase in the proportion of exams that detected lesions that after resection were all found to be benign, compared with unassisted colonoscopy.
“The potential implications include increased procedural risks, as well as costs, such as pathology costs and other healthcare expenditures, without any additional colorectal cancer prevention benefit,” said lead author Tessa Herman, MD, chief resident of internal medicine at the University of Minnesota, Minneapolis, and Minneapolis Veterans Affairs Health Care System.
In a previous implementation trial at the Minneapolis VA Medical Center, Herman and colleagues compared ADR between a group of patients undergoing AIAC and a historical cohort of patients who had non–AI-assisted colonoscopy.
In this subsequent study, the research team conducted an ad hoc analysis of data from the previous trial to determine the proportion of colonoscopies for screening, surveillance, and positive fecal immunochemical tests which detect lesions that after resection are all found to be benign. They excluded colonoscopies conducted for diagnostic indications or inflammatory bowel disease, as well as incomplete colonoscopies, and for those with inadequate bowel preparation.
Overall, they studied 441 non-AIAC colonoscopies (between November 2022 and April 2023) and 599 AIAC colonoscopies (between May 2023 and October 2023). The groups were balanced, and there were no significant differences in patient demographics, endoscopists, AI technology, procedure time, or average number of polyps detected.
In the non-AIAC cohort, 37 cases (8.4%) had polypectomies that revealed only benign lesions, as compared with 74 cases (12.4%) in the AIAC cohort. The most common resected lesions were benign colonic mucosa, lymphoid aggregates, and hyperplastic polyps.
Applied to the 15 million colonoscopies conducted in the United States per year, the findings indicate that full adoption of AIAC could result in about 600,000 more colonoscopies in which only benign, nonadenomatous lesions are removed, compared with traditional colonoscopy, Herman said.
More study of AIAC is needed, said Daniel Pambianco, MD, managing partner of GastroHealth-Charlottesville in Virginia and the 2023 ACG president. “This technology is in a fledging stage, and the more data we have, the more helpful it’ll be to know if we’re removing the right lesions at a better rate.”
“There’s a hope that assistance will improve detection, removal of polyps, and ultimately, colon cancer,” added Pambianco, who comoderated the session on colorectal cancer prevention.
Future longitudinal studies should monitor both ADR and benign lesion resection rates with AIAC, and modeling studies could determine the benefits and costs of the technology, Herman said. In addition, development of hybrid CADe and computer-aided diagnosis systems could mitigate concerns about excessive benign lesion resection with AI tools.
Clinicians already are able to find colon mucosa that are polypoid or lymphoid aggregates during colonoscopy without AI assistance, said the session’s comoderator, Sita Chokhavatia, MD, AGAF, a gastroenterologist with Valley Medical Group in Ridgewood, New Jersey.
“Instead, we need a tool that can help us to not remove these polyps that are not neoplastic,” she said. “With future developments, we may be able to take it to the next step where the algorithm tells us that it’s benign and not to touch it.”
The study was named an ACG Newsworthy Abstract. Herman, Pambianco, and Chokhavatia reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the annual meeting of the American College of Gastroenterology (ACG).
In particular, AIAC led to a statistically and clinically significant increase in the proportion of exams that detected lesions that after resection were all found to be benign, compared with unassisted colonoscopy.
“The potential implications include increased procedural risks, as well as costs, such as pathology costs and other healthcare expenditures, without any additional colorectal cancer prevention benefit,” said lead author Tessa Herman, MD, chief resident of internal medicine at the University of Minnesota, Minneapolis, and Minneapolis Veterans Affairs Health Care System.
In a previous implementation trial at the Minneapolis VA Medical Center, Herman and colleagues compared ADR between a group of patients undergoing AIAC and a historical cohort of patients who had non–AI-assisted colonoscopy.
In this subsequent study, the research team conducted an ad hoc analysis of data from the previous trial to determine the proportion of colonoscopies for screening, surveillance, and positive fecal immunochemical tests which detect lesions that after resection are all found to be benign. They excluded colonoscopies conducted for diagnostic indications or inflammatory bowel disease, as well as incomplete colonoscopies, and for those with inadequate bowel preparation.
Overall, they studied 441 non-AIAC colonoscopies (between November 2022 and April 2023) and 599 AIAC colonoscopies (between May 2023 and October 2023). The groups were balanced, and there were no significant differences in patient demographics, endoscopists, AI technology, procedure time, or average number of polyps detected.
In the non-AIAC cohort, 37 cases (8.4%) had polypectomies that revealed only benign lesions, as compared with 74 cases (12.4%) in the AIAC cohort. The most common resected lesions were benign colonic mucosa, lymphoid aggregates, and hyperplastic polyps.
Applied to the 15 million colonoscopies conducted in the United States per year, the findings indicate that full adoption of AIAC could result in about 600,000 more colonoscopies in which only benign, nonadenomatous lesions are removed, compared with traditional colonoscopy, Herman said.
More study of AIAC is needed, said Daniel Pambianco, MD, managing partner of GastroHealth-Charlottesville in Virginia and the 2023 ACG president. “This technology is in a fledging stage, and the more data we have, the more helpful it’ll be to know if we’re removing the right lesions at a better rate.”
“There’s a hope that assistance will improve detection, removal of polyps, and ultimately, colon cancer,” added Pambianco, who comoderated the session on colorectal cancer prevention.
Future longitudinal studies should monitor both ADR and benign lesion resection rates with AIAC, and modeling studies could determine the benefits and costs of the technology, Herman said. In addition, development of hybrid CADe and computer-aided diagnosis systems could mitigate concerns about excessive benign lesion resection with AI tools.
Clinicians already are able to find colon mucosa that are polypoid or lymphoid aggregates during colonoscopy without AI assistance, said the session’s comoderator, Sita Chokhavatia, MD, AGAF, a gastroenterologist with Valley Medical Group in Ridgewood, New Jersey.
“Instead, we need a tool that can help us to not remove these polyps that are not neoplastic,” she said. “With future developments, we may be able to take it to the next step where the algorithm tells us that it’s benign and not to touch it.”
The study was named an ACG Newsworthy Abstract. Herman, Pambianco, and Chokhavatia reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the annual meeting of the American College of Gastroenterology (ACG).
In particular, AIAC led to a statistically and clinically significant increase in the proportion of exams that detected lesions that after resection were all found to be benign, compared with unassisted colonoscopy.
“The potential implications include increased procedural risks, as well as costs, such as pathology costs and other healthcare expenditures, without any additional colorectal cancer prevention benefit,” said lead author Tessa Herman, MD, chief resident of internal medicine at the University of Minnesota, Minneapolis, and Minneapolis Veterans Affairs Health Care System.
In a previous implementation trial at the Minneapolis VA Medical Center, Herman and colleagues compared ADR between a group of patients undergoing AIAC and a historical cohort of patients who had non–AI-assisted colonoscopy.
In this subsequent study, the research team conducted an ad hoc analysis of data from the previous trial to determine the proportion of colonoscopies for screening, surveillance, and positive fecal immunochemical tests which detect lesions that after resection are all found to be benign. They excluded colonoscopies conducted for diagnostic indications or inflammatory bowel disease, as well as incomplete colonoscopies, and for those with inadequate bowel preparation.
Overall, they studied 441 non-AIAC colonoscopies (between November 2022 and April 2023) and 599 AIAC colonoscopies (between May 2023 and October 2023). The groups were balanced, and there were no significant differences in patient demographics, endoscopists, AI technology, procedure time, or average number of polyps detected.
In the non-AIAC cohort, 37 cases (8.4%) had polypectomies that revealed only benign lesions, as compared with 74 cases (12.4%) in the AIAC cohort. The most common resected lesions were benign colonic mucosa, lymphoid aggregates, and hyperplastic polyps.
Applied to the 15 million colonoscopies conducted in the United States per year, the findings indicate that full adoption of AIAC could result in about 600,000 more colonoscopies in which only benign, nonadenomatous lesions are removed, compared with traditional colonoscopy, Herman said.
More study of AIAC is needed, said Daniel Pambianco, MD, managing partner of GastroHealth-Charlottesville in Virginia and the 2023 ACG president. “This technology is in a fledging stage, and the more data we have, the more helpful it’ll be to know if we’re removing the right lesions at a better rate.”
“There’s a hope that assistance will improve detection, removal of polyps, and ultimately, colon cancer,” added Pambianco, who comoderated the session on colorectal cancer prevention.
Future longitudinal studies should monitor both ADR and benign lesion resection rates with AIAC, and modeling studies could determine the benefits and costs of the technology, Herman said. In addition, development of hybrid CADe and computer-aided diagnosis systems could mitigate concerns about excessive benign lesion resection with AI tools.
Clinicians already are able to find colon mucosa that are polypoid or lymphoid aggregates during colonoscopy without AI assistance, said the session’s comoderator, Sita Chokhavatia, MD, AGAF, a gastroenterologist with Valley Medical Group in Ridgewood, New Jersey.
“Instead, we need a tool that can help us to not remove these polyps that are not neoplastic,” she said. “With future developments, we may be able to take it to the next step where the algorithm tells us that it’s benign and not to touch it.”
The study was named an ACG Newsworthy Abstract. Herman, Pambianco, and Chokhavatia reported no relevant disclosures.
A version of this article first appeared on Medscape.com.
FROM ACG 2024
Short-Course Vasoconstrictors After EVL: Time for a New Standard of Care?
PHILADELPHIA — without raising the risk for rebleeding, if the initial ligation successfully controls bleeding.
“This approach would allow earlier discharge from the hospital and reduce the risk of adverse events, all without sacrificing treatment efficacy or compromising patient safety,” Sushrut Ingawale, MD, MBBS, Quinnipiac University School of Medicine, North Haven, and St. Vincent’s Medical Center, Bridgeport, both in Connecticut, said in a presentation at the annual meeting of the American College of Gastroenterology (ACG).
Ingawale called for a “re-evaluation of existing protocols, emphasizing the potential to update current protocols to reflect shorter, more personalized” duration of vasoconstrictor therapy in these patients.
Commenting on this research, Nancy Reau, MD, AGAF, of Rush University in Chicago, Illinois, said: “We should always question the standard of care.”
“Vasoconstrictors for 5 days is the standard of care, but this could lead to prolonged hospitalization in patients who are otherwise doing well after endoscopic intervention. Recognizing that a shorter course of vasoconstrictor treatment may have equal outcome is very important though it may not be appropriate for all patients, especially those at high risk for rebleeding,” said Reau.
Outdated Guidelines?
In his presentation, Ingawale noted that current guidelines that recommend continuing vasoconstrictors, like octreotide or terlipressin, for at least 3-5 days after EVL for acute variceal bleeding are based primarily on old studies in which sclerotherapy was the primary hemostatic method.
The study team assessed comparative outcomes based on the duration of vasoconstrictors after EVL for acute variceal bleeding in a systematic review and network meta-analysis of 11 randomized controlled trials.
The studies had a total of 816 patients who were grouped based on the duration vasoconstrictor therapy: 24 hours or less (group 1), 24-72 hours (group 2), and 72-120 hours (group 3).
There was no significant difference in the risk for rebleeding in group 1 (risk ratio [RR], 1.36; 95% CI, 0.48-3.52) and group 2 (RR, 1.34; 95% CI, 0.42-4.54) vs group 3.
“This finding was even consistent when we compared individual durations” of 0, 12, 24, 48, and 72 hours vs 120 hours, Ingawale said.
There was also no statistically significant difference in the 5-day mortality risk between group 1 (RR, 0.66; 95% CI, 0.09-2.52) and group 2 (RR, 1.08; 95% CI, 0.15-6.43) or the 30-day mortality risk between group 1 (RR, 1.18; 95% CI, 0.51-2.51) and group 2 (RR, 0.98; 95% CI, 0.36-2.52) vs group 3.
Rapidly Evolving Area
“Our network meta-analysis did not show any benefit of continuing vasoconstrictors after EVL,” the researchers wrote in their conference abstract. Despite historical precedent, shorter durations may be adequate, “potentially enabling earlier hospital discharge without compromising patient outcomes.”
Ingawale suggested future research should look to identify the subset of patients at a risk for failure to control bleeding who might benefit from the continuation of vasoconstrictors.
“Management of complications of portal hypertension are rapidly evolving and this study will add to the data that drives our guidelines. Seeing this data in a peer reviewed publication will add the necessary validity to impact a change in the treatment paradigm,” Reau said.
The study had no specific funding. Ingawale had no relevant financial relationships. Reau disclosed various relationships with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — without raising the risk for rebleeding, if the initial ligation successfully controls bleeding.
“This approach would allow earlier discharge from the hospital and reduce the risk of adverse events, all without sacrificing treatment efficacy or compromising patient safety,” Sushrut Ingawale, MD, MBBS, Quinnipiac University School of Medicine, North Haven, and St. Vincent’s Medical Center, Bridgeport, both in Connecticut, said in a presentation at the annual meeting of the American College of Gastroenterology (ACG).
Ingawale called for a “re-evaluation of existing protocols, emphasizing the potential to update current protocols to reflect shorter, more personalized” duration of vasoconstrictor therapy in these patients.
Commenting on this research, Nancy Reau, MD, AGAF, of Rush University in Chicago, Illinois, said: “We should always question the standard of care.”
“Vasoconstrictors for 5 days is the standard of care, but this could lead to prolonged hospitalization in patients who are otherwise doing well after endoscopic intervention. Recognizing that a shorter course of vasoconstrictor treatment may have equal outcome is very important though it may not be appropriate for all patients, especially those at high risk for rebleeding,” said Reau.
Outdated Guidelines?
In his presentation, Ingawale noted that current guidelines that recommend continuing vasoconstrictors, like octreotide or terlipressin, for at least 3-5 days after EVL for acute variceal bleeding are based primarily on old studies in which sclerotherapy was the primary hemostatic method.
The study team assessed comparative outcomes based on the duration of vasoconstrictors after EVL for acute variceal bleeding in a systematic review and network meta-analysis of 11 randomized controlled trials.
The studies had a total of 816 patients who were grouped based on the duration vasoconstrictor therapy: 24 hours or less (group 1), 24-72 hours (group 2), and 72-120 hours (group 3).
There was no significant difference in the risk for rebleeding in group 1 (risk ratio [RR], 1.36; 95% CI, 0.48-3.52) and group 2 (RR, 1.34; 95% CI, 0.42-4.54) vs group 3.
“This finding was even consistent when we compared individual durations” of 0, 12, 24, 48, and 72 hours vs 120 hours, Ingawale said.
There was also no statistically significant difference in the 5-day mortality risk between group 1 (RR, 0.66; 95% CI, 0.09-2.52) and group 2 (RR, 1.08; 95% CI, 0.15-6.43) or the 30-day mortality risk between group 1 (RR, 1.18; 95% CI, 0.51-2.51) and group 2 (RR, 0.98; 95% CI, 0.36-2.52) vs group 3.
Rapidly Evolving Area
“Our network meta-analysis did not show any benefit of continuing vasoconstrictors after EVL,” the researchers wrote in their conference abstract. Despite historical precedent, shorter durations may be adequate, “potentially enabling earlier hospital discharge without compromising patient outcomes.”
Ingawale suggested future research should look to identify the subset of patients at a risk for failure to control bleeding who might benefit from the continuation of vasoconstrictors.
“Management of complications of portal hypertension are rapidly evolving and this study will add to the data that drives our guidelines. Seeing this data in a peer reviewed publication will add the necessary validity to impact a change in the treatment paradigm,” Reau said.
The study had no specific funding. Ingawale had no relevant financial relationships. Reau disclosed various relationships with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — without raising the risk for rebleeding, if the initial ligation successfully controls bleeding.
“This approach would allow earlier discharge from the hospital and reduce the risk of adverse events, all without sacrificing treatment efficacy or compromising patient safety,” Sushrut Ingawale, MD, MBBS, Quinnipiac University School of Medicine, North Haven, and St. Vincent’s Medical Center, Bridgeport, both in Connecticut, said in a presentation at the annual meeting of the American College of Gastroenterology (ACG).
Ingawale called for a “re-evaluation of existing protocols, emphasizing the potential to update current protocols to reflect shorter, more personalized” duration of vasoconstrictor therapy in these patients.
Commenting on this research, Nancy Reau, MD, AGAF, of Rush University in Chicago, Illinois, said: “We should always question the standard of care.”
“Vasoconstrictors for 5 days is the standard of care, but this could lead to prolonged hospitalization in patients who are otherwise doing well after endoscopic intervention. Recognizing that a shorter course of vasoconstrictor treatment may have equal outcome is very important though it may not be appropriate for all patients, especially those at high risk for rebleeding,” said Reau.
Outdated Guidelines?
In his presentation, Ingawale noted that current guidelines that recommend continuing vasoconstrictors, like octreotide or terlipressin, for at least 3-5 days after EVL for acute variceal bleeding are based primarily on old studies in which sclerotherapy was the primary hemostatic method.
The study team assessed comparative outcomes based on the duration of vasoconstrictors after EVL for acute variceal bleeding in a systematic review and network meta-analysis of 11 randomized controlled trials.
The studies had a total of 816 patients who were grouped based on the duration vasoconstrictor therapy: 24 hours or less (group 1), 24-72 hours (group 2), and 72-120 hours (group 3).
There was no significant difference in the risk for rebleeding in group 1 (risk ratio [RR], 1.36; 95% CI, 0.48-3.52) and group 2 (RR, 1.34; 95% CI, 0.42-4.54) vs group 3.
“This finding was even consistent when we compared individual durations” of 0, 12, 24, 48, and 72 hours vs 120 hours, Ingawale said.
There was also no statistically significant difference in the 5-day mortality risk between group 1 (RR, 0.66; 95% CI, 0.09-2.52) and group 2 (RR, 1.08; 95% CI, 0.15-6.43) or the 30-day mortality risk between group 1 (RR, 1.18; 95% CI, 0.51-2.51) and group 2 (RR, 0.98; 95% CI, 0.36-2.52) vs group 3.
