User login
Lifesaving future seen for electronic cigarettes
A switch from cigarettes to e-cigarettes has the potential to prevent almost 90,000 premature deaths in the United States in the year 2026, according to a study examining e-cigarette substitution scenarios.
The investigators’ “optimistic scenario” – in which new smokers use e-cigarettes instead of cigarettes, smoking prevalence falls to 5% over a 10-year period, and e-cigarettes have a 5% excess risk over regular cigarettes – projects 380,832 premature deaths from smoking in the year 2026. Under a “status quo scenario,” which projected current cigarette initiation and cessation rates and did not include e-cigarettes or other tobacco products, there would be 470,743 deaths, reported David T. Levy, PhD, and his associates (Tob Control. 2017 Oct 2. doi: 10.1136/tobaccocontrol-2017-053759).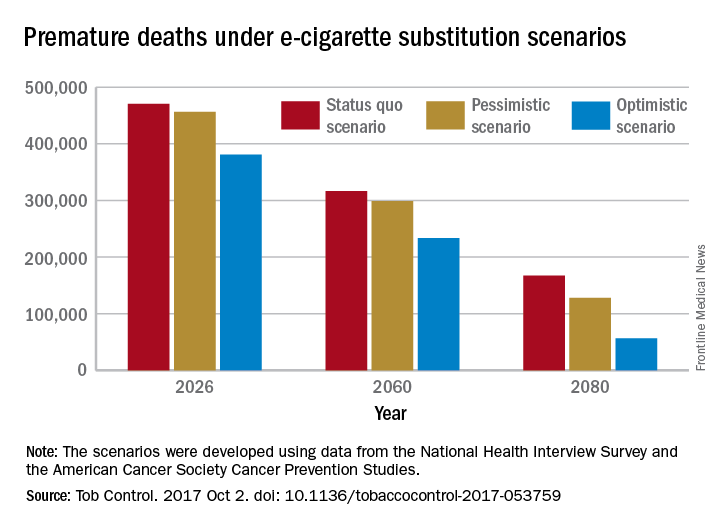
Further projections suggest that the optimistic scenario could result in almost 6.6 million fewer premature deaths and 86.7 million years of life gained by the year 2100, compared with the status quo scenario, while the pessimistic scenario would prevent 1.6 million deaths and add an extra 20.8 million years of life, they noted.
Since “a strategy of replacing cigarette by e-cigarette use can yield substantial gains, even with conservative assumptions about related risks … an endgame scenario for cigarettes might well be within reach, if new technologies for delivering nicotine with substantially less harm, but sufficient satisfaction, are harnessed with sufficient passion and political will to aggressively phase out tobacco cigarettes,” Dr. Levy and his associates wrote.
The study was funded by grants from the National Institute on Drug Abuse and the National Cancer Institute. One investigator received a research grant from Pfizer and served as an advisory board member to Johnson & Johnson, which manufactures smoking cessation medications. No other conflicts of interest were declared.
A switch from cigarettes to e-cigarettes has the potential to prevent almost 90,000 premature deaths in the United States in the year 2026, according to a study examining e-cigarette substitution scenarios.
The investigators’ “optimistic scenario” – in which new smokers use e-cigarettes instead of cigarettes, smoking prevalence falls to 5% over a 10-year period, and e-cigarettes have a 5% excess risk over regular cigarettes – projects 380,832 premature deaths from smoking in the year 2026. Under a “status quo scenario,” which projected current cigarette initiation and cessation rates and did not include e-cigarettes or other tobacco products, there would be 470,743 deaths, reported David T. Levy, PhD, and his associates (Tob Control. 2017 Oct 2. doi: 10.1136/tobaccocontrol-2017-053759).
Further projections suggest that the optimistic scenario could result in almost 6.6 million fewer premature deaths and 86.7 million years of life gained by the year 2100, compared with the status quo scenario, while the pessimistic scenario would prevent 1.6 million deaths and add an extra 20.8 million years of life, they noted.
Since “a strategy of replacing cigarette by e-cigarette use can yield substantial gains, even with conservative assumptions about related risks … an endgame scenario for cigarettes might well be within reach, if new technologies for delivering nicotine with substantially less harm, but sufficient satisfaction, are harnessed with sufficient passion and political will to aggressively phase out tobacco cigarettes,” Dr. Levy and his associates wrote.
The study was funded by grants from the National Institute on Drug Abuse and the National Cancer Institute. One investigator received a research grant from Pfizer and served as an advisory board member to Johnson & Johnson, which manufactures smoking cessation medications. No other conflicts of interest were declared.
A switch from cigarettes to e-cigarettes has the potential to prevent almost 90,000 premature deaths in the United States in the year 2026, according to a study examining e-cigarette substitution scenarios.
The investigators’ “optimistic scenario” – in which new smokers use e-cigarettes instead of cigarettes, smoking prevalence falls to 5% over a 10-year period, and e-cigarettes have a 5% excess risk over regular cigarettes – projects 380,832 premature deaths from smoking in the year 2026. Under a “status quo scenario,” which projected current cigarette initiation and cessation rates and did not include e-cigarettes or other tobacco products, there would be 470,743 deaths, reported David T. Levy, PhD, and his associates (Tob Control. 2017 Oct 2. doi: 10.1136/tobaccocontrol-2017-053759).
Further projections suggest that the optimistic scenario could result in almost 6.6 million fewer premature deaths and 86.7 million years of life gained by the year 2100, compared with the status quo scenario, while the pessimistic scenario would prevent 1.6 million deaths and add an extra 20.8 million years of life, they noted.
Since “a strategy of replacing cigarette by e-cigarette use can yield substantial gains, even with conservative assumptions about related risks … an endgame scenario for cigarettes might well be within reach, if new technologies for delivering nicotine with substantially less harm, but sufficient satisfaction, are harnessed with sufficient passion and political will to aggressively phase out tobacco cigarettes,” Dr. Levy and his associates wrote.
The study was funded by grants from the National Institute on Drug Abuse and the National Cancer Institute. One investigator received a research grant from Pfizer and served as an advisory board member to Johnson & Johnson, which manufactures smoking cessation medications. No other conflicts of interest were declared.
FROM TOBACCO CONTROL
FDA approves higher dose brigatinib tablet for advanced ALK+ NSCLC
The Food and Drug Administration has approved 180 mg brigatinib (Alunbrig) tablets for treatment of anaplastic lymphoma kinase–positive (ALK+) metastatic non–small cell lung cancer (NSCLC), expanding on previously available 30-mg tablets.*
“With the approval of a 180-mg tablet, Alunbrig has become the only ALK inhibitor available as a one tablet per day dose that can be taken with or without food,” Ryan Cohlhepp, PharmD, vice president, U.S. Commercial, at Takeda Oncology said in a press release.
Approval of the regimen was based on objective response in the ongoing, two-arm, open-label, multicenter phase 2 ALTA trial, which enrolled 222 patients with metastatic or locally advanced ALK+ NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily or 180 mg once daily following a 7-day lead-in at 90 mg once daily. Of those in the 180-mg arm, 53% had an objective response, compared with 48% in the 90-mg arm.
Adverse reactions occurred in 40% of the patients in the 180-mg arm, compared with 38% in the 90-mg arm. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. Fatal adverse reactions occurred in 3.7% of the patients, caused by pneumonia (two patients), sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis and urosepsis (one patient each).
The ALTA trial is ongoing, and updated results will be presented at the World Conference on Lung Cancer in Yokohama, Japan, on Oct. 15-18, the company said in the press release.
*Correction, 10/4/17: An earlier version of this article misstated the previously available tablet sizes.
The Food and Drug Administration has approved 180 mg brigatinib (Alunbrig) tablets for treatment of anaplastic lymphoma kinase–positive (ALK+) metastatic non–small cell lung cancer (NSCLC), expanding on previously available 30-mg tablets.*
“With the approval of a 180-mg tablet, Alunbrig has become the only ALK inhibitor available as a one tablet per day dose that can be taken with or without food,” Ryan Cohlhepp, PharmD, vice president, U.S. Commercial, at Takeda Oncology said in a press release.
Approval of the regimen was based on objective response in the ongoing, two-arm, open-label, multicenter phase 2 ALTA trial, which enrolled 222 patients with metastatic or locally advanced ALK+ NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily or 180 mg once daily following a 7-day lead-in at 90 mg once daily. Of those in the 180-mg arm, 53% had an objective response, compared with 48% in the 90-mg arm.
Adverse reactions occurred in 40% of the patients in the 180-mg arm, compared with 38% in the 90-mg arm. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. Fatal adverse reactions occurred in 3.7% of the patients, caused by pneumonia (two patients), sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis and urosepsis (one patient each).
The ALTA trial is ongoing, and updated results will be presented at the World Conference on Lung Cancer in Yokohama, Japan, on Oct. 15-18, the company said in the press release.
*Correction, 10/4/17: An earlier version of this article misstated the previously available tablet sizes.
The Food and Drug Administration has approved 180 mg brigatinib (Alunbrig) tablets for treatment of anaplastic lymphoma kinase–positive (ALK+) metastatic non–small cell lung cancer (NSCLC), expanding on previously available 30-mg tablets.*
“With the approval of a 180-mg tablet, Alunbrig has become the only ALK inhibitor available as a one tablet per day dose that can be taken with or without food,” Ryan Cohlhepp, PharmD, vice president, U.S. Commercial, at Takeda Oncology said in a press release.
Approval of the regimen was based on objective response in the ongoing, two-arm, open-label, multicenter phase 2 ALTA trial, which enrolled 222 patients with metastatic or locally advanced ALK+ NSCLC who had progressed on crizotinib. Patients were randomized to brigatinib orally either 90 mg once daily or 180 mg once daily following a 7-day lead-in at 90 mg once daily. Of those in the 180-mg arm, 53% had an objective response, compared with 48% in the 90-mg arm.
Adverse reactions occurred in 40% of the patients in the 180-mg arm, compared with 38% in the 90-mg arm. The most common serious adverse reactions were pneumonia and interstitial lung disease/pneumonitis. Fatal adverse reactions occurred in 3.7% of the patients, caused by pneumonia (two patients), sudden death, dyspnea, respiratory failure, pulmonary embolism, bacterial meningitis and urosepsis (one patient each).
The ALTA trial is ongoing, and updated results will be presented at the World Conference on Lung Cancer in Yokohama, Japan, on Oct. 15-18, the company said in the press release.
*Correction, 10/4/17: An earlier version of this article misstated the previously available tablet sizes.
Retrospective review: No difference in PFS, OS with radiation before PD-1/PD-L1 inhibition
CHICAGO – Exposure to radiation therapy prior to PD-1/PD-L1 therapy was not associated with improved outcomes in a retrospective review of 66 lung cancer patients.