Rapidly Evolving Area
“Our network meta-analysis did not show any benefit of continuing vasoconstrictors after EVL,” the researchers wrote in their conference abstract. Despite historical precedent, shorter durations may be adequate, “potentially enabling earlier hospital discharge without compromising patient outcomes.”
Ingawale suggested future research should look to identify the subset of patients at a risk for failure to control bleeding who might benefit from the continuation of vasoconstrictors.
“Management of complications of portal hypertension are rapidly evolving and this study will add to the data that drives our guidelines. Seeing this data in a peer reviewed publication will add the necessary validity to impact a change in the treatment paradigm,” Reau said.
The study had no specific funding. Ingawale had no relevant financial relationships. Reau disclosed various relationships with AbbVie, Gilead, Arbutus, Intercept, and Salix.
A version of this article first appeared on Medscape.com.
FROM ACG 2024
Guselkumab Efficacy in Crohn’s Disease Unaffected by Prior Biologic Use
VIENNA — according to a pooled analysis of the two phase 3 double-blind GALAXI 2 and 3 studies.
“We found that guselkumab was effective in both biologic-naive and biologic-inadequate subpopulations,” said coinvestigator Bruce E. Sands, MD, AGAF, gastroenterologist from Icahn School of Medicine at Mount Sinai, New York City.
These latest results add to the primary results of these studies reported earlier in 2024 that guselkumab was shown to be superior to both placebo and ustekinumab in the same patient population with moderately to severely active CD.
Sands reported the new data in a presentation at the United European Gastroenterology (UEG) Week 2024.
Guselkumab potently blocks interleukin (IL)–23 and binds to CD64, a receptor on cells that produce IL-23. The dual-acting IL-23p19 subunit inhibitor agent is currently under review by the Food and Drug Administration (FDA) for moderately to severely active CD. In September, guselkumab (Tremfya, Johnson & Johnson) was approved for use in moderately to severely active ulcerative colitis.
GALAXI 2 and 3 Pooled Dataset
In the two independent, identically designed GALAXI 2 and 3 studies, patients were randomized to guselkumab treatment at either 200 mg intravenous (IV) induction at weeks 0, 4, and 8, followed by 200 mg subcutaneous maintenance every 4 weeks, starting at week 12, or 200 mg IV induction at weeks 0, 4, and 8, followed by 100 mg subcutaneous maintenance every 8 weeks, starting at week 16; or to ustekinumab; or to placebo.
Participants were required to remain on their treatment of initial randomization for a long-term extension study (up to 5 years) looking at clinical, endoscopic, and safety outcomes, except for participants on placebo who were allowed to switch to ustekinumab if clinical response was not met at week 12.
Inclusion criteria for the studies comprised a Crohn’s Disease Activity Index score between 220 and 450, a mean daily stool frequency count > 3 or an abdominal pain score > 1, and a simple endoscopic score for CD score ≥ 6. Participants were also required to have shown an inadequate response or intolerance to oral corticosteroids, 6-mercaptopurine/azathioprine/methotrexate, or biologic therapies.
The pooled dataset included patients on either dose of guselkumab and patients on placebo (total n = 730). Of these, 52% of participants had shown a prior inadequate response to a biologic, 42% were biologic naive, and 6% had prior exposure to biologics but no documented failure. Patients on ustekinumab were not included in this analysis.
Almost all patients (97%) in the biologic-inadequate response group had previously received at least one anti–tumor necrosis factor agent, and around 15% had received vedolizumab. As expected, the biologic-inadequate responders were a lot sicker than the biologic-naive patients, Sands reported.
The composite co–primary endpoints for each guselkumab regimen vs placebo were clinical response at week 12 plus clinical remission at week 48, and clinical response at week 12 plus endoscopic response at week 48.
The major secondary endpoints comprised clinical remission at week 12 and endoscopic response also at week 12.
Short- and Long-Term Endpoints in Both Subgroups
In the biologic-naive subgroup, 54.7% of patients receiving the 200-mg dose regimen of guselkumab and 51.7% of those receiving the 100-mg dose regimen showed a clinical response at week 12 plus clinical remission at week 48, compared with 11.5% in the placebo group (P < .001 for both compared with placebo).
In the biologic-inadequate response group, 49.7% of those receiving the 200-mg dose regimen of guselkumab and 45.8% on the 100-mg dose regimen reached the composite endpoint, compared with the placebo response of 12.8% (P < .001 for both compared with placebo).
“You can see a slight decrease in response in the biologic-inadequate responders, but on the whole, the confidence intervals are highly overlapping,” said Sands.
Turning to major secondary endpoints at week 12, clinical remission was reached by 49.6% of the biologic-naive group on the 200-mg guselkumab regimen vs 16.4% on placebo, and by 46.0% of the biologic-inadequate group on the 200-mg regimen vs 19.2% on placebo (P < .001 for both subgroups). Endoscopic response was achieved by 46.3% of patients in the biologic-naive group and 29.0% in the biologic-inadequate group on the 200-mg regimen vs 18.0% and 6.4%, respectively, on placebo (P < .001 for both subgroups).
Sands noted that the drug has an excellent safety profile.
“These data show the drug works for naive patients who have failed conventional therapies, as well as for those who have failed biologic therapies,” so it could be used as a first- or second-line biologic, he added.
Sands reported potential conflicts of interest with AbbVie, Abivax, Adiso Therapeutics, Agomab, Alimentiv, Amgen, AnaptysBio, Arena Pharmaceuticals, Artugen Therapeutics, AstraZeneca, Biora Therapeutics, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Calibr, Celgene, Celltrion, ClostraBio, Equillium, Enthera, Evommune, Ferring, Fresenius Kabi, Galapagos, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Index Pharmaceuticals, Innovation Pharmaceuticals, Inotrem, Janssen, Kaleido, Kallyope, Lilly, Merck, Microbiotica, Mobius Care, Morphic Therapeutic, MRM Health, Pfizer, Nexus Therapeutics, Nimbus Discovery, Odyssey Therapeutics, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Rasayana Therapeutics, Recludix Pharma, Reistone Biopharma, Sun Pharma, Surrozen, Target RWE, Takeda, Teva, Theravance Biopharma, TLL Pharmaceutical, Tr1X, UNION Therapeutics, and Ventyx Biosciences.
A version of this article appeared on Medscape.com.
VIENNA — according to a pooled analysis of the two phase 3 double-blind GALAXI 2 and 3 studies.
“We found that guselkumab was effective in both biologic-naive and biologic-inadequate subpopulations,” said coinvestigator Bruce E. Sands, MD, AGAF, gastroenterologist from Icahn School of Medicine at Mount Sinai, New York City.
These latest results add to the primary results of these studies reported earlier in 2024 that guselkumab was shown to be superior to both placebo and ustekinumab in the same patient population with moderately to severely active CD.
Sands reported the new data in a presentation at the United European Gastroenterology (UEG) Week 2024.
Guselkumab potently blocks interleukin (IL)–23 and binds to CD64, a receptor on cells that produce IL-23. The dual-acting IL-23p19 subunit inhibitor agent is currently under review by the Food and Drug Administration (FDA) for moderately to severely active CD. In September, guselkumab (Tremfya, Johnson & Johnson) was approved for use in moderately to severely active ulcerative colitis.
GALAXI 2 and 3 Pooled Dataset
In the two independent, identically designed GALAXI 2 and 3 studies, patients were randomized to guselkumab treatment at either 200 mg intravenous (IV) induction at weeks 0, 4, and 8, followed by 200 mg subcutaneous maintenance every 4 weeks, starting at week 12, or 200 mg IV induction at weeks 0, 4, and 8, followed by 100 mg subcutaneous maintenance every 8 weeks, starting at week 16; or to ustekinumab; or to placebo.
Participants were required to remain on their treatment of initial randomization for a long-term extension study (up to 5 years) looking at clinical, endoscopic, and safety outcomes, except for participants on placebo who were allowed to switch to ustekinumab if clinical response was not met at week 12.
Inclusion criteria for the studies comprised a Crohn’s Disease Activity Index score between 220 and 450, a mean daily stool frequency count > 3 or an abdominal pain score > 1, and a simple endoscopic score for CD score ≥ 6. Participants were also required to have shown an inadequate response or intolerance to oral corticosteroids, 6-mercaptopurine/azathioprine/methotrexate, or biologic therapies.
The pooled dataset included patients on either dose of guselkumab and patients on placebo (total n = 730). Of these, 52% of participants had shown a prior inadequate response to a biologic, 42% were biologic naive, and 6% had prior exposure to biologics but no documented failure. Patients on ustekinumab were not included in this analysis.
Almost all patients (97%) in the biologic-inadequate response group had previously received at least one anti–tumor necrosis factor agent, and around 15% had received vedolizumab. As expected, the biologic-inadequate responders were a lot sicker than the biologic-naive patients, Sands reported.
The composite co–primary endpoints for each guselkumab regimen vs placebo were clinical response at week 12 plus clinical remission at week 48, and clinical response at week 12 plus endoscopic response at week 48.
The major secondary endpoints comprised clinical remission at week 12 and endoscopic response also at week 12.
Short- and Long-Term Endpoints in Both Subgroups
In the biologic-naive subgroup, 54.7% of patients receiving the 200-mg dose regimen of guselkumab and 51.7% of those receiving the 100-mg dose regimen showed a clinical response at week 12 plus clinical remission at week 48, compared with 11.5% in the placebo group (P < .001 for both compared with placebo).
In the biologic-inadequate response group, 49.7% of those receiving the 200-mg dose regimen of guselkumab and 45.8% on the 100-mg dose regimen reached the composite endpoint, compared with the placebo response of 12.8% (P < .001 for both compared with placebo).
“You can see a slight decrease in response in the biologic-inadequate responders, but on the whole, the confidence intervals are highly overlapping,” said Sands.
Turning to major secondary endpoints at week 12, clinical remission was reached by 49.6% of the biologic-naive group on the 200-mg guselkumab regimen vs 16.4% on placebo, and by 46.0% of the biologic-inadequate group on the 200-mg regimen vs 19.2% on placebo (P < .001 for both subgroups). Endoscopic response was achieved by 46.3% of patients in the biologic-naive group and 29.0% in the biologic-inadequate group on the 200-mg regimen vs 18.0% and 6.4%, respectively, on placebo (P < .001 for both subgroups).
Sands noted that the drug has an excellent safety profile.
“These data show the drug works for naive patients who have failed conventional therapies, as well as for those who have failed biologic therapies,” so it could be used as a first- or second-line biologic, he added.
Sands reported potential conflicts of interest with AbbVie, Abivax, Adiso Therapeutics, Agomab, Alimentiv, Amgen, AnaptysBio, Arena Pharmaceuticals, Artugen Therapeutics, AstraZeneca, Biora Therapeutics, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Calibr, Celgene, Celltrion, ClostraBio, Equillium, Enthera, Evommune, Ferring, Fresenius Kabi, Galapagos, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Index Pharmaceuticals, Innovation Pharmaceuticals, Inotrem, Janssen, Kaleido, Kallyope, Lilly, Merck, Microbiotica, Mobius Care, Morphic Therapeutic, MRM Health, Pfizer, Nexus Therapeutics, Nimbus Discovery, Odyssey Therapeutics, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Rasayana Therapeutics, Recludix Pharma, Reistone Biopharma, Sun Pharma, Surrozen, Target RWE, Takeda, Teva, Theravance Biopharma, TLL Pharmaceutical, Tr1X, UNION Therapeutics, and Ventyx Biosciences.
A version of this article appeared on Medscape.com.
VIENNA — according to a pooled analysis of the two phase 3 double-blind GALAXI 2 and 3 studies.
“We found that guselkumab was effective in both biologic-naive and biologic-inadequate subpopulations,” said coinvestigator Bruce E. Sands, MD, AGAF, gastroenterologist from Icahn School of Medicine at Mount Sinai, New York City.
These latest results add to the primary results of these studies reported earlier in 2024 that guselkumab was shown to be superior to both placebo and ustekinumab in the same patient population with moderately to severely active CD.
Sands reported the new data in a presentation at the United European Gastroenterology (UEG) Week 2024.
Guselkumab potently blocks interleukin (IL)–23 and binds to CD64, a receptor on cells that produce IL-23. The dual-acting IL-23p19 subunit inhibitor agent is currently under review by the Food and Drug Administration (FDA) for moderately to severely active CD. In September, guselkumab (Tremfya, Johnson & Johnson) was approved for use in moderately to severely active ulcerative colitis.
GALAXI 2 and 3 Pooled Dataset
In the two independent, identically designed GALAXI 2 and 3 studies, patients were randomized to guselkumab treatment at either 200 mg intravenous (IV) induction at weeks 0, 4, and 8, followed by 200 mg subcutaneous maintenance every 4 weeks, starting at week 12, or 200 mg IV induction at weeks 0, 4, and 8, followed by 100 mg subcutaneous maintenance every 8 weeks, starting at week 16; or to ustekinumab; or to placebo.
Participants were required to remain on their treatment of initial randomization for a long-term extension study (up to 5 years) looking at clinical, endoscopic, and safety outcomes, except for participants on placebo who were allowed to switch to ustekinumab if clinical response was not met at week 12.
Inclusion criteria for the studies comprised a Crohn’s Disease Activity Index score between 220 and 450, a mean daily stool frequency count > 3 or an abdominal pain score > 1, and a simple endoscopic score for CD score ≥ 6. Participants were also required to have shown an inadequate response or intolerance to oral corticosteroids, 6-mercaptopurine/azathioprine/methotrexate, or biologic therapies.
The pooled dataset included patients on either dose of guselkumab and patients on placebo (total n = 730). Of these, 52% of participants had shown a prior inadequate response to a biologic, 42% were biologic naive, and 6% had prior exposure to biologics but no documented failure. Patients on ustekinumab were not included in this analysis.
Almost all patients (97%) in the biologic-inadequate response group had previously received at least one anti–tumor necrosis factor agent, and around 15% had received vedolizumab. As expected, the biologic-inadequate responders were a lot sicker than the biologic-naive patients, Sands reported.
The composite co–primary endpoints for each guselkumab regimen vs placebo were clinical response at week 12 plus clinical remission at week 48, and clinical response at week 12 plus endoscopic response at week 48.
The major secondary endpoints comprised clinical remission at week 12 and endoscopic response also at week 12.
Short- and Long-Term Endpoints in Both Subgroups
In the biologic-naive subgroup, 54.7% of patients receiving the 200-mg dose regimen of guselkumab and 51.7% of those receiving the 100-mg dose regimen showed a clinical response at week 12 plus clinical remission at week 48, compared with 11.5% in the placebo group (P < .001 for both compared with placebo).
In the biologic-inadequate response group, 49.7% of those receiving the 200-mg dose regimen of guselkumab and 45.8% on the 100-mg dose regimen reached the composite endpoint, compared with the placebo response of 12.8% (P < .001 for both compared with placebo).
“You can see a slight decrease in response in the biologic-inadequate responders, but on the whole, the confidence intervals are highly overlapping,” said Sands.
Turning to major secondary endpoints at week 12, clinical remission was reached by 49.6% of the biologic-naive group on the 200-mg guselkumab regimen vs 16.4% on placebo, and by 46.0% of the biologic-inadequate group on the 200-mg regimen vs 19.2% on placebo (P < .001 for both subgroups). Endoscopic response was achieved by 46.3% of patients in the biologic-naive group and 29.0% in the biologic-inadequate group on the 200-mg regimen vs 18.0% and 6.4%, respectively, on placebo (P < .001 for both subgroups).
Sands noted that the drug has an excellent safety profile.
“These data show the drug works for naive patients who have failed conventional therapies, as well as for those who have failed biologic therapies,” so it could be used as a first- or second-line biologic, he added.
Sands reported potential conflicts of interest with AbbVie, Abivax, Adiso Therapeutics, Agomab, Alimentiv, Amgen, AnaptysBio, Arena Pharmaceuticals, Artugen Therapeutics, AstraZeneca, Biora Therapeutics, Boehringer Ingelheim, Boston Pharmaceuticals, Bristol-Myers Squibb, Calibr, Celgene, Celltrion, ClostraBio, Equillium, Enthera, Evommune, Ferring, Fresenius Kabi, Galapagos, Genentech (Roche), Gilead Sciences, GlaxoSmithKline, Gossamer Bio, Index Pharmaceuticals, Innovation Pharmaceuticals, Inotrem, Janssen, Kaleido, Kallyope, Lilly, Merck, Microbiotica, Mobius Care, Morphic Therapeutic, MRM Health, Pfizer, Nexus Therapeutics, Nimbus Discovery, Odyssey Therapeutics, Progenity, Prometheus Biosciences, Prometheus Laboratories, Protagonist Therapeutics, Q32 Bio, Rasayana Therapeutics, Recludix Pharma, Reistone Biopharma, Sun Pharma, Surrozen, Target RWE, Takeda, Teva, Theravance Biopharma, TLL Pharmaceutical, Tr1X, UNION Therapeutics, and Ventyx Biosciences.
A version of this article appeared on Medscape.com.
FROM UEG 2024
Patients With IBD More Likely to Develop, or Have Prior, T1D
VIENNA —
Their findings showed that patients with IBD had a moderately increased risk for T1D and higher odds of having prior T1D than the general population. These bidirectional associations were partially independent of shared familial factors.
Although the absolute risk for T1D is low in patients with IBD, these findings suggest that if there are nonspecific symptoms, such as weight loss and fatigue, which are typical of T1D but not of IBD, then it might be reasonable to test for diabetes, lead researcher Jiangwei Sun, PhD, postdoctoral researcher at the Karolinska Institutet, Stockholm, Sweden, told this news organization.
“Patients with IBD and T1D also tend to have worse disease outcomes for both diseases, but these two diseases are not recognized as comorbidities in the clinical guidelines,” he said.
Anecdotally, “many clinicians believe there is a higher risk of autoimmune disease in patients with IBD but not much attention is paid to type 1 diabetes,” he added.
Sun presented the study at United European Gastroenterology (UEG) Week 2024. It was also published recently in The Lancet.
Exploring the Bidirectional Relationship
Prior research in the form of a systematic review found no association between IBD and T1D, which was surprising, Sun said. Further studies found an association between IBD and incident T1D; however, these studies did not explore bidirectionality between the two diseases.
These studies also did not take shared genetic and environmental factors into consideration, though “there is known to be familial co-aggregation of IBD and T1D based on previous findings,” he said.
In this current study, Sun and colleagues compared patients with IBD with the general population, as well as with siblings without IBD to consider the potential influence of shared genetics and earlier environmental factors.
The research used two approaches to look for a bidirectional association: A nationwide matched cohort study (IBD and incident T1D) and a case-control study (IBD and prior T1D).
The cohort study included 20,314 patients with IBD aged ≤ 28 years, who were identified between 1987 and 2017. Of these, 7277 had Crohn’s disease, 10,112 had ulcerative colitis, and 2925 had unclassified IBD. There were 99,200 individually matched reference individuals.
The case-control study included 87,001 patients with IBD (without age restriction) and 431,054 matched control individuals.
Risk ratios were calculated using an adjusted hazard ratio (aHR) of incident T1D in the cohort study and an adjusted odds ratio (aOR) of prior T1D in the case-control study.
In the cohort study, the median follow-up was 14 years. Over that time, 116 patients with IBD and 353 reference individuals developed T1D. The aHR for a patient with IBD developing T1D was 1.58 (95% CI, 1.27-1.95). For patients with ulcerative colitis, the aHR of developing T1D increased to 2.02 (95% CI, 1.51-2.70); however, the association was not found for Crohn’s disease or unclassified IBD possibly because of the sample size of these latter categories, noted Sun.
In the case-control study, Sun and colleagues identified 1018 (1.2%) patients with IBD and 3496 (0.8%) control individuals who had been previously diagnosed with T1D. Patients with IBD had higher odds of having prior T1D than those without IBD (aOR, 1.36; 95% CI, 1.26-1.46). This positive association was observed in all IBD subtypes, said Sun, who added that the sample size was larger in this analysis than in the cohort analysis.
Upon comparing patients with IBD with their siblings without IBD, analyses showed similar associations between IBD and T1D; the aHR was 1.44 (95% CI, 0.97-2.15) for developing T1D, and the aOR was 1.32 (95% CI, 1.18-1.49) for prior T1D.
That these positive associations between IBD and T1D exist even when comparing patients with IBD with their siblings without IBD suggests genetics and shared environmental factors do not fully explain the association, and that later environmental factors might play a role, said Sun.
“I’m not surprised with these results,” he added. “They make sense because we know that both IBD and T1D are immunity-related diseases and have some shared pathways.”
Commenting on the study, Tine Jess, MD, director, Center for Molecular Prediction of Inflammatory Bowel Disease, PREDICT, Aalborg University in Copenhagen, Denmark, said: “The really interesting finding here is that type 1 diabetes may precede IBD, which points toward common etiologies rather than one disease leading to the other.”
“This is in line with mounting evidence that IBD is measurable at the molecular level years prior to diagnosis,” she added.
Awareness of the bidirectional association may facilitate early detection of both conditions, Sun and his colleagues noted.
Sun reported no relevant financial relationships. Jess reported receiving consultancy fees from Ferring and Pfizer.
A version of this article appeared on Medscape.com.
VIENNA —
Their findings showed that patients with IBD had a moderately increased risk for T1D and higher odds of having prior T1D than the general population. These bidirectional associations were partially independent of shared familial factors.
Although the absolute risk for T1D is low in patients with IBD, these findings suggest that if there are nonspecific symptoms, such as weight loss and fatigue, which are typical of T1D but not of IBD, then it might be reasonable to test for diabetes, lead researcher Jiangwei Sun, PhD, postdoctoral researcher at the Karolinska Institutet, Stockholm, Sweden, told this news organization.
“Patients with IBD and T1D also tend to have worse disease outcomes for both diseases, but these two diseases are not recognized as comorbidities in the clinical guidelines,” he said.
Anecdotally, “many clinicians believe there is a higher risk of autoimmune disease in patients with IBD but not much attention is paid to type 1 diabetes,” he added.
Sun presented the study at United European Gastroenterology (UEG) Week 2024. It was also published recently in The Lancet.
Exploring the Bidirectional Relationship
Prior research in the form of a systematic review found no association between IBD and T1D, which was surprising, Sun said. Further studies found an association between IBD and incident T1D; however, these studies did not explore bidirectionality between the two diseases.
These studies also did not take shared genetic and environmental factors into consideration, though “there is known to be familial co-aggregation of IBD and T1D based on previous findings,” he said.
In this current study, Sun and colleagues compared patients with IBD with the general population, as well as with siblings without IBD to consider the potential influence of shared genetics and earlier environmental factors.
The research used two approaches to look for a bidirectional association: A nationwide matched cohort study (IBD and incident T1D) and a case-control study (IBD and prior T1D).
The cohort study included 20,314 patients with IBD aged ≤ 28 years, who were identified between 1987 and 2017. Of these, 7277 had Crohn’s disease, 10,112 had ulcerative colitis, and 2925 had unclassified IBD. There were 99,200 individually matched reference individuals.
The case-control study included 87,001 patients with IBD (without age restriction) and 431,054 matched control individuals.
Risk ratios were calculated using an adjusted hazard ratio (aHR) of incident T1D in the cohort study and an adjusted odds ratio (aOR) of prior T1D in the case-control study.
In the cohort study, the median follow-up was 14 years. Over that time, 116 patients with IBD and 353 reference individuals developed T1D. The aHR for a patient with IBD developing T1D was 1.58 (95% CI, 1.27-1.95). For patients with ulcerative colitis, the aHR of developing T1D increased to 2.02 (95% CI, 1.51-2.70); however, the association was not found for Crohn’s disease or unclassified IBD possibly because of the sample size of these latter categories, noted Sun.
In the case-control study, Sun and colleagues identified 1018 (1.2%) patients with IBD and 3496 (0.8%) control individuals who had been previously diagnosed with T1D. Patients with IBD had higher odds of having prior T1D than those without IBD (aOR, 1.36; 95% CI, 1.26-1.46). This positive association was observed in all IBD subtypes, said Sun, who added that the sample size was larger in this analysis than in the cohort analysis.
Upon comparing patients with IBD with their siblings without IBD, analyses showed similar associations between IBD and T1D; the aHR was 1.44 (95% CI, 0.97-2.15) for developing T1D, and the aOR was 1.32 (95% CI, 1.18-1.49) for prior T1D.
That these positive associations between IBD and T1D exist even when comparing patients with IBD with their siblings without IBD suggests genetics and shared environmental factors do not fully explain the association, and that later environmental factors might play a role, said Sun.
“I’m not surprised with these results,” he added. “They make sense because we know that both IBD and T1D are immunity-related diseases and have some shared pathways.”
Commenting on the study, Tine Jess, MD, director, Center for Molecular Prediction of Inflammatory Bowel Disease, PREDICT, Aalborg University in Copenhagen, Denmark, said: “The really interesting finding here is that type 1 diabetes may precede IBD, which points toward common etiologies rather than one disease leading to the other.”
“This is in line with mounting evidence that IBD is measurable at the molecular level years prior to diagnosis,” she added.
Awareness of the bidirectional association may facilitate early detection of both conditions, Sun and his colleagues noted.
Sun reported no relevant financial relationships. Jess reported receiving consultancy fees from Ferring and Pfizer.
A version of this article appeared on Medscape.com.
VIENNA —
Their findings showed that patients with IBD had a moderately increased risk for T1D and higher odds of having prior T1D than the general population. These bidirectional associations were partially independent of shared familial factors.
Although the absolute risk for T1D is low in patients with IBD, these findings suggest that if there are nonspecific symptoms, such as weight loss and fatigue, which are typical of T1D but not of IBD, then it might be reasonable to test for diabetes, lead researcher Jiangwei Sun, PhD, postdoctoral researcher at the Karolinska Institutet, Stockholm, Sweden, told this news organization.
“Patients with IBD and T1D also tend to have worse disease outcomes for both diseases, but these two diseases are not recognized as comorbidities in the clinical guidelines,” he said.
Anecdotally, “many clinicians believe there is a higher risk of autoimmune disease in patients with IBD but not much attention is paid to type 1 diabetes,” he added.
Sun presented the study at United European Gastroenterology (UEG) Week 2024. It was also published recently in The Lancet.
Exploring the Bidirectional Relationship
Prior research in the form of a systematic review found no association between IBD and T1D, which was surprising, Sun said. Further studies found an association between IBD and incident T1D; however, these studies did not explore bidirectionality between the two diseases.
These studies also did not take shared genetic and environmental factors into consideration, though “there is known to be familial co-aggregation of IBD and T1D based on previous findings,” he said.
In this current study, Sun and colleagues compared patients with IBD with the general population, as well as with siblings without IBD to consider the potential influence of shared genetics and earlier environmental factors.
The research used two approaches to look for a bidirectional association: A nationwide matched cohort study (IBD and incident T1D) and a case-control study (IBD and prior T1D).
The cohort study included 20,314 patients with IBD aged ≤ 28 years, who were identified between 1987 and 2017. Of these, 7277 had Crohn’s disease, 10,112 had ulcerative colitis, and 2925 had unclassified IBD. There were 99,200 individually matched reference individuals.
The case-control study included 87,001 patients with IBD (without age restriction) and 431,054 matched control individuals.
Risk ratios were calculated using an adjusted hazard ratio (aHR) of incident T1D in the cohort study and an adjusted odds ratio (aOR) of prior T1D in the case-control study.
In the cohort study, the median follow-up was 14 years. Over that time, 116 patients with IBD and 353 reference individuals developed T1D. The aHR for a patient with IBD developing T1D was 1.58 (95% CI, 1.27-1.95). For patients with ulcerative colitis, the aHR of developing T1D increased to 2.02 (95% CI, 1.51-2.70); however, the association was not found for Crohn’s disease or unclassified IBD possibly because of the sample size of these latter categories, noted Sun.
In the case-control study, Sun and colleagues identified 1018 (1.2%) patients with IBD and 3496 (0.8%) control individuals who had been previously diagnosed with T1D. Patients with IBD had higher odds of having prior T1D than those without IBD (aOR, 1.36; 95% CI, 1.26-1.46). This positive association was observed in all IBD subtypes, said Sun, who added that the sample size was larger in this analysis than in the cohort analysis.
Upon comparing patients with IBD with their siblings without IBD, analyses showed similar associations between IBD and T1D; the aHR was 1.44 (95% CI, 0.97-2.15) for developing T1D, and the aOR was 1.32 (95% CI, 1.18-1.49) for prior T1D.
That these positive associations between IBD and T1D exist even when comparing patients with IBD with their siblings without IBD suggests genetics and shared environmental factors do not fully explain the association, and that later environmental factors might play a role, said Sun.
“I’m not surprised with these results,” he added. “They make sense because we know that both IBD and T1D are immunity-related diseases and have some shared pathways.”
Commenting on the study, Tine Jess, MD, director, Center for Molecular Prediction of Inflammatory Bowel Disease, PREDICT, Aalborg University in Copenhagen, Denmark, said: “The really interesting finding here is that type 1 diabetes may precede IBD, which points toward common etiologies rather than one disease leading to the other.”
“This is in line with mounting evidence that IBD is measurable at the molecular level years prior to diagnosis,” she added.
Awareness of the bidirectional association may facilitate early detection of both conditions, Sun and his colleagues noted.
Sun reported no relevant financial relationships. Jess reported receiving consultancy fees from Ferring and Pfizer.
A version of this article appeared on Medscape.com.
FROM UEG 2024
IBS: Understanding a Common Yet Misunderstood Condition
Irritable bowel syndrome (IBS) is one of the most common conditions encountered by both primary care providers and gastroenterologists, with a pooled global prevalence of 11.2%. This functional bowel disorder is characterized by abdominal pain or discomfort, diarrhea and/or constipation, and bloating.
Unfortunately, , according to Alan Desmond, MB, consultant in gastroenterology and general internal medicine, Torbay Hospital, UK National Health Service.
Desmond regularly sees patients who either haven’t been accurately diagnosed or have been told, “Don’t worry, it’s ‘just’ irritable bowel syndrome,” he said at the recent International Conference on Nutrition in Medicine.
A 2017 study involving nearly 2000 patients with a history of gastrointestinal (GI) symptoms found that 43.1% of those who met the criteria for IBS were undiagnosed, and among those who were diagnosed, 26% were not receiving treatment.
“Many clinicians vastly underestimate the impact functional GI symptoms have on our patients in lack of productivity, becoming homebound or losing employment, the inability to enjoy a meal with friends or family, and always needing to know where the nearest bathroom is, for example,” Desmond said in an interview.
IBS can profoundly affect patients’ mental health. One study found that 38% of patients with IBS attending a tertiary care clinic contemplated suicide because they felt hopeless about ever achieving symptom relief.
Today, several dietary, pharmacologic, and psychological/behavioral approaches are available to treat patients with IBS, noted William D. Chey, MD, AGAF, chief of the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan.
“Each individual patient may need a different combination of these foundational treatments,” he said. “One size doesn’t fit all.”
Diagnostic Pathway
One reason IBS is so hard to diagnose is that it’s a “symptom-based disorder, with identification of the condition predicated upon certain key characteristics that are heterogeneous,” Chey said in an interview. “IBS in patient ‘A’ may not present the same way as IBS in patient ‘B,’ although there are certain foundational common characteristics.”
IBS involves “abnormalities in the motility and contractility of the GI tract,” he said. It can present with diarrhea (IBS-D), constipation (IBS-C), or a mixture or alternation of diarrhea and constipation (IBS-M).
Patients with IBS-D often have an exaggerated gastro-colonic response, while those with IBS-C often have a blunted response.
Beyond stool abnormalities and abdominal pain/discomfort, patients often report bloating/distension, low backache, lethargy, nausea, thigh pain, and urinary and gynecologic symptoms.
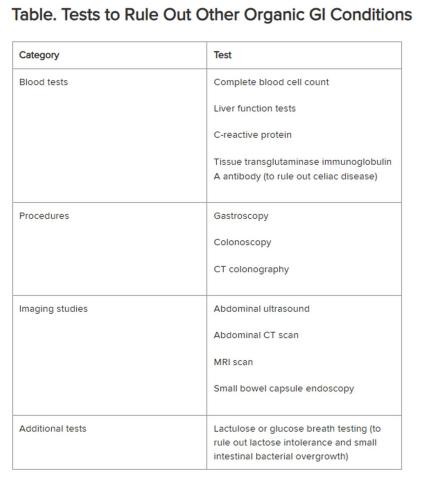
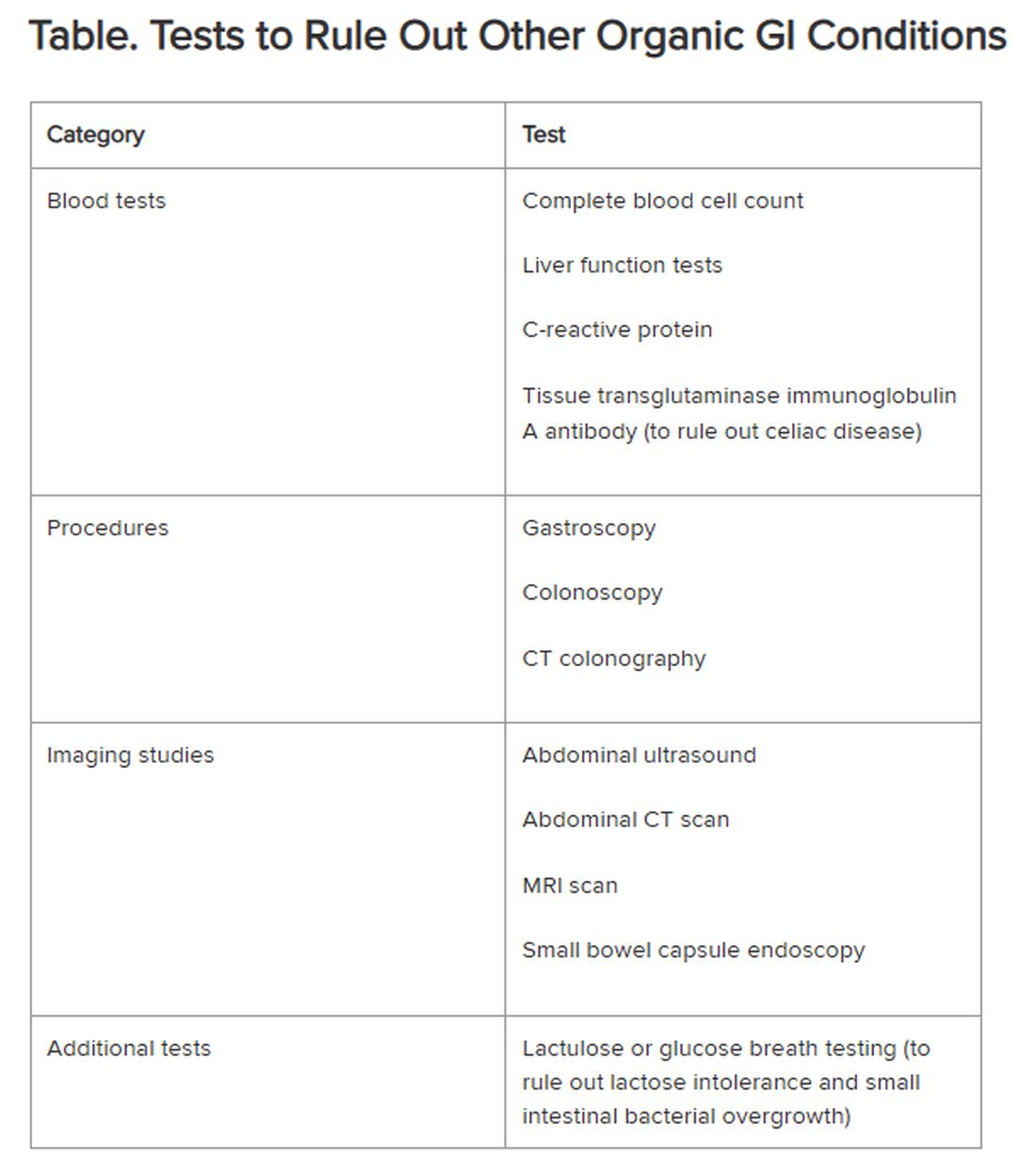
Historically, IBS has been regarded as a “diagnosis of exclusion” because classic diagnostic tests typically yield no concrete findings. Desmond noted that several blood tests, procedures, imaging studies, and other tests are available to rule out other organic GI conditions, as outlined in the Table.