The patients had stage IIIB or IV non–small cell lung cancer, median age of 64 years, received at least 6 weeks of single-agent anti-PD-1/PD-L1 therapy in the second-line setting or beyond, and had survived at least 8 weeks from immunotherapy initiation. Compared with 13 patients who received no radiation therapy, the 53 who received any prior radiation therapy – including 44 with extracranial radiation and 22 with intracranial radiation – did not differ significantly with respect to progression-free survival (median 4-5 months; hazard ratio, 0.83), or overall survival (median of about 12 months in both groups; HR, 0.96), Christopher Strouse, MD, of the University of Iowa, Iowa City, reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
There also were no significant differences in the outcomes between those who had extracranial radiation and those who had intracranial radiation (HRs for PFS and OS, respectively, 0.91 and 1.19), or (on univariate analysis), between those receiving any vs. no intracranial radiation therapy (HRs for PFS and OS, respectively, 0.92 and 0.98), Dr. Strouse said.
The patients who received extracranial radiation therapy had lower lymphocyte counts at the time of anti-PD-1/PD-L1 therapy initiation vs. those who received only radiation therapy (mean lymphocyte count, 809 vs. 1,519), and those who received intracranial radiation therapy were younger than those who did not (median age, 59 vs. 65 years), but the groups were similar with respect to other variables, including gender, histology, performance status, smoking history, KRAS mutation, and number of prior lines of systemic therapies. Anti-PD-1/PD-L1 therapies are promising treatment options for metastatic non–small cell lung cancer, and combining these agents with other immune-modulating therapies may enhance their efficacy and lead to a greater proportion of patients with responses to these treatments, Dr. Strouse noted.
“It’s known that immune response depends on a lot of steps, even beyond the PD-1/PD-L1 axis, and one possible explanation for some of these patients [not responding] may be that there is some failure along the way in some other step,” he said. “Our hypothesis was that radiation therapy would be helpful in overcoming some of these barriers.”
However, in this study, which is limited by small sample size and single-institution retrospective design, no such effect was identified.
The findings conflict with some larger studies, including the recently-reported PACIFIC study, which showed a significant PFS benefit in lung cancer patients who received chemoradiation therapy followed by treatment with the PD-L1 inhibitor durvalumab.
Dr. Strouse said he looks forward to seeing further reports looking into the effects of radiation therapy at different doses and timing.
Invited discussant Heather Wakelee, MD, of Stanford (Calif.) University, also stressed the limitations of the University of Iowa study, and noted that while there are many unanswered questions, findings such as those from the PACIFIC trial show that radiation and PD-L1 inhibition is here to stay.
“It appears safe; there will be more coming,” she said.
Dr. Strouse reported having no disclosures. Dr. Wakelee has been the institutional principal investigator for studies of nivolumab, tocilizumab, and other agents. She has consulted for Peregrine, ACEA, Pfizer, Helsinn, Genentech/Roche, Clovis, and Lilly, and received research/grant support from Clovis, Exelixis, AstraZeneca/Medimmune, Genentech/Roche, BMS, Gilead, Novartis, Xcovery, Pfizer, Celgene, Gilead, Pharmacyclics, and Lilly.
CHICAGO – Exposure to radiation therapy prior to PD-1/PD-L1 therapy was not associated with improved outcomes in a retrospective review of 66 lung cancer patients.
The patients had stage IIIB or IV non–small cell lung cancer, median age of 64 years, received at least 6 weeks of single-agent anti-PD-1/PD-L1 therapy in the second-line setting or beyond, and had survived at least 8 weeks from immunotherapy initiation. Compared with 13 patients who received no radiation therapy, the 53 who received any prior radiation therapy – including 44 with extracranial radiation and 22 with intracranial radiation – did not differ significantly with respect to progression-free survival (median 4-5 months; hazard ratio, 0.83), or overall survival (median of about 12 months in both groups; HR, 0.96), Christopher Strouse, MD, of the University of Iowa, Iowa City, reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
There also were no significant differences in the outcomes between those who had extracranial radiation and those who had intracranial radiation (HRs for PFS and OS, respectively, 0.91 and 1.19), or (on univariate analysis), between those receiving any vs. no intracranial radiation therapy (HRs for PFS and OS, respectively, 0.92 and 0.98), Dr. Strouse said.
The patients who received extracranial radiation therapy had lower lymphocyte counts at the time of anti-PD-1/PD-L1 therapy initiation vs. those who received only radiation therapy (mean lymphocyte count, 809 vs. 1,519), and those who received intracranial radiation therapy were younger than those who did not (median age, 59 vs. 65 years), but the groups were similar with respect to other variables, including gender, histology, performance status, smoking history, KRAS mutation, and number of prior lines of systemic therapies. Anti-PD-1/PD-L1 therapies are promising treatment options for metastatic non–small cell lung cancer, and combining these agents with other immune-modulating therapies may enhance their efficacy and lead to a greater proportion of patients with responses to these treatments, Dr. Strouse noted.
“It’s known that immune response depends on a lot of steps, even beyond the PD-1/PD-L1 axis, and one possible explanation for some of these patients [not responding] may be that there is some failure along the way in some other step,” he said. “Our hypothesis was that radiation therapy would be helpful in overcoming some of these barriers.”
However, in this study, which is limited by small sample size and single-institution retrospective design, no such effect was identified.
The findings conflict with some larger studies, including the recently-reported PACIFIC study, which showed a significant PFS benefit in lung cancer patients who received chemoradiation therapy followed by treatment with the PD-L1 inhibitor durvalumab.
Dr. Strouse said he looks forward to seeing further reports looking into the effects of radiation therapy at different doses and timing.
Invited discussant Heather Wakelee, MD, of Stanford (Calif.) University, also stressed the limitations of the University of Iowa study, and noted that while there are many unanswered questions, findings such as those from the PACIFIC trial show that radiation and PD-L1 inhibition is here to stay.
“It appears safe; there will be more coming,” she said.
Dr. Strouse reported having no disclosures. Dr. Wakelee has been the institutional principal investigator for studies of nivolumab, tocilizumab, and other agents. She has consulted for Peregrine, ACEA, Pfizer, Helsinn, Genentech/Roche, Clovis, and Lilly, and received research/grant support from Clovis, Exelixis, AstraZeneca/Medimmune, Genentech/Roche, BMS, Gilead, Novartis, Xcovery, Pfizer, Celgene, Gilead, Pharmacyclics, and Lilly.
CHICAGO – Exposure to radiation therapy prior to PD-1/PD-L1 therapy was not associated with improved outcomes in a retrospective review of 66 lung cancer patients.
The patients had stage IIIB or IV non–small cell lung cancer, median age of 64 years, received at least 6 weeks of single-agent anti-PD-1/PD-L1 therapy in the second-line setting or beyond, and had survived at least 8 weeks from immunotherapy initiation. Compared with 13 patients who received no radiation therapy, the 53 who received any prior radiation therapy – including 44 with extracranial radiation and 22 with intracranial radiation – did not differ significantly with respect to progression-free survival (median 4-5 months; hazard ratio, 0.83), or overall survival (median of about 12 months in both groups; HR, 0.96), Christopher Strouse, MD, of the University of Iowa, Iowa City, reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
There also were no significant differences in the outcomes between those who had extracranial radiation and those who had intracranial radiation (HRs for PFS and OS, respectively, 0.91 and 1.19), or (on univariate analysis), between those receiving any vs. no intracranial radiation therapy (HRs for PFS and OS, respectively, 0.92 and 0.98), Dr. Strouse said.
The patients who received extracranial radiation therapy had lower lymphocyte counts at the time of anti-PD-1/PD-L1 therapy initiation vs. those who received only radiation therapy (mean lymphocyte count, 809 vs. 1,519), and those who received intracranial radiation therapy were younger than those who did not (median age, 59 vs. 65 years), but the groups were similar with respect to other variables, including gender, histology, performance status, smoking history, KRAS mutation, and number of prior lines of systemic therapies. Anti-PD-1/PD-L1 therapies are promising treatment options for metastatic non–small cell lung cancer, and combining these agents with other immune-modulating therapies may enhance their efficacy and lead to a greater proportion of patients with responses to these treatments, Dr. Strouse noted.
“It’s known that immune response depends on a lot of steps, even beyond the PD-1/PD-L1 axis, and one possible explanation for some of these patients [not responding] may be that there is some failure along the way in some other step,” he said. “Our hypothesis was that radiation therapy would be helpful in overcoming some of these barriers.”
However, in this study, which is limited by small sample size and single-institution retrospective design, no such effect was identified.
The findings conflict with some larger studies, including the recently-reported PACIFIC study, which showed a significant PFS benefit in lung cancer patients who received chemoradiation therapy followed by treatment with the PD-L1 inhibitor durvalumab.
Dr. Strouse said he looks forward to seeing further reports looking into the effects of radiation therapy at different doses and timing.
Invited discussant Heather Wakelee, MD, of Stanford (Calif.) University, also stressed the limitations of the University of Iowa study, and noted that while there are many unanswered questions, findings such as those from the PACIFIC trial show that radiation and PD-L1 inhibition is here to stay.
“It appears safe; there will be more coming,” she said.
Dr. Strouse reported having no disclosures. Dr. Wakelee has been the institutional principal investigator for studies of nivolumab, tocilizumab, and other agents. She has consulted for Peregrine, ACEA, Pfizer, Helsinn, Genentech/Roche, Clovis, and Lilly, and received research/grant support from Clovis, Exelixis, AstraZeneca/Medimmune, Genentech/Roche, BMS, Gilead, Novartis, Xcovery, Pfizer, Celgene, Gilead, Pharmacyclics, and Lilly.
AT A SYMPOSIUM IN THORACIC ONCOLOGY
Key clinical point:
Major finding: PFS and OS did not differ significantly between patients who did and did not receive prior radiation therapy (HRs for PFS and OS, respectively, 0.83 and 0.96).
Data source: A retrospective review of 66 patients.
Disclosures: Dr. Strouse reported having no disclosures. Dr. Wakelee has been the institutional principal investigator for studies of nivolumab, tocilizumab, and other agents. She has consulted for Peregrine, ACEA, Pfizer, Helsinn, Genentech/Roche, Clovis, and Lilly, and received research/grant support from Clovis, Exelixis, AstraZeneca/Medimmune, Genentech/Roche, BMS, Gilead, Novartis, Xcovery, Pfizer, Celgene, Gilead, Pharmacyclics, and Lilly.
Motesanib flops again in lung cancer
Motesanib has flunked another phase III trial in advanced nonsquamous non–small-cell lung cancer, this time in East Asian patients.
Compared with placebo, the investigational oral vascular endothelial growth factor (VEGF) inhibitor did not significantly improve progression-free survival (PFS) or the secondary endpoint, overall survival (OS), when added to paclitaxel and carboplatin (P/C), reported Kaoru Kubota, MD, of Nippon Medical School, Tokyo, and his associates. “The findings are consistent with overall findings of the phase III MONET1 study but do not replicate those of the subgroup analysis of Asian patients,” they wrote in Journal of Clinical Oncology.
Motesanib is a small-molecular inhibitor of VEGF receptors 1, 2, and 3. In a phase II trial of patients with advanced nonsquamous non–small-cell lung cancer, motesanib resembled the anti-VEGF-A monoclonal antibody bevacizumab in terms of objective response rate, median PFS, and OS when added to paclitaxel and carboplatin. In the subsequent phase III MONET1 trial, however, motesanib plus P/C did not improve PFS over placebo plus P/C, except in a preplanned subgroup analysis of 227 East Asian patients, where it was associated with a 6.4-month greater median PFS (P = .02) and a 1.7-month greater OS (P = .001).
Based on those findings, Dr. Kubota and his associates randomly assigned 401 patients with advanced nonsquamous non–small-cell lung cancer to receive oral motesanib (125 mg) or placebo once daily plus paclitaxel (200 mg/m2 IV) and carboplatin (area under the concentration-time curve, 6 mg/mL per min IV) for up to six 3-week cycles. Patients were from Hong Kong, Korea, Japan, and Taiwan; averaged 65 years of age; and 72% were male (J Clin Oncol. 2017 Sep 13. doi: 10.1200/JCO.2017.72.7297).
After a median follow-up of 10 months, median PFS was 6.1 months in the motesanib plus P/C arm and 5.6 months in the placebo plus P/C arm (hazard ratio, 0.81; P = .08). Respective objective response rates were 60% and 42% (P less than .001), median times to tumor response were 1.4 and 1.6 months, and median durations of response were 5.3 and 4.1 months. Motesanib was associated with a higher rate of serious adverse events (87% versus 68%) and a higher rate of treatment discontinuation due to adverse events (33% versus 14%). Motesanib most often caused gastrointestinal disorders, hypertension, cholecystitis, gallbladder enlargement, and liver disorders.
Takeda Pharmaceuticals makes motesanib and sponsored the trial. Dr. Kubota disclosed honoraria and research funding from numerous pharmaceutical companies excluding Takeda. Four coinvestigators disclosed research funding from Takeda and two coinvestigators reported employment with the company. The remaining five researchers had no conflicts.
Motesanib has flunked another phase III trial in advanced nonsquamous non–small-cell lung cancer, this time in East Asian patients.
Compared with placebo, the investigational oral vascular endothelial growth factor (VEGF) inhibitor did not significantly improve progression-free survival (PFS) or the secondary endpoint, overall survival (OS), when added to paclitaxel and carboplatin (P/C), reported Kaoru Kubota, MD, of Nippon Medical School, Tokyo, and his associates. “The findings are consistent with overall findings of the phase III MONET1 study but do not replicate those of the subgroup analysis of Asian patients,” they wrote in Journal of Clinical Oncology.
Motesanib is a small-molecular inhibitor of VEGF receptors 1, 2, and 3. In a phase II trial of patients with advanced nonsquamous non–small-cell lung cancer, motesanib resembled the anti-VEGF-A monoclonal antibody bevacizumab in terms of objective response rate, median PFS, and OS when added to paclitaxel and carboplatin. In the subsequent phase III MONET1 trial, however, motesanib plus P/C did not improve PFS over placebo plus P/C, except in a preplanned subgroup analysis of 227 East Asian patients, where it was associated with a 6.4-month greater median PFS (P = .02) and a 1.7-month greater OS (P = .001).
Based on those findings, Dr. Kubota and his associates randomly assigned 401 patients with advanced nonsquamous non–small-cell lung cancer to receive oral motesanib (125 mg) or placebo once daily plus paclitaxel (200 mg/m2 IV) and carboplatin (area under the concentration-time curve, 6 mg/mL per min IV) for up to six 3-week cycles. Patients were from Hong Kong, Korea, Japan, and Taiwan; averaged 65 years of age; and 72% were male (J Clin Oncol. 2017 Sep 13. doi: 10.1200/JCO.2017.72.7297).
After a median follow-up of 10 months, median PFS was 6.1 months in the motesanib plus P/C arm and 5.6 months in the placebo plus P/C arm (hazard ratio, 0.81; P = .08). Respective objective response rates were 60% and 42% (P less than .001), median times to tumor response were 1.4 and 1.6 months, and median durations of response were 5.3 and 4.1 months. Motesanib was associated with a higher rate of serious adverse events (87% versus 68%) and a higher rate of treatment discontinuation due to adverse events (33% versus 14%). Motesanib most often caused gastrointestinal disorders, hypertension, cholecystitis, gallbladder enlargement, and liver disorders.
Takeda Pharmaceuticals makes motesanib and sponsored the trial. Dr. Kubota disclosed honoraria and research funding from numerous pharmaceutical companies excluding Takeda. Four coinvestigators disclosed research funding from Takeda and two coinvestigators reported employment with the company. The remaining five researchers had no conflicts.
Motesanib has flunked another phase III trial in advanced nonsquamous non–small-cell lung cancer, this time in East Asian patients.
Compared with placebo, the investigational oral vascular endothelial growth factor (VEGF) inhibitor did not significantly improve progression-free survival (PFS) or the secondary endpoint, overall survival (OS), when added to paclitaxel and carboplatin (P/C), reported Kaoru Kubota, MD, of Nippon Medical School, Tokyo, and his associates. “The findings are consistent with overall findings of the phase III MONET1 study but do not replicate those of the subgroup analysis of Asian patients,” they wrote in Journal of Clinical Oncology.
Motesanib is a small-molecular inhibitor of VEGF receptors 1, 2, and 3. In a phase II trial of patients with advanced nonsquamous non–small-cell lung cancer, motesanib resembled the anti-VEGF-A monoclonal antibody bevacizumab in terms of objective response rate, median PFS, and OS when added to paclitaxel and carboplatin. In the subsequent phase III MONET1 trial, however, motesanib plus P/C did not improve PFS over placebo plus P/C, except in a preplanned subgroup analysis of 227 East Asian patients, where it was associated with a 6.4-month greater median PFS (P = .02) and a 1.7-month greater OS (P = .001).
Based on those findings, Dr. Kubota and his associates randomly assigned 401 patients with advanced nonsquamous non–small-cell lung cancer to receive oral motesanib (125 mg) or placebo once daily plus paclitaxel (200 mg/m2 IV) and carboplatin (area under the concentration-time curve, 6 mg/mL per min IV) for up to six 3-week cycles. Patients were from Hong Kong, Korea, Japan, and Taiwan; averaged 65 years of age; and 72% were male (J Clin Oncol. 2017 Sep 13. doi: 10.1200/JCO.2017.72.7297).
After a median follow-up of 10 months, median PFS was 6.1 months in the motesanib plus P/C arm and 5.6 months in the placebo plus P/C arm (hazard ratio, 0.81; P = .08). Respective objective response rates were 60% and 42% (P less than .001), median times to tumor response were 1.4 and 1.6 months, and median durations of response were 5.3 and 4.1 months. Motesanib was associated with a higher rate of serious adverse events (87% versus 68%) and a higher rate of treatment discontinuation due to adverse events (33% versus 14%). Motesanib most often caused gastrointestinal disorders, hypertension, cholecystitis, gallbladder enlargement, and liver disorders.
Takeda Pharmaceuticals makes motesanib and sponsored the trial. Dr. Kubota disclosed honoraria and research funding from numerous pharmaceutical companies excluding Takeda. Four coinvestigators disclosed research funding from Takeda and two coinvestigators reported employment with the company. The remaining five researchers had no conflicts.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Belying a previous subgroup analysis, motesanib, an investigational oral vascular endothelial growth factor inhibitor, did not significantly improve progression-free survival when added to paclitaxel/carboplatin in East Asian patients with advanced nonsquamous non–small-cell lung cancer.
Major finding: After a median follow-up of 10 months, median PFS was 6.1 months among motesanib recipients and 5.6 months in the placebo group (hazard ratio, 0.81; P = .08).
Data source: A double-blind, phase III trial of 401 patients.
Disclosures: Takeda Pharmaceuticals makes motesanib and sponsored the trial. Dr. Kubota disclosed honoraria and research funding from numerous pharmaceutical companies excluding Takeda. Four coinvestigators disclosed research funding from Takeda and two coinvestigators reported employment with the company. The remaining five researchers had no conflicts.
CRP may predict survival after immunotherapy for lung cancer
CHICAGO – A baseline C-reactive protein (CRP) level above 50 mg/L independently predicted worse overall survival after immunotherapy in patients with advanced non–small cell lung cancer and small cell lung cancer in a retrospective study.
In 99 patients treated with nivolumab after a first-line platinum doublet, the median baseline CRP level was 22 mg/L. After a median follow-up of 8.5 months, 50% of patients were alive, and, based on univariate and multivariate analysis, both liver involvement and having a CRP level greater than 50 mg/L were significantly associated with inferior overall survival after immunotherapy.
The median overall survival after immunotherapy was 9.3 months versus 2.7 months with a CRP level of 50 mg/L or less versus above 50 mg/L, Abdul Rafeh Naqash, MD, of East Carolina University, Greenville, N.C., reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
Notably, significant increases in CRP level, compared with baseline, were seen at the time of grade 2 to grade 4 immune-related adverse events, which occurred in 38.4% of patients. This is a hypothesis-generating finding in that it suggests there is dysregulation of the immune system, in the context of immune checkpoint blockade, that leads to a more proinflammatory state, which ultimately leads to immune-related adverse events, Dr. Naqash said.
Study subjects were adults with a median age of 65 years who were treated during April 2015-March 2017. Most were white (64.7%), were male (64.6%), and had non–small cell lung cancer (88%). Most had stage IV disease (70.7%), and the most common site for metastases was the bones (35.4%) and the liver (24.2%). Patients’ CRP levels were measured at anti-PD-1–treatment initiation and serially with subsequent doses.
The findings are important because the identification of predictive biomarkers in patients treated with anti-PD-1 therapy could provide valuable insights into underlying mechanisms regulating patient responses, elucidate resistance mechanisms, and help with optimal selection of patients for treatment with and development of patient-tailored treatment, Dr. Naqash said, noting that identifying such biomarkers has thus far been a challenge.
However, this study is limited by its retrospective design and limited follow-up; the findings require validation in prospective lung cancer trials, he concluded.
Dr. Naqash reported having no disclosures.
CHICAGO – A baseline C-reactive protein (CRP) level above 50 mg/L independently predicted worse overall survival after immunotherapy in patients with advanced non–small cell lung cancer and small cell lung cancer in a retrospective study.
In 99 patients treated with nivolumab after a first-line platinum doublet, the median baseline CRP level was 22 mg/L. After a median follow-up of 8.5 months, 50% of patients were alive, and, based on univariate and multivariate analysis, both liver involvement and having a CRP level greater than 50 mg/L were significantly associated with inferior overall survival after immunotherapy.
The median overall survival after immunotherapy was 9.3 months versus 2.7 months with a CRP level of 50 mg/L or less versus above 50 mg/L, Abdul Rafeh Naqash, MD, of East Carolina University, Greenville, N.C., reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
Notably, significant increases in CRP level, compared with baseline, were seen at the time of grade 2 to grade 4 immune-related adverse events, which occurred in 38.4% of patients. This is a hypothesis-generating finding in that it suggests there is dysregulation of the immune system, in the context of immune checkpoint blockade, that leads to a more proinflammatory state, which ultimately leads to immune-related adverse events, Dr. Naqash said.