If the patient comes from a geographical region where giardia is endemic, clinicians also should consider testing for the parasite, Chey said.
New Understanding of IBS Etiology
Now, advances in the understanding of IBS are changing the approach to the disease.
“The field is moving away from seeing IBS as a ‘wastebasket diagnosis,’ recognizing that there are other causes of a patient’s symptoms,” Mark Pimentel, MD, associate professor of medicine and gastroenterology, Cedars-Sinai, Los Angeles, said in an interview. “What’s made IBS so difficult to diagnose has been the absence of biological markers and hallmark findings on endoscopy.”
Recent research points to novel bacterial causes as culprits in the development of IBS. In particular, altered small bowel microbiota can be triggered by acute gastroenteritis.
Food poisoning can trigger the onset of IBS — a phenomenon called “postinfectious IBS (PI-IBS),” said Pimentel, who is also executive director of the Medically Associated Science and Technology Program at Cedars-Sinai. PI-IBS almost always takes the form of IBS-D, with up to 60% of patients with IBS-D suffering the long-term sequelae of food poisoning.
The types of bacteria most commonly associated with gastroenteritis are Shigella, Campylobacter, Salmonella, and Escherichia coli, Pimentel said. All of them release cytolethal distending toxin B (CdtB), causing the body to produce antibodies to the toxin.
CdtB resembles vinculin, a naturally occurring protein critical for healthy gut function. “Because of this molecular resemblance, the immune system often mistakes one for the other, producing anti-vinculin,” Pimentel explained.
This autoimmune response leads to disruptions in the gut microbiome, ultimately resulting in PI-IBS. The chain of events “doesn’t necessarily happen immediately,” Pimentel said. “You might have developed food poisoning at a party weeks or months ago.”
Acute gastroenteritis is common, affecting as many as 179 million people in the United States annually. A meta-analysis of 47 studies, incorporating 28,270 patients, found that those who had experienced acute gastroenteritis had a fourfold higher risk of developing IBS compared with nonexposed controls.
“The problem isn’t only the IBS itself, but the fact that people with PI-IBS are four times as likely to contract food poisoning again, which can further exacerbate IBS symptoms,” Pimentel said.
Diarrhea-predominant IBS can be detected through the presence of two blood biomarkers — anti-CdtB and anti-vinculin — in a blood test developed by Pimentel and his group.
“Elevation in either of these biomarkers establishes the diagnosis,” Pimentel said. “This is a breakthrough because it represents the first test that can make IBS a ‘diagnosis of inclusion.’”
The blood test also can identify IBS-M but not IBS-C.
Pimentel said that IBS-C is associated with increased levels of methanogenic archaea, which can be diagnosed by a positive methane breath test. “Methane gas slows intestinal contractility, which might result in constipation,” he said.
Diet as a Treatment Option
Diet is usually the starting point for IBS treatment, Chey said. “The standard dietary recommendations, as defined by the National Institute for Health and Care Excellence Guidance for managing IBS, are reasonable and common sense — eating three meals a day, avoiding carbonated beverages, excess alcohol, and excess caffeine, and avoiding hard-to-digest foods that can be gas producing.”
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs), which are carbohydrates that aren’t completely absorbed in the intestines, has been shown to be effective in alleviating GI distress in as many as 86% of patients with IBS, leading to improvements in overall GI symptoms as well as individual symptoms (eg, abdominal pain, bloating, constipation, diarrhea, and flatulence).
Desmond recommends the low FODMAP program delineated by Monash University in Australia. The diet should be undertaken only under the supervision of a dietitian, he warned. Moreover, following it on a long-term basis can have an adverse impact on dietary quality and the gut microbiome. Therefore, “it’s important to embark on stepwise reintroduction of FODMAPS under supervision to find acceptable thresholds that don’t cause a return of symptoms.”
A growing body of research suggests that following the Mediterranean diet can be helpful in reducing IBS symptoms. Chey said that some patients who tend to over-restrict their eating might benefit from a less restrictive diet than the typical low FODMAPs diet. For them, the Mediterranean diet may be a good option.
Pharmacotherapy for IBS
Nutritional approaches aren’t for everyone, Chey noted. “Some people don’t want to be on a highly restricted diet.” For them, medications addressing symptoms might be a better option.
Antispasmodics — either anticholinergics (hyoscine and dicyclomine) or smooth muscle relaxants (alverine, mebeverine, and peppermint oil) — can be helpful, although they can worsen constipation in a dose-dependent manner. It is advisable to use them on an as-needed rather than long-term basis.
Antidiarrheal agents include loperamide and diphenoxylate.
For constipation, laxatives (eg, senna, bisacodyl, polyethylene glycol, and sodium picosulfate) can be helpful.
Desmond noted that the American Gastroenterological Association does not recommend routine use of probiotics for most GI disorders, including IBS. Exceptions include prevention of Clostridioides difficile, ulcerative colitis, and pouchitis.
Targeting the Gut-Brain Relationship
Stress plays a role in exacerbating symptoms in patients with IBS and is an important target for intervention.
“If patients are living with a level of stress that’s impairing, we won’t be able to solve their gut issues until we resolve their stress issues,” Desmond said. “We need to calm the gut-microbiome-brain axis, which is multidimensional and bidirectional.”
Many people — even those without IBS — experience queasiness or diarrhea prior to a major event they’re nervous about, Chey noted. These events activate the brain, which activates the nervous system, which interacts with the GI tract. Indeed, IBS is now recognized as a disorder of gut-brain interaction, he said.
“We now know that the microbiome in the GI tract influences cognition and emotional function, depression, and anxiety. One might say that the gut is the ‘center of the universe’ to human beings,” Chey said.
Evidence-based psychological approaches for stress reduction in patients with IBS include cognitive behavioral therapy, specifically tailored to helping the patient identify associations between IBS symptoms and thoughts, emotions, and actions, as well as learning new behaviors and engaging in stress management. Psychodynamic (interpersonal) therapy enables patients to understand the connection between GI symptoms and interpersonal conflicts, emotional factors, or relationship difficulties.
Gut-directed hypnotherapy (GDH) is a “proven modality for IBS,” Desmond said. Unlike other forms of hypnotherapy, GDH focuses specifically on controlling and normalizing GI function. Studies have shown a reduction of ≥ 30% in abdominal pain in two thirds of participants, with overall response rates up to 85%. It can be delivered in an individual or group setting or via a smartphone.
Desmond recommends mindfulness-based therapy (MBT) for IBS. MBT focuses on the “cultivation of mindfulness, defined as intentional, nonjudgmental, present-focused awareness.” It has been found effective in reducing flares and the markers of gut inflammation in ulcerative colitis, as well as reducing symptoms of IBS.
Chey noted that an emerging body of literature supports the potential role of acupuncture in treating IBS, and his clinic employs it. “I would like to see further research into other areas of CAM [complementary and alternative medicine], including herbal approaches to IBS symptoms as well as stress.”
Finally, all the experts agree that more research is needed.
“The real tragedy is that the NIH invests next to nothing in IBS, in contrast to inflammatory bowel disease and many other conditions,” Pimentel said. “Yet IBS is 45 times more common than inflammatory bowel disease.”
Pimentel hopes that with enough advocacy and recognition that IBS isn’t “just stress-related,” more resources will be devoted to understanding this debilitating condition.
Desmond is the author of a book on the benefits of a plant-based diet. He has also received honoraria, speaking, and consultancy fees from the European Space Agency, Dyson Institute of Engineering and Technology, Riverford Organic Farmers, Ltd., Salesforce Inc., Sentara Healthcare, Saudi Sports for All Federation, the Physicians Committee for Responsible Medicine, The Plantrician Project, Doctors for Nutrition, and The Happy Pear.
Pimentel is a consultant for Bausch Health, Ferring Pharmaceuticals, and Ardelyx. He holds equity in and is also a consultant for Dieta Health, Salvo Health, Cylinder Health, and Gemelli Biotech. Cedars-Sinai has a licensing agreement with Gemelli Biotech and Hobbs Medical.
Chey is a consultant to AbbVie, Ardelyx, Atmo, Biomerica, Gemelli Biotech, Ironwood Pharmaceuticals, Nestlé, QOL Medical, Phathom Pharmaceuticals, Redhill, Salix/Valeant, Takeda, and Vibrant. He receives grant/research funding from Commonwealth Diagnostics International, Inc., US Food and Drug Administration, National Institutes of Health, QOL Medical, and Salix/Valeant. He holds stock options in Coprata, Dieta Health, Evinature, FoodMarble, Kiwi Biosciences, and ModifyHealth. He is a board or advisory panel member of the American College of Gastroenterology, GI Health Foundation, International Foundation for Gastrointestinal Disorders, Rome. He holds patents on My Nutrition Health, Digital Manometry, and Rectal Expulsion Device.
A version of this article appeared on Medscape.com.
Irritable bowel syndrome (IBS) is one of the most common conditions encountered by both primary care providers and gastroenterologists, with a pooled global prevalence of 11.2%. This functional bowel disorder is characterized by abdominal pain or discomfort, diarrhea and/or constipation, and bloating.
Unfortunately, , according to Alan Desmond, MB, consultant in gastroenterology and general internal medicine, Torbay Hospital, UK National Health Service.
Desmond regularly sees patients who either haven’t been accurately diagnosed or have been told, “Don’t worry, it’s ‘just’ irritable bowel syndrome,” he said at the recent International Conference on Nutrition in Medicine.
A 2017 study involving nearly 2000 patients with a history of gastrointestinal (GI) symptoms found that 43.1% of those who met the criteria for IBS were undiagnosed, and among those who were diagnosed, 26% were not receiving treatment.
“Many clinicians vastly underestimate the impact functional GI symptoms have on our patients in lack of productivity, becoming homebound or losing employment, the inability to enjoy a meal with friends or family, and always needing to know where the nearest bathroom is, for example,” Desmond said in an interview.
IBS can profoundly affect patients’ mental health. One study found that 38% of patients with IBS attending a tertiary care clinic contemplated suicide because they felt hopeless about ever achieving symptom relief.
Today, several dietary, pharmacologic, and psychological/behavioral approaches are available to treat patients with IBS, noted William D. Chey, MD, AGAF, chief of the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan.
“Each individual patient may need a different combination of these foundational treatments,” he said. “One size doesn’t fit all.”
Diagnostic Pathway
One reason IBS is so hard to diagnose is that it’s a “symptom-based disorder, with identification of the condition predicated upon certain key characteristics that are heterogeneous,” Chey said in an interview. “IBS in patient ‘A’ may not present the same way as IBS in patient ‘B,’ although there are certain foundational common characteristics.”
IBS involves “abnormalities in the motility and contractility of the GI tract,” he said. It can present with diarrhea (IBS-D), constipation (IBS-C), or a mixture or alternation of diarrhea and constipation (IBS-M).
Patients with IBS-D often have an exaggerated gastro-colonic response, while those with IBS-C often have a blunted response.
Beyond stool abnormalities and abdominal pain/discomfort, patients often report bloating/distension, low backache, lethargy, nausea, thigh pain, and urinary and gynecologic symptoms.
Historically, IBS has been regarded as a “diagnosis of exclusion” because classic diagnostic tests typically yield no concrete findings. Desmond noted that several blood tests, procedures, imaging studies, and other tests are available to rule out other organic GI conditions, as outlined in the Table.
If the patient comes from a geographical region where giardia is endemic, clinicians also should consider testing for the parasite, Chey said.
New Understanding of IBS Etiology
Now, advances in the understanding of IBS are changing the approach to the disease.
“The field is moving away from seeing IBS as a ‘wastebasket diagnosis,’ recognizing that there are other causes of a patient’s symptoms,” Mark Pimentel, MD, associate professor of medicine and gastroenterology, Cedars-Sinai, Los Angeles, said in an interview. “What’s made IBS so difficult to diagnose has been the absence of biological markers and hallmark findings on endoscopy.”
Recent research points to novel bacterial causes as culprits in the development of IBS. In particular, altered small bowel microbiota can be triggered by acute gastroenteritis.
Food poisoning can trigger the onset of IBS — a phenomenon called “postinfectious IBS (PI-IBS),” said Pimentel, who is also executive director of the Medically Associated Science and Technology Program at Cedars-Sinai. PI-IBS almost always takes the form of IBS-D, with up to 60% of patients with IBS-D suffering the long-term sequelae of food poisoning.
The types of bacteria most commonly associated with gastroenteritis are Shigella, Campylobacter, Salmonella, and Escherichia coli, Pimentel said. All of them release cytolethal distending toxin B (CdtB), causing the body to produce antibodies to the toxin.
CdtB resembles vinculin, a naturally occurring protein critical for healthy gut function. “Because of this molecular resemblance, the immune system often mistakes one for the other, producing anti-vinculin,” Pimentel explained.
This autoimmune response leads to disruptions in the gut microbiome, ultimately resulting in PI-IBS. The chain of events “doesn’t necessarily happen immediately,” Pimentel said. “You might have developed food poisoning at a party weeks or months ago.”
Acute gastroenteritis is common, affecting as many as 179 million people in the United States annually. A meta-analysis of 47 studies, incorporating 28,270 patients, found that those who had experienced acute gastroenteritis had a fourfold higher risk of developing IBS compared with nonexposed controls.
“The problem isn’t only the IBS itself, but the fact that people with PI-IBS are four times as likely to contract food poisoning again, which can further exacerbate IBS symptoms,” Pimentel said.
Diarrhea-predominant IBS can be detected through the presence of two blood biomarkers — anti-CdtB and anti-vinculin — in a blood test developed by Pimentel and his group.
“Elevation in either of these biomarkers establishes the diagnosis,” Pimentel said. “This is a breakthrough because it represents the first test that can make IBS a ‘diagnosis of inclusion.’”
The blood test also can identify IBS-M but not IBS-C.
Pimentel said that IBS-C is associated with increased levels of methanogenic archaea, which can be diagnosed by a positive methane breath test. “Methane gas slows intestinal contractility, which might result in constipation,” he said.
Diet as a Treatment Option
Diet is usually the starting point for IBS treatment, Chey said. “The standard dietary recommendations, as defined by the National Institute for Health and Care Excellence Guidance for managing IBS, are reasonable and common sense — eating three meals a day, avoiding carbonated beverages, excess alcohol, and excess caffeine, and avoiding hard-to-digest foods that can be gas producing.”
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs), which are carbohydrates that aren’t completely absorbed in the intestines, has been shown to be effective in alleviating GI distress in as many as 86% of patients with IBS, leading to improvements in overall GI symptoms as well as individual symptoms (eg, abdominal pain, bloating, constipation, diarrhea, and flatulence).
Desmond recommends the low FODMAP program delineated by Monash University in Australia. The diet should be undertaken only under the supervision of a dietitian, he warned. Moreover, following it on a long-term basis can have an adverse impact on dietary quality and the gut microbiome. Therefore, “it’s important to embark on stepwise reintroduction of FODMAPS under supervision to find acceptable thresholds that don’t cause a return of symptoms.”
A growing body of research suggests that following the Mediterranean diet can be helpful in reducing IBS symptoms. Chey said that some patients who tend to over-restrict their eating might benefit from a less restrictive diet than the typical low FODMAPs diet. For them, the Mediterranean diet may be a good option.
Pharmacotherapy for IBS
Nutritional approaches aren’t for everyone, Chey noted. “Some people don’t want to be on a highly restricted diet.” For them, medications addressing symptoms might be a better option.
Antispasmodics — either anticholinergics (hyoscine and dicyclomine) or smooth muscle relaxants (alverine, mebeverine, and peppermint oil) — can be helpful, although they can worsen constipation in a dose-dependent manner. It is advisable to use them on an as-needed rather than long-term basis.
Antidiarrheal agents include loperamide and diphenoxylate.
For constipation, laxatives (eg, senna, bisacodyl, polyethylene glycol, and sodium picosulfate) can be helpful.
Desmond noted that the American Gastroenterological Association does not recommend routine use of probiotics for most GI disorders, including IBS. Exceptions include prevention of Clostridioides difficile, ulcerative colitis, and pouchitis.
Targeting the Gut-Brain Relationship
Stress plays a role in exacerbating symptoms in patients with IBS and is an important target for intervention.
“If patients are living with a level of stress that’s impairing, we won’t be able to solve their gut issues until we resolve their stress issues,” Desmond said. “We need to calm the gut-microbiome-brain axis, which is multidimensional and bidirectional.”
Many people — even those without IBS — experience queasiness or diarrhea prior to a major event they’re nervous about, Chey noted. These events activate the brain, which activates the nervous system, which interacts with the GI tract. Indeed, IBS is now recognized as a disorder of gut-brain interaction, he said.