Study subjects were adults with a median age of 65 years who were treated during April 2015-March 2017. Most were white (64.7%), were male (64.6%), and had non–small cell lung cancer (88%). Most had stage IV disease (70.7%), and the most common site for metastases was the bones (35.4%) and the liver (24.2%). Patients’ CRP levels were measured at anti-PD-1–treatment initiation and serially with subsequent doses.
The findings are important because the identification of predictive biomarkers in patients treated with anti-PD-1 therapy could provide valuable insights into underlying mechanisms regulating patient responses, elucidate resistance mechanisms, and help with optimal selection of patients for treatment with and development of patient-tailored treatment, Dr. Naqash said, noting that identifying such biomarkers has thus far been a challenge.
However, this study is limited by its retrospective design and limited follow-up; the findings require validation in prospective lung cancer trials, he concluded.
Dr. Naqash reported having no disclosures.
CHICAGO – A baseline C-reactive protein (CRP) level above 50 mg/L independently predicted worse overall survival after immunotherapy in patients with advanced non–small cell lung cancer and small cell lung cancer in a retrospective study.
In 99 patients treated with nivolumab after a first-line platinum doublet, the median baseline CRP level was 22 mg/L. After a median follow-up of 8.5 months, 50% of patients were alive, and, based on univariate and multivariate analysis, both liver involvement and having a CRP level greater than 50 mg/L were significantly associated with inferior overall survival after immunotherapy.
The median overall survival after immunotherapy was 9.3 months versus 2.7 months with a CRP level of 50 mg/L or less versus above 50 mg/L, Abdul Rafeh Naqash, MD, of East Carolina University, Greenville, N.C., reported at the Chicago Multidisciplinary Symposium in Thoracic Oncology.
Notably, significant increases in CRP level, compared with baseline, were seen at the time of grade 2 to grade 4 immune-related adverse events, which occurred in 38.4% of patients. This is a hypothesis-generating finding in that it suggests there is dysregulation of the immune system, in the context of immune checkpoint blockade, that leads to a more proinflammatory state, which ultimately leads to immune-related adverse events, Dr. Naqash said.
Study subjects were adults with a median age of 65 years who were treated during April 2015-March 2017. Most were white (64.7%), were male (64.6%), and had non–small cell lung cancer (88%). Most had stage IV disease (70.7%), and the most common site for metastases was the bones (35.4%) and the liver (24.2%). Patients’ CRP levels were measured at anti-PD-1–treatment initiation and serially with subsequent doses.
The findings are important because the identification of predictive biomarkers in patients treated with anti-PD-1 therapy could provide valuable insights into underlying mechanisms regulating patient responses, elucidate resistance mechanisms, and help with optimal selection of patients for treatment with and development of patient-tailored treatment, Dr. Naqash said, noting that identifying such biomarkers has thus far been a challenge.
However, this study is limited by its retrospective design and limited follow-up; the findings require validation in prospective lung cancer trials, he concluded.
Dr. Naqash reported having no disclosures.
AT A SYMPOSIUM IN THORACIC ONCOLOGY
Key clinical point:
Major finding: Median overall survival after immunotherapy: 9.3 months vs. 2.7 months with CRP of 50 mg/L or less vs. above 50 mg/L.
Data source: A retrospective study of 99 patients.
Disclosures: Dr. Naqash reported having no disclosures.
Statins linked to lower death rates in COPD
Receiving a statin prescription within a year after diagnosis of chronic obstructive pulmonary disease was associated with a 21% decrease in the subsequent risk of all-cause mortality and a 45% drop in risk of pulmonary mortality, according to the results of a large retrospective administrative database study.
The findings belie those of the recent Simvastatin in the Prevention of COPD Exacerbation (STATCOPE) trial, in which daily simvastatin (40 mg) did not affect exacerbation rates or time to first exacerbation in high-risk COPD patients, wrote Adam Raymakers, MSc, a doctoral candidate at the University of British Columbia, Vancouver, and his associates. Their study was observational, but the association between statin use and decreased mortality “persisted across several measures of statin exposure,” they wrote. “Our findings, in conjunction with previously reported evidence, suggests that there may be a specific subtype of COPD patients that may benefit from statin use.” The study appears in the September issue of Chest (2017;152;486-93).
To further explore the question, the researchers analyzed linked health databases from nearly 40,000 patients aged 50 years and older who had received at least three prescriptions for an anticholinergic or a short-acting beta agonist in 12 months some time between 1998 and 2007. The first prescription was considered the date of COPD “diagnosis.” The average age of the patients was 71 years; 55% were female.
A total of 7,775 patients (19.6%) who met this definition of incident COPD were prescribed a statin at least once during the subsequent year. These patients had a significantly reduced risk of subsequent all-cause mortality in univariate and multivariate analyses, with hazard ratios of 0.79 (95% confidence intervals, 0.68 to 0.91; P less than .002). Statins also showed a protective effect against pulmonary mortality, with univariate and multivariate hazard ratios of 0.52 (P = .01) and 0.55 (P = .03), respectively.
The protective effect of statins held up when the investigators narrowed the exposure period to 6 months after COPD diagnosis and when they expanded it to 18 months. Exposure to statins for 80% of the 1-year window after COPD diagnosis – a proxy for statin adherence – also led to a reduced risk of all-cause mortality, but the 95% confidence interval for the hazard ratio did not reach statistical significance (0.71 to 1.01; P = .06).
The most common prescription was for atorvastatin (49%), usually for 90 days (23%), 100 days (20%), or 30 days (15%), the researchers said. While the “possibility of the ‘healthy user’ or the ‘healthy adherer’ cannot be ignored,” they adjusted for other prescriptions, comorbidities, and income level, which should have helped eliminate this effect, they added. However, they lacked data on smoking and lung function assessments, both of which are “important confounders and contributors to mortality,” they acknowledged.
Canadian Institutes of Health Research supported the study. One coinvestigator disclosed consulting relationships with Teva, Pfizer, and Novartis. The others had no conflicts of interest.
Despite [its] limitations, the study results are intriguing and in line with findings from other retrospective cohorts. How then can we reconcile the apparent benefits observed in retrospective studies with the lack of clinical effect seen in prospective trials, particularly the Simvastatin in the Prevention of COPD Exacerbation (STATCOPE) study? Could it be that both negative and positive studies are “correct”? Prospective studies have thus far not been adequately powered for mortality as an endpoint. Perhaps the choice of the particular statin matters? While STATCOPE involved simvastatin, the majority of the cohort reported by Raymakers et al. received atorvastatin. [Or perhaps] the negative results of STATCOPE could be related to careful selection of study participants with a low burden of systemic inflammation.
This most recent study reinforces the idea that statins may play a beneficial role in COPD, but it isn’t clear which patients to target for therapy. It is unlikely that the findings by Raymakers et al. will reverse recent recommendations by the American College of Chest Physicians and Canadian Thoracic Society against the use of statins for the purpose of prevention of COPD exacerbations, but the suggestion of survival advantage related to statins certainly may breathe new life into an enthusiasm greatly tempered by STATCOPE.
Or Kalchiem-Dekel, MD, and Robert M. Reed, MD, are at the pulmonary and critical care medicine division, University of Maryland, Baltimore. Neither editorialist had conflicts of interest (Chest. 2017;152:456-7. doi: 10.1016/j.chest.2017.04.156).
Despite [its] limitations, the study results are intriguing and in line with findings from other retrospective cohorts. How then can we reconcile the apparent benefits observed in retrospective studies with the lack of clinical effect seen in prospective trials, particularly the Simvastatin in the Prevention of COPD Exacerbation (STATCOPE) study? Could it be that both negative and positive studies are “correct”? Prospective studies have thus far not been adequately powered for mortality as an endpoint. Perhaps the choice of the particular statin matters? While STATCOPE involved simvastatin, the majority of the cohort reported by Raymakers et al. received atorvastatin. [Or perhaps] the negative results of STATCOPE could be related to careful selection of study participants with a low burden of systemic inflammation.
This most recent study reinforces the idea that statins may play a beneficial role in COPD, but it isn’t clear which patients to target for therapy. It is unlikely that the findings by Raymakers et al. will reverse recent recommendations by the American College of Chest Physicians and Canadian Thoracic Society against the use of statins for the purpose of prevention of COPD exacerbations, but the suggestion of survival advantage related to statins certainly may breathe new life into an enthusiasm greatly tempered by STATCOPE.
Or Kalchiem-Dekel, MD, and Robert M. Reed, MD, are at the pulmonary and critical care medicine division, University of Maryland, Baltimore. Neither editorialist had conflicts of interest (Chest. 2017;152:456-7. doi: 10.1016/j.chest.2017.04.156).
Despite [its] limitations, the study results are intriguing and in line with findings from other retrospective cohorts. How then can we reconcile the apparent benefits observed in retrospective studies with the lack of clinical effect seen in prospective trials, particularly the Simvastatin in the Prevention of COPD Exacerbation (STATCOPE) study? Could it be that both negative and positive studies are “correct”? Prospective studies have thus far not been adequately powered for mortality as an endpoint. Perhaps the choice of the particular statin matters? While STATCOPE involved simvastatin, the majority of the cohort reported by Raymakers et al. received atorvastatin. [Or perhaps] the negative results of STATCOPE could be related to careful selection of study participants with a low burden of systemic inflammation.
This most recent study reinforces the idea that statins may play a beneficial role in COPD, but it isn’t clear which patients to target for therapy. It is unlikely that the findings by Raymakers et al. will reverse recent recommendations by the American College of Chest Physicians and Canadian Thoracic Society against the use of statins for the purpose of prevention of COPD exacerbations, but the suggestion of survival advantage related to statins certainly may breathe new life into an enthusiasm greatly tempered by STATCOPE.
Or Kalchiem-Dekel, MD, and Robert M. Reed, MD, are at the pulmonary and critical care medicine division, University of Maryland, Baltimore. Neither editorialist had conflicts of interest (Chest. 2017;152:456-7. doi: 10.1016/j.chest.2017.04.156).
Receiving a statin prescription within a year after diagnosis of chronic obstructive pulmonary disease was associated with a 21% decrease in the subsequent risk of all-cause mortality and a 45% drop in risk of pulmonary mortality, according to the results of a large retrospective administrative database study.
The findings belie those of the recent Simvastatin in the Prevention of COPD Exacerbation (STATCOPE) trial, in which daily simvastatin (40 mg) did not affect exacerbation rates or time to first exacerbation in high-risk COPD patients, wrote Adam Raymakers, MSc, a doctoral candidate at the University of British Columbia, Vancouver, and his associates. Their study was observational, but the association between statin use and decreased mortality “persisted across several measures of statin exposure,” they wrote. “Our findings, in conjunction with previously reported evidence, suggests that there may be a specific subtype of COPD patients that may benefit from statin use.” The study appears in the September issue of Chest (2017;152;486-93).
To further explore the question, the researchers analyzed linked health databases from nearly 40,000 patients aged 50 years and older who had received at least three prescriptions for an anticholinergic or a short-acting beta agonist in 12 months some time between 1998 and 2007. The first prescription was considered the date of COPD “diagnosis.” The average age of the patients was 71 years; 55% were female.
A total of 7,775 patients (19.6%) who met this definition of incident COPD were prescribed a statin at least once during the subsequent year. These patients had a significantly reduced risk of subsequent all-cause mortality in univariate and multivariate analyses, with hazard ratios of 0.79 (95% confidence intervals, 0.68 to 0.91; P less than .002). Statins also showed a protective effect against pulmonary mortality, with univariate and multivariate hazard ratios of 0.52 (P = .01) and 0.55 (P = .03), respectively.
The protective effect of statins held up when the investigators narrowed the exposure period to 6 months after COPD diagnosis and when they expanded it to 18 months. Exposure to statins for 80% of the 1-year window after COPD diagnosis – a proxy for statin adherence – also led to a reduced risk of all-cause mortality, but the 95% confidence interval for the hazard ratio did not reach statistical significance (0.71 to 1.01; P = .06).
The most common prescription was for atorvastatin (49%), usually for 90 days (23%), 100 days (20%), or 30 days (15%), the researchers said. While the “possibility of the ‘healthy user’ or the ‘healthy adherer’ cannot be ignored,” they adjusted for other prescriptions, comorbidities, and income level, which should have helped eliminate this effect, they added. However, they lacked data on smoking and lung function assessments, both of which are “important confounders and contributors to mortality,” they acknowledged.
Canadian Institutes of Health Research supported the study. One coinvestigator disclosed consulting relationships with Teva, Pfizer, and Novartis. The others had no conflicts of interest.
Receiving a statin prescription within a year after diagnosis of chronic obstructive pulmonary disease was associated with a 21% decrease in the subsequent risk of all-cause mortality and a 45% drop in risk of pulmonary mortality, according to the results of a large retrospective administrative database study.
The findings belie those of the recent Simvastatin in the Prevention of COPD Exacerbation (STATCOPE) trial, in which daily simvastatin (40 mg) did not affect exacerbation rates or time to first exacerbation in high-risk COPD patients, wrote Adam Raymakers, MSc, a doctoral candidate at the University of British Columbia, Vancouver, and his associates. Their study was observational, but the association between statin use and decreased mortality “persisted across several measures of statin exposure,” they wrote. “Our findings, in conjunction with previously reported evidence, suggests that there may be a specific subtype of COPD patients that may benefit from statin use.” The study appears in the September issue of Chest (2017;152;486-93).
To further explore the question, the researchers analyzed linked health databases from nearly 40,000 patients aged 50 years and older who had received at least three prescriptions for an anticholinergic or a short-acting beta agonist in 12 months some time between 1998 and 2007. The first prescription was considered the date of COPD “diagnosis.” The average age of the patients was 71 years; 55% were female.
A total of 7,775 patients (19.6%) who met this definition of incident COPD were prescribed a statin at least once during the subsequent year. These patients had a significantly reduced risk of subsequent all-cause mortality in univariate and multivariate analyses, with hazard ratios of 0.79 (95% confidence intervals, 0.68 to 0.91; P less than .002). Statins also showed a protective effect against pulmonary mortality, with univariate and multivariate hazard ratios of 0.52 (P = .01) and 0.55 (P = .03), respectively.
The protective effect of statins held up when the investigators narrowed the exposure period to 6 months after COPD diagnosis and when they expanded it to 18 months. Exposure to statins for 80% of the 1-year window after COPD diagnosis – a proxy for statin adherence – also led to a reduced risk of all-cause mortality, but the 95% confidence interval for the hazard ratio did not reach statistical significance (0.71 to 1.01; P = .06).
The most common prescription was for atorvastatin (49%), usually for 90 days (23%), 100 days (20%), or 30 days (15%), the researchers said. While the “possibility of the ‘healthy user’ or the ‘healthy adherer’ cannot be ignored,” they adjusted for other prescriptions, comorbidities, and income level, which should have helped eliminate this effect, they added. However, they lacked data on smoking and lung function assessments, both of which are “important confounders and contributors to mortality,” they acknowledged.
Canadian Institutes of Health Research supported the study. One coinvestigator disclosed consulting relationships with Teva, Pfizer, and Novartis. The others had no conflicts of interest.
FROM CHEST
Key clinical point: Statins might reduce the risk of death among patients with chronic obstructive pulmonary disease.
Major finding: Statin use was associated with a 21% decrease in risk of all-cause mortality and a 45% decrease in risk of pulmonary mortality.
Data source: A retrospective cohort study of 39,678 patients with COPD, including 7,775 prescribed statins.
Disclosures: Canadian Institutes of Health Research supported the study. One coinvestigator disclosed consulting relationships with Teva, Pfizer, and Novartis. The others had no conflicts of interest.
Lobectomy shown safe after concurrent chemo and radiation
Lobectomy can be done safely after concurrent chemotherapy and high-dose radiation in patients with resectable N2-positive stage IIIA non–small cell lung cancer, according to study findings presented by Jessica S. Donington, MD.
Dr. Donington of New York University and her colleagues presented analysis of two prospective trials conducted by NRG Oncology, RTOG 0229 and RTOG 0839 at the AATS Annual Meeting. Both trials’ primary endpoints were mediastinal node sterilization after concurrent chemotherapy and full-dose radiation and those results were previously reported.
Dr. Donington and her fellow investigators specifically examined short-term surgical outcomes, given the significant controversy regarding the safety of resection after full-dose thoracic radiation. In both trials, patients received weekly carboplatin and paclitaxel. Those in the 0229 trial underwent 61.2 Gy of radiation in 34 fractions, while patients in the 0839 trial underwent 60 Gy in 30 fractions. In addition, patients in the 0839 trial were randomized 2:1 to receive weekly panitumumab, an EGFR monoclonal antibody, with their induction therapy.
Surgical expertise was considered essential to this treatment strategy. Therefore, all surgeons were certified by RTOG prior to enrolling patients, and all patients were surgically evaluated before beginning induction therapy to determine resectability and appropriateness for trimodality therapy. Of 125 eligible patients enrolled in the two trials, 93 patients (74%) underwent anatomic resection. A total of 77 patients underwent lobectomy, 8 underwent pneumonectomy, 6 underwent bilobectomy, and 2 patients had sleeve lobectomy.
Medical contraindication and persistent nodal disease found during post-induction invasive staging were the most common reasons patients didn’t undergo resection. Eighty-five of the 93 surgical patients had R0 resections (91%). Surgeons attempted 14 minimally invasive resections (15%), 2 of which uneventfully converted to open resection. Just over one-quarter (28%) of patients suffered greater than Grade 3 adverse events (AEs) related to surgery, the majority of which were pulmonary in nature.
The 30-day mortality rate was 4%, and all four deaths were linked to pulmonary adverse events, including acute respiratory distress syndrome, bronchopleural fistula, pulmonary artery hemorrhage, and respiratory failure. Multivariable analysis for mortality identified the addition of panitumumab and use of an extended resection to be associated with an increased risk for operative mortality.
In the patients undergoing lobectomy, rates for greater than Grade 3 adverse events and 30-day mortality were 26%, and 1.3%, respectively. Those are similar to rates reported for lobectomy without induction therapy in the National Inpatient Sample (NIS) and the STS General Thoracic Surgery Database over the same time period.
These two RTOG trials are the first to prospectively demonstrate that trimodality therapy with full-dose neoadjuvant radiation therapy is safe in a multi-institutional setting, Dr. Donington said.
As her conclusions, she listed that “we have demonstrated the safety of resection following full-dose concurrent CRT in a multi-insitutional setting, that EGFR-AB and T2/T3 stage was associated with decreased nonfatal morbidity, but that extended resection and EGFR-AB were associated with excessive surgical mortality.” Dr. Donington added that the morbidity and mortality for lobectomy compared favorably with large national databases.She pointed out several limitations of the study, which included the relatively small size, and the unexpected toxicity of the EGFR antibody, “which severely limited our ability to assess trimodality therapy.”
She added that “our short-term outcomes tell us nothing about the long-term oncologic benefit.
Dr. David R. Jones, chief of thoracic surgery at the Memorial Sloan-Kettering Cancer Center, New York, was the invited discussant of the paper. “Despite combining these [2] trials, there are still only 93 patients in the analysis and 77 of these had a lobectomy,” Dr. Jones stated. He pointed out that “there was a significant increase in 30- and 90-day mortality in the 16 patients who had more than a straightforward lobectomy,” and asked whether this increased mortality could be due to the use of radiation in the induction therapy.
Dr. Donington replied that there was a wide variation in causes of death, but that “the fact that these patients had induction therapy, be that radiation or chemo, and then went on to this bigger operation, it was overall a difficult thing for them to overcome.” Dr. Donington also agreed with a comment by Dr. Jones that it would be important to follow predicted postoperative diffusion capacity in these patients to potentially help determine the amount of lung to be taken and would be a valuable preoperative consideration.
Dr. Donington reported that two of her coauthors received grant support from NCI and AMGen for the work she was reporting, but that there were no other related disclosures. Dr. Jones reported that he had no disclosures.
Lobectomy can be done safely after concurrent chemotherapy and high-dose radiation in patients with resectable N2-positive stage IIIA non–small cell lung cancer, according to study findings presented by Jessica S. Donington, MD.
Dr. Donington of New York University and her colleagues presented analysis of two prospective trials conducted by NRG Oncology, RTOG 0229 and RTOG 0839 at the AATS Annual Meeting. Both trials’ primary endpoints were mediastinal node sterilization after concurrent chemotherapy and full-dose radiation and those results were previously reported.
Dr. Donington and her fellow investigators specifically examined short-term surgical outcomes, given the significant controversy regarding the safety of resection after full-dose thoracic radiation. In both trials, patients received weekly carboplatin and paclitaxel. Those in the 0229 trial underwent 61.2 Gy of radiation in 34 fractions, while patients in the 0839 trial underwent 60 Gy in 30 fractions. In addition, patients in the 0839 trial were randomized 2:1 to receive weekly panitumumab, an EGFR monoclonal antibody, with their induction therapy.
Surgical expertise was considered essential to this treatment strategy. Therefore, all surgeons were certified by RTOG prior to enrolling patients, and all patients were surgically evaluated before beginning induction therapy to determine resectability and appropriateness for trimodality therapy. Of 125 eligible patients enrolled in the two trials, 93 patients (74%) underwent anatomic resection. A total of 77 patients underwent lobectomy, 8 underwent pneumonectomy, 6 underwent bilobectomy, and 2 patients had sleeve lobectomy.