“We now know that the microbiome in the GI tract influences cognition and emotional function, depression, and anxiety. One might say that the gut is the ‘center of the universe’ to human beings,” Chey said.
Evidence-based psychological approaches for stress reduction in patients with IBS include cognitive behavioral therapy, specifically tailored to helping the patient identify associations between IBS symptoms and thoughts, emotions, and actions, as well as learning new behaviors and engaging in stress management. Psychodynamic (interpersonal) therapy enables patients to understand the connection between GI symptoms and interpersonal conflicts, emotional factors, or relationship difficulties.
Gut-directed hypnotherapy (GDH) is a “proven modality for IBS,” Desmond said. Unlike other forms of hypnotherapy, GDH focuses specifically on controlling and normalizing GI function. Studies have shown a reduction of ≥ 30% in abdominal pain in two thirds of participants, with overall response rates up to 85%. It can be delivered in an individual or group setting or via a smartphone.
Desmond recommends mindfulness-based therapy (MBT) for IBS. MBT focuses on the “cultivation of mindfulness, defined as intentional, nonjudgmental, present-focused awareness.” It has been found effective in reducing flares and the markers of gut inflammation in ulcerative colitis, as well as reducing symptoms of IBS.
Chey noted that an emerging body of literature supports the potential role of acupuncture in treating IBS, and his clinic employs it. “I would like to see further research into other areas of CAM [complementary and alternative medicine], including herbal approaches to IBS symptoms as well as stress.”
Finally, all the experts agree that more research is needed.
“The real tragedy is that the NIH invests next to nothing in IBS, in contrast to inflammatory bowel disease and many other conditions,” Pimentel said. “Yet IBS is 45 times more common than inflammatory bowel disease.”
Pimentel hopes that with enough advocacy and recognition that IBS isn’t “just stress-related,” more resources will be devoted to understanding this debilitating condition.
Desmond is the author of a book on the benefits of a plant-based diet. He has also received honoraria, speaking, and consultancy fees from the European Space Agency, Dyson Institute of Engineering and Technology, Riverford Organic Farmers, Ltd., Salesforce Inc., Sentara Healthcare, Saudi Sports for All Federation, the Physicians Committee for Responsible Medicine, The Plantrician Project, Doctors for Nutrition, and The Happy Pear.
Pimentel is a consultant for Bausch Health, Ferring Pharmaceuticals, and Ardelyx. He holds equity in and is also a consultant for Dieta Health, Salvo Health, Cylinder Health, and Gemelli Biotech. Cedars-Sinai has a licensing agreement with Gemelli Biotech and Hobbs Medical.
Chey is a consultant to AbbVie, Ardelyx, Atmo, Biomerica, Gemelli Biotech, Ironwood Pharmaceuticals, Nestlé, QOL Medical, Phathom Pharmaceuticals, Redhill, Salix/Valeant, Takeda, and Vibrant. He receives grant/research funding from Commonwealth Diagnostics International, Inc., US Food and Drug Administration, National Institutes of Health, QOL Medical, and Salix/Valeant. He holds stock options in Coprata, Dieta Health, Evinature, FoodMarble, Kiwi Biosciences, and ModifyHealth. He is a board or advisory panel member of the American College of Gastroenterology, GI Health Foundation, International Foundation for Gastrointestinal Disorders, Rome. He holds patents on My Nutrition Health, Digital Manometry, and Rectal Expulsion Device.
A version of this article appeared on Medscape.com.
Irritable bowel syndrome (IBS) is one of the most common conditions encountered by both primary care providers and gastroenterologists, with a pooled global prevalence of 11.2%. This functional bowel disorder is characterized by abdominal pain or discomfort, diarrhea and/or constipation, and bloating.
Unfortunately, , according to Alan Desmond, MB, consultant in gastroenterology and general internal medicine, Torbay Hospital, UK National Health Service.
Desmond regularly sees patients who either haven’t been accurately diagnosed or have been told, “Don’t worry, it’s ‘just’ irritable bowel syndrome,” he said at the recent International Conference on Nutrition in Medicine.
A 2017 study involving nearly 2000 patients with a history of gastrointestinal (GI) symptoms found that 43.1% of those who met the criteria for IBS were undiagnosed, and among those who were diagnosed, 26% were not receiving treatment.
“Many clinicians vastly underestimate the impact functional GI symptoms have on our patients in lack of productivity, becoming homebound or losing employment, the inability to enjoy a meal with friends or family, and always needing to know where the nearest bathroom is, for example,” Desmond said in an interview.
IBS can profoundly affect patients’ mental health. One study found that 38% of patients with IBS attending a tertiary care clinic contemplated suicide because they felt hopeless about ever achieving symptom relief.
Today, several dietary, pharmacologic, and psychological/behavioral approaches are available to treat patients with IBS, noted William D. Chey, MD, AGAF, chief of the Division of Gastroenterology and Hepatology, University of Michigan, Ann Arbor, Michigan.
“Each individual patient may need a different combination of these foundational treatments,” he said. “One size doesn’t fit all.”
Diagnostic Pathway
One reason IBS is so hard to diagnose is that it’s a “symptom-based disorder, with identification of the condition predicated upon certain key characteristics that are heterogeneous,” Chey said in an interview. “IBS in patient ‘A’ may not present the same way as IBS in patient ‘B,’ although there are certain foundational common characteristics.”
IBS involves “abnormalities in the motility and contractility of the GI tract,” he said. It can present with diarrhea (IBS-D), constipation (IBS-C), or a mixture or alternation of diarrhea and constipation (IBS-M).
Patients with IBS-D often have an exaggerated gastro-colonic response, while those with IBS-C often have a blunted response.
Beyond stool abnormalities and abdominal pain/discomfort, patients often report bloating/distension, low backache, lethargy, nausea, thigh pain, and urinary and gynecologic symptoms.
Historically, IBS has been regarded as a “diagnosis of exclusion” because classic diagnostic tests typically yield no concrete findings. Desmond noted that several blood tests, procedures, imaging studies, and other tests are available to rule out other organic GI conditions, as outlined in the Table.
If the patient comes from a geographical region where giardia is endemic, clinicians also should consider testing for the parasite, Chey said.
New Understanding of IBS Etiology
Now, advances in the understanding of IBS are changing the approach to the disease.
“The field is moving away from seeing IBS as a ‘wastebasket diagnosis,’ recognizing that there are other causes of a patient’s symptoms,” Mark Pimentel, MD, associate professor of medicine and gastroenterology, Cedars-Sinai, Los Angeles, said in an interview. “What’s made IBS so difficult to diagnose has been the absence of biological markers and hallmark findings on endoscopy.”
Recent research points to novel bacterial causes as culprits in the development of IBS. In particular, altered small bowel microbiota can be triggered by acute gastroenteritis.
Food poisoning can trigger the onset of IBS — a phenomenon called “postinfectious IBS (PI-IBS),” said Pimentel, who is also executive director of the Medically Associated Science and Technology Program at Cedars-Sinai. PI-IBS almost always takes the form of IBS-D, with up to 60% of patients with IBS-D suffering the long-term sequelae of food poisoning.
The types of bacteria most commonly associated with gastroenteritis are Shigella, Campylobacter, Salmonella, and Escherichia coli, Pimentel said. All of them release cytolethal distending toxin B (CdtB), causing the body to produce antibodies to the toxin.
CdtB resembles vinculin, a naturally occurring protein critical for healthy gut function. “Because of this molecular resemblance, the immune system often mistakes one for the other, producing anti-vinculin,” Pimentel explained.
This autoimmune response leads to disruptions in the gut microbiome, ultimately resulting in PI-IBS. The chain of events “doesn’t necessarily happen immediately,” Pimentel said. “You might have developed food poisoning at a party weeks or months ago.”
Acute gastroenteritis is common, affecting as many as 179 million people in the United States annually. A meta-analysis of 47 studies, incorporating 28,270 patients, found that those who had experienced acute gastroenteritis had a fourfold higher risk of developing IBS compared with nonexposed controls.
“The problem isn’t only the IBS itself, but the fact that people with PI-IBS are four times as likely to contract food poisoning again, which can further exacerbate IBS symptoms,” Pimentel said.
Diarrhea-predominant IBS can be detected through the presence of two blood biomarkers — anti-CdtB and anti-vinculin — in a blood test developed by Pimentel and his group.
“Elevation in either of these biomarkers establishes the diagnosis,” Pimentel said. “This is a breakthrough because it represents the first test that can make IBS a ‘diagnosis of inclusion.’”
The blood test also can identify IBS-M but not IBS-C.
Pimentel said that IBS-C is associated with increased levels of methanogenic archaea, which can be diagnosed by a positive methane breath test. “Methane gas slows intestinal contractility, which might result in constipation,” he said.
Diet as a Treatment Option
Diet is usually the starting point for IBS treatment, Chey said. “The standard dietary recommendations, as defined by the National Institute for Health and Care Excellence Guidance for managing IBS, are reasonable and common sense — eating three meals a day, avoiding carbonated beverages, excess alcohol, and excess caffeine, and avoiding hard-to-digest foods that can be gas producing.”
A diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs), which are carbohydrates that aren’t completely absorbed in the intestines, has been shown to be effective in alleviating GI distress in as many as 86% of patients with IBS, leading to improvements in overall GI symptoms as well as individual symptoms (eg, abdominal pain, bloating, constipation, diarrhea, and flatulence).
Desmond recommends the low FODMAP program delineated by Monash University in Australia. The diet should be undertaken only under the supervision of a dietitian, he warned. Moreover, following it on a long-term basis can have an adverse impact on dietary quality and the gut microbiome. Therefore, “it’s important to embark on stepwise reintroduction of FODMAPS under supervision to find acceptable thresholds that don’t cause a return of symptoms.”
A growing body of research suggests that following the Mediterranean diet can be helpful in reducing IBS symptoms. Chey said that some patients who tend to over-restrict their eating might benefit from a less restrictive diet than the typical low FODMAPs diet. For them, the Mediterranean diet may be a good option.
Pharmacotherapy for IBS
Nutritional approaches aren’t for everyone, Chey noted. “Some people don’t want to be on a highly restricted diet.” For them, medications addressing symptoms might be a better option.
Antispasmodics — either anticholinergics (hyoscine and dicyclomine) or smooth muscle relaxants (alverine, mebeverine, and peppermint oil) — can be helpful, although they can worsen constipation in a dose-dependent manner. It is advisable to use them on an as-needed rather than long-term basis.
Antidiarrheal agents include loperamide and diphenoxylate.
For constipation, laxatives (eg, senna, bisacodyl, polyethylene glycol, and sodium picosulfate) can be helpful.
Desmond noted that the American Gastroenterological Association does not recommend routine use of probiotics for most GI disorders, including IBS. Exceptions include prevention of Clostridioides difficile, ulcerative colitis, and pouchitis.
Targeting the Gut-Brain Relationship
Stress plays a role in exacerbating symptoms in patients with IBS and is an important target for intervention.
“If patients are living with a level of stress that’s impairing, we won’t be able to solve their gut issues until we resolve their stress issues,” Desmond said. “We need to calm the gut-microbiome-brain axis, which is multidimensional and bidirectional.”
Many people — even those without IBS — experience queasiness or diarrhea prior to a major event they’re nervous about, Chey noted. These events activate the brain, which activates the nervous system, which interacts with the GI tract. Indeed, IBS is now recognized as a disorder of gut-brain interaction, he said.
“We now know that the microbiome in the GI tract influences cognition and emotional function, depression, and anxiety. One might say that the gut is the ‘center of the universe’ to human beings,” Chey said.
Evidence-based psychological approaches for stress reduction in patients with IBS include cognitive behavioral therapy, specifically tailored to helping the patient identify associations between IBS symptoms and thoughts, emotions, and actions, as well as learning new behaviors and engaging in stress management. Psychodynamic (interpersonal) therapy enables patients to understand the connection between GI symptoms and interpersonal conflicts, emotional factors, or relationship difficulties.
Gut-directed hypnotherapy (GDH) is a “proven modality for IBS,” Desmond said. Unlike other forms of hypnotherapy, GDH focuses specifically on controlling and normalizing GI function. Studies have shown a reduction of ≥ 30% in abdominal pain in two thirds of participants, with overall response rates up to 85%. It can be delivered in an individual or group setting or via a smartphone.
Desmond recommends mindfulness-based therapy (MBT) for IBS. MBT focuses on the “cultivation of mindfulness, defined as intentional, nonjudgmental, present-focused awareness.” It has been found effective in reducing flares and the markers of gut inflammation in ulcerative colitis, as well as reducing symptoms of IBS.
Chey noted that an emerging body of literature supports the potential role of acupuncture in treating IBS, and his clinic employs it. “I would like to see further research into other areas of CAM [complementary and alternative medicine], including herbal approaches to IBS symptoms as well as stress.”
Finally, all the experts agree that more research is needed.
“The real tragedy is that the NIH invests next to nothing in IBS, in contrast to inflammatory bowel disease and many other conditions,” Pimentel said. “Yet IBS is 45 times more common than inflammatory bowel disease.”
Pimentel hopes that with enough advocacy and recognition that IBS isn’t “just stress-related,” more resources will be devoted to understanding this debilitating condition.
Desmond is the author of a book on the benefits of a plant-based diet. He has also received honoraria, speaking, and consultancy fees from the European Space Agency, Dyson Institute of Engineering and Technology, Riverford Organic Farmers, Ltd., Salesforce Inc., Sentara Healthcare, Saudi Sports for All Federation, the Physicians Committee for Responsible Medicine, The Plantrician Project, Doctors for Nutrition, and The Happy Pear.
Pimentel is a consultant for Bausch Health, Ferring Pharmaceuticals, and Ardelyx. He holds equity in and is also a consultant for Dieta Health, Salvo Health, Cylinder Health, and Gemelli Biotech. Cedars-Sinai has a licensing agreement with Gemelli Biotech and Hobbs Medical.
Chey is a consultant to AbbVie, Ardelyx, Atmo, Biomerica, Gemelli Biotech, Ironwood Pharmaceuticals, Nestlé, QOL Medical, Phathom Pharmaceuticals, Redhill, Salix/Valeant, Takeda, and Vibrant. He receives grant/research funding from Commonwealth Diagnostics International, Inc., US Food and Drug Administration, National Institutes of Health, QOL Medical, and Salix/Valeant. He holds stock options in Coprata, Dieta Health, Evinature, FoodMarble, Kiwi Biosciences, and ModifyHealth. He is a board or advisory panel member of the American College of Gastroenterology, GI Health Foundation, International Foundation for Gastrointestinal Disorders, Rome. He holds patents on My Nutrition Health, Digital Manometry, and Rectal Expulsion Device.
A version of this article appeared on Medscape.com.
Weight Loss Surgery, Obesity Drugs Achieve Similar Results but Have Different Safety Profiles
PHILADELPHIA — according to a meta-analysis comparing the efficacy and safety of the different treatment options.
However, tirzepatide, a long-acting glucose-dependent insulinotropic polypeptide (GIP) receptor agonist and glucagon-like peptide 1 receptor agonist (GLP-1 RA), produces comparable weight loss and has a favorable safety profile, reported principal investigator Jena Velji-Ibrahim, MD, MSc, from Prisma Health–Upstate/University of South Carolina School of Medicine in Greenville.
In addition, there was “no significant difference in percentage total body weight loss between tirzepatide when comparing it to one-anastomosis gastric bypass (OAGB), as well as laparoscopic sleeve gastrectomy,” she said.
All 11 interventions studied exerted weight loss effects, and side-effect profiles were also deemed largely favorable, particularly for endoscopic interventions, she added.
“When we compare bariatric surgery to bariatric endoscopy, endoscopic sleeve gastroplasty and transpyloric shuttle offer a minimally invasive alternative with good weight loss outcomes and fewer adverse events,” she said.
Velji-Ibrahim presented the findings at the annual meeting of the American College of Gastroenterology (ACG).
Comparing Weight Loss Interventions
Many of the studies comparing weight loss interventions to date have been limited by relatively small sample sizes, observational designs, and inconsistent results. This prompted Velji-Ibrahim and her colleagues to conduct what they believe to be the first-of-its-kind meta-analysis on this topic.
They began by conducting a systematic search of the literature to identify randomized controlled trials (RCTs) that compared the efficacy of Food and Drug Administration–approved bariatric surgeries, bariatric endoscopies, and medications — against each other or with placebo — in adults with a body mass index of 25-45, with or without concurrent type 2 diabetes.
A network meta-analysis was then performed to assess the various interventions’ impact on percentage total weight loss and side-effect profiles. P-scores were calculated to rank the treatments and identify the preferred interventions. The duration of therapy was 52 weeks.
In total, 34 eligible RCTs with 15,660 patients were included. Overall, the RCTs analyzed 11 weight loss treatments, including bariatric surgeries (four studies), bariatric endoscopies (three studies), and medications (four studies).
Specifically, the bariatric surgeries included RYGB, laparoscopic sleeve gastrectomy, OAGB, and laparoscopic adjustable gastric banding; bariatric endoscopies included endoscopic sleeve gastroplasty, transpyloric shuttle, and intragastric balloon; and medications included tirzepatide, semaglutide, and liraglutide.
Although all interventions were associated with reductions in percentage total weight loss compared with placebo, RYGB led to the greatest reductions (19.29%) and was ranked as the first preferred treatment (97% probability). It was followed in the rankings by OAGB, tirzepatide 15 mg, laparoscopic sleeve gastrectomy, and semaglutide 2.4 mg.
Tirzepatide 15 mg had a slightly lower percentage total weight loss (15.18%) but a favorable safety profile. There was no significant difference in percentage total weight loss between tirzepatide 15 mg and OAGB (mean difference, 2.97%) or laparoscopic sleeve gastrectomy (mean difference, 0.43%).