Medical contraindication and persistent nodal disease found during post-induction invasive staging were the most common reasons patients didn’t undergo resection. Eighty-five of the 93 surgical patients had R0 resections (91%). Surgeons attempted 14 minimally invasive resections (15%), 2 of which uneventfully converted to open resection. Just over one-quarter (28%) of patients suffered greater than Grade 3 adverse events (AEs) related to surgery, the majority of which were pulmonary in nature.
The 30-day mortality rate was 4%, and all four deaths were linked to pulmonary adverse events, including acute respiratory distress syndrome, bronchopleural fistula, pulmonary artery hemorrhage, and respiratory failure. Multivariable analysis for mortality identified the addition of panitumumab and use of an extended resection to be associated with an increased risk for operative mortality.
In the patients undergoing lobectomy, rates for greater than Grade 3 adverse events and 30-day mortality were 26%, and 1.3%, respectively. Those are similar to rates reported for lobectomy without induction therapy in the National Inpatient Sample (NIS) and the STS General Thoracic Surgery Database over the same time period.
These two RTOG trials are the first to prospectively demonstrate that trimodality therapy with full-dose neoadjuvant radiation therapy is safe in a multi-institutional setting, Dr. Donington said.
As her conclusions, she listed that “we have demonstrated the safety of resection following full-dose concurrent CRT in a multi-insitutional setting, that EGFR-AB and T2/T3 stage was associated with decreased nonfatal morbidity, but that extended resection and EGFR-AB were associated with excessive surgical mortality.” Dr. Donington added that the morbidity and mortality for lobectomy compared favorably with large national databases.She pointed out several limitations of the study, which included the relatively small size, and the unexpected toxicity of the EGFR antibody, “which severely limited our ability to assess trimodality therapy.”
She added that “our short-term outcomes tell us nothing about the long-term oncologic benefit.
Dr. David R. Jones, chief of thoracic surgery at the Memorial Sloan-Kettering Cancer Center, New York, was the invited discussant of the paper. “Despite combining these [2] trials, there are still only 93 patients in the analysis and 77 of these had a lobectomy,” Dr. Jones stated. He pointed out that “there was a significant increase in 30- and 90-day mortality in the 16 patients who had more than a straightforward lobectomy,” and asked whether this increased mortality could be due to the use of radiation in the induction therapy.
Dr. Donington replied that there was a wide variation in causes of death, but that “the fact that these patients had induction therapy, be that radiation or chemo, and then went on to this bigger operation, it was overall a difficult thing for them to overcome.” Dr. Donington also agreed with a comment by Dr. Jones that it would be important to follow predicted postoperative diffusion capacity in these patients to potentially help determine the amount of lung to be taken and would be a valuable preoperative consideration.
Dr. Donington reported that two of her coauthors received grant support from NCI and AMGen for the work she was reporting, but that there were no other related disclosures. Dr. Jones reported that he had no disclosures.
Lobectomy can be done safely after concurrent chemotherapy and high-dose radiation in patients with resectable N2-positive stage IIIA non–small cell lung cancer, according to study findings presented by Jessica S. Donington, MD.
Dr. Donington of New York University and her colleagues presented analysis of two prospective trials conducted by NRG Oncology, RTOG 0229 and RTOG 0839 at the AATS Annual Meeting. Both trials’ primary endpoints were mediastinal node sterilization after concurrent chemotherapy and full-dose radiation and those results were previously reported.
Dr. Donington and her fellow investigators specifically examined short-term surgical outcomes, given the significant controversy regarding the safety of resection after full-dose thoracic radiation. In both trials, patients received weekly carboplatin and paclitaxel. Those in the 0229 trial underwent 61.2 Gy of radiation in 34 fractions, while patients in the 0839 trial underwent 60 Gy in 30 fractions. In addition, patients in the 0839 trial were randomized 2:1 to receive weekly panitumumab, an EGFR monoclonal antibody, with their induction therapy.
Surgical expertise was considered essential to this treatment strategy. Therefore, all surgeons were certified by RTOG prior to enrolling patients, and all patients were surgically evaluated before beginning induction therapy to determine resectability and appropriateness for trimodality therapy. Of 125 eligible patients enrolled in the two trials, 93 patients (74%) underwent anatomic resection. A total of 77 patients underwent lobectomy, 8 underwent pneumonectomy, 6 underwent bilobectomy, and 2 patients had sleeve lobectomy.
Medical contraindication and persistent nodal disease found during post-induction invasive staging were the most common reasons patients didn’t undergo resection. Eighty-five of the 93 surgical patients had R0 resections (91%). Surgeons attempted 14 minimally invasive resections (15%), 2 of which uneventfully converted to open resection. Just over one-quarter (28%) of patients suffered greater than Grade 3 adverse events (AEs) related to surgery, the majority of which were pulmonary in nature.
The 30-day mortality rate was 4%, and all four deaths were linked to pulmonary adverse events, including acute respiratory distress syndrome, bronchopleural fistula, pulmonary artery hemorrhage, and respiratory failure. Multivariable analysis for mortality identified the addition of panitumumab and use of an extended resection to be associated with an increased risk for operative mortality.
In the patients undergoing lobectomy, rates for greater than Grade 3 adverse events and 30-day mortality were 26%, and 1.3%, respectively. Those are similar to rates reported for lobectomy without induction therapy in the National Inpatient Sample (NIS) and the STS General Thoracic Surgery Database over the same time period.
These two RTOG trials are the first to prospectively demonstrate that trimodality therapy with full-dose neoadjuvant radiation therapy is safe in a multi-institutional setting, Dr. Donington said.
As her conclusions, she listed that “we have demonstrated the safety of resection following full-dose concurrent CRT in a multi-insitutional setting, that EGFR-AB and T2/T3 stage was associated with decreased nonfatal morbidity, but that extended resection and EGFR-AB were associated with excessive surgical mortality.” Dr. Donington added that the morbidity and mortality for lobectomy compared favorably with large national databases.She pointed out several limitations of the study, which included the relatively small size, and the unexpected toxicity of the EGFR antibody, “which severely limited our ability to assess trimodality therapy.”
She added that “our short-term outcomes tell us nothing about the long-term oncologic benefit.
Dr. David R. Jones, chief of thoracic surgery at the Memorial Sloan-Kettering Cancer Center, New York, was the invited discussant of the paper. “Despite combining these [2] trials, there are still only 93 patients in the analysis and 77 of these had a lobectomy,” Dr. Jones stated. He pointed out that “there was a significant increase in 30- and 90-day mortality in the 16 patients who had more than a straightforward lobectomy,” and asked whether this increased mortality could be due to the use of radiation in the induction therapy.
Dr. Donington replied that there was a wide variation in causes of death, but that “the fact that these patients had induction therapy, be that radiation or chemo, and then went on to this bigger operation, it was overall a difficult thing for them to overcome.” Dr. Donington also agreed with a comment by Dr. Jones that it would be important to follow predicted postoperative diffusion capacity in these patients to potentially help determine the amount of lung to be taken and would be a valuable preoperative consideration.
Dr. Donington reported that two of her coauthors received grant support from NCI and AMGen for the work she was reporting, but that there were no other related disclosures. Dr. Jones reported that he had no disclosures.
FROM THE AMERICAN ASSOCIATION FOR THORACIC SURGERY ANNUAL MEETING
FDA approves biosimilar to bevacizumab
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
The Food and Drug Administration has approved a biosimilar to bevacizumab (Avastin) for the treatment of certain colorectal, lung, brain, kidney, and cervical cancers.
Bevacizumab-awwb is the first biosimilar approved in the United States for the treatment of cancer, the FDA said in a press release.
Approval is based on structural and functional characterization, animal study data, human pharmacokinetic and pharmacodynamics data, clinical immunogenicity data, and other clinical safety and effectiveness data that demonstrate bevacizumab-awwb is biosimilar to bevacizumab, the FDA said.
• Metastatic colorectal cancer, in combination with intravenous 5-fluorouracil-based chemotherapy for first- or second-line treatment.
• Metastatic colorectal cancer, in combination with fluoropyrimidine-irinotecan–based or fluoropyrimidine-oxaliplatin–based chemotherapy for the second-line treatment of patients who have progressed on a first-line bevacizumab product–containing regimen.
• Non-squamous non–small cell lung cancer, in combination with carboplatin and paclitaxel for first line treatment of unresectable, locally advanced, recurrent, or metastatic disease.
• Glioblastoma with progressive disease following prior therapy, based on improvement in objective response rate.
• Metastatic renal cell carcinoma, in combination with interferon alfa.
• Cervical cancer that is persistent, recurrent, or metastatic, in combination with paclitaxel and cisplatin or paclitaxel and topotecan.
Common expected side effects of the biosimilar include epistaxis, headache, hypertension, rhinitis, proteinuria, taste alteration, dry skin, hemorrhage, lacrimation disorder, back pain, and exfoliative dermatitis.
Serious expected side effects include perforation or fistula, arterial and venous thromboembolic events, hypertension, posterior reversible encephalopathy syndrome, proteinuria, infusion-related reactions, and ovarian failure. Women who are pregnant should not take bevacizumab-awwb.
The biosimilar to bevacizumab carries a similar boxed warning regarding the increased risk of gastrointestinal perforations; surgery and wound healing complications; and severe or fatal pulmonary, gastrointestinal, central nervous system, and vaginal hemorrhage.
The biosimilar approval was granted to Amgen, which will market the drug under the trade name Mvasi.
PACIFIC: Durvalumab extends PFS in stage 3 NSCLC
MADRID – For patients with locally advanced, unresectable non-small cell lung cancer, consolidation therapy with the anti-programmed death ligand 1 (PD-L1) inhibitor durvalumab after chemoradiation was associated with significantly better progression-free survival (PFS) than placebo, results of an interim analysis of the phase 3 PACIFIC trial showed.
Among 713 patients with stage III NSCLC treated with definitive chemoradiotherapy, the median PFS from randomization was 16.8 months for patients assigned to durvalumab compared with 5.6 months for patients assigned to placebo, reported Luis Paz-Ares, MD, from the University of Madrid, Spain.
“Overall, we think durvalumab is a promising option for patients with stage III non-small cell lung cancer treated with chemoradiation,” he said at a briefing at the European Society of Medical Oncology (ESMO) Congress.
Approximately 25% to 30% of patients with NSCLC have locally advanced disease at the time of presentation. Patients with unresectable disease and good performance status are treated with chemoradiotheraoy consisting of a platinum doublet with concurrent radiation, but median PFS in these patients is generally short, on the order of 8 months. The 5-year overall survival (OS) rate is 15%, he said.
Given the lack of major advances in the care of patients with stage 3 disease, investigators have been looking to newer therapies such as immune checkpoint inhibitors to see whether they could improve outcomes.
PACIFIC is a phase 3 trial in which patients with stage III NSCLC who did not have disease progression after a minimum of two cycles of platinum-based chemotherapy were assigned on a 2:1 basis to receive intravenous durvalumab 10 mg/kg or placebo every 2 weeks for up to 12 months. Patients were stratified by age, sex, and smoking history.