There was also no significant difference in percentage total weight loss between semaglutide 2.4 mg, compared with endoscopic sleeve gastroplasty and transpyloric shuttle.
Endoscopic sleeve, transpyloric shuttle, and intragastric balloon all resulted in weight loss > 5%.
When compared with bariatric surgery, “endoscopic interventions had a better side-effect profile, with no increased odds of mortality and intensive care needs,” Velji-Ibrahim said.
When it came to the medications, “the most common side effects were gastrointestinal in nature, which included nausea, vomiting, diarrhea, and constipation,” she said.
Combining, Rather Than Comparing, Therapies
Following the presentation, session co-moderator Shivangi T. Kothari, MD, assistant professor of medicine and associate director of endoscopy at the University of Rochester Medical Center in New York, shared her thoughts of what the future of obesity management research might look like.
It’s not just going to be about percentage total weight loss, she said, but about how well the effect is sustained following the intervention.
And we might move “away from comparing one modality to another” and instead study combination therapies, “which would be ideal,” said Kothari.
This was the focus of another meta-analysis presented at ACG 2024, in which Nihal Ijaz I. Khan, MD, and colleagues compared the efficacy of endoscopic bariatric treatment alone vs its combined use with GLP-1 RAs.
The researchers identified three retrospective studies with 266 patients, of whom 143 underwent endoscopic bariatric treatment alone (either endoscopic sleeve gastroplasty or intragastric balloon) and 123 had it combined with GLP-1 RAs, specifically liraglutide.
They reported that superior absolute weight loss was achieved in the group of patients receiving GLP-1 RAs in combination with endoscopic bariatric treatment. The standardized mean difference in body weight loss at treatment follow-up was 0.61 (P <.01).
“Further studies are required to evaluate the safety and adverse events comparing these two treatment modalities and to discover differences between comparing the two endoscopic options to various GLP-1 receptor agonists,” Khan noted.
Neither study had specific funding. Velji-Ibrahim and Khan reported no relevant financial relationships. Kothari reported serving as a consultant for Boston Scientific and Olympus, as well as serving as an advisory committee/board member for Castle Biosciences.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a meta-analysis comparing the efficacy and safety of the different treatment options.
However, tirzepatide, a long-acting glucose-dependent insulinotropic polypeptide (GIP) receptor agonist and glucagon-like peptide 1 receptor agonist (GLP-1 RA), produces comparable weight loss and has a favorable safety profile, reported principal investigator Jena Velji-Ibrahim, MD, MSc, from Prisma Health–Upstate/University of South Carolina School of Medicine in Greenville.
In addition, there was “no significant difference in percentage total body weight loss between tirzepatide when comparing it to one-anastomosis gastric bypass (OAGB), as well as laparoscopic sleeve gastrectomy,” she said.
All 11 interventions studied exerted weight loss effects, and side-effect profiles were also deemed largely favorable, particularly for endoscopic interventions, she added.
“When we compare bariatric surgery to bariatric endoscopy, endoscopic sleeve gastroplasty and transpyloric shuttle offer a minimally invasive alternative with good weight loss outcomes and fewer adverse events,” she said.
Velji-Ibrahim presented the findings at the annual meeting of the American College of Gastroenterology (ACG).
Comparing Weight Loss Interventions
Many of the studies comparing weight loss interventions to date have been limited by relatively small sample sizes, observational designs, and inconsistent results. This prompted Velji-Ibrahim and her colleagues to conduct what they believe to be the first-of-its-kind meta-analysis on this topic.
They began by conducting a systematic search of the literature to identify randomized controlled trials (RCTs) that compared the efficacy of Food and Drug Administration–approved bariatric surgeries, bariatric endoscopies, and medications — against each other or with placebo — in adults with a body mass index of 25-45, with or without concurrent type 2 diabetes.
A network meta-analysis was then performed to assess the various interventions’ impact on percentage total weight loss and side-effect profiles. P-scores were calculated to rank the treatments and identify the preferred interventions. The duration of therapy was 52 weeks.
In total, 34 eligible RCTs with 15,660 patients were included. Overall, the RCTs analyzed 11 weight loss treatments, including bariatric surgeries (four studies), bariatric endoscopies (three studies), and medications (four studies).
Specifically, the bariatric surgeries included RYGB, laparoscopic sleeve gastrectomy, OAGB, and laparoscopic adjustable gastric banding; bariatric endoscopies included endoscopic sleeve gastroplasty, transpyloric shuttle, and intragastric balloon; and medications included tirzepatide, semaglutide, and liraglutide.
Although all interventions were associated with reductions in percentage total weight loss compared with placebo, RYGB led to the greatest reductions (19.29%) and was ranked as the first preferred treatment (97% probability). It was followed in the rankings by OAGB, tirzepatide 15 mg, laparoscopic sleeve gastrectomy, and semaglutide 2.4 mg.
Tirzepatide 15 mg had a slightly lower percentage total weight loss (15.18%) but a favorable safety profile. There was no significant difference in percentage total weight loss between tirzepatide 15 mg and OAGB (mean difference, 2.97%) or laparoscopic sleeve gastrectomy (mean difference, 0.43%).
There was also no significant difference in percentage total weight loss between semaglutide 2.4 mg, compared with endoscopic sleeve gastroplasty and transpyloric shuttle.
Endoscopic sleeve, transpyloric shuttle, and intragastric balloon all resulted in weight loss > 5%.
When compared with bariatric surgery, “endoscopic interventions had a better side-effect profile, with no increased odds of mortality and intensive care needs,” Velji-Ibrahim said.
When it came to the medications, “the most common side effects were gastrointestinal in nature, which included nausea, vomiting, diarrhea, and constipation,” she said.
Combining, Rather Than Comparing, Therapies
Following the presentation, session co-moderator Shivangi T. Kothari, MD, assistant professor of medicine and associate director of endoscopy at the University of Rochester Medical Center in New York, shared her thoughts of what the future of obesity management research might look like.
It’s not just going to be about percentage total weight loss, she said, but about how well the effect is sustained following the intervention.
And we might move “away from comparing one modality to another” and instead study combination therapies, “which would be ideal,” said Kothari.
This was the focus of another meta-analysis presented at ACG 2024, in which Nihal Ijaz I. Khan, MD, and colleagues compared the efficacy of endoscopic bariatric treatment alone vs its combined use with GLP-1 RAs.
The researchers identified three retrospective studies with 266 patients, of whom 143 underwent endoscopic bariatric treatment alone (either endoscopic sleeve gastroplasty or intragastric balloon) and 123 had it combined with GLP-1 RAs, specifically liraglutide.
They reported that superior absolute weight loss was achieved in the group of patients receiving GLP-1 RAs in combination with endoscopic bariatric treatment. The standardized mean difference in body weight loss at treatment follow-up was 0.61 (P <.01).
“Further studies are required to evaluate the safety and adverse events comparing these two treatment modalities and to discover differences between comparing the two endoscopic options to various GLP-1 receptor agonists,” Khan noted.
Neither study had specific funding. Velji-Ibrahim and Khan reported no relevant financial relationships. Kothari reported serving as a consultant for Boston Scientific and Olympus, as well as serving as an advisory committee/board member for Castle Biosciences.
A version of this article first appeared on Medscape.com.
PHILADELPHIA — according to a meta-analysis comparing the efficacy and safety of the different treatment options.
However, tirzepatide, a long-acting glucose-dependent insulinotropic polypeptide (GIP) receptor agonist and glucagon-like peptide 1 receptor agonist (GLP-1 RA), produces comparable weight loss and has a favorable safety profile, reported principal investigator Jena Velji-Ibrahim, MD, MSc, from Prisma Health–Upstate/University of South Carolina School of Medicine in Greenville.
In addition, there was “no significant difference in percentage total body weight loss between tirzepatide when comparing it to one-anastomosis gastric bypass (OAGB), as well as laparoscopic sleeve gastrectomy,” she said.
All 11 interventions studied exerted weight loss effects, and side-effect profiles were also deemed largely favorable, particularly for endoscopic interventions, she added.
“When we compare bariatric surgery to bariatric endoscopy, endoscopic sleeve gastroplasty and transpyloric shuttle offer a minimally invasive alternative with good weight loss outcomes and fewer adverse events,” she said.
Velji-Ibrahim presented the findings at the annual meeting of the American College of Gastroenterology (ACG).
Comparing Weight Loss Interventions
Many of the studies comparing weight loss interventions to date have been limited by relatively small sample sizes, observational designs, and inconsistent results. This prompted Velji-Ibrahim and her colleagues to conduct what they believe to be the first-of-its-kind meta-analysis on this topic.
They began by conducting a systematic search of the literature to identify randomized controlled trials (RCTs) that compared the efficacy of Food and Drug Administration–approved bariatric surgeries, bariatric endoscopies, and medications — against each other or with placebo — in adults with a body mass index of 25-45, with or without concurrent type 2 diabetes.
A network meta-analysis was then performed to assess the various interventions’ impact on percentage total weight loss and side-effect profiles. P-scores were calculated to rank the treatments and identify the preferred interventions. The duration of therapy was 52 weeks.
In total, 34 eligible RCTs with 15,660 patients were included. Overall, the RCTs analyzed 11 weight loss treatments, including bariatric surgeries (four studies), bariatric endoscopies (three studies), and medications (four studies).
Specifically, the bariatric surgeries included RYGB, laparoscopic sleeve gastrectomy, OAGB, and laparoscopic adjustable gastric banding; bariatric endoscopies included endoscopic sleeve gastroplasty, transpyloric shuttle, and intragastric balloon; and medications included tirzepatide, semaglutide, and liraglutide.
Although all interventions were associated with reductions in percentage total weight loss compared with placebo, RYGB led to the greatest reductions (19.29%) and was ranked as the first preferred treatment (97% probability). It was followed in the rankings by OAGB, tirzepatide 15 mg, laparoscopic sleeve gastrectomy, and semaglutide 2.4 mg.
Tirzepatide 15 mg had a slightly lower percentage total weight loss (15.18%) but a favorable safety profile. There was no significant difference in percentage total weight loss between tirzepatide 15 mg and OAGB (mean difference, 2.97%) or laparoscopic sleeve gastrectomy (mean difference, 0.43%).
There was also no significant difference in percentage total weight loss between semaglutide 2.4 mg, compared with endoscopic sleeve gastroplasty and transpyloric shuttle.
Endoscopic sleeve, transpyloric shuttle, and intragastric balloon all resulted in weight loss > 5%.
When compared with bariatric surgery, “endoscopic interventions had a better side-effect profile, with no increased odds of mortality and intensive care needs,” Velji-Ibrahim said.
When it came to the medications, “the most common side effects were gastrointestinal in nature, which included nausea, vomiting, diarrhea, and constipation,” she said.
Combining, Rather Than Comparing, Therapies
Following the presentation, session co-moderator Shivangi T. Kothari, MD, assistant professor of medicine and associate director of endoscopy at the University of Rochester Medical Center in New York, shared her thoughts of what the future of obesity management research might look like.
It’s not just going to be about percentage total weight loss, she said, but about how well the effect is sustained following the intervention.
And we might move “away from comparing one modality to another” and instead study combination therapies, “which would be ideal,” said Kothari.
This was the focus of another meta-analysis presented at ACG 2024, in which Nihal Ijaz I. Khan, MD, and colleagues compared the efficacy of endoscopic bariatric treatment alone vs its combined use with GLP-1 RAs.
The researchers identified three retrospective studies with 266 patients, of whom 143 underwent endoscopic bariatric treatment alone (either endoscopic sleeve gastroplasty or intragastric balloon) and 123 had it combined with GLP-1 RAs, specifically liraglutide.
They reported that superior absolute weight loss was achieved in the group of patients receiving GLP-1 RAs in combination with endoscopic bariatric treatment. The standardized mean difference in body weight loss at treatment follow-up was 0.61 (P <.01).
“Further studies are required to evaluate the safety and adverse events comparing these two treatment modalities and to discover differences between comparing the two endoscopic options to various GLP-1 receptor agonists,” Khan noted.
Neither study had specific funding. Velji-Ibrahim and Khan reported no relevant financial relationships. Kothari reported serving as a consultant for Boston Scientific and Olympus, as well as serving as an advisory committee/board member for Castle Biosciences.
A version of this article first appeared on Medscape.com.
FROM ACG 2024
Low-Volume Bowel Prep Easier, as Effective as Standard Prep in Hospitalized Patients
PHILADELPHIA — according to a study presented at the annual meeting of the American College of Gastroenterology (ACG).
Patients who received MoviPrep (2L of polyethylene glycol and ascorbic acid) reported higher tolerability and willingness to repeat colonoscopy preparation in the future than those taking GoLYTELY (4L of polyethylene glycol and electrolytes). In addition, the rates of electrolyte abnormalities and acute kidney injury were low and similar between the two groups.
“Bowel preparation remains a challenge in the inpatient setting, where 20%-50% of all colonoscopies can have inadequate bowel preparation,” said lead author Karen Xiao, MD, assistant professor in the section of digestive diseases at Yale School of Medicine, New Haven, Connecticut.
Previous studies have indicated that low-volume (2L), split-dose preparations are noninferior to high-volume (4L), split-dose regimens, and patients generally prefer low-volume options, she said. However, the current standard of care for inpatients continues to include high-volume polyethylene glycol electrocyte lavage, which may be less tolerable.
“Similar to prior studies, our study supports that MoviPrep may be a suitable alternative to traditional high-volume bowel preparation in hospitalized patients undergoing colonoscopy,” she said.
In a single-blind, multi-site, randomized controlled trial, Xiao and colleagues in the Yale–New Haven Health System randomly assigned inpatients undergoing colonoscopy to MoviPrep or GoLYTELY between January 2022 and July 2024. They excluded patients with prior small or large bowel resection, foreign body removal, or medical contraindications, such as obstruction, pregnancy, phenylketonuria, or glucose-6-phosphate dehydrogenase deficiency.
After bowel prep but before colonoscopy, patients took the Mayo Clinic Bowel Preparation survey. Colonoscopies were then recorded, and videos were scored by a single-blinded central reviewer. The primary outcome included the adequacy of bowel prep as defined by a Boston Bowel Preparation Scale score of 6 or higher, with each segment scoring 2 or higher.
In the final analysis, 202 patients received MoviPrep and 210 received GoLYTELY. In both groups, the average age was 62; about 60% were men; and 66% were White, about 22% Black, and 13%-15% Hispanic. About 65% of patients in both arms had an American Society of Anesthesiologists (ASA) score of 3, with another 20% in each group having an ASA score of 4, “reflective of a sicker inpatient population,” Xiao said.
Inpatient colonoscopy was indicated for gastrointestinal bleeding (55%), diarrhea (15%-20%), abnormal imaging (10%-13%), inflammatory bowel disease (4%), or other (35%-41%). Patients could have more than one indication for colonoscopy.
Overall, bowel preparation was scored as adequate in 111 patients with MoviPrep (55%) and 111 patients with GoLYTELY (52.9%), and was inadequate in 91 patients with MoviPrep (45%) and 99 patients with GoLYTELY (47.1%). With a rate difference of 2.1% and a P value of .007, MoviPrep was considered noninferior to GoLYTELY for adequate bowel preparation.
In terms of secondary outcomes, there wasn’t a significant difference in the length of hospital stay, with a median stay of 6 days. Similarly, there were no differences in the rates of adverse events, including acute kidney injury and electrolyte abnormalities, with rates ranging from 1% to 9%. MoviPrep patients were slightly more likely to need additional bowel prep but also had a slightly shorter time to colonoscopy.
Ease of Use Is a Plus
On the basis of the Mayo Clinic Bowel Preparation survey, there wasn’t a difference between the groups in how much bowel prep solution was left in the bottle. However, more than twice as many patients who took MoviPrep said the prep was “easy,” and more MoviPrep patients called it “acceptable,” whereas more GoLYTELY patients said prep was “somewhat difficult” or “very difficult.”
In addition, significantly more MoviPrep patients (49.7% vs 33.7%) said they were “mostly willing” to drink the same prep again if they needed another colonoscopy in the future, while more GoLYTELY patients said they were “somewhat willing” (44.7% vs 34.6%) or “not willing at all” (21.6% vs 15.7%).
“Bowel prep, particularly in hospitals, is important because we do it so often. When you think about what our patients in the hospital are going through, they’re very sick and often have multiple comorbidities, so how can we give them a bowel prep that is safe for them, easiest for them, easy for our nursing staff who are experiencing shortages, and as good as the traditional bowel prep,” said the session’s moderator, Amy Oxentenko, MD, AGAF, professor of medicine and gastroenterologist at Mayo Clinic, Rochester, Minnesota.
“Here we’ve seen great data that we can provide half the volume of the prep, still get amazing results in terms of adequacy of preparation, and the patients had a better experience,” said Oxentenko, the incoming ACG president. “That’s important because they likely may need another colonoscopy in the future, and we would hate for the bowel prep in the hospital to potentially dissuade them from a future colonoscopy.”
Future studies could stratify patients on the basis of colonoscopy indication or patient history, including conditions such as chronic constipation or neurogenic bowel, where some patients may still need a high-volume prep, Oxentenko said.
“Also, in the hospital setting, we don’t always know when a patient is going to the endoscopy suite due to other patient cases that may get prolonged or pushed in,” she said. “So how do you time the second dose of the split dose in anticipation of when that patient will go to the endoscopy suite to maintain that great preparation with a smaller volume prep?”
The study was awarded the ACG Governors Award for Excellence in Clinical Research (Trainee). Xiao and Oxentenko reported no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the annual meeting of the American College of Gastroenterology (ACG).
Patients who received MoviPrep (2L of polyethylene glycol and ascorbic acid) reported higher tolerability and willingness to repeat colonoscopy preparation in the future than those taking GoLYTELY (4L of polyethylene glycol and electrolytes). In addition, the rates of electrolyte abnormalities and acute kidney injury were low and similar between the two groups.
“Bowel preparation remains a challenge in the inpatient setting, where 20%-50% of all colonoscopies can have inadequate bowel preparation,” said lead author Karen Xiao, MD, assistant professor in the section of digestive diseases at Yale School of Medicine, New Haven, Connecticut.
Previous studies have indicated that low-volume (2L), split-dose preparations are noninferior to high-volume (4L), split-dose regimens, and patients generally prefer low-volume options, she said. However, the current standard of care for inpatients continues to include high-volume polyethylene glycol electrocyte lavage, which may be less tolerable.
“Similar to prior studies, our study supports that MoviPrep may be a suitable alternative to traditional high-volume bowel preparation in hospitalized patients undergoing colonoscopy,” she said.
In a single-blind, multi-site, randomized controlled trial, Xiao and colleagues in the Yale–New Haven Health System randomly assigned inpatients undergoing colonoscopy to MoviPrep or GoLYTELY between January 2022 and July 2024. They excluded patients with prior small or large bowel resection, foreign body removal, or medical contraindications, such as obstruction, pregnancy, phenylketonuria, or glucose-6-phosphate dehydrogenase deficiency.
After bowel prep but before colonoscopy, patients took the Mayo Clinic Bowel Preparation survey. Colonoscopies were then recorded, and videos were scored by a single-blinded central reviewer. The primary outcome included the adequacy of bowel prep as defined by a Boston Bowel Preparation Scale score of 6 or higher, with each segment scoring 2 or higher.
In the final analysis, 202 patients received MoviPrep and 210 received GoLYTELY. In both groups, the average age was 62; about 60% were men; and 66% were White, about 22% Black, and 13%-15% Hispanic. About 65% of patients in both arms had an American Society of Anesthesiologists (ASA) score of 3, with another 20% in each group having an ASA score of 4, “reflective of a sicker inpatient population,” Xiao said.
Inpatient colonoscopy was indicated for gastrointestinal bleeding (55%), diarrhea (15%-20%), abnormal imaging (10%-13%), inflammatory bowel disease (4%), or other (35%-41%). Patients could have more than one indication for colonoscopy.
Overall, bowel preparation was scored as adequate in 111 patients with MoviPrep (55%) and 111 patients with GoLYTELY (52.9%), and was inadequate in 91 patients with MoviPrep (45%) and 99 patients with GoLYTELY (47.1%). With a rate difference of 2.1% and a P value of .007, MoviPrep was considered noninferior to GoLYTELY for adequate bowel preparation.
In terms of secondary outcomes, there wasn’t a significant difference in the length of hospital stay, with a median stay of 6 days. Similarly, there were no differences in the rates of adverse events, including acute kidney injury and electrolyte abnormalities, with rates ranging from 1% to 9%. MoviPrep patients were slightly more likely to need additional bowel prep but also had a slightly shorter time to colonoscopy.
Ease of Use Is a Plus
On the basis of the Mayo Clinic Bowel Preparation survey, there wasn’t a difference between the groups in how much bowel prep solution was left in the bottle. However, more than twice as many patients who took MoviPrep said the prep was “easy,” and more MoviPrep patients called it “acceptable,” whereas more GoLYTELY patients said prep was “somewhat difficult” or “very difficult.”
In addition, significantly more MoviPrep patients (49.7% vs 33.7%) said they were “mostly willing” to drink the same prep again if they needed another colonoscopy in the future, while more GoLYTELY patients said they were “somewhat willing” (44.7% vs 34.6%) or “not willing at all” (21.6% vs 15.7%).
“Bowel prep, particularly in hospitals, is important because we do it so often. When you think about what our patients in the hospital are going through, they’re very sick and often have multiple comorbidities, so how can we give them a bowel prep that is safe for them, easiest for them, easy for our nursing staff who are experiencing shortages, and as good as the traditional bowel prep,” said the session’s moderator, Amy Oxentenko, MD, AGAF, professor of medicine and gastroenterologist at Mayo Clinic, Rochester, Minnesota.
“Here we’ve seen great data that we can provide half the volume of the prep, still get amazing results in terms of adequacy of preparation, and the patients had a better experience,” said Oxentenko, the incoming ACG president. “That’s important because they likely may need another colonoscopy in the future, and we would hate for the bowel prep in the hospital to potentially dissuade them from a future colonoscopy.”
Future studies could stratify patients on the basis of colonoscopy indication or patient history, including conditions such as chronic constipation or neurogenic bowel, where some patients may still need a high-volume prep, Oxentenko said.
“Also, in the hospital setting, we don’t always know when a patient is going to the endoscopy suite due to other patient cases that may get prolonged or pushed in,” she said. “So how do you time the second dose of the split dose in anticipation of when that patient will go to the endoscopy suite to maintain that great preparation with a smaller volume prep?”
The study was awarded the ACG Governors Award for Excellence in Clinical Research (Trainee). Xiao and Oxentenko reported no relevant disclosures.
A version of this article appeared on Medscape.com.
PHILADELPHIA — according to a study presented at the annual meeting of the American College of Gastroenterology (ACG).
Patients who received MoviPrep (2L of polyethylene glycol and ascorbic acid) reported higher tolerability and willingness to repeat colonoscopy preparation in the future than those taking GoLYTELY (4L of polyethylene glycol and electrolytes). In addition, the rates of electrolyte abnormalities and acute kidney injury were low and similar between the two groups.
“Bowel preparation remains a challenge in the inpatient setting, where 20%-50% of all colonoscopies can have inadequate bowel preparation,” said lead author Karen Xiao, MD, assistant professor in the section of digestive diseases at Yale School of Medicine, New Haven, Connecticut.
Previous studies have indicated that low-volume (2L), split-dose preparations are noninferior to high-volume (4L), split-dose regimens, and patients generally prefer low-volume options, she said. However, the current standard of care for inpatients continues to include high-volume polyethylene glycol electrocyte lavage, which may be less tolerable.
“Similar to prior studies, our study supports that MoviPrep may be a suitable alternative to traditional high-volume bowel preparation in hospitalized patients undergoing colonoscopy,” she said.
In a single-blind, multi-site, randomized controlled trial, Xiao and colleagues in the Yale–New Haven Health System randomly assigned inpatients undergoing colonoscopy to MoviPrep or GoLYTELY between January 2022 and July 2024. They excluded patients with prior small or large bowel resection, foreign body removal, or medical contraindications, such as obstruction, pregnancy, phenylketonuria, or glucose-6-phosphate dehydrogenase deficiency.
After bowel prep but before colonoscopy, patients took the Mayo Clinic Bowel Preparation survey. Colonoscopies were then recorded, and videos were scored by a single-blinded central reviewer. The primary outcome included the adequacy of bowel prep as defined by a Boston Bowel Preparation Scale score of 6 or higher, with each segment scoring 2 or higher.
In the final analysis, 202 patients received MoviPrep and 210 received GoLYTELY. In both groups, the average age was 62; about 60% were men; and 66% were White, about 22% Black, and 13%-15% Hispanic. About 65% of patients in both arms had an American Society of Anesthesiologists (ASA) score of 3, with another 20% in each group having an ASA score of 4, “reflective of a sicker inpatient population,” Xiao said.
Inpatient colonoscopy was indicated for gastrointestinal bleeding (55%), diarrhea (15%-20%), abnormal imaging (10%-13%), inflammatory bowel disease (4%), or other (35%-41%). Patients could have more than one indication for colonoscopy.
Overall, bowel preparation was scored as adequate in 111 patients with MoviPrep (55%) and 111 patients with GoLYTELY (52.9%), and was inadequate in 91 patients with MoviPrep (45%) and 99 patients with GoLYTELY (47.1%). With a rate difference of 2.1% and a P value of .007, MoviPrep was considered noninferior to GoLYTELY for adequate bowel preparation.
In terms of secondary outcomes, there wasn’t a significant difference in the length of hospital stay, with a median stay of 6 days. Similarly, there were no differences in the rates of adverse events, including acute kidney injury and electrolyte abnormalities, with rates ranging from 1% to 9%. MoviPrep patients were slightly more likely to need additional bowel prep but also had a slightly shorter time to colonoscopy.
Ease of Use Is a Plus
On the basis of the Mayo Clinic Bowel Preparation survey, there wasn’t a difference between the groups in how much bowel prep solution was left in the bottle. However, more than twice as many patients who took MoviPrep said the prep was “easy,” and more MoviPrep patients called it “acceptable,” whereas more GoLYTELY patients said prep was “somewhat difficult” or “very difficult.”
In addition, significantly more MoviPrep patients (49.7% vs 33.7%) said they were “mostly willing” to drink the same prep again if they needed another colonoscopy in the future, while more GoLYTELY patients said they were “somewhat willing” (44.7% vs 34.6%) or “not willing at all” (21.6% vs 15.7%).
“Bowel prep, particularly in hospitals, is important because we do it so often. When you think about what our patients in the hospital are going through, they’re very sick and often have multiple comorbidities, so how can we give them a bowel prep that is safe for them, easiest for them, easy for our nursing staff who are experiencing shortages, and as good as the traditional bowel prep,” said the session’s moderator, Amy Oxentenko, MD, AGAF, professor of medicine and gastroenterologist at Mayo Clinic, Rochester, Minnesota.
“Here we’ve seen great data that we can provide half the volume of the prep, still get amazing results in terms of adequacy of preparation, and the patients had a better experience,” said Oxentenko, the incoming ACG president. “That’s important because they likely may need another colonoscopy in the future, and we would hate for the bowel prep in the hospital to potentially dissuade them from a future colonoscopy.”
Future studies could stratify patients on the basis of colonoscopy indication or patient history, including conditions such as chronic constipation or neurogenic bowel, where some patients may still need a high-volume prep, Oxentenko said.
“Also, in the hospital setting, we don’t always know when a patient is going to the endoscopy suite due to other patient cases that may get prolonged or pushed in,” she said. “So how do you time the second dose of the split dose in anticipation of when that patient will go to the endoscopy suite to maintain that great preparation with a smaller volume prep?”
The study was awarded the ACG Governors Award for Excellence in Clinical Research (Trainee). Xiao and Oxentenko reported no relevant disclosures.
A version of this article appeared on Medscape.com.
FROM ACG 2024
Breath Gas Patterns Predict Response to Low FODMAP Diet
PHILADELPHIA — , according to a new study.
The low FODMAP diet is the most evidence-based dietary therapy for patients with IBS, but we know that “only about 50% of our patients respond to it,” said principal investigator Prashant Singh, MD, assistant professor at the University of Michigan in Ann Arbor, Michigan. “Exhaled breath gases represent bacterial fermentation of dietary carbohydrates. These measurements could provide a simple biomarker for response to low FODMAP diets.”
Even before starting the low FODMAP diet, “you could see notable differences in breath test patterns between responders and nonresponders,” he said. “We saw that low FODMAP responders had higher hydrogen (H2) and lower methane (CH4) at baseline than nonresponders and had a greater drop in hydrogen following FODMAP restriction vs nonresponders.”
He added that these results imply that responders to this diet may exhibit differences in baseline microbiota composition regarding saccharolytic capacity and/or methanogens.
Singh presented the findings at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
Breaths That Can Predict Response
To determine if pre-intervention non-fasting breath patterns are associated with a clinical response to low FODMAP diets, Singh and colleagues enrolled 284 self-selected participants (mean age, 45.2 years) with mild to moderate gastrointestinal (GI) symptoms. Participants used an app-connected breath analyzer to record hourly, non-fasting H2 and CH4 levels during waking hours, in addition to logging meal content and symptom severity (bloating, abdominal pain, and flatulence) on a 0-10 scale.
Patients were directed to consume their habitual diet for 1 week, before following an app-directed low FODMAP diet for 1 week. Responders were defined as those with a ≥ 30% reduction in at least one mean symptom score. The researchers then compared average hourly H2 and CH4 levels and symptom scores at baseline between low FODMAP diet responders and nonresponders.
Of the participants, 111 were classified as responders and 173 as nonresponders. There were no significant differences between the groups in gender, age, body mass index, or FODMAP per calorie.
Following FODMAP restriction, responders had consistently lower abdominal pain throughout the day and lower bloating and flatulence predominantly in the latter part of the day. Nonresponders experienced no significant changes in key abdominal symptoms after adopting the low FODMAP diet.
The researchers found that breath tests taken at baseline revealed predictive trends between the groups, even though average FODMAP consumption did not significantly differ between them. Baseline H2 levels were higher among responders than among nonresponders, especially in the morning and evening. However, responders had lower baseline CH4 levels throughout the day.
Following FODMAP restrictions, responders had a significant drop in non-fasting H2 but not CH4, whereas nonresponders did not have a significant drop in either.
The study was limited by the fact that participants were not clinically diagnosed with IBS, their GI symptoms were mild overall, and no data were available on stool consistency/frequency or fecal microbiome composition for correlation with exhaled breath gas levels.
A Potential New Biomarker
Session co-moderator Kyle Staller, MD, MPH, director of the Gastrointestinal Motility Laboratory at Mass General and associate professor of medicine at Harvard Medical School in Boston, Massachusetts, said in an interview that if validated, these findings provide hope for better directing low FODMAP diets to those patients who may benefit.
There are some patients who may or may not respond to a FODMAP diet, for reasons we don’t yet know, possibly related to fermentation of gas, and it’s helpful to know before starting treatment, he said. It may help us with more of “a precision medicine approach before we really torture people with diets that can be very difficult to adhere to.”
Staller, who was not involved in the study, added that, “People tend to really focus on small intestinal bacteria overgrowth when it comes to hydrogen and methane production, but in reality, this is really a very agile day-to-day, meal-to-meal responsiveness.
“It’s a different paradigm,” he continued. “I’d also like to see more data as to why we see the diurnal rhythm” and whether potential factors such as intestinal transit times are playing a role.
Singh reported receiving royalties from UpToDate. Staller reported receiving research support from Ardelyx and Restasis and serving as a consultant to Anji, Ardelyx, GI Supply, Mahana, Restasis, and Sanofi. Funding associated with the study was not available at the time of publication.
A version of this article appeared on Medscape.com.
PHILADELPHIA — , according to a new study.
The low FODMAP diet is the most evidence-based dietary therapy for patients with IBS, but we know that “only about 50% of our patients respond to it,” said principal investigator Prashant Singh, MD, assistant professor at the University of Michigan in Ann Arbor, Michigan. “Exhaled breath gases represent bacterial fermentation of dietary carbohydrates. These measurements could provide a simple biomarker for response to low FODMAP diets.”
Even before starting the low FODMAP diet, “you could see notable differences in breath test patterns between responders and nonresponders,” he said. “We saw that low FODMAP responders had higher hydrogen (H2) and lower methane (CH4) at baseline than nonresponders and had a greater drop in hydrogen following FODMAP restriction vs nonresponders.”
He added that these results imply that responders to this diet may exhibit differences in baseline microbiota composition regarding saccharolytic capacity and/or methanogens.
Singh presented the findings at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
Breaths That Can Predict Response
To determine if pre-intervention non-fasting breath patterns are associated with a clinical response to low FODMAP diets, Singh and colleagues enrolled 284 self-selected participants (mean age, 45.2 years) with mild to moderate gastrointestinal (GI) symptoms. Participants used an app-connected breath analyzer to record hourly, non-fasting H2 and CH4 levels during waking hours, in addition to logging meal content and symptom severity (bloating, abdominal pain, and flatulence) on a 0-10 scale.
Patients were directed to consume their habitual diet for 1 week, before following an app-directed low FODMAP diet for 1 week. Responders were defined as those with a ≥ 30% reduction in at least one mean symptom score. The researchers then compared average hourly H2 and CH4 levels and symptom scores at baseline between low FODMAP diet responders and nonresponders.
Of the participants, 111 were classified as responders and 173 as nonresponders. There were no significant differences between the groups in gender, age, body mass index, or FODMAP per calorie.
Following FODMAP restriction, responders had consistently lower abdominal pain throughout the day and lower bloating and flatulence predominantly in the latter part of the day. Nonresponders experienced no significant changes in key abdominal symptoms after adopting the low FODMAP diet.
The researchers found that breath tests taken at baseline revealed predictive trends between the groups, even though average FODMAP consumption did not significantly differ between them. Baseline H2 levels were higher among responders than among nonresponders, especially in the morning and evening. However, responders had lower baseline CH4 levels throughout the day.
Following FODMAP restrictions, responders had a significant drop in non-fasting H2 but not CH4, whereas nonresponders did not have a significant drop in either.
The study was limited by the fact that participants were not clinically diagnosed with IBS, their GI symptoms were mild overall, and no data were available on stool consistency/frequency or fecal microbiome composition for correlation with exhaled breath gas levels.
A Potential New Biomarker
Session co-moderator Kyle Staller, MD, MPH, director of the Gastrointestinal Motility Laboratory at Mass General and associate professor of medicine at Harvard Medical School in Boston, Massachusetts, said in an interview that if validated, these findings provide hope for better directing low FODMAP diets to those patients who may benefit.
There are some patients who may or may not respond to a FODMAP diet, for reasons we don’t yet know, possibly related to fermentation of gas, and it’s helpful to know before starting treatment, he said. It may help us with more of “a precision medicine approach before we really torture people with diets that can be very difficult to adhere to.”
Staller, who was not involved in the study, added that, “People tend to really focus on small intestinal bacteria overgrowth when it comes to hydrogen and methane production, but in reality, this is really a very agile day-to-day, meal-to-meal responsiveness.
“It’s a different paradigm,” he continued. “I’d also like to see more data as to why we see the diurnal rhythm” and whether potential factors such as intestinal transit times are playing a role.
Singh reported receiving royalties from UpToDate. Staller reported receiving research support from Ardelyx and Restasis and serving as a consultant to Anji, Ardelyx, GI Supply, Mahana, Restasis, and Sanofi. Funding associated with the study was not available at the time of publication.