Dr. Paz-Ares presented PFS results from the interim analysis, planned when about 367 events had occurred. Data on the co-primary endpoint of OS were not mature at the time of the data cutoff.
Median PFS from randomization according to blinded independent central review for 476 patients treated with durvalumab was 16.8 months, compared with 5.6 months for 237 patients who received the placebo. This translated into a stratified hazard ratio (HR) of 0.52 (P less than .0001).
The respective PFS rates for durvalumab and placebo, were 59.9% vs. 35.3% at 12 months, and 44.2% vs. 27% at 18 months.
Among 443 patients on durvalumab and 213 on placebo who were evaluable for objective responses (OR), the respective OR rates were 28.4% vs. 16.
There were 6 complete responses (CR), 120 partial responses (PR), and 233 cases of stable disease in the durvalumab arm, compared with one CR, 33 PR, and 119 cases of stable disease in the placebo arm. In the durvalumab arm, 73 patients (16.5%) had progressive disease, compared with 59 patients (27.7%) in the placebo arm.
The median duration of response was not reached in the durvalumab arm, compared with 13.8 months in the placebo arm.
The PD-L1 inhibitor was also associated with a lower incidence of any new lesion among the intention-to-treat population (20.4% vs. 32.1%).
Grade 3 or 4 toxicities from any cause were slightly higher with durvalumab, at 29.9% vs. 26.1%. Events leading to discontinuation were also higher with the active drug, at 15.4% vs. 9.8% for placebo.
There were 21 deaths (4.4%) of patients treated with durvalumab, and 13 deaths (5.6%) of patients treated with placebo.
“In my opinion, this is a very well-designed study, and the results are very promising,” commented Enriqueta Felip, MD, who was not involved in the PACIFIC trial.
“We need to wait for the overall survival results, but in my opinion this is a very valuable trial in a group of patients [for whom] we need new strategies,” she said.
Dr. Felip was invited to the briefing as an independent commentator.
Results of the interim analysis of the PACIFIC trial were also published online in the New England Journal of Medicine.
The PACIFIC trial was funded by AstraZeneca. Dr. Paz-Ares has received consultancy fees from the company. Dr. Felip disclosed financial relationships with multiple companies not including AstraZeneca.
MADRID – For patients with locally advanced, unresectable non-small cell lung cancer, consolidation therapy with the anti-programmed death ligand 1 (PD-L1) inhibitor durvalumab after chemoradiation was associated with significantly better progression-free survival (PFS) than placebo, results of an interim analysis of the phase 3 PACIFIC trial showed.
Among 713 patients with stage III NSCLC treated with definitive chemoradiotherapy, the median PFS from randomization was 16.8 months for patients assigned to durvalumab compared with 5.6 months for patients assigned to placebo, reported Luis Paz-Ares, MD, from the University of Madrid, Spain.
“Overall, we think durvalumab is a promising option for patients with stage III non-small cell lung cancer treated with chemoradiation,” he said at a briefing at the European Society of Medical Oncology (ESMO) Congress.
Approximately 25% to 30% of patients with NSCLC have locally advanced disease at the time of presentation. Patients with unresectable disease and good performance status are treated with chemoradiotheraoy consisting of a platinum doublet with concurrent radiation, but median PFS in these patients is generally short, on the order of 8 months. The 5-year overall survival (OS) rate is 15%, he said.
Given the lack of major advances in the care of patients with stage 3 disease, investigators have been looking to newer therapies such as immune checkpoint inhibitors to see whether they could improve outcomes.
PACIFIC is a phase 3 trial in which patients with stage III NSCLC who did not have disease progression after a minimum of two cycles of platinum-based chemotherapy were assigned on a 2:1 basis to receive intravenous durvalumab 10 mg/kg or placebo every 2 weeks for up to 12 months. Patients were stratified by age, sex, and smoking history.
Dr. Paz-Ares presented PFS results from the interim analysis, planned when about 367 events had occurred. Data on the co-primary endpoint of OS were not mature at the time of the data cutoff.
Median PFS from randomization according to blinded independent central review for 476 patients treated with durvalumab was 16.8 months, compared with 5.6 months for 237 patients who received the placebo. This translated into a stratified hazard ratio (HR) of 0.52 (P less than .0001).
The respective PFS rates for durvalumab and placebo, were 59.9% vs. 35.3% at 12 months, and 44.2% vs. 27% at 18 months.
Among 443 patients on durvalumab and 213 on placebo who were evaluable for objective responses (OR), the respective OR rates were 28.4% vs. 16.
There were 6 complete responses (CR), 120 partial responses (PR), and 233 cases of stable disease in the durvalumab arm, compared with one CR, 33 PR, and 119 cases of stable disease in the placebo arm. In the durvalumab arm, 73 patients (16.5%) had progressive disease, compared with 59 patients (27.7%) in the placebo arm.
The median duration of response was not reached in the durvalumab arm, compared with 13.8 months in the placebo arm.
The PD-L1 inhibitor was also associated with a lower incidence of any new lesion among the intention-to-treat population (20.4% vs. 32.1%).
Grade 3 or 4 toxicities from any cause were slightly higher with durvalumab, at 29.9% vs. 26.1%. Events leading to discontinuation were also higher with the active drug, at 15.4% vs. 9.8% for placebo.
There were 21 deaths (4.4%) of patients treated with durvalumab, and 13 deaths (5.6%) of patients treated with placebo.
“In my opinion, this is a very well-designed study, and the results are very promising,” commented Enriqueta Felip, MD, who was not involved in the PACIFIC trial.
“We need to wait for the overall survival results, but in my opinion this is a very valuable trial in a group of patients [for whom] we need new strategies,” she said.
Dr. Felip was invited to the briefing as an independent commentator.
Results of the interim analysis of the PACIFIC trial were also published online in the New England Journal of Medicine.
The PACIFIC trial was funded by AstraZeneca. Dr. Paz-Ares has received consultancy fees from the company. Dr. Felip disclosed financial relationships with multiple companies not including AstraZeneca.
MADRID – For patients with locally advanced, unresectable non-small cell lung cancer, consolidation therapy with the anti-programmed death ligand 1 (PD-L1) inhibitor durvalumab after chemoradiation was associated with significantly better progression-free survival (PFS) than placebo, results of an interim analysis of the phase 3 PACIFIC trial showed.
Among 713 patients with stage III NSCLC treated with definitive chemoradiotherapy, the median PFS from randomization was 16.8 months for patients assigned to durvalumab compared with 5.6 months for patients assigned to placebo, reported Luis Paz-Ares, MD, from the University of Madrid, Spain.
“Overall, we think durvalumab is a promising option for patients with stage III non-small cell lung cancer treated with chemoradiation,” he said at a briefing at the European Society of Medical Oncology (ESMO) Congress.
Approximately 25% to 30% of patients with NSCLC have locally advanced disease at the time of presentation. Patients with unresectable disease and good performance status are treated with chemoradiotheraoy consisting of a platinum doublet with concurrent radiation, but median PFS in these patients is generally short, on the order of 8 months. The 5-year overall survival (OS) rate is 15%, he said.
Given the lack of major advances in the care of patients with stage 3 disease, investigators have been looking to newer therapies such as immune checkpoint inhibitors to see whether they could improve outcomes.
PACIFIC is a phase 3 trial in which patients with stage III NSCLC who did not have disease progression after a minimum of two cycles of platinum-based chemotherapy were assigned on a 2:1 basis to receive intravenous durvalumab 10 mg/kg or placebo every 2 weeks for up to 12 months. Patients were stratified by age, sex, and smoking history.
Dr. Paz-Ares presented PFS results from the interim analysis, planned when about 367 events had occurred. Data on the co-primary endpoint of OS were not mature at the time of the data cutoff.
Median PFS from randomization according to blinded independent central review for 476 patients treated with durvalumab was 16.8 months, compared with 5.6 months for 237 patients who received the placebo. This translated into a stratified hazard ratio (HR) of 0.52 (P less than .0001).
The respective PFS rates for durvalumab and placebo, were 59.9% vs. 35.3% at 12 months, and 44.2% vs. 27% at 18 months.
Among 443 patients on durvalumab and 213 on placebo who were evaluable for objective responses (OR), the respective OR rates were 28.4% vs. 16.
There were 6 complete responses (CR), 120 partial responses (PR), and 233 cases of stable disease in the durvalumab arm, compared with one CR, 33 PR, and 119 cases of stable disease in the placebo arm. In the durvalumab arm, 73 patients (16.5%) had progressive disease, compared with 59 patients (27.7%) in the placebo arm.
The median duration of response was not reached in the durvalumab arm, compared with 13.8 months in the placebo arm.
The PD-L1 inhibitor was also associated with a lower incidence of any new lesion among the intention-to-treat population (20.4% vs. 32.1%).
Grade 3 or 4 toxicities from any cause were slightly higher with durvalumab, at 29.9% vs. 26.1%. Events leading to discontinuation were also higher with the active drug, at 15.4% vs. 9.8% for placebo.
There were 21 deaths (4.4%) of patients treated with durvalumab, and 13 deaths (5.6%) of patients treated with placebo.
“In my opinion, this is a very well-designed study, and the results are very promising,” commented Enriqueta Felip, MD, who was not involved in the PACIFIC trial.
“We need to wait for the overall survival results, but in my opinion this is a very valuable trial in a group of patients [for whom] we need new strategies,” she said.
Dr. Felip was invited to the briefing as an independent commentator.
Results of the interim analysis of the PACIFIC trial were also published online in the New England Journal of Medicine.
The PACIFIC trial was funded by AstraZeneca. Dr. Paz-Ares has received consultancy fees from the company. Dr. Felip disclosed financial relationships with multiple companies not including AstraZeneca.
AT ESMO 2017
Minimally invasive esophagectomy may mean less major morbidity
COLORADO SPRINGS – Minimally invasive esophagectomy was associated with a significantly lower rate of postoperative major morbidity as well as a mean 1-day briefer length of stay than open esophagectomy in a propensity-matched analysis of the real-world American College of Surgeons-National Quality Improvement Program database, Mark F. Berry, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
However, both of the study’s discussants questioned whether the reported modest absolute reduction in major morbidity was really attributable to the minimally invasive approach or could instead have resulted from one of several potential confounders that couldn’t be fully adjusted for, given inherent limitations of the ACS-NSQIP database.
“There was a statistically significant difference in morbidity,” replied Dr. Berry of Stanford (Calif.) University. “It was a 4% absolute difference, which I think is probably clinically meaningful, but certainly it’s not really, really dramatic.”
“What I think we found is that it’s safe to do a minimally invasive esophagectomy and safe for people to introduce it into their practice. But it’s not necessarily something that’s a game changer, unlike what’s been seen with minimally invasive approaches for some other things,” said Dr. Berry, who added that he didn’t wish to overstate the importance of the observed difference in morbidity.
Studies from high-volume centers show that minimally-invasive esophagectomy (MIE) reduces length of stay, postoperative major morbidity, and features equivalent or even slightly lower mortality than traditional open esophagectomy, the generalizability of these findings beyond such centers is questionable. That’s why Dr. Berry and his coinvestigators turned to the ACS-NSQIP database, which includes all esophagectomies performed for esophageal cancer at roughly 700 U.S. hospitals, not just those done by board-certified thoracic surgeons.
He presented a retrospective cohort study of 3,901 esophagectomy patients during 2005-2013 who met study criteria, 16.4% of whom had MIE. The use of this approach increased steadily from 6.5% of all esophagectomies in 2005 to 22.3% in 2013. A propensity-matched analysis designed to neutralize potentially confounding differences included 638 MIE and 1,914 open esophagectomy patients.
The primary outcome was the 30-day rate of composite major morbidity in the realms of various wound, respiratory, renal, and cardiovascular complications. The rate was 36.1% in the MIE group and 40.5% with open esophagectomy in the propensity-matched analysis, an absolute risk reduction of 4.4% and a relative risk reduction of 17%. Although rates were consistently slightly lower in each of the categories of major morbidity, those individual differences didn’t achieve statistical significance. The difference in major morbidity became significant only when major morbidity was considered as a whole.
Mean length of stay was 9 days with MIE and 10 days with open surgery.
There was no significant difference between the two study groups in 30-day rates of readmission, reoperation, or mortality.
Discussant Donald E. Low said “esophagectomy is being analysed regarding its place in all sorts of presentations, stages, and situations, so the aspect of making sure that we’re delivering the services as efficiently as possible is going to become more important, not less important.”
That being said, he noted that there is no specific CPT code for MIE. That raises the possibility of an uncertain amount of procedural misclassification in the ACS-NSQIP database.
Also, the only significant difference in major morbidity between the two study groups was in the subcategory of intra- or postoperative bleeding requiring transfusion, which occurred in 10.8% of the MIE and 16.7% of the open esophagectomy groups, observed Dr. Low, director of the Esophageal Center of Excellence at Virginia Mason Medical Center, Seattle.
“Some of us believe that blood utilization and transfusion requirement is really a quality measure and not a complication,” the surgeon said. And if that outcome is excluded from consideration, then there is no significant difference in major morbidity.
Discussant Douglas E. Wood, MD, professor and chair of the department of surgery at the University of Washington, Seattle, took the opportunity to share a self-described “pet peeve” about analyses of national surgical databases: these databases typically don’t contain key details necessary to correct for provider and hospital characteristics.
“The small differences that you demonstrate could easily have been completely driven by providers who choose to do minimally invasive esophagectomy and are in higher-volume, more specialized centers,” he said. “I’m not convinced of your conclusion that MIE produces less morbidity based on a 4% difference and no analysis of provider characteristics.”
COLORADO SPRINGS – Minimally invasive esophagectomy was associated with a significantly lower rate of postoperative major morbidity as well as a mean 1-day briefer length of stay than open esophagectomy in a propensity-matched analysis of the real-world American College of Surgeons-National Quality Improvement Program database, Mark F. Berry, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
However, both of the study’s discussants questioned whether the reported modest absolute reduction in major morbidity was really attributable to the minimally invasive approach or could instead have resulted from one of several potential confounders that couldn’t be fully adjusted for, given inherent limitations of the ACS-NSQIP database.
“There was a statistically significant difference in morbidity,” replied Dr. Berry of Stanford (Calif.) University. “It was a 4% absolute difference, which I think is probably clinically meaningful, but certainly it’s not really, really dramatic.”
“What I think we found is that it’s safe to do a minimally invasive esophagectomy and safe for people to introduce it into their practice. But it’s not necessarily something that’s a game changer, unlike what’s been seen with minimally invasive approaches for some other things,” said Dr. Berry, who added that he didn’t wish to overstate the importance of the observed difference in morbidity.
Studies from high-volume centers show that minimally-invasive esophagectomy (MIE) reduces length of stay, postoperative major morbidity, and features equivalent or even slightly lower mortality than traditional open esophagectomy, the generalizability of these findings beyond such centers is questionable. That’s why Dr. Berry and his coinvestigators turned to the ACS-NSQIP database, which includes all esophagectomies performed for esophageal cancer at roughly 700 U.S. hospitals, not just those done by board-certified thoracic surgeons.
He presented a retrospective cohort study of 3,901 esophagectomy patients during 2005-2013 who met study criteria, 16.4% of whom had MIE. The use of this approach increased steadily from 6.5% of all esophagectomies in 2005 to 22.3% in 2013. A propensity-matched analysis designed to neutralize potentially confounding differences included 638 MIE and 1,914 open esophagectomy patients.
The primary outcome was the 30-day rate of composite major morbidity in the realms of various wound, respiratory, renal, and cardiovascular complications. The rate was 36.1% in the MIE group and 40.5% with open esophagectomy in the propensity-matched analysis, an absolute risk reduction of 4.4% and a relative risk reduction of 17%. Although rates were consistently slightly lower in each of the categories of major morbidity, those individual differences didn’t achieve statistical significance. The difference in major morbidity became significant only when major morbidity was considered as a whole.
Mean length of stay was 9 days with MIE and 10 days with open surgery.
There was no significant difference between the two study groups in 30-day rates of readmission, reoperation, or mortality.
Discussant Donald E. Low said “esophagectomy is being analysed regarding its place in all sorts of presentations, stages, and situations, so the aspect of making sure that we’re delivering the services as efficiently as possible is going to become more important, not less important.”
That being said, he noted that there is no specific CPT code for MIE. That raises the possibility of an uncertain amount of procedural misclassification in the ACS-NSQIP database.
Also, the only significant difference in major morbidity between the two study groups was in the subcategory of intra- or postoperative bleeding requiring transfusion, which occurred in 10.8% of the MIE and 16.7% of the open esophagectomy groups, observed Dr. Low, director of the Esophageal Center of Excellence at Virginia Mason Medical Center, Seattle.
“Some of us believe that blood utilization and transfusion requirement is really a quality measure and not a complication,” the surgeon said. And if that outcome is excluded from consideration, then there is no significant difference in major morbidity.
Discussant Douglas E. Wood, MD, professor and chair of the department of surgery at the University of Washington, Seattle, took the opportunity to share a self-described “pet peeve” about analyses of national surgical databases: these databases typically don’t contain key details necessary to correct for provider and hospital characteristics.
“The small differences that you demonstrate could easily have been completely driven by providers who choose to do minimally invasive esophagectomy and are in higher-volume, more specialized centers,” he said. “I’m not convinced of your conclusion that MIE produces less morbidity based on a 4% difference and no analysis of provider characteristics.”
COLORADO SPRINGS – Minimally invasive esophagectomy was associated with a significantly lower rate of postoperative major morbidity as well as a mean 1-day briefer length of stay than open esophagectomy in a propensity-matched analysis of the real-world American College of Surgeons-National Quality Improvement Program database, Mark F. Berry, MD, reported at the annual meeting of the Western Thoracic Surgical Association.
However, both of the study’s discussants questioned whether the reported modest absolute reduction in major morbidity was really attributable to the minimally invasive approach or could instead have resulted from one of several potential confounders that couldn’t be fully adjusted for, given inherent limitations of the ACS-NSQIP database.
“There was a statistically significant difference in morbidity,” replied Dr. Berry of Stanford (Calif.) University. “It was a 4% absolute difference, which I think is probably clinically meaningful, but certainly it’s not really, really dramatic.”
“What I think we found is that it’s safe to do a minimally invasive esophagectomy and safe for people to introduce it into their practice. But it’s not necessarily something that’s a game changer, unlike what’s been seen with minimally invasive approaches for some other things,” said Dr. Berry, who added that he didn’t wish to overstate the importance of the observed difference in morbidity.
Studies from high-volume centers show that minimally-invasive esophagectomy (MIE) reduces length of stay, postoperative major morbidity, and features equivalent or even slightly lower mortality than traditional open esophagectomy, the generalizability of these findings beyond such centers is questionable. That’s why Dr. Berry and his coinvestigators turned to the ACS-NSQIP database, which includes all esophagectomies performed for esophageal cancer at roughly 700 U.S. hospitals, not just those done by board-certified thoracic surgeons.
He presented a retrospective cohort study of 3,901 esophagectomy patients during 2005-2013 who met study criteria, 16.4% of whom had MIE. The use of this approach increased steadily from 6.5% of all esophagectomies in 2005 to 22.3% in 2013. A propensity-matched analysis designed to neutralize potentially confounding differences included 638 MIE and 1,914 open esophagectomy patients.
The primary outcome was the 30-day rate of composite major morbidity in the realms of various wound, respiratory, renal, and cardiovascular complications. The rate was 36.1% in the MIE group and 40.5% with open esophagectomy in the propensity-matched analysis, an absolute risk reduction of 4.4% and a relative risk reduction of 17%. Although rates were consistently slightly lower in each of the categories of major morbidity, those individual differences didn’t achieve statistical significance. The difference in major morbidity became significant only when major morbidity was considered as a whole.
Mean length of stay was 9 days with MIE and 10 days with open surgery.
There was no significant difference between the two study groups in 30-day rates of readmission, reoperation, or mortality.
Discussant Donald E. Low said “esophagectomy is being analysed regarding its place in all sorts of presentations, stages, and situations, so the aspect of making sure that we’re delivering the services as efficiently as possible is going to become more important, not less important.”
That being said, he noted that there is no specific CPT code for MIE. That raises the possibility of an uncertain amount of procedural misclassification in the ACS-NSQIP database.
Also, the only significant difference in major morbidity between the two study groups was in the subcategory of intra- or postoperative bleeding requiring transfusion, which occurred in 10.8% of the MIE and 16.7% of the open esophagectomy groups, observed Dr. Low, director of the Esophageal Center of Excellence at Virginia Mason Medical Center, Seattle.
“Some of us believe that blood utilization and transfusion requirement is really a quality measure and not a complication,” the surgeon said. And if that outcome is excluded from consideration, then there is no significant difference in major morbidity.
Discussant Douglas E. Wood, MD, professor and chair of the department of surgery at the University of Washington, Seattle, took the opportunity to share a self-described “pet peeve” about analyses of national surgical databases: these databases typically don’t contain key details necessary to correct for provider and hospital characteristics.
“The small differences that you demonstrate could easily have been completely driven by providers who choose to do minimally invasive esophagectomy and are in higher-volume, more specialized centers,” he said. “I’m not convinced of your conclusion that MIE produces less morbidity based on a 4% difference and no analysis of provider characteristics.”
AT WTSA 2017
Key clinical point:
Major finding: The 30-day rate of major morbidity was 36.1% in patients who underwent minimally invasive esophagectomy, significantly lower than the 40.5% rate with open esophagectomy in a propensity-matched analysis.
Data source: This retrospective cohort study included 3,901 patients who underwent esophagectomy for esophageal cancer as recorded in the American College of Surgeons-National Quality Improvement Program database for 2005-2013.
Disclosures: The study presenter reported having no financial conflicts of interest.