A version of this article appeared on Medscape.com.
PHILADELPHIA — , according to a new study.
The low FODMAP diet is the most evidence-based dietary therapy for patients with IBS, but we know that “only about 50% of our patients respond to it,” said principal investigator Prashant Singh, MD, assistant professor at the University of Michigan in Ann Arbor, Michigan. “Exhaled breath gases represent bacterial fermentation of dietary carbohydrates. These measurements could provide a simple biomarker for response to low FODMAP diets.”
Even before starting the low FODMAP diet, “you could see notable differences in breath test patterns between responders and nonresponders,” he said. “We saw that low FODMAP responders had higher hydrogen (H2) and lower methane (CH4) at baseline than nonresponders and had a greater drop in hydrogen following FODMAP restriction vs nonresponders.”
He added that these results imply that responders to this diet may exhibit differences in baseline microbiota composition regarding saccharolytic capacity and/or methanogens.
Singh presented the findings at the American College of Gastroenterology (ACG) 2024 Annual Scientific Meeting.
Breaths That Can Predict Response
To determine if pre-intervention non-fasting breath patterns are associated with a clinical response to low FODMAP diets, Singh and colleagues enrolled 284 self-selected participants (mean age, 45.2 years) with mild to moderate gastrointestinal (GI) symptoms. Participants used an app-connected breath analyzer to record hourly, non-fasting H2 and CH4 levels during waking hours, in addition to logging meal content and symptom severity (bloating, abdominal pain, and flatulence) on a 0-10 scale.
Patients were directed to consume their habitual diet for 1 week, before following an app-directed low FODMAP diet for 1 week. Responders were defined as those with a ≥ 30% reduction in at least one mean symptom score. The researchers then compared average hourly H2 and CH4 levels and symptom scores at baseline between low FODMAP diet responders and nonresponders.
Of the participants, 111 were classified as responders and 173 as nonresponders. There were no significant differences between the groups in gender, age, body mass index, or FODMAP per calorie.
Following FODMAP restriction, responders had consistently lower abdominal pain throughout the day and lower bloating and flatulence predominantly in the latter part of the day. Nonresponders experienced no significant changes in key abdominal symptoms after adopting the low FODMAP diet.
The researchers found that breath tests taken at baseline revealed predictive trends between the groups, even though average FODMAP consumption did not significantly differ between them. Baseline H2 levels were higher among responders than among nonresponders, especially in the morning and evening. However, responders had lower baseline CH4 levels throughout the day.
Following FODMAP restrictions, responders had a significant drop in non-fasting H2 but not CH4, whereas nonresponders did not have a significant drop in either.
The study was limited by the fact that participants were not clinically diagnosed with IBS, their GI symptoms were mild overall, and no data were available on stool consistency/frequency or fecal microbiome composition for correlation with exhaled breath gas levels.
A Potential New Biomarker
Session co-moderator Kyle Staller, MD, MPH, director of the Gastrointestinal Motility Laboratory at Mass General and associate professor of medicine at Harvard Medical School in Boston, Massachusetts, said in an interview that if validated, these findings provide hope for better directing low FODMAP diets to those patients who may benefit.
There are some patients who may or may not respond to a FODMAP diet, for reasons we don’t yet know, possibly related to fermentation of gas, and it’s helpful to know before starting treatment, he said. It may help us with more of “a precision medicine approach before we really torture people with diets that can be very difficult to adhere to.”
Staller, who was not involved in the study, added that, “People tend to really focus on small intestinal bacteria overgrowth when it comes to hydrogen and methane production, but in reality, this is really a very agile day-to-day, meal-to-meal responsiveness.
“It’s a different paradigm,” he continued. “I’d also like to see more data as to why we see the diurnal rhythm” and whether potential factors such as intestinal transit times are playing a role.
Singh reported receiving royalties from UpToDate. Staller reported receiving research support from Ardelyx and Restasis and serving as a consultant to Anji, Ardelyx, GI Supply, Mahana, Restasis, and Sanofi. Funding associated with the study was not available at the time of publication.
A version of this article appeared on Medscape.com.
FROM ACG 2024
GLP-1 RAs Reduce Early-Onset CRC Risk in Patients With Type 2 Diabetes
PHILADELPHIA — according to the results of a retrospective study.
“This is the first large study to investigate the impact of GLP-1 RA use on EO-CRC risk,” principal investigator Temitope Olasehinde, MD, resident physician at the University Hospitals Cleveland Medical Center, Case Western Reserve University in Cleveland, Ohio, said in an interview.
The results indicate the GLP-1 RAs have a potentially protective role to play in combating EO-CRC, the incidence of which is notably rising in younger adults, with a corresponding increase in associated mortality.
Previous studies investigating the link between GLP-1 RAs and CRC did not capture patients aged younger than 50 years; thus, it was unknown if these results could be extrapolated to a younger age group, said Olasehinde.
The researcher presented the findings at the annual meeting of the American College of Gastroenterology.
Retrospective Database Analysis
Olasehinde and colleagues analyzed data from TriNetX, a large federated deidentified health research network, to identify patients (age ≤ 49 years) with diagnosed T2D subsequently prescribed antidiabetic medications who had not received a prior diagnosis of CRC. Additionally, patients were stratified on the basis of first-time GLP-1 RA use.
They identified 2,025,034 drug-naive patients with T2D; of these, 284,685 were subsequently prescribed GLP-1 RAs, and 1,740,349 remained in the non–GLP-1 RA cohort. Following propensity score matching, there were 86,186 patients in each cohort.
Patients who received GLP-1 RAs had significantly lower odds of developing EO-CRC than those who received non–GLP-1 RAs (0.6% vs 0.9%; P < .001; odds ratio [OR], 0.61; 95% CI, 0.54-068).
Furthermore, a sub-analysis revealed that patients who were obese and taking GLP-1 RAs had significantly lower odds of developing EO-CRC than patients who were obese but not taking GLP-1 RAs (0.7% vs 1.1%; P < .001; OR, 0.58; 95% CI, 0.50-067).
A Proposed Protective Effect
Although GLP-1 RAs are indicated for the treatment of T2D and obesity, recent evidence suggests that they may play a role in reducing the risk for CRC as well. This protective effect may be produced not only by addressing T2D and obesity — both important risk factors for CRC — but also via cellular mechanisms, Olasehinde noted.
“GLP-1 receptors are widely expressed throughout the gastrointestinal tract, with various effects on tissues in the stomach, small intestine, and colon,” she explained. Specifically, activation of these receptors in the proximal and distal colon promotes the release of “important factors that protect and facilitate healing of the intestinal epithelium” and “regulate the gut microbiome.”
This is particularly relevant in EO-CRC, she added, given its greater association with T2D and obesity, both factors that “have been shown to create dysbiosis in the gut microbiome and low-grade inflammation via release of free radicals/inflammatory cytokines.”
These results provide more evidence that EO-CRC “is clinically and molecularly distinct from late-onset colorectal cancer,” which is important for both clinicians and patients to understand, said Olasehinde.
“It is imperative that we are all aware of the specific signs and symptoms this population presents with and the implications of this diagnosis in younger age groups,” she added. “Patients should continue making informed dietary and lifestyle modifications/choices to help reduce the burden of EO-CRC.”
Hypothesis-Generating Results
Aasma Shaukat, MD, MPH, who was not affiliated with the research, called the results promising but — at this stage — primarily useful for stimulating future research.
"We do need more studies such as this to generate hypotheses that can be studied prospectively," Shaukat, professor of medicine and population health, and director of GI Outcomes Research at NYU Langone Health in New York City, told Medscape Medical News.
She referred to another study, published in JAMA Oncology, that also used the TriNetX research network, which showed that GLP-1 RAs were associated with reduced CRC risk in drug-naive patients with T2D.
Shaukat also noted that the current analysis has limitations that should be considered. "The study is retrospective, and confounding is a possibility,” she said.
“How the groups that did and did not receive GLP-1 RAs differ in other risk factors that could be the drivers of the cancers is not known. Whether cancers were detected through screening or symptoms, stage, and other features that may differ are not known. Finally, since we don’t know who did or did not have colonoscopy, undiagnosed cancers are not known," she explained.
Shaukat, who was the lead author of the ACG 2021 Colorectal Cancer Screening Guidelines, added that the field would benefit from studies providing "biological plausibility information, such as animal studies to understand how GLP-1 RAs may modulate risk of colon cancer; other population-based cohort studies on the incidence of colon cancer among GLP-1 RA users and non-users; and prospective trials on chemoprevention."
The study had no specific funding. Olasehinde reported no relevant financial relationships. Shaukat reported serving as a consultant for Freenome, Medtronic, and Motus GI, as well as an advisory board member for Iterative Scopes Inc.
A version of this article appeared on Medscape.com.
PHILADELPHIA — according to the results of a retrospective study.
“This is the first large study to investigate the impact of GLP-1 RA use on EO-CRC risk,” principal investigator Temitope Olasehinde, MD, resident physician at the University Hospitals Cleveland Medical Center, Case Western Reserve University in Cleveland, Ohio, said in an interview.
The results indicate the GLP-1 RAs have a potentially protective role to play in combating EO-CRC, the incidence of which is notably rising in younger adults, with a corresponding increase in associated mortality.
Previous studies investigating the link between GLP-1 RAs and CRC did not capture patients aged younger than 50 years; thus, it was unknown if these results could be extrapolated to a younger age group, said Olasehinde.
The researcher presented the findings at the annual meeting of the American College of Gastroenterology.
Retrospective Database Analysis
Olasehinde and colleagues analyzed data from TriNetX, a large federated deidentified health research network, to identify patients (age ≤ 49 years) with diagnosed T2D subsequently prescribed antidiabetic medications who had not received a prior diagnosis of CRC. Additionally, patients were stratified on the basis of first-time GLP-1 RA use.
They identified 2,025,034 drug-naive patients with T2D; of these, 284,685 were subsequently prescribed GLP-1 RAs, and 1,740,349 remained in the non–GLP-1 RA cohort. Following propensity score matching, there were 86,186 patients in each cohort.
Patients who received GLP-1 RAs had significantly lower odds of developing EO-CRC than those who received non–GLP-1 RAs (0.6% vs 0.9%; P < .001; odds ratio [OR], 0.61; 95% CI, 0.54-068).
Furthermore, a sub-analysis revealed that patients who were obese and taking GLP-1 RAs had significantly lower odds of developing EO-CRC than patients who were obese but not taking GLP-1 RAs (0.7% vs 1.1%; P < .001; OR, 0.58; 95% CI, 0.50-067).
A Proposed Protective Effect
Although GLP-1 RAs are indicated for the treatment of T2D and obesity, recent evidence suggests that they may play a role in reducing the risk for CRC as well. This protective effect may be produced not only by addressing T2D and obesity — both important risk factors for CRC — but also via cellular mechanisms, Olasehinde noted.
“GLP-1 receptors are widely expressed throughout the gastrointestinal tract, with various effects on tissues in the stomach, small intestine, and colon,” she explained. Specifically, activation of these receptors in the proximal and distal colon promotes the release of “important factors that protect and facilitate healing of the intestinal epithelium” and “regulate the gut microbiome.”
This is particularly relevant in EO-CRC, she added, given its greater association with T2D and obesity, both factors that “have been shown to create dysbiosis in the gut microbiome and low-grade inflammation via release of free radicals/inflammatory cytokines.”
These results provide more evidence that EO-CRC “is clinically and molecularly distinct from late-onset colorectal cancer,” which is important for both clinicians and patients to understand, said Olasehinde.
“It is imperative that we are all aware of the specific signs and symptoms this population presents with and the implications of this diagnosis in younger age groups,” she added. “Patients should continue making informed dietary and lifestyle modifications/choices to help reduce the burden of EO-CRC.”
Hypothesis-Generating Results
Aasma Shaukat, MD, MPH, who was not affiliated with the research, called the results promising but — at this stage — primarily useful for stimulating future research.
"We do need more studies such as this to generate hypotheses that can be studied prospectively," Shaukat, professor of medicine and population health, and director of GI Outcomes Research at NYU Langone Health in New York City, told Medscape Medical News.
She referred to another study, published in JAMA Oncology, that also used the TriNetX research network, which showed that GLP-1 RAs were associated with reduced CRC risk in drug-naive patients with T2D.
Shaukat also noted that the current analysis has limitations that should be considered. "The study is retrospective, and confounding is a possibility,” she said.
“How the groups that did and did not receive GLP-1 RAs differ in other risk factors that could be the drivers of the cancers is not known. Whether cancers were detected through screening or symptoms, stage, and other features that may differ are not known. Finally, since we don’t know who did or did not have colonoscopy, undiagnosed cancers are not known," she explained.
Shaukat, who was the lead author of the ACG 2021 Colorectal Cancer Screening Guidelines, added that the field would benefit from studies providing "biological plausibility information, such as animal studies to understand how GLP-1 RAs may modulate risk of colon cancer; other population-based cohort studies on the incidence of colon cancer among GLP-1 RA users and non-users; and prospective trials on chemoprevention."
The study had no specific funding. Olasehinde reported no relevant financial relationships. Shaukat reported serving as a consultant for Freenome, Medtronic, and Motus GI, as well as an advisory board member for Iterative Scopes Inc.
A version of this article appeared on Medscape.com.
PHILADELPHIA — according to the results of a retrospective study.
“This is the first large study to investigate the impact of GLP-1 RA use on EO-CRC risk,” principal investigator Temitope Olasehinde, MD, resident physician at the University Hospitals Cleveland Medical Center, Case Western Reserve University in Cleveland, Ohio, said in an interview.
The results indicate the GLP-1 RAs have a potentially protective role to play in combating EO-CRC, the incidence of which is notably rising in younger adults, with a corresponding increase in associated mortality.
Previous studies investigating the link between GLP-1 RAs and CRC did not capture patients aged younger than 50 years; thus, it was unknown if these results could be extrapolated to a younger age group, said Olasehinde.
The researcher presented the findings at the annual meeting of the American College of Gastroenterology.
Retrospective Database Analysis
Olasehinde and colleagues analyzed data from TriNetX, a large federated deidentified health research network, to identify patients (age ≤ 49 years) with diagnosed T2D subsequently prescribed antidiabetic medications who had not received a prior diagnosis of CRC. Additionally, patients were stratified on the basis of first-time GLP-1 RA use.
They identified 2,025,034 drug-naive patients with T2D; of these, 284,685 were subsequently prescribed GLP-1 RAs, and 1,740,349 remained in the non–GLP-1 RA cohort. Following propensity score matching, there were 86,186 patients in each cohort.
Patients who received GLP-1 RAs had significantly lower odds of developing EO-CRC than those who received non–GLP-1 RAs (0.6% vs 0.9%; P < .001; odds ratio [OR], 0.61; 95% CI, 0.54-068).
Furthermore, a sub-analysis revealed that patients who were obese and taking GLP-1 RAs had significantly lower odds of developing EO-CRC than patients who were obese but not taking GLP-1 RAs (0.7% vs 1.1%; P < .001; OR, 0.58; 95% CI, 0.50-067).
A Proposed Protective Effect
Although GLP-1 RAs are indicated for the treatment of T2D and obesity, recent evidence suggests that they may play a role in reducing the risk for CRC as well. This protective effect may be produced not only by addressing T2D and obesity — both important risk factors for CRC — but also via cellular mechanisms, Olasehinde noted.
“GLP-1 receptors are widely expressed throughout the gastrointestinal tract, with various effects on tissues in the stomach, small intestine, and colon,” she explained. Specifically, activation of these receptors in the proximal and distal colon promotes the release of “important factors that protect and facilitate healing of the intestinal epithelium” and “regulate the gut microbiome.”
This is particularly relevant in EO-CRC, she added, given its greater association with T2D and obesity, both factors that “have been shown to create dysbiosis in the gut microbiome and low-grade inflammation via release of free radicals/inflammatory cytokines.”
These results provide more evidence that EO-CRC “is clinically and molecularly distinct from late-onset colorectal cancer,” which is important for both clinicians and patients to understand, said Olasehinde.
“It is imperative that we are all aware of the specific signs and symptoms this population presents with and the implications of this diagnosis in younger age groups,” she added. “Patients should continue making informed dietary and lifestyle modifications/choices to help reduce the burden of EO-CRC.”
Hypothesis-Generating Results
Aasma Shaukat, MD, MPH, who was not affiliated with the research, called the results promising but — at this stage — primarily useful for stimulating future research.
"We do need more studies such as this to generate hypotheses that can be studied prospectively," Shaukat, professor of medicine and population health, and director of GI Outcomes Research at NYU Langone Health in New York City, told Medscape Medical News.
She referred to another study, published in JAMA Oncology, that also used the TriNetX research network, which showed that GLP-1 RAs were associated with reduced CRC risk in drug-naive patients with T2D.
Shaukat also noted that the current analysis has limitations that should be considered. "The study is retrospective, and confounding is a possibility,” she said.
“How the groups that did and did not receive GLP-1 RAs differ in other risk factors that could be the drivers of the cancers is not known. Whether cancers were detected through screening or symptoms, stage, and other features that may differ are not known. Finally, since we don’t know who did or did not have colonoscopy, undiagnosed cancers are not known," she explained.
Shaukat, who was the lead author of the ACG 2021 Colorectal Cancer Screening Guidelines, added that the field would benefit from studies providing "biological plausibility information, such as animal studies to understand how GLP-1 RAs may modulate risk of colon cancer; other population-based cohort studies on the incidence of colon cancer among GLP-1 RA users and non-users; and prospective trials on chemoprevention."
The study had no specific funding. Olasehinde reported no relevant financial relationships. Shaukat reported serving as a consultant for Freenome, Medtronic, and Motus GI, as well as an advisory board member for Iterative Scopes Inc.
A version of this article appeared on Medscape.com.
FROM ACG 2024